Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/29/01. The contractual start date was in April 2011. The draft report began editorial review in May 2012 and was accepted for publication in October 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Carroll et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Varicose veins are enlarged, visibly lumpy knotted veins, usually in the legs. They are produced by reflux, principally in the great saphenous vein (GSV), sometimes also called great or long saphenous vein, but also in the short saphenous vein (SSV) of this lower limb. 1,2 Venous reflux is when blood flows backwards (in the direction from the heart to the foot) because the valve in the vein has failed. Clinically important reflux lasts for > 0.5–1.0 second. 3 Chronic venous disease (CVD) is the result of such venous incompetence. The clinical signs and symptoms of the disease are usually classified by clinicians using the clinical status, aetiology, anatomy, pathophysiology (CEAP) classification. 4–6 This ranges from C0 (no signs of venous disease) to C6 (active venous ulcer). C2 indicates varicose veins. The degree of severity of each class (clinical sign or symptom) on the scale (i.e. absent, mild, moderate and severe), as well as the pain experienced by the patient, can be measured according to the Venous Clinical Severity Score (VCSS). 7,8 The VCSS may be used to gauge clinical severity before and after intervention (i.e. to measure the efficacy of an intervention). 9 The tool is administered by clinicians but components are scored based on patient responses. 9
The presence of reflux is identified principally by duplex ultrasound. The criteria usually taken as indicating pathological reflux are the presence of venous flow reversal for > 0.5–1.0 second with proximal compression, the Valsalva manoeuvre, or distal compression and release. 3,10 Uncomplicated varicose veins can cause pain, discomfort, aching, throbbing, fatigue, heaviness, swelling and itching. 3,11 Complications can include superficial thrombophlebitis, external bleeding, lipodermatosclerosis, eczema and ulceration. 12 They can also lead to ‘skin changes, such as hyperpigmentation and induration, with eventual ulceration’. 13 CVD is also reported to have a substantial negative impact on health-related quality of life (HRQoL) if left untreated. 14
Varicose veins have been reported to affect approximately one-third of the adult population,15 with various UK studies reporting prevalence between 20% and 40% in adults. 1,13,16,17 Prevalence has been found to increase with age13,17 and may vary by sex with reported prevalence in women in the range of 25–32%, and rates in men ranging from 15% to 40%. 13,16 These figures are in part based on different random samples of approximately 1500 participants from the UK, so offer good external validity despite being a relatively small sample limited to the 18–70 years age group.
The NHS in England and Wales reported performing more than 33,000 surgical procedures in 2010–11 to treat varicose veins,18 although this figure may be affected by economic considerations. It has also been reported that treatment of the condition has required, in the past, approximately 2% of national health-care resources. 19 However, more recent examinations of Hospital Episode Statistics (HES) indicate that the number of procedures performed in the NHS is declining and there is an increasing number of day cases among those procedures that are being done. 15,20
Current service provision
Conventional surgery (ligation and stripping) remains the most frequently performed procedures in the NHS,15,18 although there are regional variations in the type of procedures performed, with some regions not performing procedures other than conventional stripping and ligation. 15 Published National Institute for Health and Care Excellence (NICE) guidance supports the use of both endovenous laser ablation (EVLA) and radiofrequency ablation (RFA) ‘provided that the normal arrangements are in place for consent, audit and clinical governance,’21,22 but supports the use of foam sclerotherapy (FS) and transilluminated-powered phlebectomy (TIPP) only under certain circumstances (i.e. ‘with special arrangements for consent and for audit or research’). 23,24 However, a number of relevant randomised controlled trials (RCTs) assessing each procedure has been published since these guidance documents were produced. More recent consensus statements from North America suggest that the minimally invasive techniques are considered to offer viable alternatives to standard stripping and ligation and sclerotherapy. 3,25,26
Traditional treatments for varicose veins involve surgical ligation and stripping, liquid sclerotherapy (LS) and conservative management of symptoms. However, the principal intervention, ligation and stripping, has been associated with a range of adverse effects such as wound infection, haematoma, lymph leaks, pain, scarring, nerve injury and deep-vein thrombosis (DVT), and long post-operative recovery. 27–32 The second principal intervention used, conventional LS, is considered faster but less effective than surgical stripping. 33
The principal outcomes associated with treatment for varicose veins are symptom relief and symptom severity, recurrence of varicosities, as well as the occurrence of new varicosities in the same limb, and retreatment. Reported recurrence rates vary widely depending on the nature of the surgical technique performed and method of assessment. For conventional stripping and ligation surgery, 2-year recurrence rates of up to 33% have been reported,34,35 rising to 41% for 5 years and up to 70% at over 10 years. 36,37 Surgical procedures for recurrence can therefore place considerable demand on the health services. Other outcomes of interest are HRQoL, patient treatment satisfaction and the occurrence of related post-operative complications.
New minimally invasive treatments offer alternative methods of ablating the vein. These treatments typically involve use of laser, radiofrequency probe or foam sclerosant. They are EVLA,12 RFA38 and FS. 12 TIPP does not treat the GSV but does remove varicosities. 39 These treatments are increasingly widely used and offer potential benefits such as faster recovery, reduced complications, fewer physical limitations and increased HRQoL. They are also reported to have reduced costs and lower recurrence rates compared with surgical stripping or LS, while being equally effective. 40–45
There has been no recent assessment by NICE of the effectiveness of these minimally invasive techniques relative to standard treatments such as stripping and ligation, LS and phlebectomy. A series of recently published reviews in peer-reviewed journals have evaluated either individual techniques or a combination of EVLA, RFA and FS. 46–51 All reviews have suggested that these treatments may offer viable alternatives to traditional techniques; there is a non-significant difference in favour of surgery in terms of recurrence, but a significant difference in favour of the minimally invasive techniques in terms of technical failure. Serious adverse events were found to be rare. However, only one of these reviews exclusively analysed RCT data (and only included five such trials),51 whereas the remainder pool data from multiple study designs. The follow-up of most included studies in these reviews was also short (< 1 year).
Many new relevant RCTs have been published in recent years, including head-to-head trials of the minimally invasive techniques. The objective of this report therefore is to undertake an up-to-date evaluation of the clinical effectiveness and cost-effectiveness of these minimally invasive techniques in comparison with conventional surgery for managing varicose veins.
The national reference cost data for 2009/1052 show a very slightly lower level of activity than shown in Table 1 for that period. A total of 35,885 varicose vein procedures were recorded as inpatient procedures, which also includes day cases. At 2011/12 costs this represents a total expenditure of £44M on the procedures alone, exclusive of outpatient and primary care. The costings of the different procedures are detailed in Chapter 4, Costs. They show FS to be the least expensive procedure at £634, and RFA to be the most expensive at £2635.
Description of technology under assessment
Endovenous laser ablation
Endovenous laser ablation involves insertion and activation of a laser fibre into the refluxing vein. Wavelengths are used to target deoxygenated haemoglobin and/or water, which result in heating and thrombosis or occlusion of the vein. 53 Patients with either GSV or SSV incompetence might receive this intervention.
Radiofrequency ablation
Radiofrequency ablation involves insertion of a catheter into the varicose vein. Electrodes at the end of the catheter omit high radiofrequency energy, which heats tissue at the site, causing collagen shrinkage, denudation of endothelium (the cells that line the blood vessels) and obliteration of the venous lumen (space inside the vein). 38 This includes techniques such as VNUS Closure,® VNUS ClosureFast® (VNUS Medical Technologies, Inc., San Jose, CA)54 and Olympus RFiTT® (Olympus Surgical Technologies Europe, Hamburg, Germany). 55 Patients with either GSV or SSV incompetence might receive this intervention.
Foam sclerotherapy
Foam sclerotherapy involves the mixing of air with liquid sclerosing solution to create foam. The foam is injected into the affected vein guided by ultrasound. 12 Patients with either GSV or SSV incompetence might receive this intervention.
Transilluminated phlebectomy
Transilluminated phlebectomy offers an alternative to multiple phlebectomies. It involves hydrodissection of the varicosities, transillumination facilitating direct visualisation of the varicosities, and varicosity removal using a powered endoscopic tissue dissector. 39 This includes techniques such as powered phlebectomy (TriVex™; InaVein, Lexington, MA). 56 Patients would only receive this intervention if there was no GSV incompetence.
Current usage in the NHS
Conventional surgery and injection sclerotherapy remain the most frequently performed procedures in the NHS,15 but the relative proportion of use of the various techniques is changing. Since 2006 all of the minimally invasive procedures have been assigned codes and their use has been recorded. 20 The numbers receiving surgery have declined, and injection sclerotherapy and the various minimally invasive techniques have increased greatly, with numbers for RFA and EVLA doubling from 2006–7 to 2007–8; EVLA is the most frequently preformed of these procedures. 20
| Procedures/codes | 2000–1 | 2001–2 | 2002–3 | 2003–4 | 2004–5 | 2005–6 | 2006–7 | 2007–8 | 2008–9 | 2009–10 | 2010–11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Day case | |||||||||||
| EVLA/L88 | NR | 2104 | 4005 | 6781 | 9914 | 10,369 | 9490 | |||||
| RFA/L88 | 454 | |||||||||||
| Conventional surgery/L84, L85 and L87 | 43,991 | 40,663 | 44,374 | 40,766 | 35,701 | 33,940 | 30,486 | 26,869 | 23,795 | 19,968 | 17,417 | 13,477 |
| aInjection sclerotherapy/L86 | 1336 | 1824 | 1536 | 1718 | 2195 | 3197 | 3824 | 5495 | 6235 | 6327 | 5707 | 5592 |
More recent data reinforce these trends, with traditional surgical techniques currently accounting for more than 50% of procedures (more than 17,000), EVLA and RFA approximately 10,000 episodes, and liquid or foam sclerotherapy approximately 5000 episodes. 18
Chapter 2 Definition of the decision problem
Decision problem
The assessment will address the question of what is the clinical effectiveness and cost-effectiveness of different minimally invasive methods of managing varicose veins compared with conventional surgery, liquid sclerotherapy (LS) or conservative management.
Intervention
New minimally invasive methods of managing varicose veins: EVLA, ultrasound-guided foam sclerotherapy (just FS), RFA and TIPP.
Population and relevant subgroups
Adults aged ≥ 16 years who are being treated specifically for varicose veins.
Relevant comparators
Although any comparator was considered, the reviews focused principally on surgical treatment. Other comparators included LS, not non-FS, etc., and conservative management. Head-to-head trials comparing the minimally invasive techniques were also included.
Surgical treatments
Traditional surgical treatment of the GSV typically involves ligation at the saphenofemoral junction followed by stripping to the knee. Treatment of the SSV typically involves ligation at the saphenopopliteal junction only. 12
Non-foam sclerotherapy
Sclerotherapy involves injecting the vein with a substance (usually liquid) that causes it to collapse and be absorbed into the surrounding tissue. 57
Conservative management
Conservative management of varicose veins includes use of compression stockings, elevating the legs and regular exercise.
Overall aims and objectives of assessment
-
To evaluate the clinical effectiveness and cost-effectiveness of new minimally invasive techniques compared with other techniques, including traditional surgical techniques, LS and conservative management, in the management of varicose veins.
-
To evaluate the safety of new minimally invasive techniques compared with surgical techniques, LS and conservative management, in the management of varicose veins.
-
To identify any key areas for further research.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
A systematic review of the literature and (network) meta-analysis (where appropriate) was undertaken to evaluate the clinical effectiveness of minimally invasive techniques to manage varicose veins. The review of the clinical evidence was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 58
Identification of studies
A comprehensive search was undertaken to identify systematically clinical effectiveness literature comparing different methods for the management of varicose veins. The search involved combining terms for the population (varicose veins) with terms for the interventions of interest (i.e. the minimally invasive techniques). This highly sensitive search strategy (i.e. not using terms for comparators, outcomes or study design) was possible because scoping searches retrieved relatively small and manageable numbers of citations. An example MEDLINE search strategy is reported in Appendix 1. The aim of the strategy was to identify all studies comparing the techniques of interest with each other, conventional surgery, LS or conservative management (no RCT filter was used). All searches were performed by an information specialist (AC) in July 2011.
The following electronic databases were searched from inception for published and unpublished research evidence:
-
MEDLINE (Ovid) 1946–
-
EMBASE (Ovid) 1980–
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO) 1982–
-
the Cochrane Library including the Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA) database and NHS Economic Evaluation Database (NHS EED) 1991–
-
Biological Abstracts (BIOSIS Previews) (via ISI Web of Science) 1969–
-
Science Citation Index (SCI) (via ISI Web of Science) 1900–
-
Social Science Citation Index (SSCI) (via ISI Web of Science) 1956–
-
Conference Proceedings Citation Index-Science – (via ISI Web of Science) 1990–
-
UK Clinical Trials Research Network
-
Current Controlled Trials
-
ClinicalTrials.gov.
All citations were imported into Reference Manager version 12 (Thomson ResearchSoft, San Francisco, CA, USA) and duplicates deleted. Titles and abstracts of all unique citations were then screened independently by two reviewers (JL, EEH) using the inclusion criteria outlined below after a test screen on a sample of citations. Disagreements or queries were resolved by consensus or with reference to a third team member (CC or JM) where necessary. The full papers of all potentially relevant citations were then retrieved so that an in-depth assessment concerning inclusion could be made. Reference tracking of all included studies and relevant reviews was also performed to identify additional, relevant studies not retrieved by the search of electronic databases. Clinical advisors were also contacted about relevant RCTs that might have been missed.
Inclusion and exclusion criteria
Study design
Randomised controlled trials only. These represented the optimal study design for assessing intervention effectiveness, and scoping of the review indicated the availability of a substantial number of published RCTs. No minimum duration of follow-up was applied.
Interventions
Endovenous laser ablation, RFA, FS and TIPP.
Population
Adults aged ≥ 16 years who are being treated specifically for varicose veins. Diagnostic criteria were recorded, where given. Trials were excluded if the focus was the management of a varicose vein complication rather than the treatment of varicose veins specifically (i.e. the trial evaluated the management of complications such as ulceration and the principal outcome related to the complication, e.g. leg ulcer healing, rather than the clinical outcomes defined in Outcomes).
Comparator
Any form of varicose veins management, including traditional surgical stripping/ligation, conservative treatment, such as the use of compression stockings, phlebectomy or an alternative minimally invasive technique, such as LS. Trials were excluded if they compared different forms of the same intervention (e.g. EVLA using 810 nm laser compared with EVLA using 980 nm laser). Such comparisons were excluded because these ‘within intervention’ studies were considered less pertinent to the decision problem than trials comparing one of the interventions with an alternative, especially the principal comparator of conventional surgery. The near absence of any statistically significant or clinical difference between different versions of the same intervention was supported by both the literature59,60 and clinical opinion.
Outcomes
The unit of assessment was a single system in a single leg, so the presence of reflux in non-treated veins in a treated limb was considered as a recurrence. The outcomes of the clinical effectiveness review included:
-
Failure of the procedure (i.e. the procedure was incomplete, or occlusion or obliteration was not achieved or was not sustained for more than 1 month).
-
Second or further procedures on account of such failure (given as ‘early reoperation’ in the protocol).
-
-
Technical recurrence (as distinct from initial episode) [i.e. the presence of reflux, recanalisation or new varicose veins in a treated limb as diagnosed by duplex ultrasound scanning (DUS)].
-
Second or further procedures on account of recurrence (given as ‘late reoperation’ in the protocol).
-
-
Symptomatic recurrence (i.e. patient presentation with symptoms of varicose veins, the diagnosis of which is validated by DUS).
-
Clinical symptoms, as measured by the VCSS (including pain, oedema, inflammation and hyperpigmentation).
-
Pain.
-
Time to return to work or normal activity. This was not in the original protocol but was included as a potentially relevant outcome, missed when scoping the report.
-
Post-operative complications (adverse events). These may include but were not limited to the following: nerve damage, skin burns, deep-venous thermal injury, DVT, pulmonary embolism (PE), transient ischaemic attacks, stroke, bleeding, infection, thrombophlebitis, headache, visual disturbance, skin staining, pain at injection site, back pain, anaphylaxis, lymph leak and cellulitis.
Settings
Secondary care.
Data abstraction strategy
Data abstraction was performed by one reviewer into a standardised data extraction form (see Appendix 2) and independently checked for accuracy by a second. Discrepancies were resolved by discussion between the two reviewers and, if agreement could not be reached, a third reviewer was consulted.
Critical appraisal strategy
The quality assessment of included RCTs was performed by one reviewer, using appropriate quality assessment criteria adapted from a published checklist for surgical interventions (see Appendix 3), and independently checked for accuracy by a second. Discrepancies were resolved by discussion between the two reviewers and, if agreement could not be reached, a third reviewer was consulted. Blinding of patients and outcome assessors were not retained as criteria because the techniques generally did not permit such blinding, so the risk of detection bias was often inherently high. Other amendments to the tool are described in Appendix 3. The 5% level of attrition specified in the original tool was retained, as this proportion has been reported to be the level least likely to affect outcomes adversely. 61
Methods of data synthesis
Technical recurrence, VCSS and pain score data were tabulated, and included studies were combined in a formal network meta-analysis. A network meta-analysis allows a comprehensive comparison of all interventions that are linked with respect to at least one common intervention without breaking the randomisation within studies. A network meta-analysis makes the same assumptions as standard pairwise meta-analyses. In particular, that there is consistency of direct and indirect evidence about treatment effects across the network.
The summary statistics that were analysed were the number of patients who had an event for technical recurrence, and the mean VCSS and mean pain score. In each case, the data were analysed using a random effects model (to allow for heterogeneity in treatment effects across studies) using Markov chain Monte Carlo simulation implemented in the WinBUGS (MRC Biostatistics Unit, Cambridge, UK) and OpenBUGS (Members of the OpenBUGS Project Management Group) software packages. The analysis was conducted using a Bayesian framework in order to quantify the joint distribution about uncertain parameters as required for the economic model.
For technical recurrence, the statistical model accounted for the variation in the duration of follow-up between studies using a complimentary log–log link function assuming that the underlying survivor functions follow Weibull distributions with separate shape and scale parameters to allow for the possibility of non-proportional hazards (see Appendix 4). Results of the network meta-analyses are reported in terms of the hazard ratios and 95% credible intervals (CrIs) relative to the baseline intervention (i.e. stripping) at 6 months, 1 year and 2 years. The posterior medians of the between-study standard deviations (SDs) for the shape and scale parameters together with their 95% CrIs are also presented.
For VCSS and pain scores, the statistical model used an identity link by assuming a normal distribution for the observed sample means (see Appendix 5). Results of the network meta-analyses are reported in terms of the mean difference (MD) and 95% CrIs relative to the baseline intervention (i.e. stripping). The posterior median of the between-study SD together with the 95% CrI was also presented.
Convergence of the models to their posterior distributions was assessed using the Gelman–Rubin convergence statistic. 62 Convergence occurred after 200,000 iterations for technical recurrence, after 10,000 iterations for VCSS and after 30,000 iterations for pain. There was some suggestion of high autocorrelation between successive iterations of the Markov chains; to compensate for this the Markov chains were thinned every 25 iterations for technical recurrence, every 10 iterations for VCSS and every 20 iterations for pain. Parameter estimates were estimated based on 20,000 iterations of the Markov chains for technical recurrence, 20,000 iterations for VCSS and 30,000 for pain.
The total residual deviance was used to assess formally whether or not the statistical model provided a reasonable representation of the sample data. The total residual deviance is the mean of the deviance under the current model minus the deviance for the saturated model, so that each data point should contribute about to the deviance. 63
To enable the estimation of intervention-specific survivor functions for the technical recurrence data as required for the economic model, a separate random effects meta-analysis was conducted on the stripping intervention arms. Absolute estimates of survivor functions (no technical recurrence function) and population mean times to technical recurrence were estimated for each intervention by projecting the estimates of treatment effect from the network meta-analysis onto the baseline survivor function.
The method of analysis for technical recurrence differed from what was described in the protocol (i.e. an analysis if binary data with results presented as odds ratios) to enable an adjustment for variation between studies in the duration of follow-up.
Results
Quantity and quality of research available
Characteristics of included studies
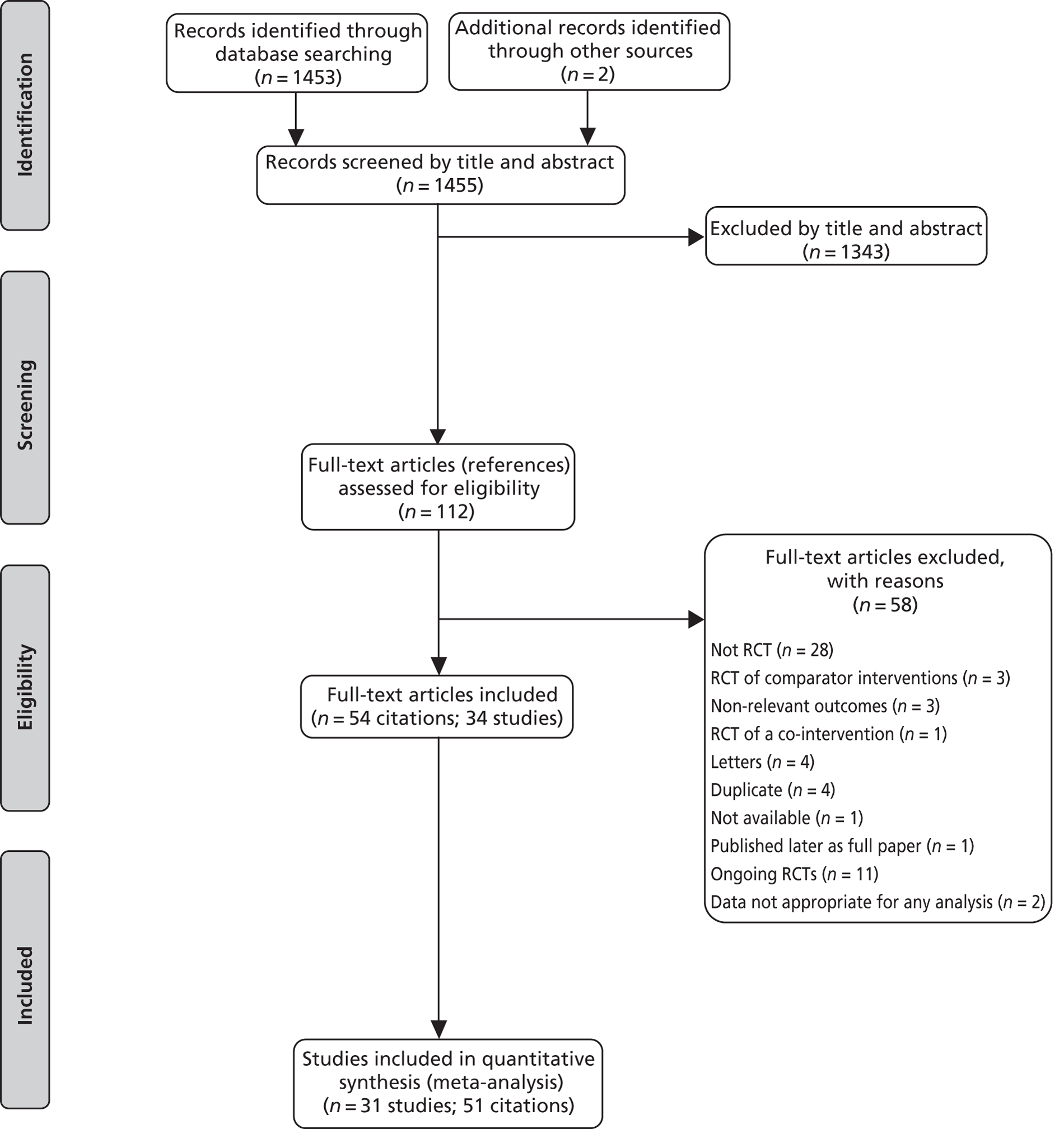
The searches identified 1453 unique citations. One hundred and twelve full papers were retrieved as being potentially relevant. Forty-five of these papers were excluded for at least one of the following reasons: not a RCT; correspondence relating to a relevant RCT; RCTs of comparator interventions only; RCT of co-interventions; duplicate publications' or not available (see Appendix 6). Eleven citations represented relevant ongoing trials64–74 and none of the available data were appropriate for analysis in one study. 75,76 Fifty-four citations, representing 34 different studies, therefore provided data for analysis (see the PRISMA flow chart, Figure 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart.
There was a total of 3873 participants across all trials in which randomised numbers in each arm were reported. The number of randomised participants in a single trial ranged from 2845 to 710. 77 Where diagnostic information was reported, all participants received a diagnosis using duplex scanning. Only three papers failed to report this information; all were abstracts. 78–80
The mean age of participants ranged from 3381 to 54 years. 82,83 There was a majority of female participants in every trial; the percentage of female participants ranged from 54%81 to 95%. 84 In all trials participants were required to have varicose veins diagnosed by duplex scanning and categorised according to the CEAP score. The vast majority of participants in any trial were C2 on the CEAP score (varicose veins). This was not the case in only 3 of the 34 trials, in which the majority were C3,83 C478 or C5. 85 The UK was the single most frequent location (12 trials39,79,83,86–94); the remainder were conducted in centres across 14 other countries (USA, Brazil, China, Egypt, Austria, Denmark, Finland, France, Germany, Ireland, the Netherlands, Spain, Sweden and Switzerland) (see Tables 2–9 for a summary of the included studies' characteristics).
Fourteen trials53,55,84,87,88,95–103 evaluated EVLA, but Rasmussen et al. 95 was a multiarm trial with more than one comparator. Eight compared the intervention with a form of conventional surgery (Table 2):84,86,87,95–100 six with RFA (Table 3);53,55,88,95,101,102 Disselhoff et al. 103 with cryostripping; and Rasmussen et al. 95 with FS (Table 4).
| Study and location | Unit of randomisation | n | Age (mean in years) | Sex (female/male) | CEAP score (C2–C6) | GA/LA |
|---|---|---|---|---|---|---|
| Carradice 2011,86,96 UK | Patient | I: 139 | I: 49 | I: 85/54 | I: C2 = 95; C3–6 = 43; unknown = 1 | I: NR |
| C: 137 | C: 49 | C: 90/47 | C: C2 = 96; C3–6 = 41 | C: NR | ||
| Total: 276 | ||||||
| Christenson 2010,97 Switzerland | Limb (mixed unilateral and bilateral) | I: 100 | I: 45 | I: 67/33 | I: C2 = 34; C3 = 58; C4 = 7; C5 = 1; C6 = 0 | I: NR |
| C: 100 | C: 47 | C: 71/29 | C: C2 = 26; C3 = 51; C4 = 18; C5 = 2; C6 = 3 | C: NR | ||
| Total: 200 | ||||||
| Darwood 2008,87 UK | Patient (unilateral and bilateral) | I1: 47 | I1: 42 | I1: 22/16 | I1: C2 = 37; C3 = 4; C4 = 2; C5 = 3; unknown = 1 | I: LA |
| I2: 33 | I2: 52 | I2: 16/11 | I2: C2 = 24; C3 = 6; C4 = 1; C5 = 0; unknown = 2 | |||
| C: 34 | C: 49 | C: 16/14 | C: C2 = 23; C3 = 9; C4 = 0; C5 = 1; unknown = 1 | C: GA | ||
| Total: 114 | ||||||
| De Medeiros 2005,84 Brazil | Limb (bilateral) | Total: 20 | 46 | 19/1 | I: C2 = 9; C3 = 2; C4 = 3; C5 = 4; C6 = 2 | I: LA |
| C: C2 = 11; C3 = 5; C4 = 3; C5 = 1; C6 = 0 | C: LA | |||||
| Kalteis 2008,98,104 Austria | Patient | I: 47 | I: 46 | I: 37/10 | I: C2 = 74%; C3 = 19%; C4 = 7% | I: NR |
| C: 48 | C: 47 | C: 34/14 | C: C2 = 69%; C3 = 27%; C4 = 4% | C: NR | ||
| Total: 95 | ||||||
| Rasmussen 2007,44 2009,105 2010,99 Denmark | Patient | I: 69 | I: 53 | I: 41/21 | I: C2 = 50; C3 = 3; C4 = 9 | I: LA |
| C: 68 | C: 54 | C: 43/16 | C: C2 = 51; C3 = 5; C4 = 3 | C: LA | ||
| Total: 137 | ||||||
| Rasmussen 2011,95 Denmark | Patient | I: 125 | I: 52 | I: 72% female | I: C2–3 = 95%; C4–6 = 5% | I: LA |
| C: 124 | C: 50 | C: 77% female | C: C2–3 = 97%; C4–6 = 3% | C: LA | ||
| Total: 249 | ||||||
| Pronk 2010,100,106 Netherlands | Patient | I: 62 | I: 49 | I: 46/16 | I: C2 = 29; C3 = 29; C4 = 4; C5 = 0 | I: LA |
| C: 68 | C: 50 | C: 53/15 | C: C2 = 26; C3 = 36; C4 = 5; C5 = 1 | C: LA | ||
| Total: 130 |
| Study and location | Unit of randomisation | n | Age (mean in years) | Sex (female/male) | CEAP score (C2–C6) | GA/LA |
|---|---|---|---|---|---|---|
| Nordon 2011,88 UK | Patient | I: 80 | I: 47 | I: 54/26 | I: C2 = 68; C3 = 3; C4–6 = 9 | I: GA |
| C: 79 | C: 47 | C: 45/34 | C: C2 = 68; C3 = 2; C4–6 = 9 | C: GA | ||
| Total: 159 | ||||||
| Gale 2009,107 2010,53,108 USA | Patient | I: 48 | I: 49 | I: 36/12 | NR | I: LA |
| C: 46 | C: 46 | C: 29/17 | NR | C: LA | ||
| Total: 94 | ||||||
| Goode 2008,92,109,110 2010,55 UK | Bilateral: limb Unilateral: patient |
I: Bilateral 17; unilateral 22 | I: Bilateral 47; unilateral: 48 | I: Bilateral 15/2; unilateral 15/7 | C2 only | I: GA |
| C: Bilateral 17; unilateral 23 | C: Bilateral 47; unilateral 45 | C: Bilateral 15/2; unilateral:15/8 | C2 only | C: GA | ||
| Total: 79 | ||||||
| Rasmussen 2011,95 Lawaetz 2010,111 Denmark | Patient | I: 125 | I: 52 | I: 72% female | I: C2–3 = 95%; C4–6 = 5% | I: LA |
| C: 125 | C: 51 | C: 70% female | C: C2–3 = 92%; C4–6 = 8% | C: LA | ||
| Total: 250 | ||||||
| Morrison 2005,101 USA | Bilateral | Total: 50 | NR | NR | NR | NR |
| Shepherd 2009,112 2010,93,102 UK | Limb | I: 64 | I: 48 | I: 42/22 | I: C1–2 = 26; C3–4 = 36; C5–6 = 2 | I: GA |
| C: 67 | C: 49 | C: 47/20 | C: C1–2 = 23; C3–4 = 39; C5–6 = 5 | C: GA | ||
| Total: 131 |
| Study and location | Intervention | Control | Unit of randomisation | n | Age (mean in years) | Sex (female/male) | CEAP score (C2–C6) | GA/LA |
|---|---|---|---|---|---|---|---|---|
| Disselhoff 2008,103 2011,113 Netherlands | EVLA | Cryostripping | Patient | I: 60 | I: 46 | I: 41/19 | C2 only | LA or GA |
| C: 60 | C: 49 | C: 42/18 | ||||||
| Total: 120 | ||||||||
| Rasmussen 2011,95 Lawaetz 2010,111 Denmark | EVLA | FS | Patient | I: 125 | I: 52 | I: 72% female | I: C2–3 = 95%; C4–6 = 5% | I: LA |
| C: 124 | C: 51 | C: 76% female | C: C2–3 = 96; C4–6 = 4 | C: LA | ||||
| Total: 249 |
Thirteen trials42,45,53,55,80,81,83,88,89,95,101,102,114 evaluated RFA, one of which, Rasmussen et al. ,95 had more than one comparator. Six trials compared the intervention with a form of conventional surgery (Table 5),42,45,81,83,89,95 six with EVLA (see Table 3),53,55,88,95,101,102 Stötter et al. 114 with invagination cryostripping and Rasmussen et al. 95 with FS, and Lin et al. 80 compared RFA in combination with TriVex with conventional surgery and TriVex (Table 6).
| Study and location | Unit of randomisation | n | Age (mean in years) | Sex (female/male) | CEAP score (C2–C6) | GA/LA |
|---|---|---|---|---|---|---|
| ElKaffas 2011,81 Egypt | Patient | I: 90 | I: 33 | I: 48/42 | I: C2 = 51; C3 = 27; C4 = 9; C5 = 3 | I: LA |
| C: 90 | C: 35 | C: 45/45 | C: C2 = 45; C3 = 27; C4 = 12; C5 = 6 | C: GA | ||
| Total: 180 | ||||||
| Hinchliffe 2006,83 UK | Bilateral: limb | Total: 16 | 54 | 12/4 | C2 = 1; C3 = 14; C4 = 1 | I: GA |
| C: GA | ||||||
| Rasmussen 2011,95 Lawaetz 2010,111 Denmark | Patient | I: 125 | I: 51 | I: 76% female | I: C2–3 = 92%; C4–6 = 8% | I: LA |
| C: 124 | C: 50 | C: 77% female | C: C2–3 = 97%; C4–6 = 3% | C: LA | ||
| Total: 249 | ||||||
| Rautio 2002,45 Perala 2005,43 Finland | Patient | I: 15 | I: 33 | I: 14/1 | I: NR | I: NR |
| C: 13 | C: 38 | C: 12/1 | C: NR | C: NR | ||
| Total: 28 | ||||||
| Lurie 2003,42 2005,115 Multicentre | Patient | I: 44a | I: 49 | I: 32/13 | I: C2 = 36; C3 = 4; C4 = 4 | LA or GA |
| C: 36 | C: 47 | C: 26/10 | C: C2 = 28; C3 = 4; C4 = 4 | |||
| Total: 81 | ||||||
| Subramonia 2010,89 Balakrishnan 2008,116 UK | Patient | I: 47 | I: 47 | I: 34/13 | I: C2 = 37; C3 = 9; C4–6 = 1 | I: GA |
| C: 41 | C: 45 | C: 27/14 | C: C2 = 33; C3 = 7; C4–6 = 1 | C: GA | ||
| Total: 88 |
| Study and location | Intervention | Control | Unit of randomisation | n | Age (mean in years) | Sex (male/female) | CEAP score | GA/LA |
|---|---|---|---|---|---|---|---|---|
| Lin 2009,80 China | RFA and TriVex | Stripping and TriVex | Patient | I: 75 | I: NR | I: NR | I: NR | I: NR |
| C: 75 | C: NR | C: NR | C: NR | C: NR | ||||
| Total: 150 | ||||||||
| Stötter 2005,114 Germany | RFA | Invagination or cryostripping | Patient | I: 20 | I: 43 | I: 14/6 | I: NR | I: NR |
| C1: 20 | C1: 53 | C1: 15/5 | C1: NR | C1: NR | ||||
| C2: 20 | C2: 42 | C2: 14/6 | C2: NR | C2: NR | ||||
| Total: 60 | ||||||||
| Rasmussen 2011,95 Laaetz 2010,111 Denmark | RFA | FS | Patient | I: 125 | I: 51 | I: 70% female | I: C2–3 = 92%; C4–6 = 8% | I: LA |
| C: 124 | C: 51 | C: 76% female | C: C2–3 = 96%; C4–6 = 4% | C: LA | ||||
| Total: 249 |
Thirteen trials77–79,85,90,91,95,117–122 evaluated FS, one of which, Rasmussen et al. ,95 had more than one comparator. Ten trials77–79,85,90,91,95,117,118,122 compared the intervention with a form of conventional surgery (Table 7), one of which, Liamis et al. ,79 used ‘reverse’ FS. Three trials119–121 compared the intervention with LS (Table 8), while Rasmussen et al. 95 compared it with both EVLA and RFA.
| Study and location | Intervention | Control | Unit of randomisation | n | Age (mean in years) | Sex (female/male) | CEAP score | GA/LA |
|---|---|---|---|---|---|---|---|---|
| Abela 2008,91 UK | ‘Reverse’ FS | Stripping | NR | I: 30 | I: 45 | I: 22/8 | All C2 and C3 | I: NR |
| C1: 30 | C1: 46 | C1: 17/13 | C1: NR | |||||
| C2: 30 | C2: 47 | C2: 15/15 | C2: NR | |||||
| Total: 30 | ||||||||
| Bountouroglou 2004,123 2006,90 UK | FS and SFJ ligation | Stripping | Patient | I: 30 | I: 42 | I: 14/16 | I: C2 = 11; C3 = 8; C4 = 7; C5 = 3; C6 = 1 | I: NR |
| C: 30 | C: 43 | C: 18/12 | C: C2 = 8; C3 = 14; C4 = 6; C5 = 1; C6 = 1 | C: NR | ||||
| Total: 58 | ||||||||
| Figuereido 2010,85 Brazil | FS | Stripping | NR | I: 27 | I: 53 | I: 23/4 | C5 only | I: NR |
| C: 29 | C: 49 | C: 23/6 | C: NR | |||||
| Total: 56 | ||||||||
| Jia 2010,78 China | FS and SFJ ligation | Stripping | Patient | I: NR | I: NR | I: NR | Median C4 in both groups | I: NR |
| C: NR | C: NR | C: NR | C: NR | |||||
| Total: 60 | ||||||||
| Kalodiki 2008,94 2011,117 UK | FS and SFJ ligation | Stripping | NR | I: 43 | I: 49 | I: 32/11 | All C2–C6, similar between groups | I: LA |
| C: 39 | C: 47 | C: 23/16 | C: GA | |||||
| Total: 82 | ||||||||
| Liamis 2005,79 UK | ‘Reverse’ FS and SFJ ligation | Stripping | Limb | I: 30 | I: NR | I: NR | I: NR | I: NR |
| C: 30 | C: NR | C: NR | C: NR | C: NR | ||||
| Total: 60 | ||||||||
| Rasmussen 2011,95 Lawaetz 2010,111 Denmark | FS | Stripping | Patient | I: 124 | I: 51 | I: 76% female | I: C2–3 = 96%; C4–6 = 4% | I: LA |
| C: 124 | C: 50 | C: 77% female | C: C2–3 = 97%; C4–6 = 3% | C: LA | ||||
| Total: 248 | ||||||||
| Shadid 2010,122 Netherlands | FS | Stripping | Patient | I: 227 | I: NR | I: NR | I: NR | I: NR |
| C: 198 | C: NR | C: NR | C: NR | C: NR | ||||
| Total: 425 | ||||||||
| Wright 2006,77 Europe | FS | Stripping | Patient | I: 178 | I: 50 | I: 112/66 | I: C2 = 144; C3 = 14; C4 = 20 | I: NR |
| C: 94 | C: 49 | C: 60/34 | C: C2 = 73; C3 = 11; C4 = 10 | C: NR | ||||
| Total: 272 |
| Study and location | Intervention | Control | Unit of randomisation | n | Age (mean in years) | Sex (female/male) | CEAP score (C2–C6) | GA/LA |
|---|---|---|---|---|---|---|---|---|
| Alos 2006,119 Spain | FS | LS | Limb/region – bilateral | Total: 75 | 59 | Total: 69/6 | Total: NR | Total: NR |
| Hamel-Desnos 2006,120 Ouvry 2008,82 France | FS | LS | Patient | I: 45 | I: NR | I: NR | I: NR | I: NR |
| C: 43 | C: NR | C: NR | C: NR | C: NR | ||||
| Total: 88 | ||||||||
| Rabe 2008,121 Germany | FS | LS | Patient | I: 54 | I: 51 | I: 35/19 | I: C2 = 26; C3 = 15; C4 = 12; C5 = 1 | I: NR |
| C: 52 | C: 50 | C: 39/13 | C: C2 = 26; C3 = 14; C4 = 8; C5 = 4 | C: NR | ||||
| Total: 106 |
Finally, Aremu et al. 124 compared TIPP with conventional surgery and Chetter et al. 39 compared TIPP with standard multistab incision phlebectomy (MSIP) (Table 9).
| Study and location | Intervention | Control | Unit of randomisation | n | Age (mean in years) | Sex (male/female) | CEAP score | GA/LA |
|---|---|---|---|---|---|---|---|---|
| Aremu 2004,124 Ireland | TIPP | Stripping | Limb (unilateral and bilateral) | I: NR | I: NR | I: NR | I: C2 = 53%; C3 = 47% | I: NR |
| C: NR | C: NR | C: NR | C: C2 = 61%; C3 = 39% | C: NR | ||||
| Total: 141 | ||||||||
| Chetter 2005,39 UK | TIPP | MSIP | Patient | I: 29 | I: 48 | I: 19/10 | I: C2 = 27; C5 = 2 | I: NR |
| C: 33 | C: 50 | C: 24/9 | C: C2 = 29; C4 = 4; | C: NR | ||||
| Total: 62 |
No trial included conservative management as a comparator. Only three trials119–121 included LS as a comparator (with FS) (see Table 8). The principal common comparator was therefore surgery (i.e. ligation and stripping).
