Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/74/01. The contractual start date was in January 2013. The draft report began editorial review in March 2013 and was accepted for publication in June 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Kaltenthaler et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the health problem
Severe mental illness (SMI) describes a wide range of major psychiatric disorders (including schizophrenia and bipolar disorder)1 which persist over time and cause extensive disability leading to impairments in social and occupational functioning. 2 There are occasional inconsistencies in the use of the term and some studies may include severe anxiety and depressive symptoms and borderline personality. Schizophrenia is estimated to affect approximately 180,4713 to 220,0004 people in the UK and bipolar disorder approximately 136,4403 to 297,000. 5 The incidence of schizophrenia is higher in men than in women6 whereas bipolar disorder is equally distributed. 5 Incidence rates among black and ethnic minority groups are higher than in a comparable white population. 5,6 About 45% of people who receive a diagnosis of schizophrenia experience recovery after one or more episodes, but about 20% show continuous symptoms and increasing disability and the remaining 35% show a mixed pattern with varying periods of remission and relapse. 7 For most patients, bipolar disorder is chronic and recurrent. Recovery may or may not be complete between episodes. Considerable variability exists in the pattern of remissions and relapses; however, remissions tend to get shorter as time goes on and depressions become more frequent and longer lasting. 5
People with SMI have higher morbidity and mortality due to physical illness than the general population. 8,9 De Hert et al. 10 reported prevalence rates of several physical illnesses in people with SMI. They found nutritional and metabolic, cardiovascular, viral, respiratory tract and musculoskeletal diseases as well as sexual dysfunction, pregnancy complications, stomatognathic diseases and possibly obesity-related cancers to be more prevalent among people with SMI. Levels of obesity are extremely high among people with SMI – nearly twice those of the overall population. 11 This can have a perceived effect on sexual attractiveness and consequent functioning. Daumit et al. 11 conducted a randomised controlled trial (RCT) to address this serious problem. The study of overweight or obese adults with SMI used a behavioural weight loss intervention programme and resulted in significantly reduced weight over a period of 18 months in the group of patients receiving the intervention.
Reasons for physical health problems in people with SMI were explored by Robson and Gray,9 who found that limitations in health services, the effects of having a SMI, health behaviours and the effects of psychotropic medication may contribute to disparities in health. A systematic review by Tosh et al. 12 found no evidence from RCTs that physical health-care monitoring for people with SMI is useful in preventing deterioration in physical health and maintaining quality of life, although the authors add that this does not mean that physical health monitoring does not affect the physical health of people with SMI.
People with SMI are often on medications, which may have an impact on sexual function. The European Schizophrenia Outpatient Health Outcome (SOHO) study,13 a large prospective, observational study of the health outcomes associated with antipsychotic treatment in approximately 11,000 outpatients with schizophrenia in 10 European countries, found that sexually related adverse events were frequent at baseline before commencing medication and included erectile dysfunction in 40% of men and loss of libido in 50% of both male and female patients. The study also found that some second-generation atypical antipsychotics such as olanzapine (ZypAdhera®, Eli Lilly and Company), clozapine (Zaponex®, TEVA UK) and quetiapine (Seroquel®, AstraZeneca) were significantly less likely to result in drug-induced sexual dysfunction after six months of treatment than other atypical antipsychotics [risperidone (Risperdal Consta®, Janssen-Cilag Ltd) and amisulpride (Solian®, Sanofi-aventis)]. Montejo et al. 14 report in a study of 243 patients with a psychotic disorder that 46% of patients exhibited drug-related sexual dysfunction, which included 50% of the males and 37% of females in the study. A review by Baggaley15 that looked at the relative impact of antipsychotics on sexual dysfunction found that risperidone was associated with the greatest level of sexual dysfunction and aripiprazole (Abilify®, Bristol-Myers Squibb) with the least. Sexual dysfunction included reduced libido, erectile dysfunction, ejaculatory difficulties and impaired orgasm in men and menstrual irregularity/amenorrhoea, reduced libido, impaired orgasm and decreased vaginal lubrication in women. Various strategies have been suggested for the management of sexual dysfunction due to antipsychotic drug therapy, including dose reduction, drug holidays, adjunctive medication and switching to another drug. These options were reviewed by Schmidt et al.,16 who found little evidence to support these strategies, although sildenafil (Viagra®, Pfizer; Revatio®, Pfizer), a drug typically prescribed to men to treat erectile dysfunction, was considered to be a useful treatment option in men with schizophrenia. Although antipsychotic medication is an important cause of sexual dysfunction in SMI, Aizenberg et al. 17 found that even patients with a diagnosis of schizophrenia who were not receiving medication exhibited decreased sexual desire. Few studies have examined diagnostic differences and sexual activity and results have been inconclusive. 18
People with SMI are more likely to engage in high-risk sexual behaviour (e.g. unprotected intercourse, having multiple partners, involvement in the sex trade and illicit drug use), putting them at risk of poorer sexual health outcomes, including sexually transmitted infections (STIs), e.g. with human immunodeficiency virus (HIV). 2,19,20 HIV infection rates among people with SMI have been estimated to be approximately 6.9%, much higher than rates for the general US population for the same time period, estimated to be 0.4%. 18 The HIV infection range among those with SMI is reported to be 3–23%, with the highest rates found among those with SMI and substance abuse disorders. 21 Homeless people with SMI were also reported to have higher rates. Among people with SMI, the age and ethnic distributions of those infected with HIV appear to be similar to those in the general population, although women with SMI are as likely as males to be infected with HIV, in contrast to the general US population, in which the ratio of men to women is 5 : 1. 18 A number of risk behaviours are thought to contribute to the higher rates of HIV among people with SMI, and these include comorbid alcohol and drug use disorders; more frequent same-sex intercourse than the non-psychiatric population; multiple sex partners; lack of condom use; trading sex for drugs or money; and transient living circumstances, among others. 18 A review of the risk behaviour associated with people with SMI explored this more fully. 2 The authors found 52 studies which showed that the majority of adults with SMI were sexually active and engaged in risk behaviours associated with HIV transmission. Correlates of HIV risk were organised into the domains of psychiatric illness, substance use, childhood abuse, cognitive behavioural factors, social relationships and demographics. Most studies identified in this review found that people with a schizophrenia spectrum disorder were significantly less likely to be sexually active compared with those with other major psychiatric disorders. Although many correlates of HIV risk factors were identified in this review, the authors suggest caution in view of the cross-sectional design of many studies and the heavy reliance on univariate analyses. Most studies also recruited participants from centres where they were receiving treatment; thus, the results may not be generalisable to people with SMI who do not seek or receive mental health care. Meade et al. 22 reviewed the evidence regarding the relationship between substance abuse and HIV sexual risk behaviour among people with SMI. The authors concluded that little is known about how substance misuse contributes to sexual health risk. Gray et al. 19 reported that, although people with schizophrenia are more likely than the general population to engage in high-risk behaviours (e.g. men having sex with men, illicit drug use and having multiple sexual partners), many health policy reports do not address the risk of HIV in people with schizophrenia and recommend that research, policy and clinical practice need to be developed to address this issue.
Many individuals with SMI will also have co-existing drug and alcohol problems23 and difficulties in establishing stable and sexual relationships. 9 O’Cleirigh and Safren24 suggest that those with SMI and substance use disorders present complexities that may be better suited to adapted or modified models of cognitive behavioural therapy to support HIV prevention. The authors suggest that these models need to incorporate design elements of efficacy (such as random assignment) and design elements that support external validity (such as usual treatment comparison). Individuals with SMI report high rates of childhood sexual abuse, which is associated with sexual risk behaviour in individuals with SMI as well as in the general population. 25
Sexual health promotion interventions (such as educational and behavioural interventions, motivational exercises, counselling and service delivery), developed and implemented for people with SMI, may improve participants’ knowledge, attitudes, beliefs or behavioural practices (including assertiveness skills) and could lead to a reduction in risky sexual behaviour. 20 McCann26 states that although there have been many initiatives in the UK to make health and social care more responsive and inclusive, the sexual needs of individuals with psychosis appear to remain marginalised and neglected. In this study of 30 people with schizophrenia living in community, 90% of clients felt some need in relation to sexual expression and only 10% of staff recognised sexual expression as a need in their clients.
Two narrative reviews of interventions to improve the sexual health of people with SMI were identified. Johnson-Masotti et al.,27 in their review of the clinical effectiveness and cost-effectiveness studies of HIV prevention interventions, report that the studies identified in their review have shown only limited success in helping people with SMI reduce their HIV risk. The cost-effectiveness literature they identified also showed mixed results. Higgins et al. 20 undertook a review on sexual health education. The authors identified six studies that were descriptive or anecdotal, three using post-evaluation or pre–post-evaluation designs and five studies with a randomised intervention design. The review found that people who attended sexual health education programmes which were facilitated in a sensitive and supportive manner developed attitudes more favourable to condom use, improved intention to avoid unsafe sexual practices, reduced the number of casual partners and were less likely to engage in unprotected vaginal intercourse. Education tended to produce a self-reported reduction in sexual risk behaviour as opposed to complete cessation. Small-group interventions combining information giving, motivational exercises and skills acquisition were found to be effective in reducing sexual risk behaviour and raising awareness of personal risk behaviour. The authors highlight the limitations of the included studies such as small sample size, lack of quality tools tested for reliability and validity and short follow-up periods. Most outcomes were self-reported and participants were self-selected. Studies did not describe method of random allocation or provide information on refusal or attrition rates and were mostly conducted in the USA.
Other studies identified in the literature explored different aspects of sexual health interventions. Melo et al. 28 conducted a cross-sectional national multicentre study throughout Brazil to measure HIV/acquired immunodeficiency syndrome (AIDS) knowledge among patients with mental illness. The study included 2475 patients from 26 mental health institutions. The authors found that psychiatric patients in Brazil lag behind the general population with regard to HIV/AIDS knowledge scores. Sikkema et al. 21 report a pilot study involving a community level HIV prevention programme for people with SMI living in supportive housing. This study of 28 residents involved a cognitive behavioural skills training HIV risk reduction programme and a 4-month follow-up period. The results were considered promising and demonstrated significant improvements in psychosocial risk factors with indications of sexual behaviour change at follow-up. DiFranceisco et al. 29 looked at differences between completers and early dropouts from two HIV intervention trials, including one with severely mentally ill participants. Non-attendees in the SMI group were younger than completers, more likely to associate condom use with positive outcomes and somewhat more confident of their ability to negotiate safe sex with their partners. Johnson-Masotti et al. 30 assessed the cost-effectiveness of a RCT by Kelly et al.,31 which had three interventions: single session, one-on-one, multisession small group and multisession small-group advocates. For men, all three were found to be cost-effective, with advocacy training the most cost-effective. Cost-effectiveness was measured using cost–utility analysis in terms of the quality-adjusted life-years saved by the intervention as a result of preventing participants from becoming infected with AIDS. For women, only the single-session intervention was cost-effective.
Blank and Hennessy32 explored the feasibility of a reasoned action approach for this population through the use of two interventions: Preventing AIDS Through Health (PATH), delivered by case managers to persons with mental illnesses who were HIV negative, and PATH PLUS, delivered by nurses to persons with mental illness who were HIV positive. The authors concluded that this approach may be useful for changing behaviour and intentions in people with SMI. As part of the same study, Tennille et al. 33 conducted focus groups and interviews with case managers and administrators to determine changes in the service and the case managers as a result of their skills training and experience. Case managers felt transformed by their training experience and that this improved their understanding of the issues around sexual health faced by their clients. In a related study by Tennille et al.,34 the researchers held focus groups with people with mental illnesses to focus on HIV risks and condom use. Participants discussed the sexual side effects of medication and how this may be associated with HIV risk behaviours, such as not using a condom. A final qualitative study by Solomon et al. 35 used rapid assessment procedures to look at HIV prevention services, and found that case managers had little formalised training on HIV prevention and felt that doctors or nurses would be better placed to deliver HIV prevention interventions.
The studies described above highlight the challenges and risk of poor sexual health outcomes (including establishing relationships) encountered by people with SMI, which may be exaggerated by co-existing drug and alcohol problems. The purpose of this review is to evaluate the effectiveness of sexual health interventions that may be suitable for people with SMI in the UK.
Current service provision
Health promotion interventions, whether brief interventions or longer-term programmes, are used to improve various aspects of health and health behaviours. Interventions can be delivered at different levels (individual, group and community) through an action or group of actions intended to change the knowledge, attitudes, beliefs or behavioural practices of individuals and populations to reduce their sexual health risk. 36 Individual-level interventions focus on one individual at a time in an attempt to help change behaviour and may include partner notification, individual risk counselling by professionals and detached education and outreach (for those not accessing mainstream services). Group-level interventions are delivered to small groups of individuals. Group-level interventions use peer and non-peer models involving a range of skills, information and support. Community-level interventions are delivered by or within a defined ‘community’. They seek to improve the risk conditions and influence behaviour by changing social norms, policies or characteristics of the environment. 36,37
Mental health nurses and other professionals working with people with SMI would be well placed to include sexual health as part of their contribution to health promotion. 20 A study by Apantaku-Olajide et al. 38 used a survey questionnaire to determine mental health patients’ attitude to psychotropic medication for sexual dysfunction and found that participants were willing to discuss their sexual problems with health-care professionals. However, staff may require specific training in order to deliver such interventions. In a qualitative study of 27 mental health nurses in Ireland, Higgins et al. 39 found that the main concerns reported by the nurses around sexuality were related to feelings of personal and professional vulnerability, due to a lack of competence, comfort and confidence in this area. Penna and Sheehy40 administered questionnaires to occupational therapists involved in the care of people with SMI in the UK to determine their attitudes towards sex education for people with schizophrenia. The majority of occupational therapists thought sex education was within the domain of their profession but were not providing sex education currently. The study found that the length of time in practice and knowledge of employers’ policies may influence this. In NHS Shetland all mental health staff working with people with psychiatric illnesses receive sexual health training,36 although it is not clear exactly what this training includes and whether or not this varies with different types of staff.
Overall aims and objectives of assessment
The review will aim to evaluate the following objectives:
-
evaluate the effectiveness of sexual health interventions for people with SMI compared with usual care and their applicability to the UK NHS setting
-
identify key areas for primary research.
Chapter 2 Assessment of clinical effectiveness
A systematic review of the literature was undertaken to evaluate the effectiveness of sexual health interventions for people with SMI compared with usual care. A review of the evidence was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (www.prisma-statement.org).
