Notes
Article history
This issue of Health Technology Assessment contains a project originally commissioned by the MRC but managed by the Efficacy and Mechanism Evaluation Programme. The EME programme was created as part of the National Institute for Health Research (NIHR) and the Medical Research Council (MRC) coordinated strategy for clinical trials. The EME programme is funded by the MRC and NIHR, with contributions from the CSO in Scotland and NISCHR in Wales and the HSC R&D, Public Health Agency in Northern Ireland. It is managed by the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC) based at the University of Southampton.
Declared competing interests of authors
FGS and GDP have received an investigator-led research grant from GlaxoSmithKline. GDP and DFM have consulted for, sat on advisory boards for, and received lecture fees from GlaxoSmithKline, and have received lecture fees from AstraZeneca for educational meetings (all unrelated to β-agonists). All other authors declare that they have no competing interests.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Gates et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Description of acute respiratory distress syndrome
Acute respiratory distress syndrome (ARDS) is a condition characterised by a failure of pulmonary oxygen exchange due to increased alveolar–capillary permeability and resultant pulmonary oedema. It can be caused by primary lung conditions such as aspiration pneumonitis, or can arise as a complication of non-pulmonary conditions such as severe sepsis. The syndrome was first described by Ashbaugh and colleagues in 19671 in a group of 12 patients with acute onset of dyspnoea, tachypnoea, refractory hypoxaemia, reduced pulmonary compliance and diffuse alveolar shadowing on their chest radiographs. All patients required positive-pressure mechanical ventilation with positive end-expiratory pressure to maintain arterial oxygenation. The term ‘adult respiratory distress syndrome’ was initially used to describe the condition,2 but it was subsequently renamed as ARDS because it may also occur in children. 3 The current definition arose from the American–European Consensus Conference in 19944 which recognised two grades of disease, based on the degree of hypoxaemia. ARDS was reserved for the more severe grade, with acute lung injury (ALI) being used to describe the less severe form. The definition of ALI/ARDS requires:
-
acute onset
-
bilateral infiltrates on chest radiographs
-
pulmonary artery occlusion pressure < 18 mmHg (if measured), or absence of clinical signs of left atrial hypertension
-
ratio of partial pressure of oxygen in arterial blood (PaO2) to the fraction of inspired oxygen (FiO2) < 200 mmHg (26.7 kPa) for ARDS, or PaO2–FiO2 ratio < 300 mmHg (40 kPa) for ALI.
Incidence and burden of disease
The population incidence of ARDS in Europe and Scandinavia has been estimated by several studies at between 7.8 and 28 cases per 100,000 population per year. 5–9 This translates as up to 16,800 cases per year in the UK, similar to all new cases of lymphomas and leukaemias combined. ARDS is a common condition among intensive care unit (ICU) patients, affecting 6–8% of all ICU admissions, and patients with ARDS have a very high risk of death. Recent multicentre cohort studies from Europe, the USA and Australia have given mortality estimates of between 34% and 61% (measured over different timescales). 5,7,8,10,11 Two studies conducted in the UK and Europe, both in 1999, found the highest of these mortality figures, with rates of death in hospital of 61% and 58%. 5,7 There has been a trend to reduced mortality in epidemiological and clinical trials in recent years. 9 Nevertheless, there may be 7000 deaths per year in the UK in patients with ARDS. As well as a high mortality, ARDS causes long-term health problems and reduction in quality of life (particularly physical activity) for survivors. 12 A recent study in survivors of ARDS found evidence of exercise limitation, physical and psychological sequelae and decreased physical quality of life up to 5 years later. 13 In addition, ARDS has significant resource implications as it prolongs ICU and hospital stay and requires convalescence in hospital and subsequent rehabilitation in the community. 14
Existing evidence
A systematic review published in 2004, based on an electronic search of seven electronic databases and a hand search of reference lists from review articles and relevant papers,15 found three clinical studies (one randomised, controlled, cross-over trial16 and two non-randomised studies17,18) using β2-agonists in patients with ARDS. These studies examined the effects of nebulised16,17 or intravenous (i.v.)18 β2-agonists on the respiratory mechanics of artificially ventilated patients with ARDS and found that β2-agonists reduced airway resistance and peak and plateau airway pressures. There were no clinical studies addressing the effects of β2-agonists on alveolar fluid clearance or on outcome.
A single-centre Phase II trial [Beta-Agonist Lung injury TrIal (BALTI)-1] investigating the efficacy of i.v. salbutamol on in vivo fluid clearance through serial measurement of extravascular lung water in 40 patients with ARDS was conducted between 2001 and 2003. 19 An initial dose-ranging study determined that the maximum infusion rate for salbutamol that did not cause tachydysrhythmias in patients with ARDS was 15 μg/kg ideal body weight (IBW)/hour. This is the maximal recommended dose for the treatment of airflow obstruction in acutely ill patients. The trial showed that an infusion of salbutamol over 7 days significantly reduced lung water [day 7 lung water mean (standard deviation; SD), 9.2 (6) vs. 13.2 (3) ml/kg; p = 0.038] and plateau airway pressures [day 7 plateau airway pressures mean (SD) 23.9 (3.8) vs. 29.5 (7.2) cmH2O; p = 0.049], providing proof of concept that treatment with i.v. β2-agonists can influence alveolar fluid clearance, but it was not designed to address important clinical outcomes and a subsequent larger trial was needed to evaluate this therapy.
As part of the BALTI-2 funding application, the investigators updated the literature search (unpublished) using the same keywords combined with terms to identify randomised controlled trials (RCTs). No studies using i.v. salbutamol infusion were identified. The only relevant additional publication was a retrospective case review of 86 patients with ALI suggesting that high-dose nebulised salbutamol may be superior to a low dose. 20
At the time that BALTI-2 was initiated, the only treatment of proven effectiveness for ARDS was use of a lung-protective (pressure and volume limited) strategy of mechanical ventilation. 21 There were no additional treatments known to improve outcome. A Cochrane review of pharmacological treatments that included 22 studies of 14 different drugs concluded that ‘Effective pharmacotherapy for ALI and ARDS is extremely limited, with insufficient evidence to support any specific intervention’. 22 Recently, a single trial has been published showing a reduction in mortality in ARDS patients treated with neuromuscular blocking agents. 23 This is the only trial that has demonstrated an effective pharmacological treatment for ARDS.
Rationale for beta-agonists in acute respiratory distress syndrome
There is good evidence from in vivo and in vitro human and animal studies that β2-agonists may have a range of important beneficial effects in ARDS patients. 15 First, they can affect epithelial and endothelial function to reduce alveolar–capillary permeability, accelerate alveolar fluid clearance and increase surfactant secretion, all of which may help to reduce pulmonary oedema. Second, they modulate the inflammatory cascades and regulate neutrophil recruitment, activation and apoptosis. 24 This may improve outcomes, as high titres and persistence of inflammatory cytokines are associated with poor outcome and stimulating neutrophil apoptosis may lead to reduced lung injury and improved survival. Third, they enhance epithelial wound repair and promote alveolar–capillary healing. 25,26
Salbutamol is a low-cost treatment and is readily available from generic drug manufacturers. A 7-day infusion is cheap compared with the cost of ICU care (the NHS reference cost for a day in ICU care is £1390). 27
Nebulised compared with intravenous salbutamol
The optimal route for delivering β2-agonists in patients with ARDS with a goal of increasing alveolar fluid clearance has not been determined. Nebulising drugs into the breathing circuits of mechanically ventilated patients appears attractive as it results in high lung concentrations but low blood concentrations and so may reduce the incidence of systemic side effects compared with parenteral treatment. 16 However, nebulised drugs might not reach the alveolar space in the consolidated and poorly ventilated lungs found in patients with ARDS. A trial [ALbuterol for the Treatment of ALI (ALTA)] conducted in the US concurrently with BALTI-2 evaluated the effects of nebulised salbutamol (albuterol) in patients with ALI. Recruitment was terminated early by the Data Monitoring and Ethics Committee (DMEC) on the grounds of ‘futility’, with no clear differences between the albuterol and placebo groups. 28
Chapter 2 Methods
Trial summary
Beta-agonist Lung Injury Trial-2 was a multicentre, pragmatic, randomised, double-blind, placebo-controlled clinical trial. Patients fulfilling the American–European Consensus Conference definition of ARDS were randomised in a 1 : 1 ratio to receive an i.v. infusion of either salbutamol (15 µg/kg IBW/hour) or placebo (0.9% sodium chloride solution), for a maximum of 7 days. Allocation to randomised groups used minimisation to ensure balance with respect to hospital of recruitment, age group and PaO2–FiO2 ratio. The trial was fully blinded and all drugs were packaged identically, so that patients, clinicians and investigators did not know which patients were in each arm. The primary outcome was mortality at 28 days after randomisation, with follow-up for mortality and quality of life to 12 months. The target sample size was 1334 patients, to be recruited from about 50 ICUs in the UK. The trial protocol has been published. 29
Pilot and main study
The trial was structured into a pilot phase and a main trial. The pilot phase was conducted at five hospitals in the West Midlands, and was conducted while substantive funding was obtained. There were no changes to the trial protocol between the pilot and main trial phases, except that, for resource reasons, the pilot phase did not include long-term follow-up at 6 and 12 months. However, patients recruited later in the pilot phase, whose follow-up points fell within the main trial period, were followed up. As there were no substantial differences in the trial between the pilot and main periods, we included all patients in the final analysis.
Objectives
The primary objective of the trial was to assess whether or not an i.v. infusion of salbutamol given at 15 μg/kg IBW/hour for up to 7 days reduces 28-day all-cause mortality in patients with ARDS compared with a placebo (0.9% sodium chloride) infusion.
The secondary objectives were:
-
to evaluate the effects of i.v. salbutamol on mortality in ICU, mortality in hospital, ventilator-free days (VFDs), organ failure-free days, length of ICU and hospital stay, mortality up to 12 months after randomisation and health-related quality of life at 6 and 12 months after randomisation
-
to evaluate the safety of i.v. salbutamol for ARDS patients
-
to evaluate the cost-effectiveness of i.v. salbutamol for patients with ARDS
-
to explore whether or not the effects of salbutamol vary between patients of different age, initial disease severity, mortality risk at ICU admission and ARDS aetiology.
Outcome measures
-
Primary outcome.
-
All-cause mortality 28 days after randomisation.
-
-
Secondary outcomes.
-
Mortality at (first) discharge from ICU.
-
Mortality at (first) discharge from hospital.
-
Number of VFDs.
-
Number of organ failure-free days.
-
Mortality at 12 months post randomisation.
-
Side effects (tachycardia/new arrhythmia/lactic acidosis) sufficient to stop treatment with trial drug.
-
Serious adverse events (SAEs) and suspected unexpected serious adverse reactions (SUSARs).
-
Health-related quality of life: European Quality of Life-5 Dimensions (EQ-5D) and Short Form questionnaire-12 items (SF-12) at 6 and 12 months after randomisation.
-
Length of stay in critical care unit.
-
Length of stay in hospital.
-
-
Economic outcomes.
-
Health service contacts up to 12 months after randomisation.
-
Patient out-of-pocket expenditure and time away from work.
-
Ventilator-free days were defined as the number of calendar days after initiating unassisted breathing to day 28 after randomisation, assuming a patient survived for at least 48 consecutive hours after initiating unassisted breathing. 30 For example, if a patient initiated unassisted breathing on day 16 and survived to day 28, he/she was assigned a value of 12 VFDs. If a similar patient began unassisted breathing on day 16 but died on day 25, the number of VFDs was 9. If a patient survived for > 48 consecutive hours of unassisted breathing but required assisted breathing (for any reason) before day 28, the number of VFDs was the number of days of unassisted breathing before day 28. Patients who died without initiating unassisted breathing or before 48 consecutive hours of unassisted breathing were assigned a value of zero VFDs. Patients transferred to another hospital or other health-care facility prior to day 28 (intermediate care, nursing home, etc.) while still on positive pressure ventilation were followed to assess this outcome.
In the assessment of VFDs, unassisted breathing was defined as:
-
extubated with face mask, nasal prong oxygen, or room air; or
-
T-tube breathing; or
-
tracheostomy mask breathing; or
-
continuous positive airway pressure = 5 cmH2O without pressure support or invasive mechanical ventilation assistance. 30
Organ failure-free days was defined as the number of days in the first 28 days after randomisation that the patient had no cardiovascular support, renal support, hepatic support or neurological support, according to Critical Care Minimum Data Set definitions. 8
Inclusion/exclusion criteria
Inclusion criteria
Patients were eligible for randomisation into the trial if they met the following criteria:
-
Patient intubated and ventilated.
-
Within 72 hours of onset of ARDS.
-
ARDS according to American–European Consensus Conference definition:
-
acute onset
-
severe hypoxaemic respiratory failure [PaO2–FiO2 ≤ 26.7 kPa (200 mmHg)]
-
bilateral infiltrates on the chest radiograph in the absence of clinical evidence of left atrial hypertension.
-
-
Aged ≥ 16 years.
The reason for restricting eligibility to patients within 72 hours of onset is that ARDS is classically divided into two distinct phases: an early exudative phase followed by a later fibroproliferative repair phase. 31 Experimental evidence suggests that β2-agonists would be most effective during the early exudative phase during which acute alveolar inflammation, alveolar–capillary barrier damage and alveolar flooding predominate. The time of onset was judged by the clinicians caring for the patient.
Exclusion criteria
-
Patient known to be pregnant.
-
Current treatment with i.v. β2-agonists or requirement for ongoing regular nebulised/inhaled β2-agonists.
-
Current treatment with β-adrenergic antagonists (‘β-blockers’).
-
Treatment withdrawal imminent.
-
Chronic liver disease, defined as Child–Pugh grade C32 (assessed at the time of consideration for trial eligibility).
-
Enrolled in another clinical trial of an investigational medicinal product in the last 28 days.
-
Patient or personal legal representative or professional legal representative unwilling to give informed consent.
Receipt of nebulised/inhaled β2-agonists during a patient's initial resuscitation and stabilisation did not render a patient ineligible for BALTI-2. Patients were only excluded if they had an ongoing requirement for regular nebulised/inhaled β2-agonists, for example a patient with an acute exacerbation of asthma or chronic obstructive pulmonary disease (COPD). Patients enrolled in the trial were not prevented from having nebulised/inhaled bronchodilators if their clinical status deteriorated. This was recorded on the case report form (CRF).
Consent
In the majority of cases patients were unable to consent for themselves, and consent was initially sought from the patient's ‘personal legal representative’, who was a relative, partner or close friend. The representative was informed about the trial by the responsible clinician and provided with a copy of the patient information sheet and additional information for personal legal representatives. If the representative decided that the patient would have no objection to participating in the trial they were asked to sign three copies of the consent form which were then counter-signed by the responsible clinician. The representative retained one copy of the signed consent form, one copy was placed in the patient's medical records and one copy was retained in the trial site file. If no personal legal representative was available, a doctor who was not connected with the conduct of the trial acted as a professional legal representative.
Patients for whom consent was given by a personal legal representative or professional legal representative were informed of their participation in the trial by the responsible clinician once they regained capacity to understand the details of the trial. The patient was asked for consent to continue participation in the trial and to sign the consent to continue form. Patients were specifically asked whether or not they were happy for data collection to continue, to receive follow-up questionnaires and for data already collected to be used.
If a patient or their representative requested termination of infusion of the trial drug during the treatment period, the drug infusion was stopped but the patient continued to be followed up. If a patient or their representative withdrew consent for trial participation during trial treatment, the trial drug was stopped but permission was sought to access medical records for data related to the trial. If a patient or their representative withdrew from the trial after completion of the trial treatment, permission to access medical records for trial data was sought.
Randomisation
Patients were randomised using a 24-hour telephone randomisation service located at the University of Aberdeen.
Randomisation was minimised by centre, PaO2–FiO2 ratio (≤ 6.7, 6.8–13.2, ≥ 13.3 kPa) and age (≤ 64, 65–84, ≥ 85 years). The minimisation criteria were used to ensure balance within centres, and within strata that were expected to differ in mortality risk, according to published reviews. 5,10
The randomisation service used a computer-generated random number sequence, and allocated a numbered treatment pack to each patient. Each pack contained all of the drugs necessary for giving a complete course of trial treatment to one patient.
Each patient was allocated a unique six-digit patient trial number that was used throughout the trial as their unique identifier.
Trial treatments
The trial drug boxes contained 50 × 5-ml ampoules containing either salbutamol (VentolinTM solution, GlaxoSmithKline) for i.v. infusion 1 mg/ml, or placebo (sodium chloride injection BP, Hameln Pharmaceuticals Ltd) 0.9% weight/volume.
Drug pack preparation and supply
All trial drugs were packaged identically and identified only by number. Patient drug packs were prepared by Bilcare Global Clinical Supplies (Europe) Ltd (Elvicta Business Park, Crickhowell, Powys, UK). Each ampoule of salbutamol or placebo had a black out label applied, and 50 ampoules of either salbutamol or placebo were packaged in a white cardboard box in 10 trays containing five ampoules each. Each box contained sufficient material for the treatment of one patient for 7 days. The outside of the boxes were labelled only with the drug box number and Medicines and Healthcare Products Regulatory Agency (MHRA)-approved labelling identifying the contents as BALTI-2 study drugs. The drug packs were stored by Bilcare and dispatched by them to participating hospital pharmacies, as required. All clinical and trial personnel were blind to study treatment, and all assessment of outcomes was done without knowledge of treatment allocations.
Hospital pharmacies dispensed the trial drugs to their ICU. Because patients could be recruited outside normal pharmacy opening hours, two or more patient drug packs (at least one each of salbutamol and placebo) were kept available on each ICU at all times. When a patient was recruited, the randomisation service informed the recruiting clinician of the drug pack number to be allocated to the patient and the number of another drug pack, of the same treatment allocation, to be obtained from pharmacy. This ensured that there was always at least one drug pack of each allocation available in the ICU.
Administration of trial drug
Prior to infusion, two ampoules of trial drug were diluted with 40 ml of saline in a 50-ml syringe. Infusion syringes were made up immediately prior to use.
Salbutamol and placebo infusions were administered through a dedicated i.v. line at a rate of 0.075 ml/kg IBW/hour (equivalent to 15 µg salbutamol/kg IBW/hour). IBW was calculated from the patient's height; the patient was measured from heel to vertex using a soft tape measure and the IBW and infusion rate were obtained from the conversion table (Table 1). Trial drug infusions were started immediately after randomisation.
| Height (cm) | Male IBW (kg) | Infusion rate (ml/hour) | Female IBW (kg) | Infusion rate (ml/hour) |
|---|---|---|---|---|
| 146 | 44.2 | 3.3 | 39.7 | 3.0 |
| 148 | 46.0 | 3.5 | 41.5 | 3.1 |
| 150 | 47.8 | 3.6 | 43.3 | 3.2 |
| 152 | 49.6 | 3.7 | 45.1 | 3.4 |
| 154 | 51.5 | 3.9 | 47.0 | 3.5 |
| 156 | 53.3 | 4.0 | 48.8 | 3.7 |
| 158 | 55.1 | 4.1 | 50.6 | 3.8 |
| 160 | 56.9 | 4.3 | 52.4 | 3.9 |
| 162 | 58.7 | 4.4 | 54.2 | 4.1 |
| 164 | 60.6 | 4.5 | 56.1 | 4.2 |
| 166 | 62.4 | 4.7 | 57.9 | 4.3 |
| 168 | 64.2 | 4.8 | 59.7 | 4.5 |
| 170 | 66.0 | 5.0 | 61.5 | 4.6 |
| 172 | 67.8 | 5.1 | 63.3 | 4.7 |
| 174 | 69.7 | 5.2 | 65.2 | 4.9 |
| 176 | 71.5 | 5.4 | 67.0 | 5.0 |
| 178 | 73.3 | 5.5 | 68.8 | 5.2 |
| 180 | 75.1 | 5.6 | 70.6 | 5.3 |
| 182 | 76.9 | 5.8 | 72.4 | 5.4 |
| 184 | 78.8 | 5.9 | 74.3 | 5.6 |
| 186 | 80.6 | 6.0 | 76.1 | 5.7 |
| 188 | 82.4 | 6.2 | 77.9 | 5.8 |
| 190 | 84.2 | 6.3 | 79.7 | 6.0 |
| 192 | 86.0 | 6.5 | 81.5 | 6.1 |
| 194 | 87.9 | 6.6 | 83.4 | 6.3 |
| 196 | 89.7 | 6.7 | 85.2 | 6.4 |
| 198 | 91.5 | 6.9 | 87.0 | 6.5 |
| 200 | 93.3 | 7.0 | 88.8 | 6.7 |
Alteration of infusion rate
Sinus tachycardia or arrhythmias are known side effects of i.v. salbutamol administration. If a patient receiving a trial drug infusion had tachycardia [heart rate (HR) > 140 beats/minute] or any new arrhythmia, the drug infusion rate was adjusted according to a prespecified protocol (Figure 1). Standard antiarrhythmic therapy was given if indicated in addition to alteration of infusion rate.
FIGURE 1.
Protocol for adjustment of study drug infusion rate. ECG, electrocardiogram.
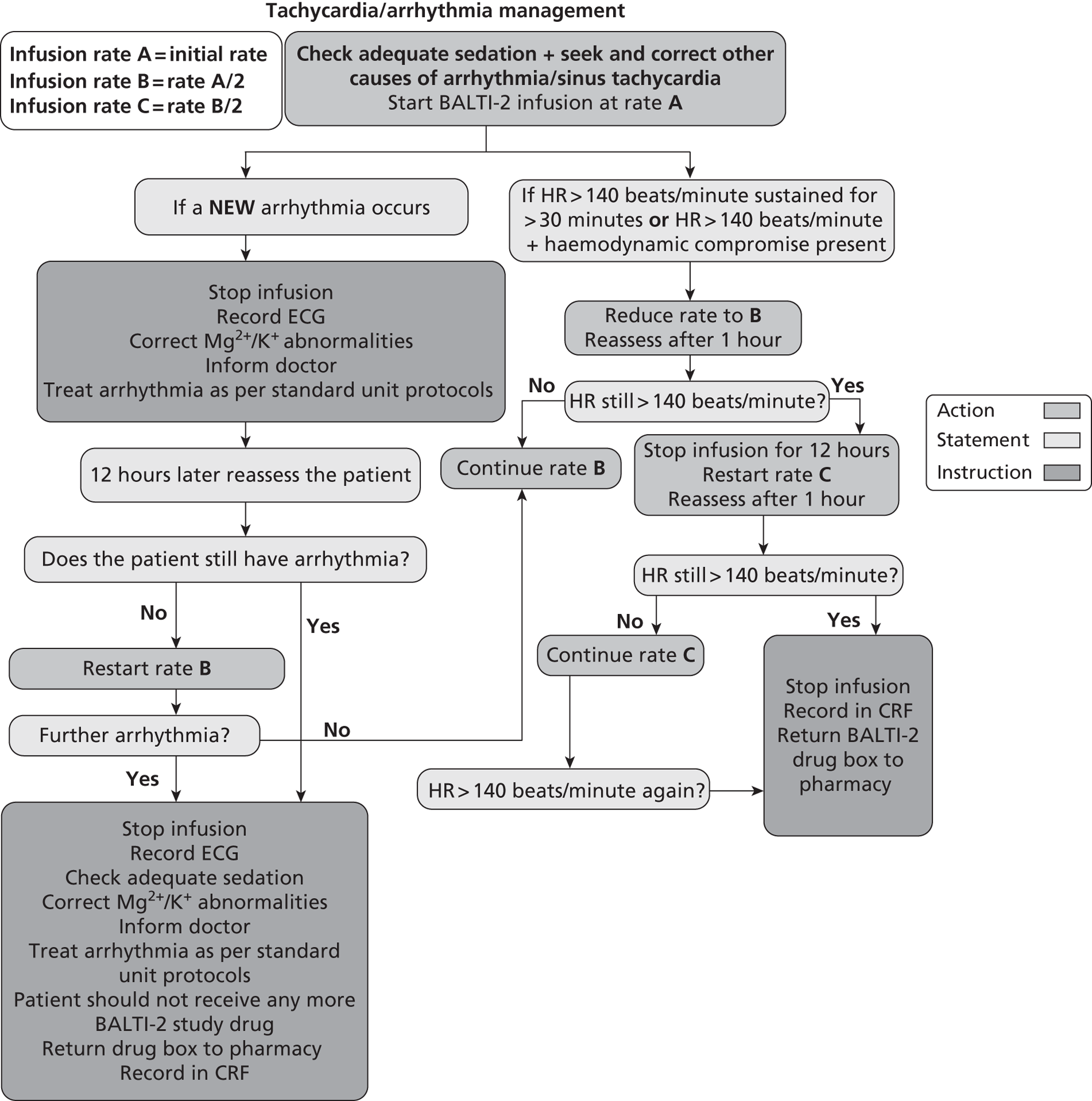
Infusion termination criteria
The trial drug infusion was terminated before 168 hours in the following circumstances:
-
death
-
HR > 140 beats/minute despite two adjustments in infusion rate
-
new arrhythmias despite adjustment in infusion rate
-
development of a significant lactic acidosis, which in the opinion of the treating clinician was attributable to infusion of the trial drug
-
24 hours after discontinuation of mechanical ventilation (of any sort)
-
discharge from critical care environment
-
discontinuation of active treatment
-
request to withdraw from personal legal representative or patient
-
decision by the attending clinician that the infusion should be discontinued on safety grounds.
Otherwise, the infusion was terminated 7 days (168 hours) after randomisation. Reasons for early termination of the infusion were recorded in the CRF.
Clinical management of patients in the trial
Patients involved in BALTI-2 were managed according to best practice established locally on each unit. The only specific requirement was that patients were not routinely administered nebulised β2-agonists or other i.v. β2-agonists such as isoprenaline. The uncontrolled use of nebulised bronchodilators in the control group would limit the ability of the trial to detect a difference in outcomes and their use in the treatment group would expose patients to a risk of toxicity.
Serious adverse events and suspected unexpected serious adverse reactions
A SAE is defined as an adverse event that fulfils one or more of the following criteria:
-
results in death
-
is immediately life-threatening
-
requires hospitalisation or prolongation of existing hospitalisation
-
results in persistent or significant disability or incapacity
-
results in congenital abnormality or birth defect
-
requires medical intervention to prevent one of the above, or is otherwise considered medically significant.
Suspected unexpected serious adverse reactions are SAEs that are also unexpected, i.e. their nature or severity is not consistent with the Summary of Product Characteristics, and are considered to be caused by the study drug.
As BALTI-2 recruited a population that was already in a life-threatening situation, many of the participants were expected to experience SAEs. Events that were expected in this population and those that were collected as outcomes of the trial were not reported as SAEs. This included death and organ failure. SUSARs and side effects of salbutamol sufficiently severe to be fatal or immediately life-threatening were reported, using a specific SAE reporting form.
Data collection
Hospital data
All data for each patient were entered by staff at participating hospitals onto the trial CRF. Data were collected from the time the patient was considered for entry into the trial through to their discharge from hospital. If a patient was transferred to another hospital, the trial team contacted the receiving hospital to request data for the remainder of that patient's hospital stay.
The Acute Physiology and Chronic Health Evaluation (APACHE) II scores were used as part of the description of the trial population. For centres that participated in the Intensive Care National Audit and Research Centre (ICNARC) Case Mix Programme (CMP), the APACHE II scores were obtained from ICNARC; therefore, these centres supplied only the CMP number for BALTI-2 participants. Centres that did not participate in the CMP were asked to collect all of the data to allow calculation of the APACHE II score.
Data were collected in duplicate using non-carbon required forms. Once a patient had been discharged from hospital and all data entered into the CRF, the top copy of each form was returned to the trial co-ordinating centre and the bottom copy was retained at the recruiting centre.
Submitted data were reviewed for completeness on receipt at the trial co-ordinating centre, and entered onto a secure, backed-up custom database. Entries on the CRF that were ambiguous, unintelligible or incomplete were queried with the hospital that completed the CRF.
Follow-up at 6 and 12 months
Survivors were followed up at 6 and 12 months after randomisation by postal questionnaire. Deaths after discharge from hospital were identified by checking patients' status and flagging with the NHS Information Centre, via the Medical Research Information Service. The follow-up questionnaire collected data on resource use and health-related quality of life, using the EQ-5D and SF-12 questionnaires. If questionnaires were not returned, a maximum of two telephone contacts was made to the patient to check that the questionnaire had been received and the patient was happy to complete it, followed by a second copy of the questionnaire and telephone contacts in the event of non-return. If the second questionnaire was not returned the patient was contacted and the outcome data collected over the telephone, where possible. During recruitment, we changed the procedures to send a £5 gift voucher with the questionnaire, as there is good evidence that this is effective in increasing the proportion returned. 33
Statistical methods
Sample size calculation
Published estimates of the mortality rate among ARDS patients range from about 34% to 60%. Two cohort studies that included UK data estimated that hospital mortality was 53.9% [95% confidence interval (CI) 49.0% to 58.7%] and 60.9% (95% CI 55.9% to 65.9%). 5,7 However, it is likely that mortality has declined since these studies were conducted (1999) because of the introduction of protective ventilation strategies after the publication of a large RCT in 2000. From unpublished ICNARC data for 2005, the hospital mortality among 37,726 patients with ARDS in the UK was 41.2%. The primary outcome for BALTI-2 was 28-day mortality, which was expected to be similar to or slightly higher than hospital mortality because most deaths will occur within a short period after randomisation and most patients leave hospital before 28 days; 28-day mortality may therefore include a few post-hospital deaths. In our earlier trial of i.v. salbutamol (BALTI) the placebo group 28-day mortality was 67% (95% CI 45% to 83%). To calculate the target sample size for BALTI-2 we used expected mortality in the placebo group of 40–50%.
Losses to follow-up for the primary outcome were expected to be low. For example, in the PAC-Man (assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care) trial,34 2.4% of recruited patients were lost (mainly because of withdrawal of consent) between randomisation and hospital discharge. We therefore conservatively assumed a 3% loss of patients for the primary outcome. Table 2 shows the sample sizes necessary for 80% and 90% power if the real risk ratio between the salbutamol and placebo arms was 0.80, using a significance level of 0.05.
| Placebo mortality (%) | Salbutamol mortality (%) | 80% power | 90% power |
|---|---|---|---|
| 40 | 32 | 1164 | 1558 |
| 42 | 33.6 | 1076 | 1440 |
| 44 | 35.2 | 998 | 1334 |
| 46 | 36.8 | 926 | 1238 |
| 48 | 38.4 | 860 | 1148 |
| 50 | 40 | 798 | 1068 |
The target sample size adopted was 1334, which gave 90% power to detect a risk ratio of 0.8 if the placebo group mortality rate was 44%, > 85% power if it was 40% and > 90% if it exceeded 44%.
Statistical analysis
All analyses were conducted as far as possible by intention to treat, i.e. all patients were analysed in their randomised group regardless of the treatment actually received, and we sought to include all randomised patients in the analyses. We did not impute values for missing data.
