Notes
Article history paragraph text
The research reported in this issue of the journal was funded by the HTA programme as project number 09/107/01. The contractual start date was in July 2010. The draft report began editorial review in May 2012 and was accepted for publication in August 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Pandor et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the health problem
Heart failure (HF) is a complex condition in which cardiac abnormality or dysfunction impairs the capacity of the heart to maintain output without a rise in filling pressures. Clinical presentation typically includes dyspnoea, fatigue, effort intolerance and signs of fluid retention (such as swelling in the extremities). 1 HF is often defined as impaired left ventricular ejection fraction (LVEF) of ≤ 40%. However, uncertainties remain concerning the appropriate threshold for diagnosis. 2,3 For formal diagnosis, clinical examination is usually supplemented with objective assessments of the underlying structural or functional abnormality of the heart and severity of the syndrome, using techniques including electrocardiography, chest radiography and laboratory tests. 1,4
Aetiology, pathology and prognosis
In Western countries, hypertension and coronary artery disease (CAD) are the most common causes of HF, whereas nutritional cardiac disease and valvular heart disease are more common in the developing world. 5 In one Scottish survey, hypertension and CAD (alone or in combination) were identified as the cause of HF in > 90% of cases. 6 HF has also been associated with neurohormonal changes7 – in particular, to brain natriuretic peptide and noradrenaline. Elevated levels of each of these hormones is an independent predictor of morbidity and mortality among HF patients. 8 Behavioural factors, such as smoking and chronic alcoholism, were strongly associated with HF in a large cohort study of men residing in the USA (n = 20,900), with men not adhering to any of the six measured health behaviours (normal weight, not smoking, regular exercise, moderate alcohol intake, consumption of breakfast cereals, consumption of fruit and vegetables) being at the highest risk [21.2%, 95% confidence interval (CI) 16.8 to 25.6%]. 9 Finally, socioeconomic status appears to play a role in the development of HF: a national HF audit commissioned by the NHS10 found that people with a home address in the highest quintile of deprivation are admitted to hospital with HF on average 5 years earlier than those in the lowest quintile (most affluent). A brief list of causal factors is included in Table 1.
| CAD | Myocardial infarction, ischaemia |
| Hypertension | |
| Cardiomyopathy | Dilated (congestive), hypertrophic/obstructive, restrictive (e.g. amyloidosis, sarcoidosis, haemochromatosis) |
| Valvular and congenital heart disease | Mitral valve disease, aortic valve disease, atrial septal defect, ventricular septal defect |
| Arrhythmias | Atrial fibrillation |
| Alcohol and drugs | Alcohol, cardiac depressant drugs (beta-blockers, calcium antagonists) |
| ‘High output’ failure | Anaemia, thyrotoxicosis, arteriovenous fistulae, Paget's disease |
| Pericardial disease | Constrictive pericarditis, pericardial effusion |
| Primary right HF | Pulmonary hypertension, tricuspid incompetence |
Severity of HF is usually assessed using the New York Heart Association (NYHA) system. This system classifies HF as mild (stage I–II), moderate (stage III) or severe (stage IV) based on symptomatic markers (Table 2). 11 Although the NYHA class system does not necessarily reflect the severity of underlying heart dysfunction, it is a useful clinical tool which provides a standardised description of symptom severity that can be used to guide clinical management. Furthermore, NYHA class has been shown to be a strong independent predictor of quality of life (QoL) for patients with HF. 12
| NYHA class | Severity | Symptoms |
|---|---|---|
| I | Mild | No limitations. No fatigue, breathlessness or palpitations in response to ordinary levels of physical activity |
| II | Mild | Comfortable at rest with slight limitation of physical activity. Ordinary physical activity leads to breathlessness, fatigue or angina pectoris |
| III | Moderate | Marked limitation of physical activity. Comfortable at rest but less than ordinary physical activity leads to symptoms |
| IV | Severe | Inability to carry out any physical activity without discomfort. Symptoms of cardiac insufficiency present at rest |
Patients with a new HF diagnosis have a 40% risk of mortality within the first year. 13,14 However, post-discharge mortality incidence varies substantially according to the care setting to which patients are admitted. A recent UK HF audit (between April 2010 and March 2011)14 found annual post-discharge mortality rates of 26.2%, 38.2% and 42.0% for cardiovascular, general medical and other wards respectively (p < 0.001). On average, patients who are discharged have an approximately 28% risk of mortality within the first year after HF discharge. 14 The highest risk period for further complications is immediately after an acute decompensation. 15 Between 20% and 30% of patients are readmitted within 30 days, rising to 50% at 6 months. 16 Prognosis is poor even among people receiving optimal pharmaceutical therapy and so preventative strategies should ideally be pursued with at-risk patients. 17
Epidemiology
Given the ongoing debate around appropriate HF diagnostic criteria,2,3 it is difficult to provide confident estimates of incidence and prevalence of HF. Early epidemiological studies used unreliable diagnostic criteria,18 and although some later surveys incorporated objective assessments of LVEF using echocardiogram6,19 they were limited by excluding adults > 85 years of age and by restricting HF to those with left ventricular systolic dysfunction (LVSD). 20 More recently, a UK population survey in 2009, drawing on an audit of GP registries, estimated total all-age prevalence of HF to be 0.9% for men and 0.7% for women. 10 The largest recent community-based survey, the Echocardiographic Heart of England Screening (ECHOES) study,21 utilised objective European Society of Cardiology (ESC) criteria to determine the presence of HF. A LVEF < 40% was found in 1.8% of the population > 45 years of age (95% CI 1.4 to 2.3%), and definite HF in 2.3% (95% CI 1.9 to 2.8%). In those > 75 years of age, prevalence of LVEF of < 40% and definite HF rose to 3.7% and 6.9% respectively. A recent British Heart Foundation (BHF) survey of the General Practice Research Database (GPRD) found an all-age prevalence rate of 0.9% for the UK, which was lowest in England (0.9%) and highest in Northern Ireland (1.1%). In those > 75 years of age, prevalence rose to 13.7% and 15.3% respectively. 10
Cowie et al. 22 found a crude incidence rate of 1.3 cases per 1000 population in a large West London cohort, rising to 11.6 cases per 1000 population in those > 85 years of age. The age-adjusted incidence was higher among men than among women (incidence ratio 1.75, 95% CI 1.34 to 2.29). 22,23 However, there are important regional variations in incidence. Using data from the GPRD, the BHF found that the incidence of HF in the UK was highest in Northern Ireland and lowest in England (58.1 per 100,000 and 37.5 per 100,000 respectively), with the overall incidence rate being approximately 75% higher among men than among women. 10 The Rotterdam cohort study found similar patterns, with an overall incidence rate of 14.4 per 1000 person-years (95% CI 13.4 to 15.5) rising to 47.4 per 1000 person-years among those aged > 90 years, and a higher incidence rate for men than women (17.6 per 1000 man-years, 95% CI 15.8 to 19.5 and 12.5 per 1000 woman-years, 95% CI 11.3 to 13.8 respectively). 23 The Rochester epidemiology project in the USA also found a higher incidence among men than among women (3.78 per 1000, 95% CI 3.61 to 3.95 and 2.89 per 1000, 95% CI 2.77 to 3.00). 24 HF incidence and prevalence in the UK are set to increase in conjunction with life expectancy as medical therapies for cardiac conditions such as hypertension and myocardial infarction improve. 25
Impact of the health problem
Heart failure is associated with high levels of morbidity and mortality, particularly among those aged > 60 years. 10 One-year mortality for HF patients > 75 years may be twice as high as for those < 75 years. 26 The illness trajectory of HF is unpredictable: NYHA functional classification can improve as well as deteriorate, and sometimes changes unevenly over time. 10 HF also has a substantial impact on patient QoL. In one German cohort study, the Short Form questionnaire-36 items (SF-36) measure was administered to 205 HF inpatients. 12 Using multiple regressions to control for confounders, this research found that NYHA functional class was the only consistent independent predictor of QoL. Evidence suggests a 25–50% prevalence of anxiety and a 18–47% prevalence of depression among HF patients, depending on age, time since diagnosis and other prognostic indicators. 27,28
Heart failure imposes a significant burden on NHS resources. The cost of inpatient bed-days for HF alone has been estimated at £563M per year,29 whereas total HF-related costs have been estimated at £625M per year. 30 HF is a leading cause of hospitalisation in the UK, with 58,164 admissions recorded for HF (as first diagnoses) between April 2009 and March 2010 in England and Wales. 30 Around 90% of HF admissions are to emergency departments,31 with hospitalisations lasting a median of 9 days. 30 As the proportion of people > 60 years of age in the UK continues to increase, and improvements are made in treatment for and survival from cardiac disorders, the burden of HF on the NHS looks set to increase. 32,33
Current service provision
Optimal HF treatment can vary depending on aetiology and severity. Evidence-based treatment guidelines from the National Institute for Health and Care Excellence (NICE)34 advocate pharmaceutical treatment of HF with angiotensin-converting enzyme (ACE) inhibitors and beta-blockers as first line (Table 3). These should be administered initially at a low dose and up-titrated at short intervals until the optimal dosage, or tolerance limit, is reached. If the patient remains symptomatic despite optimal treatment with these agents, a second line of treatment comprising one of three options, an aldosterone antagonist, an angiotensin receptor blocker (ARB) or hydralazine in combination with nitrate, should be considered. 39 For stable patients without clinical contraindications to exercise, supervised, exercise-based group rehabilitation programmes for HF should be offered. Finally, patients should be regularly monitored, although the frequency depends on the clinical status of the patient. Stable patients should be monitored at least every 6 months whereas those with recent changes to medication and/or clinical status should be monitored every few days to every 2 weeks39 (see Table 3).
| Issuing body | Country | Drug therapya | Outpatient monitoring | Device based/surgical |
|---|---|---|---|---|
| NICE34 | England and Wales | First line: ACE inhibitors; beta-blockers. Second line: aldosterone antagonist; angiotensin II receptor antagonist;b hydralazine in combination with nitrateb | Clinical assessment of functional capacity, fluid status, cardiac rhythm and BNP; regular medication review; serum urea, electrolytes, creatinine and eGFR; review at least every 6 months for stable outpatients or every few days to 2 weeks if recent medication change/clinical deterioration; education, support and group-based exercise rehabilitation | Coronary revascularisation, heart transplantation, CRT |
| SIGN35 | Scotland | First line: ACE inhibitors; beta-blockers. Second line: ARBs;b aldosterone antagonists;c diuretics/loop diuretics/metolazone (to relieve symptoms of congestion/fluid retention); digoxin;d hydralazine and isorbide dinitrateb,c | Patient education and communication including a nurse-led, home-based element; behaviour change (smoking cessation, limiting alcohol, supervised exercise training, dietary change); pharmacist input to assess knowledge of drugs and compliance; tailored self-management advice; patient support groups | CRT; assisted ventilation; left ventricular assist devices; cardiac transplantation; intra-aortic balloon counterpulsation |
| CREST36 | Northern Ireland | First line: ACE inhibitors; beta-blockers. Second line: aldosterone antagonists;c diuretics/loop diuretics (for congestion/fluid overload); digoxin;c nitrates and hydralazineb | Multidisciplinary, nurse-led management; action plan for all patients; education (exercise training, sexual activity, smoking cessation, alcohol intake, fluid intake, salt intake, daily weighing, obesity, cachexia, immunisations, travel, medication advice); psychological management | Heart transplantation; CRT; coronary revascularisation |
| ESC37 | Europe | First line: ACE inhibitors; beta-blockers. Second line: aldosterone antagonists;c,d ARBs;b,d hydralazine and isorbide dinitrate;b,d digoxin (for arrhythmias); diuretics (to relieve congestions); statins (for systolic dysfunction caused by CAD) | Multidisciplinary approach led by HF nurses; early follow-up post-discharge; patient education with emphasis on self-care; physical activity training; self-monitoring of weight, symptoms, diet, fluid intake and alcohol; involve patient in symptom monitoring and flexible diuretic use; remote monitoring; psychosocial support | Revascularisation; valvular surgery; CRT; heart transplantation; left ventricular assist devices and artificial hearts; ultrafiltration (to relieve congestion) |
| AHA38 | USA | First line: ACE inhibitors; beta-blockers. Second line: ARBs;b aldosterone antagonist;c hydralazine and nitrates;b,c digitalis; diuretics/loop diuretics and salt restriction (for congestion/fluid overload); vasodilators (to relieve congestion if adequate blood pressure); intravenous inotropes (only in patients with low blood pressure and cardiac output who can be closely monitored) | Close observation and follow-up; exercise training; written educational materials (activity level, diet, discharge medications, follow-up appointment, weight monitoring and response to symptoms); discharge planning with emphasis on medication compliance; psychosocial support; access to palliative services | CRT; coronary revascularisation; cardiac transplantation; left ventricular assist device; pulmonary artery catheter placement; ultrafiltration (if pharmacological diuretic strategies unsuccessful) |
Ideally, inpatient treatment should be provided by a specialist centre. Patients admitted to specialist cardiology wards have better survival rates while hospitalised and in the first year post discharge – a relationship that remains when age, HF aetiology, echocardiology, heart rhythm, sex and symptoms are adjusted for. 29 Overall, patients admitted to specialist centres are around half as likely to die in hospital as those admitted to general wards. 10 Post-discharge, multidisciplinary disease management programmes comprising patient education, optimal medical treatment and psychosocial care have been associated with decreased hospitalisation and improved clinical outcomes. 40–43 However, access to these services is limited because of funding-related barriers or geographical location. 44,45 Furthermore, there are inequalities in access to services in terms of sex and age. Only 38% of people referred to the UK's HF liaison service between 2008 and 2009 were women and, although approximately 70% of patients < 45 years of age were referred to the liaison service, this figure fell across the age groups to < 21% in those ≥ 95 years. 30
Description of technology under assessment
Telemedicine is an emerging approach utilising remote monitoring (RM) of prognostic indicators (e.g. weight, arrhythmias, blood pressure, intrathoracic impedance, heart rates during rest and exertion) to facilitate early detection of clinically significant changes, prevent emergency admissions and avoid complications. 46 Guidelines from the ESC currently recommend RM for patients with HF (see Table 3). Because the highest risk period for rehospitalisation is the first few weeks after discharge, RM interventions should be performed at least once in the first 28 days following discharge. RM encompasses a range of approaches depending on what physiological data are transferred to clinicians, how the data are transferred (e.g. automatically or manually, by telephone contact or through a secure web server) and how these data are utilised. Broadly speaking, two main approaches have emerged: telemonitoring (TM), in which physiological data are electronically transmitted to a health-care team, and structured telephone support (STS), that is, the use of telephone calls, usually by specialist nurses, to deliver self-care support and/or management. 47,48 For STS, support can be provided by human-to-human contact (HH) or through a human-to-machine interface (HM), that is, STS with an interactive response system. For TM, support can be provided during office hours (9 am to 5 pm, Monday to Friday) only or 24 hours a day, 7 days a week (24/7), although few studies have used the latter approach. Further details are provided in Table 4. Cardiovascular implanted monitoring devices such as modern pacemakers, implantable cardioverter defibrillators or cardiac resynchronisation devices are also capable of delivering remote physiological monitoring, often without the need for a patient to trigger the transmission of data. 53 The equipment and personnel requirements vary according to the type of RM and a number of systems have been described. 54
| TM | STS | |||
|---|---|---|---|---|
| Office hours only | 24/7 | HH | HM | |
| Description | Patients take measurements (manual or automated) of vital parameters (most commonly weight, BP and HR) at home, which are transmitted to a health-care team or HF specialist centre by telephone, mobile telephone, cable network or broadband technology. Transmitted data are reviewed by medical staff (in some cases readings outside of prespecified limits may generate automated warnings) during office hours (including provision of medical support) | Same as TM during office hours but constant presence of medical personnel required to operate the support system, i.e. 24 hours a day, 7 days a week | Patients followed up with regular telephone calls by a care provider. Calls typically from HF specialist nurses and include advice on self-care and medication. STS may also incorporate basic monitoring of physiological parameters (e.g. weight) | Patients monitored by automated telephone-based interactive response system. May include questions about HF symptoms to which patients can respond on telephone keypad |
| Example | Cleland et al.49 provided patients with a scale and sphygmomanometer. Patients took twice daily measurements of vital parameters (weight, BP, HR and single lead ECG using wristband electrodes). Results were encrypted and sent via a secure web server to a computer at each investigator site. Medical support was provided during office hours | Koehler et al.50 provided a wireless Bluetooth system with a personal digital assistant and three integrated devices: an ECG lead, a BP cuff and weighing scales. Encrypted measurements were sent via a secure server to participating sites. These sites provided physician-led medical support 24 hours a day, 7 days a week | Angermann et al.51 provided telephone-based structured monitoring delivered by trained nurses (supervised by a cardiologist and a psychologist), which included educational material/self-monitoring schemes and multidisciplinary advice | Chaudhry et al.52 used an interactive system that required patients to provide daily readings of vital parameters, which were sent to a secure internet site and reviewed by clinicians on weekdays |
Use of information communication technology may help provide wider access to HF programmes for a larger number of patients, including those constrained by geography, transport or infirmity. 15 When a care plan has been agreed with a patient, TM and STS interventions can promote a rapid response when vital clinical signs fall outside agreed parameters, for example by up-titrating medication or arranging for a clinical visit. RM could also minimise the incidence of difficult-to-treat complications, and use early warning signs to avoid hospitalisation. Conversely, RM may generate false alerts leading to inappropriate hospitalisation55 and it may not be feasible for health-care providers to contact all patients regularly or provide specialist equipment to all patients who may potentially benefit.
Telemedical interventions for a variety of chronic conditions are currently being investigated and rolled out by a number of UK NHS trusts. For example, NHS North Yorkshire and York (NY&Y) has seen approximately 500 patients with long-term conditions including HF receive a TM intervention. HF patients were supplied with RM equipment, which generated medication prompts, along with weighing scales and a blood pressure and pulse meter (Julie Ryan, Telehealth Project Manager, NHS NY&Y, 2 April 2012, personal communication). NY&Y are in the process of rolling out the initiative to cover 2000 people. 56 In addition, the UK Department of Health released headline findings from the Whole System Demonstrator (WSD) programme for telehealth in December 2011. 57 This randomised evaluation of the impact of telehealth for people with chronic conditions [diabetes, HF and chronic obstructive pulmonary disease (COPD)] included over 6000 patients from sites in Newham, Kent and Cornwall, and reported strongly positive results, including a 45% reduction in mortality. However, these results should be interpreted with caution. The Department of Health's release of these findings before peer review makes their robustness difficult to evaluate, and data on potential confounding factors, such as face-to-face clinical contact, are not yet publicly available. Nevertheless, the early release of these findings underscores the enthusiasm for telehealth among some quarters of the UK health-care authorities.
Two recent meta-analyses demonstrated significant benefits of RM interventions in terms of mortality and hospitalisation48,58 [it is noteworthy that shortly following approval of this review protocol the original Cochrane systematic review published by Clark et al. 59 (search date from January 2002 to May 2006) was superseded by that by Inglis et al. 48 (search date from January 2006 to November 2008)]. However, since the publication of the latest of these reviews, randomised controlled trials (RCTs) demonstrating minimal or no clinical benefits have been published. 50,52,60 Furthermore, neither the review by Klersy et al. 58 nor that of Clark et al. 59 (including the recent update by Inglis et al. 48) included an economic evaluation of telemedicine.
Chapter 2 Definition of the decision problem
Purpose of the decision to be made
The aim of this assessment was to investigate the clinical effectiveness and cost-effectiveness of home TM or STS programmes for adults who have been recently discharged (within 28 days) from an acute care setting after a recent exacerbation of HF (including subgroups such as those with transiently or persistently severe HF).
Clear definition of the intervention
Telemonitoring, defined as the use of information and communication technologies to monitor and transmit items related to patient health status between geographically separated individuals,54 permits home monitoring of patients (living at home or in nursing or residential care homes) using external electronic devices in conjunction with a telecommunications system (landline or mobile telephone, cable network or broadband technology). TM allows frequent or continuous assessment of HF signs and symptoms measured by patients, family or caregivers at home, while allowing patients to remain under close supervision. 37,59 Symptoms reported by patients can be remotely reviewed by a health-care professional and appropriate action can be initiated. Telephone support is another form of remote management that can be provided through structured telephone contact between patients and health-care providers (with or without home visits) and reporting of symptoms and/or physiological data. 58,59 Cardiovascular implanted monitoring devices such as modern pacemakers, implantable cardioverter defibrillators or cardiac resynchronisation devices are also capable of delivering remote physiological monitoring, often without the need for a patient to trigger the transmission of data. 53
The highest risk period for rehospitalisation is in the first few weeks after discharge from hospital. 15 STS and/or home TM interventions should be performed at least once within the first 28 days following discharge from hospital and must be targeted towards patients and intended to address patient concerns and problems and not those of caregivers. 59 The optimum time period for TM is unclear; however, it is likely that services will provide TM or STS for at least 4–6 months following discharge from hospital with its usefulness evaluated at 30-day intervals thereafter. The review focuses on the use of home TM or STS programmes for patients who have been discharged from an acute care setting after a recent exacerbation of HF.
Population and relevant subgroups
The population included any adults (defined as ≥ 18 years of age) of either sex or any ethnic group with a diagnosis of HF and discharged from an acute care setting (including emergency departments and 1-day stay procedures) to home (including a relative's home, nursing home or residential care home). The identification of subgroups of patients for whom home TM or STS programmes are appropriate or inappropriate was governed by the available evidence; however, on a priori grounds, information was sought for people with transiently or persistently severe HF.
Relevant comparators
The relevant comparator was considered to be usual care. This involves standard post-discharge multidisciplinary care without regular follow-up and may include (1) in-person follow-up visits to a primary care physician, (2) attendance at a clinic-based chronic heart failure (CHF) disease management programme and (3) any visits at home by a specialised CHF health-care professional (referred to as enhanced conventional care). 58,59
Outcomes.
The outcomes of the review were mortality (all-cause), all-cause admission to hospital, HF-related admission to hospital, length of stay (days in hospital), health-related quality of life (HRQoL) and acceptability of interventions to patients.
Overall aims and objectives of the assessment
The review had the following objectives:
Chapter 3 Assessment of clinical effectiveness
A systematic review of the literature and (network) meta-analysis (where appropriate) was undertaken to evaluate the clinical effectiveness of home TM or STS strategies compared with usual care for adults who have been recently discharged (within 28 days) from an acute care setting after a recent exacerbation of HF.
A review of the evidence was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (www.prisma-statement.org/).
Methods for reviewing effectiveness
Identification of studies
Electronic databases
Studies were identified by searching the following electronic databases and research registers:
-
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R) (Ovid) 1948–January 2012
-
EMBASE (Ovid) 1980–January 2012
-
Science Citation Index Expanded (Web of Science) 1899–January 2012
-
Conference Proceedings Citation Index – Science (Web of Science) 1990–January 2012
-
Cochrane Database of Systematic Reviews (Wiley Online Library) 1996–January 2012
-
Cochrane Central Register of Controlled Trials (Wiley Online Library) 1898–January 2012
-
Health Technology Assessment database (Wiley Online Library) 1995–January 2012
-
Database of Abstracts of Reviews of Effects (Wiley Online Library) 1995–January 2012
-
PsycINFO (Ovid) 1806–January 2012
-
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost) 1982–January 2012
-
Allied and Complementary Medicine Database (Ovid) 1985–January 2012
-
UK Clinical Research Network (CRN) Portfolio Database [National Institute for Health Research (NIHR)] 2001–January 2012
-
ClinicalTrials.gov (US NIH) 2000–January 2012
-
Institute of Electrical and Electronics Engineers/Institution of Engineering and Technology (IEEE/IET) Electronic Library (IEEE Xplore) 1988–January 2012.
Sensitive keyword strategies using free text and, where available, thesaurus terms using Boolean operators and database-specific syntax were developed to search the electronic databases. Synonyms relating to the condition (e.g. HF) were combined with terms for TM. No language restrictions were used on any database; however, the clinical effectiveness searches were restricted by date. The current review updated two existing systematic reviews48,58 of TM or STS programmes for patients with HF (within the scope of the current review). In the review by Inglis et al. 48 the searches examined the period from January 2006 to November 2008 and in Klersy et al. 58 the searches examined the period from January 2000 to October 2008. As the search strategies from the existing systematic reviews were of good quality (and clearly reported) it was assumed that all studies prior to 2008 should have been identified. Thus, the clinical effectiveness searches were limited by date from 2008 to January 2012. An example of the MEDLINE search strategy is provided in Appendix 1.
Other resources
To identify additional published, unpublished and ongoing studies, the reference lists of all relevant studies (including existing systematic reviews) were checked. Citation searches of relevant articles using the Web of Science Science Citation Index was also undertaken to identify articles that cite the relevant articles. addition, key experts in the field were contacted.
All identified citations from the electronic searches and other resources were imported into and managed using Reference Manager bibliographic software (version 12; Thomson Reuters, Philadelphia, PA).
Inclusion and exclusion criteria
The inclusion of potentially relevant articles was undertaken using a two-step process. First, all titles were examined for inclusion by one reviewer. Any citations that clearly did not meet the inclusion criteria, that is, non-human, unrelated to TM and/or HF, were excluded. Second, all abstracts and full-text articles were examined independently by two reviewers. Any disagreements in the selection process were resolved through discussion. The relevance of each article for the systematic review was assessed according to the following criteria.
Study design
All RCTs or observational cohort studies with a contemporaneous control group published from 2008 to January 2012 (as well as those identified by the existing systematic reviews) that evaluated home TM or STS programmes compared with usual post-discharge multidisciplinary care for adults who have been recently discharged (within 28 days) from an acute care setting to home (including a relative's home, nursing home or residential care home) after a recent exacerbation of HF were included. Before-and-after studies without a concurrent control group were excluded because the absence of a control group to record concurrent changes over time means that changes due to the intervention or due to temporal trends, concurrent changes or a Hawthorne effect would be conflated. Such studies therefore represent very weak evidence of effectiveness.
Reviews of primary studies were not included in the analysis but were retained for discussion and identification of additional studies. Moreover, the following publication types were excluded from the review: animal models; preclinical and biological studies; narrative reviews, editorials, opinions; non-English-language papers and reports published as meeting abstracts only when insufficient methodological details are reported to allow critical appraisal of study quality.
Population
The population comprised adults (defined as ≥ 18 years of age) with a diagnosis of HF and discharged from an acute care setting to home (including a relative's home, nursing home or residential care home).
Interventions
The following interventions were included: (1) remote home TM using patient-initiated external electronic devices or cardiovascular implanted monitoring devices, with transfer of physiological data from the patient to the health-care provider by landline or mobile telephone, cable network or broadband technology, (2) STS including regularly scheduled telephone contact between patients and health-care providers and reporting of symptoms and/or physiological data. In addition, STS and/or home TM interventions were required to be performed at least once within the first 28 days following discharge from hospital, and to be targeted towards patients and intended to address patient concerns and problems and not those of caregivers.
Relevant comparators
The relevant comparator was considered as usual care. This involved standard post-discharge multidisciplinary care without regular follow-up and may include (1) in-person follow-up visits to a primary care physician, (2) attendance at a clinic-based CHF disease management programme and (3) any visits at home by a specialised CHF health-care professional (referred to as enhanced conventional care). 58,59
Outcomes
The outcomes of the review included mortality (all-cause), all-cause admission to hospital, HF-related admission to hospital, length of stay (days in hospital), HRQoL and acceptability of interventions to patients.
Data abstraction strategy
Data abstraction was performed by one reviewer into a standardised data extraction form and independently checked for accuracy by a second. Discrepancies were resolved by discussion between the two reviewers; if agreement could not be reached a third reviewer was consulted. When multiple publications of the same study were identified, data were extracted and reported as a single study. Moreover, as this was an update of two existing reviews,48,58 all relevant data were extracted from the reviews in the first instance and cross-checked for accuracy with the original papers. When necessary, additional data were extracted from the original papers or, in cases in which information was missing from the articles, authors of the respective studies were contacted to provide further details.
The following information was extracted for all studies when reported: study characteristics (e.g. author, year of publication, country, study design, setting, duration of follow-up, funding), participant details (e.g. inclusion and exclusion criteria, age, sex, autonomy, comorbidities), intervention and comparator details (e.g. description, system activity, frequency of measurement, parameters measured) and outcomes (including definitions).
Quality assessment strategy
The methodological quality of each included study was assessed by one reviewer and independently checked by another. Disagreements were resolved by discussion between the two reviewers and, if agreement could not be reached, a third reviewer was consulted. The study quality characteristics were assessed according to (adapted) criteria based on those proposed by Verhagen et al. 61 for RCTs and by Wells et al. 62 for observational studies. Further details are provided in Appendix 2.
Methods of data synthesis
Primary analyses (recently discharged patients with heart failure)
The extracted data and quality assessment variables were presented for each study both in structured tables and as a narrative description. Mortality (all-cause), all-cause admission to hospital and HF-related admission to hospital were subjected to formal (network) meta-analyses. A network meta-analysis (NMA) allows a comprehensive comparison of all interventions that are linked with respect to at least one common intervention without breaking the randomisation within studies. The summary statistics that were analysed were the number of patients who had an event. In each case the data were analysed using a random-effects model (to allow for heterogeneity in treatment effects across studies) implemented using the WinBUGS software package, version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK). 63,64 The statistical model accounted for the variation in follow-up between studies using a complementary log-log link function (see Appendix 3). This model assumed that the parameter being analysed was the event rate (i.e. hazard) from an exponential survivor function and that an intervention effect relative to the baseline treatment was the (log-) hazard ratio (HR). Convergence of the model to its posterior distribution was assessed using the Gelman–Rubin convergence statistic. 65 In each case, convergence occurred within 20,000 iterations so the final analysis used a burn-in of 20,000. There was some suggestion of high autocorrelation between successive iterations of the Markov chains; to compensate for this the Markov chains were thinned every 10 iterations. Parameter estimates were estimated based on 10,000 iterations of the Markov chains. The total residual deviance was used to formally assess whether or not the statistical model provided a reasonable representation of the sample data. The total residual deviance is the mean of the deviance under the current model minus the deviance for the saturated model, so that each data point should contribute about 1 to the deviance. Results of the NMA were reported in terms of the HR and 95% credible interval (CrI) relative to the baseline intervention (i.e. usual care). The posterior median of the between-study standard deviation together with the 95% CrI was also presented. To account for potential heterogeneity in intervention effects between studies, the posterior predictive distribution for the HR of a new study was also presented.
The original intention was to use meta-regression in an attempt to explain any heterogeneity in the effects of the interventions amongst the studies. Potential treatment effect modifiers were quality of usual care, different telehealth intervention settings, adherence, age, sex and autonomy (i.e. single vs couple). However, because of the lack of availability of data on these study-level covariates, meta-regressions were not performed.
Additional analyses (patients with stable heart failure)
Following advice from clinical experts, additional analyses were undertaken to assess whether or not the results from the primary analysis differed markedly from results in those with stable HF who were managed in the community. In this supplementary analysis the following studies were included: RCTs comparing HF management delivered via STS, TM or cardiovascular implanted monitoring devices with HF management delivered via usual post-discharge care in stable HF patients (defined as having no acute event or deterioration in the past 28 days) who were managed in the community setting (ambulatory or outpatient care). Studies that included intensified management with additional home or clinic visits were excluded. Although no formal critical appraisal of these studies was undertaken, the results were meta-analysed (as per the methods of the primary analysis) and presented for information only. All studies published before 2008 were identified from Inglis et al. 48 and Klersy et al. ,58 whereas more recent studies (meeting these criteria) were identified using the current review.
Results of the clinical effectiveness review
This section first provides a brief overview of the evidence from the two existing systematic reviews48,58 of RM for HF [including a methodological quality assessment using a measurement tool developed by Shea et al. 66 for the ‘assessment of multiple systematic reviews’ (AMSTAR)]. Second, this section presents the results of the current systematic review, including additional analyses.
Overview of existing systematic reviews
The first of the two existing systematic reviews and meta-analyses was published by Clark et al. in 200759 and later updated by Inglis et al. in 2010. 48 The review included RCTs comparing HF management strategies delivered via STS or TM with usual post-discharge care in HF patients recently discharged from an acute care setting to home or while managed in the community setting. Any interventions that included home visits by specialised HF health-care professionals or study personnel for the purpose of education or clinical assessment, other than an initial visit to set up equipment, were excluded. The primary outcomes of interest were all-cause mortality, HF-related admission to hospital and all-cause readmissions to hospital. Secondary outcomes included length of stay, QoL, health-care cost savings in patients with HF and acceptability of the intervention to patients with HF. Overall, 30 RCTs of STS and TM were identified (25 peer-reviewed publications and five abstracts). Of the 25 peer-reviewed studies, 11 evaluated TM and 16 evaluated STS, with two testing both STS and TM in separate intervention arms compared with usual care.
The second review was conducted by Klersy et al. 58 and was published in December 2009. The review included RCTs and observational studies comparing HF management strategies delivered via STS (with or without home visits), TM or cardiovascular implanted monitoring devices with usual post-discharge care in HF patients. Overall, 32 studies were identified (20 RCTs and 12 cohort studies). Of the 20 RCTs, 11 evaluated STS, 7 evaluated TM (including cardiovascular implanted monitoring devices) and 2 tested both STS and TM. Of the 12 cohort studies, 6 were between-arm studies. The outcomes of interest included all-cause mortality, all-cause hospitalisation, HF-related hospitalisation and a composite end point comprising all-cause hospitalisation or death from any cause. Despite ostensibly being reviews of the same literature, as their objectives would suggest (except for studies of cardiovascular implanted monitoring devices), there was limited overlap between the two reviews in terms of the primary studies that were included. The lack of overlap of included studies may be largely explained by the differences in their search strategies (including search dates) and inclusion/exclusion criteria. For example, Inglis et al. 48 searched 15 electronic databases (including research registers) from 1966 to November 2008 and included RCTs that had interventions without home visits or intensified clinic follow-up. In contrast, Klersy et al. 58 searched five electronic databases from January 2000 to October 2008 and included RCTs and observational studies that had interventions with or without home visits. A summary of all of the included studies in both reviews (including discordance) is presented in Appendix 4.
The methodological quality of both systematic reviews was judged to be high, indicating low risk of bias: Inglis et al. 48 met 9 of the 11 criteria whereas Klersy et al. 58 met 7 of the 11 criteria (see Appendix 5). Both reviews provided an ‘a priori’ design, had at least two authors conduct data extraction independently, provided lists and comprehensive details of included studies, assessed the likelihood of publication bias and assessed the scientific quality of the meta-analysed trials. In addition, Klersy et al. 58 utilised appropriate methods for data synthesis and Inglis et al. 48 conducted a comprehensive literature search and reported potential conflicts of interest from both the review authors and the authors of the included trials. However, Klersy et al. 58 did not report supplementing their literature search by consulting current contents, reviews, textbooks, specialised registers and reference lists of identified literature nor did they explicitly state that they searched for reports regardless of their publication type. In addition, they did not refer to the quality of the synthesised literature when formulating recommendations and did not refer to the sources of support received by authors of the included trials. Inglis et al. 48 also did not refer to the quality of the included trials when making recommendations and did not use appropriate statistical methods for synthesising heterogeneous results (all analyses were performed using a fixed-effects model). It is also worth noting that Klersy et al. 58 did not specify their HF patient population of interest. Inglis et al. 48 included both recent discharge HF patients and stable HF patients managed in the community, although they did not specify their interpretation of recent discharge.
Quantity and quality of research available in the current (and existing48,58) systematic reviews
Number of studies identified/included
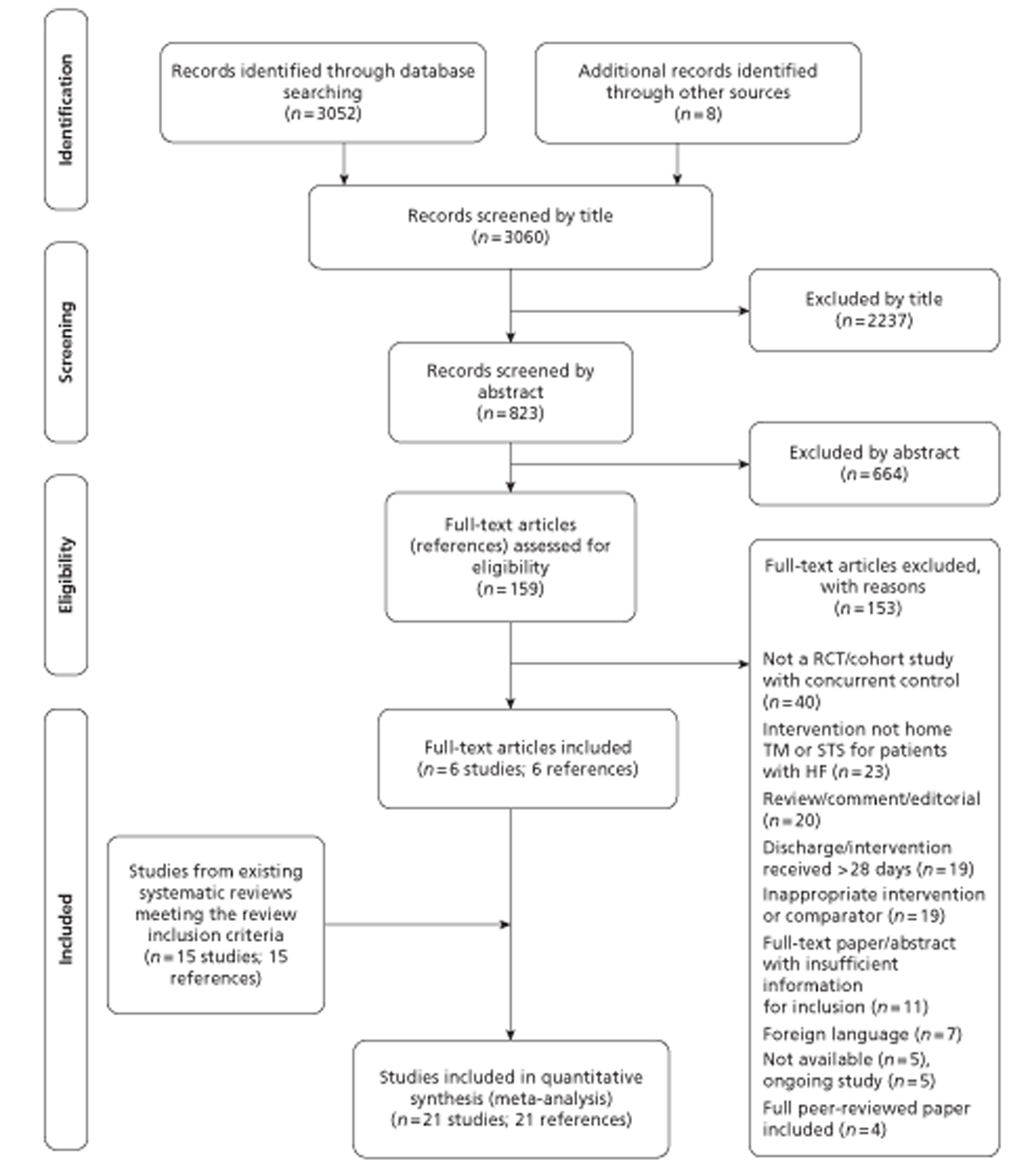
The literature searches identified 3060 citations. Of these, six RCTs52,60,67–70 met the inclusion criteria and were added to the 15 trials49,51,71–83 from the previous systematic reviews. 48,58 No trials of cardiovascular implanted monitoring devices or observational studies met the inclusion criteria of the current review. A flow chart describing the process of identifying relevant literature can be found in Figure 1.
Number and type of studies excluded
A total of 153 full-text articles were excluded as they did not meet all of the prespecified inclusion criteria. The majority of the articles were excluded primarily on the basis of inappropriate study design (not RCTs or cohort studies without concurrent controls), incorrect intervention (not home TM or STS for patients with HF) or unsuitable publication type (reviews, commentaries or editorials). A full list of excluded studies with reasons for exclusion is presented in Appendix 6.