Methods for reviewing effectiveness
Identification of studies
Electronic databases
Studies were identified by searching the following electronic databases and research registers:
-
MEDLINE(R) In-Process & Other Non-Indexed Citations and MEDLINE(R) (Ovid) 1948 to December 2012
-
EMBASE (Ovid) 1980 to December 2012
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL; EBSCO) 1982 to December 2012
-
Cochrane Database of Systematic Reviews (Wiley Online Library) 1996 to December 2012
-
Cochrane Central Register of Controlled Trials (CENTRAL; Wiley Online Library) 1898 to December 2012
-
Health Technology Assessment Database (HTA; Wiley Online Library) 1995 to December 2012
-
Database of Abstracts of Reviews of Effects (DARE; Wiley Online Library) 1995 to December 2012
-
Psychological Information Database (PsycINFO; Ovid) 1806 to December 2012
-
Conference Proceedings Index-Science (Web of Science) 1990 to December 2012
-
Conference Proceedings Citation Index-Social Science & Humanities (Web of Science) 1990 to December 2012
-
UK Clinical Research Network (CRN) Portfolio Database [National Institute for Health Research (NIHR)] (www.crncc.nihr.ac.uk)
-
ClinicalTrials.gov (US NIH) (www.clinicaltrials.gov)
-
The metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com/mrct/).
Sensitive keyword strategies using free text and, where available, thesaurus terms using Boolean operators and database-specific syntax were developed to search the electronic databases. Synonyms relating to the condition (e.g. severe mental illness) were combined with terms for sexual health interventions (e.g. sexual behaviour, sexually transmitted diseases and sexual health). A pre-defined validated methodological filter aimed at restricting search results to controlled trials was used in the searches of MEDLINE, EMBASE, CINAHL and PsycINFO. Date and language restrictions were not used on any database. An example of the MEDLINE search strategy is provided in Appendix 2.
Other resources
To identify additional published, unpublished and ongoing studies, the reference lists of all relevant studies (including identified reviews) were checked for relevant studies and a citation search of relevant articles (using the Web of Knowledge’s Science Citation Index and Social Science Citation Index) was undertaken to identify articles that cite the relevant articles. In addition, systematic keyword searches of the internet and organisational websites, especially in the context of the UK (e.g. Royal College of Psychiatrists, British Psychological Society, Department of Health, Welsh Assembly Government, International AIDS Society, the Campbell Collaboration and National Collaborating Centre for Mental Health) were undertaken and key experts in the field were contacted.
All identified citations from the electronic searches and other resources were imported into and managed using the Reference Manager bibliographic software, version 12.0 (Thomson Reuters, Philadelphia, PA, USA).
Inclusion and exclusion criteria
The inclusion of potentially relevant articles was undertaken using a two-step process. First, all titles were examined for inclusion by one reviewer. Any citations that clearly did not meet the inclusion criteria (i.e. non-human, unrelated to sexual health interventions for people with SMI) were excluded. Second, all abstracts and full-text articles were examined independently by two reviewers. Any disagreements in the selection process were resolved through discussion. The relevance of each article for the systematic review was assessed according to the following criteria.
Study design
All controlled trials (randomised or non-randomised) that evaluated sexual health interventions with usual care for adults with SMI were included. Before-and-after studies without a concurrent control group were excluded because the absence of a control group to record concurrent changes over time means that changes due to the intervention or due to temporal trends, concurrent changes or a Hawthorne effect would be conflated. Such studies therefore represent very weak evidence of effectiveness. 41,42
Reviews of primary studies were not included in the analysis, but were retained for discussion and identification of additional studies. Moreover, the following publication types were excluded from the review: animal models; preclinical and biological studies; editorials; opinions; non-English-language papers; and reports published as meeting abstracts only, where insufficient methodological details are reported to allow critical appraisal of study quality. Studies from developing countries were also excluded as it is difficult to generalise (e.g. transferability and acceptability) the characteristics of the effective interventions to developed countries.
Population
The population comprised adults (defined as ≥ 18 years of age) with SMI living in the community and their carers or staff. SMI was defined as people who have received a diagnosis of schizophrenia or bipolar disorder. 1 Adults with dementia, personality disorder, chronic depression or intellectual disability were excluded as they are not included in the definition of SMI.
Interventions
Any health promotion intervention or combination of interventions (e.g. educational, behavioural, psychological, counselling, etc. delivered at individual, group and community levels) intended to change the knowledge, attitudes, beliefs, behaviours or practices of individuals and populations to improve their sexual health were included. Interventions that focused on sexual dysfunction and sexual violence or prescribed drugs were excluded.
Relevant comparators
The relevant comparator was considered as standard usual care in the community. This may involve ad hoc advice on health risks by health-care professionals working with people with SMI, but is not integrated into routine practice. Studies including active control groups were also included.
Outcomes
The outcomes of the review included the following:
-
biological: STIs (including HIV), unintended pregnancy
-
behavioural: numbers of partners, use of contraception/condoms, uptake of screening or treatment services
-
proxy: knowledge, attitudes and beliefs, barriers and facilitators, intentions, skills.
Data abstraction strategy
Data abstraction was performed by one reviewer into a standardised data extraction form and independently checked for accuracy by a second. Discrepancies were resolved through discussion. Where multiple publications of the same study were identified, data were extracted and reported as a single study. Where necessary, authors of the included studies were contacted to provide further details or clarification.
The following information was extracted for all studies when reported: study characteristics (e.g. author, year of publication, country, duration of follow-up, funding); participant details (e.g. age, sex, diagnosis, level of education, marital status, comorbidities); intervention and comparator details (e.g. description, frequency of measurement, parameters measured); and outcomes (including definitions).
Quality assessment strategy
The methodological quality of each included study was assessed by one reviewer and independently checked by another. Disagreements were resolved through discussion. The study quality characteristics were assessed according to (adapted) criteria based on those proposed by the Effective Public Health Practice Project (EPHPP) (www.ephpp.ca/Tools.html). 43 This is a generic tool used to evaluate a variety of intervention study designs such as controlled trials and observational studies. This tool has been judged suitable for use in systematic reviews of effectiveness44 and has been reported to have content and construct validity. 43,45 Consideration of study quality included the following six criteria: (1) selection bias – the extent to which study participants were representative of the target population; (2) study design; (3) control of confounders; (4) blinding – whether outcome assessors, intervention providers and participants were aware of the research question; (5) data collection methods; and (6) withdrawals and dropouts. The six domain-based criteria were each rated as strong, moderate or weak depending on the characteristics of each criterion reported in the included study. An overall assessment of study quality was based on the following ratings: studies with at least four criteria rated as ‘strong’ and with no criteria rated as ‘weak’, were given an overall rating of ‘strong’. Those studies receiving less than four ‘strong’ ratings and only one ‘weak’ rating were given an overall rating of ‘moderate’. A rating of ‘weak’ was given if two or more criteria were rated as ‘weak’. Additional study quality criteria included an assessment of intervention integrity, statistical analysis and generalisability to the UK. Further details of the assessment tool (and dictionary) are provided in Appendix 3.
Methods of data synthesis
Data were tabulated and discussed in a narrative review. A meta-analysis was not possible owing to the heterogeneity of study designs, interventions and types of outcome data available.
Results
Quantity and quality of research available
Number of studies identified and included
The literature searches identified 2590 citations. Of these, 13 studies (representing 14 references)31,46–58 met the inclusion criteria. These studies were all defined by the authors as RCTs. A flow chart describing the process of identifying relevant literature can be found in Figure 1.
FIGURE 1.
Study flow chart (adapted PRISMA diagram).
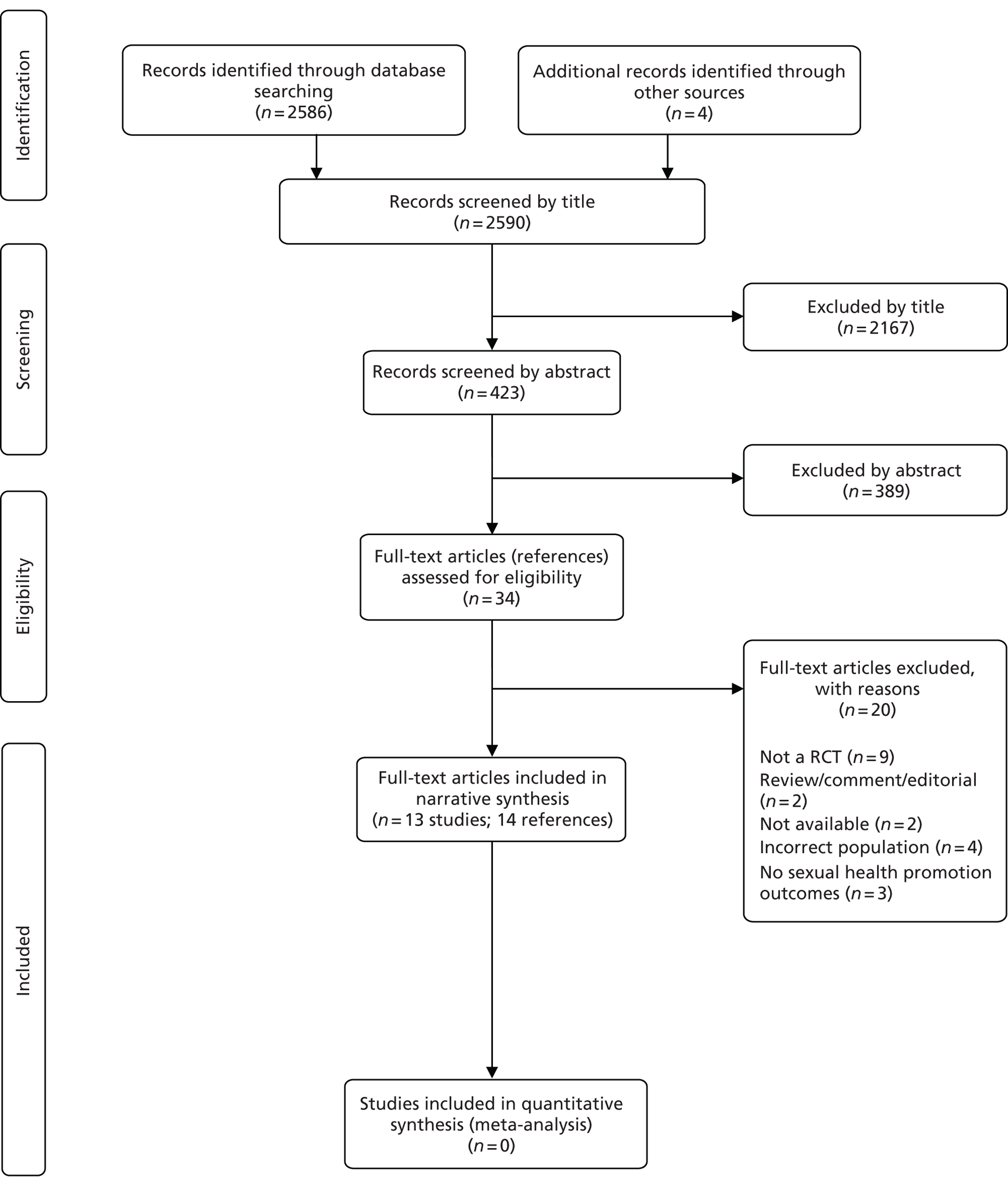
Number and type of studies excluded
A total of 20 full-text articles were excluded as they did not meet all the pre-specified inclusion criteria. The majority of the articles were excluded primarily on the basis of inappropriate study design (not controlled clinical trials),21,28,32–35,40,59,60 incorrect population (adolescents,61 inpatients only62 or patients without SMI),63,64 outcomes not identified in the protocol,65–67 not available68,69 or unsuitable publication type (reviews, commentaries or editorials). 70,71 Of the two studies that were not available, one study appeared to be a conference abstract69 of an included study (Susser et al. 56). The other study,68 which was identified on a trials register, was a planned open-label RCT that was designed to evaluate the effectiveness of motivational interviewing plus skill-building exercises compared with behavioural skill-building exercises alone in reducing HIV risk behaviour in people with SMI. Although the authors of the study were contacted, no further details were forthcoming, including information on study completion (and if the results were unpublished) or recruitment failure. A full list of excluded studies with reasons for exclusion is presented in Appendix 4.
Description of included studies (design and study characteristics)
The study characteristics of the included studies are shown in Table 1 and presented as described in the studies. All studies were published between 1996 and 2012, included populations with mental illness and were conducted in the USA. It should be noted that not all studies used the term ‘severe mental illness’, with other descriptions used including ‘chronically mentally ill outpatients’ (Katz et al. 52 and Kalichman et al. 51). The population groups are briefly described in Table 1. Sample sizes ranged from 20 (Weinhardt et al. 58) to 408 (Carey et al. 49) patients. Study populations were recruited from different settings and included homeless shelters (Berkman et al. ,46,47 Linn et al. 53 and Susser et al. 56), outpatient psychiatric clinics [Berkman et al. ,48 Carey et al. ,49 Kalichman et al. ,51 Kelly et al. ,31 National Institute of Mental Health (NIMH),57 Otto-Salaj et al. 55 and Weinhardt et al. 58], residential facilities in a community setting (Collins et al. 50), a drop-in socialisation centre (Katz et al. 52), and a treatment programme for substance misusers (Malow et al. 54). Length of follow-up ranged from 2 weeks (Katz et al. 52) to 18 months (Susser et al. 56).
