Notes
Article history paragraph text
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 10/11/01. The protocol was agreed in November 2010. The assessment report began editorial review in June 2011 and was accepted for publication in March 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors'report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Note
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed commercial-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence removed and replaced by the statement ‘commercial-in-confidence information removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Hoyle et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Abstract
Background
Colorectal cancer is the third most commonly diagnosed cancer in the UK after breast and lung cancer. People with metastatic disease who are sufficiently fit are usually treated with active chemotherapy as first- or second-line therapy. Recently, targeted agents have become available including anti-epidermal growth factor receptor (EGFR) agents, for example cetuximab and panitumumab, and anti-vascular endothelial growth factor (VEGF) receptor agents, for example bevacizumab.
Objective
To investigate the clinical effectiveness and cost-effectiveness of panitumumab monotherapy and cetuximab (mono- or combination chemotherapy) for Kirsten rat sarcoma (KRAS) wild-type (WT) patients, and bevacizumab in combination with non-oxaliplatin chemotherapy, for the treatment of metastatic colorectal cancer after first-line chemotherapy.
Data sources
The assessment comprises a systematic review of clinical effectiveness and cost-effectiveness studies, a review and critique of manufacturer submissions and a de novo cohort-based economic analysis. For the assessment of effectiveness, a literature search was conducted in a range of electronic databases, including MEDLINE, EMBASE and The Cochrane Library, from 2005 to November 2010.
Review methods
Studies were included if they were randomised controlled trials (RCTs) or systematic reviews of RCTs of cetuximab, bevacizumab or panitumumab in participants with EGFR-expressing metastatic colorectal cancer with KRAS WT status that has progressed after first-line chemotherapy (for cetuximab and panitumumab) or participants with metastatic colorectal cancer that has progressed after first-line chemotherapy (bevacizumab). All steps in the review were performed by one reviewer and checked independently by a second. Synthesis was mainly narrative. An economic model was developed focusing on third-line and subsequent lines of treatment. Costs and benefits were discounted at 3.5% per annum. Probabilistic and univariate deterministic sensitivity analyses were performed.
Results
The searches identified 7745 titles and abstracts. Two clinical trials (reported in 12 papers) were included. No data were available for bevacizumab in combination with non-oxaliplatin-based chemotherapy in previously treated patients. Neither of the included studies had KRAS status performed prospectively, but the studies did report retrospective analyses of the results for the KRAS WT subgroups. Third-line treatment with cetuximab plus best supportive care or panitumumab plus best supportive care appears to have statistically significant advantages over treatment with best supportive care alone in patients with KRAS WT status. For the economic evaluation, five studies met the inclusion criteria. The base-case incremental cost-effectiveness ratio (ICER) for KRAS WT patients for cetuximab compared with best supportive care is £98,000 per quality-adjusted life-year (QALY), for panitumumab compared with best supportive care is £150,000 per QALY and for cetuximab plus irinotecan compared with best supportive care is £88,000 per QALY. All ICERs are sensitive to treatment duration.
Limitations
In the specific populations of interest, there is a lack of evidence on bevacizumab, cetuximab and cetuximab plus irinotecan used second line and on bevacizumab and cetuximab plus irinotecan used third line. For cetuximab plus irinotecan treatment for KRAS WT people, there is no direct evidence on progression-free survival, overall survival and duration of treatment.
Conclusions
Although cetuximab and panitumumab appear to be clinically beneficial for KRAS WT patients compared with best supportive care, they are likely to represent poor value for money when judged by cost-effectiveness criteria currently used in the UK. It would be useful to conduct a RCT for patients with KRAS WT status receiving cetuximab plus irinotecan.
Funding
The National Institute for Health Research Health Technology Assessment programme.
Chapter 1 Background
Description of health problem
Colorectal cancer is a malignant neoplasm arising from the lining of the large intestine (colon and rectum). Over 95% of colon and rectal cancers are adenocarcinomas, cancers that start in the cells that line the inside of the colon and rectum. Cancer cells eventually spread to nearby lymph nodes (local metastases) and subsequently to more remote lymph nodes and other organs in the body. The liver and the lungs are common metastatic sites of colorectal cancer.
Vascular endothelial growth factor and epidermal growth factor receptor
Two key elements in the growth and dissemination of tumours are the vascular endothelial growth factor (VEGF) and the epidermal growth factor receptor (EGFR); both pathways are closely related, sharing common downstream signalling pathways. 1 VEGF and EGFR play important roles in tumour growth and progression through the exertion of both indirect and direct effects on tumour cells. 1 Biological agents targeting the VEGF and EGFR pathways have shown clinical benefit in several human cancers, either alone or in combination with standard cytotoxic therapies. Inhibition of VEGF-related pathways is thought to contribute to the mechanism of action of agents targeting the EGFR. 2 Conversely, (over)activation of VEGF expression independent of EGFR signalling is thought to be one way that tumours become resistant to anti-EGFR therapy. 3 Specific ongoing point mutations in the EGFR gene are also thought to convey resistance to anti-EGFR tyrosine kinase inhibitors. 4 The possibility that combined VEGF and EGFR pathway blockade could further enhance antitumour efficacy and help prevent resistance to therapy is currently being evaluated in clinical trials. 1
Kirsten rat sarcoma
Kirsten rat sarcoma (KRAS) is a gene that codes for a protein that plays an important role in the EGFR pathway, a complex signalling cascade that is involved in the development and progression of cancer.
The KRAS protein regulates other proteins, downstream in the EGFR signalling pathway, that are associated with tumour survival, angiogenesis, proliferation and metastasis. 5 Thereare different types of the KRAS gene found in tumours that either code for a ‘normal’, non-mutated KRAS protein known as KRAS wild type (WT), or an abnormal, mutated protein known as mutant KRAS. The KRAS ‘status’ (KRAS WT vs KRAS mutant) may be indicative of prognosis and predictive of response to certain drugs including those under consideration in this review. In tumours with KRAS WT status, the protein is only temporarily activated in response to certain stimuli such as EGFR signalling. This tight regulation warrants a close control of downstream effects. In tumours with the mutated version of the KRAS gene, the KRAS protein is permanently ‘turned on’ even without being activated by the upstream EGFR-mediated signalling. As a result the downstream effects that lead to tumour growth and spread continue unregulated.
The KRAS test is performed on a sample of tumour tissue that is sent to a laboratory for analysis of the KRAS mutation status – WT or mutant. The process helps to enable the most effective treatment to be selected for the individual patient. There are multiple methods for determining the KRAS mutation status of a tumour (Table 1);6 all appear to have adequate clinical sensitivity to detect patients unlikely to respond to cetuximab or panitumumab. The limitation of sequencing technologies is the requirement of > 5–10% mutant alleles for pyrosequencing and > 20% for Sanger sequencing, although newer approaches are being developed to increase the sensitivity of sequencing methods. 6
| Method | Sensitivity of mutant alleles (%) | Strengths | Weaknesses |
|---|---|---|---|
| Sanger sequencing | 20 |
|
|
| Pyrosequencing | 5–10 |
|
|
| Allele-specific real-time PCR | 1 |
|
|
| Post-PCR fluorescent melting curve analysis with specific probes | 5–10 |
|
|
| PCR clamping method | 1 |
|
|
In colorectal cancer, up to 65% of patients are KRAS WT status; the remaining 35% are KRAS mutant. 7
Epidemiology
Incidence and prevalence
Colorectal cancer is a common form of malignancy in developed countries but occurs much less frequently in the developing world. It is the third most commonly diagnosed cancer in the UK, with around 39,991 new cases registered in the UK in 2008 (32,644 cases registered in England and Wales). 8 The number of cases of colorectal cancer and the incidence rates in England and Wales are shown in Table 2.
| England | Wales | ||
|---|---|---|---|
| Men | Cases | 18,040 | 1311 |
| Crude rate per 100,000 | 71.2 | 89.8 | |
| ASR per 100,000 population (95% CI) | 57.0 (56.2 to 57.8) | 64.4 (60.9 to 67.9) | |
| Women | Cases | 14,604 | 89.8 |
| Crude rate per 100,000 | 55.9 | 64.6 | |
| ASR per 100,000 population (95% CI) | 36.9 (36.3 to 37.5) | 39.3 (36.9 to 41.8) | |
| Total | Cases | 32,644 | 2300 |
| Crude rate per 100,000 | 63.4 | 76.9 | |
| ASR per 100,000 population (95% CI) | 46.1 (45.6 to 46.6) | 50.7 (48.6 to 52.8) |
Data for colorectal cancer patients diagnosed in England in 2000–4 did show a deprivation gradient for male patients with incidence rates 11% higher in the most deprived groups than in the affluent groups. 8
The occurrence of colorectal cancer is strongly related to age, with 86% of cases arising in people aged 60+ years. 8 Until age 50, men and women have similar rates for colorectal cancer, but in later life the incidence rate for men is higher. In numerical terms there are more cases of colorectal cancer in men among almost all age groups up to the age of 84, after which cases of colorectal cancer in women are in the majority, even though their rates are lower, as women make up a larger proportion of the elderly population. 8 Overall, the male-to-female ratio is 11 : 10. 8 The lifetime risk of being diagnosed with colorectal cancer in the UK is estimated to be 1 in 16 for men and 1 in 20 for women. 8
Pathology
Colorectal cancer includes malignant growths from the mucosa of the colon and rectum. Cancer cells eventually spread to nearby lymph nodes (local metastases) and subsequently to more remote lymph nodes and other organs in the body. The liver and the lungs are common metastatic sites of colorectal cancer.
The pathology of the tumour is usually determined by analysis of tissue taken from a biopsy or surgery. Colorectal cancer stage can be described using the modified Dukes'staging system (based on postoperative findings – a pathological staging based on resection of the tumour and measuring the depth of invasion through the mucosa and bowel wall) or the more precise TNM staging system, which is based on the depth of tumour invasion (T), nodal involvement (N) and metastatic spread (M) assessed preoperatively by radiological examination (Table 3). 9
| Staging group | TNM staging and sites involved | Modified Dukes' stage |
|---|---|---|
| Stage 0 | Carcinoma in situ (Tis, N0, M0) | |
| Stage I | No nodal involvement, no distant metastases | A |
| Tumour invades submucosa (T1, N0, M0) | ||
| Tumour invades muscularis propria (T2, N0, M0) | ||
| Stage II | No nodal involvement, no distant metastases | B |
| Tumour invades muscularis propria into pericolorectal tissues (T3, N0, M0) | ||
| Tumour penetrates surface of visceral peritoneum or directly invades or is adherent to other organs or structures (T4a/b, N0, M0) | ||
| Stage III | Nodal involvement, no distant metastases (any T, any N, M0) | C |
| Stage IV | Distant metastases (any T, any N, M1a/M1b) | D |
Knowing the stage of colon cancer is important for several reasons, including helping the physician to define an appropriate treatment plan and in predicting prognosis. In the UK, approximately 11% of patients are diagnosed at TNM stage I, 32% at stage II, 26% at stage III (lymph node involvement) and 30% at stage IV (metastatic disease). It is estimated that around 30% of patients present with metastatic disease and a further 20% may eventually develop metastatic disease. 10 Metastatic disease often develops first in the liver but metastases may also occur at other sites, including the lungs. 10
Prognosis
The treatment, prognosis and survival rate depend on the stage of disease at diagnosis.
The 5-year relative survival rates for both men and women with colorectal cancer have doubled between the early 1970s and the mid-2000s. 11 Five-year survival rates for men with colorectal cancer rose from 25% in the early 1970s to 51% in the mid-2000s and from 27% to 55% for men with colon cancer. 11 These improvements are a result of earlier diagnosis and better treatment but there is still much scope for further progress. 11 Ten-year survival rates are only a little lower than those at 5 years indicating that most patients who survive for 5 years are cured from this disease. 11
Patients who are diagnosed at an early stage have a much better prognosis than those who present with more extensive disease. 11 Over 93% of patients diagnosed with stage A on the modified Dukes'classification system (the earliest stage of the disease) survived for 5 years compared with < 7% of patients with advanced disease (stage D) (Table 4). 11
| Modified Dukes' stage at diagnosis | Percentage of cases | 5-year relative survival (%)a |
|---|---|---|
| A | 9 | 93 |
| B | 24 | 77 |
| C | 24 | 48 |
| D | 9 | 7 |
| Unknown | 34 | 35 |
Treatment of colorectal cancer may be curative or palliative depending on the location of the tumour and the degree to which the tumour has penetrated the bowel and spread to other organs in the body. Treatment options differ considerably for colon and rectal tumours. Recurrence of colorectal cancer may be local or metastatic; however, local recurrence is less commonly reported in patients with colon cancer. Treatments of metastatic recurrence of colorectal cancer are typically palliative; however, hepatic resection and pulmonary resection may offer a chance of cure in a small proportion of patients. The mainstay of treatment for metastatic colorectal cancer involves chemotherapy; cytotoxic agents include 5-fluorouracil (5-FU), capecitabine, oxaliplatin, irinotecan, tegafur with uracil, and mitomycin. Again, these may be given according to a variety of regimens across different lines of therapy.
Impact of the health problem
Significance for patients in terms of ill-health (burden of disease)
Colorectal cancer is a significant cause of morbidity and mortality. When treating patients with metastatic colorectal cancer, the main aims of treatment are to relieve symptoms and to improve health-related quality of life (HRQoL) and survival. 12 In 2008 there were 14,233 deaths from colorectal cancer in England and Wales. The majority of deaths occurred in older people: around 80% in people aged 65+ years and almost 40% in those aged 80+ years (Table 5). 12
| England | Wales | |
|---|---|---|
| Deaths | ||
| Men | 7178 | 499 |
| Women | 6138 | 418 |
| Total | 13,316 | 917 |
| Crude rate per 100,000 population | ||
| Men | 28.4 | 34.1 |
| Women | 23.5 | 27.3 |
| Total | 25.9 | 30.6 |
| ASR (European) per 100,000 population (95% CI) | ||
| Men | 21.8 (21.3 to 22.3) | 23.6 (21.5 to 25.6) |
| Women | 13.6 (13.3 to 14.0) | 14.6 (13.2 to 16.0) |
| Total | 17.3 (17.0 to 17.6) | 18.6 (17.4 to 19.8) |
Quality of life
Assessment of HRQoL has become an important feature of cancer trials, enabling evaluation of treatment effectiveness from the perspective of the person with the condition and facilitating improved clinical decision-making.
There are several general HRQoL instruments for people with cancer that can be used to assess quality of life (QoL) both in research studies and in clinical practice, for example the National Comprehensive Cancer Network Functional Assessment of Cancer Therapy Colorectal Cancer Symptom Index (NCCN FCSI) and the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ-C30).
Significance for the NHS
Current service provision
National guidelines
The National Institute for Health and Clinical Excellence (NICE) has issued the following guidance:
-
Technology appraisal (TA) No. 93 – Irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: review of technology appraisal No. 3314
-
TA61– Guidance on the use of capecitabine and tegafur with uracil for metastatic colorectal cancer15
-
TA118 – Bevacizumab and cetuximab for the treatment of metastatic colorectal cancer16
-
TA176 – Cetuximab for the first-line treatment of metastatic colorectal cancer17
-
TA105 – Laparoscopic surgery for colorectal cancer: review of NICE technology appraisal No. 11718
-
TA212 – Bevacizumab in combination with oxaliplatin and either fluorouracil plus folinic acid or capecitabine for the treatment of metastatic colorectal cancer19
-
Guidance for Commissioning Cancer Services: Improving Outcomes in Colorectal Cancers: Research Evidence for the Manual Update 13
-
Clinical Guideline 131– Colorectal cancer: The diagnosis and management of colorectal cancer20
In addition, the Association of Coloproctology of Great Britain and Ireland (ACPGBI) has published guidelines for the management of colorectal cancer. 21
Current management
The treatment and prognosis for colorectal cancer depend on the stage of the cancer. For early cancer, treatment may consist of surgery alone. 10 Surgery to remove the primary tumour is the principal first-line treatment for approximately 80% of patients, after which about 40% will remain disease free in the long term. 20 In 20–30% of cases the disease is too far advanced at initial presentation for any attempt at curative intervention; many of these patients die within a few months. 20
The most frequent site of metastases is the liver. In as many as 50% of patients with advanced disease the liver may be the only site of spread, and for these patients surgical resection may be the only chance of a cure. 10,20 Reported 5-year survival rates for resection of liver metastases range from 16% to 48%, which is considerably better than those for systemic chemotherapy. 10
Individuals with metastatic disease who are sufficiently fit (normally those with World Health Organization performance status ≤ 2) are usually treated with active chemotherapy as first- or second-line therapy. 20 The backbone to treatment across all lines of therapy is composed of fluoropyrimidine, irinotecan or oxaliplatin. More recently, targeted agents have become available, including anti-EGFR agents, for example cetuximab and panitumumab, and anti-VEGF agents, for example bevacizumab (see National guidelines).
Based on a literature review and elicitation of expert opinion, Shabaruddin and colleagues22 identified predominant treatment pathways within NHS colorectal cancer specialties as first-line treatment with oxaliplatin-based regimens, second-line treatment with irinotecan-based regimens and third-line treatment with mitomycin-based regimens. Current evidence indicates that the use of 5-FU, oxaliplatin and irinotecan at any sequence within a patient's care pathway has survival advantages. 23
A treatment algorithm for colorectal cancer in England and Wales developed by Tappenden and colleagues10 (TA11816) estimates that up to 85% of patients with advanced metastatic colorectal cancer not amenable to resection receive active first-line therapy. Of these, approximately 50% go on to receive second-line therapy with 5% of those estimated to go on to receive third-line therapy. This treatment algorithm showed that roughly 300 patients receive a third-line chemotherapy in England and Wales. However, there is a paucity of data based on modern clinical oncology practice documenting the proportions of patients on the various pathways of disease once metastatic colorectal cancer has occurred, and this is often estimated via expert advisory groups.
First-line treatment
First-line active chemotherapy options include oxaliplatin plus infusional 5-FU/folinic acid (FA) (FOLFOX) and irinotecan plus infusional 5-FU/FA (FOLFIRI) (TA93). 14 Additionally, TA93 did not recommend raltitrexed for those with advanced colorectal cancer unless they were taking part in a clinical trial. Oral analogues of 5-FU (capecitabine and tegafur with uracil) may also be used instead of infusional 5-FU (TA61). 15
Cetuximab in combination with FOLFOX or in combination with FOLFIRI is also recommended by NICE as an option for the first-line treatment of metastatic colorectal cancer when the metastatic disease is confined to the liver and the aim of treatment is to render the metastases resectable (TA176). 17
In 2009 NICE did not recommend bevacizumab in combination with oxaliplatin and either 5-FU/FA or capecitabine for those with metastatic colorectal cancer. 16
Second-line treatment
For those patients first receiving FOLFOX, irinotecan may be a second-line treatment option, whereas for patients first receiving FOLFIRI, oxaliplatin may be a second-line treatment option (TA93). 14 Patients receiving 5-FU/FA or an oral analogue as first-line treatment may be offered FOLFOX or FOLFIRI as second-line and subsequent therapies.
Technology appraisal No. 118 did not recommend cetuximab in combination with irinotecan for the treatment of those with metastatic colorectal cancer previously treated with irinotecan. 16
Third-line treatment
In the third-line setting the majority of patients will receive best supportive care.
Description of technologies under assessment
Bevacizumab (Avastin®, Roche)
Bevacizumab is a recombinant humanised monoclonal antibody that acts as an angiogenesis inhibitor. It targets the biological activity of human VEGF, which stimulates new blood vessel formation in the tumour. 24 Depriving tumours of VEGF has several effects that are relevant to the therapeutic use of bevacizumab. These include preventing the development of new tumour blood vessels, causing the regression of existing vasculature and normalising the function of the remaining tumour blood vessels resulting in enhanced delivery of concomitantly administered cytotoxic drugs. 25
Bevacizumab is licensed in combination with 5-fluoropyrimidine-based chemotherapy and is indicated for treatment of patients with metastatic colorectal cancer. 24 The original European Medicines Agency (EMA) marketing authorisation for bevacizumab in metastatic colorectal cancer restricted it to use in the first-line setting in combination with 5-FU-based chemotherapy with or without irinotecan, based on the Phase III trial data then available. The EMA granted a broader marketing authorisation in 2010 licensing bevacizumab in combination with fluoropyrimidine-based chemotherapy for treatment of patients with metastatic cancer of the colon and rectum. The extension to the marketing authorisation followed publication of further studies showing that bevacizumab added to combinations of 5-FU or capecitabine in the first- or second-line setting also improved treatment outcomes. 26,27
Bevacizumab is contraindicated in patients who are pregnant or who have hypersensitivity to products derived from Chinese hamster ovary cell cultures or other recombinant human or humanised antibodies. Special warnings and precautions for use include gastrointestinal perforations, wound healing complications, hypertension, proteinuria, arterial thromboembolism, haemorrhage and congestive heart failure/cardiomyopathy. 24
The most common adverse events with bevacizumab (incidence > 10% and at least twice the control arm rate) are epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, rectal haemorrhage, lacrimation disorder and exfoliative dermatitis.
Bevacizumab must be administered under the supervision of a clinician experienced in the use of antineoplastic medicinal products. 24 It is administered over 90 minutes as an intravenous infusion at a dose of 5 mg/kg body weight once every 14 days, and is recommended until there is underlying disease progression. 24
Cetuximab (Erbitux®, Merck Serono Pharmaceuticals)
Cetuximab is a recombinant monoclonal antibody that blocks the human EGFR. EGFR is found on the surface of some cells and plays a role in regulating cell growth. Cetuximab is believed to interfere with the growth of cancer cells by binding to EGFR so that the normal epidermal growth factors cannot bind and stimulate the cells to grow.
Cetuximab, used in combination with irinotecan, is indicated for the treatment of EGFR-expressing KRAS WT metastatic colorectal cancer either in combination with chemotherapy or as a single agent in patients who have failed oxaliplatin- and irinotecan-based therapy and who are intolerant to irinotecan. 28 The Summary of Product Characteristics recommends that cetuximab must be administered under the supervision of a physician experienced in the use of antineoplastic medicinal products. Close monitoring is required during the infusion and for at least 1 hour after the end of the infusion in a setting with resuscitation equipment and other agents necessary to treat anaphylaxis. Patients requiring treatment should be monitored for longer. 28
Special warnings and precautions for use include hypersensitivity reactions, dyspnoea and skin reactions. Only patients with adequate renal and hepatic function have been investigated to date (serum creatinine ≤ 1.5-fold, transaminases ≤ 5-fold and bilirubin ≤ 1.5-fold the upper limit of normal). 28 Cetuximab has not been studied in patients presenting with one or more of the following laboratory parameters:28
-
haemoglobin < 9 g/dl
-
leucocyte count < 3000/mm3
-
absolute neutrophil count < 1500/mm3
-
platelet count < 100,000/mm3.
The most common adverse events with cetuximab (incidence ≥ 25%) are cutaneous adverse reactions (including rash pruritus and nail changes), headache, diarrhoea and infection.
The recommended initial dose, either as monotherapy or in combination with irinotecan, is 400 mg/m2 administered as a 120-minute intravenous infusion (maximum infusion rate 10 mg/ minute). The recommended subsequent weekly dose, either as monotherapy or in combination with irinotecan, is 250 mg/m2 infused over 60 minutes (maximum infusion rate 10 mg/minute) until disease progression or unacceptable toxicity.
There is limited experience in the use of cetuximab in combination with radiation therapy in colorectal cancer. 28
Panitumumab (Vectibix®, Amgen)
Panitumumab is a recombinant monoclonal antibody that targets the EGFR receptor, thereby inhibiting the growth of EGFR-expressing tumours. Panitumumab is licensed as monotherapy for treating patients with EGFR-expressing metastatic colorectal cancer with KRAS WT status after failure of previous chemotherapy regimens containing fluoropyrimidine, irinotecan and oxaliplatin.
Panitumumab treatment should be supervised by a physician experienced in the use of anticancer therapy. 29 The recommended dose of panitumumab is 6 mg/kg of body weight given once every 2 weeks. 29 Before infusion, panitumumab should be diluted in 0.9% sodium chloride injection to a final concentration not to exceed 10 mg/ml. 29
Panitumumab is contraindicated in patients with a history of severe or life-threatening hypersensitivity reactions to the active substance or to any of the excipients. 29 The most common adverse events (incidence ≥ 20%) are skin toxicities (i.e. erythema, dermatitis acneiform, pruritus, exfoliation, rash and fissures), paronychia, hypomagnesaemia, fatigue, abdominal pain, nausea, diarrhoea and constipation.
Chapter 2 Definition of the decision problem
Decision problem
The purpose of this report is to assess the clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy. 30
Population including subgroups
The population for this assessment is adults with metastatic colorectal cancer who have failed first-line chemotherapy. This is further restricted to patients with EGFR-expressing metastatic colorectal cancer with KRAS WT status for cetuximab and panitumumab in line with the marketing authorisations for these treatments.
Interventions
This technology assessment report will consider three pharmaceutical interventions:
-
bevacizumab in combination with non-oxaliplatin-based chemotherapy
-
cetuximab monotherapy and in combination with chemotherapy
-
panitumumab monotherapy.
Each should be being used in accordance with the marketing authorisation and in the populations indicated in Population including subgroups.
Relevant comparators
Any clinically relevant alternative treatment for the population in question, but particularly including:
-
irinotecan- or oxaliplatin-based chemotherapy regimens (in the case of second-line treatment)
-
best supportive care (in the case of third-line or later treatment) consisting of pain control, antiemetics, appetite stimulants (steroids) and, in some cases, radiotherapy
-
one of the other interventions under consideration.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
The clinical effectiveness of bevacizumab, cetuximab and panitumumab for metastatic colorectal cancer was assessed by a systematic review of research evidence. The review was undertaken following the principles published by the NHS Centre for Reviews and Dissemination (CRD). 31
Identification of studies
Electronic databases were searched using terms related to the population and the interventions only, without recourse to methodological or outcome filters. The sensitivity here allowed for the multiple requirements of the review.
Appendix 1 shows the search strategies undertaken databases searched; these included MEDLINE, EMBASE (both via Ovid), The Cochrane Library, Web of Science (ISI) (including Conference Proceedings Citation Index) and EconLit (EBSCOhost). ClinicalTrials.gov, Current Controlled Trials, the US Food and Drug Administration (FDA) website and the EMA website were also searched. The search initially used as its basis a previous multiple technology assessment (MTA) by Tappenden and colleagues10 to construct the population aspect of the search. Searches were not limited by language but were limited by date (2005–17 November 2010), as stated in the protocol (see Appendix 2).
Included studies and industry submissions were analysed to ensure the saturation of relevant studies.
All references were exported into EndNote X4 (Thomson Reuters CA, USA) for conversion to RIS format before being uploaded into EPPI Reviewer (version 4, EPPI-Centre, Institute of Education, London, UK) where manual deduplication was performed.
Relevant studies were then identified in two stages. Titles and abstracts returned by the search strategy were examined independently by two researchers (LC and TJH) and screened for possible inclusion. Disagreements were resolved by discussion. Full texts of the identified studies were obtained. Two researchers (LC and TJH) examined these independently for inclusion or exclusion, and disagreements were again resolved by discussion.
Inclusion and exclusion criteria
Study design
Inclusion criteria
For the review of clinical effectiveness, only randomised controlled trials (RCTs) and systematic reviews of RCTs were considered. The review protocol made provision for broadening the search criteria to include some observational evidence if insufficient systematic reviews or RCTs were identified.
Systematic reviews were used as a source for finding further RCTs and to compare with our systematic review. For the purpose of this review, a systematic review was defined as one that has:
-
a focused research question
-
explicit search criteria that are available to review, either in the document or on application
-
explicit inclusion/exclusion criteria, defining the population(s), intervention(s), comparator(s) and outcome(s) of interest
-
a critical appraisal of included studies, including consideration of the internal and external validity of the research
-
a synthesis of the included evidence, whether narrative or quantitative.
The preliminary screening of titles and abstracts did not discriminate according to KRAS status, to ensure that trials were not excluded in error. However, during the full-text screening process it became apparent that no clinical trials existed with prospective analysis of KRAS status. Because this relatively recent understanding of KRAS WT status and intervention efficacy is key to this review, trials that retrospectively analysed outcomes according to this subgroup were included.
Exclusion criteria
Studies were excluded if they did not match the inclusion criteria and in particular were:
-
non-randomised studies (except for adverse events)
-
animal models
-
preclinical and biological studies
-
narrative reviews, editorials, opinions
-
non-English-language papers
-
reports published as meeting abstracts only, in which insufficient methodological details were reported to allow critical appraisal of study quality.
Population
Randomised controlled trials were included for panitumumab and cetuximab if they reported clinical outcomes for an adult population with EGFR-expressing metastatic colorectal cancer with KRAS status assessed that has progressed after first-line chemotherapy. The justification for including only studies in which the population had their KRAS status assessed revolves around recent evidence indicating that anti-EGFR-targeted antibodies, such as cetuximab and panitumumab, are effective only in patients with KRAS WT as opposed to KRAS mutant oncogenes. 32
For bevacizumab, studies were included if the population with metastatic colorectal cancer had progressed after first-line chemotherapy. No stipulation for EGFR expression or KRAS status was required as this has been shown to have no influence on bevacizumab activity. 33
Interventions and comparators
Studies were included if the technologies they assessed fulfilled the following criteria:
-
after first-line therapy with cetuximab as monotherapy or in combination with chemotherapy
-
after first-line therapy with bevacizumab in combination with non-oxaliplatin-based chemotherapy
-
after first-line therapy with panitumumab as monotherapy.
Alternative treatments for the population in question (clinically relevant comparators) were:
-
irinotecan- or oxaliplatin-based chemotherapy regimens
-
one of the interventions under consideration
-
best supportive care.
We have also considered the validity of indirect comparisons between interventions when appropriate.
Outcomes
Studies were included if they reported data on one or more of the following outcomes:
-
overall survival
-
progression-free survival
-
tumour response rate
-
adverse effects of treatment
-
HRQoL
-
liver resection rates.
Data extraction strategy
Data were extracted by one reviewer (TJH) using a standardised data extraction form in Microsoft Access 2007 (Microsoft Corporation, Redmond, WA, USA) and checked by a second reviewer (LC). Disagreements were resolved by discussion, with involvement of a third reviewer if necessary. Data extraction forms for each included study can be found in Appendix 3.
Critical appraisal strategy
The methodological quality of the studies was assessed according to criteria specified by the CRD. 31 Quality was assessed by one reviewer and judgements were checked by a second. Any disagreement was resolved by discussion, with involvement of a third reviewer as necessary. The instrument is summarised below. Results were tabulated and the relevant aspects described in the data extraction forms.
Internal validity
The instrument sought to assess the following:
-
Was the assignment to the treatment groups really random?
-
Was the treatment allocation concealed?
-
Were the groups similar at baseline in terms of prognostic factors?
-
Were the eligibility criteria specified?
-
Were outcome assessors blinded to the treatment allocation?
-
Was the care provider blinded?
-
Was the patient blinded?
-
Were point estimates and a measure of variability presented for the primary outcome measure?
-
Did the analyses include an intention-to-treat (ITT) analysis?
-
Were withdrawals and dropouts completely described?
In addition, methodological notes were made for each included study, with the reviewer's observations on sample size and power calculations, participant attrition, methods of data analysis, and conflicts of interest.
External validity
External validity was judged according to the ability of a reader to consider the applicability of findings to a patient group and service setting. Study findings can be generalisable only if they provide enough information to consider whether or not a cohort is representative of the affected population at large. Therefore, studies that appeared to be typical of the UK metastatic colorectal cancer population with regard to these considerations were judged to be externally valid.
Methods of data synthesis
Details of the extracted data and quality assessment for each individual study are presented in structured tables and as a narrative description. Any possible effects of study quality on the effectiveness data are discussed. Survival data (overall survival and progression-free survival) are presented as hazard ratios where available.
When data on head-to-head comparisons between interventions were not available, we performed adjusted indirect comparisons using an adaption of the method described by Bucher and colleagues. 34 This method aims to overcome the potential problems of a simple direct comparison (i.e. comparison of simple arms of different trials), in which the benefit of randomisation is lost leaving the data subject to the biases associated with observational studies. The method is valid only when the characteristics of patients are similar between the different studies being compared. Further details of the methods used can be found in Appendix 4.
Use of manufacturers'submissions to the National Institute for Health and Clinical Excellence
A description of the search strategy employed in each of the manufacturers'submissions and a comment on whether or not it was appropriate is detailed in Appendix 5. All of the clinical effectiveness data included in the manufacturers'submissions were assessed. When these met the inclusion criteria and had not already been identified from published sources, they were included in the systematic review of clinical effectiveness. However, it became apparent that the manufacturers'submissions were dependent on evidence that did not include KRAS status and would not fulfil the inclusion criteria for this part of the report. Therefore, to maintain consistency, the papers reported in the manufacturers'submissions are briefly critiqued here, with a more detailed discussion in Chapter 5.
Interpreting the results from the clinical trials
Effectiveness
Most of the clinical trials in which the efficacy of these interventions has been evaluated report results in terms of hazard ratios – the ratio of hazard rates in two groups, such as a treatment group and a control group. The hazard rate describes the number of events per unit time per number of people exposed (i.e. the slope of the survival curve, or the instantaneous rate of events in the group). A hazard ratio of ≥ 1 indicates that the event of interest is happening faster in the treatment group, whereas a hazard ratio of ≤ 1 indicates that the event of interest is happening more slowly in the treatment group. A hazard ratio of 1 suggests that there is no difference between the groups.
Adverse drug effects
The National Cancer Institute Common Terminology Criteria (NCI-CTC) (Table 6) are frequently used by trials to report drug toxicities. 35 For each adverse event, grades are assigned using a scale from 0 to 5. Grade 0 is defined as absence of an adverse event or within normal limits for values. Grade 5 is defined as death associated with an adverse event.
| Grade | Description |
|---|---|
| 0 | No adverse event or within normal limits |
| 1 | Mild adverse event |
| 2 | Moderate adverse event |
| 3 | Severe or medically significant adverse event but not immediately life-threatening |
| 4 | Life-threatening or disabling adverse event |
| 5 | Death related to an adverse event |
Results of the clinical effectiveness review
The results of the assessment of clinical effectiveness are presented as follows:
-
An overview of the quantity and quality of available evidence together with a table summarising all included trials (see Table 7) and a summary table of key quality indicators (see Table 9).
-
A critical review of the available evidence for each of the stated research questions (see Study characteristics), covering:
-
the quantity and quality of available evidence
-
a summary table of the study characteristics
-
a summary table of the baseline population characteristics
-
comparison of the baseline populations in the included trials
-
study results presented in narrative and tabular form
-
comparison of the results in terms of effectiveness and safety.
-
-
A summary of evidence for clinical effectiveness used in the manufacturers'submissions. This is included to address the trials used by the manufacturers, none of which meet the inclusion criteria for this systematic review (see Study characteristics).
| Study | Year | Study type | n | Intervention | Comparator | Supplementary publications |
|---|---|---|---|---|---|---|
| CET + BSC vs BSC after first-line therapy | ||||||
| Jonker et al.37 | 2007 | R, O, C, BR, Phase III, international, multicentre | 572 | CET + BSC | BSC | 8, 13, 49–51 |
| PAN + BSC vs BSC after first-line therapy | ||||||
| Van Cutsem et al.7 | 2007 | R, O, C, BR, Phase III, international, multicentre | 463 | PAN + BSC | BSC | 9, 11, 52, 54 |
| PAN after first-line therapy – supplement to main trial (above) | ||||||
| Van Cutsem et al.38 | 2008 | ES, O, single-arm supplement | 176 | PAN | NA | – |
Quantity and quality of research available
Number of studies identified
The electronic searches retrieved a total of 7745 titles and abstracts. No additional papers were found by searching the bibliographies of included studies. A total of 7690 papers were excluded based on screening the title and abstract. The full text of the remaining 55 papers was requested for more in-depth screening, resulting in a total of 13 papers being included in the review. The process of study selection is shown in Figure 1.
FIGURE 1.
Summary of study selection.
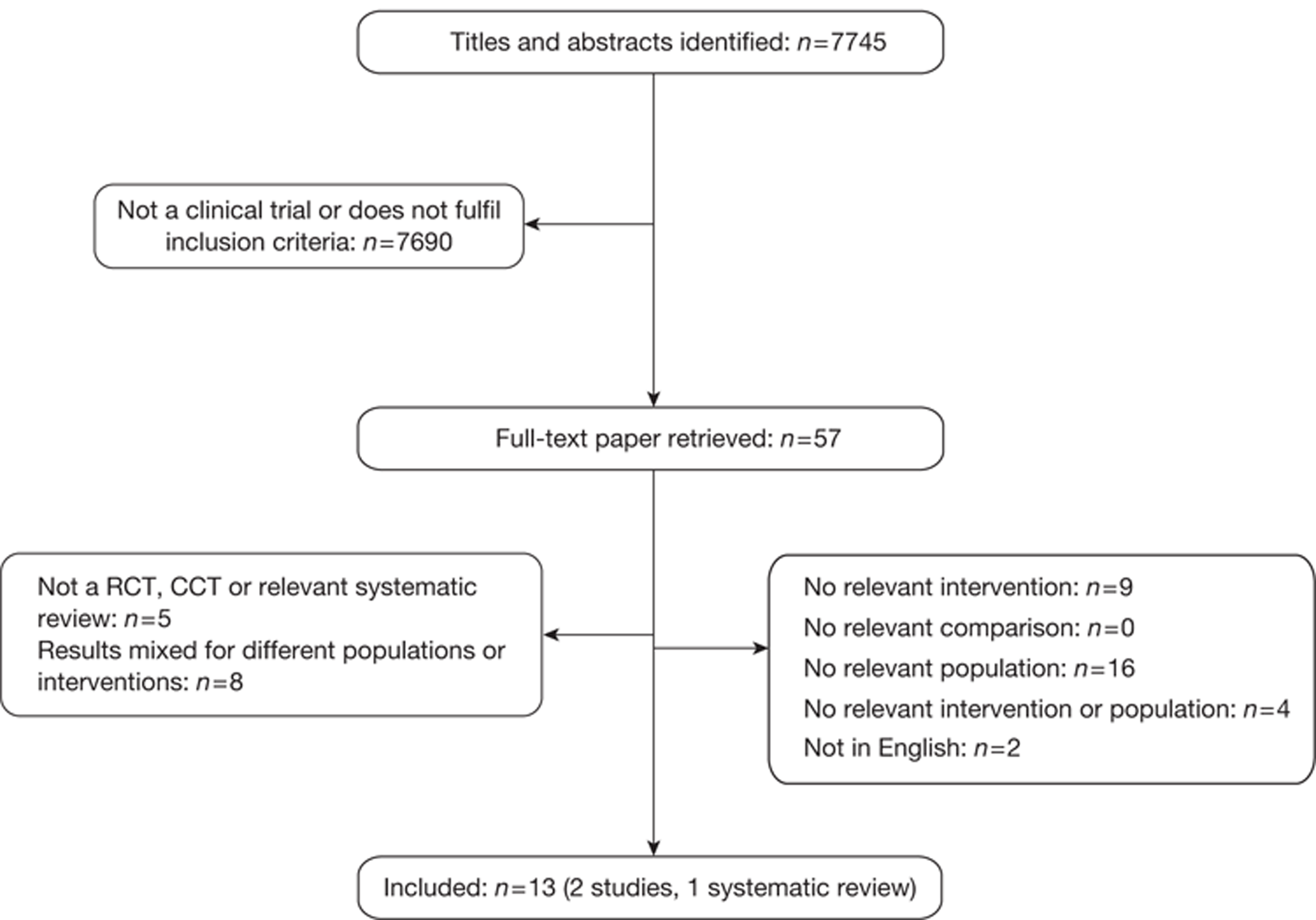
Number of studies excluded
Papers were excluded for at least one of the following reasons: duplicate publication, narrative review, uncontrolled study (when evidence from controlled trials was available for the research question) and publication (systematic reviews and individual studies) not considering the relevant interventions, population, comparisons or outcomes. The bibliographic details of studies retrieved as full papers and subsequently excluded, along with the reasons for their exclusion, are detailed in Appendix 6.
Number and description of included studies
Two main clinical trials and one single-arm extension reported in 12 papers were included in the review for cetuximab plus best supportive care and panitumumab plus best supportive care. One systematic review was also included. 36 Both trials had retrospective KRAS status determination after the study had been completed. All included clinical effectiveness studies are detailed in Table 7. It should be noted that no studies used in the earlier NICE report10 that reviewed bevacizumab and cetuximab for the treatment of metastatic colorectal cancer met the inclusion criteria in this instance, as the included trials for bevacizumab were first line and KRAS status was not established for cetuximab.
We were unable to identify any suitable data on the clinical effectiveness of bevacizumab with non-oxaliplatin-based chemotherapy; however, a clinical trial is currently under way comparing bevacizumab with FOLFIRI against panitumumab with FOLFIRI after first-line treatment (Appendix 7). 39 No data have yet been published.
Study characteristics
Bevacizumab
There is currently no clinical evidence for the effectiveness of bevacizumab with non-oxaliplatin therapy after first-line treatment in metastatic colorectal cancer, although the EMA has granted marketing authorisation for its use in this clinical setting (see Description of technologies under assessment, Bevacizumab). However, Roche reports three trials that it considers relevant to the consideration of bevacizumab for first-line use in patients with metastatic colorectal cancer, which were not included in the review. These are the ‘966 Study’ by Saltz and colleagues40 on oxaliplatin-combined therapy, the study by Hurwitz and colleagues on irinotecan-combined therapy26 and the study by Kabbinavar and colleagues27 on 5-FU/FA combined therapy. For second-line combined treatment, Roche refers to the ‘E3200 Study’ by Giantonio and colleagues41 on bevacizumab with oxaliplatin therapy. Further details can be found in Chapter 5; however, the outcomes will be briefly discussed in this chapter for consistency.
Cetuximab plus best supportive care compared with best supportive care
Jonker and colleagues37 report the results of the CO.17 trial, an open-label RCT in which 572 patients across Canada and Australia with advanced colorectal cancer expressing EGFR were randomised to receive either cetuximab plus best supportive care or best supportive care alone. Note that this primary paper does not analyse results according to KRAS status. The trial has been reported in one publication37 and four supplementary papers,42–45 one of which addresses the retrospective analysis of tissue samples for KRAS mutations with the others looking at cost-effectiveness, QoL and subgroup analysis.
The aim of the study was to demonstrate the effectiveness of cetuximab for survival and QoL in patients with advanced colorectal cancer. To that end, the primary end point was overall survival, defined as time from randomisation until death from any cause. Secondary outcomes investigated were progression-free survival, QoL and response rates. Objective tumour response was evaluated using the modified Response Evaluation Criteria in Solid Tumours (RECIST)46 and QoL was assessed using the EORTC QLQ-C30.
To be eligible for entry into the trial participants had to have advanced colorectal cancer expressing EGFR that was detectable by immunohistochemical methods in a central reference laboratory. The participants must have experienced tumour progression, unacceptable adverse events or contraindications to treatment with fluoropyrimidine, irinotecan or oxaliplatin.
Randomisation was performed centrally in a 1 : 1 ratio to cetuximab plus best supportive care or best supportive care alone, with participants stratified according to Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1 vs 2) and centre. Patients in the treatment arm received intravenously administered cetuximab over 120 minutes with an initial dose of 400 mg/m2 of body surface area, followed by a weekly maintenance infusion of 250 mg/m2 over 60 minutes. An antihistamine was given 30–60 minutes before each dose of cetuximab. Treatment was continued until death in the absence of unacceptable adverse events, tumour progression, worsening symptoms of the cancer or request by the patient.
The median duration of follow-up was reported as 14.6 months, although no range is given and it is not clear if this is for both arms. The median duration of cetuximab treatment was only 8.1 weeks (range 1–60 weeks), largely because of disease progression. The median dose intensity after the initial dose was 247 mg/m2/week and the relative dose intensity, that is, the ratio of the dose administered to the planned dose, was ≥ 90% in 75% of patients.
The first supplementary paper by Karapetis and colleagues47 considers the association between KRAS status and clinical benefit from cetuximab. The rationale for this investigation revolved around evidence suggesting that KRAS mutation rendered EGFR inhibitors, in this case cetuximab, ineffective. 36,48 The retrospective analysis was performed on 394 tumour samples obtained from the 572 participants of the CO.17 trial, with KRAS status then correlated with overall survival, progression-free survival and QoL.
The examination of tissue samples was performed by blinded assessors, with all statistical analysis performed in accordance with a protocol written before the assessment of KRAS status was performed. 47 The primary and secondary outcomes were consistent with those of the main trial report. 37
Au and colleagues44 focused on HRQoL in patients participating in the CO.17 trial to assess the influence of KRAS status in predicting benefit of cetuximab. The primary HRQoL analyses were defined prospectively as a comparison of the change of scores on the EORTC QLQ-C30 from baseline to 8 and 16 weeks for the physical function and global health status scales. Secondary HRQoL analyses included comparisons of the proportions of patients with worsened physical function and global health status at 8 and 16 weeks. A 10-unit change in score was predefined as clinically important.
The paper by Asmis and colleagues43 considered the relationships between comorbidity, age and performance status as predictors of outcome. The Charlson Comorbidity Index (CCI) was used to measure comorbidity, with the score determined by two physician reviewers. Variables of participant age and CCI score were dichotomised: age < 65 years compared with ≥ 65 years and CCI score 0 compared with ≥ 1, with higher scores indicating greater comorbidity. Univariate analysis was also performed for the association between age group and baseline characteristics.
Finally, the study by Mittmann and colleagues42 evaluated the cost-effectiveness of cetuximab with some preference-based health utility values, using the Health Utility Index (HUI) Mark 3 (HUI3).
In addition to the CO.17 trial, Merck Serono provided details of the BOND trial49 through De Roock and colleagues. 48 This is a retrospective analysis of cetuximab plus best supportive care compared with cetuximab plus irinotecan according to KRAS status. Data are used from the following four trials: BOND,49 EVEREST,50 SALVAGE51 and BABEL. Because De Roock and colleagues are not reporting a trial, or a systematic review, this study is not formally included in this review of clinical effectiveness. However, the de novo model relies heavily on this evidence; therefore, relevant information will be included throughout this chapter. Further details with a more substantial critique may be found in Chapter 5.
Panitumumab plus best supportive care compared with best supportive care
Van Cutsem and colleagues7 present the results of an open-label, Phase III, international (Western Europe, Central Europe, Eastern Europe, Canada, Australia, New Zealand) multicentre RCT in which 463 patients with metastatic colorectal cancer were randomised to receive either panitumumab and best supportive care or best supportive care alone. The trial has been reported in one main publication7 and four supplementary publications,32,38,52,53 which are summarised in Table 8.
Eligibility criteria included pathological diagnosis of metastatic colorectal adenocarcinoma and radiological documentation of disease progression during treatment or within 6 months following the last administration of fluoropyrimidine, irinotecan and oxaliplatin.
| Study | Description | Median (range) follow-up (months) |
|---|---|---|
| Van Cutsem et al.7 | Main trial of PAN + BSC vs BSC | 8.8 (3.8–19) |
| Siena et al. 53 | Analysis of association of progression-free survival with colorectal cancer symptoms, HRQoL and overall survival | 18 (13–28.3) |
| Van Cutsem et al. 38 | Crossover extension study | 15.3 (4.5–25.8) |
| Amado et al. 32 | Retrospective KRAS analysis of main trial | 14.1 (for 36 patients remaining at time of analysis) |
| Peeters et al. 52 | Analysis of association of skin toxicity severity with efficacy of PAN | 18 (13–28.3) |
The aim of the study was to evaluate the effect of panitumumab monotherapy in patients with chemorefactory metastatic colorectal cancer. The primary outcome was progression-free survival assessed by blinded central radiology. Secondary outcomes were best objective response, overall survival, time to response and duration of response.
The study was designed to have 90% power for a two-sided 1% significance level test given a hazard ratio of 0.67 (panitumumab relative to best supportive care).
Patients were randomly assigned in the ratio 1 : 1 to receive panitumumab plus best supportive care or best supportive care alone; however, details of the randomisation procedure are not given. Random assignment was stratified by ECOG performance status (0 or 1 vs 2) and region (Western Europe vs Central and Eastern Europe vs the rest of the world). Patients allocated to the intervention arm received panitumumab via a 60-minute intravenous infusion of 6 mg/kg once every 2 weeks until progression or unacceptable toxicity developed.
All patients were followed for survival every 3 months for up to 2 years after randomisation; however, median follow-up reported in this paper was approximately 35 weeks (range 15–76 weeks) in the panitumumab arm.
In the best supportive care group, 176 (76%) patients received panitumumab in a crossover protocol, which is reported in a supplementary paper. 38 The median time to crossover was 7 weeks (range 6.6–7.3 weeks) and the median follow-up after crossover was 61 weeks (range 18–103 weeks). The median duration of treatment and dose intensity were not reported.
Siena and colleagues53 examine the association of progression-free survival with colorectal cancer symptoms, HRQoL and overall survival for the panitumumab trial. Patient-reported outcomes were measured using the NCCN FCSI for colorectal cancer symptoms and HRQoL was measured using the European Quality of Life-5 Dimensions (EQ-5D) Health Index Scale, the EQ-5D visual analogue scale (VAS) and the EORTC QLQ-C30 global health status/QoL scale. In this paper median follow-up time for survival (enrolment to data cut-off for analysis) for all patients was reported as 72 weeks (range 52–113 weeks).
The efficacy and safety findings for panitumumab from the extension study of the main trial, that is, the crossover from best supportive care to panitumumab, are presented by Van Cutsem and colleagues;7,38 hence, this was a multicentre, open-label, single-arm trial. To be eligible, participants must have documented disease progression and were required to have completed the last assessment on the Phase III study not more than 3 months before enrolment in the extension study. During the interim participants could not have received systemic chemotherapy, radiotherapy, investigational agents or antitumour therapies.
Patients were followed for survival approximately every 3 months for up to 2 years from the randomisation date into the Phase III study. 7 The primary end point was safety and, although not prespecified in the protocol, progression-free survival, objective response rate, time to and duration of response, duration of stable disease and survival were explored.
The sample size was limited to the patients enrolled in the best supportive care arm of the Phase III study who met the eligibility criteria. Assuming a true event rate of 1%, the probability of at least one patient experiencing a given adverse event was 87% for a sample size of 200. Median follow-up time was reported as 61 weeks (range 18–103 weeks).
Amado and colleagues32 reported on a retrospective study assessing the predictive role of KRAS status in the main panitumumab trial. Of the 463 patients originally enrolled, 427 were included in the KRAS analysis, although the assessable sample size was 380 because of unavailable or poor-quality samples. The primary objective was to determine whether or not the effect of panitumumab plus best supportive care on progression-free survival differed between patients with KRAS mutant status and those with KRAS WT status. Secondary outcomes included whether or not panitumumab improved progression-free survival, overall survival and response rate in the KRAS WT group compared with the best supportive care group.
Estimating a 60% KRAS WT status prevalence, power was calculated at more than 99% if the hazard ratio was 1.0 in the KRAS mutant group and at 87% if the hazard ratio was 0.80 in the KRAS mutant group, assuming an overall hazard ratio of 0.54 among all patients.
The final supplementary paper by Peeters and colleagues52 uses data from the main trial to investigate the association of skin toxicity severity and patient-reported skin toxicity with progression-free survival, overall survival, disease-related symptoms and HRQoL. Associations by KRAS status were also evaluated.
Assessment of study quality
A summary of the quality assessment of studies included in this review is shown in Table 9; study characteristics are summarised in the narrative below and in Appendix 3.
| Jonker et al.37 | Van Cutsem et al.7 | Van Cutsem et al.38 | |
|---|---|---|---|
| Study design | RCT | RCT | Single arm/crossover |
| Is a power calculation provided? | Yes | Yes | N/A |
| Is the sample size adequate? | Yes | Yes | N/A |
| Was ethical approval obtained? | ? | Yes | Yes |
| Were the study eligibility criteria specified? | Yes | Yes | N/A |
| Were the eligibility criteria appropriate? | Yes | Yes | N/A |
| Were patients recruited prospectively? | Yes | Yes | N/A |
| Was assignment to the treatment groups really random? | Yes | ? | N/A |
| Was the treatment allocation concealed? | No | No | No |
| Were adequate baseline details presented? | Yes | Yes | Yes |
| Were the participants representative of the population in question? | Yes | Yes | Yes |
| Were the groups similar at baseline? | Yes | Yes | N/A |
| Were baseline differences adequately adjusted for in the analysis? | N/A | N/A | N/A |
| Were the outcome assessors blind? | ? | Yes | N/A |
| Was the care provider blind? | No | No | N/A |
| Are the outcome measures relevant to the research question? | Yes | Yes | Yes |
| Is compliance with treatment adequate? | Yes | ? | ? |
| Are withdrawals/dropouts adequately described? | Yes | Yes | Yes |
| Are all patients accounted for? | Yes | Yes | Yes |
| Is the number randomised reported? | Yes | Yes | N/A |
| Are protocol violations specified? | No | No | No |
| Are data analyses appropriate? | Yes | Yes | Yes |
| Is analysis conducted on an ITT basis? | Yes | Yes | N/A |
| Are missing data appropriately accounted for? | Partial | No | Yes |
| Were any subgroup analyses justified? | Yes | Yes | No |
| Are the conclusions supported by the results? | Yes | Yes | Yes |
Cetuximab plus best supportive care compared with best supportive care
The CO.17 trial reported by Jonker and colleagues37 is a good-quality, open-label, randomised Phase III trial. The evaluation of the trial in relation to study quality is shown in Table 9.