Assessment of effectiveness
Description of included studies (design and patient characteristics)
The design and patient characteristics of the 21 included studies that evaluated home TM or STS programmes for adults who have been recently discharged (within 28 days) from an acute care setting after a recent exacerbation of HF are summarised in Tables 5 and 6 respectively. Of these, 11 studies evaluated STS [10 used standard telephone equipment using HH51,69,72,74,77–82 and one provided support via an automated telephone interactive response system (HM) with an alert system52], nine studies assessed TM,60,67,68,70,71,73,75,76,83 and one study assessed both STS and TM compared with usual care. 49 Almost all of the studies used different measures and devices as part of the STS and TM interventions.
| Author, year | Country (sites) | Total patients | Intervention | Comparator | Follow-up duration | Primary outcome | Funding |
|---|---|---|---|---|---|---|---|
| STS HH vs TM vs usual care | |||||||
| bCleland et al. 2005 (TEN-HMS)49 | Germany, Netherlands, UK (16 sites) | 258 | Structured (monthly) telephone-based monitoring (of symptoms and current medication) and education (n = 173). Managed by a HF specialist nurse | Standard care. Followed up by GP with pharmacological treatment according to individualised patient management plan (n = 85) | 240 days and 450 days | Composite of any hospital admission or mortality | Joint European Union (Trans-European Network) and Philips Medical Systems |
| 253 | Home TM. Twice-daily measurement, automatic transmission of weight, BP, HR and single-lead ECG (n = 168). Specialist nurse-led support (following automated alert, nurses provided advice directly or through GP for long-term changes in therapy) together with monthly telephone calls to assess the patient's symptoms and current medication. Management of patients according to preset guidelines | Standard care. Followed up by GP with pharmacological treatment according to individualised patient management plan (n = 85) | 240 days and 450 days | Composite of any hospital admission or mortality | Joint European Union (Trans-European Network) and Philips Medical Systems | ||
| STS HM (e.g. telephone-based interactive response system) vs usual care | |||||||
| Chaudhry et al. 2010 (Tele-HF)52 | USA (33 cardiology practices) | 1653 | Structured (daily) telephone-based monitoring (of symptoms and weight) using an interactive voice response system (n = 826). Reviewed by a clinician every weekday except on holidays. Guideline-based therapy | Standard optimal care. Followed by local physician. Guideline-based therapy (n = 827) | 6 months | Composite of readmission for any reason or death | National Heart, Lung, and Blood Institute, USA |
| STS HH vs usual care | |||||||
| aAngermann et al. 2011 (INH)51 | Germany (nine hospitals) | 708 | Structured (weekly during the first month, then individualised: fortnightly in NYHA class III and IV, monthly in NYHA class I and II) telephone-based monitoring (of symptoms and current medication) and modular education (n = 352). Managed by trained nurses who were supervised by a cardiologist and a psychologist | Standard care. Followed up by GP plus 6-monthly visits to a HF clinic (n = 363) | 6 months | Composite of time to all-cause death or rehospitalisation | German Ministry of Education and Research, German Competence Network Heart Failure, University of Wurzburg, Germany |
| a,bBarth 200172 | USA (one hospital) | 34 | Structured (at 72 hours, 144 hours and then fortnightly) telephone-based monitoring (of signs, symptoms and weight) and education (n = 17). Nurse-managed | Standard care (no details provided) (n = 17) | 3 months | NR | NR |
| a,bDeBusk et al. 200474 | USA (five hospitals) | 462 | Structured (weekly for 6 weeks, biweekly for 8 weeks and then monthly and bimonthly) telephone-based HF lifestyle education and medication management (n = 228). Physician-directed nurse-managed case management | NR; however, standard care appeared to involve a high frequency of all kinds of follow-up clinic visit (13 in 12 months following hospitalisation) (n = 234) | 12 months | Composite of rehospitalisation for HF or all-cause rehospitalisation | National Heart, Lung, and Blood Institute, USA |
| Domingues et al. 201169 | Brazil (one tertiary hospital) | 120 | Structured (weekly for first month, every 15 days for following 2 months) telephone-based education and monitoring of signs and symptoms of decompensation (n = 57). Nurse managed | Standard care (no details provided) (n = 63) | 3 months | Level of HF awareness and self-care knowledge | Fundação Instituto de Pesquisas Econômicas (FIPE) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil |
| a,bLaramee et al. 200377 | USA (one hospital) | 287 | Structured (weekly for first 4 weeks, then biweekly) telephone-based monitoring (of signs and symptoms) and education (n = 141). Nurse managed | Standard care. Followed up by local physician (44% received some home care services) (n = 146) | 3 months | All-cause readmission | University of Vermont General Clinical Research Centre and Novartis Pharmaceuticals |
| aRainville 199978 | USA (one site) | 38 | Structured (at days 3, 7, 30 and 90 and 12 months) telephone-based education, medication review and management and weight monitoring (n = 19). Pharmacist led | Standard care. Followed up by pharmacist at 30 days, 90 days and 12 months to determine readmissions (n = 19) | 12 months | Composite of hospital readmission for HF or mortality | NR |
| a,bRiegel et al. 200279 | USA (two hospitals) | 358 | Structured (at day 5 and thereafter at a frequency guided by the software and case manager) telephone-based education and monitoring of signs and symptoms (e.g. weight, fluid retention, dyspnoea) (n = 130). Nurse managed with guidance and liaison with primary care physician | Non-standardised care (no details provided) (n = 228) | 6 months | HF rehospitalisations | Pfizer Inc., USA |
| a,bRiegel et al. 200680 | USA (two hospitals) | 135 | Structured (at day 5 and thereafter at a frequency guided by the software and case manager) telephone-based education and monitoring of signs and symptoms indicating worsening illness (n = 70). Nurse managed with guidance and liaison with primary care physician | Non-standardised care (no details provided) (n = 65) | 6 months | HF rehospitalisations | American Heart Association, USA |
| aTsuyuki et al. 2004 (REACT)81 | Canada (10 hospitals) | 276 | Structured (at 2 and 4 weeks and then monthly for 6 months) telephone-based education and monitoring of signs and symptoms (including salt and fluid restriction and weight) (n = 140). Managed by local research co-ordinator with recommended follow-up by local physician for pharmacological therapy | Standardised care (no details provided) (n = 136) | 6 months | Medication adherence | Pfizer, Canada and University of Alberta Hospital Foundation, Canada |
| aWakefield et al. 200882 | USA (one hospital) | 148 | Structured (three times in first week, then weekly for 11 weeks) telephone- or videophone-based education and monitoring of signs and symptoms (including weight, BP and ankle circumference) (n = 99). Managed by trained nurses | NR; however, subjects contacted their primary care nurse case manager by telephone if needed (n = 49) | 12 months | Readmission rates | Veterans Health Administration, Health Services Research & Development Service, USA |
| TM vs usual care | |||||||
| aAntonicelli et al. 200871 | Italy (one hospital) | 57 | Home TM. Weekly measurement, manual transmission of weight, BP, HR, 12-lead ECG, 24-hour urine output (n = 28). Reviewed by HF team at least once per week and patient management (including therapeutic regimens) modified accordingly | Standard care. Followed by a HF specialist team (including routinely scheduled clinic visits) (n = 29) | 12 months | Composite of mortality and hospitalisation | Italian Ministry of Health, Italy |
| aCapomolla et al. 200473 | Italy (one hospital) | 133 | Home TM. Daily measurement, manual transmission (using touchpad of home or mobile telephone to an interactive voice response system) of weight, systolic BP, HR and symptoms (n = 67). Automated alert (via computer software) prompting a telephone call by nurse or physician to the patient at home. Individualised care plan designed by the physician | Standard care. Followed up by GP with support of a cardiologist. During follow-up the process of care was governed by different providers with a heterogeneous range of strategies: emergency room management, hospital admission and outpatient access (n = 66) | 12 months | Composite of rehospitalisation, emergency room access and total mortality | Ministero della Salute, Italy |
| Dar et al. 2009 (Home-HF)67 | UK (three acute hospitals) | 182 | Home TM. Daily measurement, manual transmission of weight, BP, HR, oxygen saturation and symptoms (n = 91). Data were reviewed daily (Monday to Friday) by HF nurses. Variation of parameters outside predefined limits triggered an alert and resulted in a telephone call for further assessment | Standard care. Each site had a specialist HF service including at least one cardiologist or physician with an interest in HF and at least one HF specialist nurse. Regular clinical follow-ups were scheduled at the discretion of the HF team and telephone support was available during office hours (n = 91) | 6 months | Days alive and outside of hospital | Honeywell HomMed, UK |
| Dendale et al. 2011 (TEMA-HF 1)68 | Belgium (seven hospitals) | 160 | Home TM. Daily measurement, automatic transmission of weight, BP, HR (n = 80). Automated email alert to GP and HF clinic. Followed up by GP visit or contact. Guideline-based therapy | Standard care. Followed up by GP (with referral to specialist cardiologist if needed). Guideline-based therapy. No intervention by study nurse or HF clinical team (n = 80) | 6 months | All-cause mortality | Belgian Government Health Insurance Institute and Leo Pharma, Belgium |
| a,bGoldberg et al. 2003 (WHARF)75 | USA (16 sites) | 280 | Home TM. Daily measurement, manual transmission of weight and symptoms (n = 138). Data reviewed daily by nurses (7 days a week, 365 days a year; however, system was not active 24 hours a day) and concerns reported to a treating physician. Guideline-driven HF care (with additional nursing resources) | Standard care. Followed up by treating physician (at discretion) in a dedicated outpatient HF programme with additional nursing resources. In addition, patients undertook daily weight measurements and were instructed to contact their physician for weight increases of more than a prespecified amount or if their symptoms of HF worsened (n = 142) | 6 months (mean) | Hospital readmission | Alere Medical, Inc., USA |
| a,bKielblock et al. 200776 | Germany (sites NR) | 502 | Home TM. Daily measurement, automatic transmission of weight (n = 251). Automated alert to the HF specialist team, which prompted a telephone call by a designated personal adviser. Followed up by GP (24-hour on-demand telephone service including medical support) | Standard care (no details provided) (n = 251) | 12 months | Hospital stay | NR |
| aKulshreshtha et al. 201060 | USA (one hospital) | 110 | Home TM. Daily measurement, manual transmission of weight, BP, pulse and pulse oximetry (n = 68). Nurse-led support (including physician or cardiologist notification, referral to emergency room) together with weekly telephone calls to provide additional information (including evaluation of home TM readings and telephone assessment) and monitor adherence | Standard care (no details provided) (n = 42) | 6 months | All-cause rehospitalisation rate | Partners Healthcare, USA |
| Scherr et al. 2009 (MOBITEL)70 | Austria (eight centres) | 120 | Home TM. Daily measurement, manual transmission of weight, BP, HR and dosage of HF medication (n = 66). Automated e-mail alert prompting a telephone call from a physician via mobile telephone. Guideline-based therapy | Standard care. Pharmacological treatment according to guideline-based therapy (n = 54) | 6 months | Composite of cardiovascular mortality/hospital readmission for worsening HF | Novartis Pharma, Roche Pharma and Mobikom, Austria |
| a,bWoodend et al. 200883 | Canada (one site) | 121 | Home TM. Daily measurement, manual transmission of weight, BP and 12-lead ECG (periodic) (n = 62). Reviewed weekly by telehome-care nurse via video conference (frequent in first few weeks and tapered over 3 months). Triage protocol-based management | Standard care. Followed up by community physician or cardiologist (no further details provided) (n = 59) | 3 and 12 months | NR | Richard Ivey Foundation, Change Foundation and Merck-Frosst Canada |
| Author, year | Population | Mean age (years) | Male (%) | NYHA class | Autonomy (living alone) (%) | LVEF for inclusion | Period between remote intervention and hospital discharge |
|---|---|---|---|---|---|---|---|
| STS HH vs TM vs usual care | |||||||
| a,bCleland et al. 2005 (TEN-HMS)49 | Patients (aged ≥ 18 years) with a recent admission for HF and LVEF < 40% | 67 | 77 | I–IV | NR | < 40% | < 6 weeks (assumed majority received intervention < 4 weeks from discharge) |
| STS HM (e.g. telephone-based interactive response system) vs usual care | |||||||
| Chaudhry et al. 2010 (Tele-HF)52 | Patients recently hospitalized for HF | NR | 58 | I–IV (6% I; 37% II; 51% III; 7% IV) | NR | NR | < 30 days (assumed majority received intervention < 4 weeks from discharge) |
| STS HH vs usual care | |||||||
| aAngermann et al. 2011 (INH)51 | Patients (aged ≥ 18 years) hospitalised with signs and symptoms of decompensated (systolic) HF with evidence of pulmonary congestions on chest radiography and LVEF ≤ 40% (echocardiography) | 69 | 71 | I–IV (2% I; 58% II; 36% III; 4% IV) | 33 | ≤ 40% | < 28 days |
| a,bBarth 200172 | Patients discharged from acute care to home with primary diagnosis of HF | 75 | 47 | NR | 32 | NR | < 72 hours |
| a,bDeBusk et al. 200474 | Patients hospitalised with a provisional diagnosis of HF (based on clinical signs and symptoms or evidence of pulmonary congestions on chest radiography) | 72 | 51 | I–IV (49% I–II; 51% III–IV) | NR | NR | < 2 weeks |
| Domingues et al. 201169 | Hospitalised patients (aged ≥ 18 years) with HF (diagnosed using Boston diagnostic criteria) and LVEF ≤ 45% | 63 | 64 | NR | NR | ≤ 45% | < 1 week |
| a,bLaramee et al. 200377 | Patients admitted to hospital with primary or secondary diagnosis of HF (based on clinical signs and symptoms, left ventricular dysfunction < 40% or radiological evidence of pulmonary oedema and symptomatic improvement following diuresis) | 71 | 54 | I–IV (16% I; 43% II; 33% III; 2% IV; missing, 6%) | NR | < 40% | 1–3 days |
| aRainville 199978 | Patients (aged ≥ 50 years) discharged from hospital with HF | 70 | 50 | II–IV | NR | NR | < 3 days |
| a,bRiegel et al. 200279 | Patients discharged from hospital with HF | 74 | 49 | II–IV (3% II; 38% III; 59% IV) | NR | NR | < 5 days |
| a,bRiegel et al. 200680 | Hospitalised Hispanic patients with a primary or secondary diagnosis of HF, living in the community | 72 | 46 | II–IV (19% II; 46% III; 35% IV) | NR | NR | < 5 days |
| aTsuyuki et al. 2004 (REACT)81 | Patients (aged > 18 years) discharged from hospital with HF | 72 | 58 | I–IV (13% I; 50% II; 33% III; 4% IV) | NR | NR | < 2 weeks |
| aWakefield et al. 200882 | Patients hospitalised for HF exacerbation (e.g. volume overload, pulmonary oedema) | 69 | 99 | II–IV (28% II; 65% III; 7% IV) | NR | NR | < 1 week |
| TM vs usual care | |||||||
| aAntonicelli et al. 200871 | Patients (aged ≥ 70 years) hospitalised for worsening symptoms and signs of HF (NYHA classes II–IV) with evidence of pulmonary congestion on chest radiography and ejection fraction on echocardiography | 78 | 61 | II–IV (58% II; 37% III; 5% IV) | NR | NR | < 1 week |
| aCapomolla et al. 200473 | Patients discharged from specialist HF unit to home | 57 | 88 | II–IV (67% II; NR III; NR IV) | NR | NR | < 1 day |
| Dar et al. 2009 (Home-HF)67 | Patients discharged after hospitalisation with HF (defined by ESC criteria: either a new diagnosis or an acute decompensation of CHF) and NYHA class II–IV symptoms | 71 | 66 | II–IV | NR | NR | Mean 4 days (after randomisation) |
| Dendale et al. 2011 (TEMA-HF 1)68 | Patients hospitalised for fluid overload due to HF requiring an increase or initiation of diuretic therapy (treated with ACE inhibitor or angiotensin II receptor antagonist with beta-blocker, if tolerated) | 76 | 65 | ≥ III | NR | NR | < 1 day |
| a,bGoldberg et al. 2003 (WHARF)75 | Patients admitted to hospital with decompensated advanced HF (NYHA classes III–IV) secondary to systolic dysfunction (LVEF < 35%, measured within 6 months of enrolment) | 59 | 68 | III–IV (74% III; 26% IV) | 20 | ≤ 35% | < 1 day |
| a,bKielblock et al. 200776 | Patients discharged after hospitalisation with HF or with a confirmed diagnosis from ICD codes from hospital insurance data | 74 | 51 | I–IV (NR I; NR II, 17% III; 28% IV) | NR | NR | < 2 weeks |
| aKulshreshtha et al. 201060 | Hospitalised (current admission or recently discharged within previous 2 weeks) or high risk for readmission (cardiac-related reasons or ejection fraction ≤ 20%), non-homebound patients (age > 18 years) with HF | 68 | 64 | NR | NR | ≤ 20% | < 14 days |
| Scherr et al. 2009 (MOBITEL)70 | Patients (aged 18–80 years) with acute worsening of HF (acute cardiac decompensation) with hospitalisation > 24 hours in the last 4 weeks | NR | 71 | NR | NR | NR | < 4 weeks |
| a,bWoodend et al. 200883 | Patients with symptomatic HF (NYHA class II or greater) | 68 | 72 | II–IV | NR | NR | < 2 days |
STS programmes generally included regular scheduled telephone contact between patients and health-care providers (usually on a weekly/monthly basis) and incorporated telephone-based education and monitoring of signs and symptoms of worsening HF. The TM programmes generally used patient-initiated external electronic devices with daily transfer of physiological data (mainly weight, blood pressure and heart rate) from the patient to the health-care provider using a landline, a mobile telephone or broadband technology. With the exception of one study (which provided 24/7 medical support),76 all transmitted data (including alerts) in TM programmes were reviewed by medical staff (nurses and/or physicians) and support provided during office hours (in one study75 nurses reviewed transmitted data on a daily basis 7 days a week, 365 days a year; however, the system was not active 24/7).
All studies were published between 1999 and 2011. The studies were carried out in a variety of countries and regions including Europe [Austria, n = 1; Belgium, n = 1; Germany, n = 2; Italy, n = 2; UK, n = 1; and a combination of countries (Germany, Netherlands and the UK), n = 1], North America (USA, n = 10; Canada, n = 2) and South America (Brazil, n = 1). The duration of follow-up ranged from 3 months69,72,77,83 to 15 months. 49 Although all of the included studies were required to perform STS and/or home TM interventions at least once within the first 28 days following discharge from hospital, two studies performed the intervention outside this period (within 30 days52 or within 6 weeks). 49 For both of these studies it was assumed that the majority of patients would have received the intervention within 28 days of discharge. Of the 21 studies, 10 received funding from one or more commercial sponsors. 49,60,67,68,70,75,77,79,81,83 The sample sizes of the included studies ranged from 3472 to 165352 patients, with the mean age of participants ranging from 57 years73 to 78 years. 71 Only three studies recruited more women than men,72,79,80 with the number of male participants ranging from 46%80 to 99%. 82 One trial was restricted to patients with a LVEF of < 35%,75 three trials were restricted to patients with a LVEF of ≤ 40%,49,51,77 one trial was restricted to patients with a LVEF of < 45%69 and the LVEF inclusion criterion was not reported in 16 studies. 52,60,67,68,70–74,76,78–83
Quality characteristics
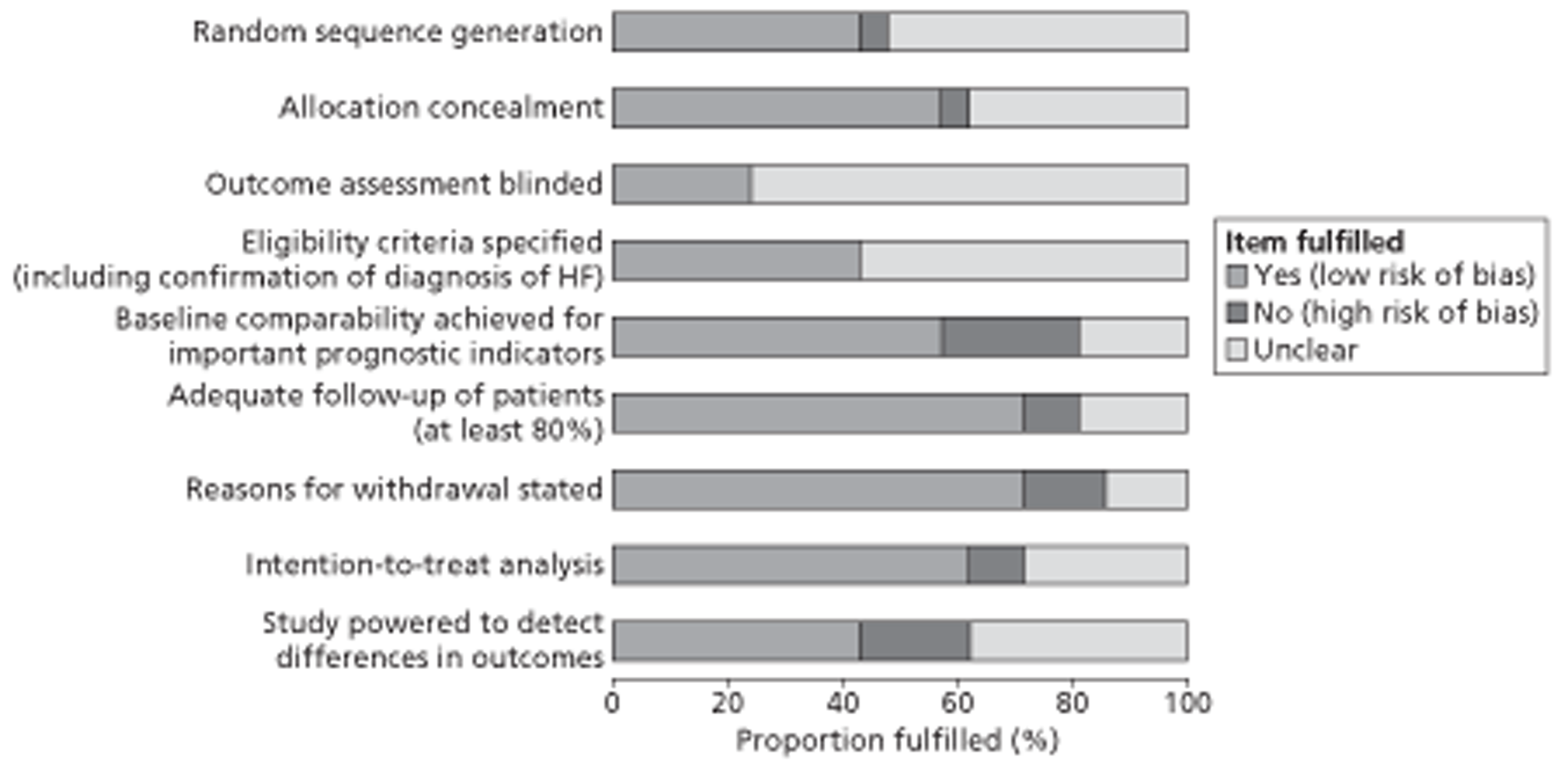
The overall methodological quality of the 21 included studies is summarised in Figure 2 and Table 7. Generally, nine studies performed well,49,51,52,67,68,74,75,80,81 receiving a positive assessment of at least six out of nine methodological quality items. Potential sources of high bias most frequently identified in studies concerned baseline comparability of important prognostic factors (24%), adequate power to detect differences in the primary outcome (19%) and reporting of numbers and reasons for loss to follow-up (14%). The majority of publications poorly described the following aspects: random sequence generation (52%), allocation concealment (38%), blinding of outcome assessment (76%) and intention-to-treat analysis (29%). Although all studies specified eligibility criteria for study entry, the majority (57%) poorly described the definition and confirmation of diagnosis of HF.
FIGURE 2.
Methodological quality graph: review authors' judgements about each methodological quality item as percentages across all included studies.
| Author, year | Methodological assessment criteriaa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| bAngermann et al. 2011 (INH)51 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Antonicelli et al. 200871 | U | U | U | Y | Y | U | N | U | Y |
| Barth 200172 | U | U | U | U | Y | U | N | U | U |
| Capomolla et al. 200473 | U | U | U | U | Y | Y | U | U | U |
| bChaudhry et al. 2010 (Tele-HF)52 | Y | Y | Y | U | Y | Y | Y | Y | Y |
| bCleland et al. 2005 (TEN-HMS)49 | Y | Y | U | U | U | Y | Y | Y | Y |
| bDar et al. 2009 (Home-HF)67 | Y | Y | U | Y | Y | Y | Y | Y | N |
| bDeBusk et al. 200474 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| bDendale et al. 2011 (TEMA-HF 1)68 | Y | U | U | U | Y | Y | Y | Y | Y |
| Domingues et al. 201169 | U | U | U | Y | Y | Y | Y | N | U |
| bGoldberg et al. 2003 (WHARF)75 | U | Y | Y | Y | Y | Y | Y | Y | U |
| Kielblock et al. 200776 | N | U | U | Y | N | U | U | U | U |
| Kulshreshtha et al. 201060 | U | N | U | U | Y | Y | Y | Y | U |
| Laramee et al. 200377 | U | U | U | Y | N | N | Y | U | U |
| Rainville 199978 | U | Y | U | U | N | Y | Y | N | U |
| Riegel et al. 200279 | U | Y | U | U | N | U | U | U | N |
| bRiegel et al. 200680 | U | Y | Y | U | U | Y | Y | Y | Y |
| Scherr et al. 2009 (MOBITEL)70 | Y | Y | U | U | U | Y | Y | Y | N |
| bTsuyuki et al. 2004 (REACT)81 | Y | Y | U | U | N | Y | Y | Y | Y |
| Wakefield et al. 200882 | Y | Y | U | U | Y | N | Y | Y | N |
| Woodend et al. 200883 | U | U | U | Y | U | Y | N | Y | Y |
Effects of interventions
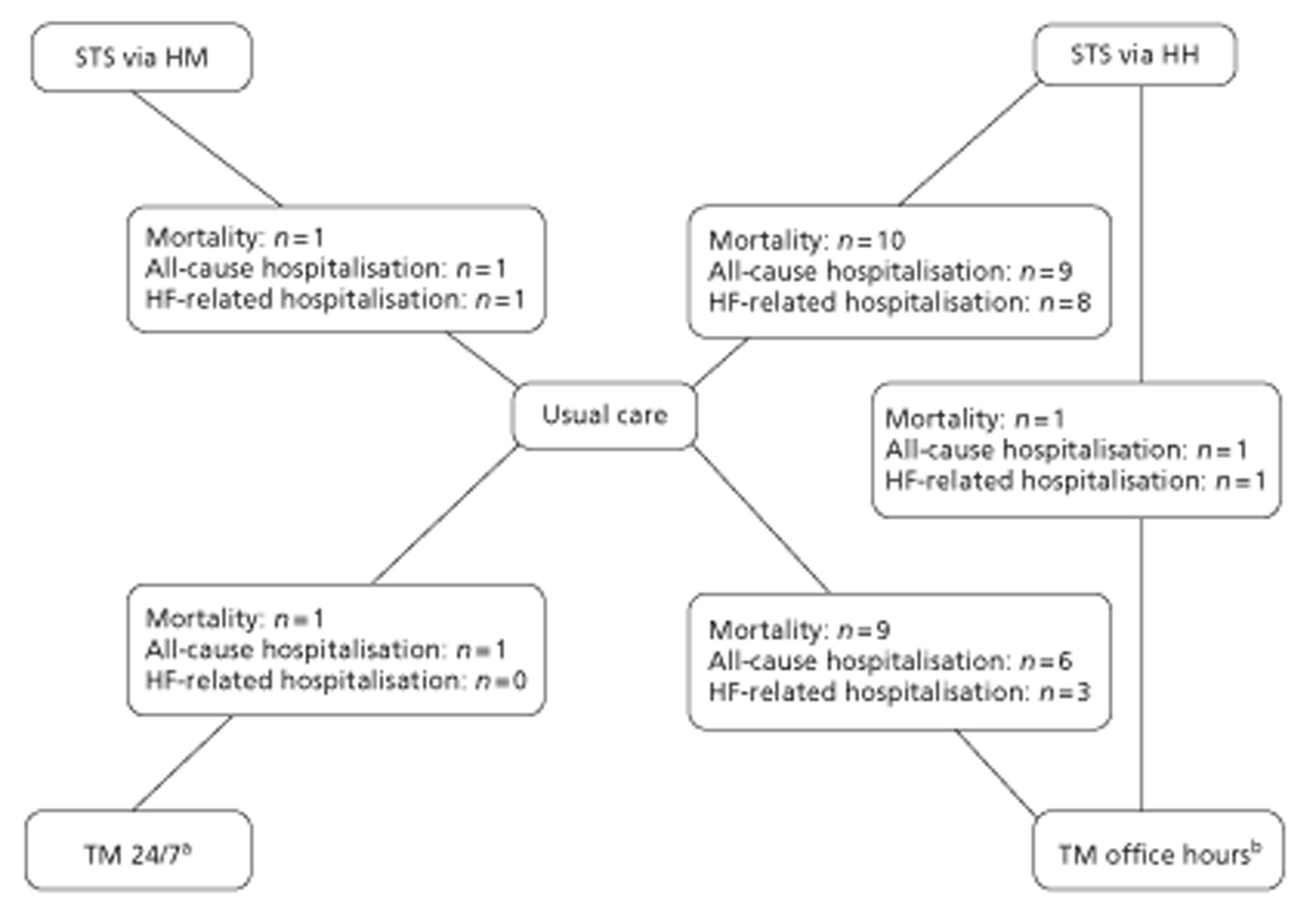
A NMA was undertaken to compare the comparative efficacy of RM (STS or TM) and usual care. Figure 3 presents the network of evidence. A total of 21 studies comparing different pairs or triplets of interventions provided information on at least one of the outcomes being analysed, although not all studies provided information on each outcome. One study72 was excluded from the network analysis because there were no events in each intervention arm and, as a consequence, it provided no information about the intervention effects. 86 A sensitivity analysis was performed excluding data from Dar et al. 67 (Home Heart Failure Study; Home-HF) because it provided better-than-usual support and optimal medical treatment to patients in the control groups and also appeared to be inconsistent with the data from the remaining studies (i.e. an outlier). A summary of all of the trials (data) included in the base-case NMA is presented in Appendix 7.
FIGURE 3.
Network diagram of different RM programmes compared with usual care in recently discharged patients with HF. The nodes are the interventions. The numbers against each outcome represent the numbers of times that each pair of interventions has been compared. There was one multiarm study comparing STS via HH, TM during office hours and usual care. a, Transmitted data reviewed by medical staff (or medical support provided) 24 hours per day, 7 days per week. b, Transmitted data reviewed by medical staff (or medical support provided) during office hours.
Primary analyses (recently discharged patients with heart failure)
All-cause mortality data were available from 20 studies,49,51,52,60,67–71,73–83 including one three-arm study (STS HM, n = 1; STS HH, n = 10; TM with medical support provided during office hours, n = 9; TM 24/7, n = 1). Table 8 summarises the all-cause mortality data for the NMA of RM compared with usual care.
| Treatment | HR and CrI | Predictive interval | |||
|---|---|---|---|---|---|
| Median | 2.5% | 97.5% | 2.5% | 97.5% | |
| STS | |||||
| HM | 0.98 | 0.41 | 2.33 | 0.30 | 3.23 |
| HH | 0.77 | 0.55 | 1.08 | 0.31 | 1.86 |
| TM | |||||
| Office hoursa | 0.76 | 0.49 | 1.18 | 0.30 | 1.91 |
| 24/7b | 0.49 | 0.20 | 1.18 | 0.14 | 1.73 |
| Usual care | |||||
| Reference | Reference | Reference | Reference | Reference | Reference |
| Between-study standard deviation (log-HR scale) | 0.34 | 0.03 | 0.75 | – | – |
The NMA model fitted the data reasonably well, with a residual deviance close to the total number of data points included in the analysis. The total residual deviance was 42.87, which compared favourably with the 40 non-zero data points being analysed. However, the model did not represent the data from the Dar et al. (Home-HF)67 and Dendale et al. (TEMA-HF 1; TElemonitoring in the MAnagement of Heart Failure)68 studies particularly well. The between-study standard deviation was estimated to be 0.34 (95% CrI 0.03 to 0.75). This indicated that there was small to moderate heterogeneity between studies in the treatment effect. All interventions showed a beneficial trend in reducing all-cause mortality compared with usual care. The intervention that exhibited the greatest effect was TM 24/7 (HR 0.49; 95% CrI 0.20 to 1.18); however, this result should be treated with caution because of the poor methodological quality of the only included study in this network. 76 STS HH (HR 0.77; 95% CrI 0.55 to 1.08) and TM during office hours (HR 0.76; 95% CrI 0.49 to 1.18) both had similar effects on all-cause mortality. In addition, the heterogeneity in the effect of RM between studies means that the intervention effects in a randomly chosen study vary substantially depending on the characteristics of the study.
A sensitivity analysis was performed excluding the Home-HF study67 (Table 9). The heterogeneity in intervention effects between studies was considerably reduced. The interventions that exhibited the greatest effects were TM 24/7 (HR 0.49; 95% CrI 0.26 to 0.88) (although this result should be treated with caution because of the poor methodological quality of the only included study in this network76), TM during office hours (HR 0.62; 95% CrI 0.42 to 0.89) and STS HH (HR 0.75; 95% CrI 0.59 to 0.96).
| Treatment | HR and CrI | Predictive interval | |||
|---|---|---|---|---|---|
| Median | 2.5% | 97.5% | 2.5% | 97.5% | |
| STS | |||||
| HM | 0.98 | 0.58 | 1.62 | 0.49 | 1.95 |
| HH | 0.75 | 0.59 | 0.96 | 0.45 | 1.27 |
| TM | |||||
| Office hoursa | 0.62 | 0.42 | 0.89 | 0.35 | 1.09 |
| 24/7b | 0.49 | 0.26 | 0.88 | 0.23 | 1.04 |
| Usual care | |||||
| Reference | Reference | Reference | Reference | Reference | Reference |
| Between-study standard deviation (log-HR scale) | 0.14 | 0.01 | 0.47 | – | – |
All-cause hospitalisation data were available from 16 studies,49,51,52,67,69,70,71,74–77,79–83 including one three-arm study (STS HM, n = 1; STS HH, n = 9; TM with medical support provided during office hours, n = 6; TM 24/7, n = 1). Table 10 summarises the all-cause hospitalisation data for the NMA of RM compared with usual care.
| Treatment | HR and CrI | Predictive interval | |||
|---|---|---|---|---|---|
| Median | 2.5% | 97.5% | 2.5% | 97.5% | |
| STS | |||||
| HM | 1.06 | 0.44 | 2.53 | 0.31 | 3.61 |
| HH | 0.97 | 0.70 | 1.31 | 0.38 | 2.43 |
| TM | |||||
| Office hoursa | 0.75 | 0.49 | 1.10 | 0.28 | 1.91 |
| 24/7b | 0.81 | 0.33 | 2.00 | 0.23 | 2.85 |
| Usual care | |||||
| Reference | Reference | Reference | Reference | Reference | Reference |
| Between-study standard deviation (log-HR scale) | 0.38 | 0.13 | 0.74 | – | – |
The NMA model fitted the data reasonably well, with a residual deviance (36.85) close to 33, the total number of data points included in the analysis. However, the model did not represent the data from Antonicelli et al. 71 particularly well. The between-study standard deviation was estimated to be 0.38 (95% CrI 0.13 to 0.74). This indicated that there was small to moderate heterogeneity between studies in the treatment effect. The intervention that exhibited the greatest effect was TM with medical support provided during office hours (HR 0.75; 95% CrI 0.49 to 1.10). In addition, the heterogeneity in the effect of RM between studies means that the intervention effects in a randomly chosen study vary substantially depending on the characteristics of the study.
A sensitivity analysis was performed excluding the Home-HF study67 (Table 11). There was little impact on the heterogeneity in intervention effects between studies. As before, the intervention that exhibited the greatest effect was TM with medical support provided during office hours (HR 0.67; 95% CrI 0.42 to 0.97), although the heterogeneity in the effect of RM between studies means that the intervention effects in a randomly chosen study vary substantially depending on the characteristics of the study.
| Treatment | HR and CrI | Predictive interval | |||
|---|---|---|---|---|---|
| Median | 2.5% | 97.5% | 2.5% | 97.5% | |
| STS | |||||
| HM | 1.06 | 0.48 | 2.32 | 0.35 | 3.22 |
| HH | 0.96 | 0.72 | 1.27 | 0.42 | 2.18 |
| TM | |||||
| Office hoursa | 0.67 | 0.42 | 0.97 | 0.26 | 1.53 |
| 24/7b | 0.81 | 0.36 | 1.81 | 0.27 | 2.50 |
| Usual care | |||||
| Reference | Reference | Reference | Reference | Reference | Reference |
| Between-study standard deviation (log-HR scale) | 0.33 | 0.08 | 0.69 | – | – |
Heart failure-related hospitalisation data were available from 11 studies,49,51,52,67,74,75,77–81 including one three-arm study (STS HM, n = 1; STS HH, n = 8; TM with medical support provided during office hours, n = 3). Table 12 summarises the HF-related hospitalisation data for the NMA of RM compared with usual care.
| Treatment | HR and CrI | Predictive interval | |||
|---|---|---|---|---|---|
| Median | 2.5% | 97.5% | 2.5% | 97.5% | |
| STS | |||||
| HM | 1.03 | 0.66 | 1.54 | 0.58 | 1.77 |
| HH | 0.77 | 0.62 | 0.96 | 0.50 | 1.19 |
| TM | |||||
| Office hoursa | 0.95 | 0.70 | 1.34 | 0.59 | 1.62 |
| 24/7b | NA | NA | NA | NA | NA |
| Usual care | |||||
| Reference | Reference | Reference | Reference | Reference | Reference |
| Between-study standard deviation (log-HR scale) | 0.11 | 0.00 | 0.42 | – | – |
The NMA model fitted the data reasonably well, with a residual deviance (22.18) close to 23, the total number of data points included in the analysis. However, the model did not represent the data from the Dar et al. (Home-HF)67 and Rainville78 studies particularly well. The between-study standard deviation was estimated to be 0.11 (95% CrI 0.00 to 0.42). This indicated that there was small heterogeneity between studies in the treatment effect. The intervention that exhibited the greatest effect was STS HH (HR 0.77; 95% CrI 0.62 to 0.96).
A sensitivity analysis was performed excluding the Home-HF study67 (Table 13). There was little impact on the heterogeneity in intervention effects between studies. As before, the intervention that exhibited the greatest effect was STS HH (HR 0.76; 95% CrI 0.61 to 0.94).
| Treatment | HR and CrI | Predictive interval | |||
|---|---|---|---|---|---|
| Median | 2.5% | 97.5% | 2.5% | 97.5% | |
| STS | |||||
| HM | 1.02 | 0.70 | 1.49 | 0.61 | 1.69 |
| HH | 0.76 | 0.61 | 0.94 | 0.51 | 1.13 |
| TM | |||||
| Office hoursa | 0.86 | 0.61 | 1.21 | 0.54 | 1.38 |
| 24/7b | NA | NA | NA | NA | NA |
| Usual care | |||||
| Reference | Reference | Reference | Reference | Reference | Reference |
| Between-study standard deviation (log-HR scale) | 0.10 | 0.00 | 0.39 | – | – |
Of the 11 studies reporting on STS intervention programmes compared with usual care,51,52,69,73,74,77–82 six studies reported length of stay data. 52,77,79–82 Of these, only the study by Tsuyuki et al. 81 reported a statistically significant reduction in the length of stay in hospital between the STS programme and the usual care group (total: 627 vs 1082 days respectively, p < 0.001; average: 6.6 vs 11.0 days respectively, p < 0.001). Of the nine studies reporting on TM intervention programmes compared with usual care, two studies reported length of stay data. 67,83 These studies found no significant differences between the groups in the number of days spent in hospital at 180 days (17 vs 13 days respectively, p = 0.99)67 or in the first year post discharge (7.13 vs 6.71 days respectively, p = not significant). 83 The study that assessed both STS and TM intervention programmes reported no significant differences in the length of stay for hospital admissions between groups during 240 days of follow up (p = not significant for all comparisons). 49
Quality of life was a secondary outcome measure in eight of the 21 included studies. 51,67,71,72,75,80,82,83 These were either a direct comparison between intervention and control groups at study conclusion51,67,80,83 or a comparison between baseline and study conclusion within the study arm. 71,72,75,82 A range of psychometric measures were used including both generic and HF-specific measures: SF-36,51,71,83 the Short Form questionnaire-12 items (SF-12),76 the Health Distress Score (HDS),75 the Minnesota Living with Heart Failure Questionnaire (MLHFQ),67,72,75,80,82,83 and the European Quality of Life-5 Dimensions (EQ-5D). 67,80
Three of the four STS studies reported improvements in QoL, with significant improvements in physical (p = 0.03)51 and overall (MLHFQ, p < 0.001)72,82 measures. One study found no significant differences between groups in either the MLHFQ or the EQ-5D measure. 80 Four TM studies measured QoL. 67,71,75,83 Of these, two reported improvements in QoL (SF-36 health perception, p = 0.046;71 MLHFQ, p = 0.025 and SF-36, p < 0.0583). Although Goldberg et al. 75 observed improvements in QoL, none of the measures was significant (MLHFQ, p = 0.22; SF-12, p > 0.05; HDS, p = 0.57). Similarly, Dar et al. 67 found no significant differences between groups in either the MLHFQ (p = 0.6) or the EQ-5D (p = 0.5) measure over a 6-month follow-up period.
Only 5 of the 21 included studies reported adherence (compliance) rates to the intervention. 51,52,67,73,75 Adherance was measured at 55.1%52–84.0%51 for STS and 81.0%73–98.5%75 for TM. Some further data were available on system acceptability and patient satisfaction. Cleland et al. 49 reported an overall acceptance rate of 91.2% with 96% of participants expressing satisfaction with the system and 97% reporting that the device was easy to use. Riegel et al. 79 reported significantly higher satisfaction among patients receiving TM than among those receiving usual care (p = 0.01), and Laramee et al. 77 reported higher satisfaction among STS patients than among usual care patients (p < 0.01). Kielblock et al. 76 reported very high satisfaction among TM patients, with 57% rating the programme ‘very good’ and the remaining 43% rating it as ‘quite good’. Woodend et al. 83 reported satisfaction scores of between 92 and 97 out of 100 on a 10-item checklist. Scherr et al. 70 reported early termination of their study because of an increasing number of patients who were unable to operate the TM equipment, with 12 participants (10%) failing to transmit any readings and a further four requesting early termination. Finally, Kulshreshtha et al. 60 reported that, of 82 patients offered TM, 40 refused participation: 24 patient refusals and 16 physician refusals.
Additional analyses (patients with stable heart failure)
Additional analyses were undertaken to assess whether or not the results from the primary analysis differed markedly from the results in those with stable HF who were managed in the community. In this supplementary analysis the following studies were included: RCTs comparing HF management delivered via STS, TM or cardiovascular implanted monitoring devices with HF management delivered via usual post-discharge care in stable HF patients (defined as having no acute event or deterioration in the past 28 days) who were managed in the community setting (ambulatory or outpatient care). Studies that included intensified management with additional home or clinic visits were excluded. Although no formal critical appraisal of these studies was undertaken, the results were meta-analysed (as per the methods of the primary analysis) and are presented in this section for information only. All studies published before 2008 were identified from Inglis et al. 48 and Klersy et al. 58 whereas more recent studies (meeting these criteria) were identified from the current review. The design and patient characteristics of the 21 included studies that evaluated home TM (including cardiovascular implanted monitoring devices) or STS programmes for adults with stable HF are briefly summarised in Appendix 8. A network of 18 studies comparing different pairs or triplets of treatments is shown in Figure 4 (data included in the base-case NMA are presented in Appendix 9).
FIGURE 4.
Network diagram of different RM programmes compared with usual care in patients with stable HF. The nodes are the interventions. The numbers against each outcome represent the number of times that each pair of interventions has been compared. There was one multiarm study comparing STS via HH, TM during office hours and usual care. a, Transmitted data reviewed by medical staff (or medical support provided) 24 hours per day, 7 days per week. b, Transmitted data reviewed by medical staff (or medical support provided) during office hours.
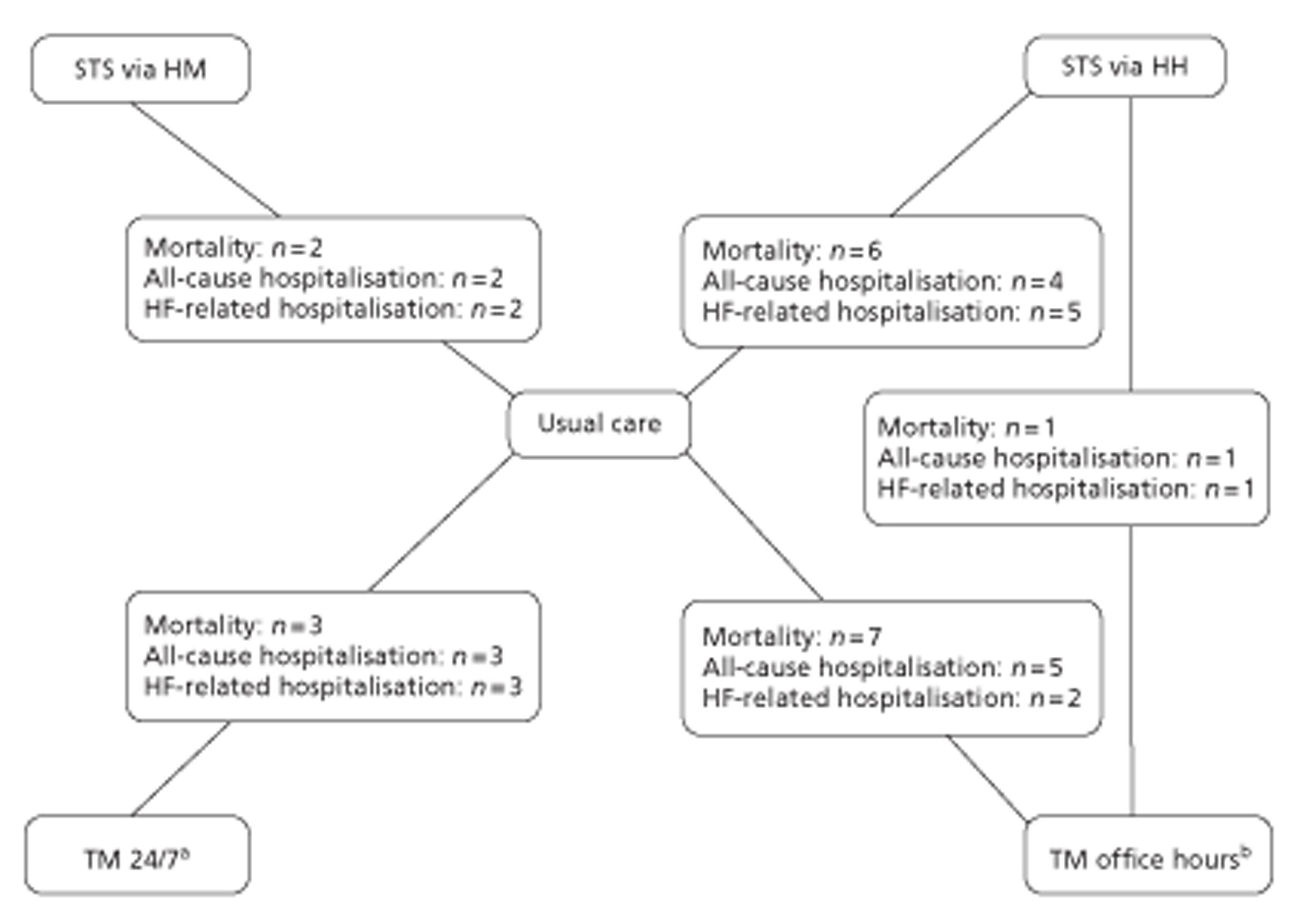
All-cause mortality data were available from 17 studies,50,87–102 including one three-arm study (STS HM, n = 2; STS HH, n = 6; TM with medical support provided during office hours, n = 7; TM 24/7, n = 3). Table 14 summarises the all-cause mortality data for the NMA of RM compared with usual care.
| Treatment | HR and CrI | Predictive interval | |||
|---|---|---|---|---|---|
| Median | 2.5% | 97.5% | 2.5% | 97.5% | |
| STS | |||||
| HM | 1.35 | 0.78 | 2.36 | 0.71 | 2.67 |
| HH | 0.87 | 0.69 | 1.14 | 0.57 | 1.42 |
| TM | |||||
| Office hoursa | 0.85 | 0.59 | 1.20 | 0.52 | 1.37 |
| 24/7b | 0.85 | 0.58 | 1.27 | 0.47 | 1.39 |
| Usual care | |||||
| Reference | Reference | Reference | Reference | Reference | Reference |
| Between-study standard deviation (log-HR scale) | 0.12 | 0.01 | 0.38 | – | – |
The residual deviance, 28.76, was < 35, the total number of data points included in the analysis, suggesting that the meta-analysis model may not be a good representation of the data. The between-study standard deviation was estimated to be 0.12 (95% CrI 0.01 to 0.38). This indicated that there was little heterogeneity between studies in the treatment effect. All interventions except for STS HM showed a beneficial trend in reducing all-cause mortality compared with usual care, although these effects were not conclusive.
Of the three studies103–105 that compared cardiovascular implanted monitoring devices with non-monitoring cardiovascular implanted monitoring devices (usual care), there was an indication of small to extreme heterogeneity between the studies in the treatment effect.
The between-study standard deviation was estimated to be 0.26 (95% CrI 0.01 to 1.63). Although cardiovascular implanted monitoring devices appeared to be associated with a reduction in mortality compared with usual care, this result was inconclusive (Table 15). In addition, the heterogeneity in the effect of cardiovascular implanted monitoring devices between studies means that the intervention effect in a randomly chosen study varies substantially depending on the characteristics of the study.
| Treatment | HR and CrI | Predictive interval | |||
|---|---|---|---|---|---|
| Median | 2.5% | 97.5% | 2.5% | 97.5% | |
| Cardiovascular implanted monitoring devices | 0.90 | 0.31 | 2.49 | 0.12 | 5.21 |
| Usual carea | |||||
| Reference | Reference | Reference | Reference | Reference | Reference |
| Between-study standard deviation (log-HR scale) | 0.26 | 0.01 | 1.63 | – | – |
All-cause hospitalisation data were available from 13 studies,50,88,90–93,95,97–102 including one three-arm study (STS HM, n = 2; STS HH, n = 4; TM with medical support provided during office hours, n = 5; TM 24/7, n = 3). Table 16 summarises the all-cause hospitalisation data for the NMA of RM compared with usual care.
| Treatment | HR and CrI | Predictive interval | |||
|---|---|---|---|---|---|
| Median | 2.5% | 97.5% | 2.5% | 97.5% | |
| STS | |||||
| HM | 0.87 | 0.54 | 1.29 | 0.44 | 1.74 |
| HH | 0.86 | 0.62 | 1.17 | 0.45 | 1.62 |
| TM | |||||
| Office hoursa | 1.17 | 0.89 | 1.59 | 0.62 | 2.18 |
| 24/7b | 0.84 | 0.54 | 1.15 | 0.40 | 1.47 |
| Usual care | |||||
| Reference | Reference | Reference | Reference | Reference | Reference |
| Between-study standard deviation (log-HR scale) | 0.23 | 0.07 | 0.49 | – | – |
The NMA model fitted the data well, with a residual deviance (27.11) close to 27, the total number of data points included in the analysis. The between-study standard deviation was estimated to be 0.23 (95% CrI 0.07 to 0.49). This indicated that there was little heterogeneity between studies in the treatment effect. All interventions except for TM with medical support provided during office hours showed a beneficial trend in reducing all-cause hospitalisation compared with usual care, although these effects were not conclusive.