Quality of included studies
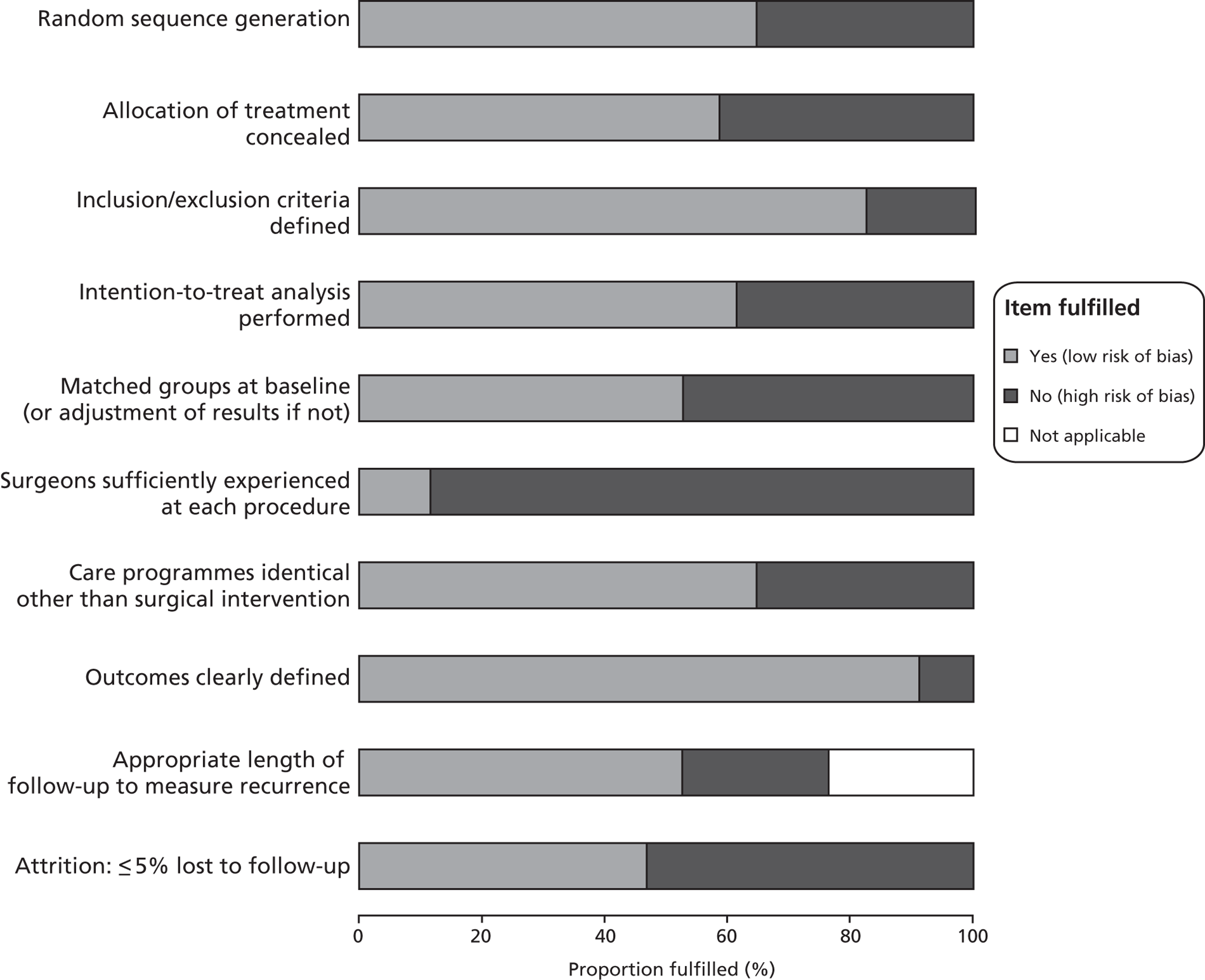
The methodological quality assessment of each included study is summarised in Figure 2 and Table 10. Only the findings affecting the most pertinent criteria are outlined here. Twelve of the 34 included trials failed adequately to report methods of either randomisation or allocation concealment, or reported inadequate methods. 77–80,83,98,101,114,117,120–122 Two further trials reported adequate randomisation but inadequate allocation concealment. 97,100 These studies, as reported, therefore had a high risk of selection bias. Thirteen studies either clearly failed to conduct an intention-to-treat analysis or left it unclear whether or not they had done so, and so were categorised as not clearly conducting such an analysis;42,53,55,77,79,80,88,91,102,114,117,122,124 six of these also failed to report adequate methods of randomisation and allocation concealment,77,79,80,114,117,122 all but one of which were abstracts only. 114
FIGURE 2.
Methodological quality graph: review authors' judgements about each methodological quality item as percentages across all included.
| Study | Randomisation? | Allocation concealment? | Inclusion/exclusion criteria defined? | Intention-to-treat analysis performed? | Matched groups at baseline (or adjustment of results if not)? | Surgeons sufficiently experienced at each procedure? | Care programmes identical other than surgical intervention? | Outcomes clearly defined? | Appropriate length of follow-up to measure recurrence? | Attrition: ≤5% lost to follow-up? |
|---|---|---|---|---|---|---|---|---|---|---|
| Abela 200891 | Y | Y | Y | N | Y | N | Y | Y | NA | N |
| Alos 2006119 | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Aremu 2004124 | Y | Y | N | N | N | N | Y | Y | Y | N |
| Bountouroglou 200690 | Y | Y | Y | Y | Y | N | Y | Y | N | N |
| Carradice 201186,96 | Y | Y | Y | Y | Y | N | Y | Y | Y | N |
| Chetter 200639 | Y | Y | Y | Y | N | N | Y | Y | NA | Y |
| Christenson 201097 | Y | N | Y | Y | N | N | Y | Y | Y | Y |
| Darwood 200887 | Y | Y | Y | Y | N | N | N | Y | Y | N |
| De Medeiros 200584 | Y | Y | Y | Y | Y | N | Y | Y | NA | Y |
| Disselhoff 2008,103 2011113 | Y | Y | Y | Y | Y | Y | Y | Y | Y | N |
| ElKaffas 201181 | Y | Y | Y | Y | N | N | N | Y | Y | N |
| Figueiredo 200985 | Y | Y | Y | Y | N | N | N | Y | NA | N |
| Gale 201053 | Y | Y | Y | N | Y | N | Y | Y | Y | N |
| Goode 201055 | Y | Y | Y | N | N | N | Y | Y | N | Y |
| Hamel-Desnos 2003120 | N | N | Y | Y | Yb | N | Y | Y | Y | N* |
| Hinchliffe 200683 | N | N | Y | Y | N | N | Y | Y | NA | Y |
| aJia 201078 | N | N | N | Y | N | N | N | N | N | N |
| aKalodiki 2008117 | N | N | N | N | Y | N | N | Y | Y | N |
| Kalteis 200898 | N | N | Y | Y | Y | N | Y | Y | NA | Y |
| aLiamis 200579 | N | N | N | N | N | N | N | N | N | N |
| aLin 200980 | N | N | N | N | N | N | N | N | N | N |
| Lurie 200342 | Y | Y | Y | N | Y | N | Y | Y | Yb | Nb |
| aMorrison 2005101 | N | N | N | Y | N | N | N | Y | Y | Y |
| aNordon 201188 | Y | Y | Y | N | Y | N | Y | Y | N | Y |
| Perala 2005,43 Rautio 200245 | Y | Y | Y | Y | N | N | Y | Y | NA | Y |
| Pronk 2010100 | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Rabe 2007121 | N | N | Y | Y | Y | N | Y | Y | N | Y |
| Rasmussen 2007, 201044,99 | Y | Y | Y | Y | Y | Y? | Y | Y | Y* | Y |
| Rasmussen 201195 | Y | Y | Y | Y | Y | N | N | Y | Y | N |
| aShadid 2010122 | N | N | Y | N | N | N | N | Y | Y | Y |
| Shepherd 2010102 | Y | Y | Y | N | Y | Y | Y | Y | NA | N |
| Stötter 2006114 | N | N | Y | N | N | N | N | Y | Y | Y |
| Subramonia 201089 | Y | Y | Y | Y | Y | N | Y | Y | N | Y |
| Wright 200677 | N | N | Y | N | N | N | N | Y | Y | N |
Two further key criteria were assessed that had the greatest potential to confound the results of this report: the non-comparability of groups at baseline and non-identical care programmes post intervention. Sixteen of the trials reported statistically significant or substantial differences between within-study groups in terms of potential confounders (such as age or CEAP score),39,45,55,77–81,83,85,87,97,101,114,122,124 while 12 of 34 trials either reported non-identical care programmes post intervention or did not make it clear what occurred. 77–81,85,87,95,101,114,117,122 An assessment of reporting bias was deemed not to be possible because no published protocol could be identified for any included trial. This criterion is therefore omitted from the tables.
Studies published as abstracts only would obviously potentially be considered to be at higher risk of bias than studies published as full papers because details of study conduct were often not reported in that abbreviated format. Twelve studies reported in full papers were at risk of two or more of these forms of bias,55,77,81,83,85,87,97,98,114,120,121,124 as were six studies reported as abstracts only. 78–80,101,117,122 The remaining 16 trials were at risk of either one or none of these forms of bias. 39,42,45,53,84,86,88–91,95,99,100,102,113,119
The majority of the trials used in the network meta-analyses (e.g. those reporting technical recurrence data for EVLA vs. stripping or EVLA vs. RFA, etc.) were at risk of either selection or attrition bias due to inadequate randomisation, allocation concealment or intention-to-treat analysis. Given that these types of bias potentially affected almost all studies included in the analysis, no sensitivity analysis was performed based on these quality appraisal criteria.
Assessment of effectiveness
First, a narrative synthesis is provided for all outcomes: failure of procedure, recurrence, VCSS, pain score, return to work or normal activity, and adverse events. Second, Quantitative analysis will present a quantitative synthesis using a formal network meta-analysis approach for those outcomes for which sufficient and appropriate data were available: technical recurrence, VCSS and pain score. Randomisation and analysis in the primary studies was described as being by patient or by limb when patients were unilateral; when patients were bilateral, randomisation was by limb. Data were therefore all per limb or per patient; no data were per procedure (i.e. there were no data where multiple procedures were conducted on the same limb).
Narrative synthesis
Failure of procedure
Twelve trials42,44,55,81,88,89,90,95,97,98,119,112 reported data on the failure of the initial procedure (i.e. the intervention failed to strip the vein successfully or the vein was not occluded or obliterated, or open segments remained) within the first few days post operation up to 1 month (see Table 11). The assumption is that the procedure did not work at all. Following this definition, 5/467 (1%) procedures were reported to be failures for EVLA; 16/431 (4%) for RFA; 21/295 (7%) for FS; and 20/681 (3%) for stripping and ligation (see relevant numerators and denominators reported in Table 11). Only Rasmussen et al. ,95 ElKaffas et al. 81 and Bountouroglou et al. 90 specifically reported the performance and type of retreatment for these failures: 9/174 additional foam sessions for FS failures; 6/90 stripping and ligation sessions for RFA failures; and 2/118 surgery failures received LS sessions.
Recurrence
The principal outcome reported by trials was technical recurrence, as defined above. Thirty of the 34 trials42,45,53,55,77,78,81,83–89,90,92–103,114,117–122 reported this outcome; only Abela et al. ,91 Chetter et al. ,39 Liamis et al. 79 and Lin et al. 80 did not do so. Seventeen trials43,44,77,78,83,84,87,88,90,96,98,115,119,121,122,124,125 reported data on technical recurrence within 6 months of treatment (see Table 12). Twenty-one trials43,53,55,77,81,82,85,87,95–97,99–101,115,117,120,122,124–126 reported technical recurrence data for follow-ups of ≥ 6 months (see Table 13). Data from all follow-up time points in the following trial arms were included in the analysis (see Assessment of effectiveness): EVLA, RFA, FS and conventional surgery (ligation and stripping). The following data were excluded from the analysis: LS,119–121 cryostripping103,113 and TIPP. 39,124 These data were excluded either because the population receiving the treatment was different from the other populations (e.g. for TIPP) or because the comparator was not deemed relevant to this analysis.
However, only Christenson et al. ,97 De Medeiros and Luccas,84 Lurie et al. ,42 Perala et al. 43 and Pronk et al. 100 reported numbers of follow-up patients experiencing symptoms of varicose veins. The number of patients reporting symptomatic recurrence for any intervention was very small, with no significant difference between treatment arms.
Venous Clinical Severity Score
Thirteen trials reported baseline and follow-up scores for the VCSS (see Table 15);44,45,53,80,86,87,90,97,102,117,127 the data reported by Figueiredo et al. 85 and Rasmussen et al. 95 were not appropriate for analysis because they did not report mean and SDs, or figures to enable the calculation of these data.
Pain score
Eleven trials45,83,87,88,89,95,97,98,100,102,103 reported measuring pain using a form of visual analogue scale (VAS) (1–10 or 1–100) for a period between 3 and 14 days post operation and were included in the network meta-analysis (see Table 16). Sixteen other trials all measured pain using different scales or measures (e.g. amount or duration of analgesic use), but the heterogeneous nature of the data measurement rendered them unsuitable for inclusion in any analysis. 39,55,78–80,84–87,89,91,97,98,119,124,128
Details of the outcomes data are given in Tables 11–16. The results of the meta-analyses, where meta-analysis was possible for these outcomes, are reported in Quantitative analysis.
| Study | Intervention | Control | ≤ 1 month | Definition of failure if reported | I: n/N | C: n/N | Definition of reoperation, if reported | I: n/N | C: n/N |
|---|---|---|---|---|---|---|---|---|---|
| Rasmussen 200744 | EVLA | Stripping | 12 days | Vein not successfully stripped | 0/67 | 2/68 | NR | NR | |
| Rasmussen 201195 | 1 month | GSV with reflux | 1/144 | 4/135 | NR | NR | |||
| Christensen 201097 | 12 days | Absent/closed GSV/reflux | 1/100 | 0/100 | NR | NR | |||
| Kalteis 200898 | EVLA and HL/S | 1 week | Technical failure/not occluded | 1/47 | 1/48 | NR | NR | ||
| 4 weeks | 0/47 | 0/48 | |||||||
| Rasmussen 201195 | RFA | Stripping | 1 month | GSV with reflux | 0/141 | 4/135 | NR | NR | |
| Lurie 200342 | Immediate | Reflux | 2/44 | 0/36 | NR | NR | |||
| 3 days | GSV flow/reflux | 7/43 | 0/36 | ||||||
| 1 week | Open segments | 4/43 | 0/36 | ||||||
| Subramonia 201089 | HL/S | 1 week | 4/47 | 7/41 | NR | NR | |||
| Elkaffas 201181 | Immediate | Vein not occluded | 6/90 | 0/90 | GSV stripping with SF ligation | 6/90 | 0/90 | ||
| Rasmussen 201195 | RFA | EVLA | 1 month | GSV with reflux | 0/141 | 1/144 | NR | NR | |
| Goode 201055 | 10 days | Not occluded | 2/40 | 2/39 | NR | NR | |||
| Nordon 201188 | 1 week | Not occluded | 0/70 | 0/68 | NR | NR | |||
| Hamel-Desnos 2003120 | FS | LS | 3 weeks | GSV with reflux | 7/45 | 26/43 | NR | NR | |
| Alos 2006119 | 15 days | Not totally occluded | 9/75 | 39/74 | NR | NR | |||
| 30 days | 5/74 | 34/74 | |||||||
| Bountouroglou 200690 | FS | Stripping | 3 weeks | Not fully obliterated | 4/30 | 2/28 | Additional foam (foam group) or liquid (surgery group) | 4/30 | 2/28 |
| Rasmussen 201195 | 1 month | GSV with reflux | 5/144 | 4/135 | Foam sessions | 5/144 | 0/135 | ||
| Rasmussen 201195 | FS | EVLA | 1 month | GSV with reflux | 5/144 | 1/144 | Foam sessions | 5/144 | 0/144 |
| Rasmussen 201195 | FS | RFA | 1 month | GSV with reflux | 5/144 | 0/141 | Foam sessions | 5/144 | 0/141 |
| Study | Intervention | Control | < 6 months follow-up | Definition of recurrence | I: n/N | C: n/N |
|---|---|---|---|---|---|---|
| Carradice 201196 | EVLA | Stripping | 6 weeks | Initial technical success | 1/137 | 10/132 |
| Rasmussen 200744 | 3 months | 0/63 | 1/63 | |||
| Darwood 200887 | 3 months | 11/71 | 8/32 | |||
| Kalteis 200898 | EVLA and HL/S | 16 weeks | Success rate of surgery | 0/47 | 0/48 | |
| De Medeiros 200584 | 60 days | GSV reopening | 1/20 | 0/20 | ||
| Disselhoff 2008126 | EVLA | Cryostripping | 6 months | GSV not ablated | 3/60 | 0/60 |
| Hinchliffe 200683 | RFA | Stripping | 6 weeks | 3/16 | 2/16 | |
| Lurie 2005115 | 4 months | Not occluded | 4/43 | 0/34 | ||
| Perala 2005,43 Rautio 2002125 | 7–8 weeks | 0/15 | 1/13 | |||
| Nordon 201188 | RFA | EVLA | 3 months | Patent vein | 2/70 | 3/68 |
| Alos 2006119 | FS | LS | 90 days | Not totally occluded | 4/71 | 33/71 |
| Rabe 2008121 | 3 months | GSV not occluded 3 cm below SFJ | 24/53 | 49/55 | ||
| Bountouroglou 200690 | FS and SFJ ligation | Stripping | 3 months | Partial obliteration without reflux | 0/29 | 0/23 |
| Wright 200677 | FS | 3 months | Occlusion of trunk vein and elimination of reflux | 72/435 | 12/94 | |
| Jia 201078 | FS and SFJ ligation | 3 months | 3/28 | 3/28 | ||
| Shadid 2010122 | 3 months | Recurrence of reflux | 11/217 | 1/177 | ||
| Aremu 2004124 | TIPP | SFJ ligation and stripping | 26 weeks | Recurrence of veins in same and new areas | 6/57 | 6/69 |
| Study | Intervention | Control | ≥ 6 months follow-up | Definition of recurrence | I: n/N | C: n/N |
|---|---|---|---|---|---|---|
| Carradice 201196 | EVLA | Stripping | 1 year | Technical recurrence | 5/124 | 23/113 |
| Pronk 2010100 | 1 year | Reflux in a vein | 5/49 | 5/56 | ||
| Rasmussen 201099 | 2 years | Technical recurrence | 18/69 | 25/68 | ||
| Rasmussen 201195 | 1 year | ‘Recurrent varicose veins’ | 14/121 | 16/108 | ||
| Darwood 200887 | 1 year | GSV and SFJ reflux and reverse flow | 13/49 | 1/12 | ||
| Christenson 201097 | EVLA and HL/S | 1 year | Reflux | 4/99 | 1/100 | |
| Disselhoff 2008126 | EVLA | Cryostripping | 1 year | 0/58 | 0/57 | |
| 2 years | 0/56 | 0/55 | ||||
| 3 years | 25/41 | 18/35 | ||||
| Rasmussen 201195 | RFA | Stripping | 1 year | ‘Recurrent varicose veins’ | 9/124 | 16/108 |
| ElKaffas 201181 | 2 years | 12/88 | 9/90 | |||
| Lurie 2005115 | 2 years | 4/43 | 3/34 | |||
| Perala 2005,43 Rautio 2002125 | 3 years | Surgeon-identified recurrence | 5/15 | 3/13 | ||
| Rasmussen 201195 | RFA | EVLA | 1 year | ‘Recurrent varicose veins’ | 9/124 | 14/121 |
| Gale 201053 | 1 year | Reflux | 11/46 | 2/48 | ||
| Goode 201055 | 9 months | Not occluded | 9/34 | 7/32 | ||
| Shepherd 2010102 | 6 months | Reflux or recanalisation | 6/76 | 1/76 | ||
| Morrison 2005101 | 1 year | GSV not completely ablated | 10/50 | 17/50 | ||
| Hamel-Desnos 2003,120 Ouvry 200882 | FS | LS | 1 year | Recanalisation | 2/45 | 6/43 |
| 2 years | 22/47 | 29/33 | ||||
| Figuereido 200985 | FS | Stripping | 6 months | Presence of reflux or residual varicose veins | 6/27 | 3/29 |
| Wright 200677 | 1 year | 92/435 | 13/94 | |||
| Kalodiki 2008117 | FS and SFJ ligation | 3.4 years | Residual or recurrent reflux | 18/38 | 16/34 | |
| Jia 201078 | FS and SFJ ligation | 6 months | Needing further sessions of FS vs. non-obliteration rate for surgery | 5/25 | 3/26 | |
| Shadid 2010122 | FS | 1 year | 43/221 | 50/188 | ||
| Rasmussen 201195 | 1 year | ‘Recurrent varicose veins’ | 17/123 | 16/108 | ||
| Rasmussen 201195 | FS | EVLA | 1 year | GSV with reflux | 17/123 | 14/121 |
| Rasmussen 201195 | FS | RFA | 1 year | GSV with reflux | 17/123 | 9/124 |
| Aremu 2004124 | TIPP | Stripping | 52 weeks | 7/37 | 2/34 |
| Study | Intervention | Control | Follow-up | Symptomatic recurrence | I: n/N | C: n/N | Reoperations | I: n/N | C: n/N |
|---|---|---|---|---|---|---|---|---|---|
| Carradice 201186,96 | EVLA | Stripping | 1 year | NR | NR | NR | Additional procedures included phlebectomy with or without additional perforator ligation under LA (10 surgery, 7 EVLA). Two patients in the surgery group had EVLA of residual GSV | 7/124 | 12/113 |
| Christenson 201097 | 1 year | GSV reopened ‘with symptoms’ | 2/100 | 0/100 | At the 1-year follow-up in the EVLT group, two GSVs had reopened (with symptoms, reoperated on, and lost to further follow-up). At 2 years, an additional two GSVs had partially reopened, one with symptoms, and underwent subsequent surgical ablation | 2/100 | 0/100 | ||
| 2 years | 1/98 | 0/99 | 2/98 | 0/99 | |||||
| Darwood 200887 | 1 year | NR | NR | NR | The leg with GSV reflux following surgery had groin neovascularisation on ultrasonography and an incompetent segment of mid-thigh GSV; this was subsequently treated with EVLA | 0/47 | 1/34 | ||
| De Medeiros 200584 | EVLA and HL/S | 60 days | Paraesthesia, not symptoms of varicose veins, but checked with DUS | 1/20 | 0/20 | NR | NR | NR | |
| Pronk 2010100 | 1 year | ‘Clinically visible’ | 3/49 | 3/56 | NR | 1/20 | 0/20 | ||
| Rasmussen 2007,44 201099 | 6 months | ‘Observed by patient’ | NR | NR | FS for recanalisations in EVLA group | ||||
| 2 years | 8/69 | 9/68 | 3/69 | 0/68 | |||||
| Disselhoff 2008103 | EVLA | Cryostripping | 6 months | NR | NR | NR | Recurrences were treated with sclerotherapySclerotherapy (and) two patients in each group developed small saphenous veins requiring saphenopopliteal ligation | 4/56 | 0/55 |
| 1 year | 7/56 | 9/55 | |||||||
| 2 years | 13/56 | 19/55 | |||||||
| Lurie 200342 | RFA | Stripping | 4 months | These limbs were all asymptomatic at 4-month follow-up | 0/43 | 0/34 | NR | NR | NR |
| Perala 200543 | 3 years | Recurrence as established by the patients themselves | 4/15 | 2/13 | One patient in each group underwent reoperation for treatment of recurrent varicose veins | 1/15 | 1/13 | ||
| Jia 201078 | FS and SFJ ligation | Stripping | 6 months | NR | NR | NR | After 6 months, in the FS group, five patients needed further sessions of FS resulting in a short-term closure rate of 80% | 5/25 | 0/26 |
| Study | Follow-up | Intervention | N | Control | N | Data | Baseline | Follow-up | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |||||||
| Carradice 201186,96 | 3 months | EVLA | 125 | Stripping | 119 | Mean, SD | 4.13 (1.95) | 4.15 (1.90) | 0.59 (1.23) | 0.7 (1.09) |
| 1 year | 124 | 113 | 0.49 (0.88) | 0.6 (1.11) | ||||||
| Christenson 201097 | 1 year | 99 | 100 | Mean, SD | 5.2 ± 2.5 | 5.2 ± 2.7 | 0.23 ± 0.59 | 0.26 ± 0.68 | ||
| 2 years | 95 | 99 | 0.23 ± 0.54 | 0.23 ± 0.57 | ||||||
| Darwood 200887 | 3 months | 71 | 32 | Median (IQR) | 4 (3–5) | 4 (3–5) | 0 (0–1) | 0 (0–1) | ||
| Rasmussen 200744 | 3 months | 63 | 63 | Mean (range) | 2.8 (1–8) | 2.4 (2–12) | 0.4 (0–7) | 0.2 (0–2) | ||
| Disselhoff 2008,103 2011113 | 6 months | EVLA | 60 | Cryostripping | 60 | Mean (range) | 3.2 (0–6) | 3.4 (0–6) | 1.0 (0–3) | 1.0 (0–3) |
| 1 year | 58 | 57 | 0.7 (0–4) | 0.9 (0–2) | ||||||
| 2 years | 56 | 55 | 0.6 (0–4) | 0.8 (0–2) | ||||||
| 5 years | 41 | 35 | 1.0 (0–3) | 1.0 (0–3) | ||||||
| Kalodiki 201194 | 3 years | FS and SFJ ligation | 38 | Stripping | 34 | Mean, SD | 5.18 (2.86) | 5.52 (2.25) | 1.43 (1.81) | 2.71 (3.00) |
| Bountouroglou 200690 | 3 months | 29 | 23 | Median (range) | 5 (2–13) | 7 (2–16) | 1 (0–5) | 3 (0–4) | ||
| Gale 201053 | 1 week | RFA | 69 | EVLA | 72 | Mean, SD | 6.4 (2.2) | 5.9 (2.5) | 4.4 (2.0) | 5.0 (2.1) |
| 1 month | 69 | 71 | 1.9 (1.7) | 2.1 (1.7) | ||||||
| 1 year | 59 | 67 | 1.4 (1.5) | 1.3 (1.8) | ||||||
| Shepherd 2010102 | 6 weeks | 60 | 55 | Mean, SD | 5.1 (2.1) | 4.7 (2.1) | 1.7 (1.7) | 1.5 (1.8) | ||
| 6 months | 55 | 52 | 1.4 (1.8) | 1.4 (1.7) | ||||||
| Rautio 2002,45 Perala 200543 | 50 days | RFA | 5 | Stripping | 13 | Mean, SD | 5/4–9 (median/range) | 4/4–6 (median/range) | 5.1 (1.5) | 4.4 (1.1) |
| 3 years | 15 | 13 | 4.3 (2.3) | 4 (1.2) | ||||||
| Lin 200980 | 4 weeks | RFA and TIPP | 75 | Stripping and TIPP | 75 | Mean, SD | 6.2 ± – 3.1 | 6.1 ± 3.5 | 1.6 ± 1.7 | 1.8 ± 1.9 |
| Study | Follow-up | Intervention | N | Control | N | Data | Intervention | Control |
|---|---|---|---|---|---|---|---|---|
| Kalteis 201198 | 7 days | EVLA and HL/S | 47 | Stripping | 48 | Median (IQR) | 2.13 (1.17–3.61) | 2.52 (1.24–4.19) |
| Rasmussen 201195 | 10 days | EVLA | 124 | 123 | Mean, SD | 2.58 (2.41) | 2.25 (2.23) | |
| Christenson 201097 | 1 day | 100 | 100 | Mean | 4.3 | 4.6 | ||
| 3 days | 2.2 | 2.9 | ||||||
| 12 days | 1.7 | 1.8 | ||||||
| Pronk 2010100 | 7 days | 62 | 68 | Mean, SD | 3.74 (2.72) | 1.78 (1.94) | ||
| 10 days | 2.65 (2.21) | 1.18 (1.49) | ||||||
| 14 days | 1.66 (2.04) | 0.77 (1.46) | ||||||
| Darwood 200887 | 7 days | 52 | 49 | Median (IQR) | 5 (4–29) | 8 (0–40) | ||
| Disselhoff 2008103 | 10 days | EVLA | 60 | Cryostripping | 60 | Mean (range) | 2.9 (0–8) | 4.4 (0–8.5) |
| Rasmussen 201195 | 10 days | RFA | 124 | Stripping | 123 | Mean, SD | 1.21 (1.72) | 2.25 (2.23) |
| Subramonia 201089 | 7 days | 47 | 41 | Median (IQR) | 1.70 (0.50–4.30) | 4.00 (2.35–6.05) | ||
| Hinchcliffe 200683 | 10 days | 16 | 16 | Median (IQR) | 1.7 (0.2–4) | 3.8 (0.6–6.3) | ||
| aRautio 200245 | 14 days | 15 | 13 | Mean, SD | 1.8 (0.8) | 3.0 (1.8) | ||
| Rasmussen 201195 | 10 days | FS | 123 | Stripping | 123 | Mean, SD | 1.60 (2.04) | 2.25 (2.23) |
| Norden 201188 | 3 days | RFA | 76 | EVLA | 78 | Median | 6 | 23.5 |
| 7 days | 0 | 13.5 | ||||||
| Rasmussen 201195 | 10 days | 69 | 72 | Mean, SD | 1.21 (1.72) | 2.58 (2.41) | ||
| Shepherd 2010102 | 3 days | 66 | 61 | Mean, SD | 26.4 (22.1) | 36.8 (22.5) | ||
| 10 days | 22.0 (19.8) | 34.3 (21.1) | ||||||
| Rasmussen 201195 | 10 days | RFA | 124 | FS | 123 | Mean, SD | 1.21 (1.72) | 1.60 (2.04) |
| Rasmussen 201195 | 4 weeks | EVLA | 124 | FS | 123 | Mean, SD | 2.58 (2.41) | 1.60 (2.04) |
Return to work or normal activity
Twelve trials42,44,45,77,88–90,95,97,98,100,102 evaluated the time to return to work or normal activity for participants exposed to different interventions (Table 17). In all cases except three, the comparator was always surgery: Nordon et al. 88 and Shepherd et al. 102 both compared EVLA with RFA and reported no difference between the interventions, and Rasmussen et al. 95 compared RFA, EVLA and FS both with surgery and with one another. No statistically significant difference was reported across any of these comparisons by Rasmussen et al. ,95 but participants did on average return to work or normal activity more quickly with these interventions than with surgery. Significantly quicker return to work or normal activity was, however, reported by other studies for every intervention compared with surgery: Bountouroglou et al. 90 for FS; Lurie et al. ,42 Rautio et al. 45 and Subramonia and Lees. 89 for RFA; and Christenson et al. ,97 Carradice et al. 86 and Kalteis et al. 98 (p = 0.054) for EVLA. Only Pronk et al. 100 and Rasmussen et al. 105 did not report a significant difference in favour of EVLA compared with surgery.
| Study | Data | Time to return to work or normal activity (days) | p-value | |
|---|---|---|---|---|
| EVLA | Stripping | |||
| Christenson 201097 | Mean (SD) | 6.9 (± 2.7) | 6.6 (± 2.1) | > 0.5 |
| Pronk 2010100 | Mean (SD) | 4.38 (± 5.43) | 4.14 (± 3.72) | 0.80 |
| Rasmussen 200744 | Mean (SD) | 7.0 (± 6.0) | 7.6 (± 4.9) | NR |
| Carradice 201186 | Median (IQR) | 4 (2–14) | 14 (13–28) | < 0.001 |
| Kalteis 200898 | Median (IQR) | 14.0 (12.8–25.0) | 20.00 (14.0–25.5) | 0.054 |
| Rasmussen 201195 | Median (IQR) | 3.6 (0–46) vs. 4.3 (0–42) | NR | |
| RFA | Stripping | |||
| Rautio 200245 | Mean (SD) | 6.5 (± 3.3) | 15.6 (± 6.0) | < 0.001 |
| Subramonia 201089 | Mean (range) | 3 (2–5) | 12.5 (4–21) | < 0.001 |
| Lurie 200342 | Mean (95% CI) | 4.7 (1.16 to 8.17) | 12.4 (8.66 to 16.23) | < 0.05 |
| Rasmussen 201195 | Median (IQR) | 2.9 (0–14) | 4.3 (0–42) | NR |
| FS | Stripping | |||
| Bountouroglou 200690 | Median (IQR) | 2 (0–6) | 8 (5–20) | < 0.001 |
| Wright 200677 | Median | 2 | 13 | < 0.001 |
| Rasmussen 201195 | Median (IQR) | 2.9 (0–33) | 4.3 (0–42) | NR |
Adverse events
A summary of the adverse event data related to the presence of DVT or PE is presented below. In general, these events were rare. Eleven studies53,82,83,88,90,95,97,100,102,120,122 reported on these outcomes, but only five studies (Gale et al. ,53 Rasmussen et al. ,95 Shepherd et al. ,102 Shadid et al. 122 and Wright et al. 77) reported that any such complication actually occurred (Table 18): 13 DVTs in the FS arms in three trials, of which 11 were in the trial reported by Wright et al. ;77 as well as one DVT in an EVLA arm, and one in a conventional surgery arm. There was one PE in a RFA arm102 and one in the FS arm in each of two different trials. The three trials reporting the highest numbers of these adverse events (i.e. Wright et al. ,77 Rasmussen et al. 95 and Shadid et al. 122) also had the largest sample sizes of all included studies in the review. This might suggest that these outcomes are rare events that the smaller studies were not powerful enough to detect, although the event rate in Wright et al. 77 was substantially higher than in any other study. However, this disproportionate rate can be explained by the intervention. The ‘Varisolve’ technique applied in this trial was new and the amount of foam used was altered part-way through the trial because of the high DVT rate: the initial amount of foam, 60 ml, was reduced to 30 ml. No DVT was reported for the 95 participants who subsequently received this lower dose.
| Study | Intervention | n | DVT | PE |
|---|---|---|---|---|
| Gale 201053 | EVLA | 49 | 1 | NR |
| Shepherd 2010102 | EVLA | 48 | 0 | 0 |
| RFA | 49 | 0 | 1 | |
| Wright 200677 | FS | 435 | 11 | 0 |
| Shadid 2010122 | FS | 227 | 1 | 1 |
| Rasmussen 201195 | FS | 124 | 1 | 1 |
| EVLA or RFA | 250 | 0 | 0 | |
| Surgery | 124 | 1 | 0 |
The complications of bruising and skin discoloration, haematoma, paraesthesia, infection and phlebitis were reported most frequently by trials. Two trials90,117 also reported on ulcers as outcomes, but only in one study90 was this reported as an adverse event or complication: one patient developed a skin ulcer following LS injection in the Bountouroglou et al. 90 study. In the study by Kalodiki et al. ,117 the ulcers of five patients ‘remained healed’ after 3 years' follow-up. For all adverse events the number of events was very small and statistically significant differences were not often reported.
Eleven trials reported data on varying degrees and discomfort due to bruising. 39,53,55,77,79,82,83,86,91,97,124 Aremu et al. ,124 Carradice et al. 86 and Ouvry et al. 82 reported no significant differences between groups for bruising, but Abela et al. 91 and Liamis et al. (p < 0.0001)79 both reported significantly better outcomes for FS than for surgery. Christenson et al. 97 reported better outcomes for EVLA than for surgery (p = 0.002) and Hinchliffe et al. 83 reported better outcomes for RFA than for surgery (p < 0.02). Gale et al. 53 and Goode et al. 55 reported different outcomes for EVLA from RFA in terms of bruising, but this difference disappeared over ≤ 1 month in both trials. Chetter et al. 39 reported worse outcomes for TIPP than for MSIP.
Twelve trials recorded haematoma outcomes,39,42,45,77,85,86,90,97,98,102,120,121 but only five trials reported p-values with significant differences between groups. Carradice et al. ,86 Rautio et al. 45 and Kalteis et al. 98 reported a significant difference between groups in favour of EVLA compared with surgery (p < 0.05), although this disappeared by 12 weeks in the Kalteis et al. 98 trial. Rabe et al. 121 reported more haematoma events in the LS group than in the FS group. Lurie et al. 42 reported significantly fewer cases of haematoma in the RFA group than in the surgery group at each follow-up (3 days, 1 week and 3 weeks; p < 0.05 for all time points).
Twelve trials recorded outcomes relating to paraesthesia. 42,45,84,88,89,95,97,98,100,102,121,122 Lurie et al. ,42 Nordon et al. ,88 Shepherd et al. ,102 Christenson et al. ,97 Pronk et al. ,100 De Medeiros and Luccas84 and Rasmussen et al. 95 reported no p-value or significant differences for this outcome. Rautio et al. 45 and Subramonia and Lees89 (p < 0.05) reported substantially more events in the surgery than the RFA trial arms, though this difference disappeared at 5 weeks in the Subramonia and Lees trial. 89 Shadid et al. 122 reported a similar favourable result for FS compared with surgery, and Kalteis et al. 98 for EVLA compared with surgery (p < 0.001), although this difference also disappeared over time.
There were no reported significant differences in any type of infection across six trials. 42,85,90,97,100,102 However, Carradice et al. 86 reported significantly fewer infections in the EVLA group than for surgery (p < 0.05) and Rasmussen et al. 95 reported higher infection rates for FS than for EVLA, RFA and surgery, whereas Shadid et al. 122 reported significantly fewer infection events for FS than for surgery.
Nine studies reported on forms of phlebitis,45,86,88,90,95,97,102,121,122 but only Shadid et al. 122 and Rasmussen et al. 95 reported any substantial differences between groups with FS and RFA, both producing much higher rates of phlebitis than surgery or EVLA.
The only other complications reported by more than one study were nerve injury39,43,83,90,100,124 and skin changes, in terms of hyperpigmentation, pigmentation and skin staining or discolouration. 42,77,86,89,90,95,98,102,119,121 Only Perala et al. 43 reported a statistically significant difference at the 5% level between treatment arms for nerve injury, which favoured EVLA over surgery. Only Alos et al. 119 reported a statistically significant difference at the 1% level between treatment arms for pigmentation, which favoured LS over FS. Only Carradice et al. 86 reported a statistically significant difference between treatment arms for any other adverse event, with fewer incidents of sensory disturbance in the EVLA arm than the surgery arm (2 vs. 13; p = 0.02).
Quantitative analysis
Technical recurrence
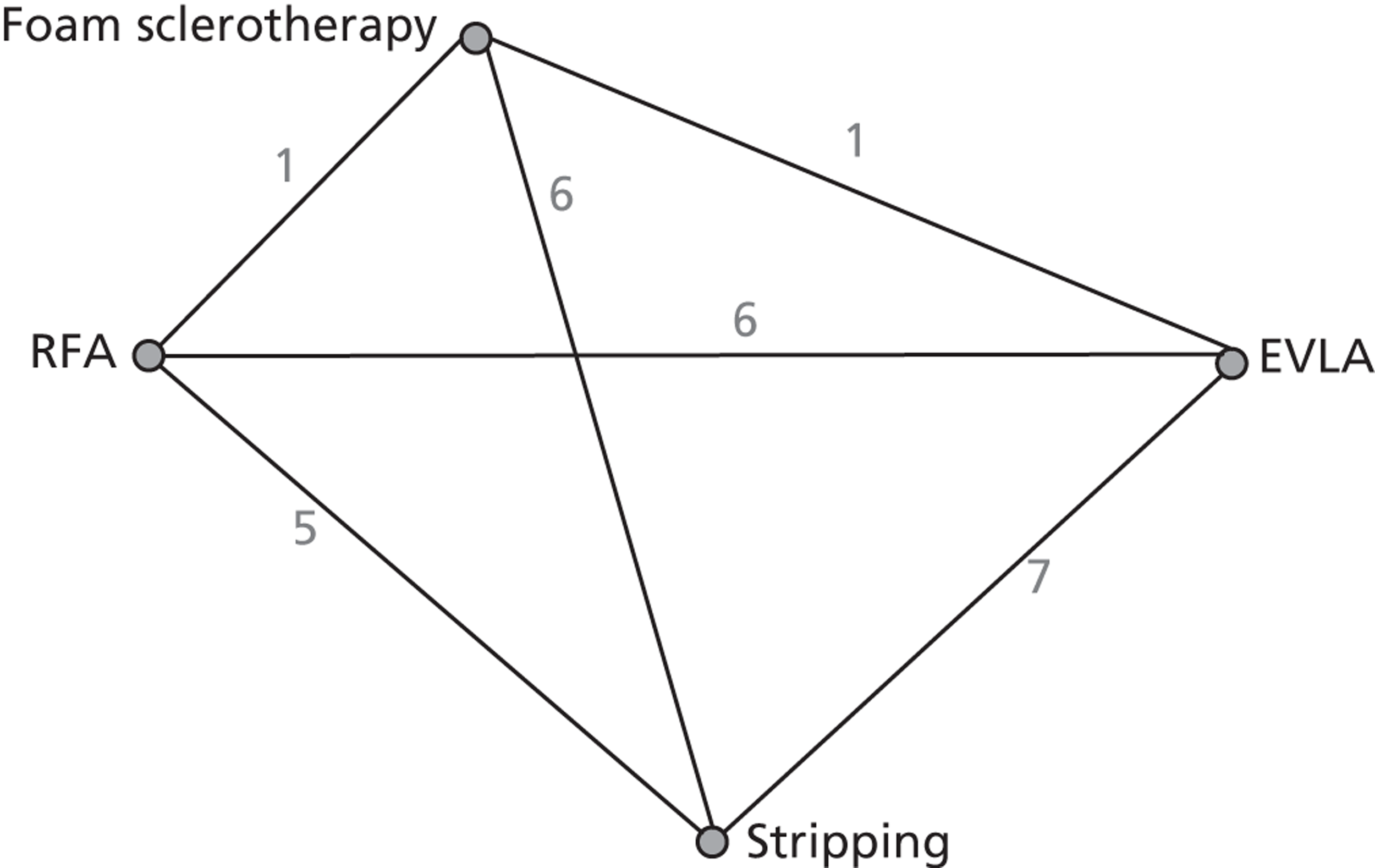
A network meta-analysis was used to compare the hazard of having technical recurrence when treating with EVLA, RFA and FS compared with stripping for 6 months, 1 year and 2 years. These durations were a pragmatic decision in that we were looking at short- and medium-term results and there was not expected to be a great difference between the data for 6 months and 2 years. It was the only viable approach for generating a network because different trials used different lengths of follow-up. A total of 23 studies42,45,53,55,71,72,75,77,78,80–86,88,95,96,99,100,110,114 comparing pairs or quadruplets of interventions provided information at various follow-up times. Bountouroglou et al. 90 and Kalteis et al. 98 were excluded from the analysis because there were no events in either intervention arm and, as a consequence, these studies provided no information about the intervention effects. 129
Figure 3 presents the network of evidence. A summary of all the trials (data) included in the base-case network meta-analysis for technical recurrence is presented in Appendix 7.
