Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/158/01. The contractual start date was in February 2011. The draft report began editorial review in November 2011 and was accepted for publication in July 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Armstrong et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Anaphylaxis is a severe, life-threatening, generalised or systemic hypersensitivity reaction. It is characterised by rapidly developing life-threatening problems involving the airway (pharyngeal or laryngeal oedema) and/or breathing (bronchospasm with tachypnoea) and/or circulation (hypotension and/or tachycardia).
There is considerable geographic variation in both practice and service provision for anaphylaxis, specifically in reviews after emergency treatment for anaphylaxis and decisions about when and whether or not to refer to a specialist service (SS). There are professional guidelines on the emergency treatment and management of anaphylaxis, but there is currently no relevant national guidance for England and Wales on assessment after the event to confirm an anaphylactic episode or on the decision to refer after emergency treatment.
There are approximately 20 anaphylaxis deaths reported each year in the UK, although this may be a substantial underestimate. There are observational data that the risk of death is increased by delayed use of adrenaline. In order to reduce the delay, adrenaline injectors (AIs) are often prescribed following anaphylaxis, but there is a perception that they are often not used in time or correctly.
Chapter 2 Definition of the decision problem
For the National Institute for Health and Care Excellence (NICE) clinical guideline CG134 ‘Anaphylaxis: assessment to confirm an anaphylactic episode and the decision to refer after emergency treatment for a suspected anaphylactic episode’, we, as the Technology Assessment Group, were asked to address six questions:
-
In adults, young people and children who receive emergency treatment for suspected anaphylaxis, which people are at high risk of anaphylactic episodes? For which people would further anaphylactic episodes have significant impact? Which people can be identified as needing special consideration?
-
What are the effects of history-taking, including signs and symptoms, and physical examination in identifying the possible cause?
-
What are the effects of providing adrenaline auto-injectors, including by whom?
-
After assessment, when should referral take place?
-
What is the cost-effectiveness of referral to specialist allergy clinics for the diagnosis of anaphylaxis and for the prevention of future episodes and the reduction in morbidity and mortality from future episodes?
-
What is the cost-effectiveness of adrenaline auto-injectors for the treatment of anaphylaxis including the cost implications of training in the use of the auto-injectors?
Questions 1–4 are addressed in Chapter 3 and questions 5 and 6 are addressed in Chapter 4 of this report.
Chapter 3 Assessment of clinical effectiveness
Note: this chapter is reproduced from the original project protocol. See also Chapter 2 and Chapter 4, Methods of cost-effectiveness analysis.
Methods for reviewing effectiveness
Research questions
This section addresses the four research questions:
-
In adults, young people and children who receive emergency treatment for suspected anaphylaxis, which people are at high risk of anaphylactic episodes? For which people would further anaphylactic episodes have significant impact? Which people can be identified as needing special consideration?
-
What are the effects of history-taking, including signs and symptoms, and physical examination in identifying the possible cause?
-
What are the effects of providing adrenaline auto-injectors, including by whom?
-
After assessment, when should referral take place?
Identification of studies
The evidence reviews used to develop the guideline recommendations were underpinned by systematic literature searches, following the methods described in ‘The guidelines manual’ (2009). 1 The aim of the systematic searches was to comprehensively identify the published evidence to answer the review questions developed by the Guideline Development Group (GDG) and Short Clinical Guidelines Technical Team.
The search strategies for the review questions were developed by the information specialist with advice from the systematic review team. Structured questions were developed using the PICO (population, intervention, comparison, outcome) model and translated into search strategies using subject heading and free-text terms. The strategies were run across a number of databases, with no date restrictions applied to the searches.
The NHS Economic Evaluation Database (NHS EED) was searched for economic evaluations. A search filter for economic evaluations was used on bibliographic databases. There were no date restrictions applied to the searches.
The searches were undertaken between 17 January and 17 March 2011.
Scoping searches
Scoping searches were undertaken in January 2011 using the following websites and databases (listed in alphabetical order) shown in Table 1; browsing or simple search strategies were used. The search results were used to provide information for scope development and project planning.
| Systematic reviews/economic evaluations | Guidance/guidelines |
|---|---|
| Cochrane Central Register of Controlled Trials (CENTRAL) | Guidelines International Network (GIN) |
| Cochrane Database of Systematic Reviews (CDSR) | National Guidelines Clearinghouse |
| Database of Abstracts of Reviews of Effects (DARE) | |
| Health Technology Assessment database (HTA) | |
| NHS Economic Evaluation Database (NHS EED) |
Main searches
The following sources were searched for the topics presented in the sections below:
-
Cochrane Database of Systematic Reviews (CDSR) (Wiley)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley)
-
Database of Abstracts of Reviews of Effects (DARE) [Centre for Reviews and Dissemination (CRD)]
-
Health Technology Assessment database (HTA) (CRD)
-
NHS Economic Evaluation Database (NHS EED) (CRD)
-
Science Citation Index (SCI) (Web of Science)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost)
-
EMBASE (OvidSP)
-
MEDLINE (OvidSP)
-
MEDLINE In-Process & Other Non-Indexed Citations and MEDLINE Daily Update (OvidSP).
Identified references were downloaded in EndNote X4 software (Thomas Reuters, CA, USA) for further assessment and handling.
Inclusion and exclusion criteria
Participants
Adults, young people and children who received emergency treatment for suspected anaphylaxis or severe allergic reactions (that may have developed into anaphylaxis without treatment).
Setting
Relevant settings were primary, secondary or tertiary care.
Interventions/diagnostic assessments
-
History-taking.
-
Physical examination.
-
Provision of adrenaline auto-injectors.
-
Referral to specialist allergy clinics.
Comparators
-
Elements of history-taking compared with each other and compared with not considering those elements.
-
Elements of physical examination compared with each other and compared with not considering these elements.
-
Provision of auto-injectors by different health-care professionals.
-
No provision of adrenaline auto-injectors.
-
Referral to other specialists.
-
No referral.
Outcomes
Any or all of the following outcomes were considered:
-
impact of testing/predictors on clinical outcome, (e.g. subsequent episodes, morbidity, mortality), correlations between tests and clinical outcomes
-
impact of adrenaline auto-injectors on clinical outcome (e.g. subsequent episodes, morbidity, mortality)
-
impact of referral on clinical outcome (e.g. subsequent episodes, morbidity, mortality)
-
indeterminacy (test failure rate)
-
impact of testing/predictors on treatment plan (e.g. referral or not or to whom), where information on the appropriateness of the final treatment plan is also reported.
For included studies reporting any of the above outcome measures, the following outcomes were also considered if reported:
-
acceptability of tests to patients
-
adverse events associated with testing.
Study designs
The following types of studies were included:
-
randomised controlled trials (RCTs) or non-RCTs
-
observational studies reporting change to treatment plan or clinical outcome subsequent to intervention or testing
-
prognostic studies that have included a multivariable analysis (evaluating risk factors or signs in an analysis that includes other relevant factors or signs, rather than an unadjusted correlation).
The following study/publication types were excluded:
-
pre-clinical, animal studies
-
reviews, editorials, and opinion pieces
-
case reports
-
studies reporting only technical aspects of the test
-
studies with < 20 participants.
Data abstraction strategy
Included studies were summarised using evidence tables for prognostic studies (see appendix K3 of the NICE guidelines manual). 1 These tables can be found in Chapter 3 (see Results). Extraction of one reviewer was checked by another. Furthermore, Grading of Recommendations Assessment, Development and Evaluation (GRADE) summary of findings tables2 were prepared. Any disagreement was discussed with a third reviewer.
Critical appraisal strategy
Quality and strength of evidence of included studies was assessed using the methodology checklist for prognostic studies (see appendix J of the NICE guidelines manual). 1 These tables can be found in Appendix 2. In addition, quality was assessed using the GRADE methodology. 2
Methods of data synthesis
Not applicable.
Results
Quantity and quality of research available
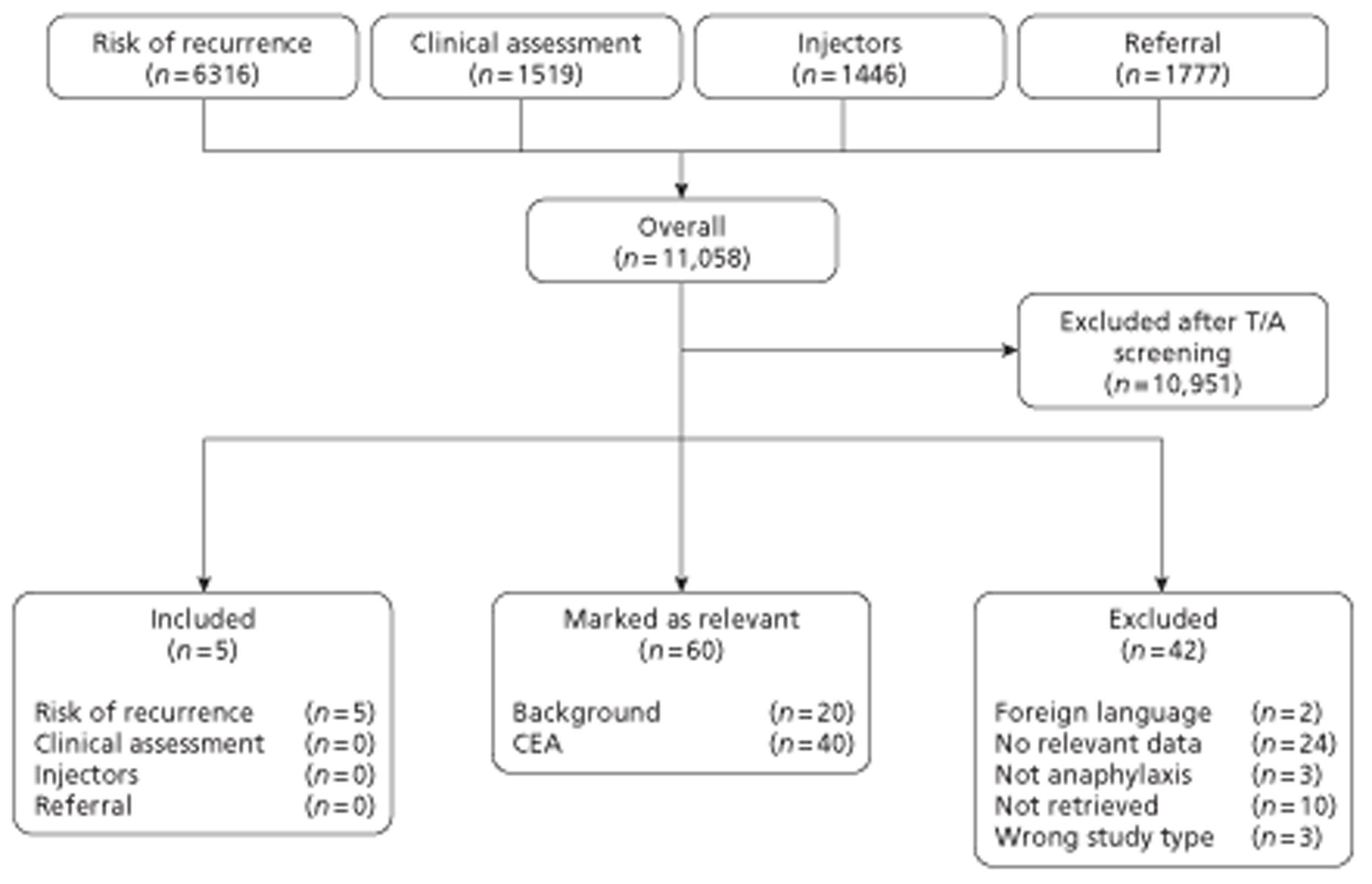
The searches of electronic searches yielded in 11,058 references. After screening of titles and abstracts, 10,951 references were excluded. The remaining 107 references were obtained and the full texts were screened. Five studies were included, with another 60 studies highlighted as possibly being relevant for the background and/or the cost-effectiveness analysis (CEA). A flow chart of the screening process is presented in Figure 1.
FIGURE 1.
Flow chart of study identification. T/A, title and abstract.
Assessment of clinical effectiveness
All five included studies were prospective observational studies reporting on risk of recurrence. 3–7 The studies, conducted in five countries (Australia, Germany, Italy, Spain and the USA), included 1725 patients overall.
The risk of bias using the NICE methodology checklist for prognostic studies1 was rated as low for three studies3,4,7 or medium (two studies)5,6 (Table 2). Two of the studies were published only as abstracts, which limited the amount of methodological details reported in these studies. 4,6 Overall, problems included unclear definition of recurrence (three studies),4,5,7 unclear patient selection (one study),3 insufficient details on role of funding source (one study)5 and missing details on included patients (one study). 6 The quality assessment is presented in Appendix 2.
| Study | Bibliographic reference | Study type | Study quality | Outcome measures | Length of follow-up | Source of funding |
|---|---|---|---|---|---|---|
| Cianferoni 20043 | Cianferoni A, Novembre E, et al. Anaphylaxis: a 7-year follow-up survey of 46 children. Ann Allerg Asthma Immunol 2004;92:464–8 | Observational retrospective | Low risk of bias, but unclear how patients were selected | Recurrence defined as the presence of another anaphylaxis episode: at least two of the main indicators of anaphylactic reaction (hypotension, inspiratory dyspnoea, and urticaria/angio-oedema) within 2 hours after exposure to one of the most probable causative agents | 7 years (SD 1 year, range 5–8.6 years) | N/R |
| Defined risk factors for recurrence: history of atopic dermatitis, current urticaria/angio-oedema, history to sensitivity to one food allergen | ||||||
| Decker 20084 | Decker KW, Bellolio MF, et al. Recurrent anaphylaxis events in patients presenting to the emergency department over a 10-year period. Ann Emerg Med 2008;51: 214 | Observational prospective | Low risk of bias, but no definition of recurrence given | No details provided | Mean 1.1 years (range 7 days to 13 years) | N/R |
| Mehl 20055 | Mehl A, Wahn U, et al. Anaphylactic reactions in children: a questionnaire-based survey in Germany. Allergy 2005;60:1440–5 | Observational retrospective | Medium risk of bias as no definition of recurrence was given. Role of funding source unclear | Questionnaire covering demographic data, symptoms and physical findings of the episode, place of occurrence, suspected allergen, diagnostic tests, treatment modalities, such as use of drugs, route of application, and drug-administering person, hospitalisation and prescribed emergency set after the episode | 1 year (patients identified over a period of 12 months retrospectively) | Industry: InfectoPharm Arzneimittel und Consilium GmbH, Heppenheim, Germany (‘financial support’) |
| Múgica Garcia 20106 | Múgica Garcia M, Tejedor Alonso M, et al. (2010). A study of the recurrence of anaphylaxis. Allergy: European Journal of Allergy and Clinical Immunology. 29th Congress of the European Academy of Allergy and Clinical Immunology, EAACI London, UK. Conference Publication: (various pages). 65 (p. 587), 2010. Published June 2010 | Observational retrospective | Medium risk of bias as only 58.7% of previous cohort was included and no details on age, sex, weight and ethnicity were reported | Recurrence defined as any new episode of anaphylaxis, irrespective of the cause of the first episode and whether the recurrence was the same or different | N/R | N/R |
| The recurrence of the same subtype of anaphylaxis was considered when the same subtype of anaphylaxis (e.g. food, drugs, exercise) was responsible for both the first episode and the recurrence | ||||||
| Mullins 20037 | Mullins RJ. Anaphylaxis: risk factors for recurrence. Clin Exp Allergy 2003;33:1033–40 | Observational prospective | Low risk of bias, but no definition of recurrence given | Recurrence presented as proportion of patients relapsing | 2.2 years | N/R |
| Rate of recurrence per 100 patient-years of observation: calculated by dividing the cumulative length of observation by the number of recurrences involving that trigger |
Using the GRADE methodology,2 quality of evidence extracted from the included studies was rated as ‘very low’. It should be noted that using the GRADE approach quality of evidence from observational studies is initially rated as ‘low’. During further assessment, certain areas can lead to upgrading or downgrading of the quality. Application of the GRADE methodology to the included studies is shown below (see Table 4). Each row of the table reports on outcomes that are addressed by included studies, and highlights problems with any of the included studies in relation to each outcome. Footnotes identify specific threats to validity identified. As can be seen in the table, the main reasons for downgrading of included evidence were missing details on blinding, as well as size of studies, i.e. number of included participants. Readers should note that the different systems of identifying bias (NICE methodology checklist for prognostic studies vs GRADE) yield slightly different conclusions on the levels of threat to validity and therefore the quality of studies is described differently (see Tables 2 and 4).