Heart failure-related hospitalisation data were available from 11 studies,50,88,90–93,97,99,100,102,106 including one three-arm study (STS HM, n = 2; STS HH, n = 5; TM with medical support provided during office hours, n = 2; TM 24/7, n = 3). Table 17 summarises the HF-related hospitalisation data for the NMA of RM compared with usual care.
| Treatment | HR and CrI | Predictive interval | |||
|---|---|---|---|---|---|
| Median | 2.5% | 97.5% | 2.5% | 97.5% | |
| STS | |||||
| HM | 0.69 | 0.34 | 1.43 | 0.23 | 2.11 |
| HH | 0.67 | 0.37 | 1.05 | 0.22 | 1.75 |
| TM | |||||
| Office hoursa | 0.70 | 0.34 | 1.50 | 0.19 | 2.30 |
| 24/7b | 0.64 | 0.34 | 1.14 | 0.23 | 1.89 |
| Usual care | |||||
| Reference | Reference | Reference | Reference | Reference | Reference |
| Between-study standard deviation (log-HR scale) | 0.31 | 0.03 | 1.05 | – | – |
The NMA model fitted the data reasonably well, with a residual deviance (26.50) close to 23, the total number of data points included in the analysis. The between-study standard deviation was estimated to be 0.31 (95% CrI 0.03 to 1.05). This indicated that there was small to extreme heterogeneity between studies in the treatment effect. All interventions showed a beneficial trend in reducing all-cause hospitalisation compared with usual care, although these effects were not conclusive. In addition, the heterogeneity in the effect of interventions between studies means that the effect in a randomly chosen study varies substantially depending on the characteristics of the study.
Of the three studies103–105 that compared cardiovascular implanted monitoring devices with non-monitoring cardiovascular implanted monitoring devices (usual care), there was an indication of small to moderate heterogeneity between studies in the treatment effect. The between-study standard deviation was estimated to be 0.24 (95% CrI 0.01 to 0.64). Although cardiovascular implanted monitoring devices appeared to be associated with a reduction in HF-related hospitalisation, this result was inconclusive (Table 18).
| Treatment | HR and CrI | Predictive interval | |||
|---|---|---|---|---|---|
| Median | 2.5% | 97.5% | 2.5% | 97.5% | |
| Cardiovascular implanted monitoring devices | 0.72 | 0.32 | 1.37 | 0.14 | 3.01 |
| Usual carea | |||||
| Reference | Reference | Reference | Reference | Reference | Reference |
| Between-study standard deviation (log-HR scale) | 0.24 | 0.01 | 0.64 | – | – |
Discussion
The NMA showed that, compared with usual care, STS HH, TM with medical support provided during office hours and TM 24/7 were associated with a 23%, 24% and 51% reduction in all-cause mortality, respectively, among adults who have been recently discharged (< 28 days) from an acute care setting after a recent exacerbation of HF. However, the results for TM 24/7 should be treated with caution because of the poor methodological quality of the only included study in this network. 76 No beneficial effect on mortality was observed with STS HM. TM with medical support during office hours and TM 24/7 were associated with a 25% and 19% reduction in all-cause hospitalisations, respectively, whereas there was no major effect of STS HM or STS HH. STS HH was associated with a reduction of 23% in HF-related hospitalisations. There was no major effect of STS HM and TM with medical support during office hours on HF-related hospitalisations. In addition, despite the limited data, STS and TM generally improved QoL and were acceptable to patients.
Although the present findings broadly support the conclusions of the latest review and meta-analysis by Inglis et al. ,48 there were some points on which the results differed. Despite differences between the two reviews in the classification of the RM strategies and in the statistical approaches to conducting the meta-analyses, STS HH was found to have a larger effect on mortality reduction than the pooled results of STS trials in the Inglis et al. 48 review [HR 0.77, 95% CrI 0.55 to 1.08 vs risk ratio (RR) 0.88, 95% CI 0.76 to 1.01]. Effects on all-cause hospitalisation (HR 0.97; 95% CrI 0.70 to 1.31 vs RR 0.92, 95% CI 0.85 to 0.99) and HF-related hospitalisation (HR 0.77, 95% CrI 0.62 to 0.96 vs RR 0.77, 95% CI 0.68 to 0.87) were similar between the two reviews. However, the findings from the analysis of STS HM were less favourable than those of Inglis et al. 48 for all-cause mortality (HR 0.98, 95% CrI 0.41 to 2.33 vs RR 0.88, 95% CI 0.76 to 1.01), all-cause hospitalisation (HR 1.06, 95% CrI 0.44 to 2.53 vs RR 0.92, 95% CI 0.85 to 0.99) and HF-related hospitalisation (HR 1.03, 95% CrI 0.66 to 1.54 vs RR 0.77, 95% CI 0.68 to 0.87). In addition, the present results were less favourable for TM during office hours (i.e. transmitted data reviewed by medical staff or medical support provided during office hours) for all-cause mortality (HR 0.76, 95% CrI 0.49 to 1.18 vs RR 0.66, 95% CI 0.54 to 0.81, respectively), but more favourable for all-cause hospitalisation (HR 0.75, 95% CrI 0.49 to 1.10 vs RR 0.91, 95% CI 0.84 to 0.99), and worse for HF-related hospitalisation (HR 0.95, 95% CrI 0.70 to 1.34 vs RR 0.79, 95% CI 0.67 to 0.94). Notably, when a sensitivity analysis excluding the results from the Home-HF study67 was conducted, the findings for TM effectiveness were similar to those observed in the Inglis et al. 48 review.
When interpreting these diverging results, a number of differences in the methodology and data sets used in the respective reviews should be borne in mind. Most importantly, the present NMA distinguished between two types of STS (HH and HM) and two types of TM (transmitted data reviewed by medical staff or support provided during office hours, or transmitted data reviewed by medical staff or support provided 24/7). As the analysis showed, effectiveness varied substantially according to the type of system used, with, in particular, greater favourability towards STS HH than STS HM. Furthermore, the present analysis included the Home-HF study,67 which was excluded from the Inglis et al. review48 because of the use of an initial nurse visit (for equipment installation and use) as part of the care package. Inclusion of this trial in the analysis substantially reduced TM clinical effectiveness. However, given the low mortality rate in the control group of the Home-HF trial,67 the results of this study may not be generalisable to the wider HF population. This review also had a more stringent definition of the population of interest than the Inglis review48 (i.e. patients who commenced RM ≤ 28 days post discharge). Given what is known about the risk of mortality following decompensation,15 it may be that the present review focused on a patient population for whom RM is particularly efficacious. If this assumption holds, it might appear surprising that the NMA did not find substantially greater benefits of RM than those observed in the Inglis et al. review. 48 However, it should be noted that the standard and quality of usual care for HF continues to evolve (generally this was poorly reported in all included studies); thus, the impact of the age of the study on the treatment effects may have been an important confounding factor in the observed results.
In addition to the studies from the Inglis et al. review,48 six new studies52,60,67–70 of RM were included in the present review. These trials were of variable methodological quality with only three studies52,67,68 performing well and receiving a positive assessment of at least six out of nine methodological quality items. Perhaps most notable was the inclusion of the largest trial of STS to date (n = 1653),52 which has already generated considerable debate. 107–109 The Tele-HF trial52 delivered STS using HM and found no benefit over usual care. Although this trial was well designed and reported (see Table 7), a low patient adherence rate was observed (55%) and the control group received good quality of care. In response to such criticisms of Tele-HF, Chaudhry et al. 52 have pointed out that patients were given individual counselling to support engagement with RM and thus the 55% adherence rate probably represents the ‘best case scenario’ for real-world clinical practice. 109 These investigators further argued that the > 50% event rate (rehospitalisation or death) in the usual care group did not suggest an excellent standard of care. There may, however, be further questions raised by the Tele-HF trial. For example, it is possible that interpersonal interaction with a care provider is an important active component of STS. It seems plausible that regular telephone contact with a care provider provides psychosocial benefits that feed into self-care practices and QoL, particularly among socially isolated older people. Similarly, Anker et al. 46 suggested that remote contact between patients and care providers could help detect depression, which is associated with poor outcomes in HF. Furthermore, the mortality rate of 11.4% in the usual care group of the Tele-HF study was low compared with usual clinical practice. 14
The Home-HF study67 was a RCT that compared TM with usual care. The trial included 182 patients from the UK with a recent hospital admission for HF and in NYHA classes II–IV. There was a higher incidence of mortality among the TM group than among the usual care group (17 vs 5 using the intention-to-treat approach or 14 vs 4 after TM equipment installation). These results may appear surprising at first glance and could even be read as showing a detrimental effect of TM compared with usual care. However, the 6-month mortality rate in the usual care group (5.5%) was substantially lower than would be expected in a HF cohort receiving care outside the context of a clinical trial (i.e. between 13% and 21%). 14 The authors stated that the standard of usual care was of high quality in the Home-HF trial, consisting of an initial home visit from a specialist nurse and access to telephone support during working hours. In addition, most patients were receiving optimal medical treatment including ACE inhibitors (70%), beta-blockers (56%) and loop diuretics (93%). Whatever the reason for the lack of effectiveness in the Home-HF67 and Tele-HF52 trials, the results at least serve as caution that all RM interventions (i.e. packages of care) are not necessarily effective in all contexts.
One TM study from Germany,76 which was conducted in collaboration with a health insurance company, reported provision of round-the-clock support to address participants' questions about medication and the TM system. The 24-hour call centre approach has been reccomended elsewhere on the grounds that HF is a dynamic illness and so patients may need quick medical response 24 hours a day. 110 In comparison with office hours-only services, the 24-hour provision appeared to confer additional benefits for mortality but not for all-cause hospitalisation or HF-related hospitalisation. However, the results from the trial of 24/7 monitoring should be treated with caution as the study had serious methodological shortcomings (see Table 7). In particular, the method used to assign groups (i.e. by date of birth) was not ideally random and the intervention group (n = 251) was significantly younger than the control group (n = 251) (73 years vs 78 years, p < 0.001). Even if the results from this study are interpreted as a reduction in short- and medium-term mortality arising from maximisation of medical therapy, it is unlikely that out-of-hours events were sufficiently frequent to result in alterations of therapy that would not have occurred in an office hours-only system. Moreover, in the UK, an existing round-the-clock response system is available through the 999 emergency response route; thus, RM interventions provided by the NHS have been during office hours only.
Additional analyses were performed to assess whether or not the findings from the present review (primary analysis) differed markedly in patients with stable HF (i.e. defined as having no acute event or detrioration in the past 28 days and managed in an ambulatory or outpatient care setting). These analyses suggested that inclusion of stable patients reduced the effectiveness of STS (both HH and HM) for mortality but provided additional reductions in both all-cause hospitalisation and HF-specific hospitalisation. With respect to TM during office hours, inclusion of stable patients yielded a marginally greater hazard reduction for mortality, a substantially greater reduction for HF-related hospitalisation and a substantially worse outcome for all-cause hospitalisation. Inclusion of stable patients in the 24/7 TM interventions yielded a substantially lower hazard reduction for all-cause mortality, a marginally lower hazard reduction for all-cause hospitalisation and a greater hazard reduction for HF-related hospitalisation. It is not clear how these apparently contradictory results should be interpreted, particularly given that no formal assessment of study quality was conducted on the studies involving stable patients. Finally, inclusion of stable patients allowed an analysis of RM effectiveness in three studies of implanted cardiac devices. 103–105 The findings showed a trend towards a reduction in all-cause mortality (HR 0.90, 95% CrI 0.31 to 2.49) and HF-related hospitalisation (HR 0.72, 95% CrI 0.32 to 1.37).
There are a number of limitations to the findings of this meta-analysis. Perhaps most importantly, the interventions were heterogeneous in terms of the physiological parameters remotely transmitted and the type of RM system utilised (see Table 4), so it may be argued that this review is a meta-analysis of a family of similar interventions rather than a single standardised intervention. For the 10 included TM studies, the most commonly monitored parameters were weight (n = 10) followed by blood pressure (n = 8) and heart rate (n = 7). Of the two UK trials, the TEN-HMS (Trans-European Network – Home-Care Management System) study49 monitored weight, blood pressure, electrocardiogram and heart rate, whereas the Home-HF study67 monitored weight, blood pressure, heart rate, oxygen saturation and symptoms (breathlessness, orthopnoea, dizziness and ankle swelling). However, this meta-analysis was unable to establish whether or not monitoring different parameters provided different levels of clinical benefit. STS interventions were somewhat more homogeneous in terms of monitored parameters, with the majority including an educational component and questions about worsening symptoms. However, the frequency of monitoring varied widely in these studies, from three times in the first week82 to monthly. 49 In addition, it is important to note that usual care for HF has improved over recent decades. Diagnosis may occur earlier because of initiatives to improve HF awareness among primary care physicians and because of the increased availability of diagnostic tests. Furthermore, HF self-management programmes led by specialist nurses, which have been shown to reduce mortality and morbidity, are now widely used in HF care. 45 The estimated 6-month survival rate for HF in the UK rose from 74.5% in 1996–7 (95% CI 70.6% to 77.9%) to approximately 85.7% in 2004–5 (95% CI 81.8% to 88.8%). 111 With these improvements, it is possible that present usual care delivery may confound the effects of RM. A final issue for trial comparability was the diagnostic criteria used to confirm HF. More than half of the included trials did not report how the presence of HF was assessed,49,52,60,68,70,72,73,78–82 and among the remaining trials a variety of criteria were used.
The difficulty in interpreting the findings from these trials is further compounded by the fact that few studies presented outcomes in such a way as to allow stratification by age and sex in meta-regression – a problem also noted in the previous Inglis et al. review. 48 Hence, the analysis was unable to establish patient subgroups in which RM is particularly effective. Another limitation concerns the quality of reporting in the included studies, which varied widely (see Table 7). In particular, a substantial number of studies were underpowered to detect differences in the primary outcome measures,60,67,69,70,72,73,75–79,82 although this is not a concern in terms of the meta-analysis. However, there was evidence of several further potential sources of bias among the included studies. In particular, either outcome assessors were unblinded or blinding status was unclear in 16 trials. 49,60,67–73,76–79,81–83 Another issue for external validity was the commercial funding reported by 10 studies49,60,67,68,70,75,77,79,81,83 as receipt of such funding has also been shown to systematically bias trials in favour of the products made by the companies that fund the research. 112
Further, foreign language studies were excluded and no cohort studies met the inclusion criteria for this review. Although RCTs are generally viewed as representing the most robust form of evidence for treatment efficacy, they have been criticised for a narrow focus on highly selected populations and outcomes. 113 Cohort studies, on the other hand, are more open to potential sources of bias but may offer a more realistic representation of how outcomes play out in the complex real world of clinical practice. 114 A previous meta-analysis by Klersy et al. 58 included 12 cohort studies of RM. The pooled results showed that RM was associated with a significantly lower number of deaths (n = 6 studies, RR 0.53, 95% CI 0.29 to 0.96, p < 0.001) and hospitalisations (n = 3 studies, RR 0.52, 95% CI 0.28 to 0.96, p < 0.001). However, the included studies had a number of internal/external validity issues. In particular, half of the studies used a pre/post-test design without a concurrent control (which could result in a Hawthorne effect being mistaken for a genuine clinical effect) and several included a programme of home visits in the RM intervention (further details are provided in Overview of existing systematic reviews and Appendix 4). The literature search for this meta-analysis identified one cohort study of implanted RM devices, which included stable HF patients115 and was therefore not eligible for inclusion in the meta-analysis. All of the cohort studies from the Klersy et al. 58 review were also excluded because of the application of more stringent inclusion criteria. Clearly, well-designed cohort studies with concurrent control groups are lacking among the RM evidence base. Another limitation of the present meta-analysis was that, given the heterogeneity in the RM systems, the analysis was unable to establish the precise ‘active ingredients’ of RM. Given that RM is a complex intervention (i.e. made up of a variety of interconnected, socially situated factors), it is important to understand not only whether RM works, but also how, why and under what circumstances. 116 One way to explore these issues would be to include qualitative research on patient experiences of RM in subsequent updates.
Finally, it should be noted that this review did not include data from the Department of Health's WSD programme. 117 The WSD programme is the largest randomised trial of RM to date, including 6191 patients from 238 GP practices across three areas: Newham, Kent and Cornwall. The trial included people with one of three chronic conditions (HF, diabetes and COPD) and the headline results57 suggest an even more dramatic reduction in mortality (45%) than the pooled results reported here. An effect size as large as this has the potential to substantially alter the point estimates of the NMA. However, until data from the WSD programme become publicly available in peer-reviewed publications, it is difficult to evaluate the true magnitude and direction of effect in recently discharged patients with HF (or people with stable HF). As one perceptive commentator has noted, the Department of Health report states that telehealth can deliver the stated 45% mortality reduction ‘if used correctly’118 – and at present it is unclear what correct usage entails.
Chapter 4 Assessment of cost-effectiveness
This chapter details the methods and results of the health economic model, which has been developed to compare different strategies for adult patients who have been discharged from an acute care setting after a recent exacerbation of HF. It includes a brief review of existing economic evaluations and a detailed explanation of the methods and results of a de novo economic model. The first section presents the results of the systematic review of economic literature. The modelling approach adopted to estimate the cost-effectiveness of RM interventions is then presented followed by the results of the analysis. The chapter concludes with a discussion of the results.
Review of cost-effectiveness evidence
The objective of this review was to identify and evaluate studies exploring the cost-effectiveness of TM or STS programmes for patients with HF.
Identification of studies
Electronic databases
Studies were identified by searching the following electronic databases:
-
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R) (Ovid) 1948–January 2012
-
EMBASE (Ovid) 1980–January 2012
-
Science Citation Index Expanded (Web of Science) 1899–January 2012
-
Conference Proceedings Citation Index – Science (Web of Science) 1990–January 2012
-
NHS Health Economic Evaluation Database (Wiley Online Library) 1995–January 2012
-
Health Technology Assessment database (Wiley Online Library) 1995–January 2012
-
Database of Abstracts of Reviews of Effects (Wiley Online Library) 1995–January 2012
-
PsycINFO (Ovid) 1806–January 2012
-
Cumulative Index to Nursing and Allied Health Literature (EBSCOhost) 1982–January 2012
-
Allied and Complementary Medicine Database (Ovid) 1985–January 2012
-
Health Economic Evaluations Database (OHE-IFPHA) 1967–January 2012.
Sensitive keyword strategies using free text and, where available, thesaurus terms using Boolean operators and database-specific syntax were developed to search the electronic databases. Synonyms relating to the condition (e.g. heart failure) were combined with sensitive economic evaluations (where applicable) or QoL search filters aimed at restricting results to economic and cost-related studies (used in the searches of MEDLINE, Cumulative Index to Nursing and Allied Health Literature and EMBASE). Essentially, the cost-effectiveness search strategy is the same as the clinical effectiveness search strategy albeit with the addition of an economic filter. The MEDLINE search strategy is provided in Appendix 10.
Other resources
To identify additional published, unpublished and ongoing studies, the reference lists of all relevant studies (including existing systematic reviews) were hand searched. A citation search of relevant articles (using the Web of Science Science Citation Index Expanded) was also undertaken. All identified citations from the electronic searches and other resources were imported into and managed using Reference Manager 12.
Inclusion and exclusion criteria
Studies were selected for inclusion according to predetermined inclusion and exclusion criteria. Studies were included if they reported an economic evaluation of disease management strategies or RM strategies for HF patients and estimated the benefits in terms of life-years gained (LYG) or quality-adjusted life-years (QALYs).
Studies that performed economic evaluations alongside trials were excluded if they did not extrapolate the outcomes beyond the trial duration, as these economic analyses are valid only for the trials under consideration. Studies that were considered to be methodologically unsound, that were not reported in sufficient detail to extract costs and outcome estimates (including abstracts) or that did not report an estimate of cost-effectiveness (e.g. costing studies) were also excluded. Papers not published in the English language were also excluded.
The inclusion of potentially relevant articles was undertaken using a two-step process. First, all titles were examined for inclusion by one reviewer. Any citations that clearly did not meet the inclusion criteria, that is, non-human, unrelated to TM and/or HF, were excluded. Second, all abstracts and full-text articles were examined independently by two reviewers. Any disagreements in the selection process were resolved through discussion.
Quality assessment strategy
The methodological quality of each included study was assessed using a combination of key components of the Drummond and Jefferson checklist for economic evaluations119,120 and the Eddy checklist for mathematical models used in technology assessments. 121 The use of the checklist ensured a consistent approach to assessing the quality of each economic evaluation.
Results of the cost-effectiveness review
The electronic literature searches identified 1696 potentially relevant publications. Of these, two studies122,123 met the inclusion criteria. A flow chart describing the process of identifying relevant literature can be found in Figure 5. A full list of excluded studies is presented in Appendix 11. Further details of the included studies including an assessment of methodological quality are provided below.
Miller et al. 122 developed a Markov model to assess the long-term effect of a STS programme compared with usual care for patients diagnosed with systolic HF. The model considered three levels of severity corresponding to (1) NYHA class I, (2) NYHA class II and (3) a combination of NYHA classes III and IV. The input data for the model were abstracted from an 18-month trial in 1069 HF patients in south Texas. 87 This study was not included in the NMA as the STS programme included HF disease management from registered nurses. The authors used SF-36 data collected from the trial to calculate the utilities for each severity class using the methods suggested by Brazier et al. 124 The study was carried out from the health-care system perspective and the analysis estimated the incremental cost-effectiveness ratio (ICER) of the disease management group against usual care. STS compared with usual care had an estimated ICER equal to $43,650 per QALY gained, and the univariate sensitivity analysis suggested that the incremental cost per QALY gained varied from $28,691 (with the use of different death rates for the control and intervention groups) to $129,738 (with an increased disease management programme cost of $246 per patient per month).
The study by Miller et al. 122 performed satisfactorily on the majority of items in the critical appraisal checklists119–121 used to assess the overall methodological quality of the model. Costs were extracted from the trial data by estimating the resource usage of different patient groups classified by morbidity and health state. Most of the costs were presented in a detailed and systematic way; however, the authors provided only an average cost of the STS programme and did not provide the breakdown of the individual cost components. One-way sensitivity analysis was performed; however, the authors did not perform a probabilistic sensitivity analysis (PSA). Both costs and QALYs were discounted at a rate of 3% per year.
Klersy et al. 123 assessed the cost-effectiveness of remote home TM for HF compared with usual care from a third payer perspective. A decision tree approach was used to compare the two strategies and the only outcome measured was admission for HF. To evaluate the clinical effectiveness of RM, a meta-analysis of 21 clinical trials assessing RM was carried out for this study. A budget impact analysis was presented and the different diagnosis-related group (DRG) reimbursement tariffs were considered with cost savings per patient ranging between €306.80 and €992.94.
Klersy et al. 123 focused mainly on the effectiveness rather than the costs and used a time horizon of 1 year. The study was limited to the budget impact and the cost-effectiveness was evaluated in this study by comparing the differences in costs and QALYs between RM and usual care. The effectiveness of RM was based on a meta-analysis of diverse studies evaluating interventions ranging from TM with home visits to STS. The authors used utilities of 0.612 and 0.662 for the usual care and RM groups respectively, based on a RCT reported by Hebert et al. 125 However, the study by Hebert et al. 125 was undertaken in an ethnically diverse urban community in Harlem, New York, and so the results might not be applicable to the UK HF population. Regarding costs, only hospitalisation costs were included and other costs such as RM costs, outpatient visits and drug costs were not considered. The authors stated that the monitoring costs were not considered because of the heterogeneity in the costs of RM. The authors used the DRG reimbursement tariff for HF hospitalisations as a proxy for real-life costs of hospitalisations. The authors performed scenario analyses using different DRG costs as part of the budget analysis to address the uncertainty in the hospitalisation costs, but neither deterministic sensitivity analysis nor PSA was performed.
Cost-effectiveness review summary
Although two cost-effectiveness analysis studies of RM were identified through the literature searches, there are a number of limitations associated with generalising the findings of these studies. The analysis reported by Miller et al. 122 was based on a single trial of STS, whereas Klersy et al. 123 included data from a meta-analysis of a wide range of studies of RM and the analysis did not differentiate between the different RM approaches. It is important to consider different RM approaches separately as they have different clinical effectiveness and costs associated with them.
There was heterogeneity in terms of the components of both usual care and RM interventions reported in the cost-effectiveness studies; this was also evident within the clinical effectiveness review (see Chapter 3, Results of the clinical effectiveness review). This made the identification of the parameters (e.g. costs) associated with the interventions difficult. Standard/specific RM approaches need to be described before estimating the cost-effectiveness of the interventions. Potential uncertainty in the description of interventions can be overcome by performing scenario analyses and sensitivity analyses.
The review also identified different approaches to the modelling of disease progression in HF patients. The analysis reported by Miller et al. 122 used the NYHA classification system to model disease progression whereas Klersy et al. 123 applied a Markov model with constant probabilities for mortality and hospitalisation. A systematic review by Goehler et al. 126 identified another approach that uses the number of rehospitalisations to model the disease progression pathway in HF. Hospital readmission- and NYHA classification-based models have significant data requirements (such as transition probabilities between NYHA classes) and this information was not reported in all of the RM studies included in the meta-analysis in Chapter 3 (see Results of the clinical effectiveness review). For hospital readmission- or NYHA classification-based models, a few selected studies that report the transition probabilities will have to be chosen to provide data for the models. This is in conflict with the aim of performing a robust analysis that takes all relevant evidence into account. Because all of the studies included in the meta-analysis provided mortality and/or hospitalisation rates for each type of remote disease management for HF, a two-state Markov model consisting of an ‘alive’ state and a ‘death’ state with a constant probability of rehospitalisation and changing mortality rate over time was chosen as the preferred approach for the de novo economic model detailed in the following section.
Independent economic assessment methods
This section details the methods and assumptions of the de novo economic model constructed to evaluate the cost-effectiveness of several strategies for RM compared with usual care for patients recently discharged with HF.
Overview of modelling methodology and objectives
A Markov model using a UK NHS perspective was developed to explore the costs and health outcomes associated with RM interventions for patients recently discharged with HF. Scenarios for costs of usual care and the RM interventions were developed through discussions with an expert advisory group (including clinicians and RM experts) and a review of the published literature and other sources that report details of resource use and unit costs of equipment, infrastructure and staff time. Data from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) study127 were used to estimate baseline mortality rates for patients in usual care, and the baseline risks associated with hospitalisation were estimated from Klersy et al. 123 The results from the NMA in Chapter 3 (see Results of the clinical effectiveness review) were used to model the HRs of event rates for patients with RM, separating HRs for mortality, HF-related hospitalisations and all-cause hospitalisations. Utilities were identified from evidence reported in the literature. Input parameters were assigned probability distributions to reflect their imprecision and Monte Carlo simulations were performed to produce expected incremental costs and QALYs for each strategy. Results were presented in terms of expected discounted QALYs and costs for each strategy, discounted incremental costs per QALY over a lifetime and net benefits using a threshold of £20,000 to value QALYs.
The objectives of the cost-effectiveness analysis were to:
-
estimate the cost-effectiveness of strategies for monitoring recently discharged HF patients, in terms of the incremental cost-effectiveness of each strategy, and to estimate the subsequent rates of death and hospitalisation among the modelled study population
-
identify the strategy that is most likely to be cost-effective for monitoring recently discharged HF patients in the NHS, defined as the most cost-effective strategy at a willingness-to-pay (WTP) threshold of £20,000–30,000 per QALY gained
-
identify the expected cost of uncertainty in the monitoring of HF patients and whether or not future research would be valuable by estimating the expected value of perfect information (EVPI) using a target population of 54,779 (number of first admissions for HF estimated from the National Heart Failure Audit for April 2010–March 201114).
A brief description of the key aspects of the economic analysis is provided in the following sections.
Model structure
A Markov model was developed to estimate the costs and health outcomes associated with different strategies for a hypothetical cohort of patients discharged in the last 28 days with HF-related hospitalisations. The model took a lifetime horizon and the economic perspective of the model was the NHS in England and Wales. In the model, as shown in Figure 6, two different states were considered:
-
alive at home
-
dead.
FIGURE 6.
Markov model of recently discharged HF patients.
The Markov model used a monthly cycle length with half-cycle correction and assigned each patient a monthly probability of death based on the time since discharge and the type of treatment. In each period the patients who were alive were under the risk of an average number of monthly rehospitalisations, that is, readmissions to a hospital for HF-related complications or other causes. Each patient then accrued lifetime QALYs and health-care costs according to their hospitalisation and treatment status.
Population
To address the research question laid out in the scope, the economic model utilised a hypothetical cohort of HF patients, discharged from hospital within 28 days. Patient age was not explicitly modelled because it was assumed that HF mortality was the dominant factor and other-cause mortality was implicitly included in the all-cause mortality. However, the mean age of HF patients as reported by the authors of the National Heart Failure Audit for 201030 was 75.85 years, which is similar to the mean age of 77.3 years at first HF admission reported in the National Heart Failure Audit for April 2010–March 2011. 14
Intervention
The systematic review presented in Chapter 3 identified considerable heterogeneity across studies with respect to how the RM activities were performed. As multiple alternative specifications of the RM approaches were reported in the studies included within the systematic review, the interventions were classified and specified as reported in Chapter 3. In the economic model, the following strategies were evaluated compared with current usual care in the NHS:
-
STS HH
-
STS HM
-
TM during office hours (i.e. TM with transmitted data reviewed by medical staff or medical support provided during office hours).
A base-case cost scenario, low-cost scenario and high-cost scenario were developed for each of these strategies based on discussions with an expert advisory group, as described in Costs.
Comparator
The comparator was usual care for patients recently discharged after HF hospitalisation in the NHS. Detailed reporting of the resources involved in usual care was severely lacking in most of the clinical trials. The base case for usual care in the economic model was estimated from the TEN-HMS study49 as it included patients from the UK. Following discussions with the expert advisory group a high-cost scenario for usual care was also developed.
Time horizon
A lifetime time horizon of 30 years was used. Patients progress through the model until they either die or reach the end of the 30-year time horizon. The proportion of patients alive in the usual care arm after 15 years predicted from the model is 5.67%; < 0.5% of the patients were alive after 30 years, suggesting that a 30-year time horizon was adequate.
Treatment duration
It was assumed that the interventions and usual care were provided for the first 6 months following discharge from hospital. At the end of 6 months all patients were assumed to receive usual care as per the NICE clinical guideline for the management of adults with HF,34 irrespective of whether or not they received the intervention or post-discharge usual care during the treatment period.
It was assumed that the treatment costs and effectiveness last only for the treatment duration of 6 months, after which the cost of usual care (as recommended by the NICE guideline on HF34) was applied along with the baseline risks of hospitalisation and mortality.
Perspective
A UK NHS perspective was used throughout; hence, productivity lost through illness or costs incurred directly by patients were not included.
Discount rate
Both the costs and QALYs were discounted at a monthly discount rate of 0.28709%, which was estimated from the annual discount rate of 3.5%, recommended by NICE, using the formula 1 + (monthly discount rate/100) = [1 + (annual rate/100)]1/12. 128
The key modelling methods together with the evidence sources and assumptions used to populate the model are discussed in detail in the following sections.
Selecting the classification of remote monitoring strategies
The systematic review in Chapter 3 identified heterogeneity in the components of interventions within the broad class of RM. Aspects of heterogeneity included the equipment available in the patient's home, the physiological measures monitored (e.g. weight, blood pressure and heart rate), the method of communicating the patient information to the RM team, the extent and timing of routine communication from the RM team to the patient, the use of automated computerised assessment of information to screen for prespecified alert levels, the method of assessment by the team and the staffing levels and types of staff.
As shown in Figure 7, the generalised structure of any remote disease management model includes monitoring, triage and a protocol for response/follow-up of the patient.
FIGURE 7.
Generalised patient pathway for RM.
The strategies can differ in terms of monitoring type, frequency and mode. Monitoring type relates to the data transmitted; this can include vital signs, physiological symptom monitoring and questionnaires. Frequency relates to how often the data are transmitted and is usually instantaneous, daily or weekly. The mode of input varies according to the intervention; it can be via the telephone verbally or via a telephone keypad, television or electronic device. The transmission can be via cables/wires or wireless (telephone lines, modem, 3G, broadband).
Triage involves investigation of the patient's alert/problem once it is discovered by the RM system. A problem could be discovered by software using prespecified algorithms or by manual examination of patient data by health professionals. Nurses or physicians will then determine whether it is a false alarm or whether the patient needs to be followed up based on the perceived severity of the problem.
If the problem is not labelled as a false alarm, a formal follow-up process is initiated by the health professional as shown in Figure 8. Depending on the diagnosis of the alarm, the follow-up process could vary from no further action to an emergency admission to the hospital. Based on the severity of diagnosis, other forms of follow-up include adjustment of medicine, adjustment of disease management protocol or an outpatient clinic visit.
FIGURE 8.
Follow-up process of RM for HF patients.
Variation in the RM interventions in terms of differences in the arrangements for monitoring, triage and a protocol for response/follow-up of the patient was used to develop a subclassification. RM was classified into three distinct categories: (1) STS HH, (2) STS HM and (3) TM during office hours (Table 19 and Figure 9). Cost-effectiveness analysis was performed for each of these intervention strategies compared with usual care and with each other.
| TM activity | STS HH | STS HM | TM during office hours |
|---|---|---|---|
| Monitoring | Undertaken via timetabled structured telephone calls from the service to the patient | Undertaken by the patient according to a predefined schedule related to their wake-up time | Undertaken via transmission of electronic data from the equipment in the patient's home to the monitoring centre |
| Triage | Most of the time the triage is carried out in real time while gathering information from the patient | Undertaken daily (5–7 days a week) by the nurse at a predefined time | Undertaken daily (5–7 days a week) by the nurse at a predefined time |
| Protocol for response/follow-up | According to the severity of the case, either the case is handled by the nurse or the physician is consulted | ||
FIGURE 9.
Interventions for remote disease management for patients with HF.
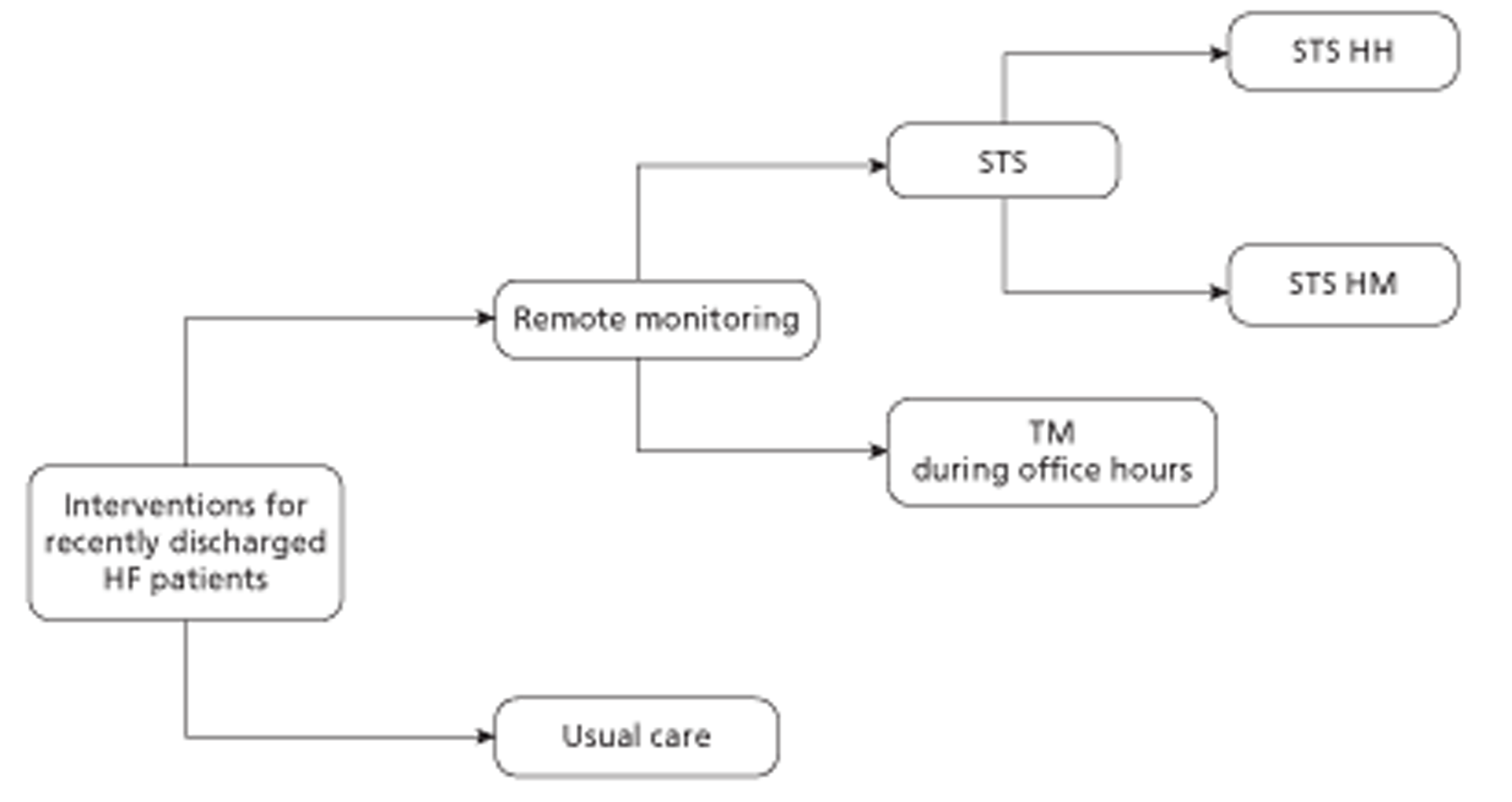
Although a fourth subclassification of 24/7 TM (i.e. transmitted data are reviewed by medical staff or medical support is provided 24 hours a day, 7 days a week) was examined separately as part of the NMA in Chapter 3, this strategy was not included in the economic analysis. There were two reasons for this. First, there were no UK-based 24/7 TM trials identified in the systematic review and the data for 24/7 TM would have been based on a single study. 76 This study76 was subject to methodological weaknesses as described in Chapter 3 (see Results of the clinical effectiveness review). Second, the expert advisory group suggested that the 24/7 home monitoring is currently not a realistic option for the UK setting as an existing round-the-clock response system is already available via the 999 emergency response route.
Baseline mortality and hospitalisation risks related to time since discharge
Patients with HF are at increased risk of both fatal and non-fatal major adverse cardiovascular events. The main outcomes of interest were all-cause mortality and hospitalisations. The model estimated the subsequent prognosis of each patient by using a monthly probability of death and monthly risks for hospitalisation (both HF-related and other causes) depending on the patient characteristics and the type of treatment. This section details the baseline risks of hospitalisation and death (i.e. for usual care without RM) estimated using data from the literature.
Mortality risk
The influence of time from non-fatal hospitalisation on subsequent mortality rates for HF patients was estimated based on the data from the CHARM study,127 which included 7572 patients followed up for 38 months. The monthly probability of death was also estimated from the CHARM study,127 which showed that the mortality risk was highest immediately after hospital discharge and then decreased over time. This section describes the process of estimation of the mortality risk for HF patients from the data reported in the CHARM study. 127
The HRs of all-cause mortality of hospitalised HF patients compared with non-hospitalised HF patients in the CHARM127 study are reported and are replicated in Table 20. The mortality risk of non-hospitalised patients was estimated from the CHARM study,127 which reported 1233 deaths in 4884 non-hospitalised patients over 38 months. The instantaneous mortality hazard rate (r) for non-hospitalised patients was calculated as 0.00765 using the formula r = –[ln(1 – Pd)]/t assuming a constant instantaneous rate, where Pd(= 0.252, i.e. 1233/4884) was the probability of death for non-hospitalised patients over a period of time (t = 38 months).
| Time since discharge (months) | HR | 95% CI |
|---|---|---|
| 0–1 | 6.18 | 4.81 to 7.93 |
| > 1–3 | 4.39 | 3.50 to 5.50 |
| > 3–6 | 3.54 | 2.86 to 4.39 |
| > 6–12 | 3.11 | 2.59 to 3.75 |
| > 12–24 | 2.46 | 2.06 to 2.94 |
| > 24 | 1.93 | 1.48 to 2.52 |
The instantaneous mortality hazard rates for hospitalised HF patients in different time periods since discharge from HF-related hospitalisation, as shown in Table 21, were estimated by multiplying the HRs in Table 20 by the constant mortality hazard rate of non-hospitalised HF patients.
The monthly mortality probabilities were then estimated from the instantaneous hazard rates using the formula p = 1 – exp(– rate). The risk of death is greatest in the early period after discharge after a hospitalisation for HF and subsequently declines over time as seen in Table 22. The probability of death in the first month after discharge is an estimated threefold higher than the probability of mortality beyond 2 years from discharge.
| Time since discharge (months) | Mortality hazard | 95% CI |
|---|---|---|
| 0–1 | 0.04732 | 0.03683 to 0.06072 |
| > 1–3 | 0.03361 | 0.02680 to 0.04211 |
| > 3–6 | 0.02711 | 0.02190 to 0.03361 |
| > 6–12 | 0.02381 | 0.01983 to 0.02871 |
| > 12–24 | 0.01884 | 0.01577 to 0.02251 |
| > 24 | 0.01478 | 0.01133 to 0.01930 |
| Time since discharge (months) | Mortality probability per month | 95% CI |
|---|---|---|
| 0–1 | 0.04622 | 0.03616 to 0.05891 |
| > 1–3 | 0.03306 | 0.02644 to 0.04124 |
| > 3–6 | 0.02674 | 0.02166 to 0.03306 |
| > 6–12 | 0.02353 | 0.01964 to 0.02831 |
| > 12–24 | 0.01866 | 0.01565 to 0.02226 |
| > 24 | 0.01467 | 0.01127 to 0.01911 |
The survival curve showing the proportion of patients alive (under usual care) over time is shown in Figure 10. It can be seen that most of the deaths occur in the initial period after discharge, which is in line with the assumptions that the mortality risk is higher in the time immediately after discharge and the effect of the intervention lasts only for the first 6 months. The survival rate changes at 1 month, 3 months, 6 months, 12 months and 24 months as expected from Table 22. Finally, the patients are assumed to have a constant mortality rate beyond the 24-month period, as seen in the smooth exponential curve.
FIGURE 10.
Baseline survival curve of recently discharged HF patients.
According to the survival curve, 4.7%, 10.8% and 17.7% of HF patients would have died by 1 month, 3 months and 6 months respectively. Furthermore, 28% of the patients would have died by the end of 1 year and 43% would have died at 2 years post discharge. This is in line with the findings from the HF audit as reported in Chapter 1 (see Aetiology, pathology and prognosis).
Risk of hospitalisation
The other main outcomes included in the model are HF-related and other-cause hospitalisations. The other-cause hospitalisations are modelled as all-cause hospitalisations minus the HF-related hospitalisations. The mean number of annual hospitalisations were estimated from the meta-analysis reported by Klersy et al. 123 and are presented in Table 23. For HF hospitalisations, Klersy et al. 123 reviewed 17 trials from different countries (2089 patients) and reported an annual incidence rate of 42.1 per 100 patients. For all-cause hospitalisations the authors reviewed 18 trials (2332 patients) and reported an annual incidence rate of 105.1 per 100 patients. These rates of hospitalisation were divided by 12 to estimate the monthly risk of hospitalisations in the economic model. Table 23 shows the parameters used in the model per patient in usual care.
Effect of the interventions
Hazard ratios for all-cause mortality, all-cause hospitalisations and HF-related hospitalisations were used as effectiveness parameters in the model during the treatment period (i.e. the first 6 months following discharge from the hospital). It should be noted that the clinical systematic review identified considerable heterogeneity in the manner in which RM and usual care were performed. Clear descriptions of the interventions were not provided in many of the studies identified in the systematic review, which made it difficult to understand exactly what was provided as part of the intervention and what was provided as part of usual care. This lack of detail meant that the HR estimates from the meta-analyses were a conglomeration of estimates from heterogeneous comparisons. For example, study 1, which compared RM variant 1 with usual care variant 1, was pooled with study 2, which compared RM variant 2 with usual care variant 2, and so on. There was insufficient information regarding how usual care variant 1 differed from usual care variant 2 to make adjustments to effectiveness difference estimates. Furthermore, as some of the studies were undertaken across multiple centres, usual care variant 1 itself could be a pooled estimate of usual care variant 1a, usual care variant 1b and usual care variant 1c. Thus, this lack of detail in research studies concerning the design of the RM and especially the usual care arm has implications for the robustness of any analysis of effectiveness. The statistical analysis of the extent of heterogeneity was discussed fully in Chapter 3.
