Notes
This monograph is based on the Diagnostic Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed commercial-in-confidence and/or academic-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence and/or academic-in-confidence data removed and replaced by the statement ‘commercial-in-confidence and/or academic-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
Article history paragraph text
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 10/125/01. The protocol was agreed in May 2011. The assessment report began editorial review in October 2011 and was accepted for publication in March 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Ward et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Scientific summary
Background
Prognostic tools such as the Nottingham Prognostic Index (NPI) and Adjuvant! Online are currently used in the UK to assist decision-making relating to adjuvant chemotherapy for women with early breast cancer at intermediate or high risk of recurrence following primary surgery. These tools use pathological parameters, for example tumour size, grade and lymph node status in the case of NPI, with the addition of oestrogen receptor (ER) status, age and comorbidity for Adjuvant! Online. Such tools are imperfect and some women with early breast cancer may be over- or undertreated, resulting in unnecessary use of chemotherapy for some women or avoidable deaths in women for whom chemotherapy was withheld.
Gene expression profiling (GEP) and expanded immunohistochemistry (IHC) (or protein expression) tests aim to improve the targeting of chemotherapy by more accurately identifying patients who will gain most benefit from it. These tests either aim to more accurately measure the risk of cancer recurrence by incorporating a wider range of biomarkers than standard clinicopathological algorithms or seek to identify breast cancer subtypes, which provide information on recurrence risk.
Nine tests were included in this assessment, as per the National Institute for Health and Care Excellence (NICE) scope. Six use GEP technology: the Randox Breast Cancer Array (Randox Laboratories, Crumlin, UK), MammaPrint® (Agendia, Amsterdam, the Netherlands), BluePrint™ (Agendia, Amsterdam, the Netherlands), the PAM50 gene expression assay (ARUP Laboratories, Salt Lake City, UT, USA), OncotypeDX™ (Genomic Health Inc., Redwood City, CA, USA) and the Breast Cancer IndexSM (bioTheranostics Inc., San Diego, CA, USA); and three use IHC technology: IHC4 [The National Institute for Health Research (NIHR) Specialist Biomedical Research Centre (BRC) for Cancer is a partnership between The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research (ICR); see http://www.cancerbrc.org/Highlights/Breast_Cancer_highlights/index.shtml], Mammostrat® (Clarient Inc., Aliso Viejo, CA) and NPI plus (NPI+) (University of Nottingham, Nottingham, UK).
Objective
The objective of this study was to evaluate the clinical effectiveness and cost-effectiveness of GEP and expanded IHC tests compared with existing prognostic tools in guiding the use of adjuvant chemotherapy in women with early breast cancer in England and Wales.
Methods
A systematic review of the evidence on the clinical effectiveness of nine GEP and expanded IHC tests to guide the use of chemotherapy in breast cancer management was conducted. For two of the tests (OncotypeDX and MammaPrint) the review updated two existing systematic reviews. Several electronic databases (including MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE and The Cochrane Library) were searched from January 2002 to May 2011 (for the OncotypeDX and MammaPrint tests searches were conducted from January 2009).
Outcome measures included analytical validity, clinical validity and clinical utility. The study by Altman (2001) was used to assess the methodological quality of included studies (Altman D. Systematic reviews in health care: systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224–8).
A systematic review of economic evaluations was also undertaken. In addition, two economic evaluations were submitted by Genomic Health and Clarient for the use of OncotypeDX and Mammostrat in the UK respectively.
A probabilistic model was developed by the External Assessment Group (EAG) using a lifetime horizon. Following a review of the evidence available, only four of the nine tests were included in the economic evaluation. Analysis was undertaken for women with ER-positive (ER+), lymph node-negative (LN−) and human epidermal growth factor receptor type 2-negative (HER2–) early breast cancer from a NHS perspective. These tests were assessed as an addition to existing prognostic tools. A subgroup analysis was conducted in women with a NPI score ≤ 3.4 and women with a NPI score > 3.4. The model used UK-specific data where possible.
In the comparator arm of the economic model, the proportion of patients receiving chemotherapy under current practice was informed by cancer registry data, reflecting the use of current prognostic tools such as NPI and Adjuvant! Online to guide the use of chemotherapy. In the intervention arm the targeting of patients to receive chemotherapy was dependent on the classification of risk by the new test. The natural history of breast cancer was then simulated using a cohort state transition model, taking into account the reduction in the risk of recurrence associated with chemotherapy. Evidence for the benefit of chemotherapy (reduction in the risk of recurrence) by risk group for the new tests was taken directly from the studies identified through the systematic review of the literature, despite the identified limitations of the studies. Patients were able to move between five possible health states – recurrence free, distant recurrence, local recurrence, long-term adverse events and death (from breast cancer, adverse events or other causes). Results were reported in terms of cost per quality-adjusted life-year (QALY).
Results
Nature, description and quality of the available evidence
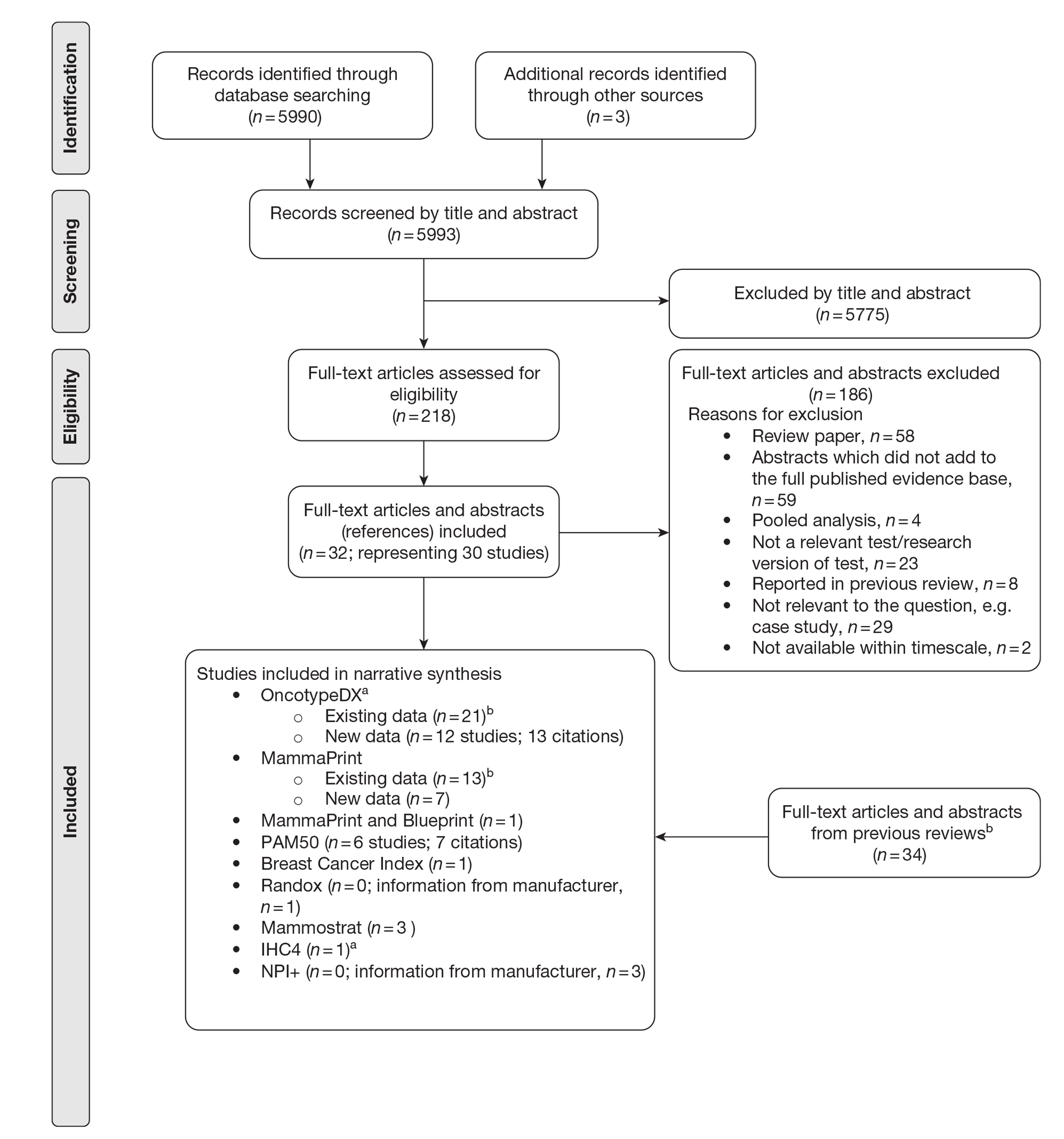
The literature searches identified 5993 citations, of which 32 full-text papers or abstracts (representing 30 studies) were included in the review. Supplementary information submitted by the manufacturers was also presented. This evidence was summarised but was not subjected to the systematic review process. Additional studies that did not meet the inclusion criteria for the systematic review were used to populate the economic model.
The study populations were generally heterogeneous in the nature of their inclusion criteria although the majority of evidence examined ER+, LN− populations. Most studies included a small number of participants, although a few studies included over 1000 patients. Follow-up was short or not reported for a large number of studies. Only six studies were specific to a UK population (three for OncotypeDX, one for NPI+, one for IHC4 and one for Mammostrat).
Summary of the benefits and risks of gene expression profiling and expanded immunohistochemistry tests
OncotypeDX
Clinical
Previous systematic reviews
OncotypeDX was reported to be furthest along the validation pathway. In terms of clinical validity these reviews reported evidence that the OncotypeDX recurrence score was significantly correlated with disease-free-survival and overall survival. One study was reported that reported a significant benefit from the use of chemotherapy in the OncotypeDX high-risk group, although it was highlighted within the review that the study may have been subject to bias.
Current review
The current review identified 12 additional studies on the OncotypeDX test. Further larger studies have now reported, which support the prognostic capability of the OncotypeDX test. In particular, one large-scale UK-based study, in postmenopausal women with ER+, LN− early breast cancer, reported that an increase in risk score was significantly associated with an increased risk of distant recurrence. Furthermore, the evidence base has been extended to include the LN+ population, and there are the beginnings of an evidence base for the validity of OncotypeDX in different populations such as Japanese patients. Four studies presented evidence on the impact of OncotypeDX on clinical decision-making, indicating that the use of OncotypeDX leads to changes in decision-making for between 31.5% and 38% of patients. However, only one of these studies was UK based and limitations in relation to study design were identified.
Economic
Two economic studies were identified. Both studies compared the use of OncotypeDX with Adjuvant! Online. These studies were non-UK studies and were not considered generalisable to the UK setting. The economic evaluation submitted by Genomic Health estimated the incremental cost for treatment guided using OncotypeDX to be £6232 per QALY gained compared with current clinical practice in the UK, although a number of limitations with regard to the analysis were highlighted.
A de novo economic model was built by the EAG and estimated the cost per QALY gained to be £29,502 compared with current clinical practice, assuming that the test was offered to all woman with ER+, LN−, HER2– early breast cancer, under our base-case assumptions. This analysis assumed OncotypeDX to be predictive of the benefit of chemotherapy, based on evidence from the Paik et al. study, although weaknesses relating to this study are highlighted. (Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726–34.) A subgroup analysis was performed and showed that the incremental cost-effectiveness ratio (ICER) for OncotypeDX compared with current clinical practice was reduced to £9774 per QALY gained if OncotypeDX was to be offered to women with a (NPI > 3.4) only. Compared with current clinical practice, OncotypeDX had a 12.4% (all women) and 91.6% (NPI > 3.4) probability of being considered cost-effective when using a threshold of £20,000 per QALY gained respectively, although the quality of the data in the model was considered relatively weak. Key areas of uncertainly relate to assumptions about the benefits of chemotherapy in terms of relative risk reduction by risk group, the risk of recurrence over time and the impact of the new test on decision-making. The ICER increased substantially and was greater than £20,000 per QALY gained for both analyses when assuming the same relative reduction in the risk of recurrence from chemotherapy for all patients irrespective of the OncotypeDX recurrence score classification, that is, assuming that the test is prognostic only.
MammaPrint
Clinical
Previous systematic reviews
There is a range of studies evaluating the prognostic ability of MammaPrint in heterogeneous populations; however, the previous reviews indicated that evidence relating to the clinical validity of MammaPrint was not always conclusive or supportive of the prognostic value of the test. In terms of clinical utility, the previous reviews identified one non-UK study which suggested that MammaPrint had an impact on clinical decision-making.
Current review
Our review identified seven additional studies on the MammaPrint test. Four studies reported that the MammaPrint score is a strong independent prognostic factor and may provide additional value to standard clinicopathological measures, although the populations in all of these studies were relatively small. Six non-UK studies evaluated the clinical utility of MammaPrint. Five of the studies reported on test reclassification against currently used guidelines and one reported that treatment advice for 40% of patients would change, assuming that all patients classified as high risk and no patients classified as low risk would receive chemotherapy. However, none of the studies provided evidence of actual changes in treatment decisions following introduction of the test. A study on the benefit of chemotherapy by MammaPrint risk group was identified but omitted from the systematic review because it was based on a pooled analysis of six primary studies.
Economic
An analysis was carried out by the EAG to evaluate the use of MammaPrint in England and Wales but because of the limitations in the evidence available this was considered exploratory only and no base-case ICER was presented.
PAM50
Clinical
The evidence base for PAM50 is still relatively immature. The current review identified two analytical validity studies (reported in abstract form only) comparing the PAM50 test with standard IHC measurements. Four studies evaluated the clinical validity of PAM50; two of these are as yet unpublished. No evidence on clinical utility was identified.
Economic
The EAG did not model treatment guided using PAM50 because of gaps in the evidence base.
Mammostrat
Clinical
The current review identified three studies that provided data to support the use of the Mammostrat test as an independent prognostic tool for women with ER+, tamoxifen-treated breast cancer. Although the evidence base for the Mammostrat test is relatively immature, these studies included a large sample size, appeared to be of reasonable quality and provided data from a UK setting (one study). One study was identified for clinical utility but limitations were identified relating to this study.
Economic
The EAG conducted an exploratory analysis using the same model structure as for the OncotypeDX evaluation and unpublished data from a small sample from a non-UK population; however, because of the limitations in the evidence base, any conclusions drawn from this analysis are subject to significant uncertainty.
IHC4
Clinical
No studies on analytical validity of the test were identified. The current review identified one study on the clinical validity of IHC4, which reports that the IHC4 score is a highly significant predictor of distant recurrence. This study was based on a large sample size and detailed the development of the test in one cohort and the external validation of the test in an independent cohort. The study also reported evidence comparing IHC4 with OncotypeDX. The review did not identify any published evidence on the clinical utility of IHC4 in terms of its impact on treatment decisions or its ability to predict chemotherapy benefit by risk group.
Economic
The EAG evaluated the cost-effectiveness of IHC4 in parallel with that of OncotypeDX as there was direct evidence between the two tests in a UK population from the same data source used to evaluate the cost-effectiveness of OncotypeDX. The IHC4 test was predicted to be dominant compared with current clinical practice in patients with ER+, LN−, HER2– early breast cancer, providing more QALYs at a lower cost. An incremental analysis was conducted comparing OncotypeDX, IHC4 and current clinical practice. When the treatment decision using OncotypeDX was compared with that using IHC4, the ICER for OncotypeDX increased to £64,111 per QALY gained if the tests were to be offered to all women and £31,125 if the tests were to be offered to women with a NPI > 3.4 only. IHC4 was predicted to remain dominant assuming the test to be prognostic only, that is, all women receiving chemotherapy derive the same relative benefit in terms of reduction in distant recurrences. However, because the evidence base for IHC4 is less developed than that for OncotypeDX, additional assumptions were required and the results are subject to greater uncertainty.
Nottingham Prognostic Index plus, Breast Cancer Index, BluePrint and Randox Breast Cancer Array
Clinical
Based on the limited available data identified for these tests, no firm conclusions can be drawn about their analytical validity, clinical validity (prognostic ability) and clinical utility. Further evidence on the prognostic and predictive ability of all of these tests is required.
Economic
No studies were identified in the systematic review of the economic literature. The EAG did not model treatment guided using these tests because of significant gaps in the evidence base.
Discussion
Strengths and limitations of the analyses and uncertainties
Clinical
Two of the tests (OncotypeDX and MammaPrint) have a reasonably large evidence base, although there are some methodological weaknesses relating to this evidence in terms of heterogeneity of patient cohorts and issues arising from the retrospective nature of the evidence, such as the relevance of the evidence to current methods of diagnosis, treatment and standards of care. The evidence base for OncotypeDX is considered to be the most robust. The MammaPrint evidence is typically based on observational data (small cohort studies) rather than randomised data, increasing the risk of selection bias. Both IHC4 and Mammostrat present early evidence of the prognostic ability of the tests based on large UK-based validation cohorts. Further evidence is required on the clinical utility of all of these tests, and on UK-based populations where this is not currently available. The evidence base for the remaining five tests has significant gaps and is considered less robust.
Economic
Four of the nine tests were included in the economic evaluation by the EAG. The model used UK-specific evidence where possible, including the baseline use of chemotherapy, the risk of distant recurrence/recurrence and reclassification with the new test, so that its conclusions would be relevant to the UK setting. Our analysis focused on patients with ER+, LN−, HER2– early breast cancer as use of the tests in this population is supported by the most robust clinical evidence. Women with a NPI ≤ 3.4 and women with a NPI > 3.4 were modelled separately to account for the prognostic value of the current treatment decision based on clinicopathological parameters and to allow a scenario assuming that the test was offered to a subgroup of the population at intermediate risk to be conducted.
However, there are significant limitations with regard to the economic analyses. Results of all of the analyses have to be interpreted with caution and the results cannot be compared directly between tests. Given that no studies following patients from initial diagnosis through to final health outcomes were identified for any of the tests, the economic model needed to combine clinical data from several different sources in order to model how the results from the new tests translate into final outcomes in the form of QALYs. This resulted in significant uncertainties that were not adequately captured with the probabilistic sensitivity analysis – data used in the model were not always based on UK populations and were not always specifically based on the ER+, LN−, HER2– population of interest. Differences in the age of the study populations and the endocrine and chemotherapy regimens used in the studies compared with those in the model introduced further uncertainty. One key area of uncertainty is whether the tests are prognostic or predictive of the benefit of chemotherapy (i.e. do they allow identification of high-risk patients who would derive a greater relative benefit from chemotherapy). The ICER was very sensitive to this assumption. There were particular concerns relating to the studies used to estimate the benefit associated with chemotherapy for patients categorised by risk group by the new tests, in relation to both the study design and the populations included in these studies. The evidence base on the impact of the new tests on the selection of patients to receive chemotherapy was also lacking or not considered generalisable to the UK population. Univariate sensitivity analyses indicated that the ICER was sensitive to these assumptions.
A greater number of assumptions were required to model IHC4 compared with OncotypeDX because of data limitations for IHC4. There were more significant gaps in the evidence for MammaPrint and Mammostrat, and any conclusions that can be drawn from these exploratory analyses are subject to considerable uncertainty.
Conclusions
The OncotypeDX and MammaPrint tests have a reasonably large evidence base, although there are some methodological weaknesses relating to this evidence in terms of heterogeneity of patient cohorts and the use of retrospective data. The evidence base for OncotypeDX is considered to be the most robust. Two of the tests (IHC4 and Mammostrat) have presented early evidence of the prognostic ability of the tests, based on large UK-based validation cohorts, but further research is required. The clinical utility evidence for GEP and expanded IHC tests is limited by the lack of large prospective studies in UK populations. PAM50, BluePrint, Breast Cancer Index, NPI+ and Randox Breast Cancer Array have only limited clinical evidence to date.
The economic analysis suggests that the use of the new tests may result in small increases in QALYs compared with currently used prognostic tools, but current limitations in the evidence base introduce significant uncertainty in the results. A key area of uncertainty is whether tests are prognostic only or identify high-risk patients who will benefit more relatively from chemotherapy (from reductions in the risk of recurrence) than low-risk patients. The economic analyses suggested that, of the four tests considered, treatment guided using IHC4 has the greatest potential to be cost-effective at a £20,000 threshold, given the low cost of the test; however, further evidence on IHC4 is needed and the exact cost of using the test in the NHS needs to be investigated further. Although the OncotypeDX test has been shown to have the potential to be cost-effective at the £20,000 threshold for patients with a NPI > 3.4, further evidence is needed on the impact on decision-making in the UK and to clarify the predictive ability of the test specifically in an ER+, LN−, HER– population receiving current endocrine and chemotherapy regimens.
Implications for service provision
The impact of sending large numbers of samples to central testing facilities for pathology services, in terms of tissue tracking, pathologist and technical staff time, data input on receipt, etc., would need to be explored. Tests requiring the use of fresh tissue require a major change in practice with regard to the handling of tissue, with significant implications for service configuration and costs. The addition of expanded IHC-based tests is likely to fit more easily with current practice in the NHS. Quality assurance issues would need to be addressed, for example for the Ki-67 component of the IHC4 test, before these tests could be considered for use in clinical practice in the NHS.
The main research priorities relate to the reliability and reproducibility of the IHC4 test, along with further evidence of the prognostic ability of IHC4 compared with NPI and Adjuvant! Online. Further evidence on the predictive ability of all of the tests is also required. In addition, evidence to improve the understanding of the impact of these tests (for tests that provide a risk score/category and tests that provide subtype information only) on the management of patients in a UK population is urgently needed.
Study registration
This study is registered as PROSPERO 2011:CRD42011001361, available from www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42011001361.
Funding
Funding for this study was provided by the Health Technology Assessment programme of the National Institute for Health Research.
Chapter 1 Background and definition of the decision problem
Condition and aetiology
Breast cancer is the most commonly diagnosed cancer in women in England and Wales. In 2009 there were 42,305 new cases diagnosed. Treatment usually involves surgery to remove the primary tumour and any involved lymph nodes; this may be followed by radiation therapy, endocrine therapy and/or chemotherapy with or without trastuzumab depending on tumour and patient variables.
Aetiology, pathology and prognosis
Aetiology
The causes of breast cancer are not completely understood. A range of risk factors have been identified including genetic, hormonal and lifestyle factors. 1
It has been estimated that 12% of women with breast cancer have one affected family member and 1% have two or more affected family members. 2 Genetic predisposition is mediated by high-penetrance genes such as breast cancer 1 gene (BRCA1) and breast cancer 2 gene (BRCA2), responsible for around 80–90% of hereditary cancers, and low-penetrance genes, which confer increased and decreased risk. 1
Environmental and lifestyle factors as well as genetic factors influence breast cancer risk. Asian migrants to the West have increased levels of risk compared with the indigenous population, whereas Asian Americans born in the West have incidence rates approximating the US average. 3
Lifestyle and environmental factors thought to increase risk include hormonal factors such as taking the oral contraceptive pill or hormone replacement therapy, higher age of menopause, early age of menarche, late age of first birth and not giving birth. Factors that decrease risk include higher folate intake, higher number of pregnancies, breastfeeding and younger age at first birth. 1
Obesity increases the risk of breast cancer in postmenopausal women. 4 The picture is less clear for premenopausal women, in whom risk may be lower but prognosis poorer. Physical activity in adolescence and young adulthood confers a decreased risk of breast cancer,5 which may be mediated hormonally.
Pathology
Breast cancer starts with genetic changes in a single cell or a small group of cells in the epithelia of the ducts or the lobules of the breast. The genetic change allows cells to reproduce uncontrollably, creating a tumour. Tumours that have not yet spread to surrounding tissue are known as carcinoma in situ and may be ductal (DCIS) or lobular (LCIS). Once spread to surrounding tissue begins, a tumour is known as invasive. More rapid growth and spread occurs once a blood supply is secured. Cancer spreads via the lymphatic system or the bloodstream. Lymphatic spread is usually first to the axillary lymph nodes. Spread via the bloodstream can lead to distant metastases in the bone or viscera that are incurable.
The presence or absence of axillary metastases is a key indicator of stage of disease and prognosis, and adjuvant therapy is planned, in part, based on their presence and extent. 6 They are caused when a single or small number of cells detach from the main tumour, travel via the lymphatic system and establish themselves in the tissue of the lymph nodes. Axillary metastases occur in approximately 41% of cases7 and prognosis is better when there is no axillary spread. When metastases are present, axillary clearance is indicated to prevent further spread and ensure local disease control.
Prognosis
Overall, 5-year, age-standardised breast cancer survival rates are around 80%. 8 Survival varies with age (Table 1) and stage of disease (Table 2). 9
| Age (years) | ||||||
|---|---|---|---|---|---|---|
| 15–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–99 | |
| 5-year survival rate (%) | 81 | 86 | 89 | 87 | 78 | 64 |
| Stage of disease | ||||
|---|---|---|---|---|
| I | II | III | IV | |
| 5-year survival rate (%) | 88 | 69 | 43 | 12 |
Other factors can affect prognosis. Clinicians may use tools such as the Nottingham Prognostic Index (NPI),10 which takes into account grade as well as size and spread, or Adjuvant! Online,11 which uses patient data such as age, tumour size, nodal involvement, hormonal receptor status and histological grade to predict disease course and treatment options. Good prognosis is associated with small tumour size, lymph node-negative (LN−) status, younger age, oestrogen receptor-positive (ER+) status and progesterone receptor-positive (PR+) status. Human epidermal growth factor receptor type 2 (HER2) overexpression is associated with poor prognosis.
Epidemiology and incidence
Incidence varies most with gender. Women are far more likely to get breast cancer than men. For both genders, incidence varies with age (Table 3). Just over 80% of cases occur in women aged ≥ 50 years. In England and Wales, 2006 data demonstrate highest rates for women in the 60- to 70-year age range. 12
| Age (years) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | 75–79 | 80–84 | 85+ | |
| Women | ||||||||||||||
| Wales | 0 | 2 | 21 | 64 | 123 | 186 | 256 | 286 | 324 | 328 | 254 | 201 | 199 | 213 |
| England | 0 | 8 | 20 | 53 | 141 | 185 | 270 | 274 | 321 | 327 | 252 | 190 | 183 | 202 |
| Men | ||||||||||||||
| Wales | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 2 | 1 | 0 |
| England | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 2 | 0 | 5 | 1 | 3 | 2 |
Incidence also varies with ethnicity. Asian, Chinese and black ethnic groups and those with mixed heritage have a lower incidence than the white ethnic group in England. Compared with the white group the rate ratios are 0.65, 0.75, 0.49 and 0.58 respectively. 13
In both England14 and Wales15 those who are classed as most deprived have a lower incidence of breast cancer. However, there is some evidence to suggest that the trend for mortality is reversed, with better survival for those from more affluent areas. It is unclear why this is but it may be due to lower levels of screening compliance, worse overall general health status and lower levels of treatment because of limited access to health care16 and poorer compliance with treatment regimens.
Significance in terms of ill-health (burden of disease)
Breast cancer is the second largest cause of cancer death in women after lung cancer, with an age-standardised mortality rate of 26 per 100,000 women. In 2008 this constituted 10,716 deaths for women in England and Wales. 17
Measurement of disease
Breast cancer has few obvious symptoms and can easily go undetected for a few years. Among the more noticeable symptoms are a palpable lump in the breast, a change in breast shape and skin appearance or changes to the nipple such as inversion, a rash or discharge.
A suspicious breast mass may be identified through screening or through presentation to a GP. Women between the ages of 50 and 70 years are routinely invited to attend regular screening (age range in the UK is changing to 47–73 years between 2010 and 2013). Screening is thought to have reduced breast cancer deaths in the 50–69 years age category by an estimated 6.4% in addition to the effects of tamoxifen, chemotherapy and earlier presentation outside of screening. 18 Screening increases the proportion of tumours detected in the early, more curable stages.
The breast mass and axillary areas are investigated clinically by palpation and mammography or ultrasound for younger women, and the status of the tumour confirmed by histology of biopsied tissue. Staging of the disease depends on tumour size, the number of involved lymph nodes and the presence or absence of distant metastases. Tumour size and axillary metastases can be estimated by clinical examination and imaging techniques, but definitive status is achieved through surgery. Those with small tumours and no axillary metastases have the best prognosis, whereas those with distant metastases are considered incurable.
Current methods for staging of breast cancer
Three main factors are used to stage breast cancer – tumour size, metastases to the regional lymph nodes and distant metastases. The tumour/node/metastases (TNM) staging system was developed and is maintained by the Union Internationale Contre le Cancer (UICC)19 and the American Joint Committee on Cancer (AJCC). 20 T stage is classified according to the size of the tumour and degree of local infiltration; N stage is classified according to the number and location of metastases to the lymph nodes in the axilla, between the ribs (internal mammary nodes) and above or below the collarbone (supraclavicular and infraclavicular nodes); and M stage is classified by the presence of metastases beyond the breast and regional lymph nodes (Table 4).
| Description | |
|---|---|
| T: tumour stage | |
| Tx | Primary tumour cannot be assessed |
| T0 | No evidence of primary tumour |
| Tis | Carcinoma in situ |
| T1 | Tumour ≤ 2 cm across |
| T2 | Tumour 2–5 cm across |
| T3 | Tumour > 5 cm across |
| T4 | Tumour of any size with direct extension to skin or chest wall, or inflammatory breast cancer |
| N: lymph node stage | |
| Nx | Nodal stage cannot be assessed |
| N0 | No metastases to any ipsilateral lymph nodes |
| N1 | Metastases to one to three axillary nodes or axillary nodes that are mobile |
| N2 | Metastases to four to nine axillary nodes, or axillary nodes that are fixed to one another or other structures, or clinically apparent metastases to internal mammary nodes |
| N3 | Metastasis to nodes above or below the collarbone (supraclavicular/infraclavicular), or to both axillary and internal mammary nodes, or to 10+ axillary nodes |
| M: metastasis stage | |
| Mx | Presence of metastases cannot be assessed |
| M0 | No distant metastases |
| M1 | Distant metastases |
The overall TNM stage of the cancer is defined as in Table 5. Early breast cancer is generally defined as cancer that has not spread beyond the breast or the ipsilateral axillary lymph nodes and which is confined to stages I, II or IIIA. 21–23
Current service provision
Management of early breast cancer
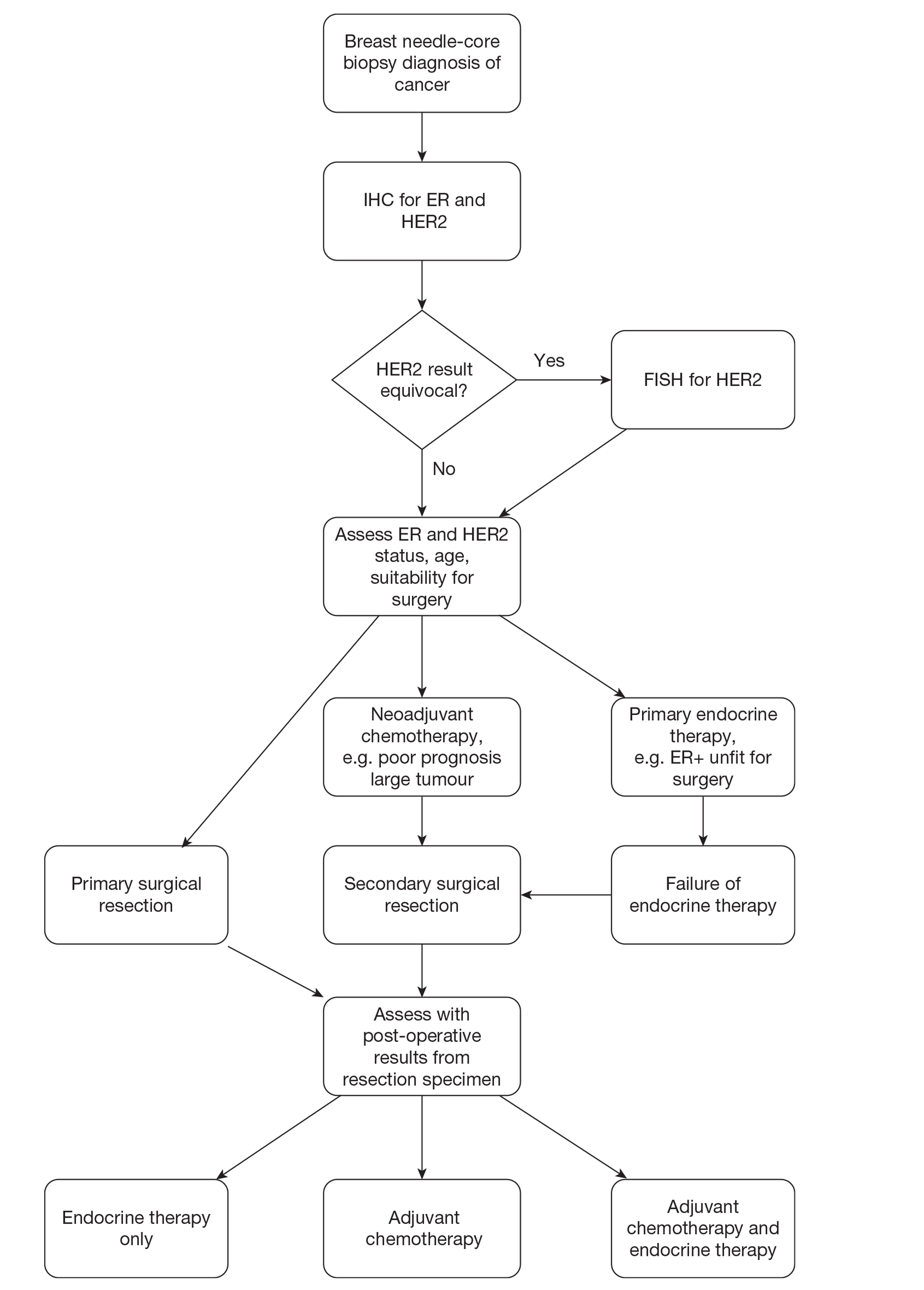
Patients diagnosed with early breast cancer currently follow the diagnosis/treatment pathway described in Figure 1.
FIGURE 1.
Diagnosis and management pathway in breast cancer. FISH, fluorescence in situ hybridisation; IHC, immunohistochemistry.
Current guidelines
Current National Institute for Health and Care Excellence (NICE) clinical guidelines (CG80)7 indicate that adjuvant therapy should be considered for all patients with early invasive breast cancer after surgery, based on assessment of the prognostic and predictive factors and the potential benefits and side effects of the treatment. These guidelines do not make specific reference to the use of gene expression profiling (GEP) or expanded immunohistochemistry (IHC) tests to aid decision-making. The guidelines do indicate that decisions should be made following discussion of these factors with the patient and recommend consideration of the use of Adjuvant! Online to support estimations of individual prognosis and the absolute benefit of adjuvant treatment for patients with early invasive breast cancer. 7 The NPI is also commonly used as the basis for many local guidelines on the management of chemotherapy for patients with early breast cancer.
Adjuvant! Online
The Adjuvant! Online computer program is designed to provide estimates of the benefits of adjuvant endocrine therapy and chemotherapy. The current version of Adjuvant! Online does not include HER2 status and the potential benefit of trastuzumab. Patient and tumour characteristics are entered into the programme and provide an estimate of the baseline risk of mortality or relapse for patients without adjuvant therapy. Information about the efficacy of different therapy options is derived from Early Breast Cancer Trialists' Collaborative Group (EBCTCG) meta-analyses in order to provide estimates of the reduction in risk at 10 years of breast cancer-related death or relapse for selected treatments. These estimates are then provided on printed sheets in simple graphical and text formats to be used in consultations.
Nottingham Prognostic Index
The NPI is a composite prognostic parameter involving both time-dependent factors and aspects of biological aggressiveness. The NPI score is based on a mix of grade, lymph node involvement and tumour size. The score is calculated as follows: add numerical grade (1, 2 or 3), lymph node score (negative = 1, one to three nodes = 2, more than three nodes = 3) and 0.2 × tumour size in cm. Patients can be divided into three prognostic groups on the basis of the NPI score: a good prognostic group (NPI < 3.4), a moderate prognostic group (3.4 < NPI < 5.4) and a poor prognostic group (NPI > 5.4).
Clinical opinion suggests that there is wide variation in clinical practice between trusts in the UK, with some centres using Adjuvant! Online and/or NPI, in addition to other clinical parameters.
Description of technologies under assessment
Gene expression profiling and expanded IHC tests aim to improve the use of chemotherapy in breast cancer by improving the stratification of patients and identification of those patients who will gain most benefit from chemotherapy. These tests typically report two types of information – breast cancer subtype and/or risk of recurrence. Tests developed to provide information on subtypes can be used either before surgery for informing decisions on neoadjuvant therapy or after primary surgery for informing decisions on adjuvant chemotherapy. Tests predicting the risk of recurrence in a specific population are likely to be used after surgery, in conjunction with other information available about tumour size, grade, etc., to guide the use of adjuvant chemotherapy. These tests are typically indicated for women with ER+ and LN− tumours (and sometime LN+ tumours if number of nodes is small).
In conjunction with other information available about tumour size, grade, etc., test results are likely to be used to guide the decision on which patients should be offered adjuvant chemotherapy. Tests that require samples to be sent away for central review following surgery may introduce a short delay (of up to 2–3 weeks) before the decision can be taken on whether or not to offer chemotherapy.
Nine tests were identified in the NICE scope25 and are included in this assessment: six are based on GEP and three on IHC (protein expression profiling) technology.
Gene expression profiling
Gene expression profiling tests assess the identity and number of messenger ribonucleic acid (mRNA) transcripts in a specific tissue sample. As only a fraction of the genes encoded in the genome of a cell are expressed by being transcribed into mRNA, gene expression profiling provides information about the activity of genes that give rise to these mRNA transcripts. Given that mRNA molecules are translated into proteins, changes in mRNA levels are ultimately related to changes in the protein composition of the cells, and consequently to changes in the properties and functions of tissues and cells (both normal and malignant) in the body.
Various assays are used in the management of breast cancer. These assays investigate the expression of specific panels of genes (also known as a gene profile or gene signature). They work by making use of different techniques to measure mRNA levels in breast cancer specimens, including real-time reverse transcription-polymerase chain reaction (RT-PCR) and deoxyribonucleic acid (DNA) microarrays. Many of these assays have been designed to measure the risk of cancer recurrence. Other uses of the assays include breast cancer subtyping (using molecular classification systems), predicting the likely benefit from certain types of therapy (e.g. chemotherapy) and diagnosing breast cancer.
There are various ways of preparing the RNA and different protocols are used to prepare the specimens [e.g. formalin-fixed paraffin-embedded (FFPE), snap-frozen and fresh samples]. Most UK hospitals currently base their pathology services around FFPE tissue and therefore the use of tests requiring fresh samples would raise major service configuration issues. Furthermore, there are varying algorithms that can be used to combine the raw data to obtain a summary measure. All of these factors can affect the reproducibility and reliability of GEP tests.
These tests provide an estimate of the risk of recurrence and/or information about the intrinsic molecular subtype of cancer. The definition of risk group varies between tests, that is, patients classified as high risk by the OncotypeDX test will be at a different level of risk from patients classified as high risk by the Mammostrat test. The definition of subtype is typically based on the classification system first described by Perou et al. 26 in 2000 and refined to include five groups – luminal A, luminal B, HER2 amplified, basal-like and unclassified. Subtype information can potentially be used to provide an indication of risk. For instance, cancers identified as luminal A typically have better prognosis than those identified as luminal B and this information may therefore aid in the risk stratification of ER+ tumours.
The six gene expression profiling tests that are included are as follows:
-
The Randox Breast Cancer Array (BCA) (Randox Laboratories, Crumlin, UK) is a complementary DNA (cDNA)-based expression biochip assay that aims to accurately define the clinical subtypes of breast cancer tumours before initiating treatment. The target population is all individuals with diagnosed breast cancer.
-
MammaPrint® (Agendia, Amsterdam, the Netherlands) is based on microarray technology and uses a 70-gene expression profile. MammaPrint is intended as a prognostic test for women of all ages, LN− and LN+ (up to three nodes positive), with a tumour size of ≤ 5.0 cm. MammaPrint is used to determine the risk of distant recurrence of early breast cancer. Patients are stratified into two distinct groups – low risk (good prognosis) or high risk (poor prognosis) of distant recurrence. It is cleared by the Food and Drug Administration as an in vitro diagnostic multivariate index assay.
-
BluePrint™ (Agendia, Amsterdam, the Netherlands) is used in addition to the MammaPrint test for molecular subtyping. It is an 80-gene microarray with a target population of patients with early-stage (stage I or II), LN− or LN+ (up to three nodes positive), ER+ or ER− breast cancer. BluePrint provides information on breast cancer subtype using three categories: basal-type, luminal-type and ERBB2-type cancers.
-
The PAM50 gene expression assay (ARUP Laboratories, Salt Lake City, UT, USA) identifies the major intrinsic biological subtypes of breast cancer. The current version of the test provides classification of breast cancer subtype and quantitative values for (gene/protein) ESR1/ER, PGR/PR, ERBB2/HER2, proliferation score and luminal score (ER pathway). The PAM50 Breast Cancer Intrinsic Classifier test is recommended for all patients diagnosed with invasive breast cancer, regardless of stage or ER status.
-
OncotypeDX™ (Genomic Health Inc., Redwood City, CA, USA) quantifies gene expression for 21 genes in breast cancer tissue using RT-PCR. It predicts the likelihood of recurrence in women of all ages with newly diagnosed stage I or II, ER+, LN− or LN+ (up to three nodes) breast cancer treated with tamoxifen. The test assigns the breast cancer a recurrence score (RS) and a risk category: low (RS < 18), intermediate (18 ≤ RS ≤ 30) or high (RS ≥ 31). The test also reports ER, PR and HER2 status.
-
The Breast Cancer Index (BCI)SM (bioTheranostics Inc., San Diego, CA, USA) is a RT-PCR assessment of the ratio of expression of two genes, HOXB13 and IL17BR, combined with the five gene Molecular Grade Index (MGI) and gives an indication of recurrence risk. The target population is those with ER+ and LN− early breast cancer. The BCI RS ranges from 0 to 10 and divides patients into three risk groups: low risk is defined as a score < 5, intermediate risk is a score of 5–6.3 and high risk is a score ≥ 6.4.
Key details of the individual GEP tests are provided in Table 6.
| OncotypeDX | MammaPrint | PAM50 | BCI | BluePrint | Randox BCA | |
|---|---|---|---|---|---|---|
| Function | Risk of recurrence | Risk of recurrence | Subtyping | Risk of recurrence | Subtyping – to be used after MammaPrint | Subtyping |
| Technology | RT-PCR (21 genes) | Microarray (70 genes) | Microarray (55 genes) | RT-PCR, HOXB13:IL17BR ratio and Molecular Grade Index (seven genes) | Microarray (80 genes) | Low-density biochip array |
| Location of testing | Central testing – USA | Central testing – Amsterdam and Irvine, USA | Central | Central | Central testing – USA | Local – purchase of array processing unit |
| Type of sample | FFPE | Fresh (use of FFPE to be introduced in 2012) | FFPE | FFPE | Fresh | Fresh |
| Staining material | Resection/core biopsy | Resection | Resection/core biopsy | Resection | Resection/core biopsy | Resection/core biopsy |
| Population | ER+, LN−; also LN+ (one to three nodes) | ER+ (or ER−), LN− and LN+ (one to three nodes) | All women | ER +, LN− | All – previously split into risk group by MammaPrint | All women |
| Key output of test | RS score – point estimate of the 10-year risk of recurrence | Risk of recurrence score – high/low (based on distant recurrence at 5 years) | Five subtypes: luminal A, luminal B, HER2, basal-like and normal-like | BCI RS | Three subtypes: basal-type, luminal-type and ERBB2-type cancers | Five subtypes: luminal A, luminal B, HER2, basal-like and normal-like |
| Presentation of results | RS and risk group (low < 18, intermediate 18–30, high ≥ 31) | Two categories: low and high risk | Subtype and quantitative values for proliferation, luminal gene expression, ESR1, PGR and ERBB2 | Risk score: 0–10. Three risk groups: (low ≤ 5, intermediate 5–6.3, high ≥ 6.4) and 10-year risk of distant recurrence | Subtype | Unknown |
| Commercially available in the UK | Yes | Yes | Yes | Yes | Yes | No |
| Cost | £2580 | £2675 | US$3200 | US$3200 (assuming 20% discount) | No additional cost (over and above MammaPrint) | Unknown |
Expanded immunohistochemistry (protein expression profiling) tests
Immunohistochemistry markers are being developed to provide similar information to that given by the GEP tests. Some of these tests offer the advantage of using existing IHC technology (such as ER and HER2 markers), which is routinely available in all UK pathology departments.
The three included expanded IHC tests for protein expression are:
-
The IHC4 test (academic sponsor: Royal Marsden Hospital and Queen Mary, University of London) assesses the levels of four key proteins in a breast cancer sample: ER, PR, HER2 and Ki-67. The IHC4 score is calculated based on the percentage of cells positive for Ki67 and PR (0–100%); the Histoscore (a measure of the percentage of cells positive multiplied by the intensity, range 0–300) for ER status; and the tumour HER2 status, expressed as a binary measure (positive/negative). The final algorithm for IHC4 calculates a risk score for distant recurrence based on ER, PR, HER2 and Ki-67 in addition to classical clinical and pathological variables (composite risk score IHC4 + clinical score referred to as IHC4 in our report). Of note, an online calculator is expected to be available at the beginning of 2012 (Professor Mitch Dowsett, Royal Marsden Hospital, London, July 2011, personal communication).
-
The Mammostrat test uses five immunohistochemical markers [solute carrier family 7 (amino acid transporter light chain, L system), member 5 (SLC7A5), HpaII tiny fragments locus 9c protein (HTF9C), protein 53 (p53), N-myc downstream regulated 1 (NDRG1) and carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5)] to stratify patients into risk groups to inform treatment decisions. These markers are independent of one another and do not directly measure either proliferation or hormone receptor status. The test calculates a relative risk of recurrence through the use of a weighted algorithm, which is interpreted in the context of published clinical studies of appropriate patient populations. Patients are classified into three risk categories: prognostic index ≤ 0, defined as the low-risk group; prognostic index > 0 and ≤ 0.7, defined as the moderate-risk group; and prognostic index > 0.7, defined as the high-risk group.
-
NPI plus (NPI+) (University of Nottingham) is a biomarker-based prognostic assay that integrates 10 predictive biomarkers [ER, PR, HER2, cytokeratin s/b (CK5/6), CK7/8, epidermal growth factor receptor (EGFR), HER3, HER4, p53, mucin 1 (MUC1; cell surface associated)] of long-term survival and therapeutic response with existing clinical and molecular pathology knowledge to support individualised clinical decision-making. This test is under development and outputs/presentation are not yet finalised.
Key details of the individual IHC tests are provided in Table 7.
| IHC4 | NPI+ | Mammostrat | |
|---|---|---|---|
| Function | Risk of recurrence | Subtyping and risk of recurrence | Subtyping and risk of recurrence |
| Technology | Combines four IHC tests and clinical parameters to derive prognostic score | Uses 10 biomarkers to derive prognostic score (plus others – to be defined) | Uses five biomarkers to derive risk score |
| Location of testing | Local? (but quality assurance issues need to be addressed) | Not known | Central |
| Type of sample | FFPE | FFPE | FFPE |
| Staining material | Resection/core biopsy | Resection/core biopsy | Resection/core biopsy |
| Population | Postmenopausal, ER+, LN− | All women, age 18–79 years | ER+, LN−, tamoxifen treated |
| Key output of test | Continuous IHC4 score | Not yet finalised. To include biological class and projected survival | Risk index and risk group |
| Presentation of results | IHC4 risk score | Not yet finalised. Likely to be similar to Adjuvant! Online | Risk groups: high, moderate and low |
| Commercially available in the UK | Algorithm available. Quality assurance issues to be addressed | No | No |
| Cost | Approx. £100–200 | Approx. £500 | Approx. £1120–1620 |
Current usage of gene expression profiling and expanded immunohistochemistry tests in the NHS
Use of these tests has been limited within the NHS to date. The OncotypeDX test has been available in the UK since 2007. 27 There are two ongoing clinical trials for OncotypeDX with some UK recruitment. Outside of this the use of OncotypeDX in the NHS appears to be relatively limited, with a small amount of self-funding by NHS patients, occasional primary care trust funding and charitable funding. Private health insurers offer reimbursement on a case-by-case basis. Use of the other GEP and expanded IHC tests appears to be negligible.
Cost of the tests
The cost of each test is included in Tables 6 and 7.
Fresh tissue collection is not routine in the NHS and so there will be additional costs associated with tests requiring fresh tissue samples. These costs could be considerable at hospitals where the dissection facilities are already filled to capacity (which is likely to be a significant proportion of hospitals) and where explicit staffing for collection of fresh tissue is not already in place. This is discussed further in Chapter 3, Model inputs: general.
Description of the decision problem
Background
Since 2002 NICE has recommended that women at intermediate or high risk of recurrence who have not had neoadjuvant chemotherapy should normally be offered a multiagent chemotherapy that includes anthracyclines. 28 Chemotherapy is defined as the use of cytotoxic medications with the intention of preventing cancer recurrence in patients. It should be noted that, for the purposes of this assessment, chemotherapy does not include other forms of systemic therapy such as endocrine treatments or targeted biological therapy (trastuzumab).
Meta-analyses of randomised controlled trials (RCTs) by the EBCTCG have indicated that the use of adjuvant chemotherapy (chemotherapy following surgery) is associated with a reduction in the risk of relapse and death in women with early-stage breast cancer. 29 Although chemotherapy can reduce the likelihood of cancer recurrence and death for women with breast cancer, it has considerable adverse effects. Short-term and long-term adverse events will affect a proportion of patients receiving chemotherapy, imposing costs and reducing quality of life. Short-term adverse events that occur during chemotherapy are usually temporary and reversible. The most common side effects include nausea, vomiting, mouth soreness, diarrhoea, tiredness, hair loss and temporary lowering of the blood counts. Long-term side effects such as damage to the heart and a small increase in the risk of leukaemia are not reversible. Although chemotherapy may prevent relapse in some, not all women with early-stage breast cancer will benefit and many women remain recurrence free at 10 years without chemotherapy. However, a subset of patients with a ‘good’ prognosis may still develop recurrence after curative surgery and adjuvant therapy. This presents a great challenge to clinicians in estimating prognosis and making the most appropriate therapeutic decisions relating to whether or not to use adjuvant chemotherapy in women with early-stage breast cancer.
Recommendations about which patients should receive chemotherapy are typically based on estimations of recurrence risk and expected benefit of therapy. Historically, clinicopathological factors, such as patient age, tumour size, nodal involvement, histological grade, ER expression, HER2 overexpression and comorbidities, have been assessed and considered alongside patient preference. In the UK, guidelines based on NPI and Adjuvant! Online have been developed to assist decision-making relating to adjuvant chemotherapy. These guidelines assist clinicians in deciding the benefits of prescribing chemotherapy for a particular patient. NPI provides information about prognosis that is largely based on pathological parameters (e.g. tumour size, grade and lymph node status), with the addition of ER receptor status, age and comorbidity for Adjuvant! Online. However, these clinicopathological tools are imperfect; different guidelines can give different results and it has been suggested that a proportion of women with early-stage breast cancer are over- or undertreated. This may result in unnecessary use of toxic and expensive chemotherapy for women who derive little or no benefit, or avoidable deaths in women for whom chemotherapy was withheld.
Role of new tests
Gene expression profiling and expanded IHC tests aim to improve the targeting of chemotherapy in breast cancer by improving the stratification and identification of patients who will gain most benefit from chemotherapy. The new tests will provide an indication of the risk of recurrence of patients (based on the results of an algorithm to estimate risk of recurrence or indirectly by identifying the cancer subtype). This is based on the knowledge that certain biological features of cancers may indicate an increased likelihood of rapid growth and metastatic potential. The management of these patients, that is, the decision whether or not to prescribe chemotherapy, will be influenced by the test results, and this may result in a change of management of patients compared with current practice (a decision made based on NPI and/or Adjuvant Online). By more accurately guiding the selection of patients to receive adjuvant chemotherapy in early breast cancer management, the use of GEP or expanded IHC tests in patients with early-stage breast cancer may improve health outcomes and quality of life compared with currently used decision-making protocols.
Comparators
The comparator is standard UK practice. This varies between trusts and encompasses the use of Adjuvant! Online and/or guidelines based on NPI to guide decisions on which patients with early breast cancer should be offered adjuvant chemotherapy.
Identification of important subgroups
The NICE scope25 identifies the population under assessment as people diagnosed with early breast cancer. However, many of the GEP and expanded IHC tests have been developed for use in a specific subpopulation or currently have evidence of efficacy only within a specific subpopulation. For tests providing a risk of recurrence output, the majority of evidence relates to populations with ER+, LN− early breast cancer. Some of these tests also have more limited evidence in LN+ populations (for patients with one to three nodes involved) and in patients with ER− disease.
These tests will have an impact on the health of patients only if they lead to changes in patient management. This is most likely to happen in populations in which the decision on whether or not to offer chemotherapy is currently uncertain. One such group is patients with ER−, LN−, HER2− early breast cancer for whom prognostic factors suggest that they are at intermediate risk. The definition of this ‘intermediate group’ is not clear-cut. Clinical advice suggests that patients with a NPI score of ≤ 3.4 are typically considered at low risk either using current prognostic tools (except for a few very young women with aggressive early breast cancer) or based on the new tests and are unlikely to receive chemotherapy; therefore, their management is unlikely to change. Few patients with ER−, LN−, HER2− early breast cancer will have a NPI score > 5.4 and therefore those with a NPI score > 3.4 can be considered as being at intermediate risk.
Current treatment protocols indicate that women with HER2+, ER− early breast cancer or with several positive nodes are likely to receive chemotherapy in most centres in England and Wales. Although the use of GEP or expanded IHC tests might be able to spare chemotherapy in a proportion of these patients, the evidence base for the use of these tests in this population is more limited and clinical opinion therefore considered the assessment of these tests in this population to be a lower priority.
Patients with ER+ LN−, HER2− early breast cancer are therefore considered to be an important population in which to assess these tests, given the current evidence base. Within this population those at intermediate risk for whom the decision about whether or not to offer chemotherapy is not clear cut are considered to be an important subgroup.
Outcomes
The clinical effectiveness review will consider the clinical effectiveness of the tests in relation to:
-
Analytical validity (i.e. the ability of the test to accurately and reliably measure the expression of mRNA or proteins by breast cancer tumour cells).
-
Clinical validity (i.e. the degree to which the test could accurately predict the risk of an outcome such as disease recurrence and discriminate patients with different outcomes). This relates to the prognostic ability of the test.
-
Clinical utility (i.e. the ability of the test to discriminate between those who will have more or less benefit from a therapeutic intervention). This includes evidence relating to how the tests will influence decision-making in terms of which patients will be offered chemotherapy and evidence relating to the predictive ability of the test, that is, the extent to which the test identifies those patients who will benefit most in terms of the relative reduction in the risk of recurrence from treatment.
The outcomes of interest for the economic evaluation are the morbidity and mortality associated with invasive breast cancer and its treatment. Outcomes from the model are expressed in terms of cost per quality-adjusted life-year (QALY).
Aim and objectives of the assessment
The overall aim of the assessment is to assess the clinical effectiveness, effect on patient outcomes and cost-effectiveness of the new GEP and expanded IHC tests.
The objectives of the assessment are:
-
To conduct a systematic review of the published evidence on the clinical effectiveness and cost-effectiveness of the nine GEP and expanded IHC tests. In relation to clinical effectiveness, evidence relating to the following outcomes will be sought:
-
analytical validity – the ability of the test to accurately and reliably measure the expression of mRNA or proteins by breast cancer tumour cells
-
clinical validity – the degree to which the test can accurately predict the risk of an outcome (typically distant recurrence) and discriminate patients with different outcomes; this relates to the prognostic ability of the test
-
clinical utility – the ability of the test to discriminate between those who will have more or less benefit from a therapeutic intervention.
-
-
To develop a decision model to investigate the benefits, harms and cost-effectiveness of the GEP and expanded IHC tests compared with current prognostic tools to guide the use of adjuvant chemotherapy in early breast cancer. Outcomes from the model are expressed in terms of cost per QALY.
Note
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Chapter 2 Assessment of clinical effectiveness
A systematic review of the evidence on the clinical effectiveness of nine GEP and expanded IHC tests to guide the use of adjuvant chemotherapy in breast cancer management was undertaken according to the general principles recommended in the Centre for Reviews and Dissemination (CRD) guidance for undertaking systematic reviews,30 the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement31 and the NICE Diagnostic Assessment Programme Interim Methods Statement. 32 The review protocol can be accessed at www.nice.org.uk/nicemedia/live/13283/54425/54425.pdf and is registered as PROSPERO 2011:CRD4201100136, available from www.crd.york.ac.uk/PROSPERO/full_doc.asp?ID=CRD42011001361.
In addition to the systematic review evidence, a separate section summarising supplementary evidence provided by the manufacturers of the tests will be presented within the section relating to each test. This evidence will simply be summarised and will not be subject to the stages of the systematic review as it is not evidence derived as part of the systematic review process.
Methods for reviewing effectiveness
Background context
The present review evaluates nine prognostic tests for guiding chemotherapy treatment decisions in early-stage breast cancer.
For two of the nine tests (OncotypeDX and MammaPrint) the current review updates an existing systematic review of GEP tests for breast cancer. Two previous systematic reviews33,34 reviewed the literature relating to both OncotypeDX and MammaPrint (one34 is an update of the other33). In the Marchionni et al. 33 review the authors conducted an exhaustive literature review of various electronic databases (covering biomedical literature) between 1990 and 2006. Additional sources included the grey literature (conference proceedings), hand searching the reference lists of included studies and pertinent reviews, contacting the manufacturers of the two tests and regulatory authorities and querying experts in the field. In the Smartt review,34 the authors updated the Marchionni et al. 33 review by updating the search strategy to include all relevant available literature between January 2007 and December 2009. Further details are provided in Overview of existing systematic reviews of the OncotypeDX and MammaPrint tests.
In the present review, new search strategies were developed for all of the nine tests based on scoping searches (and strategies reported in the two existing systematic reviews for the OncotypeDX and MammaPrint tests).
Identification of studies
Electronic databases
Studies were identified by searching the following electronic databases:
-
MEDLINE (via Ovid SP) 1950–May 2011
-
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid SP) 1950–May 2011
-
EMBASE (via Ovid SP) 1980–May 2011
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via Cochrane Library Issue 3, 2011)
-
Cochrane Database of Systematic Reviews (CDSR) (via Cochrane Library Issue 8, 2011)
-
NHS Database of Abstracts of Reviews of Effects (DARE) (via Cochrane Library Issue 3, 2011)
-
Health Technology Assessment (HTA) database (via Cochrane Library Issue 3, 2011)
-
BIOSIS previews (via Ovid SP) 1926–May 2011
-
Web of Science (includes Science Citation Index and Conference Proceedings Citation Index) (via WOK) 1899–May 2011.
Extensive searches were undertaken to identify all literature relating to the clinical effectiveness of GEP and expanded IHC tests to guide the use of chemotherapy in breast cancer management. Sensitive keyword strategies using free text and, where available, thesaurus terms using Boolean operators and database-specific syntax were developed to search the electronic databases. Synonyms related to the condition (i.e. breast cancer) were combined with synonyms related to the test (i.e. MammaPrint, OncotypeDX, Randox BCA, BluePrint, PAM50, BCI, IHC4, NPI+).
Searches were not restricted by publication type or language; however, all searches were limited by date. For the OncotypeDX and MammaPrint tests, the searches were restricted to January 2009–May 2011 as the search strategies from the existing systematic reviews appear to be of good quality and are clearly reported and, as a result, all studies up to 2009 would have been identified. For the remaining seven tests, the searches were restricted to January 2002–May 2011. The first evidence for the GEP and expanded IHC tests was reported in 2002 for OncotypeDX and MammaPrint. As these are the most established tests and the furthest along the validation pathway, evidence for subsequent tests will not predate this. An example of the MEDLINE search strategy is provided in Appendix 1.
Other resources
To identify additional published, unpublished and ongoing studies, the reference lists of all relevant studies (including existing systematic reviews) and information received by the manufacturers were hand searched and key experts in the field were contacted.
All identified citations from the electronic searches and other resources were imported into and managed using the Reference Manager bibliographic software version 12.0 (Thomson ResearchSoft, San Francisco, CA, USA).
Inclusion and exclusion criteria
The inclusion of potentially relevant articles was undertaken using a two-stage process. First, one experienced systematic reviewer screened all titles and abstracts and excluded any citations that clearly did not meet the inclusion criteria. Second, the full manuscripts of all potentially eligible articles were assessed for inclusion by the same reviewer. At each step, articles that did not satisfy the inclusion criteria were excluded. Any uncertainties in the selection process were resolved through discussion with a second reviewer. The relevance of each article for the clinical effectiveness review was assessed according to the following criteria.
Population
All people diagnosed with early invasive breast cancer being treated in the adjuvant setting were included. People diagnosed with early invasive breast cancer being treated in the neoadjuvant setting were excluded.
Index test
The following GEP tests or expanded IHC tests (that guide treatment decisions in early breast cancer management) were included:
-
OncotypeDX
-
MammaPrint
-
BluePrint
-
PAM50
-
BCI
-
Randox BCA
-
Mammostrat
-
IHC4
-
NPI+.
Reference standard
There was no existing reference standard for the index tests.
Comparator
For studies of clinical validity and clinical utility, relevant comparators were those used in current UK clinical practice. Specifically, studies with Adjuvant! Online and/or NPI as comparators to predict risk of recurrence and survival for patients with early breast cancer were sought, although studies including other comparators and those without a comparator were eligible for inclusion. Further details of the comparators are included in Description of technologies under assessment (it should be noted that, by definition, no comparator was necessary for studies of analytical validity).
Outcomes
The following outcome measures (where reported) were included:
-
Analytical validity – the ability of the test to accurately and reliably measure the expression of mRNA or proteins by breast cancer tumour cells, that is, repeatability and reproducibility.
-
Clinical validity – the degree to which the test can accurately predict the risk of an outcome (typically distant recurrence) and discriminate patients with different outcomes. This relates to the prognostic ability of the test – does the test have evidence on clinical validity and has this been externally validated (in an independent data set).
-
Clinical utility – the ability of the test to discriminate between those who will have more or less benefit from a therapeutic intervention.
Clinical utility relates to improvements in clinical outcomes such as overall survival (OS), disease-free survival (DFS), chemotherapy toxicity or quality of life. Based on the conclusion of previous reviews it is not anticipated that prospective studies reporting on long-term outcomes such as OS will be available. In the absence of such studies the following outcomes were to be included:
-
Reclassification of risk compared with existing prognostic variables (correlations between test score and score on existing measures such as NPI, Adjuvant! Online), that is, how does the test change the classification of risk for patients.
-
Impact of the test results on clinical decision-making – how do the tests results translate into changes in decision-making, for example changes in the proportion of patients receiving adjuvant chemotherapy.
-
Predictive ability of the test – does the test accurately predict patients who will benefit most from chemotherapy, that is, do patients classified as high risk benefit more in relative terms than patients classified as low risk.
-
Quality of life – directly as a result of knowledge of the test score (e.g. reduction in anxiety) or indirectly through changes in the use of chemotherapy (and consequent changes in quality of life).
Study design
All study designs were included. For the outcome of analytical validity, studies incorporating any pathology method were included. For the outcomes of clinical validity and clinical utility, priority was given to prospective RCT data if available. In the absence of these data prospective and retrospective cohort studies and case–control studies with and without a comparator were eligible for inclusion.
Reviews of primary studies were not included in the review of clinical effectiveness but were retained for discussion and identification of additional studies. The following publication types were excluded from the review: animal models, preclinical and biological studies, editorials, opinions, studies applied only to breast cancer biology, studies published only in languages other than English (unless no other comparable data exist) and non-peer-reviewed reports in which insufficient methodological details are reported to allow critical appraisal of the study quality.
Data abstraction strategy
Data abstraction was performed by one reviewer into a standardised data extraction form and independently checked for accuracy by another reviewer. Discrepancies were resolved by discussion and if agreement could not be reached a third reviewer was consulted. When multiple publications of the same study were identified, data were extracted and reported as a single study. Where appropriate, the authors of the studies (or the manufacturer/sponsor of the test) were contacted to provide further details in cases in which information was missing from the articles.
The following information was extracted for all studies when reported: study details [author, year of publication, country, study design, number of eligible patients, number of included patients, follow-up time, evidence type (analytical validity, clinical validity, clinical utility), funding], patient characteristics (age, lymph node status, ER status, tumour size, grade, HER2 status, mean NPI score, and treatment) and results [outcomes/end points, results (in the format presented in the study), authors' conclusions]. Numerical data extracted from the studies were varied and included the following: numbers and percentages of patients having a change in management as a result of the test, association between test score and risk of outcomes [distant recurrence, time to distant recurrence (TTDR)] [p-values and associated hazard ratios and 95% confidence intervals (CIs)], correlation between test score and comparator score, differences (p-values) between cases and control subjects on test score.
Critical appraisal strategy
There are no validated (or widely agreed) tools for the assessment of prognostic (predictive factor) studies and there is little empirical evidence to support the importance of particular study features affecting the reliability of findings, including the avoidance of bias. Although there are several published quality assessment checklists for assessing prognostic studies in cancer,35,36 they vary considerably, both in their content and complexity. For this review a generic list of important methodological features recommended by Altman37 was deemed to be the most appropriate (useful) to assess the internal validity of the included studies. Further details on the methodological assessment tool are provided in Appendix 2.
The methodological quality of each included study was assessed by one reviewer and checked by another reviewer using the criteria recommended by Altman. 36 Any discrepancies were resolved by discussion, with involvement of a third reviewer when necessary. Blinding of the quality assessor to author, institution or journal was not considered necessary. The quality assessment items recommended by Altman employed six dimensions relating to the risks of bias of prognostic studies and included the following: sample of participants, follow-up of participants, outcome, prognostic variable, analysis and treatment subsequent to inclusion in cohort. Study quality was assessed with each item scored as ‘yes’, ‘no’ or ‘unclear’. When a study was reported in more than one publication, its quality was assessed on the basis of the combined data from all relevant publications. Studies were rated as high quality if they received a positive assessment of at least 17 out of 21 methodological quality items.
As the current review updates two existing systematic reviews of GEP tests for breast cancer (OncotypeDX and MammaPrint tests), the methodological quality of these two systematic reviews was assessed using the criteria recommended by Shea et al. 38 (assessment of multiple systematic reviews – AMSTAR). The quality assessment checklist for assessing systematic reviews included items on a priori design, data extraction, literature searching, quality assessment, data synthesis, publication bias and conflicts of interest. Further details on the methodological assessment tool together with the details of the assessment of each review are provided in Appendix 3.
Methods of data synthesis
Studies that met the entry criteria were eligible for inclusion in meta-analyses if this was appropriate in terms of comparability of the study populations, outcomes and diagnostic thresholds, and if the studies were unlikely to be biased. However, because of the degree of heterogeneity, meta-analysis was not considered appropriate. The presentation of results is therefore limited to a narrative review. The results were grouped in separate sections by test. For each test a summary of the evidence in terms of evidence type, overall quality and key findings was presented in table form at the beginning of the results section. More detailed summaries of the evidence were presented in narrative form in the subsequent sections, arranged by evidence type. Studies relating to analytical validity were detailed first, followed by those relating to clinical validity and then those relating to clinical utility. The studies relating to clinical utility were further divided when possible by those relating to the predictive ability of the test (benefit of chemotherapy), reclassification of risk against existing prognostic variables, changes in treatment recommendations, quality of life and patient anxiety. A summary of the evidence was then presented, again by evidence type.
Results
This section will first provide an overview of the evidence from the two existing systematic reviews of GEP tests (OncotypeDX and MammaPrint) for breast cancer. Second, this section will present the results of the current systematic review of each of the nine tests. Where applicable, supplementary evidence (from the manufacturers and other sources) will also be provided.
Overview of existing systematic reviews of the OncotypeDX and MammaPrint tests
In January 2008, Marchionni et al. 33 published a systematic review of the impact of GEP tests on breast cancer outcomes. The objective of the review was to examine the available evidence relating to the analytical and clinical validity of breast cancer GEP in predicting disease recurrence and the clinical utility of these tests in improving chemotherapy choices and patient outcomes. Three gene signatures and their commercially available tests were reviewed: the OncotypeDX test, the MammaPrint test and the two-gene ratio test (HOXB13:IL17BR) (not the subject of this review). In 2010, Smartt34 updated this systematic review and included all relevant evidence from January 2007 to December 2009.
Although a number of other systematic reviews examining GEP tests have been reported, it was felt that the Marchionni et al. 33 and Smartt34 reviews were the most appropriate reviews to update. Other reviews predated those of the Marchionni et al. 33 and Smartt34 reviews were considered to be of lower quality as they did not describe the search strategy and processes of the systematic review in as much detail or did not report the findings in as much detail.