| Study, year, country | Sample size (at baseline) | Population | Intervention | Control | Duration of intervention and follow-up | Power calculation | Funding |
|---|---|---|---|---|---|---|---|
| Berkman et al., 2006,46,47 New York, USA | 92 | Men attending a homeless shelter with SMI | Social skills intervention (SexG-Brief: six sessions). Interventions included the following:46
|
Standard (2-hour) HIV educational session (n = 42, of whom 23 sexually active) |
Intervention: Two 60-minute sessions per day, every other day (total six sessions) Control: One 120-minute session Follow-up: 6 months |
Details not reported but authors state study underpowered | Not reported |
| Berkman et al., 2007,48 New York, USA | 149 | Men with SMI attending an outpatient psychiatric clinic | Social skills intervention (enhanced SexG: 10 sessions). Interventions included the following:
|
Money-management group with matched treatment for dosage and format of the intervention group (n = 76) | Intervention: Ten 60-minute sessions. First eight sessions twice a week for 4 weeks, followed by 4-week break and then final two sessions. Booster sessions at 3, 6 and 9 months Follow-up: 12 months |
Details not reported but authors state study underpowered | NIMH, USA |
| Carey et al., 2004,49 New York, USA | 408 | Men and women receiving outpatient psychiatric care for a mental illness | HIV group: Standard care plus attending a 10-session HIV risk reduction programme. Sessions included enhancing knowledge about HIV transmission, and prevention, motivation for behaviour change and strengthening behavioural skills and self-management training (n = 142) SUR intervention group: Standard care plus attending a 10-session reduction (substance abuse) programme. Sessions included enhancing knowledge, motivation and skills to reduce caffeine consumption, smoking, and alcohol use (n = 140) |
Standard care which included HIV and substance use education, if needed (n = 126) | HIV and SUR groups: Twice weekly for 5 weeks (total 10 sessions) Follow-up: 6 months |
Not reported | NIMH, USA |
| Collins et al., 2011,50 New York, USA | 79 | Women with SMI residing in an urban community setting | HIV prevention intervention with social cognitive theory, with focus on self-efficacy and skills training Sessions included increasing knowledge and awareness of HIV and STI risk, and sense of personal risk; HIV prevention methods and contraceptive options; asserting oneself and negotiating sexual encounters safely; condom use skills; negotiating use of condoms, identifying and problem-solving barriers to condom use and making commitment to self-protection (n = 39) |
Money-management group (10-session workshop on managing finances and making money last through the month) (n = 40) |
Twice weekly for 5 weeks (total 10 sessions) Follow-up: 6 months |
Details not reported but authors state study underpowered | NIMH and Robert Wood Johnson Harold Amos Medical Faculty Development Program, USA |
| Kalichman et al., 1995,51 WI, USA | 52 | Men and women receiving outpatient psychiatric community support for a chronic mental illness | HIV prevention intervention based on behavioural skills training (risk education and skills instruction). Intervention included education on risk reduction, sexual assertiveness, negotiation skills (risk-related behavioural self-management), condom use and problem-solving skills (n = 23) |
Waiting list group (who later received the intervention) (n = 29) |
Four weekly 90-minute sessions Follow-up: 2 months |
Details not reported but authors suggest study underpowered | NIMH, USA |
| Katz et al., 1996,52 CA, USA | 27 | Men and women from a drop-in socialisation centre for psychiatric outpatients with a chronic mental illness | AIDS education and risk reduction training programme. Intervention included education about HIV and AIDS, refusal skills training and problem-solving skills (n = 15) |
No treatment (n = 12) |
Four 2-hour (8 hours) group training over 4 consecutive days Follow-up: 2 weeks |
Details not reported but authors suggest study underpowered | Not reported |
| Kelly et al., 1997,31 WI, USA | 104 | Men and women with SMI receiving outpatient psychiatric care | Intervention 1: Cognitive–behavioural intervention that focused on behaviour changes to reduce the risk of contracting HIV Interventions included education on risk reduction, sexual assertiveness, negotiation skills (risk-related behavioural self-management), condom use and problem-solving skills (n = 34) Intervention 2: Cognitive–behavioural therapy combined with advocacy training (to act as a risk reduction advocate to their friends and acquaintances) (n = 42) |
A single 60-minute AIDS education session (n = 28) | Cognitive–behavioural therapy included seven 90-minute group sessions Follow-up: 3 months |
Details not reported but authors suggest study underpowered | NIMH, USA |
| Linn et al., 2003,53 Nashville, USA | 257 | Men with chronic disabling mental illnesses attending a homeless shelter | SexG: Sessions 1 and 2 – Say the word: create environment where men feel comfortable talking about sex Session 3 – A quick fix: sex with female commercial and casual partners Session 4 – All you need is love: sex with special partners Session 5 – Peanut butter: anal sex with men and women Session 6 – Graduation: receive ID cards as HIV prevention specialists (n = 130) |
A 6-session intervention including HIV and sexually transmitted diseases information and basic instruction on condom use (n = 127) |
Six sessions, 3 days per week over a 6-week period Follow-up: 6 months |
Not reported | Research Center in Minority Institutions Program (RCMI), Minority Biomedical Research Support (MBRS) and Center for Medicare and Medicaid Services (CMS) grants to Tennessee State University |
| Malow et al., 2012,54 FL, USA | 290 | Severely mentally ill men and women with a diagnosis of substance misuse attending outpatient psychiatric treatment centres | E-CB: Sessions 1 and 2: HIV education, personalised HIV risk Sessions 3 and 4: condom use, safe sex Sessions 5 and 6: high-risk situations, communication skills (n = 164) |
HPC: Session 1: HIV information Session 2: information on heart attacks Session 3: good food habits Session 4: exercise Session 5: smoking Session 6: stress (n = 126) |
Six 90-minute sessions over 6 weeks Follow-up period: 6 months |
Not reported | Not reported |
| NIMH, 2006,57 New York City and Los Angeles, USA | 99 | Men with mental health problems (who had unprotected sexual intercourse and at high HIV risk) attending outpatient mental health clinics | Project LIGHT: Small group intervention in seven sessions covering knowledge of HIV, personal triggers for risk behaviour, use of problem solving skills, condom use, assertiveness, negotiation strategies and relapse prevention (n = 52) |
One-session video intervention (n = 47) |
Seven 90-minute sessions, twice a week Follow-up period: 12 months |
Not reported | NIMH, USA |
| Otto-Salaj et al., 2001,55 WI, USA | 189 | Men and women with SMI attending community mental health outpatient clinics | HIV prevention intervention: Sessions 1 and 2: HIV risk reduction Session 3: condom use Session 4: problem solving strategies Session 5: discussion and role play Session 6: negotiation and assertiveness skills Session 7: review of individual’s plan for behaviour change Two booster sessions 1 and 2 months later (n = not reported) |
Health promotion comparison intervention: Seven sessions and booster sessions with same use of educational discussion and skills-building exercises but focused on personal relationships, stress, nutritional health, cancer, heart disease and general sexual health (n = not reported) |
Seven sessions held twice weekly plus two booster sessions, 1 and 2 months later Follow-up period: 12 months |
Not reported | NIMH, USA |
| Susser et al., 1998,56 New York, USA | 97 (59 sexually active) | Homeless sexually active men with SMI | SexG: Interactive intervention with storytelling, competitive games and acting scenes with true-to-life scenarios (n = 52, of whom 33 sexually active) |
Two sessions with information on HIV, sexually transmitted diseases and condom use (n = 45, of whom 26 sexually active) |
15 sessions, 2 days per week over an 8-week period Follow-up: 18 months |
Not reported | NIMH and Centers for Disease Control and Prevention, USA |
| Weinhardt et al., 1998,58 New York, USA | 20 | Women with SMI receiving outpatient psychiatric care | Sexual assertiveness intervention: Sessions 1 to 3: HIV-related information and risk-behaviour reduction Sessions 4 to 7: skill acquisition and fluency building Sessions 8 to 10: generalisation of skills to actual interactions (n = 9) |
Assessments completed during the same periods as intervention participants but no treatment (n = 11) |
Ten treatment sessions over 2 weeks Follow-up period: 4 months |
Not reported | Not reported |
The content of the health promotion interventions for improving sexual health varied and included strategies to increase knowledge, assess and reduce sexual health risk, change behaviour and develop condom skills. These are more fully described in Table 1. Sex, Games and Videotapes (SexG) was used in two studies (Linn et al. 53 and Susser et al. 56), while variations of SexG were used in two additional studies including SexG-Brief (Berkman et al. 46,47) and enhanced SexG (Berkman et al. 48). Other studies included HIV risk reduction programmes (Carey et al. ,49 Katz et al. 52 and Kelly et al. 31), HIV prevention interventions (Collins et al. ,50 Kalichman et al. 51 and Otto-Salaj et al. 55), enhanced cognitive behavioural skill building (Malow et al. 54), a sexual assertiveness intervention (Weinhardt et al. 58) and Project LIGHT (Living in Good Health Together), covering HIV prevention and reduction (NIMH57). The duration of the intervention sessions ranged from four (Kalichman et al. 51 and Katz et al. 52) to 15 sessions (Susser et al. 56).
The standard care in the control groups included educational sessions on HIV (Berkman et al. ,46,47 Kelly et al. ,31 Linn et al. 53 and NIMH57), money management (Berkman et al. ,48 Collins et al. 50 and Susser et al. 56) or HIV and substance misuse (Carey et al. 49), waiting list or no treatment (Kalichman et al. ,51 Katz et al. 52 and Weinhardt et al. 58), or health promotion covering a variety of topics (Malow et al. 54 and Otto-Salaj et al. 55).
Sources of funding were not reported in four studies (Berkman et al. ,46,47 Katz et al. 52 and Weinhardt et al. 58). The majority of studies were funded at least in part by the NIMH in the USA.
Patient characteristics of included studies
The patient characteristics of the included studies are shown in Table 2. With regard to the gender of study participants, five studies included males only (Berkman et al. ,46,47 Berkman et al. ,48 Linn et al. ,53 NIMH57 and Susser et al. 56) and two included females only (Collins et al. 50 and Weinhardt et al. 58). The remaining studies included both men and women and ranged from 45% (Malow et al. 54) to 52% male (Kalichman et al. 51) One study did not report the percentage of male and female participants although the authors stated that the ratio of male to female participants was 2 : 1 (Katz et al. 52).
| Study and year | % male | Diagnosis | Mean age (years) ± SD (range) | Ethnicity | Mean education (years) ± SD (range) | Marital status | Comorbidities |
|---|---|---|---|---|---|---|---|
| Berkman et al., 200646,47 | 100 | Major depressive disorder (10%) Schizophrenia or schizoaffective disorder (72%) Bipolar affective disorder (3%) |
38.0 ± 9.2 (25–45) | 65% African American 21% Latino 14% other |
Not reported | Not reported | 18% alcohol dependent only 16% drug dependent only 25% alcohol and drug dependent |
| Berkman et al., 200748 | 100 | Major depressive disorder (5.4%) Schizophrenia (49.0%) Schizoaffective disorder (22.8%) Bipolar affective disorder (9.4%) |
Enhanced SexG: 37.2 ± 8.8 (range not reported) Control: 40.0 ± 9.1 (range not reported) |
53.7% African American 27.5% Latino 11.4% White 7.4% other |
Not reported Additional data: Less than high school (44.3%) High school graduate (36.9%) Some college or college graduate (18.1%) |
8.7% married | Lifetime dependency on alcohol (34.2%), marijuana (24.8%) and cocaine (34.9%) |
| Carey et al., 200449 | 46% | Major depressive disorder (49%) Schizophrenia (18%) Schizoaffective disorder (15%) Bipolar affective disorder (19%) |
36.5 ± 9.5 (range not reported) | 67% European American 21% African American 12% other |
Not reported Additional data: Less than high school (33%) High school graduate (34%) Some college or college graduate (33%) |
28% married | Not reported |
| Collins et al., 201150 | 0 | Schizophrenia (50%) Schizoaffective disorder/psychosis not otherwise specified (14%) Mood disorder with psychosis (13%) Mood disorder without psychosis (23%) |
42.3 ± 8.3 (range not reported) | 61% Black 20% Latino 11% White 8% other |
Not reported Additional data: Less than 12th Grade (43%) 12th Grade or General Educational Development (32%) Greater than 12th Grade (25%) |
9% married | Lifetime substance abuse, use or dependency (77%) Current substance use (past month) (9%) |
| Kalichman et al., 199551 | 52 | Schizophrenia (62%) Schizoaffective disorder (23%) Major affective disorder including bipolar disorder (13%) |
39.2 ± 8.0 (range not reported) | 19% African American 73% White 8% other |
12.3 ± 2.5 Additional data: < 12 (67%) |
37% married | Alcohol and drug treatment programmes (31%) |
| Katz et al., 199652 | Not reported (male : female ratio 2 : 1) | Not reported (majority of patients diagnosed with schizophrenia and bipolar disorder) |
Not reported (22–59) | Not reported | Not reported | Not reported | Assorted personality disorders, chemical dependencies or both |
| Kelly et al., 199731 | 47 | Schizophrenia (19%) Mood disorder (58%) Anxiety disorder (11%) Substance use or personality disorder (11%) |
33.7 ± 6.4 (range not reported) | 39% African American 52% White 9% other |
Not reported Additional data: High school education (61%) |
Not reported | Not reported |
| Linn et al., 200353 | 100 | Schizophrenia/schizoaffective disorder: 58% in SexG and 64% in control Major depression/bipolar: 37% in SexG and 14% in control Other: 5% in SexG and 22% in control |
Not reported < 35 years: 38% in SexG and 47% in control ≥ 35 years: 62% in SexG and 53% in control |
African American: 54% in SexG and 64% in control Caucasian: 42% in SexG and 28% in control Hispanic: 4% in SexG and 8% in control |
Not reported Additional data: Less than high school: 53% in SexG and 61% in control |
Not reported | Cocaine and/or alcohol dependence: 25% in SexG and 27% in control |
| Malow et al., 201254 | 45 | Major depressive disorder (21.2%) Schizophrenia (15.7%) Bipolar affective disorder (9.6%) Schizoaffective disorder (8.4%) |
39.59 ± 10.42 (range not reported) | 24% non-Hispanic White 55% African American 20% Hispanic 1% other |
11.47 ± 2.64 (range not reported) | 5% married | All patients were substance abusers |
| NIMH, 200657 | 100 | Not reported but authors stated that people with SMI were eligible | Not reported (78% > 31 years) |
72.4% African American 14.3% Caucasian 6.1% Hispanic African American 2.0% Hispanic 5.1% other |
Not reported Additional data: 66.3% completed high school |
Not reported but 60% never been married | 56.1% drug use in previous 3 months |
| Otto-Salaj et al., 200155 | 46 | Schizophrenia (35%) Affective disorder (34%) Schizoaffective disorder (18%) Other disorders (13%) |
38.4 ± 10.1 (range not reported) | 35% White 51% African American 6% Hispanic/Latino 8% other |
11.9 ± 2.0 (range not reported) | Not reported | Not reported |
| Susser et al., 199856 (data on sexually active only) |
100 | Schizophrenia or schizoaffective disorder: 58% in intervention and 65% in control Major depression or bipolar: 36% in intervention and 16% in control Other: 6% in intervention and 19% in control |
Not reported (39% < 35 years in intervention and 46% < 35 years in control) |
African American: 52% in intervention and 65% in control Latino: 42% in intervention and 27% in control Other: 6% in intervention and 8% in control |
Not reported Additional data: Educational level less than high school: 52% in intervention and 62% in control |
Not reported | 33% in intervention group and 27% in control group with no substance abuse |
| Weinhardt et al., 199858 | 0 | Schizophrenia spectrum disorders (50%) Bipolar disorder (30%) Major depressive disorder (20%) |
36 ± SD (not reported) (range not reported) | 65% Caucasian | Not reported Additional data: Average 11th Grade education |
Not reported | Not reported |
Most studies, apart from two (Katz et al. 52 and NIMH57) reported the percentage of participants with psychiatric diagnoses which included schizophrenia, schizoaffective disorder, bipolar affective disorder and major depressive disorder. Although the Katz et al. 52 study did not include percentages, the authors stated that the majority of patients were diagnosed with schizophrenia or bipolar disorder. It is worth noting that some studies included other diagnoses, such as mood, anxiety and personality disorders31 and other undefined illnesses. 53,55,56 The percentage of participants with schizophrenia or schizoaffective disorder varied widely between the studies and ranged from 15.7% (Malow et al. 54) to 85% (Kalichman et al. 51).
The studies varied in how they reported the age of the study participants. Eight of the 13 studies reported the mean age of the participants which varied from 33.7 (Kelly et al. 31) to 42.3 years (Collins et al. 50). The age range in the included studies was only reported in two studies, with the widest range (22–59 years) reported by Katz et al. 52 Three studies (Linn et al. ,53 NIMH57 and Susser et al. 56) reported only the percentage of participants above or below a certain age.