The HRs estimated from the NMA, as reported in Chapter 3 (see Results of the clinical effectiveness review), for the different categories (i.e. STS HM, STS HM and TM during office hours) are presented in Tables 24a and b. All analyses were performed using an intention-to-treat analysis, that is, all patients were analysed in the groups to which they were allocated, regardless of whether or not they received the treatment.
| Intervention | All-cause mortality | HF-related hospitalisations | All-cause hospitalisations | |||
|---|---|---|---|---|---|---|
| Median HR | 95% CrI | Median HR | 95% CrI | Median HR | 95% CrI | |
| STS HH | 0.77 | 0.55 to 1.08 | 0.77 | 0.62 to 0.96 | 0.97 | 0.70 to 1.31 |
| STS HM | 0.98 | 0.41 to 2.33 | 1.03 | 0.66 to 1.54 | 1.06 | 0.44 to 2.53 |
| TM during office hours | 0.76 | 0.49 to 1.18 | 0.95 | 0.70 to 1.34 | 0.75 | 0.49 to 1.10 |
| Intervention | All-cause mortality | HF-related hospitalisations | All-cause hospitalisations | |||
|---|---|---|---|---|---|---|
| Median HR | 95% PrI | Median HR | 95% PrI | Median HR | 95% PrI | |
| STS HH | 0.77 | 0.31 to 1.86 | 0.77 | 0.50 to 1.19 | 0.97 | 0.38 to 2.43 |
| STS HM | 0.98 | 0.30 to 3.23 | 1.03 | 0.58 to 1.77 | 1.06 | 0.31 to 3.61 |
| TM during office hours | 0.76 | 0.30 to 1.91 | 0.95 | 0.59 to 1.62 | 0.75 | 0.28 to 1.91 |
A sensitivity analysis was performed excluding the Home-HF study67 as described in Chapter 3 (see Results of the clinical effectiveness review) and the HRs are presented in Tables 25a and b. For decision-makers deciding which of these scenarios is most representative of their setting, the key questions relate to the inclusion of the Home-HF study67 in the effectiveness meta-analyses. If one believes that usual care is best represented by the usual care arm in the Home-HF study,67 which is the only study showing a statistically significant difference in effectiveness of usual care over RM, then perhaps the results including the Home-HF study67 might be considered more relevant than those without. If, on the other hand, one believes that the performance of usual care is better represented by the other studies and that usual care in the Home-HF study67 is not representative of current usual care, then the results excluding the Home-HF study67 might be considered more relevant.
| Intervention | All-cause mortality | HF-related hospitalisations | All-cause hospitalisations | |||
|---|---|---|---|---|---|---|
| Median HR | 95% CrI | Median HR | 95% CrI | Median HR | 95% CrI | |
| STS HH | 0.75 | 0.59 to 0.96 | 0.76 | 0.61 to 0.94 | 0.96 | 0.72 to 1.27 |
| STS HM | 0.98 | 0.58 to 1.62 | 1.02 | 0.70 to 1.49 | 1.06 | 0.48 to 2.32 |
| TM during office hours | 0.62 | 0.42 to 0.89 | 0.86 | 0.61 to 1.21 | 0.67 | 0.42 to 0.97 |
| Intervention | All-cause mortality | HF-related hospitalisations | All-cause hospitalisations | |||
|---|---|---|---|---|---|---|
| Median HR | 95% PrI | Median HR | 95% PrI | Median HR | 95% PrI | |
| STS HH | 0.75 | 0.45 to 1.27 | 0.76 | 0.51 to 1.13 | 0.96 | 0.42 to 2.18 |
| STS HM | 0.98 | 0.49 to 1.95 | 1.02 | 0.61 to 1.69 | 1.06 | 0.35 to 3.22 |
| TM during office hours | 0.62 | 0.35 to 1.09 | 0.86 | 0.54 to 1.38 | 0.67 | 0.26 to 1.53 |
The monthly mortality probabilities for the interventions were estimated by applying the HRs to the baseline mortality probability using the formula Pintervention = 1 – (1 – Pbaseline)HR, where Pintervention is the monthly mortality probability of the intervention and Pbaseline is the baseline monthly mortality probability. This is equivalent to multiplying the HR of the intervention with the baseline hazard rate to estimate the hazard rate of the intervention, and then converting the hazard rate into a monthly probability.
Health-related quality of life
This section provides a discussion of the evidence available for four aspects of QoL: baseline HRQoL for HF patients under usual care, the impact caused by hospital readmission for HF, the impact caused by hospital readmission for other causes and whether or not there is any evidence that patients who are not readmitted experience better HRQoL with RM than with usual care.
To estimate the HRQoL for recently discharged HF patients under usual care, a rapid review was conducted and four studies were found. Capomolla et al. 73 reported HRQoL for recently discharged CHF patients as 0.63 and Calvert et al. 129 reported the utility to be 0.6 for advanced HF patients (NYHA class III or IV). Iqbal et al. 130 reported the utility of HF patients as 0.57 but the population had multiple comorbidities. Miller and Cox131 estimated the utilities from SF-36 data collected during the trial using the methods suggested by Brazier et al. 124 and reported them as 0.58 for advanced HF (NYHA class III or IV) and 0.67 for the weighted average for patients in NYHA class I or II.
Reviewing the evidence showed that there was uncertainty about the difference in patients' QoL in different arms, that is, whether or not HRQoL is different for patients in the usual care and RM groups. In a previous economic model of RM, Klersy et al. 123 used utilities of 0.612 and 0.662 for the usual care and RM groups respectively. However, the utilities used by Klersy et al. 123 were based on a RCT by Herbert et al. ,125 which was undertaken in an ethnically diverse urban community in Harlem, New York; hence, the results might not be applicable to the UK HF patient population. Furthermore, none of the studies identified in the systematic review in Chapter 3 reported any difference in the utility of patients in the usual care and RM groups. As there was no quantified evidence on the extent to which RM improves HRQoL of patients, the same utility values were used for HF patients receiving both usual care and (each of the three) RM strategies in the economic model.
Evidence on the disutility caused by rehospitalisation for HF was not clear. A disutility of 0.1 was incorporated for every HF-related hospitalisation based on a study by Yao et al. ,132 who estimated the disutility to be equivalent to the utility of one health state lower in terms of NYHA class. The disutility was assumed to last for 1 year.
Evidence on the disutility caused by rehospitalisation for other causes (not directly HF related) was also limited. In the absence of evidence it was assumed that there was no disutility caused by rehospitalisation for other causes.
In the economic model, different values of utility were used for unstable patients (i.e. recently discharged patients) and stable patients (i.e. 1 year since hospitalisation). A utility score of 0.58 was used for patients in the first year since discharge and a utility of 0.67 was used after the first year. Any HF-related hospitalisation was assumed to result in a disutility of 0.1 for a whole year, that is, the utility of the patient for that year was 0.67 – 0.1 = 0.57. Within the PSA, the uncertainty in the utility values was represented using a normal distribution using the deterministic value as mean with a standard deviation of 0.015, estimated based on the difference between utilities reported by Capomolla et al. 73 and Iqbal et al. ,130 and the disutility was represented using a triangular distribution with (–0.08,0.11) as the range with –0.1 as the mode.
Costs
The costs in the model are described in detail in the following sections.
Clear descriptions of the interventions and usual care were not provided in many of the studies identified in the systematic review. This lack of detail concerning the design of the RM interventions and especially the usual care arms (e.g. communication protocols, routine staff visits, resources used) has implications for estimating the costs of interventions and usual care. As the resources used in each intervention variant were not always clear, three different cost scenarios for RM interventions (base case, lower and upper) and two different cost scenarios for usual care (base case and upper) were developed as described in Costs of remote monitoring care interventions and Usual care costs.
Costs of remote monitoring care interventions
The RM costs were highly variable because of the heterogeneity in devices, monitoring and follow-up processes. The costs of RM comprised three main components:
-
costs of the RM devices and equipment within the patient's home, including the device hub, peripherals and communication costs
-
maintenance/monitoring costs in the RM centre
-
medical care costs to deal with events/alerts, for example GP and nurse visit costs or further hospital-based outpatient visits (excludes rehospitalisation costs).
The costs of the RM devices were elicited from the expert advisory group. The maintenance/monitoring costs were estimated using activity-based costing for the resources spent by staff on triage and follow-up based on evidence from the literature. The costs of medical care were estimated from the TEN-HMS study,49 which reported the medical care received in the usual care, STS and TM arms.
The RM device, responsible for the collection and transmission of data (which can include vital signs, symptoms and questionnaires), can take different levels of complexity and the cost of the device is based on this complexity. Each monitoring device consists of a hub and can have a number of peripherals (medical devices with specific functionality that measure the vital signs and transmit them to the hub). These peripherals can be wired or wireless with the costs of wireless peripherals being higher than the costs of wired peripherals. The communication costs include the cost of data transfer along with server costs for the management of patient data.
In terms of monitoring, the characteristics of the system/infrastructure and the composition of the monitoring team also have an impact on the cost of the monitoring through triage costs. Triage costs also vary depending on whether the triage is performed by individuals (such as technicians, nurses or physicians) or a dedicated clerical triage team that monitors the patients. Triage costs also depend on the type of software used.
The medical care costs associated with the follow-up of patients consist of A&E visits, GP/cardiologist visits and nurse visits (both home and office visits). These were examined in two stages: the mean number of visits for each patient and the unit costs per visit.
Medical care costs to deal with events/alerts: evidence and assumptions used
The evidence used to estimate the mean numbers of medical care visits is taken from the TEN-HMS study,49 conducted across 16 hospitals in Germany, the Netherlands and the UK between August 2000 and March 2002. A total of 426 patients were assigned randomly to home TM, nurse telephone support and usual care in a 2 : 2 : 1 ratio. This study reported the frequency of different health visits for each of the three arms over a 240-day period as shown in Table 26.
| Medical care visits | Usual care | STS | TM |
|---|---|---|---|
| Number of patients | 85 | 170 | 163 |
| Total days at risk | 16,089 | 33,803 | 33,641 |
| Emergency room visits | |||
| Visits | 8 | 54 | 60 |
| Total/1000 days at risk (95% CI) | 0.5 (0.2 to 0.8) | 1.6 (1.2 to 2.0) | 1.8 (1.3 to 2.2) |
| Office visits | |||
| Family practitioner | 119 | 602 | 454 |
| Specialist | 34 | 117 | 100 |
| Nurse and other | 36 | 104 | 100 |
| Total | 189 | 823 | 654 |
| Total/1000 days at risk (95% CI) | 11.7 (10.1 to 13.4) | 24.3 (22.7 to 26.0) | 19.4 (18.0 to 20.9) |
| Home visits | |||
| Family practitioner | 42 | 185 | 162 |
| Specialist | 0 | 3 | 1 |
| Nurse and other | 27 | 206 | 128 |
| Total | 69 | 394 | 291 |
| Total/1000 days at risk (95% CI) | 4.3 (3.3 to 5.9) | 11.7 (10.5 to 12.8) | 8.7 (7.7 to 9.6) |
| All face-to-face patient contacts | |||
| Total | 300 | 1388 | 1115 |
| Total/1000 days at risk (95% CI) | 18.6 (16.6 to 20.7) | 41.1 (38.9 to 43.2) | 33.1 (31.2 to 35.1) |
| Telephone calls | |||
| Total | 90 | 914 | 1180 |
| Total/1000 days at risk (95% CI) | 5.6 (4.4 to 6.8) | 27.0 (25.3 to 28.8) | 35.1 (33.1 to 37.0) |
| All patient contacts | |||
| Total contacts | 390 | 2302 | 2295 |
| Total/1000 days at risk (95% CI) | 24.2 (21.9 to 26.6) | 68.1 (65.4 to 70.8) | 68.2 (65.5 to 70.9) |
The unit costs of staff time were estimated based on data from the Personal Social Services Research Unit (PSSRU)133 and NHS Reference Costs 2009–10. 134 The unit costs of staff time used in estimating the intervention costs are shown in Table 27.
| Medical staff | Unit of time | Cost (£) | Source |
|---|---|---|---|
| GP | One office visit | 46 | PSSRU 2011,133 section 10.8b, p. 149a |
| Specialist | One office visit | 46 | PSSRU 2011133b |
| GP | One home visit | 104 | PSSRU 2011,133 section 10.8b, p. 150c |
| Community nurse | One home visit | 38 | Department of Health,134 District Nursing Services: Adult: Face To Face, Currency Code: CN301AF |
| Community nurse | One office visit | 25 | PSSRU 2011,133 section 10.7, p. 147d |
| Hospital nurse | 1 hour | 40 | PSSRU 2011,133 section 14.3, p. 193e |
| Clinical support worker (hospital) | 1 hour | 20 | PSSRU 2011,133 section 14.5, p. 195 |
Scale of typical remote monitoring service
Three alternative scenarios for each RM classification (i.e. STS HM, STS HH and TM during office hours) were developed and their costs were estimated after discussions with the expert advisory group. The three alternative scenarios for each RM classification correspond to a base-case scenario, a low-cost scenario and a high-cost scenario. These scenarios were designed to reflect the different configurations of the RM systems present in the UK.
The costs of RM interventions were estimated for a RM centre that monitored 250 patients for a period of 6 months. This was based on the median size of NHS foundation trusts in the UK and the proportion of those people eligible for RM. According to the National Heart Failure Audit for 2010–11,14 the median number of HF patients discharged annually with HF as their primary diagnosis from the different hospital foundation trusts in England and Wales was 380. Hull Foundation Trust, which had 380 HF patients admitted in 2011, has 250 CHF patients under RM. Thus, taking into consideration that the number of HF patients admitted to the Hull and East Yorkshire Hospitals NHS Trust was equal to the median number of HF patients admitted to different foundation trusts in England and Wales, it was assumed that a typical RM centre would have an average capacity to monitor 250 patients. This number was also deemed sensible by the expert advisory group.
Cost of the structured telephone support human-to-machine interface intervention
The breakdown of the costs of the device, maintenance/monitoring costs and medical care costs for the base-case STS HM intervention is shown in Table 28.
| Resource usage | Source | Unit cost (£) | Source | Cost per 6 months (£) | |
|---|---|---|---|---|---|
| Breakdown of device costs | |||||
| Cost of telephone + peripherals (scale and blood pressure cuff) | 0.5 (for 6 months) | Based on advice from clinical experts | 78 (per year) | Expert advisory input | 39 |
| Total cost of the device per patient for 6 months | 39 | ||||
| Breakdown of monitoring costs | |||||
| Triage, decision-making by nurse | 7 hours | Boyne et al.135 | 40 (per hour) | PSSRU 2011133 | 280 |
| Data management software | 1/1500a | 5000 (per site licence) | 3 | ||
| Total STS HM monitoring cost per patient for 6 months | 283 | ||||
| Frequency per patient | Source | Unit cost per contact (£) | Source | Cost per 6 months (£) | |
| Breakdown of medical care costs | |||||
| Emergency room visits | 0.30 | Cleland et al. (TEN-HMS)49 | 130 | Department of Health134 | 39 |
| Office visits: family practitioner | 3.37 | Cleland et al. (TEN-HMS)49 | 46 | PSSRU 2011133 | 155 |
| Office visits: specialist | 0.66 | Cleland et al. (TEN-HMS)49 | 46 | PSSRU 2011133 | 30 |
| Office visits: nurse and other | 0.58 | Cleland et al. (TEN-HMS)49 | 25 | PSSRU 2011133 | 15 |
| Home visits: family practitioner | 1.04 | Cleland et al. (TEN-HMS)49 | 104 | PSSRU 2011133 | 108 |
| Home visits: specialist | 0.02 | Cleland et al. (TEN-HMS)49 | 104 | PSSRU 2011133 | 2 |
| Home visits: nurse and other | 1.15 | Cleland et al. (TEN-HMS)49 | 38 | Department of Health134 | 43 |
| Total medical care costs per patient for 6 months | 392 | ||||
| Total cost of STS HM intervention per patient for 6 months | 715 | ||||
The costs of the telephone and peripherals for STS HM were elicited from the expert advisory group and a baseline yearly cost of £78 was used. For the low-cost STS HM scenario a yearly cost of £32 was used for a basic telephone device, and for the high-cost STS HM scenario a yearly cost of £235 was used for a telephone device with more peripherals (see Table 31).
The costs of monitoring for STS HM were estimated from Boyne et al. ,135 who conducted a RCT at three hospitals in the South-Limburg area of the Netherlands. The study included 382 patients with 197 in the RM group and 185 in the usual care group. Boyne et al. 135 reported that an average time of 2 minutes and 20 seconds was dedicated by the triage nurse each day for monitoring an individual patient. This time spent daily was multiplied by 182.5 to estimate the staff time spent for each patient over 6 months; this gave a total of 7 hours. The costs associated with monitoring over 6 months were estimated by multiplying this nurse time by their hourly rate. It was assumed in the model that the triage is performed by a hospital nurse and a £40 per hour rate was used according to the PSSRU. 133 Also, the cost of the patient management software (£5000) for the RM centre was converted into a cost per patient based on a 3-year depreciation period (i.e. assuming that new software will be required in 3 years) and sharing the overall cost of the software out amongst the number of patients who would benefit from the service over 3 years (estimated as 1500 patients, i.e. 250 every 6 months, assuming a 6-month treatment duration and no delays for removal and installation of the monitoring device).
The costs of medical care (excluding hospital readmissions) were estimated based on the data from the TEN-HMS trial. 49 The numbers of visits reported in the trial for patients in the nurse telephone support arm (n = 170) over a 240-day period (see Table 26) were used to estimate the average numbers of visits per patient over 6 months (see Table 28). These average numbers of visits were multiplied by the corresponding unit cost per contact, based on the staff involved, to estimate medical costs of £392 per patient over 6 months.
Thus, the total cost per patient for the STS HM intervention over 6 months was estimated to be £715, that is, a monthly cost of £119 per patient.
Cost of the structured telephone support human-to-human contact intervention
For STS HH intervention costs, it was assumed that only the monitoring costs were different between the STS HM and STS HH interventions. The costs of monitoring for STS HH were estimated from Riegel et al. ,79 who reported the workload of the staff responsible for implementing a STS intervention to be equal to 16 hours per patient for 6 months. Using the same device and medical costs as for STS HM, the total base-case cost per patient receiving the STS HH intervention for 6 months was estimated to be £1075, that is, a monthly cost of £179 per patient (Table 29).
| Resource usage | Source | Unit cost (£) | Source | Cost per 6 months (£) | |
|---|---|---|---|---|---|
| Breakdown of device costs | |||||
| Cost of telephone + peripherals (scale and blood pressure cuff) | 0.5 (for 6 months) | Based on advice from clinical experts | 78 (per year) | Expert advisory input | 39 |
| Total cost of the device per patient for 6 months | 39 | ||||
| Breakdown of monitoring costs | |||||
| Triage, decision-making by nurse | 16 hours | Riegel et al.79 | 40 (per hour) | PSSRU 2011133 | 640 |
| Data management software | 1/1500a | 5000 (per site licence) | Expert advisory input | 3 | |
| Total STS HH monitoring cost per patient for 6 months | 643 | ||||
| Frequency per patient | Source | Unit cost per contact (£) | Source | Cost per 6 months (£) | |
| Breakdown of medical care costs | |||||
| Emergency room visits | 0.30 | Cleland et al. (TEN-HMS)49 | 130 | Department of Health134 | 39 |
| Office visits: family practitioner | 3.37 | Cleland et al. (TEN-HMS)49 | 46 | PSSRU 2011133 | 155 |
| Office visits: specialist | 0.66 | Cleland et al. (TEN-HMS)49 | 46 | PSSRU 2011133 | 30 |
| Office visits: nurse and other | 0.58 | Cleland et al. (TEN-HMS)49 | 25 | PSSRU 2011133 | 15 |
| Home visits: family practitioner | 1.04 | Cleland et al. (TEN-HMS)49 | 104 | PSSRU 2011133 | 108 |
| Home visits: specialist | 0.02 | Cleland et al. (TEN-HMS)49 | 104 | PSSRU 2011133 | 2 |
| Home visits: nurse and other | 1.15 | Cleland et al. (TEN-HMS)49 | 38 | Department of Health134 | 43 |
| Total medical care costs per patient for 6 months | 392 | ||||
| Total cost of STS HH intervention per patient for 6 months | 1075 | ||||
Cost of telemonitoring during office hours
The total costs of TM during office hours were broken down into the costs of the device, maintenance/monitoring costs and medical care costs for the TM during office hours as shown in Table 30.
| Resource usage | Source | Unit cost (£) | Source | Cost per 6 months (£) | |
|---|---|---|---|---|---|
| Breakdown of device costs | |||||
| Cost of hub + peripherals | 0.5 (for 6 months) | Based on advice from clinical experts | 703 (per year) | Based on advice from clinical experts | 352 |
| Communication (patient to centre) | 0.5 (for 6 months) | Based on advice from clinical experts | 160 (per year) | Based on advice from clinical experts | 80 |
| Total cost of the device per patient for 6 months | 433 | ||||
| Breakdown of monitoring costs | |||||
| Triage, decision-making | 7 hours | Boyne et al.135 | 40 (per hour) | PSSRU 2011133 | 280 |
| Data management software | 1/1500a | 5000 | 3 | ||
| Total monitoring cost per patient for 6 months | 283 | ||||
| Frequency per patient | Source | Unit cost per contact (£) | Source | Cost per 6 months (£) | |
| Breakdown of medical care costs | |||||
| Emergency room visits | 0.35 | Cleland et al. (TEN-HMS)49 | 130 | Department of Health134 | 45 |
| Office visits: family practitioner | 2.65 | Cleland et al. (TEN-HMS)49 | 46 | PSSRU 2011133 | 122 |
| Office visits: specialist | 0.58 | Cleland et al. (TEN-HMS)49 | 46 | PSSRU 2011133 | 27 |
| Office visits: nurse and other | 0.58 | Cleland et al. (TEN-HMS)49 | 25 | PSSRU 2011133 | 15 |
| Home visits: family practitioner | 0.95 | Cleland et al. (TEN-HMS)49 | 104 | PSSRU 2011133 | 98 |
| Home visits: specialist | 0.01 | Cleland et al. (TEN-HMS)49 | 104 | PSSRU 2011133 | 1 |
| Home visits: nurse and other | 0.75 | Cleland et al. (TEN-HMS)49 | 38 | Department of Health134 | 28 |
| Total medical care costs per patient for 6 months | 336 | ||||
| Total costs | 1051 | ||||
The cost of the TM device was elicited from the expert advisory group – a baseline yearly cost of £708 was used. For the high-cost TM scenario a yearly cost of £1176 was used for a TM device with more peripherals, as detailed in Table 31.
| Scenario | Assumption | Source | Total cost (£) |
|---|---|---|---|
| STS HM scenarios | |||
| Base-case scenario | – | – | 715 |
| Low-cost scenario | Telephone cost of £31.60 per year | Clinical input | 623 |
| High-cost scenario | Telephone cost of £235 per year (with more peripherals) | Clinical input | 794 |
| STS HH scenarios | |||
| Base-case scenario | – | – | 1075 |
| Low-cost scenario | Telephone cost of £31.60 per year | Clinical input | 1051 |
| High-cost scenario | Telephone cost of £235 per year (with more peripherals) | Clinical input | 1152 |
| TM during office hours scenarios | |||
| Base-case scenario | – | – | 1051 |
| Low-cost scenario | 1 hour of triage and follow-up per patient | Clinical input/Dar et al.67 | 801.20 |
| High-cost scenario | Device cost of £1176 per year (with more peripherals) | Clinical input | 1287.50 |
The costs of monitoring were assumed to be the same as the STS HM monitoring costs estimated from Boyne et al. 135 as the triage and follow-up process was similar for both interventions. The cost of the software was also assumed to be the same and was estimated using a 3-year depreciation period for 1500 patients (i.e. for six cohorts of 250 patients each). For the low-cost TM scenario it was assumed that the time spent by the hospital nurse on triage and follow-up was 1 hour for each patient for 6 months.
The data from the TEN-HMS trial49 were used to estimate the medical costs. The numbers of visits reported in the trial for patients in the home TM arm (n = 163) over a 240-day period, reproduced in Table 26, were used to estimate the average numbers of visits per patient for 6 months as reported in Table 30. These average numbers of visits were multiplied by the corresponding unit cost per contact, based on the staff involved, to estimate medical costs of £336 per patient over 6 months.
The total cost per patient for the office hours TM intervention for 6 months was estimated to be £1051, that is, a monthly cost of £173 per patient (see Table 30).
Cost scenarios for the remote monitoring interventions
It was not possible to validate the robustness of the costs of the base-case scenarios as clear descriptions of the interventions were not provided in the trials of RM. Furthermore, large variation was observed in the costs of the devices from the pricing data accessed and there was uncertainty in the monitoring resources used within studies reporting different estimates. This heterogeneity made it difficult to provide a single description (and cost) of the interventions in each RM classification.
To this end, two further costing scenarios were developed for each RM classification, that is, STS HM, STS HH and TM during office hours, and their costs were estimated. These costing scenarios evaluate a high and low estimate of the costs for each RM classification to understand the impact of cost on the cost-effectiveness of the interventions. The differences in assumptions between the new scenarios and the base-case scenarios along with the new estimated costs (per patient for 6 months) are shown in Table 31.
Usual care costs
There is variation between different local settings in the usual care applied in current clinical practice, as described in Chapter 1 (see Current service provision). The current NICE guidelines34 provide recommendations for the management of adults with HF; however, a clear description of the current service is not available. The recommendations in the NICE guidelines state that: ‘The frequency of monitoring should depend on the clinical status and stability of the patient. The monitoring interval should be short (days to 2 weeks) if the clinical condition or medication has changed, but is required at least 6-monthly for stable patients with proven heart failure’.
Furthermore, the studies identified in the systematic review presented in Chapter 3 did not report clearly or in detail what was involved in the usual post-discharge care, thus making it difficult to estimate the costs of usual care. The key resource costs for usual care for HF patients are the visits to the GP and the nurse visits immediately after discharge. As the usual post-discharge care was not reported clearly in the studies within the systematic review, it was assumed that the cost of the base-case usual post-discharge care was the same as that described in the TEN-HMS study. 49 A high-cost usual post-discharge care scenario was also developed based on discussions with the expert advisory group.
Base-case usual post-discharge care cost scenario
Data from the TEN-HMS study49 were used to estimate the costs of usual post-discharge care in the base-case scenario, which consisted of nurse visits, GP/cardiologist visits and A&E visits. The numbers of visits reported in the trial for patients in the usual care arm (n = 85) over a 240-day period, reproduced in Table 26, were used to estimate the average numbers of visits per patient for 6 months (Table 32). These average numbers of visits were multiplied by the corresponding unit cost per contact, based on the staff involved, to estimate usual care costs of £161 per patient for 6 months.
| Medical care | Frequency per patient | Source | Unit cost per contact (£) | Source | Cost per 6 months (£) |
|---|---|---|---|---|---|
| Emergency room visits | 0.09 | Cleland et al. (TEN-HMS)49 | 130 | Department of Health134 | 12 |
| Office visits: family practitioner | 1.33 | Cleland et al. (TEN-HMS)49 | 46 | PSSRU 2011133 | 61 |
| Office visits: specialist | 0.38 | Cleland et al. (TEN-HMS)49 | 46 | PSSRU 2011133 | 18 |
| Office visits: nurse and other | 0.40 | Cleland et al. (TEN-HMS)49 | 25 | PSSRU 2011133 | 10 |
| Home visits: family practitioner | 0.47 | Cleland et al. (TEN-HMS)49 | 104 | PSSRU 2011133 | 49 |
| Home visits: specialist | – | Cleland et al. (TEN-HMS)49 | 104 | PSSRU 2011133 | – |
| Home visits: nurse and other | 0.30 | Cleland et al. (TEN-HMS)49 | 38 | Department of Health134 | 11 |
| Total medical care costs per patient for 6 months | 161 | ||||
High-cost usual post-discharge care cost scenario
As the usual care was not reported clearly in the studies in the systematic review, a high-cost usual post-discharge care scenario was developed after discussions with the expert advisory group. It was assumed that on discharge one HF hospital nurse visits the patient at home on a weekly basis for 3–4 weeks, and subsequently the district nurse visits a further three to four times in the period between week 4 and week 8 after discharge. It was assumed that patients also have monthly GP consultations. It was also assumed that monitoring costs include the medical costs of patients such as the costs of diagnostic and pathology laboratory tests once every 6 months. These numbers of visits were multiplied by the corresponding unit cost per contact, based on the data from PSSRU 2011133 reported in Table 27, to estimate a cost for each patient of £592 for 6 months (Table 33).
Usual care beyond the treatment duration of 6 months
It was assumed that at the end of the 6-month treatment period all patients receive usual care as recommended in the NICE clinical guidelines for the management of adults with HF,34 irrespective of whether they received the intervention or post-discharge usual care during the treatment period. Usual care for patients beyond the 6-month treatment period, based on the description in the NICE guidelines, is shown in Table 34.
| Medical care | Frequency per patient | Source | Unit cost per contact (£) | Source | Cost per 6 months (£) |
|---|---|---|---|---|---|
| Clinical assessment | 1 | aNICE clinical guidelines34 | 46 | bPSSRU 2011133, section 10.8b, p. 149 | 46 |
| Laboratory testsc | 1 | dNICE clinical guidelines34 | 3 | eDepartment of Health134 | 3 |
| Total medical care costs per patient for 6 months | 49 | ||||
Hospitalisation costs
The hospitalisation costs are one of the major cost drivers for HF; the estimated mean costs of the hospitalisations are presented in Table 35. The mean inpatient admission cost for HF-related hospitalisations was calculated from the weighted average of the costs for the Healthcare Resource Group (HRG) ‘Heart Failure or Shock’ (EB03H, EB03I) based on the data obtained from the NHS Reference Costs 2009–10. 134 For hospital admissions for any cause other than HF it was assumed that the cost was the same as the mean cost of hospital admission for the general population. This was estimated as a weighted average of elective inpatient admissions and non-elective inpatient admissions (including both short and long stay) based on data from the NHS Reference Costs 2009–10. 134
| Hospitalisation | Average cost (lower and upper quartile) (£) |
|---|---|
| HF-related hospitalisationsa | 2514.49 (1857.10 to 2809.95) |
| Other-cause hospitalisationsb | 1529.79 (1129.84 to 1709.55) |
Drug costs
Drug costs were not clearly reported for many of the trials and there was little description of the difference in drug use or drug costs between usual care and RM amongst studies identified in the systematic review; thus, it was assumed in the model that the drug costs were the same in the usual care and RM groups.
Summary of modelling input parameters
The Markov model assigned each patient a monthly probability of death, and in each monthly period the patients who are alive were under monthly risks of HF-related hospitalisations or other-cause hospitalisations. The risks of death and hospitalisation for RM interventions were estimated by applying the HRs from the meta-analysis to the baseline risks of mortality and hospitalisation. The effect of the intervention was assumed to last for a period of 6 months and after this it was assumed that patients reverted back to usual care. Each patient alive accumulated costs and QALYs every period based on their hospitalisation and treatment status. The model used a 30-year time horizon and the economic perspective of the model was the NHS in England and Wales. A summary of the model parameters is provided in Table 36.
| Parameter | Central estimate | Distribution | Source |
|---|---|---|---|
| Monthly mortality probability | |||
| Months 0–1 | 0.04622a | 0.005716b | Solomon et al. (CHARM)127 |
| Months > 1–3 | 0.03306a | 0.003719b | Solomon et al. (CHARM)127 |
| Months > 3–6 | 0.02674a | 0.002864b | Solomon et al. (CHARM)127 |
| Months > 6–12 | 0.02353a | 0.002178b | Solomon et al. (CHARM)127 |
| Months > 12–24 | 0.01866a | 0.001661b | Solomon et al. (CHARM)127 |
| Months > 24 | 0.01467a | 0.001970b | Solomon et al. (CHARM)127 |
| Number of monthly hospitalizations | |||
| HF-related | 0.0350a | 0.001256b | Klersy et al.123 |
| All-cause | 0.0875a | 0.001700b | Klersy et al.123 |
| HR for mortality | |||
| STS HM | 0.98c | Samples | NMA |
| STS HH | 0.77c | Samples | NMA |
| TM | 0.76c | Samples | NMA |
| HR for HF-related hospitalisations | |||
| STS HM | 1.03c | Samples | NMA |
| STS HH | 0.77c | Samples | NMA |
| TM | 0.95c | Samples | NMA |
| HR for all-cause hospitalisations | |||
| STS HM | 1.06c | Samples | NMA |
| STS HH | 0.97c | Samples | NMA |
| TM | 0.75c | Samples | NMA |
| HRQoL | |||
| Year 1 | 0.58a | 0.015b | Miller et al.131 |
| > Year 1 | 0.67a | 0.015b | Miller et al.131 |
| Disutility for HF-related hospitalisation | −0.1a | Triangular (−0.08, −0.1, 0.11) | Yao et al.132 |
| Cost (£) per 6 months | |||
| Usual care | 161d | 592e | Cleland et al. (TEN-HMS),49 clinical input |
| STS HM | 715d | 623–794f | Clinical input |
| STS HH | 1075d | 1051–1152f | Clinical input |
| TM | 1051d | 801–1288f | Clinical input |
| Cost (per month) (£) after 6 months | |||
| Usual care after 6 months | 8.23 | – | NICE clinical guidelines34 |
Methods to estimate cost-effectiveness
The cost-effectiveness of the different interventions was estimated using both the ICER and the net benefit approaches. Uncertainty was incorporated in the modelling by performing PSA. Descriptions of these terms and approaches are provided in the following sections.
Definitions of cost-effectiveness terms
The ICER measures the relative value of two strategies and is calculated as the mean incremental cost divided by the mean incremental benefits. A strategy is dominated when another strategy accrues more QALYs for less cost. Extended dominance occurs when a combination of two alternative strategies can produce the same QALYs as a chosen strategy but at a lower cost. Strategies that are neither dominated nor extendedly dominated constitute the cost-effectiveness frontier, and the ICER is reported for these strategies compared with the next least effective strategy.
The WTP threshold is the amount of money that the decision-maker is willing to pay to gain 1 additional QALY. The usual threshold for decision-making at NICE is considered to be around £20,000–30,000 per QALY.
The net monetary benefit (NMB) is defined as the QALYs multiplied by a value for the QALYs (e.g. £20,000) minus the costs of obtaining them, that is, NMB = QALYs × lambda – cost, where lambda is the WTP threshold. The NMB approach is simpler to calculate and gives equivalent findings (but requires an explicit assumption regarding the value of lambda).
Uncertainty analysis
The results presented in the following section include the effects of accounting for uncertainty in the model parameters (the costs, utilities, risks and HRs for mortality, HF-related hospitalisations and all-cause hospitalisations), characterised as probability distributions. PSA is undertaken whereby the model is rerun (10,000 times), each time with a different value for the risks, HRs, costs and utilities, which is sampled from the probability distributions.
The cost-effectiveness plane shows the incremental costs (y-axis) and incremental QALYs (x-axis) compared with usual care. In this chart, if a model run for a strategy had exactly the same costs and QALYs as usual care then the ‘sample’ for that model run would appear at the origin. Samples plotted to the right of the y-axis have more QALYs than usual care and samples plotted above the x-axis have more costs. Samples plotted to the right of a straight line with slope lambda passing through the origin are cost-effective whereas those plotted to the left are not. The cost-effectiveness acceptability curve (CEAC) shows the proportion of model runs for which each strategy is cost-effective over a range of potential WTP thresholds (i.e. lambda).
Another measure of uncertainty is the overall EVPI. This calculation is carried out based on the theory that the decision-maker will choose the most cost-effective option but could acquire additional evidence to reduce the uncertainties in the decision, for example know exactly what the HRs for mortality and hospitalisations are for each treatment. In the EVPI calculation it can be estimated how often making the decision based on current evidence could be wrong, and also how many QALYs (and costs) would be lost by choosing the strategy that is expected to be most cost-effective given current evidence when in fact one of the other strategies is truly the most cost-effective. One can estimate a monetary value lost by making a ‘wrong’ decision to choose a strategy based on current evidence by valuing the QALYs using the WTP threshold for this possible loss, that is, the net benefit lost on each of the occasions when another strategy would be optimal. This can be multiplied by the number of patients per year and the expected lifetime of the decision to estimate the population EVPI. The interpretation of this number is that if one could fund research to eliminate the uncertainty in effectiveness for all of the HRs for each strategy (e.g. by a large or infinitely large four-arm clinical trial) then the value of eliminating the uncertainty through such research would be expected to be the population EVPI.
Results of the independent economic assessment
This section details the results of the cost-effectiveness analyses estimated for a single HF patient as mean values of 10,000 PSA runs, each PSA run with a different estimate for the risks, HRs, costs and utilities sampled from the probability distributions reported in Table 36.
Results are presented using different effectiveness parameters (i.e. HRs) estimated from the NMA summarised in Tables 24 and 25. The cost-effectiveness analysis was performed using the base-case costs shown in Table 31 for these four estimates of effectiveness, that is, HRs based on CrIs and predictive distributions from the NMA, both including and excluding data from the Home-HF study. 67 Results are also presented for five cost scenarios: (1) higher usual care cost scenario, (2) lower cost scenario of TM during office hours, (3) higher cost scenario of TM during office hours, (4) lower STS cost scenario and (5) higher STS cost scenario. The cost-effectiveness for each of these scenarios was performed using the four estimates of effectiveness, that is, HRs based on CrIs and predictive distributions from the NMA, both including and excluding data from the Home-HF study. 67
This approach was taken to address the uncertainty in the cost and effectiveness evidence. For decision-makers deciding which of these sets of results is most representative of their setting, the key questions relate to the inclusion of the Home-HF study in the effectiveness meta-analyses. If one believes that usual care is best represented by the usual care arm in the Home-HF study,67 which is the only study showing a statistically significant difference in effectiveness between usual care over RM, then perhaps the results including the Home-HF study67 might be considered more relevant than those without. If, on the other hand, one believes that the performance of usual care is better represented by the other studies and that usual care in the Home-HF trial67 is not representative of current usual care, then the results excluding this trial might be considered more relevant. Similarly, the users of the results should decide which cost scenario best reflects their local practice.
In each case, the expected estimates of cost-effectiveness and the uncertainty around them are presented, along with the probability that each of the four strategies, STS HM, STS HH, TM during office hours and usual care, is the most cost-effective. The EVPI, a measure of how valuable it would be to eliminate all of the existing uncertainty, is also provided for each scenario.
Results of the primary analyses
Results for base-case costs
The results of the NMA with HRs based on CrIs and including the Home-HF study67 suggest that both TM during office hours and STS HH are similar in terms of mean HRs for mortality (0.779 for TM during office hours vs 0.780 for STS HH). STS HH is the most effective in terms of HF-related hospitalisation reduction with TM during office hours being the second most effective (mean HR 0.778 for STS HH vs 0.966 for TM during office hours). However, STS HH is the second most effective in terms of all-cause hospitalisation reduction after TM during office hours (mean HR 0.977 for STS HH vs 0.761 for TM during office hours) (Table 37). All mean HRs for STS HM are > 1, suggesting that STS HM is not effective in reducing mortality or hospitalisations. It should be noted that the mean HRs are calculated as an average of the 10,000 PSA HR samples provided by the NMA for input into the model.
| Usual care | STS HM | STS HH | TM during office hours | |
|---|---|---|---|---|
| Central estimates of HRs used as input | ||||
| Mortality HR | 1.000 | 1.074 | 0.780 | 0.779 |
| HF-related hospitalisation HR | 1.000 | 1.045 | 0.778 | 0.966 |
| All-cause hospitalisation HR | 1.000 | 1.173 | 0.977 | 0.761 |
| Treatment costs assumed per month (£) | 27 | 119 | 179 | 175 |
| Survival results (undiscounted) | ||||
| 1 year (%) | 72.14 | 71.45 | 75.34 | 75.38 |
| 5 years (%) | 33.75 | 33.43 | 35.25 | 35.27 |
| Life expectancy over 30 years (years) | 4.710 | 4.666 | 4.909 | 4.912 |
| Difference in life expectancy vs usual care (years) | – | −0.04 | 0.199 | 0.202 |
| Cost results (£) | ||||
| Discounted cost of usual care | 491 | 343 | 362 | 362 |
| Discounted cost of treatment | 0 | 632 | 978 | 957 |
| Discounted cost of HF-related hospitalisations | 4187 | 4169 | 4257 | 4348 |
| Discounted cost of other hospitalisations | 3800 | 3858 | 4007 | 3803 |
| Total costs | 8478 | 9001 | 9604 | 9470 |
| Difference in costs from usual care (£) | ||||
| Discounted cost of usual care | 0 | −148 | −129 | −129 |
| Discounted cost of treatment | 0 | 632 | 978 | 957 |
| Discounted cost of HF-related hospitalisations | 0 | −19 | 70 | 161 |
| Discounted cost of other hospitalisations | 0 | 57 | 207 | 3 |
| Total difference in costs | 0 | 523 | 1126 | 992 |
| Overall cost rank (1 = lowest cost) | 1 | 2 | 4 | 3 |
| Discounted QALY results | ||||
| QALYs with no hospitalisation decrement | 2.5748 | 2.5509 | 2.6834 | 2.6848 |
| HF-related hospitalisation decrement | −0.1611 | −0.1604 | −0.1638 | −0.1673 |
| Total discounted QALYs | 2.4137 | 2.3905 | 2.5196 | 2.5175 |
| Difference in QALYs from usual care | ||||
| QALYs with no hospitalisation decrement | 0.0000 | −0.0239 | 0.1086 | 0.1100 |
| HF-related hospitalisation decrement | 0.0000 | 0.0007 | −0.0027 | −0.0062 |
| Total difference in discounted QALYs | 0.0000 | −0.0232 | 0.1059 | 0.1038 |
| Total discounted QALYs rank (1 = highest) | 3 | 4 | 1 | 2 |
| Probabilistic ICER vs usual care (£/QALY) | Dominated | 10,629 | 9552 | |
| Probabilistic sequential ICER (£/QALY) | Dominated | 63,240a | 9552 | |
| Uncertainty analyses using net benefit at £20,000 per QALY | ||||
| Probability that strategy is most cost-effective (%) | 2 | 18 | 36 | 44 |
| Expected total costs from PSA (£) | 8478 | 9001 | 9604 | 9470 |
| Expected total QALYs from PSA | 2.4137 | 2.3905 | 2.5196 | 2.5175 |
| Expected net benefit from PSA (£) | 39,795 | 38,809 | 40,788 | 40,880 |
| Difference from usual care costs (£) | 0 | 523 | 1126 | 992 |
| Difference from usual care QALYs | 0.0000 | −0.0232 | 0.1059 | 0.1038 |
| Difference from usual care net benefit (£) | 0 | −986.75 | 993 | 1084 |
| Net benefit rank (1 = highest) | 3 | 4 | 2 | 1 |
| Overall EVPI per patient at WTP of 20,000 per QALY (£) | 826 | |||
| Population EVPI (£) | 45,247,202 | |||
Structured telephone support with human-to-human contact is the most costly strategy over a 6-month period (average monthly cost over treatment duration of first 6 months = £179 for STS HH compared with £175 for TM during office hours). Thus, it is necessary to estimate the incremental cost-effectiveness compared with the other interventions to answer the question, ‘Is the additional effect estimated for STS HH using the NMA worth the additional costs of the strategy?’
The survival results suggest that the lower HR for mortality would result in an estimated survival gain for TM during office hours over usual care of 0.202 years (mean undiscounted life expectancy = 4.912 years for TM vs 4.710 for usual care). This 0.202 life-years (just over 10 weeks, i.e. 73 days) mean additional survival for TM during office hours is slightly higher than the additional 0.199 LYG with STS HH, which has a mean life expectancy of 4.909 years. Similar survival benefits for STS HH and TM during office hours can be attributed to their similar mean HRs for mortality as seen in Table 37.
However, the QALY results show a reverse pattern to those for survival, with STS HH showing a higher QALY gain over usual care of 0.1059 compared with an additional 0.1038 QALYs gained with TM during office hours (equivalent to an additional 37.7 and 38.6 quality-adjusted days average gain for STS HH and TM respectively). This is because of fewer QALYs lost to HF hospitalisation by STS HH (−0.1638) than TM (−0.1673), in line with the higher effectiveness of STS HH in terms of HF-related hospitalisation reduction (HR of 0.778 for STS HH vs 0.966 for TM).
The expected costs over a lifetime (30-year time horizon) differ for each strategy, with STS HH having the highest costs at £9604 followed by TM during office hours (£9470), STS HM (£9001) and usual care (£8478). The main contribution to the overall costs comes from the hospitalisation costs, with the intervention and usual care costs being significantly lower.
Compared with usual care, STS HH has an additional discounted cost of approximately £1126; the majority of this cost difference was due to the difference in the costs of treatment in the treatment period, that is, the first 6 months (it was assumed that all of the patients revert to standard usual care after 6 months). There were also slightly higher hospitalisation costs, which were dependent on the number of people alive and the risks of hospitalisation for each intervention, that is, the HRs for mortality and hospitalisation respectively. For example, STS HH has an additional HF-related hospitalisation cost of around £70 per person over and above that of usual care and an additional £207 for other-cause hospitalisations. This is because STS HH patients live longer than usual care patients and, despite the lower risk of HF-related hospitalisation per month (HR 0.778) and the slightly lower risk of all-cause hospitalisation (HR 0.977), this leads to higher hospitalisation costs.
The additional discounted cost of £1126 for STS HH is higher than that of TM during office hours, which has an additional expected lifetime cost over and above that of usual care of £992 per person. This is because the higher HF-related hospitalisation costs of TM during office hours are offset by lower costs of other hospitalisations.
To assess whether or not the additional costs are worthwhile, the incremental cost per QALY gained is estimated. Comparing STS HH with usual care, the incremental cost per QALY gained is £1126/0.1059 = £10,629, which is below the typical NICE threshold of £20,000–30,000 per QALY gained. The ICER for TM during office hours compared with usual care is £992/0.1038 = £9552, which is also below the threshold of £20,000–30,000 per QALY gained.