Randomisation methods and withdrawal were adequately reported. As previously mentioned, dose intensity was also noted to be adequate at 90%. However, blinding of assessors was not reported. A reason for the open-label nature of the study was also not given, although this may be due to the anticipated skin toxicity of anti-EGFR agents. The assessment of tissue samples for KRAS status was confirmed as performed in a blinded manner. 47
The De Roock study,48 not formally included in this review but cited by Merck Serono for clinical effectiveness, analyses KRAS status from several cetuximab-based studies. The data reveal several key issues:
-
of the relatively small sample size (n7 = 113), a total of 67 patients had KRAS WT status: 40% of the patients (n = 27) from the BOND trial,49 42% (n = 28) from the EVEREST trial,50 15% (n = 10) from SALVAGE51 and 3% (n = 2) from BABEL
-
in the BOND trial, 50% of patients from the cetuximab plus best supportive care arm received irinotecan after disease progression, indicating potential crossover and subsequent underestimation of overall survival in those treated with cetuximab plus irinotecan49
-
the EVEREST trial is described as a RCT comparing cetuximab plus irinotecan, escalating doses of cetuximab plus irinotecan and cetuximab plus best supportive care; however, it is unclear why only data from cetuximab plus irinotecan patients are included in De Roock and colleagues48,50,54,55
-
the SALVAGE study is a non-comparative study of patients receiving cetuximab plus best supportive care only who have received at least two previous lines of therapy51
-
the BABEL study appears to be investigating the effect of tetracycline to alleviate a rash in cetuximab therapy, although further details on this study have been difficult to identify
-
patients were included on the basis of availability of formalin-fixed paraffin-embedded tumour tissue; however, there are no details on what this percentage was for each of the four studies contributing patient data.
As such, there are concerns regarding the disease progression and effectiveness estimates calculated using De Roock and colleagues. 48 The estimates are likely to be subject to high levels of bias and confounding, although it is unclear what impact this will have.
Panitumumab plus best supportive care compared with best supportive care
The trial by Van Cutsem and colleagues7 is a large, good-quality, open-label, international, multicentre, randomised Phase III study. The lack of participant and clinician/investigator blinding due to expected skin toxicity is discussed; however, to mitigate this, tumour assessments were performed by blinded central review. Unfortunately, it is unclear whether or not randomisation was performed centrally. Further details of the quality assessment can be found in Table 9.
Population baseline characteristics
For the main trial37 the demographic characteristics and disease status were well matched (Table 10). The baseline characteristics were re-examined by Karapetis and colleagues47 according to KRAS status, which is also included in Table 10. Of the original 572 samples, 394 were available for analysis. Fifty-eight per cent were revealed to be WT, with 51% assigned to cetuximab plus best supportive care and 49% to best supportive care. The relative proportions of each characteristic remained similar between arms.
| Jonker et al.37 | Karapetis et al.17 | Van Cutsem et al.7 | Amado et al.32 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CET | BSC | CET | BSC | PAN | ||||||||
| CET | BSC | Mutant | WT | PAN | BSC | Mutant | WT | BSC mutant | BSC WT | |||
| n a | 287 | 285 | 81 | 83 | 117 | 113 | 231 | 232 | 84 | 124 | 100 | 119 |
| Diagnosis | Advanced CRC expressing EGFR | Chemorefractory mCRC | ||||||||||
| Age (years), median (range) | 63 (29–88) | 64 (29–86) | 62 (37–88) | 64 (29–86) | 62 (27–82) | 63 (27–83) | 62 (27–79) | 63 (29–82) | 62 (27–83) | 63 (32–81) | ||
| Male, n (%) | 186 (65) | 182 (64) | 101 (62) | 156 (68) | 146 (63) | 148 (64) | 47 (56) | 83 (67) | 64 (64) | 76 (64) | ||
| ECOG performance status, n (%) | ||||||||||||
| 0 | 72 (25) | 64 (23) | 34 (21) | 56 (24) | 107 (46) | 80 (34) | 43 (51) | 53 (43) | 37 (37) | 40 (34) | ||
| 1 | 148 (52) | 154 (54) | 94 (57) | 127 (55) | 94 (41) | 115 (50) | 28 (33) | 56 (45) | 47 (47) | 62 (52) | ||
| 2 | 67 (23) | 67 (24) | 26 (22) | 47 (20) | 29 (13) | 35 (15) | 13 (15)b | 15 (12)b | 16 (16)b | 17 (14)b | ||
| 3 | – | – | – | – | 1 (0) | 2 (1) | – | – | – | – | ||
| Site, n (%) | ||||||||||||
| Colon only | 171 (60) | 161 (57) | 108 (66) | 137 (60) | 153 (66) | 157 (68) | 53 (63) | 86 (69) | 65 (65) | 82 (69) | ||
| Rectum only | 63 (22) | 70 (25) | 32 (20) | 50 (22) | 78 (34) | 75 (32) | 31 (37) | 38 (31) | 35 (35) | 37 (31) | ||
| Colon and rectum | 53 (19) | 54 (19) | 24 (15) | 43 (19) | – | – | – | – | – | – | ||
| Previous adjuvant chemotherapy, n (%) | 108 (38) | 99 (35) | 50 (31) | 77 (34) | 86 (37) | 78 (34) | 27 (32) | 50 (40) | 40 (40) | 32 (27) | ||
| No. of regimens, n (%) | ||||||||||||
| 1 or 2 | 50 (17) | 54 (19) | 27 (17) | 46 (20) | 230 (100) | 232 (100) | 54 (64) | 79 (64) | 74 (74) | 63 (53) | ||
| 3 | 109 (38) | 108 (38) | 69 (42) | 86 (37) | 84 (36) | 88 (38) | 23 (27) | 41 (33) | 24 (24) | 49 (41) | ||
| 4 | 87 (30) | 72 (25) | 46 (28) | 63 (27) | – | – | – | – | – | – | ||
| ≥ 5 | 41 (14) | 51 (18) | 22 (13) | 35 (15) | – | – | – | – | – | – | ||
| TSI | 287 (100) | 285 (100) | 164 (100) | 230 (100) | – | – | – | – | – | – | ||
| Irinotecan | 277 (97) | 273 (96) | 161 (98) | 219 (95) | – | – | – | – | – | – | ||
| Oxaliplatin | 281 (98) | 278 (98) | 163 (99) | 222 (97) | – | – | – | – | – | – | ||
| Site of metastatic disease, n (%) | ||||||||||||
| Liver | 230 (80) | 233 (82) | 129 (79) | 189 (82) | – | – | – | – | – | – | ||
| Lung | 188 (66) | 180 (63) | 98 (60) | 144 (63) | – | – | – | – | – | – | ||
| Lymph nodes | 130 (45) | 117 (41) | 64 (39) | 103 (45) | – | – | – | – | – | – | ||
| Peritoneal cavity (ascites) | 45 (16) | 41 (14) | 23 (14) | 38 (17) | – | – | – | – | – | – | ||
| No. of sites of metastatic disease, n (%) | ||||||||||||
| 1 | 40 (14) | 53 (19) | 27 (17) | 40 (17) | – | – | – | – | – | – | ||
| 2 | 84 (29) | 69 (24) | 45 (27) | 63 (27) | – | – | – | – | – | – | ||
| 3 | 84 (29) | 89 (31) | 42 (26) | 75 (33) | – | – | – | – | – | – | ||
| ≥ 4 | 79 (28) | 74 (26) | 50 (31) | 52 (23) | – | – | – | – | – | – | ||
At baseline, the two groups were well matched in the original trial. 7 A slight difference was noted with disease status, with the best supportive care arm having 34% of participants with an ECOG performance status of 0 and 50% with an ECOG performance status of 1, and the treatment arm having 46% of participants with an ECOG performance status of 0 and 41% with an ECOG performance status of 1 (see Table 10). The supplementary study32 ascertained KRAS status in 92% of the original participants, showing the distribution of KRAS WT and KRAS mutant status between arms and ECOG performance status to be broadly similar.
Participants in the two main trials of cetuximab plus best supportive care compared with best supportive care were similar in terms of age, gender distribution and site of primary cancer. 45 However, as is usually the case with cancer trials, the study populations were significantly younger than the general population presenting with colorectal cancer, with the peak in number of cases in the UK, for example, between 70 and 79 years for men and 75–85+ years for women as opposed to a median of 62–64 years shown in Table 10.
Reporting of disease status in the panitumumab trial was limited to only ECOG performance status rather than providing details of primary or metastatic sites. A higher proportion of participants was noted to have an ECOG performance status of 0 in the treatment arm than in the best supportive care arm (46% vs 25%), which equates to ‘fully active, able to carry on all pre-disease performance without restriction’; therefore, this suggests a fitter population in the intervention arm.
Assessment of clinical effectiveness
Overall survival (Table 11)
There is currently no clinical evidence for the effectiveness of bevacizumab with non-oxaliplatin therapy after first-line treatment in metastatic colorectal cancer. However, the trials reported by Roche, which are not included in this review, will be briefly mentioned here.
| Study | Interventiona | n | Median OS (months) | HR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Jonker et al.37 | All: CET + BSC | 287 | 6.1 | 0.77 | 0.64 to 0.92 | 0.005 |
| All: BSC | 285 | 4.6 | ||||
| Karapetis et al.47 | WT: CET + BSC | 117 | 9.5 | 0.55b | 0.41 to 0.74 | < 0.001 |
| WT: BSC | 113 | 4.8 | – | – | – | |
| Van Cutsem et al.7 | All: PAN + BSC | 231 | NR | 1.00 | 0.82 to 1.22 | 0.81 |
| All: BSC | 232 | NR | – | – | – | |
| Amado et al.32 | WT: PAN + BSC | 124 | 8.1 | 0.99 | 0.75 to 1.29 | NR |
| WT: BSC | 119 | 7.6 | – | – | – |
Saltz and colleagues40 conducted a RCT for first-line bevacizumab combined with oxaliplatin in 1401 patients, 75% of whom had not previously received chemotherapy. Treatment arms were capecitabine plus oxaliplatin (XELOX) plus bevacizumab, XELOX plus placebo, 5-FU, FA and oxaliplatin (FOLFOX-4) plus bevacizumab or FOLFOX-4 plus placebo. The hazard ratio for overall survival for bevacizumab compared with placebo was not statistically significant (hazard ratio 0.89, 97.5% CI 0.76 to 1.03, p = 0.077), with a median overall survival of 21.3 months for bevacizumab and 19.9 months in the placebo arm.
Hurwitz and colleagues26 randomised 813 people to receive either irinotecan, bolus fluorouracil and leucovorin (IFL) plus bevacizumab or IFL alone for first-line treatment (28% of IFL patients and 24% of IFL plus bevacizumab patients had received previous adjuvant chemotherapy). ITT analyses showed that median survival was 20.3 months for those treated with IFL plus bevacizumab and 15.6 months for those receiving IFL alone (hazard ratio 0.6, p< 0.001).
Kabbinavar and colleagues27 randomised 209 patients to receive fluorouracil and leucovorin (FU/LV) plus bevacizumab or FU/LV only. Twenty-one per cent of the FU/LV plus bevacizumab patients and 19% of the FU/LV patients had prior adjuvant chemotherapy. The primary end point of overall survival produced a non-statistically significant hazard ratio of 0.76 (95% CI 0.56 to 1.10) for FU/LV plus bevacizumab compared with FU/LV, with a median overall survival of 16.6 months in the FU/LV plus bevacizumab arm and 12.9 months in the FU/LV arm. The authors argue that the large number of patients receiving postprogression treatment could partly explain the lack of statistical significance in the primary end point of overall survival. A similar percentage of patients from both treatment arms received irinotecan, oxaliplatin or both post progression (39% of the FU/LV plus bevacizumab patients and 46% of the FU/LV patients).
Giantonio and colleagues41 report a RCT with 820 patients previously treated with a fluoropyrimidine and irinotecan randomised to one of three arms: FOLFOX-4 plus bevacizumab, FOLFOX-4 or bevacizumab alone. Median overall survival was greater in the FOLFOX-4 plus bevacizumab arm: 12.9 months compared with 10.8 months for FOLFOX-4 and 10.2 months for bevacizumab alone. The hazard ratio for overall survival associated with FOLFOX-4 plus bevacizumab compared with FOLFOX-4 was 0.75 (p = 0.01).
Overall survival, defined as the time between date of randomisation and death from any cause, was the primary end point in the CO.17 trial. 37 The analysis was performed on an ITT basis, with the final analysis conducted after at least 445 patients were known to have died.
The median overall survival was 6.1 months in the cetuximab group and 4.6 months in the best supportive care group, with a hazard ratio of 0.77 (95% CI 0.64 to 0.92, p = 0.005). Seven percent of patients receiving best supportive care were administered cetuximab after crossover; however, this would be unlikely to bias the results against treatment. 37
No significant differences were seen for the benefit of cetuximab on the basis of ECOG performance status at baseline, age or sex in subgroup analysis. However, unplanned analysis indicated that grade of rash in patients receiving cetuximab was correlated with overall survival, with median survival of 2.6 months in patients with no rash, 4.8 months in patients with grade 1 rash and 8.4 months is patients with grade 2 rash (p < 0.001). 37
For patients with KRAS mutant status, analysis by Karapetis and colleagues47 showed a median overall survival of 4.5 months for cetuximab and 4.6 months for best supportive care, with a hazard ratio of 0.98 (95% CI 0.70 to 1.37, p = 0.89). Among patients with WT status, the median overall survival was 9.5 months in the cetuximab group compared with 4.8 months in the best supportive care group, with an hazard ratio of 0.55 (95% CI 0.41 to 0.74, p < 0.001). Subsequent to adjustment for potential prognostic factors, which are reported to be as specified in the protocol but are not described in the paper, the hazard ratio increases to 0.62 (95% CI 0.44 to 0.87, p = 0.006); however, these results remain favourable towards cetuximab plus best supportive care.
Panitumumab plus best supportive care compared with best supportive care
At the time of analysis, the study had achieved the event rate required for 90% power. 7 Overall survival was calculated from the day of random assignment until death, censoring patients at the last day known to be alive. Median overall survival values were not given; however, it was reported that no significant difference was found between arms (hazard ratio of 1.00, 95% CI 0.82 to 1.22, p = 0.81). The authors cite confounding because of the rapid crossover of 76% of patients from the best supportive care arm to receive active treatment for the lack of significant difference between arms, 32, which would bias the results against treatment.
Exploratory analysis of skin toxicity demonstrated a longer overall survival in patients with a skin toxicity of grade 2–4 than in those with a skin toxicity of grade 1, resulting in an hazard ratio of 0.59 (95% CI 0.42 to 0.85).
The retrospective investigation of panitumumab efficacy and KRAS status revealed no statistically significant overall survival difference between treatment arms in either of the KRAS groups. 32 The hazard ratios for overall survival were 1.02 (95% CI 0.75 to 1.39) and 0.99 (95% CI 0.75 to 1.29) for the KRAS mutant and WT groups, respectively, which is in contrast to the analysis for skin toxicity, which apparently favours overall survival and only occurs in WT patients. 52
Median overall survival is also unclear. Patients with KRAS WT status treated with panitumumab show a median survival of 8.1 months compared with 7.6 months for those treated with best supportive care; and patients with KRAS mutant status treated with panitumumab show a median overall survival of 4.9 months compared with 4.4 months for those treated with best supportive care. 32
Progression-free survival (Table 12)
Roche mentions a number of papers that did not fulfil the inclusion criteria for this review, but their results will be briefly summarised here for completeness. Further details can be found in Chapter 5.
| Study | Interventiona | n | Median PFS (months) | HR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Jonker et al.37 | All: CET + BSC | 287 | NR | 0.68 | 0.57 to 0.80 | < 0.001 |
| All: BSC | 285 | NR | – | – | – | |
| Karapetis et al.47 | WT: CET + BSC | 117 | 3.7 | 0.40b | 0.30 to 0.54 | < 0.001 |
| WT: BSC | 113 | 1.9 | – | – | – | |
| Van Cutsem et al.7 | PAN + BSC | 231 | 2 | 0.54 | 0.44 to 0.66 | – |
| BSC | 232 | 1.8 | – | – | – | |
| Amado et al.32 | PAN + BSC | 124 | 3.1 | 0.45 | 0.34 to 0.59 | – |
| BSCd | 119 | 1.8 | – | – | – |
Saltz and colleagues40 report a hazard ratio for progression-free survival for bevacizumab compared with placebo of 0.83 (97.5% CI 0.72 to 0.95), with median progression-free survival of 9.4 months for bevacizumab and 8 months for placebo.
The hazard ratio for progression-free survival determined by Hurwitz and colleagues26 was 0.54 (p < 0.001), with patients treated with IFL plus bevacizumab having a progression-free survival of 10.6 months and those in the IFL arm having a progression-free survival of 6.2.
Kabbinavar and colleagues27 showed a statistically significantly longer progression-free survival in the FU/LV plus bevacizumab arm (9.2 months) than in the FU/LV alone arm (5.5 months) (hazard ratio 0.5, 95% CI 0.34 to 0.73).
Finally, Giantonio and colleagues41 showed median progression-free survival of 7.3 months for FOLFOX-4 plus bevacizumab, 4.7 months for FOLFOX-4 and just 2.7 months for bevacizumab alone.
Progression-free survival was defined in the CO.17 study37 as the time from randomisation until the first objective observation of disease progression or death from any cause. It should be noted that it was not reported whether or not assessors were blinded. Treatment with cetuximab significantly improved progression-free survival, with a hazard ratio of 0.68 (CI 0.57 to 0.80, p < 0.001). No median progression-free survival is reported for either arm; however, the estimated proportions of patients who were alive without documented objective progression of the disease at 3 and 6 months were 41% and 15%, respectively, in the cetuximab group and 24% and 3%, respectively, in the best supportive care group.
For patients with KRAS mutant status, median progression-free survival was 1.8 months in both the cetuximab and the best supportive care groups (hazard ratio 0.99, 95% CI 0.73 to 1.35, p = 0.96). 47 For patients with KRAS WT status, median progression-free survival was 3.7 months in the cetuximab group and 1.9 months in the best supportive care group, with a hazard ratio of 0.40 (95% CI 0.30 to 0.54, p < 0.001).
Progression-free survival (primary end point) for this trial was calculated from the day of random assignment until determination by blinded assessors of radiological progression or death. 7 A statistically significant improvement was observed in patients receiving panitumumab, giving a hazard ratio of 0.54 (95% CI 0.44 to 0.66). The difference in median progression-free survival time was statistically significant at 2 months (95% CI 7.9 to 8.4) for panitumumab and 1.8 months (95% CI 7.1 to 7.7) for best supportive care; however, the difference in mean progression-free survival time was more substantial at 3.5 months [standard error (SE) 0.2] for panitumumab and 2.1 months (SE 0.1) for best supportive care.
The supplementary KRAS analysis revealed a beneficial effect of panitumumab for patients with KRAS WT status (hazard ratio 0.45, 95% CI 0.3 to 0.59), with a median progression-free survival of 12.3 weeks in contrast to 7.3 weeks for best supportive care. 32
Tumour response (Table 13)
In the cetuximab RCT,37 response rates (defined according to RECIST criteria) were assessed; however, it is unknown whether or not the assessors were blinded. All patients were assessed every 4 weeks. Chest radiographs and cross-sectional imaging were performed at baseline and every 8 weeks in both study groups until tumour progression occurred. In the cetuximab group, 23 patients (8%) had partial responses, with none in the best supportive care group (p< 0.001). Stable disease occurred in 90 patients in the cetuximab group (31.4%) and 31 patients in the best supportive care group (10.9%, p< 0.001). No data were provided on time to response and duration.
| Objective response rate, % (n) | |||||||
|---|---|---|---|---|---|---|---|
| Study | Interventiona | n | OR | CR | PR | SD | p-value for OR |
| Jonker et al.37 | All: CET + BSC | 287 | 8.0 (23) | 0 | 8.0 (23) | 31.4 (90) | < 0.001 |
| All: BSC | 285 | 0 | 0 | 0 | 10.9 (31) | – | |
| Karapetis et al.47 | WT: CET + BSC | 117 | – | 0 | 12.8 (15) | NR | < 0.001 |
| WT: BSC | 113 | – | 0 | 0 | NR | – | |
| Van Cutsem et al.7b | All: PAN + BSC | 231 | 10 (22) | 0 | 10 (22) | 27 (62) | < 0.0001 |
| All: BSC | 232 | 0 | 0 | 0 | 10 (23) | – | |
| Amado et al.32 | WT: PAN + BSC | 124 | 17 (21) | 0 | 17 (21) | 34 (42) | NR |
| WT: BSC | 119 | – | 0 | 0 | 12 (14) | – | |
Subsequent KRAS assessment revealed that, for patients with KRAS WT status in the cetuximab group, the response rate was 12.8%, whereas only 1.2% with KRAS mutant status displayed a response. 47
Objective response was evaluated by blinded central review using modified RECIST at weeks 8, 12, 16, 24, 32, 40 and 48 and every 3 months thereafter until disease progression. 7 At the discretion of the investigator, patients could be evaluated for radiographic tumour assessment after developing symptoms consistent with disease progression.
Objective response rates were greater in those treated with panitumumab than in those treated with best supportive care. After a 12-month minimum follow-up, 10% of patients in the panitumumab arm had an objective response, whereas no patients in the best supportive care group had an objective response (p< 0.0001). Median time to response was 7.9 weeks (range 6.7–15.6 weeks) and median duration of response was 17.0 weeks (range 7.9–76.7 weeks). Twenty-seven per cent of patients in the panitumumab group and 10% of patients in the best supportive care group had a best response of stable disease. 7
According to Amado and colleagues,32 best overall response data were unassessable or missing for 15% of patients receiving panitumumab and 23% of best supportive care patients, although this was not reported in the main trial report. For the KRAS-assessable patients receiving panitumumab, response was 10%, stable disease was 25% and disease progression was 50%; in the best supportive care arm, 0% had a response and 10% had stable disease. In the panitumumab group with KRAS WT status, 21 patients (17%, 95% CI 11% to 25%) had a partial response and 34% had stable disease; no responders were identified in the panitumumab group with KRAS mutant status. Median time to response was 7.9 weeks (range 7.0–15.6 weeks) and median duration of response was 19.7 weeks (range 7.9–88.7 weeks). 32
Health-related quality of life
A summary of the HRQoL results for cetuximab plus best supportive care compared with best supportive care is shown in Table 14.
| Study | Interventiona | n | Mean change from baseline | SD | 95% CI | p-valueb | |
|---|---|---|---|---|---|---|---|
| Jonker et al.37 | All: CET + BSC | Week 8 PF | NR | −3.9 | – | – | < 0.05 |
| Week 16 PF | −5.9 | – | – | 0.03 | |||
| Week 8 GHS | −0.5 | – | – | 0.008 | |||
| Week 16 GHS | −3.6 | – | – | < 0.001 | |||
| All: BSC | Week 8 PF | −8.6 | – | – | – | ||
| Week 16 PF | −12.5 | – | – | – | |||
| Week 8 GHS | −7.1 | – | – | – | |||
| Week 16 GHS | −15.2 | – | – | – | |||
| Karapetis et al.45 | WT: CET + BSC | Week 8 GHS | NR | 3.2 | – | 4.2 to 17.6 | 0.002 |
| Week 16 GHS | −0.2 | – | 7.6 to 28.2 | < 0.001 | |||
| WT: BSC | Week 8 GHS | −7.7 | – | – | – | ||
| Week 16 GHS | −18.1 | – | – | – | |||
| Au et al.44 | CET + BSC | Week 8 PF | |||||
| All | 185 | −3.9 | 15.6 | – | 0.046 | ||
| WT | 90 | −0.69 | 13.59 | – | 0.11 | ||
| Week 8 GHS | |||||||
| All | 185 | −0.5 | 20.4 | – | 0.008 | ||
| WT | 88 | 3.22 | 19.63 | – | 0.0016 | ||
| Week 16 PF | |||||||
| All | 125 | −5.9 | 17.7 | – | 0.027 | ||
| WT | 69 | −3.43 | 17.93 | – | 0.0078 | ||
| Week 16 GHS | |||||||
| All | 128 | −3.6 | −3.6 | – | < 0.001 | ||
| WT | 70 | −0.24 | −0.24 | – | < 0.001 | ||
| BSC | Week 8 PF | ||||||
| All | 147 | −8.6 | 20.4 | – | – | ||
| WT | 62 | −7.15 | 20.26 | – | – | ||
| Week 8 GHS | |||||||
| All | 149 | −7.1 | 22.4 | – | – | ||
| WT | 63 | −7.67 | 21.34 | – | – | ||
| Week 16 PF | |||||||
| All | 76 | −12.5 | 21.6 | – | – | ||
| WT | 36 | −13.8 | 21.47 | – | – | ||
| Week 16 GHS | |||||||
| All | 75 | −15.2 | 25.8 | – | – | ||
| WT | 36 | −18.1 | 27.64 | – | – | ||
| Mittmann et al.42 | All: CET + BSC | Baseline | 263 | 0.72 | 0.23 | – | – |
| Week 4 HUI | 220 | 0.73 | 0.26 | – | – | ||
| Week 8 HUI | 190 | 0.73 | 0.24 | – | – | ||
| Week 16 HUI | 119 | 0.73 | 0.24 | – | – | ||
| Week 24 HUI | 82 | 0.77 | 0.33 | – | – | ||
| All: BSC | Baseline | 260 | 0.71 | 0.24 | – | – | |
| Week 4 HUI | 184 | 0.68 | 0.26 | – | – | ||
| Week 8 HUI | 149 | 0.66 | 0.28 | – | – | ||
| Week 16 HUI | 72 | 0.63 | 0.30 | – | – | ||
| Week 24 HUI | 36 | 0.70 | 0.24 | – | – | ||
| Odom et al.56 | All: PAN + BSC | NCCN FCSI | 188 | 3.60 | – | 0.90 to 6.30 | ≤ 0.05 |
| EQ-5D | 0.17 | – | 0.09 to 0.25 | – | |||
| WT: PAN + BSC | NCCN FSCI | 112 | 5.62 | – | 2.38 to 8.86 | ≤ 0.05 | |
| EQ-5D | 0.22 | – | 0.12 to 0.32 | ≤ 0.05 |
HRQoL for the CO.17 trial37 was reported in several papers; however, because this study was not blinded there is the potential for bias in the QoL measures.
Quality of life reported in the main trial paper was assessed by the EORTC QLQ-C30 at baseline and at 4, 8, 16 and 24 weeks after randomisation. 37 Compliance with the questionnaire reduced from 94% at baseline in both groups to 67% at 16 weeks in the cetuximab group and 43% at 16 weeks in the best supportive care group. It is acknowledged that there was a systematic difference in compliance between the treatment groups and HRQoL data were not missing at random. Au and colleagues44 suggest that patients in the best supportive care arm were subject to worse progression-free survival and overall survival and therefore were less able to complete questionnaires. Lack of blinding was also recognised as a potential bias, because of the placebo effect.
In comparison with best supportive care, the reported results indicate that cetuximab was associated with reduced deterioration in physical function at 8 weeks (mean change score −3.9 vs −8.6, p = 0.05) and 16 weeks (mean change score −5.9 vs −12.5, p = 0.03). The cetuximab arm also demonstrated less deterioration in global health status at 8 weeks (mean change score −0.5 vs −7.1, p = 0.008) and 16 weeks (mean change score −3.6 vs −15.2, p < 0.001). 37 The data for HRQoL at 24 weeks are not reported.
According to Karapetis and colleagues,47 patients with KRAS WT status in the cetuximab arm had an improvement in global health status at 8 weeks, whereas those in the best supportive care group deteriorated (mean change score 3.2 vs −7.7, 95% CI 4.2 to 17.6, p = 0.002). Patients with KRAS WT status in the cetuximab group also had less deterioration at 16 weeks than those in the best supportive care group (mean change score −0.2 vs −18.1, 95% CI 7.6 to 28.2, p < 0.001).
Mittmann and colleagues42 report on HUI3 data collected during the CO.17 trial, in which patients assess their own health attributes: vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain. The assessments were performed at 4, 8, 16 and 24 weeks after randomisation, and the results show that the scores were relatively unchanged for the cetuximab arm, but declined for best supportive care. However, these results are not displayed according to KRAS status; in addition, bias is likely as patients in the best supportive care arm may deteriorate more quickly than those in the cetuximab arm, and therefore be less able to complete the questionnaire.
Patient-reported outcomes were analysed using the EQ-5D VAS, the NCCN FCSI, EORTC QoL subscales and a dermatology question. Results in the main trial report by Van Cutsem and colleagues7 were included in an online appendix. No data were given, just a summary concluding that patients were more concerned by their skin condition in the cetuximab group than in the best supportive care group. It was reported that no clinically meaningful differences in overall QoL were observed between the groups.
A subsequent study by Odom and colleagues56 reported more information on this study. They report on data collected using the NCCN FCSI and the EQ-5D. The results were analysed according to KRAS status, which was ascertained in 92% of participants. The authors acknowledged the large amount of missing patient-reported data and attributed this to declining health, as approximately 50% of patients in the best supportive care arm, and patients with KRAS mutant status in the panitumumab plus best supportive care arm, had progressed by week 8. Sensitivity analysis was performed to evaluate the effect of attrition on QoL between treatment arms.
Overall, information on KRAS status together with post-baseline patient-reported outcome data were available for 78% of the study population. Baseline characteristics were broadly similar across groups, other than the panitumumab plus best supportive care group which had a slightly higher percentage of people with an ECOG performance status of 0. Less deterioration was observed in the FCSI score and EQ-5D index in the panitumumab plus best supportive care group than in the best supportive care group alone, both overall and for those with KRAS WT status.
For the FCSI score, an average least squares mean difference between treatment groups across all weeks favoured panitumumab by 3.60 (95% CI 0.90 to 6.30) overall and by 5.62 (95% CI 2.38 to 8.86) for patients with KRAS WT status. The EQ-5D index results also favoured panitumumab, with an average least squares mean difference between treatment groups across all weeks of 0.17 (95% CI 0.09 to 0.25) overall and 0.22 (95% CI 0.12 to 0.32) for those with KRAS WT status. Analysis of the KRAS mutant group did not show any significant differences in QoL between those treated with panitumumab plus best supportive care and those treated with best supportive care. Odom and colleagues56 suggest a limitation of the study concerning skin toxicity, which was associated with higher HRQoL, as the rash may be seen as a predictor of benefit by patients. However, the majority of KRAS mutant patients on panitumumab who also experienced a rash did not report this benefit.
Indirect comparison of cetuximab and panitumumab
There are no RCTs directly comparing the effectiveness of cetuximab with that of panitumumab. However, an indirect comparison between the two treatments can be made if it is assumed that the best supportive care arms of Karapetis and colleagues45 and Amado and colleagues32 are equivalent in terms of the care and treatment received. Based on this assumption the hazard ratios for overall survival and progression-free survival can be calculated for an indirect comparison of cetuximab and panitumumab. 34 Details of the method used can be found in Appendix 4.
Two sets of results are given in Table 15, those using the unadjusted overall survival and progression-free survival hazard ratios from Karapetis and colleagues and those using the KRAS-adjusted overall survival and progression-free survival hazard ratios. Given that KRAS status is assessed retrospectively in Karapetis and colleagues and that KRAS status was not determined for all participants, there may be some selection bias in the study (even though the authors report that there were similarities between patient characteristics for those with KRAS WT status and those with KRAS mutant status); therefore, it would seem reasonable to attach more importance to the hazard ratios adjusted for patient characteristics than those not adjusted. Note that Amado and colleagues32 do not report hazard ratios adjusted for patient characteristics, only the unadjusted hazard ratios. It is therefore possible that the unadjusted hazard ratios from Amado and colleagues are subject to selection bias as well, but the magnitude of this bias is difficult to quantify.
| Outcome | HR from Karapetis et al.45 | CET + BSC vs BSC45 | PAN + BSC vs BSC32 | CET + BSC vs PAN + BSC (calculated by PenTAG34) |
|---|---|---|---|---|
| Progression-free survival | Unadjusted | 0.40 (0.30 to 0.54) | 0.45 (0.34 to 0.59) | 0.89 (0.59 to 1.33) |
| Adjusted | 0.42 (0.30 to 0.58) | 0.93 (0.61 to 1.43) | ||
| Overall survival | Unadjusted | 0.55 (0.41 to 0.74) | 0.99 (0.75 to 1.29) | 0.56 (0.37 to 0.83) |
| Adjusted | 0.62 (0.44 to 0.87) | 0.63 (0.41 to 0.97) |
The indirect comparisons indicate that there is no statistically significant difference in the hazard for progression-free survival between those receiving cetuximab plus best supportive care and those receiving panitumumab plus best supportive care, regardless of whether the adjusted or unadjusted hazard ratio from Karapetis and colleagues45 is used. On the other hand, the results suggest that there is a statistically significant difference in hazard for overall survival between cetuximab plus best supportive care and panitumumab plus best supportive care, with patients receiving cetuximab plus best supportive care having longer overall survival. However, the study by Amado and colleagues32 is subject to a large number of patients randomised to receive best supportive care actually receiving panitumumab plus best supportive care during the progressed disease stage, potentially biasing the results against panitumumab. Thus, the hazard ratio for overall survival from this study is subject to confounding. No published analyses have addressed this issue of crossover in the study by Amado and colleagues. In Amgen's submission, analyses were undertaken to address the crossover (see Chapter 5), but the results are not presented in terms of hazard ratios and so are not included in the indirect comparisons described here.
Adverse events (Table 16)
Adverse events were graded in the cetuximab plus best supportive care and panitumumab plus best supportive care studies using the NCI-CTC version 2.0, other than for selected dermatological/skin toxic effects, which, for panitumumab plus best supportive care, were graded using the NCI-CTC version 3.0 with modifications.
| Jonker et al.37 | Van Cutsem et al.7 | Van Cutsem et al.38 | |||
|---|---|---|---|---|---|
| CET + BSC | BSC | PAN + BSC | BSC | PAN + BSC | |
| n | 288 | 274 | 229 | 234 | 176 |
| Grade 3/4 adverse events (%) | |||||
| Erythema | – | – | 5 | 0 | 6 |
| Dermatitis acneiform | – | – | 7 | 0 | 6 |
| Pruritus | – | 2 | 0 | 1 | |
| Skin exfoliation | – | – | 2 | 0 | 1 |
| Fatigue | 33 | 30 | 4 | 3 | – |
| Paronychia | – | – | 1 | 0 | 2 |
| Abdominal pain | 13 | 16 | 7 | 4 | – |
| Anorexia | 8 | 6 | 3 | 2 | – |
| Nausea | 6 | 6 | 1 | 0 | – |
| Diarrhoea | – | – | 1 | 0 | 1 |
| Rash | 12 | 0 | 1 | 0 | 5 |
| Skin fissures | – | – | 1 | 0 | – |
| Constipation | 3 | 5 | 3 | 1 | – |
| Vomiting | 6 | 6 | 2 | 1 | – |
| Dyspnoea | 16 | 12 | 5 | 3 | – |
| Pyrexia | – | – | 0 | 2 | – |
| Asthenia | – | – | 3 | 2 | – |
| Cough | – | – | 0 | 0 | – |
| Back pain | – | – | 2 | 0 | – |
| Oedema | 5 | 6 | 1 | 0 | – |
| Conjunctivitis | – | – | – | – | 1 |
| General physical health deterioration | – | – | 7 | 2 | – |
| Other paina | 15 | 7 | – | – | – |
| Non–neutropenic infection | 13 | 6 | – | – | – |
| Confusion | 6 | 2 | – | – | – |
| Hypomagnesaemia | 6 | 0 | 3 | – | 4 |
| Infusion reactions | 5 | 0 | – | – | – |
Comparison between the cetuximab and the panitumumab trials indicates a disparity in fatigue. For example, although the population characteristics between the studies are similar, Merck Serono (cetuximab) reports that 30% of patients suffered grade 3 or higher fatigue in the best supportive care arm, whereas Amgen (panitumumab) reports that only 3% of patients in the best supportive care arm experienced the same level of fatigue.
Although different criteria were used for skin toxicity, for example the cetuximab trial combined all skin-related adverse events as a rash whereas the panitumumab trial employed a variety of conditions, such as erythema and pruritus, the numbers were similar.
For consistency, the following is a brief overview of papers cited by Roche, which were not applicable for inclusion in the review. Further details are available in Chapter 5.
Saltz and colleagues40 report that 30% of patients in the bevacizumab arm discontinued treatment because of adverse events compared with 21% in the placebo arm. Hurwitz and colleagues26 report statistically significantly more grade 3 or 4 adverse events in the IFL plus bevacizumab arm than in the IFL arm (p < 0.01), because of hypertension. Kabbinavar and colleagues27 also experienced an increase in grade 3 or 4 adverse events with bevacizumab (87% for FU/LV plus bevacizumab vs 71% for FU/LV), as did Giantonio and colleagues41 (75% for FOLFOX-4 plus bevacizumab vs 61% for FOLFOX-4).
Safety analysis for the CO.17 trial37 was conducted on an on-treatment basis, contrasting patients who had at least one dose of cetuximab (including those who crossed over) with patients assigned to supportive care alone, and omitting patients who withdrew consent before any intervention. Grades were determined according to the NCI-CTC version 2.0.
It should be noted that the data are presented with the patient as the unit of measurement. No information is given as to whether or not patients experienced more than one adverse event.
No statistically significant differences were apparent between the cetuximab group and the best supportive care group in the incidence of grade 3 or higher adverse events, with the exception of rash (11.8% for cetuximab vs 0.4% for best supportive care, p < 0.001), infection without neutropenia (12.8% vs 5.5%, p = 0.003), confusion (5.6% vs 2.2%, p = 0.05) and pain defined as other according to the NCI-CTC (14.9% vs 7.3%, p = 0.005). Hypomagnesaemia was also more common in the cetuximab group than in the best supportive care group (5.8% vs 0.0%, p < 0.001). 37
Grade 3 or 4 infusion reactions occurred in 4.5% of patients assigned to cetuximab. A total of 11 patients had an adverse event leading to discontinuation of cetuximab, most frequently because of an infusion reaction. Patients in the cetuximab group also had a higher incidence of rash of any grade than patients in the best supportive care group (88.6% vs 16.1%, p < 0.001). 37
In total, 59 patients died within 30 days after the last date of the cetuximab infusion. All died of colorectal cancer except for one patient who had a pulmonary embolus.
Unfortunately, safety data were not retrospectively analysed for cetuximab in relation to KRAS status.
It should be noted that the safety data are presented with the patient as the unit of measurement, as opposed to the number of adverse events in each arm. No information is given as to whether or not patients experienced more than one adverse event.
Skin-related toxicities occurred in 90% of patients in the panitumumab group and in 9% of the best supportive care group. One patient in the panitumumab group discontinued treatment because of grade 2 dermatitis acneiform and another due to a grade 2 hypersensitivity reaction. 7 Grade 3 or 4 hypomagnesaemia occurred in 3% of patients in the panitumumab group.
Eighty-one per cent of patients in the panitumumab group and 84% in the best supportive care group died during the study, none of which was treatment related. Nearly all deaths were related to disease progression.
Specific adverse events according to KRAS status in each arm were not reported. However, in the group with KRAS WT status, 100% of patients receiving panitumumab and 90% of patients receiving best supportive care had an adverse event, although no level of statistical significance is given. 32 Consistent with previous reports,7 patients with the worst grade skin toxicity in the KRAS WT group appeared to experience better progression-free survival and overall survival. The other main adverse event in the panitumumab arm was a higher incidence of diarrhoea of any grade (KRAS WT 24%, KRAS mutant 19%). Amado and colleagues32 report the incidence of adverse events leading to withdrawal in the panitumumab arm to be 7% for the group with KRAS WT status, with 2% withdrawing for panitumumab-related events.
With regard to the single-arm extension study, 92% of patients experienced adverse events considered related to panitumumab, 16% had a grade 3 treatment-related adverse event and 2% had a grade 4 treatment-related adverse event (acute renal failure, pulmonary embolism, erythema and pustular acne). 38 There were no fatal adverse events related to panitumumab.
Fifty-three per cent of patients had at least one serious adverse event, most of which were typical of metastatic colorectal cancer disease progression, with 6% experiencing serious adverse events considered at least possibly related to panitumumab. Adverse events leading to discontinuation of the treatment phase occurred in 11% of patients, with 4% discontinuing treatment because of skin and subcutaneous tissue disorders that were possibly related to panitumumab. 38
All deaths were attributed to disease progression, with 10% of patients dying during the treatment period, 20% within 30 days of treatment discontinuation and 52% after 30 days of receiving the last panitumumab infusion.
Summary of safety data
Skin toxicity is the most common adverse event associated with EGFR inhibitors, although it has been shown that skin toxicity severity may be associated with efficacy. 52 With panitumumab treatment this association was seen only for patients with KRAS WT status; however, the trial authors advise caution on the apparent correlation because the analysis was not a randomised comparison and patients remaining for longer on the study because of benefit of treatment are more likely to develop skin toxicity. 52
Overall conclusion
From the limited clinical data available, treatment with both interventions (cetuximab plus best supportive care and panitumumab plus best supportive care) appears to have clinically relevant and statistically significant advantages over treatment with best supportive care alone (Table 17). In both trials, median progression-free survival in patients with KRAS WT status appears to almost double as a result of active treatment. For cetuximab, median progression-free survival increases from approximately 2 months to approximately 4 months and for panitumumab, median progression-free survival increases from approximately 2 months to approximately 3 months (hazard ratio for cetuximab vs best supportive care 0.40, 95% CI 0.30 to 0.54; hazard ratio for panitumumab vs best supportive care 0.45, 95% CI 0.34 to 0.59). 32,47
| CET + BSC vs BSC | Significantly greater progression-free survival for cetuximab |
| Significantly greater overall survival for cetuximab | |
| PAN + BSC vs BSC | Significantly greater progression-free survival for panitumumab |
| No significant difference in overall survivala | |
| CET + BSC vs PAN + BSCb | No significant difference in progression-free survival |
| Significantly greater overall survival for cetuximaba |
For median overall survival in the clinically relevant KRAS WT population, the cetuximab arm exhibits a statistically significant improvement of 9.5 months compared with 4.8 months for best supportive care (hazard ratio 0.55, 95% CI 0.41 to 0.75). The evidence for panitumumab is less convincing. Although a median overall survival of 8.1 months compared with 7.6 months for best supportive care is presented, the hazard ratio of 0.99 (95% CI 0.75 to 1.29) indicates a lack of significant difference. The rapid crossover of 76% of patients (median time to crossover 7.1 weeks) is likely to have had an extensive confounding effect. 7,32
Tumour response indicated that stable disease occurred in 31.4% of patients in the cetuximab group and 10.9% of patients in the best supportive care group (p < 0.001); however, it is unclear whether or not the assessors were blinded. 37 Partial response was seen in 8% of patients in the cetuximab group, with no partial response seen in the best supportive care group. Subsequent KRAS analysis revealed that, for patients with KRAS WT status in the cetuximab group, the partial response rate was 12.8%, whereas only 1.2% with KRAS mutant status had a response. 47 Stable disease was not reported.
Objective response for the panitumumab study was evaluated by blinded central review and was shown to favour panitumumab over best supportive care. After a 12-month minimum follow-up, 10% of patients in the panitumumab group had a partial response, whereas no patients in the best supportive care group had an objective response (p < 0.0001). In the panitumumab group with KRAS WT status, 17% had a partial response and 34% had stable disease, whereas no responders were identified in the panitumumab group with KRAS mutant status.
Data on adverse events are difficult to compare between the interventions. The panitumumab trial does not confirm the adverse event scale used; therefore, it is unclear if these adverse event data are analogous with those on cetuximab.
Skin toxicity was clearly an issue for both treatments, although, again, it was reported differently. Patients in the cetuximab group had an 88% incidence of rash of any grade compared with skin toxicity of 90% for panitumumab. There appears to be a correlation between extent of skin toxicity and treatment efficacy, although one paper suggests exercising caution with these results as a patient remaining longer on a treatment because of its benefit is more likely to develop skin toxicity at some point. 52
Health-related quality of life was at risk of bias in both trials because of lack of blinding and the knowledge that skin toxicity may also have been a predictor of benefit. There may also have been systematic differences in compliance between treatment groups, for example patients in the best supportive care arm were subject to worse progression-free survival and overall survival and therefore were less able to complete questionnaires. For patients receiving cetuximab plus best supportive care, a slower deterioration in global health status and physical function was noted in comparison with best supportive care alone. According to Karapetis and colleagues,45 patients with KRAS WT status in the cetuximab plus best supportive care arm had an improvement in global health status at 8 weeks, whereas those in the best supportive care group deteriorated.
For patients receiving panitumumab plus best supportive care, it was initially reported that no clinically meaningful differences in overall QoL were observed between the groups;7 however, subsequent analysis revealed less deterioration in the FCSI score and EQ-5D index in the panitumumab plus best supportive care group than in the best supportive care alone group, both overall and for those with KRAS WT status. 56
As there are no head-to-head comparison data available for cetuximab compared with panitumumab, we carried out an indirect comparison to consider which intervention might be the most clinically effective. The results indicate that there is no statistically significant difference in the hazard for progression-free survival between those receiving cetuximab plus best supportive care and those receiving panitumumab plus best supportive care. In contrast, there is a statistically significant difference in hazard for overall survival between cetuximab plus best supportive care and panitumumab plus best supportive care, with patients receiving cetuximab plus best supportive care having longer overall survival. However, the study by Amado and colleagues32 is subject to a large number of patients randomised to receive best supportive care actually receiving panitumumab plus best supportive care during the progressive disease stage; thus, the hazard ratio for overall survival from this study is subject to confounding.
Chapter 4 Assessment of cost-effectiveness: systematic review
The cost-effectiveness of bevacizumab, cetuximab and panitumumab compared with relevant comparators within their licensed indications for the treatment of metastatic colorectal cancer after first-line chemotherapy was assessed in a systematic review of the literature. An outline discussion is presented on the literature searching undertaken in the general literature on metastatic colorectal cancer, covering the costs associated with the treatment of metastatic colorectal cancer, HRQoL associated with metastatic colorectal cancer states, and the modelling of disease progression in metastatic colorectal cancer.
Systematic review of existing cost-effectiveness evidence
Cost-effectiveness evidence that supported existing guidance
A review by Tappenden and colleagues10 evaluated the clinical effectiveness and cost-effectiveness of bevacizumab and cetuximab for the treatment of individuals with metastatic colorectal cancer; this informed NICE guidance TA118. 16 Both were identified in the literature searches and are summarised in Table 18 and Considerations for cetuximab from technology appraisal No. 118. The assessment of bevacizumab in the Tappenden review falls beyond the scope of this current appraisal as it considered evidence of the use of bevacizumab in untreated metastatic colorectal cancer patients (i.e. first-line treatment). The review of cetuximab is relevant to this appraisal as it considered patients with EGFR-expressing metastatic colorectal cancer who had failed irinotecan-including therapy. It was not, however, restricted to patients with KRAS WT status as this was not part of the cetuximab licence at the time that TA118 was developed.
| Study purpose | To assess the clinical effectiveness and cost-effectiveness of bevacizumaba and cetuximab in the treatment of individuals with mCRC | |
| Country setting | UK | |
| Base-year prices | Not stated explicitly but manufacturer's submissions are 2005 | |
| Intervention/comparator | CET + IRIN vs ASC/BSC | |
| Line of treatment | Second or subsequent line (CET) | |
| Study type | Threshold analysis; based on Merck Serono's submission to NICE in 2005 | |
| Model duration/cycle length | Unclear | |
| Number of health states | Unclear. Description of utilities suggests two alive health states: stable disease and progressive disease | |
| Study group | Patients with EGFR-expressing mCRC after failure of irinotecan-including cytotoxic therapy | |
| Perspective | NHS/PSS perspective | |
| Discount rate per annum | Not included; distribution of costs incurred over time is not included in the model although given the short time horizon this omission is unlikely to have a substantial impact on the cost-effectiveness or cost–utility estimates | |
| Source of funding | Funding for this review was provided by the NIHR | |
| Base-case findings | Mean overall survival durations for ASC/BSC treatment groups range from 0.60 LYs to 0.77 LYs. Based on these estimates of overall survival the cost per LY G for CET + IRIN, given according to the proposed continuation rule, may be as low as £58,048 or as high as £462,889. When health outcomes are measured in terms of QALYs, the equivalent range is likely to be £77,210–335,358 per QALY gained. When the proposed continuation rule is not applied the cost per LYG may be as low as £77,345 or as high as £375,487, or between £104,747 and £370,044 per QALY gained (again, depending on calculation of mean overall survival duration for ASC/BSC groups). Minimum overall survival advantage required by CET + IRIN over ASC/BSC is 0.65 years assuming the continuation rule | |
| Scenario | Min. overall survival (years) advantage for CET + IRIN over comparator to have a cost per QALY gained of £30,000 | |
|---|---|---|
| Sensitivity analyses | Base case (with continuation rule) | 0.65 |
| Base case (without continuation rule) | Not possible for the incremental cost–utility of CET + IRIN vs ASC/BSC to be < £30,000 per QALY gained | |
| Alternative HRQoL data source (MABEL) | 0.6 | |
| Comparator is oxaliplatin + 5-FU/FA | Approx. 0.8 | |
| Comparator is BSC alone | Not possible for CET + IRIN to have a cost per QALY that is < £30,000 | |
| Including indirect effectiveness evidence | 0.14 | |
Considerations for cetuximab from technology appraisal No. 11816
Main guidance
Cetuximab in combination with irinotecan is not recommended for the second-line or subsequent treatment of mCRC after the failure of an irinotecan-containing chemotherapy regimen. People currently receiving ... cetuximab should have the option to continue therapy until they and their consultants consider it appropriate to stop. 16
Key economic considerations
Effectiveness data representative of third-line not second-line usage:
The Committee considered the cost-effectiveness for cetuximab. It noted that the economic modelling from both the manufacturer and the assessment group had been completed using effectiveness data from the RCT of cetuximab where approximately 80% of patients received cetuximab plus irinotecan as a third-line or subsequent therapy. It [the Appraisal Committee] was also aware that the comparator used in both models was ASC/BSC, which meant the modelled scenario and corresponding estimates of cost-effectiveness more closely resembled third-line or subsequent use of cetuximab rather than second-line use. 16
Uncertainties surrounding the utility estimates were discussed:
The Committee discussed the uncertainties around the estimates of utility for patients with mCRC. The manufacturer had provided estimates between 0.95 and 0.71, both constant over the lifetime of the patient. The Committee considered that the utility for a patient with mCRC was likely to reflect the lower end of this range, based on additional data submitted by the manufacturer from the MABEL study. The Committee concluded that, using the most realistic utility estimates, the cost effectiveness estimates provided by both the manufacturer and the assessment group were not compatible with the best use of NHS resources. The Committee also noted that these estimates were associated with a high level of uncertainty because they were based on indirect comparisons. 16
Results from the threshold analysis were considered:
The Committee therefore considered threshold analyses completed by the assessment group, where the survival in the comparator arm was held as unknown. The base-case threshold analysis suggested that, with the application of the continuation rule, a cost per quality-adjusted life-year (QALY) gained of £30,000 could only be achieved if survival with ASC/BSC is less than two months. A sensitivity analysis adjusting the assumptions to reflect utility values from the MABEL study did not materially alter the results. The Committee noted that the manufacturer had provided an estimate of mean survival of 5.6 months for patients receiving ASC/BSC in their economic model, while studies of ASC/BSC identified in their assessment report provided estimates of median survival ranging from six to nine months. The Committee therefore considered that an estimate of mean survival while receiving ASC/BSC of approximately two months was an unrealistic underestimate. Considering all the available evidence on clinical and cost effectiveness, the Committee therefore concluded that cetuximab, either as a second-line or a subsequent line treatment for mCRC would not be a cost-effective use of NHS resources. 16
Methods
Electronic databases were searched using population and intervention sets only, without restricting to methodological or outcome filters; see Chapter 3, Identification of studies and Appendix 1.
Study selection criteria and procedures
The inclusion and exclusion criteria for the systematic review of economic evaluations are similar to those for the systematic review of clinical effectiveness (see Chapter 3, Inclusion and exclusion criteria), subject to the following exceptions:
-
non-randomised studies were included (e.g. decision model-based analyses, or analyses of patient-level cost and effectiveness data alongside observational studies)
-
full cost-effectiveness, cost–utility, cost–benefit and cost–consequence analyses were included (economic evaluations that report only average cost-effectiveness ratios were included if the incremental ratios could be easily calculated from the published data)
-
standalone cost analyses based in the UK NHS were also sought and appraised.
Relevant studies to the cost-effectiveness analysis were identified in two stages based on the above inclusion/exclusion criteria. Titles and abstracts returned by the search strategy were examined independently by two researchers (CH and LC) and screened for possible inclusion. Disagreements were resolved by discussion. Full texts of the identified studies were obtained. Two researchers (CH and LC) examined these independently for inclusion or exclusion, and disagreements were again resolved by discussion.
Study quality assessment
The methodological quality of included economic evaluations was assessed according to internationally accepted criteria such as the Consensus on Health Economic Criteria (CHEC) questions developed by Evers and colleagues57 and the critical appraisal checklist developed by Drummond and colleagues. 58 The studies were assessed by one reviewer (LC) and checked by a second reviewer (CH).
Data extraction strategy
For those studies that were of relevance to the current decision problem, data were extracted by one researcher (LC) into a summary table describing the study design and main results. The table includes author and year; model type or trial based; study design (e.g. cost-effectiveness analysis or cost–utility analysis); service setting/country; study population; comparators; research question; perspective; time horizon and discounting; main costs included; base-case findings; sensitivity analyses conducted; and other notable design features. Finally, the reviewer's comments on study quality and generalisability (in relation to the final scope) of the results were recorded (see Tables 21 and 22 and Appendix 9).
Synthesis of extracted evidence
Narrative synthesis supported by abridged data extraction tables (see Appendix 3) was used to summarise the available evidence base.
Results
The systematic search of electronic databases produced 7745 titles and abstracts, of which 7670 items did not meet the specified inclusion criteria. Of 77 full papers screened, five were included in the review (further details and references for these excluded papers are available in Appendix 10). 42,59–62 A flow chart of the study selection process is shown in Figure 2.
FIGURE 2.
Flow diagram for the study selection process for the review of the cost-effectiveness of bevacizumab, cetuximab and panitumumab for the treatment of metastatic colorectal cancer.