FIGURE 3.
Technical recurrence: network diagram of different interventions vs. stripping. The nodes represent the interventions. Lines between nodes indicate when interventions have been compared. The numbers against each line represent the number of times that each pair of interventions has been compared.
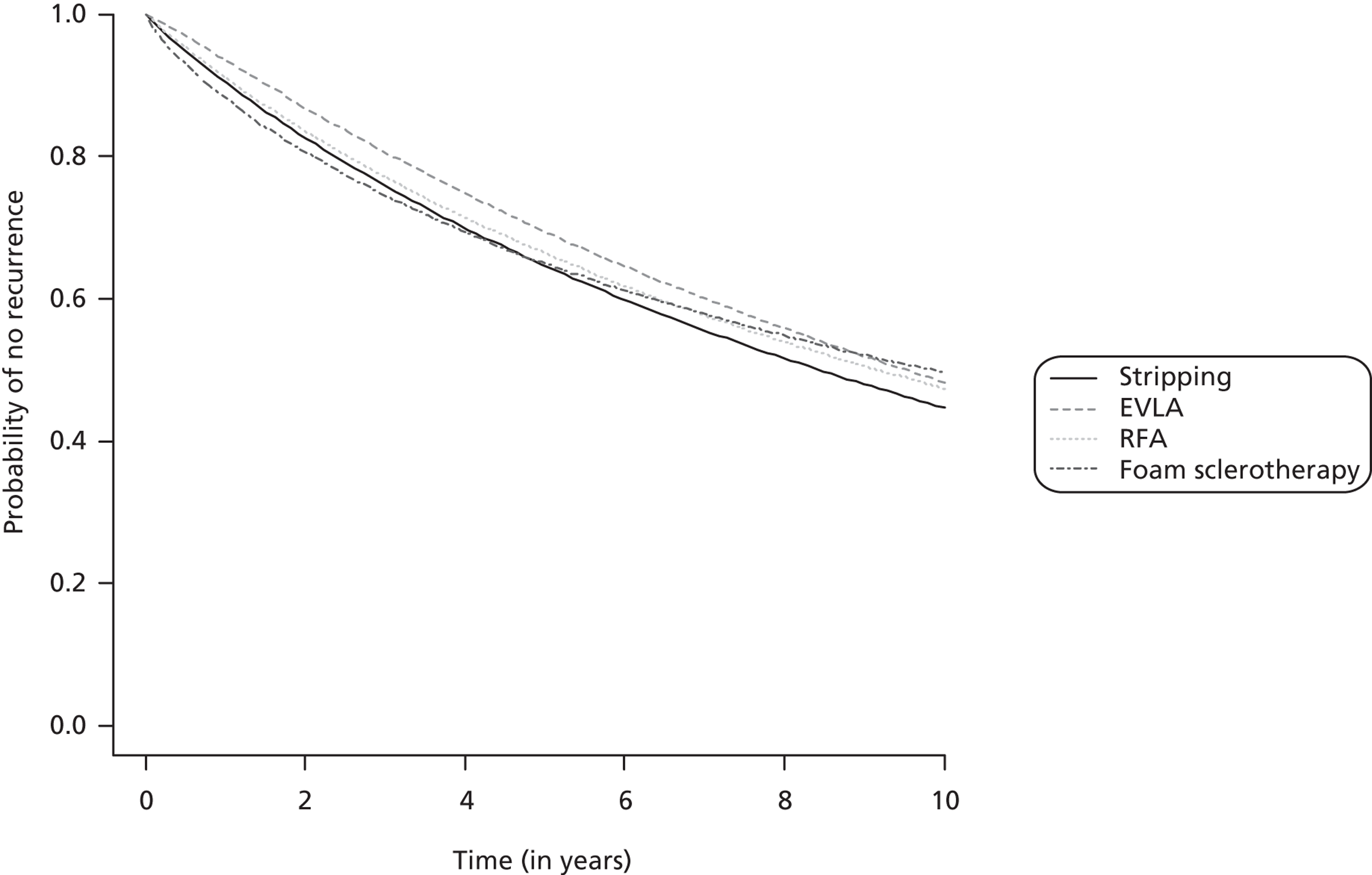
The network meta-analysis model fitted the data reasonably well, with a total residual deviance close to the total number of data points included in the analysis. The total residual deviance was 64.11, which compared favourably with the 60 non-zero data points being analysed. Figure 4 presents the fitted survivor function (i.e. probability of no technical recurrence) for each intervention.
FIGURE 4.
Technical recurrence: survivor function (no technical recurrence function) of each intervention (median).
The results suggested that there was mild heterogeneity between studies in the shape parameter, but that there was mild to potentially moderate heterogeneity between studies in the scale parameter (Table 19).
| Parameter | Median (95% CrI) |
|---|---|
| Between-study SD (Weibull shape parameter – natural scale) | 0.17 (0.01 to 0.45) |
| Between-study SD (Weibull scale parameter – log scale) | 0.26 (0.02 to 0.91) |
Endovenous laser ablation exhibited the greatest effect on technical recurrence relative to stripping, although there was some evidence that the benefit decreases over time (2-year HR 0.84, 95% CrI 0.44 to 1.81) (Table 20). RFA was associated with a small and relatively constant effect on technical recurrence over time relative to stripping (2-year HR 0.94, 95% CrI 0.42 to 2.51). FS was worse than stripping over the first year, although there was a small benefit after 2 years (2-year HR 0.92, 95% CrI 0.43 to 1.60). In each case there was considerable uncertainty about which intervention was the most beneficial.
| Comparison | Median (95% CrI) [probability hazard ratio > 1] | ||
|---|---|---|---|
| 6 months | 1 year | 2 years | |
| EVLA vs. stripping | 0.70 (0.27 to 1.45) [0.150] | 0.77 (0.37 to 1.54) [0.182] | 0.84 (0.44 to 1.81) [0.257] |
| RFA vs. stripping | 0.92 (0.39 to 2.11) [0.409] | 0.93 (0.42 to 2.22) [0.411] | 0.94 (0.42 to 2.51) [0.421] |
| FS vs. stripping | 1.12 (0.53 to 2.27) [0.659] | 1.02 (0.49 to 1.84) [0.524] | 0.92 (0.43 to 1.60) [0.359] |
Venous Clinical Severity Score
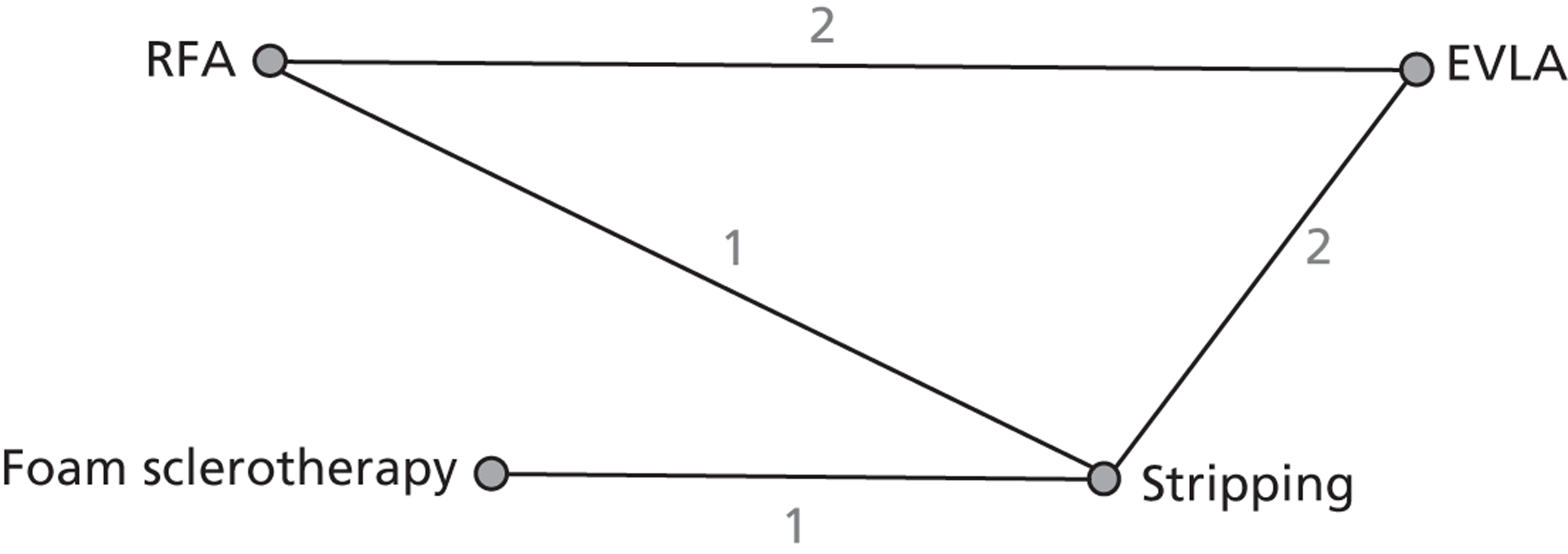
Venous Clinical Severity Score was analysed based on the data available at 1 year. However, for studies that did not provide 1-year data, the 6-month data were used, or the first available value after 1 year. A total of six studies43,53,86,97,102,117 were selected for the analysis. All studies were two-arm trials. Among all the studies, Carradice et al. 86,96 and Christenson et al. 97 compared EVLA with stripping; Perala et al. 43 compared RFA with stripping; Kalodiki et al. 94,117 compared FS with stripping; Gale et al. 53 and Shepherd et al. 102 compared RFA with EVLA. Four out of six studies reported one of median, interquartile range (IQR) or range and it was not possible to estimate the sample mean and sample SD as required for the network meta-analysis. 44,87,90,113 The authors were contacted and asked to provide the sample means and sample SDs from their studies; three authors provided the required sample means and sample SDs (see Table 15). 53,94,96 The missing SD from the Perala et al. study43 was treated as another uncertain parameter in the analysis. 130
Figure 5 presents the network of evidence. A summary of all the trials (data) included in the base-case network meta-analysis is presented in Appendix 8.
FIGURE 5.
Venous Clinical Severity Score: network diagram of different interventions vs. stripping. The nodes represent the interventions. Lines between nodes indicate when interventions have been compared. The numbers against each line represent the number of times that each pair of interventions has been compared.
The network meta-analysis model fitted the data well, with the residual deviance close to the total number of data points included in the analysis. The total residual deviance was 11.47, which compared favourably with the 12 data points being analysed. The between-study SD was estimated to be 0.22 (95% CrI 0.01 to 1.79) (Table 21). The intervention that exhibited the greatest effect relative to stripping was FS (MD –1.63, 95% CrI –2.90 to –0.42).
| Comparison and parameter | Median (95% CrI) | Probability of MD > 0 |
|---|---|---|
| EVLA vs. stripping | –0.10 (–0.94 to 0.73) | 0.324 |
| RFA vs. stripping | 0.15 (–0.50 to 0.95) | 0.739 |
| FS vs. stripping | –1.63 (–2.90 to –0.42) | 0.015 |
| Between-study SD | 0.22 (0.01 to 1.79) |
Pain
The effect of interventions on pain was assessed using a VAS based on data available within 7–14 days of treatment; all but two of the studies (see Table 16) measured pain scores at either 7 or 10 days (Christenson et al. 97 measured the pain score at 12 days and Rautio et al. 45 measured it at 14 days). Data were available from nine studies83,87–89,95,98,100,102,103 comparing pairs or quadruplets of interventions.
Among all the studies, four studies compared EVLA with stripping;87,95,97,98 three studies compared RFA with stripping;45,83,89 two studies compared RFA with EVLA;88,102 and one study had all four intervention arms. 95 Four studies reported a median, lower quartile and upper quartile. 83,87,89,98 To estimate the sample mean and sample SD from these studies, while acknowledging the skewness in the distribution, a gamma distribution was fitted to the median and interquartiles using least squares. Christenson et al. 97 reported only the mean and Nordon et al. 88 reported only the median, which we take as an estimate of the mean assuming that the data are normally distributed. The missing SDs were treated as additional uncertain parameters. 130
Figure 6 presents the network of evidence. A summary of all the trials (data) included in the base-case network meta-analysis is presented in Appendix 9.
FIGURE 6.
Pain score: network diagram of different interventions vs. stripping. The nodes represent the interventions. Lines between nodes indicate when interventions have been compared. The numbers against each line represent the number of times that each pair of interventions has been compared.
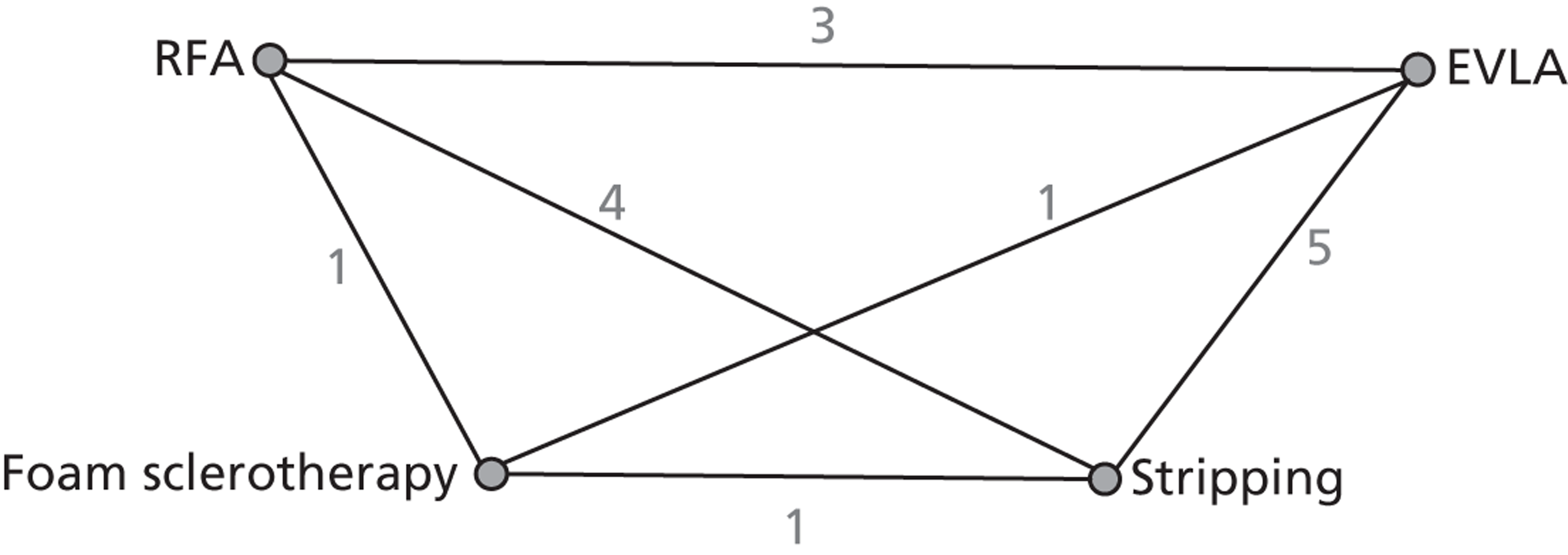
The network meta-analysis model represented the data well, with the residual deviance close to the total number of data points in the analysis. The total residual deviance was 22.29, which compares favourably with the 22 data points being analysed. The between-study SD was estimated to be 0.48 (95% CrI 0.06 to 1.12) (Table 22). The interventions that exhibited the greatest effects on pain relative to stripping were RFA (MD –1.26, 95% CrI –1.95 to –0.61) and FS (MD –0.80, 95% CrI –1.93 to 0.30).
| Comparison and parameter | Median (95% CrI) | Probability of MD > 0 |
|---|---|---|
| EVLA vs. stripping | 0.10 (–0.49 to 0.64) | 0.653 |
| RFA vs. stripping | –1.26 (–1.95 to –0.61) | 0.001 |
| FS vs. stripping | –0.80 (–1.93 to 0.30) | 0.062 |
| Between-study SD | 0.48 (0.06 to 1.12) |
Discussion
The analysis of the technical recurrence data suggested that the treatment effects for EVLA and FS were not constant over time. In particular, the early benefit associated with EVLA was much less, relative to stripping, after 2 years (15% reduction) than it was at 6 months (30% reduction). However, the results were inconclusive in determining which intervention was the most effective.
A benefit of the analysis of the technical recurrence data was that it did not assume proportional hazards; this is particularly important in terms of the assessment of cost-effectiveness as it does not assume that any treatment effect continues indefinitely. There were several limitations associated with the analysis of the technical recurrence data. In general, studies did not account for all patients at each follow-up time so that the technical recurrence response rates did not increase monotonically. Although authors often reported that theirs was an intention-to-treat analysis, some authors reported results as the number of events out of the number of patients randomised, whereas others reported results as the number of events out of the number of patients for which there were data. Some patients were assessed for their varicose veins in both limbs and results were often reported by limb rather than by patient; results will be correlated with patients and the analysis of the patient-level data should allow for variation within patients, which we were unable to do at the aggregate level. We fitted a Weibull model to the data, which effectively assumes that all patients will have a technical recurrence at some stage in the future; in practice, it is likely that a proportion of patients would never have a technical recurrence, and that a more appropriate model would be a ‘cure’ model in which the time to recurrence is conditional on not being ‘cured’, although it was not possible to do this with the data that were available. Some studies presented response rates at more than one time, which meant that we could estimate more than one parameter (i.e. the shape and scale parameter in the Weibull distribution). However, our model assumed that the observations were independent, which may have led to an overestimation of uncertainty.
The analysis of the VCSS data suggested that FS was the most effective intervention and was more effective than stripping. The analysis of the pain score data suggested that RFA was the most effective treatment and was more effective than stripping.
Chapter 4 Assessment of cost-effectiveness
This section presents the results of a review of the cost-effectiveness evidence and the development of an independent economic model.
Systematic review of existing cost-effectiveness evidence
Identification of studies
A comprehensive search was undertaken to identify systematically cost-effectiveness literature comparing the different interventions for managing varicose veins. The search involved combining terms for the population (varicose veins) with terms for the interventions of interest (i.e. the minimally invasive techniques) and a filter designed to retrieve cost-effectiveness studies. An example MEDLINE search strategy is reported in Appendix 1. The aim of the strategy was to identify all studies that reported on costs and related analyses associated with the techniques of interest. Initial cost-effectiveness searches were performed by an information specialist (AC) in July 2011. Additional cost-effectiveness searches were completed in October 2011 and September 2012 to identify studies with costs associated with varicose veins. An example search strategy is reported in Appendix 1.
The following electronic databases were searched from inception for published and unpublished research evidence:
-
MEDLINE (Ovid) 1950–
-
EMBASE (Ovid) 1980–
-
CINAHL (EBSCO) 1982–
-
the Cochrane Library limited to HTA and NHS EED databases 1991–
-
BIOSIS Previews (via ISI Web of Science) 1969–
-
SCI (via ISI Web of Science) 1900–
-
SSCI (via ISI Web of Science) 1956–
-
EconLit (Ovid) 1961–.
Searches for utilities associated with the interventions of interest for treating varicose veins were performed by an information specialist (AC) in July 2011. An example search strategy is provided in Appendix 1.
The following databases were searched from inception for published and unpublished research evidence:
-
MEDLINE (Ovid) 1950–
-
EMBASE (Ovid) 1980–
-
CINAHL (EBSCO) 1982–
-
the Cochrane Library including the CDSR, CENTRAL, DARE, HTA and NHS EED databases 1991–
-
Biological Abstracts (BIOSIS Previews) (via ISI Web of Science) 1969–
-
SCI (via ISI Web of Science) 1900–
-
SSCI (via ISI Web of Science) 1956–
-
EconLit (Ovid) 1961–
-
the Cost-Effectiveness Analysis Registry 1976–.
All citations were imported into Reference Manager Version 12 and duplicates deleted. Titles and abstracts of all unique citations were then screened independently by the cost-effectiveness reviewer (SH) using the inclusion criteria outlined below after a test screen on a sample of citations. Disagreements or queries were resolved by consensus or with reference to a second team member (CC or JM) where necessary. The full papers of all potentially relevant citations were then retrieved so that an in-depth assessment concerning inclusion could be made.
Methods
Study inclusion criteria were the same as for the clinical effectiveness review in terms of treatments and populations, but no limitation was put on study design. Additionally, studies had to report economic outcomes in terms of cost-effectiveness, cost–utility or cost–benefit. Search results were sifted, with the number of studies retained at each stage shown in Figure 7. Included studies were quality assessed according to study design. Primary economic analyses conducted alongside clinical trials were assessed using the checklist by Drummond et al. ;131 modelling studies using a checklist modified from Eddy. 132 These evaluations are reported in full in Appendix 10.
FIGURE 7.
Cost-effectiveness PRISMA flow chart.
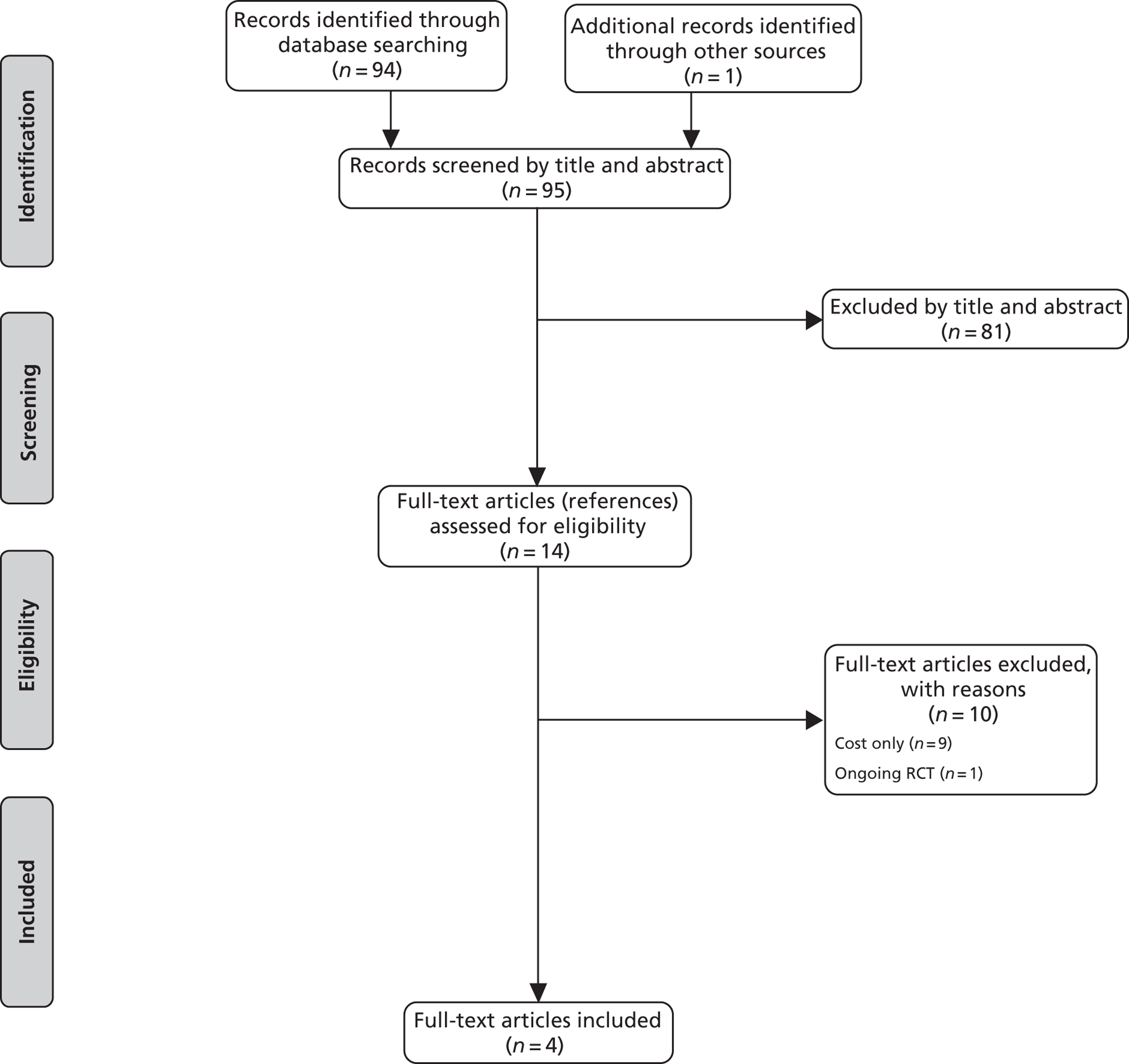
Results
In total, four relevant economic studies were identified, two economic analyses conducted alongside RCTs133,134 and two modelling analyses. 135,136
The RCT compared cryostripping with EVLA in patients with primary symptomatic varicose veins, CEAP clinical class 2 (Disselhoff et al. 133). The second RCT compared RFA with surgery in primary or recurrent lower limb varicose veins, CEAP classes 2–6, though approximately 80% were C2 (Subramonia and Lees134).
The study by Disselhoff et al. 133 is of poor quality (see Appendix 5) and with a major flaw: the incremental cost-effectiveness ratios (ICERs) have been calculated incorrectly, calculating the cost per quality-adjusted life-year (QALY) for individual treatments before subtracting one from the other, instead of calculating the incremental cost per incremental QALY. However, given the limited evidence and sufficient data for recalculation of the ICERs the study is included in the economic review.
Details of disease recurrence, further treatments and utility [measured by Short Form questionnaire-6 Dimensions (SF-6D)] were recorded over 2 years following the initial intervention. The costs of lost productivity were also included in the analysis, but sufficient data are presented to allow recalculation of ICERs excluding these, for a health costs perspective. Given the very small differences in total costs and QALYs between the two treatments, the assumption that treatment costs for cryostripping and EVLA are the same apart from equipment costs is a limitation of this study. A further limitation is that patients chose whether or not to be treated as a day case or outpatient, with their costs of treatment inferred from this choice. Eighty-two per cent of cryostripping patients and 66% of EVLA patients had a day-case procedure, which was assumed to be more costly than an outpatient procedure.
Table 23 shows costs, QALYs and ICERs for EVLA in comparison with cryostripping, with the costs in the original units (it is assumed that costs are for 2003, but this is unclear). When costs were converted to pounds sterling (assuming the current exchange rate of €0.787 to £1) and inflated to 2011/12 prices using Health and Community Health Services inflation indices (1.27), the conversion factor was equal to 1.000. Therefore, the costs and ICERs shown in Table 23 can also be interpreted as £2011/12. The source data are from Disselhoff et al. ,133 but with the following adjustments:
-
Costs of sick leave are excluded, to give the analysis a health cost perspective.
-
An all-patient cost, taking into account the proportion of patients in each trial arm having day or outpatient care, was not calculated (see above).
-
The QALYs shown in Table 23 are for all patients in each arm. Given the small patient numbers (n = 120) and uncertainty in the mean values reported by Disselhoff et al. ,133 distinguishing between those for outpatient and day care is not useful.
| Treatment scenario | Cryostripping | EVLA | Difference | ICER (€) | |||
|---|---|---|---|---|---|---|---|
| Costs (€) | QALYS | Costs (€) | QALYS | Costs (€) | QALYS | ||
| Day case | 2405 | 1.59 | 2728 | 1.60 | 323 | 0.01 | 32,265 |
| Outpatient | 2088 | 1.59 | 2411 | 1.60 | 323 | 0.01 | 32,265 |
| Outpatient and reduced price EVLA kit | 2088 | 1.59 | 2242 | 1.60 | 154 | 0.01 | 15,365 |
These results show EVLA to be both marginally more effective and more expensive than cryostripping over 2 years. The ICER for EVLA in comparison with cryostripping is €32,265, but the costs of EVLA fell during the course of the study, and if the lower cost is used the ICER falls to €15,365. However, the differences in costs and QALYs are very small relative to their uncertainties, so effectively the conclusion of this study is that the treatments are similar in terms of both costs and outcomes.
In the study by Subramonia and Lees134 resource use was collected alongside a clinical trial comparing RFA (n = 47) with surgery (n = 41). Costs include hospital costs (theatre and staff time, overheads, day ward, scans, non-protocol outpatient visits), primary health care and costs to patients. Protocol-driven costs that were the same for both treatments were excluded. These comprised hospital visits, scans and district nurse visits. The number of days of work lost by patients following the procedures was also recorded. A summary of the costs and outcomes for RFA and surgery is shown in Table 24.
| Costs | RFA | Surgery | Difference (RFA – surgery) |
|---|---|---|---|
| Hospital costs (£) | 1275.90 | 559.12 | 716.78 |
| Primary care (GP, practice and district nurses) (£) | 9.53 | 20.12 | –10.59 |
| Total health-care costs (£) | 1285.43 | 579.24 | 706.19 |
| Patient cost (£) | 3.40 | 7.79 | –4.39 |
| Societal cost (excluding lost work days) (£) | 1288.83 | 587.03 | 701.80 |
| Lost work days (5 days) (£) | –384.15 | ||
| Total societal cost (£) | 317.65 | ||
| Benefits | |||
| Residual reflux (duplex scan) | 0 | 7 | –7 |
| Change in AVVQ score | –9.12 | –8.24 | –0.88 |
| Lost work days (median) | 10 | 18.5 | Assume 5 working days (39 hours) |
Although the study generally appears to be of good quality (see Appendix 5), the incremental cost-effectiveness result quoted for RFA in comparison with surgery (incremental societal cost per work hour gained of £8.14) is clearly incorrect, including a valuation of the work hours gained in the numerator, and actual hours in the denominator. In fact, with an incremental health-care cost of £706, the incremental health costs per work hour gained are £18.11. If the work hours are valued, the total additional cost to society of RFA in comparison with surgery is £318. From a health-care perspective there was an incremental cost of £706 for an incremental improvement in the Aberdeen Varicose Veins Questionnaire (AVVQ) score at a median of 37 days of 0.88, which gives a cost of £806 per incremental unit improvement in AVVQ score. The health-care costs for RFA and surgery inflated to £2011/12 are shown in Table 25 and Costs. The cost of RFA is £1525 and surgery £687, giving a cost differential of £838.
Two modelling studies were identified (Adi et al. 135 and Gohel et al. 136). That by Adi et al. 135 is a very simple attempt to estimate cost-effectiveness of RFA compared with surgery, based on the results of a single poor-quality RCT (see Chapter 3, Quality of included studies, Rautio et al. 45) with very limited follow-up (2 weeks), so no long-term outcomes were included. Utilities were estimated from mean pain VAS scores for the two treatment arms, using a relationship between pain VAS and EQ-5D utility sourced from a study of back pain. 137 The latter gave a much steeper gradient in utility loss with increasing pain than that derived from a study of varicose veins (a utility decrement of 0.035 per 1% increase in pain VAS compared with 0.0026;86 see Utility values), suggesting that the QALY benefit of RFA compared with surgery reported by Adi et al. 135 may be overestimated by over 10 times. Costs were taken from the Finnish trial. 45 The difference in costs was £380 (year 2000 prices) and QALYs 0.016. The estimated ICER of RFA compared with surgery was £23,750, but with enormous uncertainty for the reasons described (see Appendix 5).
Gohel et al. 136 developed a Markov model to compare endovenous treatments for varicose veins (just FS, not UGFS, RFA and EVLA) with surgery over a 5-year time horizon. Model states include treatment success, residual varicosity, incomplete occlusion, recurrence of reflux and residual reflux. The study generally follows good modelling and reporting guidelines (see Appendix 5). Clinical effectiveness is based on two systematic literature reviews,29,48 and cost and utility data are adequately sourced, although the cost estimates are relatively simple. However, no information is reported (neither values nor source) regarding the likely important recurrence rates of reflux for treatments other than surgery. If it is assumed that the rate is the same for all treatments this is not made clear, nor the assumption discussed. Also the odds ratios for incomplete occlusion following treatment were from studies with different length of follow-up. The authors state that the ‘data suggested that the odds ratios for incomplete occlusion did not differ during follow-up’. However, the review (which does include non-randomised comparative studies) by van den Bos et al. ,49 which included a meta-regression of treatment success with time, indicates that relative success rates do vary with time from treatment. A further questionable assumption, which is not tested in sensitivity analysis, is that patients with residual varicosities and incomplete occlusion have the same utility value as untreated patients. In fact, the literature shows limited correlation between technical treatment success and patient symptoms. For example Merchant et al. 138 presents a chart indicating that 78% of limbs with anatomic failure following RFA were asymptomatic, compared with 90% of limbs which were classed as anatomic successes.
Costs of treatment (£2008/9) over 5 years varied from £0 for conservative treatment and £2000 for inpatient surgery. For active treatments the 5-year QALYs were fairly similar, ranging from 3.836 for UGFS performed under local anaesthetic (LA) to 3.958 for RFA with general anaesthetic (GA). Various ICERs are presented for different comparisons, but are not particularly informative. The probabilities of the treatments being cost-effective at a willingness-to-pay threshold of £20,000 are also reported. Those with the highest probabilities were EVLA – LA (35%), day-case surgery (29%) and RFA – LA (24%). The authors conclude that these are all likely to be cost-effective strategies for the treatment of varicose veins, although acknowledging considerable uncertainty in the results.
The uncertainty can be demonstrated by the monetary net benefits for each procedure. The results reported by Gohel et al. 136 were used to calculate these at a threshold of £20,000 (Table 26). This demonstrates very clearly that there is little difference in expected benefits between treatments, especially EVLA and RFA conducted under LA and day surgery.
| Vein | Bounterologou 200690 | Subramonia 2010134 | Michaels 20062 | Lattimer 2012154 | ||||
|---|---|---|---|---|---|---|---|---|
| Patient characteristics | ||||||||
| Primary/recurrent varicose veins | Primary only | Primary or recurrent | Recurrent included, but only if redo saphenofemoral or saphenopopliteal ligation not required | Primary only | ||||
| Single vein? | Varicosities including the LSV system (if both LSV and SSV excluded) | Isolated GSV incompetence (no deep venous or SSV incompetence) | No apparent restriction | No apparent restriction | ||||
| Other | Not tortuous above knee (i.e. suitable for catheterisation). GSV diameter > 3 mm, < 12 mm | GSV diameter < 12 mm, excluded saphenopopliteal incompetence | ||||||
| Treatment | ||||||||
| Unilateral treatment? | Yes | Not stated | Yes | Yes | ||||
| FS (preceded by SFJ ligation) | Stripping (preceded by SFJ ligation) plus phlebectomy | RFA | Stripping (including SF disconnection) | LS | Stripping | FS Primarily for incompetent GSV, significant tributaries treated if required at a further session |
EVLA Incompetent saphenous tributaries treated concurrently with phlebectomy hooks |
|
| Anaesthetic | LA | GA | GA | GA | None described | GA | None described | LA (tumescent) |
| Setting | Day surgery unit | Day surgery unit | Day case | Day case | Outpatient | Uncomplicated – day case More complex cases included overnight admission |
Day surgery clinical (outpatient) room | Day surgery theatre |
Although sensitivity analysis was undertaken by Gohel et al. 136 some key assumptions were not tested (same recurrence rate for all treatments, utility value for clinically failed treatments). However, those that were undertaken illustrate the sensitivity of the results to model assumptions and uncertainty in model parameters.
Conclusions of economic review
The two economic studies carried out alongside clinical trials both had seriously flawed economic analyses, limiting the value of their conclusions. 133,134 Two studies compared RFA with surgery, both with short-term outcomes (< 40 days) (Subramonia and Lees134 and Adi et al. 135). The costs for both were based on resource use in a clinical trial, one in the UK134 and one in Finland. 45 Both showed the costs of RFA to be greater than surgery, but the cost difference was much greater in the UK study (£838, 2011/12) than that derived from the Finnish study45 by Adi et al. (£451, 2011/12). 135 Adi et al. 135 estimated a QALY gain derived from differences in mean pain VAS scores at 2 weeks after the procedures, giving an ICER for RFA compared with surgery of £23,750, although this result was highly uncertain. From a societal perspective, the additional costs of RFA compared with surgery were reduced (Subramonia and Lees134) or eliminated (Rautio et al. ,45 Adi et al. 135) by taking into account lost work days.
Results derived from Disselhoff et al. 133 show EVLA to be both marginally more effective and more expensive than cryostripping over 2 years. The ICER for EVLA in comparison with cryostripping is €32,265, but the differences in costs and QALYs are very small relative to their uncertainties, so effectively the conclusion of this study is that the treatments are similar in terms of both costs and outcomes.
One modelling study compared the principal endovenous treatments (FS, EVLA and RFA) with surgery. 136 Some key assumptions were not justified, or tested in sensitivity analysis. The modelled costs and benefits were very similar for all treatments, and demonstrate the sensitivity of the results to all assumptions.
Overall, the economic analyses of endovenous treatments in comparison with conventional treatment for varicose veins have been of limited scope and quality. They do demonstrate, however, that the differences in costs and benefits between treatments are small and sensitive to assumptions, and therefore the cost-effectiveness of the different procedures in relation to each other is likely to be uncertain, and vary by local costs.
The economic model
Methods
The model structure
Outcomes of varicose vein procedures are complex. Several disease-specific quality-of-life measures have been developed for varicose veins in recognition of the fact that, although symptom relief is associated with clinical or anatomical outcomes, these are poor predictors of operative success from the patient's perspective. 139,140 In the model it is therefore not assumed that technical success equates to the patient being asymptomatic. Instead patients with technically successful and technically failed procedures have differing probabilities of being asymptomatic, with differing utility values attached to symptomatic and asymptomatic states. Ideally, direct HRQoL data would be used to model the outcomes of treatment with time, but there were insufficient data from the effectiveness literature with which to do this (see Chapter 3,Venous Clinical Severity Score).
The model structure is illustrated in Figure 8. Ovals represent events (numbered 1–3) and oblongs health states (A–D). Treatments included in the model are surgical stripping, FS, EVLA and RFA (Event 1). Costs and a loss of utility from the short-term adverse effects of treatment are assigned according to the treatment. Treatment may result in technical immediate (anatomical) success (states A and B) or failure (states C and D). If a failure, it is assumed that all patients will have further treatment with foam until technical success is achieved (Event 2; see Additional treatment to achieve a successful outcome). Patients with a successful clinical outcome nevertheless still have a probability of remaining symptomatic (state B). Thus, initial treatment may result in one of two health states (see Figure 8).
FIGURE 8.
Diagram of the model structure.
Patients whose treatment is successful nevertheless have a risk of failure over time (technical recurrence, Event 3). The proportion asymptomatic after recurrence is lower than for those whose treatment remains successful. It is assumed that only symptomatic patients with technical recurrence are retreated. It is assumed there is a delay of 6 months between treatment recurrence and retreatment as the development of symptoms may be gradual (‘time to retreatment’). The model was developed as a discrete event simulation (DES) model in Simul8® to simulate the experience of patients undergoing treatment for varicose veins. The baseline model has a perspective of 10 years. This was chosen as a reasonable time over which to extrapolate the time to failure data. Of the studies included in the analysis most had a follow-up of a few months, with the longest at 2–3 years. Some cohort studies not included in the systematic review have followed patients for up to 10 years. The treatments for varicose veins considered in the model are for symptom relief and are assumed not to affect mortality, and therefore a lifetime time horizon is not necessarily the most appropriate. A DES model was chosen to allow non-constant hazard in the time to treatment failure/technical recurrence (i.e. not necessarily assume these are exponentially distributed). This method also obviates the need for arbitrary time cycles.
Patients may die at any time for any reason (all-cause mortality). 141 When patients enter the model the age-specific life expectancy distribution is sampled to determine their time to death. Patients with asymptomatic technical recurrence stay in the same state until their date of death. Patients whose treatment is successful might experience later disease recurrence if their sampled time to failure is less than their time to death. Symptomatic recurrences are retreated, after which they may be symptomatic or asymptomatic.
Treatments included are stripping, FS, EVLA and RFA. LS was omitted from the model because trials comparing LS and surgery were not reviewed (this was not a comparison of interest for this report). Consequently, the clinical effectiveness review only considered a part of the published LS data (from studies comparing LS with FS), which represents only a small amount of the potentially relevant data on LS. The data on LS reviewed in this report were therefore extremely limited and not included in the model.
The model can be run for cohorts of different ages entering the model, from age 40 to 80 years, in increasing decades. The methods for sourcing and deriving model parameters are described in the following section. The economic analysis is from the perspective of the UK NHS. All costs and benefits are discounted at a rate of 3.5%. A complete list of parameter values and the distributions used in the probabilistic sensitivity analysis are shown in Appendix 6.
Model data
Treatment effectiveness and adverse effects of treatment
Disease recurrence is labelled as Event 3 in Figure 8. Disease recurrence data were sourced from the meta-analysis of the effectiveness literature (see Chapter 3, Assessment of effectiveness). Uncertainty about the true time to technical failure was represented as probability distributions. These were computed by taking samples for the shape and scale parameters from the Weilbull distribution at each iteration of the Markov chain (see Chapter 3, Assessment of effectiveness) and using these as inputs.to the economic model. Correlated samples of alpha and beta parameters of the Weibull curves used to describe time to recurrence were generated for individuals from the mixed-treatment comparison of the failure data sourced from the effectiveness review. Treatment failure (technical recurrence) is defined as before. Initial treatment failure was defined as treatment failure within 1 month of the procedure, as determined by the failure curves (Event 2; see Figure 8). Figure 9 shows the survivor function for the different treatments (note it differs from Figure 4 in that it presents the plot of the mean of the individual Weibull parameters, rather than the median).