The methodological quality of both systematic reviews was reasonably high (as assessed using the criteria recommended by Shea et al. ;38 for further details see Appendix 3). Both reviews provided an a priori design, details of a comprehensive literature search and details of conflicts of interest both for the review and for the included studies and combined the findings of the studies in an appropriate way. Marchionni et al. 33 provided details of duplicate study selection and detailed that data extraction had been performed by one reviewer and checked by a second, whereas this information was not provided for the Smartt review. 34 Marchionni et al. 33 stated that they had searched for and included grey literature as appropriate; however, although Smartt33 stated that the same procedure had been followed as for Marchionni et al. ,33 no specific reference was made to searching or including grey literature. A list of included studies was provided by both reviews; however, a list of excluded studies was provided only for the Marchionni et al. review. 33 In both reviews, characteristics tables for included studies were not clearly presented and appeared only in the appendices in the case of the Smartt review. 34 Both reviews presented the methods used for quality assessment, although how this was actually carried out was presented in more detail in Smartt,33 and both reviews used study quality when formulating conclusions. Neither review assessed publication bias.
In total, 21 studies on the OncotypeDX test and 13 on the MammaPrint test were identified and included by Marchionni et al. 33 and Smartt. 34 A summary of the evidence type and overall quality of each study is provided for OncotypeDX and MammaPrint in Tables 8 and 9 respectively.
| Author (year) | Evidence type | Overall qualitya |
|---|---|---|
| bCronin et al. (2004)39 | Analytical validity | Not reported |
| bCronin et al. (2007)40 | Analytical validity | Not reported |
| bHabel et al. (2006)41 | Analytical validity/clinical validity (prognosis) | Not reported |
| bPaik et al. (2004)42 | Analytical validity/clinical validity (prognosis) | Not reported |
| bCobleigh et al. (2005)43 | Analytical validity/clinical validity (prognosis) | Not reported |
| bEsteva et al. (2005)44 | Analytical validity/clinical validity (prognosis) | Not reported |
| Bryant (2005)45 (poster)b | Clinical validity (prognosis) | Not reported |
| Hornberger et al. (2005)46 (poster)b | Clinical validity (prognosis) | Not reported |
| Paik et al. (2004)42 (poster)b | Clinical validity (prognosis) | Not reported |
| bOratz et al. (2007)48 | Analytical validity/clinical utility (indirect evidence only) | Not reported |
| bPaik et al. (2006)49 | Analytical validity/clinical utility (indirect evidence only) | Not reported |
| cGoldstein et al. (2008)50 | Clinical validity | Reasonably sound evidence |
| cWolf et al. (2008)51 | Clinical validity | Low-quality evidence |
| cAsad et al. (2008)52 | Clinical utility (indirect evidence only) | Low-quality evidence |
| cHenry et al. (2009)53 | Clinical utility (indirect evidence only) | Low-quality evidence |
| cLi et al. (2009)54 | Clinical utility (indirect evidence only) | Low-quality evidence |
| cRayhanabad et al. (2008)55 | Clinical utility (indirect evidence only) | Low-quality evidence |
| Erb et al. (2007)56 (abstract)c | Clinical utility (indirect evidence only) | Not reported |
| Gold et al. (2009)57 (abstract)c | Clinical utility (indirect evidence only) | Not reported |
| Lo et al. (2007)58 (abstract)c | Clinical utility (indirect evidence only) | Not reported |
| Shak et al. (2009)59 (abstract)c | Clinical validity | Not reported |
| Author (year) | Evidence type | Overall qualitya |
|---|---|---|
| bAch et al. (2007)60 | Analytical validity | Not reported |
| bBuyse et al, (2006)61 | Analytical validity/clinical validity | Not reported |
| bGlas et al. (2006)62 | Analytical validity/clinical validity | Not reported |
| bVan't Veer et al. (2002)63 | Clinical validity | Not reported |
| bvan de Vijver et al. (2002)64 | Clinical validity | Not reported |
| cMook et al. (2009)65 | Clinical validity | Reasonably sound evidence |
| cWittner et al. (2008)66 | Clinical validity | Low-quality evidence |
| cBueno-de-Mesquita et al. (2007)67 | Clinical utility | Reasonably sound evidence |
| Bender et al. (2009)68 (abstract)c | Clinical utility | Not reported |
| de Snoo et al. (2009)69 (abstract)c | Clinical validity | Not reported |
| Glas et al. (2008)70 (abstract)c | Clinical validity | Not reported |
| Knauer et al. (2009)71 (abstract)c | Clinical validity | Not reported |
| Saghatchian et al. (2009)72 (abstract)c | Clinical validity | Not reported |
Summary of evidence: Marchionni et al.33
OncotypeDX
Marchionni et al. 33 reported that OncotypeDX was furthest along the validation pathway, with strong retrospective evidence that it predicts distant metastasis and chemotherapy benefit to a clinically relevant extent over standard predictors in a well-defined clinical subgroup with clear treatment implications. A more detailed summary of the main results is provided in Appendix 4.
Marchionni et al. 33 reported a number of studies on analytical validity and overall success rate of OncotypeDX. They concluded that evidence existed for some of the operational characteristics of this test but that there was limited evidence for the reproducibility of the test in terms of reproducibility across different samples of the same block and across samples from different blocks. No direct evidence was available about the effect of sample preparation. There was indirect evidence that the overall success rate of extracting analysable mRNA was fairly high. Centralisation was considered to be a current strength of OncotypeDX with regard to reproducibility.
Marchionni et al. 33 reported fairly strong support for the clinical validity of OncotypeDX over and above that of standard clinical predictors in ER+, LN− and tamoxifen-treated patients, with a clear treatment indication for adjuvant chemotherapy. Paik et al. 47 showed that RS was significantly correlated with DFS (p = < 0.001) and OS (p = < 0.001). RS alone was a better predictor of distant recurrence at 10 years than traditional clinicopathological predictors.
Marchionni et al. 33 concluded that the Paik et al. 49 study represented the strongest evidence for the clinical utility of the OncotypeDX test. Using data from ER+, LN− patients in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B20 trial, Paik et al. 49 compared a group of patients treated with tamoxifen and chemotherapy with a group treated with tamoxifen only. RS was found to be correlated with chemotherapy benefit, defined in terms of 10-year distant recurrence-free survival (DRFS), with a significant benefit from the use of chemotherapy in the high RS group (p = 0.001). However, in a multivariate analysis the benefit from chemotherapy was unclear because of large CIs in the low- and intermediate-risk groups. Marchionni et al. 33 noted that, although prospective confirmation of these findings was required, this evidence provided reasonable justification in the interim for the use of the test by ER+, LN− women.
MammaPrint
The evidence reported by Marchionni et al. 33 for MammaPrint was more limited. A more detailed summary of the main results is provided in Appendix 5.
Two technical studies60,62 provided evidence relating to the analytical validity of MammaPrint. Repeated gene expression measurements over time, within and across individual microarrays and across different laboratories, protocols, instruments and operators provided data on the variability and reproducibility of the test. Buyse et al. 61 reported an overall success rate of the assay of 80.9%. Marchionni et al. 33 concluded that, although these studies suggested that MammaPrint could be used in a clinical setting, they could not be considered to be direct validations of the assay. The review also noted that evidence underpinning the analytical validity of the test was obtained from a limited number of patients and a moderate number of replications. The only validation study using the MammaPrint assay (rather than the underlying 70-gene signature) showed that only about 80% of fresh-frozen specimens were analysable.
Marchionni et al. 33 concluded that, overall, the available published evidence supported MammaPrint as a better predictor of the 5-year risk of distant recurrence than traditional clinical predictors. 61 Buyse et al. 61 compared MammaPrint with Adjuvant! Online for prediction of distant metastases within 5 years and for death within 10 years. Similar sensitivities were found for both methods but a higher specificity was demonstrated for MammaPrint. However, the cohorts used were clinically heterogeneous, meaning that generalisations of the findings to a particular patient group are more difficult.
No evidence on the clinical utility of the test was reported.
Summary of evidence: Smartt34
The updated systematic review by Smartt34 found that the additional studies (published between January 2007 and December 2009) on OncotypeDX and MammaPrint addressed some but not all of the outstanding issues relating to the clinical validity and clinical utility of these tests. A summary of the main results is provided in Appendices 4 and 5.
OncotypeDX
No further evidence was reported.
Smartt34 identified two further studies50,51 on the clinical validity of OncotypeDX. Goldstein et al. 50 reported that OncotypeDX was a more accurate predictor of relapse than standard clinical features for hormone receptor-positive, chemotherapy/hormonal therapy-treated patients and provides complementary information to standard clinicopathological measures. Wolf et al. 51 assessed the correlation between standard clinical and pathological breast cancer characteristics and the RS in a cohort of Israeli breast cancer patients and compared the stratification of patients using the RS with that using commonly used clinical guidelines. Neither standard clinicopathological features nor the chosen clinical guidelines/assessment tools could reliably predict the RS among referred breast cancer patients. The clinical utility of these comparisons was not made clear.
Smartt34 identified four studies52,55,73,74 on the clinical utility of OncotypeDX. Smartt reported that the studies examined the ability of the test to predict response to treatment or its impact on clinical decision-making. The studies all reported a positive impact of the test on clinical decision-making and generally claimed that there was a reduction in the number of patients who were or would have been considered for chemotherapy. However, the studies generally had methodological weaknesses and were likely to have overestimated the effect/influence of the test and they were not designed to assess the effect of the test on clinical outcomes.
MammaPrint
No further evidence was reported.
Smartt34 identified two studies66,75 on the clinical validity of MammaPrint. Mook et al. 75 reported that MammaPrint predicted disease outcome better than traditional clinical prognostic factors in patients with one to three positive nodes and was able to accurately identify node-positive patients with an excellent prognosis. The potential clinical utility of MammaPrint was demonstrated in 72 (34%) clinically high-risk patients with a good prognosis signature who had a 10-year breast cancer disease-specific survival of 94% and therefore might be spared chemotherapy. Wittner et al. 66 reported a study on LN− patients. MammaPrint had a high negative predictive value (NPV) and provided some information that was additional to that provided by Adjuvant! Online. However, with an extremely low positive predictive value (PPV) and non-significant differences in OS between MammaPrint high- and low-risk patients, the prognostic utility of MammaPrint in this population remained unproven. Moreover, although MammaPrint classified a significant proportion of study patients as high risk, few of these developed metastatic disease.
Smartt34 identified one study on the clinical utility of MammaPrint. Bueno-de-Mesquita et al. 67 reported a prospective study of 427 patients with a MammaPrint profile. The study demonstrated a lack of congruence with well-known clinical guidelines for risk assessment in breast cancer; in approximately one-third of patients there was discordance. The addition of MammaPrint to the standard Dutch clinical assessment of risk (modified by patient preference) increased by 20 the number of patients receiving adjuvant systemic therapy. Follow-up was not long enough to provide evidence of its effect on clinical end points such as distant metastasis-free survival (DMFS) or its utility in predicting treatment benefit.
Key evidence gaps identified by these reviews
OncotypeDX
-
Analytical validity – there is limited evidence for the reproducibility of the tests in terms of reproducibility across different samples of the same block and across samples from different blocks. Centralisation was considered to be a current strength of OncotypeDX with regard to reproducibility.
-
Clinical validity (prognostic ability of the tests) – there is fairly strong support for OncotypeDX over and above standard clinical predictors, but only in a well-defined population (ER+, LN−). Evidence is required to assess the stability of risk categories in other populations.
-
Clinical utility – very few of the studies, particularly in isolation, provided compelling evidence of the test's clinical utility.
MammaPrint
-
Analytical validity – there were limited data on variability and reproducibility, with a limited number of patients and a moderate number of replications.
-
Clinical validity (prognostic ability of the tests) – evidence was based on retrospective data using clinically heterogeneous cohorts; evidence from RCTs is needed.
-
Clinical utility – very limited evidence was available on clinical utility; robust evidence on the prediction of chemotherapy benefit is required.
Marchionni et al. 32 concluded (at the time of publication) that for both tests the relationship of predicted to observed risk in different populations needed further study, as did their incremental contribution, optimal implementation and relevance to patients on current therapies. Smartt34 concluded that the largest volume of evidence related to the OncotypeDX test.
Studies included in the current systematic review
The literature searches identified 5993 potentially relevant citations. Of the titles and abstracts screened, 218 relevant full papers or abstracts were retrieved and assessed for inclusion. A flow chart describing the process of identifying relevant literature is shown in Figure 2. A total of 32 citations evaluating the effectiveness of nine prognostic tests (for guiding chemotherapy treatment decisions in early-stage breast cancer) met the inclusion criteria. Figure 2 also shows the numbers of studies included for each prognostic test. Studies excluded from the review are listed in Appendix 6 (only those citations that were excluded after a full-text reading for reasons not immediately apparent from the full text).
OncotypeDX
OncotypeDX quantifies gene expression for 21 genes in breast cancer tissue using RT-PCR. It is intended to predict the likelihood of recurrence in women of all ages with newly diagnosed stage I or II, ER+, LN− or LN+ (up to three nodes) breast cancer treated with tamoxifen. The test assigns the breast cancer a RS and a risk category: low (RS ≤ 18), intermediate (18 ≤ RS ≤ 30) or high (RS ≥ 31). The test also reports ER, PR and HER2 status and can provide an indication of how responsive the cancer is likely to be to hormonal therapy. Further details are provided in Table 6.
Description of included studies
The present review identified an additional 12 studies (13 citations) for the OncotypeDX test. This included 11 fully published peer-reviewed papers and two meeting abstracts. Of these citations five were related to clinical validity and the remaining eight to clinical utility.
The design and patient characteristics of the 12 included studies are provided in Tables 10 and 11 respectively. Most of the studies used a retrospective analysis of archived tumour samples together with a database of patient characteristics and prognostic information. Only three studies stated that the design was prospective. 76–78 The majority of participants analysed in the studies were ER+, LN−, and the mean age was around 50–60 years. Most studies included a small number of participants (range 25–367), although three analysed relatively large cohorts [Dowsett et al. 79 (n = 1231), Mamounas et al. 80 and Tang et al. 81 (both n = 1674 – analyses of the B14 and B20 trials)]. Follow-up was short or not reported for a number of studies; again, the exceptions were the studies by Dowsett et al. 79 (9 years) and Mamounas et al. 80 and Tang et al. 81 (minimum of 10 years).
| Author (year) Country | Study design | Number of patients | Follow-up | Outcomes/end points | Evidence type | Funding |
|---|---|---|---|---|---|---|
| Ademuyiwa et al. (2011)82 USA |
Observational, retrospective, consecutive series (2005–9) | Eligible sample: 276 Sample included: 276 |
NR | Impact on clinical decision-making in terms of recommending chemotherapy | Clinical utility – reclassification against existing prognostic variables – and changes in treatment recommendations | NR |
| Albain et al. (2010)83 USA, Canada |
Retrospective cohort (1989–95) from a Southwest Oncology Group intergroup trial (SWOG- 8814, INT 0100) | Eligible sample: 413 Sample included: 367 Samples excluded because of exhaustion of invasive tumour in block, no submission of primary tumour or technical issues |
Maximum 13 (median 8.94) years | The degree to which the test could accurately predict the risk of an outcome and discriminate patients with different outcomes | Clinical utility – predictive ability (benefit of chemotherapy) | National Cancer Institute and Genomic Health |
| Cuzick et al.84 (2011) Multinational including UK |
Retrospective cohort from the TransATAC trial (1990–8) FFPE |
Eligible sample: 1125 Sample included: 1125 |
Follow-up: 100-month median follow-up | Distant recurrence (within 10 years), TTDR | Clinical validity | Royal Marsden National Institute for Health Research Biomedical Research Centre, Cancer Research UK, Breakthrough Breast Cancer and AstraZeneca |
| Dowsett et al. (2010)79 Multinational including UK |
Retrospective cohort (dates NR) from TransATAC trial | Eligible sample: 1372 Sample included: 1231 Samples excluded because of unsuccessful RT-PCR analysis (n = 64) and clinical characteristics (n = 77) |
9 years | Degree to which the test could accurately predict the risk of an outcome and discriminate patients with different outcomes | Clinical validity | Breakthrough Breast Cancer and AstraZeneca |
| Geffen et al. (2009)77 Israel |
Prospective cohort, consecutive patients (2002–6) Subset who received test: NR |
Eligible sample: 328 Sample included: 25 Samples excluded as the test was not available for the majority of patients |
Subset: NR Whole cohort: NR |
Impact on clinical decision-making | Clinical utility – changes in treatment recommendations | Unfunded |
| Holt et al. (2011)78 (abstract only) UK |
Prospective, cohort (dates NR) | Eligible sample: 107 Sample included: 106 One patient excluded because of inadequate sample |
NR | Impact on clinical decision-making | Clinical utility – changes in treatment recommendations | NR |
| Kelly et al. (2010)85 USA |
Observational, consecutive patients (2004–8), retrospective analysis of prospective database | Eligible sample: 309 Sample included: 309 |
NR (states that it was short) | Correlation with Adjuvant! Online and risk prediction | Clinical utility – reclassification against existing prognostic variables | NR |
| Lo et al. (2010)76 USA |
Observational, consecutive (2005–6), prospective | Eligible sample: 93 Sample included: 89 Four samples excluded as these patients did not complete both pre- and post-RS assay questionnaires |
Up to 12 months | Impact of the 21-gene RS assay on clinical decision-making and patient preferences. End points include (1) changes in physician treatment recommendations, (2) physician self-assessed changes in long-term adjuvant treatment, (3) patient anxiety, (4) quality of life, (5) relapse data | Clinical utility – changes in treatment recommendations – and quality of life and patient anxiety | Genomic Health |
| Tang et al. (2011)81 Mamounas et al. (2010)80 USA |
Retrospective, tissue from patients in two trials [NSABP B14 (1982–8) and B20 (1988–93)] | Eligible sample: 1349 Sample included: 1319 Tamoxifen (TAM) treated: n = 895; TAM + chemotherapy treated: n = 424 30 samples excluded because of unsuccessful RT-PCR: B14: n = 11; B20: n = 19 |
Median for distant recurrence-free interval: B14 (n = 668): 14.3 years; B20 (n = 651): 10.6 years | The degree to which the test could accurately predict the risk of an outcome and discriminate patients with different outcomes | Clinical validity (Mamounas et al.); clinical utility – predictive ability (benefit of chemotherapy) (Tang et al.) | National Cancer Institute |
| Tang et al. (2010)86 abstract only) USA |
Retrospective cohort (dates NR) of patients from the randomised NSABP B20 trial | Eligible sample: NR Sample included: 625 (with RS and ER score ≥ 6.5) |
NR | Distant recurrence Value of the integration of RS and clinicopathological factors in the prediction of chemotherapy benefit in reducing risk of recurrence |
Clinical utility – predictive ability (benefit of chemotherapy) | NR |
| Toi et al. (2010)87 Japan |
Retrospective cohort (1992–8) | Eligible sample: 325 Sample included: 200 Samples excluded because of LN+ disease (exclusion criteria) (n = 80); limited or no clinical data (n = 12); ineligible by pathology evaluation (n = 27); insufficient RNA (n = 1); failed RT-PCR (n = 5) |
NR | The degree to which the test could accurately predict the risk of an outcome and discriminate patients with different outcomes | Clinical validity | Japanese Ministry of Health, Labor, and Welfare |
| Yorozuya et al. (2010)88 Japan |
Case–control, retrospective (2000–8) | Eligible sample: 40 Sample included: 40 (10 cases, 30 control subjects) |
Cases: 53.4 months; control subjects: 55 months | The degree to which the test could accurately predict the risk of an outcome and discriminate patients with different outcomes | Clinical validity | NR |
| Author (year) | Age (years), mean (SD) | LN status | ER status | Tumour size | Grade | HER2 status | Mean NPI score | Treatment |
|---|---|---|---|---|---|---|---|---|
| Ademuyiwa et al. (2011)82 | 54.8 (range 29–82) | All LN− | All ER+ | ≤ 1 cm: 63 (22.8%); 1.1–2 cm: 159 (57.6%); > 2 cm: 54 (19.6%) Mean 1.6 cm (range 0.3–4.5 cm); median 1.4 cm |
I: 104 (37.7%); II: 139 (50.4%); III: 33 (12.0%) | All HER2− | Excellent or good: 220 (79.7%); moderate: 56 (20.3%) | Chemotherapy: 88 (31.9%); no chemotherapy: 188 (68.1%) |
| Albain et al. (2010)83 | Overall: 60.4 (7.5) (range 42–81) 30–54: 90 (24.5%); 55–64: 169 (46.0%); ≥ 65: 108 (29.4%) |
All LN+ | All ER+ | < 2 cm: 120 (32.7%); 2–5 cm: 230 (62.7%); > 5 cm: 17 (4.6%) | I: 131 (35.7%); II: 194 (52.9%); III: 42 (11.4%) | HER2+: 43 (11.7%) | NR | Tamoxifen alone: 148; chemotherapy, then tamoxifen: 219 |
| Cuzick et al.84 (2011)a | G1: NR Median 64 (IQR 57–70) |
−/+/unknown 793 (70%)/299 (26%)/44 (4%) Those with unknown nodal status taken to be node negative in analyses |
NR (reported to be ER and/or PR positive) | ≤ 1 cm: 177 (16%); 1–2 cm: 574 (51%); > 2–3 cm: 272 (24%) | Poor: 206 (18%); moderate: 690 (61%); well differentiated: 229 (21%); unknown: 49 (4%) | HER2+: 116 (10%) | NR | Tamoxifen: 565 (50%); anastrozole: 560 (50%) |
| Dowsett et al. (2010)79 | 64.3 (NR) | Negative: 71%; positive: 25%; unknown: 4.3% (Note 0.7% unaccounted for) |
100% hormone receptor positive; does not state if progesterone or oestrogen | ≤ 2 cm: 67%; 2–5 cm: 31%; > 5 cm: 1.5%; unknown: 0.3% | Well: 27%; moderate: 52%; poor: 16%; unknown 4.6% | NR | NR | Radiotherapy: 68%; received HRT: 36%; tamoxifen before surgery: 3.9% Tamoxifen: 609/1231; anastrozole: 622/1231 |
| Geffen et al (2009)77 | Subset: NR Whole cohort: < 35: 5 (1.5%); 35–55: 107 (32.6%); 56–75: 190 (58.0%); > 75: 26 (7.9%) |
All LN− | Subset: NR Whole cohort: 288/328 (87.8%) |
NR | Subset: NR Whole cohort: low: 70 (21.3%); intermediate: 144 (43.9%); high: 63 (19.2%); undetermined: 51 (15.5%) |
Subset: NR Whole cohort: HER2 overexpression: 21 (6.4%) |
Subset: NR Whole cohort: NR |
Subset: NR Whole cohort: lumpectomy: 297; mastectomy: 31; local therapy: 328; endocrine therapy: 283 (57 had chemotherapy as well); no systemic therapy: 20; chemotherapy: 25 |
| Holt et al. (2011)78 (Abstract only) |
NR | Pathological negative or pathological stage N1 % NR |
All ER+ | NR | NR but states ‘early stage’ | NR | NR | Patient choice after OncotypeDX: no chemotherapy: 74/106 (69.8%); chemotherapy: 32/106 (30.2%) |
| Kelly et al. (2010)85 | Mean NR Median (IQR) at diagnosis: 54 (47–62) |
0 nodes: 292 (95%); one to three nodes: 16 (5%); four nodes: 1 (0.3%) | NR but states that all are hormone receptor positive (unclear if this refers to progesterone or oestrogen) | All: grade I and grade III NR Grade II (n = 191) median tumour size: 1.3 (IQR 1.0–1.8) cm |
I: 45 (15%); II: 191(62%); III: 72 (23%)a | HER2: negative: 307 (99%); positive: 2 (0.7%) Median Ki-67: 10 (IQR 5–20) |
NR | Adjuvant or neoadjuvant therapy: 84 (27%) |
| Lo et al. (2010)76 | 55 (NR) (range 35–77) | All LN− | All ER+ | Mean 1.7 cm (SD NR) (range 0.6–3.5) | Low: 19/89 (21.3%); intermediate: 58/89 (65.2%); high: 12/89 (13.5%) | HER2: negative: 6 (7%) | NPI NR RS: < 18: 38 (42.7%); 18–30: 42 (47.2%); ≥ 31: 9 (10.1%) |
Treatment option chosen by patients after test: chemotherapy and hormone therapy: 20; hormone therapy: 65; observation: 3; equipoise: 1 |
| Tang et al. (2011)81 Mamounas et al. (2010)80 |
Trial B14: < 50: 194 (29%); 50 to ≤ 60: 173 (26%); ≥ 60: 301 (45%) Trial B20: < 50: 289 (44%); 50 to ≤ 60: 166 (26%); ≥ 60: 196 (30%) |
All LN− | All ER+ | Trial B14: 0–1.0 cm: 112 (17%); 1.1–2.0 cm: 303 (45%); 2.1–4.0 cm: 218 (33%); ≥ 4.1 cm: 35 (5%) Trial B20: 0–1.0 cm: 83 (13%); 1.1–2.0 cm: 313 (48%); 2.1–4.0 cm: 226 (35%); ≥ 4.1 cm: 29 (4%) |
NR | NR | NR | Trial B14: lumpectomy plus irradiation: 393 (38%); mastectomy: 630 (62%) Trial B20: lumpectomy plus irradiation: 277 (43%); mastectomy: 374 (57%) |
| Tang et al. (2010)86 (abstract only) |
NR | All N− | All ER+ | NR | NR | NR | NR | Tamoxifen with or without adjuvant chemotherapy |
| Toi et al. (2010)87 | < 50: 68 (34%); ≥ 50: 132 (66%) | All LN− | All ER+ | ≤ 2 cm: 92 (46%); > 2 cm: 108 (54%) | I: 30 (15%); II: 80 (40%); III: 36 (18%); unknown: 54 (27%) | NR | NR | Mastectomy: 143 (72%); breast conservation: 57 (29%); tamoxifen: 200/200 (100%) |
| Yorozuya et al. (2010)88 | Cases: 49.1 (12.9) (range 37–76) Control subjects: 50.9 (11.9) (range 37–78) |
All LN− | All ER+ | Cases: 18.9 (SD 4.6) (95% CI 15.7 to 22.2) mm Control subjects: 15.8 (SD 6.3) (95% CI 13.4 to 18.2) mm |
Cases: I: 1 (10%); II: 1 (10%); III: 8 (80%); unknown 0 (0%) Control subjects: I: 15 (50%); II: 9 (30%); III: 5 (17%); unknown: 1 (3%) |
Cases: negative: 7 (70%); positive: 2 (20%); unknown: 1 (10%) Control subjects: negative: 27 (90%); positive: 0 (0%); unknown: 3 (10%) |
NR | Cases: mastectomy: 8 (80%); partial mastectomy: 2 (20%); adjuvant hormone therapy: 9 (90%); adjuvant chemotherapy: 1 (10%) Control subjects: mastectomy: 11 (37%); partial mastectomy: 19 (63%); adjuvant hormone therapy: 26 (87%); adjuvant chemotherapy: 4 (13%) |
Quality of included studies: OncotypeDX
The methodological quality of the 12 included studies76–88 is summarised in Figure 3 (further details are provided in Appendix 7). Generally, only three studies (four citations) performed well, receiving a positive assessment for at least 17 out of 21 methodological quality items. 80–82,84
FIGURE 3.
OncotypeDX test: methodological quality graph (review authors' judgements about each methodological quality item presented as percentages across all studies).
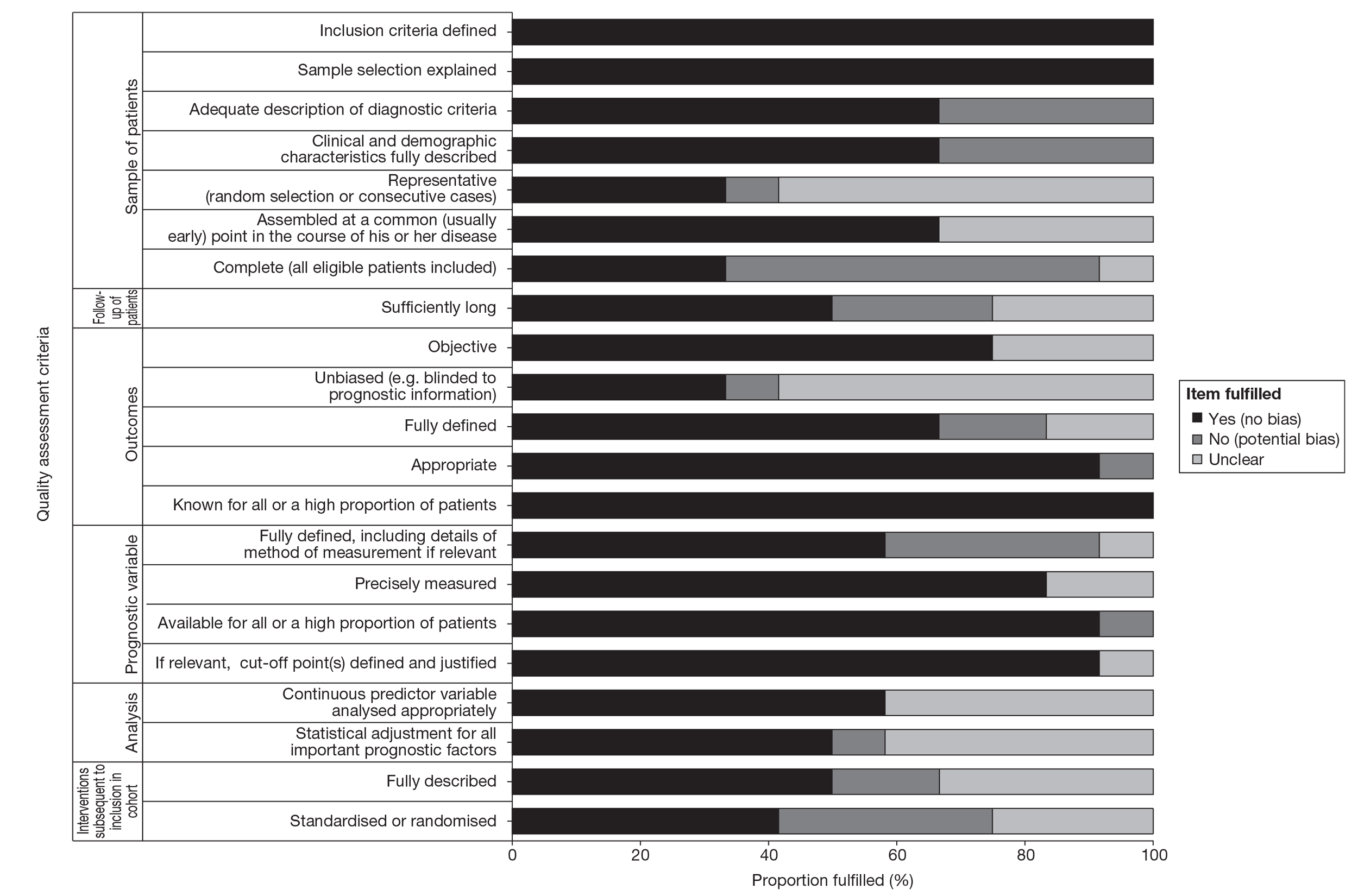
Although 9 of the 12 studies used a retrospective study design,79–88 other potential sources of bias were generally related to the following domains: sample of patients (inadequate description of diagnostic criteria, clinical/demographic characteristics not fully described and not including all eligible patients with tumour samples in the study), follow-up of patients, prognostic variables (not fully defined) and interventions subsequent to inclusion in the study (interventions were not described or standardised, thus precluding an unbiased assessment of the prognostic ability of the test). 30
The assessment of study quality was further hampered by poor reporting of the following methodological items: whether or not sample of patients was representative and assembled at an early point in the course of their disease, whether or not outcomes were fully defined, objective and unbiased and whether or not appropriate statistical analyses were undertaken (continuous predictor variables analysed appropriately and statistical adjustment made for all prognostic factors). Overall, the risk of bias from the 12 included studies was judged to be moderate.
Results: OncotypeDX
In this section a summary of the clinical evidence for OncotypeDX is presented (Table 12) followed by a narrative summary of each study. Full data extraction tables are provided in Appendix 7.
| Author (year) | Evidence type | Overall quality | Key findings |
|---|---|---|---|
| Ademuyiwa et al. (2011)82 | Clinical utility – reclassification against existing prognostic variables – and changes in treatment recommendations | High | 276 ER+, LN− patients from two cancer centres in the USA. Impact on clinical decision-making in terms of recommending CT based on clinicopathological characteristics. 37 fewer patients received CT using RS to help decide CT use. 38% of patients had a change in management as a result of the RS. Authors reported a significant association between RS risk group and NPI (p< 0.001). Conclusion: RS score had a significant impact on the receipt of adjuvant CT. Limitations: sample size relatively small, use of retrospective chart review |
| Albain et al. (2010)83 | Clinical utility – predictive ability (benefit of chemotherapy) | Medium | 367 postmenopausal ER+ and LN+ US and Canadian patients from the SWOG-9914 trial. RS is prognostic for tamoxifen-treated patients with positive nodes and predicts significant benefit of CAF in tumours with a high RS. Conclusion: a low score identifies women who might not benefit from anthracycline-based chemotherapy, despite positive nodes. Limitations: moderate sample size, time over which tumour samples were collected not reported, therefore they may be differences in diagnostic criteria being applied |
| Cuzick et al.84 (2011) | Clinical validity | High | 1125 patients, majority LN− and hormone receptor positive; multinational including UK. The authors reported the mean change in (likelihood ratio chi-squared) for the addition of the RS to the classical score (higher values indicate more added prognostic information) for TTDR and TR (all recurrences). For TTDR the (likelihood ratio chi-squared) for all patients was 25.3 (95% CI 25.2 to 25.9) and for LN− patients was 20.9 (95% CI 20.7 to 21.6). For TR the LR-x2 for all patients was 25.6 (95% CI 25.2 to 25.9) and for LN− patients was 25.7 (95% CI 25.4 to 26.4). The authors report that the OncotypeDX RS adds prognostic information to traditional clinicopathological measures. This study has been rated as high quality and benefits from a large sample of patients |
| Dowsett et al. (2010)79 | Clinical validity | Medium | 1231 UK, postmenopausal, hormone receptor-positive, LN− patients. Increase in RS significantly associated with an increased risk of distant recurrence. RS was also significantly associated with TTDR. Correlation between RS-predicted distant recurrence and Adjuvant! Online-predicted recurrence was low but statistically significant. Conclusion: RS is an independent predictor of distant recurrence in LN− and LN+ hormone receptor-positive patients treated with anastrozole, adding value to estimates using standard clinicopathological features. Large sample size, UK-based patients |
| Geffen et al. (2009)77 | Clinical utility – changes in treatment recommendations | Medium | 25 LN− Israeli patients. Each patient had a RS assay. Study reported findings on the impact of the OncotypeDX RS on clinical decision-making. Nine patients (36%) had their treatment recommendations changed based on the score, six from CT to no CT. Limitations: very small sample size |
| Holt et al. (2011)78 (Abstract only) | Clinical utility – changes in treatment recommendations | Medium | 106 UK, ER+ and either LN− or N1 patients. 35 patients (33%) had their initial recommendation changed as a result of the RS; for 71 patients (67%) there was no change. RS added prognostic information beyond that from NPI alone. Conclusion: authors concluded that early results suggest that OncotypeDX is applicable and feasible to perform in the UK setting with a reduction in the use of adjuvant CT. Limitations: although UK based only conducted in one centre, small sample size, abstract data |
| Kelly et al. (2010)85 | Clinical utility – reclassification against existing prognostic variables | Medium | 309 hormone receptor-positive, LN− patients at clinically intermediate risk. Of these, 52% were assigned a low risk on RS, 9% high risk and 39% intermediate risk. Conclusion: findings suggest that OncotypeDX has utility in reclassifying clinically intermediate patients into the three OncotypeDX risk groups. Employed recently diagnosed patients. Limitations: small sample size and a short follow-up time |
| Lo et al. (2010)76 | Clinical utility – changes in treatment recommendations – and quality of life and patient anxiety | Medium | 89 ER+, LN− patients. Prospective US-based study of RS effects on physician and patient adjuvant treatment selection and satisfaction, and quality of life. Changes in physician treatment recommendations for 28 patients (31.5%); 24 patients (27.0%) changed their own treatment decision. Most of the treatment changes were from CHT to HT alone for both physicians and patients. DCS score and state anxiety were significantly reduced across time points (pre and post RS), and the FACT-G score was marginally significantly reduced. Trait anxiety and the FACT-B score were not significantly different. Limitations: small sample size, only 16 physician self-reports |
| Tang et al. (2011)81 Mamounas et al. (2010)80 |
Clinical validity (Mamounas et al.); clinical utility – predictive ability (benefit of chemotherapy) (Tang et al.) | High | 1319 ER+, LN− patients from two large US trials (NSABP B14 and B20). Tang et al. – both RS and Adjuvant! Online provided strong independent prognostic information in tamoxifen-treated patients. In the B20 cohort RS was significantly predictive of CT benefit (for DRFI, OS and DFS) but Adjuvant! Online was not. In the larger B20 subcohort, Adjuvant! Online was significantly predictive of CT benefit for OS but not for DRFI or DFS. Conclusion: prognostic estimates can be optimised by combining RS and Adjuvant! Online. RS should be used for estimating relative CT benefit. Mamounas et al. – in the tamoxifen-treated patients, RS was a significant predictor of locoregional recurrence. Large sample size with a long follow-up. Limitation: relatively old tumour samples, may be differences in diagnostic criteria applied |
| Tang et al. (2010)86 (Abstract only) |
Clinical utility – predictive ability (benefit of chemotherapy) | Medium | 625 ER+, LN−, US patients from the NSABP B20 trial. Examined the value of the SPC (integration of RS and clinicopathological factors) in the prediction of CT benefit in reducing risk of recurrence. Authors concluded that RS used alone remains the best predictor of CT benefit in ER+, LN− breast cancer. Large sample size. Limitations: abstract data, Tang et al.80 and Mamounas et al.79 also used the NSABP cohorts – limitations in using the same data because of risks of double counting in the evidence base as a whole |
| Toi et al. (2010)87 | Clinical validity | Medium | 200 ER+, LN−, Japanese patients. Patients categorised as low risk had a significantly lower risk of distant recurrence than patients in the high-risk category. Continuous RS was significantly associated with the risk of distant recurrence. Conclusion: OncotypeDX has value in providing prognostic information in Asian populations with ER+, LN− breast cancer. Limitation: small sample, Japanese patients so generalisability to UK practice may be limited |
| Yorozuya et al. (2010)88 | Clinical validity | Medium | 40 ER+, LN−, Japanese patients. Compared those who had metastases after surgery with those who did not develop metastases. Significant differences were shown between the groups in terms of mean RS and there were significant differences in the proportions assigned to the different OncotypeDX risk categories. Conclusion: both histological grade and risk score classification were effective in identifying women at risk of developing distant metastases after initial therapy. Limitations: very small sample size, may not be generalisable to the UK setting |
Analytical validity
No new data examined analytical validity.
Clinical validity
Using 1231 tissue samples from the UK TransATAC (Arimidex, Tamoxifen, Alone or in Combination trial) trial, Dowsett et al. 79 assessed postmenopausal, hormone receptor-positive and majority LN− patients. They demonstrated that a 50-point increase in RS in all LN− patients (e.g. RS = 55 vs. RS = 5) was significantly associated with an increased risk of distant recurrence [hazard ratio (HR) 3.92, 95% CI 2.08 to 7.39; p < 0.001] when adjusted for the effects of tumour size, local grade (grade derived from case record forms), age and treatment. They also reported that, when local grade was replaced with central grade (assessed using the Elston and Ellis system) in multivariate analysis, adjusted RS was also significantly associated with risk of distant recurrence (HR 5.25, 95% CI 2.84 to 9.73; p < 0.001). RS was also significantly associated with TTDR in both node-negative (HR 5.25, 95% CI 2.84 to 9.73; p < 0.001) and node-positive patients (HR 3.47, 95% CI 1.64 to 7.38; p < 0.002). Correlation between RS-predicted distant recurrence and Adjuvant! Online-predicted recurrence was low but statistically significant by central grade (Spearman rank correlation = 0.23; p < 0.001) or local grade (Spearman rank correlation = 0.22; p < 0.001). Only approximately 5% of the variability in the estimates of recurrence using either of these scores was explained by the other. The authors concluded that the findings demonstrated that RS is an independent predictor of distant recurrence in LN− and LN+ hormone receptor-positive patients treated with anastrozole, adding value to estimates with standard clinicopathological features. As the patients were recruited as part of a large-scale trial this study benefits from a large sample size of UK-based patients and has a relatively long follow-up time (9 years).
Yorozuya et al. 88 reported a very small case–control study (10 cases, 30 control subjects) of ER+, LN− Japanese patients. The cases were those who had metastases after surgery; control subjects did not develop metastases. Significant differences were shown between the groups in terms of mean RS (cases: mean RS = 40.0, 95% CI 21.1 to 58.9; control subjects: mean RS = 17.8, 95% CI 13.8 to 21.9; p < 0.001). The study found significant differences between cases and control subjects in the proportions assigned to different risk categories [low: 3 (30%) cases vs. 19 (63%) control subjects; intermediate: 1 (10%) vs. 8 (27%); high: 6 (60%) vs. 3 (10%); p = 0.005]. Multivariate logistical regression analysis of age, ER score, PR score, RS, histological grade and lymphatic invasion compared with distant metastases showed that RS was not significant [RS ≥ 50 vs. RS < 50, p = 0.579, odds ratio (OR) 2.85, 95% CI 0.07 to 115.552] although the authors conclude that the OR indicates that it has value. The authors concluded that both histological grade and RS classification were effective in identifying women at risk of developing distant metastases after initial therapy for ER+, LN− stage I or IIA breast cancer. There are significant limitations in terms of the generalisation of the findings because of the extremely small sample size used in this study; furthermore, as the study was Japan based, generalisations to the UK setting are limited.
Toi et al. 87 examined the prognostic ability of OncotypeDX in 200 ER+, LN− Japanese patients. They showed that patients categorised as low risk had a significantly lower risk of distant recurrence than patients in the high-risk category (p < 0.001, log-rank test). No recurrences were identified in the intermediate recurrence group. Continuous RS was significantly associated with the risk of distant recurrence for a 50-point increase in RS (HR 6.20, 95% CI 2.27 to 17.0). In multivariate analyses the continuous RS maintains statistical significance when adjusting for age and clinical tumour size (HR 6.03, 95% CI 2.17 to 16.7). For risk of recurrence the HR was 3.38 (95% CI 1.32 to 8.69), for risk of recurrence or death the HR was 2.09 (95% CI 0.84 to 5.20) and for risk of death the HR was 2.67 (95% CI 0.93 to 7.62). The authors concluded that OncotypeDX has value in providing prognostic information in Asian populations with ER+, LN− breast cancer. This study had a small sample size and as it was conducted using Japanese patients generalisability to UK practice may be limited; however, the study does benefit from the fact that the tumour samples used were from patients who presented and were treated relatively recently (1992–8).
Cuzick et al. 84 reported data that aimed to assess how much of the information in the RS is contained in standard IHC markers (data from this report relating to the IHC4 test is presented in IHC4 test). Patients comprised a retrospective cohort from the TransATAC trial (multinational including the UK). The 1125 patients were mainly LN− and hormone receptor positive, and there were a total of 195 recurrences of which 145 were distant recurrences. In LN− women there were 101 recurrences of which 67 were distant recurrences. The authors reported the mean change in likelihood ratio chi-squared for the addition of GHI-RS (Genomic Health Recurrence Score) v to the classical score in the validation halves of 100 random splits of the data (higher values indicate more added prognostic information) for TTDR and time to recurrence (all recurrences). For TTDR the likelihood ratio chi-squared for all patients was 25.3 (95% CI 25.2 to 25.9) and for LN− patients was 20.9 (95% CI 20.7 to 21.6). For time to recurrence the LR-x2 for all patients was 25.6 (95% CI 25.2 to 25.9), and for LN− patients was 25.7 (95% CI 25.4 to 26.4). The authors report that the OncotypeDX RS adds prognostic information to traditional clinicopathological measures. This study has been rated as high quality and benefits from a large sample of patients.
Mamounas et al. 80 (and Tang et al. ,81 reported in the following section) undertook a retrospective analysis of ER+, LN− patients who had been recruited into two large US trials (NSABP B14 and B20). They showed a significant association between RS and the proportion of patients with locoregional recurrence at 10 years for 355 placebo-treated patients (NSABP B14), 895 tamoxifen-treated patients (NSABP B14 and B20) and 424 tamoxifen plus chemotherapy-treated patients (NSABP B20). Multivariate Cox regression analysis in the cohort of 895 tamoxifen-treated patients showed that RS was a significant predictor of locoregional recurrence (HR 2.16, 95% CI 1.26 to 3.68; p < 0.005). The authors concluded that a significant association exists between RS and risk for locoregional recurrence. This information has biologic consequences and potential clinical implications relative to locoregional therapy decisions for patients with LN− and ER+ breast cancer. These studies appeared to be of reasonable quality and, as the patients were recruited as part of two large-scale trials, the studies benefit from a large sample size with a long follow-up. However, across the two trials tumour samples were collected as long ago as 1982 until 1993. This means that there may be differences in diagnostic criteria applied and this may limit the generalisability of these findings to current practice.
Clinical utility
Tang et al. 81 (and Mamounas et al. ,80 as reported in the previous section) undertook a retrospective analysis of the NSABP B14 and B20 trial data on ER+, LN− patients. They compared the prognostic and predictive utility of the OncotypeDX RS and Adjuvant! Online, with an end point of distant recurrence-free interval (DRFI). Cox proportional hazards models were used to compare the prognostic and predictive utility of RS and Adjuvant! Online. Both RS (p < 0.001) and Adjuvant! Online (p = 0.002) provided strong independent prognostic information in tamoxifen-treated patients. Combining RS and individual clinicopathological characteristics provided greater prognostic discrimination than combining RS and the composite Adjuvant! Online. In the B20 cohort with RS results (n = 651), RS was significantly predictive of chemotherapy benefit (interaction p = 0.031 for DRFI, p = 0.011 for OS, p = 0.082 for DFS) but Adjuvant! Online was not. However, in the larger B20 subcohort (n = 1952), Adjuvant! Online was significantly predictive of chemotherapy benefit for OS (interaction p = 0.009) but not for DRFI or DFS. The authors concluded that prognostic estimates can be optimised by combining RS and Adjuvant! Online. RS should be used for estimating relative chemotherapy benefit. As stated above, these studies appeared to be of reasonable quality and, as the patients were recruited as part of two large-scale trials, the studies benefit from a large sample size with a long follow-up. However, across the two trials tumour samples were collected as long ago as 1982 until 1993. This means that there may be differences in diagnostic criteria applied and this may limit the generalisability of these findings to current practice.
Albain et al. 83 reported findings for 367 postmenopausal ER+ and LN+ US and Canadian patients from the SWOG-9914 trial. They aimed to investigate whether or not RS is prognostic in women treated with tamoxifen alone and whether or not it identified those who might not benefit from anthracycline-based chemotherapy despite higher risks of recurrence. RS was prognostic in the tamoxifen alone group (HR 2.64, 95% CI 1.33 to 5.27; p = 0.006) using a 50-point difference in RS as a threshold. There was no benefit of chemotherapy with cyclophosphamide, doxorubicin and 5-fluorouracil (CAF) in patients with a low RS but an improvement in DFS for those with a high RS (score ≥ 31) (HR 0.59, 95% CI 0.35 to 1.01; p = 0.033), after adjustment for number of positive nodes. The RS by treatment interaction was significant in the first 5 years (p = 0.029), with no additional prediction beyond 5 years, although cumulative benefit remained at 10 years. There were similar findings for OS and breast cancer-specific survival (BCSS). The authors concluded that RS is prognostic for tamoxifen-treated patients with positive nodes and predicts significant benefit of CAF in tumours with a high RS. A low score identifies women who might not benefit from anthracycline-based chemotherapy, despite positive nodes. This study employed a moderate sample size. The authors did not report the length of time over which tumour samples were collected; therefore, it is unclear whether or not it is likely that there were differences in the diagnostic criteria applied.
Tang et al. 86 reported a study in abstract form that included 625 ER+, LN− US patients treated with tamoxifen with or without adjuvant chemotherapy from the NSABP B20 trial. They aimed to examine the value of the integration of RS and clinicopathological factors (RSPC) in the prediction of chemotherapy benefit in reducing risk of recurrence. They reported that in 60 of the 625 patients distant recurrence occurred. The RS showed a significant interaction with chemotherapy treatment (p = 0.037) with a standardised HR of 0.836. The interaction of RSPC with treatment was not significant (p = 0.10) although there was a trend in the same direction as for RS (HR 0.833). The authors concluded that RS used alone remains the best predictor of chemotherapy benefit in ER+, LN− breast cancer. This study benefits from having a large sample size. However, there are significant limitations in making any interpretations from this evidence as it is derived only from an abstract. It has been shown that there may be discrepancies between data made available in abstracts and the reporting of results in subsequently published full-length articles. 89 Because of incomplete reporting the methodological quality of studies cannot be confidently assessed by systematic reviewers. It should also be noted that Tang et al. 81 and Mamounas et al. 80 also used the NSABP cohorts. There are limitations in using the same data because of the risks of double counting in the evidence base as a whole.
Kelly et al. 85 considered the correlation between OncotypeDX and Adjuvant! Online in a US population of 309 consecutive patients with hormone receptor-positive, majority LN− early breast cancer of clinically intermediate risk. They demonstrated a low correlation between Adjuvant! Online risk prediction and RS, and between death after 5 years of tamoxifen therapy and RS. Of these patients considered to be of clinically intermediate risk, 52% (n = 160) were assigned a low risk on RS, 9% (n = 27) a high risk and 39% (n = 122) an intermediate risk. The authors concluded that OncotypeDX yielded potentially informative risk assignments in patients who may be considered at indeterminate risk by routine clinical variables. However, 40% of the time they remain as intermediate risk using RS thresholds; this increases to 66% when using thresholds that have been revised for an ongoing trial of OncotypeDX [Trial Assigning Individualized Options for Treatment (TAILORx) – which will be described in Ongoing trial: the Trial Assigning Individualized Options for Treatment] (the revised thresholds are as follows: low risk ≤ 10; intermediate risk 11–25; high risk ≥ 26). These findings suggest that OncotypeDX has utility in reclassifying clinically intermediate patients into the three OncotypeDX risk groups. The study benefits from the fact that all patients had been diagnosed relatively recently (2004–8), although it also has limitations, including a small sample size and a short follow-up time (actual follow-up time was not reported). The authors were not able to report recurrence and survival results because of the short follow-up time.
Ademuyiwa et al. 82 reported a study on 276 ER+, LN− patients from two cancer centres in the USA. They reported a significant association between RS risk group and NPI (p < 0.001), although there were a number of discordant cases (comparisons are difficult because NPI and RS have two and three risk categories respectively). This was only a brief report and it therefore lacked the detail necessary to make adequate judgements about quality. Furthermore, the sample size was relatively small. Further data on clinical decision-making from this study are reported in the following section.
Geffen et al. 77 reported findings on the impact of the OncotypeDX RS on clinical decision-making in 25 LN− patients in Israel. Nine patients (36%) had their treatment recommendations changed based on the scores, six of these from chemotherapy to no chemotherapy. The generalisability of these findings is limited, primarily because of the very small sample size. Furthermore, as this study was conducted in Israel, generalisability to the UK is limited.
Lo et al. 76 reported a prospective US-based study of 89 ER+, LN− patients to examine whether or not RS affects physicians' and patients' adjuvant treatment selection and satisfaction. They reported changes in physician treatment recommendations for 28 (31.5%) patients; 24 (27.0%) patients changed their own treatment decision. The largest change after RS results was conversion in 20 (22.5%) cases from physicians' pretest recommendation of chemotherapy plus hormone therapy to a post-test recommendation of hormone therapy. Nine (10.1%) patients changed their treatment decision from chemotherapy plus hormone therapy to hormone therapy. The authors concluded that the RS assay impacts significantly on physician and patient adjuvant treatment decision-making. Most of the treatment changes were from a pretreatment recommendation of chemotherapy plus hormone therapy to hormone therapy alone, for both physicians and patients. In addition, Lo et al. 75 reported, based on physician self-reports, that RS results have an enduring impact on physician confidence in their treatment recommendation. The generalisability of these findings is limited because of the very small sample size of 89 patients and only 16 physician self-reports.
Ademuyiwa et al. 82 investigated the impact on the use of clinicopathological features (based on patient records with oncologists blind to RS) in decision-making for chemotherapy utilisation. The study included 276 ER+, LN− patients from two cancer centres in the USA. In total, 37 fewer patients received chemotherapy using RS to help decide chemotherapy use; 38% of the patients had a change in management as a result of RS. The authors reported a significant association between RS risk group and NPI (p < 0.001), although there were a number of discordant cases (comparisons are made difficult because NPI and RS have two and three risk categories respectively). They concluded that the RS had a significant impact on the receipt of adjuvant chemotherapy. This was only a brief report and therefore lacked the detail necessary to make adequate judgements about quality. Furthermore, the sample size was relatively small and there may also be significant limitations from the use of retrospective chart review.
In a conference poster Holt et al. 78 reported a study investigating the impact of RS on clinical decision-making in Wales. The 106 patients included in the study were ER+ and either LN− or N1. The authors reported data on change in recommendations pre RS assay to post RS assay.
They demonstrated that 35 patients (33.0%) had their initial recommendation changed as a result of RS [change chemotherapy to no chemotherapy: 25 (23.6%); change no chemotherapy to chemotherapy: 10 (9.4%)] whereas for 71 patients (66.9%) there was no change [no change no chemotherapy: 49 (46.2%); no change chemotherapy: 22 (20.8%)]. The Spearman's rank correlation comparing RS with individual components of the NPI showed that, of size, LN status and grade, only grade was significantly correlated. The authors concluded that early results suggest that OncotypeDX is applicable and feasible to perform in the UK setting, with a reduction in the use of adjuvant chemotherapy consistent with the findings of other studies. RS added prognostic information beyond that from NPI alone. Although the study was UK based it was conducted in only one centre with a very small sample size, making generalisations of the findings difficult. Furthermore, because more chemotherapy was given in the comparator arm, more benefits are likely to be derived from the use of OncotypeDX. In addition, there are significant limitations in making any interpretations from this evidence as it is derived only from an abstract. It has been shown that there may be discrepancies between data made available in abstracts and the reporting of results in subsequently published full-length articles. 89 Because of incomplete reporting the methodological quality of studies cannot be confidently assessed by systematic reviewers.
Lo et al. 76 also reported quality of life and patient anxiety data for 89 ER+, LN− patients. Patients were asked to complete standardised measures to assess decisional conflict and personal perceptions of decision-making [Decisional Conflict Scale (DCS)] pre and immediately post RS; anxiety – state anxiety refers to a transitory emotional state or condition and trait anxiety denotes relatively stable individual differences in anxiety proneness [State-Trait Anxiety Inventory (STAI)] pre RS, immediately post RS and 12 months post RS; and quality of life [Functional Assessment of Cancer Therapy (FACT)-B, which is specific to breast cancer, and FACT-G, which is the general scale] pre RS and 12 months post RS. The results showed that DCS score was significantly reduced post RS compared with pre-RS (p < 0.001); the STAI demonstrated that state anxiety was significantly reduced across the three time points (p = 0.007) whereas trait anxiety was not significantly different across the three time points. For quality of life the FACT-B score pre RS was not significantly different from the score at 12 months post RS; however, the FACT-G score was marginally significantly reduced at 12 months compared with pre RS (p = 0.49). The authors concluded that patient anxiety and decisional conflict were significantly lower after RS results. The small sample size used in this study limits the generalisability of the findings and further research in this area is necessary before definitive conclusions on quality of life improvements and reductions in patient anxiety can be formed.
Summary of evidence: OncotypeDX
Analytical validity of OncotypeDX
In the earlier systematic reviews evidence exists on the technical and operational aspects of the test and on assay variability and reproducibility. Studies showed reasonable within-laboratory replicability.
Our findings indicated no new evidence.
Clinical validity (prognostic ability) of OncotypeDX
In earlier systematic reviews the evidence shows that RS was significantly correlated with DFS and OS. RS alone was shown to be a better predictor of distant recurrence at 10 years than traditional clinicopathological predictors. 42 Key gaps relate to the stability of risk categories in populations other then ER+, LN− patients.
Our findings indicate that further larger studies now exist which support the prognostic capability of OncotypeDX. In particular, a large UK study in 1231 postmenopausal women with hormone receptor-positive, LN− early breast cancer concluded that an increase in risk score was significantly associated with an increased risk of distant recurrence. 90 This study and the Mamounas et al. 80 study provide new evidence on the clinical validity of OncotypeDX, which employs cohorts of patients from large-scale RCTs and is rated as high quality. Furthermore, the evidence base has been extended to include the LN+ population83 and there are the beginnings of an evidence base for the validity of OncotypeDX in different populations such as in Japanese patients. 87,88
Clinical utility of OncotypeDX
In the earlier systematic reviews, evidence on clinical utility is limited. Paik et al. 49 demonstrated a significant benefit from the use of chemotherapy in the OncotypeDX high-risk group, although the review highlighted that the study may have been subject to bias as some patients in the validation data set were also in the training data set. Clinical experts indicated that more effective chemotherapy regimes are currently used in the UK. In total, > 44% of patients were aged < 50 years. The benefit of chemotherapy (reduction in distant recurrence) was greater in this population than in women aged > 50 years. The HR for the benefit of chemotherapy (reduction in distant recurrence) in women aged > 50 years compared with younger women was 2.02 (95% CI 0.75 to 5.47; p = 0.162).
Further supporting evidence was needed. Key gaps relate to the extent to which the test added to the management of patients and the proportion of patients who would benefit from the test. The role of the OncotypeDX test in guiding treatment of HER2-positive patients was unclear, as most of these patients were classified in the high-risk RS group in the initial trials. Prospective confirmation of the clinical utility of OncotypeDX was required.
Our findings indicate that there are no prospective studies reporting the impact of OncotypeDX on long-term outcomes such as OS. Four new studies76–78,82 presented further evidence on the impact of OncotypeDX on clinical decision-making. These indicate that the use of OncotypeDX leads to changes in decision-making for between 31.5% and 38% of patients. However, only one of these studies was UK based, and limitations in relation to study design were identified for this study. Specifically, these data were based on a small sample size (n = 106) and were derived from a conference poster,78 which was lacking the detail necessary to make judgements about the quality of the evidence. Two new studies (with three related citations81,83,86) provided evidence supporting the case that OncotypeDX predicts chemotherapy benefit. The Tang et al. 81,86 studies were based on ER+, LN− patients and Albain et al. 82 reported evidence for ER+, LN+ patients. These studies were based on trial data and the sample sizes were moderate in the case of Albain et al. 83 (n = 367) and large in the Tang et al. 81,86 analyses (n = 625–1319). These studies also had long follow-up times. Study quality was judged to be medium83,86 or high,81 although as Tang et al. 86 was a conference abstract we were unable to access the detail necessary to make adequate judgements about the quality of the evidence.
The first evidence relating to improvements in quality of life and reductions in patient anxiety as a result of using the test have been reported, although generalisations should be made with caution because of the small sample sizes employed. Further research in this area is required.
Key gaps in the evidence remain:
-
Few of the studies were considered to be of high quality (n = 3). A number of studies in the current review were judged to provide medium-quality (although retrospective) evidence for OncotypeDX (n = 9). One of the most characteristic features of the studies was their heterogeneity. The studies varied considerably in their size, study design, patient populations and objectives. A large proportion of the OncotypeDX studies were small and retrospective. Many studies used old archived tumour samples and included the use of retrospective chart review to elicit treatment recommendations before and after OncotypeDX testing. There was a lack of standardised decision-making tools both within and between studies, and non-standardised methods of patient selection for OncotypeDX testing were used.
-
Further direct evidence of the clinical utility of OncotypeDX is still required. This will be addressed by the ongoing TAILORx trial.
-
The generalisability of the findings may be limited because of the small number of studies that were conducted in the UK setting and because a number of the studies were funded by the manufacturer, giving rise to possible conflicts of interest and publication bias.
Overall summary
The OncotypeDX evidence is the furthest along the validation pathway compared with other similar tests, and the evidence base, in particular in relation to the prognostic ability of the test, was reasonably sound. This review has identified further studies supporting the prognostic ability (clinical validity) of the test. These are generally of moderate to high quality. Our findings indicate that there are no prospective studies reporting the impact of OncotypeDX on long-term outcomes such as OS. Four additional studies on the impact of OncotypeDX on decision-making indicate that the use of OncotypeDX leads to changes in decision-making for 31.5–38% of patients, but only one of these relates to the UK setting. Two further studies on the predictive benefit of the test were identified, one for LN+ patients. The first evidence relating to improvements in quality of life and reductions in patient anxiety as a result of using the test has been reported, but this is based on small patient numbers and further evidence is required.
Ongoing trial: the Trial Assigning Individualized Options for Treatment
The TAILORx trial commenced in April 2006 and is due to complete primary outcomes in April 2014. It aims to demonstrate that endocrine treatment alone is non-inferior to chemoendocrine treatment in women with an intermediate OncotypeDX score (11–25). Patients aged 18–75 years with ER+ and/or PR+, HER2/neu-negative tumours who are LN− (and who will be treated with tamoxifen) are eligible for inclusion. All patients receive OncotypeDX profiling and are then allocated to risk groups. Those at low risk (≤ 10) will receive endocrine therapy alone and those at high risk (≥ 26) will receive endocrine therapy and adjuvant chemotherapy. Those at intermediate risk (11–25) will receive endocrine therapy and be randomly assigned to chemotherapy or no chemotherapy. The trial is closed for recruitment. 91 Funding for the study is provided by the National Cancer Institute. Further details of this trial are included in Appendix 8.
MammaPrint
MammaPrint is based on microarray technology and uses a 70-gene expression profile. MammaPrint is intended as a prognostic test for women of all ages, LN− and LN+ (up to three nodes positive) with a tumour size of ≤ 5.0 cm. MammaPrint is used to determine the risk of distant recurrence of early breast cancer. Patients are stratified into two distinct groups – low risk (good prognosis) or high risk (poor prognosis) of distant recurrence. Further details are provided in Table 6.
Description of included studies
The present review identified an additional seven studies for the MammaPrint test. This included six full published peer-reviewed papers and one dissertation chapter.
The design and patient characteristics of the seven included studies are provided in Tables 13 and 14 respectively. Most of the studies included retrospective analyses of archived tumour samples together with a database of patient characteristics and prognostic information. Only one study stated that the design was prospective. 92 The populations used in the studies were somewhat heterogeneous, with some using only LN− patients and others using a mixture of LN− and LN+ patients. There was a similar pattern relating to ER status. The mean age was around 50 years.
| Author (year) Country | Study design | Number of patients | Follow-up | Outcomes/end points | Evidence type | Funding |
|---|---|---|---|---|---|---|
| Bueno-de-Mesquita et al. (2009)93 Netherlands |
Consecutive cohort (1996–9) Fresh frozen tumour samples (pT1–2, LN−) |
Eligible sample: NR Sample included: 123 G1: 64 low-risk prognostic signature G2: 59 high-risk prognostic signature |
Median: 5.8 (range 0.1–9.0) years | Time from surgery to distant metastasis as first event (counted as failures); OS (defined as time from surgery to death) | Clinical validity; clinical utility – reclassification against existing prognostic variables | NR |
| Gevensleben et al. (2010)94 Germany |
Consecutive cohort (2005–8) Frozen tumour samples (evaluates concordance) |
Eligible sample: 170 Sample included: 140 G1: 78 good prognosis signature G2: 62 poor prognosis signature Samples excluded because of inadequate RNA extraction (n = 30) |
NR | Comparison of risk prediction using the MammaPrint test with those of St Gallen criteria95 and Adjuvant! Online | Clinical utility – reclassification against existing prognostic variables; changes in treatment recommendations | NR |
| Ishitobi et al. (2010)96 Japan |
Retrospective cohort (1998– 2001) Frozen tumour samples |
Eligible sample: 117 Sample included: 102 G1: 20 low-risk prognostic signature G2: 82 high-risk prognostic signature Samples excluded because of failure of microarray profiling (n = 15) |
Median: 7.1 (range 0.5–9.8) years | DMFS (not defined); correlation between the MammaPrint test risk category and clinicopathological parameters (St Gallen criteria97,98) | Clinical validity; clinical utility – reclassification against existing prognostic variables | NR |
| Kok et al. (2010)99 Netherlands |
Two datasets: G1: 1985–94; G2: 1982–96 Adjuvant tamoxifen (G1): retrospective, frozen tumour samples No adjuvant systemic treatment (G2): consecutive series from van de Vijver63 (n = 100) and Mook et al.64 (n = 51), FFPE samples |
Eligible sample: NR Sample included: 272 G1: 121 (83 low-risk, 38 high-risk prognostic signature) G2: 151 (85 low-risk, 66 high-risk prognostic signature) |
Median: G1: 9.6 years; G2: 11.1 years | BCSS (defined as time from surgery to breast cancer-related death) | Clinical validity | NR |
| Kunz et al. (2011)92 Germany |
Prospective cohort (2004–8) Fresh tumour samples (T1–3, NO-3) (evaluates concordance) |
Eligible sample: 56 Sample included: 44 Samples excluded because of insufficient sample (n = 6); lost in transit, (n = 4); not eligible because of metastases (n = 2) |
NR | Comparison of risk prediction using the MammaPrint test with that of the St Gallen guidelines97 (2007/9) and Adjuvant! Online | Clinical utility – reclassification against existing prognostic variables | NR |
| Mook et al. (2010)75 Netherlands |
Consecutive series (1984–6) Frozen tumour samples (T1–2, LN−) |
Eligible sample: 173 Sample included: 148 G1: 91 good prognosis signature G2: 57 poor prognosis signature Samples excluded because of insufficient sample (n = 22); poor RNA quality, (n = 3) |
Median: 11.6 years | DMFS (defined as time from surgery to distant metastasis as first event: counted as failures); BCSS (defined as time from surgery to breast cancer-related death); comparison of risk prediction using the MammaPrint test with that of Adjuvant! Online | Clinical validity; clinical utility – reclassification against existing prognostic variables | European Commission Framework Program VI-TRANSBIG; Dutch National Genomics Initiative Cancer Genomics Center; Agendia BV |
| Na et al. (2011)100 Republic of Korea |
Retrospective cohort (2008–9) Fresh tumour samples (T1–2, LN−, M0) (evaluates concordance) |
Eligible sample: 48 Sample included: 36 G1: 5 low-risk prognostic signature G2: 31 high-risk prognostic signature Samples excluded because of sampling failure (n = 10); not eligible because of metastases (n = 2) |
NR | Comparison of risk prediction using the MammaPrint test with those of the St Gallen criteria,95 National Institutes of Health guideline101 and Adjuvant! Online | Clinical utility – reclassification against existing prognostic variables | NR |
| Author (year) | Age (years), mean (range) | LN status | ER status | Tumour size | Grade | HER2 status | Mean NPI score | Treatment |
|---|---|---|---|---|---|---|---|---|
| Bueno-de-Mesquita et al. (2009)93 | 47 (27–55) | All LN− | +/−: G1: 62/2; G2: 32/27 | pT1 (≤ 2 cm): G1: 46; G2: 30 pT2 (2.1–5 cm): G1: 18; G2: 29 Mean: 2 cm; range 0.5–5 cm |
I: G1: 18; G2: 2 II: G1: 35; G2: 18 III: G1: 11; G2: 39 |
+/−: G1: 2/61; G2: 7/52 | NR | Adjuvant chemotherapy: G1: 1; G2: 17 Adjuvant endocrine therapy: G1: 9; G2: 5 Both: G1: 9; G2: 4 None: G1: 45; G2: 33 |
| Gevensleben et al. (2010)94 | NR | +/−: G1: 24/54; G2: 22/40 | +/−: G1: 77/1; G2: 39/23 | ≤ 1 cm: G1: 4; G2: 3 > 1 to ≤ 2 cm: G1: 39; G2: 18 > 2 to ≤ 5 cm: G1: 34; G2: 38 > 5 cm: G1: 1; G2: 3 |
NR | +/−/unknown: G1: 3/74/1; G2: 6/55/1 | NR | Adjuvant systemic therapy: 134 (chemotherapy: 23; endocrine therapy: 59; both: 52); no treatment: 2; unknown: 4 |
| Ishitobi et al. (2010)96 | NR (but < 70 years) | All LN− | +/−: G1: 19/1; G2: 33/38a | ≤ 2 cm: G1: 9;a G2: 40 > 2 cm: G1: 9;a G2: 42 |
I: G1: 11; G2: 9a II: G1: 6; G2: 23a III: G1: 3; G2: 49a |
NR | NR | Chemotherapy: G1: 2; G2: 27 Hormone therapy: G1: 17; G2: 57 |
| Kok et al. (2010)99 | NR | G1: LN−: 20; N1–3: 74; N > 3: 20; unknown: 7 G2: LN−: 138; N1–3: 10; N > 3: 3 |
All ER+ | ≤ 2 cm: G1: 55; G2: 96 > 2 cm: G1: 65; G2: 55 Unknown: G1: 1 |
I: G1: 36; G2: 52 II: G1: 63; G2: 54 III: G1: 18; G2: 45 Unknown: G1: 1 |
NR | NR | G1: adjuvant tamoxifen monotherapy (about 70% for at least 2 years) and no neoadjuvant therapy G2: no adjuvant systemic treatment |
| Kunz et al. (2011)92 | 44 (32–56) | LN−: 27 N1: 16 N2/N3: 3 |
+/−: 36/8 (intermediate: 2) |
pT1: 26 (pT1b: 2; pT1c: 24) pT2: 19 pT3: 1 Mean: 2.06 cm |
I: 18 II: 18 III: 10 |
+/−: 5/41 | NR | Chemotherapy: 32; no chemotherapy: 14; peritumoural invasion: 14; no invasion: 32 |
| Mook et al. (2010)75 | NR (55–70) | All LN− | +/−: G1: 88/3; G2: 28/29 | pT1 (≤ 2 cm): G1: 59; G2: 24 pT2 (> 2 to 5 cm): G1: 32; G2: 33 |
I: G1: 52; G2: 3 II: G1: 28; G2: 15 III: G1: 11; G2: 39 |
NR | NR | Adjuvant endocrine (tamoxifen) therapy: G1: 17; G2: 10 Note: inclusion criteria specified no adjuvant chemotherapy |
| Na et al. (2011)100 | 47 (23–68) | All LN− | +/−: G1: 4/1; G2: 25/6 | T1 (≤ 2 cm): G1: 3; G2: 20 T2 (> 2 to 5 cm): G1: 2; G2: 11 Mean: 2.0 cm |
I: G1: 3; G2: 4 II: G1: 2; G2: 15 III: G1: 0; G2: 12 |
+/−: G1: 1/4; G2: 7/24 | NR | No neoadjuvant treatment. No other details provided |
Most studies included a small number of participants (range 36–272). Follow-up was either short (< 10 years and in some cases < 5 years) or not reported for a number of studies.