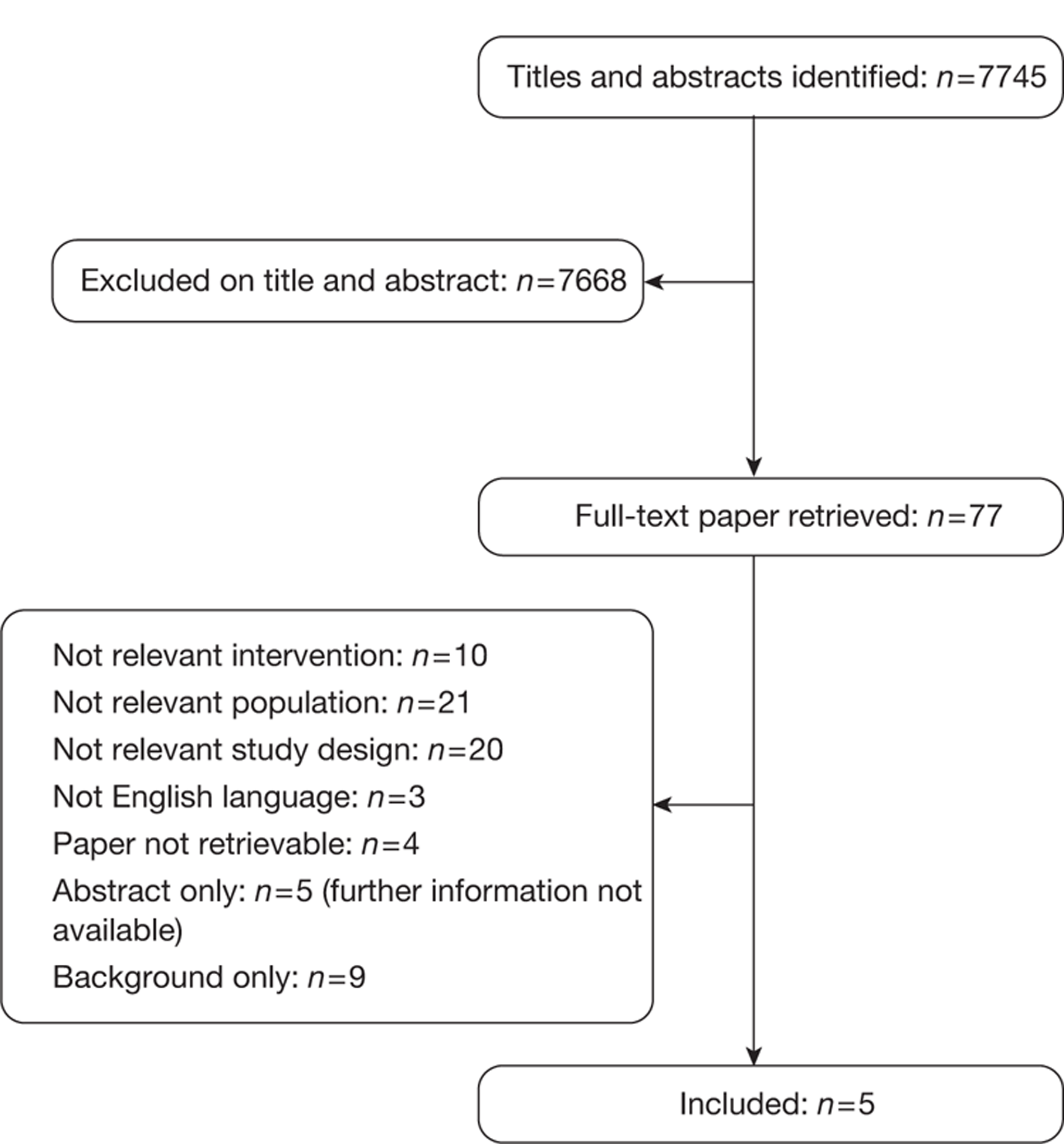
One published systematic review10 was identified in the literature search, which informed previous guidance (TA118,16 also identified in the review). These were not included in the main review but are summarised in Table 18 and Cost-effectiveness evidence that supported existing guidance.
Five abstracts63–67 were identified that met the specified inclusion criteria. Additional information was requested from the corresponding authors of each of the abstracts, but at the time of writing no responses had been received. Three of the abstracts63–65 are referred to in the discussion in this section.
Five published full economic evaluations meeting the inclusion criteria were included. 42,59–62
Summary of cost-effectiveness studies
Table 19 gives a summary of the included cost-effectiveness studies.
| Author | Intervention | Comparator | Location | Notes |
|---|---|---|---|---|
| Mittmann et al.42 | CET + BSC | BSC | Canada | Based on CO.17 study37 |
| Annemans et al.59 | CET + IRIN | BSC | Belgium | Based on BOND study49 |
| Norum60 | CET + IRIN | BSC | Norway | – |
| Starling et al.61 | CET + IRIN | BSC | UK | – |
| Wong et al.62 | Treatment sequences to measure the cost implications of treatments that include chemotherapy for the treatment of metastatic colorectal cancer; five sequences consider either CET + BSC or CET + IRIN use as a third-line treatment option | USA | – | |
Cetuximab plus best supportive care compared with best supportive care
One study addressed the cost-effectiveness of cetuximab plus best supportive care compared with best supportive care (Table 20). 42 The study is quality assessed in Tables 21 and 22. This quality assessment suggests that the study was generally well conducted.
| Mittmann et al.42 | |||
|---|---|---|---|
| Study purpose | To investigate the cost-effectiveness of CET in metastatic colorectal cancer | ||
| Country setting | Canada | ||
| Base-year prices | 2007 | ||
| Intervention/comparator | CET vs BSC | ||
| Line of treatment | Third line | ||
| Study type | Trial-based cost-effectiveness analysis; CO.17 study | ||
| Model duration/cycle length | 18–19 months | ||
| Number of health states | Not applicable | ||
| Study group | Participants in the CO.17 study: 572 patients with chemorefractory colorectal cancer | ||
| Perspective | Payer perspective: Canadian government | ||
| Discount rate per annum | Not used | ||
| Source of funding | Funding for the CO.17 study was provided by NCIC CTG, AGIGT, B-MS and ImClone Systems | ||
| Base-case findings | For all patients the incremental cost of CET compared with BSC was C$23,969. The ICER was C$199,742 per LYG (95% CI C$125,973 to C$652,492 per LYG) and the incremental cost–utility ratio was C$299,613 per QALY gained (95% CI C$187,440 to C$898,201 per QALY gained). | ||
| For patients with KRAS WT tumours, the incremental cost of CET compared with BSC was $33,617 and the ICER was C$120,061 per LYG (95% CI C$88,679 to C$207,075 per LYG), The incremental cost–utility ratio was C$186,761 per QALY gained (95% CI C$130,326 to C$334,940 per QALY gained). | |||
| Sensitivity analyses | A sensitivity analysis was performed on every cost, resource and effectiveness variable. The ICERs were most sensitive to changes in the cost of CET and patient survival | ||
| Variable | Range | ICER over range (C$/LYG) | |
| Entire study population | |||
| Cost of CET (C$) (base case 3.24/kg) | 2.94–6.73/kg | 188,734–384,823 | |
| Overall survival with CET [base case 7.7 months (0.64 years)] | 6.2 months (0.52 years)–9.2 months (0.77 years) | 166,451–249,677 | |
| KRAS WT patients | |||
| Cost of CET (C$) (base case 3.24/kg) | 2.94–6.73/kg | 112,939–228,591 | |
| Overall survival with CET [base case 9.5 months (0.79 years)] | 7.6 months (0.63 years)–11.4 months (0.95 years) | 100,051–150,076 | |
| Item | Yes/no | |
|---|---|---|
| 1 | Is the study population clearly described? | Yes. The results of all study patients from the CO.17 study were used in this analysis: a total of 572 patients with chemorefractory colorectal cancer all had received prior chemotherapy with a fluoropyrimidine, 98% of patients had received prior treatment with oxaliplatin and 96% had received prior treatment with irinotecan |
| 2 | Are competing alternatives clearly described? | Yes. The study prospectively evaluated the cost-effectiveness of cetuximab when given in addition to best supportive care |
| 3 | Is a well-defined research question posed in answerable form? | Yes. Cetuximab plus best supportive care vs best supportive care for patients with chemorefractory colorectal cancer |
| 4 | Is the economic study design appropriate to the stated objective? | Yes. A trial-based cost-effectiveness analysis |
| 5 | Is the chosen time horizon appropriate to include relevant costs and consequences? | Yes. The time horizon of the analysis was the duration of the clinical trial (i.e. 18–19 months) because > 77% of the patients on cetuximab and 82% of those on best supportive care alone had died by the end of the collection period |
| 6 | Is the actual perspective chosen appropriate? | Yes. The study was calculated from a payer perspective – Canadian government |
| 7 | Are all important and relevant costs for each alternative identified? | Yes. Most main categories of cost were captured (drug, outpatient visits, hospitalisation and surgical procedures, serious adverse events, laboratory tests and diagnostic procedures). KRAS testing was not, however, included |
| 8 | Are all costs measured appropriately in physical units? | Yes. For instance, the total dosage of cetuximab used by each patient in the trial was the basis for drug cost calculations |
| 9 | Are costs valued appropriately? | Yes. For instance, cetuximab drug cost/mg was the median value in the countries that were reviewed by the Patented Medicines Prices Review Board |
| 10 | Are all important and relevant outcomes for each alternative identified? | Yes |
| 11 | Are all outcomes measured appropriately? | Yes. Treatment benefit was defined in terms of mean survival gain after random assignment. Self-reported HUI3 was prospectively collected to assess preference-based measures of health status throughout the study (baseline and weeks 4, 8, 16 and 24 after random assignment) |
| 12 | Are outcomes valued appropriately? | Yes – quality-adjusted life-years |
| 13 | Is an incremental analysis of costs and outcomes of alternatives performed? | Yes, and subjected to sensitivity analysis |
| 14 | Are all future costs and outcomes discounted appropriately? | No. No discounting appears to have been carried out |
| 15 | Are all important variables whose values are uncertain appropriately subjected to sensitivity analysis? | Yes. One-way deterministic sensitivity analyses to test the robustness of the incremental ratios were performed on every cost, resource and effectiveness variable. Uncertainty surrounding the estimates of cost-effectiveness was illustrated by means of cost-effectiveness acceptability curves |
| 16 | Do the conclusions follow from the data reported? | Yes. The ICERs were acknowledged to be ‘high’, which is consistent with the ICERs reported |
| 17 | Does the study discuss the generalisability of the results to other settings and patient/ client groups? | Yes. The authors state that the results may not be generalisable to all patients under routine care for advanced colorectal cancer |
| 18 | Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | Funding for the CO.17 study was provided by the National Cancer Institute of Canada Clinical Trials Group, the Australasian Gastrointestinal Trials Group, Bristol-Myers Squibb and ImClone Systems |
| 19 | Are ethical and distributional issues discussed appropriately? | Yes. Fully explored in discussion section |
| Item | Critical appraisal | Reviewer comment |
|---|---|---|
| Is there a well-defined question? | ✓ | Cetuximab plus best supportive care vs best supportive care for patients with chemorefractory colorectal cancer. Whole population and KRAS WT population analysed |
| Is there a clear description of alternatives (i.e. who did what to whom, where and how often)? | ✓ | Patients in the CO.17 study; cetuximab vs best supportive care in patients with chemorefractory colorectal cancer |
| Has the correct patient group/population of interest been clearly stated? | ✓ | Yes. The study prospectively evaluated the cost-effectiveness of cetuximab when given in addition to best supportive care |
| Is the correct comparator used? | ✓ | Best supportive care, reflective of current standard of care |
| Is the study type reasonable? | ✓ | Trial-based economic evaluation |
| Is the perspective of the analysis clearly stated? | ✓ | Health-care payer perspective – Canadian government |
| Is the perspective employed appropriate? | ✓ | Costs and benefits are appropriate to the perspective |
| Is the effectiveness of the intervention established? | ✓ | The CO.17 study population demonstrated a clinically and statistically significant overall survival advantage for cetuximab vs best supportive care in chemorefractory colorectal cancer patients (median survival 6.1 months vs 4.6 months, hazard ratio for death 0.77, p = 0.005). The survival advantage was even greater in the subset of patients with KRAS WT status (median overall survival for cetuximab vs best supportive care 9.5 months vs 4.8 months, hazard ratio for death 0.55, p < 0.001) |
| Has a lifetime horizon been used for analysis or if not has a shorter time horizon been justified? | ✓ | The time horizon of the study was the duration of the clinical trial (i.e. 18–19 months) because > 77% of the patients on cetuximab and 82% of those on best supportive care alone had died by the end of the data collection period. Median survival time in the study was < 1 year |
| Are the costs and consequences consistent with the perspective employed? | ✓ | The main categories of cost were drugs, outpatient visits, hospitalisation and surgical procedures, serious adverse events, laboratory tests and diagnostic procedures. This is consistent with the health-care payer perspective |
| Is differential timing considered? | ✗ | No. No discounting appears to have been carried out |
| Is incremental analysis performed? | ✓ | Yes |
| Is sensitivity analysis undertaken and presented clearly? | ✓ | One-way deterministic sensitivity analyses were performed and presented in table 20 |
This study by Mittmann and colleagues was a trial-based cost-effectiveness analysis based on the CO.17 study,37 a Phase III, multicentre, open-label, randomised study37 in which resource utilisation and utility values were collected prospectively for 572 patients with advanced colorectal cancer expressing EGFR who received cetuximab plus best supportive care or best supportive care alone. The CO.17 study is also included in the clinical effectiveness review (see Chapter 3, Study characteristics, Cetuximab plus best supportive care compared with best supportive care).
Mean improvement in overall survival for the entire study population was 0.12 years and mean improvement in quality-adjusted survival was 0.08 QALYs. The incremental cost of cetuximab (all patients) compared with best supportive care was C$23,969. The incremental cost-effectiveness ratio (ICER) for cetuximab plus best supportive care compared with best supportive care alone was C$199,742 per life-year gained (LYG) (95% CI C$125,973 to C$652,492 per LYG) and the incremental cost–utility ratio was C$299,613 per QALY gained (95% CI C $187,440 to C$898,201 per QALY gained).
For patients with KRAS WT status, the incremental cost of cetuximab was C$33,617 and mean gains in overall and quality-adjusted survival were 0.28 years and 0.18 QALYs respectively. The ICER was C$120,061 per LYG (95% CI C$88,679 to C$207,075 per LYG) and the incremental cost–utility ratio was C$186,761 per QALY gained (95% CI C$130,326 to C$334,940 per QALY gained). Updating this to 2011, converting to pounds sterling and using the current UK price of cetuximab we estimate the cost–utility ratio to be approximately equivalent to £101,000 per QALY.
Cetuximab plus irinotecan compared with best supportive care
Three studies addressed the cost-effectiveness of cetuximab plus irinotecan compared with best supportive care (Table 23).
Of the three included studies evaluating cetuximab plus irinotecan compared with best supportive care, one was a model-based cost-effectiveness analysis and the other two were trial-based cost-effectiveness analyses. Unfortunately, none of the studies considered patients with KRAS WT status, reducing their relevance to this assessment.
| Annemans et al.59 | Norum60 | Starling et al.61 | |
|---|---|---|---|
| Study purpose | To investigate the cost-effectiveness of CET + IRIN with current approaches to treatment | To compare the cost-effectiveness of including CET in the treatment of metastatic colorectal cancer in Norway | To investigate the cost-effectiveness of CET + IRIN vs BSC |
| Country setting | Belgium | Norway | UK |
| Base-year prices | Not reported, but study that analysis is based on was carried out in 2007 | 2005 | Not reported but study carried out in 2004 |
| Intervention/comparator | CET + IRIN vs current carea | CET + IRIN vs BSC | CET + IRIN vs BSC |
| Line of treatment | Third line | Third line | Third line |
| Study type | Trial-based cost-effectiveness analysis; BOND study49 | Model-based cost-effectiveness analysis | Trial-based cost-effectiveness analysis49,68 |
| Model duration/cycle length | 6-week and 12-week ruleb | Unclear | Lifetime |
| Number of health states | Not applicable | Unclear but likely two states | Not applicable |
| Study group | Belgian participants from the BOND trial (n = 218) vs eligible participants in the three largest study centres (Belgium, France and Italy) who fell outside the recruitment period (retrospective; n = 66) | Patients with metastatic colorectal cancer | Metastatic colorectal cancer patients with disease progression following IRIN failure; patients in the BOND trial49 |
| Perspective | Health-care perspective | Third-party payer | NHS perspective |
An analysis by Annemans and colleagues59 compared the cost-effectiveness of cetuximab plus irinotecan with that of current care in the treatment of EGFR-expressing metastatic colorectal cancer that has failed irinotecan-containing therapy. Treatment outcomes and medical resource use data for patients receiving cetuximab plus irinotecan from the BOND study49 (Phase III, multicentre, open-label, randomised study) were compared with those from a matched group of patients (current care). Current care was based on a retrospective review of treatment received by patients in the three largest BOND study centres (Belgium, France, Italy) who met the eligibility for inclusion in the BOND study but who fell outside the recruitment period for the study. Fifteen per cent had received one prior line of chemotherapy and 77% at least two prior lines of chemotherapy for metastatic disease. Approximately 80% of these patients went on to receive chemotherapy, including capecitabine, 5-FU, raltitrexed and rechallenge with irinotecan or oxaliplatin. Annemans and colleagues considered two scenarios in which cetuximab was discontinued at 6 weeks or at 12 weeks if there was no tumour response at those time points. The ICERs were €17,000 per LYG and €40,000 per LYG for the 6- and 12-week rule respectively compared with current care. Sensitivity analyses showed an acceptable robustness of the results, with generally acceptable ICERs for the 6-week rule, even in the worst-case scenario for cetuximab (defined as higher end survival and lower end cost for the control group). The study concluded that cetuximab plus irinotecan was cost-effective compared with current care when treatment was stopped in the case of non-response after 6 weeks.
Norum60 explored the cost-effectiveness of including cetuximab plus irinotecan in the treatment of metastatic colorectal cancer in Norway using a model-based cost-effectiveness analysis. Based on randomised trial data the increased lifetime gain was between 1.7 and 2.0 months, in addition to the 18–21 months of expected lifetime with standard chemotherapy. The median cost per patient treated was calculated as €34,256–45,764, yielding a cost per LYG in the range €205,536–323,040. Sensitivity analysis documented the price of cetuximab and survival gain to be the major factors influencing the ICER. The efficacy data for this analysis were based on one RCT and single-arm Phase II or III studies; the randomised study did not measure overall survival as its primary end point and had a crossover following progressive disease, with 50% of the patients crossing over from cetuximab alone to cetuximab plus irinotecan. The study, funded by the Norwegian Cancer Union, concluded that cetuximab plus irinotecan was a promising but expensive treatment.
Starling and colleagues61 compared the cost-effectiveness of cetuximab plus irinotecan with that of best supportive care from an NHS perspective for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Effectiveness estimates for the treatment groups were modelled from key clinical trials: Cunningham and colleagues49 compared cetuximab plus irinotecan with cetuximab plus best supportive care, and Cunningham and colleagues68 compared irinotecan monotherapy in a second-line setting with supportive care. The discounted life expectancy was 0.91 years for patients treated with cetuximab plus irinotecan compared with 0.47 years for patients receiving best supportive care. Patients treated with cetuximab plus irinotecan accumulated mean additional costs of £18,901 per patient relative to best supportive care, with £11,802 attributable to drug costs of cetuximab. The incremental cost per LYG of cetuximab plus irinotecan compared with best supportive care was £42,975. The incremental cost per QALY gained was £57,608. The study concluded that the incremental cost per LYG for cetuximab plus irinotecan is relatively high compared with that for other interventions.
Cetuximab compared with best supportive care and cetuximab plus irinotecan compared with best supportive care
One included study62 evaluated the cost implications of treatment with sequential regimens that include chemotherapy and/or monoclonal antibodies. Nine possible treatment sequences were selected to reflect the sequential advances in colorectal cancer treatment. Of these, five treatment sequences involving cetuximab third line were considered relevant to this review. The general characteristics of this study are given in Table 24.
| Wong et al.62 | |
|---|---|
| Publication type | Full paper |
| Study purpose | To measure the cost implications of treatment with sequential regimens that include chemotherapy and/or monoclonal antibodies |
| Country setting | USA |
| Base-year prices | 2008 |
| Intervention/comparator | Nine possible treatment strategies selected to reflect the sequential advances in colorectal cancer treatment. Of these, five treatment sequences involving CET third line were considered relevant to this reviewa |
| Line of treatment | Sequences of relevance to the review consider third-line treatment |
| Study type | Model-based cost-effectiveness analysis |
| Model duration/cycle length | Unclear |
| Number of health states | Unclear but likely four states |
| Study group | A hypothetical cohort of 1000 patients with newly diagnosed metastatic colorectal cancer. Patients supposedly received up to three lines of treatment before supportive care and death (as stated above, only the five treatment sequences that considered CET third line were considered relevant to this review) |
| Perspective | Third-party payer |
| Discount rate per annum | Life expectancyb and costs at 3% per year |
| Source of funding | Three authors had acted as consultants for industry (Amgen, Sanofi-Aventis, B-MS, Pfizer and Genentech) and one author had received a research grant from B-MS and was supported during the research by funding from the ASCO Young Investigator Award |
| Base case findings | The ICER per discounted LYG for adding the modern chemotherapy agents is approximately US$100,000. The benefits of adding monoclonal antibodies come at a higher cost (US$170,000 per discounted LYG). The modest additional benefits of the most effective regimen (using both CET and IRIN in the third-line setting) come at an even higher cost (US$240,00 per discounted LYG) |
| Sensitivity analyses | One-way sensitivity analyses on progression, toxicity, drug cost and second progression demonstrated that the ICERs for sequences containing monoclonal antibodies are very high. The most significant changes in the ICERs occurred when the parameters for first-line treatment were changed |
Wong and colleagues62 used a Markov model to evaluate a hypothetical cohort of 1000 patients with newly diagnosed metastatic colorectal cancer. Patients received up to three lines of treatment before supportive care and subsequent death. Data were obtained from published, multicentre, Phase III clinical trials. The study considered nine possible treatment sequences; of these, five were considered relevant to this review (i.e. drug of interest in the third-line setting: three sequences of cetuximab plus best supportive care and two sequences of cetuximab plus irinotecan). Based on drug costs alone, treatment that included the new chemotherapeutic agents increased survival at an ICER of US$100,000 per discounted life-year. The addition of monoclonal antibodies improved survival at an ICER of > US$170,000 per discounted life-year. The results were most sensitive to changes in the initial regimen. Even with significant improvements in clinical characteristics (efficacy and toxicity), treatments with the most effective regimens have very high ICERs. The authors concluded that treatment of metastatic colorectal cancer with the most effective regimens came at very high incremental costs.
Of the three abstracts identified in the review, one65 examined the cost-effectiveness of cetuximab use among metastatic colorectal cancer patients. Despite further information being requested from the authors none was received. It should be noted that the title of the abstract suggests that the analysis was undertaken in an elderly patient population. In the patients with metastatic colorectal cancer, the incremental cost per QALY was $336,218 for cetuximab and $318,609 for cetuximab plus irinotecan in comparison with best supportive care. Probabilistic sensitivity analysis demonstrated that best supportive care is more cost-effective than cetuximab treatments until the willingness-to-pay threshold is raised to $240,000. The authors conclude that cetuximab is not cost-effective, either in monotherapy or in combination with irinotecan, as the cost-effectiveness ratios are far beyond the accepted threshold of $50,000 per QALY gained.
Kirsten rat sarcoma testing and cetuximab treatment
Mittmann and colleagues42 concluded that restricting cetuximab to patients with KRAS WT status reduced the ICER, resulting in a more efficient use of health-care resources. However, Mittmann and colleagues also note that, as the KRAS WT patient group had a greater survival gain with cetuximab compared with best supportive care (3–4 months or 0.25–0.33 years) than the overall group (1.5 months or 0.13 years), the drug cost was also greater in the group with KRAS WT status because cetuximab was used for a longer time. The ICER was still high for the group with KRAS WT status (C$120,061 per LYG and C$189,761 per QALY gained) and would generally be considered unfavourable. The authors hypothesise that, to achieve a generally accepted level of cost-effectiveness, the survival gain would need to be in the order of 6–8 months.
One of the abstracts identified63 examined the cost–utility of using KRAS mutation testing before initiating monotherapy for patients with metastatic colorectal cancer from a US payer perspective. Although further information was requested none was received and more detailed assessment was not possible. It is worth noting, however, that the results suggest that the use of KRAS testing to select patients for cetuximab treatment in metastatic colorectal cancer can reduce costs (US$10,037) with a negligible impact on QALYs compared with using cetuximab for all patients.
Panitumumab plus best supportive care compared with best supportive care
Graham and colleagues64 assessed the cost-effectiveness of panitumumab plus best supportive care compared with best supportive care alone in chemorefractory metastatic colorectal cancer patients with KRAS WT status in the Netherlands. In the base-case analysis, the ICERs for metastatic colorectal cancer patients with KRAS WT status receiving panitumumab plus best supportive care compared with best supportive care alone were €51,314 per LYG and €59,440 per QALY gained. Univariate sensitivity analyses and probabilistic sensitivity analyses showed the results to be robust to assumptions around input parameters. We requested more information on this abstract from the authors but none was received to allow a more detailed assessment of the study for inclusion. Interestingly, despite a number of Amgen-linked authors, no mention was made of this abstract in Amgen's submission.
No other studies looking at the cost-effectiveness of panitumumab in the relevant patient population were found in the literature review.
Bevacizumab plus non-oxaliplatin-containing regimens
No studies looking at the cost-effectiveness of bevacizumab in the relevant patient population were found in the literature review.
Conclusions
There were five studies included in the review that considered cetuximab or cetuximab plus irinotecan in third-line therapy. In addition, three abstracts were identified for which we received no further information; of these, one considered the cost-effectiveness of KRAS testing prior to cetuximab treatment,63 one considered the cost-effectiveness of cetuximab (monotherapy and combination therapy)65 and one considered panitumumab in third-line therapy. 64
Of the full papers, Annemans and colleagues59 concluded that, in comparison with best supportive care, cetuximab was a cost-effective treatment option in one of the scenarios tested (6-week rule, i.e. cetuximab was discontinued at 6 weeks if there was no tumour response at that time point). The other studies42,43,60–63 concluded that, although clinically effective, cetuximab is an expensive intervention. The study by Wong and colleagues,62 which evaluated treatment strategies that included one, two, three or four therapies, concluded that, in general, the treatment of metastatic colorectal cancer with the most effective regimens comes at very high incremental costs.
Of the studies identified in the review, the study by Mittmann and colleagues42 was the only full paper to consider metastatic colorectal cancer patients with KRAS WT status. They concluded that, although the ICER for cetuximab compared with best supportive care in metastatic colorectal cancer patients was high and sensitive to drug cost, it was lower when analysis was limited to patients with KRAS WT tumours. We consider this in greater detail and in comparison with the PenTAG cost-effectiveness model in Chapter 6. In addition, results from a study by Carlson and colleagues63 also suggest that the use of KRAS testing to select patients for cetuximab treatment in metastatic colorectal cancer can reduce costs compared with using cetuximab for all patients, with a negligible impact on QALYs.
One abstract64 identified in the review found panitumumab to be a cost-effective treatment option in metastatic colorectal cancer patients with KRAS WT status; however, no further information was made available for analysis.
Most of the available studies were supported by grants from industry. In some cases, the cost-effectiveness studies received independent funding, for example that by Norum,60 yet many of the RCTs on which they were based had received funding, either in full or in part, from industry.
Chapter 5 Assessment of industry submissions to the National Institute for Health and Clinical Excellence
Introduction
Three manufacturer submissions were potentially available for this MTA; however, only one full economic model was submitted, which was by Merck Serono for cetuximab. 69 Roche submitted some basic cost calculations in its report of a comparison between bevacizumab plus FOLFIRI and cetuximab plus FOLFIRI,70 whereas Amgen did not provide any details of a cost-effectiveness model, nor make any comment on the likely cost-effectiveness of panitumumab. 71 In this section the full economic model submitted by Merck Serono, the cost calculations presented by Roche and the trial analysis submitted by Amgen are critiqued. The material in this chapter which has been reproduced from these manufacturers'submissions in text, tables and figures has been done so with the permission of Merck Serono, Roche and Amgen.
Industry submission critique 1: Merck Serono, cetuximab
The decision problem: cetuximab
Merck Serono restricts the evaluation of cetuximab to third and subsequent lines of treatment. Bevacizumab is disregarded by Merck Serono as an inappropriate treatment comparator for cetuximab because of the lack of published clinical data on the effectiveness of bevacizumab for metastatic colorectal cancer patients with KRAS WT status in third-line treatment. In summary, Merck Serono reports estimates of cost-effectiveness for the following four comparisons:
-
cetuximab plus best supportive care compared with best supportive care
-
cetuximab plus irinotecan compared with best supportive care
-
cetuximab plus best supportive care compared with panitumumab plus best supportive care
-
cetuximab plus irinotecan compared with panitumumab plus best supportive care.
There are three points of interest relating to these comparisons. First, the pairwise cost-effectiveness comparisons are selective in the sense that several relevant comparisons, although possible, are not presented. Figure 3 shows the data available to Merck Serono (the bold lines) and the cost-effectiveness comparisons that it has undertaken (the dashed lines). It has not compared cetuximab plus irinotecan with cetuximab plus best supportive care despite the data being available.
FIGURE 3.
Diagram of the available data and the cost-effectiveness comparisons modelled by Merck Serono. Bold lines represent published effectiveness data, dashed lines represent the cost-effectiveness comparisons made by Merck Serono. BSC, best supportive care; CET, cetuximab; IRIN, irinotecan; PAN, panitumumab.
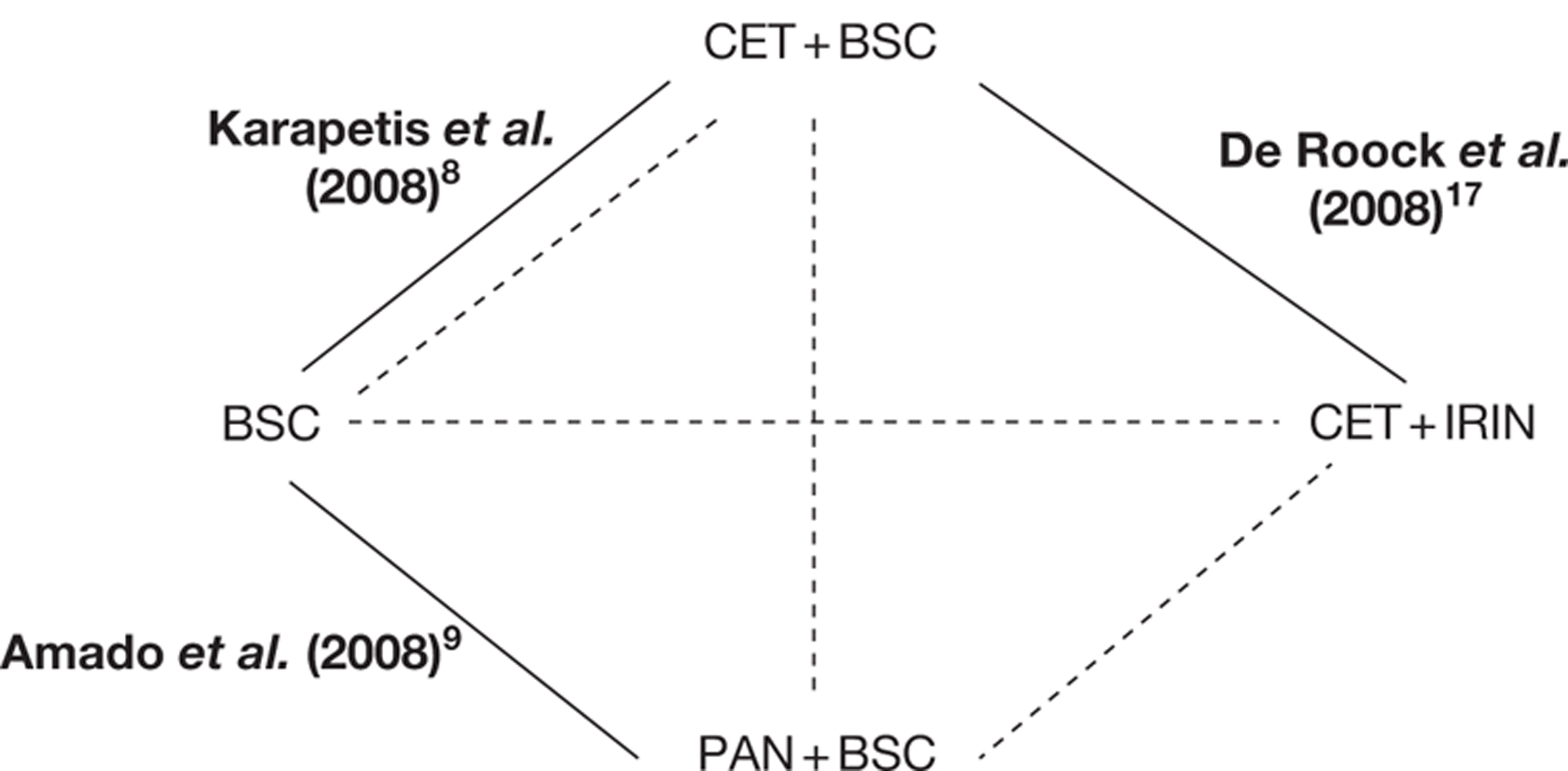
Second, depending on the active comparator arm, the best supportive care arm is modelled slightly differently (hence, post hoc incremental analysis could not be undertaken by the assessment group). This difference in the modelling of the best supportive care arms appears to depend on data availability and flexibility in modelling and is discussed in Modelling progression-free survival and overall survival: cetuximab. Third, Merck Serono assesses cost-effectiveness only in third-line treatment and does not consider the second-line scenario, which the scope of this guidance also covers (see Line of treatment: cetuximab).
Overview of model design: cetuximab
A three-state Markov model, with health states defined as progression free, progressive disease and death, is used by Merck Serono to compare best supportive care with cetuximab plus best supportive care and cetuximab plus irinotecan in third-line treatment for metastatic colorectal cancer patients with KRAS WT status (Figure 4). Treatment-specific utilities are assigned to the progression-free and progressive disease states (discussed further in Utilities: cetuximab). The costs associated with KRAS testing, drug acquisition and administration, best supportive care and adverse events are accounted for in the model (discussed further in Utilities: cetuximab). Merck Serono states that all patients in the progression-free state are assumed to receive active treatment whereas those in the progressive disease state receive best supportive care only (see p. 94 of Merck Serono's submission69). As will be discussed in Drug acquisition costs and dose intensity: cetuximab, this is not how the base-case analysis is modelled and so the reported base-case ICERs are misleading.
FIGURE 4.
Three-state Markov model used by Merck Serono.
Summary of results: cetuximab
The base-case results are reproduced from the Merck Serono submission in Tables 25 and 26 for cetuximab plus best supportive care compared with best supportive care and cetuximab plus irinotecan compared with best supportive care respectively. Merck Serono shows that the biggest cost component for the cetuximab plus best supportive care and the cetuximab plus irinotecan arms is the drug acquisition costs, followed by the best supportive care and drug administration costs. The QALYs gained for cetuximab plus best supportive care are similar between the progression-free and the progressive disease health states, as is also the case for cetuximab plus irinotecan. Merck Serono conducted a probabilistic sensitivity analysis of its base-case assumptions. It reports that the probability that cetuximab plus best supportive care is cost-effective compared with best supportive care is 0.1% at a willingness to pay of £30,000 per QALY gained and 65% at a willingness to pay of £50,000 per QALY gained. It also reports a 16% probability that cetuximab plus irinotecan is cost-effective compared with best supportive care at a willingness to pay of £30,000 per QALY gained, with a probability of 68% of cetuximab plus irinotecan being cost-effective compared with best supportive care at a willingness to pay of £50,000 per QALY gained. Merck Serono argues that the end of life (EoL) criteria are appropriate for both cetuximab plus best supportive care and cetuximab plus irinotecan.
Merck Serono has followed NICE's reference case as far as is possible given the evidence limitations. A NHS and Personal Social Services (PSS) perspective has been taken, with costs and outcomes discounted at an annual rate of 3.5% (Table 27). The only exception is for the health-related utility values for which HUI3 rather than EQ-5D data were used. The impact of this on the results is unknown.
| Comparators | Total costs (£) | Total LYG | Total QALYs | Incremental costs (£) | Incremental LYG | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|---|---|
| BSC | 7580 | 0.512 | 0.359 | ||||
| CET + BSC | 21,836 | 0.829 | 0.662 | 14,256 | 0.317 | 0.303 | 47,095 |
| Comparators | Total costs (£) | Total LYG | Total QALYs | Incremental costs (£) | Incremental LYG | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|---|---|
| BSC | 7947 | 0.547 | 0.391 | ||||
| CET + IRIN | 37,248 | 1.325 | 1.059 | 29,301 | 0.779 | 0.668 | 43,887 |
| Element | NICE reference case72 | Merck Serono's model |
|---|---|---|
| Perspective on costs | NHS and PSS | ✓ |
| Perspective on outcomes | All health effects on individuals | ✓ |
| Type of economic evaluation | Cost-effectiveness | ✓ Cost–utility |
| Synthesis of evidence on outcomes | Based on systematic review | ✓ Except that data relating to cetuximab plus irinotecan vs cetuximab plus best supportive care48 are not from a RCT and are of questionable quality |
| Measure of health effects | QALYs; EQ-5D preferred measure of HRQoL | ✓ QALYs used, but utilities based on HUI3 |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | ✓ Reported directly by patients. Valuation based on Canadian public preferences42 |
| Discount rate | Annual rate of 3.5% for costs and health effects | ✓ |
| Equity weighting | Same QALY weight regardless of other characteristics of the individuals receiving the health benefit | ✓ |
Sensitivity analyses suggest that the important drivers for the cost-effectiveness analysis are the cetuximab acquisition and administration costs, the utility values assigned to progression-free and progressive disease and the cost of best supportive care. In Critique of Merck Serono's model for cetuximab a number of uncertainties regarding the cetuximab administration and best supportive care costs and the utilities used in the Merck Serono model are discussed. Furthermore, Merck Serono did not assess the sensitivity of the model to the effectiveness data.
Line of treatment: cetuximab
Merck Serono argues that it considers only third-line use of cetuximab in its submission because this is ‘based on clinical need, the strength of the evidence, expert opinion and current usage’ (p. 21, Merck Serono's submission69). Accordingly, the studies informing estimates of effectiveness in the model mainly involve patients who have previously received two or more lines of treatment. The EPIC study73–75 is available to model second-line treatment comparing cetuximab plus irinotecan with irinotecan. Merck Serono argues that irinotecan is an appropriate second-line treatment comparator, and our clinical expert agrees that it is part of current practice in second-line treatment. There are, however, a number of issues related to the EPIC study: first, 46.9% of patients randomised to irinotecan crossed over to receive cetuximab at some stage and, second, KRAS status is not known for the participants. It is understood that the manufacturer did not have access to the individual patient data and could not address this accurately; however, it would have been possible for some assumptions to have been made to find some indication of the likely cost-effectiveness of cetuximab plus irinotecan compared with irinotecan in second-line therapy.
Peninsula Technology Assessment Group corrections for errors: cetuximab
In this section all ICERs reported from the Merck Serono model have been corrected for the following three logical errors identified in Merck Serono's Excel (Microsoft Corporation, Redmond, WA, USA) spreadsheet:
-
cetuximab plus irinotecan cost of administration: a cost of £180 is modelled when Merck Serono reports in its submission that this value should be £196
-
incorrect proportion in progression-free state at cycle 0 for best supportive care arm in comparison with cetuximab plus best supportive care
-
cost of non-serious adverse events in best supportive care arm: cost of £200 is modelled when Merck Serono reports in its submission that this value should be £174.
Note that correction for these errors leads to slightly increased base-case ICERs compared with those reported by Merck Serono – from £47,095 to £48,238 per QALY for cetuximab plus best supportive care compared with best supportive care and from £43,887 to £44,429 per QALY for cetuximab plus irinotecan compared with best supportive care.
Critique of Merck Serono's model for cetuximab
In this section we detail our main concerns over the assumptions made in the model submitted by Merck Serono. Where possible we present the impact on the ICERs of alternative assumptions. In Impact on the ICERs: cetuximab the cumulative effect of different assumptions on Merck's base-case results are presented.
Drug acquisition costs and dose intensity: cetuximab
Merck Serono assumes a guaranteed NHS price of £136.50 for a 20-ml (100-mg) vial of cetuximab. We believe that this price is that which would be available nationally. Merck assumes a cost for generic irinotecan of £120.30 for a 5-ml (100-mg) vial from the British National Formulary(BNF) 61. 76
The manufacturer states that dose intensity is included in the model and presents details in table 81 of its submission69 for dose intensity of cetuximab plus best supportive care, cetuximab plus irinotecan, and irinotecan (Table 28). However, examination of the Microsoft Excel model indicates that only the calculations for the cost of cetuximab include consideration of dose intensity. The value used in the Excel model is 94% but the value reportedly used in the submission is 98% (‘based on CO.17 study’; see p. 110, Merck Serono's submission69). Thus, for cetuximab plus best supportive care, a lower dose intensity is assumed in the model than is specified in the report. For irinotecan, 100% dose intensity is modelled even though the report indicates that a dose intensity of 90% is modelled. Adjusting the drug costs for these discrepancies has negligible impact on the PenTAG corrected base-case ICERs.
| Treatment | Reported (%) | Modelled (%) | Impact |
|---|---|---|---|
| CET (in CET + BSC) | 98 | 94 | Negligible |
| CET (in CET + IRIN) | 94 | 94 | Negligible |
| IRIN | 90 | 100 | Negligible |
Merck Serono assumes vial wastage for cetuximab in its base-case analysis but does not assume wastage for treatment with irinotecan, instead assuming vial sharing. Although inconsistent, this is unlikely to have much of an impact on the ICERs as the cost of irinotecan is very small relative to the cost of cetuximab; however, vial sharing of irinotecan does not happen in the UK.
Administration costs: cetuximab
Merck Serono assumes that administration takes place in the day-care setting, with a cost of £180 for cetuximab plus best supportive care for ‘deliver simple parenteral chemotherapy at first attendance’ from the NHS reference costs for 2008–2009,77 and £196 for cetuximab plus irinotecan (the average of £180 for delivery of cetuximab plus best supportive care and £213 for ‘deliver more complex chemotherapy at first attendance’).
The costs of administration of cetuximab used by Merck Serono could be too low as they refer to drugs at first delivery, whereas ‘delivery of subsequent elements of chemo cycle’ is likely to be more relevant. This would incur costs of £227 per administration at 2008–9 prices. Note that this is the cost of drug administration assumed by Roche in its cost calculations for bevacizumab (see Costs: bevacizumab). Merck Serono does not account for pharmacy preparation costs in its model. In the PenTAG model we assume a pharmacy preparation cost of £15 per infusion informed by data from a pharmacist at the Royal Devon and Exeter Hospital (Kate Copland, Chief Technician Aseptic Services in Pharmacy, Royal Devon & Exeter Hospital, personal communication, 2011; see Chapter 6). Although these pharmacy preparation costs are small, they are incurred for every administration. Thus, assuming an administration cost of £242 (£227 + £15) for cetuximab plus best supportive care at 2008–9 costs (consistent with Merck's model) increases the ICER slightly (Table 29).
| Treatment | Cost used by Merck | Alternative cost value | Impact |
|---|---|---|---|
| CET (in CET + BSC) | £180 | £242 | Alternative cost value increases ICER from £43,238 to £50,624 per QALY gained |
| CET (in CET + IRIN) | £196 | £242 per CET infusion; £128.50 per IRIN infusion | Alternative cost value increases ICER from £44,429 to £47,624 per QALY gained |
It is difficult to assess the administration costs of irinotecan in addition to those of cetuximab. In the PenTAG model a best estimate of the average cost of no additional administration time being required (£0) and the same administration time as that for cetuximab being required (£227) is assumed (see Chapter 6, Pharmacy drug preparation costs). This leads to an administration cost for irinotecan, including pharmacy preparation time, of £128.50 per infusion (£113.50 + £15). Using this cost estimate, the ICER for cetuximab plus irinotecan compared with best supportive care increases slightly (see Table 29).
In the sensitivity analyses Merck Serono examines the impact of assuming different dosing regimens for irinotecan for the cetuximab plus irinotecan compared with best supportive care comparison. It is reported that the ICER increases when assuming 350 mg/m2 administration every 3 weeks, and decreases when assuming 125 mg/m2 every 3 weeks. However, the base-case analysis assuming that administration of irinotecan is every 2 weeks at a dose of 180 mg/m2 nationally is reasonable.
Treatment duration: cetuximab
In the Excel model, for the base-case analyses, drug acquisition and administration costs are modelled for all patients in the progression-free state until week 13 for cetuximab plus best supportive care and week 24 for cetuximab plus irinotecan. At these times approximately 60% of patients are still in the progression-free state for all active treatments. After these times, no drug acquisition or administration costs are assumed for patients but they remain in the progression-free health state. In the Excel model the 13- and 24-week cut-offs imply an estimated mean time on drug treatment of 11.4 weeks and 19.4 weeks for cetuximab plus best supportive care and cetuximab plus irinotecan respectively. This is our main concern with Merck Serono's model.
In its report, Merck Serono gives no explanation for the value of these cut-offs for cetuximab (plus best supportive care or irinotecan). Instead, it states in the list of model assumptions (see table 59, Merck Serono's submission69) that ‘the model is adjusted with clinical data related to the mean number of weeks on chemotherapy’. However, Merck Serono does not report the mean number of weeks on treatment for cetuximab plus best supportive care or cetuximab plus irinotecan in its submission nor does it provide sufficient detail concerning the proper justification for these very important assumptions. The rationale for this assumption cited in the report is ‘The model offers the option to adjust the number of chemotherapy cycles in order to (1) generate the mean number of cycles corresponding to the estimated mean number of doses in clinical studies, (2) ensure that the chemotherapy costs stay within plausible ranges in the probabilistic sensitivity analysis when the parameters of the progression-free survival can be sampled from a distribution and generate an unrealistic amount of time spent on active treatment’ (p. 97, Merck Serono's submission69).
We asked Merck Serono to clarify its assumptions regarding mean time on treatment as it was not sufficiently reported in the submission. In its response it states that only the median time on treatment for the total cetuximab plus best supportive care population (KRAS WT and KRAS mutant status) is available, and this is 8.1 weeks. 37 It is reported in the panitumumab plus best supportive care compared with best supportive care study by Amado and colleagues32 that a mean of 10 panitumumab infusions was received by patients with KRAS WT status. For all patients (KRAS WT and KRAS mutant status combined) the mean number of panitumumab infusions is reported to be seven. Using these data from the panitumumab plus best supportive care compared with best supportive care study, Merck Serono multiplies the median 8.1 weeks on cetuximab for all patients by 10/7 to estimate the mean number of infusions of cetuximab for patients with KRAS WT status. This gives a value of 11.57 infusions for patients with KRAS WT status. Merck Serono then adjusts the mean number of weeks on treatment in the model to 11.4, which corresponds ‘as close as possible to 11.57’, assuming one infusion per week. 69 This approach assumes that the relative difference between the mean number of infusions for patients with KRAS WT status and the mean number of infusions for all patients in panitumumab plus best supportive care is the same as the difference between the ‘median weeks on treatment’ for patients with KRAS WT status and the ‘median weeks on treatment’ for all patients. First, there is no evidence to suggest that the relative difference between the mean number of infusions for patients with KRAS WT status and for all patients in the panitumumab plus best supportive care arm can be assumed for cetuximab plus best supportive care. Second, there is no evidence to suggest that the relative difference in means is the same as the relative difference in medians. Furthermore, the median 8.1 weeks on cetuximab treatment from Jonker and colleagues37 is very similar to the median progression-free survival in this study (8.2 weeks). This suggests but does not prove that treatment is received throughout the progression-free state, which our clinical expert believes is reasonable. For more information on this see Chapter 6, Time on cetuximab plus irinotecan treatment.
For cetuximab plus irinotecan, Merck Serono states in its response to our request that in the cetuximab plus irinotecan compared with cetuximab plus best supportive care study (BOND49) the mean number of infusions for all patients (KRAS WT and KRAS mutant status combined) receiving cetuximab plus best supportive care and cetuximab plus irinotecan was 7 and 18 respectively. In fact, these figures refer to the median not mean number of infusions. 49 Second, it is not clear how this mean of 18 infusions was then translated by Merck Serono into its assumed mean of 19.4 weeks of treatment for cetuximab plus irinotecan. Note again that, as for cetuximab plus best supportive care, the trial data suggest that treatment duration is very similar to progression-free survival. It is reported in BOND that median progression-free survival for all patients on cetuximab plus best supportive care is 6.5 weeks whereas the median number of infusions of cetuximab was seven, corresponding to 7 weeks of treatment. 49 For more information on this see Chapter 6, Time on cetuximab plus irinotecan treatment.
Merck Serono's assumption that drug treatment ceases at the cut-off times (of 13 and 24 weeks) even if patients have not progressed has a large impact on the base-case ICERs, as the costs associated with drug treatment are reduced yet there is no impact on time to progression or death and therefore on QALYs. This contradicts another of Merck Serono's assumptions, that ‘Patients in the progression free state are assumed to be on therapy whereas patients in the progressive disease health state are assumed to receive no active treatment’ (see p. 94, Merck Serono's submission69). Assuming that all patients continue to receive active treatment as long as they remain in the progression-free state increases the ICERs substantially, as shown in Table 30.
| CET + BSC vs BSC | CET + IRIN vs BSC | |
|---|---|---|
| ICER (£/QALY) | 75,417 | 67,429 |
| Incremental costs (£) | 22,289 | 45,018 |
| Incremental QALYs | 0.296 | 0.668 |
| Total costs (CET + BSC/IRIN) (£) | 29,868 | 52,927 |
| Total costs (BSC) (£) | 7580 | 7909 |
| Total QALYs (CET + BSC/IRIN) | 0.662 | 1.059 |
| Total QALYs (BSC) | 0.367 | 0.391 |
These ICERs are much larger than those reported by Merck Serono (£47,000 vs £75,417 per QALY gained for cetuximab plus best supportive care vs best supportive care and £44,000 vs £67,429 per QALY gained for cetuximab plus irinotecan vs best supportive care). The estimates in Table 30 are likely to be slight overestimates as some patients in the progression-free state may discontinue active treatment for reasons other than disease progression. However, the trial data indicate that very few patients discontinue treatment because of adverse events [e.g. Jonker and colleagues37 report that 11 patients (4%) receiving cetuximab plus best supportive care withdrew because of adverse events]. Merck Serono argues that ‘patient[s] dropping out of treatment are not doing so due to an injection site reaction but more probably due to non efficacious treatment. At this point patients are in PD’ (see table 59, Merck Serono's submission69). Therefore, the ICERs in Table 30 are more likely to be closer to reality than those presented in Merck Serono's base case, in which it is assumed that treatment ceases for all patients at 13 or 24 weeks.
In Merck Serono's submission it is stated that ‘patients dropping out of active treatment are allocated to the progressive disease state’ (table 59, Merck Serono's submission69). However, in Merck Serono's model patients remaining in the progression-free state after stopping active treatment continue to receive the utilities for being progression free, not those associated with progressive disease as this assumption would indicate.
Best supportive care costs: cetuximab
Merck Serono uses a monthly value of £785 for best supportive care costs in its model. It cites Remak and Brazil78 for the source of this cost but does not provide the actual value extracted from Remak and Brazil. It is therefore assumed that the cost of best supportive care of £675 is extracted (from table 578). We believe that Merck Serono has made two errors in its use of the Remak and Brazil data: first, by the methods of inflating this value and, second, by its use of this value in both the progression-free and progressive disease health states. Inflating £675 from 2004 to 2008–9 costs using the Retail Price Index,79 as reported by Merck Serono, gives the value used by the manufacturer of £785. However, the manufacturer's inflating of the best supportive care costs are incorrect as it assumes that the costs reported by Remak and Brazil are at 2004 prices when in fact they are at 2000 prices. 78 Using Merck's method of inflating using the Retail Price Index from 2000 leads to a monthly cost of best supportive care of £856 (rather than £785). In addition to this, Merck Serono has used the incorrect index to inflate this value. It uses the Retail Price Index as opposed to the Hospital and Community Health Service Index from Curtis,79 which is widely used in health technology assessment (HTA) economic analyses as it refers specifically to hospital and health service costs. Using the Hospital and Community Health Service Index and inflating the Remak and Brazil value of £675 from 2000 to 2008–9 gives a cost of £917 a month, a 17% increase on the value used by Merck Serono. This increase in the cost of best supportive care leads to slight increases in the ICERs: £49,938 per QALY gained for cetuximab plus best supportive care compared with best supportive care, and £46,276 per QALY gained for cetuximab plus irinotecan compared with best supportive care (assuming PenTAG's corrections to Merck Serono's base-case ICERs as reported in Peninsula Technology Assessment Group correction for errors: cetuximab).
This value is not appropriate to use for both the progression-free and the progressed disease health states as Merck Serono does. Remak and Brazil78 present costs for four periods of stage IV breast cancer treatment: (1) active drug treatment, (2) follow-up after active treatment until disease progression, (3) active supportive care after disease progression and (4) EoL care. The value assumed to have been extracted by Merck Serono, £675, relates to active supportive care after disease progression; however, Merck Serono applies this value to all patients in all treatment arms regardless of whether they are in the progression-free or progressive disease health state. Remak and Brazil report the combined monthly cost for the progression-free state [covering periods (1) and (2) above]. This cost is £679, which is very similar to the cost of active supportive care that Merck Serono uses from Remak and Brazil for the progression-free state. However, the progression-free state costs from Remak and Brazil included active treatment (including drugs specifically for breast cancer) and so are likely to be an overestimate of the best supportive care costs appropriate to the progression-free state in the Merck Serono model, because this should reflect non-drug-related costs. This will lead to the total costs of the best supportive care arms being overestimated with the consequence that the ICERs for cetuximab plus best supportive care compared with best supportive care and cetuximab plus irinotecan compared with best supportive care are underestimated. Given these issues related to the best supportive care costs that Merck Serono has used from Remak and Brazil, we believe that the best supportive care cost assumed by Merck Serono is an underestimate for the progressive disease state and an overestimate for the progression-free state.