All included studies reported the number of patients with recurrent anaphylactic episodes. Risk of recurrence was estimated to be between 30% and 42.8%. Overall, 497 of 1386 patients (35.9%) had a recurrent anaphylactic episode (see Table 4). One study suggested the rate of a third event to be 5.2%, with a higher risk of recurrence for women [relative risk (RR) 2.14, 95% confidence interval (CI) 1.17 to 3.9]. 4 In children of < 12 years, an overall recurrence of 27% was reported with food being the most frequent allergen (71%). 5 One larger study (432 patients) reported serious recurrences in 45 patients (10.4%) of whom 18 (40%) received adrenaline. 7 This study also presented findings on mortality and reported no deaths. 7 Another study presented results for sex, age, and race (see Table 4 for details). 4
Characteristics and findings of the included studies are presented below. Table 2 shows characteristics of the five included studies. The findings of these studies are presented in Tables 3 and 4.
| Study | Setting | No. of patients | Patient characteristics | Results |
|---|---|---|---|---|
| Cianferoni 20043 | Primary care, Italy | 46 (of 76 from a previous cohort study, re-evaluated after a mean of 7 years) | Diagnosed anaphylaxis. Mean age 14 (SD 4.92, range 7–26) years | Risk of recurrence: 30% (14/46) |
| Inclusion for previous study: patients with anaphylaxis referred to an allergy unit (Florence, Italy) who had at least two of the main indicators of anaphylactic reaction (hypotension, inspiratory dyspnoea, and urticaria/angio-oedema) within 2 hours after exposure to one of the most probable causative agents | Age at first episode: 5.8 (SD 4.9, 1–18) years | |||
| 61% male. No details on weight and ethnicity | ||||
| Aetiology: food 19.5% (9/46), exercise 4.4% (2/46), drug 2.2% (1/46) and idiopathic 4.4% (2/46) | ||||
| Decker 20084 | Primary care, USA | 211 (visiting an ED) | Mean age: 29.3 (SD 18.2) years. 44.1% male. No further details | Second event in 45/211 (21.3%). Median time of presentation: 395 days (range 7 days to 13 years). Third event in 11/211 (5.2%) |
| Diagnosed anaphylaxis criteria from the National Institutes of Health/Food and Allergy and Anaphylaxis network | ||||
| Risk of recurrence for women higher (RR 2.14, 95% CI 1.17 to 3.9). No difference in age (p = 0.535) or race (p = 0.743) for a subsequent event | ||||
| Mehl 20055 | Primary care, Germany | 103 children (< 2 years) | Median age 5 years (range 3 months to 12 years) | ‘No significant difference was found for allergens looking only at severe reactions (grades III and IV)’ (no data reported) |
| Inclusion: reported accidental anaphylactic reactions occurring during 12 months in infants and children of < 12 years of age. Reports reviewed individually by two paediatric allergologists | 58% male | Age differences:
|
||
| No details on weight and ethnicity | ||||
| Causative allergen was known or strongly suspected in 95/103 (92%) of all patients | ||||
| Exclusion: reported cases excluded if the reported episode was not accidental (e.g. occurred after diagnostic provocation) or if the patient was not < 12 years | Overall: food 57% (59/103), insect sting 13% (13/103), SIT 12% (12/103), medication 6% (6/103), othera 4% (4/103) and unknown 8% (9/103) | |||
| Foods only: 57% (59/103): peanut 20% (12/59), tree nut 20% (12/59), cow's milk 14% (8/59), fish 14% (8/59), hen's egg 7% (4/59) and othera 25% (15/59) | ||||
Recurrence:
|
||||
| Same allergen as episode(s) in medical history 50% (14/28) | ||||
| Múgica Garcia 20106 | Primary care, Spain | 933 (original cohort of 1590) | Diagnosed anaphylaxis. Mainly urban community. No details on age, sex, weight and ethnicity | Overall risk 325/933 (34.8%) |
| Presented anaphylaxis and were followed in allergy unit (no further details) | Same type as first episode: | |||
|
||||
| Mullins 20037 | Primary care, Australia | 432 patients referred for evaluation of possible anaphylaxis to community-based specialist medical practice between February 1995 and July 2000 | Mean age 27.4 (SD 19.5, range 1–82) years 48% male | 130/304 (42.8%) have experienced 386 episodes of recurrent symptoms (median 2, range 0–18) |
| No details on weight and ethnicity | Risk of overall recurrence: 57/100 patient-years; risk of severe recurrence: 10/100 patient-years | |||
| First episode during study course/before study: 71%/29% | Risk factors for recurrence: exercise and idiopathic cause, female sex | |||
| No deaths | ||||
| Serious recurrences: 10.4% (45/432); had adrenaline: 40% (18/45) | ||||
| No serious recurrences: 19.7 (85/432); had adrenaline: 1.2% (1/85) |
| Quality assessment | Summary of findings | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients with recurrent anaphylactic episodes (unless stated otherwise) | Quality | |||||||
| No. of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | n/N | |
| No. of patients with recurrent anaphylactic episodes (follow-up mean 1–7 yearsa) | ||||||||
| Five (all included studies3–7)b | Observational studiesc | Seriousd | No serious inconsistency | No serious indirectness | Seriouse | None | 497/1386 (35.9%) | ⊕000 VERY LOW |
| Mortality: no. of patients who died owing to anaphylactic reactions (follow-up mean 2.2 years) | ||||||||
| One7 | Observational studies | Seriousf | No serious inconsistency | No serious indirectness | No serious imprecision | None | 0/304 (0%)g | ⊕000 VERY LOW |
| No. of patients with second recurrent anaphylactic episodes (follow-up mean 1.1 years) | ||||||||
| One4 | Observational studies | Seriousf | No serious inconsistency | No serious indirectness | Serioush | None | 45/211 (21.3%) | ⊕000 VERY LOW |
| No. of patients with third recurrent anaphylactic episodes (follow-up mean 1.1 years) | ||||||||
| One4 | Observational studies | Seriousf | No serious inconsistency | No serious indirectness | Serioush | None | 11/211 (5.2%) | ⊕000 VERY LOW |
| Sex comparison for anaphylactic recurrent episodes (follow-up mean 1.1 years) | ||||||||
| One4 | Observational studies | Seriousf | No serious inconsistency | No serious indirectness | Very serioush,j | None | RR 2.14 (95% CI 1.17 to 3.9)k | ⊕000 VERY LOW |
| Age comparison for anaphylactic recurrent episodes (follow-up mean 1.1 years) | ||||||||
| One4 | Observational studies | Seriousf | No serious inconsistency | No serious indirectness | Very serioush,j | None | p = 0.535j,1 | ⊕000 VERY LOW |
| Race comparison for anaphylactic recurrent episodes (follow-up mean 1.1 years) | ||||||||
| One4 | Observational studies | Seriousf | No serious inconsistency | No serious indirectness | Very serioush,j | None | p = 0.743j,m | ⊕000 VERY LOW |
No studies that fulfilled inclusion criteria for objectives 2–4 were identified.
Summary
Overall, five prospective observational studies reporting on risk of recurrence were included. 3–7 No studies were found for the questions on history-taking, adrenaline auto-injectors, and referral.
The included studies reported a recurrent anaphylactic episode for 497 of 1386 patients (35.9%), indicating that recurrent episodes are relatively common for anaphylactic patients (see Table 4). Findings of single studies suggested that women have a higher risk of recurrence. Around one-quarter (27%) of recurrences in children of < 12 years are caused by food.
Limitations and implications for future research
Although a comprehensive search was undertaken to identify relevant studies (see Quantity and quality of research available), only five studies were included (see Assessment of clinical effectiveness). All of these studies are observational studies with low or medium risk of bias assessing the risk of recurrence. The studies were relatively small (1725 patients) and assessed the risk of recurrence in various patient groups. This should be taken into account when formulating recommendation based on these studies.
No studies addressing any of the other clinical research questions in terms of history-taking, physical examination, provision of adrenaline auto-injectors or referral to specialist allergy clinics for those with anaphylaxis were identified.
Lack of good data to inform the effectiveness of anaphylaxis interventions means that RCTs or at least well-designed observational studies of the components of care in SSs should be conducted. Ideally, these should report findings based on large numbers of participants, if possible divided into relevant subgroups.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
A search strategy was designed in order to retrieve any economic evaluation or cost study in the population of allergy or anaphylaxis (refer to Appendix 1 for how this was applied to each database). Forty papers were retrieved from title and abstract screening and three met the inclusion criteria for design and population.
Two studies8,9 were published that reported on economic evaluations in the form of decision-analytic models (DAMs) of the use of AIs (n = 2) in a general allergy population8 and in patients with a mild venom anaphylaxis9 in the USA. Another American study evaluated the treatment and its related costs in patients with idiopathic anaphylaxis. 10 All studies (Table 5) reported the costs in US dollars (US$). To assess the quality of reporting of these economic evaluations the British Medical Journal (BMJ) checklist was used, including 35 items (http://resources.bmj.com/bmj/authors/checklists-forms/health-economics). The BMJ checklist showed that 11 out of 35 criteria were satisfactorily reported for the study by Krasnick et al. ,10 and 18 out of 35 for the study reported by Shaker 2007. 9 The study published by Desai and Carroll 20098 was reported only as a congress abstract, which unsurprisingly resulted in many missing sections of the BMJ checklist (30 out of 35). Full details are in Appendix 4.
| Study | Design | Population | Comparators |
|---|---|---|---|
| Krasnick et al. 201010 | Cost description | Idiopathic anaphylaxis | Before AI implementation compared with after AI implementation |
| Shaker 20079 | DAM for CEA | Children with mild venom anaphylaxis | Treatment of mild venom anaphylaxis with AI compared with treatment of mild venom anaphylaxis without AI use |
| Desai and Carroll 20098 | DAM | Users of AI | Conventional AI [EpiPen (Meda Pharmaceuticals Ltd, Bishop's Stortford, UK) compared with a new AI device (Intelliject, Intelliject Llc., Richmond, VA, USA)] |
In the following paragraphs the details of the three studies are presented.
Krasnick et al. 1996
This study10 was designed to determine the efficacy of a specialist treatment in a University Allergy-Immunology Division using oral corticosteroids, antihistamines, and sympathomimetics for patients with idiopathic anaphylaxis. A total of 225 patients, diagnosed with idiopathic anaphylaxis and treated in one university hospital from 1971 to 1990, were retrospectively reviewed. The costs of both emergency care [physician fees, medications (intravenous corticosteroids, subcutaneous adrenaline and intramuscular diphenhydramine), pulse oximetry and cardiac monitoring] and hospitalisation (general medical floor hospital admission and intensive care unit admission with and without need of intubation and mechanical ventilation) were estimated on the basis of costs of services at Northwestern Memorial Hospital, Chicago, IL, USA, during the year 1995 (no details on unit costs were reported). Optimal discriminant analyses (ODAs) were used to determine whether or not the treatment protocol made a significant decrease in hospital costs for four subgroups of patients with idiopathic anaphylaxis. Significant decreases in emergency room visits occurred for three of the four subgroups of patients with idiopathic anaphylaxis. Significant decreases in the number of hospitalisations (p< 0.022) and intensive care unit admissions (p< 0.009) occurred for the patients with idiopathic anaphylaxis with generalised symptoms (two subgroups). Overall, there were 165 emergency room evaluations, 17 hospitalisations and 18 intensive care unit admissions (five admissions requiring intubation) before patients received the specialist treatment at a cost of US$225,000. There were 51 emergency room visits, three hospitalisations, and no intensive care unit admissions after patients received the SS at an estimated cost of US$40,260, producing a saving of US$184,740.
Shaker 2007
This study9 was designed to evaluate the cost-effectiveness of the prophylactic self-injectable adrenaline in mild childhood venom anaphylaxis from a societal perspective, although the only cost data included in the model were the market costs of an AI (US$50 per year). A Markov model evaluated two scenarios: one using an AI and another not using an AI for the treatment of venom anaphylaxis. The base case in each scenario was represented by a 6-year-old child. The year ‘2007’ was used as the baseline cost year and a discount rate of 3% was used for future costs and years. Literature sources were used to estimate mortality but the model assumed that all deaths would be prevented by the AI, regardless of time between trigger and death or success in use. One-way sensitivity analysis was performed of the following parameters: age, fatality rates of anaphylaxis and duration of use of AI after prescription.
The main findings were as follows: the incremental cost of prophylactic AI for mild childhood venom anaphylaxis was US$469,459 per year of life saved and US$6,882,470 per death prevented when evaluated at a 40-year time horizon. The sensitivity analysis revealed that the use of AI might become cost-effective at US$97,146 per life-year saved only if the annual fatality rate exceeded 2 per 100,000 persons at risk. The conclusion of this study was that the use of prophylactic AI to prevent fatalities in children with mild venom anaphylaxis is not cost-effective if the annual venom-associated fatality rate is < 2 per 100,000 persons at risk. The source of financial support of this study was not reported.