There was also variation in the ethnicity of participants between the studies, with seven studies reporting the majority of participants to be African Americans (Berkman et al. ,46,47 Berkman et al. ,48 Linn et al. ,53 Malow et al. ,54 NIMH,57 Otto-Salaj et al. 55 and Susser et al. 56). One study did not report information on the ethnicity of the participants. 52 Only four studies reported information regarding the marital status of participants (Berkman et al. ,48 Carey et al. ,49 Kalichman et al. 51 and Malow et al. 54). Most of the studies provided at least some information on the educational level of participants, apart from two studies (Berkman et al. 46,47 and Katz et al. 52) which provided no information. Nine of the 13 studies reported some information regarding comorbidities, which was usually substance misuse, and four studies (Carey et al. ,49 Kelly et al. ,31 Otto-Salaj et al. 55 and Weinhardt et al. 58) provided no information.
Quality characteristics
The overall methodological quality of the 13 included studies is summarised in Table 3 and Appendix 5. Generally, only two studies (Linn et al. 53 and Susser et al. 56) were considered as having very few methodological limitations. All studies, except one (NIMH57), selected participants who were ‘somewhat likely’ to be representative of the target population; however, most studies (54%) did not report the number of individuals who were eligible to participate (Berkman et al. ,47 Berkman et al. ,48 Kalichman et al. ,51 Malow et al. ,54 Otto-Salaj et al. 55 and NIMH57) or reported very low numbers of eligible individuals who agreed to participate in the study (Carey et al. 49).
| Author, year | Selection bias | Study design | Confounders | Blinding | Data collection | Withdrawals and dropouts | Overall rating |
|---|---|---|---|---|---|---|---|
| Berkman et al., 200646,47 | Moderate | Strong | Weak | Moderate | Strong | Strong | Moderate |
| Berkman et al., 200748 | Moderate | Strong | Weak | Moderate | Strong | Strong | Moderate |
| Carey et al., 200449 | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate |
| Collins et al., 201150 | Moderate | Strong | Weak | Moderate | Strong | Strong | Moderate |
| Kalichman et al., 199551 | Moderate | Strong | Strong | Moderate | Strong | Weak | Moderate |
| Katz et al., 199652 | Moderate | Strong | Weak | Moderate | Strong | Weak | Weak |
| Kelly et al., 199731 | Moderate | Strong | Weak | Moderate | Weak | Weak | Weak |
| Linn et al., 200353 | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Malow et al., 201254 | Moderate | Strong | Strong | Moderate | Strong | Moderate | Moderate |
| NIMH, 200657 | Weak | Strong | Weak | Moderate | Weak | Strong | Weak |
| Otto-Salaj et al., 200155 | Moderate | Strong | Strong | Moderate | Weak | Strong | Moderate |
| Susser et al., 199856 | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Weinhardt et al., 199858 | Moderate | Strong | Strong | Moderate | Strong | Weak | Moderate |
Although all the studies were described as RCTs, only three studies reported the method of randomisation (Berkman et al. ,48 Collins et al. 50 and Susser et al. 56). In seven studies confounders were well controlled (Carey et al. ,49 Kalichman et al. ,51 Linn et al. ,53 Malow et al. ,54 Otto-Salaj et al. ,55 Susser et al. 56 and Weinhardt et al. 58); however, in the remaining studies (Berkman et al. ,47 Berkman et al. ,48 Collins et al. ,50 Katz et al. ,52 Kelly et al. ,31 and NIMH57) no details were provided on baseline compatibility or whether a variable was associated with the intervention or exposure and causally related to the outcome of interest. None of the studies was graded as ‘strong’ for blinding. Only five studies (Berkman et al. ,48 Carey et al. ,49 Linn et al. ,53 Malow et al. 54 and Susser et al. 56) blinded the outcome assessors and protected against detection bias.
All studies failed to provide details on whether or not study participants were aware of the research question (reporting bias). Reliable and valid outcome measures were used in most (77%) of the studies (Berkman et al. ,47 Berkman et al. ,48 Carey et al. ,49 Collins et al. ,50 Kalichman et al. ,51 Katz et al. ,52 Linn et al. ,53 Malow et al. ,54 Susser et al. 56 and Weinhardt et al. 58). While three studies (Kalichman et al. ,51 Kelly et al. 31 and Weinhardt et al. 58) failed to provide details of withdrawals and dropouts, the follow-up rate was 80% or greater in eight studies (Berkman et al. ,47 Berkman et al. ,48 Carey et al. ,49 Collins et al. ,50 Linn et al. ,53 Otto-Salaj et al.,55 Susser et al. 56 and NIMH57). Intervention integrity (assurance that the intervention was delivered according to plan) is an important part of programme delivery. Only five studies (Collins et al. ,50 Kalichman et al. ,51 Linn et al. ,53 Otto-Salaj et al. 55 and Susser et al. 56) reported that > 80% of participants received the allocated intervention and eight studies measured the consistency of the intervention (Carey et al. ,49 Collins et al. ,50 Kalichman et al. ,51 Kelly et al. ,31 Linn et al. ,53 Otto-Salaj et al. ,55 Susser et al. 56 and NIMH57), which was considered satisfactory. Contamination or co-intervention was unlikely in three studies (Berkman et al. ,47 Kalichman et al. 51 and Linn et al. 53), likely in one study (Collins et al. 50) and not reported in nine studies (Berkman et al. ,48 Carey et al. ,49 Katz et al. ,52 Kelly et al. ,31 Malow et al. ,54 Otto-Salaj et al. ,55 Susser et al. ,56 NIMH57 and Weinhardt et al. 58).
No studies reported a sample size calculation. Many of the studies had small sample sizes so it is likely they had inadequate statistical power to detect between-group differences, even if these were present. The statistical analysis in most studies was appropriate and used intention-to-treat analysis. All the included studies were conducted in the USA, thus making generalisability of the findings to the UK setting uncertain.
Study outcomes
Sexual risk behaviours were stated to be the main outcome of the studies apart from Otto-Salaj et al. ,55 which listed HIV risk behaviours as the primary outcome (Table 4). How sexual risk behaviours were measured varied between the studies. Five studies (Berkman et al. ,46,47 Berkman et al. ,48 Collins et al. ,50 Linn et al. 53 and Susser et al. 56) included vaginal episode equivalent (VEE) scores in the reported outcomes. The VEE score is weighted on the basis of statistical estimates of the relative risk of various sexual behaviours (unprotected vaginal, anal and oral sex). A greater weight is assigned to unprotected anal sex (2 points) than unprotected vaginal sex (1 point), and also allows for some contribution from unprotected oral sex (0.1 points). A VEE score is calculated by summing VEE points for various sexual behaviours over a designated period of time. 72 A wide range of other outcomes included the total number of sexual partners, number of casual partners, unprotected anal, vaginal or oral sex, self-report of sexually transmitted diseases, condom use, knowledge, attitudes, intentions, assertiveness and motivation.
| Study | Primary outcome | Included outcomes | Completion of programme | Findings |
|---|---|---|---|---|
| Berkman et al., 200646,47 | Sexual risk behaviours with casual partners (measured by VEE score)a | Unprotected anal, vaginal, oral sex with casual partners (women or men) | For the intervention, 28 men (56%) attended all six sessions, 16 (32%) attended four sessions, and six (12%) attended two sessions | Mean VEE score – sexually active group: The mean VEE score of the 56 sexually active men at 6-month follow-up was less in the SexG-Brief (1.64 ± 4.06) than in the control group (3.50 ± 5.90), which was not a significant difference Mean VEE score – non-sexually active group: The mean VEE score of the 36 men who were not sexually active at baseline was similar, although the variance was even higher (SexG-Brief, 0.26 ± 0.58 vs. control group, 0.63 ± 2.35) |
| Berkman et al., 200748 | Sexual risk behaviours with casual partners (measured by VEE score)a | Unprotected anal, vaginal, oral sex with casual partners (women or men) | Not reported | Mean VEE score: During the first and second follow-up intervals, there were no significant differences in sexual risk behaviours with casual partners as measured by VEE Additional analyses demonstrated a trend towards sexual risk reduction at 6 months post intervention (p = 0.06) but not at 12 months |
| Carey et al., 200449 | Sexual risk behaviours | Frequency of unprotected vaginal sex, total number of sex partners, total number of casual partners, number of safer sex communications before intercourse and self-report of STIs Other secondary measures included HIV knowledge, personal condom attitudes and intentions and behavioural skills relative to the SUR and control groups |
HIV group: 103 (73%) patients attended at least one session, and 95 (67%) completed five or more sessions (overall average 5.7 sessions) SUR group: 106 (76%) patients attended at least one session, and 94 (67%) completed five or more sessions (overall average 6.0 sessions) |
HIV vs. control: Compared with the control group, the HIV risk reduction intervention (over time) reported less unprotected sex (p = 0.004), fewer casual sex partners (p = 0.001), fewer partners (p = 0.037), more safer sex communications (p = 0.053), improved HIV knowledge (p = 0.001), more positive condom attitudes (p = 0.003), stronger condom use intentions (p = 0.001), and improved behavioural skills as indicated by the role playing ratings (p = 0.002) HIV vs. SUR: Compared with the SUR group, the HIV risk reduction intervention (over time) reported less unprotected sex (p = 0.001), fewer casual sex partners (p = 0.015), fewer new STIs (p < 0.013), more safer sex communications (p = 0.001), improved HIV knowledge (p = 0.001), more positive condom attitudes (p = 0.045), stronger condom use intentions (p = 0.001), and improved behavioural skills (p = 0.005) SUR vs. control: Compared with the control group, the SUR intervention (over time) reported fewer casual sex partners (p = 0.001), more positive condom attitudes (p = 0.003), stronger condom use intentions (p = 0.002), and improved behavioural skills (p = 0.001) No differences were observed in the number of incident STIs, the overall frequency of unprotected sex over time (p = 0.864), the number of communications about safer sex (p = 0.081) and HIV knowledge (p = 0.291) Additional analyses: Exploratory analyses suggested that female patients and patients diagnosed with a major depressive disorder were more likely to benefit from the HIV risk reduction intervention. Women were more responsive than men to the intervention with regard to the total frequency of unprotected sex and men improved their HIV knowledge more than women |
| Collins et al., 201150 | Sexual risk behaviours (measured by VEE score)a | Unprotected anal, vaginal, oral sex with sexual partners (casual, steady, exchange) | HIV group: Two patients did not attend any sessions, and 34 (89.5%) completed five or more sessions Control group: Five patients did not attend any sessions, and 29 (72.5%) completed five or more sessions |
Women in the HIV prevention intervention showed a threefold reduction in mean VEE score at the 3-month follow-up compared with the control group [9.94 vs. 31.89, but the difference was not significant (p = 0.37)]. At 6 months no differences were observed in the mean VEE scores between the intervention and control groups (12.23 vs. 11.58; p = 0.91) These women were significantly more likely to know about female condoms (p = 0.1) and to have inserted one (p = 0.0001) and used it with a sexual partner (p = 0.04) at the 3-month follow-up, and to have inserted one (p = 0.02) at 6 months, compared with controls |
| Kalichman et al., 199551 | Sexual risk behaviours | Knowledge, condom use, behaviour change interventions | Eight patients did not complete the programme and 44 (85%) patients completed two of the four intervention sessions | Compared with the control group, the intervention group demonstrated significant gains in knowledge (p < 0.001) and intention to change risk behaviour (p < 0.05). At 1-month follow-up, the intervention group reported reduced rates of unprotected sex (p < 0.01) and increased frequency of condom use (p < 0.05) |
| Katz et al., 199652 | Sexual risk behaviours | Knowledge, behaviour change interventions | Not reported | Compared with the control group, the intervention group demonstrated improvements in their knowledge about AIDS after training (p = 0.005). This was maintained at the 2-week follow-up (p = not reported). Similarly, compared with the control group, participants in the intervention group were more confident to deal with high-risk situations after training (p = 0.005). This was maintained at the 2-week follow-up (p = not reported). Although no significant differences were observed at follow-up, behavioural measures of coping in high-risk situations were significantly different to the control group (p = 0.0002) after training |
| Kelly et al., 199731 | Sexual risk behaviours | AIDS risk behaviour (knowledge and safer-sex practices) and condom use: barriers to behaviour change and perceived risk reduction, self-efficacy for use | Not reported; however, all patients attended at least one session of the intervention to which they were assigned. Mean 5.6 sessions in the cognitive behavioural intervention and 5.9 sessions in the cognitive behavioural intervention plus advocacy | Although all participants exhibited change at follow-up in some risk-related psychological characteristics and sexual risk behaviours, participants who received the cognitive–behavioural intervention that included the advocacy training reported greater reductions in rates of unprotected sex (p < 0.01) and had fewer sexual partners at follow-up (p < 0.02) compared with cognitive–behavioural training alone. In addition, a non-significant reduction was observed in the number of casual and new sexual partners (p < 0.1) and unprotected sexual activity (p < 0.09) Improvements in AIDS risk behaviour knowledge for all groups, with the greatest improvement in both intervention groups (p < 0.001 for within-group change from baseline for both intervention groups) |
| Linn et al., 200353 | Sex risk activity (sexual risk index) | VEE sexual risk index; total number of sexual episodes and percentage protected by condom use | All participants available at 6-month follow-up | Mean VEE score: significantly lower in SexG group compared with control (p = 0.01) Number of sex encounters: no difference between groups Percentage protected by condom use: the proportion of all encounters that were protected by condom use was greater (p-value not reported) in SexG (0.74) than in the control group (0.51) and was specific to sex with women (p-value not reported) |
| Malow et al., 201254 | Sex risk behaviours | HIV knowledge, perceived susceptibility, AIDS-related anxiety, personal condom attitudes, peer and partner sexual attitudes, condom use skills, sexual self-efficacy, total number of unprotected vaginal sex acts, proportion of unprotected vaginal sex acts, total number of sex partners | All participants attended at least four sessions; 72% of E-CB and 63% of HPC attended all six sessions | HIV knowledge and perceived susceptibility: no significant difference for I × G × T or I × T analyses for either (all p-values > 0.10) AIDS-related anxiety: significant time effect reflecting reduction in levels of HIV anxiety across intervention and gender (p < 0.001) Condom attitudes: women demonstrated more favourable attitudes across interventions and times (p = 0.013) Sexual attitudes: males in E-CB demonstrated an improvement in intentions and males had greater improvements than females in E-CB group (p = 0.013) Condom use skills: males in E-CB group demonstrated greater condom use skills than females in E-CB and both males and females in HPC (all p-values < 0.05) Sexual self-efficacy: women had modestly higher levels than men (p = 0.02) Total number of unprotected vaginal sex acts: no I × G × T or I × T or gender effects (all p-values > 0.10) Proportion of unprotected vaginal sex acts: males in E-CB demonstrated reduction in proportion of unprotected vaginal sex acts at follow-up (p = 0.01) Total number of sex partners: reduction from baseline but not significant |
| NIMH, 200657 | Sexual risk behaviours | Number of partners, number of risky sexual acts, proportion with condom use, consistent condom use | 88% completed 3-month follow-up; 85.5% completed 6-month and 82% completed 12-month follow-up | Number of partners: no significant difference between groups Number of risky sex acts: decreased significantly more in intervention group than in control group (p = 0.001) Condom use: significantly greater condom use among African Americans only in the intervention group compared with the control (p = 0.0004) Consistent condom use: significantly greater among African Americans only in the intervention group compared with the control (p = 0.016) |
| Otto-Salaj et al., 200155 | HIV risk behaviours | HIV risk knowledge, attitudes towards condom use, risk reduction behavioural intentions, frequency of protected and unprotected intercourse, intercourse occasions protected by condoms, number of partners | 84.1% attended all of the sessions; 80.4% completed 12-month follow-up | HIV risk knowledge: men only in intervention group showed significant increase in HIV knowledge at 3, 6, 9 and 12 months compared with control group (p < 0.05 for all time points) Attitudes towards condom use: in intervention group, women only showed significant improvement in attitudes towards condom use at 6 and 9 months (p < 0.05 for both) but not at 12 months (p < 0.1) compared with control HIV risk reduction behaviour intentions: no significant differences between groups Frequency of protected intercourse: mean frequency of condom-protected intercourse for women only in intervention group increased significantly compared with control group at 3 months (p < 0.05), 6 months (p < 0.01) and 9 months (p < 0.05) but not at 12 months Frequency of unprotected intercourse: no significant difference between groups Intercourse protected by condoms: significant percentage of condom-protected intercourse occasions for women only in intervention group at 3 months (p < 0.05) and 6 months (p < 0.005) but decline at 12 months Number of sexual partners: no significant differences between groups |
| Susser et al., 199856 | Sexual risk behaviours (measured by VEE score)a | Unprotected anal, vaginal, oral sex with casual and occasional partners (women or men) | All participants completed the initial 6 months and 95% were available for 18-month follow-up | Mean VEE score at 6 months: significantly lower in intervention group (p = 0.01) Condom use: episodes protected by condom use higher in the intervention group (p = 0.04) High-risk behaviours (multiple partners, unprotected anal or vaginal sex): significantly lower in the intervention group (p = 0.01) |
| Weinhardt et al., 199858 | Sexual risk behaviour | Sexual assertiveness, knowledge, motivation, HIV risk behaviour | All intervention participants completed at least 6 of 10 sessions (mean 8.2) | Sexual assertiveness: intervention group improved more than control at each assessment period (p < 0.001 at 2 months and p < 0.005 at 4 months) Knowledge: intervention group exhibited greater knowledge at each assessment period (p < 0.01 at 2 months and p < 0.05 at 4 months) Motivation: no difference between groups on perceived risk or behavioural intentions HIV risk behaviour: intervention group showed significant increase in protected sex at 2 months (p < 0.01) but not at 4 months. No significant differences for frequency of unprotected intercourse |
Three studies (Berkman et al. ,48 Katz et al. 52 and NIMH57) reported no information regarding number of participants completing at least part of the intervention. It was unclear in Linn et al. 53 how many participants completed all sessions although the authors stated that all participants were available for follow-up at 6 months. Kelly et al. 31 reported the mean number of sessions only and Susser et al. 56 reported that all participants completed the initial 6-month study period. The remaining studies reported the percentage of participants who completed either all sessions or at least some of the sessions, as shown in Table 4.