Primary outcome
Mortality at 28 days post randomisation was compared between the trial arms by the risk ratio and 95% CI. Time to death was also analysed, using survival analysis methods, comparing the groups using the hazard ratio and its 95% CI from a Cox-proportional hazards model, and illustrated with a Kaplan–Meier curve.
Secondary outcomes
Dichotomous outcomes (death in ICU, death in hospital, tachycardia, arrhythmia and other side effects) were compared using risk ratios and 95% CIs.
For continuous outcomes (duration of ICU and hospital stay, VFDs and organ failure-free days), mean differences and 95% CI were calculated.
Mortality over 12 months after randomisation was analysed in the same way as the short-term data, using the risk ratio of mortality up to 12 months and a survival analysis, including data up to 12 months for all participants.
The SF-12 physical and mental component scores were calculated from the raw data according to standard methods35 and the trial treatment groups were compared using the mean difference and 95% CI. The EQ-5D was scored according to the UK valuation model36,37 and presented as the difference in means between the groups with 95% CI.
Subgroup analyses
Four subgroup analyses were prespecified, analysing whether or not the treatment effect was modified by:
-
age
-
severity of hypoxaemia before randomisation
-
aetiology of ARDS
-
APACHE II mortality risk.
Subgroup analyses were conducted for the primary outcome only.
The APACHE II score was originally specified as a subgrouping variable, but this was replaced, before the start of any analysis, by the APACHE II mortality risk. 38 APACHE II scores do not correlate well with mortality, as similar scores may occur in patients with different conditions, who have different risks of mortality. This means that APACHE II score is unlikely to be predictive of outcome or of treatment effect. The mortality risk incorporates the underlying condition and is a better measure of a patient's ‘sickness’. It is therefore more plausible that mortality risk could have a treatment-modifying effect, and it is preferable to explore this variable's relationship to treatment effect. APACHE II score was retained in the table of baseline characteristics as a descriptor of the population recruited to the trial.
Methods for subgroup analyses were modified during the trial to take account of improved methods for continuous subgrouping variables (age, severity of hypoxaemia and APACHE II mortality risk). For the dichotomous subgrouping variable, aetiology of ARDS, we calculated the ratio of risk ratios in the direct and indirect aetiology subgroups, with its 95% CI. 39
For continuous subgrouping variables, the potential treatment-modifying factors were not categorised but included as continuous variables in regression models, modelling the interaction between the continuous baseline variables and outcomes in the salbutamol and placebo groups. Categorisations of age, severity of hypoxaemia and APACHE II score were retained in the table of participants' characteristics, to facilitate comparison of the randomised groups. 40
Sensitivity analyses
We undertook sensitivity analyses to adjust for baseline variables, to explore the effects of adjustment for any imbalances between the randomised groups.
Interim analyses
Interim analyses were planned to be conducted every 12 months during the period of recruitment, or more frequently if requested by the DMEC. The DMEC used the Haybittle–Peto stopping guideline:41,42 a difference of 3 standard errors would be required before considering recommending stopping a trial for benefit at an interim analysis, but a less stringent criterion would be used for stopping for harm.
Causes of death
Data on causes of death (as recorded on the death certificate) were sought from participating hospitals for all patients who died up to day 28 post randomisation. Death certification in the UK is undertaken by the treating clinician in most cases. The cause of death is usually based on antemortem clinical/radiological information as post-mortems are relatively rare in the UK. The death certificate assigns a primary/immediate cause of death (referred to as 1a). Conditions leading to the primary/immediate cause of death may be listed as 1b followed by 1c. Section 2 is used to record other diseases present but not directly related to the cause of death. For example, a patient with ARDS secondary to pneumonia with a background of COPD would be recorded as 1a ARDS, 1b pneumonia, 2 COPD.
Ethics and regulatory approvals
Ethics approval was given for the study by the Oxfordshire A Research Ethics Committee in September 2006. Local Research Ethics Committee (LREC) approval and permission from the research and development (R&D) department of each participating NHS Trust were required until April 2009, when the system was changed. LREC approval was no longer required after this date.
Funding and registration
The pilot phase of the trial was funded by the Intensive Care Society. The main phase of the trial was funded by the Medical Research Council (grant number 84730). Authorisation was given by the MHRA in 2006 (24698/0004/001). The trial was registered with the EudraCT (European Union Drug Regulatory Authorities Clinical Trials) database (2006-002647-86) and with the International Standardised Randomised Controlled Trial Number database (ISRCTN38366450).
Chapter 3 Results
Overview of recruitment
Patients were recruited between November 2006 and March 2010. Initially five hospitals in the West Midlands were recruited to the pilot phase, prior to acquisition of substantive funding, which allowed further centres across the UK to be opened. The main trial commenced recruiting in August 2008. Recruitment was terminated following the second interim analysis, in March 2010, when the DMEC reviewed the results for 273 patients. Owing to a significant adverse effect of salbutamol on 28-day mortality, the DMEC recommended closing recruitment to BALTI-2. The Trial Steering Committee endorsed the DMEC recommendation and closed recruitment on 23 March 2010. All patients receiving study drug at that time had their infusion discontinued (one salbutamol, two placebo).
Recruitment of centres
The pilot study was conducted at five centres in the West Midlands, which opened to recruitment between September and December 2006. The first patient was recruited in November 2006. The pilot study continued until July 2007, when it was terminated at four sites but continued at a single site. Following award of funding for the main trial in September 2007, a new supply of study drugs was ordered and further sites were opened. Recruitment to the main phase of the trial began in September 2008. Further sites were opened throughout recruitment and in total 46 ICUs in the UK recruited to the trial. A further 25 ICUs obtained approvals to start the trial but were unable to do so before recruitment was stopped.
At the start of the study, approval was required from a LREC and the R&D department of each participating NHS Trust before recruitment could commence. The requirement for LREC approval was removed in April 2009, so sites initiated after this date needed only R&D approval. As well as the approvals process, each site needed to sign a site agreement with the sponsor of the trial (University of Warwick) and the co-ordinating centre needed to organise training of the relevant clinicians and arrange drug supplies with the hospital pharmacy and Bilcare.
The process of setting up sites was extremely time consuming (Table 3). The average time for LREC and R&D approval was 92 days. In most cases the R&D approval was the rate-limiting step; submissions to LRECs and R&D were normally done concurrently and LREC took an average of only 34 days. It took an average of 123.5 days from the date of approval to the recruitment of the first patient. This was partly owing to time taken to get the site ready to recruit (training, site agreement and drug delivery) and partly due to time taken to identify, approach and consent an eligible patient once the site was open. The average time from submission of LREC and R&D approvals to recruitment of the first patient was about 218 days, with a range from 84 to 452 days.
| n | Mean (SD) | Minimum | Maximum | |
|---|---|---|---|---|
| Time from local ethics submission to approval (days) | 36a | 34.1 (23.8) | 4 | 105 |
| Time from R&D submission to approval (days) | 58b | 92.2 (58.9) | 2 | 284 |
| Total time for local ethics and R&D approval (days) | 58 | 95.7 (56.2) | 2 | 284 |
| Time from approval to first patient (days) | 43c | 123.5 (85.1) | 6 | 356 |
| Time from submission to first patient (days) | 43 | 217.7 (97.6) | 84 | 452 |
Participants
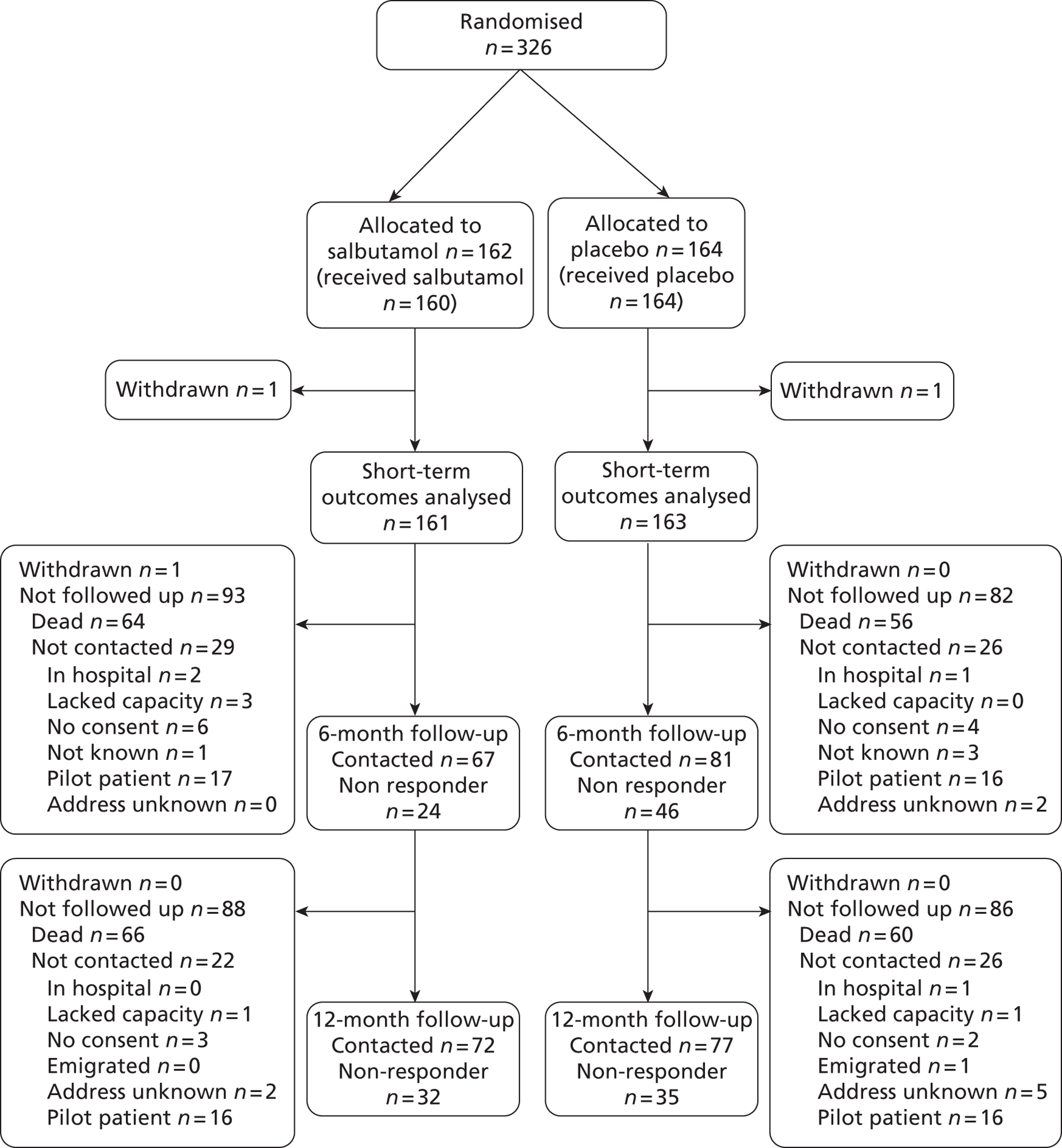
A total of 326 patients were enrolled from December 2006 to March 2010. Of the 326 patients, 162 were randomly assigned to salbutamol and 164 to placebo. During the pilot phase, up to September 2008, 63 patients were recruited, and 263 were recruited to the main phase of the trial. One patient in each arm withdrew consent before assessment of the primary outcome and hence no outcome data were available for these patients. The statistical analysis of the primary outcome and other short-term outcomes was therefore based on 161 patients in the salbutamol arm and 163 patients in the placebo arm (Figure 2). One additional patient in the salbutamol arm withdrew from the follow-up. Survival status at 12 months could not be determined for 13 patients and in the survival analysis these patients were censored at the last time they were known to be alive (discharge from hospital or 6-month follow-up). A relatively small number of patients were followed up for quality-of-life outcomes at 6 and 12 months to evaluate quality of life and record economic data. This was for several reasons: a high proportion of patients died, some patients recruited to the pilot phase before the start of the main trial could not be followed up for resource reasons, some were not contacted because it was considered inappropriate by clinicians or carers and the response rate of those who were contacted was poor (Figure 3).
FIGURE 2.
Flow chart for 12-month mortality.
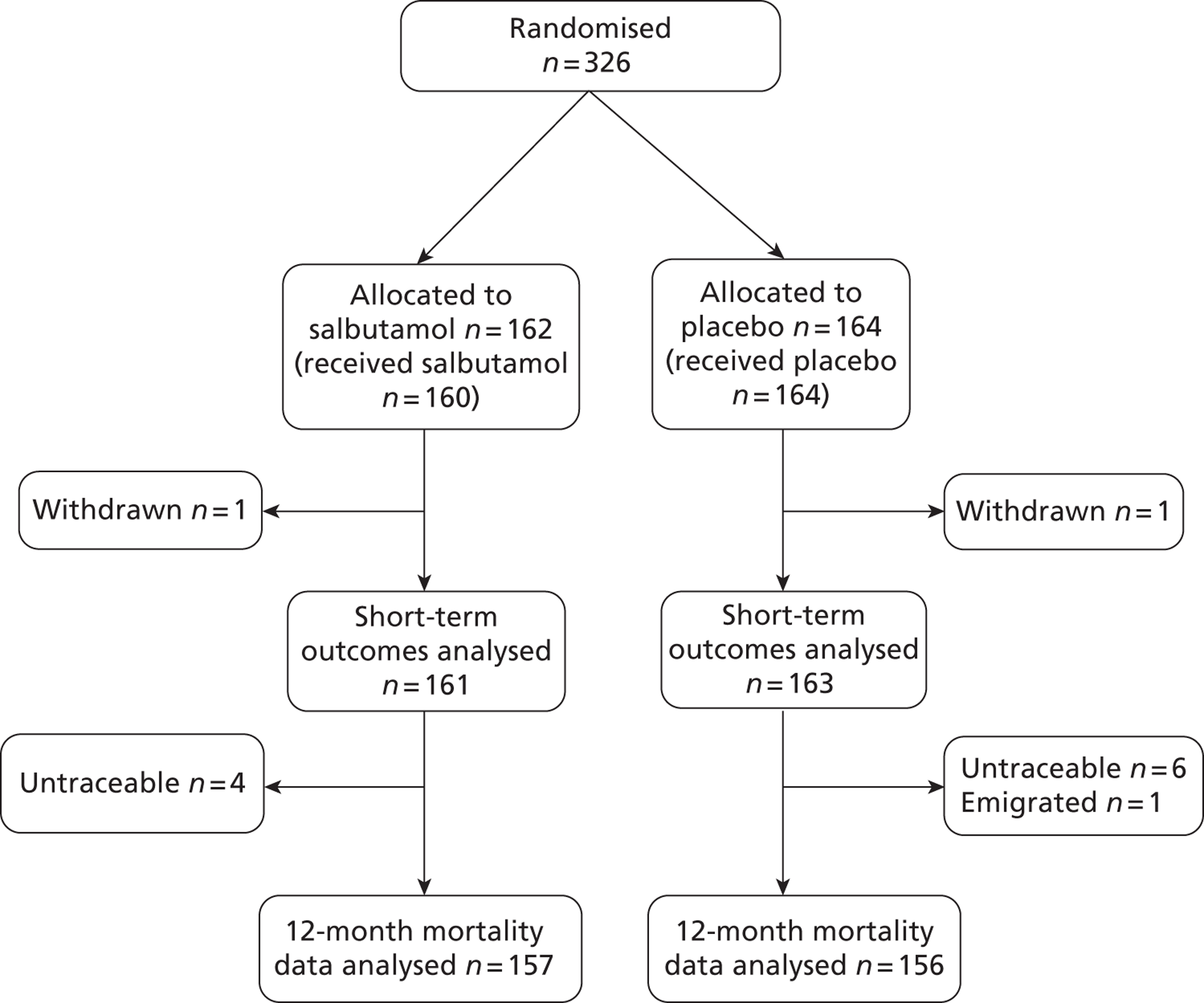
FIGURE 3.
Flow chart for 6- and 12-month follow-up for quality-of-life outcomes.
One centre (Birmingham Heartlands Hospital) recruited about 25% of the trial population, far more than any other centre (most of which recruited < 10 patients over the course of the trial) (Figure 4). This was achieved by the hospital recruiting for a longer period of time than any other centre and also as a result of a high monthly recruitment rate.
FIGURE 4.
Number of recruits for each participating centre.
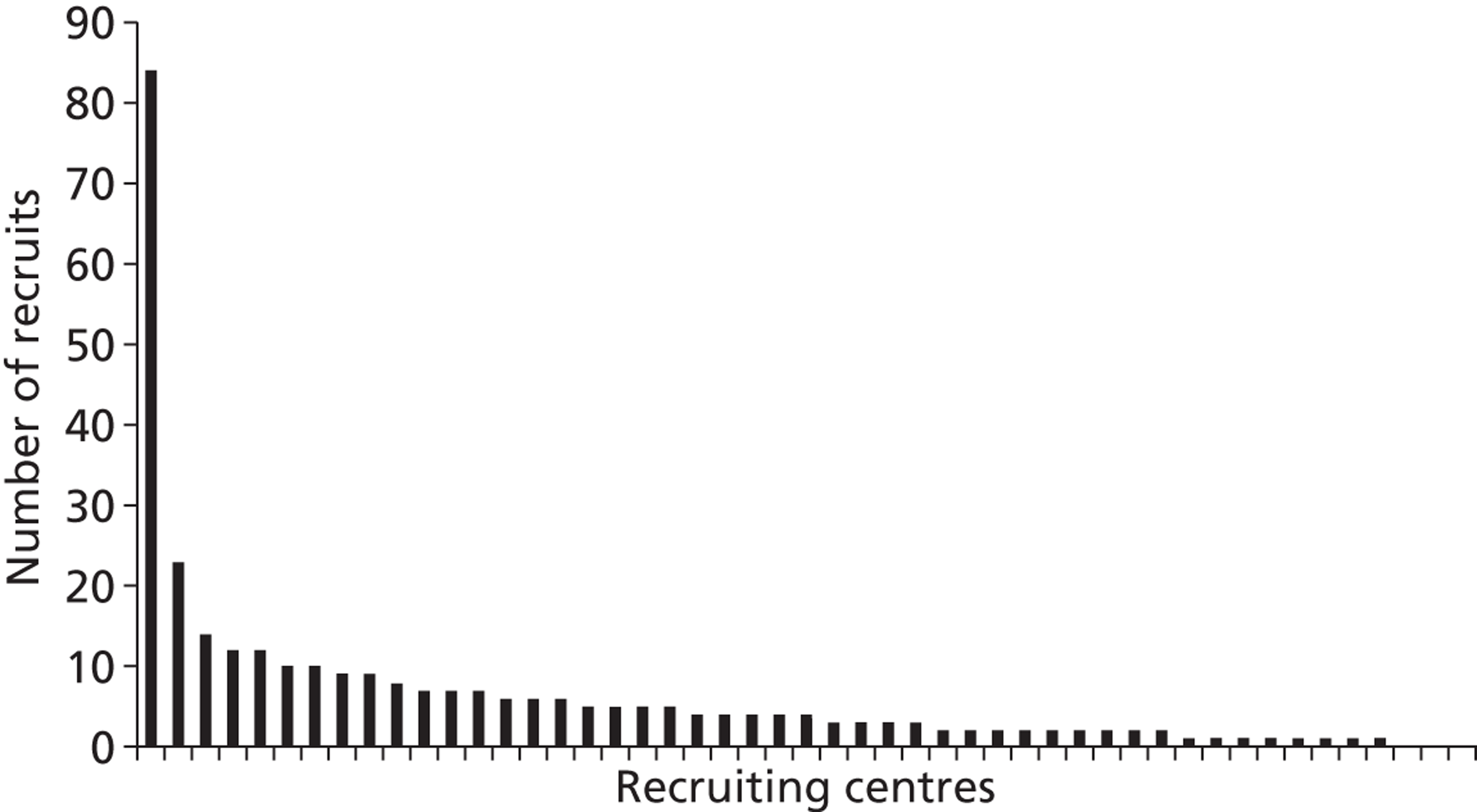
The recruitment rate projected in the study protocol was one patient/centre/month. We therefore estimated that 37 centres would be needed to complete recruitment of 1334 patients in 36 months. It was planned to recruit in up to 50 centres, to give extra capacity in case of poor recruitment and to allow for the fact that some smaller units would be unlikely to recruit one patient/month. It became clear during recruitment that in addition to substantial delays in starting recruitment, few centres were achieving the target recruitment. Only six centres achieved a recruitment rate (after recruiting their first patient) above one patient/month (Figure 5). Additional centres were added to the trial to make up for the shortfall in recruitment and 72 centres were eventually involved.
FIGURE 5.
Number of recruits per month for each participating centre. The recruitment rate was calculated over the period from the centre's first recruit to the end of recruitment. Centres that recruited for < 30 days were omitted from Figure 5 (n = 2).
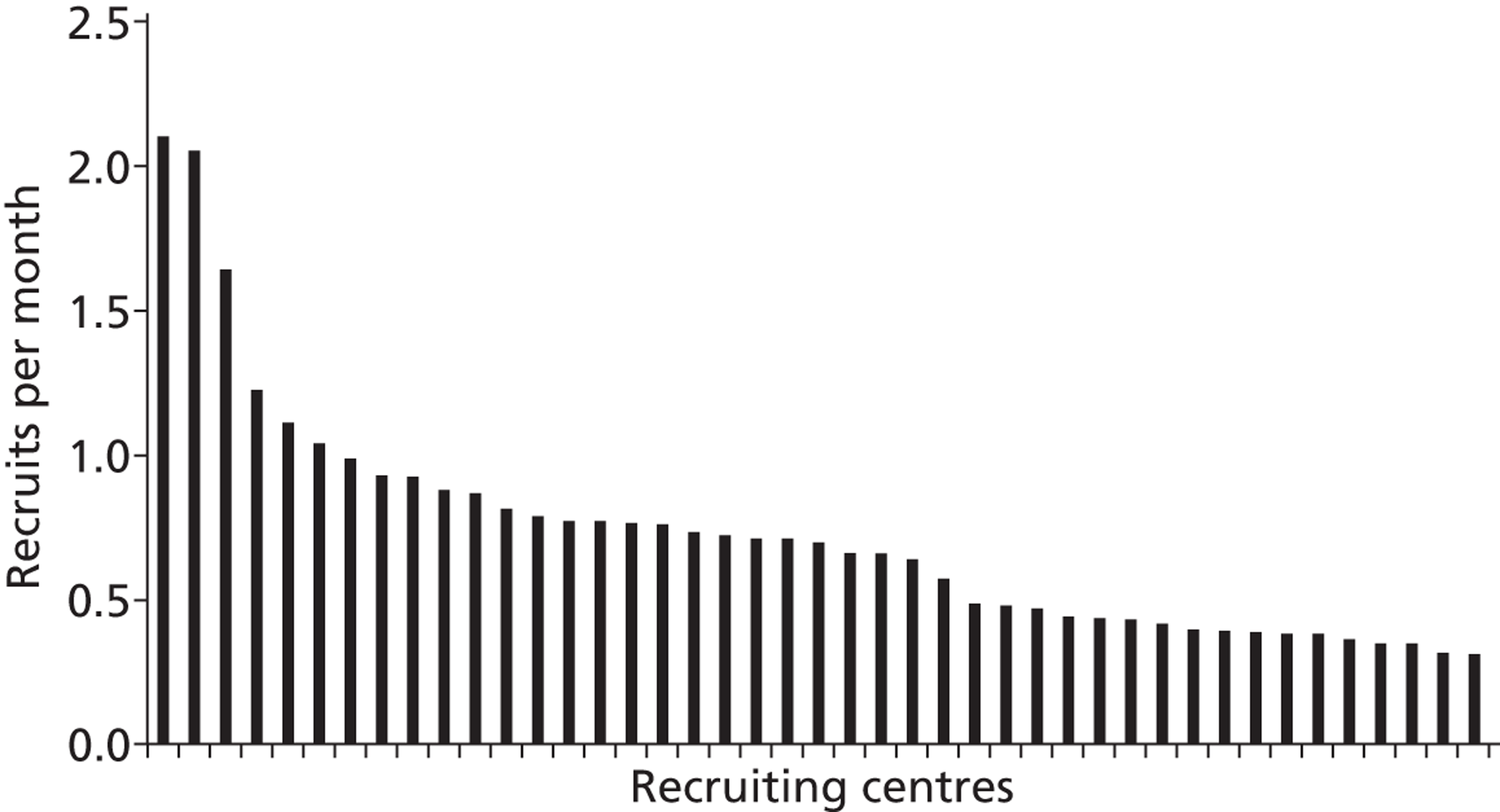
There appear to be several contributory reasons for poor recruitment. First, in some sites there were considerable delays from receiving approvals to recruitment of the first patient (see Table 3). These were caused by various factors, including organising drugs supplies with the pharmacy and supplier, signing site agreements with the sponsor, dissemination of information about the trial and training of staff, and developing systems for identifying eligible patients. Second, it is likely that in most sites a high proportion of eligible patients were missed. At one centre, Birmingham Heartlands Hospital, there was sustained careful screening of all patients against the eligibility criteria and it is unlikely that more than a few eligible patients were missed. This unit recruited at a rate of more than two patients/month. This suggests that most other sites would not have been limited by a shortage of patients and probably failed to recruit to target because many patients who were eligible were not identified.
Baseline characteristics
Baseline characteristics were, as expected, similar between the randomised groups (Table 4). There were very few patients in the oldest age stratum (aged ≥ 85 years), or the most severe stratum of PaO2–FiO2 ratio. Collection of end-expiratory tidal volume was only started about halfway through recruitment, hence the large number of participants with missing data for this variable. As the variable was collected continuously after its introduction, there is no concern that any selection bias could affect the comparison between the groups.
Acute Physiology and Chronic Health Evaluation II mortality risks could not be calculated for about 20% of participants. This was for several reasons, including failure of centres to provide data for calculation of the APACHE II score, unknown primary reason for admission, patient could not be identified in the ICNARC database and reason for admission was excluded from calculation of mortality risk.
| Salbutamol (%) (n = 162) | Placebo (%) (n = 164) | |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 55.8 (17.2) | 54.2 (17.5) |
| Range | 17–93 | 17–86 |
| ≤ 64 | 104 (64.2) | 110 (67.1) |
| 65–84 | 55 (34.0) | 53 (32.3) |
| ≥ 85 | 3 (1.9) | 1 (0.6) |
| APACHE II score | ||
| Mean (SD) | 19.5 (6.2) | 18.9 (6.7) |
| Median (IQR) | 19 (16–23) | 18.5 (14–24) |
| Range | 6–42 | 5–39 |
| Missing | 8 | 10 |
| APACHE II mortality risk | ||
| Mean (SD) | 0.43 (0.20) | 0.42 (0.21) |
| Median (IQR) | 0.43 (0.29–0.57) | 0.40 (0.25–0.57) |
| Range | 0.02–0.95 | 0.05–0.93 |
| Missing | 36 | 37 |
| PaO2–FiO2 ratio | ||
| Mean (SD) | 13.8 (4.9) | 13.8 (4.9) |
| Range | 4.9–26.0 | 5.0–25.3 |
| 13.3–26.7 kPa | 82 (50.6) | 81 (49.4) |
| 6.8–13.2 kPa | 74 (45.7) | 78 (47.6) |
| ≤ 6.7 kPa | 6 (3.7) | 4 (2.4) |
| Missing | 0 | 1 |
| End-expiratory tidal volume (ml/kg IBW) | ||
| Mean (SD) | 8.6 (2.1) | 8.7 (2.3) |
| Median (IQR) | 8.4 (7.1–9.5) | 8.1 (7.0–10.0) |
| Range | 4.1–14.9 | 5.4–17.1 |
| Missing | 85 | 81 |
| Gender | ||
| Male | 102 (63.0) | 110 (67.1) |
| Female | 59 (36.4) | 54 (32.9) |
| Missing | 1 (0.6) | 0 |
| Height (cm) | ||
| Mean (SD) | 168.8 (10.8) | 169.0 (12.2) |
| Missing | 1 | 0 |
| Aetiology of ARDS | ||
| Direct | 103 (63.6) | 105 (64.0) |
| Smoke/toxin inhalation | 1 | 2 |
| Gastric content aspiration | 6 | 9 |
| Near drowning | 1 | 0 |
| Thoracic trauma | 5 | 9 |
| Pneumonia | 86 | 79 |
| Drug related | 2 | 1 |
| Other | 2 | 5 |
| Indirect | 58 (35.8) | 59 (36.0) |
| Sepsis | 39 | 47 |
| Cardiopulmonary bypass | 1 | 1 |
| Pancreatitis | 6 | 4 |
| Non-thoracic trauma | 2 | 6 |
| Transfusion related | 6 | 1 |
| Other | 4 | 0 |
| Missing | 1 | 0 |
| Time from ICU admission to randomisation (days) | ||
| Mean (SD) | 2.7 (2.9) | 2.5 (2.6) |
| Median (IQR) | 2 (1–3) | 2 (1–3) |
| Range | 0–18 | 0–18 |
| Missing | 1 | 0 |
Treatment with study drug
The study drug infusion was not given to two patients in the salbutamol arm. One of these required a β-blocker between randomisation and starting the study drug and the other patient's next of kin refused to have a separate i.v. line inserted for the study drug infusion, after initially giving consent.
Patients in the salbutamol group were more likely to have their infusion terminated early than patients in the placebo arm. This was mainly due to death (14/161 vs. 8/163), or the development of significant side effects (47/161 vs. 13/163). The duration of infusion was on average 24.5 hours shorter in the salbutamol group [mean (SD), 114.1 (62.7) hours vs. placebo group 138.6 (47.9) hours; p < 0.01]. The risks of developing a tachycardia, new arrhythmia or lactic acidosis severe enough to warrant stopping the study drug were substantially higher in the salbutamol group (Table 5).
A small number of patients had infusions that were given in error for more than 168 hours. Most of these were terminated soon after 168 hours when the error was noticed, but a few infusions were continued for considerably longer. Infusions of > 168 hours were more common in the placebo group (10 vs. 5 patients), presumably because more patients in the salbutamol group had already had their infusion terminated before reaching 168 hours and therefore the chance of accidentally exceeding 168 hours was lower.
