Notes
Article history paragraph text
The research reported in this issue of the journal was funded by the HTA programme as project number 09/111/01. The contractual start date was in August 2010. The draft report began editorial review in February 2012 and was accepted for publication in October 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Beynon et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Executive summary
Background
During the course of a year, 36–48% of British adults recall having low back pain, with 58–62% of adults experiencing low back pain at some point in their lives. In the UK, the economic burden of back pain in terms of health-care costs and lost productivity is around £12B. In most cases, the pain will resolve after a few days or weeks, but in some patients pain may not resolve and becomes chronic. Patients with chronic pain often develop significant disability and have impaired quality of life. Patients may develop referred symptoms including pain, sensory disturbance (e.g. numbness) and weakness extending to the leg. In some patients, lower limb symptoms are caused by inflammation or compression of a spinal nerve root and this is termed lumbar radiculopathy (LR).
The exact cause of low back and leg pain may be difficult to diagnose. The distinction between radiculopathy and other types of referred lumbar spine pain is crucial for treatment planning. In carefully selected patients, decompressive lumbar surgery (e.g. discectomy) is more effective than conservative care in rapidly relieving leg pain and reducing disability. In most patients, the diagnosis of radiculopathy is made by careful correlation of clinical signs and symptoms (e.g. pain distribution, paresis, straight-leg raising test) and imaging findings (e.g. evidence of disc herniation and nerve root compression) from magnetic resonance imaging or computed tomography scans. Neither clinical findings nor anatomical imaging have perfect diagnostic accuracy and, not infrequently, the clinical and imaging findings are discordant. In patients with suspected LR in whom the clinical and imaging findings are equivocal or discordant, diagnostic uncertainty remains. Before embarking on invasive therapy (e.g. surgery) to decompress the lumbar nerve root, additional diagnostic tests such as selective nerve root blocks (SNRBs) are used to help clinicians decide between surgical and conservative care.
Objectives
This project aimed to:
-
Conduct a systematic review (SR) to determine the diagnostic performance of SNRB in patients with probable radicular pain that is not fully concordant with the imaging findings prior to lumbar decompression surgery.
-
Evaluate whether or not the diagnostic accuracy of SNRB varies by patient subgroup (e.g. patients with suspected radiculopathy at more than one level of the lumbar spine).
-
Conduct a SR to summarise the evidence on the incidence of procedure-related complications of diagnostic SNRB.
-
Conduct a SR of previous economic studies of the use of SNRB in patients with suspected LR and develop a cost-effectiveness model to evaluate the cost-effectiveness of using SNRB in patients with discordant clinical and imaging findings, including value of information analysis.
Methods
We developed and followed a protocol for all stages of the review. Studies were identified through searches of electronic databases, internet searches and scanning reference lists of included papers. Published and unpublished studies in any language were eligible for inclusion. Two reviewers screened titles and abstracts for relevance. Full papers of potentially relevant studies were obtained and assessed for inclusion by one reviewer and checked by a second. To be eligible for the diagnostic accuracy review, studies had to report on patients with low back pain and symptoms in a lower limb, and the diagnostic accuracy of SNRB administered under radiological guidance had to be assessed against a reference standard for the diagnosis of LR: studies had to report sufficient data to allow extraction of a 2 × 2 table of test performance. To be eligible for the review of adverse events, studies had to report the administration of a diagnostic SNRB in patients with LR. Data extraction was performed by one reviewer and checked by a second. Four reviewers independently assessed the quality of diagnostic accuracy studies using the quality assessment of diagnostic accuracy studies (QUADAS)-2 checklist and discrepancies were resolved through discussion. The methodological quality of studies reporting on adverse events was not formally assessed. Data were extracted to populate 2 × 2 tables of test performance and were used to calculate sensitivity, specificity and 95% confidence intervals (CIs). Study estimates of sensitivity and specificity were plotted in summary receiver operating characteristic space. Random-effects meta-analysis was used to calculate summary sensitivity and specificity separately for diagnostic cohort studies that used intraoperative findings and those that used post-surgical follow-up as a reference standard. Owing to the substantial differences between the control injections used in the within-patient case–control studies, we did not pool data from these studies. Data from studies on adverse events were combined in a narrative summary.
Based on data on diagnostic accuracy from the SR, we developed a decision tree and Markov model to estimate the incremental costs and effects of adding SNRB to the diagnostic work-up of suspected LR. The effectiveness and post-treatment costs of surgery and conservative care were taken from randomised controlled trial (RCT) evidence. Evidence about additional parameters of the model was identified from the medical literature and routine data sources. We developed two models to estimate the incremental cost per quality-adjusted life-year (QALY) in patients with suspected single- and two-level nerve root compression.
Results of the diagnostic accuracy systematic review
The searches identified 11,211 titles and abstracts; of these, 138 were considered potentially relevant, retrieved and screened as full papers. Five studies (n = 241 patients; range 15–83 per study) were included in the review: two within-patient case–control studies, one prospective diagnostic cohort study and two retrospective diagnostic cohort studies. In all patients in the case–control studies, the source of the radiculopathy was confirmed by concordant clinical and radiological or surgical findings prior to the use of SNRB. These concordant findings formed the reference standard against which the results of injections at the symptomatic nerve root and adjacent asymptomatic sites were compared. The diagnostic cohort studies recruited patients with suspected LR but equivocal or discordant clinical and radiological findings. These studies used intraoperative findings and/or outcome following surgery as the reference standard.
There was substantial variation in the results of the studies: sensitivity ranged from 57% to 100% and specificity from 10% to 86%. All studies were judged to be at a high risk of bias. Both within-patient case–control studies selected patients with concordant clinical and imaging findings and, therefore, their findings were judged to have poor applicability to patients with discordant clinical and imaging findings. All three cohort studies were judged to be at a high risk of bias, as the decision to perform surgery (the reference standard) was not independent of the SNRB result. The reference standard was judged to be at a high risk of bias in all five studies, as there was no gold standard for the diagnosis of radiculopathy.
Based on the two cohort studies that used an intraoperative reference standard the sensitivity was 93.5% (95% CI 84.0% to 97.6%) and specificity was 50.0% (95% CI 16.8% to 83.2%). Summary sensitivity was similar in the two studies that used post surgery as the reference standard at 93.3% (95% CI 85.8% to 97.0%), but specificity was lower at 25.6% (95% CI 5.4% to 67.5%). Owing to the differences in patient selection, type of control injection and reference standards between within-patient case–control studies and the diagnostic cohort studies we decided that it would be inappropriate to statistically combine the results of these studies.
Results of the selective nerve root block-related adverse events systematic review
Seven studies reported on SNRB-related adverse events. Only one study reported on the complications of SNRBs as the primary outcome of interest. This study found that minor and transient complications were encountered in 98 of the 1777 total patient visits (during which 2217 injections were delivered to 1203 patients), giving an overall per-patient visit complication rate of 5.5%. One other study reported that complications were encountered in four patients (3.8%) who experienced aggravated pain for 1–2 days following SNRB. The remaining five studies (range n = 15–117) reported that there were no complications. None of the studies reported major or permanent complications resulting from SNRB.
Results of the economic evaluation
Our economic model estimated that, for patients with suspected single-level nerve root compression, the addition of SNRB to the diagnostic work-up was not cost-effective, with an incremental cost per QALY gained of £1,576,000, which is greater than conventional thresholds for acceptable cost-effectiveness. SNRB was not cost-effective even when the societal savings of earlier return to work were included. The use of SNRB for suspected multilevel nerve root compression was less cost-effective. A range of probabilistic and deterministic sensitivity analyses confirmed that SNRB was unlikely to be a cost-effective method for diagnosis and planning surgical therapy. However, our conclusions were sensitive to assumptions about the continuing clinical effectiveness and cost savings of surgery beyond 1 year. Under the optimistic assumption that the economic benefits of surgery reported by RCTs at 1 year continue undiminished in subsequent years, then SNRB became cost-effective from the perspective of society, despite relatively poor diagnostic accuracy.
Discussion
There were few studies that estimated the diagnostic accuracy of SNRB in patients with low back pain and radiculopathy who have discordant or equivocal clinical and imaging findings. Research on this topic is hampered by the lack of a diagnostic gold standard against which to compare tests such as SNRB. We identified five diagnostic accuracy studies, all at high risk of bias. Of particular concern was the fact that many studies were at risk of verification bias as patients with a positive SNRB were more likely to undergo surgery (the reference standard) than those testing negative. There was substantial variation in estimates of sensitivity and specificity across studies; sensitivity ranged from 57% to 100% and specificity from 10% to 86%. Based on the two cohort studies that used post-surgery outcomes as the reference standard, the summary sensitivity was 93.3% (95% CI 85.8% to 97.0%) and summary specificity was 25.6% (95% CI 5.4% to 67.5%). However, conclusions based on these data should be tempered because of the large CIs around specificity and the high risk of bias which affects these studies.
Two previous SRs on the topic have been supportive of the diagnostic use of SNRB. The more recent review concluded that there was ‘moderate evidence for SNRBs in the preoperative evaluation of patients with negative or inconclusive imaging studies, but with clinical findings of nerve root irritation’. Based on our review of the evidence, we believe that these conclusions are too strong. The differences in interpretation between our review and those conducted previously may be partly owing to our use of more rigorous eligibility criteria, restricting analysis to studies that provided sufficient data to construct estimates of sensitivity and specificity.
Despite case reports of serious adverse events associated with SNRB, our SR confirmed that these were very rare events. Of the seven studies identified that reported on complications and adverse events of SNRB (n > 1500 patients), no serious adverse events were reported. The largest case series reported minor and transient complications in 5.5% of patient visits, but no major or permanent complications.
Our economic model indicated that, in patients with suspected single-level nerve root compression, SNRB does increase the proportion of patients with an accurate diagnosis of the presence or absence of nerve root compression (59.5% vs 50%) and the proportion of patients with nerve root compression who undergo surgery (20.1% vs 18%). However, these benefits do not appear to be justified by the additional costs of testing. The incremental cost per additional case accurately diagnosed was £2674 and the incremental cost per QALY gained was £1,576,007. In comparison with other health interventions, reviewed by the National Institute for Health and Care Excellence on behalf of the NHS, this does not represent good value for money. This conclusion was the same for patients with suspected two-level nerve root compromise and was not altered in sensitivity analyses varying several key assumptions of the model, including prevalence, the diagnostic accuracy of SNRB and the impact of the SNRB result on the probability of performing surgery. The model was sensitive to assumptions about the long-term costs and benefits of surgery. If the residual improvement in quality of life (utility) scores and the savings in costs observed in the surgical arm of trials at 12 months post randomisation continues, rather than diminishes over time, then SNRB has the potential to be cost-effective, despite low specificity. However, we conclude that it is unlikely based on the current evidence that SNRB is a cost-effective method for informing the decision to operate in patients with low back and leg pain where there is doubt about the localisation of the lesion.
Conclusions
There were few studies that estimated the diagnostic accuracy of SNRB in patients with radiculopathy and discordant or equivocal imaging findings. All studies were limited by the difficulty of making a reference standard diagnosis in all patients who were tested. The evidence that is available suggests that the specificity of SNRB is relatively low. Therefore, based on current weak evidence, it is unlikely that SNRB is a cost-effective method for determining which patients will benefit from lumbar surgery.
Implications for service provision
Our review highlights the uncertain value of SNRBs when used for diagnostic purposes to establish whether or not clinical symptoms result from a particular nerve root. However, the distinction between diagnostic and therapeutic SNRBs is often not straightforward. Many centres combine local anaesthetic and periradicular steroid injections in order to gain both diagnostic information and, potentially, longer-term pain relief for the patient. Evidence collated in SRs confirms that transforaminal epidural steroid injections can be an effective and cost-effective part of a treatment strategy for patients with radicular pain.
Better evidence is needed to inform practice in centres that currently rely on SNRB for diagnostic information to help decide whether, or at which level, to perform lumbar decompressive surgery. These centres could perform SNRB procedures as part of research projects to improve the evidence base.
Suggested research priorities
Our recommendations for future research are:
-
A large rigorous diagnostic cohort study to determine the diagnostic accuracy of SNRB in predicting the short-term outcome of lumbar surgery in patients with suspected radiculopathy but equivocal or discordant clinical and radiological findings.
-
Separate or nested diagnostic cohort studies to identify the optimal SNRB technique (e.g. optimal anaesthetic dose, the value of needle provocation and control injections at adjacent sites).
-
A RCT to measure the impact of diagnostic SNRB on treatment decisions and the costs and outcomes of care for (subgroups of) patients with discordant or equivocal clinical and imaging findings of nerve root compression.
Funding
Funding for this study was provided by the Health Technology Assessment programme of the National Institute for Health Research.
Chapter 1 Background
Prevalence and cost of low back pain
On any single day, 15–19% of adults in the UK report symptoms of low back pain. 1,2 During each year 36–48% of adults recall having low back pain1–3 and 58–62% of the people will have low back pain at some point in their lives. 2–4 The economic burden of back pain in the UK, including health-care costs and lost productivity, is approximately £12B. 5 In most acute cases seen in primary care the pain is limited to the lower back and will resolve after a few days or weeks. However, some patients develop chronic pain and disability. 6 Others have referred symptoms of pain, sensory disturbance (e.g. numbness) and weakness in the leg. In a small group of patients with low back pain, the underlying cause of symptoms is lumbar radiculopathy (LR), and this can be due to inflammation or compression of a spinal nerve root.
Frequency of lumbar decompressive surgery, clinical effectiveness and cost-effectiveness
Substantial numbers of patients with persistent low back pain are treated surgically. In 2009–10 there were > 9300 primary excisions of a lumbar intervertebral disc performed on NHS inpatients in England. A further 12,500 patients underwent other primary decompression operations on the lumbar spine. 7
Randomised trial evidence on the clinical effectiveness and cost-effectiveness of lumbar decompressive surgery in patients with radiculopathy and intervertebral disc herniation is not definitive. A recent systematic review (SR) of the topic identified five randomised controlled trials (RCTs) comparing surgery with conservative care (n = 4) or epidural injections (n = 1). 8 The review authors did not pool trial results in a meta-analysis because of clinical heterogeneity and poor reporting of data. One Dutch RCT, considered by the review authors to be at low risk of bias, randomised 283 patients who had had severe sciatica for 6–12 weeks and radiologically confirmed disc herniation to early lumbar discectomy (within 2 weeks) or prolonged conservative treatment with surgery if needed. 9,10 This trial concluded that surgery led to faster pain relief (2 and 8 weeks post randomisation), but there were no differences between the groups after 1 year. Another trial conducted in the USA, also considered to be at low risk of bias, randomised 501 patients with persistent radiculopathy and imaging evidence of lumbar disc herniation (LDH) to open discectomy or non-operative care. 11 The intention-to-treat analysis showed no statistically significant differences in any of the primary outcome measures, but there was considerable crossover between the randomly assigned groups. Interpretation of the trial was thus hampered by substantial non-compliance with treatment allocation (57% of patients randomised to surgery received it within 6 months, whereas 39% of patients randomised to non-operative care also received surgery within 6 months).
Cost-utility analysis conducted alongside the Dutch trial suggested that the cost of surgery was not offset by reductions in other health services resulting in net additional health service costs of 1819 (or £1449). 12 Nevertheless, the early surgery group experienced a greater increase in quality-adjusted life-years (QALYs) owing to faster relief of symptoms, and the cost per QALY gained of surgery was 41,000 (approximately £32,930). Given that the National Institute for Health and Care Excellence (NICE) threshold for defining cost-effective use of health service money is £20,000–30,000 per QALY,13 the economic case for surgery seems finely balanced. However, the Dutch trial found that if the productivity savings and other non-health-care costs are factored into the economic analysis, early surgery becomes marginally cost-saving and probably cost-effective from the perspective of society.
Observational work demonstrates that the pain, function and mental health status of patients with LR improves significantly after lumbar discectomy. 14 However, surgery is not universally successful. In the Maine Lumbar Spine Study, only 70% of surgically treated patients reported improved pain, 63% were satisfied with the outcome and 19% had had at least one reoperation at 5-year follow-up. 15 In 2009–10 there was one revision lumbar discectomy for every nine primary lumbar discectomies performed on NHS patients. 7 Improved diagnosis could help identify patients most likely to benefit from surgery and minimise the cost and risks associated with unsuccessful back surgery.
Diagnosis of the cause of low back pain and radicular symptoms
A timely and accurate diagnosis of the cause of low back pain is important, as it is occasionally an early symptom of a serious systemic disease or more complex spinal disease including tumour and infection. 16 However, the exact cause of low back pain is often difficult to diagnose. The distinction between radiculopathy and other types of referred lumbar spine pain is crucial for treatment planning. Radiculopathy is almost always caused by inflammation and/or compression of a nerve root, and the most common causes of compression are herniation of an intervertebral disc or stenosis of the lumbar canal usually in the lateral recess or occasionally in the neural foramen. Patients with compressive radiculopathy stand to benefit the most from surgical decompression of the nerve root (e.g. lumbar discectomy).
In most patients, the diagnosis of radiculopathy is made by careful correlation of clinical signs and symptoms (e.g. pain distribution, paresis, straight leg raising test) and imaging findings [e.g. evidence of disc herniation and nerve root compression on magnetic resonance imaging (MRI) or computed tomography (CT) scanning]. Neither clinical findings17 nor radiological imaging have perfect diagnostic accuracy. Patients often find it difficult to define the boundaries of their leg pain, sensory disturbance or weakness. MRI studies on volunteers have demonstrated surprisingly high rates of asymptomatic disc protrusions and extrusions with associated nerve root compression. 18 Therefore, clinical and imaging evidence of nerve root compression may not be concordant. In a prospective study of patients with clinical findings of low back pain without lower limb symptoms (n = 150) or with LR (n = 96), Modic et al. 19 found MRI evidence of nerve root compression in both groups (27% of low back pain patients and 46% of radiculopathy patients). Diagnosis may be further complicated in the subgroup of patients who have nerve root anomalies20 or bony malformations of the lumbosacral junction. 21 For patients with suspected LR in whom the clinical and imaging findings are equivocal or discordant, diagnostic uncertainty remains about the nature and source of the symptoms and, therefore, whether or not the patient is a good candidate for surgery to decompress the lumbar nerve root. In these cases, additional diagnostic tests such as selective nerve root blocks (SNRBs) could help clinicians and patients to choose between surgical and conservative care.