However, when there are multiple possible strategies, one needs to calculate ICERs between different pairs, comparing each strategy with the next most effective strategy. Strategies that are dominated (or extendedly dominated) are removed from the cost-effectiveness frontier and the ICER is reported for these strategies compared with the next least effective strategy. Here, STS HM is dominated as usual care resulted in better health outcomes (2.4137 QALYs) and lower costs (£8478) than STS HM (2.3905 QALYs and £9001). As TM during office hours is the next most effective strategy compared with usual care, the ICER of TM during office hours against usual care is estimated at £9522 per QALY. The ICER of STS HH compared with TM during office hours is estimated as (£9604−£9470)/(2.5196−2.5175) = £136/0.0021 = £63,240 per QALY, which suggests that STS HH cannot be considered more cost-effective than TM during office hours at the typical NICE threshold of £20,000–30,000 per QALY gained. In this situation, TM during office hours is estimated to be the most cost-effective option with an ICER of £9522 per QALY.
Another way to present these results is to calculate the NMB of each strategy. The NMB of TM during office hours is (2.5175 × £20,000) – £9470 = £40,880. This approach takes away the need to calculate the ICER and simplifies the interpretation for decision-makers as the strategy with the highest expected incremental NMB is the most cost-effective. Using a threshold value of £20,000 per QALY, the estimated incremental NMB of TM during office hours compared with usual care is estimated to be £40,880 – £39,795 = £1084. Mathematically, as this difference is positive (i.e. > 0), the ICER must be < £20,000 (the ICER of TM during office hours compared with usual care is £9522 per QALY).
In terms of NMB then, if one accepts a cost-effectiveness threshold of £20,000 per QALY, the results show that TM during office hours is estimated to be the most cost-effective, with STS HH second and STS HM the least cost-effective.
In the cost-effectiveness plane shown in Figure 11, the samples to the right of the dotted line through the origin would have an incremental cost per QALY compared with usual care of < £20,000 and so would be considered cost-effective compared with usual care. Figure 11 shows that the majority of the TM during office hours and STS HH samples fall to the right of the dotted line, suggesting that they have a high chance of being cost-effective compared with usual care. The figure shows that the uncertainty in QALYs is larger for STS HM than it is for the other two strategies, because of the greater uncertainty in the HRs reported. There is less uncertainty in costs than QALYs in this base-case scenario. This is because in this scenario we have assumed that the monthly costs of the interventions are fixed at £119, £179 and £175 during the treatment period of 6 months for STS HM, STS HH and TM during office hours respectively. Thus, the uncertainty in costs shown in Figure 11 is actually a function of the uncertainty in the HRs for hospitalisations (more or fewer hospitalisations occurring, which are then multiplied by fixed unit costs) and mortality (more or less time alive, during which there is a risk of hospitalisation per month).
FIGURE 11.
Cost-effectiveness plane for the economic analysis using base-case costs with effectiveness data from the NMA including the Home-HF study. 67
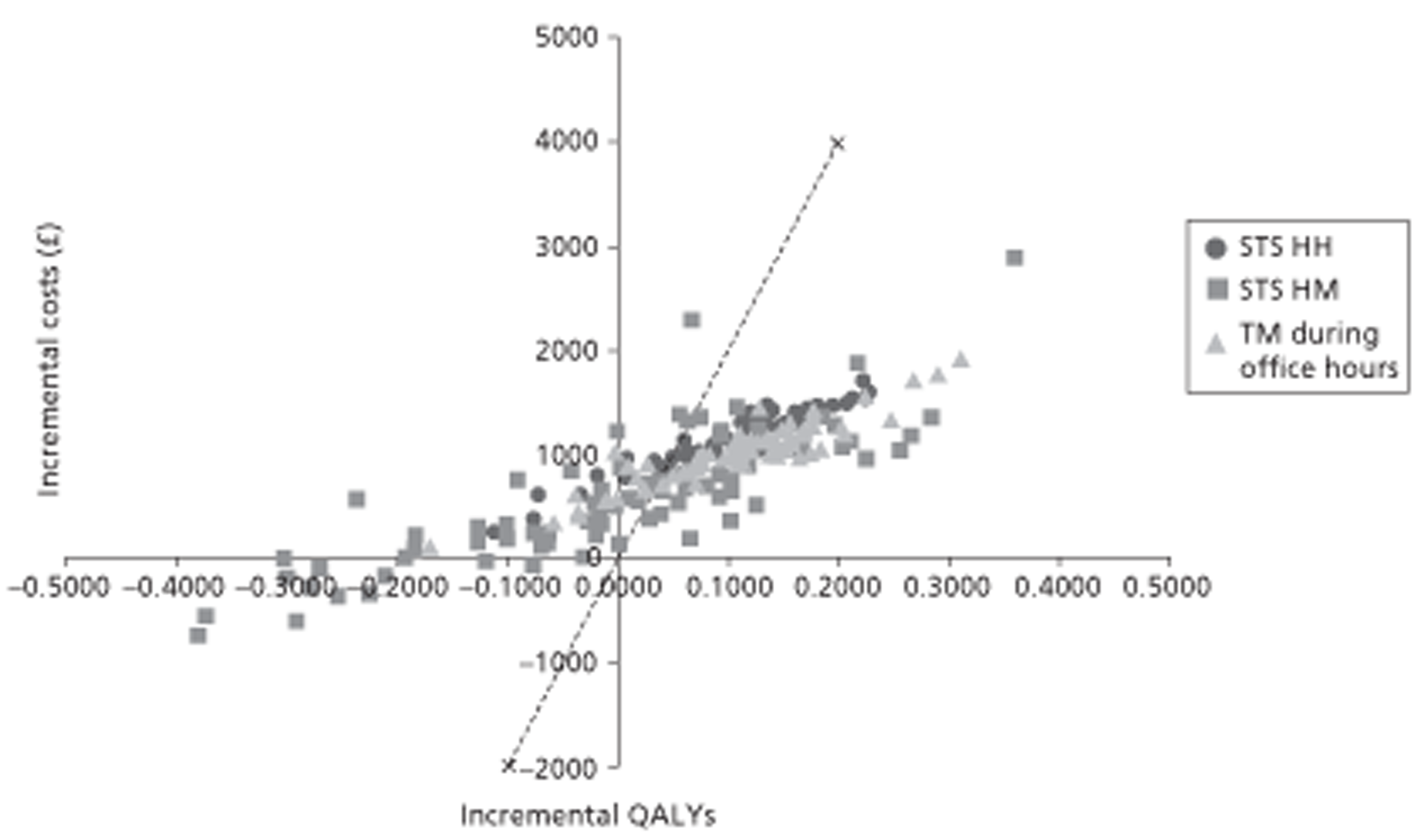
The model is rerun 10,000 times, each time with a different value for the HRs, costs and utilities sampled from the probability distribution, and in some of the sampled model runs TM during office hours could be more effective than STS HH because of the overlap in the probability distributions of their HRs. There is a chance that the HR for TM during office hours could be lower, that is, better, than that for STS HH. The CEAC in Figure 12 shows the proportion of model runs for which each strategy is cost-effective over a range of potential WTP thresholds. The percentage of model runs in which TM during office hours was the most cost-effective strategy (at a £20,000 per QALY threshold) was 44%, with the percentage of model runs in which STS HH, STS HM and usual care were the most cost-effective being 36%, 18% and 2% respectively.
FIGURE 12.
Cost-effectiveness acceptability curve for the economic analysis using base-case costs.
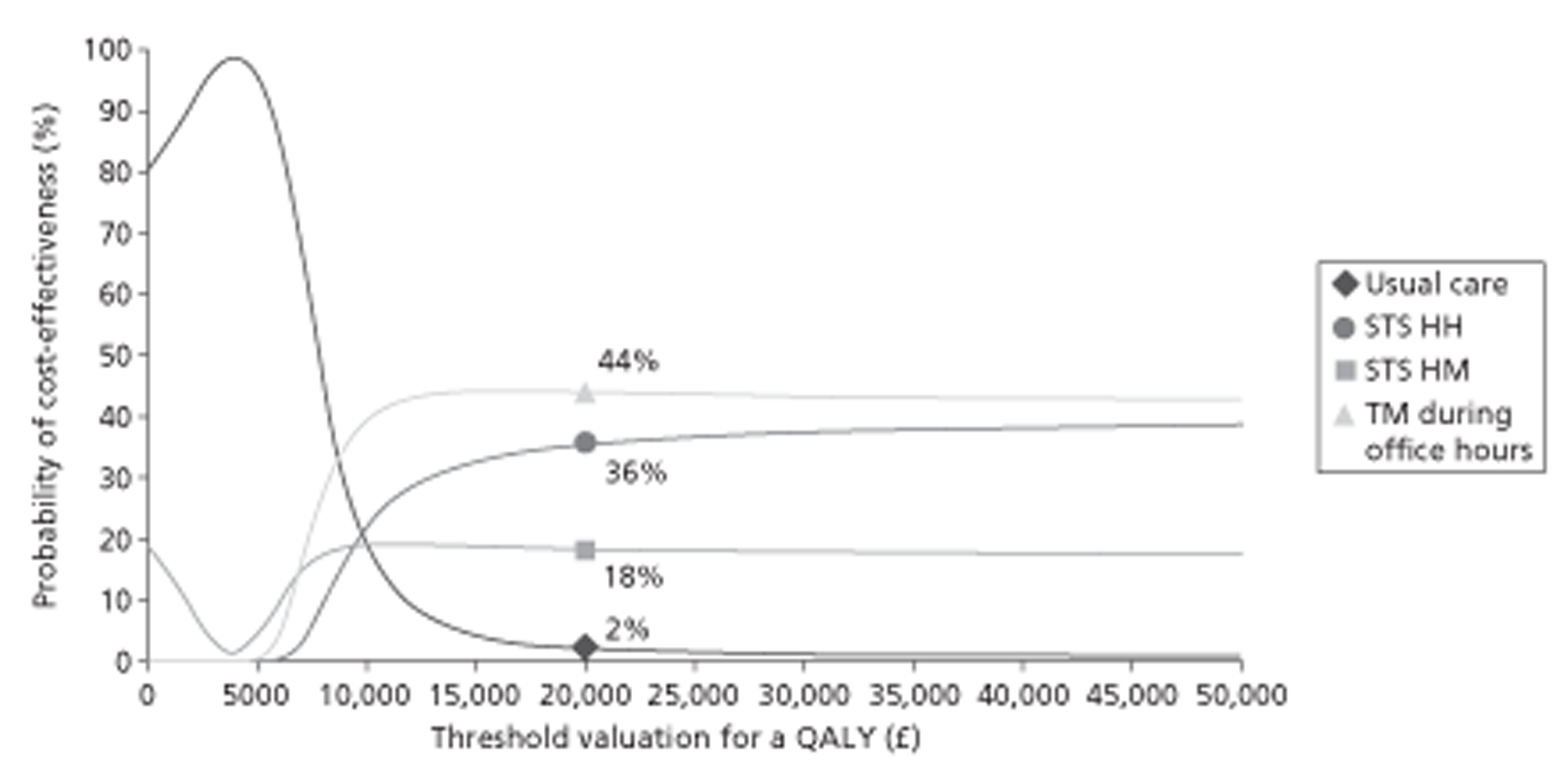
A CEAC in which the best strategy is cost-effective less than half of the time (44%) indicates that there is substantial uncertainty as to which strategy is the optimal in terms of net benefit. This uncertainty can also be measured as the overall EVPI, which is the average of the net benefits lost by making the decision now to choose TM during office hours. The EVPI in this case is £826 per patient for whom the decision is made, and the population EVPI per annum was estimated at £45,247,202 by multiplying the EVPI per patient with the annual incidence of first HF-related hospital admissions in England and Wales (i.e. £826 × 54,779).
Results for base-case costs using effectiveness data from predictive distributions
The same analyses were performed using HRs from predictive distributions of the NMA including the Home-HF study67 and the results are presented in Table 38. As explained in Chapter 3 (see Results of the clinical effectiveness review), the predictive distributions place more emphasis on heterogeneity between studies and provide wider estimates than CrIs. In this analysis, the most effective strategy in terms of the mortality HR is again TM during office hours (mean HR 0.843), with STS HH being the second best strategy (mean HR 0.849).
| Usual care | STS HM | STS HH | TM during office hours | |
|---|---|---|---|---|
| Central estimates of HRs used as input | ||||
| Mortality HR | 1.000 | 1.180 | 0.849 | 0.843 |
| HF-related hospitalisation HR | 1.000 | 1.063 | 0.790 | 0.982 |
| All-cause hospitalisation HR | 1.000 | 1.302 | 1.074 | 0.835 |
| Treatment costs assumed per month (£) | 27 | 119 | 179 | 175 |
| Survival results (undiscounted) | ||||
| 1 year (%) | 72.14 | 70.61 | 74.58 | 74.66 |
| 5 years (%) | 33.75 | 33.04 | 34.89 | 34.93 |
| Life expectancy over 30 years (years) | 4.710 | 4.614 | 4.862 | 4.867 |
| Difference in life expectancy vs usual care (years) | – | −0.10 | 0.151 | 0.157 |
| Cost results (£) | ||||
| Discounted cost of usual care | 491 | 339 | 358 | 359 |
| Discounted cost of treatment | 0 | 627 | 972 | 951 |
| Discounted cost of HF-related hospitalisations | 4187 | 4130 | 4221 | 4316 |
| Discounted cost of other hospitalisations | 3800 | 3869 | 4023 | 3811 |
| Total costs | 8478 | 8965 | 9574 | 9437 |
| Difference in costs from usual care (£) | ||||
| Discounted cost of usual care | 0 | −152 | −132 | −132 |
| Discounted cost of treatment | 0 | 627 | 972 | 951 |
| Discounted cost of HF-related hospitalisations | 0 | −57 | 33 | 129 |
| Discounted cost of other hospitalisations | 0 | 69 | 223 | 11 |
| Total difference in costs | 0 | 487 | 1096 | 959 |
| Overall cost rank (1 = lowest cost) | 1 | 2 | 4 | 3 |
| Discounted QALY results | ||||
| QALYs with no hospitalisation decrement | 2.5748 | 2.5223 | 2.6575 | 2.6605 |
| HF-related hospitalisation decrement | −0.1611 | −0.1589 | −0.1624 | −0.1661 |
| Total discounted QALYs | 2.4137 | 2.3633 | 2.4950 | 2.4944 |
| Difference in QALYs from usual care | ||||
| QALYs with no hospitalisation decrement | 0.0000 | −0.0525 | 0.0827 | 0.0857 |
| HF-related hospitalisation decrement | 0.0000 | 0.0022 | −0.0013 | −0.0050 |
| Total difference in discounted QALYs | 0.0000 | −0.0504 | 0.0814 | 0.0808 |
| Total discounted QALYs rank (1 = highest) | 3 | 4 | 1 | 2 |
| Probabilistic ICER vs usual care (£/QALY) | Dominated | 13,473 | 11,873 | |
| Probabilistic sequential ICER (£/QALY) | Dominated | 228,035a | 11,873 | |
| Uncertainty analyses using net benefit at £20,000 per QALY | ||||
| Probability that strategy is most cost-effective (%) | 6 | 19 | 35 | 40 |
| Expected total costs from PSA (£) | 8478 | 8965 | 9574 | 9437 |
| Expected total QALYs from PSA | 2.4137 | 2.3633 | 2.4950 | 2.4944 |
| Expected net benefit from PSA (£) | 39,795 | 38,301 | 40,327 | 40,452 |
| Difference from usual care costs (£) | 0 | 487 | 1096 | 959 |
| Difference from usual care QALYs | 0.0000 | −0.0504 | 0.0814 | 0.0808 |
| Difference from usual care net benefit (£) | 0 | −1494.07 | 531 | 656 |
| Net benefit rank (1 = highest) | 3 | 4 | 2 | 1 |
| Overall EVPI per patient at ICER of £20,000 per QALY (£) | 1831 | |||
| Population EVPI (£) | 100,299,791 | |||
In terms of NMB, TM during office hours has the highest followed by STS HH, with mean incremental NMBs of £656 and £534, respectively, compared with usual care, suggesting that the ICERs for TM during office hours and STS HH compared with usual care are < £20,000 per QALY. In terms of incremental analysis, again TM during office hours is the most cost-effective option assuming a NICE cost-effectiveness threshold of £20,000 per QALY gained, with an ICER for TM during office hours compared with usual care of £11,873 per QALY gained, whereas STS HH has an ICER of £228,035 per QALY gained compared with TM during office hours.
The cost-effectiveness results estimated here were similar to the results with effectiveness data from the CrIs of the NMA including the Home-HF study. 67 However, the ICER for TM during office hours compared with usual care using HRs from the predictive distributions is £11,873, which is slightly higher than the ICER of £9522 estimated using HRs from the CrIs. This is due to the higher estimates of HRs in the predictive distributions (mean mortality HR 0.843) than the HRs based on CrIs (mean mortality RR 0.779).
Furthermore, the results using HRs from the predictive distributions are also more uncertain, as seen in the wider distribution of the samples in the cost-effectiveness plane shown in Figure 13 than in the cost-effectiveness plane shown in Figure 11, estimated using HRs based on CrIs. This is because the HRs estimated from the predictive distributions of the NMA have more uncertainty than those estimated from HRs based on CrIs.
FIGURE 13.
Cost-effectiveness plane for the economic analysis using base-case costs with effectiveness data from predictive distributions.
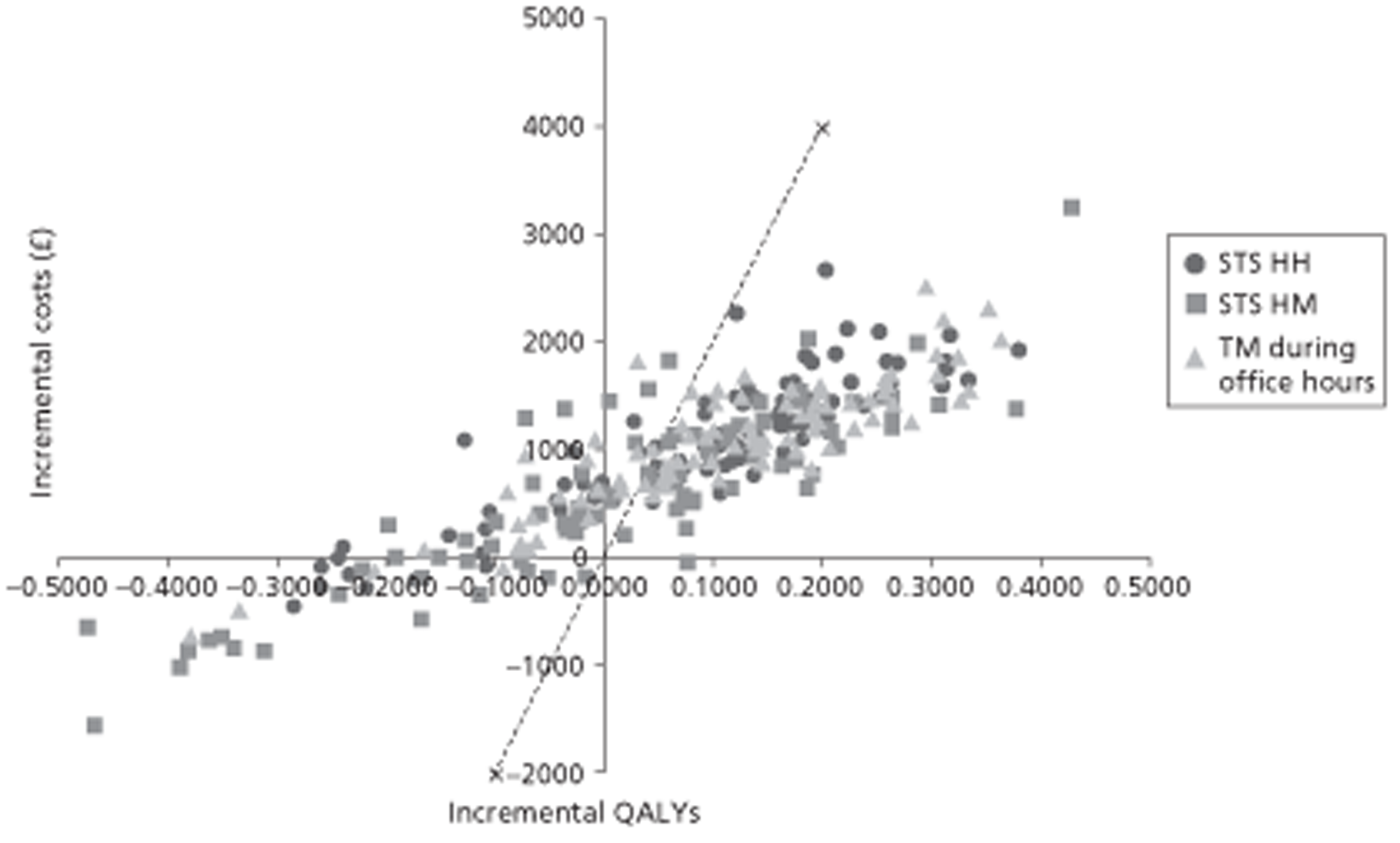
The proportion of model runs in which TM during office hours was the most cost-effective strategy (at a £20,000 per QALY threshold) was 40%, with the proportion of model runs in which STS HH, STS HM and usual care were the most cost-effective being 35%, 19% and 6% respectively (Figure 14). As described earlier, the results are slightly more uncertain when HRs from predictive distributions are used [the proportion of model runs in which TM during office hours was the most cost-effective strategy (at a £20,000 per QALY threshold) when HR estimates from CrIs were used was 44%]. This uncertainty is also reflected in the higher EVPI of £1831 per patient when HR estimates from predictive distributions were used compared with £826 when HR estimates from CrIs were used (as reported in Results for base-case costs). The population EVPI per annum was £100M compared with £45M when HR estimates from CrIs were used.
FIGURE 14.
Cost-effectiveness acceptability curve for the economic analysis using base-case costs with effectiveness data from predictive distributions.
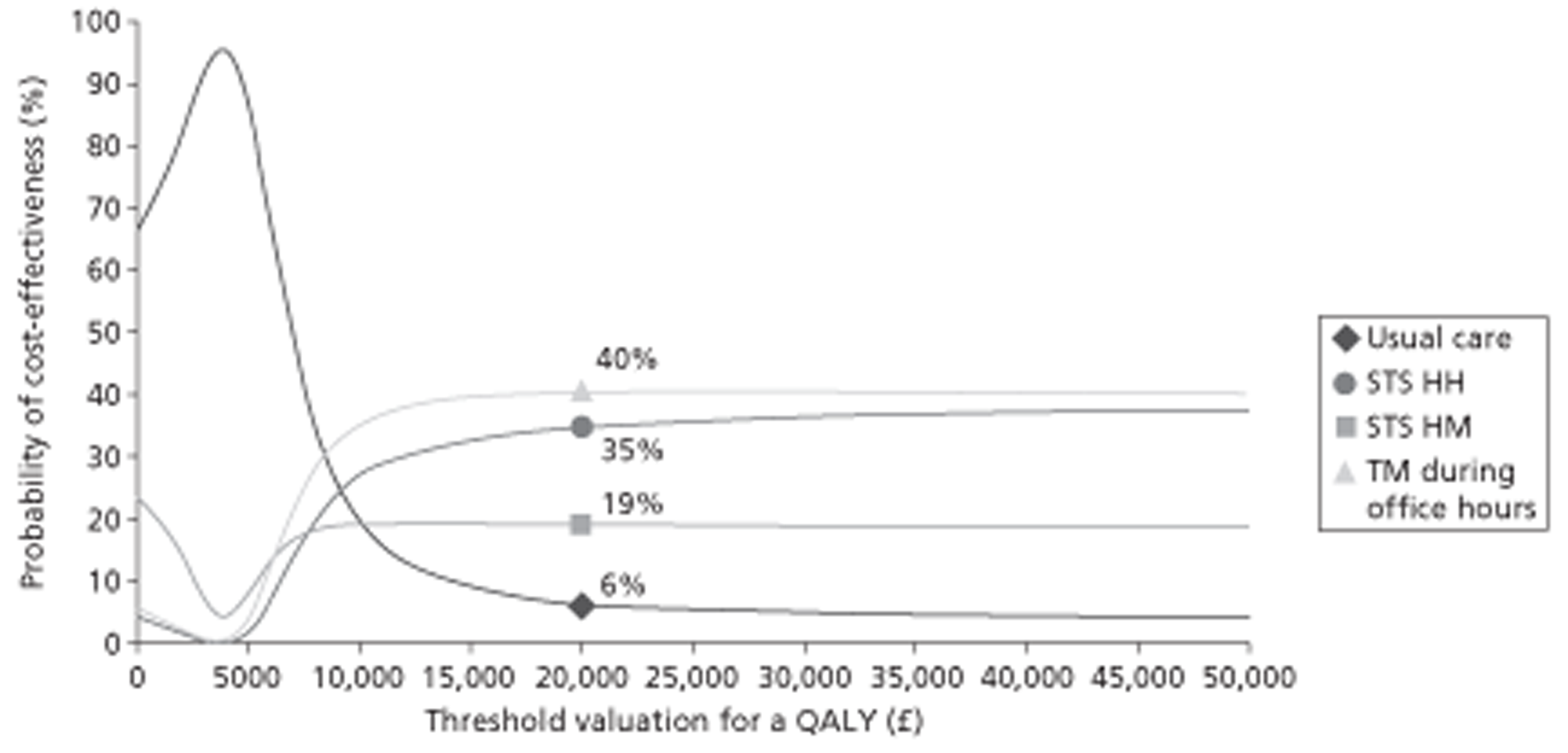
Results for base-case costs using effectiveness data excluding the Home-HF study67
The results of the NMA excluding the Home-HF study67 suggest that TM during office hours is substantially more effective in terms of mortality risk reduction (HR 0.627) than the second most effective strategy STS HH (HR 0.757), with STS HM slightly worse than usual care (HR 1.007). Cost-effectiveness analysis was performed with these HR estimates and the results are presented in Table 39.
| Usual care | STS HM | STS HH | TM during office hours | |
|---|---|---|---|---|
| Central estimates of HRs used as input | ||||
| Mortality HR | 1.000 | 1.007 | 0.757 | 0.627 |
| HF-related hospitalisation HR | 1.000 | 1.042 | 0.766 | 0.872 |
| All-cause hospitalisation HR | 1.000 | 1.134 | 0.969 | 0.678 |
| Treatment costs assumed per month (£) | 27 | 119 | 179 | 175 |
| Survival results (undiscounted) | ||||
| 1 year (%) | 72.14 | 72.14 | 75.67 | 77.63 |
| 5 years (%) | 33.75 | 33.75 | 35.40 | 36.32 |
| Life expectancy over 30 years (years) | 4.710 | 4.7103 | 4.930 | 5.052 |
| Difference in life expectancy vs usual care (years) | – | 0.0003 | 0.220 | 0.342 |
| Cost results (£) | ||||
| Discounted cost of usual care | 491 | 346 | 363 | 373 |
| Discounted cost of treatment | 0 | 635 | 981 | 972 |
| Discounted cost of HF-related hospitalisations | 4187 | 4206 | 4269 | 4426 |
| Discounted cost of other hospitalisations | 3800 | 3873 | 4021 | 3879 |
| Total costs | 8478 | 9060 | 9635 | 9650 |
| Difference in costs from usual care (£) | ||||
| Discounted cost of usual care | 0 | −144 | −127 | −118 |
| Discounted cost of treatment | 0 | 635 | 981 | 972 |
| Discounted cost of HF-related hospitalisations | 0 | 18 | 82 | 239 |
| Discounted cost of other hospitalisations | 0 | 73 | 221 | 79 |
| Total difference in costs | 0 | 582 | 1157 | 1172 |
| Overall cost rank (1 = lowest cost) | 1 | 2 | 3 | 4 |
| Discounted QALY results | ||||
| QALYs with no hospitalisation decrement | 2.5748 | 2.5747 | 2.6949 | 2.7612 |
| HF-related hospitalisation decrement | −0.1611 | −0.1619 | −0.1643 | −0.1703 |
| Total discounted QALYs | 2.4137 | 2.4128 | 2.5306 | 2.5908 |
| Difference in QALYs from usual care | ||||
| QALYs with no hospitalisation decrement | 0.0000 | −0.0002 | 0.1200 | 0.1864 |
| HF-related hospitalisation decrement | 0.0000 | −0.0007 | −0.0032 | −0.0092 |
| Total difference in discounted QALYs | 0.0000 | −0.0009 | 0.1169 | 0.1772 |
| Total discounted QALYs rank (1 = highest) | 3 | 4 | 2 | 1 |
| Probabilistic ICER vs usual care (£/QALY) | Dominated | 9897 | 6616 | |
| Probabilistic sequential ICER (£/QALY) | Dominated | Extendedly dominated | 6616a | |
| Uncertainty analyses using net benefit at £20,000 per QALY | ||||
| Probability that strategy is most cost-effective (%) | 0 | 5 | 12 | 83 |
| Expected total costs from PSA (£) | 8478 | 9060 | 9635 | 9650 |
| Expected total QALYs from PSA | 2.4137 | 2.4128 | 2.5306 | 2.5908 |
| Expected net benefit from PSA | 39,795 | 39,196 | 40,976 | 42,167 |
| Difference from usual care costs (£) | 0 | 582 | 1157 | 1172 |
| Difference from usual care QALYs | 0.0000 | −0.0009 | 0.1169 | 0.1772 |
| Difference from usual care net benefit (£) | 0 | −599.54 | 1181 | 2371 |
| Net benefit rank (1 = highest) | 3 | 4 | 2 | 1 |
| Overall EVPI per patient at ICER of £20,000 per QALY (£) | 133 | |||
| Population EVPI (£) | 7,285,566 | |||
Telemonitoring during office hours was estimated to be the most cost-effective option with an ICER of £6616 per QALY gained, with STS HM and STS HH being dominated and extendedly dominated respectively. In terms of the incremental NMB compared with usual care, again TM during office hours was the best strategy with an incremental NMB of £2371; STS HH was the second best strategy with an incremental NMB of £1181. These estimates for TM during office hours show improved cost-effectiveness (i.e. lower ICER and higher incremental NMB) than the estimates when the Home-HF study67 was included in the NMA (ICER of £9522 per QALY and NMB of £1084). This is because the heterogeneity in intervention effects between studies was considerably reduced when the Home-HF study67 was excluded, resulting in better effectiveness in terms of mortality risk reduction than when the Home-HF study67 was included (HR 0.627 vs HR 0.779).
This can also be observed in the cost-effectiveness plane, with the samples based on estimates of effectiveness when the Home-HF study67 was excluded from the NMA shifting to the right in the cost-effectiveness plane, as shown in Figure 15, compared with the samples in the cost-effectiveness plane shown in Figure 11, which is based on the estimates of effectiveness when the Home-HF study67 was included in the NMA.
FIGURE 15.
Cost-effectiveness plane for the economic analysis using base-case costs with effectiveness data excluding the Home-HF study. 67
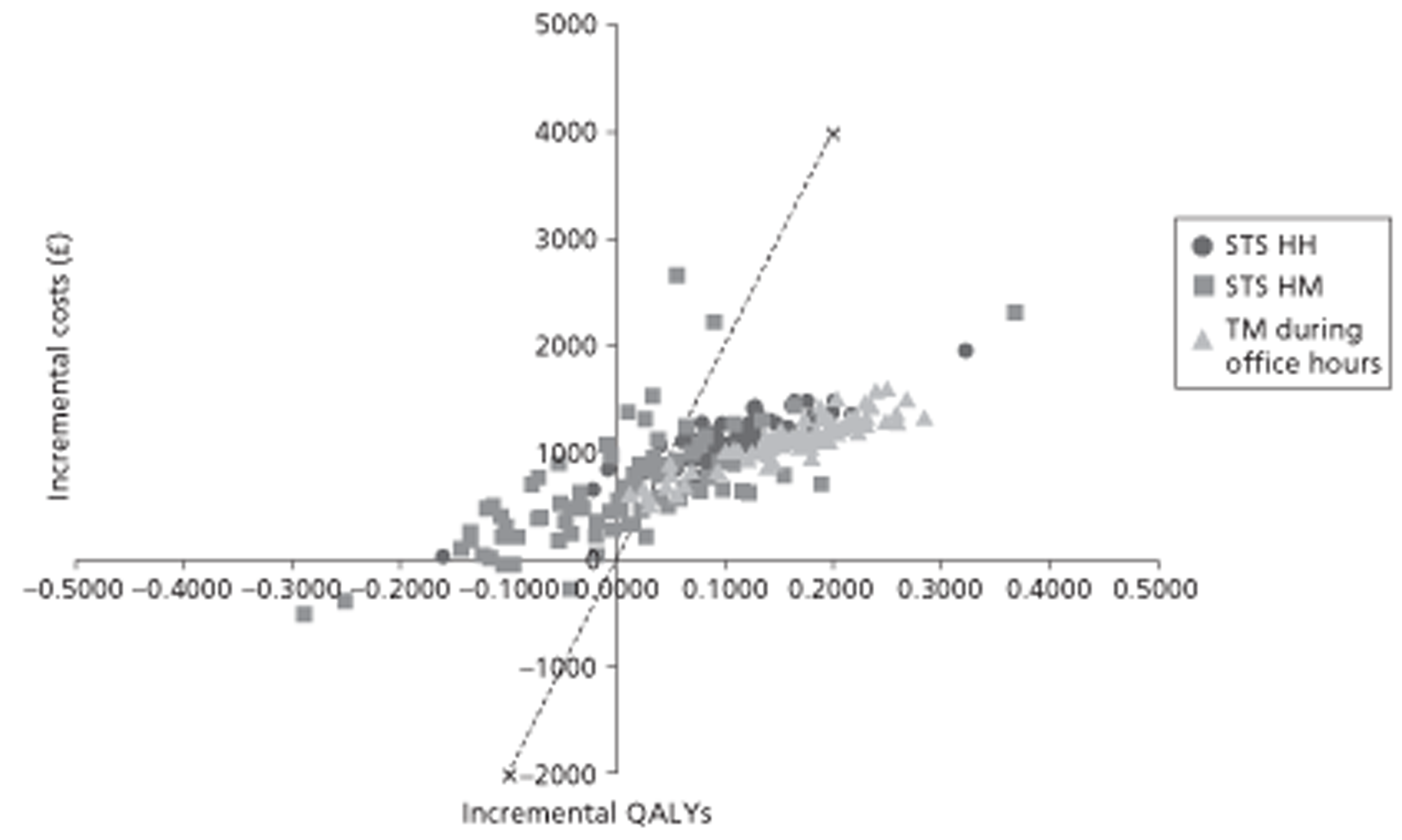
The proportion of model runs in which TM during office hours was the most cost-effective strategy (at a £20,000 per QALY threshold) was 83%, with STS HH at 12%, STS HM at 5% and usual care at 0% (Figure 16). This proportion of model runs (83%) in which TM was the most cost-effective strategy at £20,000 per QALY is much higher than the proportion of model runs in which TM was the most cost-effective strategy (44%) when the Home-HF study67 was included in the NMA. This reduction in uncertainty is also reflected in the lower EVPI of £133 per patient when the Home-HF study67 was excluded from the NMA compared with £826 when the Home-HF study67 was included (as reported in Results for base-case costs).
FIGURE 16.
Cost-effectiveness acceptability curve for the economic analysis using base-case costs with effectiveness data excluding the Home-HF study. 67
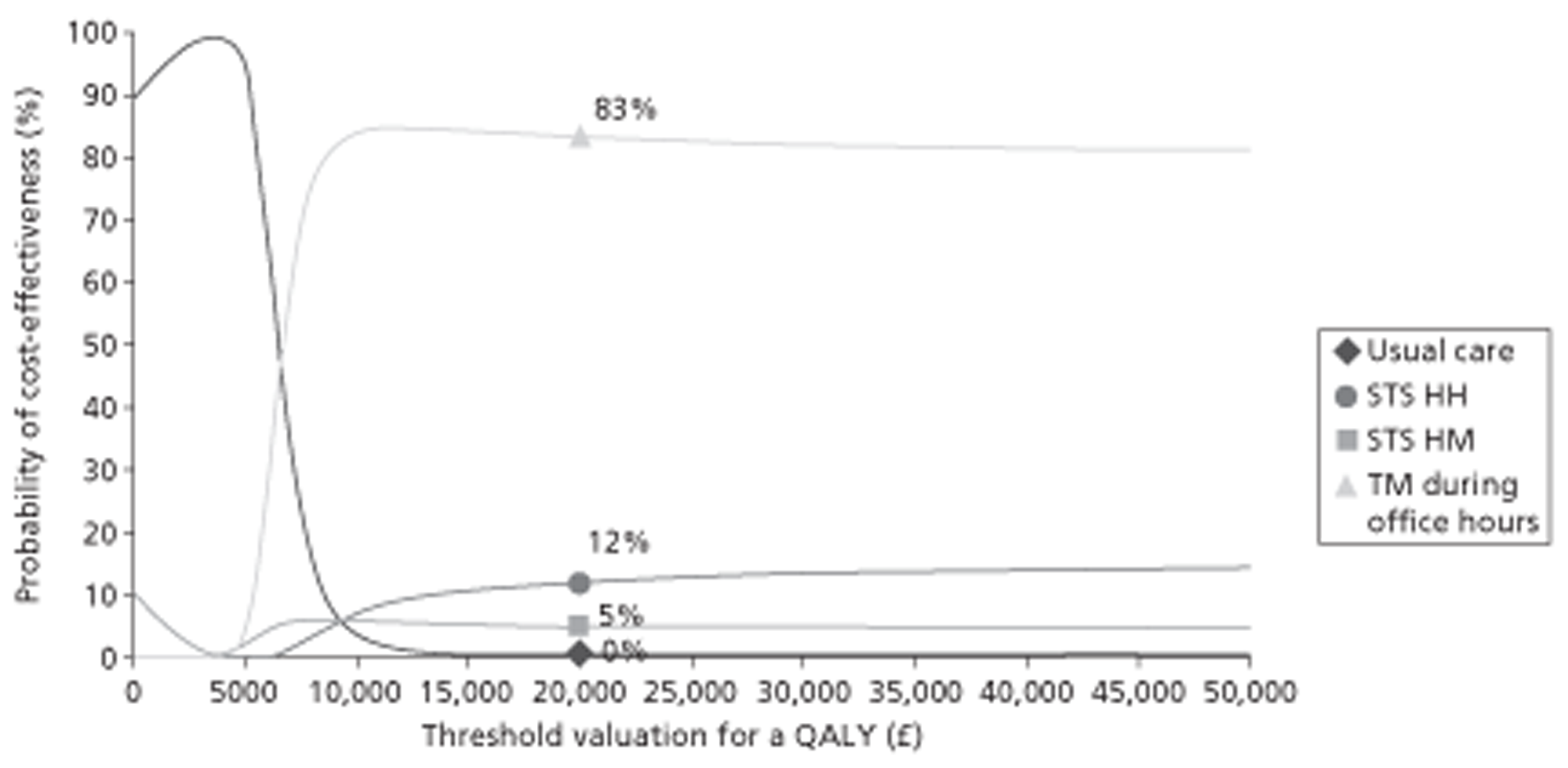
Therefore, excluding the Home-HF study67 from the NMA suggests that TM during office hours is the most cost-effective strategy and that there is less uncertainty involved in suggesting that this is the most cost-effective strategy than when the Home-HF study67 is included in the NMA. Users can decide which of these analyses is most representative of the UK setting, that is, whether or not the usual care in the Home-HF study67 is representative of usual care in the UK and whether or not this study should be included in the meta-analysis.
Results for base-case costs with effectiveness data from predictive distributions excluding the Home-HF study67
The results of the cost-effectiveness analysis using HRs from predictive distributions of the NMA excluding the data from the Home-HF study67 suggest that the most effective strategy in terms of mortality reduction is TM during office hours (mean mortality HR 0.642), with STS HH the second most effective strategy (mean mortality HR 0.776) and STS HM worse than usual care (mean mortality HR 1.032). The cost-effectiveness analysis was performed with these HRs and the results are presented in Table 40.
| Usual care | STS HM | STS HH | TM during office hours | |
|---|---|---|---|---|
| Central estimates of HRs used as input | ||||
| Mortality HR | 1.000 | 1.032 | 0.776 | 0.642 |
| HF-related hospitalisation HR | 1.000 | 1.058 | 0.778 | 0.883 |
| All-cause hospitalisation HR | 1.000 | 1.235 | 1.048 | 0.731 |
| Treatment costs assumed per month (£) | 27 | 119 | 179 | 175 |
| Survival results (undiscounted) | ||||
| 1 year (%) | 72.14 | 71.89 | 75.44 | 77.44 |
| 5 years (%) | 33.75 | 33.63 | 35.30 | 36.23 |
| Life expectancy over 30 years (years) | 4.71 | 4.69 | 4.92 | 5.04 |
| Difference in life expectancy vs usual care (years) | 0.00 | −0.02 | 0.21 | 0.33 |
| Cost results (£) | ||||
| Discounted cost of usual care | 491 | 345 | 362 | 372 |
| Discounted cost of treatment | 0 | 634 | 979 | 971 |
| Discounted cost of HF-related hospitalisations | 4187 | 4200 | 4262 | 4422 |
| Discounted cost of other hospitalisations | 3800 | 3909 | 4055 | 3901 |
| Total costs | 8478 | 9087 | 9658 | 9665 |
| Difference in costs from usual care (£) | ||||
| Discounted cost of usual care | 0 | −145 | −128 | −119 |
| Discounted cost of treatment | 0 | 634 | 979 | 971 |
| Discounted cost of HF-related hospitalisations | 0 | 12 | 75 | 234 |
| Discounted cost of other hospitalisations | 0 | 108 | 255 | 101 |
| Total difference in costs | 0 | 609 | 1180 | 1187 |
| Overall cost rank (1 = lowest cost) | 1 | 2 | 3 | 4 |
| Discounted QALY results | ||||
| QALYs with no hospitalisation decrement | 2.5748 | 2.5659 | 2.6870 | 2.7548 |
| HF-related hospitalisation decrement | −0.1611 | −0.1616 | −0.1640 | −0.1701 |
| Total discounted QALYs | 2.4137 | 2.4043 | 2.5230 | 2.5847 |
| Difference in QALYs from usual care | ||||
| QALYs with no hospitalisation decrement | 0.0000 | −0.0089 | 0.1122 | 0.1800 |
| HF-related hospitalisation decrement | 0.0000 | −0.0004 | −0.0029 | −0.0090 |
| Total difference in discounted QALYs | 0.0000 | −0.0093 | 0.1093 | 0.1710 |
| Total discounted QALYs rank (1 = highest) | 3 | 4 | 2 | 1 |
| Probabilistic ICER vs usual care (£/QALY) | Dominated | 10,798 | 6942 | |
| Probabilistic sequential ICER (£/QALY) | Dominated | Extendedly dominated | 6942a | |
| Uncertainty analyses using net benefit at £20,000 per QALY | ||||
| Probability that strategy is most cost-effective (%) | 1 | 7 | 19 | 73 |
| Expected total costs from PSA (£) | 8478 | 9087 | 9658 | 9665 |
| Expected total QALYs from PSA | 2.4137 | 2.4043 | 2.5230 | 2.5847 |
| Expected net benefit from PSA (£) | 39,795 | 39,000 | 40,801 | 42,029 |
| Difference from usual care costs (£) | 0 | 609 | 1180 | 1187 |
| Difference from usual care QALYs | 0.0000 | −0.0093 | 0.1093 | 0.1710 |
| Difference from usual care net benefit (£) | 0 | −795.73 | 1006 | 2233 |
| Net benefit rank (1 = highest) | 3 | 4 | 2 | 1 |
| Overall EVPI per patient at ICER of £20,000 per QALY (£) | 410 | |||
| Population EVPI (£) | 22,459,265 | |||
In terms of incremental analysis, STS HM and STS HH are dominated and extendedly dominated respectively, with TM during office hours being the most cost-effective option with an ICER of £6942 per QALY. In terms of NMB, TM during office hours is again the most cost-effective strategy with a NMB of £2233; STS HH is the second most cost-effective strategy with a NMB of £1006.
The cost-effectiveness results estimated here were similar to the results using effectiveness data based on CrIs of the NMA excluding the Home-HF study. 67 However, the central estimates are slightly higher with an ICER of £6942 for TM during office hours compared with usual care using HRs from predictive distributions compared with an ICER of £6616 using HRs based on CrIs. This is due to the higher estimates of the HRs in the predictive distributions (mean mortality HR 0.642) than the HRs based on CrIs (mean mortality RR 0.627).
Furthermore, the results using predictive distributions are also more uncertain, as seen in the wider distribution of the samples in the cost-effectiveness plane shown in Figure 17 compared with the cost-effectiveness plane shown in Figure 15, estimated using HRs from CrIs. This is because the HRs for the predictive distribution of a new study are more uncertain than the HRs for the population mean of the studies.
FIGURE 17.