Kirsten rat sarcoma testing costs: cetuximab
Merck Serono accounts only for the KRAS costs associated with those patients who subsequently receive cetuximab, that is, those who are KRAS WT. In practice, all patients considered for cetuximab will have a KRAS test and therefore it is appropriate that the cost of the KRAS test should also include those patients who are tested but who are KRAS mutant. Previous cost–utility models for cetuximab have assumed a KRAS testing cost of £300;17 however, data from the All Wales Medical Genetics Service suggest that the cost of a KRAS test is £160. 80 The real cost of KRAS testing (including the cost of those identified to be KRAS mutant) is likely to be £296 (£160/0.54) (see Chapter 6, Costs of epidermal growth factor receptor and Kirsten rat sarcoma testing). Updating Merck's model with this assumption increases the ICER very slightly.
Utilities: cetuximab
Merck Serono uses utilities from the cost-effectiveness analysis of CO.17 by Mittmann and colleagues,42 who report the analysis of HUI3 scores according to values taken from the CO.17 study and valued by the general Canadian population. As Merck Serono states, the utility analysis by Mittmann and colleagues is presented at baseline and 2, 8, 16 and 24 weeks after random assignment and is therefore not useful for the Merck Serono model in which the health states are defined as progression free and progressed disease. Thus, Merck Serono has reanalysed the utility data from the CO.17 study according to these health states. The reanalysis produces mean HUI values that are generally greater than those reported by Mittmann and colleagues (Table 31).
| Study | Stage of disease | Treatment arm | No. of patients | Utility values |
|---|---|---|---|---|
| Ramsey et al.81 | Stage IV | N/A | 13 | 0.76–0.95 |
| Mittmann et al.42 | Study baseline | CET + BSC | 263 | 0.72 |
| BSC | 260 | 0.71 | ||
| 24 weeks from baseline | CET + BSC | 82 | 0.77 | |
| BSC | 36 | 0.70 | ||
| Merck Serono69 | Progression-free state | CET + BSC | 294 | 0.81 |
| BSC | 170 | 0.75 | ||
| Progressive disease state | CET + BSC | 83 | 0.79 | |
| BSC | 85 | 0.69 |
Assessment of the CO.17 study indicates that at baseline all patients were progression free. 37,47 Therefore, we can assume that the baseline mean HUI values reported by Mittmann and colleagues could give some idea of the likely progression-free utility values that the manufacturer may obtain in its analysis (utility cetuximab plus best supportive care utility = 0.72, best supportive care = 0.71). However, the utility values that the manufacturer reports are considerably greater for the progression-free state (utility cetuximab plus best supportive care = 0.81, best supportive care = 0.75).
The numbers of patients included in the calculations of the utility values differ between the analysis by Mittmann and colleagues42 and the manufacturer's reanalysis (see Table 31). There is no explanation as to why this has occurred. We asked Merck to clarify the population base on which its utility estimates are based. It reported that the utility estimates are restricted to patients with KRAS WT status (see Appendix 11). However, given the numbers of patients contributing to the utility estimates, it is unlikely that this is the case. For example, 294 patients receiving best supportive care and 170 patients receiving cetuximab contribute to Merck's reanalysis of utilities in the progression-free health state (see table 80, Merck Serono's submission69); however, in Karapetis and colleagues45 it is reported that the total numbers of patients with KRAS WT status receiving best supportive care and cetuximab plus best supportive care are 113 and 117 respectively. By considering all patients (KRAS WT and KRAS mutant status combined), the mean utility for those receiving cetuximab could have been underestimated by Merck Serono, as patients with KRAS mutant status would not experience a treatment effect. It would be difficult to quantify this underestimation without access to the individual patient data. Moreover, it is highly unlikely that the utility values used by Merck Serono are larger than would be expected in the absence of all sources of bias, as is now discussed.
The progressive disease utility estimates from the analysis by Mittmann and colleagues42 and the Merck Serono reanalysis are based on a much lower number of patients than the number used for the progression-free state (see Table 31). Furthermore, there is likely to be unequal dropout between the treatment arms, with those in the best supportive care arm more likely to drop out because of a lack of treatment effect, meaning that they progress more quickly and are perhaps less able to complete the questionnaire. In addition, the CO.17 study37 is not blinded and so there is the potential for bias in the QoL measures. The unequal dropout and lack of blinding in the CO.17 study could lead to overestimates of the utilities in both treatment arms. Placing these utility values in context, the HUI values from Ramsey and colleagues81 are of a similar magnitude to, or even greater than, those reported by Merck Serono: 0.76–0.95 for stage IV depending on time since diagnosis compared with 0.69–0.81 (see Table 31). However, Ramsey and colleagues note that the study design is likely to have excluded more severely ill patients and the utility values are based on just 13 patients; therefore, these values are likely to be overestimates. This seems probable when the UK EQ-5D norm is 0.73 for those aged ≥ 75 years. 82 EQ-5D values collected alongside the MABEL study61 for metastatic colorectal cancer patients receiving cetuximab plus irinotecan also suggest such high values: mean utility of 0.746.
In the Merck Serono cost–utility model, although the utility data are from the cetuximab plus best supportive care compared with best supportive care study (CO.1737), all active treatments are assumed to have utilities as reported for cetuximab plus best supportive care. There is a concern here that irinotecan is a particularly toxic chemotherapy and therefore it would seem unreasonable to assume that the utility for cetuximab plus best supportive care is equivalent to that for cetuximab plus irinotecan. In fact, our clinical expert agrees that QoL is unlikely to be equivalent for cetuximab plus best supportive care and cetuximab plus irinotecan; therefore, the utilities associated with cetuximab plus irinotecan in the Merck Serono model are likely to be overestimates. Merck Serono's assumption of equivalent utilities leads to a more favourable ICER for cetuximab plus irinotecan over any comparator but, in the absence of data, it is difficult to quantify the extent to which this is an overestimate.
As can be seen in Table 31, the utility values calculated by Merck Serono associated with cetuximab plus best supportive care are greater than those for best supportive care, regardless of the health state. Au and colleagues44 found a similar pattern in their analysis of the EORTC QLQ-C30 data from CO.17: higher QoL for cetuximab than for best supportive care. For the progression-free state this finding appears reasonable given that patients are more likely to be responding with cetuximab plus best supportive care than with best supportive care and therefore more likely to have a greater QoL, even though they are more likely to experience treatment-related adverse events (see Chapter 3, Indirect comparison of cetuximab and panitumumab, Adverse events).
The progressive disease utilities are also different between treatment arms, with cetuximab plus best supportive care utilities higher than best supportive care utilities. This is difficult to explain as it is assumed in Merck Serono's model that all progressed patients cease cetuximab plus best supportive care and therefore they will no longer be responding to treatment. The magnitude of this difference in utility is difficult to explain. The mean utility value in the progression-free state for those on cetuximab plus best supportive care is 0.063 greater than that for those on best supportive care alone. In the progressive disease state this difference is even greater: 0.097. This is inconsistent as patients are receiving the same care once in progressive disease regardless of whether they received cetuximab plus best supportive care or best supportive care alone in the progression-free state. Given the possibilities for bias in the analysis of these data (lack of blinding, unequal dropout and healthier patients more likely to complete the QoL survey), it does not seem reasonable that QoL in progressive disease should differ between the treatment arms. By assuming higher utilities in the progressive disease state for those receiving cetuximab plus best supportive care in the progression-free state, greater total QALYs will be associated with cetuximab plus best supportive care than with best supportive care.
As a sensitivity analysis, if the utility of 0.693 is assumed for both treatment arms in the progressive disease state and leaving unchanged Merck Serono's utility assumptions in the progression-free state, the following ICERs are obtained by PenTAG rerunning Merck Serono's model:
-
cetuximab plus best supportive care compared with best supportive care: £56,132 per QALY gained (vs £48,238 per QALY gained)
-
cetuximab plus irinotecan compared with best supportive care: £49,233 per QALY gained (vs £44,439 per QALY gained).
It is worth noting that Merck Serono is aware that ‘there are uncertainties around whether the utilities are truly representative of likely quality of life when in progression free and PD states’ (scenarios 3a and b, p. 136, Merck Serono's submission69), and that the utilities used in both health states were found to be important drivers in its model. The trial contributing evidence to the utility estimates is open label, which introduces the possibility of bias in favour of cetuximab plus best supportive care for the QoL estimates but which is difficult to avoid.
Impact on the incremental cost-effectiveness ratios: cetuximab
In the above sections the impact of individual issues on the PenTAG corrected base-case ICERs has been discussed. In this section the simultaneous impacts on the ICER of a number of the important assumptions are presented. As can be seen in Tables 32 and 33, the simultaneous adjustment of a number of the assumptions regarding the costs associated with treatment and care for patients with metastatic colorectal cancer has quite a large impact on the PenTAG corrected base-case ICERs: from £48,238 per QALY gained to £95,238 per QALY gained for cetuximab plus best supportive care compared with best supportive care and from £44,429 per QALY gained to £83,215 per QALY gained for cetuximab plus irinotecan compared with best supportive care. However, removing Merck's cap on the mean duration of treatment and instead assuming that patients are treated throughout the progression-free state has the greatest impact on the ICERs (see Treatment duration: cetuximab).
| Total cost (£) | Total QALYs | ||||||
|---|---|---|---|---|---|---|---|
| ICER (£/QALY) | Incremental cost (£) | Incremental QALYs | CET + BSC | BSC | CET + BSC | BSC | |
| Merck Serono base case | 47,095 | 14,256 | 0.303 | 21,836 | 7580 | 0.662 | 0.359 |
| PenTAG corrected base casea | 48,238 | 14,256 | 0.296 | 21,836 | 7580 | 0.662 | 0.367 |
| Drug administration costs £242; BSC costs £917; KRAS test cost £296; treated throughout progression-free state | 81,922 | 24,211 | 0.296 | 32,601 | 8390 | 0.662 | 0.367 |
| Drug administration costs £242; BSC costs £917; KRAS test cost £296; treated throughout progression-free state; PD utilities 0.693 | 95,328 | 24,211 | 0.254 | 32,601 | 8390 | 0.621 | 0.367 |
| Total cost (£) | Total QALYs | ||||||
|---|---|---|---|---|---|---|---|
| ICER (£/QALY) | Incremental cost (£) | Incremental QALYs | CET + IRIN | BSC | CET + IRIN | BSC | |
| Merck Serono base case | 43,887 | 29,301 | 0.668 | 37,248 | 7947 | 1.059 | 0.391 |
| PenTAG corrected base casea | 44,429 | 29,663 | 0.668 | 37,571 | 7909 | 1.059 | 0.391 |
| Drug administration costs £306; BSC costs £917; KRAS test cost £296; treated throughout progression-free state | 75,015 | 50,083 | 0.668 | 58,857 | 8774 | 1.059 | 0.391 |
| Drug administration costs £306; BSC costs £917; KRAS test cost £296; treated throughout progression-free state; PD utilities 0.693 | 83,125 | 50,083 | 0.603 | 58,857 | 8774 | 0.994 | 0.391 |
Effectiveness evidence: cetuximab
Karapetis and colleagues (CO.17)47
Merck Serono uses the individual patient data from the CO.17 study to inform time to disease progression and death for patients receiving cetuximab plus best supportive care compared with best supportive care. The data used are from the retrospective analysis of the CO.17 study by Karapetis and colleagues,47 which stratifies by KRAS status (see also Chapter 3, Study characteristics, Cetuximab plus best supportive care compared with best supportive care). Karapetis and colleagues present both hazard ratios adjusted for patient characteristics for progression-free survival and overall survival and unadjusted hazard ratios (Table 34). In the Merck Serono model, the unadjusted hazard ratios are used. However, given that KRAS status is assessed retrospectively in Karapetis and colleagues and that KRAS status was not determined for all participants, there may be some selection bias in the study (even though the authors report that there were similarities between patient characteristics for those identified to be KRAS WT and those KRAS mutant). Therefore, it would seem more reasonable for Merck Serono to have used the adjusted hazard ratios rather than the unadjusted hazard ratios. The adjusted hazard ratios from Karapetis and colleagues47 are less favourable to the effectiveness of cetuximab plus best supportive care compared with best supportive care (i.e. the adjusted hazard ratio is closer than the unadjusted hazard ratio to 1) than Merck Serono suggests in its submission (table 52, Merck Serono's submission69).
| Unadjusted | Adjusted for potential prognostic factors | |
|---|---|---|
| Overall survival | 0.55 (0.41 to 0.74) | 0.62 (0.44 to 0.87) |
| Progression-free survival | 0.40 (0.30 to 0.54) | 0.42 (0.30 to 0.58) |
De Roock and colleagues48
Although details of the BOND trial49 are presented throughout the submission by Merck Serono, the BOND trial only impacts on its submission through De Roock and colleagues. 48 De Roock and colleagues48 is a retrospective analysis of cetuximab plus best supportive care compared with cetuximab plus irinotecan using data from four studies (BOND,49 EVEREST,50,54,55 SALVAGE51 and BABEL) based at four centres in Belgium, in which KRAS status has been retrospectively determined for a selection of patients. Note that the four studies included in De Roock and colleagues48 did not distinguish KRAS status; therefore, these studies are not covered in the clinical effectiveness systematic review (see Chapter 3) but are briefly described below.
Restricting the De Roock and colleagues48 data to only those patients with KRAS WT status (as done by the manufacturer) leads to a rather small sample of 67 patients. A total of 40% of the patients (n = 27) in De Roock and colleagues are from the BOND trial (whereas the total number of patients in BOND is 329), 42% (n = 28) are from the EVEREST trial, 15% (n = 10) are from the SALVAGE trial and 3% (n = 2) are from the from BABEL trial (Table 35).
| Original study | Treatment arms in original study | Total no. (%) of patients contributing to De Roock et al.48 | No. (%) of patients receiving CET + BSC | No. (%) of patients receiving CET + IRIN |
|---|---|---|---|---|
| BOND49 | CET + IRIN vs CET + BSC | 27 | 8 | 19 |
| EVEREST50,54,55 | CET + IRIN vs CET + BSC | 28 | 0 | 28 |
| SALVAGE51 | CET + BSC | 10 | 10 | 0 |
| BABEL | Unclear | 2 | 0 | 2 |
| Total | – | 67 (100) | 18 (27) | 49 (73) |
The BOND trial49 consists of 329 patients randomly assigned to cetuximab plus best supportive care (n = 111) or cetuximab plus irinotecan (n = 218), with 80% of those randomised having received at least two previous lines of therapy. Importantly, 50% (n = 56) of patients from the cetuximab plus best supportive care arm received irinotecan after disease progression; thus, there is a great deal of crossover in the BOND trial, which does not appear to have been dealt with, or even discussed, by De Roock and colleagues48 or Merck Serono. Ignoring this crossover underestimates the overall survival effectiveness of cetuximab plus irinotecan compared with cetuximab plus best supportive care.
In its submission, Merck Serono categorises the EVEREST trial50,54,55 as relating to second-line treatment yet comments that the study is ‘not fully published. It is unclear what proportion of patients received the experimental treatment in the second-line compared with the third-line’ (p. 42, Merck Serono's submission69). Although EVEREST is described as a RCT comparing cetuximab plus irinotecan, escalating doses of cetuximab plus irinotecan and cetuximab plus best supportive care, it is surprising that only data from cetuximab plus irinotecan patients are included in De Roock and colleagues (see Table 35).
The SALVAGE study,51 although representing only 15% of patients in De Roock and colleagues,48 is not mentioned in the manufacturer's submission. Our investigations indicate that this is a non-comparative study of patients receiving cetuximab plus best supportive care only who have received at least two previous lines of therapy. The BABEL study also appears to be a single-arm study, although further details have been difficult to find.
More generally, De Roock and colleagues comment that patients were included on the basis of availability of formalin-fixed paraffin-embedded tumour tissue; however, there are no details on what this percentage was for each of the four studies contributing patient data. In addition to this, there are uncertainties as to the inclusion of some patients and the exclusion of others. For instance, EVEREST is a three-arm trial [cetuximab plus irinotecan, cetuximab (escalating) plus irinotecan and cetuximab plus best supportive care] but only data from patients receiving cetuximab plus irinotecan have been included and it is unclear if this is from the escalating or non-escalating cetuximab plus irinotecan arm. Given these issues, there are concerns that the disease progression and effectiveness estimates calculated using De Roock and colleagues are likely to be subject to high levels of bias and confounding. This is in addition to the possibility of chance findings given that only 18 patients with KRAS WT status contribute to the cetuximab plus best supportive care arm of De Roock and colleagues. 48
The manufacturer used data from the Kaplan–Meier curves reported in De Roock and colleagues48 to calculate hazard ratios for progression-free survival and overall survival for cetuximab plus irinotecan compared with cetuximab plus best supportive care. As described in the next section, Merck Serono used data from De Roock and colleagues83 in the calculation of the indirect hazard ratios. This study is a non-comparative data set consisting of 773 cetuximab plus irinotecan-treated patients from 11 European centres. In total, 87% of patients had two or more previous lines of therapy, with 58% of patients (n = 448) found to have KRAS WT status. Note that De Roock and colleagues83 point out that progression-free survival and overall survival are not appropriate outcomes given the differences in the studies.
Indirect comparison: cetuximab
Merck Serono uses the Bucher method34 to calculate indirect comparisons for progression-free survival and overall survival hazard ratios (see Figure 3 for comparison network). There are a number of concerns with the method employed by Merck Serono to calculate the indirect hazard ratios: (1) randomised and non-randomised evidence is combined, (2) no assessment is made of the similarities/appropriateness for comparison between the patient populations of the studies, (3) the indirect hazard ratio for overall survival is adjusted using De Roock and colleagues,83 (4) unadjusted hazard ratios from CO.1747 are used and (5) there is no accounting for crossover in De Roock and colleagues48 (i.e. BOND49).
With respect to the first concern, in the indirect comparisons, because of limitations in the available evidence, data from a RCT47 and a non-RCT48 have been combined without considering the fact that different study designs are being used. These different study designs are subject to different sources of bias and confounding; in particular, we have serious concerns over the use of De Roock and colleagues. 48 It is difficult to ascertain what impact the synthesis of randomised and non-randomised data may have on the subsequent hazard ratio estimates. Related to this is the second point, that Merck Serono makes no assessment of the patient populations from the different studies involved in the indirect comparison. In any form of evidence synthesis, there should be some consideration of whether or not the populations and interventions are comparable across studies. Although Merck Serono reports the baseline characteristics for CO.1747 and for De Roock and colleagues,48 it does not explicitly evaluate the appropriateness of combining these studies. A quick assessment of the baseline characteristics suggests that patients in De Roock and colleagues are slightly younger and more likely to have had fewer lines of therapy than those in the CO.17 study (Table 36). 47 It is unclear what impact this may have on the results of the indirect comparison.
| Characteristic | CET + BSC vs BSC47 | CET + IRIN vs CET + BSC48 |
|---|---|---|
| Male (%) | 67.8 | 58.1 |
| Age (years), median (range) | 63.5 (28.6–85.9) | 61 (22–86) |
| ECOG status (%) | ||
| 0 | 24.3 | – |
| 1 | 55.2 | – |
| 2 | 20.4 | – |
| Number of lines of previous therapy (%) | ||
| ≤ 2 | 20.0 | 62.2 |
| 3 | 37.4 | 24.0 |
| 4 | 27.4 | 9.2 |
| ≥ 5 | 15.2 | 3.6 |
The third concern with the indirect comparison is specific to the calculation of the overall survival hazard ratio for cetuximab plus irinotecan compared with best supportive care. The manufacturer uses data from De Roock and colleagues48 and CO.1747 for this comparison (see table 46, Merck Serono's submission69). After calculating the hazard ratio using the Bucher approach, plots of the observed Kaplan–Meier curves and fitted parametric curves for this comparison are shown (see figure 29, Merck Serono's submission69), but Merck Serono states that advice from clinical experts indicated that the modelled curves were not a good fit. As a consequence, the manufacturer uses data from the non-comparative study by De Roock and colleagues83 to adjust the hazard ratio obtained from the indirect comparison from 0.29 (95% CI 0.14 to 0.59) to 0.32 (95% CI 0.14 to 0.59). It is unclear why model fit was determined by clinical experts as the submission suggests rather than by statistical methods. There is no explanation from Merck Serono as to how exactly this adjustment was made, regardless of the fact that De Roock and colleagues83 is a non-comparative study. Given that, as stated in the previous section, De Roock and colleagues83 themselves state that progression-free survival and overall survival are not the best outcomes to assess given the differences in the studies, there are further issues of bias and confounding associated with the indirect estimates obtained by the manufacturer for the overall survival hazard ratio for cetuximab plus irinotecan compared with best supportive care.
The adjustment made to the indirect hazard ratio using data from De Roock and colleagues83 is less favourable to cetuximab plus irinotecan than the initial indirect comparison results (from a hazard ratio of 0.29 to 0.32). {Interestingly, a similar estimate of hazard ratio to that using the data from De Roock and colleagues83 is obtained if the adjusted hazard ratio from CO.1747 is used in this indirect comparison instead of the unadjusted hazard ratio [0.33, 95% CI 0.16 to 0.68] [as discussed in Effectiveness evidence: cetuximab, Karapetis and colleagues (CO.17)47].} After adjustment of the hazard ratio using data from De Roock and colleagues,83 Merck Serono assumes the same 95% CI for the hazard ratio adjusted by the data from De Roock and colleagues as that from the initial indirect comparison. This will lead to more favourable estimates for cetuximab in the probabilistic sensitivity analysis even though the mean value is slightly different. It is very unusual to externally adjust an indirect comparison as Merck Serono has reported for the overall survival of cetuximab plus irinotecan compared with best supportive care. Within the other indirect comparisons reported by Merck Serono there is no indication that such an assessment of the Kaplan–Meier curves using clinical experts was undertaken. Although this adjustment leads to a less favourable estimate for cetuximab plus irinotecan, it is unclear whether or not the manufacturers went through a similar process including experts for the other calculations and outcomes.
The fourth concern is that already expressed in Effectiveness evidence: cetuximab, Karapetis and colleagues (CO.17)47, that unadjusted hazard ratios from CO.1747 were used by the manufacturer in the indirect comparison when adjusted hazard ratios would have been more appropriate. Consequently, this means that the effectiveness of cetuximab in all comparisons is likely to be overestimated, particularly for overall survival for which the difference between the adjusted hazard ratio and the unadjusted hazard ratio is most pronounced.
The fifth point for concern is that there appears to be no accounting for the crossover in the BOND data used in De Roock and colleagues. 48 Such crossover during progressive disease will underestimate the effectiveness of cetuximab plus irinotecan in terms of overall survival.
Note further that Merck Serono did not investigate uncertainty surrounding the effectiveness within its sensitivity analyses.
Modelling progression-free survival and overall survival: cetuximab
Cetuximab plus best supportive care compared with best supportive care
Merck Serono uses the individual patient data from Karapetis and colleagues45 to model disease progression for cetuximab plus best supportive care and best supportive care: ‘[individual patient data] allows the progression-free health state (over time) for cetuximab monotherapy and BSC arms (from CO.17 study) to be determined by two processes based on death before progression and ‘real progression’ (p. 98, Merck Serono's submission69). Merck Serono fits four parametric functions to the individual patient data: exponential, Weibull, lognormal and log-logistic. The Akaike information criterion (AIC) was used to identify the best fitting function. For both time to ‘real progression’ and time to ‘death before progression’, a log-logistic function was the best fit (although the AIC results for ‘real progression’ are illegible in their report). The parametric log-logistic curves have a reasonable fit to the Kaplan–Meier curves (see figure 35, Merck Serono's submission69).
For overall survival, the same four parametric functions are fitted to the cetuximab plus best supportive care and best supportive care individual patient data. The Weibull function was found to give the best fit according to the AIC and appears to give a reasonable fit to the Kaplan–Meier data. Note, however, that 20% and 10% of patients receiving cetuximab plus best supportive care and best supportive care, respectively, have not died before the end of the study; therefore, the appropriateness of the Weibull function to extrapolate beyond the data cannot be assessed.
Cetuximab plus irinotecan compared with best supportive care
Merck Serono fits a Weibull curve to the progression-free survival and overall survival individual patient data for the best supportive care arm of the study by Karapetis and colleagues. 47 Using the indirect progression-free survival and overall survival hazard ratios for cetuximab plus irinotecan compared with best supportive care calculated as described and critiqued in Indirect comparison: cetuximab, Merck Serono assumes proportional hazards and uses the following equation to calculate the corresponding Weibull function for progression-free survival and overall survival in the cetuximab plus irinotecan arm: S(t) = exp(–(λHR)tγ), where S(t) is the survival function at time t, HR is the indirect hazard ratio for cetuximab plus irinotecan compared with best supportive care, and λ and γ are the parameters of the Weibull distribution.
Merck Serono presents the Kaplan–Meier curves of the best supportive care arm from Karapetis and colleagues45 and the cetuximab plus irinotecan arm from De Roock and colleagues48 alongside the Weibull fitted curves. Because the indirect hazard ratio has been used for the cetuximab plus irinotecan Weibull fit, one would not necessarily expect a good fit to the Kaplan–Meier curve from De Roock and colleagues. 48 Nevertheless, the parametric curve seems to fit reasonably well to the Kaplan–Meier curve for both progression-free survival and overall survival. There is no explanation as to why a Weibull curve was used to model the best supportive care progression-free survival and overall survival data and no statistical assessment of the goodness of fit of this curve (e.g. using the AIC). Presumably, a Weibull model was used for simplicity to allow calculation of the cetuximab plus irinotecan curve using the indirect hazard ratio. Given the lack of direct evidence for cetuximab plus irinotecan compared with best supportive care, Merck Serono has taken a reasonable approach to calculate the fitted curves for progression-free survival and overall survival in the cetuximab plus irinotecan arm, but we still have concerns regarding the use of data from De Roock and colleagues48 and the ‘adjustment’ to the indirect overall survival hazard ratio using data from De Roock and colleagues83 (see Indirect comparison: cetuximab). Note that the Weibull curve does not appear to be a particularly good fit to the best supportive care progression-free survival Kaplan–Meier data; however, this is likely to lead to an underestimate of the progression-free survival in the best supportive care arm and can therefore be considered conservative. The Weibull fit to the best supportive care overall survival data, however, appears to be reasonable.
Finally, note that Merck's log-logistic modelling of ‘real progression’ and ‘death before progression’ in the best supportive care arm for the cetuximab plus best supportive care compared with best supportive care comparison and Weibull parametric modelling of progression-free survival in the best supportive care arm for cetuximab plus irinotecan compared with best supportive care are the source of the difference in the best supportive care arm depending on the comparator (see The decision problem: cetuximab)
Adverse events: cetuximab
Merck Serono includes in the model the costs associated with treatment for grade 3 or 4 adverse events (Table 37). The manufacturer assumes that the utilities used in the model reflect the impact of any adverse events experienced, and so does not calculate disutilities for adverse events. As Merck Serono's results suggest, the cost of the adverse events has very little impact on the ICERs. Yet for completeness, we report the assessment of the costing and inclusion of adverse events in the Merck model having identified a number of points worth highlighting. The adverse event data used by Merck Serono are taken from the subset of patients with KRAS WT status and an ECOG performance status of 0 or 1 from the CO.17 study. 47 Merck Serono assumes that all grade 1 or 2 adverse events are minor and do not require treatment; therefore, they are not associated with any costs. Grade 3/4 adverse events reported in CO.17 are divided into three categories: non-serious (requiring outpatient treatment only), serious but not leading to hospitalisation, and serious leading to, or prolonging, hospitalisation.
| CET + BSC | CET + IRIN | BSC (in CET + BSC) | BSC (in CET + IRIN) | |
|---|---|---|---|---|
| No. (%) of non-serious adverse events | 245 (63) | 245 (63) | 129 (58) | 129 (58) |
| No. (%) of serious (no hospitalisation) adverse events | 21 (5) | 21 (5) | 7 (3) | 7 (3) |
| No. (%) of serious (hospitalised) adverse events | 126 (32) | 126 (32) | 88 (40) | 88 (40) |
| Total no. (%) of adverse events | 392 (100) | 392 (100) | 224 (100) | 224 (100) |
| Total no. of patients | 97 | 97 | 87 | 87 |
| Cost of non-serious adverse event (£) | 174 | 174 | 174 | 200a |
| Cost of serious (no hospitalisation) adverse event (£) | 165 | 165 | 165 | 165 |
| Cost of serious (hospitalised) adverse event (£) | 2460 | 2460 | 2460 | 2460 |
Merck Serono has calculated the cost of adverse events by assigning each type of adverse event experience in CO.17 (non-serious, serious but not requiring hospitalisation, serious requiring hospitalisation) to a body type/system. For the non-serious and serious but not requiring hospitalisation adverse events, the corresponding body type/system is matched to appropriate Healthcare Resource Group (HRG) codes and a mean cost (from the NHS reference costs 2008–200977) is assigned to the body type/system adverse event. The average cost per body type/system ranges from £106 for ocular adverse events to £259 for infection and influenza-like symptom adverse events. Note that the adverse events from the best supportive care and cetuximab plus best supportive care arms are combined to obtain a cost per non-serious or serious but not requiring hospitalisation adverse event across treatment arms. For serious adverse events requiring hospitalisation, the adverse events were also assigned to a body type/system but were allocated inpatient procedure costs based on the HRG codes. Note that the adverse events are assigned to body type/system not reported by the actual adverse event experienced.
Based on the CO.17 data analysed by Merck Serono, the cost of a non-serious adverse event is slightly higher than that of a serious adverse event not requiring hospitalisation (£175 compared with £165). There is a greater percentage of serious adverse events requiring hospitalisation (the adverse event associated with the most costs) in the best supportive care arm than in the cetuximab plus best supportive care arm: 32% of adverse events in the cetuximab plus best supportive care arm require hospitalisation compared with 40% in the best supportive care arm. Both of these findings appear unintuitive.
Merck Serono assumes the same proportion of different types of adverse events for cetuximab plus irinotecan as its analysis suggests for cetuximab plus best supportive care; however, given that irinotecan is known to have significant toxicities, the assumption made by Merck Serono is likely to underestimate the costs of adverse events associated with cetuximab plus irinotecan. Given the lack of available evidence it is difficult to quantify what the level of adverse events should be for cetuximab plus irinotecan. Nevertheless, we have no better evidence for the adverse events associated with best supportive care, cetuximab plus best supportive care or cetuximab plus irinotecan.
Summary of cost-effectiveness
Cetuximab monotherapy
We have major concerns over a number of Merck Serono's important assumptions including the adjustment for modelling mean time on cetuximab plus best supportive care, the utilities used and the costs associated with best supportive care. Simultaneously modelling alternative assumptions for these concerns leads to an ICER of £82,000–95,000 per QALY gained for cetuximab plus best supportive care compared with best supportive care.
Cetuximab combined therapy
We have major concerns over a number of important assumptions including the adjustment that Merck Serono makes for modelling mean time on treatment, the utilities used, the costs associated with best supportive care and the effectiveness data for cetuximab plus irinotecan. Simultaneously modelling alternative assumptions for these concerns leads to an ICER of £75,000–83,000 per QALY gained for cetuximab plus irinotecan compared with best supportive care. However, there is a great deal of uncertainty regarding the impact of the questionable effectiveness data from De Roock and colleagues48 on which the cetuximab plus irinotecan arm is modelled; thus, we have very little confidence in the ICER estimated by Merck for cetuximab plus irinotecan compared with best supportive care.
Industry submission critique 2: Roche, bevacizumab
Roche did not submit a decision model for the cost-effectiveness of bevacizumab in combination with non-oxaliplatin chemotherapy for the treatment of metastatic colorectal cancer after first-line therapy. Instead, it presents some cost calculations for bevacizumab plus FOLFIRI compared with cetuximab plus FOLFIRI. Below we critique the effectiveness evidence submitted by Roche and its basic cost calculations.
Effectiveness evidence: bevacizumab
Although there is no clinical evidence for the effectiveness of bevacizumab with non-oxaliplatin therapy after first-line treatment in metastatic colorectal cancer, the main focus of Roche's argument for the effectiveness of bevacizumab in this setting is that there is evidence for the effectiveness of bevacizumab in first-line treatment and for second-line treatment in combination with oxaliplatin. From this, Roche argues that the benefits of bevacizumab in addition to chemotherapy are therefore not dependent on regimen or line of therapy, suggesting that there is no reason to expect that bevacizumab plus non-oxaliplatin therapy after first-line treatment would not provide added benefits. This argument was adequate for the EMA to grant marketing authorisation for bevacizumab with non-oxaliplatin therapy after first-line treatment in metastatic colorectal cancer; however, this does not make a case for the cost-effectiveness of bevacizumab in the scope of this review.
Roche reports three trials relevant to the consideration of bevacizumab for first-line use in patients with metastatic colorectal cancer: the 966 study by Saltz and colleagues40 for oxaliplatin combined therapy, Hurwitz and colleagues26 for irinotecan combined therapy and Kabbinavar and colleagues27 for 5-FU/FA combined therapy. For second-line combined treatment, Roche refers to the E3200 study by Giantonio and colleagues41 for bevacizumab with oxaliplatin therapy.
Saltz and colleagues40 conducted a 2 × 2 factorial design RCT and randomised 1401 patients, 75% of whom had not previously received chemotherapy. Treatment arms were XELOX plus bevacizumab, XELOX plus placebo, FOLFOX-4 plus bevacizumab or FOLFOX-4 plus placebo. In ITT analyses, patients receiving XELOX plus bevacizumab or FOLFOX-4 plus bevacizumab were pooled, as were those receiving XELOX plus placebo or FOFLOX-4 plus placebo, as no statistically significant treatment difference was identified between XELOX and FOLFOX-4. The hazard ratio for progression-free survival for bevacizumab compared with placebo was 0.83 (97.5% CI 0.72 to 0.95), with median progression-free survival of 9.4 months for bevacizumab and 8 months for placebo. The hazard ratio for overall survival for bevacizumab compared with placebo was not statistically significant (hazard ratio 0.89, 97.5% CI 0.76 to 1.03), with a median overall survival for bevacizumab of 21.3 months compared with 19.9 months in the placebo arm. Saltz and colleagues40 report that 30% of patients in the bevacizumab arm discontinued treatment because of adverse events compared with 21% in the placebo arm. Furthermore, they note that the percentage of patients receiving treatment until progression (as defined in the protocol) was particularly low: 29% of the bevacizumab arm and 47% of the placebo arm.
Hurwitz and colleagues26 randomised 813 patients from the USA, Australia and New Zealand to either irinotecan with FU/LV plus bevacizumab or irinotecan with FU/LV alone for first-line treatment (28% of irinotecan with FU/LV patients and 24% of irinotecan with FU/LV plus bevacizumab patients had previously received adjuvant chemotherapy). ITT analyses showed that median survival was 20.3 months for those treated with irinotecan with FU/LV plus bevacizumab and 15.6 months for those receiving irinotecan with FU/LV alone (hazard ratio 0.6, p< 0.001). The hazard ratio for progression-free survival was 0.54 (p< 0.001), with patients treated with irinotecan with FU/LV plus bevacizumab having a progression-free survival of 10.6 months compared with 6.2 months for patients in the irinotecan with FU/LV arm. The authors report statistically significantly more grade 3 or 4 adverse events in the irinotecan with FU/LV plus bevacizumab arm than in the irinotecan with FU/LV arm (p< 0.01), mainly explained by differences in hypertension rates between the arms.
Kabbinavar and colleagues27 randomised 209 patients to FU/LV plus bevacizumab or FU/LV only. Twenty-one percent of the FU/LV plus bevacizumab patients and 19% of the FU/LV patients had previous adjuvant chemotherapy. The primary end point of overall survival was associated with a hazard ratio of 0.76 (95% CI 0.56 to 1.10) for FU/LV plus bevacizumab compared with FU/LV, with a median overall survival of 16.6 months in the FU/LV plus bevacizumab arm and 12.9 months in the FU/LV arm. Progression-free survival, however, was statistically significantly longer in the FU/LV plus bevacizumab arm (9.2 months) than in the FU/LV arm (5.5 months) (hazard ratio 0.5, 95% CI 0.34 to 0.73). Treatment with FU/LV plus bevacizumab was associated with a greater incidence of grade 3 or 4 adverse events than treatment with FU/LV (87% for FU/LV plus bevacizumab vs 71% for FU/LV). The authors argue that the large number of patients receiving postprogression treatment could partly explain the lack of statistical significance in the primary end point of overall survival. A similar percentage of patients from both treatment arms received irinotecan, oxaliplatin or both post progression (39% of the FU/LV plus bevacizumab patients and 46% of the FU/LV patients).
Giantonio and colleagues41 report the ITT analyses of a RCT with 820 patients previously treated with fluoropyrimidine and irinotecan randomised to one of three arms: FOLFOX-4 plus bevacizumab, FOLFOX-4 or bevacizumab alone. Median overall survival was greater in the FOLFOX-4 plus bevacizumab arm: 12.9 months compared with 10.8 months for FOLFOX-4 and 10.2 months for bevacizumab alone. The hazard ratio for overall survival associated with FOLFOX-4 plus bevacizumab compared with FOLFOX-4 was 0.75 (p= 0.01). Median progression-free survival was also greater in the FOLFOX-4 plus bevacizumab arm: 7.3 months compared with 4.7 months for FOLFOX-4 and just 2.7 months for bevacizumab alone. A greater number of grade 3/4 adverse events were reported in the FOLFOX-4 plus bevacizumab arm (75%) than in the FOLFOX-4 arm (61%).
As Roche points out in its submission, these four trials suggest that bevacizumab in combination with therapies is associated with benefit for progression-free survival and overall survival, which is statistically significant for progression-free survival in all four trials. None of these trials is included in our systematic review of clinical effectiveness in Chapter 3 as they do not meet the inclusion criteria for this review.
The decision problem: bevacizumab
The manufacturer argues that, because of the lack of clinical evidence on the effectiveness of bevacizumab after first-line therapy, a decision model comparing bevacizumab plus FOLFIRI with FOLFIRI ‘would be subject to sizeable uncertainty’ (p. 8, Roche's submission70). Therefore, no economic evaluation or cost calculations for bevacizumab plus FOLFIRI compared with FOLFIRI are presented by the manufacturer. In comparison with cetuximab, Roche argues that bevacizumab is likely to be less expensive given the purchase prices of each drug. The manufacturer does, however, provide very basic cost calculations for a comparison of bevacizumab plus FOLFIRI with cetuximab plus FOLFIRI in patients who have failed one previous line of therapy, and these are critiqued in the sections below. Note that even though Roche states that there is considerable uncertainty over the bevacizumab plus FOLFIRI compared with cetuximab plus FOLFIRI comparison, cost calculations are still presented; it is reasonable to query why such basic cost calculations were not performed for the bevacizumab plus FOLFIRI compared with FOLFIRI comparisons with the same caveat of ‘sizeable uncertainty’.
Costs: bevacizumab
Roche focuses on the incremental cost differences between bevacizumab plus FOLFIRI and cetuximab plus FOLFIRI over a seven-cycle (14-week) treatment regime. Roche accounts for differences in KRAS testing and drug acquisition and administration costs between bevacizumab plus FOLFIRI and cetuximab plus FOLFIRI. Roche uses the ‘guaranteed NHS cost’ for cetuximab as reported in the submission from Merck Serono. 69 Roche assumes a cost of £462 for KRAS testing associated with each KRAS WT patient as in TA176. 17 This cost is defined to account for the costs of all KRAS testing including patients who are KRAS mutant (who would not go on to receive cetuximab treatment). However, Roche's assumption for KRAS test costs may be too high as it is based on a KRAS test cost of £300. Data indicate that the test cost is £160;80 thus, the cost for testing for KRAS, accounting for those identified as KRAS mutant (54%), is likely to be around £296 (as discussed in KRAS testing costs: cetuximab).
The drug preparation and administration costs assumed by Roche include an additional hospital visit for cetuximab plus FOLFIRI per cycle for administration at £218 per visit (from NHS reference costs 2008–9, SB15Z77). However, this value is actually reported as £227 in NHS reference costs 2008–2009. 77 This is based on the assumption that bevacizumab plus FOLFIRI requires one administration per 2-week cycle, whereas cetuximab plus FOLFIRI requires two administrations per 2-week cycle. Similarly, an additional pharmacy preparation cost of £9 (12 minutes of pharmacy time) per cycle is assumed for cetuximab plus FOLFIRI. Thus, Roche assumes an incremental cost per cycle for drug preparation and administration associated with cetuximab plus FOLFIRI of £227 when in fact this value should be £236 if the £9 pharmacy cost is assumed. Data given to PenTAG on pharmacy preparations suggest that pharmacy costs are £15 (see Appendix 12). The impact of changing the preparation and administration costs from £227 to £242 (£227 + £15) is assessed below.
To calculate the dose of bevacizumab required per administration Roche assumes a mean weight of 75 kg, referencing TA118,16 and to calculate the of dose of cetuximab required it assumes a mean body surface area of 1.75 m2 (although this estimate of body surface area is not referenced). Using the body surface area-to-weight calculations used by Merck Serono in its submission (p. 112, Merck Serono's submission69), it is assumed that a weight of 75 kg is equivalent to a body surface area of 1.91 m2. Therefore, Roche's estimate of cetuximab dose required per administration could be an underestimate if we are to accept the equations used by Merck. The impact of this is assessed below.
The total incremental cost of cetuximab plus FOLFIRI over bevacizumab plus FOLFIRI calculated and reported by Roche is therefore £5408 (KRAS testing costs of £462 plus drug costs of £3357 plus administration costs of £1589).
Threshold analysis assumptions and results: bevacizumab
To undertake threshold analyses on the incremental costs for bevacizumab plus FOLFIRI compared with cetuximab plus FOLFIRI, Roche assumes a utility of 0.6 for progressive disease. This is taken from TA11816 anddoes not appear to be based on any evidence. The threshold analyses reported by Roche using this utility value indicate that cetuximab plus FOLFIRI would have to provide a survival advantage of 3.6 months over bevacizumab plus FOLFIRI to be considered cost-effective at a willingness to pay of £30,000 per QALY gained.
Adjusting the drug preparation, administration and KRAS test costs and body surface area estimates for cetuximab plus FOLFIRI has no impact on the estimated survival advantage of 3.6 months required by cetuximab plus FOLFIRI to be considered cost-effective compared with bevacizumab plus FOLFIRI at a willingness to pay of £30,000 per QALY gained. Note that the progressive disease utility of 0.6 is lower than that used by Merck Serono for either best supportive care or active treatment. If a utility of 0.693 is assumed in addition to the updated KRAS testing and administration costs and body surface area, a survival advantage of 3.2 months would be required.
Summary of cost calculations: bevacizumab
As Roche states, these are very basic cost calculations and, given the lack of effectiveness evidence for bevacizumab plus FOLFIRI, they do not really help with the decision-making. Only KRAS test, drug acquisition and administration costs associated with a seven-cycle treatment regimen are accounted for. Roche assumes that no patients (either those receiving cetuximab plus FOLFIRI or those receiving bevacizumab plus FOLFIRI) progress or die within that 14-week (seven-cycle) period. Furthermore, by assuming a utility for progressive disease in the threshold analysis, Roche is implicitly assuming that time progression free is the same for bevacizumab plus FOLFIRI as for cetuximab plus FOLFIRI. The differential cost of treating adverse events between cetuximab plus FOLFIRI and bevacizumab plus FOLFIRI is not considered. Given the lack of evidence for adverse events associated with bevacizumab plus FOLFIRI, it is difficult to state what impact they may have on the cost calculations, but it is likely to be slight.
Roche conducted a comparison only of bevacizumab plus FOLFIRI with cetuximab plus FOLFIRI, but another appropriate comparison would be between bevacizumab plus FOLFIRI and cetuximab plus best supportive care. This can be crudely estimated by subtracting the costs of irinotecan from the cetuximab plus FOLFIRI costs calculated by Roche. We assume, as assumed by Merck, that irinotecan is administered every 2 weeks at a dose of 180 mg/m2, and that the mean body surface area is 1.75 m2 as Roche assumes. At a cost of £1.23 per mg of irinotecan (from Merck Serono) and assuming wastage, the cost of one cycle of irinotecan is £385. Over seven cycles this gives a cost of £2695. Thus, the incremental cost of cetuximab plus FOLFIRI is adjusted by subtracting £2695 from £5408 to roughly estimate the incremental cost of cetuximab monotherapy compared with bevacizumab plus FOLFIRI (£2713). Assuming this incremental cost and a willingness-to-pay threshold of £30,000 per QALY leads to the estimation of incremental QALYs of 0.09. Assuming a progressive disease utility of 0.6 leads to cetuximab monotherapy requiring an additional 1.8 months of survival over bevacizumab plus FOLFIRI. As with the threshold analysis undertaken by Roche, this is a very basic calculation that considers only the costs of the first 14 weeks of treatment, and it is difficult to evaluate the likelihood of the finding that cetuximab would need 1.8 months of survival over bevacizumab plus FOLFIRI to be considered cost-effective at a willingness to pay of £30,000 per QALY gained.
Industry submission critique 3: Amgen, panitumumab
Amgen did not submit an economic model for this appraisal and does not argue that panitumumab could be a cost-effective treatment option for patients with KRAS WT status after first-line treatment in metastatic colorectal cancer. Its submission consists of an analysis of the only RCT of panitumumab after first-line treatment in which KRAS status is known, that by Amado and colleagues. 32 Here, we critique Amgen's analysis of this trial data, in particular its adjustment for the large proportion of patients who crossed over from best supportive care to panitumumab plus best supportive care at disease progression.
Effectiveness evidence: panitumumab
An important feature in Amado and colleagues32 is that 76% of patients randomised to best supportive care received panitumumab plus best supportive care once they had progressed. Thus, estimates of overall survival are confounded by this crossover, and no effect of panitumumab plus best supportive care over best supportive care was found. The manufacturer has made adjustments to the calculation of overall survival to account for this crossover. As it notes, there are a number of techniques available for adjusting for crossover, but, given the specific nature of the relationship between the effectiveness of panitumumab plus best supportive care and KRAS status, Amgen uses a simple method for adjustment.
Amgen estimates overall survival for patients with KRAS WT status in the best supportive care treatment arm adjusted for crossover as equal to that for patients with KRAS mutant status in the best supportive care treatment arm, regardless of whether or not patients crossed over at progression (Table 38).
| BSC | Panitumumab arm |
|---|---|
| KRAS mutant patients randomised to receive BSC (n = 100) | KRAS WT patients randomised to receive panitumumab (n = 124) |
In coming to this approach, Amgen argues that including all patients as they were randomised (i.e. ignoring the fact that many best supportive care patients crossed over to panitumumab plus best supportive care at progression) will underestimate the effectiveness of panitumumab plus best supportive care relative to best supportive care in terms of overall survival. Similarly, it argues that censoring all patients who crossed over from best supportive care to panitumumab plus best supportive care at progression would overestimate the effectiveness of panitumumab plus best supportive care, because patients who crossed over were generally fitter with a better prognosis than those patients who did not cross over. It further argues that just censoring those who crossed over and achieved stable disease or a complete or partial response would also lead to an overestimate of the effectiveness of panitumumab plus best supportive care, for the same reason.
The approach used by Amgen depends on two main assumptions. First, to be able to include KRAS mutant patients randomised to the best supportive care arm as a substitute for KRAS WT patients in the best supportive care arm, even though they may have crossed over to panitumumab plus best supportive care at progression, it must be assumed that panitumumab plus best supportive care is not effective for patients with KRAS mutant status. Second, to use only patients with KRAS mutant status randomised to best supportive care (see Table 38), it must be assumed that survival in patients with KRAS mutant status assigned to best supportive care is similar to survival in patients with KRAS WT status assigned to best supportive care (had these patients with KRAS WT status not crossed over to receive panitumumab).
The first assumption is based on the retrospective analysis of effectiveness by KRAS status by Amado and colleagues. 32 As reviewed in Chapter 3, Panitumumab plus best supportive care compared with best supportive care, the evidence indicates that this is a fair assumption, that is, that panitumumab plus best supportive care is not effective for patients with KRAS mutant status.
The second assumption is also based on data from Amado and colleagues32 and involves an additional important assumption that similarities in progression-free survival between patients with KRAS mutant and KRAS WT status randomised to best supportive care can predict similarities in overall survival between these two groups of patients. Amgen states that there are few differences between the Kaplan–Meier curves for progression-free survival for patients with KRAS mutant and those with KRAS WT status randomised to best supportive care.
We agree that there is very little difference between the two curves; in fact, Amgen reports that mean progression-free survival is 71 days for patients who are KRAS mutant and 64 days for those who are KRAS WT. Thus, if anything, its assumption for the survival benefit of panitumumab plus best supportive care may be biased against panitumumab plus best supportive care. In further analyses, Amgen compares baseline characteristics across the four groups (treatment × KRAS status) to identify any statistically significant differences between the groups. Amgen then carries out Cox regression on three groups of patients [(1) all patients, (2) patients with KRAS mutant status and (3) patients with KRAS mutant status receiving best supportive care and patients with KRAS WT status receiving panitumumab plus best supportive care] to determine whether or not any of the baseline variables are statistically significant predictors of survival. In doing this, Amgen is evaluating whether or not any differences in the survival between the KRAS mutant best supportive care arm and the KRAS WT panitumumab plus best supportive care arm can be attributed to factors other than treatment.
Amgen appears to have taken a reasonable approach to this evaluation of characteristics; however, its focus on the 5% level of statistical significance does not help to fully assess whether or not any variables important to predicting time to death are different across the groups. For instance, there may be important factors that Amgen has not included because they were found to have a p-value above the p = 0.05 cut-off used (i.e. p = 0.051).
The additional point that the similarity in progression-free survival in patients with KRAS mutant status receiving best supportive care and in patients with KRAS WT status receiving best supportive care translates to a similarity in overall survival is difficult to assess given the limited data available. However, there is evidence that could shed some light on this. In an evaluation of the impact of KRAS status on response to bevacizumab for metastatic colorectal cancer, Ince and colleagues84 and Hurwitz and colleagues33 reported a greater median overall survival for patients with KRAS WT status treated with placebo than for patients with KRAS mutant status (17.6 vs 13.6 months). Hurwitz and colleagues do not report whether or not any patients randomised to best supportive care received bevacizumab; however, as KRAS status has no impact on the effectiveness of bevacizumab, these data suggest that in this trial overall survival was not similar between KRAS WT and mutant patients. However, neither was progression-free survival similar between the KRAS subgroups (7.4 months for patients with KRAS WT status vs 5.5 months for patients with KRAS mutant status), plus the sample sizes are small (67 patients with KRAS WT status and 34 patients with KRAS mutant status).
Quality of life data: panitumumab
Amgen summarises the analyses of QoL data from the Van Cutsem and colleagues7 and Amado and colleagues32 studies published by Odom and colleagues. 56 It is reported that HRQoL, as measured by the EQ-5D and NCCN FCSI, was greater for patients receiving panitumumab plus best supportive care than for patients receiving best supportive care alone (a mean difference of 0.22 on the EQ-5D scale). Furthermore, average estimates from both measures were greater than the minimum clinically important differences reported in the published literature. Note that Odom and colleagues report that HRQoL was reported only for patients in the progression-free survival health state, and that there was a great deal of patient dropout. Amgen reports that the analysis suggested that dropout was treatment related, being much higher in patients in the best supportive care arm. Note that neither Odom and colleagues56 nor Amgen report the absolute EQ-5D utility value for the best supportive care arm or the panitumumab plus best supportive care arm, only the mean difference between them (0.22).
Safety data: panitumumab
Amgen reports the number of patients experiencing adverse events from the Van Cutsem study,7 in which KRAS status was unknown, and from the retrospective analysis by Amado and colleagues,32 in which KRAS status was available for 92% of patients. In total, 100% of panitumumab patients in Amado and colleagues32 with KRAS WT status developed an adverse event and 90% of best supportive care patients developed an adverse event. Table 39 is taken from Amgen's report detailing the percentages of patients with KRAS WT status and KRAS mutant status receiving panitumumab who experienced grade 3 or 4 adverse events or who withdrew because of adverse events.
| % developing adverse event | ||
|---|---|---|
| Adverse event | KRAS WT | KRAS mutant |
| Grade 3/4 | 44 | 28 |
| Treatment related (grade 3) | 25 | 12 |
| Withdrawal because of adverse event: non-specified | 7 | 5 |
| Withdrawal because of adverse event: panitumumab related | 2 | 1 |
Liver resection: panitumumab
Amgen reports on a Delphi consultation of 15 clinical specialists in the UK on the expected rates of liver resection in patients with metastatic colorectal cancer after first-line therapy. It suggests that 5–9% of these patients could be expected to undergo liver resection. Amgen summarises the results of the Delphi consultation, which include that ‘a majority of the clinical experts agreed that treating chemorefractory patients with panitumumab as a single agent may lead to downsizing of liver metastases’ (p. 43, Amgen's submission71) and ‘mean survival following successful liver resection in a chemorefractory patient on panitumumab was three years; estimates of five years and 10 years were expected in 20–24% and 10–14% of patients respectively’ (p. 43, Amgen's submission71).
Overall summary of industry submissions
The base-case ICERs reported by Merck Serono (£47,000 per QALY gained for cetuximab plus best supportive care compared with best supportive care and £44,000 per QALY gained for cetuximab plus irinotecan compared with best supportive care) are highly likely to be underestimates given the concerns that we have over the estimate of treatment duration and the costs assumed for best supportive care, drug administration and KRAS testing. An alternative ICER using Merck Serono's model of £82,000 per QALY gained for cetuximab plus best supportive care compared with best supportive care is more likely.
The very basic cost calculations submitted by Roche for bevacizumab plus FOLFIRI compared with cetuximab plus FOLFIRI are reasonably robust to alternative drug acquisition, drug administration and KRAS test cost estimates, but offer very little information for the decision-making process.
Amgen makes no claims for the cost-effectiveness of panitumumab, but its analysis of the crossover in the study by Amado and colleagues32 is reasonable, suggesting an overall survival advantage of 2.74 or 3.13 months (depending on the method used) for panitumumab plus best supportive care compared with best supportive care.