FIGURE 9.
Technical recurrence: survivor function (no technical recurrence function) of each intervention (mean).
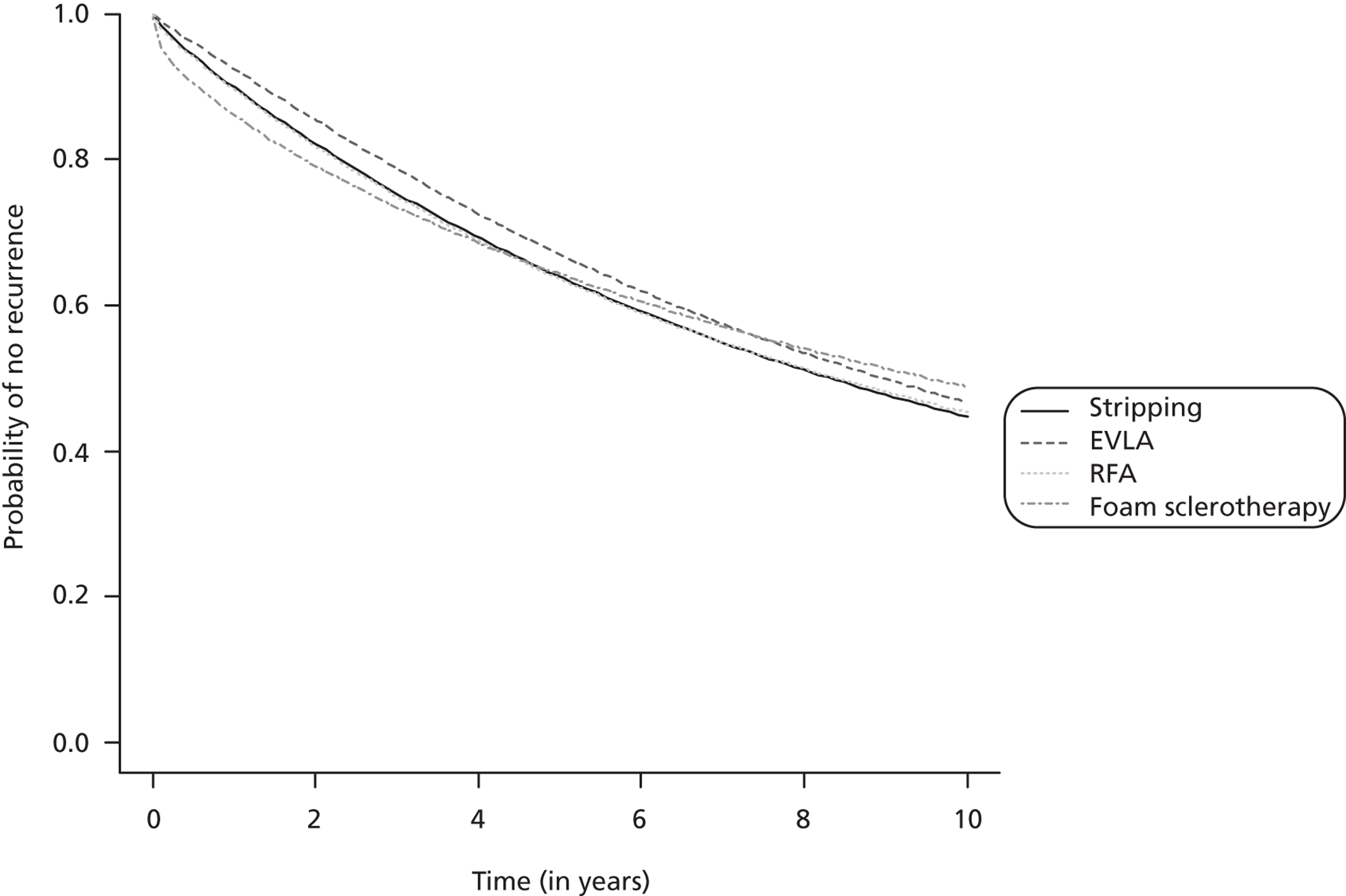
These proportions determine whether or not patients are in state A or B (see Figure 8) following treatment, or states C or D following disease recurrence. Very few included studies report symptomatic recurrence and none report asymptomatic recurrence (see Table 13); in fact, since the majority of procedures are technically successful it requires large cohorts, more likely achieved in observational studies, to identify the proportion asymptomatic in technically failed procedures. Two papers were identified with relevant data (Merchant and Pichot140 and Darvall et al. 142). The study by Merchant and Pichot140 is based on a multicentre prospective registry of patients treated with RFA, with up to 5 years of follow-up. A total of 1222 limbs were treated, of which 518 were available for follow-up at 6 months. Anatomical failure was identified using duplex imaging and defined as flow in a segment or all of treated vein, or groin reflux despite a completely occluded GSV trunk. The authors report: ‘clinical symptom improvement was seen in 70% to 80% of limbs with anatomical failures and in 85% to 94% of limbs with anatomical success from 6 months to 5 years after the radiofrequency obliteration’. From a figure showing the proportion of technical successes and failures that are symptomatic or not over the 5 years, the average proportion asymptomatic for the two groups was estimated. There is no indication that the proportion changes with time. The average proportion of technical successes with asymptomatic limbs is 89.3% and for technical failures 78.7%.
Darvall et al. 142 carried out a prospective cohort study on 246 patients having FS. 142 They examined the association between normalisation of venous refill time (a measure of technical success) and patient symptoms on the Aberdeen Varicose Vein Symptom Score (AVSS) scale 6 months after treatment. The proportion of patients with each symptom according to success or failure varies between symptoms and, in fact, only itching and swelling were significantly more common in patients with abnormal venous refill time. Overall, however, ‘relief of all symptoms was significantly more likely when there was normalisation of previously abnormal venous refill time (80% vs. 65%)’. 142
The somewhat lower rates of asymptomatic patients reported by Darvall et al. 142 than Merchant and Pichot140 may be due to consideration of more symptoms. Merchant and Pichot140 consider pain, fatigue and swelling, whereas Darvall et al. 142 also include itching, tingling, cramp, restless legs and heaviness, and are therefore likely to give a more sensitive measure of the patient being asymptomatic. However, duplex imaging, as used by Merchant and Pichot,140 to identify technical success is considered the gold standard. Venous refilling time was clearly somewhat problematical in the Darvall et al. 142 analysis as not all treated patients had abnormal venous refilling time prior to treatment, and therefore these patients had no potential for improvement on this measure. Although the two studies are measuring slightly different things, there is no clear reason for choosing one over the other. Moreover, their results are reasonably similar, so the results of both studies were combined by adding the number of patients in the two categories from both studies to give a proportion of asymptomatic for technical successes [0.88, standard error (SE) 0.014] and a relative risk of being asymptomatic if a technical failure (0.84, SE 0.048), resulting in the proportion asymptomatic for technical failures of 0.74. The former was characterised by a beta distribution based on the estimated numbers in each category, the latter with a gamma distribution based on the calculated log-normal distribution, but which prevented the implausible situation arising in probabilistic sensitivity analysis (PSA) runs of more failures being asymptomatic than successes. Note that there was no a priori reason or data to support different assumptions regarding the proportion of asymptomatic patients associated with technical treatment success or failure, so the same proportions were applied to all patients regardless of treatment. Technical failures are reassigned to asymptomatic or symptomatic at the time of failure independently of their originating state.
Adverse events of treatment are presented in Chapter 3, Characteristics of included studies. Most adverse events of treatment are relatively mild and of short duration and require no treatment. They are potentially considered in the model in terms of the loss of utility (HRQoL) associated with each treatment (see Utility values). An exception is DVT, which can occasionally lead to death. DVT was therefore considered for inclusion in the model. However, the effectiveness review shows that DVT following treatment for varicose veins is very rare (see Chapter 3, Characteristics of included studies). There were insufficient data for meta-analysis. The lifetime discounted costs and QALYs associated with DVT can be estimated from an economic analysis of diagnostic tests for DVT. 143 It reports the total lifetime QALYs and costs for persons suffering from DVT with different diagnostic testing scenarios (see Appendix 5 and table 44 in Goodacre et al. 143). Total QALYs for a person with no DVT were 11.580, and with DVT 11.558 (scenarios 9 and 10, recommended in report as maximising net financial benefit, i.e. a QALY difference of 0.022). The total costs of DVT for scenarios 9 and 10 (including all subsequent treatment) were £248 and £245 respectively. So, for example, an absolute difference of 0.5% in DVT rate following different procedures would result in a QALY difference of 0.0001 and cost difference of £1.24. Thus, the effects of DVT on the cost-effectiveness of treatments is negligible and omitted from the economic model.
Costs
National reference costs are available for varicose vein procedures, but they do not differentiate between different treatment methods. The latest available NHS activity data (2010/11) in Table 1 show that surgery is still the most common procedure (52%), although the others (EVLA, RFA and sclerotherapy) are also commonly provided. Of the total 36,000 varicose vein procedures captured in the 2009/10 national reference costs, almost 80% were conducted as day cases, the majority of which were a primary unilateral procedure without complications (84%). 52 The average cost of such procedures, inflated to 2011/12 with an inflation factor of 1.064,144 is £1155 (IQR £765–1355). 52
A total of 15 studies2,45,81,90,95,122,126,134,135,145–149 reporting costs of treatments for varicose veins were identified from the economic searches, with one further study identified in the evidence review. Several of the studies, including one from the UK (Gohel et al. 136), estimated the costs of treatment from standard charges/reimbursement costs with additional costs added for equipment and consumables particular to each treatment mode. These studies were not considered useful in informing the costs of treatment. All the 16 studies2,45,81,90,95,122,126,134–136,145–149 identified are listed in Appendix 11, together with a brief comment on the costing approach.
Two studies are primary studies which collected resource and cost data alongside RCTs in the UK (Bountouroglou et al. 90 and Subramonia and Lees134). The costs they report are presented below, together with those reported by Michaels et al. 2,150–152 from a RCT comparing traditional treatments for varicose veins in the UK. Patient characteristics and summary treatment details are show in Table 25. Note that Table 25 includes one further UK cost study which was identified in a later search (see below).
| Treatment | Net benefits at £20,000 (£) |
|---|---|
| Conservative treatment | 70,440 |
| UGFS (LA) | 76,291 |
| EVLA (LA) | 77,769 |
| RFA (LA) | 77,770 |
| Surgery (day case) | 77,778 |
| EVLA (GA) | 77,165 |
| RFA (GA) | 77,196 |
| Surgery (inpatient) | 77,020 |
The studies are quite heterogeneous in terms of varicose vein inclusion criteria, and procedures may have varied, but all were unilateral treatments, with the possible exception of Subramonia and Lees,89 where it is not stated. In all studies stripping was done under GA as a day case, but some of the other procedures (FS, EVLA) were done either under LA or the type of anaesthesia was not reported.
Table 27 shows the costs of varicose vein treatments reported in RCTs that conducted primary collection of resource and cost data in the UK. All costs have been inflated to 2011/12 prices. 144 The Michaels et al. study2 had much longer follow-up (2 years) than the other two studies (< 3 months) and the total health-care costs include retreatment. The total initial hospital costs of treatment are more similar. Each of the three studies compares a different treatment (FS, RFA and LS) with stripping.
| Item | Bountouroglou 200690 | Subramonia 2010134 | Michaels 20062 | Lattimer 2012153 | |||||
|---|---|---|---|---|---|---|---|---|---|
| FS | Surgery | RFA | Surgery | LS | Surgery | FS | EVLA | ||
| Number of patients | 30 | 30 | 47 | 41 | 41 | 36 | 54 | 56 | |
| Surgeon | 52.06 | 118.99 | 102.32 | 68.16 | |||||
| Assistant | 21.80 | 49.83 | 49.62 | 33.06 | |||||
| Anaesthetist | 0.00 | 118.99 | 102.32 | 68.16 | |||||
| Anaesthetic assistant | 0.00 | 25.42 | 36.86 | 24.56 | |||||
| Nursing | 30.26 | 69.16 | 66.24 | 44.14 | |||||
| Portering | 5.90 | 5.90 | |||||||
| Subtotal staff time | 104.11 | 382.39 | 363.25 | 243.98 | |||||
| Consumables | Stripper (surgery)/sodium tetradecyl sulphate (foam)/catheter (RFA) | 5.96 | 10.87 | 652.45 | 0.00 | ||||
| Antiembolism stockings | 22.25 | 8.90 | |||||||
| Unspecified | 59.31 | 59.31 | |||||||
| Sterile supplies | 102.41 | 102.41 | |||||||
| Anaesthetic | 3.61 | 10.63 | |||||||
| Subtotal consumables | 134.23 | 132.81 | 711.77 | 59.31 | |||||
| Theatre recovery | 50.85 | 105.93 | |||||||
| Ward time | 328.40 | 625.03 | |||||||
| Day-case ward | 250.07 | 250.07 | |||||||
| Subtotal facilities | 379.25 | 730.96 | 250.07 | 250.07 | |||||
| Ultrasound | 63.56 | 0.00 | 43.89 | 0.00 | |||||
| Medical attendance/non-protocol outpatient visits | 10.11 | 0.00 | 3.89 | 8.91 | |||||
| Subtotal miscellaneous | 73.67 | 0.00 | 47.78 | 8.91 | |||||
| Capital and overhead | 91.15 | 178.44 | 140.70 | 101.00 | |||||
| Total initial treatment hospital costs | 782.41 | 1424.60 | 1513.58 | 663.27 | 251.14 | 895.75 | |||
| Retreatment | 52.84 | 27.12 | |||||||
| Hospital admissions/visits | 0.00 | 12.56 | |||||||
| GP/practice nurse/district nurse visits | 11.31 | 23.87 | 2.09 | 2.38 | |||||
| Total health-care costs | 1524.88 | 687.14 | 306.06 | 937.80 | 230.24 | 724.72 | |||
It can be seen that there is considerable variation between the three studies2,89,90 in the reported costs of stripping, varying from £663 to £1425. In fact, only that of Michaels et al. 2 falls within the IQR of the national reference costs (£765–1355). 52 The study characteristics shown in Table 25 do not explain these variations. All the studies compared different treatments with stripping, so their costs relative to stripping can be calculated. Table 28 shows the estimated costs of varicose vein procedures, assuming the cost of stripping is the national reference cost for primary unilateral day-case procedures,52 and the relative costs for other procedures based on the data from the RCTs shown in Table 27. The validity of the assumption that the national reference cost for all procedures was reasonably representative of the costs of stripping, although accounting for only 52% of activity, was explored using the limited HES activity data (see Table 1) and the cost ratios shown in Table 28. The limitation of the HES data is that EVLA and RFA activity are combined, as is that for FS and LS. Assuming an equal split in activity between treatments for both these groups gives an estimate for the cost of stripping of £1076. The effect on the differences in costs between the newer treatments and stripping (FS +£35, RFA –£100, EVLA –£80) is insignificant in the context of their magnitude and the lack of effect on the results (see Costs).
| Procedure | Cost relative to surgery | Initial procedure cost (£) | Source |
|---|---|---|---|
| Surgery | – | 1155 | National reference costs52 |
| FS | 0.55 | 634 | Bountouroglou 200690 |
| RFA | 2.28 | 2635 | Subramonia 2010134 |
| EVLA | 2.02a | 2338 | See text below |
None of the UK RCTs, or any detailed costing studies from other countries, included EVLA. One study shows the procedure times for EVLA and RFA to be the same,88 and a survey of expert opinion also indicates that the duration of procedure and facilities required are very similar to that for RFA, the key difference being the specialist catheter kits and generators that are used for the two procedures. 154 The differential between the costs for these two items was used to estimate the cost difference between EVLA and RFA. Prices for catheter kits vary between manufacturers. Most are in the range £200–300 for optical fibre kits (including access kits – EVLA) and £460–578 for RFA catheter and access kits (2009). 154 In the clinical trials, Biolitec laser kits (Biolitec AG, CeramOptec GmbH, Bonn, Germany) were commonly used for EVLA (£255) and VNUS Closure catheters for RFA (£578), including Subramonia and Lees;134 these were the costs used in the model. Subramonia and Lees134 do not explicitly cost the RFA generator, but rather use a standard formula for calculation of overhead costs, including capital charges, of 30% direct theatre costs. 134 However, as a laser generator costs approximately twice as much as a RFA generator (£20,000 compared with £10,000),154 an allowance needs to be made for the additional capital cost when comparing the costs of the two procedures. Assuming capital costs are depreciated over 5 years, an interest rate of 3.5%, and 50 patients are treated per year, the additional capital cost for EVLA compared with RFA is £44. This cost is clearly sensitive to the assumed number of patients treated per year. The activity data in the national reference costs show that the average number of day-case unilateral procedures per unit is 151,52 so, if EVLA were to be widely adopted, 50 patients per year seems a reasonable assumption. This gives an overall cost difference between RFA and EVLA of £279 (2009/10 prices) or £297 at 2011/12 prices.
Given the limited information on the costs of EVLA in particular, the costs literature search was rerun in August 2012 to identify any additional literature. One further UK RCT was identified that collected and reported cost data for EVLA and FS. 153 These were £725 and £230 respectively (cost year unspecified, 2012?). These costs are considerably lower than those reported in the other studies (see Table 27), and also inconsistent with the mean national reference cost for varicose vein day procedures presented earlier. However, the ratio of costs of FS to EVLA at 0.31 is similar to that derived from Table 28 (0.55/2.02 = 0.27).
There are limited data on the uncertainty in procedure costs. Only one study (Michaels et al. 2) reports the SD with the mean costs. These data were used to calculate the SE of the mean of the costs for LS and stripping. Assuming the SE of the distributions of mean costs are described by gamma distributions, the mean cost distributions for LS and stripping were each sampled 1000 times to give a distribution of the cost ratio for LS compared with stripping. This had a SE of 11% of the mean ratio value, and was normally distributed. It was assumed that this distribution applied to the cost ratios of other treatments compared with stripping.
It has been assumed that patients whose treatment is an initial failure (by 1 month – determined from the time to failure distribution) are retreated with FS at least once. Sclerotherapy (FS or LS) was the most commonly reported method of short-term retreatment for incomplete occlusion in the effectiveness studies (Table 29). Two studies also report additional treatment81,103 not for unsuccessful occlusion, but for ‘residual side branches and accessory saphenous veins’. 103 This form of additional treatment is referred to here as top-up treatments. Very few studies report on further treatment within approximately 1 month of the initial procedure. All studies reporting further treatment within this time scale, whether for incomplete occlusion (retreatment) or not (top-up treatment) are shown in Table 29. It has been assumed that all of these studies are informative for top-up treatments, with zero being assumed for those cohorts for whom all retreatment was for incomplete occlusion. Note that this table differs from Table 11 in that it only includes studies that report retreatment, and not just treatment failure. In two studies (Bounteroglou et al. 90 and ElKaffas et al. 81) all treatment failures were retreated, so the numbers match those shown in Table 11, whereas in Rasmussen et al. 95 not all failures were retreated, and so the numbers are not the same. Furthermore, Table 29 includes Disselhoff et al. ,126 although the follow-up period is 6 weeks rather than 1 month (the inclusion criteria for Table 11) because of the paucity of data on top-up treatment.
| Study | Initial procedure | Time success defined | Retreatment for incomplete occlusion | n | Retreated | Top-up treatment | Top-up treatment (%) |
|---|---|---|---|---|---|---|---|
| Bountouroglou 200690 | FS (+ SJL) | 3 weeks | Additional foam | 30 | 4 | 0 | 0.0 |
| Stripping | 3 weeks | Additional liquid | 28 | 2 | 0 | 0.0 | |
| Disselhoff 2008103 | EVLA | 6 weeks | LS (n = 36) or phlebectomy (n = 1) | 56 | 37 | 66.1 | |
| Cryostripping | 6 weeks | LS (n = 33) or phlebectomy (n = 2) | 55 | 35 | 63.6 | ||
| ElKaffas 201181 | RFA | Immediate | GSV stripping with SF ligation | 90 | 6 | ||
| Post-intervention sclerotherapy | 90 | 24 | 26.7 | ||||
| Stripping | Immediate | 90 | 0 | 0 | 0.0 | ||
| Rasmussen 201195 | FS | 1 month | Foam sessions | 144 | 5 | 0 | 0.0 |
| EVLA | 1 month | Foam sessions | 144 | 0 | 0 | 0.0 | |
| Stripping | 1 month | Foam sessions | 144 | 0 | 0 | 0.0 | |
| Combined results top-up treatment | Stripping | 262 | 0 | 0.0 | |||
| FS | 174 | 0 | 0.0 | ||||
| EVLA | 200 | 37 | 18.5 | ||||
| RFA | 90 | 24 | 26.7 | ||||
| EVLA/RFA | 290 | 61 | 21.0 |
The numbers for each treatment procedure were combined, but the variation between studies in top-up treatment rates suggests differences in populations/practice. However, the derived rates do, overall, reflect expected differences, although there is clearly uncertainty in the rates. Clinical experience suggests that it is very rare for any sort of top-up treatment to be required after stripping. This is very different from the case with EVLA and RFA. If these are carried out under LA, which is the usual situation, it is almost impossible to get full clearance of all the varicose veins at the time of the initial procedure. The low top-up treatment rates in some studies may reflect a policy decision not to retreat some residual varicosities. A combined rate for EVLA and RFA was calculated as in principle the top-up treatment rate might be expected to be similar.
From Table 29 it can be seen that sclerotherapy is the most common mode of immediate retreatment and top-up treatment. It is assumed in the model that all immediate retreatment and top-up treatments are FS. Ongoing time to failure following initial failure or top-up treatment is determined in the model by the initial treatment mode.
Taking in to account top-up procedures, the total initial procedure costs used in the model are shown in Table 30.
| Procedure | Total cost (£) |
|---|---|
| Stripping | 1154.91 |
| FS | 634.29 |
| EVLA | 2471.54 |
| RFA | 2768.91 |
It is assumed the costs of retreatment itself are the same as the cost of treatment, but clinical opinion suggests that patients will also see their general practitioner (GP) on average 2.5 times, and attend two outpatient visits, including a duplex scan. The costs are shown in Table 31.
| Item | Cost/number | Source |
|---|---|---|
| GP visit cost (£) | 32.10 | PSSRU144 |
| Outpatient first attendance vascular surgery cost (£) | 172.34 | National reference costs52 |
| Outpatient second (or further) attendance vascular surgery cost (£) | 118.13 | National reference costs52 |
| Duplex scan cost (£) | 59.04 | National reference costs52 |
| GP visits, n | 2.5 | Author estimate |
| Outpatient visits, n | 2 | Author estimate |
| Duplex scans, n | 1 | Author estimate |
| Total additional retreatment cost (£) | 429.76 |
Assumptions need to be made in the model regarding the mode of retreatment following late treatment failure. Data from the trials included in the effectiveness review were extracted on retreatment mode for each initial treatment, but shows no consensus for retreatment modes, although FS was the most commonly used method (see Appendix 6). In many trials only one mode of retreatment was reported, suggesting that the choice was procedure driven rather than patient driven.
In practice, patients have a scan, the results of which determine further treatments. In terms of conventional methods, the general principle is that if there is recurrent reflux at the saphenofemoral junction (SFJ) then stripping is required, whereas for those who have reflux in the long saphenous vein without saphenofemoral incompetence or have some recurrent varicosities it is more likely that sclerotherapy, either LS or FS, would be used. The newer modalities of EVLA and RFA are possible as repeat procedures only where there is an incompetent long saphenous vein that can be treated in this way. None of the effectiveness studies reports the use of RFA for secondary procedures, but two do report the use of EVLA following stripping. 87,96 It has been assumed that 60% of retreatments are surgical procedures and 40% are FS.
Utility values
The model requires (1) the utility associated with symptomatic varicose veins; and (2) the short-term post-operative loss of utility reflecting adverse effects of each procedure. To inform these parameters a search was undertaken to identify utility values for varicose veins in the literature (see Identification of studies). This identified 975 unique references. The literature identified as being relevant to these two sets of parameters is discussed in turn.
(1) Utility of symptomatic varicose veins
Pre-operative utility was interpreted as reflecting the utility of symptomatic varicose veins. Six unique studies reported EQ-5D utility values in this population (Browne et al. ,155 Carradice et al. ,86,156 Durkin et al. ,157 Michaels et al. 2 and Norden et al. 88) and three SF-6D (Carradice et al. ,86 Disselhoff et al. 133 and Michaels et al. 2), two of which also reported EQ-5D (Carradice et al. 86 and Michaels et al. 2). Note that some of the data in Michaels et al. 2 are also reported elsewhere. 150–152 One further study reported 15D utility. 158 As the NICE methods manual159 recommends the use of European Quality of Life-5 Dimensions (EQ-5D), and there are more EQ-5D data, EQ-5D utility was used.
To derive an estimate of the utility associated with symptomatic varicose veins a meta-analysis was undertaken of all studies reporting baseline (pre-treatment) EQ-5D. Six studies2,86,88,155–157 with 1177 unique patients were included. [Note that Carradice et al. 156 report data on patients prior to EVLT, which are also included in two other papers (Carradice et al. 86,160). There are more patients in the 2010 paper,156 and so this was used. Carradice et al. 86 also has a stripping arm: data for this arm of the study were retained.] The studies included, and their reported utility values, are shown in Appendix 7. Age-independent estimates were calculated by dividing the reported values by the population average utility for the mean study population ages. 161 This gave a utility value of 0.88 (SE 0.009) for patients with symptomatic varicose veins. Asymptomatic patients are assumed to have the same utility as the general population of their age, so the state utility value is 1. In the model, age-specific utility is calculated by multiplying the state utility by the age-dependent utility.
(2) Loss of utility from treatment
There are two main issues to consider:
-
Loss of utility in the first few days following treatment due to adverse effects of treatment and, in particular, pain.
-
In the model there is no time delay between operative procedure and procedural outcome, although the literature suggests outcomes continue to improve over the first few weeks. Any differences between treatments in the time for quality-of-life benefits to be realised can be included in the model using the utility loss parameter for each treatment.
The two issues will be considered in turn.
Loss of utility in the first few days following treatment
Only one relevant study reports utility values in the first month following treatment. Carradice et al. 86 report a reduction in median EQ-5D utility 1 week after treatment of 0.040 and 0.052 for stripping and EVLA respectively (difference not statistically significant). Many studies, however, report pain on a VAS in the days following treatment, and VAS pain has been shown to be associated with EQ-5D utility in another disease area. 137 In a regression analysis of patients with low back pain, Kovacs et al. 137 found, for an absolute 1% increase in pain, a decrease of 0.035 in EQ-5D utility. Carradice et al. ,86 as well as reporting EQ-5D utility at baseline and after 1 week, report VAS pain over the same time scale (data from chart), allowing the relationship between change in EQ-5D and VAS pain to be estimated in varicose vein patients, albeit with very limited data. The reduction in EQ-5D utility for each absolute 1% increase in VAS pain was 0.00242 and 0.00274 for stripping and EVLA patients, respectively, or an average of 0.0026. This result is a different order of magnitude from that reported for back pain.
Differences in pain following treatment relative to surgery were obtained from the mixed-treatment comparison of pain data described in Chapter 3. Baseline utility loss for surgery was sourced from Carradice et al. 86 It was assumed the difference in pain endured for a mean of 14 days, an estimate based on studies which reported pain over time. Maximum disutility associated with treatment was for EVLA (– 0.04009) and minimum with RFA (–0.03878) (Table 32).
| Item | Absolute utility value | Age-adjusted utility value (age 50 years) |
|---|---|---|
| Asymptomatic varicose veins | 1.000 | 0.8831 |
| Symptomatic varicose veins | 0.8781 | 0.7755 |
| Post-operative pain associated with: | ||
| Stripping | –0.0400 | –0.0353 |
| FS | –0.0392 | –0.0346 |
| EVLA | –0.0401 | –0.0354 |
| RFA | –0.0388 | –0.0343 |
Differences between treatments in the time for quality-of-life benefits to be realised
Eight studies included in the effectiveness review report quality of life at more than one time point following treatment. 44,53,85,86,87,95,113,115 Measures include AVSS, VCSS, Aberdeen Varicose Vein Severity Score (AAVSSS), Short Form questionnaire-36 items, SF-6D and EQ-5D. Many studies indicate continuing improvement in quality of life up to 3 months following treatment53,85,86 (with some showing some lesser improvement between 3 months and 1 year44,87). However, only two of the eight studies report any differences between treatments in the rate of improvement, and the differences are limited to 1–3 weeks. 53,115 Thus, since there are no important differences between treatments in the time for quality-of-life benefits to be realised it is not considered in the model.
A summary of the absolute and age-adjusted utility values used in the model is shown in Table 32.
A summary of the model parameters is shown in Table 33.
| Parameter | Mean | Distribution | Parameter 1 | Parameter 2 | Source |
|---|---|---|---|---|---|
| Procedure costs | |||||
| Cost: stripping (£) | 1154.91 | Normal | 36.03 | National reference costs52 | |
| Cost ratio: FS/stripping | 0.55 | Normal | 0.06 | Bountouroglou 200690 | |
| Cost ratio: LS/stripping | 0.28 | Normal | 0.03 | Michaels 20062 | |
| Cost ratio: EVLA/stripping | 2.02 | Normal | 0.22 | See Costs | |
| Cost ratio: RFA/stripping | 2.28 | Normal | 0.25 | Subramonia 2010134 | |
| Proportion patients requiring top-up treatment following | |||||
| Stripping | 0.00 | Meta-analysis | |||
| FS | 0.00 | Meta-analysis | |||
| EVLA | 0.19 | Beta | 37 | 163 | Meta-analysis |
| RFA | 0.27 | Beta | 24 | 66 | Meta-analysis |
| EVLA/RFA | 0.21 | Beta | 61 | 229 | Meta-analysis |
| Total costs of treatment | |||||
| Stripping | £1154.91 | Calculated from above | |||
| FS | £634.29 | Calculated from above | |||
| EVLA | £2471.54 | Calculated from above | |||
| RFA | £2768.91 | Calculated from above | |||
| Costs associated with retreatment | |||||
| GP visit cost | £32.10 | Normal | 3.21 | PSSRU144 | |
| Outpatient first attendance vascular surgery cost | £172.34 | Normal | 7.87 | National reference costs52 | |
| Outpatient second (or further) attendance vascular surgery cost | £118.13 | Normal | 5.90 | National reference costs52 | |
| Duplex scan cost | £59.04 | Normal | 1.59 | National reference costs52 | |
| GP visits, n | 2.5 | Gamma | 25 | 0.10 | Author estimate |
| Outpatient visits, n | 2 | Gamma | 25 | 0.08 | Author estimate |
| Duplex scans, n | 1 | Fixed | Author estimate | ||
| Total additional retreatment cost | £429.76 | Calculated from above | |||
| Utility | |||||
| Utility symptomatic | 0.88 | Beta | 1239 | 172 | Meta-analysis |
| Surgery baseline disutility post-operative | –0.040 | –Beta | 740 | 17,753 | Carradice 201186,96 |
| Disutility for post-operative difference in pain score of 1/10 | –0.0252 | –Beta | 694 | 26,851 | Carradice 201186,96 |
| Duration pain (days) | 14 | Normal | 14 | 2 | Author estimate |
| Pain scores (VAS) relative to surgery | |||||
| FS | –0.81 | NA: individual samples output from network meta-analysis | Meta-analysis: see Chapter 3, Pain | ||
| EVLA | 0.09 | ||||
| RFA | –1.26 | ||||
| Disutility FS | –0.03922 | Calculated from above | |||
| Disutility EVLA | – 0.04009 | Calculated from above | |||
| Disutility RFA | – 0.03878 | Calculated from above | |||
| Other | |||||
| Time to retreatment after failure | 0.50 | Gamma | 36 | 0.01 | Author estimate |
| Retreatment mode distribution | 0.60 | Beta | 60 | 40 | Author estimate |
| Proportion asymptomatic, technical success | 0.88 | Beta | 441.31 | 59.26 | Merchant 2005,138 Darvall 2010142 |
| Relative risk asymptomatic technical fails/success | 0.84 | Gamma | 309.05 | 0.0027 | Merchant 2005,138 Darvall 2010142 |
Note, individual samples of correlated Weibull parameters for time to failure were output from the network meta-analysis, and used in the model to predict time to failure for each individual entering the model. Figure 9 shows the time to failure curves for the different treatments.
Results
The results of the PSA are presented first as they are considered the most reliable as they take into account the distribution of the uncertainty in the model parameters, which is important particularly for the survival distributions, which are skew. The deterministic results are presented later for the purpose of univariate sensitivity analysis only. The results of the PSA with costs and QALYs discounted at a rate of 3.5% are shown in Table 34.
| Procedure | Discounted | Incremental | ICER (£) | ||
|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | ||
| Stripping | 1334 | 8.0347 | – | – | – |
| FS | 804 | 8.0362 | – 530 | 0.0015 | NAa |
| EVLA | 2637 | 8.0372 | 1302 | 0.0025 | 518,462 |
| RFA | 2952 | 8.0359 | 1617 | 0.0012 | 1,352,992 |
Although there is an element of retreatment, the total costs of treatment primarily comprise the initial treatment cost, and are similarly ordered, with RFA the most expensive procedure and foam the least costly option. All the novel treatments result in more QALYs than stripping at 10 years, but the QALY differences between stripping, EVLA and RFA are negligible: equivalent to less than a day in full health for EVLA compared with stripping.
Foam sclerotherapy is less costly than stripping and marginally more effective, and can thus be said to dominate stripping. The ICERs for EVLA and RFA in comparison with stripping show they are not cost-effective at usually accepted levels. 159 EVLA dominates RFA, as can be seen from a plot of the mean costs and QALYs of each treatment (Figure 10).
FIGURE 10.
Mean costs and QALYs at 10 years.
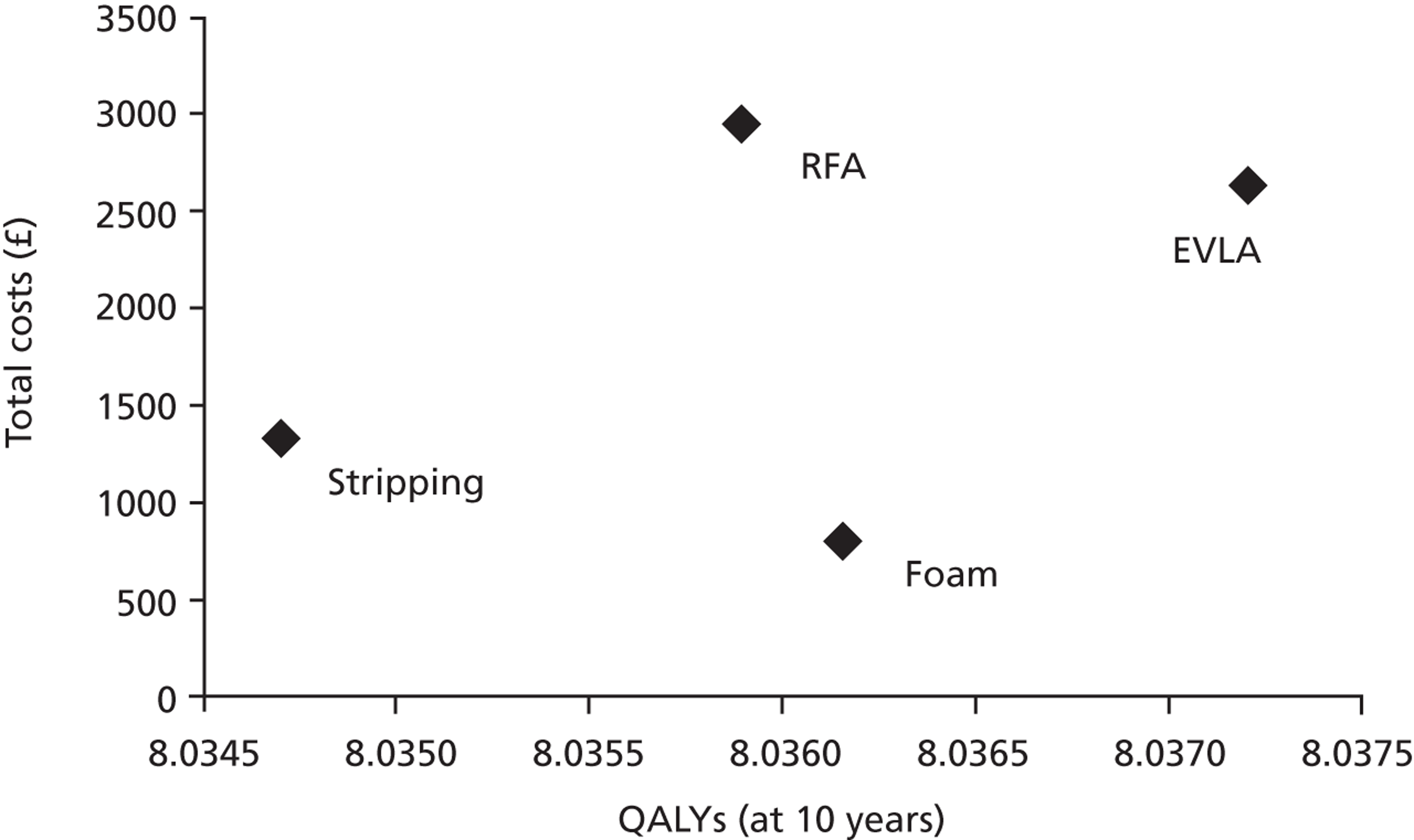
Given the negligible differences in QALYs between the treatments, the incremental net benefits primarily reflect the total cost differences between them (Table 35). At thresholds between £20,000 and £50,000 foam is the most cost-effective treatment, with a small probability of error.
| Procedure | MAICER = £20,000 | MAICER = £30,000 | MAICER = £50,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Net benefit (£) | Incremental net benefit (£) | Probability cost-effective (%) | Net benefit (£) | Incremental net benefit (£) | Probability cost-effective (%) | Net benefit (£) | Incremental net benefit (£) | Probability cost-effective (%) | |
| Stripping | 159,359 | – | 2 | 239,706 | – | 3 | 400,400 | 8 | |
| FS | 159,919 | 560 | 99 | 240,281 | 574 | 97 | 401,004 | 603 | 92 |
| EVLA | 158,108 | –1252 | 0 | 238,480 | –1227 | 0 | 399,224 | –1176 | 0 |
| RFA | 157,766 | –1593 | 0 | 238,125 | –1581 | 0 | 398,843 | –1557 | 0 |
Univariate sensitivity analysis
The full results of the univariate sensitivity analysis are shown in Appendix 12. Key results are presented and discussed in this section. As the analyses shown in Table 35 and Appendix 12 indicate, the results are not sensitive to most model parameters when varied between their IQRs.
The results for FS are, however, sensitive to the time horizon of the model. The loss of utility associated with post-operative pain varies for the different procedures, and the time to failure curves cross, resulting in the incremental QALYs evolving over time, as shown in Table 36.
| Model time span (years) | Incremental costs (£) | Incremental QALYs | ICERS (£) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FS | EVLA | RFA | FS | EVLA | RFA | FS | EVLA | RFA | |
| 2 | –515.12 | 1307.98 | 1608.95 | 0.0001 | 0.0008 | 0.0017 | NA | 1,696,843 | 962,673 |
| 5 | –516.61 | 1300.92 | 1603.04 | –0.0006 | 0.0030 | 0.0033 | NA | 437,325 | 490,390 |
| 10 | –522.86 | 1297.28 | 1597.67 | –0.0010 | 0.0068 | 0.0061 | NA | 190,348 | 264,055 |
| Life | –537.10 | 1301.09 | 1593.03 | 0.0097 | 0.0094 | 0.0132 | NA | 138,172 | 120,403 |
Note that the deterministic analysis shows FS resulting in fever QALYs than surgery, contrary to the results of the probabilistic analysis previously discussed. This apparent discrepancy is due to the skewness of the time to failure distributions combined with the small difference between the treatments. Thus, the analysis is useful only for exploring changes in the economic results with the model time span, rather than definitive results for the different scenarios. For EVLA and RFA the incremental QALYs are greater and the costs lower with increasing time span as their failure rates are lower than for stripping (hazard ratio at 1 year is 0.77 for EVLA and 0.93 for RFA); therefore, the ICERs are lower the longer the model time horizon, but even run for lifetime the ICERs do not approach £30,000. RFA results in less post-operative pain than EVLA, so RFA results in more QALYs at 2 years than EVLA, but by 10 years EVLA has overtaken RFA because of lower failure rates. For FS the picture is more complex. The pain associated with treatment is lower than for stripping, resulting initially in higher QALYs. However, the rate of failure in the first few years is higher than for stripping (hazard ratio at 1 year is 1.02 for FS; see Figure 4), potentially resulting in fewer QALYs for intermediate model time spans. In the long term (between 10 years and life) FS has a lower failure rate than stripping and leads to a small QALY gain.
Sensitivity analysis on disutility associated with treatment showed that only the results for FS in comparison with stripping were sensitive to this parameter at 10 years, due to the incremental QALYs changing from negative with mean FS disutility to positive at the upper IQR of the distribution. The sensitivity of the results to treatment disutility at 2 years is shown in Table 37.
| Scenario | Disutility associated with treatment | Incremental QALYs | ICERS (£) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stripping | FS | EVLA | RFA | FS | EVLA | RFA | FS | EVLA | RFA | |
| Baseline | –0.0400 | –0.0392 | –0.0401 | –0.0388 | 0.0001 | 0.0008 | 0.0017 | NA | 1,696,843 | 962,673 |
| Lower IQR | – | –0.0403 | –0.0411 | –0.0398 | –0.0009 | –0.0002 | 0.0007 | NA | NA | 2,391,758 |
| Upper IQR | – | –0.0382 | –0.0391 | –0.0378 | 0.0012 | 0.0018 | 0.0055 | NA | 744,241 | 292,422 |
Uncertainty in the disutility associated with treatment is sufficient to affect whether the QALY difference with stripping is positive or negative for both FS and EVLA with a time horizon of 2 years, the typical length of follow-up of the studies included in the effectiveness review. However, the uncertainty affects only the decision for FS, as for EVLA the ICER is considerably greater than a threshold of £30,000 when the incremental QALYs are positive.