Effects of interventions
This section provides a narrative summary of the results by the following sexual health-related outcomes: biological, behavioural and proxy. Further details are provided in Tables 4 and 5.
| Outcome type | Specific outcome | Studies |
|---|---|---|
| Biological | STI (including HIV) | Carey et al., 200449 |
| Unintended pregnancy | None | |
| Behavioural | Number of partners or episodes of unprotected intercourse | Berkman et al., 200646,47 Berkman et al., 200748 Carey et al., 200449 Collins et al., 201150 Kelly et al., 199731 Linn et al., 200353 Malow et al., 201254 NIMH, 200657 Otto-Salaj et al., 200155 Susser et al., 199856 |
| Use of contraception | None | |
| Use of condoms | Kalichman et al., 199551 Linn et al., 200353 Malow et al., 201254 NIMH, 200657 Otto-Salaj et al., 200155 Susser et al., 199856 |
|
| Uptake of screening or treatment services | None | |
| Proxy | Knowledge, attitudes and beliefs | Carey et al., 200449 Kalichman et al., 199551 Katz et al., 199652 Kelly et al., 199731 Malow et al., 201254 Otto-Salaj et al., 200155 Weinhardt et al., 199858 |
| Barriers and facilitators | None | |
| Intentions | Carey et al., 200449 Kalichman et al., 199551 Otto-Salaj et al., 200155 Weinhardt et al., 199858 |
|
| Skills | Carey et al., 200449 Weinhardt et al., 199858 |
Biological outcomes
The biological outcomes included in the protocol were STIs (including HIV) and unintended pregnancy. Only one study (Carey et al. 49) reported information on STIs, with the intervention group reporting significantly fewer new STIs than the control group. No studies reported information on unintended pregnancies.
Behavioural outcomes
Four behavioural outcomes were included in this review: number of partners, use of contraception, use of condoms and uptake of screening or treatment services. No studies were identified reporting information on the use of contraception or uptake of screening or treatment services. Seven studies reported information on the number of partners (Carey et al. ,49 Kelly et al. ,31 Linn et al. ,53 Malow et al. ,54 NIMH,57 Otto-Salaj et al. 55 and Susser et al. 56), with two of these studies49,31 reporting patients having significantly fewer partners in the intervention groups than the control groups. The other studies showed no difference between the intervention and control groups with regard to the number of partners.
Indirectly related to number of partners is the number of episodes of unprotected intercourse, which was reported in six studies. Kelly et al. 31 found significant changes in risk-related behaviours such as unprotected sex and number of sexual partners in the intervention group, which included advocacy training. Three studies found no significant improvements or differences in the reported VEE scores (which included the number of unprotected episodes of intercourse) between the intervention and control groups (Berkman et al. ,46,47 Berkman et al. 48 and Collins et al. 50). In contrast, Linn et al. 53 and Susser et al. 56 found significant improvements in VEE scores in the intervention groups compared with the control groups.
With regard to use of condoms, six studies reported some information on condom use. Kalichman et al. 51 and Susser et al. 56 both reported a significant increase in the frequency of condom use in the intervention groups, and Linn et al. 53 also reported a greater proportion of encounters protected by condom use. Malow et al. 54 reported significant improvements in condom use skills in males only in the intervention group, whereas Otto-Salaj et al. 55 found a significant percentage of intercourse occasions protected by condoms for females only in the intervention group. The NIMH57 study found significantly greater condom use for African Americans only in the intervention group.
Proxy outcomes
The proxy outcomes included in the review were knowledge, attitudes and beliefs; intentions; skills; and barriers and facilitators. No studies reported outcomes that were related to barriers and facilitators to sexual health interventions.
Carey et al. 49 reported more positive condom attitudes and stronger condom use intentions, improved HIV knowledge and improved behavioural skills in the intervention group. Kalichman et al. 51 reported that the intervention group demonstrated significant gains in knowledge and intention to change risk behaviour. Kelly et al. 31 reported improvements in AIDS risk behaviour knowledge for all groups with the greatest improvement in both intervention groups. Malow et al. ,54 however, found no improvement in HIV knowledge but did find an improvement in intentions regarding sexual attitudes for males only in the intervention group. Katz et al. 52 also found that the intervention group demonstrated significant improvement in knowledge about AIDS. Otto-Salaj et al. 55 reported improvements in HIV knowledge for men only, improvements in attitudes for condom use for women only and no significant change in HIV risk reduction behaviour intentions. Weinhardt et al. 58 found improvements in sexual assertiveness, knowledge and HIV risk reduction behaviour although no difference was found with regard to behavioural intentions.
Chapter 3 Discussion
Main findings
This is the first comprehensive systematic review of sexual health interventions for people with SMI. Despite wide variations in the study populations, interventions (e.g. programme content and duration), comparators and outcomes, four studies49,51,52,56 showed significant improvements in all measured sexual risk behaviour outcomes (e.g. HIV knowledge and behaviour change) compared with the control groups. In contrast, four studies31,50,53,58 found significant improvements in the intervention groups for some outcomes only and three studies found significant improvements in subgroups only, based on either gender54,55 or ethnicity. 57 Finally, two studies46–48 reported no significant differences in any sexual risk behaviour outcomes between the intervention and control groups. Moreover, positive findings were not consistently sustained at follow-up in many studies. These mixed results are similar to those reported in other previous reviews of the literature. 20,27 In addition, in a systematic review of sexual health improvement interventions, conducted by Fullerton and Burtney73 for NHS Scotland, the authors concluded that there was insufficient evidence to fully support or reject the identified sexual health interventions for people with SMI. The findings from this systematic review reinforce this view.
Strengths and limitations of the assessment
Although an extensive literature search was conducted, it is possible that some relevant studies may have been missed. However, such omissions are likely to have been minimal, as the search included all identifiable publications in the grey literature (including contact with clinical experts in the field). Another limitation is that the initial screening was undertaken by only one reviewer and relevant studies may have potentially been missed.
Many of the studies included in the systematic review included participants with major depression, which was not included in the definition of SMI used for this review, which only included bipolar disorder and schizophrenia. Many studies also included participants with schizoaffective disorder, which again was not part of our initial definition of SMI. It was not possible to differentiate results for patients with different diagnoses reported in the studies. Patients with major depression may respond differently to interventions than those with bipolar disorder or schizophrenia, or indeed there may be differences between these two diagnoses. Another issue related to diagnosis is the lack of uniformity in determining how the diagnoses were made.
Little detail was provided in the studies regarding the content of interventions, how they were delivered and by whom, making replication or generalisability difficult. The control groups used in the studies often included components of the intervention such as education or were not appropriate controls; for example, the control group may have received one session where the intervention group received six or more. This makes it difficult to assess the true effectiveness of the intervention. It was not always clear in the studies what the primary outcomes were and whether or not data from all recorded outcomes were reported. This could potentially result in outcome reporting bias. 74 In addition, the follow-up periods reported in the studies varied and were often quite short, ranging from 2 weeks to 18 months, and positive results were often diminished at follow-up assessments. The main threats to validity in this group of patients relate to study outcomes and participants. Most outcomes were measured by self-reporting and participants self-selecting, thus raising the possibility of bias and recall problems. 20
Although none of the studies reported that it had been designed to have adequate power to assess differences in subgroups, some reported differences in results between male and female participants. This issue needs to be explored further as there may be differences between male and female responses to sexual health interventions. There was also a lack of data and analysis reported in the studies regarding the relationship between education levels and outcomes, as well as that between the age of the participants and outcomes, both of which are important variables in the context of sexual health.
In general, the studies identified were mostly considered to be of moderate quality although there were issues in the methods of randomisation, which were often not fully described. Other limitations were: limited blinded assessment of outcomes; little information provided regarding loss of participants to follow-up; no power calculations reported; and small sample sizes in most studies. Only some studies used validated instruments to collect data on outcomes and most relied on self-report of these outcomes. Little information was provided in any of the studies regarding acceptability to participants or those delivering the interventions. There was also little information provided regarding the feasibility of the interventions and how much they would cost to implement. None of the studies provided any information on unintended pregnancies, use of contraception, uptake of screening or treatment services, or facilitators and barriers to providing interventions.
Assessment of factors relevant to the NHS and other parties
There are considerable uncertainties around the generalisability of these findings to the UK setting as the evidence is based on studies from the USA. In addition, the ethnic groups represented in the included studies are not directly comparable to those in the UK. Nevertheless, several factors discussed above are relevant to implementing sexual health interventions for people with SMI in the NHS. These include consideration of who will deliver the interventions and where they will be delivered. There needs to be an assessment of whether or not sexual health interventions could be integrated into the current care provision provided for people with SMI on the NHS. White et al. 75 described an ongoing study to assess ways that physical comorbidity in SMI could be addressed by mental health nurses working in NHS community mental health teams. Lessons may be learned from this research that could be incorporated into sexual health intervention programmes.
The choices made regarding location of sexual health services for people with SMI will have resource implications. Likewise, whether or not interventions are delivered to groups of participants or individually will have resource implications and the potential to impact on the effectiveness of the intervention. The follow up of patients also needs to be taken into account as many of the included studies in the review showed a diminished impact at follow-up. Higgins et al. 20 suggested the need for an ongoing process rather than a single intervention.
Chapter 4 Conclusions
Implications for service provision
Despite the methodological limitations and variations in the populations, sexual health interventions, use of control groups and outcomes measured in the included studies, the overall findings were mixed, with only 4 of the 13 studies showing significant benefits in sexual risk behaviour interventions (e.g. HIV knowledge and behaviour change) compared with control groups in people with SMI living in the community. In view of study heterogeneity, issues with generalisability and the methodological quality of the included studies, the findings need to be interpreted with caution. Recommendations for further research are listed below.
Suggested research priorities
Primary research
Further research in this area should incorporate the following.
-
UK-based trials of sexual health interventions for people with SMI should be conducted. These need to be well designed (use clear methods of randomisation and blinded outcome assessment) and appropriately powered.
-
Care needs to be given to the selection of patients and their diagnoses need to be clearly recorded. Sample sizes need to be large enough to ensure that analyses for each relevant subgroup of patients are possible or studies need to be conducted with recruitment of patients with a single diagnosis. Methods of recruitment need to be standardised, transparent and clearly reported. Comorbidities and other treatments need to be reported.
-
Interventions should be clearly described and could potentially include behavioural skills, relationship development, condom use and other issues. Controls also need to be clearly described and should include usual care. If active control groups are included, then the components of these groups need to be clearly described.
-
Reliable and valid tools for measuring outcomes are needed. Alternatives to measures of self-report need to be considered.
-
Follow-up periods need to be long enough to determine how long change can be maintained. The optimal duration of studies requires further research. The provision of ‘booster’ sessions and long-term interventions needs to be considered.
Other issues
-
Staff training should include support for communicating sexual health messages and attitudes towards such work. Research is needed to find the most cost-effective way of providing this.
-
The location of services needs to be given consideration and whether or not sexual health interventions can be incorporated into existing services.
-
The costs of providing sexual health interventions need to be addressed and should include the incorporation of any savings from reduced incidence of STIs.
-
The acceptability of sexual health interventions to people with SMI needs to be researched.
Acknowledgements
Many thanks to our clinical advisors for providing support and advice for this review: Dr Shubulade Smith, Consultant Psychiatrist and Clinical Senior Lecturer, Department of Forensic and Neurodevelopmental Science, Institute of Psychiatry, King’s College London; and Professor Kevan Wylie, Porterbrook Clinic, Sexual Medicine, Sheffield.
Thanks to Professor Agnes Higgins, Professor of Mental Health, School of Nursing and Midwifery, Trinity College Dublin and Dr Karen Lorimer, School of Health and Life Sciences, Glasgow Caledonian University for reviewing the draft report.
