Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/78/02. The contractual start date was in September 2010. The draft report began editorial review in December 2012 and was accepted for publication in April 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
PA has been a consultant and done research for manufacturers of smoking-cessation products. RW has undertaken research and consultancy for companies that develop and manufacture smoking-cessation medications. He is co-director of the National Centre for Smoking Cessation and Training and a trustee of the stop-smoking charity, QUIT. He has a share of a patent on a novel nicotine delivery device. All other authors have declared no competing interests.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Taylor et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
During the course of this pilot trial a number of methodological issues arose and these were resolved. A specific section in the discussion chapter within the main report considers discrepancies in reported methods (e.g. inclusion/exclusion criteria, sample size, outcome measurement) between different sections such as the protocol (see Appendix 2), methods (see Chapter 2) and results (see Chapter 3). Sequentially the protocol and methods describe what we planned to do, and the results and discussion chapters describe what was achieved.
Scientific background
Current treatment/management options for smoking cessation and reduction, especially among disadvantaged smokers
Health service priorities for helping people to quit smoking focus on identifying a quit date with associated abrupt cessation, involving pharmacological and behavioural support. 1 After 1 year, only about 4% of those who attempt to quit without support succeed,2 whereas in the UK that figure is almost doubled (7%) with NHS support in primary care and almost quadrupled (15%) with the support of the NHS specialist Stop Smoking Service (SSS). 3 In recent years greater resources have been directed towards helping disadvantaged groups (e.g. unemployed people, low-skilled manual workers and people with mental health problems) to quit in an attempt to address growing health inequalities. 4 The rate of those attempting to quit is constant across social groups, but those from more disadvantaged backgrounds are less likely to succeed in remaining abstinent. 5 This results in a growing disparity in smoking prevalence rates between social groups and therefore in consequent health inequalities. For example, from 2007 to 2008, among those in social class grades C2–E smoking prevalence rates reduced by only 1.3% compared with 2.3% for grades AB–C1. 6
Good-quality evidence for the effectiveness of smoking-cessation interventions for disadvantaged groups is limited. 7 Further research is needed on how best to increase the reach of interventions to those who are less attracted to smoking-cessation services, and increase smoking-cessation success among such groups to reduce health inequalities. It is likely that a range of options may be needed to increase the reach of services and to reduce smoking prevalence, such as locating services in community settings with most need,4 developing roles for NHS outreach workers [e.g. health trainers (HTs)],8 and developing complex behaviour-change interventions that are specifically designed for disadvantaged groups. 9
Abrupt cessation is the preferred treatment approach for quitting because, in theory, smokers who cut down prior to quitting may gain greater reward from each cigarette as they become fewer and farther between and hence find quitting more difficult. 1 Yet in the English Smoking Toolkit Study, 57% of current smokers reported that they were in the process of cutting down,6 with a variety of approaches being used. 10 While nicotine replacement therapy (NRT) is popular as an aid for smoking reduction, another study revealed that 31% of smokers believed that sustained use of NRT was ‘very’ or ‘quite’ harmful to health. 11 Furthermore, stop-smoking advisors and managers have expressed concern that combining NRT with smoking may have negative health consequences. 12 There is clearly a need for further research on supporting smoking reduction for those who do not wish to use NRT, among both those who do wish to quit and those who do not. Among those who do wish to quit, smoking reduction using pharmacotherapy and behavioural support appears to be equally as effective as abruptly quitting. 13
In a US survey, interest in reduction was highest among those who were less interested in quitting and among heavier smokers. 14 However, epidemiological studies suggest that cutting down is associated with an increased probability of trying to quit. Also, smokers who do not intend to quit in the next month, but do cut down (with NRT), are more likely to make a quit attempt and remain abstinent at follow-up. 15 Smoking reduction may increase the motivation to quit, which is highly predictive of quit attempts, and reduce smoking dependence, which is related to successful quitting. 16 Motivational advice (without NRT) can increase quit attempts lasting at least 24 hours and 7-day point prevalence abstinence at 6 months. 17 Behavioural support aims to increase confidence in smokers so that they can cope with cravings and withdrawal symptoms, reduce smoking, and ultimately remain abstinent. 18
Evidence for the effectiveness of exercise for smoking cessation and related outcomes
There is strong evidence that a single session of physical activity (PA) can reduce both cigarette cravings19 and withdrawal symptoms. 20 It can also delay smoking21,22 and decrease puff volume during temporary abstinence. 22,23 A review of exercise interventions (vs. usual care) as an aid for long-term smoking cessation24 identified 16 studies. However, most were methodologically limited, with seven involving fewer than 25 participants in each arm. Of the seven that were adequately powered, three supported significant increases in abstinence at the end of treatment, but only one supported increased abstinence rates at 12-month follow-up. Variation in study length, type (e.g. structured group-based exercise, and PA counselling) and content of the control condition complicated comparison of the studies in the review. The timing of the introduction of PA also varied across studies, with some studies promoting involvement in PA several weeks before a quit attempt. All these studies were among smokers who wished to quit, and none with those who wished only to cut down. Almost all studies focused on the use of prescriptive exercise sessions supervised by an exercise professional, with only a few promoting changes in daily lifestyle activity as a way to manage cigarette cravings and withdrawal symptoms. Coupled with epidemiological data suggesting that physically active smokers are more likely to attempt to quit,24 there is therefore scope to explore if PA could facilitate smoking reduction and cessation induction.
Possible mechanisms for how exercise might influence smoking
There are several ways in which an increase in PA may putatively facilitate smoking reduction and cessation induction. 25 In addition to smokers explicitly using short bouts of PA to cope with cravings and withdrawal symptoms (see above), it may also help to reduce the substantial weight gain associated with cessation. 26 On average, smokers experience almost 5 kg of weight gain, with 13% gaining over 10 kg, in the year after quitting. 26 In a limited number of studies, increasing PA has been shown to be a useful strategy to prevent weight gain among those quitting smoking,27 and its promotion is popular with smoking-cessation practitioners. 28 PA may be effective by both increasing energy expenditure and enhancing self-regulation of emotional food snacking associated with low mood. 29,30 Of relevance to the present study, systematic reviews and prospective cohort studies suggest that people of lower socioeconomic status and heavier smokers (among other characteristics) are at increased risk of weight gain. 31
Smoking prevalence is greater among people with mental health problems, perhaps because nicotine may improve concentration and cognition, relieve stress, improve depressive affect and increase pleasurable sensations. 32 PA can reduce depression33 and anxiety34 and (speculatively) may replace the need to smoke. In laboratory studies, a single session of exercise appears to reduce attentional bias to smoking cues,35 and reduce activation in areas of the brain associated with reward seeking while viewing smoking-related images. 36 Finally, it may be reasonable to speculate that undertaking more PA may help or reinforce a shift from the identity of a smoker to that of an exerciser, with the potential for a reduced exposure to environments and cues associated with smoking. In a cross-sectional survey the negative association between PA and smoking was mediated by having a physically active identity. 37 Thus, by simply increasing PA there may be implicit positive effects on smoking habits.
At least 50 cross-sectional surveys have assessed the association between self-reported PA and smoking status,38 with most reporting a negative association. Physically active smokers are more likely than inactive smokers to have attempted cessation in the past year. 39 However, RCTs to assess the effects of a primary care intervention to promote PA have shown that increases in PA were not associated with concurrent reductions in smoking among the subsample of smokers in the study. 40,41 This evidence questions the idea that simply increasing PA will lead to a spontaneous change in smoking behaviour.
Promoting physical activity among disadvantaged groups
The relationship between PA and socioeconomic status among adults is type dependent. Disadvantaged groups undertake less leisure-time PA but undertake more activity associated with work and active transport (in part due to low rates of car ownership). 42,43 This relationship has implications for the effectiveness of interventions to generally increase PA. 44 Systematic reviews have found that interventions that use a set of established behaviour change techniques (especially self-regulation techniques) are more likely to produce increases in PA than those that do not. 45
The role of health trainers in supporting behaviour change among disadvantaged populations
In the UK, NHS HTs were introduced in the ‘Choosing Health’ White Paper,46 and were established to facilitate health behaviour change in disadvantaged communities. The role of a HT fits within a broader role family called ‘health-related lifestyle advisors’, which also includes lay or peer workers or volunteers. Their predominant function is to support health behaviour change in a way that is acceptable to target populations and provides appropriate support to increase motivation and engage in action planning to facilitate healthy lifestyles. HTs are trained to help smokers to develop the motivation to quit smoking (and adopt other health behaviours) and then typically refer their clients to SSS for support to quit abruptly. At the time of initiating this research, there was no synthesis of the effectiveness and cost-effectiveness of HTs or ‘health-related lifestyle advisors’. A recent review of 26 studies (across a wide range of targeted health behaviours, from promoting immunisation and breastfeeding to smoking cessation and PA) suggested that those working in such a role, in general, have little impact on healthy lifestyle. 47 Overall, there was little evidence for effectiveness that interventions could change smoking and PA among disadvantaged communities and the available studies lacked individuals, while methodological rigor and reporting was not always clear.
The challenge, therefore, is to design a PA promotion intervention that explicitly helps a smoker to build a connection between doing PA and smoking reduction among a disadvantaged population. Within the UK, such an integrated intervention was piloted among smokers who were attempting to quit with the help of smoking-cessation practitioners. 48 There are mixed views on whether multiple behaviour changes (e.g. increases in PA and dietary change) should be tackled simultaneously or sequentially when smokers quit. 1 Anecdotal evidence suggests that attempting to modify diet and PA while quitting is not detrimental to successfully quitting and can be facilitative. 28,49 However, an integrative approach has not been developed or evaluated for disadvantaged smokers who do not wish to quit abruptly, but do want to reduce smoking.
Aims and objectives
The aim of this pilot randomised controlled trial (RCT) was to evaluate the feasibility and acceptability of a novel PA and smoking reduction counselling intervention for disadvantaged smokers who do not wish to quit in the immediate future but do want to reduce their smoking. The pilot study also seeks to inform the design of a full RCT of Exercise Assisted Reduction then Stop (EARS) among disadvantaged smokers.
The present research comprised the following to address those aims:
-
the development of a theoretically based PA intervention designed to increase PA levels in smokers, in a way that may complement and support smoking reduction and increase quit attempts and smoking cessation
-
the conduct of a pilot RCT in which the PA intervention (in addition to usual care) was compared with usual care alone
-
an examination of the feasibility and acceptability of the collection of secondary outcomes
-
estimation of the intervention effect on the primary outcome
-
an examination of the acceptability and feasibility of the intervention and its delivery
-
an examination of the acceptability and feasibility of the trial design and methods
-
an evaluation of the intervention fidelity from taped intervention sessions
-
identification of good practice within the intervention sessions to inform future training and delivery of the intervention
-
an evaluation of the cost of providing the PA intervention, and the conducting of exploratory modelling to assess the framework needed for future cost-effectiveness analyses alongside a full trial.
Development of the Exercise Assisted Reduction then Stop intervention
The aim was to develop a pragmatic intervention that could improve the reach of SSS to offer another option to reduce smoking for ‘hard to reach’ or disadvantaged smokers not ready to make an abrupt quit attempt.
Four steps were taken prior to initiating a pilot RCT, as follows:
-
individual and focus group discussions with smoking-cessation practitioners, researchers, public health consultants, community workers (including volunteers) and smokers to inform the EARS intervention structure and delivery
-
reviewing literature on using exercise as an aid to quitting, and consulting with academic experts on behaviour change for PA, smoking reduction and cessation, alone and in combination to inform the EARS intervention principles and theoretical basis
-
development of an EARS-specific training manual to build on the existing HT manual (www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_085779)
-
training EARS HTs, piloting the intervention with smokers who did not wish to quit in the immediate future and, in response to the reviewing of recorded intervention sessions and discussions with the HTs and smokers, adapting the intervention over a 4-month period prior to the pilot RCT for delivery in the targeted neighbourhoods of Devonport and Stonehouse (Plymouth, UK).
Exercise Assisted Reduction then Stop intervention structure and delivery
The EARS intervention was designed to involve up to 8 weeks of one-to-one support from a HT, in person or by telephone, after an initial face-to-face session. The HT provided no supervised PA sessions but offered subsidised access to PA opportunities (e.g. swimming, gym admission and transport subsidies to walking events), subject to individual preference. The focus was always on making any change in PA sustainable through motivational support.
Participants were given up to 8 weeks to cut down until they were ready to make a quit attempt. To count as abstinent a participant needed to have set a quit date within 12 weeks of randomisation to provide confirmation of a successful 4-week cessation by the final assessment at 16 weeks within the pilot RCT. Anyone who was ready to set a quit date was encouraged to attend and referred to the local SSS for support if they wished to get support. After quitting they were also offered weekly counselling to support ongoing PA from the HT for up to a further 6 weeks.
Exercise Assisted Reduction then Stop intervention principles and theoretical basis
The intervention was client centred in that smokers (who wanted to reduce but not quit in the immediate future) set the speed of reduction and their level of engagement in PA. The HT worked with the participant using client-centred motivational interviewing (MI) techniques50 throughout the intervention. The intervention was further informed by self-determination theory (SDT)51,52 which suggests that changing smoking behaviour will be facilitated by helping the smoker to fulfil three core human needs: a sense of competence or mastery, autonomy or control, and relatedness or companionship. Enhancing autonomy and competence motivations has been shown in prior research to increase abstinence rates and lead to greater cessation. 53 Links between54 MI and SDT have been made in the literature,55–57 suggesting that there may be good synergies in combining these two intervention approaches: both focus on helping the client to develop a sense of ownership of any change and empowerment. In addiction research, there is evidence that a client-centred counselling approach is effective for engaging with clients, building commitment to change,58 and increasing cognitive dissonance59 which can predict treatment outcomes. 60 There is some evidence that MI is effective in treating substance abuse61 and for smoking cessation,62 albeit from generally low-quality studies with questions about treatment fidelity. MI has also been shown to be an effective intervention for increasing PA. 63 The EARS intervention drew from principles of MI but also drew on SDT and other theories of behaviour change (as below).
In particular, MI does not focus on social influences on behaviour change, but SDT-founded interventions seek to help clients fulfil a need for a sense of relatedness. In the EARS intervention, techniques are described that were used to help participants to find social support for smoking reduction and increasing PA.
The EARS intervention was also informed by social cognitive theory64 and control theory,65 in that it sought to promote self-regulation by helping clients to build confidence over time to reduce smoking and increase PA. The self-regulation processes we specifically targeted were action planning, self-monitoring, review of progress, problem solving and review of goals – together these represent a process of experiential learning. Uniquely, this intervention also sought to help participants to use PA to self-regulate smoking by identifying situations where it may be possible to reduce withdrawal symptoms and desire to smoke, enhance positive mood, and break the link between environment and smoking behaviours. A range of behaviour change techniques was matched to the above theoretical processes of change (Table 1) and these were all included in the EARS training programme.
| Intervention process/objective | Intervention strategy | Behaviour change techniques (see Table 2 for description) | Theoretical domains |
|---|---|---|---|
| Active participant involvement | Use MI principles and communication skills. Exhibit empathy using Open questions, Affirmation, Reflections, Summaries (OARS) | RC1, RC2, RC4, RC7, RC8, RC9, RC10 | Knowledge; skills; identity (e.g. social identity); capability beliefs; beliefs about consequences; reinforcement; intentions; goals; memory or attention; context/resources; social influences; emotion; behavioural regulation |
| Develop rapport, building trust, and shared respect and empower the participant to be the primary agent of change | Individual tailoring of techniques and responses to the individual participant’s existing knowledge, skills, needs or preferences | RD1, RD2 | |
| Explore initial beliefs about cutting down (importance and confidence, triggers for smoking) | Use OARS (as above) to explore current and past smoking behaviour, the pros and cons of cutting down. 0–10 questions to explore importance and confidence. Use OARS to develop discrepancies (e.g. by exploring possible futures) | RI1, RI2, BM3, BM9 | Knowledge; capability beliefs; beliefs about consequences; intentions; context/resources; social influences; emotion |
| Build/enhance motivation and confidence for cutting down | Identify strengths and barriers (e.g. by exploring past experiences of success and failure or asking ‘what might stop you?’). Identify possible solutions to barriers | RC6, RI3, RI4, A2, BM2, BS2 | |
| Desire to quit may also be discussed | Exchange information on pros and cons of cutting down and barrier-solutions using the elicit–provide–elicit (Ask–Tell–Discuss) technique | RC2, A2, BM2, BS2 | |
| Explore initial beliefs about PA and using it as an aid to cutting down (importance and confidence, barriers to PA) | Use OARS (as above) to explore pros and cons. Decisional balance tool, 0–10 questions to explore importance and confidence about introducing additional physical activities. Use OARS to develop discrepancies | C37 | Knowledge; capability beliefs; beliefs about consequences; intentions; context/resources; social influences; emotion |
| Identify strengths and barriers (e.g. by exploring past experiences of success and failure or asking ‘what might stop you?’). Identify possible solutions to barriers | C18, C37 | ||
| Build/enhance motivation and confidence for PA | Exchange information on pros and cons of PA and on barriers/solutions using the elicit–provide–elicit (Ask–Tell–Discuss) technique | C8, C31, C37 | |
| Set goals and discuss strategies to reduce smoking | Set SMART goals with smoker to reduce smoking. Discuss/offer a choice of specific strategies | BS3, BS4, BS6, BS7, BS8, BS9 | Intentions; goals; behavioural regulation |
| Negotiate strategy and rate of smoking reduction (over following 1 and 4 weeks) | C12, C23 | ||
| Encourage self-monitoring of daily smoking | BS6 | ||
| Set goals and discuss strategies for PA | Set SMART goals with smoker to increase PA/introduce new physical activities. Discuss preferences and smoker to choose activities. Signpost to relevant PA/exercise opportunities | C5, C7, C9, C23, C26, C24 | Intentions; goals; behavioural regulation; context/resources |
| Encourage self-monitoring of daily or weekly physical activity (e.g. using a pedometer) | C16 | ||
| Review and reflect on efforts to cut down smoking to build confidence gradually and perceptions of control and ability to self-regulate | Smoker and HT review progress with smoking reduction. Any successes are reflected on and reinforced | RC7, RC8, BM3, BS5 | Skills; identity (e.g. social identity); capability beliefs; beliefs about consequences; memory or attention; context/resources; social influences; emotion; behavioural regulation |
| Smoker and HT discuss any setbacks (reframing to normalise them, identifying social, environmental or other barriers and exploring ways to overcome them) | A2, RI4, RC6, BS1, BM5, BS8 | ||
| Set new targets (perhaps to quit) | BS3, BS4, BS5, BS6, BS7, BS9 | ||
| Reflection on/reinforcement of the smoker’s skills in avoiding or managing relapse | BM2, BM3 | ||
| Reassessment/checking of motivation/perceived benefits of reducing smoking and also of making an attempt to quit | BM2, BM9 | ||
| Review and reflect on efforts to increase PA to build confidence gradually and perceptions of control and ability to self-regulate | Smoker and HT review and reflect on successes in increasing PA/introducing new physical activities | C11 | Skills; identity (e.g. social identity); capability beliefs; beliefs about consequences; memory or attention; context/resources; social influences; emotion; behavioural regulation |
| Smoker and HT discuss any setbacks (reframing to normalise them, identifying social, environmental or other barriers and exploring ways to overcome them) | C8, C28, C29, C35 | ||
| Set new targets for PA | C10, C6, C7, C16 | ||
| Reassessment/checking of motivation/perceived benefits of physical activity in relation to smoking reduction, but also discussing other personal benefits | C37, C15 | ||
| Integration of concepts: building an association between PA and smoking reduction | The HT introduces PA as a healthy behaviour and aid to cutting down and quitting. A clear rationale is presented for how PA might be relevant to reducing smoking (as a distraction, as a way to reduce withdrawal symptoms such as stress or cravings) | RD1, RC2, RC8, R6 | Beliefs about consequences; emotion |
| The HT and smoker agree to experiment with using PA. The smoker reflects on use of PA and relates it to smoking urges and/or to number of cigarettes smoked | C6, C11 | ||
| Engage social support to facilitate behaviour change (both for reducing smoking and for physical activity) | Exploring the possible role of social influences as potential barriers to change and as potential facilitators of change is encouraged during the motivation, action-planning and review stages above | A2 | Social influences; emotion |
| Social support is conceptualised as being either informational (e.g. helping to make plans) practical (e.g. providing transport), or emotional (e.g. encouraging) | C29 | ||
| Identify and reinforce any identity shifts towards being a more ‘healthy person’ or ‘healthy living’. This represents a generalisation of the specific desire to stop smoking or to be more active into a more general self-concept of being someone who is healthy | Recognise and reinforce any identity change talk using reflective listening techniques | RC2, RC7, RC8, C30 NB: Explicitly encouraging/reinforcing positive changes in social identity is not currently a recognised BCT |
Identity (e.g. social identity); emotion |
| Referral to NHS Stop Smoking Services if needed | Ask if ready to quit and refer to NHS SSS if desired | RC2, RD1 | Context/resources |
Finally, EARS also drew from research on stage-matched interventions66,67 to help focus the use of specific behavioural change techniques at the appropriate time. Following an assessment of readiness to change and perceived importance of and confidence about cutting down, intervention techniques were used, as needed, to shift participants from pre-contemplation, contemplation and planning stages to action and maintenance, in terms of smoking reduction and quitting, and increasing PA as a way to facilitate changes in smoking behaviour.
Behavioural targets and support for action planning
The study inclusion/exclusion criteria meant that we could assume that smokers were ready to cut down but not quit in the next month. Given the addictive nature of smoking behaviour, part of the challenge of reducing smoking was deciding what to do and specifically how to do it. Having a clear and consciously regulated plan was considered to be helpful in disrupting habitual, automated patterns of smoking behaviour, and for extinguishing cigarette cravings associated with conditioned cues and environments. 68,69 Initial pilot work, prior to the trial, highlighted that no two smokers had identical personal situations or smoking and PA experiences, and that any intervention would require flexibility and tailoring to an individual’s needs.
We therefore created a set of materials to help participants to think about and discuss different possible strategies for cutting down (see Appendix 1). Participants were encouraged to set an initial smoking reduction goal of 50% during the first 4 weeks, using one of four different reduction strategies70 as follows:
-
Hierarchical reduction: this involves identifying the easiest to the hardest cigarettes to give up during the course of a typical day, and then systematically giving up either the easiest or hardest cigarettes over time until a goal is reached.
-
Smoke-free periods: this involves identifying blocks of time through the day where the participant will not smoke, progressively increasing the length of these periods over time.
-
Scheduled reduction: this involves spacing cigarettes evenly through the day (e.g. smoking every 30 minutes) and progressively increasing the time between each cigarette.
-
Planned reduction: this involves setting a target of a maximum number of cigarettes to smoke per day, and progressively decreasing this number over time.
The strategy chosen was not fixed but was used by participants in an exploratory way to discover which was most suitable. HTs recorded any reduction plans and used these to review and update further goals in subsequent sessions with participants as a way of encouraging self-monitoring and self-regulation. For each strategy, the aims were to build participants’ confidence to reduce smoking, to allow choice in how they achieved this and to encourage participants to seek support from others as appropriate.
Increasing physical activity
In the initial session HTs initiated a dialogue about how PA may influence smoking and may help any reduction. This was expected to include reduction of cravings, stress reduction and using PA as a distraction. Pilot work suggested that it was easier for the HT to focus on smoking reduction initially, and then introduce and develop goals for PA as a facilitating behaviour, though this was open for negotiation with the participant. As we did not exclude people who were already physically active, we expected participants to vary greatly in the amount of PA they were already doing and hence the intervention needed to be responsive to this variation.
The initial aim was to increase motivation and confidence to increase PA and to build beliefs in the reinforcing value of PA to aid smoking reduction. Later in the sessions, PA facilitation focused mainly on encouraging the selection of options that were likely to be sustainable and accessible for the individual participant. The focus was on moderate lifestyle activity (including walking, active transport or activities with few barriers to engagement) and activities that were enjoyable to the participant. The HT had a number of options to help participants increase PA, including a free low-cost MP3 player preloaded with a 10-minute spoken isometric exercise instruction track;71 a free rubber exercise band for home use; a free pedometer (self-monitoring with pedometers has been shown to increase PA);72 and free, or subsidised, access to local leisure and exercise facilities (e.g. for swimming or gym use). Participants were encouraged to self-monitor the number of daily steps they achieved and set goals (both important behaviour change techniques73), identify their use of PA in managing smoking cravings (or providing a distraction) and elicit any other positive associations that they recognised. The focus was not only on increasing the volume of PA and using this as an aid to reduce smoking, but also on helping participants to build a more generalised sense of competence, control and companionship through the activities they engaged in.
Training the health trainers
Health trainers were initially trained, using an intervention manual, in PA counselling (see Appendix 1) to achieve the above aims. Table 2 shows a list of behaviour change techniques (BCTs)74,75 that the HTs were trained to use and are linked to the main theoretical constructs (see Table 1) that underpinned the intervention.
| Behaviour addressed | BCT (modified for the EARS protocol of delivery) |
|---|---|
| aSmoking reduction74 | BM2 (boost motivation and self-efficacy) |
| BM3 (offer feedback on current behaviour) | |
| BM5 (offer normative information about others’ behaviour and experiences) | |
| BM9 (elicit reasons for wanting and not wanting to stop smoking or cut down) | |
| BM11 (measure CO) | |
| BS1 (facilitate barrier identification and problem solving) | |
| BS2 (facilitate relapse prevention and coping) | |
| BS3 (facilitate action planning/develop treatment plan) | |
| BS4 (facilitate goal setting) | |
| BS5 (prompt review of goals) | |
| BS6 (prompt self-recording) | |
| BS7 (offer to provide support with techniques for changing behaviour) | |
| BS8 (prompt thoughts on environmental restructuring) | |
| BS9 (help set graded tasks) | |
| A2 (advise on/facilitate use of social support) | |
| RD1 (tailor interventions appropriately) | |
| RD2 (emphasise choice) | |
| RI1 (assess current and past smoking behaviour) | |
| RI2 (assess current readiness and ability to quit cut down) | |
| RI3 (assess past history of quit attempts) | |
| RI4 (assess withdrawal symptoms) | |
| RC1 (build general rapport) | |
| RC2 (elicit and answer questions) | |
| RC4 (explain expectations regarding treatment programme) | |
| RC6 (provide information where appropriate on withdrawal symptoms) | |
| RC7 (use reflective listening) | |
| RC8 (elicit client views) | |
| RC9 (summarise information/confirm client decisions) | |
| RC10 (provide reassurance) | |
| bPA75 | C5 (goal setting – behaviour) |
| C6 (goal setting – to achieve possible benefits from increasing PA) | |
| C7 (action planning) | |
| C8 (barrier identification/problem solving) | |
| C9 (set graded tasks) | |
| C10 (prompt review of behavioural goals) | |
| C11 (prompt review of achievement of benefits from PA) | |
| C12 (prompt rewards contingent on progress) | |
| C15 (prompting generalisation of a target behaviour) | |
| C16 (prompt self-monitoring of behaviour) | |
| C18 (prompting focus on past success) | |
| C23 (teach to use prompts/cues) | |
| C24 (environmental restructuring) | |
| C26 (prompt practice) | |
| C28 (facilitate social comparison) | |
| C29 (plan social support) | |
| C30 (prompt identification as role model) | |
| C31 (prompt anticipated regret from not changing current behaviour) | |
| C35 (relapse prevention/coping planning) | |
| C37 (motivational interviewing) |
Intervention developmental phase with stakeholders and test participants
Context familiarisation and initial research activity
Previous research48 had helped to build a positive working relationship with the Plymouth NHS SSS. From the very beginning of the research and intervention development phase, meetings were held with those offering support for smoking cessation within the SSS and public health teams. Further afield the research team met with HTs and with those responsible for training and their management in the south-west. We focused questions on how HTs identified and engaged with clients to promote PA and smoking cessation. Plymouth itself did not employ HTs but similar outreach work was undertaken by public health specialists. One stop smoking advisor who we consulted with, based in the deprived area of Devonport, had gained extensive experience in trying to recruit smokers into a SSS. Devonport itself had received over £50M via the Devonport Regeneration Community Partnership, over a 10-year period, to support a broad range of environmental-, social- and health-related initiatives. We interviewed a wide range of formal and informal community leaders about how the local community engaged in lifestyle change support initiatives, such as employees in local leisure services and representatives of Voice of Devonport and the Pembroke St. Housing Association.
The aim of the information gathering was to develop a strong understanding of how the communities of Devonport and adjoining Stonehouse may respond to our planned intervention to facilitate changes in smoking and PA. We also developed an initial compendium of PA opportunities in the local community and further afield. All this was done over several months prior to the employment of HTs, and continued with their input throughout the training and intervention delivery. Each HT also worked in other part-time employment in and around Devonport and Stonehouse, and they were encouraged to work as a team, sharing information on opportunities for PA and community recruitment. The employment of three part-time HTs (2 × 0.5 and 1 × 0.4), with one common afternoon for weekly meetings and supervision, provided opportunities for team building, sharing good practice, and supervision.
Training
The training manual and programme for initial training are shown in Appendix 1 and Appendix 5a. We began by covering generic material in the Department of Health Training Manual for HTs, which provided a framework for the role and for developing core competencies for supporting behaviour change. The training then progressed to developing an understanding of the options available to aid smoking cessation with NHS support, and behaviour change for smoking reduction and increasing PA (in its broadest sense). The manual was largely completed prior to the beginning of training but did become more refined during and after the training. The version shown in the appendices would require further adaptation based on the findings from the pilot trial, ahead of a larger trial.
Towards the end of the training, and leading into the start of recruitment for the pilot trial, we opportunistically identified six test participants (through local contacts) who did not wish to quit smoking in the immediate future. They were able to help us resolve issues such as how best to describe the study and intervention to potential participants, how to adapt the intervention to individual differences and needs, how to maintain a client-centred approach and encourage goal setting, how to sequence multiple behaviour change, how to conduct assessments and how to stay in touch with participants. The experiences of working with six test participants are shown in Table 3. In total, they attended 35 sessions consisting of face-to-face and telephone contacts. Most sessions were digitally recorded, eight sessions directly observed, and most were discussed with the three HTs, by AHT, TT and CGVS, with field notes recorded throughout. The sessions informed the learning process and intervention development, and increasingly, leading up to the start of the actual pilot trial, increased uniform practice across the HTs.
| Issues | Participants | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Initial smoking behaviour | History: failed quitter. 20–25 cigarettes daily | Smokes c. 20 a day (mainly at home). Smokes when bored and more at weekends with friends | 20–30 cigarettes daily. Has given up for 3 years in the past. Smokes when bored and more at weekends | 25–30 cigarettes daily. Would not have got involved if we were ‘preaching about stopping’ | c. 40 roll-ups daily. History of serious drug abuse | 12–15 cigarettes daily. Smokes a lot more when out socialising with friends |
| Initial PA levels | No transport so walks a lot. A few back problems, which means they can be sedentary for extended periods | Not a ‘gym person’, but does a bit of (enjoyable) walking to break day up. Asthmatic and back trouble. c. 7500 steps per day | Walks a lot at work (12–17,000 steps per day). Weekend is a time to relax/be sedentary (4000–5000 steps). Does not enjoy gyms. Desires to be as active as wife | Steps range from 7000–16,000 daily. Busy home life with young children and a dog | Walks to most places – has no transport. Has health concerns. Heavily asthmatic | Walks dog daily, about 1 mile. Pedometer steps between 6000 and 20,000. Is lightly involved in youth football coaching |
| Smoking behaviour change processes and outcome for smokers | Processes: hierarchical reduction moved into planned reduction. Enlisted friend. Saved money in jar. Smokes less of each cigarette. New job with youths changed smoking habits | Processes: initial self-monitoring of smoking, and motives/pleasure. Goal to halve smoking. Tried smoking every hour and extending the time between cigarettes by roughly 15 minutes each week. Changed to loose tobacco to reduce smoking. ‘Come this far, can go further’ (self-efficacy) | Processes: goal to delay morning cigarettes. Attempted to increase times between cigarettes (scheduled reduction) without much success. Switched to five cigars a day instead of cigarettes. Health concerns a motive. ‘I do not know why I do it, it’s a disgusting habit.’ Very important to reduce but low confidence | Processes: initial smoking diary – shocked by number smoked. Goal to reduce two a day in week 1, by distraction. Treated reduction as an experiment, with PA. Planned to avoid cues to smoking such as waiting for children to finish school | Processes: planned reduction by pre-rolling fixed number of daily cigarettes, smoke-free periods at work, and support from boss. Goal to avoid social cues to smoking. Self-monitoring with smoking diary | Processes: motivated by health and money (saved in jar). Supported by girlfriend and father. Used hierarchical reduction to cut out the ‘easier’ cigarettes, with the intention to develop strategies for cutting out the ‘harder’ cigarettes |
| Outcomes: stopped smoking in the morning (easy!). Down to eight a day during week and up to 15 a day at weekends | Outcomes: reduced to 10 per day. Stopped chain smoking in mornings | Outcomes: only limited reduction. Failed to identify why it is easier on some days | Outcomes: reduced to 10 a day. Explicitly aware of own patterns of smoking and cues to smoking | Outcomes: reduced to c.20 roll-ups a day on average. Still prone to smoke more when out drinking/socialising | Outcomes: reduced to c. seven a day on average. Well placed to progress further, but lost contact | |
| PA behaviour change processes and outcome for smokers | Processes: looked for walking group (to enlist support). Uses pedometer for self-monitoring. Enlisted a friend to walk with as well as cut down with. Feels ‘healthier’ | Processes: set goals and self-monitored daily steps (> 8000 per day) – useful. Enlisted family to walk with. Aware of reduced smoking when on long walks. Explicit use of walking to lower cravings | Processes: goal to start swimming classes and work on allotment more often with wife (enlist support). Self-monitored PA with pedometer (sometimes). Linked more pedometer steps to less smoking | Processes: goals to walk more. Then thinking about jogging with friend (enlist support). ‘Walking more gave a sense of achievement, more energy, less breathless, skin better, fewer chest infections, and thought about smoking less’. Treated link between PA and smoking as experiment | Processes: already very active so hard to increase. Conscious effort made to walk at a brisker pace and take longer routes. Thought of using a gym, but no plans emerged (low importance/confidence) | Processes: goal to do minimum – 10,000–15,000 daily steps (including cricket and football coaching twice a week). Chose longer routes to walk places. Explicitly used PA to reduce smoking – by distraction |
| Outcomes: used cross-trainer at home and walked regularly | Outcomes: chest not so tight. Increased PA (walking) | Outcomes: failed to get to swimming classes. Completing more work on the allotment at weekends to break up sedentary time | Outcomes: achieved > 16,000 steps most days. Weight loss through extra PA | Outcomes: continued to walk a lot, possibly increasing, but difficult to know due to loss of contact | Outcomes: ‘just feel better mentally’. Maintained higher average number of steps and additional activities | |
| Challenges for individuals and for HTs to engage | Struggled with plans and goals by 3rd/4th week due to unsettled personal circumstances (new active job, daughter at home) and chaotic lifestyle. Low confidence from previous failed attempts to quit. Seven sessions held | Low confidence to cope with social cues to smoking. Boredom a real challenge due to unemployment. Reduction becoming progressively harder. Seven sessions held | Strong social cues to smoke. Active work undermined ‘selling’ activity to reduce smoking. Scheduled reduction did not fit work. Little commitment to goals. Weight-gain concerns. Five sessions held | Close family member diagnosed with cancer: presented high levels of stress and complicated adherence to sessions. Became increasingly difficult to keep in contact with. Six sessions held | Smokes a lot when binge drinking – up to 20 pints a night. Hard to avoid others smoking. Became very difficult to keep in touch due to unpredictable lifestyle. Completed six sessions in person (no telephone!) | Keeping in contact became difficult quite early on. Unknown reasons for loss of contact. Four sessions held |
The HTs gained encouragement and confidence from these experiences, which complemented their own working practice in their other part-time roles as a cardiac nurse, drug and alcohol service practitioner and trainer, and occupational health promotion practitioner.
In summary, valuable lessons were learnt during this pre-pilot developmental phase. The general aims and application of theory to practice were confirmed as appropriate, with some fine tuning of implementation of the intervention and methods for conducting assessments with test participants. With input from AHT, CGVS and TT, and drawing on the past experiences of the three HTs, the training provided an opportunity for team building and common achievement of the core aims of the pilot study.
Chapter 2 Trial design and methods
Study design
The EARS smoking study was a pragmatic, pilot, two-arm RCT into which disadvantaged smokers were recruited by three distinct approaches: (1) through primary care via general practitioner (GP) invitation letter with reminder telephone calls; (2) through NHS SSS invitation letter (aimed at those who have previously failed to quit) with reminder telephone calls; or (3) through a variety of other community-based approaches without a personalised invitation letter. The study compared the effect of individual PA and smoking counselling with brief advice (on SSS support to quit) among disadvantaged smokers not wishing to quit in the next month (see Appendix 1). Those who expressed a desire to quit post randomisation were referred to the SSS if they wished, and continued in the study with PA support if they were in the intervention arm. Following baseline, follow-up assessments were conducted at 4, 8 and 16 weeks in both groups. Ethical approval for the study was granted by the NHS National Research Ethics Service Committee South West, in the UK.
Eligibility criteria
Participants were eligible to enter the study if they were over 18 years old, smoked at least 10 cigarettes per day (and had done so for at least 2 years), did not want to quit in the next month, were able to engage in moderate-intensity PA (walk without stopping for at least 15 minutes), were registered with a GP, and did not wish to use NRT to reduce smoking. The study focus was on initially reducing smoking, not on quitting, and so those who expressed an immediate desire to quit were referred directly to the SSS without entering the study. Those wishing to use NRT were excluded to avoid any confounding of the effects of PA on their smoking behaviour. We excluded those with severe mental health problems and ongoing substance misuse due the potential difficulties of engaging them in the intervention given the large uncertainties and complexities of its delivery, and the fact that they may have put the safety of the research team at risk. Given the exploratory nature of the study, participants were required to be able to converse in English.
Sample size
Given the lack of research involving behavioural smoking reduction interventions, the effect of our intervention was uncertain. While the sample size of the study was primarily chosen to undertake the feasibility objectives of the study, we also sought to obtain an estimate of the intervention impact (relative to control). 76 Using data from a recent meta-analysis of trials of smoking cessation15 we undertook a scenario analysis in order to examine the precision of the effect size estimation based on different pilot trial sample sizes and plausible effect sizes (Table 4). A sample size target of 120 (60 per group) was initially selected for this pilot.
| Control quit ratea | Sample sizeb (control–intervention ratio) | Effect size; relative risk | Precision of effect size estimate (95% CI)c |
|---|---|---|---|
| 5% | 60 (1 : 1); 60 (1 : 1) | 2.00; 4.00 | 0.19 to 20.89; 0.47 to 33.72 |
| 5% | 120 (1 : 1); 120 (1 : 1) | 2.00; 4.00 | 0.52 to 7.63; 1.18 to 13.46 |
| 5% | 160 (1 : 1); 160 (1 : 1) | 2.00; 4.00 | 0.63 to 6.38; 1.28 to 11.41 |
Recruitment
Recruitment for the study was over a 12-month period between May 2011 and May 2012. We planned to recruit 50% of participants through primary care via GP invitation letters from three identified GP surgeries (on the basis that this has been shown to be a successful approach77) in two very socially deprived wards in Plymouth (Indices of Multiple Deprivation 2010 values = 52–59.9, placing them in within the 3% ‘most deprived’ in England). GP practice lists were searched based on cursory inclusion/exclusion criteria [including smoking status, incidents of physical health conditions (especially in the previous 12 months) that may contraindicate moderate-intensity PA and current serious mental illness or drug dependence] (see Appendix 3). A list of potential participants was generated and invitation letters sent with a postal reminder 1 week later. To ensure that nobody was discriminated against on the grounds of illiteracy, telephone calls were made to non-responders to check that they had received and understood the invitation. If there was no reply on the first call a message was left to enquire if the invitation had been received and to leave a contact number for further information. Up to four more calls were made but no further messages were left, to avoid harassment. Interested participants were screened for eligibility (see inclusion/exclusion criteria) by telephone before being invited to attend a baseline assessment.
The aim in the pilot RCT was to recruit participants of whom at least 75% were in social class C2–E (www.nrs.co.uk/lifestyle.html), 30% were single parents and 20% had a mental health problem. This operational definition of disadvantaged was based on the high prevalence of smoking among these subpopulations. 5
We planned to recruit the other 50% of participants by a variety of community-based approaches to maximise the chances of reaching disadvantaged smokers. One approach involved sending invitation letters (with a postal reminder 1 week later) to those on the database of the Plymouth SSS who had failed to successfully quit with the service within the past 2 years. Other planned community recruitment approaches included media engagement, attendance and networking with local community centre groups including housing trusts and parent and toddler groups, distribution and display of flyers, cascading information through workplaces, and opportunistic recruitment of people with a mental health problem [through the local Improving Access to Psychological Therapies (IAPT) service]. Interested participants contacted the research team directly (in person or by telephone) or indirectly by returning contact details with a request for further information. Following screening to determine eligibility, a time for attending a baseline session was arranged.
As an incentive for completing data collection at baseline, and at weeks 8 and 16, participants were offered a small financial incentive on each occasion for returning an accelerometer after wearing it for a 7-day period. Initially this was £10 for each occasion, but was increased to £30 later in the study to investigate whether or not this improved data collection and the likelihood of participants returning the device.
Randomisation and concealment
Following screening and baseline data collection, the researcher phoned the trial manager who then allocated smokers using a password-protected web-based randomisation system set up and managed by the UKCRC accredited Peninsula Clinical Trials Unit (PenCTU). Randomisation was 1 : 1 and minimised (in order to ensure balance between the two arms) by age (30 years and under/over 30), sex, HT (one of three), and smoking dependence (high = smokes first cigarette within 30 minutes of waking; low = smokes first cigarette later). To maintain concealment, the minimisation algorithm retained a stochastic element. On occasion when two potential participants were closely acquainted, at random, only one of the pair was selected for inclusion in the study to avoid contamination. If the individual was randomised to the intervention arm then both were permitted to attend sessions together but only the individual randomised provided data.