Cost-effectiveness plane using base-case costs with effectiveness data from predictive distributions excluding the Home-HF study. 67
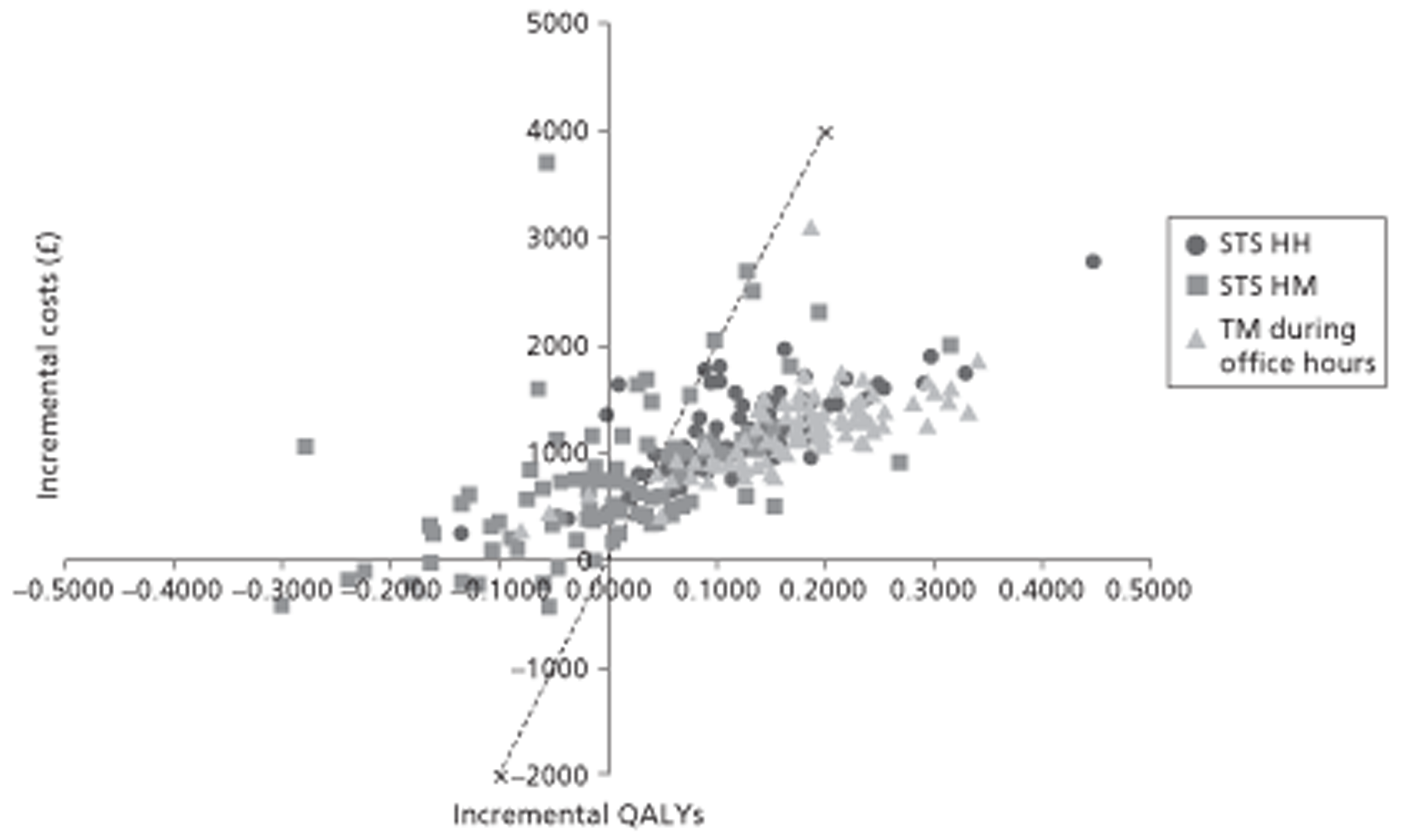
The percentage of model runs in which TM during office hours was the most cost-effective strategy (at a £20,000 per QALY threshold) was 73%, with the percentage of model runs in which STS HH, STS HM and usual care were the most cost-effective being 19%, 7% and 1% respectively (Figure 18). The lower percentage of model runs in which TM during office hours was the most cost-effective strategy when estimates from the predictive NMA were used compared with when estimates from CrIs were used (83%) reflects the higher uncertainty in the HRs estimated from the predictive NMA than in those estimated based on CrIs.
FIGURE 18.
Cost-effectiveness acceptability curve for the economic analysis using base-case costs with effectiveness data from predictive distributions excluding the Home-HF study. 67
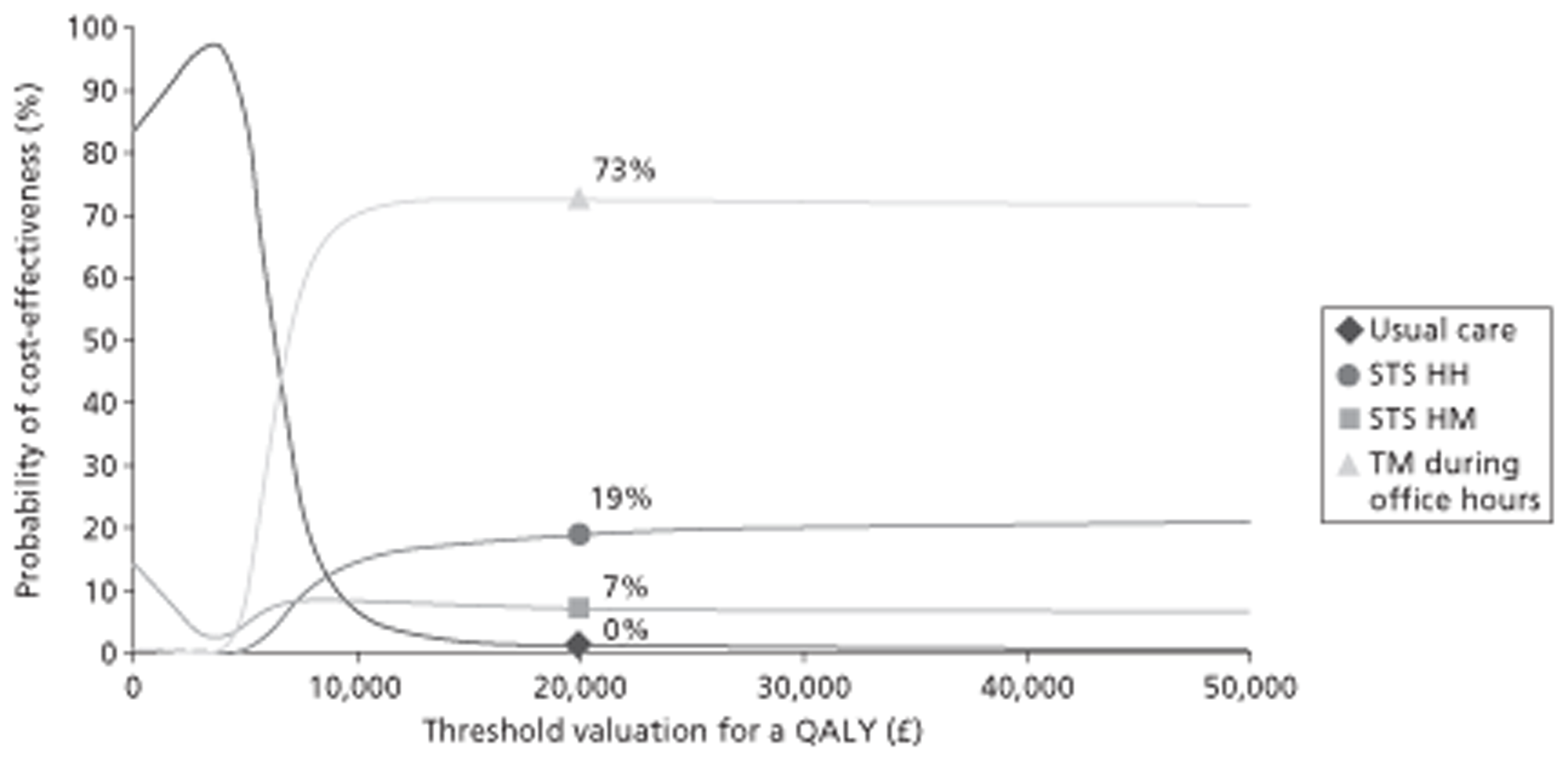
Summary of the results for base-case costs
Table 41 provides the summary of the cost-effectiveness results using the base-case costs. TM during office hours appears to be the most cost-effective strategy at a threshold of £20,000 per QALY in all four analyses, that is, HRs based on CrIs and predictive distributions of the NMA, including and excluding the Home-HF study. 67 TM during office hours is also the most effective strategy (i.e. highest QALYs gained) in the analyses that excluded the Home-HF study. 67 TM during office hours is not the most effective strategy in the analyses that included the Home-HF study,67 with STS HH providing the highest number of expected QALYs. However, the additional QALYs gained by STS HH are not worth the additional costs of the strategy as seen in the ICERs (compared with TM during office hours), which are greater than the threshold of £20,000 per QALY.
| Usual care | STS HM | STS HH | TM during office hours | |
|---|---|---|---|---|
| Total costs (£) | ||||
| CrI | 8478 | 9001 | 9604 | 9470 |
| PrI | 8478 | 8965 | 9574 | 9437 |
| CrI excluding Home-HF67 | 8478 | 9060 | 9635 | 9650 |
| PrI excluding Home-HF67 | 8478 | 9087 | 9658 | 9665 |
| Difference in costs (£) | ||||
| CrI | – | 523 | 1126 | 992 |
| PrI | – | 487 | 1096 | 959 |
| CrI excluding Home-HF67 | – | 582 | 1157 | 1172 |
| PrI excluding Home-HF67 | – | 609 | 1180 | 1187 |
| Total QALYs | ||||
| CrI | 2.4137 | 2.3905 | 2.5196 | 2.5175 |
| PrI | 2.4137 | 2.3633 | 2.4950 | 2.4944 |
| CrI excluding Home-HF67 | 2.4137 | 2.4128 | 2.5306 | 2.5908 |
| PrI excluding Home-HF67 | 2.4137 | 2.4043 | 2.5230 | 2.5847 |
| Difference in QALYs | ||||
| CrI | – | −0.0232 | 0.1059 | 0.1038 |
| PrI | – | −0.0504 | 0.0814 | 0.0808 |
| CrI excluding Home-HF67 | – | −0.0009 | 0.1169 | 0.1772 |
| PrI excluding Home-HF67 | – | −0.0093 | 0.1093 | 0.1710 |
| ICER (£/QALY) | ||||
| CrI | – | Dominated | 63,240a | 9552 |
| PrI | – | Dominated | 228,035a | 11,873 |
| CrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 6616a |
| PrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 6942a |
| Expected incremental NMB (£) | ||||
| CrI | – | −986.75 | 993 | 1084 |
| PrI | – | −1494.07 | 531 | 656 |
| CrI excluding Home-HF67 | – | −599.54 | 1181 | 2371 |
| PrI excluding Home-HF67 | – | −795.73 | 1006 | 2233 |
| Probability of cost-effectiveness (%) | ||||
| CrI | 2 | 18 | 36 | 44 |
| PrI | 6 | 19 | 35 | 40 |
| CrI excluding Home-HF67 | 0 | 5 | 12 | 83 |
| PrI excluding Home-HF67 | 1 | 7 | 19 | 73 |
In the analyses that included the Home-HF study,67 the cost-effectiveness of TM during office hours has high uncertainty as there is a 44% and 40% chance of TM during office hours being cost-effective for analyses using HR estimates from CrIs and predictive distributions respectively. However, this uncertainty is lower in the analyses using HRs from the NMA that excluded the Home-HF study,67 with TM during office hours having an 83% and 73% chance of being cost-effective for analyses using estimates from CrIs and predictive distributions respectively.
Scenario analyses
Results for the high usual care cost scenario
A high cost usual care scenario in which the cost of usual care was £98.70 per patient per month during the treatment period (compared with the base-case usual care cost of £27), as described in Usual care costs, was incorporated to address the heterogeneity and uncertainty in the usual care cost data. All of the other parameters in this analysis were the same and the results summarised in Table 42 are also presented in detail in Appendix 12.
| Usual care | STS HM | STS HH | TM during office hours | |
|---|---|---|---|---|
| Total costs (£) | ||||
| CrI | 8861 | 9001 | 9604 | 9470 |
| PrI | 8861 | 8965 | 9574 | 9437 |
| CrI excluding Home-HF67 | 8861 | 9060 | 9635 | 9650 |
| PrI excluding Home-HF67 | 8861 | 9087 | 9658 | 9665 |
| Difference in costs (£) | ||||
| CrI | – | 140 | 743 | 609 |
| PrI | – | 104 | 713 | 576 |
| CrI excluding Home-HF67 | – | 199 | 774 | 789 |
| PrI excluding Home-HF67 | – | 226 | 797 | 804 |
| Total QALYs | ||||
| CrI | 2.4137 | 2.3905 | 2.5196 | 2.5175 |
| PrI | 2.4137 | 2.3633 | 2.4950 | 2.4944 |
| CrI excluding Home-HF67 | 2.4137 | 2.4128 | 2.5306 | 2.5908 |
| PrI excluding Home-HF67 | 2.4137 | 2.4043 | 2.5230 | 2.5847 |
| Difference in QALYs | ||||
| CrI | – | −0.0232 | 0.1059 | 0.1038 |
| PrI | – | −0.0504 | 0.0814 | 0.0808 |
| CrI excluding Home-HF67 | – | −0.0009 | 0.1169 | 0.1772 |
| PrI excluding Home-HF67 | – | −0.0093 | 0.1093 | 0.1710 |
| ICER (£/QALY) | ||||
| CrI | – | Dominated | 63,240a | 5864 |
| PrI | – | Dominated | 228,035a | 7133 |
| CrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 4455a |
| PrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 4703a |
| Expected incremental NMB (£) | ||||
| CrI | – | −603.90 | 1375 | 1467 |
| PrI | – | −1111.22 | 914 | 1039 |
| CrI excluding Home-HF67 | – | −216.70 | 1564 | 2754 |
| PrI excluding Home-HF67 | – | −412.88 | 1389 | 2616 |
| Probability of cost-effectiveness (%) | ||||
| CrI | 1 | 18 | 36 | 44 |
| PrI | 4 | 19 | 35 | 41 |
| CrI excluding Home-HF67 | 0 | 5 | 12 | 83 |
| PrI excluding Home-HF67 | 1 | 7 | 19 | 73 |
In general, the higher usual care cost makes only a small difference to the results. For the high usual care cost scenario analysis, all of the intervention strategies showed an increase in cost-effectiveness. The ICER for TM during office hours compared with usual care decreased from £9522 per QALY in the base-case cost scenario to £5864 per QALY in the high usual care cost scenario estimated using HRs from CrIs of the NMA. Similarly, the probability of TM being cost-effective increased from 44% in the base-case cost scenario to 45% in the high usual care cost scenario, and the probability of usual care being cost-effective decreased from 2% to 1%. Similar patterns were observed in the other analyses (using HRs based on CrIs excluding the Home-HF study67 as well as predictive distributions of the NMA including and excluding the Home-HF study67). This is because the difference in costs between the interventions and usual care decreases as the cost of usual care increases, resulting in better cost-effectiveness for the interventions.
Results of the lower cost of telemonitoring during office hours scenario
Similar scenario analysis using a lower cost for TM during office hours of £133.50 per patient per month was repeated using effectiveness evidence from all four NMAs (HRs based on CrIs as well as predictive distributions of NMA, including and excluding the Home-HF study67). The results of this analysis are presented in Appendix 13 and summarised in Table 43.
| Usual care | STS HM | STS HH | TM during office hours | |
|---|---|---|---|---|
| Total costs (£) | ||||
| CrI | 8478 | 9001 | 9604 | 9243 |
| PrI | 8478 | 8965 | 9574 | 9211 |
| CrI excluding Home-HF67 | 8478 | 9060 | 9635 | 9420 |
| PrI excluding Home-HF67 | 8478 | 9087 | 9658 | 9435 |
| Difference in costs (£) | ||||
| CrI | – | 523 | 1126 | 765 |
| PrI | – | 487 | 1096 | 733 |
| CrI excluding Home-HF67 | – | 582 | 1157 | 942 |
| PrI excluding Home-HF67 | – | 609 | 1180 | 957 |
| Total QALYs | ||||
| CrI | 2.4137 | 2.3905 | 2.5196 | 2.5175 |
| PrI | 2.4137 | 2.3633 | 2.4950 | 2.4944 |
| CrI excluding Home-HF67 | 2.4137 | 2.4128 | 2.5306 | 2.5908 |
| PrI excluding Home-HF67 | 2.4137 | 2.4043 | 2.5230 | 2.5847 |
| Difference in QALYs | ||||
| CrI | – | −0.0232 | 0.1059 | 0.1038 |
| PrI | – | −0.0504 | 0.0814 | 0.0808 |
| CrI excluding Home-HF67 | – | −0.0009 | 0.1169 | 0.1772 |
| PrI excluding Home-HF67 | – | –0.0093 | 0.1093 | 0.1710 |
| ICER (£/QALY) | ||||
| CrI | – | Dominated | 170,629a | 7367 |
| PrI | – | Dominated | 605,112a | 9080 |
| CrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 5315a |
| PrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 5595a |
| Expected incremental NMB (£) | ||||
| CrI | – | −986.75 | 993 | 1311 |
| PrI | – | −1494.07 | 531 | 882 |
| CrI excluding Home-HF67 | – | −599.54 | 1181 | 2602 |
| PrI excluding Home-HF67 | – | −795.73 | 1006 | 2463 |
| Probability of cost-effectiveness (%) | ||||
| CrI | 2 | 17 | 31 | 50 |
| PrI | 6 | 18 | 33 | 44 |
| CrI excluding Home-HF67 | 0 | 4 | 9 | 87 |
| PrI excluding Home-HF67 | 1 | 6 | 16 | 77 |
For the lower cost of TM during office hours scenario analysis, TM during office hours showed an increase in cost-effectiveness. The ICER of TM during office hours compared with usual care estimated using HRs from CrIs of the NMA decreased from £9522 per QALY in the base-case cost scenario to £7367 per QALY in the low-cost TM scenario. Similarly, the probability of TM during office hours being cost-effective increased from 44% in the base-case cost scenario to 50% in the low-cost TM scenario. Similar patterns were observed in the other analyses (using HRs based on CrIs excluding the Home-HF study67 as well as predictive distributions of the NMA including and excluding the Home-HF study67). Again, this is because delivering the same health outcomes at a lower cost increases the cost-effectiveness.
Results of the higher cost of telemonitoring during office hours scenario
Similar scenario analysis using a higher cost of TM during office hours of £215 per patient per month was repeated using effectiveness evidence from all four NMAs (HRs based on CrIs as well as predictive distributions of the NMA, including and excluding the Home-HF study67). Results of these analyses are presented in Appendix 14 and are summarised in Table 44.
| Usual care | STS HM | STS HH | TM during office hours | |
|---|---|---|---|---|
| Total costs (£) | ||||
| CrI | 8478 | 9001 | 9604 | 9686 |
| PrI | 8478 | 8965 | 9574 | 9652 |
| CrI excluding Home-HF67 | 8478 | 9060 | 9635 | 9870 |
| PrI excluding Home-HF67 | 8478 | 9087 | 9658 | 9884 |
| Difference in costs (£) | ||||
| CrI | – | 523 | 1126 | 1207 |
| PrI | – | 487 | 1096 | 1174 |
| CrI excluding Home-HF67 | – | 582 | 1157 | 1392 |
| PrI excluding Home-HF67 | – | 609 | 1180 | 1406 |
| Total QALYs | ||||
| CrI | 2.4137 | 2.3905 | 2.5196 | 2.5175 |
| PrI | 2.4137 | 2.3633 | 2.4950 | 2.4944 |
| CrI excluding Home-HF67 | 2.4137 | 2.4128 | 2.5306 | 2.5908 |
| PrI excluding Home-HF67 | 2.4137 | 2.4043 | 2.5230 | 2.5847 |
| Difference in QALYs | ||||
| CrI | – | −0.0232 | 0.1059 | 0.1038 |
| PrI | – | −0.0504 | 0.0814 | 0.0808 |
| CrI excluding Home-HF67 | – | −0.0009 | 0.1169 | 0.1772 |
| PrI excluding Home-HF67 | – | −0.0093 | 0.1093 | 0.1710 |
| ICER (£/QALY) | ||||
| CrI | – | Dominated | £10,629a | Dominated |
| PrI | – | Dominated | £13,473a | Dominated |
| CrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 7854a |
| PrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 8223a |
| Expected incremental NMB (£) | ||||
| CrI | – | −986.75 | 993 | 869 |
| PrI | – | −1494.07 | 531 | 442 |
| CrI excluding Home-HF67 | – | −599.54 | 1181 | 2152 |
| PrI excluding Home-HF67 | – | −795.73 | 1006 | 2014 |
| Probability of cost-effectiveness (%) | ||||
| CrI | 3 | 19 | 40 | 38 |
| PrI | 7 | 20 | 37 | 37 |
| CrI excluding Home-HF67 | 0 | 6 | 16 | 78 |
| PrI excluding Home-HF67 | 1 | 8 | 23 | 68 |
The probability of TM during office hours being cost-effective decreased from 44% in the base-case cost scenario to 38% in the high-cost TM during office hours scenario estimated using HRs from CrIs of the NMA. Furthermore, TM during office hours is dominated by STS HH when the expected values of the ICERs are estimated. The reason for this difference is the similarity in the estimates of the effectiveness parameters, which means that the ICERs are estimated based on very small differences in benefits (STS HH results in 0.0021 QALYs more than TM during office hours). Thus, a small change in the incremental costs of TM during office hours compared with STS HH (from −£134 to +£82) led to a marked change in the ICER of TM during office hours compared with STS HH.
In the analyses performed using HRs from the predictive distributions of the NMA that excluded the Home-HF study,67 TM during office hours is still the most cost-effective strategy with an ICER of £8223 per QALY compared with usual care (STS HH is extendedly dominated by a combination of usual care and TM during office hours). Threshold analysis suggested that the monthly cost of TM during office hours needs to be > £390 for it not to be cost-effective, that is, to have an ICER > £20,000 per QALY compared with STS HH. At this monthly cost of £390, TM during office hours has an ICER of £13,357 per QALY compared with usual care.
Results of the high-cost structured telephone support human-to-human contact scenario
Scenario analysis was also performed using a higher STS HH cost of £192 per patient per month during the treatment period. This scenario analysis was performed with effectiveness evidence from all four NMAs (HRs based on CrIs as well as predictive distributions of the NMA, including and excluding the Home-HF study67). The results of this analysis are presented in Appendix 15 and summarised in Table 45.
| Usual care | STS HM | STS HH | TM during office hours | |
|---|---|---|---|---|
| Total costs (£) | ||||
| CrI | 8478 | 9001 | 9675 | 9470 |
| PrI | 8478 | 8965 | 9645 | 9437 |
| CrI excluding Home-HF67 | 8478 | 9060 | 9706 | 9650 |
| PrI excluding Home-HF67 | 8478 | 9087 | 9729 | 9665 |
| Difference in costs (£) | ||||
| CrI | – | 523 | 1197 | 992 |
| PrI | – | 487 | 1167 | 959 |
| CrI excluding Home-HF67 | – | 582 | 1228 | 1172 |
| PrI excluding Home-HF67 | – | 609 | 1251 | 1187 |
| Total QALYs | ||||
| CrI | 2.4137 | 2.3905 | 2.5196 | 2.5175 |
| PrI | 2.4137 | 2.3633 | 2.4950 | 2.4944 |
| CrI excluding Home-HF67 | 2.4137 | 2.4128 | 2.5306 | 2.5908 |
| PrI excluding Home-HF67 | 2.4137 | 2.4043 | 2.5230 | 2.5847 |
| Difference in QALYs | ||||
| CrI | – | −0.0232 | 0.1059 | 0.1038 |
| PrI | – | −0.0504 | 0.0814 | 0.0808 |
| CrI excluding Home-HF67 | – | −0.0009 | 0.1169 | 0.1772 |
| PrI excluding Home-HF67 | – | −0.0093 | 0.1093 | 0.1710 |
| ICER (£/QALY) | ||||
| CrI | – | Dominated | 97,300a | 9552 |
| PrI | – | Dominated | 346,341a | 11,873 |
| CrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 6616a |
| PrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 6942a |
| Expected incremental NMB (£) | ||||
| CrI | – | −986.75 | 922 | 1084 |
| PrI | – | −1494.07 | 460 | 656 |
| CrI excluding Home-HF67 | – | −599.54 | 1110 | 2371 |
| PrI excluding Home-HF67 | – | −795.73 | 935 | 2233 |
| Probability of cost-effectiveness (%) | ||||
| CrI | 2 | 19 | 34 | 46 |
| PrI | 6 | 19 | 34 | 41 |
| CrI excluding Home-HF67 | 0 | 5 | 11 | 84 |
| PrI excluding Home-HF67 | 1 | 7 | 18 | 74 |
For the high-cost STS scenario analyses, the probability of STS HH being cost-effective decreased whereas the probability of TM during office hours being cost-effective increased compared with the analysis with base-case costs. Similar patterns were also observed in the other analyses (using HRs based on CrIs excluding the Home-HF study67 as well as predictive distributions of the NMA including and excluding the Home-HF study67).
Results of the low-cost structured telephone support human-to-human contact scenario
Scenario analysis was also performed using a lower cost of STS HH of £175 per patient per month during the treatment period. This scenario analysis was performed using the effectiveness evidence from all four NMAs (HRs based on CrIs as well as predictive distributions of NMA, including and excluding the Home-HF study67). The results of this analysis are presented in Appendix 16 and summarised in Table 46.
| Usual care | STS HM | STS HH | TM during office hours | |
|---|---|---|---|---|
| Total costs (£) | ||||
| CrI | 8478 | 9001 | 9582 | 9470 |
| PrI | 8478 | 8965 | 9553 | 9437 |
| CrI excluding Home-HF67 | 8478 | 9060 | 9613 | 9650 |
| PrI excluding Home-HF67 | 8478 | 9087 | 9636 | 9665 |
| Difference in costs (£) | ||||
| CrI | – | 523 | 1104 | 992 |
| PrI | – | 487 | 1075 | 959 |
| CrI excluding Home-HF67 | – | 582 | 1135 | 1172 |
| PrI excluding Home-HF67 | – | 609 | 1158 | 1187 |
| Total QALYs | ||||
| CrI | 2.4137 | 2.3905 | 2.5196 | 2.5175 |
| PrI | 2.4137 | 2.3633 | 2.4950 | 2.4944 |
| CrI excluding Home-HF67 | 2.4137 | 2.4128 | 2.5306 | 2.5908 |
| PrI excluding Home-HF67 | 2.4137 | 2.4043 | 2.5230 | 2.5847 |
| Difference in QALYs | ||||
| CrI | – | −0.0232 | 0.1059 | 0.1038 |
| PrI | – | −0.0504 | 0.0814 | 0.0808 |
| CrI excluding Home-HF67 | – | −0.0009 | 0.1169 | 0.1772 |
| PrI excluding Home-HF67 | – | −0.0093 | 0.1093 | 0.1710 |
| ICER (£/QALY) | ||||
| CrI | – | Dominated | 52,951a | 9552 |
| PrI | – | Dominated | 193,206a | 11,873 |
| CrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 6616a |
| PrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 6942a |
| Expected incremental NMB (£) | ||||
| CrI | – | −986.75 | 1014 | 1084 |
| PrI | – | −1494.07 | 553 | 656 |
| CrI excluding Home-HF67 | – | −599.54 | 1203 | 2371 |
| PrI excluding Home-HF67 | – | −795.73 | 1028 | 2233 |
| Probability of cost-effectiveness (%) | ||||
| CrI | 2 | 18 | 36 | 43 |
| PrI | 6 | 19 | 35 | 40 |
| CrI excluding Home-HF67 | 0 | 5 | 12 | 83 |
| PrI excluding Home-HF67 | 1 | 7 | 19 | 72 |
Structured telephone support with human-to-human contact is still not cost-effective at a threshold of £20,000 per QALY, although the ICER of STS HH compared with TM during office hours estimated using the HRs from CrIs of the NMA decreased from £63,240 per QALY in the base-case scenario to £52,951 per QALY in the low-cost STS HH scenario. Assuming that the effectiveness parameters are constant, STS HH has to cost < £163 per month to be cost-effective at a threshold of £20,000 per QALY compared with TM during office hours. In the analyses excluding the Home-HF study,67 STS HH has to cost < £105 per month to not be extendedly dominated by a combination of usual care and TM during office hours.
Scenario analysis using 12 months' treatment duration
Scenario analysis using 12 months' treatment duration was performed using effectiveness evidence from all four NMAs (HRs based on CrIs as well as predictive distributions of the NMA, including and excluding the Home-HF study67). The results of this analysis are presented in Appendix 17 and summarised in Table 47.
| Usual care | STS HM | STS HH | TM during office hours | |
|---|---|---|---|---|
| Total costs (£) | ||||
| CrI | 8562 | 9571 | 10,603 | 10,353 |
| PrI | 8562 | 9564 | 10,582 | 10,326 |
| CrI excluding Home-HF67 | 8562 | 9645 | 10,655 | 10,663 |
| PrI excluding Home-HF67 | 8562 | 9708 | 10,707 | 10,698 |
| Difference in costs (£) | ||||
| CrI | – | 1009 | 2040 | 1791 |
| PrI | – | 1002 | 2019 | 1764 |
| CrI excluding Home-HF67 | – | 1082 | 2093 | 2101 |
| PrI excluding Home-HF67 | – | 1146 | 2145 | 2136 |
| Total QALYs | ||||
| CrI | 2.4137 | 2.3857 | 2.5935 | 2.5898 |
| PrI | 2.4137 | 2.3536 | 2.5589 | 2.5576 |
| CrI excluding Home-HF67 | 2.4137 | 2.4155 | 2.6117 | 2.7159 |
| PrI excluding Home-HF67 | 2.4137 | 2.4044 | 2.6005 | 2.7065 |
| Difference in QALYs | ||||
| CrI | – | −0.0280 | 0.1798 | 0.1761 |
| PrI | – | −0.0601 | 0.1452 | 0.1439 |
| CrI excluding Home-HF67 | – | 0.0019 | 0.1980 | 0.3022 |
| PrI excluding Home-HF67 | – | −0.0093 | 0.1868 | 0.2928 |
| ICER (£/QALY) | ||||
| CrI | – | Dominated | 68,189a | 10,167 |
| PrI | – | Dominated | 205,812a | 12,257 |
| CrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 6953a |
| PrI excluding Home-HF67 | – | Dominated | Extendedly dominated | 7296a |
| Expected incremental NMB (£) | ||||
| CrI | – | −1568.71 | 1555 | 1732 |
| PrI | – | −2202.89 | 884 | 1114 |
| CrI excluding Home-HF67 | – | −1045.41 | 1868 | 3942 |
| PrI excluding Home-HF67 | – | −1331.28 | 1590 | 3720 |
| Probability of cost-effectiveness (%) | ||||
| CrI | 2 | 18 | 35 | 44 |
| PrI | 7 | 19 | 34 | 40 |
| CrI excluding Home-HF67 | 0 | 5 | 12 | 83 |
| PrI excluding Home-HF67 | 1 | 7 | 19 | 73 |
In general, the 12-month treatment duration scenario produced similar results. The probability of the different interventions being cost-effective remained the same as in the 6-month treatment duration scenario. The ICER of TM during office hours compared with usual care estimated using the HRs from CrIs of the NMA increased from £9522 per QALY in the base-case 6-month treatment duration scenario to £10,353 per QALY in the 12-month treatment duration scenario. Similar patterns were observed in the other analyses (using HRs based on CrIs excluding the Home-HF study67 as well as predictive distributions of the NMA including and excluding the Home-HF study67).
Telemonitoring during office hours for 12 months was also compared with TM during office hours for 6 months to identify whether or not it was cost-effective to keep the patients on TM during office hours beyond 6 months. In the analysis using HRs from CrIs of the NMA, TM during office hours for 12 months was still cost-effective compared with TM during office hours for 6 months with an ICER of £12,213 per QALY. Similar patterns were observed in the other analyses as shown in Table 48 (£8097 per QALY using HRs based on CrIs excluding the Home-HF study,67 £14,066 per QALY using predictive distributions of the NMA including the Home-HF study67 and £8481 per QALY using predictive distributions of the NMA excluding the Home-HF study67).
| TM during office hours for 6 months | TM during office hours for 12 months | ICER (TM for 12 months vs TM for 6 months) (£/QALY) | |||
|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | ||
| CrI | 9470 | 2.5175 | 10,353 | 2.5898 | 12,213 |
| PrI | 9437 | 2.4944 | 10,326 | 2.5576 | 14,066 |
| CrI excluding Home-HF67 | 9650 | 2.5908 | 10,663 | 2.7159 | 8097 |
| PrI excluding Home-HF67 | 9665 | 2.5847 | 10,698 | 2.7065 | 8481 |
More importantly, given the potential capacity constraints for the TM devices, health organisations might choose to treat double the number of patients with TM during office hours for 6 months rather than using TM during office hours for 12 months. For example, assuming a capacity of 100 TM devices at a local health organisation, 200 patients could be treated in 1 year using TM during office hours for 6 months compared with 100 patients treated using TM during office hours for 12 months with the rest of the 100 patients under usual care (because of the lack of TM devices). This scenario was evaluated to find the most cost-effective strategy. In the analysis using HRs from CrIs of the NMA, treating 2n patients with TM during office hours for 6 months was cost-effective with an ICER of £793 per QALY compared with a combination of treating n patients with TM during office hours for 12 months and treating n patients under usual care. Again, similar patterns were observed in the other analyses as shown in Table 49. These results suggest that, in situations with a limited number of TM devices, it is cost-effective to treat patients with TM during office hours for 6 months rather than with TM during office hours for 12 months with the rest of the patients under usual care.
| 2n patients on TM during office hours for 12 monthsa | 2n patients on TM during office hours for 6 monthsb | ICER (TM for 6 months vs TM for 12 months) (£/QALY) | |||
|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | ||
| CrI | 18,915 | 5.0035 | 18,940 | 5.0350 | 793 |
| PrI | 18,888 | 4.9713 | 18,874 | 4.9888 | Dominant |
| CrI excluding Home-HF67 | 19,225 | 5.1296 | 19,300 | 5.1816 | 1442 |
| PrI excluding Home-HF67 | 19,260 | 5.1202 | 19,330 | 5.1694 | 1423 |
Discussion of the cost-effectiveness results
The effectiveness parameters (HRs of mortality and hospitalisation) are the key drivers in the model. Mortality reduction leads to a gain in QALYs whereas reduction in hospitalisations leads to fewer costs and less disutility. As the intervention costs are only a small part of the overall costs (hospitalisation costs are the main contributor), RM is likely to be cost-effective if it can save lives and reduce hospitalisations.
The results of the base-case cost-effectiveness analyses suggest that TM during office hours is expected to be the most cost-effective strategy at a threshold of £20,000 per QALY. However, there is uncertainty involved in suggesting that TM during office hours is the most probable cost-effective strategy and, in particular, there is higher uncertainty when the Home-HF study67 is included in the NMA than when it is excluded. This uncertainty also increased marginally when the HRs from the predictive distributions of the NMA were used instead of the HRs based on the CrIs of the NMA.
Scenario analyses performed using a higher usual care cost, lower TM during office hours cost and higher STS cost did not substantially change the conclusions regarding the relative cost-effectiveness of TM during office hours.
In the scenario analysis performed using a higher cost for TM during office hours (£215 per month) with HRs based on the predictive distributions of the NMA that included the Home-HF study,67 TM during office hours is dominated by STS HH. This is because a small change in the difference between the cost of TM during office hours and the cost of STS HH led to a marked change in the ICER, given the small difference in expected QALYs (0.0006) between STS HH and TM during office hours. However, the same scenario analysis (i.e. a higher cost of TM during office hours of £215 per month) performed using the HRs from the NMA that excluded the data from the Home-HF study67 suggested that TM during office hours is still the most cost-effective strategy. This is because of the much larger difference in the expected QALYs between STS HH and TM during office hours (0.0617), meaning that the small change in the difference between the cost of TM during office hours and the cost of STS HH did not lead to a marked change in the ICER.
Scenario analysis using a 12-month treatment duration produced similar results as in the 6-month treatment duration scenarios. The ICER of TM during office hours compared with usual care increased from £11,873 per QALY in the base-case 6-month treatment duration scenario to £12,257 per QALY in the 12-month treatment duration scenario. TM during office hours for 12 months was also cost-effective compared with TM during office hours for 6 months with an ICER of £14,066 per QALY, which suggests that it is cost-effective to keep patients on TM during office hours beyond 6 months. However, in situations with a limited number of TM devices, it is cost-effective to treat patients with TM during office hours for 6 months rather than 12 months with the rest of the patients under usual care.
Users can decide which of these base-case analyses is most representative of the UK setting, that is, whether or not the usual care in the Home-HF study67 is representative of usual care in the UK. If the usual care in the Home-HF study67 is not representative of usual care in the UK, then the modelling suggests that TM during office hours becomes the most cost-effective strategy with much reduced uncertainty.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Chronic conditions are set to be the major challenge for the NHS over coming years, and already account for approximately 70% of health-care expenditure in the UK. 137 RM may be an opportunity to optimise care quality while controlling costs by bringing care to patients in a way that would be difficult to achieve in conventional hospital-based clinical pathways. For example, in the clinic, collection of vital signs tends to be organised around hospital routine rather than patient needs, and the information is sometimes left in handwritten notes until the patient has another consultation with a senior clinician. Also contrary to usual clinical care, in which follow-up appointments tend to be organised for a prespecified time, RM can be more responsive to important changes in physiological parameters. Furthermore, RM is gradually being shown to be a viable addition to conventional service delivery for chronic conditions, with more than 100 telehealth pilots currently taking place in the NHS. 137 For instance, in Sheffield, 30 high-risk patients with COPD were offered a TM intervention for a period of 5 months. Throughout that time, patients measured their own vital signs, which were remotely transmitted to the care provider. The use of RM decreased hospital admissions by around 50%, saving the trust between £35,000 and £40,000. 37 As previously discussed, early results from the largest trial of RM (the WSD study) also seem promising. 117 However, a number of issues need to be considered if the NHS is to roll out RM as standard care for HF.
First, NHS purchasers need to consider the business model by which RM is provided. As pointed out by Inglis et al. ,48 purchasing RM equipment will involve large start-up costs and relatively low running costs, whereas renting the equipment would involve relatively low start-up costs and high running costs. Another relevant consideration is the speed with which RM equipment is changing and developing, which brings a series of further challenges and opportunities for care provision. Purchasing RM equipment may offer the benefits of stability with the risk of equipment rapidly becoming outdated. Conversely, renting may allow the NHS to maintain up-to-date service provision while running the risk of uncertainty, high costs of new technologies and start-up difficulties for new systems. More generally, the logistical and cost challenges of rolling out RM should not be underestimated. 138 The WSD trial cost over £30M to run, and provision of RM as standard for HF patients would require considerable reorganisation of services in the short term.
Second, selection of appropriate patients for RM is an important consideration. Although the acceptability of RM technologies was generally high in the synthesised literature, they will not be suited to everyone. Nor will RM necessarily be effective among those for whom it is acceptable: in the meta-analysed trials, compliance was inconsistently reported, with one large, high-quality trial reporting a low rate. 52 Best practice patient selection methods are therefore critical to guarantee the success of RM interventions. These might include selection of patients who are keen to incorporate RM into their care, and using physiological parameters, such as those described by Fonarow et al. ,139 to identify particularly at-risk patients prior to discharge. Indeed, it has been argued that null results in some trials [Telemedical Interventional Monitoring in Heart Failure (TIM-HF50) and Tele-HF52] may have been attributable to patients with less severe, well-controlled HF. 140
The NHS should also consider the duration of RM interventions, as this will have important implications for clinical effectiveness and cost-effectiveness. The highest risk period for mortality and rehospitalisation for patients with a new diagnosis of HF is the period immediately following hospitalisation,15 so offering early RM is likely to deliver the maximum benefit. What is less clear is the time period for which use of RM could continue to confer benefits. The duration of the RM interventions included in this meta-analysis varied from 2 months72 to 12 months. 74,78,104 However, because of inconsistent reporting of intervention duration, it was not possible to evaluate the relative efficacy of RM interventions by duration in a meta-regression. Further research is required to inform NHS decisions on how long to offer RM to patients with HF.
Finally, as Kaplan and Litewka141 note, RM ‘is not only a technological improvement, but a reengineering of healthcare processes requiring consideration of socio-technical aspects of their design and development’ (p. 402). This raises two important issues. First, health-care providers will require appropriate training to ensure stable and high-quality provision. Experience from the system-wide use of RM technologies in the US Veterans Health Administration suggests national or common training support facilities could be one viable way to achieve this. 137 Second, by further shifting the onus of health care from hospital to home, RM has the potential to fundamentally change what it means to be a patient with a chronic condition, which raises ethical issues that go beyond confidentiality and secure data transfer. 141 It is beyond the scope of this review to comprehensively address these issues here but, at the very least, frameworks and guidelines are required to ensure that RM is conducted to deliver benefits to patients in an equitable and genuinely empowering way. 142
Chapter 6 Discussion
Statement of principal findings
For adults who have recently (< 28 days) been discharged from an acute care setting after a recent exacerbation of HF, the NMA found that, compared with usual care, RM was beneficial in reducing all-cause mortality by 23%, 24% and 51% for STS HH (HR 0.77, 95% CrI 0.55 to 1.08), TM with medical support provided during office hours (HR 0.76, 95% CrI 0.49 to 1.18) and TM 24/7 (HR 0.49, 95% CrI 0.20 to 1.18) respectively. However, the results for TM 24/7 should be treated with caution because of the poor methodological quality of the only included study in this network. No beneficial effect on mortality was observed with STS HM. TM with medical support during office hours and TM 24/7 were associated with a 25% (HR 0.75, 95% CrI 0.49 to 1.10) and a 19% (HR 0.81, 95% CrI 0.33 to 2.00) reduction in all-cause hospitalisations, respectively, whereas there was no major effect of STS HM (HR 1.06, 95% CrI 0.44 to 2.53) or STS HH (HR 0.97, 95% CrI 0.70 to 1.31). Although there was no major effect of STS HM (HR 1.03, 95% CrI 0.66 to 1.54) and TM with medical support during office hours (HR 0.95, 95% CrI 0.70 to 1.34) on HF-related hospitalisation, STS HH (HR 0.77, 95% CrI 0.62 to 0.96) was associated with a reduction of 23%. No trials of cardiovascular implanted monitoring devices or observational studies met the inclusion criteria of the current review. Although data were limited, care packages that included STS and TM generally improved QoL and were acceptable to recently discharged patients with HF.
A sensitivity analysis, which excluded data from the Home-HF trial67 (as it appeared to be inconsistent with the data from the remaining studies, i.e. an outlier), found that TM with medical support provided during office hours was generally more effective than STS HH for all-cause mortality (TM during office hours: HR 0.62, 95% CrI 0.42 to 0.89; STS HH: HR 0.75, 95% CrI 0.59 to 0.96) and all-cause hospitalisations (TM during office hours: HR 0.67, 95% CrI 0.42 to 0.97; STS HH: HR 0.96, 95% CrI 0.72 to 1.27) but not HF-related hospitalisations (TM during office hours: HR 0.86, 95% CrI 0.61 to 1.21; STS HH: HR 0.76, 95% CrI 0.61 to 0.94). By excluding this study from the NMA, larger reductions in effect were observed for all-cause mortality, all-cause hospitalisations and HF-related hospitalisations for TM during office hours.
Additional analyses were undertaken to assess whether or not the results from the primary analysis differed markedly from the results in those with stable HF who were managed in the community. Of the 21 included studies of TM (including cardiovascular implanted monitoring devices) or STS programmes for adults with stable HF, 18 studies contributed to the network comparing different pairs or triplets of treatment using TM or STS programmes and usual care. For all-cause mortality, the NMA found that the effects of STS HH and TM during office hours were similar to the effects in patients who have recently (< 28 days) been discharged from an acute care setting after a recent exacerbation of HF. In terms of all-cause hospitalisations and HF-related hospitalisations, RM appears to be beneficial, although the effects of each intervention are not consistent relative to adults who were recently discharged. An analysis of the effect of cardiovascular implanted monitoring devices compared with cardiovascular implanted non-monitoring devices (n = 3 studies) found a trend in favour of a reduction in all-cause mortality (HR 0.90, 95% CrI 0.31 to 2.49) and HF-related hospitalisations (HR 0.72, 95% CrI 0.32 to 1.37). However, these effects were not conclusive.
Base-case monthly costs per patient were estimated using microcosting methods as £27 for usual care, £119 for STS HM, £179 for STS HH and £175 for TM during office hours. Five cost scenarios were also developed to calculate lower and higher estimates of costs of STS HH (£175 and £192 per month respectively) and TM during office hours (£133.50 and £215 per month respectively) along with a higher estimate of usual care costs (£92 per month).
The results of the full incremental cost-effectiveness analysis using the base-case costs suggest that TM during office hours is likely to be the most cost-effective strategy at a threshold of £20,000 per QALY for both analysis using CrIs and PrIs of the NMA as HRs in the model. In the analysis performed using PrIs from NMA as HRs, TM during office hours had an estimated ICER of £11,873 per QALY, compared with usual care whereas STS HH had an ICER of £228,035 per QALY compared with TM during office hours. STS HM was dominated by usual care. Thus, although STS HH is the most effective strategy providing the highest number of expected QALYs (2.4950), with TM the second most effective (2.4944 QALYs), the additional QALYs gained by STS HH are not worth the additional costs of the strategy, as seen in the ICER, which is greater than the threshold of £20,000 per QALY.
The PSA showed substantial uncertainty over the most probable cost-effective strategy. TM during office hours was the most cost-effective strategy in 40% of the PSA runs whereas STS HH was most cost-effective in 35% of the PSA runs. STS HM and usual care were the most cost-effective in 19% and 6% of the runs respectively. The EVPI per patient was estimated at £1831 and the population EVPI per annum was estimated at £100,299,791 assuming an annual incidence of first HF admissions in England and Wales of 54,779.