Thank you to Andrea Shippam for providing administrative support and preparing and formatting the report.
The final report and any errors remain the responsibility of the University of Sheffield. Eva Kaltenthaler and Matt Stevenson are guarantors.
Contributions of authors
Eva Kaltenthaler and Abdullah Pandor carried out the systematic review and quality assessment of the studies and wrote the report. Ruth Wong carried out the searches.
About ScHARR
The School of Health and Related Research (ScHARR) is one of the nine departments that comprise the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research, and the application of health economics and decision science to the development of health services and the improvement of public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the NIHR Health Technology Assessment Programme on behalf of a range of policy-makers, including the National Institute for Health and Care Excellence (NICE). ScHARR-TAG is part of a wider collaboration of a number of units from other regions including Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; Aberdeen Health Technology Assessment Group (Aberdeen HTA Group), University of Aberdeen; Liverpool Reviews & Implementation Group (LRiG), University of Liverpool; Peninsular Technology Assessment Group (PenTAG), University of Exeter; the NHS Centre for Reviews and Dissemination, University of York; Warwick Evidence, University of Warwick; the BMJ Group and Kleijnen Systematic Reviews.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- No Health Without Mental Health: A Cross-Government Mental Health Outcomes Strategy for People of all Ages. London: Department of Health; 2010.
- Meade C, Sikkema K. HIV risk behavior among adults with severe mental illness: a systematic review. Clin Psychol Rev 2005;25:433-57. http://dx.doi.org/10.1016/j.cpr.2005.02.001.
- Kirkbride JB, Errazuriz A, Croudace TJ, Morgan C, Jackson D, McCrone P, et al. Systematic Review of the Incidence and Prevalence of Schizophrenia and Other Psychoses in England, 1950–2009. Cambridge: University of Cambridge; 2011.
- The Abandoned Illness: A Report from the Schizophrenia Commission. London: Rethink Mental Illness; 2012.
- The Management of Bipolar Disorder in Adults, Children and Adolescents, in Primary and Secondary Care. London: National Institute for Health and Care Excellence; 2006.
- McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med 2004;2.
- Barbato A. Schizophrenia and Public Health. Geneva: World Health Organization; 1998.
- Dixon L, Postrado L, Delahanty J, Fischer P, Lehman A. The association of medical comorbidity in schizophrenia with poor physical and mental health. J Nerv Ment Dis 1999;187:496-502. http://dx.doi.org/10.1097/00005053-199908000-00006.
- Robson D, Gray R. Serious mental illness and physical health problems: a discussion paper. Int J Nurs Stud 2007;44:457-66. http://dx.doi.org/10.1016/j.ijnurstu.2006.07.013.
- De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen DAN, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52-77.
- Daumit G, Dickerson F, Wang N-Y, Dalcin A, Jerome G, Anderson C, et al. A behavioralweight-loss intervention in persons with serious mental illness. New Engl J Med 2013;368:1594-602. http://dx.doi.org/10.1056/NEJMoa1214530.
- Tosh G, Clifton A, Mala S, Bachner M. Physical health care monitoring for people with serious mental illness. Cochrane Database Syst Rev 2010;3.
- Haro JM, Salvador-Carulla L. The SOHO (Schizophrenia Outpatient Health Outcome) study: implications for the treatment of schizophrenia. CNS Drugs 2006;20:293-301. http://dx.doi.org/10.2165/00023210-200620040-00003.
- Montejo AL, Majadas S, Rico-Villademoros F, Llorca G, de la Gandara J, Franco M, et al. Frequency of sexual dysfunction in patients with a psychotic disorder receiving antipsychotics. J Sex Med 2010;7:3404-13. http://dx.doi.org/10.1111/j.1743-6109.2010.01709.x.
- Baggaley M. Sexual dysfunction in schizophrenia: focus on recent evidence. Hum Psychopharmacol 2008;23:201-9. http://dx.doi.org/10.1002/hup.924.
- Schmidt HM, Hagen M, Kriston L, Soares-Weiser K, Maayan N, Berner MM, et al. Management of sexual dysfunction due to antipsychotic drug therapy. Cochrane Database Syst Rev 2012;11. http://dx.doi.org/10.1002/14651858.CD003546.pub3.
- Aizenberg D, Zemishlany Z, Dorfman-Etrog P, Weizman A. Sexual dysfunction in male schizophrenic patients. J Clin Psychiatry 1995;56:137-41. http://dx.doi.org/10.4088/JCP.v62n07a07.
- McKinnon K, Cournos F, Herman R. HIV among people with chronic mental illness. Psychiatr Q 2002;73:17-31.
- Gray R, Brewin E, Noak J, Wyke-Joseph J, Sonik B. A review of the literature on HIV infection and schizophrenia: implications for research, policy and clinical practice. J Psychiatr Ment Health Nurs 2002;9:405-9. http://dx.doi.org/10.1046/j.1365-2850.2002.00511.x.
- Higgins A, Barker P, Begley CM. Sexual health education for people with mental health problems: what can we learn from the literature?. J Psychiatr Ment Health Nurs 2006;13:687-97. http://dx.doi.org/10.1111/j.1365-2850.2006.01016.x.
- Sikkema KJ, Meade CS, Doughty-Berry JD, Zimmerman SO, Kloos B, Snow DL. Community-level HIV prevention for persons with severe mental illness living in supportive housing programs: a pilot intervention study. J Prev Interv Community 2007;33:121-35. http://dx.doi.org/10.1300/J005v33n01_10.
- Meade CS, Weiss R. Substance abuse as a risk factor for HIV sexual risk behavior among persons with severe mental illness: review of evidence and exploration of mechanisms. Clin Psychol Sci Pract 2007;14:23-3. http://dx.doi.org/10.1111/j.1468-2850.2007.00059.x.
- Marshall J, Farell M. Substance use and psychiatric comorbidity. Psychiatry 2007;6:23-6. http://dx.doi.org/10.1053/j.mppsy.2006.10.004.
- O’Cleirigh C, Safren S. Breaking the mold or business as usual? Meeting the challenges of HIV prevention in people with serious mental illness and substance use disorders. Clin Psychol Sci Pract 2007;14:34-8. http://dx.doi.org/10.1111/j.1468-2850.2007.00060.x.
- Senn T, Carey M. HIV, STD, and sexual risk reduction for individuals with a severe mental illness: review of the intervention literature. Curr Psychiatry Rev 2008;4:87-100. http://dx.doi.org/10.2174/157340008784529313.
- McCann E. The sexual and relationship needs of people who experience psychosis: quantitative findings of a UK study. J Psychiatr Ment Health Nurs 2010;17:295-303. http://dx.doi.org/10.1111/j.1365-2850.2009.01522.x.
- Johnson-Masotti AP, Weinhardt LS, Pinkerton SD, Otto-Salaj LL. Efficacy and cost-effectiveness of the first generation of HIV prevention interventions for people with severe and persistent mental illness. J Ment Health Policy Econ 2003;6:23-35.
- Melo AP, Cesar CC, Acurcio FA, Campos LN, Ceccato MG, Wainberg ML, et al. Individual and treatment setting predictors of HIV/AIDS knowledge among psychiatric patients and their implications in a national multisite study in Brazil. Community Ment Health J 2010;46:505-16. http://dx.doi.org/10.1007/s10597-010-9303-7.
- DiFranceisco W, Kelly JA, Sikkema KJ, Somlai AM, Murphy DA, Stevenson LY. Differences between completers and early dropouts from 2 HIV intervention trials: a health belief approach to understanding prevention program attrition. Am J Public Health 1998;88:1068-73. http://dx.doi.org/10.2105/AJPH.88.7.1068.
- Johnson-Masotti AP, Pinkerton SD, Kelly JA, Stevenson LY. Cost-effectiveness of an HIV risk reduction intervention for adults with severe mental illness. AIDS Care 2000;12:321-32.
- Kelly JA, McAuliffe TL, Sikkema KJ, Murphy DA, Somlai AM, Mulry G, et al. Reduction in risk behavior among adults with severe mental illness who learned to advocate for HIV prevention. Psychiatr Serv 1997;48:1283-8.
- Blank M, Hennessy M. A reasoned action approach to HIV prevention for persons with serious mental illness. Ann Am Acad Polit Soc Sci 2012;640:173-88. http://dx.doi.org/10.1177/0002716211424711.
- Tennille J, Solomon P, Blank M. Case managers discovering what recovery means through an HIV prevention intervention. Community Ment Health J 2010;46:486-93. http://dx.doi.org/10.1007/s10597-010-9326-0.
- Tennille J, Solomon P, Fishbein M, Blank M. Elicitation of cognitions related to HIV risk behaviors in persons with mental illnesses: implications for prevention. Psychiatr Rehabil J 2009;33:32-7. http://dx.doi.org/10.2975/33.1.2009.32.37.
- Solomon P, Tennille J, Lipsitt D, Plumb E, Metzger D, Blank M. Rapid assessment of existing HIV prevention programming in a community mental health center. J Prev Interv Community 2007;33:137-50. http://dx.doi.org/10.1300/J005v33n01_11.
- Burtney E, Fullerton D. Sexual Health Improvement Interventions in Scotland. Mapping exercise. Edinburgh: NHS Health Scotland; 2011.
- Ellis S, Grey A. Prevention of Sexually Transmitted Infections (STIs): A Review of Reviews into the Effectiveness of Non-clinical Interventions. Evidence briefing. London: Health Development Agency; 2004.
- Apantaku-Olajide T, Gibbons P, Higgins A. Drug-induced sexual dysfuntion and mental health patients’ attitude to psychotropic medications. Sex Relation Ther 2011;26:145-55. http://dx.doi.org/10.1080/14681994.2011.567259.
- Higgins A, Barker P, Begley CM. ‘Veiling sexualities’: a grounded theory of mental health nurses’ responses to issues of sexuality. J Adv Nurs 2008;62:307-17. http://dx.doi.org/10.1111/j.1365-2648.2007.04586.x.
- Penna S, Sheehy K. Sex education and schizophrenia: should occupational therapists offer sex education to people with schizophrenia?. Scand J Occup Ther 2000;7:126-31.
- Armstrong R, Waters E, Jackson N, Oliver S, Popay J, Shepherd J, et al. Guidelines for Systematic Reviews of Health Promotion and Public Health Interventions. Melbourne: Melbourne University; 2007.
- Cook TD, Campbell DT. Quasi Experimentation. Design & Analysis Issues for Field Settings. Boston, MA: Houghton Mifflin Company; 1979.
- Thomas B, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004;1:176-84. http://dx.doi.org/10.1111/j.1524-475X.2004.04006.x.
- Deeks J, Dinnes J, D’Amico R, Soeden A, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7.
- Jackson N, Waters E. Criteria for the systematic review of health promotion and public health interventions. Health Promot Int 2005;20:367-74. http://dx.doi.org/10.1093/heapro/dai022.
- Berkman A. Reducing sexual risk behaviors of men with severe mental illness. Psychiatr Serv 2006;57. http://dx.doi.org/10.1176/appi.ps.57.3.417.
- Berkman A, Cerwonka E, Sohler N, Susser E. A randomized trial of a brief HIV risk reduction intervention for men with severe mental illness. Psychiatr Serv 2006;57:407-9. http://dx.doi.org/10.1176/appi.ps.57.3.407.
- Berkman A, Pilowsky DJ, Zybert PA, Herman DB, Conover S, Lemelle S, et al. HIV prevention with severely mentally ill men: a randomised controlled trial. AIDS Care 2007;19:579-88. http://dx.doi.org/10.1080/09540120701213989.
- Carey M, Carey K, Maisto S, Gordon C, Schroder K, Vanable P. Reducing HIV-risk behavior among adults receiving outpatient psychiatric treatment: results from a randomized controlled trial. J Consult Clin Psychol 2004;72:252-68. http://dx.doi.org/10.1037/0022-006X.72.2.252.
- Collins PY, von Unger H, Putnins S, Crawford N, Dutt R, Hoffer M, et al. Adding the female condom to HIV prevention interventions for women with severe mental illness: a pilot test. Community Ment Health J 2011;47:143-55. http://dx.doi.org/10.1007/s10597-010-9302-8.
- Kalichman SC, Sikkema KJ, Kelly JA, Bulto M. Use of a brief behavioral skills intervention to prevent HIV infection among chronic mentally ill adults. Psychiatr Serv 1995;46:275-80.
- Katz RC, Westerman C, Beauchamp K, Clay C. Effects of AIDS counseling and risk reduction training on the chronic mentally ill. AIDS Educ Prev 1996;8:457-63.
- Linn JG, Neff JA, Theriot R, Harris JL, Interrante J, Graham ME. Reaching impaired populations with HIV prevention programs: a clinical trial for homeless mentally ill African-American men. Cell Mol Biol 2003;49:1167-75.
- Malow RM, McMahon RC, Devieux J, Rosenberg R, Frankel A, Bryant V, et al. Cognitive behavioral HIV risk reduction in those receiving psychiatric treatment: a clinical trial. AIDS Behav 2012;16:1192-202. http://dx.doi.org/10.1007/s10461-011-0104-y.
- Otto-Salaj LL, Kelly JA, Stevenson LY, Hoffmann R, Kalichman SC. Outcomes of a randomized small-group HIV prevention intervention trial for people with serious mental illness. Community Ment Health J 2001;37:123-44.
- Susser E, Valencia E, Berkman A, Sohler N, Conover S, Torres J, et al. Human immunodeficiency virus sexual risk reduction in homeless men with mental illness. Arch Gen Psychiatry 1998;55:266-72. http://dx.doi.org/10.1001/archpsyc.55.3.266.
- The National Institute of Mental Health (NIMH) Multisite HIV Prevention Trial Group . HIV prevention with persons with mental health problems. Psychol Health Med 2006;11:142-54.
- Weinhardt LS, Carey MP, Carey KB, Verdecias RN. Increasing assertiveness skills to reduce HIV risk among women living with a severe and persistent mental illness. J Consult Clin Psychol 1998;66:680-4. http://dx.doi.org/10.1037//0022-006X.66.4.680.
- Goldberg RW, Tapscott SL, Calmes CA, Wolfe RS. HIV and hepatitis C knowledge among individuals with serious mental illness. Psychiatr Rehabil J 2009;33:47-9. http://dx.doi.org/10.2975/33.1.2009.47.49.
- Weinhardt LS, Carey MP, Carey KB. HIV risk reduction for the seriously mentally ill: pilot investigation and call for research. J Behav Ther Exp Psychiatry 1997;28:87-95. http://dx.doi.org/10.1016/S0005-7916(97)00002-5.
- Brown LK. HIV Prevention for Youth with Severe Mental Illness 2009.
- Collins PY, Geller PA, Miller S, Toro P, Susser ES. Ourselves, our bodies, our realities: an HIV prevention intervention for women with severe mental illness. J Urban Health 2001;78:162-75. http://dx.doi.org/10.1093/jurban/78.1.162.