Desai and Carroll 2009
This study8 compared the costs and consequences of using an established device (probably the EpiPen) compared with a novel device (Intelliject) for treatment of a uniphasic anaphylactic reaction. The decision tree model evaluated the two scenarios from a health-payer perspective, but no information was provided on the baseline cost year, length of the time horizon and a discount rate used. The consequences included recovering without visiting the emergency department (ED), ED use and hospitalisations. The costs included in the model were costs of device use, ED use and hospitalisations. Data were obtained from literature, an online query tool for health care cost (HCUPnet) and clinical study data of the company that developed the new AI (Intelliject Inc., Richmond, VA, USA). One-way sensitivity analyses were conducted for patients' probabilities of carrying the device, using it correctly and of recovery and death after using the device incorrectly. The base-case results per 100 patients indicate that the new device would lead to more patients recovering without visiting the ED (57 vs 35), similar rates of ED use without hospitalisation (7) and fewer hospitalisations (2 vs 4). The results also indicated higher device costs (US$15,837 vs US$6291) and the same ED use costs (US$9375), but lower costs for hospitalisations (US$15,303 vs US$30,606), leading to lower total costs of the new device (US$40,515 vs US$46,272) (no statistical analyses on outcomes and costs were reported). Sensitivity analyses indicated that the new device would have lower total costs and lead to better consequences under most tested assumptions. The authors stated that the assumed price premium (not reported) of the new device provided lower total costs, and a higher recovery rate, as well as fewer hospitalisations.
Summary
None of these studies is useful in directly addressing the questions regarding SSs. However, the study by Krasnick et al. 10 does provide useful data in terms of the time to remission in idiopathic anaphylaxis and this is used in the de novo CEA described below. The study by Shaker9 does address the question regarding AI but the model is too simplistic, assuming that protection is guaranteed. Also, the population is those who have had a ‘mild’ reaction, which is not directly comparable with our definition of anaphylaxis, which is life-threatening. The study by Desai and Carroll 20098 was unfortunately too poorly reported to be useful.
Methods of cost-effectiveness analysis
Research questions
The analysis aimed to inform the following two questions:
-
What is the cost-effectiveness of referral to specialist allergy clinics for the diagnosis of anaphylaxis and for the prevention of future episodes and the reduction in morbidity and mortality from future episodes?
-
What is the cost-effectiveness of adrenaline auto-injectors for the treatment of anaphylaxis including the cost implications of training in the use of the auto-injectors?
Population
The population of interest is all patients with anaphylaxis (irrespective of the cause) who needed emergency treatment.
However, as the title ‘… suspected anaphylaxis’ suggests, there is a problem with diagnosis,11 which includes the definition of anaphylaxis. For example, Stewart and Ewan12 use the term ‘severe’ anaphylaxis and associate it with loss of consciousness or fainting. On this basis, they count 9 out of 55,000 emergency admissions. They then included 15 others to make 24 with ‘generalized reactions involving hypotension and/or respiratory difficulty’. The rate of referral to SSs was [through the general practitioner (GP)] 4 out of 24. In a study by El-Shanawany et al. 13 in Wales, the 77 cases identified in 6 months implied a rate out of a population of about 500,000 of 30.8 per 100,000 people-years. This was much higher than the 6.7 in the UK previously estimated by Sheikh et al. 14 However, a more recent study in the UK by Gonzalez-Perez et al. 15 produced an estimate of 34.38. The El-Shanawany study13 also revealed that the rate of referral to SSs was zero. Erlewyn-Lajeunesse et al. 11 selected cases of asthma, and urticarial and allergic reaction, as well as anaphylaxis according to physician diagnosis (in the absence of a gold standard) to test diagnostic criteria. This could imply that the suspected population is composed essentially of those suffering an allergic reaction albeit less severe as well as those with asthma. However, this Guideline definition rules this out by including: ‘… rapidly developing life-threatening airway, breathing and/or circulation problems …’ (http://www.nice.org.uk/nicemedia/live/12346/52120/52120.pdf, p. 1).
This fits with the definition used by Brown et al. 16 and, therefore, implies that, in the absence of a known trigger, other conditions that cause such life-threatening problems might be included in the population and might thus be referred to SSs. Indeed, in the absence of further information on the nature of those patients seen in a SS, an increase in referral, as is being considered, might actually increase the prevalence of patients not suffering from anaphylaxis.
However, in the latest UK guidelines for emergency treatment17 there is a recommendation that all of those who are suffering from anaphylaxis should be referred to a SS and there is no mention of any difficulty in diagnosing anaphylaxis. Indeed, the suggestion is that the diagnosis of anaphylaxis has been made in the vast majority of cases by discharge, other possible diagnoses having been ruled out. It is on this basis that the comparison is between SSs and standard care (SC), given definite diagnosis of anaphylaxis.
Comparators
The following combinations were considered in the model:
-
SC, no AI: SC plus no prescription of AIs where SC is defined as the absence of referral to a SS. It is not defined any further but is expected to consist of no more than GP consultation. AIs come in the form of either EpiPen or Anapen (Lincoln Medical Ltd, Salisbury, UK) [British National Formulary (BNF) no. 6118] and in several doses, recommended as 500, 300 and 150 g for adults, children aged 6–12 years and children aged < 6 years, respectively. 17 There is little variation in cost and so the cost of AI was based on the current cost of EpiPen of £26.45 (note that this value includes a price reduction that, at time of writing, had not been reflected in BNF 6118). Based on expert opinion, it was assumed that AIs should be replaced every 12 months, that adults require two AIs at any one time, and that children require four (because it is common practice to keep two at school and two at home).
-
SC plus AI: Injectors are recommended in the latest guidelines by the Resuscitation Council UK, to be prescribed for all patients with ‘… life-threatening features’ (p. 162). 17
-
SS no AI: All patients with suspected anaphylaxis are referred to a SS in accordance with the same guidelines: ‘All those who are suspected of having had an anaphylactic reaction should be referred to a specialist in allergy’ (p. 158). 17 The same guideline goes on to state: ‘All patients presenting with anaphylaxis should be referred to an allergy clinic to identify the cause, and thereby reduce the risk of future reactions and prepare the patient to manage future episodes themselves’ (p. 166). 17
-
SS plus AI: All patients both attend a SS and are prescribed AIs.
Framework
Given the lack of CEA evidence, a cost–utility analysis19 was undertaken with costs and quality-adjusted life-years (QALYs) considered over patients' lifetimes from a UK NHS perspective in accordance with NICE methods guidance. 20 Costs were in 2011 GB pounds (£) and an annual discount rate of 3.5% was used. Despite these treatments being for short-term use, a lifetime horizon is most appropriate to capture the full impact of treatment.
Model structure
A Markov model21 was constructed with mutually exclusive health states. The model simulated the course of events in a hypothetical cohort of persons with anaphylaxis who had been treated in an emergency care setting in the UK, aged ≥ 6 years. The model initially divides the cohort according to their relative incidence (referred to as ‘trigger probability’), into the four main causes of anaphylaxis: drugs (including medication, biologics, vaccines and anaesthetics), insects (stings), food and idiopathic origin11 (see Trigger probability). In the model, as time progresses, persons move from one state to another state according to a set of transition probabilities (see sections on model parameters below: Rate of recurrence, Mortality rate, Idiopathic treatment and Venom immunotherapy). The cycle length of the model was set to 3 months.
A cycle length of 3 months was chosen for convenience in modelling rates of recurrence as probability of a single recurrence event, as it can be shown that the longer the period the greater the error. Intuitively, this can be understood by considering that the longer the period then the greater the probability of more than one event occurring. For example, using the probability density function of the Poisson distribution, the probability of one event in 3 months with an annual rate of 0.28 (that of idiopathic cause, which is the highest of all causes used in the model) is 0.065. Although actually more than one event could occur in this time, the probability of two events is only 0.002 and that of more events is extremely small at only about 0.00005. Given the large amount of uncertainty in all parameter estimates, it was believed to be acceptably close and all other rates (for food, drug and insect causes) are no larger than about 0.12, which produces even less of an error. A shorter cycle length could have been used but there would still have been an error, although smaller, and this would have only increased model calculation time.
The health states are ‘death’, ‘at risk’ (of recurrence), ‘recurrence’ and, for idiopathic cause only, ‘remission’ (Figure 2). All members of the cohort begin in the ‘at-risk’ state and move in the next 3 months to the ‘recurrence’ state, with a probability according to the rate of recurrence (see explanation above), except if the cause was not known (i.e. idiopathic cause), where recurrence could occur only if remission had not.
FIGURE 2.
Diagram showing the health states and transitions between them; transitions can occur from any live state to the death state.
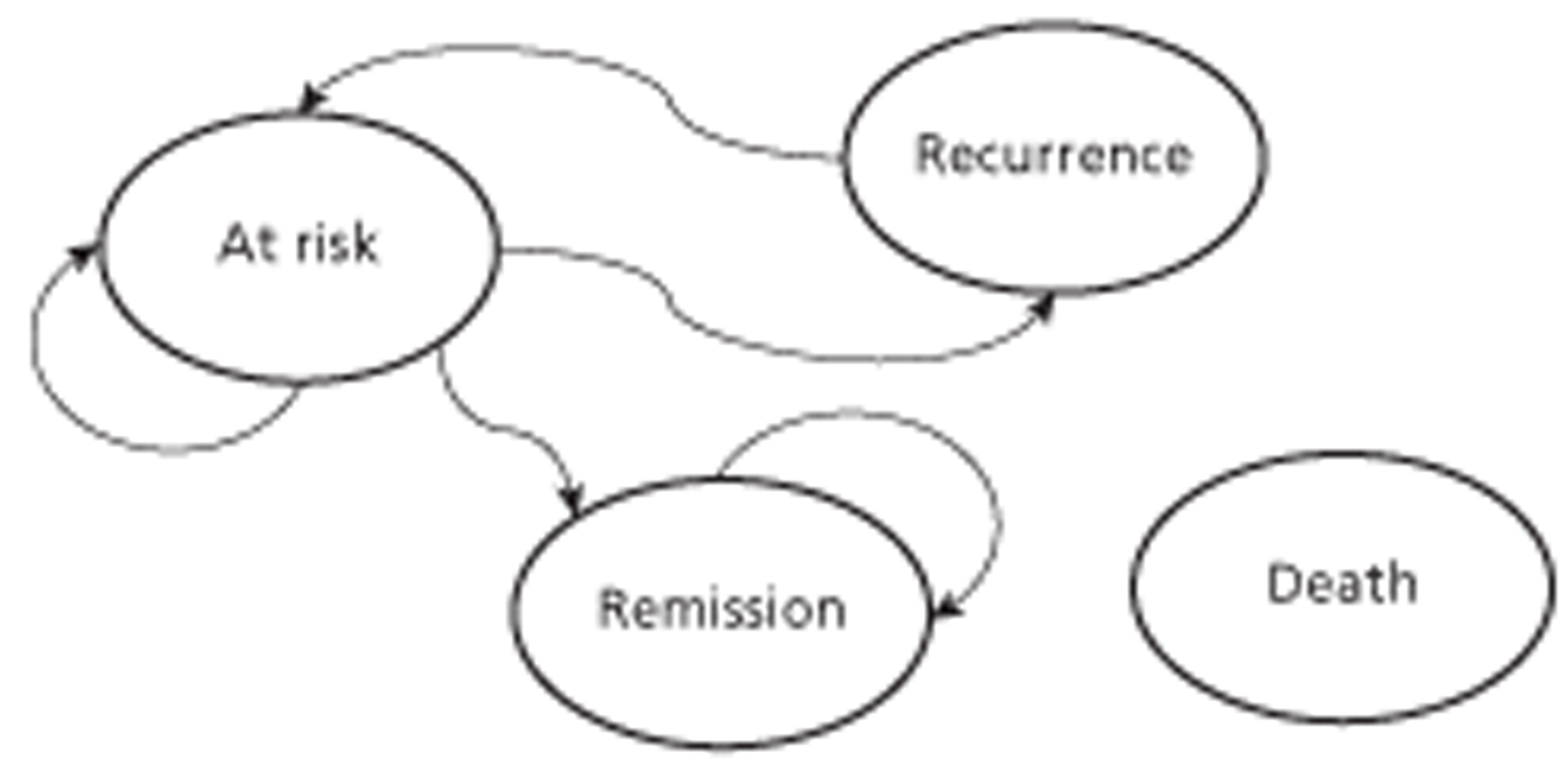
Those in the ‘at-risk’ or ‘remission’ states (idiopathic and insect only) were assumed to have general population age and sex-specific mortality. 22 Those in the ‘recurrence’ state had this mortality plus an additional probability. First, they were divided into those who used an AI or not, according to a probability for correct use (see Mortality). For both SS and SC plus AI, this probability was greater than zero, as all patients were assumed to be prescribed two injectors, each of which has a 6-month life. It was then assumed that all would continue be supplied and thus incur the cost until death, unless there was remission. In the ‘no AI comparators’ the probability was zero.
Under SC, unless there was remission (idiopathic and insect only), the recurrence rate was assumed to be constant. This was based on a lack of evidence to the contrary presented in any of the guidelines or the systematic review. The effectiveness of SSs, therefore, was partly mediated by a change in recurrence rate, which depends on trigger and is explained below.
Food and drug
Based on the various guidelines and expert opinion it was assumed that the effect of SSs on recurrence was mediated by the identification and then advice to avoid the trigger, which then reduced the rate of recurrence.
Idiopathic
The possibility of remission for idiopathic was based on two international guidelines,23,24 in which it is suggested that it will occur spontaneously, although those patients classed as having ‘frequent’ recurrences (more than two in 2 months or more than six in 1 year) are recommended to be prescribed prednisolone. It was therefore assumed that the effect of SSs on recurrence was mediated by treatment (with prednisolone) of those suffering from frequent episodes of recurrence (see Idiopathic treatment). This implies an advantage of SSs over SC, as with SSs remission can occur in both the frequent and the infrequent, whereas with SC remission was assumed to occur only in the infrequent.
Insect
It was assumed that effect of SSs on recurrence was mediated by remission due to identification and then treatment with venom immunotherapy (VIT) in accordance with an international guideline,25 guided by expert opinion as to regime (see Venom immunotherapy). This involved a total period of treatment of about 3 months with an initial ‘build-up’ phase of about 10 weeks. Not everyone is offered this treatment: some refuse and some drop out. Therefore, the recurrence rate is a function of probability of uptake, dropout and effectiveness.
The effect of SSs was also mediated through greater compliance (correct use) of AIs on the basis that training should be better, and thus reduced mortality.
Finally, the effect of both SSs and AI also included an increase in utility in the ‘at-risk’ state in order, in accordance with expert opinion, to capture the general improvement in well-being.
Parameterisation
All parameter values were estimated using the best evidence available and according to best practice. 20,26 Unfortunately, the systematic review revealed only few and generally poor-quality studies on rates of recurrence by trigger and none comparing the effectiveness of SSs versus SC or the effectiveness of AIs (by any measure, e.g. reduction in rate of recurrence), which is confirmed by other recent reviews. 17,27–29 All other parameter estimates were chosen in order to be as UK relevant as possible, based on evidence that was either directly cited by recent UK or international guidelines or found by citation searching from these sources. This method was chosen in order to maximise the efficiency of obtaining high-quality relevant estimates.
In accordance with best practice and the principle that expert opinion proxies for the beliefs of the decision-maker, which, in effect, is NICE, expert opinion from the GDG was sought for all parameters. This was done either to provide an estimate in the absence of evidence or to provide an estimate based where possible on the presentation of some evidence. Practically, it involved asking during a GDG meeting for consensus as to the ‘most likely’, ‘lowest’ and ‘highest’ values of parameters. In order to facilitate this, where possible data from the literature were presented and in these cases, the source is given as ‘expert opinion and based on [data]’.