Diagnostic selective nerve root blocks
Selective nerve root blocks have been employed since the 1930s as a method of confirming the source of radicular pain prior to surgery. 22 Diagnostic SNRB consists of injection of local anaesthetic (e.g. 1 ml of 2% lidocaine) or other substances (e.g. corticosteroids) around a spinal nerve under imaging guidance. Both provocative responses (replicating the patient's symptoms during needle placement) and analgesic responses (significant reduction of symptoms after injection of anaesthetic) to SNRB may be diagnostically useful in confirming or ruling out a nerve root as the source of clinical symptoms. The diagnostic role of SNRBs has narrowed with the advent of imaging techniques such as MRI, which depict in exquisite detail the bony and soft tissue structures of the lumbar spine. Nevertheless, SNRBs are still used to identify the putative symptomatic nerve root in patients with probable radicular pain that is not fully concordant with the radiological findings or who have nerve root anomalies or transitional vertebrae. 23 Recent international consensus statements have concluded that properly performed diagnostic SNRBs ‘... are useful when the location of symptoms seems to conflict with abnormalities identified with imaging findings ...’,24 although the evidence on this topic was categorised as being of only moderate quality. The diagnostic value of SNRB should be weighed against the costs and the small risk of complications associated with the procedure such as leg weakness or exacerbation of pain. 25 Very rarely, there have been case reports of more serious complications, such as paraplegia. 26
The diagnostic accuracy of selective nerve root blocks
Researchers have evaluated the diagnostic role of SNRB for > 30 years. 27,28 However, many of the early studies are more correctly described as ‘technical performance’ rather than ‘diagnostic accuracy’ studies. Technical performance studies evaluate the most valid processes for performing SNRB (e.g. amount of anaesthetic used or positioning of needle tip) rather than formal measures of diagnostic accuracy such as sensitivity or specificity. The diagnostic accuracy of SNRB is difficult to evaluate because of a lack of an obvious reference standard against which to compare it.
Studies have used either a diagnostic within-patient case–control design or a diagnostic cohort design to evaluate the diagnostic accuracy of SNRB. In the within-patient case–control design, patients have clear clinical signs of radiculopathy and imaging findings of nerve root compression at the corresponding lumbar spine level. These patients are given a SNRB at that ‘case’ level in the expectation that, if the test is sensitive, then radicular symptoms will be temporarily relieved by the anaesthetic. A ‘control’ injection is also performed on the same patient at a different site in the lumbar spine (e.g. an adjacent nerve root), in the expectation that, if the test is specific, the control injection will not affect radicular symptoms. Standard case–control studies are criticised for inducing spectrum bias as the cases and controls are considered not to be representative of patients in whom SNRB would be used in actual practice. 29 This criticism also applies to the within-patient case–control design, in which patients are selected based on concordance between clinical and imaging findings. In practice, in patients who receive SNRB, there is likely to be some discordance in the clinical and MRI findings.
In the diagnostic cohort study design, a group of patients with suspected radiculopathy undergo the index test (SNRB) and the result of this index test is compared with a reference standard. For LR, the reference standard is usually some combination of surgical findings, surgical outcomes and outcomes of conservative care. All of these standards fall well short of being a gold standard (e.g. a poor surgical outcome might result from poor surgical technique rather than incorrect diagnosis of radiculopathy).
The potential therapeutic impact and cost-effectiveness of selective nerve root blocks
Lumbar spine nerve root injections with ‘therapeutic’ rather than ‘diagnostic’ intent may have an effect on reducing leg pain and the proportion of patients who eventually have surgery. 30 A SR of the topic, conducted in 2009, including nine RCTs, concluded that there is fair evidence that transforaminal epidural steroid injections are superior to placebo for treating radicular symptoms and good evidence that these injections can be used to avoid surgery. 31 However, the independent role of the anaesthetic and the steroid in this overall treatment effect is less clear. A double-blinded RCT comparing up to four nerve root injections using either anaesthetic alone (1 ml of 0.25% bupivacaine) or anaesthetic plus steroid [1 ml betamethasone (6 mg/ml)] in 55 patients with lumbar radicular pain found that the addition of betamethasone increased the proportion of patients deciding not to have surgery from 33% to 71% (p < 0.01). 32 A further publication on this study followed up the patients who had avoided surgery for 5 years and found that the majority of patients who avoided an operation for at least 1 year after receiving a nerve root injection with bupivacaine alone or in combination with betamethasone continued to avoid operative intervention for a minimum of 5 years. 33 Conversely, a larger (n = 150) double-blind RCT conducted in the UK randomised patients with unilateral leg pain and MRI-confirmed nerve root compromise to receive 2 ml of 0.25% bupivacaine alone or with 40 mg of methylprednisolone (Depomedrone®, Pfizer). The trial found no significant differences between groups in Oswestry Disability Index or leg pain visual analogue scale (VAS) scores at 6 or 12 weeks after randomisation. There was no strong evidence that rates of surgery at 1 year were different between the anaesthetic and anaesthetic plus corticosteroid arms (21.5% vs 14.1%; p = 0.38). 34
The impact of ‘diagnostic’ SNRB results on treatment decisions is less well studied, but the potential for diagnostic and therapeutic impact is large. Primary excisions of lumbar intervertebral disc procedures involve a mean inpatient stay of 3.2 days, totalling 30,738 days in NHS hospitals in England annually. 7 This use of acute-care resources, combined with additional NHS costs and productivity losses associated with rehabilitation from surgery, suggest that a minimally invasive test that accurately differentiates patients who will or will not benefit from surgery has the potential to be cost-effective. However, in order to evaluate this, unbiased evidence on the diagnostic accuracy of SNRB, procedure-related complications and the impact of diagnosis on surgical management and the cost and outcomes of therapy should be combined in a formal decision analysis.
Chapter 2 Research questions
This evidence synthesis aimed to determine whether or not SNRBs result in more accurate diagnosis in patients considered for lumbar decompression surgery where there is doubt about the localisation of the lesion based on clinical signs and imaging findings (e.g. MRI). We developed an economic model to evaluate the extent to which improvements in diagnostic accuracy lead to more cost-effective care for this patient group and subgroups within it. Specifically, the project addressed the following objectives:
-
Conduct a SR to determine the diagnostic performance of SNRB in patients with probable radicular pain that is not fully concordant with the imaging findings prior to lumbar decompression surgery.
-
Evaluate whether or not the diagnostic accuracy of SNRB varies by patient subgroups (e.g. patients with suspected radiculopathy at more than one level of the lumbar spine).
-
Conduct a SR to summarise the evidence on the incidence of procedure-related complications of diagnostic SNRB.
-
Conduct a SR of previous economic studies of the use of SNRB in patients with suspected LR and develop a cost-effectiveness model to evaluate the cost-effectiveness of using SNRB in patients with discordant clinical and imaging findings, including value of information analysis.
Chapter 3 Systematic review methods
Search strategy
Studies were identified by searching the following databases from inception to 18 August 2011: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Science Citation Index, Bioscience Information Service (BIOSIS) and Latin American and Caribbean Health Sciences Literature (LILACS). In addition, information on studies in progress, unpublished research or research reported in the grey literature was sought from a range of relevant databases including Inside Conferences, Dissertation Abstracts and National Technical Information Service (NTIS). We combined terms for SNRB [e.g. ‘exp Nerve Block/’ or ‘(nerve adj3 block$).tw.’ or ‘SNRB.tw.’ or ‘(neural adj3 block$).tw.’ or ‘(nerve adj3 injection$).tw.’ or ‘(nerve adj3 infiltration).tw.’] with terms for the target condition [e.g. ‘radiculopath$.tw.’ or ’radiculitis.tw.’ or ‘(radicular adj3 pain).tw.‘ or ‘Sciatica/’ or sciatica.tw.’]. We did not use a methodological search filter to identify diagnostic accuracy studies as such filters result in the omission of relevant studies. 35,36 There was no restriction of study by country of origin, language or publication date. Attempts were made to identify further studies by examining the reference lists of all retrieved articles and previous reviews. Full details of the search strategies and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist are given in Appendices 1 and 2, respectively.
Inclusion and exclusion criteria
Two reviewers independently screened titles and abstracts for relevance to any of the three SRs: any disagreements were resolved by consensus or referral to a third reviewer. The full text of potentially relevant studies was obtained and assessed for inclusion by one reviewer and checked by a second. Full-text articles were also assessed for inclusion in the review of economic evaluations by a health economist. Articles were selected according to the criteria in Table 1. In cases where we were unable to extract 2 × 2 tables of test performance from otherwise eligible diagnostic accuracy studies, we contacted study authors to request this information.partial expected value of perfect information.
| Review of diagnostic accuracy | Review of procedure related complications | Review of economic evaluations | |
|---|---|---|---|
| Population | Patients with low back pain and symptoms in a lower limb | ||
| Target condition | LR | ||
| Index test | Diagnostic SNRB administered under radiological guidance | ||
| Reference standard | Any reported reference standard, e.g. surgical findings and/or clinical outcomes | N/A | N/A |
| Outcome(s) | Sufficient data to construct 2 × 2 contingency tables | Transient and permanent adverse events | Cost-effectiveness, cost-utility, cost-benefit, cost-consequence study |
| Study design | Diagnostic cohort or (within-patient) case-control studies | Any study design with at least 15 patients | RCTs, controlled studies, decision analyses |
Data extraction
Data extraction was performed by one reviewer and checked by a second. Microsoft Access (Microsoft Corporation, Redmond, WA, USA) data extraction forms were developed and piloted on two studies. The following data were extracted, where reported: study details (identifier, study design, location); participant details (age, sex, previous surgery, duration of radicular symptoms, inclusion criteria); SNRB details (criteria for a positive test, needle gauge, site of injection, anaesthetic name and dose, corticosteroid name and dose, method of imaging guidance, contrast agent, lumbar levels evaluated); and reference standard details (if applicable) (intraoperative findings, outcome after follow-up or other). For diagnostic accuracy studies we extracted 2 × 2 data on test performance [i.e. number of true-positives (TPs), false-negatives (FNs), false-positives (FPs), true-negatives (TNs)]. For studies of adverse events, we extracted data on the type, number, severity and duration (acute/chronic) of adverse events. Data were extracted at the patient level, unless unavailable, and then injection level was used.
Quality assessment
Diagnostic accuracy studies were assessed for methodological quality using the quality assessment of diagnostic accuracy studies (QUADAS)-2 tool. 37 This tool assessed study quality in terms of risk of bias and concerns regarding applicability and includes domains covering patient selection, index test, reference standard and patient flow.
Bias occurs if the results of a study are distorted by systematic flaws or limitations in its design or conduct (e.g. knowledge of the index test result when interpreting the reference standard). Applicability may be reduced if patient demographic and clinical features, or the use or interpretation of the index test in the diagnostic accuracy study differ from those specified in the SR research question. Reviewers rate concerns regarding applicability and risk of bias as low, high or unclear.
The first section of QUADAS-2 asks reviewers to state their review question in terms of the relevant patient group, index test, target condition and reference standard(s). This is to aid with judgements of applicability – for example, if a study enrols a slightly different patient group, then it would be judged as having high concerns regarding applicability. We defined the review question for the diagnostic accuracy review as:
Patients Patients with low back pain and radiculopathy (or sciatica) with non-congruent imaging and clinical findings who might benefit from lumbar decompression surgery.
Index test SNRB including injection of anaesthetic close to the lumbar nerve root under guidance by fluoroscopy or other imaging.
Target condition Radiculopathy (or sciatica) amenable to surgery.
Reference standard Outcome following surgery.
Full details of the QUADAS-2 checklist, adapted for our review, are provided in Appendix 3. QUADAS-2 forms were developed in Microsoft Access. We did not formally assess the quality of studies of adverse events or economic evaluations. Quality assessment was carried independently by four reviewers and the responses compared. Disagreements were resolved through consensus.
Data analysis
We calculated sensitivity and specificity together with 95% confidence intervals (CIs) for each set of 2 × 2 data. We plotted estimates of sensitivity and specificity from individual studies in summary receiver operating characteristic space. For the cohort studies, we estimated summary sensitivity and specificity together with associated CIs using univariate logistic regression random-effects meta-analysis. There were insufficient data to allow use of the more statistically robust bivariate/hierarchical summary receiver operating characteristic models. Analyses were stratified according to whether findings at surgery or outcome following surgery were used as the reference standard. Owing to the small number of studies that assessed adverse events and economic evaluations, a narrative synthesis was used to combine findings.
Chapter 4 Results of the systematic review of diagnostic accuracy
Details of included studies
The searches identified 11,211 titles and abstracts; of these, 138 were considered potentially relevant, retrieved and screened as full papers and five studies (n = 241 patients; range 15–83 per study) were included (see Appendix 5 and Figure 1). Details of studies excluded following full-paper screening are included in Appendix 4.
FIGURE 1.
Flow chart of study selection process.
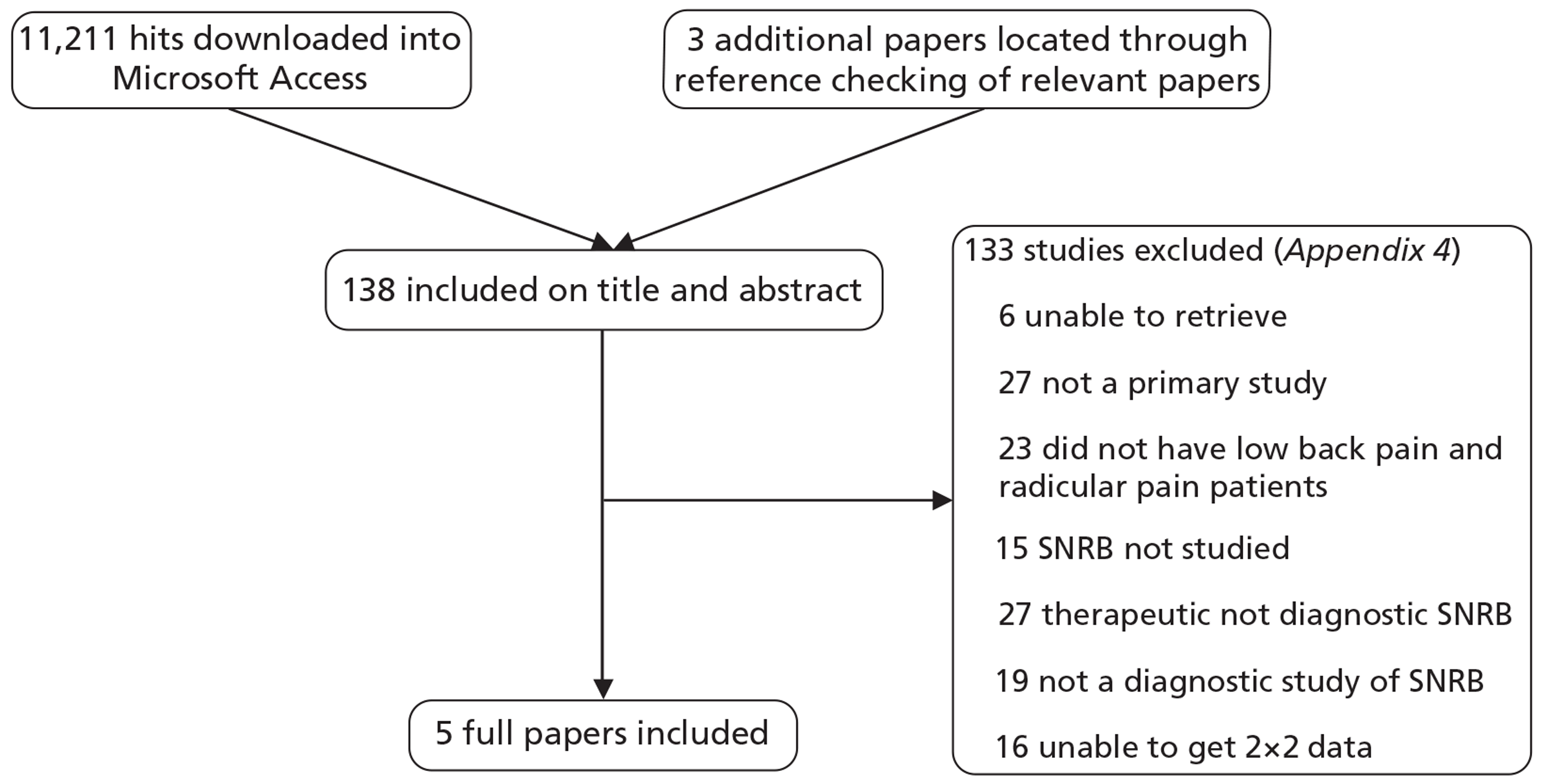
Two studies were within-patient case–control studies,38,39 one was a prospective diagnostic cohort study28 and two were retrospective diagnostic cohort studies40,41 (Table 2). Publication dates ranged from 1973 to 2008. All studies were conducted in secondary care and enrolled small numbers of patients (range 15–83 patients). Where reported, the mean age of patients ranged from 46 to 47 years, the majority were male, most had experienced symptoms for at least 3 months, and the proportion who had undergone previous surgery ranged from 0% to 48%.
| Author (year), country | n analysed/n recruited | Inclusion criteria | Exclusion criteria | Description of included patients | Details of previous surgery | Mean age (years) | % male | Recruitment years | Mean symptom duration (range) |
|---|---|---|---|---|---|---|---|---|---|
| Within patient case-control studies | |||||||||
| Yeom38 (2008), NR | 47/83 | Patients who were to undergo a lumbar spine operation with single-level, unilateral lumbosacral radiculopathy confirmed by clinical, radiographic and MRI findings | Multilevel or bilateral neural compression on MRI, prolonged pain relief after first injection that precluded evaluation of next block; operations cancelled because of persistent pain relief after SNRB | Patients with established pure radiculopathy from a single level. Affected roots were L4 in 3, L5 in 31, S1 in 13. Concordant imaging and clinical findings | No history of lumbar surgeries | 47 (18–76) | 60 | 2005–6 | 3 months (15 days–3 years) |
| North39 (1996), USA | 33/33 | Patients with sciatica with or without low back pain, attributed to spinal pathology | Active issues of secondary gain or compensation; prominent signs of somatisation. Symptoms indicating a lesion in sciatic nerve or its branches, or piriformis entrapment | Established sciatica patients with or without low back pain. All had L5 or S1 radiculopathy. 52% had diagnostic imaging findings of ongoing nerve root compression. The remaining 48% had a well-documented history of root compression which had been corrected surgically | 48% history of root compression corrected surgically | 46 (24–70) | 73 | NR | > 6 months |
| Prospective diagnostic cohort studies | |||||||||
| Schutz28 (1973), Canada | 15/23 | Patients with current sciatica | NR | Patients with sciatica Investigation undertaken only at a time when sciatica symptoms actually present | One patient had previous surgery, unsuccessful SNRB and excluded from analysis. Unclear if patients included in analysis had previous surgeries | NR | NR | NR | NR |
| Retrospective diagnostic cohort studies | |||||||||
| Sasso40 (2005), USA | 83/83 | Patients who underwent selective nerve root injections, MRI and nerve root decompression surgery and had a follow-up evaluation > 12 months post surgery | NR | Patients with cervical or LR. Discordant imaging and clinical findings | Unclear how many previous lumbar surgeries. Twenty patients with cervical or lumbar symptoms had previous surgery [15 patients at same and/or adjacent level(s) and five patients at non-adjacent levels] | NR | NR | 1996–9 | 4.7 months (1.5–27) |
| Dooley41 (1988), Canada | 62/73 | Patients who underwent NRI | NR | Patients with radicular pain who underwent NRI | 32 patients with one or more previous surgeries Three patients had four surgeries | NR | NR | 1982–3 | NR |
The two within-patient case-control studies confirmed the symptomatic nerve root in all enrolled patients by concordant clinical and radiological or surgical findings prior to the use of SNRB. The specificity of SNRB in these two studies was evaluated through control injections; in the Yeom et al. 38 study these were given at adjacent asymptomatic nerve roots, whereas in the North et al. 39 study three other anatomical sites in the lumbar spine were injected (sciatic nerve, facet joint and subcutaneous). However, North et al. 39 present diagnostic accuracy data based only on the control injection at the sciatic nerve, and the other two control injection sites are not considered further in this review. The three diagnostic cohort studies recruited patients with suspected LR where some doubt remained because of equivocal or discordant clinical and radiological findings. These studies used intraoperative findings and/or outcome following surgery as reference standards.