- Mansergh G, Koblin BA, McKirnan DJ, Hudson SM, Flores SA, Wiegand RE, et al. An intervention to reduce HIV risk behavior of substance-using men who have sex with men: a two-group randomized trial with a nonrandomized third group. PLoS Med 2010;7. http://dx.doi.org/10.1371/journal.pmed.1000329.
- Markowitz JC, Spielman LA, Sullivan M, Fishman B. An exploratory study of ethnicity and psychotherapy outcome among HIV-positive patients with depressive symptoms. J Psychother Pract Res 2000;9:226-31.
- Colom F, Vieta E, Martinez-Aran A, Reinares M, Goikolea JM, Benabarre A, et al. A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission. Arch Gen Psychiatry 2003;60:402-7. http://dx.doi.org/10.1001/archpsyc.60.4.402.
- Craig TK, Garety P, Power P, Rahaman N, Colbert S, Fornells-Ambrojo M, et al. The Lambeth Early Onset (LEO) Team: randomised controlled trial of the effectiveness of specialised care for early psychosis. BMJ 2004;329. http://dx.doi.org/10.1136/bmj.38246.594873.7C.
- Hafner RJ, Badenoch A, Fisher J, Swift H. Spouse-aided versus individual therapy in persisting psychiatric disorders: a systematic comparison. Fam Process 1983;22:385-99.
- Brady SM. Improving HIV Prevention Skills in People with Serious Mental Illnesses 2008.
- Collins P, Susser E, Valencia E, Miller S, Saez H, Geller P. HIV prevention for mentally ill men and women n.d.
- Holder S, Susser E, Valencia E, Berkman A, . Human immunodeficiency virus sexual risk reduction in homeless men with mental illness. Arch Gen Psychiatry 1998;55:266-72. http://dx.doi.org/10.1136/ebn.2.1.15.
- Peters AT, Nierenberg AA. Stepping back to step forward: lessons from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). J Clin Psychiatry 2011;72:1429-31. http://dx.doi.org/10.4088/JCP.11ac07357.
- Susser E, Desvarieux M, Wittkowski KM. Reporting sexual risk behavior for HIV: a practical risk index and a method for improving risk indices. Am J Public Health 1998;88:671-4. http://dx.doi.org/10.2105/AJPH.88.4.671.
- Fullerton D, Burtney E. An Overview of the Effectiveness of Sexual Health Improvement Interventions. Edinburgh: NHS Health Scotland; 2010.
- Kirkham J, Dwan K, Altman D, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c365.
- White J, Gray R, Swift L, Barton G, Jones M. The serious mental illness health improvement profile (HIP): study protocol for a cluster randomised controlled trial. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-167.
Appendix 1 Study protocol
HTA REFERENCE NO. 12/74 SEXUAL HEALTH OF PEOPLE WITH SEVERE MENTAL ILLNESS
29 January 2013.
1. Title of the project:
What is the effectiveness of sexual health interventions for people with severe mental illness?
2. Name of TAR team and project ‘lead’
School of Health and Related Research (ScHARR), The University of Sheffield.
Project lead:
Dr Eva Kaltenthaler, Reader in Health Technology Assessment, Health Economics and Decision Science, ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield S1 4DA
3. Plain English Summary
Severe mental illness describes a wide range of major psychiatric disorders (including schizophrenia and bipolar disorder)1 which persist over time and cause extensive disability leading to impairments in social and occupational functioning. 2 People with severe mental illness have higher morbidity and mortality due to chronic disease than the general population,3 and are more likely to engage in high-risk sexual behaviour (e.g. unprotected intercourse, multiple partners, sex trade and injection drug use), putting them at risk of worse sexual health outcomes including sexually transmitted infections, including HIV. 2,4 Many of these individuals will also have coexisting drug and alcohol problems5 and have difficulties in establishing stable and sexual relationships. 6 Sexual health promotion interventions (such as educational and behavioural interventions, motivational exercises, counselling and service delivery), developed and implemented for people with severe mental illness, may improve participants’ knowledge, attitudes, beliefs or behavioural practices (including assertiveness skills) and could lead to a reduction in risky sexual behaviour. 4 The aim of this review is to evaluate the effectiveness of sexual health interventions for people with severe mental illness, its applicability to the UK NHS setting, and identify key areas for primary research.
4. Decision problem
Purpose of the decision to be made
The assessment will address the question ‘What is the effectiveness of sexual health interventions for people with severe mental illness?’
Clear definition of the intervention
Health promotion interventions, whether brief interventions or longer term programmes are used to improve various aspects of health and health behaviours. Interventions can be delivered at different levels (individual, group and community) through an action or group of actions intended to change the knowledge, attitudes, beliefs, or behavioural practices of individuals and populations to reduce their sexual health risk. 7 Individual level interventions focus on one individual at a time in an attempt to help change behaviour and may include partner notification, individual risk counselling by professionals and detached education and outreach (for those not accessing mainstream services). Group level interventions are delivered to small groups of individuals. Group level interventions use peer and non-peer models involving a range of skills, information and support. Community level interventions are delivered by or within a defined ‘community’. They seek to improve the risk conditions and influence behaviour by changing social norms, policies or characteristics of the environment. 7,8
Place of the intervention in the treatment pathway(s)
Mental health nurses and other professionals working with people with SMI would be well placed to include sexual health as part of their contribution to health promotion. 4 However, staff may require specific training in order to deliver such interventions.
Relevant comparators
The relevant comparator is considered as usual care in the community. Professionals working with people with severe mental illness may currently provide ad hoc advice on health risks, but this is not integrated into routine practice.
Population and relevant sub-groups
The population will include any adults (defined as ≥ 18 years of age) of either gender living in the community with severe mental illness and their carers/staff. As recommended in the vignette (produced by NETSCC HTA), severe mental illness is defined as people who have received a diagnosis of schizophrenia or bipolar disorder. 1 The identification of subgroups will be governed by the available evidence. However, on a priori grounds, information will be sought from people with dual diagnoses, that is, those with severe mental illness plus substance abuse.
Key factors to be addressed
The review will aim to evaluate the following objectives:
-
Evaluate the effectiveness of sexual health interventions for people with severe mental illness compared with usual care and its applicability to the UK NHS setting.
-
Identify key areas for primary research
5. Report methods for synthesis of evidence of clinical effectiveness
A review of the evidence for clinical effectiveness will be undertaken systematically following the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (http://www.prisma-statement.org/). The review will assess the effectiveness of sexual health interventions for people with severe mental illness compared with usual care.
Inclusion/Exclusion criteria:
Population
The population will comprise adults (defined as ≥ 18 years of age) with severe mental illness living in the community and their carers/staff. Severe mental illness is defined as people who have received a diagnosis of schizophrenia or bipolar disorder. Adults with dementia, personality disorder, or mental retardation will be excluded as they are not included in our definition of severe mental illness
Interventions
Any health promotion intervention or combination of interventions (e.g. educational, behavioural, psychological, counselling etc. delivered at the individual, group and community level) intended to change the knowledge, attitudes, beliefs, behaviours or practices of individuals and populations to improve their sexual health will be considered. Interventions that focus on sexual dysfunction and sexual violence or prescribed drugs will be excluded.
Comparators
Usual care (defined as standard community care)
Outcomes
The outcomes of the review will include the following:
-
Biological: sexually transmitted infections (including HIV), unintended pregnancy
-
Behavioural: numbers of partners, use of contraception/condoms, uptake of screening or treatment services
-
Proxy: knowledge, attitudes and beliefs, barriers and facilitators, intentions, skills
Search strategy
A comprehensive search will be undertaken to systematically identify clinical effectiveness literature on sexual health interventions for people with severe mental illness. The search strategy will comprise the following main elements:
-
Searching of electronic databases
-
Contact with experts in the field
-
Scrutiny of bibliographies of all retrieved papers
A list of the electronic databases and grey literature sources that will be searched is provided in Table 1. The search strategy will be adapted across the databases and language and date restrictions will not be applied. However, due to the large number of potentially relevant citations, searches in the major databases will be restricted by study type (i.e. controlled trials). An example of the MEDLINE search strategy is provided in Section 9.
| Electronic database sources |
|---|
|
| Grey literature and internet sources |
|
Inclusion criteria
All controlled trials (randomised or non-randomised) that evaluate sexual health interventions with usual care for adults with severe mental illness will be included. In the absence of trial evidence, other study designs such as observational cohort studies with a contemporaneous control group will be considered and identified via iterative searching methods. 9 Before and after studies without a concurrent control group will be excluded because the absence of a control group to record concurrent changes over time means that changes due to the intervention or due to temporal trends, concurrent changes or a Hawthorne effect would be conflated. Such studies therefore represent very weak evidence of effectiveness. 10,11
The inclusion of potentially relevant articles will be undertaken using a two-step process. First all titles will be examined for inclusion by one reviewer (any citations that clearly do not meet the inclusion criteria i.e. non-human, unrelated to sexual health interventions for people with severe mental illness will be excluded). Second, all abstracts and full text articles will be examined independently by two reviewers. Any disagreements in the selection process will be resolved through discussion.
Exclusion criteria
Reviews of primary studies will not be included in the analysis, but will be retained for discussion and identification of additional studies. Moreover, the following publication types will be excluded from the review: animal models; preclinical and biological studies; narrative reviews, editorials, opinions; non-English language papers and reports published as meeting abstracts only, where insufficient methodological details are reported to allow critical appraisal of study quality. Studies from developing countries will also be excluded as it will be difficult to generalise (e.g. transferability and acceptability) the characteristics of the effective interventions to developed countries. Details of all full text excluded papers (including non-English language citations) will be provided in the review.
Data extraction strategy
Data will be extracted by one reviewer using a standardised data extraction form and independently checked for accuracy by a second. Uncertainties will be resolved by discussion. Where multiple publications of the same study are identified, data will be extracted and reported as a single study.
Quality assessment strategy
The methodological quality of each included study will be assessed according to (adapted) criteria based on those proposed by Effective Public Health Practice Project (http://www.ephpp.ca/Tools.html). This is a generic tool used to evaluate a variety of intervention study designs such as RCTs and observational studies. This tool has been judged suitable to be used in systematic reviews of effectiveness12 and has been reported to have content and construct validity. 13,14 Consideration of study quality will include the following factors: (1) selection bias; (2) study design; (3) confounders; (4) blinding; (5) data collection method; (6) withdrawals/dropouts; (7) intervention integrity; (8) statistical analysis; and (9) generalisability.
Methods of analysis/synthesis
Data will be tabulated and discussed in a narrative review. If appropriate (i.e. populations, interventions and outcomes are comparable), meta-analysis will be employed to estimate a summary measure of effect on relevant outcomes based on intention to treat analyses. Meta-analysis will be undertaken using fixed or random effects models, using STATA Statistical Software or the Cochrane Collaboration Review Manager Software, for example. Heterogeneity will be explored through consideration of the study populations, methods and interventions, by visualisation of results and, in statistical terms, by the χ2 test for homogeneity and the I2 statistic. However, it is anticipated that heterogeneity of study designs and interventions, and the type of data available, may mean that it is not appropriate to perform meta-analysis. The likely form of analysis will be a narrative synthesis.
6. Expertise in this TAR team
TAR Centre
The ScHARR Technology Assessment Group (ScHARR-TAG) undertakes reviews of the effectiveness and cost-effectiveness of healthcare interventions for the NHS R&D Health Technology Assessment Programme on behalf of a range of policy makers in a short timescale, including the National Institute for Health and Clinical Excellence. A list of our publications can be found at: http://www.sheffield.ac.uk/scharr/sections/heds/collaborations/scharr-tag/reports.
Much of this work, together with our reviews for the international Cochrane Collaboration, underpins excellence in healthcare worldwide.
7. Competing interests of authors
The following authors do not have any competing interest: Eva Kaltenthaler, Abdullah Pandor, Ruth Wong, Professor Wylie and Dr Smith.
Professor Gray has received speaker fees and honoraria from companies (Wyeth, BMS, Otsuka, Janssen-Cilag, Eli Lilly and AstraZeneca) that manufacture products that can potentially impact on sexual functioning. He is also funded by the National Institute of Health Research to undertake a trial to promote physical (including sexual) health in patients with mental illness.
8. Timetable/milestones
| Milestone | |
|---|---|
| Draft protocol | 14 December 2012 |
| Final protocol | 14 January 2013 |
| Progress report | 1 March 2013 |
| Assessment report | 29 March 2013 |
9. Appendices
9.1 Draft search strategy (Ovid MEDLINE)
-
((chronic$ or sever$ or persist$) and mental$ and (ill$ or disorder$)).mp.
-
exp Schizophrenia/
-
(schizo$ or hebephreni$ or oligophreni$ or psychotic$ or psychosis or psychoses).tw.
-
Paranoid Disorders/
-
exp Psychotic Disorders/
-
(paranoia or paranoid disorders or psychotic disorders or psychosis).tw.
-
exp Bipolar Disorder/
-
((bipolar or bi polar) adj5 (disorder$ or depress$)).tw.
-
(hypomania$ or mania$ or manic$).tw.
-
(((cyclothymi$ or rapid or ultradian) adj5 cycl$) or RCBD).tw.
-
or/1-10
-
exp Sexual Behavior/
-
(sex* and (health or safe or safer or unsafe or risk or high-risk or unprotected or abstinence or behaviour* or behavior* or activit* or partner*)).mp.
-
exp Sexually Transmitted Diseases/
-
((STI or STIs or STD or STDs) and (incidence or prevalen* or prevent* or control* or risk* or reduc*)).mp.
-
((sexually transmitted disease* or sexually transmitted infection*) and (incidence or prevalen* or prevent* or control* or risk* or reduc*)).mp.
-
or/12-16
-
Randomized controlled trials as Topic/
-
Randomized controlled trial/
-
Random allocation/
-
randomized controlled trial.pt.
-
Double blind method/
-
Single blind method/
-
Clinical trial/
-
exp Clinical Trials as Topic/
-
controlled clinical trial.pt.
-
or/18-26
-
(clinic$ adj25 trial$).ti,ab.
-
((singl$ or doubl$ or treb$ or tripl$) adj (blind$ or mask$)).tw.
-
Placebos/
-
Placebo$.tw.
-
(allocated adj2 random).tw.
-
or/28-32
-
27 or 33
-
Case report.tw.
-
Letter/
-
Historical article/
-
35 or 36 or 37
-
exp Animals/
-
Humans/
-
39 not (39 and 40)
-
38 or 41
-
34 not 42
-
11 and 17 and 43
9.2 Team members’ contributions
Eva Kaltenthaler, Reader in Health Technology Assessment. EK has extensive experience in systematic reviews of health technologies. EK will undertake the systematic review. She will coordinate the review process, protocol development, abstract assessment for eligibility, quality assessment of trials, data extraction, data entry, data analysis and review development of background information and clinical effectiveness.
Abdullah Pandor, Senior Research Fellow. AP has extensive experience in systematic reviews of health technologies. AP will assist EK with the project and undertake the systematic reviewing. He will be involved in assessing abstracts for eligibility, quality assessment of trials, data extraction, data entry, data analysis and review development of background information and clinical effectiveness.