Data collection
Baseline data collection
At baseline the following data were collected: participant demographic information (i.e. age, sex, marital status, cohabiting with other smokers, parental status, employment status, age of leaving full-time education, ethnicity, weight and height), smoking history (age started smoking, longest period of cessation in last year, attempts at cutting down, cessation aids used in past year, use of SSS) (Table 5). A more detailed schedule and content of assessments is shown in the study protocol (see Appendix 2).
| Variable | ||
|---|---|---|
| Cognitive | Physical activity | Confidence for undertaking PA |
| SOC to use PA to control smoking | ||
| Smoking | Confidence for and importance of quitting | |
| Behavioural | Physical activity | Accelerometer data |
| 7-day PA recall | ||
| Smoking | Self-reported cigarettesa | |
| Expired CO | ||
| Number of quit attempts made | ||
| Cessation aids used | ||
| Alcohol consumption | Alcohol consumption | |
| Emotional/affective | Withdrawal symptoms | MPSS |
| Strength of desire to smoke | Self-reported cravings and strength of urge to smoke | |
| Quality of life | EQ-5D | |
| Nicotine dependence | FTND | |
| Subjective stress | PSS | |
| Smoking satisfaction | mCEQ | |
| Weight | ||
Given that baseline and follow-up assessments were conducted by a researcher who also provided the intervention, it was not possible to blind outcome assessment. This decision was made to provide a more seamless interaction with participants who had no prior experience of engaging in a research study and with whom it may have been particularly challenging to remain in contact. One of the aims of the process evaluation was to examine whether or not this was indeed important for participants.
Feasibility outcomes
The following feasibility outcomes were identified: participant recruitment rate (the proportion of those invited, via different methods, who agreed to take part), follow-up and outcome data collection rates, flow of smokers through the trial from each location [e.g. numbers of smokers recruited, number ineligible (with reasons), number declined, numbers failing to continue with data collection]; number of those deciding to quit who chose to use a SSS; number who chose to quit who could be tracked on a weekly basis [and hence inform the number with 4 weeks’ expired air carbon monoxide (CO) confirmed abstinence], and demographic characteristics of the sample relative to a disadvantaged definition.
Primary outcome
The primary outcome is expired air CO-confirmed abstinence [< 10 parts per million (p.p.m.)] using a Micro+ Smokerlizer (Bedfont Scientific Ltd, Maidstone, Kent, UK) using standardised procedures, with a self-reported time since last cigarette, at 4 weeks post quit. The outcome is binary, that is to say abstinent or not abstinent (those lost to follow-up are assumed to be still smoking78). Participants with an expired air CO reading ≥ 10 p.p.m. and those whose self-report data did not confirm their quit status were assumed to have started smoking again. The EARS intervention aimed to reduce smoking with the hope that a quit attempt and successful quitting might follow.
Secondary outcomes
Secondary outcomes included the number of participants reducing their self-reported smoking levels by at least 50% along with several other cognitive, behavioural and emotional outcomes outlined in Table 5.
Cognitive variables
Participants were asked at baseline, 8 weeks and 16 weeks how confident they were in their ability to (1) do at least 30 minutes of moderate-intensity PA on most days of the week over the next 6 months and (2) walk continuously for 15 minutes at a brisk pace, using a 7-point response scale from ‘not at all confident’ (1) to ‘extremely’ (7). Stage of readiness to use PA to control smoking was assessed by participants ticking one from five options as follows: (1) I do not use physical activity as a way of controlling my cigarette smoking and I do not intend to start; (2) I do not use physical activity as a way of controlling my cigarettes smoking but I’m thinking of starting; (3) I use physical activity once in a while as a way of controlling my cigarette smoking, but not regularly; (4) I use physical activity regularly as a way of controlling my cigarette smoking, but only started within the past 6 months; (5) I use physical activity regularly as a way of controlling my cigarette smoking and have been doing so for longer than 6 months. Responses reflected being in the pre-contemplation, contemplation, planning, action, or maintenance stage, respectively. All the above questions have been previously used. 67
To assess beliefs about quitting smoking the following questions were asked using a 7-point response scale from ‘not at all’ (1) to ‘extremely’ (7): How important is it for you to stop smoking permanently and completely in the next six months?; how confident are you that you can stop smoking permanently and completely in the next six months?; an important person in your life thinks you should quit smoking; how confident are you that over the next week (except at baseline when we referred to ‘over the next 4 weeks’) you will smoke only half the number of cigarettes you smoked at the time of entry into the study?
Behavioural measures
Physical activity data were collected using a tri-axial GT3X accelerometer (Actigraph, Pensacola, FL, USA). Data were recorded using a 1-second epoch, over a 7-day period. Self-reported PA was collected using the 7-day PA recall questionnaire. 79 Minutes of moderate to vigorous PA (MVPA) and average nightly sleep were recorded, and minutes of light activity were derived. Minutes of MVPA and energy expenditure were calculated. Smoking status was recorded by the question (1) How many cigarettes, cigars, or pipes, have you usually smoked each day over the past week (recorded in numbers smoked or grams/ounces of loose tobacco)? Those who reported no smoking in the past week (with expired air CO confirmation of < 10 p.p.m.) at 16 weeks were defined as not smoking at that point and we used this to report 16-week point prevalence abstinence. Alcohol consumption was assessed to monitor changes in another lifestyle behaviour often associated with smoking, using two questions taken from the World Health Organization’s (WHO) validated AUDIT alcohol screening tool:80 (1) How often do you have a drink containing alcohol? (0, ≤ 1 per month; 2–4 times per month; 2–3 times per week; ≥ 4 times per week); (2) How many drinks containing alcohol do you have on a typical day when you are drinking? (1–2; 3–4; 5–6; 7–9; ≥ 10); and the addition of the third question: how many drinks containing alcohol have you had in the past week? (1–2; 3–4; 5–6; 7–9; ≥ 10).
Emotional/affective measures
Strength of urge to smoke and time spent with those urges over the past week were assessed using two items (1–6 point response scale)81 and withdrawal symptoms assessed using an adapted 9-item Mood and Physical Symptoms Scale (MPSS). 82 Quality of life is assessed using the European Quality of Life-5 Dimensions (EQ-5D). 83 Smoking dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND). 84,85 Subjective stress was assessed using the 4-item Perceived Stress Scale (PSS),86 to monitor any changes associated with smoking reduction or increasing PA. Satisfaction from smoking was assessed using the 10-item modified Cigarette Evaluation Questionnaire (mCEQ),87 as we expected that the pleasure derived from smoking may reduce with increased PA participation.
All questionnaire data were scanned and manually checked using Teleform Scanning software (Cardiff Software, Digital Vision, Highland Park, IL, USA). A second researcher double-checked 10% of the entered data against the raw data and if more than a 1% error was found, all questionnaire data would have been manually rechecked. The data were stored on a secure institutional server.
Statistical analysis
We report the flow of participants through this study according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for non-pharmaceutical interventions. 88 Given that this was a pilot study, the primary approach to data analysis is descriptive (i.e. proportions, means and standard deviations, or median and interquartile ranges) for primary and secondary outcomes at baseline and follow-up for each of the two groups reported. However, to inform the power of a future definitive trial we also calculated an estimate of the intervention (vs. control) effect size [relative risk (RR)] and its precision [95% confidence interval (CI)] for the primary outcome (i.e. continuous expired air CO-confirmed abstinence at 4 weeks post quit), and other dichotomous smoking-related outcomes (e.g. point prevalence abstinence and proportion reducing by at least 50% at 16 weeks). Participants lost to follow-up were assumed to still be smoking the same amount as at baseline.
Qualitative data collection and analysis
The methods, procedures, data analysis and findings are reported in Chapter 5.
Cost-effectiveness data collection, analysis and modelling
Within-trial data collection was used to estimate resource use associated with the delivery of the EARS intervention. Items of resource use were combined with published unit cost data to estimate the cost of the EARS intervention, per participant. Evidence review, on modelling methods used to assess cost-effectiveness of smoking-cessation interventions, informed the development of a framework for cost-effectiveness analyses. We present the initial stages of development of a decision-analytic model to compare EARS with brief advice over the longer term. Exploratory cost-effectiveness analyses are undertaken, using assumptions on inputs for effectiveness of EARS. The methods, procedures, data analysis and findings are reported in Chapter 6.
Chapter 3 Trial results: quantitative results
This chapter reports on:
-
participant recruitment
-
participant characteristics for the total sample and across treatment arms
-
study attrition and associated factors
-
bias in self-reported PA
-
changes in outcomes over time, by treatment arm
-
intervention adherence and its association with outcomes.
Participant recruitment
The flow of participants from invitation letter via GP surgery and SSS, and via other community approaches, is shown in the CONSORT diagrams (Figure 1, up to randomisation, and Figure 2, after randomisation).
FIGURE 1.
Consolidated Standards of Reporting Trials chart showing recruitment approaches, and participant flow, up to randomisation. DNA, did not attend.
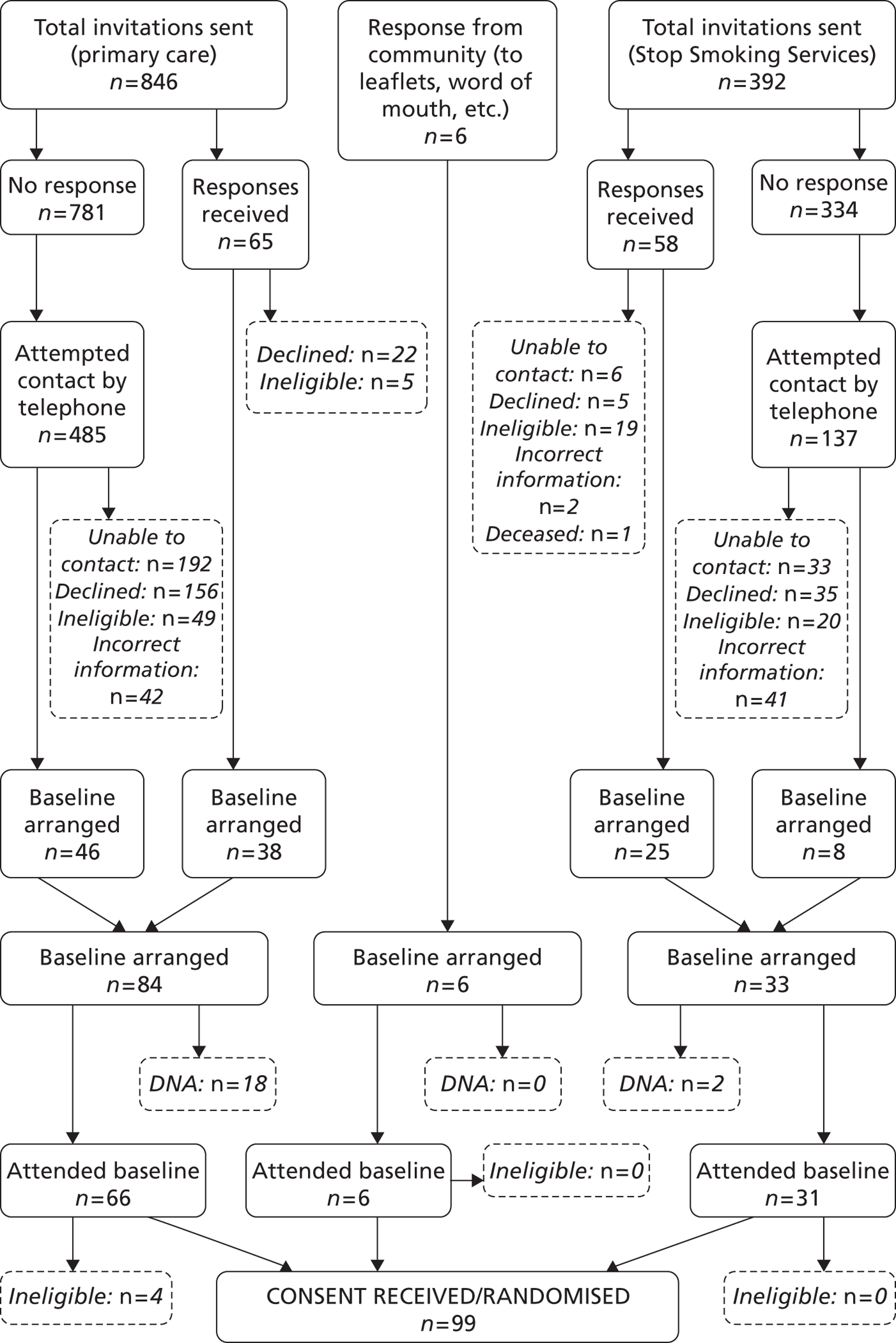
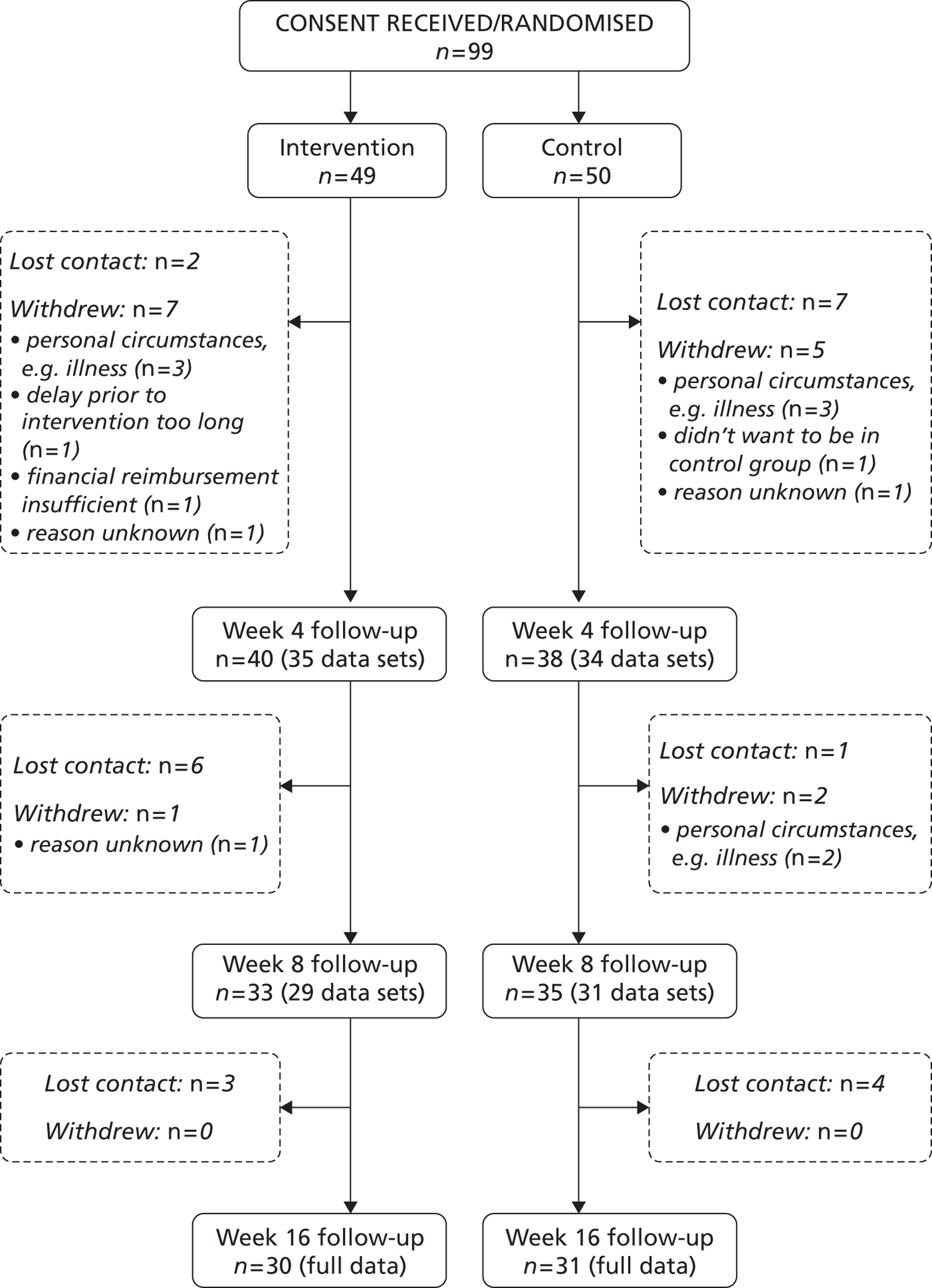
FIGURE 2.
Consolidated Standards of Reporting Trials chart showing recruitment approaches, and participant flow, after randomisation. DNA, did not attend.
Process of recruitment
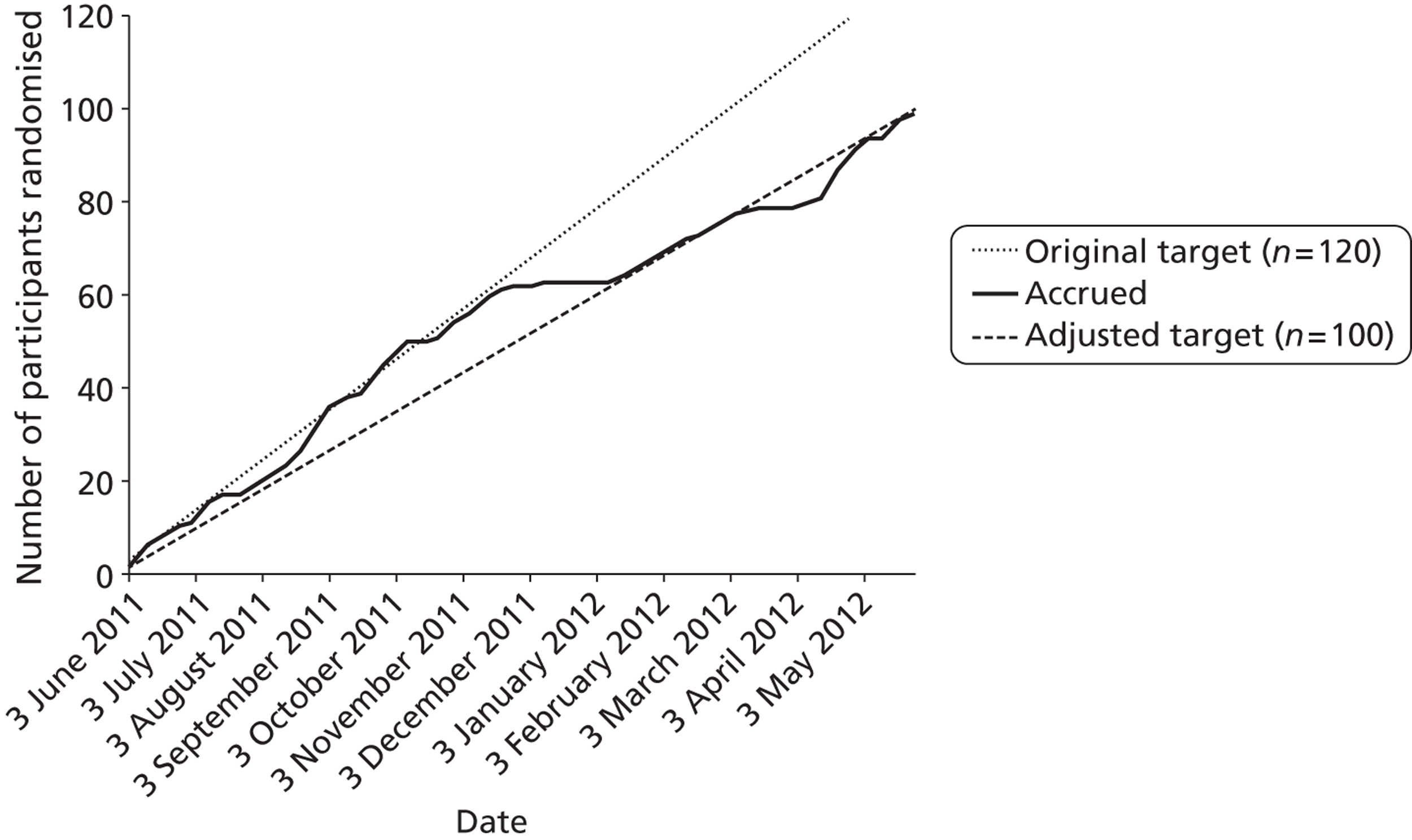
We began by focusing recruitment on mailed invitations from GP practices and the SSS. This was done to ensure that the research team initially gained confidence in the trial methods and intervention delivery, including the invitation and recruitment rate. Mailed invitations were done in batches of about 100 from each GP practice, or the SSS, to reduce the burden on associated GP practice screening and administration time, and to establish a steady flow of willing participants into the study. The aim was to recruit 60 participants from GP mailed invitations: 30 from SSS-mailed invitations and 30 from other community approaches. As Figure 3 shows, we remained on target during the first 6 months for recruiting the planned 120 participants. By this time we had exhausted the patient database of eligible smokers in one GP surgery and began recruiting from another surgery. Recruitment slowed prior to the Christmas period, and the new surgery also wanted to screen all participants before mailing an invitation rather than waiting to screen any willing participants who had responded to the invitation. This required additional time between identifying potential participants and mailing invitations. At this time we also allocated one HT/researcher to other community recruitment activity, which we anticipated would have greater uncertainty with recruitment rates. We revised our recruitment target, as shown in Figure 3, to 100 participants at this point.
FIGURE 3.
Participant recruitment accrual graph over the duration of the study.
Overall, there was a higher percentage of smokers contacted through the SSS who were ineligible to enter the study, likely due to the extra level of screening that took place through GP practice recruitment. The reasons for ineligibility were similar across recruitment methods and are shown in Table 6. The most common reason for ineligibility was due to the individual having already quit smoking, suggesting out-of-date records in both GP practices and the SSS.
| Reasons | Primary care | SSS |
|---|---|---|
| Health/physical (%) | 15.8 | 20.5 |
| Already quit (%) | 57.9 | 53.8 |
| Smokes < 10 cigarettes per day (%) | 10.5 | 10.4 |
| Close friend or relative of somebody already in the trial (%) | 0.0 | 5.1 |
| Currently using NRT (%) | 5.3 | 5.1 |
| Under 18 years old (%) | 0.0 | 5.1 |
| Wants to quit immediately (%) | 10.5 | 0.0 |
Different amounts of effort were involved in following up participants invited by letter from the three GP surgeries and the SSS. The maximum effort involved telephoning, on up to five occasions, all smokers who had not responded to an initial invitation letter and postal reminder. If there was no response a message was left with contact details for the study on the first call, but not on subsequent calls to avoid harassment. The percentage of the total sample recruited, by recruitment method, is shown in Table 7.
| Recruitment method | n (%) |
|---|---|
| Primary care | 62 (62.6) |
| Letter only | 31 (31.3) |
| Letter plus reminder telephone calls | 31 (31.3) |
| SSS | 31 (31.3) |
| Letter only | 24 (24.2) |
| Letter plus reminder telephone calls | 7 (7.1) |
| Community (without invitation letter) | 6 (6.1) |
Recruitment by letter and reminder telephone calls (from GP or SSS)
In terms of response rates, we were able to substantially increase the proportion of people invited by letter who were randomised, from about 7% to 11%, by making up to five reminder telephone calls. A lack of availability of staff prevented us from making reminder telephone calls to all those initially invited, particularly to those invited from the SSS. The associated researcher time to recruit one participant ranged from approximately 20 minutes for those who responded directly to the letter invitation up to approximately 150 minutes for completing reminder telephone calls to those who did not initially respond to the letter invitation.
Community recruitment without invitation letter
A variety of approaches were attempted to recruit participants other than by letter as summarised in Table 8. Our efforts focused on workplaces with a high proportion of manual and unskilled workers, educational sites (in an effort to recruit single parents), local media, opportunistic referral through the IAPT to target people with depression and anxiety, and a wide range of other community sites and organisations in Devonport and Stonehouse. We estimate that at least 46 hours of dedicated time by the HTs/researchers resulted in the six participants recruited via these approaches.
| Recruitment sites | Recruitment activity |
|---|---|
| Workplace site | |
| Local adult education and training provider | Flyers and packs in the refectory. Contact at the centre distributed packs |
| Post office MDEC | Information cascaded through managers to all employees in team briefings |
| Educational site | |
| Local primary school | Article in parent newsletter |
| Mother/toddler groups; several local children’s centres | Mother/toddler groups visited through Sure Start. Posters and packs left, packs given out during groups |
| Community site/organisation | |
| Job centre (Devonport) | 100 packs given out over several periods in a week |
| Local community hub cafe | Local health promotion sessions and food bank session attended |
| Local community co-operative organisation | Flyers and posters given out for the Guildhall and workers based there |
| YMCA (community-run gym) | Posters on display. Fitness manager promoted study to users of the Stonehouse gym. HTs attended a children’s session; one pack given out. |
| Local gym | Gym instructors gave out flyers and packs |
| Local social club | Central contact gave out several packs and reply sheets |
| Public health | Posters and packs given to the local health club in Devonport |
| Three local housing associations | 180 flyers distributed through mailboxes in housing association residences in Plymouth; flyers distributed and attendance at residents’ meetings. Posters, flyers and packs left at site for visitors |
| Neighbourhood managers (city council) | HT met with managers in Devonport and Stonehouse. Information distributed |
| Local community learning centre | Information and flyers displayed. HT attended information sessions |
| Other | |
| Local library | Flyers and posters on display. |
| Heart Radio/Plymouth Sound/Radio Devon/newspaper | Radio chat about the study and news advert in paper |
| Word of mouth | First 60 trial participants asked to invite friends/acquaintances to join study |
| Individual contacts (e.g. Church of England minister, local day support facility member, publican) | Posters displayed by contacts |
| IAPT Service, Plymouth | Met and encouraged psychological well-being practitioners. Left flyers to be distributed. Encouraged by e-mail |
Participant characteristics across recruitment methods
Table 9 shows the demographic characteristics of those recruited by GP and SSS invitation letter and via other community approaches without an invitation letter. Given the small number of participants recruited via other community approaches, any comparison with approaches involving an invitation letter are meaningless. Comparisons of the characteristics of participants recruited by invitation letter from the GP versus the SSS appear to show little difference.
| Baseline characteristic | Primary care (N = 62) | SSS (N = 31) | Community (N = 6) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 28 (45.2) | 13 (41.9) | 2 (33.3) |
| Female | 34 (54.8) | 18 (58.1) | 4 (66.7) |
| Age (years), mean (SD); median (IQR) | 45.9 (11.4); 47.1 (38.0 to 55.0) | 47.1 (11.7); 48.9 (38.3 to 56.9) | 50.8 (8.6); 49.6 (44.3 to 58.2) |
| Ethnicity, n (%) | |||
| White British | 58 (93.6) | 31 (100) | 6 (100) |
| Other | 4 (6.5) | 0 (0) | 0 (0) |
| Cohabiting status, n (%) | |||
| Cohabiting | 35 (56.5) | 12 (38.7) | 3 (50.0) |
| Not cohabiting | 27 (43.6) | 19 (61.3) | 3 (50.0) |
| Children under 16 years, n (%) | |||
| Yes | 16 (25.8) | 9 (29.0) | 3 (50.0) |
| No | 46 (74.2) | 22 (71.0) | 3 (50.0) |
| Single parent,a n (%) | |||
| Yes | 2 (3.2) | 2 (6.5) | 2 (33.3) |
| Employment status, n (%) | |||
| Employed | 37 (59.7) | 13 (41.9) | 4 (66.7) |
| Not employed | 25 (40.3) | 18 (58.1) | 2 (33.3) |
| Job status, n (%) | |||
| A to C1 | 5 (8.1) | 2 (6.5) | 2 (33.3) |
| C2 to E | 32 (51.6) | 11 (35.5) | 2 (33.3) |
| Unemployed | 25 (40.3) | 18 (58.1) | 2 (33.3) |
| Age (years) on leaving education, mean (SD); median (IQR) | 16.3 (2.1); 16.0 (15.0 to 16.3) | 16.2 (1.6); 16 (15 to 16) | 16.0 (1.3); 15.5 (15.0 to 17.3) |
| Age (years) on starting smoking, mean (SD); median (IQR) | 14.5 (3.6); 14.0 (13.0 to 16.0) | 14.6 (3.1); 15.0 (12.0 to 16.0) | 16.8 (3.0); 17.0 (13.8 to 20.0) |
| Does partner or other cohabitant smoke, n (%) | |||
| Yes | 20 (32.3) | 10 (32.3) | 1 (16.7) |
| No | 20 (32.3) | 5 (16.1) | 2 (33.3) |
| Not applicable | 22 (35.5) | 16 (51.6) | 3 (50.0) |
| BMI (kg/m2), mean (SD), n; median (IQR) | 27.6 (6.5), 62; 26.5 (22.2 to 31.3) | 28.9 (6.6), 30; 27.7 (22.5 to 35.0) | 29.9 (4.1), 6; 30.2 (26.5 to 33.3) |
| Indicated mental health problem,b n (%) | |||
| Yes | 24 (38.7) | 16 (51.6) | 1 (16.7) |
| No | 38 (61.3) | 15 (48.4) | 5 (83.3) |
| Duration of smoking (years), mean (SD); median (IQR) | 31.5 (12.2); 33.1 (22.2 to 40.0) | 32.5 (13.0); 36.4 (23.3 to 43.1) | 34.0 (8.9); 34.1 (29.9 to 42.2) |
| Previous use of SSS, n (%) | |||
| Yes | 17 (27.4) | 22 (71.0) | 2 (33.3) |
| No | 45 (72.6) | 9 (29.0) | 4 (66.6) |
| Satisfaction with previous use of SSS (if used) (scale 1–11); mean (SD), n | 7.3 (3.28), 17 | 8.9 (2.3), 21 | 10.5 (0.7), 2 |
| Did the participant make a quit attempt lasting 24 hours or more in the past year, n (%) | |||
| Yes | 17 (27.4) | 18 (58.1) | 2 (33.3) |
| No | 45 (72.6) | 13 (47.9) | 4 (66.6) |
| Did the participant cut down before previous cessation,c n (%) | |||
| Yes | 1 (5.9) | 4 (22.2) | 0 (0.0) |
| No | 16 (94.1) | 14 (77.8) | 2 (100.0) |
| Total n | 17 | 18 | 2 |
| Used cessation aids as part of a quit attempt in previous 12 months,d n (%) | |||
| Yes | 11 (64.7) | 17 (94.4) | 1 (50.0) |
| No | 6 (35.3) | 1 (5.6) | 1 (50.0) |
| Total n | 17 | 18 | 2 |
| Used cessation aids not as part of a quit attempt in previous 12 months, n (%) | |||
| Yes | 8 (17.8) | 10 (26.3) | 3 (75.0) |
| No | 37 (82.2) | 3 (73.7) | 1 (25.0) |
| Total n | 45 | 13 | 4 |
We also compared the characteristics of those recruited after an immediate response to the invitation letter with those recruited in response to a subsequent reminder letter and reminder telephone call as shown in Table 10. The numbers were fairly small for some categories of response but there appeared to be little difference between contact methods, suggesting that making the additional effort to recruit participants does not necessarily increase the reach of the intervention to more disadvantaged participants or increase generalisability.
| Baseline characteristic | Contact methode | |
|---|---|---|
| Letter (N = 55) | Letter and reminder telephone call (N = 38) | |
| Sex, n (%) | ||
| Male | 25 (45.5) | 16 (42.1) |
| Female | 30 (54.6) | 22 (57.9) |
| Age (years), mean (SD); median (IQR) | 47.3 (11.7); 48.9 (38.0 to 55.5) | 44.9 (11.0); 46.0 (38.1 to 53.6) |
| Age (years), n (%) | ||
| 30 and under | 6 (10.9) | 7 (18.4) |
| 31 and over | 49 (89.1) | 31 (81.6) |
| Ethnicity, n (%) | ||
| White British | 53 (96.4) | 36 (94.7) |
| Other | 2 (3.6) | 2 (5.3) |
| Cohabiting, n (%) | 27 (49.1) | 20 (52.6) |
| Children under 16, n (%) | 15 (27.3) | 10 (26.3) |
| Single parent,a n (%) | 2 (3.6) | 2 (5.3) |
| Employment status, n (%) | ||
| Employed | 29 (52.7) | 21 (55.3) |
| Not employed | 26 (47.3) | 17 (44.7) |
| Job status, n (%) | ||
| A to C1 | 5 (9.1) | 2 (5.6) |
| C2 to E | 24 (43.6) | 19 (50.0) |
| Unemployed | 26 (47.3) | 17 (44.7) |
| Age (years) on leaving education, mean (SD); median (IQR) | 16.2 (1.8); 16.0 (15.0 to 16.0) | 16.4 (2.1); 16.0 (15.0 to 16.3) |
| BMI (kg/m2), mean (SD), n; median (IQR) | 28.0 (6.0), 54; 27.3 (22.5 to 33.0) | 28.1 (7.3), 38; 26.4 (22.2 to 31.3) |
| Indicated mental health problem,b n (%) | ||
| Yes | 22 (40.0) | 18 (47.4) |
| No | 33 (60) | 20 (52.6) |
| Age (years) on starting smoking, mean (SD); median (IQR) | 14.9 (3.5); 14 (13 to 16) | 14.1 (3.3) 14 (12 to 16) |
| Does partner or other cohabitant smoke, n (%) | ||
| Yes | 20 (36.4) | 10 (26.4) |
| No | 11 (20.0) | 14 (36.8) |
| Not applicable | 24 (43.6) | 14 (36.8) |
| Duration of smoking (years), mean (SD); median (IQR) | 32.4 (12.8); 35.8 (22.6 to 42.3) | 30.9 (11.9); 32.0 (24.8 to 40.0) |
| Previous use of SSS, n (%) | ||
| Yes | 28 (50.9) | 11 (28.9) |
| No | 27 (49.1) | 27 (71.1) |
| Satisfaction with previous use of SSS (if used) (scale 1–11), mean (SD), n | 8.6 (2.6), 27 | 7.2 (3.3), 11 |
| Did the participant make a quit attempt lasting 24 hours or more in the past year, n (%) | ||
| Yes | 25 (45.5) | 10 (26.4) |
| No | 30 (54.5) | 28 (73.6) |
| Did the participant cut down before previous cessation,c n (%) | ||
| Yes | 3 (12.0) | 2 (20.0) |
| No | 22 (88) | 8 (80.0) |
| Total n | 25 | 10 |
| Used cessation aids as part of a quit attempt in previous 12 months,d n (%) | ||
| Yes | 20 (80.0) | 8 (80.0) |
| No | 5 (20.0) | 2 (20.0) |
| Total n | 25 | 10 |
| Used cessation aids not as part of a quit attempt in previous 12 months, n (%) | ||
| Yes | 10 (33.3) | 8 (28.6) |
| No | 20 (66.7) | 20 (71.4) |
| Total n | 30 | 28 |
Participant characteristics for the total sample and across treatment arms
Table 11 shows the demographic characters of those randomly assigned to the control and intervention arms. There appeared to be minimal difference between the groups.
| Baseline characteristic | Control, n (%) | Intervention, n (%) |
|---|---|---|
| Sex | ||
| Male (N = 43) | 21 (48.8) | 22 (51.2) |
| Female (N = 56) | 29 (51.2) | 27 (48.8) |
| Age (years), mean (SD); median (IQR) | 46.0 (11.1); 47.0 (38.2 to 54.3) | 47.2 (11.6); 47.8 (38.5 to 56.5) |
| Ethnicity | ||
| White British (N = 95) | 48 (50.5) | 47 (49.5) |
| Other (N = 4) | 2 (50.0) | 2 (50.0) |
| Cohabiting status | ||
| Cohabiting (N = 50) | 29 (58.0) | 21 (42.0) |
| Not cohabiting (N = 49) | 21 (42.9) | 28 (57.1) |
| Children under 16 years | ||
| Yes (N = 28) | 17 (60.7) | 11 (39.3) |
| No (N = 71) | 33 (46.5) | 38 (53.5) |
| Single parenta | ||
| Yes (N = 6) | 3 (50.0) | 3 (50.0) |
| Employment status | ||
| Employed (N = 54) | 28 (51.9) | 26 (48.1) |
| Not employed (N = 45) | 22 (48.9) | 23 (51.1) |
| Job status | ||
| A to C1 (N = 9) | 4 (44.4) | 5 (55.6) |
| C2 to E (N = 45) | 24 (53.3) | 21 (46.7) |
| Unemployed (N = 45) | 22 (48.9) | 23 (51.1) |
| Age left education (years), mean (SD); median (IQR) | 16.4 (2.2); 16.0 (15.0 to 16.3) | 16.2 (1.5); 16.0 (15.0 to 16.0) |
| Indicated mental health problemb | ||
| Yes (N = 41) | 19 (46.3) | 22 (53.7) |
| No (N = 58) | 31 (53.4) | 27 (46.6) |
| Does partner or other cohabitant smoke? | ||
| Yes (N = 31) | 21 (67.7) | 10 (32.3) |
| No (N = 27) | 13 (48.1) | 14 (51.9) |
| Not applicable (N = 41) | 16 (39.0) | 25 (61.0) |
| Age on starting smoking (years), mean (SD); median (IQR) | 14.7 (4.1); 14.0 (12.0 to 16.0) | 14.6 (2.8); 14.0 (13.0 to 16.0) |
| Duration of smoking (years), mean (SD); median (IQR) | 31.3 (12.3); 32.7 (21.7 to 40.0) | 32.6 (12.2); 34.7 (24.9 to 43.6) |
| Previous use of SSS | ||
| Yes (N = 41) | 20 (48.8) | 21 (51.2) |
| No (N = 58) | 30 (51.7) | 28 (48.3) |
| Satisfaction with previous use of SSS (if used) (scale 1–11), mean (SD), n | 7.9 (3.3), 19 | 8.6 (2.3), 21 |
| Did participant make a quit attempt lasting 24 hours or more in past year? | ||
| Yes (N = 37) | 20 (54.1) | 17 (45.9) |
| No (N = 62) | 30 (48.4) | 32 (51.6) |
| Did the participant cut down before previous cessation?c | ||
| Yes (N = 5) | 4 (80.0) | 1 (20.0) |
| No (N = 32) | 16 (50.0) | 16 (50.0) |
| Total n | 20 | 17 |
| Used cessation aid as part of a quit attempt in previous 12 monthsd | ||
| Yes (N = 29) | 17 (58.6) | 12 (41.4) |
| No (N = 8) | 3 (37.5) | 5 (62.5) |
| Total n | 20 | 17 |
| Used cessation aid not as part of a quit attempt in previous 12 months | ||
| Yes (N = 21) | 9 (42.9) | 12 (57.1) |
| No (N = 41) | 21 (51.2) | 20 (48.8) |
| Total n | 30 | 32 |
Overall, the sample was 57% female and 96% white British; 50% were living with someone else, 28% had a child under 16 and 91% were in social class C2–E (of whom 46% were unemployed). On average, the sample was 47 years of age, left school at 16 years and started smoking at 14.7 years and had thus been smoking for 32 years. In the past, 41% had used a SSS, with a generally high level of satisfaction with the support received. In the past year, 37% had made a quit attempt lasting 24 hours or more, of whom very few had cut down beforehand, and most had used some kind of cessation aid.