Details of the injections used in the included studies are given in Table 3. The type of local anaesthetic given differed between studies (lidocaine, bupivacaine, procaine or mepivacaine), with doses ranging from 1 ml to 3 ml: none of the studies combined the anaesthetic with a steroid. Four studies reported using fluoroscopic guidance for needle placement;38–41 one study did not specify the guidance method. 28 Four studies reported using a contrast agent. 28,38,40,41 Needle provocation of the nerve root was conducted in four studies. 28,38,40,41 Similarly, three studies28,38,39 carried out up to three control injections at adjacent asymptomatic levels (two38,39 being the within-patient case-control studies). The third28 used the control injections in order to try to increase the sensitivity and specificity of patient responses to injections at the level thought to be symptomatic. Post-procedure assessment of pain response was often not well described and varied from immediate pain relief to detailed assessment every 15 minutes for the first 3 hours.
| Author (year) | Clinician | Needle gauge | Needle position | Needle level | Anaesthetic name | Anaesthetic concentration | Anaesthetic dose | Steroid used | Guided method | Contrast agent used | Needle provocation | Control injections | Time to pain measurement | Time before surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yeom38 (2008) | Spinal radiologist | 22 | Neural foramen near target nerve root | L3, L4, L5, S1 | Lidocaine | 2% | 1 ml | No | Fluoroscopy | Yes – 1 ml iohexol myelographic | No | 1 or 2 | 30 minutes | 1–2 days |
| North39 (1996) | Individual with extensive experience | NR | Lumbosacral root at L5 or S1 foramen | L5, S1 | Bupivacaine | 0.5% | 3 ml | NR | Fluoroscopy | NR | Yes | 3 | Every 15 minutes for 3 hours | NR |
| Schutz28 (1973) | NR | NR | The superior level of the intervertebral foramen around the nerve root | L4, L5, S1 | Procaine | NR | 1 ml | NR | Guided but method NR | Yes – 1 ml ethodian | Yes | 1 or 2 | Immediate | NR |
| Sasso40 (2005) | NR | 22, 21 or 20 | The anterosuperior aspect of the neuroforamen of the selected nerve root | NR | Lidocaine | 2% | 0.5–0.75 ml | NR | Fluoroscopy | Yes – 0.25–0.75 ml Iohexol | Yes | NR | Immediate | 1–3 months |
| Dooley41 (1988) | NR | 18 | The proximal end of the intravertebral foramen for lumbar nerve roots. Needle introduced vertically through the posterior foramen for the first sacral nerve root | L3, L4, L5, S1 | Mepivacaine or lidocaine | 1% | 1 ml | NR | Fluoroscopy | Yes – ethyl iodophenyl undecylate | Yes | NR | Immediate | NR |
There was no consistency in the degree of post-test pain relief, which was defined as indicating a positive SNRB result (see Table 5). Three studies28,38,39 used a quantification of pain relief ranging from 50% relief to 100% relief. Sasso et al. 40 defined a positive SNRB as a post-injection visual analogue scale (VAS; 0–10) pain score of 0 or 1 and immediate relief of > 95% of the patient's extremity pain, even when pain-provoking manoeuvres were performed. Dooley et al. 41 reported response to SNRB in four groups based on all four permutations of whether or not typical pain had been recreated when the needle was inserted (yes/no) and whether or not the complete pain relief was achieved after injection of anaesthetic (yes/no). Data from this study can, therefore, be combined in different ways giving results at each threshold. We selected patients who had either a group 1 (typical pain on needle insertion and relief of pain following SNRB) or a group 3 (no typical pain on needle insertion, but relief of pain following SNRB) response as our threshold for a positive test. This definition is most similar to the other studies that did not use pre-injection pain provocation when interpreting the index test result; it is also most applicable to centres that do not perform needle provocation, relying solely on the response to anaesthesia to reach a diagnosis.
Two studies28,41 used intraoperative findings as the reference standard (see Table 5). One of these studies41 also used outcome following surgery as a second reference standard. Sasso et al. 40 used outcome at 12 months following surgery. The two within-patient case-control studies used concordant symptoms and imaging evidence of nerve root compression (or lack of) as the reference standard for injections given at the symptomatic (or adjacent) sites.
Quality of included studies
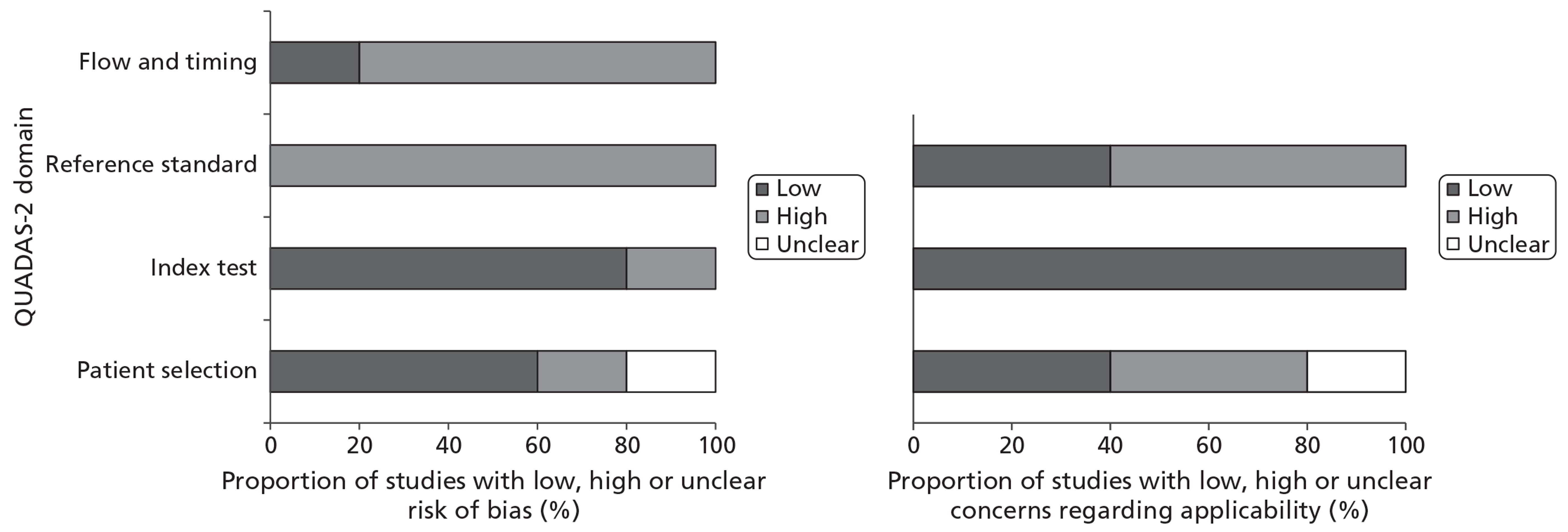
All studies were judged to be at high risk of bias on two or more domains (Table 4). All studies were judged to be at high risk of bias for reference standard, the three cohort studies28,40,41 were at high risk of bias for flow and timing and the two within-patient case-control studies38,39 were at high risk of bias for patient selection. Two cohort studies40,41 were judged as low concerns regarding applicability on all domains. There were high concerns regarding the applicability of the third cohort study28 as the reference standard consisted of intraoperative findings alone. Both within patient case-control studies38,39 were judged as high concerns regarding applicability for patient selection. Figure 2 shows the proportions of studies rated as having a high, low or unclear risk of bias or concerns regarding applicability for each domain (patient selection, index test, reference standard, and flow and timing).
| Author (year) | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Within-patient case-control studies | |||||||
| Yeom38 (2008) | ☹ | ☹ | ☹ | ☹ | ☹ | ☺ | ☹ |
| North39 (1996) | ☹ | ☺ | ☹ | ☺ | ☹ | ☺ | ☹ |
| Diagnostic cohort studies | |||||||
| Sasso40 (2005) | ☺ | ☺ | ☹ | ☹ | ☺ | ☺ | ☺ |
| Schutz28 (1973) | ? | ☺ | ☹ | ☹ | ? | ☺ | ☹ |
| Dooley41 (1988) | ☺ | ☺ | ☹ | ☹ | ☺ | ☺ | ☺ |
FIGURE 2.
Proportion of included studies fulfilling each QUADAS-2 domain.
Patient selection
Two cohort studies40,41 were judged to be at low risk of bias as they enrolled patients with low back pain and radiculopathy in whom the radiculopathy level was not confirmed, with no further restriction. The third cohort study28 was judged to be at unclear risk of bias as it did not provide details on how patients were selected. The two within-patient case-control studies38,39 enrolled patients with clinically and radiologically confirmed radiculopathy from a single level and so were judged to be at high risk of bias.
There were high concerns regarding applicability for the two within-patient case-control studies38,39 because the included patients did not have discordant imaging and clinical findings. One of the cohort studies28 was judged as having unclear applicability, as the details on the included patients were limited. The other two diagnostic cohort studies40,41 were judged as low concerns regarding applicability.
Index test
Four studies29,39–41 were judged to be at low risk of bias. All three cohort studies28,40,41 performed the SNRB before the reference standard was applied and one of the within-patient case-control studies39 blinded patients to the nature of the individual blocks delivered and so test review bias could be ruled out; all studies29,39–41 pre-specified the threshold for a positive SNRB test or provided a breakdown of individual patient results. The other within-patient case-control study38 was judged to be at high risk of bias, despite blinding patients to the nature of the blocks, as it used the pain relief threshold with the highest diagnostic accuracy to determine a positive SNRB result, although sensitivity and specificity values at other thresholds were also reported.
All studies were judged as low concern regarding applicability as all diagnostic SNRBs were adequately described as being localised to the nerve root and administered under imaging guidance.
Reference standard
All of the reference standards used were imperfect. Intraoperative findings are sometimes equivocal. Decompression takes place in order to expose the nerve root and only the proximal part of the root is normally seen. Most decompression procedures in the lumbar spine involve the disc and lateral recess but rarely expose the nerve root in the foramen. If the surgeon is not blinded to the result of the SNRB, then the intraoperative judgement of nerve root compression is particularly susceptible to bias. A post-surgical outcome reference standard is also problematic as these outcomes will be affected by the technical quality of the surgical procedure and any concomitant therapy the patient has in the interim period between index test and reference standard. Therefore, a poor surgical outcome might not purely or even predominantly be the result of an incorrect diagnosis at SNRB. The within-patient case-control studies which use the concordant clinical and imaging findings at the ‘case’ and ‘control’ injection site will also be flawed if the clinical and imaging findings are both wrong. For these reasons, all reference standards applied in the included studies were judged to be at risk of bias. Furthermore, in all three cohort studies28,40,41 the clinical or research teams were not blinded to the SNRB findings when recording the intraoperative or post-surgical outcomes.
We considered studies that used outcome following surgery as the reference standard to be most applicable, as this is the outcome of most importance to patients. The two cohort studies40,41 that used this reference standard were, therefore, judged as low concerns regarding applicability.
Flow and timing
Verification bias was a major risk in all three cohort studies. 28,40,41 All three selected patients to undergo surgery based on the SNRB result, with patients testing positive more likely to receive surgery. It is likely that the patients with negative SNRB results who, despite this, were selected for surgery were a biased subset of those testing negative as these are likely to have been the patients in whom the clinicians suspected a FN result. One of the within-patient case-control studies38 was also judged to be at high risk of bias for this domain, as 15 patients were excluded after the SNRB had been delivered and reasons for exclusion were not reported.
Summary of test accuracy results
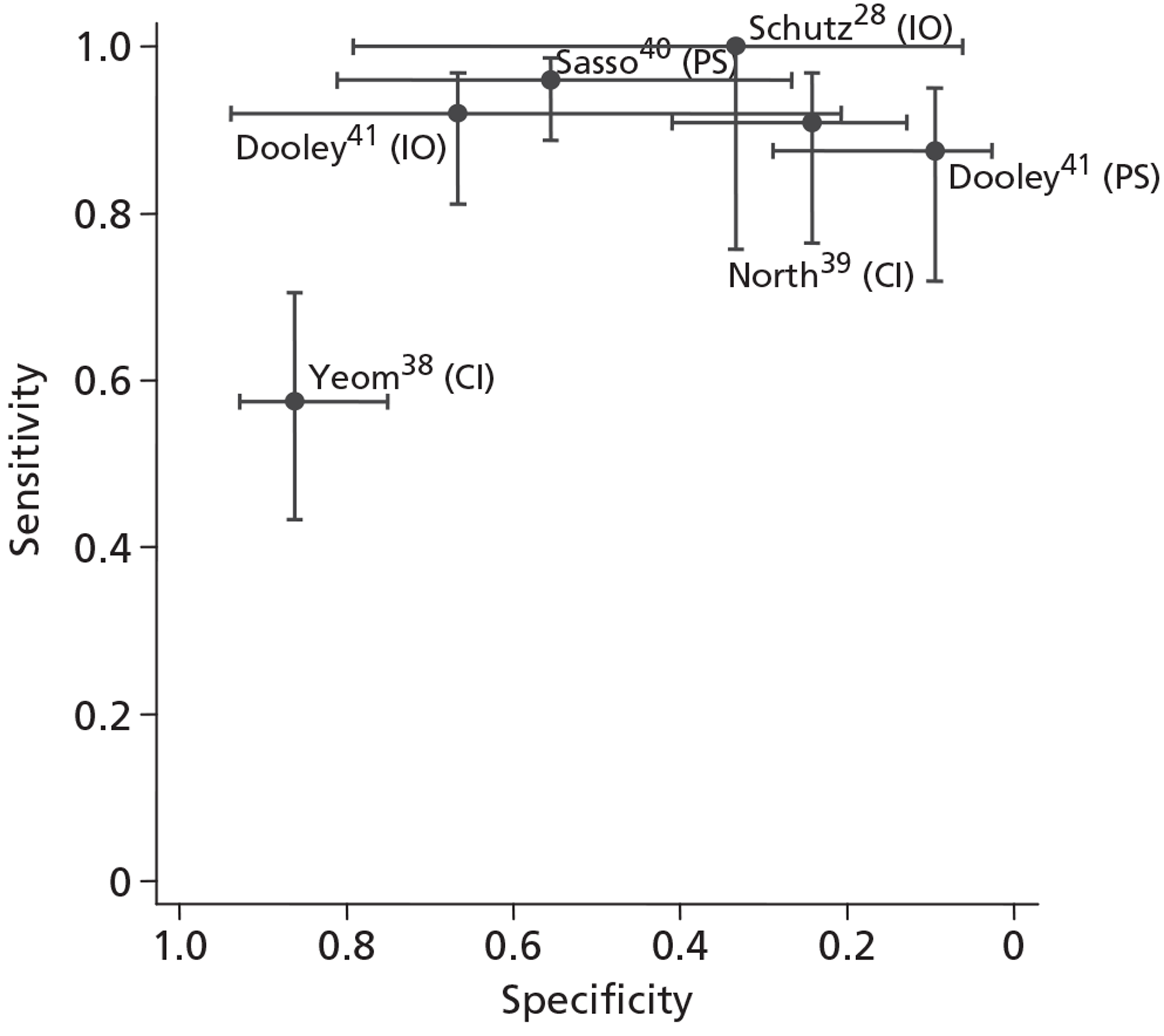
The diagnostic cohort studies reported sensitivity and specificity based on per-patient analyses, whereas sensitivity and specificity from the within-patient case-control studies were based on per-injection analyses. There was substantial variation in estimates of sensitivity and specificity across studies: sensitivity ranged from 57% to 100% and specificity from 10% to 86% (Table 5 and Figure 3). Most studies reported relatively high sensitivity (in excess of 87%), with the exception of that by Yeom et al. ,38 in which sensitivity was much lower (57%). A similar divergence was also observed for specificity, which was low (< 75%) in four studies,28,39–41 but higher (86%) in the Yeom et al. study. 38 This was unlikely to be purely a threshold effect, as the 70% pain reduction threshold used by Yeom et al. 38 to define a positive SNRB result was lower than most of the other studies, which would be expected to increase sensitivity and decrease specificity, whereas the reverse was found.
| Author (year) | Threshold | Reference standard | TP | FN | Sensitivity (%) (95% CI) | TN | FP | Specificity (%) (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Within-patient case-control studiesa | ||||||||
| Yeom38 (2008) | 70% pain relief – several other thresholds also evaluated | Concordant symptoms and imaging evidence of nerve root compression (or lack thereof) | 27 | 20 | 57 (43 to 70) | 50 | 8 | 86 (75 to 93) |
| North39 (1996) | 50% reduction in baseline pain following block | Concordant symptoms and imaging evidence of nerve root compression (or lack thereof) | 30 | 3 | 91 (76 to 97) | 8 | 25 | 24 (12 to 41) |
| Diagnostic cohort studies | ||||||||
| Schutz28 (1973) | 100% pain relief. Full trunk flexion and straight leg raising possible | Intraoperative findings | 12 | 0 | 100 (76 to 100) | 1 | 2 | 33 (6 to 79) |
| Sasso40 (2005) | VAS score 0–1 and immediate relief of > 95% pain | Outcome 12 months following surgery | 71 | 3 | 96 (89 to 99) | 5 | 4 | 56 (27 to 81) |
| Dooley41 (1988) | Pain relief | Intraoperative surgical confirmation of root pathology | 46 | 4 | 92 (81 to 98) | 2 | 1 | 67 (9 to 99) |
| Outcome following surgery (follow-up range 24–36 months) | 28 | 4 | 88 (71 to 96) | 2 | 19 | 10 (1 to 30) | ||
FIGURE 3.