Chapter 6 The Peninsula Technology Assessment Group's cost-effectiveness analysis
Independent economic assessment
Scope of the economic evaluation
Our economic evaluation is restricted to patients with KRAS WT status on third-line or further lines of treatment for metastatic colorectal cancer for reasons described below. We estimate the cost-effectiveness of cetuximab plus best supportive care compared with best supportive care, panitumumab plus best supportive care compared with best supportive care and cetuximab plus irinotecan compared with best supportive care. We are not able to model cost-effectiveness as a function of the line of treatment because we do not have access to the required underlying individual patient data from the clinical trials.
The scope of our analysis is reduced relative to the original NICE scope in two respects. First, although the NICE scope refers to second-line treatment, we do not model the cost-effectiveness of any drugs for second-line treatment because of the lack of relevant clinical data. There is only one RCT of any of the assessed drugs for second-line use, the RCT of cetuximab plus irinotecan compared with irinotecan (EPIC trial);74 however, the clinical results are not stratified according to KRAS status. In addition, we note that Merck Serono does not model the cost-effectiveness of cetuximab plus best supportive care or cetuximab plus irinotecan for second-line treatment and that there appears to be little clinical demand for second-line use. Second, because of the lack of clinical evidence, we did not model treatment with bevacizumab in combination with chemotherapy not containing oxaliplatin for those on second or subsequent lines of treatment. Both of these issues were agreed with NICE during the preparation of this report.
The following section describes the structure of our cost-effectiveness model. Subsequent sections describe the parameterisation of the model and a comparison of the results of our model with those of other relevant models, including that submitted by Merck Serono, the manufacturer of cetuximab.
Model structure
The cost-effectiveness model, implemented in Microsoft Excel 2007, simulates a cohort of people with KRAS WT metastatic colorectal cancer on third or subsequent lines of treatment. The structure of the model was informed by a review of the literature and expert opinion. The basic design of the model is simple and has been used previously to simulate the progression of metastatic cancers, for example for metastatic renal cell carcinoma. 85 There are three health states: progression-free survival, progressive disease and death (Figure 5).
FIGURE 5.
Structure of the PenTAG cost-effectiveness model.
In Figure 5, arrows represent the possible transitions between health states. Circular arrows denote that patients can remain in a state at the end of each model cycle. During each cycle, a patient is assumed to be in one of the states. Patients are assumed to move between states once at the end of each cycle.
Although our model closely resembles a Markov state-transition approach, it differs in that an ‘area under the curve’/‘cohort partition’ method is used to determine state populations at each cycle of the model (rather than using transition probabilities). In this method, the number of people in each health state at each successive model cycle is determined by using survival curve data to apportion the overall cohort between the states. This approach has been used in previous HTAs. 85 Using this method, there is no requirement to calculate the probabilities of transition between health states (depicted by the arrows in Figure 5) as estimates of populations for each health state are derived directly from the survival curves.
Differences in clinical effectiveness between treatments are represented by the differences between progression-free survival and overall survival curves (and hence the respective populations of each disease state at each successive cycle of the model). Estimates of cost and utility per cycle are assigned to the progression-free survival and progressive disease states, and these provide an aggregated output over the modelled time horizon for the total costs and utility per person for each treatment. The main economic outcome presented is the incremental cost per QALY gained.
The model cycle length is 1 month and the model time horizon is 10 years, after which time virtually all people in all cohorts have died. A model half-cycle correction is applied.
Future costs and benefits are discounted at 3.5% per annum and the perspective is that of the NHS and PSS in accordance with the NICE reference case. 72
After treatment with any of the comparator drugs, in common with Merck Serono, we assume no further lines of drug treatment.
Sensitivity of the Kirsten rat sarcoma test
We assume that everyone tested as KRAS WT is indeed WT, that is, the sensitivity of the KRAS test (to test for KRAS mutant status) in routine clinical practice is 100%. However, if the sensitivity of the test used in routine clinical practice is < 100%, some people may be incorrectly diagnosed with KRAS WT status. These people will then receive panitumumab plus best supportive care or cetuximab plus best supportive care even though they will not benefit from these drugs. Therefore, these drugs compared with best supportive care will actually have higher costs per QALY than the figures produced from our model. To be more precise, we should consider the relative sensitivities of the tests for patients with KRAS mutant status as used in the RCTs of panitumumab plus best supportive care and cetuximab plus best supportive care and the sensitivity of the test used in the routine testing of patients. If the sensitivities are equal, the cost-effectiveness of these drugs will be the same as calculated in our model. If the sensitivities of the tests used in the RCTs are greater than the sensitivity of the test used in routine practice, the cost-effectiveness of these drugs will actually be worse than predicted by our model. In this case, we should model the clinical effectiveness for those who are assessed as KRAS mutant status from the RCTs in addition to that for people assessed as KRAS WT status.
We asked Merck Serono and Amgen for the sensitivity of KRAS tests used in routine practice. Amgen replied that the probability of a patient with KRAS mutant status being incorrectly diagnosed as KRAS WT ‘using the standard KRAS test assured by appropriate external quality assurance’ is 14 in every 10,000 tests, that is, 0.14%. 71 In summary, Merck Serono does not quantify the probability of incorrectly diagnosing a patient as KRAS WT but instead claims that it is ‘slim’. If insufficient tumour sample is available for testing then the test result is reported as not available.
Progression-free survival and overall survival
The distribution of progression-free survival and overall survival times across those in:
-
the best supportive care treatment group is taken directly from the RCT of cetuximab plus best supportive care compared with best supportive care47
-
the cetuximab plus best supportive care treatment group is also taken directly from the RCT of cetuximab plus best supportive care compared with best supportive care47
-
the panitumumab plus best supportive care treatment group is taken from the RCT of panitumumab plus best supportive care compared with best supportive care,32 adjusted for the indirect comparison with best supportive care and cetuximab plus best supportive care
-
the cetuximab plus irinotecan treatment group is taken from a variety of sources and is adjusted for the indirect comparison with best supportive care and cetuximab plus best supportive care.
Details are given in Evidence to inform model parameters, Overall survival, progression-free survival and treatment duration.
Time on treatment
The mean duration of drug treatment is a key determinant of the mean drug acquisition costs and therefore of cost-effectiveness. Ideally, we would model the mean duration of drug treatment as experienced in the RCTs. This is reported as a mean of 10 treatment cycles for patients with KRAS WT status on panitumumab plus best supportive care,32 but is not reported for patients with KRAS WT status on cetuximab plus best supportive care or for cetuximab plus irinotecan. However, in the pivotal trials of cetuximab plus best supportive care compared with best supportive care, panitumumab plus best supportive care compared with best supportive care and cetuximab plus irinotecan compared with cetuximab (BOND), all drugs were taken until disease progression, the occurrence of intolerable adverse events or death. In the RCT of cetuximab plus best supportive care compared with best supportive care,37 treatment with cetuximab was additionally stopped because of ‘worsening symptoms of the cancer, or request by the patient’. Therefore, we modelled treatment duration by treatment group (Table 40). We have good corroborating evidence that it is reasonable to assume that KRAS WT patients randomised to cetuximab took the drug until disease progression (see Time on cetuximab treatment).
| Drug | Treatment duration modelled | Source/rationale |
|---|---|---|
| PAN + BSC | Mean of 20 weeks (one dose every 2 weeks; mean of 10 doses) | As reported in Amado et al.32 for patients with KRAS WT status |
| CET + BSC | Until disease progression | Assume main reason for stopping treatment is disease progression |
| Based on median treatment duration in Jonker et al.37 trial being very similar to median progression-free survival in this trial (8.1 weeks vs 8.2 weeks) and clinical opinion | ||
| CET + IRIN | Until disease progression | Based on median treatment duration in BOND being very similar to median progression-free survival (7 weeks vs 6.5 weeks)49 |
Details are given in Evidence to inform model parameters, Overall survival, progression-free survival and treatment duration.
Postprogression survival
Postprogression survival is calculated as overall survival minus progression-free survival.
Severe adverse events
We do not model disutilities due to adverse events associated with drug treatment directly. Instead, as Merck Serono, we allow for disutilities indirectly in that we use utilities specific to each treatment.
We base our estimates of the costs of treating adverse events on those calculated by Merck Serono; see Costs of treating adverse events for details.
Evidence to inform model parameters
Overall survival, progression-free survival and treatment duration
Given that there is no single RCT with all treatment groups, it was necessary to perform an indirect comparison between some pairs of treatments. For progression-free survival, overall survival and time on drug treatment, we chose the baseline treatment for the indirect comparison to be best supportive care taken from the RCT of cetuximab plus best supportive care compared with best supportive care. 47 The clinical effectiveness for those on best supportive care is also available from the RCT of panitumumab plus best supportive care compared with best supportive care. 32 However, this was not considered an appropriate estimate for the baseline treatment for best supportive care because the effectiveness in this treatment group was confounded by substantial crossover (76% of patients).
Best supportive care
We have based our estimate of progression-free survival for best supportive care on the analysis of the individual patient data by Merck Serono. In particular, we have assumed the same mean time in progression-free survival as Merck Serono, namely 2.72 months. This is the most important summary statistic of progression-free survival given that cost-effectiveness is a function of mean values. We did not use precisely the same progression-free survival curve as Merck Serono, because this function is commercial-in-confidence. We specified that progression-free survival follows a Weibull distribution, as this is a flexible function, widely used in cancer survival analysis. We read off the progression-free survival probabilities at monthly intervals from the published Kaplan–Meier graphs for patients with KRAS WT status. 47 We then fitted a Weibull curve to these data by minimising the sums of squares of differences between actual and fitted probabilities. We estimated the shape parameter, γ, of the Weibull from this fit to the Kaplan–Meier curve. However, note that the shape is not important, because cost-effectiveness is almost completely insensitive to it. Instead, it is the mean progression-free survival that is critical, which we fix to be the same as that reported by Merck Serono. Finally, given that we have specified the mean, this then specifies the scale parameter, λ, of the Weibull, given that the mean of the Weibull is:
FIGURE 6.
Peninsula Technology Assessment Group and Merck Serono progression-free survival for patients with KRAS WT status in the best supportive care group.
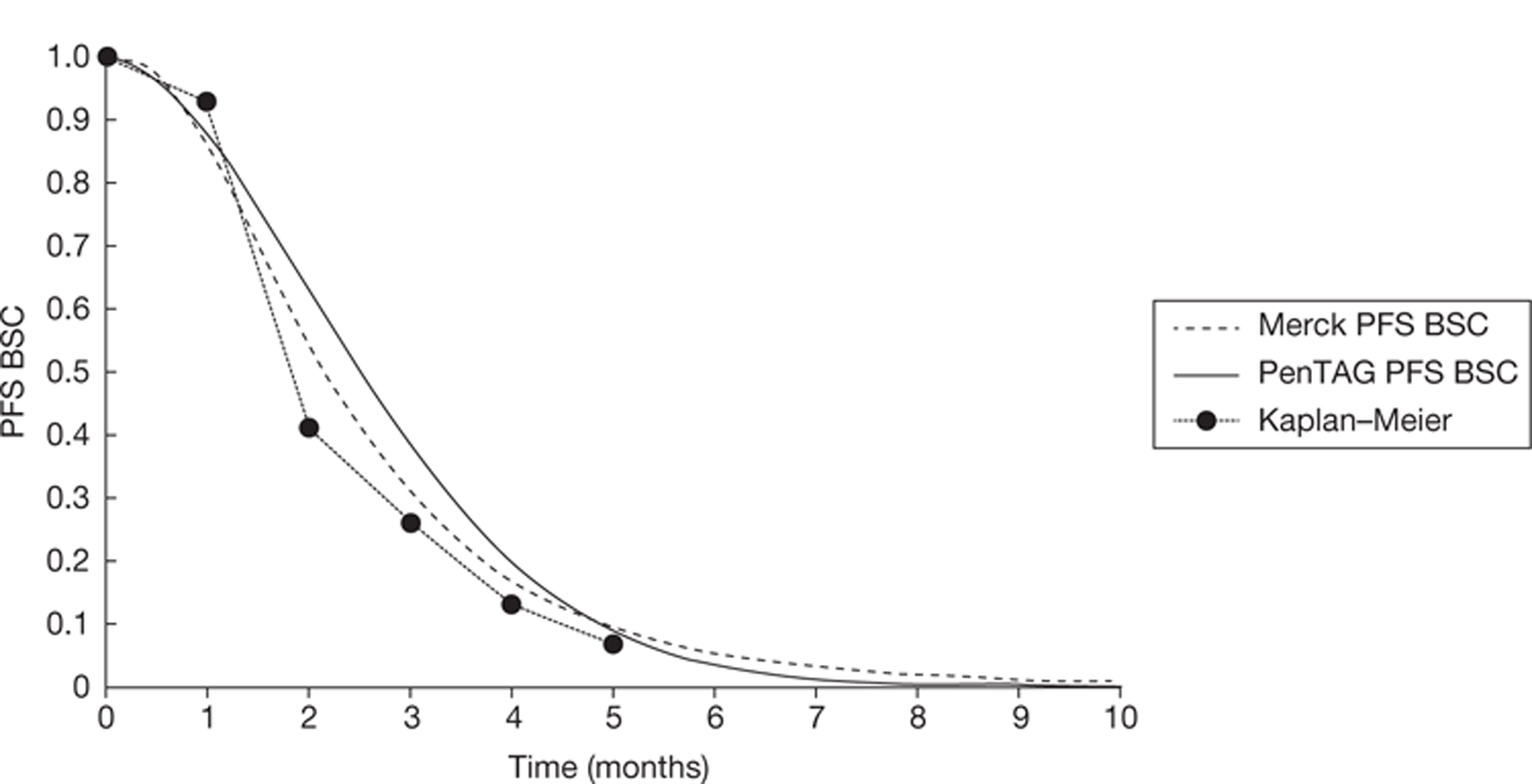
We estimated the uncertainty in progression-free survival for the probabilistic sensitivity analysis purely by modelling the uncertainty in the mean progression-free survival. This is valid given that it is the mean progression-free survival that drives cost-effectiveness. First, for simplicity, we fixed the shape value, γ, of the Weibull. Next, we specified that the mean progression-free survival follows a gamma distribution with a mean of 2.72 months, as above. We then estimated the standard error of the mean progression-free survival by making two simplifying assumptions. The first was that progression-free survival approximately follows an exponential distribution. We can then say that the standard deviation of progression-free survival across patients approximately equals the mean progression-free survival, as this is a property of the exponential distribution. The second simplifying assumption was that no patients in the best supportive care arm in the RCT of cetuximab plus best supportive care compared with best supportive care were censored, which is probably approximately true, given that progression occurs quickly. In this case, the standard error of the mean progression-free survival equals:
Finally, the scale parameter, λ, of the Weibull is back-calculated from the fixed gamma and variable mean, using the formula of the mean of the Weibull described above.
As for progression-free survival for best supportive care, we based our estimate of overall survival for best supportive care on the analysis of the individual patient data by Merck Serono. In particular, we assume the same mean overall survival as that calculated by Merck Serono of 6.2 months. Next, we again specified that overall survival for best supportive care follows a Weibull function, and we read off the overall survival probabilities at monthly intervals from the published Kaplan–Meier graphs for patients with KRAS WT status. 47 We then fitted a Weibull curve to these data by minimising the sums of squares of differences between actual and fitted probabilities. We estimated the shape parameter, γ, of the Weibull from the shape parameter of this fit to the Kaplan–Meier curve. Finally, given that we have specified the mean, this then specifies the scale parameter, λ, of the Weibull (Figure 7).
FIGURE 7.
Peninsula Technology Assessment Group and Merck Serono overall survival for patients with KRAS WT status in the best supportive care group.
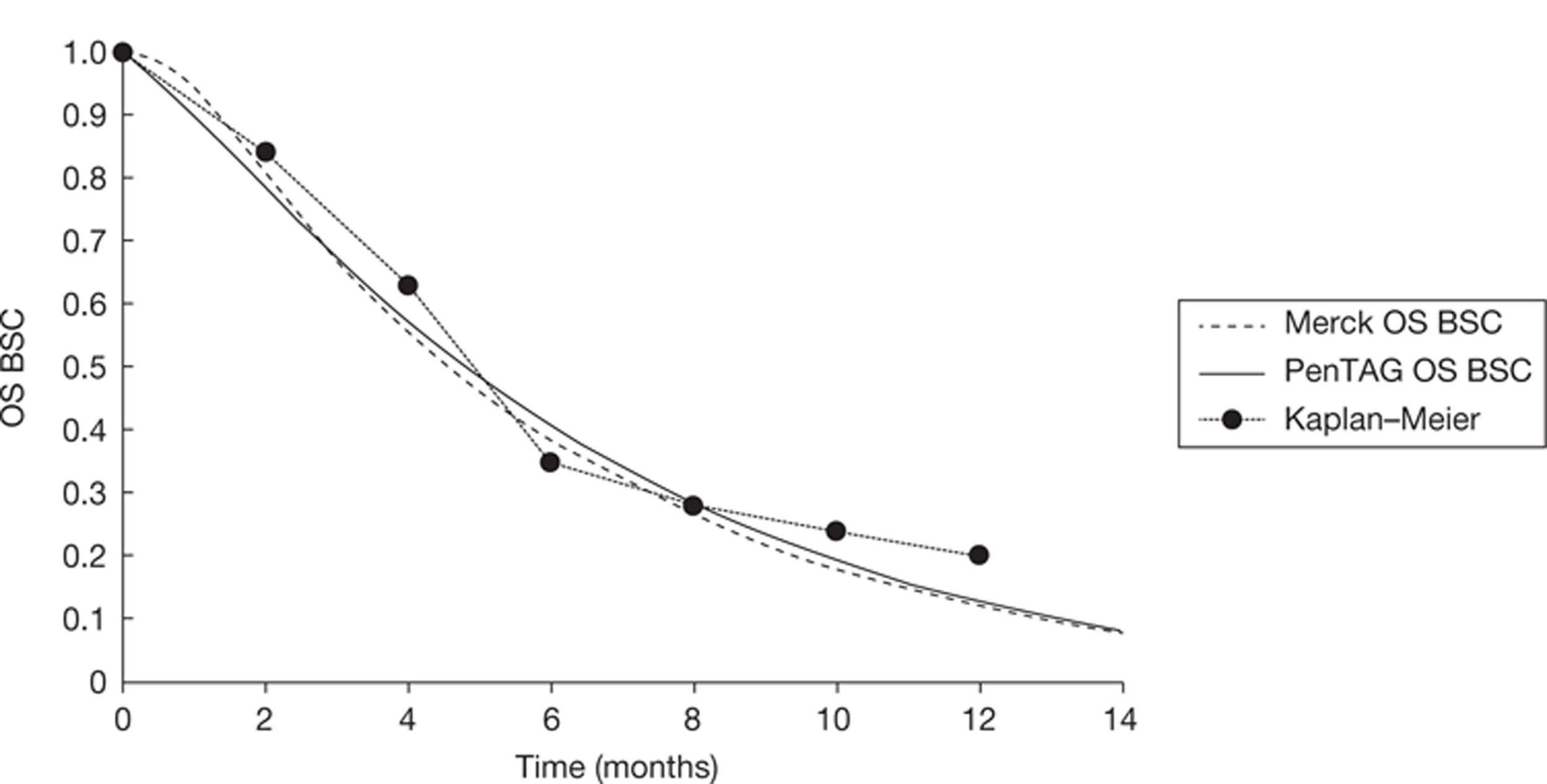
We estimated the uncertainty in overall survival for the probabilistic sensitivity analysis in exactly the same way as for the uncertainty in progression-free survival on best supportive care, as explained in the last section. Our estimation of the standard error of mean overall survival assumes that no patients were censored in the best supportive care arm of the cetuximab plus best supportive care compared with best supportive care RCT. This assumption is less likely to hold for overall survival than for progression-free survival, but given the lack of further data, and the need for simplicity, this was again our assumption.
It is impossible to know the correlation between overall survival and progression-free survival for best supportive care without access to the underlying individual patient data from the RCT of cetuximab plus best supportive care compared with best supportive care. Nonetheless, it seems intuitive that these quantities will be highly correlated. Therefore, given the lack of further evidence, we assumed that overall survival and progression-free survival are perfectly correlated. This was implemented in the model by using the same random number to draw values for mean progression-free survival and overall survival.
Cetuximab monotherapy
We modelled the time on cetuximab treatment, progression-free survival and overall survival for the cetuximab plus best supportive care group directly from the RCT of cetuximab plus best supportive care compared with best supportive care. 47
As in the best supportive care group, given that Merck Serono have the underlying individual patient data from this trial, we have based our estimate of progression-free survival for cetuximab plus best supportive care on the analysis of the individual patient data by Merck. In particular, we assume the same mean progression-free survival for cetuximab plus best supportive care as that calculated by Merck (4.78 months or 0.40 years). Next, we again specified that progression-free survival for cetuximab plus best supportive care follows a Weibull function, and again we read off the progression-free survival probabilities at monthly intervals from the published Kaplan–Meier graphs for patients with KRAS WT status. 47 We then fitted a Weibull curve to these data by minimising the sums of squares of differences between actual and fitted probabilities. We estimated the shape parameter, γ, of the Weibull from the shape parameter of this fit to the Kaplan–Meier curve. Finally, given that we have specified the mean, this then specifies the scale parameter, λ, of the Weibull (Figure 8).
FIGURE 8.
Peninsula Technology Assessment Group and Merck Serono progression-free survival for patients with KRAS WT status in the cetuximab plus best supportive care group.
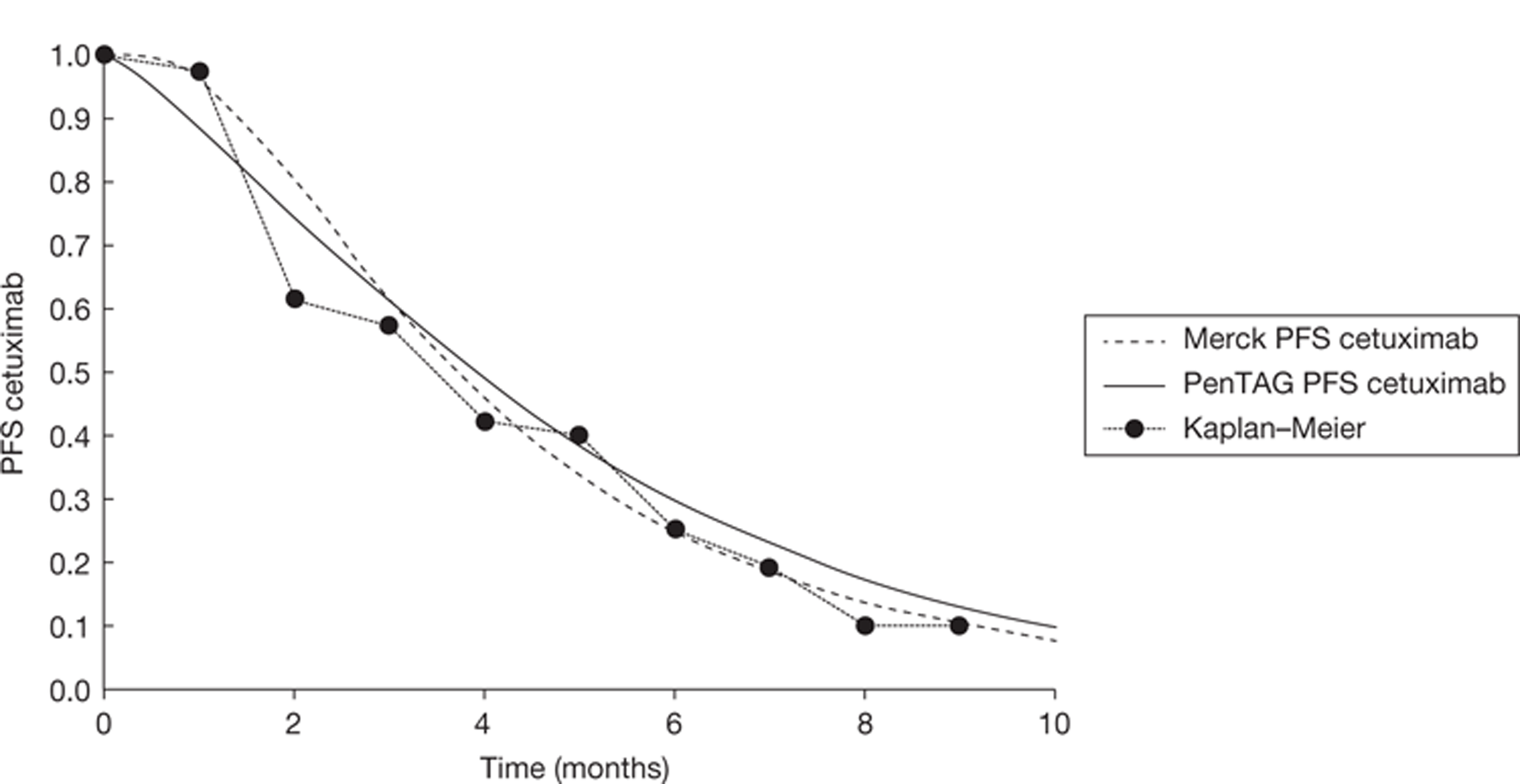
We estimated the uncertainty in progression-free survival for the probabilistic sensitivity analysis in exactly the same way as we estimated the uncertainty in progression-free survival on best supportive care, as described above. Again, we modelled the uncertainty in the mean progression-free survival by specifying that the mean progression-free survival follows a gamma distribution with a mean of 4.78 months, as above. In this case, the standard error of the mean progression-free survival equals:
Time on cetuximab treatment is an extremely important quantity because it affects the total mean cost of cetuximab acquisition and administration per person, and the former in particular is a critical driver of the cost-effectiveness of cetuximab compared with best supportive care.
Ideally, we would model the mean total dose of cetuximab per patient with KRAS WT status, allowing for wastage of cetuximab. However, these data are not published. Alternatively, we would model the dose intensity of cetuximab and the mean number of doses of cetuximab per patient with KRAS WT status (as for panitumumab; see Time on panitumumab treatment). Unfortunately, this information is also not published and could not be made available on request. We have therefore assumed that those in the cetuximab plus best supportive care compared with best supportive care RCT received cetuximab for the entire duration of progression-free survival. This is mainly informed by the finding that the median time on cetuximab treatment for patients with KRAS WT and KRAS mutant status combined was 8.1 weeks in the RCT of cetuximab plus best supportive care compared with best supportive care, which is virtually identical to the median progression-free survival for all patients (i.e. KRAS WT and KRAS mutant status combined) of 8.2 weeks. 37 Both our model and Merck Serono's model predict that patients with KRAS WT status are progression free for a median of approximately 16 weeks. Therefore, we predict that patients with KRAS WT status also took cetuximab for the entire duration of progression-free survival, for a median of 16 weeks and a mean of 21 weeks. We have corroborating evidence that it is indeed reasonable to assume that WT patients took cetuximab until disease progression. Dr Nicole Mittman, who coauthored a paper on the cost-effectiveness of cetuximab compared with best supportive care42 with some of the authors of the publications describing the cetuximab compared with best supportive care RCT,37,47 informed us that the duration of cetuximab treatment for KRAS WT patients, for those who had at least one dose of cetuximab, was approximately 19 weeks. Given that almost all patients randomised to cetuximab had at least one dose of cetuximab, the mean duration of cetuximab treatment for all patients was approximately 19 weeks. This is very close to the mean duration of progression-free survival, at 21 weeks.
In the RCT of cetuximab plus best supportive care compared with best supportive care, patients took cetuximab until death, the occurrence of serious adverse events, progression, worsening symptoms of the cancer or request by the patient, with or without the withdrawal of consent for continued follow-up. 37 Although our assumption that patients took cetuximab until progression allows for only the ‘death’ and ‘progression’ causes for cetuximab cessation, cetuximab treatment was rarely discontinued because of serious adverse events, given that Merck Serono notes that ‘In the CO.17 study 11 patients discontinued cetuximab therapy among the 287 treated subjects, and only 3 patients amongst the 117 KRAS WT patients taking cetuximab stopped the therapy due to adverse events.’69 We have no data on cetuximab cessation because of worsening symptoms of the cancer or request by the patient, with or without the withdrawal of consent for continued follow-up; therefore, it is impossible to quantify cessation resulting from these factors.
For the probabilistic sensitivity analysis, the mean time on cetuximab treatment was assumed to equal the mean time in progression-free survival, which we varied stochastically as explained in Cetuximab monotherapy, Progression-free survival for cetuximab plus best supportive care.
As for progression-free survival for cetuximab plus best supportive care (see Cetuximab monotherapy, Progression-free survival for cetuximab plus best supportive care), we have based our estimate of overall survival for cetuximab plus best supportive care on the analysis of the individual patient data by Merck Serono. In particular, we assume the same mean overall survival for cetuximab plus best supportive care as that calculated by Merck Serono (10.0 months). Next, we again specified that overall survival for cetuximab plus best supportive care follows a Weibull function, and we read off the overall survival probabilities at monthly intervals from the published Kaplan–Meier graphs for patients with KRAS WT status. 47 We then fitted a Weibull curve to these data by minimising the sums of squares of differences between actual and fitted probabilities. We estimated the shape parameter, γ, of the Weibull from the shape parameter of this fit to the Kaplan–Meier curve. Finally, given that we have specified the mean, this then specifies the scale parameter, λ, of the Weibull (Figure 9).
FIGURE 9.
Peninsula Technology Assessment Group and Merck Serono overall survival for patients with KRAS WT status in the cetuximab plus best supportive care group.
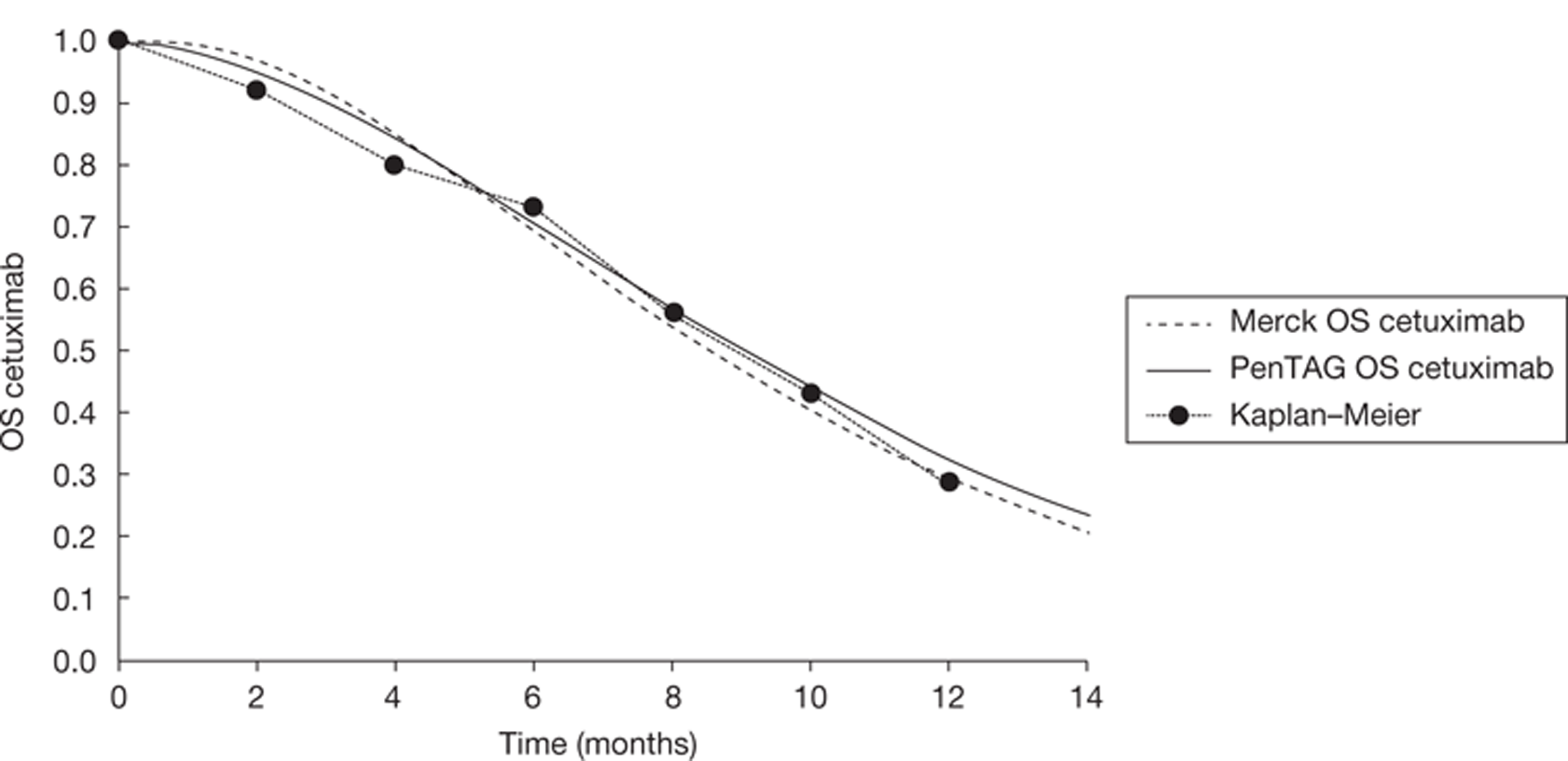
We estimated the uncertainty in overall survival for the probabilistic sensitivity analysis in exactly the same way as for the uncertainty in progression-free survival on cetuximab plus best supportive care, as explained above (see Cetuximab monotherapy, Progression-free survival for cetuximab plus best supportive care). Our estimation of the standard error of mean overall survival assumes that few patients were censored in the cetuximab plus best supportive care arm of the cetuximab plus best supportive care compared with best supportive care RCT. This assumption is less likely to hold for overall survival than for progression-free survival, but given the lack of further data, and the need for simplicity, this was again assumed.
Given the lack of further evidence we set overall survival and progression-free survival for cetuximab plus best supportive care to be perfectly correlated, just as we did for overall survival and progression-free survival for best supportive care.
Panitumumab plus best supportive care
As explained earlier (see Evidence to inform model parameters, Overall survival, progression-free survival and treatment duration), for the purposes of the indirect comparison, we chose the best supportive care treatment group from the cetuximab plus best supportive care compared with best supportive care RCT to represent the clinical effectiveness of the best supportive care treatment group. Therefore, in modelling the clinical effectiveness of panitumumab plus best supportive care (time on treatment, progression-free survival and overall survival), it is necessary to adjust the clinical effectiveness of panitumumab plus best supportive care as reported in the RCT of panitumumab plus best supportive care compared with best supportive care32 in the manner of the Bucher indirect comparison. 34 The implicit assumption is that the baseline patient characteristics in the two RCTs are reasonably similar, and indeed this is true (see Table 10).
In this section we estimate progression-free survival for panitumumab plus best supportive care for the indirect comparison. First, we estimated the mean progression-free survival for the best supportive care group as 2.2 months from the panitumumab plus best supportive care compared with best supportive care RCT, by calculating the area under the best supportive care progression-free survival Kaplan–Meier curve, using probabilities at 4-weekly intervals. Next, we estimated the mean progression-free survival for the panitumumab plus best supportive care group as 4.0 months from the panitumumab plus best supportive care compared with best supportive care RCT, by calculating the area under the panitumumab plus best supportive care progression-free survival Kaplan–Meier curve, using probabilities at 4-weekly intervals. Adjusting progression-free survival for panitumumab plus best supportive care for the indirect comparison using the Bucher method34 yields a mean of:
This is our estimate of the mean progression-free survival for the panitumumab plus best supportive care group if panitumumab plus best supportive care had been included as a third treatment group in the cetuximab plus best supportive care compared with best supportive care RCT.
Next, we specified that progression-free survival for panitumumab plus best supportive care follows a Weibull distribution. We then estimated the shape parameter, γ, of the Weibull by fitting a Weibull curve to the Kaplan–Meier panitumumab plus best supportive care progression-free survival curve at 4-weekly intervals, by minimising the sums of squares of differences between actual and expected progression-free survival.
Finally, given that we have specified the mean progression-free survival for panitumumab plus best supportive care and the shape parameter, γ, this then specifies the scale parameter, λ, of the Weibull, given the formula for the mean of the Weibull.
We estimated the uncertainty in progression-free survival on panitumumab plus best supportive care for the probabilistic sensitivity analysis in exactly the same way as for best supportive care and cetuximab plus best supportive care (see Progression-free survival for best supportive care and Progression-free survival for cetuximab and best supportive care). Again, we modelled the uncertainty in the mean progression-free survival by specifying that the mean progression-free survival follows a gamma distribution, given that this appropriately models positive random variables, with a mean of 5.1 months. In this case, the estimated standard error of the mean panitumumab plus best supportive care progression-free survival equals:
The mean time on panitumumab is a very important parameter in the estimation of the cost-effectiveness of panitumumab plus best supportive care compared with best supportive care. The RCT of panitumumab compared with best supportive care reports a mean of 10 doses of panitumumab per patient for those with KRAS WT status. 32 However, for the indirect comparison, we require the estimated number of doses of panitumumab if panitumumab plus best supportive care had been a treatment group in the cetuximab compared with best supportive care RCT. This is estimated by the Bucher indirect comparison method34 as:
For the deterministic analysis, this quantity equals 10 × (5.06/4.0) = 12.7 doses.
Given that panitumumab is taken every 2 weeks, this corresponds to a treatment duration of 5.8 months or 0.49 years. Discounting has only a very small impact on the total drug acquisition costs given that progression-free survival is of such a short duration. Nonetheless, we approximated for discounting in the cost of panitumumab acquisition by assuming that all panitumumab doses were taken at the mean time in progression-free survival. We also used the adjusted number of panitumumab doses of 12.7 to estimate the total per person mean administration cost of panitumumab, as described below.
To estimate the mean number of doses of panitumumab for the probabilistic sensitivity analysis, we needed two further assumptions. First, we modelled the mean number of doses from the RCT of panitumumab plus best supportive care compared with best supportive care as a normal distribution, which is appropriate given the relatively small coefficient of variation, with a mean of 10 doses and a standard error of 10% of the mean, given that the standard error of mean progression-free survival for panitumumab plus best supportive care is approximately 10% of the mean progression-free survival. Second, we modelled the mean progression-free survival from the panitumumab plus best supportive care compared with best supportive care RCT as a normal distribution, with a mean of 4.0 months and a standard error of 0.36, with standard error estimated with two simplifying assumptions. The first was that progression-free survival for panitumumab plus best supportive care from the panitumumab plus best supportive care compared with best supportive care RCT approximately follows an exponential distribution. Indeed, we find this to be approximately true (gamma of Weibull = 1.2). We can then say that the standard deviation of progression-free survival across patients equals the mean progression-free survival, as this is a property of the exponential distribution. The second simplifying assumption is that no patients who started treatment with panitumumab plus best supportive care in the RCT of panitumumab plus best supportive care compared with best supportive care were censored. Indeed, this is also approximately true: only 7% of patients were censored. 32 In this case, the standard error of mean panitumumab plus best supportive care progression-free survival equals:
The estimated number of doses of panitumumab if panitumumab plus best supportive care had been a treatment group in the cetuximab plus best supportive care compared with best supportive care RCT for the probabilistic sensitivity analysis is then calculated as described above for the deterministic case.
In this section we estimate overall survival for panitumumab plus best supportive care for the indirect comparison using a similar method as for the estimation of progression-free survival for panitumumab plus best supportive care for the indirect comparison. First, we fitted a Weibull curve to the overall survival for the panitumumab plus best supportive care group from the panitumumab plus best supportive care compared with best supportive care RCT, by minimising the sums of squares of differences between the actual and estimated survival probabilities, using survival probabilities at 4-weekly intervals. This gives a mean overall survival of 9.9 months. Next, we estimated the mean overall survival as 9.4 months for best supportive care from the panitumumab plus best supportive care compared with best supportive care RCT,32 again by fitting a Weibull curve and by minimising the sums of squares of differences between the actual and estimated survival probabilities, using survival probabilities at 4-weekly intervals. This is the mean overall survival for best supportive care without adjustment for the substantial crossover of patients from the best supportive care group to the panitumumab plus best supportive care group. Amgen's analysis of the individual patient data suggested that, after adjusting for crossover, the mean overall survival in the best supportive care group is 2.7 months less than for the panitumumab plus best supportive care group. This is discussed in detail in our critique of Amgen's submission (see Chapter 5, Industry submission critique 3: Amgen, panitumumab) and assumes that we can model overall survival for best supportive care patients with KRAS mutant status (including some people who cross over) as an approximation for overall survival for best supportive care patients with KRAS WT status. We therefore estimate the mean overall survival for the best supportive care group as the mean overall survival for the panitumumab plus best supportive care group minus 2.7 months: 9.9 – 2.7 = 7.2 months.
Adjusting mean overall survival for panitumumab plus best supportive care for the indirect comparison using the Bucher method yields:34
This is our estimate of the mean overall survival for the panitumumab plus best supportive care group if panitumumab plus best supportive care had been included as a treatment group in the cetuximab plus best supportive care compared with best supportive care RCT. 47
Next, we specify that overall survival for panitumumab plus best supportive care follows a Weibull distribution. We then use the shape parameter, γ, of the Weibull from our fit to the Kaplan–Meier panitumumab overall survival curve, described above. Finally, given that we have specified the overall survival mean and the shape parameter, γ, this then specifies the scale parameter, λ, of the Weibull.
We estimated the uncertainty in overall survival for panitumumab plus best supportive care for the probabilistic sensitivity analysis in exactly the same way as for the uncertainty in progression-free survival on panitumumab plus best supportive care (see Progression-free survival for panitumumab). Our estimate of the standard error of mean overall survival assumes that few patients were censored in the panitumumab plus best supportive care arm of the panitumumab plus best supportive care compared with best supportive care RCT: 14% of patients were censored. 32
Given the lack of further evidence, we set overall survival and progression-free survival for panitumumab plus best supportive care to be perfectly correlated, just as we did for overall survival and progression-free survival for best supportive care and for cetuximab plus best supportive care.
Cetuximab plus irinotecan
The pivotal BOND trial49 and supportive MABEL trial used to confirm the clinical efficacy of cetuximab in combination with irinotecan in the pretreated metastatic colorectal cancer setting did not have KRAS status as a prerequisite for recruitment, and no retrospective KRAS analysis has been systematically undertaken. Given that we do not have direct randomised evidence for progression-free survival, time on treatment and overall survival for patients with KRAS WT status on cetuximab plus irinotecan, some assumptions to estimate these quantities have to be made. These complex assumptions are critical to an understanding of the estimation of the cost-effectiveness of cetuximab plus irinotecan, and the associated uncertainty. Details of the methods used are given in Appendix 14 (progression-free survival) and Appendix 15 (overall survival).
Progression-free survival is estimated in three stages:
-
first, we estimate the median progression-free survival for patients with KRAS WT status on cetuximab plus irinotecan in the BOND RCT of cetuximab plus irinotecan compared with cetuximab plus best supportive care (as this is not reported)
-
next, we adjust this to estimate the median progression-free survival for patients with KRAS WT status on cetuximab plus irinotecan for our model
-
finally, we assume that progression-free survival follows a Weibull distribution (as for cetuximab plus best supportive care) with the same shape parameter as for cetuximab plus best supportive care.
A detailed description of these three stages and the assumptions made is given in Appendix 14. We estimate a mean progression-free survival of 8.8 months for patients receiving cetuximab plus irinotecan. This is similar to Merck Serono's estimated mean of 7.8 months (Figure 10).
FIGURE 10.
Peninsula Technology Assessment Group and Merck Serono progression-free survival for patients with KRAS WT status in the cetuximab plus irinotecan group.
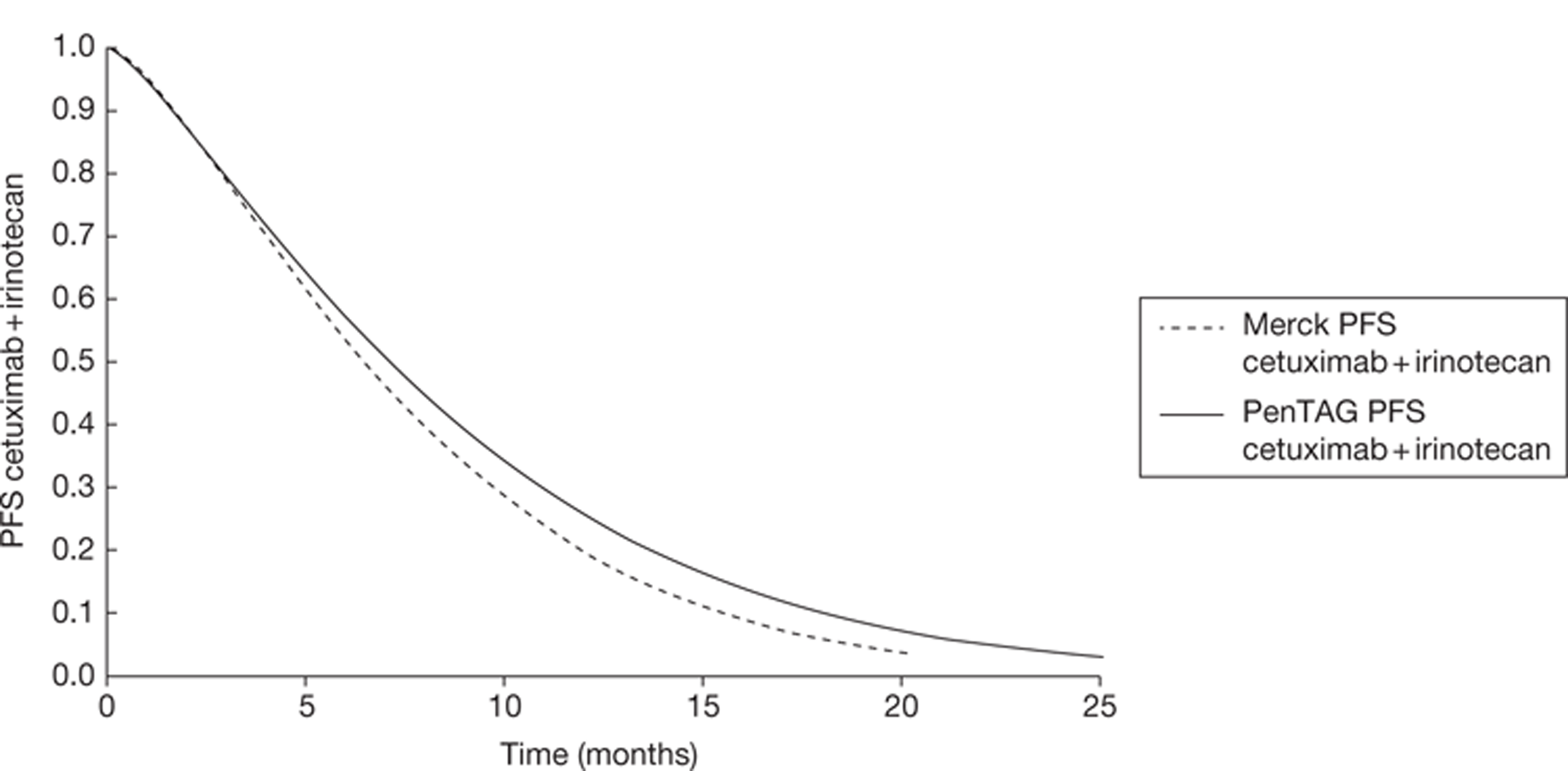
We note briefly that Merck Serono estimates progression-free survival for patients with KRAS WT status on cetuximab plus irinotecan by applying the hazard ratio of 0.47 between cetuximab plus irinotecan (patients with KRAS WT status) and cetuximab monotherapy (patients with KRAS WT status) from De Roock and colleagues48 to its curve fit to progression-free survival for cetuximab monotherapy (patients with KRAS WT status) (which it estimated from the cetuximab plus best supportive care compared with best supportive care RCT) (p. 104, Merck Serono's submission69). Merck Serono states that it estimated the hazard ratio by reading off survival data from the progression-free survival curves published in De Roock and colleagues;48 however, it is difficult to verify the hazard ratio because it is not published in this study. Furthermore, the method used by Merck Serono introduces a good deal of uncertainty because it relies on data on cetuximab monotherapy (patients with KRAS WT status) for which there are only 18 people.
We estimated the uncertainty in progression-free survival on cetuximab plus irinotecan for the probabilistic sensitivity analysis in a similar but slightly different way to that used for uncertainty in progression-free survival for cetuximab plus best supportive care and panitumumab plus best supportive care. In this case, we modelled the uncertainty in the median progression-free survival, not the mean progression-free survival, given that our estimation of progression-free survival for cetuximab plus irinotecan is based on the median. First, for simplicity, we fixed the shape value gamma of the Weibull for progression-free survival. Next, we specified that the median (not the mean) progression-free survival follows a gamma distribution, which is appropriate for positive random variables, with a mean of 7.1 months (see stage 2, Appendix 14). We then estimated the standard error of the median progression-free survival as simply equal to 20% of the mean: 1.4 months. This method was chosen as being the most pragmatic, given the lack of evidence on this quantity. Note that the ratio of the standard error to the mean of the median, at 20%, is greater than the corresponding ratio for the mean progression-free survival for best supportive care, cetuximab and panitumumab (all approximately 10%), to reflect the extra uncertainty in progression-free survival for cetuximab plus irinotecan. Finally, the scale parameter, λ, of the Weibull for progression-free survival is back-calculated from the fixed gamma and variable median, using the following formula for the median t* of the Weibull: 0.5 = exp(–λt*γ).
The time on cetuximab plus irinotecan treatment is an extremely important quantity because it affects the total mean cost of cetuximab plus irinotecan acquisition per person, which is a critical driver of the cost-effectiveness of cetuximab plus irinotecan compared with best supportive care.
Unfortunately, the mean duration of cetuximab plus irinotecan treatment for patients with KRAS WT status in the BOND RCT is not reported. In the absence of this crucial information, we assume that all patients take cetuximab plus irinotecan treatment for the entire duration of progression-free survival. This gives a median duration of cetuximab plus irinotecan treatment of 31 weeks, and a mean duration of 38 weeks.
Given the importance and uncertainty of the mean duration of cetuximab plus irinotecan treatment, we vary the mean duration of irinotecan treatment and the mean duration of cetuximab treatment in our sensitivity analyses.
There is some support for our base-case assumption that patients in the cetuximab plus best supportive care and cetuximab plus irinotecan treatment groups in the BOND RCT took cetuximab until progression. The median progression-free survival for all patients (KRAS WT and KRAS mutant status combined) in the cetuximab plus best supportive care treatment group was 1.5 months (6.5 weeks), and the median number of cetuximab doses in the cetuximab plus best supportive care treatment group was seven. 49 Given that cetuximab was given once per week in the BOND RCT, this suggests that it was taken until disease progression. Similarly, the median progression-free survival for all patients (KRAS WT and KRAS mutant status combined) in the cetuximab plus irinotecan treatment group was 4.1 months (17.8 weeks), and the median number of cetuximab doses in the cetuximab plus irinotecan treatment group was virtually identical, at 18. 49
As for treatment with cetuximab (see Cetuximab monotherapy), Merck Serono forced the mean time on cetuximab plus irinotecan treatment in its model to equal its estimate of the mean time on cetuximab plus irinotecan treatment for patients with KRAS WT status in the BOND RCT of cetuximab plus irinotecan compared with cetuximab plus best supportive care (see table 59, Merck Serono's submission69). However, we believe that it was a very serious limitation that Merck Serono did not state this, their derivation of the mean time on cetuximab plus irinotecan treatment of 19 weeks (4.4 months) (although this was stated in the model), and also a very serious omission that it did not explain the derivation of this figure in its report. We questioned Merck Serono on the derivation of the 19 weeks and received the following reply: ‘The BOND study compared cetuximab plus irinotecan compared with cetuximab monotherapy, but was undertaken before KRAS status was identified as a marker for response; hence, the mean number of infusions is not available for the KRAS WT population. For the ITT analysis, the mean number of infusions was 18 for those on cetuximab plus irinotecan and 7 for those on cetuximab monotherapy (Cunningham et al. 2004) ... The mean number of cetuximab and irinotecan combination therapy infusions within the model for the KRAS WT population was not increased proportionately as per cetuximab monotherapy. The increasing side effects with combination therapy are likely to limit the treatment duration.’
We strongly disagree with Merck Serono's derivation of the mean duration of cetuximab plus irinotecan treatment for three important reasons. First, it seems highly unlikely that patients with KRAS WT status would take cetuximab plus irinotecan for the same time as those patients with KRAS mutant status. It is far more likely that the duration of treatment would be longer for patients with KRAS WT status than for those with KRAS mutant status, and therefore longer for patients with KRAS WT status than for all patients combined. This is because cetuximab is known to improve progression-free survival for patients with KRAS WT status but not for those with KRAS mutant status. Furthermore, in BOND, drug treatment was given until disease progression or the occurrence of adverse events, and three sources cite a substantially longer progression-free survival time for cetuximab plus irinotecan treatment for patients with KRAS WT status than for those with KRAS mutant status: 7.8 months compared with 2.8 months,48 7.4 months compared with 2.1 months86 and 5.5 months compared with 2.8 months. 83 Second, Merck Serono has equated means with medians: it sets the mean duration of cetuximab plus irinotecan treatment for patients with KRAS WT status equal to the median duration of cetuximab plus irinotecan treatment for all patients. Given that the mean is usually greater than the median, for example by a factor of 1.44 for the exponential distribution, Merck Serono's estimate for the mean treatment duration of cetuximab plus irinotecan for all patients in BOND is probably an underestimate. Third, Merck Serono has made no attempt to adjust the treatment duration from BOND for the indirect comparison with best supportive care.
For the probabilistic sensitivity analysis, the mean time on cetuximab plus irinotecan treatment was assumed to equal the mean time in progression-free survival, which we varied stochastically as explained in the previous section.
Here, we estimate overall survival for cetuximab plus irinotecan for patients with KRAS WT status for the purposes of the indirect comparison. We first note that both our estimate and Merck Serono's estimate of overall survival are highly uncertain given the lack of randomised evidence, and that we have both had to make substantial assumptions. Furthermore, we believe that the uncertainty in overall survival for cetuximab plus irinotecan is considerably greater than that for progression-free survival.