Quality of included studies: MammaPrint
The methodological quality of the seven included studies75,92–94,96,99,100 is summarised in Figure 4 (further details are provided in Appendix 9). Generally, only two studies75,93 performed well, receiving a positive assessment for at least 17 out of 21 methodological quality items. Although the majority of the studies (as reported by the authors) used a retrospective study design,75,96,99,100 other potential sources of bias were generally related to the following domains: prognostic variable (inadequate reporting and justification of cut points used), statistical analysis (lack of statistical adjustment of all prognostic factors and inappropriate analysis of continuous predictor variables, for example categorising of continuous variables leads to loss of statistical power, and data-dependent categorisation leads to overoptimism)30 and interventions subsequent to inclusion in the study (interventions were not described or standardised). In the majority of studies, the assessment of study quality was hampered by poor reporting of the following methodological items: length of follow-up of patients, whether or not the sample of patients was representative and assembled at an early point in the course of the disease and whether or not outcomes were fully defined and appropriate (including whether or not the outcome assessment was unbiased). Overall, the risk of bias from the seven included studies was judged to be moderate.
FIGURE 4.
MammaPrint test: methodological quality graph (review authors' judgements about each methodological quality item presented as percentages across all studies).
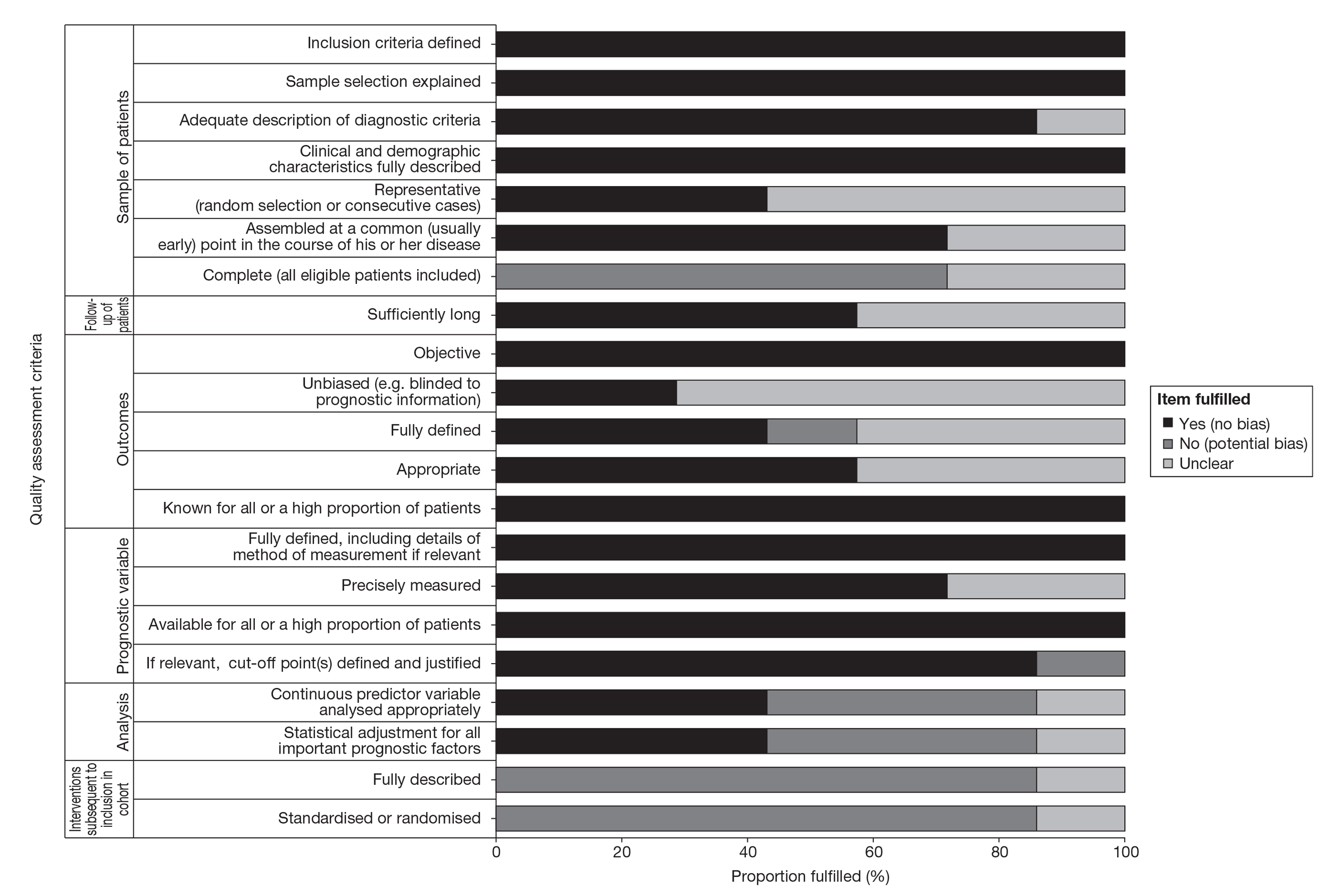
Results: MammaPrint
A summary of the clinical evidence on MammaPrint is presented in Table 15, followed by a narrative summary of each study. Full data extraction tables are provided in Appendix 9.
| Author (year) | Evidence type | Overall quality | Key findings |
|---|---|---|---|
| Bueno-de-Mesquita et al. (2009)93 | Clinical validity; clinical utility – reclassification against existing prognostic variables | High | 123 LNx, majority ER+ patients from the Netherlands. The rates of discordance between MammaPrint and four different standard clinicopathological measures were relatively high (38%, 41%, 26%, 30%). OS probability was 97% for good and 82% for poor prognosis signature patients with an estimated HR of 3.4 (95% CI 1.2 to 9.6; p = 0.021). The probability of remaining free of distant metastasis (as first event) was 98% for good and 78% for poor prognosis signature patients with an estimated HR of 5.7 (95% CI 1.6 to 20; p = 0.007). MammaPrint was shown to be a strong independent prognostic factor in multivariate analyses, outperforming the clinicopathological risk indexes. Limitations: small sample size, follow-up limited to 5 years |
| Gevensleben et al. (2010)94 | Clinical utility – reclassification against existing prognostic variables; changes in treatment recommendations | Moderate | 140 LN− and ER+ patients from Germany. MammaPrint and Adjuvant! Online were concordant in 83 cases and discordant in 57 cases (41%). A retrospective analysis of treatment given (where available) compared with treatment indication by MammaPrint was performed showing that, according to MammaPrint, 40% of patients had been either undertreated or overtreated. Limitation: small sample size |
| Ishitobi et al. (2010)96 | Clinical validity; clinical utility – reclassification against existing prognostic variables | Moderate | 102 LN−, majority ER+ patients from Japan. NPV for time to distant metastasis was high (100%), indicating that all patients were correctly classified, whereas PPV was low (9.8%), indicating that many of the cases classified as high risk were incorrectly classified. The relatively young patient population and 5-year follow-up may also explain why the probability of DMFS was also very high for the high-risk group. Limitations: small sample size, particularly in the low-risk group, findings may not be generalisable to the UK setting |
| Kok et al. (2010)99 | Clinical validity | Moderate | 272, all ER+ patients from the Netherlands. Inpatients treated with adjuvant tamoxifen (mainlyLN+), both MammaPrint and the endocrine response categories were associated with BCSS at 10 years. Inpatients treated with tamoxifen, combined analysis of MammaPrint and ER/PR revealed additional value. Inpatients who did not receive tamoxifen, only MammaPrint was associated with outcome. Limitation: small sample size |
| Kunz et al. (2011)92 | Clinical utility – reclassification against existing prognostic variables | Moderate | 44 LN− and majority ER+ patients from Germany. Comparison of numbers of patients classified into risk groups using St Gallen,97 Adjuvant! Online and MammaPrint. The authors concluded that gene expression analysis as an additional tool can accurately separate patients with an intermediate clinical risk into low- and high-risk groups. Limitation: very small sample size |
| Mook et al. (2010)75 | Clinical validity; clinical utility – reclassification against existing prognostic variables | High | 148 ER+, LN−, postmenopausal patients from the Netherlands. Distant metastasis-free survival at 5 years was 93% in the low-risk group and 72% in the high-risk group (p = 0.07) with an associated HR of 4.6 (95% CI 1.8 to 12.0; p = 0.001). At 10 years the difference was not significant. BCSS at 5 years was 99% in the low-risk group and 80% in the high-risk group (p = 0.036) with an associated HR of 19.1 (95% CI 2.5 to 148; p = 0.005). At 10 years the difference was not significant. MammaPrint and Adjuvant! Online were concordant in 107 cases and discordant in 41 cases. The authors concluded that the MammaPrint signature can accurately select postmenopausal patients at low risk of breast cancer in terms of related death within 5 years of diagnosis and can be of clinical use in selecting postmenopausal women for adjuvant chemotherapy. Limitations: small sample size, assessed only postmenopausal women |
| Na et al. (2011)100 | Clinical utility – reclassification against existing prognostic variables | Moderate | 36 LN−, majority ER+ patients from Republic of Korea. Clinical risk concordant with the prognostic signature for 29 (81%) patients according to the St Gallen guidelines;95 30 (83%) patients according to the National Institutes of Health guidelines and 23 (64%) patients according to Adjuvant! Online. Limitations: very small sample size, no follow-up data, may not be generalisable to the UK setting |
Analytical validity
Our searches did not reveal any studies that examined analytical validity.
Clinical validity (prognostic ability)
Kok et al. 99 assessed whether or not analysing both MammaPrint score and hormone receptors provides superior prediction of outcome than hormone receptors alone in 272 Dutch patients. One group comprised LN+, ER+, tamoxifen-treated patients and a second group comprised LN−, ER+ patients who had received no adjuvant systemic treatment. Hormone receptors were evaluated using the St Gallen consensus recommendations102 (highly endocrine responsive: ER and PR ≥ 50%; incompletely endocrine responsive: ER and/or PR low or with either one absent). In patients treated with adjuvant tamoxifen (mainly LN+), both MammaPrint (adjusted for endocrine response categories, HR 2.78; 95% CI 1.30 to 5.94) and the endocrine response categories (adjusted for MammaPrint, HR 7.22; 95% CI 2.17 to 24.0) were associated with BCSS at 10 years. Also, in patients treated with tamoxifen for metastatic disease, combined analysis of MammaPrint and ER/PR revealed additional value (multivariate Cox regression; p = 0.013). In patients who did not receive tamoxifen, only MammaPrint was associated with outcome. The authors concluded that both methods provide independent information on outcome after tamoxifen for LN+ breast cancer. There are a number of limitations to this study: the second patient group comprised patients included in two previously reported evaluations, the overall sample size was small and tumour samples had been collected over a number of years (1982–97), which has implications for changes in diagnosis, treatment and standards of care. The study did benefit from an adequate follow-up time of 10 years.
Ishitobi et al. 96 examined risk classification using MammaPrint and disease outcome for 102 LN−, majority ER+, relatively young breast cancer patients in Japan. The results relating to clinical validity are presented here and the results relating to clinical utility are presented in the relevant section below. Among the 102 patients, 20 (20%) were classified as low risk and 82 (80%) as high risk. The authors reported that the probability of DMFS at 5 years was 100% for the low-risk group and 94% for the high-risk group. They did not report a HR. The NPV for time to distant metastasis was high (100%, 20/20), whereas the PPV was quite low (9.8%, 8/82). The NPV indicates the proportion of patients classified as low risk who were correctly classified using MammaPrint, whereas the small PPV indicates that many of the cases classified as high risk were incorrectly classified. The authors concluded that the 70-gene prognosis signature accurately identified Japanese breast cancer patients as being at low risk of developing recurrences, as 100% of the individuals in the low-risk group remained metastasis free for the duration of the observation period. The authors suggest that the low number of individuals in the low-risk group is consistent with previous findings on patient groups of ≤ 54 years. However, these low numbers make any generalisations of the findings limited. The young patient population may also explain why the probability of DMFS was also very high for the high-risk group, together with the fact that this was assessed at only 5 years, given that the majority of distant recurrences and deaths from breast cancer occur > 5 years after diagnosis. This study employed a very small sample size and, furthermore, as this study was performed in a Japanese population any generalisations to the UK population are significantly limited.
In a Netherlands-based study, Bueno-de-Mesquita et al. 93 assessed 123 LN−, majority ER+ patients who had been assigned MammaPrint risk categories. They reported risk classification and probability of disease outcome (time from surgery to distant metastasis and OS). OS probability was 97% (±2%) for good and 82% (±5%) for poor prognosis signature patients (p-value not reported) with an estimated HR of 3.4 (95% CI 1.2 to 9.6; p = 0.021). The probability of remaining free of distant metastasis (as first event) was 98% (±2%) for good and 78% (±6%) for poor prognosis signature patients (p-value not reported) with an estimated HR of 5.7 (95% CI 1.6 to 20; p = 0.007). In multivariate analysis, the authors demonstrated that MammaPrint was a strong independent prognostic factor, outperforming the clinicopathological risk indexes. They concluded that the 70-gene prognosis signature is also an independent prognostic factor in LN− breast cancer patients for women diagnosed in recent years. Again, as this study used a small sample size and the follow-up assessment was limited to 5 years, generalisations of the findings are limited. This study also reported reclassification findings, which are detailed in the relevant section below.
Mook et al. 75 examined 148 LN− and majority ER+, specifically postmenopausal patients. The study, conducted in the Netherlands, investigated disease outcome (DMFS and BCSS at 5 years), and prediction of early breast cancer-specific death (BCSD) using MammaPrint risk categories. The authors also assessed reclassification and these findings will be presented below. DMFS at 5 years was 93% in the low-risk group and 72% in the high-risk group (p = 0.07) with an associated HR of 4.6 (95% CI 1.8 to 12.0; p = 0.001). At 10 years it was 80% in the low-risk group and 67% in the high-risk group (HR not reported, p-value not reported). Over the entire follow-up period the HR was 1.8 (95% CI 0.9 to 3.5; p = 0.07). BCSS at 5 years was 99% in the low-risk group and 80% in the high-risk group (p = 0.036) with an associated HR of 19.1 (95% CI 2.5 to 148, p = 0.005). At 10 years it was 90% for the low-risk group and 69% for the high-risk group (HR not reported, p-value not reported). Over the entire follow-up period the HR was 2.0 (95% CI 1.0 to 4.0; p = 0.04). In terms of the prediction of early BCSD, the HR for BCSS at 5 years was 14.4 (95% CI 1.7 to 122.2; p = 0.01) and at 10 years was 4.4 (95% CI 1.4 to 13.6; p = 0.01). Subgroup analyses showed that the HR for BCSS in hormonal therapy-naive patients (untreated) at 5 years was 10.8 (95% CI 1.2 to 94.7; p = 0.03). The authors concluded that the MammaPrint signature can accurately select postmenopausal patients at low risk of breast cancer in terms of related death within 5 years of diagnosis, although not at 10 years, and can be of clinical use in selecting postmenopausal women for adjuvant chemotherapy. Again this study employed a very small sample size and was based on postmenopausal women, limiting the applicability of the findings.
Clinical utility
Kunz et al. 92 compared the MammaPrint result with St Gallen criteria97 and Adjuvant! Online and conducted risk assessment using MammaPrint according to nodal status in 44 women in Germany. The majority of patients were LN− and ER+. MammaPrint classified 29 patients as low risk and 15 patients as high risk. St Gallen criteria classified four patients as low risk, 34 patients as intermediate risk and six patients as high risk. In the group of women with intermediate risk according to St Gallen, MammaPrint assigned 23 patients to low risk and 11 to high risk. Adjuvant! Online classified 19 patients as low risk and 25 patients as high risk (for Adjuvant! Online, patients were classified as having low clinical risk when the 10-year OS rate as predicted by Adjuvant! Online was > 88% for ER+ tumours and > 92% for ER− tumours). MammaPrint classified 13 patients with LN+ disease as low risk and five as high risk. For those with LN− disease, 17 were classified as low risk and nine as high risk. The authors concluded that, by using gene expression analysis as an additional tool, patients with an intermediate clinical risk can be accurately separated into low- and high-risk groups. Follow-up data would be required to verify the authors' conclusions that the gene expression analysis provides more accurate information on recurrence risk than conventional clinicopathological criteria. This was a reasonable quality study with a prospective design although the interpretation of the findings is limited because of the very small sample size. Studies on larger sample sizes would be required to verify these conclusions.
Na et al. 100 compared MammaPrint with the St Gallen criteria,95 the National Institutes of Health (NIH) guidelines101 and Adjuvant! Online in 36 LN− and majority ER+ Korean patients. The 70-gene prognosis signature identified 5 (14%) patients with a low-risk prognosis signature and 31 (86%) patients with a high-risk prognosis signature. Clinical risk was concordant with the prognostic signature for 29 (81%) patients according to the St Gallen guidelines, 30 (83%) patients according to the NIH guidelines and 23 (64%) patients according to Adjuvant! Online. The authors concluded that the 70-gene prognostic signature gave somewhat different results in Korean patients with breast cancer from those in European patients. They suggested that further studies should assess whether or not a gene disparity between Asians and Europeans influenced the results. Further large-scale studies with a follow-up evaluation are required to assess whether or not the use of the 70-gene prognostic signature can predict the prognosis of Korean patients with breast cancer. (Note that as St Gallen has three risk categories and MammaPrint has two, a calculation of concordance is not possible.) This study had a very small sample size and, as the results could have been influenced by a gene disparity between European and Asian patients, any generalisations to the UK population are significantly limited. Furthermore, as there was no follow-up evaluation, no conclusions regarding the prognostic value of MammaPrint compared with clinicopathological guidelines can be made.
Ishitobi et al. 96 examined risk classification using MammaPrint and disease outcome for breast cancer in 102 LN−, majority ER+ patients in Japan. The results relating to clinical validity have been presented in the previous section. Among the 102 patients, 20 (20%) were classified as low risk and 82 (80%) as high genomic risk. Based on the 1998 St Gallen criteria,103 all patients were classified as intermediate or high risk. The 2009 St Gallen criteria97 use more refined criteria to define the low-risk group and they classified 7 (of 100) patients as low risk compared with 20 (of 102) patients identified as low risk by MammaPrint (p = 0.009). The authors concluded that the 70-gene prognosis signature accurately identified Japanese breast cancer patients at low risk of developing recurrences, as 100% of the individuals in the low-risk group remained metastasis free for the duration of the observation period. Overall, this study employed a very small sample size; furthermore, as this study was performed using a Japanese population, any generalisations to the UK population are significantly limited.
Bueno-de-Mesquita et al. 93 made a comparison between MammaPrint risk categories and risk assessment based on Adjuvant Online!, St Gallen guidelines,103,104 NPI and Dutch Institute for Healthcare Improvement (CBO) guidelines (2004)105,106 in 123 LN− and majority ER+ Dutch patients. Discordance between the measures was 38% (47/123), 41% (50/123), 26% (32/123) and 30% (37/123) respectively. These rates of discordance appear relatively high although we do not know which predictor is classifying correctly. Again, as this study used a small sample size and the follow-up assessment was limited to 5 years, generalisations of the findings are limited.
Mook et al. 75 examined 148 LN− and majority ER+, specifically postmenopausal patients. The study, conducted in the Netherlands, investigated classification using MammaPrint and disease outcome (the results of the latter are presented in the earlier section). MammaPrint risk categories of high and low were compared with Adjuvant! Online categories of high and low (low clinical risk: predicted 10-year BCSS > 88% for ER+ tumours and > 92% for ER− tumours). MammaPrint and Adjuvant! Online were concordant in 107 cases and discordant in 41 cases, although again we cannot make any assertions regarding which indicator is more accurate. The authors concluded that the 70-gene prognosis signature can accurately select postmenopausal patients at low risk of breast cancer. Again, these findings were based on a very small sample size and assessed only postmenopausal women, limiting the applicability of the findings to younger women.
A German-based study95 investigating 140 majority LN− and ER+ patients was reported by Gevensleben et al. 94 The authors made a comparison between risk prediction using the 70-gene prognostic signature and risk prediction using the St Gallen criteria95 and Adjuvant! Online (Adjuvant! Online risk classification according to Ravdin et al. 107). MammaPrint and Adjuvant! Online were concordant in 83 cases and discordant in 57 cases (41%). The authors concluded that MammaPrint provides improved prediction of recurrence risk compared with currently used guidelines. The generalisability of the findings is limited because of the small sample size employed.
Gevensleben et al. 94 in their study investigating 140 LN− and ER+ German patients, also examined treatment advice. For 59 patients (out of 62) with poor prognosis identified by the 70-gene prognosis signature, the clinical treatment was recorded. In total, 19 (32%) of these patients did not receive adjuvant systemic treatment other than endocrine therapy and were potentially undertreated. In contrast, 35 (out of 77) patients who were classified as having a good prognosis by the 70-gene prognosis signature, and for whom treatment was known, received chemotherapy and were potentially overtreated. As a result, the authors concluded that the 70-gene prognosis signature would have resulted in altered treatment advice in 40% of the patients, based on the assumption that all high-risk patients would receive chemotherapy and all low-risk patients would not. There are limitations to this study, including that it was based on a small sample of patients and that the data relating to changes in treatment recommendations are retrospective and relate only to potential and not actual changes.
Supplementary evidence: MammaPrint
Four further citations108–111 were excluded from the review as they did not meet the inclusion criteria on the basis that they are pooled analyses of existing data. This evidence was not in the form of meta-analyses of the separate studies. This approach is methodologically inappropriate. However, because of the paucity of data on the clinical utility of MammaPrint, a number of these studies have been used to inform the economic model and therefore they will be summarised here for completeness.
These studies suffer from several major limitations. First, they are based on pooled analyses and it is unclear how individual patient data have been combined from the various primary studies incorporated. Furthermore, there is likely to be significant heterogeneity in the chemotherapy used, standards of care and diagnosis as patients were recruited over a period of > 20 years (1984–2006). This makes any generalisability of the conclusions to current practice difficult.
Knauer et al. 112 evaluated the predictive value of the 70-gene prognostic signature for response to chemotherapy. They created a pooled database of patients from six previous studies, including 541 women with unilateral stage T1–3, N-1, M0 invasive breast cancer diagnosed between 1984 and 2006. Each tumour had been classified as having a high- or low-risk signature using the MammaPrint test: 252 (47%) as low risk and 289 (53%) as high risk. Median follow-up was 7.1 years, but all analyses were censored at 5 years. The signature was prognostic: women with a low-risk tumour signature had a 5-year BCSS of 97% and a 5-year DMFS of 95% whereas women with a high-risk tumour signature had a 5-year BCSS of 87% and a 5-year DMFS of 82%. However, women in both risk categories appeared to benefit from chemotherapy, although the estimates were not statistically significant in the low-risk group. For BCSS the unadjusted HR for chemotherapy was 0.58 (95% CI 0.07 to 5.0) in the low-risk group and 0.21 (95% CI 0.07 to 0.59) in the high-risk group. The p-value for interaction between use of chemotherapy and the risk signature was not statistically significant (p = 0.45). For DMFS the unadjusted HR for chemotherapy was 0.26 (95% CI 0.03 to 2.0) in the low-risk group and 0.35 (95% CI 0.17 to 0.71) in the high-risk group. The p-value for the interaction was not reported, but in this case the trend was towards greater relative benefit from chemotherapy in the low-risk group. This study, however, has some major statistical flaws. For instance, data were truncated arbitrarily at 5 years, despite that fact that the median follow-up was 7.1 years. Censoring the follow-up at 5 years biased the results in favour of the utility of the prognostic signature because the association between the 70-gene signature and recurrent disease falls quickly after 5 years of follow-up. 113 As the majority of distant recurrences and deaths from breast cancer occur > 5 years after diagnosis, this is a significant limitation. 113
Knauer et al. 110 investigated whether or not MammaPrint identifies HER2-positive patients with a favourable outcome. A total of 168 T1–3, N-1, HER2-positive patients were identified from a pooled database, classified by the MammaPrint test as having a good or a poor prognosis, and correlated with long-term outcome. A total of 89 of these patients did not receive adjuvant chemotherapy. In these patients, after a median follow-up of 7.4 years, 35 (39%) distant recurrences and 29 (33%) BCSDs occurred. The test classified 20 (22%) patients as having a good prognosis, with 10-year distant disease-free survival (DDFS) of 84%, compared with 69 (78%) poor prognosis patients with a 10-year DDFS of 55%. The estimated HRs were 4.5 (95% CI 1.1 to 18.7, p = 0.04) and 3.8 (95% CI 0.9 to 15.8, p = 0.07) for DDFS and BCSS respectively. In multivariate analysis adjusted for known prognostic factors and hormone therapy, HRs were 5.8 (95% CI 1.3 to 26.7, p = 0.03) and 4.7 (95% CI 1.0 to 21.7, p = 0.05) for DDFS and BCSS respectively. The authors concluded that the test is an independent prognostic indicator that identified a subgroup of HER2-positive early breast cancer patients with a favourable long-term outcome.
Mook et al. 108 aimed to evaluate the accuracy of MammaPrint in T1 breast cancer. They selected 964 patients with pT1 tumours (≤ 2 cm) from a pooled database. The samples had been previously analysed and classified as having good or poor prognosis. The median follow-up was 7.1 years. The 10-year DMFS and BCSS probabilities were 87% [standard error (SE) 2%] and 91% (SE 2%), respectively, for the good prognosis group (n = 525) and 72% (SE 3%) and 72% (SE 3%), respectively, for the poor prognosis group (n = 439). The signature was an independent prognostic factor for BCSS at 10 years (multivariate HR 3.25; 95% CI 1.92 to 5.51; p < 0.001]). Moreover, the test predicted DMFS at 10 years for 139 patients with pT1ab cancers (HR 3.45; 95% CI 1.04 to 11.50; p = 0.04). The authors concluded that the test is an independent prognostic factor in patients with pT1 tumours and can help to individualise adjuvant treatment recommendations.
Bueno-de-Mesquita et al. 109 evaluated the additional prognostic value of MammaPrint compared with a combination of established prognostic guidelines. A total of 701 patients from a pooled database with an existing MammaPrint result were evaluated. Only 6% (10/156) of ER− tumours had a good prognosis signature. The signature was not useful for ER+ tumours with a concordant high Adjuvant! Online, high St Gallen and/or high NPI risk (n = 139). The 10-year OS estimate for the good prognosis group with these characteristics was < 80% and adjuvant systemic treatment (AST) would therefore be appropriate irrespective of the signature result. In contrast, for patients with a concordant low Adjuvant! Online, low St Gallen and/or low NPI risk and in discordant clinical risk patients, the signature identified low-risk patients in whom AST could be safely withheld (10-year OS < 90%). The authors concluded that the MammaPrint signature provides additional prognostic information, especially in ER+, LN− breast cancer patients with a predominantly low or discordant clinical risk on the basis of Adjuvant! Online, the St Gallen guidelines and/or NPI.
Summary of evidence: MammaPrint
Analytical validity of MammaPrint
In the earlier systematic reviews limited data are available on variability and reproducibility, with a limited number of patients and a moderate number of replications. The only validation study using the MammaPrint assay (rather than the underlying 70-gene signature) showed that only about 80% of fresh-frozen specimens were analysable.
Our review identified no new evidence.
Clinical validity (prognostic ability) of MammaPrint
Earlier systematic reviews identified a range of studies providing evidence on the prognostic ability of the test in heterogeneous populations. The evidence relating to the clinical validity of MammaPrint was not always conclusive nor supportive of the prognostic value of the test. Four studies suggested that the test could predict prognosis, one study failed to verify the prognostic utility of the test and in another the methods and results were at variance with those of other studies.
The current review identified four additional studies that contain data on clinical validity. Of these, two were rated as high quality and two as moderate quality. These studies demonstrated that the MammaPrint score is a strong independent prognostic factor and may provide additional value to standard clinicopathological measures. The majority of the evidence suggests that the test is reliable at predicting outcome at 5 years. 74 However, the population in all of these studies was relatively small, ranging between 102 and 272 patients. One of the studies was conducted in a Japanese population, making generalisation to UK practice difficult. Follow-up was limited to only 5 years in two of the studies.
Clinical utility of MammaPrint
Earlier systematic reviews identified one study on clinical utility, which demonstrated that MammaPrint had an impact on clinical decision-making. The addition of MammaPrint to the standard Dutch clinical assessment of risk (modified by patient preference) in a cohort of 427 patients increased the number of patients receiving adjuvant systemic therapy by 20. However, the follow-up was not long enough to provide evidence of its effect on clinical end points such as DMFS or its utility in predicting treatment benefit.
The current review identified six studies that contained data on the clinical utility of MammaPrint. Of these, two were rated as high quality and four as moderate quality. Five of the six studies reported on how the MammaPrint test reclassifies patients into high- and low-risk groups compared with the risk assigned in current practice. None of these was based in a UK setting. These studies reported that there was a high level of discordance between MammaPrint and current practice, although the studies did not demonstrate how this would impact on treatment decisions. One study reported that the use of MammaPrint would result in altered treatment advice for 40% of patients, but this was based on the assumption that all patients classified as high risk would receive chemotherapy and no patients classified as low risk would receive chemotherapy rather than by providing evidence of actual changes in practice. A study on the benefits of chemotherapy by MammaPrint risk group was identified but omitted from the systematic review because it was based on a pooled analysis of six primary studies (which were included in the review in their own right). This study reports findings on chemotherapy benefit for MammaPrint high-and low-risk groups but the findings are not considered to be robust as the authors do not reanalyse the tumour samples and it is unclear how individual patient data were combined. All of the studies on clinical utility were based on small sample sizes.
Key gaps in the evidence base remain:
-
Robust evidence of clinical utility is needed. It is not yet clear whether or not the use of the MammaPrint test will change the management of patients, for example reduce the number of patients unnecessarily treated with chemotherapy or improve patient outcomes through increases in DFS and OS. The ongoing Microarray In Node-negative Disease may Avoid ChemoTherapy (MINDACT) trial, which is summarised in Ongoing randomised trial: the Microarray In Node-negative Disease may Avoid ChemoTherapy trial (see Appendix 8 for further detail of the MINDACT trial), will provide this evidence.
-
Only two studies were considered to be of high quality. The rest of the studies in the current review were judged to provide moderate-quality (although retrospective) evidence for MammaPrint. All of the included studies employed very small sample sizes. One of the most characteristic features of the studies was their heterogeneity, particularly with respect to patient populations. All but one92 of the MammaPrint studies were retrospective, and many used old archived tumour samples and non-standardised methods of patient selection. In addition to patient heterogeneity, there is likely to be significant heterogeneity in the chemotherapy treatment as patients were diagnosed with breast cancer over a period of > 20 years (1984–2006) and the standards of care have changed considerably.
-
Further issues that may limit the extent to which we can generalise the findings include publication bias and the fact that no studies were conducted in the UK setting.
Overall summary
The evidence base, in particular in relation to the prognostic ability of the test, is developing but is based on small sample sizes (≤ 272). None of the studies used UK-based patients and the data were all based on cohort studies. The test appears to be prognostic at 5 years although the validity of the test to predict longer-term outcomes does not seem to have been established. Robust evidence of clinical utility is needed as it is not yet clear to what extent the use of the MammaPrint test will change the management of patients and to what extent chemotherapy would be offered to patients classified as having a good or a poor prognosis with MammaPrint. It is also unclear to what extent MammaPrint risk groups are predictive of chemotherapy benefit or how the use of MammaPrint will improve patient outcomes through increases in DFS and OS. The evidence for MammaPrint to date is mainly derived from premenopausal women, but younger women are more likely to be classified as having a poor prognosis using MammaPrint, which might overestimate the benefit of the test.
Ongoing randomised trial: the Microarray In Node-negative Disease may Avoid ChemoTherapy trial
The MINDACT trial started recruiting patients in 2006 and has an estimated completion date of 2019. It is a partially randomised, open-label, prospective, multicentre clinical trial that aims to assess the value of the 70-gene prognostic signature in predicting which patients would benefit from chemotherapy compared with Adjuvant! Online, which is based on clinical characteristics. Women > 18 years (the upper age limit of 70 years was recently removed) with histologically confirmed unilateral invasive breast cancer, T1–3 operable disease, up to three positive lymph nodes and no distant metastases are eligible for enrolment. The target for enrolment recently increased to 6600 from 6000. This is predicted to result in 55% of patients assessed as high risk by both methods, who will go on to have chemotherapy, and 13% of patients assessed as low risk by both methods, who will go on to have no chemotherapy. The 32% who are assessed as high risk by one method and low risk by the other will then be randomised to follow the treatment indicated by MammaPrint or the treatment indicated by Adjuvant! Online. Two further objectives of the trial relating to the efficacy of different chemotherapy agents and endocrine treatment strategies are addressed by two further stages of randomisation. Regardless of previous randomisation and risk categorisation, patients who are to receive chemotherapy are randomised to docetaxel or capecitabine regimens and patients who are hormone receptor-positive are randomised to a single-agent upfront aromatase inhibitor (letrozole) for 7 years or tamoxifen for 2 years followed by letrozole for 5 years. The primary outcome measures are DMFS at 5 years and DFS, followed up for a minimum of 15 years. As of October 2012, the study had enrolled 6700 patients. Further details of this trial are included in Appendix 8.
MammaPrint and BluePrint
BluePrint is used in addition to MammaPrint for molecular subtyping. It is an 80-gene microarray and the target population is patients with early-stage (stage I or II), LN− or LN+ (up to three nodes), ER+ or ER− breast cancer. BluePrint provides information on breast cancer subtype using three categories: basal-type, luminal-type and ERBB2-type cancers.
Description of included studies
The searches did not identify any full peer-reviewed papers relating to the BluePrint test; however, one meeting abstract by Stork-Sloots et al. 114 met the inclusion criteria. This study related to clinical validity, the design was retrospective and the sample size was moderate (n = 469). No further details of the design or the study populations were reported.
Quality of the included study: MammaPrint and Blueprint
Although the assessment of study quality was hindered by poor reporting in the domains of outcome, prognostic variable, analysis and interventions subsequent to inclusion in the cohort, the overall methodological quality of the included study114 was judged to be low, indicating a high risk of bias (the study received a positive assessment of at least two out of 21 methodological quality items).
Results: MammaPrint and Blueprint
Full data extraction tables are provided in Appendix 10.
Analytical validity
No available evidence.
Clinical validity
Stork-Sloots et al. 114 compared BluePrint directly with the intrinsic subtyping using the original intrinsic gene set as developed by Perou et al. ,26 from which the PAM50 gene set has originated. Using 469 independent samples and two publicly available data sets (n = 215, n = 159), the authors reported 5-year survival estimates for the subtypes and for the groups further separated by high-and low-risk MammaPrint categories. They reported that samples with a ERBB2-like or basal-like gene profile showed equally poor 5-year survival rates of ∼65%. However, the ERBB2-like subset of MammaPrint low-risk patients (15%) showed an 89% (95% CI 71% to 100%) survival rate without trastuzumab treatment. When the luminal-like subtype was separated into high and low risk by MammaPrint the survival rate was 56% (95% CI 46% to 68%) for high-risk luminal-like samples and 94% (95% CI 90% to 99%) for low-risk luminal-like samples. The authors concluded that the developed multigene profile can classify breast tumours into luminal-, ERBB2- and basal-like subgroups. By combining this molecular subtyping with MammaPrint risk classification, specific groups of patients can be recognised that are at high risk of recurrence. The low-risk patients within the luminal- and ERBB2-like subclasses have a very low risk of recurrence. There are significant limitations in making any interpretations from this evidence as it is derived only from an abstract. It has been shown that there may be discrepancies between data made available in abstracts and the reporting of results in subsequently published full-length articles. 89 Because of incomplete reporting the methodological quality of studies cannot be confidently assessed by systematic reviewers.
Clinical utility
No available evidence.
Summary of evidence: MammaPrint and BluePrint
Because of the limited available data (reported in abstract form only), no firm conclusions can be drawn about the clinical validity (prognostic ability) of the MammaPrint and BluePrint test, although it does appear that the test has been validated in an independent cohort. No published evidence is yet available on the clinical utility of the test in combination with MammaPrint. Further evidence for this test is required.
PAM50 test
The PAM50 gene expression assay identifies the major intrinsic biological subtypes of breast cancer. The current version of the test provides classification of breast cancer subtype and quantitative values for (gene/protein) ESR1/ER, PGR/PR, ERBB2/HER2, proliferation score and luminal score (ER pathway). The current version does not provide a risk of recurrence score or category. The PAM50 Breast Cancer Intrinsic Classifier test is recommended for all patients diagnosed with invasive breast cancer, regardless of stage or ER status. Further details are provided in Table 6.
Description of included studies
The searches identified six studies for the PAM50 test. This included two full peer-reviewed papers,115,116 three meeting abstracts117–120 and an unpublished manuscript provided by the manufacturer. 121
The design and patient characteristics of the six included studies are provided in Tables 16 and 17 respectively. All of the reported studies had a retrospective design analysing archived tumour samples. The populations used in the studies were somewhat heterogeneous, although in most studies more patients were LN+ and ER+ than LN− and ER−. The ages of the patients varied across the studies, from a median age of 47.5 years in one paper121 to a median of 67 years in another. 116 Most studies included a moderate number of tumour samples. Follow-up was not reported for a number of studies but was around a median of 10 years in those that did report a follow-up time.
| Author (year) Country | Study design | Number of patients | Follow-up (years) | Outcomes/end points | Evidence type | Funding |
|---|---|---|---|---|---|---|
| Bernard et al. (2011)119 (abstract) Additional data from Martin et al.120 (abstract) Country NR |
Retrospective cohort (dates not reported) from randomised prospective GEICAM 9906 trial FFPE blocks with invasive breast tissues |
Eligible sample: NR Sample included: 793 Tumour samples analysed using PAM50 by RT-qPCR method |
8.7 | Analytical outcomes including accuracy, reproducibility, etc. | Analytical validity; clinical utility – predictive ability (benefit of chemotherapy) Comparison of PAM50 with IHC by RT-qPCR and identification of potential predictive markers of taxane clinical benefit |
NR |
| Cheang et al. (2011)117 (abstract) Additional data from unpublished manuscript Canada |
Retrospective cohort (dates not reported) from randomised NCIC.CTG MA.5 trial | Eligible sample: 476 Sample included: 476 Tumour samples analysed using PAM50 |
NR | Responsiveness of intrinsic subtypes to adjuvant anthracyclines vs. non-anthracyclines – tumour classification and correlation with RFS and OS | Clinical validity; clinical utility – predictive ability (benefit of chemotherapy) | NR |
| Chia et al. (2011)121 Canada, USA |
[Academic-in-confidence (AIC) information has been removed] | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | Clinical validity; clinical utility – predictive ability (benefit of chemotherapy) (AIC information has been removed) |
Canadian Breast Cancer Foundation and National Cancer Institute |
| Ebbert et al. (2011)118 (abstract) USA |
Retrospective (dates not reported) FFPE blocks with invasive (n = 155) and normal (n = 16) breast tissues (training set) |
Eligible sample: NR Sample included: 171 Tumour samples analysed using PAM50 by RT-qPCR method |
NR | Analytical outcomes including accuracy, reproducibility, etc. | Analytical validity | NR |
| Nielsen et al. (2010)116 Unpublished supplementary data from the manufacturer Canada |
Retrospective cohort (1986–1992) FFPE, minimum two tumour cores extracted |
Eligible sample: 991 Sample included: 786 (archived tumour samples) Samples excluded because of insufficient yield (n = 180), technically insufficient, (n = 25) |
Median: 11.7 | Numbers assigned to each intrinsic subtype, risk of recurrence score Comparator: clinical, IHC (ER, PR, HER2, Ki-67) Adjuvant! Online used to generate breast cancer RFS and DSS estimates for each patient |
Clinical validity – comparison of clinical, IHC and GEP models of prognosis | NR |
| Parker et al. (2009)115 Canada, USA |
Retrospective cohort (dates not reported) Fresh-frozen and FFPE tissues Independent publicly available data sets including data sets previously reported by Loi, Wang, and Ivs.hima |
Eligible sample: NR Sample included: 950 G1: 189 (training set for development of the subtype predictor); G2: 761 |
NR | Distribution of intrinsic subtypes compared with clinical marker status risk of relapse models for prognosis in LN− breast cancer | Clinical validity | NR |
| Author (year) | Age (years), mean (SD) | LN status | ER status | Tumour size | Grade | HER2 status | Mean NPI score | Treatment |
|---|---|---|---|---|---|---|---|---|
| Bernard et al. (2011)119 (abstract) Additional data from Martin et al.120 (abstract) |
NR | All LN+ | NR | NR | NR | NR | NR | NR |
| Cheang et al. (2011)117 Additional data from unpublished manuscript |
NR (AIC information has been removed) |
All LN+ | +/−/missing: (AIC information has been removed) | NR | I: (AIC information has been removed); II: (AIC information has been removed); III: (AIC information has been removed); unknown: (AIC information has been removed) | NR | NR | Adjuvant cyclophosphamide, methotrexate and 5-fluorouracil (CMF) or adjuvant cyclophosphamide, epirubicin and 5-fluorouracil (CEF) chemotherapy after randomization |
| Chia et al. (2011)121 | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) |
| Ebbert et al. (2011)118 (abstract) | NR | NR | NR | NR | NR | NR | NR | NR |
| Nielsen et al. (2010)116 | Median 67 (range NR) | +/−/unknown: 511/222/53 | +/−/missing: 768/9/9 | Median 2.1 cm | I: 34; II: 338; III: 370; unknown: 44 | +/−/unknown: 75/696/15 | NR | All patients had adjuvant tamoxifen |
| Parker et al. (2009)115 | G1: 58 (15); G2: 53 (13) | +/−: G1: 96/100;aG2: 35/710a | +/−: G1: 114/77;aG2: 544/195a | ≤/> 2 cm: G1: 63/136;a G2: 408/339a | Low/medium/high: G1: 12/56/127;a G2: 133/218/286a | +/−: G1: NA; G2: 66/192a | NR | G1: training set G2: no adjuvant systemic therapy |
Quality of included studies: PAM50
The methodological quality of the six included studies (seven citations)115–121 is summarised in Figure 5 (further details are provided in Appendix 11). Of these, two studies performed well,117,121 receiving a positive assessment for at least 17 out of 21 methodological quality items. Although all studies used a retrospective study design, other potential sources of bias were generally related to the following domains: sample of patients (all eligible patients were not included), outcomes (not fully defined) and interventions subsequent to inclusion in the cohort (interventions were not fully described or standardised). Overall, the risk of bias from these studies was judged to be moderate.
FIGURE 5.
PAM50 test: methodological quality graph (review authors' judgements about each methodological quality item presented as percentages across all studies).
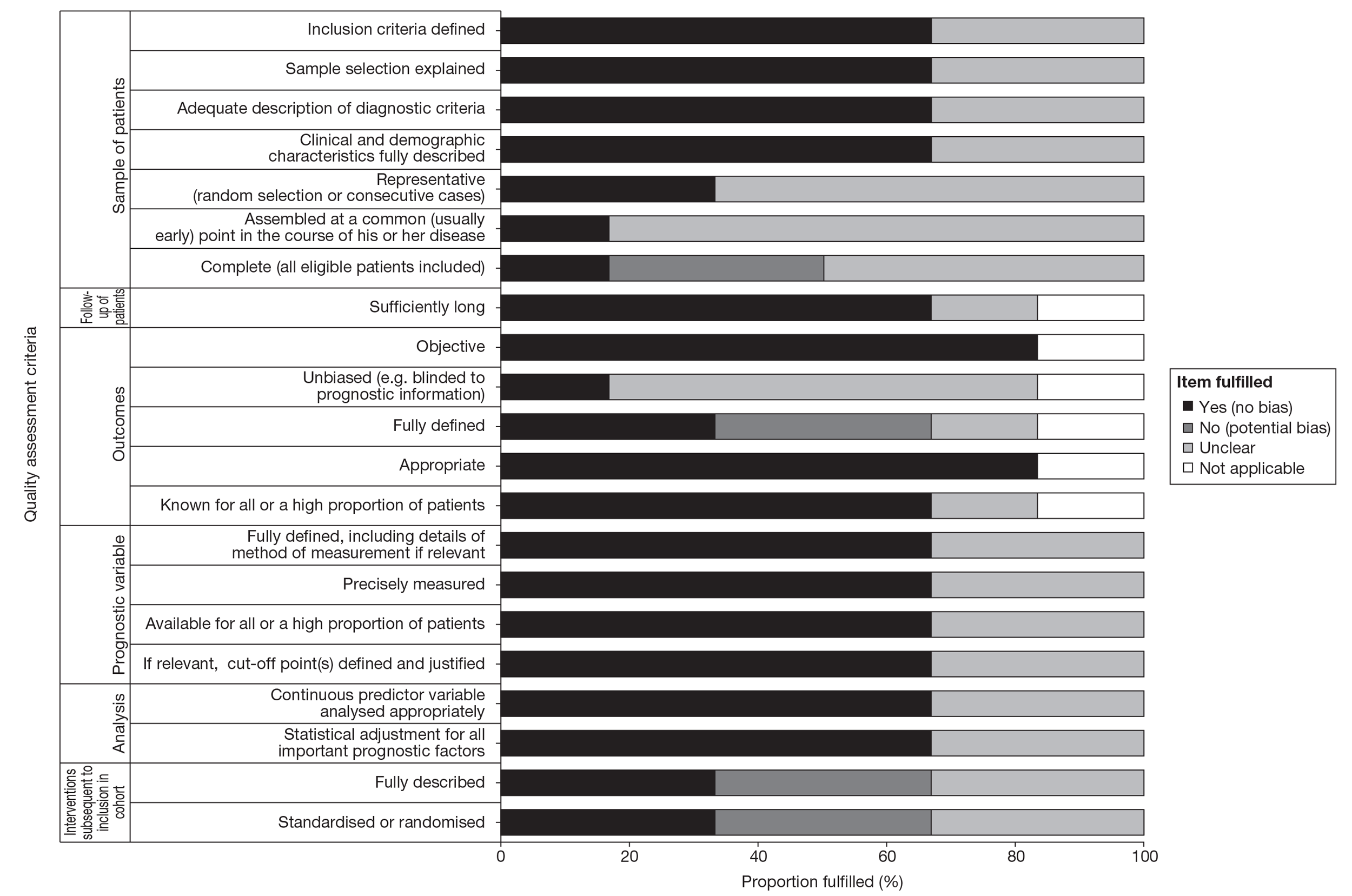
Results: PAM50
A summary of the clinical evidence for PAM50 is presented in Table 18 followed by a narrative summary of each study. Full data extraction tables are provided in Appendix 11.
| Author (year) | Evidence type | Overall quality | Key findings |
|---|---|---|---|
| Bernard et al. (2011)119 (abstract) and Martin et al.120 (abstract) | Analytical validity; clinical utility – predictive ability (benefit of chemotherapy) Comparison of PAM50 by RT-qPCR with IHC and identification of potential predictive markers of taxane clinical benefit |
Low | 793 LN+ patients from the GEICAM 9906 randomised trial. Bernard et al. reported agreement between RT-qPCR gene expression and IHC scoring for clinical markers. They showed that there was good agreement between (gene/protein) ESR1/ER, PGR/PR and ERBB2/HER2. The accuracy was significantly lower for MKI67/Ki-67, EGFR/EGFR and KRT5/CK5/6. The authors concluded that calling cut-points based on RT-qPCR expression across subtypes is reproducible across data sets and has good agreement with expression by IHC for clinically used biomarkers. Martin et al. reported (AIC information has been removed). The authors concluded that (AIC information has been removed). Limitations: abstract data |
| Cheang et al. (2011)117 (AIC information has been removed) | Clinical validity; clinical utility – predictive ability (benefit of chemotherapy) (AIC information has been removed) |
High | (AIC information has been removed) |
| Chia et al. (2011)121 | Clinical validity; clinical utility – predictive ability (benefit of tamoxifen) (AIC information has been removed) |
High | (AIC information has been removed) |
| Ebbert et al. (2011)118 (abstract) | Analytical validity | Low | 171 US patients. Reported that within-platform cross-validation of the clinical subtype predictor showed 91.6% concordance. There was 100% reproducibility of subtype predictions across 46 runs testing different subtypes. Subtype predictions across platforms showed 88.1% concordance. The authors concluded that the PAM50 Breast Cancer Intrinsic Classifier is highly reproducible within and across platforms. Limitations: small sample size, abstract data |
| Nielsen et al. (2010)116 (additional data from unpublished version of the manuscript) | Clinical validity Comparison of clinical, IHC and GEP models of prognosis |
Moderate | 786 LN+ or higher-risk LN−, ER+ Canadian patients. Assessed numbers of patients assigned to each intrinsic subtype and risk of relapse score against IHC (ER, PR, HER2, Ki-67). Reported that the included patients were considered to be high risk with overall 10- year RFS of 62% and DSS of 72%. Those assigned to luminal A tumours had significantly better outcomes (10-year RFS 74%; DSS 83%) than those assigned to luminal B, HER2-enriched and basal-like tumours. The authors concluded that IHC approaches do work and provide significant prognostic information; however, PAM50 is superior to these in terms of adding significant additional information and in its capacity to identify a particularly low-risk group. Incorporated a relatively large sample |
| Parker et al. (2009)115 | Clinical validity | Moderate | 950 majority LN−, ER+ Canadian and US patients. Reported that the intrinsic subtypes showed prognostic significance (for RFS) in untreated (no systemic therapy) patients (p< 0.0001) and remained significant in multivariable analyses that incorporated clinical covariates (ER status, histological grade, tumour size and LN status) (p< 0.0001). The authors concluded that the intrinsic subtype and risk predictors based on the PAM50 gene set add significant prognostic and predictive value to pathological staging, histological grade and standard clinical molecular markers |
Analytical validity
Ebbert et al. 118 reported an analytical validity study using 171 tumour samples from US patients. They reported that within-platform cross-validation of the clinical subtype predictor showed 91.6% concordance. There was 100% reproducibility in subtype predictions across 46 runs testing different subtypes. Subtype predictions across platforms showed 88.1% concordance. Dilution experiments, introducing ‘normal’ breast tissue RNA into breast cancer RNA, showed a systematic switch towards the ‘normal’ signature, with luminal A and luminal B subtypes being most susceptible. The authors concluded that the PAM50 Breast Cancer Intrinsic Classifier is highly reproducible within and across platforms and that the clinical test has utility in the management of ER+ and ER− invasive breast cancer of all stages. The quality of this report was judged as low. Furthermore, the study was based on a small number of tumour samples and full details of the patient characteristics were not provided. In addition, there are significant limitations in making any interpretations from this evidence as it is derived only from an abstract. It has been shown that there may be discrepancies between data made available in abstracts and the reporting of results in subsequently published full-length articles. 89 Because of incomplete reporting the methodological quality of studies cannot be confidently assessed by systematic reviewers.
Bernard et al. ,119 using a cohort of 793 LN+ tumour samples from the GEICAM 9906 randomised trial, reported agreement between reverse transcription-quantitative polymerase chain reaction (RT-qPCR) gene expression and IHC scoring for clinical markers. They showed that there was good agreement between (gene/protein) ESR1/ER, PGR/PR and ERBB2/HER2. The accuracy was significantly lower for MKI67/Ki-67, EGFR/EGFR and KRT5/CK5/6. Discrepancies between the Hercep test and chromogenic in situ hybridisation (CISH) procedure for 2+ and 3+ staining-intensity samples showed that RT-qPCR agreed better with the Herceptest [area under the curve (AUC): 0.95 vs. 0.93). The authors concluded that calling cut-points based on RT-qPCR expression across subtypes is reproducible across data sets and has good agreement with expression by IHC for clinically used biomarkers. The quality of this report was judged as low. Although the study benefits from a relatively large sample size, as these data were reported in abstract form only there are significant limitations in using these results to make generalisations.
Clinical validity
Parker et al. 115 investigated the distribution of intrinsic subtypes in comparison with clinical marker status and risk of relapse models for prognosis in a cohort of 950 Canadian and US majority LN−, ER+ breast cancer patients. They reported that the intrinsic subtypes showed prognostic significance (for recurrence-free survival, RFS) in untreated (no systemic therapy) patients (p < 0.0001) and remained significant in multivariable analyses that incorporated clinical covariates (ER status, histological grade, tumour size and node status) (p < 0.0001). The authors concluded that the intrinsic subtype and risk predictors based on the PAM50 gene set add significant prognostic and predictive value to pathological staging, histological grade and standard clinical molecular markers. The quality of this study was judged to be moderate and the sample size was relatively large.
Nielsen et al. 116 used a cohort of 786 LN+ or higher-risk LN−, ER+ Canadian patients with tumours collected between 1986 and 1992 to assess the numbers of patients assigned to each intrinsic subtype and risk of relapse score against IHC (ER, PR, HER2, Ki67). Adjuvant! Online was used to generate breast cancer recurrence-free survival and disease-specific survival estimates for each patient. They reported that the included patients were considered to be high risk, with overall 10-year RFS of 62% and disease-specific survival of 72%. Those assigned to luminal A tumours had significantly better outcomes (10-year RFS 74%; disease-specific survival 83%) than those assigned to luminal B, HER2-enriched and basal-like tumours. In Cox models incorporating standard prognostic variables, HRs for breast cancer disease-specific survival over the first 5 years of follow-up, relative to the most common luminal subtype, were 1.99 (95% CI 1.09 to 3.64) for the luminal B subtype, 3.65 (95% CI 1.64 to 8.16) for the HER2-enriched subtype and 17.71 (95% CI 1.71 to 183.33) for the basal-like subtype (p = 0.0018). The authors concluded that IHC approaches do work and provide significant prognostic information; however, PAM50 is superior to these in terms of adding significant additional information and in its capacity to identify a particularly low-risk group. This study was judged to be of moderate quality and incorporated a relatively large sample size.
Chia et al. 121 (AIC information has been removed).
Cheang et al. 117 (AIC information has been removed).
Clinical utility
In addition to the clinical validity data reported above, Chia et al. 121 (AIC information has been removed).
(AIC information has been removed.) Using the same data reported by Bernard et al. 119 for a cohort of 793 LN+ tumour samples, Martin et al. 120 (AIC information has been removed). The quality of this report was judged as low. Although the study benefits from a relatively large sample size, as these data were reported in abstract form only there are significant limitations in using these results to make generalisations.
Cheang et al. 117 (AIC information has been removed).
Summary of evidence: PAM50
Analytical validity of PAM50
Two abstracts118,119 reported data on analytical validity, both rated as low quality. One118 employed a relatively small sample (n = 171) whereas the other119 was based on a much larger sample (n = 793). These studies provide a comparison of PAM50 against standard IHC measurements. There are significant limitations in making any interpretations from this evidence as it is derived only from abstracts. It has been shown that there may be discrepancies between data made available in abstracts and the reporting of results in subsequently published full-length articles. 89 Because of incomplete reporting the methodological quality of studies cannot be confidently assessed by systematic reviewers.
Clinical validity (prognostic ability) of PAM50
Four studies,115–117,121 two rated as high quality and two rated as low quality, were identified that contain data on clinical validity. Two of these are as yet unpublished. These studies demonstrate that the intrinsic subtypes are significantly associated with outcome, provide additional information to IHC approaches and standard clinicopathological measures and can identify a particularly low-risk group. They demonstrate that prognostic ability has been validated in external cohorts. However, the population in most of the studies was LN+, with the exception of that by Parker et al. ,115 who assessed LN− patients; therefore, the generalisability of these findings to LN−, ER+ patients is limited. Furthermore, no studies were UK based, limiting the generalisation of the findings to UK practice. Finally, as two of these studies were unpublished there are significant limitations in drawing conclusions from this evidence.
Clinical utility of PAM50
(AIC information has been removed).
Key gaps in the evidence base remain and are summarised below:
-
The evidence base to date is still immature as the majority of the evidence presented here is in abstract or unpublished form only. Only two studies were considered to be of high quality (these presented clinical validity and clinical utility data), two were of moderate quality and two were of low quality, although it should be noted that only two studies were full peer-reviewed papers.
-
Further evidence around analytical validity is required as the current evidence is based on abstracts only.
-
Robust evidence of clinical utility is needed. It is not yet clear whether or not the use of the PAM50 test will change the management of patients, for example reduce the number of patients unnecessarily treated with chemotherapy or improve patient outcomes through increases in DFS and OS.
-
The fact that no studies were conducted in a UK setting may limit the extent to which we can generalise the findings.
Overall summary
The evidence base, in particular in relation to the prognostic ability of the test, is developing.
None of the studies used UK-based patients and the data were all based on cohort studies. Most of the evidence is in LN+ patients. The main limitation is that currently most of the evidence is unpublished or is in abstract form only. Robust evidence of clinical utility is needed as it is not yet clear to what extent the use of the PAM50 test will change the management of patients, to what extent PAM50 subtypes are predictive of chemotherapy benefit or how the use of PAM50 will improve patient outcomes through increases in DFS and OS.
Breast Cancer Index
The BCI is a RT-PCR assessment of the ratio of expression of two genes, HOXB13 and IL17BR, combined with the MGI and gives an indication of recurrence risk. The target population is those with ER+ and LN− early breast cancer. The BCI RS ranges from 0 to 10 and divides patients into three risk groups. Low risk is defined as a score < 5; intermediate risk as a score of 5–6.3; and high risk as a score ≥ 6.4. Further details are included in Table 6.
Description of included studies
The searches identified one full peer-reviewed study relating to the BCI. 122 The design and patient characteristics of the included study are provided in Tables 19 and 20.
| Author (year) Country | Study design | Number of patients | Follow-up (years) | Outcomes/end points | Evidence type | Funding |
|---|---|---|---|---|---|---|
| Jerevall et al. (2011)122 Sweden |
Retrospective cohort (1976–1990) from the randomised, prospective Stockholm trial FFPE RT-PCR |
Eligible sample: 808 Sample included: 588 G1: 314; G2: 274 Samples excluded because of insufficient tumour (n = 37); failure of RT-PCR, (n = 2); ER− cases (n = 181) (exclusion criterion of study) |
Mean: 17 | Time to distant metastasis; DMFS; BCSD | Clinical validity | Swedish Cancer Society, Swedish Research Council, King Gustaf V Jubilee Fund, National Cancer Institute, Avon Foundation and bioTheranostics |
| Author (year) | Age (years), mean (SD) | LN status | ER status | Tumour size | Grade | HER2 status | Mean NPI score | Treatment |
|---|---|---|---|---|---|---|---|---|
| Jerevall et al. (2011)122 | NR (all postmenopausal) | All LN− | All ER+ | G1: ≤ 2 cm: 256 (82%); > 2 cm: 55 (18%); unknown: 3 (1%) G2: ≤ 2 cm: 223 (81%); > 2 cm: 49 (18%); unknown: 2 (1%). |
G1: I: 67 (21%); II: 209 (67%); III: 38 (12%) G2: I: 67 (24%); II: 172 (63%); III: 35 (13%) |
G1: +/−/unknown: 14 (4%)/272 (87%)/28 (9%) G2: +/−/unknown: 13 (5%)/238 (87%)/23 (8%) |
NR | G1: tamoxifen G2: systemically untreated |
Quality of included studies: Breast Cancer Index
Although the assessment of study quality was hindered by poor reporting of whether or not outcomes were unbiased, the overall methodological quality of the included study was judged to be high, indicating a low risk of bias (received a positive assessment for at least 19 out of 21 methodological quality items).
Results: Breast Cancer Index
Full data extraction tables are presented in Appendix 12.
Analytical validity
No available evidence.
Clinical validity (prognostic ability)
Jerevall et al. 122 reported a retrospective analysis of (a subcohort of) a randomised prospective trial cohort. The 588 patients were all postmenopausal, LN− and ER+. The authors reported the development and testing of HOXB13:IL17BR plus MGI as a continuous index (BCI) in a training set (G1) and a test set (G2) of the same trial data. In the training set (G1) BCI classified 59% of patients as low risk. The rate of distant recurrence for the low-risk group was 7.1% (95% CI 0% to 3.5%) and the rate of death was 1.1% (95% CI 0% to 2.6%). In total, 22% were classified as intermediate risk, with a rate of distant recurrence of 17.8% (95% CI 7.6% to 26.8%) and a rate of death of 14.5% (95% CI 5.2% to 22.9%), and 18.4% were classified as high risk, with a rate of distant recurrence of 20.0% (95% CI 8.7% to 30.0%) and a rate of death of 14.7% (95% CI 4.7% to 23.6%). In the test set (G2) 53% of patients were classified as low risk (rate of distant recurrence 8.3%, 95% CI 4.7% to 14.4%; rate of death 5.1%, 95% CI 1.3% to 8.7%), 27% were intermediate risk (rate of distant recurrence 22.9%, 95% CI 14.5% to 35.2%; rate of death 19.8%, 95% CI 10.0% to 28.6%) and 20% were high risk (rate of distant recurrence 28.5%, 95% CI 17.9% to 43.6%; rate of death 28.8%, 95% CI 15.3% to 40.2%). The authors concluded that the BCI was a strong prognostic factor for distant recurrence and BCSD, independent of tumour size, grade, HER2 status and PR status (although tumour size did contribute prognostic value to distant recurrence). The prognostic utility of the BCI was also assessed compared with Adjuvant! Online for the test set (G2). Both BCI and Adjuvant Online were significant predictors of BCSD (BCI: HR 2.3, 95% CI 1.5 to 3.7, p < 0.001; Adjuvant Online: HR 1.4, 95% CI 1.0 to 1.8, p = 0.04) and distant recurrence (BCI: HR 2.0, 95% CI 1.3 to 3.1, p = 0.001; Adjuvant! Online: HR 1.4, 95% CI 1.0 to 1.8, p0.03). The authors concluded that the retrospective analysis of this randomised, prospective trial cohort validated the prognostic utility of HOXB13:IL17BR plus MGI and it was used to develop and test a continuous risk model that enables prediction of distant recurrence risk at the patient level. The study had a long mean follow-up time and a moderate sample size. The study used tumour samples that dated back to 1976, introducing differences in the diagnostic criteria applied to patients.
Clinical utility
No available evidence.
Summary of evidence: Breast Cancer Index
Based on the limited available data, no firm conclusions can be made about the BCI. Further evidence on analytical validity, clinical validity and clinical utility is required. The test has also not been validated in an external cohort.
Randox Breast Cancer Array
The Randox BCA is a cDNA-based expression biochip assay that aims to accurately define the clinical subtype of breast cancer tumour before initiating treatment. The target population is all individuals with diagnosed breast cancer.
Description of included studies
The searches did not identify any relevant full peer-reviewed papers or meeting abstracts relating to the Randox assay. Supplementary evidence was provided by the manufacturer of the test.
Results: the Randox assay
Supplementary evidence
The manufacturer submitted a description of the data gathered on the Randox assay up to August 2011. 123 A summary of this information is provided here.
The objective of the study was to improve the discrimination, to include basal and unclassified (triple-negative) types, subdivide luminal groups into A and B and assign samples to an ERBB2 group. The main indicator of correct typing is whether the samples are correctly typed as ER+ or ER−. A total of 150 archived tumour samples were collected and used on the Randox biochip array. Exclusion criteria included a lack of ER status information or one or both of the housekeeping genes failing to reach adequate expression levels, preventing normalisation of the remaining gene set on the chip. The sample set included information on the following: DFS, OS ER status, PR status and other standard clinical measurements. However, HER2 (ERBB2) status was not available for any patients; thus, hormonal status was either luminal positive or negative. The initial summary, using a patient cohort of 78 individuals, shows an overall agreement of 79% between hormonal status (primarily ER) and the multiplex biochip assay. The authors concluded that these findings were encouraging.
Methodological detail was lacking for the summarised study and the sample size was very small. Quality assessment could not be undertaken because of the limited detail available. It should also be noted that, although Randox separates luminal A and luminal B groups, the overall reported agreement of 79% was based on luminal A and B combined and hormonal status, and did not differentiate between the subtypes.
Summary of evidence: the Randox assay
No conclusions can be drawn from this evidence. Further evidence is required.
Mammostrat test
The Mammostrat test uses five IHC markers (SLC7A5, HTF9C, p53, NDRG1 and CEACAM5) to stratify patients into risk groups to inform treatment decisions. These markers are independent of one another and do not directly measure either proliferation or hormone receptor status. The current version of the test provides classification into one of five breast cancer subtypes, and quantitative values for (gene/protein) ESR1/ER, PGR/PR, ERBB2/HER2, proliferation, and luminal score (ER pathway), along with a RS and category (low, moderate and high). For further information see Table 7.