Study attrition and associated factors
This section refers to capturing complete data at follow-up assessments. Table 12 shows the number of participants who we were able to assess after baseline at week 4, 8 and 16 weeks, by treatment arm. The 4-week assessment was conducted by telephone (or in person for those in the intervention arm, if still in contact) and focused only on capturing a limited data set. There was a minimal difference in attrition between the two arms of the trial. Much of the attrition occurred within the first 4 weeks, and then levelled off over time. At the longest follow-up of 16 weeks we were able to collect data from 61% and 62% of participants in the intervention and control arms, respectively. The figures were 55% and 58% for collecting follow-up data at both 8 and 16 weeks. In subsequent tables showing data collected over time the values were derived from the number of participants shown below or fewer if there were any missing data for individual variables, as shown.
| Attendance | Baseline | Week 4 | Week 8 | Week 16 | Attendance at baseline, week 8 and week 16 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (N = 49) | Control (N = 50) | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Attend, n (%) | 49 (100) | 50 (100) | 35 (71.4) | 34 (68.0) | 29 (59.2) | 32 (64.0) | 30 (61.2) | 31 (62.0) | 27 (55.1) | 29 (58.0) |
| Non-attend, n (%) | 0 (0) | 0 (0) | 14 (28.6) | 16 (32.0) | 20 (40.8) | 18 (36.0) | 19 (38.8) | 19 (38.0) | 22 (44.9) | 21 (42.0) |
We examined what, if any, factors influenced study attrition. Table 13 shows that attrition was about double for those recruited by a reminder telephone call through GP practice invitation compared with those who responded to the initial invitation letter.
| Recruitment method | Withdrawal before final follow-up, n (%) |
|---|---|
| Primary care (N = 62) | 25 (40.3) |
| Letter (N = 31) | 8 (25.8) |
| Telephone reminder (N = 31) | 17 (54.8) |
| SSS (N = 31) | 11 (35.5) |
| Letter (N = 24) | 9 (37.5) |
| Telephone reminder (N = 7) | 2 (28.6) |
| Community (N = 6) | 2 (33.3) |
| Overall (N = 99) | 38 (38.4) |
Table 14 shows that those completing data collection at 16 weeks were fairly similar to those who did not. Any apparent differences were probably due to small numbers in different categories for categorical variables.
| Baseline characteristic | Withdrew, n (%) | Did not withdraw, n (%) |
|---|---|---|
| Sex | ||
| Male (N = 43) | 14 (32.6) | 29 (67.4) |
| Female (N = 56) | 24 (42.9) | 32 (57.1) |
| Age (years), mean (SD), n; median (IQR) | 44.2 (10.9), 38; 43.7 (37.5 to 53.6) | 48.1 (11.4), 61; 50.0 (39.6 to 56.3) |
| Age (years) | ||
| ≤ 30 (N = 13) | 5 (38.5) | 8 (61.5) |
| ≥ 31 (N = 86) | 33 (38.4) | 53 (61.6) |
| Ethnicity | ||
| White British (N = 95) | 37 (39.0) | 58 (61.1) |
| Other (N = 4) | 1 (25.0) | 3 (75.0) |
| Cohabiting status | ||
| Cohabiting (N = 50) | 18 (36.0) | 32 (64.0) |
| Not cohabiting (N = 49) | 20 (40.8) | 29 (59.2) |
| Children under 16 years | ||
| Yes (N = 28) | 13 (46.4) | 15 (53.6) |
| No (N = 71) | 25 (35.2) | 46 (64.8) |
| Single parent | ||
| Yes (N = 6) | 4 (66.7) | 2 (33.3) |
| Employment status | ||
| Employed (N = 54) | 20 (37.0) | 34 (63.0) |
| Not employed (N = 45) | 18 (40.0) | 27 (60.0) |
| Job status | ||
| A to C1 (N = 9) | 3 (33.3) | 6 (66.7) |
| C2 to E (N = 45) | 17 (37.8) | 28 (62.2) |
| Unemployed (N = 45) | 18 (40.0) | 27 (60.0) |
| Age on leaving education (years), mean (SD), n | 16.1 (1.2), 38 | 16.4 (2.2), 61 |
| BMI (kg/m2), mean (SD), n; median (IQR) | 28.3 (7.0), 38; 27.6 (22.2 to 33.5) | 28.0 (6.0), 60; 27.0 (22.5 to 31.9) |
| Indicated mental health problemb | ||
| Yes | 18 (43.9) | 23 (56.1) |
| No | 20 (34.5) | 38 (65.5) |
| Age on starting smoking (years), mean (SD); median (IQR) | 14.5 (4.3); 14 (12 to 16) | 14.8 (2.9); 14 (13 to 16) |
| Does partner or other cohabitant smoke? | ||
| Yes (N = 31) | 9 (29.0) | 22 (71.0) |
| No (N = 27) | 14 (51.9) | 13 (48.1) |
| Not applicable (N = 41) | 15 (36.6) | 26 (63.4) |
| Duration of smoking (years), mean (SD); median (IQR) | 29.7 (12.6); 30.2 (22.2 to 39.9) | 33.3 (11.9); 35.8 (23.4 to 43.1) |
| Previous use of SSS | ||
| Yes (N = 41) | 15 (36.6) | 26 (63.4) |
| No (N = 58) | 23 (39.7) | 35 (60.3) |
| Satisfaction with previous use of SSS (if used) (scale 1–11), mean (SD), n | 7.3 (2.8), 15 | 8.9 (2.7), 25 |
| Did the participant make a quit attempt lasting 24 hours or more in the past year? | ||
| Yes (N = 37) | 14 (37.8) | 23 (62.2) |
| No (N = 62) | 24 (38.7) | 38 (61.3) |
| Did the participant cut down before previous cessation?c | ||
| Yes (N = 5) | 2 (40.0) | 3 (60.0) |
| No (N = 32) | 12 (37.5) | 20 (62.5) |
| Total n | 14 | 23 |
| Used cessation aids as part of a quit attempt in previous 12 monthsd | ||
| Yes (N = 29) | 9 (31.0) | 20 (69.0) |
| No (N = 8) | 5 (62.5) | 3 (37.5) |
| Total n | 14 | 23 |
| Used cessation aids not as part of a quit attempt in previous 12 months | ||
| Yes (N = 21) | 6 (28.6) | 15 (71.4) |
| No (N = 41) | 18 (43.9) | 23 (56.1) |
| Total n | 24 | 38 |
Table 15 shows that those completing data collection for PA and smoking-related variables at 16 weeks were fairly similar to those who did not. Any apparent differences were probably due to small numbers in different categories for categorical variables.
| Variable | Withdrew (N = 38) | Did not withdraw (N = 61) |
|---|---|---|
| Smoking and PA data | ||
| Self-reported cigarettes smoked per day, mean (SD), n; median (IQR) | 21.2 (15.2), 38; 19.7 (14.3 to 25.3) | 21.8 (13.8), 61; 18.9 (14.7 to 24.5) |
| Expired air CO (p.p.m.), mean (SD), n | 19.5 (8.4), 37 | 17.1 (7.8), 61 |
| FTND, mean (SD); median (IQR) | 6.0 (2.0); 6.5 (4.0 to 7.0) | 5.3 (2.1); 6 (3.0 to 7.0) |
| Readiness to use PA as a way of controlling smoking, ACTION and MAINTENANCE stage, n (%) | 3 (7.9) | 6 (9.8) |
| Self-reported minutes of moderate and vigorous physical activity over previous 7 days, mean (SD), n; median (IQR) | 415 (530.5), 38; 268 (0 to 594) | 573 (694.3), 60; 338 (173 to 540) |
| Accelerometer data | ||
| Minutes spent in moderate/vigorous/very vigorous activity per day, mean (SD), n; median (IQR) | 34 (26.8), 19; 33 (9 to 48) | 31 (23.7), 47; 27 (42 to 16) |
| Step counts, mean (SD), n; median (IQR) | 8200 (3667.6), 19; 7303 (5570 to 10541) | 7500 (3501.8), 47; 7426 (4534 to 9853) |
Bias in self-reported physical activity
Baseline data were available from both accelerometer-measured and self-reported minutes of PA for 65 participants (across both arms). Of these, 41 participants (63%) self-reported a greater number of minutes of MVPA than their equivalent accelerometer-measured data indicated. Figure 4 plots the difference between mean accelerometer-measured and mean self-reported MVPA by the overall mean MVPA derived from the means of both measures. Over-reporting was greatest for those participants completing the most daily MVPA based on the average of both methods.
FIGURE 4.
Plot of the difference (accelerometer minus recall diary) between MVPA as recorded by accelerometer and by self-report against the mean of both measures.
Changes in outcomes over time, by treatment arm
Table 16 presents data for the primary smoking-related outcomes. A greater proportion of those in the intervention arm made a quit attempt and had reduced their smoking by at least 50% at week 16. The RR for being abstinent at 4 weeks after quitting and at 16 weeks (point prevalence) was 3.57 and 2.55, respectively, but the CI included 1.0. Also, the RR for having reduced smoking by at least 50% by week 8 was 1.91 but the CI included 1.0.
| Smoking | Intervention (N = 49) | Control (N = 50) | RR (95% CI) |
|---|---|---|---|
| Self-reported quit attempt during study,a n (%) | |||
| Yes | 11 (22.5) | 3 (6.0) | 3.74 (1.11 to 12.60) |
| No | 38 (77.6) | 47 (94.0) | |
| Confirmed quit at 4 weeks post quit date,a,b n (%) | |||
| Yes | 7 (14.3) | 2 (4.0) | 3.57 (0.78 to 16.35) |
| No | 42 (85.7) | 48 (96.0) | |
| Self-reported non-smoking at week 16,a,c n (%) | |||
| Yes | 5 (10.2) | 2 (4.0) | 2.55 (0.52 to 12.53) |
| No | 44 (89.8) | 48 (96.0) | |
| Reduction of smoking by 50% or more by week 8,a n (%) | |||
| Yes | 15 (30.6) | 8 (16.0) | 1.91 (0.89 to 4.10) |
| No | 34 (69.4) | 42 (84.0) | |
| Reduction of smoking by 50% or more by week 16,a n (%) | |||
| Yes | 19 (38.8) | 10 (20.0) | 1.94 (1.01 to 3.74) |
| No | 30 (61.2) | 40 (80.0) | |
Table 17 presents data for smoking-related variables at each assessment by treatment condition, for only those completing assessments. We did not plan to conduct inferential statistics so none are presented. With no differences at baseline, there appeared to be greater reductions among the intervention group in cigarettes smoked, expired air CO, cravings and withdrawal symptoms (MPSS) for cigarettes, strength of desire to smoke and withdrawal symptoms, and increases in importance and confidence in quitting, and confidence in reducing cigarettes smoked by 50%. Nicotine dependence (FTND) and satisfaction with smoking scales (mCEQ) also appeared to reduce more in the intervention arm.
| Smoking-related variable | Baseline | Week 4 | Week 8 | Week 16 |
|---|---|---|---|---|
| Self-reported cigarettes per day, mean (SD), n; median (IQR) | ||||
| Intervention | 21.7 (14.2), 49; 19.1 (13.6 to 25.9) | 12.7 (6.2), 34; 13.1 (8.0 to 15.7) | 10.8 (7.0), 26; 9.7 (5.6 to 16.0) | 9.1 (8.1), 30; 7.4 (0.4 to 15.2) |
| Control | 21.5 (14.6), 50; 19.4 (15.0 to 23.2) | 15.0 (6.1), 34; 14.7 (10.0 to 18.1) | 14.0 (6.0), 32; 15.3 (9.8 to 17.3) | 13.6 (7.5), 31; 13.3 (8.9 to 15.8) |
| FTND, mean (SD), n; median (IQR) | ||||
| Intervention | 5.5 (2.0), 49; 6.0 (4.0 to 7.0) | Not collected | 3.1 (2.4), 24; 3.5 (1.0 to 5.0) | 3.1 (2.2), 22; 3.0 (1.0 to 5.0) |
| Control | 5.6 (2.1), 50; 6.0 (4.0 to 7.0) | 4.9 (2.1), 30; 4.5 (3.0 to 7.0) | 4.2 (2.5), 29; 4.0 (2.5 to 6.0) | |
| CO (p.p.m.), mean (SD), n; median (IQR) | ||||
| Intervention | 17.4 (7.5), 49; 16.0 (12.0 to 23.0) | Not collected | 12.4 (7.4), 27; 14.0 (6.0 to 16.0) | 12.3 (8.3), 30; 13.0 (3.8 to 18.3) |
| Control | 18.5 (8.6), 49; 18.0 (12.0 to 22.0) | 15.2 (6.2), 32; 15.0 (12.0 to 19.0) | 15.8 (7.8), 31; 15.0 (11.0 to 21.0) | |
| Importance of quitting in next 6 months (scale 1–7), mean (SD), n; median (IQR) | ||||
| Intervention | 5.4 (1.7), 49; 6.0 (4.0 to 7.0) | 5.3 (1.8), 34; 6.0 (4.0 to 7.0) | 5.5 (1.4), 26; 6.0 (4.8 to 7.0) | 5.8 (1.4), 26; 6.0 (5.0 to 7.0) |
| Control | 5.2 (1.7), 50; 5.0 (4.0 to 7.0) | 5.3 (1.7), 34; 6.0 (4.0 to 7.0) | 5.2 (1.8), 30; 6.0 (4.0 to 7.0) | 5.6 (1.6), 29; 6.0 (5.0 to 7.0) |
| Confidence of quitting in next 6 months (scale 1–7), mean (SD), n; median (IQR) | ||||
| Intervention | 3.5 (1.6), 47; 3.0 (2.0 to 5.00) | 4.4 (1.8), 34; 5.0 (3.8 to 6.0) | 4.8 (1.4), 26; 5.0 (4.0 to 6.0) | 4.7 (1.9), 26; 5.0 (3.0 to 6.3) |
| Control | 3.5 (1.7), 50; 4.0 (2.0 to 5.0) | 3.3 (1.6), 34; 3.0 (2.0 to 4.3) | 3.7 (1.8), 30; 4.0 (2.0 to 5.0) | 4.0 (1.5), 29; 4.0 (3.0 to 5.0) |
| Important person thinks should quit (scale 1–7), mean (SD), n; median (IQR) | ||||
| Intervention | 5.4 (2.4), 49; 7.0 (3.0 to 7.0) | 5.5 (2.4), 33; 7.0 (4.5 to 7.0) | 5.2 (2.5), 26; 7.0 (2.0 to 7.0) | 5.0 (2.6), 24; 7.0 (1.5 to 7.0) |
| Control | 5.9 (2.0), 50; 7.0 (5.8 to 7.0) | 5.9 (2.0), 34; 7.0 (5.8 to 7.0) | 5.9 (2.1), 30; 7.0 (5.0 to 7.0) | 5.8 (2.1), 29; 7.0 (5.0 to 7.0) |
| Confidence to cut down by half in 4 weeks (scale 1–7), mean (SD), n; median (IQR) | ||||
| Intervention | 4.0 (1.3), 49; 4.0 (3.0 to 5.0) | 4.7 (2.1), 34; 5.0 (3.0 to 7.0) | 5.7 (2.0), 26; 7.0 (4.0 to 7.0) | 5.7 (1.8), 24; 6.5 (4.0 to 7.0) |
| Control | 4.0 (1.7), 50; 4.0 (3.0 to 5.0) | 3.5 (2.0), 34; 4.0 (2.0 to 5.0) | 3.2 (1.8), 30; 3.5 (1.8 to 5.0) | 4.0 (2.0), 29; 4.0 (2.0 to 6.0) |
| Self-reported cravings in past week (scale 1–7), mean (SD), n; median (IQR) | ||||
| Intervention | 3.1 (1.3), 49; 3.0 (2.0 to 4.0) | 2.2 (0.7), 34; 2.0 (2.0 to 3.0) | 2.0 (1.1), 29; 2.0 (1.0 to 2.5) | 1.8 (0.9), 30; 2.0 (1.0 to 2.3) |
| Control | 2.9 (1.2), 50; 3.0 (2.0 to 4.0) | 3.0 (1.3), 34; 3.0 (2.0 to 4.0) | 2.7 (1.1), 32; 3.0 (2.0 to 3.0) | 2.4 (1.0), 31; 2.0 (2.0 to 3.0) |
| Strength of urge in past week (scale 1–7), mean (SD), n; median (IQR) | ||||
| Intervention | 2.7 (1.2), 49; 3.00 (2.00 to 3.00) | 2.2 (0.9), 34; 2.00 (2.00 to 3.00) | 2.3 (1.1), 29; 2.00 (1.00 to 2.50) | 2.0 (0.9), 29; 2.00 (1.00 to 2.50) |
| Control | 2.7 (1.2), 50; 3.0 (2.0 to 3.0) | 2.7 (1.2), 34; 3.0 (2.0 to 4.0) | 2.3 (1.0), 32; 3.0 (2.0 to 3.0) | 2.2 (1.0), 31; 2.0 (2.0 to 3.0) |
| MPSS, mean (SD), n; median (IQR) | ||||
| Intervention | 2.5 (1.0), 49; 2.2 (1.8 to 3.0) | 2.1 (0.8), 34; 2.0 (1.5 to 2.6) | 2.2 (0.7), 29; 2.0 (1.7 to 2.6) | 2.1 (0.9), 30; 1.9 (1.6 to 2.7) |
| Control | 2.4 (0.8), 50; 2.6 (1.8 to 3.11) | 2.6 (1.0), 34; 2.5 (1.6 to 3.6) | 2.6 (1.0), 34; 1.9 (1.5 to 2.8) | 2.3 (1.0), 31; 2.0 (1.6 to 3.1) |
| mCEQ satisfaction, mean (SD), n; median (IQR) | ||||
| Intervention | 3.8 (1.4), 49; 4.0 (2.8 to 4.7) | 3.0 (1.4), 31; 3.3 (2.0 to 4.0) | 3.0 (1.2), 24; 3.3 (2.8 to 4.0) | 2.9 (1.2), 24; 2.7 (2.3 to 3.6) |
| Control | 3.9 (1.5), 50; 3.7 (2.7 to 5.0) | 3.7 (1.6), 34; 3.7 (2.6 to 4.8) | 3.3 (1.4), 30; 3.0 (2.0 to 4.0) | 3.0 (1.1), 29; 3.0 (2.3 to 4.0) |
| mCEQ respiratory sensations, mean (SD), n; median (IQR) | ||||
| Intervention | 2.3 (1.5), 49; 2.0 (1.0 to 3.5) | 2.3 (1.8), 31; 1.0 (1.0 to 4.0) | 2.2 (1.4), 24; 2.0 (1.0 to 3.0) | 1.9 (1.2), 24; 1.5 (1.0 to 2.0) |
| Control | 2.7 (1.9), 49; 2.0 (1.0 to 4.0) | 2.7 (1.9), 34; 2.0 (1.0 to 4.0) | 2.5 (1.8), 30; 2.0 (1.0 to 4.0) | 2.0 (1.3), 29; 1.0 (1.0 to 3.5) |
| mCEQ reward, mean (SD), n; median (IQR) | ||||
| Intervention | 3.3 (1.3), 49; 3.2 (2.2 to 4.4) | 2.7 (1.3), 31; 2.6 (1.8 to 3.5) | 2.6 (1.0), 24; 2.4 (2.2 to 3.0) | 2.2 (1.2), 24; 1.9 (1.3 to 2.5) |
| Control | 3.4 (1.2), 50; 3.3 (2.6 to 3.9) | 2.9 (1.2), 34; 3.0 (1.9 to 3.8) | 2.7 (1.1), 30; 2.7 (1.8 to 3.5) | 2.4 (1.2), 29; 2.2 (1.6 to 2.9) |
| mCEQ cravings, mean (SD), n; median (IQR) | ||||
| Intervention | 4.6 (2.1), 49; 5.0 (3.0 to 7.0) | 4.6 (1.4), 31; 5.0 (4.0 to 6.0) | 4.6 (1.9), 24; 5.0 (4.0 to 6.0) | 4.1 (1.8), 23; 5.0 (3.0 to 5.0) |
| Control | 5.2 (1.9), 50; 5.5 (4.0 to 7.0) | 5.1 (1.7), 34; 5.5 (4.0 to 6.3) | 5.1 (1.5), 30; 5.0 (4.8 to 6.0) | 5.2 (1.5), 29; 5.0 (4.0 to 7.0) |
Data for item 2 (‘How soon after waking up do you smoke your first cigarette?’) of the FTND, at baseline and follow-up assessments, are shown by condition in Table 18.
| Response, n (%) | Baseline | Week 8 | Week 16 | |||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| Within 5 minutes | 20 (40.8) | 24 (48.0) | 3 (12.5) | 9 (30.0) | 3 (13.6) | 11 (37.9) |
| 6–30 minutes | 20 (40.8) | 18 (36.0) | 10 (41.7) | 11 (36.7) | 8 (36.4) | 8 (27.6) |
| 31–60 minutes | 7 (14.3) | 4 (8.0) | 3 (12.5) | 7 (23.3) | 4 (18.2) | 6 (20.7) |
| After 60 minutes | 2 (4.1) | 4 (8.0) | 8 (33.3) | 3 (10.0) | 7 (31.8) | 4 (13.8) |
| Total, N | 49 | 50 | 24 | 30 | 22 | 29 |
Table 19 shows the number (%) of participants at each stage of readiness to use PA as a way of controlling smoking, in each arm of the trial over time. In summary, as expected, few reported that they were actually using PA (i.e. in action and maintenance stage) as a way of controlling their smoking at baseline, but at 4, 8 and 16 weeks the percentage in the intervention arm versus control were 36% versus 23%, 55% versus 22% and 37% versus 16%, respectively, among those completing follow-up assessments.
| Stage, n (%) | Baseline | Week 4 | Week 8 | Week 16 | ||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Pre-contemplation | 6 (12.2) | 3 (6.0) | 2 (6.1) | 5 (14.7) | 3 (10.3) | 6 (18.8) | 6 (20.0) | 6 (19.4) |
| Contemplation | 34 (69.4) | 36 (72.0) | 8 (24.2) | 14 (41.2) | 5 (17.2) | 12 (37.5) | 6 (20.0) | 10 (32.3) |
| Planning | 3 (6.1) | 8 (16.0) | 11 (33.3) | 7 (20.6) | 5 (17.2) | 7 (21.9) | 7 (23.3) | 10 (32.3) |
| Action | 4 (8.2) | 2 (4.0) | 11 (33.3) | 3 (8.8) | 16 (55.2) | 6 (18.8) | 11 (36.7) | 4 (12.9) |
| Maintenance | 2 (4.1) | 1 (2.0) | 1 (3.0) | 5 (14.7) | 0 (0) | 1 (3.1) | 0 (0) | 1 (3.2) |
| Total, N | 49 | 50 | 33 | 34 | 29 | 32 | 30 | 31 |
Table 20 presents data for body mass index (BMI), weight, sleep and PA-related variables at each assessment by treatment condition, for only those completing assessments. The mean weight for the intervention arm increased by 5.3 kg over 16 weeks, while the mean weight in the control arm did not change. There appeared to be a difference in median minutes of daily self-reported MVPA between the intervention and control arms of 21 minutes (60 minutes vs. 39 minutes) and 5 minutes (35 minutes vs. 30 minutes) at 8 and 16 weeks, respectively. The proportion of participants who self-reported that they did 30 minutes or more of MVPA was 21 percentage points greater in the intervention arm versus the control arm (76% vs. 55%) at 8 weeks, and 5 percentage points greater (57% vs. 52%) at 16 weeks. There were small increases in confidence to exercise for 30 minutes per day for the next 6 months at 8 weeks but not at 16 weeks in the intervention relative to the control arm.
| Variable | Baseline | Week 4 | Week 8 | Week 16 |
|---|---|---|---|---|
| BMI (kg/m2), mean (SD), n; median (IQR) | ||||
| Intervention | 28.6 (5.9), 49; 27.9 (23.7 to 32.5) | Not collected | 28.8 (6.1), 26; 28.3 (23.6 to 32.6) | 29.6 (6.8), 27; 27.9 (24.6 to 32.8) |
| Control | 27.7 (6.8), 49; 26.8 (22.1 to 31.6) | 26.3 (5.3), 32; 24.5 (22.1 to 27.9) | 26.9 (5.5), 29; 26.45 (22.30 to 28.42) | |
| Weight (kg), mean (SD), n; median (IQR) | ||||
| Intervention | 82.3 (19.3), 49; 79.0 (68.9 to 94.9) | Not collected | 85.2 (22.6), 26; 82.9 (67.7 to 96.7) | 87.6 (22.8), 27; 83.7 (69.2 to 104.2) |
| Control | 77.5 (17.8), 49; 75.2 (62.5 to 87.7) | 74.9 (17.9), 32; 71.4 (62.2 to 82.9) | 77.3 (18.6), 29; 72.6 (64.5 to 86.5) | |
| Average hours of sleep per night, mean (SD), n | ||||
| Intervention | 6.2 (1.7), 48 | Not collected | 6.1 (1.7), 27 | 6.1 (1.7), 29 |
| Control | 6.3 (1.5), 48 | 6.7 (2.4), 29 | 6.7 (1.4), 30 | |
| N (%) doing some self-reported moderate PA over past 7 days | ||||
| Intervention | 41 (83.7) | Not collected | 26 (89.7) | 21 (70.0) |
| Control | 40 (80.0) | 25 (80.7) | 20 (64.5) | |
| Self-reported minutes of moderate PA over past 7 days, mean (SD), n; median (IQR) | ||||
| Intervention | 508 (600.9), 49; 315 (128 to 540) | Not collected | 510 (517.0), 29; 365 (170 to 660) | 389 (558.7), 30; 243 (0 to 455) |
| Control | 476 (643.3), 49; 330 (35 to 540) | 381 (500.1), 31; 260 (60 to 435) | 331 (467.1), 31; 180 (0 to 420) | |
| Self-reported minutes of moderate PA (1-day mean), mean (SD), n; median (IQR) | ||||
| Intervention | 73 (85.8), 49; 45 (18 to 77) | Not collected | 73 (73.9), 29; 52 (24 to 94) | 56 (79.8), 30; 35 (0 to 65) |
| Control | 68 (91.9), 49; 47 (5 to 77) | 54 (71.4), 31; 37 (9 to 62) | 47 (66.7), 31; 26 (0 to 60) | |
| N (%) doing some self-reported vigorous PA over past 7 days | ||||
| Intervention | 2 (4.1) | Not collected | 8 (27.6) | 3 (10.0) |
| Control | 8 (16.0) | 5 (16.1) | 4 (12.9) | |
| Self-reported minutes of vigorous PA over past 7 days, mean (SD), n; median (IQR) | ||||
| Intervention | 20 (128.7), 49; 0 (0 to 0) | Not collected | 40 (109.8), 29; 0 (0 to 25) | 11 (36.7), 30; 0 (0 to 0) |
| Control | 20 (58.3), 50; 0 (0 to 0) | 36 (132.9), 31; 0 (0 to 0) | 47 (188.6), 31; 0 (0 to 0) | |
| Self-reported minutes of vigorous PA (1-day mean), mean (SD), n; median (IQR) | ||||
| Intervention | 3 (18.4), 49; 0 (0 to 0) | Not collected | 6 (15.7), 29; 0 (0 to 4) | 2 (5.2), 30; 0 (0 to 0) |
| Control | 3 (8.3), 50; 0 (0 to 0) | 5 (19.0), 31; 0 (0 to 0) | 7 (26.9), 31; 0 (0 to 0) | |
| Self-reported minutes of MVPA over past 7 days, mean (SD), n; median (IQR) | ||||
| Intervention | 528 (611.2), 49; 315 (128 to 563) | Not collected | 551 (511.4), 29; 420 (210 to 725) | 400 (559.6), 30; 243 (0 to 458) |
| Control | 496 (668.9), 49; 330 (35 to 563) | 418 (589.2), 31; 270 (60 to 465) | 379 (517.2), 31; 210 (0 to 540) | |
| Self-reported minutes of MVPA (1-day mean), mean (SD), n; median (IQR) | ||||
| Intervention | 75 (87.3), 49; 45 (18 to 80) | Not collected | 79 (73.1), 29; 60 (30 to 104) | 57 (79.9), 30; 35 (0 to 65) |
| Control | 71 (95.6), 49; 47 (5 to 80) | 60 (84.2), 31; 39 (9 to 66) | 54 (73.9), 31; 30 (0 to 77) | |
| Proportion completing 30 minutes or more self-reported moderate/vigorous activity, N/n; (%) | ||||
| Intervention | 33/49 (67.3) | 22/29 (75.9) | 17/30 (56.7) | |
| Control | 31/49 (63.3) | 17/31 (54.8) | 16/31 (51.6) | |
| Confidence to exercise for 30 minutes/day for next 6 months (scale 1–7), mean (SD), n; median (IQR) | ||||
| Intervention | 5.5 (1.7), 50; 6.0 (4.0 to 7.0) | 5.6 (1.7), 33; 6.0 (4.0 to 7.0) | 6.0 (1.6), 29; 7.0 (5.0 to 7.0) | 5.4 (1.9), 30; 6.5 (4.0 to 7.0) |
| Control | 5.2 (1.9), 49; 6.0 (5.0 to 7.0) | 5.1 (1.9), 34; 5.0 (4.0 to 7.0) | 5.0 (2.2), 32; 6.00 (3.25 to 7.00) | 5.1 (2.0), 31; 6.0 (4.0 to 7.0) |
| Confidence to walk briskly for 15 minutes per day (scale 1–7), mean (SD), n; median (IQR) | ||||
| Intervention | 6.0 (1.7), 49; 7.0 (5.5 to 7.0) | 6.0 (1.7), 33; 7.0 (6.0 to 7.0) | 6.3 (1.5), 29; 7.0 (6.5 to 7.0) | 5.5 (2.2), 31; 7.0 (6.0 to 7.0) |
| Control | 5.6 (2.0), 50; 7.0 (4.0 to 7.0) | 5.5 (2.2), 34; 7.0 (3.8 to 7.0) | 5.8 (2.2), 32; 7.0 (6.0 to 7.0) | 6.0 (1.8), 30; 7.0 (3.0 to 7.0) |
Table 21 presents data for PA, measured by accelerometer, at each assessment by treatment condition, for only those completing assessments. There appeared to be a difference in median minutes of objectively assessed MVPA between the intervention and control arms of 14 minutes (36 minutes vs. 22 minutes) at 8 weeks but none at 16 weeks (20 minutes vs. 23 minutes). Comparing the two arms, 62% in the intervention arm versus 35% in the control arm did 30 minutes or more of MVPA per day at 8 weeks and 38% versus 33%, respectively, did so at 16 weeks.
| Variable | Baseline | Week 4 | Week 8 | Week 16 |
|---|---|---|---|---|
| Minutes of sedentary time, mean (SD), n; median (IQR) | ||||
| Intervention | 510 (98.3), 34; 511 (429 to 585) | Not collected | 519 (142.9), 21; 532 (392 to 603) | 516 (132.7), 21; 520 (443 to 596) |
| Control | 496 (110.0), 32; 477 (428 to 582) | 535 (120.1), 23; 556 (470 to 622) | 490 (88.0), 18; 487 (414 to 555) | |
| Minutes of moderate physical activity, mean (SD), n; median (IQR) | ||||
| Intervention | 31 (22.7), 34; 26 (13 to 46) | Not collected | 35 (21.0), 21; 36 (17 to 50) | 26 (20.0), 21; 20 (12 to 45) |
| Control | 31 (25.3), 32; 27 (11 to 39) | 22 (16.7), 23; 21 (9 to 33) | 26 (18.8), 18; 23 (12 to 43) | |
| Minutes of vigorous physical activity, mean (SD), n; median (IQR) | ||||
| Intervention | 1 (3.9), 34; 0 (0 to 0) | Not collected | 1 (2.0), 21; 0 (0 to 0) | 1 (2.8), 21; 0 (0 to 0) |
| Control | 0 (0.7), 32; 0 (0 to 0) | 0 (0.3), 23; 0 (0 to 0) | 0 (0.8), 18; 0 (0 to 1) | |
| Minutes of very vigorous physical activity, mean (SD), n; median (IQR) | ||||
| Intervention | 0 (0.3), 34; 0 (0 to 0) | Not collected | 0 (0.7), 21; 0 (0 to 0) | 0 (0.1), 21; 0 (0 to 0) |
| Control | 0 (0.1), 32; 0 (0 to 0) | 0 (0.1), 23; 0 (0 to 0) | 0 (0.0), 18; 0 (0 to 0) | |
| Minutes of MVPA, mean, (SD), n; median (IQR) | ||||
| Intervention | 33 (23.7), 34; 31 (13 to 46) | Not collected | 35 (21.1), 21; 36 (17 to 50) | 27 (21.0), 21; 20 (12 to 48) |
| Control | 31 (25.6), 32; 27 (11 to 41) | 22 (16.8), 23; 22 (9 to 33) | 26 (19.0), 18; 23 (12 to 43) | |
| Step counts per day, mean (SD), n; median (IQR) | ||||
| Intervention | 7408 (2851.2), 34; 7246 (4940 to 9300) | Not collected | 7142 (3124.9), 21; 7296 (4079 to 9007) | 6720 (3552.8), 21; 6810 (3950 to 8649) |
| Control | 8014 (4167.9), 32; 7654 (4628 to 11,390) | 6265 (3089.5), 22; 6810 (3787 to 8228) | 7613 (3814.0), 18; 7872 (5193 to 10,188) | |
| Proportion completing 30 minutes or more moderate/vigorous activity, n/N (%) | ||||
| Intervention | 17/34 (50.0) | 13/21 (61.9) | 8/21 (38.1) | |
| Control | 15/32 (46.9) | 8/23 (34.8) | 6/18 (33.3) | |
Table 22 presents data for EQ-5D and perceived stress at each assessment by treatment condition, for only those completing assessments. There appeared to be little or no difference between the treatment conditions over time.
| Variable | Baseline | Week 8 | Week 16 |
|---|---|---|---|
| EQ-5D, mean (SD), n | |||
| Intervention | 0.75 (0.29), 49 | 0.78 (0.24), 29 | 0.75 (0.29), 30 |
| Control | 0.75 (0.27), 50 | 0.80 (0.24), 31 | 0.75 (0.29), 31 |
| PSS, mean (SD), n; median (IQR) | |||
| Intervention | 6.0 (4.07), 49; 4.0 (2.0 to 6.3) | 4.3 (3.8), 28; 2.5 (1.3 to 5.0) | 5.1 (4.8), 30; 2.50 (0.0 to 6.0) |
| Control | 5.5 (4.2), 50; 4.0 (2.0 to 6.0) | 4.6 (4.2), 32; 3.0 (1.0 to 5.0) | 4.2 (4.5), 31; 2.0 (1.0 to 4.0) |
Table 23 presents data for alcohol-related variables at each assessment by treatment condition, for only those completing assessments. There appeared to be no difference between the treatment conditions over time, though any interpretation was difficult given the small sample size.
| Variable | Baseline | Week 8 | Week 16 | |||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| How often do you have a drink containing alcohol?, n (%) | ||||||
| Never | 9 (18.4) | 6 (12.0) | 4 (13.8) | 4 (12.5) | 6 (20.0) | 4 (12.9) |
| Once a month or less | 11 (22.5) | 15 (30.0) | 10 (34.5) | 10 (31.3) | 9 (30.0) | 10 (32.3) |
| 2–4 times per month | 17 (34.7) | 9 (18.0) | 11 (37.9) | 4 (12.5) | 7 (23.3) | 7 (22.6) |
| 2–3 times per week | 6 (12.2) | 14 (28.0) | 1 (3.5) | 12 (37.5) | 4 (13.3) | 6 (19.4) |
| 4 or more times per week | 6 (12.2) | 6 (12.0) | 3 (10.3) | 6 (2.3) | 4 (13.3) | 4 (12.9) |
| Total N | 49 | 50 | 29 | 32 | 30 | 31 |
| How many drinks containing alcohol do you have on a typical day when you are drinking?, n (%) | ||||||
| One or two | 10 (25.0) | 13 (29.6) | 8 (32.0) | 9 (32.1) | 10 (41.7) | 10 (37.0) |
| Three or four | 10 (25.0) | 10 (22.7) | 7 (28.0) | 9 (32.1) | 8 (33.3) | 9 (33.3) |
| Five or six | 9 (22.5) | 8 (18.2) | 5 (20.0) | 3 (10.7) | 2 (8.33) | 5 (18.5) |
| Seven to nine | 7 (17.5) | 8 (18.2) | 2 (8.0) | 4 (14.3) | 3 (12.5) | 1 (3.7) |
| 10 or more | 4 (10.0) | 5 (11.4) | 3 (12.0) | 3 (10.7) | 1 (4.2) | 2 (7.4) |
| Total N | 40 | 44 | 25 | 28 | 24 | 27 |
| How many drinks containing alcohol have you had in the past week?, n (%) | ||||||
| None | 11 (27.5) | 14 (31.8) | 9 (36.0) | 8 (28.6) | 6 (25.0) | 10 (37.0) |
| One or two | 7 (17.5) | 7 (15.9) | 2 (8.0) | 4 (14.3) | 5 (20.8) | 4 (14.8) |
| Three or four | 3 (7.5) | 4 (9.1) | 6 (24.0) | 5 (17.9) | 3 (12.5) | 5 (18.5) |
| Five or six | 5 (12.5) | 1 (2.3) | 2 (8.0) | 2 (7.1) | 2 (8.3) | 3 (11.1) |
| Seven to nine | 3 (7.5) | 3 (6.8) | 0 (0) | 1 (3.6) | 1 (4.2) | 2 (7.4) |
| Ten or more | 11 (27.5) | 15 (34.1) | 6 (24.0) | 8 (28.6) | 7 (29.2) | 3 (11.1) |
| Total N | 44 | 44 | 25 | 28 | 24 | 27 |
Intervention adherence and its association with outcomes
The number of sessions attended by the 49 participants in the intervention arm is shown in Table 24. Overall, the mean [standard deviation (SD)] number of sessions attended was 4.2 (2.7) support sessions (56% face to face, 44% by telephone), with a range from 0 to 8.
| Number of sessions | Frequency |
|---|---|
| 0 | 6 |
| 1 | 5 |
| 2 | 4 |
| 3 | 5 |
| 4 | 6 |
| 5 | 6 |
| 6 | 4 |
| 7 | 6 |
| 8 | 7 |
Using a median split, 20 participants were identified as low attenders (0–3 sessions) and 29 as high attenders (4–8 sessions). The demographic characteristics are shown in Table 25 and the baseline smoking and PA-related variables are shown in Table 26. The sample size was too small to statistically identify demographic and baseline determinants of intervention adherence.
| Baseline characteristic | Low attendance [0–3 sessions; n (%)] | High attendance [4–8 sessions; n (%)] |
|---|---|---|
| Sex | ||
| Male (N = 22) | 7 (31.8) | 15 (68.2) |
| Female (N = 27) | 13 (48.2) | 14 (51.9) |
| Age (years), mean (SD), n; median (IQR) | 44.4 (13.5), 20; 47.6 (33.6 to 55.9) | 49.2 (9.9), 29; 50.0 (40.8 to 58.5) |
| Age (years) | ||
| ≤ 30 (N = 6) | 4 (66.7) | 2 (33.3) |
| ≥ 31 (N = 43) | 16 (37.2) | 27 (62.8) |
| Ethnicity | ||
| White British (N = 47) | 20 (42.6) | 27 (57.5) |
| Other (N = 2) | 0 (0) | 2 (100) |
| Cohabiting status | ||
| Cohabiting (N = 21) | 7 (33.3) | 14 (66.7) |
| Not cohabiting (N = 28) | 13 (46.4) | 15 (53.6) |
| Children under 16 years | ||
| Yes (N = 11) | 3 (27.3) | 8 (72.7) |
| No (N = 38) | 17 (44.7) | 21 (55.3) |
| Single parenta | ||
| Yes (N = 3) | 1 (33.3) | 2 (66.7) |
| Employment status | ||
| Employed (N = 26) | 11 (42.3) | 15 (57.7) |
| Not employed (N = 23) | 9 (39.1) | 14 (60.9) |
| Job status | ||
| A to C1 (N = 5) | 1 (20.0) | 4 (80.0) |
| C2 to E (N = 21) | 10 (47.6) | 11 (52.4) |
| Unemployed (N = 23) | 9 (39.1) | 14 (60.9) |
| Age on leaving education (years), mean (SD), n; median (IQR) | 16.0 (0.9), 20; 16.0 (15.0 to 16.0) | 16.3 (1.8), 29; 16.0 (15.0 to 16.5) |
| BMI (kg/m2), mean (SD), n; median (IQR) | 29.7 (5.8), 20; 29.4 (24.8 to 33.9) | 27.8 (6.0), 29; 26.7 (22.2 to 32.3) |
| Indicated mental health problemb | ||
| Yes (N = 23) | 8 (34.8) | 15 (65.2) |
| No (N = 26) | 12 (46.2) | 14 (53.8) |
| Age on starting smoking (years), mean (SD); median (IQR) | 14.8 (3.4); 13.5 (13.0 to 16) | 14.5 (2.4); 14 (13 to 16) |
| Does partner or other cohabitant smoke? | ||
| Yes (N = 9) | 5 (55.6) | 4 (44.4) |
| No (N = 14) | 4 (28.6) | 10 (71.4) |
| Not applicable (N = 25) | 11 (44.0) | 14 (56.0) |
| Duration of smoking (years), mean (SD); median (IQR) | 29.6 (14.2); 33.6 (16.6 to 41.0) | 34.7 (10.4); 34.7 (26.6 to 44.4) |
| Previous use of SSS | ||
| Yes (N = 21) | 9 (42.9) | 12 (57.1) |
| No (N = 28) | 11 (39.3) | 17 (60.7) |
| Satisfaction with previous use of SSS (if used) (scale 1–11), mean (SD), n | 8.1 (2.6), 9 | 9.0 (2.1), 12 |
| Did the participant make a quit attempt lasting 24 hours or more in the past year? | ||
| Yes (N = 17) | 10 (58.8) | 7 (41.2) |
| No (N = 32) | 10 (31.3) | 22 (68.7) |
| Did the participant cut down before previous cessation?c | ||
| Yes (N = 1) | 1 (100.0) | 0 (0.0) |
| No (N = 16) | 9 (56.3) | 7 (43.7) |
| Total n | 10 | 7 |
| Used cessation aids as part of a quit attempt in previous 12 monthsd | ||
| Yes (N = 12) | 7 (58.3) | 5 (41.7) |
| No (N = 5) | 3 (60.0) | 2 (40.0) |
| Total n | 10 | 7 |
| Used cessation aids not as part of a quit attempt in previous 12 months | ||
| Yes (N = 12) | 4 (33.3) | 8 (66.7) |
| No (N = 20) | 6 (30.0) | 14 (70.0) |
| Total n | 10 | 22 |
| Baseline characteristic | Low attendance (0–3 sessions; N = 20) | High attendance (4–8 sessions; N = 29) |
|---|---|---|
| Self-reported composite cigarettes per day, mean (SD), n; median (IQR) | 20.0 (9.4), 20; 19.6 (13.4 to 29.2) | 22.8 (16.8), 29; 17.0 (12.5 to 25.9) |
| Expired air CO (p.p.m.), mean (SD) | 20.3 (7.5) | 15.5 (7.0) |
| FTND, mean (SD); median (IQR) | 5.9 (1.9); 6.5 (5 to 7) | 5.3 (2.1); 5 (4 to 7) |
| Readiness to use PA as a way of controlling smoking, ACTION and MAINTENANCE stage, n (%) | 3 (15.0) | 3 (10.3) |
| Self-reported moderate PA, n (%) | ||
| Yes (N = 41) | 17 (41.5) | 24 (58.5) |
| No (N = 8) | 3 (37.5) | 5 (62.5) |
| Self-reported MVPA over previous 7 days (minutes), mean (SD) n; median (IQR) | 614 (628.0), 20; 343 (120 to 1205) | 468 (603.2), 29; 310 (128 to 490) |
| Time spent in objectively assessed MVPA per day (minutes), mean (SD) n; median (IQR) | 31 (34.7), 10; 15 (10 to 49) | 33 (18.3), 24; 33 (18 to 45) |
| Step counts per day, mean (SD), n; median (IQR) | 7555 (3306.2), 10; 7090 (4531 to 10,001) | 7347 (2715.3), 24; 7307 (5126 to 8860) |
The relationship between level of intervention participation and smoking-related variables is shown in Tables 27 and 28. There appeared to be a greater proportion of participants who reduced the number of cigarettes smoked by at least 50% among those who attended more sessions of the intervention, and a trend towards a greater proportion of high attenders making a quit attempt. Comparison of the number of cigarettes smoked and FTND at follow-up assessments was limited because of the small numbers of participants who completed assessments in the low-treatment adherers.
| Variable | Intervention high attenders (N = 29) | Intervention low attenders (N = 20) | RR (95% CI) |
|---|---|---|---|
| Self-reported quit attempt during study,a n (%) | |||
| Yes | 10 (34.5) | 1 (5.0) | 6.90 (0.96 to 49.71) |
| No | 19 (65.5) | 19 (95.0) | |
| Confirmed quit at 4 weeks post quit date,a,b n (%) | |||
| Yes | 6 (20.7) | 1 (5.0) | 4.14 (0.54 to 31.8) |
| No | 23 (79.3) | 19 (95.0) | |
| Self-reported non-smoking at week 16,a,c n (%) | |||
| Yes | 4 (13.8) | 1 (5.0) | 2.76 (0.33 to 22.89) |
| No | 25 (86.2) | 19 (95.0) | |
| Reduction of smoking by 50% or more by week 8,a n (%) | |||
| Yes | 13 (44.8) | 2 (10.0) | 4.48 (1.13 to 17.74) |
| No | 16 (55.2) | 18 (90.0) | |
| Reduction of smoking by 50% or more by week 16,a n (%) | |||
| Yes | 18 (62.1) | 1 (5.0) | 12.41 (1.80 to 85.65) |
| No | 11 (37.9) | 19 (95.0) | |
| Baseline characteristic | Baseline | Week 4 | Week 8 | Week 16 |
|---|---|---|---|---|
| Self-reported cigarettes per day, mean (SD), n; median (IQR) | ||||
| Low attendance | 20.0 (9.3), 20; 19.6 (13.4 to 29.2) | 17.4 (4.9), 7; 15.6 (15.0 to 20.0) | 7.6 (7.1), 4; 7.7 (0.8 to 14.3) | 11.6 (9.2), 5; 14.0 (2.0 to 20.0) |
| High attendance | 22.8 (16.8), 29; 17.0 (12.5 to 25.9) | 11.5 (5.9), 27; 10.0 (7.1 to 15.6) | 11.4 (7.0), 22; 9.7 (6.5 to 17.7) | 8.5 (8.0), 25; 7.0 (0.3 to 14.5) |
| FTND, mean (SD), n; median (IQR) | ||||
| Low attendance | 5.9 (1.9), 20; 6.5 (5.0 to 7.0) | Not collected | 3.3 (2.1), 3; 4.0 (1.0 to 5.0) | 4.5 (1.0), 4; 5.0 (3.5 to 5.0) |
| High attendance | 5.3 (2.1), 29; 5.0 (4.0 to 7.0) | 3.1 (2.5), 21; 3.0 (1.0 to 5.0) | 2.8 (2.3), 18; 2.5 (1.0 to 4.5) | |
Chapter 4 Trial results: discussion of quantitative data
Overall summary
The main aim of this pilot study (to conduct a pilot RCT in which a PA intervention in addition to usual care was compared with usual care alone, in a largely disadvantaged sample) was achieved. We were able to recruit a largely socially disadvantaged sample, and conduct follow-up assessments with over 60% of those randomised. Recruitment by invitation letter (from the GP and the SSS) was the most effective and efficient approach. Our efforts to recruit a sample of 120 over 12 months proved to be slightly ambitious relative to the 1.4 full-time equivalent HTs/researchers available and challenges faced in recruiting participants other than by invitation letter.