Receiver operating characteristic plot displaying diagnostic accuracy results of included studies. The CIs for sensitivity and specificity for each result are shown by the lines extending from the point estimates. CI, control injection reference standard; IO, intraoperative reference standard; PS, post-surgical reference standard.
Yeom et al. 38 were the only researchers not to include needle provocation prior to injection of anaesthetic. However, this also is unlikely to explain the lower sensitivity reported in their study; less stringent criteria for defining test positivity (i.e. not requiring pain to be reproduced on needle provocation) typically result in an increase in sensitivity. Nevertheless, it is possible that the provocation of nerve root pain during the procedure makes patients with genuine nerve root compression better able to judge any subsequent pain relief from the anaesthetic.
Interpretation of specificity was particularly hampered by verification bias in the cohort studies. Because surgeons were not blinded to the SNRB results, very few patients with negative test findings had surgery. Schutz et al. ,28 Sasso et al. 40 and Dooley et al. 41 contribute a total of just eight TN cases to the analyses. The higher specificity reported by Yeom et al. 38 could be a manifestation of patient selection bias as ‘control’ injections were performed at a level of the spine where the patients had no symptoms and no imaging findings suggestive of pathology.
Owing to the patient selection bias inherent in within-patient case-control designs we decided that it would be inappropriate to combine the results of these studies with those of the diagnostic cohort studies to give an overall estimate of the accuracy of SNRB. Based on differences in the type of control injection used in the two within-patient case-control studies38,39 (see Table 5), we did not pool their results. Owing to the incomparability of the different reference standards used, we decided not to pool results of cohort studies that used different reference standards. Based on the two cohort studies28,41 that used an intraoperative reference standard, the pooled sensitivity was 93.5% (95% CI 84.0% to 97.6%) and specificity was 50.0% (95% CI 16.8% to 83.2%); in contrast, for the two studies40,41 that used post surgery as the reference standard the summary sensitivity was 93.3% (95% CI 85.8% to 97.0%) and summary specificity was 25.6% (95% CI 5.4% to 67.5%). In both cases, specificity was low, implying that a high proportion of patients without nerve root compression might still have a positive SNRB result. However, conclusions based on these data should be tempered because of the large CIs around specificity and the high risk of bias, which affected all three diagnostic cohort studies. 28,40,41
None of the five included studies reported data on patients with suspected multiple nerve root compression separately from those with suspected single nerve root compression and, therefore, we were unable to perform a subgroup analysis on this group of patients.
Chapter 5 Review of complications of diagnostic selective nerve root block
Details of included studies
Seven studies25,28,42–46 assessed complications and/or adverse events (see Appendix 6 and Table 6). One study was a diagnostic cohort study,28 one was a RCT42 and five studies were case series. 25,43–46 Publication dates ranged from 1973 to 2010. Only one study25 reported on the complications of SNRBs in the lumbar spine as the primary outcome of interest. This study included all patients (n = 1203) who received one or more therapeutic or diagnostic SNRBs in a radiology department with no details provided on their pre-test symptoms. The remaining six studies28,42–46 were all conducted among participants with radicular pain in a lower limb but were generally small (15–117 participants analysed).
| Author (year), country | nrecruited | nanalysed | Inclusion criteria | Mean age (years) | % male |
|---|---|---|---|---|---|
| Case series | |||||
| Stalcup25 (2006), USA | 1203 | 1203 | All adult patients who underwent a SNRB in the lumbar spine in the radiology department from 1 April 1997 to 31 May 2002 | 57.8 | 45 |
| Ng44 (2004), UK | 125 | 117 | Consecutive patients with clinical evidence of unilateral radicular pain that lasted despite at least 6 weeks of conservative management, MRI confirmation of nerve root compression secondary to LDH or peripheral degenerative spinal stenosis | 52 | 52 |
| Jonsson43 (1988), Sweden | 78 | 78 | Patients with unilateral sciatic pain, severe enough for them to consider operation. Sciatic pain in one specific dermasome but with normal findings on myelography, CT and or MRI. Sciatic pain and minor radiographic findings according to myelography, CT or MRI. Pathological radiographic findings on multiple levels | 44 | 34.6 |
| Quinn45 (1988), USA | 33 | 33 | Patients with a herniated disc (n = 31) or foraminal stenosis (n = 2) as identified by CT or MRI | NR | NR |
| Tajima46 (1980), Japan | 106 | 106 | Patients undergoing lumbosacral radiculography and block who had lumbosacral diseases considered to be manifested by radicular symptoms | 46 | 56 |
| Prospective diagnostic cohort study | |||||
| Schutz28 (1973), Canadaa | 23 | 15 | Patients with current sciatica | NR | NR |
| RCT | |||||
| Ghahreman42 (2010), Australia | 150 | 27 | Adult patients with pain radiating into the lower limb; associated with limitation of straight-leg raise to < 30°; demonstration of a disc herniation by CT or MRI at a segmental level consistent with the clinica features. Pain of appropriate quality was the primary indication for treatment and surgery would be the next intervention if the injections did not relieve the pain. Only data for single arm of trial in which patients received anaesthetic was included in the current review | 43 | 63 |
Table 7 gives full details of the SNRB injection methods and the adverse events reported. The needle gauge was reported in five25,43–46 out of seven studies and ranged from 20 to 25. The needle length, reported in three studies,43,45,46 ranged from 8 cm to 15 cm. Where reported, injected nerve root levels were L4–S1 in two studies43,46 and L2–S1 in one study. 42 The local anaesthetic injected varied between the studies, anaesthetic volume ranged from 1 ml to 6 ml, and anaesthetic concentration, reported in five studies,25,42,44–46 ranged from 0.25% to 1%. Three studies25,44,46 used an injectable steroid as well as the anaesthetic. All SNRB procedures were guided and six studies25,28,42,44–46 reported use of a contrast agent. Five of seven studies reported that there were no complications. 28,42–45 Tajima et al. 46 reported that ‘... pain in the lower extremity was aggravated for 1–2 days following selective radiculography and block in four patients. There was no other complication’. The largest study25 reported that minor and transient complications were encountered in 98 of the 1777 total patient visits (during which 2217 injections were delivered to 1203 patients), giving an overall per patient visit complication rate of 5.5%. Complications occurred in 134 of the 2217 total injections (6% complication rate per injection). There were no major or permanent complications resulting from SNRB in this large case series. Stalcup et al. 25 also present data on the complication rate by needle tip position in an analysis limited to the patients who only received one SNRB. For needle tip positions that had been used in > 50 injections, the complication rate ranged from 3.5% (7/199) to 7.4% (4/54) for needles placed in a posterior-superior-lateral position and an anterior-inferior-lateral position, respectively.
| Author (year) | Needle gauge | Needle length (cm) | Needle tip position | Needle levels | Anaesthetic name | Anaesthetic concentration (%) | Anaesthetic volume (ml) | Steroid used | Guided method | Contrast agent | Needle provocation used? | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stalcup25 (2006) | 22 | NR | Adjacent or into the intervertebral foramen | NR | Bupivacaine | 0.25 | 1–2 | Yes – celestone (Betamethasone Sodium Phosphate, Betamethasone Acetate®, Shering-Plough) early in study, depomedrol later in study | Fluoroscopy | Yes | NR | Numbers given in injections: leg weakness n = 77 (3.5%); pain increased n = 51 (2.3%); other n = 6 (0.3%); total n = 134 (6.0%) |
| Ng44 (2004) | 22–25 | NR | Superiorly to pedicle, medially to nerve and laterally to vertebral body | NR | Bupivacaine | 0.25 | 2 | Yes – methylprednisolone (Depo-medrone®, Pharmacia) | Assumed fuoroscopy | Yes | NR | No adverse events |
| Jonsson43 (1988) | 20 | 9 | Just lateral to the opening of the intervertebral foramen | L4, L5, S1 | Carbocaine | NR | 3–6 | NR | Fluoroscopy | NR | NR | No adverse events |
| Quinn45 (1988) | 22 | 9 or 15 | An attempt was made to pierce the nerve or to have the needle tip within 1−2 mm of the nerve | NR | Lidocaine or bupivacaine | 1 or 0.5, respectively | 2.5–5 | NR | CT | Yes | Yes | No adverse events |
| Schutz28 (1973)a | NR | NR | Superior level of interverebral foramen. Introduced about 2 inches from the midline | NR | Procaine | NR | 1 | NR | Guided but method NR | Yes | Yes | No adverse events |
| Ghahreman42 (2010) | NR | NR | Placed in the intervertebral foramen of the target level | L2, L3, L4, L5, S1 | Bupivacaine | 0.5 | 2 | No (steroid used in different randomised group) | Assumed fluoroscopy | Yes | NR | No complications occurred that could be attributed to the treatment |
| Tajima46 (1980) | 21 | 8–10 | Approximately 4 cm lateral to upper margin of lumbar spinous process corresponding to nerve root to be radiographed | L4, L5, S1 | Lidocaine | 1 | 3 | Yes – water-soluble corticosteroid | Radiography | Yes | Yes | Pain in the lower extremity was aggravated for 1–2 days following selective radiculography and block in four patients. There was no other complication |
Chapter 6 Assessment of cost-effectiveness evidence
Review of existing cost-effectiveness evidence
The 138 titles and abstracts of studies selected by the reviewers as being potentially relevant (see Figure 1) were assessed for inclusion in the economic review. None of the studies met the inclusion criteria. We identified two studies evaluating the cost-effectiveness of other types of spinal nerve injections [zygapophyseal (facet) joint injections and medial branch (facet nerve joint) blocks]. 47,48 Although these studies provide indirect evidence that diagnostic injections in the spine can influence the percentage of patients receiving surgery and the cost of care, they do not provide direct evidence on the cost-effectiveness of diagnostic SNRB in patients with LR. We therefore constructed an economic model based on evidence from our SR on the diagnostic accuracy of SNRB and the wider literature on the costs and outcomes of diagnosis and treatment of radiculopahy.
Model overview
Perspective
We calculated cost-effectiveness from the perspective of the NHS and personal social services (PSS). We subsequently broadened the analysis to the societal perspective by including patient expenses and the costs of lost productivity resulting from back and leg pain.
Patient groups
Two hypothetical patient groups were considered in our economic evaluation: (1) individuals with suspected single-level nerve root compression considered for lumbar decompression surgery where there were discordant clinical and imaging findings; and (2) individuals with suspected two-level nerve root compression where there were discordant clinical and imaging findings. Two decision-analytic models were developed to estimate the cost-effectiveness of diagnostic SNRB in these groups.
Intervention and comparator
The economic model consisted of two arms: those patients who received only imaging and clinical work-up and those patients who received an additional SNRB to assess whether or not their symptoms were related to nerve root compression.
Outcomes
The model estimated the incremental cost per correct diagnosis and per QALY of SNRB.
Model structure
In developing the models, we aimed for the best-practice principles suggested by Buxton et al. :49 (1) the models were kept as simple as possible to aid understanding; (2) the presentation of methods and results was as transparent as possible; (3) the quality of all data used in the models was explicitly discussed; (4) uncertainty in the models was explored using probabilistic sensitivity analysis (PSA) where possible; and (5) the models were internally verified and validated against other models and epidemiological studies where possible.
The short-run model considered the incremental cost per correct diagnosis of SNRB. The model for patients with suspected single-level symptoms is depicted in Figure 4. The model combined estimates of sensitivity and specificity with the pre-test prevalence of nerve root compression to generate post-test probabilities of accurate diagnosis. The short-run model allows for a proportion of patients to undergo repeat SNRB examinations at the same level if the initial test results are equivocal or are thought to need confirmation. The second model, in patients in whom two-level nerve root compression is suspected, repeats the diagnostic pathway so that SNRB is performed at both levels regardless of the SNRB result at the first level tested.
FIGURE 4.
Model: incremental cost per correct diagnosis, suspected single-level nerve root compression. For patients with suspected two-level nerve root compression, the diagnostic pathway is repeated, so a patient may end up with any permutation of the four diagnostic results at the two levels investigated.
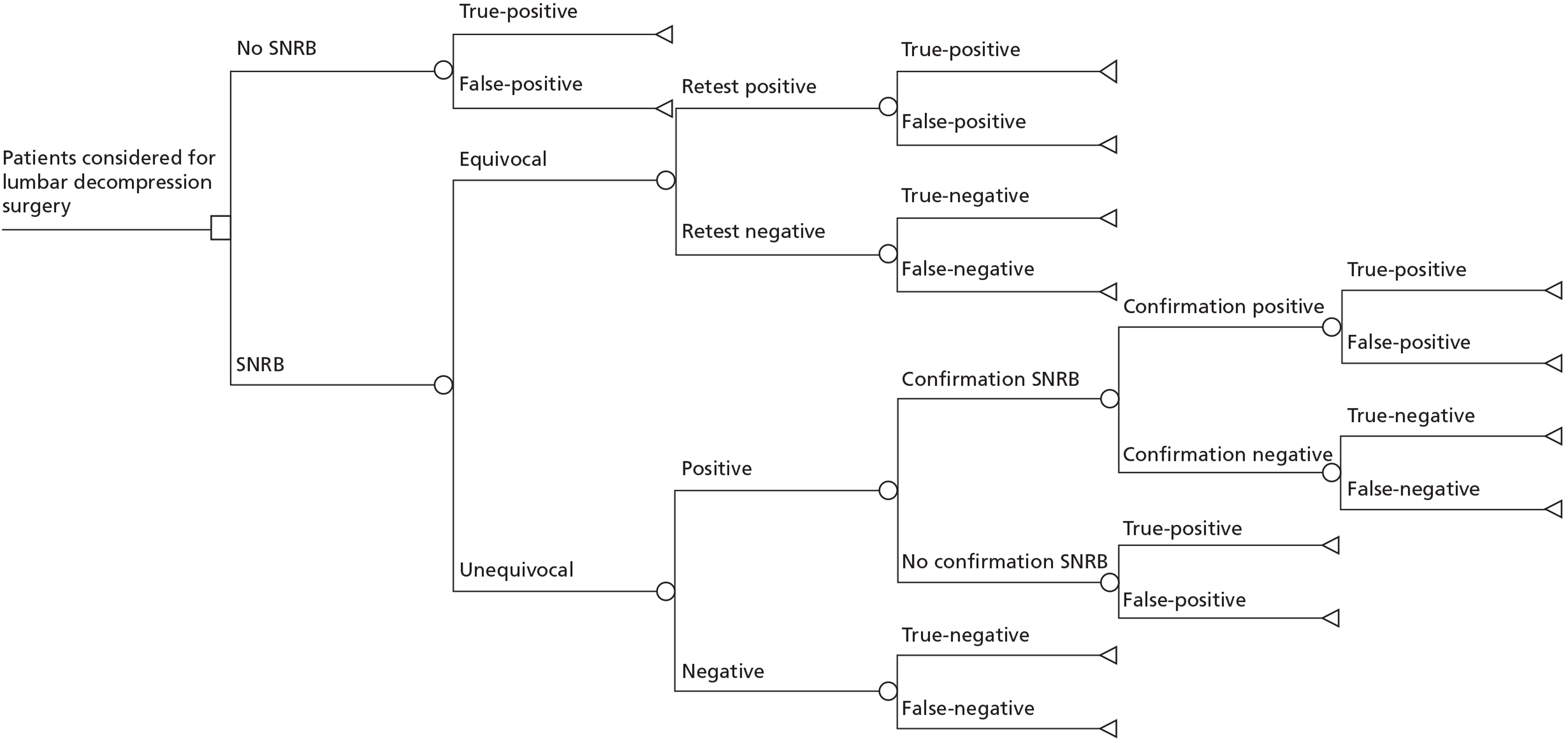
There are four possible diagnostic outcomes following SNRB: (1) positive SNRB response in a patient with symptoms caused by nerve root compression at that level (TP); (2) positive SNRB response in a patient whose symptoms are not caused by nerve root compression at that level (FP); (3) negative SNRB response in a patient with symptoms not caused by nerve root compression at that level (TN); and (4) negative SNRB response in a patient with symptoms caused by nerve root compression at that level (FN). Patients who undergo SNRB at two levels to evaluate possible two-level nerve root compression will receive a correct overall diagnosis only if a correct diagnosis is made at both levels. If the diagnosis is incorrect at either level this is considered a false diagnosis (Table 8).
| Nerve root A | Nerve root B | Overall diagnostic result | Impact on decision to perform surgery | Explanation |
|---|---|---|---|---|
| FP | FP | False | FP | More likely to have surgery at two levels, both without NR compression |
| TN | False | FP | More likely to have surgery at one level, without NR compression | |
| TP | False | TP | More likely to have surgery at two levels, one of which has NR compression | |
| FN | False | FP | More likely to have surgery at one level, but not the one that has NR compression | |
| TN | TN | True | TN | More likely to have conservative care at both levels, without NR compression |
| TP | True | TP | More likely to have surgery at one level, with NR compression | |
| FN | False | FN | More likely to have conservative care at both levels, one of which has NR compression | |
| TP | TP | True | TP | More likely to have surgery at two levels, with NR compression |
| FN | False | FN | More likely to have surgery at one level, but both levels have NR compression | |
| FN | FN | False | FN | More likely to have conservative care at both levels, both of which have NR compression |
We assumed that the working diagnosis was nerve root compression, based on clinical and imaging findings. Therefore, in the absence of SNRB (i.e. the top branch of Figure 4) this would be either a TP or FP diagnosis. Patients with suspected two-level nerve root compression who did not undergo SNRB have a TP diagnosis only if in fact they have nerve root compression at both levels. Otherwise, the working diagnosis is considered to be FP.
The long-term phase of the model estimated the costs and health effects of SNRB post diagnosis and treatment (Figures 5 and 6). This element of the model tracks patients as they have surgery or conservative care, as they incur costs and as their health-related quality of life evolves. Not all patients with positive SNRB results will go on to have lumbar spine surgery to decompress the nerve root. Patients may decline surgery because of resolving pain or despite ongoing pain if they prefer to continue with conservative care. Patients with negative SNRB results will also not necessarily avoid surgery. Surgery to decompress the nerve root investigated by SNRB might be pursued, despite the negative SNRB result, if persistent symptoms and imaging findings convince the clinician and patient that surgery is worthwhile.
FIGURE 5.
Diagnosis and treatment pathway – single-level nerve root compromise. Ⓜdenotes start of the Markov process.
FIGURE 6.
Diagnosis and treatment pathway – two-level nerve root compromise. Ⓜdenotes start of the Markov process.