Patients who withdrew from the study were specifically asked for permission to continue data collection, including the 6- and 12-month follow-ups. Two patients (one in each arm) withdrew and did not allow further data collection; hence, no outcome data were available for these patients. One patient withdrew from treatment only and was happy for data collection to continue, a further patient withdrew from the follow-up only and is therefore included in the analysis of short-term outcomes and long-term mortality.
| Salbutamol (%) (n = 162) | Placebo (%) (n = 164) | |
|---|---|---|
| Study drug given | 160 | 164 |
| Study drug not given | 2 | 0 |
| Time from randomisation to start of study drug (hours) | ||
| Mean (SD) | 2.0 (2.5) | 1.9 (2.6) |
| Median (IQR) | 1.3 (0.6–2.5) | 1.1 (0.6–2.2) |
| Range | 0–17 | 0–21.3 |
| Study drug not given | 2 | 0 |
| Missing | 1 | 0 |
| Duration of treatment with study drug (hours) | ||
| Mean (SD) | 114.1 (62.7) | 138.6 (47.9) |
| Median (IQR) | 144 (54–168) | 167.5 (118.75–168) |
| Range | 0–234 | 3–186 |
| < 168 | 106 | 82 |
| > 168 | 5 | 10 |
| Missing | 1 | 0 |
| Reasons for termination of study drug | ||
| 168 hours since randomisation | 64 | 96 |
| Death | 14 | 8 |
| Tachycardia | 23 | 2 |
| New arrhythmia | 14 | 3 |
| Lactic acidosis | 10 | 1 |
| 24 hours after discontinuation of mechanical ventilation | 11 | 22 |
| Discharge from critical care | 1 | 3 |
| Discontinuation of active treatment | 4 | 5 |
| Request by patient or legal representative | 2 | 1 |
| Stopped early in error | 8 | 9 |
| Stopped late in error | 1 | 0 |
| Clinical need for β2-agonists | 0 | 2 |
| Transfer to another ICU | 2 | 1 |
| Study suspended | 1 | 2 |
| No reason for early termination given | 3 | 4 |
| Other | 1 | 5 |
| Study drug infusion not started | 2 | 0 |
| Missing | 1 | 0 |
| Given non-trial β2-agonists | 63 | 55 |
| Days of non-trial β2-agonists, mean (SD) | 1.9 (4.1) | 1.6 (3.8) |
| Range | 0–24 | 0–23 |
| Missing | 1 | 1 |
| Protocol violations | ||
| Post-randomisation withdrawal | 2 | 2 |
| Withdrew from treatment only, all data collected | 0 | 1 |
| Withdrew and refused use of all data | 1 | 0 |
| Withdrew, allowed use of existing data but no further data collection | 0 | 1 |
| Withdrew from follow-up only, all other data collected | 1 | 0 |
| Missing outcome data due to withdrawal | 1 | 1 |
| Ineligible patient | 0 | 0 |
| Did not receive allocated treatment | 2 | 0 |
| Received treatment of other group | 0 | 0 |
Outcomes
Primary outcome
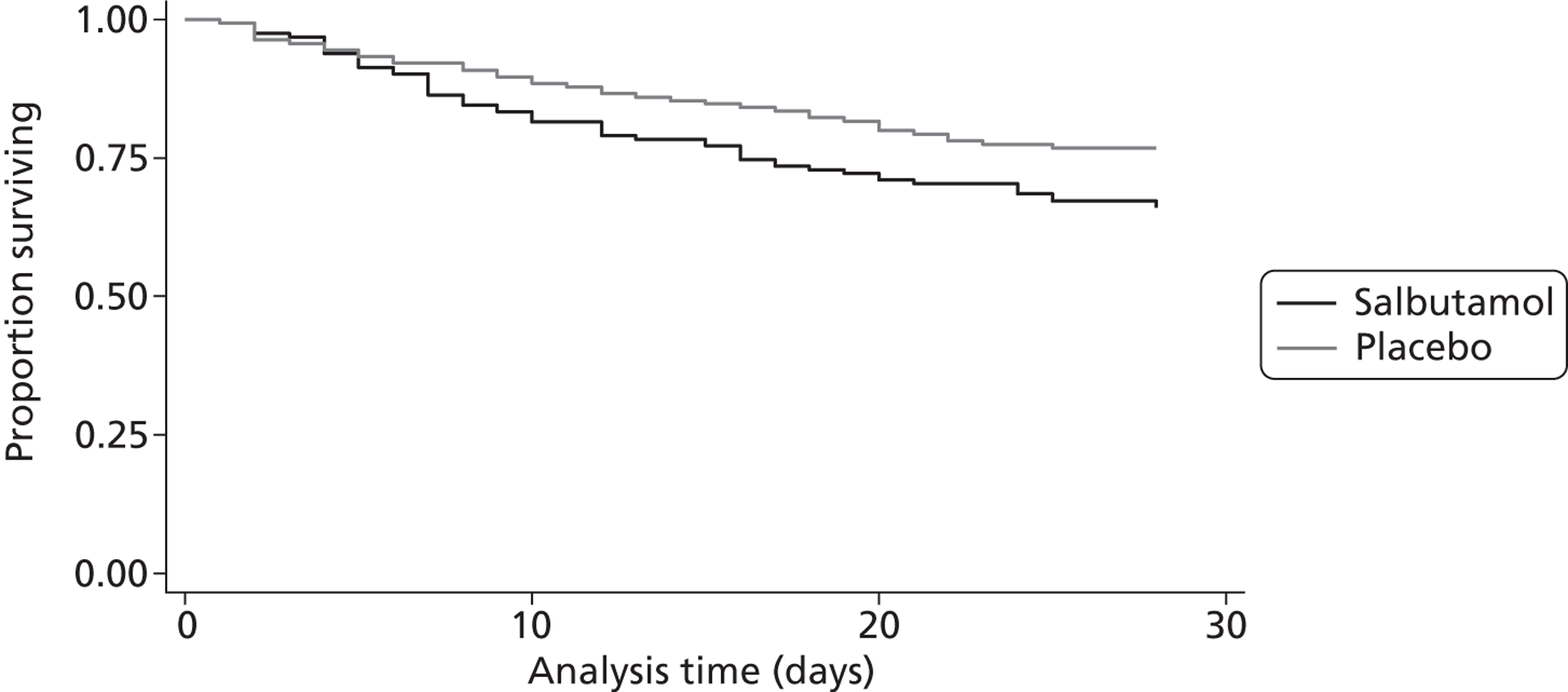
The risk ratio for death at 28 days in the salbutamol group compared with the placebo group was 1.47 (95% CI 1.03 to 2.08; p = 0.03) (Table 6). Salbutamol resulted in a 10.9% (95% CI 1.0% to 20.4%) absolute increase (34.2% vs. 23.3%) in 28-day mortality (see Table 6). There was one additional death for every 9.2 (95% CI 4.9 to 100.9) ARDS patients treated with salbutamol (number needed to treat for harm). Survival analysis for the primary outcome (Figure 6) showed a hazard ratio of 1.56 (95% CI 1.03 to 2.36).
| Salbutamol (%) (n = 162) | Placebo (%) (n = 164) | Statistics (95% CI) | |
|---|---|---|---|
| Primary outcome; mortality at 28 days post randomisation | 55 (34.0) | 38 (23.2) | RR = 1.47 |
| Missing | 1 | 1 | (1.03 to 2.08) |
| Death before discharge from ICU | 58 (35.8) | 45 (27.4) | RR = 1.31 |
| Missing | 1 | 1 | (0.95 to 1.80) |
| Death before discharge from hospital | 62 (38.3) | 53 (32.3) | RR = 1.18 |
| Missing | 1 | 1 | (0.88 to 1.59) |
| Tachycardia sufficient to stop treatment with study drug | 23 (14.2) | 2 (1.2) | RR = 11.71 |
| Missing | 1 | 0 | (2.81 to 48.88) |
| New arrhythmia sufficient to stop treatment with study drug | 14 (8.6) | 3 (1.8) | RR = 4.75 |
| Missing | 1 | 0 | (1.39 to 16.23) |
| Other side effects sufficient to stop treatment with study drug | 10 (6.2) | 1 (0.6) | RR = 10.19 |
| Missing | 1 | 0 | (1.32 to 78.66) |
| VFDs | |||
| Mean (SD) | 8.46 (8.83) | 11.14 (9.32) | Difference –2.68 |
| Median (IQR) | 6 (0–16) | 13 (0–20) | (–4.67 to –0.70) |
| Range | 0–26 | 0–27 | |
| Missing | 1 | 1 | |
| Organ failure-free days | |||
| Mean (SD) | 16.2 (10.7) | 18.5 (9.8) | Difference –2.30 |
| Median (IQR) | 19 (5–26) | 23 (12–26) | (–4.54 to –0.06) |
| Range | 0–28 | 0–28 | |
| Missing | 1 | 1 | |
| High-frequency oscillatory ventilationa | 10 | 7 | RR = 1.53 |
| Missing | 81 | 77 | (0.61 to 3.84) |
| Duration of ICU stay (days) | |||
| Mean (SD) | 17.6 (14.3) | 17.1 (14.0) | Difference +0.5 |
| Median (IQR) | 15 (8–23) | 13 (7.5–21.5) | (–2.6 to 3.6) |
| Range | 0–85 | 0–91 | |
| Missing | 1 | 1 | |
| Duration of hospital stay (days) | |||
| Mean (SD) | 32.5 (35.9) | 34.9 (36.3) | Difference –2.4 |
| Median (IQR) | 23 (12–39) | 22 (14–42) | (–10.3 to 5.5) |
| Range | 0–191 | 0–243 | |
| Missing | 1 | 1 | |
| Duration of ICU stay excluding deaths (days) | n = 103 | n = 118 | |
| Mean (SD) | 20.5 (15.3) | 17.1 (12.6) | Difference +3.4 |
| Median (IQR) | 17 (11–23.5) | 13 (8–22) | (–0.3 to 7.1) |
| Range | 3–85 | 1–82 | |
| Duration of hospital stay excluding deaths (days) | n = 99 | n = 110 | |
| Mean (SD) | 42.4 (37.8) | 40.7 (38.9) | Difference +1.7 |
| Median (IQR) | 32 (20–49) | 26 (17–49) | (–8.8 to 12.2) |
| Range | 4–277 | 7–243 | |
| Missing | 1 | 1 | |
| Days level 3 care | |||
| Mean (SD) | 14.1 (8.0) | 13.2 (8.0) | Difference +0.9 |
| Median (IQR) | 13 (7–20) | 11 (7.5–19) | (–0.85 to 2.65) |
| Range | 1–28 | 1–28 | |
| Missing | 1 | 1 | |
FIGURE 6.
Kaplan–Meier plot for survival over the first 28 days.
The result for the primary outcome at the second interim analysis, when the DMEC took the decision to recommend stopping recruitment based on data from 273 patients, was a risk ratio of 1.55 (95% CI 1.07 to 2.24) and the 99.8% CI excluded a benefit for salbutamol of the size anticipated in the protocol.
Short-term secondary outcomes
Consistent with 28-day mortality, the risk ratio for death within ICU was 1.31 (95% CI 0.95 to 1.80; p = 0.10) and for death within hospital was 1.18 (95% CI 0.88 to 1.59; p = 0.26) (see Table 6). There was an 8.4% absolute increase (95% CI –1.7% to 18.3%) in ICU mortality and 6.0% increase in hospital mortality (95% CI –4.4% to 16.2%) in the salbutamol group.
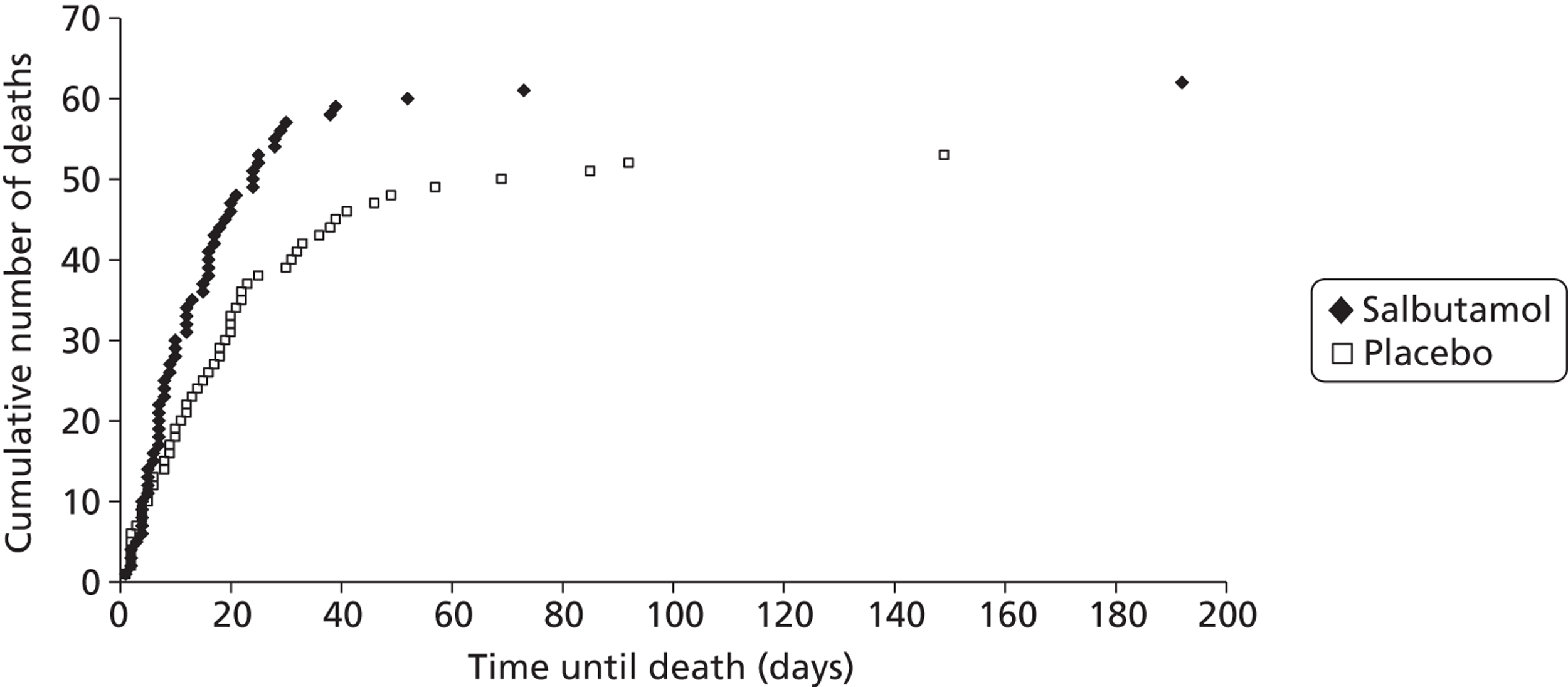
The differences between the groups in ICU and (especially) hospital mortality were smaller than the difference in 28-day mortality. This was because the deaths in the salbutamol group occurred earlier, although the final proportions that eventually died in hospital were fairly similar (Figure 7). Deaths in hospital continued to occur up to nearly 200 days after randomisation.
FIGURE 7.
Cumulative numbers of deaths in hospital with time from randomisation.
Ventilator-free days and organ failure-free days during the first 28 days after randomisation were both reduced in the salbutamol group (see Table 6). No clear differences were detected between the groups in lengths of ICU and hospital stays, or in level 3-care bed days (see Table 6). ARDS survivors in the salbutamol group required on average 3.4 more days in ICU than those in the placebo group (95% CI of difference –0.3 to 7.1 days).
Long-term outcomes
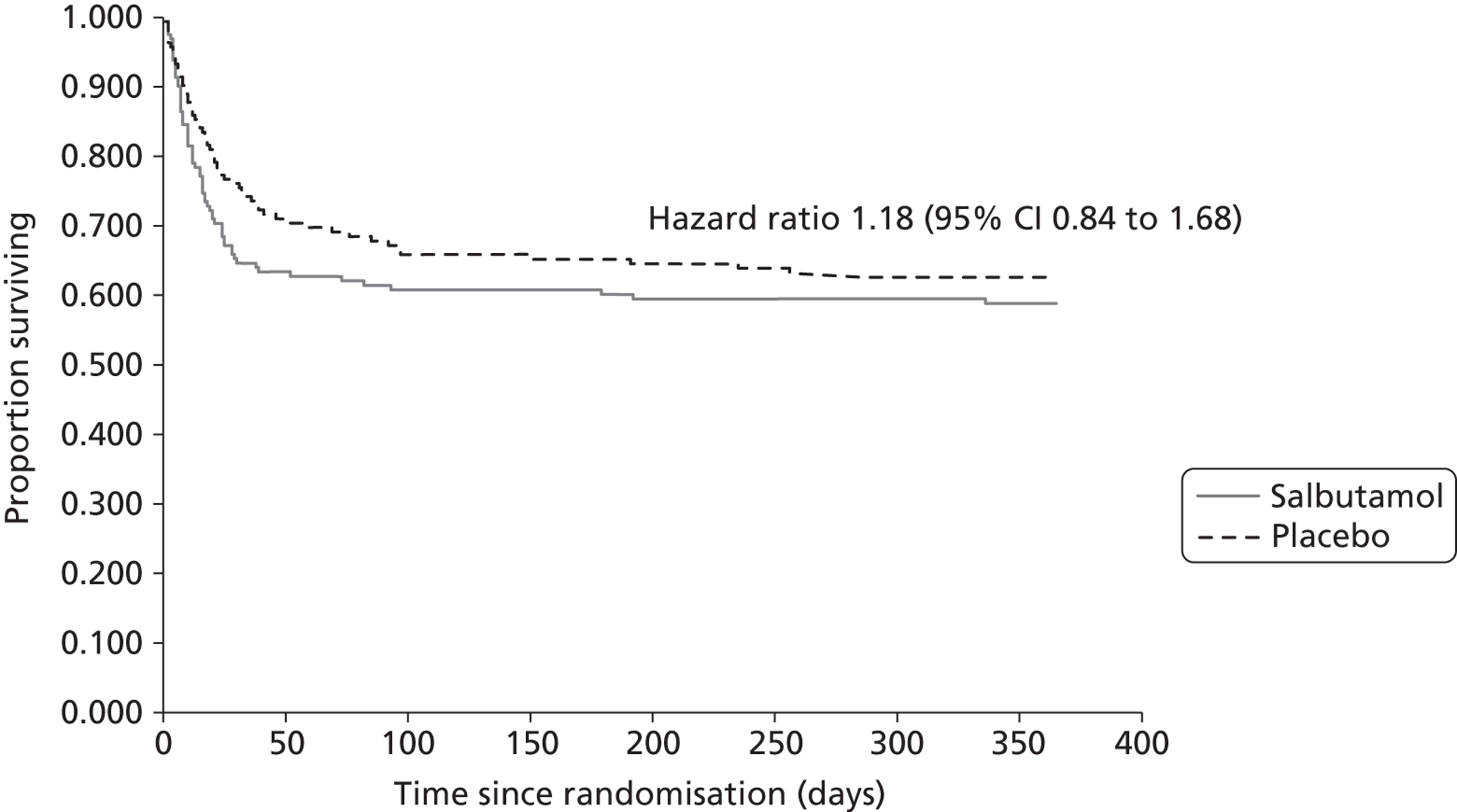
Mortality at 12 months was higher in the salbutamol group than in the placebo group (42.0% vs. 38.5%), but the 95% CI included 1, and the data are compatible with a small reduction in 12-month mortality or an increase (Table 7). The survival analysis of death up to 12 months after randomisation gave a hazard ratio of 1.18 (95% CI 0.84 to 1.68); the Kaplan–Meier plot showed that although the final difference in the proportions that died at 12 months was moderate, the majority of deaths occurred earlier in the salbutamol group (Figure 8).
FIGURE 8.
Kaplan–Meier plot for 12-month survival.
The proportion followed up at 6 and 12 months was disappointingly low (see Figure 3). This was for a combination of reasons. First, owing to a lack of resources the pilot study did not include the follow-up; the intention was to begin following up patients when the main study started and resources allowed. Hence, 33 patients were not included in the follow-up. Second, 22 patients at 6 months and 16 patients at 12 months were not contacted for a range of other reasons (see Figure 3). Third, the proportion of questionnaires returned was low (52.7% and 55.0% of those contacted at 6 and 12 months, respectively).
No clear differences were found in the SF-12 and EQ-5D scores at 6 months, though all were lower in the salbutamol group. A similar pattern was found at 12 months; all of the scores were lower in the salbutamol group and for the SF-12 physical component score the 95% CI did not include zero. These results suggest that, consistent with the short-term outcomes, salbutamol may be associated with a lower quality of life 6 and 12 months after randomisation, but these results should be interpreted with considerable caution because of the high number of missing data.
| Salbutamol (%) (n = 162) | Placebo (%) (n = 164) | Statistics (95% CI) | |
|---|---|---|---|
| Death in 12 months after randomisation | 66 (40.7) | 60 (36.6) | RR = 1.09 |
| Missing (status unknown) | 5 | 8 | (0.83 to 1.43) |
| Health-related quality of life at 6 months | |||
| n = 42 | n = 32 | ||
| SF-12 physical component score; mean (SD) | 34.9 (11.0) | 38.9 (11.3) | Difference −3.61 (−8.85 to 1.63) |
| SF-12 mental component score; mean (SD) | 42.1 (13.4) | 44.4 (12.5) | Difference −2.25 (−8.30 to 3.79) |
| n = 43 | n = 33 | ||
| EQ-5D | 0.52 (0.39) | 0.60 (0.33) | Difference −0.09 (−0.25 to 0.08) |
| Health-related quality of life at 12 months | |||
| n = 37 | n = 37 | ||
| SF-12 physical component score; mean (SD) | 37.8 (12.3) | 43.6 (12.6) | Difference −5.78 (−11.56 to −0.01) |
| SF-12 mental component score; mean (SD) | 45.0 (12.4) | 50.4 (11.7) | Difference −5.39 (−10.97 to 0.19) |
| n = 39 | n = 38 | ||
| EQ-5D | 0.59 (0.37) | 0.76 (0.22) | Difference −0.14 (−0.28 to 0.01) |
Sensitivity analyses
We performed sensitivity analyses to explore adjustment of the analysis of the primary outcome for baseline variables (age, gender, PaO2–FiO2 ratio and aetiology). No adjustment for any of the baseline factors alone or in combination made a substantial difference to the estimate of the treatment effect of salbutamol or altered the conclusions. The unadjusted odds ratio (OR) was 1.71 (95% CI 1.05 to 2.78) and the adjusted ORs were between 1.70 and 1.76, and all had a lower 95% confidence limit > 1.
Subgroup analyses
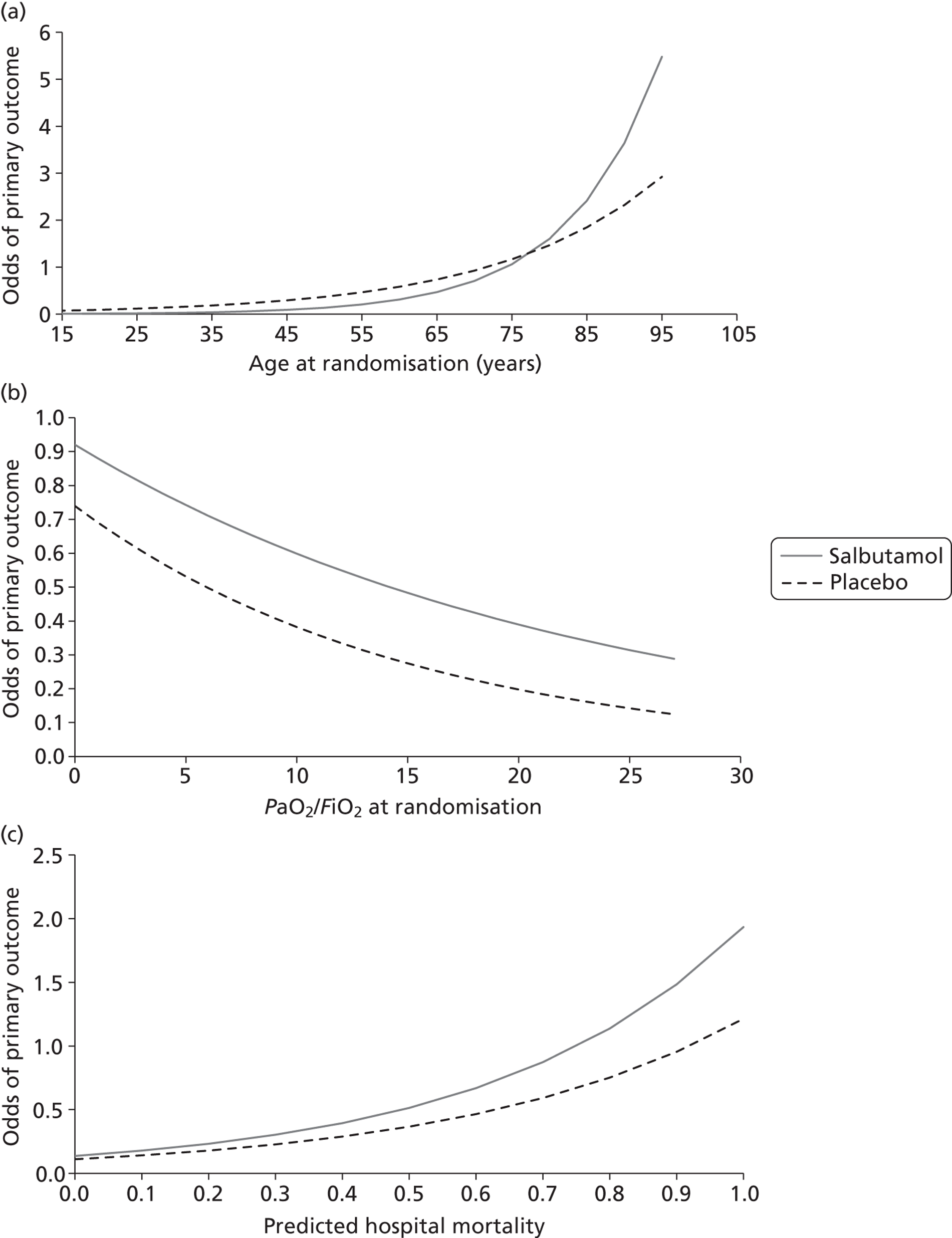
We investigated modification of the treatment effect by four prespecified factors: aetiology, age, severity of hypoxaemia and APACHE II mortality risk. Subgroup analyses did not suggest that the effects of salbutamol were modified by any of the variables investigated. For aetiology (categorical subgrouping variable) the ratio of risk ratios was 0.96 (95% CI 0.46 to 2.01) (Table 8). The analysis by age suggested weak evidence of a possible interaction effect, whereby salbutamol is superior to placebo in the oldest patients [ratio of ORs 0.97 (95% CI 0.93 to 1.00); p = 0.07 (Table 9)]. However, as the effect is small and there were very few patients in the oldest age stratum (n = 4 aged > 85 years) this is likely to be a chance finding. For the other continuous variables, there was no evidence of an interaction (Tables 10 and 11 and Figure 9). The ratios of ORs for each variable investigated were: severity of hypoxaemia 1.02 (95% CI 0.92 to 1.14); p = 0.66 and mortality risk 1.29 (95% CI 0.08 to 22.04); p = 0.86.
| Primary outcome (28-day mortality) | Statistics | |||
|---|---|---|---|---|
| Salbutamol | Placebo | |||
| Direct | 35/102 (34.3%) | 25/106 (23.6%) | RR = 1.46 (95% CI 0.94 to 2.25) | Ratio of RRs 0.96 (95% CI 0.46 to 2.01) |
| Missing | 0 | 1 | ||
| Indirect | 20/58 (34.5%) | 13/57 (22.8%) | RR = 1.51 (95% CI 0.83 to 2.74) | |
| Missing aetiology | 0/2 | 0/0 | ||
| Primary outcome (28-day mortality) | ||
|---|---|---|
| Age (years) | Salbutamol | Placebo |
| ≤ 42 | 6/39 (15.4%) | 4/47 (8.5%) |
| 43–57 | 8/40 (20.0%) | 3/36 (8.3%) |
| Missing | 0 | 1 |
| 58–69 | 23/42 (54.8%) | 10/44 (22.7%) |
| Missing | 1 | 0 |
| ≥ 70 | 18/40 (45.0%) | 21/36 (58.3%) |
| PaO2–FiO2 | Primary outcome (28-day mortality) | |
|---|---|---|
| Salbutamol | Placebo | |
| > 17.3 | 8/37 (21.6%) | 9/45 (20.0%) |
| 13.2–17.3 | 18/47 (38.3%) | 5/35 (14.3%) |
| 9.8–13.2 | 14/37 (37.8%) | 11/41 (26.8%) |
| Missing | 1 | 1 |
| < 9.8 | 15/40 (37.5%) | 13/41 (31.7%) |
| Missing PaO2–FiO2 | 0 | 1 |
| APACHE II mortality risk | Primary outcome (28-day mortality) | |
|---|---|---|
| Salbutamol | Placebo | |
| 0.00–0.25 | 4/26 (15.4%) | 2/32 (6.3%) |
| 0.25–0.50 | 19/55 (34.5%) | 11/48 (22.9%) |
| 0.50–0.75 | 10/38 (26.3%) | 15/38 (39.5%) |
| 0.74–1.00 | 6/7 (85.7%) | 3/10 (30.0%) |
| Missing APACHE II mortality risk | 36 | 36 |
FIGURE 9.
Subgroup analyses: variation of estimated odds of primary outcome with (a) age; (b) severity of hypoxaemia; and (c) APACHE II mortality risk.
Serious adverse events
Serious adverse event reports were received for 14 patients, 10 in the salbutamol group and 4 in the placebo group (see Appendix 1).
Causes of death
Data on cause of death were returned for 91/93 patients who died by day 28 (97.8%): 55/55 in the salbutamol group and 36/38 in the placebo group.
Owing to the diversity of individual diagnoses, causes of death results were grouped according to organ system. Respiratory system diagnoses were the most common primary cause of death in both groups (salbutamol 50.9% vs. placebo 52.6%), followed by multiorgan failure (salbutamol 21.8% vs. placebo 36.8%). ARDS was recorded on the death certificate for 11/55 patients in the salbutamol group and 8/38 patients in the placebo group.
Respiratory system diagnoses most commonly accounted for the primary (1a) cause of death in both groups, 50% of the salbutamol group and 52.6% of the placebo group (Table 12). Pneumonia/lower respiratory tract infection was the single most frequent cause of death recorded in 1a in the salbutamol group (28.6%), while multiorgan failure was most commonly recorded in the placebo group (36.8%). Respiratory system diagnoses predominated in cause of death 1b in both groups (30.4% vs. 29%; Table 13). The single most frequent diagnosis in the salbutamol group was pneumonia/lower respiratory tract infection (19.6%) compared with sepsis/septicaemia in the placebo group (23.7%).
| Cause of death | Salbutamol, n (%) (N = 55) | Placebo, n (%) (N = 38) |
|---|---|---|
| Respiratory system | 28 (50.9) | 20 (52.6) |
| Cardiovascular system | 1 (1.9) | 1 (2.6) |
| Neurological system | 2 (3.6) | 1 (2.6) |
| Gastrointestinal system | 3 (5.5) | 0 (0) |
| Multiorgan failure | 12 (21.8) | 14 (36.8) |
| Sepsis/septicaemia | 7 (12.7) | 2 (5.3) |
| Malignancy | 2 (3.6) | 1 (2.6) |
| Missing | 1 (1.8) | 2 (5.3) |
| Cause of death | Salbutamol, n (%) (N = 55) | Placebo, n (%) (N = 38) |
|---|---|---|
| Respiratory system | 16 (29.1) | 11 (29.0) |
| Cardiovascular system | 4 (7.3) | 0 (0) |
| Neurological system | 0 (0) | 1 (2.6) |
| Gastrointestinal system | 6 (10.9) | 5 (13.2) |
| Malignancy | 1 (1.8) | 0 (0) |
| Orthopaedic | 2 (3.6) | 0 (0) |
| Genitourinary system | 1 (1.8) | 0 (0) |
| Othera | 13 (23.6) | 12 (31.6) |
| Nil stated | 14 (25.4) | 8 (21.1) |
| Missing | 1 (1.8) | 2 (5.3) |
In the majority of patients in both groups no entry was recorded under cause of death 1c. Respiratory system diagnoses continued to predominate in both groups where a diagnosis was recorded in this section of the certificate; 10.7% in the salbutamol group and 13.2% in the placebo group. Pneumonia/lower respiratory tract infection was the commonest single diagnosis in both groups (8.9% and 10.5%).