Description of included studies
The searches identified three full peer-reviewed studies relating to the Mammostrat test. 124–126 All studies contained data relating to clinical validity. Ross et al. 126 also reported on clinical utility in terms of the predictive ability of the test by risk group. The studies are described in Tables 21 and 22.
| Author (year) Country | Study design | Number of patients | Follow-up (years) | Outcomes/end points | Evidence type | Funding |
|---|---|---|---|---|---|---|
| Bartlett et al. (2010)124 UK |
Retrospective, consecutive sample series (1981–98) Microarray |
Eligible sample: 1540 Sample included: 1540 G1 (all ER+): 1189; G2 (ER+, tamoxifen only): 831; G3 (ER+, N−, tamoxifen only): 657 |
Minimum 9 | DRFS; RFS; OS | Clinical validity | Sarah Percy Endowment Fund |
| Ring et al. (2006)125 [Commercial-in-confidence (CIC) information has been removed] USA |
Retrospective cohort (G1: 1989–2002; G2: 1995–6; G3: 1974–95) | Eligible sample: NR Sample included: 1109 G1: 466 (also training cohort); G2: 299; G3: 344 |
G1: NR; G2: 5; G3: mean 11.7 | DFS at 5 years | Clinical validity | NR |
| Ross et al. (2008)126 NR |
Retrospective cohort (dates not specified) from the randomised, prospective NSABP B14 and B20 trials | Eligible sample: NR Sample included: 1267 Placebo: 287; B14 tamoxifen: 550; B20 tamoxifen: 161; B20 chemotherapy: 269 |
Minimum 10 | Recurrence-free interval; DRFI; BCSD | Clinical validity; clinical utility – predictive ability (benefit of chemotherapy) | NR |
| Author (year) | Age (years) | LN status | ER status | Tumour size | Grade | HER2 status | Mean NPI score | Treatment |
|---|---|---|---|---|---|---|---|---|
| Bartlett et al. (2010)124 | < 50/> 50/missing All cases: 660 (42.8%)/879 (57.1%)/1 (0.1%) G1: 505 (42.5%)/683 (57.4%)/1 (0.1%) G2: 284 (34.2%)/547 (65.8%)/0 G3: 243 (37.0%)/414 (63.0%)/0 |
LN−/1–3/4–9/10+/missing All cases: 1164 (75.6%)/321 (20.8%)/44 (2.9%)/9 (0.8%)/38 (2.5%) G1: 889 (74.8%)/264 (22.2%)/29 (2.4%)/5 (0.4%)/2 (0.2%) G2: 657 (79.1%)/154 (18.5%)/17 (2.0%)/3 (0.4%) G3: 657 (100%) |
Allred score < 2/3–5/6–8/missing All cases: 278 (18.1%)/347 (22.5%)/823 (53.4%)/92 (6.0%) G1: 19 (1.6%)/347 (29.2%)/823 (69.2%) G2: 15 (1.8%)/213 (25.6%)/603 (72.6%) G3: 14 (2.1%)/174 (26.5%)/469 (71.4%) |
< 2 cm/> 2 cm/missing All cases: 1150 (74.7%)/314 (20.4%)/76 (4.9%) G1: 903 (75.9%)/224 (18.8%)/62 (5.2%) G2: 648 (78.0%)/148 (17.8%)/35 (4.2%) G3: 531 (80.8%)/99 (15.1%)/27 (4.1%) |
I/II/III/missing All cases: 411 (26.7%)/710 (46.1%)/381 (24.7%)/38 (2.5%) G1: 359 (30.2%)/581 (48.9%)/233 (19.6%)/16 (1.3%) G2: 269 (32.4%)/416 (50.1%)/135 (16.2%)/11 (1.3%) G3: 215 (32.7%)/323 (49.2%)/109 (16.6%)/10 (1.5%) |
NR | NR | Breast-conservation surgery, axillary node sampling or clearance, and whole-breast radiotherapy 1102 treated with adjuvant tamoxifen without chemotherapy, 92 with other hormone therapy and 197 no adjuvant hormone therapy or chemotherapy |
| Ring et al. (2006)125 | < 50/≥ 50 G2: 74 (25%)/225 (75%)G1: 135 (29%)/327 (71%) G3: not available |
LN−/N1/N2/N3/unknown G1: 264 (58%)/184 (40%)/8 (2%)/0/10 G2: 170 (62%)/68 (25%)/24 (9%)/12 (4%)/25 G3: 200 (66%)/0/103 (34%)/0/0 |
+/+LN−/− G1: 325 (70%)/195 (42%)/141 (30%) G2: 232 (78%)/137 (46%)/67 (22%) G3: 273 (79%)/159 (46%)/71 (21%) |
T1/T2/T3/T4/unknown G1: 242 (54%)/173 (39%)/21 (5%)/12 (3%)/18 G2:167 (57%)/96 (33%)/19 (6%)/13 (4%)/4 G3: not available |
I/II/III/unknown G1: 59 (16%)/168 (45%)/148 (39%)/91 G2: 45 (18%)/120 (48%)/83 (33%)/51 G3: not available |
+/−/unknown (+ = 2–3 nodes; − = 0–1 nodes) G1: 378 (85%)/68 (15%)/20 G2: 253 (88%)/34 (12%)/12 G3: not available |
Good/moderate/poor/unknown G1: 120 (36%)/156 (47%)/55 (17%)/135 G2: not available G3: 69 (38%)/164 (58%)/31 (11%)/80 |
NR |
| Ross et al. (2008)126 | NR (% of cohort only) < 50/50–59/> 60: 32/27/41 |
NR | NR (% of cohort only – fmol/mg) 10–49/50–99/> 100: 37/22/41 |
NR (% of cohort only) < 2 cm/2.1– 4.0 cm/> 4.1 cm: 59/35/5 |
NR | NR | NR | Placebo: 287; tamoxifen treated (B14 and B20): 711; chemotherapy (B20): 269 |
Quality of included studies: Mammostrat
The methodological quality of the three included studies124–126 is summarised in Figure 6 (further details are provided in Appendix 13). Of these, two studies performed well,124,126 receiving a positive assessment for at least 17 out of 21 methodological quality items. Although all studies used a retrospective study design, other potential sources of bias were generally related to the following domains: sample of patients (clinical/demographic characteristics not fully described) and interventions subsequent to inclusion in the cohort (interventions were not fully described or standardised). The assessment of study quality was generally hampered by poor reporting of the following methodological items: whether or not all eligible patients were included, representative and assembled at an early point in the course of their disease and whether or not outcomes were unbiased and known for all or a high proportion of patients. Overall, the risk of bias from these studies was judged to be low.
FIGURE 6.
Mammostrat test: methodological quality graph (review authors' judgements about each methodological quality item presented as percentages across all studies).
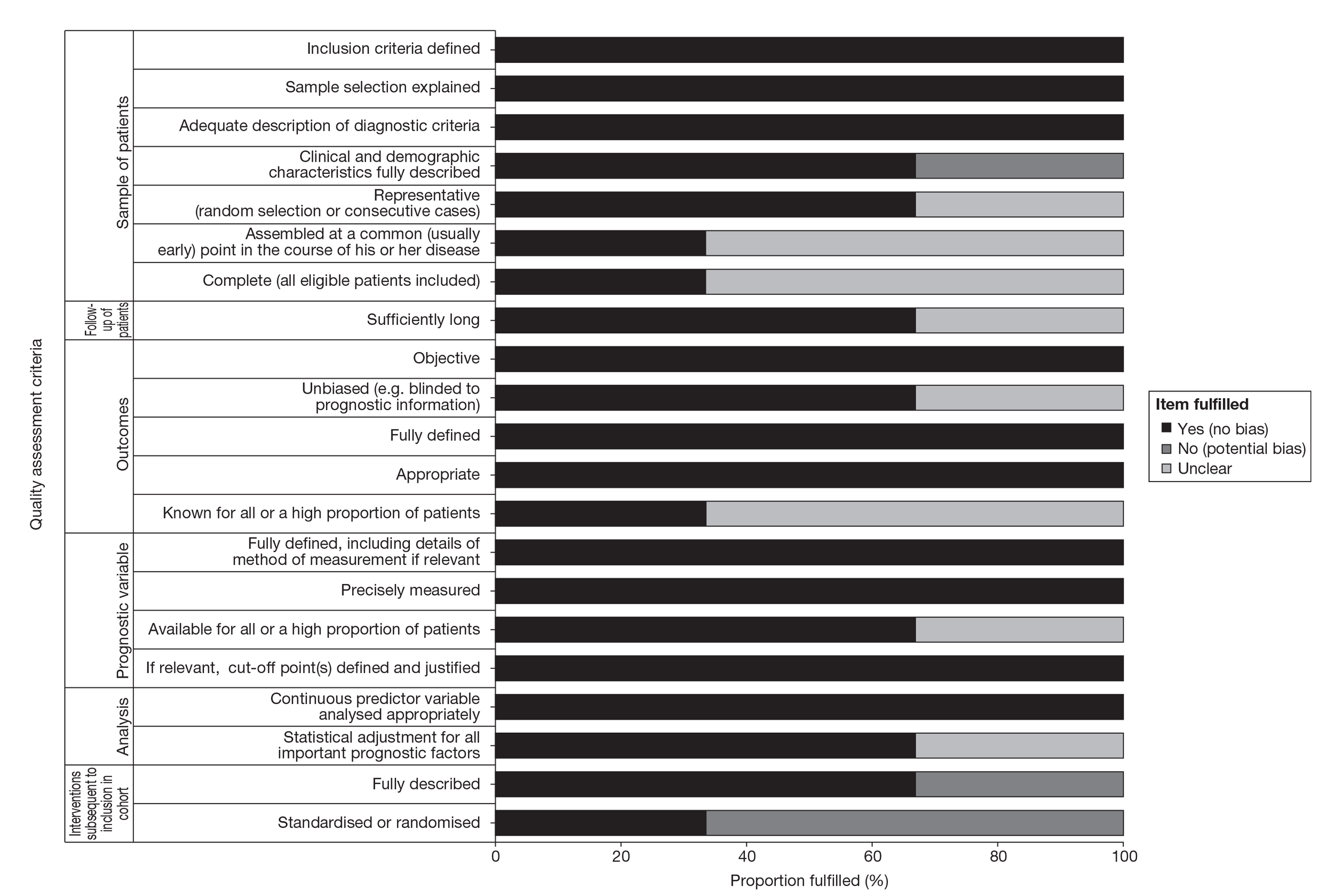
Results: Mammostrat
A summary of the clinical evidence for the Mammostrat test is presented in Table 23 followed by a narrative summary of each study. Full data extraction tables are provided in Appendix 13.
| Author (year) | Evidence type | Overall quality | Key findings |
|---|---|---|---|
| Bartlett et al. (2010)124 | Clinical validity | High | 1540 LN− UK patients. Also looked at associations between risk score and outcomes across the different groups: all cases, all ER+, all ER+ treated with tamoxifen only and ER+, LN− treated with tamoxifen only. For all cases and across the three groups there were significant associations between risk score and RFS, DRFS and OS (with the exception of the ER+, LN− treated with tamoxifen only group for which there was only a trend for OS). The authors concluded that Mammostrat can act as an independent prognostic tool for ER+, tamoxifen-treated breast cancer and that there is a possible association with outcome regardless of node status and ER− tumours. This study had a large sample size overall, a relatively long follow-up of 9 years and employed UK patients. Limitations: subsets analysed had relatively small numbers of patients within each; the samples used in these analyses were relatively old, dating back to 1981, and therefore there may be differences between the patients in terms of stage at presentation and diagnosis |
| Ring et al. (2006)125 | Clinical validity | Moderate | 1109 majority LN−, ER+ US patients. Cox model was able to identify patients classified in different risk categories based on outcomes. In both independent cohorts the model was independent of stage, grade and LN status. The authors concluded that the test can significantly improve on traditional prognosticators in predicting outcome for ER+ breast cancer patients. Large sample of patients. Validated in an external cohort. Limitation: tumour samples used in these analyses dated back as far as 1974 and therefore there may be differences between the patients in terms of stage at presentation and diagnosis |
| Ross et al. (2008)126 | Clinical validity; clinical utility – predictive ability (benefit of chemotherapy) | High | 711 ER+, LN−, tamoxifen-treated patients taken from the NSABP B14 and B20 trials. In multivariate analyses the Mammostrat test had significant prognostic power independent of age and tumour size (HR 1.3; 95% CI 1.1 to 1.6; p = 0.007). Concluded that the risk index was significantly associated with clinical outcome among the ER+, LN−, tamoxifen-treated patients. Clinical utility: in terms of recurrence-free interval, patients in the low-risk group improved by 5% from 86% to 91% (HR 0.4; 95% CI 0.2 to 0.8) and patients in the high-risk group improved by 21% from 64% to 85% (HR 0.4; 95% CI 0.2 to 0.9), showing that these groups benefited from chemotherapy, whereas the patients in the intermediate risk group did not. Limitation: data from two trials were used but it was unclear how the data were combine |
Analytical validity
No available evidence.
Clinical validity
Bartlett et al. 124 investigated assignment to risk groups using Mammostrat for 1540 UK patients with LN− tumours. They demonstrated that significantly more cases were assigned to high-risk groups for ER− than for ER+ cases (45% vs. 16%; p < 0.001), but there were no differences between other groups. They also looked at associations between risk scores and DRFS, RFS and OS across the different groups: all cases, all ER+ cases, all ER+ cases treated with tamoxifen only and ER+, LN− cases treated with tamoxifen only. For all groups there were significant associations between risk score and RFS, DRFS and OS (with the exception of the ER+, LN− treated with tamoxifen only group for which there was a trend only for OS). Multivariate analyses for each of the four groups showed that risk score was a significant independent predictor of RFS, DRFS and OS, along with clinicopathological predictors, for all cases and all ER+ cases. Risk score was a significant independent predictor of DRFS and OS with a trend for RFS for ER+ cases treated with tamoxifen only, and there was a trend towards significance for Mammostrat score to predict RFS and DRFS in ER+, LN− cases treated with tamoxifen only. The authors concluded that Mammostrat can act as an independent prognostic tool for ER+, tamoxifen-treated breast cancer and that there is a possible association with outcome regardless of LN status and ER− tumours. This study was rated as being of high quality on the basis of the quality assessment employed here. It also benefits from a large sample size overall, although it should be noted that the subsets analysed had relatively small numbers of patients within each. It had a relatively long follow-up of 9 years, and as it employed UK patients the findings should be applicable to UK practice. The samples used in these analyses were relatively old, dating back to 1981; therefore, there may be differences between the patients in terms of stage at presentation and diagnosis.
In a US-based study, Ring et al. 125 assessed 1109 majority LN−, ER+ patients. Using a training cohort the authors demonstrated that a Cox model identified a group of patients as having either poor or moderate prognosis, with a 5-year DFS rate of approximately 75%, as opposed to patients classified as having good prognosis, who had a 5-year DFS rate of approximately 95% (p < 0.001). In the first independent cohort the model identified poor prognosis patients with a 5-year DFS rate of 50%, compared with approximately 70% for patients classified as having moderate prognosis and 87% for patients classified as having good prognosis (p = 0.008). In the second independent cohort the model distinguished ER+ patients classified as having poor prognosis with OS rates of 55%, compared with 75% for patients classified as having moderate prognosis and 90% for patients classified as having good prognosis (p = 0.0039). In both independent cohorts the model was independent of stage, grade and LN status. In the combined independent cohort, for patients with poor or good prognosis (82%), sensitivity for poor prognosis in predicting disease progression was 38%, whereas specificity was 88%. The PPV of poor prognosis was 38% (95% CI 32% to 44%), whereas the NPV was 88% (95% CI 84% to 92%). The authors concluded that the test can significantly improve on traditional prognosticators in predicting outcome for ER+ breast cancer patients. The quality assessment indicated that this study was of moderate quality and it used a large sample of patients. The test was also validated in an external cohort. However, there are limitations in that the tumour samples used in these analyses date back as far as 1974; therefore, there may be differences between the patients in terms of stage at presentation and diagnosis.
Ross et al. 126 examined the association between the clinical outcomes recurrence-free interval (RFI), DRFI and BCSD and stratification by the Mammostrat test in 711 ER+, LN− tamoxifen-treated patients taken from the NSABP B14 and B20 trials. Of this group approximately 58% were classified as low risk, 21% as moderate risk and 21% as high risk. There was a significant association between patients stratified by the Mammostrat test and RFI (HR 1.3; 95% CI 1.1 to 1.6; p = 0.006). This was not significant in the low-risk group compared with the moderate-risk group (log-rank, p = 0.05) but was significant in the low-risk group compared with the high-risk group (HR 1.8; 95% CI 1.2 to 2.6). The authors reported a significant association between patients stratified by the test and DRFI (HR 1.4, 95% CI 1.1 to 1.7; p = 0.001). In the low-risk group compared with the moderate-risk group this was not significant whereas in the high-risk group compared with the low-risk group it was significant (HR 2.1, 95% CI 1.4 to 3.1; p = 0.0004). They also reported a significant association between patients stratified by the test and BCSD (HR 1.5, 95% CI 1.2 to 1.9; p = 0.0003). In the low-risk group compared with the moderate-risk group this was not significant whereas in the high-risk group compared with the low-risk group it was significant (HR 2.3; 95% CI 1.5 to 3.5; p < 0.0001). The Kaplan–Meier estimate of the proportion of patients recurrence free after 10 years was 82% (95% CI 79% to 85%) for the group overall, 85% (95% CI 81% to 88%) for the low-risk group, 85% (95% CI 80% to 91%) for the moderate-risk group and 73% (95% CI 65% to 80%) for the high-risk group. In multivariate analyses the Mammostrat test had significant prognostic power independent of age and tumour size (HR 1.3; 95% CI 1.1 to 1.6; p = 0.007). The authors concluded that the risk index was significantly associated with clinical outcome among the ER+, LN− tamoxifen-treated patients. Ross et al. 126 also reported data relating to clinical utility, which are detailed in the following section.
Clinical utility
Ross et al. 126 also presented evidence on the ability of the test to identify patients who have greater absolute benefit from adjuvant chemotherapy compared with unstratified patient populations. These analyses were based on the tamoxifen- and cytotoxic chemotherapy-treated patients (n = 269) and the B20 tamoxifen only-treated patients (n = 161) from the trial data. In terms of RFI patients in the low-risk group improved by 5% from 86% to 91% (HR 0.4; 95% CI 0.2 to 0.8) and patients in the high-risk group improved by 21% from 64% to 85% (HR 0.4; 95% CI 0.2 to 0.9), showing that these groups benefited from chemotherapy, whereas the patients in the intermediate-risk group did not. This study was rated as high quality although data from two different trials were used and it was unclear how the data were combined.
Supplementary evidence
(CIC information has been removed.)
Summary of evidence: Mammostrat
Analytical validity of Mammostrat
No evidence was found on the analytical validity of the test.
Clinical validity (prognostic ability) of Mammostrat
The three studies identified suggest that Mammostrat can act as an independent prognostic tool for ER+, tamoxifen-treated breast cancer. Two of the studies were rated as high quality and one as moderate quality. The test has been validated in an external cohort. Although the evidence base for Mammostrat is relatively immature, these initial studies include a large sample size, appear to be of reasonable quality and, in the case of Bartlett et al. ,127 use a UK-based population.
Clinical utility of Mammostrat
One study reported on clinical utility and was rated as high quality. Initial evidence suggests that low- and high-risk groups benefited from chemotherapy, with high-risk patients benefitting more than low-risk patients. The moderate-risk group did not appear to benefit. There was no published evidence on reclassification of risk groups compared with conventional risk classifiers, and no evidence on the impact of the test on decision-making. Further evidence is required.
Overall summary
The evidence base, in particular in relation to the prognostic ability of the test, was of reasonably high quality. Further evidence of analytical validity and clinical utility is required.
IHC4 test
IHC4 assesses the levels of four key proteins (ER, PR, HER2 and Ki-67) in a breast cancer sample. The IHC4 score is calculated based on the percentage of cells positive for Ki-67 and PR (0–100%); the Histoscore for ER status (a measure of the percentage of cells positive multiplied by the intensity, range 0–300); and the tumour HER2 status, expressed as a binary measure (positive/negative). The final algorithm for IHC4 calculates a risk score for distant recurrence based on ER, PR, HER2 and Ki-67 in addition to classical clinical and pathological variables (composite risk score IHC4 + clinical). No risk category is given. Further details are included in Table 7.
Description of included study
The searches did not identify any relevant full peer-reviewed papers relating to IHC4. One relevant meeting abstract was identified, but had been superseded by a full paper. 83 The study design and patient characteristics are detailed in Tables 24 and 25 respectively. The investigators also provided further information on the test (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication) and this information is detailed in the supplementary evidence section.
| Author (year) Country | Study design | Number of patients | Follow-up (months) | Outcomes/end points | Evidence type | Funding |
|---|---|---|---|---|---|---|
| Cuzick et al. (2011)84 G1: multinational, including UK G2: UK |
G1: retrospective, cohort study of TransATAC trial G2: cohort study IHC4 assessed in a group of hormone receptor-positive patients with a GHI-RS score FFPE |
Eligible sample: 1911 Sample included: 1911 G1: 1125 hormone receptor-positive patients who did not receive adjuvant chemotherapy, had a GHI-RS and adequate tissue G2: 786 patients. A predictive model using classical variables and the four IHC markers (IHC4 score) was created and assessed in a separate cohort |
Median: 100 | Distant recurrence (within 10 years); TTDR | Clinical validity To determine how much of the information contained in the GHI-RS is contained in standard IHC markers |
Royal Marsden NIHR Biomedical Research Centre, Cancer Research UK, Breakthrough Breast Cancer and AstraZeneca |
| Author (year) | Age (years), median (IQR) | LN status | ER status | Tumour size | Grade | HER2 status | Mean NPI score | Treatment |
|---|---|---|---|---|---|---|---|---|
| Cuzick et al. (2011)84 | G1: 64 (57–70) G2: 55 (48–63) |
G1: −/+/unknown: 793 (70%)/288 (26%)/44 (4%) Those with unknown nodal status taken to be node negative in analyses G2: −/+: 487 (62%)/299 (38%) |
NR (reported to be ER+ and/or PR+) Reported to be ER+ and/or PR+ |
G1: ≤ 1 cm: 177 (16%); 1–2 cm: 574 (51%); > 2–3 cm: 272 (24%) G2: ≤ 1 cm: 105 (13%); 1–2 cm: 415 (53%); > 2–3 cm: 190 (24%) |
G1: poor: 206 (18%); moderate: 690 (61%); well differentiated: 229 (21%) G2: poor: 178 (23%); moderate: 336 (43%); well differentiated: 272 (34%) |
G1: +: 116 (10%) G2: +: 41 (5%) |
NR | G1: tamoxifen: 565 (50%); anastrozole: 560 (50%) G2: no endocrine treatment: 410 (52%); tamoxifen: 376 (48%) |
Quality of included studies: IHC4
Although the assessment of study quality was hindered by poor reporting of whether or not outcomes were fully defined and unbiased, the overall methodological quality of the included study was judged to be high, indicating a low risk of bias (received a positive assessment for at least 19 out of 21 methodological quality items).
Results: IHC4
Full data extraction tables are presented in Appendix 14.
Clinical validity
Cuzick et al. 83 reported a study assessing the prognostic value of IHC4. The IHC4 score was created and validated in one cohort (G1) and further validated in an independent cohort (G2). G1 was a retrospective cohort comprising patients from the TransATAC trial (a multinational trial, including the UK). The majority of the 1125 patients in G1 were LN− and hormone receptor positive. In this cohort there was a total of 195 recurrences of which 145 were distant recurrences. In LN− women there were 101 recurrences of which 67 were distant recurrences. The authors determined the value of each of the four IHC markers in three ways: univariately, as an addition to a model containing the classical variables, and when added to a model containing the classical variables and the other three IHC markers; this was carried out for all women and separately for LN− women only. They found that each of the four variables added a significant amount of information. Ki-67 was the most powerful univariately, but not in multivariate analyses because of its correlation with grade. For the multivariate models PR was most prognostic overall, but less so in LN− patients, in whom ER, HER2 and Ki-67 had similar values. The overall contribution of the IHC measurements for distant recurrence was highly significant [χ2 (4 degrees of freedom, df) = 39.1; p < 0.0001], and it was reported that the median IHC4 score for all patients was −4.2 and the interquartile range (IQR) −29.9 to 29.9. The HR for a change from the 25th to the 75th percentile of the IHC4 score for all patients was 5.7 (95% CI 3.4 to 9.7) in univariate analysis and 3.9 (95% CI 2.4 to 6.7) when added to clinical score. In a second validation cohort of 786 ER+ younger patients treated in the UK (G2), the authors demonstrated that IHC4 score was highly significantly predictive of outcome (HR 4.8; 95% CI 2.2 to 10.2) for a change from the 25th to the 75th percentile in univariate analysis, and gave similar results when added to clinical score (HR 4.4; 95% CI, 2.0 to 9.3; p < 0.0001). The authors concluded that they have created a prognostic model that integrates IHC information with classical clinical and pathological variables and may prove helpful in managing early ER+ breast cancer in postmenopausal patients, but that additional studies are needed to determine the general applicability of the IHC4 score. This study was rated as high quality on the basis of the quality assessment checklist. It has employed a large sample size and the test has been validated in an external cohort of UK patients.
Supplementary information
Supplementary data were provided by the co-investigators of this study84 after request by the External Assessment Group (EAG) (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication). Two analyses were conducted in the TransATAC trial – among women with LN−, ER+, HER2− early breast cancer (n = 707) and among all women (n = 1117).
Discussion with the co-investigators of the study (Professor Mitch Dowsett, Royal Marsden Hospital, London, July 2011, personal communication) indicated that the test was meant to be used in conjunction with clinicopathological parameters and therefore data for the final algorithm (using age, grade and tumour size) were used in the economic model. They provided data on the reclassification and risk of distant recurrence of patients using IHC4 plus clinical score (for simplicity the term IHC4 will be used in the report but the data refer to the use of the test in conjunction with clinicopathological parameters). Although cut-offs are not available for IHC4, for the purpose of the economic assessment investigators provided risk classification evidence of IHC4 based on low, intermediate and high risk of distant recurrence. Cut-offs were defined using a similar approach to the classification with OncotypeDX (< 10%, 10–20% and > 20% risk of distant recurrence). The cut-offs used for IHC4 are, however, exploratory and were defined only to populate the economic model. More details are available in Chapter 3.
Among the 707 women with LN−, ER+, HER2− early breast cancer, 85.3% of patients (n = 603) were classified as having a low risk of distant recurrence using IHC4. The proportions of patients classified as having an intermediate and a high risk of distant recurrence were 9.9% (n = 70) and 4.8% (n = 34). The risk of distant recurrence for patients classified as low, intermediate or high risk of distant recurrence by IHC4 is shown in Figure 7.
FIGURE 7.
Time to distant recurrence by IHC4 + clinical score group for patients who were LN−, ER+, HER2−.
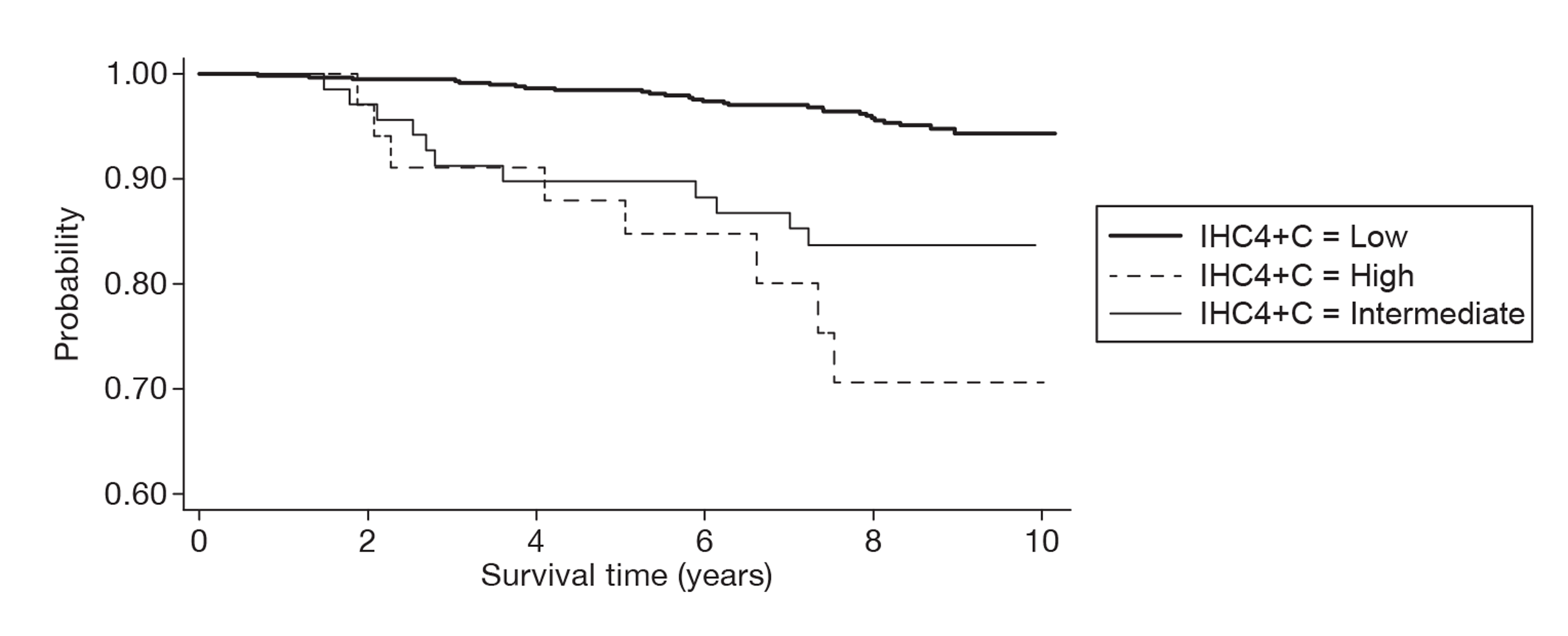
The reclassification of the three IHC4 risk groups against the two NPI risk groups used in the economic model (NPI ≤ 3.4 and NPI > 3.4) is presented in Table 26.
| Low risk IHC4, n (%) | Intermediate risk IHC4, n (%) | High risk IHC4, n (%) | Total, n (%) | |
|---|---|---|---|---|
| NPI ≤ 3.4 | 437 (97.3) | 12 (2.7) | 0 | 449 (100) |
| NPI > 3.4 | 166 (64.6) | 58 (22.6) | 33 (12.8) | 257 (100) |
| Total | 603 (85.4) | 70 (9.9) | 33 (4.7) | 706 (100) |
Summary of evidence: IHC4
Analytical validity of IHC4
We found no evidence on analytical validity. Although the use of IHC4 may be extended for use in other laboratories, the included paper suggests that there may be a lack of reproducibility of the test in relation to Ki-67. The authors suggest that, because IHC4 offers the possibility of carrying out the test in local laboratories, full validation would require evaluation of the IHC4 score when carried out in a range of local laboratories. Reproducibility of the test would need to be confirmed and quality assurance programmes put in place.
Clinical validity (prognostic ability) of IHC4
One study on the clinical validity of IHC4 was available, which claims that the IHC4 score is a highly significant predictor of distant recurrence. This initial study included a large sample size and detailed the development of the test in one cohort and the external validation of the test in an independent cohort. The study has been rated as high quality on the basis of the quality assessment employed.
Clinical utility of IHC4
There is currently no evidence on the clinical utility of IHC4 in terms of its ability to change treatment decisions or its ability to predict chemotherapy benefit. Although there are no published data on clinical utility, unpublished data were obtained to populate the economic model.
Overall summary
The evidence base for IHC4 is currently limited to clinical validity (prognostic ability), although the evidence for clinical validity is relatively strong given that the test has been developed using a large cohort of patients and has been validated in an external cohort. Further evidence is required on the analytical validity and clinical utility of IHC4.
Nottingham Prognostic Index plus
NPI+ is a biomarker-based prognostic assay that integrates 10 predictive biomarkers of long-term survival and therapeutic response with existing clinical and molecular pathology knowledge to support individualised clinical decision-making. This test is under development and outputs/presentation are not yet finalised. Further details are provided in Table 7.
Description of included studies
The searches did not identify any relevant full peer-reviewed papers or meeting abstracts relating to NPI+. Supplementary evidence was provided by the manufacturer of the test.
Supplementary evidence
The manufacturers submitted two draft full papers based on the same data and one draft abstract (of a full paper). The study design and patient characteristics included in these documents are presented in Tables 27 and 28.
| Author (year) Country | Study design | Number of patients | Follow-up (years) | Outcomes/end points | Evidence type | Funding |
|---|---|---|---|---|---|---|
| Green et al. (unpublished) and Nottingham Prognostics (2011)128 (AIC information has been removed) | (AIC information has been removed) | Eligible sample: (AIC information has been removed) Sample included: (AIC information has been removed) |
(AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) |
| Nottingham Prognostics (2011) (abstract)128 (AIC information has been removed) (AIC information has been removed) |
(AIC information has been removed) | Eligible sample: (AIC information has been removed) Sample included: (AIC information has been removed) |
(AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) |
| Author (year) | Age (years), mean (SD) | LN status | ER status | Tumour size | Grade | HER2 status | Mean NPI score | Treatment |
|---|---|---|---|---|---|---|---|---|
| (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) |
| (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) | (AIC information has been removed) |
Quality of included studies: Nottingham Prognostic Index plus
The overall methodological quality of the two (unpublished) included studies is provided in Appendix 15. Both studies were deemed to be of (AIC information has been removed).
Results: Nottingham Prognostic Index plus
A summary of the evidence provided is present in Table 29 followed by a narrative summary. Full data extraction tables are presented in Appendix 15.
| Author (year) | Evidence type | Overall quality | Key findings |
|---|---|---|---|
| (AIC information has been removed) | (AIC information has been removed) | Moderate | (AIC information has been removed) |
| (AIC information has been removed) | (AIC information has been removed) | Low | (AIC information has been removed) |
(AIC information has been removed.)
Summary of evidence: Nottingham Prognostic Index plus
The evidence base for NPI+ is currently insufficient to draw any firm conclusions regarding the analytic and clinical validity of the test, and as yet there is no available evidence on the clinical utility of the test. Further evidence on the prognostic ability of the test is required. According to the unpublished abstract from the manufacturers of the test, validation in an external cohort is ongoing but as yet results are not available.
Chapter 3 Economic analysis
A systematic review of existing cost-effectiveness evidence is reported in the following section. This is followed by reviews of the economic evaluations submitted by two of the manufacturers/sponsors of the tests in response to the request for information issued by NICE at the start of the assessment process. The relevance of existing cost-effectiveness evidence for NICE decision-making is then summarised. This is followed by a description of the independent economic model and its results, and a comparison of the independent economic model with the evaluations from the two manufacturers/sponsors. Finally, the independent economic model results are discussed.
Systematic review of existing cost-effectiveness evidence
This section of the report describes a review of the existing published evidence on the cost-effectiveness of GEP and expanded IHC (or protein expression profiling) tests to guide the use of adjuvant chemotherapy in breast cancer management.
Methods
A systematic search of the existing literature evaluating the cost-effectiveness of the nine GEP and expanded IHC tests identified by NICE (OncotypeDX, MammaPrint, Mammostrat, PAM50, BluePrint in combination with MammaPrint, IHC4, Randox BCA, BCI and NPI+) to guide adjuvant chemotherapy treatment decision-making in the management of early breast cancer was undertaken. Only full economic evaluations published in English addressing the cost-effectiveness of those tests compared with NPI, Adjuvant! Online or any adaptations of these tools in clinical practice were included in the review. Cost-effectiveness studies that used St Gallen, the National Comprehensive Cancer Network (NCCN)129 and NIH guidelines101 were excluded from the review because of time and resource constraints as these comparators are not directly relevant to the UK context, but such studies were scanned by the reviewers to inform the model development.
The following databases were searched for relevant published literature: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations (Ovid), CINAHL, EMBASE, NHS Economic Evaluation Database and HTA (via the Cochrane Library), Web of Science (which includes the Science Citation Index) and BIOSIS. In addition, literature searches were undertaken for the clinical effectiveness review (see Chapter 2, Methods for reviewing effectiveness) and relevant cost papers were identified from these searches. In addition, the reference lists of relevant articles were hand searched. Full details of the search strategies used in MEDLINE are presented in Appendix 1 (these have been adapted for use in other databases). Searches were not restricted by language.
Studies were selected for inclusion through a two-stage process. Titles and abstracts were examined for inclusion by one reviewer. Full manuscripts of selected citations were then retrieved and assessed by the same reviewer. The quality of the cost-effectiveness studies was assessed using a critical appraisal checklist adapted from the Drummond and Jefferson130 and Eddy131 checklists.
The aim of the review was to identify published economic evaluations and summarise the main limitations of the existing models. Because of time constraints it was not possible to provide a detailed direct comparison of the models.
Results
Identified studies
The search retrieved 72 citations relating to cost-effectiveness (Figure 8) and two additional references were known by the authors. Fifty-six articles were excluded at title stage and four articles were excluded at abstract level. Thirteen studies (corresponding to 14 references) were examined at full-text level and four studies (corresponding to five references) were identified as meeting the inclusion criteria of the systematic review of economic evaluations. 132–136 This included an economic evaluation developed as part of the Ontario Health Technology Assessment (OHTA) described in a report134 and PowerPoint presentation slides. 136
FIGURE 8.
Flow diagram of economic evaluation selection/exclusion.
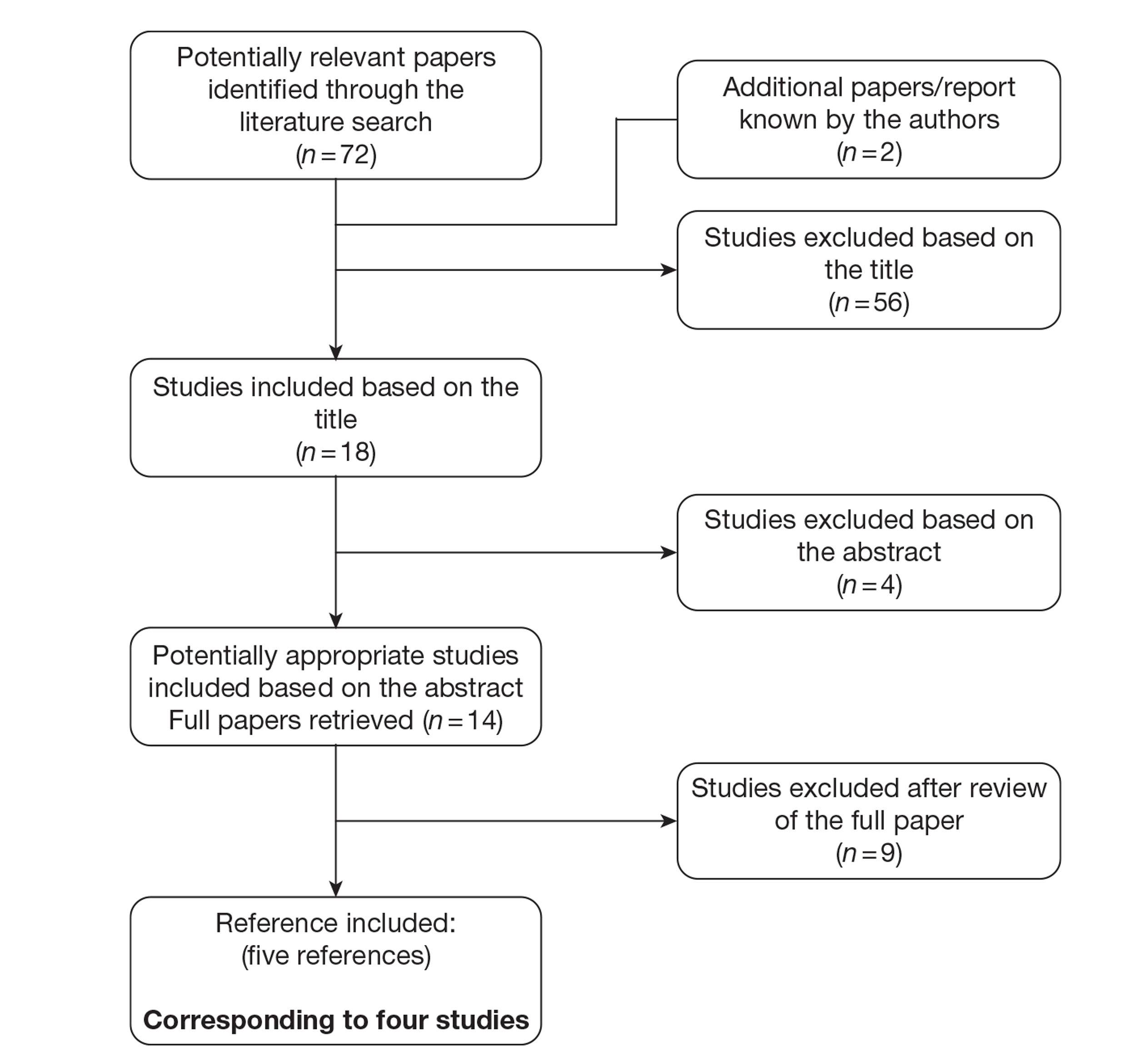
Nine articles were excluded after retrieving the full text because there were insufficient details to assess the validity of assumptions,137 the economic evaluation was available only in abstract form,138–140 the study used a different comparator141 or for other reasons. 143–145 Klang et al. 141 was excluded from the review as the exact nature of the comparator defined as clinical practice in Israel was unclear.
Of the four identified economic studies (corresponding to five references), two compared MammaPrint against Adjuvant! Online132,133 and two compared OncotypeDX against Adjuvant! Online. 134–136 None of the four published economic evaluations was conducted in a UK setting.
Description of published cost-effectiveness studies evaluating the use of MammaPrint
Two economic evaluations compared treatment guided using the MammaPrint test with treatment guided using Adjuvant! Online and used a health-care payer perspective. 132,133 A Markov approach was employed in both economic evaluations but the populations considered in the models differed slightly. Retel et al. 133 addressed the cost-effectiveness of MammaPrint in women with LN−, ER+, HER2+/− early breast cancer. Chen et al. 132 included ER− early breast cancer but excluded HER2+ early breast cancer.
A tabulated summary of the key features and data sources and a quality assessment for the two studies included in the cost-effectiveness review of MammaPrint are presented in Appendices 16 and 17 respectively. It was not possible for the EAG to check the economic models as only the publications were available in the public domain.
A narrative description and assessment of each economic evaluation is presented in the following sections.
Description and critique of Retel et al.:146 cost-effectiveness of the 70-gene signature compared with St Gallen guidelines and Adjuvant! Online for early breast cancer
The aim of the study was to estimate the cost-effectiveness of MammaPrint compared with that of the St Gallen guidelines and Adjuvant! Online to guide adjuvant treatment decisions in Dutch women with LN−, ER+, HER2 +/− early breast cancer.
The model used a Markov approach and followed women over 20 years in four possible health states: disease free, relapse (local, regional or contralateral relapse), distant metastasis and death. The study adopted the perspective of the Dutch health-care payer and costs and QALYs were discounted at 4.0% and 1.5% per annum respectively. The mean age of patients entering the model was 50 years.
For the strategy using MammaPrint and Adjuvant! Online, the sensitivity and specificity were calculated and patients were classified into four risk groups of developing distant metastasis: true high, true low, false high and false low. The sensitivity and specificity were calculated from a pooled analysis of three validation studies60,63,93 using 10-year BCSS as a final outcome (thus, patients were classified as low or high risk according to the probability of survival rather than the probability of developing distant metastasis).
The study classified patients as low risk with Adjuvant! Online if the predicted 10-year survival was > 88%. MammaPrint classified patients into two categories: low (good prognosis) and high (poor prognosis) risk of developing distant recurrence. Patients classified as high risk either by MammaPrint or by Adjuvant! Online were assumed to receive chemotherapy in addition to endocrine therapy. Low-risk patients were assumed to receive endocrine therapy alone.
Patients classified as true low and false high had a zero probability of relapse and distant metastasis. The probability of relapse and distant metastasis for true high-risk patients was based on an analysis conducted in a sample of 20,624 Swedish breast cancer patients derived from Lidgren et al. ,147 with a constant risk within three time periods: 1–5, 5–10 and 10–20 years. Patients classified as false low were assumed to have a 100% probability of developing distant metastases.
Patients could have only one relapse (and then possibly move to distant metastasis). In total, 10% of high-risk patients were assumed to be HER2+, with a risk twice as high as observed for HER2− patients. A relative risk reduction with a HR of 0.64 (95% CI 0.54 to 0.76) was applied for patients treated with trastuzumab. Adverse events associated with chemotherapy were included for chronic heart failure only.
Finally, utilities (see Appendix 16) were measured using the European Quality of Life-5 Dimensions (EQ-5D). 148
The costs of the health states (see Appendix 16) were based on Lidgren et al. 148 Drug costs for chemotherapy and hormonal therapies were based on Dutch sources. Chemotherapy costs included drug costs, day-care costs (including administration), laboratory and diagnostic imaging costs (mammography, tumour markers) and prevention. The cost of MammaPrint was assumed to be €2675. Costs were expressed in 2005 euros.
The results for the base case are presented in Table 30. Treatment guided using MammaPrint was associated with a cost per QALY gained of €4614 compared with Adjuvant! Online.
| Life-years | QALYs | Cost (€) | Cost/QALY gained (€) | Cost/life-year gained (€) | |
|---|---|---|---|---|---|
| Adjuvant! Online | 15.68 | 12.20 | 26,915 | ||
| MammaPrint | 15.88 | 12.44 | 28,045 | 4614 | 5736 |
The impact of key model parameters was examined in one-way univariate sensitivity analysis and probabilistic sensitivity analysis (PSA) and showed that the results were sensitive to data used to calculate the sensitivity and specificity of the tests (5-year risk of distant metastasis instead of 10-year risk of BCSS, and using data for each individual validation study) and the cost of chemotherapy.
Based on the description of the model, this appears to be a reasonably well-conducted cost-effectiveness analysis, although it has a number of limitations. The generalisability of the results from this study to the UK context is limited.
The study used sensitivity/specificity of the tests to reclassify patients into risk group categories. Patients were classified according to their risk of developing distant metastasis, but the sensitivity/specificity were calculated using the 10-year BCSS risk in the base-case analysis. Using the 5-year risk of distant metastasis instead of the 10-year of risk of BCSS was tested in sensitivity analysis. An assumption has also been made when calculating the sensitivity/specificity of the tests that low-risk patients cannot die from breast cancer. Although low-risk patients are less likely to die from breast cancer, they could still die from their cancer. There are also some limitations associated with the use of the sensitivity/specificity for tests providing a continuous risk score (especially for Adjuvant! Online).
The evaluation assumes that the decision to receive chemotherapy will be based on the test results for MammaPrint and Adjuvant! Online alone. However, it is likely that MammaPrint would be used in conjunction with other clinical parameters to inform the treatment recommendation. The assumption that the prognostic test results and treatment guidelines would be followed in all cases (patients and physicians are 100% compliant) is simplistic. Furthermore, it is unclear if the cut-off of ≤ 88% used to identify high-risk patients with Adjuvant! Online reflects actual clinical practice. Discussions with clinical experts indicated that the risk score estimated using Adjuvant! Online on its own is less informative than the complete output, which includes estimates of reduction in risk at 10 years of breast cancer-related death or relapse for selected treatments.
The test was assumed to be administered to women with both HER2+ and HER2− early breast cancer; however, UK clinical opinion indicated that the vast majority of patients with HER2+ early breast cancer are typically offered chemotherapy and the MammaPrint test may therefore be considered unnecessary.
The authors also assumed that patients classified as low risk (true low or false high) have a zero probability of having a relapse or distant metastasis. This seems to be a very simplistic assumption. Furthermore, the authors modelled only one relapse but acknowledge that about 30% of patients develop more than one relapse.
Many assumptions have also been made about the probability of moving between health states, and the impact of chemotherapy is unclear. The risk of recurrence for patients treated with endocrine therapy was extracted from studies of patients receiving tamoxifen only; however, more effective agents are now available, potentially reducing the risk of recurrence. The starting age of the cohort was low (50 years) given that the majority of breast cancers are diagnosed in women > 50 years of age. Finally, the use of fresh tissue samples for MammaPrint will have service configuration implications for UK pathology laboratories.
Description and critique of Chen et al.:132 cost-effectiveness of the 70-gene MammaPrint signature in node-negative breast cancer
The aim of the study was to estimate the cost-effectiveness of MammaPrint compared with Adjuvant! Online to guide the adjuvant treatment decision in US patients aged ≤ 60 years with ER+/−, T1 or T2, LN−, HER2− breast cancer. The model used a Markov approach and followed patients over their lifetime in three possible health states: disease free, death from cancer and death from other causes. The study adopted the perspective of the US payer, and costs and QALYs were discounted at 3.0% per annum.
Two separate models were constructed using effectiveness data from a validation study61 and Surveillance, Epidemiology and End Results (SEER) data149 to reflect US clinical practice (the alternative model). This was carried out as no low-risk patients with ER− tumours were included in the Buyse et al. 61 study.
In the base-case model, the risk classification and 10-year OS were extracted from Buyse et al. 61 In the alternative model, the risk reclassification was adapted from SEER and Buyse et al. ,61 assuming the same rate of cross-classification between high- and low-risk patients as observed in the Buyse et al. study, as data for MammaPrint were unavailable. A range of assumptions was necessary to use the SEER data.
Patients with ER+ early breast cancer were assumed to receive endocrine therapy (tamoxifen) whereas ER− patients were not; chemotherapy was given to patients classified as high risk only. The benefit of chemotherapy was extracted from a meta-analysis of RCTs (EBCTCG 1998),150 applying a reduction in all-cause deaths of 26% in ER+ and 32% in ER− patients.
Utilities used to calculate quality of life were extracted from the published literature. 46,151 A utility of 0.70 was applied for patients undergoing chemotherapy for 6 months and 0.98 for patients after completion of chemotherapy or disease free. The authors did not report the valuation method or the quality of life instrument used to estimate the utility values.
Costs are presented in 2007 US dollars. Costs included the costs of endocrine therapy, chemotherapy, administration, treatment-related toxic effects and breast cancer surveillance. The cost of recurrence and terminal care (with cancer) was included for women dying from cancer. A cost of terminal care was included for women dying from other causes.
The cost of chemotherapy was derived from insurance claims152 and included the costs of chemotherapy medication, hospitalisation and emergency room for chemotherapy adverse events or all causes, ambulatory encounters and prescription. The study included patients receiving alkylating agents (58%), anthracyclines (51%), taxanes (25%) and antimetabolites (18%). The cost of the MammaPrint test was $4200.
Results for the base-case and alternative model are presented in Table 31. The incremental cost-effectiveness ratio (ICER) was also presented by ER status subgroup – US$5908 per QALY gained (US$6167 per life-year) for ER+ patients. MammaPrint was dominated in ER− patients in the base-case model.
| Life-years | QALYs | Cost ($) | Cost/QALY gained ($) | Cost/life-year gained ($) | |
|---|---|---|---|---|---|
| Base-case model | |||||
| Adjuvant! Online | 21.596 | 21.065 | 162,140 | ||
| MammaPrint | 21.739 | 21.218 | 163,580 | 9428 | 10,059 |
| Alternative model | |||||
| Adjuvant! Online | 20.659 | 21.191 | 163,108 | ||
| MammaPrint | 21.230 | 21.751 | 163,509 | 702 | 716 |
The impact of the main model parameters was examined in one-way univariate sensitivity analysis, which showed that the results were mostly sensitive to the proportion of ER+ patients classified as high risk by MammaPrint, the estimate of OS, the cost of MammaPrint and the cost of chemotherapy.
Based on the description of the model, this appears to be a reasonable cost-effectiveness analysis. The generalisability of the results from this study is limited given that it is based on the US health-care system.
The model is simplistic – patients either stay alive or die. The impact of recurrence is incorporated only in terms of the cost for patients dying from breast cancer. This ignores the health effect. Furthermore, a proportion of patients will have a relapse but not die from breast cancer. The authors also did not discuss the selected cut-off for Adjuvant! Online. It was unclear how the benefit of chemotherapy was applied to breast cancer deaths.
The benefit of chemotherapy was extracted from a meta-analysis and was assumed to be the same whether patients were classified as low or high risk with MammaPrint or Adjuvant! Online.
Furthermore, there were limitations in the data used. As highlighted by the authors, no low-risk patients with ER− early breast cancer were included in the Buyse et al. study. 61 Therefore, an alternative model was constructed using SEER data. However, a series of assumptions were necessary in order to make use of the SEER data, increasing the uncertainty relating to these results.
The authors stated that patients can experience local, regional or distant recurrence before death. It is unclear what the relative contribution of each of the types of relapse was on breast cancer survival. This can have implications in terms of costs and health effects if included in the economic model. Likewise, the authors report neither the valuation method nor the quality of life instrument used to estimate utilities. No PSA was conducted.
Furthermore, the risk of recurrence for patients treated with endocrine therapy was extracted from patients receiving tamoxifen only; however, more effective agents are now available, reducing the risk of recurrence. The use of fresh tissue samples associated with MammaPrint will have service configuration and cost implications for UK pathology laboratories.
The health-state utility value for the recurrence-free health state was high (0.98). Evidence indicates that the utility in the general population for a similar age cohort would be lower. 153 Less gain would be accrued in the model by preventing a recurrence if a lower value was used.
The mean age of patients entering the model is unclear. The economic evaluation considered only patients aged ≤ 60 years. MammaPrint is now licensed for both younger and older women with breast cancer; however, the cost-effectiveness of the test in an older population is not known.
Description of published cost-effectiveness studies evaluating the use of OncotypeDX
Two economic evaluations134–136 compared treatment guided using OncotypeDX with that guided using Adjuvant! Online and used a health-care perspective. The same model structure was used in both studies, with the model developed by Tsoi et al. 135 being made available to the OHTA and adapted. 134,136 Both studies addressed the cost-effectiveness of OncotypeDX in Canada in women with LN−, ER+, HER2− early breast cancer. The mean age of women entering the model was 50 years.
A tabulated summary of the key features and data sources and a quality assessment for the two studies included in the cost-effectiveness review of OncotypeDX are presented in Appendix 18. It was not possible for the EAG to check the economic models as only the publications were available in the public domain.
A narrative description and assessment of each economic evaluation is presented in the following sections.
Description and critique of Tsoi et al.:135 cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer
The aim of the study was to estimate the cost-effectiveness of OncotypeDX compared with Adjuvant! Online to guide the adjuvant treatment decision in Canadian patients with LN−, ER+, HER2 early breast cancer. The model used a Markov approach using a monthly cycle and followed patients over their lifetime in four possible health states: chemotherapy, recurrence free, distant recurrence and death. The study adopted the perspective of Canadian health care, and costs and QALYs were discounted at 5.0% per annum. The mean age of patients entering the model was 50 years.
The probability of reclassification was based on Bryant et al. 45 High and intermediate risks defined by OncotypeDX were grouped together. High-risk patients according to Adjuvant! Online were defined as patients with a 10-year mortality ≤ 91%. Patients were first classified according to Adjuvant! Online (low vs. high). For the strategy including OncotypeDX, patients classified as low or high risk using Adjuvant! Online were further reclassified into low and intermediate/high risk using OncotypeDX. Patients were assumed to receive chemotherapy if they were considered at intermediate/high risk and entered the chemotherapy state for 6 months during which they might experience toxicity. The probability of developing toxicity (major and minor) was obtained from the literature. 154,155 Patients in the recurrence-free state received tamoxifen for 5 years. Patients could develop distant metastases, remain disease free or die. Death from other causes than breast cancer was included.
The 10-year risk of recurrence was obtained from Paik et al. 49 for each risk group category (for both the Adjuvant! Online and OncotypeDX arms). A relative risk reduction of 30%, taken from a meta-analysis conducted by the EBCTCG,29 was applied for patients classified in the high-risk group to represent the effect of chemotherapy. The median survival after distant metastasis was assumed to be 21 months. The probabilities were assumed to follow an exponential distribution.
Utility values were extracted from the published literature and were estimated using different approaches, including standard gamble and a visual analogue scale.
Costs were reported in 2008 Canadian dollars. The cost of chemotherapy was obtained from the Sunnybrook Odette Cancer Centre pharmacy, Toronto, Ontario. In the base case, patients were assumed to receive four cycles of doxorubicin and cyclophosphamide (AC). Other chemotherapy regimes were considered in sensitivity analysis [four cycles of docetaxel and cyclophosphamide (TC) and six cycles of 5-flurouracil, epirubicin and cyclophosphamide-docetaxel (FEC-D)]. The costs of chemotherapy included the costs of the chemotherapeutic agent, supportive medications, laboratory evaluation and human resources.
No costs were assumed for minor toxicities as it was assumed that they were already included in the cost of supportive medication. The cost of major toxicities included the cost for the management of febrile neutropenic complications and growth factor support. The model included the cost of fatal toxicities. The cost of hormonal treatment was applied to all patients for 5 years or until death. In addition to the costs of the health state (recurrence free and recurrence), the model included the cost for terminal care.
The cost of OncotypeDX was assumed to be C$4404.
Results for the base-case analysis are presented in Table 32.
| Life-years | QALYs | Cost (C$) | Cost/QALY gained (C$) | Cost/life-year gained (C$) | |
|---|---|---|---|---|---|
| Adjuvant! Online | 13.933 | 13.573 | 15,645 | ||
| OncotypeDX + Adjuvant! Online | 13.997 | 13.638 | 19,747 | 63,064 (approx. £39,917a) | 63,911 (approx. £40,466a) |
The impact of changes in the main model parameters was examined in one-way univariate sensitivity analysis, which showed that the results were sensitive to the reclassification probabilities, recurrence rates used, discounting, baseline age and cost of OncotypeDX.
Based on the description of the model, this appears to be a reasonably well-conducted cost-effectiveness analysis. The generalisability of the results from this study to the UK context is, however, limited.
The study assumed that the decision to receive chemotherapy will be based on OncotypeDX or Adjuvant! Online alone; however, it is likely that both tools will be used in clinical practice to inform the treatment recommendation. It is also assumed that the prognostic test results and treatment guidelines would be followed in all cases (patients and physician are 100% compliant). This is unlikely to be the case in clinical practice.
High- and intermediate-risk group patients identified by OncotypeDX were grouped together and assumed to receive chemotherapy. However, it is unclear from existing studies whether or not patients classified in the intermediate-risk group would benefit from chemotherapy. Furthermore, the benefit of chemotherapy was assumed to be the same irrespective of OncotypeDX risk score. There is some evidence to suggest that high-risk patients gain a greater proportionate benefit, although this evidence has a number of weaknesses.
There was also an issue with the definition of risk groups. Bryant et al. 45 rank order outputs from Adjuvant! Online so that a similar proportion of cases would be categorised as low risk (50%) as for OncotypeDX. This is arbitrary and therefore may introduce biases into the analysis.
Local and regional recurrences were not included in the model. No long-term adverse events were included.
The risk of recurrence for patients treated with endocrine therapy was extracted from data on patients receiving tamoxifen only; however, more effective agents are now available, reducing the risk of local and systemic recurrence.
Finally, utilities were extracted from a variety of sources using different valuation methods. This might bias the cost-effectiveness results. The starting age of the cohort was low (50 years) compared with the average age of patients presenting with early breast cancer in the UK.
Description and critique of OHTA analysis:134,136 gene expression profiling for guiding adjuvant chemotherapy decisions in women with breast cancer
The aim of the study was to estimate the cost-effectiveness of Adjuvant! Online in combination with OncotypeDX compared with Adjuvant! Online alone to guide the adjuvant treatment decision in Canadian patients (Ontario) with LN−, ER+, HER2− early breast cancer. The analysis was built on the economic model developed by Tsoi et al. 135
Compared with the original model,135 the OHTA analysis classified patients into low, intermediate and high risk using OncotypeDX or Adjuvant! Online, analysed all possible combination to give OncotypeDX to specific group of patients according to the Adjuvant! Online score (all patients, low, intermediate or high only, intermediate and high), modelled different chemotherapy regimens and conducted a PSA.
The majority of costs have been inflated from Tsoi et al. 135 to reflect 2010 prices. The cost of OncotypeDX was updated to C$4191. The authors also stated that the cost of chemotherapy was updated but this was not reported. The benefit of chemotherapy was assumed to be different between risk group categories, based on evidence from the Paik et al. study. 48 As in the original analysis, the risk reclassification and the probability of distant recurrence were derived from Bryant et al. 45 and Paik et al. 49
Incremental analysis was conducted comparing the most effective strategy with the next most effective strategy (Table 33). Assuming that OncotypeDX was provided only to high-risk patients classified by Adjuvant! Online resulted in an estimated ICER of C$518 per QALY gained compared with not using OncotypeDX. The ICER was C$795 per QALY gained if OncotypeDX was given to intermediate- and high-risk patients compared with high-risk patients only classified by Adjuvant! Online. Finally, giving OncotypeDX to all patients is associated with higher benefit and costs. The ICER comparing this strategy (OncotypeDX to all patients) with OncotypeDX given only to patients classified as high and intermediate risk by Adjuvant! Online was C$23,983 per QALY gained.
| Cost (C$) | QALYs | ICER (C$) | |
|---|---|---|---|
| No patients | 13,298 | 13.34 | |
| Adjuvant! Online high risk | 13,660 | 14.04 | 518 (approx. £328a) |
| Adjuvant! Online intermediate/high risk | 13,961 | 14.42 | 795 (approx. £503a) |
| All patients | 17,466 | 14.64 | 23,983 (approx. £15,179a) |
Univariate sensitivity analysis was performed. PSA was also performed using Monte Carlo simulation. The PSA indicated that, at the willingness-to-pay threshold of $75,000 per QALY gained, the probability that OncotypeDX is cost-effective is 83.5% for patients identified as Adjuvant! Online low risk, 99.8% for patients identified as Adjuvant! Online intermediate risk and 65.8% for patients identified as Adjuvant! Online high risk.
Based on the description of the model, this appears to be a reasonably well-conducted cost-effectiveness analysis. The generalisability of the results from this study to the UK context are, however, limited.
The description of the model and its assumptions is minimal in the report, but this is explained by the fact that this is an adaptation of a previously published cost-effectiveness evaluation. The authors were also contacted and a greater description of the model and results are due for publication soon. Despite the adaptations, key limitations remain regarding the data used to reclassify patients and for utility estimates, the probability of distant metastases, the type of relapse modelled, long-term adverse events after chemotherapy and the benefit of chemotherapy.
Assessment of the economic evaluation submitted by Genomic Health
An economic evaluation was submitted by Genomic Health27 comparing the use of OncotypeDX with current clinical practice in the UK and included a full report and an electronic model submitted in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). The economic model was reviewed to check that the parameters presented in the report corresponded to those used in the economic model and assessed using a critical appraisal checklist adapted from the Drummond and Jefferson130 and Eddy131 checklists (Table 34).
| Modelling assessments should include: | Economic evaluation submitted by Genomic Health26 | |
|---|---|---|
| 1 | A statement of the problem | Yes |
| 2 | A discussion of the need for modelling vs. alternative methodologies | Yes |
| 3 | A description of the relevant factors and outcomes | Yes |
| 4 | A description of the model including reasons for this type of model and a specification of the scope, including time frame, perspective, comparators and setting. Note: n = number of health states within submodel | Yes |
| 5 | A description of data sources (including subjective estimates) with a description of the strengths and weaknesses of each source, with reference to a specific classification or hierarchy of evidence | Yes |
| 6 | A list of assumptions pertaining to the structure of the model (e.g. factors included, relationships and distributions) and the data | Yes |
| 7 | A list of parameter values that will be used for a base-case analysis and a list of the ranges in those values that represent appropriate confidence limits and which will be used in a sensitivity analysis | Yes |
| 8 | The results derived from applying the model for the base case | Yes |
| 9 | The results of the sensitivity analyses: unidimensional, best/worst case, multidimensional (Monte Carlo/parametric), threshold | Yes |
| 10 | A discussion of how the modelling assumptions might affect the results, indicating both the direction of the bias and the approximate magnitude of the effect | Yes |
| 11 | A description of the validation undertaken including concurrence of experts, internal consistency, external consistency, predictive validity | Unclear |
| 12 | A description of the settings to which the results of the analysis can be applied and a list of factors that could limit the applicability of the results | Unclear |
| 13 | A description of research in progress that could yield new data that could alter the results of the analysis | Unclear |
Description of the economic model submitted by Genomic Health
Overview
The economic model submitted by Genomic Health27 used a Markov approach with individuals moving between three possible health states: recurrence free, distant recurrence and death (from breast cancer or other causes) (Figure 9). The model compared the cost-effectiveness of the addition of OncotypeDX to clinical and pathological parameters (using NPI and Adjuvant! Online; termed usual care in the economic model submitted by Genomic Health) with that of clinical and pathological parameters alone in women with ER+ and LN− or single node-positive early breast cancer in the UK. The starting age in the model was 60.55 years and patients were followed up for 30 years. The study adopted the perspective of the UK NHS, with costs and QALYs discounted at 3.5%. A tabulated summary of the key features and data sources of the economic model submitted by Genomic Health is presented in Table 35.
FIGURE 9.
Overview of the OncotypeDX cost-effectiveness model structure submitted by Genomic Health (reproduction of figure 6–2 in the report submitted by Genomic Health). 27
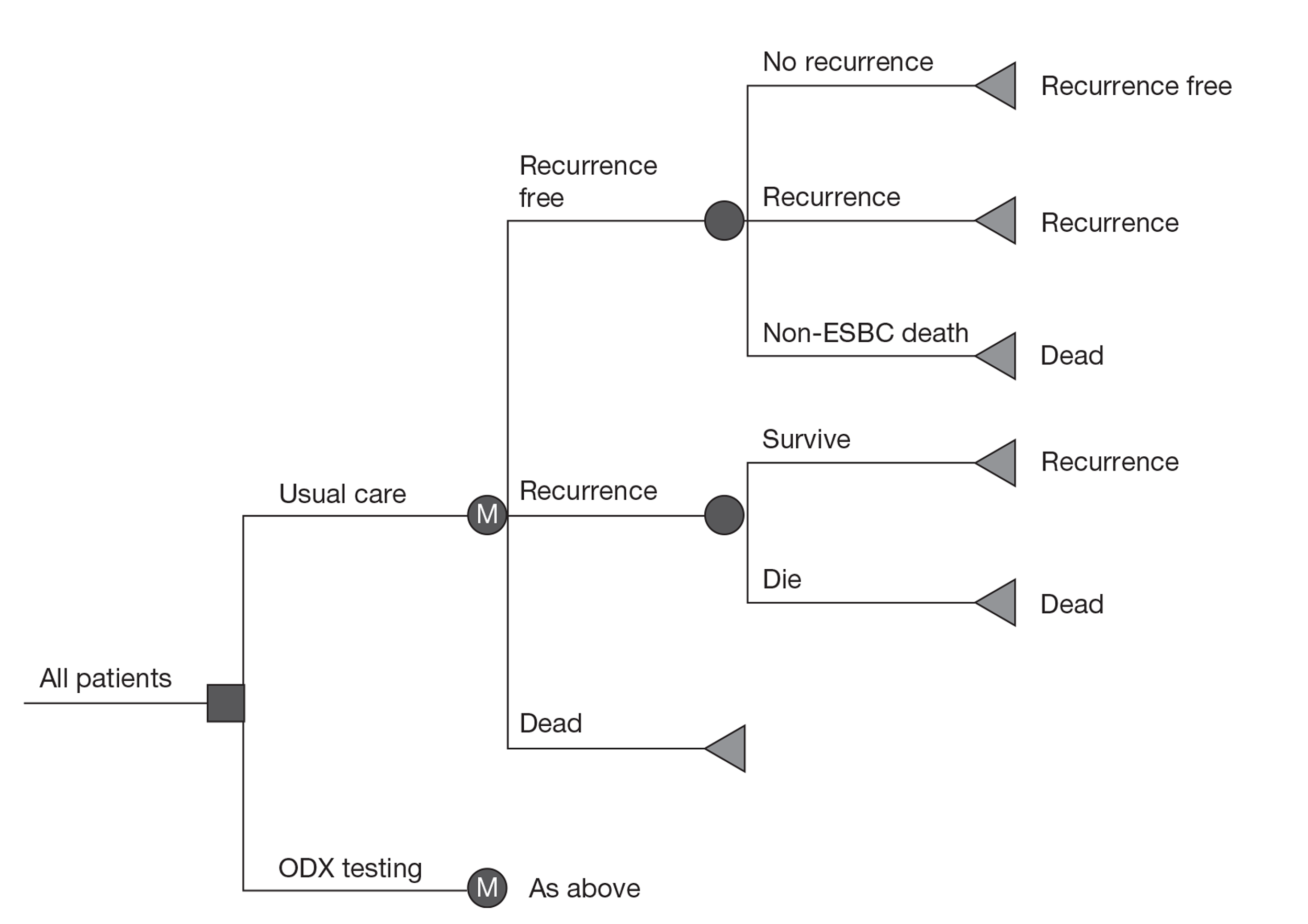
| Parameter | Key features/data |
|---|---|
| Country | UK |
| Perspective (costs) | NHS and PSS |
| Comparators (NPI, Adjuvant Online!) | Usual care (NPI and Adjuvant Online!) |
| Starting age in the model | 60.55 years |
| Population | ER+, LN− (or single node-positive) early breast cancer |
| Model structure (type, health states) | Markov model with three health states (recurrence free, distant recurrence and death) |
| Definition of relapse | Distant recurrence only |
| Time horizon | 30 years |
| Endocrine therapy regime | Mixed – tamoxifen and aromatase inhibitors – according to NICE guidance (see text) |
| Chemotherapy regime | Six cycles of 5-fluorouracil, epirubicin and cyclophosphamide (FEC75) |
| Benefit of chemotherapy by RS risk group | Low-risk group: no benefit (assumed); intermediate group: 39%; high risk: 74% |
| Adverse events | Short-term adverse events included in the cost and disutility associated with chemotherapy |
| Other assumptions | The probability of dying after distant metastases was the same irrespective of risk classification. Transitional probabilities are assumed to be exponential |
| Definition of high risk | In the usual care arm patients were offered chemotherapy based on the treatment decision taken using NPI and Adjuvant Online! In the OncotypeDX arm patients were offered chemotherapy based on the treatment decision taken using NPI and Adjuvant Online! and knowledge of the OncotypeDX test result. Note that the results of the test were not always followed |
| Quality of life | Different sources, valuation methods. Recurrence free = 0.78; decrement from chemotherapy = 0.07; distant recurrence = 0.6 |
| Costs and resources used | 2010 UK pounds OncotypeDX test: £2580; chemotherapy (all cycles): £3194 (chemotherapy) + £4534 (adverse events associated with chemotherapy and use of G-CSF); recurrence free (yearly): £0; endocrine therapy (mixed): £853 (first 5 years) + £40 (adverse events first 5 years) + £123 (adverse events 6–8 years); DM (3.3 years): £916 monthly; terminal care (last 3 months): £0 |
| Discounting (per annum) | 3.5% for both costs and benefits |
| Uncertainty | One-way and probabilistic sensitivity analysis |
| % of HER2+ | Unclear from the submission |
| Cost per QALY | £6232 |
The structure was based on an original model by Hornberger et al. 46 Patients with ER+ and LN− or single node-positive [pN1(mic)] early breast cancer with no contraindications for adjuvant chemotherapy are assigned adjuvant therapy based on:
-
clinical and pathological parameters alone (using NPI and Adjuvant! Online) or
-
the addition of OncotypeDX RS to usual care [terminology used in the Sponsor submission (SS)].