For patients with suspected two-level compression, the diagnostic nerve root block may inform both the decision to perform surgery and the level(s) at which the surgery will be performed (see Figure 6). In these patients it is possible for the overall diagnostic result to be incorrect but the impact on the decision to perform surgery to be correct. For example, a patient who has a FP SNRB result on nerve root ‘A’ and a TP SNRB finding on nerve root ‘B’ would be more likely to undergo surgery to decompress both nerve roots. Therefore, the patient does undergo decompressive surgery on the symptomatic nerve root, which should relieve symptoms, albeit the surgery is more extensive than it need be because a second nerve root is also decompressed. A full description of each permutation of diagnostic result and the likely impact on the decision to perform surgery is provided in Table 8.
The long-term model (see Figures 5 and 6) estimated the initial costs and outcomes of care in the first year after diagnosis and treatment based on RCT evidence on the costs and outcomes of surgery and conservative care. After the first year, subsequent costs and utility (health-related quality-of-life) scores for patients were extrapolated over a period of 20 years using a two-state Markov process (recovering from low back pain and radiculopathy or death). Although evidence suggests that the majority of the benefits of surgery occur within the first year, this extrapolation allows us to estimate any residual benefits after 1 year and estimate QALYs as the cohort ages.
Model parameters
All model parameters are listed in Table 9 and data sources for the parameters are described below.
| Variable (variable name) | Point estimate | Lower bounda | Upper bounda | Source of information | PSA distribution |
|---|---|---|---|---|---|
| Prevalence | |||||
| True nerve compression | 50% | 25% | 75% | Clinical Opinion | Discrete estimates (0.25, 0.5, 0.75)b |
| Sensitivity/specificity | |||||
| Sensitivity SNRB | 93.3% | 87.3% | 96.5% | Accompanying SR | Beta(112.86, 9.12) |
| Specificity SNRB | 25.6% | 5.4% | 61.4% | Accompanying SR | Beta(2.35, 5.88) |
| Probability surgery | |||||
| No SNRB | 18% | Sasso40 | Interdependent | ||
| SNRB positive | 21% | 17.9% | 25.6% | Sasso40 | Beta(94, 341) |
| SNRB negative | 8% | 4.5% | 13.5% | Sasso40 | Beta(12, 130) |
| Probability repeat SNRB | |||||
| Equivocal SNRB leading to repeat | 1.0% | 0.5% | 2.3% | Sasso40 | Beta(7, 568) |
| Confirmation SNRB following positive result | 1.9% | 1.0% | 3.8% | Sasso40 | Beta(9, 404) |
| QALYs during the first year after treatment | |||||
| Surgery – in TPs/FNs | 0.78 | 0.56 | 1 | van den Hout12 | Log-normal (0.78, 0.17) |
| Surgery – in FPs/TNs | 0.72 | 0.52 | 1 | van den Hout12 | Log-normal (0.72, 0.17) |
| Conservative care in TPs/FNs | 0.73 | 0.53 | 1 | van den Hout12 | Log-normal (0.73, 0.16) |
| Conservative care in FPs/TNs | 0.73 | 0.53 | 1 | van den Hout12 | Log-normal (0.73, 0.16) |
| Costs (£) | |||||
| SNRB | 247 | 133 | 366 | NHS Reference Costs50 | Triangular(100, 400) |
| Surgery | 3159 | 1932 | 5435 | NHS Reference Costs50 | Triangular(1500, 6000) |
| Non-surgical costs in the year post surgery | 1514 | 0 | 8511 | van den Hout12 | Gamma(0.39, 3875) |
| Non-surgical costs in the year after conservative care | 1785 | 0 | 9696 | van den Hout12 | Normala (0.42, 4237) |
| Societal costs (£) | |||||
| Non-surgical societal costs in the year post surgery | 12,860 | 640 | 42,822 | van den Hout12 | Gamma(1.27, 10,089) |
| Non-surgical societal costs in the year after conservative care | 14,350 | 1140 | 43,849 | van den Hout12 | Gamma(1.58, 9109) |
| Discount rate | |||||
| Discount rate costs | 3.5% | NICE13 | |||
| Discount rate outcomes | 3.5% | NICE13 | |||
Initial prevalence of nerve root compression
The prevalence of true nerve root compression is, to some extent, under the control of the clinician requesting the SNRB. Some clinicians may choose to use SNRB predominantly in patients whose symptoms they believe, based on clinical and imaging findings, are due to surgically amenable causes. In such a setting, prevalence will be low and the purpose of SNRB will be primarily to reassure the patient and clinician that surgery is not necessary. Alternatively, other clinicians may be more inclined to use SNRB as a final confirmatory test in patients whom they believe do have nerve root compression and will benefit from surgery. In this setting, the pre-test prevalence will be high. In order to take into account this variability in the use of SNRB, we conducted a deterministic sensitivity analysis (sensitivity analysis A) to evaluate the cost-effectiveness of SNRB at three levels of pre-test prevalence selected a priori: (1) a base-case moderate prevalence of nerve root compression (50%); (2) low prevalence of nerve root compression (25%); and (3) high prevalence of nerve root compression (75%). For patients with suspected two-level nerve root compression, the probability of compression at either level was assumed to be the same and these probabilities were assumed to be independent of each other.
Cost of the selective nerve root block diagnostic test
Our SR and broader searches identified no published sources on the cost of diagnostic SNRB injections. We therefore estimated the cost from NHS reference costs 2009–10 using the Healthcare Resource Group (HRG) codes' code of AB06Z ‘minor pain procedure’. 50 SNRBs are generally performed on either a day-case or outpatient basis. We used the weighted average of outpatient and day case costs of £247 (see Table 9).
The marginal cost of each additional SNRB was assumed to be identical to that of the initial SNRB as it typically requires a separate appointment.
The probability of selective nerve root block-related complications
Adverse events associated with diagnostic SNRB were reviewed as part of our SR. The largest study was conducted by Stalcup et al. 25 Although their study of 1777 patient visits found a 5.5% complication rate, the complications were minor and transient in nature. Given this, we decided not to model any quality-of-life decrements associated with these minor SNRB-related complications. However, very rarely case reports of much more serious complications are described in the literature. 26,51,52 We therefore included a small probability (1 in 10,000) of permanent paraplegia per SNRB injection. We assumed that, in this event, paralysis would occur immediately and reduce quality of life in the model to 51.6% of that of the treated group, adding a cost of £18,919 per year. 53
The sensitivity and specificity of selective nerve root block
Sensitivity and specificity for the model were taken from our SR. We selected the pooled estimate of the two-cohort studies40,41 that used post-surgery outcomes as the reference standard as this was considered to be the most applicable reference standard. In sensitivity analysis (sensitivity analysis B), we used estimates of diagnostic accuracy from two diagnostic accuracy studies to provide a representation of the range of high sensitivity/low specificity40 and low sensitivity/high specificity38 results that have been reported in the literature.
Likelihood of equivocal selective nerve root block results and repeating selective nerve root block on the same nerve root
Selective nerve root blocks are sometimes repeated at the same level when findings are equivocal, if the procedure was performed inadequately (i.e. incorrect needle positioning) or if continuing symptoms prompt reinvestigation of the same nerve root. The proportion of patients with equivocal SNRB tests was estimated from Sasso et al. ,40 where 6 of 573 patients had an equivocal initial SNRB and went on to receive a repeat SNRB at the same level. Of the 411 patients who had a positive finding at their initial SNRB, eight (1.9%) patients received a repeat confirmatory SNRB on the same nerve root. For individuals with a negative SNRB result at the initial level tested, none (0/156) received a repeat SNRB at the same level.
The impact of the selective nerve root block result on the decision to perform surgery
The same study by Sasso et al. 40 was the only one identified by our search that reported on the therapeutic impact of a positive and negative SNRB result on the probability of receiving surgery. Of the 573 patients, 433 patients had a positive SNRB result at one or more vertebral level (411 at the initial level tested and 22 at a subsequent level), of whom 21% (93/433) went on to receive decompression surgery at that level within 3 months. Of the 140 patients with equivocal or negative SNRB results, 8% (11/140) had decompression surgery at that level, despite the negative SNRB finding. We used these probabilities to estimate the likelihood of surgery after a positive or negative SNRB result. We assumed that the probability of surgery in the no SNRB arm would be the weighted average of the negative and positive SNRB probabilities [18% (104/573)].
The association between the SNRB result (positive or negative) and the probability of having surgery may differ between the USA and the UK. We therefore performed a service evaluation of all diagnostic SNRBs conducted at Cambridge University Hospitals NHS Foundation Trust between 1 April 2009 and 1 April 2010. Patients were followed up to see if lumbar spine surgery had been performed within 1 year of the SNRB and whether or not it had been performed at the level indicated by the SNRB. A positive result was defined as moderate or substantial patient-reported pain relief post SNRB. We used data from patients receiving SNRB at a single level (n = 69).
Forty-four patients had a positive SNRB result and, of these, 12 (27%) went on to receive surgery at the level investigated within 1 year. Of the 19 patients with a negative SNRB result, one patient (5%) went on to receive surgery at the level investigated within 1 year. The remaining six patients did not have their SNRB result recorded in the medical records although none went on to receive lumbar surgery within 1 year. In a sensitivity analysis (sensitivity analysis C) we used a threshold analysis to determine how large the therapeutic impact of SNRB would have to be in order for it to be cost-effective.
The initial (1-year) effectiveness of surgical and conservative therapy in patients with nerve root compression
Jacobs et al. 8 published a SR that compared surgery with conservative/non-surgical care in the management of sciatica in adult patients with lumbar herniated disc, consisting of five RCTs10,11,54–56 published before October 2009. The RCTs were of variable methodological quality and heterogeneous in terms of the interventions compared (e.g. early vs delayed discectomy, discectomy vs epidural steroid injection, or discectomy vs conservative care). The narrative synthesis of the review suggested there was evidence that early surgical care is better for short-term (3-month) relief of leg pain, although only one study assessed this. Overall, the SR concluded there were no significant differences between surgery and conservative/non-operative care at 1- or 2-year follow-ups, although the evidence was scarce and interpretation was hampered in many studies because of non-compliance with surgery and crossover from conservative to surgical care.
Two of the most recent RCTs included in Jacobs et al. 's review,8 both considered by the review authors to be at low risk of bias, also collected cost-effectiveness data. Peul et al. 9,10,12 evaluated the effects of early lumbar surgery (n = 141) compared with prolonged conservative care (n = 142) among Dutch patients who had sciatica for between 6 and 12 weeks. Early surgery consisted of operative treatment within 2 weeks of diagnosis by experienced surgeons, while prolonged conservative care comprised 6 months of non-surgical care administered by a family practitioner. After 6 months, surgical care was allowed in the conservative care arm if it was considered necessary. Over a 2-year follow-up, the study found no difference in the scores between the two treatment arms in the Roland–Morris Disability Index57 (p = 0.25), but did find a difference in leg pain which favoured early surgery (p = 0.05) at the 8- and 26-week follow-ups, although the differences between treatment groups diminished and were similar at 1 and 2 years post randomisation.
van den Hout et al. 12 evaluated the economic outcomes alongside the Peul et al. trial. 9,10 The study found that early surgery resulted in higher utility scores (European Quality of Life-5 Dimensions; EQ-5D) during the first 6 months. By 12 months the difference in QALYs between the surgery arm (0.78 QALYs) and prolonged conservative care arm (0.73 QALYs) was 0.044 (95% CI 0.005 to 0.083). This study may provide conservative estimates of the incremental QALYs of surgical care, as not all patients randomised to surgery received it (11% did not) and a proportion of patients randomised to prolonged conservative care (approximately 30%) received surgery within 6 months of randomisation. However, as this was a recent RCT, with a low risk of bias, conducted in a European health-care system, we used it as the primary source of data on QALY gains in our model (see Table 9).
A second RCT, the Spine Patient Outcomes Research Trial (SPORT), compared surgical with conservative management of sciatica due to LDH. 11,58,59 The SPORT LDH trial involved 501 patients with imaging-confirmed LDH and radiculopathy symptoms for at least 6 weeks. Patients were randomised either to surgery (n = 245) or to non-operative care (n = 256); a further 743 patients declined randomisation and were entered into an observational cohort. Secondary outcome measures included the EQ-5D and resource utilisation. 58 Non-adherence with randomisation was problematic, with only 59% of those randomised to surgery having received surgery by 1 year compared with 43% in the non-operative group.
The SPORT LDH economic evaluation has been reported on only an ‘as treated’ analysis of costs and outcomes in the randomised (n = 501) and observational (n = 743) cohorts combined and is therefore potentially affected by selection bias. 58 Of the combined cohort of patients (n = 1191), 775 patients received surgery at some point during the 2-year follow-up period. After adjusting for numerous baseline covariates, these patients were estimated to have mean discounted QALYs of 1.64 (95% CI 1.62 to 1.67) compared with 1.44 (95% CI 1.41 to 1.47) in patients (n = 416) treated non-surgically, a difference of 0.21 (95% CI 0.16 to 0.25). We used the relative differences between 1-year estimate of QALYs gained from surgery in the SPORT trial in a sensitivity analysis (sensitivity analysis D).
The initial (1-year) effectiveness of surgical and conservative therapy in patients without nerve root compression
The outcomes for patients whose symptoms were not related to the nerve root investigated by SNRB are difficult to estimate as these patients may have a wide range of causes for their symptoms. As most low back pain and referred symptoms in the lower limbs are self-limiting,60 we assumed that outcomes in these patients would be the same as in patients with nerve root symptoms treated conservatively and that decompression surgery would not improve the prognosis (see Table 9).
If decompression surgery is performed on a patient without nerve root compression, this will have a cost to the health service and a detrimental effect on health (at least in the short term). Post-surgery patients in the van den Hout et al. 12 study experienced lower quality of life between baseline and 2 weeks (utility score 0.425 vs 0.471). We assumed that patients without nerve root compression who had surgery would have this perisurgical dip in quality of life and then recover, and have outcomes identical to patients without nerve root compression treated conservatively. This dip in quality of life was equivalent to a reduction of 0.0017 QALYs over the first year of the model (see Table 9).
The cost of the surgical procedure
We estimated the cost of lumbar decompression surgery for radiculopathy based on HRG code. HRG codes group together spells of inpatient care for similar patients that have similar resource implications. The HRG code assigned is based on a number of variables including the primary diagnosis, major procedures performed during the admission, complications, comorbidity and length of stay. Using hospital episode statistics data for 2008–9, we ascertained the frequency with which specific HRG codes were assigned to patients who had surgery to treat lumbar nerve compression. We identified patients whose primary procedure indicated an excision of a lumbar intervertebral disc with or without laminectomy (Table 10). HRG code R02 (Surgery for Prolapsed Intervertebral Disc) was most commonly used, accounting for > 90% of the inpatient spells (see Table 10). Therefore, we used the elective spell tariff (£3159) for HRG code R02 as the estimate for the initial cost of surgery (see Table 9). 50
| OPCS 4.4 procedure code | Procedure description | HRG 3.5 code | Frequency | HRG name | % total |
|---|---|---|---|---|---|
| V331 | Primary laminectomy excision of lumbar intervertebral disc | R02 | 1271 | Surgery for prolapsed intervertebral disc | 86.8 |
| V331 | R03 | 128 | Decompression and effusion for degenerative spinal disorders | 8.7 | |
| V331 | Other | 65 | Intracranial procedures except trauma – category 3 | 4.4 | |
| V332 | Primary fenestration excision of lumbar intervertebral disc | R02 | 1084 | Surgery for prolapsed intervertebral disc | 88.3 |
| V332 | R03 | 97 | Decompression and effusion for degenerative spinal disorders | 7.9 | |
| V332 | Other | 46 | Intracranial procedures except trauma – category 3 | 3.7 | |
| V337 | Primary microdiscectomy of lumbar intervertebral disc | R02 | 4252 | Surgery for prolapsed intervertebral disc | 94.7 |
| V337 | R03 | 155 | Decompression and effusion for degenerative spinal disorders | 3.5 | |
| V337 | Other | 85 | Intracranial Procedures Except Trauma – category 2 | 1.9 |
Initial non-surgical costs of care in patients with nerve root compression
The medical and societal costs during the first year following surgical and conservative care in patients with nerve root compression were taken from van den Hout et al. 12 Patients completed resource-use diaries to record admissions to hospital, medical visits, home help, domestic help (both professional and unpaid), drugs, other medical aids, absenteeism from work and out-of-pocket costs. van den Hout et al. estimated that the annual non-surgical health-care costs were 2021 (£1785) and 1714 (£1514) for prolonged conservative care and early surgical care, respectively. Societal costs in the first year, excluding the initial surgical costs, were estimated by van den Hout et al. 12 to be 16,270 (£14,350) and 14,581 (£12,860), respectively.