Recruitment closed with 99 participants having been randomised. The study provided valuable information on the feasibility and acceptability of data collection methods and intervention. There was evidence that the intervention was able to reduce smoking and increase short-term PA, albeit without inferential statistics.
Information on changes in related outcomes suggests that the intervention impacted in expected ways to some extent. Data collected provide valuable information for conducting a phase 3 trial, and for making minor refinements of the intervention and methods. The following discussion provides a more detailed analysis and reflection on the research methods and intervention, to inform future research.
Design and methods
Recruitment
Table 29 shows a summary of our targets for recruiting a ‘hard to reach’ or disadvantaged sample against what we achieved and indicates that we exceeded our targets. Most of the sample were unemployed or in social class C2–E. Despite considerable effort (see Methods) fewer single parents entered the study than expected. The target was possibly unrealistic, but there was also some resistance from single parents who anecdotally found it difficult to give up the time to engage in the study, particularly during school holiday time. We did not have ethical approval or the resources to systematically identify the participants’ mental health status at the time of entering the study, though we asked the GP surgery to screen out possible participants with an unstable mental illness who may have placed the HTs at risk. As a result, we used two criteria (i.e. items from the EQ-5D and MPSS) to determine to the extent to which our sample had a mental health problem, as shown in Table 29.
| Target samples | Target for recruitment | Actually recruited |
|---|---|---|
| Unemployed or social class C2-E | 75% | 91% |
| Single parent | 30% | 17%a |
| Mental health problem (EQ-5D item) | 20% | 41%b |
| Mental health problem (MPSS item) | 20% | 19%c |
We believe that this target would not have been achieved in less socially deprived parts of Plymouth. Efforts to recruit through the local IAPT service resulted in only one participant being recruited who had mild to moderate depression. Success with this approach may depend on the personnel involved in the service and operational procedures. At the time we engaged with the IAPT service, it was facing a period of significant disruption and change as it was being taken out of the NHS. Also, a single meeting with the psychological well-being practitioners was insufficient for them to make EARS recruitment a priority. Finally, we set no specific target but aimed to recruit an ethnically diverse sample. Plymouth has very low ethnic diversity and the four participants in the sample we did recruit were of Eastern European origin. One attended sessions with her husband who acted as a translator, and the others had no difficulties engaging in the study.
There were several other indicators that the sample were disadvantaged and treatment resistant: (1) the mean school leaving age was 16 years; (2) they were, on average, heavy smokers (smoking over 21 cigarettes per day) with a long history of smoking (over 32 years) and high nicotine dependence; and (3) almost 60% reported that they had not used NHS SSS support to attempt to quit in the past. Also, the EQ-5D sample mean of 0.75 indicated a generally poor health status.
Our recruitment involved two methods: by invitation letter (from the GP and the SSS) and by other community-based approaches. We initially aimed to recruit 60 by GP invitation letter, 30 by SSS invitation letter and 30 by other community-based approaches. Considerable effort went into trying other community-based approaches, but this resulted in few participants being recruited. We had hoped to contrast the characteristics of those recruited by these two approaches (invitation letter vs. community-based approaches) but insufficient numbers made this impossible. We recruited only participants who were registered with a GP and this may have limited the recruitment of homeless people and the most disadvantaged smokers. We felt that this was necessary to minimise risk to the participant (from increasing PA) and to the HT, and ensure that we were able to keep in contact with participants over the duration of the study. Invitation letters from the GP and SSS led to similar response rates and sample characteristics. We therefore believe that disadvantaged smokers can be recruited, from areas with high social deprivation, by invitation letter and reminder telephone calls (to ensure that those with low levels of literacy are not excluded). There did appear to be little difference in demographic characteristics between those responding to the initial letter and those drawn into the trial through an additional reminder telephone call. Given the additional effort required to complete reminder telephone calls, recruitment of a sample may be possible by a single letter if the target population is large enough. Recruitment rates were similar across three GP surgeries and the local NHS SSS, which provides confidence that at least 5–8% of those invited can be recruited by a single mailed invitation.
The response and recruitment rates are reported with an assumption that all those invited were smoking (at least 10 cigarettes) and still living at the address to which the invitation was sent. However, it appeared that we had incorrect information for about 10% of those we invited, and a further 10–15% were ineligible, so there is uncertainty about the correct denominator to use in calculating recruitment rates. Only 10–30% (varying across letter and telephone invitation from the GP and SSS) actually declined to take part, so the 5–8% figure for recruitment rate could be higher or lower depending on the quality of patient records kept with regard to smoking status. We identified participants from the respective databases that had been recorded as smoking in the past 2 years, and this may have introduced some inaccuracy. We did consider asking the GP surgery team to identify potential participants but the practice managers advised against this owing to time pressures. If the intervention became accepted as standard practice it may well be appropriate to opportunistically recruit smokers in surgeries.
Self-reported data were somewhat verified by accelerometer data, though, as is often the case, participants typically over-reported their involvement in moderate-intensity PA. This was especially true of those reporting the most daily MVPA. At baseline the sample participated in virtually no vigorous intensity PA; nevertheless, almost 50% and 65% of the participants achieved ≥ 30 minutes of at least moderate-intensity PA per day recorded by accelerometer and self-report respectively (albeit the two measures captured data over 2 different weeks). Based on self-reported accounts, this volume of activity was largely due to daily walking and involvement in manual work, with little leisure-time PA. We did not exclude participants from the study who were already physically active (e.g. achieving at least 150 minutes of weekly MVPA), but did exclude those who were unable to walk for at least 15 minutes. This may have led to a more active sample than in the general population of smokers with a similar demographic background but we were keen to recruit only those who could increase their activity in response to the intervention. We made an effort to avoid highlighting that the study was about exercise and PA in recruitment materials and the participant information sheet, to avoid recruiting only those more interested in PA and run the risk of failing to meet our recruitment targets. In summary, we think that the sample was probably representative of smokers living in the catchment areas in terms of PA.
Randomisation
At the end of the baseline assessment, randomisation took place by the researcher telephoning either TT or AT, who were in a different location and expecting the call. They were logged into a website managed by the Peninsula Clinical Trials Unit and quickly entered minimisation details and revealed a unique ID number and assigned the participant to a treatment arm within the trial. There were no difficulties in this procedure. Randomisation procedures resulted in no baseline differences in the sample characteristics. In order to minimise the number of sessions with participants for data collection, the accelerometer (to objectively assess PA) was given to participants post randomisation, with a request to return it, after wearing it for 1 week, in a stamped addressed envelope or in person when attending their first intervention session. There were no differences between treatment arms in the amount of PA recorded by accelerometer at baseline, suggesting that this approach did not introduce any bias.
Study attrition (assessment completion)
Considerable effort went into maintaining contact with participants in both arms, particularly at the 16-week follow-up. Although for a disadvantaged sample capturing data from over 60% of the sample was encouraging, we do feel that this could be improved with the involvement of a trial administrator to make telephone calls and possibly manage a technology reminder system with messages sent to mobile or home telephones prior to appointments. This would not work for a proportion of participants who changed their telephones on a regular basis and those who had no telephone, but could be successful for the majority. In our pilot study we had budgeted for 0.3 full-time equivalent (fte) of administrative support over the duration of the study, but with complexities associated with managing the trial 45 miles away from Exeter, we ended up employing a 1.0 fte administrator for the final third of the project, who was based in Exeter. The HTs were responsible for arranging all appointments and any rearrangements for all assessments and intervention sessions. With only 1.4 fte of HT resource budgeted for the study, the attrition rate was very encouraging.
Of the 38 participants who did not complete the 16-week assessment, 30 had dropped out by week 4, and these were evenly split between the control and intervention arms. Those failing to complete 4-week follow-up assessments in the intervention arm also attended few intervention sessions. Also, there is some anecdotal evidence that those in the control arm who failed to complete the 4-week assessment were disappointed that they were not going to receive the intervention. Thus, reasons for study attrition appeared to be different in each arm of the study. Later in the trial we did offer to provide a single session with control participants after the week 16 assessment to provide them with information given to those in the intervention arm. This would not be possible in a study with an even longer follow-up assessment, and so additional messages to highlight the importance of completing follow-up assessments, in the participant information sheet prior to entering the study, may be needed.
In our analysis of factors associated with study attrition (following recruitment by GP letter), the withdrawal rate was twice as high among those recruited through reminder telephone calls as among those recruited by just an initial letter. This was not the case for those recruited by a SSS invitation letter or reminder call and the relatively small numbers of participants involved may limit our confidence in this finding. One may expect that the effort required to recruit disadvantaged participants would be linked to study attrition as suggested by the GP invitation figures. The only other indication of any bias in attrition rates was that 33% of males and 43% of females failed to complete the 16-week assessment. Overall, though, attrition was similar from both arms of the trial.
Measures and measurement issues
Primary outcome
The original intention was to encourage all participants who wished to quit smoking to agree to be referred to the local NHS SSS. Support for those in the intervention arm from the HT was also planned. We allowed participants to begin any quit attempt up until week 12 to ensure that we had a further period of 4 weeks (up until week 16) to have an expired air CO verified 4-week period of abstinence. Participants in the control group, at baseline and subsequent assessments, were also reminded that if they did plan to quit they should notify the HT/researcher and that the SSS was available to support them. These planned procedures were to ensure some control over capturing quit attempts and post-quit monitoring. In fact, we identified only two participants who contacted and used the support of the NHS SSS. Also, some participants wished to quit without further support from the HT. This created a challenge for collecting data on our flexible primary outcome, namely expired air CO continuous confirmed abstinence (captured on a weekly basis).
Thus, data we report for our primary outcome (i.e. number achieving 4 weeks’ expired air CO-confirmed continuous abstinence) are not as rigorous as we had planned. We had expected all participants to attend the SSS and be monitored weekly. However, those reported to have quit fitted the following criteria: (1) we were aware of their planned quit date and could therefore prospectively follow their progress; and (2) we recorded an expired air CO value of < 10 p.p.m. between 4 and 8 weeks after the quit date and participants self-reported that they had not smoked more than five cigarettes since quitting.
Point prevalence data at week 16 confirmed the data reported for 4-week continuous abstinence, with the exception of two intervention participants who had lapsed in week 15.
The difficulty of capturing data for our primary outcome was also due to the nature of the sample: scheduled assessments were routinely missed and rearranged (for a wide range of reasons). We make recommendations in the final chapter to address this issue, but this pilot study has provided valuable information on how to work with disadvantaged smokers and capture a flexible outcome.
Although participants were excluded from the study if they wanted to quit in the coming month at the time of entry into the study, one participant decided to quit only 1 week after starting the intervention. On average, it was 7 weeks before those participants who decided to quit began their attempt and it is not clear if the participants’ awareness that the intervention contact was due to finish after 8 weeks influenced this. Some participants needed longer than 8 weeks but, overall, a target to reduce (and, if desired, to quit) over 8 weeks was appropriate. The client-centred approach of the intervention certainly aimed to allow participants to decide if and when they wished to quit.
Other smoking-related measures
We aimed to assess the proportion in each arm of the trial who reduced the number of cigarettes smoked by at least 50% at 8 and 16 weeks. In our sample, approximately 60% smoked only, or predominantly, self-rolled cigarettes, with no one smoking pipes, and approximately 35% smoking only, or predominantly, factory-made cigarettes. The remaining 5% smoked a roughly equal combination of self-rolled and factory-made cigarettes. Given the intention to assess this outcome we decided to nevertheless create a common variable, namely number of cigarettes smoked at each assessment. We decided to base our conversion from grams of tobacco to number of cigarettes based on Laugeson89 who reported that 0.45 g was equivalent to one factory-made cigarette, following a rigorous laboratory assessment of number of puffs and nicotine inhalation. Also, for those reporting the number of factory-made cigarettes smoked, there was inevitably some random measurement error. Participants were encouraged to use a range of recall prompts to indicate how many cigarettes were typically or had recently been smoked (see detailed description of measures in Chapter 2), including number of packs consumed each day, number of cigarettes smoked in the morning, afternoon and evening, or in specific situations. The precise number of cigarettes smoked in the present study therefore has measurement error but data collection and conversion procedures were consistent across time and treatment arms.
Physical activity measures
We used the self-reported 7-day recall of PA to capture the type, intensity, frequency and volume, which has been widely used in trials and has been shown to be sensitive to changes as a result of behaviour change interventions. This measure tended to show over-reporting of MVPA when compared with accelerometer data. This was particularly true among the most active, perhaps because the most active tended to be those in manual labour who reported doing up to 10 hours of MVPA per day, whereas in reality this probably included some periods of inactivity. The value of using the self-report measure is that we were able to capture data from 60 participants at weeks 8 and 16. In contrast, just 44% and 39% participants provided a minimum of 3 days of accelerometer data during weeks 8 and 16, respectively. These accelerometer compliance figures are comparable with those from previous research with adult populations.
After recruiting the first 60 participants, we realised that a number of accelerometers had not been returned, at a cost of c. £250 each. In an attempt to avoid this, and to maximise compliance with wearing it, we initially provided a payment of £10 in exchange for returning the device after each complete week of wearing it: a total of £30 for wearing it for a period of 7 days at baseline, week 8 and week 16. This was then increased to £30 after each week of wearing. Overall, 13 were not returned, and all of the missing accelerometers were administered before the increase in payment for its return. It seems reasonable to assume that the increase in payment did impact on the successful returning of the accelerometers. The logistics of reimbursing participants was also complex and challenging. Some participants did not have bank accounts or preferred cash payments, and the research team had to set up a cash float. Eventually we worked out systems with the university for processing claims (in bulk) and reimbursing expenses from the float.
In any future study the use of 24-hour wrist-worn GENEActiv (Activinsights, Cambridgeshire, UK) accelerometers (c. £120 each) is recommended to increase compliance and minimise financial loss.
Both self-report and accelerometer data suggested that both groups were less active at week 16 than at baseline. Reactivity in trials is common, with participants tending to do more PA, possibly due to a heightened awareness of researchers’ interest in this behaviour. These findings must be interpreted with caution given that alternative explanations such as withdrawal of more active participants may have influenced the results.
Intervention engagement
There was clearly considerable variability in participant engagement in the intervention, with 40% having fewer than four sessions, including 12% who had none, and 58% having four or more sessions. In this pilot trial we did not specify an a priori intervention dose in order to conduct sensitivity analysis, but did complete some exploratory analyses of the effects of dose received on smoking outcomes. The sample size limited our confidence in suggesting a dose–response relationship but the data suggest the presence of a relationship between intervention attendance and smoking reduction at 8 and 16 weeks.
Our decision to be flexible in offering sessions face to face or by telephone was clearly justified, with only a slightly larger proportion involving the former.
Nine participants claimed subsidies relating to PA, ranging from £1.50 to £91. Activities subsidised included paying for public transport to help them reach (or return from) walking routes, enrolling in gym memberships and paying for swimming sessions at a local facility. Also, one received money to purchase a pair of training shoes.
The factors influencing intervention engagement are examined in much more detail in Chapter 5, and we provide further overall discussion in the final chapter.
Outcomes among control and intervention participants
As a pilot study we did not seek to contrast outcomes between the control and intervention participants at follow-up but we were keen to identify whether or not there were indications of changes in smoking and PA outcomes that related to our intervention aims.
Smoking outcomes
Prior to the study we presented a scenario analysis in which 5% in the control arm would be expired air CO-confirmed abstinent at 4 weeks post quit. The present study revealed that 6% attempted to quit and 4% were abstinent 4 weeks later (and at 16 weeks) in the control arm, thus supporting our estimation. As Table 4 shows, different scenarios were created to show the impact of sample size, effect size (RR) and precision of effect size estimate. On this basis we initially aimed to recruit 120 participants. Owing to previously discussed difficulties in recruitment and on the basis of an interim analysis of our accrual chart over the first 8 months of recruitment, a new recruitment target of 100 participants was set. With a final sample of 99 participants, the RR of having expired air CO-confirmed abstinent at 4 weeks post quit, making a quit attempt, and reducing the number of cigarettes smoked by at least 50% at 16 weeks also provided support for positive changes in smoking in favour of the intervention arm. There appears to be a good case for conducting a larger study to confirm these effects with greater confidence.
Other smoking-related outcomes
There were also reductions in mean expired air CO in the intervention arm, as well as more favourable beliefs about importance of quitting and confidence to quit, and cutting down smoking by 50% within 4 weeks, across the study. There were also favourable changes in cigarette cravings and withdrawal symptoms and satisfaction from smoking. While the study was not powered to conduct inferential statistical analyses to compare changes relative to the control these findings suggest that the intervention could be effective. The intervention aimed to increase the importance of quitting and confidence to quit through participant-centred support, without directly focusing on quitting. We expected that by increasing confidence to reduce smoking, participants would develop the motivation to quit. Smoking reduction has not been promoted in the past for fear of increasing cravings and withdrawal symptoms between cigarettes, hence increasing satisfaction with the fewer cigarettes smoked. The present data provide no evidence of this; in fact, the opposite appears to be the case. The study was not powered to statistically examine the mediating role of changes in PA on smoking-related outcomes but the link between PA and smoking is further discussed in Chapter 5.
Physical activity and related outcomes
The intervention aimed to both increase PA in general but also enhance the perceived value of doing PA as an aid for reducing smoking. Results from both the accelerometer and the self-report measures show that over 50% of the sample were already doing at least 150 minutes per week of MVPA at baseline. This may have reduced the scope of the intervention to substantially increase PA, but self-reported and accelerometer data for moderate PA at the 8-week assessment did appear to be greater in the intervention arm compared with the control participants (albeit among only those providing data). Also, a greater proportion in the intervention arm reported doing at least 30 minutes per day of MVPA at 8 weeks. The proportion who reported using PA as an aid for controlling smoking also increased considerably more in the intervention arm than in the control. Everson-Hock, Taylor and Ussher67 reported that 22% of smokers attempting to quit, with the support of a NHS SSS, were using PA as a smoking-cessation aid. The present study suggests that an identical proportion of control participants (who wanted to reduce but not quit) were using PA as an aid. In contrast, at 4 and 8 weeks in the intervention arm, 36% and 55% respectively were using PA as an aid. Everson et al. 67 also reported a significant association between self-efficacy to do PA and stage of readiness to use it as an aid for cessation. The present study suggested that confidence to do 30 minutes of PA over the next 6 months and to walk briskly for 15 minutes per day also tended to be greater in the intervention arm than in control participants at weeks 4 and 8. The study was not powered to explicitly link changes in PA with changes in smoking-related cognitions but a larger study may permit this. Overall, the present study does suggest that some changes in PA and related outcomes, such as a sense of competence, did occur, which links well with the change in processes we expected to see as a result of the intervention.
In terms of duration of apparent changes in PA after the intervention, all measures of PA indicated a return to baseline levels, albeit from means and proportions among only those who completed a 16-week assessment. An alternative explanation is that the most active did not return to complete the 16-week assessment. Given that most study attrition took place up to week 4, this explanation seems unlikely. We may therefore conclude that any changes in PA were short-lived and had dissipated by week 16, though this was not statistically confirmed. While this may be regarded as disappointing and similar to other PA intervention evaluations, it also provides some evidence that the EARS intervention did lead to short-term increases in PA. The HTs did focus on increasing PA to aid smoking reduction and cessation induction rather than long-term sustained increases in PA. Also, because the sample had relatively high levels of PA at baseline, it is perhaps not surprising that longer-term changes would be not be sustained.
There appears to be differences in weight at follow-up between the groups and in terms of change over time. These results could be due to differential withdrawal from the study. Given the potential importance of weight gain as a deterrent to quitting and remaining abstinent, further analyses in a larger study should explore changes in weight while controlling for baseline factors and missingness.
Intervention adherence and its impact on outcomes
Although limited by small numbers, the finding that those who attended more sessions were more likely to have favourable smoking outcomes does suggest that the intervention worked for those who were willing and able to engage. Of the 29 participants who did have at least four sessions with the HT, 45% and 62% had reduced by at least 50% by weeks 8 and 16, respectively, and 34% made a quit attempt. This compares with no more than 10% of participants who had fewer than four intervention sessions, who either quit or reduced by at least 50%.
Finally, the duration of the intervention, for those who did attempt to quit, ranged from 1 to 12 weeks, with a mean of 7 weeks. We designed the intervention and study with an expectation that participants would engage in up to eight weekly sessions, with the belief that if they had not decided to quit by the end of that period then they probably would not quit at all. However, in the interests of giving intervention participants a sense of autonomy, the period over which the intervention was offered was increased so that a quit attempt could occur no later than 4 weeks prior to the final 16-week assessment. A few participants were not ready to quit after 8 weeks, but were ready in later weeks. Extending or being flexible with the intervention period should therefore be considered in any future study.
Chapter 5 Process and qualitative evaluation
Introduction
A range of qualitative approaches were used in the early pre-trial phase of the study to capture information to inform the development of the intervention and trial methods. These were described in Chapter 1.
The focus of this chapter is on deriving qualitative information from a range of sources to assess the feasibility and acceptability of the intervention and trial methods, and suggest how the intervention and trial methods could be improved. Within the constraints of completing this report, it was not possible to report a full assessment of intervention processes, but these will be examined in future outputs.
The main aims and forms of data collection are shown in Table 30, with a letter Y indicating which aim was matched against which method. Further details about the respective methods are described in the text below. The findings will then be presented for each method of data collection. The final section will summarise and discuss the findings in relation to our stated aims.
We recognised a two-stage change process in which (1) HTs were asked to deliver a multicomponent intervention to facilitate behaviour change, and (2) participants engage in, and respond to, the intervention. The qualitative evaluation described below sought to identify, through the various methods, if the multicomponent intervention was appropriate in the first instance, and secondly whether or not what was offered was effective.
| Aims | Data collection methods | |||
|---|---|---|---|---|
| Taped sessions between HTs and intervention participants | Interviews with control and intervention participants at the end of the study | Interviews with HTs both early and late in the study | Describe the story of a participant who successfully engaged in the intervention | |
| Identify the acceptability of the trial methods (across trial arms) | Y | Y | ||
| Identify the acceptability of the intervention and possible adaptations | Y | Y | ||
| Identify the components of the intervention perceived to be effective | Y | Y | Y | Y |
| Examine treatment fidelity in relation to the planned intervention described in the manual | Y | Y | ||
| Generate ideas to inform future training of practitioners | Y | Y | Y | |
Taped sessions between health trainers and intervention participants
Background/rationale
Detailed description of what was delivered is important in the evaluation of complex interventions. 90 In order for such interventions to work, they must be well delivered and poor delivery may lead to underestimation of intervention effects. Understanding the fidelity compared with the manual is important for interpretation. For behavioural interventions in particular, variations in delivery quality can strongly mediate intervention effectiveness. 91,92 Knowledge about what was delivered allows one to test and refine the underlying theory of behaviour change or process model. Understanding fidelity allows refinement of the intervention and training procedures for future use by identifying elements that are less well delivered and where there is scope for improvement. Variations in delivery can also be positive and such practitioner innovations can be used to enhance the intervention. A range of qualitative and quantitative methods have been developed for assessment of delivery, including the use of qualitative research methods and the use of checklists to score transcripts or recordings of intervention consultations. 92–94
Aims
We sought to use recordings of consultations between participants and HTs to (a) check the quality of intervention delivery (what content was delivered and how it compared with the manual) and (b) identify specific areas for improvement in the EARS intervention and its training course.
Methods
Design
The intended intervention processes for the EARS intervention are described in Chapter 1. These were used as a basis for generating items for a checklist to assess intervention delivery and fidelity. Following a brief scoring-standardisation procedure, the checklist was applied to a purposive selection of consultation recordings and descriptive analyses were used to summarise the data.
A more detailed qualitative analysis, linking data from all of the above data sources (individual participant feedback with feedback from the HTs and the consultation recordings), will be conducted to examine the relationships between intervention delivery and smoking reduction and to refine the process model. However, this work is outwith the remit of the Health Technology Assessment (HTA)-funded research and so is not reported here.
Sampling frame
All consultation sessions were audio recorded subject to informed consent. Consent for this was taken on the main study consent form and this was checked verbally prior to starting the first consultation. A sample of four participants for each of the three HTs (12 participants in total) was selected to provide examples from early, late and in the middle of the study period (to smooth out any HT practice effects). For each client, three (out of a possible eight) consultations were selected for coding to provide examples of intervention techniques from early-stage motivation through to later-stage progress reviewing/relapse prevention.
Measures and procedure
To assess intervention fidelity (and at the same time quantify delivery in terms of predefined manualised elements), we used the Dreyfus system for assessing skill acquisition (Figure 5)95 to score recorded consultations with respect to a HT’s skill in delivering each of the 12 intervention processes (see Table 31). A scoring checklist and instructions were developed and these are provided in Appendix 5c. The checklist was applied initially by three researchers with expertise in behaviour change (AT, TT, CGVS) to a sample of six consultations from two participants. Scores were compared and reasons for any discrepancies were discussed to produce a consensus about how to apply the scoring system.
Two researchers (TT, CGVS) then each scored consultation data from two participants for each of the three HTs (using three consultations per participant) to produce an overall intervention fidelity rating for each item and for each HT (see Table 31). This was done by listening to the set of (three) recorded consultations for each participant, reading the transcripts of the same consultations and then rating the fidelity for each item on the checklist. Because of limitations in time and resources, we did not conduct formal inter-rater reliability checks; this would have required both researchers to rate fidelity for around 20–30 participants each. However, we did split the coding for each HT between the two researchers, so that each researcher coded two participants for each HT. The average score for the HT is therefore the average of the scores given by two coders.
Analysis and interpretation
Descriptive data were extracted and reported to highlight areas of good or bad practice in delivering the intended intervention processes. Owing to the clear descriptions associated with each score (see checklist scoring instructions in Appendix 5c) and the steps taken to establish a consensus between coders on the approach to scoring, interpretation of scores is relatively straightforward. Scores of 0 or 1 represented poor delivery (or no delivery) of the intended process. A score of 3 or more was considered to represent reasonable quality of intervention delivery. Scores of 5 or 6 represented very high (expert-level) quality, which we were not expecting to see very often with our trainers delivering this novel intervention for the first time. It was accepted that for item 9 (seeking to identify and reinforce shifts in identity), the opportunities to deliver this process would be scarce and so a lower score (1.5 or more) was considered acceptable for this item. Item 12 (referral to smoking-cessation services) was scored as either 0 or 1 (yes or no) and so is reported separately.
Examples of good intervention practice were highlighted as the coders went through the consultation transcripts and these will be collated and used as examples in future training for the EARS intervention (either in their current form as audio recordings and transcripts, or by conversion into video example using actors to play the roles of practitioner and participant).
Results
Table 31 shows the intervention fidelity scores for each item on the checklist, broken down by HT and by coder.
| Mean score | IF1 | IF2 | IF3 | IF4 | IF5 | IF6 | IF7 | IF8 | IF9 | IF10 | IF11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.0 | 3.3 | 2.2 | 3.6 | 2.9 | 3.4 | 2.6 | 2.9 | 1.5 | 1.8 | 1.3 | |
| HT1 mean | 3.9 | 3.5 | 2.4 | 3.9 | 2.9 | 3.5 | 2.6 | 3.0 | 2.4 | 2.3 | 1.5 |
| HT2 mean | 4.3 | 2.8 | 1.9 | 3.0 | 2.9 | 3.3 | 2.4 | 2.5 | 0.8 | 1.4 | 1.3 |
| HT3 mean | 4.0 | 3.6 | 2.4 | 4.0 | 3.0 | 3.5 | 2.8 | 3.3 | 1.5 | 1.8 | 1.0 |
| Coder 1 mean | 3.6 | 3.6 | 2.4 | 3.3 | 2.8 | 3.2 | 2.4 | 3.2 | 1.9 | 1.8 | 1.3 |
| Coder 2 mean | 4.5 | 3.0 | 2.0 | 3.9 | 3.1 | 3.7 | 2.8 | 2.7 | 1.2 | 1.8 | 1.2 |
The average scores for each item for each HT differed by –0.9 to +0.8 points (out of 6) with an average difference of 0.0. Hence, there seemed to be a reasonable level of agreement between coders about the quality of intervention delivery for all of the intervention processes.
One additional item (IF12: referral to smoking-cessation services if appropriate) was coded either as Yes or No (and so is not shown above). In all but one of the 12 cases examined this was scored as Yes, indicating high fidelity for this intended process.
Overall, intervention fidelity was deemed to be acceptable, but with clear room for improvement in some areas:
-
All three HTs demonstrated a high level of skill in the use of client-centred counselling techniques. However, the delivery intervention elements related to promoting PA (IF3, IF5 and IF7) were generally scored lower than elements relating to promoting smoking reduction (IF2, IF4, IF6). This probably reflects the primary aim of the intervention and difficulties in introducing ideas about PA in this context (see sections below on this).
-
The sample was more active than expected and this created uncertainty for the HTs regarding how best to further increase PA.
-
The scores for IF10 and IF11 (identifying and seeking to manage social influences) were considerably lower than expected, falling well below the criterion (3 or more) for good delivery. This was due to a lack of exploration of social influences, rather than poor delivery style.
-
For IF9 (reinforcing any changes in identity), the score met our lower criterion of 1.5: this was expected, as (a) this was not a key focus of the training and (b) few opportunities arose to do this. When such opportunities did arise, however, they were not always reinforced and so the training could be improved to increase sensitivity of the HTs to this issue.
The mean overall scores across the eleven scales for HT1, HT2 and HT3 were 2.9, 2.4 and 2.8, respectively, suggesting no large differences in overall fidelity scores. However, although HT2 performed well on IF1, she did slightly less well on six of the other 10 scores relative to HT1 and HT3. It would be inappropriate to explore these differences in more detail for risk of breaking anonymity but the scores do support the sensitivity of the scales used to assess intervention fidelity. In the future the scales could be used to highlight training and supervision needs and in a larger study to link HT performance with smoking and PA outcomes.
Interviews with control and intervention participants
Aims
The aims of conducting interviews with control and intervention participants at the end of the study were to:
-
identify the acceptability of the trial methods (across trial arms)
-
identify the acceptability of the intervention and possible adaptations
-
identify the components of the intervention perceived to be effective.
We were particularly aware that this disadvantaged population would be largely new to participating in research, and to procedures such as randomisation and to different forms of data collection. The methods described below aimed to consider acceptability and feasibility with this in mind.
Methods
Recruitment and sampling
All trial participants had consented at baseline to being approached by an independent qualitative researcher to capture their experiences associated with the study and with the intervention, for those in that arm of the study.
During the delivery of the intervention, the research team regularly discussed the progress of individual participant progress and the nature of engagement with the intervention. We were particularly keen to identify intervention participants who appeared to have benefitted from the intervention and be examples of good practice, possibly for future use in training. As many participants as possible who engaged with the intervention were interviewed by TT.
Control participants were selected at random. Participants sampled were contacted by telephone and a convenient time to conduct the interview by telephone was arranged. No participants who were contacted declined to be interviewed. Verbal consent was obtained for the interviewed to be digitally recorded. All interviews were digitally recorded and transcribed verbatim for further analysis.
Interviews
Participants were interviewed within 16 weeks of completing the study. The interviews followed the guide shown in Figure 6 for control and intervention participants, respectively.
FIGURE 6.
Generic and trial arm-specific interview guide.
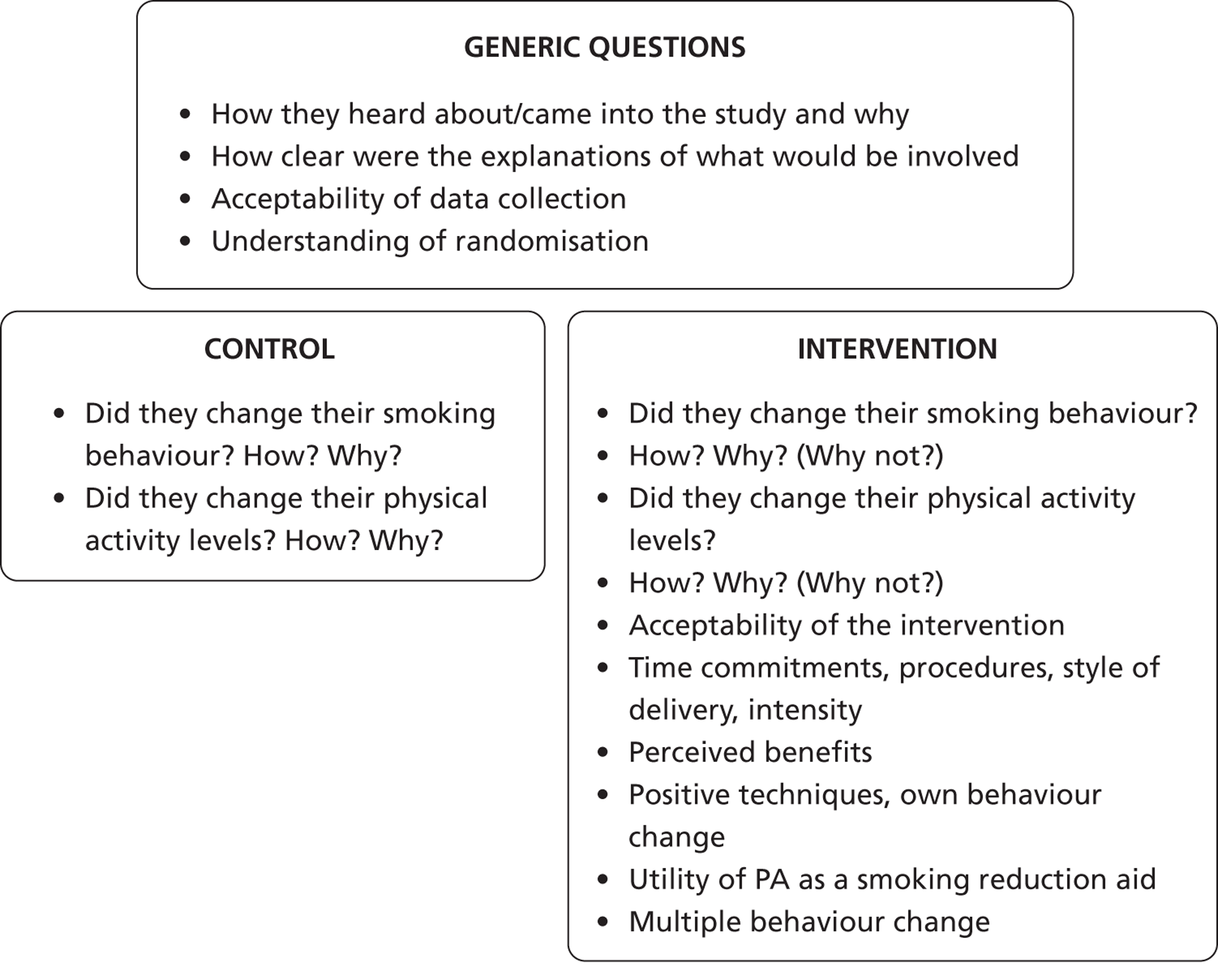
Data analysis
Interview transcripts were analysed used the qualitative software package NVivo (version 9.2, QSR International, Southport, UK). The data were organised using a basic thematic analysis to provide a simple descriptive-level overview of the participants’ views and experiences. In-depth qualitative analysis procedures were not used here. However, these data will be analysed in more depth alongside the participant interviews and consultation recordings, using framework analysis6 to generate an integrated analysis of processes of behaviour change in the EARS intervention. This in-depth analysis will be reported as part of the PhD of one of the researchers (TT). The participant characteristics of those interviewed from both arms of the trial are shown in Table 32.
| Characteristics | Control (n = 10) (20% of sample) | Intervention (n = 15) (30% of initial sample) |
|---|---|---|
| Demographics | ||
| Age, mean (SD) | 46 (11) years | 52 (11) years |
| Sex (male/female) | 5/5 | 8/7 |
| Deprivation indicators | ||
| Social class | 50% unemployed (5/10) | 40% unemployed (6/15) |
| 50% in C2–E work (5/10) | 60% in C2–E work (9/15) | |
| 0% in A–C1 work (0/10) | 0% in A–C1 (0/15) | |
| Single parenthood | 10% (1/10) | 0% (0/10) |
| Baseline data | ||
| Smoking characteristics | ||
| Mean (SD) tobacco per day | 17.4 (8.7) g | 18.4 (12.5) g |
| FTND (SD) | 5.3 (1.9) | 4.9 (1.7) |
| MVPA (self-report) | ||
| Mean (SD) minutes per week | 595 (757) | 401 (644) |
| 20% at 0 (2/10) | 27% at 0 (4/15) | |
| 40% at 100–499 (4/10) | 60% at 100–499 (9/15) | |
| 30% at 500–999 (3/10) | 7% at 500–999 (1/15) | |
| 10% at > 2000 (1/10) | 7% at >2000 (1/15) | |
| Quit attempt in past year | 50% (5/10) | 33% (5/15) |
| Outcomes | ||
| Quit attempt | 0% (0/10) | 33% (5/15) |
| 4 weeks’ CO-confirmed abstinent | NA | 80% (4/5) |
| 50% reduction | 20% (2/10) | At least 33% (4/11)a |
| No change | 80% (8/10) | At least 26% (4/15)a |
| Recruitment | ||
| Avenue (GP : SSS : community) | 5 : 5 : 0 (50% : 50% : 0%) | 7 : 6 : 2 (47% : 40% : 13%) |
| Type (letter : telephone : other) | 7 : 3 : 0 (70% : 30% : 0%) | 6 : 7 : 2 (40% : 47% : 13%) |
Results
The aim of this section is to give participants a voice in the research through quotes that may capture the rich diversity of perceptions in this sample of hard-to-reach smokers. We engaged with a wide variety of smokers in terms of literacy and ability to grasp the methods and aims of the intervention. Nowhere else in the document is this captured. This portrayal of diversity also serves to provide important information for future researchers and intervention trainers.
Acceptability and feasibility of methods used to recruit, randomise and assess participants
Clarity of invitation to take part in the trial
Targeting a population with expected low levels of literacy meant keeping the invitation as simple as possible without compromising the individuals’ understanding of what was being offered to them. Overall, the responses revealed a clear and concise understanding of what was being offered. For example:
So how clear was the information that you had?
Very. Very clear.
So how much did the information that you had read, how much did it match what you actually received in terms of the support and things like that?
Oh erm exactly . . . .