Patients are categorised as low, intermediate or high risk according to the OncotypeDX classification. Among each risk group category patients are further divided according to the treatment they received (either hormonal therapy alone or hormonal therapy in addition to chemotherapy). In each cycle of the model, the risk of recurrence was evaluated for each simulated patient based on their RS-defined category of low, intermediate or high risk as reported for the NSABP B20 cohort. 49 The risk was then adjusted for patients who received chemotherapy, based on whether or not patients received chemotherapy as per the initial recommendation (in the arm termed usual care) and based on the recommendation following the additional information provided by the OncotypeDX RS. The benefit of chemotherapy varied by OncotypeDX risk group, based on Paik et al. 49 For PSA, recurrence risks and relative risk reductions for chemotherapy were sampled from normal distributions, with the assumed variance derived from published data. Non-breast cancer death was captured as a competing risk in the model, based on UK life tables for women in 2007–9. 156 For patients experiencing distant recurrence, the median survival was assumed to be 3.3 years. 157
Summary of effectiveness data
The impact of OncotypeDX on treatment recommendations was obtained from the preliminary results from a Welsh cohort study by Holt et al. 78 reporting on the first 107 patients. 27 The study considered the treatment recommendations made based on usual care (chemotherapy or no chemotherapy) and then the treatment recommendations made following the additional knowledge of the OncotypeDX test result. In this study, 33% of patients had their initial treatment recommendations changed following OncotypeDX testing. 27,78 Not all treatment decisions were directly influenced by the high- or low-risk category from the report, that is, some high-risk patients did not receive chemotherapy and vice versa (Table 36).
| Initial recommendation | Post ODX | |||
|---|---|---|---|---|
| RS Group | HT | CT | RS Group | HT |
| Low | 30.5% | 23.8% | Low | 30.5% |
| Int. | 16.2% | 10.5% | Int. | 16.2% |
| High | 8.6% | 10.5% | High | 8.6% |
| All | 55.24% | 44.76% | All | 55.24% |
The risk of recurrence for patients in the low-, intermediate- and high-risk groups assessed by OncotypeDX and the impact of chemotherapy on risk of recurrence by risk group was taken from Paik et al. 49 The risk of recurrence was assumed to be constant over time, modelled by an exponential distribution. All patients within each OncotypeDX risk category were assumed to have the average risk of recurrence for that group.
The probability of dying from distant metastasis was derived from Thomas et al. ,157 assuming a median life expectancy of 3.3 years. Again, this was assumed to be the same for all risk groups.
Utilities were extracted from the published literature. The quality of life associated with recurrence (0.60) was taken from Milne et al. ,158 who reported an analysis in New Zealand women with advanced breast cancer and assumed treatment with endocrine therapy. The disutility associated with chemotherapy (−0.07) was taken from Peasgood et al. 159 The health utility associated with 1 year in the recurrence-free state (0.78) was assumed to be the same during and after endocrine therapy. 160
Summary of resource utilisation and cost data
All drug costs were taken from the British National Formulary (BNF). 161 Five endocrine therapy regimes were considered in line with NICE guidelines: (1) tamoxifen for 5 years, (2) anastrozole for 5 years, (3) letrozole for 5 years, (4) tamoxifen for 2 years plus exemestane for the final 3 years and (5) tamoxifen for 5 years followed by extended therapy with letrozole for a further 3 years. The probability that a patient was treated with each regime was taken from NICE TA112. 162 The annual cost over the first 5 years was £669.03 and the annual cost over the following 3 years was £108.40. All patients were assumed to be 100% compliant with endocrine therapy. Adverse event probabilities and costs of adverse events associated with endocrine therapies were derived from Hind et al. 163 and inflated to 2010 prices.
Patients treated with chemotherapy were assumed to receive six cycles of FEC75 (5-fluorouracil, epirubicin and cyclophosphamide) based on the regime description of FEC75 given by Avon, Somerset and Wiltshire Cancer Services in its chemotherapy protocol documents. 27 Administration costs were taken from the National Schedule of Reference Costs 2009–10164 for NHS trusts on an outpatient basis. The costs of adverse events and the probability of their occurrence was taken from Wolowacz et al. 165 for patients treated with CAF in the absence of sufficient evidence for FEC. The model did not include long-term adverse events associated with chemotherapy.
Finally, it was assumed that all patients were treated with granulocyte colony-stimulating factor (G-CSF) in order to prevent neutropenia.
Summary of cost-effectiveness
The base-case analysis is presented in Table 37. The addition of OncotypeDX to current practice (clinical and pathological parameters) resulted in an ICER of £6231.91 per QALY gained.
| Usual care | OncotypeDX testing | Difference | |
|---|---|---|---|
| Cost (£) | 11,847.24 | 12,734.93 | 887.69 |
| QALYs | 11.39 | 11.54 | 0.14 |
| Life expectancy (years) | 14.73 | 14.89 | 0.16 |
| ICER (£/QALY gained) | 6231.91 | ||
| ICER (£/life-year gained) | 5633.30 |
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
|---|---|---|
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
One-way sensitivity analysis showed that the base-case outcomes were most sensitive to variation in patient age, the cost of OncotypeDX testing and the change in chemotherapy recommendations for low-risk patients. The PSA results indicated that, at a willingness-to-pay threshold of £20,000 per QALY, there was a 99.6% probability that OncotypeDX would be cost-effective compared with current clinical practice (Figure 10).
FIGURE 10.
Cost-effectiveness acceptability curve for the probability of OncotypeDX being cost-effective at various willingness-to-pay thresholds estimated by Genomic Health. 27
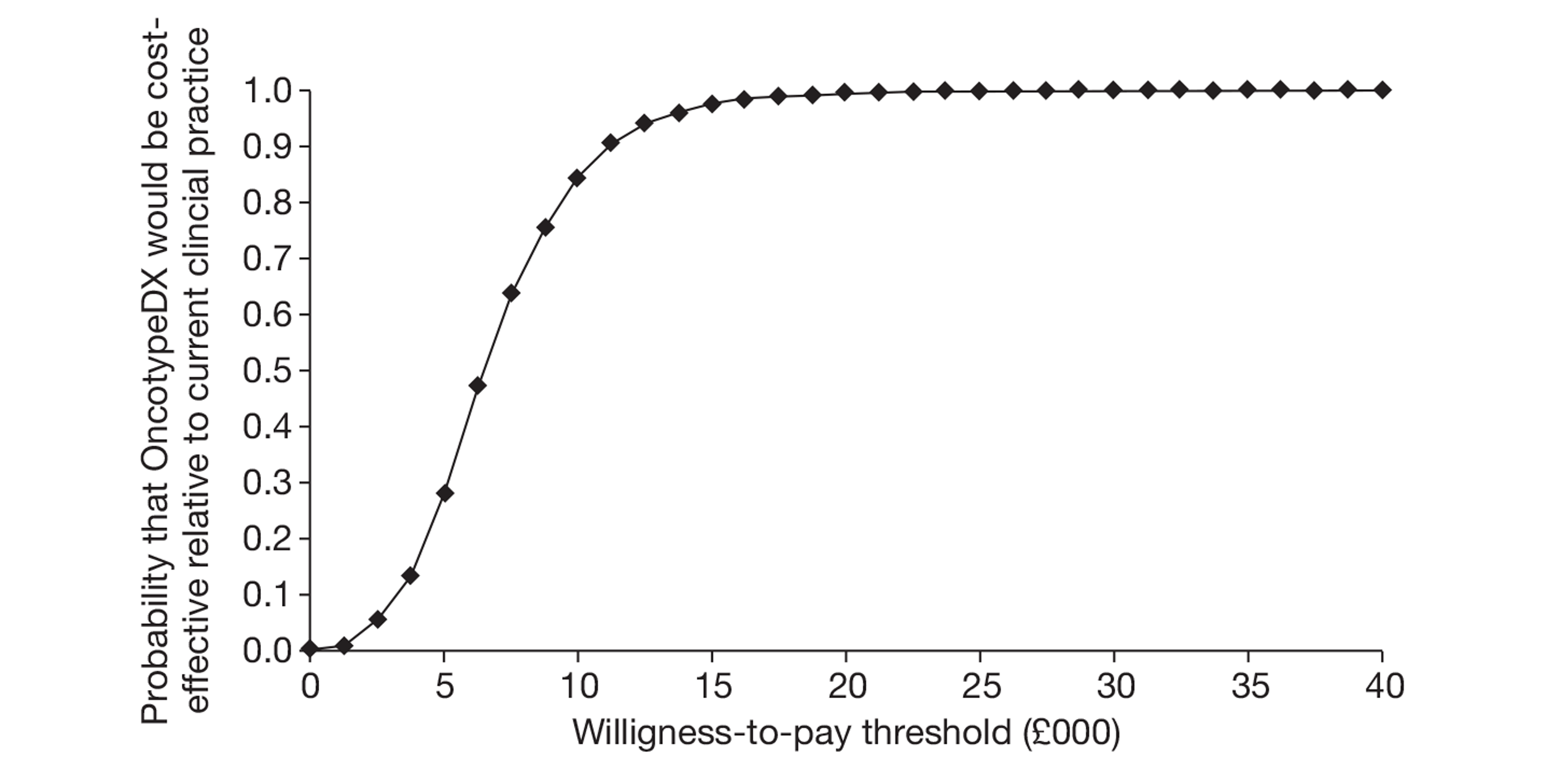
A scenario analysis was also presented for node-positive patients using data from treatment decisions in node-positive patients in the German setting. 166 (Commercial in-confidence information has been removed.)
Critique of the economic evaluation submitted by Genomic Health
The EAG has reviewed the economic model and report submitted by Genomic Health. A detailed critique is presented below. In summary, the model was considered to be of a good standard given the evidence available; however, there are a number of limitations with the model structure, assumptions and data inputs that need further consideration.
Impact of OncotypeDX on chemotherapy decision-making in the UK
The economic model used data from Holt et al. 78 to reflect current practice in England and Wales and the impact of OncotypeDX on treatment recommendations. 27 The study was conducted in a Welsh cohort and is the only identified evidence of the impact of the test on UK decision-making.
The EAG have several concerns regarding the use of the Holt et al. study. These concerns have been discussed in Chapter 3, Results: OncotypeDX and are further detailed in Model inputs: test-specific parameters. Briefly:
-
Data used to populate the economic model were taken from a preliminary analysis conducted in a small sample of 106 patients.
-
The study was conducted in Wales in two centres and it is unclear to what extent results are generalisable to the rest of England and Wales.
-
It is unclear how the decision to recommend chemotherapy was made.
-
There are concerns that patients may not be representative of patients seen in clinical practice in England and Wales. In Model inputs: test-specific parameters, the NPI distribution of patients included in the Holt et al. study78 is compared with the NPI distribution of patients from two registries [Eastern Cancer Registration and Information Centre (ECRIC) and West Midland Cancer Intelligence Unit (WMCIU)], which shows that patients included in the Holt et al. study were more severe – with larger tumours and a higher proportion of grade II and III tumours (analysis conducted by the EAG).
-
The proportion of patients recommended for chemotherapy under current practice in the Holt et al. study appears to be overestimated when compared with the proportion of patients who are actually offered chemotherapy, based on data from two cancer registries in England and Wales (see Model inputs: test-specific parameters). If this is the case, use of these data in the model may increase the predicted benefits derived from the use of OncotypeDX, resulting in a potential overestimation of the ICER.
Because of these limitations, the EAG did not consider the Holt et al. study to be an appropriate study to reflect current practice in England and Wales.
Risk of recurrence
In the absence of follow-up in the Holt et al. study,27,78 a separate data source was used to estimate the risk of recurrence for patients classified as being of low, intermediate or high risk of distant recurrence with OncotypeDX. The 10-year risk of distant recurrence used in the model for patients on endocrine therapy (tamoxifen) was 3.2% for the low RS group, 9.1% for the intermediate RS group and 39.5% for the high RS group. 49 The EAG expresses three main concerns with these data:
-
Data were taken from a US cohort of women prior to 2006 and therefore the results might not be transferable to current treatment practice for women in England and Wales.
-
The Paik et al. study49 is based on pre- and postmenopausal women who received tamoxifen only; however, a mixture of different endocrine therapies is now used in the UK.
-
Biases could have been introduced because two separate sources of data were used for the risk classification and risk of recurrence. Although the EAG acknowledges the rationale of the manufacturer to use two separate data sources, the EAG considers this approach to be inappropriate because of the high correlation between the two parameters. This is particularly important as the ICER is likely to be sensitive to these assumptions. Data from a previous US study indicated a 10-year risk of distant recurrence of 6.8%, 14.3% and 30.5% for the low, intermediate and high RS groups, respectively, in women treated with tamoxifen only. The TransATAC trial conducted in the UK79 showed a 10-year risk of recurrence of 4.0%, 12.0% and 25.0% for women classified in the low, intermediate and high RS groups, respectively, based on postmenopausal, LN−, HER2+/− women treated with tamoxifen and anastrozole. In sensitivity analyses, the manufacturer shows that using data for the risk of recurrence from the TransATAC trial increases the ICER from £6232 to about £9160 per QALY gained.
In addition, the manufacturer assumed the risk of recurrence to be constant over time. Evidence shows that the hazard of distant recurrence declines with time, with a plateau after approximately 15 years. 167 Assuming a reduction in recurrences over time, and certainly beyond 15 years, would have resulted in a higher ICER as the use of OncotypeDX would prevent fewer recurrences.
Finally, the risk of recurrence was applied according to the OncotypeDX classification, that is, patients recommended chemotherapy or not using current clinical practice have the same risk of recurrence. However, it seems likely that patients who are recommended chemotherapy within current clinical practice (based on the use of the NPI and/or Adjuvant Online) have a higher risk of recurrence than patients who are not recommended chemotherapy (even within the same RS group). For example, as shown in Model inputs: test-specific parameters, the risk of distant recurrence for patients classified using OncotypeDX is different from the risk of distant recurrence for patients classified by NPI. Ignoring the prognostic value of the treatment decision using clinicopathological parameters is likely to produce a more favourable ICER.
Patients who are offered the test
The model assumes that OncotypeDX is given to all women with ER+ and LN− or single node-positive early breast cancer. However, clinical opinion indicates that in the UK only a subgroup of patients might be offered OncotypeDX – those patients at intermediate risk for whom the decision for adjuvant treatment is uncertain. Assuming that all women receive the test is considered to be a conservative assumption and the ICER is likely to be more favourable if only selected patients receive the test.
Benefit of chemotherapy
Data from the Paik et al. study49 were used to determine the benefit of chemotherapy. Although the study showed a consistent benefit in women classified as being of intermediate or high risk of distant recurrence with OncotypeDX, there are some concerns with the study design. Indeed, data from the training set (used to develop the test) were used to estimate the benefit of chemotherapy. This is likely to positively bias the observed effect of chemotherapy. More discussion is available in Chapter 2, OncotypeDX test. Given that the Paik et al. study is based on patients who received tamoxifen only, it is not clear how this evidence relates to women in the UK who currently receive a mixture of different endocrine therapies. This impact is not, however, expected to be large as the use of different endocrine therapies does not generate large differences in OS. In addition, the study included women with HER2+ early breast cancer. Those women are likely to have a high risk of distant recurrence (and are more likely to be classified with a high RS) and derive a greater benefit from chemotherapy.
The chemotherapy regimen used to define the impact of chemotherapy by risk group in Paik et al. 49 was CMF (cyclophosphamide, methotrexate and 5-fluorouracil) or MF (methotrexate and 5-fluorouracil). Discussion with clinical experts indicated that newer and more effective regimens are used in the UK. The economic model assumes the use of FEC75 in the UK. The impact of this assumption (on both efficacy and cost) has not been discussed. It is not known how this will influence the impact of chemotherapy on distant recurrence.
Time between recurrence and death
The economic model submitted by Genomic Health assumed that the time between distant recurrence and death was the same irrespective of the risk group. Discussion with clinical experts indicated that it is likely that the time between recurrence and death may be shorter for patients at high risk. This has not been discussed by the manufacturer and it is unclear how this would affect the ICER.
Exclusion of local and regional recurrences
The economic model submitted by Genomic Health included only distant metastases. The omission of local and regional recurrences is likely to produce a less favourable ICER as additional benefits might be accrued by the use of the new test with no additional cost.
Cost and utility associated with recurrence
The cost of recurrence was taken from the study by Thomas et al. ,157 which was conducted in a mixture of patients, some with ER−, HER2+ and LN− early breast cancer. The manufacturer discussed the limitations of using data from this study.
There are concerns that the cost of recurrence is applied as a one-off cost, which has implication when discounting costs. It is further assumed that patients remain in the recurrence health state for 3.3 years, whether they are aged 60 years or 90 years. However, in reality, older women are likely to spend less time in the recurrence health state. It is unclear how this would affect the ICER as it potentially results in an overestimation of the cost of distant recurrence but also of the QALYs gained whilst in the recurrence health state.
Adverse events
Long-term adverse events associated with chemotherapy, such as cardiotoxicity and secondary cancers, are not captured in the model. This is likely to produce a less favourable ICER if the use of OncotypeDX reduces the proportion of patients receiving chemotherapy, as it does in the Genomic Health model.
Short-term adverse events were included in the model. Costs relating to the use of G-CSF to prevent neutropenia were considered to be overestimated as it was assumed that all patients receive G-CSF for each of the six cycles. This is a concern given the high cost of G-CSF in the model. The cost of G-CSF accounts for £4118 (53%) of the total cost of chemotherapy (drug, administration, monitoring, adverse events) in the model (£7728). Discussion with clinical experts indicated that in the UK G-CSF is typically used for the secondary prevention of febrile neutropenia (i.e. after an event or following a dose delay due to neutropenia); it is given only to a proportion of patients (approximately 25%) as secondary prophylaxis for all subsequent cycles (five or fewer) following an episode of febrile neutropenia or dose delay. This assumption used in the Genomic Health model is likely to produce a lower (more favourable) ICER.
Cost of endocrine therapy and chemotherapy
Finally, the economic model submitted by Genomic Health assumed no wastage and a dosage per body surface area (BSA) of 1.8 mg/m2. A UK study reported that the mean dosage per BSA for women with breast cancer in the UK was 1.75 mg/m2. 168 This is likely to overestimate the drug cost based on the BSA, such as the cost of chemotherapy or endocrine therapy, and therefore produce a more favourable ICER.
Probabilistic sensitivity analysis
Finally, the EAG had some concerns about the PSA conducted by the manufacturer. The benefit of chemotherapy for patients classified as low risk with OncotypeDX was not varied in the PSA. Although no benefit (reduction in distant recurrence) was observed for this group of patients (HR 1.31), the CI was wide enough (95% CI 0.46 to 3.78) that a benefit is not impossible. 49 Costs were varied in the PSA assuming a normal distribution and an arbitrary SE of 10% around the mean. Neither the proportion of patients receiving chemotherapy under clinical practice nor the classification of patients was varied in the PSA. The change in treatment allocation after knowledge of the OncotypeDX result was, however, varied using a normal distribution using an arbitrary SE of 10%. The EAG did not consider this approach appropriate as this ignores the correlation between the risk reclassification used for both the comparator and the intervention arms and the change in treatment allocation for the intervention arm.
Assessment of the economic evaluation submitted by Clarient
An economic evaluation for the use of Mammostrat in the UK (report only) was submitted by Clarient late in the appraisal process shortly before the finalisation of the EAG report. 169 Because of its direct relevance to the UK, the EAG felt it useful to report the method and main finding. Because of the late submission and time constraints, only a brief description and critique is reported thereafter.
The submission169 included a full report only and therefore it was not possible for the EAG to check the economic model.
Brief description of the economic model submitted by Clarient
Description of the method and data inputs
The model compared the cost-effectiveness of treatment guided using Mammostrat with that of treatment guided using the NPI in women with ER+, LN− early breast cancer in the UK, including both pre- and postmenopausal women. Patients were followed up for 10 years and the study adopted the perspective of the UK NHS, with costs and QALYs discounted at 3.5%. The economic model used a Markov approach with individuals moving between three possible health states: recurrence free, recurrence (all recurrences) and death (from breast cancer or other causes).
(CIC information has been removed.)
Summary of results
(CIC information has been removed.)
(CIC information has been removed.)
Critique of the economic evaluation submitted by Clarient
It was not possible for the EAG to check the economic model as only the report was provided by the manufacturer. Based on the description of the model alone the robustness of the model cannot be verified. A large number of assumptions were made to link the evidence available and it is not possible to fully assess the impact of this. Therefore, the results from this study should be interpreted with caution.
Because of time and resource constraints and the absence of the Microsoft Excel model, it was not possible to provide a detailed critique of the economic evaluation submitted by Clarient; however, the main limitations/concerns are highlighted below:
-
The model uses a 10-year time horizon. This is believed to be very short given that recurrences can usually occur after 10 years.
-
(CIC information has been removed.)
(CIC information has been removed.)
Relevance of existing cost-effectiveness evidence for NICE decision-making
The existing cost-effectiveness evidence has limited relevance for the UK setting. Only two of the nine tests (OncotypeDX and MammaPrint) have any published cost-effectiveness evidence to date132,134–136,146 and each presented a number of limitations (see Systematic review of existing cost-effectiveness evidence).
Genomic Health and Clarient each submitted an economic evaluation considering the cost-effectiveness of OncotypeDX26 and Mammostrat169 in the UK, respectively, and the submitted economic evaluations are therefore potentially more relevant for UK decision-making. However, there were a number of issues in the evaluations that require further consideration:
-
the assumption about the baseline level of chemotherapy in clinical practice in England and Wales
-
the assumption about the risk of distant recurrence in a UK population
-
the assumption about the proportion of patients who would be offered chemotherapy after reclassification with the new test in England and Wales
-
the assumption about who would be offered the test in England and Wales
-
the assumptions about the cost of chemotherapy and the treatment of adverse events generated by the chemotherapy in England and Wales.
Independent economic model: methods
This section of the report describes the development of the de novo economic model. The following sections describe the population under assessment, the interventions to be modelled, the comparators and the subgroups of interest. The economic model is described in Description of the de novo economic model. This gives an overview of the model and a more detailed description of the model structure, followed by a description of the model input parameters – first, those common to all models (including costs and utilities) and, second, those that are test specific (clinical parameters).
The key objective of the economic assessment is to address the cost-effectiveness of the use of GEP and expanded IHC tests compared with current practice to guide adjuvant chemotherapy decision-making in women with early breast cancer in England and Wales. Only two of the tests, OncotypeDX and MammaPrint, have published evidence about their economic value134–136,146 but these evaluations are not UK specific. Two UK economic evaluations were submitted by Genomic Health (OncotypeDX)27 and Clarient (Mammostrat)169 as part of the NICE request for additional information to the manufacturers. The review of the published cost-effectiveness evidence and the critique of the economic evaluations submitted by the manufacturers for this appraisal revealed a number of limitations that need to be addressed.
Therefore, a de novo economic model was constructed to address these limitations where possible and to estimate the cost-effectiveness of a wider range of GEP and expanded IHC tests. Notably, the EAG economic assessment uses UK-specific data and addresses limitations over the proportion of patients who currently receive chemotherapy in England and Wales and the risk of distant recurrence in a UK population; carries out a subgroup analysis offering the test to patients who are considered the most likely to benefit from the test; and seek to undertake a more accurate estimation of the cost of chemotherapy in England and Wales.
The economic model considers the selection of patients for chemotherapy using the new tests (intervention arm) compared with the selection of patients for chemotherapy using current prognostic tools (comparator arm). Patients who receive chemotherapy are assumed to experience a reduction in the risk of recurrence (and subsequent deaths) compared with those patients who receive endocrine therapy only. The costs of chemotherapy, along with the costs and the reduction in quality of life resulting from the adverse events associated with the chemotherapy, are taken into account within the model.
Population under assessment
The NICE scope25 identifies the population under assessment as people diagnosed with early breast cancer. However, most of the GEP and expanded IHC tests have been developed for use in a specific subpopulation or have evidence of efficacy only within a specific subpopulation (see Chapter 2, Results). The economic assessment focuses on women with ER+, LN−, HER2− early breast cancer. This subgroup was selected after review of the evidence available (see Chapter 2, Results) and the indications of the tests (see tables 6 and 7), discussion with clinical experts and the perceived likelihood of the use of the test resulting in a change in current clinical practice. 170 This was considered to be the population for which the new tests had the most robust evidence base and the population in which the tests were most likely to be used in the first instance in England and Wales. Patients with HER2+ early breast cancer or with positive nodes were not considered in this assessment because of time and resource constraints and lack of evidence, but they should be the subject of future research. Of particular note, the role and cost-effectiveness of GEP and expanded IHC tests in LN+ women may be explored as part of the planned Optimal Personalised Treatment of breast cancer using Multi-parameter Analysis (OPTIMA) trial, although funding for this trial is not yet confirmed. The proposed aim of the OPTIMA trial is to identify an effective method, using multiparameter analysis, to target women with ER+, HER2 normal primary breast cancer who are likely to benefit or not from chemotherapy. A health economic evaluation is planned as part of the study (Dr Peter Hall, Clinical Research Fellow, University of Leeds, July 2011, personal communication).
Interventions
Nine tests were identified by NICE in the scope25 (OncotypeDX, MammaPrint, Mammostrat, IHC4, PAM50, BCI, Randox BCA, NPI+ and BluePrint). These tests are described in detail in Chapter 1, Description of technologies under assessment. Our systematic review of the evidence (see Chapter 2, Results) indicated considerable differences in the level, quality and reporting of evidence between tests. Although some of the included tests have a relatively well-developed evidence base, some tests are still under development or have a relatively immature evidence base (e.g. NPI+, Randox BCA). Furthermore, there are differences in the output of the tests. Many of the tests predict the likelihood of distant recurrence, providing either a risk of recurrence score (as a continuous scale) or a risk category (e.g. high/low), but three of the tests (Randox BCA, current version of the PAM50 test and BluePrint) provide information about subtyping alone. The impact of information on subtype on the management of patients with early breast cancer is not yet clearly understood. No evidence on the impact of subtype information on clinical decision-making in early breast cancer in England and Wales was identified. This makes the potential comparison between tests particularly difficult.
To allow a sensible comparison between tests based on the available evidence, and given the time and resource constraints of the project, the EAG defined four minimum criteria that a test had to fulfil to be included in the economic evaluation. These criteria have been defined after consideration of the NICE scope,25 discussion with clinical experts and consideration of the review of the existing cost-effectiveness evidence:
-
The test has been validated in an external cohort (clinical validity).
-
There is evidence about risk reclassification against one of the comparators defined by NICE within the scope (i.e. NPI, Adjuvant! Online or clinical practice in the UK). 25 In other words, there is evidence on how the new test reclassifies patients into risk groups relative to their initial risk group as defined by current practice.
-
The test provides an estimate of risk of recurrence in the form of a risk score or risk category. Following discussion with clinical experts tests that provide only information about subtyping were excluded as it is not yet clear how this knowledge will impact on the treatment decision-making process.
-
The outputs of the test, which will be used to inform the decision about whether or not to offer chemotherapy, are well defined.
A summary of these criteria for each of the nine tests considered for this appraisal is presented in Table 39.
| Test | External validation of the test in an independent cohort | Evidence about risk reclassification | Final version of the test provides risk of recurrence | Clear use of the test | Other comments |
|---|---|---|---|---|---|
| Included | |||||
| OncotypeDX | ✓ | ✓ Adjuvant! Online, NPI, clinical practice | ✓ | ✓ | |
| MammaPrint | ✓ | ✓ Adjuvant! Online, NPI | ✓ | ✓ | |
| Mammostrat | ✓ | ✓ NPI | ✓ | ✓ | |
| IHC4 | ✓ | ✓ NPI (OncotypeDX) | ✓ | ✓ | |
| PAM50 | ✓ | ✓ OncotypeDX, Adjuvant! Online | ✗ In vitro diagnostic version in development | ✓ | Reclassification evidence in a mix of LN+/− |
| Excluded | |||||
| BluePrint | ✗ | ✗ | ✗ | ✓ | |
| NPI+ | ✗ Nearing completion | ✓ Unpublished | ✓ | ✗ | |
| Randox CA | ✗ | ✗ | ✗ | ✗ | |
| BCI | ✗ | ✗ | ✓ | ✓ | |
Overall, only a subset of these tests met the criteria for inclusion in the economic evaluation defined by the EAG: OncotypeDX, MammaPrint, IHC4 and Mammostrat.
Although the PAM50 test has evidence about risk reclassification against OncotypeDX and Adjuvant! Online,171 this test was excluded for the following reasons:
-
PAM50 was not available in the UK at the time of writing of the report. Furthermore, the current version of the commercialised test (not available in the UK) does not provide a risk score but only information about subtyping. Following discussion with clinical experts it remained unclear how subtyping would be used to inform treatment decisions. An in vitro diagnostic version of the test is expected to be commercialised and this version will calculate a risk score; however, this is still under development.
-
The evidence for risk reclassification was derived from a cohort in which the majority of women had positive nodes and therefore fall outside the subgroup of interest for this assessment.
The evidence base for NPI+ is developing. External validation of the test in an independent cohort is currently underway but has not yet been published. At the time of writing this report there was no published evidence on risk reclassification with NPI+.
There is no published evidence on risk reclassification against any of the comparators defined in the scope for BluePrint, Randox BCA and BCI. Furthermore, BluePrint and Randox BCA provide subtyping only.
Interventions to be assessed in the economic evaluation
Four tests were evaluated: OncotypeDX, MammaPrint, IHC4 and Mammostrat. These tests are described in detail in Chapter 1, Description of technologies under assessment. However, as indicated in the systematic review of the literature conducted as part of this project, the level and quality of evidence for these tests varies considerably.
The primary analysis evaluated the cost-effectiveness of adjuvant chemotherapy guided using OncotypeDX and IHC4. The systematic review of the evidence indicated that OncotypeDX is the furthest along the validation pathway compared with other similar tests, and the evidence base, in particular in relation to the prognostic ability of the test, was considered to be reasonably sound. The evidence for IHC4 is less developed; however, there is evidence relating to the performance of IHC4 compared with OncotypeDX and this allowed both tests to be modelled within the same model structure. A number of additional assumptions were, however, necessary to evaluate the cost-effectiveness of IHC4.
The final algorithm for IHC4 calculates a risk score for distant recurrence based on ER, PR, HER2 and Ki-67 in addition to classical clinical and pathological variables (composite risk score IHC4 + clinical score). This version of the algorithm was considered in the economic analysis (the term IHC4 will be used for simplicity but it refers to the composite risk score IHC4 + clinical score). Of note, an online calculator is expected to be made available (Professor Mitch Dowsett, Royal Marsden Hospital, London, July 2011, personal communication).
Analyses were performed for MammaPrint and Mammostrat but these were considered to be exploratory as there were significant gaps and/or limitations in the evidence base available for both tests (see Model inputs: test-specific parameters).
Comparators
Description of potentially relevant comparators
NICE CG807 indicates that adjuvant therapy should be considered for all patients with early invasive breast cancer after surgery, based on assessment of the prognostic and predictive factors alongside the potential benefits and side effects of the treatment. The guidelines recommend consideration of the use of Adjuvant! Online to support estimation of individual prognosis and the absolute benefit of adjuvant treatment for patients with early invasive breast cancer. 7 In addition, guidelines based on NPI are widely used in England and Wales. Clinical opinion suggests that there is wide variation in clinical practice between centres in the UK, with some centres using Adjuvant! Online, some using NPI-based guidelines and some using a combination of the two.
Adjuvant! Online
Despite NICE recommendations to use Adjuvant! Online,7 clinical experts indicated that it is not comprehensively used in the UK for a number of reasons:
-
It is based on a US population and there are some difficulties in applying the Adjuvant! Online data to the UK population.
-
Although it is a useful aid for discussing risk of recurrence and benefits of chemotherapy with patients, it is viewed by some as complex to use and interpret for decision-making purposes.
-
It cannot be used by all NHS trusts as access is blocked by some trusts for information technology security reasons.
The published evidence reports outcomes based only on the risk of recurrence estimated using Adjuvant! Online. However, both the risk of recurrence and predicted impact of adjuvant treatments would be used to inform treatment decisions.
Nottingham Prognostic Index
Nottingham Prognostic Index-based guidelines are widely used in some parts of the UK to inform decisions about adjuvant chemotherapy. The NPI forms part of the National Cancer Dataset for breast cancer so the NPI score should be given in the report of every invasive breast cancer case in the UK. It is simple to use although it may be considered to be less informative and therefore potentially less useful than Adjuvant! Online, particularly when discussing prognosis and potential treatments with patients.
Comparator used in the economic model
The comparator used in the model was current clinical practice. Clinical opinion indicated that, although NPI and Adjuvant Online! are used to aid the decision-making process, the decision whether or not to offer adjuvant chemotherapy to a specific patient is complex and includes other demographic and pathological parameters. Consequently, the EAG economic assessment used cancer registry data to reflect current clinical practice in England and Wales in terms of the proportion of women who currently receive chemotherapy. Summary data from ECRIC and WMCIU were obtained to populate the economic model (West Midland Cancer Intelligence Unit, July 2011, personal communication; Eastern Cancer Registration and Information Centre, July 2011, personal communication). The use of registry data reflects decision-making based on actual clinical practice, using NPI and/or Adjuvant! Online or other prognostic information.
The ECRIC registers all malignant tumours and some precancerous lesions occurring in people resident in the East of England at the time of diagnosis. Analyses for this assessment were constrained to women with ER+, LN−, HER2− early breast cancer (stage I or II) aged < 75 years at diagnosis. An age cut-off was applied to reflect the fact that older women are likely to benefit less from the test (with a high proportion ineligible or unwilling to undergo chemotherapy because of frailty, comorbidities, etc.). It is acknowledged that there is no specific age cut-off but in practice the proportion of women receiving chemotherapy falls significantly for women aged ≥ 70 years and is very low for women aged ≥ 75 years. 172 Overall, 4475 patients were included in the analysis from 2007 onwards. Of these, around 800 had unknown HER2 status. The mean (median) age of included patients was 58.3 (60.0) years. The mean (median) tumour size of included patients was 16.9 (14.0) mm and 23.7% had grade I breast cancer, 56.0% grade II breast cancer and 20.2% grade III breast cancer.
The WMCIU registers all malignant tumours and some precancerous lesions occurring in people resident in the West Midlands. Again, analyses were constrained to women with ER+, LN−, HER2− invasive breast cancer and who were aged < 75 years at diagnosis. The WMCIU had incomplete information on stage; therefore, early breast cancer was defined as women with no metastases and having had surgery (mastectomy or breast-conserving surgery). Data for the years 2007 and 2008 were available but data from 2007 only were used in the economic model as this was believed to be more accurate as the data were supplemented by national audit data (West Midland Cancer Intelligence Unit, July 2011, personal communication). Overall, 1214 patients with ER+, LN−, HER2− early breast cancer, who were diagnosed in 2007, were included. The mean (median) age of included patients was 58.0 (60.0) years. The mean (median) tumour size of included patients was 17 (15) mm and 26.6% had grade I breast cancer, 56.5% grade II breast cancer and 16.5% grade III breast cancer.
Cancer registry data from ECRIC and WMCIU were combined by the EAG and used in the base-case analysis to reflect the current levels of chemotherapy in England and Wales for women with ER+, LN−, HER2− early breast cancer. Data were obtained for patients with a NPI score ≤ 3.4 and patients with a NPI score > 3.4 to allow a subgroup analysis to be performed and to take account of the prognostic value of the current treatment decision based on clinicopathological parameters. Registry data reflect how both NPI or Adjuvant! Online are used currently in the decision-making process; however, it is not known which particular tools/guidelines (e.g. NPI or Adjuvant! Online, both, other tools) were used to inform adjuvant treatment decisions in the trusts within these cancer registry areas. For the purposes of the economic model it is assumed that data from these two areas are representative of all trusts in England and Wales. The term ‘clinical practice’ is used to define the comparator selected for this appraisal (i.e. current levels of adjuvant chemotherapy, based on the use of current prognostic tools, such as NPI and Adjuvant! Online).
Subgroups for whom the new tests are most likely to be used
Previous economic evaluations have typically assumed that the new tests will be offered to all women with ER+, LN−, HER2− early breast cancer. However, after discussion with clinical experts, it seems likely that, in England and Wales, the new tests may be targeted at a subgroup of this population – those at intermediate risk (and typically those aged < 75 years) for whom the decision about whether or not to give chemotherapy is most uncertain. The definition of this ‘intermediate group’ is not clear-cut (see Chapter 1, Identification of important subgroups) but clinical advice suggested that usually typically patients with a NPI score of ≤ 3.4 are unlikely to receive chemotherapy (except for a few very young women with aggressive early breast cancer).
Consequently, two analyses are presented:
-
The new test is given to all women with ER+, LN−, HER− breast cancer.
-
The new test is given only to women with a NPI score > 3.4 (based on the assumption that the vast majority of women with a NPI score ≤ 3.4 would not be considered for chemotherapy).
Of note, this subgroup is a proxy for the intermediate-risk group that might benefit the most from the test, but this may subgroup also include patients at the top end of the NPI distribution for whom the decision of chemotherapy is more certain. This subgroup was used as it was not possible, because of data restrictions, to create an intermediate-only group by separating out the high NPI risk group. However, because our population is ER+, LN−, HER2− it is rare to have a patient with a NPI score > 5.4 and therefore the number of high-risk patients is expected to be low.
Finally, the EAG acknowledges that the cut-off is arbitrary and, although NPI is used in clinical practice to guide treatment decisions in some centres in England and Wales, treatment decision will not be based on NPI alone.
Description of the de novo economic model
Overview
A probabilistic decision-analytic model was developed to estimate the costs and QALYs of adjuvant chemotherapy guided by GEP and expanded IHC tests compared with current clinical practice (using cancer registry data) in England and Wales. The economic model was programmed using Microsoft Excel software (2011) and used a 6-monthly cycle length and followed patients over a lifetime horizon (100 years as the upper age limit) in the base case. Shorter time horizons were examined in sensitivity analyses. In accordance with NICE's interim methods guide for diagnostics,32 the economic model adopted the perspective of the UK NHS and Personal Social Services (PSS) with costs and benefits discounted at 3.5% per annum.
No prospective studies that follow patients from initial diagnosis through to final health outcomes have been identified for any of the tests. Two prospective studies, MINDACT (MammaPrint) and TAILORx (OncotypeDX), are ongoing but not due to report for several years (see Chapter 2, Results). The economic model therefore needed to combine clinical data from several different sources in order to model how the results from the new tests translated into final outcomes in the form of QALYs.
Four tests were selected for the economic evaluation (OncotypeDX, IHC4, MammaPrint and Mammostrat). It is envisaged that these tests will be used as an addition to existing prognostic tools. As indicated in the systematic review, there are differences in the level and quality of evidence supporting each of the tests. Three separate analyses were performed using the best direct sources of data available for each test and these should not be directly compared. This was carried out because the EAG considered that combining evidence from different studies, based on different methodologies and with different patient characteristics (see Chapter 2, Results), limited the conclusions that could be drawn from the analyses and, in particular, the comparisons that could be made between the analyses.
The primary analysis compared current clinical practice with the adjuvant treatment decision based on the addition of OncotypeDX to current clinical practice and the addition of IHC4 to current clinical practice. Two exploratory analyses were undertaken to compare current clinical practice with the addition of MammaPrint and Mammostrat to current clinical practice. These analyses were considered to be exploratory only because of significant limitations in the evidence base.
Model structure
The key objective of the economic assessment is to address the cost-effectiveness of the use of GEP and expanded IHC tests to guide adjuvant chemotherapy decisions in women with early breast cancer in England and Wales. The model takes into account the reduction in the risk of relapses (and subsequent deaths) associated with the use of adjuvant chemotherapy. It also takes into account the costs and reduction in quality of life resulting from the adverse events associated with the chemotherapy.
All patients in the model are assumed to receive endocrine therapy. A proportion of patients in the comparator arm (current practice) received chemotherapy, based on cancer registry data. In the intervention arm (addition of new test) patients were assigned into a risk category using the new test and this additional information influenced the decision to prescribe chemotherapy.
The economic model comprises three key components:
-
Patients were assigned to risk categories according to the assigned risk score/group using the new test.
-
Women who would receive chemotherapy, as well as endocrine therapy, were identified, using the additional knowledge of the assigned risk group.
-
The natural history of breast cancer for patients treated with endocrine therapy alone or with the addition of chemotherapy was then simulated using a state transition model.
These three components are described in detail in the following sections.
Assignment of patients into different risk groups
OncotypeDX, MammaPrint and Mammostrat assign women into risk groups – high, intermediate and low risk (OncotypeDX and Mammostrat) or good and poor prognosis (MammaPrint). The IHC4 test provides a risk score only; however, patients have been allocated into risk groups, similar to the OncotypeDX risk groups, for the purposes of this assessment (see Model inputs: test-specific parameters for more details).
In the economic model, women were first stratified into two NPI groups (women with a NPI score ≤ 3.4 and women with a NPI score > 3.4). This was carried out to allow the use of the test with different subgroups of patients to be explored and to allow adjustment of non-UK clinical evidence to reflect the NPI distribution in the UK population. This also takes into account the prognostic value of the treatment decision using clinicopathological parameters. Indeed, within the current treatment decision-making process based on clinicopathological parameters, it is possible to identify patients who are at a higher risk of distant recurrence. Within these two NPI groups, patients were further reclassified into low, intermediate or high risk (or low and high risk in the case of MammaPrint) of recurrence according to the outputs of the new tests.
In simple terms, patients are assigned into different boxes, each with a different prognosis. Patients are assigned to the same boxes for the comparator (current practice) arm or the intervention arm (GEP and expanded IHC tests) as the diagnostic tool does not affect the prognosis of those patients if there is no change in the adjuvant treatment.
Identification of women receiving adjuvant chemotherapy on the basis of the test results
Once women have been assigned into the different boxes (with different prognosis) the next step is to identify which women would receive adjuvant chemotherapy.
The aim of categorising patients into risk groups based on distant recurrence with the GEP and expanded IHC tests is to identify patients who have a greater chance of developing a distant recurrence/recurrence. The risk groups identified by the new tests are therefore expected to influence the targeting of chemotherapy. However, other factors will also influence the decision regarding chemotherapy, including clinical and pathological factors, along with patient choice. In clinical practice a proportion of women classified as low risk of distant recurrence using GEP and expanded IHC tests may still receive chemotherapy; similarly, a proportion of women considered to be at high risk may not receive chemotherapy, as shown in Spain173 or in the USA174 for OncotypeDX.
In the intervention arm of the economic model the proportion of patients who would receive chemotherapy is based on the expected interpretation of the test, for example women categorised as high risk of recurrence are more likely to receive chemotherapy than women categorised as low risk. Some previous analyses have assumed that chemotherapy is received based on the risk group only. For instance, all women defined as high risk receive chemotherapy. However, in clinical practice other issues are likely to impact on this decision (clinicopathological factors, age of patient, patient choice, etc.) and it is unlikely that 100% of high-risk patients will receive chemotherapy. An adjustment for such factors was therefore used in the model.
In the comparator (current practice) arm, the proportion of women receiving chemotherapy is based on cancer registry data. Two subgroups are considered: women with a NPI score ≤ 3.4 and women with a NPI score > 3.4. Because the model categorised women into boxes (defined by the new test a posteriori) and the oncologist is blind to the results of the new test, we assumed that the probability of receiving chemotherapy was the same in the current practice arm whether patients were reclassified as low, intermediate or high using GEP and expanded IHC tests. However, it is likely that patients who are classified as high risk by the new test are more likely to have been identified as high risk under current practice and, therefore, are more likely to have received chemotherapy than those patients classified as low or intermediate risk by the new test. To further explore this assumption, data from the Holt et al. study78 were analysed by the EAG to approximate the proportion of patients who were recommended chemotherapy by RS group before knowledge of the OncotypeDX score (analysis conducted by the EAG using individual patient-level data submitted by Genomic Health).
Overall, 30.43%, 30.30% and 68.42% of patients with a low, intermediate and high RS score were recommended chemotherapy before knowledge of the OncotypeDX test results. Preliminary analyses suggested that the proportion is likely to be higher for patients with a high RS, but the sample size was too small (69 in low RS, 33 in intermediate RS and 19 in high RS) to draw any definitive conclusion. While preliminary, this analysis suggested that our assumption (that the probability of receiving chemotherapy in the comparator is constant irrespective of the RS group) might be conservative as current practice using clinicopathological parameters does appear to add some prognostic value.
Natural history of breast cancer
The final part of the model was a Markov model. Patients were able to move between five possible health states: recurrence free (A), distant recurrence (B), local recurrence (C), long-term adverse events after chemotherapy (D) and death (from breast cancer, long-term adverse events or general causes – E).
As shown in Figure 11, patients enter the model in the recurrence-free survival health state (A) and remain in that health state until they develop a distant recurrence (B), have an adverse event after chemotherapy (D) or die from breast cancer or general causes or from their adverse event (E). After a distant recurrence (B), patients remain in this health state until they die from either breast cancer or general causes (E) or develop an adverse event for women treated with chemotherapy (D). Patients developing an adverse event after chemotherapy can remain in that health state, die from their adverse event or die from general causes (E). The estimation of long-term adverse events is simplistic. No distinction was made between patients developing long-term adverse events after a recurrence (B) and patients developing long-term adverse events in the recurrence-free health state (A). Furthermore, patients with a long-term adverse event were assumed to remain in that health state (D) until death (E) and were not allowed to move to other health states.
FIGURE 11.
Schematic of the model structure.
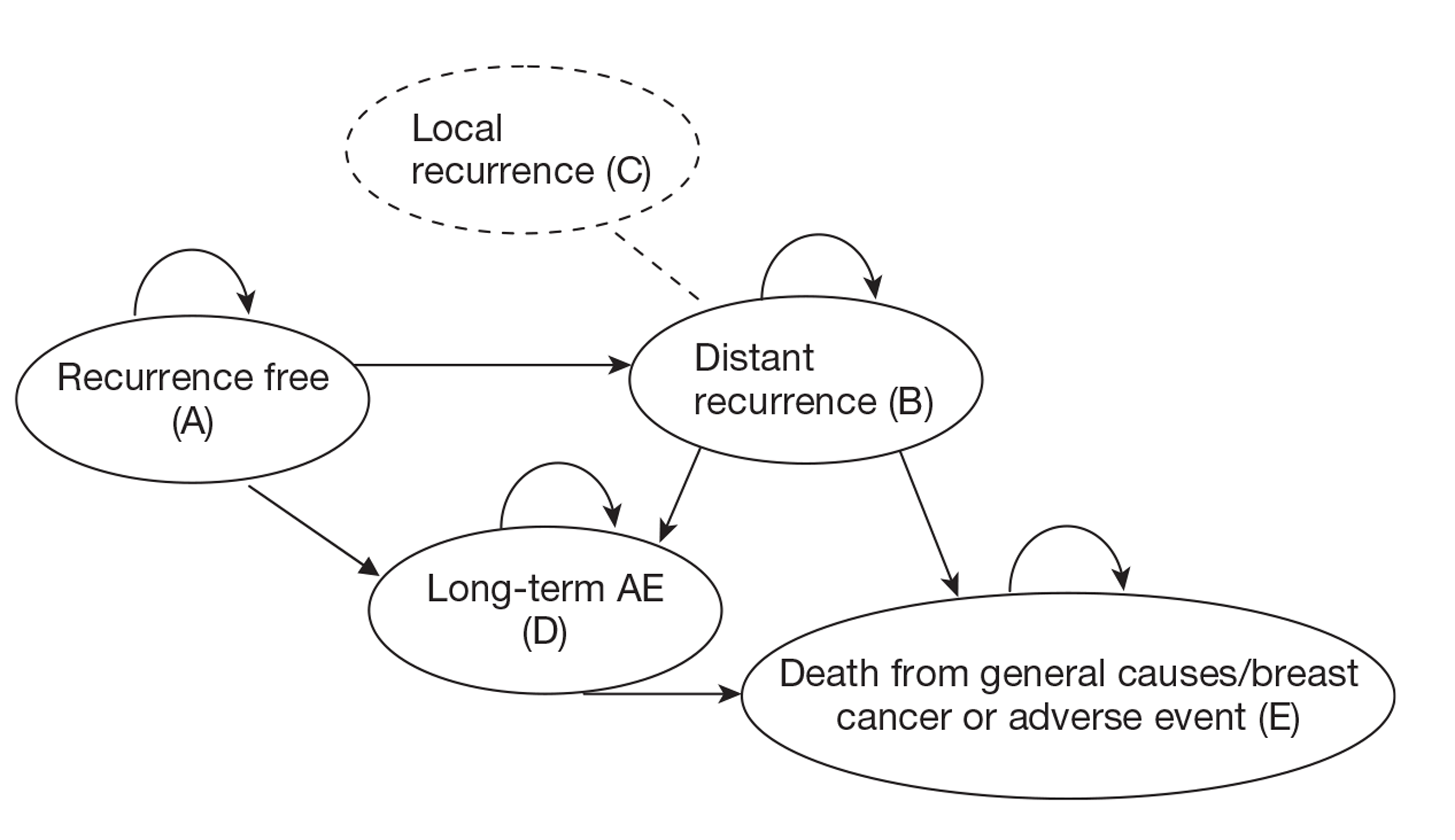
Local/regional recurrences have been modelled by considering the costs and quality of life decrements (disutility) assuming that a proportion of patients entering the distant recurrence state (B) have previously experienced a local recurrence (C). No transition probabilities were used between this health state and death or adverse events. This is simplistic but justified by the fact that the risk categories (used in the economic model) defined by the new tests (OncotypeDX, MammaPrint, IHC4) have been defined according to the risk of developing distant recurrence and there is no robust evidence to accurately model the development of local recurrence and the different transitions between health states for patients reclassified as low, intermediate or high risk with GEP and expanded IHC tests.
Model inputs: general
Model inputs that were common to the assessment of each of the four tests are described below. Model inputs that are test specific, such as clinical parameters, are described in Model inputs: test-specific parameters.
Mean age of patients entering the model
The EAG economic assessment focuses on women with ER+, LN−, HER2− who are aged ≤ 75 years. Patients were assumed to enter the economic model at a mean age of 58.3 years based on the average age in the ECRIC dataset of women with ER+, LN−, HER2− aged < 75 years (Eastern Cancer Registration and Information Centre, July 2011, personal communication). Although patient age is not used to determine treatment selection, experts suggested that women aged > 70–75 years are much less likely to be offered chemotherapy because of issues of frailty and comorbidities. Sensitivity analysis was conducted varying the mean baseline age.
Of note, the model does not separate pre- and postmenopausal women but most of the evidence was taken from postmenopausal women. In addition, it was not possible to explore different age thresholds as we did not have access to patient-level data.
Baseline Nottingham Prognostic Index distribution in England and Wales
The economic assessment separates women with a NPI score ≤ 3.4 and women with a NPI score > 3.4. The baseline NPI distribution was extracted from the combined (EAG analysis) ECRIC (2007 onwards) and WMCIU (2007 only) data (West Midland Cancer Intelligence Unit, July 2011, personal communication; Eastern Cancer Registration and Information Centre, July 2011, personal communication). Approximately two-thirds of patients had a NPI score ≤ 3.4 (Table 40).
| Cohort | NPI ≤ 3.4, n (%) | NPI > 3.4, n (%) | Total, n |
|---|---|---|---|
| ECRICa (2007 onwards) | 2602 (65.6) | 1365 (34.4) | 3967 |
| WMCIU (2007 only) | 819 (68.2) | 382 (31.8) | 1201 |
| Combined data used in the economic model | 3421 (66.2) | 1747 (33.8) | 5168 |
For the scenario assuming that the test is given to all women with ER+, LN−, HER2− early breast cancer, we modelled patients with a NPI score of ≤ 3.4 and patients with a NPI score > 3.4 separately to account for the prognostic value of the treatment decision using clinicopathological parameters.
Additional sources of evidence for the baseline distribution of NPI were considered in sensitivity analysis (Table 41). These included women with ER+, LN−, HER2− early breast cancer from the TransATAC trial (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication) and the ER+, LN−, HER2− population from the Holt et al. study78 (analysis conducted by the EAG using individual patient-level data submitted by Genomic Health).
| Source | NPI ≤ 3.4, n (%) | NPI > 3.4, n (%) |
|---|---|---|
| TransATAC | 449 (63.6) | 257 (36.4) |
| Holt (EAG analysis) | 70 (57.8%) | 51 (42.2%) |
Proportion of patients receiving chemotherapy in current clinical practice in England and Wales
As described in Comparator used in the economic model, cancer registry data were used to reflect the proportion of women with ER+, LN−, HER2− early breast cancer who currently receive chemotherapy in England and Wales. Data from ECRIC (2007 onwards; Eastern Cancer Registration and Information Centre, July 2011, personal communication) and WMCIU (2007 only) (West Midland Cancer Intelligence Unit, July 2011, personal communication; Eastern Cancer Registration and Information Centre, July 2011, personal communication) were combined (EAG analysis) and showed that overall about 14.4% of women aged < 75 years with ER+, LN−, HER2− early breast cancer received chemotherapy. When separating patients with a NPI score of ≤ 3.4 and a NPI score > 3.4, 4.6% and 33.6% of women received chemotherapy respectively (Table 42).
| Cohort | Entire cohort (%) | NPI ≤ 3.4 (%) | NPI > 3.4 (%) |
|---|---|---|---|
| ECRICa (2007 onwards) | 13.97 | 4.23 | 32.53 |
| WMCIU (2007) | 15.90 | 5.86 | 37.43 |
| Combined data used in the economic model | 14.42 | 4.62 | 33.60 |
Death from breast cancer causes after a distant recurrence
In the base case, the hazard rate of death after a distant recurrence was taken from Thomas et al. 157 Thomas et al. 157 analysed the time to death from relapse among 77 relapsed breast cancer patients. The first site of relapse was distant in 51 patients (66%) with the remaining patients having locoregional recurrences. The study included a mix of patients with regard to ER status (55% ER+), nodal involvement (66% LN−) and HER2 status (75% HER2−). The study reported a median survival of 40.1 months (equating to an annual hazard rate of about 0.30) and this value was used in the base case. Sensitivity analyses were conducted varying the time spent in the distant recurrence health state within the reported CI in this study. 155
In the base case we assumed that the risk of death after a recurrence was independent of the prognosis of the patient, because of the lack of more informative data. Discussion with clinical experts indicated that it is likely that low- and high-risk patients will spend a different amount of time in the distant recurrence health state. High-risk patients are likely to have more aggressive cancer and are likely to spend less time in recurrence before death. A scenario analysis was therefore explored assuming different times in recurrence between risk groups. Because there are no published data to our knowledge on survival after distant metastasis for patients with different prognosis, assumptions were made to examine the impact of this assumption on the ICER.
Costs
All costs are in 2010 prices.
No costs were assumed for treatment guided by current clinical practice as pathological parameters that are currently used to guide the adjuvant treatment decision will continue to be collected after the introduction of the new tests. The new tests are considered to be add-ons to the existing tests.
The costs of performing the OncotypeDX, MammaPrint and Mammostrat tests in the UK were assumed to be £2580, £2675 and £1120–1620 (£1135 was used in the economic model) respectively (data received from the respective manufacturers) (Table 43). The IHC4 algorithm is free; therefore, the only costs will be the additional time required for analysis of Ki-67 and PR (measured in some centres) and quantitative H scoring (a immunohistochemical approach used in the assessment of markers for breast cancer prognosis, by assessing the intensity and distribution of positive staining) of ER. The investigators of IHC4 were contacted to provide an estimate of the likely additional cost to the NHS. Although no formal costings have been made it was estimated that the additional cost of IHC4 would range between £100 and £200, including the pathologist's time for scoring (Professor Mitch Dowsett, Royal Marsden Hospital, London, July 2011, personal communication). In the base case we assumed that it cost an additional £150 to the NHS to run an IHC4 test (see Table 43). This figure is varied in the sensitivity analysis.
| Cost of the new test (£) | Cost of the additional NHS time (£) | Cost of handling fresh tissue (£) | |
|---|---|---|---|
| OncotypeDX | 2580 | ||
| IHC4 | 100–200 | ||
| Mammostrat | 1135 | ||
| MammaPrint | 2675 | 250 |
The MammaPrint test can be performed on fresh tissue preserved in RNARetain® (Asuragen, Austin, TX) or fresh frozen tissue (note: use of FFPE to be introduced in 2012). In addition to the cost of the test, we assumed an additional cost of £250 per patient for MammaPrint for the cost of handling fresh tissue. A sensitivity analysis was conducted assuming no additional cost. Fresh tissue collection is not routine in the NHS (only a few research centres currently have this working arrangement) so there will be additional costs, which would be considerable at hospitals where the dissection facilities are running at capacity (which is likely to be a significant proportion of hospitals) and where explicit staffing for collection of fresh tissue is not in place. Discussion with local clinicians indicates that capital costs could be at least £75,000 per hospital if new dissection tables are required, which is likely to be the case in many hospitals where routine fresh tissue sampling is not in place, and additional staff costs for biomedical scientists and histopathologists will be incurred. If a full fresh tissue service is required and needed to cover all theatre time then additional staff costs could be £20,000–50,000 per year (Simon Cross, Reader and Honorary Consultant, Royal Hallamshire Hospital, Sheffield, July 2011, personal communication). Experts indicated that a charge of about £250 per sample would be necessary to take a fresh tissue sample for a research study because of the time-critical staff-intensive work required. However, this assumes that a fresh tissue sample is collected only in a small number of patients under the current service configuration. A reconfiguration of the entire pathology service would be necessary if fresh tissue samples had to be collected routinely for all patients, which would incur additional costs.
We did not incorporate the additional cost associated with the failure of a test. This was considered to be minimal as contact with the manufacturers indicated that another sample could be sent for free or a refund issued in case of failure of the test.
Endocrine therapy costs
The economic model considers only women with ER+ early breast cancer and assumes that all patients receive endocrine therapy. Five endocrine therapy regimens were considered as per NICE Technology Appraisal 112:162 (1) tamoxifen for 5 years, (2) anastrozole for 5 years, (3) letrozole for 5 years, (4) tamoxifen for 2 years plus exemestane for the final 3 years and (5) tamoxifen for 5 years followed by extended therapy with letrozole for a further 3 years.
Drug costs were taken from BNF 61. 161 It was assumed that each endocrine therapy was given once daily at a 20-mg, 1-mg, 2.5-mg and 25-mg dosage for tamoxifen, anastrozole, letrozole and exemestane respectively. The annual cost for each drug is presented in Table 44.
| Dose (mg) | Tablets per pack | Price per pack (£) | Annual cost (£) | |
|---|---|---|---|---|
| Tamoxifen | 20 | 30 | 2.09 | 25.45 |
| Anastrozole | 1 | 28 | 68.56 | 894.34 |
| Letrozole | 2.5 | 28 | 84.86 | 1106.97 |
| Exemestane | 25 | 30 | 88.80 | 1081.14 |
The probability that a patient will be treated with each regimen was taken from the costing template accompanying TA112. 162 It was assumed that 40% of patients received tamoxifen for 5 years, 20% anastrozole for 5 years, 20% letrozole for 5 years and 20% tamoxifen for 2 years plus exemestane for the final 3 years. It was further assumed that 10% of patients received tamoxifen for 5 years followed by extended therapy with letrozole for a further 3 years. After weighting, the annual drug cost was calculated to be £668.90 for the first 5 years and £110.70 for the remaining 3 years.
In addition to drug costs, monitoring cost were included. We assumed that patients treated with endocrine therapy have two follow-up appointments in the first year and one follow-up appointment every subsequent year (£129 based on NHS reference costs 2009/10,175 370 Medical Oncology). We further assumed that patients had one mammogram every year (£46.37 based on Campbell et al. 176) for a maximum of 5 years.
Chemotherapy costs
It was assumed that all patients received FEC75 as clinical opinion indicated that this is the most commonly used chemotherapy regime for this population (ER+, LN−, HER2−). Sensitivity analysis was carried out varying the cost to explore the sensitivity of the results to this assumption. Note that the choice of chemotherapy (FEC75) in the economic model impacts on cost only as the effect of chemotherapy was taken from a separate source of data49 that uses CMF/MF regimes. No effectiveness data were available for FEC75 for this group of patients.
The cost of the chemotherapy drugs was calculated according to the regime description of FEC75 given by Avon, Somerset and Wiltshire Cancer Services in their chemotherapy protocol documents. 177 All drug costs are taken from BNF 61. 161 We also assumed a dosage per BSA of 1.75 mg/m2 based on the value reported by Sacco et al. ,168 estimated in women with breast cancer in the UK.
The chemotherapy drug cost per cycle is summarised in Table 45. No drug wastage was assumed.
| Dose (mg) | Total dose per cycle (mg) | Cost per cycle (£) | |
|---|---|---|---|
| Fluorouracil | 600 | 1050 | 16.00 |
| Epirubicin | 75 | 131 | 168.78 |
| Cyclophosphamide | 600 | 1050 | 16.32 |
| Total | 201.10 |
Discussion with clinical experts indicated that FEC75 is usually given for six cycles. The number of cycles was varied in sensitivity analysis. In addition to drug costs, we assumed an additional pharmacy cost of £38162 per cycle to account for the chemopharmacy/aseptic costs.
Furthermore, administration costs were assumed to be £270.60 for the first cycle of treatment (NHS reference costs 2009/210,175 S13Z: Deliver more complex Parenteral Chemotherapy at first attendance) and £284.50 for the remaining cycles (NHS reference costs 2009/10,175 SB15Z: Deliver subsequent elements of a Chemotherapy cycle). Patients were also assumed to have a separate outpatient appointment before administration of each cycle of the chemotherapy (NHS reference costs 2009/10,175 370 Medical Oncology – £129).
Patients were also assumed to be monitored and to receive one liver function test (£12.68), a test of urea and electrolytes (£12.30) and a full blood count (£5.81) at each cycle based on 2008/9 data from the Sheffield Teaching Hospital (Sheffield Teaching Hospital Trust, 2007–8, personal communication), uplifted to 2010 prices. 178 Finally, it was further assumed that 25% of patients would have an echocardiogram before starting chemotherapy (NHS reference costs 2009/10,175 RA60Z – £59).
The total cost of chemotherapy including drug acquisition cost, administration and monitoring was calculated to be £4099 for the entire course of chemotherapy (six cycles of treatment).
Costs of short-term adverse events associated with chemotherapy
Short-term adverse events were included for patients receiving chemotherapy.
The probability of short-term grade 3 and grade 4 adverse events was extracted from the PACS-01 clinical trial for patients treated with FEC100 as no data were available for FEC75. 179 This included anaemia, thrombocytopenia, neutropenic infection, nausea/vomiting and stomatitis. Short-term grade 3 and grade 4 adverse events were costed using the NHS reference costs where appropriate. 175 The total cost of treating short-term adverse events was estimated to be £275.61 per patient (Table 46). This excluded the cost associated with the secondary prevention of febrile neutropenia using G-CSF prophylaxis, included separately (see next section).
| Proportion of grade 3/4 adverse events (%) | HRG code175 | HRG | Cost of HRG (£) | Cost per patient (£) | |
|---|---|---|---|---|---|
| Anaemia | 1.4 | SA09F | Other Red Blood Cell Disorders without CC | 1529 | 21.41 |
| Thrombocytopenia | 0.3 | SA12F | Thrombocytopenia without CC | 1355 | 4.06 |
| Neutropenic infection | 1.6 | 2286 | 36.58 | ||
| Nausea/vomiting | 24.2 | FZ48C | Malignant General Abdominal Disorders with length of stay 1 day or less | 588 | 142.34 |
| Stomatitis | 4.0 | CZ24Q | Complex/Major Head, Neck and Ear Disorders without CC | 1781 | 71.22 |
| Cost of adverse event per patient | 275.61 |
Costs of the secondary prevention of febrile neutropenia
We assumed that G-CSF prophylaxis was given for the secondary prevention of neutropenia to women receiving chemotherapy only. In the base case it was assumed that about 25% of patients would receive G-CSF prophylaxis (Dr Matthew Winter, Consultant in Medical Oncology, Sheffield Teaching Hospitals NHS Foundation Trust, September 2011, personal communication) and that G-CSF would be given for an average of three cycles. Patients were assumed to receive filgrastim at a dose of 500,000 units/kg daily for six days after each cycle of chemotherapy (maximum three cycles). A mean weight of 66 kg was assumed and the drug cost per injection of 30 million units was assumed to be £59. 161 Filgrastim was assumed to be administrated by a district nurse (£39 per injection) using the cost from the Personal Social Services Research Unit (PSSRU). 178
Overall, the cost associated with the secondary prevention of adverse event for patients receiving chemotherapy was estimated to be £485.30 per patient.
Costs associated with long-term adverse events
Potential long-term adverse events include secondary malignancies and congestive heart failure (CHF). Although CHF is more common than secondary malignancies, the development of cancer is likely to have more serious consequences and to be associated with a higher impact on health-care resources than the management of CHF.
The base-case economic model included acute myeloid leukaemia (AML) as a long-term adverse event after chemotherapy. The probability of developing AML was based on the 8-year cumulative probability of developing AML in women treated with epiribucin extracted from a meta-analysis of 19 trials conducted in early breast cancer. 180
The meta-analysis showed that the 8-year cumulative probability of AML was 0.37% (95% CI 0.13 to 0.61%) in women receiving a cumulative dose of cyclophosphamide < 6300 mg/m2 and a cumulative dose of epirubicin < 720 mg/m2 (n = 4760). 180
We further assumed that patients spend 8 months on average in the AML health state at a mean cost of £11,500 based on the approximate mean life-years and mean costs estimated by the manufacturer for the NICE technology appraisal of azacitidine for myelodysplastic syndromes. 181 These assumptions were varied in sensitivity analysis.
Costs associated with the management of distant recurrence
The costs associated with distant recurrence were derived from Thomas et al. 157 using a sample of 77 patients with relapsed breast cancer. Costs included active supportive care and end-of-life care. Costs specifically associated with terminal care were removed to avoid double counting as these were included separately in the economic model. (Note that only cost items described as supportive/terminal care were removed.)
After removing cost items that were specific to terminal care, we estimated the 6-monthly cost to be approximately £4082 (uplifted to 2010 prices). 178 We assumed that the cost was constant over time. This is very simplistic as evidence shows that the cost is higher in the first 2 years and decreases thereafter. 182 However, the impact of this assumption is likely to be minimal because this affects both the comparator arm and the intervention arm in the model.
Costs associated with the management of local recurrence
The cost of local recurrence was taken from Karnon et al. 182 and was estimated at £14,132 (uplifted to 2010 prices). 178
Costs associated with the management of death from breast cancer
Finally, the cost associated with terminal care/end of life was taken from Campbell et al. 176 and was assumed to be about £4038. This cost was applied as a one-off in the economic model, immediately before death from breast cancer.
