Notes
Article history paragraph text
The research reported in this issue of the journal was funded by the HTA programme as project number 09/78/01. The contractual start date was in July 2010. The draft report began editorial review in April 2012 and was accepted for publication in July 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
AH has been an investigator on trials of immunotherapy products sponsored by ALK-Abelló and has received sponsorship from ALK-Abelló for providing allergy training meetings
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Meadows et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Seasonal allergic rhinitis
Allergic rhinitis (AR) is an immunoglobulin E (IgE)-mediated inflammation of the nasal mucosa following allergen exposure. Symptoms include rhinorrhoea, nasal obstruction, nasal itching and sneezing. AR is often comorbid with allergic conjunctivitis and is a risk factor for asthma. 1
Depending on the nature of the triggering allergen, AR has traditionally been categorised as either seasonal allergic rhinitis (SAR, e.g. induced by pollen) or perennial allergic rhinitis (PAR, e.g. induced by animals, dust mites, etc.). More recently, an alternative classification of either intermittent or persistent AR has been proposed [Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update]. 1 The disease can further be categorised as either ‘mild’ or ‘moderate/severe’, depending on the severity of symptoms and impact on quality of life (QoL). 1 ‘Hay fever’ is the common name classically given to SAR or rhinoconjunctivitis.
Diagnosis is based on symptom history and examination, and could include investigations such as peak nasal inspiratory flow or nasal endoscopy. Skin prick tests should be carried out routinely in order to determine whether rhinitis is allergic or non-allergic. 2
Epidemiology and natural history
Allergic rhinitis is a global health problem and affects patients from all ethnic groups, all socioeconomic conditions and all ages; in many countries the prevalence of allergic sensitisation is > 50% in some age groups. 1 It is more common in developed countries. The prevalence of AR based on questionnaire studies has been found to range from 1% to 40% worldwide and between 3% and 29% in the UK (18%, 14.9%, 3%, 11.9%, 29%, 16.5% and 18.9%, based on sample sizes of between 813 and > 12,000, including adults and children). 1 A clinical definition is difficult to use in surveys of large populations, and a questionnaire-only approach may therefore overestimate or underestimate the prevalence of AR. In a study using the ARIA definition of AR,3 the prevalence of clinically confirmable AR in the UK was found to be 26% in adults [95% confidence interval (CI) 20.3% to 31.7%]. A 2009 report4 estimated that SAR affected approximately 16 million people in the UK, with grass pollen allergy the most common form, affecting around 95% of sufferers, followed by sensitivity to tree pollen (25%), weed pollen (20%) and fungal spores. Many people are sensitised to more than one allergen. A large international survey in children5 found a UK prevalence of AR of around 10% in 6- to 7-year-olds and 15–19% in 13- to 14-year-olds. Although rates of AR are increasing in countries with low prevalence, rates may be plateauing or decreasing in countries with high prevalence. 1 However, based on climate change predictions, the prevalence of SAR is likely to increase, with general practitioner (GP) consultations for SAR forecast to rise by 30–40% by 2020. 4
There are few data on the prognosis of AR, although symptoms tend to become milder with age, and allergic skin reactivity decreases in the elderly. 1 Some studies have investigated the incidence and remission of AR in the same general population; a Danish study6 found that remission from symptoms was relatively infrequent and remission from both symptoms and IgE sensitisation was rare; a Swedish study7 found that overall prevalence increased over an 8-year period (from 12.4% to 15.0%), whereas in a proportion of cases (23%) symptoms ceased to be reported.
Allergic rhinitis and asthma are frequently comorbid conditions. Both disorders affect the mucosal lining of the respiratory tract and are linked by common underlying cellular processes and, thus, may be considered as part of the same allergic disease (‘united airways’ approach). 8 There is now widespread evidence to suggest that AR in children often predicts development of asthma. A large longitudinal study9 (n = 8275) found that childhood AR was associated with a significant two- to sevenfold increased risk of incident asthma later in life.
Burden of disease
The burden on primary and secondary care from allergic diseases, particularly asthma, is high, as are the associated costs. A 2004 review10 of UK databases found that:
-
Six per cent of all GP consultations were for allergic disease, with allergic rhinoconjunctivitis being the third most common reason for consultation after eczema and asthma (1991 data).
-
Eleven per cent of community prescriptions were for asthma and other allergic problems, including nasal allergy (4.3 million prescriptions in 2000–1 for nasal allergy).
-
Most hospital admissions for allergic conditions were due to asthma (87% or 92.5/100,000 in 2000–1); 1.6 per 100,000 admissions were for AR (2000–1).
-
Cost estimates for GP consultations for allergic problems range from £211M to £311M per year.
-
Asthma and other allergic diseases accounted for 11% (£0.7B) of all primary care prescribing costs.
-
Allergic problems are responsible for over 183,000 bed-days each year, with an estimated cost of £68M per year (sensitivity analysis limits £56–83M).
Conventional treatment
Conventional treatment of SAR includes oral or topical antihistamines and intranasal corticosteroids as required, with the goal of treatment being symptomatic relief. Occasionally, systemic corticosteroids are prescribed (see Current guidelines). However, some patients are unable to tolerate pharmacotherapy and a substantial number – up to two-thirds in a UK study of patients in 16 general practices11 – report only partial or poor symptom control, particularly of systemic symptoms. Pharmacotherapy has no enduring effect following discontinuation and is not thought to influence the course of disease.
Allergen immunotherapy
Allergen immunotherapy (IT) involves administering gradually increasing doses of a specific allergen, or part of the allergen, to an allergic subject, with the aim of reducing sensitivity and minimising future symptomatic reaction on natural exposure to the causative agent. 12 Delivery of specific allergen immunotherapy (SIT) has traditionally been by subcutaneous injection. 13 A number of other routes of administration have now been investigated, but only subcutaneous and sublingual administration are currently in general use. 13
The mechanisms by which allergen-specific IT modulates the immune response have not been fully elucidated; however, IT has been shown to increase serum levels of allergen-specific IgG, which correlates closely with an IgE-blocking activity and may be partly responsible for the therapeutic effect. 14 IT also appears to alter the balance of helper T-cells, consequently decreasing production of proallergenic cytokines. 14
In contrast with the use of conventional symptom relief medication, the clinical benefits of both subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) appear to be sustained following cessation of treatment. An uncontrolled cross-sectional study15 of SIT for Japanese cedar pollinosis (mean treatment duration 3.6 years; n = 485) reported duration of effect at 10 years post completion, with 42% of patients remaining symptom free even in the year with the highest pollen count. A small prospective, open, controlled study16 (treatment duration 3 years; n = 28) recorded ongoing clinical benefit 12 years after discontinuation of treatment. However, this study was not randomised, with treatment allocation based on patient or parent preference.
Few studies have conducted long-term follow-up while maintaining double-blind conditions. One study17 of SCIT for grass pollen allergy demonstrated that, following 3–4 years of treatment, clinical benefits were maintained over the next three allergy seasons, and did not differ from a continued active treatment arm. A 3-year trial of SLIT for grass pollen allergy with 2 years of blinded follow-up has also shown sustained effects of IT for all clinical and patient-reported outcomes measured. 18
Subcutaneous IT must be delivered in a clinical setting owing the increased risk of severe allergic reactions. Full resuscitation facilities must be available and, in the UK, a minimum of 60 minutes post-injection supervision is required. 19,20 SCIT thus requires a considerable time commitment from patients, as well as substantial use of clinical resources. SLIT appears to be safe even at very high doses (up to 500 times the usual monthly subcutaneous dose) and is associated with fewer adverse events (AEs). 21 Thus, SLIT can normally be self-administered outside of a clinical setting, and is therefore more time efficient for the patient, as well as reducing resource utilisation. It should be noted, however, that maintenance doses for SLIT generally range from 20 to 200 times the dose used in SCIT, with implications for treatment cost. 21
Treatment schedule
Owing to the risk of adverse reactions to allergen injection in sensitised patients, conventional SCIT treatment schedules involve a gradual increase in the allergen content of injections, usually involving one or two injections per week over a 3- to 6-month period. 22 Once a prespecified maximum treatment dose has been achieved, or the maximum tolerated dose for any given patient attained, treatment continues with this maintenance dose at regular intervals, usually monthly, for the duration of therapy. 21 Optimal maintenance dosing for a given product is often prespecified by the manufacturer, although substantial evidence suggests that a maintenance dose in the range of 5–20 μg of major allergen per injection is associated with significant clinical improvement. 23 However, the maximum tolerated dose varies between individual patients and may be lower than the target therapeutic dose.
A number of studies have investigated accelerated updosing schedules for SCIT. For example, rush IT involves administering increasing doses of allergen at intervals of between 15 and 60 minutes over a 1- to 3-day period, until the target therapeutic dose is achieved. 22 An alternative form of accelerated schedule is cluster IT, whereby two to three incremental doses are administered on non-consecutive days. Maintenance dose is usually reached at between 4 and 8 weeks. 22
In contrast, treatment schedules for SLIT may or may not include an updosing period, and following initial treatment administration under medical supervision, maintenance dosing is undertaken by the patient in a non-clinical setting. Typically, dosing continues daily for the period of treatment – up to 3 years. However, studies21,24–26 have shown that shorter treatment periods, with SLIT administered for a few months before and during the pollen season, or during the pollen season only, may be as effective as year-round treatment, in terms of symptom and medication reduction and improved QoL.
Optimal treatment schedules for SLIT have yet to be definitely established, and a wide variety of practices are used. 19 Updosing may or may not be necessary, and maintenance schedules ranging from once per day to once per week have been used,19 although daily dosing is the most common.
More recently, rush or cluster regimens for SLIT have been used. A recent meta-analysis27 of individual patient data (IPD) from three open, prospective studies of high-dose SLIT, totalling 1052 adult and paediatric pollen-allergic patients, found no significant difference in rhinoconjunctivitis symptom scores (SSs) or use of rescue medication between perennial or coseasonal schedules, or standard or ultrarush titration. The rate of AEs was also similar between the different treatment schedules. Thus, the major benefit of accelerated IT schedules appears to be in terms of patient convenience. As inconvenience is one of the major reasons for treatment discontinuation,28 accelerated schedules may increase both adherence and therapy uptake. 22
A recent review29 found that accelerated schedules in SCIT may be associated with a higher risk of systemic reactions but also suggested that premedication, for example with antihistamines or corticosteroids, may result in a risk profile similar to that of conventional treatment schedules.
A number of studies25,30,31 have reported that the clinical effects of SIT are additive over time, with increasing benefit following subsequent years of treatment. Based on evidence of sustained clinical benefits after treatment cessation following studies with 3 years of active treatment,17,32 current guidelines recommend this duration of treatment for both SCIT and SLIT. 19 However, there are few double-blind discontinuation studies, and none comparing the long-term effects after different lengths of active treatment for SAR. One prospective controlled study33 evaluated relapse rates following between 12 and 96 months of SCIT treatment in 40 adult and paediatric patients with house dust mite allergy. All patients were symptom free at completion of treatment, but 55% relapsed over the following 3 years. Relapse rate was significantly related to treatment duration, with 62% of those treated for 35 months or less experiencing a recurrence of symptoms, compared with 48% of those treated for > 36 months (p < 0.04).
Specific (allergen) immunotherapy formulations
The immunomodulatory effect of SIT is specific to the allergen used. Although single-allergen IT has proven effective in reducing symptoms on exposure to the specific allergen in polysensitised patients, no additional benefit is obtained in respect of the other allergic triggers. However, there is some indication that SIT may prevent the onset of new sensitisations in monosensitised children,16,34,35 possibly due to cross-reactivity between related allergen species. For example, there is strong reactivity between members of the Festucoideae family of grasses, which includes timothy grass (Phleum pratense L. ), rye grass and orchard grass, and there is extensive cross-reactivity within and between a number of subfamilies of tree pollen. 21
In contrast, mixtures of unrelated allergens have failed to show efficacy in double-blind, placebo-controlled (DBPC) trials of either SCIT or SLIT in multisensitised populations, possibly due to potential interactions between the different enzymatic components and/or dilution of individual allergen dosage. 21,36 For example, extracts from Alternaria species reduce the immunogenicity of timothy grass extract, and studies have shown that extracts of moulds and fungi significantly reduce the potency of grass pollens, some weeds, trees, and a number of perennial allergens when mixed together. 21 Thus, concurrent treatment of multiple sensitivities is not recommended. However, mixtures of related and cross-reacting allergens (e.g. antigens from more than one species of grass pollen) are effective, and a number of commercial products of this type are currently available. 19 It should be noted that multiallergen treatment is commonplace in the USA, where vaccines are formulated for individual use by the treating clinician, but separate vaccines may need to be given for each allergen. 37
A number of modifications in formulation procedures have been made in recent years to improve treatment convenience and/or safety, particularly for SCIT products. The development of depot formulations by adsorption of allergen extract on to depot materials, for example aluminium hydroxide, L-tyrosine or calcium phosphate, is now common practice. 23 This results in prolonged, gradual release of the allergen at the injection site, allowing for a larger maintenance dose to be given at each injection and reducing the number and frequency of maintenance injections required. Another important modification involves chemical modification of the allergen extract with adjuvants such as glutaraldehyde or formaldehyde. 23 The resultant allergoid has reduced specific IgE-binding capacity and therefore lower allergenicity, reducing the risk of treatment-emergent AEs. The reduced allergenicity of allergoid compounds again allows for larger doses to be used, making treatment schedules more convenient. The effect of these modifications on the immunogenicity of the allergoid is unchanged and, hence, clinical efficacy is maintained. Again, this situation differs from that in the USA, where the use of unmodified aqueous allergen extracts is standard practice. 37 More recently, genetically modified allergens or allergen derivatives, or use of allergens conjugated with immunostimulatory molecules has been reported. 12,38
Standardisation
The production of allergen extracts derived from natural allergens can result in highly variable potency of the end product to be used in SIT. Individual manufacturers have therefore developed in-house standardisation procedures for the purpose of quality control and consistency between batches. 23 In addition, European regulations now specify requirements for starting materials, production processes and quality control. 39 Nevertheless, in-house reference standards are based on units of biological activity obtained from immunological assays and/or skin prick tests in a representative population. Thus, sensitivity of the test population, sample size, and the immunological methodologies used may result in differences in potency between products with the same nominal activity. Further, manufacturers use a range of specific units to measure biological response and these are not readily comparable between different commercial products. 23 Given these differences, optimal dosages are product specific and cannot be generalised. Nevertheless, the degree of clinical improvement appears to be dose dependent in both SLIT and SCIT. 40 In injection IT, increased efficacy with higher doses must be balanced with increased risk of systemic reactions. 41 In contrast, a meta-analysis of 25 studies42 in SLIT found that this route of administration did not result in a dose-dependent increase in AEs. These findings were confirmed by a 2011 report from the European Academy of Allergy and Clinical Immunology (EAACI) task force on dose–response relationships in SIT. 40
It has been recommended that manufacturers state the major allergen content (MAC) of their products in mass units (g/ml),43,44 although differences in assay methods may still limit comparability, and the variable contribution of minor allergen content to total biological potency is not accounted for. The use of recombinant allergen products may improve standardisation in the future but these are not yet widely available and few have been tested in large-scale randomised controlled trials (RCTs). 23
Commercial products in the UK
The only aeroallergen SLIT product licensed in the UK for adults and children (5 years) is Grazax® (75,000 SQ-T oral lyophilisate; ALK-Abelló Ltd, Hørsholm, Denmark), a standardised allergen extract of grass pollen from timothy grass. Tablets (one per day) are placed under the tongue and allowed to disperse. Treatment is ideally initiated 4 months before the grass pollen season and continued for a period of 3 years. Where no improvement in symptoms is observed during the first pollen season, there is no indication for continuing the treatment. 45 Grazax costs £66.77 for 30 tablets [source: British National Formulary (BNF) (2012)]. 46
The only SCIT product licensed in the UK is Pollinex® Allergy Therapeutics, Worthing, UK), a standardised L-tyrosine-adsorbed allergoid of grass or tree pollens. Pollinex for grass allergy contains allergen extracts of 12 grass species plus rye, and the tree pollen vaccine contains birch, alder and hazel. Both vaccines may be prescribed to adults and children (≥ 6 years) and are given in six preseasonal injections. 19 An initial treatment set (three vials) and extension course treatment (one vial) of Pollinex costs £450 [source: BNF (2012)46].
A variety of unlicensed products may be prescribed by specialists on an individual ‘named-patient’ basis [see the 2011 British Society for Allergy and Clinical Immunology (BSACI) guidelines for AR19 for an overview].
Non-standard therapies
A number of researchers have investigated highly truncated SIT schedules, including single-injection treatment47,48 and the Rinkel method. 49,50 These are not considered to be standard IT, have proven ineffective in double-blind placebo-controlled studies49,50 and, therefore, have not been included in this review. More recent developments in IT formulations have included the use of peptides fragments of relevant T-cell epitopes of an allergen, as opposed to whole allergens; vaccination with immunostimulatory compounds without a specific allergen attached; and the use of recombinant wild allergens or allergen fragments. 51 Genetically engineered allergens have the potential to reduce allergenicity while maintaining immunogenicity52,53 and are thus a promising avenue for future research. However, these products are generally in the early stages of development and are therefore not currently used in standard practice. These therapies have also not been included in this review.
The role of specific (allergen) immunotherapy in asthma prevention
As well as treating symptoms of AR (and allergic asthma), there is evidence that SIT can prevent disease progression, development of new sensitisations, and onset of asthma. 8,19 The review by Fiocchi and Fox8 identifies a number of studies54–56 demonstrating the preventative effect of SIT, including the Preventative Allergy Treatment (PAT) study. The PAT study54–56 was an open-label RCT (n = 205) of SCIT compared with control, which followed children aged 6–14 years for up to 7 years after a 3-year treatment period. Symptomatic rescue medication was allowed in both treatment arms. At 10 years, the number of patients who had developed asthma was 16 out of 64 (25%) in the SCIT group and 24 out of 53 (45%, control OR = 2.5, 95% CI 1.1 to 5.9; p-value not reported) in the control group. 54 Loss to follow-up at this point was 23% in the SCIT and 33% in the control group. When adjusted for bronchial hyper-responsiveness and asthma status at baseline, the treatment effect was found to be statistically significant (OR for no asthma = 4.6, 95% CI 1.5 to 13.7; p = 0.0075).
Fiocchi and Fox8 identify a number of additional studies that support these findings. Overall, they showed that SCIT or SLIT is beneficial compared with medication only in the prevention of new asthma cases and/or new sensitisations, or for reducing asthma severity or the number of asthma cases. The studies included in the review are mainly open-label RCTs or use non-randomised designs, so are likely to be subject to greater bias than blinded RCTs, which, in turn, may influence the effect size. Loss to follow-up may also be an issue in those studies with long follow-up periods and fairly small numbers of patients.
Three studies54–58 report on the preventative effect of SIT in the development of new asthma cases; these are summarised in Table 1.
| Study | Participants | Study design | Outcome |
|---|---|---|---|
| Novembre 200457 | Children (n = 113, aged 5–14 years) with AR (grass) and fewer than three episodes of asthma per season | Open-label RCT, 3 years of SLIT or medication only | At 3 years, asthma development less frequent in active group (OR = 3.8, 95% CI 1.5 to 10) |
| Polosa 200458 | Adults (n = 30, aged 20–54 years) with AR (grass) and no asthma | Double-blind RCT, 3 years of SCIT or placebo | At 3 years, 7/15 (47%) in the placebo group developed asthma symptoms compared with 2/14 (14%) in the SCIT group (p = 0.0056) |
| PAT study54–56 (2002, 2006, 2007) | Children (n = 205, aged 6–14 years) with AR (grass/birch) and no asthma needing daily treatment | Open-label RCT, 3 years of SCIT or medication only, follow-up up to 10 years | For those with no asthma at baseline, lower incidence of new asthma in SCIT group at 3, 5 and 10 years:
|
An ongoing RCT, which may be able to further substantiate these findings, is the Grazax Asthma Prevention (GAP) RCT,59 which commenced in 2010 and is due to finish in 2015. It randomised children aged 6–12 years with grass pollen-induced AR and no asthma to receive SLIT with Grazax or a placebo tablet. The primary outcome measure is the evaluation of allergy and asthma symptoms.
Current guidelines
Guidance for the management of patients with AR and non-allergic rhinitis prepared by the Standards of Care Committee (SOCC) of BSACI in 20082 canbe summarised as follows. Following diagnosis, first-line treatment of AR is allergen avoidance (where possible and practicable). The nature and severity of symptoms determines the type of medication offered; if symptoms are mild, a non-sedating oral or topical H1-antihistamine is given. Where symptoms are moderate to severe, first-line therapy is with a topical intranasal steroid. If these treatments fail, further agents may be added according to the troublesome symptom: ipratropium for watery rhinorrhoea, a non-sedating H1-antihistamine for itch or sneeze, or a leukotriene-receptor antagonist for catarrh if asthmatic. Blockage of the nose may require a decongestant, oral corticosteroids or a long-acting non-sedating H1-antihistamine.
If there is further treatment failure, and if the symptoms are predominantly due to one allergen, then IT may be considered. Specific guidelines from BSACI on the use of allergen IT for AR,19 published in 2011, conclude that both injection and SLIT are effective in patients with IgE-mediated seasonal pollen-induced rhinitis and/or conjunctivitis whose symptoms respond inadequately to usual therapy, although the relative efficacy of SCIT and SLIT has still to be determined. The BSACI highlights the need for both head-to-head trials of SCIT compared with SLIT, and for long-term studies that include pharmacoeconomic evaluation comparing SIT with antiallergic drugs. 19 The 2011 BSACI guidelines also update the position on the use of SIT in asthmatic patients. SIT has been shown to improve symptoms in atopic, asthmatic adults and children clinically sensitised to seasonal and perennial allergens, and treatment is generally considered safe in patients with pollen-induced seasonal allergic asthma (SAA), provided any updosing is conducted out of season. 19 However, owing to the slightly elevated risk of severe systemic reactions in asthmatic patients, perennial, unstable or uncontrolled asthma is still considered a relative contraindication for SIT.
The ARIA guidelines (2010 revision)60 on the role of SIT in the treatment of AR make the following recommendations (a summary is shown in Table 2). The guidelines suggest that both SLIT and SCIT can be used in both adults and children for treating AR, but note that a higher value is placed on relieving symptoms, and a lower value on avoiding AEs and resource expenditure.
| Treatment | Recommendation | Underlying values/preferences |
|---|---|---|
| SCIT for adults with AR and without asthma | Suggest use in adults with seasonal AR (moderate-quality evidence) and persistent AR caused by house dust mites (low-quality evidence) | Relatively high value placed on symptom relief; relatively low value placed on avoidance of AEs and resource expenditure |
| SCIT for children with AR and without asthma | Suggest use in children (low-quality evidence) | Relatively high value placed on probable reduction in symptoms and potential prevention of development of asthma; relatively low value placed on avoidance of AEs and resource expenditure |
| SLIT for adults with AR and without asthma | Suggest use in adults with rhinitis caused by pollen (moderate-quality evidence) or house dust mites (low-quality evidence) | Relatively high value placed on symptom relief; relatively low value placed on avoidance of AEs and resource expenditure |
| Local AEs are relatively frequent (around 35%) | ||
| SLIT for children with AR and without asthma | Suggest use in children with rhinitis caused by pollen (moderate-quality evidence), but not in children with AR caused by house dust mites outside clinical trials (very low-quality evidence) | Relatively high value placed on symptom relief; relatively low value placed on avoidance of AEs and resource expenditure |
| Local AEs are relatively frequent (around 35%) | ||
| SCIT or SLIT in patients with AR and asthma | Suggest use of SCIT or SLIT to treat asthma and/or rhinitis (moderate-quality evidence) | Relatively high value placed on symptom relief; relatively low value placed on avoidance of AEs and resource expenditure |
No guidelines from the National Institute for Health and Care Excellence (NICE) regarding IT for AR were identified. The British Guidelines on the Management of Asthma,61 produced by the British Thoracic Society and the Scottish Intercollegiate Guidelines Network (SIGN), mention IT in the context of primary prevention of asthma, but find that more studies are required to establish this role and no recommendations are made.
UK clinical practice
A 2010 report61 from the Royal College of Physicians reported an increasing trend in the use of SLIT compared with figures from 2007. Sales figures for SCIT products have remained relatively unchanged over this time. The authors estimate that approximately 2000 patients per year are receiving each treatment.
One possible explanation for the relatively low uptake of SIT is the perception of risk associated with the practice following reports of serious AEs in early studies of SCIT. 62,63 Consequently, outside of specialist centres, there remains an unwillingness to utilise SIT in clinical practice. Knowledge of safety improvements in SCIT preparations and the relatively favourable safety profile of SLIT remains lacking. 62 In addition, primary care trust funding for SIT is still uncommon, leading to wide geographic variations in treatment access. The shortage of trained specialists and the absence of clinical guidelines from NICE may be compounding factors in this matter. 62
With increasing evidence that SIT may result in the prevention of new sensitisations and incidence of asthma, the uptake of SIT in paediatric allergy sufferers is likely to have long-term clinical and economic impacts. However, although children attending specialist centres are more likely to be treated with SIT than those in non-specialist practices,62,64 a recent audit of NHS paediatricians offering pollen IT in England and Wales65 identified only 20 centres, all of which were in England, with three located in London. Further, absolute numbers of children treated with SIT were still low, although the trend was for increasing numbers over time. Approximately twice as many children had been treated with SLIT (n = 363 courses) than with SCIT (n = 165 cycles) over the 10-year audit period. The most commonly used SCIT products were Pollinex Quattro (Allergy Therapeutics, 53%), Pollinex (Allergy Therapeutics, 32%), Allergovit® (Allergopharma, Reinbek, Germany, 8%) and Alutard SQ® P. pratense (ALK-Abelló, 8%). Only two SLIT products were used in these centres: Staloral® (Stallergènes, Antony Cedex, France) made up the majority of treatment courses (70%), with the remainder accounted for by Grazax®. 65 Despite earlier guidelines that asthma was a contraindication for SIT in children,2 49% of children receiving SCIT and 58% of those receiving SLIT had a diagnosis of asthma. Of these, nearly three-quarters had perennial asthma (126/174, 72%),65 which remains a contraindication to SIT in the updated BSACI guidelines. 19
Patient perspective
A survey was conducted in 2005 by Allergy UK, in conjunction with the General Practice Airways Group;66 1000 individuals with AR were asked about their symptoms and the impact of SAR on their lives. It should be noted that there were no details on how patients were sampled and it is unclear whether or not patients across the whole of the severity spectrum are represented. The results are shown in Table 3.
| Impact | Percentage |
|---|---|
| AR symptoms for > 2 months/year | 92 |
| AR symptoms for > 10 years | 73 |
| AR symptoms affect school/work moderately to severely | 49 |
| AR symptoms affect how social/leisure time is spent | 80 |
| AR symptoms disrupt sleep | 85 |
| Disrupted sleep affects school/work | 56 |
| Disrupted sleep affects planned social activities | 33 |
The same survey66 found that over half of those patients taking medication felt that their symptoms were not fully controlled, and that one in four patients had tried more than five different oral antihistamines.
Another UK report67 found that students who have AR symptoms are 40% more likely to drop a grade in their General Certificate of Secondary Education (GCSE) examinations, with the figure rising to 70% if they were taking antihistamines. Onset of hay fever peaks in adolescence and GCSE examinations run from mid-May to the end of June, coinciding with the height of the grass pollen season.
For this report, a patient representative, Lynne Deason (LD), shared her experiences of living with hay fever and other allergies, and receiving treatment with SCIT at Birmingham Heartlands Hospital, Birmingham, UK. These are summarised below.
Patient experience
Lynne Deason developed allergies to different moulds and dog dander in her mid-teens. In her mid-20s she also suffered increasingly with SAR (mainly birch) and allergic reactions to fruit (oral allergy syndrome). In addition, she regularly experienced episodes of anaphylaxis, for which she sought help on several occasions from the accident and emergency (A&E) department; the allergen responsible for these episodes has to date not been identified. No treatment was initiated as a result of visits to the A&E department. As a teenager, LD's parents had paid for her to have private allergy testing and standard medication was recommended (antihistamines and nasal sprays). LD ‘managed’ her anaphylaxis by quickly taking antihistamines whenever signs appeared that an episode was imminent, such as an itching sensation in her ears. More recently, conventional medication provided reasonable relief for SAR, although this still impacted negatively on daily activities, particularly work. Symptoms from both SAR and the oral allergies included puffy eyes, not being able to see very clearly, changes in voice quality, looking like ‘someone had punched me in the face’, feeling ‘groggy’ and an itchy throat; these symptoms made giving presentations at work difficult.
After seeing a nurse at her local GP practice and describing her history of oral allergies, SAR and regular anaphylaxis, LD was referred to hospital and was eventually placed under consultant care for treatment. The nurse expressed disbelief that there had been no earlier referral. Treatment with SCIT was time intensive as it initially involved weekly 2-hour appointments, which then decreased to monthly appointments for approximately 3 years. It also involved a travelling distance of around 25 miles to the hospital. Undergoing treatment was facilitated by having an employer willing to allow time off work. LD did not experience the treatment itself as being particularly unpleasant and felt that professional members of staff who provided good explanations of the procedure were a positive aspect of treatment. LD stated that treatment would be more difficult to incorporate into daily life for parents, as it would involve additional childcare. Additional positive aspects of undergoing SCIT included meeting people with similar experiences at hospital, sharing tips on managing symptoms, feeling less isolated and feeling that people were being empathic. LD also started carrying an EpiPen® (Mylan Speciality L.P., Basking Ridge, NJ, USA) for the first time.
Subcutaneous IT significantly improved LD's SAR symptoms, which became both milder and less frequent. Four years after completing the treatment, significant improvement is still noticeable, with only mild irritation experienced in response to particularly high pollen counts. Medication use also decreased and LD now rarely takes antihistamines. No occurrences of anaphylaxis have arisen since treatment and LD no longer carries an EpiPen. The lessening of symptoms also had a positive knock-on effect on general QoL.
Side effects of the treatment included tiredness (particularly after updosing) and some fairly mild swelling and redness of the arm immediately afterwards. LD noted that other patients had more severe swellings. The treatment has had no effect on the oral allergies. A single allergen was used in the treatment (birch), and LD now knows she is also allergic to almond and hazel. LD felt that there was generally little awareness of severe allergy and treatments with SCIT or SLIT and little empathy for affected individuals. Although, in her own estimation, LD was not among the worst affected, she felt that other people might benefit even more from SCIT or SLIT, for example individuals who are confined indoors during peak allergen times.
Existing evidence for allergen immunotherapy
Subcutaneous immunotherapy
To date, the most comprehensive systematic review of SCIT for SAR is a Cochrane review,68 with searches up to February 2006. The review identified 51 RCTs including a total of 2871 participants (1645 active, 1226 placebo), with only one study69 including children of < 12 years. There was significant heterogeneity in treatment durations (3 days to 3 years) but, on average, participants received 18 injections each. Pooled standardised mean differences (SMDs) from meta-analyses found statistically significant results in favour of active treatment across all outcomes [symptoms scores, rescue medication use, combined symptom and medication scores (SMS) and QoL]. AE reporting was highly variable, making comparisons difficult. Local reactions at site of injection were the most commonly reported event, with the majority resolving without treatment. Systemic reactions occurred in over half of studies, with more severe reactions occurring less frequently than milder reactions. No deaths were reported.
However, evidence from surveillance studies in the USA suggests that fatal reactions still occur following SCIT in clinical practice. 70 Between 1973 and 2007, 82 direct or indirect reports of fatal reactions were identified, although the frequency appears to be decreasing – only six of these deaths occurred between 2001 and 2007, presumably due to improved vaccines, protocols and safety measures. No fatalities were identified between 2008 and 2010.
A 2008 systematic review71 of the paediatric literature included only four randomised, placebo-controlled studies72–75 of SCIT for seasonal allergens, all of which were conducted between 1966 and 1986. Adequacy of blinding could be ascertained in only one study,72 although this may be due to less transparent reporting practices in earlier studies. Overall, only one study72 reported positive effects of SCIT on symptoms, but medication use was not monitored in this study. None of the other studies73–75 reported benefits of SCIT on either SSs, or, when reported, medication scores (MSs). The authors concluded that there was insufficient good-quality evidence to draw conclusions regarding the effectiveness of SCIT in this patient group.
Sublingual immunotherapy
A number of systematic reviews evaluating the effectiveness of SLIT for SAR have been published,71,76–82 the largest and most recent of which was the Cochrane review of SLIT for AR. 83 Although this review included studies in both SAR and PAR, subgroup analysis was performed for both types of allergen. The review identified 39 studies24,26,35,84–119 conducted in patients with SAR, comprising a total of 4084 participants (2081 active, 2003 placebo). Meta-analysis of both symptom and MS data suggested a moderate effect size in favour of SLIT, with similar results reported for MS outcomes. Subgroup analyses were conducted in adults or children; short-, medium- and long-term duration; low, medium and high levels of MAC; and type of allergen. All of the subgroup analyses included studies of both SAR and PAR, but all reported pooled effect sizes favouring the active treatment. The majority of these findings were statistically significant, although a few were not: MSs in children, SSs in studies using IT with < 5 μg MAC, MSs in studies with > 20 μg MAC and MSs in ragweed pollen. QoL was reported in only two studies,102,112 both involving seasonal allergens, and combined SMD was in favour of SLIT. As for SCIT, AEs for SLIT are reported quite variably between studies. The majority of systemic reactions were of mild to moderate severity; none required administration of adrenaline and no fatalities were reported. Discontinuations due to AE were rare and more often associated with unpleasant local side effects than with systemic reactions.
One recent meta-analysis77 has been conducted for studies of SLIT for SAR only, specifically grass pollen AR. This meta-analysis included 19 RCTs (compared with 23 studies in grass allergen in the Cochrane review) and produced almost identical results. Subgroup analysis by age showed that effect sizes were greater in adult populations than in children, with neither symptom nor MSs reaching statistical significance in the five included paediatric studies.
Several reviews of the paediatric literature have been published. 71,78–80,82 Although all included both SAR and PAR, results are often separable for the two types of allergen. The most recent, and inclusive, of these (Larenas-Linneman 2009)78 included 1084–89,120–122 double-blind studies of SLIT in children with SAR (as well as three with SAA), which reported clinical outcomes. All of these studies were identified in the 2010 Cochrane review. 83 Interestingly, earlier studies deemed to be of high quality84,89 (total n = 192) failed to report statistically significant effects of SLIT on rhinitis outcomes, whereas studies deemed to be of lower quality86,88,121,122 (total n = 158) favoured SLIT. In contrast, three26,85,90 of the four studies conducted since 2006 (total n = 560)26,84,85,90 reported significant improvements in both SSs and MSs with active treatment. It is not clear whether this change was due to improvement in SLIT treatments over time or to the larger study sizes. One recent study87 (n = 168) did not find an advantage for SLIT treatment in grass allergy. However, this study87 was conducted in a primary care setting, inclusion criteria did not specify objective diagnosis of AR, and dropout rates were high (44%).
Sublingual immunotherapy has a good safety profile. One report, based on 41 studies, identified 1047 AEs in an estimated 386,149 doses, equivalent to 2.7 AEs per 1000 doses. 123 Based on 49 studies, approximately 12% (529/4378) patients experienced at least one AE, although most of these were local reactions in the oral cavity or gastrointestinal symptoms, also considered a local reaction in SLIT. 124 Systemic reactions occurred in 169 of 314,959 doses, or 0.54 per 1000 doses. Only 14 treatment-related serious adverse events (SAEs) were recorded in 5377 treatment-years, mostly involving asthma or gastrointestinal symptoms, equivalent to one SAE per 384 treatment-years. A 2010 Cochrane review83 reported no occurrences of anaphylaxis in six trials reporting this outcome (n = 579) and no reports of adrenaline use for systemic reactions following active treatment. Again, the vast majority of AEs were of mild to moderate severity, and gastrointestinal symptoms were the only systemic reactions reported more frequently in patients receiving active treatment than in placebo (88 events in 630 patients vs 10 events in 561 patients, respectively). Discontinuations due to AEs were more often associated with unpleasant local side effects than with systemic reactions, and were reported in 5% of active patients (41/824) in 15 studies.
Outcome measures in randomised controlled trials of specific (allergen) immunotherapy
Outcome measures used in trials of SIT are highly variable. SSs are the most widely used, and often the only outcome measure used in older trials. Although no individual scoring system has been thoroughly validated for clinical trials,64 the vast majority of RCTs conducted in the last 20 years utilise a four-point scoring scale for describing symptom severity, ranging from a score of ‘0’ to indicate absence of symptoms to a score of ‘3’ representing severe symptoms that interfere with activities of daily living. Despite this common system of measuring symptom severity, there is significant heterogeneity in the actual number of symptoms that are scored in any given trial, meaning that maximum possible scores vary between studies. Indeed, the six major European manufacturers (ALK-Abelló, Allergopharma, Allergy Therapeutics, HAL Allergy, Leiden, the Netherlands, Laboratorios LETI/Novartis, Barcelona, Spain, and Stallergènes) all use different systems. 125 There are also differences in the way that SS data are reported, for example as mean daily score, cumulative score over 1 week or an entire season, differences from baseline, or area under the curve (AUC). In addition, some studies record outcomes over an entire pollen season (EPS), whereas others use values for a 1- or 2-week period around the peak recorded pollen value. These differences make comparisons between studies very difficult.
One limitation of the use of SSs as the sole outcome measure is that most trials allow the use of rescue medication on an as-needed basis, under varying conditions of stringency. As placebo patients might be expected to use more rescue medication, the use of SSs alone may underestimate the effects of treatment. 125 Reporting of MSs, a measure of rescue medication use, is intended to address this problem. However, although a 2008 report126 from the World Allergy Organization (WAO) taskforce on standardisation of clinical trials suggested a three-point scoring system for anti-allergy medications, scoring of this outcome remains somewhat arbitrary and highly variable between trials. As with SSs, methods for reporting MSs also vary. In addition, the use of separate SSs and MSs does not account for the interdependence of these two measures. 125
Thus, the WAO taskforce also recommended that weighted SSs and MSs be combined into a SMS, and that this combined score should be used as the primary outcome in clinical trials. 126 Indeed, an increasing number of recent trials have reported SMSs (15 out of 28 trials in this report). Further, the ‘Allergy-Control-SCORE©’, a combined symptom and MS, has recently been formally evaluated, and was found to be a valid and reliable tool for assessing and monitoring allergy severity. 127 However, there is currently no standardised method for calculating SMSs, and the methods used are frequently not reported. As with SSs and MSs, the units of statistical analysis of SMS differ between studies, and are often not stated explicitly. Again, the six major European manufacturers of IT products use different protocols and scoring systems for usage of rescue medication, and different methods of weight symptom and MSs into a combined measure. 125 Thus, despite the increasing convergence in outcome measurements used in clinical trials, between-trial comparison is still problematic.
Other outcomes that have been recommended by the WAO126 and European Medicines Agency (EMA)64 include responder analysis – the percentage of patients with a combined SMS below a prespecified level, visual analogue scales (VASs) for long-term treatment outcomes, number of ‘well-days’ – i.e. SSs below a predefined threshold and no requirement for rescue medication, and patient-reported outcomes, such as overall impact on health-related QoL, which may provide more useful information on the impact of treatment than measuring organ-specific SSs. However, data on these outcomes are available in only a small proportion of the SIT literature.
It has been argued in an ARIA-GA(2)LEN statement128 that RCTs in IT cannot be interpreted in the same way as RCTs in drug treatment. One of the factors that has been criticised in SIT RCTs is the relatively low level of efficacy compared with medications, which may prevent regulatory bodies from recommending SIT. One of the reasons for this apparently lower efficacy may be that exposure to allergens varies over the pollen season, yet the averaged score is presented for the whole season. It is for this reason that the concept of ‘worst-days’,129 i.e. days with severe symptoms as an outcome measure, has been introduced, as it may better reflect the impact of IT compared with placebo on days when pollen counts are high and symptoms are severe.
The majority of studies reporting on QoL have used the validated disease-specific Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ),130–132 although there are a number of different versions of this instrument. A few studies have used more global measures, such as the Short Form questionnaire-36 items (SF-36). Generic tools are broad and are likely to be less sensitive to measuring changes in AR, where disease-specific instruments may be more appropriate. 125 QoL is a difficult parameter to be measured in trials of IT: patients do not have impaired QoL at inclusion, but this will deteriorate during the course of the trial. The difference in QoL between IT and placebo groups will depend on the pollen exposure on the day(s) that QoL is measured. If pollen exposure is low then QoL in the placebo group may appear relatively high compared with that of the IT group. 128
Even within a single trial, results may be difficult to interpret clinically. So, for example, a difference in reduction in symptoms scores may be statistically significant but not necessarily clinically significant. Malling has proposed ranges of improvement in SSs or MSs to discriminate between effective and non-effective therapy (no effect, improvement of < 30%; little effect, improvement of 30–44%; moderate effect, improvement of 45–59%; strong effect, improvement of ≥ 60%). 133 This outcome is not at present reported consistently. In contrast, an expert group134 has estimated that an improvement of 20% is clinically relevant given that SIT trials may show relatively lower effectiveness (than drug trials) given the reasons outlined above. There is no consensus on a minimum meaningful difference.
Chapter 2 Aim of the review
The aim of this systematic review and cost-effectiveness analysis (CEA) was to:
-
update the Cochrane review68 on the clinical effectiveness of SCIT based on double-blind RCTs of SCIT compared with placebo
-
update the Cochrane review83 on the clinical effectiveness of SLIT based on double-blind RCTs of SLIT compared with placebo
-
more specifically, to update the meta-analyses (including those for prespecified subgroups including adults and children) undertaken in the Cochrane reviews in order to provide up-to-date summary estimates
-
evaluate the clinical effectiveness of SCIT compared with SLIT using both direct and indirect comparisons
-
undertake a systematic review and critical appraisal of existing economic evaluations (EEs) of SCIT or SLIT compared with placebo or SCIT compared with SLIT
-
develop a de novo cost-effectiveness model, based where possible on clinical data from this report
-
estimate cost-effectiveness separately for SCIT compared with placebo, SLIT compared with placebo, SCIT compared with SLIT, and for adults and children.
This report did not aim to address questions relating to the optimum dosing schedules (e.g. rush or cluster compared with conventional dosing) or optimum length of treatment of SCIT or SLIT. It also did not address other methods of administration, such as epicutaneous or intralymphatic IT.
Chapter 3 Clinical effectiveness
Methods
The original protocol can be found in Appendix 1.
Searches
Randomised controlled trials of SCIT compared with placebo and SLIT compared with placebo were sought, as were any existing RCTs of head-to-head comparisons (SCIT vs SLIT). A sensitive search strategy, based broadly on those employed in the Cochrane reviews, but with no restriction on routes of IT administration or dates, was used in order to cover both the update and the search for head-to-head trials. There were no language restrictions. Appropriate filters for study design were used where possible. Searches were carried out during April 2011. See Appendix 2 for full details of the search strategies.
The following resources were searched:
-
bibliographic databases: MEDLINE (Ovid) 1948–April week 2 2011; EMBASE (Ovid) 1980–April week 15 2011; The Cochrane Library [Cochrane Central Register of Controlled Trials (CENTRAL)] 2011 Issue 1; Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost) 1982–2011; Science Citation Index (Web of Knowledge) 1900–2011. Searches were based on index and text words that encompassed the population and intervention (e.g. ‘seasonal allergic rhinitis’, ‘immunotherapy’)
-
ClinicalTrials.gov, UK Clinical Research Network Portfolio Database and metaRegister of Current Controlled Trials (mRCT) (http://controlled-trials.com) were searched for ongoing studies, as well as the lists of ongoing trials identified in the Cochrane reviews
-
references lists of relevant reviews and included studies
-
consultation with clinical advisors
-
selected websites.
Study selection
The following inclusion and exclusion criteria were used (Table 4). These are broadly consistent with those listed in the Cochrane reviews.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Study design | |
| Double-blind RCTs of SCIT compared with placebo, or SLIT compared with placebo or SCIT compared with SLIT | Single-blind or open-label RCTs. Any other study design |
| Population | |
| Treatment-naive adults or children with a confirmed diagnosis and symptoms of SAR (hay fever). Patients with comorbidities such as (seasonal allergic) asthma will be included. All or majority (≥ 90%) of included patients (adults or children) with SAR | Adults or children with a different allergic disease (e.g. food allergy, perennial rhinitis). Population (or > 10%) with SAA only |
| Intervention | |
| Allergen-specific subcutaneous (injection) or SLIT in any setting. Any allergen responsible for inducing SAR (e.g. grass, tree). No restrictions regarding a particular dose or dosing regimen | Any other route of administration [e.g. oral (swallowed rather than sublingual), nasal, epicutaneous, intralymphatic]; specific allergen IT with other allergens (e.g. house dust mite, cat dander). Non-standard therapy protocols or products, e.g. Rinkel method, peptide IT |
| Comparator | |
| Placebo [with or without conventional (rescue) medication], SCIT or SLIT | Any other route of administration (e.g. oral, nasal). Studies comparing different doses or schedules of IT that did not include a placebo arm |
| Outcomes | |
| At least one of the following: symptom severity, medication use, combined SMSs, frequency of exacerbations, QoL, AEs, the prevention of new asthma cases | RCTs not reporting any of the listed outcomes (e.g. laboratory parameters, such as IgE levels only). Studies evaluating clinical effectiveness without natural exposure (e.g. allergen chamber) |
The brief specified adults and children (examined separately) with severe hay fever, which does not respond to conventional treatment. We anticipated that not all trials would provide this information, or that they would use different classifications for ‘severe’ or ‘not responding to conventional treatment’. We therefore did not restrict inclusion by severity. We did restrict inclusion to treatment-naive patients; where this was not explicitly stated we noted this but included the study.
Titles and abstracts of retrieved studies underwent an initial screen by one reviewer, and studies that were clearly not relevant were excluded. The remaining studies were independently screened for inclusion by two reviewers. Where it was unclear whether or not studies met the inclusion criteria on the basis of title and abstract, full copies were obtained for assessment. Any discrepancy between reviewers was resolved through discussion or referral to a third reviewer. Reference Manager software, version 11 (Thompson ResearchSoft, San Francisco, CA, USA) was used to track and record study selection decisions and reasons for exclusion. Foreign-language papers were translated, where necessary, by the authors or colleagues.
Assessment of trial validity
Cochrane collaboration guidelines were followed for risk of bias assessment. 135 The following criteria were considered: adequate sequence generation, concealment of allocation, blinding of patients and personnel, completeness of outcome data, selective reporting of outcomes and IT treatment history. This last point is relevant as it is known that SIT can have long-lasting effects. Blinding of outcome assessors was not assessed, as outcomes were largely patient reported. Each item was classified as having a low risk of bias, high risk of bias or unclear risk of bias. Assessment of trial validity was at study level rather than outcome level.
Data extraction
Data extraction, including quality assessment, was conducted by one reviewer and checked by another using a standard, piloted, extraction form. There were no discrepancies that could not be resolved through discussion. Data were extracted on main study characteristics, main patient characteristics, study quality and all included outcomes. Data previously reported in the Cochrane reviews and included in this report were not checked.
Analysis
Where possible, we updated the meta-analyses, including subgroup analyses, from the existing Cochrane reviews. Meta-analysis assumes similarity between trials, and this was explored for both population and study characteristics in the newer studies identified. Studies included in meta-analyses in the Cochrane reviews were assumed to satisfy the similarity criteria.
Meta-analysis was limited to the following outcomes that were consistently reported across a high proportion of trials, and where data suitable for use in meta-analysis were provided or calculable: SSs, MSs, combined SMS and QoL scores. Where not reported, standard deviations (SDs) were calculated from other appropriate measures of variance (e.g. standard error, 95% CIs) according to Cochrane guidelines. 136 Results not suitable for meta-analyses were tabulated and described.
All meta-analyses were undertaken in RevMan software version 5.1 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) using a random-effects model. SMDs were presented, as there was little overlap between outcome measures across trials. So, for example, although AR symptoms were frequently measured, the number of symptoms measured was different across trials, as was the maximum score that could be achieved. As a rule of thumb, SMDs (effect sizes) can be described as small (< 0.4), moderate (0.4–0.7) or large (> 0.7). 137
In studies with more than one active intervention arm receiving different dosage IT, data for the group receiving the highest dose were used in meta-analysis, consistent with the Cochrane reviews. Where data were reported at different time points, data for the longest treatment duration were used. Outcome values were sometimes reported both for peak pollen season (PPS) and averaged over an EPS. Consistent with the Cochrane review, the mean values for the EPS were used when available.
Indirect comparison meta-analysis (ICMA) and indirect comparison meta-regression (ICMR) were used to compare the efficacy of SCIT and SLIT for each of the four outcomes (SSs, MSs, combined SMSs and QoL). Similarity of trial and population characteristics within (1) SCIT compared with placebo and (2) SLIT compared with placebo trials was assessed qualitatively and statistically (to test the assumption of homogeneity138); similarity between population and trial characteristics between SCIT compared with placebo and SLIT compared with placebo trials was also assessed (to test the assumption of similarity138). Possible sources of heterogeneity were explored and adjusted for using meta-regression, with a number of trial, population and reporting characteristics being used as covariates; specifically, these comprised participant age (adult/child), treatment duration, MAC of IT product and type of allergen (covariates as prespecified in subgroup analyses in the Cochrane review). We also conducted a post hoc exploration using year of publication and number of separate symptoms for which outcome data had been obtained as covariates. Improvement in model fit was expressed using the deviance information criterion (DIC), a compound measure of model fit and complexity, and extent of residual variation was monitored. As scores were measured on different scales, standardised score differences were calculated, except for RQLQ scores. Analyses were conducted in WinBUGS 1.4 (MRC Biostatistics Unit, Cambridge, UK). 139 Full methodological details for the indirect comparison can be found in Appendix 3.