Female, aged 60–65 years, unemployed, moderate smoker, intervention
There were a couple of examples of where a participant reported misunderstanding, or elements of the study that surprised them. This included explanations of the requirements to wear an accelerometer, and confusion over the randomisation procedure:
The leaflet was kind of vague, it told you what it was actually based on but it didn’t tell you about you were going to get split into two groups. I don’t think it mentioned anything about wearing the accelerometer – was it called . . .?
No I didn’t know that, I thought ‘cause [the HT] rang up somebody else and they said, ‘You are in this section [trial arm],’ so I don’t know if they’d done any background research and said, ‘Well, she’ll be good in this group,’ or, ‘she’ll be good in that group’.
OK, right. So it doesn’t sound like that was explained to you properly then.
No, no not at all.
Female, aged 25–30 years, employed part-time, moderate smoker, intervention
Another example of confusion arose surrounding the payment for wearing and returning the accelerometer where the individual thought that rather than them getting paid for wearing and returning it, they had to pay the study. [Note: during the course of the study, a number of accelerometers were not being returned so we increased the reward for returning them from £10 to £30 in exchange for wearing it for 1 week and returning it].
Yeah, well, because [the HT] hadn’t mentioned it at the beginning, so I didn’t know anything about it, and then when it had been changed [the HT] said, ‘Oh, just to let you know that it’s gone from £10 to £30’. And I thought that I had to pay her and [the HT] said, ‘Why?!’
So that wasn’t made very clear to you.
No!
Female, aged 30–35 years, unemployed, moderate smoker, control
While this may demonstrate a high level of motivation for taking part in the study, it suggests that explanation of the reimbursement structure and randomisation may need a clearer explanation. This was the first time that most participants had engaged in a research trial and more practical explanations to participants of the need for and process of randomisation are suggested by these findings.
Appeal of the invitation and support for reduction
The novel approach of actively promoting support for reduction in smoking was shown to be well received by the majority of the sample. Many framed the appeal of reduction against the alternative of stopping abruptly. The appeal of reducing appeared to stem from an underlying desire to change behaviour but, owing to a lack of confidence in stopping abruptly, reduction seemed like a much more manageable approach to tackling their smoking behaviour:
Yeah, I think yes, I think that was probably it, the reduction thinking. Well you know, rather than sort of go cold turkey and completely stop I thought, ‘Oh you know, you could help me reduce it,’ which you did, so you know that obviously it worked.
Female, aged 60–65 years, unemployed, moderate smoker, intervention
What was it about that then that appealed to you?
Erm [coughs] it’s easier than cutting it out altogether, I guess it should be easier actually. But . . . well I needed to try something as opposed to nothing, that’s what appealed to me.
Male, aged 60–65 years, unemployed, moderate smoker, intervention
Well it . . . I didn’t feel as if I was undermined in any way you know, until it’s to stop altogether you know. I felt that it was, well, maybe an easier solution.
Female, aged 60–65 years, employed full-time, moderate smoker, intervention
One individual explicitly expressed this notion as reason for coming into the study, revealing that cutting down would be a good idea at a time when they feel they are not ready to stop:
Cutting down, is that something that perhaps appealed to you?
Yeah it did as well, because I thought, ‘I don’t really want to, I am not ready to stop yet,’ and I thought cutting down is quite good. I tend to smoke if I am a bit stressed. I use it as an excuse to smoke so [laughs] I thought, ‘You know, that’s quite a good way to do it really is just cut down bit by bit’.
Female, aged 35–40 years, employed part-time, moderate smoker, control
For some, past experiences of failed quitting heightened the appeal of support for reduction as a novel approach to tackling their smoking behaviour:
Well, for three or four years I’ve been trying to give up smoking [and] last month [I] done ten months, and then I had a smoke. The year before six months, the year before that was four months, and it’s always a quit, you know, stop, go on to nicotine gum and lozenges and patches and whatsoever. This one appealed to me because you cut down, you know, every week you cut down two cigarettes a week and you just cut down and cut down and I eventually got down to none.
Male, aged 60–65 years, unemployed, moderate smoker, intervention, successful quitter
The message of support to cut down, in the trial invitation, did not appear to threaten people’s sense of control over their own behaviour compared with a message around abrupt quitting. For some, it was clear that a pervading message of the need to ‘stop smoking’ would have completely alienated them from engaging in the study:
Well, [the HT] said to me, ‘We are not here to make you stop smoking, make you stop smoking. We are here to at least try and cut you back, if we can help you just to cut down, cut down by whatever, just to help you cut down, we are happy with just helping you cut down.’ And I thought, ‘Well, this is someone talking to me that’s not saying, ‘We can stop you smoking. We can do this,’ ’ but [the HT] was saying, ‘We can help you to cut down. We may in time be able to stop you smoking,’ and I said, ‘Well, that’s a very sensible attitude to take,’ because someone telling me, ‘I’m going to stop you smoking,’ I’d tell them to . . . go away! So that’s what made me do it initially, because they weren’t threatening me that they could stop me smoking. But even at this time, there is no one that can tell me, ‘I can stop you smoking,’ you know what I mean?
Male, aged 55–60 years, unemployed, heavy smoker, intervention
There was also evidence to suggest that smokers can feel saturated and alienated by the ‘hard sell’ of the abrupt quit message. The invitation was designed, as was the intervention, to be supportive and client centred and a step away from traditional services, and the supportive and pressure-free nature of the invitation was well received:
You know, I say, when [the HT] gave me the leaflet I thought, ‘Yeah, all right, I’ve heard all this before,’ and I thought, ‘Well, here we go with the hard sell’. But [they were] totally different. [They were] so relaxed, so friendly, and that’s what pushed me towards it. If [they] had tried to come across with the hard sell I would most probably have just ignored [them] and said cheerio. But I think just approaching people in a friendly manner . . . I mean, sometimes it helps.
Male, aged 55–60 years, employed full-time, heavy smoker, intervention
Oh, I expected the trial to be like, ‘Oh, you’ve got to do this, you’ve got to do that,’ you know! And it wasn’t nothing like that because I said to my chap, I said, ‘If I go down there and they say, ‘You’ve got to give up smoking now,’ I’m saying no, I can’t do that!’ But [the HT] wasn’t nothing like it, [the HT] was really nice. I said to her, ‘I can do what I can do.’ I said, ‘You can set me out some tasks and I will try and do them.’ So that’s what [the HT] done for me.
Female, aged 50–55 years, employed full-time, moderate smoker, intervention, failed quitter
The intention of providing a supportive and pressure-free message seems to have been achieved and effective in engaging with the targeted population.
For one individual, they recognised both the reduction and the PA support side of the intervention as being something they would like to take advantage of:
I was actually thinking about doing something to help myself so when it came up and then it said about the smoking reduction I thought it was like a bonus that I can get healthy and reduce my smoking at the same time.
Female, aged 25–30 years, employed part-time, moderate smoker, intervention
This was a relatively isolated case in responding to the appeal of the invitation, but this was to be expected due to the intentional ‘down-playing’ of the PA side of the intervention in an attempt not to alienate the less active, or to recruit only those most interested in PA.
Effect of invitation
It became apparent that the invitation itself had a motivating effect on people’s desire for change. As was expected, most smokers were already contemplating, and had been for some time, the idea of changing their smoking behaviour. The invitation acted as a prompt to change their smoking habits:
Oh exactly, exactly, it actually gave me the push that I needed [laughs] just getting the letter and then, you know, yeah it just gave me the kick that I needed. I’d been thinking about it for a long time but never doing anything about it. As I say, the first getting the letter was the kick that I needed. I mean I could really have stopped in the next couple of years, I don’t know about that one but I hope I would have done, but it was just that initial for the letter coming through the door and I thought, ‘Yeah, I would do that’.
Female, aged 60–65 years, unemployed, moderate smoker, intervention
Um, well, because I recently tried to give up smoking a few months before I started and obviously it didn’t happen, but I was on the smoking replacement stuff, and I just thought, ‘You know, I’ll give anything a try,’ if you know what I mean. So when I got the letter I just thought, ‘Oh yeah, I’ll go for that’. You know, it was just something that I immediately thought, ‘Yeah, I want to do it’.
Female, aged 35–40 years, employed part-time, moderate smoker, intervention
Motivations for taking part
Not surprisingly, a common motivation was health related, either through personal health or through ill health of family or friends. Although there is little evidence to suggest that cutting down smoking has significant health benefits, it was perceived by people to offer health benefits with quitting still an underlying long-term goal:
Because I have had a few friends who have died of cancer and that recently, see? So it’s getting more of a thing to, like, try [cutting down].
Male, aged 45–50 years, employed full-time, moderate smoker, control
Because I keep on having a permanent cough and it won’t go away. And the doctor has given me this, that and the other but he said, ‘Your cough ain’t going unless you give up smoking’. So it really is . . . it irritates my throat and that’s why I cough all the time.
Female, aged 50–55 years, employed full-time, moderate smoker, intervention, failed quitter
Oh, I’d been thinking for about a year I must give up smoking, it’s expensive and it’s not healthy, it’s antisocial and you know, all these things, but never quite doing anything about it.
Female, aged 60–65 years, unemployed, moderate smoker, intervention
The last person also mentioned the financial cost of smoking as a motivating factor, a motive that was borne out in other dialogues:
So you weren’t necessarily interested in quitting altogether, but just the idea of reducing was quite a good idea for you, was it?
Yeah, I was averaging about 20 a day and being on a pension I went over it and I thought, ‘This is getting too expensive. I am going to have to knock it on the head’.
Male, aged 60–65 years, employed full-time, heavy smoker, intervention, successful quitter
Unsuccessful and negative previous experiences of using NRT and other medicinal therapies emerged as a strong theme linked positively to motivation for taking part. It was envisaged that the intervention would offer a novel alternative to the use of NRT, and people’s description of past experiences seemed to confirm this particular aspect of the intervention.
Umm [sighs] well I suppose the fact that I’d been on the 3-month course [varenicline] . . . I started smoking again [on holiday] and when I got back and this cropped up I thought, ‘Well you know, anything I can do to help,’ you know, ‘stop people smoking including myself,’ I thought, ‘Well I’ll take part in it,’ you know and whether it was going to be of any use to me personally or not at the end of the day I thought I’d just wait and find out.
Male, aged 55–60 years, employed full-time, heavy smoker, control
Oh I’ve tried a couple of times to cut out smoking totally ‘cause I’ve tried the smoking aids and all the things you know, the puffer and the patches and that hasn’t worked, so I thought, ‘Oh well, I’ll give this a try then try and cut down,’ yeah.
Male, aged 40–45 years, employed part-time, very heavy smoker, intervention
There were a couple of reports of people taking part through altruistic motivation and the interest of being part of a research study:
I just thought I’d see. I don’t know, it just sort of appealed really, and also I suppose out of interest of a scientific enquiry.
Male, aged 45–50 years, employed full-time, moderate smoker, intervention
Well, I’m up at the uni anyway, and helping out with studies is a good idea.
Female, aged 30–35 years, unemployed, moderate smoker, control
There was an isolated example of somebody coming forward to enter the study because of the incentives offered to the intervention group in terms of subsidised leisure facility access:
Well obviously I thought I might be one of the lucky ones that would get the support, obviously they had gym membership and obviously I was still wanting to do all of that as well so, and obviously where I am it costs too much a month to actually go to the gym, so . . .
Female, aged 35–40 years, unemployed, moderate smoker, control
It appeared that some participants were only taking part for the financial incentive offered for returning the accelerometer:
Well, I’m not sure. I mean, I’ve spoken to a few people who’s been on it and when it was first mentioned, somebody said, ‘Oh you get £30 at the end of the 3 months.’ I said, ‘So you did it for the £30?’ He said, ‘Well, yeah.’ I said, ‘Well, how sad is that? That’s really sad. So are you going to pack up smoking?’ ‘Oh, no.’ You know, which I think is rather sad. I would just cut out the incentive of any money.
Male, aged 55–60 years, employed full-time, heavy smoker, intervention
We are also aware of one participant who withdrew because the financial incentive was ‘not enough’.
Randomisation: understanding the process and acceptability
Participating in a RCT was a new experience for the majority of the participants. With the exception of the one example discussed earlier, the procedure was well understood by those interviewed, albeit explained and understood in a variety of terminology:
[The HT] said to me that there’s going to be two groups and after we’d had the discussion about the whole thing, they [phoned] somebody in Exeter and they would allocate me to a particular group.
Female, aged 50–55 years, employed full-time, moderate smoker, control
Yeah, yeah. No, the person who was mentoring me at the time, he said, ‘You may be lucky, you may not. It depends’.
Male, aged 60–65 years, employed full-time, heavy smoker, intervention, successful quitter
The effect of the randomisation procedure on motivation to remain in the study (reluctance to remain in the study if not receiving any support) showed no particular influence either way. Some people showed no disappointment in being allocated to the control arm, while some did:
Right, and how was that for you? Was it a disappointment or was it . . .?
No, no, not at all, no, it was fine. I was just interested to see what would happen actually!
Female, aged 50–55 years, employed full-time, moderate smoker, control
Yeah, I was a bit gutted by it but like you say, they did explain to me beforehand that that may be the case.
Male, aged 25–30 years, unemployed, heavy smoker, control
The HTs also revealed that participants were on the whole ‘disappointed’ to be allocated to the control condition, with one participant immediately withdrawing from the study as a result.
Acceptability and feasibility of data collection methods
Within our methods we carefully considered the amount and type of data collection, given the nature of the target population, with a few participants with low levels of literacy. As a result all data collection forms were administered and completed by the HT. Particularly intrusive questions (e.g. probing too deeply about mental health) were eliminated in an attempt not to alienate or cause suspicion or defensiveness in the participants.
On the whole, the feeling was that the number and type of questions was acceptable, and that the HTs/researchers helped them understand the questions.
Yeah, no, it was fine, it was all good. Nothing I found offensive or anything, or you know, noseyfied or anything like that, no, it was fine.
Female, aged 35–40 years, employed part-time, moderate smoker, intervention
No if they had all stayed at the same amount of time that I was there for the first one [baseline assessment] then yeah I would have been like, ‘Oh my God,’ but no, they got better as they went on.
Female, aged 25–30 years, employed part-time, moderate smoker, intervention
They explained it really quite well and that and yeah, it was good. It was good, I quite enjoyed it.
Male, aged 45–50 years, employed part-time, moderate smoker
Well I mean yeah, the questions were all right, you were given enough time to answer and I think some of them gave you a choice of answers so you only had to pick at the relevant one that matched my own situation, so it was pretty easier to answer.
Male, aged 55–60 years, employed full-time, heavy smoke, control
There were some expressed difficulties with Likert scales and multiple option questions:
Um, yeah, I found them all right. They were quite . . . I think there was probably like maybe too many options within the answers, sort of 1 to 10 or something like that. And I was either sort of right at the end, in the middle, or right at the beginning. I was either one rather than sort of anything in between.
Female, aged 30–35 years, unemployed, moderate smoker, control
I’m just trying to think. I think some of the answers I didn’t fall into any of the categories as such because I fell into one between two of them, if you know what I mean, which wasn’t there.
Male, aged 60–65 years, employed full-time, heavy smoker, intervention, successful quitter
Some questions appeared individually inappropriate, perhaps due to a misunderstanding of the question or because some of the questions were rather abstract and difficult for some participants; feedback from the HTs implied that some of the questions were more difficult to ask and explain than others (e.g. with the mCEQ):
Well, some of them I thought quite amusing but I said to [the HT] on a couple of occasions, ‘How can I answer this? I don’t do things like that!’
Male, aged 60–65 years, employed full-time, heavy smoker, intervention, successful quitter
If I remember the questions, yeah, I think they were very random! I mean, some of them I had a bit of a chuckle at, what [the HT] would ask me, like!
Male, aged 25–30 years, unemployed, heavy smoker, control
One difficulty we faced was tailoring the questionnaires to include the situation of making a quit attempt. This was difficult as several of the questions became non-applicable in the light of a quit attempt, but also varied depending on their smoking behaviour since they made their quit attempt. This was reflected in one participant’s observations following a quit attempt:
The only one grouse I had was in the forms that you’ve got, that you fill in saying if you have depression and if you have [this and that] and all the rest, [but] there is no form for when you quit smoking. So half the forms when I quit smoking were no good to me at all [ . . . ] so you want us to quit and when you do quit, there’s no form saying, how do you feel? That’s the only thing that I feel was wrong with it.
Male, aged 60–65 years, unemployed, moderate smoker, intervention, successful quitter
Methods for measuring PA and potential problems associated with wearing an accelerometer on an elasticated waistband was of interest to the study. Most described it as comfortable and wearing it became a routine:
Do you know what, surprisingly, it wasn’t as uncomfortable as I thought it was, although the belt itself is really comfortable, the elasticated belt. I mean, you couldn’t even feel it was there most of the time. I mean, you didn’t even know it was there until you hit against it or brushed against it with your wrist.
Male, aged 25–30 years, unemployed, heavy smoker, control
A few participants had an issue with remembering to put it on, or returning it to the researchers:
Remembering to put it on as well, sometimes I would walk around for an hour then think, ‘Ah! I’ve got to put that thing on,’ like, you know?
Male, aged 55–60 years, unemployed, heavy smoker, intervention
Yeah, yeah, it was fine. The only sort of thing, as I say, I was like, I’d wear it for the day and then I’d put it on my bedside and then forget all about it! And because you’ve rung, I’ve now remembered I need to send it back.
Female, aged 30–35 years, unemployed, moderate smoker, control
The possibility of daily automated text message reminders to accompany the accelerometer was explored with those who expressed trouble remembering to put it on. The responses highlighted that it would be acceptable and useful, but should be optional:
Would maybe a text in the morning have been useful or something like that, or would that have been a bit intrusive?
Um, I’m not sure really. I mean, I would say, it sounds like something that’s intrusive but when you have got a memory like mine, it would have helped, so it’s a bit of a hard one. I suppose that one’s an individual case. Maybe it’s a question that should be asked at the beginning of the thing when you get given the band to wear.
Female, aged 30–35 years, unemployed, moderate smoker, control
A few participants in active professions found that the accelerometer could become obstructive:
Yeah, I wore one I think for the first couple of weeks and then [the HT] gave me another one but where I was working it kept on catching, so I didn’t wear the second one. See I work on a building site, see, so there was a lot of bending over and like I just climbed down from the loft now, it was getting quite irritable like, on my side. So I didn’t wear the second one.
Male, aged 45–50 years, employed full-time, moderate smoker, control
One participant thought that it was frustrating to wear the accelerometer and get no feedback on how they had done. Perhaps the use of it could be better described and understood in the future with an example of the type of graphical information it provided (on a sheet of paper) rather than individual feedback. Overall, the pedometer provided some feedback. The administrative challenge of downloading individual information from the accelerometer would be considerable.
You wear it for a week, you give it to them . . . no, no, no, they get feedback, obviously, but it would help or it would have been nice. You wear it for a week, what for? I don’t know. It monitors my body. OkayOK, thank you. But monitors what? Monitors . . . I wore it three times and I don’t know why.
Male, aged 55–60 years, unemployed, heavy smoker, intervention
Locality of assessments
Assessments took place in a centralised and well-known health service building and, where appropriate, at the participant’s GP surgery. From those interviewed there were no complaints about the location of sessions.
One of the successful quitters in the study praised the location of the sessions as the SSS was based in the same building, and so when he did decide to make a quit attempt the process was relatively seamless. Only two of the quitters did make use of the SSS, so this was not relevant for many participants.
Yeah. I would just like to mention that at the [centre] where it was held, to actually stop smoking and then getting the nicotine aids things, it’s made a damn sight easier because you are in the hospital and they have got a non-smoking unit there. So I saw this [stop smoking advisor], prescription, straight away, for gum, nicotine gum, patches and all the rest of it, you know. Because the location is brilliant because you did not have to go away and find somewhere, go to my surgery.
Male, aged 60–65 years, unemployed, moderate smoker, intervention, successful quitter
Acceptability and feasibility of the intervention
Intensity of support
Behavioural support for helping people who do not want to quit in the immediate future to cut down is a new area of research and little is known about the amount of support required. A major consideration was the frequency, duration and length of time over which the support should take place, and the mode of delivery. The pre-pilot development work resulted in a very flexible style of intervention delivery, with a mix of face-to-face sessions and telephone support sessions.
There was support for weekly sessions, which some participants found to be acceptable:
Oh no, no that was fine. I think once a week was brilliant and that’s what’s helped me.
Male, aged 60–65 years, unemployed, moderate smoker, intervention, successful quitter
I think it was probably about right, I don’t think, you know, it didn’t seem onerous particularly being part of the study.
Male, aged 45–50 years, employed full-time, moderate smoker, intervention
Well I think I, all the ideas were there but you know I thought it was probably enough.
Male, aged 60–65 years, unemployed, moderate smoker, intervention
No, it was just right, I saw [the HT] on a Tuesday morning at 10 and I was more or less out of there by 11, just before. Yeah, no, I was happy with that, that was fine.
Female, aged 35–40 years, employed part-time, moderate smoker, intervention
The lengths of the sessions were generally acceptable as well:
No, I think it was OK. It was mostly an hour a week, sometimes it went a bit longer, but no it was fine.
Male, aged 40–45 years, employed part-time, very heavy smoker, intervention
The mixture of face-to-face contact and telephone support was generally well received by those interviewed, allowing for increased flexibility and engagement with the support around continually changing circumstances for some:
[The HT] always used to ring me. Because some days I couldn’t make it and I just said to [them], ‘Can I speak to you on the phone instead?’ And then we’d do the paperwork over the phone because I was working quite a bit then.
Female, aged 50–55 years, employed full-time, moderate smoker, intervention, failed quitter
Having the telephone contact appeared to play an important part of the perceived support participants experienced, whether or not they used it, having been provided with the HT’s telephone number and being told they could always telephone if they had any problems.
I always had the mobile number anyway, not that I needed it, but they said you know whenever I did need it for anything.
Male, aged 40–45 years, employed part-time, very heavy smoker, intervention
I think it worked really well because I always had [the HT] on the other end of the phone, if I needed her, do you know what I mean? You know, [the HT] was always there for me and so they really helped me a lot.
Female, aged 50–55 years, employed full-time, moderate smoker, intervention, failed quitter
No, no it was fine it was fine. I mean when, well, they were always there at the end of the phone whoever you were dealing with, that was quite nice, if you had to change an appointment or what have you they were always there.
Female, aged 60–65 years, employed full-time, moderate smoker, intervention
The overall feeling regarding the intensity of the intervention was generally mixed. There was no report from those interviewed that the support was too intensive or overbearing, but there was a recurring theme that the support could have been more intensive than once a week:
Maybe erm a little more often to see somebody. I mean as I say I was fine, but maybe some people would like that little bit more . . . even a phone call maybe just to see how you’re getting on [in between sessions].
Female, aged 60–65 years, unemployed, moderate smoker, intervention
We were doing regular meetings but I found if there had have been a little bit more contact like maybe just a phone call like halfway through the week . . . I think as well the weekly meetings maybe like a midweek telephone call to give you that little bit of encouragement and everything.
Female, aged 25–30 years, employed part-time, moderate smoker, intervention
Some participants reported that not only was the support not intensive enough, but it could have gone on for longer to support further behaviour change:
I would say the only thing is, I mean like now I’ve done it and everything, it was too short and it wasn’t intense enough . . . I mean, [the HT] was excellent and I am personally saying my idea is that if it was more intense it would be more helpful. It would be better.
Male, aged 55–60 years, unemployed, heavy smoker, intervention
Erm I think . . . if you got people to give up smoking I think it would be, you know, a longer period of time would be better.
Male, aged 60–65 years, unemployed, moderate smoker, intervention
There was some evidence to suggest that participants were not receiving the weekly level of support that was intended.
Well, when I say that, without being rude, I mean . . . my counsellor was very good but I saw [them] once every few weeks, of a couple of weeks maybe or maybe I saw [them] like next week, then I wouldn’t see [them] for two weeks, or three weeks maybe, or two weeks, you know what I mean?
Male, aged 55–60 years, unemployed, heavy smoker, intervention
There was a period when it was sort of four, eight and sixteen weeks was it I can’t remember what the time was but erm, yeah, maybe say every two weeks would have been helpful to some people.
Female, aged 60–65 years, unemployed, moderate smoker, intervention
Some instances of limited contact were due to staff illness, the three HTs all working part-time and on different days, and HTs being fairly rigidly assigned to individual participants (to maintain continuity). Field and meeting notes did reveal a large variability in participants’ abilities to stick to regular scheduled meetings depending on unpredictable and changeable personal circumstances. One participant withdrew from the study before receiving any intervention support sessions due to a delay between the baseline assessment and the first intervention session. Fortunately, these difficulties were few and participants generally appreciated the intensity and flexibility of support received.
Style of delivery
The EARS client-centred intervention was designed to maximise adherence among a traditionally service resistant group. The HTs were trained to establish a strong rapport and engage participants in taking control of any behaviour change. We were keen to understand how this worked and was perceived by participants.
There was good support for the HTs and the strong rapport they developed with the participants interviewed, with discussions around the targeted behaviours moving forwards in an individualised way.
Yeah, fine. [The HT’s] all right. Yeah, [they’re] lovely. I used to go down and see them or they used to ring me if I was too busy at work or something. Yeah, they was fine. I got on really well with them. They was a good help.
Female, 50–55 years, employed full-time, moderate smoker, intervention, failed quitter)
But [the HT] was very good at what they done, you know what I mean? [The HT] was, I mean, like first, like most people with a doctor or something like that, ‘How are you feeling? How’s your problem?’ Well with [the HT], with their consulting ways, it was like, ‘How’s your week been? Have you had a good time? Did you do anything?’ then you had a 5-minute chat, only about basics like, you know, blah blah blah, and then slowly they brought in the smoking, you know, and what I was doing and the conversation. They was very good at what they done.
Male, aged 55–60 years, unemployed, heavy smoker, intervention
[The HT] was a brilliant help, [they were] fantastic to be honest.
Male, aged 45–50 years, unemployed, heavy smoker, intervention
The skill of the HTs in developing and maintaining good relationships with the participants was widely acknowledged among those interviewed. Advanced interpersonal skills were a high priority for the appointment of the HTs within the trial and they appeared to be effective:
Well I mean, there was never any question, you know, it was always like, ‘You know it’s down to you,’ you know, so no, I found it, you know . . . really quite good actually you know, I thought they were there was no question but lots of support I guess, and interest.
Male, aged 60–65 years, unemployed, moderate smoker, intervention
[The HT] said it was entirely up to me, what I was doing, and yeah, they were a good help, that’s all I can say, really. [The HT] was a good help.
Female, aged 50–55 years, employed full-time, moderate smoker, intervention, failed quitter
Oh totally, like my decisions, I wasn’t pressurised into it at all. I was encouraged and I was supported but I wasn’t pressurised.
Did you feel like you were in control of your decisions then about how much to cut down?
All the time, yeah, all the time.
Female, aged 60–65 years, unemployed, moderate smoker, intervention
Oh yeah, I was in control. I mean, I told [The HT] how many I was going to cut down a week or a day and they said, ‘OK, well that’s the goal you are going for.’
Male, aged 60–65 years, unemployed, moderate smoker, intervention, successful quitter
Oh no, not at all, [the HT] gave me some useful, like, suggestions and how to plan what I was going to do. But no, [the HT] was never like suggesting, ‘You are going to do this, you’re going to do that,’ no, never like that, no.
Female, aged 25–30 years, employed part-time, moderate smoker, intervention
Participants experienced a very client-centred style of delivery, one which was not threatening and placed them firmly in control of the decision-making process. There was also repeated evidence of people responding well to the strongly supportive and non-directive nature of the intervention delivery:
Well, no one likes people telling them what to do really, do they, unless it was discussed, so it was a study about me and my smoking habits then obviously it’s got to come from me and not someone else, ‘cause everyone’s different ain’t they with smoking and everything.
Female, aged 25–30 years, employed part-time, moderate smoker, intervention
It’s all down to the person but as I say, I’m not weak-minded and people can’t tell me to stop smoking but [the HT], the way they put things across to me, explained things, and, you know? They brought me down to earth in a nice way.
Male, aged 55–60 years, unemployed, heavy smoker, intervention
[The HT] was just, you know, not advising me but . . . [the HT] wasn’t even telling me, [the HT] was just saying, ‘You do what you think best,’ and ‘Do what you can do,’ they said. But they did say to me to get out a bit more because I never did go out very much. No, it was from me. They didn’t force it on to me, they just advised.
Female, aged 50–55 years, employed full-time, moderate smoker, intervention, failed quitter
Yeah, I mean, it was advice, it was good advice. [The HT] used to say, ‘We are not pushing you to stop smoking, but if you want to cut down, this is the ways you can do it.’ I had the forms each week down there each time like, and it was always good advice, it was always pleasant. There was no hard pressure, no hard sell.
Male, aged 55–60 years, employed full-time, heavy smoker, intervention
I wonder how well would the intervention have worked if it was a bit more directive?
Yeah I, yeah I probably wouldn’t have reacted you know in the same way, yeah. Because I think it’s something, it’s nice to talk to somebody one to one but at the end of the day it’s yourself that must be in control and be able to say, you know, ‘Well yeah, this is okayOK, I can do this,’ or, ‘I can do that,’ but what do you know sort of to be told that you should be do this, you should do that, because it’s up to each and every one of us to decide.
Female, aged 60–65 years, employed full-time, moderate smoker, intervention
Key to promoting ownership and supporting people in taking control of their own behaviour change is eliciting the individuals’ own solutions to problems. The HTs mostly prompted participants to reflect on behaviours and consequences, and identify solutions:
Yes I did, yes, and as I said you know you didn’t, they weren’t intrusive, they weren’t saying, ‘You must do this,’ or . . . they were helpful and just made suggestions, but it’s probably things that you would have thought about yourself but their suggestions were what you were thinking really, you know, the ones that reacted, but it was nice to know that they weren’t sort of saying, ‘Well you should do this, you should do that,’ you know, it was a one-to-one thing, it was very good.
Female, aged 60–65 years, employed full-time, moderate smoker, intervention
Support was voiced for the non-judgemental nature of the intervention, which was designed to avoid failure and always reframe any failures as learning experiences or opportunities for reflection:
The next fortnight that I saw [the HT], they said, ‘How did you get on?’ and I showed them the sheet and they said, ‘Well, you kept to your goal.’ And that was it. They didn’t tell me off a lot or belabour me or . . . I did try and keep to my goal [but] a couple of days I missed out, you know.
Male, aged 60–65 years, unemployed, moderate smoker, intervention, successful quitter
Yeah I think, you know, there wasn’t, I didn’t have any sort of great pressure or anything, you know, I mean there was no, ‘You are a bad person, you must quit,’ sort of thing or attitude if you know what I mean.
Male, aged 45–50 years, employed full-time, moderate smoker, intervention
One participant favourably compared the different approach with other support they have received in the past:
I wasn’t told, you know, ‘You should be doing, this you should be doing that,’ like when I have, when I’ve gone to other places to try and give up smoking [laughs] yeah, ordered to do things, you know what I mean, we just talked about things, talked about ideas.
Male, aged 40–45 years, employed part-time, very heavy smoker, intervention
Multiple behaviour change
Fundamental to the intervention structure was its aim of addressing two behaviours simultaneously, an approach that is not widely advocated in behaviour change literature, and in most cases avoided, for those wishing to quit. The complementary nature of the two behaviours (one a ‘stopping’ and one a ‘starting’ behaviour) meant that it was postulated that addressing the two behaviours alongside each other could be acceptable.
Resulting from the pre-pilot work and work with early intervention participants, the behaviours were introduced sequentially with smoking behaviour taking precedence over PA behaviours. How acceptable this was to participants and how much they engaged with the idea was of particular interest, especially how they viewed the utility of using PA to influence their smoking behaviour.
While it was difficult to explicitly elicit from participants how difficult or challenging they found it to try changing two behaviours simultaneously, there was qualitative evidence that participants actively engaged in the process of using PA as a way of managing and influencing their smoking habits:
I did make some changes, yeah, and when I do go out for a walk with the dog I don’t take my cigarettes with me and I could be out for hours, so I don’t bother taking my fags with me.
Female, aged 50–55 years, employed full-time, moderate smoker, intervention, failed quitter
Well, there was the exercise thing, getting out and being more active and stuff, which I am quite an active person anyway. I walk everywhere and you know, I am not a sitty-home person. I might want to do it once a week but I’m sort of like an active person anyway. Yeah, things like that helped me. You know, the gym helped.
Female, aged 35–40 years, employed part-time, moderate smoker, intervention
Physical activity was important in highlighting the negative health consequences of smoking and this appeared to change the way some people thought about their smoking habits:
As I say, doing exercises and that, you feel healthier and then obviously if you are doing exercises and silly things like that, you feel healthier and then when you go to put a cigarette in your mouth, it’s obviously psychological, you’re going, ‘Oh no, I am wasting myself going in the gym for that hour,’ if you know what I mean, ‘to go and have a cigarette now’.
Male, aged 55–60 years, unemployed, heavy smoker, intervention
There was also support for an ‘identity shift’ from a smoker to an exerciser, and the value attached to being active:
Yeah, well as I say, it did help and not only that, you see, getting confident was . . . in this gym, it was state-of-the-art, you know, I tell you if I was still smoking there is no way I could use some of those machines that I’ve been using, the rowing machine and the boxing machine thing. No way I could use those if I was smoking! I’d just be coughing my lungs up.
Male, aged 60–65 years, unemployed, moderate smoker, intervention, successful quitter
And I found out it’s a drug in a way ‘cause when you go to a gym you come back out you feel great for it and then smoking, you don’t even think about smoking ‘cause you’ve gone in there and you’ve done like an hour and a half workout, the last thing you want to do is come out and have a cigarette.
Female, aged 25–30 years, employed part-time, moderate smoker, intervention
Physical activity was an acceptable way for some of distracting themselves from smoking and coping with the cravings:
Erm, well it’s still difficult to cut down the cigarettes I’ve got to agree, but if I walk more then I don’t need to, you know, I don’t want to smoke, put it that way.
Female, aged 60–65 years, employed full-time, moderate smoker, intervention
Well, I get about a bit more now because I never used to get out very much but yeah, I get out a bit more now with the dog and that, and [the HT] said, you know, [the HT] said, ‘Go out walking and try and keep your mind off cigarettes,’ which I did do . . . Yeah, I take the dog out and I do things, you know? Definitely. Yeah, it works, most definitely works, yeah. It’s something there to stop me . . . yeah, to stop me from thinking about it, distraction, yeah.
Female, aged 50–55 years, employed full-time, moderate smoker, intervention, failed quitter
I’m doing my exercises three afternoons a week. And that takes my mind off the smoking. It takes your mind off of smoking actually, the exercise. It really does.
Male, aged 60–65 years, unemployed, moderate smoker, intervention, successful quitter
When [the HT] was trying to put across all the activity side, I found [that when] I was engrossed in doing something, which may have been whatever, I didn’t smoke so much [ . . .]. But then, if I wasn’t doing anything I was smoking.
Male, aged 60–65 years, employed full-time, heavy smoker, intervention, successful quitter
It did become clear that PA was not necessarily the primary way in which people would control their smoking behaviour. It was continually aligned with other ‘distraction’ activities which participants would engage in to manage their smoking behaviour. PA was, for many, just another way to keep busy:
Well I like walking so you know, I’d go out for a walk you know or something like that or, you know, read a book; anything to take your mind off it really, break the routine, ‘cause that’s what smoking is really.
Erm well, keeping myself busy basically, because if I am doing other things I am not smoking so much. I go swimming too you know, but first thing I do when I get up in the morning is have a cigarette you know, so if you can cut that one out you know a good part of the, it is a different sort of day so instead of, you know, having a cup of tea and having a cigarette I would do something for an hour or so.
Male, aged 60–65 years, unemployed, moderate smoker, intervention
Some participants did see more benefit in PA as a way of distracting themselves from smoking than other activities:
It’s more effective because if you’re sat down reading or anything you’ve still got that opportunity to think about a cigarette, whereas when I’m like huffing and puffing along to the aerobics the last thing I want to do is stick a cigarette in my mouth.
Female, aged 25–30 years, employed part-time, moderate smoker, intervention
In one case, the participant had made explicit links between PA and the potential for gaining weight while cutting down their smoking:
I made myself cut down but I haven’t put on any weight, you know, and I think that’s how I explain it [PA], you know, ‘cause some of my friends who have cut down smoking the first thing you hear about is putting on weight, putting on weight, putting on weight, and I haven’t, so I’ve cut down on my cigarettes – well, I have and I haven’t put on any weight whatsoever you know, nothing like that has changed at all.
Male, aged 40–45 years, employed part-time, very heavy smoker, intervention
On the whole, those interviewed found the idea of addressing behaviours simultaneously acceptable, to varying degrees. There were cases where the promotion of PA was not completely appropriate, and people failed to see how the two behaviours linked together. This was particularly the case if the participant already viewed himself or herself as being highly active.
No I didn’t you see, you know [the HT] was telling me about exercises and I mean I do dance and I do swim and I do walk you know this sort of thing, and I am a pretty sort of active person anyway to do an exercise to, ‘cause I think that was one of the sort of objectives of it to up your exercise to lessen your smoking, but with me that didn’t or wouldn’t have worked because if I’d really wanted a cigarette I’d have done my exercise and then had a cigarette, so [coughs] it didn’t stop me from smoking if you know what I mean, to do more exercise.
Female, aged 60–65 years, unemployed, moderate smoker, intervention
For one individual, PA may have reduced their desire for a cigarette while actually doing it, but heightened their desire for a cigarette afterwards:
I said to [the HT] you know, the truth of the matter is that whenever I actually have a cigarette, whenever I did physical exercise at the end of it I would sort of in some case smoke more [laughs] if that makes sense. It was sort of a reward you know.
OK, that’s interesting. So for you, you don’t find that sort of doing something active tends to lessen your desire for a cigarette then.
Well when I’m doing it, but not afterwards, no.
Male, aged 45–50 years, employed full-time, moderate smoker, intervention
Possible further adaptations
When invited to offer thoughts on possible adaptations to the intervention to help improve the experience or further support behaviour change, on the whole very little was suggested. Most participants interviewed were very satisfied with what they had received. As discussed earlier, the possibility for more intensive support over a longer time period was the most pervading suggestion.
One individual suggested that group support, for both smoking reduction and increasing PA, might be helpful:
I think the only thing I could suggest really is if you did it in a group format so you’ve got other people to keep up with as well.
Male, aged 60–65 years, unemployed, moderate smoker, intervention
One of those interviewed, who was a successful quitter, highlighted that they felt a lack of support, or perhaps interest, when they had actually made a quit attempt. Implying more dedicated support for those who do quit could be involved in the intervention. This was part of the intervention design, but was possibly not executed as well as it could have been:
So you want us to quit and when you do quit, there’s no form saying, How do you feel? That’s the only thing that I feel was wrong with it.
Male, aged 60–65 years, unemployed, moderate smoker, intervention, successful quitter
The idea of more monitoring and feedback was something that appealed to one participant:
I guess I quite like the, you know, being able to monitor how much you walked in a day and that sort of thing . . . more of that maybe, you know, more of the science.
OK that’s interesting. More of the science, more of the sort of numbers and figures and changes and things like that.
Yeah, absolutely.
Male, aged 45–50 years, employed full-time, moderate smoker, intervention
Similarly, another participant suggested the use of more informative materials, as well as materials for providing options of things that people could try, with information around the benefits, pros and cons of different activities:
I know like a lot of people are able to get out and about, like to be active, so when people think, ‘Oh,’ when we sort of, something about like your fitness people always assume like when the gym and everything . . . but there are other ways you can stop smoking so I think like giving them some suggestion about how to, ‘cause I think that would be a good thing.
OK, like a big list of options of different things that people could try something like that?
Yes, just like put next to it like the benefits you get from it, like a 10-minute walk, ‘This could do this for you,’ and then maybe just put in what smoking would do. Say like you done a 20-minute walk and then say you’ve got more oxygen in your lungs and something, and then give them a chart like at the end that, ‘If you have a cigarette this will come down by this much.’
Female, aged 25–30 years, employed part-time, moderate smoker, intervention
While this was something that was intentionally avoided in this study for fear of overloading participants with information and taking away a sense of control over their decisions, it would perhaps be worth exploring ways of introducing this in future research.
Dual role of the researcher/health trainer
We intended to maximise retention in the intervention and trial by streamlining recruitment, data collection and intervention delivery through contact with one HT/researcher throughout the trial. While this resulted in a less rigorous trial without the possibility of blinding outcome assessors, we wanted to assess the possible merits of this. The feedback was in favour of a dual dole and thus a single person to deliver the intervention and conduct assessments.
Two people doing different things, no, I think it would be better with just one person, yeah.
Female, aged 50–55 years, employed full-time, moderate smoker, intervention, failed quitter
I wouldn’t, yeah, I wouldn’t have liked that no, it was nice because the girls were very friendly and so no, I think the one person doing everything was better from my point of view.
Female, aged 60–65 years, unemployed, moderate smoker, intervention
Yeah, the first [HT] I met that was fine because I didn’t see them again [due to sickness] I saw another [HT], I didn’t, yeah I didn’t mind that but I think personally I would have liked to have seen the same person each time.
Female, aged 60–65 years, employed full-time, moderate smoker, intervention
No, I liked the thought that it was [the HT], you know, every week. I wouldn’t have . . . because I got used to [the HT], you know, do you know what I mean? I wouldn’t like the thought of seeing somebody new every week or anything like that. That was nice.
Female, aged 35–40 years, employed part-time, moderate smoker, intervention
Some participants went further, expressing why they felt it would be more productive to just have one person completing all the roles, expressing that some of the data collection involved quite personal aspects and this helped in building confidence and rapport in the relationship:
I don’t think it would have been fair, because obviously the questions could become a bit more personal to you instead of a whole general kind of thing. So I think the way that it was run like by the researcher and the intervention I think that was actually quite good.