Cost-effectiveness analysis performed using the HRs from the predictive distributions of the NMA that excluded the data from the Home-HF trial67 showed an improvement in the cost-effectiveness of TM during office hours. STS HM and STS HH were dominated and extendedly dominated, respectively, with the ICER for TM during office hours compared with usual care estimated as £6492 per QALY. In this analysis, TM during office hours is also the most effective strategy (2.5847 QALYs for TM vs 2.5230 QALYs for STS HH). Furthermore, the results from the uncertainty analysis suggest that TM during office hours is cost-effective in 73% of the runs whereas STS HH and STS HM are cost-effective in 19% and 7% of the runs respectively. This reduction in the uncertainty was also reflected in the lower EVPI per patient, estimated as £410, and the lower population EVPI per annum, estimated as £22,459,265.
Scenario analysis performed using a higher cost of TM during office hours (£215 per month) increased the uncertainty. Both TM during office hours and STS HH were cost-effective in 37% of the PSA runs. But, TM during office hours is dominated by STS HH. This is because the estimated ICER is based on very small differences in benefits (STS HH results in 0.0006 QALYs more than TM during office hours) and so a small increase in the difference between costs of TM during office hours and STS HH leads to a marked change in the ICER. The same scenario analysis (i.e. a higher cost of TM during office hours of £215 per month), performed using the HRs from the NMA that excluded the data from the Home-HF trial,67 suggested that TM during office hours would still be the most cost-effective strategy with an ICER of £8223 per QALY compared with usual care (STS HH is extendedly dominated by a combination of usual care and TM during office hours). Threshold analysis suggested that the monthly cost of TM during office hours has to be > £390 to produce an ICER > £20,000 per QALY compared with STS HH. At a monthly cost of £390, the ICER of TM during office hours compared with usual care is £13,357 per QALY.
Scenario analyses performed using a higher cost of usual care, a higher cost of STS HH and a lower cost of TM during office hours do not substantially change the conclusions. TM during office hours was estimated to be the most cost-effective strategy in all of these scenarios.
Strengths and limitations of the assessment
Although an extensive literature search was conducted, it is possible that some relevant studies may have been missed. However, such omissions are likely to have been minimal as the search included all identifiable publications in the grey literature (including contact with clinical experts in the field).
The data were analysed by assuming a binomial likelihood function for the sample data. The statistical model acknowledged the fact that events accumulate over time by adjusting for the varying durations of each study using a complementary log-log links function. Parameter estimates, including the between-study standard deviation, were estimated using Markov chain Monte Carlo (MCMC), which allows for uncertainty in the estimate of the between-study standard deviation; it also allowed the estimation of the predictive distribution of the effect of each intervention in a new study.
The clinical effectiveness findings had a number of limitations. In particular, the RM interventions were heterogeneous in terms of monitored parameters and selection criteria for HF. This was the case even within each of the four specific types of RM (STS HH, STS HM, TM with medical support during office hours, TM with medical support 24/7). Clear descriptions of the RM interventions were not provided in many of the studies included in the systematic review, making it difficult to understand exactly what was provided as part of the intervention. In addition, a number of trials were underpowered to detect the clinical outcome of interest and did not report blinding of outcome assessors. A limitation of the statistical model (as a consequence of having only one observation from each study) was that it assumed that the hazards and relative intervention effects were constant over time; nevertheless, this is better than assuming that study duration has no impact on the data. Moreover, because of the differences in the HF populations (e.g. definition of HF, LVEF inclusion criteria) of the included studies the true estimate of treatment effect may be unclear. However, the NMA analysis used a random-effects distribution together with 95% CrIs to reflect the uncertainty associated with the population mean. In addition, the predictive distribution of a randomly chosen study in the population was presented. This reflects not only uncertainty in the population mean but also the heterogeneity in treatment effects between studies. Unfortunately, it was not possible to model the heterogeneity between studies using a meta-regression technique because of the lack of suitable data on potential treatment effect modifiers.
The cost-effectiveness analysis has been undertaken assuming that the NMA results represent the best knowledge regarding the relative uncertainty between treatments. Therefore, although the treatment effects estimated from the NMA were statistically inconclusive, the joint uncertainty about these effectiveness parameters was used to populate the economic model. The expected values of costs and QALYs produced, which were used to estimate the cost-effectiveness of the RM interventions, thus are also aligned with the best knowledge on relative effectiveness. The uncertainty within the cost-effectiveness results was quantified by estimating the probability of each intervention being the most cost-effective at different WTP thresholds, and the EVPI was calculated to explicitly quantify the cost of reducing the decision uncertainty by undertaking further research.
Any limitations in the evidence base also manifest as limitations of the cost-effectiveness model. Most of the included studies in the NMA provided information on mortality and/or hospitalisation rates, which allowed synthesis using meta-analytical methods, but only a few studies reported any data about other potentially relevant states/events (such as stroke, having a pacemaker fitted), which did not extend to reporting any differences between the usual care and RM arms. Given the lack of evidence, it was deemed prudent to use a two-state Markov model even though it involved simplifications and assumptions that may not exactly reflect clinical practice. An advantage of using this simple model is that it can be easily updated to include other states or events should there be future evidence demonstrating differences between the usual care and RM arms.
A limitation of the cost-effectiveness model was that there was no age-specific analysis. Another limitation was that the constant hazards and relative intervention effects over time were applied to the time-dependent baseline mortality hazard (which is greatest in the early period after discharge after a hospitalisation for HF and subsequently declines over time) and constant risk of hospitalisation. If the studies reported observations at different time points, time-dependent effectiveness parameters can be estimated and used in the cost-effectiveness model. Furthermore, the optimal duration for each of the RM interventions can also be identified.
None of the studies identified in the review provided an estimate for the utility of the patients and whether or not there was a difference between the RM and usual care groups. Thus, in the economic model, similar utility values were used for HF patients in both the RM and usual care groups; however, the validity of this assumption is unclear. Furthermore, the lack of detail provided in research studies concerning the components of RM packages and usual care (e.g. communication protocols, routine staff visits and resources used) made it difficult to estimate costs. Costing scenarios for different RM classifications were developed and their costs were estimated using microcosting methods. Although the users can decide which of these analyses is most representative of their setting, uncertainties still remain about the assumptions made in the estimation of these costs. This uncertainty in the costs is a limitation, especially as, given the small difference in QALYs between STS HH and TM during office hours, a small change in the difference between the cost of TM during office hours and the cost of STS HH can lead to marked changes in the ICER. A further limitation is that the effectiveness remained the same for the different cost scenarios whereas in reality there might be some correlation between the cost and the effectiveness of different RM strategies.
Uncertainties
In the cost-effectiveness model, the HRs of mortality and hospitalisation were the key drivers as mortality reductions lead to a gain in QALYs whereas reductions in hospitalisations lead to fewer costs and more QALYs. The intervention costs were only a small part of the overall costs (hospitalisation costs are the main contributor); thus, RM is likely to be cost-effective if it can save lives and reduce hospitalisations to a large enough extent. However, there was still some uncertainty in the effectiveness parameters as suggested in the EVPI analysis.
At the time of writing, the long-awaited results of the WSD programme117 had not been published in a peer-reviewed journal. This study is a large UK-based RCT of telehealth compared with usual care, which included over 6000 patients with HF, diabetes mellitus or COPD. Although early headline results, published by the UK Department of Health,57 suggest a substantial reduction in mortality by 45%, the magnitude and direction of effect in recently discharged patients with HF is unclear (including people with stable HF). Given the large sample size, it is anticipated that the effectiveness results from the WSD programme will help reduce some of the uncertainty reported in the model results for recently discharged patients with HF.
Chapter 7 Conclusions
Implications for service provision
In general, although the effectiveness of the interventions varied widely according to the type of RM system used, STS HH and TM with medical support provided during office hours showed beneficial effects, particularly in reducing all-cause mortality for recently discharged patients with HF; however, these effects were statistically inconclusive.
Given the variation in usual care and RM strategies, the cost-effectiveness analysis was performed using a set of costing scenarios. These scenarios were designed to reflect the different configurations of usual care and RM interventions present in the UK. The cost-effectiveness analyses suggest that TM during office hours was an optimal strategy in most of the scenarios.
Suggested research priorities
Despite the growing evidence base for RM, a number of key questions are yet to be addressed. First, it would be helpful to have more direct comparisons of STS and TM. To our knowledge, only one study of recently discharged patients (TEN-HMS49) has made this comparison. The results of this trial suggested that TM was somewhat more beneficial than STS – in particular, TM had a substantially greater effect on reducing the duration of hospitalisation and the number of home or clinical visits. This broadly coheres with our findings (particularly from the sensitivity analyses excluding the Home-HF study67) that TM interventions showed a greater risk reduction than STS for mortality and hospitalisation. In addition, patients with HF are at increased risk of atrial fibrillation, which can lead to deterioration and hospitalisation. Further research on the precipitants of admission for HF (including atrial fibrillation, infection and non-compliance) and how they might be detected and managed early is required.
Given the complex nature of RM interventions, new research should seek to examine the ‘active ingredients’ of RM. For instance, the NMA was unable to compare the effectiveness of TM interventions that monitored different physiological parameters. Well-known risk factors such as low LVEF, NYHA class and heart rate perform well in predicting mortality but it is not yet clear which factors in which combination can provide optimal clinical benefit for RM. As a complex intervention (i.e. made up of multiple, socially meaningful, interconnected factors), it is important to understand the processes by which RM works, and qualitative research on patient experiences of RM may help throw light on the issue. 116 In relation to STS, one interesting question is whether contact with a care professional is required to deliver benefits, or whether it can work as an automated human-to-machine interface. RCTs of STS that manipulate the presence of a human caregiver as the primary experimental variable could help address this issue. It also remains unclear how RM affects clinical decision-making. Further research should seek to establish in what ways RM might improve such decision-making, and conversely whether it may, in some circumstances, act as an impediment to good care. More importantly, it is worth echoing the recommendation made by Inglis et al. 48 that future RM studies should publish data in such a way as to identify which patient subgroups benefited most from the intervention. For example, there might be differential effectiveness in different age groups and future trials should explore these issues. If particular groups tend to benefit more, the potential for RM to exacerbate health inequalities should be carefully considered, and strategies should be pursued to minimise this.
Furthermore, to aid robust cost-effectiveness estimations, the costs associated with usual care and RM interventions need to be reported in detail (including the costs of HF treatment pathways). The costs need to be linked to the activities or items involved in the intervention using activity-based costing or unit costing approaches respectively. In addition, QoL, patient severity status transitions (e.g. NYHA class) and hospitalisations need to be reported with observations at specific time points to enable the estimation effectiveness of RM over time and also to identify the optimal duration of RM interventions.
Implementation costs (such as set-up costs, staff training costs, costs for dual running of usual care and RM services) were often missing from the studies in the review. Future studies should provide greater detail of the costs of reconfiguration and link more clearly with the financial impact (e.g. cost variation with scale and over time) on provider organisations. Wider adaptation of RM in the NHS can be facilitated by providing financial impact data along with the cost-effectiveness information.
Acknowledgements
We would like to thank the following people for their help with this project:
-
Dr Abdallah Al-Mohammad, Consultant Cardiologist, Sheffield Teaching Hospitals NHS Foundation Trust; Dr Ameet Bhaki, Consultant Cardiologist, Barnet General Hospital; Professor John Cleland, Professor of Cardiology, University of Hull; Mr Tim Ellis, Research Fellow, University of Sheffield; Professor Mark Hawley, Professor of Health Services Research, University of Sheffield; Hazel Marsh, Research Nurse, Barnsley Hospital NHS Foundation Trust; and Dr Rachel O'Hara, Lecturer in Public Health, University of Sheffield for providing clinical expertise
-
Professor Robyn A Clark, Queensland University of Technology, Brisbane, Australia; Professor Martin R Cowie, Professor of Cardiology, Imperial College London; Dr Eva Kaltenthaler, Reader in Health Technology Assessment, University of Sheffield; Dr Jason Madan, Research Associate, University of Bristol; and Dr Paul Tappenden, Senior Research Fellow, University of Sheffield for peer reviewing the draft report.
Thanks also to Gill Rooney and Andrea Shippam for providing administrative support in the preparation and formatting of the report.
The views expressed in this report are those of the authors and not necessarily those of the NIHR HTA Programme. Any errors are the responsibility of the authors.
Contribution of authors
Abdullah Pandor (Senior Research Fellow) co-ordinated the review and was responsible for the acquisition of data, analysis and interpretation of data (for the systematic reviews) and drafting and revision of the final report.
Praveen Thokala (Research Fellow) and Hassan Baalbaki (Research Associate) were responsible for the acquisition of data, analysis and interpretation of data and model construction (for the health economic evaluation) and drafting and revision of the final report.
Tim Gomersall (Research Associate) was responsible for the acquisition of data, analysis and interpretation of data (for the systematic reviews) and drafting and revision of the final report.
John Stevens (Senior Lecturer in Statistics) and Jenny Wang (Research Assistant Statistician) were responsible for the statistical analyses, interpretation of data and drafting and revision of the final report.
Ruth Wong (Information Specialist) was responsible for developing and undertaking the electronic literature searches.
Alan Brennan (Professor of Health Economics and Decision Modelling) oversaw the modelling and reviewed the final report.
Patrick Fitzgerald was responsible for the conception and design of the study.
About the School of Health and Related Research
The School of Health and Related Research (ScHARR) is one of the nine departments that constitute the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research and the application of health economics and decision science to the development of health services and the improvement of public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the NIHR Health Technology Assessment programme on behalf of a range of policy-makers, including NICE. ScHARR-TAG is part of a wider collaboration of a number of units from other regions including Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; Aberdeen Health Technology Assessment Group (Aberdeen HTA Group), University of Aberdeen; Liverpool Reviews & Implementation Group (LRiG), University of Liverpool; Peninsula Technology Assessment Group (PenTAG), University of Exeter; the NHS Centre for Reviews and Dissemination, University of York; Warwick Evidence, University of Warwick; the BMJ Group and Kleijnen Systematic Reviews.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001;22:1527-60. http://dx.doi.org/10.1053/euhj.2001.2783.
- Mahadevan G, Davis RC, Frenneaux MP, Hobbs FD, Lip GY, Sanderson JE, et al. Left ventricular ejection fraction: are the revised cut-off points for defining systolic dysfunction sufficiently evidence based?. Heart 2008;94:426-8. http://dx.doi.org/10.1136/hrt.2007.123877.
- Petrie M, McMurray J. Changes in notions about heart failure. Lancet 2001;358:432-4. http://dx.doi.org/10.1016/S0140-6736(01)05664-1.
- Cowie MR, Zaphiriou A. Management of chronic heart failure. BMJ 2002;325:422-5. http://dx.doi.org/10.1136/bmj.325.7361.422.
- Lip GY, Gibbs CR, Beevers DG. ABC of heart failure: aetiology. BMJ 2000;320:104-7. http://dx.doi.org/10.1136/bmj.320.7227.104.
- McDonagh TA, Morrison CE, Lawrence A, Ford I, Tunstall-Pedoe H, McMurray JJ, et al. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet 1997;350:829-33. http://dx.doi.org/10.1016/S0140-6736(97)03033-X.
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 2000;35:569-82. http://dx.doi.org/10.1016/S0735-1097(99)00630-0.
- Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation 2003;107:1278-83. http://dx.doi.org/10.1161/01.CIR.0000054164.99881.00.
- Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA 2009;302:394-400. http://dx.doi.org/10.1001/jama.2009.1062.
- Scarborough P, Bhatnagar P, Wickramasinghe K, Smolina K, Mitchell C, Rayner M. Coronary heart disease statistics 2010 edition. London: British Heart Foundation; 2010.
- American Heart Association . AHA Medical/Scientific Statement. 1994 revisions to classification of functional capacity and objective assessment of patients with diseases of the heart. Circulation 1994;90:644-5.
- Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart 2002;87:235-41. http://dx.doi.org/10.1136/heart.87.3.235.
- Cowie MR, Wood DA, Coats AJ, Thomson SG, Suresh V, Poole-Wilson PA, et al. Survival of patients with a new diagnosis of heart failure: a population based study. Heart 2000;83:505-10. http://dx.doi.org/10.1136/heart.83.5.505.
- National Institute for Cardiovascular Outcomes Research . National Heart Failure Audit, April 2010–March 2011 n.d. www.ucl.ac.uk/nicor/audits/heartfailure/additionalfiles/pdfs/annualreports/annual11.pdf (accessed 29 March 2012).
- Riley JP, Cowie MR. Telemonitoring in heart failure. Heart 2009;95:1964-8. http://dx.doi.org/10.1136/hrt.2007.139378.
- Hasan A, Paul V. Telemonitoring in chronic heart failure. Eur Heart J 2011;32:1457-64. http://dx.doi.org/10.1093/eurheartj/ehr005.
- McKelvie RS. Heart failure. Clin Evid (Online) 2011;pii.
- Marantz PR, Tobin JN, Wassertheil-Smoller S, Steingart RM, Wexler JP, Budner N, et al. The relationship between left ventricular systolic function and congestive heart failure diagnosed by clinical criteria. Circulation 1988;77:607-12. http://dx.doi.org/10.1161/01.CIR.77.3.607.
- Morgan S, Smith H, Simpson I, Liddiard GS, Raphael H, Pickering RM, et al. Prevalence and clinical characteristics of left ventricular dysfunction among elderly patients in general practice setting: cross sectional survey. BMJ 1999;318:368-72. http://dx.doi.org/10.1136/bmj.318.7180.368.
- Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess 2009;13.
- Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet 2001;358:439-44. http://dx.doi.org/10.1016/S0140-6736(01)05620-3.
- Cowie MR, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, Suresh V, et al. Incidence and aetiology of heart failure; a population-based study. Eur Heart J 1999;20:421-8. http://dx.doi.org/10.1053/euhj.1998.1280.
- Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure. The Rotterdam Study. Eur Heart J 2004;25:1614-19. http://dx.doi.org/10.1016/j.ehj.2004.06.038.
- Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA 2004;292:344-50. http://dx.doi.org/10.1001/jama.292.3.344.
- Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, et al. Congestive heart failure in the community: trends in incidence and survival in a 10-year period. Arch Intern Med 1999;159:29-34. http://dx.doi.org/10.1001/archinte.159.1.29.
- Cleland JG, Coletta AP, Buga L, Ahmed D, Clark AL. Clinical trials update from the American College of Cardiology meeting 2010: DOSE, ASPIRE, CONNECT, STICH, STOP-AF, CABANA, RACE II, EVEREST II, ACCORD, and NAVIGATOR. Eur J Heart Fail 2010;12:623-9. http://dx.doi.org/10.1093/eurjhf/hfq083.
- Delville CL, McDougall G. A systematic review of depression in adults with heart failure: instruments and incidence. Issues Ment Health Nurs 2008;29:1002-17. http://dx.doi.org/10.1080/01612840802274867.
- Dekker RL, Peden AR, Lennie TA, Schooler MP, Moser DK. Living with depressive symptoms: patients with heart failure. Am J Crit Care 2009;18:310-18. http://dx.doi.org/10.4037/ajcc2009672.
- Cleland JG, McDonagh T, Rigby AS, Yassin A, Whittaker T, Dargie HJ. The national heart failure audit for England and Wales 2008–2009. Heart 2011;97:876-86. http://dx.doi.org/10.1136/hrt.2010.209171.
- NHS Information Centre . National Heart Failure Audit 2010 n.d. www.npc.nhs.uk/rapidreview/?p=2479 (accessed 30 March 2012).
- HES Online . Hospital Episode Statistics 2008–2009 n.d. www.hesonline.nhs.uk (accessed 29 March 2012).
- Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, et al. The EuroHeart Failure survey programme – a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J 2003;24:442-63. http://dx.doi.org/10.1016/S0195-668X(02)00823-0.
- Stewart KJ, Badenhop D, Brubaker PH, Keteyian SJ, King M. Cardiac rehabilitation following percutaneous revascularization, heart transplant, heart valve surgery, and for chronic heart failure. Chest 2003;123:2104-11. http://dx.doi.org/10.1378/chest.123.6.2104.
- Chronic heart failure: the management of chronic heart failure in adults in primary and secondary care. London: National Clinical Guideline Centre; 2012.
- Guideline 95. Edinburgh: SIGN; 2007.
- Guidelines on the management of chronic heart failure in Northern Ireland. Belfast: CREST; 2005.
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008;29:2388-442. http://dx.doi.org/10.1093/eurheartj/ehn309.
- Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:1977-2016. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.192064.
- Al-Mohammad A, Mant J, Laramee P, Swain S. Chronic Heart Failure Guideline Development Group . Diagnosis and management of adults with chronic heart failure: summary of updated NICE guidance. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4130.
- Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart 2005;91:899-906. http://dx.doi.org/10.1136/hrt.2004.048389.
- Roccaforte R, Demers C, Baldassarre F, Teo KK, Yusuf S. Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta-analysis. Eur J Heart Fail 2005;7:1133-44. http://dx.doi.org/10.1016/j.ejheart.2005.08.005.
- Gonseth J, Guallar-Castillon P, Banegas JR, Rodriguez-Artalejo F. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: a systematic review and meta-analysis of published reports. Eur Heart J 2004;25:1570-95. http://dx.doi.org/10.1016/j.ehj.2004.04.022.
- McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004;44:810-19.
- Murphy JJ, Chakraborty RR, Fuat A, Davies MK, Cleland JG. Diagnosis and management of patients with heart failure in England. Clin Med 2008;8:264-6. http://dx.doi.org/10.7861/clinmedicine.8-3-264.
- Jaarsma T, Strömberg A, De Geest S, Fridlund B, Heikkila J, Màrtensson J, et al. Heart failure management programmes in Europe. Eur J Cardiovasc Nurs 2006;5:197-205. http://dx.doi.org/10.1016/j.ejcnurse.2006.04.002.
- Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. Lancet 2011;378:731-9. http://dx.doi.org/10.1016/S0140-6736(11)61229-4.
- Casas JP, Kwong J, Ebrahim S. Telemonitoring for chronic heart failure: not ready for prime time. Cochrane Database Syst Rev 2010;8.
- Inglis SC, Clark RA, McAlister FA, Ball J, Lewinter C, Cullington D, et al. Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane Database Syst Rev 2010;8.
- Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol 2005;45:1654-64. http://dx.doi.org/10.1016/j.jacc.2005.01.050.
- Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Bohm M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation 2011;123:1873-80. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.018473.
- Angermann CE, Störk S, Gelbrich G, Faller H, Jahns R, Frantz S, et al. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circulation Heart Fail 2011;5:25-3. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.111.962969.
- Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, et al. Telemonitoring in patients with heart failure. N Engl J Med 2010;363:2301-9. http://dx.doi.org/10.1056/NEJMoa1010029.
- Sticherling C, Kuhne M, Schaer B, Altmann D, Osswald S. Remote monitoring of cardiovascular implantable electronic devices: prerequisite or luxury?. Swiss Med Wkly 2009;139:596-601.
- Maric B, Kaan A, Ignaszewski A, Lear SA. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail 2009;11:506-17. http://dx.doi.org/10.1093/eurjhf/hfp036.
- Zhang J, Goode KM, Cuddihy PE, Cleland JG. Predicting hospitalization due to worsening heart failure using daily weight measurement: analysis of the Trans-European Network-Home-Care Management System (TEN-HMS) study. Eur J Heart Fail 2009;11:420-7. http://dx.doi.org/10.1093/eurjhf/hfp033.
- Yorkshire & Humber Health Innovation and Education Cluster . Telemonitoring for Long Term Conditions: A Workbook for Implementing New Service Models 2012. http://yhhiec.org.uk/wp-content/uploads/2011/10/11070604_Tele_Moni_Workbk.pdf (accessed 6 January 2012).
- Department of Health . Whole System Demonstrator Programme: Headline Findings – December 2011 2011. www.dh.gov.uk/dr_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_131689.pdf (accessed 6 January 2012).
- Klersy C, De Silvestri A, Gabutti G, Regoli F, Auricchio A. A meta-analysis of remote monitoring of heart failure patients. J Am Coll Cardiol 2009;54:1683-94. http://dx.doi.org/10.1016/j.jacc.2009.08.017.
- Clark RA, Inglis SC, McAlister FA, Cleland JG, Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ 2007;334. http://dx.doi.org/10.1136/bmj.39156.536968.55.
- Kulshreshtha A, Kvedar JC, Goyal A, Halpern EF, Watson AJ. Use of remote monitoring to improve outcomes in patients with heart failure: a pilot trial. Int J Telemed Appl 2010. http://dx.doi.org/10.1155/2010/870959.
- Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235-41. http://dx.doi.org/10.1016/S0895-4356(98)00131-0.
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Insititute; 2008.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37. http://dx.doi.org/10.1023/A:1008929526011.
- McCullah P, Nelder J. Generalized linear models. London: Chapman and Hall; 1989.
- Brooks S, Gelman A. Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-45.
- Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62:1013-20. http://dx.doi.org/10.1016/j.jclinepi.2008.10.009.
- Dar O, Riley J, Chapman C, Dubrey SW, Morris S, Rosen SD, et al. A randomized trial of home telemonitoring in a typical elderly heart failure population in North West London: results of the Home-HF study. Eur J Heart Fail 2009;11:319-25. http://dx.doi.org/10.1093/eurjhf/hfn050.
- Dendale P, De Keulenaer G, Troisfontaines P, Weytjens C, Mullens W, Elegeert I, et al. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: the TEMA-HF 1 (TElemonitoring in the MAnagement of Heart Failure) study. Eur J Heart Fail 2012;14:333-40. http://dx.doi.org/10.1093/eurjhf/hfr144.
- Domingues FB, Clausell N, Aliti GB, Dominguez DR, Rabelo ER. Education and telephone monitoring by nurses of patients with heart failure: randomized clinical trial. Arq Bras Cardiol 2011;96:233-9. http://dx.doi.org/10.1590/S0066-782X2011005000014.
- Scherr D, Kastner P, Kollmann A, Hallas A, Auer J, Krappinger H, et al. Effect of home-based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: randomized controlled trial. J Med Internet Res 2009;11. http://dx.doi.org/10.2196/jmir.1252.
- Antonicelli R, Testarmata P, Spazzafumo L, Gagliardi C, Bilo G, Valentini M, et al. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare 2008;14:300-5. http://dx.doi.org/10.1258/jtt.2008.071213.
- Barth V. A nurse-managed discharge program for congestive heart failure patients: outcomes and costs. Home Health Care Manage Pract 2001;13:436-43. http://dx.doi.org/10.1177/108482230101300604.
- Capomolla S, Pinna GD, La Rovere MT, Maestri R, Ceresa M, Ferrari M. Heart failure case disease management program: a pilot study of home telemonitoring versus usual care. Eur Heart J 2004;6:F91-8. http://dx.doi.org/10.1016/j.ehjsup.2004.09.011.
- DeBusk RF, Miller NH, Parker KM, Bandura A, Kraemer HC, Cher DJ. Care management for low-risk patients with heart failure: a randomised, controlled trial. Ann Intern Med 2004;141:606-13.
- Goldberg LR, Piette JD, Walsh MN. Randomized trial of a daily electonic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J 2003;146:705-12. http://dx.doi.org/10.1016/S0002-8703(03)00393-4.
- Kielblock B, Frye C, Kottmair S, Hudler T. Impact of telemetric management on overall treatment costs and mortality rate among patients with chronic heart failure. Dtsc Med Wochenschr 2007;132:417-22. http://dx.doi.org/10.1055/s-2007-970350.
- Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: a randomized controlled trial. Arch Intern Med 2003;163:809-17. http://dx.doi.org/10.1001/archinte.163.7.809.
- Rainville EC. Impact of pharmacist intervention on hospital readmissions for heart failure. Am J Health Syst Pharm 1999;56:1339-42.
- Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med 2002;162:705-12. http://dx.doi.org/10.1001/archinte.162.6.705.
- Riegel B, Carlson B, Glaser D, Romero T. Randomized controlled trial of telephone case management in Hispanics of Mexican origin with heart failure. J Cardiac Fail 2006;12:211-19. http://dx.doi.org/10.1016/j.cardfail.2006.01.005.
- Tsuyuki RT, Fradette M, Johnson JA, Bungard TJ, Eurich DT, Ashton T, et al. A multicenter disease management program for hospitalized patients with heart failure. J Cardiac Fail 2004;10:473-80. http://dx.doi.org/10.1016/j.cardfail.2004.02.005.
- Wakefield BJ, Ward MM, Holman JE, Ray A, Scherubel M, Burns TL, et al. Evaluation of home telehealth following hospitalization for heart failure: a randomized trial. Telemed J E Health 2008;14:753-61. http://dx.doi.org/10.1089/tmj.2007.0131.
- Woodend AK, Sherrard H, Fraser M, Stuewe L, Cheung T, Struthers C, et al. Telehome monitoring in patients with cardiac disease who are at high risk of readmission. Heart Lung 2008;37:36-45. http://dx.doi.org/10.1016/j.hrtlng.2007.04.004.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000097.
- Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A, et al. Telephone follow-up by nurses reduces hospital readmissions among people with chronic heart failure. Evid Based Healthcare 2002;6:152-3. http://dx.doi.org/10.1054/ebhc.2002.0550.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials 2011. www.nicedsu.org.uk (accessed 6 January 2012).
- Galbreath AD, Krasuski RA, Smith B, Stajduhar KC, Kwan MD. Long-term healthcare and cost outcome of disease management in a large, randomized, community-based population with heart failure. Circulation 2004;110:3518-26. http://dx.doi.org/10.1161/01.CIR.0000148957.62328.89.
- Mortara A, Pinna GD, Johnson P, Maestri R, Capomolla S, La Rovere MT, et al. Home telemonitoring in heart failure patients: the HHH study (Home or Hospital in Heart Failure). Eur J Heart Fail 2009;11:312-18. http://dx.doi.org/10.1093/eurjhf/hfp022.
- DeWalt DA, Malone RM, Bryant ME, Kosner MC, Corr KE, Rothman RL. A heart failure self-management program for patients of all literacy levels: a randomised, controlled trial. Health Serv Res 2006;13.
- Gattis WA, Hasselblad V, Whellan DJ, O’Conner CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team. Arch Intern Med 1999;159:1939-45. http://dx.doi.org/10.1001/archinte.159.16.1939.
- GESICA investigators . Randomised trial of telephone intervention in chronic heart failure: DIAL trial. BMJ 2005;331:425-7. http://dx.doi.org/10.1136/bmj.38516.398067.E0.
- Sisk JE, Herbert PL, Horowitz CR, McLaughlin MA, Wang JJ, Chassin MR. Effects of nurse management on the quality of heart failure care in minority communities: a randomized trial. Ann Intern Med 2006;145:273-83.
- Tonkin A, Yallop J, Driscoll A, Forbes A, Davidson PM. Does telephone support of the rural and remote patients with heart failure improve clinical outcomes?: results of the Chronic Heart Failure Assistance by Telephone (CHAT) study. Heart Lung Circ 2009;185. http://dx.doi.org/10.1016/j.hlc.2009.05.239.
- Balk AH, Davidse W, Dommelen P, Klaassen E, Caliskan K, van der Burgh P, et al. Tele-guidance of chronic heart failure patients enhances knowledge about the disease. A multi-centre, randomised controlled study. Eur J Heart Fail 2008;10:1136-42. http://dx.doi.org/10.1016/j.ejheart.2008.08.003.
- Blum K, Gottlieb SS. Morbidity and mortality benefits of reliable instrumental support. J Cardiac Fail 2007;13. http://dx.doi.org/10.1016/j.cardfail.2007.06.581.
- de Lusignan S, Wells S, Johnson P, Meredith K, Leatham E. Compliance and effectiveness of 1 year's home telemonitoring: the report of a pilot study of patients with chronic heart failure. Eur J Heart Fail 2001;3:723-30. http://dx.doi.org/10.1016/S1388-9842(01)00190-8.
- Giordano A, Scalvini S, Zanelli E, Corra U, Longobardi GL, Ricci VA, et al. Multicenter randomised trial on home-based telemanagement to prevent hospital readmission of patients with chronic heart failure. Int J Cardiol 2009;131:192-9. http://dx.doi.org/10.1016/j.ijcard.2007.10.027.
- Soran OZ, Pina IL, Lamas GA, Kelsey SF, Selzer F, Pilotte J, et al. A randomized clinical trial of the clinical effects of enhanced heart failure monitoring using a computer-based telephonic monitoring system in older minorities and women. J Card Fail 2008;14:711-17. http://dx.doi.org/10.1016/j.cardfail.2008.06.448.
- Villani A, Malfatto G, Della RF, Branzi G, Boarin S, Borghi C, et al. Disease management for heart failure patients: role of wireless technologies for telemedicine. The ICAROS project. G Ital Cardiol 2007;8:107-14.
- Boyne JJJ, Vrijhoef HJM, Nieman FHN, De Wit R, De Weerd GJ, Kragten J, et al. Tailored telemonitoring in patients with heart failure: the TEHAF-study. Eur J Heart Fail Suppl 2011;10.
- Wade MJ, Desai AS, Spettell CM, Snyder AD, McGowan-Stackewicz V, Kummer PJ, et al. Telemonitoring with case management for seniors with heart failure. Am J Manag Care 2011;17:e71-9.
- Zugck C, Frankenstein I, Nelles M, Frochlich H, Schellberg D, Cebola R. Telemedicine reduces hospitalisation rates in patients with chronic heart failure: results of the randomised HiTel trial. Eur J Heart Fail Suppl 2008;7. http://dx.doi.org/10.1016/S1567-4215(08)60024-7.
- Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658-66. http://dx.doi.org/10.1016/S0140-6736(11)60101-3.
- Adamson PB, Gold MR, Bennett T, Bourge RC, Stevenson LW, Trupp R, et al. Continuous hemodynamic monitoring in patients with mild to moderate heart failure: results of the Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients With Chronic Heart Failure (REDUCEhf) trial. Congest Heart Fail 2011;17:248-54. http://dx.doi.org/10.1111/j.1751-7133.2011.00247.x.
- Bourge RC, Abraham WT, Adamson PB. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol 2008;51:1073-9. http://dx.doi.org/10.1016/j.jacc.2007.10.061.
- Ramachandran K, Husain N, Maikhuri R, Seth S, Vij A, Kumar M, et al. Impact of a comprehensive telephone-based disease management programme on quality-of-life in patients with heart failure. Natl Med J India 2007;20:67-73.
- Swedberg K, Wolf A, Ekman I. Telemonitoring in patients with heart failure. N Engl J Med 2011;364. http://dx.doi.org/10.1056/NEJMc1100395.
- Inglis SC, Clark RA, Cleland JG. Cochrane Systematic Review Team . Telemonitoring in patients with heart failure. N Engl J Med 2011;364:1078-9. http://dx.doi.org/10.1056/NEJMc1100395.
- Chaudhry SI, Mattera JA, Krumholz HM. The authors reply. N Engl J Med 2011;364:1079-80.
- Silber H. The challenge of comprehensive disease management in heart failure. Isr Med Assoc J 2011;13:494-5.
- Mehta PA, Dubrey SW, McIntyre HF, Walker DM, Hardman SM, Sutton GC, et al. Improving survival in the 6 months after diagnosis of heart failure in the past decade: population-based data from the UK. Heart 2009;95:1851-6. http://dx.doi.org/10.1136/hrt.2008.156034.
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326:1167-70. http://dx.doi.org/10.1136/bmj.326.7400.1167.
- Murray MD, Callaghan CM. Improving medication use for older adults: an integrated research agenda. Ann Intern Med 2003;139:425-9.
- Rochon PA, Gurwitz JH, Sykora K, Mamdani M, Streiner DL, Garfinkel S, et al. Reader's guide to critical appraisal of cohort studies: 1. Role and design. BMJ 2005;330:895-7. http://dx.doi.org/10.1136/bmj.330.7496.895.
- Conraads VM, Tavazzi L, Santini M, Oliva F, Gerritse B, Yu CM, et al. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE-HF trial. Eur Heart J 2011;32:2266-73. http://dx.doi.org/10.1093/eurheartj/ehr050.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. http://dx.doi.org/10.1136/bmj.321.7262.694.
- Bower P, Cartwright M, Hirani SP, Barlow J, Hendy J, Knapp M, et al. A comprehensive evaluation of the impact of telemonitoring in patients with long-term conditions and social care needs: protocol for the whole systems demonstrator cluster randomised trial. BMC Health Serv Res 2011;11. http://dx.doi.org/10.1186/1472-6963-11-184.
- Phillips J. Reducing admissions for long-term conditions: is telehealth the answer?. Br J Community Nurs 2012;17.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Eddy DM. Assessing medical technology. Washington DC: National Academy Press; 1985.
- Miller G, Randolph S, Forkner E, Smith B, Galbreath AD, Miller G, et al. Long-term cost-effectiveness of disease management in systolic heart failure. Med Decis Making 2009;29:325-33. http://dx.doi.org/10.1177/0272989X08327494.
- Klersy C, De Silvestri A, Gabutti G, Raisaro A, Curti M, Regoli F, et al. Economic impact of remote patient monitoring: an integrated economic model derived from a meta-analysis of randomized controlled trials in heart failure. Eur J Heart Fail 2011;13:450-9. http://dx.doi.org/10.1093/eurjhf/hfq232.
- Brazier JE, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. http://dx.doi.org/10.1016/S0167-6296(01)00130-8.
- Herbert PL, Sisk JE, Wang JJ, Tuzzio L, Casabianca JM, Chassin MR. Cost-effectiveness of nurse-led disease management for heart failure in an ethnically diverse urban community. Ann Intern Med 2008;149:540-8.
- Goehler A, Geisler BP, Manne JM, Jahn B, Conrads-Frank A, Schnell-Inderst P, et al. Decision-analytic models to simulate health outcomes and costs in heart failure: a systematic review. Pharmacoeconomics 2011;29:753-69. http://dx.doi.org/10.2165/11585990-000000000-00000.
- Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007;116:1482-7. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.696906.
- The Treasury, New Zealand . Cost Benefit Analysis Primer 2012. www.treasury.govt.nz/publications/guidance/planning/costbenefitanalysis/primer (accessed March 2012).
- Calvert MJ, Shankar A, McManus RJ, Ryan R, Freemantle N. Evaluation of the management of heart failure in primary care. Fam Pract 2009;26:145-53. http://dx.doi.org/10.1093/fampra/cmn105.
- Iqbal J, Francis L, Reid J, Murray S, Denvir M. Quality of life in patients with chronic heart failure and their carers: a 3-year follow-up study assessing hospitalization and mortality. Eur J Heart Fail 2010;12:1002-8. http://dx.doi.org/10.1093/eurjhf/hfq114.
- Miller LC, Cox KR. Case management for patients with heart failure: a quality improvement intervention. J Gerontol Nurs 2005;31:20-8.
- Yao G, Freemantle N, Flather M, Tharmanathan P, Coats A, Poole-Wilson PA. Long-term cost-effectiveness analysis of nebivolol compared with standard care in elderly patients with heart failure: an individual patient-based simulation model. Pharmacoeconomics 2008;26:879-89. http://dx.doi.org/10.2165/00019053-200826100-00007.
- Unit costs of health and social care 2011. Canterbury: PSSRU, University of Kent; 2011.
- NHS reference costs 2009–10. London: Department of Health; 2011.
- Boyne J, Vrijhoef HJ, Nieman FH, De Wit R, Kragten J, De Weerd GJ, et al. Telemonitoring in patients with heart failure: results from a multicenter randomized controlled trial (the TEHAF study). J Am Coll Cardiol 2011;57. http://dx.doi.org/10.1016/S0735-1097(11)60389-6.
- Guide to the methods of technology appraisals. London: NICE; 2008.
- Cruickshank J, Winpenny E. Telehealth: what can the NHS learn from experience at the US Veterans Health Administration?. London: 2020health.org; 2012.
- Lyndon H, Tyas D. Telehealth enhances self care and independence in people with long term conditions. Nurs Times 2010;106:12-3.
- Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure. JAMA 2005;293:572-80. http://dx.doi.org/10.1001/jama.293.5.572.
- Stoyanov N, Paul V. Clinical use of telemonitoring in chronic heart failure: keeping up with the times or misuse of time?. Curr Heart Fail Rep 2012;9:75-80. http://dx.doi.org/10.1007/s11897-011-0074-4.
- Kaplan B, Litewka S. Ethical challenges of telemedicine and telehealth. Cambridge Quarterly of Healthcare Ethics 2008;17:401-16. http://dx.doi.org/10.1017/S0963180108080535.
- Iserson KV. Telemedicine: a proposal for an ethical code. Camb Q Healthc Ethics 2000;9:404-6. http://dx.doi.org/10.1017/S0963180100003133.
- Pinna GD, Maestri R, Andrews D, Witkowski T, Capomolla S, Scanferlato JL. Home telemonitoring of vital signs and cardiorespiratory signals in heart failure patients: system architecture and feasibility of the HHH model. Int J Cardiol 2007;120:371-9. http://dx.doi.org/10.1016/j.ijcard.2006.10.029.
- Angermann CE, Stork S, Gelbrich G, Faller H, Jahns R, Frantz S. A prospective randomised trial comparing the efficacy of a standardised supraregionally transferable program for monitoring and education of patients with systolic heart failure with usual care: the Interdisciplinary Network for Heart Failure (INH) study. Circulation 2007;116.
- Clark RA. Adherence, adaptation and acceptance of elderly chronic heart failure patients to receiving healthcare via telephonemonitoring. Eur J Heart Fail 2007;9:1104-11. http://dx.doi.org/10.1016/j.ejheart.2007.07.018.
- Yallop J, Chan B, Piterman P, Tonkin A, Forbes A, Davidson PM. The Chronic Heart-failure Assistance by Telephone (CHAT) study: a new model of care to support aging rural patients with chronic disease. Asia Pac Fam Med 2006;5.
- Blum K, Janowick F, Gottlieb SS. One year changes in quality of life for heart failure patients in a home telemonitoring program. J Cardiac Fail 2006;12. http://dx.doi.org/10.1016/j.cardfail.2006.06.422.
- Zugck C, Cebola R, Frankenstein L, Nelles M, Taeger T, Pribe R, et al. Telemedicine reduces hospitalisation rates in patients with chronic heart failure – results of the randomized HiTel trial. Eur J Heart Fail Suppl 2008;7. http://dx.doi.org/10.1016/S1567-4215(08)60024-7.
- Blue L, Lang E, McMurray JJ. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ 2001;323:715-18. http://dx.doi.org/10.1136/bmj.323.7315.715.
- Dunagan WC, Littenberg B, Ewald GA, Jones CA, Emery VB, Waterman BM, et al. Randomized trial of a nurse-administered, telephone-based disease management program for patients with heart failure. J Cardiac Fail 2005;11:358-65. http://dx.doi.org/10.1016/j.cardfail.2004.12.004.
- Jerant AF, Azari R, Nesbitt TS. Reducing the cost of frequent hospital admissions for congestive heart failure: a randomized trial of a home telecare intervention. Med Care 2001;39:1234-45. http://dx.doi.org/10.1097/00005650-200111000-00010.
- Kashem A, Droogan MT, Santamore WP, Wald JW, Bove AA. Managing heart failure care using an internet-based telemedicine system. J Card Fail 2008;14:121-6. http://dx.doi.org/10.1016/j.cardfail.2007.10.014.
- Kasper EK, Gerstenblith G, Hefter G. A randomised trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol 2002;39:471-80. http://dx.doi.org/10.1016/S0735-1097(01)01761-2.
- Krumholz HM, Amatruda J, Smith GL. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. Am Coll Cardiol 2002;39:83-9. http://dx.doi.org/10.1016/S0735-1097(01)01699-0.
- McDonald K, Ledwidge K, Cahill J. Heart failure management: multidisciplinary care has intrinsic benefit above the optimization of medical care. J Card Fail 2002;8:142-8. http://dx.doi.org/10.1054/jcaf.2002.124340.
- Schwarz KA, Mion LC, Hudock D, Litman G. Telemonitoring of heart failure patients and their caregivers: a pilot randomized controlled trial. Prog Cardiovasc Nurs 2008;23:18-26.
- Adamson PB, Magalski A, Braunschweig F. Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol 2003;41:565-71. http://dx.doi.org/10.1016/S0735-1097(02)02896-6.
- Gambetta M, Dunn P, Nelson D, Herron B, Arena R. Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort. Prog Cardiovasc Nurs 2007;22:196-200. http://dx.doi.org/10.1111/j.0889-7204.2007.06483.x.
- Hudson LR, Hamar GB, Orr P, Johnson JH, Neftzger A, Chung RS, et al. Remote physiological monitoring: clinical, financial, and behavioral outcomes in a heart failure population. Dis Manag 2005;8:372-81. http://dx.doi.org/10.1089/dis.2005.8.372.
- Myers S, Grant RW, Lugn NE, Holbert B, Kvedar JC. Impact of home-based monitoring on the care of patients with congestive heart failure. Home Health Care Manag Pract 2006;18:444-51. http://dx.doi.org/10.1177/1084822306289991.
- Morguet AJ, Kuhnelt P, Kallel A, Jaster M, Schultheiss HP. Impact of telemedical care and monitoring on morbidity in mild to moderate chronic heart failure. Cardiology 2008;111:134-9. http://dx.doi.org/10.1159/000119701.
- Oeff M, Kotsch P, Gosswald A, Wolf U. Monitoring multiple cardiovascular parameters using telemedicine in patients with chronic heart failure. Herzschrittmacherther Elektrophysiol 2005;16:150-8. http://dx.doi.org/10.1007/s00399-005-0483-8.