Results
Quantity of evidence (subcutaneous immunotherapy versus placebo studies)
Searches identified 84 publications of DBPC RCTs of SCIT (see PRISMA flow diagram in Appendix 4). Of these, 48 were included in the relevant Cochrane review. 68 Of the remaining 36 publications, 10 pre-dated the Cochrane searches but were not included in that review, and a further four had been excluded by the Cochrane review, despite appearing to meet the inclusion criteria. Details of excluded studies are presented in Appendix 5. The remaining 22 publications,30,140–160 reporting on 18 distinct RCTs, post-dated the final search date listed in the Cochrane review (February 2006). Two151,156 of these publications provided additional data relating to the trial reported in Frew et al. in 2006,161 which was included in the Cochrane review. Thus, 20 publications30,140–150,152–155,157–160 relating to 17 new RCTs were identified. As the purpose of this report was to update, rather than repeat, the Cochrane review, results are presented only for those 17 trials initially published from 2006 onwards. However, all relevant studies have been included in the meta-analyses.
Main study characteristics and risk of bias (subcutaneous immunotherapy versus placebo studies)
The main study and population characteristics, and assessment of risk of bias are detailed for each of the 17 newly identified RCTs30,142–146,148–150,152–155,157–160 in Appendix 5. All studies were DBPC RCTs. Approximately half of the studies (nine trials30,142–144,146,148,152,158,160) involved fewer than 65 participants, but the remaining trials included at least 100 patients, and the largest145 represented over 1000 participants. Skin prick tests were performed in all patients to demonstrate specific sensitivity. Allergy symptoms were described as moderate to severe in 2 out of 17 trials,145,146 whereas level of severity was not stated in 15 out of 17. 30,142–144,148–150,152–155,157–160 Ten studies30,142,143,148–150,152,154,155,160 stated that some patients also had asthma symptoms. Patients with previous IT were excluded in five studies,30,142,152,154,160 four trials143,145,157,159 allowed patients who had not received SIT in the last 3–5 years or ‘recently’, and previous treatment status was unclear in eight trials. 144,146,148–150,153,155,157 Outcomes included SSs, MSs, combined symptom and medication scores, QoL and AEs (note: only outcomes consistent with the inclusion criteria for this report have been listed). Approximately half of the studies (930,145,148,149,152,153,155,157,160 out of 1713,30,142–146,148–150,152–155,157,159,160) reported a combined score, either alone or in addition to individual SSs and MSs. Although SSs were consistently reported across trials, the number and types of symptom assessed varied widely (see Appendix 7 – SSs across studies). Similarly, there were differences in how SMSs were calculated.
Tree and mixed-grass allergens were the most commonly investigated (eight30,142,148,149,154,155,158,160 and three trials,145,150,157 respectively), followed by two trials each of timothy grass (P. pratense)146,153 and ragweed,144,159 one trial in Alternaria142 and one in Russian thistle143 (Salsola kali L. ). The main study characteristics are summarised in Table 5.
| Study ID | Size | Previous SIT | Stated that symptoms moderate to severe | Patients with asthma allowed/included | Type of allergen | Administration schedule | Outcomes |
|---|---|---|---|---|---|---|---|
| Casale 2006159 | n = 159 | Not ‘recently’ but 19.5% had previous SIT | No details on severity | No (patients with asthma excluded) | Ragweed | Pretreatment with active/placebo omalizumab, rush IT (six injections over 3–5 hours), 4 weeks updosing, 8 weeks maintenance; coseasonal | AEs |
| Ceuppens 2009160 | n = 62 | No | No details on severity | Yes (mild only) | Birch | Weekly, then fortnightly induction doses, followed by monthly maintenance dose, total of 18–22 months | SMSs, SSs, MSs, AEs |
| Chakraborty 200630 | n = 35 | No | No details on severity | Yes | Sugar date palm | Weekly induction phase for 24 weeks, maintenance phase for 18 months at 2-weekly intervals. Dose reduced 20–40% in symptomatic patients during pollen season | SMSs, global measure of severity, spirometry, AEs |
| Charpin 2007142 | n = 40 | No | No details on severity | Yes | Cypress | Induction phase with fortnightly injections followed by maintenance phase at maximum tolerated dose for 15 months (frequency not reported), covering two pollen seasons | SSs, MSs, days with asthma, AEs, QoL |
| Colas 2006143 | n = 63 | Not in last 4 years | No details on severity | Yes | Russian thistle | Cluster schedule: first day: 0.1, 0.25 and 0.5 ml × 450 g extract/ml; 1 week later, 0.1, 0.25 and 0.5 ml × 450 g/ml; then starting 1 month later one injection per month totalling 12 maintenance doses 0.5 ml × 450 g/ml | SSs, MSs, QoL, global assessment of health, AEs |
| Creticos 2006144 | n = 25 | Unclear | No details on severity | No details | Ragweed | Preseasonal, six weekly injections | SS, QoL, AE |
| DuBuske 2011 145 | n = 1028 | Not in last 3 years | Yes | No details | Thirteen grass mix (Grass MATA MPL Pollinex Quattro) | Ultrashort course SCIT: four increasing dose injections given at approximately weekly intervals preseason | SMSs, number of well-days, number of bad-days, number of well patients, AEs |
| Francis 2008146 | n = 18 | Unclear | Yes, with poor symptom control | No details | Timothy grass (Alutard SQ) | Modified cluster regimen: weekly visits for 2 months, with two injections per visit in increasing dosage. Maintenance dose monthly up to 1 year | Overall clinical assessment, AEs |
| Hoiby 2010148 | n = 61 | Unclear | No details on severity | Yes | Birch (Depigoid®, Laboratorios LETI SL, Barcelona, Spain) | Updosing at 7-day intervals, maintenance dose every 6 weeks for 18 months | SMSs, SSs, MSs, AEs, QoL |
| Kettner 2007149 (A) | n = 211 | Unclear | No details on severity | Yes | Birch | Updosing then maintenance for 1.5 years (frequency of injections not stated) | SMSs, AEs |
| Klimek 2010150 (A) | n = 148 | Unclear | No details on severity | Yes | Grasses and rye (Alutard SQ) | Coseasonal. Updosing with six injections with one to three injection intervals then two injections after 14 and 28 days | AEs |
| Kuna 2011152 | n = 50 | No | No details on severity | Yes | Alternaria | Updosing: 14 injections weekly or fortnightly. Maintenance dose every 4–6 weeks for up to 3 years | SMSs, SSs, MSs, AEs, QoL |
| Ljorring 2009153 (A) | n = 162 | Unclear | No details on severity | No details | Grass (Alutard SQ) | One year. No further details on treatment schedule. | SMSs |
| Pauli 2008154 | n = 147 | No | No details on severity | Yes | Birch | Build up starting 6 months before pollen season by weekly injections. Maintenance dose reached at least 7 weeks before pollen season then given monthly for 2 years | SSs, MSs, AEs |
| Pfaar 2010155 | n = 184 | Unclear | No details on severity | Yes | Birch, hazel, alder (Depigoid) | Updosing at 7-day intervals. Maintenance dose every 6 weeks for 18 months | SSs, MSs, SMSs, responder analysis, AEs |
| Sahin 2011157 (A) | n = 121 | Not in last 5 years | No details on severity | No details | Grass and rye | Two injections/day for initiation phase (cluster schedule) then once/month for maintenance (length of treatment not clear) | SMSs, symptom-free day, global evaluation by patients, AEs |
| Ventura 2009158 | n = 20 | Unclear | No details on severity | No details | Cypress | Twelve-week induction phase with weekly injections and maintenance phase of 9 months with monthly injections | SSs |
Similarity between trials was explored for a range of study and population characteristics. Trial duration and type and amount of allergen used varied between trials; however, this was explored as a source of heterogeneity in subgroup analyses. Where reported, inclusion criteria were very similar across trials, with the majority stipulating a minimum of 2 years' clinical history of moderate to severe SAR, incompletely controlled by standard medication, and no prior experience of SIT. Rates of asthma across trials were largely consistent, comprising between one-quarter and one-third of participants. Prevalence of asthma was higher in one paediatric trial122 (38 out of 50 patients). All studies excluded patients with severe or perennial asthma.
Results of the risk of bias assessment are summarised in Table 6. Full details are given in Appendix 5.
| Study | Sequence generation | Allocation concealment | Blinding | Data completeness | Selective reporting | Patients treatment naive |
|---|---|---|---|---|---|---|
| Casale 2006159 | ? | ? | + | + | + | ? |
| Ceuppens 2009160 | ? | ? | + | + | + | + |
| Chakraborty 200630 | ? | ? | + | + | + | + |
| Charpin 2007142 | + | ? | + | ? | + | + |
| Colas 2006143 | ? | ? | + | + | + | ? |
| Creticos 2006144 | + | + | + | – | ? | ? |
| DuBuske 2011145 | + | + | + | + | + | + |
| Francis 2008146 | ? | ? | + | + | + | ? |
| Hoiby 2010148 | + | ? | + | ? | + | ? |
| Kettner 2007149 (A) | ? | ? | ? | ? | ? | ? |
| Klimek 2010150 (A) | ? | ? | ? | ? | + | ? |
| Kuna 2011152 | + | + | + | ? | + | + |
| Ljorring 2009153 (A) | ? | ? | ? | ? | + | ? |
| Pauli 2008154 | ? | ? | + | + | + | + |
| Pfaar 2010155 | ? | ? | + | – | + | ? |
| Sahin 2011157 (A) | ? | ? | ? | ? | + | ? |
| Ventura 2009158 | + | ? | + | + | + | ? |
The risk of bias was low in three or more areas of potential bias for 1230,142–146,148,152,154,158,160 out of 17 studies. A lack of detail in the published reports, most notably for those reported in abstract form only, meant that the risk of bias was unclear in many cases. Lack of detail related most often to allocation concealment, sequence generation and whether or not patients were treatment naive. In all studies, apart from those reported as abstracts, there were details on blinding. Study authors were not contacted and an ‘unclear’ rating may be due to a lack of reporting rather than a reflection of poor trial quality. There were only two instances for which a high risk of bias was identified, in the area of data completeness. Only one114 of these studies contributed to any meta-analyses (QoL data); owing to its small sample size (n = 25) it is unlikely to have a large influence on overall results. It should be noted that a certain degree of subjectivity remains in assigning a rating of low/high/unclear risk of bias.
Effectiveness of subcutaneous immunotherapy compared with placebo
Of the 17 newly identified RCTs, only five142–144,152,154 reported data in a form suitable for meta-analyses. Of these, three studies provided new SS and MS data,142,152,154 and two provided QoL data143,144 (Table 7).
| Study ID | Not in meta-analyses | In meta-analyses |
|---|---|---|
| Casale 2006159 | AEs | None |
| Ceuppens 2009160 | SSs, MSs, SMSs, AEs | None |
| Chakraborty 200630 | SMSs, global measure, spirometry, AEs | None |
| Charpin 2007142 | Days with asthma, AEs, QoL | SSs, MSs |
| Colas 2006143 | SSs, MSs, global assessment, AEs | QoL |
| Creticos 2006144 | SSs, AEs | QoL |
| DuBuske 2011145 | SMSs, well-days, bad-days, well patients, AEs | None |
| Francis 2008146 | Overall clinical assessment, AEs | None |
| Hoiby 2010148 | SMSs, SSs, MSs, AEs, QoL | None |
| Kettner 2007149 (A) | SMSs, AEs | None |
| Klimek 2010150 (A) | AEs | None |
| Kuna 2011152 | SMSs, AEs, QoL | SSs, MSs |
| Ljorring 2009153 (A) | SMSs | None |
| Pauli 2008154 | AEs | SSs, MSs |
| Pfaar 2010155 | SSs, MSs, SMSs, responder analysis, AEs | None |
| Sahin 2011157 (A) | SMSs, symptom-free days, global evaluation, AEs | None |
| Ventura 2009158 | SSs | None |
Symptom scores
Of the 15 studies91,162–174 included in the Cochrane review meta-analysis, one162 was excluded from this study as patients were not treatment naive. Searches for this report identified 10 new studies142–144,148,152,154,155,157,158,160 reporting this outcome, of which only three142,152,154 provided data suitable for inclusion in meta-analysis. Thus, a total of 17 studies, comprising 659 active and 525 placebo patients, were included (Figure 1).
Figure 1.
Subcutaneous immunotherapy vs placebo: SSs.
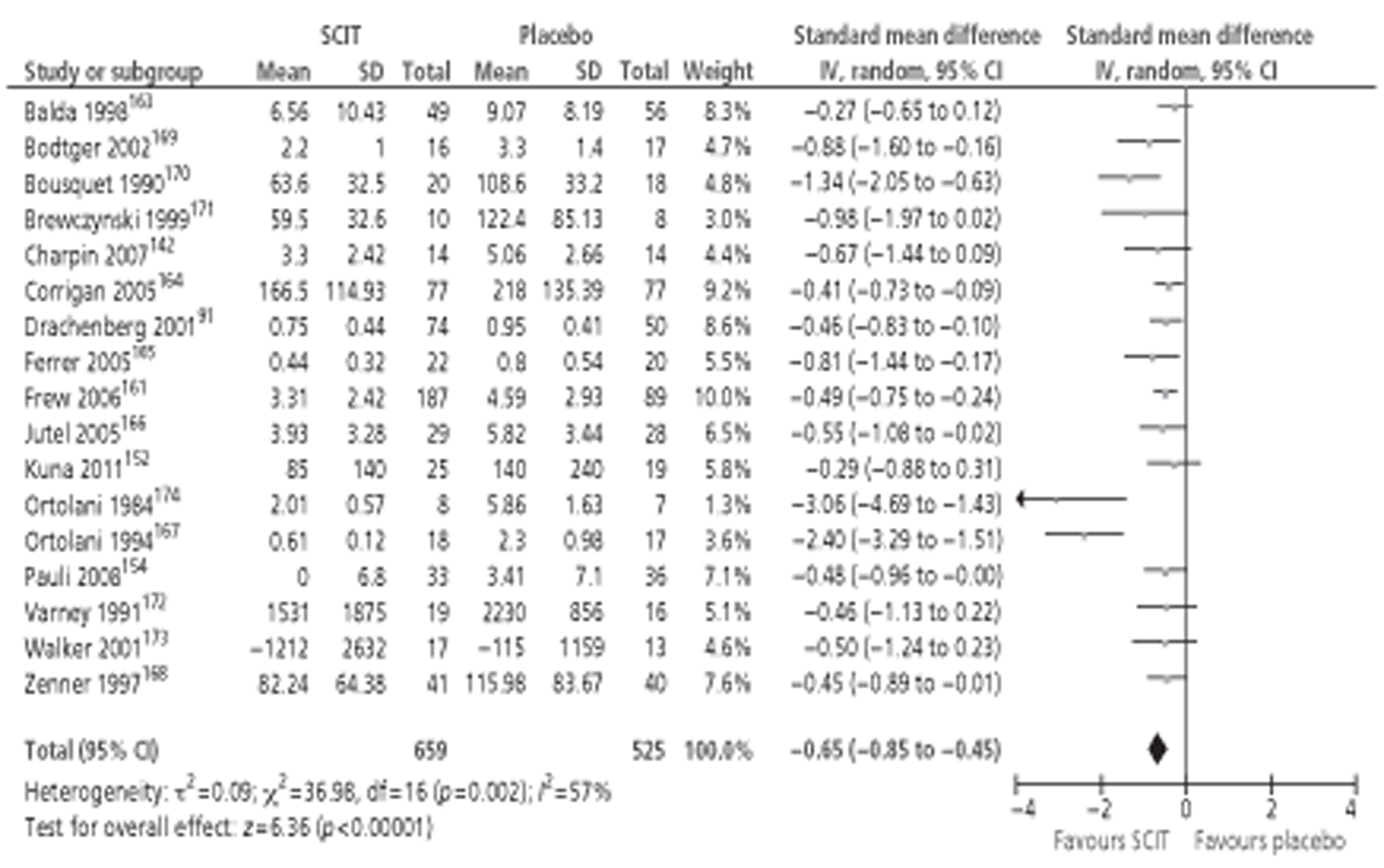
The combined SMD was −0.65 (95% CI −0.85 to −0.45; p < 0.00001) favouring SCIT, with evidence of moderate heterogeneity, similar to the findings of the Cochrane review68 (SMD −0.73, 95% CI −0.97 to −0.50; p < 0.00001, based on 15 trials).
Subgroup analyses were conducted by age, study duration, MAC and type of allergen. All favoured the active treatment and were statistically significant. Results of subgroup analyses are shown in Table 8. Forest plots of all subgroup analyses are shown in Appendix 7. Note that the Cochrane review of SCIT did not include subgroup analyses, but that studies identified in that review are included in subgroup analyses here.
| Subgroup | No. of studies | Total n | SMD (IV, random 95% CI) |
|---|---|---|---|
| Age | |||
| Adults | 16 | 1140 | −0.68 (−0.89 to −0.47) |
| Duration (months) | |||
| < 6 | 5 | 274 | −1.29 (−2.10 to −0.49) |
| 6–12 | 2 | 309 | −0.54 (−0.78 to −0.29) |
| > 12 | 8 | 442 | −0.51 (−0.70 to −0.32) |
| MAC | |||
| < 5 μg | 3 | 228 | −0.43 (−0.69 to −0.16) |
| 5–20 μg | 5 | 231 | −0.54 (−0.80 to −0.27) |
| > 20 μg | 3 | 341 | −1.06 (−2.08 to −0.05) |
| Allergen | |||
| Grass | 9 | 552 | −0.64 (−0.91 to −0.37) |
| Parietaria | 3 | 353 | −1.15 (−2.09 to −0.21) |
| Tree | 4 | 235 | −0.46 (−0.72 to −0.20) |
Sixteen142,154,161,163–174 of the 17 studies were conducted in an adult population and the results did not differ from those of the entire sample. Only one study152 involved a paediatric population. In this study,152 rhinitis, conjunctivitis and asthma SSs were all significantly lower following 3 years of active treatment than with placebo.
Analysis by treatment duration found that studies of ≥ 6 months in duration resulted in similar effect sizes to the sample as a whole. All three142,152,154 of the more recent trials lasted for > 12 months. Shorter studies gave a larger effect size; this was associated with a high degree of between-study heterogeneity.
In line with current guidelines, all three of the newer studies142,152,154 used vaccines with between 5 and 20 μg MAC. Subgroup analyses (see Appendix 7, Figures 31–95) suggest that effectiveness increases with increasing MAC; this finding should be interpreted cautiously, as it is not based on a randomised comparison.
Immunotherapy with grass allergens made up the largest subgroup (47% total sample). Combined effect size was similar to that of the entire sample, with a moderate degree of heterogeneity. Similar effect sizes were found for tree pollen allergy. Only three studies involved Parietaria pollen. 161,165,167 Combined effect size was quite large, but was associated with wide CIs and a high degree of heterogeneity. None of the studies conducted in ragweed that were identified in the Cochrane review were suitable for meta-analysis, and no new studies were found. Only one study152 was conducted in Alternaria.
Details of SS data from the seven studies not included in the meta-analysis143,144,148,155,157,158,160 are presented in Table 9. All favoured the active treatment over placebo.
| Study ID | Results |
|---|---|
| Ceuppens 2009160 | Median nasal SSs (IQR) 0.3 (0.1–0.6) vs 0.7 (0.5–1.1) in the active and placebo groups, respectively (p = 0.041) |
| Colas 2006143 | Over the EPS, median (IQR) TSSs were 4.3 (3.4–4.6) and 6.4 (4.0–8.4) in the IT and placebo groups, respectively (p < 0.001) |
| Creticos 2006144 | During the first ragweed season post treatment, mean rhinitis visual analogue score was 13.8 in the AIC-treated group, vs 35.1 in the placebo group (p = 0.01). Daily nasal SSs were also lower in the active group (p = 0.03) |
| Hoiby 2010148 | Median SS was not significantly different between treatment groups (p = 0.375), despite a reduction of 25% in the SCIT group compared with placebo |
| Pfaar 2010155 | Median SSs were 0.54 (IQR 0.27–0.77) for Depigoid-treated patients completing the study and 0.61 (IQR 0.48–0.75) for placebo [median difference −0.1 (95% CI −0.20 to −0.02); p < 0.01] |
| Sahin 2011157 (A) | Compared with placebo, the overall SS in the active group was significantly reduced by 36% (p = 0.006) |
| Ventura 2009158 | Not possible to extract data from graphs. Clinical improvements were noted with active treatment compared with placebo |
Medication scores
Medication scores suitable for meta-analysis were available in 16 studies (13 from the Cochrane review,91,161,163–166,169–173,17,176 three142,152,154 more recent), which together included 621 active and 483 placebo patients. The combined SMD was −0.55 (95% CI −0.75 to −0.34; p < 0.00001); this was very similar to that reported in the Cochrane review (SMD −0.57, 95% CI −0.82 to −0.33; p < 0.00001). Heterogeneity was reduced slightly, but remained statistically significant (Figure 2).
FIGURE 2.
Subcutaneous immunotherapy vs placebo: MSs.
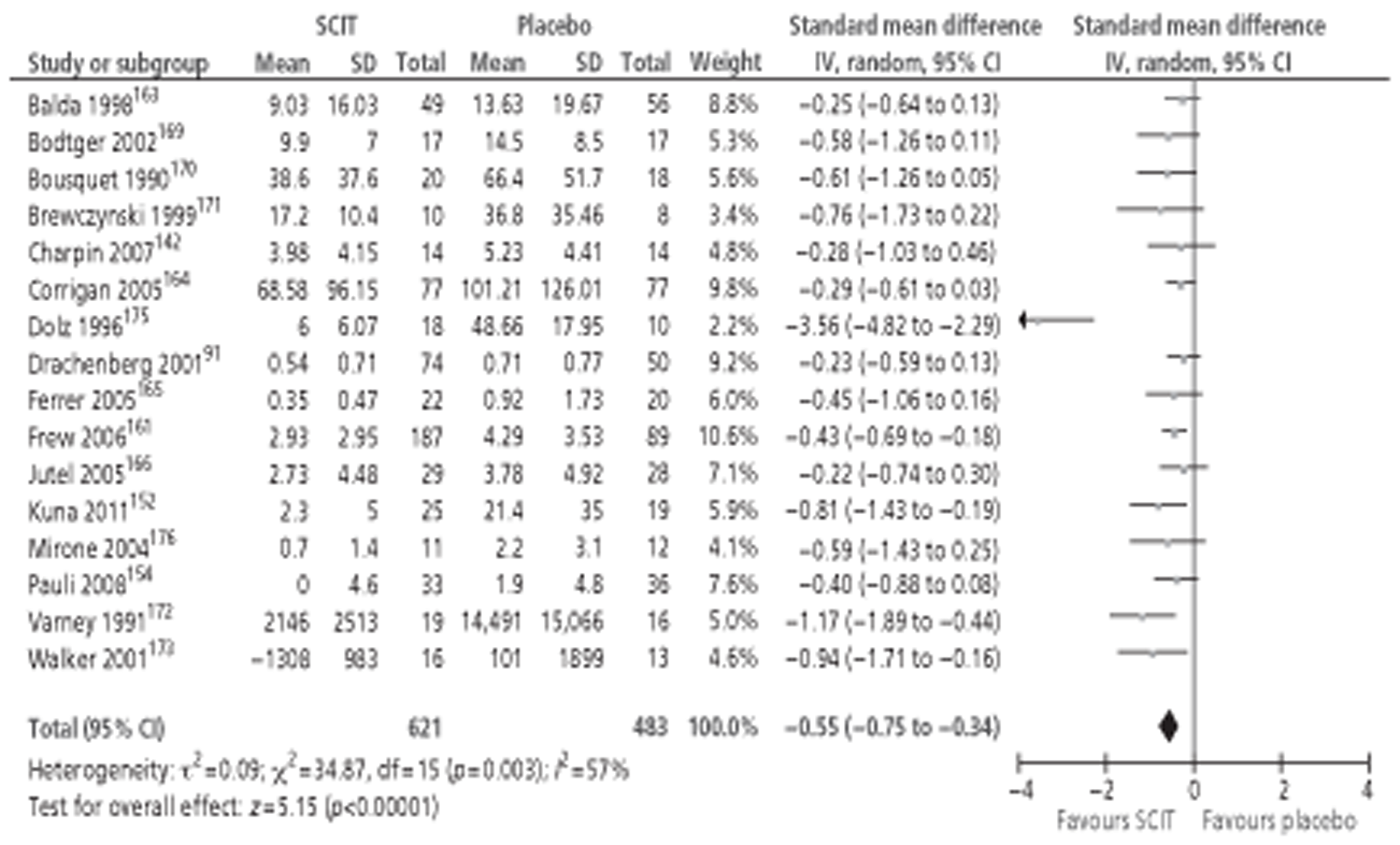
Subgroup analyses were conducted by age, study duration, MAC and type of allergen. All favoured the active treatment and, with the exception of studies using a < 5 μg MAC, were statistically significant. However, this non-significant result was based on only two trials163,165 totalling 147 patients. Results of the subgroup analyses are shown in Table 10.
| Subgroup | No. of studies | Total n | SMD (IV, random 95% CI) |
|---|---|---|---|
| Age | |||
| Adults | 15 | 1059 | −0.53 (−0.75 to −0.32) |
| Duration (months) | |||
| < 6 | 2 | 143 | −0.34 (−0.68 to −0.01) |
| 6–12 | 3 | 332 | −0.46 (−0.69 to −0.23) |
| > 12 | 9 | 469 | −0.67 (−1.06 to −0.29) |
| MAC | |||
| < 5 μg | 2 | 147 | −0.31 (−0.63 to 0.02) |
| 5–20 μg | 6 | 254 | −0.45 (−0.70 to −0.20) |
| > 20 μg | 2 | 305 | −0.55 (−0.96 to −0.13) |
| Allergen | |||
| Grass | 8 | 483 | −0.77 (−1.22 to −0.33) |
| Parietaria | 2 | 318 | −0.43 (−0.67 to −0.20) |
| Tree | 4 | 235 | −0.34 (−0.60 to −0.09) |
Fifteen91,126,142,154,161,163–166,169–173,176 of the 16 trials91,126,142,152,154,161,163–166,169–173,176 reporting MSs were conducted in adults, and the results did not differ greatly from the sample as a whole. Only one study152 was conducted in children, and also reported that active treatment resulted in statistically improved MSs compared with placebo.
Nine126,142,152,154,164–16,171,173 of the 14 studies126,142,152,154,161,163–166,169–171,173,176 in the meta-analysis for which treatment duration could be determined lasted for > 12 months. All subgroups favoured active treatment and were statistically significant, although effect size appeared to increase with treatment duration.
Six142,152,154,166,169,176 of the 10 included studies,142,152,154,161,163,165,166,169,173,176 including all three of the recent trials, utilised vaccines with between 5 and 20 μg MAC. Again, combined SMD increased with increasing dosage. Improvements in MS in the lowest dosage group were not significantly better than placebo, but this finding was based on only two studies163,165 (total n = 147).
The most commonly investigated allergen was grass pollen, representing 483 subjects across eight trials,91,126,164,166,170–173 and this was associated with the largest effect size, but also with a high degree of between-study heterogeneity. Tree pollen allergy was studied in four trials,142,154,163,169 and Parietaria (a plant of the nettle family) in two trials. 161,165 Combined SMD favoured active treatment and were statistically significant in all allergen subgroups. Only one study152 was performed in Alternaria (a fungus) and thus meta-analysis was not possible. No studies in ragweed reported MSs suitable for meta-analysis.
Medication score results from five recent studies143,148,155,157,160 were not suitable for inclusion in meta-analysis, and details are shown in Table 11. Two studies reported quite large reductions in MSs in actively treated patients (43%157 and 52%148); however, three studies143,155,160 reported no significant difference between the groups. These contrasting results cannot be explained by differences in sample size, treatment duration, MAC or type of allergen.
| Study ID | Results |
|---|---|
| Ceuppens 2009160 | No significant differences between groups (p = 0.155) |
| Colas 2006143 | Over the EPS, median (IQR) MSs were 0.8 (0.7–0.8) and 0.9 (0.5–1.1) in the IT and placebo groups, respectively (p = 0.115) |
| Hoiby 2010148 | Median MS were significantly different between treatment groups after 18 months: median (IQR) 2.1 (0.6–3.7) in the SCIT group and 4.4 (1.9–14.0) in the placebo group (p = 0.016), a reduction of 51.9% in the SCIT group compared with placebo |
| Pfaar 2010155 | Median MSs were 1.36 (IQR 0.4–2.9) for Depigoid-treated patients completing the study and 2.95 (IQR 1.7–3.9) for placebo [median difference −1.3 (95% CI −1.87 to −0.34); p = 0.09] |
| Sahin 2011157 (A) | Compared with placebo, the overall MS in the verum group was significantly reduced by 43% (p = 0.002) |
Symptom and medication scores
Only the eight studies91,163–168 (total n = 617) previously reported in the Cochrane review reported this outcome in a manner suitable for meta-analysis. Thus, the combined effect size calculated in that review remains valid (SMD −0.48; 95% CI −0.67 to −0.29; p < 0.00001) (Figure 3). 68 However, it was possible to conduct a number of subgroup analyses on this sample.
FIGURE 3.
Subcutaneous immunotherapy vs placebo: SMSs.
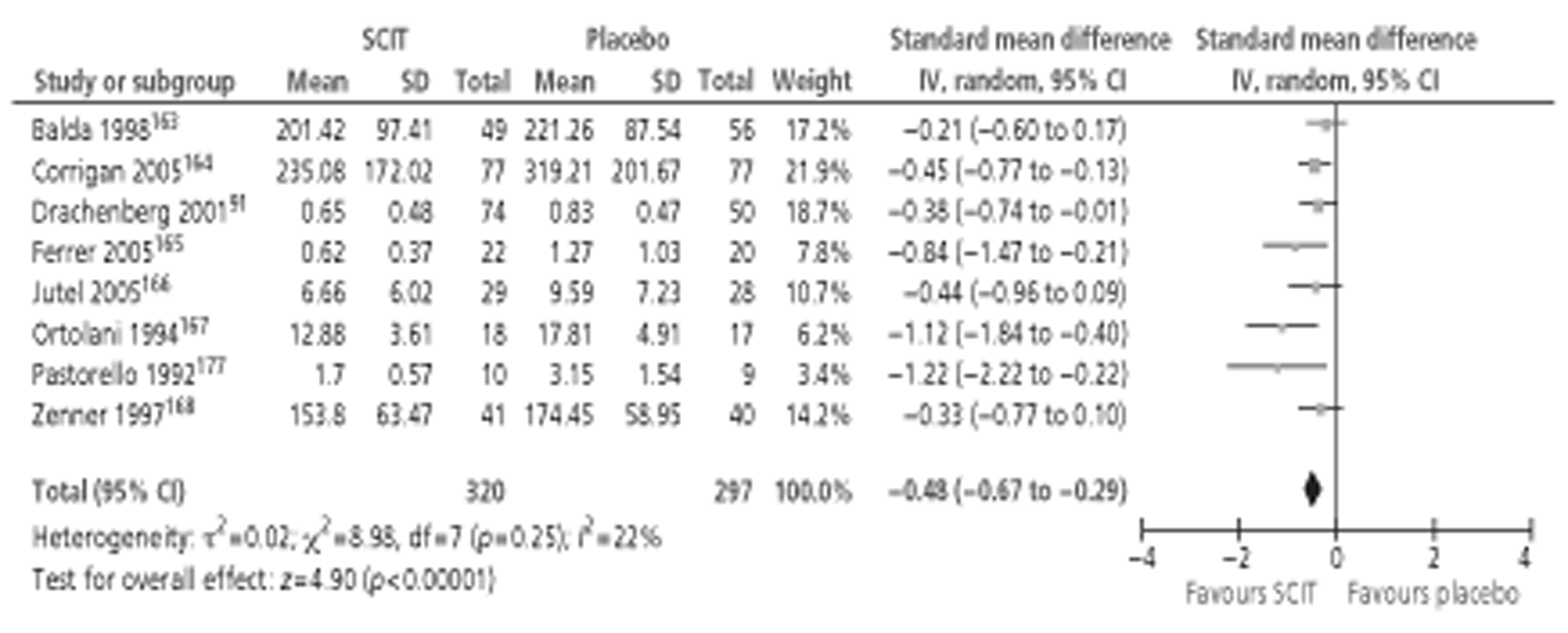
All of the eight included studies91,163–169 were conducted in adults. Thus, subgroup analyses were conducted for study duration, MAC and type of allergen. All favoured the active treatment and were statistically significant. Results of subgroup analyses are shown in Table 12.
| Subgroup | No. of studies | Total n | SMD (IV, random, 95% CI) |
|---|---|---|---|
| Duration (months) | |||
| < 6 | 3 | 221 | −0.47 (−0.91 to −0.02) |
| > 12 | 3 | 253 | −0.51 (−0.76 to −0.25) |
| MAC | |||
| < 5 μg | 3 | 228 | −0.39 (−0.70 to −0.07) |
| Allergen | |||
| Grass | 5 | 435 | −0.43 (−0.62 to −0.24) |
| Parietaria | 2 | 77 | −0.96 (−1.44 to −0.49) |
Treatment duration could not be determined for two studies. 91,177 Three of the included studies164–166 were of < 6 months' duration. Combined effect size was similar to that in the overall sample, although significantly more heterogeneity was indicated in this subgroup (I2 = 59% vs 22%). A further three studies163,167,168 lasted over 12 months, and effect sizes were again similar. However, these studies163,167,168 were more homogeneous (I2 = 0%). None of the studies reporting this outcome lasted between 6 and 12 months in length.
Major allergen content could not be determined for three studies. 91,164,177 Three studies163,165,168 utilised a dose of < 5 μg major allergen. Effect size was a little smaller than for the sample as a whole but remained significant. Only one study each used a vaccine with 5–20 μg166 and > 20 μg167 major allergen, and thus meta-analysis was not possible in these subgroups.
Again, the largest subgroup of studies was for grass pollen: five studies,91,164,166,168,177 (total n = 435) resulted in a moderate effect size in favour of active treatment. Two studies165,167 were conducted with Parietaria allergen, with the combined effect size strongly favouring the active treatment. Only one study164 was conducted with tree allergen and meta-analysis was therefore not possible.
None of the nine newer studies30,145,148,149,152,153,155,157,160 reporting combined SMSs was suitable for meta-analysis. Results from all nine studies30,145,148,149,152,153,155,157,160 favoured active SCIT; details of the data from these studies are shown in Table 13. Studies that involved >1 year of treatment reported that the effect size increased with each year of active treatment.
| Study ID | Results |
|---|---|
| Chakraborty 200630 | The SIT group had a 33.5% (p < 0.01) and 57% (p < 0.001) decrease in the SMS during the first and second treatment seasons of 2000 and 2001 when compared with the baseline peak month. There were no significant changes in the control group |
| Ceuppens 2009160 | Clinical index score (a combined score of symptoms and medication use) was reduced in the verum group by 24% compared with placebo [median (IQR) 0.5721 (0.2759–1.2830) vs 1.0322 (0.5882–1.6080), respectively; p = 0.055] |
| DuBuske 2011145 | Median (SD) combined SMS during the four peak weeks of the grass pollen season was 6.00 (5.57) in the Grass MATA MPL-treated subjects and 7.06 (5.57) in the placebo-treated subjects. In the ITT analysis (n = 1028), the least squares mean combined SMS was reduced by 13.4% with Grass MATA MPL compared with placebo (p = 0.0038). Similar differences were seen during the EPS |
| Hoiby 2010148 | After 18 months, the median (IQR) combined SMS of the SCIT and placebo groups were 8.0 (5.8–10.3) and 12.6 (8.6–16.2), respectively (p = 0.004) – a 36.5% reduction in the SCIT group compared with the placebo |
| Kettner 2007149 (A) | After 1.5 years of therapy, a highly significant and clinically relevant reduction in the median AUC of the SMS from 389.6 to 207.8 in the active group compared with the placebo group (from 382.5 to 306.5; p = 0.0137) was observed in the full analysis set |
| Kuna 2011152 | Reductions in combined SMS after therapy, compared with placebo, were 10.8%, 38.7%, and 63.5% after the first, second, and third years of SIT, respectively (p = 0.73, 0.102, < 0.001 and < 0.001 for baseline, 1, 2 and 3 years of SIT, respectively, active therapy vs placebo, one-way ANOVA test) |
| Ljorring 2009153 (A) | The estimated treatment effect on combined SMS over peak season was −4.45 (95% CI −6.84 to −2.06) in favour of active treatment. (Abstract; patient numbers in each group not reported) |
| Pfaar 2010155 | At 18 months, the median AUC for the combined SMS was 2.3 (IQR 0.9−3.2) for the actively treated patients and 2.6 (IQR 2.2–4.4) for placebo-treated patients [median difference −0.4 (95% CI −1.22 to −0.03); p < 0.04] for the ITT population |
| Sahin 2011157 (A) | There was a significant reduction in total combined scores in the active group compared with the placebo group (p = 0.005) |
Quality of life
Quality-of-life data suitable for meta-analysis were available for eight RCTs (five from the Cochrane review,161,164–166,173 three more recent;143,144,146 Figure 4). The addition of the three newer studies resulted in a nearly 70% increase in sample size for this outcome (total n = 955). Nevertheless, the results (SMD −0.53, 95% CI −0.66 to −0.39; p <0.00001) were almost identical to those reported in the Cochrane review (SMD −0.52, 95% CI −0.69 to −0.34; p <0.00001). No heterogeneity between studies was found.
FIGURE 4.
Subcutaneous immunotherapy vs placebo: QoL.
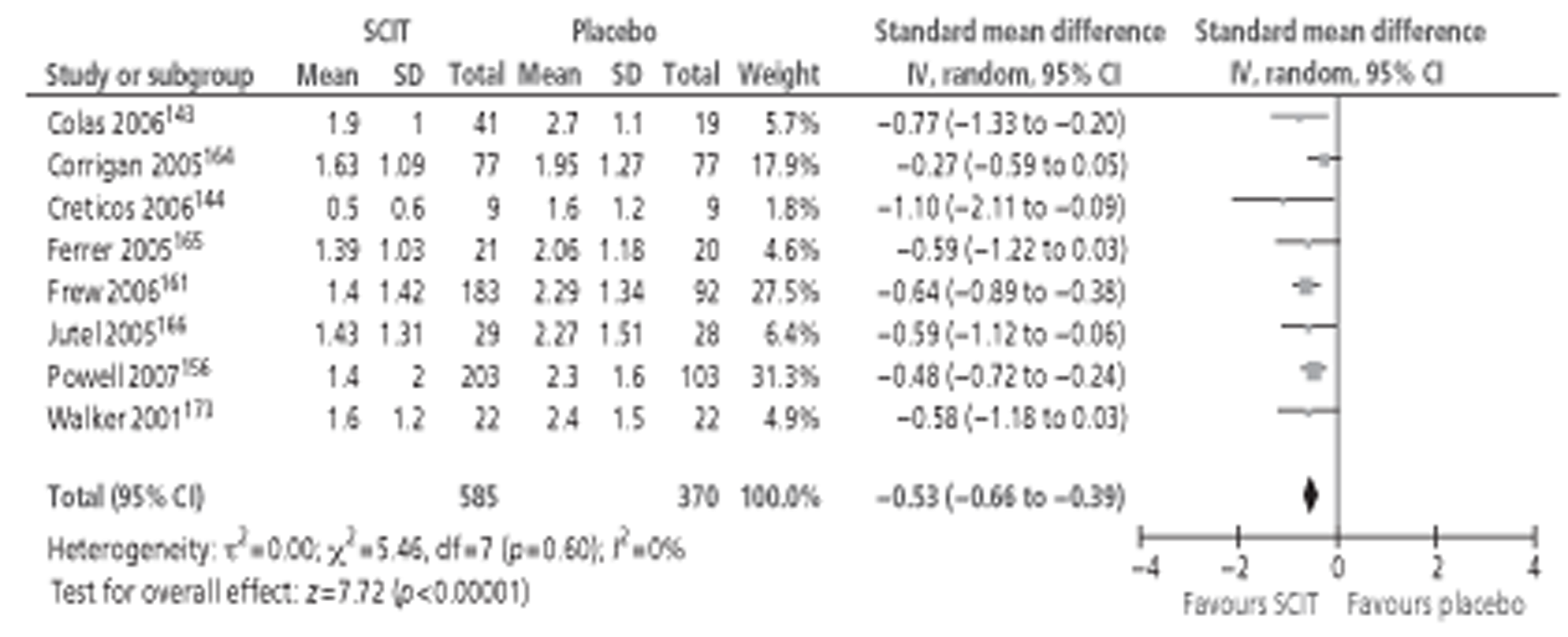
Where subgroup analyses were possible, treatment duration, MAC and type of allergen did not appear to affect the outcome (Table 14). All of the studies included in the meta-analysis were conducted in adults and thus analysis by participant age was not possible. However, based on results from one paediatric study,152 SCIT appears to be effective for the improvement of QoL in children and adolescents when used long term.
| Subgroup | No. of studies | Total n | SMD (IV, random, 95% CI) |
|---|---|---|---|
| Duration (months) | |||
| > 12 | 6 | 662 | −0.47 (−0.63 to −0.31) |
| MAC (μg) | |||
| 5–20 | 3 | 381 | −0.52 (−0.74 to −0.31) |
| > 20 | 3 | 379 | −0.65 (−0.87 to −0.43) |
| Allergen | |||
| Grass | 4 | 561 | −0.44 (−0.61 to −0.26) |
| Parietaria | 2 | 316 | −0.63 (−0.87 to −0.39) |
As all eight of the included studies143,144,156,161,164–166,173 assessed QoL using the Juniper RQLQ, an additional meta-analysis was conducted to calculate weighted mean difference (MD) (Figure 5). Active treatment had a significant positive effect on QoL, equivalent to a 0.74 unit reduction in RQLQ score compared with placebo.
FIGURE 5.
Subcutaneous immunotherapy vs placebo: QoL (RQLQ scores).
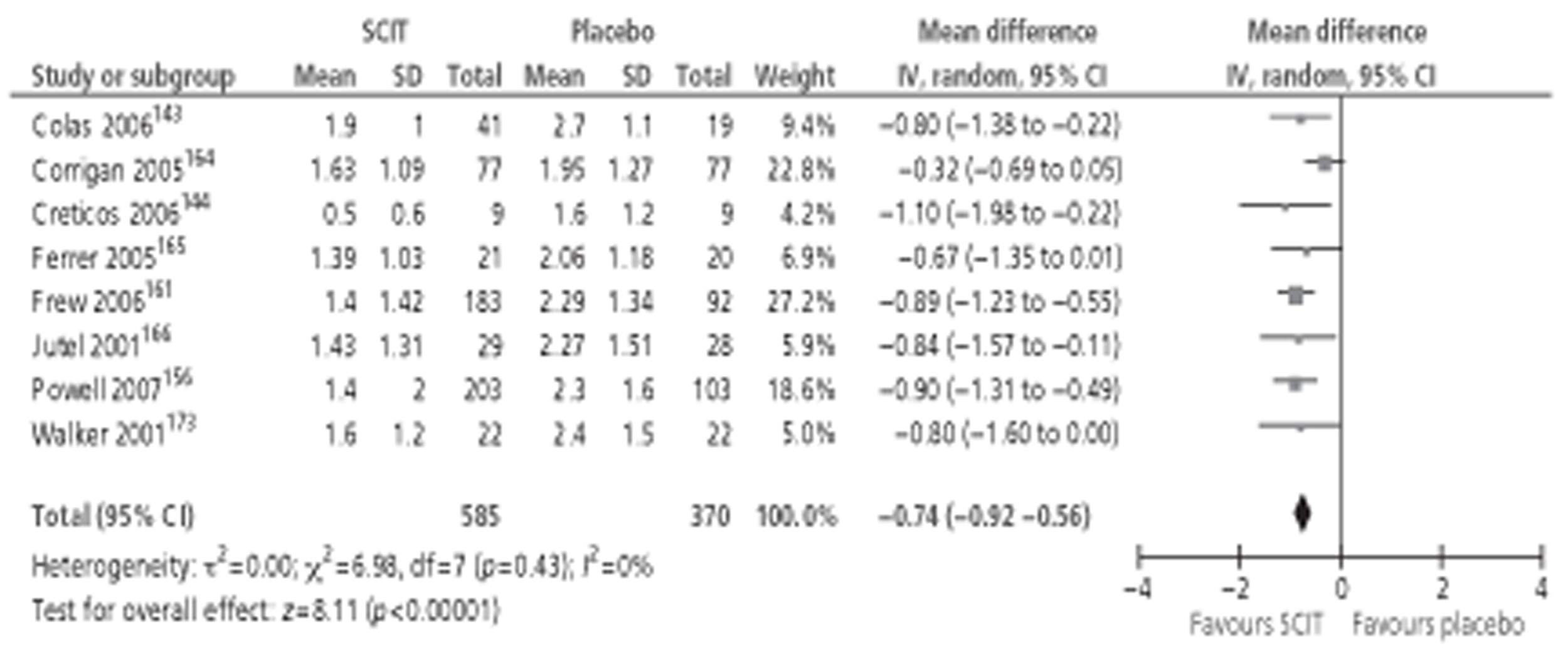
Three new studies142,148,152 reported QoL data in a manner not suitable for inclusion in meta-analysis. Details of data from these studies142,148,152 are shown in Table 15. The smallest142 of the three studies (total n = 28) found no difference in overall QoL between treatment groups using SF–36. The other two studies148,152 used disease-specific instruments (age-appropriate versions of the RQLQ) and both reported clinically significant improvements in QoL in active- but not placebo-treated subjects. However, in the 3-year paediatric study,152 improvements became statistically significant only in later years of treatment.
| Study ID | Results |
|---|---|
| Charpin 2007142 | Assessment measure was SF–36. There was no significant difference between the two treatment groups except for variations in social function after the first season |
| Hoiby 2010148 | At baseline, there was a statistically significant difference between the two treatment groups in the mean RQLQ (SCIT: 2.58; placebo: 2.7; p < 0.0001). During the study, RQLQ improved in both groups. The change in mean RQLQ between baseline and assessment during pollen season 2005 after 6 months of treatment was significantly different between the two groups (SCIT −3.8; placebo −0.1; p < 0.0001) |
| Kuna 2011152 | Active treatment was associated with an improvement in QoL for children (up to 12 years of age) with rhinoconjunctivitis. The mean baseline RQLQ score was 1.7 and decreased significantly in consecutive years of therapy to 1.4, 1.0, and 0.7 (p = 0.009, 0.008 and 0.003 after the first, second and third years of SIT, respectively). In the comparable group of children receiving placebo, the baseline QoL score was 2.0 and increased in consecutive years of therapy to 2.3, 2.3 and 2.7 (p = 0.08, 0.09 and 0.019, respectively). The group of adolescents who received active treatment also showed significant improvements in QoL: a baseline QoL score of 2.7 decreased significantly in consecutive years of therapy to 2.0, 1.4 and 0.9 (p = 0.0018, 0.0006 and 0.0006, respectively). The comparable placebo group showed no change during the entire study, with a baseline mean QoL score of 2.0, and scores of 1.9, 1.9 and 2.2 after the first, second, and third years, respectively (p = 0.034, 0.5 and 0.68, respectively). Comparisons of the actively treated and placebo groups showed statistically significant differences for children after the second and third years of SIT (p = 0.015 and 0.001, respectively) and for adolescents after the third year of SIT (p = 0.03) |
Other clinical outcomes
Recent guidelines64,134 have recommended the use of a number of secondary efficacy outcomes, including patient-reported assessments of improvement, symptom control (well-/bad-days) and responder analysis. Seven of the recent SCIT studies30,142,143,145,146,155,157 reported additional clinical outcomes, all of which favoured the active treatment (Table 16). No studies reported on the development of new asthma cases.
| Study ID | Results |
|---|---|
| Chakraborty 200630 | Pulmonary function tests: After 2 years of treatment, % predicted FEV1 during pollen season in patients with both AR and asthma (n = 8 active, n = 6 placebo) was better in patients receiving active treatment, and was also significantly better than baseline values in patients receiving SCIT (p < 0.001) but not the control group (p > 0.05) |
| New sensitivities: During the 2-year treatment period, two patients in the placebo group and none in the active group developed new allergic sensitivities | |
| Charpin 2007142 | Days with asthma: Six active and four placebo patients suffered with comorbid asthma. During the first pollen season after commencement of treatment, patients receiving active SCIT experienced 0.4 days with asthma symptoms vs 2 days for placebo patients. During the second pollen season, the figures were 0.8 days vs 4 days, respectively. These differences were not statistically significant |
| Colas 2006143 | SMFDs: During the EPS, 368/1230 (29.9%) patient-days were symptom and medication free in patients receiving active treatment, compared with 50/570 (8.7%) patient-days in patients receiving placebo (p < 0.01). Similar results were reported for the active group during the PPS but fewer SMFD were reported in the placebo group (29.1% vs 1.4%; p < 0.01) |
| Visual scale of health situation related to symptomatology (100 mm VAS, from ‘very poor’ to ‘very good’): No differences in scores between active and placebo groups were apparent before or after the pollen season – median (cm) (IQR) 9.00 (8.20–9.30) vs 8.75 (6.80–9.58) and 9.35 (8.50–10.00) vs 8.20 (7.10–9.25), respectively; both p > 0.05. During the pollen season, scores decreased in both groups, but were significantly better in the active group compared with placebo – 7.00 (6.50–7.70) vs 4.30 (3.43–4.90), respectively; p < 0.001 | |
| DuBuske 2011145 | No. of well-days (days without rescue medication and low TSS): |
| Maximum TSS = 24. Total n = 491 active, 499 placebo | |
| TSS ≤ 2: 78% more well-days in active group – median 16 days vs 9 days n active and placebo, respectively; p = 0.007 | |
| TSS ≤ 3: 28% more well-days in active group – median 32 days vs 25 days; p = 0.008 | |
| TSS ≤ 4: 22% more well-days in active group – median 39 days vs 32 days; p = 0.004 | |
| No. of bad-days (combined SMS ≥ 8 or 10): | |
| SMS ≥ 8: 35% fewer bad-days in active group – median 20 days vs 27 days in active and placebo, respectively; p = 0.008 | |
| SMS ≥ 10: 86% fewer bad-days in active group – median 7 days vs 13 days; p = 0.0046 | |
| No. of well subjects (overall median combined SMS ≤ 2 during the 4-week PPS). Total n = 485 active, 488 placebo | |
| 85% more well subjects in active group compared with placebo – median 78 vs 42 active and placebo subjects, respectively; p = 0.0005 | |
| Francis 2008146 | Overall assessment: On completion of 1-year treatment, subjects answered question ‘How has your hay fever been this year compared with previous years?’ on a numerical scale of −3, a lot worse, to +3, a lot better; mean (standard error) 2.42 (0.22) in active group vs 1.50 (0.34) in placebo group; p < 0.05 |
| Pfaar 2010155 | Responder analysis: Based on receiver operating curves, responders were defined as those subjects with AUC of at least 30% less than median AUC for placebo group during the pollen season; 64% (87/137) of the active group were defined as treatment responders compared with 32% (15/47) of the placebo group; p < 0.01 |
| Sahin 2011157(A) | Well-days (symptom-free days): Significantly more well-days in active group than placebo group |
| Global evaluation: 85% active SCIT group improved (p = 0.002), and 91% were satisfied with the treatment (p < 0.001) |
Adverse events
Adverse event data were available from 15 of the newly identified trials. 30,142–146,148–150,152,154,155,157,159,160 Comparison of AE data between trials was not straightforward as methods of reporting varied considerably. Most studies reported only the number of patients experiencing events rather than the number of events; some only counted patients once, by their worst-case event, whereas others counted all events; and some studies only reported events considered to be treatment related. In addition, the amount of information regarding AEs varied greatly between trials, from brief narrative reports to detailed analyses of events. The following results are based on both newly identified trials and those in the Cochrane review. 68
At least one AE was experienced by 79% (667 out of 849) of patients receiving active injections, compared with 57% (418 out of 729) of patients receiving placebo in the nine trials that reported this outcome. 30,142–145,148,154,155,159
Incidence of local reactions was reported in eight trials,30,142,143,148,152,154,155,160 comprising 352 SCIT and 222 placebo patients in total. The active and control groups experienced a total of 138 and 39 localised AEs, respectively. Where actual number of injections delivered was reported, the rates of injection site reactions were 2.8% (67 events in 2428 injections),155 1.1% (11 events in 987 active injections),152 0.5% (29 events in 1672 injections)160 and 0.02% (five events in 2095 injections). 30 In the four trials143,144,148,152 (total n = 116 active, 80 placebo) that reported on treatment of local reactions, 103 local reactions occurred after active treatment, compared with 84 after placebo, none of which required treatment.