Female, aged 25–30 years, employed part-time, moderate smoker, intervention
I am not sure because some of the questions are quite personal aren’t they on there, and it’s how you are feeling and things, you know, and I don’t know, I just liked it was the one person to do everything, you build up a bit of a rapport.
Male, aged 40–45 years, employed part-time, very heavy smoker, intervention
However, none of those interviewed had experienced research with an independent researcher and practitioner, so it is difficult to evaluate their comments when there is a lack of an experiential frame of reference.
Feedback from health trainers
Aims
Our aims were:
-
to assess acceptability and feasibility by capturing the experiences of the HTs in delivering the research and the intervention with a focus on what is working well/badly and what could be improved and identifying barriers to delivery
-
to capture the HTs understanding of the fundamental driving principles behind the intervention in terms of:
-
how the intervention is supposed to work (the process model)
-
participant engagement (i.e. client centred, non-judgemental, self-paced reduction) and
-
engagement of the HTs and participants with the idea of using PA to assist smoking-reduction approaches to eliciting change (i.e. specific BCTs and processes involved).
-
Methods
Design
Qualitative research was conducted using a basic thematic analysis of individual face-to-face interviews to elicit and describe the HTs’ experiences and views about delivering the intervention.
Sampling frame
An opportunity sample consisting of all three part-time HTs (with a dual role of also collecting data) employed in the study was used to maximise the diversity of opinions in the data. Interviews were conducted both early in the study (1–2 months after starting to deliver the intervention in the pilot trial, to capture experiences of the training course while they were still fresh in the HTs’ memories), and in the last 1–2 months of the 16-month pilot trial (to capture any changes in practice or opinion following extended experience of delivering the intervention).
Measures and procedure
Semi-structured, individual, face-to-face interviews were conducted within a few months of completing the training and at the end of the intervention period using topic guides developed by AT, CGVS and TT, designed to elicit data relating to the aims above. The first interview started with general questions about the HTs’ experiences of delivering the intervention and then asked specific questions about the training course, recruitment processes, intervention delivery (what was working well or badly) and the HTs’ understanding of the different intervention processes. The second interview (at the end of the intervention phase) asked about their ongoing experiences in delivering the intervention and how these might have changed since the initial interview. The interviews were digitally recorded and transcribed verbatim.
Analysis
The data were organised using a basic thematic analysis to provide a simple descriptive-level overview of the HTs’ views. In-depth qualitative analysis procedures were not used here. However, these data will be analysed in more depth alongside the participant interviews and consultation recordings, using framework analysis96 to generate an integrated analysis of processes of behaviour change in the EARS intervention. This in-depth analysis will be reported as part of the PhD of one of the researchers (TT).
Results
Feedback was organised under three main themes (training, recruitment and intervention, and trial delivery). A summary is provided below and a selection of quotes pertaining to each section is presented in Appendix 5a–c. Ideas for improvement in training, recruitment and the intervention are collated at the end of the Results section.
The training course (see Appendix 5)
Overall, the training was well received and valued in building the necessary skills for delivering both the research and the intervention. The training was an exploratory process to help formulate the intervention, drawing on the individual skills and experiences that the HTs brought to the training; they were all involved in some form of health promotion work in their other part-time employment. The developmental nature of the training gave the HTs an impression that the training process was somewhat disjointed, but equally one HT valued the opportunity to be able to contribute to the intervention development.
When asked ‘what worked well?’ the HTs identified practice sessions (with volunteer clients) and reflecting on recorded consultations, having an intervention manual, and getting formative feedback from a health psychologist as important. There were differences of opinion about how many practice sessions were useful, apparently based on the differences in individual learning styles.
One aspect that the HTs found particularly difficult was the initial assessment of their ‘instincts’ for the intervention (a simulated client interview in session 2 of the training). The intention was to allow the training team to adapt the training to the existing skill levels of the trainees. However, this was perceived to be challenging and disconcerting by the HTs but useful, nonetheless, for the research team.
The several months between the end of training and the start of intervention delivery, due to the logistics of taking occupancy of an office in an NHS facility and delays caused by co-ordinating the recruitment process, was a frustration. However, it was clear that the HTs’ confidence in using the techniques increased greatly with practice and their use of these became more ‘automatic’ as time went on.
Ideas for improvement in the training course included:
-
having more content for what to do beyond the initial motivation stage
-
having a longer session on motivational interviewing or client-centred approaches and techniques
-
practising the protocol for collecting the research data/baseline assessments
-
having a short self-reflection period built in at the end of each session
-
having the fidelity scale available from the outset to provide a steer on what was expected
-
having the trainers model the intended delivery style
-
providing a much clearer structure to the training to ‘put the cogs right in the correct order’
-
guidance on working with smokers with other substance misuse issues
-
including training on how to how to rein people in if they go off track
-
reinforcing the idea that a client-centred approach still allows the HT the opportunity, with the participant’s consent, to offer (or exchange) information
-
guidance on how to deal with people who are already active
-
including more supervision and formative feedback, particularly in the early stages of delivery.
These suggested changes will be very useful in designing the training course for future implementations of the EARS intervention.
Recruitment (see Appendix 5)
The HTs recognised the relative efficiency of recruiting participants through GP and SSS invitation letters after identifying smokers on surgery databases. There were minor initial teething problems in working with surgeries. In contrast, recruiting without an invitation letter from the community was much harder, with many of the strategies used producing few participants. There was a belief that approaching existing group leaders as advocates (rather than directly approaching individuals) could be more promising but that it may take more time to build relationships within the community. The study timeline may therefore have placed constraints on what could be achieved.
When asked ‘What attracted participants to the study?’, the HTs identified a desire to reduce smoking as the primary factor, along with an interest in the research itself and the idea of getting one-to-one support. All the HTs felt that potential participants were ‘not hearing the physical activity side of it’. Reasons reported for non-participation included having other priorities, a lack of time, illness or a lack of interest in reducing smoking.
Making contact with potential participants by telephone was reported to be acceptable, but there were mixed views from different HTs: one felt she was intruding into people’s lives ‘like a salesman’. All HTs reported that this process was time-consuming, as they often needed to make several calls to get hold of the participant.
The most difficult part of the recruitment process was the disappointment expressed by participants who were allocated to the control group. It was noted that increasing the study-completion payment later in the study might have helped with this. No major problems were reported regarding access, non-attendance or workload for participants after they were enrolled.
Suggestions for improvement included:
-
offering the intervention to controls at the end of the study to counteract the sense of disappointment (possibly in a group format or as a condensed version)
-
developing a strategy for what to say if someone else answers the telephone
-
not focusing on PA during the recruitment stage as it ‘sort of confuses people’
-
having a more prolonged engagement with workplaces, perhaps via occupational health professionals or existing public health team contacts, which could help recruitment.
Delivering the intervention (see Appendix 5)
The HTs reported no problems in using most of the intended intervention techniques, including exploring a typical day; encouraging self-monitoring (which was seen as particularly useful); problem solving; empathy-building/person-centred counselling; exploring importance (including pros and cons and using 1 to 10 scales); exploring confidence; using motivational interviewing techniques (including affirmation and reflective listening); reviewing progress; assessing existing smoking; offering alternative strategies for smoking reduction; setting realistic/SMART (Specific, Measurable, Attainable, Realistic, Time-referenced) goals (usually verbally, or with the HT writing them down) and making coping plans.
When asked ‘what worked well?’, the HTs identified regular contact, encouraging self-monitoring, MI (client-centred) techniques, pedometers and offering a choice of clear behavioural strategies for smoking reduction.
In terms of delivering the PA aspect of the intervention, the HTs felt that that most people were willing to try to do some PA. However, making the link between PA and smoking reduction was easier for some participants than others. The idea that PA could help to reduce cravings was not generally well understood or accepted. However, the idea that PA could provide a distraction was felt to be more useful/more easily accepted by participants. The HTs also tried to encourage people to do experiments to test the link, with mixed success. Other strategies were sometimes useful (e.g. focusing on the general health benefits, addressing misconceptions about what PA entails). The need for individually tailored strategies was highlighted by all three HTs:
Everybody’s very, very different aren’t they, sort of, receptive to different things.
Tailoring the intervention applied to assessing motivation, identifying and addressing barriers, trying to make the link between PA and smoking reduction and deciding which behavioural strategy (or mix of strategies) to use. One HT reported that making the link to PA was often easier for these participants, although it was not clear why this might be.
For the ‘harder-to-reach’ participants (e.g. those with higher levels of mental health problems or low literacy or analytic skills), flexible tailoring of the intervention seemed to be particularly important although this did not seem to diminish the chances of a successful intervention.
There were mixed views about delivering the process measures alongside intervention sessions (NB: this was also noted as being a potentially difficult process when reviewing the consultation recordings). Some HTs felt that this was not a problem, but others identified a ‘tension between the HT and the researcher role’.
All the HTs felt that the short timescale of the intervention (8 weeks) was a limitation and would have liked to have more flexibility to maintain contact with participants who were starting to make progress.
Encouraging engagement of social support (which was identified by the intervention fidelity analysis as largely lacking) was identified as being potentially problematic/provocative as not everyone had good sources of (positive) support. This was also identified as not being high on the agenda, perhaps reflecting a lack of emphasis on this during the training. Similarly, the issue of identity change was not considered to be a major element of the intervention process.
Overall, the HTs reported feeling very positive about their experiences in being part of the research study and delivering the intervention. They felt that this was a job they would enjoy doing and would apply for it again if it the opportunity arose.
Ideas for improvement included:
-
Consider using a solution-focused approach rather than a problem-solving approach.
-
Possibly encourage participants to take a longer-term personal appraisal of the benefits of PA, rather than any acute effects.
-
Include training on how to avoid or minimise dependence/reliance on the HT.
-
Use text reminders as a useful way to remind people about appointments.
-
Include training on how to deal with passive resistance (participants who avoid engagement by agreeing/going along with the HT, but then do not make the changes discussed).
-
There was a further suggestion that some type of debriefing supervision might be useful, where the HTs could discuss difficult cases or their own feelings about certain difficult clients.
-
Finally, there was also a suggestion from one HT that at the baseline interviews, there was a tension between the need to build empathy at this stage and the need not to engage therapeutically. This might result in some contamination of the control group (albeit quite low level).
The intervention at work: John’s story
This case study illustrates an individual who fully engaged with the intervention and was successful in changing both smoking and PA. The identity of the individual referred to is concealed through careful removal or modification of any information that may break this anonymity, while also maintaining a true story captured from taped sessions and reference to other data collected at the respective assessments. The case was selected to provide an example of how smoking reduction could be facilitated by PA, both implicitly and explicitly, and how a HT supported the process of changing two behaviours concurrently.
Participant description
John is a male aged between 55 and 65 years old who, at the start of the study, was unemployed and smoking around 20 roll-your-own cigarettes per day. He reported walking for around 1 hour per day, mainly first thing in the morning to go to the shops, and also to see friends who lived nearby. Walking was John’s main form of activity as he suffers from some health problems (joint related) that mean he finds it difficult to do much else. He once worked as a painter and decorator but had to stop because of these limitations. John looks after younger members of his family on one day of the week and has a partner who also smokes, but lives across town.
When he was younger, John used to take part in several types of activity, such as swimming and weight lifting, but had not done anything similar in many years.
John heard about the study via a letter invitation from the SSS and responded directly to the invitation. The invitation appealed to him thanks to the approach of cutting down, as he had experienced numerous attempts to quit abruptly over the previous years with varying degrees of success.
Early engagement with the intervention
Coming in to the first intervention session, John had completed a week of self-monitoring his smoking behaviour, recording when and how many he smoked. He found this activity to be particularly thought-provoking, highlighting patterns in his smoking behaviour he had not previously considered:
And with further exploration of perceived challenges for smoking reduction:
The HT gave John a chance to reflect on his smoking behaviour:
After discussion about techniques and approaches to cut down, John adapted his own way to approach cutting down, which did not exactly fit any of the four strategies the HT had identified. He broke his day down into morning, afternoon and evening and began thinking about which cigarettes would be easiest to cut down (hierarchical reduction) and times of the day he would not smoke (smoke-free periods), and talked about extending the time between when he smoked (scheduled reduction).
John lacked confidence to cut down the first three cigarettes in the morning, and recognised the strong association between his morning coffee and cigarettes. The morning cigarettes would be the last ones John would tackle, and the ones he was least confident about cutting out.
Early discussions about PA revolved around John’s walking habits. He did not smoke when he walked, but he had not made this connection, and there was little engagement in any idea to increase PA at this stage. The HT provided him with a pedometer and he agreed to monitor the step counts. Surprisingly, John raised the possibility of using a gym.
John left the first session with a goal, after the first three cigarettes in the morning, to extend the period between cigarettes to at least an hour and a half and cut out smoking in the evening. He also planned to obtain information about opportunities at the local sports centre.
Early progress
John returned to the next session having completed another week of self-monitoring and achieved his smoking reduction goals, having smoked only about 13 cigarettes a day.
I always buy 25 grams. I used to have, um, two packets but I cut down to one packet. Well, it’s only since I’ve been here with you, is that I have reduced my smoking.
John revealed he now had no intentions of using any kind of gym, despite the discussion of the first session, and was happy to continue walking:
But he expressed an interest in swimming:
Um, when the weather gets a bit more better, I go down to Devil’s Point [in the sea] and swim and it’s free.
With some prompted reflection on the use of the pedometer, the HT did well in reframing John’s perception that he wasn’t particularly active:
He set and discussed strategies and goals for how to cut down further, making plans on how to deal with the cigarettes early in the morning:
Well, one cup of coffee, one cigarette, and carry on . . . Yeah, so what I am going to do is just have one cup of coffee and then get in the shower a bit quicker, get into the bathroom a bit quicker.
When John came back for the next session, he had cut down to 11 cigarettes a day, and had bought only a 12.5-g packet of tobacco over the past week. He was still breaking his daily smoking routine down into morning, afternoon and evening blocks:
So this is Tuesday, four [morning], three [afternoon], four [evening].
A breakthrough was revealed at this point where he managed to smoke only two cigarettes first thing in the morning despite his early convictions these were the ones he enjoyed the most and would find the hardest:
I’d say you sort of get a jolt – ‘Oh, I could do with a cigarette!’ – and then if you can get over it you don’t think about it until the next jolt. But this morning it did! For my cups of coffee in the morning. So I had two [cigarettes].
From here John’s confidence changed as he began to break habits he thought he would not be able to. Later, though, he discussed a day when he had smoked three cigarettes immediately in the morning, and he expressed guilt:
I felt . . . well, I enjoyed them, I have got to admit. I enjoyed them but I felt I’d let myself down.
The change in the way John appraised his smoking behaviour was starting to shift. He had continued his walking routines, and made decisions to try and increase his walking:
Yeah. I mean, instead of going [a] library, I suppose I could walk up to [a] library [further away] . . . I suppose I could do that, you can just use the same card.
Every week, John would take diary sheets from the HT to record his smoking and his pedometer steps (which he would often have to remind the HT to get for him):
The goal setting and self-monitoring complemented each other well for John, and reviewing the records he had kept at the beginning of each session allowed the HT to focus on achievements and explore reasons for any setbacks:
John’s desire to take on any more activity was limited, but with some skilful probing by the HT he reflected on when he had quit smoking or reduced, and had been able to be more active and feel healthier:
Changes in confidence/importance
By week 5 of the intervention John had cut down to about seven cigarettes a day, and was rolling thinner ones. In the mornings he had cut down to simply one cigarette with his morning coffee. When asked what he would like to achieve over the next 4 weeks he revealed that he would like to stop completely, but expressed concern about gaining ‘about three-quarters of a stone at the moment’.
At this point the HT identified opportunities to revisit the link between PA and smoking again:
Without any explicit prompting from the HT, at the next session, John revealed that he had attended the local swimming pool:
The support and interest from the HT, and greater awareness of improving health, appeared to give John confidence to try activities he had done in the past.
At the following session (6 weeks after baseline), John made the decision that he would like to quit, triggered by a health warning:
Sunday, we run for a bus you know . . . and we run for the bus and I was coughing and spluttering and I said, ‘That’s it, I’m quitting from tomorrow.’ I was really coughing and spluttering, and that was just running for a bus. [He did not smoke again that day].
This prompted him to discuss options for quitting with the HT, and John was one of the few participants who took up support from the SSS:
John did engage with the SSS and made it successfully to 4 weeks post quit without smoking. He did not use any NRT prior to quitting but did with the SSS. While being referred to the SSS, an opportunity came up to join a local gym as part of a local health initiative. In confirming how far John’s confidence had changed and progressed, he joined along with others:
So I said, ‘A couple of us, us and myself, would you take me on?’ And they said, ‘We don’t advertise it but yes, you can join, two pounds a month.’ And I said, ‘I’ll have some of that!’ So I go three times a week now, three mornings a week, and I’m doing my exercises three afternoons a week. And that takes my mind off the smoking.
The use of the gym and the new exercise routine adopted by John helped him to reinforce his identity of a non-smoker:
Yeah, well as I say, it did help and not only that, you see, getting confident was . . . in this gym, it was state-of-the-art, you know, I tell you if I was still smoking there is no way I could use some of those machines that I’ve been using, the rowing machine and the boxing machine thing. No way I could use those if I was smoking! I’d just be coughing my lungs up.
John also found a sense of relatedness and companionship through starting exercise classes, which would have supported his identity shift:
But I must admit, it’s going to these classes with other people, it gives you a goal, a dream, to get fitter and fitter!
The change was further emphasised by John going out and buying his own exercise equipment for use in his home:
Yeah. I’d been down to Argos to buy one of these blow-up balls where you put your back on it and do sit-ups, and I bought some of these weights that you can alter. It’s doing something.
And towards the end, John explicitly made the link between smoking and exercise:
When asked if the two behaviours worked for him, he responded very positively:
They fit together very well, you know. Because I mean, I’ve always swum in the sea all my life but as I say, I’ve been smoking for 51 years so yeah. To be honest with you, it’s habit. I find myself now going to get a cigarette and I think, oh, flippin’ heck, I don’t smoke! And it’s just habit, it’s 51 years of habit.
Although it was a struggle for John to engage with the activity side of the intervention at the beginning, he voiced strong support for the effect it did have:
Well no, I do two miles every morning and I have done for ages, for years, I walk two miles every morning but now I am doing it in half the time since I’ve been going to the gym. But I would never have thought of going to a gym or these exercises, pilates they call it? Pilates classes, I wouldn’t be doing any of that, I would never have thought of doing it, until I got involved with you people, and this healthy heart thing.
And not only that, but after engaging in PA John reported weight loss:
And finally, the change in habit and desire was perhaps best illustrated in the following lines:
And I get that urge now, not as strong at all as I used to. I mean, I don’t wake up in the morning and get my coffee and think I need a fag. I just don’t do it any more.
Reflection
In a case study such as John’s, one is never sure if such a change would have occurred without the intervention. Nevertheless, the story portrays the person’s priorities for smoking reduction, and strategies used, and the limited initial success with introducing PA. By promoting thoughts about the link between PA and smoking John appeared to leave the sessions with ideas to think about and develop on his own. The client-centred HT support is also identified, and this helped to build John’s motivation and confidence to reduce smoking and find and enjoy PA behaviour within a different personal identity.
Chapter summary
The overall aims of the qualitative work were to capture as much information, from participants and the vicarious and personal experiences of HTs, about the acceptability and feasibility of the trial methods and intervention delivered in this pilot study to inform a larger study.
Acceptability of the trial methods (across trial arms)
Overall the trial methods were acceptable for the participants (in both arms of the trial) and the HTs largely endorsed the procedures.
Recruitment through mailed invitation was the preferred recruitment method by the HTs as it was less time intensive, and was well received and understood by interviewed participants.
There was scope for a refinement of a few questions in the assessments, but overall the data collection was largely acceptable across both arms.
While there was support from the participants for a dual role of the researcher and HT (with little or no experience of other procedures), the HTs found the dual role to be challenging at times, and possibly detrimental to intervention delivery. In appraising intervention fidelity during recorded sessions, a noticeable change in session dynamics occurred, which seemed to interrupt any therapeutic relationship with participants receiving the intervention. Specifically, a tension was identified between the patient-centred style of intervention and the more rigid structure of questioning associated with the researcher role.
A limitation is that we know less about the views of those participants who withdrew from the study or were unable to be contacted for interview.
Acceptability of the intervention and possible adaptations
On the whole, the intervention, offered in a central NHS facility (within 1 mile of most participants’ residences), was acceptable. The HTs did suggest that other locations may target specific hard-to-reach groups (e.g. single parents), but this may introduce contamination across trial arms if several people who were closely acquainted came into the study together and were randomised to different treatment arms.
The intensity of the intervention and type of support being offered was very well received by those engaging in it. Telephone support was shown to be particularly valued as a flexible option, but the quality of a session may have been interrupted if the call was made in undesirable locations. Strong support was found for the client-centred approach for engaging with those who otherwise may have been more service resistant.
An important adaptation which emerged from both the HTs and the participants interviewed is that extending the duration of the intervention could be effective in producing further behaviour change.
A number of suggestions were made for improving the training, intervention and trial delivery procedures.
What were the perceived effective components of the intervention?
Across both behaviours, self-monitoring and individual tailoring of techniques to the individual’s circumstances and preference were frequently reported as being the most effective tools for promoting and eliciting change.
In Nicotine Assisted Reduction then Stop (NARS) studies,15 a reduction strategy is rigidly adopted in conjunction with NRT use. In EARS we introduced the different reduction strategies to enable participants to choose how to reduce and in time to use PA to support this reduction.
Support to reduce smoking and the promotion of different behavioural strategies for cutting down appeared to be one of the most effective components of the intervention. Although people may not have engaged with the reduction strategies precisely as they were intended, and they were not specifically prescribed to people, they took what meaning they could from the strategies and applied it to their own circumstances.
There was a strong focus on smoking reduction over PA promotion, which was reflected in all three components of this qualitative work. The intervention was primarily promoted as a smoking reduction study to avoid recruiting only those interested in PA and exercise. There was evidence that PA and smoking behaviour were not always linked together in the way that was envisaged, and the HTs did find this difficult for some participants, especially those who were already physically active. This will be explored in greater depth outside this report. Quite often, the HTs expressed difficulty in promoting PA when participants’ main motivations were to cut down smoking, at least initially. In terms of the fidelity scores, increasing motivation for smoking change was greater than for PA and linking PA and smoking also had a low fidelity rating. PA was outweighed by a focus on smoking behaviour, but was one of the many ways participants kept themselves busy to distract themselves from smoking. For some participants, a failure to support an increase in PA may not have resulted in changes in smoking, but for many this was not the case.
Did the intervention delivered match that described in the intervention manual (i.e. treatment fidelity)?
Intervention fidelity was examined and deemed to be acceptable overall in the context of a pilot study. The intervention fidelity scores for the different process elements indicated a need to modify the training course to (a) increase the emphasis on identification and management of social influences, (b) sensitise the HTs to recognise and reinforce shifts in identity and (c) to reinforce techniques for introducing and integrating PA more into the intervention process.
The examination of intervention fidelity was facilitated by the development of a clear process model (see Table 1) and was useful in highlighting specific areas where the intervention training could be improved. However, a limitation is that we were not able to formally test the inter-rater reliability or validity of the intervention fidelity checklist. The existing data could be used to do this, but further resources and time would be required. An additional limitation was the fidelity measure’s limited scope in for judging the style and process of engagement, which other data revealed the participants were very pleased with. A valid and reliable measure of intervention fidelity would be very useful for both training and quality assurance purposes if the EARS intervention is used in future projects or implemented more widely.
What can we learn from a case study?
The case study highlighted the issues surrounding the promotion of PA to support reduction and eventually cessation. This example shows that the effect of PA on an individual’s smoking behaviour may be unpredictable but can be complementary to an attempt to reduce and then quit smoking. For this individual PA was simply a distraction technique to begin with but grew to represent a shift in the participant’s identity away from that of a smoker. The use of this and other case studies would help in future training to help understand how this subtle process can be supported.
Chapter 6 Economic analysis
Introduction
In this chapter we present preliminary research on the estimation of intervention costs, economic outcomes, and the development of (an analytical framework) for future cost-effectiveness analyses (CEAs). The research on aspects of the economic analysis are undertaken and presented here to support further development of, and research on, the EARS intervention. The primary questions most relevant for the economic analysis are:
-
What is the estimated resource use and cost associated with the EARS intervention?
-
What is the estimated mean cost of EARS per participant?
-
What type of framework is best suited to the assessment of cost-effectiveness of EARS in a full future economic evaluation alongside a trial?
-
What recommendations to future research in economic evaluation can be made on the basis of the pilot trial results?
In the following sections we consider each of these above areas of research.
Estimating the resource use and subsequent cost of the Exercise Assisted Reduction then Stop intervention
The EARS intervention has been described in detail in previous sections of this report (see Chapter 1). Within the pilot RCT, and exploratory research alongside the trial, data have been collected to inform on the resource use associated with delivery of the intervention, compared with brief advice (control).
The main components of resource use for EARS intervention are HT time, supervisory time input (supervision of HT) from more senior/experienced staff, consumables (e.g. exercise aids), subsidies for PA related opportunities, intervention-related resources required for training of HTs in the delivery of the EARS intervention, and the ongoing costs associated with recruitment of participants (clients) for the EARS intervention. These main components were determined by earlier development work on the format and structure of the EARS intervention and its delivery. A further area of potential resource use, although uncertain, is the use of the NHS SSS and other smoking-cessation aids.
Resource use data collection on Exercise Assisted Reduction then Stop delivery
Health trainer time input to Exercise Assisted Reduction then Stop
Health trainers are the primary delivery point for the EARS intervention. For the current analyses, and based on experience within development research, and within the pilot RCT, HTs on delivery of EARS are expected to be employed on Agenda for Change Band 497 (salary mid point assumed for base-case analyses). It is assumed here that the EARS intervention, delivered by HTs, would be implemented in a way that placed EARS within a broader provider environment, where EARS was one of the interventions available, for example integrated with other NHS SSSs or health promotion services.
Health trainer time input is for specific EARS-related contact time, participant-level contacts (e.g. face-to-face meetings, telephone contacts), participant-related non-contact time/activities (e.g. planning, organising and preparation for participant contact), supervision time, and other more general activities related to recruitment of caseload and service development. Within the pilot trial, electronic records were kept by HTs to record the duration of each contact as well as the frequency of administrative contacts specific to participants (e.g. email, text messaging, written letter). HTs were also asked to record those who did not attend, and the uptake of financial subsidies. To assess the level of participant-related HT time for non-contact activities, a work-sampling approach can be used. 98 In the pilot trial a work-sampling form was developed, in consultation and with design input from the trial co-ordinator and the three HTs employed on the trial, in order to improve ease of use and acceptability. The work-sampling form was piloted and tested over a 2-week period in the trial (completed by two of the three HTs); HTs recorded the number of minutes they spent on each of the specified areas of work activity over the days worked in the data collection period (see Appendix 9 for the form used).
In order to assess the level of HT time unrelated to intervention delivery (e.g. recruitment activity, service development), activity logs for trial recruitment activities and training activity were reported/recorded by the trial co-ordinator.
Other resource use in Exercise Assisted Reduction then Stop intervention
Data on supervisory time input, assumed to be from more senior staff (Agenda for Change Band 6), exercise aids, and resources required for training of HTs in the EARS approach, were recorded by the trial co-ordinator (trial team).
Estimated costs associated with delivery of Exercise Assisted Reduction then Stop intervention
Data on units of resource use associated with the EARS intervention are combined with unit costs, or cost estimates, in order to estimate the mean participant-level costs for the EARS intervention. For base-case analyses an assumption is made on annual caseload per HT (EARS), at 250 participants, assuming that a participant is allocated to an EARS HT for approximately 8 weeks (see Appendix 9). This assumption is based on experience within the pilot trial, and estimates from the trial team.
Table 33 reports the summary detail associated with resource use for delivery or the EARS intervention. Using these data (see Appendix 9 for further details) results in an estimate of cost at £192.17 per person entering the EARS intervention/service.
| Resource use (type) | Mean units of resource per participant | Unit cost (£) | Mean cost (£) |
|---|---|---|---|
| Traininga | NA | 6.12 | |
| Recruitment (assuming 1.25 hours’ HT time per participant)a | 75 minutes | 27 per hourb | 33.75 |
| Intervention deliverya | |||
| HT to participant contact time | 136 minutesc | ||
| HT to participant-related non-contact time (assuming 1 : 1 ratio of contact–non contact time) | 136 minutesc | ||
| Total estimated participant-level HT time | 272 minutes | 27 per hourb | 122.40 |
| HT supervision (by HT manager/locality lead trainer) | 10 minutesd | 38 per houre | 6.33 |
| HT supervision (HT time) | 10 minutesd | 27 per hourb | 4.50 |
| Exercise aidsf | NA | 19.07 | |
| Estimated total mean cost | 192.17 | ||
Sensitivity analysis (Exercise Assisted Reduction then Stop intervention cost)
Where allowance is made for difference in use of the NHS SSS, and pharmacotherapies, assuming that 50% of those making a quit attempt use these interventions (see Appendix 9 for detail/assumptions) the estimate of the EARS intervention cost is £242.62 per participant (i.e. an increase of £50.45 per participant). However, there is no strong evidence in the pilot trial of a difference (significant difference) in use of services, with two EARS participants and no controls reporting use of services/interventions. A future full RCT will further inform this point.
Limitations with estimate of Exercise Assisted Reduction then Stop intervention cost
Data on resource use are from the pilot RCT, and subject to uncertainty. Data collection methods via HT electronic records have successfully recorded specific contact activity of HTs at a participant level. However, in the pilot trial, while the methods for work sampling were developed (for future use), the data collection was limited. Therefore, work-sampling data, although indicating that a significant proportion of time is required for non-contact and general activities, do not accurately report on the magnitude of time to allocate to the EARS intervention. Training logs have been used, together with the records/input of the trial co-ordinator, to estimate the resource use for training, assuming a caseload of 250 participants per year per full-time HT; however, there is uncertainty in this estimate. Of note, the training cost per participant is a small component (∼3%) of the estimated intervention cost, and therefore the level of uncertainty introduced here is not likely to impact on any findings from this exploratory research.
Framework for estimating the cost-effectiveness of the Exercise Assisted Reduction then Stop intervention
Introduction
In this section we present a narrative summary of key published studies informing on modelling methods for CEAs in a smoking-cessation context.
Evidence review: decision-analytic models for use in cost-effectiveness analyses on smoking cessation (Exercise Assisted Reduction then Stop-type intervention)
To inform on the framework for future CEAs for the EARS intervention, as part of a full RCT with economic evaluation, here we set out a general overview of the current literature reporting on modelling methods used in the context of CEAs in this area (smoking cessation).
The aim of this review is to provide an outline of the key studies in this area, to inform the structure and development of a modelling framework suitable for assessing the cost-effectiveness of the EARS intervention, and to inform on data required to populate such a model.
A recent review by Bolin99 has reported on economic evaluations for smoking-cessation interventions, providing helpful insights on modelling methods used to assess cost-effectiveness. An earlier review by Woolacott et al. ,100 undertaken as part of a study assessing NRT, is also helpful in outlining methods used to model smoking cessation. A number of other prominent HTA studies have also reported on modelling methods for smoking cessation. 15,101 Here we present a summary of these reviews/studies. In addition, to supplement the insights from these studies, we have undertaken a literature search (from 2002 to September 2012) to identify any further models in smoking cessation published in the cost-effectiveness literature (see Appendix 9 for detail on the literature search strategy). We identify 11 studies that we set out in this evidence review (see Table 34), nine of which are discussed in summary detail.
Summary of reviews
Woolacott et al. 100 present a narrative review of fifteen economic evaluations (published 1986–2001) of smoking cessation. Included studies considered pharmacological aids delivered as part of face-to-face interventions, that is to say in addition to advice or counselling. The review of methods used in the included studies (n = 15) offers guidance in terms of potential data requirements (and sources); however, recommendations by the authors do not extend to modelling methods. The review highlights some of the salient issues associated with undertaking economic evaluation in this field, particularly challenges and limitations in the data available to inform CEAs studies.
Bolin99 documents a formal systematic review that identifies 30 studies (identified from 1995) using a mathematical modelling framework, applying simulation techniques, to assess smoking-cessation therapies. Most of the identified studies used a Markov-type modelling approach, and most were cohort-based models rather than individual-level simulation models. The review charts the development of the included mathematical models chronologically. Particular emphasis is given to a widely adapted model, the Benefits of Smoking Cessation on Outcomes (BENESCO) model,102 which was used in 10 of the 30 modelling studies identified. The BENESCO model is used by Bolin et al. 99 as a point of reference to frame the review of the studies identified, with commentary presented/organised around central considerations of ‘model structure’, ‘data’ used to drive and to populate the model, and on the approach taken towards assessing ‘uncertainty/consistency’. The critique of models offered by Bolin et al. 99 concludes that, while the modelling methods published to date are reasonable, there is scope for improvement. The suggestion is that a more complex modelling framework (than that currently reported) may be helpful, and that models could consider heterogeneity in more detail.
A further review from Ronckers et al. 103 was identified as potentially useful. This review considered the results from 14 model-based economic evaluations, reanalysing cost-effectiveness ratios, by applying standardised methods for estimating costs and effects. Ronckers et al. 103 do not offer commentary on modelling methods (in terms of model type and structure) and so this study is not considered further here.
Summary of model-based cost-effectiveness analysis studies in smoking cessation
Table 34 presents summary detail on 11 studies using modelling methods in CEAs for smoking-cessation interventions. Here we provide an outline on nine of these studies. Further detail is provided in Appendix 9d. The models by Feenstra et al. 104 and Hurley and Matthews105 are not discussed further here, given the particular perspective in these studies, that is to say from a Dutch and Australian perspective, respectively, and their focus on national evaluations. The BENESCO model, presented by Howard et al. 102 has been used in multiple settings and populations and is presented, albeit in slightly different forms, in other papers. Given that these papers document the same underlying model, the focus in the current review is on the original paper.
| Study (country/setting) | Year | Perspective | Intervention(s) considered | Modelling (evaluation) methods | Summary measure of cost-effectiveness |
|---|---|---|---|---|---|
| Fiscella and Franks106 (UK) | 1996 | US payer | Transdermal NRT, counselling alone | Decision tree (stochastic) | Cost per QALY |
| Orme et al.107 (UK) (HECOS model) | 2001 | UK NHS | Pharmacological, GP advice, group therapy, willpower alone | Cohort-level Markov (deterministic) | Cost/LYS and cost per death averted and costs, LYS and deaths averted are presented in disaggregated form (cost consequence) |
| Woolacott et al.100 (UK) | 2002 | England and Wales NHS | NRT, bupropion vs. advice | Decision tree (deterministic) | Cost per LYS, cost per QALY |
| Godfrey et al.108 (UK) | 2005 | English NHS | Actual practice: different service configurations, staffing, interventions | Decision tree (deterministic) and uses the model by Orme – cohort-level Markov (deterministic) to estimate cost saving of smoking-related disease | Cost per LYS |
| Feenstra et al.104 (Netherlands) | 2005 | Third-party payer | Five face-to-face smoking-cessation interventions including current practice | Cohort-level Markov continuous time, (deterministic) | Cost per 1000 quitters |
| Hurley et al.105 (Australia) | 2007 | Australian health care | National tobacco campaign, no campaign | Cohort-level Markov (deterministic) | Cost savings and LYG (dominance of intervention) |
| Howard et al.102 (USA/multicountry) (BENESCO model) | 2008 | US health care | Varenicline vs. US pharmacological aids | Cohort-level Markov (deterministic) | Cost per QALY |
| NICE109 (UK) | 2008 | UK NHS | Brief intervention and referral for smoking cessation | Decision tree (deterministic) | Cost per quitter |
| Wang et al.15 (UK) | 2008 | NHS/Personal and Social Services | Cut down to quit (CDTQ) with NRT | Decision tree (deterministic), final QALY outcome using continuous analytical methods | Cost per QALY |
| Coleman et al.101 (UK) | 2010 | UK NHS | NRT, bupropion, varenicline, no intervention | Cohort-level Markov (deterministic) | Cost per QALY |
| Bauld et al.110 (UK) | 2011 | UK NHS | Pharmacy and group-based counselling, no intervention/self-help | Trial outcomes: decision tree (deterministic) alongside trial. Final outcomes: Markov extrapolation for estimation of final outcomes | Cost per QALY, cost per additional quitter |
Fiscella and Franks106
Fiscella and Franks106 undertake CEA on NRT (patches) using a decision tree model, populated with effectiveness data from a meta-analysis of published RCTs. This study considers mortality by smoking status, using observational data from Doll et al. 111 on mortality rates for British doctors. The analysis applies health-state values (from the US National Health Interview survey) to estimate quality-adjusted life-years (QALYs). This early model considers the impact of abstinence on smoking status by estimating the rates of mortality among former smokers and using these estimated rates with those of current smokers (from national data) to determine life expectancy by the age and sex profile of interest. Both life extension (in terms of life-years gained) and the QALY gains associated with abstinence are considered.
The model presents its results in terms of age and sex, and the base-case results presented account for both background rate of quit (2.5% per annum) and lifetime probability of relapse (35%).
Woolacott et al.100
Woolacott et al. 100 use the same methods as Fiscella and Franks106 to determine the benefits relating to quitting (i.e. LYGs from Doll et al. 111 and the health-related quality of life values reported by Fiscella and Franks106). This study evaluates the cost-effectiveness of NRT and bupropion sustained release versus advice in the England and Wales NHS, using a simple decision-tree framework to model outcomes and associated costs. The options considered in the model comprise advice alone, counselling alone, advice plus NRT, advice plus bupropion sustained release, and advice plus NRT plus bupropion sustained release.
The modelled effectiveness outcome for each intervention was the 12-month continuous quit rate. The approach for estimating the relative effectiveness of each intervention was to apply the odds ratio (OR) of the intervention relative to the control rate for advice or counselling alone. In the main, ORs were estimated using appropriate, high-quality methods of evidence synthesis including meta-analysis of RCT data, statistical pooling and adjusted indirect comparisons. The authors include all relevant costs associated with the intervention, which are presented with units of resource use. The methods used for converting to life-year and QALY gains are similar to those of Fiscella and Franks. 106
Health and Economic Consequences of Smoking model, Orme et al.107
Orme et al. 107 present the Health and Economic Consequences of Smoking (HECOS) model developed for WHO. It is a Markov model estimating the costs and consequences (QALYs) associated with a quit attempt over time. The model uses a hypothetical cohort of the UK smoking population, moving through annual cycles, with their costs and QALYs model using health states by smoking status (current smoker, recent quitter, long-term quitter), a long term quitter being defined as someone who has been abstinent from smoking for more than 1 year. The model uses a lifetime time horizon, and applies the relative risk of five main smoking diseases [chronic obstructive pulmonary disease (COPD), asthma, coronary heart disease (CHD), stroke, and lung cancer] to calculate smoking-related mortality. 112 The model uses a set of equations to model smoking-related mortality, and death from non-smoking related causes are not considered in the model.
Godfrey et al.108
Godfrey et al. 108 report CEAs on NHS SSS, post implementation, considering how variation in process measures (e.g. different service configurations, staffing, intensity of interventions) impact on cost per client, cost per life-year gained and cost per life-year saved. The focus of the analyses is on cost and resource use at the level of the individual service. Participant-level costs (in terms of cost per person setting a quit date and cost per life-year gained) are estimated from aggregate data using regression methods.
The analysis is primarily based on survey data on the cost of various SSS providers, with data from 58 out of 92 SSSs and outcomes (biochemically validated 4-week cessation rates) from routine Department of Health monitoring. Data from Doll et al. 113 are used to determine the gains due to life extension of a long-term quitter at 4 weeks. Interestingly, Godfrey et al. 108 make use of the HECOS epidemiological model107 to calculate the future health-care savings attributable to cessation. The authors conduct regression analyses (using simple ordinary least squares methods) to examine the impact of deprivation levels on cost per client setting a quit date and on the cost-effectiveness ratio (cost per life-year saved).
Howard et al.102
Howard et al. 102 use a cohort-level Markov model structure with annual cycles to simulate the lifetime costs and consequences of a one-time course of varenicline versus existing smoking-cessation strategies (bupropion, NRT and unaided quitting) from a US payer perspective. The resulting BENESCO model (which is based on the HECOS model107) is currently one of the most frequently adapted models for other populations and settings. 114 The model simulates the movement of a hypothetical cohort of male or female smokers aged 18–34, 35–64 and 65+ and cost-effectiveness is estimated over a range of potential time horizons (2, 5, 10 and 20 years) in addition to lifetime. The model uses epidemiological data and hazard ratios for smokers and non-smokers from the Cancer Prevention study II trial112 to determine smoking-attributable morbidity and mortality (determined as a proportion of total population morbidity/mortality). Disease events are mutually exclusive in any given year (i.e. the model allows only one diagnosis per year), but relevant comorbid states and recurrent events (stroke, CHD) are allowed. The published study (to our knowledge) does not provide technical detail on how the modelling framework is operationalised.
Health-state values (QALY weights) for the non-morbid health states are taken from Fiscella and Franks;106 however, disease-specific health-state values are used to calculate the QALYS associated with years of life in each of the morbid health states (COPD, asthma, CHD, stroke, and lung cancer). Only direct medical costs relating to the intervention were included in the model.
Unlike the HECOS model, which was restricted to deterministic sensitivity analysis, the BENESCO model was set out and populated with data to run in a probabilistic way (to consider uncertainty in parameter inputs simultaneously). Later studies applying the BENESCO model to different settings include an update of the US BENESCO model to account for an extended course of treatment. 115
National Health and Care Excellence model, to inform National Health and Care Excellence Public Health Guidance109
The model employed/presented by the National Institute for Health and Care Excellence (NICE)109 employed a decision-tree approach to evaluate brief interventions in smoking referral. The model is used by NICE to evaluate the cost-effectiveness of opportunistic brief advice by GPs and brief (30-minute) nurse-led interventions both in primary care and in hospitals. Both interventions were considered as stand-alone interventions with the option of subsequent referral to secondary smoking services. The perspective of the analysis is NHS and Personal and Social Services and the model adopts a lifetime time horizon. The decision-tree model is specifically set out to consider an initial 12-month model cycle, after with the model adopts the approach used by Fiscella and Franks106 and Godfrey et al. 108 to estimate life expectancy using data from Doll,111,113 and QALYs using data from an unpublished survey of 15,000 smokers (obtained via administration of the EQ-5D).