Because the latest NICE guidance20 demands probabilistic sensitivity analysis (PSA),30 parameters to estimate distributions were also estimated. Where the source was deemed to be good enough the sampling distributions of the probabilities (beta for binomial and Dirichlet for multinomial) were used. 31 In most other cases, a triangular distribution was used, based on expert opinion elicited as the lowest, most likely and highest values. In order to make the expected value the same as the most likely, all triangular distributions were symmetrical. The table containing the estimates and summarising the sources is split into several tables between sections in order to facilitate explanation, although it is also presented in full in Appendix 5.
Population characteristics
For the population in the model the following two (Table 6, parameters 1–2) assumptions have been made: 50% of the patients in the model are male and the starting age is 30 years. Although there is a little variation between studies as to what age defines someone as a child, we assume that it is < 17 years.
| No. | Parameter | Name parameter in model | Distribution type | Base case | Sources |
|---|---|---|---|---|---|
| 1 | Cohort start age | startage | N/A | 30 | Assumption |
| 2 | Proportion of cohort male | pmale | N/A | 0.5 | HES 201032 (see General model assumptions) |
Rate of recurrence
For the model, the annual rate of recurrence of anaphylaxis caused by drugs after referral to SSs was based on expert opinions (Table 7, parameter 3). This rate will probably be very low based on the idea that it is very unlikely that the same drugs which caused the first anaphylactic reaction will be prescribed for the same patient again.
| No. | Parameter | Name parameter in model | Distribution type | Minimum | Most likely | Maximum | Sources |
| 3 | Annual rate of recurrence of anaphylaxis due to drugs with SSs | dprecurdrugSS | Triangular | 0 | 0.001 | 0.002 | Expert opinion |
| 4 | Annual rate of recurrence of anaphylaxis due to food with SSs | dprecurfoodSS | Triangular | 0 | 0.01 | 0.02 | Expert opinion and based on Ewan et al. 2001,33 p. 753, text |
| Paragraph heading: ‘Severity of follow-up reaction’ | |||||||
| No one with a severe initial reaction (n = 49) had a further severe reaction | |||||||
| Ewan et al. 200534 | |||||||
| Table 1, p. 112: Severe follow-up reaction grade 5 | |||||||
| r = 3 (0.5%), n = 567 (100%) | |||||||
| 5 | Annual rate of recurrence of anaphylaxis due to food with SC | drecurfood | Triangular | 0.05 | 0.11 | 0.16 | Expert opinion and based on Mullins 2003,7 figure 1, p. 1037 |
| 6 | Annual rate of recurrence of idiopathic anaphylaxis with SC | drecuridio | Triangular | 0.05 | 0.28 | 0.51 | Expert opinion and based on Mullins 2003,7 figure 1, p. 1037 |
| 7 | Annual rate of recurrence of anaphylaxis due to drugs with SC | drecurdrug | Triangular | 0.05 | 0.12 | 0.19 | Expert opinion and based on Mullins 2003,7 figure 1, p. 1037 |
| 8 | Annual rate of recurrence of anaphylaxis due to insect sting with SC | drecurinsect | Triangular | 0.05 | 0.10 | 0.15 | Expert opinion and based on Gonzalez-Perez 2010,15 pp. 1101–2 |
| Last paragraph, p. 1101: ‘Anaphylaxis is associated with high risk of recurrence but is highly unpredictable. Estimated rate: 0.06 to 0.11 episodes per year’ |
Parameter 4, the annual rate of recurrence of anaphylaxis due to food in SSs, was based on the data of two longitudinal prospective observational studies on the effectiveness of a management programme providing advice on nut avoidance and emergency medication in the UK. These two studies reported only three recurrences out of over 13,000 observation months, which is equivalent to a rate of about 0.003 per patient-year in adults and/or children who were diagnosed for peanut or tree nut. 33,34 However, these studies were not controlled trials. Furthermore, nut allergy patients are only a subgroup of all anaphylactic patients who will be referred after emergency treatment to specialist allergy care. Therefore, based on expert opinion, a more conservative estimate of 0.01 was chosen, although the minimum of 0 allowed for the possibility of very effective treatment.
Under SC, the most likely values for the annual rate of recurrence of anaphylaxis due to food or drugs and idiopathic (see Table 7, parameters 5 and 6) in current practice were based on the findings of a prospective study of 432 patients who were referred to a community-based specialist practice in Australia. 7 This was the only study from the systematic review that reported rates of recurrence by cause and the results had to be read off a graph (figure 1, p. 1037). The rate of anaphylaxis due to food was calculated by a combination of figures on incidence of anaphylaxis due to food and exercise-induced anaphylaxis (as these were not separated in the report) (Table 8).
| Food | Rate (no. of episodes per person at risk per year) | No. of persons at risk (n) | No. of episodes per year (r)a |
|---|---|---|---|
| Meat | 0 | 7 | 0 |
| Soy | 12 | 8 | 96 |
| Cow's milk | 11 | 19 | 209 |
| Crustaceans | 7 | 27 | 189 |
| Fish | 3 | 22 | 66 |
| Wheat plus exercise | 40 | 29 | 1160 |
| Fruit/vegetables plus exercise | 15 | 48 | 720 |
| Egg | 10 | 49 | 490 |
| Nuts | 9 | 112 | 1008 |
The average across all foods was calculated by dividing the total annual number (sum across all r per year in the table) by the total number at risk (summing across all n in the table). The annual rate of recurrence of anaphylaxis due to insect sting (see Table 7, parameter 8) was based on the findings of the most recent (2010; 343 with anaphylaxis) UK study (Gonzalez-Perez et al. 15), as figures for the Australian population are not likely to resemble those for the UK population because the risk of experiencing an insect bite or sting is much higher in Australia than in the UK. Gonzalez-Perez et al. 15 reported a range from about 0.05 to 0.1 for any cause and so, given expert opinion, the higher rate was chosen as the most likely.
Based on expert opinion, 0.05 was chosen as the lowest value for all causes and the highest value followed from making the distributions symmetrical.
Trigger probability
As stated in literature, it is difficult to calculate the exact incidence rates of anaphylaxis as a result of difficulties with coding, diagnosis and reporting (Sampson et al. 35) and actual rates remain unclear.
In the model, the figures for probability of anaphylaxis due to insect sting and idiopathic anaphylaxis (Table 9, parameters 9 and 10) were estimated based on a 1-year study analysing The Health Improvement Network (THIN) database on 2.3 million patients (age 10–79 years) who had been enrolled with a GP in the UK for at least 1 year (Gonzalez-Perez et al. 15).
In the model, the probabilities that anaphylaxis was due to drug were specified for adults and children (see Table 9, parameter 11) using the figures of a retrospective study on emergency calls for allergic reactions within greater Manchester, also in a 1-year period, by the North West Ambulance Service in the UK (Capps et al. 36).
As can be seen, all probabilities were converted from multi- to binomial (essentially from marginal to conditional), which produces exactly the same result as if they had been treated as multinomial. This was done for ease of use in the model software (TreeAge Software, Inc., Williamstown, MA, USA, 2009). This means that the probability of idiopathic anaphylaxis is calculated first from r/n (103/343). Then, the probability of insect given not idiopathic is calculated given that idiopathic is ruled out from 46/240.
| No. | Parameter | Name of parameter in model | Distribution type | n | r | Sources |
| 9 | Probability that anaphylaxis idiopathic | didio | Betaa | 343 | 103 | Gonzalez-Perez 2010,15 table V, p. 1104 = 30% |
| 10 | Probability that trigger was insect, given not idiopathic | dinsect | Betaa | 240 | 46 | Gonzalez-Perez 2010,15 table V, p. 1104 = 13.41% |
| 11 | Probability that trigger was drug, given not idiopathic and not insect in child | ddrugchild | Betaa | 87 | 19 | Capps et al. 2010,36 table 1, p. 655 = 12.4% |
| 12 | Probability that trigger was drug, given not idiopathic and not insect in adult | ddrugadult | Betaa | 303 | 236 | Capps et al. 2010,36 table 1, p. 655 = 44.1% |
| Probability that trigger was food, given not idiopathic, not insect nor drug in child | – | – | – | – | = 44.2% | |
| Probability that trigger was food, given not idiopathic, not insect nor drug in adult | – | – | – | – | = 12.5% |
Next, the probability of drug given being not idiopathic or insect is calculated from 19/87 or 236/303, depending on whether child or adult. The probability of food given being not idiopathic or insect or drug is then simply 1 – probability of drug.
Table 9 gives a description of the model inputs, which imply the following marginal probabilities (r/all anaphylaxis = r/343): idiopathic 30.03%; insect 13.41%; food 44.21% (children) and 12.51% (adults); and drug 12.35% (children) and 44.05% (adults).
Mortality
Details of mortality from anaphylaxis are shown in Table 10.
| No. | Parameter | Name of parameter in model | Distribution type | n | r | Sources |
|---|---|---|---|---|---|---|
| 13 | Annual probability of dying given anaphylaxis and presence of emergency services and current AI use | ddieanaph | Beta | 3517 | 20 | Soar et al. 200817 |
| HES 201032 |
The number of deaths due to anaphylaxis in the UK was estimated from the findings reported by the working group of the Resuscitation Council (Soar et al. 17). This was based partly on a set of studies using a register of deaths due to anaphylaxis compiled by Pumphrey,37 Pumphrey and Gowland,38 and Pumphrey and Roberts. 39 The number of anaphylaxis cases was estimated by figures for the period of 2009–10 from the Department of Health Hospital Episode Statistics (HES)32 (www.hesonline.nhs.uk). As already stated in the section on incidence rates, both of the reported figures are likely to be underestimates, as it is difficult to diagnose and correctly code anaphylaxis, so the mortality rate will probably not vary much from this. These figures imply an annual probability of dying of 20/3517 = 0.005687, i.e. about 0.5%.
Effect of adrenaline injectors on mortality
In order to estimate the effect of the AIs, it is necessary to ‘subtract’ out the effect of the injectors in order to estimate the probability of death with no AI. Put another way, the estimate of mortality shown above is lower than the mortality rate due to anaphylaxis in the presence of both the use of emergency services (referred to as ‘ambulance’) and AIs. Therefore, to estimate the effect of AI, we first need to estimate an ‘underlying’ rate plus ambulance effect only. Note that all of the calculations to estimate the probability of dying given no AI were performed in TreeAge from the parameters for death given emergency services and current AI use and parameters for time to death and ambulance response times shown below (Table 11).
| Intervention | Percentage of anaphylaxis cases that result in death | Relative risk of death (vs no intervention) | No. of deaths per year (from 3517 cases of anaphylaxis) |
|---|---|---|---|
| No intervention | 6.473 | 1 | 228 |
| Ambulance only | 0.838 | 0.129 | 29 |
| Ambulance plus AI (current practicea) | 0.569 | 0.096 | 20 |
| Ambulance plus perfect useb of AI | 0.025 | 0.004 | 1 |
Having calculated the probability with no AI, the effect of AIs can be applied, either with SC or with SSs. As will be explained below, the parameter in the model that estimates the effect of SC or SSs is the proportion of correct use, which would be expected to be higher with SSs than with SC.
In the absence of direct evidence as to how many deaths have actually been prevented by AIs, there are several steps in the calculation, which implies the need to use several parameters and, thus, the need to make some assumptions. However, it will be attempted to make these explicit and justified where possible. Also, as with all parameters, they were all subject to sensitivity analysis. Before the exposition, in order to improve clarity, the result of the calculations is first summarised by intervention (presence of AIs or ambulance service) in Table 11.
First, it was assumed that the effect of ambulance or AI depended on the time between exposure to trigger and death. Of course, with idiopathic this would be impossible, as there is no trigger. Indeed, the register by Pumphrey,37 and summarised by Soar et al. ,17 does contain these data for food, drug [oral and injected (although only ‘oral’ used, as ‘injected’ most likely to be administered in a health-care setting)] and insect. However, the total number of observations (111) is small. Therefore, time to death was estimated, making the assumption that the average across these three groups would apply to any cause including idiopathic. In practice, all of these times were times to first cardiac arrest, but, given that all individuals died, it is assumed that, in order to prevent death, adrenaline must be administered before this point. It was also assumed that the time to death observed in those who died was similar to that in those avoided by either the emergency services (referred to as ‘ambulance’) or AI.
Therefore, first, the proportions dying in each of the categories reported by Soar et al. 17 (2.1–4.5, 4.6–9.9, 10–20 and > 20 minutes) was estimated, as shown in Table 12.
| No. | Parameter | Name of parameter in model | Distribution type | r in categories (2.1–4.5, 4.6–9.9, 10–20 and > 20 minutes) | Sources |
|---|---|---|---|---|---|
| 14 | Time to die, food | dtimediefood | Dirichlet | (0; 0; 9; 50) | Soar et al. 200817 |
| 15 | Time to die, drug | dtimediedrug | Dirichlet | (0; 2; 4; 7) | Soar et al. 200817 |
| 16 | Time to die, insect | dtimedieinsect | Dirichlet | (2; 4; 20; 13) | Soar et al. 200817 |
‘Drug’ only included oral and not injected, on the basis that injected would have been administered by a health-care professional with little need for AI. These values, which were inputs in the model, imply the following proportions in each of the time categories shown in Table 13.
| Trigger | Categories (minutes) | |||
| 2.1–4.5 | 4.6–9.9 | 10–20 | > 20 | |
| Food | 0 | 0 | 0.152542 | 0.847458 |
| Drug (oral) | 0 | 0.153846 | 0.307692 | 0.538462 |
| Sting | 0.051282 | 0.051282 | 0.512821 | 0.384615 |
| Any trigger | 0.003889 | 0.096853 | 0.273614 | 0.625645 |
Using the probabilities of each trigger (excluding idiopathic) from the same sources as used above allows calculation of the probabilities of time to death for any trigger.
For example, about 62% of cases of patients with anaphylaxis from any cause would still be alive for up to 20 minutes, which means that death might be prevented by the arrival of an ambulance within that time. Therefore, to calculate the deaths that could be prevented by AI, one needs to first estimate the effect of the ambulance service. For example, if 100% of response times were < 4.5 minutes then there would be no need for AI but also there would be no deaths, which, of course, is not the case.