Systemic AEs were relatively uncommon, with 129 events occurring after 2909 injections (4.4%) in six trials that reported this statistic. 155,166,178–181 Six trials30,143,144,148,155,157 gave some indication of the event severity (Table 17). The majority (81%) were of mild or moderate intensity. However, severe AEs constituted 19% of the total. Three per cent of patients receiving active treatment withdrew owing to AEs.
| Severity of reaction | No. studies reporting | SCIT | Placebo | ||
|---|---|---|---|---|---|
| n | No. of events | n | No. of events | ||
| Mild | 4 | 227 | 38 | 113 | 8 |
| Moderate | 4 | 227 | 25 | 113 | 25 |
| Severe | 6a | 302 | 12 | 183 | 11 |
| AE leading to study withdrawal | 2 | 536 | 15 | 532 | 4 |
In addition, eight trials142,146,148,152,154,155,159,160 reported systemic reactions by type of event. Table 18 shows systemic events reported in more than one trial. Thirteen studies (n = 557)23,161,164–167,175,176,178,182–185 reported using adrenalin after 19 of 14,085 injections (0.13%).
| Type of reaction | No. studies reporting | SCIT | Placebo | ||
|---|---|---|---|---|---|
| n | No. of events | n | No. of events | ||
| Wheezing/asthma | 6 | 271 | 9 | 169 | 7 |
| Urticaria/oedema | 5 | 241 | 19 | 138 | 2 |
| Rhinitis | 4 | 223 | 44 | 131 | 22 |
| Conjunctivitis | 4 | 223 | 25 | 131 | 17 |
| Headache | 3 | 91 | 18 | 81 | 8 |
| Flushing | 3 | 91 | 16 | 73 | 51 |
| Light-headedness | 2 | 69 | 8 | 68 | 2 |
Post-injection anaphylaxis was reported in one trial,159 and was experienced in 10 patients receiving active SCIT (n = 39), with eight requiring administration of adrenaline, and in one patient receiving placebo (n = 37).
Occurrence of SAEs was reported by seven studies. 142,144,148–150,155,159 Sixteen SAEs occurred in 770 patients, none of which was considered treatment related. Treatment of AEs was not widely reported. Eleven trials126,143,144,148,152,163,168,169,186–188 (total active n = 324) reported 21 local reactions that required treatment. In contrast, 28 trials (total active n = 1023) reported 936 local reactions that did not require any treatment. Approximately one-third of systemic reactions (69 out of 206 events, based on 20 studies, n = 558) required treatment, with antihistamines, bronchodilators and glucocorticoids being the most commonly used. Administration of adrenaline is reported above.
Summary of findings: subcutaneous immunotherapy
A summary is shown below in Box 1.
-
Treatment with SCIT resulted in a statistically significant reduction in SSs, MSs and combined SMSs compared with placebo. Moderate effect sizes were observed in most cases, and these were largely unrelated to treatment duration and type of allergen. All but one152 trials were conducted in adults; the one small (n = 50) trial122 in children found a statistically significant reduction in SSs and MSs with SCIT after 3 years of treatment. Larger effect sizes observed in some analyses were always associated with wide CIs and a very high degree of between-study heterogeneity. Subgroup analyses suggested an increased benefit with greater allergen content
-
Subcutaneous immunotherapy treatment had a moderate effect on QoL scores, independent of treatment duration, MAC or type of allergen. Results from the one trial in children152 are suggestive of a benefit from SCIT in the long term
-
A range of other clinical outcomes were reported in a number of trials, and all favoured the active treatment
-
The majority of AEs were local injection site reactions and were more prevalent in participants receiving active treatment. Local reactions occurred in between 0.02% and 2.8% of injections (based on four studies30,152,155,160)
-
Most (81%) of systemic reactions were graded as mild or moderate; however, one-fifth were classified as severe
-
Discontinuation rates due to AEs were around 3% (reported in two trials, total n = 1068)
-
Post-injection anaphylaxis was reported in only one small trial159 (total n = 76) but was considerably more frequent following active treatment, occurring in approximately 10 of 39 patients (compared with 1 of 37 receiving placebo); 8 of the 10 patients were treated with adrenaline
Quantity of evidence (sublingual immunotherapy vs placebo studies)
Searches identified 85 publications of DBPC RCTs of SLIT (see PRISMA flow diagram in Appendix 4). Of these, 52 publications relating to 44 RCTs were already included in the relevant Cochrane review83 (note: the other RCTs in the Cochrane review related to dust mites or animal dander). Of the remaining 33 publications, 15 were within the Cochrane search period but appear not to have been identified and two had been excluded from the Cochrane review despite apparently meeting the inclusion criteria. Details of the excluded studies are presented in Appendix 5. Sixteen publications25,32,158,188–199 post-dated the Cochrane searches; thirteen25,158,188–199 of these related to 11 new RCTs, three were updates of trials previously included in the Cochrane review: two32,200 related to the Dahl et al. (GT–08) trial201 and one202 provided additional data from the trial described in Didier et al. 24 As the purpose of this report was to update, rather than repeat, the Cochrane review, results are presented for only the 11 studies25,158,189–199 published from 2009 onwards, although all relevant studies were included in the meta-analyses.
Main study characteristics and risk of bias (sublingual immunotherapy compared with placebo studies)
The main study and population characteristics, and assessment of risk of bias, are detailed for each of the 11 new studies25,158,189–199 in Appendix 6. All studies were double-blind placebo-controlled RCTs. The largest four trials25,189,192,195 had between 276 and 633 participants, with the number of participants in the smaller trials ranging from 20 to 115. Skin prick tests were performed in all patients to demonstrate specific sensitivity. Allergy symptoms were described as moderate to severe in 6 out of 11 trials,189–191,195–197 with no indication of severity given in 5 out of 11 trials. 25,158,192–194 Seven studies25,189,190,192,193,195,197 stated that some patients also had asthma symptoms. Two trials specified that patients were treatment naive,190,191 four reported no details,25,158,189,197 and in five trials192–196 it was stated that patients had not received SIT within the last 3–5 years, although it was unclear if any patients had ever been treated with SIT. This may be important, as it is known that SIT can have long-term effects. Outcomes included SSs, MSs, combined SMSs, QoL and AEs (note: only outcomes consistent with the inclusion criteria of this report have been listed). Six25,189–192,196 of the eleven studies reported SMSs compared with none of the studies in the Cochrane review. As for SCIT compared with placebo trials, the number and types of symptom assessed varied widely (see Appendix 7 – SSs across studies).
The most commonly tested allergen was timothy grass (P. pratense), investigated in four trials,174,177–179 followed by tree pollen (three trials),158,191,197 a mix of several grasses (two trials),25,194 one trial196 with ragweed and one190 with Alternaria. Length of treatment varied between 8 and 10 weeks and over three pollen seasons, and there was also variation in treatment schedules (e.g. daily or weekly dosing). One study189 was in children and adolescents, one190 in both children and adults, with the remaining studies all conducted in adults. The main study characteristics are summarised in Table 19.
| Study | Size | Previous SIT | Stated that symptoms moderate to severe | Patients with asthma allowed/ included | Type of allergen | Administration schedule | Outcomes |
|---|---|---|---|---|---|---|---|
| Blaiss 2011189 | n = 345 | Unclear | Yes | Yes | Timothy grass (Grazax) | Once-daily tablet for 23 weeks; pre-and coseasonal | SSs, MSs, SMSs, QoL, AEs, effects on asthma |
| Cortellin 2010190 | n = 27 | No | Yes | Yes | Alternaria (seasonal mould) | Fifteen days build-up phase (one to five drops daily); five drops every other day for 10 months; pre-, co-and post-seasonal | SSs, MSs, SMSs, AEs |
| Didier 201125 | n = 633 | Unclear | No details on severity | Yes | Five-grass mix | Once-daily tablet, 2 or 4 months preseasonal then over three consecutive pollen seasons | SMSs, SSs, MSs, SMFDs, QoL, AEs |
| Fujimura 2011 191 | n = 103 | No | Yes | No details on asthma | Tree (cedar) | Approximately 3-week build-up phase (unclear from report), followed by 18 months of maintenance with once-weekly dosing of 1 ml 2000 JAU/ml drops | SMSs, AEs, QoL |
| Nelson 2011192 | n = 438 | Not in last 5 years | No details on severity | Yes | Timothy grass | Once-daily tablet for 23–24 weeks; pre-and coseasonal | SS, MS, SMS, QoL, AEs, effects on asthma |
| Panizo 2010193 | n = 78 | Not in last 5 years | No details on severity | Yes | Timothy grass (Grazax) | Once daily tablet for approximately 22 weeks; pre-and coseasonal | AEs |
| Pfaar 2011194 | n = 80 | Not in last 3 years | No details on severity | No details on asthma | Twelve-grass mix | High-dose group: dose escalation to maximum 19.04 µg Phl p1/dose + 52.5 µg MPL; daily dosing with 0.21 ml sublingual drops for a total of 11 weeks | AEs |
| Reich 2011180 | n = 276 | Not in last 5 years | Yes | Yes | Timothy grass (Grazax) | Once-daily tablet (active or placebo) for 8–10 weeks; treatment initiated during pollen season and administered coseasonally | Days with medication, AEs, global evaluation, spirometry |
| Skoner 2010196 | n = 115 | Not in last 3 years | Yes | No details on asthma | Ragweed | Once-daily tablet for 14–20 weeks; pre- and coseasonal | SSs, MSs, SMSs, AEs |
| Ventura 2009158 | n = 20 | Unclear | No details on severity | No details on asthma | Tree (juniper) | 30-day induction, then drops given three times per week for 11 months | SSs |
| Voltolini 2010197 | n = 24 | Unclear | Yes | Yes | Tree (birch) | Induction over 11 days then daily drops 300 IR over 4 months | SSs, AEs, asthma days/severity |
Similarity between trials was explored for a range of study and population characteristics. Trial duration and type and amount of allergen used varied between trials; however, this was explored as a source of heterogeneity in subgroup analyses. Where reported, inclusion criteria were very similar across trials, with the majority stipulating a minimum of 2 years' clinical history of moderate to severe SAR, incompletely controlled by standard medication; actual SAR history of included patients ranged from approximately 5 to 18 years. Four studies158,191,194,196 did not report whether or not patients with previous SIT were included. Rates of asthma across trials were largely consistent, with none greater than one-quarter (range 7–26%). All studies excluded patients with severe or perennial asthma.
Results of the risk of bias assessment are summarised in Table 20 (full details are given in Appendix 5).
| Study | Sequence generation | Allocation concealment | Blinding | Data completeness | Selective reporting | Patients treatment naive |
|---|---|---|---|---|---|---|
| Blaiss 2011189 | + | + | + | ? | + | ? |
| Cortellini 2010190 | + | ? | + | + | + | + |
| Didier 201125 | ? | ? | + | ? | + | ? |
| Fujimura 2011191 | + | + | ? | +a | + | + |
| –b | ||||||
| Nelson 2011192 | + | + | + | + | + | ? |
| Panizo 2010193 | ? | ? | + | + | + | ? |
| Pfaar 2011194 | ? | ? | + | + | + | ? |
| Reich 2011195 | + | + | + | + | + | ? |
| Skoner 2010196 | + | + | + | ? | + | ? |
| Ventura 2009158 | + | ? | + | + | + | ? |
| Voltolini 2010197 | + | ? | + | + | – | ? |
Overall, the risk of bias was low for three or more areas of potential bias in most of the studies, although a lack of detail in the published reports meant that risk of bias was unclear in many cases. Study authors were not contacted, and an ‘unclear’ rating may be due to lack of reporting rather than a reflection of poor trial quality. All but one study191 indicated that endeavours were made to maintain blinding. There were only two instances191,197 in which a high risk of bias was identified; neither of these studies contributed to any meta-analyses. It should be noted that a certain degree of subjectivity remains in assigning a rating of low/unclear/high.
Effectiveness of sublingual immunotherapy compared with placebo
Of the 11 newly identified RCTs,25,158,189–197 only five reported data in a form suitable for meta-analysis. 25,189,190,192,196 All five studies provided SS, MS and combined SMS data,25,189,190,192,196 and three additionally provided QoL data25,189,192 (Table 21).
| Study ID | Not in meta-analyses | In meta-analyses |
|---|---|---|
| Blaiss 2011189 | AEs, effects on asthma | SSs, MSs, SMSs, QoL |
| Cortellini 2010190 | AEs | SSs, MSs, SMSs |
| Didier 201125 | SMFDs, AEs | SSs, MSs, SMSs, QoL |
| Fujimura 2011191 | SMSs, AEs, QoL | None |
| Nelson 2011192 | AEs, effects on asthma | SSs, MSs, SMSs, QoL |
| Panizo 2010193 | AEs | None |
| Pfaar 2011194 | AEs | None |
| Reich 2011195 | Days with medication, AEs, global evaluation, spirometry | None |
| Skoner 2010196 | AEs | SSs, MSs, SMSs |
| Ventura 2009158 | SSs | None |
| Voltolini 2010197 | SSs, AEs, asthma days/severity | None |
Symptom scores
A total of 39 SAR studies24,26,38,84–91,94–118,197,200 were included in the meta-analysis in the Cochrane review. Of these, two were excluded by this review,35,92 one92 reported combined results for patients with SAR and SAA, and one35 was restricted to patients with SAA. Further, 3-year results of the GT–08 trial200 superseded the 1-year results reported by Dahl,93 which was consequently removed from the meta-analysis. Searches for this review identified eight25,158,189–191,196,197,200 new studies reporting this outcome, of which six25,189,190,192,196,200 were included in the meta-analysis. In total, 2440 active (SLIT) and 2379 patients receiving placebo were included in 42 studies.
The combined SMD following SLIT was −0.33 (95% CI −0.42 to −0.25; p < 0.00001) favouring active treatment (Figure 6). This result is very similar to that found in the Cochrane review (SMD −0.34, 95% CI −0.44 to −0.25; p < 0.00001). Heterogeneity was also similar to the earlier review.
FIGURE 6.
Sublingual immunotherapy vs placebo: SSs.
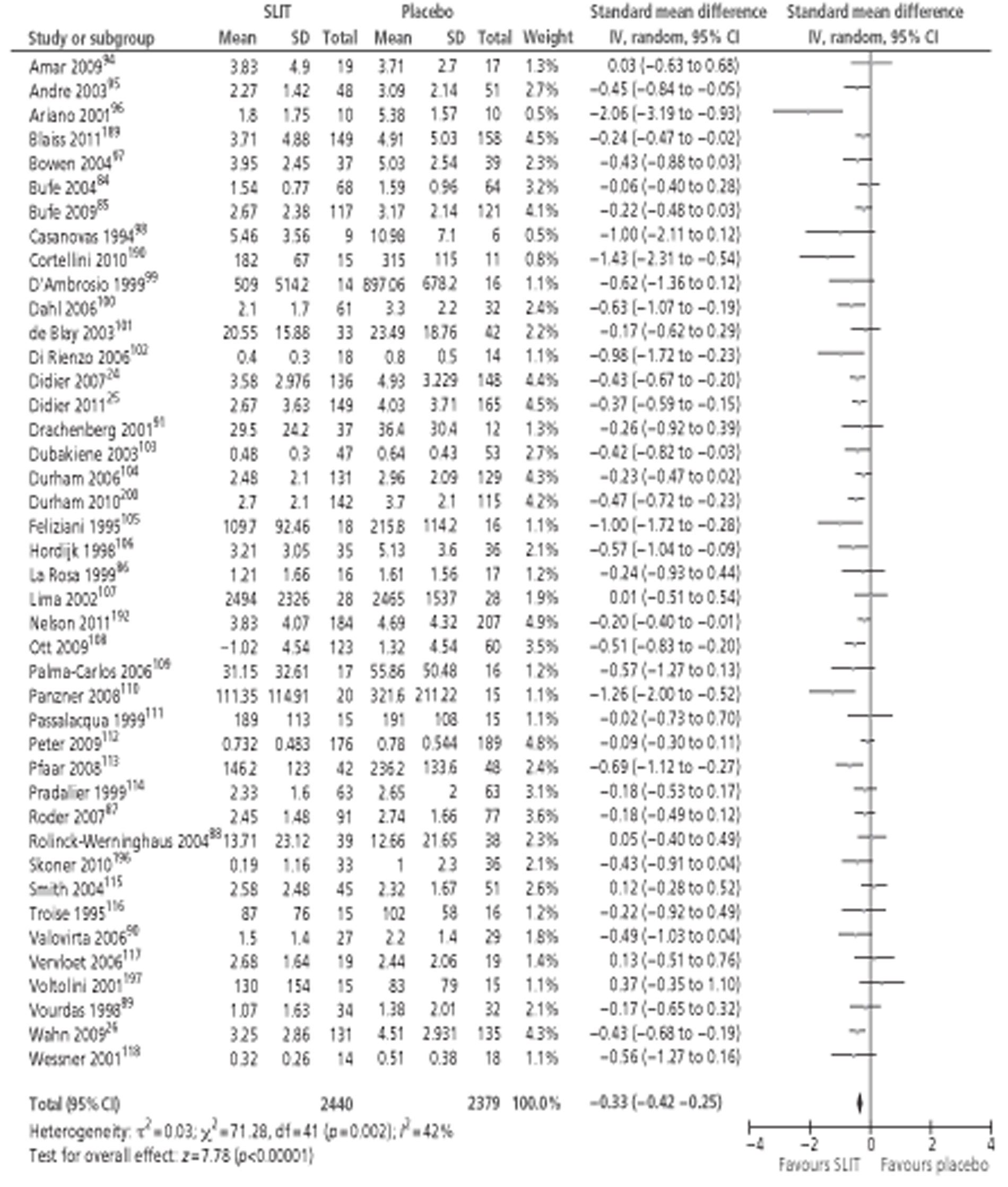
Subgroup analyses in this report were limited to studies in SAR, whereas data from the earlier Cochrane review included studies of SLIT for the treatment of PAR. Small to moderate effect sizes favouring active SLIT were found in all subgroup analyses, and these did not differ significantly with age, study duration, MAC or type of allergen. All were statistically significant, with the exception of the four studies in Parietaria allergy;99,110,115,125 however, this was based on a total of only 124 participants. Results of subgroup analyses are shown below in Table 22. Forest plots of all subgroup analyses are shown in Appendix 6.
| Subgroup | No. of studies | Total n | SMD (IV, random, 95% CI) | Comparison with CR |
|---|---|---|---|---|
| Age group | ||||
| Adults | 33 | 3476 | −0.38 (−0.49 to −0.27) | Similar |
| Children | 9 | 1343 | −0.24 (−0.35 to −0.13) | ES smaller, but remained statistically significant |
| Duration (months) | ||||
| < 6 | 15 | 1882 | −0.34 (−0.47 to −0.20) | ES smaller, but remained statistically significant |
| 6–12 | 15 | 1539 | −0.31 (−0.47 to −0.16) | Similar |
| > 12 | 12 | 1398 | −0.35 (−0.52 to −0.18) | ES smaller, but remained statistically significant |
| MAC | ||||
| < 5 μg | 6 | 229 | −0.53 (−1.03 to −0.03) | ES larger; becomes statistically significant |
| 5–20 μg | 13 | 2287 | −0.29 (−0.37 to −0.20) | Similar |
| > 20 μg | 12 | 1088 | −0.33 (−0.48 to −0.18) | Similar |
| Allergen | ||||
| Grass | 25 | 4042 | −0.31 (−0.39 to −0.24) | 34% increase n; ES similar |
| Ragweed | 3 | 244 | −0.44 (−0.69 to −0.18) | Similar |
| Parietaria | 4 | 124 | −0.27 (−0.62 to 0.09) | ES smaller and became non-significant |
| Tree | 9 | 380 | −0.42 (−0.77 to 0.06) | Data as per Cochrane review |
Compared with the Cochrane review, subgroup analysis by age resulted in very similar effect sizes in adult participants but a much smaller effect in children (SMD −0.24 compared with −0.52 in the Cochrane review), although this remained statistically significant. Although only nine paediatric studies have been included here,26,84–90,189 compared with 15 in the Cochrane review,26,35,84–90,92,203–207 total participant numbers were very similar (1343 vs 1392 children, respectively) and heterogeneity was significantly reduced (I2 = 0%, compared with 92% in the Cochrane review).
Analysis by treatment duration found reduced effect sizes in trials lasting < 6 months and over 12 months (the latter associated with a 69% increase in sample size) compared with the Cochrane review, but all remained statistically significant.
Few differences were found compared with the earlier review in subgroup analysis by allergen content, with the exception of studies using < 5 μg of major allergen. 88,99,111,116,190 Despite a small reduction in both study number and total sample size, effect size was larger in the present review (SMD −0.53, compared with SMD −0.32 in the Cochrane review), and became statistically significant. There was no apparent dose–response relationship for SLIT.
Subgroup analysis by allergen type gave effect sizes very similar to those in the earlier review, despite a 34% rise in sample size in the grass allergen subgroup. This latter was associated with a reduction in heterogeneity, which became non-significant. Effect size decreased slightly in Parietaria allergen, becoming non-significant, but total participant numbers were small (total n = 124 in present review).
Two studies158,197 reported results in a manner not suitable for meta-analysis and details are shown in Table 23. Data could be extracted from only one study,197 which showed a significant improvement in SSs compared with placebo.
| Study ID | Results |
|---|---|
| Ventura 2009158 | Not possible to extract data from graphs. Clinical improvements were noted with active treatment compared with placebo |
| Voltolini 2010197 | Median baseline rhinorrhoea score in both groups was 2 (5–95 percentiles: 1–3); after 1 year IT median scores were 1 (5–95 percentiles: 0–2) with active SLIT and 1.5 (5–95 percentiles: 0–2) with placebo (p < 0.05) |
Medication scores
A total of 32 SAR studies26,35,84–86,88–109,111,114,116,117,197 were included in the meta-analysis in the Cochrane review. Of these, two35,92 were excluded by this review, as described above. Further 3-year results of the GT−08 trial200 superseded the 1-year results reported by Dahl et al. ,93 which was consequently removed from the meta-analysis. Six new studies25,189,190,192,196,200 reported this outcome and were included in the meta-analysis.
In total, 1934 active and 1845 placebo patients were included in 35 studies. 25,26,84–86,88–91,94–109,111,114,116,117,189,190,192,196,197,200 The combined SMD was −0.27 (95% CI −0.37 to −0.17; p < 0.00001) favouring active treatment (Figure 7). This result is very similar to that found in the Cochrane review (−0.30, 95% CI −0.41 to −0.19; p < 0.00001). Heterogeneity was also similar to the earlier review.
FIGURE 7.
Sublingual immunotherapy vs placebo: MSs.
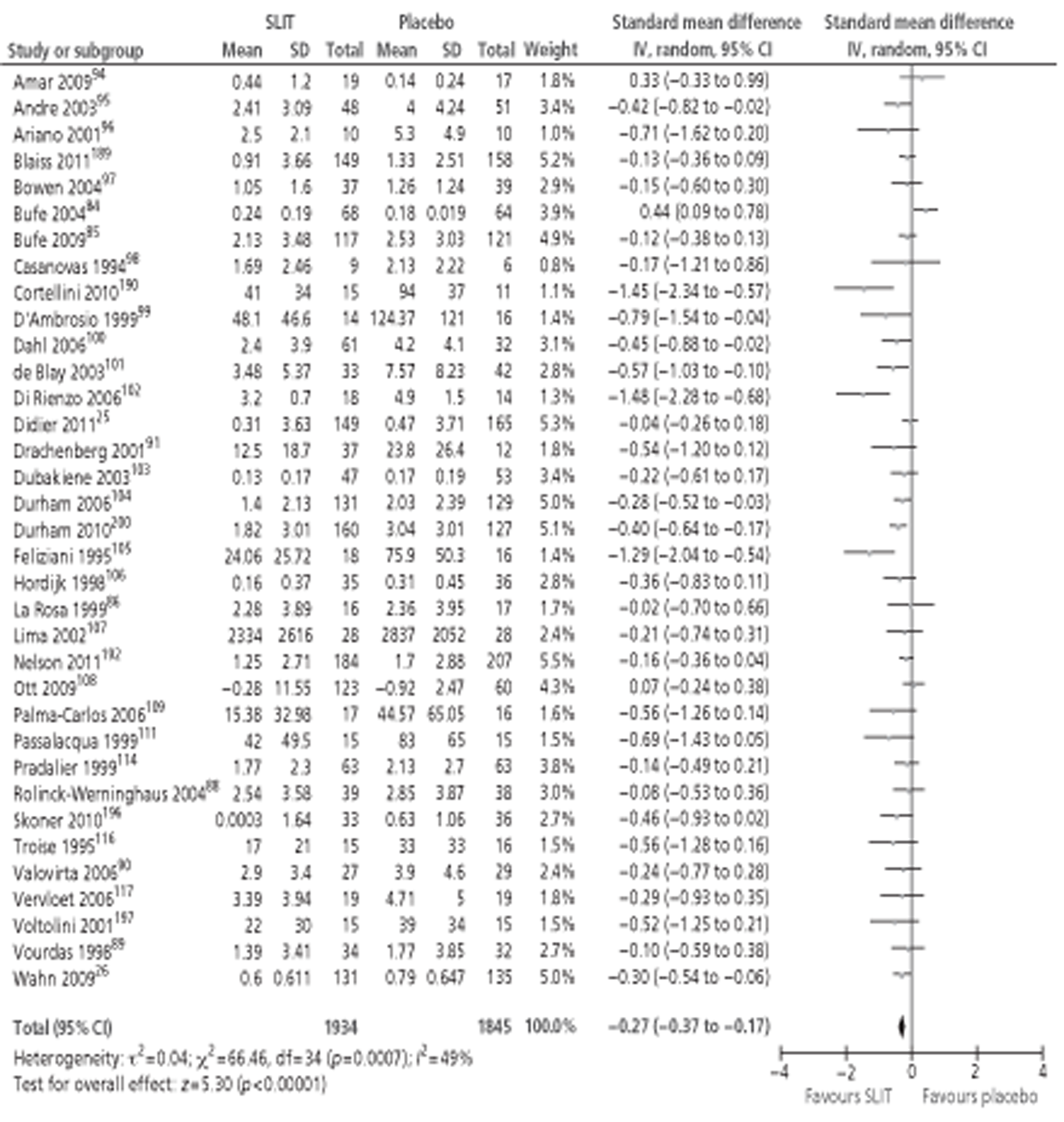
Small to moderate effect sizes favouring active SLIT were found in all subgroup analyses. MSs in children were not significantly better than with placebo treatment. This finding was consistent with that of the earlier Cochrane review, and effect size was decreased further with the addition of the more recent studies. Of the eight included studies,26,84–86,88–90,93 only one favouring placebo treatment was statistically significant. All others favoured active SLIT, but did not reach significance either alone or when combined. All other subgroup analyses were statistically significant, and most did not differ greatly from effect sizes reported in the earlier review, despite sometimes large increases in participant numbers (e.g. 31% increase in grass allergen; 69% increase in studies with duration of > 12 months). Analyses in two subgroups – > 20 μg major allergen and ragweed pollen SLIT – became statistically significant in the present review. Results of subgroup analyses are shown in Table 24.
| Subgroup | No. of | Total n | SMD (IV, random, 95% CI) | Comparison with CR |
|---|---|---|---|---|
| Age group | ||||
| Adults | 27 | 2604 | −0.35 (−0.47 to −0.23) | Similar |
| Children | 8 | 1175 | −0.08 (−0.25 to 0.08) | ES smaller; remained non-significant |
| Duration (months) | ||||
| < 6 | 14 | 1517 | −0.33 (−0.47 to −0.19) | Similar |
| 6–12 | 13 | 1223 | −0.31 (−0.53 to −0.08) | Similar |
| > 12 | 8 | 1039 | −0.16 (−0.31 to −0.01) | 69% increase n; ES smaller, but remained significant |
| MAC | ||||
| < 5 μg | 5 | 194 | −0.63 (−1.08 to −0.18) | Similar |
| 5–20 μg | 12 | 2285 | −0.18 (−0.30 to −0.05) | Similar |
| > 20 μg | 10 | 708 | −0.26 (−0.47 to −0.06) | ES similar; however, became statistically significant |
| Allergen | ||||
| Grass | 19 | 3028 | −0.20 (−0.32 to −0.08) | 31% increase in n; ES similar |
| Ragweed | 3 | 244 | −0.34 (−0.60 to −0.09) | ES similar; however, became statistically significant |
| Parietaria | 4 | 124 | −0.49 (−0.85 to −0.13) | No new studies; one study from CR excluded;34 ES smaller, but remained statistically significant |
| Tree | 9 | 380 | −0.38 (−0.62 to −0.13) | Data as per CR |
Symptom and medication scores
The Cochrane review did not report on this outcome. We identified seven new studies25,189–192,196,200 that reported combined SMS, of which six25,189,190,192,196,200 were suitable for meta-analysis. Details of the remaining study191 are shown in Table 25. Although inadequate reporting means the results are difficult to interpret, active treatment appeared to result in improved outcomes in the second year of treatment.
| Study ID | Results |
|---|---|
| Fujimura 2011191 | Data presented in graphical form only; summary statistic used unclear. No apparent difference in scores between treatment groups during first pollen season, but in second season better scores were reported in the active group |
The six studies25,189,190,192,196,200 included in the meta-analysis represented a total of 690 patients who received active treatment and 704 receiving placebo (Figure 8). Combined SMD was −0.40 (95% CI −0.55 to −0.25; p < 0.00001) in favour of SLIT. Some heterogeneity between studies was indicated, but this was not statistically significant.
FIGURE 8.
Sublingual immunotherapy vs placebo: SMSs.
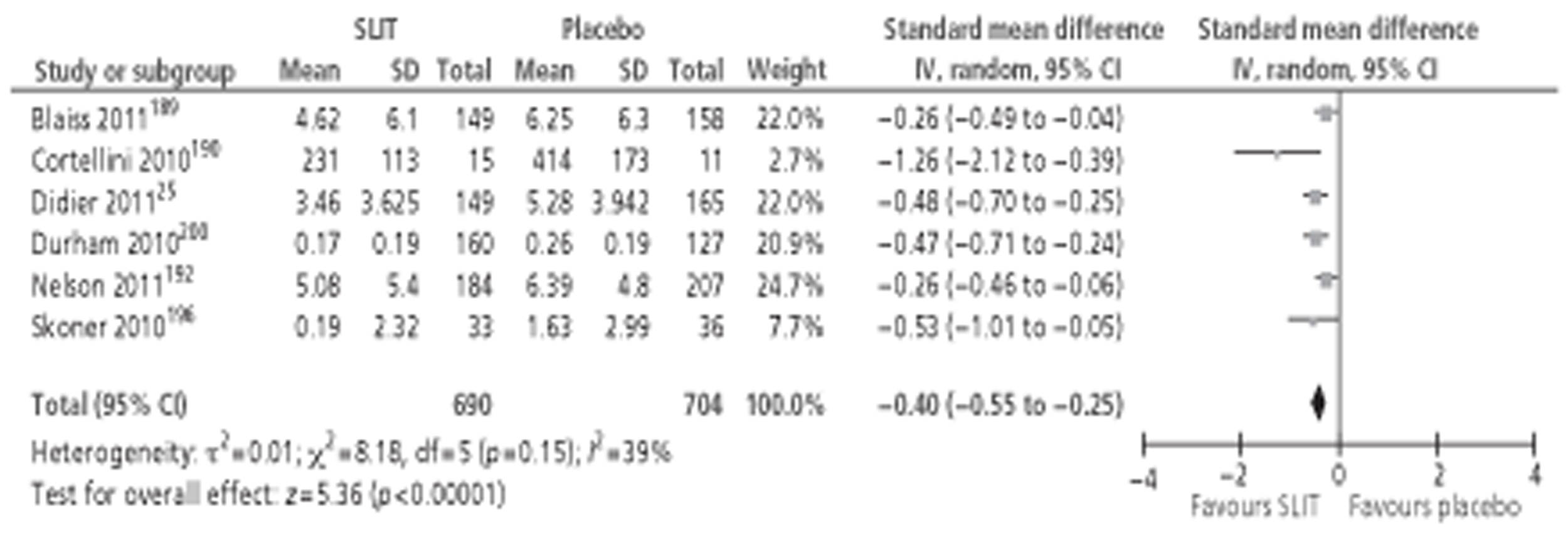
Moderate effect sizes favouring active SLIT were found in all subgroup analyses conducted (Table 26), and these were similar between studies. Combined SMD in the two studies190,191 lasting between 6 and 12 months was larger but a high degree of heterogeneity was indicated and this result was not statistically significant. Only one study189 conducted in children (n = 307) reported SMS and, therefore, meta-analysis was not possible. However, SMD for this study favoured active treatment and was statistically significant. Only one study190 was conducted using < 5 μg of major allergen and in Alternaria allergy, and only one study196 used > 20 μg of major allergen and was conducted in ragweed. Meta-analyses were, therefore, not possible in these subgroups. However, both of these studies190,196 favoured active treatment. No studies of SLIT for tree allergy reported usable data and no new studies were conducted in Parietaria allergy.
| Subgroup | No. of studies | Total n | SMD (IV, random, 95% CI) |
|---|---|---|---|
| Age group | |||
| Adults | 5 | 1087 | −0.44 (−0.62 to −0.27) |
| Duration (months) | |||
| < 6 | 2 | 376 | −0.31 (−0.51 to −0.11) |
| 6–12 | 2 | 417 | −0.66 (−1.63 to 0.30) |
| > 12 | 2 | 601 | −0.48 (−0.64 to −0.31) |
| MAC | |||
| 5–20 μg | 3 | 985 | −0.32 (−0.45 to −0.19) |
| Allergen | |||
| Grass | 4 | 1299 | −0.36 (−0.48 to −0.24) |
Quality of life
Eight studies25,102,112,189,191,192,200,202 reported QoL data (three from Cochrane review,102,112,200 five new25,189,191,192,202). All assessed QoL using versions of the disease-specific RQLQ.
Seven studies25,102,189,192,200,202 suitable for meta-analysis included 927 active and 951 placebo patients in total, a 473% increase from the Cochrane review (Figure 9). Nevertheless, the effect size (SMD −0.37; 95% CI −0.52 to −0.22; p < 0.00001) was very similar to that in the earlier review (SMD −0.42; 95% CI −0.73 to −0.12; p = 0.0063). Heterogeneity between studies was reduced, but remained significant (χ2 = 14.62; p = 0.02; I2 = 59%).
FIGURE 9.
Sublingual immunotherapy vs placebo: QoL.
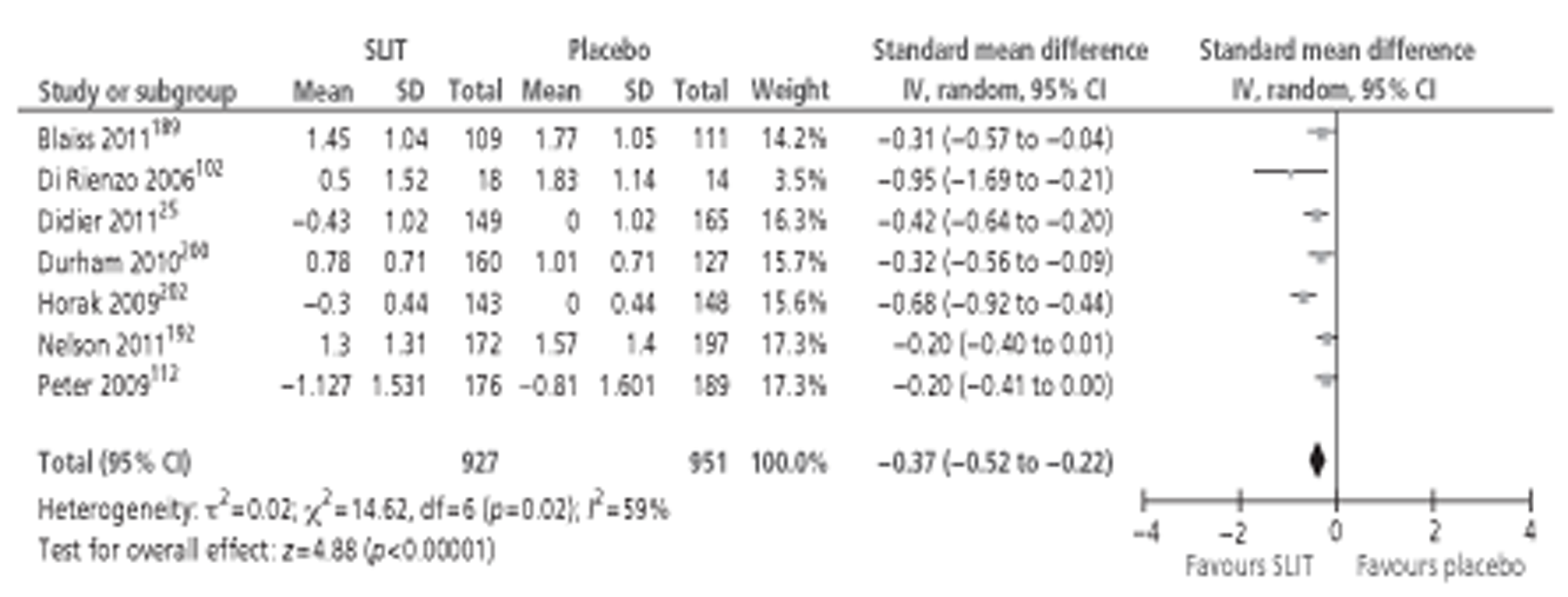
Summary of subgroup analyses are shown in Table 27.
| Subgroup | No. of studies | Total n | SMD (IV, random, 95% CI) |
|---|---|---|---|
| Age group | |||
| Adults | 6 | 1658 | −0.37 (−0.52 to −0.22) |
| Duration (months) | |||
| < 6 | 4 | 908 | −0.45 (−0.74 to −0.17) |
| > 12 | 2 | 601 | −0.37 (−0.54 to −0.21) |
| MAC | |||
| 5–20 μg | 2 | 507 | −0.32 (−0.49 to −0.14) |
| > 20 μg | 2 | 323 | −0.70 (−0.93 to 0.48) |
One189 of the new studies reported the first QoL results for children and adolescents, and these were statistically significant in favour of SLIT. Subgroup analysis of the remaining six studies25,102,112,192,200,202 did not lead to significantly different results from those reported above.
Four102,112,189,202 of the seven studies25,102,112,189,192,200,202 reporting QoL data lasted for < 6 months. Subgroup analysis found a moderate effect size in favour of SLIT, although heterogeneity between these studies was high (χ2 = 11.54, p = 0.009, I2 = 74%). One study192 was of between 6 and 12 months duration and, therefore, meta-analysis of this subgroup was not performed; however, the SMD was small and failed to reach statistical significance. In contrast, two studies25,200 presented data from trials lasting > 12 months, comprising a total of 601 participants. These longer studies25,200 reported a moderate effect size for QoL, which was statistically significant and no between-study heterogeneity was detected (I2 = 0%).
None of the studies included in the meta-analysis used vaccines with < 5 μg MAC. Two studies each used medium and high doses, and results are suggestive of a positive correlation between dose and effect size, although, given the small number of studies involved, particularly at the higher dose, these results should be interpreted with caution.
Four of the included studies25,102,200,202 used the full version of the disease-specific RQLQ to measure QoL, and random-effect meta-analysis was conducted for these studies (Figure 10). Two studies182,192 used alternate versions of the RQLQ (age-specific or standardised activities), which do not use the same scale or domains as the original, and these could therefore not be included in the meta-analysis. It was not possible to identify the instrument used in one study. 112
FIGURE 10.
Sublingual immunotherapy vs placebo: QoL (RQLQ scores).
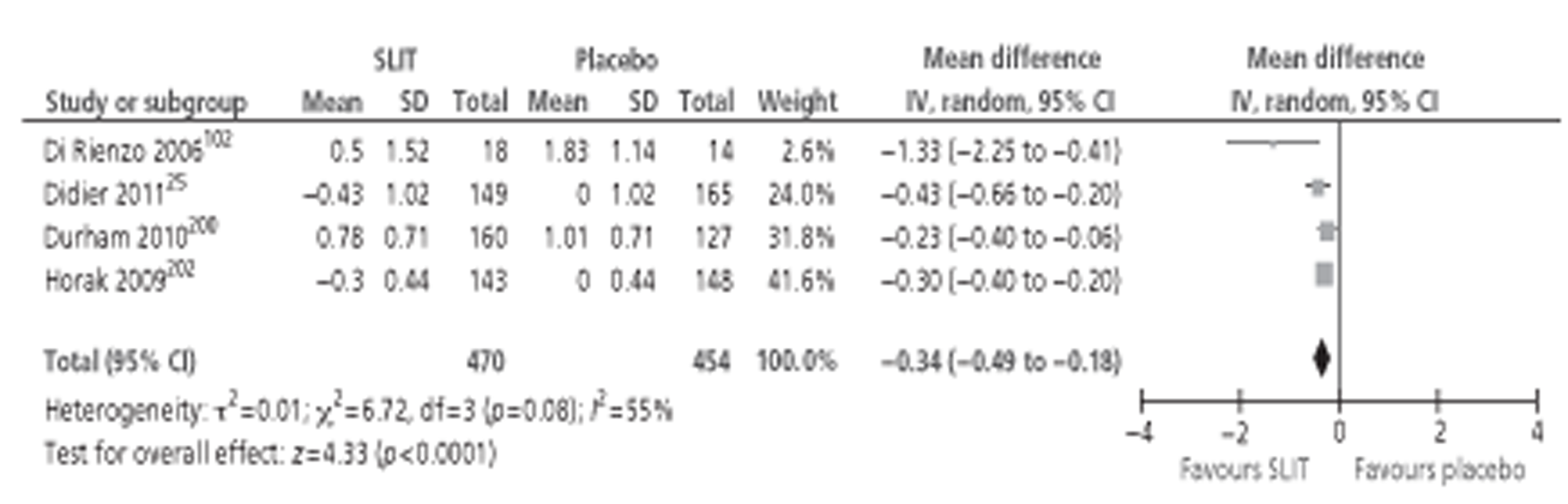
Weighted MD indicated an overall reduction in RQLQ scores of 0.34 units in the active SLIT group, indicating a positive effect of treatment.
One study191 did not present data in a manner suitable for meta-analysis (Table 28), but active treatment resulted in significantly improved QoL scores compared with placebo during the second year of treatment.
| Study ID | Results |
|---|---|
| Fujimura 2011191 | Data presented in graphical form only; summary statistic used unclear. Total scores on the Japanese Allergic Rhinitis QoL Standard Questionnaire No. 1 were significantly better in the active group (p <0.01) during the second pollen season. Data are not presented for the first pollen season |
Other clinical outcomes
Five of the recent SLIT studies189,192,195,197,202 reported additional clinical outcomes, as did the updates from the GT–08200 and Didier 200724 trials (Table 29). All trials reported that active treatment resulted in significantly better AR symptom control. Asthma SSs tended to be lower in actively treated patients, but the use of asthma medication appeared to be significantly lower in active groups. In addition, active SLIT may reduce the severity of asthma during the pollen season. One study195 reported no significant differences between active treatment in terms of medication use, spirometry or global assessment. This trial195 was of quite short duration, lasting only for 8–10 weeks, with treatment initiated during the pollen season. No studies reported development of new cases of asthma.
| Study ID | Results |
|---|---|
| Blaiss 2011189 | Effects on asthma: Both treatment groups (asthmatic subjects: n = 46 active, n = 44 placebo) reported low mean daily asthma SSs during the pollen season – 0.86 vs 1.08, respectively, out of a maximum of 12 (21% lower in the active group). The difference was not statistically significant |
| Didier 201125 | Proportion SMFD: Active group (n = 188) experienced mean 37.9% SMFD vs 26.4% in placebo group (n = 205) |
| Durham 2010200/2011129 | Proportion SMFD: After third treatment-year, subjects in active group experienced a mean 34.1% SMFD, compared with 24.1% in placebo group (p = 0.0035). This difference remained at the 1-year follow-up, with 35.2% vs 27.6% SMFD in the two groups, respectively (p = 0.0384) |
| No. of severe days: Severe days defined as having a maximum score on at least two of four nasal SSs measured; maximum score on at least one of two ocular SSs; or an overall SS > 9 out of a maximum 18. Mean number of severe days was 55.3% lower in Grazax-treated patients than in placebo; it is unclear to which time point or period this figure refers | |
| Horak 2009202 | Global assessment: Patient global assessment after first pollen season, compared with pre-treatment season, was significantly better than placebo (p < 0.0001). Details of assessment and absolute values not reported |
| Nelson 2011192 | Effects on asthma: Both treatment groups (asthmatic subjects: n = 44 active, n = 59 placebo) reported low mean daily asthma SSs over the EPS – 0.84 vs 1.10, respectively, out of a maximum of 12 (24% lower in the active group, p = 0.04). During the PPS, the difference between the groups was not statistically significant. However, the mean daily asthma MSs were 46% lower in the active than the placebo group (p = 0.01). In addition, only two patients in the actively treated group required treatment for worsening asthma (defined as four or more inhalations of short-acting β2-agonist per day, at any time) compared with 13 patients in the placebo group. The number of patients requiring initiation of treatment with inhaled corticosteroids during the pollen season was similar between the groups – six patients in the active group and five patients in the placebo group |
| Reich 2011195 | Use of rhinitis/asthma rescue medication: Use of rescue medication was similar between treatment groups – 176 out of 219 (81%) active and 51 out of 57 (89%) placebo subjects used allergy or asthma medication. Number of days with medication during the 10-week trial were 34.9 and 37.5 days, respectively |
| Spirometry: No pronounced changes in FEV1 occurred during the trial in either group | |
| Global evaluation: ‘Compared to your rhinoconjunctivitis symptoms in the previous grass pollen season, how have you felt overall in this grass pollen season: much worse, worse, the same, better, much better?’ Results were similar between the treatment groups: 68% active and 72% placebo patients reported improvements (better/much better); no change was reported in 28% and 26% of patients, respectively; only 4% (9/219) active and 2% (1/57) placebo patients felt worse or much worse | |
| Voltolini 2010197 | Days with asthma: During the second pollen season, reduction in the median number of days with asthma from visit three to visit six was much greater in the active than placebo group – from 10 (range 0–27) to 2 (0–6) days in the active group compared with 13 (0–29) to 7 (0–15) days in the placebo group (difference between groups, p < 0.05) |
| Asthma severity: Ten of the thirteen active patients stepped down an asthma severity grading level (GINA criteria) following treatment, compared with none of the nine placebo patients |
Adverse events
Again, reporting of AEs was highly variable between studies, making comparisons difficult. It should be noted that SLIT is usually self-administered outside the clinic, and thus AE reporting will depend on non-clinical judgement and patient recall. 134 Only one158 of the 11 new RCTs did not report AE data.