The number of patients with comorbid conditions recorded under cause of death 2 on the certificate was significantly greater overall in the salbutamol group (62.5%) compared with the placebo group (36.8%). Cardiovascular system diseases were most frequently recorded in the salbutamol group (23.2%) but rarely in the placebo group (5.3%). There were no other major differences between individual or system diagnoses between the two groups.
Acute respiratory distress syndrome was recorded on the death certificate for 11/56 patients in the salbutamol group and 8/38 patients in the placebo group. In the salbutamol group, the death certificate indicated a direct aetiology for ARDS in seven patients and an indirect aetiology in four patients. In the placebo group the death certificate indicated a direct aetiology for ARDS in six patients and an indirect aetiology in two patients.
Chapter 4 Economic analysis
Aim and perspective
Initially the aim of the BALTI-2 economic analysis was to assess the within-trial cost-effectiveness of a 7-day continuous infusion of salbutamol at 12 months and to construct a cost-effectiveness model with a lifetime horizon. However, owing to the limitations of available data (particularly follow-up data post hospital discharge) resulting from the trial being stopped early, the economic analyses were changed from those described in the original protocol. In this chapter we present the following:
-
analysis 1: health-care costs at 28 days
-
analysis 2: cost-effectiveness analysis at 28 days based on life-years gained
-
analysis 3: cost–utility analyses at 6 and 12 months.
Although analyses 1 and 2 allow a larger sample to be included in the evaluation, giving more robust results, they do not provide any information on longer-term cost-effectiveness or quality of life. Analysis 3, including follow-up data at 6 and 12 months, gives a more comprehensive picture of cost-effectiveness; however, there is greater uncertainty in the estimates because of the considerably smaller sample size.
Description of methods of analyses
Analysis 1: health-care costs at 28 days
Analysis 1 calculates the costs to the health sector (based on the cost of inpatient days and of salbutamol) at 28 days. The analysis uses the trial-reported survival data and prospectively collected patient-specific hospital data to calculate the cost to the NHS for participants who survived 28 days and those who did not. The results are presented for each arm of the trial.
Non-parametric and parametric tests (independent t-tests) were run to determine whether or not there were significant differences in NHS resource use between the groups.
Analysis 2: cost-effectiveness analysis at 28 days
The aim of the second analysis is to determine whether or not there were any differences in the costs to the NHS and life-years gained at 28 days between the salbutamol and placebo arms of the trial.
A cost-effectiveness analysis was undertaken from the perspective of the health-care provider; the expected incremental cost-effectiveness ratio (ICER) is presented. 43 As per analysis 2, this analysis uses the trial-reported survival data and prospectively collected patient hospital data for participants who survived 28 days and those who did not. The ICER represents the additional cost per life-year gained. Scatterplots on the cost-effectiveness plane are presented illustrating the uncertainty surrounding the cost-effectiveness estimates. The cost-effectiveness planes were derived using bootstrapping with replacement. This stochastic uncertainty analysis uses 10,000 bootstrapped estimates of the incremental costs and life-years gained. Cost-effectiveness acceptability curves (CEACs) were also generated.
Analysis 3: cost–utility analysis at 6 and 12 months
The aim of the third analysis is to assess the cost-effectiveness of salbutamol compared with the placebo at 6 and 12 months post randomisation. A cost–utility analysis was undertaken from the perspective of the health- and social-care sectors; an ICER was produced. In this case the ICER represents the additional cost per quality-adjusted life-year (QALY) gained for each intervention compared with its next best alternative. 43,44 As a guideline rule, the National Institute for Health and Care Excellence (NICE) accepts as cost-effective those interventions with an ICER of < £20,000 per QALY. NICE states that, in general, if a treatment costs > £30,000 per QALY, then it would not be considered cost-effective. As in analysis 2, we also present scatterplots on the cost-effectiveness planes illustrating the uncertainty surrounding the cost-effectiveness estimates using the same methods.
Participant health-related quality of life was assessed using the EQ-5D. 45,46 EQ-5D data were collected by postal questionnaire at 6 and 12 months post randomisation. Differences between the randomised groups at follow-up with respect to EQ-5D scores were investigated using non-parametric Mann–Whitney U-tests. Participant responses to the EQ-5D questionnaire were converted to health-state utility values using the UK tariff values47 and then combined with the survival data to construct QALYs. Non-parametric tests were run on the utility values to determine whether or not there was any significant difference between the utility scores of the salbutamol and placebo arms.
Baseline utility values were not collected within the study because of the nature of the intervention, with patients residing in ICU following admittance to the hospital. We have used the utility value for an unconscious patient (–0.402) for the baseline utility, as this is a good proxy for the patients who are being treated. 37
In order to maximise the sample size and make appropriate use of the 12-month data, missing data were imputed using the method of last observation carried forward (LOCF). This process was completed for all patients bar one who had died in the 6–12 months following randomisation (this patient was assigned a zero utility score).
In addition to the prospective resource use data used in analyses 1 and 2, analysis 3 also uses retrospective resource use data collected from patients by postal questionnaire. No resource use data were available post discharge for those patients who survived 28 days but died within 6 months. For this group we have assumed costs equivalent to those in analysis 1. Where data at 12 months were missing, but the individual was still alive, we have assumed that their costs between 6 and 12 months were equivalent to the average costs for this time frame for the arm of the trial that they were allocated to.
Time frame
Costs were calculated for the duration of the initial hospital stay up to 28 days post randomisation for analyses 1 and 2 and for a 6- and 12-month time frame for analysis 3.
Sample size
The sample sizes that were used for the analyses are provided in Table 14. For the cost analysis and cost-effectiveness analyses at 28 days (analyses 1 and 2) the total sample included 162 patients who had been randomised to the salbutamol arm of the trial and 164 patients who were randomised to placebo. For the cost–utility analysis (analysis 3) a sample of 75 patients had completed the health-related quality of life questionnaire at 6 months. Only 54 patients completed both the 6- and 12-month questionnaires. As highlighted earlier, in order to maximise the sample size we imputed missing values using the LOCF method for all those patients who were alive at 12 months and had completed the questionnaire at 6 months but had not completed the questionnaire at 12 months.
| Total sample | Total sample that completed the 6-month self-completion questionnaires | ||
|---|---|---|---|
| Salbutamol | Placebo | Salbutamol | Placebo |
| 162 | 164 | 43 | 32 |
Resource use
Resource use relating to participants' initial hospital episode was collected prospectively from patients' hospital records. The mean values and the SDs of the individual items in the prospectively collected hospital data are reported in Table 15. These data are reported for the full sample and for a subsample of those with follow-up data at 6 months. Non-parametric tests found no significant differences in resource use between the two arms of the trial.
| Outcome | Total sample (collected using patient records), mean (SD) | Total sample that completed the self-completion questionnaire at 6 months, mean (SD) | ||
|---|---|---|---|---|
| Salbutamol (n = 162) | Placebo (n = 164) | Salbutamol (n = 43) | Placebo (n = 32) | |
| Number of days in hospital | 30.6 (30.1) | 33.59 (34.24) | 42.81 (30.71) | 37.15 (28.23) |
| Number of days in ICU | 17.68 (14.31) | 17.30 (14.12) | 21.88 (16.54) | 16.91 (9.90) |
| Days of advanced respiratory support (ventilation) | 14.01 (8.09) | 12.93 (8.06) | 15.23 (8.30) | 13.19 (6.90) |
| Days of advanced cardiovascular support | 4.34 (4.78) | 3.87 (4.36) | 3.62 (4.34) | 2.56 (3.90) |
| Duration of study drug infusion (hours) | 114.80 (62.10) | 138.60 (47.92) | 108.70 (62.06) | 151.13 (32.52) |
| Days patient received non-trial β2-agonists | 2.01 (0.47) | 1.63 (0.45) | 2.11 (3.53) | 1.75 (4.13) |
| Days patient received high-frequency oscillatory ventilation | 0.47 (1.38) | 0.45 (1.68) | 0.62 (1.65) | 0.00 (0.00) |
| Days of level 3 care | 14.07 (8.02) | 13.20 (7.99) | 15.72 (8.47) | 12.81 (6.98) |
| Days of level 2 care | 1.76 (2.93) | 1.96 (2.93) | 2.46 (3.04) | 3.06 (2.99) |
| Days patient received liver support | 0.38 (0.43) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| Days patient received neurological support | 0.47 (1.90) | 0.54 (1.60) | 0.84 (2.34) | 0.23 (0.96) |
| Days patient received renal support | 3.11 (5.81) | 2.92 (5.86) | 3.79 (6.59) | 2.68 (4.93) |
Post-discharge use of health and social care was collected retrospectively by way of postal patient self-completed questionnaires at 6 and 12 months. Table 16 shows the resource use from discharge to 6 months and Table 17 shows the resource use for 6–12 months.
| Resource item | Salbutamol | Placebo | ||
|---|---|---|---|---|
| Total patients | Face-to-face contact (counts) | Total patients | Face-to-face contact (counts) | |
| GP, surgery visit | 39 | 190 | 34 | 109 |
| GP, home visit | 32 | 33 | 30 | 10 |
| District nurse | 36 | 576 | 31 | 309 |
| Health visitor | 29 | 32 | 26 | 28 |
| NHS walk-in centre | 30 | 4 | 25 | 3 |
| Social worker | 30 | 4 | 26 | 25 |
| Physiotherapist | 36 | 174 | 24 | 100 |
| Home help or care worker | 30 | 234 | 26 | 272 |
| Occupational therapist | 33 | 14 | 23 | 5 |
| Outpatient visits | 41 | 25 | 30 | 20 |
| Inpatient days | 35 | 14 | 28 | 4 |
| Attendance at A&E | 32 | 7 | 28 | 5 |
| Day care attendance | 31 | 2 | 28 | 1 |
| Resource item | Salbutamol | Placebo | ||
|---|---|---|---|---|
| Total patients | Face-to-face contact (counts) | Total patients | Face-to-face contact (counts) | |
| GP, surgery visit | 29 | 19 | 27 | 14 |
| GP, home visit | 28 | 3 | 26 | 0 |
| District nurse | 28 | 2 | 28 | 2 |
| Health visitor | 28 | 0 | 26 | 0 |
| NHS walk-in centre | 27 | 0 | 26 | 3 |
| Social worker | 27 | 1 | 26 | 0 |
| Physiotherapist | 27 | 7 | 25 | 3 |
| Home help or care worker | 27 | 1 | 25 | 0 |
| Occupational therapist | 27 | 3 | 25 | 0 |
| Outpatient visits | 27 | 16 | 25 | 7 |
| Inpatient days | 27 | 4 | 25 | 3 |
| Attendance at A&E | 27 | 3 | 25 | 3 |
| Day care attendance | 27 | 0 | 25 | 0 |
Unit costs
The analyses assume price year of 2010. The cost of salbutamol was derived from the British National Formulary. Within the trial patients received an i.v. infusion of salbutamol given at 15 μg/kg IBW/hour for up to 7 days compared with a placebo (0.9% sodium chloride) infusion. The economic analysis assumes an average body weight of 70 kg. Given a net price of £2.48 for a 5-ml ampoule [Ventolin® (A&H) solution for i.v. infusion, salbutamol (as sulphate) 1 mg/ml] this equates to a cost per hourly infusion of £0.62 as shown below:
It is assumed that each 5-ml ampoule will allow for 4 hours of treatment with some wastage.
Cost per hour = £2.48/4 = £0.62 (2012 price) (deflated using Consumer Price Index to 2010 price = £0.58).
The unit costs for hospital resources collected prospectively through patient records during participants' initial hospital episodes are provided in Table 18. The unit costs used in the study for health- and social-care services collected retrospectively by way of patient-completed questionnaire inputs are provided in Table 19.
| Resource item | Cost per day (£) | Source |
|---|---|---|
| Days of level 2 and 3 care (intensive care) | 1390 | NICE clinical guideline 83 costing report48 (2009–10 prices), p. 20 |
| Ward only days | 267 | NHS reference costs (2009–10)27 (VC40Z), rehabilitation for respiratory disorders – level 1 bed |
| Resource item | Face-to-face cost (£) | Telephone call cost (£) | Source |
|---|---|---|---|
| GP, surgery visit | 36 | 22 | PSSRU 2010,49 p. 167 with qualification – based on average visit time/telephone call |
| GP, home visit | 120 | N/A | PSSRU 2010,49 p. 167 with qualification – based on average time |
| District nurse | 27 | N/A | PSSRU 2010,49 p. 159 – per home visit including qualifications |
| Health visitor | 19 | 10 | NHS reference costs (2009–10)27 (CN403FO) |
| NHS walk-in centre | 97 | N/A | PSSRU 2010,49 p. 119 – walk-in services |
| Social worker | 53 per hour | N/A | PSSRU 2010,49 p. 172 – per hour of client-related work |
| Physiotherapist | 45 | N/A | PSSRU 201049 |
| Home help or care worker | 25 | N/A | PSSRU 2010,49 p. 176 |
| Occupational therapist | 17 | N/A | PSSRU 2010,49 p. 152 |
| Outpatient visits | 152 | N/A | PSSRU 2010,49 p. 119 |
| Inpatient days | 149 | N/A | PSSRU 2010,49 p. 31 – assumed rehabilitation |
| Attendance at A&E | 144.05 | N/A | PSSRU 2010,49 p. 119 – assumed admitted |
| Day care attendance | 36 | N/A | PSSRU 2010,49 p. 176 |
Results
Analysis 1: cost analysis at 28 days
The cost analysis is based on the survival rates reported in the trial at 28 days (Table 20). The data shows that 76.83% of those receiving the placebo survived for 28 days compared with 66.05% of those receiving salbutamol.
| Salbutamol | Placebo | |||
|---|---|---|---|---|
| Survived 28 days | Died before 28 days | Survived 28 days | Died before 28 days | |
| Number | 107 | 55 | 126 | 38 |
| Percentage | 66.05 | 33.95 | 76.83 | 23.17 |
The results (Table 21) show that the mean costs for those who survived 28 days following randomisation are higher in the salbutamol arm of the trial than in the placebo arm. The difference in costs was statistically significant (p < 0.05) for those who were alive at 28 days but not for those patients who died within 28 days.
| Resource item | Salbutamol, mean (SD) | Placebo, mean (SD) | ||
|---|---|---|---|---|
| Survived 28 days | Died before 28 days | Survived 28 days | Died before 28 days | |
| Days in ICU | 18.03 (7.70) | 11.47 (7.37) | 16.37 (8.14) | 11.13 (7.39) |
| Days not in level 3 or level 2 care | 22.93 (28.38) | 0.00 (0.00) | 24.23 (32.55) | 0.21 (1.02) |
| Cost (£) up to 28 days per patient (2010 prices) | 26,965.99 (9973.28) | 15,173.44 (9464.59) | 24,569.70 (10,309.09) | 15,529.11 (10,233.98) |
Analysis 2: cost-effectiveness analysis at 28 days
The data on life-years gained at 28 days for both arms of the trial are shown in Table 22. An individual who was alive after 28 days was allocated 0.0767 (28/365) of a life-year gained. In the case of those patients who died within 28 days, the values are based on number of days the patient survived post randomisation.
| Life-years gained, mean (SD) | |
|---|---|
| Salbutamol | Placebo |
| 0.061 (0.024) | 0.066 (0.021) |
Costs to the NHS for the 28-day period were calculated based on patient resource use (days in hospital by level of care and drug use) (see Table 18). The mean costs are shown in Table 23.
| Total cost (£), mean (SD) | |
|---|---|
| Salbutamol | Placebo |
| 23,083.97 (11,248.64) | 22,462.07 (10,953.14) |
The ICER shown in Table 24, calculated using deterministic values and the results from bootstrapping, shows salbutamol to be dominated by the placebo as it is associated with higher resource costs and is less effective (results in fewer life-years gained).
| Sample mean | Bootstrapped estimated mean | |||
|---|---|---|---|---|
| Salbutamol | Placebo | Salbutamol | Placebo | |
| Sample size | 162 | 164 | 10,000 | |
| Expected incremental life-years (effectiveness) | −0.0047 | −0.005 | ||
| Expected incremental cost (£) | 623.13 | 701.00 | ||
| Expected ICER (£) | Dominated (−131,385.83) | Dominated (−142,297.73) | ||
In order to explore the levels of uncertainty surrounding this result, Figure 10 presents the cost-effectiveness plane. This shows a large degree of uncertainty surrounding the results of the ICER calculation. The CEAC (Figure 11) shows for each threshold of willingness to pay the probability that salbutamol would be cost-effective.
FIGURE 10.
Incremental cost-effectiveness plane (28 days).
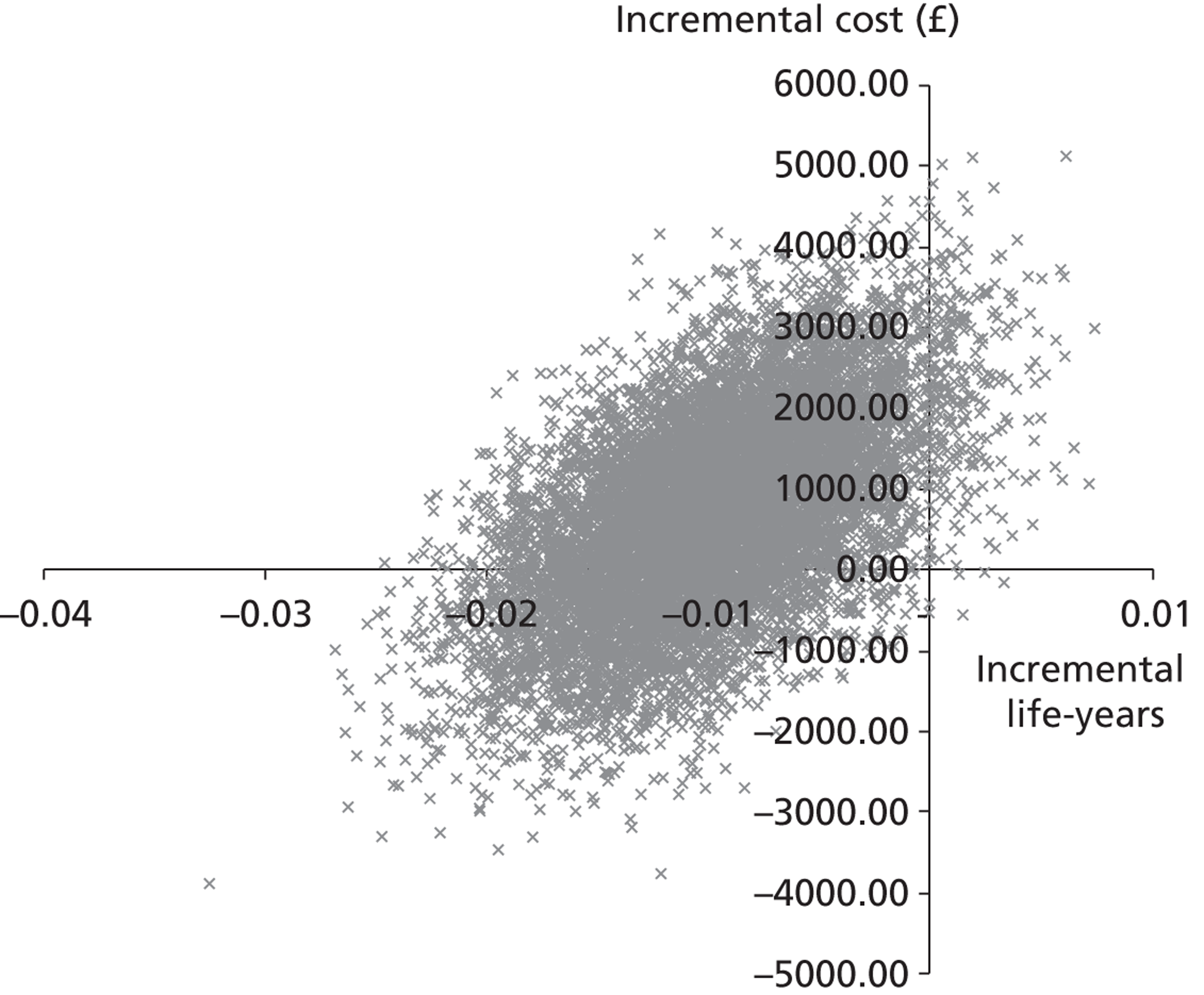
FIGURE 11.
Cost-effectiveness acceptability curve (28 days).
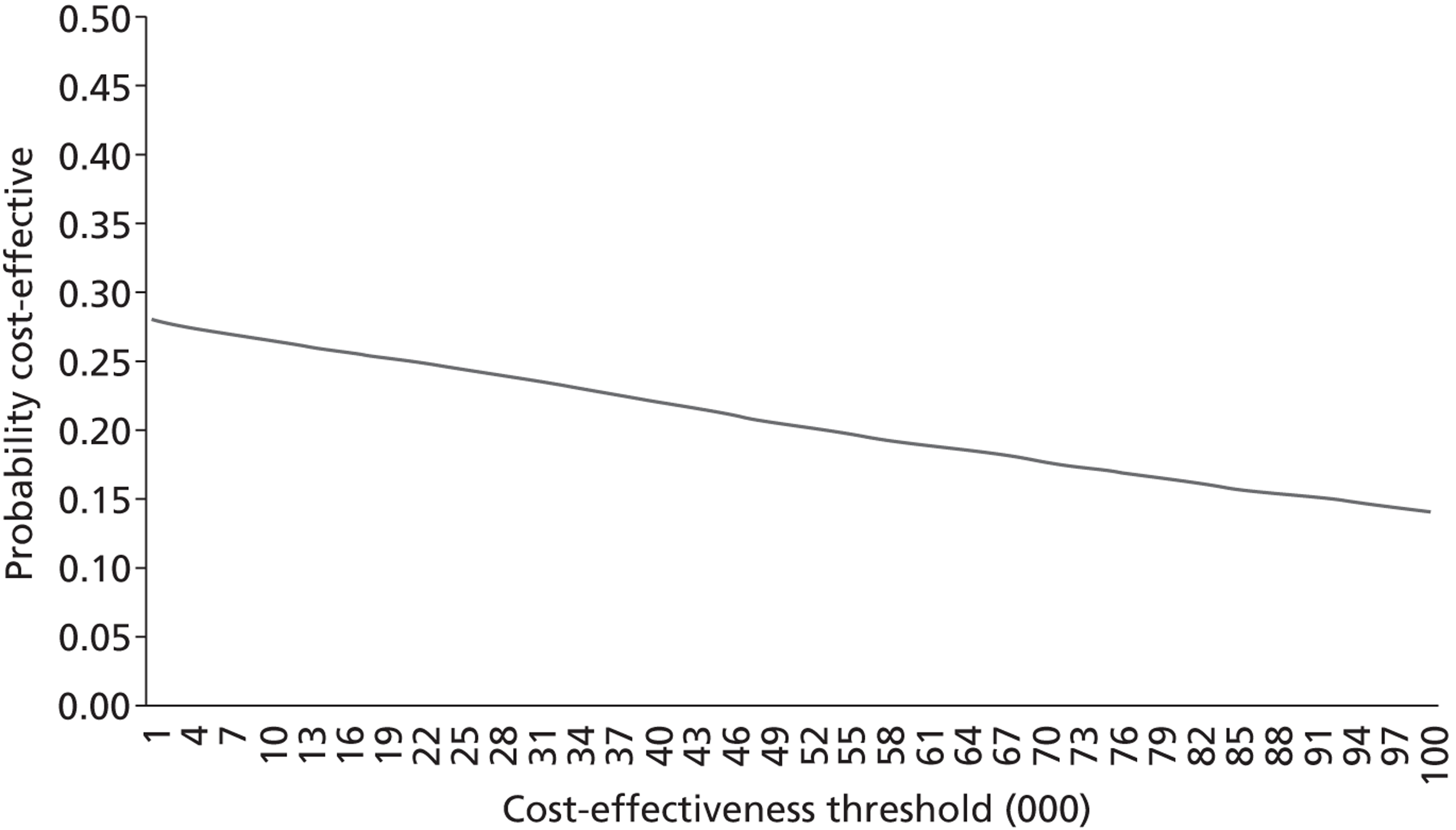
Analysis 3: cost–utility analysis at 6 and 12 months
Table 25 provides the mean utility scores. It indicates that in this sample those patients randomised to the salbutamol arm of the trial had a lower utility score at 6 months than those randomised to the placebo arm. This was still the case at 12 months, although the sample was small. The non-parametric tests on the utility values showed no significant difference between the utility scores for the salbutamol and placebo arms. However, despite the lack of statistical significance, it should be noted that a difference of 0.08 in expected utility will support a cost premium in a cost-effectiveness analysis of approximately £2600 for a threshold of £30,000.
| 6-month data | 6–12-month data | 6–12-month data with imputed missing values | ||||
|---|---|---|---|---|---|---|
| Salbutamol | Placebo | Salbutamol | Placebo | Salbutamol | Placebo | |
| Sample size | 43 | 33 | 29 | 27 | 43 | 32 |
| Mean utility score | 0.5174 (0.3916) | 0.6058 (0.3294) | 0.5855 (0.3809) | 0.7507 (0.2539) | 0.5030 (0.4077) | 0.7075 (0.2607) |
Health and social service costs
Table 26 shows the mean cost per patient under the two arms of the trial calculated using the prospectively collected patient hospital data and the patient self-completed questionnaires at 6 and 12 months.
| Mean costs (£) of resource use from randomisation to 6 months (SD) | Mean costs (£) of resource use between 6 and 12 months (SD) (without imputed values) | |
|---|---|---|
| Salbutamol | 34,689.86 (15,544.52) | 513.75 (817.82) |
| Placebo | 30,319.16 (14,907.23) | 302.40 (459.60) |
A simple decision tree was developed using the health states of dead and survived. The survival rates collected as part of the trial (Table 27) were applied to the model to determine the probabilities that a patient would be in each health state during the time frame.
| Time period | Salbutamol | Placebo | ||
|---|---|---|---|---|
| Died | Survived | Died | Survived | |
| 0–6 months | 64 | 98 | 56 | 108 |
| 6–12 months | 2 | 96 | 4 | 104 |
Incremental cost-effectiveness ratios
The ICERs showing the ratios of the incremental cost and incremental benefits (QALYs) between the salbutamol and placebo arms over 6 and 12 months are given in Table 28. The results indicate that the salbutamol arm of the trial is more costly and less effective than the placebo arm of the trial over both 6 and 12 months (i.e. dominated by the placebo).
| 6-month data (baseline = −0.402) | 12-month data (baseline = −0.402), using 6-month data for missing data | 6 months (baseline = −0.402) | 12 months (baseline = −0.402), including 6 months data | |||
|---|---|---|---|---|---|---|
| Salbutamol | Placebo | Salbutamol | Placebo | Simulation | Simulation | |
| Based on sample size | 43 | 33 | 43 | 32 | 10,000 | 10,000 |
| Difference in QALYs | −0.021 | −0.081 | −0.021 | −0.079 | ||
| Difference in costs (£) | 1873.83 | 1798.80 | 1488.51 | 1854.00 | ||
| ICER (£) | −88,878.63 | −22,176.99 | −72,062.85 | −23,478.09 | ||
In order to explore the levels of uncertainty surrounding the result, Figures 12–14 present CEACs and cost-effectiveness planes. The data indicate, using the mean values for both the 6- and 12-month analyses, that the placebo is more cost-effective. Over a 12-month period (see Figure 12), while there are some points on the right-hand side of the quadrants, indicating that there is a possibility in the longer term that salbutamol could be more cost-effective, the majority of points are still on the left-hand side of the quadrants. Similarly, Figure 13 shows that there are few examples at a 6-month level where salbutamol is more effective than the placebo, with the majority of the simulated points in the left two quadrants.
FIGURE 12.
Cost-effectiveness plane for salbutamol vs. placebo (bootstrapping output) 12-month time frame with imputed values.
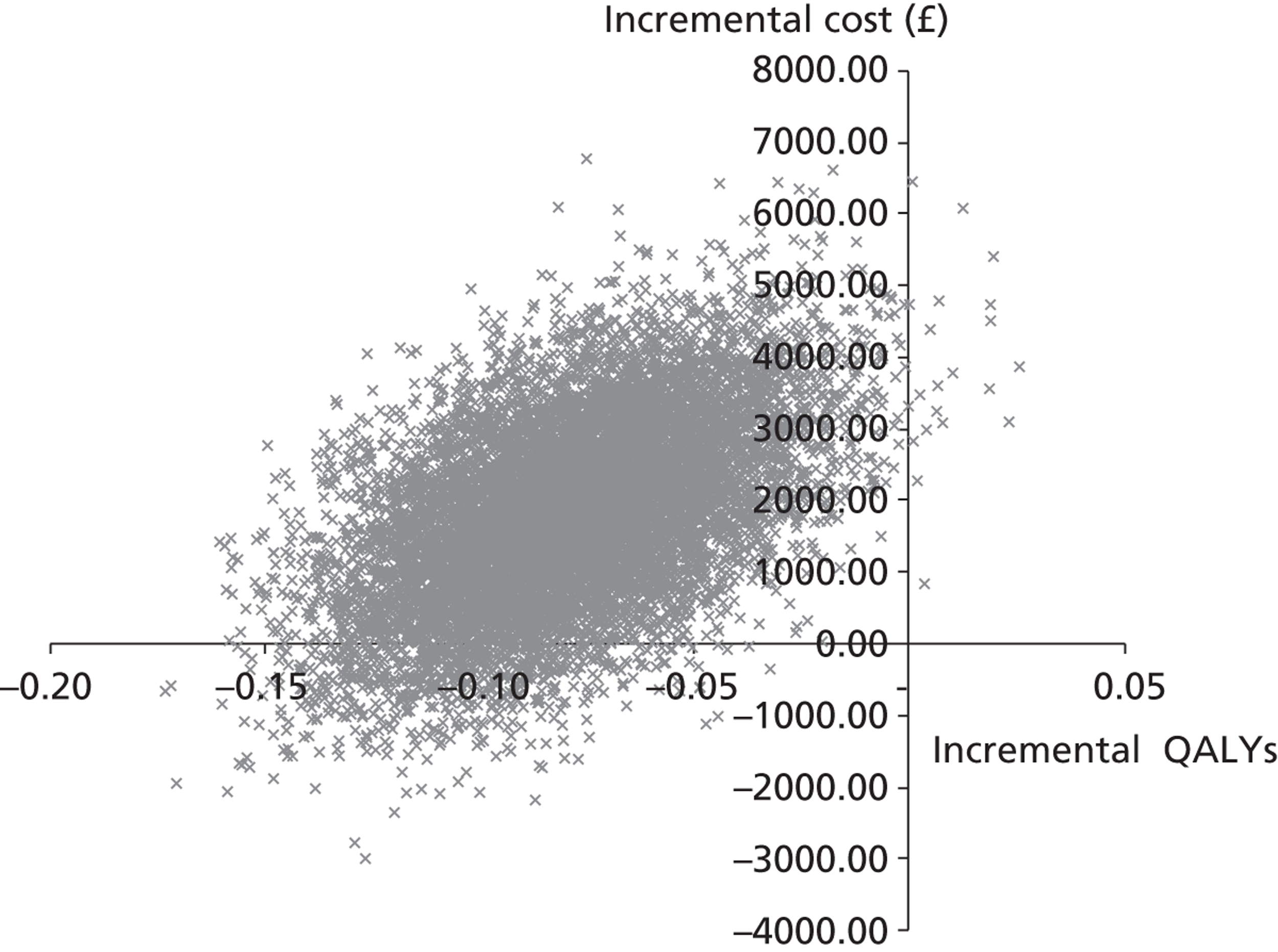
FIGURE 13.