Initial non-surgical costs of care in patients without nerve root compression
We assumed that during the first year after surgical or conservative therapy, individuals with no nerve root compression would incur non-surgical costs identical to those incurred by patients with nerve root compression who had prolonged conservative care in the van den Hout et al. trial. 12
The long-term costs of care
The costs (health service, social service and productivity costs) observed during the first year of the van den Hout et al. trial12 were extrapolated over 20 years. The quarterly costs of care reported by van den Hout et al. 12 declined rapidly in both the surgical and prolonged conservative care groups over the 1-year follow-up period, although the rate of decline decreased over time (Table 11). In our primary extrapolation (convergence), we assumed that the average decline in quarterly costs observed in the last two quarters of the first year continued thereafter. Effectively, this assumption means that the costs of care in the surgery and prolonged conservative care arms of the trial converge after approximately 6 years.
| Year | Conservative | Surgery | Probability of death | ||||
|---|---|---|---|---|---|---|---|
| NHS + PSS costs (£) | Societal costs (£) | Utility | NHS + PSS costs (£) | Societal costs (£) | Utility | ||
| 0.25a | 838 | 6826 | 0.68 | 862 | 7659 | 0.77 | N/A |
| 0.5a | 409 | 4063 | 0.80 | 302 | 2529 | 0.83 | N/A |
| 0.75a | 322 | 2422 | 0.81 | 201 | 1555 | 0.82 | N/A |
| 1a | 210 | 1402 | 0.83 | 141 | 1087 | 0.86 | N/A |
| 2 | 396 | 1758 | 0.86 | 238 | 1693 | 0.88 | 0.001 |
| 3 | 106 | 209 | 0.88 | 52 | 315 | 0.89 | 0.002 |
| 4 | 29 | 25 | 0.89 | 11 | 59 | 0.89 | 0.002 |
| 5 | 8 | 3 | 0.89 | 2 | 11 | 0.89 | 0.002 |
| 6 | 2 | 0 | 0.89 | 1 | 2 | 0.89 | 0.002 |
| 7b | 1 | 0 | 0.89 | 0 | 0 | 0.89 | 0.002 |
| Total costs and QALYs (years 2–20) | 541 | 1996 | 16.86 | 304 | 2081 | 16.89 | 0.074 |
As a secondary extrapolation (parallel – sensitivity analysis E), we assumed that the costs observed in the final quarter of the first year would be constant in all subsequent quarters for 20 years. Effectively, this assumption locks in differences in costs observed in the final quarter of the trial and represents the most optimistic assumption for cost savings achieved by correctly selecting surgical decompression in a patient with nerve root compromise (Table 12).
| Year | Conservative | Surgery | Probability of death | ||||
|---|---|---|---|---|---|---|---|
| NHS + PSS costs (£) | Societal costs (£) | Utility | NHS + PSS costs (£) | Societal costs (£) | Utility | ||
| 0.25a | 838 | 6826 | 0.68 | 862 | 7659 | 0.77 | N/A |
| 0.5a | 409 | 4063 | 0.80 | 302 | 2529 | 0.83 | N/A |
| 0.75a | 322 | 2422 | 0.81 | 201 | 1555 | 0.82 | N/A |
| 1a | 210 | 1402 | 0.83 | 141 | 1087 | 0.86 | N/A |
| 2 | 841 | 5607 | 0.83 | 563 | 4347 | 0.86 | 0.001 |
| 3 | 841 | 5607 | 0.83 | 563 | 4347 | 0.86 | 0.002 |
| 4 | 841 | 5607 | 0.83 | 563 | 4347 | 0.86 | 0.002 |
| 5 | 841 | 5607 | 0.83 | 563 | 4347 | 0.86 | 0.002 |
| 6 | 841 | 5607 | 0.83 | 563 | 4347 | 0.86 | 0.002 |
| 7b | 841 | 5607 | 0.83 | 563 | 4347 | 0.86 | 0.002 |
| Total costs and QALYs (years 2–20) | 15,984 | 106,540 | 16.33 | 10,701 | 82,597 | 16.85 | 0.074 |
Malpractice litigation may substantially increase the long-term costs of surgery. However, recent evidence suggests that in the 9-year period from 2002 to 2010 there were just 13 cases of successful litigation against the NHS for ‘wrong level’-, ‘incorrect diagnosis’- or ‘on-going pain’-related elective spinal surgery (which is < 1 in 11,000 procedures). 61 We estimated the potential impact of litigation on the cost of surgery performed without SNRB assuming total damages and legal costs of £250,000 per successful claim in sensitivity analyses (sensitivity analysis F).
The long-term utility scores and mortality following surgical and conservative therapy
The utility values from the van den Hout et al. trial12 were extrapolated over 20 years using the same extrapolation methods [converging (see Table 11) and parallel (see Table 12)] as described for cost extrapolations. The probability of death was based on lifetable survival data and was assumed to match that of the general population. 12 The age of patients at the outset of our model was assumed to be equal to the average age of patients recruited in the van den Hout trial et al. (42 years). 12
Discounting
All costs and QALYs after the first year of the model have been discounted at 3.5% per annum consistent with guidance from NICE. 13
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was carried out to estimate the overall uncertainty in the model results due to the combined uncertainty stemming from each of the parameters used to construct the model. Parameters were randomly sampled from a probability distribution assigned to each variable (see Table 9). The results of these analyses are presented as cost-effectiveness acceptability curves (CEACs).
In addition, five deterministic sensitivity analyses were performed as noted in the preceding paragraphs: (1) sensitivity analysis A varied the pre-test prevalence of nerve root compression to a lower 25% and a higher 75%; (2) sensitivity analysis B used high-sensitivity/low-specificity and low-sensitivity/high-specificity estimates of diagnostic accuracy; (3) sensitivity analysis C conducted threshold analyses to examine how large the therapeutic impact of SNRB would have to be in order for it to be cost-effective; (4) sensitivity analysis D based the outcomes of surgery on ‘as treated’ QALY results from the SPORT LDH analysis; and (5) in sensitivity analysis E we used the parallel extrapolation to model costs and utilities after the initial year.
Partial expected value of perfect information
The model is a simplification of the reality of diagnostic testing with SNRB, based on imperfect data. This creates a possibility that the model will reach the ‘wrong’ conclusion favouring a technology that would be proven to be not cost-effective were more data collected. Partial expected value of perfect information (PEVPI) analysis allows a comparison of the value of potential future research projects. A research project is less valuable if it will provide evidence on a parameter that is already precisely known from existing research or a parameter that is unimportant for the conclusion of the model. We use PEVPI to look at the value of a potential diagnostic accuracy study to more precisely estimate the sensitivity and specificity of SNRB. We used 10,000 iterations and a willingness to pay per QALY of £30,000 to calculate PEVPI per patient.
Model verification and validation
The project economists and clinicians jointly reviewed the structure of the model to ensure that it reflected clinically plausible diagnostic and therapeutic transitions. We verified the internal validity of the model using extreme value analyses for every parameter to check that the results of the model were correlated with each parameter of the model in the expected direction. We double coded the model in TreeAge Pro (TreeAge Software, Inc., Williamstown, MA, USA) and Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA) to identify any discrepancies or miscoding in the model.
Chapter 7 Results of cost-effectiveness study
Suspected single-level nerve root compression – cost per case detected
When the pre-test prevalence of nerve root compression is 50% and patients have suspected single-level nerve root compression, adding SNRB to the diagnostic work-up results in an additional cost of £254 (Table 13). SNRB also leads to an increase in patients receiving a correct diagnosis to 59.5%. Adding SNRB to the diagnostic work-up has an incremental cost of £2684 per correct diagnosis although the CI is very wide.
| Cost/outcome measure | No SNRB | SNRB | Difference | 95% CI |
|---|---|---|---|---|
| Cost of SNRB testing (£) | 0 | 254 | 254 | 141 to 374 |
| Percentage receiving correct diagnosis | 50.0 | 59.5 | 9.5 | −1.6 to 26.7 |
| Incremental cost per correct diagnosis (£) | 2684 | 780 to dominated |
Suspected single-level nerve root compression – cost per quality-adjusted life-year
Although SNRB does result in a lower proportion of patients incorrectly receiving surgery (17.6% vs 18.0%; Table 14) and does increase surgery in patients with nerve root compression (20.1% vs 18.0%; see Table 14), these differences are small because of the low specificity of SNRB and the high percentage of patients who do not receive surgery despite a positive SNRB result. This marginal improvement achieved by targeting surgery at those most likely to benefit from it does lead to some savings in costs because of faster recovery during the first year. However, this does not outweigh the cost of the SNRB test plus the additional cost of surgery in the higher proportion of patients who receive it after SNRB. In terms of NHS and PSS costs, the SNRB strategy is £304 per patient more expensive. The incremental gain in QALYs due to more accurate diagnosis with SNRB is very small (0.0002; see Table 14). Hence, the incremental cost per QALY gained of using SNRB in patients with suspected nerve root compression at a single level is very high (£1,576,000; see Table 14). This is well above conventional cost-effectiveness thresholds.
| Cost/outcome measure | No SNRB | SNRB | Difference | 95% CI |
|---|---|---|---|---|
| Percentage receiving surgery | 18.0 | 18.9a | 0.87 | −2.60 to 5.22 |
| Percentage of patients with nerve root compression receiving surgery | 18.0 | 20.1 | 2.11 | −0.93 to 6.53 |
| Percentage of patients without nerve root compression receiving surgery | 18.0 | 17.6 | −0.38 | −5.33 to 4.67 |
| NHS and PSS costs (£) | 2824 | 3127 | 304 | −119 to 558 |
| Societal costs | 18,467 | 18,728 | 261 | −277 to 859 |
| QALYs | 12.6229 | 12.6231 | 0.0002 | −0.0069 to 0.0071 |
| NHS PSS cost per QALY gained (£) | 1,576,007 | |||
| Societal cost per QALY gained (£) | 1,356,638 |
If societal costs are included (e.g. lost productivity because of back and leg pain), the costs associated with both diagnostic strategies increase markedly. However, the incremental cost of SNRB is relatively similar (£261; see Table 14) despite this broader perspective and the cost per QALY gained is still well in excess of conventional thresholds.
There is considerable uncertainty around this estimate of cost per QALY, in large part because of the broad CIs around diagnostic accuracy identified in our SR. The CEAC (Figure 7) indicates a low, but not negligible, probability (2.4%) that the addition of SNRB to the diagnostic work-up will be cost-effective at a cost per QALY threshold of £30,000.
FIGURE 7.
Cost-effectiveness acceptability curve – one-level nerve root compression.
Suspected two-level nerve root compression – cost per case detected
For patients with suspected two-level nerve root compression, adding SNRB to the diagnostic work-up costs £507 (Table 15). Compared with single-level SNRB the diagnostic accuracy is reduced, in both arms, as both levels need to be diagnosed accurately to get a correct diagnosis. As for suspected single-level compression the addition of SNRB leads to a higher proportion patients being correctly diagnosed. Adding SNRB to the diagnostic work-up has an incremental cost-effectiveness of £4903 per case correctly diagnosed. However, the CIs are again wide, reflecting the uncertainty around the diagnostic accuracy of SNRB.
| Cost/outcome measure | No SNRB | SNRB | Difference | 95% CI |
|---|---|---|---|---|
| Cost of SNRB testing (£) | 0 | 507 | 507 | 280 to 763 |
| Percentage receiving correct diagnosis | 25.0 | 35.3 | 10.3 | −1.3 to 10.9 |
| Incremental cost per correct diagnosis (£) | 4903 | 1264 to dominated |
Suspected two-level nerve root compression – cost per quality-adjusted life-year
The use of SNRB in patients with suspected nerve root compression at two levels was not cost-effective (Table 16). This was because of the increased cost of testing and the low specificity of SNRB, resulting in a large number of patients with a FP result on at least one nerve root, potentially leading to inappropriate surgery. The incremental NHS and PSS costs (£1040) were greater when SNRB was used for suspected two-level nerve root compression, while the incremental QALYs gained were negative (−0.0002). Hence, SNRB was dominated (more costly, less effective) by no additional testing in patients with suspected two-level nerve root compression.
| Cost/outcome measure | No SNRB | SNRB | Difference | 95% CI |
|---|---|---|---|---|
| Percentage receiving surgery | 18.0 | 20.7a | 2.7 | −3.70 to 2.57 |
| NHS and PSS costs (£) | 2838 | 3878 | 1040 | 545 to 1923 |
| QALYs | 12.6268 | 12.6265 | −0.0002 | −0.0825 to 0.0774 |
| NHS PSS cost per QALY gained | Dominated |
Deterministic sensitivity analyses
There were a number of structural uncertainties and variance in patient characteristics that we chose to explore through deterministic sensitivity analysis. These sensitivity analyses were conducted on the single-level nerve root compromise model and are listed below.
-
Sensitivity analysis about the pre-test prevalence of nerve root compression.
-
Sensitivity analysis about the possible values of sensitivity and specificity of SNRB.
-
Threshold sensitivity analyses to examine how large the therapeutic impact of SNRB would have to be in order for it to be cost-effective.
-
Sensitivity analysis about the maximum benefit of surgery based on ‘as treated’ rather than ‘intention to treat’ estimates.
-
Sensitivity analysis about the extrapolation of costs and outcomes beyond 1 year.
-
Sensitivity analysis including malpractice litigation.
Pre-test prevalence
As the prevalence of nerve root compression increases, both the incremental costs and the incremental QALYs gained by SNRB increase (Table 17). In a patient group with high prevalence, a test such as SNRB, which has high sensitivity but low specificity, makes more correct diagnoses and more patients appropriately receive surgery. SNRB becomes more cost-effective as prevalence increases, but even at a prevalence of 75% the incremental cost-effectiveness ratio (ICER) (£552,734) is not efficient compared with conventional thresholds of cost-effectiveness. Our conclusions are relatively insensitive to the pre-test prevalence of nerve root compression.
| Cost/outcome measure | 25% | 75% | ||
|---|---|---|---|---|
| No SNRB | SNRB | No SNRB | SNRB | |
| NHS and PSS costs (£) | 2846 | 3132 | 2801 | 3122 |
| Societal costs (£) | 18,543 | 18,793 | 18,391 | 18,633 |
| QALYs | 12.6189 | 12.6188 | 12.6268 | 12.6273 |
| NHS PSS cost per QALY gained (£) | Dominated | 552,734 | ||
| Societal cost per QALY gained (£) | Dominated | 460,579 | ||
Sensitivity and specificity of selective nerve root block
Replacing the pooled estimate of sensitivity and specificity with data from two diagnostic accuracy studies in our SR towards the extremes of the receiver operating characteristic space (see Figure 3) did not change our conclusion that SNRB is unlikely to be cost-effective (Table 18) as increases in diagnostic accuracy are tempered by the moderate impact of the SNRB result on the decision to perform surgery.
| Cost/outcome measure | Yeom et al.38 (sensitivity 57%, specificity 86%) | Sasso et al.40 (sensitivity 96%, specificity 56%) | ||
|---|---|---|---|---|
| No SNRB | SNRB | No SNRB | SNRB | |
| NHS and PSS costs (£) | 2824 | 2938 | 2824 | 3070 |
| Societal costs (£) | 18,467 | 18,567 | 18,467 | 18,669 |
| QALYs | 12.6229 | 12.6217 | 12.6229 | 12.6234 |
| NHS PSS cost per QALY gained (£) | Dominated | 471,171 | ||
| Societal cost per QALY gained (£) | Dominated | 386,317 | ||
The therapeutic impact of selective nerve root block
At a willingness to pay per QALY of £30,000 there is no probability of surgery that would lead to SNRB being cost-effective keeping all other variables constant. Even if all patients with positive SNRB results had surgery and no patients with negative SNRB results received surgery, the ICER (£87,023) is still above conventional thresholds defining cost-effectiveness.
Benefit of surgery in the first year
Using the relative difference in QALYs between surgery and conservative care from the ‘as treated’ group in the SPORT LDH trial and keeping our QALY gained from surgery estimate constant, the cost-effectiveness of SNRB increases with an ICER of £236,778 per QALY (Table 19).
| Cost/outcome measure | As treated | |
|---|---|---|
| No SNRB | SNRB | |
| NHS and PSS costs (£) | 2824 | 3127 |
| Societal costs (£) | 18,467 | 18,728 |
| QALYs | 12.5300 | 12.5312 |
| NHS PSS cost per QALY gained (£) | 236,778 | |
| Societal cost per QALY gained (£) | 203,820 | |
Extrapolation of costs and outcomes beyond 1 year
Extrapolating the results out over 20 years using a parallel rather than a converging extrapolation assumption increases the cost-effectiveness of SNRB because the value of correctly selecting surgery in a patient with nerve root compression is increased. SNRB remains more expensive and, from the NHS and PSS perspective, the ICER remains above conventional thresholds defining efficiency (Table 20). However, from a societal perspective, the extra costs of SNRB are justified by the benefits of surgery and the NHS and productivity savings associated with performing surgery in those likely to benefit most. The interpretation of the model is relatively sensitive to assumptions about the long-term costs and benefits of surgery vis à vis conservative care.
| Cost/outcome measure | Parallel | |
|---|---|---|
| No SNRB | SNRB | |
| NHS and PSS costs (£) | 13,252 | 13,518 |
| Societal costs (£) | 90,063 | 90,138 |
| QALYs | 11.9192 | 11.9230 |
| NHS PSS cost per QALY gained (£) | 68,481 | |
| Societal cost per QALY gained (£) | 19,862 | |
Malpractice litigation
The net impact of including malpractice litigation costs on our model findings was negligible. The NHS and societal incremental cost per QALY gained still exceeded £1M.
Partial expected value of perfect information
For all values of sensitivity and specificity, holding all other variables constant, the conclusion that SNRB is not cost-effective does not change. This indicates that without further research into other variables there would be little value to undertaking further research on the diagnostic accuracy of SNRB. Even when the willingness to pay per QALY is increased to £200,000, research around the diagnostic accuracy of SNRB still has a PEVPI per patient of only £0.03, suggesting that further research would not be a high priority.
Chapter 8 Discussion
Statement of principal findings
There were few studies that estimated the diagnostic accuracy of SNRB in patients with low back pain and radiculopathy in whom clinical and imaging findings are discordant or equivocal. Research on this topic is hampered by the lack of a diagnostic gold standard against which to compare tests such as SNRB. We identified five diagnostic accuracy studies,28,38–41 all at high risk of bias. Of particular concern was the fact that many studies were at risk of verification bias, as patients with a positive SNRB were more likely to undergo surgery (the reference standard) than those testing negative. There was substantial variation in estimates of sensitivity and specificity across studies: sensitivity ranged from 57% to 100% and specificity from 10% to 86%. Based on the two cohort studies40,41 that used post-surgery outcomes as the reference standard, the summary sensitivity was 93.3% (95% CI 85.8% to 97.0%) and summary specificity was 25.6% (95% CI 5.4% to 67.5%). However, conclusions based on these data should be tempered because of the large CIs around specificity and the high risk of bias which affects these studies.
Despite case reports26,51,52 of serious adverse events associated with SNRB, our SR confirmed that these were very rare events. Of the seven studies25,28,42–46 (n > 1500 patients) identified that reported on complications and adverse events of SNRB, no serious adverse events were reported. The largest case series25 (n = 1203 adult patients) reported minor and transient complications in 5.5% of patient visits, but no major or permanent complications.
Our economic model indicated that, in the case of patients with suspected single-level nerve root compression, SNRB does increase the proportion of patients in whom presence or absence of nerve root compression (59.5% vs 50%) is accurately diagnosed and the proportion of patients with nerve root compression who undergo surgery (20.1% vs 18%). However, these benefits do not appear to be justified by the additional costs of testing. The incremental cost per additional case accurately diagnosed was £2684 and the incremental cost per QALY gained was £1,576,007. In comparison with other health interventions, reviewed by NICE on behalf of the NHS, this does not represent good value for money. This conclusion was the same for patients with suspected two-level nerve root compromise and was not altered in sensitivity analyses varying several key assumptions of the model, including prevalence, the diagnostic accuracy of SNRB, and the impact of the SNRB result on the probability of performing surgery. The model was sensitive to assumptions about the benefits of surgery beyond the 1-year follow-up reported in RCTs examining cost-effectiveness. If the residual improvement in quality-of-life (utility) scores and the savings in costs observed in the surgical arm of trials at 12 months post randomisation continues, rather than diminishes over time, then SNRB has the potential to be cost-effective, despite low specificity. However, we conclude that it is very unlikely based on the current evidence that SNRB is a cost-effective method for informing the decision to operate in patients with low back and leg pain where there is doubt about the localisation of the lesion.