Overall, the incidence of AE was quite high, with 65% (420 out of 646) of patients receiving active treatment experiencing at least one AE, compared with 42% (194/467) of patients receiving placebo in the six trials25,189,193,195–197 that reported on AEs. The most commonly reported local reactions were itching, swelling and burning in the oral cavity. Four trials189,190,192,194 (total n = 890) reported oral pruritus in 39% of active and 5% of placebo patients; two trials189,192 (total n = 782) reported throat irritation in 33% of active patients compared with 4% of control patients, and mild erythema in 11% of active patients compared with 1% of control patients; and three trials189,192,194 (total n = 863) reported oral paraesthesia in 10% of SLIT patients compared with 2% of placebo patients, and mouth oedema in 9% of SLIT patients compared with 1% of placebo patients. The numbers of events were generally not reported.
Six trials88,189,192,194,195,208 reported systemic events by severity (Table 30). The vast majority (73%) of systemic AEs in these trials were of mild intensity, 24% were of moderate intensity and 3% were graded as severe. Anaphylaxis was reported in two trials192,195 and occurred in 4 of 427 patients receiving active treatment and in none of 282 patients receiving placebo. Only two trials189,192 (total n = 782) reported on adrenaline use. In each study, one instance of an AE in response to SLIT administration was treated with adrenaline. In both cases, the patients were receiving active treatment. Two instances of hospitalisation were reported,88,208 both for asthma attacks.
| Severity of reaction | No. of studies reporting | SLIT | Placebo | ||
|---|---|---|---|---|---|
| n | No. of events | n | No. of events | ||
| Mild | 5 | 669 | 830 | 458 | 314 |
| Moderate | 5 | 669 | 253 | 458 | 129 |
| Severe | 6 | 744 | 30 | 498 | 12 |
| AEs leading to study withdrawal | 5 | 879 | 30 | 686 | 14 |
Five trials25,189,192,194,195 reported a total of 20 SAEs in a total of 1565 study participants, of which only one, abdominal pain in a placebo-treated patient, was considered likely to be treatment related.
Only four small trials107,118,175,209 (total active n = 107) reported on treatment of AEs. Based on this small data set, 109 out of 1000 (11%) AEs required treatment, whereas 891 out of 1000 (89%) resolved spontaneously without treatment. This includes both local and systemic reactions, which were usually not reported separately. Most reports did not specify the nature of treatment required but based on one study209 (n = 43) the most commonly used treatments were salbutamol [used in 49% of cases (73 out of 149 treated AEs)] and analgesics (used in 40% of treated cases). Antiseptics, cold sore medication cold medication and glucocorticoids made up only 9% of all treatments between them. Details of adrenaline administration and hospitalisations are noted above.
Five studies189,190,192,194,195 reported discontinuations due to AEs. Thirty patients (3.4%) out of a total of 879 receiving active treatment were withdrawn for this reason. This number is similar to the 5% withdrawal rate reported in the Cochrane review.
Summary of findings: sublingual immunotherapy
A summary is shown below in Box 2.
-
Treatment with SLIT resulted in statistically significant reductions in symptom, medication and SMSs compared with placebo, and these effects were largely unrelated to participant age, treatment duration or type of allergen. SMD for SS improvement was not statistically significant in SLIT with Parietaria allergen, but this was based on only four studies86,99,111,116 (total n = 124). MSs were not significantly improved in paediatric patients compared with placebo (eight studies,26,84–86,88–90,174 total n = 1175)
-
Seven trials (total n = 1878) reported on QoL. Overall, SLIT had a statistically significant effect on QoL. Six of these trials25,112,190,192,200,202 were conducted in adults and there remains a shortage of paediatric QoL data; the one study189 in children reported a statistically significant difference in favour of SLIT
-
SLIT also appeared to result in better overall symptom control compared with placebo, and may reduce asthma severity and medication requirements
-
AEs were relatively common and more frequent in patients receiving active treatment. Local reactions in the oral cavity were the most frequently reported event and the majority of systemic reactions were of mild or moderate severity. Only one serious AE deemed to be treatment related occurred in five trials25,189,192,194,195 reporting this outcome (total n = 1565). Two trials192,195 (total n = 709) reported on anaphylaxis, which occurred in 0.9% patients receiving active treatment, compared with none receiving placebo. Two trials189,192 (total n = 782) reported on adrenaline use, which was required for only one treatment-related event; this event occurred in a patient receiving active SLIT. Discontinuations due to AEs occurred in 3.4% of participants receiving active treatment in five trials189,190,192,194,195 reporting this outcome (SLIT n = 879)
Quantity of evidence sublingual immunotherapy compared with subcutaneous immunotherapy
Searches identified only one randomised, double-blind, double-dummy comparison study210 of SLIT with SCIT (n = 71). Three further studies158,211,212 were initially identified as potentially relevant, but were subsequently excluded. One study158 (n = 40) was not a double-blind, double-dummy comparison; it included a comparison of SCIT with placebo and SLIT with placebo but no adequately blinded direct comparison of SCIT with SLIT, and results for SLIT compared with SCIT were not reported (this study is included in the relevant sections on placebo-controlled studies in this report). A second study211 (n = 47) compared a SLIT with a SCIT treatment arm, but there was no blinding (no treatment with placebo). Finally, a small, double-blind, double-dummy study (n = 20) by Quirino et al. 212 used 10 ‘matched pairs’ and does not appear to have used random allocation. The three studies that reported results for SCIT compared with SLIT found no significant differences between the two. Results for the one study210 meeting inclusion criteria are reported in more detail below.
Main study characteristics sublingual immunotherapy compared with subcutaneous immunotherapy
The main study and population characteristics of the included study, and the assessment of risk of bias are detailed in Appendix 5.
The main characteristics are summarised in Table 31.
| Study | Size | Previous SIT | Stated that symptoms moderate to severe | Patients with asthma allowed or included | Type of allergen | Administration schedule SCIT | Administration schedule SLIT | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Khinchi 2004210 | n = 71 | No SIT within last 5 years | No, but stated that rhinoconjunctivitis uncontrolled by conventional pharmacotherapy | No details | Birch | Twelve-week induction phase (weekly injections) with 0.0164 µg; monthly maintenance phase 3.28 µg | Thirty-day induction phase, maintenance phase 21–23 months. Drops every other day held under tongue for 2 minutes. Dose between 0.0164 and 49.2 µg | SSs, MSs, QoL, AEs |
Results of the risk of bias assessment are summarised in Table 32 (full details are given in Appendix 5).
| Study | Sequence generation | Allocation concealment | Blinding | Data completeness | Selective reporting | Patients treatment naive |
|---|---|---|---|---|---|---|
| Khinchi 2004210 | + | ? | + | ? | ? | ? |
As in many of the placebo-controlled studies, details of randomisation and blinding are reported but a lack of detail on other aspects of quality make it difficult to judge the overall quality.
Effectiveness of subcutaneous immunotherapy compared with sublingual immunotherapy
Results were reported for SSs, MSs, QoL and AEs and are summarised below (Box 3). Results were not presented in a way that is consistent with most other RCTs in this area, in that absolute mean SSs or MSs post treatment are not presented; instead median changes relative to the preceding pollen season are given. Nevertheless, the findings are consistent with other studies showing a benefit in terms of symptoms and MSs with both SCIT and SLIT compared with placebo. No significant differences between SLIT and SCIT groups were identified in this small study. 210 This does not mean that such differences do not potentially exist.
Rhinoconjunctivitis SSs
Method 1: subtraction of pre-treatment values from treatment season values
Median rhinoconjunctivitis SS (0–3 scale) improved by 0.36 score points (95% CI 0.18 to 0.86) in the SLIT arm, by 0.75 score points (95% CI 0.02 to 1.31) in the SCIT arm and decreased by 0.2 score points (95% CI −1.05 to 0.22) in the placebo arm. Significant difference in change between SLIT/SCIT and placebo (both p < 0.002). No significant difference in change between SLIT and SCIT
MSs
Method 1: subtraction of pre-treatment values from treatment season values
Median MS increased by 0.29 score points (95% CI −2.57 to 0.82) in the SLIT arm, were unchanged in the SCIT arm (95% CI −1.52 to 2.65) and increased by 1.35 score points (95% CI −4.04 to 0.12) in the placebo arm. Significant difference in change between SLIT/SCIT and placebo (p < 0.02 and p < 0.002, respectively). No significant difference in change between SLIT and SCIT
Rhinoconjunctivitis SSs
Method 2: values of first treatment season relative to pre-treatment season
Deterioration in SS by a factor of 1.45 (95% CI 0.87 to 2.09) in placebo group compared with an improvement in the SLIT group (by a factor of 0.78; 95% CI 0.6 to 1.06, p < 0.01) and the SCIT group (by a factor of 0.48; 95% CI 0.28 to 1.02, p < 0.001). No significant difference between SLIT and SCIT groups
MSs
Method 2: values of first treatment season relative to pre-treatment season
Increase in MS by a factor of 2.01 (95% CI 1.02 to 3.56) in placebo group compared with a change by a factor of 1.03 (95% CI 0.77 to 1.75; p < 0.05) in the SLIT group and a decrease by a factor of 0.78 (95% CI 0.3 to 2.0, p < 0.02) in the SCIT group. No significant difference between SLIT and SCIT groups
Indirect comparison of subcutaneous immunotherapy with sublingual immunotherapy
Given the paucity of direct comparisons (head-to-head trials) between SCIT and SLIT, it was decided to undertake an indirect comparison of SCIT with SLIT using data from the separate meta-analyses of SCIT compared with placebo and SLIT compared with placebo. Indirect comparison analyses rely on the assumptions (explored below) that there is sufficient similarity both within the SCIT compared with placebo and the SLIT compared with placebo trials (homogeneity assumption138), and that the true treatment effect comparing any two interventions would be similar across all trials, irrespective of whether they included one or both of those interventions (similarity assumption138).
The direct (head-to-head) evidence from the one small SCIT compared with SLIT trial210 included in this report could not be incorporated into this analysis, as the outcomes were not reported in a way that would allow this.
This section presents the most relevant results of the statistical analysis. The full list of parameter estimates and modelling approach is reported in Appendix 3.
Homogeneity assumption
For both SCIT compared with placebo and SLIT compared with placebo trials, heterogeneity was explored qualitatively by looking at patient and study characteristics, and statistically through meta-analysis (with I2 giving an indication of the extent of heterogeneity). Where reported, inclusion criteria were very similar across trials and it is likely that included populations had similar severity of AR. There was also not much variation in asthma rates where these were reported. All studies excluded patients with severe or perennial asthma. There was variation between trials in trial duration and type and amount of allergen. There was statistical evidence of moderate heterogeneity in the meta-analyses for some outcome measures (I2 of 57% for SS and MS for SCIT compared with placebo; I2 of 59% for QoL for SLIT compared with placebo). Statistical heterogeneity was less than moderate for the other outcome measures.
Similarity assumption
This was explored qualitatively. Overall, patient characteristics appeared to be similar between SCIT and SLIT populations (in terms of SAR history, severity, prior experience of SIT and prevalence of asthma). There was variation between trials in trial duration and type and amount of allergen. An attempt was made to assess whether placebo rates between SCIT and SLIT populations were similar. If populations are comparable, then placebo rates could also be expected to be similar. In this particular set of trials, placebo is administered in different ways (sublingual or subcutaneous) and given at different frequencies (daily for sublingual and weekly, then monthly for subcutaneous). There is evidence from different clinical areas that placebo rates may differ according to how the placebo is administered. 213
Although clinical opinion suggests that placebo rates between SCIT and SLIT are likely to be comparable (Stephen Durham, Imperial College London, 2 March 2012, personal communication), placebo rates could not be compared directly between SLIT and SCIT trials owing to (1) the use of different outcome measures and (2) the fact that any differences between baseline and follow-up measures are likely to differ between studies conducted at different times and in different geographical areas owing to variations in pollen count. However, failing to make this assumption would have precluded any indirect comparison analysis.
Given that there was some evidence of heterogeneity, a number of variables (treatment duration and type and amount of allergen, as well as age of participants – adults or children) were explored and adjusted for using ICMR.
A random-effects model was a better fit for the data and was therefore used in all further modelling (see Appendix 3 for methodology and full results). Standardised score differences were calculated and indicate the difference in effect size between SCIT and SLIT; positive values favour SCIT and negative values favour SLIT. When interpreting results, the 95% credible intervals (CrIs) need to be considered,214 with wider intervals indicating greater uncertainty. Best estimate probabilities were also presented. These show the probability of either SLIT or SCIT being the best treatment; however, the probability does not give any information on how much better one treatment is likely to be (i.e. they are not a measure of effectiveness). Note that even where there is a very high probability of one treatment being best, the 95% CrIs around the pooled standardised score differences may include zero and the results will therefore not be statistically significant.
According to the DIC measure, random-effects modelling fitted the data better than fixed-effects modelling (see Appendix 3), indicating a degree of unexplained heterogeneity in the data. Therefore, only results from random-effects modelling are presented in the following sections.
Symptom scores
When covariates were not included in the model (unadjusted model), the standardised score difference was 0.351 (95% CrI 0.127 to 0.586), a statistically significant result in favour of SCIT. Probabilistic analysis suggests that SCIT has a greater probability of being the best treatment compared with SLIT overall (unadjusted model) and also when participant age, study duration, MAC and type of allergen are accounted for (Table 33). However, not all the standardised score differences were statistically significant. For Alternaria allergy, the best estimate probability suggests that SLIT is the preferred treatment; however, this is based on only a single study for each intervention and a non-significant standardised score difference. Residual heterogeneity in the model could not be accounted for by participant age or type of allergen, while including MAC as a covariate reduced heterogeneity slightly (as shown by a decrease in σ2).
| Covariate | SCIT, no.of trials (A/P) | SLIT, no. of trials (A/P) | Probability that treatment is best (%) | ICMR SCIT compared with SLIT | |||
|---|---|---|---|---|---|---|---|
| Placebo | SCIT | SLIT | Standardised score difference (95% CrI) | Heterogeneity, σ2 (95% CrI) | |||
| None | 17 (659/529) | 42 (2440/2379) | 00.0 | 99.9 | 00.1 | 0.351 (0.127 to 0.586) | 0.089 (0.027 to 0.586) |
| Age group | |||||||
| Adult | 16 (634/506) | 33 (1768/1708) | 00.0 | 99.6 | 00.4 | 0.328 (0.088 to 0.579) | 0.091 (0.028 to 0.192) |
| Child | 1 (25/23) | 9 (672/671) | 00.9 | 54.9 | 44.2 | 0.059 (−0.837 to 0.966)a | |
| Trial duration (months) | |||||||
| < 6 months | 5 (136/138) | 15 (942/940) | 00.0 | > 99 | 00.0 | 0.822 (0.379 to 1.299) | 0.1112 (0.040 to 0.221) |
| 6–12 | 2 (203/106) | 15 (759/780) | 00.0 | 80.1 | 19.9 | 0.252 (−0.357 to 0.862)a | |
| > 12 | 8 (227/215) | 12 (739/659) | 00.0 | 83.4 | 16.6 | 0.187 (−0.199 to 0.577)a | |
| MAC (µg) | |||||||
| < 5 | 3 (112/116) | 6 (118/111) | 00.0 | 47.4 | 52.6 | −0.016 (−0.516 to 0.483)a | 0.053 (0.005 to 0.13) |
| 5–20 | 5 (117/114) | 13 (1163/1124) | 00.0 | 92.0 | 08.0 | 0.262 (−0.108 to 0.634)a | |
| > 20 | 3 (222/119) | 12 (564/524) | 00.0 | 99.7 | 00.3 | 0.582 (0.167 to 1.060) | |
| Type of allergenb | |||||||
| Grass | 9 (295/257) | 25 (2050/1995) | 00.0 | 99.4 | 00.6 | 0.396 (0.090 to 0.721) | 0.096 (0.030 to 0.20) |
| Parietaria | 3 (227/126) | 4 (60/64) | 00.0 | 99.1 | 00.9 | 0.775 (0.140 to 1.446) | |
| Ragweed | 0 (0/0) | 3 (118/126) | 01.2 | 50.0 | 48.8 | 0.011 (−197.0 to 196.70)a | |
| Tree | 4 (112/123) | 9 (197/183) | 00.0 | 64.3 | 35.7 | 0.092 (−0.408 to 0.597)a | |
| Alternaria | 1 (25/19) | 1 (15/11) | 00.1 | 04.6 | 95.3 | −1.133 (−2.460 to 0.199)a | |
The ICMR model included comparisons where there were no studies (no data) in either the SCIT or the SLIT arm (e.g. no SCIT trials for ragweed compared with three SLIT trials95,97,196 for ragweed); here the extremely large CrIs around the standardised score difference reflect the uncertainty resulting from this absence of data.
In order to explore unexplained residual heterogeneity further, two post hoc meta-regressions were conducted. In the first analysis, year of publication was used as a covariate in the model, and a strong effect for year was identified. An improvement in the fit of the model to the data was also observed (DIC 502, compared with 508 for the null model) and the heterogeneity parameter estimate was also lower [σ2 0.067 (95% CrI 0.017 to 0.147) compared with 0.089 (95% CrI 0.027 to 0.187), respectively].
Figure 11a shows pooled SMD point estimates for placebo-controlled trials and suggests that results from older studies show a greater benefit for SCIT, with more recent studies finding a decreasing benefit (more negative SMD values indicate a greater improvement in SSs compared with placebo). In contrast, SMD estimates for SLIT compared with placebo appear to remain more stable over time. The ICMR with year as a covariate (Figure 11b) finds that from approximately 2007 there is an increased probability that SLIT is more beneficial than SCIT. Note that standardised score differences at different time points are not all statistically significant (see Appendix 3).
FIGURE 11.
Symptoms scores: year of publication as covariate in the model. (a) theoretical and observed SMD and credible boundaries by year of publication for SCIT vs placebo and SLIT vs placebo; (b) probability of intervention being superior.
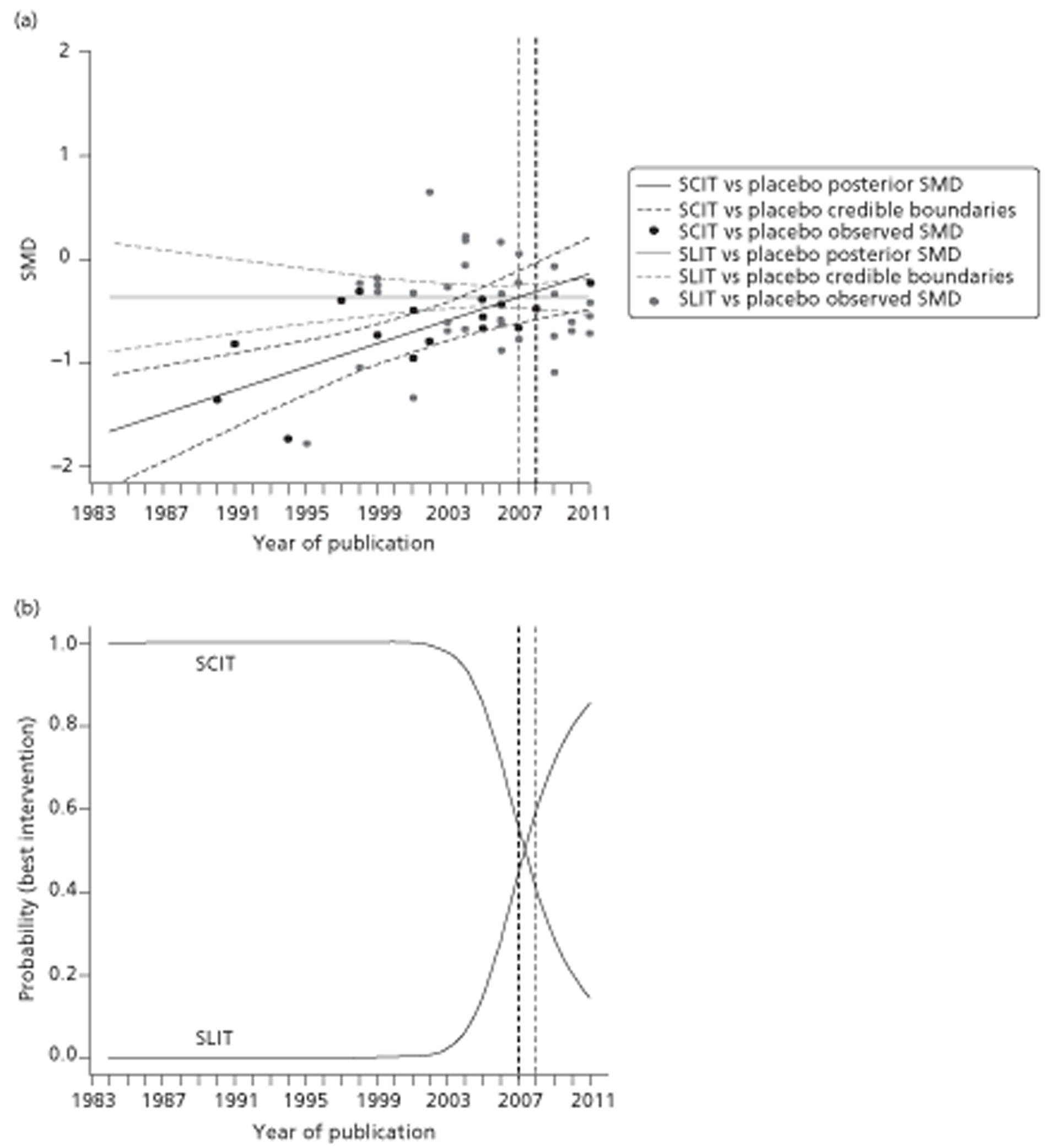
Given the huge variability in symptoms recorded between trials (see Appendix 7 for types and numbers of symptoms scored in different trials), a second post hoc analysis was conducted to explore any effect of this variable on trial outcome. It was found that increasing numbers of symptoms being measured in a trial appeared to favour SCIT over SLIT (Figure 12). Again, standardised score differences are not all statistically significant (see Appendix 3) and residual heterogeneity increases in this analysis.
FIGURE 12.
Number of symptoms measured as covariate in the model. (a) theoretical and observed SMD and credible boundaries by year of publication for SCIT vs placebo and SLIT vs placebo; (b) probability of intervention being superior.
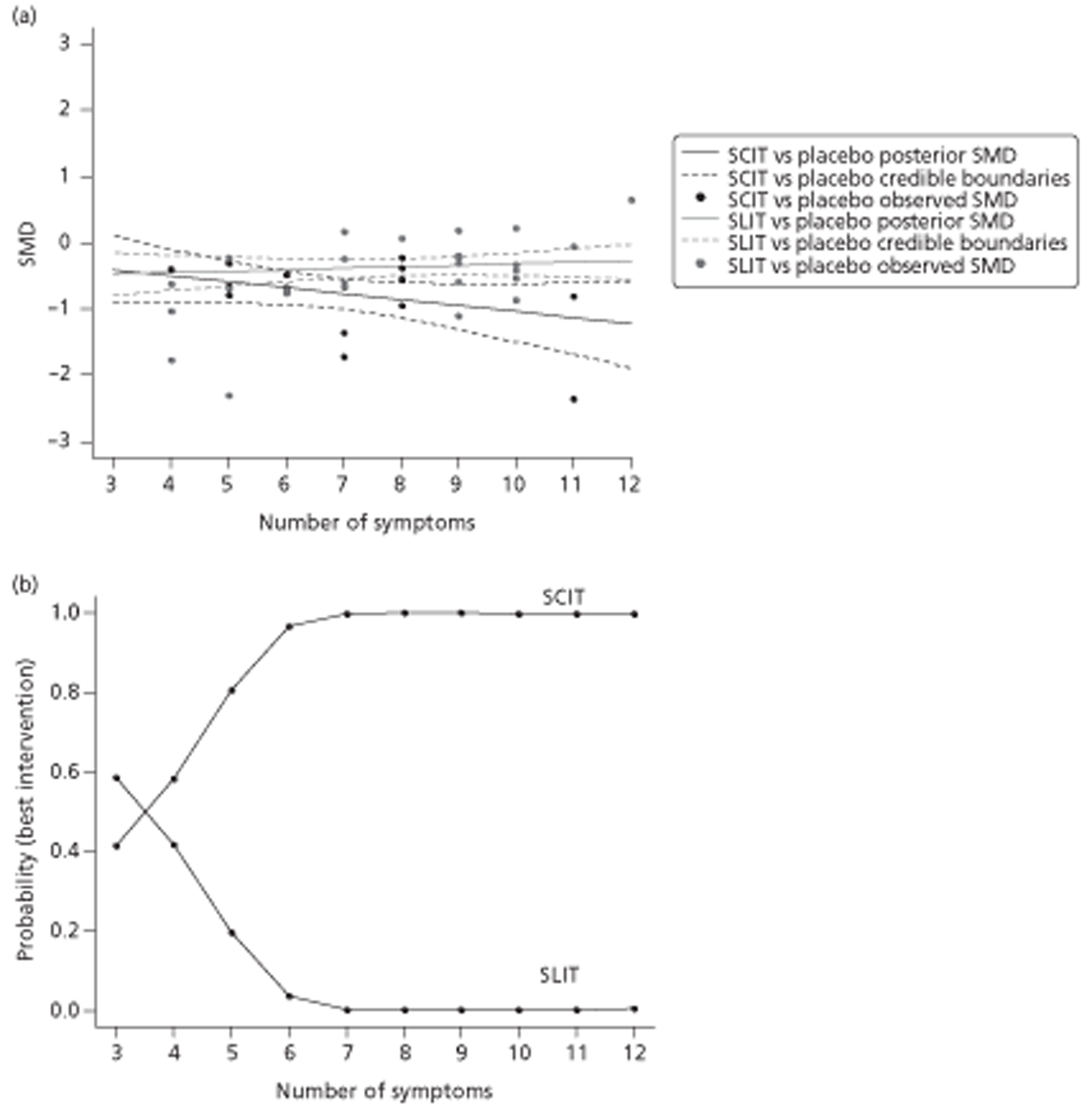
Medication scores
The unadjusted model (no covariates included), found a standardised score difference of 0.273 (95% CrI 0.027 to 0.529) in favour of SCIT (Table 34). This was associated with a > 99% chance of SCIT being the best treatment. Where meta-regressions resulted in standardised score differences in favour of SLIT, these were not statistically significant. It appears that some of the heterogeneity could be reduced by introducing MAC and particularly age (adult/child) as a covariate.
| Covariate | SCIT, no.of trials (A/P) | SLIT, no. of trials (A/P) | Probability (%) that treatment is best | ICMR SCIT vs SLIT | |||
|---|---|---|---|---|---|---|---|
| Placebo | SCIT | SLIT | Standardised score difference (95% CrI) | Heterogeneity, σ2 (95% CrI) | |||
| None | 16 (621/483) | 35 (1934/1845) | 00.0 | > 99 | 00.0 | 0.273 (0.027 to 0.529) | 0.099 (0.030 to 0.211) |
| Age group | |||||||
| Adult | 15 (596/464) | 27 (1353/1251) | 01.8 | 43.1 | 55.1 | −0.009 (−0.124 to 0.106)a | 0.002 (0.001 to 0.005) |
| Child | 1 (25/19) | 8 (581/594) | 00.0 | 68.1 | 31.9 | 0.009 (−0.031 to 0.050)a | |
| Trial duration (months) | |||||||
| < 6 | 2 (69/74) | 14 (766/751) | 00.0 | 50.9 | 49.1 | 0.006 (−0.634 to 0.634)a | 0.120 (0.039 to 0.255) |
| 6–12 | 3 (215/118) | 13 (609/614) | 00.0 | 75.6 | 24.3 | 0.192 (−0.376 to 0.750)a | |
| > 12 | 3 (244/225) | 8 (559/480) | 00.0 | 99.1 | 00.9 | 0.511 (0.094 to 0.950) | |
| MAC (µg) | |||||||
| < 5 | 2 (71/76) | 5 (98/96) | 00.0 | 15.1 | 84.9 | −0.286 (−0.849 to 0.266)a | 0.042 (0.006 to 0.108) |
| 5–20 | 6 (129/126) | 12 (1167/1118) | 00.0 | 95.3 | 04.7 | 0.283 (−0.049 to 0.622)a | |
| > 20 | 2 (203/102) | 10 (383/325) | 00.0 | 90.4 | 09.6 | 0.298 (−0.164 to 0.765)a | |
| Type of allergenb | |||||||
| Grass | 8 (263/220) | 19 (1544/1461) | 00.0 | 99.8 | 00.2 | 0.504 (0.169 to 0.873) | 0.094 (0.024 to 0.209) |
| Parietaria | 2 (209/109) | 4 (60/64) | 00.1 | 42.1 | 57.9 | −0.068 (−0.761 to 0.619)a | |
| Ragweed | 1 (11/12) | 3 (118/126) | 00.8 | 67.8 | 31.4 | 0.251 (−0.809 to 1.325)a | |
| Tree | 4 (113/123) | 8 (197/183) | 00.0 | 43.0 | 57.0 | −0.45 (−0.550 to 0.453)a | |
| Alternaria | 1 (25/35) | 1 (15/11) | 00.0 | 18.0 | 82.0 | −0.608 (−1.933 to 0.710)a | |
As for SSs above, adjusting for year of publication found changes in benefit from SCIT and SLIT over time, although the effect was not as pronounced and more difficult to interpret (Figure 13).
FIGURE 13.
Medication scores: year of publication as covariate in the model. (a) theoretical and observed SMD and credible boundaries by year of publication for SCIT vs placebo and SLIT vs placebo; (b) probability of intervention being superior.
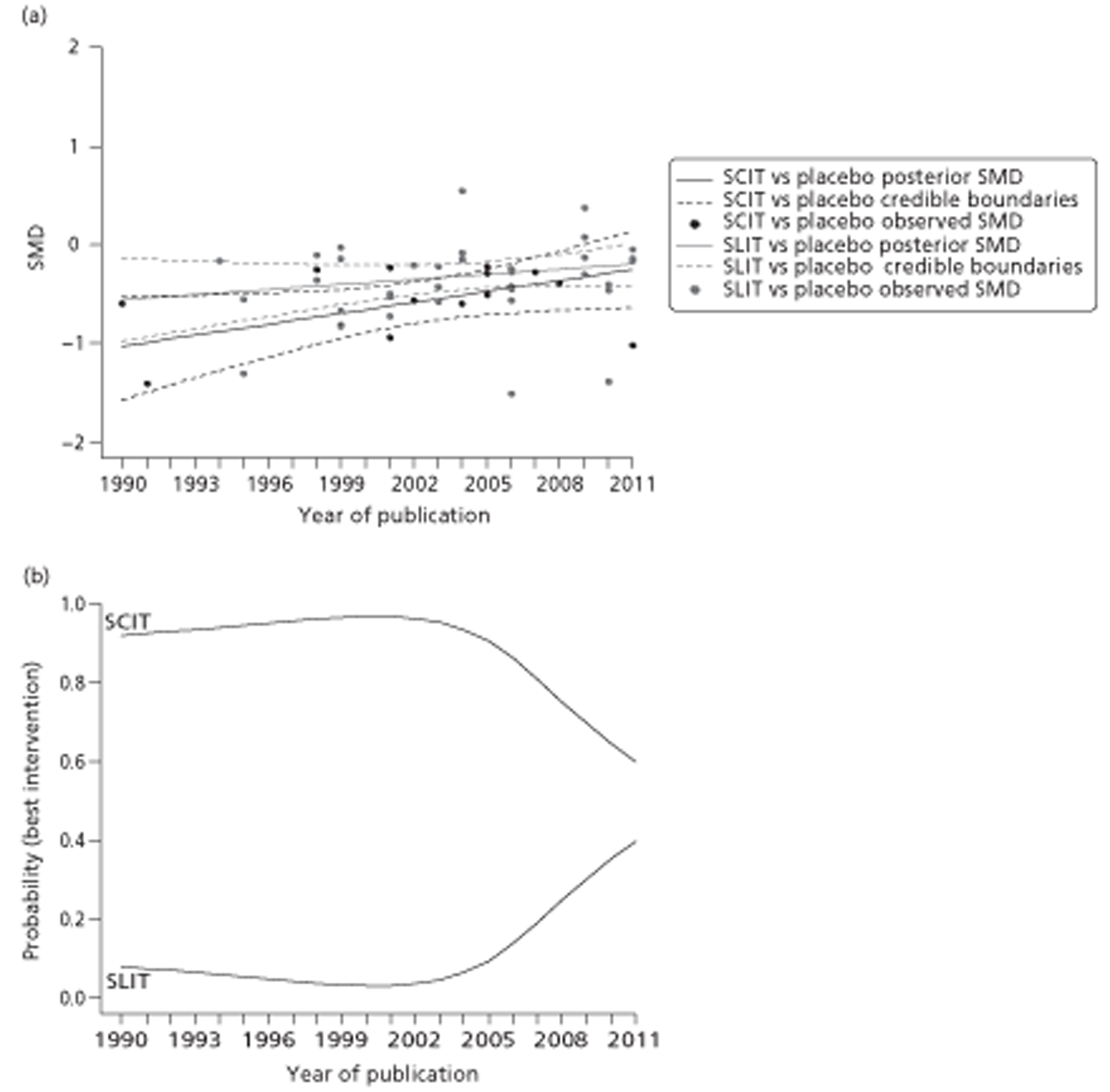
Symptom and medication scores
No significant difference between SCIT or SLIT could be shown in this analysis (Table 35), and this is associated with a large degree of uncertainty, as reflected in the wide CrIs. The combined SMS may be seen as a more robust outcome measure compared with the SS or MS alone, and this analysis could arguably be given more weight. Heterogeneity is lower for this analysis (compared with SSs and MSs separately) and is further reduced when type of allergen is used as a covariate.
| Covariate | SCIT, no. of trials (A/P) | SLIT, no. of trials (A/P) | Probability (%) that treatment is best | ICMR SCIT vs SLIT | |||
|---|---|---|---|---|---|---|---|
| Placebo | SCIT | SLIT | Standardised score difference (95% CrI) | Heterogeneity, σ2 (95% CrI) | |||
| None | 8 (320/297) | 6 (690/704) | 00.0 | 50.2 | 49.8 | 0.313 (−195.80 to 194.10)a | 0.017 (0.0 to 0.086)a |
| Age group | |||||||
| Adult | 8 (320/297) | 5 (541/546) | 00.0 | 50.2 | 49.8 | 0.307 (−196.40 to 196.70)a | 0.019 (0.0 to 0.097)a |
| Child | 0 (0/0) | 1 (159/158) | 02.9 | 47.1 | 50.0 | 0.089 (−277.30 to 274.70)a | |
| Trial duration (months) | |||||||
| < 6 | 3 (108/113) | 2 (182/194) | 00.1 | 50.2 | 49.7 | 0.676 (−195.70 to 198.10)a | 0.037 (0.0 to 0.210)a |
| 6–12 | 0 (0/0) | 2 (199/218) | 00.5 | 49.8 | 49.8 | 0.583 (−274.60 to 279.40)a | |
| > 12 | 3 (128/125) | 2 (309/292) | 00.0 | 50.4 | 49.6 | 0.874 (−276.10 to 279.20)a | |
| MAC (µg) | |||||||
| < 5 | 3 (112/116) | 1 (15/11) | 00.2 | 50.0 | 49.9 | 0.505 (−196.90 to 198.40)a | 0.061 (0.0 to 0.338)a |
| 5–20 | 1 (29/28) | 3 (493/492) | 00.7 | 49.2 | 50.0 | 0.148 (−278.0 to 277.80)a | |
| > 20 | 1 (18/17) | 1 (33/36) | 00.2 | 49.8 | 50.0 | 0.843 (−276.50 to 280.90)a | |
| Type of allergenb | |||||||
| Grass | 5 (231/204) | 4 (642/657) | 00.0 | 50.3 | 49.7 | 0.623 (−194.90 to 195.70)a | 0.011 (0.0 to 0.059)a |
| Parietaria | 2 (40/37) | 0 (0/0) | 00.0 | 50.4 | 49.6 | 1.568 (−275.70 to 277.10)a | |
| Ragweed | 0 (0/0) | 1 (33/36) | 01.0 | 49.5 | 49.5 | 1.189 (−278.60 to 278.40)a | |
| Tree | 1 (49/56) | 1 (51/37) | 00.7 | 49.5 | 49.8 | 0.633 (−277.90 to 278.20)a | |
| Alternaria | 0 (0/0) | 1 (15/11) | 00.1 | 50.3 | 49.7 | 1.341 (−274.70 to 278.70)a | |
Quality of life
Results of adjusted indirect comparison of QoL scores find that, although there is a high probability of SCIT being the best treatment, the standardised score difference is not statistically significant and is associated with a high degree of heterogeneity (Table 36). None of the adjusted standardised score differences are statistically significant. The analysis was repeated with trials using only the RQLQ for measuring QoL, and the result is therefore expressed as a difference in RQLQ units (Table 37). The results are very similar.
| Covariate | SCIT, no. of trials (A/P) | SLIT, no. of trials (A/P) | Probability (%) that treatment is best | ICMR SCIT vs SLIT | |||
|---|---|---|---|---|---|---|---|
| Placebo | SCIT | SLIT | Standardised score difference (95% CrI) | Heterogeneity, σ2 (95% CrI) | |||
| None | 8 (583/370) | 7 (927/951) | 00.0 | 96.4 | 03.6 | 0.383 (−0.042 to 0.804)a | 0.132 (0.041 to 0.342) |
| Age group | |||||||
| Adult | 8 (583/370) | 6 (818/840) | 00.0 | 95.9 | 04.1 | 0.402 (−0.062 to 0.864)a | 0.149 (0.043 to 0.402) |
| Child | 0 (0/0) | 1 (109/111) | 10.5 | 50.0 | 39.5 | 0.161 (−196.0 to 196.40)a | |
| Trial duration (months) | |||||||
| < 6 | 1 (9/9) | 4 (446/462) | 01.3 | 92.6 | 06.1 | 0.983 (−0.363 to 2.288)a | 0.197 (0.045 to 0.612) |
| 6–12 | 1 (183/92) | 1 (172/197) | 02.6 | 76.1 | 21.3 | 0.436 (−0.872 to 1.742)a | |
| > 12 | 6 (393/269) | 2 (309/292) | 00.2 | 66.8 | 33.0 | 0.152 (−0.617 to 0.925)a | |
| MAC (µg) | |||||||
| < 5 | 1 (21/20) | 0 (0/0) | 10.9 | 68.3 | 20.9 | 37.220 (−60.470 to 136.0)a | 0.260 (0.015 to 1.0) |
| 5–20 | 3 (241/140) | 3 (269/238) | 00.6 | 83.3 | 16.2 | 0.375 (−0.508 to 1.315)a | |
| > 20 | 3 (246/133) | 2 (161/162) | 01.2 | 91.3 | 07.5 | 0.718 (−0.393 to 1.670)a | |
| Type of allergenb | |||||||
| Grass | 4 (331/230) | 6 (909/937) | 00.5 | 90.2 | 09.3 | 0.334 (−0.197 to 0.868)a | 0.143 (0.038 to 0.420) |
| Parietaria | 2 (204/112) | 0 (0/0) | 01.2 | 49.1 | 49.8 | 0.597 (−194.80 to 195.30)a | |
| Ragweed | 1 (9/9) | 0 (0/0) | 01.5 | 48.9 | 49.6 | 0.933 (−194.70 to 198.0)a | |
| Tree | 0 (0/0) | 1 (18/14) | 01.5 | 49.7 | 48.8 | −0.875 (−196.70 to 194.80)a | |
| S. kali | 1 (41/19) | 0 (0/0) | 02.5 | 47.8 | 49.7 | 0.622 (−195.10 to 196.60)a | |
| Alternaria | 0 (0/0) | 0 (0/0) | 25.0 | 37.4 | 37.6 | −0.283 (−275.80 to 277.0)a | |
| Covariate | SCIT, no. of trials (A/P) | SLIT, no. of trials (A/P) | Probability (%) that treatment is best | ICMR SCIT vs SLIT | |||
|---|---|---|---|---|---|---|---|
| Placebo | SCIT | SLIT | Standardised score difference (95% CrI) | Heterogeneity, σ2 (95% CrI) | |||
| None | 8 (585/370) | 4 (470/454) | 00.0 | 96.2 | 03.8 | 0.517 (−0.071 to 1.045)a | 0.155 (0.033 to 0.494) |
| Age group | |||||||
| Adult | 8 (585/370) | 4 (470/454) | 00.0 | 96.0 | 04.0 | 0.517 (−0.085 to 1.051)a | 0.158 (0.033 to 0.506) |
| Child | 0 (0/0) | 0 (0/0) | 24.7 | 37.8 | 37.5 | 0.493 (−276.0 to 278.70)a | |
| Trial duration (months) | |||||||
| < 6 | 1 (9/9) | 2 (161/162) | 01.5 | 88.9 | 09.6 | 0.950 (−0.686 to 2.364)a | 0.226 (0.006 to 0.946) |
| 6–12 | 1 (183/92) | 0 (0/0) | 01.9 | 48.3 | 49.8 | 0.536 (−194.3 to 195.2)a | |
| > 12 | 6 (393/269) | 2 (309/292) | 00.1 | 85.8 | 14.0 | 0.374 (−0.454 to 1.228)a | |
| MAC (µg) | |||||||
| < 5 | 1 (21/20) | 0 (0/0) | 24.9 | 37.7 | 37.4 | 0.375 (−195.10 to 196.20)a | 0.467 (0.003 to 2.323) |
| 5–20 | 3 (241/140) | 2 (178/141) | 00.9 | 86.3 | 12.8 | 0.692 (−0.046 to 1.281)a | |
| > 20 | 3 (246/133) | 1 (143/148) | 00.8 | 85.0 | 14.2 | 1.015 (−337.90 to 341.10)a | |
| Type of allergenb | |||||||
| Grass | 4 (331/230) | 3 (452/440) | 00.3 | 94.7 | 04.9 | 0.576 (−0.154 to 1.316)a | 0.196 (0.032 to 0.771) |
| Parietaria | 2 (204/112) | 0 (0/0) | 00.9 | 49.6 | 49.5 | 1.407 (−194.60 to 196.6)a | |
| Ragweed | 1 (9/9) | 0 (0/0) | 01.9 | 48.4 | 49.7 | 0.852 (−194.50 to 195.5)a | |
| Tree | 0 (0/0) | 1 (18/14) | 01.0 | 49.7 | 49.3 | −0.518 (−196.60 to 195.3)a | |
| S. kali | 1 (41/19) | 0 (0/0) | 03.0 | 47.3 | 49.8 | 0.440 (−195.70 to 195.2)a | |
| Alternaria | 0 (0/0) | 0 (0/0) | 24.8 | 37.9 | 37.3 | 1.001 (−276.20 to 276.1)a | |
Discussion of the indirect comparisons
There was some evidence of heterogeneity within trials of SCIT or SLIT compared with placebo and between placebo-controlled trials. Therefore, possible sources of heterogeneity were explored through ICMR. No substantial reduction in heterogeneity when adjusting for type of allergen, allergen content or duration of treatment in the analyses for SSs, MSs or QoL scores was found. A smaller amount of heterogeneity was evident in the analysis for combined SMSs [σ2 of 0.017 (95% CrI 0.0 to 0.086), no covariates included], and this was further reduced when type of allergen was introduced as a covariate [σ2 of 0.011 (95% CrI 0.000 to 0.059)]. It should be noted that this is based on a smaller number of studies compared with the SS and MS analyses, which may also have an effect on heterogeneity. In the indirect comparison based on combined SMSs, the standardised score difference was not statistically significant and was associated with very wide credibility intervals. This is reflected in the best estimate probability, which found that SCIT and SLIT had roughly equal probability of being the best treatment. The analysis using combined SMS is based on fewer studies compared with the SS and MS analyses, but it has been argued that it is a more appropriate outcome measure as it takes into account the relationship between symptoms and medication use. The overall results are consistent with the results from small and mainly poor-quality studies directly comparing SCIT to SLIT (see Chapter 3, Effectiveness of subcutaneous immunotherapy versus sublingual immunotherapy), which found no significant difference between the two treatments.
Unadjusted analyses (no covariates) using SSs and MSs found significant differences in standardised scores favouring SCIT but, given the high residual heterogeneity, these results need to be interpreted cautiously. Using RQLQ, the standardised score difference was non-significant and the result was associated with substantial heterogeneity.
Using ‘year of publication’ and ‘number of symptoms recorded in a trial’ as covariates in the ICMR suggested a decreasing benefit from SCIT over time (with SLIT appearing relatively more effective) and an increasing benefit from SCIT the more symptoms were measured. These covariates were not prespecified and findings should be seen as suggestive only, and interpreted very cautiously. Possible explanations for changes over time include changes in treatment protocols (e.g. use of standardised products) or reporting/publication bias. With regard to an increasing benefit for SCIT the more symptoms are measured, it could be speculated that SCIT is better at alleviating a broader range of symptoms. It is also possible that these findings are due to chance.
Owing to the differences in outcome measures used, results were expressed as standardised score differences (except for the RQLQ). As with the meta-analyses of placebo-controlled studies, these results are difficult to interpret clinically, and statistical differences may not be consistent with clinically important differences.
There are several relevant trials that have not been included in the meta-analyses or indirect comparison meta-analyses, as data were not reported in a suitable manner (see Tables 37 and 21). The results are therefore not based on all of the available data; the effect of this is uncertain.
It is difficult to draw firm conclusions from these results as (1) they vary depending on which outcome measure is used and (2) they are associated in some instances with substantial residual heterogeneity. A more useful data set would include studies using validated standardised outcome measures and treatment regimens.
Ongoing trials
Searches identified 22 (12 SLIT, 10 SCIT) Phase III double-blind, randomised placebo-controlled trials that are still ongoing or had recently finished but for which no published results were yet available (see Appendix 9). The majority of trials are being conducted in adults (some including adolescents), comprising over 4500 adult subjects in total, with only two SLIT trials (n = 1450) and one SCIT trial (number of participants not stated) recruiting children and adolescents specifically. We identified only one Phase II/III double-blind, double-dummy study of SLIT compared with SCIT, initiated in March 2011 and due for completion September 2014. 215 Four studies216–219 are investigating the efficacy and safety of SCIT with recombinant allergens, of which one includes off-treatment follow-up:216 a SCIT trial will collect data for one season after 2 years of treatment; a further trial will look at long-term allergy and asthma outcomes over a 5-year period in 1000 children aged 5–12 years (the Grazax Asthma Prevention study59). However, in general, the majority of trials do not appear to differ extensively from previously published studies in terms of patients and treatment regimens. Although beyond the scope of this report, it would be of interest to investigate the type of outcome measures being used given recent recommendations in this area.
Chapter 4 Cost-effectiveness
This chapter is divided into the following sections: (1) a systematic review of published EEs on SCIT and SLIT for the treatment of SAR; (2) a description of our preferred Markov models (for adults and children separately) that were constructed to assess the cost-effectiveness of SCIT and SLIT for the treatment of SAR; (3) challenges met when trying to identify data to populate the preferred Markov models; and (4) results of an EE of SCIT and SLIT for treating SAR based on a simpler decision model.
Systematic review of existing evidence
This section reports the results of a systematic review of published EEs evaluating the costs and benefits of SCIT and/or SLIT compared with standard care [symptomatic treatment (ST)], or of SCIT compared with SLIT. The purpose of the systematic review was to (1) gain an overview of existing evidence in this area and (2) identify any suitable data (e.g. costs, utilities, transition probabilities) with which to populate a new Markov model.
Methods
Searches
Studies on costs, cost-effectiveness, modelling and QoL were sought in The Cochrane Library [NHS Economic Evaluation Database (NHS EED)] 2011 Issue 1, MEDLINE (Ovid) 1948–April week 2 2011 and EMBASE (Ovid) 1980–week 15 2011. Quality-of-life studies were sought in MEDLINE (Ovid) 1948–June week 5 2011. Reference lists of included studies were also checked. There were no language restrictions. See Appendix 2 for full details of the search strategies.