Ruth Wong, Information Specialist. RW has extensive experience of undertaking literature searches for the ScHARR Technology Assessment Group systematic reviews and other external projects. RW will develop the search strategy and undertake the electronic literature searches.
Andrea Shippam, Project Administrator. AS will assist in the retrieval of papers and in preparing and formatting the report.
Professor Richard Gray, Professor of Mental Health and Honorary Consultant, University of the West of England, Bristol, UK. RG will assist with protocol development (advisor), help interpret data, provide a methodological, policy and clinical perspective on data and review development of background information and clinical effectiveness.
Professor Kevan Wylie, Consultant in Sexual Medicine, NHS, Sheffield, UK. KW will assist with protocol development (advisor), help interpret data, provide a methodological, policy and clinical perspective on data and review development of background information and clinical effectiveness.
Dr Shubulade Smith, Consultant Psychiatrist and Clinical Senior Lecturer, South London & Maudsley NHS Foundation Trust, UK. SS will assist with protocol development (advisor), help interpret data, provide a methodological, policy and clinical perspective on data and review development of background information and clinical effectiveness.
References
- HM Government . No health without mental health: a cross-government mental health outcomes strategy for people of all ages. Department of Health 2010.
- Meade C, Sikkema K. HIV risk behavior among adults with severe mental illness: a systematic review. Clinical Psychology Review 2005;25:433-57.
- Dixon L, Postrado L, Delahanty J, Fischer P, Lehman A. The association of medical comorbidity in schizophrenia with poor physical and mental health. J Nerv Ment Dis 1999;187:496-502.
- Higgins A, Barker P, Begley C. Sexual health education for people with mental health problems: what can we learn from the literature?. Journal of Psychiatric and Mental Health Nursing 2006;13:687-9.
- Marshall J, Farell M. Substance use and psychiatric comorbidity. Psychiatry 2007;6:23-6.
- Robson D, Gray R. Serious mental illness and physical health problems: a discussion paper. International Journal of Nursing Studies 2007;44:457-66.
- Burtney E, Fullerton D. Sexual Health Improvement Interventions in Scotland. Mapping Exercise. NHS Health Scotland 2011.
- Ellis S, Grey A. Prevention of sexually transmitted infections (STIs): a review of reviews into the effectiveness of non-clinical interventions. Evidence briefing. Health Development Agency 2004.
- Kaltenthaler E, Tappenden P, Paisley S, Squires H. NICE DSU Technical Support Document 13: Identifying and reviewing evidence to inform the conceptualisation and population of cost-effectiveness models. NICE DSU 2011.
- Armstrong R, Waters E, Jackson N, Oliver S, Popay J, Shepherd J, et al. Australia: Melbourne University; 2007.
- Cook TD, Campbell DT. Quasi Experimentation. Design & Analysis Issues for Field Settings. Boston, MA, USA: Houghton Mifflin Company; 1979.
- Deeks J, Dinnes J, D’Amico R, Soeden A, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technology Assessment 2003;7:3-173.
- Jackson N, Waters E. Criteria for the systematic review of health promotion and public health interventions. Health Promotion International 2005;20:367-74.
- Thomas B, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews on Evidence-Based Nursing 2004;1:176-84.
Appendix 2 Literature search strategy: a MEDLINE example
| Database searched: | Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) |
| Platform or provider used: | Ovid SP |
| Date of coverage: | 1948 to December 2012 |
| Search undertaken: | 6 December 2012 |
-
((chronic$ or sever$ or persist$) and mental$ and (ill$ or disorder$)).mp.
-
exp Schizophrenia/
-
(schizo$ or hebephreni$ or oligophreni$ or psychotic$ or psychosis or psychoses).tw.
-
Paranoid Disorders/
-
exp Psychotic Disorders/
-
(paranoia or paranoid disorders or psychotic disorders or psychosis).tw.
-
exp Bipolar Disorder/
-
((bipolar or bi polar) adj5 (disorder$ or depress$)).tw.
-
(hypomania$ or mania$ or manic$).tw.
-
(((cyclothymi$ or rapid or ultradian) adj5 cycl$) or RCBD).tw.
-
or/1-10
-
exp Sexual Behavior/
-
(sex* and (health or safe or safer or unsafe or risk or high-risk or unprotected or abstinence or behaviour* or behavior* or activit* or partner*)).mp.
-
exp Sexually Transmitted Diseases/
-
((STI or STIs or STD or STDs) and (incidence or prevalen* or prevent* or control* or risk* or reduc*)).mp.
-
((sexually transmitted disease* or sexually transmitted infection*) and (incidence or prevalen* or prevent* or control* or risk* or reduc*)).mp.
-
or/12-16
-
Randomized controlled trials as Topic/
-
Randomized controlled trial/
-
Random allocation/
-
randomized controlled trial.pt.
-
Double blind method/
-
Single blind method/
-
Clinical trial/
-
exp Clinical Trials as Topic/
-
controlled clinical trial.pt.
-
or/18-26
-
(clinic$ adj25 trial$).ti,ab.
-
((singl$ or doubl$ or treb$ or tripl$) adj (blind$ or mask$)).tw.
-
Placebos/
-
Placebo$.tw.
-
(allocated adj2 random).tw.
-
or/28-32
-
27 or 33
-
Case report.tw.
-
Letter/
-
Historical article/
-
35 or 36 or 37
-
exp Animals/
-
Humans/
-
39 not (39 and 40)
-
38 or 41
-
34 not 42
-
11 and 17 and 43
Appendix 3 Quality assessment tool43
Reproduced with the permission of Thomas B, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004;1:176–84.
Appendix 4 Table of excluded studies with rationale
| Author, year | Reason for exclusion |
|---|---|
| Brady, 200868 | Not available (from Trials register – author contacted but no reply) |
| Brown, 200961 | Adolescents |
| Blank et al., 201232 | Not RCT (pooled analysis) |
| Collins et al., 199669 | Not available |
| Collins et al., 200162 | Inpatients only |
| Colom et al., 200365 | No sexual health promotion outcomes |
| Craig et al., 200466 | No sexual health promotion outcomes |
| Goldberg et al., 200959 | Not RCT |
| Hafner et al., 198367 | No sexual health promotion outcomes |
| Holder, 199970 | Comment/discussion |
| Mansergh et al., 201063 | Not SMI |
| Markowitz et al., 200064 | Not SMI |
| Melo et al., 201028 | Not RCT |
| Penna and Sheehy, 200040 | Not RCT |
| Peters and Nierenberg, 201171 | Review |
| Sikkema et al., 200721 | Not RCT |
| Solomon et al., 200735 | Not RCT |
| Tennille et al., 200934 | Not RCT |
| Tennille et al., 201033 | Not RCT |
| Weinhardt et al., 199760 | Not RCT |
Appendix 5 Methodological quality summary
The following presents a review of the authors’ judgements about each methodological quality item for each included study. The questions asked about each study, and the answers available for each question, are detailed in Appendix 3.
| Author, year | 1. Selection bias | 2. Study design | 3. Confounders | 4. Blinding | 5. Data collection | 6. Withdrawals and dropouts | 7. Intervention integrity | 8. Analyses | 9. Generalisability to UK setting | Overall rating (items 1–6 only) |
|---|---|---|---|---|---|---|---|---|---|---|
| Berkman et al., 200646,47 | Moderate | Strong | Weak | Moderate | Strong | Strong | Weak | Strong | Weak | Moderate |
| (Q1: 2; Q2: 5) | (CCT, method of randomisation NR)a | (Q1: 1; Q2: 4) | (Q1: 3; Q2: 3) | (Q1: 1; Q2: 1) | (Q1: 2; Q2: 1) | (Q1: 3; Q2: 3; Q3: 5) | (Q4: 1; ITT) | (USA only) | ||
| Berkman et al., 200748 | Moderate | Strong | Weak | Moderate | Strong | Strong | Weak | Strong | Weak | Moderate |
| (Q1: 2; Q2: 5) | (RCT, block random, allocation concealed) | (Q1: 1; Q2: 4) | (Q1: 2; Q2: 3) | (Q1: 1; Q2: 1) | (Q1: 2; Q2: 1) | (Q1: 4; Q2: 3; Q3: 6) | (Q4: 1; ITT) | (USA only) | ||
| Carey et al., 200449 | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate | Strong | Weak | Moderate |
| (Q1: 2; Q2: 3) | (CCT, method of randomisation NR)a | (Q1: 2; Q2: 1) | (Q1: 2; Q2: 3) | (Q1: 1; Q2: 1) | (Q1: 2; Q2: 1) | (Q1: 2; Q2: 1; Q3: 6) | (Q4: 1; ITT) | (USA only) | ||
| Collins et al., 201150 | Moderate | Strong | Weak | Moderate | Strong | Strong | Weak | Strong | Weak | Moderate |
| (Q1: 2; Q2: 2) | (RCT, block random) | (Q1: 1; Q2: 4) | (Q1: 3; Q2: 3) | (Q1: 1; Q2: 1) | (Q1: 2; Q2: 1) | (Q1: 1; Q2: 1; Q3: 4) | (Q4: 1; ITT) | (USA only) | ||
| Kalichman et al., 199551 | Moderate | Strong | Strong | Moderate | Strong | Weak | Strong | Weak | Weak | Moderate |
| (Q1: 2; Q2: 5) | (CCT, method of randomisation NR)a | (Q1: 2; Q2: 1) | (Q1: 3; Q2: 3) | (Q1: 1; Q2: 1) | (Q1: 3; Q2: 4) | (Q1: 1; Q2: 1; Q3: 5) | (Q4: 2) | (USA only) | ||
| Katz et al., 199652 | Moderate | Strong | Weak | Moderate | Strong | Weak | Weak | Weak | Weak | Weak |
| (Q1: 2; Q2: 2) | (CCT, method of randomisation NR)a | (Q1: 3; Q2: 4) | (Q1: 3; Q2: 3) | (Q1: 1; Q2: 1) | (Q1: 3; Q2: 3) | (Q1: 4; Q2: 3; Q3: 6) | (Q4: 2) | (USA only) | ||
| Kelly et al., 199731 | Moderate | Strong | Weak | Moderate | Weak | Weak | Weak | Moderate | Weak | Weak |
| (Q1: 2; Q2: 2) | (CCT, method of randomisation NR)a | (Q1: 1; Q2: 4) | (Q1: 3; Q2: 3) | (Q1: 3; Q2: 3) | (Q1: 3; Q2: 4) | (Q1: 4; Q2: 1; Q3: 6) | (Q4: 3) | (USA only) | ||
| Linn 200353 | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong | Strong | Weak | Strong |
| (Q1: 2; Q2: 1) | (CCT, method of randomisation NR)a | (Q1: 2; Q2: 1) | (Q1: 2; Q2: 3) | (Q1: 1; Q2: 1) | (Q1: 1; Q2: 1) | (Q1: 1; Q2: 1; Q3: 5) | (Q4: 1; ITT) | (USA only) | ||
| Malow et al., 201254 | Moderate | Strong | Strong | Moderate | Strong | Moderate | Moderate | Weak | Weak | Moderate |
| (Q1: 2; Q2: 5) | (CCT, method of randomisation NR)a | (Q1: 2; Q2: 1) | (Q1: 2; Q2: 3) | (Q1: 1; Q2: 1) | (Q1: 2; Q2: 2) | (Q1: 2; Q2: 3; Q3: 6) | (Q4: 2) | (USA only) | ||
| NIMH 200657 | Weak | Strong | Weak | Moderate | Weak | Strong | Weak | Moderate | Weak | Weak |
| (Q1: 3; Q2: 5) | (CCT; method of randomisation NR)a | (Q1: 3; Q2: 4) | (Q1: 3; Q2: 3) | (Q1: 3; Q2: 3) | (Q1: 2; Q2: 1) | (Q1: 4; Q2: 1; Q3: 6) | (Q4: 3) | (USA only) | ||
| Otto-Salaj 200155 | Moderate | Strong | Strong | Moderate | Weak | Strong | Moderate | Weak | Weak | Moderate |
| (Q1: 2; Q2: 5) | (CCT, method of randomisation NR)a | (Q1: 2; Q2: 1) | (Q1: 3; Q2: 3) | (Q1: 3; Q2: 3) | (Q1: 2; Q2: 1) | (Q1: 1; Q2: 1; Q3: 6) | (Q4: 2) | (USA only) | ||
| Susser 199856 | Moderate | Strong | Strong | Moderate | Strong | Strong | Moderate | Strong | Weak | Strong |
| (Q1: 2; Q2: 1) | (RCT, randomly ordered sealed envelopes) | (Q1: 2, Q2: 1) | (Q1: 2; Q2: 3) | (Q1: 1; Q2: 1) | (Q1: 2; Q2: 1) | (Q1: 1; Q2: 1; Q3: 6) | (Q4: 1; ITT) | (USA only) | ||
| Weinhardt 199858 | Moderate | Strong | Strong | Moderate | Strong | Weak | Weak | Moderate | Weak | Moderate |
| (Q1: 2; Q2: 1) | (CCT; method of randomisation NR)a | (Q1: 2; Q2: 1) | (Q1: 3; Q2: 3) | (Q1: 1; Q2: 1) | (Q1: 2; Q2: 4) | (Q1: 4; Q2: 3; Q3: 6) | (Q4: 3) | (USA only) |
Glossary
- Severe mental illness
- Severe and long-lasting mental illness associated with functional impairment that typically involves psychosis (losing touch with reality or experiencing delusions). Someone with a severe mental illness may nevertheless also have long periods when they are well and are able to manage their illness. Examples of severe mental illnesses include schizophrenia and bipolar disorder.
- Bipolar disorder
- A severe mental illness with a long course usually characterised by episodes of depression and also periods of elation and increased activity (mania or hypomania). However, for many people the predominant experience is of low mood. In its more severe forms, bipolar disorder is associated with significant impairment of personal and social functioning.
- Schizophrenia
- A major psychiatric disorder, or cluster of disorders, characterised by psychotic symptoms that alter a person’s perceptions and thoughts and affect their behaviour. Each person with the disorder will have a unique combination of symptoms and experiences.
- Proxy outcome
- Outcomes used to demonstrate change where a direct measure is not feasible. A proxy outcome would be used when the outcome of interest cannot feasibly be calculated or when a large sample size may be required to detect change, e.g. changes in sexual health knowledge, attitudes and intentions or self-efficacy.
List of abbreviations
- AIDS
- acquired immunodeficiency syndrome
- EPHPP
- Effective Public Health Practice Project
- HIV
- human immunodeficiency virus
- NIHR
- National Institute for Health Research
- NIMH
- National Institute of Mental Health
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-analyses
- RCT
- randomised controlled trial
- ScHARR
- School of Health and Related Research
- SexG
- Sex, Games and Videotapes
- SMI
- severe mental illness
- STI
- sexually transmitted infection
- VEE
- vaginal episode equivalent