Also worth consideration is the role of top-up treatments in the additional costs of EVLA and RFA in comparison with stripping. The percentage of patients who required top-up treatment was 21% for EVLA and RFA and zero for FS and stripping, thereby adding an additional £134 to the cost of the former treatments. However, if a zero differential in the requirement for top-up treatments is assumed, the incremental costs for EVLA and RFA still result in ICERs considerably beyond the usually acceptable threshold.
Endovenous laser ablation and RFA are relatively novel treatments and in future their costs may fall relative to stripping. With expected QALYS slightly higher for EVLA and RFA than for stripping, then, if the additional costs of EVLA and RFA were no more than £50 and £24, respectively, relative to stripping they would be considered cost-effective at a threshold of £20,000.
Discussion
The analysis shows that any differences in benefits (QALYs) between the different procedures are negligible, but marginally favour the novel treatments relative to stripping. The time to treatment failure curves sourced from the mixed-treatment comparison described in Chapter 3, Quantitative analysis are all very similar. Disutility associated with post-operative pain, although not severe and limited to a few days' duration, affects the results in the short term (2 years), demonstrating the limited effects of time to failure on differential QALYs. There are differences in treatment costs and, although these are somewhat uncertain, with little differences in QALYs the incremental net benefits are primarily driven by costs. Our central estimate is that FS costs £530 less than stripping, and is marginally more effective, with a probability of being the most cost-effective treatment above 90% for willingness-to-pay thresholds in the range £20,000–50,000. This result is sensitive to the model time horizon. With FS having a higher failure rate (initially) than stripping, a shorter time horizon may result in fewer QALYs than stripping. With a short model time horizon (2 years) the result is also sensitive to the disutility associated with treatment. This parameter was derived from the mixed-treatment comparison of reported pain at approximately 10 days (see Table 22). By 10 days post-operative pain has already subsided, and therefore the analysis may not fully reflect differences between the treatments. Also the relationship between post-operative pain and utility was based on limited data. 86 However, the best possible estimate of these parameters has been used given the available data.
Endovenous laser ablation and RFA both cost more than surgery, and with very little difference in QALYs they cannot be considered cost-effective at the usual threshold of £20,000–30,000,159 a result that is robust to parameter variation and model time horizon. If their costs approach that for surgery they would be considered cost-effective.
There is uncertainty in the cost differentials between treatments, and in fact these are likely to vary with setting, and may vary over time. However, the differences in clinical effectiveness (time to recurrence, post-operative pain) are small. Threshold analysis shows that the additional costs of EVLA and RFA would have to be no more than £50 and £24, respectively, to be considered cost-effective at a threshold of £20,000.
In the model QALYs are determined by initial disutility associated with treatment and treatment failure. Treatment failure is not assumed to be necessarily symptomatic, but for those who are they are assumed to have a lower utility for a period of 6 months prior to retreatment. There is also a further disutility associated with the retreatment procedure, as for the initial procedure. Asymptomatic and symptomatic utility is assumed to be the same following all treatments. There is no direct evidence of relevant differences in utility following treatment. Nordon et al. 88 report the same increase in utility from baseline for EVLA and RFA at 3 months, and Carradice et al. 86 report the same utility for EVLA and surgery patients preoperatively, at 1 week and 1 year following treatment.
However, the mixed-treatment comparison of VCSS scores at approximately 1 year indicates slightly lower VCSS scores (i.e. less severe symptoms) in FS patients than in patients treated with stripping, despite a higher failure rate at this time (see Table 21). Studies reporting analyses of the relationship between disease-specific quality of life in varicose veins and generic measures have mixed results. Shepherd et al. 139 and Kahn162 found poor correlation between measures [AVVQ, Specific Quality-of-life and Outcome Response–Venous (SQOR-V) and SF-12 for the former, Venous Insufficiency Epidemiological and Economic Study (VEINES)-QOL (quality of life), VEINES-sym (symptoms) and SF-12 for the latter]. One study does report a significant relationship between VCSS and EQ-5D in a multivariate model which also included CEAP score, AVVQ and population characteristics. For a unit decrement in VCSS there is an increase in EQ-5D of 0.02. 163 If this relationship holds, the QALY difference between FS and stripping may be greater than shown by the model results, but does not change the conclusion from the baseline results as FS dominates stripping. It potentially does affect the QALY difference with surgery with shorter model time scales, and would mean that FS results in more QALYs than surgery irrespective of the time scale. The differences in the VCSS scores between EVLA and RFA with stripping were much smaller than for FS, and their inclusion does not change the cost-effectiveness of these treatments.
The model results are consistent with other studies in finding that QALY differences between treatments are very small. Only one relevant study was identified that reported incremental QALYs from trial data. 133 It found a difference of 0.01 QALYs between EVLA and cryostripping 2 years after treatment. In the modelling analysis by Gohel et al. 136 the maximum relevant difference in QALYs at 5 years was 0.115 between day-case surgery and FS. 136 However, in other respects the results of this model are different from those of Gohel et al. 136 Gohel et al. 136 estimated the costs of treatments from basic units of resource (day case, outpatient, equipment costs) and reports day-case surgery to be more costly than any of the novel treatments, contrary to more recently published cost studies showing the costs of EVLA and RFA to be greater than those for surgery. 90,134 Gohel et al. 136 also find surgery to be more effective than the novel treatments, on the basis of much more limited effectiveness data than used in the current analysis.
The strength of the model described in this report is that the treatment failure rates are derived from a comprehensive systematic review and meta-analysis using mixed-treatment comparison. Furthermore, the analysis of effectiveness allowed the shapes of the time to failure curves to vary, thereby avoiding any assumption about indefinite treatment effects. Nevertheless, the limitations of the failure data previously described are recognised, as well as the limited evidence of differences between treatments in post-procedure utility.
The economic analysis was undertaken from a UK NHS perspective. The effectiveness review included time to return to work or usual activities, which showed this to be quicker for FS and RFA than for stripping, and possibly also quicker for EVLA. This means that from both an NHS and a societal perspective FS is the most cost-effective option.
Chapter 5 Assessment of factors relevant to the NHS and other parties
This assessment of the currently available evidence suggests there is little to choose between the minimally invasive techniques in terms of efficacy or cost, and each offers a viable, clinically effective and cost-effective alternative to stripping.
There are a number of issues that need to be considered in the interpretation of the evidence and implementation of the findings in clinical practice. Varicose vein treatment by conventional surgery would appear to be cost-effective within the usual criteria used in the NHS. 2 Despite this, the perceived low priority of varicose veins has resulted in many commissioners introducing limitations on its availability,164,165 which may explain the recent reductions in varicose vein activity in England and Wales. This also means that the population who are receiving treatment are likely to be those with more severe symptoms or complications, particularly skin changes, who were less well represented in the clinical trials. Those people with varicose veins recruited to clinical trials will have been suitable for more than one technique, whereas there are likely to be a further group of patients who are less suitable for EVLA or RFA because of the size, depth, tortuosity or partial occlusion of the GSV.
The new techniques require routine use of duplex scanning to identify the vein and assess suitability for the treatments, and current practice in this regard is variable. Where this is not routine it may be an additional cost associated with the new treatments. Most of the studies provide results based on the technical assessment of recurrent reflux using Doppler studies. However, follow-up in this way is not routine in clinical practice, and the relationship between technical and symptomatic recurrence is based on relatively sparse data and the assumption that this is consistent between treatments.
The new treatments have additional implications for training and the availability of equipment. It is possible that there are learning curve effects because the technology is continuing to develop and there are various options for some aspects of the treatment, such as timing and dosage of energy exposure, which are continuing to be investigated. Some of the earlier studies used devices or techniques that have already been superseded and it is possible that greater experience and more widespread adoption will result in improved outcomes and reduced complications. However, there may also be issues of the availability of the necessary skills and equipment, with the resource implications of providing training in the new methods.
In view of the small absolute differences in costs and outcomes between the techniques, other issues of importance to patients, such as the less invasive nature of some options, the opportunity to avoid larger scars and general anaesthesia, and potential reduction in recovery time or earlier return to work, may be important in the choice of procedure.
Chapter 6 Discussion
Statement of principal findings
The systematic review identified a total of 34 trials (54 papers) for the assessment of clinical effectiveness. No studies were identified comparing any minimally invasive technique with conservative management. Approximately half of the included studies reported inadequate randomisation, allocation concealment, between-group comparability or intention-to-treat analyses.
The reported proportion of initial failures was very small for all techniques. Where reported, retreatment consisted of stripping and ligation for RFA, or further sessions of sclerotherapy for FS or stripping. Where appropriate data were available, a network meta-analysis was performed for technical recurrence, VCSS and pain, to compare each intervention (EVLA, RFA and FS) with the common comparator of conventional surgery (ligation and stripping).
The risk of experiencing a technical recurrence of varicose veins over time was lower for EVLA (hazard ratios: 6 months 0.70; 1 year 0.77; 2 years 0.84) and RFA (hazard ratios: 6 months 0.92; 1 year 0.93; 2 years 0.94) than for ligation and stripping. The risk of experiencing a technical recurrence of varicose veins over time was initially higher for FS (hazard ratios: 6 months 1.12; 1 year 1.02) than for ligation and stripping, but lower after 2 years (hazard ratio 0.92). The estimates of absolute risk of technical recurrence are presented in Figure 4. There was some indication of heterogeneity in the effect of treatments between studies, although this was not extreme. An examination of potential treatment effect modifiers would normally be explored using meta-regression. However, there was insufficient information about these modifiers to do so on this occasion.
Very few studies reported symptomatic recurrence or reoperation rates beyond 1-month follow-up. Meta-analysis found lower post-intervention VCSS for both FS and EVLA than for stripping, but a slightly higher score for RFA than for stripping. There was significantly lower post-operative pain for RFA than for stripping, as well as reduced pain for FS and a slightly increased level of pain for EVLA than for stripping. Although pain is part of the VCSS, this scale is measuring many additional components also and is used at much longer follow-up, which would explain the difference in the results for RFA on these two measures.
Where the outcome was reported, significantly quicker return to work or normal activity was reported by all relevant studies for both FS and RFA than for stripping. Studies comparing EVLA and stripping reported either no difference or more rapid return to work for participants in the EVLA trial arm. There were no consistent or statistically significant differences between any of the interventions in terms of complications or adverse events. The FS treatment arms of trials were associated with a relatively higher incidence of DVT than any other intervention, but the number of such events was very small. Other important outcomes such as ulceration were rarely reported.
Six reviews,46–48,50,51,166 a clinical practice guideline26 and a cost-effectiveness analysis136 have been published since 2007 on this topic. One meta-analysis, by Leubke et al. ,47 evaluated RFA alone and that by Jia et al. 46 evaluated the efficacy and safety of FS alone. The meta-analyses published by Murad et al. ,50 Luebke et al. 48 and van den Bos et al. 167 considered all three principal minimally invasive techniques, as well as LS, but found data only from 9, 12 and 8 relevant RCTs, respectively, with substantial duplication of included studies. Large numbers of observational studies, and some non-comparative studies48,50,166 were also included in the analyses.
Jia et al. 46 reported that FS was less effective than stripping but more effective than LS, albeit with the acknowledgement that the available data had limitations. This meta-analysis also reported the absence of any significant side effects with FS. Leubke et al. 48 and van den Bos et al. 166 reported that EVLA and RFA were more effective than either surgery or FS. Murad et al. 50 reported that surgery was more effective than both LS and endovenous interventions for preventing recurrence.
All of these results differ from the findings of this report that FS, EVLA and RFA offer potentially equally effective alternatives to stripping and, in the case of FS, a cost-effective alternative also. This difference can be explained by the inclusion of much more RCT evidence in the present report (approximately three times as many relevant RCTs than any previous review, despite broader criteria in the majority of the previous reviews), the exclusion of non-RCT and non-comparative evidence, and the analysis methods used.
Nesbitt et al. 51 reported no significant differences between EVLA and RFA compared with stripping based on five RCTs only (all included in this review, and only with short-term follow-up). This Cochrane review included studies evaluating interventions for the GSV only and excluded trials with combined interventions (e.g. FS and ligation). However, further relevant RCTs might have been included, such as Rabe et al. ,121 Hinchcliffe et al. ,83 Gale et al. ,53 Goode et al. 55 and Rasmussen et al. 95 The recently published (2011) clinical practice guidelines of the Society for Vascular Surgery and American Venous Forum also recommend EVLA, RFA and FS as effective alternatives to stripping and other modalities, but cite only a small number of RCTs with short-term follow-up, and one or two of the reviews cited here. 26 None of the previously published reviews or analyses acknowledged the limitation presented by exclusively technical recurrence, rather than symptomatic technical recurrence as an outcome.
There is limited literature on the cost-effectiveness of novel varicose vein treatments. Two analyses were carried out alongside clinical trials, but only one of these was a cost–utility analysis. In a comparison of EVLA with cryostripping it reports EVLA achieving an additional 0.01 QALYs for an additional cost of €323 (ICER €32,265) (Disselhoff et al. 133). Recently a cost-effectiveness analysis by Gohel et al. 136 has been published of a model comparing treatments for varicose veins. Gohel et al. estimated the costs of treatments from basic units of resource (day case, outpatient, equipment costs) and reports day-case surgery to be more costly than any of the novel treatments, contrary to more recently published cost studies showing the costs of EVLA and RFA to be greater than those for surgery. Gohel et al. also find surgery to be more effective than the novel treatments, on the basis of much more limited effectiveness data than used in the current analysis.
The long-term risk of a technical recurrence is less for all the minimally invasive treatments than for stripping, although the time to treatment failure curves are quite similar. The cost-effectiveness model shows that any differences in benefits (QALYs) between the different procedures are negligible, but marginally favour the novel treatments relative to stripping. Disutility associated with post-operative pain, although not severe and limited to a few days' duration, affects the results in the short term (2 years), demonstrating the limited effects of time to failure on differential QALYs. There are differences in treatment costs, however, and, with little differences in QALYs, incremental net benefits are primarily driven by costs. Our central estimate is that total FS costs are £530 less than stripping, and it is marginally more effective (+ 0.0015 QALYs), with a probability of being the most cost-effective treatment above 90% for willingness-to-pay thresholds in the range £20,000–50,000. This result is, however, sensitive to the model time horizon.
Endovenous laser ablation and RFA both cost more than stripping (total costs +£1302 and + £1617, respectively, cost differences primarily from initial procedure costs) and show very little difference in QALYs (+ 0.0025 and + 0.0012, respectively) compared with stripping. With ICERs of £518,000 and £1,353,000, respectively, they cannot be considered cost-effective at the usual threshold of £20,000–30,000, results that are robust to parameter variation and model time horizon. There is considerable uncertainty in the cost differences between treatments arising from different reported costs of the procedures, and in fact these are likely to vary with setting, and may also vary over time. Threshold analysis shows that the additional total costs of EVLA and RFA would have to be no more than £50 and £24, respectively, to be considered cost-effective at a threshold of £20,000.
Strengths and limitations of the assessment
The clinical effectiveness review identified almost three times the number of relevant RCTs of any previously published review. All stages of the review were conducted independently by at least two reviewers.
A benefit of the analysis of the technical recurrence data was that it did not assume proportional hazards; this is particularly important in terms of the assessment of cost-effectiveness as it does not assume that any treatment effect continues indefinitely. There were several limitations associated with the analysis of the technical recurrence data. In general, studies did not account for all patients at each follow-up time so that the technical recurrence response rates did not increase monotonically. Although authors often reported that theirs was an intention-to-treat analysis, some authors reported results as the number of events out of the number of patients randomised whereas others reported results as the number of events out of the number of patients for which there were data. Some patients were assessed for their varicose veins in both limbs and results were often reported by limb rather than by patient. However, data on each limb within patients are correlated and an analysis of the patient-level data should have acknowledged this correlation rather than treating observations as independent. Our analysis used aggregate data and we were not able to adjust for the fact that the observations were not independent.
A Weibull model was fitted to the data, which effectively assumes that all patients will have a technical recurrence at some stage in the future; in practice, it is likely that a proportion of patients would never have a technical recurrence, and that a more appropriate model would be a ‘cure’ model in which the time to recurrence is conditional on not being ‘cured’, although it was not possible to do this with the data that were available. Some studies presented response rates at more than one time, which meant that we could estimate more than one parameter (i.e. the shape and scale parameter in the Weibull distribution). However, our model assumed that the observations were independent, which may have led to an overestimation of uncertainty.
The findings of this report should be verified with evidence from a well-conducted RCT with independent unilateral and bilateral data, longer follow-up and appropriately defined outcome measures. For the purposes of a more meaningful comparison of effectiveness and costs, trial arms should have equally experienced surgeons and comparable groups in terms of CEAP score and follow-up interventions, and report all details of ‘top-up’ treatments, reoperations and symptomatic as well as technical recurrence. The relative efficacy of the interventions compared with stripping might be underestimated if surgeons are insufficiently experienced in performing the more recent minimally invasive techniques. 167
Uncertainties
All of the effectiveness analyses presented here used only technical rather than symptomatic recurrence data, so the true proportion of treated individuals who are likely to present with symptoms of recurrence requiring retreatment is not certain. The rates of technical recurrence reported here are therefore higher than those likely to be encountered in clinical practice. Although rates of symptomatic recurrence relative to technical recurrence were identified from the literature and used in the model, it was assumed that the rate of symptomatic recurrence was the same across all treatment modalities, which might not be the case. The majority of trials were at risk of either selection bias (inadequate randomisation or allocation concealment) or attrition bias (inadequate intention-to-treat analyses), which adversely affect the internal validity of the studies and their findings. The results of individual studies and the review are therefore affected by uncertainty on account of the relatively high risk of bias present. However, despite this, the findings were largely consistent (i.e. network meta-analysis found no statistically significant differences in the technical recurrence outcome between the principal treatment modalities of EVLA, RFA, FS and stripping).
Those people with varicose veins recruited to the clinical trials assessed in this report will have been suitable for more than one technique, whereas there is likely to be a further group of patients who are less suitable for EVLA or RFA owing to the size, depth, tortuosity or partial occlusion of the GSV. The relative effectiveness of the treatments considered here is not known for this group. The issue of bilateral varicose veins has not been considered as there were no studies specifically addressing this point, but the potential for bilateral surgical treatment under a single anaesthetic may alter the balance between costs and benefits in these cases. Indeed, most published trial data relate to unilateral procedures, so there is a question as to the relative effectiveness and cost-effectiveness of simultaneous and sequential procedures for bilateral disease. There were also no data on progress to ulceration from the included trials. This would require longer trials with placebo or non-intervention groups to determine whether or not treatments reduce the likelihood of ulceration. We did not identify any such trials.
The analyses compared minimally invasive treatments with the principal comparator currently provided in the NHS (i.e. stripping). No formal analysis was undertaken to compare these techniques with the less frequently performed comparators of LS and conservative management. This is because no head-to-head trials were identified comparing the minimally invasive techniques with conservative management, and only three trials compared FS with LS, which does not represent a closed network. However, the three relevant included trials all found that FS was superior to LS and a previous large trial has suggested that stripping is more effective than either LS or conservative management but costs more, although this was within normal bounds of cost-effectiveness. 2 There is also a potential clinical issue that might explain the absence of trials comparing conservative management and other techniques (i.e. the patient population prepared to accept randomisation to conservative management is not representative of the population who are likely to choose or want surgery or the new modalities). 2 This makes the conduct of trials directly comparing such interventions difficult. Nevertheless, in the absence of a specific analysis of clinical effectiveness and cost-effectiveness for these comparators, the actual effectiveness of the minimally invasive techniques relative to these less frequently performed interventions is uncertain.
The model was not sensitive to most model parameters. The results are dependent on the time to survival curves derived from the meta-analysis of results, which was limited by the quality of the data and the considerable uncertainty in the failure rate hazard ratios. The results for FS are sensitive to the time horizon of the model, owing to its initially higher failure rate than surgery and, for short model time horizons (2 years), also sensitive to the initial disutility associated with treatment. The results for EVLA and RFA are more robust as their incremental costs in comparison with stripping mean that they are not cost-effective in any scenario. There is considerable uncertainty in the cost differences between treatments. Treatment costs primarily comprised the initial procedure costs and, in fact, these are likely to vary with setting, and may vary over time. However, the difference in QALYs between treatments was negligible, so the additional costs of EVLA and RFA would have to be no more than £50 and £24 relative to stripping, respectively, to be considered cost-effective at a threshold of £20,000.
Differences in QALYs in the model were determined by disutility associated with treatment (reflecting post-operative pain) and failure which might result in a period of being symptomatic and retreatment. There is little evidence in the literature on the utility of patients following varicose vein treatment, and what there is shows no difference between treatments. However, the meta-analysis of VCSS scores suggests that at 1 year patients treated with FS may have fewer symptoms than patients treated by other methods, despite a higher failure rate at this time. This may mean that the incremental QALYs for FS were underestimated, but, because FS dominates stripping in the baseline model, then the result does not change. The VCSS scores for EVLA and RFA were very close to surgery, and at the current costs for these treatments minor changes in QALYs would not affect the results. However, the VCSS score was slightly lower for EVLA than stripping, so, if the costs of EVLA do fall, evidence of higher post-treatment utility for EVLA in comparison with stripping would affect its cost-effectiveness.
Other relevant factors
The new treatments have additional implications for training and the availability of equipment. It is possible that there are learning curve effects, the technology is continuing to develop and there are various options for some aspects of the treatment, such as timing and dosage of energy exposure, which are continuing to be investigated.
Other than the differences in costs and outcomes between the techniques, there are additional issues of importance to patients. These include the less invasive nature of the techniques being assessed here, the opportunity to avoid larger scars and general anaesthesia, and the potential reduction in recovery time or earlier return to work. Patient preferences might therefore be an issue in terms of the choice of procedure if the relative cosmetic and time merits of each treatment are explained by a patient's vascular surgeon. 168
Finally, the NHS may have broader economic considerations, which might shape policy decisions based on the clinical need for treatment of uncomplicated varicose veins.
Chapter 7 Conclusions
Implications for service provision
This assessment of the currently available evidence suggests that there is little to choose between the minimally invasive techniques in terms of efficacy, and each offers a viable, clinical alternative to stripping. Based on the data reviewed, only FS offers a cost-effective alternative to stripping. Training and experience in the minimally invasive techniques might be required before more substantial, relative clinical benefits are apparent.
Suggested research priorities
The results of individual studies and the review are affected by uncertainty on account of the relatively high risk of bias present. These sources of bias need to be minimised. Additional trials are indicated when the cost of conducting such trials is offset by the value of the information in reducing the decision uncertainty.
Any further trials should measure and report outcomes in a standardised format, which would permit more efficient pooling of their results (e.g. mean and SD of all validated and commonly used and recommended measures26) such as VCSS and EQ-5D.
Larger and longer trials are also indicated to offer sufficient data and follow-up, beyond the standard duration of published trials of 1–2 years, to better judge rates of recurrence, retreatment and progress to important outcomes such as ulceration. The required sample size will depend on the outcomes to be measured. Some consideration should be given to the definition of the patient population to ensure that the inclusion criteria reflect the characteristics of patients who present in practice but have been excluded from existing, published trials. Trials with a non-intervention or conservative management arm, as well as intervention arms, are also indicated to give the fullest picture of the clinical effectiveness and cost-effectiveness of the interventions. Possible subgroups for analysis in future trials might be based on symptoms and severity (e.g. those with skin changes or cosmetic problems), as well as anatomical features (e.g. SSV reflux vs. LSV reflux or bilateral veins).
Any future trials should be analysed according to the way in which patients were randomised to treatments and acknowledge the patient as the ‘block’ or experimental unit. Previous trials have often considered legs to be the experimental unit, whereas it is well known that outcomes measured on the same patient will be correlated. An appropriate analysis would treat the data on legs as bivariate. It should also take into account the longitudinal repeated measure nature of the data and also appropriately deal with missing data and patients who are lost to follow-up.
Trial authors should also report both technical and symptomatic recurrence to permit assessment of likely retreatment rates and costs, and utilise surgeons with adequate experience of the minimally invasive techniques, if the comparison with stripping (currently the most common procedure performed by all surgeons) is to be internally valid. 167 In addition, most trial data currently relate to unilateral procedures, so there is a question as to the relative effectiveness and cost-effectiveness of simultaneous and sequential procedures for bilateral disease.
Procedure costs reported in four UK RCTs are quite variable, and the national reference costs do not distinguish between procedures, and therefore there is uncertainty in the costs used in the cost-effectiveness analysis. However, with the clinical effectiveness data currently available showing very limited differences between procedures the costs of EVLA and RFA would need to be very close to those for stripping (no more than £50 and £24 more expensive, respectively) to be cost-effective. Only if new clinical evidence becomes available showing greater differences between treatments, or the cost differential between EVLA/RFA and stripping approaches the reported threshold costs, would a cost study be worthwhile. As costs are likely to vary between Hospital Trusts, a survey of several will yield more accurate estimates of the expected costs of the different procedures than costing studies alongside RCTs.
Future reviews should also make an assessment of how far all of these requirements are satisfied by the evidence base.
Acknowledgements
The authors would like to thank the advisors that have contributed to the project: Mr Dominic Dodd (consultant vascular surgeon, Sheffield Teaching Hospitals) for his advice at an early stage in the project; Dr Sarah Davis (Deputy Director of the NICE Decision Support Unit); Mr Edward Mulkern (consultant vascular surgeon, Sheffield Teaching Hospitals); and Abdullah Pandor [Senior Research Fellow, School of Health and Related Research (ScHARR), University of Sheffield] for their comments on a draft of this report.
The authors would also like to thank Andrea Shippam, Programme Administrator, ScHARR, for her help in preparing and formatting the report.
Contributions of authors
Christopher Carroll led the review and was responsible for managing the project.
Silvia Hummel conducted the review of the economic literature and the economic modelling.
Joanna Leaviss and Emma Everson-Hock contributed to the review.
John Stevens and Shijie Ren provided statistical support and undertook the meta-analyses.
Matt Stevenson oversaw the modelling and reviewed the final report.
Anna Cantrell was responsible for developing and undertaking the electronic literature searches.
Jonathan Michaels provided expert clinical advice throughout the project.
All authors were involved in drafting and reviewing the final report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Evans CJ, Allan PL, Lee AJ, Bradbury AW, Ruckley CV, Fowkes FG. Prevalence of venous reflux in the general population on duplex scanning: the Edinburgh vein study. J Vasc Surg 1998;28:767-76. http://dx.doi.org/10.1016/S0741-5214(98)70051-5.
- Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al. Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial). Health Technol Assess 2006;10.
- Khilnani NM, Grassi CJ, Kundu S, D’Agostino HR, Khan AA, McGraw JK, et al. Multi-society consensus quality improvement guidelines for the treatment of lower-extremity superficial venous insufficiency with endovenous thermal ablation from the Society of Interventional Radiology, Cardiovascular Interventional Radiological Society of Europe, American College of Phlebology, and Canadian Interventional Radiology Association. J Vasc Interv Radiol 2010;21:14-31.
- Eklof B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004;40:1248-52. http://dx.doi.org/10.1016/j.jvs.2004.09.027.
- Kistner R, Eklof B, Masuda E. Diagnosis of chronic venous disease of the lower extremities: the ‘CEAP’ classification. Mayo Clin Proc 1996;71:338-45. http://dx.doi.org/10.4065/71.4.338.
- Labropoulus N. CEAP in clinical practice. Vasc Surg 1997;31:224-5.
- Rutherford R, Padberg F, Comerota A, Kistner R, Meissner M, Moneta G. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg 2000;31:1307-12. http://dx.doi.org/10.1067/mva.2000.107094.
- Kakkos SK, Rivera MA, Matsagas MI, Lazarides MK, Robless P, Belcaro G, et al. Validation of the new venous severity scoring system in varicose vein surgery. J Vasc Surg 2003;38:224-8. http://dx.doi.org/10.1016/S0741-5214(03)00323-9.
- Vasquez MA, Munschauer CE. Venous Clinical Severity Score and quality-of-life assessment tools: application to vein practice. Phlebolymphology 2010;17:108-15. http://dx.doi.org/10.1258/phleb.2008.008018.
- Min R, Khilnani NM, Golia P. Duplex ultrasound evaluation of lower extremity venous insufficiency. J Vasc Interv Radiol 2003;14:1233-41. http://dx.doi.org/10.1097/01.RVI.0000092663.72261.37.
- Michaels JA, Campbell WB, King B, Palfreyman SJ, Shackley P, Stevenson M. A randomised controlled trial and cost-effectiveness analysis of antimicrobial silver antimicrobial dressings for venous leg ulcers: the VULCAN Trial. Br J Surg 2009;96:1147-56.
- Nijsten T, van den Bos RR, Goldman MP, Kockaert MA, Proebstle TM, Rabe E, et al. Minimally invasive techniques in the treatment of saphenous varicose veins. J Am Acad Dermatol 2009;60:110-19. http://dx.doi.org/10.1016/j.jaad.2008.07.046.
- Evans CJ, Fowkes FG, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh vein study. J Epidemiol Commun Health 1999;53:149-53. http://dx.doi.org/10.1136/jech.53.3.149.
- Andreozzi GM, Cordova RM, Scomparin A, Martini R, D’Eri A, Andreozzi F, et al. Quality of life in chronic venous insufficiency: an Italian pilot study of the Triveneto Region. Int Angiol 2005;24:272-7.
- Lim C, Gohel M, Shepherd A, Davies A. Secondary care treatment of patients with varicose veins in National Health Service England: at least how it appeared on a National Health Service website. Phlebology 2010;25:184-9. http://dx.doi.org/10.1258/phleb.2009.009035.
- Callam MJ. Epidemiology of varicose veins. Br J Surg 1994;81:167-73. http://dx.doi.org/10.1002/bjs.1800810204.
- Franks PJ, Wright DD, Moffatt CJ, Fletcher AE, Bulpitt CJ. Prevalence of venous disease: a community study in west London. Eur J Surg 1992;158:143-7.
- Department of Health . HES Statistics 2010–2011 2011. www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=215 (accessed December 2010).
- Laing W. Chronic venous diseases of the leg. London: Office of Health Economics; 1992.
- Kanwar A, Hansrani M, Lees T, Stansby G. Trends in varicose vein therapy in England: radical changes in the last decade. Ann R Coll Surg Engl 2010;92:341-6. http://dx.doi.org/10.1308/003588410X12518836440649.
- Endovenous laser treatment of the long saphenous vein. London: NICE; 2004.
- Radiofrequency ablation of varicose veins. 2003; Interventional Procedure Guidance 8. London: NICE; 2003.
- Ultrasound-guided foam sclerotherapy for varicose veins. 2009; Interventional procedure guidance 314. London: NICE; 2009.
- Transilluminated powered phlebectomy for varicose veins. London: NICE; 2004.
- Kundu S, Lurie F, Millward SF, Padberg F, Vedantham S, Elias S, et al. Recommended reporting standards for endovenous ablation for the treatment of venous insufficiency: joint statement of the American Venous Forum and the Society of Interventional Radiology. J Vasc Surg 2007;46:582-9. http://dx.doi.org/10.1016/j.jvir.2007.07.022.
- Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Gloviczki ML, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2011;53:2S-48S. http://dx.doi.org/10.1016/j.jvs.2011.01.079.
- Cox SJ, Wellwood JM, Martin A. Saphenous neuritis following varicose vein surgery. BMJ 1974;1:415-17.
- Docherty JG, Morrice JJ, Bell G. Saphenous neuritis following varicose vein surgery. Br J Surg 1994;81. http://dx.doi.org/10.1002/bjs.1800810523.
- Morrison C, Dalsing MC. Signs and symptoms of saphenous nerve injury after greater saphenous vein stripping: prevalence, severity, and relevance for modern practice. J Vasc Surg 2003;38:886-90. http://dx.doi.org/10.1016/S0741-5214(03)00790-0.
- Wood JJ, Chant H, Laugharne M, Chant T, Mitchell DC. A prospective study of cutaneous nerve injury following long saphenous vein surgery. Eur J Vasc Endovasc Surg 2005;30:654-8. http://dx.doi.org/10.1016/j.ejvs.2005.06.009.
- Holme JB, Skajaa K, Holme K. Inidence of lesions of the saphenous nerve after partial or complete stripping of the long saphenous vein. Acta Chir Scand 1990;156:145-8.
- Sam RC, Silverman SH, Bradbury AW. Nerve injuries and varicose vein surgery. Eur J Vasc Endovasc Surg 2004;27:113-20. http://dx.doi.org/10.1016/j.ejvs.2003.11.007.
- Rutgers PH, Kitslaar PJ. Randomized trial of stripping versus high ligation combined with sclerotherapy in the treatment of the incompetent greater saphenous vein. Am J Surg 1994;168:311-15. http://dx.doi.org/10.1016/S0002-9610(05)80155-2.
- Fischer R, Chandler JG, Stenger D, Puhan MA, De Maeseneer MG, Schimmelpfennig L. Patient characteristics and physician-determined variables affecting saphenofemoral reflux recurrence after ligation and stripping of the great saphenous vein. J Vasc Surg 2006;31. http://dx.doi.org/10.1016/j.jvs.2005.09.027.
- Winterborn RJ, Foy C, Heather BP, Earnshaw JJ. Randomised trial of flush saphenofemoral ligation for primary great saphenous varicose veins. Eur J Vasc Endovasc Surg 2008;36:477-84. http://dx.doi.org/10.1016/j.ejvs.2008.06.022.
- Campbell WB, Kumar AV, Collin TW, Allington KL, Michaels JA. The outcome of varicose vein surgery at 10 years: clinical findings, symptoms and patient satisfaction. Ann R Coll Surg Engl 2003;85:52-7. http://dx.doi.org/10.1308/003588403321001462.
- Winterborn RJ, Foy C, Earnshaw JJ. Causes of varicose vein recurrence: late results of a randomized controlled trial of stripping the long saphenous vein. J Vasc Surg 2004;40:634-9. http://dx.doi.org/10.1016/j.jvs.2004.07.003.
- Schmedt C-G, Sroka R, Steckmeier S, Meissner OA, Babaryka G, Hunger K, et al. Investigation on radiofrequency and laser (980 nm) effects after endoluminal treatment of saphenous vein insufficiency in an ex-vivo model. Eur J Vasc Endovasc Surg 2006;32:318-25. http://dx.doi.org/10.1016/j.ejvs.2007.03.009.
- Chetter IC, Mylankal KJ, Hughes H, Fitridge R. Randomized clinical trial comparing multiple stab incision phlebectomy and transilluminated powered phlebectomy for varicose veins. Br J Surg 2006;93:169-74. http://dx.doi.org/10.1002/bjs.5261.
- Almeida JI, Raines JK. Radiofrequency ablation and laser ablation in the treatment of varicose veins. Ann Vasc Surg 2006;20:547-52. http://dx.doi.org/10.1007/s10016-006-9098-8.
- Puggioni A, Kalra M, Carmo M, Mozes G, Gloviczki P. Endovenous laser therapy and radiofrequency ablation of the great saphenous vein: analysis of early efficacy and complications. J Vasc Surg 2005;42:488-93. http://dx.doi.org/10.1016/j.jvs.2005.05.014.
- Lurie F, Creton D, Eklof B, Kabnick LS, Kistner RL, Pichot O, et al. Prospective randomized study of endovenous radiofrequency obliteration (closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS Study). J Vasc Surg 2003;38:207-14. http://dx.doi.org/10.1016/S0741-5214(03)00228-3.
- Perala J, Rautio T, Biancari F, Ohtonen P, Wiik H, Heikkinen T, et al. Radiofrequency endovenous obliteration versus stripping of the long saphenous vein in the management of primary varicose veins: 3-year outcome of a randomized study. Ann Vasc Surg 2005;19:669-72.
- Rasmussen LH, Bjoern L, Lawaetz M, Blemings A, Lawaetz B, Eklof B. Randomized trial comparing endovenous laser ablation of the great saphenous vein with high ligation and stripping in patients with varicose veins: short-term results. J Vasc Surg 2007;46:308-15. http://dx.doi.org/10.1016/j.jvs.2007.03.053.
- Rautio T, Ohinmaa A, Perala J, Ohtonen P, Heikkinen T, Wiik H, et al. Endovenous obliteration versus conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of the costs. J Vasc Surg 2002;35:958-65. http://dx.doi.org/10.1067/mva.2002.123096.
- Jia X, Mowatt G, Burr JM, Cassar K, Cook J, Fraser C. Systematic review of foam sclerotherapy for varicose veins. Br J Surg 2007;94:925-36. http://dx.doi.org/10.1002/bjs.5891.
- Luebke T, Gawenda M, Heckenkamp J, Brunkwall J. Meta-analysis of endovenous radiofrequency obliteration of the great saphenous vein in primary varicosis. J Endovasc Ther 2008;15:213-23. http://dx.doi.org/10.1583/07-2287.1.
- Luebke T, Brunkwall J. Systematic review and meta-analysis of endovenous radiofrequency obliteration, endovenous laser therapy, and foam sclerotherapy for primary varicosis. J Cardiovasc Surg 2008;49:213-33.
- van den Bos R, Arends L, Kockaert M, Neumann M, Nijsten T. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg 2009;49:230-9. http://dx.doi.org/10.1016/j.jvs.2008.06.030.
- Murad MH, Coto-Yglesias F, Zumaeta-Garcia M, Elamin MB, Duggirala MK, Erwin PJ, et al. A systematic review and meta-analysis of the treatments of varicose veins. J Vasc Surg 2011;53:49S-65S. http://dx.doi.org/10.1016/j.jvs.2011.02.031.
- Nesbitt C, Eifell R, Coyne P, Badri H, Bhattacharya V, Stansby G. Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus conventional surgery for varicose veins. Cochrane Database Syst Rev 2011;10.
- Department of Health . National Schedule of Reference Costs 2010–11 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_131140 (accessed 20 January 2012).
- Gale SS, Lee JN, Walsh ME, Wojnarowski DL, Comerota AJ. A randomized, controlled trial of endovenous thermal ablation using the 810-nm wavelength laser and the ClosurePLUS radiofrequency ablation methods for superficial venous insufficiency of the great saphenous vein. J Vasc Surg 2010;52:645-50. http://dx.doi.org/10.1016/j.jvs.2010.04.030.
- Shepherd AC, Gohel MS, Brown LC, Metcalfe MJ, Hamish M, Davies AH. Early results of a randomised clinical trial (RCT) comparing VNUS (R) ClosureFAST (TM) ablation and laser for varicose veins (VALVV). Br J Surg 2010;97.
- Goode SD, Chowdhury A, Crockett M, Beech A, Simpson R, Richards T, et al. Laser and radiofrequency ablation study (LARA study): a randomised study comparing radiofrequency ablation and endovenous laser ablation (810 nm). Eur J Vasc Endovasc Surg 2010;40:246-53.
- Cheshire N, Elias SM, Keagy B, Kolvenbach R, Leahy AL, Marston W, et al. Powered phlebectomy (TriVex (TM)) in treatment of varicose veins. Ann Vasc Surg 2002;16:488-94.
- Rigby KA, Palfreyman SJ, Beverley C, Michaels JA. Surgery for varicose veins: use of tourniquet. Cochrane Database Syst Rev 2002;4. http://dx.doi.org/10.1002/14651858.CD001486.
- Moher D, Liberati A, Tetzlaff J, Altman DG. the PRISMA Group . Preferred Reporting items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. Ann Intern Med 2009;151:264-9. http://dx.doi.org/10.7326/0003-4819-151-4-200908180-00135.
- Ceulen RP, Bullens-Goessens YI, Pi-Van De Venne SJ, Nelemans PJ, Veraart JC, Sommer A. Outcomes and side effects of duplex-guided sclerotherapy in the treatment of great saphenous veins with 1% versus 3% polidocanol foam: results of a randomized controlled trial with 1-year follow-up. Dermatol Surg 2007;33:276-81. http://dx.doi.org/10.1111/j.1524-4725.2007.33062.x.
- Pannier F, Rabe E, Maurins U. 1470 nm diode laser for endovenous ablation (EVLA) of incompetent saphenous veins – a prospective randomized pilot study comparing warm and cold tumescence anaesthesia. Vasa 2010;39:249-55. http://dx.doi.org/10.1024/0301-1526/a000037.
- Schulz K, Grimes D. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002;359:781-5. http://dx.doi.org/10.1016/S0140-6736(02)07882-0.
- Brooks SP, Gelman A. Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-45.
- McCullagh PNJA. Generalized linear models. London: Chapman & Hall/CRC; 1989.
- FOAM-study. Cost minimization study comparing surgery versus duplex guided foam sclerotherapy of varicose veins: a randomized controlled study (Project record). Hague, Netherlands: The Netherlands Organisation for Health Research and Development (ZonMw); 2005.
- Mekako A. EVLT for Sapheno-Popliteal Incompetence and SS Reflux: A RCT 2008. www.controlled-trials.com/ISRCTN56883670 (accessed 12 November 2012).
- Braithwaite BD. Randomised Controlled Comparison Study of Endoleuminal Laser Versus Foam Sclerotherapy in Treatment of Varicose Veins 2007. www.controlled-trials.com/ISRCTN52077851 (accessed 12 November 2012).
- Surgery or Noninvasive Therapy for Varicose Veins (Magna) 2007. http://clinicaltrials.gov/ct2/show/NCT00529672 (accessed 12 November 2012).