Study selection
Inclusion and exclusion criteria were used as outlined in Table 38. These were slightly broader than those for clinical effectiveness in order to cover the breadth of available evidence. Titles and abstracts of retrieved studies were screened for inclusion and exclusion by one reviewer. Full texts were obtained for any potentially relevant studies. All uncertainties around study selection were resolved through discussion with two other reviewers. Reference Manager was used to track and record study selection decisions and reasons for exclusion.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Study design | |
|
Any study not including or reporting on an EE or reporting data that could potentially be used in a model-based EE |
| Population | |
| Adults or children with SAR, with or without asthma. Where populations have included some patients with PAR, the study has been included | Adults or children with a different allergic disease, such as food allergy, or with PAR or asthma only |
| Intervention | |
| Allergen-specific subcutaneous (injection) or sublingual immunotherapy in any setting. Where SIT covered both seasonal and perennial AR (e.g. dust mite allergy), the study was included | Any other route of administration [e.g. oral (swallowed rather than sublingual), nasal, epicutaneous, intralymphatic] |
| Comparator | |
| Placebo [with or without conventional (rescue) medication], SCIT or SLIT | Any other route of administration (e.g. oral, nasal) |
| Outcomes | |
| Any measure of cost, cost-effectiveness, resource use, QoL or utility associated with SCIT or SLIT | Outcomes relating to clinical effectiveness only with no cost or quality-of-life/utility data suitable for use in a model |
Data extraction
Data extraction was performed by one reviewer using a standard extraction form and checked by a second reviewer. Data were extracted on type of EE, study population, intervention and comparator, perspective, time horizon, model structure and model assumptions if applicable, resource and cost data and main findings.
Assessment of quality of included studies
The methodology of all included EEs was critically appraised using checklists recommended by the Cochrane Collaboration, i.e. the Philips checklist220 for model-based EEs and the Evers checklist221 for non-model-based EEs. For the Philips checklist,220 this involved assessment of a range of factors relating to objectives and structure of the model, the theory underpinning the model, assumptions and treatment of uncertainty as well as the appropriateness and evaluation of the data used to populate the model. The Evers checklist221 assesses details around the interventions being compared, assumptions made when valuing costs and benefits as well as the generalisability of the results obtained. Reviews of EEs were used as a source for identifying primary studies and were not formally quality assessed.
Quantity of evidence
Searches identified 406 potentially relevant publications, of which 330 were excluded at the title/abstract stage. Of the 76 publications that were potentially relevant, full texts could not be obtained for eight. Full-text copies of 68 papers were examined, of which 52 were excluded (reasons for exclusion are detailed in Appendix 5). Sixteen publications were included. Of these, 14 were primary EEs and two were reviews of EE studies. In addition, three studies were identified that reported utility-based outcomes for SAR.
Main characteristics of economic evaluations
The main study characteristics and findings are shown below. Further details can be found below (see Table 40) and Appendix 9.
| Study ID | Type of Study | Population | Type of AR | Intervention and comparator | Outcomes assessed | Cost perspective | Type of costs included | Key findings | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Berto 2008247 | Review of economic studies | USA, northen and southern European Countries | SAR or PA R | SCIT, SLIT vs ST | QALYs and other unreported outcomes | Societal, NHS and patient | Direct and indirect costs | Costs per patient/year varied from €96 to €348.50. The average costs per patient for IT ranged from €288 to €1182, whereas those for pre-IT/control subjects ranged from €116 to €2672. In EEs, IT was found to be more cost-effective than ST | The search strategy used in the review was not reported in detail. One study103 included in our systematic review was not reported in this review |
| Hankin 2011237 | Review of economic studies | USA, Germany, France, Italy, Denmark and northern Europe | SAR or PA R | SCIT, SLIT vs ST | QALYs, net benefits and other natural outcomes | NHS, societal and patient | Direct and indirect costs | IT provided cost benefits ranging from $96 to $5465. Average annual costs for IT per patient ranged from US$247 to US$10,200; Average annual costs for per patient ranged from US$1335 to $24,243; mean cost of allergy medications per patient year varied from US$23 to US$37 and costs per QALY gained ranged from US$14,536 to US$38,695 | The search strategy used in the review was not reported |
| Five studies224,225,228–230 reported in our systematic review were not included in this review |
Type of economic studies
Different types of EEs were identified:
-
one study reported both a CEA and CUA analysis233
-
one study reported both a CEA and cost–benefit analysis (CBA)234
-
one study reported both a CEA and CCA analysis. 235
The different forms of EEs differ in the way outcomes are measured and/or expressed. Results from CEAs, CBAs and CUAs are typically reported in terms of a single measure of economic benefit, the incremental cost-effectiveness ratio (ICER), whereas results (costs and outcomes) from CCA are expressed in a disaggregated way.
Population
All of the primary studies were based on European populations, whereas the two reviews236,237 additionally considered US populations. Sample sizes ranged from 30 to 2230 (for cohorts used in model-based analyses). Populations considered were those with SAR only (nine studies222,224–226,228,230–233) or SAR/PAR (five studies223,227,229,234,235 and the two reviews238,239), either with or without asthma. Where patients had PAR, this related to dust mite allergy.
Routes of immunotherapy and comparators
Five studies222,229,233–235 compared SCIT with standard care, six studies223,226,228,230–232 compared SLIT with standard care and two studies225,227 compared both SCIT and SLIT with standard care. One study224 compared different forms of SCIT (short and long term and with an adjuvant) to SLIT and standard care.
Cost perspectives and costs included
Six studies223,228,230–232,234 were undertaken from a purely societal perspective, and two224,227 were from a health insurer perspective. Five studies considered a combination of cost perspectives: two222,225 considered societal, health service and patient perspectives, one226 used societal and NHS (Italy) perspectives, one used societal and third-party payer points of view,229 and one235 used societal, NHS (Germany) and health insurer perspectives. The perspective used was not stated in one study. 233
Costs associated with IT were divided into direct medical costs [e.g. costs related to GP visits, hospital visits (inpatient and outpatient), drugs and specialist examinations/tests] and indirect costs (e.g. cost related to productivity losses owing to time missed from work as a result of AR, or costs associated with productivity gains through reduced number of working days lost). Most EEs included both direct and indirect costs.
Outcomes within economic evaluations
A number of effectiveness or cost-effectiveness outcomes were reported in the EEs. These are listed by type of EE below.
For studies with a CCA, outcomes included SSs,222 number of asthma and rhinitis exacerbations and number of nursery/school days lost223 and VAS for allergic symptoms. 223 Other outcomes were number of medical visits and health-care use,223,225 RQLQ and symptomatic medication reduction225 and break-even points of costs/expenses per patient. 101 One study201 included development of asthma and new sensitisation.
The following outcomes were reported in the CEA studies: cost per number of patients improved and number of asthma cases avoided,226 cost per well-day and symptom-free day,233 number of additional patients free from asthma symptoms,235 and cost per asthma case avoided. 227
Only six studies228–233 conducted CUAs, and therefore reported outcomes based on utility-based measures. For the four studies228,230–232 based on the GT–08 GRAZAX trial,93 the chosen measure of utility was the European Quality of Life-5 Dimensions (EQ-5D), which was then converted into quality-adjusted life-years (QALYs). The instrument or outcome measure on which the utilities, subsequently converted into QALYs, were based was not specified in Brüggenjürgen et al. 229 In the fifth CUA study,233 QALY estimates were based on EQ-5D values mapped from RQLQ scores.
Model-based economic evaluations
Five studies224,226,227,229,234 reported analyses based on decision-analytic models. Of these, two224,229 used Markov models, whereas three226,227,235 were based on decision tree models. Six main health states were considered in Brüggenjürgen et al. ,229 including mild AR, moderate to severe AR, severe AR and mild allergic asthma, severe AR and moderate to severe allergic asthma, no symptoms, and dead. Transition probabilities depicting movement between these health states and annual costs were obtained from published sources. In Claes et al. ,224 three main health states were modelled: SAR, non-SAR and asthma. Model inputs were not clearly described, but included data based on the PAT study. 54–56 Assumptions were made for effectiveness, adherence and discontinuation rates.
Berto et al. 226 considered five main health states in their decision tree: improvement, stabilisation, aggravation, inadequate response and asymptomatic. Data for the model came from the Retrospective Observation Physician Panel (ROPP). The same health states were considered in Omnes et al. ,227 with most of the model inputs coming from a Delphi panel of 10 allergologists and one epidemiologist. In Schädlich and Brecht,235 two main health states were considered and divided by type of intervention (ST compared with specific IT): patients without asthma symptoms and patients with asthma symptoms. Data to populate this model came from a number of sources including observational studies and epidemiological information, statutory health insurance data, Uniform Assessment Standard information and cost-of-illness studies in Germany. 238–240
Quality of economic evaluations
Quality assessment of the EEs identified a number of methodological issues, which are outlined below (see Appendix 10 for completed checklists and see Table 39 for key issues for each study).
| Study | Intervention | Cost per QALY | Comments |
|---|---|---|---|
| Bachert 2007228 | SLIT (Grazax) | Cost per QALY gained between €12,930 and €18,263 for different northern European countries, including the UK | SLIT was cost-effective at an annual cost of €2200 [tablet < €6 (£4) and based on a threshold of €29,000 (£20,000) per QALY] |
| Beriot-Mathiot 2007232 | SLIT (Grazax) | Cost per QALY gained between €7894 and €47,844 for different northern European countries, including UK | WHO-recommended SLIT pattern was cost-effective if sustained effect after treatment is ≥ 2 years based on a threshold of €29,000 (£20,000) per QALY, whereas seasonal SLIT pattern was cost-effective regardless of time horizon with ICER of €21,829 |
| Brüggenjürgen 2008229 | SCIT (product not stated) | From a third-party payer's perspective, SCIT + ST is associated with a cost per QALY of €8308 | From a societal point of view break-even point is reached after 10 years, after 15 years SCIT dominates ST (i.e. is cheaper and more effective) |
| Canonica 2007230 | SLIT (Grazax) | Cost per QALY gained between €13,870 and €21,695 for different European countries (Spain, France, Italy and Austria) | Cost-effective at annual cost of Grazax of between €1500 and €1900 |
| Keiding and Jørgensen 2007233 | SCIT (Alutard SQ) | Cost per QALY gained between €9716 and €25,863 per QALY (without indirect costs) | With indirect costs included, SCIT dominates ST or has low ICERs |
| Nasser 2008231 | SLIT (Grazax) | Cost per QALY gained between £4319 and £11,769 (UK only) | Cost-effective up to an annual cost of Grazax of £1850. The highest cost per QALY in sensitivity analyses was £11,769 |
Sources of clinical effectiveness data
Some studies222,225,228,230–232 were based on randomised trials and therefore were able to use robust resource use and outcome data. Four of the CUAs228,230–232 were based on findings from the GT–08 trial. 93 In other studies, however, there were limitations associated with the data sources. The use of self-reported data relying on patient recall over very long periods of time introduced imprecision in the calculating of parameters; for example, the recall period was 6 years in Petersen et al. 234 Other studies, such as that by Omnes et al. ,227 did not use patient-level data but instead used expert panels to estimate efficacy and health resource use data, which could lead to biases. In the Claes et al. study,224 assumptions were made regarding clinical effectiveness parameters.
Assumptions made
Some of the assumptions made in the studies did not appear realistic. In Schädlich and Brecht,235 for instance, non-adherence to IT was not accounted for as the model assumed 100% compliance. A number of studies made the assumption that resource use would be sustained over a period of extrapolation; Bachert et al. ,228 for example, extrapolated 3-year outcome and resource use estimates to a 9-year period, whereas Keiding and Jørgensen 2007233 extrapolated 1-year data to a 9-year period.
Cost data
Overall, many studies provided sufficient detail in terms of resource use and unit cost data to allow for the potential use of these data in other studies. Some costs may have been underestimated owing to the non-inclusion of certain resource use data in the estimation of both direct and indirect costs, for example costs of hospitalisations222,223 or costs for non-allergy-related resource use. 233
Discounting
The majority of studies discounted their costs by between 3% and 6%.
Generalisability of studies
It is unclear to what extent results (assuming that they are otherwise robust) can be generalised to wider populations. Some evaluations were based on small sample sizes222 or restricted to a single allergy centre. 223 Selection bias may be an issue, as in one evaluation226 clinicians from different centres retrospectively reported data for 100 patients each. Where assumptions have been made regarding base-line and post-treatment effectiveness,223,227 results are unlikely to be representative of real-life populations.
Reporting
Inadequate or incomplete reporting hampered analysis of the studies included in this review. For instance, parameter estimates used to populate decision tree or Markov models were either partly reported222 or not reported at all. 229 In particular, actual EQ-5D and QALY estimates, although used in the analyses, were not reported. 228,230–232 One study233 did not report the size of the sample analysed, making it difficult to assess the validity of the analysis. Some studies did not clearly describe the sources of the data used (e.g. sources of health-care resource data for Berto et al. 223), making it difficult to comment on the appropriateness of the costs estimates. Furthermore, some studies222,223 did not provide information on whether or not they had discounted their costs, making it difficult to ascertain the accuracy of these costs.
Sensitivity analyses
No sensitivity analyses were conducted in three studies,222,223,233 although this would have been appropriate. Sensitivity analyses in the other 11 studies took the form of univariate deterministic sensitivity analyses, varying a number of parameters, such as costs,224–231 disease and severity levels,226,239 thresholds for cost-effectiveness,228,230 cost perspectives,226,229 IT treatment time,229,232 excluding certain patient groups,230 time horizon of model224,225,231 and discount rates. 224,229 These analyses enabled the robustness of the study results to be tested. However, no probabilistic sensitivity analyses were undertaken for the model-based studies as is usual practice. 241 This, therefore, meant that uncertainty around model inputs was not accounted for in the model results.
Results of economic evaluations
Seven studies134,223,226–228,231,232 compared SLIT with standard care, and all found that SLIT was more cost-effective. Studies reporting results of EEs based on data from randomised studies134,222,225,228,231,232 seemed to have been the most robust. The quality of reporting for resource use and unit cost data in these trial-based studies also appeared to be adequate. Four studies228,230–232 reported cost per QALY (Table 39); all were based on the same multinational trial (GT–0893) and included populations from different European countries in the respective analyses. All found that Grazax was cost-effective (below a threshold of £20,000), providing that annual costs of Grazax remain below £2200. The current annual cost in the UK is £814 (at £2.23 per tablet). 46 The evaluations appear to be well conducted; however, there was a general lack of detailed reporting on the outcome side, for example lack of disaggregated baseline and follow-up EQ-5D values and QALY gains. All four studies were funded by the manufacturer of Grazax.
Six studies222,227,229,233–235 compared SCIT with standard treatment. All found that SCIT was associated with better outcomes and/or lower long-term costs. Two studies229,233 calculated a cost per QALY for SCIT (see Table 39). Brüggenjürgen et al. 229 found a low ICER, but the robustness of this result is uncertain, as there was insufficient detail on the instrument used to derive utilities, and on cost and resource use data. The evaluation by Keiding and Jørgensen233 found that SCIT either dominated ST or was associated with very low ICERs, although robust sensitivity analyses were not conducted.
Where SCIT and SLIT were directly compared against each other, SCIT was found to be both more effective and more cost-effective over the long term. The sample size of the only trial225 that has directly compared the cost-effectiveness of SCIT and SLIT was, however, small (n = 64), and therefore more studies based on larger samples are needed. As this was a CCA, there is no combined cost-effectiveness measure. Assumptions made in the other study224 that directly compared the two interventions were not robust enough and variations of such assumptions should ideally be tested within a sensitivity analysis.
Other useful studies
Three studies242–244 were identified that reported utility-based outcomes for AR. Tamayama et al. 242 used the rating scale and time trade-off methods to estimate utility weights for four AR severity levels (mild, moderate, severe and severest) based on a Japanese sample. From mild to severest, time-trade-off estimates were 0.96, 0.94, 0.89 and 0.83, respectively. The rating scale counterpart weights were 0.82, 0.71, 0.56 and 0.43. Chen et al. 243 collected EQ-5D data for three groups in the USA and reported the following values: 0.76 (for individuals with asthma and rhinitis), 0.76 (for those with asthma only) and 0.92 (for individuals with rhinitis only). In Wasserfallen et al. ,244 the responsiveness of the EQ-5D when used on asthma patients with AR was compared with that of the McMaster Asthma Quality of Life Questionnaire (MAQOL). The EQ-5D was found to be less responsive than the MAQOL.
Two abstracts245,246 reporting EEs of Grazax based on populations of children with AR were identified at a late stage of writing this report and have thus not been formally included. A detailed appraisal was not possible, as the information was in abstract form only, but the results of these studies were consistent with those found for adults in that Grazax was found to be cost-effective compared with ST below a £20,000 threshold.
Reviews of economic evaluations
Both reviews of EEs237,247 considered studies that applied simple cost analyses as well as full EEs from societal, health service and patient perspectives (Table 40).
Nine EEs222,223,225–228,230,233,235 included in Berto et al. 247 were also identified in the present review. All of the EEs reported in Hankin et al. 237 were included in our review with the exception of Buchner and Siepe,248 which seems to be a cost analysis rather than a CBA. Neither review presents a detailed search strategy. An additional seven EEs224,225,229–232,234 were identified for this report.
Consistent with our findings, both reviews found that IT (SCIT or SLIT) was (just) more effective or, in some cases, both more effective and cost-effective when compared with standard care. Hankin et al. 237 also highlighted limitations associated with the data sources used in the EEs, a finding similar to ours. In particular, there were issues around generalisability and short time horizons associated with trial-based studies, and concerns about the quality of data obtained from observational studies were also raised.
Conclusions
Economic evaluations varied widely in the type of analysis used, outcome measures, sources for cost and effectiveness data, and adequacy of reporting for different parameters (Table 41). They did find consistently that where either SCIT or SLIT was compared with standard therapy, IT was (just) more effective or, in some cases, both more effective and cost-effective. The most robust studies228,230–232 found that SLIT is likely to be cost-effective at thresholds of £20,000; these studies did not, however, report all of the utility data in a disaggregated form and all were funded by a manufacturer of SIT products. SCIT was also found to be cost-effective at a threshold of £20,000 (based on two studies229,233); however, these results were associated with slightly greater uncertainty.
| Study ID | Type of EE | Country | Type of AR | Intervention and comparator | Outcomes assessed | Cost perspective | Type of costs included | Type of model where applicable | Key findings | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Ariano 2006222 | CCA | Italy | SAR | SCIT vs ST | Symptom and satisfaction scores | Societal, NHS, patient | Direct costs | N/A | SCIT associated with better symptom and satisfaction scores. Net savings started at year 3 and were €623 per year at year 6 | Not all relevant costs appear to have been identified, valued and discounted appropriately. There were no appropriate sensitivity analyses |
| Bachert 2007228 | CUA | UK, Sweden, Germany, Netherlands, Denmark, Norway and Finland | SAR | SLIT vs ST | EQ-5D, QALYs | Societal | Direct and indirect costs | N/A | SLIT (Grazax) had more QALY gains associated with it compared with standard treatment. SLIT was cost-effective at an annual cost of €2000 and based on a threshold of €29,000 (£20,000) per QALY. Cost per QALY across seven countries was between €12,930 and €18,263 | Good range of values used in sensitivity analyses. Actual EQ-5D inputs and QALY estimates were not presented, which makes it impossible to assess the robustness of these, or to apply the values in another model. Study funded by manufacturer of SIT products |
| Beriot-Mathiot 2007232 | CUA | UK, Netherlands, Denmark and Sweden | SAR | SLIT vs ST | EQ-5D, QALYs | Societal | Direct and indirect costs | N/A | SLIT (Grazax) had more QALY gains associated with it compared with standard treatment. In the seasonal scenario, SLIT was more cost-effective, regardless of time horizon, with an ICER of €21,829 | Limited sensitivity analysis performed. Some EQ-5D inputs were presented, but not disaggregated according to SLIT or ST. Actual QALY estimates were not presented, which makes it impossible to assess the robustness of these, or to apply the values in another model. Study funded by manufacturer of SIT products |
| In the WHO-recommended scenario, SLIT was more cost-effective than standard care (at a threshold of €29,200) if the sustained effect of treatment is over 2 years or more. The ICER decreased from €47,844 at the 1-year time horizon to €7894 at the 9-year time horizon. The WHO-recommended SLIT scenario was the most cost-effective, with a time horizon of ≥ 6.3 years | ||||||||||
| Berto 2005223 | CCA | Italy | SAR or PAR | SLIT vs no SLIT control (assumed to be standard care) | Number of asthma and rhinitis exacerbations, medical visits, absence from nursery or school | Societal | Direct and indirect costs | N/A | SLIT associated with substantial reductions in the number of asthma and rhinitis exacerbations, number of school/ nursery days lost and number of medical visits (overall and in the SAR and PAR groups). Costs per patient (SAR group) were €2723 pre-SLIT and €643 during SLIT | Effectiveness and costs compared within the same group, before and during treatment with SLIT. Costs associated with the different treatment options have not been described. There were no details on funding. One of the author's affiliations was a manufacturer of SIT products |
| Berto 2006226 | CEA | Italy | SAR | SLIT vs ST | No. of patients improved, number of asthma cases avoided | NHS, societal | Direct and indirect costs | Decision tree model | SLIT was more effective and cheaper than standard therapy (i.e. dominates) from both cost perspectives in terms of costs per additional improved patient and costs per additional asthma cases avoided | There was some uncertainty about representativeness and similarity of patients in different treatment arms, also a lack of details on the data used in the model. Study funded by manufacturer of SIT products |
| Brüggenjürgen 2008229 | CUA | Germany | SAR or PAR | SCIT vs ST | Utilities, QALYs | Societal, third-party payer | Direct and indirect costs | Markov model | From a societal point of view at 15 years, SCIT + ST dominates ST with break-even point reached after 10 years. From a third-party payer's perspective, SCIT + ST was associated with an ICER of €8308 per QALY (cost-effective at a threshold of £20,000) | Overall there was insuffcient detail on the instrument used to derive utilities, and on cost and resource use data. Several assumptions were based on expert consensus. Study funded by manufacturer of SIT products |
| Canonica 2007230 | CUA | Spain, France, Italy and Austria | SAR | SLIT vs ST | EQ-5D, QALYs | Societal | Direct and indirect costs | N/A | SLIT (Grazax) had more QALY gains and was more cost-effective than ST (at annual Grazax cost of between €1500 and €1900). Cost per QALY gained was between €13,870 and €21,695 for different European countries | Overall, this CUA seemed to be well conducted; however, no actual QALY or EQ-5D inputs for each treatment arm were given in the paper. Study funded by manufacturer of SIT products |
| Claes 2009224 | CCA | Germany | SAR | SLIT, SCIT (short and long term), ST, adjuvant-supported allergoid (allergoid + MPL) | No. of asthma cases and new sensitisations | Health insurer | Direct costs | Markov model | Three-year average direct costs for the five forms of IT were: €2584 (long-term SCIT), €4269 (SLIT), €1533 (short-term SCIT), €2523 (short-term SCIT + maintenance injections) and €2080 (short-term SCIT + MPL). The direct costs of the short-term SCIT + MPL arm, compared with those of other forms SCIT and SLIT, are higher at the beginning of therapy. Other scenarios show that, compared with other forms of IT, short-term SCIT and short-term SCIT + MPL have favourable sustainability and adherence-related outcomes. Standard therapy is the cheapest option at 3 years and the most expensive option at 15 years | There was substantial uncertainty over sources and appropriateness of data feeding into model. An assumption of 100% effectiveness for the base case was made, which seems infeasible. The study was funded by a manufacturer of SIT products |
| Keiding 2007233 | CEA and CUA | Austria, Denmark, Finland, Germany, Netherlands and Sweden | SAR | SCIT vs ST | Symptom-free days and well-days (RQLQ), VAS, EQ-5D, QALYs | Not stated | Direct and indirectindirect costs | N/A | SCIT associated with ICERs of between €9716 and €25,863 per QALY (without indirect costs). With indirect costs, SCIT dominates ST or has low ICERs (€6458 per QALY for the Netherlands and €5024 per QALY for Sweden). SCIT also has more favourable costs per symptom-free day and costs per well-day | Overall, this CUA seemed to be well conducted, however, no sensitivity analyses were conducted so it is diffcult to assess the robustness of results. Utility values were obtained by mapping RQLQ values to the EQ-5D. The study was funded by a manufacturer of SIT products |
| Nasser 2008231 | CUA | UK, Germany, Netherlands, Denmark, Sweden, Spain, Italy and Austria | SAR | SLIT vs ST | EQ-5D, QALYs | Societal | Direct and indirect costs | N/A | SLIT (Grazax) had more QALY gains and was more cost-effective than ST (ICER of £4319 per QALY gained). Grazax remained cost-effective up to an annual cost of £1850. The highest cost per QALY in sensitivity analyses was £11,769 | Actual EQ-5D inputs were not presented. The study was funded by a manufacturer of SIT products |
| Omnes 2007227 | CEA | France | SAR or PAR | SLIT, SCIT vs ST | No. of improved patients and number of asthma cases | Health insurers | Direct and indirect costs | Decision tree model | When compared with ST, both SCIT and SLIT were associated with favourable costs per number of improved patients and costs per number of asthma cases. ICERs for SCIT were lower than those for SLIT. Costs per asthma case avoided were €1327 (SCIT) and €1708 (SLIT) | Most of the effectiveness inputs for the model came from estimates from a Delphi panel. There were no details on funding; however, one of the author's affiliations was a manufacturer of SIT products |
| Petersen 2005234 | CBA and CEA | Denmark | SAR or PAR | SCIT vs ST | Monetary benefits and measure of psychological well-being | Societal | Direct and indirect costs | N/A | SCIT was associated with an ICER of DKK 2784 per patient/year of improved well-being. From a CBA perspective, SCIT was shown to be net beneficial | Unvalidated measure of psychological well-being used and the methodology used to determine CBA estimates was not clearly described |
| Pokladnikova 2007225 | CCA | Czech Republic | SAR | SLIT, SCIT vs ST | RQLQ, VAS, Symptomatic MS | Third-party payer, patient and societal | Direct and indirect costs | N/A | Clinical benefits for SLIT comparable to those for SCIT, but SCIT patients showed a slightly better improvement especially in VAS and symptomatic MSs. Compared with SCIT, SLIT was associated with lower costs (from all perspectives). No comparisons with ST | Based on small (n = 64) open-label trial. As CCA, no combined measure of cost-effectiveness |
| Schädlich 2000235 | CEA and CCA | Germany | SAR or PAR | SCIT vs ST | No. of additional patients free from asthma symptoms and break-even points of costs or expenses per patient | Societal, NHS, statutory health insurance provider | Direct and indirect costs | Decision tree model | Break-even point reached between 6 and 8 years after commencement of SCIT and net savings associated with SCIT were between DM650 and DM1190 per patient after 10 years. Further, SCIT was associated with ICERs of between DM3640 and DM7410 per additional patient free from asthmatic symptoms | Some uncertainty around estimates of benefit. Study funded by manufacturers of SIT products |
Only two studies224,225 looked at the cost-effectiveness of SCIT compared with SLIT. Although suggestive of greater cost-effectiveness for SCIT, both studies had some methodological concerns associated with them and neither presented combined cost-effectiveness measures.
Preferred adult and child Markov models
To estimate the comparative long-term cost-effectiveness of SCIT and SLIT when used to treat SAR, two Markov models were constructed (adults and children) in TreeAge Pro 2009 (TreeAge Software, Inc., Williamstown, MA, USA). Both models were built to combine costs and outcomes associated with three different treatment pathways, SCIT, SLIT or standard (symptomatic) treatment.
Briefly, this entailed dividing a patient's possible course of disease progression into a number of health states with transition probabilities assigned for the movement between these states over a discrete time period (Markov cycle). Long-term costs and health outcomes are assessed by attaching estimates of resource use and health outcomes to the states in the model and then running the model over a large number of cycles to evaluate patient movement between states. Clinical advice was taken into account for the model structure (e.g. types of health states).
Adult Markov model
The long-term progress of a hypothetical cohort of AR patients moving along the three alternative pathways of care (SCIT, SLIT and ST) was to be compared. The treatment schedules for SCIT, SLIT and ST are presented in more detail in Chapter 1 (see Allergen immunotherapy). Briefly, patients would receive weekly subcutaneous injections (SCIT) for 16 weeks in a clinical setting, followed by monthly injections for up to 3 years. Patients receiving SLIT would take daily tablets or drops at home, also for up to 3 years. Symptomatic treatments may be oral, topical or intranasal. Occasionally, systemic corticosteroids may be prescribed.
Patients would then follow clinical pathways designed to mirror the natural progression of the condition in the population, with health resources use for all three groups also modelled. The aim was to enable model-based predictions of costs and outcomes to be compared for the SCIT, SLIT and ST groups in a CUA from the UK NHS and patient perspectives.
Adult Markov model structure and model inputs
The structure of the adult Markov model is shown in Figures 14–16. Only health states for the SCIT and ST arms are presented. The health states for the SLIT arm are identical to those in the SCIT arm.
FIGURE 14.
Adult Markov model structure: SLIT arm. Note that the health states for the SLIT arm are identical to those in the SCIT arm.
FIGURE 15.
Adult Markov model structure: SCIT arm. [+], same structure as the first expanded structure directly above but with appropriate changes in probabilities.
FIGURE 16.
Adult Markov model structure: ST arm. [+], same structure as the first expanded structure directly above but with appropriate changes in probabilities.
Subcutaneous immunotherapy arm
The Markov process for the SCIT arm begins with the initial health state called ‘Start IT’ representing individuals with SAR eligible for treatment with SCIT. Following the ‘Start IT’ health state, the model then distinguishes between patients who, in addition to SCIT, would receive treatment to control all, or some, of their symptoms (symptoms controlled and symptoms part controlled), as well as patients who would not receive any ST at all as they are assumed to be symptom free (no ST).
In the second or third year, patients who complete the previous year of SCIT (with or without ST) may then continue with, or quit, SCIT. In total, patients can move to one of 10 possible health states. The first three represent variations of patients continuing with SCIT with or without ST (‘on IT no ST’, ‘on IT symptoms controlled’ or ‘on IT symptoms partly controlled’). The next three health states are for patients who have completed SCIT and then continue with or without ST (i.e. ‘complete IT no ST’, ‘complete IT symptoms controlled’ or ‘complete IT symptoms partly controlled’). Patients who remained on SCIT for 3 years move to one of these states at the end of the third year. Another three health states depict options for patients who continue with or without ST having quit SCIT in the first or second year (i.e. ‘Early quit IT no ST’, ‘Early quit IT symptoms controlled’ or ‘Early quit IT symptoms partly controlled’). The last health state is ‘Dead’. Moving on from nine of these 10 health states (excluding ‘Dead’), the model also allowed for a change in ST. For instance, given that an adult with AR completed treatment with SCIT in the first or second year and was not on any other treatment, what was the probability that they would add treatments to control all, or some, of their symptoms at some point within the following year?
The symptomatic treatment-only arm
The starting point for the Markov process in the ST-only arm is the health state called ‘Start Symptomatic Rx only’ representing individuals with SAR eligible for treatment with SCIT or SLIT, but only receiving treatment to relieve their AR symptoms. Following the ‘Start Symptomatic Rx only’ health state, the model then distinguishes between patients who continue with treatment to control all or some of their symptoms (symptoms controlled and symptoms part controlled) as well as patients who stop receiving any ST completely (no ST). In the second or third year, patients who survive the previous year with or without ST can then move to one of four possible health states: have no ST (No ST), have treatment to control all of their symptoms (Symptoms controlled), have treatment to control some of their symptoms (Symptoms partly controlled) or die (Dead).
Child Markov model
Methods of delivering SIT were similar to those in the adult model, but with appropriate adjustments in dosages. The resultant clinical pathways were also designed to mirror the natural progression of AR in the children with health resources use for all three groups also modelled. The goal for this model was also to enable model-based predictions of costs and outcomes to be compared for the SCIT, SLIT and ST groups in a CUA from the UK NHS and patient perspectives.
Child model structure and model inputs
The structure of the child Markov model is similar to that for adults except it incorporates asthma in all outcomes in order to account for the probability of developing the condition among children with AR (Figures 17–20). Only health states for the SCIT and ‘Symptomatic treatment’ arms are presented. The health states for the ‘SLIT’ arm are identical to those in the ‘SCIT’ arm.
FIGURE 17.
Child Markov model structure. Note that the health states for the SLIT arm are identical to those in the SCIT arm.
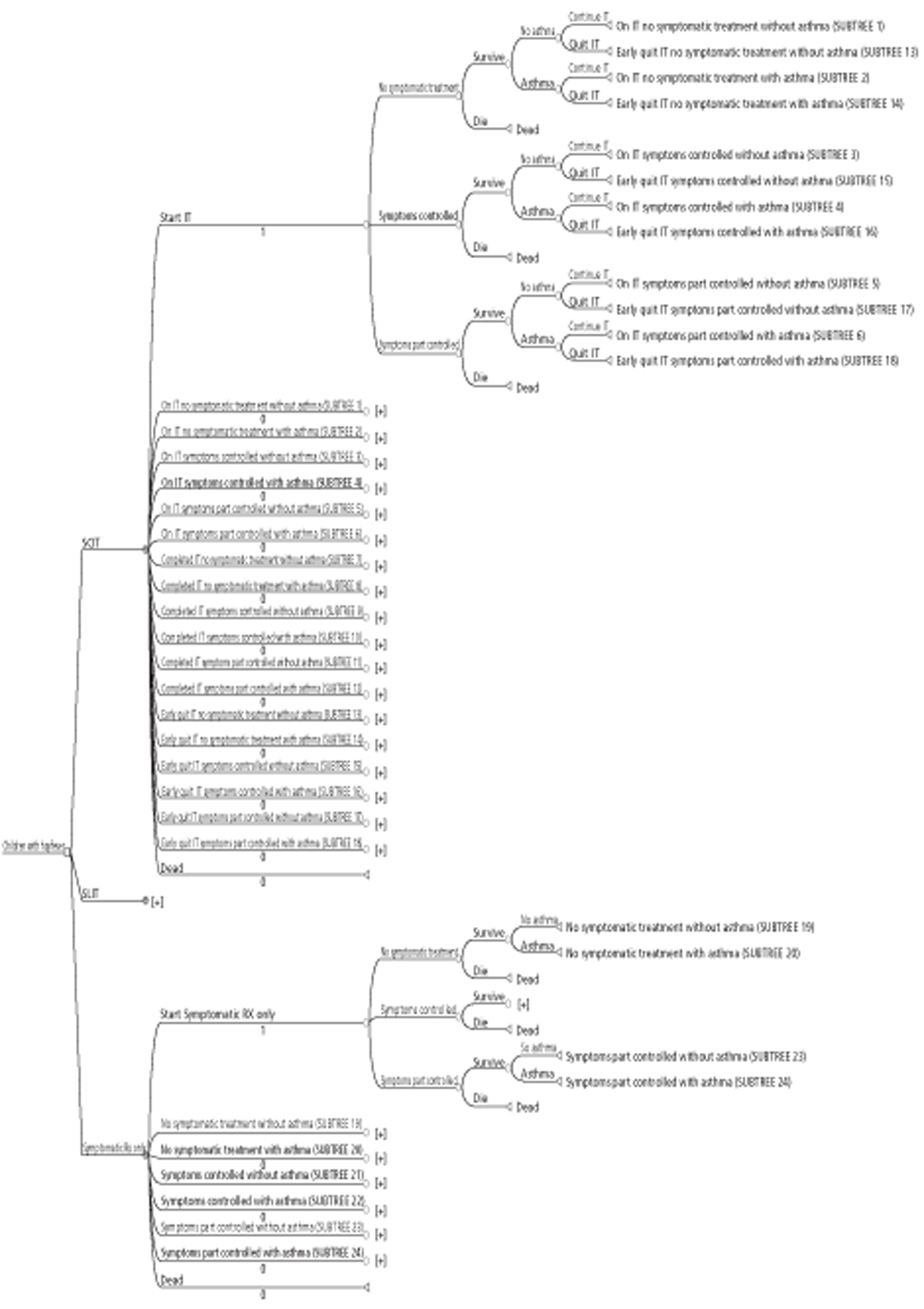
FIGURE 18.
Child Markov model structure: SCIT arm (subtrees 1–6). [+], same structure as the first expanded structure directly above but with appropriate changes in probabilities.
FIGURE 19.
Child Markov model structure: SCIT arm (subtrees 7–18). [+], same structure as the first expanded structure directly above but with appropriate changes in probabilities.
FIGURE 20.
Child Markov model structure: ST arm. [+], same structure as the first expanded structure directly above but with appropriate changes in probabilities.
Subcutaneous immunotherapy arm
The Markov process for the SCIT arm begins with the initial health state called ‘Start IT’, representing children with SAR who are eligible for treatment with SCIT. Following the ‘Start IT’ health state, the model then distinguishes between children who, in addition to SCIT, would receive treatment to control all, or some of their symptoms (symptoms controlled and symptoms part controlled), as well as children who would not receive any ST at all (no ST). In the second or third year, asthmatic and non-asthmatic children who complete the previous year following SCIT (with or without ST) may then continue with, or quit, SCIT. Therefore, patients can move to 1 of 19 possible health states. The first six represent variations of asthmatic and non-asthmatic children continuing with SCIT with or without ST (‘on IT no ST with asthma’, ‘on IT no ST without asthma’, ‘on IT symptoms controlled with asthma’, ‘on IT symptoms controlled without asthma’, ‘on IT symptoms partly controlled with asthma’ or ‘on IT symptoms partly controlled without asthma’). The next six present states for asthmatic and non-asthmatic children who complete SCIT and then continue with or without ST (i.e. ‘complete IT no ST with asthma’, ‘complete IT no ST without asthma’, ‘complete IT symptoms controlled with asthma’, ‘complete IT symptoms controlled without asthma’, ‘complete IT symptoms partly controlled with asthma’ or ‘complete IT symptoms partly controlled without asthma’). Patients who remained on SCIT for 3 years move to one of these states at the end of the third year. Another six health states depict options for asthmatic and non-asthmatic children who continue with or without ST having quit SCIT in the first or second year (i.e. ‘Early quit IT no ST with asthma’, ‘Early quit IT no ST without asthma’, ‘Early quit IT symptoms controlled with asthma’, ‘Early quit IT symptoms controlled without asthma’, ‘Early quit IT symptoms partly controlled with asthma’ or ‘Early quit IT symptoms partly controlled without asthma’). The last health state is ‘Dead’. Moving from 18 of these 19 health states (excluding ‘Dead’), the model also allowed for a change in ST. For instance, given that an asthmatic child with AR completed treatment with SCIT in the first or second year and was not on any other treatment, what was the probability that they would add treatments to control all or some of their symptoms at some point within the following year?
The symptomatic treatment-only arm
The starting point for the Markov process in the ST-only arm is the health state called ‘Start Symptomatic Rx only’, representing children with SAR who are eligible for treatment with SIT but who are receiving treatment to relieve only their AR symptoms. Following the ‘Start Symptomatic Rx only’ health state, the model then distinguishes between children who continue with treatment to control all or some of their symptoms (symptoms controlled and symptoms part controlled), as well as children who would not receive any ST at all (no ST). In the second or third year, both asthmatic and non-asthmatic patients who complete the previous year with or without ST can then move to one of seven possible health states. These include states for asthmatic children who have no ST (‘No ST with asthma’), non-asthmatic children who have no ST (‘No ST without asthma’), asthmatic children who have treatment to control all of their symptoms (‘Symptoms controlled with asthma’) and non-asthmatic children who have treatment to control all of their symptoms (‘Symptoms controlled without asthma’). The rest of health states are for asthmatic children who have treatment to control some of their symptoms (‘Symptoms partly controlled with asthma’), non-asthmatic children who have treatment to control some of their symptoms (‘Symptoms partly controlled without asthma’) and ‘Dead’.
Inputs for both the adult and child Markov models
To conduct long-term comparative cost-effectiveness analyses of SCIT and SLIT when used to treat AR, a number of parameters (costs, outcomes and probabilities) were to be identified from the systematic review of EEs, systematic review of clinical effectiveness and other relevant studies.
Resource use and costs
All costs were to be reported in UK pounds at 2011–12 unit prices and, where appropriate, discounted at 3.5% as recommended by the UK NICE. 249 Resource use data were to be obtained from studies reviewed, as well as from expert opinion. Unit cost data were to be derived from nationally representative sources, such as the BNF (2012),250 the National Schedule for Reference Costs and the Unit Costs of Health and Social Care (PSSRU). 251
Utility values
As the primary outcome was to be expressed in terms of costs per QALYs gained, studies were of interest if they reported utility scores to reflect the health-related QoL associated with each health state in the model.
Transition probabilities
To complete the process of populating the model, it is necessary to obtain transition probabilities governing movement between the different health states in both adult and child models from studies in this review. A full list of transition probabilities required for the two models is provided in Appendix 11.
Challenges met when identifying model inputs for the preferred Markov models
One purpose of conducting the systematic review was to identify data associated with IT interventions or that on health states within AR pathways that could be used to populate the preferred Markov models described above. Both model- and non-model-based studies were considered in this respect. In particular, four types of model inputs and information were sought:
-
UK costs associated with IT strategies. Where UK cost data were not available, other data from countries with similar or transferable characteristics were sought.
-
Utility-based outcome data associated with health states within AR pathways.
-
Markov model structures that could be populated with UK specific data to enable the modelling of long-term cost-effectiveness of different types of IT treatments. Where possible, data on health states (from both Markov and decision tree models) that could be adapted and used in the preferred Markov models.
-
Information on transition probabilities governing movements between health states in Markov and decision tree models.
It was not possible to identify much suitable data from the 16 included EEs and reviews. The reasons are discussed below by type of model input/information.
Cost data
Two studies228,231 were conducted using samples that included subjects from the UK, both of which compared SLIT with standard care. They presented detailed UK costs associated with AR, based on resource use data such as visits to the GP, allergy specialist, and A&E department. In addition, costs of symptomatic and asthma medication, as well as indirect costs associated with production loss, were also presented. The cost estimates reported in these studies lend themselves to replication in our model and we would therefore consider these estimates to be credible inputs to our model. Despite these cost estimates all being based on the GT–08 GRAZAX trial,93 it is likely that these results would be transferable to the UK generally. Other potentially useful cost estimates were identified in four other European studies. 225,226,230,234
Outcome measures
Over 15 different types of outcome measures have been reported in the studies evaluated.
As described above (see Quality of economic evaluations), the vast number of these outcomes were not expressed as utilities but rather as ‘natural’ outcomes, and, as such, cannot be used in the Markov model. These include the number of asthma and rhinitis exacerbations, number of hospital visits and absence from nursery or school, SSs and MSs or the RQLQ.
Some studies did report utility-based outcome measures that could be converted into QALYs. As outlined above (see Main characteristics of economic evaluations), these utility-based outcomes were based on EQ-5D for four of the studies228,230–232 and on an unspecified instrument for one study. 229 Studies whose QALYs were based on EQ-5D did not present EQ-5D data in sufficient detail to allow for their replication in our model. In particular, no baseline or follow-up values were reported in three of these studies,228,230,231 with only the final results being reported in terms of cost per QALY gained. In the Beriot-Mathiot et al. study,232 EQ-5D could be read off a diagram, but these were not disaggregated according to SLIT or ST. Brüggenjürgen et al. 229 reported utility scores for the following health states: mild AR (0.7579); moderate to severe AR (0.7378); severe AR and mild allergic asthma (0.7317); severe AR and moderate to severe allergic asthma (0.6985); no symptoms (0.7841); and death (0.0). Much higher utility scores were reported by Tamayama et al. 242 for similar AR health states: 0.96 (mild), 0.94 (moderate), 0.89 (severe) and 0.83 (severest). Provided that information on transition probabilities could have been obtained, these values could potentially have been used. In order to explore the possibility of using utility data based on a UK population, study authors from the GT–08 trial93 were contacted, but it was not possible to obtain the necessary data.
Model structure and transition probabilities
Only five studies224,226,227,229,235 were model-based studies (EEs or effectiveness studies): three226,227,235 were based on decision tree models, whereas the other two224,229 used Markov models.
Berto et al. 226 compared SLIT (with standard care) to standard care only, in a decision tree framework based on a sample of 2230 patients. A model structure was presented but the health states analysed are not the same or adaptable to those in our model. More importantly, information of transition probabilities depicting movements between health states is not presented in the study,226 making it impossible for the model to be replicated using UK cost data.
The second decision tree model-based evaluation235 was based on a cohort of 2000 patients and compared SCIT with ST. This study235 presented some potentially useful data on probabilities in terms of asthma symptoms: the proportion of patients with AR and asthma compared with those with AR only; patients with asthma symptoms compared with those without asthma symptoms following either symptomatic or specific IT treatment; proportion of patients who develop asthma compared with proportion who do not among patients previously without asthma; and the proportion of patients who stop having asthma symptoms compared with proportion who continue with the symptoms among patients previously with asthma. The target outcome was the number of additional patients free from asthma symptoms after 10 years. However, it was difficult to ascertain how robust these probabilities were as the sources were not described in detail. Changes in symptoms or severity of AR were not incorporated into this analysis.
The third evaluation based on a decision tree model226 compared SCIT, SLIT and ST for adults and children with AR and asthma. Some potentially important transition probabilities were also presented in this study. 226 However, most of these were based on expert opinion from a Delphi panel, whereas some information was obtained from an unpublished report. All epidemiological data (‘hypotheses’) for children were determined by the Delphi panel. For both children and adults, for example, the study227 gives proportions that help determine the following probabilities: having rhinitis only as opposed to having rhinitis and asthma; having moderate compared with having severe rhinitis; and having mild compared with moderate asthma, as well as the percentage decrease in ST associated with IT by type of allergy and by SCIT or SLIT. However, attempts to replicate the model using UK cost data are not possible, as not all of the probabilities required to populate the model are presented, for example the proportion of patients who were either asymptomatic, who had an improvement in symptoms or those for whom the response to IT was inadequate.
Brüggenjürgen et al. 229 presented an analysis based on a Markov model. This study229 was based on a hypothetical cohort of 2000 patients and compared SCIT in addition to ST to ST alone. An overview of the Markov model was given in the paper but the actual structure or the parameter estimates are not presented. This makes it difficult to assess the suitability of the model inputs for use in our model or for replicating the model using UK cost data.
The second Markov model-based study224 was a cost analysis comparing SCIT, SLIT and variations of SCIT with standard care. The structure of the model was presented in the paper showing various pathways taken by patients following different treatment options. The health states presented in this model are not the same as those in our preferred model, and, more importantly, there was a lack of detail on the transition probabilities governing movement between health states, which precluded attempts to replicate the model using UK cost data.
Suitable data on transition probabilities, i.e. proportion of patients moving between different health states or levels of disease severity depending on treatment, were also not identified in any of the clinical effectiveness studies reviewed for this report.
Summary
Detailed information was available on cost data. A number of studies (both UK and non-UK) reported direct and indirect costs in sufficient detail to allow for their replication in our model. In particular, data from Bachert et al. 228 and Nasser et al. 231 were useful, supplemented with those obtained from expert opinion. Furthermore, data on utility scores associated with some severity levels of AR were also available based on German229 and Japanese230 populations. It may therefore have been possible to adapt these data for use in our models.
The biggest challenge faced, however, was the lack of information on transition probabilities. Although some model structures were presented in sufficient detail in some studies,224,227 and utility data were provided in others,229 not enough information on transition probabilities was given in all of the studies to allow for model replication using UK cost data.
Economic evaluation of immunotherapy
Because of the challenges faced with obtaining model inputs for the preferred Markov models, it was decided that an alternative approach to estimating the cost-effectiveness of SCIT, SLIT and ST would be taken. A novel EE was conducted, with results expressed in terms of ICERs calculated as costs per unit improvement in RQLQ and costs per QALY gain.
Methods
Costs estimation
Total costs were estimated by combining resource use data on staff, medication, supplies and productivity loss with unit costs. Resource use data are reported in Table 42, and unit costs are shown in Table 43.
| Staff costs, consumables and productivity loss | Units | Source |
|---|---|---|
| Staff (hours per visit) | ||
| SLIT clinic visits | ||
| Consultant | 0.33a | Expert opinion |
| Band 8 nurse | 0.33a | Expert opinion |
| SCIT clinic visits | ||
| Consultant | 0.27b | Expert opinion |
| Band 8 nurse | 0.27b | Expert opinion |
| Supplies (no. of items per SCIT injection/visit) | ||
| Swabs | 1 | Expert opinion |
| Syringes | 1 | Expert opinion |
| Needles | 2 | Expert opinion |
| Gloves | 1 | Expert opinion |
| Medication | ||
| SLIT medication | ||
| Grazaxc | One tablet daily | BNF (2012)46 |
| SCIT medication | ||
| Alutardd | 10–100,000 units/ml | Personal communication |
| Symptomatic medicinee | ||
| Desloratadine | 5 mg/daily | BNF (2012)46 |
| Budesonide | 0.1–0.8 mg twice dailyf | BNF (2012)46 |
| Productivity loss (SLIT) | ||
| Hours missed from work | 2.12 | Nasser et al. (2007)231 |
| Hours at work with reduced productivity | 4.73 | Nasser et al. (2007)231 |
| Productivity loss (SCIT)g | ||
| Hours missed from work | 5.17 | Pokladnikova et al. (2008),225 Nasser et al. (2007)231 |
| Hours at work with reduced productivity | 9.89 | |
| Productivity loss (ST) | ||
| Hours missed from work | 6.27 | Nasser et al. (2007)231 |
| Hours at work with reduced productivity | 14.04 | Nasser et al. (2007)231 |
| Staff costs, consumables and productivity loss | Units | Source | |
|---|---|---|---|
| Staff (wage per hour) | |||
| Consultant | £137.00 | Curtis (2011)251 | |
| Band 8 nurse | £40.69a | Curtis (2011)251 | |
| Supplies (cost per item) | |||
| Swabs | £0.01 | Medisave (2011)252 | |
| Syringes | £0.10 | Medisave (2011)252 | |
| Needles | £0.03 | Medisave (2011)252 | |
| Gloves | £0.03 | Medisave (2011)252 | |
| Medication | |||
| SLIT medication | |||
| Grazax | £2.23 per tablet | BNF (2012)46 | |
| SCIT medication | |||
| Alutard | £61.97–93.82/mlb | Manufacturer communication | |
| Symptomatic medicine | |||
| Desloratadine | £0.02/mg | BNF (2012)46 | |
| Budesonide | £0.44/mg | BNF (2012)46 | |
| Productivity loss | |||
| Average wage/hour | £9.62c | Office for National Statistics (2011)253 | |
Resource use for the sublingual immunotherapy group
The number of clinic visits for patients receiving SLIT was assumed to be 13 over a 3-year period, with an initial visit before commencing SLIT, followed by four outpatient visits per year (A Huissoon, Birmingham Heartlands Hospital, 9 March 2012, personal communication). Each visit for SLIT was assumed to last 20 minutes (0.33 hours) and patients would be seen by a consultant and a grade 8 nurse. Estimates of SLIT medication were based on Grazax, with one tablet taken daily throughout the year. 46 Over a 3-year period, the total number of tablets taken was 1095. Information on doses for two STs [desloratadine (Neoclarityn®, Schering-Plough) and budesonide (Budelin Novolizer®, Meda)] was obtained from the BNF (2012);46 the dose of desloratadine was 5 mg daily, whereas 0.1–0.8 mg of budesonide was given twice daily. In line with the BSACI guidelines2 (2008), ST was assumed to be administered for 3.5 months per year (2 weeks before, and then during the EPS). Estimates of productivity loss due to hours missed from work and hours at work with reduced productivity were obtained from a trial231 that assessed the cost-effectiveness of Grazax in the UK.