Therefore, to estimate the response times, the data from an audit of ambulance services were used;40 the proportions of responses in each of the reported categories (< 8, 8–18 and > 18 minutes) were estimated for each of the emergency categories, A (essentially life-threatening) and B, shown in Table 14.
| No. | Parameter | Name of parameter in model | Distribution type | r in categories (< 8, 8–18 and > 18 minutes) or n | r | Sources |
|---|---|---|---|---|---|---|
| 17 | Ambulance response time, category A | DtimeA | Dirichlet | (1, 442, 519; 437, 973; 60, 160) | N/A | NHS Information Centre 201040 |
| 18 | Ambulance response time, category B | Dtime19B | Beta | 2, 559, 126 | 2, 322, 793 | NHS Information Centre 201040 |
The category ‘< 8 minutes’ is not reported for ‘B’ and so it was assumed to be zero. This is unlikely to be a problem, as the proportion of calls to anaphylaxis in category B is likely to be very small. Indeed the figures used were from Capps et al.,36 where there were < 10% in ‘B’ (referred to as ‘amber’ in that study). ‘Purple’ and ‘red’ were assumed to be equivalent to ‘A’. The category ‘< 8 minutes’ was assumed to correspond to 4.6–9.9 minutes, assuming that response time would never be < 4.6 minutes. The categories ‘8–18 minutes’, ‘10–20 minutes’, ‘> 20 minutes’ and ‘> 18 minutes’ were assumed to be equivalent. These r and n values, used as inputs in the model, imply the proportions shown in Table 15.
| Ambulance | Categories (minutes) | ||
|---|---|---|---|
| < 8 | 8–18 | > 18 | |
| Category A | 0.743316 | 0.171142 | 0.085542 |
| Category B | 0 | 0.907651 | 0.092349 |
| Any category | 0.672829 | 0.240983 | 0.086187 |
The proportions for any category are calculated by taking the average, weighted by the total numbers in each of the categories.
This, therefore, permitted the estimation of the proportion of all deaths that would not be saved by ambulance and thus could be saved only by correct and timely use of AI. For example, all of those with a time to death of < 4.6 minutes would not be prevented, whereas the proportion who would still die in the ‘10–20 minutes’ category would be only those for whom the ambulance response time was in the > 18-minute category. The formula is:
where Propnotamb is the proportion of deaths that would occur as a result of anaphylaxis, which are not prevented by ambulance, which depends on the response time distribution so that:
where Propdie is the proportion who die in each time period, shown in Table 13, and Propresp is the proportion who respond within that time period, shown in Table 15. It can be seen that Propnot (2.1–4.5 minutes) = Propdie (2.1–4.5 minutes), because it is assumed that the ambulance never arrives that early. It can also be seen that a factor of 0.5 is used for some proportions; these are where the response time period is the same as the time period for death. Multiplying by 0.5 implies that only 50% of response times are less than time to die. This is an assumption given the lack of more precise data within each period.
From the data in the tables:
This means that about 13% of anaphylaxis deaths are not prevented by ambulance.
Now to calculate the effect of AIs it was assumed that all AIs, if used successfully, would be used within the ‘4.6–9.9 minutes’ category. This implies that all of those deaths not prevented by ambulance in < 4.6 minutes would still not be prevented. However, this does not imply that all deaths in the time window of 4.6 minutes or longer would be prevented, as this applies to only those who actually use the injector correctly; there is another parameter, which is the proportion who do this, which might be < 100%. Indeed, in the Capps et al. study,36 only about 44% (53/119) of those who eventually were given adrenaline (by ambulance or injector) received an adrenaline by AI. This means that the proportion of deaths not saved by either ambulance or AI can be estimated:
i.e. the proportion of deaths prevented by AI in each time period is the probability of correct use, Pcorrect (53/119) multiplied by the proportion that would not have been prevented by ambulance with a correction factor of 0.5 for the period 4.6–9.9 minutes only. Therefore, it can be calculated that:
i.e. about 9% of deaths are prevented by both ambulance and AI use. This is therefore the proportion of deaths from anaphylaxis (without any intervention) that would remain in the event of current ambulance service provision and current AI use. Therefore, to calculate the overall (no intervention) mortality rate, Pdeath, use:
where ‘Nintervention’ is the number of deaths with current service and AI use, which is 20 (see Table 10); ‘Pintervention’ is the proportion of deaths not saved, which was calculated to be 0.087852; and ‘Nno intervention’ is the number of deaths that would have occurred and ‘Nanaphylaxis’ is the number of cases of anaphylaxis, which is 3517 (see above).
Substituting (7) into (6) gives:
i.e. the probability of dying from anaphylaxis without any treatment would be about 6%, which would result in 3517 × 0.064729 = about 228 deaths per year.
Therefore, we can now fulfil the aim of this section and calculate the probability of dying with ambulance and no AI, which is:
‘PdeathnoAI’ is the probability of death used in the model for no injector use. This means that ‘Pdeath AI’ is the probability of death with correct AI use (recall that those deaths at < 4.6 minutes would not be prevented even with correct use), which can be calculated by assuming that the proportion given AI is 100%:
This is because the only deaths not prevented by 100% correct AI use are those that occur within 4.5 minutes. This means that, whereas current AI use (44%) saves about nine deaths per year, if AI use was 100% correct, there would be only about one death per year, saving an extra eight lives per year.
In the model, ‘Pdeath’ is calculated by using ‘Pcorrect’ from Capps et al.,36 53/116 (about 44%) (Table 16).
| No. | Parameter | Name of parameter in model | Distribution type | n | r | Sources |
|---|---|---|---|---|---|---|
| 19 | Probability of correct use of AI with SC | dpinjector | Beta | 116 | 53 | Capps et al. 201036 |
| n = table 3, p. 655 | ||||||
| at any time | ||||||
| r = before ambulance arrived |
This is not the value used to estimate the probability of correct use in the model, i.e. during the cohort simulation, as Capps et al. 36 also presented separate values for children (< 15 years) and adults (shown with the value for SS in Table 17).
| No. | Parameter | Name parameter in model | Distribution type | n | r | Sources |
|---|---|---|---|---|---|---|
| 20 | Probability use injector correctly with SC in child | dinjectorchild | Beta | 15 | 10 | Capps et al. 201036 |
| n = table 3, p. 655 | ||||||
| at any time | ||||||
| r = before ambulance arrived (child) | ||||||
| 21 | Probability use injector correctly with SC in adult | dinjectoradult | Beta | 101 | 43 | Capps et al. 201036 |
| n = table 3, p. 655 | ||||||
| r = before ambulance arrived (adult) |
The probability of using an AI given SC (see Table 17, parameters 20 and 21) was based on the figures of use of AIs before arrival of the North West Ambulance Service (Capps et al. 36) and the total number of patients who received adrenaline. These figures are much lower than the 514 patients (adults and children) who eventually presented with symptoms that might be consistent with anaphylaxis, i.e. this implies that not all patients who had the symptoms of an anaphylactic reaction needed/received an adrenaline injection for treatment.
It is expected that the compliance of patients who received education (in SSs) will increase (Table 18, parameter 22). Compliance with AIs is mainly dependent on the knowledge of how to use it in a correct way, as well as the will to ensure that it is easily accessible and to use it when necessary. However, no estimate could be found of the effect of SS on compliance. Therefore, in the base case, 90% correct use was assumed, although recall that this means that those with a very short time to die (‘< 4.6 minutes’ category from Soar et al. 17) will still die (see Table 18). This makes the estimate more conservative.
| No. | Parameter | Name parameter in model | Distribution type | Minimum | Most likely | Maximum | Sources |
|---|---|---|---|---|---|---|---|
| 22 | Probability use injector correctly with SSs | dpinjectorSS | Triangular | 0.8 | 0.9 | 1 | Assumption |
Idiopathic treatment
Estimates to calculate probability of remission came from an observational study by Krasnick et al. ,10 which was used because it was the only study that could be found that included any time to event data to enable the probability of remission to be estimated. Data on years of follow-up and years in remission were provided, from which time to remission could be calculated by subtraction. Table 19 shows the data extracted for frequent and infrequent recurrence categories.
| Recurrence | |||||
|---|---|---|---|---|---|
| Frequent | Infrequent | ||||
| Years of follow-up | Years in remission | Time to remission | Year of follow-up | Years in remission | Time to remission |
| 7 | 4 | 3 | 6 | 2 | 4 |
| 8 | 2 | 6 | 5 | 5 | 0 |
| 8 | 4 | 4 | 3 | 2 | 1 |
| 8 | 8 | 0 | 6 | 4 | 2 |
| 12 | 11 | 1 | 5 | 4 | 1 |
| 7 | 6 | 1 | 6 | 5 | 1 |
| 10 | 2 | 8 | 6 | 6 | 0 |
| 6 | 2 | 4 | 5 | 4 | 1 |
| 5 | 3 | 2 | 12 | 9 | 3 |
| 9 | 9 | 0 | 10 | 3 | 7 |
| 6 | N/R | 6 | 6 | 0 | 6 |
| 18 | N/R | 18 | 9 | 1 | 8 |
| 7 | N/R | 7 | 6 | N/R | 6 |
| 9 | N/R | 9 | |||
| 5 | N/R | 5 | |||
Only those and all of those experiencing frequent episodes received treatment with prednisolone. As this implies specialist provision, the probability of remission with SSs is the sum of that with frequent episodes (plus treatment) and infrequent episodes (no treatment), whereas the probability of remission with SC is only that of the infrequent episodes.
Because data on rate of recurrence were not available separately for those experiencing frequent or infrequent episodes, it was assumed that the same (average) rate (see parameter 6, Table 7) applied to both. Thus, when remission occurs, the average rate would decrease, as for those in remission the rate is zero. Therefore, the advantage of SSs over SC can be explained in the following way. The probability of recurrence given SSs is the sum across both the frequent, some of whom go into remission due to treatment, and the infrequent, some of whom go into remission spontaneously. However, the probability of recurrence given SC is the sum across the frequent, none of whom go into remission, and the infrequent, some of whom go into remission spontaneously.
From the data in Table 19 the median of time to remission was calculated, which was then used to inform the probability of remission (per cycle length, i.e. 6 months) in the model where, according to the definition of the median, the probability of remission per cycle (median time) = 0.5 and a constant rate (exponential model) assumed. The median was estimated by assuming that censoring (no remission at follow-up) indicated remission. This is a conservative estimate of time to remission. However, excluding the censored data produced a lower estimate and so the estimates of 4 and 1.5 for frequent are probably not too low. These estimates were used to form the most likely with assumptions as to the low and high (Table 20).
| No. | Parameter | Name of parameter in model | Distribution type | Low | Most likely | High | Sources |
|---|---|---|---|---|---|---|---|
| 23 | Median time to remission in frequent idiopathic | dmedianfreq | Triangular | 2 | 4 | 6 | Based on data from Krasnick et al. 199610 |
| 24 | Median time to remission in infrequent idiopathic | dmedianinfreq | Triangular | 1 | 1.5 | 2 | Based on data from Krasnick et al. 199610 |
It was assumed that the rate of recurrence among those who did not go into remission would remain the same, which is probably an underestimate as the median time to remission is longer in those with frequent recurrence. Remission is still allowed to occur with SC, although only in those with infrequent recurrence, but also with no rise in the remaining rate so that there should be little bias towards either SC or SSs.
The proportion of frequent anaphylaxis (0.5) was also taken from the study by Krasnick et al. 10, which uses the same definition of frequent as the guideline, shown in Table 21.
| No. | Parameter | Name of parameter in model | Distribution type | n | r | Sources |
|---|---|---|---|---|---|---|
| 25 | Proportion of idiopathic that are frequent | dfreqidio | Beta | 56 | 28 | Krasnick et al. 199610 |
Venom immunotherapy
Venom immunotherapy is indicated for patients who have a history of severe systemic reaction to a sting. 41 The effectiveness of VIT (Table 22, parameter 26) is estimated to be 85%; this is based on several studies that report a range of effectiveness of 75–95% (Krishna and Huissoon). 42 There is also a potential risk of anaphylaxis with VIT and, thus, increased cost and reduced utility but these are assumed to be negligible, especially given that the therapy is administered in a clinic where there is access to adrenaline and other emergency care (based on Cox 2011). 25 As VIT is time-consuming in terms of both frequency of treatments and total duration of therapy, and there is also the possibility of adverse reactions caused by VIT, we presume that not all patients will continue immunotherapy for 3 years (parameter 27, see Table 22). This figure is based on the finding of Goldberg et al. ,43 who reported a dropout rate of 40% in a study evaluating the attitudes of patients in Israel with insect venom allergy regarding after-sting behaviour and proper administration of adrenaline. We assumed that in the UK, 10 years later, the dropout rate of VIT would be much lower (about 20%) as a result of better care and fewer adverse events. Also, because of knowledge of these problems and the fact that, depending on the results of skin and anti-immunoglobulin E (IgE) testing, not everyone is eligible (as low as 65% according to Cox et al. )25 it was conservatively estimated that uptake would be about 60% (parameter 28; see Table 22).
| No. | Parameter | Name of parameter in model | Distribution type | Base case | Range | Sources | |
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | ||||||
| 26 | Effectiveness of VIT | dpeffectVIT | Triangular | 0.75 | 0.85 | 0.95 | Expert opinion and based on Krishna 201142 |
| 27 | Dropout | dropout | Triangular | 0.1 | 0.2 | 0.3 | Expert opinion and based on Goldberg43 |
| 28 | Uptake of VIT | duptakeVIT | Triangular | 0.4 | 0.6 | 0.8 | Expert opinion and based on Cox et al. 201125 |
Health valuation estimation
For the calculation of QALYs of the NICE reference case20 we needed an estimate of utility values (usually between 0 and 1), ideally obtained using the EuroQoL (EQ-5D index) instrument.
Utility (with no adjustment for anaphylaxis) was estimated as a function of age from a large recent EQ-5D US population study. 44 Decrements were then applied to each state except for that of ‘remission’.
For the estimation of the utility decrement due to being at risk of recurrence of anaphylaxis, the study by Voordouw et al. 45 was used. This case–control study45 using a postal survey was designed to evaluate the household costs associated with food allergy and also reported EQ-5D index data of 125 patients. The utility decrement was estimated as 0.08 (based on the difference between the values reported of 0.887 for cases and 0.803 for control subjects; p < 0.05) (Table 23, parameter 29).
| No. | Parameter | Name of parameter in model | Distribution type | Low | Most likely | High | Sources |
|---|---|---|---|---|---|---|---|
| 29 | Utility decrement due to at risk | duatrisk | Triangular | 0.00 | 0.08 | 0.1 | Expert opinion and based on Voordouw 201045 |
| 30 | Duration of recurrence | ddurationrecur | Uniform | 1 | N/A | 9 | Expert opinion and based on Neuner et al. 200346 |
| 31 | Utility factor with SSs | duSSimprove | Triangular | 0 | 0.25 | 0.5 | Assumption based on expert opinion |
| 32 | Utility factor with AI | duAIimprove | Triangular | 0 | 0.25 | 0.5 | Assumption based on expert opinion |
We presumed that the impact of anaphylaxis will be very short but profound. The estimation of mean duration of having recurrence of anaphylaxis (parameter 30; see Table 23) was based on the finding that the mean loss of about 9 whole quality-adjusted life-days for severe allergic reaction due to penicillin is equivalent to utility decrement of the whole of the age-dependent utility for 1–9 days, reported in another CEA. 46 Unfortunately, this value was no obtained using the EQ-5D instrument, but appeared to be based on an assumption. Indeed, the mean length of hospital stay reported in the HES32 is only about 1 day, but this is likely to be an underestimate of the duration of the effect on well-being of recurrence. Therefore, a value half-way between these extremes was chosen, which is the expected value of a uniform distribution bounded by 1 and 9.