Cost-effectiveness plane – 6-month time data.
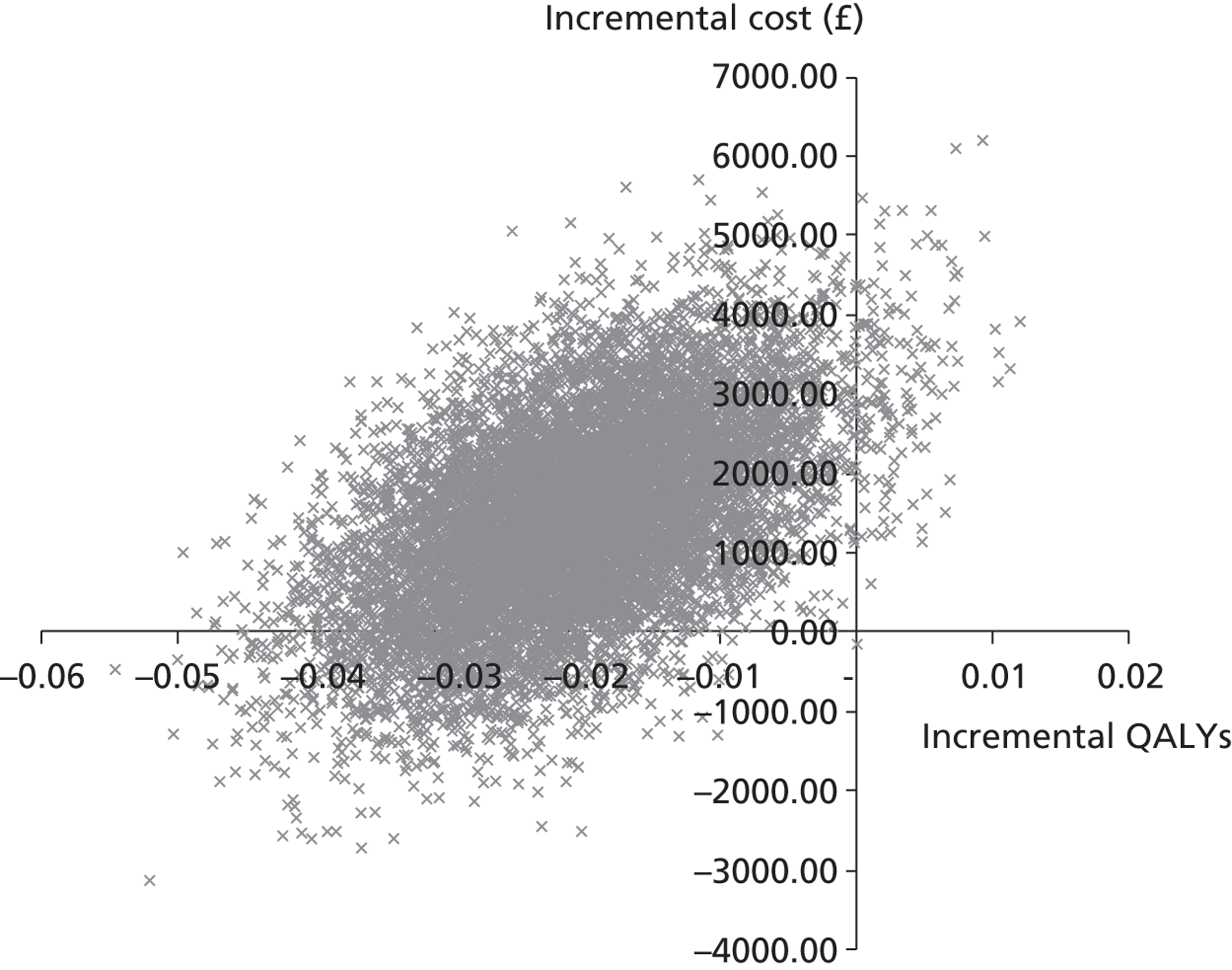
FIGURE 14.
Cost-effectiveness acceptability curves – 12-month data (with imputed values).
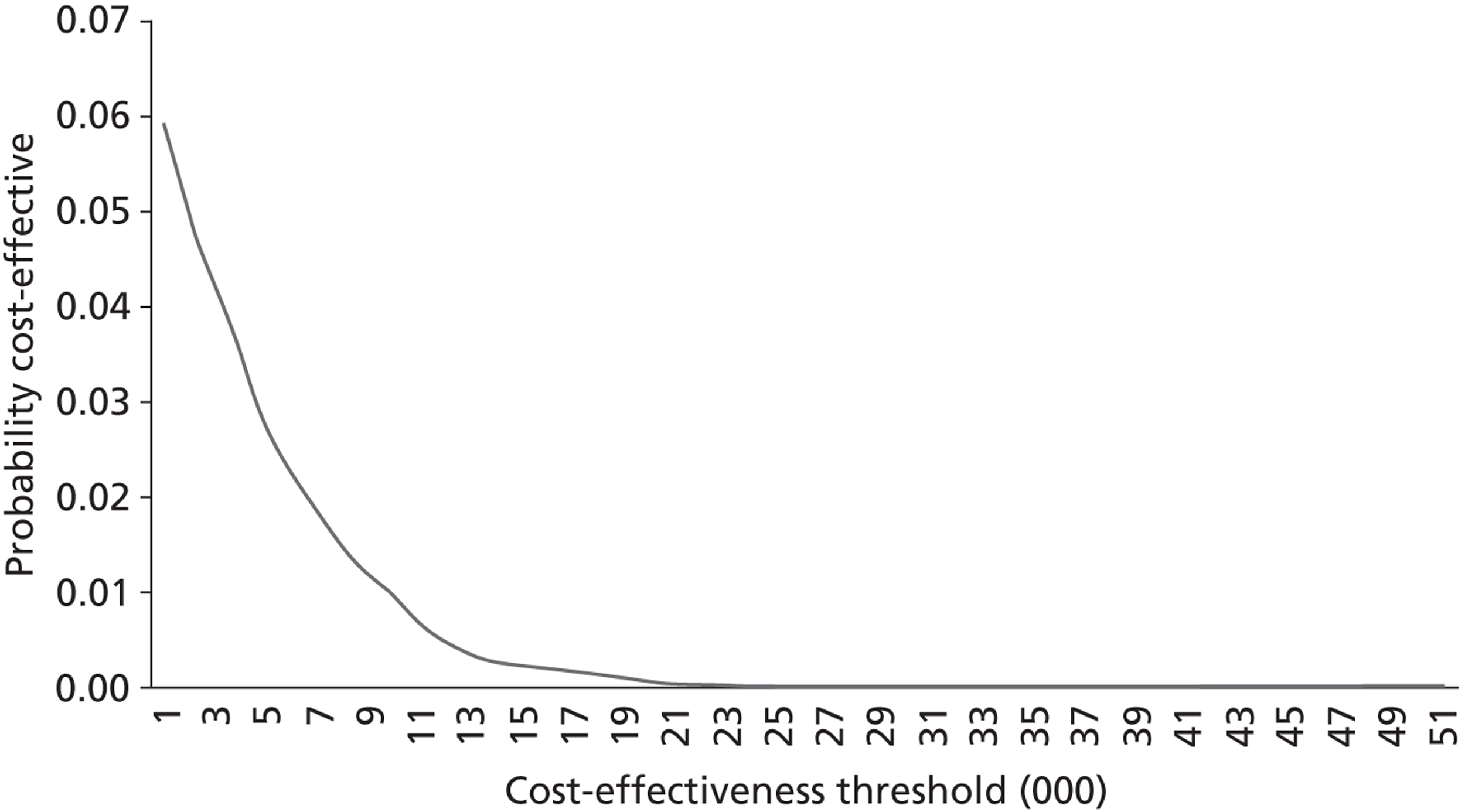
Summary
There were several limitations in this economic evaluation. The analysis at 28 days (analysis 1) indicates that mean cost was higher for patients in the salbutamol arm of the trial than in the placebo arm, both for those who survived 28 days from randomisation and those who died within 28 days. Analysis 2 also shows that the mean costs to the NHS were higher and the life-years gained were lower for the salbutamol arm of the trial. Both these analyses are based on data from the patients' initial hospital episode and as such do not include health- and social-care use between discharge and 28 days (for those participants who survived and were discharged within the 28-day period). Also, no additional costs were included for administering the infusion. As such, the costs are likely to be underestimated. It was not originally intended to undertake analysis at 28 days and while data were collected on health- and social-care use post discharge, and have been included in analysis 3, it was not possible to disaggregate those relating to this period for analyses 1 and 2.
We made assumptions regarding the baseline utility values. Baseline utility value was not collected within the study because of the nature of the intervention, with patients residing in ICU following admittance to the hospital. Within analysis 3 we have assumed a baseline utility value of –0.402 representing ‘unconscious’, which is in line with previous studies. 37
In order to facilitate analysis of the longer-term cost-effectiveness it was necessary to impute missing data given a large proportion of patients not completing the follow-up questionnaires (full details of the follow-up quality-of-life data have been given in the results section of the main trial). Zero utility scores were assigned to participants who had died and we used the method of LOCF for the remaining missing values. The advantage to this approach is that it minimises the number of subjects who are eliminated from the analysis, and it allows the analysis to examine the trends over time, rather than focusing simply on the end point. Other studies have shown a gradual improvement in quality-of-life scores over 12 months following admission to ICU50 so LOCF is likely to be a conservative way of dealing with missing utility data.
As highlighted earlier, in addition to the prospective resource use data used in analyses 1 and 2, analysis 3 also uses retrospective resource use data collected from patients by postal questionnaire. No resource use data were available post discharge for those patients who survived 28 days but died within 6 months. For this group we have assumed costs equivalent to those in analysis 1. This is likely to be an underestimate as no cost is applied for use of health or social care post discharge. If data at 12 months were missing but the individual was still alive, we have assumed that their costs between 6 and 12 months are equivalent to the average costs for this time frame for the arm of the trial that they were allocated to. However, this also has the potential to bias the results based on the small sample size.
The analysis 3 results indicate that patients who were randomised to the salbutamol arm of the trial had worse health outcomes at 6 months (lower utility values) and incurred a higher mean cost to the health- and social-care sector; however, given the restrictions owing to the small sample size and limited follow-up data, the results should be treated with caution.
Chapter 5 Discussion
Overview of the trial findings
The Beta-Agonist Lung injury TrIal-2 found that i.v. administration of salbutamol to patients with ARDS increased 28-day mortality and reduced VFDs and organ failure-free days compared with treatment with placebo. 51 The findings were unexpected, but the trial provided a definitive answer to the question of whether or not i.v. infusion of β2-agonists should be used in patients with ARDS.
Internal validity and methodological limitations
This evaluation used a randomised, placebo-controlled design, which should ensure that the result is as reliable as possible. Very small numbers of patients did not receive the planned interventions (2/326; 0.6%) or had missing data for the primary outcome (2/326; 0.6%); these are very unlikely to have affected the estimated treatment effect.
The most notable methodological issue is that the trial was terminated at a smaller sample size than originally intended, on the recommendation of the DMEC, because of excess mortality in the salbutamol group found at an interim analysis. This has a number of potential consequences. First, the precision of treatment effect estimates is inevitably lower than anticipated, because of the smaller sample size. This is likely to make the effects of the intervention on some outcomes less clear than would be the case if the trial had reached its planned sample size. A larger sample size and narrower CIs would probably clarify salbutamol's effects on some outcomes such as mortality in ICU and in hospital. Second, there is a small possibility that the increased risk of death in the salbutamol group was a chance finding, arising because the interim analysis happened to coincide with a time when more deaths had occurred in the salbutamol group. The trial was stopped when < 25% of the planned sample size had been recruited, and the treatment effect of salbutamol could have changed substantially if the trial had continued to recruit up to its original target. The excess of deaths in the salbutamol group might then have been small or non-existent, and it is even possible, though unlikely, that the treatment effect could have been reversed into a benefit for salbutamol. Thus, there is a remote possibility that the early termination of the trial may have led to a beneficial treatment effect being missed.
A substantial number of earlier studies, including one clinical trial, indicated potential benefit for salbutamol, and there was sufficient clinical interest in β2-agonists to initiate trials in the UK and North America. 28 However, the final result of BALTI-2 was in the opposite direction. This may have occurred partly because of the different outcomes measured by different studies. The early studies were concerned primarily with physiological outcomes, and it is possible that these are simply poor surrogates for substantive clinical outcomes and were inaccurate predictors of salbutamol's effects on mortality. Alternatively, the results on physiological parameters may have been misleading because of chance and bias. There are numerous examples in many fields of medicine where a treatment that seemed promising based on preliminary studies was found by a subsequent RCT to be ineffective or harmful. There is currently a huge investment of time and resources in large-scale RCTs that fail to show any treatment benefit, which suggests that current ways of selecting interventions for evaluation in large-scale RCTs may not be optimal. More rigorous selection of promising interventions could increase the number of large trials that provide evidence of benefit, and hence speed up improvements in health care.
We were successful in following up a high percentage of the population for 12-month mortality. The survival status at 12 months could not be ascertained for only 13 patients (4%) and most of these provided some data to the survival analysis, censored at the last point they were known to be alive. Follow-up for mortality used routinely collected data systems and did not require a response from the individual participants. Eleven patients could not be traced for mortality; one was known to have emigrated, another was from overseas and did not have a permanent address in the UK, and the remaining nine had insufficient identifying details for tracing because the recruiting hospitals were unable to supply them, despite efforts by the study team to retrieve this information. For these nine patients, the only information provided was initials, gender and date of birth, whereas address and preferably NHS number are also needed for successful and accurate tracing. Inability to trace participants was therefore mainly due to problems in data collection rather than the tracing system. A recommendation for future trials is to set up mechanisms to collect identifying details, especially NHS numbers, to facilitate long-term follow-up. A possible weakness in the data collection system of BALTI-2 was that it relied on hospital staff to provide contact information; if this could be collected directly from participants or their relatives, it would probably be more complete and would allow collection of alternative addresses and modes of contact (such as mobile telephone or e-mail). Achieving this in a multicentre trial is, however, challenging.
Previous intensive care trials have found it difficult to achieve high rates of long-term data collection52 and the experience of BALTI-2 confirms that this is a difficult population to follow-up. Only about 40% of survivors were successfully followed up at 6 and 12 months for quality-of-life outcomes. This was due to a high level of participants who were not contacted, plus a very poor return rate for postal questionnaires, despite the use of evidence-based strategies to improve the response rate. The protocol for chasing questionnaires that were not returned has been used successfully in previous trials,53 but was not noticeably successful in BALTI-2. In part its usefulness was limited by lack of contact information; telephone numbers were only supplied by hospitals for a limited number of participants. Telephone data collection was therefore not possible in many cases. A combination of factors was probably responsible for the low response rate; many of the population were elderly or disabled, and their motivation to return questionnaires may have been low because they were recruited to the trial while unconscious and did not feel any sense of personal investment. The follow-up questionnaires incorporated both clinical and economic data collection, and may have appeared long and time-consuming, discouraging completion. A possible strategy in future trials would be to separate the economic and clinical parts of the questionnaire. This would increase the administrative burden of follow-up and increase the number of questionnaires that participants were expected to complete, but might promote higher return rates of a shorter clinical questionnaire, although it could have a negative impact on return of the economic (or both) questionnaires. So far this idea has not been tested.
Obviously, the large number of missing data means that conclusions can only be very tentative because of the risk of bias, but the data suggested that quality of life may be lower at 12 months in the salbutamol group. This was in the same direction as the results for mortality.
A possible limitation is that we did not measure tidal volume after randomisation. It is possible that the use of salbutamol may have made low tidal volume ventilation more difficult, and this may have influenced the outcomes. This could occur through stimulation of metabolic activity and increased production of carbon dioxide, although there was no evidence of such an effect in the earlier BALTI-1.
Salbutamol and increased mortality
The finding of excess mortality in the salbutamol group mirrors that of a study of early goal-directed therapy in which critically ill patients were treated with i.v. dobutamine, which has mixed beta 1 and dopaminergic receptor actions. 54 The mechanisms underlying the increase in mortality because of salbutamol are unclear. BALTI-2 was designed as a pragmatic trial and hence did not collect data that might help to explain the increase in mortality, such as cardiovascular comorbidity and causes of death. It may be significant that the survival curves for salbutamol and placebo appear to continue to diverge after the end of the study drug infusion (see Figures 6 and 8). This suggests that the mechanisms may be complex, and involve indirect effects. Several possible causes of increased mortality can be suggested. First, salbutamol is known to cause arrhythmia and tachycardia, and as many patients with critical illness have comorbid cardiovascular disease, it may cause adverse cardiovascular events, leading to greater mortality in these patients. Second, known side effects of salbutamol include hypokalaemia, hypomagnesaemia and lactic acidosis, which may adversely affect outcomes in some patients. Third, the dose of salbutamol used in BALTI-2 (15 µg/kg IBW/hour) was selected after an early dose-ranging study identified it to be the maximum dose that critically ill patients could receive without an increase in ventricular or atrial tachycardia or ectopy. The dose is at the higher end of the manufacturer's recommended dosing regimen, and it is possible that a lower dose might have been better tolerated and caused fewer adverse outcomes.
External validity and generalisability
The intention was for BALTI-2 to be a pragmatic trial, embedded as far as possible in usual UK practice for caring for patients with ARDS. Therefore, apart from the trial intervention, patients were treated according to their hospital's standard care. The inclusion criteria were not tightly controlled, but allowed scope for slightly different practice in different centres in, for example, definition of the time of onset of ARDS and interpretation of chest radiographs. This is appropriate, because it should ensure that the population recruited to the trial is representative of ARDS patients treated in UK hospitals. The trial should have involved minimal change to the way patients were identified, although it is possible that in some centres screening of patients for the trial may have identified ARDS patients more quickly than would have been the case without the trial.
Two other randomised trials investigating the effects of β2-agonists in patients with ALI/ARDS have been published. The first BALTI19 used the same intervention as BALTI-2, but was designed as a Phase II trial and was not powered to address clinical outcomes. It suggested an advantage to salbutamol based on physiological outcome; however, mortality was considerably higher than we found in BALTI-2 (58% of the salbutamol group and 66% of the placebo group died). The patient characteristics were similar to those recruited to BALTI-2, so the reasons for higher mortality in the earlier trial are unclear.
ALbuterol for the Treatment of ALI was a multicentre RCT of nebulised salbutamol in patients with ALI, conducted in the USA. 28 Patients were randomised to receive either salbutamol 5 mg every 4 hours or saline placebo, for up to 10 days. The primary outcome was VFDs. Recruitment started in August 2007 with a target sample size of 1000 patients, but was terminated by the Data and Safety Monitoring Board after 282 patients had been enrolled, on the grounds of futility. There was no clear difference in VFDs between the salbutamol and placebo arms (14.4 vs. 16.6 days; 95% CI –4.7 to 0.3 days) or hospital mortality (salbutamol 23.0% vs. placebo 17.7%; 95% CI –4.0% to 14.7%). Although the intervention was delivered by a different route in ALTA, and the early termination of recruitment means that the CIs are wide, the results are similar to those of BALTI-2 (there was an increase in mortality and decrease in VFDs in the salbutamol group).
The mortality in the placebo group (23.7%) was considerably lower than assumed in the original sample size calculation. This is likely to have been, at least in part, due to a reduction in the mortality caused by ARDS in recent years due to improvements in treatments. 55 We used a lower estimate of mortality than was found in earlier published studies, because it was likely that mortality had improved since these data were collected in 1999. However, mortality was also considerably lower than was suggested by ICNARC data from 2005 (23.7% vs. 41.2%). It is possible that the unexpectedly low mortality in the BALTI-2 population may be partly owing to selection effects; it is very likely that at most centres, only a fraction of the eligible patients were recruited to the trial, and it is possible that patients with the highest risk of mortality tended not to be recruited. There is little direct evidence of this, but it could have implications for the generalisability of the results.
Recruitment of centres and patients
Recruitment to BALTI-2 was more difficult than anticipated, for two main reasons: (1) the process of obtaining approvals and setting up sites was time-consuming; and (2) once recruitment had started, few centres were able to recruit as many patients as was anticipated.
Many trials experience significant delays in obtaining NHS approvals. We found that the main delay was with NHS Trust R&D approvals, a step that is supposed to be a simple assessment of whether or not there are particular local reasons that would prevent that hospital from running the research project. We also found substantial delays from granting of NHS R&D approval to recruiting the first patient. In part, this was a consequence of the delays in R&D approval; the trial co-ordinating centre was not willing to commit resources to centres before R&D approval had been given because of the uncertainty about the amount of time this would take. Time spent in training staff could easily be wasted effort if recruitment was not able to begin until several months later.
Only a small number of centres achieved the target of one recruit/month once they had commenced recruitment (and fewer would achieve the target if delays in starting recruitment were included). The centres that did manage to recruit to target were generally not centres with particularly large or high-risk populations, and we therefore speculate that the main reason for slow recruitment was that eligible patients were not recruited, rather than that there was a lack of eligible patients. There may be several reasons why patients were not recruited: they may not have been recognised as eligible, they may have been identified as eligible but not approached or they may have been approached but not given consent. A further possibility is that the eligibility criteria were applied in different ways in different centres. Although they were as objective as possible, there remains some subjectivity within the definition of ARDS, and it is possible that some patients would have been regarded as eligible in some centres but not others. Recruiting centres were asked to complete screening logs of potentially eligible patients, but these were not well completed, so we do not have enough information to perform any analysis of the reasons for non-recruitment. It is likely to be true that greater resources for participating centres might allow them to screen patients more effectively, to ensure that relatives of eligible patients are always contacted about randomisation, and that initial contacts are followed up. It would also be helpful for trial co-ordination to ensure that detailed logs of eligible patients are kept by participating centres, which should record the number of patients fulfilling the eligibility criteria, the number excluded and the number actually recruited, with reasons for non-recruitment. This would enable the trial co-ordination team to see where problems in recruitment were occurring. However, most centres found that screening was time consuming and with limited resources they were unable to do it consistently. Obtaining detailed screening and eligibility information is therefore likely to be expensive.
Chapter 6 Conclusions
Implications for health care
The results of this trial showed that i.v. salbutamol at a dose of 15 µg/kg IBW/hour, early in the course of ARDS, was poorly tolerated, is unlikely to be beneficial and could worsen outcomes. The evidence from this and other trials suggests that salbutamol is unlikely to be a beneficial treatment for ARDS.
Implications for research
Further trials of β-agonists in patients with ARDS are unlikely to be conducted. Some questions remain, such as whether or not there may be benefit at a different dose or in specific populations, but any studies investigating these would require a very strong rationale.
Acknowledgements
The trial was funded by the Medical Research Council (UK, project number 84730). The drug supply was funded by the Department of Health and the internal pilot was funded by the Intensive Care Foundation. This project benefited from facilities funded through Birmingham Science City Translational Medicine Clinical Research and Infrastructure Trials Platform, with support from Advantage West Midlands. We thank the patients and families who participated in BALTI-2.
Contribution of authors
Simon Gates (Principal Research Fellow): design of study, funding application, supervision of project, statistical analysis and writing study report.
Gavin D Perkins (Professor of Critical Care): concept and design of study, funding application, supervision of project and writing study report.
Sarah E Lamb (Professor of Rehabilitation, Director of Clinical Trials Unit): design of study, funding application, supervision of study and writing study report.
Charlotte Kelly (Senior Research Fellow, Health Economics): health economic analysis and writing study report.
David R Thickett (Wellcome Intermediate Clinical Fellow): supervision of study and clinical expertise.
Duncan Young (Consultant in Anaesthetics and Critical Care): concept and design of study, funding application and writing study report.
Daniel F McAuley (Professor of Critical Care): recruitment, interpretation of results and writing study report.
Catherine Snaith (Clinical Fellow): data collection and analysis for causes of death and writing study report.
Christopher McCabe (Professor of Health Economics): design of study and supervision of health economic analysis.
Claire T Hulme (Senior Lecturer, Health Economics): supervision of health economic analysis and writing study report.
Fang Gao Smith (Professor of Anaesthesia, Critical Care and Pain): concept and design of study, funding application, supervision of project and writing study report.
BALTI-2 Study Group
Trial Management Group: F Gao (chief principal investigator), G Perkins, S Gates (statistician), S Lamb, D Young, V Barber, C McCabe (health economist), S Duggan and T Melody.
Trial Steering Committee: S Baudouin (chairperson), B Cuthbertson, K Rowan, B Williams, F Gao, S Gates, S Lamb and D Young.
DMEC: K Wheatley, J Bion and G Bellingan.
Statistics: S Gates and JL Smith.
Health economics: C McCabe, CE Hulme and C Kelly.
Trial management: S Duggan and T Melody.
Trial co-ordinators: T Latif, J Bell, R Ezra and B Hoddell.
Administration and data entry: H Johnson, I Ruders and C Snaith.
Recruitment facilitators: S Dale, H Reay, A McLaughlin and V Gordon.
Clinical Collaborators
Birmingham City Hospital: Z Khan.
Birmingham Heartlands Hospital: F Gao.
Queen Elizabeth Hospital Birmingham: W Tunnicliffe.
Selly Oak Hospital: W Tunnicliffe.
Royal Blackburn Hospital: A Krige.
Good Hope Hospital: J Hull.
Ipswich Hospital: R Howard-Griffin.
Royal Berkshire Hospital: C Danbury.
Hereford County Hospital: R Harding.
Broomfield Hospital: E Makings.
Watford General Hospital: S Afolami.
University Hospital of Wales: S Shah.
Royal Preston Hospital: S Laha.
Royal Victoria Hospital: D McAuley.
Ulster Hospital: J Trinder.
Papworth Hospital: A Vuylsteke.
University Hospital of Coventry and Warwickshire: D Watson.
Dumfries and Galloway Royal Infirmary: P Jefferson.
Whiston Hospital: J Wood.
Arrowe Park Hospital: H Black.
University Hospital Aintree: G Dempsey.
Solihull Hospital: F Gao.
Cheltenham General Hospital: R Orme.
Sandwell General Hospital: A Arora.
Hammersmith Hospital: S Brett.
Charing Cross Hospital: S Brett.
Russells Hall Hospital: J Sonksen.
Barnet General Hospital: R Schoub.
Bedford General Hospital: S Snape.
Southend University Hospital: D Higgins.
Norfolk and Norwich Hospital: J Nortje.
James Paget Hospital: K Blenk.
Nevill Hall Hospital: M Martin.
Worcester Royal Hospital: S Graystone.
Yeovil District Hospital: R Daum.
Glan Clwyd Hospital: R Pugh.
Hull Royal Infirmary: I Smith.
Bristol Royal Infirmary: J Bewley.
Addenbrookes Hospital: A Johnston.
Edinburgh Royal Infirmary: M Beatty.
Great Western Hospital: M Watters.
Southampton General Hospital: M Grocott.
North Middlesex Hospital: JM Cuesta.
Royal Derby Hospital: D Rogerson.
James Cook University Hospital: J Park.
Frenchay Hospital: J Soar.
George Eliot Hospital: M Ranganathan.
Frimley Park Hospital: S Tote.
Warwick Hospital: T Long.
Wythenshawe Hospital: P Alexander.
Walsall Manor Hospital: M Khalil.
Warrington Hospital: J Little.
Royal Hallamshire Hospital: G Mills.
Macclesfield Hospital: J Hunter.
Northern General Hospital: G Mills.
Salford Royal Hospital: M Ghrew.
Kings Mill Hospital: L Milligan.
Antrim Area Hospital: R Ballie.
Royal United Hospital Bath: A Padkin.
Royal Victoria Infirmary: S Wright.
Freeman Hospital: S Wright.
St Johns Hospital: M Beatty.
Newcastle General Hospital: S Wright.
Chase Farm Hospital: R Schoub.
Scunthorpe Hospital: R Sharawi.
Southmead Hospital: J Soar.
Royal Lancaster Infirmary: D Highley.
Hairmyres Hospital: D Allen.
Aberdeen Royal Infirmary: D Noble.
Monklands Hospital: J Ruddy.
West Middlesex Hospital: M Popescu.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the HTA programme, the EME programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme, the EME programme or the Department of Health.
References
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2:319-23. http://dx.doi.org/10.1016/S0140-6736(67)90168-7.
- Fowler AA, Hamman RF, Good JT, Benson KN, Baird M, Eberle DJ, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med 1983;98:593-7. http://dx.doi.org/10.7326/0003-4819-98-5-593.
- Petty TL. Acute respiratory distress syndrome (ARDS). Dis Mon 1990;36:1-58. http://dx.doi.org/10.1164/ajrccm.163.3.16331.
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care 1994;9:72-81. http://dx.doi.org/10.1016/0883-9441(94)90033-7.
- Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 2004;30:51-6. http://dx.doi.org/10.1007/s00134-003-2136-x.
- Luhr OR, Antonsen K, Karlsson M, Aardal S, Thorsteinsson A, Frostell CG, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med 1999;159:1849-61. http://dx.doi.org/10.1164/ajrccm.159.6.9808136.