Resource use for the subcutaneous immunotherapy group
Subcutaneous immunotherapy medication costs were based on Alutard. Although Pollinex is the only licensed product in the UK, treatment using Alutard is more representative of clinical practice in the UK (A Huissoon, personal communication). Pollinex is a preseasonal treatment consisting of six injections each year for 3 years, whereas Alutard is given throughout the year. After an updosing period (weekly injection for 12 weeks, followed by one injection after 2 weeks), maintenance injections are given once every 4 weeks. Thus, the total number of injections with Alutard over a 3-year period was assumed to be 46, i.e. 20 in year 1, and 13 in both years 2 and 3. Treatment with Alutard using 6-weekly, rather than 4-weekly, maintenance injections was also explored in an additional analysis. A clinic visit to receive an injection was assumed to last 0.27 hours, on the basis that a typical clinic, staffed by a consultant and a grade 8 nurse, would last about 4 hours, during which time 15 patients would be seen (A Huissoon, personal communication). Supplies used during each clinic visit were assumed to be one swab, one syringe, two hypodermic needles and a pair of gloves for each patient. Proportional differences in doses of symptomatic medication between SCIT and SLIT were taken from the study by Pokladnikova et al. ,225 which assessed the comparative cost-effectiveness of SCIT and SLIT; these proportions were 204/241 (SCIT/SLIT). Estimates of productivity loss for SCIT and SLIT were calculated based on data in the Pokladnikova et al. study225 and the Nasser et al. study231 Hours missed from work and hours at work with reduced productivity for the SCIT group were 14.03/5.75 and 16.25/7.77 those for SLIT, respectively.
Resource use for the symptomatic treatment group
The only resources considered in this group were symptomatic medication and estimates of productivity loss. Proportional differences in symptomatic medication doses between SLIT and ST observed in Nasser et al. 231 were used in estimating incremental doses for SLIT and ST. Desloratadine and budesonide doses for ST were therefore assumed to be 15.88/13.22 and 22.43/15.68 those of SLIT, respectively. The estimates of productivity loss due to hours missed from work and hours at work with reduced productivity were obtained from Nasser et al. 231
Unit costs for the sublingual immunotherapy, subcutaneous immunotherapy and symptomatic treatment groups
These are shown in Table 43. Hourly wages for consultants (£137) and averaged hourly wages for band 8 nurses (£40.69) were obtained from Curtis. 251 The costs of supplies (swabs, syringes, needles and gloves) were estimated from a commercial website. 252 Drug costs for SLIT and symptomatic medications were based on the BNF (2012)46 or were obtained from the manufacturer of Alutard (A Young, ALK-Abelló, 29 March 2012, personal communication). The opportunity costs associated with productivity loss were estimated as the gross hourly rate for the UK in 2011 minus tax, pension and national insurance contributions valued at 35% of the average gross rate. 254 As this hourly rate was £14.82 (Office for National Statistics253), the opportunity cost associated with productivity loss was assumed to be £9.62.
Outcome estimation
Two outcomes were assessed in this EE: the RQLQ and QALYs.
Rhinoconjunctivitis Quality of Life Questionnaire
Two sets of mean RQLQ changes (and associated CIs) were used in this model. The first set was based on meta-analyses of SLIT compared with ST, and SCIT compared with ST (direct comparisons; see Chapter 3, Results). The second set was based on results from the ICMR (SLIT compared with ST, SCIT compared with ST and SCIT compared with SLIT (see Chapter 3, Indirect comparison subcutaneous immunotherapy versus sublingual immunotherapy).
Quality-adjusted life-years
These were based on changes in the EQ-5D, with an assumption that the EQ-5D changes applied to a 3-month period during the pollen season. To calculate EQ-5D, a mapping algorithm was used to convert mean RQLQ changes associated with either SCIT or SLIT to mean EQ-5D changes. The most appropriate method of developing a mapping equation between two measures is to apply both measures to a common group of patients. As no data set based on such a comparison was available, nor the result of any mapping exercise based on such a comparison, it was necessary to make a number of assumptions in calculating the EQ-5D values. The RQLQ scale is from 0 (best) to 6 (worst). It is assumed that the top end of the scale maps to the EQ-5D state representing no problems in any of the five dimensions. By definition, this state has a QoL score of ‘1’. The bottom end of the RQLQ scale was mapped to the EQ-5D state representing maximum problems with usual activities, pain/discomfort and anxiety/depression, but no problems with mobility or self-care, which are assumed to be unaffected by SAR. This state has a QoL score of −0.07 on the standard UK tariff. Therefore, it was assumed that going from worst to best was a six-point reduction in RQLQ and a 1.07-point increase in the EQ-5D score. As a result, each unit decrease (improvement) in RQLQ was assumed to map to a 0.178-point increase in QoL score (assuming that a unit decrease has the same value at all points on the scale).
Incremental cost-effectiveness ratio calculation
The generic formula for an ICER255 was used to calculate cost-effectiveness estimates. For an outcome based on costs per QALY in a comparison between SCIT and ST, for example, the formula would be given by:
Discounting
All costs are reported in UK pounds at 2010–11 unit prices. All future costs and outcomes, where appropriate, were discounted at 3.5% as recommended by NICE. 249
Duration of analysis
Based on expert clinical opinion and observations from clinical trials (the GT–08 trial93), it was assumed that 3 years of IT would result in a sustained effect of a further 3 years. Therefore, a 6-year time horizon was used in the base-case analysis of this study. 93 For illustration purposes, however, a time horizon of up to 10 years (7 years post IT) was also considered. Costs included from years 4 to 10 were only those associated with ST and productivity loss. As SLIT and SCIT medication was assumed to be given for only 3 years, only 3-year cumulative costs were incorporated in the analysis of costs beyond 3 years.
Results
Costs
The annual and cumulative costs associated with SLIT, SCIT and ST are shown in Tables 44–46. The annual costs of SCIT were higher than those for either SLIT or ST. Over a 3-year period, the cumulative costs for SCIT were £2869 and £5537 higher than those for SLIT and ST, respectively. The major cost drivers for the SCIT group were staff and SCIT medication costs; this was the same in the SLIT group, although not to the same extent as for SCIT. Costs associated with productivity loss were highest in the ST group (about 35% and 30% higher than for the SCIT and SLIT groups, respectively).
| Staff costs, consumables and productivity loss | Costs | ||
|---|---|---|---|
| Year 1 | Year 2a | Year 3a | |
| Staff during clinic visits | |||
| Consultant | £226.05 | £174.72 | £168.82 |
| Band 8 nurse | £67.14 | £51.89 | £50.14 |
| SLIT medication | |||
| Grazax | £813.95 | £786.42 | £759.83 |
| Symptomatic medicine | |||
| Desloratadine | £20.44 | £19.75 | £19.08 |
| Budesonide | £35.43 | £34.24 | £33.08 |
| Productivity loss | |||
| Hours missed from work | £20.39 | £19.70 | £19.04 |
| Hours at work with reduced productivity | £45.50 | £43.96 | £42.48 |
| Total annual costs | £1228.91 | £1130.70 | £1092.46 |
| Total cumulative costs | £1228.91 | £2359.61 | £3452.07 |
| Staff costs, consumables and productivity loss | Costs | ||
|---|---|---|---|
| Year 1 | Year 2a | Year 3a | |
| Staff during clinic visits | |||
| Consultant | £739.80 | £464.61 | £448.90 |
| Band 8 nurse | £219.73 | £137.99 | £133.33 |
| Supplies/consumablesb | £4.00 | £2.51 | £2.43 |
| SCIT medication | |||
| Allergy medicine | |||
| Alutard | £1239.46 | £1178.42 | £1138.57 |
| Symptomatic medicine | |||
| Desloratadine | £24.15 | £23.33 | £22.54 |
| Budesonide | £41.86 | £40.45 | £39.08 |
| Productivity loss | |||
| Hours missed from work | £49.74 | £48.05 | £46.43 |
| Hours at work with reduced productivity | £95.14 | £91.92 | £88.82 |
| Total annual costs | £2413.88 | £1987.29 | £1920.08 |
| Total cumulative costs | £2413.88 | £4401.16 | £6321.25 |
| Consumables and productivity loss | Costs | ||
|---|---|---|---|
| Year 1 | Year 2a | Year 3a | |
| Medication | |||
| Symptomatic medicine | |||
| Desloratadine | £24.55 | £23.72 | £22.92 |
| Budesonide | £50.68 | £48.97 | £47.31 |
| Productivity loss | |||
| Hours missed from work | £60.32 | £58.28 | £56.31 |
| Hours at work with reduced productivity | £134.78 | £130.22 | £125.82 |
| Total annual costs | £270.33 | £261.19 | £252.35 |
| Total cumulative costs | £270.33 | £531.52 | £783.87 |
A similar hierarchy of costs could be observed when costs were extrapolated from 3 years to a time horizon of between 4 and 10 years (Tables 47–49). However, the differences between SCIT and ST and between SLIT and ST reduced gradually with time, whereas those between SCIT and SLIT increased.
| Staff costs, consumables and productivity loss a,b | Costs | ||||||
|---|---|---|---|---|---|---|---|
| Year 4 | Year 5 | Year 6 | Year 7 | Year 8 | Year 9 | Year 10 | |
| Staff during clinic visits | |||||||
| Consultant | – | – | – | – | – | – | – |
| Band 8 nurse | – | – | – | – | – | – | – |
| SLIT medication | |||||||
| Grazax | – | – | – | – | – | – | – |
| Symptomatic medicine | |||||||
| Desloratadine | £18.44 | £17.81 | £17.21 | £16.63 | £16.07 | £15.52 | £15.00 |
| Budesonide | £31.96 | £30.88 | £29.83 | £28.83 | £27.85 | £26.91 | £26.00 |
| Productivity loss | |||||||
| Hours missed from work | £18.39 | £17.77 | £17.17 | £16.59 | £16.03 | £15.49 | £14.96 |
| Hours at work with reduced productivity | £41.04 | £39.65 | £38.31 | £37.02 | £35.76 | £34.56 | £33.39 |
| Total annual costs | £109.83 | £106.12 | £102.53 | £99.06 | £95.71 | £92.48 | £89.35 |
| Total cumulative costs b | £3561.91 | £3668.02 | £3770.55 | £3869.61 | £3965.33 | £4057.80 | £4147.15 |
| Staff costs, consumables and productivity loss | Costs | ||||||
|---|---|---|---|---|---|---|---|
| Year 4 | Year 5 | Year 6 | Year 7 | Year 8 | Year 9 | Year 10 | |
| Staff during clinic visits | |||||||
| Consultant | – | – | – | – | – | – | – |
| Band 8 nurse | – | – | – | – | – | – | – |
| SCIT medication | |||||||
| Alutard | – | – | – | – | – | – | – |
| Symptomatic medicine | |||||||
| Desloratadine | £21.78 | £21.05 | £20.33 | £19.65 | £18.98 | £18.34 | £17.72 |
| Budesonide | £37.76 | £36.48 | £35.25 | £34.06 | £32.90 | £31.79 | £30.72 |
| Productivity loss | |||||||
| Hours missed from work | £44.86 | £43.34 | £41.88 | £40.46 | £39.09 | £37.77 | £36.49 |
| Hours at work with reduced productivity | £85.81 | £82.91 | £80.11 | £77.40 | £74.78 | £72.25 | £69.81 |
| Total annual costs | £190.21 | £183.78 | £177.56 | £171.56 | £165.76 | £160.15 | £154.74 |
| Total cumulative costsa,b | £6511.46 | £6695.24 | £6872.80 | £7044.36 | £7210.12 | £7370.27 | £7525.01 |
| Consumables and productivity loss | Costs | ||||||
|---|---|---|---|---|---|---|---|
| Year 4 | Year 5 | Year 6 | Year 7 | Year 8 | Year 9 | Year 10 | |
| Medication | |||||||
| Symptomatic medicine | |||||||
| Desloratadine | £22.15 | £21.40 | £20.67 | £19.97 | £19.30 | £18.65 | £18.02 |
| Budesonide | £45.71 | £44.17 | £42.67 | £41.23 | £39.84 | £38.49 | £37.19 |
| Productivity loss | |||||||
| Hours missed from work | £54.40 | £52.56 | £50.79 | £49.07 | £47.41 | £45.81 | £44.26 |
| Hours at work with reduced productivity | £121.56 | £117.45 | £113.48 | £109.64 | £105.93 | £102.35 | £98.89 |
| Total annual costs | £243.82 | £235.58 | £227.61 | £219.91 | £212.48 | £205.29 | £198.35 |
| Total cumulative costsa,b | £1027.69 | £1263.27 | £1490.88 | £1710.79 | £1923.26 | £2128.55 | £2326.90 |
Cost-effectiveness results
Results based on both direct comparisons and indirect comparisons of the difference in RQLQ between the three groups are presented below.
Direct comparisons
As shown in Table 50, the mean change in RQLQ for the comparison between SLIT and ST was 0.340 (95% CI 0.18 to 0.49) in favour of SLIT. Based on a cost difference between the two groups of £2668, an ICER of £7848 per unit improvement in RQLQ was obtained. Table 50 also shows that between 3 and 10 years after the start of IT, the cost difference between SLIT and ST reduces from £2668 to £1820. Over the same period, QALY gains favouring SLIT increase from 0.0440 to 0.1305. The ICERs based on QALYs gained over this period therefore reduce from £60,704 to £13,951. An ICER of £27,269 was obtained 6 years after the start of IT. This reduction in costs per QALY gained is shown graphically in Figure 21.
| Cost difference (£) at | |
| 3 years | 2668 |
| 4 years | 2534 |
| 5 years | 2405 |
| 6 years | 2280 |
| 7 years | 2159 |
| 8 years | 2042 |
| 9 years | 1929 |
| 10 years | 1820 |
| RQLQ difference | 0.340 (95% CI 0.180 to 0.490) |
| ICER (£): costs/unit improvement in RQLQ | 7848 (95% CI 5445 to 14,823) |
| Total QALY gain in 3 years | 0.0440 (95% CI 0.0233 to 0.0633) |
| ICER (£): costs per QALY | 60,704 (95% CI 42,121 to 114,663) |
| Total QALY gain in 4 years | 0.0576 (95% CI 0.0305 to 0.0830) |
| ICER (£): costs per QALY | 43,977 (95% CI 30,514 to 83,067) |
| Total QALY gain in 5 years | 0.0708 (95% CI 0.0305 to 0.0830) |
| ICER (£): costs per QALY | 33,948 (95% CI 23,556 to 64,124) |
| Total QALY gain in 6 years | 0.0836 (95% CI 0.0443 to 0.1205) |
| ICER (£): costs per QALY | 27,269 (95% CI 18,921 to 51,508) |
| Total QALY gain in 7 years | 0.0959 (95% CI 0.0508 to 0.1383) |
| ICER (£): costs per QALY | 22,504 (95% CI 15,615 to 42,508) |
| Total QALY gain in 8 years | 0.1078 (95% CI 0.0571 to 0.1554) |
| ICER (£): costs per QALY | 18,935 (95% CI 13,139 to 35,767) |
| Total QALY gain in 9 years | 0.1194 (95% CI 0.0632 to 0.1720) |
| ICER (£): costs per QALY | 16,164 (95% CI 11,216 to 30,532) |
| Total QALY gain in 10 years | 0.1305 (95% CI 0.0691 to 0.1880) |
| ICER (£): costs per QALY | 13,951 (95% CI 9680 to 26,351) |
FIGURE 21.
Cost-effectiveness of SLIT vs ST (direct comparisons).
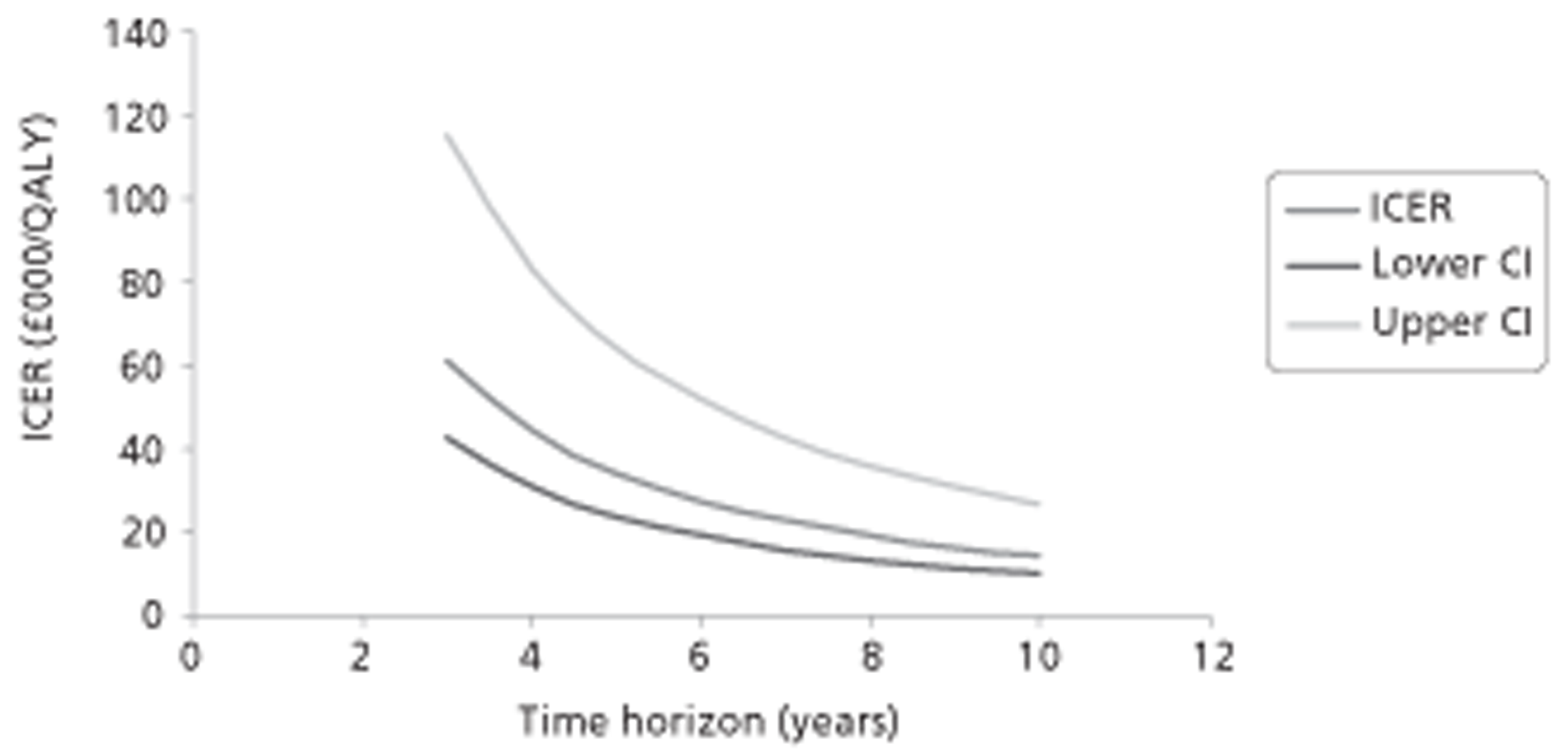
Table 51 shows the results comparing SCIT with ST. The mean change in RQLQ in favour of SCIT was 0.740 (95% CI 0.56 to 0.92) and this resulted in an ICER of £7483 per unit improvement in RQLQ (based on a cost difference of £5537). Between 3 and 10 years after starting IT, the cost difference between SCIT and ST reduced from £5537 to £5198, whereas the QALY gains in favour of SCIT increased from 0.0957 to 0.2840. Consequently, the cost per QALY gained between the same time period reduced from £57,883 to £18,304 as shown in Figure 22. The value of this ICER was £29,579 6 years after the start of IT.
| Cost difference (£) at | |
| 3 years | 5537 |
| 4 years | 5484 |
| 5 years | 5432 |
| 6 years | 5382 |
| 7 years | 5334 |
| 8 years | 5287 |
| 9 years | 5242 |
| 10 years | 5198 |
| RQLQ difference | 0.740 (95% CI 0.560 to 0.920) |
| ICER (£): costs/unit improvement in RQLQ | 7483 (95% CI 6019 to 9889) |
| Total QALY gain in 3 years | 0.0957 (95% CI 0.0724 to 0.1189) |
| ICER (£): costs per QALY | 57,883 (95% CI 46,558 to 76,488) |
| Total QALY gain in 4 years | 0.1254 (95% CI 0.0949 to 0.1559) |
| ICER (£): costs per QALY | 43,722 (95% CI 35,168 to 57,776) |
| Total QALY gain in 5 years | 0.1542 (95% CI 0.1167 to 0.1917) |
| ICER (£): costs per QALY | 35,233 (95% CI 28,340 to 46,558) |
| Total QALY gain in 6 years | 0.1820 (95% CI 0.1377 to 0.2262) |
| ICER (£): costs per QALY | 29,579 (95% CI 23,792 to 39,087) |
| Total QALY gain in 7 years | 0.2088 (95% CI 0.1580 to 0.2596) |
| ICER (£): costs per QALY | 25,545 (95% CI 20,547 to 33,756) |
| Total QALY gain in 8 years | 0.2347 (95% CI 0.1776 to 0.2918) |
| ICER (£): costs per QALY | 22,524 (95% CI 18,117 to 29,764) |
| Total QALY gain in 9 years | 0.2598 (95% CI 0.1966 to 0.3230) |
| ICER (£): costs per QALY | 20,178 (95% CI 16,230 to 26,664) |
| Total QALY gain in 10 years | 0.2840 (95% CI 0.2149 to 0.3531) |
| ICER (£): costs per QALY | 18,304 (95% CI 14,723 to 24,188) |
FIGURE 22.
Cost-effectiveness of SCIT vs ST (direct comparisons).
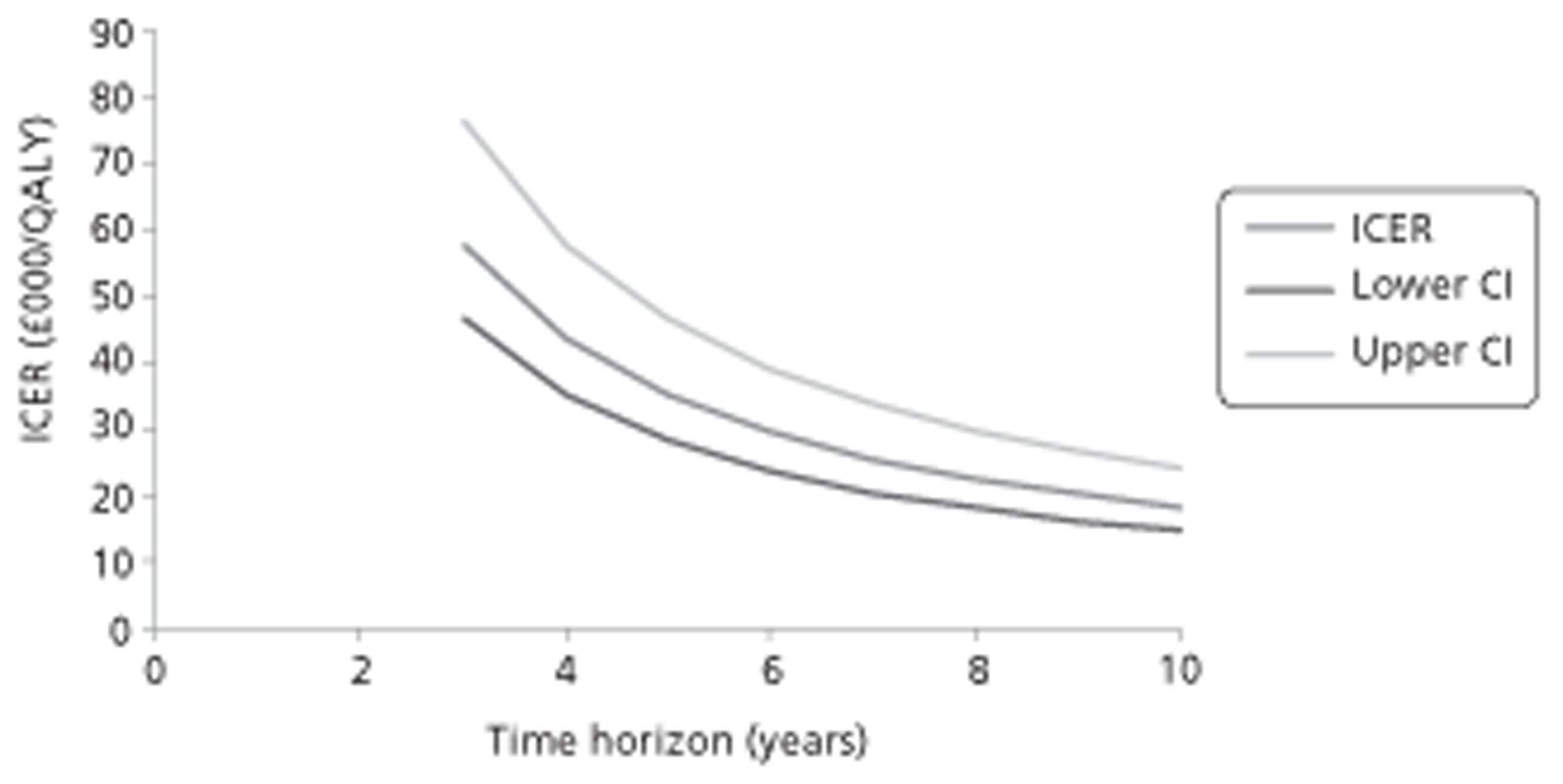
Indirect comparisons
Table 52 presents the results of the comparison between SLIT and ST. The mean change in RQLQ favouring SLIT was 0.247 (95% CrI −0.156 to 0.729) and this resulted in an ICER of £10,802 per unit improvement in RQLQ (based on a cost difference of £2668). The cost differences between the two groups reduced from £2668 to £1820 over a period of 3–10 years post commencement of IT. As in the direct comparison, the QALY gains favouring SLIT increased from 0.0319 to 0.0948 over the same period. This resulted in a reduction in the costs per QALY gained from £83,560 to £19,203 over the same period of time as depicted in Figure 23. The value of the ICER 6 years after the start of IT was £37,537.
| Cost difference (£) at | |
| 3 years | 2668 |
| 4 years | 2534 |
| 5 years | 2405 |
| 6 years | 2280 |
| 7 years | 2159 |
| 8 years | 2042 |
| 9 years | 1929 |
| 10 years | 1820 |
| RQLQ difference | 0.247 (95% CI −0.1560 to 0.729) |
| ICER (£): costs/unit improvement in RQLQ | 10,802 (3660 to ST dominates) |
| Total QALY gain in 3 years | 0.0319 (95% CI −0.0202 to 0.0942) |
| ICER (£): costs per QALY | 83,560 (28,312 to ST dominates) |
| Total QALY gain in 4 years | 0.0419 (95% CI −0.0264 to 0.1236) |
| ICER (£): costs per QALY | 60,535 (20,510 to ST dominates) |
| Total QALY gain in 5 years | 0.0515 (95% CI −0.0325 to 0.1519) |
| ICER (£): costs per QALY | 46,730 (15,833 to ST dominates) |
| Total QALY gain in 6 years | 0.0607 (95% CI −0.0384 to 0.1792) |
| ICER (£): costs per QALY | 37,537 (12,718 to ST dominates) |
| Total QALY gain in 7 years | 0.0697 (95% CI −0.0440 to 0.2057) |
| ICER (£): costs per QALY | 30,977 (10,496 to ST dominates) |
| Total QALY gain in 8 years | 0.0783 (95% CI −0.0495 to 0.2312) |
| ICER (£): costs per QALY | 26,065 (8831 to ST dominates) |
| Total QALY gain in 9 years | 0.0867 (95% CI −0.0548 to 0.2559) |
| ICER (£): costs per QALY | 22,250 (7539 to ST dominates) |
| Total QALY gain in 10 years | 0.0948 (95% CI −0.0599 to 0.2798) |
| ICER (£): costs per QALY | 19,203 (6506 to ST dominates) |
FIGURE 23.
Cost-effectiveness of SLIT vs ST (indirect comparisons).
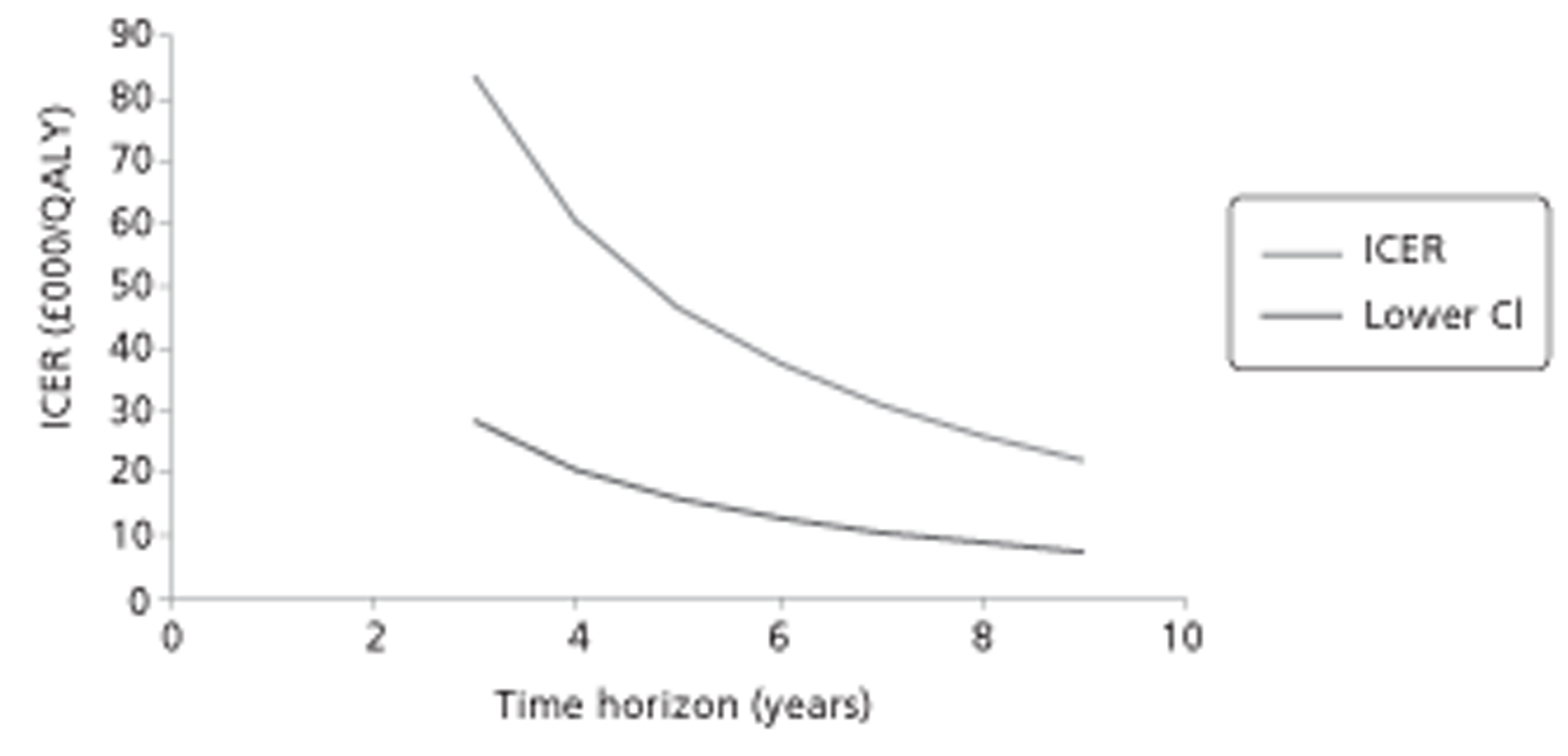
In the comparison between SCIT and ST (Table 53), the mean change in RQLQ favouring SCIT was 0.764 (95% CrI 0.425 to 1.116). Based on a cost difference of £5537, an ICER of £7248 per unit improvement in RQLQ was found. As in the direct comparisons, the cost difference between the two groups reduced over a period of 8 years (3–10 years after the start of IT) from £5537 to £5198. The QALY gains in favour of SCIT increased from 0.0988 to 0.2932 over the same period. As depicted in Figure 24, the resultant ICERs therefore decreased from £56,064 to £17,729 over this period. The ICER, 6 years after the start of IT, was £28,650 per QALY gained.
| Cost difference (£) at | |
| 3 years | 5537 |
| 4 years | 5484 |
| 5 years | 5432 |
| 6 years | 5382 |
| 7 years | 5334 |
| 8 years | 5287 |
| 9 years | 5242 |
| 10 years | 5198 |
| RQLQ difference | 0.764 (95% CI 0.425 to 1.116) |
| ICER (£): costs/unit improvement in RQLQ | 7248 (95% CI 4962 to 13,029) |
| Total QALY gain in 3 years | 0.0988 (95% CI 0.0549 to 0.1443) |
| ICER (£): costs per QALY | 56,064 (95% CI 38,381 to 100,784) |
| Total QALY gain in 4 years | 0.1295 (95% CI 0.0720 to 0.1892) |
| ICER (£): costs per QALY | 42,349 (95% CI 28,992 to 76,128) |
| Total QALY gain in 5 years | 0.1592 (95% CI 0.0885 to 0.2325) |
| ICER (£): costs per QALY | 34,126 (95% CI 23,362 to 61,347) |
| Total QALY gain in 6 years | 0.1879 (95% CI 0.1045 to 0.2744) |
| ICER (£): costs per QALY | 28,650 (95% CI 19,613 to 51,502) |
| Total QALY gain in 7 years | 0.2156 (95% CI 0.1199 to 0.3149) |
| ICER (£): costs per QALY | 24,743 (95% CI 16,939 to 44,479) |
| Total QALY gain in 8 years | 0.2423 (95% CI 0.1348 to 0.3540) |
| ICER (£): costs per QALY | 21,816 (95% CI 14,935 to 39,218) |
| Total QALY gain in 9 years | 0.2682 (95% CI 0.1492 to 0.3918) |
| ICER (£): costs per QALY | 19,544 (95% CI 13,380 to 35,133) |
| Total QALY gain in 10 years | 0.2932 (95% CI 0.1631 to 0.4283) |
| ICER (£): costs per QALY | 17,729 (95% CI 12,137 to 31,871) |
FIGURE 24.
Cost-effectiveness of SCIT vs ST (indirect comparisons).
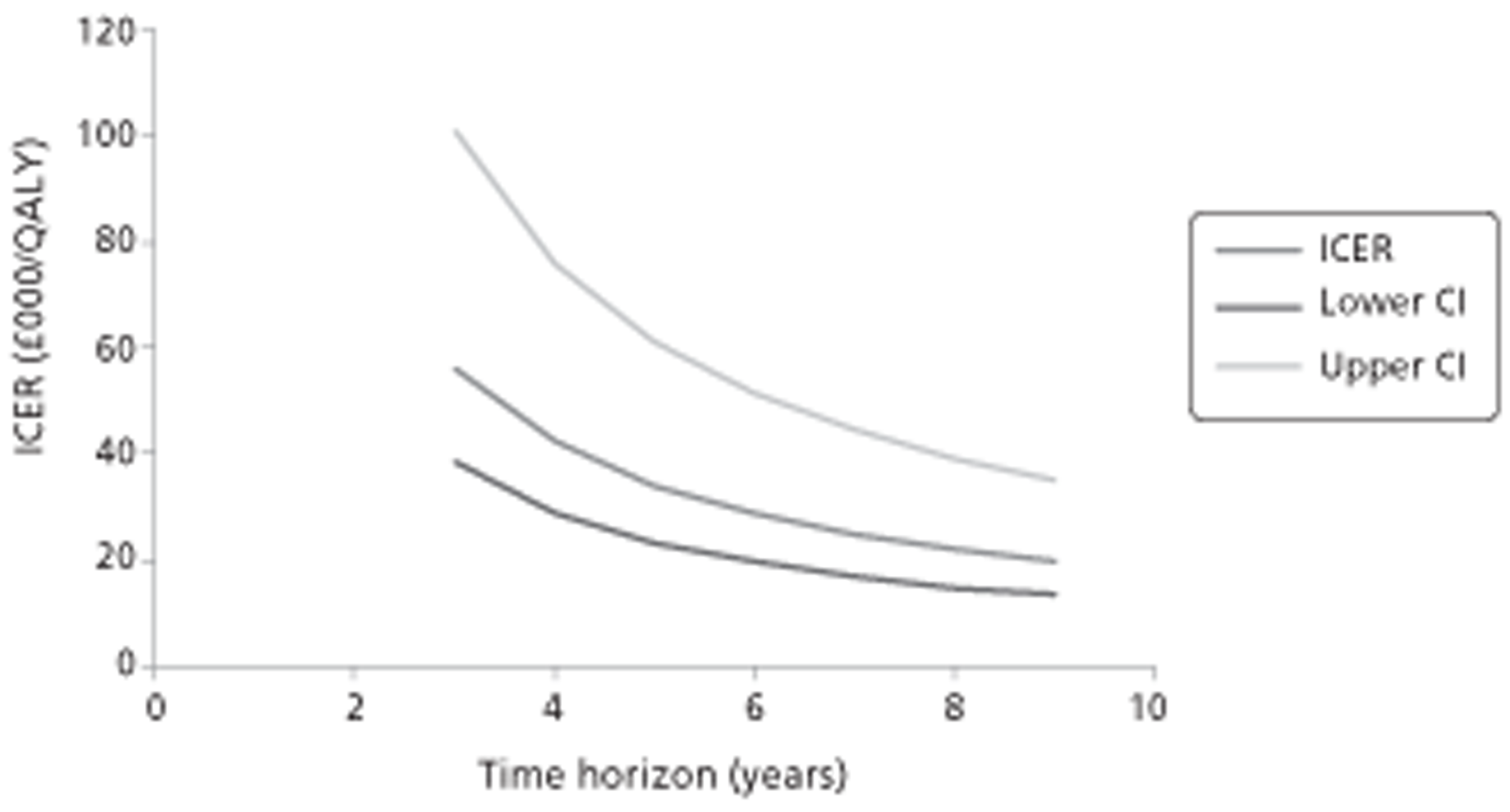
The last comparison was between SCIT and SLIT (Table 54). The mean change in RQLQ (favouring SCIT) was 0.517 (95% CrI −0.0710 to 1.045) resulting in an ICER of £5550 per unit improvement in RQLQ, based on a cost difference of £2869. The cost differences between the two groups reduced from £2869 to £3378 over a period of 3–10 years following commencement of IT. QALY gains favouring SCIT increased from 0.0668 to 0.1984 over the same period. This resulted in a reduction in the costs per QALY gained from £42,928 to £17,025 over the same period of time as depicted in Figure 25. The value of the ICER, 6 years after the start of IT, was £24,404.
| Cost difference (£) at | |
| 3 years | 2869 |
| 4 years | 2950 |
| 5 years | 3027 |
| 6 years | 3102 |
| 7 years | 3175 |
| 8 years | 3245 |
| 9 years | 3312 |
| 10 years | 3378 |
| RQLQ difference | 0.517 (95% CI −0.0710 to 1.045) |
| ICER (£): costs/unit improvement in RQLQ | 5550 (2746 to SLIT dominates) |
| Total QALY gain in 3 years | 0.0668 (95% CI −0.0092 to 0.1351) |
| ICER (£): costs per QALY | 42,928 (21,238 to SLIT dominates) |
| Total QALY gain in 4 years | 0.0876 (95% CI −0.0120 to 0.1771) |
| ICER (£): costs per QALY | 33,661 (16,653 to SLIT dominates) |
| Total QALY gain in 5 years | 0.1077 (95% CI −0.0148 to 0.2177) |
| ICER (£): costs per QALY | 28,105 (13,904 to SLIT dominates) |
| Total QALY gain in 6 years | 0.1271 (95% CI −0.0175 to 0.2569) |
| ICER (£): costs per QALY | 24,404 (12,074 to SLIT dominates) |
| Total QALY gain in 7 years | 0.1459 (95% CI −0.0200 to 0.2948) |
| ICER (£): costs per QALY | 21,764 (10,768 to SLIT dominates) |
| Total QALY gain in 8 years | 0.1640 (95% CI −0.0225 to 0.3315) |
| ICER (£): costs per QALY | 19,787 (9789 to SLIT dominates) |
| Total QALY gain in 9 years | 0.1815 (95% CI −0.0249 to 0.3668) |
| ICER (£): costs per QALY | 18,251 (9030 to SLIT dominates) |
| Total QALY gain in 10 years | 0.1984 (95% CI −0.0272 to 0.4010) |
| ICER (£): costs per QALY | 17,025 (8423 to SLIT dominates) |
FIGURE 25.
Cost-effectiveness of SCIT vs SLIT.
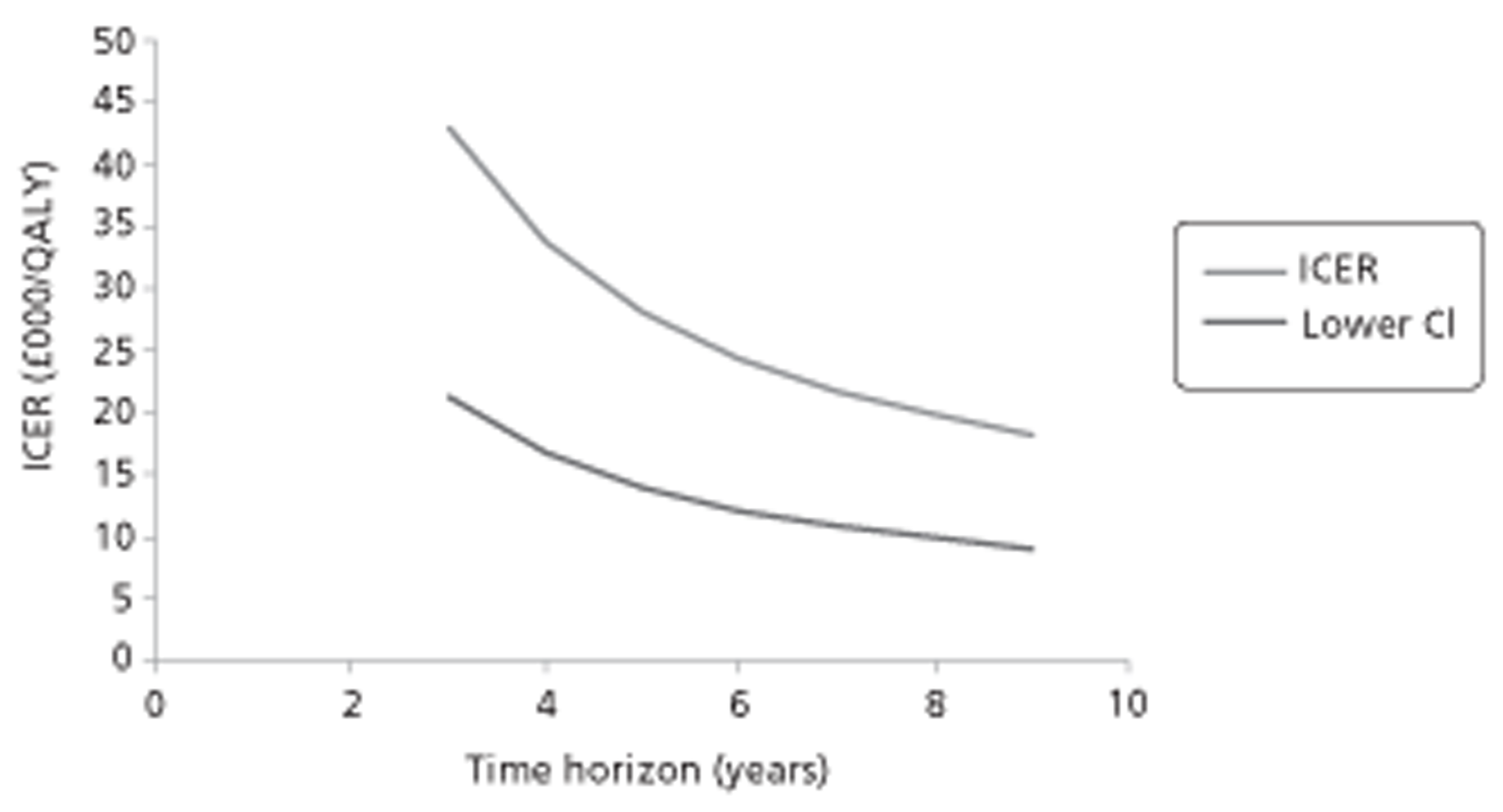
Six-weekly treatment schedule
Using a 6-weekly maintenance schedule instead of a 4-weekly one resulted in lower costs (fewer clinic visits and fewer injections) (Table 55). The effectiveness was assumed to be the same, therefore the cost-effectiveness was increased. The differences in ICERs (at 6 years) between the two maintenance schedules are shown below (full details are shown in Appendix 12).
| Comparison | Alutard maintenance | |
|---|---|---|
| 4-weekly | 6-weekly | |
| SCIT vs ST (direct comparisons) | £29,579 | £21,599 |
| SCIT vs ST (indirect comparisons) | £28,650 | £20,920 |
| SCIT vs SLIT | £24,404 | £12,982 |
NHS perspective only
Costs were considered from the NHS and patient perspectives because of the burden that AR places on both the NHS and patients. For purposes of reimbursement, however, only NHS costs are important. We therefore also conducted a CEA (results not shown) based on NHS costs only, and the results were not altered significantly. IT (when compared with ST) was found to be cost-effective around 7 years after the start of treatment (1 year later than when productivity costs are included). The only exception was the comparison between SLIT and ST based on indirect comparison results where this threshold increased to more than 10 years after the start of IT. In the comparison between SCIT and SLIT, SCIT was shown to be more cost-effective as early as 4 years after the start of treatment (1 year earlier than when productivity costs are included).
Interpretation of the cost-effectiveness results
Staff costs for SCIT were higher compared with those for SLIT owing to the greater number of clinic visits made by patients in this group over a 3-year period, i.e. 46 compared with 13. The costs of SCIT medication per unit were also higher than those for SLIT thereby driving the costs for SCIT further up. As would be expected, SCIT was associated with higher productivity losses owing to the nature of treatment that warranted absence from work. Individuals in the ST group had highest overall productivity losses. This finding is consistent with those found in other studies. 228,230,231 Costs associated with asthma and AE medication were not included in the analysis because the cost differences in medications between SCIT, SLIT and ST were assumed to be negligible. This was based on expert clinical opinion and a review of the literature.
In the results based on direct and indirect comparisons, SCIT and SLIT were both found to be more effective than ST as shown by the difference in RQLQ. The results from the indirect comparison also suggest that SCIT may be more effective than SLIT; however, this is associated with uncertainty (a non-significant result) and must be interpreted cautiously (see Chapter 3, Indirect comparison of subcutaneous immunotherapy versus sublingual immunotherapy). As the QALYs used in this analysis were based on an algorithm that mapped the RQLQ to EQ-5D, the same direction of effect observed in the RQLQ was also seen in the QALY gains.
In terms of cost per unit improvement in RQLQ, ICERs of £7848 and £10,802 were obtained in the comparison between SLIT and ST, whereas ICERS of £7483 and £7248 were estimated for the comparison between SCIT and ST. As IT in the two sets of comparisons was both more costly and effective, it would be considered to be cost-effective only if decision-makers were willing to pay at least £11,000 for each unit improvement in RQLQ. For the comparison between SCIT and SLIT, SCIT would be considered a more cost-effective alternative if decision-makers were willing to pay at least £5600 for a similar improvement in RQLQ. A unit change of 0.5 may be considered clinically significant.