- Prospective Randomized Trial Comparing the New Endovenous Procedures Versus Conventional Surgery for Varicose Veins Due to Great Saphenous Vein Incompetence (RAFPELS) 2008. http://clinicaltrials.gov/show/NCT00621062 (accessed 12 November 2012).
- Randomized, Single Blind, Placebo Controlled, to Evaluate Efficacy and Safety of Polidocanol Endovenous Microfoam for Treatment of Symptomatic, Visible Varicose Veins With SFJ Incompetence (Pilot) 2008. http://clinicaltrials.gov/ct2/show/NCT00758420 (accessed 12 November 2012).
- Efficacy and Safety Study of Varisolve™ Polidocanol Endovenous Microfoam (PEM) for the Treatment of Saphenofemoral Junction (SFJ) Incompetence (VANISH-1) 2010. http://clinicaltrials.gov/ct2/show/NCT01072877 (accessed 12 November 2012).
- Endovenous Ablation With and Without Polidocanol Endovenous Microfoam Treatment for Patients With Great Saphenous Vein Incompetence and Visible Varicosities (017) 2010. http://clinicaltrials.gov/ct2/show/NCT01197833 (accessed 12 November 2012).
- Polidocanol Endovenous Microfoam (PEM) Versus Vehicle for the Treatment of Saphenofemoral Junction (SFJ) Incompetence (VANISH-2) 2010. http://clinicaltrials.gov/show/NCT01231373 (accessed 12 November 2012).
- Comparison of Treatments in Venous Insufficiency 2011. http://clinicaltrials.gov/show/NCT01298908 (accessed 10 October 2013).
- Randomised Controlled Trial Comparing Foam Sclerotherapy, Alone or in Combination With Endovenous Laser Therapy, With Conventional Surgery As a Treatment for Varicose Veins 2008. http://www.controlled-trials.com/ISRCTN51995477 (accessed 12 November 2012).
- Belcaro G, Cesarone MR, Di RA, Brandolini R, Coen L, Acerbi G, et al. Foam-sclerotherapy, surgery, sclerotherapy, and combined treatment for varicose veins: a 10-year, prospective, randomized, controlled, trial (VEDICO trial). Angiology 2003;54:307-15. http://dx.doi.org/10.1177/000331970305400306.
- Belcaro G, Cesarone M, Di RA, Brandolini R, Coen L, Acerbi G, et al. Treatments for varicose veins: surgery, sclerotherapy, foamsclerotherapy and combined (surgery + sclerotherapy) options. A 10-year, prospective, randomised, controlled, follow-up study. The VEDICO* trial and EST (European Sclerotherapy Trial). Angeiologie 2003;55:29-36.
- Wright D, Gobin J, Bradbury A, Coleridge-Smith P, Spoelstra H, Berridge D, et al. Varisolve polidocanol microfoam compared with surgery or sclerotherapy in the management of varicose veins in the presence of trunk vein incompetence: European randomized controlled trial. Phlebology 2006;21:180-90. http://dx.doi.org/10.1258/026835506779115807.
- Jia X, Liu XP, Xiong J, Zhang HP, Liu M, Du X, et al. [Foam sclerotherapy of the great saphenous vein with sapheno-femoral ligation compared to standard stripping: a prospective randomized controlled trial.]. Chung-Hua Wai Ko Tsa Chih 2010;48:1731-4.
- Liamis A, Prionidis I, Mathai J, Gorton L, Browne T, Panayiotopoulos YP. Long saphenous vein reverse foam sclerotherapy with saphenofemoral junction ligation compared with head and invagination stripping: a prospective randomized trial. Phlebology 2005;20.
- Lin SM, Zhang ZH, Yao YD, Xiao JB. [Experience of endovenous radiofrequency combined with TriVex in treatment of chronic venous insufficiency in lower extremity.]. Chung-Hua Wai Ko Tsa Chih 2009;47:271-4.
- ElKaffas KH, ElKashef O, ElBaz W. Great saphenous vein radiofrequency ablation versus standard stripping in the management of primary varicose veins – a randomized clinical trial. Angiology 2011;62:49-54.
- Ouvry P, Allaert FA, Desnos P, Hamel-Desnos C. Efficacy of polidocanol foam versus liquid in sclerotherapy of the great saphenous vein: a multicentre randomised controlled trial with a 2-year follow-up. Eur J Vasc Endovasc Surg 2008;36:366-70. http://dx.doi.org/10.1016/j.ejvs.2008.04.010.
- Hinchliffe RJ, Ubhi J, Beech A, Ellison J, Braithwaite BD. A prospective randomised controlled trial of VNUS closure versus surgery for the treatment of recurrent long saphenous varicose veins. Eur J Vasc Endovasc Surg 2006;31:212-18. http://dx.doi.org/10.1016/j.ejvs.2005.07.005.
- De Medeiros CAF, Luccas GC. Comparison of endovenous treatment with an 810 nm laser versus conventional stripping of the great saphenous vein in patients with primary varicose veins. Dermatol Surg 2005;31:1685-94.
- Figueiredo M, Araujo S, Barros N, Miranda F. Results of surgical treatment compared with ultrasound-guided foam sclerotherapy in patients with varicose veins: a prospective randomised study. Eur J Vasc Endovasc Surg 2009;38:758-63. http://dx.doi.org/10.1016/j.ejvs.2009.07.015.
- Carradice D, Mekako AI, Mazari FA, Samuel N, Hatfield J, Chetter IC. Randomized clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. Br J Surg 2011;98:501-10. http://dx.doi.org/10.1002/bjs.7615.
- Darwood RJ, Theivacumar N, Dellagrammaticas D, Mavor AI, Gough MJ. Randomized clinical trial comparing endovenous laser ablation with surgery for the treatment of primary great saphenous varicose veins. Br J Surg 2008;95:294-301. http://dx.doi.org/10.1002/bjs.6101.
- Nordon IM, Hinchliffe RJ, Brar R, Moxey P, Black S, Thompson MM, et al. A prospective double-blind randomized controlled trial of radiofrequency versus laser treatment of the great saphenous vein in patients with varicose veins. Ann Surg 2011;254:876-81. http://dx.doi.org/10.1097/SLA.0b013e318230af5a.
- Subramonia S, Lees T. Randomized clinical trial of radiofrequency ablation or conventional high ligation and stripping for great saphenous varicose veins. Br J Surg 2010;97:328-36. http://dx.doi.org/10.1002/bjs.6867.
- Bountouroglou DG, Azzam M, Kakkos SK, Pathmarajah M, Young P, Geroulakos G. Ultrasound-guided foam sclerotherapy combined with sapheno-femoral ligation compared to surgical treatment of varicose veins: early results of a randomised controlled trial. Eur J Vasc Endovasc Surg 2006;31:93-100. http://dx.doi.org/10.1016/j.ejvs.2005.08.024.
- Abela R, Liamis A, Prionidis I, Mathai J, Gorton L, Browne T, et al. Reverse foam sclerotherapy of the great saphenous vein with sapheno-femoral ligation compared to standard and invagination stripping: a prospective clinical series. Eur J Vasc Endovasc Surg Surgery 2008;36:485-90. http://dx.doi.org/10.1016/j.ejvs.2008.06.029.
- Goode S, Crockett M, Richards T, Braithwaite B, Chowdhury A. The bilateral laser and radiofrequency ablation (BLARA) trial. Phlebology Conference: Report on the 9th Meeting of the European Venous Forum Barcelona Spain. Phlebology 2008;23.
- Shepherd AC, Gohel MS, Brown LC, Davies AH. Randomized clinical trial comparing VNUS closure fast versus laser for varicose veins: duplex and quality of life outcomes at six months. Phlebology Conference: 11th Meeting of the European Venous Forum, EVF 2010 Antwerp Belgium. Phlebology 2010;25:305-6.
- Kalodiki E, Azzam M, Lattimer CR, Shawish E, Zambas N, Geroulakos G. Randomized controlled trial of ultrasound guided foam sclerotherapy combined with sapheno-femoral ligation compared to surgical treatment of varicose veins: five-year results. Journal of Vascular Surgery Conference: 23rd Annual Meeting of the American Venous Forum San Diego, CA United States. J Vasc Surg 2011;53:259-60. http://dx.doi.org/10.1016/j.jvs.2010.11.025.
- Rasmussen LH, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg 2011;98:1079-87. http://dx.doi.org/10.1002/bjs.7555.
- Carradice D, Mekako AI, Mazari FA, Samuel N, Hatfield J, Chetter IC. Clinical and technical outcomes from a randomized clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. Br J Surg 2011;98:1117-23. http://dx.doi.org/10.1002/bjs.7615.
- Christenson JT, Gueddi S, Gemayel G, Bounameaux H. Prospective randomized trial comparing endovenous laser ablation and surgery for treatment of primary great saphenous varicose veins with a 2-year follow-up. J Vasc Surg 2010;52:1234-41. http://dx.doi.org/10.1016/j.jvs.2010.06.104.
- Kalteis M, Berger I, Messie-Werndl S, Pistrich R, Schimetta W, Polz W, et al. High ligation combined with stripping and endovenous laser ablation of the great saphenous vein: early results of a randomized controlled study. J Vasc Surg 2008;47:822-9. http://dx.doi.org/10.1016/j.jvs.2007.10.060.
- Rasmussen LH, Bjoern L, Lawaetz M, Lawaetz B, Blemings A, Eklof B. Randomised clinical trial comparing endovenous laser ablation with stripping of the great saphenous vein: clinical outcome and recurrence after 2 years. Eur J Vasc Endovasc Surg 2010;39:630-5.
- Pronk P, Gauw SA, Mooij MC, Gaastra MT, Lawson JA, van Goethem AR, et al. Randomised controlled trial comparing sapheno-femoral ligation and stripping of the great saphenous vein with endovenous laser ablation (980 nm) using local tumescent anaesthesia: one year results. Eur J Vasc Endovasc Surg 2010;40:649-56. http://dx.doi.org/10.1016/j.ejvs.2010.08.007.
- Morrison N. Saphenous ablation: what are the choices, laser or RF energy. Semin Vasc Surg 2005;18:15-8. http://dx.doi.org/10.1053/j.semvascsurg.2004.12.006.
- Shepherd AC, Gohel MS, Brown LC, Metcalfe MJ, Hamish M, Davies AH. Randomized clinical trial of VNUS ClosureFAST radiofrequency ablation versus laser for varicose veins. Br J Surg 2010;97:810-18. http://dx.doi.org/10.1002/bjs.7091.
- Disselhoff BCVM, Kinderen DJD, Kelder JC, Moll FL. Randomized clinical trial comparing endovenous laser with cryostripping for great saphenous varicose veins. Br J Surg 2008;95:1232-8. http://dx.doi.org/10.1002/bjs.6351.
- Kalteis M, Berger I, Hoarding Werndl S, Pistrich R, Schimetta W, . High ligation combined with stripping and endovenous laser ablation of the great saphenous vein: early results of a randomized controlled study. J Vasc Surg 2008;47:822-9. http://dx.doi.org/10.1016/j.jvs.2007.10.060.
- Rasmussen LH, Lawaetz M, Bjoern L, Lawaetz B, Blemings A, Eklof B. Medium-term follow-up of a randomised trial comparing laser ablation with stripping of the great saphenous vein: recurrence rate and pattern after two years. Phlebology 2009;24.
- Pronk P, Gauw SA, Mooij MC, Gaastra MTW, Lawson JA, Van Vlijmen-Van Keulen CJ. A prospective recovery study after high ligation and stripping or endovenous treatment of the insufficient great saphenous vein using local anaesthesia. Phlebology Conference: 11th Meeting of the European Venous Forum, EVF 2010 Antwerp Belgium. Phlebology 2010;25:304-5.
- Gale SS, Lee JN, Walsh ME, Wojnarowski DL, Comerota AJ. A randomized trial of endovenous thermal ablation for superficial venous insufficiency of the great saphenous vein: radiofrequency ablation versus endovenous laser therapy. Phlebology 2009;24.
- Gale SS, Lee JN, Walsh E, Wojnarowski KL, Comerota AJ. A randomized, controlled trial of endovenous thermal ablation using the 810-nm wavelength laser and Closure PLUS radiofrequency ablation methods for superficial venous insufficiency of the great saphenous vein. Phlebology Conference: 11th Meeting of the European Venous Forum, EVF 2010 Antwerp Belgium. Phlebology 2010;25.
- Goode S, Crockett M, Richards T, Braithwaite B. Preliminary results for the bilateral laser and radiofrequency ablation study. Phlebology Conference: Spring Meeting of the Venous Forum London United Kingdom. Phlebology 2008;23:196-7.
- Goode S. n.d.
- Lawaetz M, Rasmussen LH, Bjoern L, Blemings A, Eklf B. 2010.
- Leslie-Mazwi TM, Avery LL, Sims JR. Intra-arterial air thrombogenesis after cerebral air embolism complicating lower extremity sclerotherapy. Neurocrit Care 2009;11:247-50. http://dx.doi.org/10.1007/s12028-009-9211-2.
- Disselhoff BC, der Kinderen DJ, Kelder JC, Moll FL. Five-year results of a randomized clinical trial comparing endovenous laser ablation with cryostripping for great saphenous varicose veins. Br J Surg 2011;98:1107-11. http://dx.doi.org/10.1002/bjs.7542.
- Stötter L, Schaaf I, Bockelbrink A. Comparative outcomes of radiofrequency endoluminal ablation, invagination stripping, and cryostripping in the treatment of great saphenous vein insufficiency. Phlebology 2006;21:60-4. http://dx.doi.org/10.1258/026835506777304692.
- Lurie F, Creton D, Eklof B, Kabnick LS, Kistner RL, Pichot O, et al. Prospective randomised study of endovenous radiofrequency obliteration (closure) versus ligation and vein stripping (EVOLVeS): two-year follow-up. Eur J Vasc Endovasc Surg Surgery 2005;29:67-73. http://dx.doi.org/10.1016/j.ejvs.2004.09.019.
- Balakrishnan A, Mylankal K, Nalachandran S, Subramonia S, Lees T. A randomized controlled trial of radiofrequency ablation and conventional surgery for primary long saphenous varicose veins. Phlebology Conference: Spring Meeting of the Venous Forum London United Kingdom. Phlebology 2008;23.
- Kalodiki E, Azzam M, Kakkos SK, Zambas N, Bountouroglou D, Geroulakos G. Ultrasound-guided foam sclerotherapy combined with saphenofemoral ligation versus surgical treatment of varicose veins: intermediate results of a randomized controlled trial. Phlebology 2008;23:242-3.
- Darvall KA, Bate GR, Adam DJ, Bradbury AW. Recovery after ultrasound-guided foam sclerotherapy compared with conventional surgery for varicose veins. Br J Surg 2009;96:1262-7. http://dx.doi.org/10.1002/bjs.6754.
- Alos J, Carreno P, Lopez JA, Estadella B, Serra-Prat M, Marinel-Lo J. Efficacy and safety of sclerotherapy using polidocanol foam: a controlled clinical trial. Eur J Vasc Endovasc Surg Surgery 2006;31:101-7. http://dx.doi.org/10.1016/j.ejvs.2005.08.018.
- Hamel-Desnos C, Desnos P, Wollmann JC, Ouvry P, Mako S, Allaert FA. Evaluation of the efficacy of polidocanol in the form of foam compared with liquid form in sclerotherapy of the greater saphenous vein: initial results. Dermatol Surg 2003;29:1170-5. http://dx.doi.org/10.1111/j.1524-4725.2003.29398.x.
- Rabe E, Otto J, Schliephake D, Pannier F. Efficacy and safety of great saphenous vein sclerotherapy using standardised polidocanol foam (ESAF): a randomised controlled multicentre clinical trial. Eur J Vasc Endovasc Surg Surgery 2008;35:238-45. http://dx.doi.org/10.1016/j.ejvs.2007.09.006.
- Shadid N, Nelemans P, Sommer A. Duplex-guided foam sclerotherapy versus surgery for the incompetent great saphenous vein: a randomized controlled trial. Phlebology Conference: 11th Meeting of the European Venous Forum, EVF 2010 Antwerp Belgium. Phlebology 2010;25:306-7.
- Bountouroglou DG, Azzam M, Kakkos SK, Pathmarajah M, Young P, Geroulakos G. Prospective randomized study of ultrasound-guided foam sclerotherapy and adjuvant high tie under local anaesthesia versus conventional surgery for primary varicose veins: early results. Phlebology 2004;19.
- Aremu MA, Mahendran B, Butcher W, Khan Z, Colgan MP, Moore DJ, et al. Prospective randomized controlled trial: conventional versus powered phlebectomy. J Vasc Surg 2004;39:88-93. http://dx.doi.org/10.1016/j.jvs.2003.09.044.
- Rautio TT, Perala JM, Wiik HT, Juvonen TS, Haukipuro KA. Endovenous obliteration with radiofrequency-resistive heating for greater saphenous vein insufficiency: a feasibility study. J Vasc Interv Radiol 2002;13:569-75. http://dx.doi.org/10.1016/S1051-0443(07)61649-2.
- Disselhoff BC, der Kinderen DJ, Kelder JC, Moll FL. Randomized clinical trial comparing endovenous laser ablation of the great saphenous vein with and without ligation of the sapheno-femoral junction: 2-year results. Eur J Vasc Endovasc Surg Surgery 2008;36:713-18. http://dx.doi.org/10.1016/j.ejvs.2008.08.015.
- Puggioni A, Marks N, Hingorani A, Shiferson A, Alhalbouni S, Ascher E. The safety of radiofrequency ablation of the great saphenous vein in patients with previous venous thrombosis. J Vasc Surg 2009;49:1248-55. http://dx.doi.org/10.1016/j.jvs.2008.12.016.
- Janne DB, Faintuch S, Schirmang T, Lang EV. Endovenous laser ablation of the saphenous veins: bilateral versus unilateral single-session procedures. J Vasc Interv Radiol 2008;19:211-15.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials 2011. www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%20Mar2013.pdf (accessed 21 May 2013).
- Stevens JW. A note on dealing with missing standard errors in meta-analyses of continuous outcome measure in WinBUGS. Pharm Stat 2011;10:374-8. http://dx.doi.org/10.1002/pst.491.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Eddy DM. Technology assessment: the role of mathematical modeling. Section in assessing medical technologies, Institute of Medicine. Washington, DC: National Academy Press; 1985.
- Disselhoff BC, Buskens E, Kelder JC, der Kinderen DJ, Moll FL. Randomised comparison of costs and cost-effectiveness of cryostripping and endovenous laser ablation for varicose veins: 2-year results. Eur J Vasc Endovasc Surg 2009;37:357-63. http://dx.doi.org/10.1016/j.ejvs.2008.11.013.
- Subramonia S, Lees T. Radiofrequency ablation vs conventional surgery for varicose veins: a comparison of treatment costs in a randomised trial. Eur J Vasc Endovasc Surg Surgery 2010;39:104-11. http://dx.doi.org/10.1016/j.ejvs.2009.09.012.
- Adi Y, Bayliss S, Taylor R. Systematic review of clinical effectiveness and cost-effectiveness of radiofrequency ablation for the treatment of varicose veins. Birmingham: Department of Public Health and Epidemiology, University of Birmingham; 2004.
- Gohel MS, Epstein DM, Davies AH. Cost-effectiveness of traditional and endovenous treatments for varicose veins. Br J Surg 2010;97:1815-23. http://dx.doi.org/10.1002/bjs.7256.
- Kovacs F, Abraira V, Zamora J, Teresa Gil del Real M, Llobera J, Fernandez C, et al. Correlation between pain, disability, and quality of life in patients with common low back pain. Spine 2004;29:206-10. http://dx.doi.org/10.1097/01.BRS.0000107235.47465.08.
- Merchant RF, Pichot O. Closure Study Group . Long-term outcomes of endovenous radiofrequency obliteration of saphenous reflux as a treatment for superficial venous insufficiency. J Vasc Surg 2005;42:502-9. http://dx.doi.org/10.1016/j.jvs.2005.05.007.
- Shepherd AC, Gohel MS, Lim CS, Davies AH. A study to compare disease-specific quality of life with clinical anatomical and hemodynamic assessments in patients with varicose veins. J Vasc Surg 2011;53:374-82. http://dx.doi.org/10.1016/j.jvs.2010.09.022.
- Merchant RF, Pichot O. Long-term outcomes of endovenous radiofrequency obliteration of saphenous reflux as a treatment for superficial venous insufficiency. J Vasc Surg 2005;42:502-9. http://dx.doi.org/10.1016/j.jvs.2005.05.007.
- Office for National Statistics . Interim Life Tables, United Kingdom, 2007–09 2010. www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2008-2010/index.html (accessed 28 February 2012).
- Darvall KA, Sam RC, Bate GR, Silverman SH, Adam DJ, Bradbury AW. Changes in health-related quality of life after ultrasound-guided foam sclerotherapy for great and small saphenous varicose veins. J Vasc Surg 2010;51:913-20. http://dx.doi.org/10.1016/j.jvs.2009.11.045.
- Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al. Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis. Health Technol Assess 2006;10.
- Curti L. Unit costs of health and social care 2011. Canterbury: PSSRU, University of Kent; 2011.
- Eidson JL, Atkins MD, Bohannon WT, Marrocco CJ, Buckley CJ, Bush RL. Economic and outcomes-based analysis of the care of symptomatic varicose veins. J Surg Res 2011;168:5-8. http://dx.doi.org/10.1016/j.jss.2010.12.027.
- Hahn M, Schulz T, Junger M. Sonographically guided, transcatheter foam sclerotherapy of the great saphenous vein: medical and oeconomic aspects. Phlebology 2007;36:309-12.
- Vuylsteke M, Van den Bussche D, Audenaert EA, Lissens P. Endovenous laser obliteration for the treatment of primary varicose veins. Phlebology 2006;21:80-7. http://dx.doi.org/10.1258/026835506777304683.
- Medical Advisory Secretariat . Endovascular laser therapy for varicose veins: an evidence-based analysis. Ontario Health Technology Assessment Series 2010;10.
- Medical Advisory Secretariat . Endovascular radiofequency ablation for varicose veins: an evidence-based analysis. Ontario Health Technology Assessment Series 2011;11.
- Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al. Randomized trial of treatment for uncomplicated varicose veins: surgery is clinically and cost effective. Br J Surg 2005;92:506-7.
- Ratcliffe J, Brazier JE, Campbell WB, Palfreyman S, MacIntyre JB, Michaels JA. Cost-effectiveness analysis of surgery versus conservative treatment for uncomplicated varicose veins in a randomized clinical trial. Br J Surg 2006;93:182-6.
- Ratcliffe J, Brazier JE, Palfreyman SJ, Michaels JA. A Comparison of patient and population values for health states in varicose veins patients. Health Econ 2007;16:395-40. http://dx.doi.org/10.1002/hec.1170.
- Lattimer CR, Azzam M, Kalodiki E, Shawish E, Trueman P, Geroulakos G. Cost and effectiveness of laser with phlebectomies compared with foam sclerotherapy in superficial venous insufficiency: early results of a randomised controlled trial. Eur J Vasc Endovasc Surg Surgery 2012;43:594-600. http://dx.doi.org/10.1016/j.ejvs.2012.01.032.
- Rees G, Crawford D, Dale M, Williams J, Conway K, Sethi H, et al. Evidence review: endovascular treatments for varicose veins. Cardiff: Clinical Engineering and Device Assessment (CEDAR); 2009.
- Browne J, Jamieson L, Lewsey J, van der Meulen J, Copley L, Black N. Case-mix & patients' reports of outcome in Independent Sector Treatment Centres: comparison with NHS providers. BMC Health Serv Res 2008;78. http://dx.doi.org/10.1186/1472-6963-8-78.
- Carradice D, Mazari FA, Mekako A, Hatfield J, Allgar V, Chetter IC. Energy delivery during 810 nm endovenous laser ablation of varicose veins and post-procedural morbidity. Eur J Vasc Endovasc Surg Surgery 2010;40:393-8. http://dx.doi.org/10.1016/j.ejvs.2010.04.010.
- Durkin MT, Turton EP, Wijesinghe LD, Scott DJ, Berridge DC. Long saphenous vein stripping and quality of life: a randomised trial. Eur J Vasc Endovasc Surg 2001;21:545-9. http://dx.doi.org/10.1053/ejvs.2001.1364.
- Eskelinen E, Rasanen P, Alback A, Lepantalo M, Eskelinen A, Peltonen M, et al. Effectiveness of superficial venous surgery in terms of quality-adjusted life years and costs. Scand J Surg 2009;98:229-33.
- Guide to the methods of technology assessment. London: NICE; 2008.
- Carradice D, Mekako AI, Hatfield J, Chetter IC. Randomized clinical trial of concomitant or sequential phlebectomy after endovenous laser therapy for varicose veins. Br J Surg 2009;96:369-75. http://dx.doi.org/10.1002/bjs.6556.
- Ara R, Brazier J. Populating an economic model with health state utility values: moving towards better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Kahn SR. The post-thrombotic syndrome: the forgotten morbidity of deep venous thrombosis. J Thromb Thrombolysis 2006;21:41-8. http://dx.doi.org/10.1007/s11239-006-5574-9.
- Carradice D, Mazari FA, Samuel N, Allgar V, Hatfield J, Chetter IC. Modelling the effect of venous disease on quality of life. Br J Surg 2011;98:1089-98. http://dx.doi.org/10.1002/bjs.7500.
- NHS Suffolk Public Health Team . Low Priority Pocedure – Policy T6 January 2011 2011. www.suffolk.nhs.uk/LinkClick.aspx?fileticket=xIibBQNVYMk%3D&tabid=321&mid=5898 (accessed 21 May 2013).
- NHS Derby City and NHS Derbyshire County . Commissioning Policy for Procedures of Limited Clinical Value (PLCV). Version 2.1 2011. www.derbycitypct.nhs.uk/UserFiles/Documents/538%20v2%20PLCV%20Commissioning%20Policy%20version%202%201.pdf (accessed 21 May 2013).
- van den Bos R, Arends L, Kockaert M, Neumann M, Nijsten T. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg 2009;49:230-9. http://dx.doi.org/10.1016/j.jvs.2008.06.030.
- Winterborn RJ, Corbett CR. Treatment of varicose veins: the present and the future – a questionnaire survey. Ann R Coll Surg Engl 2008;90:561-4. http://dx.doi.org/10.1308/003588408X318228.
- Shepherd AC, Gohel MS, Lim CS, Hamish M, Davies AH. The treatment of varicose veins: an investigation of patient preferences and expectations. Phlebology 2010;25:54-65. http://dx.doi.org/10.1258/phleb.2009.009008.
- Parker MJ, Gurusamy KS, Azegami S. Arthroplasties (with and without bone cement) for proximal femoral fractures in adults. Cochrane Database Syst Rev 2010;6. http://dx.doi.org/10.1002/14651858.CD001706.pub2.
Appendix 1 Literature search strategies for clinical effectiveness and cost-effectiveness reviews
MEDLINE search strategy for clinical effectiveness
Database: Ovid MEDLINE(R) <1948 to June week 5 2011>
Search strategy
-
Varicose Veins/
-
varicose vein.tw.
-
varicose veins.tw.
-
vein, varicose.tw.
-
veins, varicose.tw.
-
varices.tw.
-
varix.tw.
-
varicosis.tw.
-
varicosit$.tw.
-
Saphenous Vein/
-
(saphenous adj2 vein$).tw.
-
(saphena adj2 vein$).tw.
-
or/1-12
-
laser ablation.tw.
-
evla.tw.
-
radiofrequency ablation.tw.
-
radio frequency ablation.tw.
-
radio-frequency ablation.tw.
-
rfa.tw.
-
vnus.tw.
-
closurefast.tw.
-
rfitt.tw.
-
olympus.tw.
-
foam sclero$.tw.
-
Sclerotherapy/
-
sclerotherap$.tw.
-
25 or 26
-
foam.tw.
-
27 and 28
-
ugfs.tw.
-
transilluminated phlebectomy.tw.
-
tipp.tw.
-
radiofrequency obliteration.tw.
-
radio frequency obliteration.tw.
-
radio-frequency obliteration.tw.
-
rfo.tw.
-
or/14-24,29-36
-
13 and 37
MEDLINE search strategy for cost-effectiveness
Database: Ovid MEDLINE(R) <1946 to January week 4 2012>
Search strategy
-
Varicose Veins/
-
varicose vein.tw.
-
varicose veins.tw.
-
vein, varicose.tw.
-
veins, varicose.tw.
-
varices.tw.
-
varix.tw.
-
varicosis.tw.
-
varicosit$.tw.
-
Saphenous Vein/
-
(saphenous adj2 vein$).tw.
-
(saphena adj2 vein$).tw.
-
or/1-12
-
laser ablation.tw.
-
evla.tw.
-
radiofrequency ablation.tw.
-
radio frequency ablation.tw.
-
radio-frequency ablation.tw.
-
rfa.tw.
-
vnus.tw.
-
closurefast.tw.
-
rfitt.tw.
-
olympus.tw.
-
foam sclero$.tw.
-
Sclerotherapy/
-
sclerotherap$.tw.
-
25 or 26
-
foam.tw.
-
27 and 28
-
ugfs.tw.
-
transilluminated phlebectomy.tw.
-
tipp.tw.
-
radiofrequency obliteration.tw.
-
radio frequency obliteration.tw.
-
radio-frequency obliteration.tw.
-
rfo.tw.
-
or/14-24,29-36
-
13 and 37
-
Economics/
-
"costs and cost analysis"/
-
Cost allocation/
-
Cost-benefit analysis/
-
Cost control/
-
cost savings/
-
Cost of illness/
-
Cost sharing/
-
"deductibles and coinsurance"/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
exp economics, hospital/
-
exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
exp "fees and charges"/
-
exp budgets/
-
low adj cost).mp.
-
(high adj cost).mp.
-
(health?care adj cost$).mp.
-
(fiscal or funding or financial or finance).tw.
-
(cost adj estimate$).mp.
-
(cost adj variable).mp.
-
(unit adj cost$).mp.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
or/39-69
-
38 and 70
MEDLINE additional search strategy for cost-effectiveness
Database: Ovid MEDLINE(R) <1946 to January week 4 2012>
Search strategy
-
Varicose Veins/
-
varicose vein.tw.
-
varicose veins.tw.
-
vein, varicose.tw.
-
veins, varicose.tw.
-
varices.tw.
-
varix.tw.
-
varicosis.tw.
-
varicosit$.tw.
-
Saphenous Vein/
-
(saphenous adj2 vein$).tw.
-
(saphena adj2 vein$).tw.
-
or/1-12
-
laser ablation.tw.
-
evla.tw.
-
radiofrequency ablation.tw.
-
radio frequency ablation.tw.
-
radio-frequency ablation.tw.
-
rfa.tw.
-
vnus.tw.
-
closurefast.tw.
-
rfitt.tw.
-
olympus.tw.
-
foam sclero$.tw.
-
Sclerotherapy/
-
sclerotherap$.tw.
-
25 or 26
-
foam.tw.
-
27 and 28
-
ugfs.tw.
-
transilluminated phlebectomy.tw.
-
tipp.tw.
-
radiofrequency obliteration.tw.
-
radio frequency obliteration.tw.
-
radio-frequency obliteration.tw.
-
rfo.tw.
-
or/14-24,29-36
-
13 and 37
-
Economics/
-
"costs and cost analysis"/
-
Cost allocation/
-
Cost-benefit analysis/
-
Cost control/
-
cost savings/
-
Cost of illness/
-
Cost sharing/
-
"deductibles and coinsurance"/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
exp economics, hospital/
-
exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
exp "fees and charges"/
-
exp budgets/
-
(low adj cost).mp.
-
(high adj cost).mp.
-
(health?care adj cost$).mp.
-
(fiscal or funding or financial or finance).tw.
-
(cost adj estimate$).mp.
-
(cost adj variable).mp.
-
(unit adj cost$).mp.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
or/39-69
-
38 and 70
-
13 and 70
-
72 not 71
MEDLINE search strategy for utilities
Database: Ovid MEDLINE(R) <1946 to January week 4 2012>
Search strategy
-
Varicose Veins/
-
varicose vein.tw.
-
varicose veins.tw.
-
vein, varicose.tw.
-
veins, varicose.tw.
-
varices.tw.
-
varix.tw.
-
varicosis.tw.
-
varicosit$.tw.
-
Saphenous Vein/
-
(saphenous adj2 vein$).tw.
-
(saphena adj2 vein$).tw.
-
or/1-12
-
laser ablation.tw.
-
evla.tw.
-
radiofrequency ablation.tw.
-
radio frequency ablation.tw.
-
radio-frequency ablation.tw.
-
rfa.tw.
-
vnus.tw.
-
closurefast.tw.
-
rfitt.tw.
-
olympus.tw.
-
foam sclero$.tw.
-
Sclerotherapy/
-
sclerotherap$.tw.
-
25 or 26
-
foam.tw.
-
27 and 28
-
ugfs.tw.
-
transilluminated phlebectomy.tw.
-
tipp.tw.
-
radiofrequency obliteration.tw.
-
radio frequency obliteration.tw.
-
radio-frequency obliteration.tw.
-
rfo.tw.
-
or/14-24,29-36
-
13 and 37
-
"Quality of Life"/
-
"Value of Life"/
-
quality of life.tw.
-
Health Status Indicators/
-
health status indicator$.tw.
-
Health Status/
-
health status profile$.tw.
-
health related quality of life.tw.
-
Quality-Adjusted Life Years/
-
quality adjusted life.tw.
-
(qaly$ or qald$ or qale$ or qtime$).tw.
-
disability adjusted life.tw.
-
daly$.tw.
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).tw.
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).tw.
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).tw.
-
(sf6D or sf 6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).tw.
-
(sf20 or sf 20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).tw.
-
(sf8 or sf 8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight).tw.
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
(hql or hqol or h qol or hrqol or hr qol).tw.
-
health related quality of life instrument.tw.
-
(aqol or a qol).tw.
-
assessment of quality of life instrument.tw.
-
(hye or hyes).tw.
-
health$ year$ equivalent$.tw.
-
health utilit$.tw.
-
utilit$.tw.
-
(hui or hui1 or hui2 or hui3).tw.
-
disutili$.tw.
-
rosser.tw.
-
quality of wellbeing.tw.
-
quality of well-being.tw.
-
qwb.tw.
-
willingness to pay.tw.
-
standard gamble$.tw.
-
time trade off.tw.
-
time tradeoff.tw.
-
tto.tw.
-
health impact survey$.tw.
-
or/39-78
-
13 and 79
Appendix 2 Data abstraction tables
Characteristics of included studies
| Ref Man ID | Study author, date, country | Study design | Inclusion criteria (including criteria for diagnosis) | Exclusion criteria (including number excluded) | Intervention | Intervention group characteristics (N = ) 1. Age, sex (F/M) 2. Comorbidities |
Comparator | Comparison group characteristics (N = ) 1. Age, sex (F/M) 2. Comorbidities |
|---|---|---|---|---|---|---|---|---|
Study outcomes
| Ref Man ID | Study | Follow-up | Symptoms (I vs. C) | Numbers with recurrence (I vs. C) | Numbers needing a second intervention (I vs. C) | Mortality (I vs. C) | Adverse events or complications (I vs. C) | Quality of life Cost utilisation |
|---|---|---|---|---|---|---|---|---|
Appendix 3 Quality assessment
Risk of bias assessment criteria for a surgical randomised controlled trial (from Parker et al. 2006169)
The principal aim of the tool is to gain an overall impression of quality, or to establish to risk of bias within key domains, which might confound findings, and not to sum the listed ‘scores’ for quantitative purposes.
| (1) Was there clear concealment of allocation? Score 3 (and code A) if allocation clearly concealed (e.g. numbered sealed opaque envelopes drawn consecutively). Score 2 (and code B) if there was a possible chance of disclosure before allocation. Score 1 (and code B) if the method of allocation concealment or randomisation was not stated or was unclear. Score 0 (and code C) if allocation concealment was clearly not concealed such as those using quasi-randomisation (e.g. even or odd date of birth) |
|
| (2) Were the inclusion and exclusion criteria clearly defined? Score 1 if text states type of vein incompetence and which patients were included and excluded. Otherwise score 0 |
|
| (3) Were the outcomes of participants who withdrew or excluded after allocation described and included in an intention-to-treat analysis? Score 1 if intention to treat has clearly been used (only those with an evaluated outcome are included in the analysis, or they explicitly impute these missing outcome data in some way, e.g. best/case scenario). Otherwise score 0 |
|
| (4) Were the treatment and control groups adequately described at entry and if so were the groups well matched, or an appropriate covariate adjustment made? Score 1 if at least three admission details given (e.g. age, sex, CEAP score) with either no important difference between groups or an appropriate adjustment made. Otherwise score 0 |
|
| (5) Were the surgeons assigned to perform each procedure equally experienced at both operations prior to commencement of the trial? Score 1 if text states yes or there was an introductory period or all surgeons were experienced in both operations. Otherwise score 0 |
|
| (6) Were the care programmes other than the trial options identical? Score 1 if text states they were or this can be inferred. Otherwise score 0 |
|
| (7) Were all the outcomes clearly defined in the text with a definition of any ambiguous terms encountered? Score 1 if yes. Otherwise score 0 |
|
| (8) Was the timing of assessment of recurrence appropriate? Score 1 if there was a minimum of 12-months follow-up for all surviving participants for assessing recurrence. Otherwise score 0 If recurrence rates are NR, categorise as NA |
|
| (9) Score 1 if loss to follow-up was < 5% Score 1 if text states reasons for withdrawals Therefore score 2 if answer to both is Yes Score 0 if answer to both is No |
Appendix 4 Statistical model used to analyse technical recurrence
We present the basic details for the network meta-analysis of technical recurrence described in this report. The analysis assumed that the studies are exchangeable in the sense that the investigators would be willing to assign each of the patients in the studies to any of the interventions. A random-effects network meta-analysis was conducted, with the baseline treatment being defined as stripping.
The studies presented data in terms of the number of patients who had recurrence. Define rikj as the number of events (recurrence), out of the total number of patients in each arm for arm k of study i at follow-up time fj, nikj. We assume that the data follow a binomial likelihood such that:
where pikj represents the probability of an event in arm k of study i after follow-up time fj.
To account for the variation in follow-up between studies, it was assumed that the time until an event occurs in arm k of study i, Tik, is from a Weibull distributed such that:
Therefore, the probability that there are no events by time fj in arm k of study i (i.e. the survivor function of a Weibull distribution) is:
Then for each study, i, pikj, the probability of an event in arm k of study i after follow-up time fj can be written as:
which is time dependent.
Therefore, the parameter pikj was modelled using the complimentary log–log link function such that:
vi,bk and δi,bk are the treatment effects of interest on the shape and scale parameter relative to the baseline intervention (i.e. stripping) in arm b (b = 1) respectively. αi and μi are the effects of interest on the shape and scale parameter of study i. Note that αi and vi,1k are on the absolute scale and μi and δi,1k are on the log scale.
We treat ai and μi as nuisance parameters with fixed (but known) study effects and give them weak prior distributions such that:
We assume a random treatment effects model in which vi,1k are coming from a common population distribution such that:
where the multivariate normal distribution needs to be truncated, so, when combining (vi,12⋮vi,1k) with ai to get effect of treatment i (i ≠ 1), the Weibull shape parameter y1k will always be positive.
We also assume a random treatment effects model in which δi,1k are coming from a common population distribution such that:
We give dikv and dikδ, i ≠ 1, a weak prior distribution such that:
The model is completed by giving the logarithm of the population SD of the shape and scale parameter a uniform prior distribution, respectively:
These prior distributions will have minimal influence on the posterior results in the presence of sufficient sample data.
This model includes arm-specific shape parameters that allow for time-varying hazards. It takes into account variation between studies in the duration of follow-up and acknowledges that events are accumulated over time. The model incorporates an adjustment for the correlation between treatment effects in case of multiarm trials.
Appendix 5 Statistical methods used to analyse VCSS and pain score
The analysis assumed that the studies are exchangeable in the sense that the investigators would be willing to assign each of the patients in the studies to any of the interventions.
A random-effects network meta-analysis was conducted, with the baseline treatment being defined as stripping.
We first describe the basic details for the meta-analysis of continuous data. For treatment j in study i, we have an observation vector, yij, such that:
where x¯ij is the sample mean for treatment j in study i, and sijnij is the SE for treatment j in study i.
We assume that the sample means, x¯ij, are normally distributed such that:
and that μij = Φi + θij.
Φi is the effect of study i and θij is the effect of treatment j in study i.
We treat Φi as nuisance parameters with fixed (but unknown) study effects and give them weak prior distributions such that Φi ∼ N(0,10,000).
We assume a random (treatment) effects model in which θij are assumed to come from a common population distribution such that θij∼N(μθ,τ2). To make the parameters identifiable, we set μθ1=0 so that Φi is the effect of the control group in study i, and μθj is the population mean effect of treatment j relative to treatment 1.
We give μθj,j≠1, a weak prior distribution such that μθj∼N(0,10,000).
τ represents the between-study SD, which we give a prior uniform distribution, τ ∼ U(0,100)
We assume that the sample variances, sij2, are gamma distributed such that:
The model is completed by giving the logarithm of the population SD a prior uniform distribution such that:
These prior distributions will have minimal influence on the posterior results in the presence of sufficient sample data.
The model for the network meta-analyses differs from this basic model in two particular ways. First, the estimates of treatment effect within each study are represented as functions of each treatment effect relative to placebo. Second, it is acknowledged that there will be correlation between treatment effects for the multiarm trial.
For each study it was assumed that the sample SDs were the same in each treatment arm of the study within study.