- Hughes M, MacKirdy FN, Ross J, Norrie J, Grant IS. Acute respiratory distress syndrome: an audit of incidence and outcome in Scottish intensive care units. Anaesthesia 2003;58:838-45. http://dx.doi.org/10.1046/j.1365-2044.2003.03287.x.
- Bersten AD, Edibam C, Hunt T, Moran J. the Australian and New Zealand Intensive Care Society Clinical Trials Group. AaNZICSCT . Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian states. Am J Respir Crit Care Med 2002;165:443-8.
- Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 2011;37:1932-41.
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Eng J Med 2005;353:1685-93. http://dx.doi.org/10.1056/NEJMoa050333.
- Roupie E, Lepage E, Wysocki M, Fagon JY, Chastre J, Dreyfuss D, et al. Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients. SRLF Collaborative Group on Mechanical Ventilation. Société de Réanimation de Langue Française. Intensive Care Med 1999;25:920-9. http://dx.doi.org/10.1007/s001340050983.
- Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, et al. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med 2006;32:1115-24. http://dx.doi.org/10.1007/s00134-006-0217-3.
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Eng J Med 2011;364:1293-304. http://dx.doi.org/10.1056/NEJMoa1011802.
- Rossi C, Simini B, Brazzi L, Rossi G, Radrizzani D, Iapichino G, et al. Variable costs of ICU patients: a multicenter prospective study. Intensive Care Med 2006;32:545-52. http://dx.doi.org/10.1007/s00134-006-0080-2.
- Perkins GD, McAuley DF, Richter A, Thickett DR, Gao F. Bench-to-bedside review: beta2-agonists and the acute respiratory distress syndrome. Crit Care 2004;8:25-32.
- Wright PE, Carmichael LC, Bernard GR. Effect of bronchodilators on lung mechanics in the acute respiratory distress syndrome (ARDS). Chest 1994;106:1517-23. http://dx.doi.org/10.1378/chest.106.5.1517.
- Morina P, Herrera M, Venegas J, Mora D, Rodriguez M, Pino E. Effects of nebulized salbutamol on respiratory mechanics in adult respiratory distress syndrome. Intensive Care Med 1997;23:58-64.
- Pesenti A, Pelosi P, Rossi N, Aprigliano M, Brazzi L, Fumagalli R. Respiratory mechanics and bronchodilator responsiveness in patients with the adult respiratory distress syndrome. Crit Care Med 1993;21:78-83. http://dx.doi.org/10.1097/00003246-199301000-00016.
- Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 2006;173:281-7. http://dx.doi.org/10.1164/rccm.200508-1302OC.
- Manocha S, Gordon AC, Salehifar E, Groshaus H, Walley KR, Russell JA. Inhaled beta-2 agonist salbutamol and acute lung injury: an association with improvement in acute lung injury. Crit Care 2006;10.
- Fan E, Needham D, Stewart T. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA 2005;294:2889-96. http://dx.doi.org/10.1001/jama.294.22.2889.
- Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004;8. http://dx.doi.org/10.1002/14651858.CD004477.
- Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. http://dx.doi.org/10.1056/NEJMoa1005372.
- Perkins GD, Nathani N, McAuley DF, Gao F, Thickett DR. In vitro and in vivo effects of salbutamol on neutrophil function in acute lung injury. Thorax 2007;62:36-42. http://dx.doi.org/10.1136/thx.2006.059410.
- Perkins GD, Gao F, Thickett DR. In vivo and in vitro effects of salbutamol on alveolar epithelial repair in acute lung injury. Thorax 2008;63:215-20. http://dx.doi.org/10.1136/thx.2007.080382.
- O’Kane CM, McKeown SW, Perkins GD, Bassford CR, Gao F, Thickett DR, et al. Salbutamol up-regulates matrix metalloproteinase-9 in the alveolar space in the acute respiratory distress syndrome. Crit Care Med 2009;37:2242-9. http://dx.doi.org/10.1097/CCM.0b013e3181a5506c.
- NHS reference costs (2011). Cost data for 2009–2010. London: Department of Health; 2011.
- Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, et al. Randomized, placebo-controlled clinical trial of an aerosolized β2 agonist for treatment of acute lung injury. Am J Respir Crit Care Med 2011;184:561-8.
- Perkins GD, Gates S, Lamb SE, McCabe C, Young D, Gao F. Beta Agonist Lung Injury TrIal-2 (BALTI-2) trial protocol: a randomised, double-blind, placebo-controlled of intravenous infusion of salbutamol in the acute respiratory distress syndrome. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-113.
- Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002;30:1772-7. http://dx.doi.org/10.1097/00003246-200208000-00016.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. http://dx.doi.org/10.1056/NEJM200005043421806.
- Pugh R, Murray-Lyon I, Dawson J, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. http://dx.doi.org/10.1002/bjs.1800600817.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.MR000008.pub4.
- Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet 2005;366:472-7. http://dx.doi.org/10.1016/S0140-6736(05)67061-4.
- Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to score version 2 of the SF-12v2® health survey (with a supplement documenting SF-12® health survey). Lincoln, RI: QualityMetric Incorporated; 2002.
- Szende A, Oppe M, Devlin N. EQ-5D value sets: inventory, comparative review and user guide. Series. Dordrecht: Springer; 2007.
- The measurement and valuation of health. Final report on the modelling of valuation tariffs. York: Centre for Health Economics, University of York; 1995.
- Harrison D, Brady A, Parry G, Carpenter J, Rowan K. Recalibration of risk prediction models in a large multicenter cohort of admissions to adult, general critical care units in the United Kingdom. Crit Care Med 2006;34:1378-88. http://dx.doi.org/10.1097/01.CCM.0000216702.94014.75.
- Altman D, Bland J. Interaction revisited: the difference between two estimates. BMJ 2003;326. http://dx.doi.org/10.1136/bmj.326.7382.219.
- Altman D, Royston P. The cost of dichotomising continuous variables. BMJ 2006;332. http://dx.doi.org/10.1136/bmj.332.7549.1080.
- Haybittle J. Repeated assessments of results in clinical trials of cancer treatment. Br J Radiol 1971;44:793-7. http://dx.doi.org/10.1259/0007-1285-44-526-793.
- Peto R, Pike M, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 1976;34:585-612. http://dx.doi.org/10.1038/bjc.1976.220.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. http://dx.doi.org/10.1002/hec.901.
- Brooks RG, Jendteg S, Lindgren B, Persson U, Björk S. EuroQol: health-related quality of life measurement. Results of the Swedish questionnaire exercise. Health Policy 1991;18:37-48. http://dx.doi.org/10.1016/0168-8510(91)90142-K.
- Kind P, Dolan P. The effect of past and present illness experience on the valuations of health states. Medical Care 1995;33:AS255-63.
- Koopmanschap MA, Rutten FF. A practical guide for calculating indirect costs of disease. Pharmacoeconomics 1996;10:460-6. http://dx.doi.org/10.2165/00019053-199610050-00003.
- Rehabilitation after illness costing report. London: NICE; 2009.
- Curtis L. Unit costs of health and social care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Cuthbertson BH, Scott J, Strachan M, Kilonzo M, Vale L. Quality of life before and after intensive care. Anaesthesia 2005;60:332-9. http://dx.doi.org/10.1111/j.1365-2044.2004.04109.x.
- Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, et al. Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 2012;379:229-35.
- Jackson JC, Girard TD, Gordon SM, Thompson JL, Shintani AK, Thomason JWW, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med 2010;182:183-91. http://dx.doi.org/10.1164/rccm.200903-0442OC.
- Lamb SE, Williams MA, Williamson EM, Gates S, Withers EJ, Mt-Isa S, et al. Managing Injuries of the Neck Trial (MINT): a randomised controlled trial of treatments for whiplash injuries. Health Technol Assess 2012;16.
- Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Eng J Med 1994;330:1717-22. http://dx.doi.org/10.1056/NEJM199406163302404.
- Li G, Malinchoc M, Cartin-Ceba R, Venkata CV, Kor DJ, Peters SG, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med 2011;183:59-66. http://dx.doi.org/10.1164/rccm.201003-0436OC.
Appendix 1 Serious adverse event reports
| Patient no. | Allocation | Description | Expected? | Criteria for seriousness | Related to study drug? | Follow-up information |
|---|---|---|---|---|---|---|
| 0047 | Salbutamol | Became dysrhythmic with haemodynamic compromise. Protocol followed; stop infusion, potassium checked and replaced, found to be 3.8 mmol. ECG performed. Doctor informed as per protocol | No | Life-threatening, medically important | Yes | |
| 0082 | Salbutamol | Loss of cardiac output was reported by bedside nursing staff following removal of CVC. Spontaneous circulation was restored following manual ventilation with 100% O2 and 5 × cardiac compressions. HR, rhythm and blood pressure returned to pre-event parameters without further intervention. Medical staff were in attendance | No | Life-threatening, medically important | No | Since the initial SAE on XX/XX/XX, this subject has experienced six similar episodes of loss of cardiac output requiring manual ventilation and cardiac compressions to restore spontaneous output. Each episode has lasted 1 minute or less. On XX/XX/XX the subject had a temporary pacing wire inserted and has not subsequently experienced a loss of cardiac output |
| 0178 | Salbutamol | Patient suffered deterioration in oxygenation and developed severe bronchospasm approximately 12 hours after completion of study drug infusion (as per study protocol) | No | Life-threatening, medically important | Yes | Patient was intentionally unblinded following discussion with trial co-ordinating centre |
| 0133 | Salbutamol | Patient died following withdrawal of treatment, due to a persistent deterioration of condition (multiple organ failure) following upper GI bleed, abdominal sepsis and ARDS | Yes | Death | No | |
| 0159 | Salbutamol | Patient had an episode of supraventricular tachycardia on XX/XX/XX at 0400. Infusion was reduced but tachycardia persisted. Infusion was stopped at 0600. Patient received adenosine and underlying rhythm was shown to be atrial flutter. Patient received i.v. amiodarone and sinus rhythm was established | Yes | Medically important (required intervention) | Yes | Study drug stopped but patient subsequently died on XX/XX/XX |
| 0260 | Salbutamol | Non-sustained SVT (12 beats) 1410; ST depression noted at 1435, positive troponin test 2200 – diagnosed acute MI. BALTI-infusion discontinued (2300). Patient had PMH of cardiomyopathy and moderately impaired LV function | No | Medically important | No | |
| 0534 | Salbutamol | Base deficit increased from −5 to −15 within 1 hour of commencing trial drug. Patient previously stable. Pulse increased to 145 from 115 b.p.m. Both signs resolved within 2 hours of terminating trial drug | Yes | Medically important | Yes | Originally reported by centre as unexpected but in fact is a recognised side effect |
| Drug discontinued but patient died on XX/XX/XX | ||||||
| 106 | Salbutamol | Tachycardia – sudden increase from 70/minute to 130/minute, sustained rise | Yes | Medically important | Yes | |
| Increasing lactate levels from 1.4 to 5.9 mmol/l over 4 hours and then sustained around 4.8 over the next 8 hours | ||||||
| Hyperglycaemia with increasing requirements | ||||||
| 0135 | Salbutamol | Patient found to have large extrapulmonary cavity, resulting in treatment at specialist hospital and therefore prolonging stay. In retrospect, this was present (but smaller) prior to trial drug being started | No | Prolongation of hospitalisation | No | Transferred for treatment |
| 0738 | Salbutamol | Deterioration in respiratory status requiring increased FiO2 and mandatory ventilation. GCS dropped on XX/XX/XX to 3/15. Subsequently required recommencement of adrenaline infusion/noradrenaline infusion. CT scan of head performed which showed no changes on informal report. However, formal report today shows an extensive acute infarction in the region of right cerebral artery | No | Prolongation of hospitalisation | No | |
| Persistent or significant disability/incapacity | ||||||
| 0367 | Placebo | 12/XX/XX: patient found to have pus and clots in vagina. Urine HCG positive, serum βHCG negative. Ultrasound scan trans-vaginal difficult to view. Cervical os closed | No | Protocol violation | No | |
| 13/XX/XX: BALTI-2 nurse contacted by Dr D, referred to Dr P. Trial drug stopped for 1 hour. Advised that the patient can continue in trial because she is not pregnant. Miscarriage unconfirmed. Pregnancy test not performed before starting trial. Relatives did not report a pregnancy | ||||||
| 14/XX/XX: BALTI-2 trial drug was discontinued on the request of our governance department | ||||||
| 0117 | Placebo | Episode of acute ? following patient repositioning. Endotracheal tube found to have been displaced and evidence of feed aspiration. Despite immediate [illegible] and reintubation patient suffered PEA arrests × 2. Resuscitated though remained hypoxic for some time. Family requested withdrawal from BALTI-2 study | No | Life-threatening | No | The SAE was entirely unrelated to the administration of drugs (both standard and study drugs). Withdrawal of consent for participation was not related to drug effects |
| 589 | Placebo | Cardiac arrest – cause unknown, awaiting post-mortem results | No | Resulted in death, medically important | No | SAE was not due to study drug. Cause of death was 1a pulmonary thromboembolism (secondary to deep-vein thrombosis) and multiorgan failure |
| 1b pulmonary haemorrhage and surgically treated mesenteric tear | ||||||
| 1c road traffic collision | ||||||
| 357 | Placebo | Spontaneously generating tonic–clonic seizure on day 14. Nil immediate medical intervention. Supportive nursing care given. Nothing abnormal detected on MRI scan of brain. Abnormal EEG; awaiting neuro review. Lumbar puncture performed; result pending | No | Prolongation of hospitalisation | No | Follow-up not yet received |
Appendix 2 Final study protocol
BALTI-2 PROTOCOL 15 JULY 2008
A MULTICENTRE RANDOMISED, DOUBLE-BLIND, PLACEBO-CONTROLLED TRIAL TO EVALUATE THE EFFECT OF INTRAVENOUS INFUSIONS OF SALBUTAMOL VERSUS PLACEBO ON 28-DAY MORTALITY IN PATIENTS WITH ACUTE RESPIRATORY DISTRESS SYNDROME
1. Background
1.1 Acute Respiratory Distress Syndrome
1.1.1 Terminology
The acute respiratory distress syndrome (ARDS) is a condition characterised by a failure of pulmonary oxygen exchange due to increased alveolar-capillary permeability and resultant lung oedema. It can be caused by primary lung conditions such as aspiration, pneumonitis, or can arise as a complication of non-pulmonary conditions such as severe sepsis. Ashbaugh and colleagues first described the syndrome in 19671 in a group of 12 patients with acute onset of dyspnoea, tachypnoea, refractory hypoxiaemia, reduced pulmonary compliance and diffuse alveolar shadowing on their chest radiographs. All the patients required positive pressure mechanical ventilation with positive end-expiratory pressure to maintain arterial oxygenation. Post mortem examination of the lungs of the seven patients who died revealed widespread atelectasis, vascular congestion and intra-alveolar haemorrhage, severe pulmonary oedema and formation of hyaline membranes in the alveolar space. Four years later, the term “Adult Respiratory Distress Syndrome” was used to describe the condition. 2 Subsequently the syndrome was renamed as the Acute Respiratory Distress Syndrome (ARDS) because the syndrome also occurs in children. 3 The current definition of ARDS arose from the American-European Consensus Conference in 19944 and requires an oxygen exchange deficit, typical chest radiograph changes and the absence of cardiogenic causes of pulmonary oedema.
1.1.2 Pathophysiology
The pathological findings during the acute stage of ARDS result from diffuse damage to the alveolar capillary barrier causing increased permeability and flooding of the alveolar with proteinaceous exudates. 5 Macroscopically the lungs are oedematous and heavy with a uniform solid red cut surface appearance. 6 Microscopically, there is evidence of an exudative process with extensive epithelial and endothelial barrier damage, alveolar flooding with proteinaceous liquid, inflammatory cells (neutrophils and alveolar macrophages) and fibrin. 5;7 Hyaline membrane formation is seen in the alveolar spaces. The recovery from ARDS is variable, in some patients there is rapid reabsorption of alveolar oedema fluid and repair of the injured region of the alveolar epithelium, followed by clinical recovery from respiratory failure. However, in other patients alveolar oedema persists followed by organisation of hyaline membranes and gradual appearance of intra-alveolar (interstitial) fibrosis. 7 The development of interstitial fibrosis distorts the normal architecture of the lung. The alveoli fill with cellular debris, leucocytes, red cells and fibrin and fibroblasts proliferate in the interstitial and alveolar space. These processes result in extreme narrowing or even obliteration of the airspaces and prevent normal gas exchange. With the passage of time, fibrin and cell debris are progressively replaced by collagen leading to the development of fibrosis and scarring. 8 Recent evidence suggests there is a much greater overlap of the inflammatory and fibroproliferative phases than was initially thought. 9 As early as 24 hours after the initiation of ventilation evidence of collagen turn-over and lung remodelling can be found in the lavage fluid of patients with ARDS. 10
1.1.3 Formation and Resolution of Pulmonary Oedema
The alveolar epithelium and the endothelium of the pulmonary capillaries together form the alveolar capillary barrier. The normal alveolar epithelium is composed of two different cell types. The flat type I cells make up 90 percent of the alveolar surface and are easily injured. The remaining 10 percent of cells consist of the cuboidal type II cells. These cells are more resistant to injury and have important functions such as surfactant synthesis and secretion, ion transport and proliferation and differentiation to type I cells after injury. Both tight and gap junctions couple Type I and Type II cells, providing barrier functions and pathways for intercellular communication. 11 In ARDS, alterations to alveolar-capillary permeability, pulmonary capillary hydrostatic pressures and oncotic pressure leads to flooding of the alveolus with protein rich oedema fluid and the development of non-cardiogenic pulmonary oedema, as outlined above. This interferes with the matching of ventilation to perfusion (V/Q matching) with resulting hypoxaemia, reduced pulmonary compliance and thus acute respiratory failure.
The clearance of oedema fluid is dependent on the balance between oedema formation and re-absorption processes. Oedema formation is governed by Starling forces and the integrity of the alveolar capillary barrier whilst fluid re-absorption is dependent on the active transport of sodium and electrolytes, which drives water re-absorption. Attempts to clear oedema fluid by reducing pulmonary capillary hydrostatic pressure using diuretics, vasoactive agents or extra-corporeal membrane oxygenation have been largely unsuccessful. Matthay was the first to demonstrate that alveolar fluid clearance was not governed by Starling forces but occurs via the active transport of sodium across the alveolar epithelium. 12 Sodium/potassium adenosine-triphosphatase (Na+/K+ ATPase) pumps located on the basolateral surface of type I and type II alveolar epithelial cells pump sodium out of the cell and potassium into the cell against their respective concentration gradients. The Na+/K+ ATPase consists of two sub-units, the alpha subunit, containing the catalytic activity and ion binding sites and the beta subunit, which contributes to the stability of the alpha/beta complex and its insertion into the basolateral membrane of the alveolar epithelial cell. 13 The active transport of sodium by the Na+/K+ ATPase leads to the development of a concentration gradient across the basolateral surface of the alveolar epithelial cell. Sodium then enters the cell through channels located on the apical surface of the cell. Several different types of channels have been characterised and include amiloride sensitive (e.g. non-selective cation channel, highly selective cation channels and epithelial sodium channel) and non-amiloride sensitive channels. The amiloride sensitive channels contribute towards at least 50–60% of the fluid clearance capacity of the alveolar epithelium. 14 The active transport of sodium across the alveolar epithelial cells creates an osmotic gradient which in turn drives fluid movement from the alveolar to interstitial space leading to the resolution of alveolar oedema.
1.2. Rationale for Beta Agonists in ARDS
1.2.1 A High Burden of Disease and Lack of Effective Therapies
ARDS is common, 13.3% of patients who require mechanical ventilation have ARDS, which is up to 40 times as high as previous studies have indicated. 15 ARDS is frequently fatal; in-Intensive Care Unit (ICU) mortality is estimated at 41–46%, corresponding to about 2,200 deaths per year in the UK. 1 ARDS is costly in health economics terms: these patients consume significantly more resources than matched patients without ARDS since they require a longer ICU and hospital stay (median 17 vs 8 days and 31 vs 25 days, respectively),17 and convalescence on the ward and subsequent rehabilitation in the community. 18 The quality of life after ARDS is significantly reduced with 35% unable to return to work 24 months after hospital discharge. 19;20 ARDS has no primary treatments proven to improve outcome other than supportive care with a lung-protective ventilator strategy. 21
1.2.2 Basic Science Data Support a Clinical Trial of a β2 Agonist in ARDS
Experimental studies in animals, as well as in the ex-vivo human lung, have demonstrated that β adrenergic agonists accelerate the rate of alveolar fluid clearance predominantly through stimulation of the β2 receptor on alveolar type I and II cells. 14 β2 receptor activation increases intracellular cAMP resulting in increased sodium transport across alveolar cells by up-regulation of the apical sodium and chloride pathways, Na+/K+ ATPase and probably cystic fibrosis transmembrane conductance regulator. 1 This leads to the development of a micro-osmotic gradient between the alveolar space and interstitium which drives the movement of water and accelerates alveolar fluid reabsorption.
β2 agonists have been shown to reduce neutrophil sequestration, activation and inflammatory cytokine production in-vitro and in animal models of ARDS. 22 In humans, inhaled salmeterol (long acting β2 agonist) given prior to lipopolysaccharide inhalation reduces neutrophil influx, degranulation and tumour necrosis factor-α release. 23
In ARDS, β2 agonists reduce endothelial permeability in animal models and humans24;25 and afford a degree of epithelial cytoprotection from infection related epithelial cell injury. 2 In BALTI 1, we found in-vivo evidence of reduced alveolar capillary permeability26 (fig 1) and in-vitro evidence of enhanced epithelial monolayer wound repair in patients treated with salbutamol27 (fig 2). This effect is protein kinase A dependent and occurs predominantly due to cell migration/spreading. These data suggest that, in addition to enhancing alveolar fluid clearance, β2 agonists may maintain alveolar-capillary integrity, thereby reducing alveolar flooding and the development of ARDS or promote alveolar capillary repair in those with established ARDS.
FIGURE 1.
FIGURE 2.
1.2.3 Lack of Published Randomised Controlled Trials of β2-Agonists in Patients with ARDS
In 2004, we conducted an electronic search of the on-line bibliographic databases Medline, PubMed, Current Contents, Clinical Evidence, the Cochrane Library, EBM and bmj.com for all publications in English using key words “acute lung injury” (or “ALI”), “ARDS”, “alveolar epithelium”, “β2-agonists” and “pharmacotherapy” in the title, abstract, or Medical Subject Headings. A ‘hand search’ of the full reference lists from review articles and individual relevant papers in peer reviewed English language respiratory and critical care journals was also performed in order to cross check the quality of the computer retrieval method. The results were published as a review. 22 Three clinical studies (one randomised controlled cross over trial28 and two non-randomised studies29;30) using β2-agonists in patients with ARDS were identified. These studies examined the effects of nebulised28;29 or intravenous (IV)30 β2-agonists on the respiratory mechanics of artificially-ventilated patients with ARDS and found that β2-agonists reduced airway resistance, peak and plateau airway pressures. There were no clinical studies addressing the effects of β2-agonists on alveolar fluid clearance, or on outcome.
Recently, we conducted a further literature search (unpublished), using the same keywords combined with terms to identify randomised controlled trials, to identify any recent studies of the treatment of human ALI or ARDS with β2-agonists. No studies using IV Salbutamol infusion were identified. A retrospective case review of 86 patients with ALI was the only relevant publication. 31 The patients with ALI who also received high dose nebulised Salbutamol (2.5–6.4 mg day–1) had a significantly more days alive and free of ALI (n = 22, 12.2 (4.4) days) compared with the group receiving ≤ 2.4 mg day–1 (n = 64, 7.6 (1.9) days) although both groups had similar hospital mortality rates of 48% vs 50%.
1.2.4 A Pilot Study of the β2 Agonist Salbutamol in ARDS Confirms Laboratory Findings
After an initial dose-ranging study to determine the maximum infusion rate for salbutamol that did not cause tachydysrhythmias in patients with ARDS we undertook a single centre randomised, double blind, placebo-controlled phase II study (BALTI 1) in 40 adult patients with ARDS to determine if an intravenous infusion of salbutamol 15 µg kg ideal body weight–1 hr–1 for 7 days would accelerate clearance of alveolar oedema. As shown in the figure below, salbutamol significantly reduced lung water (left) (day 7: mean (SD), 9.2 (6) (•) vs 13.2 (3) (▴) ml kg–1, P = 0.038) and plateau airway pressures (right) compared with placebo, and trend towards reduced lung injury score. 32
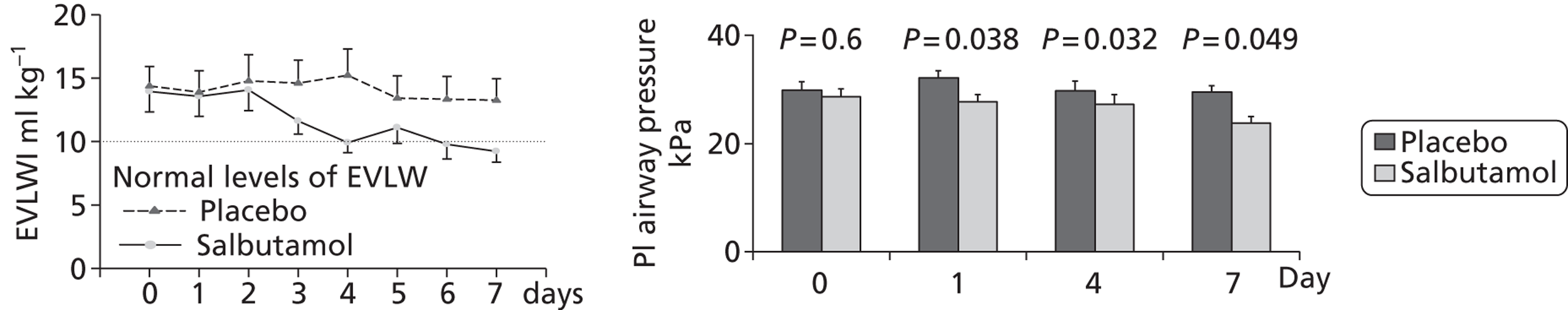
Patients with ARDS who have impaired alveolar fluid clearance have a higher hospital mortality than those with normal clearance (fig 3). 33
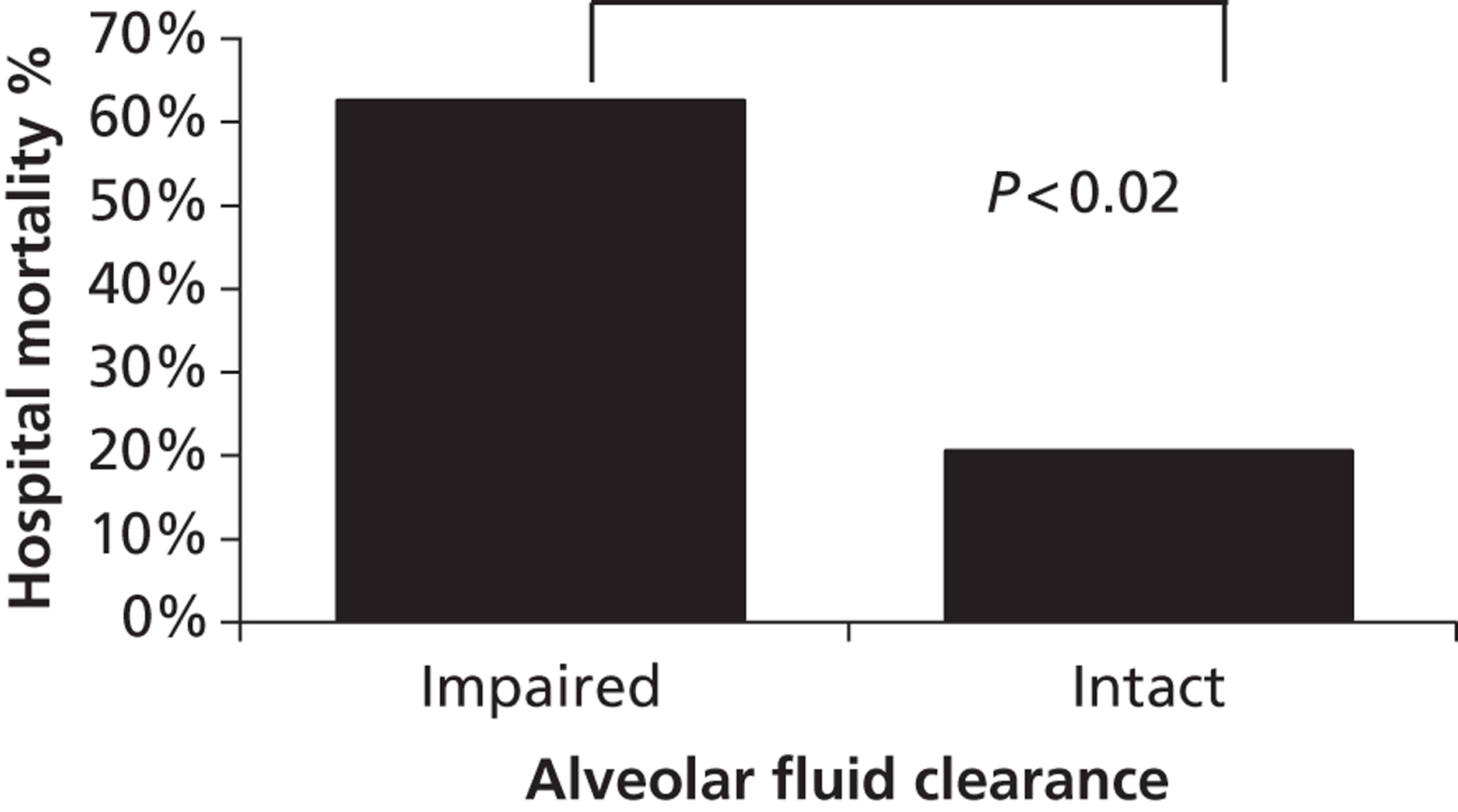
This association suggests that the improved clearance of extravascular lung water seen in the salbutamol-treated patients in the BALTI 1 study may lead to a survival benefit. We could not demonstrate this as the study was powered to detect a reduction in extravascular lung water. Therefore, a large-scale definitive trial with a survival endpoint is required.
1.2.5 The Research Proposed is Supported by the Worldwide Critical Care Community
The American-European Consensus Conference on ARDS in 199834 first supported the hypothesis that β2 agonists could accelerate alveolar fluid clearance in ARDS and called for a clinical trial to investigate if β2 agonists would alter outcomes in ARDS. The National Heart, Lung and Blood Institute Working Group considered the future research directions in ARDS in 2002 and concluded that clinical trials to investigate strategies targeting alveolar fluid clearance were required. 35 More recently, Professors Matthay and Abraham, two leading American critical care physicians, endorsed the need for a clinical trial with β2 agonists in ARDS in their editorial which accompanied the BALTI 1 publication. 36
The BALTI 1 trial was funded and heavily supported by the West Midlands Intensive Care Society. At a national critical care research strategy meeting in November 2005, held by the UK Intensive Care Society (ICS), to assess the feasibility of undertaking ICU based multi-centre randomised clinical trials, the BALTI 2 trial was most highly ranked by over 50 active ICU researchers and the expert panel. The ICS funded a feasibility and pilot study on BALTI 2 for one year which has allowed the piloting and refinement of the trial protocol.