Finally, there was expert opinion that the reassurance provided by attending an SS through, for example, diagnosis of trigger and learning how to avoid triggers, as well as the provision of AI, should reduce the utility decrement due to the condition (parameter 31; see Table 23). Therefore, in the absence of any evidence as to the extent of this effect, ranges of 0–0.5 for a factor to be multiplied by a utility increment equal to the decrement due to anaphylaxis, were chosen for each of SSs and AI (parameter 32; see Table 23). This means that, at best (factor = 0.5 for SS + 0.5 for AI = 1), the combination could completely remove the decrement and, at worst, have no effect (factor = 0).
Resource use and unit cost estimation
Table 24 gives a description of the unit costs (in £) and the resource-use data used in the model.
| No. | Parameter | Name of parameter in model | Distribution type | Low (triangular) | Mean (normal) or most likely (triangular) | Standard error (normal) or high (triangular) | Sources |
|---|---|---|---|---|---|---|---|
| 33 | Mean cost of inpatient care | dcostrecur | Normal | N/A | £469.88 | 37.585 | NHS Reference Costs 2009/10 47 |
| 34 | Mean cost of AI | cinjector | N/A | N/A | £28.97 | N/A | BNF 6118 |
| 35 | Costs of SS sessions | cSS | N/A | N/A | (initial, follow-up) | N/A | NHS Reference Costs 2009/10 47 |
| Children (£266, £234) | |||||||
| Adults (£321, £450) | |||||||
| 36 | Duration of VIT (months) | ddurationVIT | Triangular | 2 | 3 | 4 | Based on Diwakar 200848 |
| 37 | Induction phase of VIT (build-up) (weeks) | dbuildupVIT | Triangular | 8 | 10 | 12 | Based on Cox et al. 201125 |
| Expert opinion | |||||||
| 38 | Average cost for bee and wasp extract for VIT maintenance treatment | cVITmaintenance | N/A | N/A | £60 | N/A | BNF 6118 |
| 39 | Average cost for bee and wasp extract for VIT induction treatment | cVITinitial | N/A | N/A | £70 | N/A | BNF 6118 |
| 40 | No. of weeks between VIT maintenance doses | dnVITmaint | N/A | 4 | 6 | 8 | Expert opinion |
| Cox et al. 201125 | |||||||
| 41 | Cost of prednisolone per milligram | cpred | N/A | N/A | 0.02 | N/A | BNF 6118 |
| 42 | Duration of prednisolone course in months | ddurationpred | Uniform | 2 | N/A | 3 | Simons et al. 201027 |
| 43 | Start dose of prednisolone (mg) | dstartdosepred | Uniform | 60 | N/A | 100 | Simons et al. 2010,27 Lieberman et al. 201024 |
| 44 | Duration of start dose of prednisolone | dstartduration | Uniform | 1 | N/A | 2 | Simons et al. 2010,27 |
| Lieberman et al. 201024 | |||||||
| 45 | No. of follow-ups per year for those with a food trigger | nFUfoodSS | N/A | N/A | 0.5 | N/A | Expert opinion |
| 46 | Cost of follow-up for those with a food trigger | cFUfoodSS | N/A | N/A | £200 | N/A | Expert opinion |
| 47 | Cost of VIT visit | cVITvisit | N/A | N/A |
The mean cost and standard error of inpatient care was estimated from the individual Primary Care Trust data for the period of 2009–10 from the Department of Health Hospital Episode Statistics (www.hesonline.nhs.uk)32 (see Table 24, parameter 33).
The average costs of AIs were based on the costs reported in the BNF 6118 (parameter 34). The lifespan of an AI was assumed to be 6 months and two prescribed or replaced at a time, based on expert opinion. Only those who were prescribed an AI incurred that cost and this was assumed to be for the rest of their lives. The costs of treatment of patients in SS were based on the NHS reference costs. 47 All individuals with anaphylaxis, regardless of trigger, incurred the cost of two appointments (one initial and one follow-up) in the first 3-month cycle of the model. These were based on the ‘multiprofessional’ categories: for children, Paediatric Clinical Immunology and Allergy (Service code 255) and, for adults, Clinical Immunology and Allergy (Service code 316) (see Table 24, parameter 35). Expert opinion was to include the cost of training in the use of the auto-injectors, i.e. there was no additional training cost.
Only those with an insect trigger and who underwent VIT incurred those additional costs (see Table 24, parameters 36–40). Model estimates on current practice of VIT in the UK are based on an audit that evaluated the adherence to international guidelines48 and on expert opinion (Dr Pamela Ewan, Allergy Department, Addenbrookes Hospital, 9 June 2011, personal communication). Most of the VITs in the UK were given by injection of a purified extract (Pharmalgen, ALK-Abelló UK, Reading, UK), using an induction scheme of weekly injection for about 10 weeks and continuation for about 3 years, with about a 6-weekly interval during maintenance. 48 In the model, a mean duration time of VIT of 3 years with a range of 2–4 years is used48 (see Table 24, parameter 36). The average costs for bee and wasp extracts used for VIT were based on the costs reported in BNF 6118 (parameter 38–39). The number of weeks between VIT maintenance doses was based on expert opinion and on the American guideline for allergen immunotherapy25 (see Table 24, parameter 40). The duration of the build-up phase, based on expert opinion, was up to 10 weeks and, given that the next dose would not occur for at least another 4 weeks, implied that the cost in the first 3-month cycle was only that of initial treatment (£70). The cost thereafter is therefore calculated as number of maintenance doses multiplied by cost of maintenance dose (£60), where mean number of maintenance doses is duration of maintenance divided by number of weeks between doses.
Only those with idiopathic anaphylaxis with frequent episodes incurred the additional cost of prednisolone (see Table 24, parameters 41–44). The recommendation from two international guidelines23,24 for prednisolone is 1–2 weeks every day, starting at 60–100 mg, until symptoms are under control and then decreasing over a period of about 2–3 months.
Those with food triggers also incurred additional regular follow-up costs in accordance with expert opinion that would be necessary to reinforce avoidance measures. According to expert opinion, the frequency would vary depending on the specific food trigger and age, with milk trigger in children having the highest frequency. However, an average of about once every 2 years over a lifetime was assumed in the base case (see Table 24, parameter 45). The cost of each follow-up was also taken from the NHS reference costs47 (see Table 24, parameter 46).
Discount rate
The discount rate for costs and benefits was 3.5% in accordance with NICE methods guidance. 16
General model assumptions
We assumed that 50% of the population consisted of males, which is based on the HES. 32 Furthermore, we assumed that there are only four main triggers of anaphylaxis: drug, food, insect/venom and idiopathic (no known cause). We expected that in SC there is either no referral to SSs or a GP referral only after anaphylaxis, and that SSs essentially consisted of SC plus referral to SSs on the basis that the patient would probably see his/her GP as well. We also assumed, based on expert opinion, that anyone with anaphylaxis gets only two sessions with SSs unless cause of anaphylaxis is insect or idiopathic. In all causes, patients receive benefit from recurrence rate reduction, utility increase and mortality rate reduction from SSs, and from only mortality rate reductions with AI. We assumed that historic recurrence and mortality rates are due to SC only, given the likely low rate of referral to SSs: in one study the referral rate was zero. 13 Finally, we expected the cost of recurrence to be due to hospital admission only, i.e. no further follow-up costs were included, which is conservative in terms of the chances of SSs being cost-effective.
Further assumptions are explained in each of the sections on model parameters below.
Time horizon
The time horizon was lifetime in accordance with NICE methods guidance. 20
Results
Base-case results
An arbitrary age of 30 years was chosen for the base case, and Table 25 and Figure 3 show the results of the model run probabilistically (10,000 simulations).
| Strategy | Cost | Incremental cost | Effectiveness (QALYs) | Incremental effectiveness (QALYs) | Cost-effectiveness | Incremental cost-effectiveness (ICER) |
|---|---|---|---|---|---|---|
| SC no AI | 981.13 | 39.22 | 25.02 | |||
| SS no AI | 1744.40 | 763.27 | 40.25 | 1.03 | 43.34 | 742.01 |
| SC plus AI | 1879.96 | 135.56 | 39.76 | −0.48 | 47.28 | Dominated |
| SS plus AI | 2668.52 | 924.12 | 40.76 | 0.51 | 65.47 | 1819.82 |
FIGURE 3.
Base-case results (probabilistic).
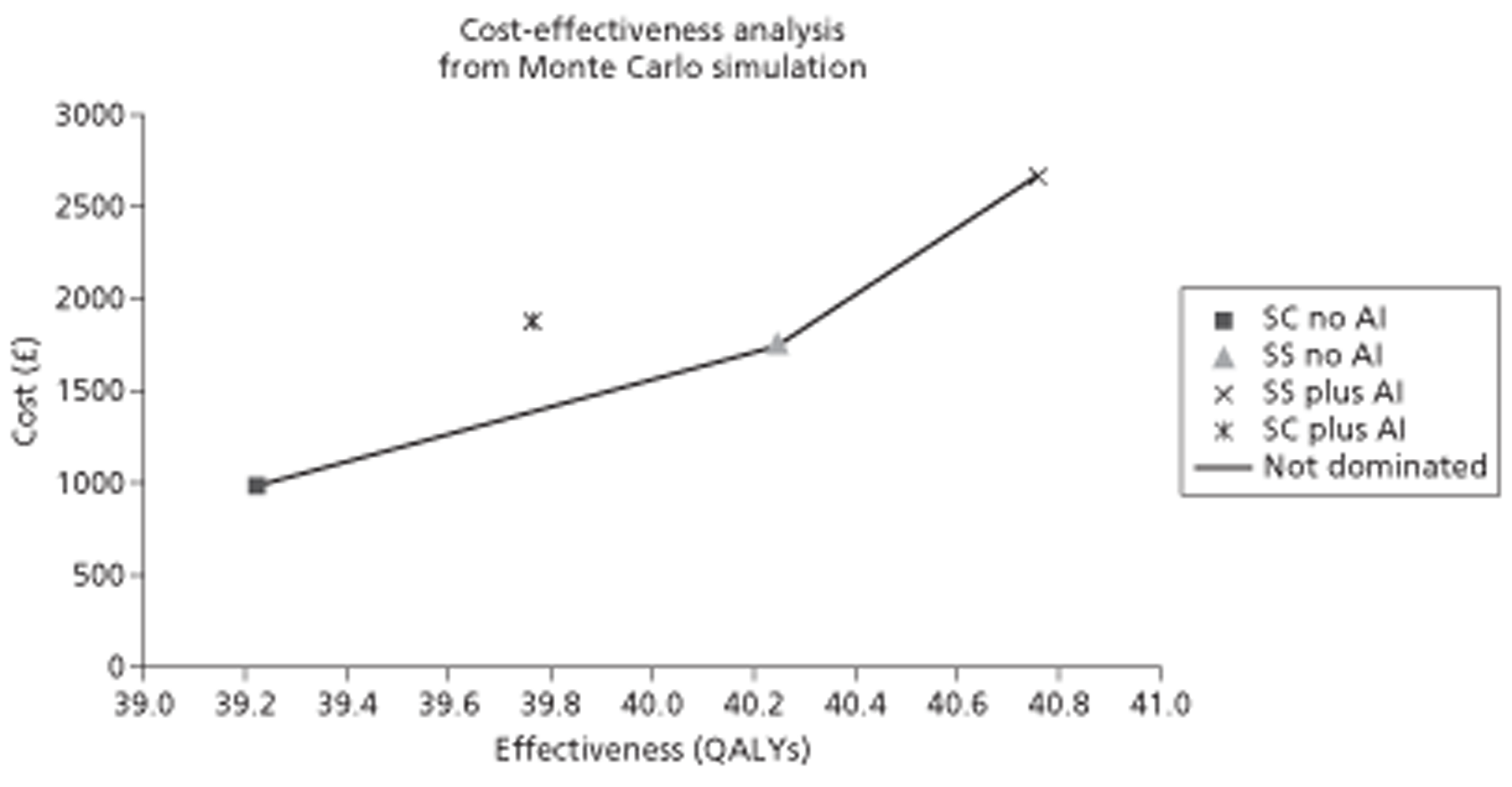
This shows that SC with AI would not be cost-effective. SS with no AI would be cost-effective if the threshold [willingness to pay (WTP) for a QALY] was greater than about £740, and SS with AI would be cost-effective if the threshold was > £1800 per QALY. Given a threshold of £20,000 this would make SS with AI cost-effective.
In order to show the effect of the uncertainty a cost-effectiveness acceptability curve (CEAC) was plotted.
Figure 4 shows that, at a threshold above about £2000 per QALY, SS with AI is most likely to be cost-effective, and, below this, SS without AI would be most likely.
FIGURE 4.
Cost-effectiveness acceptability curve: base case.
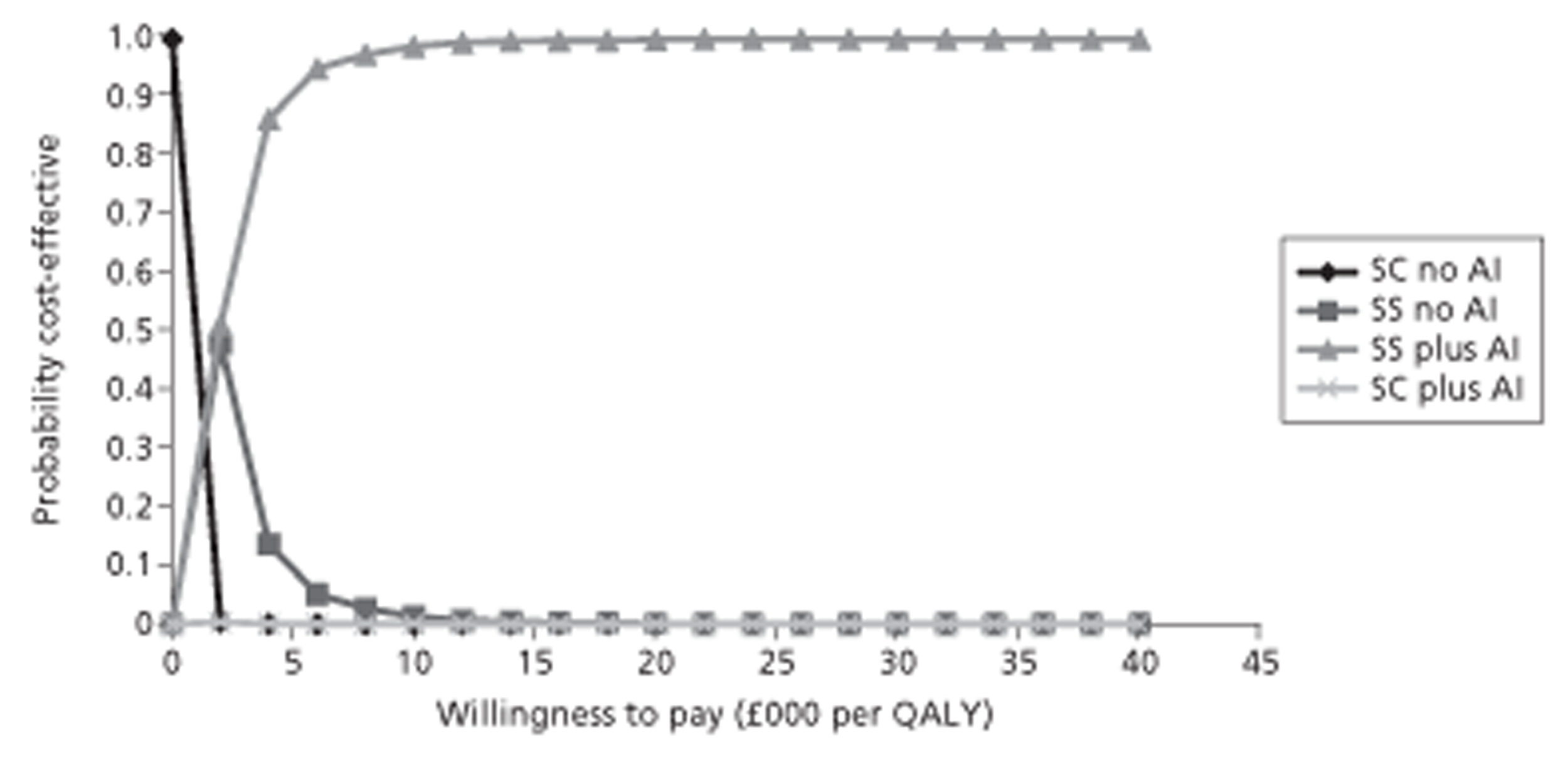
Table 26 shows the results of the deterministic (parameters at expected values) analysis.
| Strategy | Cost | Incremental cost | Effectiveness (QALYs) | Incremental effectiveness (QALYs) | Cost-effectiveness | Incremental cost-effectiveness (ICER) |
|---|---|---|---|---|---|---|
| SC no AI | 978.26 | 39.25 | 24.93 | |||
| SS no AI | 1745.19 | 766.93 | 40.25 | 1.00 | 43.36 | 763.45 |
| SC plus AI | 1875.83 | 130.64 | 39.79 | −0.46 | 47.14 | Dominated |
| SS plus AI | 2668.59 | 923.40 | 40.76 | 0.51 | 65.47 | 1808.13 |
It can easily be seen that there is virtually no difference between the results, indicating that the expected cost and QALYs are close to a linear function of the parameter values. It is for this reason and that it is much quicker to run the TreeAge software deterministically that all one way or threshold sensitivity analyses were conducted deterministically.