The initial 12-month model cycle, including modelling of the probability of successful quit at 12 months, is based on an evidence synopsis116 and effectiveness data (assumptions) that are distinct from the modelling of outcomes thereafter. The model uses effectiveness data on interventions considered, and it applies/assumes a background quit rate of 1% in the model. The authors note that relapse rates beyond 12 months were approximated using 8-year follow-up data of participants in a UK-based RCT of the nicotine patch. 117
The model is run for different age- and sex-specific cohorts/analyses (aged 30, 40, 50 and 60 years). Resource units and costs (NHS and Personal and Social Services perspective, price year 2004–5118) relevant to the delivery of the intervention are documented fully; an additional sensitivity analysis considers the discounted cost savings due to smoking related morbidity using data from Godfrey et al. 108 Extensive univariate sensitivity analysis of key parameters is undertaken, including variation by effectiveness rates, background quit rate and the length of intervention. In addition, the authors discuss how contextual factors, that is to say level of tobacco dependence, previous exposure to brief interventions, and socio-economic group, might affect projected incremental cost-effectiveness ratios (ICERs).
Wang et al.15
Wang et al. 15 use a decision-tree modelling framework to consider a range of interventional questions involving multiple scenarios, and different levels of decision-maker. The model estimates the probability that a smoker/population will reach 12 months’ continuous abstinence if NRT is offered within a ‘cut down to quit’ programme using a deterministic decision tree. Wang et al. 15 model the impact of cutting down with NRT versus an abrupt quit. The ‘full’ model developed by Wang et al. 15 allowed a simulated cohort of smokers to follow different treatment pathways prior to an ‘abrupt’ quit or ‘cut down’ attempt and provides the most complete representation of the decision problem in policy terms. Modelled pathways include NRT over the counter, prescription NRT and smokers’ clinic NRT. The model framework considers the staging of different therapies. The model uses data from Doll111 to predict life expectancy (mortality) over time.
This study extrapolates modelled outcomes to final (lifetime) outcomes by considering two components of QALY gained: those gained due to abstinence (assumed to be realised immediately), and those gained due to extension in life (considered to be at age 65). In the latter case, years of life saved is estimated by applying an arbitrary ‘socio-economic’ adjustment (subtracting 2 years from the life-years gained) projections of Doll111 to correct for deprivation indices. The two components – abstinence and life extension – are then summed to form the total QALY gain. Wang et al. 15 therefore consider quality of life over the life course.
Coleman et al.101
The HTA report presented by Coleman et al. 101 uses a Markov modelling approach, with cohort-level analyses, to examine the cost-effectiveness of relapse prevention initiatives in smoking cessation. The perspective of the model was lifetime, arranged in 6-month model cycles. The risk of smoking-related disease is calculated for current, never and former smokers using the observational data from Doll et al. 111 The epidemiologically driven model derives ratios for mortality rates (by smoking status) and uses prevalence data/rates to attribute/decompose standard population mortality rates by smoking (Figure 7).
FIGURE 7.
Example of cohort-level simulation (simple schematic) used by Coleman et al. 101 Status by age and sex category. CHD, chronic heart disease; COPD, chronic obstructive pulmonary disease; LC, lung cancer; MI, myocardial infarction.
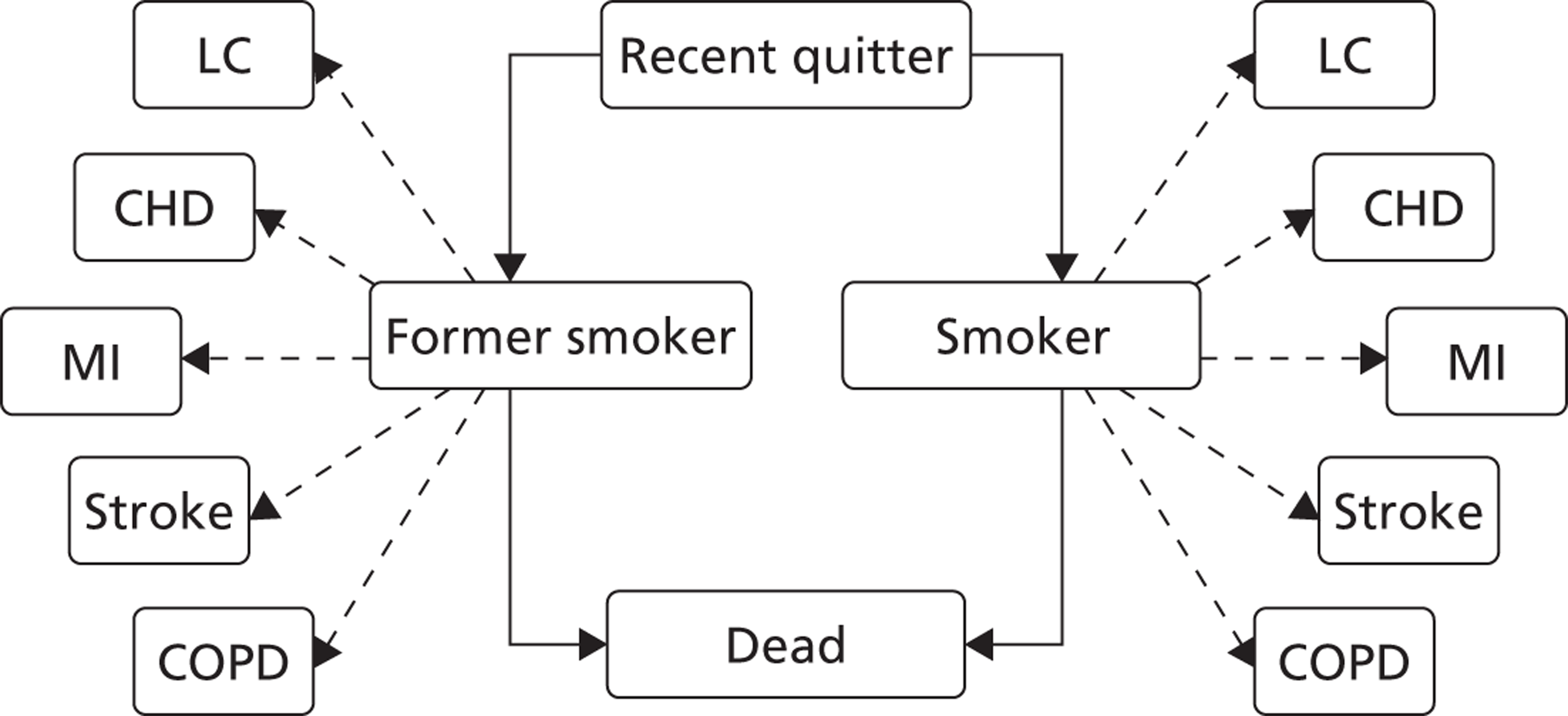
The structure of the model is based on a simple Markov process, but with some complexities. For instance, the likelihood of an individual being a smoker or former smoker and of developing comorbidities varies with their age, based on the prevalence of smoking status from the Health Survey for England. 119 RRs of diseases by smoking status are used to decompose the population-based prevalence rates of five major smoking related diseases. Further detail on prediction of mortality by smoking status is given in the next section [see Modelling mortality: mortality rate by smoking status, and projection of life expectancy (life-years) over time].
Bauld et al.110
The model by Bauld et al. 110 is a two-stage model, firstly using a decision-tree model, based on clinical evidence from a single observational study, and thereafter using a Markov model to estimate longer-term outcomes. The decision-tree framework has a time horizon of 1 year and was developed to assess the clinical and economic impact of two community-based cessation programmes delivering behavioural support with NRT. The target population consisted of current smokers who were clients of one of two Glasgow NHS SSSs. The 1-year time horizon for the decision tree corresponded to the follow-up period of the observational study for reporting sustained abstinence. The short-term decision tree is used to estimate the incremental cost per quitter for both services compared with self-quit attempts at 52 weeks. Thereafter, the model uses a four-state Markov modelling approach in cohort analyses to model outcomes and costs over time. The model uses a longer-term lifetime time horizon, and applies mortality rates from Scottish mortality life tables, relapse data from published sources, and estimates of QALYs using health-state values from a published study. 120 The Markov model uses data from Yudkin et al. ,117 giving 8-year follow-up data from a RCT of NRT versus control, to establish the number of long-term quitters.
Overview
The models identified in the literature are either decision-tree or Markov-type models, or a combination of the two frameworks. Decision-tree models have been used to model the first 12 months of the model time frame, typically an intervention related time period (and/or post intervention duration), in order to establish the probability of 12-month sustained abstinence. The complexity of the modelling of short-term abstinence was in many cases dependent upon the nature of the interventions being compared and the trial evidence available. The scope of the models (in terms of treatment strategies and referral pathways considered) tended to be more comprehensive if the model was produced to inform specific policy contexts.
A significant number of the cohort-level Markov models identified101,102,104,105,107 were population-based smoking-cessation models developed to guide budget-holders and policy makers in their decision-making. In general, these models replicated national populations, explicitly modelled individual smoking-related diseases using data from epidemiological registries and used simulation methods (typically cohort-level Markov models with multiple states and time dependency) to determine the benefits of quitting smoking.
The evidence review has been used to inform planning for model development related to EARS, and here we outline considerations on structure and data, specifically around the outcomes used, the modelling of abstinence and relapse (data inputs) and the use of available data to inform on mortality data/rates in order to model life-years and related QALYs. We also consider, in brief, issues related to data inputs on resource use and associated cost, and the way models had presented outcomes and considered issues related to uncertainty (structural and parameter uncertainty in modelling methods) and consistency (model validation).
Data inputs
For comparative purposes, we looked at the key parameters for the main studies of interest. Table 35 provides a summary of data inputs used in the model for the key effectiveness parameters from the included studies (see Appendix 9 for further details).
| Data inputs | Studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fiscella and Franks106 | Orme107 | Wang15 | Bauld110 | Woolacott100 | NICE109 | Howard102 (BENESCO) | Godfrey108 | Coleman101 | |
| Background rate of quit per year | 2.5% | 1% | Not stated | 1.5% | 1% | 1% (0.5–2%) | 5% | 2% (1–4%) | 2% |
| 12-month abstinence rate (or other short-term quit rate, as specified) | Physician counselling 4%; counselling and NRT patch 7.9% | Including background quit group advice 3%; group therapy 9% | CDTQ with NRT 5.33% (meta-analysis); abrupt quit with NRT 16% | 2.5% pharmacy intervention; 5.5% group intervention | Excluding background quit: 3% brief advice; 9% counselling; 15% NRT | Including background quit: 1.7–4% for range of brief interventions considered | With NRT 15.4% | Mean estimate = 13.6% for population attending SSSs | 23–37% for interventions considered (note: all pharmaceutical) |
| Lifetime relapse rate (applied at 12 months unless otherwise stated) | 35% | 30% | 30% relapse at 12 months | Relapse rates up to 8 years post quit from published study.117 Appears to be 36% if probabilities can be summed | 40% (30–50%) relapse at 12 months | Relapse rates up to 8 years post quit from published study117 | Relapse rates up to 10 years post quit from two different observational studies. 6.3% per year for first 2–5 years inclusive,122 2% for years 6–10 inclusive (Veteran’s affairs normative study123) | 54% relapse at 12 months | Varied assumptions in absence of long-term information about the efficacy of relapse prevention interventions long term. 2% relapse per year |
Smoking status – smoking cessation
The primary outcome in any smoking-cessation clinical effectiveness study is the quit rate (sometimes referred to as the abstinence rate) at a defined period of follow-up. In RCTs, the metric which is the preferred standard for reporting effectiveness is the expired air CO-confirmed quit; outcomes are usually reported at 4 weeks post quit as a minimum and frequently at 6 months and 1 year. However, the gold-standard criteria for smoking-cessation trials is the Russell standard,78 which identifies criteria over and beyond biochemical verification for validating a quit attempt; most notably, a sustained period of abstinence is required to provide confidence in long-term quit, and those who decline to be followed up should be treated as smokers. Reported 12-month abstinence rates varied across studies in the range of 1.7–37%.
Relapse rates
Beyond trial end points, there is a dearth of high-quality longitudinal data with regards to the risk of reuptake (relapse) or its corollary, long-term quit (abstinence) rates. Models have responded to this particular challenge by using study/trial evidence when available. The majority of studies included in this review made an assumption about the lifetime relapse rate; relapse and abstinence were considered to be time dependent in only a few cases. 102,109,110 Woolacott et al. 100 present a review of relapse rates and refer to a variation from 0% to 50% by intervention. Coleman et al. 101 report a high-quality systematic review of RCTs to inform on rate of relapse (abstinence) (short-term relapse rate). Etter and Stapleton121 report a high-quality systematic review of RCTs of NRT with follow-up of 1 year or more after start of treatment. They present findings from 12 included trials, with follow-up over 2–8 years, with an average of 4.3-years’ follow-up. The overall relapse rate reported by Etter and Stapleton,121 between 12 months and final follow-up, was 30.0% (95% CI 23.5% to 37.5%); this rate did not differ between NRT and control groups or length of initial NRT treatment.
| Outcomes | Studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fiscella and Franks106 | Orme107 | Wang15 | Bauld110 | Woolacott100 | NICE109 | Howard102 (BENESCO) | Godfrey108 | Hurley105 (QBM) | |
| Life years gained | As per Doll et al.111 | Calculated within model 0.4/quitter | Model assumed a sum total 3–10 life-years gained per quitter (age dependent, source: Doll et al.113) | Age- and sex-dependent risk of other-cause death and smoking-related death (limited to 12 years post quit) – from Scottish life tables | Model assumed 2 life-years gained per quitter | Model assumed a sum total 3–10 life-years gained per quitter (age dependent, source: Doll et al.113) | Estimated within model – between 6 and 45 life-years gained, as adjusted for different age and sex bands | After discounting 3.59 life-years gained per quitter (applied to rates from Doll et al.113) | 0.047 life-years gained (in quitters followed for 10 years) |
| Detail on QALYs (source, method) | Utility values estimated from YHL measure (US). Categorised by age, sex and smoking status | Not reported | Model used published study by Fiscella and Franks.106 CQG due to abstinence varies by age band 0.03–0.07. Utility due to life-years gained compared with smokers: 0.755 general population weights (healthy individual)124 | Tengs and Wallace:120 0.8 smokers, 0.87 ex-smokers. Model estimated a CQG of 15.17 (self-quit) –15.25 (group intervention) relative to non-quitters | Not reported | From an unpublished survey of current and ex-smokers using EQ-5D (apparently age and sex dependent): 0.805 for a male smoker aged 50–59; 0.858 for a male non-smoker aged 50–59; 0.755 for a female smoker aged 50–59; 0.837 for a non-smoker in the same age group | Fiscella and Franks106 | NA | Morbid health states range from 0.45 to 0.84. Healthy smoker and quitter given full health utilities of 1 (source: CEA registry) |
Modelling mortality: mortality rate by smoking status, and projection of life expectancy (life-years) over time
The benefits of abstinence, and quit, are clearly set out in terms of differences in life expectancy. 111,113 In the modelling literature reviewed here, many of the studies15,100,101,106,108,109 used published data from the prospective study of UK male doctors reported by Doll et al. 111,113 Doll et al. 111,113 report the smoking habits for former, non- and current smokers of male doctors at different ages and time periods, and compared the reduction in risk associated with quitting. It is the only UK study to date which has sufficiently long follow-up to allow for extrapolation; a similar approach is evidenced in Nurses’ Health Study in the USA. 125 Data from Doll et al. 111,113 provide expected differences in life expectancy by smoking status, including by time since quit.
An alternative method for extrapolating to final outcomes is to model mortality/morbidity in terms of the number of fatal/non-fatal events conditional on sustained quit (smoking status). Bauld et al. 110 use a cohort-level Markov model to provide a representation of smoking-related death, but the model does not account for morbidity (health events) directly. Non-fatal morbidity (e.g. non life-threatening asthma exacerbations, wheeze) is not considered within the model. However, the simple and parsimonious approach used by Bauld et al. ,110 while predicting outcomes appropriate for CEAs, demonstrates that methods can be extended to predict outcomes related to smoking-related health events. The model presented by Coleman et al. 101 models smoking status over time, using population mortality data adjusted via ratios for relative mortality by smoking status derived from Doll et al. 111 Coleman et al. 101 also model specific health events related to smoking status over time. Figure 7 presents the model structure used by Coleman et al. 101 In the current evidence review, the method used by Coleman et al. 101 to model mortality by smoking status is considered as a prominent and appropriate method for use in a future EARS smoking-cessation model, and is considered further in the next section of the economic analysis. Below, we outline the basic approach used by Coleman et al. 101 to model mortality rates by smoking status. This approach is adapted, for use in EARS analyses, to allow for modelling of mortality rate over time by smoking status, and by time since quit. Coleman et al. 101 apply a similar approach, to that described here for all-cause mortality, to disease-specific annual mortality rates (see Figure 7).
Coleman et al. 101 estimate mortality rates for categories of smokers (s), former smokers (f) and never smokers (n) by decomposing the population mortality estimate (referred to as Q). We outline this approach below; for clarity, we assign our own notation and let E = the actual mortality rate for each category of smoker, determined by subscripts s, f, and n for the categories of smokers above. We therefore rewrite the Coleman equation, which must be satisfied across three unknowns (Ec, En, Ef), as
where Pj = the prevalence of smokers across categories of smokers (j = s, f, n).
The RR [the correct term for these RRs is age-specific mortality rate ratios (calculated as the RR of death in former and non-smokers vs. current smokers) and not mortality odds ratios (MORs) as labelled by Coleman et al.]101 Where the probability is small, and n is large, note that the RR can approximate the MOR] of mortality for never and former smokers are defined as
Substituting in allows us to write (1) in terms of 1 unknown, Ec:
Rearranging to solve in terms of Ec:
Coleman et al. 101 illustrate this with an example for a 44-year-old smoker by substituting the prevalence of smoking across categories of smoker [Pc = 0.26, Pf = 0.21, Pn = 0.53] and actual mortality [Q = 0.002144] into (1) to give:
Substituting the RRs (where RRn = 0.7143 and RRf = 0.571):
Solving in terms of Ec:
Using the definition of RR to solve for Ef and En:
Quality-adjusted life-year weights, and estimation of quality-adjusted life-years over time
Modelling methods described here have adjusted mortality data over time using health-state values (QALY weights) in order to estimate QALYs by treatment strategy, and to compare incremental QALY gains by treatment (Table 36). Within the literature review undertaken here, we identify a recent study presenting health-state values (QALY weights) by smoking status. 126 Vogl et al. 126 present health-state values obtained by use of EQ-5D within the Health Survey for England119 by age (year of life as opposed to decile of life), sex and smoking status for the English population.
Relevance of resource use and costs
All studies discussed here considered costs directly relevant to the intervention, and the majority used a unit costing approach and reported units of resource separately from cost. The majority of included models considered the costs of treating the main smoking-related diseases, either in the base case or in the sensitivity analysis (notable exceptions being studies by Wang et al. 15 and Woolacott et al. 100). In terms of the smoking-related diseases, lung cancer, myocardial infarction, stroke, and COPD were typically included, and diseases such as CHD and asthma more seldom. The epidemiological methods for determining smoking-attributable disease differed, as discussed by Bolin et al. 99 in their review. There was a tendency for studies to report cost-effectiveness both with and without the future cost of lifetime smoking-related diseases, with discounting of costs only usually applied in the latter. No study considered the possibility of higher future lifetime costs (‘survivor’ costs) of treating former smokers relative to continuing smokers, despite this being an area of contention in the literature. 127,128
Uncertainty
All models discussed above reported some attempt to consider uncertainty in input parameters. This typically included a series of univariate analyses, but in addition many studies apply best-/worst-case scenarios to examine the potential cost-effectiveness of smoking-cessation interventions. Probabilistic sensitivity analysis (PSA) was not widely reported in the literature. Discounting of future outcomes and costs was a common consideration in sensitivity analysis, as one of the challenges facing public health interventions, where the payoff from current investment may be many years into the future, is the fact that discounting reduces the estimated magnitude of health gains. For example, Coleman et al. 101 highlight that 1 QALY today is worth approximately 0.25 QALYs in 40 years’ time, where a discount rate of 3.5% per year is applied.
Conclusions/reflections
The literature overview presented above outlines how the current literature has been used to inform the structure and development of a CEA model, and to inform the identification of data to populate a model, for application in estimating the cost-effectiveness of the EARS intervention.
The review here has not identified modelling studies that have put emphasis on interventions with a specific focus on PA as a key component of the intervention, as is the case with EARS. This may be due to the nature of the literature review and its consideration of specific smoking-cessation interventions, rather than searching more broadly on PA. However, as the main outcome of interest for EARS is the quit rate, the model set out for EARS (in the next section), draws on the previously published models for smoking cessation. It may be that in a future, more comprehensive systematic search of the literature, and in further development of the modelling framework set out in this report, the use of PA data could be integrated into a more complex model structure.
A specific consideration for EARS is that the target population is a disadvantaged target treatment population. The models identified to inform on the smoking-cessation analyses were not in disadvantaged populations, nor is there a specific focus in the evidence base around abstinence, relapse or mortality, on a hard-to-reach population. In only one of the models described above is there a focus/reference to a specific target population, with Bauld et al. 110 considering NHS smoking services in Glasgow, highlighting higher levels of social deprivation in its description of baseline characteristics of clients entering one of two community-based interventions. Therefore, the modelling development in this report draws on the current evidence that relates to a broader smoking-cessation target group.
A further feature of the EARS intervention is the targeting of smokers who do not wish to make an abrupt quit attempt (as trial inclusion criteria). In our review of the literature we identified only one study15 that explicitly examined the costs and consequences of ‘cutting down’. In that study, Wang et al. 15 did not consider the long-term benefits of cutting down, in terms of additional benefits to life-year gains and QALY gains.
In the literature considered here on modelling methods for smoking cessation, there is a reliance/emphasis on use of data on mortality by smoking status from Doll111,113 and on data from Fiscella and Franks106 to inform QALY values. The recently published study by Vogl et al. 126 provides a useful contribution/addition to the literature on health-state (QALY) values by smoking status. Vogl et al. 126 publish health-state values by age and sex and smoking status based on data obtained in the Health Survey for England119 through use of the EQ-5D (generic preference-based health status measure).
In the following section we document the development of a smoking-cessation model, a simple model to inform exploratory analyses and to set the foundations for future cost-effectiveness analyses for EARS, alongside a future full RCT. Using the findings from the EARS pilot trial (as presented in Chapter 3 and Chapter 4) we also present illustrative CEAs.
Cost-effectiveness analyses of the Exercise Assisted Reduction then Stop intervention
Development of a framework for cost-effectiveness analysis: model development
Statement of the problem/objective of research
A modelling framework is needed to predict longer-term outcomes from end points in smoking-cessation trials, in this instance to predict longer-term outcomes from effectiveness data in the trial comparing the EARS intervention with brief advice. The model set out here is developed to estimate the impact of smoking status over time on mortality and QALYs, in order to inform on estimates of the cost-effectiveness of the EARS intervention compared with brief advice. The model developed and presented here is a simple model, aligned to the current exploratory research and pilot RCT of the EARS intervention. The model development research set out here seeks to inform on a framework for CEA that will be appropriate for a future CEA alongside a full RCT of the EARS intervention.
Perspective/viewpoint
The model developed is aligned to a UK policy context, and the primary perspective of the analysis is that of the third-party payer, that is to say UK NHS and Personal and Social Services, consistent with the commonly applied analytical ‘reference case’ recommended by NICE in the UK. 129 The framework developed here can be applied to a broader analytical perspective, where additional detail, complexity and data inputs are added to the simple model presented here, for exploratory purposes.
Model type and rationale for structure
A Markov-type model has been developed to estimate the cost-effectiveness of smoking-cessation interventions. The model structure has been informed by a review of the literature describing modelling methods used in assessment of the cost-effectiveness of interventions in smoking cessation (discussed/presented above). The model developed here is a simple Markov model, a cohort simulation model, with an annual cycle length and a lifetime time horizon for analysis (up to maximum age 85 years); both of these model characteristics are informed by review of the literature and are consistent with methods used for assessment of cost-effectiveness in smoking-cessation interventions. The model includes two health states for smoking status: either ‘smoker’ or ‘former smoker’, and death/dead (Figure 8). It is assumed at the start of the model that people will be in one of the two states for smoking status, with former smokers being recent quitters (i.e. at least 4 weeks post quit and 3–4 months post EARS intervention/comparator).
FIGURE 8.
Model structure/schematic for the EARS model.
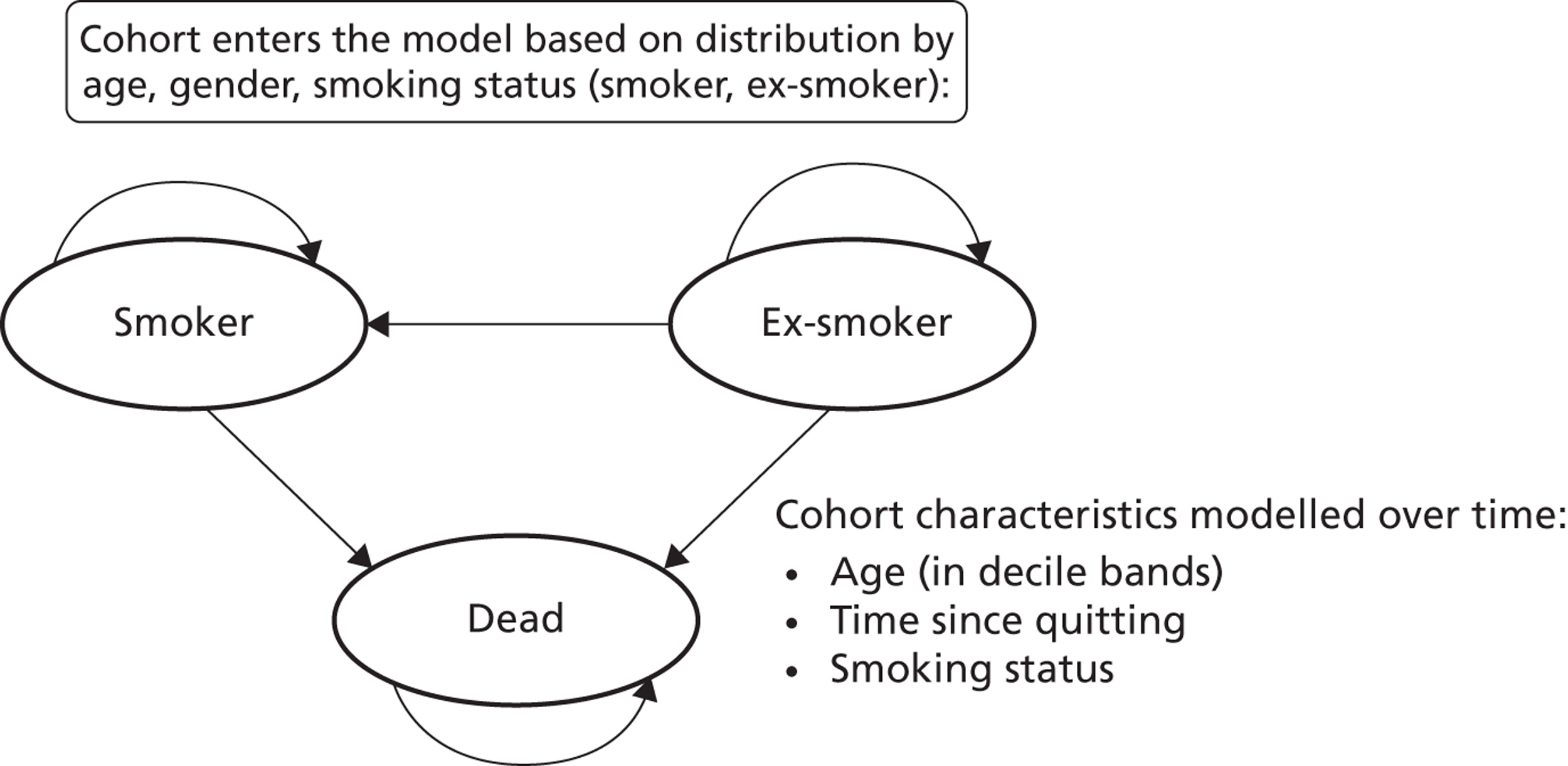
The model considers a starting cohort of people, by age group (decile age bands) and sex, with a distribution across smoking status, either smoker or former smoker, and predicts future status over time (in each cycle) in these states, or death. For the CEAs, cohorts with differing start point distributions (all other characteristics being equal) are modelled over time, with cost and consequences estimated and compared between competing interventions.
The model is structured to allow transitions from former smoker (successful quitters) to smoker, using a risk of relapse in each of the first eight 1-year cycles of the model, and people can continue to be in states for ‘smoker’ and ‘former smoker’ (continued abstinence) in each cycle of the model. The current model does not allow for transition from ‘smoker’ to ‘former smoker’ (spontaneous quit) in each cycle of the model (as evidenced in Figure 8 – this could be included, e.g. 2% quit rate in literature, Coleman et al. 101). Each person faces a risk of death in each model cycle. The main driver in the model is differential mortality rates by smoking status, with comparison of life years in each health state, and difference in life expectancy and QALYs over time, being the focus of comparisons by differing starting distribution (smoking status) for subsequent CEAs.
The primary outcomes, and economic end points, of interest in this development modelling are number of long-term quitters (12 months and beyond), that is to say cost per quitter, the cost per life-year gained, and cost per QALY gained. The model therefore aims to estimate, in the first instance, smoking status over time, life-years and QALYs in the cohorts considered for comparison in CEAs.
The model does not model smoking-related morbidity directly; however, people are given a health-state value, according to smoking status, age and sex, using published data (see discussion of Vogl et al. 126 in Health-related quality of life/quality-adjusted life-years, below), and in such a way differences in health-related quality of life/QALYs are estimated over time, by cohort distribution (smoking status). The model does not estimate costs associated with smoking-related morbidity, although there is an opportunity to develop the model in this direction for future analyses. In the present model, sensitivity analyses consider differential costs by comparison interventions through the use of a cost associated with death, smoking-related death, where estimated costs are assumed to capture additional health care expenditures associated with treating smoking-related diseases.
Modelling smoking status over time
The framework used here models smoking status over time. At the start of the model, the cohort entering the model is defined by proportions (distribution) in states for ‘smoker’ and ‘former smoker’. The model is developed here to support analyses for the EARS smoking-cessation intervention, as described in this report. In applying the modelling framework to EARS and comparators, in a decision-analytic context, we have a distribution (comparison of differing starting distributions) of people entering the model where a proportion will be recent quitters, that is to say former smokers. In the current analyses data from the end point of the EARS pilot RCT is used to inform the starting distribution of smoking status for cohorts modelled over time. In the first 12 months of the model, former smokers face a relatively high risk of relapse (to smoker); thereafter, in years 2 to 8 of the model, these former smokers face a continued, but relatively lower, risk of relapse. Data to inform transition from former smoker to smoker have been taken from the current literature,101,117,121 informed by our review of the literature on modelling methods.
Relapse/continuous quit rate at 12 months
In order to estimate the continuous quit rate at 12 months, and the related relapse rate, we use data from the published literature, applying data from Coleman et al. 101 in the base-case analyses. In practice, we do not know how many successful quitters within the EARS trial reported at 4 weeks post quit will relapse at 12 months (a future long-term trial may inform on this). Coleman et al. 101 report a high-quality systematic review, presenting estimates of abstinence rates and relapse patterns in smokers who had made pharmacologically aided quit attempts with NRT, bupropion and varenicline (see previous discussion). In the current model we use a relapse rate of 28% for the first 12-month cycle of the model. This input/assumption is informed by data from Coleman et al. 101 on relapse (continued abstinence) in RCTs reporting on NRT. Data on difference in abstinence between 3 months (post intervention) and 12 months is used to inform the current analyses (see Coleman et al. ; Table 35; Figure 7), with a relapse rate of 28% applied, taken from data on five RCTs for NRT. Coleman et al. 101 report a relapse rate of 40% (years 2–8) in combined analyses, using 16 RCTs; however, in some of these RCTs the intervention was over a duration of up to 52 weeks; this latter estimate is used in sensitivity analyses.
Relapse/long-term quitters beyond 12 months
Data to inform on longer-term smoking-cessation status, and relapse, beyond 12 months are sparse. A high-quality literature review has been reported by Etter and Stapleton. 121 From the literature review, we identified long-term continuous cessation data (8 years post quit) from a study by Yudkin et al. 117 who report a longitudinal study of 1686 trial participants (NRT) based in Oxfordshire, UK. Yudkin et al. 117 report a relapse rate of 46% for former smokers to smokers, over the period after a 1-year trial, up to 8 years (the rate was 44% for placebo and 47% for those in the NRT arm of the trial). The median time to follow-up was 8.3 (SD 0.35) years. Of the 153 participants who had stopped smoking for 1 year in the trial, 83 were still not smoking at follow-up, giving a relapse rate of 46%. The study assumed that all those lost to follow-up were still smoking. We use the data from Yudkin et al. 117 (46% relapse) in the base case of the model. In sensitivity analyses we apply data from the meta-analysis reported by Etter and Stapleton,121 which includes the study by Yudkin et al. ,117 in which a relapse rate of 30% is reported, over an average follow-up of 4.3 years (after year 1).
In order to apply these data, on rate of continued abstinence and on rate of relapse over years 2 to 8, in the framework of the decision model developed, using a 1-year model cycle, we derived a profile for continued abstinence and relapse rate by year over years 2 to 8. Data were derived using an exponential survival function for remaining smoke free (time to event analyses). It would be possible to use other functional forms here but the rationale for using the exponential function is that it is consistent with evidence suggesting that the proportion of quitters follows a decreasing trend.
We calculate the hazard or (instantaneous event rate) within the 7-year period of follow-up as h = –[ln(Sm/S0)]/(tm – t0)] (Box 1), representing a constant hazard rate over years 2 to 8. The hazard rate for relapse is applied to the number of people in the health state of ‘former smoker’ from 12 months. Applying these methods in the modelling framework provides outputs over time (number who relapse and revert to smoking) that are consistent with the implicit relapse rate used from Yudkin et al. 117
In order to estimate the proportion of the cohort who have quit at other time points, St, within the interval for which we have data (i.e. t = 2, 3, . . . 7, etc.) one option is to assume that proportion of quitters follows a decreasing trend. One of the simplest models that can be used to represent a decreasing trend is the exponential function
St = S0e–ht
where S0 = proportion of quitters at time t = 0.
In order to calculate this exponential function from data used to inform current analyses, we used the following steps:
-
From existing data sources (Yudkin et al. 117), calculate the proportion of quitters at the start and end of the follow-up period (S0 and Sm).
-
Calculate the hazard rate to be applied to the model over the time period (years 2–8), i.e. h = –[ln(Sm/S0)]/(tm – t0) = –[ln(0.051/0.094)]/(8 – 1) = –[ln(0.542)]/7 = (0.611)/7 = 0.0874 (SE 0.043).
The hazard rate is applied using the following: St = S0 e–t(0.0874), applied to the specific state occupancy (former smokers) in the model over time. We applied this to our estimate of former smokers at S0 (corresponding to 52 weeks from entry to the model). This allows us to calculate the proportion of the modelled cohort to quit at time t. This is best illustrated by example. For example, where t = 3 years, and where the proportion of non-smokers at time S0 = 0.103, St = (0.103)e–(3)(0.0874) = 0.103 (0.769) = 0.0791.
The model framework assumes that smokers who are abstinent at 8 years post quit would remain abstinent. The model does not allow non-smokers [or cut-downers (at trial follow-up)] to make a subsequent quit attempt if they failed to do so within the prior trial (prior to entering the model). Both assumptions are potential limitations with this model and studies of this type. Any future modelling, alongside a full RCT and economic evaluation, will explore the impact of these assumptions. The current literature commonly refers to the spontaneous quit rate at around 2% per year (see Coleman et al. 101). However, it is expected that in the EARS target population, of hard-to-reach smokers who are not planning a quit attempt, spontaneous quit rates will be relatively low, and the differences between comparisons (EARS vs. brief advice in current context) will be very small/negligible.
Modelling mortality over time by smoking status
The model estimates life expectancy over time, survival in each model cycle, using methods presented by Coleman et al. 101 for estimating mortality by age, sex and smoking status (see the earlier evidence review for discussion/detail of the approach used by Coleman et al.). Methods presented by Coleman et al. 101 have been adapted for the current model, in order to use data from Doll et al. 113 (Coleman et al. 101 used data reported by Doll et al. 111). As discussed earlier, Doll et al. 111 report mortality in British doctors over time by smoking status. We adapt methods in order to model mortality by smoking status, and to model mortality rates dependent on time since quit, in order to model the relation between sustained abstinence (in deciles age bands) and smoking-related mortality.
The estimation of mortality data by smoking status uses (1) current mortality rate data, by age group and sex and (2) data on the prevalence attached to smoking status; the data from Doll et al. 113 on mortality by age and smoking status is used to represent the ratio of mortality rate by smoking status, the latter analogous to a RR of mortality by smoking status and age group (and here referred to as RR). Mortality is modelled from age 35 upwards, using age groups reflecting 10-year age groups (e.g. 35–44 years), aligned to the data reported by Doll et al. 113 (Doll et al. report that mortality by smoking status below this age is subject to wide random variation. Evidence from actuary life tables suggest that total mortality prior to the age of 30 is low, in the region of < 1 per 1000, and that while mortality rates between smokers and non-smokers begin to diverge at this age the divergence in mortality rates between former and current smokers starts at age 35) (Table 37).
| Age now (years) | Status | ||||
|---|---|---|---|---|---|
| Lifelong non-smokers | Years (t) since stopped smoking | Continuing cigarette smokers | |||
| t < 10 | 10 ≤ t <20 | 20 < t | |||
| 35–44 | 1.6 | 2.0a | – | – | 2.7 |
| 45–54 | 3.8 | 5.4 | – | – | 8.5 |
| 55–64 | 8.4 | 16.4 | 9 | – | 21.4 |
| 65–74 | 18.6 | 36.4 | 31.7000 | 22.7 | 50.7 |
| 75–84 | 51.7 | – | 78.9 | 69.1 | 112.2 |
As the approach described by Coleman et al. ,101 RRs are derived from data presented by Doll et al. 113 to reflect relative mortality rates by smoking status (see Table 37). The RRs derived in the current analyses, for former smokers, distinguish between the age of the quit attempt in terms of deciles since quitting. These data are combined with mortality data reported in current life tables, sorted by age decile, in the general population (see Table 7) (www.ons.gov.uk/ons/rel/lifetables/decennial-life-tables/no-16-–2000–2002-/index.html) and data on the prevalence of smoking for each age and sex category, using data presented in the Health Survey for England,119 (see Table 40). Data were not available, to our knowledge, on prevalence of former quitters (in terms of time in deciles since quitting in general population statistics), and therefore we apply a simplifying assumption, that prevalence is uniformly distributed between quit ages 35–44, 45–54 and 55–64 years.
The above data for former smokers can be presented using years of smoking abstinence as the independent variable. This cross-tabulation facilitates the calculation of time dependent state transitions based on years since quitting.
| Age (years) | Status | ||||
|---|---|---|---|---|---|
| Current smoker | Years (t) since stopped smoking | Never smoked | |||
| t < 10 | 10 ≤ t < 20 | 20 < t | |||
| 35–44 | 1.0000 | 0.7407b | 0.5926 | ||
| 45–54 | 1.0000 | 0.6353 | 0.4471 | ||
| 55–64 | 1.0000 | 0.7664 | 0.4206 | 0.3925 | |
| 65–74 | 1.0000 | 0.7179 | 0.6252 | 0.4477 | 0.3669 |
| 75–84 | 1.0000 | 0.7119a | 0.7038 | 0.6164 | 0.4612 |
| Age now (years) | Male | Female |
|---|---|---|
| 35–44 | 0.0010 | 0.0004 |
| 45–54 | 0.0016 | 0.0010 |
| 55–64 | 0.0040 | 0.0026 |
| 65–74 | 0.0105 | 0.0064 |
| 75–84 | 0.0289 | 0.0175 |
| Smoking status | Current age (years) | ||||
|---|---|---|---|---|---|
| 35–44 | 45–54 | 55–64 | 65–74 | 75+ | |
| Prevalence of smoking (males) | |||||
| Current smoker | 0.2600 | 0.2500 | 0.1900 | 0.1000 | 0.0700 |
| Former smokera | |||||
| t < 10 | 0.2100 | 0.30 | 0.2200 | 0.1867 | 0.2033 |
| 10 ≤ t <20 | – | – | 0.2200 | 0.1867 | 0.2033 |
| 20 < t | – | 0.1867 | 0.2033 | ||
| Non-smoker | 0.5300 | 0.4400 | 0.3600 | 0.3400 | 0.3200 |
| Prevalence of smoking (females) | |||||
| Current smoker | 0.2700 | 0.2500 | 0.2000 | 0.1300 | 0.0900 |
| Former smokera | |||||
| t < 10 | 0.2100 | 0.24 | 0.1500 | 0.0967 | 0.1133 |
| 10 ≤ t <20 | 0.1500 | 0.0967 | 0.1133 | ||
| 20 < t | 0.0967 | 0.1133 | |||
| Non-smoker | 0.5300 | 0.5100 | 0.5000 | 0.5700 | 0.5700 |
Applying the methods used by Coleman et al. ,101 we estimate/derive the mortality rates for use in the model by smoking status using the above data. We model mortality data for each potential combination of age, sex and smoking status category, using the formula below. This is an adaptation of the formula, from Coleman et al. ,101 described earlier.