Cost-effectiveness results based on costs per QALY gained can be assessed against a threshold of £20,000–30,000, the conventional threshold adopted by decision-makers in the UK NHS, such as NICE. 249 The results of the analysis show that IT, when compared with ST, becomes cost-effective around 6 years after the start of treatment (7 years for NHS perspective only). The only exception is the comparison between SLIT and ST based on indirect comparisons, where this threshold increased to 7 years (10 years for NHS perspective only). In the comparison between SCIT and SLIT, SCIT was shown to be more cost-effective as early as 5 years after the start of treatment (4 years for NHS perspective only). These results are consistent with those for studies that reported outcomes in terms of ICERs (shown in Table 39), although the ICERs in our study were much higher.
In view of the many simplifications required in performing the CEA, these results must be regarded as indicative. The results using direct comparisons suggest that either SLIT or SCIT may be cost-effective compared with symptomatic treatment (ST), applying usual UK standards of cost-effectiveness. However, this tentative conclusion depends on there being a good reason to believe that clinical effectiveness will be sustained for somewhat longer than the 3 years' period following cessation of treatment.
When the results from the indirect comparisons were used, the cost-effectiveness results for SCIT compared with ST were largely unchanged. For SLIT compared with ST, however, the results for a difference in RQLQ were no longer statistically significant at the 95% confidence level. It is clear that SLIT is more costly than ST. Accordingly, there is no finite upper confidence limit for the ICER: the CI stretches into the region where ST dominates (is less costly and more effective than) SLIT. Figure 26 illustrates this point. It is shown in terms of mean improvement in RQLQ, but exactly the same principles apply to the results when effectiveness is estimated in QALYs.
FIGURE 26.
Incremental cost-effectiveness plane for SLIT vs ST using the results of the indirect comparison. The incremental cost is fixed at approximately £2700 per patient. The points L, M and U show this incremental cost combined with the lower, mean and upper limits of the CI for improvement in RQLQ, respectively. The gradient of the line from the origin to M is the point estimate of the ICER. The gradient of the line from the origin to U is the lower confidence limit of the ICER. The dashed line from the origin to L has a negative gradient indicating that, if L were the true representation of the cost and effect difference, ST would dominate (be less costly and more effective than) SLIT. In such a case, the magnitude of the slope of the dashed line is of no importance: ST would be preferred in any case. It should also be noted that the negative ICER represented by point L should be considered as higher than all positive ICERs, not lower. For the reasons given in the preceding sentences, it is not appropriate to quote a numerical upper confidence limit for the ICER, and the statement ‘ST dominates’ has been placed instead of such a limit in the results tables.
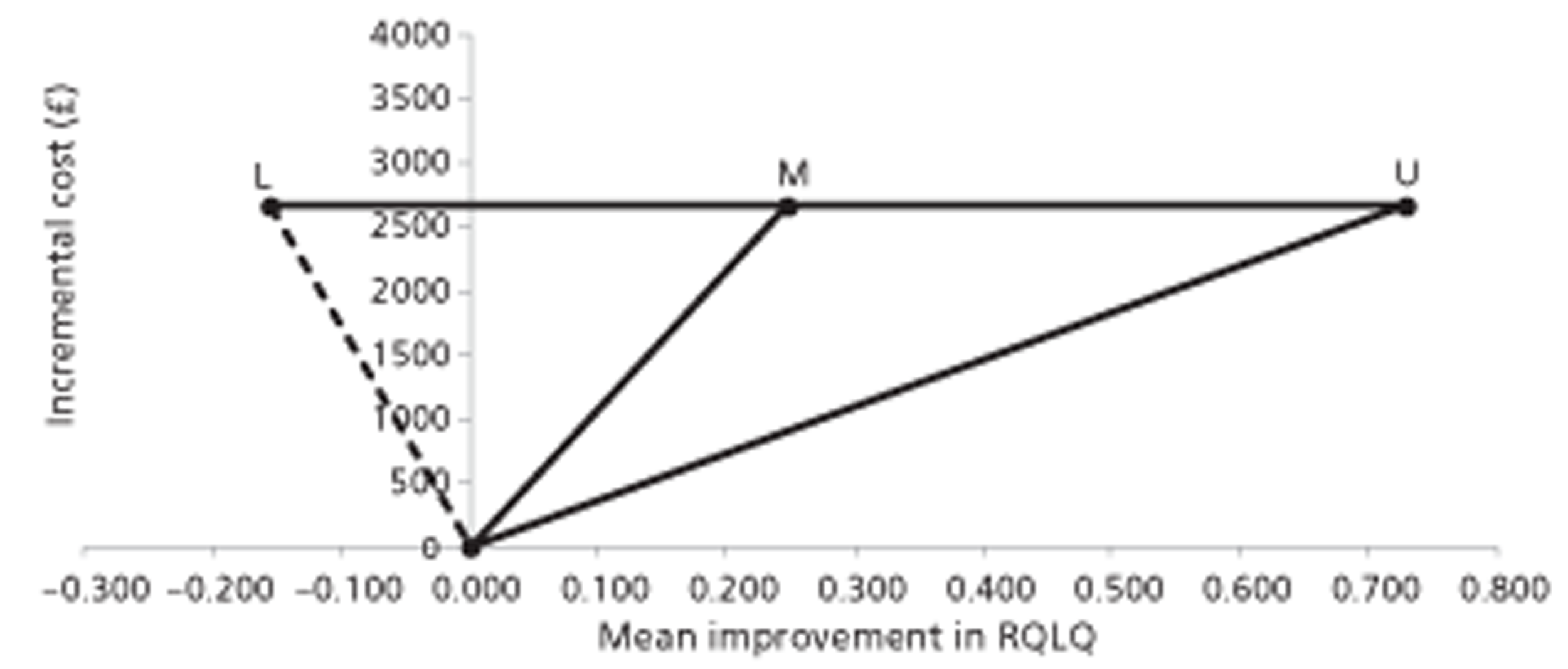
Using the point estimate of difference in effectiveness based on RQLQ, SCIT appears to be cost-effective compared with SLIT. However, as for SLIT compared with ST, the CI for difference in effectiveness crosses zero, so again there is no finite upper limit for the CI in the ICER.
It is acknowledged that there is considerable relative uncertainty in the inputs concerning costs relating to symptomatic medication and productivity loss. However, these form a sufficiently small part of the overall cost difference between treatments that making plausible changes to those values would not make any appreciable difference to the results quoted. Formal sensitivity analysis on these inputs has not been carried out, as such analysis would add nothing of value to the illustrative results already quoted, and risks being quoted out of context as giving some spurious indication of the robustness of the results. In particular, it is not possible, on the evidence available to the research team, to produce a meaningful estimate of the probability that each treatment is cost-effective at any given threshold ICER, or the value of perfect information at any such threshold.
Repeating the CEA using a 6-weekly (rather than 4-weekly) maintenance schedule for Alutard resulted in slightly lower ICERs. This was based on an assumption that clinical effectiveness remained the same and was undertaken only to illustrate the potential impact of a reduction in costs. The use of shorter treatment courses, such as preseasonal treatment using Pollinex, is likely to reduce costs even further, but there is uncertainty around the long-term effectiveness.
No analysis based on RQLQ was possible for children. One study that reported a paediatric version of the RQLQ was identified;152 however, differences compared with the adult RQLQ meant that equivalent mapping to the EQ-5D could not be undertaken.
One of the limitations of the CEA is that it does not take into account any health benefits or potential cost savings from future cases of asthma prevented. This would be particularly relevant when considering the treatment of children with SAR, as they are more likely to develop asthma than children without SAR. A simple calculation based on costs for SCIT and SLIT as outlined above, and number of asthma cases avoided based on the PAT study54–56 (for SCIT) and Novembre et al. 57 (for SLIT) is presented below [see also Chapter 1, The role of specific (allergen) immunotherapy in asthma prevention]. Both studies are in children with SAR, with no asthma at the start of treatment. The numbers needed to treat (NNT) to prevent one case of asthma were derived from the number of patients with and without asthma in the respective treatment arms, and CIs were calculated around these (method given in Armitage et al. 256). The NNT was multiplied by the cost difference (at 3 years) between SCIT (or SLIT) and symptomatic treatment only (Table 56).
| Study | NNT | Cost difference IT and ST (£) | Cost (£) per asthma case avoided (95% CI) |
|---|---|---|---|
| SCIT: PAT study,55 3-year data (n = 205) | 5 | 5537 | 27,500 (15,598 to 104,660) |
| SCIT: PAT study,56 5-year data (n = 183) | 4 | 5537 | 22,000 (14,374 to 68,807) |
| SCIT: PAT study,54 10-year data (n = 147) | 5 | 5537 | 27,500 (14,745 to 183,571) |
| SLIT: Novembre et al. 2004,57 3-year data (n = 113) | 4 | 2668 | 10,627 (6348 to 63,004) |
The results suggests that if each case of asthma costs over £10,627 (SLIT) or over £22,000–27,500 (SCIT) in treatment costs over a lifetime (appropriately discounted) then SLIT or SCIT could be potentially cost saving compared with symptomatic treatment. These results should be seen as indicative only, as they are based on effectiveness data from relatively small open-label studies. Health benefits and costs associated with a reduction in SAR symptoms are not considered in these calculations.
What is required as a top priority is to establish the extent to which the data already collected in past primary research can be used to populate a useful cost-effectiveness model, which captures all relevant benefits and costs. This means making the data available to independent researchers who will be able to analyse it using a common, and economically useful, framework. Only when this has been done will it be possible to carry out a more meaningful analysis, possibly using a value of information framework, to determine whether or not further primary research is worthwhile and to set the priorities for such research.
Chapter 5 Discussion
Clinical effectiveness
Main findings
A total of 28 new DBPC RCTs of SCIT (n = 17) or SLIT (n = 11) compared with placebo for SAR were identified, bringing the total number of relevant RCTs in this area to around 128. Assessment of the risk of bias was hampered by a lack of reporting of all relevant criteria, but, while risk of bias was often unclear, there were very few instances of high risk of bias. Overall, results were unlikely to be affected by the studies reporting instances of high risk of bias.
The updated findings are consistent with the Cochrane reviews and find statistically significant benefits for both SCIT compared with placebo and SLIT compared with placebo across all outcome measures and for the majority of subgroup analyses.
In trials of SCIT for SAR, the total number of studies and participants included in the present review was only slightly higher than those for the earlier Cochrane review, and results of meta-analyses remained very similar, with moderate effect sizes in favour of SCIT for all outcomes. Greater improvements in both SSs and MSs, compared with placebo,were found with increasing vaccine allergen content, consistent with the recognised dose–response relationship in SCIT. Note that this was based on non-randomised comparisons from subgroup analyses and should therefore be interpreted cautiously. It was beyond the scope of this report to look at randomised comparisons of allergen content, although such comparisons exist. There was only one small trial152 of SCIT in children and this found significantly lower SSs and MSs, and improved QoL, in the actively treated group (after 3 years of treatment).
Consistent with previous literature, randomised, double-blind trials of SLIT show efficacy in all major outcomes compared with placebo. Few differences were found compared with the earlier Cochrane review, despite restriction to SAR and increased sample sizes in many cases. However, a number of previously non-significant results reached statistical significance in the present review – namely, SSs in studies with < 5 μg of MAC, MSs in studies with > 20 μg of MAC, and MSs in ragweed allergy. Only one previously significant result became non-significant: of the five studies in Parietaria allergy included in the Cochrane review, removal of the study by Pajno et al. 35 (which was restricted to SAA) resulted in loss of significance; however, all of these studies had very small sample sizes (total number in remaining studies was 124).
Perhaps more importantly, despite a small increase in total sample size, and limitation to SAR, MSs in children still failed to show a significant improvement with active treatment compared with placebo. The clinical significance of this finding is unclear. The MS in isolation may not be able to reflect the effectiveness of SLIT, which is why the combined SMS is a preferred measure. It is also possible that medication use differs in children compared with adults, as it may be influenced by parental preferences. Another possibility is that SLIT is less effective in children than in adults.
Consistent with this hypothesis, the pooled SMD in SSs in children decreased by over 50% compared with the Cochrane review of SLIT. However, the result did remain statistically significant in favour of SLIT. The Cochrane review included studies of both SAR and PAR, and subgroup analysis of SAR studies only (while maintaining very similar participant numbers of > 1300 children) appears to have been associated with a reduction in treatment effect. It may be possible that SLIT treatment is more appropriate for perennial allergens in paediatric populations than for seasonal allergens. Consistent with this, exploratory subgroup analysis of SSs from paediatric studies in PAR only indicates a much larger effect size than for SAR (combined SMD −0.89 vs −0.24, respectively). Although the findings in the PAR studies were more variable than those in SAR, and the pooled result failed to reach statistical significance, this was based on a much smaller sample size overall (total n = 328, compared with n = 1343 for SAR).
None of the trials in children included in this review take into consideration future benefits from SLIT, such as avoidance of asthma or new sensitisations, although there are trials currently under way to evaluate this (see Chapter 3, Ongoing trials). Ten-year data from the PAT study54 (open-label RCT) suggests that the incidence of asthma in the treatment arm was lower than that of the control arm. Further follow-up of this study is ongoing.
In contrast with SCIT, no relationship between increased MAC and effect size was apparent from subgroup analyses, but this finding may be related to different sample sizes or due to other sources of heterogeneity in the allergen content subgroups. The estimate for the low-allergen group had wider CIs and is associated with more uncertainty. Again, these findings are not based on randomised groups and should thus be interpreted cautiously.
Overall, both SLIT and SCIT resulted in statistically significant improvements in QoL scores. Although SCIT improved RQLQ scores by 0.74 points compared with placebo, SLIT resulted in only a 0.31-point improvement compared with placebo, despite both of these meta-analyses including a similar number of participants (955 compared with 924, respectively).
All of these studies were conducted in adults (alternative versions of the RQLQ have been validated for use in paediatric and adolescent populations) and the findings are consistent with those for SSs and MSs, suggesting a greater clinical benefit from SCIT, at least in adults.
Quality-of-life data in children are scarce; eight studies of SCIT25,102,112,189,191,192,200,202 and 11 of SLIT142–144,148,152,156,161,164–166,173 reported QoL outcomes (not all included in the meta-analyses), but only one study in each intervention was restricted to paediatric or adolescent152,189 participants. SLIT is more commonly prescribed than SCIT in this population, largely due to a perceived reduced risk of potentially severe AEs. Given the possibility that efficacy of SLIT in children is poorer in terms of SSs and MSs than in adults, the presence or absence of benefit in terms of QoL in children should be further explored. In addition, more studies using the paediatric or adolescent version of the RQLQ are needed in order that clinical as well as statistical significance may be assessed. Nevertheless, both studies (one for SCIT and SLIT, respectively) found statistically significant benefits in terms of QoL in children with active treatment.
The overall incidence of AEs following treatment with SCIT was slightly higher than for SLIT (79% vs 65%, respectively, experienced at least one AE); however, as SCIT is administered under clinical supervision, reporting of AEs could be expected to be more stringent. With both routes of administration, the majority of AEs were local reactions at the site of injection (SCIT) or in the oral cavity or gastrointestinal system (SLIT), and resolved spontaneously without treatment. Where severity of systemic AEs was reported, most were graded as mild or moderate, and, again, many did not require special treatment; however, 19% of systemic AEs occurring following SCIT treatment were graded as severe, compared with only 2% following SLIT treatment.
Based on six trials155,166,179,181,185,257 that reported rates of events per injection, 129 systemic reactions occurred following 2909 administrations of active SCIT (4.4%). This number is much higher than the 0.2% suggested by a recent review of the literature. 258 However, that review included studies of both seasonal, perennial and venom IT, IT for other atopic conditions besides AR, and reports from trials, retrospective studies, surveys, and clinical observations. It seems likely that the strict reporting requirements of RCTs may provide a more realistic representation of the true rate of systemic reactions. Similar incidence-per-dose data were not available from the studies of SLIT included in the present review. No fatalities as a result of treatment were reported for either SCIT or SLIT.
Funnel plot evaluations of SSs for both SCIT and SLIT trials showed slight evidence of plot asymmetry (see Appendix 13), with larger effect sizes tending to be associated with smaller trial size. Possible sources of asymmetry include publication bias or poorer methodological quality in smaller studies, sampling variation or chance,259 and these findings should be interpreted with caution. Further, not all studies reported large effect size estimates in favour of the active treatment, and comparison of combined SMD using both fixed-effect and random-effect meta-analyses identified little difference between the two methods. Thus, any small-study effects are unlikely to impact significantly on the overall effect estimates for the interventions. 259
In contrast to the large number of placebo-controlled trials, only one small double-blinded head-to-head RCT of SCIT compared with SLIT was identified; this study did not find a significant difference between the two types of treatment, although both were better than placebo. Given the paucity of this type of data, an indirect comparison was conducted.
As there was some evidence of heterogeneity both within and between sets of placebo-controlled trials, ICMR with various covariates was performed in order to explore and potentially reduce heterogeneity. However, adjusting for type of allergen, allergen content and duration of treatment did not substantially reduce heterogeneity. Statistically significant findings favouring SCIT over SLIT (for SSs and MSs, not adjusted for covariates) were associated with substantial heterogeneity. In the analysis using combined SMS, arguably a preferred outcome measure, none of the differences in adjusted and non-adjusted analyses were statistically significant, but were associated with reduced heterogeneity. The difference in RQLQ score (0.517; 95% CrI −0.071 to 1.045) was also found to be not statistically significant. Many of the best estimate probabilities found that SCIT was most likely to be the best treatment, but this needs to be interpreted in the context of the standardised score difference results.
Year of publication appeared to account for a degree of heterogeneity (for SSs). Analyses were suggestive of earlier studies showing greater benefit for SCIT compared with studies published at a later date finding less benefit. It seems unlikely that SCIT has become less effective over time, and alternative explanations for the apparent reduction in effectiveness of SCIT include improved trial protocols and reduced dosages of SCIT being administered for safety reasons. Another possibility is that with increasingly stringent requirements for registration of clinical trials, the potential for publication bias may have reduced over time. However, these results are based on use of a post hoc defined variable and must be considered exploratory in nature.
The use of standardised units makes interpretation difficult, and where significant differences were found favouring SCIT, it is difficult to gauge how much of a clinical difference this would make.
A strength of this review is that a robust review methodology was used, including a comprehensive search strategy. It is unlikely that many relevant studies were missed in this update.
One limitation of both the Cochrane reviews and this review is that many studies do not contribute to the direct and indirect comparison meta-analyses. In this review only 5 out of 17 SCIT and 5 out of 11 SLIT RCTs contributed to at least one meta-analysis. This was partly because different outcome measures were used or that data were not presented in a way that was suitable for meta-analysis. Findings from any studies not represented in a meta-analysis were presented and were found to be consistent with the meta-analyses in their findings (benefit from IT over placebo). By far the largest of the new trials identified for SCIT compared with placebo (DuBuske et al. ,145 n = 1028) did not contribute to meta-analyses, as outcomes were expressed as number of ‘well-days’ and ‘bad-days’. This study145 evaluated the effect of an ultrashort course of SCIT and found significant benefits for SCIT over a 4-week period; longer-term outcomes were not reported.
A major limitation is the inconsistent use of outcome measures across studies [see Chapter 1, Outcome measures in randomised controlled trials of specific (allergen) immunotherapy, for further details]. Although almost all studies use the same four-point scale to assess symptom severity, the number and type of symptoms measured in different studies is so diverse as to preclude any useful comparison of results (see Appendix 7). The inconsistency in choice of outcome measure is also reflected in MSs and combined SMSs. A consequence of the highly variable outcome reporting across studies is that the pooled summary measures from meta-analyses can only be reported as SMDs, which are difficult to interpret clinically. Although effect sizes can be classified as small, moderate or large, these do not necessarily correspond to clinically meaningful changes. So, although it can be stated with some certainty that allergen IT shows consistent benefit, and that pooled results are statistically significant, there is no good estimate of the proportion of patients who, for example, have more ‘well-days’ or fewer ‘worst-days’.
The COMET (Core Outcome Measures in Effectiveness Trials) Initiative260 is attempting to develop standardised sets of outcomes (‘core outcomes’) that represent the minimum that should be measured and reported in all clinical trials of a specific condition, although this does not mean that outcomes have to be restricted to only core ones. Ultimately, this would make it easier for the results of trials to be compared and combined. Similarly, guidelines on AE reporting23,134,261 should be followed more consistently.
A further limitation was the inclusion of studies of variable methodological study design or risk of bias. This is in addition to the clinical heterogeneity observed. We did not use a quality threshold for including studies, as quality criteria were often poorly reported and inclusion would therefore have been on the basis of reporting rather than actual quality. Contacting all study authors would have been beyond the scope of this report.
The impact of IT in patients with severe, uncontrolled SAR was of particular interest when conducting this review; however, this information was not always included in the published reports of trials, and it is conceivable that there was a degree of variation in severity across trials. The concept of ‘severe chronic upper airway disease’ has recently been proposed;262 this defines patients with uncontrolled AR despite adequate pharmacological treatment based on guidelines, and could potentially be used as an inclusion criterion for future trials.
It was beyond the scope of this review to address the issue of optimum dosing and treatment regimen (see Chapter 1, Treatment schedule, for further details). Similarly, the review did not address issues around the efficacy of different SIT products [e.g. differences in depot formulations, modification of the allergen extract with adjuvants, use of allergen fragments; see Chapter 1, Specific (allergen) immunotherapy formulations and Non-standard therapies]. Routes of administration other than sublingual or subcutaneous were also not explored (e.g. epicutaneous, intralymphatic).
Cost-effectiveness
A systematic review of EEs identified 13 relevant studies of varying quality, most funded by a manufacturer of SIT products. All studies had some limitations regarding the reporting and/or robustness of data feeding into their analyses or models.
Overall, results from some of the better-quality studies suggested that SLIT was likely to be cost-effective in terms of cost per QALY compared with symptomatic treatment at thresholds of £20,000. Two studies229,233 of SCIT compared with symptomatic treatment reporting cost per QALY also found that SCIT was likely to be cost-effective at this threshold, but these studies were less transparent in their reporting. Limited evidence of SCIT compared with SLIT was suggestive of SCIT being more beneficial and less costly. None of the models included in the evaluations were described with sufficient information to be suitable for adaptation to a UK setting.
A preferred model was therefore constructed, which included health states describing patients with and without symptoms, or with partly controlled symptoms for the different treatment arms (SCIT, SLIT or symptomatic treatment). Although cost data were readily available from the literature and standard UK sources, and it may have been possible to make plausible utility estimates, it was not possible to identify suitable data on transition probabilities with which to populate the model.
Obtaining data on transition probabilities was problematic as effectiveness outcomes are almost exclusively reported as changes in a mean score (e.g. symptom and/or medication), and it is not possible to translate this into proportions (probabilities) of patients moving from one health state to another. Where this sort of data has been used in previous EEs, it has either not been adequately reported, or has been based on assumptions or expert opinion. It was also not possible to obtain EQ-5D data for different health states directly from the study authors.
An alternative cost-effectiveness model was therefore constructed based on pooled MDs in RQLQ from meta-analyses, and indirect comparison meta-analyses.
Incremental cost-effectiveness ratios (cost per QALY) varied depending on the effectiveness data used (from direct or indirect comparisons) and consistently decreased with increasing years of treatment. Up to year 6, they ranged from £28,650 (year 6) to £57,883 (year 3) for SCIT compared with symptomatic treatment, and from £27,269 to £83,560 for SLIT compared with symptomatic treatment. Thus, with increasing time, both SCIT and SLIT were found to be approaching cost-effectiveness thresholds of £20,000–30,000. Estimates of effectiveness post year 6 are associated with uncertainty as good-quality data supporting sustained effectiveness after this time are not (yet) available. These ICERs are higher than those reported in the literature.
The indirect comparison found an ICER of between £24,404 (year 6) and £42,928 (year 3) for SCIT compared with SLIT, with SCIT being both more costly and more effective. It should be noted that this is based on a difference in effectiveness obtained from an indirect comparison analysis that was non-significant, and associated with a substantial degree of heterogeneity. This finding of SCIT being more costly is in contrast with the two EEs identified, which found SCIT to be both better and less costly; however, one of these was associated with substantial uncertainty around model inputs and neither reported a combined cost-effectiveness measure.
The ICERs should be seen as indicative mainly because they are based on a very simple analysis. There are a number of other factors that limit their robustness. They are based on results from a relatively small pool of studies (eight143,144,156,161,164–166,173 for SCIT and four25,102,200,202 for SLIT vs symptomatic treatment). A large proportion of available effectiveness evidence is thus not contributing to this analysis. Furthermore, sustained effectiveness (up to 6–10 years) has been assumed and, although this has been shown in randomised trials and/or cohort studies for other effectiveness measures, the RQLQ data used here are based on more variable treatment and follow-up periods. Furthermore, the RQLQ used in isolation may not be the most appropriate outcome measure to demonstrate effectiveness of SIT; increasingly, the combined SMS or improvement during ‘worst-days’ are being recommended as outcome measures.
A number of assumptions were made when using RQLQ data in the absence of a validated mapping from RQLQ to EQ-5D based on the same patients answering both questionnaires. One was that the 0–6 point scale of the RQLQ scale could be mapped to the whole range of three of the five dimensions of the EQ-5D scale (usual activities, pain/discomfort and anxiety/depression). The effect of this was that a score of 6 (representing severe impairment in the domains of activity limitation, sleep problems, nose symptoms, eye symptoms, non-nose/eye symptoms, practical problems and emotional function) would be equivalent to a score of −0.07 on the EQ-5D (representing a state worse than death). It could be argued that these are not comparable states, and that even the most severe impairment of QoL due to AR would not be equivalent to the lowest possible EQ-5D score. If this were the case, then QALY gains would be lower than those reported, and ICERs correspondingly higher. An alternative would be to use only a proportion of the EQ-5D scale; however, no source could be found for any validated cut-off points and any point chosen would have been completely arbitrary.
Sensitivity analyses were restricted to varying the time horizon of the analysis and using the upper and lower confidence limits of the RQLQ improvement. More detailed sensitivity analysis was not performed, as the effect of plausible changes to cost inputs would have been much smaller than the effect of uncertainty in the clinical outcome and therefore would be unlikely to substantially affect the overall results. The estimates have been based on costs for a 3-year treatment schedule, which has been found to be associated with sustained benefits. Increasingly, shorter, more intensive courses of SCIT are being evaluated (rush or cluster IT), which are associated with less clinic time and are therefore less costly. However, there is as yet no evidence of long-term effectiveness and, therefore, such an analysis was not included. Should long-term effectiveness be demonstrated, this would likely result in greater cost-effectiveness.
It was not possible to model cost-effectiveness in children owing to limitations in the available data. Given the resource use associated with treating asthma, and the potential of SIT to prevent the development of asthma in this population, there is a potential for substantial cost savings. Including this in a model would likely decrease the ICER further over time. Although associated with some uncertainty, our calculations suggest that if each case of asthma costs > £10,627 (SLIT) or > £22,000–27,500 (SCIT) in treatment costs over a lifetime (appropriately discounted), then SLIT or SCIT could be potentially cost saving compared with symptomatic treatment.
Overall, the disparate nature of the existing research and the lack of reporting of key parameters have made it difficult to generate robust findings that are of use to inform reimbursement decisions. However, the results are indicative of IT being cost-effective in the longer term (6–7 years) at conventional thresholds used by decision-makers in the UK NHS.
Current guidelines suggest severity criteria for selecting patients, i.e. those uncontrolled on conventional treatment, but if the aim of treatment is asthma prevention then the initial severity is less of an issue. The presence of AR identifies a population at risk of asthma, with no evidence to suggest that the level of risk is affected by the level of severity.
Future research recommendations
Given the difficulties in comparing and combining results across studies using different outcome measures, there is a clear need for greater consistency in the use of validated outcome measures. Further research is also needed into outcomes that (1) take into consideration that the relative effectiveness of IT compared with symptomatic medication varies depending on prevailing allergen levels and (2) could best inform more meaningful EEs.
In view of the limitations of the indirect comparison analysis in this report, consideration should be given to a head-to-head trial of SCIT compared with SLIT. For both SCIT and SLIT, at present the most appropriate treatment regimens to be compared would be those with 3-year protocols, as this is where the best evidence exists in terms of long-lasting efficacy.
Ultra-short IT treatment schedules have shown promise in terms of clinical efficacy, and these protocols place considerably less burden on patients in terms of time and inconvenience. Ultra-short IT is also likely to have major cost benefits compared with conventional schedules. However, little evidence exists as to the duration of clinical benefits beyond treatment cessation and this is likely to be an important area of future research.
In terms of EEs, the main priority for future research will be to assess the extent to which results of all previous primary research can be made available to independent researchers. Only when this has been done can meaningful model-based value of information analysis be carried out to direct future primary research.
Chapter 6 Conclusions
Based on a large number of RCTs, both SCIT and SLIT have been consistently shown to be significantly more effective than symptomatic treatment only and this remains the case for the vast majority of subgroups analyses based on differences in population and treatment protocols. It is uncertain to what extent this statistical significance translates to clinically significant differences across the different types of outcome measures used. An indirect comparison is suggestive of SCIT being more beneficial than SLIT based on SSs and MSs, but no such difference could be shown for combined SMSs or QoL and firm conclusions cannot be drawn. Cost-effectiveness analyses suggest that both SCIT and SLIT may become cost-effective at a threshold of £20,000–30,000 per QALY at around 6 years (NHS and patient perspective). This is based on limited data and the use of a number of assumptions. Potential future cost savings resulting from cases of asthma avoided were not included in this analysis. There is a need for consistent reporting of validated outcome measures, ideally in head-to-head trials, which would allow for a more meaningful data synthesis and use of results to inform model-based economic analyses.
Acknowledgements
We would like to thank the following individuals:
Dr Duncan Wilson for providing input into the protocol and clinical advice.
Dr M Thirumala Krishna for input into the protocol.
Lynne Deason for advising on the patient perspective and commenting on the report.
Dr Karla Hemming for advice and comments on statistical aspects.
Contribution of authors
Angela Meadows contributed to study selection, data extraction and quality assessment, data analysis and writing of the report.
Billingsley Kaambwa undertook the systematic review of economic evaluations, and contributed to the development of new economic models and the writing of the report.
Nicola Novielli ran the indirect comparisons and contributed to the writing of the report.
Aarnoud Huissoon advised on the clinical context of the project and contributed to the clinical effectiveness analysis and economic evaluation.
Catherine Meads contributed to protocol development, study selection, project management and writing of the report.
Anne Fry-Smith devised the search strategy and carried out the searches.
Pelham Barton contributed to the development of new economic models and the writing of the report.
Janine Dretzke, principal investigator, contributed to protocol development, study selection, data extraction and quality assessment, project management and report writing.
All authors provided input to the development of the review report, commented on various drafts of the chapters and contributed to their editing.
Disclaimer
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008;63. http://dx.doi.org/10.1111/j.1398-9995.2007.01620.x.
- Scadding GK, Durham SR, Mirakian R, Jones NS, Leech SC, Farooque S, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy 2008;38:19-42. http://dx.doi.org/10.1111/j.1365-2222.2007.02888.x.
- Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 2004;24:758-64. http://dx.doi.org/10.1183/09031936.04.00013904.
- Emberlin J. Hayfever prevalence in the UK in 2020, 2040 and 2060. Worcester: National Pollen and Aerobiology Research Unit, University of Worcester; 2009.
- Asher MI, Montefort S, Bjorksten B, Lai CKW, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006;368:733-43. http://dx.doi.org/10.1016/S0140-6736(06)69283-0.
- Bodtger U, Linneberg A. Remission of allergic rhinitis: an 8-year observational study. J Allergy Clin Immunol 2004;114:1384-8. http://dx.doi.org/10.1016/j.jaci.2004.08.039.
- Nihlén U, Greiff L, Montnémery P, Löfdahl CG, Johannisson A, Persson C, et al. Incidence and remission of self-reported allergic rhinitis symptoms in adults. Allergy 2006;61:1299-304.
- Fiocchi A, Fox AT. Preventing progression of allergic rhinitis: the role of specific immunotherapy. Arch Dis Child Educ Pract 2011;96:91-100. http://dx.doi.org/10.1136/adc.2010.183095.
- Burgess JA, Haydn-Walters E, Byrnes GB, Matheson MC, Jenkins MA, Wharton CL, et al. Childhood allergic rhinitis predicts asthma incidence and persistence to middle-age: a longitudinal study. J Allergy Clin Immunol 2007;120:863-9. http://dx.doi.org/10.1016/j.jaci.2007.07.020.
- Gupta R, Sheikh A, Strachan DP, Anderson HR. Burden of allergic disease in the UK secondary analyses of national databases. Clin Exp Allergy 2004;34:520-6. http://dx.doi.org/10.1111/j.1365-2222.2004.1935.x.
- White P, Smith H, Baker N, Davis W, Frew A. Symptom control in patients with hay fever in UK general practice: how well are we doing and is there a need for allergen immunotherapy?. Clin Exp Allergy 1998;28:266-70. http://dx.doi.org/10.1046/j.1365-2222.1998.00237.x.
- Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy 2010;40:385-97. http://dx.doi.org/10.1111/j.1365-2222.2009.03443.x.
- Fitzhugh DJ, Lockey RF. History of immunotherapy: the first 100 years. Immunol Allergy Clin North Am 2011;31:149-57. http://dx.doi.org/10.1016/j.iac.2011.03.003.
- Till SJ, Francis JN, Nouri-Aria K, Durham SR. Mechanisms of immunotherapy. J Allergy Clin Immunol 2004;113:1025-34. http://dx.doi.org/10.1016/j.jaci.2004.03.024.
- Okuda M. A long-term follow-up study after discontinuation of immunotherapy for Japanese cedar pollinosis. Arerugi 2006;55:655-61.
- Eng PA, Borer-Reinhold M, Heijnen IAFM, Gnehm HPE. Twelve-year follow-up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy 2006;61:198-201. http://dx.doi.org/10.1111/j.1398-9995.2006.01011.x.
- Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med 1999;341:468-75. http://dx.doi.org/10.1056/NEJM199908123410702.
- Durham SR, Emminger W, Kapp A, deMonchy JGR, Rak S, Scadding GK, et al. SQ-standardised sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol 2012;129:717-25.e5.
- Walker SM, Durham SR, Till SJ, Roberts G, Corrigan CJ, Leech SC, et al. BSACI guidelines. Immunotherapy for allergic rhinitis. Clin Exp Allergy 2011;41:1177-200. http://dx.doi.org/10.1111/j.1365-2222.2011.03794.x.
- Committee on Safety of Medicines (CSM), Medicine Control Agency . Desensitising Vaccines: New Advice in Current Problems in Pharmacovigilance 1994.
- Cox L, Li JT, Nelson H, Lockey RF. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol 2007;120:S25-85. http://dx.doi.org/10.1016/j.jaci.2007.06.019.
- Cox L. Advantages and disadvantages of accelerated immunotherapy schedules. J Allergy Clin Immunol 2008;122:432-4. http://dx.doi.org/10.1016/j.jaci.2008.06.007.
- Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Mailing HJ, Valovirta E. Standards for practical allergen-specific immunotherapy. Allergy 2006;61:1-20. http://dx.doi.org/10.1111/j.1398-9995.2006.01219_1.x.
- Didier A, Malling HJ, Worm M, Horak F, Jager S, Montagut A, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol 2007;120:1338-45. http://dx.doi.org/10.1016/j.jaci.2007.07.046.
- Didier A, Worm M, Horak F, Sussman G, de Beaumont O, Le Gall M, et al. Sustained 3-year efficacy for pre- and coseasonal 5-grass-pollen sublingual immunotherapy tablets in patients with grass pollen-induced rhinoconjunctivitis. J Allergy Clin Immunol 2011;128:559-66. http://dx.doi.org/10.1016/j.jaci.2011.06.022.
- Wahn U, Tabar A, Kuna P, Halken S, Montagut A, de Beaumont O, et al. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol 2009;123:160-6. http://dx.doi.org/10.1016/j.jaci.2008.10.009.
- Sieber J, Koberlein J, Mosges R, Sieber J, Koberlein J, Mosges R. Sublingual immunotherapy in daily medical practice: effectiveness of different treatment schedules – IPD meta-analysis. Curr Med Res Opin 2010;26:925-32. http://dx.doi.org/10.1185/03007991003659483.
- More DR, Hagan LL. Factors affecting compliance with allergen immunotherapy at a military medical center. Ann Allergy Asthma Immunol 2002;88:391-4. http://dx.doi.org/10.1016/S1081-1206(10)62370-8.
- Cox L. Accelerated immunotherapy schedules: review of efficacy and safety. Ann Allergy Asthma Immunol 2006;97:126-37. http://dx.doi.org/10.1016/S1081-1206(10)60003-8.
- Chakraborty P, Roy I, Chatterjee S, Chanda S, Gupta-Bharracharya S. Phoenix sylvestris Roxb pollen allergy: a 2-year randomized controlled trial and follow-up study of immunotherapy in patients with seasonal allergy in an agricultural area of West Bengal, India. J Investig Allergol Clin Immunol 2006;16:377-84.
- Ott H, Sieber J, Brehler R, Folster-Holst R, Kapp A, Klimek L, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy 2009;64:1394-401. http://dx.doi.org/10.1111/j.1398-9995.2009.02194.x.
- Durham S. GT-08 investigators . Sustained effects of grass pollen AIT. Allergy 2011;66:50-2. http://dx.doi.org/10.1111/j.1398-9995.2011.02639.x.
- DesRoches A, Paradis L, Knani J, Hejjaoui A, Dhivert H, Chanez P, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. V. Duration of the efficacy of immunotherapy after its cessation. Allergy 1996;51:430-3. http://dx.doi.org/10.1111/j.1398-9995.1996.tb00155.x.
- DesRoches A, Paradis L, Menardo JL, Bouges S, Daures JP, Bousquet J. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI. Specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol 1997;99:450-3. http://dx.doi.org/10.1016/S0091-6749(97)70069-1.
- Pajno GB, Vita D, Parmiani S, Caminiti L, La Grutta S, Barberio G. Impact of sublingual immunotherapy on seasonal asthma and skin reactivity in children allergic to Parietaria pollen treated with inhaled fluticasone propionate. Clin Exp Allergy 2003;33:1641-7. http://dx.doi.org/10.1111/j.1365-2222.2003.01809.x.
- Cox L, Esch RE, Corbett M, Hankin C, Nelson M, Plunkett G. Allergen immunotherapy practice in the United States: guidelines, measures, and outcomes. Ann Allergy Asthma Immunol 2011;107:289-99. http://dx.doi.org/10.1016/j.anai.2011.06.018.
- Cox L, Calderon MA. Subcutaneous specific immunotherapy for seasonal allergic rhinitis: a review of treatment practices in the US and Europe. Curr Med Res Opin 2010;26:2723-33. http://dx.doi.org/10.1185/03007995.2010.528647.
- Passalacqua G, Compalati E, Canonica GW. Advances in allergen-specific immunotherapy. Current Drug Targets 2009;10:1255-62. http://dx.doi.org/10.2174/138945009789753237.
- Committee for Medicinal Products for Human Use (CHMP) . Guideline on Allergen Products: Production and Quality Issues 2008.
- Calderon MA, Larenas D, Kleine-Tebbe J, Jacobsen L, Passalacqua G, Eng PA, et al. European Academy of Allergy and Clinical Immunology task force report on ‘dose-response relationship in allergen-specific immunotherapy’. Allergy 2011;66:1345-59. http://dx.doi.org/10.1111/j.1398-9995.2011.02669.x.
- EAACI . Position paper: immunotherapy. Allergy 1993;48:9-35. http://dx.doi.org/10.1111/j.1398-9995.1993.tb04754.x.
- Gidaro GB, Marcucci F, Sensi L, Incorvaia C, Frati F, Ciprandi G. The safety of sublingual-swallow immunotherapy: an analysis of published studies. Clin Exp Allergy 2005;35:565-71. http://dx.doi.org/10.1111/j.1365-2222.2005.02240.x.
- Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol 1998;102:558-62. http://dx.doi.org/10.1016/S0091-6749(98)70271-4.
- Nelson HS. The use of standardized extracts in allergen immunotherapy. J Allergy Clin Immunol 2000;106:41-5. http://dx.doi.org/10.1067/mai.2000.107197.
- eMC (electronic Medicines Compendium) . Summary of Product Characteristics (SPC). GRAZAX 75,000 SQ-T Oral Lyophilisate n.d. www.medicines.org.uk/EMC/medicine/19565/SPC/GRAZAX+75%2c000+SQ-T+oral+lyophilisate/ (accessed 10 May 2011).
- British National Formulary. No. 63, March 2012. London: BMA and RPS; 2012.
- Fell P, Brostoff J. A single dose desensitization for summer hay fever. Results of a double blind study-1988. Eur J Clin Pharmacol 1990;38:77-9. http://dx.doi.org/10.1007/BF00314808.
- Di Stanislao C, Di Berardino L, Bianchi I, Bologna G. A double-blind, placebo-controlled study of preventive immunotherapy with E.P.D, in the treatment of seasonal allergic disease. Allerg Immunol 1997;30:39-42.
- Van Metre TE, Adkinson NF, Amodio FJ, Lichtenstein LM, Mardiney MR, Norman PS, et al. A comparative study of the effectiveness of the Rinkel method and the current standard method of immunotherapy for ragweed pollen hay fever. J Allergy Clin Immunol 1980;66:500-13. http://dx.doi.org/10.1016/0091-6749(80)90012-3.
- Van Metre TEJ, Adkinson NF, Lichtenstein LM, Mardiney MR, Norman PS, Rosenberg GL, et al. A controlled study of the effectiveness of the Rinkel method of immunotherapy for ragweed pollen hay fever. J Allergy Clin Immunol 1980;65:288-97. http://dx.doi.org/10.1016/0091-6749(80)90158-X.
- Casale TB, Stokes JR. Future forms of immunotherapy. J Allergy Clin Immunol 2011;127:8-15. http://dx.doi.org/10.1016/j.jaci.2010.10.034.
- Pauli G, Purohit A, Oster JP, de Blay F, Vrtala S, Niederberger V, et al. Comparison of genetically engineered hypoallergenic rBet v 1 derivatives with rBet v 1 wild-type by skin prick and intradermal testing: results obtained in a French population. Clin Exp Allergy 2000;30:1076-84. http://dx.doi.org/10.1046/j.1365-2222.2000.00869.x.
- Swoboda I, De Weerd N, Bhalla PL, Niederberger V, Sperr WR, Valent P, et al. Mutants of the major ryegrass pollen allergen, Lol p 5, with reduced IgE-binding capacity: candidates for grass pollen-specific immunotherapy. Eur J Immunol 2002;32:270-80. http://dx.doi.org/10.1002/1521-4141(200201)32:1⟨270::AID-IMMU270⟩3.0.CO;2-X.
- Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy 2007;62:943-8. http://dx.doi.org/10.1111/j.1398-9995.2007.01451.x.
- Möller C, Dreborg S, Ferdousi HA, Halken S, Høst A, Jacobsen L, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT study). J Allergy Clin Immunol 2002;109:251-6. http://dx.doi.org/10.1067/mai.2002.121317.
- Niggemann B, Jacobsen L, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy 2006;61:855-9. http://dx.doi.org/10.1111/j.1398-9995.2006.01068.x.
- Novembre E, Galli E, Landi F, Caffarelli C, Pifferi M, De Marco E, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol 2004;114:851-7. http://dx.doi.org/10.1016/j.jaci.2004.07.012.
- Polosa R, Li Gotti F, Mangano G, Paolino G, Mastruzzo C, Vancheri C, et al. Effect of immunotherapy on asthma progression, BHR and sputum eosinophils in allergic rhinitis. Allergy 2004;59:1224-8. http://dx.doi.org/10.1111/j.1398-9995.2004.00537.x.
- ClinicalTrials.gov . Grazax Asthma Prevention (GAP) n.d. http://clinicaltrials.gov/ct2/show/NCT01061203 (accessed 12 October 2011).
- Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol 2010;126:466-76. http://dx.doi.org/10.1016/j.jaci.2010.06.047.
- British Thoracic Society and Scottish Intercollegiate Guidelines Network (SIGN) . British Guidelines on the Management of Asthma. A National Clinical Guideline 2008.
- Allergy services: still not meeting the unmet need. London: RCP; 2010.
- Committee on Safety of Medicines . CSM Update. Desensitising vaccines. BMJ 1986;293. http://dx.doi.org/10.1136/bmj.293.6552.948.
- Committee for Medicinal Products for Human Use (CHMP) . Guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases 2008.
- Vance GH, Goldring S, Warner JO, Cox H, Sihra B, Hughes S, et al. A national audit of pollen immunotherapy for children in the United Kingdom: patient selection and programme safety. Clin Exp Allergy 2011;41:1313-23. http://dx.doi.org/10.1111/j.1365-2222.2011.03803.x.
- Allergy UK . Night and day. Every day report. A report based on research amongst people with allergic rhinitis n.d. www.allergyuk.org/download.aspx?File=leaflets/Night_and_Day_Report.pdf (accessed 8 June 2011).
- British Society for Allergy and Clinical Immunology (BSACI) . News Release. Under Performance During Exams Is Linked to Hay Fever n.d. www.bsaci.org/index.php?option=com_content&task=view&id=184&Itemid=195 (accessed 11 May 2011).
- Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev 2007. http://dx.doi.org/10.1002/14651858.CD001936.pub2.
- Karmakar PR, Das A, Chatterjee BP. Placebo-controlled immunotherapy with Cocos nucifera pollen extract. Int Arch Allergy Immunol 1994;103:194-201. http://dx.doi.org/10.1159/000236627.
- Bernstein DI, Epstein T. Systemic reactions to subcutaneous allergen immunotherapy. Immunol Allergy Clin North Am 2011;31:241-9. http://dx.doi.org/10.1016/j.iac.2011.02.007.
- Roder E, Berger MY, de GH, van-Wijk RG. Immunotherapy in children and adolescents with allergic rhinoconjunctivitis: a systematic review. Pediatr Allergy Immunol 2008;19:197-20. http://dx.doi.org/10.1111/j.1399-3038.2007.00648.x.
- Sanders S. The treatment of seasonal hay fever and asthma in children. A controlled trial of pre seasonal depot pollen therapy. Practitioner 1966;196:811-15.
- Dreborg S, Agrell B, Foucard T, Kjellman NI, Koivikko A, Nilsson S. A double-blind, multicenter immunotherapy trial in children, using a purified and standardized Cladosporium herbarum preparation. Clinical results. Allergy 1986;41:131-40. http://dx.doi.org/10.1111/j.1398-9995.1986.tb00289.x.
- Fontana VJ, Holt LE, Mainland D, Fontana VJ, Holt LEJ, Mainland D. Effectiveness of hyposensitization therapy in ragweed hay-fever in children. J Am Med Assoc 1966;195:985-92. http://dx.doi.org/10.1001/jama.1966.03100120053013.
- Weisnagel J. Nouvel agent hyposensibilisant dans le traitement de la rhinite allergique saisonnière (fièvre des foins) à l'herbe à poux chez l'enfant: le MRTA (modified ragweed tyrosine adsorbate). Union Med Canada 1979;108:685-90.
- Wilson D, Torres Lima M, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 2003;2.
- Di Bona D, Plaia A, Scafidi V, Leto-Barone MS, Di LG, Di Bona D, et al. Efficacy of sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a systematic review and meta-analysis. J Allergy Clin Immunol 2010;126:558-66. http://dx.doi.org/10.1016/j.jaci.2010.06.013.
- Larenas-Linnemann D. Sublingual immunotherapy in children: complete and updated review supporting evidence of effect. Curr Opin Allergy Clin Immunol 2009;9:168-76. http://dx.doi.org/10.1097/ACI.0b013e328329a2a9.
- Olaguibel JM, varez Puebla MJ. Efficacy of sublingual allergen vaccination for respiratory allergy in children. Conclusions from one meta-analysis. J Investig Allergol Clin Immunol 2005;15:9-16.
- Penagos M, Compalati E, Tarantini F, Baena-Cagnani R, Huerta J, Passalacqua G, et al. Efficacy of sublingual immunotherapy in the treatment of allergic rhinitis in pediatric patients 3 to 18 years of age: a meta-analysis of randomized, placebo-controlled, double-blind trials. Ann Allergy Asthma Immunol 2006;97:141-8. http://dx.doi.org/10.1016/S1081-1206(10)60004-X.
- Radulovic S, Calderon MA, Wilson DR, Durham SR. Cochrane systematic review: safety profile of sublingual immunotherapy (SLIT) for allergic rhinitis (AR). J Allergy Clin Immunol 2008;121. http://dx.doi.org/10.1016/j.jaci.2007.12.1105.
- Sopo SM, Macchiaiolo M, Zorzi G, Tripodi S. Sublingual immunotherapy in asthma and rhinoconjunctivitis; systematic review of paediatric literature. Arch Dis Child 2004;89:620-4. http://dx.doi.org/10.1136/adc.2003.030411.
- Radulovic S, Calderon M, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 2010;12. http://dx.doi.org/10.1002/14651858.CD002893.pub2.
- Bufe A, Ziegler-Kirbach E, Stoeckmann E, Heidemann P, Gehlhar K, Holland-Letz T, et al. Efficacy of sublingual swallow immunotherapy in children with severe grass pollen allergic symptoms: a double-blind placebo-controlled study. Allergy 2004;59:498-504. http://dx.doi.org/10.1111/j.1398-9995.2004.00457.x.