Health-state utilities
Quality of life utility scores were identified from a recent systematic review of utility values in breast cancer. 159 The utility values used in the model are given in Table 47.
| Mean utility score | Duration | Source | |
|---|---|---|---|
| Recurrence free | 0.824 (95% CI 0.785 to 0.857) | 1 year | Lidgren et al.148 |
| Distant recurrence | 0.685 (95% CI 0.62 to 0.735) | 1 year | Lidgren et al.148 |
| Local recurrence (decrement per patient) | −0.108 | NA | Campbell et al.176 |
| AML | 0.26 | 1 year | Younis et al.183 |
| Chemotherapy (decrement per patient) | −0.038 | NA | Campbell et al.176 |
| Utility for patients dying from breast cancer (final 3 months of life) | 0.159 (SE 0.04) | 3 months | Campbell et al.176 |
Utility values for patients in the recurrence-free and distant recurrence health states were extracted from Lidgren et al. 148 These were EQ-5D values and using this study allowed values for recurrence free (0.824) and distant recurrence (0.685) to be taken from the same study for consistency (see Table 47). The study followed 361 breast cancer patients attending the breast cancer outpatient clinic at Karolinska University Hospital Solna between April and May 2005. The decrement in utility per patient experiencing a local recurrence was taken from Campbell et al. 176 and assumed to be −0.108 in the base case. We assumed that patients with AML have a utility value of 0.26 based on the value used in a previous economic evaluation conducted in Canada. 183 We further assumed that patients receiving chemotherapy have a disutility of 0.038, taken from Campbell et al. 176 for women treated with E-CMF (epirubicin, cyclophosphamide, methotrexate and 5-fluorouracil)/FEC60 in the first year. This is believed to capture the decrement in utility associated with the administration of chemotherapy and related adverse events. Finally, a decrement in utility was applied for patients dying from breast cancer, derived from Campbell et al. 176 and Lidgren et al. 148 Utility values were varied in sensitivity analysis.
Death from causes other than breast cancer
The mortality rate from causes other than breast cancer was extracted from UK life tables (2007–9) for women184 after adjustment to remove death attributable to breast cancer.
Proportion of patients with distant recurrence who have previously experienced a local recurrence
In the base case we assumed that 10.5% of patients entering the distant recurrence state have previously experienced a local recurrence. This is based on an analysis conducted in 3601 women with early breast cancer enrolled in previous European Organisation for Research and Treatment in Cancer (EORTC) trials (10801, 10854 and 10902), which showed that the presence of locoregional recurrence was a significant prognostic risk factor for the occurrence of distant recurrence. 185 The analysis showed that, among the 1224 patients who developed a distant recurrence, 129 patients had a distant recurrence after a locoregional recurrence.
We did not make any assumptions about the time spent in the local recurrence health state. Local/regional recurrences have been modelled by considering the cost and quality of life decrements (disutility), assuming that a proportion of patients entering the distant recurrence state have previously experienced a local recurrence.
Model inputs: test-specific parameters
Clinical parameters specific to each test are described below. For each test clinical parameters relating to the three main components of the model are described in turn.
Clinical parameters: OncotypeDX and IHC4
The systematic review of evidence indicated that the OncotypeDX test is the furthest along the validation pathway compared with other similar tests, and the evidence base, in particular in relation to the prognostic ability of the test, was reasonably sound. The evidence base for IHC4 is less developed but there is direct evidence relating to the performance of IHC4 compared with that of OncotypeDX and so the clinical evidence relating to these two tests is described together.
The primary analysis compared current clinical practice with treatment guided using OncotypeDX or IHC4 in addition to current practice. This was carried out using evidence that directly compared the test results for OncotypeDX and IHC4. An overview of this evidence was presented in Cuzick et al. 84 However, the specific data used in the economic model for IHC4 were unpublished and were made available to the EAG for the purpose of this assessment (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication).
Most of the evidence on the ability of OncotypeDX to classify patients into the low, intermediate or high risk group is derived from US studies (see Chapter 2, OncotypeDX test). The systematic review did, however, identify two UK studies78,79 that presented classification evidence. Data from one of these studies, the TransATAC trial (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication), were used in the base-case analysis. The second study, the Holt et al. study78 included a small sample of patients recruited in Wales and reported how the test classified patients by OncotypeDX RS and how this influenced clinical decision-making. However, the systematic review of evidence showed that there are limitations with using data from this study (see Chapter 2, OncotypeDX test results). Likewise, discussion with clinical experts indicated some concerns that the patients included might not be representative of patients seen in clinical practice. To further explore this point, we compared the baseline characteristics of women with ER+, LN−, HER2− early breast cancer included in the final analysis of the Holt et al. study (n = 121) (EAG analysis) with the baseline characteristics of cohorts of patients from the cancer registry data provided by ECRIC (2007 onwards) and WMCIU (2007 only) (West Midland Cancer Intelligence Unit, July 2011, personal communication; Eastern Cancer Registration and Information Centre, July 2011, personal communication) (Table 48). Overall, the Holt cohort was generally more severe with a higher distribution of Grade 2 and 3 tumours and larger tumour size (20.4 mm vs 17.0 mm). There was also a higher proportion of patients classified as intermediate or high risk according to NPI (NPI > 3.4) in the Holt study (42.15%) compared with patients included in the ECRIC (34.4%) or WMCIU (31.8%) cohort.
| Baseline characteristic | Holt et al.78 (n = 121)a | ECRIC cohort (n = 3245) | WMCIU cohort (n = 1214) |
|---|---|---|---|
| Age (years) | |||
| Mean | 55.88 | 58.30 | 58 |
| First interquartile | 50.00 | 51.00 | 51 |
| Third interquartile | 63.00 | 66.00 | 66 |
| Median | 55.00 | 60.00 | 60 |
| Grade distribution (%) | |||
| I | 19.01 | 23.7 | 26.6 |
| II | 63.64 | 56.0 | 56.5 |
| III | 17.36 | 20.2 | 16.5 |
| Tumour size (mm) | |||
| Mean | 20.39 | 16.90 | 17 |
| First interquartile | 13.00 | 10.00 | 10 |
| Third interquartile | 23.00 | 20.00 | 20 |
| Median | 18.00 | 14.00 | 15 |
| NPI score (%) | |||
| Low (≤ 3.4) | 57.85 | 65.59 | 68.19 |
| Intermediate/high (> 3.4) | 42.15 | 34.41 | 31.81 |
The impact of using data from the Holt et al. study was explored in a scenario analysis using data used in the economic model submitted by Genomic Health (see Comparison of assumptions and results with the economic models submitted by Genomic Health and Clarient).
The TransATAC trial evaluated the efficacy and safety of 5 years of anastrozole, tamoxifen or the combination of both treatments in postmenopausal women with localised breast cancer in the UK. 79,84 The study included a much larger sample size. Furthermore, data on both the risk of distant recurrence and risk classification were available in the TransATAC trial from the same cohort for patients using OncotypeDX and IHC4, making it the most robust source to use to populate the economic model. Clinical experts supported the view that patients included in this study were more likely to be representative of patients seen in clinical practice. However, the inherent limitations of the generalisability of such trial data, such as the fact that women with comorbidities would have been excluded from the trial, need to be taken into consideration.
Data from the TransATAC trial were reanalysed by the investigators of the trial to exclude women with HER2+ cancer, to stratify patients by NPI score (≤ 3.4 and > 3.4) and to provide additional data relating to IHC4 (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication). Reclassification using the OncotypeDX and IHC4 tests compared with NPI group for women with ER+, LN−, HER2− is presented in Tables 49 and 50 respectively. The IHC4 + clinical score test provides a continuous risk score. Selected cut-offs for three IHC4 risk groups have been defined specifically to populate the economic model (using the same methodology as for OncotypeDX) and therefore this might not reflect how the test will be used in clinical practice.
| Low RS, n (%) | Intermediate RS, n (%) | High RS, n (%) | Total, n (%) | |
|---|---|---|---|---|
| NPI ≤ 3.4 | 323 (71.94) | 109 (24.28) | 17 (3.79) | 449 (100) |
| NPI > 3.4 | 133 (51.75) | 76 (29.57) | 48 (18.68) | 257 (100) |
| Total | 456 (64.59) | 185 (26.20) | 65 (9.21) | 706 (100) |
| Low IHC4, n (%) | Intermediate IHC4, n (%) | High IHC4, n (%) | Total, n (%) | |
|---|---|---|---|---|
| NPI ≤ 3.4 | 437 (97.33) | 12 (2.67) | 0 | 449 (100) |
| NPI > 3.4 | 166 (64.59) | 58 (22.57) | 33 (12.84) | 257 (100) |
| Total | 603 (85.41) | 70 (9.92) | 33 (4.67) | 706 (100) |
Among patients with a NPI score ≤ 3.4 (n = 449), significantly more patients were reclassified as intermediate/high using OncotypeDX (n = 126) than with IHC4 (n = 12). Among patients with a NPI score > 3.4 (n = 257), more patients were reclassified as having a low risk using IHC4 (n = 166) than with OncotypeDX (n = 133).
For the purposes of the economic assessment, patients classified using OncotypeDX were reclassified according to IHC4. Reclassification data for patients with a NPI score ≤ 3.4 and a NPI score > 3.4 used in the EAG economic model are presented in Tables 51 and 52 respectively.
| Low RS, n (%) | Intermediate RS, n (%) | High RS, n (%) | Total, n (%) | |
|---|---|---|---|---|
| Low IHC4 | 321 (71.49) | 103 (22.94) | 13 (2.90) | 437 (97.33) |
| Intermediate IHC4 | 2 (0.45) | 6 (1.34) | 4 (0.89) | 12 (2.67) |
| High IHC4 | 0 | 0 | 0 | 0 |
| Total | 323 (71.94) | 109 (24.28) | 17 (3.79) | 449 (100) |
| Low RS, n (%) | Intermediate RS, n (%) | High RS, n (%) | Total, n (%) | |
|---|---|---|---|---|
| Low IHC4 | 111 (43.19) | 39 (15.18) | 16 (6.23) | 166 (64.59) |
| Intermediate IHC4 | 13 (5.06) | 28 (10.89) | 17 (6.61) | 58 (22.57) |
| High IHC4 | 9 (3.50) | 9 (3.50) | 15 (5.84) | 33 (12.84) |
| Total | 133 (51.75) | 76 (29.57) | 48 (18.68) | 257 (100) |
The impact of using data from Holt27 was examined in Comparison with the economic model submitted by Genomic Health.
Note that the approach of classifying patients into risk categories has some limitations:
-
It was assumed that the new tests categorised patients into risk categories; however, both OncotypeDX and IHC4 provide a continuous risk score.
-
Although cut-offs are available for OncotypeDX to identify patients at low, intermediate or high risk of distant recurrence, these are informative and not definitive. In the economic model, patients were classified according to the original cut-offs defined by the manufacturer of the technology: low – RS < 18; intermediate – RS between 18 and 30; and high – RS ≥ 31. However, the definition of low, high and mid-range RS was modified in the TAILORx trial for OncotypeDX. A cut-off of ≤ 10 was used instead of the original < 18 to define patients at low risk of distant recurrence. The cut-off for high risk of distant recurrence was modified from ≥ 31 to ≥ 26.
-
The IHC4 test does not present cut-offs. The test is intended to be used as a continuous risk score and interpretation is at the discretion of the physician. For the purpose of the economic assessment, investigators provided risk classification evidence of IHC4 based on low, intermediate and high risk of distant recurrence. Cut-offs were defined using a similar approach to that for OncotypeDX (< 10%, 10–20% and > 20% predicted risk of distant recurrence). The cut-offs used for IHC4 are therefore exploratory and were defined only to populate the economic model.
The TransATAC trial reported the risk of distant recurrence in patients treated with anastrozole or tamoxifen. The advantage of using data from this trial is that it was possible to extract the risk of distant recurrence for patients classified using OncotypeDX and further reclassified using IHC4 from the same source of data as the reclassification data used above (see Tables 51 and 52). The proportions of patients without distant recurrence at 10 years used in the economic model for patients with a NPI score ≤ 3.4 and a NPI score > 3.4 are presented in Tables 53 and 54 respectively (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication). Note that inconsistencies can be observed because of the small sample size of patients within each box (in bold for sample size < 10).
| Low RS (%) | Intermediate RS (%) | High RS (%) | |
|---|---|---|---|
| Low IHC4 | 98 | 92 | 91 |
| Intermediate IHC4 | 100 | 100 | 100 |
| High IHC4 | – | – | – |
| Low RS (%) | Intermediate RS (%) | High RS (%) | |
|---|---|---|---|
| Low IHC4 | 92 | 89 | 93 |
| Intermediate IHC4 | 100 | 75 | 76 |
| High IHC4 | 63 | 89 | 60 |
We assumed that the risk of distant recurrence was constant over the first 10 years, using an exponential distribution. This was done in the absence of the Kaplan–Meier data for each subgroup and is acknowledged as a limitation as the risk of recurrence may vary over time.
We also assumed that the risk was reduced by half after 10 years as clinical experts indicated that patients who had not experienced a distant recurrence before 10 years have a lower risk of distant recurrence beyond 10 years. We further assumed that no recurrences would occur after 15 years. Discussion with clinical experts indicated that only a minority of recurrences are likely to occur beyond this date. This assumption was tested in sensitivity analysis.
The risk groups identified by the new tests are expected to influence the targeting of chemotherapy. However, other factors will also influence the decision regarding chemotherapy, including clinical and pathological factors, along with patient choice. In clinical practice, a proportion of women classified as having a low risk of distant recurrence may receive chemotherapy; similarly, a proportion of women classified at high risk may not receive chemotherapy, as shown in previous studies for OncotypeDX undertaken in Spain173 or in the USA. 174
To populate our economic model, data from the only identified UK study was used for OncotypeDX in the base case. 78 The Holt et al. study reported the OncotypeDX RS and the chemotherapy decision based on knowledge of the RS combined with traditional clinical and pathological parameters. Individual patient-level data were made available to the EAG and were reanalysed by NPI group. Results are presented in Table 55 (analysis conducted by the EAG using individual patient-level data submitted by Genomic Health). This study, however, has a number of limitations, discussed previously, and these assumptions were tested in sensitivity analysis.
| Entire cohort | NPI ≤ 3.4 | NPI > 3.4 | |
|---|---|---|---|
| Low RS | 1.45% | 0.00% | 4.55% |
| Moderate RS | 42.42% | 38.10% | 50.00% |
| High RS | 89.47% | 50.00% | 94.12% |
No data exist on the proportion of women who are given chemotherapy according to the results of the IHC4 test. Discussion with clinical experts indicated that interpretation of the OncotypeDX and IHC4 results is likely to be similar. We therefore assumed the same proportions for IHC4 as for OncotypeDX. This is, however, a limitation of the analysis and the assumption was tested in sensitivity analysis.
The systematic review of evidence identified one study, the Paik et al. study, evaluating the benefit of chemotherapy for LN− patients receiving chemotherapy in addition to tamoxifen compared with tamoxifen alone and classified using OncotypeDX. 49,86 In the overall population, the addition of chemotherapy compared with tamoxifen alone was estimated to reduce the risk of distant recurrence by 44% (HR 0.56, 95% CI 0.34 to 0.91). No chemotherapy benefit (reduction in distant recurrence) was found for women classified as low risk of distant recurrence with OncotypeDX (HR 1.31, 95% CI 0.46 to 3.78, p = 0.61). A reduction of 39% (HR 0.61, 95% CI 0.24 to 1.59, p = 0.39) and 74% (HR 0.26, 95% CI 0.13 to 0.53, p < 0.001) was found for the risk of distant recurrence for women receiving chemotherapy in addition to tamoxifen compared with tamoxifen alone and classified as intermediate and high risk of distant recurrence with OncotypeDX respectively. The limitations of this study are described in Chapter 2, Results: OncotypeDX test.
The base-case analysis used data from the Paik et al. study,49 assuming the test to be predictive of the benefit of chemotherapy. This may be an optimistic assumption as the effect of chemotherapy might be lower than that reported (as the Paik et al. study included younger women and women with HER2+ early breast cancer). However, this study is based on a less effective regimen than is currently used and it is unclear what the overall impact of these factors would be. Univariate sensitivity analyses were conducted varying the benefit of chemotherapy. In addition, because of the limitations of this study (see Chapter 2, Results: OncotypeDX test), the EAG explored a scenario assuming that all women receiving chemotherapy derive the same benefit in terms of reduction in distant recurrence (i.e. that the test is prognostic only). However, not all clinical experts supported this assumption and recommended the use of the predictive evidence available despite the limitations noted above.
No studies were identified for the benefit of chemotherapy in women reclassified using IHC4; therefore, we used indirect evidence. Patients classified as low, intermediate or high risk with OncotypeDX were assumed to derive the same benefit from chemotherapy irrespective of their further reclassification as low, intermediate or high with IHC4. The benefit of chemotherapy for a particular risk group for IHC4 was therefore derived from the known mix of patients with OncotypeDX RS of low, intermediate and high within the IHC4 group. In simple terms, because we know the RS classification of patients reclassified by IHC4, it is possible to apply the benefit of chemotherapy from the RS risk group. This assumes that the reclassification with IHC4 does not provide any additional benefit in terms of identifying patients who would benefit the most from chemotherapy. This may be a conservative assumption; however, it is not possible to ascertain the potential bias of such an assumption.
Clinical parameters: Mammostrat
Compared with OncotypeDX, the evidence base for Mammostrat was less developed and some gaps were identified by the systematic review of the literature (see Chapter 2, Quality of included studies: Mammostrat test). Most of the evidence available for Mammostrat related to the clinical validity (prognostic ability) of the test. No published analyses of reclassification against Adjuvant! Online or NPI or the impact of the test on decision-making were identified.
Because of the gaps and uncertainties in the data available, an exploratory analysis was carried out to assess the cost-effectiveness of Mammostrat in addition to current clinical practice compared with current clinical practice in England and Wales.
Clinical parameters relating to the three main components of the model are described in turn.
Unpublished data from a subset of women with ER+, LN− breast cancer included in the Ring et al. study169 were used to populate the economic model for the risk reclassification against NPI (Table 56). (CIC information has been removed.)
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
|---|---|---|---|---|
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
The number of recurrences in the same subset of patients (n = 245) was also obtained for patients classified by Mammostrat and NPI (Table 57). 169
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | |
|---|---|---|---|
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
The fraction of patients who recur was calculated by the EAG for patients classified using Mammostrat and NPI to provide an indication of the risk of recurrence (mean follow-up of 11.7 years) for each prognostic group (Table 58).
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | |
|---|---|---|---|---|
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
(CIC information has been removed.)
Data from this subset of patients were used in the economic model in the absence of other evidence.
Furthermore, because this study was conducted outside the UK, the risk was adjusted by calculating the ratio of the risk between patients with a NPI score < 3.4 and for patients with a NPI score > 3.4 for patients classified as low, intermediate or high using Mammostrat. The ratio was then applied to the risk of recurrence (DFS) estimated from the TransATAC trial for patients with a NPI score ≤ 3.4 and patients with a NPI score > 3.4 with ER+, LN−, HER2− breast cancer. The estimated adjusted 10-year risk of being free of distant recurrence used in the economic model is presented in Table 59. A sensitivity analysis was conducted using the direct data without adjustment.
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | |
|---|---|---|---|
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
| (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) | (CIC information has been removed) |
There is no published evidence on how Mammostrat will influence treatment decisions in the UK, or elsewhere. In the base case we assumed that the interpretation of the Mammostrat test would be the same as for patients categorised as low, intermediate or high risk of recurrence with OncotypeDX, using data from the Holt et al. study78 (see Table 55). A sensitivity analysis was conducted assuming that no patients classified as low risk, 50% of patients classified as intermediate risk and 100% of patients classified as high risk received chemotherapy.
One study has been identified by the systematic review reporting the effect of chemotherapy for patients reclassified as low, intermediate or high risk using Mammostrat. 126 This study showed that patients with a low Mammostrat risk (HR 0.4, 95% CI 0.2 to 0.8) and a high Mammostrat risk (HR 0.4, 95% CI 0.2 to 0.9) benefited from chemotherapy whereas patients in the intermediate-risk group did not. The limitations of this study are described in Chapter 2, Quality of included studies: Mammostrat test.
Data from this study by Ross et al. 126 were used in the base case. Sensitivity analyses were undertaken using the 95% CIs of this study.
Clinical parameters: MammaPrint
Compared with OncotypeDX, the evidence base for MammaPrint is less well developed and gaps were identified by the systematic review of the literature (see Chapter 2, Results: MammaPrint test). The systematic review of the literature indicated that the evidence base, in particular in relation to the prognostic ability of the test, is developing but is based on cohort studies with small sample sizes. None of the studies is based on UK patients and studies are mainly derived from premenopausal women and so are not representative of the population of women with ER+, LN−, HER2− early breast cancer. No robust evidence on clinical utility was identified. The one study identified that considered chemotherapy benefit by MammaPrint risk group had significant limitations. 111 No UK studies of the impact of the test on clinical decision-making were identified.
Because of the gaps and uncertainties in the data available, an exploratory analysis was carried out looking at the cost-effectiveness of adjuvant chemotherapy guided using MammaPrint in addition to current clinical practice compared with current clinical practice in England and Wales.
Clinical parameters relating to the three main components of the model are described in turn.
Risk classification data from Bueno de Mesquita et al. 109 were used in the economic model as this study contained a relatively large sample size (Table 60). The study included Dutch women only. In this study, data from previous cohorts were pooled and reanalysed. Women included in this study were mainly premenopausal and included ER− women. Premenopausal women and ER− women are more likely to be classified as poor prognosis with MammaPrint and this raises concerns relating to the generalisability of the reclassification data to the population considered in the economic model.
| Good prognosis using MammaPrint, n (%) | Poor prognosis using MammaPrint, n (%) | All, n (%) | |
|---|---|---|---|
| NPI ≤ 3.4 | 259 (71.75) | 102 (28.25) | 361 (100) |
| NPI > 3.4 | 84 (24.71) | 256 (75.29) | 340 (100) |
| All | 343 (48.93) | 358 (51.07) | 701 (100) |
The systematic review of evidence did not identify any studies that presented the risk of recurrence in a UK population for patients classified using MammaPrint. The risk of distant recurrence for patients receiving endocrine therapy was therefore derived using data from the same non-UK study as the risk reclassification data but from a different subset of patients. 105 This study reported the risk of recurrence (distant metastases as first event) for patients stratified by NPI, Adjuvant! Online and MammaPrint. This evidence relates to premenopausal women in a non-UK cohort and is based on patients receiving tamoxifen only. The study separated patients into those with a low NPI/low Adjuvant! Online score, an intermediate NPI/high Adjuvant! Online score or discordant results, rather than just low and intermediate NPI only (Table 61).
| Low NPI/low Adjuvant! Online score (%) | Intermediate NPI/high Adjuvant! Online score (%) | Discordant results (%) | |
|---|---|---|---|
| Good prognosis using MammaPrint | 87 | 77 | 92 |
| Poor prognosis using MammaPrint | 69 | 45 | 59 |
| All | 82 | 53 | 83 |
This study also showed, from a different subset of patients, that 94% of the discordances between NPI and Adjuvant! Online occurred in patients with a NPI ≤ 3.4. Consequently, the EAG pooled data for patients with a low NPI and low Adjuvant! Online score and patients with a discordant result between the two prognostic tools.
This non-UK study presented the risk of distant recurrence as first event (rather than time to any distant recurrence) in predominantly premenopausal women. We therefore adjusted the risk using data from the TransATAC trial to more closely reflect the expected risk within a UK population. The ratio of the risk between the good and poor prognosis groups was calculated for patients with a NPI score ≤ 3.4 and a NPI score > 3.4. This ratio was then applied to the 10-year risk of distant recurrence for patients with a NPI score ≤ 3.4 and a NPI score > 3.4 estimated from the TransATAC trial (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication) in a UK population with ER+, LN−, HER2− early breast cancer. This is acknowledged to be a limitation of the analysis.
The estimated adjusted 10-year risk of being free of distant recurrence used in the economic model is presented in Table 62.
| Good prognosis using MammaPrint (%) | Poor prognosis using MammaPrint (%) | |
|---|---|---|
| NPI ≤ 3.4 | 97.7 | 91.3 |
| NPI > 3.4 | 94.3 | 83.7 |
No UK studies were identified on the proportion of patients who receive chemotherapy based on the MammaPrint test results (see Chapter 2, Results: MammaPrint test). A Dutch study (the MircoarRAy PrognoSTics in Breast CancER study or RASTER)186 was identified that provided information on the number of patients who have been recommended and/or offered chemotherapy based on poor or good outcome classification according to the CBO105 and subsequently following knowledge of the MammaPrint test result.
The study showed that, overall, out of the 273 and 208 patients classified as having good and poor prognosis by MammaPrint, respectively, chemotherapy advice was given to 13.55% and 87.50% respectively. Among patients considered to be at low risk according to the CBO,105 1.80% and 68.42% were classified as having good and poor prognosis using MammaPrint, respectively, and were recommended chemotherapy. The figures for patients considered to be at high risk according to the CBO were 32.08% and 98.48% respectively.
Data from this study were used in the base-case analysis in the absence of other evidence, assuming that the NPI category was a proxy for the risk group defined using the CBO guidelines. 105 This is a limitation. Furthermore, the study included ER− patients and the MammaPrint test classified most ER− patients as having poor prognosis. Furthermore, there were some concerns with the study design as there were some amendments to the protocol and patients from different cohorts had been pooled. In total, 84% of patients were aged < 55 years and the population included women with ER+ and ER− early breast cancer. It was unclear how representative patients were compared with those seen in clinical practice in the UK and how this study would relate to UK clinical practice.
A study was identified providing data on the benefit of chemotherapy for ER+, LN− patients reclassified as having a good or a poor prognosis with MammaPrint. 112 This study showed that patients with both a poor and a good prognosis benefited from chemotherapy (compared with no chemotherapy) in terms of a reduction in distant metastasis, but the benefit was not significant for patients with a good prognosis. The limitations of this study are described in Chapter 2, Results: MammaPrint test.
Summary of inputs used
To summarise, we assign patients into different boxes according to the risk group predicted by the new test (Figure 12). A proportion of these patients are assumed to receive chemotherapy. In the current practice arm, the proportion is informed by registry data and is assumed to be independent of the risk group (as oncologists are blind to the results of the new test). For the intervention arm, the proportion is linked to the risk group assigned by the new test. Patients are then at risk of developing a recurrence, and die from breast cancer or other causes.
FIGURE 12.
Schematic diagram showing how the inputs were assembled.
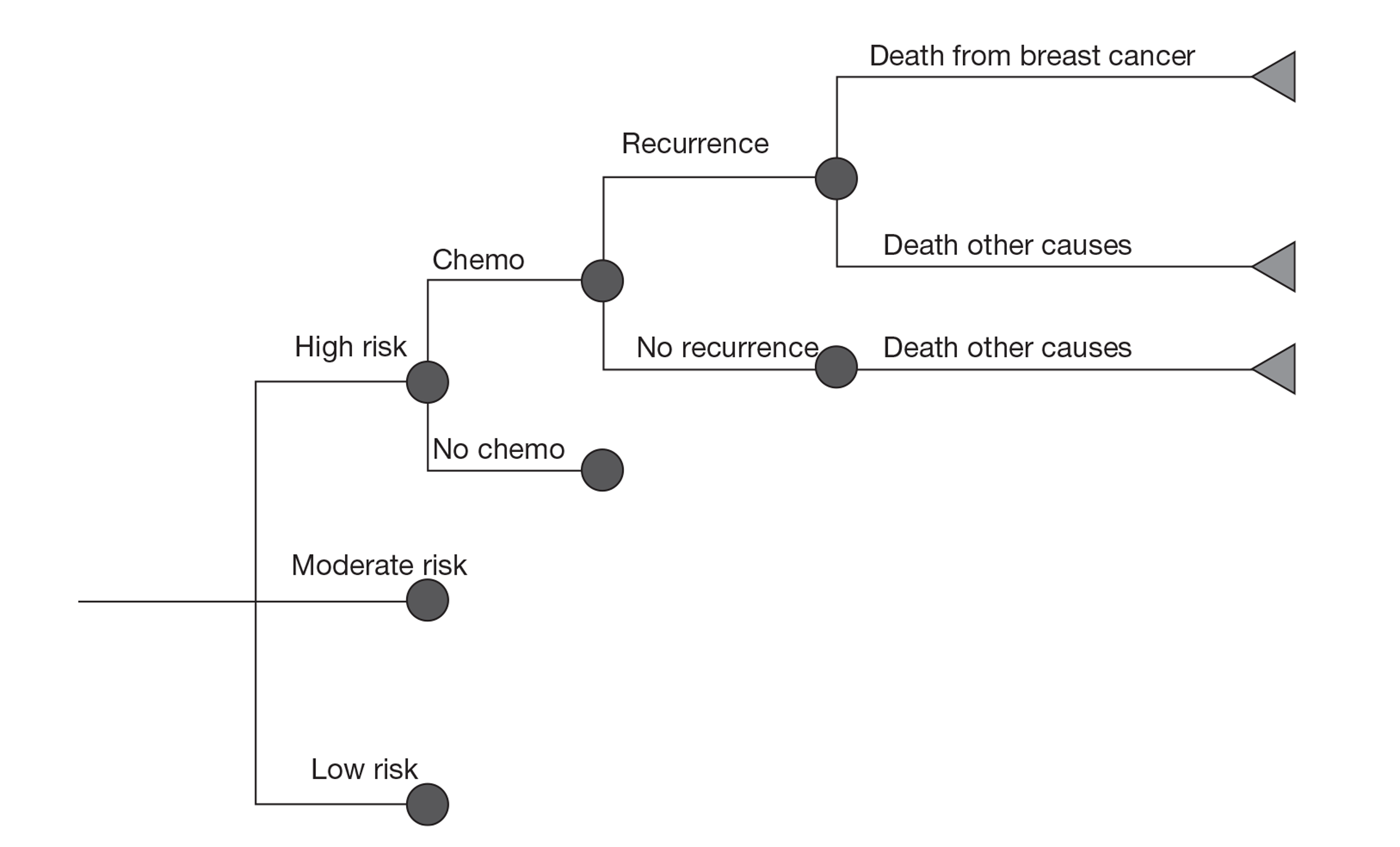
A summary of the main inputs is presented in Table 63.
| Parameter | Base-case value | Distribution | Source |
|---|---|---|---|
| Baseline age (years) | 58.3 | ECRIC (West Midland Cancer Intelligence Unit, July 2011, personal communication; Eastern Cancer Registration and Information Centre, July 2011, personal communication) | |
| Dosage per BSA (mg/m2) | 1.75 | Normal (SE 0.01) | Sacco et al.168 |
| Cost of the tests | |||
| OncotypeDX | £2580 | ||
| IHC4 | £150 | Personal communication and assumption (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011) | |
| MammaPrint | £2675 | ||
| Mammostrat | £1135 | ||
| Handling fresh tissue | £250 | Personal communication (Simon Cross, Reader and Honorary Consultant, Royal Hallamshire Hospital, Sheffield, July 2011) | |
| Endocrine therapy cost (6-monthly cost) | |||
| First 5 years | £334 | Normal | BNF161 plus NICE guidance162 and assumption |
| Remaining 3 years | £65 | ||
| Cost of monitoring in recurrence-free state (6-monthly cost) (£) | |||
| First year | £151 | Normal | NHS reference costs 2010/11175 and assumption |
| Remaining 4 years | £87 | ||
| Chemotherapy cost (one-off) | |||
| Drug, administration and monitoring | £4099 | Normal | BNF,161 NHS reference costs 2010/11175 and assumption |
| Short-term adverse events | £276 | NHS reference costs 2010/11175 and assumption | |
| G-CSF | £485 | ||
| Long-term adverse events after chemotherapy | |||
| 8-year probability of AML | 0.37% | Beta (129,1095) | Praga et al.180 |
| Time spent in AML health state | 8 months | Assumption based on NICE STA18181 | |
| Lifetime cost | £11,500 | Assumption based on NICE STA18181 | |
| Recurrence cost (6-monthly) | £4082 | Normal | Derived from Thomas et al.157 |
| End-of-life cost (one-off) – after death from breast cancer | £4038 | Normal (SE £454) | Campbell et al.176 |
| Local recurrence cost – one-off | £14,132 | Normal (SE £1853) | Derived from Karnon et al.182 and PSSRU178 |
| Health state utilities | |||
| Recurrence-free state | 0.824 | Beta (353,75) | Lidgren et al.148 |
| Distant recurrence | 0.685 | Beta (171,79) | Lidgren et al.148 |
| Local recurrence (decrement per patient) | −0.108 | Normal (SE 0.04) | Campbell et al.176 |
| AML | 0.26 | Younis et al.183 | |
| Chemotherapy (decrement per patient) | −0.038 | Campbell et al.176 | |
| Terminal care cost (final 3 months) | 0.159 | Normal (SE 0.04) | Campbell et al.176 |
| Baseline NPI score distribution | |||
| ≤ 3.4 | 66.2% | Beta (2602,819) | ECRIC and WMCIU (West Midland Cancer Intelligence Unit, July 2011, personal communication; Eastern Cancer Registration and Information Centre, July 2011, personal communication) |
| > 3.4 | 33.8% | ||
| Proportion of patients receiving chemotherapy under current clinical practice | |||
| NPI ≤ 3.4 | 4.6% | Beta (3263,158) | ECRIC and WMCIU (West Midland Cancer Intelligence Unit, July 2011, personal communication; Eastern Cancer Registration and Information Centre, July 2011, personal communication) |
| NPI > 3.4 | 33.6% | Beta (1160, 587) | ECRIC and WMCIU (West Midland Cancer Intelligence Unit, July 2011, personal communication; Eastern Cancer Registration and Information Centre, July 2011, personal communication) |
| Proportion of patients receiving chemotherapy after knowledge of the result of the new test (Holt et al. study78) | See Table 55 | EAG analysis | |
| Risk reclassification | |||
| OncotypeDX and IHC4 | See Tables 51 and 52 | Beta | TransATAC trial (personal communication) |
| MammaPrint | See Table 60 | Beta | Bueno-de-Mesquita et al.109 |
| Mammostrat | See Table 56 | Beta | Subset of the Ring et al. study125 (Clarient169) |
| Risk of recurrence | |||
| OncotypeDX and IHC4 | See Tables 53 and 54 | TransATAC trial (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication) | |
| MammaPrint | See Table 62 | Derived from Bueno-de-Mesquita et al.109 and the TransATAC trial (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication) | |
| Mammostrat | See Table 59 | Derived Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, from a subset of the Ring et al. study125 (Clarient169) and the TransATAC trial (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication) | |
| Benefit of chemotherapy (HR) | |||
| OncotypeDX | Log-normal | Paik et al.49 | |
| Low RS: 1.31 | Low RS: 95% CI 0.46 to 3.78 | ||
| Intermediate RS: 0.61 | Intermediate RS: 95% CI 0.24 to 1.59 | ||
| High RS: 0.26 | High RS: 95% CI 0.13 to 0.53 | ||
| MammaPrint | Log-normal | Knauer et al.110 | |
| Good prognosis: 0.26 | Good prognosis: 95% CI 0.03 to 2.02 | ||
| Poor prognosis: 0.35 | Poor prognosis: 95% CI 0.17 to 0.71 | ||
| Mammostrat | Log-normal | Ross et al.126 | |
| Low: 0.4 | Low: 95% CI 0.2 to 0.8 | ||
| Intermediate: 1 | Intermediate: 95% CI NA | ||
| High: 0.4 | High: 95% CI 0.2 to 0.9 | ||
The main assumptions used in the base-case economic model are summarised below:
-
The population assessed was all women with ER+, LN−, HER2− early breast cancer.
-
A subgroup analysis was carried out in women with ER+, LN−, HER2− early breast cancer with a NPI score > 3.4 only.
-
Interventions assessed were OncotypeDX, MammaPrint, IHC4 and Mammostrat.
-
The comparator was current clinical practice in England and Wales. We used registry data from the ECRIC and WMCIU to reflect current clinical practice. We assumed that data from these two registries were representative of all trusts in England and Wales.
-
Women were separated by NPI score (≤ 3.4 and > 3.4) to conduct subgroup analysis but also to capture the prognostic value of the treatment decision using clinicopathological parameters based on NPI, that is, women with a low NPI score are likely to have a lower risk than women with a NPI score > 3.4 but are also less likely to receive chemotherapy under current clinical practice.
-
The NPI score distribution was taken from data from two registries (ECRIC and WMCIU).
-
The starting age of the cohort was 58.3 years.
-
We assumed that the new test will not be considered in women aged > 75 years as the decision for chemotherapy might be limited because of frailty and comorbidities.
-
The model used a lifetime horizon.
-
The model used a 6-monthly cycle length.
-
The model adopted the perspective of the UK NHS and PSS.
-
Costs and benefits were discounted at 3.5% annually.
-
A primary analysis was carried out comparing current clinical practice alone, OncotypeDX in addition to current clinical practice and IHC4 in addition to current clinical practice as this presented the most robust evidence to populate the economic model (mainly derived from UK sources).
-
Two exploratory analyses were carried out comparing current clinical practice alone against MammaPrint or Mammostrat in addition to current clinical practice.
-
We assumed that the diagnostic tool does not affect the prognosis of patients if there is no change in the adjuvant treatment.
-
In the comparator arm (current clinical practice), the probability of receiving chemotherapy was taken from data from two registries (ECRIC and WMCIU). It was assumed that the probability of receiving chemotherapy was the same irrespective of the reclassification of patients with the new test in the low-, intermediate- or high-risk group. This is likely to be a conservative assumption.
-
The probability of receiving chemotherapy in the intervention arm was based on the assigned risk group, with a greater likelihood of receiving chemotherapy for patients classified as high risk of recurrence/distant recurrence than for patients classified as low risk.
-
The economic model used distant recurrence as the primary outcome.
-
Only locoregional recurrence that led to a distant recurrence was included, by considering the cost and quality of life decrement associated with locoregional recurrence. We assumed that 10.5% of distant recurrences were preceded by a local recurrence.
-
We assumed that patients treated with chemotherapy can develop long-term adverse events. The model included the development of AML only.
-
The risk of recurrence was assumed to be constant in the first 10 years and to be halved between 10 and 15 years; no recurrence was assumed after 15 years.
-
Median survival after a distant recurrence was assumed to be 40.1 months.
-
We assumed that all patients receive endocrine therapy. Five regimens were considered: tamoxifen for 5 years, anastrozole for 5 years, letrozole for 5 years, tamoxifen for 2 years plus exemestane for the final 3 years and tamoxifen (or other endocrine therapy regimens) for 5 years followed by extended therapy with letrozole for a further 3 years.
-
Patients were assumed to have two follow-up appointments in the first year and one follow-up appointment every subsequent year. Patients were assumed to have one mammogram every year for a maximum of 5 years.
-
Patients treated with chemotherapy receive six cycles of FEC75. We assumed that patients have a separate outpatient appointment before drug administration. Monitoring includes a liver function test, urea and electrolytes tests and a full blood count. It was further assumed that 25% of patients have an echocardiogram before starting chemotherapy.
-
Short-term adverse events after chemotherapy were included based on the probability of patients treated with FEC100 suffering from short-term adverse events, as no data were available for FEC75.
-
We assumed that 25% of patients treated with chemotherapy receive G-CSF (maximum of three cycles) for the secondary prevention of febrile neutropenia.
-
A decrement in utility was assumed for patients receiving chemotherapy and for patients dying from breast cancer-specific causes.
Assumptions specific to each test are described in Table 64.
| OncotypeDX | IHC4 | Mammostrat | MammaPrint |
|---|---|---|---|
|
|
|
|
Independent economic model: results
The primary analysis compared current clinical practice with treatment guided using OncotypeDX and IHC4. This involved using data from a direct comparison between OncotypeDX and IHC (Professor Mitch Dowsett, Royal Marsden Hospital, London, September 2011, personal communication).
In addition to the primary analysis, exploratory analyses were undertaken for Mammostrat and MammaPrint. These analyses were exploratory because of the significant limitations in the evidence base, and results need to be interpreted with consideration of the assumptions made and the robustness of the evidence used.
All analyses assumed that the new tests were used in addition to current prognostic tools. Base-case analyses assumed the tests to have predictive ability, that is, patients in the high-risk group benefit relatively more from reduction in recurrences following chemotherapy than patients in the lower-risk groups; this assumption was tested in sensitivity analysis.
Cost-effectiveness of treatment guided using OncotypeDX and IHC4
Two analyses are presented:
-
The tests were given to all women with ER+, LN−, HER2− early breast cancer.
-
The tests were given only to women with ER+, LN−, HER2− early breast cancer with a NPI score > 3.4. This subgroup analysis was undertaken to explore the impact of targeting the tests at patients with intermediate risk. It is considered likely that the majority of women with a NPI score ≤ 3.4 would be considered low risk and would not receive chemotherapy under current practice or using the new tests and therefore the tests would have a limited impact on the management of these women.
The new tests were offered to all women with ER+, LN−, HER2− early breast cancer
Deterministic results
Assuming that the tests were offered to all women with ER+, LN−, HER2− early breast cancer, treatment guided using OncotypeDX was predicted to lead to an increase in the proportion of patients receiving chemotherapy compared with current clinical practice under our base-case assumptions (19.11% vs. 14.42%). On the contrary, treatment guided using IHC4 was predicted to lead to a reduction in the proportion of patients receiving chemotherapy compared with current clinical practice under our base-case assumptions (9.57% vs. 14.42%). More women were classified as high or intermediate risk with OncotypeDX than with IHC4, and were therefore more likely to receive chemotherapy.
For a cohort of 1000 women with ER+, LN−, HER2− early breast cancer, we predicted that 76 distant recurrences would occur under current clinical practice. Treatment guided using OncotypeDX and IHC4 was predicted to reduce the number of distant recurrences to 64 and 71, respectively, under the assumptions used for the base-case analysis.
The mean discounted cost of treatment guided using current clinical practice, OncotypeDX and IHC4 was estimated to be £6519, £9094 and £6340 per patient respectively. The breakdown of costs by category is presented in Table 65. Treatment guided using OncotypeDX was estimated to reduce the costs associated with the management of recurrences (distant and local recurrences, terminal care) but incurred additional costs to perform the test (£2580) compared with current clinical practice. IHC4 also reduced the costs associated with recurrences, but also reduced the costs associated with chemotherapy, for an additional test cost of £150 per patient compared with current clinical practice.
| Cost categories | Current clinical practice (£) | OncotypeDX (£) | IHC4 (£) |
|---|---|---|---|
| Recurrence free | 926 | 928 | 927 |
| Distant recurrence | 1277 | 1081 | 1199 |
| Terminal care | 222 | 188 | 209 |
| Local recurrence | 92 | 78 | 87 |
| Endocrine therapy | 3298 | 3307 | 3302 |
| Chemotherapy | 591 | 783 | 392 |
| Short-term adverse events | 110 | 145 | 73 |
| Long-term adverse events | 3 | 4 | 2 |
| Cost of test | 0 | 2580 | 150 |
| Total cost | 6519 | 9094 | 6340 |
The mean discounted QALYs were 13.44, 13.54 and 13.49 for current clinical practice, OncotypeDX and IHC4 respectively.
Compared with current clinical practice, the incremental cost for treatment guided using OncotypeDX was estimated to be £26,940 per QALY gained. Chemotherapy treatment guided using IHC4 was dominant (i.e. provided more QALYs at a lower cost) compared with current clinical practice. These results are based on the assumption that OncotypeDX has predictive ability. This assumption was tested in sensitivity analysis.
Treatment guided using OncotypeDX, IHC4 and current clinical practice was also compared using incremental analysis, that is, the least effective strategy was compared with the next least effective strategy that was neither dominated nor extendedly dominated. The base-case costs and QALYs are shown in Table 66. Treatment guided using OncotypeDX provided the most benefit (13.54 QALYs) at the highest cost (£9094). The ICER for treatment guided using OncotypeDX compared with treatment guided using IHC4 was £55,406 per QALY gained.
| Mean cost (£) | Mean QALYs | ICER (£) | Incremental analysis (£) | |
|---|---|---|---|---|
| OncotypeDX | 9094 | 13.54 | 26,940 | 55,406 |
| IHC4 | 6340 | 13.49 | Cost saving | |
| Current clinical practice | 6519 | 13.44 |
Probabilistic results
The results of PSA using 2500 iterations are shown in Table 67. Treatment guided using IHC4 remained dominant (i.e. provided more QALYs at a lower cost) compared with current clinical practice. The incremental cost for treatment guided using OncotypeDX was £29,503 per QALY gained compared with current clinical practice and £64,111 per QALY gained compared with IHC4 (see Table 67).
| Mean cost (£) | Mean QALYs | ICER (£) | Incremental analysis (£) | |
|---|---|---|---|---|
| OncotypeDX | 9100 | 13.52 | 29,503 | 64,111 |
| IHC4 | 6332 | 13.48 | Cost saving | |
| Current clinical practice | 6507 | 13.44 |
Figure 13 shows the cost-effectiveness acceptability curve (CEAC) using results generated over a lifetime horizon. The curve shows the probability of each test being cost-effective for different monetary values that the decision-maker may be willing to pay for an additional QALY. The CEAC shows that treatment guided by IHC4 was the most cost-effective strategy compared with current clinical practice and OncotypeDX when using a willingness-to-pay threshold of £20,000 per QALY gained (in 99.48% of cases). The probability that treatment guided using OncotypeDX was cost-effective at a £20,000 threshold was 0.40% in the incremental analysis and 12.44% compared with current clinical practice alone.
FIGURE 13.
Cost-effectiveness acceptability curve for the primary analysis comparing OncotypeDX and IHC4 with current clinical practice assuming that the test is given to all women with ER+, LN−, HER2− early breast cancer in England and Wales.
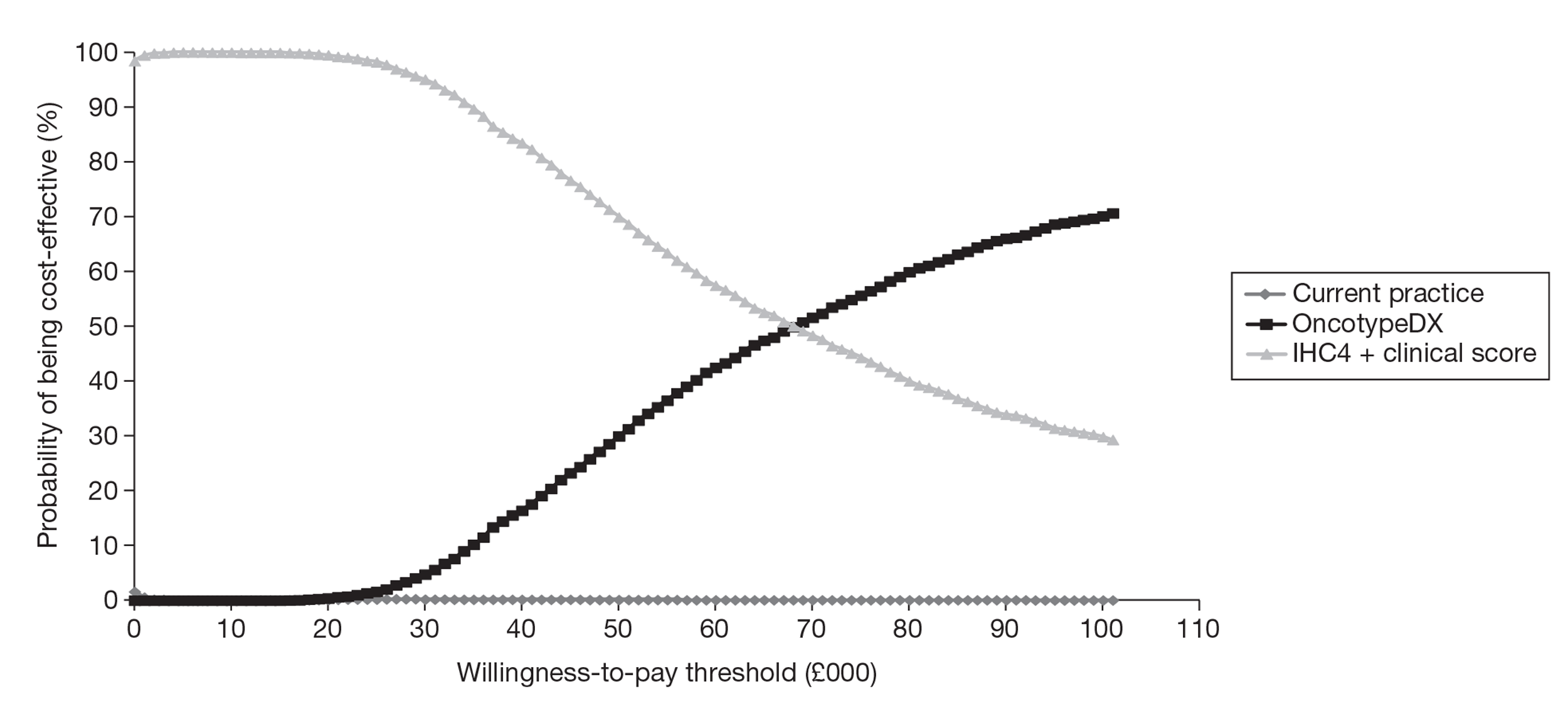
The new tests were offered only to women with ER+, LN−, HER2− early breast cancer with a Nottingham Prognostic Index score > 3.4
Deterministic results
Assuming that the tests were offered only to women with ER+, LN−, HER2− early breast cancer with a NPI score > 3.4, a greater proportion of patients were predicted to receive chemotherapy when using OncotypeDX (under our base-case assumptions) and a lower proportion were predicted to receive chemotherapy when using IHC4 compared with current clinical practice (34.72% vs. 26.31% vs. 33.60% respectively).
Treatment guided using IHC4 was dominant (i.e. provided more QALYs at a lower cost) compared with current clinical practice. The incremental cost for treatment guided using OncotypeDX was £9007 per QALY gained compared with current clinical practice and £26,859 per QALY gained compared with IHC4 (Table 68). This is based on the assumption that OncotypeDX has predictive ability. This assumption was tested in sensitivity analysis.
| Mean cost (£) | Mean QALYs | ICER (£) | Incremental analysis (£) | |
|---|---|---|---|---|
| OncotypeDX | 10,911 | 13.06 | 9007 | 26,859 |
| IHC4 | 8318 | 12.97 | Cost saving | |
| Current clinical practice | 8816 | 12.83 |
Probabilistic results
The results of PSA using 2500 iterations are shown in Table 69. Treatment guided using IHC4 remained dominant (i.e. provided more QALYs at a lower cost) compared with current clinical practice. The incremental cost for treatment guided using OncotypeDX was £9774 per QALY gained compared with current clinical practice and £31,125 per QALY gained compared with IHC4 (see Table 69).
| Mean cost (£) | Mean QALYs | ICER (£) | Incremental analysis (£) | |
|---|---|---|---|---|
| OncotypeDX | 10,924 | 13.05 | 9774 | 31,125 |
| IHC4 | 8305 | 12.96 | Cost saving | |
| Current clinical practice | 8797 | 12.83 |
The CEAC (Figure 14) shows that treatment guided by IHC4 was the most cost-effective strategy when using a willingness-to-pay threshold of £20,000 per QALY gained (in 81.24% of cases). The probability that treatment guided using OncotypeDX was cost-effective at a £20,000 threshold was 18.60% in the incremental analysis and 91.56% compared with current clinical practice alone.
FIGURE 14.
Cost-effectiveness acceptability curve for the primary analysis comparing OncotypeDX and IHC4 with current clinical practice assuming that the test is given only to women with a NPI score > 3.4.
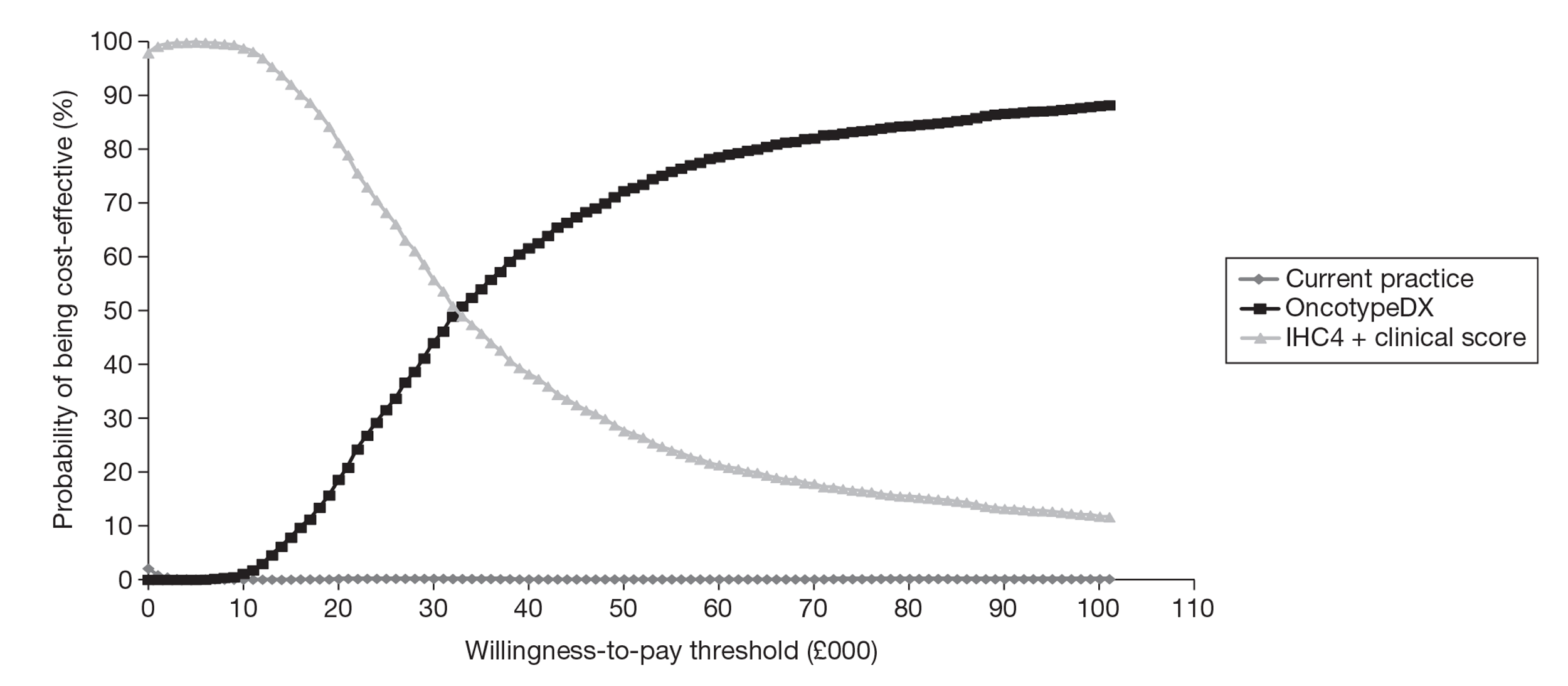
Univariate sensitivity analyses: parameters
A range of univariate sensitivity analyses were carried out to explore the impact of varying the main model parameters. Results of the univariate sensitivity analysis assuming that the tests were offered to all women with ER+, LN−, HER2− early breast cancer are presented in Table 70. Results for the univariate sensitivity analysis assuming that the tests were offered only to women with a NPI score > 3.4 are presented in Table 71.
| OncotypeDX | IHC4 | Clinical practice | ICER (£) | |||||
|---|---|---|---|---|---|---|---|---|
| QALYs | Cost (£) | QALYs | Cost (£) | QALYs | Cost (£) | OncotypeDX vs. clinical practice | IHC4 vs. clinical practice | |
| Base case | 13.54 | 9094 | 13.49 | 6340 | 13.44 | 6519 | 26,940 | Cost saving |
| Time horizon = 5 years | 4.11 | 7947 | 4.11 | 5093 | 4.10 | 5209 | 632,318 | Cost saving |
| Time horizon = 10 years | 7.05 | 8680 | 7.04 | 5883 | 7.03 | 6036 | 120,123 | Cost saving |
| Time horizon = 20 years | 11.08 | 9080 | 11.04 | 6324 | 11.01 | 6502 | 39,368 | Cost saving |
| Starting age = 50 years | 15.51 | 9166 | 15.45 | 6416 | 15.39 | 6597 | 21,632 | Cost saving |
| Starting age = 60 years | 12.97 | 9066 | 12.93 | 6310 | 12.88 | 6487 | 28,932 | Cost saving |
| Starting age = 70 years | 9.83 | 8792 | 9.80 | 6017 | 9.77 | 6184 | 47,796 | Cost saving |
| Reduction in the risk of recurrence by 10% | 13.59 | 8975 | 13.54 | 6207 | 13.50 | 6379 | 29,960 | Cost saving |
| Reduction in the risk of recurrence by 20% | 13.64 | 8853 | 13.60 | 6073 | 13.56 | 6236 | 33,784 | Cost saving |
| Increase in the risk of recurrence by 10% | 13.49 | 9212 | 13.43 | 6471 | 13.38 | 6656 | 24,494 | Cost saving |
| Increase in the risk of recurrence by 20% | 13.44 | 9328 | 13.37 | 6599 | 13.32 | 6791 | 22,473 | Cost saving |
| Recurrence up to 20 years | 13.50 | 9229 | 13.45 | 6488 | 13.40 | 6673 | 25,298 | Cost saving |
| No changed in the risk of distant recurrence between 10 and 15 years | 13.48 | 9277 | 13.42 | 6541 | 13.37 | 6728 | 24,342 | Cost saving |
| Reduction in the risk of distant recurrence by 75% between 10 and 15 years | 13.57 | 9002 | 13.52 | 6238 | 13.48 | 6411 | 28,520 | Cost saving |
| Proportion of local recurrence before a distant recurrence = 5% | 13.54 | 9053 | 13.49 | 6294 | 13.44 | 6470 | 27,034 | Cost saving |
| Proportion of local recurrence before a distant recurrence = 20% | 13.54 | 9165 | 13.49 | 6418 | 13.44 | 6602 | 26,780 | Cost saving |
| Proportion of local recurrence before a distant recurrence = 30% | 13.54 | 9239 | 13.49 | 6500 | 13.44 | 6689 | 26,611 | Cost saving |
| Time in distant recurrence health state different by prognosis group | 13.56 | 9359 | 13.51 | 6648 | 13.46 | 6772 | 26,793 | Cost saving |
| Time in distant recurrence – LCI | 13.51 | 8809 | 13.46 | 6023 | 13.41 | 6181 | 26,248 | Cost saving |
| Time in distant recurrence – UCI | 13.56 | 9354 | 13.51 | 6628 | 13.47 | 6825 | 27,629 | Cost saving |
| Proportion of patients receiving chemotherapy under current clinical practice = ECRIC only | 13.54 | 9094 | 13.49 | 6340 | 13.44 | 6493 | 26,830 | Cost saving |
| Proportion of patients receiving chemotherapy under current clinical practice = WMCIU only | 13.54 | 9094 | 13.49 | 6340 | 13.45 | 6605 | 27,428 | Cost saving |
| Proportion of patients receiving chemotherapy under current clinical practice = Holt et al. study77 | 13.54 | 9094 | 13.49 | 6340 | 13.48 | 7230 | 35,951 | Cost saving |
| Increase by 20% in the proportion of patients receiving chemotherapy under current clinical practice | 13.54 | 9094 | 13.49 | 6340 | 13.45 | 6632 | 28,346 | Cost saving |
| Increase by 30% in the proportion of patients receiving chemotherapy under current clinical practice | 13.54 | 9094 | 13.49 | 6340 | 13.45 | 6688 | 29,182 | Cost saving |
| Reduction by 20% in the proportion of patients receiving chemotherapy under current clinical practice | 13.54 | 9094 | 13.49 | 6340 | 13.43 | 6405 | 25,810 | Cost saving |
| Reduction by 30% in the proportion of patients receiving chemotherapy under current clinical practice | 13.54 | 9094 | 13.49 | 6340 | 13.43 | 6348 | 25,326 | Cost saving |
| Assumption about who would receive chemotherapy with the new tests | (CiC information has been removed) | (CiC information has been removed) | 13.49 | 6308 | 13.44 | 6519 | 23,765 | Cost saving |
| Reduction in the benefit of chemotherapy by 10% | (CiC information has been removed) | (CiC information has been removed) | 13.48 | 6350 | 13.44 | 6525 | 28,416 | Cost saving |
| Reduction in the benefit of chemotherapy by 20% | 13.52 | 9128 | 13.48 | 6359 | 13.43 | 6532 | 29,945 | Cost saving |
| Reduction in the benefit of chemotherapy by 30% | 13.51 | 9144 | 13.47 | 6368 | 13.43 | 6538 | 31,529 | Cost saving |
| Reduction in the benefit of chemotherapy by 40% | 13.51 | 9158 | 13.47 | 6376 | 13.43 | 6543 | 33,169 | Cost saving |
| Assuming the same benefit of chemotherapy for everyone = 40% | 13.49 | 9200 | 13.47 | 6374 | 13.45 | 6498 | 64,940 | Cost saving |
| Assuming the same benefit of chemotherapy for everyone = 30% | 13.46 | 9264 | 13.45 | 6421 | 13.43 | 6535 | 91,274 | Cost saving |
| NPI distribution from the Holt et al. study77 | 13.48 | 9323 | 13.42 | 6589 | 13.36 | 6808 | 22,281 | Cost saving |
| NPI distribution from the TransATAC trial | 13.52 | 9169 | 13.47 | 6421 | 13.42 | 6613 | 25,251 | Cost saving |
| Cost for IHC4 = £100 | 13.54 | 9094 | 13.49 | 6290 | 13.44 | 6519 | 26,940 | Cost saving |
| Cost for IHC4 = £200 | 13.54 | 9094 | 13.49 | 6390 | 13.44 | 6519 | 26,940 | Cost saving |
| Cost for IHC4 = £300 | 13.54 | 9094 | 13.49 | 6490 | 13.44 | 6519 | 26,940 | Cost saving |
| Cost for IHC4 = £400 | 13.54 | 9094 | 13.49 | 6590 | 13.44 | 6519 | 26,940 | 1557 |
| G-CSF is given to all patients receiving chemotherapy | 13.54 | 9373 | 13.49 | 6479 | 13.44 | 6728 | 27,655 | Cost saving |
| Five cycles of G-CSF (instead of three) | 13.54 | 9156 | 13.49 | 6371 | 13.44 | 6565 | 27,099 | Cost saving |
| Five cycles of chemotherapy (instead of three) | 13.54 | 8964 | 13.49 | 6275 | 13.44 | 6420 | 26,604 | Cost saving |
| 100% echocardiogram (instead of 25%) | 13.54 | 9103 | 13.49 | 6344 | 13.44 | 6525 | 26,961 | Cost saving |
| Increase in the cost of chemotherapy by 25% | 13.54 | 9290 | 13.49 | 6438 | 13.44 | 6666 | 27,443 | Cost saving |
| Reduction in the cost of chemotherapy by 25% | 13.54 | 8899 | 13.49 | 6242 | 13.44 | 6371 | 26,437 | Cost saving |
| Increase in the cost of endocrine therapy by 25% | 13.54 | 9921 | 13.49 | 7166 | 13.44 | 7343 | 26,964 | Cost saving |
| Reduction in the cost of endocrine therapy by 25% | 13.54 | 8268 | 13.49 | 5515 | 13.44 | 5694 | 26,916 | Cost saving |
| Increase in the cost of distant metastases by 25% | 13.54 | 9365 | 13.49 | 6640 | 13.44 | 6838 | 26,427 | Cost saving |
| Reduction in the cost of distant metastases by 25% | 13.54 | 8824 | 13.49 | 6040 | 13.44 | 6199 | 27,453 | Cost saving |
| Cost of local recurrence – LCI | 13.54 | 9070 | 13.49 | 6313 | 13.44 | 6490 | 26,986 | Cost saving |
| Cost of local recurrence – UCI | 13.54 | 9123 | 13.49 | 6372 | 13.44 | 6552 | 26,886 | Cost saving |
| Terminal care cost – LCI | 13.54 | 9053 | 13.49 | 6294 | 13.44 | 6470 | 27,018 | Cost saving |
| Terminal care cost – UCI | 13.54 | 9136 | 13.49 | 6386 | 13.44 | 6568 | 26,861 | Cost saving |
| Utility values – LCI | 12.89 | 9094 | 12.84 | 6340 | 12.80 | 6519 | 28,061 | Cost saving |
| Utility values – UCI | 14.08 | 9094 | 14.03 | 6340 | 13.98 | 6519 | 26,034 | Cost saving |
| Increase of 25% in the decrement in utility for patients dying from breast cancer | 13.53 | 9094 | 13.48 | 6340 | 13.44 | 6519 | 26,862 | Cost saving |
| Decrease of 25% in the decrement in utility for patients dying from breast cancer | 13.54 | 9094 | 13.49 | 6340 | 13.44 | 6519 | 27,018 | Cost saving |
| Increase of 25% in the decrement in utility for patients receiving chemotherapy | 13.53 | 9094 | 13.49 | 6340 | 13.44 | 6519 | 27,066 | Cost saving |
| Decrease of 25% in the decrement in utility for patients receiving chemotherapy | 13.54 | 9094 | 13.49 | 6340 | 13.44 | 6519 | 26,815 | Cost saving |
| Utility for patients with AML = 0.5 | 13.54 | 9094 | 13.49 | 6340 | 13.44 | 6519 | 26,936 | Cost saving |
| Utility for patients with AML = 0.6 | 13.54 | 9094 | 13.49 | 6340 | 13.44 | 6519 | 26,934 | Cost saving |
| Risk of long-term adverse events multiplied by 2 | 13.52 | 9096 | 13.48 | 6340 | 13.43 | 6519 | 27,954 | Cost saving |
| Risk of long-term adverse events multiplied by 3 | 13.51 | 9097 | 13.47 | 6340 | 13.42 | 6520 | 29,034 | Cost saving |
| Proportion of patients classified as intermediate with the new test undergoing chemotherapy = low-risk value | 13.50 | 8683 | 13.46 | 6215 | 13.44 | 6519 | 35,629 | Cost saving |
| Proportion of patients classified as intermediate with the new test undergoing chemotherapy = high-risk value | 13.56 | 9323 | 13.52 | 6434 | 13.44 | 6519 | 22,812 | Cost saving |
| OncotypeDX | IHC4 | Clinical practice | ICER (£) | |||||
|---|---|---|---|---|---|---|---|---|
| QALYs | Cost (£) | QALYs | Cost (£) | QALYs | Cost (£) | OncotypeDX vs. clinical practice | IHC4 vs. clinical practice | |
| Base case | 13.06 | 10,911 | 12.97 | 8318 | 12.83 | 8816 | 9007 | Cost saving |
| Time horizon = 5 years | 4.07 | 9012 | 4.07 | 6245 | 4.06 | 6544 | 170,573 | Cost saving |
| Time horizon = 10 years | 6.94 | 10,170 | 6.92 | 7501 | 6.88 | 7918 | 39,573 | Cost saving |
| Time horizon = 20 years | 10.75 | 10,884 | 10.69 | 8289 | 10.59 | 8784 | 13,108 | Cost saving |
| Starting age = 50 years | 14.93 | 11,019 | 14.81 | 8434 | 14.64 | 8941 | 7203 | Cost saving |
| Starting age = 60 years | 12.53 | 10,868 | 12.44 | 8272 | 12.32 | 8765 | 9686 | Cost saving |
| Starting age = 70 years | 9.56 | 10,456 | 9.50 | 7828 | 9.42 | 8286 | 16,152 | Cost saving |
| Reduction in the risk of recurrence by 10% | 13.16 | 10,700 | 13.07 | 8085 | 12.94 | 8559 | 10,087 | Cost saving |
| Reduction in the risk of recurrence by 20% | 13.25 | 10,485 | 13.17 | 7846 | 13.06 | 8296 | 11,440 | Cost saving |
| Increase in the risk of recurrence by 10% | 12.98 | 11,117 | 12.87 | 8547 | 12.72 | 9066 | 8125 | Cost saving |
| Increase in the risk of recurrence by 20% | 12.89 | 11,319 | 12.77 | 8770 | 12.62 | 9311 | 7392 | Cost saving |
| Recurrence up to 20 years | 13.00 | 11,146 | 12.90 | 8577 | 12.76 | 9095 | 8389 | Cost saving |
| No changed in the risk of distant recurrence between 10 and 15 years | 12.96 | 11,230 | 12.85 | 8670 | 12.71 | 9195 | 8055 | Cost saving |
| Reduction in the risk of distant recurrence by 75% between 10 and 15 years | 13.12 | 10,747 | 13.03 | 8138 | 12.90 | 8619 | 9585 | Cost saving |
| Proportion of local recurrence before a distant recurrence = 5% | 13.07 | 10,836 | 12.97 | 8235 | 12.83 | 8723 | 9086 | Cost saving |
| Proportion of local recurrence before a distant recurrence = 20% | 13.06 | 11,039 | 12.97 | 8459 | 12.83 | 8973 | 8872 | Cost saving |
| Proportion of local recurrence before a distant recurrence = 30% | 13.06 | 11,174 | 12.97 | 8609 | 12.83 | 9139 | 8729 | Cost saving |
| Time in distant recurrence health state different by prognosis group | 13.08 | 11,118 | 12.99 | 8587 | 12.84 | 8932 | 9091 | Cost saving |
| Time in distant recurrence – LCI | 13.02 | 10,391 | 12.92 | 7743 | 12.78 | 8175 | 9116 | Cost saving |
| Time in distant recurrence – UCI | 13.11 | 11,383 | 13.01 | 8840 | 12.88 | 9396 | 8900 | Cost saving |
| Proportion of patients receiving chemotherapy under current clinical practice = ECRIC only | 13.06 | 10,911 | 12.97 | 8318 | 12.83 | 8775 | 9019 | Cost saving |
| Proportion of patients receiving chemotherapy under current clinical practice = WMCIU only | 13.06 | 10,911 | 12.97 | 8318 | 12.85 | 8959 | 8967 | Cost saving |
| Proportion of patients receiving chemotherapy under current clinical practice = Holt et al. study77 | 13.06 | 10,911 | 12.97 | 8318 | 12.97 | 10,037 | 8818 | Cost saving |
| Increase by 20% in the proportion of patients receiving chemotherapy under current clinical practice | 13.06 | 10,911 | 12.97 | 8318 | 12.86 | 9066 | 8938 | Cost saving |
| Increase by 30% in the proportion of patients receiving chemotherapy under current clinical practice | 13.06 | 10,911 | 12.97 | 8318 | 12.87 | 9191 | 8907 | Cost saving |
| Reduction by 20% in the proportion of patients receiving chemotherapy under current clinical practice | 13.06 | 10,911 | 12.97 | 8318 | 12.81 | 8564 | 9082 | Cost saving |
| Reduction by 30% in the proportion of patients receiving chemotherapy under current clinical practice | 13.06 | 10,911 | 12.97 | 8318 | 12.79 | 8438 | 9121 | Cost saving |
| Assumption about who would receive chemotherapy with the new tests | 13.09 | 10,799 | 12.98 | 8192 | 12.83 | 8816 | 7761 | Cost saving |
| Reduction in the benefit of chemotherapy by 10% | 13.05 | 10,950 | 12.96 | 8347 | 12.82 | 8834 | 9459 | Cost saving |
| Reduction in the benefit of chemotherapy by 20% | 13.03 | 10,987 | 12.94 | 8374 | 12.82 | 8851 | 9923 | Cost saving |
| Reduction in the benefit of chemotherapy by 30% | 13.02 | 11,022 | 12.93 | 8399 | 12.81 | 8867 | 10,400 | Cost saving |
| Reduction in the benefit of chemotherapy by 40% | 13.00 | 11,055 | 12.92 | 8423 | 12.80 | 8882 | 10,890 | Cost saving |
| Assuming the same benefit of chemotherapy for everyone = 40% | (CiC information has been removed) | (CiC information has been removed) | 12.93 | 8419 | 12.85 | 8766 | 28,833 | Cost saving |
| Assuming the same benefit of chemotherapy for everyone = 30% | (CiC information has been removed) | (CiC information has been removed) | 12.87 | 8557 | 12.81 | 8867 | 39,579 | Cost saving |
| NPI distribution from the Holt et al. study77 | 13.06 | 10,911 | 12.97 | 8318 | 12.83 | 8816 | 9007 | Cost saving |
| NPI distribution from the TransATAC trial | 13.06 | 10,911 | 12.97 | 8318 | 12.83 | 8816 | 9007 | Cost saving |
| Cost for IHC4 = £100 | 13.06 | 10,911 | 12.97 | 8268 | 12.83 | 8816 | 9007 | Cost saving |
| Cost for IHC4 = £200 | 13.06 | 10,911 | 12.97 | 8368 | 12.83 | 8816 | 9007 | Cost saving |
| Cost for IHC4 = £300 | 13.06 | 10,911 | 12.97 | 8468 | 12.83 | 8816 | 9007 | Cost saving |
| Cost for IHC4 = £400 | 13.06 | 10,911 | 12.97 | 8568 | 12.83 | 8816 | 9007 | Cost saving |
| G-CSF is given to all patients receiving chemotherapy | 13.06 | 11,416 | 12.97 | 8701 | 12.83 | 9305 | 9077 | Cost saving |
| Five cycles of G-CSF (instead of three) | 13.06 | 11,023 | 12.97 | 8403 | 12.83 | 8924 | 9023 | Cost saving |
| Five cycles of chemotherapy (instead of three) | 13.06 | 10,674 | 12.97 | 8139 | 12.83 | 8586 | 8974 | Cost saving |
| 100% echocardiogram (instead of 25%) | 13.06 | 10,926 | 12.97 | 8330 | 12.83 | 8830 | 9009 | Cost saving |
| Increase in the cost of chemotherapy by 25% | 13.06 | 11,267 | 12.97 | 8588 | 12.83 | 9160 | 9056 | Cost saving |
| Reduction in the cost of chemotherapy by 25% | 13.06 | 10,555 | 12.97 | 8049 | 12.83 | 8471 | 8958 | Cost saving |
| Increase in the cost of endocrine therapy by 25% | 13.06 | 11,727 | 12.97 | 9132 | 12.83 | 9627 | 9031 | Cost saving |
| Reduction in the cost of endocrine therapy by 25% | 13.06 | 10,094 | 12.97 | 7504 | 12.83 | 8005 | 8984 | Cost saving |
| Increase in the cost of distant metastases by 25% | 13.06 | 11,403 | 12.97 | 8862 | 12.83 | 9421 | 8519 | Cost saving |
| Reduction in the cost of distant metastases by 25% | 13.06 | 10,419 | 12.97 | 7774 | 12.83 | 8210 | 9495 | Cost saving |
| Cost of local recurrence – LCI | 13.06 | 10,867 | 12.97 | 8269 | 12.83 | 8761 | 9051 | Cost saving |
| Cost of local recurrence – UCI | 13.06 | 10,963 | 12.97 | 8375 | 12.83 | 8879 | 8956 | Cost saving |
| Terminal care cost – LCI | 13.06 | 10,835 | 12.97 | 8235 | 12.83 | 8723 | 9082 | Cost saving |
| Terminal care cost – UCI | 13.06 | 10,986 | 12.97 | 8402 | 12.83 | 8908 | 8932 | Cost saving |
| Utility values – LCI | 12.44 | 10,911 | 12.35 | 8318 | 12.21 | 8816 | 9378 | Cost saving |
| Utility values – UCI | 13.59 | 10,911 | 13.49 | 8318 | 13.35 | 8816 | 8707 | Cost saving |
| Increase of 25% in the decrement in utility for patients dying from breast cancer | 13.06 | 10,911 | 12.97 | 8318 | 12.83 | 8816 | 8982 | Cost saving |
| Decrease of 25% in the decrement in utility for patients dying from breast cancer | 13.07 | 10,911 | 12.97 | 8318 | 12.84 | 8816 | 9032 | Cost saving |
| Increase of 25% in the decrement in utility for patients receiving chemotherapy | 13.06 | 10,911 | 12.97 | 8318 | 12.83 | 8816 | 9011 | Cost saving |
| Decrease of 25% in the decrement in utility for patients receiving chemotherapy | 13.07 | 10,911 | 12.97 | 8318 | 12.84 | 8816 | 9003 | Cost saving |
| Utility for patients with AML = 0.5 | 13.06 | 10,911 | 12.97 | 8318 | 12.83 | 8816 | 9007 | Cost saving |
| Utility for patients with AML = 0.6 | 13.07 | 10,911 | 12.97 | 8318 | 12.83 | 8816 | 9007 | Cost saving |
| Risk of long-term adverse events multiplied by 2 | 13.04 | 10,913 | 12.95 | 8318 | 12.81 | 8817 | 9061 | Cost saving |
| Risk of long-term adverse events multiplied by 3 | 13.02 | 10,915 | 12.93 | 8318 | 12.79 | 8818 | 9112 | Cost saving |
| Proportion of patients classified as intermediate with the new test undergoing chemotherapy = low risk value | 13.00 | 10,450 | 12.88 | 8045 | 12.83 | 8816 | 10,022 | Cost saving |
| Proportion of patients classified as intermediate with the new test undergoing chemotherapy = high risk value | 13.14 | 11,351 | 13.06 | 8565 | 12.83 | 8816 | 8371 | Cost saving |
OncotypeDX
The main model parameters were varied within reasonable ranges. The ICER for OncotypeDX compared with current clinical practice was mainly sensitive (defined as changes in the ICER by ≥ ±10%) to the assumptions about the time horizon, the starting age of the cohort, the risk of recurrence, the proportion of patients receiving chemotherapy after reclassification with the new test, the benefit of chemotherapy and the NPI score distribution (see Tables 70 and 71).