Strengths and limitations of the assessment
We conducted systematic and extensive literature searches in order to locate all relevant studies that met inclusion criteria. These included electronic searches in a wide variety of databases, scanning the references of included studies and previous SRs. Diagnostic accuracy studies are very difficult to identify from electronic databases as there are no specific indexing terms. Therefore, very sensitive searches were carried out to ensure that relevant studies were not missed. Previous work has shown that inclusion of methodological search filters in searches for diagnostic accuracy studies misses relevant studies;36 for this reason we did not use a search filter. Attempts were also made to identify unpublished studies. This included searching conference proceedings and grey literature. It is unlikely that any relevant published studies have been missed, although it is possible that some unpublished studies were not identified.
We used a validated tool (QUADAS-2) to assess the risk of bias and applicability to the research question of the diagnostic studies included in our review. This revealed the flaws in the primary evidence on this research topic. In particular, it highlighted the verification bias inherent in the diagnostic cohort studies, in which patients with a positive SNRB result were more likely to go on to receive surgery. It also raised concerns about the within-patient case-control studies, in which patient selection was limited to patients with clear-cut clinical and imaging findings of nerve root compression. It is unclear whether or not the diagnostic accuracy results from these studies are relevant to clinical practice, in which patients in whom clinical and imaging findings are discordant are most likely to undergo SNRB. The presence of these concerns about the risk of bias and applicability of the primary data led us to interpret the primary and pooled diagnostic accuracy data cautiously.
We have extended the diagnostic accuracy review to model the costs and effects of lumbar SNRB through its impact on treatment choices and patient outcomes. This enabled us to place the evidence on the value of SNRB in a broader context and compare it with other health interventions competing for NHS resources. However, given the scarcity of high-quality primary data, the assessment of the cost-effectiveness was inevitably speculative. The uncertainty within the economic model was high and driven primarily by (a) the uncertainty surrounding the point estimates of sensitivity and specificity; (b) the lack of information on the extent to which the SNRB results influence the subsequent choice of surgical and conservative care; and (c) the limited evidence from RCTs (with substantial non-compliance with assigned therapy) about the costs and benefits of surgery in appropriately selected patients. Despite this inherent uncertainty, we were able to conclude that it is very unlikely based on the current evidence that SNRB is a cost-effective method for informing the decision to operate in patients with low back pain and radiculopathy where there is doubt about the localisation of the lesion.
Comparison with other studies
Two previous SRs of the diagnostic utility of SNRB in patients whose pain was of spinal origin have been reported. 62,63 The first used a computerised search of MEDLINE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) databases, while the second (an update of the first) additionally searched BioMed, abstracts from scientific meetings and screened the reference lists of included studies.
The original review by Everett et al. 63 included 11 diagnostic studies and the updated review by Datta et al. 62 included 16 diagnostic studies (Table 21). Both reviews provided a narrative summary of the evidence. The original review concluded that the ‘available literature is supportive of selective nerve root injections as a diagnostic test in equivocal radicular pain’ while calling for additional research on the topic. 63 The updated review also acknowledged the need for further research and concluded that there was ‘moderate evidence for SNRBs in the preoperative evaluation of patients with negative or inconclusive imaging studies, but with clinical findings of nerve root irritation’.62 Based on our review of the evidence, we believe that these conclusions are too strong. We found limited evidence of low methodological quality indicating that the diagnostic accuracy of SNRB is uncertain and that specificity in particular may be low.
| Author (year) | Included in other reviews | ||
|---|---|---|---|
| Datta62 (2007) | Everett63 (2005) | ||
| Included in our review | |||
| Dooley41 (1988) | ✓ | ✓ | |
| North39 (1996) | ✓ | ✓ | |
| Sasso40 (2005) | ✗ | ✗ | |
| Schutz28 (1973) | ✓ | ✗ | |
| Yeom38 (2008) | ✗ | ✗ | |
| Excluded from our review | Reason for exclusion from our review | ||
| Anderberg65 (2006) | ✓ | ✓ | Cervical SNRB |
| Anderberg64 (2004) | ✓ | ✗ | Cervical SNRB |
| Slipman66 (1998) | ✓ | ✓ | Cervical SNRB |
| Haueisen67 (1985) | ✓ | ✓ | Only positive SNRBs received surgery reference standard. Cannot get 2 × 2 |
| Krempen27 (1974) | ✓ | ✓ | Only positive SNRBs received surgery reference standard. Cannot get 2 × 2 |
| Stanley69 (1990) | ✓ | ✓ | Only positive SNRBs received surgery reference standard. Cannot get 2 × 2 |
| Herron68 (1989) | ✓ | ✓ | Cannot get 2 × 2 |
| Wolff70 (2001) | ✓ | ✗ | Diagnostic block, but no diagnostic data reported. Cannot get 2 × 2 |
| Wolff71 (2006) | ✓ | ✓ | No 2 × 2 or accuracy measure. Only reports means |
| Faraj72 (2006) | ✓ | ✗ | No reference standard. Testing SNRB with and without a stimulator |
| Tajima46 (1980) | ✓ | ✓ | No reference standard |
| van Akkerveeken74 (1993) | ✓ | ✓ | Cannot separate out the group of patients of interest from those with cancer |
| Wolff73 (2006) | ✗ | ✗ | Examining epidural spread of anaesthetic, not a diagnostic study |
The differences in interpretation between our review and those conducted previously may be partly due to the smaller number of primary studies included in our review. We used rigorous eligibility criteria, restricting analysis to studies that provided sufficient data to construct estimates of sensitivity and specificity. Unlike previous reviews, we restricted our analysis to studies evaluating lumbar SNRB, and therefore studies of patients with cervical spine radiculopathy were excluded. Of the five studies28,38–41 included in our review, three28,39,41 were also included in the updated Datta et al. review. 62 Of the remaining 13 included in the Datta et al. review62 but excluded from our study, three studies64–66 were excluded as they focused on cervical spine injections (see Table 21). Six studies27,67–71 were excluded from our review because it was impossible to reconstruct sensitivity and specificity or 2 × 2 tables of index test and reference standard results. Three studies46,72,73 did not include a reference standard and were not considered diagnostic accuracy studies. In the final study,74 it was not possible to separate results for patients with malignant and non-malignant pathology.
Datta et al. 62 used an earlier version of QUADAS75 to assess the methodological quality of studies. The proportion of studies fulfilling each QUADAS item and a profile of individual studies' score on each item are not provided. Instead a combined summary score out of 14 is presented for each study, which has been shown to be an inappropriate way of summarising the results of the QUADAS assessment. 76 However, Datta et al. 62 state that methodological quality of the primary studies included in their review is a limitation. We agree with this assessment and acknowledge that the same limitation applies to our review.
Unanswered questions and future research
Our review highlights the uncertain value of SNRBs when used for diagnostic purposes to establish whether or not clinical symptoms result from a particular nerve root. However, the distinction between diagnostic and therapeutic SNRBs is often not straightforward. Many centres combine local anaesthetic and periradicular steroid injections in order to gain both diagnostic information and, potentially, longer-term pain relief for the patient. Evidence collated in SRs31,77 confirms that transforaminal epidural steroid injections can be a clinically effective and cost-effective part of a management strategy for patients with radicular pain.
Better evidence is needed to inform practice in centres that currently rely on SNRB for diagnostic information to help decide whether, or at which level, to perform lumbar decompressive surgery. These centres could perform SNRB procedures as part of research projects to improve the evidence base. Our recommendations for future research are as follows.
1. A large rigorous diagnostic cohort study to determine the diagnostic accuracy of SNRB in predicting the short-term outcome of lumbar surgery in patients with suspected radiculopathy but equivocal or discordant clinical and radiological findings.
In order to minimise bias, the SNRB result would not be made available to the surgical team and all patients would receive surgery soon after the SNRB. All SNRB results would be recorded based on a pre-specified threshold of pain reduction at fixed intervals post procedure. Similarly the outcomes of surgery (the reference standard) would be collected at a uniform period (e.g. 8 weeks) post surgery.
The research team would have to convince patients, clinicians and an ethics committee that it was acceptable to withhold potentially diagnostic SNRB information in this way, but given the poor-quality evidence on the diagnostic utility of SNRB identified by our review, this should be possible.
2. Separate or nested diagnostic cohort studies to identify the optimal SNRB technique.
In particular, lower anaesthetic volume, the use of needle provocation and the use of control injections at adjacent sites all have the potential to increase the specificity of SNRB. The variation in these parameters observed in the studies identified by our review demonstrates the lack of agreement on the optimal SNRB technique.
The diagnostic cohort studies described above would answer the question of whether or not SNRB can predict which patients will have good outcomes after surgery, which is an important first priority in demonstrating diagnostic accuracy. However, it leaves a key question unanswered, namely ‘Can SNRB predict which patients will have better outcomes after surgery than if they were treated conservatively?’
3. A RCT to measure the impact of diagnostic SNRB on treatment decisions and the costs and outcomes of care for (subgroups of) patients with discordant or equivocal clinical and imaging findings of nerve root compression.
Patients would be randomised to receive either SNRB or management based on clinical and imaging findings alone. As the number of diagnostic SNRBs conducted at any one hospital is likely to be relatively small, this would need to be a multicentre trial. Even so, it may be difficult to demonstrate a difference in patient outcomes as SNRB will alter eventual management in only a subgroup of patients in whom it is performed. It may, however, be possible to provide convincing evidence on the cost-effectiveness of diagnostic SNRB if, for instance, SNRB substantially reduces the number of patients who have surgery. Researchers might choose to focus patient recruitment to this RCT on subgroups of patients, for example those with symptoms indicating potential multiple nerve root involvement, in whom SNRB is believed to be most valuable.
Chapter 9 Conclusions
Despite being widely used for many decades as a method of confirming the source of radicular pain prior to lumbar decompressive surgery, there are few studies of the diagnostic accuracy of SNRB. Based on current weak evidence it is unlikely that SNRB is a cost-effective method for determining which patients will benefit from lumbar surgery.
Better diagnostic accuracy studies are needed to determine whether or not the use of SNRB in the diagnostic work-up of patients with discordant clinical and imaging findings of nerve root compression can identify patients who will have good outcomes after surgery. A trial randomising patients to treatment based on clinical and imaging findings alone or treatment based on these findings plus SNRB is needed to establish whether or not diagnostic SNRB can improve the process and outcomes of care.
Acknowledgements
The authors wish to thank Margaret Burke for her advice in developing and implementing the search strategy. The authors are also grateful to numerous authors of the primary RCTs and studies of diagnostic accuracy who provided additional information about their studies.
Contribution of authors
Rebecca Beynon (Research Associate, Health Services Research) conducted the reviews of diagnostic accuracy and adverse events, conducted analyses and completed the first draft of the report.
James Hawkins (Research Assistant, Health Economics) developed the economic model, analysed the model findings, conducted value of information analysis and drafted Chapter 6 and 7.
Rodney Laing (Consultant Neurosurgeon) contributed to the conception and design of the study, provided clinical expertise in neurosurgery, helped with acquisition of data for the service evaluation of SNRB and critically revised the draft report.
Nicholas Higgins (Consultant Radiologist) contributed to the conception and design of the study, provided clinical expertise in neuroradiology, helped with acquisition of data for the service evaluation of SNRB and critically revised the draft report.
Penny Whiting (Senior Research Fellow, Health Services Research) contributed to the conception and design of the study, supervised conduct of reviews of diagnostic accuracy and adverse events, and critically revised the draft report.
Catherine Jameson (Research Associate, Health Services Research) conducted the reviews of diagnostic accuracy and adverse events.
Jonathan Sterne (Professor, Medical Statistics and Epidemiology) contributed to the conception and design of the study, supervised conduct of the meta-analysis and critically revised the draft report.
Pierluigi Vergara (Clinical fellow in neurosurgery) led on the acquisition of data for the service evaluation of SNRB and critically revised the draft report.
William Hollingworth (Reader, Health Economics) was principal investigator on the project, contributed to the conception and design of the study, supervised conduct of reviews of diagnostic accuracy and adverse events, supervised construction of the economic model and critically revised the draft report.
Disclaimer
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Dodd T. The prevalence of back pain in Great Britain in 1996. A report on research for the Department of Health using the ONS Omnibus Survey. London: The Stationery Office; 1997.
- McKinnon ME, Vickers MR, Ruddock VM, Townsend J, Meade TW. Community studies of the health service implications of low back pain. Spine 1997;22:2161-6. http://dx.doi.org/10.1097/00007632-199709150-00014.
- Walsh K, Cruddas M, Coggon D. Low back pain in eight areas of Britain. J Epidemiol Community Health 1992;46:227-30. http://dx.doi.org/10.1136/jech.46.3.227.
- Epidemiology review: the epidemiology and cost of low back pain. London: HMSO; 1994.
- Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain 2000;84:95-103. http://dx.doi.org/10.1016/S0304-3959(99)00187-6.
- Von Korff M, Deyo RA, Cherkin D, Barlow W. Back pain in primary care. Outcomes at 1 year. Spine 1993;18:855-62. http://dx.doi.org/10.1097/00007632-199306000-00008.
- Hospital Episode Statistics . HES Online 2011. http://www.hesonline.nhs.uk (accessed 1 March 2012).
- Jacobs WC, van Tulder M, Arts M, Rubinstein SM, van Middelkoop M, Ostelo R, et al. Surgery versus conservative management of sciatica due to a lumbar herniated disc: a systematic review. Eur Spine J 2011;20:513-22. http://dx.doi.org/10.1007/s00586-010-1603-7.
- Peul WC, van den Hout WB, Brand R, Thomeer RT, Koes BW. Prolonged conservative care versus early surgery in patients with sciatica caused by lumbar disc herniation: two year results of a randomised controlled trial. BMJ 2008;336:1355-8. http://dx.doi.org/10.1136/bmj.a143.
- Peul WC, van Houwelingen HC, van den Hout WB, Brand R, Eekhof JA, Tans JT, et al. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med 2007;356:2245-56. http://dx.doi.org/10.1056/NEJMoa064039.
- Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Hanscom B, Skinner JS, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA 2006;296:2441-50. http://dx.doi.org/10.1001/jama.296.20.2441.
- van den Hout WB, Peul WC, Koes BW, Brand R, Kievit J, Thomeer RT. Prolonged conservative care versus early surgery in patients with sciatica from lumbar disc herniation: cost utility analysis alongside a randomised controlled trial. BMJ 2008;336:1351-4. http://dx.doi.org/10.1136/bmj.39583.709074.BE.
- Guide to the methods of technology appraisal. London: NICE Publications; 2008.
- Guilfoyle MR, Ganesan D, Seeley H, Laing RJ. Prospective study of outcomes in lumbar discectomy. Br J Neurosurg 2007;21:389-95. http://dx.doi.org/10.1080/02688690701477310.
- Atlas SJ, Keller RB, Chang Y, Deyo RA, Singer DE. Surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: five-year outcomes from the Maine Lumbar Spine Study. Spine 2001;26:1179-87. http://dx.doi.org/10.1097/00007632-200105150-00017.
- Deyo RA, Diehl AK. Lumbar spine films in primary care: current use and effects of selective ordering criteria. J Gen Intern Med 1986;1:20-5. http://dx.doi.org/10.1007/BF02596320.
- Vroomen PC, de Krom MC, Wilmink JT, Kester AD, Knottnerus JA. Diagnostic value of history and physical examination in patients suspected of lumbosacral nerve root compression. J Neurol Neurosurg Psychiatry 2002;72:630-4. http://dx.doi.org/10.1136/jnnp.72.5.630.
- Jarvik JJ, Hollingworth W, Heagerty P, Haynor DR, Deyo RA. The Longitudinal Assessment of Imaging and Disability of the Back (LAIDBack) Study: baseline data. Spine 2001;26:1158-66. http://dx.doi. org/10.1097/00007632-200105150-00014.
- Modic MT, Obuchowski NA, Ross JS, Brant-Zawadzki MN, Grooff PN, Mazanec DJ, et al. Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology 2005;237:597-604. http://dx.doi.org/10.1148/radiol.2372041509.
- Haijiao W, Koti M, Smith FW, Wardlaw D. Diagnosis of lumbosacral nerve root anomalies by magnetic resonance imaging. J Spinal Disord 2001;14:143-9. http://dx.doi. org/10.1097/00002517-200104000-00009.
- Hughes RJ, Saifuddin A. Imaging of lumbosacral transitional vertebrae. Clin Radiol 2004;59:984-91. http://dx.doi.org/10.1016/j.crad.2004.02.019.
- Wolff AP. Diagnostic segmental nerve root blocks in patients with chronic radiating low back pain: bringing light to the darkness?. Amsterdam: Radboud Universiteit; 2006.
- Huston CW, Slipman CW. Diagnostic selective nerve root blocks: indications and usefulness. Phys Med Rehabil Clin N Am 2002;13:545-65. http://dx.doi.org/10.1016/S1047-9651(02)00011-6.
- Boswell MV, Trescot AM, Datta S, Schultz DM, Hansen HC, Abdi S, et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician 2007;10:7-111.
- Stalcup ST, Crall TS, Gilula L, Riew KD. Influence of needle-tip position on the incidence of immediate complications in 2,217 selective lumbar nerve root blocks. Spine J 2006;6:170-6. http://dx.doi.org/10.1016/j.spinee.2005.08.009.
- Houten JK, Errico TJ. Paraplegia after lumbosacral nerve root block: report of three cases. Spine J 2002;2:70-5. http://dx.doi.org/10.1016/S1529-9430(01)00159-0.
- Krempen JF, Smith BS. Nerve-root injection: a method for evaluating the etiology of sciatica. J Bone Joint Surg Am 1974;56:1435-44.
- Schutz H, Lougheed WM, Wortzman G, Awerbuck BG. Intervertebral nerve-root in the investigation of chronic lumbar disc disease. Can J Surg 1973;16:217-21.
- Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM. Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem 2005;51:1335-41. http://dx.doi.org/10.1373/clinchem.2005.048595.
- Quraishi NA. Transforaminal injection of corticosteroids for lumbar radiculopathy: systematic review and meta-analysis. Eur Spine J 2012;21:214-19. http://dx.doi.org/10.1007/s00586-011-2008-y.
- Roberts ST, Willick SE, Rho ME, Rittenberg JD. Efficacy of lumbosacral transforaminal epidural steroid injections: a systematic review. PMR 2009;1:657-68. http://dx.doi.org/10.1016/j.pmrj.2009.04.008.
- Riew KD, Yin Y, Gilula L, Bridwell KH, Lenke LG, Lauryssen C, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am 2000;82–A:1589-93.