Sensitivity analysis
Table 27 shows the results for the age of 5 years.
| Strategy | Cost | Incremental cost | Effectiveness (QALYs) | Incremental effectiveness (QALYs) | Cost-effectiveness | Incremental cost-effectiveness (ICER) |
|---|---|---|---|---|---|---|
| SC no AI | 1137.78 | 61.05 | 18.64 | |||
| SC plus AI | 2551.18 | 1413.40 | 61.96 | 0.91 | 41.18 | Extendedly dominated |
| SS no AI | 3049.38 | 1911.60 | 62.96 | 1.91 | 48.44 | 999.94 |
| SS plus AI | 4501.53 | 1452.15 | 63.74 | 0.78 | 70.62 | 1850.46 |
The results are essentially very similar to those for the age of 30 years, except that SC plus AI was extendedly dominated [incremental cost-effectiveness ratio (ICER) to move from SC no AI is greater than to move from SC plus AI to SS no AI].
Table 28 shows the effect of varying the time horizon instead of using a lifetime.
| Strategy | Cost | Incremental cost | Effectiveness (QALYs) | Incremental effectiveness (QALYs) | Cost-effectiveness | Incremental cost-effectiveness (ICER) |
|---|---|---|---|---|---|---|
| Start age 30 years | ||||||
| SC no AI | 108.26 | 1.68 | 64.33 | |||
| SC plus AI | 179.48 | 71.22 | 1.71 | 0.02 | 105.20 | 3076.31 |
| SS no AI | 919.95 | 740.47 | 1.70 | 0.00 | 539.72 | Dominated |
| SS plus AI | 992.17 | 812.69 | 1.73 | 0.02 | 574.19 | 37,207.02 |
| Start age 5 years | ||||||
| SC no AI | 111.48 | 1.88 | 59.23 | |||
| SC plus AI | 253.83 | 142.34 | 1.91 | 0.02 | 133.20 | 6110.20 |
| SS no AI | 685.84 | 432.01 | 1.90 | 0.00 | 360.27 | Dominated |
| SS plus AI | 830.21 | 576.38 | 1.93 | 0.02 | 430.79 | 26,689.05 |
Table 28 shows that, as the time horizon decreases, SSs plus AI becomes less likely to be cost-effective. Indeed, threshold analysis (see next paragraph) reveals that, starting at the age of 30 years, for a range of time horizons from 1 to 3 years, assuming a WTP of £20,000 per QALY, SC plus AI would be cost-effective. This is true up to 2 years only for children.
Threshold analysis was conducted on all parameters. All probabilities were varied between 0 and 1 and, unless stated otherwise, a WTP of £20,000 was used.
No change to SS plus AI being cost-effective was observed for the following:
-
Population age (0–90 years, base case: 30 years).
-
Probability trigger was drug.
-
Probability trigger was idiopathic.
-
Probability trigger was insect.
-
Rate of recurrence with drug caused anaphylaxis with SC (base case: 0.12).
-
Rate of recurrence with drug caused anaphylaxis with SS (up to 0.12, base case: 0.001).
-
All rates of recurrence due to some trigger (drug, food or insect) with SSs (up to 10 times base case for all in multiway sensitivity analysis).
-
Cost of SSs (up to £10,000, base case: about £250 or about £400, depending on age)
-
Frequency of follow-up for food trigger (up to once per month, base case once every 2 years).
-
Proportion frequent idiopathic.
-
Probability of remission, either frequent or infrequent.
-
Cost per milligram of prednisolone (up to £1, base case: £0.02).
-
Cost of VIT (initial or maintenance) (up to £200).
-
Effectiveness of VIT (0–1, base case: 0.85).
-
Probability of correct use with SSs (0–1, base case: 0.9).
-
Probability of dying from anaphylaxis with no intervention.
-
Utility improvement factor for SSs (0–0.5, base case: 0.25).
-
Utility improvement factor for AI (0–0.5, base case: 0.25).
It was observed that there was a change from:
SS plus AI to SC plus AI above 0.35 for rate of recurrence in food-caused anaphylaxis (base case: 0.01)
SS plus AI to SS no AI above 0.03 for probability of dying with injector (correct use) (base case: 0.000252)
SS plus AI to SS no AI above £146 for cost of injector (base case: £26.45) (at start age of 30 years; less than this implies a higher threshold)
SS plus AI to SS no AI below 0.03 for utility improvement factor with AI (base case: 0.25)
SS plus AI to SC plus AI between time horizon of about one and, for adults, 3 years and, for children, 2 years (base case: lifetime)
SS plus AI to SS no AI below 0.77 for probability of correct use with a SS, no utility increment for AI use (base case: 0.9)
Therefore, in summary, that SS plus AI was cost-effective at a threshold of £20,000 per QALY was robust to all sensitivity analysis except mostly at relatively extreme values of a small number of parameters. The only exception was if it was assumed that there was no benefit to having AIs irrespective of whether or not they were used correctly: this analysis was performed given the lack of evidence to inform the utility increment for AIs (see Health valuation estimation). In this case, a threshold of 0.77 (77%) does not seem that implausible.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Given the conclusion that SSs are likely to be cost-effective, consideration would need to be given as to how to increase referral, such as by training or education. Also, any implementation would require an assessment of whether or not current SS capacity is sufficient if increased referral should occur.
Chapter 6 Discussion
Key results
The assessment of clinical effectiveness aimed to inform the following four questions:
-
In adults, young people and children who receive emergency treatment for suspected anaphylaxis, which people are at high risk of anaphylactic episodes? For which people would further anaphylactic episodes have significant impact? Which people can be identified as needing special consideration?
-
What are the effects of history-taking, including signs and symptoms, and physical examination in identifying the possible cause?
-
What are the effects of providing adrenaline auto-injectors, including by whom?
-
After assessment, when should referral take place?
The searches of electronic searches yielded 11,058 references. After screening of titles and abstracts, 10,951 references were excluded. The remaining 107 references were obtained and the full texts were screened. Five studies were included, none of which was a RCT. All five included studies were prospective observational studies reporting on risk of recurrence. 3–7 The studies, conducted in five countries (Australia, Germany, Italy, Spain and the USA), included 1725 patients overall.
Risk of recurrence was estimated to be between 30% and 42.8%. One study suggested the rate of a third event to be 5.2%, with a higher risk of recurrence for women (RR 2.14, 95% CI 1.17 to 3.9). 4 In children aged < 12 years, an overall recurrence of 27% was reported, with food being the most frequent allergen (71%). 5 One larger study (432 patients) reported serious recurrences in 45 patients (10.4%), of whom 18 (40%) received adrenaline. 7
The assessment of cost-effectiveness aimed to inform the following two questions:
-
What are the cost-effectiveness of referral to specialist allergy clinics for the diagnosis of anaphylaxis (as opposed to for the acute event) and for the prevention of future episodes and the reduction in morbidity and mortality from future episodes?
-
What is the cost-effectiveness of adrenaline auto-injectors for the treatment of anaphylaxis, including the cost implications of training in the use of the AI?
These two questions were translated into a comparison between four possible strategies:
-
SC plus no AI
-
SC plus AI
-
SS plus no AI
-
SS plus AI.
In order to avoid misunderstanding, the question was not the consequences of a change in current service configuration where there is a non-zero level of referral to SSs, i.e. there was a choice of either SC (with no SSs) or SSs. Furthermore, the population was those with a diagnosis of anaphylaxis and, therefore, did not include the possibility of misdiagnosis.
The effectiveness of AIs was mediated through reduction in mortality and a small utility improvement owing to reassurance. The effectiveness and cost reduction due to SSs was mediated through reduction in rate of recurrence and also a small utility improvement. The reduction in rate of recurrence was mediated through mechanisms that depended on trigger: avoidance of trigger with drug and food and remission with insect and idiopathic.
A Markov model was constructed to model the possibility of recurrence over a lifetime in each of the subgroups by cause of anaphylaxis: insect, food, drug and idiopathic. It modelled the effect of SSs in terms of rate reduction via a mechanism that depended on the trigger, assuming that all patients had anaphylaxis and that the trigger was identified with certainty. AI (prescription of two injectors) effect was modelled as having an effect only on mortality due to recurrence. The results showed that, in the base case of a lifetime horizon, with a discount rate of 3.5%, SS with AI had an ICER of about £1800 (model run probabilistically or deterministically, i.e. all parameters set at expected value) and, therefore, would be cost-effective according to a threshold of no less than this figure. Any SC strategy (with or without AI) was dominated, i.e. found to be less effective and more costly than another strategy. SS with no AI would be cost-effective only below a threshold of about £740. The CEAC also revealed that above a WTP of about £2000, SS plus AI was also the most likely (highest probability) strategy to be cost-effective.
Given the complexity of the model and much uncertainty in many parameters, extensive sensitivity analysis in the form of threshold analyses was performed. This revealed that, variation in most parameters would not change the strategy that would be cost-effective. Indeed, only relatively extreme values for rate of food-caused anaphylaxis following SSs could cause a change to SC. Similarly, only relatively extreme values for the cost of injector, probability of dying with the injector or utility improvement factor (essentially the proportion of the utility decrement due to living with the risk of anaphylaxis that would be restored as a result of prescription of an injector) could cause a change to SS with no injector.
Strengths
First, all systematic review methods were conducted in accordance with the standards of the Cochrane Handbook. 49 This included a comprehensive search to identify relevant studies and all included studies were appropriately quality assessed. For the CEA, the methods were those recommended in the NICE guidance,20 particularly in terms of using a lifetime horizon, discount rate of 3.5%, QALYs and costs from the perspective of the NHS. Also, PSA was used to model the uncertainty in the parameter estimates.
Second, both the model structure and parameter estimates were validated by expert opinion by presentation to the GDG, including after feedback from stakeholders. In particular, either all parameter estimates were taken directly from the literature and confirmed by expert opinion or, where literature estimates were absent or deemed not good enough, expert opinion was sought in the form of the most likely value, as well as lowest and highest plausible.
Third, all uncertain parameter estimates were subjected to sensitivity analysis, using threshold analysis, in order to check how extreme they needed to be to change the strategy that would be cost-effective. Indeed, most parameters had no effect and the small number that did had to be at quite extreme values in order to change which strategy would be cost-effective at a threshold of £20,000 per QALY. This analysis was extended to examine the effect of probability of correct use of AIs given SSs, assuming no utility increment for use of AIs. In that case, a threshold of 0.77 (base case: 0.9) was found, which might be interpreted as showing that the probability of correct use with SSs has to be ‘quite high’ or the addition of AIs might not be cost-effective. Of course, what counts as ‘quite high’ is subjective and the judgement of the experts was that 0.8 was the lowest possible value. However, this might be biased.
Fourth, the analysis takes appropriate account of inappropriate use of AIs by costing all prescriptions, but only incurring benefit by mortality reduction with correct and timely use.
Finally, a review of the extant CEA literature revealed that the cost-effectiveness of SSs had never been estimated before. One study9 had examined AI, but only in the general allergic population, as opposed to those who have had anaphylaxis, and it had not estimated QALYs. Therefore, this is the first CEA in the area of anaphylaxis treatment.
Weaknesses
First, although a comprehensive search was undertaken to identify relevant studies (see Chapter 3, Quantity and quality of research available), only five studies were included (see Chapter 3, Assessment of clinical effectiveness). All of these studies are observational studies with low or medium risk of bias assessing the risk of recurrence [and ‘very low’ quality of evidence using the GRADE approach, as detailed in Chapter 3 (see Results)]. The studies were relatively small (1725 patients) and assessed the risk of recurrence in various patient groups. This limitation should be taken account when formulating recommendations based on these studies.
Second, no studies addressing any of the other clinical research questions in terms of history-taking, physical examination, provision of adrenaline auto-injectors, or referral to specialist allergy clinics for those with anaphylaxis were identified.
Third, in terms of the cost-effectiveness model, although validation by expert opinion did occur, several assumptions were made and, although parameter values were obtained, many did rely on expert opinion. This might also be said to be subject to ‘bias’, but, by definition, it can only be subjective and was obtained by involving the whole GDG, which is, through NICE, intended to be independent (www.nice.org.uk/aboutnice/howwework/developingniceclinicalguidelines/guidelinedevelopmentgroups/guideline_development_groups.jsp). Also, there was no attempt to either elicit individual GDG uncertainty or use a formal consensus process. However, in most cases, there was no threshold at which the strategy that is cost-effective would change. For example, the proportion of incident cases that were idiopathic was estimated from study of routine UK data15 to be about 30%, but this did not differentiate by age. Two other UK studies were found that did differentiate cause by age13,36,50 but it was not clear how many had idiopathic cause, although the proportion with ‘aetiology not recorded’ was about 34% (children 27% and adults 35%) in one study50 and ‘allergen not documented’ 40% for both adults and children in the other. 36 Also, variation of this proportion by itself had no effect on the cost-effective strategy.