Our method to estimate overall survival proceeds in two stages:
-
first, we estimate the median overall survival for patients with KRAS WT status on cetuximab plus irinotecan for our model
-
next, we assume that overall survival follows a Weibull distribution (as for cetuximab) with the same shape parameter as for cetuximab plus best supportive care.
A detailed description of these two stages and the assumptions made is given in Appendix 15. We estimate a mean overall survival of 16.6 months for patients receiving cetuximab plus irinotecan. This is similar to Merck Serono's estimated mean of 16.3 months (Figure 11).
FIGURE 11.
Peninsula Technology Assessment Group and Merck Serono overall survival for patients with KRAS WT status in the cetuximab plus irinotecan group.
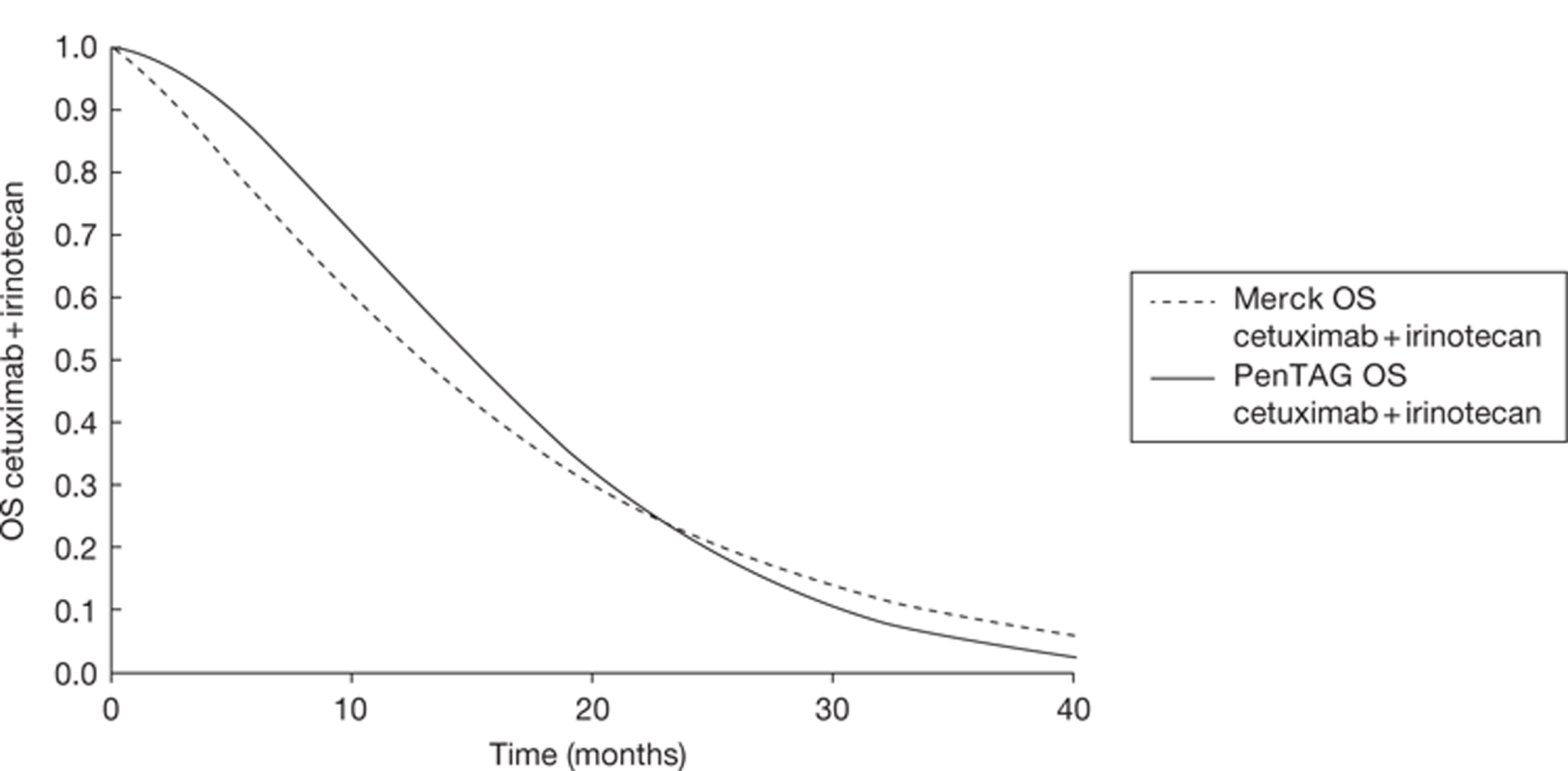
Both our estimate and Merck Serono's estimate of overall survival for patients with KRAS WT status on cetuximab plus irinotecan are highly uncertain given that we have both had to make substantial assumptions. Therefore, we also present sensitivity analyses in which we use different methods of estimating overall survival (see Appendix 15).
We estimated the uncertainty in overall survival for the probabilistic sensitivity analysis in exactly the same way as for the uncertainty in progression-free survival on cetuximab plus irinotecan, namely by modelling the uncertainty in median overall survival. We estimated the standard error of the median overall survival as simply equal to 20% of the mean of the median, to reflect the substantial uncertainty in overall survival.
Given the lack of further evidence, we set overall survival and progression-free survival for cetuximab plus irinotecan to be perfectly correlated, just as we did for best supportive care, panitumumab plus best supportive care and cetuximab plus best supportive care.
Utilities
Health-related quality of life literature
Au and colleagues44 describe the HRQoL information collected using the EORTC QLQ-C30 during the cetuximab plus best supportive care compared with best supportive care RCT.
Compliance was high at baseline (> 90%) but declined over time, particularly for best supportive care. The authors believe that this selective non-compliance may make QoL for cetuximab plus best supportive care conservative because more people on best supportive care than on cetuximab plus best supportive care who were in poor health stopped completing the questionnaire. Conversely, given that the RCT was not blinded, people's judgement of their QoL could have been biased downwards for people on best supportive care, and biased upwards for those on cetuximab (because of a potential placebo effect). Also, given that those people who complete the questionnaire are likely to be healthier, on average, than those who do not, all utilities from Au and colleagues44 are likely to be overestimates.
For patients with KRAS WT status, treatment with cetuximab plus best supportive care resulted in less physical function deterioration over time than best supportive care. Treatment with cetuximab plus best supportive care resulted in improved global health status at 8 weeks compared with baseline, whereas the global health status of patients on best supportive care worsened. At 16 weeks, the global health status of patients on cetuximab plus best supportive care was approximately unchanged from baseline, whereas the global health status of patients on best supportive care was much reduced from baseline.
The cost-effectiveness study of Mittmann and colleagues42 reports that the HUI3 was also used in the RCT of cetuximab plus best supportive care compared with best supportive care. Although the NICE reference case72 states a preference for the EQ-5D, Mittmann and colleagues present the only utility data from the RCT of which we are aware. Health was assessed at baseline and at 4, 8, 16 and 24 weeks after randomisation (Table 41). Merck Serono claims that these values relate to patients with KRAS WT status only; however, this is difficult to believe given that there were more patients in the utility data set than there were patients with KRAS WT status in the RCT.
| Time of assessment | CET + BSC, mean ± SD (n) | BSC, mean ± SD (n) |
|---|---|---|
| Baseline | 0.72 ± 0.23 (263) | 0.71 ± 0.24 (260) |
| Week 4 | 0.73 ± 0.26 (220) | 0.68 ± 0.26 (184) |
| Week 8 | 0.73 ± 0.24 (190) | 0.66 ± 0.28 (149) |
| Week 16 | 0.73 ± 0.24 (119) | 0.63 ± 0.30 (72) |
| Week 24 | 0.77 ± 0.22 (82) | 0.70 ± 0.24 (36) |
At each follow-up time, mean utility scores for cetuximab plus best supportive care were higher than those for best supportive care. For the cetuximab plus best supportive care arm, utilities remained largely unchanged over time. By contrast, utilities in the best supportive care arm generally declined over time, with the exception of the last time point, which may have been unrepresentative because of the relatively small sample size.
Merck Serono assumed the utilities from the RCT of cetuximab plus best supportive care compared with best supportive care in its economic model (given in Table 42). These utilities were calculated from the HUI3 index, although Merck Serono provides very little detail of the calculations. We agree with Merck Serono's claim that the QoL data reflect both the positive aspects of a response to treatment with cetuximab plus best supportive care and the negative aspects of treatment such as adverse events.
However, we are concerned that, although both Mittmann and colleagues42 and Merck Serono both report utilities estimated by the HUI3, the values reported by Merck do not always tally with the values quoted in Mittmann and colleagues. For example, the Mittmann values would suggest that the utility for progression-free survival for cetuximab plus best supportive care should be about 0.73, whereas Merck Serono reports 0.81.
| CET + BSC | BSC | |
|---|---|---|
| Progression-free survival | ||
| No. of patients | 294 | 170 |
| Mean utility (SE) | 0.809 (0.011) | 0.746 (0.017) |
| Progressive disease | ||
| No. of patients | 83 | 85 |
| Mean utility (SE) | 0.789 (0.025) | 0.693 (0.027) |
Merck Serono's estimated utilities for progressive disease are also a limitation of the approach. First, because of high dropout rates, there are far fewer utility observations for patients in progressive disease than for those in progression-free survival. Second, at the point of data cut-off, a large proportion of patients were still alive in both treatment arms. 37 This means that the utilities for progressive disease do not include many time points when patients are close to death. Therefore, we suspect that the true mean utilities for progressive disease for best supportive care and cetuximab plus best supportive care, averaged over the total time in progressive disease, may be lower than the values used by Merck Serono.
In the economic evaluation of bevacizumab for first-line treatment and cetuximab plus irinotecan for second-line and further treatment of metastatic colorectal cancer, Tappenden and colleagues10 assumed a utility of 0.80 in progression-free survival and 0.60 in progressive disease, independent of treatment. The progression-free survival value of 0.80 was taken from HUI3 responses from a small study of 173 people with colorectal cancer of various stages taken from the US SEER database. The progressive disease value of 0.60 appears to have been a ‘best guess’ given the dearth of relevant literature.
Odom and colleagues56 assessed the HRQoL of patients in the RCT of panitumumab plus best supportive care compared with best supportive care. QoL was assessed using the EQ-5D, NICE's preferred instrument,72 using the VAS, and the NCCN FCSI. Data were available for 208 patients with KRAS WT status (112 panitumumab plus best supportive care arm, 96 best supportive care arm). Only outcomes before disease progression up to week 17 of the study were used because of small sample sizes after this time. The QoL of patients with KRAS WT status taking panitumumab plus best supportive care was better than the QoL of those on best supportive care, with a significant difference of 0.22 in the EQ-5D utility (95% CI 0.12 to 0.32). 56 Similar to the QoL study in the cetuximab plus best supportive care compared with best supportive care RCT, there were many missing data, particularly in the later weeks, although patients receiving panitumumab plus best supportive care had a higher percentage of available data for each post-baseline week than those receiving best supportive care. This could bias against panitumumab, and this is confirmed by analysis in Odom and colleagues. 56 Also, similar to the cetuximab plus best supportive care compared with best supportive care RCT, the panitumumab plus best supportive care compared with best supportive care RCT was not blinded. Therefore, patients’ judgement of QoL could have been biased downwards for those on best supportive care and upwards for those on panitumumab plus best supportive care. Also, given that those people who complete the questionnaire are likely to be healthier, on average, than those who do not, all utilities from Odom and colleagues are likely to be overestimates.
Utilities in the Peninsula Technology Assessment Group's model
Our choice of utilities is given in Table 43 and is based on those supplied by Merck Serono. This seems appropriate because the utilities were collected in the RCT of cetuximab plus best supportive care compared with best supportive care, the same source as for our baseline efficacy estimates. These utilities are probably overestimates because people who complete HRQoL questionnaires are likely to be healthier, on average, than those who do not. In addition, given that both RCTs7,37 were not blinded, people's judgement of their QoL could have been biased upwards for those on cetuximab plus best supportive care and panitumumab plus best supportive care, because of the placebo effect. However, without further information, it is not possible to quantify the bias in either case.
| Progression-free survival | Progressive disease | |||||
|---|---|---|---|---|---|---|
| Mean (SE) | Correlation | Source | Mean (SE) | Correlation | Source | |
| BSC | 0.75 (0.08) | Baseline | MS submission | 0.69 (0.07) | Correlated with BSC progression-free survival | MS submission |
| CET + BSC | 0.81 (0.08) | Correlated with BSC progression-free survival | MS submission | 0.69 (0.07) | Set equal to BSC progressive disease | Adjusted from MS submission |
| PAN + BSC | 0.87 (0.09) | Correlated with BSC progression-free survival | Based on Odom et al.56 See also calculations in Appendix 11 | 0.69 (0.07) | Set equal to BSC progressive disease | MS submission |
| CET + IRIN | 0.75 (0.08) | Set equal to BSC progression-free survival | MS submission | 0.69 (0.07) | Set equal to BSC progressive disease | Adjusted from MS submission |
For best supportive care we use the mean utilities quoted by Merck Serono – 0.75 in progression-free survival and 0.69 in progressive disease – taken from the RCT of cetuximab plus best supportive care compared with best supportive care. 47 For cetuximab monotherapy, as Merck Serono did, we used the mean utility of 0.81, also taken from the RCT of cetuximab plus best supportive care compared with best supportive care. Our clinical advisor believes that it is indeed plausible that people taking cetuximab plus best supportive care in progression-free survival have a higher HRQoL than people in progression-free survival in the best supportive care group, as follows. First, HRQoL for cancer patients is broadly affected by two factors: tumour bulk and the degree of drug toxicity. Given that cetuximab is not particularly toxic (e.g., compared with chemotherapy drugs such as irinotecan), the HRQoL of people in progression-free survival on cetuximab plus best supportive care would be similar to that for people in progression-free survival on best supportive care. Second, tumour bulk will, on average, be lower in progression-free survival for patients taking cetuximab, because some patients respond to cetuximab, that is, their tumour shrinks.
However, the mean utility of 0.79 for patients in the cetuximab plus best supportive care treatment group in progressive disease seems too high. This value is only marginally lower than the 0.81 for patients in the cetuximab plus best supportive care group in progression-free survival, and is substantially higher than the utility of 0.69 for patients in the best supportive care group in progressive disease. This may result from differential time spent in progressive disease by those treated with best supportive care and cetuximab plus best supportive care, leading to questionnaires being completed by patients who had been in progressive disease for a longer time, on average, in the best supportive care group than in the cetuximab plus best supportive care group. This is due to the fairly short data cut-off time and the fact that patients in the best supportive care group progressed faster than those in the cetuximab plus best supportive care group. We sought clarification from Merck Serono on this point. Merck Serono did not deny this assertion, but replied: ‘The assumption in the model simplifies the detailed observations by assuming one utility weight per disease state. There may be biases caused by the fact that the model assumes the same utility in PFS [progression-free survival] and from progression until death, but it is not likely to affect the cost effectiveness.’
A mean utility of 0.69 for patients in the cetuximab plus best supportive care treatment group in progressive disease, the same as for patients in the best supportive care group in progressive disease, seems more appropriate. Indeed, we set the mean utility for patients in all groups in progressive disease equal, at 0.69, and for the probabilistic sensitivity analysis we set the utility for all patients in all groups in progressive disease equal within each simulation. Our justification is as follows: all patients in progressive disease, regardless of treatment group, are, by definition, off active drug treatment and as such there is no drug toxicity. Second, tumour bulk will be similar for all patients, regardless of treatment group in progressive disease, given that tumour bulk is a major criterion for disease progression.
The value of 0.69 for all patients in progressive disease is probably an overestimate because it is likely that many patients were alive for several months after their last HRQoL questionnaire, because of the limited data cut-off time. However, it is impossible to quantify the magnitude of any such bias without access to the detailed individual patient data.
Next, we used the utilities measured in the RCT of panitumumab plus best supportive care compared with best supportive care, published in Odom and colleagues,56 to estimate that the mean utility in progression-free survival for patients taking panitumumab plus best supportive care is 0.12 higher than for patients in progression-free survival on best supportive care. Similar to the assertion that the utility of patients receiving cetuximab plus best supportive care is higher than for patients in progression-free survival in the best supportive care group, our clinical expert is satisfied with the analogous finding for patients receiving panitumumab plus best supportive care. Odom and colleagues do not provide absolute utilities, only difference from baseline. Detailed calculations are given in Appendix 11, but, broadly, we calculate the increment in utility by weighting the progression-free survival curve for panitumumab by the decrease in utility from baseline for panitumumab plus best supportive care over time, and weighting the progression-free survival curve for best supportive care by the decrease in utility from baseline for best supportive care over time. We then estimate the mean utility for patients in progression-free survival on panitumumab plus best supportive care in the manner of an indirect comparison as the utility for patients on best supportive care from the cetuximab plus best supportive care compared with best supportive care RCT plus the difference in utility between panitumumab plus best supportive care and best supportive care from the panitumumab plus best supportive care compared with best supportive care RCT, which equals 0.75 + 0.12 = 0.87. Although this value is evidence based, we caution that it is high compared with that corresponding to the UK general population.
Finally, we assume that the mean utility for patients in progression-free survival taking cetuximab plus irinotecan is equal to 0.75, which is the utility for patients in progression-free survival in the best supportive care group. Furthermore, in the probabilistic sensitivity analysis, we assume that the progression-free survival utilities for patients in the best supportive care and cetuximab plus irinotecan groups are equal within each simulation. Conversely, Merck Serono chose a mean utility of 0.81 for patients in progression-free survival taking cetuximab plus irinotecan, the same as for patients in progression-free survival taking cetuximab plus best supportive care. As stated above, HRQoL is influenced by both tumour mass and drug toxicity. On the one hand, we might expect the HRQoL for patients in progression-free survival on cetuximab plus irinotecan to be higher than for patients in progression-free survival in the best supportive care group because the tumour mass for patients in progression-free survival on cetuximab plus irinotecan is, on average, smaller than that for patients in progression-free survival on best supportive care. On the other hand, one might expect the HRQoL for people in progression-free survival on cetuximab plus irinotecan to be lower than for patients in the best supportive care group because irinotecan is a toxic chemotherapy. On balance, our clinical advisor suggests that the mean utility for patients taking cetuximab plus irinotecan is probably lower than for patients in progression-free survival in the best supportive care treatment group. It is difficult to estimate the net effect, but note that Starling and colleagues61 report that, in the MABEL single-arm study of cetuximab plus irinotecan, the mean utility, as assessed by the EQ-5D, was 0.746, which is similar to our estimate of 0.75.
For the probabilistic sensitivity analysis, we modelled all utilities as beta distributions. We could have taken the standard errors of the utilities for cetuximab plus best supportive care and best supportive care from the RCT of cetuximab plus best supportive care compared with best supportive care; however, this would capture only uncertainty within the RCT. Instead, we attempted to capture broader uncertainty, for example to allow for the fact that utilities in progressive disease were not collected throughout the progressive disease of all patients, and to allow for extra uncertainty given that not all people completed the HRQoL questionnaires. This broader uncertainty was achieved by setting the standard errors of all utilities equal to 10% of the mean of each utility. Indeed, this is similar to Merck Serono's approach of setting the standard error equal to 20% of the mean for variables for which data on uncertainty are not available. We chose the standard error as 10% because this gave a plausible range of simulated utilities, given our experience of the utilities in other disease areas. Although the standard errors are clearly approximate, our method seems to be reasonably pragmatic.
It is impossible to accurately model the correlation between the utilities. Therefore, we took the pragmatic view of assuming correlation between all utilities, as shown in Table 43. This then captures various commonsense ideas, for example that the utility in progressive disease should always be less than the utilities in progression-free survival, and that if the utility for best supportive care progression-free survival is higher than expected, then so too should be the utility for all the other treatments in progression-free survival.
We did not model additional utility decrements associated with adverse events in the base case, as our utilities reflect the experiences of people on treatment and therefore include treatment-related adverse events that did not result in treatment discontinuation.
Costs
We model the following costs: KRAS testing, drug acquisition, drug administration, consultant outpatient visits, computerised tomography scans, best supportive care in progressive disease and treatment for adverse events. All costs are inflated to 2011–12 values where appropriate.
In addition to the cost of drug acquisition, mean drug costs per person allow for treatment duration (see Overall survival, progression-free survival and treatment duration) and dose intensity (see Dose intensities).
Costs of epidermal growth factor receptor and Kirsten rat sarcoma testing
Across the UK as a whole, to test the suitability of a patient for cetuximab or panitumumab, most patients are tested only for KRAS, not for EGFR status (Dr Ian Chandler, Consultant Histopathologist, Royal Devon & Exeter Hospital, Exeter, personal communication, 18 March 2011). Therefore, we model the cost of the KRAS test only. There are no testing costs for people on best supportive care.
When we model the mean cost of testing per EGFR-expressing metastatic colorectal cancer patient with KRAS WT status, we need to cost for all patients who take the KRAS test, not just those who are KRAS WT. This is because, although people who test negative will not receive the drug treatment, they will nonetheless incur the testing costs. The total cost of the KRAS test per person in the model is then taken as the cost of a test divided by the proportion of people who are KRAS WT. In common with Merck Serono, we assume a cost per KRAS test of £160. 80
We set the proportion of people with KRAS WT disease as 54%. This is taken from Merck Serono's submission: ‘Global clinical trial data indicates that ... approximately 30–50% have KRAS WT disease (Erbitux Summary of Product Characteristics, November 2010). A more accurate estimate for the proportion of patients with KRAS WT disease is available from local KRAS testing facilities in Wales. The figure of 54% with KRAS WT disease from the local laboratories has consequently been used throughout the submission’ (p. 16, Merck Serono's submission69).
Combining this information, we assume a cost for KRAS testing per person tested as £160/54% = £296 for all treatments apart from best supportive care, for which the cost was set at zero. This cost is very low compared with other costs, such as for drug acquisition. For the probabilistic sensitivity analysis we modelled the cost of a KRAS test as a gamma distribution, independent of all other model parameters. It is very difficult to select an appropriate standard error; therefore, we chose the pragmatic solution of setting the standard error equal to 20% of the mean.
Drug prices
Table 44 presents the drug prices, which for panitumumab and irinotecan have been taken from BNF 61. 76 The price of cetuximab was provided by Merck Serono, and NICE have instructed us to use this value.
| Cost per month model cycle | |||
|---|---|---|---|
| Dose and frequency | Price | No vial wastage | With vial wastage |
| Cetuximab (Erbitux) | |||
| Initially 400 mg/m2 body area, followed by weekly 250 mg/m2 | £136.50 per 20-ml (100-mg) vial, £682.50 per 100-ml (500-mg) viala | £3108 first month, £2730 subsequently | Initially 400 mg/m2 body area, followed by weekly 250 mg/m2 |
| Panitumumab (Vectibix) | |||
| 6 mg/kg every 2 weeks | 20 mg/ml, net price: £379.29 per 5-ml vial, £1517.16 per 20-ml vial | £3693 | 6 mg/kg every 2 weeks |
| Irinotecan (generic) | |||
| 180 mg/m2 every 2 weeks | £49.03 per 2-ml vial, 20 mg/ml | £882 | 180 mg/m2 every 2 weeks |
In common with Merck Serono, we assumed a dosage of irinotecan of 180 mg/m2 every 2 weeks. Indeed, members of Merck Serono's advisory board (see appendix 1, Merck Serono's submission69) agreed that most clinicians in the UK follow this regime. In common with Merck Serono, we assumed the generic price for irinotecan, although this is not an important assumption because the price of the branded version of irinotecan, Campto (Pfizer), is very similar.
All drugs are given intravenously in fixed vial sizes. Any unused drug left in the vials after administration is discarded, partly to avoid contamination; this is thought to be common practice across the UK (Kate Copland, personal communication). Therefore, in our base case, we assumed total wastage of all drugs that remain in vials at the end of the infusion for each patient. Also in common with Merck Serono, we assumed the smallest vial sizes for all drugs to minimise the drug costs per patient, after allowing for wastage of drugs.
The doses of cetuximab and irinotecan are given in proportion to body surface area. Merck Serono assumed a body surface area of 1.79 m2, representing the mean value from Sacco and colleagues. 87 In this study, the authors calculated the body surface area of 3613 patients receiving chemotherapy for various cancers in the UK in 2005 from the height and weight, using the Dubois and Dubois method88 (also quoted by Merck Serono): body surface area (m2) = 0.007184 × weight (kg)0.425 × height (cm)0.725. Merck Serono's value of 1.79 m2 is the mean over several cancers and over men and women.
As is standard practice in HTA, Merck Serono then assumed that the body surface areas of all patients is the same, 1.79 m2. However, in reality, weights, heights and surface areas vary and, as such, the amount of drug wastage will also vary. As suggested by Sacco and colleagues, we captured this by modelling the distribution of body surface areas in a population of people receiving palliative chemotherapy for colorectal cancer, with 66% men and 34% women, and assumed the typical sex mix in the RCTs for metastatic colorectal cancer. We then calculated the total drug used, including wastage, for each patient in this range, and then took the average of these dosages. The details are given in Appendix 12.
The mean body surface area of 1.79 m2 cited by Merck Serono refers to people with a range of cancers. To be more precise, the mean is 1.85 m2 for people receiving palliative chemotherapy for colon cancer (66% men, 34% women). If we assume that all patients have the same body surface area of 1.85 m2, the dose per administration of cetuximab for all patients, allowing for wastage, is 500 mg (after the first dose). However, using our methodology of assuming a distribution for doses across patients, the mean dose per patient per administration, allowing for wastage, is 511 mg. Similarly, the corresponding figures for irinotecan are 360 mg and 352 mg. In these cases, the effect of assuming a distribution for dosages has little effect on the mean dose per patient and hence on cost-effectiveness. However, this is coincidental; Sacco and colleagues found that cost-effectiveness can change substantially.
The dose of panitumumab is proportional to weight, not body surface area. Merck Serono assumed that the weights of all patients are the same, at 64 kg. Again, we modelled the distribution of body weights in a population receiving palliative chemotherapy for colorectal cancer, with 66% men and 34% women. The details are given in Appendix 12 and we calculate the mean weight as 74.9 kg. If we assume that all patients have the same mean weight of 74.9 kg, the dose per administration of panitumumab, allowing for wastage, is 500 mg. However, assuming a distribution for the doses across patients, the mean dose per patient per administration, allowing for wastage, is 499 mg. Again, assuming a distribution for dosages has little effect on the mean dose per person and hence on cost-effectiveness.
We assume no drug costs for patients in the best supportive care treatment arm. This reflects the experience of the cetuximab compared with best supportive care RCT, that is, that only a very small proportion of patients in the best supportive care arm took expensive drug treatments (e.g. 2.5% of patients took irinotecan before progression) (see p. 164, Merck Serono's submission69).
Dose intensities
For consistency between the costs of the drugs and the clinical outcomes, it is necessary to model the amounts of the drugs actually taken in the relevant clinical trials. The dose intensity of a drug is defined as the amount of drug administered in a trial as a proportion of the amount that would have been administered if there had been no dose reductions or dose interruptions. This does not include people who withdraw from treatment because of adverse events. Mean dose intensities per person used in our model are given in Table 45. We assume a dose intensity for panitumumab plus best supportive care of 100%.
| Drug | Treatment arm | Mean dose intensity | Standard error | Source |
|---|---|---|---|---|
| CET | CET + BSC | 98% | 0.8% | Merck Serono submission, p. 11069 |
| CET | CET + IRIN | 94% | 1.6% | Merck Serono submission, p. 11069 |
| IRIN | CET + IRIN | 90% | 2.0% | Merck Serono submission, p. 11069 |
| PAN | PAN + BSC | 100% | 0.0% | Amgen's response to PenTAG's questions (see Appendix 13) |
For the probabilistic sensitivity analysis we modelled the dose intensities as beta distributions, in which we assumed that the dose intensity for any one patient lies between 0% and 100%. The standard error for cetuximab plus best supportive care was calculated as:
where n = 287, the number of people taking cetuximab in the RCT of cetuximab plus best supportive care compared with best supportive care.
The standard error for cetuximab in combination with irinotecan was calculated in the same way, but with n = 218 from the BOND RCT49 of cetuximab plus irinotecan compared with irinotecan. Given that we assumed a mean dose intensity for panitumumab plus best supportive care of 100% and that we specify that the dose intensity per person lies between 0% and 100%, this forces the standard error of the dose intensity for panitumumab plus best supportive care to be 0%. Clearly, there should be no correlation between the dose intensities across drugs.
Drug costs per month, drug costs adjusted for dose intensity, and drug administration costs (see the following section) are shown in Figure 12.
FIGURE 12.
Drug acquisition costs, drug acquisition costs adjusted for dose intensity, and drug administration costs assumed in the PenTAG model BSC, best supportive care.
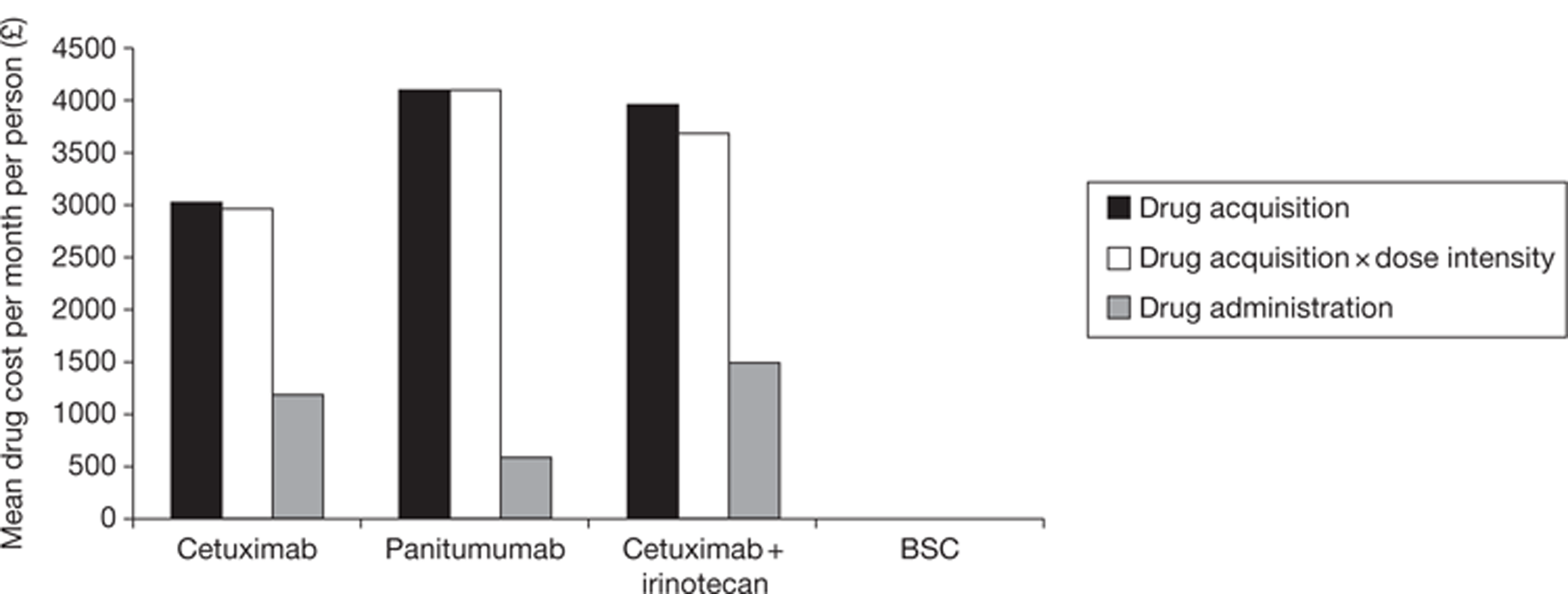
Drug administration costs
According to the Summary of Product Characteristics the administration of drugs should be as follows:
-
cetuximab: the first dose is administered as a 120-minute intravenous infusion and all subsequent doses are administered as 60-minute infusions28
-
panitumumab is administered as a 60-minute intravenous infusion29
-
irinotecan for use in combination therapy is administered as an intravenous infusion over 30–90 minutes followed by infusion with 5-FU/FA. 89
Merck Serono assumes that cetuximab and panitumumab cost £180 per infusion, corresponding to the HRG ‘Deliver simple parenteral chemotherapy at first attendance’, ‘Daycase and regular day/night’ from the NHS reference costs 2008–2009. 77 Merck Serono also assumes that an infusion of cetuximab and irinotecan, when given together, costs £213, corresponding to the HRG ‘Deliver more complex chemotherapy at first attendance’, ‘Daycase and regular day/night’, also from the NHS reference costs 2008–2009. 77
By contrast, Roche assumes a cost of £218 for the administration of bevacizumab, cetuximab and cetuximab plus irinotecan, corresponding to the HRG ‘Deliver subsequent elements of a chemotherapy cycle’, ‘Outpatients’, also from the NHS reference costs 2008–2009,77 although we note that the database lists this as £227, not £218.
In common with Roche, and unlike Merck Serono, we assume the cost of £227 in 2008–9 prices for the intravenous administration of cetuximab monotherapy and panitumumab, corresponding to the HRG SB15Z ‘Deliver subsequent elements of a chemotherapy cycle’, ‘Outpatients’ from the NHS reference costs 2008–2009. 77 We calculate that non-drug NHS costs have typically increased at approximately 4% per annum over the last 5 years, using the Hospital & Community Health Services Pay & Prices Index. 79 Inflating the administration costs over 3 years at 4% per annum, from 2008–9 to 2011–12, the date of this appraisal, gives £255 per administration.
For patients in the cetuximab plus irinotecan group, when irinotecan is administered (every 2 weeks) it is given during the same visit to the hospital as for cetuximab (every week). We assumed a cost of £255 for the administration of cetuximab and half this amount, £128, for the subsequent administration of irinotecan. We did not assume the same cost for the administration of irinotecan because, according to our clinical advisor, the patient will already be mostly set up to receive the second drug, irinotecan, after the first drug, cetuximab. At the other extreme, we do not assume £0 for the administration of irinotecan because there will still be some nursing functions to perform. In the absence of further information, we assume the average of these costs, that is, £128.
When we estimated the total acquisition cost per patient of cetuximab and cetuximab plus irinotecan, we assumed that patients took these drugs while in progression-free survival. Also, we estimated the total acquisition cost per patient of panitumumab based on the mean of 10 doses reported in the panitumumab plus best supportive care compared with best supportive care RCT. 32 For the purpose of calculating the total administration cost per patient of all these treatments, we assumed the same mean number of administrations as we did in our calculation of the total acquisition cost per patient. Figure 12 displays one important component of this calculation, the mean administration cost per person per month by treatment.
Pharmacy drug preparation costs
All drugs require preparation by a hospital pharmacist. We costed for the time of drug preparation as follows. The preparation times per infusion of bevacizumab, irinotecan and cetuximab according to the task (e.g. clinical check of prescription, drug reconstitution and labelling of product) were determined to be equal (Kate Copland, personal communication; see Appendix 12). We assume that the same schedule applies to panitumumab. Using the information in Appendix 12, we calculate the total cost of the preparation of one infusion as £15 for all drugs.
For the probabilistic sensitivity analysis, we modelled the cost of a single drug administration (including pharmacy preparation) as a gamma distribution. We tried to capture the broader uncertainty of this variable given that there may be alternative sources for this cost in addition to the source that we chose, namely, the NHS reference costs 2008–2009. This was achieved by setting the standard error equal to 20% of the mean cost. This is the same approach as that used by Merck Serono, that is, setting the standard error equal to 20% of the mean for variables for which data on uncertainty are not available. We assumed perfect correlation between cetuximab plus best supportive care and panitumumab plus best supportive care by setting the administration costs equal for each simulation. The cost of administration of cetuximab plus irinotecan was also set to correlate perfectly with the other drug administration costs, and for each simulation we set it equal to the cost for the other drugs multiplied by the ratio of the mean cost for cetuximab plus irinotecan to the mean cost for the other drugs.
Medical management costs
Our clinical expert advised us on the nature and frequency of medical management (Table 46).
| Health state | Population | Frequency | Mean cost | Mean cost per 1-month model cycle | Source |
|---|---|---|---|---|---|
| Consultant outpatient visits | |||||
| Progression-free survival | During all active drug treatmenta | 1 visit per 2 weeks | £136 per visit | £295 | £121 per visit (n = 106), NHS reference costs 2008–2009 – NHS Trusts and PCTs combined Consultant Led: Follow up Attendance Non-Admitted Face to Face Service Code 370: Medical oncology;77 £136 inflated to 2011/12 at 4% per annum79 |
| BSC group | Never | £0 | |||
| Computerised tomography scans | |||||
| Progression-free survival | During all active drug treatmenta | Every 3 months | £112 per scan | £37 | £100 (interquartile range £75–109, n = 162), NHS reference costs 2008–2009 – NHS Trusts and PCTs combined Diagnostic Imaging: Outpatient Computerised Tomography Scan, one area, no contrast. Currency code RA08Z;77 £112 inflated to 2011/12 at 4% per annum79 |
| BSC group | Never | £0 | |||
| Medication, hospitalisations, hospice stays, outpatient visits, scans and laboratory tests | |||||
| Progressive disease | All treatment groups | N/A | £1039 | Remak and Brazil78 inflated by 4% per annum from 2000 to 2011 | |
In common with Merck Serono, we took our estimate of the cost of medical management for all treatment groups in progressive disease from a study of UK patients with breast cancer, reported by Remak and Brazil. 78 Once patients are off active drug treatment, and at the end stage of metastatic cancer, resources to alleviate pain and other symptoms are similar across cancer types and therefore the data from the breast cancer study are appropriate. Table 5 from Remak and Brazil reports the monthly cost in progressive disease as £675 per patient in year 2000 prices. This represents mostly medication, hospitalisations, hospice stays, outpatient visits, scans and laboratory tests. Inflating the cost at 4% per annum over 11 years, from 2000 to 2011, gives £1039 per month. This is noticeably higher than the £785 used by Merck Serono, even though it also used Remak and Brazil to estimate this cost. We believe that this difference arises because Merck Serono incorrectly inflated the cost over a shorter time period.
Our clinical expert believes that blood tests would be performed once every 2 weeks for people taking cetuximab plus irinotecan and once per month for people on all other active drug treatments. Patients in the best supportive care group would have no blood tests. We have not modelled the cost of blood tests because the cost per test is negligible, at about £3.28 [£2.92 from NHS reference costs 2008–2009, speciality code DAP823, Haematology (excluding anticoagulant services),77 inflated by 4% per annum over 3 years].
Unlike Merck Serono, we assumed no magnetic resonance imaging scans. Our clinical advisor suggests that scans would be performed only for patients for whom tumour resection is an option. There would be very few such patients in this patient population because they are taking late-line drugs.
Merck Serono states that some patients in the RCT of cetuximab plus best supportive care compared with best supportive care received radiotherapy for palliation of symptoms in progressive disease in both treatment groups (see p. 203, Merck Serono's submission69). It calculates the total cost of radiotherapy per patient as £34 for patients in the cetuximab plus best supportive care group and £46 for patients in the best supportive care group; however, given that these figures are dwarfed by other costs in the model, we do not cost for radiotherapy received in progressive disease. Similarly, Merck Serono states that some patients in the RCT of cetuximab plus best supportive care compared with best supportive care received chemotherapy for palliation of symptoms (see p. 204, Merck Serono's submission69). It calculates the total cost of chemotherapy per patient as £359 for patients in progression-free survival in the best supportive care group, £624 in progressive disease in the cetuximab plus best supportive care group and £827 in progressive disease in the best supportive care group. We do not cost for palliative chemotherapy: first, because use of palliative chemotherapy in clinical practice may differ from that in the RCT; second, because the costs are very small compared with other costs; and, third, because the progressive disease chemotherapy costs for the two treatment arms nearly cancel each other out.
For the probabilistic sensitivity analysis, we modelled each medical management cost as a gamma distribution. We tried to capture the broader uncertainty of these variables given that there may be alternative sources for these costs in addition to the sources that we chose. This was achieved by setting the standard errors of all costs equal to 20% of the mean costs, which reflects much uncertainty and because this gives a plausible range of simulated costs. Also, we assumed that all medical management costs are independent of all other model parameters.
Costs of treating adverse events
As explained in Severe adverse events, in our base case we base our estimates of the mean costs of treating adverse events on those calculated by Merck Serono. The mean costs per person assumed by Merck Serono are £2760 for best supportive care, £3671 for cetuximab plus best supportive care, £880 for panitumumab plus best supportive care and £3671 for cetuximab plus irinotecan; however, we use only Merck Serono's values for best supportive care, cetuximab plus best supportive care and cetuximab plus irinotecan. This approach seems reasonable given that (1) Merck Serono has performed an extensive analysis of these costs from its RCT of cetuximab compared with best supportive care,47 (2) we have found no logical flaws in its calculations (see Chapter 5) and (3) the costs estimated by Merck Serono are very small compared with other costs, for example drug costs.
We have not used Merck Serono's cost of £880 for treating adverse events for panitumumab plus best supportive care because its justification for this figure seems invalid (see Chapter 5). In particular, it seems unreasonable that the cost for panitumumab plus best supportive care should be less than that for best supportive care given that the incidence of grade 3 and 4 adverse events is greater for panitumumab plus best supportive care than for best supportive care for virtually every adverse event category. 7 Instead, we set the mean cost of treating adverse events for panitumumab plus best supportive care equal to that for best supportive care, at £2760 per person. The true value for panitumumab plus best supportive care may be somewhat higher than that for best supportive care and we explore this in a sensitivity analysis.
For the probabilistic sensitivity analysis, we model all costs of treating adverse events as gamma distributions. Given the lack of an obvious choice for the standard errors, we chose the pragmatic solution of setting all standard errors equal to 20% of each mean. It is also very difficult to parameterise the correlations between these costs across treatments. For simplicity, we set the cost for panitumumab plus best supportive care equal to that for best supportive care for each simulation, and these costs were independent of the cost for cetuximab plus best supportive care, which itself was independent of the cost for cetuximab plus irinotecan.
Deterministic one-way sensitivity analysis
We performed several one-way sensitivity analyses for the cost-effectiveness of cetuximab compared with best supportive care, panitumumab compared with best supportive care and cetuximab plus irinotecan compared with best supportive care.
Probabilistic sensitivity analysis
We performed probabilistic sensitivity analysis to incorporate parameter uncertainty in the cost-effectiveness analysis. Values for each stochastic parameter in each of 1000 simulations of the cost-effectiveness model were drawn at random from a specified distribution. The distributions, standard errors and correlations for all parameters are given in Evidence to inform model parameters. The results are plotted on cost-effectiveness planes and cost-effectiveness acceptability curves, which show the probability that a treatment is the most cost-effective given a particular willingness-to-pay threshold.
The Peninsula Technology Assessment Group's cost-effectiveness results
We present our cost-effectiveness results in this section. We present and discuss first the base-case results and then the results of the sensitivity analyses.
Table 47 presents the aggregated totals for the base-case results for the four treatments and Table 48 displays the incremental results compared with best supportive care and the corresponding cost-effectiveness ratios.
| CET | PAN | CET + IRIN | BSC | |
|---|---|---|---|---|
| Life-years (mean, undiscounted) | ||||
| Time on drug treatment | 0.40 | 0.49 | 0.73a | N/A |
| Progression free | 0.40 | 0.42 | 0.73 | 0.23 |
| Post progression | 0.44 | 0.29 | 0.65a | 0.29 |
| Total (mean) | 0.84 | 0.71 | 1.38b | 0.51 |
| Total (median) | 0.75 | 0.60 | 1.25b | 0.40 |
| QALYs (mean, discounted) | ||||
| Progression free | 0.32 | 0.36 | 0.54 | 0.17 |
| Post progression | 0.29 | 0.19 | 0.43b | 0.19 |
| Total | 0.61 | 0.56 | 0.97b | 0.36 |
| Costs (£) (mean, discounted) | ||||
| KRAS test | 296 | 296 | 296 | 0 |
| Drug costs | 14,408 | 23,643 | 32,022a | 0 |
| Drug administration | 5546 | 3374 | 12,714a | 0 |
| Consultant monitoring appointment | 1397 | 1479 | 2533 | 0 |
| Computerised tomography scans | 178 | 188 | 322 | 0 |
| BSC in progressive disease | 5304 | 3473 | 7790b | 3496 |
| Adverse events | 3671 | 2760 | 3671 | 2760 |
| Total | 30,800 | 35,213 | 59,348 | 6256 |
| CET vs BSC | PAN vs BSC | CET + IRIN vs BSC | |
|---|---|---|---|
| Life-years (mean, undiscounted) | |||
| Progression free | 0.17 | 0.20 | 0.50 |
| Post progression | 0.15 | 0.00 | 0.37a |
| Total (mean) | 0.32 | 0.19 | 0.87a |
| Total (median) | 0.35 | 0.20 | 0.85a |
| QALYs (mean, discounted) | |||
| Progression free | 0.15 | 0.19 | 0.37 |
| Post progression | 0.10 | 0.00 | 0.24a |
| Total | 0.25 | 0.19 | 0.60a |
| Costs (£) (mean, discounted) | |||
| KRAS test | 300 | 300 | 300 |
| Drug costs | 14,400 | 23,600 | 32,000b |
| Drug administration | 5500 | 3400 | 12,700b |
| Consultant monitoring appointment | 1400 | 1500 | 2500 |
| Computerised tomography scans | 200 | 200 | 300 |
| BSC in progressive disease | 1800 | 0 | 4300a |
| Adverse events | 900 | 0 | 900 |
| Total | 24,500 | 29,000 | 53,100 |
| Cost per life-year gained | 78,000 | 145,000 | 64,000c |
| Cost per QALY | 98,000 | 150,000 | 88,000c |
Survival results
The relative proportions of patients in each health state for each treatment throughout the time horizon of the model are displayed in Figure 13. The mean duration in each health state for each treatment (as reported in Table 47) is represented in these graphs by the area under each curve. Accordingly, mean progression-free survival is represented by the area under the dotted line, and the area between the dotted line and the solid overall survival curve represents the mean progressive disease time. As expected, virtually all patients are predicted to have died 3 years from the start of treatment.
FIGURE 13.
Base-case cohort composition over time by treatment BSC, best supportive care; PFS, progression-free survival.
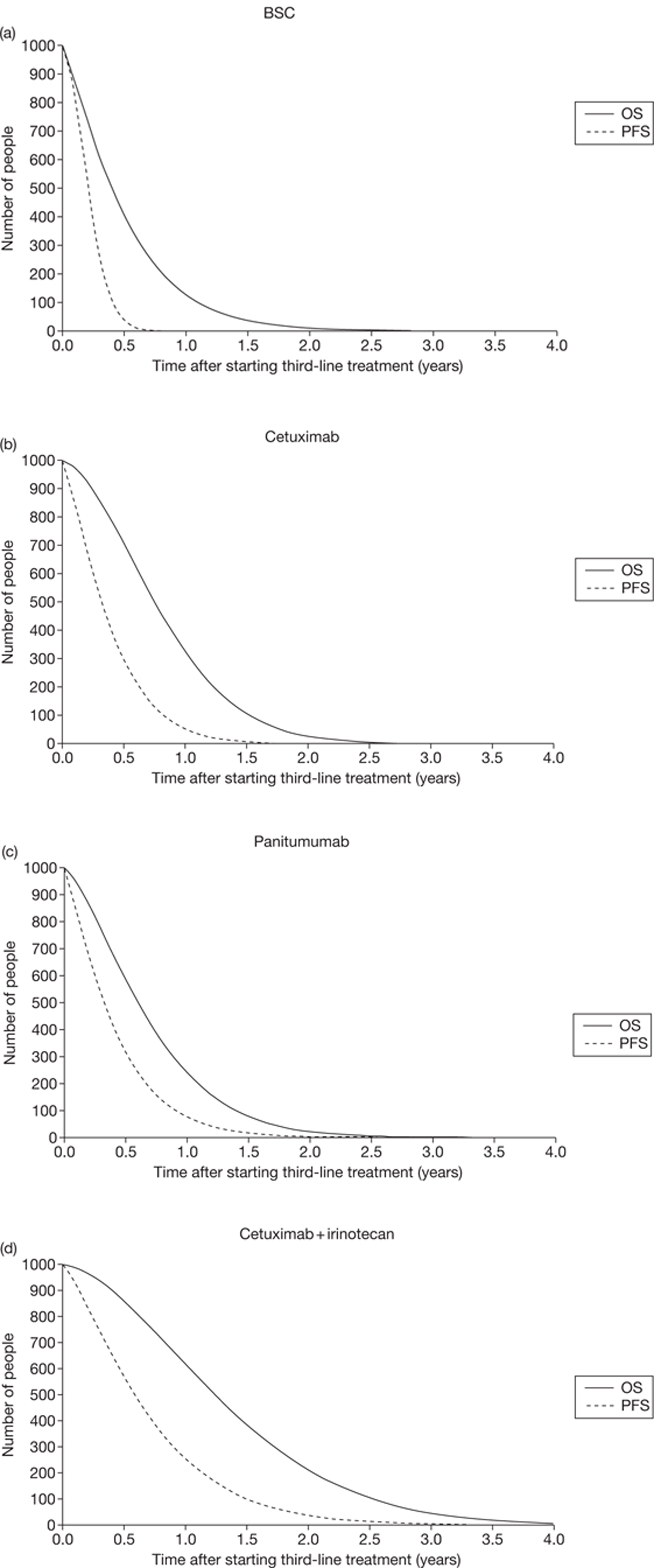
We predict that average progression-free survival is least for people under best supportive care (0.23 years = 2.7 months), greater for cetuximab plus best supportive care and panitumumab plus best supportive care [0.40 and 0.42 years respectively (approximately 5 months)] and greatest for cetuximab plus irinotecan (0.73 years = 8.8 months) (see Table 47 and Figure 13). Given that drugs are taken largely until progression, cetuximab plus best supportive care and panitumumab plus best supportive care are taken for similar times (0.40 and 0.49 years respectively) and cetuximab plus irinotecan is taken for longer (0.73 years).
Next, we predict that people spend a similar time in progressive disease on best supportive care and panitumumab plus best supportive care (0.29 years = 3.4 months), longer in progressive disease on cetuximab (0.44 years = 5.2 months) and longer still in progressive disease on cetuximab plus irinotecan (0.65 years = 7.8 months). Note that the time in progressive disease on panitumumab plus best supportive care is uncertain because it is calculated from overall survival for best supportive care in the panitumumab plus best supportive care compared with best supportive care RCT, which is confounded because of crossover of people from the best supportive care to the panitumumab plus best supportive care arm. The time in progressive disease on cetuximab plus irinotecan is even more uncertain because it is calculated from overall survival for cetuximab plus irinotecan, which is highly uncertain (see Overall survival, progression-free survival and treatment duration, Cetuximab plus irinotecan). Average overall survival, the sum of progression-free survival and progressive disease, is least for best supportive care (0.51 years = 6.2 months), greater for cetuximab plus best supportive care and panitumumab plus best supportive care (0.84 and 0.71 years or 10.0 and 8.5 months respectively) and greatest for cetuximab plus irinotecan (1.38 years = 16.6 months), which we repeat is very uncertain.
The relative QALYs in progression-free survival and progressive disease are similar to the relative life-years in progression-free survival and progressive disease. To be more precise the relative mean QALYs in progression-free survival for cetuximab plus best supportive care and panitumumab plus best supportive care compared with best supportive care (e.g. QALYs in progression-free survival for cetuximab compared with QALYs in progression-free survival for best supportive care) are greater than the relative mean life-years (e.g. progression-free survival for cetuximab compared with progression-free survival for best supportive care) because we assume that the QoL of people on these drugs is better than the QoL on best supportive care. This is not the case for cetuximab plus irinotecan because we assume that the QoL of people on this treatment is the same as the QoL for best supportive care. This is also not the case for QALYs and life-years in progressive disease because we assume that the QoL of all people is equal in progressive disease.
Cost results
We now turn to the expected costs per person. The expected drug acquisition costs are by far the largest single cost item (see Table 47) and account for the largest incremental costs compared with best supportive care (Figure 14). The acquisition costs are least for cetuximab plus best supportive care (about £14,000 per person), greater for panitumumab plus best supportive care (about £24,000 per person) and greatest for cetuximab plus irinotecan (£32,000 per person), although the last figure is particularly uncertain given that the mean duration of cetuximab plus irinotecan treatment is not reported. The expected drug acquisition costs are calculated as the product of the mean drug acquisition cost per person per unit time and the discounted mean duration of drug treatment. Figure 12 suggests that the mean drug acquisition cost per person per unit time, allowing for dose intensity, is lowest for cetuximab monotherapy (£3000 per month), greater for cetuximab plus irinotecan (£3700 per month) and greatest for panitumumab plus best supportive care (£4100 per month). From Table 47, cetuximab plus best supportive care and panitumumab plus best supportive care are taken for similar times (0.40 and 0.49 years respectively) and cetuximab plus irinotecan is taken for much longer (0.73 years). Combining these two pieces of information, the expected drug acquisition cost is least for cetuximab plus best supportive care because it is both the cheapest per person per unit time and is taken for the least time. The expected drug acquisition cost is greatest for cetuximab plus irinotecan because the acquisition cost per unit time is nearly as great as for panitumumab plus best supportive care and because we predict that it is taken for far longer than the other two treatments.
FIGURE 14.
Base-case incremental costs vs best supportive care BSC, best supportive care; CT, computerised tomography; PD, progressive disease.