- Roth A, Kajiloti I, Elkayam I, Sander J, Kehati M, Golovner M. Telecardiology for patients with chronic heart failure: the ‘SHL’ experience in Israel. Int J Cardiol 2004;97:49-55. http://dx.doi.org/10.1016/j.ijcard.2003.07.030.
- Scalvini S, Zanelli E, Volerrani M. A pilot study of nurse led home-based telecardiology for patients with chronic heart failure. J Telemed Telecare 2004;10:113-17. http://dx.doi.org/10.1258/135763304773391576.
- Scalvini S, Capomolla S, Zanelli E. Effect of home-based telecardiology on chronic heart failure: costs and outcomes. J Telemed Telecare 2005;11:16-8. http://dx.doi.org/10.1258/1357633054461688.
- Scalvini S, Zanelli E, Paletta L, Benigno M, Domeneghini D, De Giuli F, et al. Chronic heart failure home-based management with a telecardiology system: a comparison between patients followed by general practitioners and by a cardiology department. J Telemed Telecare 2006;12:46-8. http://dx.doi.org/10.1258/135763306777978461.
- Schofield RS, Kline SE, Schmaltuss CM. Early outcomes of a care coordination-enhanced telehome care program for elderly veterans with chronic heart failure. Telemed J E Health 2005;11:20-7. http://dx.doi.org/10.1089/tmj.2005.11.20.
- Anonymous . After-care for patients with heart failure: supporting the family physician with telephone nurses. MMW Fortschr Med 2009;151.
- Adamson PB, Abraham WT, Aaron M, Aranda JM, Bourge RC, Smith A, et al. CHAMPION trial rationale and design: the long-term safety and clinical efficacy of a wireless pulmonary artery pressure monitoring system. J Cardiac Fail 2011;17:3-10. http://dx.doi.org/10.1016/j.cardfail.2010.08.002.
- Adlbrecht C, Hulsmann M, Gwechenberger M, Strunk G, Khazen C, Wiesbauer F, et al. Outcome after device implantation in chronic heart failure is dependent on concomitant medical treatment. Eur J Clin Invest 2009;39:1073-81. http://dx.doi.org/10.1111/j.1365-2362.2009.02217.x.
- Al-Khatib SM, Piccini JP, Knight D, Stewart M, Clapp-Channing N, Sanders GD. Remote monitoring of implantable cardioverter defibrillators versus quarterly device interrogations in clinic: results from a randomized pilot clinical trial. J Cardiovasc Electrophysiol 2010;21:545-50. http://dx.doi.org/10.1111/j.1540-8167.2009.01659.x.
- Anand IS, Chopra VK, Reddy N, Chakravarthy N, Libbus I, Katra RP. Chronic multi-sensor monitoring of heart failure patients predicts acute heart failure decompensation events: results from the MUSIC-Asia study. Eur Heart J 2010;31.
- Anand IS, Greenberg BH, Fogoros RN, Libbus I, Katra RP, Music I, et al. Design of the Multi-Sensor Monitoring in Congestive Heart Failure (MUSIC) study: prospective trial to assess the utility of continuous wireless physiologic monitoring in heart failure. J Cardiac Fail 2011;17:11-6. http://dx.doi.org/10.1016/j.cardfail.2010.08.001.
- Antonicelli R, Mazzanti I, Abbatecola AM, Parati G. Impact of home patient telemonitoring on use of ß-blockers in congestive heart failure. Drugs Aging 2010;27:801-5. http://dx.doi.org/10.2165/11538210-000000000-00000.
- Arya A, Block M, Kautzner J, Lewalter T, Mortel H, Sack S, et al. Influence of home monitoring on the clinical status of heart failure patients: design and rationale of the IN-TIME study. Eur J Heart Fail 2008;10:1143-8. http://dx.doi.org/10.1016/j.ejheart.2008.08.004.
- Bardy GH, McCullough PA. Results of the home automatic external defibrillator trial (HAT). ACC Cardiosource Rev J 2008;17:4-8.
- Bardy GH, Lee KL, Mark DB, Poole JE, Toff WD, Tonkin AM, et al. Rationale and design of the Home Automatic External Defibrillator Trial (HAT). Am Heart J 2008;155:445-54. http://dx.doi.org/10.1016/j.ahj.2007.12.008.
- Bento VF, Brofman PR. Impact of the nursing consultation on the frequency of hospitalizations in patients with heart failure in Curitiba, Parana State. Arq Bras Cardiol 2009;92:454-60.
- Benvenuto M, Beltrami L, Pizzocri S, Rossetti G, Redaelli C, Rainoldi M, et al. Management for chronic congestive heart failure (CHF): our experience of non-invasive home telemonitoring (HTM). J Hypertension 2008;26:S380-1.
- Benvenuto M, Beltrami L, Solari D, Esposito A, Rossettti G, Pernigotti A, et al. Management for Chronic Congestive Heart Failure (CHF): our experience of non-invasive home telemonitoring (HTM). J Hypertension 2009;27.
- Berkley R, Bauer S, Rowland C. How telehealth can increase the effectiveness of chronic heart failure management. Nurs Times 2010;106:14-5.
- Biddiss E, Brownsell S, Hawley MS. Predicting need for intervention in individuals with congestive heart failure using a home-based telecare system. J Telemed Telecare 2009;15:226-31. http://dx.doi.org/10.1258/jtt.2009.081203.
- Bocchi EA, Cruz F, Guimaraes G, Pinho Moreira LF, Issa VS, Ayub Ferreira SM, et al. Long-term prospective, randomized, controlled study using repetitive education at six-month intervals and monitoring for adherence in heart failure outpatients: the REMADHE trial. Circulation Heart Fail 2008;1:115-24. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.107.744870.
- Boehmer JP, Seth M, Jones PW, Meyer TE, Saxon LA. The relationship of the LATITUDE patient symptom report to heart failure hospitalization. J Cardiac Fail 2009;15:S112-13. http://dx.doi.org/10.1016/j.cardfail.2009.06.030.
- Boriani G, Diemberger I, Mantovani V, Biffi M, Martignani C. Remote monitoring of patients with an implanted device and patients' outcomes: the potential for ‘win–win’ dynamics. J Cardiovasc Electrophysiol 2009;20:1252-4. http://dx.doi.org/10.1111/j.1540-8167.2009.01576.x.
- Boveda S, Marijon E, Jacob S, Defaye P, Winter JB, Bulava A, et al. Incidence and prognostic significance of sustained ventricular tachycardias in heart failure patients implanted with biventricular pacemakers without a back-up defibrillator: results from the prospective, multicentre, Mona Lisa cohort study. Eur Heart J 2009;30:1237-44. http://dx.doi.org/10.1093/eurheartj/ehp071.
- Bover FR, Bas VM, Perez-Castellano N, Moreno PJ, Morales PR, Perez-Villacastin J, et al. CareLink home telemonitoring system: initial clinical experience. Eur J Heart Fail Suppl 2009;8.
- Bowles KH, Horowitz DA. The advantages and disadvantages of heart failure disease management when randomly assigned to be delivered by telehealth or telephone. J Cardiac Fail 2008;14. http://dx.doi.org/10.1016/j.cardfail.2008.06.025.
- Bowles KH, Holland DE, Horowitz DA. A comparison of in-person home care, home care with telephone contact and home care with telemonitoring for disease management. J Telemed Telecare 2009;15:344-50. http://dx.doi.org/10.1258/jtt.2009.090118.
- Boxer RS, Burant CJ, DiCarlo CM, Struk C, Madigan E, Pina HL. Home telemonitoring improves health status in the post-hospitalization period for patients with heart failure. J Cardiac Fail 2010;16. http://dx.doi.org/10.1016/j.cardfail.2010.06.319.
- Boyne JJJ, Ramaekers B, De Wit R, Gorgels APM, Vrijhoef HJM. Adherence to medication and non-pharmacological recommendations in telemonitored heart failure patients. Eur J Heart Fail Suppl 2009;8.
- Boyne JJ, Vrijhoef HJ, De Wit R, Gorgels AP, Boyne JJJ, Vrijhoef HJM, et al. Telemonitoring in patients with heart failure, the TEHAF study: study protocol of an ongoing prospective randomised trial. Int J Nurs Stud 2011;48:94-9. http://dx.doi.org/10.1016/j.ijnurstu.2010.05.017.
- Boyne JJ. Gothenburg, Sweden: Heart Failure Congress; 2011.
- Brandon A, Ellison KJ, Schuessler J, Lazenby R. The effects of a clinical nurse specialist-led, telephone-based intervention on hospital readmissions, quality of life, and self-care behaviors of heart failure patients. Clin Nurse Spec 2009;23:99-100. http://dx.doi.org/10.1097/01.NUR.0000325413.53976.90.
- Brandon AF, Schuessler JB, Ellison KJ, Lazenby RB. The effects of an advanced practice nurse led telephone intervention on outcomes of patients with heart failure. Appl Nurs Res 2009;22:e1-7. http://dx.doi.org/10.1016/j.apnr.2009.02.003.
- Braunschweig F, Ford I, Conraads V, Cowie MR, Jondeau G, Kautzner J, et al. Can monitoring of intrathoracic impedance reduce morbidity and mortality in patients with chronic heart failure? Rationale and design of the Diagnostic Outcome Trial in Heart Failure (DOT-HF). Eur J Heart Fail 2008;10:907-16. http://dx.doi.org/10.1016/j.ejheart.2008.06.016.
- Broesch L, Heywood JT. Device diagnostics data by remote monitoring provides time efficient method of managing heart failure patients. J Cardiac Fail 2009;15. http://dx.doi.org/10.1016/j.cardfail.2009.06.025.
- Brotons C, Falces C, Alegre J, Ballarin E, Casanovas J, Cata T, et al. Randomized clinical trial of the effectiveness of a home-based intervention in patients with heart failure: the IC-DOM study. Rev Esp Cardiol 2009;62:400-8. http://dx.doi.org/10.1016/S0300-8932(09)70897-8.
- Burri H, Quesada A, Ricci RP, Boriani G, Davinelli M, Favale S, et al. The MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE-CARE) study: rationale and design. Am Heart J 2010;160:42-8. http://dx.doi.org/10.1016/j.ahj.2010.04.005.
- Cardozo L, Steinberg J. Telemedicine for recently discharged older patients. Telemed J E Health 2010;16:49-55. http://dx.doi.org/10.1089/tmj.2009.0058.
- Carson LK, Bella LD. Heart failure outreach pilot program (HFOPP). Heart Lung 2009;38:273-4. http://dx.doi.org/10.1016/j.hrtlng.2009.03.022.
- Catanzariti D, Lunati M, Landolina M, Zanotto G, Lonardi G, Iacopino S, et al. Monitoring intrathoracic impedance with an implantable defibrillator reduces hospitalizations in patients with heart failure. Pacing Clin Electrophysiol 2009;32:363-70. http://dx.doi.org/10.1111/j.1540-8159.2008.02245.x.
- Chen YH, Ho YL, Huang HC, Wu HW, Lee CY, Hsu TP, et al. Assessment of the clinical outcomes and cost-effectiveness of the management of systolic heart failure in Chinese patients using a home-based intervention. J Int Med Res 2010;38:242-52. http://dx.doi.org/10.1177/147323001003800129.
- Clark RA, Inglis SC, McAlister FA, Ball J, Lewinter C, Cullington D, et al. Remote (non-invasive) monitoring in heart failure: effect on length of stay, quality of life, knowledge, adherance and satisfaction in 8,323 heart failure patients: a systematic review. Eur Heart J 2010;31:944-5.
- Cleland JG, Coletta AP, Castiello T, Clark AL. Clinical trials update from the European Society of Cardiology Heart Failure meeting 2011: TEHAF, WHICH, CARVIVA, and atrial fibrillation in GISSI-HF and EMPHASIS-HF. Eur J Heart Fail 2011;13:1147-51. http://dx.doi.org/10.1093/eurjhf/hfr119.
- Copeland LA, Berg GD, Johnson DM, Bauer RL. An intervention for VA patients with congestive heart failure. Am J Manag Care 2010;16:158-65.
- Cowie MR. ‘Hospital at home’ care shows similar mortality and subsequent hospital admissions to hospital care for older patients with acutely decompensated chronic heart failure. Evid Based Med 2010;15:9-10. http://dx.doi.org/10.1136/ebm.15.1.9.
- Cowie MR, Conraads V, Tavazzi L, Yu CM. SENSE-HF Investigators . Rationale and design of a prospective trial to assess the sensitivity and positive predictive value of implantable intrathoracic impedance monitoring in the prediction of heart failure hospitalizations: the SENSE-HF study. J Cardiac Fail 2009;15:394-400. http://dx.doi.org/10.1016/j.cardfail.2008.12.004.
- Crawford K, Volkman F. Management of high-risk Medicaid congestive heart failure and comorbid patients using remote patient monitoring. Crit Pathways Cardiol 2010;9.
- Crossley G, Boyle A, Vitense H, Sherfesee L, Mead RH. Trial design of the clinical evaluation of remote notification to reduce time to clinical decision: the Clinical evaluation Of remote NotificatioN to rEduCe Time to clinical decision (CONNECT) study. Am Heart J 2008;156:840-6. http://dx.doi.org/10.1016/j.ahj.2008.06.028.
- Crossley GH, Chen J, Choucair W, Cohen TJ, Gohn DC, Johnson WB, et al. Clinical benefits of remote versus transtelephonic monitoring of implanted pacemakers. J Am Coll Cardiol 2009;54:2012-19. http://dx.doi.org/10.1016/j.jacc.2009.10.001.
- Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol 2011;57:1181-9. http://dx.doi.org/10.1016/j.jacc.2010.12.012.
- Dansky KH, Vasey J, Bowles K. Impact of telehealth on clinical outcomes in patients with heart failure. Clin Nurs Res 2008;17:182-99. http://dx.doi.org/10.1177/1054773808320837.
- Dansky KH, Vasey J, Bowles K. Use of telehealth by older adults to manage heart failure. Res Gerontol Nurs 2008;1:25-32. http://dx.doi.org/10.3928/19404921-20080101-01.
- Dansky K, Vasey J. Managing heart failure patients after formal homecare. Telemed J E Health 2009;15:983-91. http://dx.doi.org/10.1089/tmj.2009.0064.
- Dar O, Riley J, Chapman C, Dubrey SW, Morris S, Rosen S, et al. The Home Heart Failure Study (Home-HF): a randomised controlled trial of home telemonitoring of heart failure patients at high risk of readmission and death. Heart 2008;94:A106-7.
- De Vries AE, Kraai IH, Otten RM, De Jong RM, Van Dijk RB, van Veldhuisen DJ, et al. The value of innovative ICT guided disease management combined with telemonitoring in outpatient clinics for chronic heart failure patients (IN TOUCH trial). Eur J Heart Fail Suppl 2010;11:S103-4.
- Delaney C, Apostolidis B. Pilot testing of a multicomponent home care intervention for older adults with heart failure: an academic clinical partnership. J Cardiovasc Nurs 2010;25:E27-40.
- Desai AS, Stevenson LW. Connecting the circle from home to heart-failure disease management. N Engl J Med 2010;363:2364-7. http://dx.doi.org/10.1056/NEJMe1011769.
- Domingo M, Lupon J, Gonzalez B, Ramos A, Lopez R, Crespo E, et al. Noninvasive remote telemonitoring for ambulatory patients with heart failure: effect on number of hospitalizations, days in hospital, and quality of life. CARME (CAtalan Remote Management Evaluation) study. Rev Esp Cardiol 2011;64:277-85. http://dx.doi.org/10.1016/j.recesp.2010.10.032.
- Domingo M, Lupon J, Gonzalez B, Ramos A, Lopez R, Crespo E, et al. Evaluation of a telemedicine system for heart failure patients: feasibility, acceptance rate, satisfaction and changes in patient behaviour. Results from the CARME (CAtalan Remote Management Evaluation) study. Eur J Cardiovasc Nurs 2012;11:410-18.
- Duffy JR, Hoskins LM, Dudley-Brown S. Improving outcomes for older adults with heart failure: a randomized trial using a theory-guided nursing intervention. J Nurs Care Qual 2010;25:56-64. http://dx.doi.org/10.1097/NCQ.0b013e3181ad0fbd.
- Ewald GA, Gilliam FR, Sweeney RJ. Automated HF decompensation detection: results from the decompensation detection study (DECODE). J Cardiac Fail 2009;15. http://dx.doi.org/10.1016/j.cardfail.2009.06.065.
- Ewald GA, Gilliam FR, Sweeney RJ. Patient risk stratification for HF decompensation. J Cardiac Fail 2009;15. http://dx.doi.org/10.1016/j.cardfail.2009.06.054.
- Fan HH, Shi HY, Jin W, Zhu YJ, Huang DN, Yan YW, et al. Effects of integrated disease management program on the outcome of patients with heart failure. Chung-Hua Hsin Hsueh Kuan Ping Tsa Chih 2010;38:592-6.
- Ferrante D, Varini S, Macchia A, Soifer S, Badra R, Nul D, et al. Long-term results after a telephone intervention in chronic heart failure: DIAL (Randomized Trial of Phone Intervention in Chronic Heart Failure) follow-up. J Am Coll Cardiol 2010;56:372-8. http://dx.doi.org/10.1016/j.jacc.2010.03.049.
- Finkelstein J, Dennison CR. A pilot study of home automated telemanagement (HAT) system in African Americans with congestive heart failure n.d.:90-4.
- Finkelstein J, Wood J, Cha E, Orlov A, Dennison C, Finkelstein J, et al. Feasibility of congestive heart failure telemanagement using a Wii-based telecare platform. Conf Proc IEEE Eng Med Biol Soc 2010;2010:2211-14.
- Fursse J, Clarke M, Jones R, Khemka S, Findlay G. Early experience in using telemonitoring for the management of chronic disease in primary care. J Telemed Telecare 2008;14:122-4. http://dx.doi.org/10.1258/jtt.2008.003005.
- Germany R, Neisen KB, Kueffer FJ, Naftilan AJ. Integrating device diagnostic data into a disease management care model. Heart Lung 2009;38. http://dx.doi.org/10.1016/j.hrtlng.2009.03.017.
- Goernig M, Doede T, Brehm B, Figulla HR, Leder U. Ambulatory disease management in cardiac patients: 12 month follow-up of home care telemedicine in Thuringia by the Management Program Zertiva (R). Phys Med Rehab Kuror 2009;19:9-13. http://dx.doi.org/10.1055/s-0028-1112113.
- Goernig M, Kwetkat A, Brehm B, Fidorra K, Roth A, Figulla HR, et al. Feasibility and effectiveness of home care telemedicine in patients with heart failure in Thuringia. IFMBE Proc 2009;25:79-81. http://dx.doi.org/10.1007/978-3-642-03904-1_21.
- Gonzalez B, Domingo M, Lupon J, Lopez R, Ramos A, Crespo E, et al. Changes in patients' behaviour and impact on quality of life with the use of telemedicine (Motiva-Philips) in an heart failure unit: the CARME study (CAtalan Remote Management Evaluation). Eur Heart J 2010;31.
- Haddour N. Effect of moderate or intensive disease management program on outcome in patients with heart failure: the COACH study. MT Cardio 2008;4:133-6.
- Hannah J, Humphrey G, Doughty R, McGrinder H, Bos N, Bowman C. Telehealth in heart failure management: a proof of principle study. Heart Lung Circ 2010;19:S13-14. http://dx.doi.org/10.1016/j.hlc.2010.04.028.
- Remote monitoring of patients with congestive heart failure. Lansdale, PA: HAYES Inc.; 2008.
- Holden S, Yaman D, Mattina C, Feuker C, Fancelli J, Wakade O, et al. Clinical initiatives to decrease 30-day readmission rates and improve quality of care in patients with heart failure. Crit Pathw Cardiol 2011;10.
- Houston-Feenstra L, Chiong J, Jutzy K. Gender comparison of outcomes 48 months following a disease management and rehabilitation program for patients with congestive heart failure and patients followed by routine care for CHF. Chest 2008;134.
- Howlett JG, Marr D, Palmer K, O’Neill BJ, Rajda M. A comparison of primary care, home-based telemonitoring, telemonitoring with video, or specialist directed heart failure clinic care for patients with high risk heart failure. Circulation 2011;124.
- Jacobs B. Reducing heart failure hospital readmissions from skilled nursing facilities. Prof Case Manag 2011;16:18-24.
- Katra R, Chakravarthy N, Libbus I. Remote monitoring of heart failure status using an external multi-sensor device may reduce heart failure-related hospitalization. Circulation 2010;122.
- Knotter N, Meregalli PG, Kok WE, De Voogt WG, De Beurs M, Cherpanath-Paes C, et al. Home telemonitoring in congestive heart failure: patients perspective. Eur J Heart Fail Suppl 2010;9.
- Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Bohm M, et al. Telemedical Interventional Monitoring in Heart Failure (TIM-HF), a randomized, controlled intervention trial investigating the impact of telemedicine on mortality in ambulatory patients with heart failure: study design. Eur J Heart Fail 2010;12:1354-62. http://dx.doi.org/10.1093/eurjhf/hfq199.
- Konstam V, Gregory D, Chen J, Weintraub A, Patel A, Levine D, et al. Health-related quality of life in a multicenter randomized controlled comparison of telephonic disease management and automated home monitoring in patients recently hospitalized with heart failure: SPAN-CHF II trial. J Cardiac Fail 2011;17:151-7. http://dx.doi.org/10.1016/j.cardfail.2010.08.012.
- Kraai IH, Otten RM, De Vries AE, De Jong RM, Van Dijk RB, van Veldhuisen DJ, et al. INnovative ICT guided disease management and telemonitoring in out patient clinics for chronic heart failure patients (IN TOUCH study design): a disease management study with patient oriented outcomes. Eur J Cardiovasc Nurs 2010;9. http://dx.doi.org/10.1016/S1474-5151(10)60038-2.
- Kriegeskorte V. Heart failure: comparison of disease management programs. Gesundheitsokonomie Qualitatsmanagement 2008;13.
- Kulshreshtha A, Nieves R, Kvedar JC, Watson AJ. Remote monitoring program may improve outcomes for heart failure patients. Circulation 2008;118.
- Kurtz B, Lemercier M, Pouchin SC, Benmokhtar E, Vallet C, Cribier A, et al. Automated home telephone self-monitoring reduces hospitalization in patients with advanced heart failure. J Telemed Telecare 2011;17:298-302. http://dx.doi.org/10.1258/jtt.2011.100901.
- LaFramboise LM, Woster J, Yager A, Yates BC. A technological life buoy: patient perceptions of the Health Buddy. J Cardiovasc Nurs 2009;24:216-24.
- Mainardi L, Iazzolino E, Asteggiano R, Lusardi R, Varbella F, Sasso L, et al. Comparison between telephone and outpatient nursing management in patients with chronic heart failure in a large territorial area in Piedmont, Italy. G Ital Cardiol 2010;11:35-42.
- Margolis K, Kerby T, Asche S, Cooper S, Loes L, Maciosek M, et al. PS1–04: home blood pressure telemonitoring and case management to control hypertension: hyperlink trial design and baseline characteristics. Clin Med Res 2010;8. http://dx.doi.org/10.3121/cmr.2010.943.ps1-04.
- Margolis KL, Kerby TJ, Asche SE, MacIosek MV, Meyers PJ, Sperl-Hillen JM, et al. Home blood pressure telemonitoring and case management to control hypertension: hyperlink design, baseline characteristics, and intervention adherence. J Clin Hypertens 2010;12:A81-2.
- Maric B, Kaan A, Araki Y, Ignaszewski A, Lear SA. The use of the internet to remotely monitor patients with heart failure. Telemed J E Health 2010;16:26-33. http://dx.doi.org/10.1089/tmj.2009.0094.
- Masella C, Zanaboni P, Di Stasi F, Gilardi S, Ponzi P, Valsecchi S, et al. Assessment of a remote monitoring system for implantable cardioverter defibrillators. J Telemed Telecare 2008;14:290-4. http://dx.doi.org/10.1258/jtt.2008.080202.
- McEntee ML, Johnson BJ, Dennison CR, Hopkins J. Establishing feasibility of a telemanagement system to facilitate self care among African American heart failure patients. Heart Lung 2010;39:370-1. http://dx.doi.org/10.1016/j.hrtlng.2010.05.039.
- Melillo P, Pecchia L, Bracale M. Interactive voice response system for home telemonitoring of heart failure patients. World Congress on Medical Physics and Biomedical Engineering, Munich Germany, 7–12 September 2009. IFMBE Proc 2009;25:153-6. http://dx.doi.org/10.1007/978-3-642-03904-1_43.
- Merchant FM, Dec GW, Singh JP. Implantable sensors for heart failure. Circ Arrhythm Electrophysiol 2010;3:657-67. http://dx.doi.org/10.1161/CIRCEP.110.959502.
- Meriggi P, Rizzo F, Faini A, Chiarugi F, Karatzanis I, Zacharioudakis G, et al. A new simple multimodal platform for home monitoring of cardiac patients through textile technology. Comput Cardiol 2009;36:93-6.
- Metten L, Zucca F, Haver Y, Neukirch B, Rauchhaus M. Effects of intensified care for heart failure patients by telemonitoring. Eur J Cardiovasc Nurs 2011;10. http://dx.doi.org/10.1016/S1474-5151(11)60096-0.
- Morguet AJ, Kuhnelt P, Kallel A, Rauch U, Schultheiss HP. Utilization of telemedicine by heart disease patients following hospitalization. J Telemed Telecare 2008;14:178-81. http://dx.doi.org/10.1258/jtt.2007.070602.
- Mullens W, Kelly L, Verga T, Abrahams Z, Tang WHW. Feasibility and validation of clinical status with internet-based remote intrathoracic impedance monitoring in patients with heart failure. J Am Coll Cardiol 2008;51.
- Naccarella F, Enrico A, Lachetti F, Sun LL, Zhang F. Italian telemedicine and telecardiology the fragile old patient, home monitoring of chronic patients. Mediterranean J Pacing Electrophysiol 2008;10:1-7.
- Nathani A, Braun RL, Rahmatullah AF. Multidisciplinary outpatient management of chronic heart failure to reduce 30 day and 90 day readmission in high risk patients. J Cardiac Fail 2010;16. http://dx.doi.org/10.1016/j.cardfail.2010.06.332.
- Nikus K, Lahteenmaki J, Lehto P, Eskola M. The role of continuous monitoring in a 24/7 telecardiology consultation service – a feasibility study. J Electrocardiol 2009;42:473-80. http://dx.doi.org/10.1016/j.jelectrocard.2009.07.005.
- Oliveira LPJ, Verga T, Wilkoff BL, Tang WHW. Insights from intra-thoracic threshold crossings in ambulatory patients with heart failure from a remote, nurse-run, internet-based surveillance program. J Cardiac Fail 2009;15.
- Paget T, Jones C, Davies M, Evered C, Lewis C. Using home telehealth to empower patients to monitor and manage long term conditions. Nursing Times 2010;106:17-9.
- Perl S, Stiegler P, Rotman B, Prenner G, Lercher P, Anelli-Monti M, et al. Remote control of implanted cardioverter defibrillator devices in heart failure patients: a safe, efficient and cost-saving method to reduce routine device follow ups (The SAVE-Trial). Eur J Heart Fail Suppl 2011;10.
- Persson H, Lofsjogard J, Melin M, Jaraj D, Fjellner A, Straat E. Results of a randomized clinical study evaluating nurse-driven telemedicine interventions for high risk chronic heart failure patients with frequent rehospitalizations. Eur J Heart Fail Suppl 2011;10:S103-4.
- Ramaekers BLT, Janssen-Boyne JJ, Gorgels APM, Vrijhoef HJM. Adherence among telemonitored patients with heart failure to pharmacological and nonpharmacological recommendations. Telemed E Health 2009;15:517-24. http://dx.doi.org/10.1089/tmj.2009.0160.
- Raval N, Abraham W, Adamson P, Chronos N, Sachar R, Ginn G. Long-term effects of implantable hemodynamic monitoring in patients with moderate heart failure. Eur Heart J 2011;32.
- Ricci RP, Morichelli L, Santini M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: impact on medical management and health-care resource utilization. Europace 2008;10:164-70. http://dx.doi.org/10.1093/europace/eum289.
- Riley JP. Telemonitoring or structured telephone support for people with chronic heart failure reduces CHF-related hospital admissions; telemonitoring also reduces all-cause mortality. Evid Based Nurs 2011;14:27-8. http://dx.doi.org/10.1136/ebn1116.
- Riley J, Dar O, Chapman C, Dubrey S, Rosen S, Gabe J, et al. Telemonitoring in heart failure, accessibility and acceptability: the Home-HF study. Heart 2008;94.
- Riley J, Dar O, Chapman C, Dubrey SW, Rosen SD, Gabe JPN, et al. Telemonitoring in heart failure increases patients' confidence in self care but identifies additional healthcare needs. The Home-HF study. Eur J Heart Fail Suppl 2009;8.
- Rosa MA. How a heart failure home care disease management program makes a difference. Home Healthc Nurse 2008;26:483-90. http://dx.doi.org/10.1097/01.NHH.0000335607.84095.82.
- Rosati RJ. Evaluation of remote monitoring in home health care n.d.:151-3.
- Santamore WP, Homko CJ. Understanding how the patient interacts with internet intervention is key to advancing telemedicine. J Cardiovasc Nurs 2008;23:472-3.
- Scalvini S, Zanelli E, De GF, Vigliani F, Rivadossi F, Cinelli A, et al. Homecare telesurveillance program in patients with CHF: evaluation of clinical changes over time. Eur J Heart Fail Suppl 2010;9.
- Schweinzer M. Criteria – tele monitoring in chronic heart failure and after cardiac surgery. Ehealth 2009 – Med Inform Meets Ehealth 2009:147-52.
- Seibert PS, Whitmore TA, Patterson C, Parker PD, Otto C, Basom J, et al. Telemedicine facilitates CHF home health care for those with systolic dysfunction. Int J Telemed Appl 2008. http://dx.doi.org/10.1155/2008/235031.
- Seto E, Leonard KJ, Cafazzo JA, Masino C, Barnsley J, Ross HJ. Mobile phone-based remote patient monitoring improves heart failure management and outcomes: a randomized controlled trial. J Am Coll Cardiol 2011;57. http://dx.doi.org/10.1016/S0735-1097(11)61260-6.
- Smith P, May C, Santamaria N, Hendrie D, Sheehan M, Hung J, et al. Randomised comparison of remote monitoring versus enhanced standard care for heart failure. Heart Lung Circ 2011;20. http://dx.doi.org/10.1016/j.hlc.2011.05.186.
- Sonntag S, Sohn HY, Klauss V, Ziegler M, Moehlmann H, Kleber FX. The disease management and telemonitoring program CORDIVA for patients with heart failure reduces hospitalizations and costs: 2 years experience. Eur J Heart Fail Suppl 2010;9.
- Sprenger C, Oeff M. Telemonitoring in patients with chronic heart failure – prevention of hospital admissions. Eur J Heart Fail Suppl 2009;8.
- Stevenson LW. Counting performance with therapies for heart failure: aiming for quality or quantity?. Circulation 2010;122:561-6. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.970061.
- Stewart S, Carrington MJ, Lee G, Jennings G, Wong C. High risk for heart failure: diastolic dysfunction and left ventricular hypertrophy in the nurse-led intervention for less chronic heart failure (NIL-CHF) study cohort. Eur Heart J 2010;8.
- Stork S, Faller H, Schowalter M, Ertl G, Angermann CE. Evidence-based disease management in patients with heart failure (HeartNetCare-HF Wurzburg). Dtsch Med Wochenschr 2009;134:773-6.
- Strobeck JE, Wilkoff BL, Silver M. Remote cardiac monitoring in patients with heart failure. Cong Heart Fail 2008;14:4-6. http://dx.doi.org/10.1111/j.1751-7133.2008.tb00013.x.
- Takahashi PY, Hanson GJ, Pecina JL, Stroebel RJ, Chaudhry R, Shah ND, et al. A randomized controlled trial of telemonitoring in older adults with multiple chronic conditions: the Tele-ERA study. BMC Health Serv Res 2010;10. http://dx.doi.org/10.1186/1472-6963-10-255.
- Talukder RM, Pray WS. Automated external defibrillators in the community setting. US Pharm 2009;34:12-5.
- Tang WHW, Small RS, Heywood JT, Andriulli J. Device-based remote monitoring as contemporary heart failure disease management: baseline characteristics of patients enrolled in the OptiVol Care Pathway study. J Cardiac Fail 2010;16. http://dx.doi.org/10.1016/j.cardfail.2010.06.240.
- Tang WWH, Chopra VK, Chakravarthy N, Libbus I, Katra RP. External wireless monitoring of bioimpedance in heart failure patients: results from the ACUTE study. J Cardiac Fail 2010;16:S70-1. http://dx.doi.org/10.1016/j.cardfail.2010.06.244.
- Taylor M. Tele-case management lets Iowa heart patients go home and stay there. Hosp Health Netw 2008;82.
- Thompson DR. Telehome monitoring reduced readmissions and improved quality of life in heart failure or angina. Evid Based Nurs 2008;11. http://dx.doi.org/10.1136/ebn.11.3.86.
- Tompkins C, Orwat J. A randomized trial of telemonitoring heart failure patients. J Healthc Manag 2010;55:312-22.
- Trembath R, Azucena C, Stain N, Cowie MR. Remote device monitoring for CRT-D leads to substantial reduction in the need for ‘routine’ pacing clinic. Eur J Heart Fail Suppl 2009;8.
- van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011;124:1719-26. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.043042.
- Vanderheyden M, Houben R, Verstreken S, Stahlberg M, Reiters P, Kessels R, et al. Continuous monitoring of intrathoracic impedance and right ventricular pressures in patients with heart failure. Circ Heart Fail 2010;3:370-7. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.109.867549.
- Varma N, Pavri B, Michalski J, Stambler B. Do heart failure patients with ICDs managed remotely suffer increased adverse event rates? Automatic remote monitoring compared to conventional follow up in the TRUST trial. Europace 2011;13.
- Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. TRUST Investigators . Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010;122:325-32. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.937409.
- Vercauteren S, Castadot M, Vanhalewyn M, Boulanger S, Robert A, Bouvy C, et al. BELGIUM-HF (Better Efficacy in Lowering events by General practitioner's Intervention Using remote Monitoring in Heart Failure): concept and feasibility. Acta Cardiol 2009;64.
- Weintraub A, Gregory D, Patel AR, Levine D, Venesy D, Perry K, et al. A multicenter randomized controlled evaluation of automated home monitoring and telephonic disease management in patients recently hospitalized for congestive heart failure: the SPAN-CHF II trial. J Cardiac Fail 2010;16:285-92. http://dx.doi.org/10.1016/j.cardfail.2009.12.012.
- Wexler R. Early physician follow-up and 30-day readmission among older patients with heart failure. JAMA 2010;304. http://dx.doi.org/10.1001/jama.2010.1147.
- Whellan DJ, Ousdigian KT, Al-Khatib SM, Pu W, Sarkar S, Porter CB, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol 2010;55:1803-10. http://dx.doi.org/10.1016/j.jacc.2009.11.089.
- Wootton R, Gramotnev H, Hailey D. A randomized controlled trial of telephone-supported care coordination in patients with congestive heart failure. J Telemed Telecare 2009;15:182-6. http://dx.doi.org/10.1258/jtt.2009.081212.
- Zile MR, Bourge RC, Bennett TD, Stevenson LW, Cho YK, Adamson PB, et al. Application of implantable hemodynamic monitoring in the management of patients with diastolic heart failure: a subgroup analysis of the COMPASS-HF trial. J Cardiac Fail 2008;14:816-23. http://dx.doi.org/10.1016/j.cardfail.2008.07.235.
- Zucca F. Guideline based remote monitoring and 24/7 telephone support for people with heart failure: an opportunity to improve outcomes in Germany. Clin Res Cardiol 2001;100.
- Almond C, Latimer J, Brereton NJ, Chapman AM. The budgetary impact of implementing a telehealth home monitoring system for chronic heart failure patients in a typical UK primary care trust. Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.08.762.
- Benatar D, Bondmass M, Ghitelman J, Avitall B. Outcomes of chronic heart failure. Arch Intern Med 2003;163:347-52. http://dx.doi.org/10.1001/archinte.163.3.347.
- Berg GD, Wadhwa S, Johnson AE. A matched-cohort study of health services utilization and financial outcomes for a heart failure disease-management program in elderly patients. J Am Geriatr Soc 2004;52:1655-61. http://dx.doi.org/10.1111/j.1532-5415.2004.52457.x.
- Chan DC, Heidenreich PA, Weinstein MC, Fonarow GC. Heart failure disease management programs: a cost-effectiveness analysis. Am Heart J 2008;155:332-8. http://dx.doi.org/10.1016/j.ahj.2007.10.001.
- Davalos ME, French MT, Burdick AE, Simmons SC. Economic evaluation of telemedicine: review of the literature and research guidelines for benefit–cost analysis. Telemed J E Health 2009;15:933-48. http://dx.doi.org/10.1089/tmj.2009.0067.
- Eapen ZJ, Reed SD, Curtis LH, Hernandez AF, Peterson ED. Do heart failure disease management programs make financial sense under a bundled payment system?. Am Heart J 2011;161:916-22. http://dx.doi.org/10.1016/j.ahj.2011.02.016.
- Gregory D, Kimmelstiel C, Perry K, Parikh A, Konstam V, Konstam MA, et al. Hospital cost effect of a heart failure disease management program: the Specialized Primary and Networked Care in Heart Failure (SPAN-CHF) trial. Am Heart J 2006;151:1013-18. http://dx.doi.org/10.1016/j.ahj.2005.06.039.
- Postmus D, Abdul Pari AA, Jaarsma T, Luttik ML, van Veldhuisen DJ, Hillege HL, et al. A trial-based economic evaluation of 2 nurse-led disease management programs in heart failure. Am Heart J 2011;162:1096-104. http://dx.doi.org/10.1016/j.ahj.2011.09.019.
- Rojas SV, Gagnon MP. A systematic review of the key indicators for assessing telehomecare cost-effectiveness. Telemed J E Health 2008;14:896-904. http://dx.doi.org/10.1089/tmj.2008.0009.
- Scalvini S, Capomolla S, Zanelli E, Corra U, Longobardi GL, Ricci VA, et al. Effect of a home-based telecardiology on chronic heart failure: cost evaluation. Eur Heart J 2004;25.
- Seto E. Cost comparison between telemonitoring and usual care of heart failure: a systematic review. Telemed J E Health 2008;14:679-86. http://dx.doi.org/10.1089/tmj.2007.0114.
- Smith B, Hughes-Cromwick PF, Forkner E, Galbreath AD. Cost-effectiveness of telephonic disease management in heart failure. Am J Manag Care 2008;14:106-15.
- Soran OZ, Feldman AM, Pina IL, Lamas GA, Kelsey SF, Selzer F, et al. Cost of medical services in older patients with heart failure: those receiving enhanced monitoring using a computer-based telephonic monitoring system compared with those in usual care: the Heart Failure Home Care trial. J Cardiac Fail 2010;16:859-66. http://dx.doi.org/10.1016/j.cardfail.2010.05.028.
- Stafylas P, Dafoulas G, Aletras VH, Lashos V, Raptis O. Cost–utility analysis of home telemonitoring in elderly patients with chronic heart failure. Value Health 2008;11. http://dx.doi.org/10.1016/S1098-3015(10)66350-4.
- Stewart S, Blue L, Walker A, Morrison C, McMurray JJ. An economic analysis of specialist heart failure nurse management in the UK; can we afford not to implement it?. Eur Heart J 2002;23:1369-78. http://dx.doi.org/10.1053/euhj.2001.3114.
- Stone PW. Nurse-led heart failure management improved quality of life and was cost-effective. Evid Based Nurs 2009;12. http://dx.doi.org/10.1136/ebn.12.2.59.
- Van Montfort APWP, van der Helm MHJ. Telemonitoring of patients with chronic heart failure. Dis Manag Health Outcome 2006;14:33-5. http://dx.doi.org/10.2165/00115677-200614001-00009.
Appendix 1 Home telemonitoring or structured telephone support programmes for patients with heart failure: literature search strategy, a MEDLINE example
Home telemonitoring or structured telephone support programmes for patients with heart failure: literature search strategy, a MEDLINE example (PDF download)
Appendix 2 Methodological assessment (adapted) criteria for randomised controlled trials and observational studies
Methodological assessment (adapted) criteria for randomised controlled trials and observational studies (PDF download)
Appendix 3 Statistical model used to analyse the data
Appendix 4 Comparison of included studies from existing reviews
Comparison of included studies from existing reviews (PDF download)
Appendix 5 Methodological assessment tool for systematic reviews and meta-analysis
Methodological assessment tool for systematic reviews and meta-analysis (PDF download)
Appendix 6 Clinical effectiveness review: table of excluded studies with rationale
Clinical effectiveness review: table of excluded studies with rationale (PDF download)
Appendix 7 Summary of the trials included in the base-case network meta-analysis of recently discharged patients with heart failure
Summary of the trials included in the base-case network meta-analysis of recently discharged patients with heart failure (PDF download)
Appendix 8 Additional analyses: summary of the design and patient characteristics of included studies of stable patients with heart failure
Additional analyses: summary of the design and patient characteristics of included studies of stable patients with heart failure (PDF download)
Appendix 9 Summary of the trials included in the base-case network meta-analysis of patients with stable heart failure
Summary of the trials included in the base-case network meta-analysis of patients with stable heart failure (PDF download)
Appendix 10 MEDLINE search strategy for the cost-effectiveness review
MEDLINE search strategy for the cost-effectiveness review (PDF download)
Appendix 11 Table of excluded cost-effectiveness studies
Appendix 12 Results for higher usual care cost scenarios
Appendix 13 Results for lower-cost telemonitoring during office hours scenarios
Results for lower-cost telemonitoring during office hours scenarios (PDF download)
Appendix 14 Results for higher-cost telemonitoring during office hours scenarios
Results for higher-cost telemonitoring during office hours scenarios (PDF download)
Appendix 15 Results for higher-cost structured telephone support human-to-human contact cost scenarios
Results for higher-cost structured telephone support human-to-human contact cost scenarios (PDF download)
Appendix 16 Results for lower-cost structured telephone support human-to-human contact cost scenarios
Results for lower-cost structured telephone support human-to-human contact cost scenarios (PDF download)
Appendix 17 Results for 12-month treatment duration scenario
Results for 12-month treatment duration scenario (PDF download)
Appendix 18 Protocol
Glossary
Technical terms and abbreviations are used throughout this report. The meaning is usually clear from the context, but a glossary is provided for the non-specialist reader. In some cases, usage differs in the literature, but the term has a constant meaning throughout this review.
- Base-case analysis
- In modelling, the base case is the primary analysis based on the best estimates of each model input (cf. sensitivity analysis).
- Baseline risk
- The probability of an event (e.g. death) occurring in the comparator arm. This is a term used in modelling in which the baseline risk from one data source might be combined with a risk ratio from another source to estimate the probability of an event occurring for patients receiving a different intervention.
- Conservative assumption
- When there is uncertainty, modellers may have a choice of which value to give to a model input. A conservative assumption is when the modeller chooses the parameter in such a way that it cannot bias in favour of the new treatment (and is likely to be biasing in favour of the standard treatment).
- Cost-effectiveness acceptability curve
- A way of illustrating cost-effectiveness results by plotting the probability that the intervention is cost-effective (y-axis) against the maximum that society is willing to pay for an improvement in health (x-axis).
- Cost-effectiveness plane
- A way of illustrating cost-effectiveness results by plotting the mean incremental cost and effectiveness on a four-quadrant graph. Interventions that are more costly and more effective fall in the north-east quadrant.
- Incremental cost-effectiveness ratio
- The difference in costs between one intervention and an alternative divided by the difference in outcomes.
- Length of stay
- The total number of days that a participant stays in hospital.
- Meta-analysis
- A statistical technique for combining (pooling) the results of a number of studies that address the same question and report on the same outcomes to produce a summary result. The aim is to derive more precise and clear information from a large data pool. It is generally more reliably likely to confirm or refute a hypothesis than the individual trials.
- Quality-adjusted life-year
- A measure of benefit of health care combining the impact of both expected length of life and quality of life.
List of abbreviations
- ACE
- angiotensin-converting enzyme
- ARB
- angiotensin receptor blocker
- BHF
- British Heart Foundation
- CAD
- coronary artery disease
- CEAC
- cost-effectiveness acceptability curve
- CHARM
- Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity
- CHF
- chronic heart failure
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CrI
- credible interval
- DRG
- diagnosis-related group
- EQ-5D
- European Quality of Life-5 Dimensions
- ESC
- European Society of Cardiology
- EVPI
- expected value of perfect information
- GPRD
- General Practice Research Database
- HDS
- Health Distress Score
- HF
- heart failure
- HH
- human-to-human contact
- HM
- human-to-machine interface
- Home-HF
- Home Heart Failure Study
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- LVEF
- left ventricular ejection fraction
- LVSD
- left ventricular systolic dysfunction
- LYG
- life-years gained
- MCMC
- Markov chain Monte Carlo
- MLHFQ
- Minnesota Living with Heart Failure Questionnaire
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NMB
- net monetary benefit
- NY&Y
- NHS North Yorkshire and York
- NYHA
- New York Heart Association
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RM
- remote monitoring
- RR
- risk ratio
- ScHARR
- School of Health and Related Research
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- STS
- structured telephone support
- TEN-HMS
- Trans-European Network – Home-Care Management System
- TIM-HF
- Telemedical Interventional Monitoring in Heart Failure
- TM
- telemonitoring
- WTP
- willingness to pay
- WSD
- Whole System Demonstrator
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.