- Riew KD, Park JB, Cho YS, Gilula L, Patel A, Lenke LG, et al. Nerve root blocks in the treatment of lumbar radicular pain. A minimum five-year follow-up. J Bone Joint Surg Am 2006;88:1722-5. http://dx.doi.org/10.2106/JBJS.E.00278.
- Tafazal S, Ng L, Chaudhary N, Sell P. Corticosteroids in peri-radicular infiltration for radicular pain: a randomised double blind controlled trial. One year results and subgroup analysis. Eur Spine J 2009;18:1220-5. http://dx.doi.org/10.1007/s00586-009-1000-2.
- Leeflang MM, Scholten RJ, Rutjes AW, Reitsma JB, Bossuyt PM. Use of methodological search filters to identify diagnostic accuracy studies can lead to the omission of relevant studies. J Clin Epidemiol 2006;59:234-40. http://dx.doi.org/10.1016/j.jclinepi.2005.07.014.
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2011;64:602-7. http://dx.doi.org/10.1016/j.jclinepi.2010.07.006.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med 2011;155:529-36.
- Yeom JS, Lee JW, Park KW, Chang BS, Lee CK, Buchowski JM, et al. Value of diagnostic lumbar selective nerve root block: a prospective controlled study. AJNR Am J Neuroradiol 2008;29:1017-23. http://dx.doi.org/10.3174/ajnr.A0955.
- North RB, Kidd DH, Zahurak M, Piantadosi S. Specificity of diagnostic nerve blocks: a prospective, randomized study of sciatica due to lumbosacral spine disease. Pain 1996;65:77-85. http://dx.doi.org/10.1016/0304-3959(95)00170-0.
- Sasso RC, Macadaeg K, Nordmann D, Smith M. Selective nerve root injections can predict surgical outcome for lumbar and cervical radiculopathy: comparison to magnetic resonance imaging. J Spinal Disord Tech 2005;18:471-8. http://dx.doi.org/10.1097/01.bsd.0000146761.36658.45.
- Dooley JF, McBroom RJ, Taguchi T, Macnab I. Nerve root infiltration in the diagnosis of radicular pain. Spine 1988;13:79-83. http://dx.doi.org/10.1097/00007632-198801000-00019.
- Ghahreman A, Ferch R, Bogduk N. The efficacy of transforaminal injection of steroids for the treatment of lumbar radicular pain. Pain Med 2010;11:1149-68. http://dx.doi.org/10.1111/j.1526-4637.2010.00908.x.
- Jonsson B, Stromqvist B, Annertz M, Holtas S, Sunden G. Diagnostic lumbar nerve root block. J Spinal Disord 1988;1:232-5.
- Ng LC, Sell P. Outcomes of a prospective cohort study on peri-radicular infiltration for radicular pain in patients with lumbar disc herniation and spinal stenosis. Eur Spine J 2004;13:325-9. http://dx.doi.org/10.1007/s00586-003-0649-1.
- Quinn SF, Murtagh FR, Chatfield R, Kori SH. CT-guided nerve root block and ablation. AJR 1988;151:1213-16.
- Tajima T, Furukawa K, Kuramochi E. Selective lumbosacral radiculography and block. Spine 1980;5:68-77. http://dx.doi.org/10.1097/00007632-198001000-00013.
- Bogduk N, Holmes S. Controlled zygapophysial joint blocks: the travesty of cost-effectiveness. Pain Med 2000;1:24-3. http://dx.doi.org/10.1046/j.1526-4637.2000.99104.x.
- Cohen SP, Williams KA, Kurihara C, Nguyen C, Shields C, Kim P, et al. Multicenter, randomized, comparative cost-effectiveness study comparing 0, 1, and 2 diagnostic medial branch (facet joint nerve) block treatment paradigms before lumbar facet radiofrequency denervation. Anesthesiology 2010;113:395-40. http://dx.doi.org/10.1097/ALN.0b013e3181e33ae5.
- Buxton MJ, Drummond MF, Van Hout BA, Prince RL, Sheldon TA, Szucs T, et al. Modelling in economic evaluation: an unavoidable fact of life. Health Econ 1997;6:217-27. http://dx.doi.org/10.1002/(SICI)1099-1050(199705)6:3⟨217::AID-HEC267⟩3.0.CO;2-W.
- NHS reference costs 2009–10. London: Department of Health; 2010.
- Kennedy DJ, Dreyfuss P, Aprill CN, Bogduk N. Paraplegia following image-guided transforaminal lumbar spine epidural steroid injection: two case reports. Pain Med 2009;10:1389-94. http:// dx.doi.org/10.1111/j.1526-4637.2009.00728.x.
- Somayaji HS, Saifuddin A, Casey AT, Briggs TW. Spinal cord infarction following therapeutic computed tomography-guided left L2 nerve root injection. Spine 2005;30:E106-8. http://dx.doi. org/10.1097/01.brs.0000153400.67526.07.
- Blackmore CC, Ramsey SD, Mann FA, Deyo RA. Cervical spine screening with CT in trauma patients: a cost-effectiveness analysis. Radiology 1999;212:117-25.
- Buttermann GR. Treatment of lumbar disc herniation: epidural steroid injection compared with discectomy. A prospective, randomized study. J Bone Joint Surg Am 2004;86–A:670-9.
- Osterman H, Seitsalo S, Karppinen J, Malmivaara A. Effectiveness of microdiscectomy for lumbar disc herniation: a randomized controlled trial with 2 years of follow-up. Spine 2006;31:2409-14. http://dx.doi.org/10.1097/01.brs.0000239178.08796.52.
- Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine 1983;8:131-40. http://dx.doi.org/10.1097/00007632-198303000-00003.
- Roland M, Morris R. A study of the natural history of low-back pain. Part II: development of guidelines for trials of treatment in primary care. Spine 1983;8:145-50. http://dx.doi. org/10.1097/00007632-198303000-00005.
- Tosteson AN, Skinner JS, Tosteson TD, Lurie JD, Andersson GB, Berven S, et al. The cost effectiveness of surgical versus nonoperative treatment for lumbar disc herniation over two years: evidence from the Spine Patient Outcomes Research Trial (SPORT). Spine 2008;33:2108-15. http://dx.doi.org/10.1097/BRS.0b013e318182e390.
- Weinstein JN, Lurie JD, Tosteson TD, Tosteson AN, Blood EA, Abdu WA, et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine 2008;33:2789-800. http://dx.doi.org/10.1097/BRS.0b013e31818ed8f4.
- Casey E. Natural history of radiculopathy. Phys Med Rehabil Clin N Am 2011;22:1-5. http://dx.doi.org/10.1016/j.pmr.2010.10.001.
- Quraishi NA, Hammett TC, Todd DB, Bhutta MA, Kapoor V. Malpractice litigation and the spine: the NHS perspective on 235 successful claims in England. Eur Spine J 2012;21:S196-9. http://dx.doi.org/10.1007/s00586-012-2203-5.
- Datta S, Everett CR, Trescot AM, Schultz DM, Adlaka R, Abdi S, et al. An updated systematic review of the diagnostic utility of selective nerve root blocks. Pain Physician 2007;10:113-28.
- Everett CR, Shah RV, Sehgal N, Kenzie-Brown AM. A systematic review of diagnostic utility of selective nerve root blocks. Pain Physician 2005;8:225-34.
- Anderberg L, Annertz M, Brandt L, Saveland H. Selective diagnostic cervical nerve root block- -correlation with clinical symptoms and MRI-pathology. Acta Neurochir 2004;146:559-65. http://dx.doi.org/10.1007/s00701-004-0241-4.
- Anderberg L, Saveland H, Annertz M. Distribution patterns of transforaminal injections in the cervical spine evaluated by multi-slice computed tomography. Eur Spine J 2006;15:1465-71. http:// dx.doi.org/10.1007/s00586-005-0024-5.
- Slipman CW, Plastaras CT, Palmitier RA, Huston CW, Sterenfeld EB. Symptom provocation of fluoroscopically guided cervical nerve root stimulation. Are dynatomal maps identical to dermatomal maps?. Spine 1998;23:2235-42. http://dx.doi.org/10.1097/00007632-199810150-00019.
- Haueisen DC, Smith BS, Myers SR, Pryce ML. The diagnostic accuracy of spinal nerve injection studies. Their role in the evaluation of recurrent sciatica. Clin Orthop Relat Res 1985;198:179-83.
- Herron LD. Selective nerve root block in patient selection for lumbar surgery: surgical results. J Spinal Disord 1989;2:75-9. http://dx.doi.org/10.1097/00002517-198906000-00002.
- Stanley D, McLaren MI, Euinton HA, Getty CJ. A prospective study of nerve root infiltration in the diagnosis of sciatica. A comparison with radiculography, computed tomography, and operative findings. Spine 1990;15:540-3. http://dx.doi.org/10.1097/00007632-199006000-00020.
- Wolff AP, Groen GJ, Crul BJ. Diagnostic lumbosacral segmental nerve blocks with local anesthetics: a prospective double-blind study on the variability and interpretation of segmental effects. Reg Anesth Pain Med 2001;26:147-55.
- Wolff AP, Groen GJ, Wilder-Smith OH, Richardson J, van Egmond J, Crul BJ. Do diagnostic segmental nerve root blocks in chronic low back pain patients with radiation to the leg lack distinct sensory effects? A preliminary study. Br J Anaesth 2006;96:253-8. http://dx.doi.org/10.1093/bja/aei307.
- Faraj AA, Mulholland RC. The value of nerve root infiltration for leg pain when used with a nerve stimulator. Eur Spine J 2006;15:1495-9. http://dx.doi.org/10.1007/s00586-006-0137-5.
- Wolff AP, Groen GJ, Wilder-Smith OH. Influence of needle position on lumbar segmental nerve root block selectivity. Reg Anesth Pain Med 2006;31:523-30.
- van Akkerveeken PF. The diagnostic value of nerve root sheath infiltration. Acta Orthop Scand Suppl 1993;251:61-3.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3. http://dx.doi.org/10.1186/1471-2288-3-25.
- Whiting P, Harbord R, Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med Res Methodol 2005;5. http://dx.doi.org/10.1186/1471-2288-5-19.
- Lewis R, Williams N, Matar HE, Din N, Fitzsimmons D, Phillips C, et al. The clinical effectiveness and cost-effectiveness of management strategies for sciatica: systematic review and economic model. Health Technol Assess 2011;15.
Appendix 1 Literature search strategies
Appendix 2 Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist
Appendix 3 The QUADAS-2 tool for methodological assessment of diagnostic studies
Appendix 4 Table of studies excluded following full paper assessment, with reasons for exclusion
Appendix 5 Studies included in the diagnostic review
Appendix 6 Studies included in the review of complications
Appendix 7 Review protocol
Glossary
Clinical terms
- Decompression surgery
- A surgical procedure designed to relieve pressure on a lumbar nerve root usually undertaken within the lumbar spinal canal. The procedure involves surgical removal of bone, ligament and or disc material which is compressing one or more nerve roots.
- Lumbar disc herniation
- Prolapse of disc material into the spinal canal, lateral recess or neural foramen.
- Lumbar radiculopathy
- Dysfunction of one or more nerve roots in the lumbar spine, characterised by pain and sensory and motor disturbances and often caused by compression of a nerve root(s).
- Lumbar spine
- Vertebrae between the thoracic and sacral vertebrae designated L1–L5.
- Radicular pain
- Pain which radiates along a nerve and is perceived to be in the distribution of that nerve.
- Sciatica
- A lay term for radicular pain which radiates from the lower back to the buttocks, back of the thigh, and calf and foot, often associated with sensory disturbance in the same distribution.
- Selective nerve root block
- Injection of local anaesthetic with or without other substances such as a steroid around spinal nerve root.
- Spinal stenosis
- Narrowing of the spinal canal and/or lateral recesses resulting in compression of the cauda equina or individual nerve roots in the lateral recesses.
- Zygapophyseal joint
- The paired synovial joints which lie posterolateral to the canal and together with the disc constitute a spinal motion segment.(1) Determine the diagnostic accuracy of SNRB in patients with low back and radiating pain in a lower limb; (2) evaluate whether or not accuracy varies by patient subgroups; (3) review injection-related adverse events; and (4) evaluate the cost-effectiveness of SNRB.
Diagnostic accuracy terms
- Diagnostic case-control study
- Diagnostic accuracy study in which the index test results of a series of patients with an established diagnosis are compared with the index test results of a non-diseased control group.
- Within-patient case-control study
- Study in which each patient acts as his or her own control. For example, the nerve root block is performed on a nerve root clinically and radiologically confirmed to be the source of radiculopathy. A second block is performed in the same patient at a different site known not to be the source of radicular symptoms.
- Diagnostic cohort study
- Diagnostic accuracy study in which a group of individuals with suspected disease undergo both the index test and the reference standard, and the results of the two tests are compared.
- False-negative
- A test result which indicates that a person does not have the disease when that person actually does have the disease.
- False-positive
- A test result which indicates that a person does have the disease when that person actually does not have the disease.
- Index test
- New diagnostic test under examination.
- Receiver operating characteristic curve
- A receiver operating characteristic curve represents the relationship between the ‘true-positive fraction’ (sensitivity) and the ‘false-positive fraction’ (specificity). It displays the trade-offs between sensitivity and specificity as a result of varying the cut-off value for positivity in case of a continuous test result.
- Reference standard
- Established test(s) against which the accuracy of a new test for detecting a particular condition can be evaluated.
- Sensitivity (true-positive rate)
- The proportion of individuals with the target condition who are correctly identified by the index test.
- Specificity (true-negative rate)
- The proportion of individuals free of the target condition who are correctly identified by the index test.
- True-negative
- A person without the disease correctly identified as negative by the index test.
- True-positive
- A person with the disease correctly identified as positive by the index test.
Economic evaluation terms
- Cost-effectiveness acceptability curve
- A way of illustrating cost-effectiveness results by graphing the probability that the intervention is cost-effective (y-axis) against the maximum that society is willing to pay for an improvement in health (x-axis).
- Cost-effectiveness plane
- A way of illustrating cost-effectiveness results by graphing the mean incremental cost and effectiveness on a four-quadrant graph. Interventions that are more costly and more effective fall in the north-east quadrant.
- Incremental cost-effectiveness ratio
- The difference in costs between one intervention and an alternative, divided by the difference in outcomes.
- Quality-adjusted life-year
- A measure of benefit of health care combining the impact of both expected length of life and quality of life.
Clinical terms
- Decompression surgery
- A surgical procedure designed to relieve pressure on a lumbar nerve root usually undertaken within the lumbar spinal canal. The procedure involves surgical removal of bone, ligament and or disc material which is compressing one or more nerve roots.
- Lumbar disc herniation
- Prolapse of disc material into the spinal canal, lateral recess or neural foramen.
- Lumbar radiculopathy
- Dysfunction of one or more nerve roots in the lumbar spine, characterised by pain and sensory and motor disturbances and often caused by compression of a nerve root(s).
- Lumbar spine
- Vertebrae between the thoracic and sacral vertebrae designated L1–L5.
- Radicular pain
- Pain which radiates along a nerve and is perceived to be in the distribution of that nerve.
- Sciatica
- A lay term for radicular pain which radiates from the lower back to the buttocks, back of the thigh, and calf and foot, often associated with sensory disturbance in the same distribution.
- Selective nerve root block
- Injection of local anaesthetic with or without other substances such as a steroid around spinal nerve root.
- Spinal stenosis
- Narrowing of the spinal canal and/or lateral recesses resulting in compression of the cauda equina or individual nerve roots in the lateral recesses.
- Zygapophyseal joint
- The paired synovial joints which lie posterolateral to the canal and together with the disc constitute a spinal motion segment.(1) Determine the diagnostic accuracy of SNRB in patients with low back and radiating pain in a lower limb; (2) evaluate whether or not accuracy varies by patient subgroups; (3) review injection-related adverse events; and (4) evaluate the cost-effectiveness of SNRB.
Diagnostic accuracy terms
- Diagnostic case-control study
- Diagnostic accuracy study in which the index test results of a series of patients with an established diagnosis are compared with the index test results of a non-diseased control group.
- Within-patient case-control study
- Study in which each patient acts as his or her own control. For example, the nerve root block is performed on a nerve root clinically and radiologically confirmed to be the source of radiculopathy. A second block is performed in the same patient at a different site known not to be the source of radicular symptoms.
- Diagnostic cohort study
- Diagnostic accuracy study in which a group of individuals with suspected disease undergo both the index test and the reference standard, and the results of the two tests are compared.
- False-negative
- A test result which indicates that a person does not have the disease when that person actually does have the disease.
- False-positive
- A test result which indicates that a person does have the disease when that person actually does not have the disease.
- Index test
- New diagnostic test under examination.
- Receiver operating characteristic curve
- A receiver operating characteristic curve represents the relationship between the ‘true-positive fraction’ (sensitivity) and the ‘false-positive fraction’ (specificity). It displays the trade-offs between sensitivity and specificity as a result of varying the cut-off value for positivity in case of a continuous test result.
- Reference standard
- Established test(s) against which the accuracy of a new test for detecting a particular condition can be evaluated.
- Sensitivity (true-positive rate)
- The proportion of individuals with the target condition who are correctly identified by the index test.
- Specificity (true-negative rate)
- The proportion of individuals free of the target condition who are correctly identified by the index test.
- True-negative
- A person without the disease correctly identified as negative by the index test.
- True-positive
- A person with the disease correctly identified as positive by the index test.
Economic evaluation terms
- Cost-effectiveness acceptability curve
- A way of illustrating cost-effectiveness results by graphing the probability that the intervention is cost-effective (y-axis) against the maximum that society is willing to pay for an improvement in health (x-axis).
- Cost-effectiveness plane
- A way of illustrating cost-effectiveness results by graphing the mean incremental cost and effectiveness on a four-quadrant graph. Interventions that are more costly and more effective fall in the north-east quadrant.
- Incremental cost-effectiveness ratio
- The difference in costs between one intervention and an alternative, divided by the difference in outcomes.
- Quality-adjusted life-year
- A measure of benefit of health care combining the impact of both expected length of life and quality of life.
List of abbreviations
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CT
- computed tomography
- EQ-5D
- European Quality of Life-5 Dimensions
- FN
- false-negative
- FP
- false-positive
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- LDH
- lumbar disc herniation
- LR
- lumbar radiculopathy
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- PEVPI
- partial expected value of perfect information
- PSA
- probabilistic sensitivity analysis
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- QUADAS
- quality assessment of diagnostic accuracy studies
- RCT
- randomised controlled trial
- SNRB
- selective nerve root block
- SPORT
- Spine Patient Outcomes Research Trial
- SR
- systematic review
- TN
- true-negative
- TP
- true-positive
- VAS
- visual analogue scale
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.