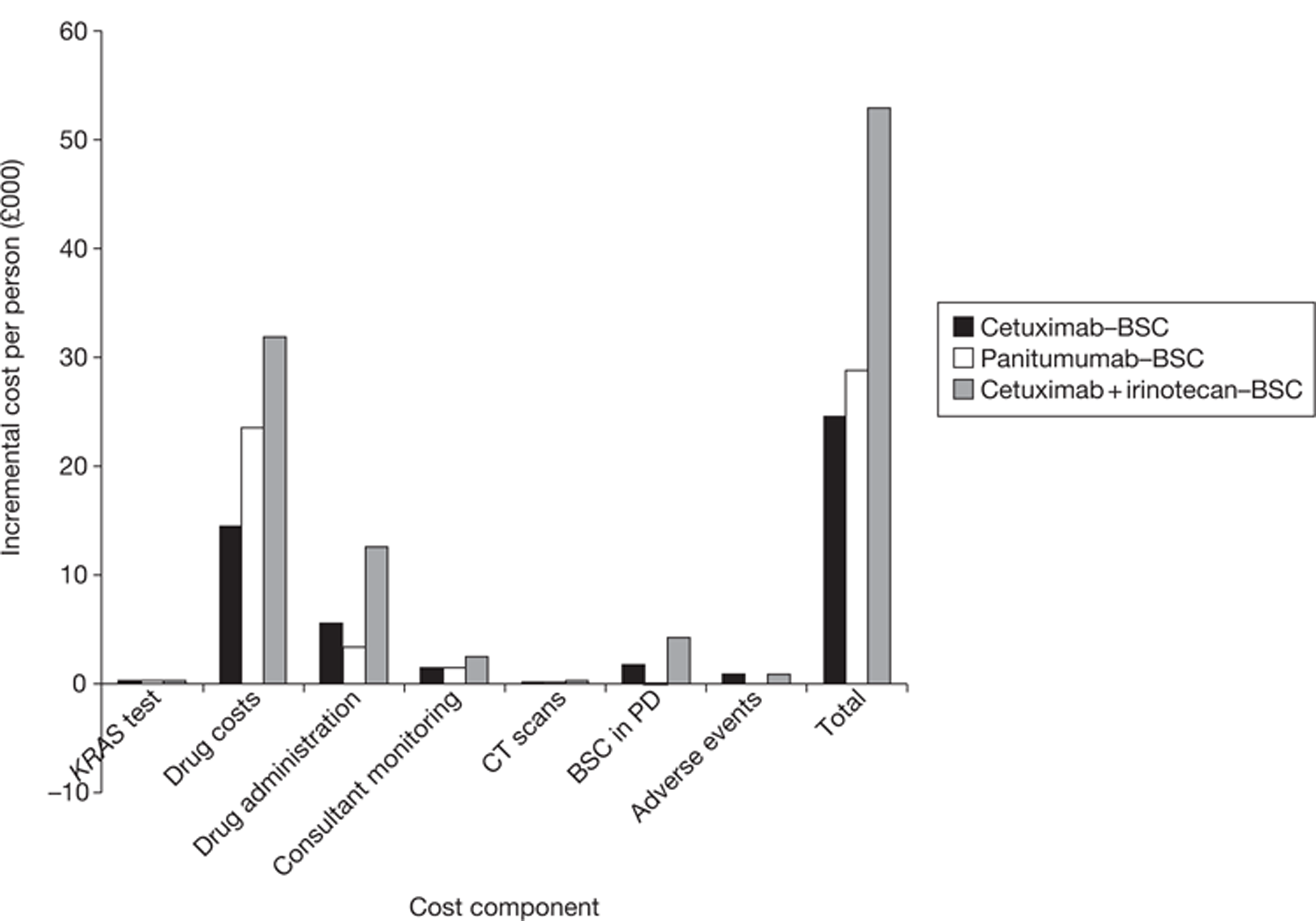
The expected drug administration costs and expected costs in progressive disease are the next largest single cost items (see Table 47); however, the cost-effectiveness of the drugs compared with best supportive care is influenced more by expected drug administration costs because these account for larger incremental costs compared with best supportive care (see Figure 14). Similar to the total per person drug acquisition costs, these are calculated as the product of the mean drug administration cost per person per unit time and the discounted mean duration of drug treatment. From Figure 12, the mean drug administration cost per person per unit time is lowest for panitumumab (£600 per month) because it is given relatively infrequently (every 2 weeks), greater for cetuximab plus best supportive care (£1200 per month) because it is given relatively frequently (once per week) and greatest for cetuximab plus irinotecan (£1500 per month) because cetuximab plus best supportive care is given every week and there are two drugs to administer every second week. From Table 47, cetuximab plus best supportive care and panitumumab plus best supportive care are taken for similar times (0.40 and 0.49 years respectively) and cetuximab plus irinotecan is taken for much longer (0.73 years). Combining this information, the expected total drug administration cost is least for panitumumab plus best supportive care because it is easily the least expensive per person per unit time and is taken for much less time than cetuximab plus irinotecan and for a similar time as cetuximab plus best supportive care. The expected total drug administration cost for cetuximab plus irinotecan is by far the greatest because it has the greatest administration cost per unit time and because we predict that it is taken for far longer than the other two treatments.
Absolute costs for best supportive care in progressive disease are fairly large for all treatments (between £3500 and £7800), but the incremental costs compared with best supportive care are small, with the exception of the cetuximab plus irinotecan group, because the mean times spent in progressive disease are fairly similar between treatments, again with the exception of cetuximab plus irinotecan (see Table 47).
All other costs (KRAS testing, consultant monitoring appointments, computerised tomography scans and treatment of serious adverse events) are much smaller and therefore have very little impact on cost-effectiveness.
Cost-effectiveness results
Combining all of the information on expected costs and QALYs per person, we estimate the following ICERs:
-
cetuximab plus best supportive care compared with best supportive care: £98,000 per QALY
-
panitumumab plus best supportive care compared with best supportive care: £150,000 per QALY
-
cetuximab plus irinotecan compared with best supportive care: £88,000 per QALY.
Our estimate of the ICER for cetuximab plus best supportive care compared with best supportive care is based on the relevant clinical effectiveness evidence from a high-quality RCT,37 although we caution that, although we have some evidence for the mean treatment duration of cetuximab, the precise information is not published. The ICER for panitumumab plus best supportive care compared with best supportive care is based on relevant clinical effectiveness evidence from another high-quality RCT;7 however, there remains some uncertainty because we rely on an adjustment for the crossing over of many people from the best supportive care to the panitumumab plus best supportive care treatment group, and there is uncertainty about the accuracy of the adjustment. The ICER for cetuximab plus irinotecan compared with best supportive care is the most uncertain because there is much uncertainty about progression-free survival on cetuximab plus irinotecan, and hence treatment duration, and even more uncertainty about overall survival (see Overall survival, progression-free survival and treatment duration, Cetuximab plus irinotecan).
The incremental costs and QALYs for cetuximab plus best supportive care and panitumumab plus best supportive care compared with best supportive care are similar, whereas these quantities are far greater for cetuximab plus irinotecan (Figure 15 and see Table 48).
FIGURE 15.
PenTAG base-case cost-effectiveness results BSC, best supportive care; WTP, willingness to pay.
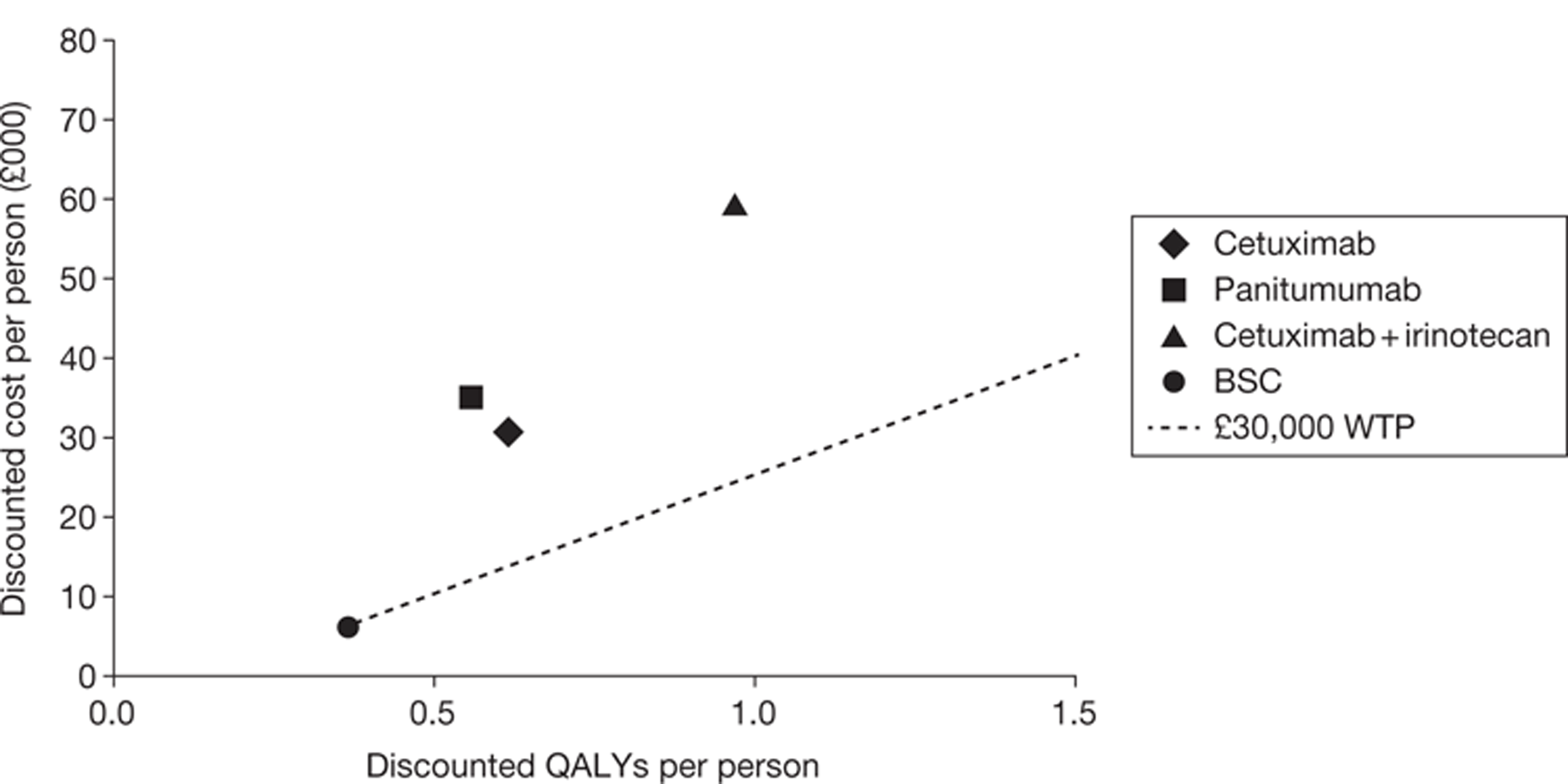
End-of-life criteria
In Tables 49–51 we assess the treatments against all of NICE's EoL criteria except that concerning the patient population size for indications that are outside the scope of this appraisal. Merck Serono considers that both cetuximab plus best supportive care and cetuximab plus irinotecan qualify for EoL criteria.
| EoL criteria | CET + BSC for mCRC | Meets criteria | Justification |
|---|---|---|---|
| Life expectancy on current standard care < 24 months | 6.2 months | Yes | Clear |
| Treatment provides extension to life expectancy compared with current standard care of > 3 months | Mean extension to life expectancy of 3.9 months. Probability life extension > 3 months = 0.78 | Yes | Clear |
| No alternative treatment with comparable benefits is available through the NHS | True | Yes | Clear |
| The treatment is licensed or otherwise indicated for small patient populations | Merck Serono estimates 260–390 people per year eligible for CET for mCRC (p. 149, Merck Serono's submission69) | Unsure | Population is small for mCRC but we do not assess use of CET for other indications |
| The estimates of the extension to life are robusta | True | Yes | Probability extension to life > 3 months = 0.78 estimated directly from high-quality RCT. No adjustment for crossover needed |
| Overall assessment | Unsure | We do not assess the size of the patient population for other indications, but all other EoL criteria are met |
| EoL criteria | CET + IRIN for mCRC | Meets criteria | Justification |
|---|---|---|---|
| Life expectancy on current standard care < 24 months | 6.2 months | Yes | Clear |
| Treatment provides extension to life expectancy compared with current standard care of > 3 months | Mean extension to life expectancy of 10.4 months. Probability life extension > 3 months = 0.99 | Yes | Clear |
| No alternative treatment with comparable benefits is available through the NHS | True | Yes | Clear |
| The treatment is licensed or otherwise indicated for small patient populations | Merck Serono estimates 260–390 people per year eligible for CET for mCRC (p. 149, Merck Serono's submission69) | Unsure | Population is small for mCRC but we do not assess use of cetuximab for other indications |
| The estimates of the extension to life are robusta | Estimate of extension to life expectancy relies on two important assumptions concerning splitting of overall survival data for KRAS WT and KRAS mutant patients and use of non-RCT data | Unsure | If ‘robust’ means that we have high confidence in our mean estimate of overall survival then evidence is not ‘robust’. If ‘robust’ means that we have high confidence that treatment increases overall survival by at least 3 months then evidence is ‘robust’ |
| Overall assessment | Unsure | We are not sure whether or not all criteria passed |
| EoL criteria | PAN + BSC for mCRC | Meets criteria | Justification |
|---|---|---|---|
| Life expectancy on current standard care < 24 months | 6.2 months | Yes | Clear |
| Treatment provides extension to life expectancy compared with current standard care of > 3 months | Indirect comparison: Mean extension to life expectancy of 2.3 months. Probability life extension > 3 months = 0.25 | No | Probability that panitumumab provides extension to life expectancy compared with current standard care of > 3 months is low |
| Direct comparison: Mean extension to life expectancy of 2.7 months. Probability life extension > 3 months = 0.39 | |||
| No alternative treatment with comparable benefits is available through the NHS | True | Yes | Clear |
| The treatment is licensed or otherwise indicated for small patient populations | Merck Serono estimates 260–390 people per year eligible for CET for mCRC (p. 149, Merck Serono's submission69) and we estimate a similar number will be eligible for panitumumab | Yes | Population small |
| The estimates of the extension to life are robusta | False | Unsure | Although extension to life expectancy comes from high-quality RCT, adjustment for crossover introduces much uncertainty |
| Overall assessment | No | Not all EoL criteria passed |
Deterministic sensitivity analyses
Cetuximab plus best supportive care compared with best supportive care
One-way deterministic sensitivity analyses for cetuximab plus best supportive care compared with best supportive care are reported in Table 52 and Figure 16, which show the impact on the deterministic ICER of various alterations in model parameters. The sensitivity analyses were chosen on the basis of general interest (e.g. assuming no discounting), plausibility (e.g. varying mean progression-free survival and overall survival by two standard errors) or Merck Serono's assumptions. None of these sensitivity analyses brings the ICER within usually accepted willingness-to-pay thresholds.
| Parameter | Base case | Sensitivity analysis | ICER (£) |
|---|---|---|---|
| Base case | N/A | N/A | 98,000 |
| General | |||
| Discounting costs and benefits | 3.5% per annum | 0% per annum | 97,000 |
| Effectiveness | |||
| Mean PFS CET + BSC | 0.40 years | 0.47 years (increased by 2 SE) | 107,000 |
| 0.32 years (decreased by 2 SE) | 89,000 | ||
| Mean PFS BSC | 0.23 years | 0.27 years (increased by 2 SE) | 101,000 |
| 0.18 years (decreased by 2 SE) | 95,000 | ||
| Mean OS CET+BSC | 0.84 years | 1.00 years (increased by 2 SE) | 74,000 |
| 0.68 years (decreased by 2 SE) | 158,000 | ||
| Mean OS BSC | 0.51 years | 0.61 years (increased by 2 SE) | 127,000 |
| 0.41 years (decreased by 2 SE) | 81,000 | ||
| Costs | |||
| Dose intensity CET | 98% | 85% | 91,000 |
| Mean mg CET per person | 511 mg CET (allowing for distribution of body surface area) | 500 mg CET (assumed all same body surface area) | 97,000 |
| KRAS test cost | £160 per test | Halve cost: £80 | 98,000 |
| Double cost: £320 | 99,000 | ||
| CET administration | £270 per administration | Halve cost: £135 | 87,000 |
| Double cost: £540 | 120,000 | ||
| Merck Serono assumption: £180 | 91,000 | ||
| Medical management costs (consultant visit, computerised tomography scans, BSC in PD) | Halve all unit costs or frequencies | 91,000 | |
| Double all unit costs or frequencies | 112,000 | ||
| Adverse event costs | CET + BSC = £3671, BSC = £2760 | CET + BSC and BSC equal | 95,000 |
| CET + BSC and BSC doubled | 102,000 | ||
| Utilities | |||
| PFS and PD | CET + BSC PFS = 0.81, PD = 0.69 ; BSC PFS = 0.75, PD = 0.69 | Merck Serono values: CET + BSC PFS = 0.81, PD = 0.79; BSC PFS = 0.75, PD = 0.69 | 84,000 |
| PFS | CET + BSC PFS = 0.81; BSC PFS = 0.75 | CET + BSC PFS = BSC PFS = 075 | 108,000 |
FIGURE 16.
Sensitivity analyses: cetuximab plus best supportive care vs best supportive care Admin., administration; AE, adverse event; BSC, best supportive care; decr., decreased by; incr., increased by; med. manage., medical management; OS, overall survival; PFS, progression-free survival; SE, standard error.
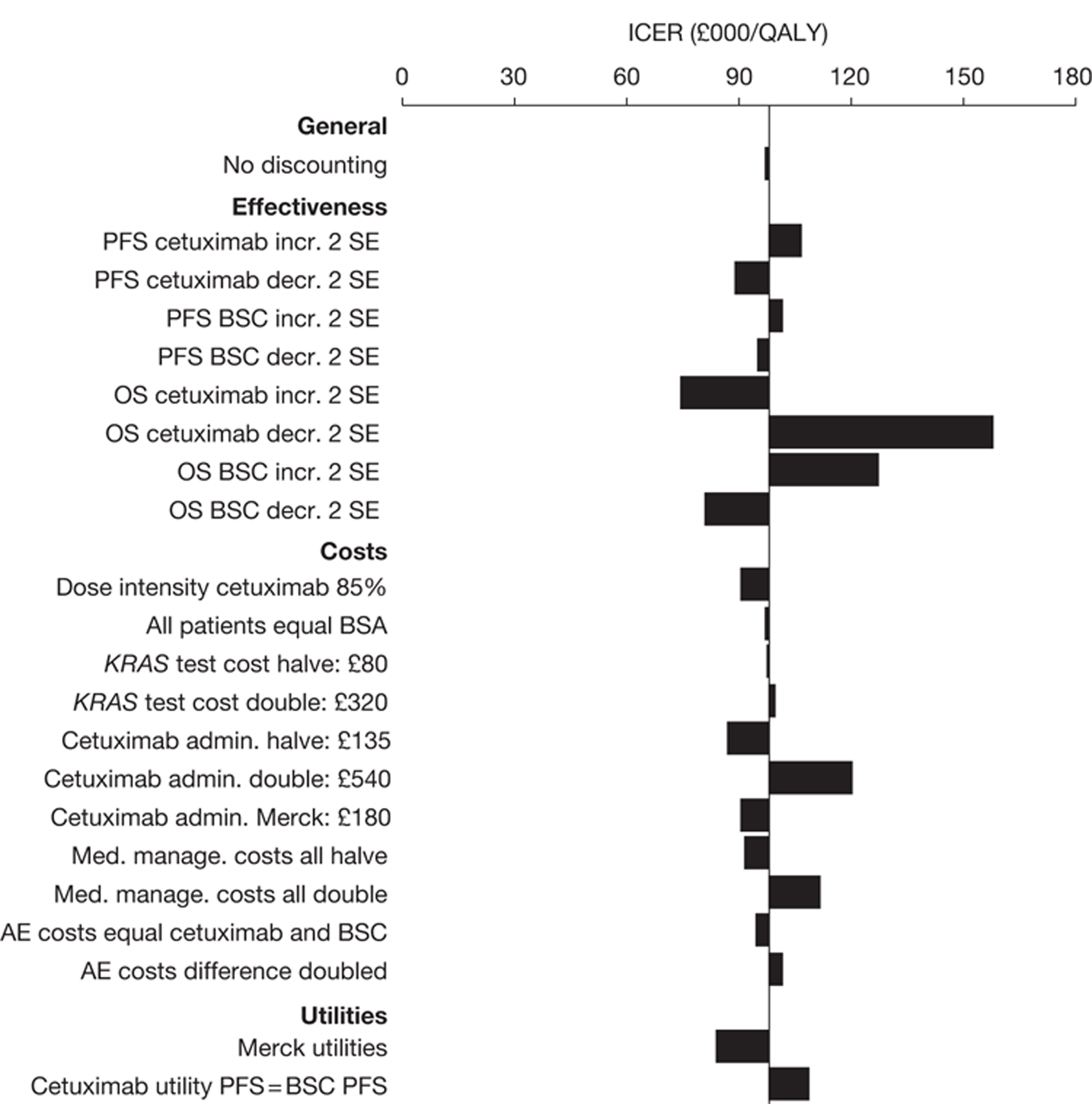
The ICER remains above £70,000 per QALY in all cases. The ICER is most sensitive to overall survival for best supportive care and cetuximab plus best supportive care, noting that we have varied these quantities to the most extreme values (two standard errors from the mean) that are consistent with the RCT of cetuximab plus best supportive care compared with best supportive care. 47 The ICER is reasonably sensitive to mean progression-free survival for cetuximab plus best supportive care because this is proportional to the mean duration of cetuximab plus best supportive care treatment and hence the cost of cetuximab acquisition, which strongly influences the ICER. The ICER is less sensitive to mean progression-free survival for best supportive care because this is not associated with any drug costs.
The ICER is fairly sensitive to the administration cost of cetuximab when this is varied within a plausible range, because cetuximab is given regularly (once per week). The ICER falls moderately when we use Merck Serono's utilities, although we disagree with these values. The ICER increases moderately when we assume that the HRQoL of people in progression-free survival is the same for those on cetuximab plus best supportive care and those on best supportive care, but we have good evidence that the QoL is higher for people on cetuximab plus best supportive care. The ICER is insensitive to all other parameters when they are varied within plausible ranges. For example, it is insensitive to the discount rate for costs and benefits because patients typically have a short life expectancy. Also, for the calculation of the mean cost of cetuximab acquisition, the ICER is insensitive to whether we assume that all people have the same body surface area or whether we more realistically assume a range of surface areas; however, as already stated, this is purely coincidental in this case and we recommend that the distribution should be routinely modelled whenever drugs are administered in patient-related doses.
BSC, best supportive care; CET, cetuximab; mCRC, metastatic colorectal cancer; PAN, panitumumab.
a And can be shown or reasonably inferred from either progression-free survival or overall survival (taking account of trials in which crossover has occurred and been accounted for in the effectiveness review).
Panitumumab plus best supportive care compared with best supportive care
One-way deterministic sensitivity analyses for panitumumab plus best supportive care compared with best supportive care are reported in Table 53 and Figure 17. None of these sensitivity analyses brings the ICER below usually accepted willingness-to-pay thresholds.
| Parameter | Base case | Sensitivity analysis | ICER (£) |
|---|---|---|---|
| Base case | N/A | N/A | 150,000 |
| General | |||
| Discounting costs and benefits | 3.5% per annum | 0% per annum | 149,000 |
| Effectiveness | |||
| Mean PFS PAN + BSC | 0.42 years | 0.50 years (increased by 2 SE) | 161,000 |
| 0.35 years (decreased by 2 SE) | 138,000 | ||
| Mean PFS BSC | 0.23 years | 0.27 years (increased by 2 SE) | 155,000 |
| 0.18 years (decreased by 2 SE) | 145,000 | ||
| Mean OS PAN + BSC | 0.708 years | 0.86 years (increased by 2 SE) | 110,000 |
| 0.58 years (decreased by 2 SE) | 254,000 | ||
| 0.709 years, so that PD PAN = PD BSC | 150,000 | ||
| Mean OS BSC | 0.51 years | 0.61 years (increased by 2 SE) | 221,000 |
| 0.41 years (decreased by 2 SE) | 116,000 | ||
| PAN + BSC and BSC effectiveness | BSC from CET + BSC vs BSC RCT, PAN from PAN + BSC vs BSC RCT, adjusted for indirect comparison | All from PAN + BSC vs BSC RCT only (direct comparison) | 142,000 |
| Costs | |||
| Dose intensity PAN | 100% | 85% | 132,000 |
| Mean mg PAN per person | 499 mg (allowing for distribution of weights) | 500 mg (all people assumed same weight) | 150,000 |
| Mean duration PAN therapy | 0.49 years | Same as mean PFS: 0.42 years | 132,000 |
| KRAS test cost | £160 per test | Halve cost: £80 | 150,000 |
| Double cost: £320 | 152,000 | ||
| PAN administration | £270 per administration | Halve cost: £135 | 142,000 |
| Double cost: £540 | 168,000 | ||
| Merck Serono assumption: £180 | 144,000 | ||
| Medical management costs (consultant visit, computerised tomography scans, BSC in PD) | Halve all unit costs or frequencies | 146,000 | |
| Double all unit costs or frequencies | 159,000 | ||
| Adverse events costs | PAN + BSC = BSC = £2760 | PAN + BSC same as CET + BSC = £3671, BSC = £2760 | 155,000 |
| Utilities | PAN + BSC PFS = 0.87; BSC PFS = 0.75 | PAN + BSC PFS = BSC PFS = 0.75 | 203,000 |
FIGURE 17.
Sensitivity analyses: panitumumab plus best supportive care vs best supportive care. Admin., administration; AE, adverse event; BSC, best supportive care; decr., decreased by; incr., increased by; med. manage., medical management; OS, overall survival; PD, progressive disease; PFS, progression-free survival; SE, standard error.
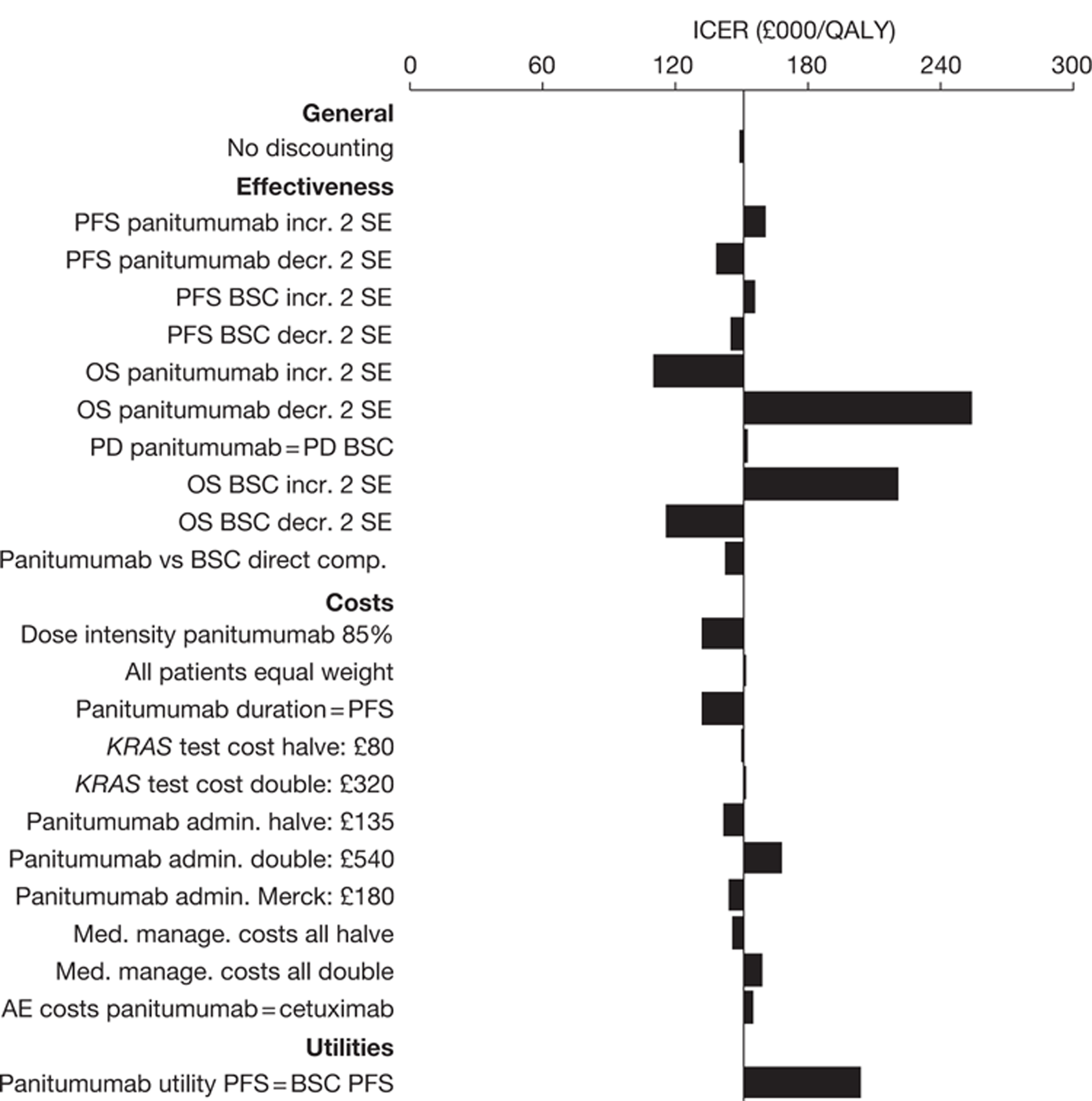
The ICER remains very high, > £110,000 per QALY in all cases. As for the comparison of cetuximab plus best supportive care compared with best supportive care, the ICER is most sensitive to overall survival for best supportive care and panitumumab plus best supportive care. As for cetuximab plus best supportive care compared with best supportive care, the ICER is reasonably sensitive to mean progression-free survival for panitumumab plus best supportive care, because this is proportional to the mean cost of panitumumab acquisition. The ICER is less sensitive to mean progression-free survival for best supportive care because this is not associated with any drug costs. Further, concerning the clinical effectiveness, we addressed the confounding due to substantial crossover of people from the best supportive care to panitumumab plus best supportive care arms in the RCT as follows. We performed a sensitivity analysis in which we varied overall survival for panitumumab plus best supportive care so that the time in progressive disease is equal for the panitumumab plus best supportive care and best supportive care arms. This models the plausible scenario in which mortality is affected only while patients are on panitumumab. Once they are off panitumumab, in progressive disease, the mortality rate is equal to that in progressive disease in the best supportive care arm. This analysis was performed by adjusting overall survival for panitumumab plus best supportive care only very slightly, from a mean of 0.708 years to 0.709 years. Not surprisingly, the ICER changed only incrementally. In the base case we took the clinical effectiveness for the best supportive care arm from the RCT of cetuximab plus best supportive care compared with best supportive care, not the RCT of panitumumab compared with best supportive care. When we use the RCT of panitumumab plus best supportive care compared with best supportive care, that is, we perform a direct comparison of panitumumab plus best supportive care compared with best supportive care using data only from the panitumumab plus best supportive care compared with best supportive care RCT, the ICER decreases only slightly.
In our base case we assume that panitumumab is typically taken for slightly longer (0.49 years) than patients are in progression-free survival for (0.42 years). Indeed, we have good evidence for the treatment duration of panitumumab for patients with KRAS WT status, as this is reported directly from the RCT. However, in cost-effectiveness analyses of drugs for terminal cancers, it is normal to assume that drugs are taken until disease progression. Under this assumption, the ICER decreases moderately. The ICER is less sensitive to the drug administration cost than the ICER for cetuximab plus best supportive care compared with best supportive care because panitumumab is taken less frequently than cetuximab (once every 2 weeks compared with once a week respectively). The ICER increases substantially when we assume that the QoL of people in progression-free survival is equal for those on panitumumab plus best supportive care and those on best supportive care, but we have good evidence that the QoL is higher for people on panitumumab plus best supportive care. The ICER is insensitive to all other parameters when they are varied within plausible ranges. For example, for the calculation of the mean cost of panitumumab acquisition, the ICER is insensitive to whether we assume that all people are the same weight or whether we more realistically assume a distribution of weights.
Cetuximab plus irinotecan compared with best supportive care
One-way deterministic sensitivity analyses for cetuximab plus irinotecan compared with best supportive care are reported in Table 54 and Figure 18.
| Parameter | Base case | Sensitivity analysis | ICER (£) |
|---|---|---|---|
| Base case | N/A | N/A | 88,000 |
| General | |||
| Discounting costs and benefits | 3.5% per annum | 0% per annum | 86,000 |
| Effectiveness | |||
| Median PFS CET + IRIN | 0.59 years | 0.82 years (increased by 2 SE) | 109,000 |
| 0.36 years (decreased by 2 SE) | 65,000 | ||
| 0.88 years, using CET vs BSC RCT (see Appendix 14, Method A) | 114,000 | ||
| 0.61 years, using De Roock et al.18 (see Appendix 14, Method B) | 90,000 | ||
| 0.59 years, using Lievre et al.86 (see Appendix 14, Method C) | 87,000 | ||
| 0.57 years, using De Roock et al.83 (see Appendix 14, Method D) | 86,000 | ||
| Mean PFS BSC | 0.23 years | 0.27 years (increased by 2 SE) | 89,000 |
| 0.18 years (decreased by 2 SE) | 86,000 | ||
| Median OS CET + IRIN | 1.25 years | 1.75 years (increased by 2 SE) | 62,000 |
| 0.75 years (decreased by 2 SE) | 191,000 | ||
| 0.91 years, so that PD CET + IRIN = PD BSC | 134,000 | ||
| 0.94 years (see Appendix 15, Method A) | 129,000 | ||
| 1.48 years (see Appendix 15, Method C) | 73,000 | ||
| 1.12 years (see Appendix 15, Method D) | 101,000 | ||
| Mean OS BSC | 0.51 years | 0.61 years (increased by 2 SE) | 96,000 |
| 0.41 years (decreased by 2 SE) | 80,000 | ||
| Costs | |||
| Dose intensity | 94% CET, 90% IRIN | 100% CET, 100% IRIN | 92,000 |
| 85% CET, 85% IRIN | 83,000 | ||
| Mean mg CET + IRIN per person | 511 mg CET, 352 mg IRIN (allowing for distribution of body surface area) | 500 mg CET, 360 mg IRIN (all people assumed same body surface area) | 87,000 |
| Mean duration therapies | 0.73 years CET, 0.73 years IRIN | 0.73 years CET, 0.42 years IRIN | 81,000 |
| 0.42 years CET + IRIN (same as all people in BOND)49 | 56,000 | ||
| Dosing schedule IRIN | 180 mg/m2 once every 2 weeks | 350 mg/m2 every 3 weeks | 90,000 |
| 125 mg/m2 for each of 4 weeks with 2 weeks'rest | 88,000 | ||
| KRAS test cost | £160 per test | Halve cost: £80 | 88,000 |
| Double cost: £320 | 88,000 | ||
| CET + IRIN administration | £270 per administration CET, £143 per administration IRIN | Halve both costs | 77,000 |
| Double both costs | 109,000 | ||
| IRIN: £0 | 83,000 | ||
| Merck Serono assumption total: £196 | 79,000 | ||
| Medical management costs (consultant visit, computerised tomography scans, BSC in PD) | Halve all unit costs or frequencies | 82,000 | |
| Double all unit costs or frequencies | 100,000 | ||
| Adverse events costs | CET = £3671, BSC = £2760 | CET and BSC equal | 86,000 |
| CET double BSC | 89,000 | ||
| Utilities | CET + IRIN PFS = 0.75, PD = 0.69; BSC PFS = 0.75, | Merck Serono values: CET + IRIN PFS = 0.81, PD = 0.79; BSC PFS = 0.75, PD = 0.69 | 75,000 |
FIGURE 18.
Sensitivity analyses: cetuximab plus irinotecan vs best supportive care. Admin., administration; AE, adverse event; BSC, best supportive care; CET, cetuximab; decr., decreased by; incr., increased by; IRIN, irinotecan; med. manage., medical management; OS, overall survival; PD, progressive disease; PFS, progression-free survival; SE, standard error.
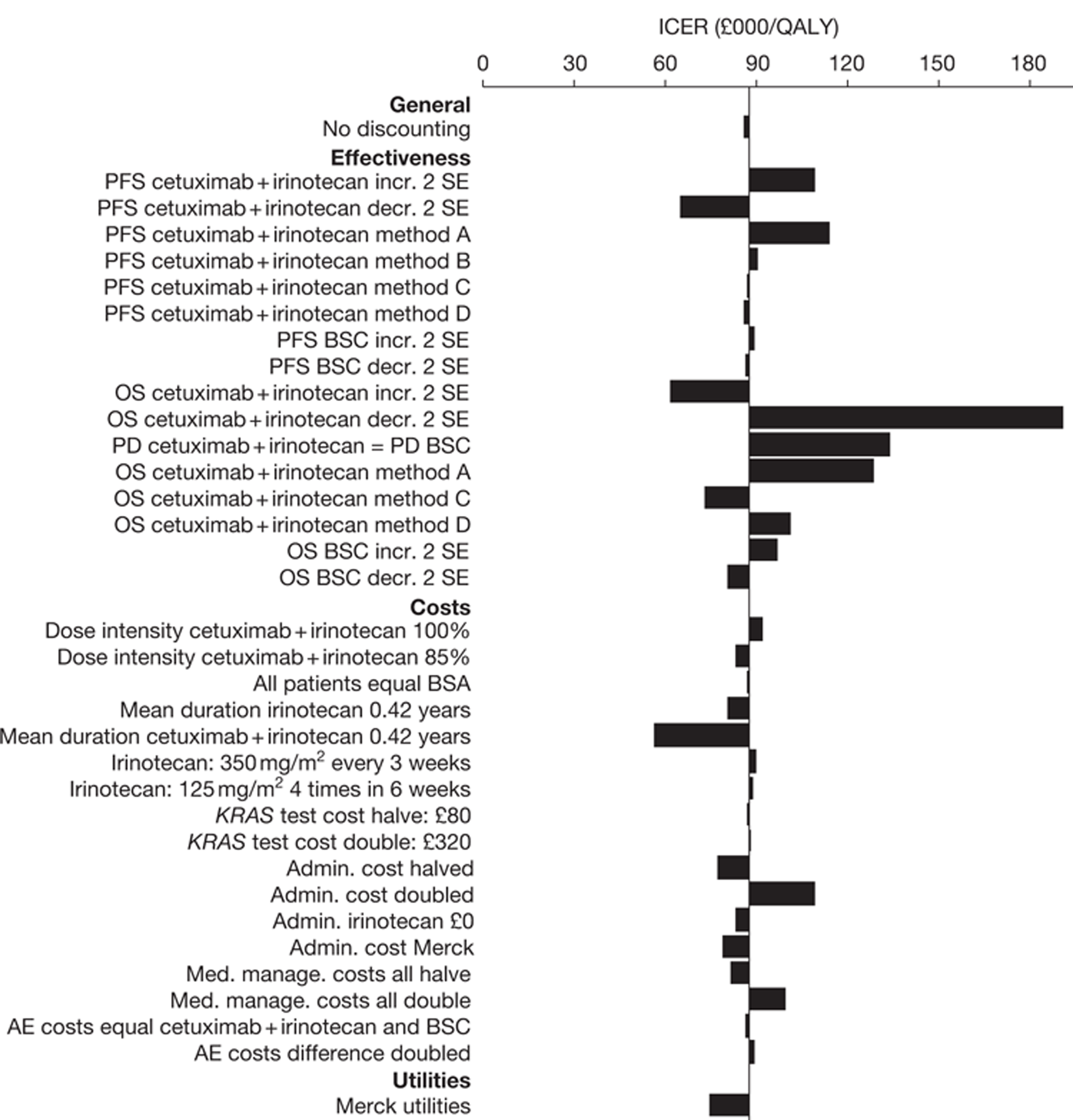
The ICER remains very high, > £55,000 per QALY in all cases. The ICER is most sensitive to overall survival for cetuximab plus irinotecan. In particular, it is more sensitive to overall survival for cetuximab plus irinotecan than to overall survival for best supportive care because we have imposed a higher coefficient of variation (ratio of standard error to mean) for overall survival for cetuximab plus irinotecan to reflect its greater uncertainty. The alternative method of estimating overall survival for cetuximab plus irinotecan, that is, in which we do not adjust for crossover in the BOND RCT, yields a substantially higher ICER (see Appendix 15, Method A). However, we do not favour this approach because we should make some adjustment for treatment crossover. The ICER changes moderately when we use two other alternative methods to estimate overall survival for cetuximab plus irinotecan, that is, when we use information on progression-free survival and when we simply set our estimate of the median overall survival for patients with KRAS WT status on cetuximab plus irinotecan for our model equal to that from the BOND RCT without adjustment for indirect comparison (see Appendix 15, Methods C and D).
The ICER increases substantially when we adjust overall survival for cetuximab plus irinotecan so that the mean time in progressive disease for cetuximab plus irinotecan equals the mean time in progressive disease for best supportive care. As explained in the last section, this models the plausible scenario in which mortality is affected only whilst people are on active treatment, in this case cetuximab plus irinotecan. We believe that this is a useful sensitivity analysis given the substantial uncertainty in overall survival for cetuximab plus irinotecan.
The ICER is sensitive to mean progression-free survival for cetuximab plus irinotecan because this is proportional to the mean cost of cetuximab plus irinotecan acquisition and because it is very uncertain. The ICER is less sensitive to mean progression-free survival for best supportive care because this is not associated with any drug costs and because it is more certain. The ICER increases substantially when we model progression-free survival for cetuximab plus irinotecan using the cetuximab plus best supportive care compared with best supportive care RCT (see Appendix 14, Method A). The ICER is largely unchanged when we model progression-free survival for cetuximab plus irinotecan using alternative methods (see Appendix 14, Methods B–D), that is, using only additional information from De Roock and colleagues,48 Lievre and colleagues86 or De Roock and colleagues83 respectively.
In our base case, we predict that people take both irinotecan and cetuximab for a mean of 0.73 years, that is, nearly 9 months. We understand that irinotecan may typically be tolerated by patients for rather less than this period, given its toxicity. Therefore, we also modelled the scenario in which irinotecan is taken for substantially less time (0.42 years or 5 months), but the treatment duration of cetuximab is unchanged at 0.73 years. In this case the ICER decreases only marginally because irinotecan is substantially less expensive than cetuximab. In a different sensitivity analysis we modelled the time on treatment for both irinotecan and cetuximab as 0.42 years, that is, 5 months. This corresponds approximately to the mean progression-free survival time for all patients (KRAS WT and KRAS mutant status combined) on cetuximab plus irinotecan in the BOND RCT, in which the median progression-free survival time is 4.1 months. However, we do not suggest that this treatment duration of 0.42 years for cetuximab plus irinotecan is realistic. Instead, it should be seen as being lower than the true value because the 0.42 years represents the treatment duration for all patients (KRAS WT and KRAS mutant status combined) whereas we are modelling patients with KRAS WT status only, and progression-free survival and hence treatment duration for patients with KRAS WT status is longer than for those with KRAS mutant status. This correlates with other sources of evidence, for example the cetuximab plus best supportive care compared with best supportive care RCT47 and De Roock and colleagues,48 De Roock and colleagues83 and Lievre and colleagues. 86
We considered the following further sensitivity analysis on the duration of cetuximab and irinotecan treatment. In this analysis we assumed that the duration of treatment is a function of response time and that the mean response time for patients taking the combination therapy is the same as that for cetuximab monotherapy. We also assumed that the difference between the mean time on the combination therapy and the mean time on the monotherapy was due entirely to the difference in the proportions of patients responding between the combination therapy and the monotherapy. The mean duration of cetuximab plus irinotecan therapy was then estimated as equal to the mean duration of cetuximab monotherapy multiplied by the estimated response rate for KRAS WT patients in the cetuximab plus irinotecan arm of the BOND RCT divided by the measured response rate for KRAS WT patients in the cetuximab arm of the cetuximab compared with best supportive care RCT. The response rate for KRAS WT patients in the cetuximab arm of the cetuximab compared with best supportive care RCT is 12.8%. 47 Also, the response rate for all patients in the cetuximab plus irinotecan arm of the BOND RCT is 22.9%. 49 We then estimate the response rate for KRAS WT patients in the cetuximab plus irinotecan arm of the BOND RCT as the response rate for all patients (22.9%) multiplied by the response rate for the cetuximab arm for all patients in the cetuximab compared with best supportive care RCT (8%)47 divided by the response rate for the cetuximab arm for all patients in the BOND RCT (10.8%). 49 This gives the estimated response rate for KRAS WT patients in the cetuximab plus irinotecan arm of the BOND RCT of 22.9% × 8%/10.8% = 17.0%. Then, the estimated mean time on cetuximab plus irinotecan = 17.0%/12.8% × 0.40 years = 0.53 years, where 0.40 years is the mean time on cetuximab monotherapy for KRAS WT patients. Assuming a mean duration of the combination therapy of 0.53 years yields an ICER of £66,000 per QALY.
The ICER is fairly sensitive to the drug administration cost. In addition, the ICER falls moderately when we use Merck Serono's utility assumptions, but we disagree with its values for cetuximab plus irinotecan. The ICER is insensitive to all other parameters when they are varied within plausible ranges. For example, the ICER is insensitive to the dosing regimen of irinotecan, partly because irinotecan is much less expensive than cetuximab, and partly because the dose of irinotecan per unit time is similar for all regimes.
Probabilistic sensitivity analysis
The scatterplots shown in Figures 19–21 depict the results of the 1000 simulations of the probabilistic sensitivity analysis in terms of the incremental cost–utility of cetuximab plus best supportive care, panitumumab plus best supportive care and cetuximab plus irinotecan compared with best supportive care. In all simulations, all treatments generated more QALYs and more costs than best supportive care. There is clearly more uncertainty in the incremental costs and QALYs of cetuximab plus irinotecan compared with best supportive care than in those of cetuximab plus best supportive care compared with best supportive care or panitumumab plus best supportive care compared with best supportive care. This is because we have assumed more uncertainty in the mean progression-free survival and overall survival of cetuximab plus irinotecan than in the mean progression-free survival and overall survival of cetuximab plus best supportive care and panitumumab plus best supportive care, to reflect the fact that we were forced to make assumptions about the effectiveness of cetuximab plus irinotecan whereas the effectiveness of the other two treatments was taken directly from RCTs. In all cases the incremental costs and QALYs are correlated. This is because we have assumed correlations between progression-free survival and overall survival for all treatments. So, for example, the longer that patients are in progression-free survival, and hence the higher the total drug cost (as this is proportional to the time in progression-free survival), the longer the overall survival, and hence the higher the total QALYs.
FIGURE 19.
Probabilistic sensitivity analysis results: incremental cost–utility per person of cetuximab plus best supportive care compared with best supportive care. BSC, best supportive care.
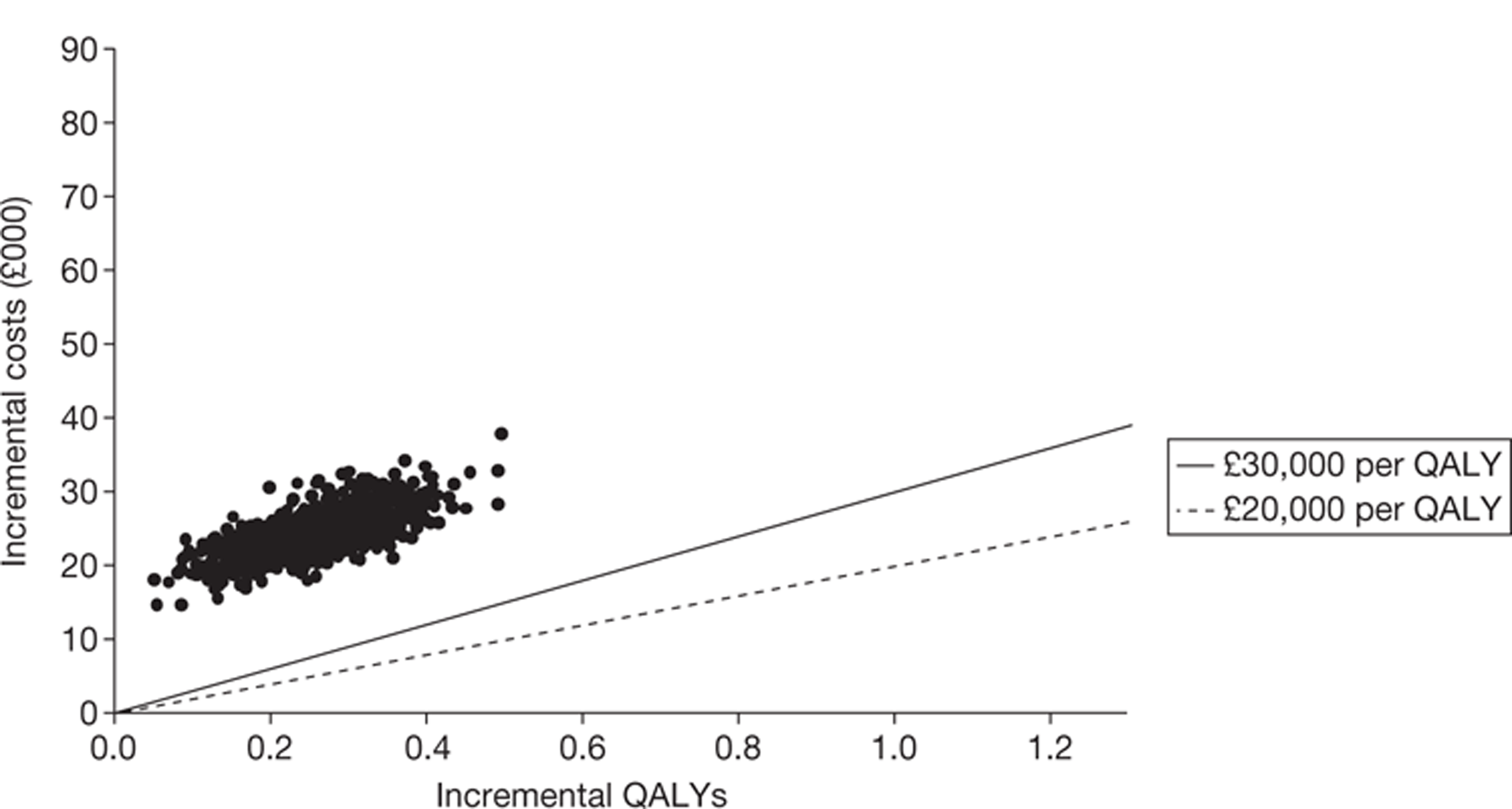
FIGURE 20.
Probabilistic sensitivity analysis results: incremental cost–utility per person of panitumumab plus best supportive care compared with best supportive care. BSC, best supportive care.
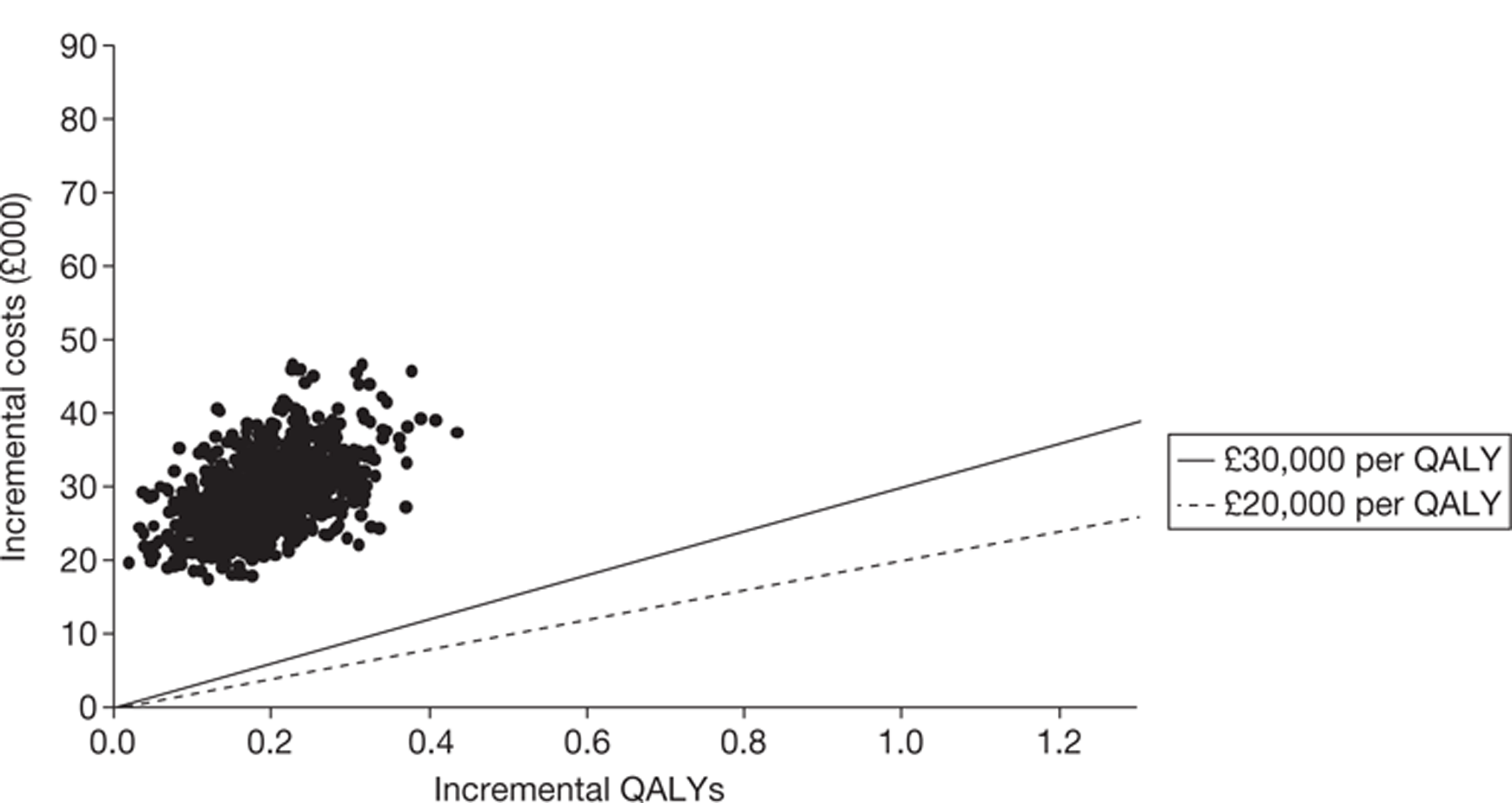
FIGURE 21.
Probabilistic sensitivity analysis results: incremental cost–utility per person of cetuximab plus irinotecan compared with best supportive care. BSC, best supportive care.