- Bufe A, Eberle P, Franke-Beckmann E, Funck J, Kimmig M, Klimek L, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol 2009;123:167-73. http://dx.doi.org/10.1016/j.jaci.2008.10.044.
- La Rosa M, Ranno C, Andre C, Carat F, Tosca MA, Canonica GW. Double-blind placebo-controlled evaluation of sublingual-swallow immunotherapy with standardized Parietaria judaica extract in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol 1999;104:425-32. http://dx.doi.org/10.1016/S0091-6749(99)70388-X.
- Roder E, Berger MY, Hop WC, Bernsen RM, de Groot H, Gerth van WR. Sublingual immunotherapy with grass pollen is not effective in symptomatic youngsters in primary care. J Allergy Clin Immunol 2007;119:892-8. http://dx.doi.org/10.1016/j.jaci.2006.12.651.
- Rolinck-Werninghaus C, Wolf H, Liebke C, Baars JC, Lange J, Kopp MV, et al. A prospective, randomized, double-blind, placebo-controlled multi-centre study on the efficacy and safety of sublingual immunotherapy (SLIT) in children with seasonal allergic rhinoconjunctivitis to grass pollen. Allergy 2004;59:1285-93. http://dx.doi.org/10.1111/j.1398-9995.2004.00627.x.
- Vourdas D, Syrigou E, Carat F, Batard T, Andre C, Papageorgiou PS. Double-blind, placebo-controlled evaluation of sublingual immunotherapy with standardized olive pollen extract in pediatric patients with allergic rhinoconjunctivitis and mild asthma due to olive pollen sensitization. Allergy 1998;53:662-72. http://dx.doi.org/10.1111/j.1398-9995.1998.tb03952.x.
- Valovirta E, Jacobsen L, Ljorring C, Koivikko A, Savolainen J. Clinical efficacy and safety of sublingual immunotherapy with tree pollen extract in children. Allergy 2006;61:1177-83. http://dx.doi.org/10.1111/j.1398-9995.2006.01190.x.
- Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduced allergic symptoms after only four preseasonal injections. Allergy 2001;56:498-505. http://dx.doi.org/10.1034/j.1398-9995.2001.056006498.x.
- Caffarelli C, Sensi LG, Marcucci F, Cavagni G. Preseasonal local allergoid immunotherapy to grass pollen in children: a double-blind, placebo-controlled, randomized trial. Allergy 2000;55:1142-7. http://dx.doi.org/10.1034/j.1398-9995.2000.00655.x.
- Dahl R, Kapp A, Colombo G, deMonchy JGR, Rak S, Emminger W, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 2006;118:434-40. http://dx.doi.org/10.1016/j.jaci.2006.05.003.
- Amar SM, Harbeck RJ, Sills M, Silveira LJ, O’Brien H, Nelson HS. Response to sublingual immunotherapy with grass pollen extract: monotherapy versus combination in multiallergen extract. J Allergy Clin Immunol 2009;124:150-6. http://dx.doi.org/10.1016/j.jaci.2009.04.037.
- Andre C, Perrin- Fayolle M, Grosclaude M, Couturier P, Basset D, Cornillon J, et al. A double-blind placebo-controlled evaluation of sublingual immunotherapy with standardized ragweed extract in patients with seasonal rhinitis. Int Arch Allergy Immunol 2003;131:111-18. http://dx.doi.org/10.1159/000070926.
- Ariano R, Spadolini I, Panzani RC. Efficacy of sublingual immunotherapy in Cupressaceae allergy using an extract of Cupressus arizonica. A double blind study. Allergol Immunopathol 2001;29:238-44.
- Bowen T, Greenbaum J, Charbonneu Y, Hebert J, Filderman R, Sussman G, et al. Canadian trial of sublingual swallow immunotherapy for ragweed rhinoconjuctivitis. Annals Allergy Asthma Immunol 2004;93:425-30. http://dx.doi.org/10.1016/S1081-1206(10)61408-1.
- Casanovas M, Guerra F, Moreno C, Miguel R, Maranon F, Daza JC. Double-blind, placebo-controlled clinical trial of preseasonal treatment with allergenic extracts of Olea europaea pollen administered sublingually. J Invest Allergol Clin Immunol 1994;4:305-14.
- D’Ambrosio F. Sublingual immunotherapy: a double-blind, placebo-controlled trial with Parietaria judaica extract standardised in mass units in patients with rhinoconjunctivitis, asthma, or both. Allergy 1999;54:968-73. http://dx.doi.org/10.1034/j.1398-9995.1999.00203.x.
- Dahl R, Stender A, Rak S. Specific immunotherapy with SQ standardized grass allergen tablets in asthmatics with rhinoconjunctivitis. Allergy 2006;61:185-90. http://dx.doi.org/10.1111/j.1398-9995.2005.00949.x.
- de Blay F, Purohit A, Kanny G, Le Sellin J, Wessel F, Leynadier F, et al. Sublingual-swallow immunotherapy with standardized 3-grass pollen extract: a double-blind placebo controlled study. Abstract n.d.
- Di Rienzo V, Pucci S, D’Alo S, Di CG, Incorvaia C, Frati F, et al. Effects of high-dose sublingual immunotherapy on quality of life in patients with cypress-induced rhinitis: a placebo-controlled study. Clin Exp Allergy Rev 2006;6:67-70. http://dx.doi.org/10.1111/j.1365-2222.2005.00102.x.
- Dubakiene R, Kleinjans HAJ. Effectiveness of specific sublingual IT (SLIT) with tree pollen. A placebo controlled study in 119 patients. Abstract n.d.
- Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 2006;117:802-9. http://dx.doi.org/10.1016/j.jaci.2005.12.1358.
- Feliziani V, Lattuada G, Parmiani S, Dall’Aglio PP. Safety and efficacy of sublingual rush immunotherapy with grass allergen extracts. A double blind study. Allergol Immunopathol 1995;23:224-30.
- Hordijk GJ, Antvelink JB, Luwema RA. Sublingual immunotherapy with a standardised grass pollen extract: a doubleblind, placebo-controlled study. Allergol Immunopathol 1998;26:234-40.
- Lima M, Wilson DR, Roberts A, Walker SM, Durham SR. Grass pollen sublingual immunotherapy (SLIT) for seasonal rhinoconjunctivitis: a randomised controlled trial. Clin Exp Allergy 2002;32:507-14. http://dx.doi.org/10.1046/j.0954-7894.2002.01327.x.
- Ott H, Sieber J, Brehler R, Fölster-Holst R, Kapp A, Klimek L, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy 2009;64:179-86. http://dx.doi.org/10.1111/j.1398-9995.2008.01875.x.
- Palma-Carlos AG, Santos AS, Branco-Ferreira M, Pregal AL, Palma-Carlos ML, Bruno ME, et al. Clinical efficacy and safety of preseasonal sublingual immunotherapy with grass pollen-carbamylated allergoid in rhinitis patients. A double-blind, placebo-controlled study. Allergol Immunopathol 2006;34:194-8. http://dx.doi.org/10.1157/13094026.
- Panzner P, Petrás M, Sýkora T, Lesná I. Double-blind, placebo controlled evaluation of grass pollen specific immunotherapy with oral drops administered sublingually or supralingually. Resp Medicine 2008;102:1296-304. http://dx.doi.org/10.1016/j.rmed.2008.03.024.
- Passalacqua G, Albano M, Riccio A, Fregonese L, Puccinelli P, Parmiani S, et al. Clinical and immunologic effects of a rush sublingual immunotherapy to Parietaria species: a double-blind, placebo-controlled trial. J Allergy Clinical Immunol 1999;104:964-8. http://dx.doi.org/10.1016/S0091-6749(99)70076-X.
- Peter R, Kleinjans H, Hecker H. Significant increase in IgG(4) levels after slit grass treatment. A double-blind, placebo-controlled, multi-center study (twin grasses study). Allergy 2009;90.
- Pfaar O, Klimek L. Efficacy and safety of specific immunotherapy with a high-dose sublingual grass pollen preparation: a double blind, placebo-controlled trial. Ann Allergy Asthma Immunol 2008;100:256-63.
- Pradalier A, Basset D, Claudel A, Couturier P, Wessel F, Galvain S, et al. Sublingual-swallow immunotherapy (SLIT) with a standardised five-grass-pollen extract (drops and sublingual tablets) versus placebo in seasonal rhinitis. Allergy 1999;54:819-28. http://dx.doi.org/10.1034/j.1398-9995.1999.00077.x.
- Smith H, White P, Annila I, Poole J, Andre C, Frew A. Randomized controlled trial of high-dose sublingual immunotherapy to treat seasonal allergic rhinitis. J Allergy Clin Immunol 2004;114:831-7. http://dx.doi.org/10.1016/j.jaci.2004.06.058.
- Troise C, Voltolini S, Canessa A, Pecora S, Negrini AC. Sublingual immunotherapy in Parietaria pollen-induced rhinitis: a double- blind study. J Invest Allergol Clin Immunol 1995;5:25-30.
- Vervloet D, Birnbaum J, Laurent P, Hugues B, Fardeau MF, Massabie-Bouchat YP, et al. Safety and efficacy of Juniperus ashei sublingual-swallow ultra-rush pollen immunotherapy in cypress rhinoconjunctivitis: a double-blind, placebo-controlled study. Int Arch Allergy Immunol 2007;142:239-46. http://dx.doi.org/10.1159/000097026.
- Wessner DB, Wessner S, Mohrnschlager M, Rakoski J, Ring J. Efficacy and safety of sublingual immunotherapy in adults with allergic rhinoconjunctivitis: results after 2 years of controlled trial. Abstract. Allergy 2001;56.
- Voltolini S, Modena P, Minale P, Bignardi D, Troise C, Puccinelli P, et al. Sublingual immunotherapy in tree pollen allergy. Double blind, placebo-controlled study with a biologically standardised extract of three pollens (alder, birch and hazel) administered by a rush schedule. Allergol Immunopathol 2001;29:103-10.
- Valovirta E. PAT: the Preventive Allergy Treatment Study design and preliminary results. Wien Med Wochenschr 1999;149:442-3.
- Wüthrich B, Bucher C, Jorg W, Bircher A, Eng P, Schneider Y, et al. Double-blind, placebo-controlled study with sublingual immunotherapy in children with seasonal allergic rhinitis to grass pollen. J Investig Allergol Clin Immunol 2003;13:145-8.
- Yuksel H, Tanac R, Gousseinov A, Demir E. Sublingual immunotherapy and influence on urinary leukotrienes in seasonal pediatric allergy. J Investig Allergol Clin Immunol 1999;9:305-13.
- Cox LS, Larenas LD, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol 2006;117:1021-35. http://dx.doi.org/10.1016/j.jaci.2006.02.040.
- Passalacqua G, Canonica GW. Sublingual immunotherapy for allergic respiratory diseases: efficacy and safety. Immunol Allergy Clin North Am 2011;31:265-77. http://dx.doi.org/10.1016/j.iac.2011.03.002.
- Pfaar O, Kleine-Tebbe J, Hormann K, Klimek L. Allergen-specific immunotherapy: which outcome measures are useful in monitoring clinical trials?. Immunol Allergy Clin North Am 2011;31:289-30. http://dx.doi.org/10.1016/j.iac.2011.02.004.
- Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy 2009;64:1-59. http://dx.doi.org/10.1111/j.1398-9995.2009.02309.x.
- Hafner D, Reich K, Matricardi PM, Meyer H, Kettner J, Narkus A. Prospective validation of ‘Allergy-Control-SCORE(TM)’: a novel symptom-medication score for clinical trials. Allergy 2011;66:629-36. http://dx.doi.org/10.1111/j.1398-9995.2010.02531.x.
- Bousquet J, Schunemann HJ, Bousquet PJ, Bachert C, Canonica GW, Casale TB, et al. How to design and evaluate randomized controlled trials in immunotherapy for allergic rhinitis: an ARIA-GA(2) LEN statement. Allergy 2011;66:765-74. http://dx.doi.org/10.1111/j.1398-9995.2011.02590.x.
- Durham SR, Birk AO, Andersen JS. Days with severe symptoms: an additional efficacy endpoint in immunotherapy trials. Allergy 2011;66:120-3. http://dx.doi.org/10.1111/j.1398-9995.2010.02434.x.
- Ciprandi G, Klersy C, Cirillo I, Marseglia GL. Quality of life in allergic rhinitis: relationship with clinical, immunological, and functional aspects. Clin Exp Allergy 2007;37:1528-35.
- Didier A, Melac M, Montagut A, Lheritier-Barrand M, Tabar A, Worm M. Agreement of efficacy assessments for five-grass pollen sublingual tablet immunotherapy. Allergy 2009;64:166-71. http://dx.doi.org/10.1111/j.1398-9995.2008.01767.x.
- Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy 1991;21:77-83. http://dx.doi.org/10.1111/j.1365-2222.1991.tb00807.x.
- Malling HJ. Immunotherapy as an effective tool in allergy treatment. Allergy 1998;53:461-72. http://dx.doi.org/10.1111/j.1398-9995.1998.tb04082.x.
- Canonica GW, Baena-Cagnani CE, Bousquet J, Bousquet PJ, Lockey RF, Malling HJ, et al. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy 2007;62:317-24. http://dx.doi.org/10.1111/j.1398-9995.2006.01312.x.
- Higgins JPT, Altman DG, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley-Blackwell; 2008.
- Higgins JPT, Deeks JJ, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley-Blackwell; 2008.
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley-Blackwell; 2008.
- Donegan S, Williamson P, Gamble C, Tudur-Smith C. Indirect comparisons: a review of reporting and methodological quality. PLoS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0011054.
- The BUGS project n.d. www.mrc-bsu.cam.ac.uk/bugs/winbugs/dicpage.shtml#q9 (accessed 29 February 2012).
- Aberer W, von Weikersthal-Drachenberg F. European outcomes amongst allergic rhinoconjunctivitis patients participating in a placebo-controlled study of ultra short course subcutaneous immunotherapy (uSCIT) conducted during the 2007 grass pollen season. Allergy 2009;64.
- Bhattacharya SG, Chakraborty P. A two-year aerobiological study and immunotherapy follow-up with Phoenix sylvestris pollen to seasonal allergic patients from West Bengal, India. J World Allergy Org 2007.
- Charpin D, Gouitaa M, Dron-Gonzalvez M, Fardeau MF, Massabie-Bouchat YP, Hugues B, et al. Immunotherapy with an aluminum hydroxide-adsorbed Juniperus ashei foreign pollen extract in seasonal indigenous cypress pollen rhinoconjunctivitis: a double-blind, placebo-controlled study. Int Arch Allergy Immunol 2007;143:83-91. http://dx.doi.org/10.1159/000098656.
- Colas C, Monzon S, Venturini M, Lezaun A. Double-blind, placebo-controlled study with a modified therapeutic vaccine of Salsola kali (Russian thistle) administered through use of a cluster schedule. J Allergy Clin Immunol 2006;117:810-16. http://dx.doi.org/10.1016/j.jaci.2005.11.039.
- Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med 2006;355:1445-55. http://dx.doi.org/10.1056/NEJMoa052916.
- DuBuske LM, Frew AJ, Horak F, Keith PK, Corrigan CJ, Aberer W, et al. Ultrashort-specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc 2011;32:239-47. http://dx.doi.org/10.2500/aap.2011.32.3453.
- Francis JN, James LK, Paraskevopoulos G, Wong C, Calderon MA, Durham SR, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol 2008;121:1120-5. http://dx.doi.org/10.1016/j.jaci.2008.01.072.
- Frew A, DuBuske L, Amersdorffer J, Holdich T. Significant benefits of combined symptom and medication scores for ultra short course subcutaneous immunotherapy compared with placebo in patients with grass pollen allergic rhinoconjunctivitis. Allergy 2009;64.
- Hoiby AS, Strand V, Robinson DS, Sager A, Rak S, Hoiby AS, et al. Efficacy, safety, and immunological effects of a 2-year immunotherapy with Depigoid birch pollen extract: a randomized, double-blind, placebo-controlled study. Clin Exp Allergy 2010;40:1062-70. http://dx.doi.org/10.1111/j.1365-2222.2010.03521.x.
- Kettner J, Meyer H, Cromwell O, Narkus A, Jost K. Specific immunotherapy with recombinant birch pollen allergen rBet v 1-FV results of 2 years of treatment (Phase II trial). Allergy 2007;62.
- Klimek L, Wolf H, Pfaar O, Wustenberg E. Intraseasonal short-time updosing with SQ-standardised subcutaneous immunotherapy in patients with intermittent allergic rhinitis: a new therapeutic option. Allergy 2010;65.
- Krishna T, Frew A, Durham S. Quality of life in specific immunotherapy with Alutard Sq grass pollen in allergic rhinoconjunctivitis: the Ukis-study results. J Allergy Clin Immunol 2006;117. http://dx.doi.org/10.1016/j.jaci.2005.12.1288.
- Kuna P, Kaczmarek J, Kupczyk M. Efficacy and safety of immunotherapy for allergies to Alternaria alternata in children. J Allergy Clin Immunol 2011;127:502-8. http://dx.doi.org/10.1016/j.jaci.2010.11.036.
- Ljorring C, Shamji MH, Wnrtzen PA, Lund K, Frew AJ, Durham SR, et al. IgE-blocking antibodies may account for a large proportion of the observed treatment effect induced by specific immunotherapy (SIT). J Allergy Clin Immunol 2009;123. http://dx.doi.org/10.1016/j.jaci.2008.12.577.
- Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol 2008;122:951-60. http://dx.doi.org/10.1016/j.jaci.2008.09.017.
- Pfaar O, Robinson DS, Sager A, Emuzyte R. Immunotherapy with depigmented-polymerized mixed tree pollen extract: a clinical trial and responder analysis. Allergy 2010;65:1614-21. http://dx.doi.org/10.1111/j.1398-9995.2010.02413.x.
- Powell RJ, Frew AJ, Corrigan CJ, Durham SR. Effect of grass pollen immunotherapy with Alutard SQ((R)) on quality of life in seasonal allergic rhinoconjunctivitis. Allergy 2007;62:1335-8. http://dx.doi.org/10.1111/j.1398-9995.2007.01455.x.
- Sahin G, Pfaar O, Klimek L. Cluster-immunotherapy with a highly polymerized pollen extract: a randomized, double-blind, placebo-controlled study. Allergy 2011;66.
- Ventura MT, Carretta A, Tummolo RA, Buquicchio R, Arsieni A, Murgia N. Clinical data and inflammation parameters in patients with cypress allergy treated with sublingual swallow therapy and subcutaneous immunotherapy. Int J Immunopathol Pharmacol 2009;22:403-13.
- Casale TB, Busse WW, Kline JN, Ballas ZK, Moss MH, Townley RG, et al. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol 2006;117:134-40. http://dx.doi.org/10.1016/j.jaci.2005.09.036.
- Ceuppens JL, Bullens D, Kleinjans H, van der Werf J. Immunotherapy with a modified birch pollen extract in allergic rhinoconjunctivitis: clinical and immunological effects. Clin Exp Allergy 2009;39:1903-9. http://dx.doi.org/10.1111/j.1365-2222.2009.03379.x.
- Frew AJ, Powell RJ, Corrigan CJ, Durham SR. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 2006;117:319-25. http://dx.doi.org/10.1016/j.jaci.2005.11.014.
- Meriney DK, Kothari H, Chinoy P, Grieco MH. The clinical and immunologic efficacy of immunotherapy with modified ragweed extract (allergoid) for ragweed hay fever. Ann Allergy 1986;56:34-8.
- Balda BR, Wolf H, Baumgarten C, Klimek L, Rasp G, Kunkel G, et al. Tree-pollen allergy is efficiently treated by short-term immunotherapy (STI) with seven preseasonal injections of molecular standardized allergens. Allergy 1998;53:740-8. http://dx.doi.org/10.1111/j.1398-9995.1998.tb03969.x.
- Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A. Efficacy and safety of preseasonal-specific immunotherapy with an aluminium-adsorbed six-grass pollen allergoid. Allergy 2005;60:801-7. http://dx.doi.org/10.1111/j.1398-9995.2005.00790.x.
- Ferrer M, Burches E, Pelaez A, Munoz A, Hernandez D, Basomba A, et al. Double-blind, placebo-controlled study of immunotherapy with Parietaria judaica: clinical efficacy and tolerance. J Investig Allergol Clin Immunol 2005;15:283-92.
- Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol 2005;116:608-13. http://dx.doi.org/10.1016/j.jaci.2005.06.004.
- Ortolani C, Pastorello EA, Incorvaia C, Ispano M, Farioli L, Zara C, et al. Double-blind, placebo-controlled study of immunotherapy with an alginate-conjugated extract of Parietaria judaica in patients with Parietaria hay-fever. Allergy 1994;49:13-21. http://dx.doi.org/10.1111/j.1398-9995.1994.tb00767.x.
- Zenner HP, Baumgarten C, Rasp G, Fuchs T, Kunkel G, Hauswald B, et al. Short-term immunotherapy: a prospective, randomized, double-blind, placebo-controlled multicenter study of molecular standardized grass and rye allergens in patients with grass pollen-induced allergic rhinitis. J Allergy Clin Immunol 1997;100:23-9. http://dx.doi.org/10.1016/S0091-6749(97)70190-8.
- Bodtger U, Poulsen LK, Jacobi HH, Malling HJ. The safety and efficacy of subcutaneous birch pollen immunotherapy: a one-year, randomised, double-blind, placebo-controlled study. Allergy 2002;57:297-305. http://dx.doi.org/10.1034/j.1398-9995.2002.1o3532.x.
- Bousquet J, Hejjaoui A, Soussana M, Michel FB. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. IV Comparison of the safety and efficacy of two dosages of a high-molecular-weight allergoid. J Allergy Clin Immunol 1990;85:490-7. http://dx.doi.org/10.1016/0091-6749(90)90160-6.
- Brewczynski PZ, Kroon AM. Efficacy and safety of immunotherapy with modified grass pollen allergens. Results of a placebo controlled study. Allergologie 1999;22:411-20.
- Varney VA, Gaga M, Frew AJ, Aber VR, Kay AB, Durham SR. Usefulness of immunotherapy in patients with severe summer hay fever uncontrolled by antiallergic drugs. BMJ 1991;302:265-9. http://dx.doi.org/10.1136/bmj.302.6771.265.
- Walker SM, Pajno GB, Torres-Lima M, Wilson DR, Durham SR. Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J Allergy Clin Immunol 2001;107:87-93. http://dx.doi.org/10.1067/mai.2001.112027.
- Ortolani C, Pastorello E, Moss RB, Hsu YP, Restuccia M, Joppolo G, et al. Grass pollen immunotherapy: a single year double-blind, placebo-controlled study in patients with grass pollen-induced asthma and rhinitis. J Allergy Clinical Immunol 1984;73:283-90. http://dx.doi.org/10.1016/S0091-6749(84)80021-4.
- Dolz I, Martinez-Cocera C, Bartolome JM, Cimarra M. A double blind, placebo-controlled study of immunotherapy with grass pollen extract Alutard SQ during a 3-year period with initial rush immunotherapy. Allergy 1996;51:489-500.
- Mirone C, Albert F, Tosi A, Mocchetti F, Mosca S, Giorgino M, et al. Efficacy and safety of subcutaneous immunotherapy with a biologically standardized extract of Ambrosia artemisiifolia pollen: a double-blind, placebo controlled study. Clin Exp Allergy 2004;34:1408-14. http://dx.doi.org/10.1111/j.1365-2222.2004.02056.x.
- Pastorello EA, Pravettoni V, Incorvaia C, Mambretti M, Franck E, Wahl R, et al. Clinical and immunological effects of immunotherapy with alum-absorbed grass allergoid in grass-pollen-induced hay-fever. Allergy 1992;47:281-90. http://dx.doi.org/10.1111/j.1398-9995.1992.tb02054.x.
- Bousquet J, Hejjaoui A, Skassa-Brociek W, Guerin B, Maasch HJ, Dhivert H, et al. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. I. Rush immunotherapy with allergoids and standardized orchard grass-pollen extract. J Allergy Clin Immunol 1987;80:591-8. http://dx.doi.org/10.1016/0091-6749(87)90013-3.
- Bousquet J, Maasch HJ, Hejjaoui A, Skassa-Brociek W, Wahl R, Dhivert H, et al. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. III. Efficacy and safety of unfractionated and high-molecular-weight preparations in rhinoconjunctivitis and asthma. J Allergy Clin Immunol 1989;84:546-56. http://dx.doi.org/10.1016/0091-6749(89)90369-2.
- Juniper EF, Roberts RS, Kennedy LK, O’Connor J, Syty-Golda M, Dolovich J, et al. Polyethylene glycol-modified ragweed pollen extract in rhinoconjunctivitis. J Allergy Clin Immunol 1985;75:578-85. http://dx.doi.org/10.1016/0091-6749(85)90033-8.
- Tari MG, Mancino M, Ghezzi E, Frank E, Cromwell O. Immunotherapy with an alum-adsorbed Parietaria-pollen allergoid: a 2-year, double-blind, placebo-controlled study. Allergy 1997;52:65-74. http://dx.doi.org/10.1111/j.1398-9995.1997.tb02547.x.
- Fling JA, Ruff ME, Parker WA, Whisman BA, Martin ME, Moss RB, et al. Suppression of the late cutaneous response by immunotherapy. J Allergy Clin Immunol 1989;83:101-9. http://dx.doi.org/10.1016/0091-6749(89)90483-1.
- Metzger WJ, Clay DH, Richerson HB, Weiler JM, Donnelly A, Moran D. Clinical and immunologic evaluation of glutaraldehyde-modified, tyrosine-adsorbed short ragweed extract: A double-blind, placebo-controlled trial. J Allergy Clin Immunol 1981;68:442-8. http://dx.doi.org/10.1016/0091-6749(81)90198-6.
- Iliopoulos O, Proud D, Adkinson NJ, Creticos PS, Norman PS, Kagey S, et al. Effects of immunotherapy on the early, late, and rechallenge nasal reaction to provocation with allergen: changes in inflammatory mediators and cells. J Allergy Clin Immunol 1991;87:855-66. http://dx.doi.org/10.1016/0091-6749(91)90134-A.
- Bousquet J, Frank E, Soussana M, Hejjaoui A, Maasch HJ, Michel FB. Double-blind, placebo-controlled immunotherapy with a high-molecular-weight, formalinized allergoid in grass pollen allergy. Int Arch Allergy Appl Immunol 1987;82:550-2. http://dx.doi.org/10.1159/000234277.
- D’Amato G, Kordash TR, Liccardi G, Lobefalo G, Cazzola M, Freshwater LL. Immunotherapy with Alpare in patients with respiratory allergy to Parietaria pollen: a two year double-blind placebo-controlled study. Clin Exp Allergy 1995;25:149-58. http://dx.doi.org/10.1111/j.1365-2222.1995.tb01020.x.
- Grammer LC, Shaughnessy MA, Suszko IM, Shaughnessy JJ, Patterson R. A double-blind histamine placebo-controlled trial of polymerized whole grass for immunotherapy of grass allergy. J Allergy Clin Immunol 1983;72:448-53. http://dx.doi.org/10.1016/0091-6749(83)90580-8.
- Lee LK, Kniker WT, Campos T. Aggressive co-seasonal immunotherapy in mountain cedar pollen allergy. Arch Otolaryngol 1982;108:787-94. http://dx.doi.org/10.1001/archotol.1982.00790600031008.
- Blaiss M, Maloney J, Nolte H, Gawchik S, Yao R, Skoner DP. Efficacy and safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. J Allergy Clin Immunol 2011;127:64-71. http://dx.doi.org/10.1016/j.jaci.2010.11.034.
- Cortellini G, Spadolini I, Patella V, Fabbri E, Santucci A, Severino M, et al. Sublingual immunotherapy for Alternaria-induced allergic rhinitis: a randomized placebo-controlled trial. Ann Allergy Asthma Immunol 2010;105:382-6. http://dx.doi.org/10.1016/j.anai.2010.08.007.
- Fujimura T, Yonekura S, Horiguchi S, Taniguchi Y, Saito A, Yasueda H, et al. Increase of regulatory T cells and the ratio of specific IgE to total IgE are candidates for response monitoring or prognostic biomarkers in 2-year sublingual immunotherapy (SLIT) for Japanese cedar pollinosis. Clin Immunol 2011;139:65-74. http://dx.doi.org/10.1016/j.clim.2010.12.022.
- Nelson HS, Nolte H, Creticos P, Maloney J, Wu J, Bernstein DI, et al. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American adults. J Allergy Clin Immunol 2011;127:72-80. http://dx.doi.org/10.1016/j.jaci.2010.11.035.
- Panizo C, Cimarra M, Gonzalez-Mancebo E, Vega A, Senent C, Martin S. In vivo and in vitro immunological changes induced by a short course of grass allergy immunotherapy tablets. J Investig Allergol Clin Immunol 2010;20:454-62.
- Pfaar O, Barth C, Jaschke C, Hormann K, Klimek L. Sublingual allergen-specific immunotherapy adjuvanted with monophosphoryl lipid A: a phase I/IIa study. Int Arch Allergy Immunol 2011;154:336-44. http://dx.doi.org/10.1159/000321826.
- Reich K, Gessner C, Kroker A, Schwab JA, Pohl W, Villesen H, et al. Immunologic effects and tolerability profile of in-season initiation of a standardized-quality grass allergy immunotherapy tablet: a phase III, multicenter, randomized, double-blind, placebo-controlled trial in adults with grass pollen-induced rhinoconjunctivitis. Clinical Therapeutics 2011;33:828-40. http://dx.doi.org/10.1016/j.clinthera.2011.06.006.
- Skoner D, Gentile D, Bush R, Fasano MB, McLaughlin A, Esch RE, et al. Sublingual immunotherapy in patients with allergic rhinoconjunctivitis caused by ragweed pollen. Int Arch Allergy Immunol 2010;125:660-6.
- Voltolini S, Troise C, Incorvaia C, Bignardi D, Di Cara G, Marcucci F, et al. Effectiveness of high dose sublingual immunotherapy to induce a stepdown of seasonal asthma: a pilot study. Curr Med Res Opin 2010;26:37-40. http://dx.doi.org/10.1185/03007990903431886.
- Blaiss M, Maloney J, Nolte H, Gawchik S, Skoner D. Efficacy and Safety of Grass Allergy Immunotherapy Tablet (AIT) in a North American Pediatric Population. Int Arch Allergy Immunol 2010;125.
- Didier A, Horak F, Worm M, Melac M, de Beaumont O, Le Gall M. An assessment of sustained efficacy and safety of a 300 IR five-grass pollen sublingual immunotherapy tablet in adults with grass pollen induced allergic rhinoconjunctivitis during a 3-year treatment study. Allergy 2010;65.
- Durham SR, Emminger W, Kapp A, Colombo G, de Monchy JGR, Rak S, et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J Allergy Clin Immunol 2010;125:131-8. http://dx.doi.org/10.1016/j.jaci.2009.10.035.
- Dahl R, Kapp A, Ribel M, Durham SR. Sublingual immunotherapy with SQ standardized grass allergen tablets 2006.
- Horak F, Jaeger S, Worm M, Melac M, Didier A. Implementation of pre-seasonal sublingual immunotherapy with a five-grass pollen tablet during optimal dosage assessment. Clin Exp Allergy 2009;39:394-400. http://dx.doi.org/10.1111/j.1365-2222.2008.03153.x.
- Tari MG, Mancino M, Madonna F, Buzzoni L, Parmiani S. Immunologic evaluation of 24 month course of sublingual immunotherapy. Allergol Immunopathol 1994;22:209-16.
- Bahceciler NN, Isik U, Barlan IB, Basaran MM. Efficacy of sublingual immunotherapy in children with asthma and rhinitis: a double blind, placebo-controlled study. Pediatr Pulmonol 2001;32:49-55. http://dx.doi.org/10.1002/ppul.1088.
- Cao LF, Lu Q, Gu HL, Chen YP, Zhang Y, Lu M, et al. Clinical evaluation for sublingual immunotherapy of allergic asthma and atopic rhinitis with Dermatophagoides farinae drops. Zhonghua Er Ke Za Zhi 2007;45:736-41.
- Hirsch T, Sahn M, Leupold W. Double-blind placebo-controlled study of sublingual immunotherapy with house dust mite extract (D. pt.) in children. Pediatr Allergy Immunol 1997;8:21-7. http://dx.doi.org/10.1111/j.1399-3038.1997.tb00138.x.
- Marcucci F, Sensi L, Di Cara G, Salvatori S, Bernini M, Pecora S, et al. Three-year follow-up of clinical and inflammation parameters in children monosensitized to mites undergoing sub-lingual immunotherapy. Pediatr Allergy Immunol 2005;16:519-26. http://dx.doi.org/10.1111/j.1399-3038.2005.00301.x.
- Ibanez MD, Kaiser F, Knecht R, Armentia A, Schopfer H, Tholstrup B, et al. Safety of specific sublingual immunotherapy with SQ standardized grass allergen tablets in children. Pediatr Allergy Immunol 2007;18:516-22. http://dx.doi.org/10.1111/j.1399-3038.2007.00556.x.
- Calderon M, Essendrop M. Specific immunotherapy with high dose SQ standardized grass allergen tablets was safe and well tolerated. J Investig Allergol Clin Immunol 2006;16:338-44.
- Khinchi MS, Poulsen LK, Carat F, Andre C, Hansen AB, Malling HJ. Clinical efficacy of sublingual and subcutaneous birch pollen allergen-specific immunotherapy: a randomized, placebo-controlled, double-blind, double-dummy study. Allergy 2004;59:45-53. http://dx.doi.org/10.1046/j.1398-9995.2003.00387.x.
- Mauro M, Russello M, Incorvaia C, Gazzola GB, Di Cara G, Frati F. Comparison of efficacy, safety and immunologic effects of subcutaneous and sublingual immunotherapy in birch pollinosis: a randomized study. Eur Ann Allergy Clin Immunol 2007;39:119-22.
- Quirino T, Iemoli E, Siciliani E, Parmiani S, Milazzo F. Sublingual versus injective immunotherapy in grass pollen allergic patients: a double blind (double dummy) study. Clin Exp Allergy 1996;26:1253-61. http://dx.doi.org/10.1111/j.1365-2222.1996.tb00522.x.
- Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev 2004;2. http://dx.doi.org/10.1002/14651858.CD003974.pub2.
- Spiegelhalter SD, Abrams KR, Myles J. Baysian approaches to clinical trials and health care evaluation. Statistics in practice. Chichester: Wiley; 2004.
- ClinicalTrials.gov website . Long-Term Effects of Sublingual Grass Therapy n.d. http://clinicaltrials.gov/show/NCT01335139 (accessed 21 November 2012).
- ClinicalTrials.gov website . 2nd Pivotal Study RPhleum: Adults and Adolescents With Rhinoconjunctivitis + − Controlled Asthma n.d. http://clinicaltrials.gov/ct2/show/NCT01353755?term=NCT01353755&rank=1 (accessed 21 November 2012).
- ClinicalTrials.gov website . Evaluation of Safety and Efficacy of Specific Immunotherapy With Recombinant Major Allergens of Timothy Grass Pollen Adsorbed onto Aluminium-Hydroxide in Patients With IgE-Mediated Allergic Rhinoconjunctivitis + – Controlled Asthma (AMETHYST) n.d. http://clinicaltrials.gov/ct2/show/NCT00671268?term=NCT00671268&rank=1 (accessed 21 November 2012).
- ClinicalTrials.gov website . Safety and Efficacy of Recombinant Grass Pollen Allergen Cocktail in the Treatment of Allergic Rhinoconjunctivitis n.d. http://clinicaltrials.gov/ct2/show/NCT00309036?term=NCT00309036&rank=1 (accessed 21 November 2012).
- ClinicalTrials.gov website . Safety and Efficacy of Recombinant Birch Pollen Allergen in the Treatment of Allergic Rhinoconjunctivitis n.d. http://clinicaltrials.gov/ct2/show/NCT00309062?term=NCT00309062&rank=1 (accessed 21 November 2012).
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Ariano R, Berto P, Tracci D, Incorvaia C, Frati F. Pharmacoeconomics of allergen immunotherapy compared with symptomatic drug treatment in patients with allergic rhinitis and asthma. Allergy Asthma Proc 2006;27:159-63.
- Berto P, Bassu N, Incorvaia C, Frati F, Puccinelli P, Giaquinto C, et al. Cost effectiveness of sublingual immunotherapy in children with allergic rhinitis and asthma. Eur Ann Allergy Clin Immunol 2005;37:303-8.
- Claes C, Mittendorf T, von der Schulenburg JM. Health economic modeling of allergen-specific immunotherapy in seasonal allergic rhinitis from a health care payer's perspective. Allergo J 2009;18:60-7.
- Pokladnikova J, Krcmova I, Vlcek J. Economic evaluation of sublingual vs subcutaneous allergen immunotherapy. Brief record. Ann Allergy Asthma Immunol 2008;100:482-9. http://dx.doi.org/10.1016/S1081-1206(10)60475-9.
- Berto P, Passalacqua G, Crimi N, Frati F, Ortolani C, Senna G, et al. Economic evaluation of sublingual immunotherapy vs symptomatic treatment in adults with pollen-induced respiratory allergy: the Sublingual Immunotherapy Pollen Allergy Italy (SPAI) study. Structured abstract. Ann Allergy Asthma Immunol 2006;97:615-21. http://dx.doi.org/10.1016/S1081-1206(10)61090-3.
- Omnes LF, Bousquet J, Scheinmann P, Neukirch F, Jasso MG, Chicoye A, et al. Pharmacoeconomic assessment of specific immunotherapy versus current symptomatic treatment for allergic rhinitis and asthma in France. Structured abstract. Allergie Immunol 2007;39:148-56.
- Bachert C, Vestenbaek U, Christensen J, Griffiths UK, Poulsen PB. Cost-effectiveness of grass allergen tablet (GRAZAX) for the prevention of seasonal grass pollen induced rhinoconjunctivitis: a Northern European perspective. Clin Exp Allergy 2007;37:772-9. http://dx.doi.org/10.1111/j.1365-2222.2007.02706.x.
- Brüggenjürgen B, Reubhold T, Brehler R, Laake E, Wiese G, Machate Uea. Cost-effectiveness of specific subcutaneous immunotherapy in patients with allergic rhinitis and allergic asthma. Ann Allergy Asthma Immunol 2008;101:316-24. http://dx.doi.org/10.1016/S1081-1206(10)60498-X.
- Canonica GW, Poulsen PB, Vestenbaek U. Cost-effectiveness of GRAZAX for prevention of grass pollen induced rhinoconjunctivitis in Southern Europe. Respir Med 2007;101:1885-94. http://dx.doi.org/10.1016/j.rmed.2007.05.003.
- Nasser S, Vestenbaek U, Beriot-Mathiot A, Poulsen PB. Cost-effectiveness of specific immunotherapy with Grazax in allergic rhinitis co-existing with asthma. Allergy 2008;63:1624-9. http://dx.doi.org/10.1111/j.1398-9995.2008.01743.x.
- Beriot-Mathiot A, Vestenbaek U, Poulsen PB. Influence of time horizon and treatment patterns on cost-effectiveness measures: the case of allergen-specific immunotherapy with Grazax. Provisional abstract. J Med Econ 2007;10:215-28. http://dx.doi.org/10.3111/13696990701438587.
- Keiding H, Jorgensen KP. A cost-effectiveness analysis of immunotherapy with SQ allergen extract for patients with seasonal allergic rhinoconjunctivitis in selected European countries. Curr Med Res Opin 2007;23:1113-20. http://dx.doi.org/10.1185/030079907X187865.
- Petersen KD, Gyrd HD, Dahl R. Health-economic analyses of subcutaneous specific immunotherapy for grass pollen and mite allergy. Allergol Immunopathol 2005;33:296-302. http://dx.doi.org/10.1016/S0301-0546(05)73246-8.
- Schadlich PK, Brecht JG. Economic evaluation of specific immunotherapy versus symptomatic treatment of allergic rhinitis in Germany. Pharmacoeconomics 2000;17:37-52. http://dx.doi.org/10.2165/00019053-200017010-00003.
- Berto P, Frati F, Incorvaia C, Cadario G, Contiguglia R, Di GM, et al. Comparison of costs of sublingual immunotherapy and drug treatment in grass-pollen induced allergy: results from the SIMAP database study. Brief record. Curr Med Res Opin 2008;24:261-6. http://dx.doi.org/10.1185/030079908X253726.
- Hankin CS, Cox L, Bronstone A. The health economics of allergen immunotherapy. Immunol Allergy Clin North Am 2011;31:325-41. http://dx.doi.org/10.1016/j.iac.2011.03.007.
- Brecht JG, Schädlich PK. Krankheitslast chronischer Krankheiten in Deutschland. Hamburg: InForMed GmbH; 1995.
- Büchner K. Die jährlichen Kosten der allergischen Rhinitis und des Asthmas in der Bundesrepublik Deutschland. Basel: Infratest-Suisse; 1993.
- Wettengel R, Volmer T. Asthma: Medizinische und ökonomische Bedeutung einer Volkskrankheit. Stuttgart: EuMe-Com; 1994.
- Andronis L, Barton P, Bryan S. Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making. Health Technol Assess 2009;13.
- Tamayama K, Kondo M, Shono A, Okubo I. Utility weights for allergic rhinitis based on a community survey with a time trade-off technique in Japan. Allergol Int 2009;58:201-7. http://dx.doi.org/10.2332/allergolint.08-OA-0032.
- Chen H, Cisternas MG, Katz PP, Omachi TA, Trupin L, Yelin EH, et al. Evaluating quality of life in patients with asthma and rhinitis: English adaptation of the rhinasthma questionnaire. Ann Allergy Asthma Immunol 2011;106:110-18. http://dx.doi.org/10.1016/j.anai.2010.10.027.
- Wasserfallen JB, Gold K, Schulman KA, Baraniuk JN. Item responsiveness of a rhinitis and asthma symptom score during a pollen season. J Asthma 1999;36:459-65. http://dx.doi.org/10.3109/02770909909087288.
- Whitehead S, Taylor MJ, Christensen J. An economic evaluation of Grazax for the treatment of grass pollen induced rhinoconjunctivitis in children. Value Health 2009;12.
- Whitehead SJ, Taylor MJ, Bech PG, Prenzler A. Cost-effectiveness of grazax in the paediatric population for the treatment of grass pollen induced rhinoconjunctivitis in Germany. Value Health 2009;12.
- Berto P, Frati F, Incorvaia C. Economic studies of immunotherapy: a review. Curr Opin Allergy Clin Immunol 2008;8:585-9. http://dx.doi.org/10.1097/ACI.0b013e32831411e9.
- Buchner K, Siepe M. Nutzen der Hyposensibilierung unter wirtschaftlichen Aspekten. Allergo J 1995;4:156-63.
- Guide to the methods of technology appraisal. London: NICE; 2004.
- British National Formulary. London: BMA and RPS; 2011.
- Curtis L. Unit Costs of Health and Social Care 2011 n.d. www.pssru.ac.uk/project-pages/unit-costs/2011/index.php (accessed 19 March 2012).
- Medisave (UK) Ltd . Consumables and General Supplies n.d. www.medisave.co.uk/consumables-general-supplies.html (accessed 17 March 2012).
- Office for National Statistics (ONS) . Annual Survey of Hours and Earnings – 2011 n.d. www.ons.gov.uk/ons/rel/ashe/annual-survey-of-hours-and-earnings/2011-provisional-results--soc-2010-/stb--ashe-results-2011--soc-2010-.html (accessed 19 March 2012).
- Robinson S, Roberts T, Barton P, Bryan S, Macleod J, McCarthy A, et al. Healthcare and patient costs of a proactive chlamydia screening programme: the Chlamydia Screening Studies project. Sex Transm Infect 2007;83:276-81. http://dx.doi.org/10.1136/sti.2006.023374.
- Drummond MF, Sculpher MJ, O’Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Armitage P, Berry G, Matthews JNS. Statistical methods in medical research. Oxford: Blackwell Science; 2002.
- Juniper EF, Roberts RS, Kennedy LK, O’Connor J, Syty Golda M, Dolovich J, et al. Polyethylene glycol-modifed ragweed; pollen extract in rhinoconjunctivitis. J Allergy Clin Immunol 1985;75:578-85. http://dx.doi.org/10.1016/0091-6749(85)90033-8.
- Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allegeren immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011;127:S1-55. http://dx.doi.org/10.1016/j.jaci.2010.09.034.
- Sterne JAC, Egger M, Moher D, Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley-Blackwell; 2008.
- The COMET Initiative n.d. www.comet-initiative.org/ (accessed 28 February 2012).
- Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol 2010;125:569-74. http://dx.doi.org/10.1016/j.jaci.2009.10.060.
- Bousquet J, Bachert C, Canonica GW, Casale TB, Cruz AA, Lockey RJ, et al. Unmet needs in severe chronic upper airway disease (SCUAD). J Allergy Clin Immunol 2009;124:428-33. http://dx.doi.org/10.1016/j.jaci.2009.06.027.
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester: Wiley; 2000.
- Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. Am J Epidemiol 2009;169:1158-65. http://dx.doi.org/10.1093/aje/kwp014.
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J Roy Stat Soc A Sta 2009;172:137-59.
- Gelman A. Prior distributions for variance parameters in hierarchical models. Bayesian Anal 2006;1:515-33. http://dx.doi.org/10.1214/06-BA117A.
- Racine-Poon A, Wakefield JC, Berry DA, Stangl DK. Bayesian biostatistics. New York, NY: Marcel Dekker; 1996.
- Ntzoufras I. Bayesian modeling using WinBUGS. Hoboken, NJ: Wiley; 2009.
- Spiegelhalter SD, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J Roy Stat Soc B Met 2002;64:583-639. http://dx.doi.org/10.1111/1467-9868.00353.
- Dahl R, Kapp A, Colombo G, de Monchy JGR, Rak S, Emminger W, et al. Sublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 years. J Allergy Clin Immunol 2008;121:512-18. http://dx.doi.org/10.1016/j.jaci.2007.10.039.
- Schramm B, Ehlken B, Smala A, Quednau K, Berger K, Nowak D. Cost of illness of atopic asthma and seasonal allergic rhinitis in Germany: 1-yr retrospective study. Eur Respir J 2003;21:116-22.
- Jacobsen L, Dreborg S, Moller C, Valovirta E, Wahn U, Urbanek R, et al. Preventive Allergy Treatment (PAT) by use of specific immunotherapy (SIT). Allergy 1995;50.
Appendix 1 Original protocol
Appendix 2 Search strategies
Appendix 3 Indirect comparison methodology and results
Appendix 4 Study selection process
Appendix 5 Reasons for exclusion and discrepancies between included/excluded studies in Cochrane reviews and this report
Reasons for exclusion and discrepancies between included/excluded studies in Cochrane reviews and this report (PDF download)
Appendix 6 Main study characteristics and risk of bias
Appendix 7 Results of subgroup analyses
Appendix 8 Symptom scores across studies
Appendix 9 Characteristics of ongoing trials
Appendix 10 Reasons for exclusion: cost-effectiveness studies and reviews
Reasons for exclusion: cost-effectiveness studies and reviews (PDF download)
Appendix 11 Characteristics of included economic studies
Appendix 12 Quality assessment of economic evaluations
Appendix 13 Transition probabilities
Appendix 14 Cost-effectiveness analysis based on six-weekly injections
Cost-effectiveness analysis based on six-weekly injections (PDF download)
Appendix 15 Funnel plots
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AR
- allergic rhinitis
- ARIA
- Allergic Rhinitis and its Impact on Asthma
- AUC
- area under the curve
- BNF
- British National Formulary
- BSACI
- British Society for Allergy and Clinical Immunology
- CBA
- cost–benefit analysis
- CCA
- cost–consequences analysis
- CEA
- cost-effectiveness analysis
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CrI
- credible interval
- CUA
- cost–utility analysis
- DBPC
- double blind, placebo controlled
- DIC
- deviance information criterion
- EAACI
- European Academy of Allergy and Clinical Immunology
- EE
- economic evaluation
- EPS
- entire pollen season
- EQ-5D
- European Quality of Life-5 Dimensions (EuroQol Group standardised instrument for use as a measure of health outcome)
- GCSE
- General Certificate of Secondary Education
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- ICMA
- indirect comparison meta-analysis
- ICMR
- indirect comparison meta-regression
- IgE
- immunoglobulin E
- IT
- immunotherapy
- MAC
- major allergen content
- MAQOL
- McMaster Asthma Quality of Life Questionnaire
- MCMC
- Monte Carlo Markov chain
- MD
- mean difference
- MS
- medication score
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NNT
- number needed to treat
- PAR
- perennial allergic rhinitis
- PAT
- Preventative Allergy Treatment
- PPS
- peak pollen season
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RQLQ
- Rhinoconjunctivitis Quality of Life Questionnaire
- SAA
- seasonal allergic asthma
- SAE
- serious adverse event
- SAR
- seasonal allergic rhinitis
- SCIT
- subcutaneous immunotherapy
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SIGN
- Scottish Intercollegiate Guidelines Network
- SIT
- specific (allergen) immunotherapy
- SLIT
- sublingual immunotherapy
- SMD
- standardised mean difference
- SMS
- combined symptom and medication score
- SOCC
- Standards of Care Committee
- SS
- symptom score
- ST
- symptomatic treatment
- VAS
- visual analogue scale
- WAO
- World Allergy Organization
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.