Many assumptions and several sources of data were required in order to estimate the mortality effect of AIs. However, only if the probability of dying with AI was raised above 0.03 (about 10 times that in the base case) would prescription of AI not be cost-effective.
Also, there was no direct evidence for the influence of AI or SSs on utility, for example owing to an increase or decrease in anxiety, but even a factor of 0 for SSs or AI had no effect on which strategy was cost-effective.
Fourth, for recurrence, only cost of hospital treatment for anaphylaxis was included, but this was conservative in relation to the effect of rate reduction by SSs and, even if reduced to zero, it would not change which strategy was cost-effective. Cost of SSs might have been too low if any capital investment was required, but even raising it to the equivalent of about 50 sessions had no effect. The only cost parameter change that had a threshold was that of the injectors, which were costed using the BNF18 at £26.45 per injector with two injectors (or four for children) at 12-monthly replacement. Only above an unrealistic £146, the strategy that would be cost-effective at an ICER threshold of £20,000 would be SS without injector.
Fifth, the population was limited to those confirmed to have a diagnosis of anaphylaxis. However, not only did the GDG consider this to be reasonable, but misdiagnosis would most likely only waste cost, the effect of which was tested by variation in cost of SSs. There were also no parameters for tests for trigger identification, but any misidentification would only have decreased effectiveness, which was tested by variation in rate of recurrence with SSs.
Sixth, cost of training in the use of AIs was not explicitly included, but expert opinion was that this could be included in the SS cost; including an extra cost with SC would only have made it less likely to be cost-effective.
Finally, the evidence used for effectiveness of SS management to reduce risk of recurrence was also very sparse, and the rate of recurrence for drug-caused anaphylaxis with SC was believed by some stakeholders to be too high. However, variation in this parameter, effectiveness of VIT or probability of remission in idiopathic anaphylaxis had no effect. It is also possible that remission might occur not only in the idiopathic group. However, in the World Allergy Organization Guidelines, published this year,23 remission is mentioned only as a possibility in idiopathic anaphylaxis. Also, the net effect of remission might not make much difference. On the one hand it would improve health outcomes of SC relative to SSs, but, on the other hand, it would also decrease the cost of SSs relative to SC as a result of reduced need for follow-up. Moreover, only raising the rate of recurrence from 0.01 to 0.35 (35 times the base case) for food cause would make SC cost-effective.
Generalisability
The results of the clinical evidence review are generally applicable. Also, overall model structure, insofar as it models the natural history of those at risk of anaphylaxis and to some extent the health-related parameter values, such as rate of recurrence with SC, will be generalisable across settings and countries. However, many parameter values, such as the probability that anaphylaxis has a particular trigger and the rates of recurrence given SSs, as well as costs, will probably be particular to the nature of health services in the UK. Therefore, the results are unlikely to be generalisable beyond the UK.
Chapter 7 Conclusions
The systematic review revealed only five studies addressing risk of recurrence. No study was found that directly addressed any of the other clinical research questions in terms of history-taking, physical examination, provision of adrenaline auto-injectors, or referral to specialist allergy clinics for those with anaphylaxis. None of the included studies was a RCT.
The results of the CEA showed that SS with AI was cost-effective at a threshold of £20,000 per QALY. However, given the lack of RCTs, the model had to be informed by observational studies and expert opinion.
Given that the results that both referral to a SS and prescription of AIs are likely to be cost-effective and that this study has been used to inform a NICE guideline, it does potentially have important implications for policy. The guideline was published in December 2011.
In addition, the lack of good data to inform the effectiveness by any measure of any anaphylaxis intervention means that we recommend consideration of RCTs, or at least well-designed observational studies, of the components of care in SSs. These components include all those that formed the CEA model, including AIs, trigger avoidance measures, VIT and idiopathic anaphylaxis treatment.
Acknowledgements
Contribution of authors
Nigel Armstrong (Health Economist, KSR) led the CEA and wrote this section of the report.
Robert Wolff (Systematic Reviewer, KSR) led the systematic review and wrote this section of the report.
Ghislaine van Mastrigt (Health Economist, Maastricht, the Netherlands) contributed to the CEA and the writing of the report.
Nahara Martinez (Systematic Reviewer, Zurich, Switzerland) and Adrian V Hernandez (Assistant Professor of Medicine, Cleveland Clinic Lerner College of Medicine, Cleveland, OH, USA) were involved in screening of title, abstracts, and full texts and preparation of GRADE summary of findings tables.
Kate Misso formulated the search strategies, carried out searches and wrote this section of the report.
Jos Kleijnen (Director, KSR) supervised the project.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- The guidelines manual. January 2009. London: NICE; 2009.
- Owens DK, Lohr K, Atkins D, Treadwell J, Reston J, Bass EB, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions. Agency for Healthcare Research and Quality and the Effective Health-Care Program. J Clin Epidemiol 2010;63:513-23. http://dx.doi.org/10.1016/j.jclinepi.2009.03.009.
- Cianferoni A, Novembre E, Pucci N, Lombardi E, Bernardini R, Vierucci A. Anaphylaxis: a 7-year follow-up survey of 46 children. Ann Allergy Asthma Immunol 2004;92:464-8. http://dx.doi.org/10.1016/S1081-1206(10)61784-X.
- Decker KW, Bellolio MF, Campbell RL, Luke A, Anderson JL, Sauver J, et al. Recurrent anaphylaxis events in patients presenting to the emergency department over a 10-year period. Ann Emerg Med 2008;51. http://dx.doi.org/10.1016/j.annemergmed.2008.01.184.
- Mehl A, Wahn U, Niggemann B. Anaphylactic reactions in children: a questionnaire-based survey in Germany. Allergy 2005;60:1440-5. http://dx.doi.org/10.1111/j.1398-9995.2005.00909.x.
- Mugica Garcia M, Tejedor Alonso M, Rojas Perez Ezquerra P, Moro Moro M, Vila Albelda C, Rosado Ingelmo A, et al. A study of the recurrence of anaphylaxis 2010:5-9.
- Mullins RJ. Anaphylaxis: risk factors for recurrence. Clin Exp Allergy 2003;33:1033-40. http://dx.doi.org/10.1046/j.1365-2222.2003.01671.x.
- Desai U, Carroll NV. A cost-consequence analysis comparing an established and a novel epinephrine auto-injector for anaphylaxis. Value Health 2009;12. http://dx.doi.org/10.1016/S1098-3015(10)73680-9.
- Shaker MS. An economic evaluation of prophylactic self-injectable epinephrine to prevent fatalities in children with mild venom anaphylaxis. Ann Allergy Asthma Immunol 2007;99:424-8. http://dx.doi.org/10.1016/S1081-1206(10)60567-4.
- Krasnick J, Patterson R, Harris KE. Idiopathic anaphylaxis: long-term follow-up, cost, and outlook. Allergy 1996;51:724-31.
- Erlewyn-Lajeunesse M, Dymond S, Slade I, Mansfield HL, Fish R, Jones OS, et al. Diagnostic utility of two case definitions for anaphylaxis a comparison using a retrospective case notes analysis in the UK. Drug Saf 2010;33:57-64. http://dx.doi.org/10.2165/11318970-000000000-00000.
- Stewart AG, Ewan PW. The incidence, aetiology and management of anaphylaxis presenting to an Accident and Emergency department. QJM 1996;89:859-64. http://dx.doi.org/10.1093/qjmed/89.11.859.
- El-Shanawany T, Seddon L, Jolles S, Carne E, Dowd H, Williams P. Patients with anaphylaxis in accident and emergency are not referred to specialised allergy services. J Clin Pathol 2010;63. http://dx.doi.org/10.1136/jcp.2009.068577.
- Sheikh A, Hippisley-Cox J, Newton J, Fenty J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. J Roy Soc Med 2008;101:139-43. http://dx.doi.org/10.1258/jrsm.2008.070306.
- Gonzalez-Perez A, Aponte Z, Vidaurre CF, García Rodríguez LA. Anaphylaxis epidemiology in patients with and patients without asthma: a United Kingdom database review. J Allergy Clin Immunol 2010;125:1098-104.e1.
- Brown SGA. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 2004;114:371-6. http://dx.doi.org/10.1016/j.jaci.2004.04.029.
- Soar J, Pumphrey R, Cant A, Clarke S, Corbett A, Dawson P, et al. Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. Resuscitation 2008;77:157-69. http://dx.doi.org/10.1016/j.resuscitation.2008.02.001.
- British national formulary. London: BMA and RPS; 2007.
- Drummond MF, Sculpher MJ, Torrance GW. Methods for the Economic Evaluation of Health care programmes. Oxford Medical Publications. Oxford: Oxford University Press; 1997.
- Guide to the methods of technology appraisal. London: NICE; 2008.
- Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998;13:397-409. http://dx.doi.org/10.2165/00019053-199813040-00003.
- Office for National Statistics (ONS) . England and Wales Interim Lifetables 2011. www.gad.gov.uk/Demography%20Data/Life%20Tables/Interim_life_tables.html (accessed 28 March 2011).
- Simons FER, Ardusso LR, Bilò MB, El-Gamal YM, Ledford DK, Ring J, et al. World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis. J Allergy Clin Immunol 2011;127:13-37.
- Lieberman P, Nicklas RA, Oppenheimer J, Kemp SF, Lang DM, Bernstein DI, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 Update. J Allergy Clin Immunol 2010;126:477-80. http://dx.doi.org/10.1016/j.jaci.2010.06.022.
- Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011;127:S1-55.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Simons KJ, Simons FER. Epinephrine and its use in anaphylaxis: current issues. Curr Opin Allergy Clin Immunol 2010;10:354-61. http://dx.doi.org/10.1097/ACI.0b013e32833bc670.
- Simons FER. Epinephrine auto-injectors: first-aid treatment still out of reach for many at risk of anaphylaxis in the community. Ann Allergy Asthma Immunol 2009;102:403-9. http://dx.doi.org/10.1016/S1081-1206(10)60512-1.
- Simpson CR, Sheikh A. Adrenaline is first line treatment for the emergency treatment of anaphylaxis. Resuscitation 2010;81:641-2. http://dx.doi.org/10.1016/j.resuscitation.2010.04.002.
- Claxton K, Sculpher M, McCabe C, Briggs A, Akehurst R, Buxton M, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ 2005;14:339-47. http://dx.doi.org/10.1002/hec.985.
- Briggs A. Probabilistic analysis of cost-effectiveness models: statistical representation of parameter uncertainty. Value Health 2005;8:1-2. http://dx.doi.org/10.1111/j.1524-4733.2005.08101.x.
- Healthcare Resource Groups 2009–10. Leeds: NHS Information Centre for health and social care; n.d.
- Ewan PW, Clark AT. Long-term prospective observational study of patients with peanut and nut allergy after participation in a management plan. Lancet 2001;357:111-15. http://dx.doi.org/10.1016/S0140-6736(00)03543-1.
- Ewan PW, Clark AT. Efficacy of a management plan based on severity assessment in longitudinal and case-controlled studies of 747 children with nut allergy: proposal for good practice. Clin Exp Allergy 2005;35:751-6. http://dx.doi.org/10.1111/j.1365-2222.2005.02266.x.
- Sampson HA, Munoz-Furlong A, Bock SA, Schmitt C, Bass R, Chowdhury BA, et al. Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol 2005;115:584-91. http://dx.doi.org/10.1016/j.jaci.2005.01.009.
- Capps JA, Sharma V, Arkwright PD. Prevalence, outcome and pre-hospital management of anaphylaxis by first aiders and paramedical ambulance staff in Manchester, UK. Resuscitation 2010;81:653-7. http://dx.doi.org/10.1016/j.resuscitation.2010.01.021.
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy 2000;30:1144-50. http://dx.doi.org/10.1046/j.1365-2222.2000.00864.x.
- Pumphrey RSH, Gowland MH. Further fatal allergic reactions to food in the United Kingdom, 1999–2006. J Allergy Clin Immunol 2007;119:1018-19. http://dx.doi.org/10.1016/j.jaci.2007.01.021.
- Pumphrey RSH, Roberts ISD. Postmortem findings after fatal anaphylactic reactions. J Clin Pathol 2000;53:273-6. http://dx.doi.org/10.1136/jcp.53.4.273.
- Ambulance services England 2008 to 2009. London: NHS; 2010.
- Golden DB. Insect sting allergy and venom immunotherapy: a model and a mystery. Curr Rev Allergy Clin Immunol 2005;115:439-47. http://dx.doi.org/10.1016/j.jaci.2005.01.005.
- Krishna MT, Huissoon AP. Clinical immunology review series: an approach to desensitization. Clin Experimental Immunology 2011;163:131-46. http://dx.doi.org/10.1111/j.1365-2249.2010.04296.x.
- Goldberg A, Confino-Cohen R. Insect sting–inflicted systemic reactions: attitudes of patients with insect venom allergy regarding after-sting behavior and proper administration of epinephrine. J Allergy Clin Immunol 2000;106:1184-9. http://dx.doi.org/10.1067/mai.2000.110927.
- Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006;26:410-20. http://dx.doi.org/10.1177/0272989X06290495.
- Voordouw J, Fox M, Cornelosse-Vermaat J, Antonides G, Mugford M, Frewer L. Household costs associated with food allergy: an exploratory study. Br Food J 2010;112:1205-15. http://dx.doi.org/10.1108/00070701011088197.
- Neuner JM, Hamel MB, Phillips RS, Bona K, Aronson MD. Diagnosis and management of adults with pharyngitis: a cost effectiveness analysis. Ann Int Med 2003;139:113-22.
- Department of Health (DoH) . NHS Reference Costs 2009–10 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591 (accessed 30 September 2010).
- Diwakar L, Noorani S, Huissoon AP, Frew AJ, Krishna MT. Practice of venom immunotherapy in the United Kingdom: a national audit and review of literature. Clin Exp Allergy 2008;38:1651-8. http://dx.doi.org/10.1111/j.1365-2222.2008.03044.x.
- Higgins JPT, Green S. The Cochrane Collaboration, Version 5.1.0. 2011.
- Alves B, Sheikh A. Age-specific aetiology of anaphylaxis. Arch Dis Child 2001;85. http://dx.doi.org/10.1136/adc.85.4.348b.
- Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427-37.
Appendix 1 Literature search strategies
Appendix 2 Quality of evidence of included studies
Appendix 3 List of studies with rationale for inclusion or exclusion
List of studies with rationale for inclusion or exclusion (PDF download)
Appendix 4 Economic evaluation quality assessment
Appendix 5 Table of model parameters
List of abbreviations
- AI
- adrenaline injector
- BMJ
- British Medical Journal
- BNF
- British National Formulary
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- DAM
- decision-analytic model
- ED
- emergency department
- GDG
- Guideline Development Group
- GIN
- Guidelines International Network
- GP
- general practitioner
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- HES
- Hospital Episode Statistics
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IgE
- immunoglobulin E
- NICE
- National Institute for Health and Care Excellence
- ODA
- optimal discriminant analysis
- PICO
- population, intervention, comparison, outcome
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SC
- standard care
- SS
- specialist service
- VIT
- venom immunotherapy
- WTP
- willingness to pay
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.