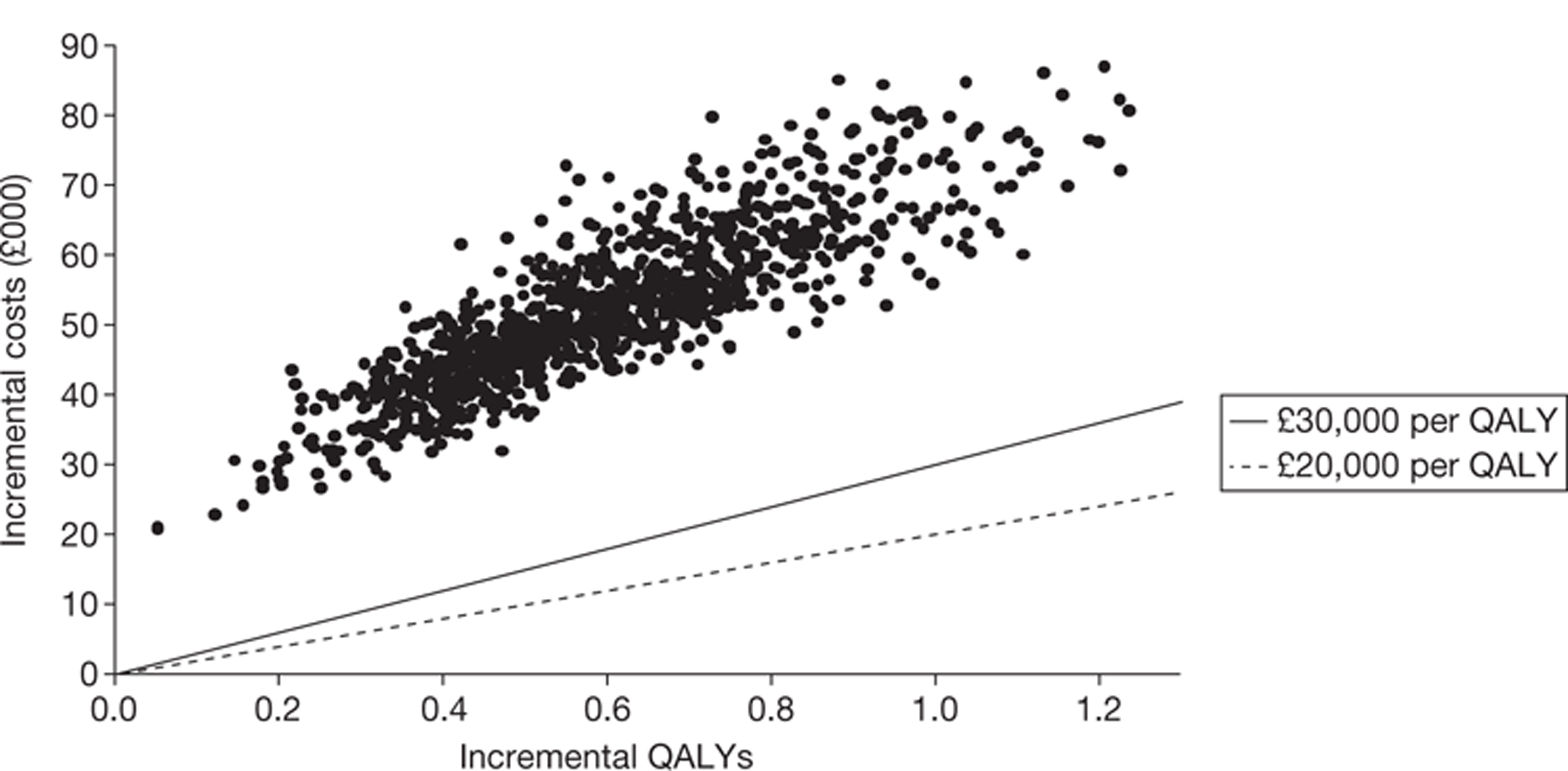
Figure 22 gives the cost-effectiveness acceptability curves for the four treatments, showing the probability that each provides best value for money given a range of willingness-to-pay thresholds. We predict that panitumumab plus best supportive care is never the most cost-effective option, and cetuximab monotherapy is unlikely to be the most cost-effective option regardless of the willingness-to-pay threshold. For willingness-to-pay values < £90,000 per QALY, best supportive care is likely to be the most cost-effective treatment, and for values > £90,000 per QALY, cetuximab plus irinotecan is likely to be most cost-effective. For willingness-to-pay values of ≤ £60,000 per QALY, we predict that the probability that best supportive care is the most cost-effective treatment is approximately 100%.
FIGURE 22.
Probabilistic sensitivity analysis results: cost-effectiveness acceptability curves.
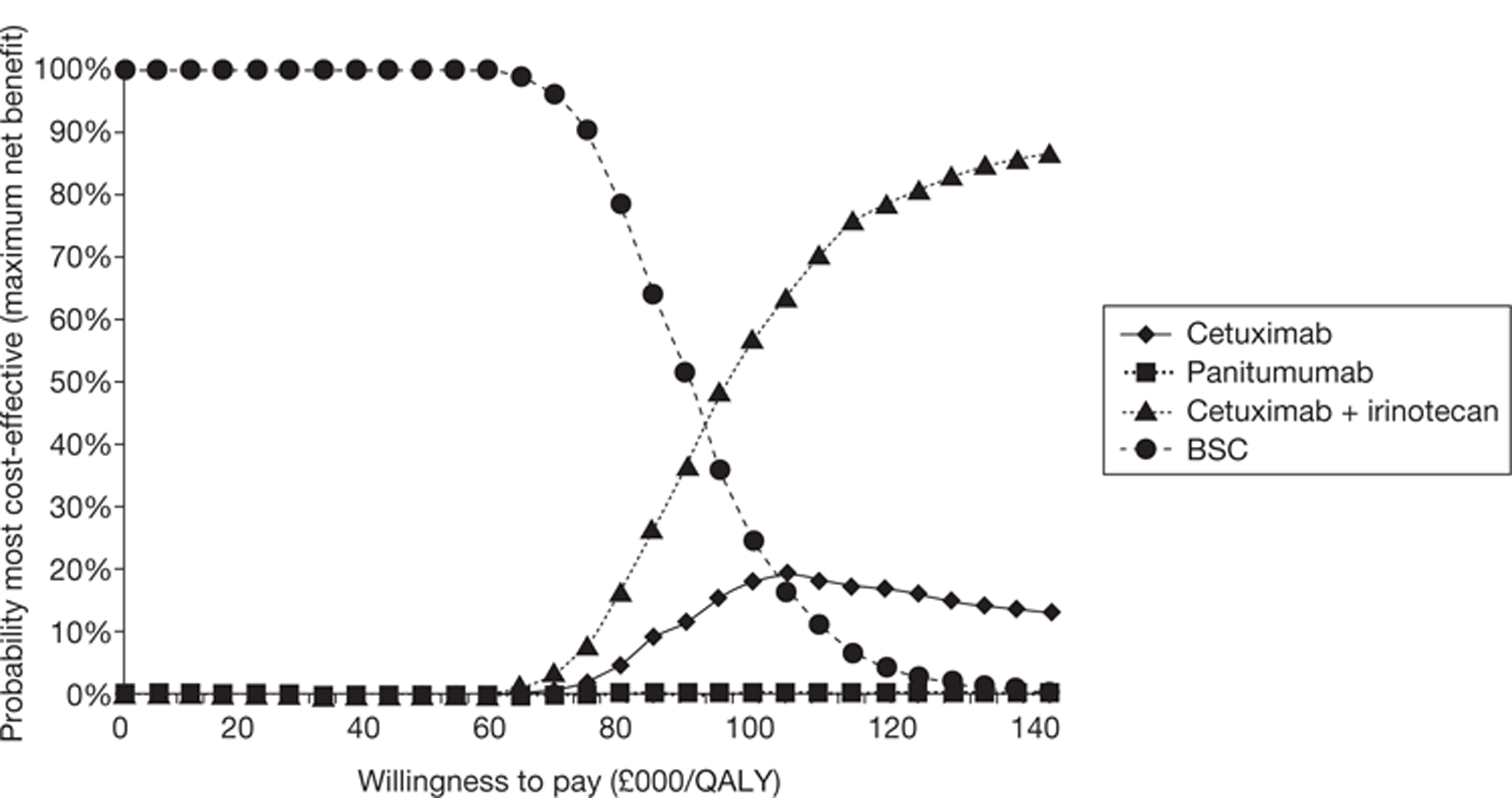
Comparison of the results of the Peninsula Technology Assessment Group, Merck Serono and Mittmann and colleagues42
In the preceding sections we described our cost-effectiveness analyses and those of Merck Serono, the manufacturer of cetuximab, and Mittmann and colleagues. 42 In this section we compare the results of these analyses and discuss the reasons for the different predictions of cost-effectiveness from the PenTAG model and the Mittmann evaluation of cetuximab plus best supportive care compared with best supportive care relative to Merck Serono's model; and from the PenTAG model of cetuximab plus irinotecan compared with best supportive care relative to Merck Serono's model.
No comparisons of the cost-effectiveness of panitumumab plus best supportive care compared with best supportive care for patients with KRAS WT status are possible because Amgen, the manufacturer of panitumumab, did not submit a cost-effectiveness analysis to NICE and we are not aware of any other relevant fully published models.
We have adjusted the results of Mittmann and colleagues'evaluation in three ways so that it is as comparable as possible with our model and Merck Serono's model. First, Mittman and colleagues report the estimated mean acquisition and administration costs of cetuximab combined. We separated out these two values. We did this by assuming the same ratio of mean drug acquisition cost to administration cost as in our model. Second, and most importantly, we used the same cost per mg of cetuximab in our model as Merck Serono used in its model, which is relevant to the UK at today's date. Mittmann and colleagues assumed a cost of C$3.24 per mg, which equals £2.05 per mg assuming an exchange rate of £1 = C$1.58 (as at 6 May 2011), whereas we use a cost of £1.37 per mg as does Merck Serono. Third, we inflated all non-drug costs at 4% per annum over 4 years because all costs reported by Mittmann and colleagues are given at 2007 prices.
Cetuximab plus best supportive care compared with best supportive care
A summary of the cost-effectiveness results for cetuximab plus best supportive care compared with best supportive care is given in Tables 55–58. We believe that Mittmann and colleagues’ results are worthy of close scrutiny because they focus on the relevant KRAS WT population and the trial-based economic evaluation appears to have been conducted to a high standard.
Importantly, the ICER for cetuximab plus best supportive care compared with best supportive care from our analysis (£98,000 per QALY) is similar to that from the adjusted results from the Mittmann analysis (£101,000 per QALY) but very different to that from Merck Serono's analysis (£48,000 per QALY).
First, note that there are very many similarities between our model and Merck Serono's model, for example we assume the same:
-
clinical effectiveness for cetuximab plus best supportive care and best supportive care
-
health states of progression-free survival and progressive disease
-
cost per mg of cetuximab
-
dose intensity for cetuximab of 98%
-
costs of treating adverse events.
In addition, we used similar utilities, the only difference being that we assume a lower utility than Merck Serono for cetuximab plus best supportive care in progressive disease.
| CET + BSC | ||||
|---|---|---|---|---|
| PenTAG | Merck Serono | Mittmann unadjusteda | Mittmann adjusteda | |
| Life-years (mean, undiscounted) | ||||
| Time on CET | 0.40 | 0.22 | NR | |
| Progression free | 0.40 | 0.40 | N/Ab | |
| Post progression | 0.44 | 0.44 | N/Ab | |
| Total (mean) | 0.84 | 0.84 | 0.79 | |
| QALYs (mean, discounted) | ||||
| Progression free | 0.32 | 0.32 | N/Ab | |
| Post progression | 0.29 | 0.34 | N/Ab | |
| Total (mean) | 0.61 | 0.66 | 0.51 | |
| Costs (£) (mean, discounted) | ||||
| KRAS testing | 300 | 200 | c | |
| Drug costs | 14,400 | 8200 | 18,500 | 8900 |
| Drug administration | 5500 | 2000 | 6000 | |
| Medical management in PFS | 1600 | 3700 | 5100 | 6000 |
| BSC in PD | 5300 | 4100 | ||
| Adverse events | 3700 | 3700 | ||
| Total | 30,800 | 21,800 | 23,600 | 20,900 |
| PenTAG | Merck Serono | Mittmann et al.42 | |
|---|---|---|---|
| Cost data | Modelled | Modelled | Directly measured |
| Discounting costs and benefits | 3.5% per annum | 3.5% per annum | None |
| Perspective | UK payer | UK payer | Canadian payer |
| Duration | Lifetime | Lifetime | 18–19 months |
| Utilities | PFS and PD from Merck Serono (CO.17 trial47) | PFS and PD from CO.17 trial47 | Baseline and follow-up from CO.17 trial47 |
| Effectiveness estimates | Same mean PFS and OS assumed for CET + BSC vs BSC | No extrapolation of OS beyond end of CO.17 trial | |
| BSC | ||||
|---|---|---|---|---|
| PenTAG | Merck Serono | Mittmann unadjusteda | Mittmann adjusteda | |
| Life-years (mean, undiscounted) | ||||
| Progression free | 0.23 | 0.23b | N/Ac | |
| Post progression | 0.29 | 0.29 | N/Ac | |
| Total (mean) | 0.51 | 0.51b | 0.51 | |
| QALYs (mean, discounted) | ||||
| Progression free | 0.17 | 0.17b | N/Ac | |
| Post progression | 0.19 | 0.20 | N/Ac | |
| Total (mean) | 0.36 | 0.37b | 0.33 | |
| Costs (£) (mean, discounted) | ||||
| KRAS testing | 0 | 0 | d | |
| Drug costs | 0 | 0 | 0 | |
| Drug administration | 0 | 0 | 0 | |
| Medical management in PFS | 0 | 2100 | 2300 | 2700 |
| BSC in PD | 3500 | 2700 | ||
| Adverse events | 2800 | 2800 | ||
| Total | 6300 | 7600 | 2300 | 2700 |
| CET + BSC | ||||
|---|---|---|---|---|
| PenTAG | Merck Serono | Mittmann unadjusteda | Mittmann adjusteda | |
| Life-years (mean, undiscounted) | ||||
| Progression free | 0.17 | 0.17 | N/Ab | |
| Post progression | 0.15 | 0.15 | N/Ab | |
| Total (mean) | 0.32 | 0.32 | 0.28 | |
| QALYs (mean, discounted) | ||||
| Progression free | 0.15 | 0.15 | N/Ab | |
| Post progression | 0.10 | 0.14 | N/Ab | |
| Total (mean) | 0.25 | 0.30 | 0.18 | |
| Costs (£) (mean, discounted) | ||||
| KRAS testing | 300 | 200 | 0 | |
| Drug costs | 14,400 | 8200 | 18,500 | 8900 |
| Drug administration | 5500 | 2000 | 6000 | |
| Medical management in PFS | 1600 | 1600 | 2800 | 3300 |
| BSC in PD | 1800 | 1400 | ||
| Adverse events | 900 | 900 | ||
| Total | 24,500 | 14,300 | 21,300 | 18,200 |
| ICER (incremental cost per QALY) | 98,000 | 48,000c | 118,000 | 101,000 |
The difference between Merck Serono's assessment of cost-effectiveness and our assessment of cost-effectiveness is almost entirely caused by the large difference in total mean cost of acquisition and administration of cetuximab: for drug acquisition we estimate £14,400 whereas Merck Serono estimates £8200, and for drug administration we estimate £5600 whereas Merck Serono estimates £2000 (see Table 55). This itself is mostly due to the fact that we estimate a far higher mean time on cetuximab treatment than Merck Serono: 0.40 years (4.8 months) compared with 0.22 years (2.6 months) (see Overall survival, progression-free survival and treatment duration, Cetuximab plus irinotecan for justification of this input). We both assume that cetuximab is taken while patients are in progression-free survival, and we assume the same mean time in progression-free survival; however, Merck Serono additionally imposes an artificial maximum time on cetuximab treatment. When we use Merck Serono's model and lift its cap on the time on treatment, its estimate of the mean time on cetuximab treatment increases from 0.22 to 0.40 years, equal to our estimate, and the ICER increases from £48,000 per QALY to £75,000 per QALY. As well as assuming a shorter mean time on treatment, Merck Serono also assumes a lower cost per administration than us, £180 compared with £270.
Mittmann and colleagues42 estimate the mean total acquisition cost per person of cetuximab as £8900 (see final column in Table 55) using the 2011 UK cost per mg of cetuximab and assuming the same proportionate split of costs between cetuximab acquisition and administration as in our model. This value is similar to that of Merck but far lower than our value of £14,400. Unfortunately, Mittmann and colleagues42 do not give their estimated mean duration of cetuximab treatment for patients with KRAS WT status; instead, they mention only that the median duration of cetuximab treatment in the RCT was 8.1 weeks for the whole population (i.e. those with KRAS WT and KRAS mutant status combined). The mean total acquisition cost of cetuximab of £8900, given a cost of cetuximab of £1.37 per mg, equates to a mean time on cetuximab of approximately 0.23 years, which is similar to that assumed by Merck and far lower than that assumed by us.
There are several further differences between Mittmann and colleagues’ evaluation and Merck Serono's model and our model (see Table 56). Mittmann and colleagues consider costs and consequences over 18–19 months, the duration of the cetuximab plus best supportive care compared with best supportive care RCT. At the end of the study 77% of patients on cetuximab plus best supportive care and 82% on best supportive care had died. However, we believe that Mittman and colleagues should have extrapolated overall survival. Mittmann and colleagues speculate that if they had extrapolated overall survival their estimated ICER would have fallen. The final major difference is that Mittmann and colleagues used different utilities even though they also took utilities from the cetuximab plus best supportive care compared with best supportive care RCT.
None of the differences in assessed cost-effectiveness between our model and Merck Serono's model is due to differences in effectiveness assumptions; we both assume the same mean progression-free survival and overall survival for cetuximab plus best supportive care and best supportive care (see Tables 55 and 56 and the survival curves shown in Figures 6–9). Given that Mittmann and colleagues42 do not separate the progression-free survival and progressive disease health states, we can compare only their estimates of mean overall survival. Their estimated overall survival of 0.79 years for cetuximab plus best supportive care is slightly less than, but very similar to, the 0.84 years used in both our model and that presented in the Merck Serono submission (see Table 55). Mittmann and colleagues’ value is probably lower because they did not extrapolate overall survival beyond the trial follow-up period. Their estimated overall survival of 0.51 years for best supportive care is exactly the same as the value used in both our model and Merck Serono's model.
Merck Serono's estimate of total discounted QALYs for cetuximab plus best supportive care, at 0.66, is slightly higher than our estimate of 0.61; this is because Merck Serono assumes a slightly higher utility for those in progressive disease. Mittmann and colleagues’ estimate of 0.51 is slightly lower than our value because they assume slightly lower mean overall survival and they use slightly different utilities to those used in our model and Merck Serono's model. Our estimate of 0.36 for total QALYs for best supportive care is virtually the same as Merck Serono's value of 0.37 because we use the same mean overall survival and utilities for best supportive care. Mittmann and colleagues’ estimate of 0.33 is similar but not the same as our value because they have used different utilities.
Merck Serono's estimate of £3700 for the total mean cost of medical management in progression-free survival for cetuximab is substantially higher than our estimate of £1600. However, as we state in Chapter 5, we believe that Merck Serono's estimated cost per unit time for those in progression-free survival is logically flawed. It has based its estimate on a value from Remak and Brazil;78 however, this refers specifically to the treatment of breast cancer, not colorectal cancer. Similarly, Merck Serono's flawed estimate of £2100 for the total mean cost of medical management in progression-free survival for best supportive care is substantially higher than our estimate of £0. However, coincidentally, we estimate the same mean incremental costs for medical management in progression-free survival for cetuximab compared with best supportive care (£1600) (see Table 58).
Our estimates for the mean costs of best supportive care in progressive disease for both treatments are slightly higher than those of Merck Serono because we have inflated the cost of best supportive care in progressive disease per unit time, quoted in Remak and Brazil,78 over a greater period of time than Merck Serono.
Cetuximab plus irinotecan compared with best supportive care
A summary of the results from our model and Merck Serono's model is given in Tables 59–61.
The ICER for cetuximab plus irinotecan compared with best supportive care from our analysis, £88,000 per QALY, is much higher than Merck Serono's ICER of £44,000 per QALY.
As for the comparison of cetuximab plus best supportive care compared with best supportive care, first note that there are many similarities between our model and Merck Serono's model, for example we assume:
-
similar progression-free survival and overall survival for best supportive care and for cetuximab plus irinotecan
-
the same health states of progression-free survival and progressive disease
-
the same cost per mg for cetuximab and irinotecan
-
the same dose intensities of 94% for cetuximab and 90% for irinotecan
-
the same costs of treating adverse events
-
the same utilities for best supportive care, although we assume lower utilities for cetuximab plus irinotecan in progression-free survival and in progressive disease.
As for the comparison of cetuximab plus best supportive care compared with best supportive care, the difference between Merck Serono's and our assessment of cost-effectiveness is almost entirely due to the large difference in total mean costs of acquisition and administration of cetuximab plus irinotecan: for drug acquisition we estimate £32,000 whereas Merck Serono estimates £17,400, and for administration we estimate £12,700 whereas Merck Serono estimates £3800.
Very little of the difference in estimated cost-effectiveness between our model and the Merck Serono model is due to differences in effectiveness assumptions because we assume very similar mean progression-free survival and overall survival for best supportive care, and similar progression-free survival and overall survival for cetuximab plus irinotecan (even though our method of estimating the effectiveness for cetuximab plus irinotecan is rather different to Merck Serono's method) (see Tables 59–61 and the survival curves for cetuximab plus irinotecan in Figures 10 and 11). Instead, the substantial differences in mean costs of drug acquisition and administration are mostly due to the fact that we estimate a far longer mean time on cetuximab plus irinotecan treatment than Merck Serono: 0.73 years (8.8 months) compared with Merck Serono's estimate of 0.37 years (4.4 months). As we explained in Overall survival, progression-free survival and treatment duration, Cetuximab plus irinotecan, we strongly disagree with Merck Serono's derivation of mean time on cetuximab plus irinotecan treatment. We both assume that cetuximab is taken while patients are in progression-free survival; however, Merck Serono additionally imposes an artificial maximum time on cetuximab plus irinotecan treatment. When we use Merck Serono's model and lift its cap on the time on treatment, its estimate of the mean time on cetuximab plus irinotecan treatment increases from 0.37 to 0.65 years (equal to mean progression-free survival), slightly below our estimate of 0.73 years, and its ICER increases from £44,000 to £67,000 per QALY. Notice also that, if we use Merck Serono's estimated mean progression-free survival for cetuximab plus irinotecan of 0.65 years, our ICER decreases slightly, from £88,000 to £82,000 per QALY. Merck Serono's estimate of the mean total administration cost per person of cetuximab plus irinotecan is lower than our estimate mostly because it assumes a far shorter mean time on treatment, but also because it assumes a lower cost per administration per month than us: £840 compared with £1480.
| CET + IRIN | ||
|---|---|---|
| PenTAG | Merck Serono | |
| Life-years (mean, undiscounted) | ||
| Time on CET + IRIN | 0.73 | 0.37 |
| Progression free | 0.73 | 0.65 |
| Post progression | 0.65 | 0.70 |
| Total (mean) | 1.38 | 1.36 |
| QALYs (mean, discounted) | ||
| Progression free | 0.54 | 0.53 |
| Post progression | 0.43 | 0.53 |
| Total (mean) | 0.97 | 1.06 |
| Costs (£) (mean, discounted) | ||
| KRAS testing | 300 | 160 |
| Drug costs | 32,000 | 17,400 |
| Drug administration | 12,700 | 3800a |
| Medical management in PFS | 2900 | 6100 |
| BSC in PD | 7800 | 6400 |
| Adverse events | 3700 | 3700 |
| Total | 59,300 | 37,600a |
BSC, best supportive care; CET, cetuximab; IRIN, irinotecan; PD, progressive disease; PFS, progression-free survival.
a After correcting for Merck Serono's error in which model cell referred to CET + BSC administration, not CET + IRIN administration.
| BSC | ||
|---|---|---|
| PenTAG | Merck Serono | |
| Life-years (mean, undiscounted) | ||
| Progression free | 0.23 | 0.24 |
| Post progression | 0.29 | 0.31 |
| Total (mean) | 0.51 | 0.55 |
| QALYs (mean, undiscounted) | ||
| Progression free | 0.17 | 0.18 |
| Post progression | 0.19 | 0.21 |
| Total (mean) | 0.36 | 0.39 |
| Costs (£) (mean, undiscounted) | ||
| KRAS testing | 0 | 0 |
| Drug costs | 0 | 0 |
| Drug administration | 0 | 0 |
| Medical management in PFS | 0 | 2200 |
| BSC in PD | 3500 | 2900 |
| Adverse events | 2800 | 2800 |
| Total | 6300 | 7900 |
| CET + IRIN vs BSC | ||
|---|---|---|
| PenTag | Merck Serono | |
| Life-years (mean, undiscounted) | ||
| Progression free | 0.50 | 0.42 |
| Post progression | 0.37 | 0.39 |
| Total (mean) | 0.87 | 0.81 |
| QALYs (mean, undiscounted) | ||
| Progression free | 0.37 | 0.35 |
| Post progression | 0.24 | 0.32 |
| Total (mean) | 0.60 | 0.67 |
| Costs (£) (mean, undiscounted | ||
| KRAS testing | 300 | 200 |
| Drug costs | 32,000 | 17,400 |
| Drug administration | 12,700 | 3800 |
| Medical management in PFS | 2900 | 3900 |
| BSC in PD | 4300 | 3500 |
| Adverse events | 900 | 900 |
| Total | 53,100 | 29,600 |
| ICER (incremental cost per QALY) | 88,000 | 44,000 |
Although we estimate slightly longer overall survival on cetuximab plus irinotecan than Merck Serono (1.38 years compared with 1.36 years), we estimate slightly lower total QALYs than Merck Serono (0.97 compared with 1.06). This is because we estimate lower utilities for cetuximab plus irinotecan in progression-free survival and progressive disease than Merck Serono. Our estimate of 0.36 for total QALYs for best supportive care is very similar to Merck's value of 0.39 because we use very similar mean overall survival and the same utilities for best supportive care.
Merck Serono's estimate of £6100 for the mean total cost of medical management in progression-free survival for cetuximab plus irinotecan is substantially higher than our estimate of £2900. However, we believe that Merck Serono's estimated cost per unit time for people on cetuximab plus irinotecan while in progression-free survival is logically flawed. Similarly, Merck Serono's flawed estimate of £2200 for the mean total cost of medical management in progression-free survival for best supportive care is substantially higher than our estimate of £0. However, coincidentally, we estimate similar mean total incremental costs for medical management in progression-free survival for cetuximab plus irinotecan compared with best supportive care.
Our estimates for the mean costs of best supportive care in progressive disease for both treatments are slightly higher than those of Merck Serono because we inflate the cost per unit time of best supportive care in progressive disease, quoted in Remak and Brazil,78 over a longer period of time than Merck Serono.
Chapter 7 Discussion
Aim
The question addressed was, ‘What is the clinical effectiveness and cost-effectiveness of cetuximab (monotherapy or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy’.
The populations of interest were limited to those with KRAS WT metastatic colorectal cancer in the cases of cetuximab and panitumumab.
The aim was addressed through a HTA comprising a systematic review of clinical effectiveness and cost-effectiveness studies, a review and critique of manufacturer submissions and a de novo economic analysis.
Strengths and limitations of the systematic review of effectiveness
The strengths of this systematic review are that it was conducted by an independent research team using the latest evidence in line with a prespecified protocol. The main limitation was the lack of evidence on bevacizumab, cetuximab and cetuximab plus irinotecan used second line in the populations of interest and lack of evidence on bevacizumab and cetuximab plus irinotecan used third line.
Strengths and limitations of the systematic review of economic evaluations
Again, the strengths of this systematic review are that it was conducted by an independent research team using the latest evidence in line with a prespecified protocol. The main limitation was the incomplete reporting of the reviews of the cost-effectiveness of panitumumab and the absence of cost-effectiveness estimates for bevacizumab.
Strengths and limitations of the critique of the manufacturers'submissions
This was conducted by an independent research team using a number of established frameworks to identify strengths and weaknesses. The scope of the submissions on bevacizumab and panitumumab, which did not directly estimate cost-effectiveness, was the main limitation.
Strengths and limitations of the Peninsula Technology Assessment Group's model
Strengths
Our assessment of the cost-effectiveness of drugs for metastatic colorectal cancer is independent. Our analysis is the second independent fully published cost-effectiveness analysis of cetuximab compared with best supportive care for KRAS WT patients, the first being that of Mittmann and colleagues. 42 Our analysis is the first independent fully published cost-effectiveness analysis of panitumumab plus best supportive care compared with best supportive care for patients with KRAS WT status and of cetuximab plus irinotecan compared with best supportive care for patients with KRAS WT status. We have carefully compared our model and the results of our analysis with those of Mittmann and colleagues42 and Merck Serono and in so doing we have highlighted areas in common and those where there is disagreement.
Our model adheres to the NICE reference case72 and has been extensively checked. In addition to our base-case analysis we also present numerous one-way sensitivity analyses, which we have chosen carefully to reflect the key areas of uncertainty and disagreements between ourselves and Merck Serono. We also present probabilistic analyses in which we vary the key parameters within plausible ranges.
Our certainty about the accuracy of our analyses for cetuximab plus best supportive care compared with best supportive care and panitumumab plus best supportive care compared with best supportive care is increased given that the effectiveness evidence that underpins these analyses is taken from high-quality RCTs whose data are mature. There is much greater uncertainty concerning the analysis of cetuximab plus irinotecan compared with best supportive care given the lack of effectiveness evidence, particularly for patients with KRAS WT status.
We have confidence in the accuracy of our utility estimates for the best supportive care, panitumumab and cetuximab treatment arms. Indeed, their accuracy is greater than is typically available for cost-effectiveness analysis, with the data being derived from direct observation of patients in trials. This is not true for the utilities for cetuximab plus irinotecan.
Limitations
There are some factors that limit the accuracy of our analysis. For example, the mean duration of drug treatment for the KRAS WT population, a vital parameter, is available in published form only for panitumumab. Indeed, the mean durations of cetuximab and cetuximab plus irinotecan treatment are not published for patients with KRAS WT status, although the mean duration of cetuximab treatment was provided to us by personal communication. These are important limitations of our analysis given that cost-effectiveness is very sensitive to these parameters.
The external validity of the results is uncertain given that we use efficacy data from RCTs in which patients are relatively young (median age approximately 63 years) and fit (an ECOG performance status of 0–2) in comparison with patients in actual clinical practice, who are typically older and less fit (some with an ECOG performance status of 3–4).
Progression-free survival and overall survival for cetuximab plus irinotecan are available only for the whole population (KRAS WT and KRAS mutant combined). Like Merck Serono we have therefore been forced to adjust these estimates using other data sources to obtain estimates of progression-free survival and overall survival in the KRAS WT population. However, we have provided several possible methods of adjustment and the ICER for cetuximab plus irinotecan compared with best supportive care remains high regardless of which estimates for progression-free survival and overall survival are used.
In common with Merck Serono we do not stratify our analysis according to the line of treatment as the necessary individual patient data were not available.
We estimate the cost of medical management in progressive disease for all treatment groups based on a study of medical management in progressive disease for women with breast cancer. 78 Like Merck Serono we believe that this is methodologically acceptable given the absence of suitable alternatives but do caution that the data from this publication are now rather old, relating to practices from the year 2000.
Main findings in the light of limitations
Effectiveness review
There is consensus about the evidence on the effectiveness of cetuximab and panitumumab for patients with KRAS WT status. Based on RCTs, both cetuximab and panitumumab are effective used third line, particularly with respect to progression-free survival. For cetuximab, median progression-free survival increases from approximately 2 months to approximately 4 months (hazard ratio 0.40, 95% CI 0.30 to 0.54). For panitumumab, median progression-free survival increases from approximately 1.8 months to approximately 3 months (hazard ratio 0.45, 95% CI 0.34 to 0.59).
We broadly agree with Merck Serono's estimate of the effectiveness of cetuximab plus irinotecan for KRAS WT people even though it has not been directly measured in a RCT.
There is an absence of RCT evidence for bevacizumab combined with non-oxaliplatin chemotherapy in second-line and further lines of therapy.
Economic evaluations
Cost-effectiveness of cetuximab compared with best supportive care
There are many similarities between Merck Serono's model for cetuximab compared with best supportive care and the PenTAG de novo model. Importantly, we assume the same mean times as Merck Serono for progression-free survival and overall survival for cetuximab and for best supportive care. Nonetheless, Merck Serono estimates a far lower ICER for cetuximab compared with best supportive care than us: £47,000 compared with £98,000 per QALY gained. This is explained almost entirely by Merck Serono's estimates of the total mean costs of cetuximab acquisition and administration, which are far lower than our estimates. These differences are due almost entirely to Merck Serono's far lower estimate of the mean time on cetuximab treatment: 2.6 months compared with our estimate of 4.8 months. Merck Serono's derivation of its estimate is based on its imposition of an artificial maximum time on cetuximab treatment. When we use Merck Serono's model and lift the cap on the time on cetuximab treatment, the ICER increases from £47,000 to £75,000 per QALY gained.
We are aware of only one other fully published cost-effectiveness analysis of any of the treatments in this appraisal for KRAS WT people, that of Mittmann and colleagues. 42 They perform a trial-based economic analysis to consider cost-effectiveness from the health-care payer perspective in Canada. After we adjust their result for the cost per mg of cetuximab appropriate in the UK in 2011, and other costs for inflation to the year 2011, we estimate that their ICER is approximately equivalent to £101,000 per QALY gained. This is very close to our estimate of £98,000 per QALY gained and much higher than Merck Serono's £48,000 per QALY gained.
Cost-effectiveness of cetuximab plus irinotecan compared with best supportive care
Again, there are many similarities between Merck Serono's model for cetuximab plus irinotecan compared with best supportive care and the PenTAG de novo model. Importantly, we assume similar mean times as Merck Serono for progression-free survival and overall survival for cetuximab plus irinotecan and for best supportive care. Merck Serono estimates a far lower ICER for cetuximab plus irinotecan compared with best supportive care: £44,000 per QALY compared with our estimate of £88,000 per QALY. Similar to the case of cetuximab compared with best supportive care, this is explained almost entirely by Merck Serono's estimates of the total mean costs of cetuximab plus irinotecan acquisition and administration, which are far lower than our estimates. These differences, in turn, are due almost entirely to Merck Serono's far lower estimate of the mean time on cetuximab plus irinotecan treatment: 4.4 compared with our estimate of 8.8 months. Merck Serono's derivation of its estimate is based on its imposition of an artificial maximum time on cetuximab plus irinotecan treatment. When we use Merck Serono's model and lift the cap on the time on treatment the ICER increases from £44,000 to £67,000 per QALY.
Cost-effectiveness of panitumumab compared with best supportive care
The estimate of cost-effectiveness from the PenTAG de novo model is £150,000 per QALY gained, with no alternative estimate being offered by the manufacturer.
Chapter 8 Conclusions
Implications
On balance we conclude that, used as third- and subsequent-line treatment relative to best supportive care, cetuximab plus best supportive care, cetuximab plus irinotecan plus best supportive care and panitumumab plus best supportive care are not cost-effective if decision thresholds of £20,000 per QALY or £30,000 per QALY are used.
There is no additional evidence on the effectiveness and cost-effectiveness of cetuximab used in second-line treatment to that informing the guidance on second-line use provided by TA118. 16
In common with the manufacturer we were not able to estimate the cost-effectiveness of bevacizumab in combination with non-oxaliplatin chemotherapy second line or beyond because of the absence of RCT evidence.
Suggested research priorities
-
Given the lack of clinical data for patients with KRAS WT status taking cetuximab plus irinotecan, it would be useful to conduct a RCT for these patients comparing cetuximab plus irinotecan with cetuximab plus best supportive care or panitumumab plus best supportive care. It would be helpful to collect HRQoL data in such a trial.
-
There is a need to have data documenting the proportions of patients on the various pathways of disease once metastatic colorectal cancer has occurred to better inform the clinical costs and overall costs.
-
We cannot model the cost-effectiveness of bevacizumab in combination with non-oxaliplatin chemotherapy because of the absence of relevant clinical evidence. Ideally, a RCT should be conducted if this is thought to be a potentially important use of the agent by the wider clinical community.
-
Given that the mean duration of cetuximab plus irinotecan treatment strongly influences cost-effectiveness, and that it is not known with certainty, further data on this parameter from the BOND RCT of cetuximab plus irinotecan compared with cetuximab would be helpful.
-
Given that the medical management cost data come from a study of women with breast cancer carried out over 10 years ago, collecting data on the medical management of metastatic colorectal cancer would be useful.
Ongoing trials identified in the course of this appraisal indicate that some of the gaps in knowledge may already be being addressed.
Acknowledgements
We would like to thank Ian Chandler (Consultant Histopathologist, Royal Devon and Exeter Hospital, Exeter, UK) for information regarding the EGFR test; Kate Copland (Chief Technician, Aseptic Services, Royal Devon and Exeter Hospital, Exeter, UK) for advice on common practices of drug wastage and on pharmacy procedures for drug preparation; and Joseph Sacco (Department of Medical Oncology, Clatterbridge Centre for Oncology, Merseyside, UK) for providing data on patients’ weights for the purpose of costing panitumumab. We would also like to thank Sue Whiffin and Jenny Lowe for their administrative support throughout the project and Dr Ruth Garside for proofreading and commenting on the final draft report.
Contributions of authors
Martin Hoyle led the design, development and execution of the economic model and wrote the sections on the design and results of the economic model.
Louise Crathorne provided overall project management, assessed abstracts and titles for inclusion and contributed to the clinical effectiveness and cost-effectiveness systematic review and the writing and editing of the report.
Jaime Peters critically appraised the industry submissions and contributed to the development of the health economic model and the writing and editing of the report.
Tracey Jones-Hughes assessed abstracts and titles for inclusion, led the systematic review of clinical effectiveness and contributed to the writing and editing of the report.
Chris Cooper designed and carried out literature searches for the systematic reviews and identification of model parameters and contributed to the writing and editing of the report.
Mark Napier provided clinical input into the design of the model, advised on clinical matters and contributed to the editing of the report.
Paul Tappenden provided background from NICE TA118 and reviewed and commented on the final draft.
Chris Hyde led the systematic review of economic evaluations, contributed to the design of the model and the writing and editing of the report and was overall director of the project and guarantor of the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publication
Hoyle M, Peters J, Crathorne L, Jones-Hughes T, Cooper C, Napier M, et al. Cost-effectiveness of cetuximab, cetuximab plus irinotecan, and panitumumab for third and further lines of treatment for KRAS wild-type patients with metastatic colorectal cancer [published online ahead of print 25 January 2013]. Value Health 2013. URL: www.valueinhealthjournal.com/article/S1098-3015(12)04203-9/abstract (accessed 1 March 2013).
References
- Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T, Kim ES, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol 2005;23:2544-55.
- Ellis LM. Epidermal growth factor receptor in tumor angiogenesis. Hematol Oncol Clin North Am 2004;18:1007-21.
- Vallbohmer D, Zhang W, Gordon M, Yang DY, Yun J, Press OA, et al. Molecular determinants of cetuximab efficacy. J Clin Oncol 2005;23:3536-44.
- Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786-92.
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 2007;67:2643-8.
- Monzon FA, Ogino S, Hammond ME, Halling KC, Bloom KJ, Nikiforova MN. The role of KRAS mutation testing in the management of patients with metastatic colorectal cancer. Arch Pathol Lab Med 2009;133:1600-6.
- Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658-64.
- Cancer Research UK . Bowel (colorectal) Cancer – UK Incidence Statistics 2011. http://info.cancerresearchuk.org/cancerstats/types/bowel/incidence (accessed 10 March 2011).
- Chapuis PH, Dixon MF, Fielding LP, Gordon PH, Hermanek P, Kyriakos M, et al. Staging of colorectal cancer. Int J Colorectal Dis 1987;2:123-38.
- Tappenden P, Jones R, Paisley S, Carroll C. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess 2007;11.
- Cancer Research UK . Bowel (colorectal) Cancer – Survival Statistics 2011. http://info.cancerresearchuk.org/cancerstats/types/bowel/survival/ (accessed 10 March 2011).
- Cancer Research UK . Bowel (colorectal) Cancer – Mortality Statistics 2011. http://info.cancerresearchuk.org/cancerstats/types/bowel/mortality/ (accessed 10 March 2011).
- Guidance for commissioning cancer services: improving outcomes in colorectal cancers. Research evidence for the manual update. London: Centre for Research and Dissemination on behalf of National Cancer Guidance Steering Group; 2004.
- Technology Appraisal 93. London: NICE; 2005.
- Technology Appraisal 61. London: NICE; 2003.
- Technology Appraisal 118. London: NICE; 2009.
- Technology Appraisal 176. London: NICE; 2008.
- Technology Appraisal 105. London: NICE; 2009.
- Technology Appraisal 212. London: NICE; 2010.
- Clinical Guideline 131 – Colorectal cancer: The diagnosis and management of colorectal cancer. London: NICE; 2004.
- Guidelines for the management of colorectal cancer. London: ACPGBI; 2007.
- Shabaruddin FH, Elliott RA, Valle JW, Newman WG, Payne K. Understanding chemotherapy treatment pathways of advanced colorectal cancer patients to inform an economic evaluation in the United Kingdom. Br J Cancer 2010;103:315-23.
- Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004;22:1209-14.
- Roche Products Ltd . Bevacizumab (Avastin) Summary of Product Characteristics 2010. www.medicines.org.uk/emc/medicine/15748/SPC/ (accessed 4 March 2011).
- Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest 2000;105:R15-24.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42.
- Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 2005;23:3697-705.
- Summary of Product Characteristics: Erbitux 5mg/ml solution for infusion. Feltham, Middlesex: Merck Serono; 2010.
- Amgen Ltd . Vectibix Summary of Product Characteristics 2010. www.medicines.org.uk/EMC/medicine/20528/SPC/Vectibix/ (accessed 4 March 2011).
- Cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal 150 and part-review of technology appraisal 118): final scope. London: NICE; 2010.
- Undertaking systematic reviews of research on effectiveness. York: NHS Centre for Reviews and Dissemination; 2001.
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman D, et al. Wild-Type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626-34.
- Hurwitz HI, Yi J, Ince W, Novotny WF, Rosen O. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist 2009;14:22-8.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91.
- National Institutes of Health, National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) 2009. evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010–06–14_QuickReference_5x7.pdf (accessed 16 February 2011).
- Qiu LX, Mao C, Zhang J, Zhu XD, Liao RY, Xue K, et al. Predictive and prognostic value of KRAS mutations in metastatic colorectal cancer patients treated with cetuximab: a meta-analysis of 22 studies. Eur J Cancer 2010;46:2781-7.
- Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040-8.
- Van Cutsem E, Siena S, Humblet Y, Canon JL, Maurel J, Bajetta E, et al. An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann Oncol 2008;19:92-8.
- Hecht JR, Cohn AL. SPIRITT: a study of second-line treatment of metastatic colorectal cancer with FOLFIRI plus panitumumab or bevacizumab. Community Oncology 2008;5:1-4.
- Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-19.
- Giantonio BJ, Catalano PJ, Meropol NJ, Oapos;Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44.
- Mittmann N, Au HJ, Tu D, O’Callaghan CJ, Isogai PK, Karapetis CS, et al. Prospective cost-effectiveness analysis of cetuximab in metastatic colorectal cancer: evaluation of National Cancer Institute of Canada Clinical Trials Group CO.17 trial. J Natl Cancer Inst 2009;101:1182-92.
- Asmis TR, Powell E, Karapetis CS, Jonker DJ, Tu D, Jeffery M, et al. Comorbidity, age and overall survival in cetuximab-treated patients with advanced colorectal cancer (ACRC) – results from NCIC CTG CO.17: a phase III trial of cetuximab versus best supportive care. Ann Oncol 2011;22:118-26.
- Au HJ, Karapetis CS, O’Callaghan CJ, Tu D, Moore MJ, Zalcberg JR, et al. Health-related quality of life in patients with advanced colorectal cancer treated with cetuximab: overall and KRAS-specific results of the NCIC CTG and AGITG CO.17 trial. J Clin Oncol 2009;27:1822-8.
- Karapetis C, Khambata-Ford S, Jonker D, O’Callaghan C, Tu D, Vachan B, et al. Kras mutation status is a predictive biomarker for cetuximab benefit in the treatment of advanced colorectal cancer – results from NCIC CTG CO.17: a phase III trial of cetuximab versus best supportive care (33rd Congress of the European Society of Medical Oncology, Stockholm). Ann Oncol 2008;19.
- Therasse P, Arbuck S, Eisenhauer E. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205-16.
- Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65.
- De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 2008;19:508-15.
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45.
- Tejpar S, Peeters M, Humblet Y. Dose escalation study using up to twice the standard dose of cetuximab in patients with metastatic colorectal cancer (mCRC) with no or slight skin reactions on cetuximab standard dose treatment (EVEREST study): preliminary data. J Clin Oncol 2006;24.
- Lenz HJ, Van Cutsem E, Khambata-Ford S, Mayer RJ, Gold P, Stella P, et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin and fluoropyrumidines. J Clin Oncol 2006;24:4914-21.
- Peeters M, Siena S, Van Cutsem E, Sobrero A, Hendlisz A, Cascinu S, et al. Association of progression-free survival, overall survival, and patient-reported outcomes by skin toxicity and KRAS status in patients receiving panitumumab monotherapy. Cancer 2009;115:1544-54.
- Siena S, Peeters M, Van C, Humblet Y, Conte P, Bajetta E, et al. Association of progression-free survival with patient-reported outcomes and survival: results from a randomised phase 3 trial of panitumumab. Br J Cancer 2007;97:1469-74.
- Tejpar S, Peeters M, Humblet Y, Vermorken JB, De Hertogh G, De Roock W, et al. Relationship of efficacy with KRAS status (wild type versus mutant) in patients with irinotecan-refractory metastatic colorectal cancer (mCRC), treated with irinotecan (q2w) and escalating doses of cetuximab (q1w): The EVEREST experience (preliminary data). J Clin Oncol 2008;26.
- Tejpar S, Peeters M, Humblet Y, Gelderblom H, Vermorken J, Viret F, et al. Phase I/II study of cetuximab dose-escalation in patients with metastatic colorectal cancer (mCRC) with no or slight skin reactions on cetuximab standard dose treatment (EVEREST): pharmacokinetic (PK), pharmacodynamic (PD) and efficacy data. J Clin Oncol 2007;25.
- Odom D, Barber B, Bennett L, Peeters M, Zhao Z, Kaye J, et al. Health-related quality of life and colorectal cancer-specific symptoms in patients with chemotherapy-refractory metastatic disease treated with panitumumab. Int J Colorectal Dis 2011;26:173-81.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Drummond M, O’Brien B, Stoddart G, Torrance G. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 1997.
- Annemans L, Van Cutsem E, Humblet Y, Van Laethem JL, Bleiberg H. Cost-effectiveness of cetuximab in combination with irinotecan compared with current care in metastatic colorectal cancer after failure on irinotecan – a Belgian analysis. Acta Clin Belg 2007;62:419-25.
- Norum J. Cetuximab in the treatment of metastatic colorectal cancer: a model-based cost-effectiveness analysis. J Chemother 2006;18:532-7.
- Starling N, Tilden D, White J, Cunningham D. Cost-effectiveness analysis of cetuximab/ irinotecan vs active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Br J Cancer 2007;96:206-12.
- Wong YN, Meropol NJ, Speier W, Sargent D, Goldberg RM, Beck JR. Cost implications of new treatments for advanced colorectal cancer. Cancer 2009;115:2081-91.
- Carlson JJ. Cost–utility of K-RAS mutation testing prior to treatment of metastatic colorectal cancer with cetuximab monotherapy. Value Health 2010;13.
- Graham CN, Borker R, Oppe M, Uyl-de Groot C, Barber B, Brogan AJ. Colorectal cancer: cost effectiveness of panitumumab plus best supportive care (BSC) compared with BSC alone in chemrefractory metastatic colorectal cancer patients with wild-type KRAS tumour status in the Netherlands. Ann Oncol 2008;19.
- Wei DB, Lin CC. The cost-effectiveness of cetuximab use among elderly metastatic colorectal cancer. Value Health 2010;13.
- Blank PR, Schewenkglenks M, Herrmann R, Moch H, Szucs TD. Cost-effectiveness of novel predictive tests in the treatment of metastatic colorectal cancer: an analysis from a Swiss perspective. J Clin Oncol 2010;28.
- Tilden D, Thurley D, White J, Aristides M. The cost-effectiveness of cetuximab in combination with irinotecan for the treatment of patients with EGFR-expressing mCRC after failure of irinotecan including cytotoxic therapy in Scotland (PCN17). Value Health 2005;8.
- Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 1998;352:1413-18.
- Report to the National Institute for Health and Clinical Excellence. Feltham: Merck Serono; 2011.
- Roche submission to the National Institute for Health and Clinical Excellence. Welwyn Garden City: Roche; 2011.
- Submission of evidence on panitumumab monotherapy by the manufacturer. Cambridge: Amgen; 2011.
- Guide to the methods of technology appraisal. London: NICE; 2008.
- Sobrero Alberto F. n.d.
- Sobrero Alberto F, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr Yousif A, Lutz Manfred P, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2311-19.
- Abubakr Y, Eng C, Pautret J. Cetuximab plus irinotecan for metastatic colorectal cancer (mCRC): safety analysis of 800 patients in a randomised phase III trial (EPIC). J Clin Oncol 2006;24.
- British national formulary. No. 61, March 2011. London: BMA and RPS; 2011.
- Department of Health . NHS Reference Costs 2008–2009 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591 (accessed 9 March 2011).
- Remak E, Brazil L. Cost of managing women presenting with stage IV breast cancer in the United Kingdom. Br J Cancer 2004;91:77-83.
- Curtis L. Unit costs of health and social care. Canterbury: PPSRU; 2010.
- All Wales Medical Genetics Service: KRAS & tumour mutation testing. Cardiff: Cardiff & Vale NHS Trust; 2011.
- Ramsey SD, Andersen MR, Etzioni R, Moinpour C, Peacock S, Potosky A, et al. Quality of life in survivors of colorectal carcinoma. Cancer 2000;88:1294-303.
- Kind P, Hardman G, Macran S. Discussion paper. York: Centre for Health Economics; 1999.
- De Roock WD, Vriendt VD, Normanno N, Ciardiello F, Tejpar S. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol 2011;12:594-603.
- Ince WL, Jubb AM, Holden SN, Holmgren EB, Tobin P, Sridhar M, et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst 2005;97:981-9.
- Thompson Coon J, Hoyle M, Green C, Liu Z, Welch K, Moxham T, et al. Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation. Health Technol Assess 2010;14.
- Lievre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 2008;26:374-9.
- Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLoS One 2010;5.
- Dubois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 1916;17:863-71.
- Summary of Product Characteristics: Irinotecan Actavis 20mg Ml Concentrate for Solution for Infusion 2010. www.medicines.org.uk/EMC/medicine/23982/SPC/Irinotecan+Actavis+20mg+ml+concentrate+for+solution+for+infusion/ (accessed 4 March 2011).
- Brookmeyer R, Crowley J. A Confidence Interval for the Median Survival Time. Biometrics 1982;38:29-41.
- NHS terms and conditions of service handbook. Leeds: NHS Employers; 2011.
Appendix 1 Literature search strategies
Appendix 2 Protocol
Appendix 3 Clinical effectiveness: data extraction forms
Appendix 4 Method of indirect comparison
Appendix 5 Critique of manufacturer's search strategy
Appendix 6 Clinical effectiveness: excluded studies
Appendix 7 Ongoing trials
Appendix 8 Clinical effectiveness: supplementary tables
Appendix 9 Cost-effectiveness: quality appraisal
Appendix 10 Cost-effectiveness: excluded studies
Appendix 11 Estimation of difference in utilities between patients taking panitumumab and those on best supportive care while in progression-free survival
Appendix 12 Estimation of mean dosages of cetuximab, irinotecan and panitumumab, including wastage, for patients of varying body surface areas and weights
Appendix 13 Requests for clarification: Amgen
Appendix 14 Calculating progression-free survival for cetuximab plus irinotecan
Appendix 15 Calculating overall survival for cetuximab plus irinotecan
List of abbreviations
- 5-FU/FA
- 5-fuorouracil plus folinic acid
- AIC
- Akaike information criterion
- BNF
- British National Formulary
- CCI
- Charlson Comorbidity Index
- CI
- confidence interval
- CRD
- Centre for Reviews and Dissemination
- ECOG
- Eastern Cooperative Oncology Group
- EGFR
- epidermal growth factor receptor
- EMA
- European Medicines Agency
- EoL
- end of life
- EORTC QLQ-C30
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30
- EQ-5D
- European Quality of Life-5 Dimensions
- FA
- folinic acid
- FDA
- US Food and Drug Administration
- FOLFIRI
- irinotecan + 5-FU/FA
- FOLFOX
- oxaliplatin + 5-FU/FA
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- health technology assessment
- HUI
- Health Utility Index
- ICER
- incremental cost-effectiveness ratio
- IFL
- irinotecan with fluorouracil and leucovorin
- ITT
- intention to treat
- KRAS
- Kirsten rat sarcoma
- LV
- leucovorin
- LYG
- life-year gained
- MTA
- multiple technology assessment
- NCCN FCSI
- National Comprehensive Cancer Network Functional Assessment of Cancer Therapy Colorectal Cancer Symptom Index
- NCI-CTC
- National Cancer Institute Common Terminology Criteria
- NICE
- National Institute for Health and Clinical Excellence
- PenTAG
- Peninsula Technology Assessment Group
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RECIST
- Response Evaluation Criteria in Solid Tumors
- SE
- standard error
- TA
- technology appraisal
- TNM
- tumour node metastases
- VAS
- visual analogue scale
- VEGF
- vascular endothelial growth factor
- WT
- wild type
- XELOX
- capecitabine with oxaliplatin
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.