The ICER was sensitive to the assumed benefit of chemotherapy. The ICER increased (less favourable to OncotypeDX) assuming a lower benefit of chemotherapy (−20% to −40%). The ICER also deteriorated (less favourable to OncotypeDX) significantly assuming that the test was prognostic only, that is, the same relative reduction in the risk of distant recurrence following chemotherapy was applied whether patients were classified as low, intermediate or high risk according to the OncotypeDX RS classification.
The ICER increased (less favourable to OncotypeDX) as the time horizon decreased or the age increased, given that less benefit can be accrued over time.
A reduction in the risk of distant recurrence increased the ICER (less favourable to OncotypeDX) whereas an increase in the risk of distant recurrence improved the ICER in favour of OncotypeDX. Given that more recurrences can be avoided if there is an increase in the risk of distant recurrence, more of the cost of the test can be offset.
Furthermore, the ICER was sensitive to the assumptions about the proportion of patients who received chemotherapy depending on the results of the test (interpretation of the test). If we assumed that chemotherapy was guided solely by the test results, so no women classified as low risk, 50% of women classified as intermediate risk and 100% of women classified as high risk with OncotypeDX receive chemotherapy (for women with both a NPI score ≤ 3.4 and a NPI score > 3.4), the ICER improved (more favourable to OncotypeDX) because chemotherapy is targeted to patients who, according to the test, are likely to benefit the most from it. In addition, the ICER was very sensitive to the assumption about the probability of chemotherapy in patients classified as intermediate risk with OncotypeDX. The ICER ranged from £22,812 to £35,629 if the test was given to all women and from £8371 to £10,022 if the test was given only to women with a NPI score > 3.4, assuming that the probability of receiving chemotherapy was the same as for patients classified as low and high risk respectively.
The ICER improved (more favourable to OncotypeDX) when using the NPI distribution from the Holt et al. study77 in the model as more patients were classified with a NPI score > 3.4. This group of patients was shown to derive a greater benefit from the new test.
IHC4
The ICER for IHC4 was sensitive to a greater number of assumptions than the ICER for OncotypeDX, such as the time spent in the distant recurrence health state, the proportion of patients receiving chemotherapy under clinical practice and the cost of chemotherapy, but remained dominant compared with current clinical practice (i.e. provided more QALYs at a lower cost) except when the cost of IHC4 was raised to £400 (ICER of £1557 per QALY gained compared with current clinical practice).
Univariate sensitivity analyses: structural assumptions
In addition to input parameter values, we also examined the impact of two structural assumptions; the exclusion of IHC4 from the model and the impact of modelling patients as a single group (instead of as two separate subgroups: NPI score ≤ 3.4 and NPI score > 3.4)
Assuming no further reclassification using IHC4 (exclusion of IHC4)
In this scenario we used data for OncotypeDX only (Table 49 presents data for the risk classification) from the TransATAC trial, assuming no further reclassification with IHC4. Therefore, we calculated the ICER only for OncotypeDX compared with current clinical practice. Patients were split into six possible risk categories (by NPI and OncotypeDX RS) compared with 18 risk categories in the base-case model (by NPI, RS and IHC4).
The impact on the ICER was minimal: a reduction in the ICER from £26,940 (base case) to £25,574 per QALY gained assuming that the test is given to all women with ER+, LN−, HER2− early breast cancer or an increase in the ICER from £9007 (base case) to £10,218 per QALY gained assuming that the test is given only to women with a NPI score > 3.4.
The results of this scenario analysis suggested that our base-case ICER for OncotypeDX was minimally affected by our choice of model structure to accommodate the evaluation of IHC4.
Assuming no further reclassification using IHC4 (exclusion of IHC4) and modelling the entire cohort as a single group (not split by Nottingham Prognostic Index score)
A second structural assumption was tested, to examine to what extent not separating patients into two subgroups (by NPI score) affected the ICER. This assumes that patients with a NPI score ≤ 3.4 and patients with a NPI score > 3.4 within the same risk group (defined by the new test) have the same prognosis.
Again, in this scenario analysis we used data for OncotypeDX only (Table 49 present data for the risk classification) from the TransATAC trial, assuming no further reclassification with IHC4. Therefore, we calculated the ICER only for OncotypeDX compared with current clinical practice. In this scenario analysis, the model separated patients into three possible risk categories (by RS only) compared with 18 risk categories in the base-case model (by NPI, RS and IHC4).
As expected, this assumption had a positive impact on the ICER (more favourable to OncotypeDX), with the ICER decreasing from £26,940 (base case) to £18,859 per QALY gained assuming that the test is given to all woman with ER+, LN−, HER2− early breast cancer.
By modelling the entire cohort as a single group, the prognostic value of current decision-making using clinicopathological parameters is ignored (i.e. that patients with a low NPI score have a lower risk of recurrence but are also less likely to receive chemotherapy compared with patients with a NPI score > 3.4 under current clinical practice). This is more favourable to OncotypeDX. This scenario assumes that patients within the defined RS risk group are homogeneous; however, it seems more likely that patients with a low RS and low NPI score would have a better prognosis than patients with a low RS and high NPI score.
Exploratory analysis: cost-effectiveness of treatment guided using Mammostrat
An exploratory analysis was carried out to assess the cost-effectiveness of Mammostrat compared with current clinical practice in England and Wales. The evidence base for Mammostrat is less well developed and a number of gaps were identified by the systematic review of the literature.
The EAG economic model was repopulated using the evidence for Mammostrat on reclassification (unpublished) and on the benefit of chemotherapy by risk group, but many assumptions were necessary because of limitations in the evidence available, especially on the impact of the test on decision-making and the extent to which the reclassification data used in the model were generalisable to the UK population. Further uncertainty is introduced given that the EAG economic model uses distant recurrence as an outcome whereas most of the evidence for Mammostrat was drawn from analyses of DFS and therefore included all recurrences.
Data from a subset of the Ring et al. study125 were used in the economic model; however, (CIC information has been removed). There is major uncertainty regarding the robustness of the reclassification data from the subset of the Ring et al. study. 125
Because of the limitations of the evidence base, any conclusions drawn from this analysis are subject to significant uncertainty.
Deterministic results
The proportion of women receiving chemotherapy was estimated to increase slightly with the use of Mammostrat compared with current clinical practice under our base-case assumptions (21.16% vs. 14.42% in all women; 34.27% vs. 33.60% in women with a NPI score > 3.4 with ER+, LN−, HER− early breast cancer).
Compared with current clinical practice, the incremental cost for treatment guided using Mammostrat was £26,598 per QALY gained under our base-case assumptions, assuming that the test is given to all women with ER+, LN−, HER− early breast cancer (Table 72).
| Mean cost (£) | Mean QALYs | ICER (£) | |
|---|---|---|---|
| All patients | |||
| Mammostrat | 9040 | 12.91 | 26,598 |
| Current clinical practice | 7699 | 12.86 | |
| Patients with a NPI score > 3.4 | |||
| Mammostrat | 10,985 | 12.29 | Dominated |
| Current clinical practice | 9717 | 12.34 | |
If Mammostrat was given only to women with a NPI score > 3.4, the Mammostrat test was dominated (i.e. provided less benefits for a higher cost). (CIC information has been removed.)
Probabilistic sensitivity analysis
The results of the PSA using 2500 iterations are shown in Table 73. Treatment guided using Mammostrat had a cost per QALY gained of £27,731 compared with current clinical practice if the test was offered to all women with ER+, LN−, HER2− early breast cancer. If the test was offered only to women with a NPI score > 3.4, Mammostrat was dominated (i.e. provided less QALYs at a higher cost).
| Mean cost (£) | Mean QALYs | ICER (£) | |
|---|---|---|---|
| All patients | |||
| Mammostrat | 9028 | 12.90 | 27,731 |
| Current clinical practice | 7683 | 12.85 | |
| Patients with a NPI score > 3.4 | |||
| Mammostrat | 10,958 | 12.29 | Dominated |
| Current clinical practice | 9685 | 12.34 | |
The CEAC shows that treatment guided by Mammostrat score is a cost-effective strategy in 36.0% of cases when using a willingness-to-pay threshold of £20,000 per QALY gained (Figure 15) if the test were to be given to all women with ER+, LN−, HER2− early breast caner. The probability of treatment guided using Mammostrat being cost-effective at a £20,000 threshold was 18.0% if the test were to be offered only to women with a NPI score > 3.4 (Figure 16).
FIGURE 15.
Cost-effectiveness acceptability curve for the exploratory analysis comparing Mammostrat with current clinical practice assuming that the test is given to all women with ER+, LN−, HER2− early breast cancer in England and Wales.
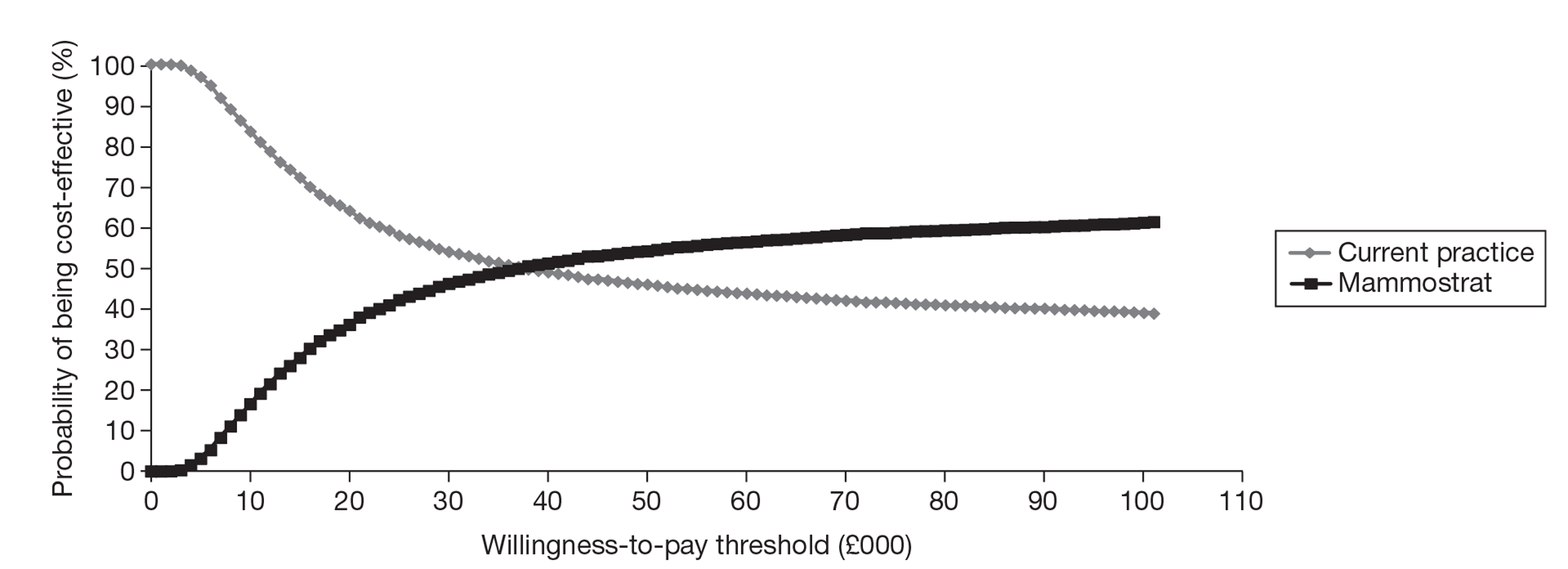
FIGURE 16.
Cost-effectiveness acceptability curve for the exploratory analysis comparing Mammostrat with current clinical practice assuming that the test is given only to women with a NPI score > 3.4.
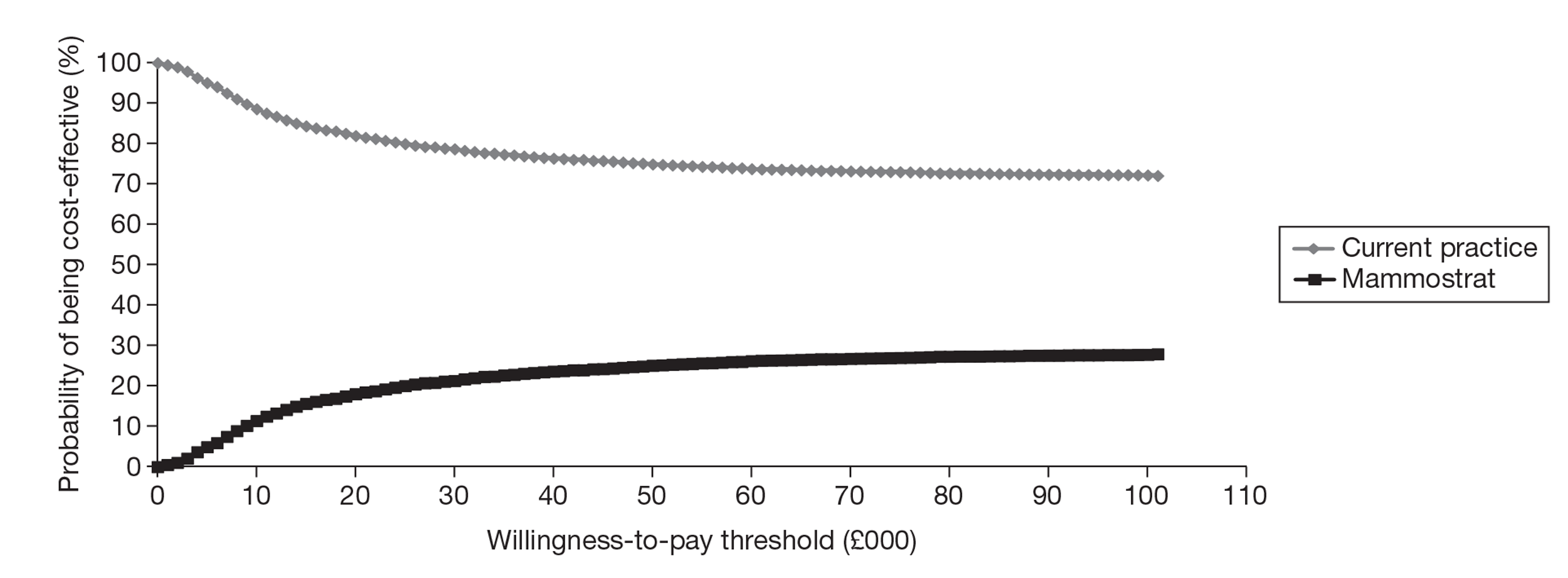
Univariate sensitivity analysis
The impact of key parameters was tested in univariate sensitivity analysis (Tables 74 and 75).
| Current clinical practice | Mammostrat | ICER (£) | |||
|---|---|---|---|---|---|
| QALYs | Cost (£) | QALYs | Cost (£) | ||
| Base case | 12.86 | 7699 | 12.91 | 9040 | 26,598 |
| No adjustment for risk of recurrence | 13.18 | 6995 | 13.23 | 8333 | 25,729 |
| Interpretation based on assumption | 12.86 | 7699 | 12.99 | 9115 | 10,407 |
| Chemotherapy benefit = LCI | 12.89 | 7626 | 12.96 | 8927 | 18,879 |
| Chemotherapy benefit = UCI | 12.78 | 7863 | 12.77 | 9329 | Dominated |
| Time in DM = 50 months | 12.91 | 8279 | 12.96 | 9593 | 27,324 |
| Time in DM = 60 months | 12.95 | 8820 | 13.00 | 10,110 | 28,071 |
| Time in DM = 70 months | 13.00 | 9319 | 13.04 | 10,586 | 28,830 |
| Utility value in recurrence = 0.7 | 12.86 | 7699 | 12.91 | 9040 | 26,696 |
| Utility value in recurrence = 0.75 | 12.87 | 7699 | 12.92 | 9040 | 27,028 |
| Reduction in cost of DM of 20% | 12.86 | 7216 | 12.91 | 8579 | 27,037 |
| Reduction in cost of DM of 40% | 12.86 | 7457 | 12.91 | 8810 | 26,817 |
| Proportion of patients classified as intermediate with the new test undergoing chemotherapy = low-risk value | 12.86 | 7699 | 12.92 | 8616 | 15,500 |
| Proportion of patients classified as intermediate with the new test undergoing chemotherapy = high-risk value | 12.86 | 7699 | 12.90 | 9287 | 34,959 |
| Current clinical practice | Mammostrat | ICER (£) | |||
|---|---|---|---|---|---|
| QALYs | Cost (£) | QALYs | Cost (£) | ||
| Base case | 12.34 | 9717 | 12.29 | 10,985 | Dominated |
| No adjustment for risk of recurrence | 13.15 | 7962 | 13.12 | 9193 | Dominated |
| Interpretation based on assumption | 12.34 | 9717 | 12.28 | 10,955 | Dominated |
| Chemotherapy benefit = LCI | 12.43 | 9523 | 12.37 | 10,815 | Dominated |
| Chemotherapy benefit = UCI | 12.15 | 10,146 | 12.10 | 11,401 | Dominated |
| Time in DM = 50 months | 12.41 | 10,528 | 12.37 | 11,819 | Dominated |
| Time in DM = 60 months | 12.48 | 11,286 | 12.43 | 12,597 | Dominated |
| Time in DM = 70 months | 12.54 | 11,984 | 12.50 | 13,314 | Dominated |
| Utility value in recurrence = 0.7 | 12.35 | 9717 | 12.30 | 10,985 | Dominated |
| Utility value in recurrence = 0.75 | 12.37 | 9717 | 12.32 | 10,985 | Dominated |
| Reduction in cost of DM of 20% | 12.34 | 9040 | 12.29 | 10,291 | Dominated |
| Reduction in cost of DM of 40% | 12.34 | 9379 | 12.29 | 10,638 | Dominated |
| Proportion of patients classified as intermediate with the new test undergoing chemotherapy = low-risk value | 12.34 | 9717 | 12.30 | 10,471 | Dominated |
| Proportion of patients classified as intermediate with the new test undergoing chemotherapy = high-risk value | 12.34 | 9717 | 12.28 | 11,484 | Dominated |
The ICER was very sensitive to the assumption about the proportion of patients who would receive chemotherapy based on the result of the new test if the test was offered to all women with ER+, LN−, HER2− early breast cancer (see Table 74). Assuming that no patients classified as low risk, 50% of patients classified as intermediate risk and 100% of patients classified as high risk would receive chemotherapy improved the ICER (more favourable to Mammostrat). Furthermore, the ICER was very sensitive to the assumption about the probability of chemotherapy in patients classified as intermediate risk with Mammostrat. The ICER ranged from £15,500 to £34,959 if the test was given to all women assuming that the probability of receiving chemotherapy was the same as for patients classified as low and high risk respectively.
The ICER ranged from £18,879 to being dominated, using the lower and upper CIs from the Ross et al. study,126 for the impact of chemotherapy in terms of reduction in risk of recurrences if the test was offered to all women with ER+, LN−, HER2− early breast cancer. The ICER was not sensitive to the assumptions about utility values, management costs and the time spent in the recurrence health state (see Table 74).
The ICERs for the use of Mammostrat remained dominated under the assumptions examined in sensitivity analysis if the test was offered only to women with a NPI score > 3.4 (see Table 75).
Exploratory analysis: cost-effectiveness of treatment guided using MammaPrint
Finally, a second exploratory analysis was carried out assessing the cost-effectiveness of MammaPrint. Although there was a greater volume of evidence for MammaPrint than for Mammostrat, there were significant gaps in the evidence available and data that were used to populate the economic model were not considered to be robust. Therefore, any conclusions that can be drawn from this analysis are subject to considerable uncertainty.
Of note, particular concerns exist about the existing evidence on the benefit of chemotherapy as this is likely to have a significant influence on the ICER. Other issues include the lack of UK data and the fact that the data available were derived mainly from premenopausal women, limiting generalisability to the UK population. Because of these issues, the EAG was not sufficiently confident to provide a single ICER but presented a range of ICERs within the CIs for the benefit of chemotherapy, as this was considered to be the main uncertainty in the model. Note that, although a range of ICERs is presented, there were also significant concerns relating to the design of studies in the evidence base, and this range does not capture this uncertainty in study design.
More assumptions have been made within this analysis than within the other analyses and the results are highly uncertain.
Only a limited number of univariate sensitivity analyses were carried out because of the nature of the analysis. A sensitivity analysis was conducted assuming no additional costs for the NHS for the use of fresh tissue. A second sensitivity analysis was conducted assuming that 5% of patients classified as having good prognosis and 95% of patients classified as having poor prognosis received chemotherapy.
In addition to the univariate sensitivity analyses, we performed a multivariate sensitivity analysis examining different values for the benefit of chemotherapy.
No PSA was conducted as there was considered to be significant uncertainties in the studies used that could not be adequately captured in the economic model (for instance limitations in study design, differences in population included in the studies being younger and at higher risk than the population in the economic model, uncertainties that could not be adequately captured by the parameter uncertainty within the PSA).
MammaPrint offers the option of three complementary tests at no additional cost. ER, PR and HER2 status can be provided in the TargetPrint report. The impact of this has not been captured in the economic model.
Deterministic results
Compared with current clinical practice, the incremental cost for treatment guided using MammaPrint was estimated to range between £12,240 and £53,058 per QALY gained, when the benefit of chemotherapy was varied by the upper and lower CI limits from the Knauer et al. study,110 assuming that the test was given to all women with ER+, LN−, HER2− early breast cancer. If MammaPrint was given only to women with a NPI score > 3.4, the ICER ranged between £6053 and £29,569 per QALY gained (Table 76).
| Mean QALYs | Mean cost (£) | ICER (£) | |
|---|---|---|---|
| All patients | |||
| Current practice | 13.49–13.39 | 6408–6629 | 12,240–53,058 |
| MammaPrint | 13.78–13.47 | 10,017–10,748 | |
| Patients with a NPI score > 3.4 | |||
| Current practice | 13.07–12.81 | 8281–8872 | 6053–29,569 |
| MammaPrint | 13.73–12.99 | 12,278–14,014 | |
Of note, the proportion of patients receiving chemotherapy increased significantly with the use of MammaPrint compared with current practice under our base-case assumptions: 44.18% vs. 14.42% in all women and 90.31% vs. 33.60% in women with a NPI score > 3.4.
Univariate sensitivity analysis
As expected, assuming no additional cost for the NHS for the use of fresh tissue samples improved the ICER (more favourable to MammaPrint) (Table 77).
| Mean QALYs | Mean cost (£) | ICER (£) | |
|---|---|---|---|
| All patients | |||
| Current practice | 13.49–13.39 | 6408–6629 | 11,392–49,838 |
| MammaPrint | 13.78–13.47 | 9767–10,498 | |
| Patients with a NPI score > 3.4 | |||
| Current practice | 13.07–12.81 | 8281–8,872 | 5675–28,131 |
| MammaPrint | 13.73–12.99 | 12,028–13,764 | |
Assuming that 5% and 95% of patients classified as having good and poor prognosis with MammaPrint, respectively, received chemotherapy improved the ICER (more favourable to MammaPrint) (Table 78).
| Mean QALYs | Mean cost (£) | ICER (£) | |
|---|---|---|---|
| All patients | |||
| Current practice | 13.49–13.39 | 6408–6629 | 12,369–48,322 |
| MammaPrint | 13.78–13.48 | 10,045–10,756 | |
| Patients with a NPI score > 3.4 | |||
| Current practice | 13.07–12.81 | 8281–8872 | 6115–23,939 |
| MammaPrint | 13.63–12.99 | 11,705–13,189 | |
Multivariate sensitivity analysis examining the benefit of chemotherapy for risk of distant recurrence
An exploratory multivariate sensitivity analysis was conducted examining the effect of different values for the benefit of chemotherapy by risk group in terms of reduction in the risk of distant recurrence, assuming that MammaPrint is given to all women with ER+, LN−, HER2− early breast cancer (Table 79).
| ICER (£) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reduction in the risk of distant recurrence in patients classified with a poor prognosis using MammaPrint | ||||||||||||||||||||||
| 0% | 5% | 10% | 15% | 20% | 25% | 30% | 35% | 40% | 45% | 50% | 55% | 60% | 65% | 70% | 75% | 80% | 85% | 90% | 95% | 100% | ||
| Reduction in the risk of distant recurrence in patients classified with a good prognosis using MammaPrint | 0% | Dominated | Dominated | 679,644 | 169,516 | 95,893 | 66,391 | 50,493 | 40,552 | 33,748 | 28,799 | 25,037 | 22,081 | 19,696 | 17,732 | 16,087 | 14,688 | 13,485 | 12,438 | 11,519 | 10,707 | 9983 |
| 5% | Dominated | Dominated | 620,556 | 165,504 | 94,569 | 65,740 | 50,107 | 40,298 | 33,568 | 28,665 | 24,933 | 21,998 | 19,629 | 17,677 | 16,040 | 14,648 | 13,450 | 12,408 | 11,493 | 10,683 | 9962 | |
| 10% | Dominated | Dominated | 570,873 | 161,673 | 93,280 | 65,102 | 49,728 | 40,046 | 33,389 | 28,531 | 24,830 | 21,916 | 19,562 | 17,621 | 15,993 | 14,608 | 13,415 | 12,377 | 11,466 | 10,659 | 9940 | |
| 15% | Dominated | Dominated | 528,515 | 158,013 | 92,024 | 64,474 | 49,353 | 39,797 | 33,213 | 28,399 | 24,728 | 21,834 | 19,495 | 17,566 | 15,946 | 14,568 | 13,381 | 12,347 | 11,440 | 10,636 | 9920 | |
| 20% | Dominated | Dominated | 491,972 | 154,510 | 90,800 | 63,858 | 48,983 | 39,552 | 33,037 | 28,268 | 24,626 | 21,753 | 19,429 | 17,511 | 15,900 | 14,528 | 13,346 | 12,317 | 11,413 | 10,613 | 9899 | |
| 25% | Dominated | Dominated | 460,125 | 151,156 | 89,607 | 63,254 | 48,619 | 39,309 | 32,864 | 28,138 | 24,525 | 21,672 | 19,363 | 17,456 | 15,854 | 14,489 | 13,312 | 12,287 | 11,387 | 10,589 | 9878 | |
| 30% | Dominated | Dominated | 432,123 | 147,941 | 88,444 | 62,659 | 48,260 | 39,068 | 32,692 | 28,009 | 24,425 | 21,592 | 19,298 | 17,401 | 15,808 | 14,449 | 13,278 | 12,258 | 11,361 | 10,566 | 9857 | |
| 35% | Dominated | Dominated | 407,310 | 144,857 | 87,309 | 62,076 | 47,905 | 38,831 | 32,522 | 27,881 | 24,325 | 21,513 | 19,233 | 17,347 | 15,762 | 14,410 | 13,244 | 12,228 | 11,334 | 10,543 | 9836 | |
| 40% | Dominated | Dominated | 385,170 | 141,896 | 86,202 | 61,502 | 47,556 | 38,595 | 32,353 | 27,755 | 24,226 | 21,434 | 19,168 | 17,293 | 15,716 | 14,371 | 13,210 | 12,198 | 11,308 | 10,520 | 9816 | |
| 45% | Dominated | Dominated | 365,293 | 139,051 | 85,121 | 60,938 | 47,211 | 38,363 | 32,186 | 27,629 | 24,128 | 21,355 | 19,104 | 17,240 | 15,671 | 14,332 | 13,177 | 12,169 | 11,282 | 10,496 | 9795 | |
| 50% | Dominated | Dominated | 347,349 | 136,314 | 84,067 | 60,384 | 46,870 | 38,133 | 32,020 | 27,504 | 24,031 | 21,277 | 19,040 | 17,186 | 15,626 | 14,293 | 13,143 | 12,140 | 11,256 | 10,473 | 9774 | |
| 55% | Dominated | Dominated | 331,070 | 133,681 | 83,036 | 59,840 | 46,534 | 37,906 | 31,856 | 27,380 | 23,934 | 21,199 | 18,976 | 17,133 | 15,581 | 14,255 | 13,110 | 12,110 | 11,231 | 10,451 | 9754 | |
| 60% | Dominated | Dominated | 316,233 | 131,145 | 82,030 | 59,304 | 46,203 | 37,680 | 31,694 | 27,257 | 23,838 | 21,122 | 18,913 | 17,080 | 15,536 | 14,216 | 13,076 | 12,081 | 11,205 | 10,428 | 9733 | |
| 65% | Dominated | Dominated | 302,656 | 128,701 | 81,047 | 58,778 | 45,876 | 37,458 | 31,532 | 27,135 | 23,742 | 21,045 | 18,850 | 17,028 | 15,491 | 14,178 | 13,043 | 12,052 | 11,179 | 10,405 | 9713 | |
| 70 | Dominated | Dominated | 290,185 | 126,343 | 80,086 | 58,260 | 45,553 | 37,238 | 31,373 | 27,014 | 23,648 | 20,969 | 18,787 | 16,975 | 15,447 | 14,140 | 13,010 | 12,023 | 11,154 | 10,382 | 9693 | |
| 75% | Dominated | Dominated | 278,689 | 124,069 | 79,147 | 57,751 | 45,234 | 37,020 | 31,215 | 26,894 | 23,553 | 20,893 | 18,725 | 16,923 | 15,403 | 14,102 | 12,977 | 11,994 | 11,128 | 10,360 | 9672 | |
| 80% | Dominated | Dominated | 268,059 | 121,872 | 78,229 | 57,250 | 44,920 | 36,804 | 31,058 | 26,775 | 23,460 | 20,818 | 18,663 | 16,871 | 15,359 | 14,064 | 12,944 | 11,966 | 11,103 | 10,337 | 9652 | |
| 85% | Dominated | Dominated | 258,200 | 119,750 | 77,330 | 56,757 | 44,609 | 36,591 | 30,902 | 26,657 | 23,367 | 20,743 | 18,601 | 16,820 | 15,315 | 14,027 | 12,912 | 11,937 | 11,078 | 10,315 | 9632 | |
| 90% | Dominated | Dominated | 249,032 | 117,698 | 76,451 | 56,272 | 44,303 | 36,380 | 30,748 | 26,540 | 23,275 | 20,669 | 18,540 | 16,768 | 15,271 | 13,989 | 12,879 | 11,908 | 11,052 | 10,292 | 9612 | |
| 95% | Dominated | Dominated | 240,484 | 115,714 | 75,591 | 55,795 | 44,000 | 36,171 | 30,596 | 26,423 | 23,183 | 20,595 | 18,479 | 16,717 | 15,228 | 13,952 | 12,847 | 11,880 | 11,027 | 10,270 | 9592 | |
| 100% | Dominated | Dominated | 232,496 | 113,793 | 74,750 | 55,325 | 43,701 | 35,964 | 30,444 | 26,308 | 23,092 | 20,521 | 18,418 | 16,667 | 15,185 | 13,915 | 12,814 | 11,852 | 11,002 | 10,247 | 9572 | |
This analysis suggested that, under base-case assumptions about the risk classification, risk of recurrence and interpretation of the test (i.e. which patients would receive chemotherapy), the ICER was < £20,000 per QALY gained if the relative risk reduction in the risk of recurrence was at least 60% for patients with a poor prognosis. However, the conclusions are likely to change if different assumptions are used for the risk classification or the proportion of patients who would receive chemotherapy according to MammaPrint.
Comparison of assumptions and results with the economic models submitted by Genomic Health and Clarient
Comparison with the economic model submitted by Genomic Health
The base-case ICER estimated by Genomic Health for treatment guided using OncotypeDX compared with current clinical practice was £6232 per QALY gained assuming that the test was given to all women with ER+, LN− or single node-positive and HER2+/− early breast cancer (Table 80).
| Genomic Health economic model | EAG economic model | |
|---|---|---|
| Cost (£) | 12,735 | 9094 |
| QALYs | 11.54 | 13.54 |
| Life expectancy (years) | 14.89 | 16.47 |
| Current clinical practice | ||
| Cost (£) | 11,847 | 6519 |
| QALYs | 11.39 | 13.44 |
| Life expectancy (years) | 14.73 | 16.35 |
| ICER (£) | 6232 | 26,940 |
The ICER estimated by the EAG was £26,940 (deterministic) assuming that the test was offered to all women with ER+, LN−, HER2− early breast cancer (see Table 80).
The main differences between the EAG's economic assessment and the economic model submitted by Genomic Health for OncotypeDX are:
-
A shorter time horizon was used in the economic evaluation submitted by Genomic Health (30 years compared with lifetime in the EAG economic assessment). The starting age of the cohort was also different (58.3 years in the EAG economic assessment compared with 60.6 years in the Genomic Health economic model). This partly explains the differences in the mean life-years and QALYs.
-
There were differences in the populations under assessment (LN−, HER− only in the EAG economic assessment compared with LN− or single positive node and HER2+/− in the economic model submitted by Genomic Health).
-
The risk of distant recurrence was taken from the TransATAC trial in the EAG economic model of a UK population treated with tamoxifen and anastrozole. Data from Paik et al. 48 from a US cohort treated with tamoxifen only was used in the Genomic Health economic model. Of note, the manufacturer examined a scenario using the risk of distant recurrence from the TransATAC trial and showed that the ICER increased from about £6232 to about £9160 per QALY gained.
-
The EAG assumed that the risk of distant recurrence was halved after 10 years and that no distant recurrences occurred after 15 years. The Genomic Health economic model assumed that the risk of distant recurrence was constant and ongoing over time. Therefore, there is the potential to avoid more recurrences in the Genomic Health economic model, resulting in a more favourable ICER.
-
The distribution of patients reclassified using OncotypeDX was derived from the TransATAC trial and cancer registry data in the UK in the EAG economic model, compared with the reclassification from the Holt et al. study78 in the Genomic Health economic model. However, the EAG had concerns regarding the representativeness of patients included in the Holt et al. study (discussed in Chapter 2, Results: OncotypeDX test).
-
The proportion of patients receiving chemotherapy under clinical practice was extracted from registry data in the EAG economic model, whereas in the Genomic Health economic model the proportion observed in the Holt et al. study78 was used. About 44% of women received chemotherapy in the manufacturer's model under current clinical practice. Registry data (used in the EAG economic model) suggested that about 14.4% of women with ER+, LN−, HER2− breast cancer received chemotherapy (4.6% among women with a NPI score ≤ 3.4 and 33.6% among women with a NPI score > 3.4). In the Genomic Health model, 43.86% of patients subsequently classified as low risk by OncotypeDX received chemotherapy under current clinical practice. Because those patients have a low risk of distant recurrence and derive limited benefit from chemotherapy, this high estimate of the proportion of patients receiving chemotherapy in the comparator arm in this subgroup is favourable to OncotypeDX.
-
There were also structural differences between the models. The EAG modelled patients with a NPI score ≤ 3.4 and patients with a NPI score > 3.4 separately in order to conduct a subgroup analysis but also to account for the prognostic value of current decision-making based on clinicopathological parameters. Indeed, as shown in cancer registry data, patients with a low NPI score are less likely to receive chemotherapy than patients with a NPI score > 3.4. But at the same time, patients with a low NPI score have a lower risk of recurrence than patients with a NPI score > 3.4. The economic model submitted by Genomic Health assumed that the risk of recurrence was constant within each OncotypeDX RS group and used the Holt et al. study78 to estimate the proportion of patients who would receive chemotherapy. The Genomic Health approach ignores the prognostic value of current treatment decision-making using clinicopathological parameters and is therefore more favourable to OncotypeDX.
-
The EAG economic model further reclassified patients according to the IHC4 test results to also evaluate the cost-effectiveness of IHC4. A scenario analysis was conducted and showed that the impact of this structural assumption was minimal.
-
The costs of chemotherapy and associated short-term adverse events were lower in the EAG economic assessment (£4866) than in the Genomic Health economic assessment (£7728).
-
There were also differences between the EAG economic assessment and the Genomic Health economic assessment in the utility estimates for the recurrence-free (0.824 vs. 0.78) and distant recurrence (0.685 vs. 0.60) health states. There were therefore more gains associated with the prevention of a distant recurrence in the Genomic Health economic model than in the EAG model (0.18 vs. 0.14).
-
Finally, the EAG economic model also included local recurrences and long-term adverse events due to chemotherapy.
To understand the differences in the results produced by the two models, the EAG economic model was repopulated using the same data inputs and assumptions as in the Genomic Health economic model. The results of this analysis are presented in Table 81. Each row shows the impact of introducing a new assumption in addition to the assumptions considered in the rows above.
| Assumption | ICER (£) |
|---|---|
| Base case | 26,940 |
| Using data for OncotypeDX only (excluding IHC4) | 25,574 |
| Using data for OncotypeDX only (excluding IHC4) and modelling the entire cohort as a single group (no split by NPI score) | 18,859 |
| Assuming the risk of distant recurrence to be constant and ongoing | 13,874 |
| 10-year risk of recurrence extracted from the Paik et al. study49 | 8311 |
| Using the classification of patients from the Holt et al. study78 | 3953 |
| Using the proportion of patients receiving chemotherapy from the Holt et al. study78 | 7032 |
| Starting age = 60.55 years as per the Genomic Health model | 7887 |
| Time horizon = 30 years as per the Genomic Health model | 8431 |
| No long-term adverse events | 8883 |
| No local recurrences | 9067 |
| Cost of chemotherapy as per the Genomic Health model | 6534 |
| Utility values as per the Genomic Health model | 6607 |
| No terminal care cost or decrement in utility | 7091 |
| Cost of distant recurrence as per the Genomic Health model | 6276 |
Using a similar model structure as the Genomic Health economic model reduced the ICER in the EAG model from £26,960 to £18,859 per QALY gained (i.e. modelling three groups of patients according to the OncotypeDX RS classification, with no split by NPI score). When we further assumed a constant risk of recurrence over time using data from the Paik et al. study,49 the ICER decreased to £8311 per QALY gained. Finally, using similar assumptions/data inputs for the proportion of patients receiving chemotherapy, starting age, time horizon, utility values and costs as in the Genomic Health economic model reduced the ICER further to £6276 (compared with £6232 in Genomic Health economic model).
This analysis suggests that the differences in the results are mainly explained by the choice of model structure, assumptions about the risk of recurrence over time and data on risk reclassification and the proportion of patients receiving chemotherapy in clinical practice and after using the new tests.
Comparison with the economic model submitted by Clarient
(CIC information has been removed.)
Both analyses had to use a large number of assumptions given the gap in the evidence available and therefore need to be interpreted with caution. The EAG economic assessment also showed that the use of Mammostrat in women with a NPI score > 3.4 is dominated (i.e. provided less benefit at a higher cost). This may reflect limitations in the reclassification data used.
(CIC information has been removed.) Because of time and resource constraints, the late submission and the nature of this analysis (exploratory), only a brief comparison of the differences is presented for completeness:
-
Time horizon: 10 years in the Clarient economic assessment compared with lifetime in the EAG economic assessment.
-
Model structure: the EAG economic assessment is simple and assumes that patients enter the recurrence state and remain in that health state until death (using data from Thomas et al. 155). The Clarient economic model is more complex and models recurrence-free survival and OS separately; however, a large number of assumptions have been made and inconsistencies were reported by the manufacturer.
-
(CIC information has been removed.)
-
(CIC information has been removed.)
Discussion of the independent economic model results
The four tests with the most well-developed clinical evidence base were considered within the economic evaluation. The EAG presented a primary analysis that compared OncotypeDX and IHC4 with current clinical practice in England and Wales. Based on the EAG model the incremental cost for adjuvant chemotherapy guided using OncotypeDX was estimated to be £29,503 per QALY gained compared with current clinical practice, assuming that the test was offered to all woman with ER+, LN−, HER2− early breast cancer under our base-case assumptions. This assumes that the test has predictive ability, that is, patients in the high-risk group benefit relatively more proportionally from chemotherapy than patients in the lower-risk groups. The IHC4 test was dominant compared with current clinical practice, providing more QALYs at a lower cost. The ICER for OncotypeDX increased substantially if the test was assumed to be prognostic only, that is, assuming the same relative reduction in the risk of recurrence from chemotherapy for all patients irrespective of the OncotypeDX RS classification. IHC4 remained dominant under this assumption. In incremental analysis, when the treatment decision using OncotypeDX was compared with the treatment decision using IHC4, the ICER increased to £64,111 per QALY gained. In a second scenario, assuming that the test was offered only to women with a NPI score > 3.4, treatment guided using IHC4 remained dominant (i.e. provided more QALYs at a lower cost) compared with current clinical practice. The incremental cost for treatment guided using OncotypeDX was £9774 per QALY gained compared with current clinical practice and £31,125 per QALY gained compared with IHC4 (assuming that the test has predictive ability). However, it should be noted that the evidence base for IHC4 is less well developed and therefore the results should be interpreted with consideration of the additional assumptions used in the evaluation. One-way sensitivity analyses indicated that the ICER was most sensitive to the assumptions about the benefit reduction associated with chemotherapy, the time horizon of the model, the starting age of the cohort, the risk of recurrence and who would receive chemotherapy depending on the result of the test. A key area of uncertainty is whether the new tests are prognostic only or offer predictive ability.
The economic analyses suggested that treatment guided using IHC4 has the greatest potential to be cost-effective at a willingness-to-pay threshold of £20,000. However, the evidence base for IHC4 is less well developed than the evidence base for OncotypeDX and a number of additional assumptions were needed to model the IHC4 test. The IHC4 test provides only a continuous risk score and so it was necessary to derive risk categories solely for the purposes of the analysis. No evidence exists on the predictive ability of the IHC4 test. The benefits of chemotherapy by IHC4 risk group were based on indirect evidence, using the OncotypeDX classification; no additional benefit was assumed for IHC4. In the absence of evidence it was assumed that the likelihood of receiving chemotherapy based on the IHC4 risk classification would be the same as for OncotypeDX RS group, that is, that physicians would interpret the results from OncotypeDX and IHC4 in the same way.
For MammaPrint and Mammostrat there were significant gaps/limitations in the evidence available and data that have been used were not considered to be robust by the EAG. For this reason the analyses that were carried out evaluating the cost-effectiveness of MammaPrint and Mammostrat compared with current clinical practice in England and Wales were considered to be exploratory. Any conclusions that are drawn from these analyses are limited and further clinical evidence will be needed to make the findings more robust. The exploratory analyses suggested that the ICER for Mammostrat was around £28,000 per QALY gained compared with current clinical practice under our base-case assumptions, assuming that the test was offered to all women with ER+, LN−, HER2− early breast cancer, but Mammostrat was dominated if the test was given only to women with a NPI score > 3.4 (i.e. provided less QALYs at a higher cost). The second exploratory analysis indicated that MammaPrint has the potential to be cost-effective, but there were too many uncertainties in the data used and the design of the clinical studies to draw any definitive conclusions. Additionally, MammaPrint offers the option of three complementary tests at no additional cost. Notably, ER, PR and HER2 status can be provided in the TargetPrint report. This has not been captured in the economic model.
We did not perform an incremental analysis including the four tests evaluated because of the heterogeneity in the data used to populate the models and the differences in the quality of evidence between the tests. These differences are not adequately reflected in the PSA. Although this may be considered a limitation, we considered that including MammaPrint and Mammastrat within an incremental analysis could potentially be misleading given the gaps in the evidence base and significant issues relating to the quality of the data used to populate the economic models for these two tests.
No prospective studies that follow patients from initial diagnosis through to final health outcomes have been identified for any of the tests. Two prospective studies, MINDACT186 (MammaPrint) and TAILORx187 (OncotypeDX), are ongoing but not due to report for several years. The economic model therefore needed to combine data from different sources to model how the results from the new tests translated into final outcomes in the form of QALYs. This resulted in significant limitations – data used in the model were not always based on UK populations and were not always specifically based on the ER+, LN−, HER2− population of interest. Differences in the ages of study populations and the endocrine and chemotherapy regimens used in the studies compared with those in the model introduced further uncertainty. In addition to the uncertainty in the data derived from each study, there are uncertainties introduced by using separate studies to represent different elements in the model.
These tests will have an impact on the health of patients only if the management of the patients changes. Evidence on how the results of tests change treatment decisions in practice in the UK is limited. We conducted two analyses, one assuming that the test was given to all women and one assuming that the test was given only to women with a NPI score > 3.4, as a proxy for those at intermediate risk. This group reflects patients for whom the decision whether or not to given chemotherapy is most uncertain. The definition of the subgroup is relatively simplistic, because of data limitations, and may include women at the top end of the NPI distribution who are likely to receive chemotherapy despite the result of the test. It does, however, suggest that generally the cost-effectiveness may be improved by focusing the test in these women (although this was not the case in the exploratory analysis for Mammostrat).
Our analysis focused on women with ER+, LN−, HER2− early breast cancer as this population is supported by the most robust clinical evidence. Other populations, such as women with a small number of positive nodes, might also benefit from the tests, and results are likely to change if the population appraised is extended to women with ER− cancer or with positive nodes.
Evidence used in the model was generally identified from the systematic review of the literature on the clinical validity and utility of each of the tests. However, for the purpose of the economic analysis of patients with ER+, LN−, HER2− early breast cancer in a UK population the published data were sometimes not available in the right format for use within the economic model or the necessary data were not presented. Therefore, on occasion, once we had identified the most relevant data source from the review, we sought additional data to populate the economic model. For instance, data on the risk reclassification and risk of recurrence for patients treated with endocrine therapy in the UK for the main analysis were taken from a reanalysis of a study (TransATAC trial79) identified through the systematic review of the literature, as the published data were not specific to the population of interest (ER+, LN−, HER2− early breast cancer). Similarly, data on the impact of OncotypeDX on decision-making were taken from a reanalysis of the Holt et al. study78 (identified through the systematic review), to provide data specific to the population of interest, that is, ER+, LN−, HER2− early breast cancer. On occasion, data outside the systematic review were used, such as UK registry data to inform the current level of chemotherapy in the UK.
Despite the strength of the analysis, there were a number of significant limitations, mostly because of the gaps in the evidence base, the quality of the evidence base in some instances (e.g. issues with trial design) and the necessity to use data taken from non-UK populations when UK data were not available. There were particular concerns with the data used to reflect the benefit associated with chemotherapy by risk group for the new tests. Methodological flaws have been highlighted for the study on the benefits of chemotherapy by MammaPrint risk group. Limitations were identified with the data for OncotypeDX and Mammostrat in terms of how this evidence should be generalised to the UK population and potential biases in the evidence base. In addition, the evidence base on the proportion of patients who would receive chemotherapy after classification with the new tests had limitations or was lacking (in the case of IHC4 and Mammostrat). OncotypeDX was the only test for which there was evidence from a UK setting; however, there were concerns relating to this study, notably the small sample size and the possibility that patients were not representative of typical patients seen in clinical practice in England and Wales. Univariate sensitivity analyses indicated that the ICER was sensitive to the assumptions about the benefit reduction associated with chemotherapy and the proportion of patients who would be offered chemotherapy after categorisation with the new test. There are particular uncertainties relating to whether or not physicians would recommend chemotherapy to patients classified as intermediate risk with the new tests, as the evidence for the benefit of chemotherapy (reduction in the risk of recurrence) in these patients is less clear. Data from the TransATAC trial show that about 26% and 10% of ER+, LN−, HER− women were classified as intermediate risk with OncotypeDX and IHC4 respectively (predicted risk of recurrence between 10% and 20%). The ICER for Mammostrat was very sensitive to the assumption about the proportion of patients who would receive chemotherapy in the intermediate-risk group.
The ICER for OncotypeDX improved if more chemotherapy was given to this intermediate-risk group. In addition, in the evaluation of OncotypeDX and IHC4, the data on risk classification using OncotypeDX followed by further reclassification using IHC4 relied on a very small number of patients and therefore biases could have been introduced.
The exploratory analyses were subject to further uncertainties in the data. The exploratory analysis for Mammostrat used data from a subset of patients included in the Ring et al. study;125 however, the tests (CIC information has been removed). The exploratory analysis for MammaPrint used a wide range of assumptions and it was not possible for the EAG to present an ICER with confidence given the lack of robustness of the data that have been used to populate the economic model.
No direct comparison between tests was possible because of the differences in quality of the evidence. This therefore limits the conclusions that can be drawn from the analysis.
Further uncertainties were introduced into the analysis because of the wide range of assumptions needed in the EAG model. These include:
-
The use of UK cancer registry data to inform the proportion of patients receiving chemotherapy in the current practice arm. The registry data allowed us to capture decision-making based on real clinical practice, using current methods (a mix of the NPI, Adjuvant! Online and/or other prognostic tools). It should be noted that NPI was not used as a comparator in the economic model. NPI was used only to separate patients into two groups – those with a NPI score ≤ 3.4 and those with a NPI score > 3.4. This was to allow a subgroup analysis to be conducted and to allow the model to take into account, at least in part, the prognostic value of the treatment decision using clinicopathological parameters. This is a limitation given that it may be less discriminatory than current practice. The decision was taken to model current practice in this way as the evidence available for each test did not reflect the current level of chemotherapy given in the UK. Furthermore, data from only two registries were used (WMCIU and ECRIC), the results are generalisable only if the centres included in these two regions are considered to be representative of the centres across England and Wales.
-
The original RS groups were used for OncotypeDX to define patients who are at low, intermediate or high risk of distant recurrence. However, cut-offs have been modified in the ongoing TAILORx trial. The impact of these revised cut-offs cannot be assessed.
-
It was assumed that the IHC4 test was reproducible; however, there are issues relating to the reproducibility of the Ki-67 element of the test, which would need to be addressed in the UK before this test could be used by local laboratories in clinical practice.
-
The risk of distant recurrence was assumed to be constant over the first 10 years. It is likely that the risk increases over the first few years and then decreases with time. Likewise, we assumed that the risk reduced after 10 years and that no recurrence would occur after 15 years. This is a simplifying assumption.
-
The impact of locoregional recurrences was included in the model by applying a one-off cost and a decrement in utility to a proportion of patients developing distant recurrence. This is simplistic but this approach was used because of data limitations.
-
Long-term adverse events were modelled using simplifying assumptions. Only AML was included as a long-term adverse event after chemotherapy. The prevalence of CHF following chemotherapy with FEC may be higher but clinical opinion suggested that modelling CHF is complex as some patients remain asymptomatic or have a reversible disease and this would have added further uncertainty into the model.
-
A significant proportion of the total cost of chemotherapy (including treatment of adverse events and prevention of febrile neutropenia) is made up of the cost of treatment to prevent febrile neutropenia, which is more uncertain than the cost of the drug or the administration costs. We assumed that 25% of women receive G-CSF for the secondary prevention of neutropenia after chemotherapy in the UK based on clinical opinion.
-
In the comparator arm (current clinical practice), the probability of receiving chemotherapy was based on registry data. It was assumed that the probability of receiving chemotherapy was the same irrespective of the reclassification of patients with the new test into the low-, intermediate- or high-risk group. This is likely to be conservative.
In addition, there are potentially some limitations relating to the structure of the model. The model is static in that individuals are separated into risk groups and are assumed to be homogeneous with similar characteristics on average within these groups. For the main analysis, we separated individuals according to NPI, OncotypeDX and IHC4 to allow us to model IHC4 using direct evidence against OncotypeDX. Revised structural assumptions were examined in sensitivity analysis: removing IHC4 from the analysis and therefore separating patients according to NPI and OncotypeDX only or assuming no further reclassification using IHC4 (exclusion of IHC4) and modelling the entire cohort as a single group (no split by NPI). Results of the scenario analysis suggested that our base-case ICER for OncotypeDX was minimally affected by our choice of model structure to accommodate the evaluation of IHC4. Results were more affected when modelling the entire cohort as a single group (no split by NPI and IHC4) as the prognostic value of current decision-making using clinicopathological parameters is ignored (i.e. that patients with a low NPI score have a lower risk of recurrence but are also less likely to receive chemotherapy than patients with a NPI score > 3.4 under clinical practice). It is unclear how the ICER would be affected if a different NPI cut-off was selected or if patients were separated using Adjuvant! Online or other prognostic tools. Additional limitations are imposed as we assumed that tests categorised patients into risk groups and that the groups are homogeneous. However, we did not have access to individual patient-level data to explore the heterogeneity within the risk groups or to explore using different thresholds to define the risk groups.
Finally, the model structure for the exploratory analyses for MammaPrint and Mammostrat was driven by the OncotypeDX analysis, which imposed some constraints in the data that have been used. No economic assessment was provided for PAM50, NPI+, Randox BCA, BluePrint and BCI because of significant gaps in the data.
Chapter 4 Assessment of factors relevant to the NHS and other parties
Central processing
Most GEP tests require samples to be sent to central processing laboratories and therefore time delays will be imposed on patient management pathways. This may also be an issue for Mammostrat, which is likely to require central processing. IHC tests and GEP tests that can be processed locally will provide faster results than assays that need to be processed centrally. There are also legal issues relating to possible litigation costs if errors occur when tests are performed in other legal jurisdictions. The impact of sending large numbers of blocks for central processing in terms of pathology services, tissue tracking, pathologist and technical staff time, data input on receipt, etc. would need to be explored.
Impact on NHS services
Tests that require the use of fresh tissue raise particular service configuration issues. Fresh tissue collection is not routine in the NHS and so there will be additional costs that would be considerable at hospitals where the dissection facilities are already filled to capacity (which is likely to be a significant proportion of hospitals) and where explicit staffing for collection of fresh tissue is not in place. Discussion with local clinicians indicated that capital costs could be at least £75,000 per hospital if new dissection tables are required, which is likely to be the case in many hospitals. If routine fresh tissue sampling is not in place (only a few research centres currently have this working arrangement) then additional staff costs for biomedical scientists and histopathologists will be incurred. If a full fresh tissue service was required and needed to cover all theatre time then additional staff costs could be £20,000–50,000 per year (Simon Cross, July 2011, personal communication).
The impact on the chemotherapy service has not been considered. For instance, if additional women were prescribed chemotherapy as a result of these tests, NHS capacity (compared with current practice) may need to expand. Services are typically already running at full capacity and therefore this might mean delays in chemotherapy or the need for additional staff and beds.
Quality issues relating to immunohistochemistry tests
Lack of reproducibility of IHC assays will need to be taken into account when considering the use of IHC4 in local UK laboratories. Differences in IHC values can occur as a result of variability in several factors, including fixation, antigen retrieval, reagents and interpretation. A quality assurance programme will need to be considered, such as the UK National External Quality Assessment Service (NEQAS), given that these have in the past been shown to lead to marked improvements in between-laboratory agreement. Validation of the IHC4 score when carried out in a range of local laboratories is required. A guideline is currently in preparation (Professor Mitch Dowsett, July 2011, personal communication) to help standardise the measurement of Ki-67. Guidelines will need to be developed through NEQAS to ensure consistency among all participating UK laboratories.
Patient anxiety
There is evidence to suggest that OncotypeDX improves patient anxiety levels and decisional conflict. This was based on a small study of 89 assessable patients with ER+, LN− breast cancer. 76 Before and after OncotypeDX testing, medical oncologists stated their adjuvant treatment recommendation and confidence in it, and patients indicated their treatment choice. Changes in oncologist treatment recommendations were evaluated and patients completed measures for decisional conflict, anxiety and quality of life. Such improvements in patient anxiety levels are not taken into account within the economic analysis.
Classification of patients in the intermediate group
Some GEP and expanded IHC tests classify a proportion of patients into an intermediate-risk category. Evidence for the benefit of chemotherapy (in terms of reduction in the risk of recurrence) in these patients is less clear. It is also less clear whether or not physicians would recommend chemotherapy in addition to endocrine therapy for patients classified as intermediate risk with GEP or expanded IHC tests. This question is being addressed by the ongoing TAILORx187 study for OncotypeDX.
Categorical risk compared with continuous risk score
Some of the GEP and expanded IHC tests (MammaPrint, Mammostrat) classify patients into risk group only (categorical) and do not calculate a continuous risk score. This is likely to be less informative than a continuous risk score as it does not differentiate between patients who are at the lower end of the distribution and those who are at the upper end or those who are borderline.
Failure of the test/wrong results
Immunohistochemistry-based tests offer the advantage that biomarker expression is interpreted in situ, which allows the pathologist to ensure that the test is not confounded by expression of biomarkers in non-tumour tissue. Gene expression assays that homogenise the tissue and measure biomarkers that may be expressed in stroma run a greater risk of confounding the interpretation of biomarker expression levels.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
Thirty studies reporting data on analytical validity, clinical validity or clinical utility of the nine included GEP and expanded IHC tests for breast cancer were identified. Thirty-four studies (on OncotypeDX and MammaPrint) that had been included in previous systematic reviews were also retrieved and summarised.
OncotypeDX
The OncotypeDX evidence is the furthest along the validation pathway compared with other similar tests and the evidence base, in particular in relation to the clinical validity (prognostic ability) of the test, was consider to be reasonably sound. This review has identified recent studies supporting the clinical validity of the test. These are generally of moderate to high quality. Our findings indicate that there are no prospective studies reporting the impact of OncotypeDX on long-term outcomes such as OS. Four studies on the impact of OncotypeDX on decision-making indicate that the use of OncotypeDX leads to changes in decision-making for 31.5–38% of patients, but only one of these studies relates to the UK setting. Two studies on the predictive benefit of the test were identified: one was based on the same data used in the Paik et al. study48 and one included LN+ patients. The first evidence relating to improvements in quality of life and reductions in patient anxiety as a result of using the test has been reported, but this is based on small patient numbers and further evidence is required. Key gaps in the evidence remain and few of the studies were considered to be of high quality (n = 3). A number of studies in the current review were judged to provide moderate-quality (although retrospective) evidence for OncotypeDX (n = 9). Further direct evidence of clinical utility of OncotypeDX is still required. This will be addressed by the ongoing TAILORx trial.
MammaPrint
The evidence base for MammaPrint, in particular in relation to the prognostic ability of the test, is developing but is based on small sample sizes (n ≤ 272). The evidence for MammaPrint is less robust than that for OncotypeDX. No MammaPrint studies used RCT data, the sample sizes were small and heterogeneous patient populations were studied, making generalisation of the findings difficult. None of the studies used UK-based patients and the data were all based on cohort studies. The test appears to be prognostic at 5 years although the validity of the test to predict longer-term outcomes does not seem to have been established. Robust evidence of clinical utility is needed as it is not yet clear to what extent the use of the MammaPrint test will change the management of patients. It is also unclear to what extent MammaPrint risk groups are predictive of chemotherapy benefit or how the use of MammaPrint will improve patient outcomes through increases in disease-free and overall survival. The evidence for MammaPrint to date is mainly derived from premenopausal women and this evidence may not be generalisable to an older population given that younger women are likely to be at higher risk of recurrence and are more likely to be classified as having poor prognosis using MammaPrint.
PAM50
The PAM50 evidence base, in particular in relation to the prognostic ability of the test, is developing. The limitations of this evidence are based primarily on the fact that currently most of the evidence is unpublished (ARUP Laboratories, Salt Lake City, UT, USA).
Mammostrat
The evidence base for Mammostrat is developing and the evidence relating to the prognostic ability of the test is of reasonably high quality. These initial studies include a large sample size and one study provided external validation of the test in a UK population. A further study provides evidence relating to the benefit of chemotherapy by risk group, indicating that both low- and high-risk groups benefit whereas those in the moderate-risk group do not. Further evidence is needed to clarify these findings. Further evidence of analytical validity and clinical utility is also required. In particular, there was no published evidence on reclassification of risk groups compared with conventional risk classifiers and no evidence on the impact of the test on decision-making.
IHC4
The evidence base for IHC4 is currently limited to one study of clinical validity (prognostic ability). However, this evidence for clinical validity is relatively strong given that the test has been developed using a large cohort of patients and has been validated in an external cohort. This study allowed direct comparison with OncotypeDX. Further evidence is required on the analytical validity and clinical utility of IHC4.
BluePrint, Breast Cancer Index, Nottingham Prognostic Index plus and Randox Breast Cancer Array
The evidence base for the MammaPrint and BluePrint test combined (use of the BluePrint test for subtyping following the MammaPrint test), BCI, NPI+ and Randox BCA is limited to date and no firm conclusions can be drawn about these tests.