1.2.6 Reliable Drug Delivery
The optimal route for delivering β2 agonists in patients with ARDS with a goal of increasing alveolar fluid clearance has not been determined. Nebulising drugs into the breathing circuits of mechanically ventilated patients appears attractive as it results in high lung concentrations but low blood concentrations and so may reduce the incidence of systemic side effects compared with parenteral treatment. 28 However, nebulised drugs might not reach the alveolar space in the consolidated and poorly ventilated lungs found in patients with ARDS.
Prior to the BALTI 1 study we conducted a dose-ranging study to determine the maximum tolerable dose of intravenous salbutamol that critically-ill patients could receive without an increase in ventricular or atrial ectopy. The maximal tolerable dose was 15 µg kg ideal body weight–1 hour–1 which is the maximal recommended dose for the treatment of airflow obstruction in acutely ill patients. This dose was used in the BALTI 1 study. This dose achieved plasma levels of salbutamol (10–6M) which are associated with 100% increase in basal alveolar fluid clearance in animal models of ARDS. 37
1.2.7 Acceptable Tolerability and Side Effects
The administration of β2-agonists can lead to important cardiovascular, metabolic and renal complications. Stimulation of cardiac and vascular β1 and β2 receptors causes tachycardia, arrhythmias, exacerbation of myocardial ischaemia, pulmonary vasodilatation and loss of hypoxic pulmonary vasoconstriction. 38;39 Metabolic sequelae include hypokalamaemia, hyperinsulinaemia and hyperglycaemia. 40 The use of intravenous β2 agonists for tocolysis during pregnancy has been associated with the development of maternal pulmonary oedema. 41;42 Studies investigating this phenomenon in vivo in rabbits and humans found that intravenous injection of β2-agonists caused reduced sodium, potassium and water excretion leading to a reduced haematocrit and intravascular hypervolaemia. 43;44 These adverse effects are usually more marked following intravenous rather than nebulised administration. However, in general, these drugs are well tolerated in the critically ill. These potentially deleterious effects may limit the potential beneficial effects of β2-agonists described in this review.
In BALTI 1 treatment was generally well tolerated. 7 There was a trend towards higher heart rates in the salbutamol group at day 4 (means (SD), 103(22) vs 88(16) beats min–1, salbutamol vs placebo, p = 0.06) and day 7 (94(14) vs 86(22), p = 0.264). 19 patients received intravenous salbutamol for a total of 2148 hours. During salbutamol or placebo infusions, seven patients (n = 5 – salbutamol, n = 2 – placebo, p = 0.164) developed new onset of supraventricular tachycardia. These arrhythmias did not cause significant hemodynamic compromise and were short lived. No patients sustained serious ventricular arrhythmias. There were no substantial differences in electrolyte concentrations or acid-base balance between salbutamol and placebo for K+, Mg++ H+ or glucose as shown in Figure 4:
FIGURE 4.
Electrolyte, pH and glucose levels in salbutamol and placebo arms.
1.2.8 Lactic acidosis
Lactic acidosis is reported in the literature as a recognised side effect of intravenous and nebulised β2 agonists. 45 This effect is probably mediated by β2-adrenoreceptors and is hypothesised as being due to an increase in skeletal muscle glycogenolysis leading to a rise in peripheral lactate production. Splanchnic glucose production and lactate extraction are also increased, probably secondary to increases in hepatic glycogenolysis and gluconeogenesis. Acidosis does not develop until the bicarbonate buffering system is saturated, and this usually does not occur until lactate concentrations exceed 5 mmol L–1. 46
There was no significant difference in lactate levels between placebo and treatment arms in the BALTI-1 study and no patients required discontinuation of the trial drug due to lactic acidosis. Two patients (out of 53) recruited to the BALTI-2 pilot study developed a lactic acidosis which the treating clinicians attributed the trial drug and discontinued the infusion. In both cases, the lactic acidosis resolved spontaneously after discontinuation of the trial drug over the next 6 hours.
1.2.9 The Intervention is Simple and Cheap
Salbutamol is a low cost treatment, and is readily available from generic drug manufacturers. A seven day infusion for a 70 kg patient will cost just £98. By comparison the NHS Reference cost for a day ICU care (2004) is £1,328.
1.3 Good Clinical Practice
The trial will be carried out in accordance with the International Conference on Harmonisation Good Clinical Practice (ICH-GCP) guidelines (www.ich.org), the EU Clinical Trials Directive and UK legislation.
1.4 CONSORT Guidelines
The trial will be reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines (www.consort-statement.org).
2. Trial Design
2.1 Trial Summary
BALTI-2 is a multicentre, pragmatic, randomised, double-blind, placebo-controlled clinical trial. Patients fulfilling the American-European Consensus Conference Definition of ARDS will be randomised in a 1 : 1 ratio to receive an IV infusion either of salbutamol (15 µg kg ideal body weight–1 hr–1) or placebo (0.9% sodium chloride solution), for a maximum of seven days. Allocation to randomised groups will use minimisation to ensure balance with respect to hospital of recruitment, age group (< 64, 65–84, > 85 years) and PaO2–FiO2 ratio (≤ 6.7, 6.8–13.2, ≥ 13.3 kPa). The trial will be fully blinded and all drugs will be packaged identically, so that neither patients, clinicians or investigators will know which patients are in each arm. Data will be recorded by participating ICUs until hospital discharge, and all surviving patients will be followed up by post at six and twelve months post randomisation. The primary outcome is mortality at 28 days after randomisation; secondary outcomes are mortality in ICU, mortality in hospital, number of ventilator-free days, number of organ failure-free days, mortality at twelve months post-randomisation, quality of life at six and twelve months, length of stay in ICU, length of stay in hospital, adverse effects (tachycardia and arrhythmia). 1334 patients will be recruited from about fifty ICUs in the UK, and an economic evaluation will be conducted alongside the trial.
2.2 Trial objectives
2.2.1 Primary Objective
The primary objective of the trial is to assess whether an intravenous (IV) infusion of salbutamol given at 15 μg (kg ideal body weight)–1 hour–1 for up to seven days reduces 28 day all-cause mortality in patients with ARDS compared with a placebo (0.9% sodium chloride) infusion.
2.2.2 Secondary Objectives
The secondary objectives of the trial are:
-
To evaluate the effects of IV salbutamol on mortality in ICU, mortality in hospital, ventilator-free days, organ failure-free days, length of ICU and hospital stay, mortality up to twelve months after randomisation, and health related quality of life six and twelve months after randomisation.
-
To evaluate the safety of IV salbutamol for ARDS patients.
-
To evaluate the cost-effectiveness of IV salbutamol for patients with ARDS.
-
To explore whether the effects of salbutamol vary between patients of different age, initial disease severity, mortality risk at ICU admission, and ARDS aetiology.
2.3 Outcome measures
2.3.1 Efficacy
Primary outcome
All cause mortality 28 days after randomisation.
Secondary outcomes
Mortality at (first) discharge from ICU.
Mortality at (first) discharge from hospital.
Number of ventilator-free days.
Number of organ failure-free days.
Mortality at twelve months post randomisation.
Ventilator-free days (VFDs) are often used as a trial outcome in addition to mortality. 47 They are defined as the number of calendar days after initiating unassisted breathing to day 28 after randomisation, assuming a patient survives for at least 48 consecutive hours after initiating unassisted breathing. 48 For example, if a patient initiates unassisted breathing on day 16 and survives to day 28, he/she will be assigned a value of 12 VFDs. If a similar patient begins unassisted breathing on day 16 but dies on day 25, the number of VFDs is 9. If a patient survives for > 48 consecutive hours of unassisted breathing but requires assisted breathing (for any reason) before day 28, the number of VFDs is the number of days of unassisted breathing before day 28. Patients who die without initiating unassisted breathing or before 48 consecutive hours of unassisted breathing will be assigned a value of zero VFDs. Patients transferred to another hospital or other health care facility prior to day 28 (intermediate care, nursing home etc.) while still on positive pressure ventilation will be followed to assess this efficacy measure.
In the assessment of VFDs, unassisted breathing is defined as:
-
Extubated with face mask, nasal prong oxygen, or room air, OR
-
T-tube breathing, OR
-
Tracheostomy mask breathing, OR
-
CPAP = 5 cm H20 without PS or IMV assistance. 48
Organ failure-free days are defined as the number of days in the first 28 days after randomisation that the patient has none of: respiratory support, cardiovascular support, renal support, or neurological support, according to Critical Care Minimum Dataset definitions.
2.3.2 Safety
-
Tachycardia sufficient to stop treatment with trial drug.
-
New arrhythmia sufficient to stop treatment with trial drug.
-
Other side effects sufficient to stop treatment with trial drug.
-
Serious adverse events and suspected unexpected serious adverse reactions.
2.3.3 Others
Health related quality of life
EQ-5D and SF-12 at six and twelve months after randomisation.
Resource use
Length of stay in Critical Care Unit.
Length of stay in Hospital.
Health service contacts up to twelve months after randomisation.
Patient out of pocket expenditure and time away from work.
2.4 Flow Diagram of Trial Design
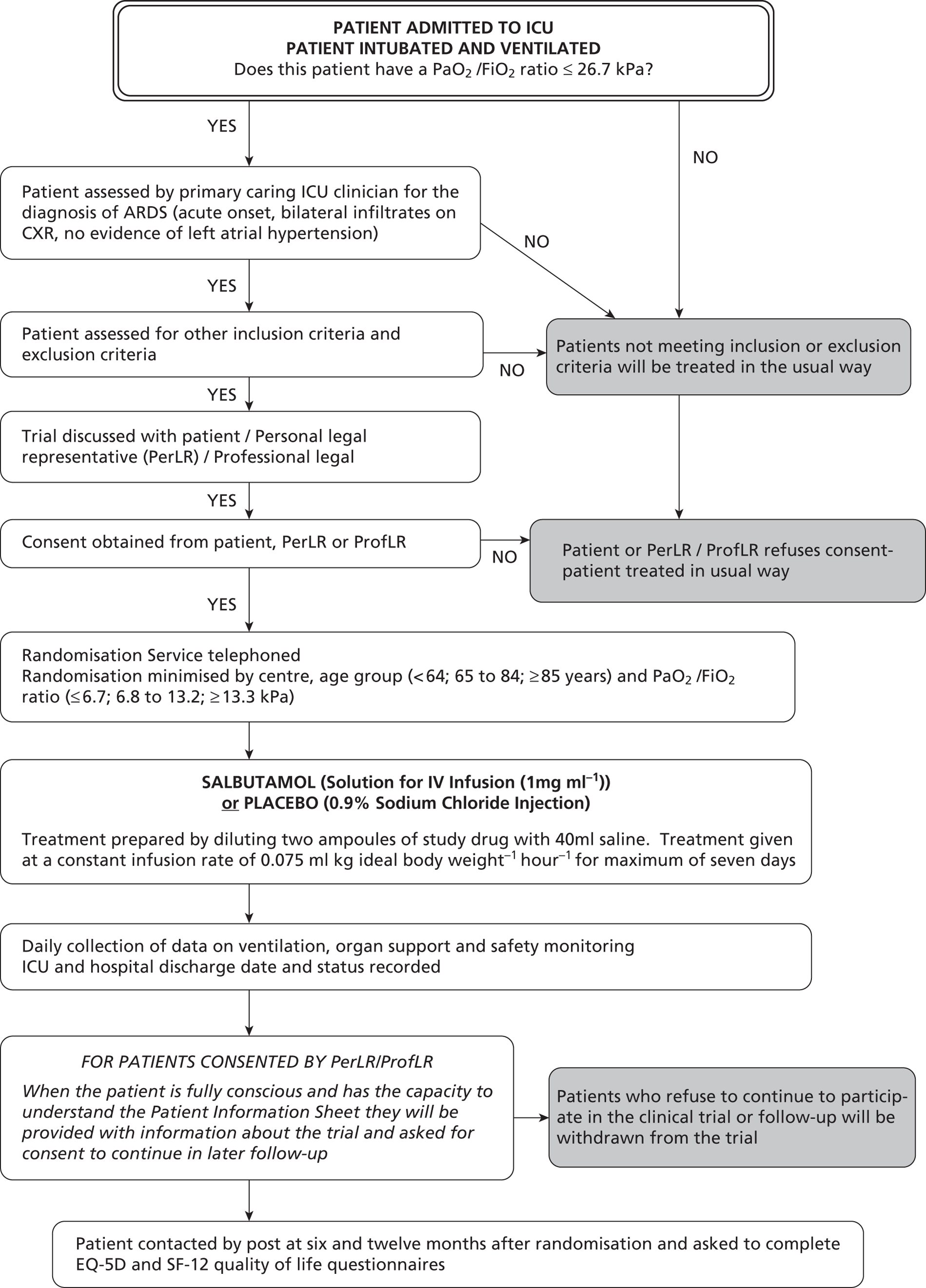
2.5 Eligibility Criteria
Patients are eligible to be included in the trial if they meet the following criteria:
2.5.1 Inclusion Criteria:
-
Patient intubated and ventilated.
-
Within 72 hours of onset of ARDS.
-
ARDS according to American European consensus conference definition.
-
(a) Acute onset.
-
(b) Severe hypoxaemic respiratory failure (PaO2/FiO2 ratio ≤ 26.7 kPa).
-
(c) Bilateral infiltrates on the chest radiograph in the absence of clinical evidence of left atrial hypertension.
-
-
Age ≥ 16 years.
2.5.2 Exclusion Criteria:
-
Patient known to be pregnant.
-
Current treatment with IV β2-agonists or requirement for on-going regular nebulised/inhaled β2-agonists (see Note below).
-
Current treatment with β-adrenergic antagonists (“β-blockers”).
-
Treatment withdrawal imminent.
-
Chronic liver disease, defined as Child–Pugh grade C (Appendix 2).
-
Enrolled in another clinical trial of an investigational medicinal product in the last 28 days.
-
Patient or Personal Legal Representative or Professional Legal Representative unwilling to give informed consent.
Note: Many critically ill patients with respiratory failure may receive nebulised/inhaled beta agonists during their initial resuscitation and stabilisation as part of their clinical care. This does not render a patient ineligible for BALTI-2. The clinician considering enrolling a patient in BALTI-2 should determine whether, at the time of assessment of eligibility, the patient has an on-going requirement for regular nebulised/inhaled beta agonists. The most common situations for this will be a patient with an acute exacerbation of asthma or COPD. If, in the opinion of the treating clinician, the patient does require on-going regular nebulised/inhaled beta agonists, then they should be excluded from the trial. If the patient is not deemed to require such treatment they may be enrolled in the trial provided all other eligibility criteria are met. Once a patient is enrolled in the trial, they are not prevented from having as required (PRN) nebulised/inhaled bronchodilators if their clinical status deteriorates. This covered in section 2.10.2 of the protocol on page 25.
Advice for management of a patient with a baseline tachycardia (heart rate (HR) > 140 beats min–1) is provided in section 2.9.7 on page 22. Tachycardia at the time of recruitment does not make a patient ineligible for the trial.
2.6 Screening of Patients Not Suitable for Trial
Brief details of all patients admitted to ICUs with, or who develop, ARDS but who do not fulfill the eligibility criteria will be recorded on a Patient Screening Log at each collaborating unit.
2.7 Consent
The Principal Investigator is responsible for ensuring that informed consent for trial participation is given by each patient or a legal representative. Appropriate signatures and dates must be obtained on the informed consent documentation prior to collection of trial data and administration of the trial drug. If no consent is given a patient cannot be randomised into the trial.
Consent will be sought from the patients themselves if this is possible, but it is recognised that in the majority of cases patients will be unable to give informed consent due to alterations in their level of consciousness caused by illness and therapeutic sedation. In this situation informed consent will be sought from a Personal Legal Representative or Professional Legal representative.
2.7.1 Patient Consent
Whenever possible, informed consent will be obtained from the patient. The patient will be informed about the trial by the responsible clinician or a member of the research team and given a copy of the Patient Information Sheet (PIS). Informed patients will be given an adequate amount of time to consider their decision on trial entry. If the patient decides to enter the trial they will be asked to sign two copies of the Patient Consent Form which will then be counter signed by the responsible clinician. The patient will retain one copy of the signed Consent Form. The second copy will be photocopied and the photocopy placed in the patient's medical records whilst the original will be retained in the Trial Site File.
2.7.2 Personal Legal Representative Consent
If the patient is unable to give consent, informed consent will be sought from the patient's ‘Personal Legal Representative’ (PerLR) who may be a relative, partner or close friend. The PerLR will be informed about the trial by the responsible clinician or a member of the research team and provided with a copy of the Covering Statement for Personal Legal Representative with an attached PIS and asked to give an opinion as to whether the patient would object to taking part in such medical research. If the PerLR decides that the patient would have no objection to participating in the trial they will be asked to sign two copies of the PerLR Consent Form which will then be counter signed by the responsible clinician. The PerLR will retain one copy of the signed Consent Form. The second copy will be photocopied and the photocopy placed in the patients' medical records whilst the original will be retained in the Trial Site File.
2.7.3 Professional Legal Representative Consent
If the patient is unable to give informed consent and no PerLR is available, a doctor who is not connected with the conduct of the trial may act as a Professional Legal Representative (ProfLR). The doctor will be informed about the trial by the responsible clinician or a member of the research team and given a copy of the PIS. If the doctor decides that the patient is suitable for entry into the trial they will be asked to sign two copies of the Professional Legal Representative Consent Form. The doctor will retain one copy of the signed Consent Form. The second copy will be photocopied and the photocopy placed in the patient's medical records; the original will be retained in the Trial Site File.
2.7.4 Retrospective Patient Information
Patients for whom consent is given by a PerLR or ProfLR will be informed of their participation in the trial by the responsible clinician or a member of the research team once they regain capacity to understand the details of the trial. The responsible clinician will discuss the study with the patient and the patient will be given a copy of the PIS to keep. The patient will be asked for consent to participate in the trial and to sign the Consent to Continue Form. If the patient does not give consent, data collected about the patient will not be entered into the analysis.
2.7.5 Withdrawal of Consent
Patients may withdraw or be withdrawn (by PerLR or ProfLR) from the trial at any time without prejudice. Data recorded up to the point of withdrawal will be included in the trial analysis. If a patient or PerLR requests termination of infusion of the trial drug during the treatment period, the drug infusion will be stopped but the patient will continue to be followed-up as part of the trial. If a patient or a PerLR withdraws consent during trial treatment, the trial drug will be stopped but permission will be sought to access medical records for data related to the trial. If a patient or PerLR wishes to withdraw from the trial after completion of trial treatment, permission to access medical records for trial data will be sought.
2.8 Randomisation
Once written, informed consent has been obtained for the patient to participate in the trial the patient will be randomised to treatment with salbutamol or placebo. Patients will be randomised using a 24-hour telephone randomisation service located at the University of Aberdeen.
Randomisation will be minimised by centre, PaO2 /FiO2 ratio (≤ 6.7, 6.8 to 13.2, ≥ 13.3 kPa), and age (< 64, 65 to 84, ≥ 85 years) because of the expected differences in mortality among these strata. The randomisation service will ask to be provided with the patients' initials, date of birth and recruitment centre, confirmation that the patient fulfills the trial entry criteria and data for minimisation. The randomisation service will allocate a numbered treatment pack to each patient. This pack will contain all drugs for giving a complete course of trial treatment to one patient.
At the time of randomisation, each patient will be allocated a unique Patient Trial Number which will be used throughout the trial for patient identification. The number will consist of six digits, the first two will correspond to the Trial Centre Number and the last four to the number of the drug box allocated.
2.9 Trial Treatments
2.9.1 Test Treatment
Active ingredient: Salbutamol Sulphate.
Trade name: VentolinTM Solution for Intravenous Infusion.
Concentration: 1 mg ml–1.
Excipient: Sodium chloride, sodium hydroxide and Water for Injection.
Container: Clear glass ampoules, 5 ml.
Pharmaceutical Form: Sterile injection.
Manufacturer: GlaxoSmithKline Manufacturing S.p.A.
2.9.2 Control (Placebo) Treatment
Name: Sodium chloride Injection BP 0.9% w/v.
Concentration: 9 mg ml–1.
Container: Clear glass ampoules, 5 ml.
Pharmaceutical Form: Sterile injection.
Manufacturer: Hameln Pharmaceuticals Ltd.
2.9.3 Diluent
Name: Sodium chloride Injection BP 0.9% w/v.
Concentration: 9 mg ml–1.
Pharmaceutical Form: Sterile injection.
2.9.4 Drug Pack Preparation and Supply
Patient drug packs will be prepared by Bilcare GCS (Europe) Limited (Elvicta Business Park, Crickhowell, Powys, UK). Salbutamol and sodium chloride ampoules will be supplied to Bilcare. Each ampoule will have a randomised black out label applied and 50 ampoules of either salbutamol or placebo will be packaged in a white cardboard box in ten trays containing five ampoules each. Boxes will be sealed and labelled. Each box will contain sufficient material for the treatment of one patient for seven days. All trial drugs will be packaged identically and identified only by number.
Study: BALTI 2 Drug pack: VV
EUDRACT No. 2006-002647-86
Salbutamol 5 mg per 5 ml / Placebo
FOR IV INFUSION ONLY
FOR CLINICAL TRIAL USE ONLY
Batch: V Expiry: V
Store between 15°C – 25°C
Chief Investigator: Prof Fang Gao
Co-ordinating Centre: Warwick Medical School
Clinical Trials Unit, University of Warwick, Coventry
CV4 7AL. Tel: 02476 575848
AMPBAL
Drug boxes will be stored by Bilcare and dispatched by them to participating Hospital pharmacies.
2.9.5 Dispensing of Drug Packs
Hospital pharmacies will dispense the trial drugs to their ICU. Because patients may be recruited into the trial outside normal pharmacy opening hours, two or more patient drug packs (at least one each of salbutamol and placebo) need to be available on each hospital ICU at all times. When a patient is recruited, the randomisation service will inform the recruiting clinician of the drug pack number to be allocated to the current patient and the number of the next drug pack to be obtained from pharmacy. A retrospective prescription will be completed by the recruiting physician when the drug pack has been allocated to a patient along with a request form for the next pack required.
2.9.6 Calculation of Infusion Rate
Salbutamol and placebo infusions will be administered through a dedicated intravenous line at a rate of 0.075 ml (kg ideal body weight)–1 hour–1 (equivalent to 15 µg salbutamol (kg ideal body weight)–1 hour–1). Ideal body weight will be calculated from the patient's height. The patient will be measured in centimetres from heel to vertex using a soft tape measure and the ideal body weight and infusion rate obtained from the conversion table on next page.
| Height (cm) | Male IBW (kg) | Infusion Rate (ml hr–1) | Female IBW (kg) | Infusion Rate (ml hr–1) | Height (cm) | Male (IBW) (kg) | Infusion Rate (ml hr–1) | Female IBW (kg) | Infusion Rate (ml hr–1) |
|---|---|---|---|---|---|---|---|---|---|
| 146 | 44.2 | 3.3 | 39.7 | 3.0 | 174 | 69.7 | 5.2 | 65.2 | 4.9 |
| 148 | 46.0 | 3.5 | 41.5 | 3.1 | 176 | 71.5 | 5.4 | 67.0 | 5.0 |
| 150 | 47.8 | 3.6 | 43.3 | 3.2 | 178 | 73.3 | 5.5 | 68.8 | 5.2 |
| 152 | 49.6 | 3.7 | 45.1 | 3.4 | 180 | 75.1 | 5.6 | 70.6 | 5.3 |
| 154 | 51.5 | 3.9 | 47.0 | 3.5 | 182 | 76.9 | 5.8 | 72.4 | 5.4 |
| 156 | 53.3 | 4.0 | 48.8 | 3.7 | 184 | 78.8 | 5.9 | 74.3 | 5.6 |
| 158 | 55.1 | 4.1 | 50.6 | 3.8 | 186 | 80.6 | 6.0 | 76.1 | 5.7 |
| 160 | 56.9 | 4.3 | 52.4 | 3.9 | 188 | 82.4 | 6.2 | 77.9 | 5.8 |
| 162 | 58.7 | 4.4 | 54.2 | 4.1 | 190 | 84.2 | 6.3 | 79.7 | 6.0 |
| 164 | 60.6 | 4.5 | 56.1 | 4.2 | 192 | 86.0 | 6.5 | 81.5 | 6.1 |
| 166 | 62.4 | 4.7 | 57.9 | 4.3 | 194 | 87.9 | 6.6 | 83.4 | 6.3 |
| 168 | 64.2 | 4.8 | 59.7 | 4.5 | 196 | 89.7 | 6.7 | 85.2 | 6.4 |
| 170 | 66.0 | 5.0 | 61.5 | 4.6 | 198 | 91.5 | 6.9 | 87.0 | 6.5 |
| 172 | 67.8 | 5.1 | 63.3 | 4.7 | 200 | 93.3 | 7.0 | 88.8 | 6.7 |
2.9.7 Treatment Preparation and Administration
Prior to infusion, two ampoules of trial drug will be diluted with 40 ml of saline in a 50 ml syringe. Infusion syringes should be made up immediately prior to use.
Trial drug infusions should be started immediately after randomisation. If at the time of attempting to commence the trial drug the patient's heart rate exceeds 140 beats min–1, the administration should be delayed until the heart rate is less than 140 beats min–1 for at least 30 minutes. Every attempt should be made to complete the treatment infusion without interruption for a maximum of seven days (i.e. until 168 hours after randomisation).
2.9.8 Alteration of Infusion Rate
Sinus tachycardia or arrhythmias are known side effects of intravenous salbutamol administration. If a patient receiving a trial drug infusion is noted to have tachycardia (HR > 140 beats min–1) or any new arrhythmia occurs, the dose rate of drug will be adjusted according to the flow diagram (page 23). Dose adjustments for renal or hepatic failure will be driven by the cardiovascular response to the infusion rather than on the degree of renal or hepatic impairment. Standard anti-arrhythmic therapy will be given if indicated in addition to alteration of infusion rate.
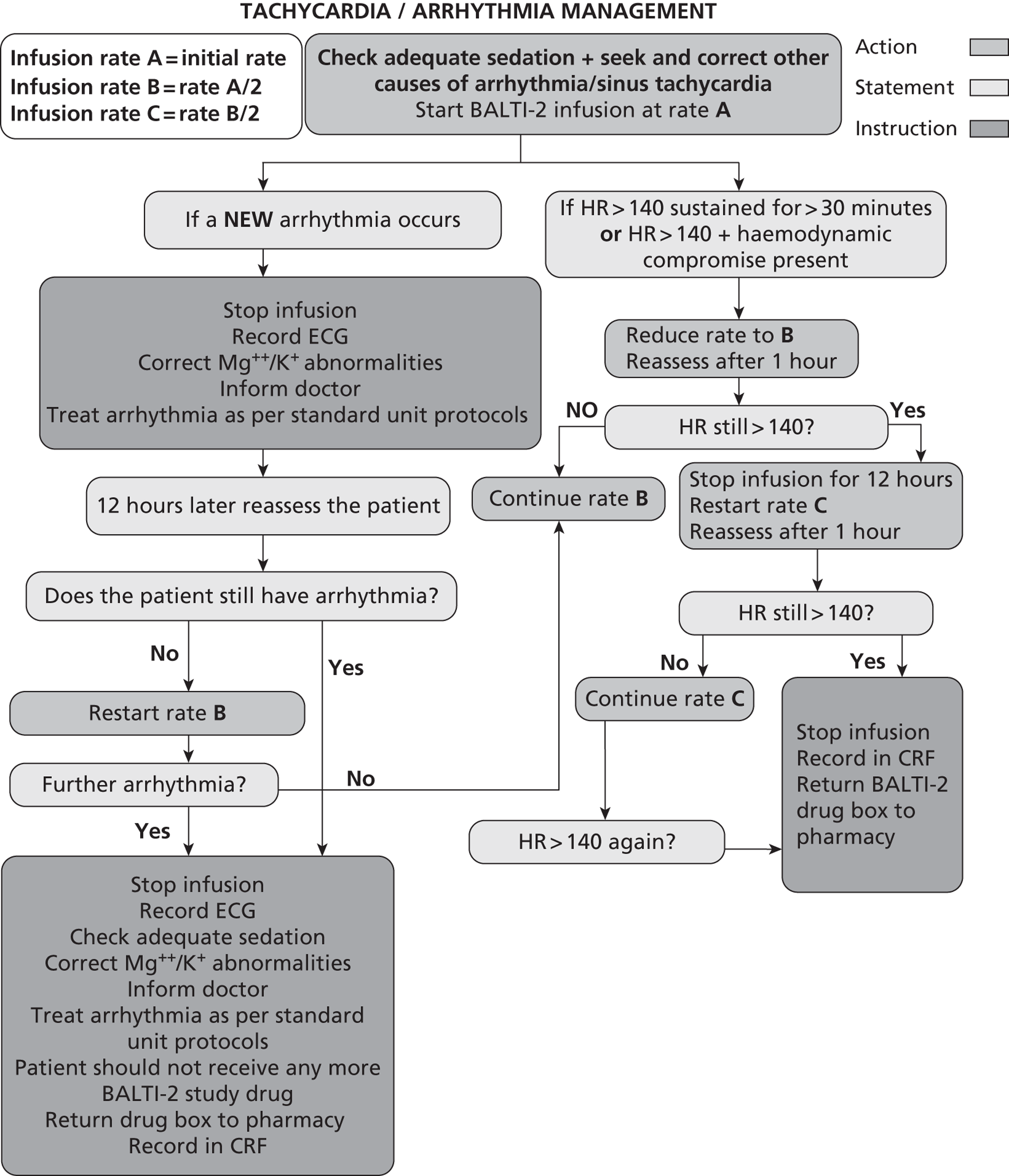
Example 1 – Patient develops sinus tachycardia rate 145 beats min–1, sustained for greater than 30 minutes without haemodynamic compromise. Infusion running at rate A. Action – sedation and analgesia requirements reviewed; other causes of tachycardia sought but not identified. Infusion reduced to rate B. One hour later HR 118 beats min–1. Infusion continued at rate B.
Example 2 – Patient with pre-existing atrial fibrillation develops tachycardia (AF) rate 160 beats min–1. Infusion running at rate A. Action – sedation and analgesia requirements reviewed; other causes of tachycardia sought but not identified. Infusion reduced to rate B. One hour later HR 148 beats min–1 (AF). Infusion stopped. Clinical decision taken to treat AF with anti-arrhythmic according to unit protocol. Patient reassessed 12 hours later. HR 80 beats min–1 (AF). Infusion restarted at rate C. If further tachycardia or new arrhythmia infusion should be stopped and remaining drug returned to pharmacy.