Appendix 6 Table of excluded studies with rationale
Excluded studies: clinical effectiveness review
Not a randomised controlled trial
-
Aksunger EH, Aikimbaev K, Akgul E. Consecutive or late foam sclerotheraphy after EVLA: which one is more effective and tolerated by patients? CardioVascular and Interventional Radiology Conference: Cardiovascular and Interventional Radiological Society of Europe, CIRSE 2009, Lisbon, Portugal. Conference Publication, September.
-
Ali S. Randomized clinical trial comparing endovenous laser ablation with surgery for the treatment of primary great saphenous varicose veins (Br J Surg 2008;95:294–301). Br J Surg 2008;95:1428.
-
Alm J. Small saphenous veins with ClosureFAST. Phlebolymphology 2010;17:65–6.
-
Alm J, Bohme J, Kensy M. VNUS Closure radiofrequency ablation of varicose veins from Closure PLUS to Closure FAST. Phlebology 2010;39:61–8.
-
Almeida JI, Raines J. II.2 radiofrequency versus laser versus chemical sclerotherapy for endoablation of the saphenous vein and when you do not need to do stab avulsions. Vascular 2005;13(Suppl. 1):S16.
-
Bhalla MI, Bhalla N. Concomitant use of endovenous laser and foamed sclerosant in the treatment of lower limb varicosities: 3 year follow-up results. Journal of Vascular and Interventional Radiology Conference: 36th Annual Scientific Meeting of the Society of Interventional Radiology, SIR 2011 – IR Rising: Leading Image-Guided Medicine Chicago, IL, USA. March.
-
Bradbury A. Foam sclerotherapy treatment in varicose veins: results from 1200 cases. Phlebolymphology 2010;17:60.
-
Brittenden J. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg 2011;98:1079–89.
-
Bush RG. Regarding ‘Laser therapy and radiofrequency ablation of the great saphenous vein: analysis of early efficacy and complications’. J Vasc Surg 2006;43:642–3.
-
Christenson JT, Bounameaux H. Regarding ‘Prospective randomized trial comparing endovenous laser ablation and surgery for treatment of primary great saphenous varicose veins with a 2-year follow-up’ reply. J Vasc Surg 2011;53:1456.
-
Darvall KA, Bate GR, Adam DJ, Bradbury AW. Recovery after ultrasound-guided foam sclerotherapy compared with conventional surgery for varicose veins. Br J Surg 2009;96:1262–7.
-
Darvall KAL, Bate GR, Adam DJ, Bradbury AW. Recovery after ultrasound-guided foam sclerotherapy compared with conventional surgery for varicose veins (Br J Surg 2009;96:1262–7) reply. Br J Surg 2010;97:457–8.
-
Demagny A. [Comparative study into the efficacy of a sclerosant product in the form of liquid or foam in echo-guided sclerosis of the arches of the long and short saphenous veins.] Phlebology 2002;55:133–7.
-
Eidson JL, Atkins MD, Bohannon WT, Marrocco CJ, Buckley CJ, Bush RL. Economic and outcomes-based analysis of the care of asymptomatic varicose veins. J Surg Res 2011;168:5–8.
-
Hoch A, Pichlmaier AM, Teebken OE, Bisdas T, Haverich A, Wilhelmi M. Effectiveness and clinical outcome following endovenous therapy of primary varicose veins: First results of a study comparing the VNUS-Closure-Fast-system, 980nm- and 1470nm-lasers and surgery. Thoracic and Cardiovascular Surgeon Conference: 39th Annual Meeting of the German Society for Cardiovascular and Thoracic Surgery Stuttgart Germany. Conference Publication.
-
Labropoulos N, Bhatti A, Leon L, Borge M, Rodriguez H, Kalman P. Neovascularization after great saphenous vein ablation. Eur J Vasc Endovasc Surg 2006;31:219–22.
-
Lewis BD. Re: Radiofrequency endovenous ClosureFAST versus laser ablation for the treatment of great saphenous reflux: a multicenter, single-blinded, randomized study (RECOVERY study). J Vasc Interv Radiol 2010;21:302–3.
-
Morris WT. Recovery after ultrasound-guided foam sclerotherapy compared with conventional surgery for varicose veins (Br J Surg 2009;96:1262–7). Br J Surg 2010;97:457–8.
-
Nael R, Rathbun S, Kirkpatrick A, Whitsett T. Effectiveness of endovenous foam sclerotherapy for treatment of varicose veins. Vasc Med 2007;12:154.
-
Neglen P. High ligation combined with stripping and endovenous laser ablation of the great saphenous vein: early results of a randomized controlled study – invited commentary. J Vasc Surg 2008;47:829.
-
Nesbitt CI, Stansby G. Regarding ‘prospective randomized trial comparing endovenous laser ablation and surgery for treatment of primary great saphenous varicose veins with a 2-year follow-up’. J Vasc Surg 2011;53:1456.
-
Roddy SP. A randomized, controlled trial of endovenous thermal ablation using the 810-nm wavelength laser and the ClosurePLUS radiofrequency ablation methods for superficial venous insufficiency of the great saphenous vein. J Vasc Surg 2010;52:796.
-
Sanchez-Ismayel A, Pujadas-Arias Z, Sanchez-Miralles R, Rodriguez-Gonzalez O, Benitez P. Crossectomy and foam sclerotherapy versus saphenectomy as treatment for varicose veins produced by reflux at the saphenofemoral. Angiologia 2007;59:367–74.
-
Sultan S, Hynes N. Comparison of endovenous upward perforate invaginate stripping, downward invaginate, and high-energy endovenous laser ablation for varicose veins. Vascular Conference: 7th Annual Western Vascular Institute Symposium Galway Ireland. Conference Publication, June 2009.
-
Theivacumar NS, Darwood R, Gough MJ. Neovascularisation and recurrence 2 years after varicose vein treatment for sapheno-femoral and great saphenous vein reflux: a comparison of surgery and endovenous laser ablation. Eur J Vasc Endovasc Surg 2009;38:203–7.
-
Theivacumar NS, Dellagrammaticas D, Mavor AI, Gough MJ. Endovenous laser ablation: does standard above-knee great saphenous vein ablation provide optimum results in patients with both above- and below-knee reflux? A randomized controlled trial. J Vasc Surg 2008;48:173–8.
-
Vuylsteke M, Van den Bussche D, Audenaert EA, Lissens P. Endovenous laser obliteration for the treatment of primary varicose veins. Phlebology 2006;21:80–7 (confirmed by author communication, though analysed as a RCT in other reviews).
-
Yamaki T, Nozaki M, Iwasaka S. Comparative study of duplex-guided foam sclerotherapy and duplex-guided liquid sclerotherapy for the treatment of superficial venous insufficiency. Dermatol Surg 2004;30:718–22.
Letters relating to randomised controlled trials
-
Darwood RJ, Gough MJ. Randomized clinical trial comparing endovenous laser ablation surgery for the treatment of primary great saphenous varicose veins (Br J Surg 2008;95:294–301) reply. Br J Surg 2008;95:1428.
-
Figueiredo M, Araujo S, Barros J, Miranda J. Corrigendum to ‘Results of surgical treatment compared with ultrasound-guided foam sclerotherapy in patients with varicose veins: a prospective randomised study’ (Eur J Vasc Endovasc Surg 2009;38:758–63). Eur J Vasc Endovasc Surg 2010;39:379.
-
Figueiredo M, Araujo S, Barros N, Miranda F. Results of surgical treatment compared with ultrasound-guided foam sclerotherapy in patients with varicose veins: a prospective randomised study. Eur J Vasc Endovasc Surg 2010;39:379.
-
Figueiredo M, Araujo S, Barros J, Miranda J. Results of surgical treatment compared with ultrasound-guided foam sclerotherapy in patients with varicose veins: a prospective randomised study. Vasomed 2010;22:248–9.
Randomised controlled trials of comparator interventions
-
Carradice D, Mekako AI, Hatfield J, Chetter IC. Randomized clinical trial of concomitant or sequential phlebectomy after endovenous laser therapy for varicose veins. Br J Surg 2009;96:369–75.
-
Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al. Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial). Health Technol Assess 2006;10(13).
-
Pares JO, Juan J, Tellez R, Mata A, Moreno C, Quer FX, et al. Varicose vein surgery stripping versus the CHIVA method: a randomized controlled trial. Ann Surg 2010;251:624–31.
Randomised controlled trial of co-intervention rather than intervention of interest
-
Disselhoff BC, der Kinderen DJ, Kelder JC, Moll FL. Five-year results of a randomised clinical trial of endovenous laser ablation of the great saphenous vein with and without ligation of the saphenofemoral junction. Eur J Vasc Endovasc Surg 2011;41:685–90.
Wrong outcome
-
Carradice D, Mekako AI, Mazari FA, Samuel N, Hatfield J, Chetter IC. Randomized clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. Br J Surg 2011;98:501–10.
-
O’Hare JL, Earnshaw JJ. Randomised clinical trial of foam sclerotherapy for patients with a venous leg ulcer. Eur J Vasc Endovasc Surg 2010;39:495–9.
-
Viarengo LMA, Poterio-Filho J, Poterio GMB, Menezes FH, Meirelles GV. Endovenous laser treatment for varicose veins in patients with active ulcers: measurement of intravenous and perivenous temperatures during the procedure. Dermatol Surg 2007;33:1234–41.
Duplicate of an included study
-
Perälä J, Rautio T, Biancari F, Ohtonen P, Wiik H, Heikkinen T, et al. Radiofrequency endovenous obliteration versus stripping of the long saphenous vein in the management of primary varicose veins: 3-year outcome of a randomized study. Ann Vasc Surg 2005;19:669–72.
-
Rasmussen LH, Bjoern L, Lawaetz M, Blemings A, Lawaetz B, Eklof B. Randomized trial comparing endovenous laser ablation of the great saphenous vein with high ligation and stripping in patients with varicose veins: short-term results. J Vasc Surg 2007;46:308–15.
-
Shepherd AC, Gohel MS, Brown LC, Metcalfe MJ, Hamish M, Davies AH. Early results of a randomised clinical trial (RCT) comparing VNUS® ClosureFAST™ ablation and laser for varicose veins (VALVV). London: The Vascular Society of Great Britain & Ireland Yearbook; 2009.
-
Stoetter L, Schaaf I, Bockelbrink A, Baurecht H. Radiofrequency obliteration, invagination or cryostripping: which is the best tolerated treatment by the patients? Phlebology 2005;34:19–24.
Published later as a full paper
-
Brittenden J. Randomised controlled trial comparing foam sclerotherapy, alone or in combination with endovenous laser therapy, with conventional surgery as a treatment for varicose veins. 2008. URL: www.controlled-trials.com/ISRCTN51995477 (last accessed 12 November 2012).
Unavailable
-
Ukritmanoroat T. Comparison of efficacy and safety between foam sclerotherapy and conventional sclerotherapy: a controlled clinical trial. J Med Assoc Thai 2011;94(Suppl. 2):S35–S40.
Appendix 7 Summary of the trials included in the base-case network meta-analysis on technical recurrence for all treatments and all follow-ups
| Author, year | Number of follow-up points | First follow-up point (years) | Second follow-up point (years) | Treatment | Number of events | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 1, time 1 | Arm 1, time 2 | Arm 2, time 1 | Arm 2, time 2 | Arm 3, time 1 | Arm 3, time 2 | Arm 4, time 1 | Arm 4, time 2 | ||||
| Carradice 201196 | 2 | 0.12 | 1 | 1 | 2 | 10 | 23 | 1 | 5 | ||||||
| Rasmussen 2007,44 201099 | 2 | 0.25 | 2 | 1 | 2 | 1 | 25 | 0 | 18 | ||||||
| Darwood 200887 | 1 | 0.25 | 1 | 2 | 8 | 11 | |||||||||
| De Medeiros84 | 1 | 0.17 | 1 | 2 | 0 | 1 | |||||||||
| Pronk 2010100 | 1 | 1 | 1 | 2 | 5 | 5 | |||||||||
| Christenson 201097 | 1 | 1 | 1 | 2 | 1 | 4 | |||||||||
| Hinchliffe 200683 | 1 | 0.12 | 1 | 3 | 2 | 3 | |||||||||
| Lurie 200342 | 2 | 0.33 | 2 | 1 | 3 | 0 | 3 | 4 | 4 | ||||||
| Perala 2005,43 Rautio 200245 | 2 | 0.14 | 3 | 1 | 3 | 1 | 3 | 0 | 5 | ||||||
| Nordon 201188 | 1 | 0.25 | 2 | 3 | 3 | 2 | |||||||||
| Shepherd 2010102 | 1 | 0.50 | 2 | 3 | 1 | 6 | |||||||||
| ElKaffas 201181 | 1 | 2 | 1 | 3 | 9 | 12 | |||||||||
| Gale 201053 | 1 | 1 | 2 | 3 | 2 | 11 | |||||||||
| Goode 201055 | 1 | 0.75 | 2 | 3 | 7 | 9 | |||||||||
| aMorrison 2005101 | 1 | 1 | 2 | 3 | 17 | 10 | |||||||||
| Figueiredo 200985 | 1 | 0.50 | 1 | 4 | 3 | 6 | |||||||||
| aKalodiki 2011117 | 1 | 3.40 | 1 | 4 | 16 | 18 | |||||||||
| aJia 201078 | 2 | 0.25 | 0.5 | 1 | 4 | 3 | 3 | 3 | 5 | ||||||
| aShadid 2010122 | 2 | 0.25 | 1 | 1 | 4 | 1 | 50 | 11 | 43 | ||||||
| Wright 200677 | 2 | 0.25 | 1 | 1 | 4 | 12 | 13 | 72 | 92 | ||||||
| bRasmussen 201195 | 1 | 1 | 1 | 2 | 3 | 4 | 16 | 14 | 9 | 17 | |||||
| cKalteis 200898 | 1 | 1.33 | 1 | 2 | 0 | 0 | |||||||||
| cBountouroglou 200690 | 1 | 0.25 | 1 | 4 | 0 | 0 | |||||||||
| Author, year | Number of patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Arm 1, time 1 | Arm 1, time 2 | Arm 2, time 1 | Arm 2, time 2 | Arm 3, time 1 | Arm 3, time 2 | Arm 4, time 1 | Arm 4, time 2 | |
| Carradice 201196 | 132 | 113 | 137 | 124 | ||||
| Rasmussen 2007,44 201099 | 63 | 58 | 63 | 65 | ||||
| Darwood 200887 | 32 | 71 | ||||||
| De Medeiros84 | 20 | 20 | ||||||
| Pronk 2010100 | 56 | 49 | ||||||
| Christenson 201097 | 100 | 99 | ||||||
| Hinchliffe 200683 | 16 | 16 | ||||||
| Lurie 200342 | 34 | 34 | 43 | 43 | ||||
| Perala 2005,43 Rautio 200245 | 13 | 13 | 15 | 15 | ||||
| Nordon 201188 | 68 | 70 | ||||||
| Shepherd 2010102 | 76 | 76 | ||||||
| ElKaffas 201181 | 90 | 88 | ||||||
| Gale 201053 | 48 | 46 | ||||||
| Goode 201055 | 32 | 34 | ||||||
| aMorrison 2005101 | 50 | 50 | ||||||
| Figueiredo 200985 | 29 | 27 | ||||||
| aKalodiki 2011117 | 34 | 38 | ||||||
| aJia 201078 | 28 | 26 | 28 | 25 | ||||
| aShadid 2010122 | 177 | 188 | 217 | 221 | ||||
| Wright 200677 | 94 | 94 | 435 | 435 | ||||
| bRasmussen 201195 | 108 | 121 | 124 | 123 | ||||
| cKalteis 200898 | 48 | 47 | ||||||
| cBountouroglou 200690 | 23 | 29 | ||||||
Appendix 8 Summary of the trials included in the base-case network meta-analysis on Venous Clinical Severity Score for all treatments and all follow-ups
| Author, year | Treatment | Arm 1 | Arm 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Mean | SD | Number of patients | Mean | SD | Number of patients | |
| aCarradice 201186 | 1 | 2 | 0.7 | 1.09 | 113 | 0.49 | 0.88 | 124 |
| Christenson 201097 | 1 | 2 | 0.23 | 0.57 | 100 | 0.26 | 0.68 | 99 |
| a,bKalodiki 2011117 | 1 | 4 | 2.73 | 2.66 | 34 | 1.11 | 1.43 | 38 |
| aGale 201053 | 2 | 3 | 1.3 | 1.8 | 70 | 1.4 | 1.5 | 59 |
| Shepherd 2010102 | 2 | 3 | 1.4 | 1.7 | 52 | 1.4 | 1.8 | 55 |
| Perala 200543 | 1 | 3 | 0 | 13 | 0.7 | 15 | ||
Appendix 9 Summary of the trials included in the base-case network meta-analysis on pain score for all treatments and all follow-ups
| Author, year | Treatment | On treatment pain score | Sample variance | Total number of patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | |
| aChristenson 201097 | 1 | 2 | 1.80 | 1.70 | 100 | 100 | ||||||||||
| Kalteis 200898 | 1 | 2 | 3.08 | 2.64 | 6.05 | 4.08 | 48 | 47 | ||||||||
| Pronk 2010100 | 1 | 2 | 1.18 | 2.65 | 2.22 | 4.88 | 68 | 62 | ||||||||
| Darwood 200887 | 1 | 2 | 2.46 | 1.79 | 12.28 | 3.26 | 49 | 94 | ||||||||
| Subramonia 201089 | 1 | 3 | 4.47 | 2.87 | 7.55 | 11.71 | 41 | 47 | ||||||||
| Hinchliffe 200683 | 1 | 3 | 4.41 | 2.95 | 25.15 | 17.31 | 16 | 16 | ||||||||
| Rautio 200245 | 1 | 3 | 3.00 | 1.80 | 3.24 | 0.64 | 13 | 15 | ||||||||
| Shepherd 2010102 | 2 | 3 | 3.43 | 2.20 | 4.45 | 3.92 | 61 | 66 | ||||||||
| aNordon 201188 | 2 | 3 | 1.35 | 0.00 | 78 | 76 | ||||||||||
| Rasmussen 201195 | 1 | 2 | 3 | 4 | 2.25 | 2.58 | 1.21 | 1.60 | 4.97 | 5.81 | 2.96 | 4.16 | 123 | 124 | 124 | 123 |
Appendix 10 Quality assessment economic studies
Drummond: critical appraisal of a published article
Disselhoff et al.133 Randomised comparison of costs and cost-effectiveness of cryostripping and endovenous laser ablation for varicose veins. 2009
| Question | Yes | No | Cannot tell |
|---|---|---|---|
| 1. Was a well-defined question posed in an answerable form? | Clear but not explicitly stated | Perspective – health-care costs and costs of lost productivity through sick leave included | |
| 2. Was a comprehensive description of the competing alternatives given? | Partly. Discussed surgery as most common treatment, although not included in this trial. Other alternatives not discussed | ||
| 3. Was there evidence that the programme's effectiveness had been established? | Both treatments equally effective on primary measure (SF-6D measured QALYs over 2 years post operative). (Note non-statistically significant improvement for each cohort and diff between treatments.) Greater difference in technical fail rates, but also statistically non-significant | ||
| 4. Were all important and relevant costs and consequences for each alternative identified? | Yes, includes treatment, equipment costs, costs of retreatment and lost productivity. Adverse events rare and costs negligible | ||
| 5. Were costs and consequences measured accurately in appropriate physical units? | Consequences measured with SF-6D, adverse events and retreatment data also presented | Claims surgery costs are representative of treatment costs for both EVLA and cryostripping, with differences only in equipment costs. Given small difference in overall costs a small difference in treatment costs would make a difference Patients were not randomised to day or outpatient procedures, which are assumed to incur different costs. 82% cryostripping patients and 66% EVLA were done as day cases |
|
| 6. Were costs and consequences valued credibly? | Mainly | Productivity loss valuation (80% of €41) not justified or referenced Year of costing not clear. Presumed 2003 |
|
| 7. Were costs and consequences adjusted for differential timing? | No – but time horizon only 2 years and most costs at T0, so effect likely negligible | ||
| 8. Was an incremental analysis of costs and consequences of alternatives performed? | ICERs were calculated, but incorrectly as (Cost T1/QALY T1) – (Cost T0/QALY T0). This ≠ the correct calculation of (Cost T1 – T0)/(QALYS T1 – T0). This explains why reported ICERs do not match source cost and QALY data | ||
| 9. Was allowance made for the uncertainty in the estimates of costs and consequences? | Bootstrapping of results to give confidence limits for ICERS (non-significant). Results also presented on cost-effectiveness plane, with some per cent of data points in different quadrants quoted in text | ||
| 10. Did the presentation and discussion of study results include all issues of concern to users? | Mainly | Generalisability to secondary treatment, CEAP 2 Did not collect actual data on treatment costs |
Drummond: critical appraisal of a published article
Subramonia and Lees.134 Radiofrequency ablation vs. conventional surgery for varicose veins – a comparison of treatment costs in a randomised trial. 2010
| Question | Yes | No | Cannot tell |
|---|---|---|---|
| 1. Was a well-defined question posed in an answerable form? | Yes – perspective (societal) not stated | ||
| 2. Was a comprehensive description of the competing alternatives given? | Yes | ||
| 3. Was there evidence that the programme's effectiveness had been established? | Yes – economic analysis conducted alongside a clinical RCT | ||
| 4. Were all important and relevant costs and consequences for each alternative identified? | Comprehensive for short-term costs (hospital, GP, patient and lost work days), but only short term | ||
| 5. Were costs and consequences measured accurately in appropriate physical units? | Yes | ||
| 6. Were costs and consequences valued credibly? | Yes – working days valued at average wages | ||
| 7. Were costs and consequences adjusted for differential timing? | Not applicable – all short term | ||
| 8. Was an incremental analysis of costs and consequences of alternatives performed? | Yes – consequences measured in terms of lost work hours, but other benefits (reduced post-operative pain) (significant), greater improvement in AVVQ score for RFA compared with surgery (non-significant) not included However, cost per work hour saved inappropriately included valuation of work hours in costs |
||
| 9. Was allowance made for the uncertainty in the estimates of costs and consequences? | No | ||
| 10. Did the presentation and discussion of study results include all issues of concern to users? | Cost per work hour saved calculation incorrect Main limitation of the study is the very limited duration of follow-up (median 37 days). Differential recurrence rates could affect the results |
Quality checklist of economic model
Gohel et al.136 Cost-effectiveness of traditional and endovenous treatments for varicose veins. 2010
| Question | Yes/no | Description |
|---|---|---|
| 1. A statement of the problem | Yes | |
| 2. A discussion of the need for modelling vs. alternative approaches | No | |
| 3. A description of the relevant factors and outcomes | No | Model outcomes described (clinical success), but no consideration of relationship between clinical outcomes and patient symptoms |
| 4. A description of the model, including reasons for this type of model and a specification of the scope, time frame, perspective, comparators and settings | Yes | |
| 5. A description of data sources (including subjective estimates) with a description of the strengths and weaknesses of each source, with reference to a specific hierarchy of evidence | Yes – but see comment ‘Key parameter’ | Most data sources described and appropriate Key parameter – the recurrence of reflux for the endovenous treatments – it is not clear whether or not the rate for surgery is assumed for all treatments. No other data for this parameter is reported (neither data nor source) |
| 6. A list of assumptions pertaining to: the structure of the model (e.g. factors included, relationships and distributions) and the data | Yes – but inadequate | Many assumptions mentioned, but fails to discuss major (apparent) assumption which is contradicted by one of the data sources used. If the recurrence rate for surgery is applied to all treatments the relative risk of treatment failure is in effect assumed to be constant over time. In fact, the review by van den Bos et al.49 [used for the baseline time to failure (surgery)] shows that this is not the case. Given the considerable uncertainty in the model results this is very likely to change the conclusions. Also assumes (and not discussed) that technical failure after treatment results in utility equal to that prior to treatment – although literature shows only a small difference in the proportion symptomatic (∼ 10%) with clinical success/failure after treatment |
| 7. A list of parameter values that will be used for the base-case analysis, and a list of the ranges of those values that represent appropriate confidence limits for use in sensitivity analysis | Mainly | Baseline values presented together with confidence intervals, except costs |
| 8. The results derived from applying the model for the base case | Yes | |
| 9. Was allowance made for the uncertainty in the estimates of costs and consequences? | ||
| 10. A discussion of how the modelling assumptions might affect the results | ||
| 11. A description of the validation undertaken including: concurrence of experts internal consistency external consistency predictive validity |
||
| 12. A description of the setting to which the results can be applied | ||
| 13. A description of research in progress that could yield new data that could alter the results of the analysis |
Quality checklist of economic model
Adi et al.135 Systematic review of clinical effectiveness and cost-effectiveness of radiofrequency ablation for the treatment of varicose veins. 2004
| Question | Yes/no | Description |
|---|---|---|
| 1. A statement of the problem | Yes | |
| 2. A discussion of the need for modelling vs. alternative approaches | Yes | |
| 3. A description of the relevant factors and outcomes | Yes | However, long-term outcomes were ignored because of lack of data |
| 4. A description of the model, including reasons for this type of model and a specification of the scope, time frame, perspective, comparators and settings | Yes | Very simple model based on a single RCT. Estimates utilities from average pain VAS scores reported in trial, and uses reported trial costs (Finland). Time horizon 2 weeks |
| 5. A description of data sources (including subjective estimates) with a description of the strengths and weaknesses of each source, with reference to a specific hierarchy of evidence | Yes | Based on a poor-quality RCT, with limited follow-up (2 weeks) and estimated utility gain. Potential differences in costs between Finland and England not discussed |
| 6. A list of assumptions pertaining to: the structure of the model (e.g. factors included, relationships and distributions) and the data | Yes | See above |
| 7. A list of parameter values that will be used for the base-case analysis, and a list of the ranges of those values that represent appropriate confidence limits for use in sensitivity analysis | Some | For utility values, not costs |
| 8. The results derived from applying the model for the base case | Yes | |
| 9. Was allowance made for the uncertainty in the estimates of costs and consequences? | None | |
| 10. A discussion of how the modelling assumptions might affect the results | Some | How small increase in RFA morbidity would reduce its cost-effectiveness. Lack of long-term outcomes |
| 11. A description of the validation undertaken including: concurrence of experts internal consistency external consistency predictive validity |
No | |
| 12. A description of the setting to which the results can be applied | No | |
| 13. A description of research in progress that could yield new data that could alter the results of the analysis |
Appendix 11 Literature reporting costs for the treatment of varicose veins
| Study | Country | Intervention | Control | Primary study? | Key assumptions/comments |
|---|---|---|---|---|---|
| Adi 2004135 | UK | RFA | Stripping | Based on Rautio et al.,45 but excludes lost work days | |
| Bountouroglou 200690 | UK | FS | Stripping | Yes | Detailed costing based on RCT |
| Disselhoff 2008126 | Netherlands | Cryostripping | EVLA | Assume procedure costs the same, with differences in equipment costs, and additional treatments within 2 years | |
| Eidson 2011146 | US | RFA | Stripping | Yes | Excludes surgeon's fee, resource use not shown, no cost breakdown |
| ElKaffas 201181 | Egypt | RFA | Stripping | Yes | Detailed costing based on RCT |
| Gohel 2010136 | UK | RFA/EVLA/FS | Stripping | Surgery – national reference costs, other day-case/outpatient attendance plus equipment costs | |
| Hahn 2007147 | Germany | FS | None | One treatment only, costing method not described | |
| Michaels 20062 | UK | LS | Stripping | Yes | Resource use collected alongside clinical trial, costed using national and local data |
| Medical Advisory Secretariat, Ontario 2010149 | Canada | EVLA | Stripping | Procedure costs assumed same, differences in anaesthetist and equipment costs | |
| Medical Advisory Secretariat, Ontario 2011150 | Canada | RFA | Stripping | Procedure costs assumed same, differences in anaesthetist and equipment costs | |
| Rasmussen 2007145 | Denmark | EVLA | Stripping | Assumed costs same apart from equipment | |
| Rasmussen 201195 | Denmark | RFA/EVLA | Stripping | Costs based on reimbursement plus equipment costs (laser, RFA) | |
| Rautio 200245 | Finland | RFA | Stripping | Yes | Detailed costing based on RCT |
| Shadid 2010122 | Netherlands | FS | Stripping | Not stated | RCT, abstract only, costing method not described |
| Subramonia 2010134 | UK | RFA | Stripping | Yes | Detailed costing based on RCT |
| Vuylsteke 2006148 | Belgium | EVLA | Stripping | Assumed costs same apart from equipment |
Appendix 12 Deterministic sensitivity analysis on economic model – inputs and results (discounted)
| Item | Baseline values | IQR | |
|---|---|---|---|
| Lower | Upper | ||
| Utility symptomatic | 0.8781 | 0.8724 | 0.8840 |
| Cost: surgery (£) | 1155 | 1131 | 1179 |
| Cost: FS (£) | 634 | 585 | 684 |
| Cost: EVLA (£) | 2472 | 2287 | 2651 |
| Cost: RFA (£) | 2769 | 2563 | 2969 |
| Retreatment extra cost (£) | 430 | 394 | 463 |
| Probability asymptomatic if success | 0.8816 | 0.8723 | 0.8916 |
| Probability asymptomatic if fail | 0.7408 | 0.7112 | 0.7700 |
| Retreatment mode distribution | 0.6000 | 0.5678 | 0.6334 |
| Time to retreatment | 0.5000 | 0.4413 | 0.5538 |
| Baseline treatment disutility (surgery) | –0.0400 | –0.0410 | –0.0390 |
| Disutility FS | –0.0392 | –0.0403 | –0.0382 |
| Disutility EVLA | –0.0401 | –0.0411 | –0.0391 |
| Disutility RFA | –0.0388 | –0.0398 | –0.0378 |
| Probability asymptomatic if success (Darvall et al.118) | 0.8000 | ||
| Probability asymptomatic if fail (Darvall et al.118) | 0.6500 | ||
| Probability asymptomatic if success (Merchant et al.138) | 0.8929 | ||
| Probability asymptomatic if fail (Merchant et al.138) | 0.7872 | ||
| Scenario | Stripping | FS | EVLA | RFA | Incremental costs (£) | Incremental QALYs | ICERs | Net benefit (£) – MAICER 20,000 | Incremental net benefit (£) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | Costs (£) | QALYs | FS | EVLA | RFA | FS | EVLA | RFA | FS | EVLA | RFA | Stripping | FS | EVLA | RFA | F FS | EVLA | RFA | ||
| Baseline | |||||||||||||||||||||||||
| Age (baseline 50 years) | 50 years | 1246.92 | 8.045 | 724.06 | 8.044 | 2544.20 | 8.052 | 2844.59 | 8.051 | – 522.86 | 1297.28 | 1597.67 | – 0.0010 | 0.0068 | 0.0061 | NA | 190,348 | 264,055 | 159,660 | 160,162 | 158,499 | 158,183 | 503 | – 1161 | – 1477 |
| 40 years | 1247.88 | 8.019 | 725.01 | 8.018 | 2545.04 | 8.025 | 2845.43 | 8.025 | – 522.87 | 1297.16 | 1597.55 | – 0.0004 | 0.0061 | 0.0060 | NA | 212,337 | 268,449 | 159,129 | 159,644 | 157,954 | 157,650 | 515 | – 1175 | – 1479 | |
| 60 years | 1244.62 | 7.978 | 721.99 | 7.976 | 2541.99 | 7.985 | 2842.70 | 7.984 | – 522.63 | 1297.37 | 1598.08 | – 0.0019 | 0.0073 | 0.0054 | NA | 177,382 | 294,666 | 158,317 | 158,802 | 157,166 | 156,828 | 485 | – 1151 | – 1490 | |
| Utility symptomatic | Lower IQR | 1246.92 | 8.039 | 724.06 | 8.038 | 2544.20 | 8.046 | 2844.59 | 8.045 | – 522.86 | 1297.28 | 1597.67 | – 0.0010 | 0.0070 | 0.0062 | NA | 184,309 | 256,846 | 159,530 | 160,032 | 158,373 | 158,056 | 502 | – 1157 | – 1473 |
| Upper IQR | 1246.92 | 8.052 | 724.06 | 8.051 | 2544.20 | 8.059 | 2844.59 | 8.058 | – 522.86 | 1297.28 | 1597.67 | – 0.0010 | 0.0066 | 0.0059 | NA | 197,026 | 271,950 | 159,794 | 160,297 | 158,628 | 158,314 | 503 | – 1166 | – 1480 | |
| Cost: surgery | Lower IQR | 1221.64 | 8.045 | 723.11 | 8.044 | 2543.43 | 8.052 | 2843.79 | 8.051 | – 498.53 | 1321.79 | 1622.15 | – 0.0010 | 0.0068 | 0.0061 | NA | 193,945 | 268,101 | 159,685 | 160,163 | 158,499 | 158,184 | 479 | – 1185 | – 1501 |
| Upper IQR | 1272.18 | 8.045 | 725.01 | 8.044 | 2544.97 | 8.052 | 2845.40 | 8.051 | – 547.17 | 1272.79 | 1573.22 | – 0.0010 | 0.0068 | 0.0061 | NA | 186,755 | 260,013 | 159,634 | 160,161 | 158,498 | 158,182 | 527 | – 1136 | – 1452 | |
| Cost: FS | Lower IQR | 1245.60 | 8.045 | 673.43 | 8.044 | 2543.16 | 8.052 | 2843.51 | 8.051 | – 572.18 | 1297.56 | 1597.91 | – 0.0010 | 0.0068 | 0.0061 | NA | 190,389 | 264,094 | 159,661 | 160,213 | 158,500 | 158,184 | 552 | – 1161 | – 1477 |
| Upper IQR | 1248.24 | 8.045 | 774.70 | 8.044 | 2545.24 | 8.052 | 2845.68 | 8.051 | – 473.54 | 1297.00 | 1597.44 | – 0.0010 | 0.0068 | 0.0061 | NA | 190,308 | 264,016 | 159,658 | 160,112 | 158,497 | 158,182 | 454 | – 1161 | – 1476 | |
| Cost: EVLA | Lower IQR | 1246.92 | 8.045 | 724.06 | 8.044 | 2359.82 | 8.052 | 2844.59 | 8.051 | – 522.86 | 1112.90 | 1597.67 | – 0.0010 | 0.0068 | 0.0061 | NA | 163,294 | 264,055 | 159,660 | 160,162 | 158,683 | 158,183 | 503 | – 977 | – 1477 |
| Upper IQR | 1246.92 | 8.045 | 724.06 | 8.044 | 2723.84 | 8.052 | 2844.59 | 8.051 | – 522.86 | 1476.92 | 1597.67 | – 0.0010 | 0.0068 | 0.0061 | NA | 216,706 | 264,055 | 159,660 | 160,162 | 158,319 | 158,183 | 503 | – 1341 | – 1477 | |
| Cost: RFA | Lower IQR | 1246.92 | 8.045 | 724.06 | 8.044 | 2544.20 | 8.052 | 2638.43 | 8.051 | – 522.86 | 1297.28 | 1391.51 | – 0.0010 | 0.0068 | 0.0061 | NA | 190,348 | 229,982 | 159,660 | 160,162 | 158,499 | 158,389 | 503 | – 1161 | – 1271 |
| Upper IQR | 1246.92 | 8.045 | 724.06 | 8.044 | 2544.20 | 8.052 | 3045.18 | 8.051 | – 522.86 | 1297.28 | 1798.26 | – 0.0010 | 0.0068 | 0.0061 | NA | 190,348 | 297,207 | 159,660 | 160,162 | 158,499 | 157,982 | 503 | – 1161 | – 1677 | |
| Retreatment extra cost | Lower IQR | 1244.53 | 8.045 | 721.73 | 8.044 | 2542.31 | 8.052 | 2842.63 | 8.051 | – 522.80 | 1297.79 | 1598.10 | – 0.0010 | 0.0068 | 0.0061 | NA | 190,422 | 264,126 | 159,662 | 160,165 | 158,500 | 158,185 | 503 | – 1161 | – 1477 |
| Upper IQR | 1249.16 | 8.045 | 726.25 | 8.044 | 2545.97 | 8.052 | 2846.44 | 8.051 | – 522.92 | 1296.81 | 1597.27 | – 0.0010 | 0.0068 | 0.0061 | NA | 190,279 | 263,989 | 159,657 | 160,160 | 158,497 | 158,181 | 503 | – 1161 | – 1476 | |
| Probability asymptomatic if success | Lower IQR | 1246.92 | 8.037 | 724.06 | 8.037 | 2544.20 | 8.044 | 2844.59 | 8.043 | – 522.86 | 1297.28 | 1597.67 | – 0.0008 | 0.0064 | 0.0058 | NA | 202,462 | 277,218 | 159,502 | 160,008 | 158,333 | 158,019 | 506 | – 1169 | – 1482 |
| Upper IQR | 1246.92 | 8.054 | 724.06 | 8.053 | 2544.20 | 8.061 | 2844.59 | 8.060 | – 522.86 | 1297.28 | 1597.67 | – 0.0010 | 0.0073 | 0.0063 | NA | 177,089 | 253,651 | 159,825 | 160,327 | 158,674 | 158,353 | 503 | – 1151 | – 1472 | |
| Probability asymptomatic if fail | Lower IQR | 1257.82 | 8.041 | 734.63 | 8.039 | 2552.72 | 8.049 | 2853.49 | 8.048 | – 523.19 | 1294.90 | 1595.67 | – 0.0010 | 0.0082 | 0.0070 | NA | 158,866 | 227,174 | 159,552 | 160,055 | 158,421 | 158,097 | 503 | – 1132 | – 1455 |
| Upper IQR | 1236.16 | 8.050 | 713.70 | 8.049 | 2535.74 | 8.056 | 2835.80 | 8.055 | – 522.46 | 1299.58 | 1599.64 | – 0.0009 | 0.0055 | 0.0051 | NA | 234,485 | 314,250 | 159,765 | 160,270 | 158,577 | 158,268 | 505 | – 1189 | – 1498 | |
| Retreatment mode distribution | Lower IQR | 1245.73 | 8.045 | 722.89 | 8.044 | 2543.37 | 8.052 | 2843.70 | 8.051 | – 522.84 | 1297.64 | 1597.97 | – 0.0009 | 0.0067 | 0.0060 | NA | 193,101 | 267,501 | 159,663 | 160,167 | 158,500 | 158,185 | 504 | – 1163 | – 1478 |
| Upper IQR | 1248.03 | 8.045 | 725.18 | 8.044 | 2545.09 | 8.052 | 2845.52 | 8.051 | – 522.85 | 1297.06 | 1597.50 | – 0.0009 | 0.0069 | 0.0062 | NA | 187,739 | 256,433 | 159,657 | 160,161 | 158,498 | 158,184 | 504 | – 1159 | – 1473 | |
| Time to retreatment | Lower IQR | 1247.51 | 8.045 | 724.61 | 8.044 | 2544.88 | 8.052 | 2845.15 | 8.051 | – 522.90 | 1297.37 | 1597.64 | – 0.0010 | 0.0067 | 0.0060 | NA | 194,444 | 265,836 | 159,660 | 160,164 | 158,497 | 158,183 | 504 | – 1164 | – 1477 |
| Upper IQR | 1246.41 | 8.045 | 723.39 | 8.044 | 2543.62 | 8.052 | 2844.09 | 8.051 | – 523.02 | 1297.21 | 1597.69 | – 0.0008 | 0.0069 | 0.0061 | NA | 188,410 | 261,744 | 159,659 | 160,166 | 158,500 | 158,184 | 507 | – 1160 | – 1476 | |
| Baseline treatment disutility (surgery) | Lower IQR | 1246.92 | 8.044 | 724.06 | 8.044 | 2544.20 | 8.052 | 2844.59 | 8.051 | – 522.86 | 1297.28 | 1597.67 | – 0.0001 | 0.0077 | 0.0070 | NA | 168,059 | 229,773 | 159,641 | 160,162 | 158,498 | 158,182 | 521 | – 1143 | – 1459 |
| Upper IQR | 1246.92 | 8.046 | 724.06 | 8.044 | 2544.20 | 8.052 | 2844.59 | 8.051 | – 522.86 | 1297.28 | 1597.67 | – 0.0019 | 0.0059 | 0.0051 | NA | 219,978 | 311,209 | 159,678 | 160,163 | 158,499 | 158,183 | 485 | – 1179 | – 1495 | |
| Disutility FS | Lower IQR | 1246.92 | 8.045 | 724.06 | 8.043 | 2544.20 | 8.052 | 2844.59 | 8.051 | – 522.86 | 1297.28 | 1597.67 | – 0.0020 | 0.0068 | 0.0061 | NA | 190,197 | 263,855 | 159,659 | 160,143 | 158,498 | 158,182 | 484 | – 1161 | – 1477 |
| Upper IQR | 1246.92 | 8.045 | 724.06 | 8.045 | 2544.20 | 8.052 | 2844.59 | 8.051 | – 522.86 | 1297.28 | 1597.67 | 0.0000 | 0.0068 | 0.0060 | NA | 190,501 | 264,257 | 159,660 | 160,182 | 158,499 | 158,183 | 522 | – 1161 | – 1477 | |
| Disutility EVLA | Lower IQR | 1246.92 | 8.045 | 724.06 | 8.044 | 2544.20 | 8.051 | 2844.59 | 8.051 | – 522.86 | 1297.28 | 1597.67 | – 0.0010 | 0.0059 | 0.0061 | NA | 219,610 | 264,055 | 159,660 | 160,162 | 158,480 | 158,183 | 503 | – 1179 | – 1477 |
| Upper IQR | 1246.92 | 8.045 | 724.06 | 8.044 | 2544.20 | 8.053 | 2844.59 | 8.051 | – 522.86 | 1297.28 | 1597.67 | – 0.0010 | 0.0077 | 0.0061 | NA | 167,806 | 264,055 | 159,660 | 160,162 | 158,517 | 158,183 | 503 | – 1143 | – 1477 | |
| Disutility RFA | Lower IQR | 1246.92 | 8.045 | 724.06 | 8.044 | 2544.20 | 8.052 | 2844.59 | 8.050 | – 522.86 | 1297.28 | 1597.67 | – 0.0010 | 0.0068 | 0.0051 | NA | 190,348 | 311,809 | 159,660 | 160,162 | 158,499 | 158,164 | 503 | – 1161 | – 1495 |
| Upper IQR | 1246.92 | 8.045 | 724.06 | 8.044 | 2544.20 | 8.052 | 2844.59 | 8.052 | – 522.86 | 1297.28 | 1597.67 | – 0.0010 | 0.0068 | 0.0070 | NA | 190,348 | 228,583 | 159,660 | 160,162 | 158,499 | 158,202 | 503 | – 1161 | – 1458 | |
| Probability asymptomatic if success/fail (Darvall et al.118) | 1280.86 | 7.962 | 757.34 | 7.961 | 2571.04 | 7.969 | 2872.61 | 7.968 | – 523.52 | 1290.18 | 1591.75 | – 0.0011 | 0.0070 | 0.0065 | NA | 183,876 | 243,075 | 157,954 | 158,455 | 156,804 | 156,493 | 501 | – 1150 | – 1461 | |
| Probability asymptomatic if success/fail (Merchant et al.138) | 1154.91 | 8.186 | 634.29 | 8.185 | 2471.54 | 8.187 | 2768.91 | 8.188 | – 520.62 | 1316.62 | 1614.00 | – 0.0005 | 0.0016 | 0.0020 | NA | 848,763 | 805,059 | 162,559 | 163,069 | 161,273 | 160,985 | 511 | – 1286 | – 1574 | |
| Model time span (baseline scenario) | |||||||||||||||||||||||||
| Baseline | 2 years | 1176.11 | 1.830 | 660.99 | 1.830 | 2484.09 | 1.831 | 2785.05 | 1.832 | – 515.12 | 1307.98 | 1608.95 | 0.0001 | 0.0008 | 0.0017 | NA | 1,696,843 | 962,673 | 35,423 | 35,940 | 34,130 | 33,847 | 517 | – 1293 | – 1576 |
| Baseline | 5 years | 1208.48 | 4.384 | 691.86 | 4.383 | 2509.39 | 4.387 | 2811.51 | 4.387 | – 516.61 | 1300.92 | 1603.04 | – 0.0006 | 0.0030 | 0.0033 | NA | 437,325 | 490,390 | 86,466 | 86,971 | 85,224 | 84,928 | 505 | – 1241 | – 1538 |
| Baseline | 10 years | 1246.92 | 8.045 | 724.06 | 8.044 | 2544.20 | 8.052 | 2844.59 | 8.051 | – 522.86 | 1297.28 | 1597.67 | – 0.0010 | 0.0068 | 0.0061 | NA | 190,348 | 264,055 | 159,660 | 160,162 | 158,499 | 158,183 | 503 | – 1161 | – 1477 |
| Baseline | Life | 1318.43 | 18.346 | 781.33 | 18.356 | 2619.52 | 18.356 | 2911.46 | 18.360 | – 537.10 | 1301.09 | 1593.03 | 0.0097 | 0.0094 | 0.0132 | NA | 138,172 | 120,403 | 365,609 | 366,341 | 364,496 | 364,280 | 732 | – 1113 | – 1328 |
Appendix 13 Protocol
Clinical and cost-effectiveness of methods for managing varicose veins
HTA 10/29/01
Protocol (also available as CRD42011001355 in the PROSPERO database)
15 March 2011
1. Title of the project:
What is the clinical and cost effectiveness of different methods of managing varicose veins based upon current evidence?