Cost-effectiveness
The four tests with the most well-developed clinical evidence base were considered within the economic evaluation. The EAG presented a primary analysis that compared OncotypeDX and IHC4 with current clinical practice in England and Wales. Based on the EAG model the incremental cost for adjuvant chemotherapy guided using OncotypeDX was estimated to be £29,503 per QALY gained compared with current clinical practice, assuming that the test was offered to all woman with ER+, LN−, HER2− early breast cancer under our base-case assumptions (assuming the test to be predictive of the benefit of chemotherapy). The IHC4 test was dominant compared with current clinical practice, providing more QALYs at a lower cost. The ICER for OncotypeDX increased substantially if the test was assumed to be prognostic only, that is, assuming the same relative reduction in the risk of recurrence from chemotherapy for all patients irrespective of the OncotypeDX RS classification. IHC4 remained dominant under this assumption. In incremental analysis, when the treatment decision using OncotypeDX was compared with that using IHC4, the ICER increased to £64,111 per QALY gained. In a second scenario, assuming that the test was offered only to women with a NPI score > 3.4, treatment guided using IHC4 remained dominant (i.e. provided more QALYs at a lower cost) compared with current clinical practice. The incremental cost for treatment guided using OncotypeDX was £9774 per QALY gained compared with current clinical practice and £31,125 per QALY gained compared with IHC4 (assuming that the test had predictive ability). A key area of uncertainty is whether tests are prognostic or also offer predictive ability.
It should be noted that the evidence base for IHC4 is less well developed and therefore the results of this analysis should be interpreted with consideration of the assumptions used in the evaluation. One-way sensitivity analysis indicated that the ICER was most sensitive to the assumptions about the benefit reduction associated with chemotherapy, the time horizon of the model, the starting age of the cohort, the risk of recurrence and who would receive chemotherapy depending on the result of the test. IHC4 remained dominant compared with current clinical practice (i.e. provided more QALYs at a lower cost) except when the cost of IHC4 was raised to £400 (producing an ICER of £1557 per QALY gained compared with current clinical practice).
The IHC4 test provides a continuous risk score and so it was necessary to assume risk categories for the purposes of analysis. The benefits of chemotherapy by risk group were based on indirect evidence using the OncotypeDX classification; no additional benefit was assumed for IHC4. In the absence of evidence it was assumed that the likelihood of receiving chemotherapy based on the IHC4 risk classification would be the same as for OncotypeDX RS group, that is, that physicians would interpret the results from OncotypeDX and IHC4 in the same way.
There are other issues that need to be considered for these new tests, such as technical and logistical issues. The implementation of the OncotypeDX test will have an impact on pathology services, and issues of tissues tracking and additional pathologist and technical staff time should be considered. There are also issues in terms of turnaround time and legal issues relating to possible litigation costs in case of errors when tests are performed in another legal jurisdiction. There is no morphological correlation and tissues included in the analysis can be heterogeneous and the results will be affected by tumour cellularity. The accuracy of HER2 measurements with OncotypeDX also needs clarification. The IHC4 test, despite the lack of evidence, is promising and can be incorporated more easily into clinical practice and should provide results more quickly. However, there are also issues of variability for this test (time to fixation, different fixatives), and the need for standardisation of the method of Ki-67 assessment and the cut-off to be used. Quality assurance issues would need to be addressed before the implementation of IHC4 in clinical practice.
For MammaPrint and Mammostrat there were significant gaps in the evidence available and data that have been used were not considered to be robust by the EAG. For this reason the analyses that were carried out evaluating the cost-effectiveness of MammaPrint and Mammostrat compared with current clinical practice in England and Wales were considered to be exploratory. Further clinical evidence will be needed to make the findings more robust.
Strengths and limitations of the assessment
Clinical effectiveness
The systematic review was conducted by an independent research team to a prespecified protocol using the latest evidence for nine GEP and expanded IHC tests. Extensive searches were undertaken to identify all literature relating to the clinical effectiveness of GEP and expanded IHC tests to guide the use of chemotherapy in breast cancer management.
The main limitation was the varied nature of the evidence base, relating to the study design for the evidence on clinical validity and clinical utility, making comparisons between tests difficult. None of the clinical studies had a prospective RCT design, although there are currently ongoing RCTs of both OncotypeDX and MammaPrint. Few studies, across all of the tests, used RCT data, with the majority of the evidence based on cohort designs. One of the most characteristic features of the studies across all tests was their heterogeneity. The studies varied considerably in their size, study design, patient populations and objectives. A large proportion of the studies were small and retrospective. Many studies used old archived tumour samples and some included the use of retrospective chart review to elicit treatment recommendations before and after testing. There was a lack of standardised decision-making tools both within and between studies and non-standardised methods of patient selection were used.
Studies relating to analytical validity, where available, appeared adequate, although for the majority of the tests the data are lacking and further studies are required. For MammaPrint and BluePrint, BCI, Randox BCA, Mammostrat, IHC4 and NPI+, no specific evidence for analytical validity has been reported, and for PAM50 the evidence for analytical validity is only in abstract form.
Economic evaluation
The economic assessment was conducted by an independent research team using the latest evidence for four GEP and expanded IHC tests. The EAG economic assessment has several strengths compared with previous evaluations. The evaluation used UK-specific data whenever possible, including for the baseline use of chemotherapy, risk of distant recurrence/recurrence and reclassification with the new test, so that its conclusions should be relevant to the UK setting. Notably, the EAG model used cancer registry data from ECRIC and WMCIU and data from the TransATAC trial, which are considered to provide the best reflection of current practice in the UK. The risk reclassification with OncotypeDX and IHC4 was taken from the TransATAC trial, and the risk of distant recurrence was taken from the same data source. The EAG economic assessment also considered an analysis of IHC4, which has not previously been undertaken, using direct evidence of the test compared with OncotypeDX. We also modelled women with a NPI score ≤ 3.4 and women with a NPI score > 3.4 separately to account for the prognostic value of the current treatment decision based on clinicopathological parameters and to allow a scenario to be conducted assuming that the test was offered to a subgroup of the population with a NPI score > 3.4. Extensive sensitivity analyses were undertaken to determine the impact of key parameter uncertainties on the cost-effectiveness ratio and a PSA was carried out to account for the joint uncertainty between parameters when appropriate.
Our analysis focuses on women with ER+, LN−, HER2− early breast cancer as this population is supported by the most robust clinical evidence. Other populations, such as women with a small number of positive nodes, might also benefit from the test, and results are likely to change if the population appraised is extended to women with ER– cancer or with positive nodes. We conducted two analyses, one assuming that the test was given to all women and one assuming that the test was given only to women with a NPI score > 3.4. This subgroup analysis was undertaken to explore the impact of targeting the tests at patients at intermediate risk. It is considered likely that the majority of women with a NPI score ≤ 3.4 would be considered low risk and would not receive chemotherapy under current practice or using the new tests and therefore the test would have a limited impact on the management of these women. Although this is relatively simplistic, and includes women at the top end of the NPI distribution who are likely to receive chemotherapy despite the result of the test, it does indicate that generally the cost-effectiveness may be improved by focusing the test in these women (although this was not the case in the exploratory analysis for Mammostrat).
Despite the strength of the analysis there were some significant limitations, mostly because of gaps in the evidence base, the quality of the studies within the evidence base in some instances and the necessity of using data from non-UK populations when UK data were not available. There were particular concerns over the data used to reflect the benefit associated with chemotherapy for the categorisation of patients with the new tests. In addition, the evidence base on the proportion of patients who would receive chemotherapy after classification with the new tests had limitations or was lacking (in the case of IHC4 and Mammostrat). There are particular uncertainties relating to whether or not physicians would recommend chemotherapy to patients classified as intermediate risk with the new tests, as the evidence for the benefit of chemotherapy (reduction in the risk of recurrence) in these patients is less clear.
The exploratory analyses for Mammostrat and MammaPrint were subject to further uncertainties in the data. The exploratory analysis for Mammostrat used data from a subset of patients included in the Ring et al. study;125 however, the tests (CIC information has been removed). The exploratory analysis for MammaPrint used a wide range of assumptions and it was not possible for the EAG to present an ICER with confidence given the perceived lack of robustness of the data that have been used to populate the economic model.We did not perform an incremental analysis because of these differences in the quality of evidence between tests. These differences are not adequately reflected in the PSA. Although this may be considered a limitation, we considered that including MammaPrint and Mammastrat within an incremental analysis could potentially be misleading given the gaps in the evidence base and significant issues relating to the quality of the data used to populate the economic models for these two tests.
Uncertainty was increased by the model structure used and the significant number of assumptions that had to be made in the EAG model. These are discussed in Chapter 3, Discussion of the independent economic model results. Extensive sensitivity analyses were carried out to determine the factors that impacted most on the ICER and to determine why the results of our model differed from those of other UK evaluations. The EAG model used UK data whenever possible and modelled patients with low and intermediate or high NPI separately. The results of the EAG analysis for OncotypeDX suggest that the ICER may be higher than that reported by the manufacturer's model. The difference in the ICER between the two models is attributable to the differences in model structure, the assumptions that have been made about the risk of recurrence and the different data sources used. The model developed by the manufacturer was built on data on changes in treatment decisions taken from the Holt et al. study. 77 However, there are issues with this study, particularly that patients might not be representative of patients seen in clinical practice in the UK. This study indicated that 36.4% of patients with ER+, LN−, HER2− breast cancer (based on EAG analysis) were offered chemotherapy under current clinical practice, which appears high. Cancer registry data (used in the EAG economic model) suggested that about 14.4% of women with ER+, LN−, HER2− breast cancer currently receive chemotherapy (5% among women with a NPI score ≤ 3.4 and 34% among women with a NPI score > 3.4). The EAG model also used data from the TransATAC trial, which is considered to provide a more robust source of evidence for the risk of distant recurrence for patients treated with endocrine therapy in the UK. This also provided risk reclassification data in a large sample of patients and a direct comparison against IHC4. A key area of uncertainty is whether tests are prognostic only or offer predictive ability, that is, whether or not they identify high-risk patients who will benefit more in relative terms from reductions in the risk of recurrence following chemotherapy than low-risk patients.
A structural assumption was also examined in sensitivity analysis, modelling the population as a single group instead of separating patients by NPI. This was shown to influence the ICER. The explanation is that modelling patients as one group ignores the prognostic value of current treatment decision-making using clinicopathological parameters and therefore will be more favourable to the new test. The base-case analysis separated patients into two subgroups by NPI; it is unclear how the ICER would be affected if patients were separated using Adjuvant! Online.
No modelling work was undertaken on tests providing outputs in terms of intrinsic breast cancer subtype rather than risk of recurrence. This will be an important area for future modelling work.
No economic assessment was provided for PAM50, NPI+, Randox BCA, BluePrint and BCI. This was because of significant gaps in the data and the uncertainty over how the tests would be used to inform clinical decision-making.
No direct comparison between tests was possible because of the differences in quality of the evidence. This therefore limits the conclusions that can be drawn from the analysis.
Uncertainties
The main uncertainties included:
-
The varied nature of the clinical evidence base, making comparisons between tests difficult.
-
The lack of prospective trials for the tests directly linking the use of the tests with final outcomes in terms of recurrence or survival. The economic model therefore needed to combine data from different sources to model how the results from the new tests translated into final outcomes in the form of QALYs, resulting in significant limitations – data used in the model were not always based on UK populations, were not always specifically taken from the ER+, LN−, HER2− population of interest and tended to be based on younger populations and populations treated with older, less effective, endocrine and chemotherapy regimens than are currently used.
-
The lack of data on the ability of the tests to classify patients in the relevant UK population.
-
The benefit of chemotherapy in terms of reduction in the risk of distant recurrence/recurrence in patients classified as low, intermediate or high risk according to the new tests. Although evidence was available for three of the tests (OncotypeDX, MammaPrint and Mammostrat), there were limitations with these studies and it is also unclear how this evidence translates specifically to the ER+, LN−, HER2− population in the UK. A key area of uncertainty is therefore whether tests are prognostic only or are predictive of the benefit of chemotherapy.
-
The lack of UK data about how the tests will impact on decision-making, that is, the proportion of patients who would receive chemotherapy according to the risk classification with the new test. One small UK study was identified for OncotypeDX but this had some limitations. Also, there is a lack of evidence on how this impact is likely to differ between tests providing a continuous risk score and tests providing only a categorical risk label.
-
Some GEP and expanded IHC tests classify a proportion of patients into an intermediate-risk category. Evidence for the benefit of chemotherapy (reduction in the risk of recurrence) in these patients is not clear. It is more uncertain whether or not physicians would recommend chemotherapy in addition to endocrine therapy for patients classified as being at intermediate risk with GEP or expanded IHC tests.
-
How the test will be used in UK clinical practice, notably the group of women who are most likely to be offered the new tests.
-
The potential acceptance and adoption of the tests by UK physicians.
Other relevant factors
Our analyses do not capture the cost implications of any service reconfiguration issues. The use of fresh tissue by some tests would require a change in practice with regard to the handling of tissues by pathology laboratories. This would have major service reconfiguration and cost issues. The impact on the chemotherapy service has also not been considered. For instance, if additional women were prescribed chemotherapy as a result of these tests, NHS capacity (compared with current practice) may need to expand. Services are typically already running at full capacity and therefore this might mean delays in chemotherapy or the need for additional staff and beds.
Most GEP tests require samples to be sent to central processing laboratories and therefore time delays will be imposed on patient management pathways. This may also be an issue for Mammostrat, which is likely to require central processing.
Currently, ER and HER2 testing is performed in most hospitals whereas PR testing is performed in a more limited number of hospitals. The potential introduction of the IHC4 test would require quality assurance issues to be addressed for the Ki-67 test. Because the IHC4 test is expected to be carried out locally, full validation would require evaluation of the IHC4 score when carried out in a range of local laboratories. A guideline is currently in preparation (Professor Mike Dowsett, July 2011, personal communication) to help standardise the measurement of Ki-67; however, reproducibility of the test would need to be confirmed and quality assurance programmes put in place.
Some of the tests (MammaPrint, Mammostrat) classify patients into risk group only (categorical) and do not calculate a continuous risk score. This is less informative than a continuous risk score as patients are classified into broad groups, for example low, intermediate or high. This does not allow differentiation between patients who are at the lower end or upper end of the distributions and those who are borderline; the impact that this additional knowledge would have on clinical decision-making is unclear.
Immunohistochemistry-based tests such as Mammostrat offer the advantage that biomarker expression is interpreted in situ, which allows the pathologist to ensure that the test is not confounded by expression of biomarkers in non-tumour tissue. Gene expression assays that require homogenisation of the tissue and measure biomarkers that may be expressed in stroma run a greater risk of confounding the interpretation of biomarker expression levels.
Chapter 6 Conclusions
Clinical effectiveness
Two of the tests (OncotypeDX and MammaPrint) have a reasonably large evidence base although there are some methodological weaknesses relating to this evidence in terms of the heterogeneity of patient cohorts and the retrospective study design. In addition, the MammaPrint evidence is typically based on observational data (small cohort studies) rather than randomised data, increasing the risk of selection bias. Further evidence is required on the clinical utility of all of the tests and specifically in UK-based populations.
The IHC4 and Mammostrat tests also demonstrate promise, presenting early evidence of the prognostic ability of the tests based on large UK-based validation cohorts. There is no predictive evidence for IHC4. PAM50 has an emerging evidence base; however, most of the evidence to date is in abstract form or unpublished. NPI+, Randox BCA, BluePrint and BCI have little evidence to date.
Cost-effectiveness
The economic analysis suggests that the use of the new tests may result in small increases in QALYs compared with currently used prognostic tools, but current limitations in the evidence base produce significant uncertainty in the results. A key area of uncertainty is whether tests are prognostic only or identify high-risk patients who will benefit from larger relative reductions in the risk of recurrence following chemotherapy than lower-risk patients. The economic analysis suggested that, of the four tests considered, treatment guided using IHC4 has the greatest potential to be cost-effective at a willingness-to-pay threshold of £20,000, given the low cost of the test. However, the evidence base to support IHC4 needs further research and the exact cost of using the test in the NHS needs to be investigated further. OncotypeDX has a more robust evidence base but further evidence on its impact on decision-making in the UK and the predictive ability of the test, specifically in an ER+, LN−, HER– population receiving current endocrine and chemotherapy regimens, is needed. For MammaPrint and Mammostrat there were significant gaps in the evidence available and the estimates of cost-effectiveness produced were not considered to be robust by the EAG.
Implications for service provision
The implications for service provision will vary by test. The impact of sending large numbers of blocks to central testing facilities in terms of pathology services, tissue tracking, pathologist and technical staff time, data input on receipt, etc. would need to be explored. The potential introduction of the IHC4 test would require quality assurance issues to be addressed for the Ki-67 element of the test. Currently, ER and HER2 testing is performed in most hospitals whereas PR testing is performed in a more limited number of hospitals. The use of tests requiring fresh tissue would be expected to have more major implications for service reconfiguration within pathology departments in England and Wales as currently only a minority of centres in the UK have the structure and staff to handle fresh tissue.
Gene expression profiling tests requiring samples to be sent to central processing laboratories will impose time delays of up to 2–3 weeks on patient management pathways. This may also be an issue for Mammostrat, which is likely to require central processing.
Suggested research priorities
Future research priorities common to all tests include:
-
Studies investigating the predictive ability of GEP and expanded IHC tests. Do tests identify patients classified at high risk who benefit more in terms of larger relative reductions in risk of recurrence following chemotherapy than those classified at lower risk?
-
Prospective studies investigating how the tests will be used in clinical practice within the current decision-making process in England and Wales. Further evidence is needed for all of the tests demonstrating how they will be used in the current decision-making process and, especially, how this will impact on patient management decisions.
-
There is a need for pilot studies demonstrating how tests could be introduced in the UK and used within the current decision-making process and highlighting issues that this would raise for the NHS.
-
Studies investigating the use of continuous compared with categorical risk scores in terms of clinicians' preferences and the potential differential impact on decision-making.
-
Studies providing evidence on how the subtyping information provided by some tests would impact on clinical decision-making in the UK.
-
The psychological impact of these tests needs more formal evaluation, in particular the impact of the test results on decision conflict, decision quality and regret for women considering chemotherapy. Quality of life data in women who have access to the tests or not would also be of value.
-
Further extension of the clinical evidence base to other populations who may benefit from the use of these tests, including patients with a small number of positive nodes.
Research specific to IHC4 includes:
-
Studies on the analytical validity of IHC4. There is a lack of data on the reproducibility of the IHC tests used to compose the IHC4 score, in particular the Ki-67 element. Studies need to investigate whether or not the incorporation of Ki-67 in clinical practice is feasible and whether or not results are reproducible.
-
Further studies to confirm the prognostic value of IHC4. There is also a need for studies directly comparing the use of IHC4 against current practice (NPI and Adjuvant! Online) in England and Wales.
Research specific to Mammostrat includes:
-
further evidence on analytical validity and risk reclassification.
Research specific to MammaPrint includes:
-
studies based on trial data – although the test is promising, most data are based on cohort studies or pooled analyses
-
research evidence to confirm the analytical validity and clinical validity of MammaPrint results based on FFPE rather than fresh samples.
Acknowledgements
The following team of clinical advisors gave substantial advice throughout the project and/or provided peer review of the report: Dr Matthew C Winter, Consultant in Medical Oncology, Weston Park Hospital, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield; Dr Simon S Cross, Reader and Honorary Consultant, Academic Unit of Pathology, Faculty of Medicine, Dentistry and Health, University of Sheffield, Sheffield; and Professor Jorge S Reis-Filho, Professor of Molecular Pathology, Breakthrough Breast Cancer Research Centre, Institute of Cancer Research, London.
The authors also wish to thank the Eastern Cancer Registration and Information Centre (ECRIC) and West Midland Cancer Intelligence Unit (WMCIU) for providing data and advice.
The authors wish to thank Gill Rooney and Andrea Shippam for their help in preparing and formatting the report, John Stevens for providing statistical advice, Alice Bessey and Hasan Basarir assisted in deriving input parameters for the economic model, and Mike Paulden, Dr Peter Hall, Dr Matt Stevenson and Dr Eva Kaltenthaler for peer-reviewing the report.
Contributions of authors
Sue Ward was the Assessment Group lead and provided advice for the conduct of the clinical effectiveness review and was involved in the development of the economic model.
Alison Scope undertook the clinical effectiveness review.
Rachid Rafia undertook the cost-effectiveness review and developed the cost-effectiveness model.
Abdullah Pandor and Sue Harnan helped undertake the clinical effectiveness review.
Pippa Evans performed the literature searches.
Lynda Wyld provided clinical advice, reviewed the report and was involved in the writing of the report.
About ScHARR
The School of Health and Related Research (ScHARR) is one of the twelve departments that comprise the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research, and the application of health economics and decision science to the development of health services and the improvement of public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the National Institute of Health Research (NIHR) Health Technology Assessment programme on behalf of a range of policy-makers, including NICE. ScHARR-TAG is part of a wider collaboration of a number of units from other regions including Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; Aberdeen Health Technology Assessment Group (Aberdeen HTA Group), University of Aberdeen; Liverpool Reviews & Implementation Group (LRiG), University of Liverpool; Peninsular Technology Assessment Group (PenTAG), University of Exeter; Centre for Reviews and Dissemination (CRD) and Centre for Health Economics (CHE), University of York; Warwick Evidence, University of Warwick; the BMJ Group; and Kleijnen Systematic Reviews.
Disclaimer text
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Dumitrescu RG, Cotarla I. Understanding breast cancer risk – where do we stand in 2005?. J Cell Mol Med 2005;9:208-21.
- Collaborative Group on Hormonal Factors in Breast Cancer . Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. Lancet 2001;358:1389-99.
- Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst 1993;85:1819-27.
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78.
- Lagerros YT, Hsieh SF, Hsieh CC. Physical activity in adolescence and young adulthood and breast cancer risk: a quantitative review. Eur J Cancer Prev 2004;13:5-12.
- Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 1983;52:1551-7.
- Clinical guideline CG80. London: NICE; 2009.
- Office for National Statistics . Survival Rates in England, Patients Diagnosed 2001–2006 Followed up to 2007 2007. www.statistics.gov.uk/StatBase/Product.asp?vlnk=14007 (accessed September 2011).
- 0–10 year relative survival for cases of breast cancer by stage diagnosed in the West Midlands 1985–1989 followed up to the end of 1999, as at January 2002. 2009.
- Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat 1992;22:207-19.
- Adjuvant! Online . Adjuvant! Online. Decision Making Tools for Health Care Professionals 2009. www.adjuvantonline.com/index.jsp (accessed September 2011).
- Cancer Research UK . Cancer Statistics: Breast Cancer UK 2009. http://publications.cancerresearchuk.org/WebRoot/crukstoredb/CRUK_PDFs/CSBREA09.pdf (accessed September 2011).
- National Cancer Intelligence Network . Cancer Incidence and Survival by Major Ethnic Group, England, 2002–2006 2009. http://publications.cancerresearchuk.org/WebRoot/crukstoredb/CRUK_PDFs/CR-UKNCIN_ETHNIC.pdf (accessed September 2011).
- National Cancer Intelligence Network . Cancer Incidence by Deprivation England, 1995–2004 n.d. www.ncin.org.uk/docs/081202-NCIN-Incidence_by_Deprivation_95_04.pdf (accessed September 2011).
- Welsh Cancer Intelligence and Surveillance Unit . Cancer Incidence, Mortality and Survival by Deprivation in Wales 2009. www.wales.nhs.uk/sites3/Documents/242/Deprivation%20 (accessed September 2011).
- Cancer Research UK . Cancer Survival and Deprivation Statistics 2005. http://info.cancerresearchuk.org/cancerstats/survival/survivaldeprivation (accessed September 2011).
- Office for National Statistics . Death Registrations in England and Wales, 2008, Causes 2009. www.statistics.gov.uk/pdfdir/dthreg0809.pdf (accessed September 2011).
- Blanks RG, Moss SM, McGahan CE, Quinn MJ, Babb PJ. Effect of NHS breast screening programme on mortality from breast cancer in England and Wales, 1990–8: comparison of observed with predicted mortality. BMJ 2000;321:665-9.
- TNM classification of malignant tumours. New York: Wiley-Liss; 1997.
- Singletary SE, Connolly JL. Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin 2006;56:37-4.
- Cancer Research UK . TNM Breast Cancer Staging 2009. www.cancerhelp.org.uk/help/default.asp?page=3316 (accessed September 2011).
- Agency for Healthcare Research and Quality . Surgery Choices for Women With Early-Stage Breast Cancer 2009. www.ahrq.gov/consumer/brcanchoice.htm (accessed September 2011).
- US National Cancer Institute . Surgery Choices for Women With Early-Stage Breast Cancer 2009. www.cancer.gov/cancertopics/breast-cancer-surgery-choices (accessed September 2011).
- American Cancer Society . How Is Breast Cancer Staged? 2009. www.cancer.org/docroot/CRI/content/CRI_2_4_3X_How_is_breast_cancer_staged_5.asp (accessed August 2011).
- Gene expression profiling and expanded immunohistochemistry tests to guide selection of chemotherapy regimes in breast cancer management. London: NICE; 2011.
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52.
- Oncotype DX. 2011.
- Guidance on cancer services. Improving outcomes in breast cancer. London: NICE; 2011.
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2011;365:1687-717.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD'S Guidance for Undertaking Reviews in Health Care. Centre for Reviews and Dissemination 2009. www.york.ac.uk/inst/crd/systematic_reviews_book.htm (accessed September 2011).
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gotzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339.
- NICE Diagnostic Assessment Programme interim methods statement (pilot). London: NICE; 2010.
- Marchionni L, Wilson R, Marinopoulos S, Wolff A, Parmigiani G, Bass E, et al. Impact of gene expression profiling tests on breast cancer outcomes. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- Smartt P. A comparison of gene expression profiling tests for breast cancer. Christchurch, New Zealand: Health Services Assessment Collaboration; 2010.
- Levine MN, Browman GP, Gent M, Roberts R, Goodyear M. When is a prognostic factor useful? A guide for the perplexed. J Clin Oncol 1991;9:348-56.
- Simon R. A checklist for evaluating reports of expression profiling for treatment selection. Clin Adv Hematol Oncol 2006;4:219-24.
- Altman D. Systematic reviews in health care: systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-8.
- Shea B, Hamel C, Wells G, Bouter L, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62:1013-20.
- Cronin M, Pho M, Dutta D, Stephans J, Shak S, Kiefer M, et al. Measurement of gene expression in archival paraffin-embedded tissues: development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assay. Am J Pathol 2004;164:35-42.
- Cronin M, Sangli C, Liu M, Pho M, Dutta D, Nguyen A, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem 2007;53:1084-91.
- Habel L, Shak S, Jacobs M, Capra A, Alexander C, Pho M, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 2006;8.
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817-26.
- Cobleigh M, Tabesh B, Bitterman P, Baker J, Cronin M, Liu M, et al. Tumor gene expression and prognosis in breast cancer patients with 10 or more positive lymph nodes. Clin Cancer Res 2005;11:8623-31.
- Esteva F, Sahin A, Cristofanilli M, Coombes K, Lee S, Baker J, et al. Prognostic role of a multigene reverse transcriptase-PCR assay in patients with node-negative breast cancer not receiving adjuvant systemic therapy. Clin Cancer Res 2005;11:3315-19.
- Bryant J. Data from the National Surgical Adjuvant Breast and Bowel Project. Toward a More Rational Selection of Tailored Adjuvant Therapy n.d.
- Hornberger J, Cosler L, Lyman G. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care 2005;11:313-24.
- Paik S, Shak S, Tang G. Risk Classification of Breast Cancer Patients by the Recurrence Score Assay: Comparison to Guidelines Based on Patient Age, Tumor Size, and Tumor Grade 2004.
- Oratz R, Paul D, Cohn A, Sedlacek S. Impact of a commercial reference laboratory test recurrence score on decision making in early-stage breast cancer. J Oncol Pract 2007;3:182-6.
- Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726-34.
- Goldstein L, Gray R, Badve S, Childs B, Yoshizawa C, Rowley S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol 2008;26:4063-71.
- Wolf I, Ben Baruch N, Shapira-Frommer R, Rizel S, Goldberg H, Yaal-Hahoshen N, et al. Association between standard clinical and pathologic characteristics and the 21-gene recurrence score in breast cancer patients: a population-based study. Cancer 2008;112:731-6.
- Asad J, Jacobson A, Estabrook A, Smith S, Boolbol S, Feldman S, et al. Does Oncotype DX recurrence score affect the management of patients with early-stage breast cancer?. Am J Surg 2008;196:527-9.
- Henry L, Stojadinovic A, Swain S, Prindiville S, Cordes R, Soballe PW. The influence of a gene expression profile on breast cancer decisions. J Surg Oncol 2009;99:319-23.
- Li L, Xu X, Zhao Y, Liu Z, Shen Z, Jin W, et al. Integrated gene expression profile predicts prognosis of breast cancer patients. Breast Cancer Res Treat 2009;113:231-7.
- Rayhanabad J, Difronzo L, Haigh P, Romero L. Changing paradigms in breast cancer management: introducing molecular genetics into the treatment algorithm. Am Surg 2008;74:887-90.
- Erb C, Fox KR, Patel M, Hook K, DeMichele A, Kaplan C, et al. Evaluation of Practice Patterns in the Treatment of Node-Negative, Hormone-Receptor Positive Breast Cancer Patients With the Use of the Oncotype DX Assay at the University of Pennsylvania 2007.
- Gold JM, Najita JS, Lester S, Richardson AL, Morganstern DE, Chen WY, et al. Personalizing treatment in early-stage breast cancer: The role of standard clinical factors and genomic information in adjuvant chemotherapy decision making. J Clin Oncol 2009;27.
- Lo SS, Norton J, Mumby PB, Smerage J, Kash J, Chew HK, et al. Prospective multicenter study of the impact of the 21-gene recurrence score (RS) assay on medical oncologist (MO) and patient (pt) adjuvant breast cancer (BC) treatment selection. J Clin Oncol 2007;25.
- Shak S, Palmer G, Baehner FL, Millward C, Watson D, Sledge GW. Molecular characterization of male breast cancer by standardized quantitative RT-PCR analysis: first large genomic study of 347 male breast cancers compared to 82,434 female breast cancers. J Clin Oncol 2009;27.
- Ach R, Floore A, Curry B, Lazar V, Glas A, Pover R, et al. Robust interlaboratory reproducibility of a gene expression signature measurement consistent with the needs of a new generation of diagnostic tools. BMC Genomics 2007;8.
- Buyse M, Loi S, van’t Veer L, Viale G, Delorenzi M, Glas AM, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 2006;98:1183-92.
- Glas A, Floore A, Delahaye L, Witteveen A, Pover R, Bakx N, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics 2006;7.
- van’t Veer LJ, Dai H, van de Vijver M, He Y, Hart A, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002;415:530-6.
- van de Vijver M, He Y, van’t Veer L, Dai H, Hart A, Voskuil D, et al. A gene-expression signature as a predictor of survival in breast cancer. N Eng J Med 2002;347:1999-200.
- Mook S, Schmidt M, Viale G, Pruneri G, Eekhout I, Floore A, et al. The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1–3 positive lymph nodes in an independent validation study. Breast Cancer Res Treat 2009;116:295-302.
- Wittner B, Sgroi D, Ryan P, Bruinsma T, Glas A, Male A, et al. Analysis of the MammaPrint breast cancer assay in a predominantly postmenopausal cohort. Clin Cancer Res 2008;14:2988-93.
- Bueno-de-Mesquita JM, van Harten W, Retel V, Van’t Veer LJ, van Dam F, Karsenberg K, et al. Use of 70-gene signature to predict prognosis of patients with node-negative breast cancer: a prospective community-based feasibility study (RASTER). Lancet Oncol 2007;8:1079-87.
- Bender RA, Knauer M, Rutgers EJ, Glas AM, de Snoo FA, Linn SC, et al. The 70-gene profile and chemotherapy benefit in 1,600 breast cancer patients. J Clin Oncol 2009;27.
- de Snoo FA, Knauer M, Bender RA, Stork-Sloots L, Rutgers EJ, Glas AM, et al. Outcome prediction by the 70-gene profile in the context of the National Comprehensive Cancer Network (NCCN) guidelines. J Clin Oncol 2009;27.
- Glas A, de Snoo F, Dreezen C, Roepman P, Bender R, Van’t Veer L. Use of MammaPrint to Identify Good Prognosis Group Within Clinically Indeterminate Risk Patients 2008. www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=58&abstractID=40205.
- Knauer M, Mook S, Retèl V, Kok M, Wesseling J, Glas AM, et al. Early determination of metastatic potential in breast cancer: The 70-gene signature in small tumors. J Clin Oncol 2009;27.
- Saghatchian M, Mook S, Pruneri G, Viale G, Glas A, Eekhout I, et al. 43LBA Combining genomic profiling (70-gene MammaPrint) with nodal status allows to classify patients with primary breast cancer and positive lymph nodes (1-9) into very distinct prognostic subgroups that could help tailor treatment strategies. Eur J Cancer Suppl 2009;7.
- Henry LR, Stojadinovic A, Swain SM, Prindiville S, Cordes R, Soballe PW. The influence of a gene expression profile on breast cancer decisions. J Surg Oncol 2009;99:319-23.
- Li LF, Xu XJ, Zhao Y, Liu ZB, Shen ZZ, Jin WR, et al. Integrated gene expression profile predicts prognosis of breast cancer patients. Breast Cancer Res Treat 2009;113:231-7.
- Mook S, Schmidt MK, Weigelt B, Kreike B, Eekhout I, van de Vijver MJ, et al. The 70-gene prognosis signature predicts early metastasis in breast cancer patients between 55 and 70 years of age. Ann Oncol 2010;21:717-22.
- Lo SS, Mumby PB, Norton J, Rychlik K, Smerage J, Kash J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol 2010;28:1671-6.
- Geffen DB, Amir N, Sion-Vardy N, Ariad S, Kachko L, Bayme M, et al. Stage I breast cancer in a regional oncology practice in Israel 2002–2006: clinicopathologic features, risk estimation and planned therapy of 328 consecutive patients. Breast 2009;18:316-21.
- Holt S, Bertelli G, Pudney D, Rolles M, Moe M, Brinkworth S, et al. Results from a prospective clinical study on the impact of Oncotype DX on adjuvant treatment decision and risk classification by Nottingham prognostic index (NPI) and recurrence score (RS). Breast 2011.
- Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010;28:1829-34.
- Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol 2010;28:1677-83.
- Tang G, Shak S, Paik S, Anderson SJ, Costantino JP, Geyer CE, et al. Comparison of the prognostic and predictive utilities of the 21-gene recurrence score assay and Adjuvant! for women with node-negative, ER-positive breast cancer: results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat 2011;127:133-42.
- Ademuyiwa FO, Miller A, O’Connor T, Edge SB, Thorat MA, Sledge GW, et al. The effects of Oncotype DX recurrence scores on chemotherapy utilization in a multi-institutional breast cancer cohort. Breast Cancer Res Treat 2011;126:797-802.
- Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55-6.
- Cuzick J, Dowsett M, Wale C, Salter J, Quinn E, Zabaglo L, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and Human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011;29:4273-8.
- Kelly CM, Krishnamurthy S, Bianchini G, Litton JK, Gonzalez-Angulo AM, Hortobagyi GN, et al. Utility of Oncotype DX risk estimates in clinically intermediate risk hormone receptor-positive, HER2-normal, grade II, lymph node-negative breast cancers. Cancer 2010;116:5161-7.
- Tang G, Constantino J, Crager M, Shak S, Wolmark N. [S4–9] Comparing the Prediction of Chemotherapy Benefit in Patients With Node-Negative, ER-Positive Breast Cancer Using the Recurrence Score and a New Measure That Integrates Clinical and Pathologic Factors With the Recurrence Score n.d.
- Toi M, Iwata H, Yamanaka T, Masuda N, Ohno S, Nakamura S, et al. Clinical significance of the 21-gene signature (Oncotype DX) in hormone receptor-positive early stage primary breast cancer in the Japanese population. Cancer 2010;116:3112-18.
- Yorozuya K, Takeuchi T, Yoshida M, Mouri Y, Kousaka J, Fujii K, et al. Evaluation of Oncotype DX recurrence score as a prognostic factor in Japanese women with estrogen receptor-positive, node-negative primary stage I or IIA breast cancer. J Cancer Res Clin Oncol 2010;136:939-44.
- Dundar Y, Dodd S, Dixkson R, Walley T, Haycox A, Williamson PR. Comparison of conference abstracts and presentations with full-text articles in the health technology assessment of rapidly evolving technologies. Health Technol Assess 2006;10.
- Dowsett M, Cuzick J, Wales C, Forbes J, Mallon L, Salter J, et al. Risk of distant recurrence using Oncotype DX in postmenopausal primary breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Cancer Res 2009;69:75S-76S.
- ClinicalTrials.gov . Hormone Therapy With or Without Combination Chemotherapy in Treating Women Who Have Undergone Surgery for Node-Negative Breast Cancer (the TAILORx Trial) 2011. http://clinicaltrials.gov/ct2/show/study/NCT00310180?term=oncotype+dx&rank=3 (accessed September 2011).
- Kunz G. Use of a genomic test (MammaPrint?) in daily clinical practice to assist in risk stratification of young breast cancer patients. Arch Gynecol Obstet 2011;283:597-602.
- Bueno-de-Mesquita JM, Linn SC, Keijzer R, Wesseling J, Nuyten DSA, van Krimpen C, et al. Validation of 70-gene prognosis signature in node-negative breast cancer. Breast Cancer Res Treat 2009;117:483-95.
- Gevensleben H, Gohring UJ, Buttner R, Heukamp LC, Kunz G, Dimpfl T, et al. Comparison of MammaPrint and TargetPrint results with clinical parameters in German patients with early stage breast cancer. Int J Mol Med 2010;26:837-43.
- Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol 2003;21:3357-65.
- Ishitobi M, Goranova TE, Komoike Y, Motomura K, Koyama H, Glas AM, et al. Clinical utility of the 70-gene MammaPrint profile in a Japanese population. Jpn J Clin Oncol 2010;40:508-12.
- Goldhirsch A, Ingle JN, Gelber R, Coates AS, Thuerlimann B. Thresholds for therapies: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer. Ann Oncol 2009;20:1319-29.
- Goldhirsch A, Glick JH, Gelber RD, Senn HJ. Meeting Highlights: international consensus panel on the treatment of primary breast cancer. J Natl Cancer Inst 1998;90:1601-9.
- Kok M, Koornstra R, Mook M, Hauptmann M, Fles R, Glas A, et al. The Breast. 2012.
- Na KY, Kim KS, Lee JE, Kim HJ, Yang JH, Ahn SH, et al. The 70-gene prognostic signature for Korean breast cancer patients. J Breast Cancer 2011;14:33-8.
- National Institutes of Health Consensus Development Panel . National Institutes of Health Consensus Development Conference statement: adjuvant therapy for breast cancer. J Natl Cancer Inst Monogr 2001;30:5-15.
- Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 2007;18.
- Goldhirsch A, Glick JH, Gelber R, Senn HJ. Meeting highlights: international consensus panel on the treatment of primary breast cancer. J Natl Cancer Inst 1998;90:1601-9.
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn HJ. Meeting highlights: International Consensus Panel on the treatment of primary breast cancer. Seventh International Conference on adjuvant therapy of primary breast cancer. J Clin Oncol 2001;19:381-727.
- Nationaal Borstkanker Overleg Nederland . Richtlijn Behandeling Van HetMammacarcinoom 2005 2005. www.oncoline.nl.
- Adjuvante Systemische Therapie voor het Operabel Mammacarcinoom. Richtlijn Behandeling van het Mammacarcinoom; 2005.
- Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 2001;19:980-91.
- Mook S, Knauer M, Bueno-de-Mesquita JM, Retel VP, Wesseling J, Linn SC, et al. Metastatic potential of T1 breast cancer can be predicted by the 70-gene MammaPrint signature. Ann Surg Oncol 2010;17:1406-13.
- Bueno-de-Mesquita JM, Sonke G, van der Vijer M, Linn S. Additional value and potential use of the 70-gene prognosis signature in node-negative breast cancer in daily clinical practice. Ann Oncol 2011;22:2012-30.
- Knauer M, Cardoso F, Wesseling J, Bedard PL, Linn SC, Rutgers EJT, et al. Identification of a low-risk subgroup of HER-2-positive breast cancer by the 70-gene prognosis signature. Br J Cancer 2010;103:1788-93.
- Knauer M, Mook S, Rutgers EJT, Bender RA, Hauptmann M, van de Vijver MJ, et al. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat 2010;120:655-61.
- Knauer M, Mook S, Linn S, van’t Veer L, Rutgers E. The 70-gene expression profile as MammaPrint predictive markers for a response to (neo) adjuvant chemotherapy in breast cancer. Onkologie 2010;33.
- Tice JA. The 70-Gene Signature (MammaPrint) As a Guide for the Management of Early Stage Breast Cancer 2010. www.ctaf.org/content/assessment/detail/1178 (accessed September 2011).
- Stork-Sloots L, Krijgsman O, Roepman P, de Snoo FA, Bender RA, Glas AM. Combining multigene profiling of molecular subtypes with the 70-gene profile for classification of breast cancer. J Clin Oncol 2009;27.
- Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160-7.
- Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 2010;16:5222-32.
- Cheang M, Voduc D, Tu D, Jiang S, Leung S, Chia SKL, et al. The responsiveness of intrinsic subtypes to adjuvant anthracyclines versus nonanthracyclines in NCIC.CTG MA.5 randomized trial. J Clin Oncol 2011;29.
- Ebbert M, Bastien R, Rowe L, Miller P, Anderson D, Boucher KM, et al. PAM50 breast cancer intrinsic classifier: clinical validation of a multianalyte laboratory developed test. J Clin Oncol 2011;29.
- Bernard PS, Davis C, Munarriz B. Determining agreement between immunohistochemistry and RT-qPCR for standard biomarkers in breast cancer: validation on GEICAM 9906 clinical trial. J Clin Oncol 2011;29.
- Martin M, Rodriguez-Lescure A, Stijleman I, Munarriz B, Ruiz-Borrego M, Davis C, et al. 2012.
- Chia SK, Bramwell VH, Tu D, Shepherd LE, Jiang S, Vickery T, et al. A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res 2012;18:4465-72.
- Jerevall PL, Ma X-J, Li H, Salunga R, Kesty NC, Erlander MG, et al. Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer 2011;104:1762-9.
- 2011.
- Bartlett JM, Thomas J, Ross DT, Seitz RS, Ring BZ, Beck RA, et al. Mammostrat as a tool to stratify breast cancer patients at risk of recurrence during endocrine therapy. Breast Cancer Res 2010;12.
- Ring B, Seitz R, Beck R, Shasteen W, Tarr S, Cheang M, et al. Novel prognostic immunohistochemical biomarker panel for estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3039-47.
- Ross D, Kim C-Y, Tang G, Bohn O, Beck R, Ring B, et al. Chemosensitivity and stratification by a five monoclonal antibody immunohistochemistry test in the NASBP B14 and B20 trials. Clin Cancer Res 2008;14:6602-9.
- Bartlett J. Biomarkers and patient selection for PI3K/Akt/mTOR targeted therapies: current status and future directions. Clin Breast Cancer 2010;10:S86-95.
- Nottingham Prognostics . NPI+ 2011.
- National Cancer Comprehensive Network (NCCN) . NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. V.2.2010 n.d. www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
- Drummond M, Jefferson T. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83.
- Eddy DM. Assessing Medical Technology. Washington, DC: National Academy Press; 1985.
- Chen E, Tong KB, Malin JL. Cost-effectiveness of 70-gene MammaPrint signature in node-negative breast cancer. Am J Manag Care 2010;16:E333-42.
- Retel VP, Joore MA, Knauer M, Linn SC, Hauptmann M, van Harten WH. Cost-effectiveness of the 70-gene signature versus St. Gallen guidelines and Adjuvant Online for early breast cancer. Eur J Cancer Suppl 2010;8:1382-91.
- Medical Advisory Secretariat . Ont Health Technol Assess Ser (Internet); December 2010. Gene Expression Profiling for Guiding Adjuvant Chemotherapy Decisions in Women With Early Breast Cancer: An Evidence-Based and Economic Analysis n.d. www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/gep_20101213.pdf (accessed September 2011).
- Tsoi DT, Inoue M, Kelly CM, Verma S, Pritchard KI, Tsoi DT, et al. Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist 2010;15:457-65.
- Paulden M, Franek J, Pham B, Krahn M. Gene Expression Profiling for Guiding Adjuvant Chemotherapy Decisions in Women With Early Breast Cancer: A Cost-Effectiveness Analysis of 1000 Strategies for the Provision of Adjuvant! Online, Oncotype DX and Adjuvant Chemotherapy 2011. http://theta.utoronto.ca/papers/theta_cadth2011_paulden-2.pdf (accessed September 2011).
- Hornberger J, Lyman GH, Chien R, Hornberger J, Lyman GH, Chien R. Economic implications of 21-gene recurrence score assay: US multicenter experience. J Clin Oncol 2010;28.
- Liaropoulos L, Galanis P, Kaitelidou D, Rovithis D, Siskou O, Theodorou P. Gross financial evaluation of the application of the 21-gene assay Oncotype DX for adjuvant chemotherapy decision-making in estrogen receptor-positive node negative breast cancer in Greece. Breast 2011;20.
- Lopes LF, Bacchi CE, Lopes LF, Bacchi CE. HER-2 status in gastrointestinal stromal tumor. Ann Diagn Pathol 2008;12:283-5.
- Tsoi DT, Inoue M, Kelly CM, Verma S, Pritchard K. Cost-effectiveness analysis of Oncotype DX-guided treatment in early breast cancer. J Clin Oncol 2009;27.
- Klang SH, Hammerman A, Liebermann N, Efrat N, Doberne J, Hornberger J. Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health 2010;13:381-7.
- Oncotype DX for prognosis of breast cancer recurrence. Lansdale, PA: HAYES, Inc.; 2010.
- Bacchi CE, Prisco F, Carvalho FM, Ojopi EB, Saad ED, Bacchi CE, et al. Potential economic impact of the 21-gene expression assay on the treatment of breast cancer in Brazil. Rev Assoc Med Bras 2010;56:186-91. www.oncologypractice.com/fileadmin/content_images/tor/issue_archives/2010/ONC_JAN_FEB_2010_lores.pdf.
- Jancin B. Oncotype DX can predict risk in node-positive patients. Oncol Rep 2010.
- Annabi BL. A MT1-MMP/NF-B signaling axis as a checkpoint controller of COX-2 expression in CD133(+) U87 glioblastoma cells. J Neuroinflammation 2008;6.
- Retel VP, Joore MA, Knauer M, Linn SC, Hauptmann M, Harten WH, et al. Cost-effectiveness of the 70-gene signature versus St Gallen guidelines and Adjuvant Online for early breast cancer. Eur J Cancer 2010;46:1382-91.
- Lidgren M, Jonsson B, Rehnberg C, Willking N, Bergh J. Cost-effectiveness of HER2 testing and 1-year adjuvant trastuzumab therapy for early breast cancer. Ann Oncol 2008;19:487-95.
- Lidgren M, Willking N, Jonsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res 2007;16:1073-81.
- National Cancer Institute . Surveillance, Epidemiology and End Results Cancer Statistics 2009. http://seer.cancer.gov/statistics/ (accessed 22 December 2009).
- Early Breast Cancer Trialists' Collaborative Group . Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet 1998;352:930-42.
- Earle CC, Chapman RH, Baker C, Bell CM, Stone PW, Sandberg EA, et al. Systematic overview of cost–utility assessments in oncology. J Clin Oncol 2000;18:3302-17.
- Hassett MJ, O’Malley AJ, Pakes JR, Newhouse JP, Earle CC. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst 2006;98:1108-17.
- Ara RM, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health 2011;14:539-45.
- Hillner BE, Smith TJ. Efficacy and cost effectiveness of adjuvant chemotherapy in women with node-negative breast cancer. N Engl J Med 1991;324:160-8.
- Partridge AH, Burstein HJ, Winer EP. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr 2001;30.
- Office for National Statistics . Interim Life Tables 2010. www.statistics.gov.uk/STATBASE/Product.asp?vlnk=14459 (accessed 6 April 2011).
- Thomas R, Williams M, Marshall C, . The total hospital and community UK costs of managing patients with relapsed breast cancer. Br J Cancer 2009;100:598-600.
- Milne R, Heaton-Brown KH, Hansen P, Thomas D, Harvey V, Cubitt A. Quality-of-life valuations of advanced breast cancer by New Zealand women. Pharmacoeconomics 2006;24:281-92.
- Peasgood T, Ward SE, Brazier J. Health-state utility values in breast cancer. Exp Rev Pharmacoecon Outcomes Res 2011;10:553-66.
- Conner-Spady BL, Cumming C, Nabholtz JM, Jacobs P, Stewart D. A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transplant 2005;36:251-9.
- British national formulary. London: BMA and RPS; 2011.
- Tappenden P, Jones R, Paisley S, Carroll C. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess 2007;11.
- Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L. Hormonal therapies for early breast cancer: systematic review and economic evaluation. Health Technol Assess 2007;11.
- NHS reference costs. London: Department of Health; 2010.
- Wolowacz SE, Cameron DA, Tate HC, Bagust A. Docetaxel in combination with doxorubicin and cyclophosphamide as adjuvant treatment for early node-positive breast cancer: a cost-effectiveness and cost–utility analysis. J Clin Oncol 2008;26:925-33.
- Blohmer JU, Kuhn T, Rezai M, Kummel S, Warm M, Eggemann H, et al. German multicentre decision impact study of Oncotype DX recurrence score (RS) on adjuvant treatment in estrogen receptor positive (ER plus) node negative (N0) and node positive (N plus) early breast cancer. Breast 2011;20.
- Demichell R. Time distribution of the recurrence risk for breast cancer patients undergoing mastectomy: further support about the concept of tumor dormancy. Breast Cancer Res Treat 1996;41:177-85.
- Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. Plos One 2010;5.
- Mammostrat. 2011.
- Rosman M, Mylander WC, Tafra L, Rosman M, Mylander WC, Tafra L. What is the value of the 21 gene recurrence score in HER2-negative patients?. J Clin Oncol 2010;28.
- PAM50. 2011.
- Breast Cancer Clinical Outcome Measures (BCCOM) Project . Analysis of the Management of Symptomatic Breast Cancers Diagnosed in 2002 2006. www.wmpho.org.uk/wmciu/documents/BCCOM_annual_report.pdf (accessed September 2011).
- Albanell J, Colomer R, Ruiz-Borrego M, Alba E, Saenz JAG, Martin M, et al. Prospective trans-GEICAM study of the impact of the 21-gene recurrence score assay and traditional clinico-pathological factors on clinical decision making in women with estrogen receptor-positive, HER2-negative, node-negative breast cancer. Breast 2011;20.
- Gregg X, Belnap T, Rowley B, Rees W. Experience with use of the Oncotype DX gene assay test in a multicenter community-based healthcare system. Cancer Res 2009;69.
- NHS reference costs 2010–2011. London: Department of Health; 2011.
- Campbell HE, Epstein D, Bloomfield D, Griffin S, Manca A, Yarnold J, et al. The cost-effectiveness of adjuvant chemotherapy for early breast cancer: a comparison of no chemotherapy and first, second and third generation regimens for patients with differing prognoses. Eur J Cancer 2011;47:2517-30.
- Fluorouracil, epirubicin and cyclophosphamide (FEC75) for early breast cancer. Avon, Somerset and Wiltshire NHS Cancer Services; 2011.
- Curtis L. Unit Costs of Health and Social Care 2010 2010. www.pssru.ac.uk/pdf/uc/uc2010/uc2010.pdf (accessed September 2011).
- Roche H, Fumoleau P, Spielmann M, Canon J, Delozier T, Serin D, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 trial. J Clin Oncol 2006;24:5664-71.
- Praga C, Bergh J, Bliss J, Bonneterre J, Cesana B, Coombes RC, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol 2005;23:4179-91.
- Edlin R, Connock M, Round J, Tubeuf S, Fry-Smith A, Hyde C, et al. Azacitidine for the treatment of myelodyplastic syndrome, chronic myelomonocytic leukaemia, and acute myeloid leukaemia. A single technology appraisal. West Midlands Health Technology Assessment Collaboration; 2009.
- Karnon J, Kerr GR, Jack W, Papo NL, Cameron DA. Health care costs for the treatment of breast cancer recurrent events: estimates from a UK-based patient-level analysis. Br J Cancer 2007;97:479-85.
- Younis T, Rayson D, Skedgel C. Adjuvant chemotherapy in breast cancer: is TC a cost-effective regimen compared to AC? 2008. www.abstracts2view.com/sabcs/viewp.php?nu=p6110 (accessed September 2011).
- Office for National Statistics . Cancer Registration Statistics England 2007 2010. www.statistics.gov.uk/StatBase/Product.asp?vlnk=7720&Pos=1&ColRank=1&Rank=272 (accessed September 2011).
- de Bock G, Putter H, Bonnema J, van der Hage J, Bartelink H, van de Velde C. The impact of loco-regional recurrences on metastatic progression in early-stage breast cancer: a multistate model. Breast Cancer Res Treat 2009;117:401-8.
- Bueno-de-Mesquita JM. Use of 70-gene signature to predict prognosis of patients with node-negative breast cancer: a prospective community-based feasibility study (RASTER). Lancet Oncol 2007;8:1079-87.
- MINDACT trial . Microarray In Node Negative Disease May Avoid ChemoTherapy. A Prospective, Randomised Study Comparing the 70-Gene Expression Signature With Common Clinical-Pathological Criteria in Selecting Patients for Adjuvant Chemotherapy in Node-Negative Breast Cancer 2011. http://www.eortc.be/services/unit/mindact/documents/MINDACT_trial_outline.pdf (accessed 11 September 2012).
- TAILORx trial . Hormone Therapy With or Without Combination Chemotherapy in Treating Women Who Have Undergone Surgery for Node-Negative Breast Cancer (the TAILORx Trial) 2011. http://clinicaltrials.gov/ct2/show/study/NCT00310180?term=oncotype+dx&rank=3 (accessed 11 September 2012).
- Early Breast Cancer Trialists' Collaborative Group . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717.
- Schor A, Carvalho F, Ojopi E, Mangabeira A, Saad E, Bacchi C. 21-gene recurrence score (RS) in 100 consecutive Brazilian Patients. Cancer Res 2009;69:842S-843S.
- Bighin C, Del Mastro L, Canavese G, Carli F, Taveggia P, Levaggi A, et al. Use in current clinical practice of 70-gene signature in early breast cancer. Int J Cancer 2010;127:2736-7.
- Espinosa E, Sanchez-Navarro I, Gamez-Pozo A, Marin AP, Hardisson D, Madero R, et al. Comparison of prognostic gene profiles using qRT-PCR in paraffin samples: a retrospective study in patients with early breast cancer. Plos One 2009;4.
- Ma XJ, Salunga R, Dahiya S, Wang W, Carney E, Durbecq V, et al. A five-gene molecular grade index and HOXB13:IL17BR are complementary prognostic factors in early stage breast cancer. Clin Cancer Res 2008;14:2601-8.
- European Organisation for Research and Treatment of Cancer . MINDACT (Microarray In Node Negative Disease May Avoid ChemoTherapy). A Prospective, Randomised Study Comparing the 70-Gene Expression Signature With Common Clinical-Pathological Criteria in Selecting Patients for Adjuvant Chemotherapy in Node-Negative Breast Cancer 2011. www.eortc.be/services/unit/mindact/documents/MINDACT_trial_outline.pdf (accessed September 2011).
Appendix 1 Search strategy
Appendix 2 Example of the quality assessment checklist applied to included studies
Example of the quality assessment checklist applied to included studies (PDF download)
Appendix 3 Assessment of multiple systematic reviews (AMSTAR): a measurement tool to assess the methodological quality of systematic reviews
Assessment of multiple systematic reviews (AMSTAR): a measurement tool to assess the methodological quality of systematic reviews (PDF download)
Appendix 4 Summary of evidence relating to OncotypeDX
Appendix 5 Summary of evidence relating to MammaPrint
Appendix 6 Studies identified by the electronic searches and other searches and excluded at the full paper stage for reasons not immediately apparent from the full text
Studies identified by the electronic searches and other searches and excluded at the full paper stage for reasons not immediately apparent from the full text (PDF download)
Appendix 7 OncotypeDX test: quality assessment and summary of results
OncotypeDX test: quality assessment and summary of results (PDF download)
Appendix 8 Ongoing trials
Appendix 9 MammaPrint test: quality assessment and summary of results
MammaPrint test: quality assessment and summary of results (PDF download)
Appendix 10 MammaPrint and BluePrint tests: quality assessment and summary of results
MammaPrint and BluePrint tests: quality assessment and summary of results (PDF download)
Appendix 11 PAM50 test: quality assessment and summary of results
PAM50 test: quality assessment and summary of results (PDF download)
Appendix 12 Breast Cancer Index: quality assessment and summary of results
Breast Cancer Index: quality assessment and summary of results (PDF download)
Appendix 13 Mammostrat test: quality assessment and summary of results
Mammostrat test: quality assessment and summary of results (PDF download)
Appendix 14 IHC4 test: quality assessment and summary of results
IHC4 test: quality assessment and summary of results (PDF download)
Appendix 15 NPI+ test: quality assessment and summary of results
NPI+ test: quality assessment and summary of results (PDF download)
Appendix 16 Tabulated summary of cost-effectiveness studies addressing the use of MammaPrint to guide the selection of adjuvant chemotherapy regimes in breast cancer management
Tabulated summary of cost-effectiveness studies addressing the use of MammaPrint to guide the selection of adjuvant chemotherapy regimes in breast cancer management (PDF download)
Appendix 17 Critical appraisal checklist of the economic model comparing MammaPrint with Adjuvant! Online
Critical appraisal checklist of the economic model comparing MammaPrint with Adjuvant! Online (PDF download)
Appendix 18 Tabulated summary of cost-effectiveness studies addressing the use of OncotypeDX to guide the selection of adjuvant chemotherapy regimes in breast cancer management
Tabulated summary of cost-effectiveness studies addressing the use of OncotypeDX to guide the selection of adjuvant chemotherapy regimes in breast cancer management (PDF download)
Appendix 19 Critical appraisal checklist of the economic model comparing OncotypeDX with Adjuvant! Online
Appendix 20 Final scope
Appendix 21 Protocol
Glossary
Technical terms and abbreviations are used throughout this report. The meaning is usually clear from the context, but a glossary is provided for the non-specialist reader.
- Adjuvant! Online
- A computer program designed to provide estimates of the benefits of adjuvant endocrine therapy and chemotherapy.
- Adjuvant therapy
- Adjuvant therapy is treatment that is given in addition to the primary (initial) treatment. It is designed to help reach the primary treatment goal (e.g. disease eradication). Adjuvant therapy for cancer usually refers to chemotherapy, hormonal therapy or radiotherapy when administered after primary surgery to help decrease the risk of the cancer recurring (coming back).
- Amplification
- In genetics, an increase in the frequency of replication of a deoxyribonucleic acid segment.
- Analytical validity
- The ability of the test to accurately and reliably measure the expression of messenger ribonucleic acid or proteins by breast cancer tumour cells.
- Axillary lymph nodes
- Located in the armpit area, they receive lymph fluid from the arm, breast and ipsilateral (same side) upper torso.
- Chemotherapy
- The use of medication(s) (drugs) that are toxic to cancer cells, given with the aim of killing the cells or preventing or slowing their growth.
- Clinical utility
- The utility of the test in relation to harm, impact on clinical decision-making, evidence of improvement in outcomes and health-care costs.
- Clinical validity
- The degree to which the test could accurately predict the risk of an outcome and discriminate patients with different outcomes.
- Cohort study
- A study that follows groups of people with and without the condition of interest over time to study outcomes.
- Endocrine therapy
- Treatment of cancer by removing and/or blocking the effects of hormones that stimulate the growth of cancer cells.
- External Assessment Group
- An independent group of researchers commissioned by the National Institute for Health and Care Excellence to review the evidence on a group of technologies. The External Assessment Group includes researchers who assess the quality of studies on the treatments, and health economists who look at whether or not the treatments are good value for money. The Diagnostics Assessment Committee bases its discussions on the diagnostics assessment report produced by the External Assessment Group.
- Gene expression
- Gene expression refers to the translation of the information encoded in a gene into an ribonucleic acid (RNA) transcript. Expressed transcripts include messenger RNAs, which are translated into proteins, as well as other types of RNA, such as transfer RNA, ribosomal RNA, micro RNA and non-coding RNA, that are not translated into protein. Gene expression is a highly specific process by which cells switch genes on and off in a timely manner, according to their state. The study of mRNA expression in a cell is an indirect way to study the protein counterpart.
- Gene expression profiling
- This term refers to any genomic techniques that measure the fraction of the genes that are expressed in a specific sample. It refers to techniques that allow the assessment of the expression of more than one gene at a time, such as microarray analysis after the use of real-time reverse transcription-polymerase chain reaction to amplify levels of genetic material to measureable levels.
- Grading
- Assessing the degree of aggressiveness of a malignant tumour based usually on the appearance of its cells under the microscope.
- Histology
- An examination of the cellular characteristics of a tissue using a microscope.
- Hazard ratio
- The hazard ratio (HR) is an estimate of the ratio of the hazard rate in two groups. It is broadly equivalent to relative risk and is useful when the risk is not constant over a given period as it uses information collected at different times. The term is typically used in the context of survival over time. If the HR = 0.5 then the relative risk of dying (or some other health event) in one group is half the risk of dying in the other group.
- Hormone receptor
- Protein molecules with a specific conformation that bind to hormones in the cell's environment and trigger hormone-dependent changes in the cell's behaviour.
- Human epidermal growth factor receptor
- A molecule on the surface of a cell that interacts with a specific growth factor and helps to control how rapidly the cells grow.
- IHC4
- The IHC4 test uses immunohistochemistry technology to assess the levels of four key proteins in a breast cancer sample – oestrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor type 2 (HER2) and Ki-67. The final algorithm for IHC4 calculates a risk score for distant recurrence based on these four proteins in addition to classical clinical and pathological variables (composite risk score IHC4 + clinical score termed IHC4 in our report).
- Immunohistochemistry
- A technique that uses antibodies to identify specific molecules in tissues, which are examined and scored by a pathologist using a microscope.
- Ki-67
- Antigen KI-67 is a molecule that can be easily detected in growing cells in order to gain an understanding of the rate at which the cells within a tumour are growing.
- Lymph nodes
- Small bean-shaped glands that are part of the lymphatic system. White blood cells in the lymph nodes attack bacteria and viruses as they pass through the node.
- Malignant
- Cancerous cells that can invade into nearby tissue and spread to other parts of the body.
- Mammography
- The process of taking a mammogram – a soft-tissue radiograph of the breast – which may be used to evaluate a lump or which may be used as a screening test in women with no signs or symptoms of breast cancer.
- Mastectomy
- Surgical removal of the breast.
- Metastases
- Deposits of cancer in the body at a site distant from the primary site.
- Nottingham Prognostic Index
- The Nottingham Prognostic Index (NPI) is a composite prognostic parameter involving both time-dependent factors and aspects of biological aggressiveness. The NPI score is based on a mix of grade, lymph node involvement and tumour size. To calculate the score, add numerical grade (1, 2 or 3), lymph node score (negative = 1, one to three nodes = 2, more than three nodes = 3) and 0.2 × tumour size in cm. Patients can be divided into three prognostic groups on the basis of the NPI score: a good prognostic group (NPI < 3.4), a moderate prognostic group (3.4 < NPI < 5.4) and a poor prognostic group (NPI > 5.4).
- Polymerase chain reaction
- The polymerase chain reaction (PCR) is a molecular biology technique for isolating and exponentially amplifying a deoxyribonucleic acid sequence of interest in vitro by enzymatic replication. This technique has been extensively modified to perform a wide array of tasks. It is a common tool in medical and biological research. PCR is now used to obtain the sequence of genes, diagnose hereditary diseases, identify genetic fingerprints (forensic medicine), detect infectious diseases and create transgenic organisms. Coupled to reverse transcription it is used to amplify ribonucleic acid molecules.
- Predictive molecular markers
- A molecule that is assessed to predict the likely response to a specific treatment, for example oestrogen receptor to predict the likely response to endocrine therapy.
- Prognosis
- A prediction of the likely outcome or course of a disease; the chance of recovery, recurrence or death.
- Prognostic factors
- Disease characteristics that are correlated with the course of the disease and which are used to predict the likely outcomes.
- Reverse transcription-polymerase chain reaction
- The reverse transcription-polymerase chain reaction (RT-PCR) is a variant of PCR, a laboratory technique commonly used in molecular biology to generate many copies of a deoxyribonucleic acid (DNA) sequence using a process termed ‘amplification’. In RT-PCR the ribonucleic acid strand of interest is first reverse transcribed into its DNA complement (complementary DNA, or cDNA) using the enzyme reverse transcriptase, and the resulting cDNA is amplified using traditional or real-time PCR.
- Staging
- Clinical description of the size and spread of a patient's tumour, allocated by internationally agreed categories.
- Systemic therapy/treatment
- Medicine, usually given by mouth or injection, to treat the whole body rather than targeting one specific area.
- Transcription
- In genetics, the process by which genetic information on a strand of deoxyribonucleic acid is used to synthesise a strand of complementary ribonucleic acid.
- Translation
- In genetics, the process by which a messenger ribonucleic acid molecule specifies the linear sequence of amino acids on a ribosome for protein synthesis.
List of abbreviations
- AC
- doxorubicin and cyclophosphamide
- AIC
- academic-in-confidence
- AML
- acute myeloid leukaemia
- AST
- adjuvant systemic treatment
- ATAC
- Arimidex, Tamoxifen, Alone or in Combination trial
- BCA
- Breast Cancer Array
- BCI
- Breast Cancer Index
- BCSD
- breast cancer-specific death
- BCSS
- breast cancer-specific survival
- BNF
- British National Formulary
- BSA
- body surface area
- CAF
- cyclophosphamide, doxorubicin and fluorouracil
- CBO
- Dutch Institute for Healthcare Improvement
- cDNA
- complementary deoxyribonucleic acid
- CEAC
- cost-effectiveness acceptability curve
- CHF
- congestive heart failure
- CG
- Clinical Guideline
- CI
- confidence interval
- CIC
- commercial-in-confidence
- CISH
- chromogenic in situ hybridisation
- CMF
- cyclophosphamide, methotrexate and 5-fluorouracil
- DARE
- Database of Abstracts of Reviews of Effects
- DCIS
- ductal carcinoma in situ
- DCS
- Decisional Conflict Scale
- DDFS
- distant disease-free survival
- DFS
- disease-free survival
- DMFS
- distant metastasis-free survival
- DNA
- deoxyribonucleic acid
- DRFI
- distant recurrence-free interval
- DRFS
- distant recurrence-free survival
- EAG
- External Assessment Group
- EBCTCG
- Early Breast Cancer Trialists' Collaborative Group
- ECRIC
- Eastern Cancer Registration and Information Centre
- EGFR
- epidermal growth factor receptor
- EORTC
- European Organisation for Research and Treatment in Cancer
- EQ-5D
- European Quality of Life-5 Dimensions
- ER
- oestrogen receptor (ER+ is ER positive and ER− is ER negative)
- FEC
- 5-fluorouracil, epirubicin and cyclophosphamide
- FEC-D
- 5-flurouracil, epirubicin and cyclophosphamide-docetaxel
- FEC-P
- 5-fluorouracil, epirubicin, cyclophosphamide and paclitaxel
- FFPE
- formalin-fixed paraffin-embedded
- G-CSF
- granulocyte colony-stimulating factor
- GEP
- gene expression profiling
- HER2
- human epidermal growth factor receptor type 2
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IHC
- immunohistochemistry
- LCIS
- lobular carcinoma in situ
- LN
- lymph node
- LR-x2
- likelihood ratio chi-square
- MF
- methotrexate and 5-fluorouracil
- MGI
- Molecular Grade Index
- MINDACT
- Microarray In Node-negative Disease may Avoid ChemoTherapy
- mRNA
- messenger ribonucleic acid
- NCCN
- National Comprehensive Cancer Network
- NICE
- National Institute for Health and Care Excellence
- NIH
- National Institutes of Health
- NPI
- Nottingham Prognostic Index
- NPI+
- Nottingham Prognostic Index Plus
- NPV
- negative predictive value
- NSABP
- National Surgical Adjuvant Breast and Bowel Project
- OHTA
- Ontario Health Technology Assessment
- OPTIMA
- Optimal Personalised Treatment of breast cancer using Multi-parameter Analysis
- OS
- overall survival
- PR
- progesterone receptor
- PPV
- positive predictive value
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RFI
- recurrence-free interval
- RFS
- recurrence-free survival
- RNA
- ribonucleic acid
- RS
- recurrence score
- RSPC
- integration of RS and clinicopathological factors
- RT-PCR
- reverse transcription-polymerase chain reaction
- SE
- standard error
- SEER
- Surveillance, Epidemiology and End Results
- STAI
- State-Trait Anxiety Inventory
- TAILORx
- Trial Assigning Individualized Options for Treatment
- TC
- docetaxel and cyclophosphamide
- TNM
- tumour, nodes, metastases classification system for cancer stage of the UICC
- TTDR
- time to distant recurrence
- WMCIU
- West Midland Cancer Intelligence Unit