Example 3 – Patient develops new onset atrial fibrillation. Infusion running at rate A. Action – Stop infusion. Check electrolytes, record 12 lead ECG. Clinical review and decision taken to treat AF with anti-arrhythmic according to unit protocol. Patient reassessed 12 hours later. HR 80 beats min–1 (sinus rhythm) Infusion restarted at rate B. If further tachycardia or new arrhythmia infusion should be stopped and remaining drug returned to pharmacy.
2.9.9 Infusion Termination Criteria
Termination of the infusion is defined as discontinuation of the trial drug infusion without intention to restart the infusion at a later time. Patients whose infusion is terminated before 7 days after randomisation are not withdrawn from the trial, but will remain in the trial until twelve months after randomisation or death. Trial drug infusion will be terminated in the following circumstances:
-
Death.
-
Heart rate > 140 beats min–1 despite two adjustments in infusion rate.
-
New arrhythmias despite adjustment in infusion rate.
-
Development of a significant lactic acidosis, which in the opinion of the treating clinician is attributable to infusion of the trial drug.
-
24 hours after discontinuation of mechanical ventilation (of any sort).
-
Discharge from Critical Care environment.
-
Discontinuation of active treatment.
-
Request to withdraw from PerLR or patient.
-
Decision by the attending clinician that the infusion should be discontinued on safety grounds.
-
7 days (168 hours) after randomisation.
2.9.10 Treatment Compliance
Treatment will be administered by site personnel with relevant training and experience at the hospital. Trial infusions will be recorded in the Case Report Forms to monitor treatment compliance.
2.9.11 Drug Accountability
Hospital pharmacies will be responsible for recording trial drug packs dispensed to the ICU.
Preparation of all drug infusions will be recorded on the Nursing Staff Drug Accountability Form and drug administration on the patients prescription chart. The trial drug packs will include a sheet on which the fate of all ampoules will be recorded (infused, opened but not infused, discarded, unused). At the end of the treatment period any remaining unused drug will be returned to the hospital pharmacy for recording and will then be destroyed.
2.10 Clinical Management of Patients in the Trial
Patients involved in the BALTI 2 trial will be managed according to best practice established locally on each unit. Particular care to monitor electrolytes (K+, Mg++) and glucose is required, with electrolyte supplementation/insulin administered as clinically indicated.
The only specific trial requirement is that patients are not routinely administered nebulised beta agonists or other intravenous beta agonists such as isoprenaline. The uncontrolled use of nebulised bronchodilators in the control group will limit the ability of the trial to detect a significant difference in outcomes and the use in the treatment group exposes the patients to a risk of toxicity. There is no definitive evidence at the current time that routine nebulisation of bronchodilators improves outcomes in patients with acute lung injury.
2.10.1 Acute Bronchospasm
In the event of acute bronchospasm, where the clinician feels that a nebulised bronchodilator is required, nebulised ipratropium bromide may be given. If nebulised ipratopium is insufficient to treat the bronchospasm, then salbutamol may be given as a rescue therapy. This will be recorded on the relevant case report form.
2.10.2 Ventilatory Management
There are no specific guidelines for ventilatory management. Clinicians will be encouraged to use a low tidal volume strategy of ventilation based on ideal body weight. Rescue therapies such as high-frequency oscillatory ventilation, nitric oxide and extracorporeal membrane oxygenation can be used according to local policy.
2.10.3 Blinding and Procedures for Unblinding Patients
As a placebo controlled, double-blind trial, patients, clinicians and investigators will be blinded to each patient's allocation. All trial drugs, whether salbutamol or placebo, will be packaged identically and identified only by number.
Emergency unblinding may be requested on grounds of safety by any Investigator. Emergency unblinding will be performed by telephone contact with the randomisation service in Aberdeen. This option may be used ONLY if the patient's future treatment requires knowledge of the treatment assignment. If a Principal Investigator decides that there is justification to unblind a patient, they should make every attempt to contact the Trial Co-ordinating Centre, who will arrange for them to discuss the necessity of unblinding with a clinical member of the study team.
2.11 Post Infusion Follow-up
Any patients who remain in the Intensive Care Unit or High Dependency Unit for more that seven days post randomisation (the end of the expected drug infusion period), will continue to be monitored on daily basis until discharged to a ward. The date and place of hospital discharge will be obtained from hospital records.
All patients discharged from hospital will be followed-up six and twelve months after randomisation by postal questionnaire. The questionnaire will collect data on disability and health-related quality of life, using the EQ-5D and SF-12 questionnaires.
2.12 Adverse Event Management
2.12.1 Definitions
2.12.1.1 Adverse Events
An adverse event is defined as any untoward medical occurrence in a patient administered a medicinal product and which does not necessarily have a causal relationship with the treatment. The following are expected adverse events and will be recorded on the CRF:
-
Termination of trial drug due to tachycardia.
-
Termination of trial drug due to new arrhythmia.
-
Termination of trial drug due to lactic acidosis.
-
Termination of the trial drug for any other reason.
These events will be included as part of the safety analysis for the trial and do not need to be reported separately to the Trial Co-ordinating Centre.
2.12.1.2 Serious Adverse Events (SAEs) and Suspected Unexpected Serious Adverse Reactions (SUSARs)
A serious adverse event is defined as an adverse event that fulfills one or more of the following criteria:
-
Results in death.
-
Is immediately life-threatening.
-
Requires hospitalisation or prolongation of existing hospitalisation.
-
Results in persistent or significant disability or incapacity.
-
Results in congenital abnormality or birth defect.
-
Requires medical intervention to prevent one of the above, or is otherwise considered medically significant.
Suspected Unexpected Serious Adverse Reactions (SUSARs) are SAEs that are also unexpected i.e. their nature or severity is not consistent with the Summary of Product Characteristics (Appendix 3), and are considered to be caused by the study drug.
Because BALTI-2 is recruiting a population that is already in a life-threatening situation, it is expected that many of the participants will experience SAEs. Events that are expected in this population and those that are collected as outcomes of the trial should not be reported as SAEs. This includes:
-
Death.
-
Organ failure.
Other SAEs or SUSARs that occur between trial entry and 30 days after the end of the trial drug infusion will be reported using the mechanism described in Section 2.12.1.3. The following events should be reported:
-
Unexpected SAEs (SUSARs)
-
Side effects of salbutamol sufficiently severe to be fatal or immediately life-threatening.
2.12.2 Reporting of SAEs and SUSARs
SAEs and SUSARs will be reported using the SAE form in the patient's CRF. The Principal Investigator in each centre must report any SAEs and SUSARs to the Trial Co-ordinating Centre within 24 hours of becoming aware of them. To do this, the SAE form should be completed and faxed to the trial's secure fax number (02476 150549). Subsequently, the Principal Investigator will be required to submit a full report on the resolution of the event. The Trial Co-ordinating Centre is responsible for reporting adverse events to the sponsor, ethics committee and MHRA within required timelines. The Principal Investigator's assessment of causality of SAEs (i.e. their relationship to trial treatment) will be reported on the Serious Adverse Event form.
2.13 End of Trial
The trial will end when 1334 patients have been recruited and completed twelve month follow-up.
The trial will be stopped prematurely if:
-
Mandated by the Ethics Committee.
-
Mandated by the Medicines and Healthcare products Regulatory Agency (MHRA).
-
Following recommendations from the Data Monitoring and Ethics Committee (DMEC).
-
Funding for the trial ceases.
The Main Research Ethics Committee (MREC) that originally gave a favourable opinion of the trial and the MHRA that issued the Clinical Trial Authorisation will be notified in writing if the trial has been concluded or terminated early.
3. Data Management
3.1 Training Issues
To ensure accurate, complete and reliable data, the Trial Co-ordinating Centre will do the following:
-
Provide instructional material to the trial site(s).
-
Provide an Initiation training session to instruct the Investigators and trial nurses. This session will give instructions on the protocol, the completion of Case Report Forms and trial procedures.
-
Make periodic visits to the trial site.
-
Be available for consultation and stay in contact with the trial site personnel by mail, telephone and/or fax.
-
Review and evaluate Case Report Form (CRF) data, detect errors in data collection and request data collection.
3.2 Data Collection and Management
All data for an individual patient will be collected by each Principal Investigator or their delegated nominees and recorded in the CRF. Patient identification in the CRF will be through their unique Patient Trial Number allocated at the time of randomisation and initials. Data will be collected from the time the patient is considered for entry into the trial through to their discharge from hospital. In the event that a patient is transferred to another hospital, the trial team will liaise with the receiving hospital to ensure complete data collection.
APACHE II scores will be used as part of the description of the trial population. For centres that participate in the Intensive Care National Audit and Research Centre (ICNARC) Case Mix Programme (CMP), the APACHE II scores can be obtained from ICNARC; therefore these centres will need to supply only the CMP number for BALTI-2 participants. Centres that do not contribute to the CMP will need to collect all of the data to allow calculation of the APACHE II score.
Data will be collected in duplicate using non-carbon required forms. Once a patient has been discharged from hospital and all data entered into the CRF, the top copy of each form will be returned to the Trial Co-ordinating Centre. The bottom copy of the CRF will be retained at the recruiting centre. The trial number, name, address and other contact details of all patients who survive will be supplied to the Trial Co-ordinating Centre at the time of hospital discharge to allow follow-up questionnaires to be posted to the patients at six and twelve months.
Submitted data will be reviewed for completeness and entered onto a secure, backed-up custom database. Due care will be taken to ensure data safety and integrity, and compliance with the Data Protection Act 1998.
3.3 Follow-up at Six and Twelve months
All survivors will be followed up at six and twelve months after randomisation by postal questionnaire. Any deaths after discharge from hospital will be identified using the NHS Strategic Tracing Service (NSTS), to avoid sending questionnaires to patients who have died. Trial patients will be asked to let the Co-ordinating Centre know if they move house at any time after hospital discharge; NSTS will enable us to locate any who move without informing the Co-ordinating Centre. The follow-up questionnaire will collect data on disability and health-related quality of life, using the EQ-5D and SF-12 questionnaires. If questionnaires are not returned a maximum of two telephone contacts will be made to the trial patient to check that the questionnaire has been received and the patient is happy to complete it, followed by a second copy of the questionnaire and telephone contacts in the event of non-return. If the second questionnaire is not returned the patient will be contacted and the outcome data collected over the telephone.
3.4 Data Storage
All essential documentation and trial records will be stored by WMSCTU in conformance with the applicable regulatory requirements and access to stored information will be restricted to authorised personnel.
3.5 Archiving
Trial documentation and data will be archived for at least five years after completion of the trial.
4. Data Analysis
4.1 Sample size calculation
The estimated sample size is 1334 patients (667 in each arm).
Published estimates of the mortality rate among ARDS patients range from about 34% to 60%. Two cohort studies that included UK data estimated that hospital mortality was 53.9% (95% CI 49.0, 58.7%) and 60.9% (95% CI 55.9, 65.9%). However, it is likely that mortality has declined since these studies were conducted (1999) because of the introduction of protective ventilation strategies after the publication of a large RCT in 2000. From unpublished ICNARC data for 2005, the hospital mortality among 37,726 patients with ARDS in the UK was 41.2%. The primary outcome for BALTI-2 is 28-day mortality, which is likely to be similar to or slightly higher than hospital mortality because most deaths will occur in ICU within a short period after randomisation, and most patients leave hospital before 28 days. In BALTI-1 the placebo group 28-day mortality rate was 67% (95% CI 0.45, 0.83). A reasonable conservative estimate of the 28-day mortality to be expected in BALTI-2 is 40–50%.
Losses to follow-up for the primary outcome are expected to be very low; in the recently-completed PAC-Man trial 2.4% of recruited patients were lost (mainly because of withdrawal of consent) between randomisation and hospital discharge. We have therefore conservatively assumed a 3% loss of patients for the primary outcome. The table below shows the sample sizes necessary for 80% and 90% power to detect a real risk ratio of 0.80 between the salbutamol and placebo arms, using a significance level of 0.05.
| Placebo mortality | Salbutamol mortality | 80% power | 90% power |
|---|---|---|---|
| 40% | 32% | 1164 | 1558 |
| 42% | 33.6% | 1076 | 1440 |
| 44% | 35.2% | 998 | 1334 |
| 46% | 36.8% | 926 | 1238 |
| 48% | 38.4% | 860 | 1148 |
| 50% | 40% | 798 | 1068 |
We will adopt a target sample size of 1334, which will give 90% power to detect a risk ratio of 0.8 if the placebo group mortality rate is 44%, over 85% power if it is 40%, and more than 90% if it exceeds 44%. The 28-day mortality in the placebo group will be monitored (via the DMEC), to ascertain whether the assumptions made in the sample size calculations are correct. If not, the DMEC will advise on modification to the sample size.
4.2 Statistical Analysis
4.2.1 General Analysis
All analyses will be by intention to treat i.e. all patients will be analysed in their randomised group regardless of the treatment actually received, and we will seek to include all randomised patients in the analyses. The primary outcome and other dichotomous outcomes will be compared using risk ratios and 95% confidence intervals. Time to event outcomes such as length of stay and will use survival analysis techniques and compare the groups using hazard ratios and 95% confidence intervals.
A detailed Statistical Analysis Plan will be written by the trial statisticians and approved by the DMEC before the end of the trial.
4.2.2 Subgroup Analysis
Subgroup analyses will use a statistical test for interaction and will be reported using 99% CI.
Four subgroup analyses are pre-specified, stratifying by:
-
APACHE II score at ICU admission: 0–16, 17–21, 22–26 and 27–49.
-
Severity of hypoxaemia; the lowest PaO2/FiO2 ratio between onset of ARDS and randomisation of ≤ 6.7, 6.8–13.2, ≥ 13.3 kPa.
-
Age: ≤ 64, 65–84 and ≥ 85 years.
-
Direct versus indirect aetiology of ARDS.
4.2.3 Frequency of Analyses
Interim analyses will be conducted every 12 months during the period of recruitment, or more frequently if requested by the DMEC.
4.3 Economic Evaluation
4.3.1 Objective
To calculate the expected incremental cost-effectiveness of IV salbutamol compared with standard care in the treatment of patients with ARDS, admitted to ICUs in the UK.
4.3.2 Economic Analyses
Two economic analyses will be undertaken:
1. A within-trial cost effectiveness analysis comparing the costs and outcomes of patients in each arm of the trial at 12 months.
The perspective for this analysis will be that of the NHS and Social Services. The primary outcome for this analysis will be the Quality Adjusted Life Years (QALY's). Utilities will be measured using the EQ-5D at 6 and 12 months follow-up. Within ICU resource use will be identified through a detailed costing study undertaken at a sample of ICUs recruiting to the trial. Use of other hospital services will be abstracted from the trial CRFs. Use of primary, community and social care services will be recorded via a patient diary completed at six and 12 months follow-up. Particular effort will be made to identify place of residence at 12 months follow-up and whether this is funded by health, social services or privately. Out of pocket expenditure and time away from work data will also be collected using the same patient diary. Unit costs will be obtained from national sources such as the NHS reference costs and the PSSRU Unit Costs of Health and Social Care. Where national costs are not available, unit costs will be identified in consultation with finance departments of trusts recruiting to the trial. Parameter uncertainty will be addressed using probabilistic sensitivity analysis. Outputs from the analysis will include the expected incremental cost effectiveness ratio (ICER), a scatterplot on the cost effectiveness plane, cost effectiveness acceptability curve and incremental net benefit assuming lambda = £20,000 per QALY.
2. As there is potential for a difference in mortality between the groups, a lifetime horizon is required to fully capture the cost and benefits of IV salbutamol compared to usual care. Therefore, we will construct a cost effectiveness model with a lifetime time horizon. This will model the expected long term difference in QALY's lived and health and social care resource utilised by two hypothetical cohorts of patients with ARDS; one treated with IV salbutamol the other not. The age distribution of these cohorts will reflect the age profile of ARDS patients actually seen in UK ICUs. Life expectancy post hospital discharge will be modeled using national age specific life expectancy data adjusted to reflect published evidence on the reduced life expectancy of ICU ‘survivors’. Long-term quality of life will be estimated using published age-specific utility data adjusted to reflect any published evidence of a divergence in health related quality of life in ICU ‘survivors’. In the absence of evidence to the contrary, the model will assume that the treatment modality does not impact upon the long terms non-ARDS-related health care costs. Costs and outcomes will be discounted in line with best practice recommendations at the time of the analysis. Parameter uncertainty will be addressed using probabilistic sensitivity analysis. Outputs from the analysis will include the expected ICER, a scatterplot on the cost effectiveness plane, cost effectiveness acceptability curve and incremental net benefit assuming lambda = £20,000 per QALY.
4.4 Publication of Results
The success of the trial depends on the collaboration of doctors, nurses and researchers from across the UK. Equal credit will be given to those who have wholeheartedly collaborated in the trial.
The results of the trial will be reported first to trial collaborators. The main report will be drafted by the trial office team, and the final version will be agreed by the Steering Committee before submission for publication, on behalf of the collaboration.
Due to limited resources, it will be not be possible to provide each surviving patient with a personal copy of the results of the trial. If the patients require a copy of the results they should contact the Principal Investigator.
5. Trial Organisation
5.1 Sponsor
The Heart of England NHS Foundation Trust acted as sponsor for the pilot trial. The Heart of England Foundation NHS Trust and University of Warwick will act as co-sponsors for the main trial.
Local agreements will be drawn up with individual participating hospitals to ensure that Investigators and patients are indemnified for against negligent harm.
5.2 Trial Steering Committee (TSC)
The trial will be guided by a group of respected and experienced critical care personnel and trialists as well as a ‘lay’ representative. Face to face meetings will be held at regular intervals determined by need but not less than once a year. Routine business is conducted by email, post or teleconferencing.
The Steering Committee, in the development of this protocol and throughout the trial will take responsibility for:
-
Major decisions such as a need to change the protocol for any reason.
-
Monitoring and supervising the progress of the trial.
-
Reviewing relevant information from other sources.
-
Considering recommendations from the DMEC.
-
Informing and advising on all aspects of the trial.
5.3 Data Monitoring and Ethics Committee (DMEC)
A DMEC will be appointed comprising two clinicians with experience in undertaking clinical trials / caring for critically ill patients and a statistician who are independent of the trial.
During the period of recruitment into the trial, interim analyses of the proportion of patients alive at 28 days and analyses of deaths from all causes at 28 days will be supplied, in strict confidence, to the chairman of the DMEC, along with any other analyses that the committee may request. The intervals for these analyses will be determined by the committee.
The DMEC will advise the Chairman of the Steering Committee if, in their view, the randomised comparisons have provided both (i) ‘proof beyond reasonable doubt’ that for all, or some, the treatment is clearly indicated or clearly contra-indicated and (ii) evidence that might reasonably be expected to materially influence future patient management.
Following a report from the DMEC, the Steering Committee will decide what actions, if any, are required. Unless the DMEC request cessation of the trial the Steering Committee and the collaborators will remain ignorant of the interim results.
5.4 Administration
The trial will be co-ordinated at the Warwick Medical School Clinical Trials Unit with support from the West Midlands Critical Care Research Network and Intensive Care Society Clinical Trials Group.
All day-to-day co-ordination of the trial will be the responsibility of the trial manager. All clinical co-ordination of the trial will be the responsibility of Professor Fang Gao.
The trial is managed by a multi-disciplinary team (page 3).
The trial office team will assist and facilitate the setting up of centres wishing to collaborate in the trial. In addition the trial office team will:
-
Distribute the standardised data collection forms to collaborators.
-
Organise the telephone randomisation service for formal trial entry.
-
Monitor the collection of data, process data and seek missing data.
-
Train local staff with regards to data collection.
-
Ensure the confidentiality and security of all trial forms and data.
-
Conduct extensive data checking and cleaning.
-
Organise any interim and main analyses.
-
Organise Steering Committee, DMEC and Collaborators meetings.
The trial office will receive completed data forms, via the postal service. Upon receipt, data forms will be checked for completeness and entered into a trial-dedicated computer programme which will check the data validity.
Patient confidentiality will be maintained at every stage and we comply with the Data Protection Act (1998).
5.5 Indemnity
NHS indemnity covers NHS staff, medical academic staff with honorary contracts, and those conducting the trial. NHS bodies carry this risk themselves or spread it through the Clinical Negligence Scheme for Trusts, which provides unlimited cover for this risk. The University of Warwick provides indemnity for any harm caused to patients by the design of the research protocol.
5.6 Monitoring and Safety Procedures
5.6.1 Safety and Well-being of Trial Patients
The safety and well-being of trial patients are protected by implementation of the sponsoring organisation's Standard Operating Procedures (SOP's) as set out in the Research Governance Framework and The Medicines for Human Use (Clinical Trials) Regulations 2004.
Individual sites will ensure that all investigators are able to demonstrate that they are qualified by education, training and experience to fulfill their roles. Systems and procedures are in place which can assure the quality of every aspect of the trial.
If new safety information becomes available, trial patient or personal legal representative will be informed of this and asked if they wish to continue in the trial. If the patient continues in the trial, a revised Patient Information Sheet and a new Consent Form will require completion.
Early termination of the trial in response to safety issues will be addressed via the DMEC.
Day to day management of the trial will be undertaken via a Trial Management group which includes the Chief Investigator. They will meet on a regular basis to discuss trial issues.
5.6.2 Safety of Investigators
Each Trust and The University of Warwick has Health and Safety Policies applicable to all employees. All personnel should also ensure they adhere to any other Health and Safety Regulations relating to their area of work. The Principal Investigator at each site will ensure that all personnel involved in the trial have been appropriately and adequately trained to undertake their specific tasks.
As the trial fits closely to standard practice, there are few risks identified which are hazardous to Investigators. Individual sites will be responsible for ensuring all staff have received Good Clinical Practice (GCP) training prior to start up.
5.6.3 Monitoring of Trial Conduct
The Trial Manager and Recruitment Facilitators will undertake site visits to ensure that the trial protocol is adhered to and that necessary paperwork (CRF's, Patient Consent) are being completed appropriately.
5.6.4 Ethics and Regulatory Approval
The trial will be conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki. Approval from a Multi-Centre Research Ethics Committee (MREC) approval and Clinical Trial Authorisation from the Medicines and Healthcare products Regulatory Agency (MHRA) are needed before the start of the trial.
Following detailed discussion of the trial, written, informed consent will be obtained from each patient. In line with The Medicines and For Human Use (Clinical Trials) Regulations 2004 and to comply with the Research Governance Framework, consenting processes are standardised and will be reinforced via training prior to trial start up.
The trial is registered with the International Standard Randomised Controlled Trial Number register, number ISRCTN38366450.
The trial has been registered with the UK National Institute for Health Research (NIHR) Clinical Research Portfolio. In order that the trial remains on the NIHR Portfolio and receives the appropriate level of support through the relevant Local Research Network, accrual data on patient recruitment will be forwarded to the UKCRN Co-ordinating Centre on a monthly basis from the Trial Co-ordinating Centre.
Reference List
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2:319-23.
- Fowler AA, Hamman RF, Good JT, Benson KN, Baird M, Eberle DJ, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med 1983;98:593-7.
- Petty TL. Acute respiratory distress syndrome (ARDS). Dis Mon 1990;36:1-58.
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24.
- Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982;3:35-56.
- Corrin B, Evans TW, Haslett C. Acute Respiratory Distress Syndrome. London: Chapman & Hall; 1996.
- Bellingan GJ. The pulmonary physician in critical care * 6: The pathogenesis of ALI/ARDS. Thorax 2002;57:540-6.
- Tomashefski JF. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med 2000;21:435-66.
- Berthiaume Y, Lesur O, Dagenais A. Treatment of adult respiratory distress syndrome: plea for rescue therapy of the alveolar epithelium. Thorax 1999;54:150-60.
- Armstrong L, Thickett DR, Mansell JP, Ionescu M, Hoyle E, Billinghurst RC, et al. Changes in collagen turnover in early acute respiratory distress syndrome. Am J Respir Crit Care Med 1999;160:1910-15.
- Crandall ED, Matthay MA. Alveolar Epithelial Transport. Basic science to clinical medicine. Am J Respir Crit Care Med 2001;163:1021-9.
- Matthay MA, Landolt CC, Staub NC. Differential liquid and protein clearance from the alveoli of anesthetized sheep. J Appl Physiol 1982;53:96-104.
- Skou JC. Nobel Lecture. The identification of the sodium pump. Biosci Rep 1998;18:155-69.
- Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569-600.
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685-93.
- Intensive Care National Audit and Research Centre (ICNARC) . Survey on Prevalence of the UK ARDS 2005.
- Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 2004;30:51-6.
- Rossi C, Simini B, Brazzi L, Rossi G, Radrizzani D, Iapichino G, et al. Variable costs of ICU patients: a multicenter prospective study. Intensive Care Med 2006;32:545-52.
- Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, et al. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med 2006;32:1115-24.
- Cheung AM, Tansey CM, Tomlinson G, az-Granados N, Matte A, Barr A, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;174:538-44.
- The Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-08.
- Perkins GD, McAuley DF, Richter A, Thickett DR, Gao F. Bench-to-bedside review: beta2-Agonists and the acute respiratory distress syndrome. Crit Care 2004;8:25-32.
- Maris NA, de Vos AF, Dessing MC, Spek CA, Lutter R, Jansen HM, et al. Antiinflammatory Effects of Salmeterol after Inhalation of Lipopolysaccharide by Healthy Volunteers. Am J Respir Crit Care Med 2005;172:878-84.
- McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med 2004;32:1470-6.
- Basran GS, Hardy JG, Woo SP, Ramasubramanian R, Byrne AJ. Beta-2-adrenoceptor agonists as inhibitors of lung vascular permeability to radiolabelled transferrin in the adult respiratory distress syndrome in man. Eur J Nucl Med 1986;12:381-4.
- Perkins GD. Intravenous salbutamol reduced alveolar-capillary permeability and extra-vascular lung water in ARDS: Results of the BALTI study. Thorax 2004;59.
- Perkins GD. Do Beta agonists promote epithelial repair?. Thorax 2005;60.
- Wright PE, Carmichael LC, Bernard GR. Effect of bronchodilators on lung mechanics in the acute respiratory distress syndrome (ARDS). Chest 1994;106:1517-23.
- Morina P, Herrera M, Venegas J, Mora D, Rodriguez M, Pino E. Effects of nebulized salbutamol on respiratory mechanics in adult respiratory distress syndrome. Intensive Care Med 1997;23:58-64.
- Pesenti A, Pelosi P, Rossi N, Aprigliano M, Brazzi L, Fumagalli R. Respiratory mechanics and bronchodilator responsiveness in patients with the adult respiratory distress syndrome. Crit Care Med 1993;21:78-83.
- Manocha S, Gordon AC, Salehifar E, Groshaus H, Walley KR, Russell JA. Inhaled beta-2 agonist salbutamol and acute lung injury: an association with improvement in acute lung injury. Crit Care 2006;10.
- Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 2006;173:281-7.
- Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1376-83.
- Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, et al. The American-European Consensus Conference on ARDS, part 2: Ventilatory, pharmacologic, supportive therapy, study design strategies, and issues related to recovery and remodeling. Acute respiratory distress syndrome. Am J Respir Crit Care Med 1998;157:1332-47.
- Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2003;167:1027-35.
- Matthay MA, Abraham E. Beta-adrenergic agonist therapy as a potential treatment for acute lung injury. Am J Respir Crit Care Med 2006;173:254-5.
- Perkins G, McAuley D, Frank J, Thickett D, Fang X, Matthay M. Clinically relevant concentrations of beta agonists may stimulate alveolar fluid clearance in acute respiratory distress syndrome. Critical Care 2004;8.
- Conover WB, Benumof JL, Key TC. Ritodrine inhibition of hypoxic pulmonary vasoconstriction. Am J Obstet Gynecol 1983;146:652-6.
- Harris L. Comparison of the effect on blood gases, ventilation, and perfusion of isoproterenol-phenylephrine and salbutamol aerosols in chronic bronchitis with asthma. J Allergy Clin Immunol 1972;49:63-71.
- Neville A, Palmer JB, Gaddie J, May CS, Palmer KN, Murchison LE. Metabolic effects of salbutamol: comparison of aerosol and intravenous administration. British Medical Journal 1977;1:413-14.
- King JF, Grant A, Keirse MJ, Chalmers I. Beta-mimetics in preterm labour: an overview of the randomized controlled trials. Br J Obstet Gynaecol 1988;95:211-22.
- Bader AM, Boudier E, Martinez C, Langer B, Sacrez J, Cherif Y, et al. Etiology and prevention of pulmonary complications following beta-mimetic mediated tocolysis. Eur J Obstet Gynecol Reprod Biol 1998;80:133-7.
- Grospietsch G, Fenske M, Girndt J, Uhlich E, Kuhn W. The renin-angiotensin-aldosterone system, antidiuretic hormone levels and water balance under tocolytic therapy with Fenoterol and Verapamil. Int J Gynaecol Obstet 1980;17:590-5.
- Grospietsch G, Ulbrich R, Saul U, Fenske M, Ensink FB, Kuhn W. Urinary excretion, osmolarity and electrolytes after bolus-injection of fenoterol in female rabbits. Gynecol Obstet Invest 1984;17:317-25.
- Stratakos G, Kalomenidis J, Routsi C, Papins S, Roussos C. Transient lactic acidosis as a side effect of inhaled salbutamol. Chest 2002;122:385-6.
- Day NP, Phu NH, Bethell DP, Mai NT, Chau TT, Hein TT, et al. The effect of dopamine and adrenaline infusion on acid-base balance and systemic haemodynamics in severe infection. Lancet 1996;348:219-23.
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685-93.
- Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002;30:1772-7.
- The ARDS Network Clinical Coordinating Center . ARDSNet Definitions and Recommendations 2005.
- Wright PE, Carmichael LC, Bernard GR. Effect of bronchodilators on lung mechanics in the acute respiratory distress syndrome (ARDS). Chest 1994;106:1517-23.
List of abbreviations
- ALI
- acute lung injury
- ALTA
- ALbuterol for the Treatment of ALI
- APACHE
- Acute Physiology and Chronic Health Evaluation
- ARDS
- acute respiratory distress syndrome
- BALTI
- Beta-Agonist Lung injury TrIal
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CMP
- Case Mix Programme
- CRF
- case report form
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- European Quality of Life-5 Dimensions
- FiO2
- fraction of inspired oxygen
- HR
- heart rate
- IBW
- ideal body weight
- ICER
- incremental cost-effectiveness ratio
- ICNARC
- Intensive Care National Audit and Research Centre
- ICU
- intensive care unit
- i.v.
- intravenous
- LOCF
- last observation carried forward
- LREC
- Local Research Ethics Committee
- MHRA
- Medicines and Healthcare Products Regulatory Agency
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PaO2
- partial pressure of oxygen in arterial blood
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SUSAR
- suspected unexpected serious adverse reaction
- VFD
- ventilator-free day
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.