2. Project lead
The University of Sheffield, School of Health and Related Research (ScHARR)
Dr Christopher Carroll
Senior Lecturer in Health Technology Assessment
ScHARR
University of Sheffield
3. Plain English Summary
Varicose veins are enlarged, visibly lumpy knotted veins, usually in the legs. Uncomplicated varicose veins can cause discomfort, aching, heaviness and itching. 1 Complications can include superficial thrombophlebitis, external bleeding, lipodermatosclerosis, eczema and ulceration. 2 Varicose veins is part of chronic venous insufficiency, which is reported to have a substantial negative impact on Health-related Quality of Life (HRQoL). 3 Prevalence of varicose veins in the UK has been reported to be between 20–40% in adult. 4–7 Reported prevalence in women is in the range of 24 and 32%, with male prevalence rates ranging from 14–19%. The NHS performs over 36,000 surgical procedures per year to treat varicose veins,8 although this figure may be affected by economic considerations.
Traditional treatments for varicose veins involve surgical stripping and ligation, non-foam sclerotherapy or conservative management of symptoms. Surgical stripping has been associated with nerve damage, scars, pain and long post-operative recovery. Traditional surgical procedures have been shown to produce a range of adverse effects such as wound infection, haematoma, lymph leaks, scarring, nerve injury and Deep Vein Thrombosis. 9–14 Conventional liquid sclerotherapy is considered faster but less effective than surgical stripping. 15 New minimally invasive treatments offer alternative methods of ablating the vein. These treatments typically involve use of laser, radiofrequency or foam scleroscant. These treatments are now widely used and offer potential benefits such as reduced postoperative downtime, reduced complications, faster recovery, fewer physical limitations, increased HRQoL, is reported to have reduced costs and lower recurrence rates compared to surgical stripping, whilst being equally effective. 16–21
The principal outcomes associated with treatment for varicose veins are symptom relief, symptom severity, quality of life, patient treatment satisfaction, retreatment, and the occurrence of related adverse effects. Recurrence of new varicosities is also considered an important outcome of treatment for varicose veins. Reported recurrence rates for vary widely depending on the nature of the surgical technique performed and method of assessment. Two-year recurrence rates of up to 33% are reported,22,23 with reported 5 year recurrence of 41% rising to up to 70% at over 10 years. 24,25 Surgical procedures for recurrence can therefore place considerable demand on the health services.
Four reviews26–29 and a cost-effectiveness analysis30 have recently been published on this topic. The meta-analysis by Leubke et al 200826 evaluated RFA alone and that by Jia et al 200728 evaluated foam sclerotherapy alone. The meta-analyses published by Luebke et al 200827 and van den Bos et al 200929 considered all three principal minimally invasive techniques but only included some data from twelve and seven relevant RCTs respectively, with substantial duplication of included studies. Large numbers of observational and case series studies were also included in the analyses. However, given that almost twenty RCTs are cited across these reviews and meta-analyses, principally for foam sclerotherapy,1,15,31–39 but also for RFA18,40–44 and EVLA,21,45–47 it is possible that both Luebke et al and van den Bos et al failed to include relevant trial data. Finally, at least six relevant RCTs published since 2008 have been identified by limited scoping searches for this report and have not been analysed in any previous review. 48–53 These include head-to-heads trial of EVLA and both ClosureFast51 and RFiTT53 RFA techniques. This proposed work would therefore be analysing new data, as well as applying more inclusive criteria and conducting analyses different from previous reviews. The recently published cost effectiveness analysis by Gohel et al 201030 uses Great Saphenous Vein (GSV) occlusion as a proxy for clinical outcomes, such as symptoms, recurrence and reoperation rates, and only employs utility data from short-term follow-up. The proposed cost-effectiveness model may therefore reach beyond this and might also employ utility data from more recent RCTs. 48,49,51
4. Decision problem
4.1 Purpose of the decision to be made
The assessment will address the question: What is the clinical and cost effectiveness of different methods of managing varicose veins based on the evidence?
4.2 Clear definition of the intervention
New minimally invasive methods of managing varicose veins: Endovenous Laser Ablation (EVLA), Ultrasound Guided Foam Sclerotherapy (UGFS), Radiofrequency Ablation (RFA) or Obliteration (RFO), and Transilluminated Phlebectomy.
4.2.1 EVLA
EVLA involves insertion and activation of a laser fibre into the varicose vein. Wavelengths used target deoxygenated haemoglobin and/or water. 54
4.2.2 UGFS
Sclerotherapy involves injecting the vein with a substance that causes it to collapse and be absorbed into the surrounding tissue. 55 UGFS involves the mixing of air with liquid sclerosing solution to create foam. The foam is injected into the affected vein guided by ultrasound. 54
4.2.3 RFA
RFA involves insertion of a catheter into the varicose vein. Electrodes at the end of the catheter omit high radiofrequency energy which heats tissue at the site, causing collagen shrinkage, denudation of endothelium and obliteration of the venous lumen. 56 This includes techniques such as VNUS Closure and VNUS ClosureFast51 and Olympus RFiTT. 53
4.2.4 Transilluminated Phlebectomy
Transilluminated Phlebectomy offers an alternative to multiple phlebectomies. It involves hydrodissection of the varicosities, transillumination facilitating direct visualization of the varicosities, and varicosity removal using a powered endoscopic tissue dissector. 57
4.3 Place of the intervention in the treatment pathway(s)
This review will focus on the use of interventions in the treatment of varicose veins.
4.4 Relevant comparators
Any. However, this is most likely to consist of surgical treatment, non-foam sclerotherapy and conservative management. Head-to-head trials comparing the minimally invasive techniques will also be included.
4.4.1 Surgical treatments
Traditional surgical treatment of the greater saphenous vein (GSV) typically involves ligation at the saphenofemoral junction followed by stripping to the knee. Treatment of the short saphenous vein (SSV) typically involves ligation at the saphenopopital junction only. 54
4.4.2 Non-foam sclerotherapy
Sclerotherapy involves injecting the vein with a substance that causes it to collapse and be absorbed into the surrounding tissue. 55
4.4.3 Conservative management
Conservative management of varicose veins includes use of compression stockings, elevating the legs, regular exercise.
4.5 Population and relevant sub-groups
Adults aged 16 years or more who are being treated specifically for varicose veins. Note: 5 July 2011: Groups: 1. Main vein incompetence (LSV) = majority (SSV) = minority – might receive all techniques; 2. No main vein incompetence would receive only 4.23 and 4.2.4.
4.6 Key factors to be addressed
-
Evaluate the clinical and cost-effectiveness of new minimally invasive techniques compared to other techniques, including traditional surgical techniques, non-foam sclerotherapy and conservative management.
-
Evaluate the safety of new minimally invasive techniques versus surgical techniques, non-foam sclerotherapy and conservative management.
-
Identify any key areas for further research.
5. Report methods for synthesis of evidence of clinical effectiveness
A review of the evidence for clinical effectiveness will be undertaken systematically following the general principles recommended in the PRISMA statement. 58 English and non-English language studies will be included and there will be no limit by date.
5.1 Population
Adults aged 16 years or more who are being treated specifically for varicose veins. Diagnostic criteria will be recorded, where given.
5.2 Intervention
Ultrasound Guided Foam Sclerotherapy (UGFS), Endovenous Laser Ablation (EVLA) and Radiofrequency Ablation (RFA) or Radiofrequency Obliteration (RFO), and Transilluminated Phlebectomy.
5.3 Comparator
Any form of varicose veins management, including traditional surgical stripping/ligation, conservative treatment, phlebectomy or other minimally invasive techniques, such as non-foam sclerotherapy.
5.4 Settings
Secondary care.
5.5 Outcomes
5.5.1 Clinical outcomes
-
Clinical symptoms, as measured by, for example, the Venous Clinical Severity Score (VCSS) (including pain, oedema, inflammation, hyperpigmentation and lipodermatosclerosis).
-
Recurrence rate (recurrence of varices or occurrence of new varices) as distinct from initial treatment episode, usually indicated by neoreflux (on duplex scanning).
-
Early and late re-operations and re-do procedures.
-
Post-operative complications, may include but are not limited to, e.g. nerve damage, skin burns, deep venous thermal injury, deep vein thrombosis, pulmonary embolism, transient ischaemic attacks, stroke, bleeding, infection, thrombophlebitis, headache, visual disturbance, skin staining, pain at injection site, back pain, anaphylaxis, lymph leak, cellulitis, etc.
5.5.2 Cost and utility outcomes
-
Cost effectiveness and cost utility.
-
Quality of Life as measured by, for example, the Aberdeen Varicose Vein Questionnaire (AVVQ) and Short Form 12 (SF-12).
5.6 Follow-up
There is to be no minimum duration of follow-up.
5.7 Study design
Randomised Controlled Trials (RCTs) only. Scoping searches and an examination of the review literature indicates that there is likely to be more than four or five relevant RCTs for each technique (see section 3, above).
5.8 Search strategy
The search strategy will comprise the following main elements:
-
Searching of electronic databases.
-
Contact with experts in the field.
-
Scrutiny of bibliographies of retrieved papers.
5.8.1 Electronic searches
A comprehensive search will be undertaken to identify systematically both clinical and cost-effectiveness literature comparing different methods of the management of varicose veins. The search will involve only combining terms for the population (varicose veins) and the interventions of interest, i.e. the new minimally invasive techniques. This highly sensitive search (i.e. not using terms for comparators, outcomes or study design) is possible because scoping searches using this strategy retrieved relatively small and manageable numbers of citations. An example MEDLINE search strategy is reported in Appendix A. The aim of the strategy is to identify all studies that report on trials or controlled studies comparing new techniques with traditional surgery, non-foam sclerotherapy or conservative management. All searches will be done by an Information Specialist (AC).
5.8.2 Databases
The following electronic databases will be searched from inception for published and unpublished research evidence:
-
MEDLINE (Ovid) 1950–;
-
EMBASE (Ovid) 1980–;
-
CINAHL (EBSCO) 1982–;
-
The Cochrane Library including the Cochrane Systematic Reviews Database, Cochrane Controlled Trials Register, DARE, HTA and NHS EED databases 1991–;
-
Biological Abstracts (via ISI Web of Science) 1969–;
-
Science Citation Index (via ISI Web of Science) 1900–;
-
Social Science Citation Index (via ISI Web of Science) 1956–;
-
Conference Proceedings Citation Index- Science (CPCI-S)- (via ISI Web of Science) 1990–
-
UK Clinical Trials Research Network (UKCRN) and the National Research Register archive (NRR);
-
Current Controlled Trials;
-
Clinical Trials.gov up.
All citations will be imported into Reference Manager software and duplicates deleted.
5.9 Inclusion criteria
The inclusion criteria are as reported in 5.1–5.7 above. Titles and abstracts of all unique citations will be screened independently by two reviewers using the inclusion criteria outlined below. Disagreement will be resolved by consensus, or with reference to a third team member when necessary. The full papers of all potentially relevant citations will be retrieved so that an in-depth assessment concerning inclusion could be made. Reference-tracking of all included studies and relevant reviews will also be performed to identify additional, relevant studies not retrieved by the search of electronic databases.
5.10 Exclusion criteria
RCTs will be excluded if the focus of the study is the management of a varicose vein complication using the minimally invasive techniques rather than the treatment of varicose veins specifically, i.e. the trial evaluates the management of complications such as ulceration and the principal outcome relates to the complication, e.g. leg ulcer healing, rather than the clinical outcomes defined above.
5.11 Data extraction strategy
Data will be extracted from all studies by one reviewer (JL) using a standardised data extraction form piloted on at least one study (see Appendix B). All extractions will be checked thoroughly by a second reviewer (CC). Discrepancies will be resolved by discussion, and with reference to a third team member if necessary.
5.12 Quality assessment strategy
The quality assessment of included RCTs will be undertaken using an appropriate quality assessment criteria. These are included in Appendix C. Critical appraisal will be performed by one reviewer and double-checked by a second reviewer. Discrepancies will be resolved by discussion, with involvement of a third team member if necessary.
5.13 Methods of analysis/synthesis
Data will be tabulated and included studies will be combined in a meta-analysis if the included trials are sufficiently similar in terms of population, intervention, comparator and outcome. Statistical heterogeneity between trials will be accounted for using a random effects meta-analysis and by calculating the I2 statistic. 59
Binary outcome measures will be analysed assuming a binomial distribution for the observed number of events; continuous outcome measures will be analysed assuming a normal distribution for sample means.
Where trials form a network of evidence in which trials compare one or more different treatments, data will be synthesised using a network meta-analysis to allow a more precise estimate of treatment effect to be calculated and to provide more information with which to estimate the between-study standard deviation. Results will be presented in terms of odds ratios (ORs) and mean difference (MD) for binary and continuous outcome measures respectively.
Absolute estimates of risk and means will be estimated for each treatment by projecting the estimates of treatment effect onto an estimate of baseline risk and an estimate of a baseline mean for binary and continuous outcome measures respectively. The absolute estimates of risk will be used to represent uncertainty about parameters in the economic model.
6. Report methods for synthesising evidence of cost-effectiveness
A systematic review of the existing literature studying the cost-effectiveness of new techniques compared to traditional surgery, non-foam sclerotherapy, and conservative management will be undertaken. In addition, a new economic model will be developed to compare a treatment strategy which incorporates novel techniques with a strategy that uses traditional surgery, non-foam sclerotherapy or conservative treatment.
6.1 Identifying and systematically reviewing published cost effectiveness studies
The search strategy and sources detailed in Section 5 will be used to identify studies of cost effectiveness. The approach described is very sensitive as no study design filters are being used and will retrieve any relevant cost-effectiveness studies. Identified economic literature will be critically appraised and assessed using the Drummond checklist. 60 Existing cost effectiveness analyses will also be used to identify sources of evidence to inform structural modelling assumptions and parameter values for the economic model.
6.2 Development of a health economic model
A de novo economic evaluation will be constructed, with the primary outcome from the model being an estimate of the incremental cost per additional quality adjusted life year (QALY) gained associated with use of novel techniques of varicose vein management. The time horizon of our analysis will be a patient’s lifetime in order to reflect the chronic nature of the condition and potential mortality. The perspective will be that of the National Health Services and Personal Social Services. Both costs and QALYs will be discounted at 3.5%. 61
The model structure will be determined in consultation with clinical experts. It is expected that a Markov model will be used to follow patient progression following initial treatment into post-treatment health states (reflecting the success or otherwise of treatment and adverse effects of treatment), as well as further recurrences and appearance of new varicosities, although the modelling team have experience in a wide range of different modelling techniques, should these be required following analyses of data. 62–64
Costs will be attached to discrete events (such as treatment of recurrences) as well as ongoing care appropriate to each disease state, allowing lifetime costs to be estimated. Utility values will be associated with each disease/adverse event state to allow total lifetime quality-adjusted-life –years (QALYs) to be calculated. This will allow an analysis of whether novel techniques are more cost effective than traditional surgery, non-foam sclerotherapy or conservative management. Clinical parameters (immediate treatment outcomes, adverse events, recurrence rates) will be taken from the systematic review and meta-analysis of the literature, supplemented by clinical expert opinion where necessary.
Ideally, health related quality of life estimates will be available from the reviewed literature. In the absence of such evidence, the economic model may use indirect evidence on quality of life from alternative sources. Quality of life data will be reviewed and used to generate the quality adjustment weights required for the model. National sources (e.g. NHS reference costs,65 national unit costs66) as well as the reviewed literature will be used to estimate resource use and costs for use in the economic model.
There will inevitably be some uncertainty around parameter estimates, which will be modelled by the use of appropriate distributions around the central estimates. This will allow probabilistic sensitivity analysis to be undertaken on the model results. Through expected value of perfect information analysis67 and, if resources allow, expected value of partial perfect information analyses68 we will identify whether further research is valuable, and in which areas further research is likely to be particularly valuable.
7. Expertise in this TAR team
TAR Centre:
The ScHARR Technology Assessment Group (ScHARR-TAG) undertakes reviews of the effectiveness and cost effectiveness of healthcare interventions for the NHS R&D Health Technology Assessment Programme on behalf of a range of policy makers in a short timescale, including the National Institute for Health and Clinical Excellence. A list of our publications can be found at:
http://www.sheffield.ac.uk/scharr/sections/heds/collaborations/scharr-tag/reports
Much of this work, together with our reviews for the international Cochrane Collaboration, underpins excellence in healthcare worldwide.
Team members' contributions:
Christopher Carroll, Senior Lecturer in Health Technology Assessment, ScHARR: has extensive experience in systematic reviews of health technologies. CC will lead the project and review of effectiveness. He will co-ordinate the review process, protocol development, abstract assessment for eligibility, quality assessment of trials, data extraction, data entry, data analysis and review development of background information and clinical effectiveness.
Silvia Hummel, Research Fellow, ScHARR: will undertake a review of health economic literature relevant to the study question, as well as design, construct, parameterise, and operate an economic model, and interpret its results.
Joanna Leaviss, Research Associate, ScHARR: will assist CC with the abstract assessment for eligibility, quality assessment of trials, data extraction, data entry and data analysis for the clinical effectiveness review.
Anna Cantrell, Systematic Reviews Information Officer, ScHARR: has experience of undertaking literature searches for the ScHARR Technology Assessment Group systematic reviews and other external projects. AC will be involved in developing the search strategy and undertake the electronic literature searches.
John Stevens,Senior Lecturer in Bayesian statistics in health economics, ScHARR: has extensive experience in the design, analysis and reporting of clinical trials for the pharmaceutical industry, and in the application of Bayesian methods to synthesise data and quantify uncertainty about parameters in economic models. He will advise on and carry out the statistical analyses, including the network meta-analysis.
Matt Stevenson,Reader in health technology assessment, ScHARR: has extensive experience in constructing mathematical models used within health technology assessments. He will provide guidance throughout the project.
Andrea Shippam, Programme Administrator: will assist in the retrieval of papers and in preparing and formatting the report.
Clinical and expert advisors:
Jonathan Michaels, Professor of Vascular Surgery, University of Sheffield: has extensive experience of treatment for varicose veins, including experience in leading a large RCT of treatments for the HTA Programme and carrying out systematic reviews for the Cochrane Collaboration.
Dominic Dodd, Consultant Vascular Surgeon, Sheffield Teaching Hospitals. Dominic Dodd is recognised as one of the leading endevenous surgeons in the UK and has over fifteen years experience in the treatment of varicose veins. In addition to conventional surgery he has expertise in the use of endovenous laser, radiofequency ablation and scelrotherapy having performed over 1000 endovenous procedures over the last seven years.
8. Competing interests of authors
The authors do not have any competing interests.
The clinical advisors do not have any competing interests. Dominic Dodd is presently a principal investigator in the CLASS trial comparing endovenous laser ablation, foam sclerotherapy and surgery for varicose veins.
9. Timetable/milestones
The project is expected to run from
| Milestone | |
|---|---|
| Draft protocol | 31 January 2011 |
| Final protocol | 31 March 2011 |
| Start review | 30 June 2011 |
| Progress report | 30 November 2011 |
| Assessment report | 30 December 2011 |
10. References
- Michaels J. A., Campbell W. B., Brazier J. E., MacIntyre J. B., Palfreyman S. J., Ratcliffe J., et al. Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial). Health Technology Assessment 2006;10:1-114.
- Nijsten T., Van Den Bos R. R., Goldman M. P., Kockaert M. A., Proebstle T. M., Rabe E., et al. Minimally invasive techniques in the treatment of saphenous varicose veins. Journal of the American Academy of Dermatology 2009;60:110-9.
- Andreozzi G. M., Cordova R. M., Scomparin A., Martini R., D’Eri A., Andreozzi F. Quality of Life Working Group on Vascular Medicine of SIAPAV . Quality of life in chronic venous insufficiency. An Italian pilot study of the Triveneto Region. Int Angiol 2005;24:272-7.
- Callam M. J. Epidemiology of varicose veins. Br J Surg 1994;81:167-73.
- Evans C. J., Allan P. L., Lee A. J., Bradbury A. W., Ruckley C. V., Fowkes F. G. Prevalence of venous reflux in the general population on duplex scanning: the Edinburgh vein study. J Vasc Surg 1998;28:767-76.
- Evans C. J., Fowkes F. G., Ruckley C. V., Lee A. J. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh vein study. J Epidemiol Commun Health 1999;53:149-53.
- Franks P. J., Wright D. D., Moffatt C. J., Fletcher A. E., Bulpitt C. J. Prevalence of venous disease: a community study in west London. Eur J Surg 1992;158:143-7.
- Cox S. J., Wellwood J. M., Martin A. Saphenous neuritis following varicose vein surgery. BMJ 1974;1:415-7.
- Docherty J. G., Morrice J. J., Bell G. Saphenous neuritis following varicose vein surgery. Br J Surg 1994;81.
- Morrison C., Dalsing M. C. Signs and symptoms of saphenous nerve injury after greater saphenous vein stripping: prevalence, severity, and relevance for modern practice. J Vasc Surg 2003;38:886-90.
- Sam R. C., Silverman S. H., Bradbury A. W. Nerve injuries and varicose vein surgery. Eur J Vasc Endovasc Surg 2004;27:113-20.
- Wood J. J., Chant H., Laugharne M., Chant T., Mitchell D. C. A prospective study of cutaneous nerve injury following long saphenous vein surgery. Eur J Vasc Endovasc Surg 2005;30:654-8.
- Holme J. B., Skajaa K., Holme K. Inidence of lesions of the saphenous nerve after partial or complete stripping of the long saphenous vein. Acta Chir Scand 1990;156:145-8.
- Rutgers P. H., Kitslaar P. J. Randomized trial of stripping versus high ligation combined with sclerotherapy in the treatment of the incompetent greater saphenous vein. Am J Surg 1994;168:311-5.
- Almeida J. I., Raines J. K. Radiofrequency ablation and laser ablation in the treatment of varicose veins. Annals of Vascular Surgery 2006;20:547-52.
- Lurie F., Creton D., Eklof B., Kabnick L. S., Kistner R. L., Pichot O., et al. Prospective randomized study of endovenous radiofrequency obliteration (closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS Study). Journal of Vascular Surgery 2003;38:207-14.
- Perala J., Rautio T., Biancari F., Ohtonen P, Wiik H., Heikkinen T. Radiofrequency endovenous obliteration versus stripping of the long saphenous vein in the management of primary varicose veins: 3-year outcome of a randomised study. Ann Vasc Surg 2005;19:669-72.
- Puggioni A., Kalra M., Carmo M., Mozes G., Gloviczki P. Endovenous laser therapy and radiofrequency ablation of the great saphenous vein: analysis of early efficacy and complications. Journal of Vascular Surgery 2005;42:488-93.
- Rasmussen L. H., Bjoern L., Lawaetz M., Blemings A., Lawaetz B., Eklof B. Randomized trial comparing endovenous laser ablation of the great saphenous vein with high ligation and stripping in patients with varicose veins: short-term results (Brief record). Journal of Vascular Surgery 2007;46:308-15.
- Rautio T., Ohinmaa A, Perala J., Ohtonen P, Heikkinen T., Wiik H. Endovenous obliteration versus conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of the costs. Journal of Vascular Surgery 2002;35:958-65.
- Fischer R, Chandler J. G., Stenger D., Puhan M. A., De Maeseneer M. G., Schimmelpfennig L. Patient characteristics and physician-determined variables affecting saphenofemoral reflux recurrence after ligation and stripping of the great saphenous vein. Journal of Vascular Surgery 2006;31.
- Winterborn R. J., Foy C., Heather B. P., Earnshaw J. J. Randomised trial of flush saphenofemoral ligation for primary great saphenous varicose veins. Eur J Vasc Endovasc Surg 2008;36:477-84.
- Campbell W. B., Kumar A. V, Collin T. W., Allington K. L., Michaels J. A. The outcome of varicose vein surgery at 10 years: clinical findings, symptoms and patient satisfaction. Ann R Coll Surg Engl 2003;85:52-7.
- Winterborn R. J., Foy C., Earnshaw J. J. Causes of varicose vein recurrence: Late results of a randomized controlled trial of stripping the long saphenous vein. Journal of Vasular Surgery 2004;40:634-9.
- Luebke T., Gawenda M., Heckenkamp J., Brunkwall J. Meta-analysis of endovenous radiofrequency obliteration of the great saphenous vein in primary varicosis. Journal of Endovascular Therapy 2008;15:213-2.
- Luebke T., Brunkwall J. Systematic review and meta-analysis of endovenous radiofrequency obliteration, endovenous laser therapy, and foam sclerotherapy for primary varicosis. Journal of Cardiovascular Surgery 2008;49:213-3.
- Jia X., Mowatt G., Burr J. M., Cassar K., Cook J., Fraser C. Systematic review of foam sclerotherapy for varicose veins. British Journal of Surgery 2007;94:925-36.
- van den Bos R., Arends L., Kockaert M., Neumann M., Nijsten T. Endovenous therapies of lower extremity varicosities: A meta-analysis. Journal of Vascular Surgery 2009;49:230-9.
- Gohel M. S., Epstein D. M., Davies A. H. Cost-effectiveness of traditional and endovenous treatments for varicose veins. British Journal of Surgery 2010;97:1815-23.
- AlòS J., Carreño P., LóPez J. A., Estadella B., Serra-Prat M., Marinel-Lo J. Efficacy and safety of sclerotherapy using polidocanol foam: a controlled clinical trial. European Journal of Vascular and Endovascular Surgery: The Official Journal of the European Society for Vascular Surgery 2006;31:101-7.
- Belcaro G., Nicolaides A., Ricci A., Dugall M., Errichi B. M., Vasdekis S. Ebdovascular sclerotherapy, surgery and surgery plus sclerotherapy in superficial venous incompetence: a randomized, 10-year follow up trial – final results. Angiology 2000;51:529-34.
- Belcaro G., Cesarone M. R., Di, Renzo A., Brandolini R., Coen L., et al. Foam-sclerotherapy, surgery, sclerotherapy, and combined treatment for varicose veins: A 10-year, prospective, randomized, controlled, trial (VEDICO* trial). Angiology 2003;54:307-15.
- Bountouroglou D. G., Azzam M., Kakkos S. K., Pathmarajah M., Young P., Geroulakos G. Ultrasound-guided foam sclerotherapy combined with sapheno-femoral ligation compared to surgical treatment of varicose veins: early results of a randomised controlled trial. European Journal of Vascular &Amp; Endovascular Surgery 2006;31:93-100.
- Hamel-Desnos C., Desnos P., Wollmann J. C., Ouvry P., Mako S., Allaert F. A. Evaluation of the efficacy of polidocanol in the form of foam compared with liquid form in sclerotherapy of the greater saphenous vein: initial results. Dermatologic Surgery 2003;29:1170-5.
- Kern P, Ramelet A.-A., Wutschert R, Bounameaux H., Hayoz D. Single-blind, randomized study comparing chromated glycerin, polidocanol solution, and polidocanol foam for treatment of telangiectatic leg veins. Dermatologic Surgery 2004;30:367-72.
- Martimbeau P. R. 2003.
- Rao J., Goldman M. P. Stability of foam in sclerotherapy: differences between sodium tetradecyl sulfate and polidocanol and the type of connector used in the double-syringe system technique. Dermatologic Surgery 2005;31:19-22.
- Wright D., Gobin J.-P., Bradbury A., Coleridge, Smith P., Spoelstra H, et al. Varisolve polidocanol microfoam compared with surgery or sclerotherapy in the management of varicose veins in the presence of trunk vein incompetence: European randomized controlled trial. Phlebology 2006;21:180-9.
- Hinchliffe R. J., Ubhi J., Beech A., Ellison J., Braithwaite B. D. A prospective randomised controlled trial of VNUS closure versus surgery for the treatment of recurrent long saphenous varicose veins. European Journal of Vascular and Endovascular Surgery 2006;31:212-8.
- Kianifard B., Holdstock J. M., Whiteley M. S. Radiofrequency ablation (VNUS closure) does not cause neo-vascularisation at the groin at one year: Results of a case controlled study. Surgeon 2006;4:71-4.
- Lurie F., Creton D., Eklof B., Kabnick L. S., Kistner R. L., Pichot O., et al. Prospective randomized study of endovenous radiofrequency obliteration (Closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS Study). Journal of Vascular Surgery 2003;38:207-14.
- Lurie F., Creton D., Eklof B., Kabnick L., Kistner R. L., Pichot O. Prospective randomised study of endovenous radiofrequency obliteration (losure) versus ligation and vein stripping (EVOLVeS): two-year follow-up. Eur J Vasc Endovasc Surg 2005;29:67-73.
- Stötter L., Schaaf I., Bockelbrink A. Comparative outcomes of radiofrequency endoluminal ablation, invagination stripping, and cryostripping in the treatment of great saphenous vein insufficiency. Phlebology 2006;21:60-4.
- Carradice D., Mekako A., Hatfield J., Chetter I. Randomized clinical trial of concomitant or sequential phlebectomy after endovenous laser therapy for varicose veins. British Journal of Surgery 2009;96:369-75.
- de Medeiros C, Luccas G. Comparison of endovenous treatment with an 810mm laser versus conventional stripping of the great saphenous vein in patients with primary varicose veins. Dermatologic Surgery 2005;31:1685-94.
- Rasmussen L. H., Bjoern L., Lawaetz M., Blemings A., Lawaetz B., Eklof B. Randomized trial comparing endovenous laser ablation of the great saphenous vein with high ligation and stripping in patients with varicose veins: short-term results. Journal of Vascular Surgery 2007;46:308-15.
- Christenson J. T., Gueddi S., Gemayel G., Bounameaux H. Prospective randomized trial comparing endovenous laser ablation and surgery for treatment of primary great saphenous varicose veins with a 2-year follow-up. Journal of Vascular Surgery 2010;52:1234-41.
- Gale S. S., Lee J. N., Walsh M. E., Wojnarowski D. L., Comerota A. J. A randomized, controlled trial of endovenous thermal ablation using the 810-nm wavelength laser and the ClosurePLUS radiofrequency ablation methods for superficial venous insufficiency of the great saphenous vein. Journal of Vascular Surgery 2010;52:645-50.
- Lin Y, Ye C, Huang X, Ye J, Yin H, Wang S. A random, comparative study on endovenous laser therapy and saphenous vein stripping for the treatment of great saphenous vein incompetence. Chinese Medical Journal 2010;87:3043-6.
- Shepherd A. C., Gohel M. S., Brown L. C., Metcalfe M. J., Hamish M., Davies A. H. Randomized clinical trial of VNUSClosureFAST radiofrequency ablation versus laser for varicose veins. British Journal of Surgery 2010;97:810-8.
- Theivacumar N. S., Dellagrammaticas D., Mavor A. I. D., Gough M. J. Endovenous laser ablation: Does standard above-knee great saphenous vein ablation provide optimum results in patients with both above- and below-knee reflux? A randomized controlled trial. Journal of Vascular Surgery 2008;48:173-8.
- Goode S. D., Chowdhury A., Crockett M., Beech A., Simpson R., Richards T., et al. Laser and radiofrequency ablation study (LARA study): A randomised study comparing radiofrequency ablation and endovenous laser ablation (810 nm). European Journal of Vascular and Endovascular Surgery 2010;40:246-53.
- Nijsten T., Van Den Bos R. R., Goldman M. P., Kockaert M. A., Proebstle T. M., Rabe E., et al. Minimally invasive techniques in the treatment of saphenous varicose veins. Journal of the American Academy of Dermatology 2009;60:110-9.
- Rigby K., Palfreyman S. S. J., Beverley C., Michaels J. A. Surgery versus sclerotherapy for the treatment of varicose veins. Cochrane Database of Systematic Reviews 2004.
- Schmedt C.-G., Sroka R., Steckmeier S., Meissner O. A., Babaryka G., Hunger K., et al. Investigation on Radiofrequency and Laser (980 nm) Effects after Endoluminal Treatment of Saphenous Vein Insufficiency in an Ex-vivo Model. European Journal of Vascular and Endovascular Surgery 2006;32:318-25.
- Chetter I., Mylankal K., Hughes H., Fitridge R. Randomized clinical trial comparing multiple stab incision phlebectomy and transilluminated powered phlebectomy for varicose veins. British Journal of Surgery 2006;93:169-74.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Annals of Internal Medicine 2009;151:264-9.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisals 2008. URL: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf.
- Stevenson M, Simpson E, Rawdin A, Papaioannou D. A review of discrete event simulation in National Coordinating Centre for Health Technology Assessment funded work and a case study exploring the cost-effectiveness of testing for thrombophilia in patients presenting with an initial idiopathic venous thromboembolism. Journal of Simulation 2010;4:14-23.
- Stevenson M, Scope A, Sutcliffe P. The cost effectiveness of group cognitive behavioural therapy compared with routine primary care for women with postnatal depression in the UK. Value in Health 2010;13:580-4.
- Michaels J. A., Campbell W. B., King B, Palfreyman S. J., Shackley P, Stevenson M. A Randomised Controlled Trial and Cost-effectiveness Analysis of Antimicrobial silver Antimicrobial Dressings for Venous Leg Ulcers: The VULCAN Trial. British Journal of Surgery 2009;96:1147-56.
- Eckermann S, Willan A. Expected Value of Information and Decision Making in HTA. Health Economics 2007;16:195-209.
- Felli JC, Hazen GB. Sensitivity analysis and the expected value of perfect information. Medical Decision Making 1998;18:95-109.
11. Appendices
A. Draft Medline search strategy
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1950 to Present>
Search Strategy:
-
Varicose Veins/ (10,432)
-
varicose vein.tw. (854)
-
varicose veins.tw. (4141)
-
vein, varicose.tw. (7)
-
veins, varicose.tw. (17)
-
varices.tw. (9734)
-
varix.tw. (915)
-
varicosis.tw. (381)
-
Saphenous Vein/ (12097)
-
(saphenous adj2 vein$).tw. (10,413)
-
(saphena adj2 vein$).tw. (39)
-
or/1-11 (33471)
-
laser ablation.tw. (2406)
-
evla.tw. (54)
-
radiofrequency ablation.tw. (5556)
-
radiofrequency ablation.tw. (379)
-
rfa.tw. (1992)
-
foam sclerotherapy.tw. (169)
-
ugfs.tw. (18)
-
illuminated phlebectomy.tw. (0)
-
tipps.tw. (8)
-
or/13-21 (8991)
-
12 and 22 (323)
Appendix B: Data extraction forms
| Ref Man ID | Study Author, date, country | Study design | Inclusion criteria (incl. criteria for diagnosis) |
Exclusion criteria (incl. number excluded) |
Intervention | Intervention group characteristics N = 1.Age, sex (f/m) 2.Co-morbidities |
Comparator | Comparison group characteristics N = 1.Age, sex (f/m) 2.Co-morbidities |
|---|---|---|---|---|---|---|---|---|
| Ref Man ID | Study | Follow-up | Symptoms (I vs C) | Numbers with recurrence (I vs C) |
Numbers needing a second intervention (I vs C) | Mortality (I vs C) | Adverse events or complications (I vs C) |
Quality of life Cost utilisation |
|---|---|---|---|---|---|---|---|---|
Appendix C: RCT Critical Appraisal quality assessment criteria
Trial quality assessment
| Phase III trial | |
|---|---|
| Was the method used to assign participants to the treatment groups really random? | |
| What method of assignment was used? | |
| Was the allocation of treatment concealed? | |
| What method was used to conceal treatment allocation? | |
| Was the number of participants who were randomised stated? | |
| Were details of baseline comparability presented? | |
| Was baseline comparability achieved? | |
| Were the eligibility criteria for study entry specified? | |
| Were any co-interventions identified that may influence the outcomes for each group? | |
| Were the outcome assessors blinded to the treatment allocations? | |
| Were the participants who received the intervention blinded to the treatment allocation? | |
| Was the success of the blinding procedure assessed? | |
| Were at least 80% of the participants originally included in the randomised process followed up in the final analysis? | |
| Were the reasons for withdrawal stated? | |
| Was an intention-to-treat analysis included? |
Glossary
- Ablation
- The removal or destruction of particular tissues (e.g. the incompetent vein).
- Duplex ultrasound
- An imaging test to investigate patients with chronic venous disease.
- European Quality of Life-5 Dimensions
- A standardised measure of health status developed by the EuroQol Group.
- Ligation and stripping
- The ‘tying-off’ of the great saphenous vein and the removal of the incompetent vein through incisions.
- Occlusion
- The creation of a blockage in a vein.
- Phlebectomy
- A procedure by which varicose tributaries are removed with small hooks with the use of local anaesthetic.
- Recanalisation
- The process by which a previously occluded vein regains patency (i.e. flow is re-established).
- Reflux
- Retrograde flow.
- Sclerosant
- The medium injected into a vein (e.g. foam or liquid).
- Sclerotherapy
- A procedure in which a medication is injected into a vein in order to occlude it.
List of abbreviations
- AVSS
- Aberdeen Varicose Vein Symptom Score
- AVVQ
- Aberdeen Varicose Veins Questionnaire
- CDSR
- Cochrane Database of Systematic Reviews
- CEAP
- clinical status, aetiology, anatomy, pathophysiology
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CrI
- credible interval
- CVD
- chronic venous disease
- DARE
- Database of Abstracts of Reviews of Effects
- DES
- discrete event simulation
- DUS
- duplex ultrasound scanning
- DVT
- deep-vein thrombosis
- EQ-5D
- European Quality of Life-5 Dimensions
- EVLA
- endovenous laser ablation
- FS
- foam sclerotherapy
- GA
- general anaesthetic
- GP
- general practitioner
- GSV
- great saphenous vein
- HES
- Hospital Episode Statistics
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- LA
- local anaesthetic
- LS
- liquid sclerotherapy
- MD
- mean difference
- MSIP
- multistab incision phlebectomy
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NS
- non-significant difference
- PE
- pulmonary embolism
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RFA
- radiofrequency ablation/obliteration
- SCI
- Science Citation Index
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-36
- Short Form questionnaire-36 items
- SFJ
- saphenofemoral junction
- SSCI
- Social Science Citation Index
- SSV
- short saphenous vein
- TIPP
- transilluminated-powered phlebectomy
- UGFS
- ultrasound-guided foam sclerotherapy
- VAS
- visual analogue scale
- VCSS
- Venous Clinical Severity Score