For each age and sex population mortality rate, Q, we use the Coleman et al. 101 formula to derive mortality rate by smoking status:
As applied in Coleman et al. ,101 in order to solve for mortality risks associated with current (Ec), non (En), and former smokers (Ef) we define ratios for mortality rates between smoking status categories as En/Ec = RRn and Ef/Ec = RRf.
We adapt this formula to estimate mortality rates for former smokers according to time since quit, using the data reported from Doll et al. 113 (see Table 37). In the younger age group(s), those aged 45–54 years (and below), former smokers will have quit smoking too recently (1 year < quit time > 10 years) to derive statistically significant variations in mortality for the current ages, while for older age groups, such as those aged 55–64 years, former smokers will include those who have quit smoking for less than 10 years (1 year < quit time < 10 years) and for between 10 and 20 years. (Note: the benefits of early quitting are realised in terms of higher mortality rates as the cohort ages within the model. See the following section for a description of how these rates are implemented in the model.)
To illustrate the approach taken, consider the example of a female who is 55–64 years old. The mortality rate for former smokers in this formula, EfPf, can be represented by the following expression:
where time since quit is represented by t1 < 10 years and 10 < t2 < 20 years.
Substituting (2) into (1) gives
Information on Q is taken from (given by) mortality life tables; in addition, data on the expected ration of mortality rates by smoking status (RR) are available from Doll et al. ,113 and therefore by adapting the methods presented by Coleman et al. ,101 it is possible to solve the equation for E (by smoking status) by replacing RRs by smoking status relations, for En and Ef, solving for Ec.
Substituting En = EcRRn and Eft1 = EcRRft1 and Eft2 = EcRRft2:
and rearrange to
Using date from the above tables (female aged 55–64 years), when Pc = 0.19, RRn = 1, Pn = 0.36, RRft1 = 0.7664, Pft1 = 0.22, RRft2 = 0.4206, Pft2 = 0.22, Pft and Q = 0.0026, then Ec = 0.004389; and substituting back into equation (13) solves for En = 0.3925, Eft1 = 0.003363 and Eft2 = 0.001846. Using these data, the mortality rate for a former smoker in this category is Ef = Eft1Pft1/Pf + Eft2Pft2/Pf.
Using the above methods, we derive estimates of mortality by smoking status for age and sex age bands, stratified by time since quit for former smokers (Table 41).
| Age (years) | Current smoker | Former smoker by age stopped | Non-smoker | ||
|---|---|---|---|---|---|
| < 10 years | < 20 years | ≥ 20 years | |||
| Males | |||||
| 35–44 | 0.001302 | 0.000964 | – | – | 0.000772 |
| 45–54 | 0.002479 | 0.001575 | – | – | 0.001108 |
| 55–64 | 0.006684 | 0.005123 | 0.002811 | – | 0.002624 |
| 65–74 | 0.018818 | 0.013510 | 0.011766 | 0.008425 | 0.006904 |
| 75–84 | 0.045752 | 0.032571 | 0.032202 | 0.028202 | 0.021101 |
| Females | |||||
| 35–44 | 0.000987 | 0.000731 | – | – | 0.000585 |
| 45–54 | 0.002460 | 0.001563 | – | – | 0.001100 |
| 55–64 | 0.006878 | 0.005271 | 0.002892 | – | 0.002700 |
| 65–74 | 0.021015 | 0.015087 | 0.013139 | 0.009409 | 0.007709 |
| 75–84 | 0.054728 | 0.038961 | 0.038520 | 0.033735 | 0.025240 |
Health-related quality of life/quality-adjusted life-years
As described above, the model developed and used here predicts mortality over the (cycles) time horizon, up to maximum age of 85 years. In addition, in order to consider health-related quality of life over time, using the QALY approach, for each year in the model a health-state value is attached by smoking status, and according to age and sex characteristics. The health-state values are used as QALY weights to reflect health-related quality of life in each smoking category. Data used on health-state values are from a recent study published by Vogl et al. 126 Vogl et al. 126 also present date by smoking intensity, and the base-case inputs used in the current analyses are for ‘heavy smokers’. Vogl et al. 126 estimate health-state values through use of the EQ-5D, the generic preference based measure recommended by NICE for their ‘reference case’ analyses. 129 Table 42 presents data on health-state valuations from Vogl et al. 126
| Smoking status/age (years) | Men | Women | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Former smokers | ||||
| 35–44 | 0.9058 | 0.0041 | 0.8872 | 0.0041 |
| 45–54 | 0.8596 | 0.0042 | 0.8479 | 0.0041 |
| 55–64 | 0.802 | 0.005 | 0.7827 | 0.0051 |
| 65–74 | 0.7802 | 0.0059 | 0.7709 | 0.0057 |
| 75–84 | 0.7358 | 0.0059 | 0.6987 | 0.0067 |
| Light smokers | ||||
| 35–44 | 0.9002 | 0.0059 | 0.8814 | 0.0059 |
| 45–54 | 0.8526 | 0.0063 | 0.8418 | 0.0062 |
| 55–64 | 0.7917 | 0.0071 | 0.7748 | 0.0071 |
| 65–74 | 0.7684 | 0.0077 | 0.7631 | 0.0073 |
| 75–84 | 0.7229 | 0.0079 | 0.6896 | 0.0082 |
| Moderate smokers | ||||
| 35–44 | 0.8899 | 0.006 | 0.8716 | 0.006 |
| 45–54 | 0.8422 | 0.0063 | 0.8317 | 0.0062 |
| 55–64 | 0.7815 | 0.007 | 0.7648 | 0.007 |
| 65–74 | 0.7575 | 0.0079 | 0.752 | 0.0076 |
| 75–84 | 0.7112 | 0.0082 | 0.6778 | 0.0087 |
| Heavy smokers | ||||
| 35–44 | 0.8728 | 0.0089 | 0.8522 | 0.0093 |
| 45–54 | 0.8529 | 0.009 | 0.813 | 0.0093 |
| 55–64 | 0.7647 | 0.0095 | 0.7466 | 0.0097 |
| 65–74 | 0.7395 | 0.0103 | 0.7336 | 0.0104 |
| 75–84 | 0.6925 | 0.0104 | 0.6586 | 0.011 |
The above rates are used to inform risk of death in each 1-year time cycle of the model, and to therefore estimate mortality over time (in a cohort analysis).
Smoking attributable (related) mortality
In sensitivity analyses, where the model is used to inform CEAs, we use an estimate of ‘smoking-related’ mortality to capture potential costs associated with smoking-related morbidity. In order to determine mortality attributable to smoking and not to other causes, the excess risk of death for a smoker over and above a non-smoker is calculated by subtracting En (risk of death non-smoker) from Ef (risk of death former smoker) and Ec (risk of death current smoker), for each category. This allows the model to estimate smoking attributable mortality. This outcome also allows calculation of the cost of smoking-related disease leading to death (see ‘Costs’).
Applying the model in a decision-analytic context
The modelling framework described above is applied here to consider the cost-effectiveness of the EARS intervention compared with brief advice, in a decision-analytic context. The perspective for the analyses is stated above, as is the model structure. While there are currently some simplifications and limitations with the modelling framework set out, and data inputs are uncertain in some areas, the analyses described here are aligned to the exploratory research being undertaken alongside the EARS pilot RCT, in preparation for, and to develop methods for, a future full RCT with economic evaluation alongside.
Intervention(s)
The interventions compared in the CEA are the EARS intervention, versus brief advice. Interventions have been described in detail earlier in this report (see Chapter 1).
Effectiveness: comparing alternative intervention strategies
The model as simply set out produces estimates of outcomes (and costs) over time for a given cohort of people described by age, sex and smoking status. The distribution by smoking status will differ where intervention strategies differ, assuming that people are subject to an intervention prior to the modelling of longer-term outcomes, and associated costs. The model is configured in this way for the development stages of research; however, it is possible to integrate intervention strategies within the first cycle of the model where appropriate. In this simple modelling framework, outcomes over time are compared in different cohort by intervention scenarios, yet it is possible, in developments of this model, to introduce other mechanisms for comparing effectiveness (e.g. applying adjustment, such as a relative risk, to transit probabilities in a base/control cohort).
Base-case cohort characteristics
The modelling framework is developed to predict outcomes, and related costs, associated with a given starting distribution for smoking status for a cohort (defined by the decision-making context) of people over time. The modelling framework estimates average (expected) outcomes against specific baseline characteristics; in the current framework it is by age and sex groups. In the current exploratory analyses we run the model for the distribution of smokers and ex-smokers reported at the final follow-up of the EARS pilot RCT, and also for a cohort with the distribution by smoking status reported in the control participants having brief advice (the EARS trial reports the number of participants achieving expired air CO-confirmed abstinence at 4–8 weeks post quit date). Table 43 presents these starting distributions for smoking status. We undertake analyses by age and sex stratification to represent these population data available to inform the model and to reflect policy-relevant analyses. However, we apply the trial end point distributions uniformly across the age-/sex-specific analyses. In future analyses it is possible to use age-/sex-specific results, and to model a weighted average result, based on stated demographics in the overall population cohort (e.g. using population weights from national data).
| Cohort | Proportion ‘former smoker’ (recent quitter/RCT; confirmed quit, in EARS pilot) | Proportion ‘smoker’ |
|---|---|---|
| Control – brief advice | 4% | 96% |
| Intervention – EARS | 14.3% | 85.7% |
Costs
The additional cost associated with delivery of the EARS intervention has been estimated at a mean cost of £192 (described in Table 33). This is the only additional cost included in this exploratory CEA. The intervention cost reflects the additional resource use and cost associated with delivery of the intervention. We introduce uncertainty around this cost estimate in the probabilistic CEA, reported in the cost-effectiveness plane and acceptability curves, through the use of a potential range of costs £154 to £230, reflected via a standard error input of £19.61.
The model framework here does not include a description of, or modelling over time of, smoking-related morbidity. These costs can be considered in future development of the model, for example using the methods applied and described by Coleman et al. 101 In the current analyses, a simple and crude estimation of costs associated with smoking-related morbidity is introduced in a sensitivity analysis where death attributable to smoking is associated with an increased cost. The cost of smoking-related death used in sensitivity analysis is a mean cost of £27,120 as reported by Bauld et al. 110 This was applied to the smoking-attributable deaths in both the comparator and intervention scenarios.
Discounting
Future outcomes and future costs (beyond the first year) are discounted at 3.5% in the base-case analyses, consistent with common practice in the UK, and with the reference case for analyses recommended by NICE. 109 Sensitivity analyses also report estimates where future costs and outcomes are not discounted. Note that intervention costs were not discounted as they were accrued in the base year.
Cost-effectiveness analyses: methods/presentation
In order to estimate the relative effectiveness and cost-effectiveness of EARS versus brief advice, we compare the outcomes from the model, quit rate over time, life-years and QALYs over time (up to a maximum age of 85 years) and we combine incremental effects with incremental costs to present the ICER associated with the comparison of interventions, for the base-case scenario and for scenarios considered in sensitivity analyses. The formula for the ICER is:
Results are presented in a tabular form for comparative analyses, showing data in a disaggregated form (by intervention), and as incremental costs and effects.
Analyses are undertaken using deterministic and probabilistic methods. 130 Probabilistic analyses are used to propagate uncertainty associated with parameter inputs simultaneously, in order to reflect joint uncertainty in model inputs. This is undertaken in an exploratory way in the current analyses/framework, applying data on distribution (standard error) by parameter input, and using assumptions on the nature of the distribution (e.g. normal, beta or gamma distributions). Appendix 9e reports these data inputs with distributions used for probabilistic analyses.
Estimates from probabilistic analyses allow presentation of CEA results via the cost-effectiveness plan, and via cost-effectiveness acceptability curves (CEACs). In these presentations of these data we use the net-benefit statistic,130 alongside assumptions on decision-maker willingness to pay per QALY gained, to represent the proportion of analyses (in simulations undertaken) where the net-benefit statistic shows the intervention to be cost-effective, that is to say where the incremental cost per QALY is equal to or lower than the decision-maker’s willingness to pay. These methods are consistent with good practice in economic evaluation, as presented by Drummond et al. 131
Sensitivity analyses/assumptions
Sensitivity analyses are reported to assess uncertainty in model parameter inputs. Probabilistic sensitivity analyses capture uncertainty in input parameters, although many input parameters have small standard errors (see Appendix 9). To address uncertainty over the choice of input parameters we set out a range of sensitivity analyses, one-way and multiway scenarios, where alternative data inputs and/or assumptions are used. The key areas for sensitivity analyses (to date, can do others) are:
-
start point distributions by smoking status (base case informed by EARS pilot RCT)
-
relapse rate in first 12 months post quit (base case at 28%)
-
relapse rate over years 2–8 (base case derived from rate of 46% over 2–8-years)
-
use of discount rate of 3.5% in base-case analyses
-
estimate of EARS intervention cost (base case at £192)
-
assumption (simplifying) that there are no smoking-related morbidity costs
-
alternative QALY weight data used.
Other key simplifying assumptions, not subject to sensitivity analyses in current exploratory modelling/results, include:
-
no spontaneous quit rate included in the model (focus on EARS effectiveness only)
-
time horizon up to maximum of age 85 years
-
no specific smoking-related morbidity events/costs included
-
simple method used for estimating smoking-attributable mortality
-
health-state (QALY) values from Vogl et al. ;126 no sensitivity analyses undertaken.
Results
The addition of the EARS intervention to support smoking cessation, compared with brief advice in current analyses, results in additional costs associated with the intervention. Here we estimate a mean participant cost of £192, assuming that the EARS intervention is delivered by HTs (as above), and that the EARS intervention is implemented as part of a model of service provision that is multifaceted and covers a range of smoking-cessation and/or health promotion public health services, that is to say not as a stand-alone EARS service. Given the difference in quit rate reported in the pilot RCT (4% vs. 14.3%), a net quit rate of 10.3%, a simple short-term cost analysis would suggest an additional investment of £1864 per additional person with confirmed quit at 4–8-week post-quit follow-up.
Table 44 presents the estimated outcomes, costs, and cost-effectiveness ratios by age and sex categories for base-case CEAs, using the decision-analytic modelling framework set out in earlier sections. In all comparative scenarios with base-case assumptions, the EARS intervention is cost-effective compared with commonly used decision-maker willingness-to-pay estimates (cost-effectiveness thresholds). 129 The difference in start point distribution by smoking status results in incremental gains in life-years, and incremental QALY gains, for a relatively low intervention cost.
| Outcomes | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| Life-years | 40 | 19.74 | 19.78 | 0.044 | 192 | 4367 |
| 50 | 16.92 | 16.97 | 0.054 | 192 | 3573 | |
| 60 | 13.35 | 13.40 | 0.047 | 192 | 4119 | |
| QALYs | 40 | 9.78 | 9.81 | 0.02 | 192 | 7705 |
| 50 | 8.51 | 8.54 | 0.03 | 192 | 6315 | |
| 60 | 6.94 | 6.97 | 0.03 | 192 | 5563 | |
| Females | ||||||
| Life-years | 40 | 20.43 | 20.46 | 0.030 | 192 | 6525 |
| 50 | 17.73 | 17.77 | 0.037 | 192 | 5267 | |
| 60 | 14.24 | 14.27 | 0.031 | 192 | 6162 | |
| QALYs | 40 | 15.89 | 15.94 | 0.05 | 192 | 3607 |
| 50 | 13.18 | 13.23 | 0.05 | 192 | 3550 | |
| 60 | 10.19 | 10.24 | 0.04 | 192 | 4342 | |
Table 44 presents estimates of cost per life-year and cost per QALY ranging from £3573 to £7705, across age and sex analyses (ages 40 years, 50 years, 60 years). Incremental life-year gains and QALY gains are relatively modest, with mean QALY gains ranging from 0.02 to 0.05, and mean life-year gains ranging from 0.031 to 0.054 across age and sex analyses. These mean differences reflect the context of the public health intervention, with a relatively large treatment group where only a small proportion of people change smoking status. However, the pay-offs associated with each successful quit (former smoker) are significant. The impact of a difference in the distribution of smoking status by group is reflected in a long-term health gain, from a relatively low cost intervention, that reflects value for money, against commonly applied thresholds for decision-maker willingness to pay per health outcome (QALY).
The assessment of uncertainty, using probabilistic analyses, presented using the cost-effectiveness plane (example in Figure 9, other age and sex analyses presented in Appendix 9e), provides a positive cost-effectiveness profile for EARS versus brief advice, across age- and sex-specific analyses, consistent with the base-case results presented above. Probabilistic estimates of mean cost per QALY are similar to the deterministic results presented above (see Table 44), and Figure 10 presents the CEAC, showing that where a decision-maker is willing to pay £20,000 per additional QALY the EARS intervention is expected to be a cost-effective intervention in greater than 87% of simulations (in the probabilistic analyses).
FIGURE 9.
Cost-effectiveness plane, showing incremental costs and QALYs for males aged 40 years.
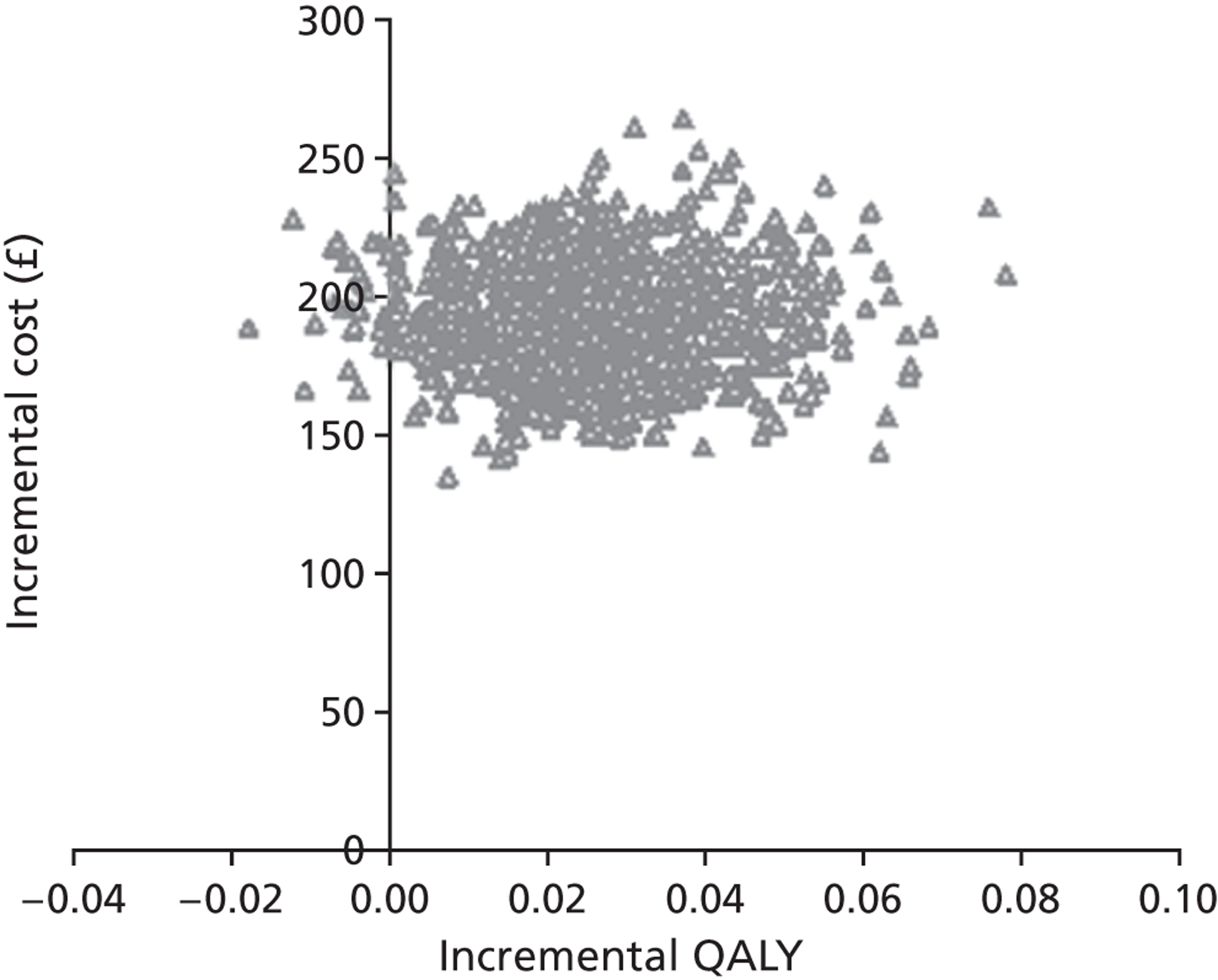
FIGURE 10.
Cost-effectiveness acceptability curve for males aged 40 years.
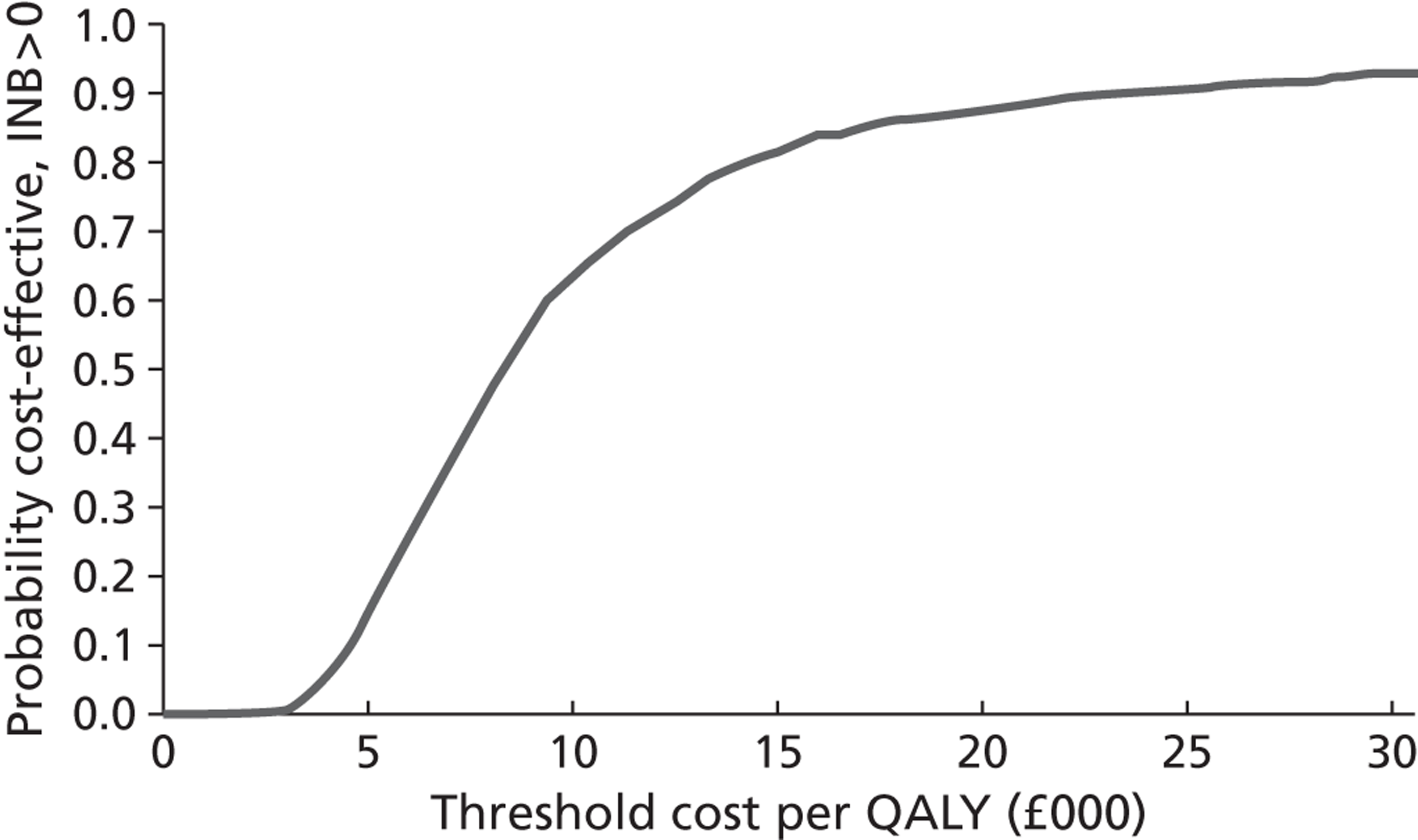
The predicted profile by smoking status over time, by age and sex, is presented in Table 45 and Table 46 (these tables report estimates for males; however, similar patterns are seen for females). In the EARS intervention (cohort), the starting distribution, using the EARS pilot results (final follow-up), shows 14.3% start in the former smoker state, compared with control at 4%. This difference narrows over the early years of the model, where relapse risk is high/higher, with a difference of 3–5% persisting over the longer term. In the longer term, the difference in the number of former smokers is relatively small, again demonstrating the public health context of the intervention and analyses.
| Time point | Control | EARS | ||||
|---|---|---|---|---|---|---|
| Former smokers | Smokers | Dead | Former smokers | Smokers | Dead | |
| Entry | 4.0% | 96.0% | 0.0% | 14.3% | 85.7% | 0.0% |
| Year 1 | 2.9% | 97.1% | 0.0% | 10.3% | 89.7% | 0.0% |
| Year 8 | 1.6% | 97.2% | 1.2% | 5.6% | 93.2% | 1.2% |
| Year 10 | 1.6% | 96.7% | 1.7% | 5.6% | 92.7% | 1.7% |
| Year 20 | 1.5% | 92.4% | 6.1% | 5.5% | 88.6% | 6.0% |
| At age 85 | 1.0% | 46.2% | 52.7% | 3.7% | 44.3% | 51.9% |
| Time point | Control | EARS | ||||
|---|---|---|---|---|---|---|
| Former smokers | Smokers | Dead | Former smokers | Smokers | Dead | |
| Entry | 4.0% | 96.0% | 0.0% | 14.3% | 85.7% | 0.0% |
| Year 1 | 2.9% | 97.1% | 0.0% | 10.3% | 89.7% | 0.0% |
| Year 8 | 1.5% | 90.5% | 8.0% | 5.5% | 86.6% | 7.9% |
| Year 10 | 1.5% | 87.1% | 11.4% | 5.4% | 83.4% | 11.2% |
| Year 20 | 1.2% | 62.7% | 36.1% | 4.4% | 60.0% | 35.6% |
| At age 85 | 1.1% | 49.6% | 49.3% | 3.8% | 47.5% | 48.7% |
Sensitivity analyses
In order to consider the sensitivity of cost-effectiveness results to inputs and assumptions, sensitivity analyses have been undertaken to assess alternative data inputs/assumptions. Tables 47 and 48 present a range of sensitivity analyses, with CEA results (i.e. a cost-effective profile) being robust to most changes/variations in model inputs and assumptions.
| Outcomes | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| Life-years | 40 | 38.25 | 38.39 | 0.143 | 192 | 1349 |
| 50 | 28.90 | 29.03 | 0.130 | 192 | 1473 | |
| 60 | 20.03 | 20.12 | 0.087 | 192 | 2203 | |
| QALYs | 40 | 29.88 | 30.03 | 0.15 | 192 | 1294 |
| 50 | 21.76 | 21.89 | 0.13 | 192 | 1467 | |
| 60 | 14.59 | 14.68 | 0.09 | 192 | 2084 | |
| Females | ||||||
| Life-years | 40 | 40.55 | 40.64 | 0.097 | 192 | 1978 |
| 50 | 30.94 | 31.03 | 0.089 | 192 | 2149 | |
| 60 | 21.74 | 21.80 | 0.059 | 192 | 3265 | |
| QALYs | 40 | 30.55 | 30.68 | 0.13 | 192 | 1494 |
| 50 | 22.55 | 22.66 | 0.11 | 192 | 1759 | |
| 60 | 15.38 | 15.45 | 0.07 | 192 | 2618 | |
| Outcomes | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| Life-years | 40 | 19.74 | 19.78 | 0.044 | 500 | 11,362 |
| 50 | 16.92 | 16.97 | 0.054 | 500 | 9296 | |
| 60 | 13.35 | 13.40 | 0.047 | 500 | 10,718 | |
| QALYs | 40 | 9.78 | 9.81 | 0.025 | 500 | 20,048 |
| 50 | 8.51 | 8.54 | 0.030 | 500 | 16,430 | |
| 60 | 6.94 | 6.97 | 0.035 | 500 | 14,474 | |
| Females | ||||||
| Life-years | 40 | 20.43 | 20.46 | 0.029 | 500 | 16,978 |
| 50 | 17.73 | 17.77 | 0.036 | 500 | 13,705 | |
| 60 | 14.24 | 14.27 | 0.031 | 500 | 16,032 | |
| QALYs | 40 | 15.89 | 15.94 | 0.053 | 500 | 9384 |
| 50 | 13.18 | 13.23 | 0.054 | 500 | 9236 | |
| 60 | 10.19 | 10.24 | 0.044 | 500 | 11,297 | |
Table 47 presents results in which no discounting of future health outcomes has been included, presenting a greater mean health gain over time, especially relevant in analyses such as these where expected gains are accrued over the longer-term time horizon.
Table 48 presents sensitivity analysis in which the mean participant cost is assumed to be much higher, at £500, compared with a base case of £192, with EARS versus control continuing to show a cost-per-QALY range of between £9400 and £20,000 across age and sex groups.
Tables 49 and 50 present sensitivity results where the effectiveness of EARS versus control has been varied by ± 25% through adjustment of expected starting in which by smoking status, compared with control with 4% former smokers. These analyses indicate that results are robust to these variations. In later scenario analyses, exploratory threshold analyses examine this area further.
| Outcomes | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| Life-years | 40 | 19.74 | 19.76 | 0.029 | 192 | 6689 |
| 50 | 16.92 | 16.95 | 0.035 | 192 | 5474 | |
| 60 | 13.35 | 13.38 | 0.031 | 192 | 6310 | |
| QALYs | 40 | 9.78 | 9.80 | 0.02 | 192 | 11,804 |
| 50 | 8.51 | 8.53 | 0.02 | 192 | 9673 | |
| 60 | 6.94 | 6.96 | 0.02 | 192 | 8522 | |
| Females | ||||||
| Life-years | 40 | 20.43 | 20.45 | 0.019 | 192 | 9996 |
| 50 | 17.73 | 17.76 | 0.024 | 192 | 8069 | |
| 60 | 14.24 | 14.26 | 0.020 | 192 | 9439 | |
| QALYs | 40 | 15.89 | 15.92 | 0.03 | 192 | 5525 |
| 50 | 13.18 | 13.21 | 0.04 | 192 | 5438 | |
| 60 | 10.19 | 10.22 | 0.03 | 192 | 6651 | |
| Outcomes | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| Life-years | 40 | 19.74 | 19.79 | 0.059 | 192 | 3241 |
| 50 | 16.92 | 16.99 | 0.072 | 192 | 2652 | |
| 60 | 13.35 | 13.41 | 0.063 | 192 | 3058 | |
| QALYs | 40 | 9.78 | 9.82 | 0.034 | 192 | 5719 |
| 50 | 8.51 | 8.55 | 0.041 | 192 | 4687 | |
| 60 | 6.94 | 6.98 | 0.047 | 192 | 4129 | |
| Females | ||||||
| Life-years | 40 | 20.43 | 20.47 | 0.040 | 192 | 4843 |
| 50 | 17.73 | 17.78 | 0.049 | 192 | 3910 | |
| 60 | 14.24 | 14.28 | 0.042 | 192 | 4574 | |
| QALYs | 40 | 15.89 | 15.96 | 0.072 | 192 | 2677 |
| 50 | 13.18 | 13.25 | 0.073 | 192 | 2635 | |
| 60 | 10.19 | 10.25 | 0.060 | 192 | 3223 | |
Tables 51 and 52 present results from sensitivity analyses in which different data sources have been used to inform the relapse rate, in the first 12 months (see Table 51) and over years 2–8 (see Table 52). The adjustment to the 12-month relapse rate, from 28% to 40%, using data from Coleman et al. 101 is a more pessimistic assumption, while the adjustment to the longer-term relapse rate, from 46% to 30%, using data from Etter and Stapleton,121 reflects a more optimistic assumption. In univariate analyses, neither of these assumptions shows a big impact on the overall estimates of cost-effectiveness. These rates are applied uniformly across comparison interventions, to a relatively small proportion of the overall cohort, and so mean estimates of difference have not shown sensitivity.
| Outcomes | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| Life-years | 40 | 19.73 | 19.77 | 0.037 | 192 | 5240 |
| 50 | 16.91 | 16.96 | 0.045 | 192 | 4288 | |
| 60 | 13.35 | 13.39 | 0.039 | 192 | 4943 | |
| QALYs | 40 | 9.78 | 9.80 | 0.02 | 192 | 9246 |
| 50 | 8.51 | 8.53 | 0.03 | 192 | 7577 | |
| 60 | 6.93 | 6.96 | 0.03 | 192 | 6676 | |
| Females | ||||||
| Life-years | 40 | 20.43 | 20.45 | 0.025 | 192 | 7830 |
| 50 | 17.73 | 17.76 | 0.030 | 192 | 6321 | |
| 60 | 14.24 | 14.27 | 0.026 | 192 | 7394 | |
| QALYs | 40 | 15.88 | 15.93 | 0.04 | 192 | 4328 |
| 50 | 13.17 | 13.22 | 0.05 | 192 | 4260 | |
| 60 | 10.19 | 10.23 | 0.04 | 192 | 5210 | |
| Outcomes | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| Life-years | 40 | 19.74 | 19.80 | 0.056 | 192 | 3427 |
| 50 | 16.92 | 16.99 | 0.068 | 192 | 2834 | |
| 60 | 13.35 | 13.41 | 0.058 | 192 | 3321 | |
| QALYs | 40 | 9.79 | 9.82 | 0.03 | 192 | 6364 |
| 50 | 8.51 | 8.55 | 0.04 | 192 | 5061 | |
| 60 | 6.94 | 6.98 | 0.04 | 192 | 4633 | |
| Females | ||||||
| Life-years | 40 | 20.43 | 20.47 | 0.037 | 192 | 5108 |
| 50 | 17.74 | 17.78 | 0.046 | 192 | 4177 | |
| 60 | 14.25 | 14.28 | 0.039 | 192 | 4971 | |
| QALYs | 40 | 15.89 | 15.96 | 0.07 | 192 | 2904 |
| 50 | 13.18 | 13.25 | 0.07 | 192 | 2876 | |
| 60 | 10.20 | 10.25 | 0.05 | 192 | 3573 | |
Table 53 presents results from sensitivity analyses in which costs associated with smoking-related morbidity have been considered via a cost payoff associated with deaths attributable to smoking-related causes. Base-case results are not sensitive to this change; it does introduce other cost inputs in addition to the EARS intervention cost, but these reflect minimal changes when applied over the cohort model comparisons, where the majority of people are similar in a ‘smoker’ health state over time.
| Outcomes | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| Life-years | 40 | 19.74 | 19.78 | 0.044 | 181 | 4116 |
| 50 | 16.92 | 16.97 | 0.054 | 161 | 2991 | |
| 60 | 13.35 | 13.40 | 0.047 | 134 | 2862 | |
| QALYs | 40 | 9.78 | 9.81 | 0.025 | 181 | 7262 |
| 50 | 8.51 | 8.54 | 0.030 | 161 | 5286 | |
| 60 | 6.94 | 6.97 | 0.035 | 134 | 3866 | |
| Females | ||||||
| Life-years | 40 | 20.43 | 20.46 | 0.029 | 181 | 6144 |
| 50 | 17.73 | 17.77 | 0.036 | 160 | 4374 | |
| 60 | 14.24 | 14.27 | 0.031 | 130 | 4179 | |
| QALYs | 40 | 15.89 | 15.94 | 0.053 | 181 | 3396 |
| 50 | 13.18 | 13.23 | 0.054 | 160 | 2947 | |
| 60 | 10.19 | 10.24 | 0.044 | 130 | 2944 | |
Tables 54 and 55 present results from sensitivity analyses in which health-state values (QALY weights) associated with smoking status have been informed from estimates for light smokers and moderate smokers (see Table 42), compared with the use of data on heavy smokers in the base-case analyses. Data for all scenarios are taken from the study presented by Vogl et al. 126 As expected, where differences between non-smoker and smoker health-state values are smaller, the estimated mean QALY gain reduces over time; however, changes still reflect ICERs that are £13,000 per QALY gained.
| Outcomes | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| QALYs | 40 | 9.97 | 9.99 | 0.015 | 192 | 12,788 |
| 50 | 8.72 | 8.74 | 0.021 | 192 | 9003 | |
| 60 | 7.20 | 7.22 | 0.021 | 192 | 9163 | |
| Females | ||||||
| QALYs | 40 | 16.47 | 16.49 | 0.026 | 192 | 7322 |
| 50 | 13.68 | 13.71 | 0.030 | 192 | 6355 | |
| 60 | 10.61 | 10.63 | 0.024 | 192 | 8011 | |
| Outcomes | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| QALYs | 40 | 9.85 | 9.87 | 0.021 | 192 | 9156 |
| 50 | 8.61 | 8.64 | 0.027 | 192 | 7128 | |
| 60 | 7.10 | 7.13 | 0.026 | 192 | 7360 | |
| Females | ||||||
| QALYs | 40 | 16.26 | 16.30 | 0.036 | 192 | 5366 |
| 50 | 13.50 | 13.54 | 0.039 | 192 | 4941 | |
| 60 | 10.45 | 10.48 | 0.031 | 192 | 6101 | |
Multivariate sensitivity analysis
In addition to the above one-way sensitivity analyses, we present here two multiway analyses, reflecting a representation of a ‘pessimistic’ and an ‘optimistic’ scenario, where there is uncertainty over data inputs.
Table 56 presents results where the pessimistic scenario reflects adjusted effect size (–25% of EARS effect), a relatively high relapse rate over year 1, a higher intervention cost, and health-state values reflecting ‘moderate smokers’, with other base-case assumptions remaining the same. This scenario shows the potential for the EARS intervention to be at an estimated cost per QALY between £23,000 and £43,000. These assumptions are in some places arbitrary, and for illustrative purposes, but indicate potential boundaries for CEAs.
| Outcomes | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| Life-years | 40 | 19.73 | 19.76 | 0.024 | 500 | 20,886 |
| 50 | 16.91 | 16.94 | 0.029 | 500 | 17,090 | |
| 60 | 13.35 | 13.37 | 0.025 | 500 | 19,702 | |
| QALYs | 40 | 9.85 | 9.86 | 0.011 | 500 | 43,794 |
| 50 | 8.61 | 8.62 | 0.015 | 500 | 34,095 | |
| 60 | 7.10 | 7.11 | 0.014 | 500 | 35,201 | |
| Females | ||||||
| Life-years | 40 | 20.43 | 20.45 | 0.016 | 500 | 31,210 |
| 50 | 17.73 | 17.75 | 0.020 | 500 | 25,194 | |
| 60 | 14.24 | 14.26 | 0.017 | 500 | 29,472 | |
| QALYs | 40 | 16.26 | 16.28 | 0.019 | 500 | 25,663 |
| 50 | 13.49 | 13.52 | 0.021 | 500 | 23,631 | |
| 60 | 10.45 | 10.47 | 0.017 | 500 | 29,181 | |
Table 57 presents results with a more optimistic scenario reflecting a greater effect size (+25%) compared with EARS base case (pilot RCT data), a 20% relapse rate over year 1 (vs. base case of 28%), a relapse rate of 30% over years 2–8 (base case at 46%), and a lower intervention cost at mean cost of £150 (base case at £192), with other base-case assumptions remaining the same. This scenario shows the potential for the EARS intervention to be at an estimated cost per QALY between £1500 and £3400. These assumptions are in some places arbitrary and for illustrative purposes, but indicate potential boundaries for CEAs.
| Outcome | Age (years) | Brief advice | EARS | Difference | Cost difference (£) | ICER (£) |
|---|---|---|---|---|---|---|
| Males | ||||||
| Life-years | 40 | 19.74 | 19.83 | 0.084 | 150 | 1787 |
| 50 | 16.93 | 17.03 | 0.102 | 150 | 1478 | |
| 60 | 13.36 | 13.44 | 0.087 | 150 | 1732 | |
| QALYs | 40 | 9.79 | 9.83 | 0.045 | 150 | 3319 |
| 50 | 8.52 | 8.57 | 0.057 | 150 | 2639 | |
| 60 | 6.94 | 7.00 | 0.062 | 150 | 2416 | |
| Females | ||||||
| Life-years | 40 | 20.44 | 20.49 | 0.056 | 150 | 2663 |
| 50 | 17.74 | 17.81 | 0.069 | 150 | 2178 | |
| 60 | 14.25 | 14.30 | 0.058 | 150 | 2592 | |
| QALYs | 40 | 15.89 | 15.99 | 0.099 | 150 | 1514 |
| 50 | 13.18 | 13.28 | 0.100 | 150 | 1500 | |
| 60 | 10.20 | 10.28 | 0.081 | 150 | 1863 | |
Scenario/threshold analysis
In addition to the other sensitivity analyses, Tables 58 and 59 present threshold analyses to indicate at what level of effectiveness (difference in distribution by smoking status) and intervention cost the EARS intervention would be cost-effective, compared with a decision-maker willingness to pay of £20,000 per QALY gain. For example, for males aged 40 years a quit rate of 8% compared with control at 4% would provide a basis for suggestion that EARS is cost-effective, using the modelling framework and base-case assumptions applied here.
| Age (years) | Males, % | Females, % |
|---|---|---|
| 40 | 8.0 | 5.8 |
| 50 | 7.2 | 5.8 |
| 60 | 6.9 | 6.2 |
| Age (years) | Males, £ | Females, £ |
|---|---|---|
| 40 | 499 | 1066 |
| 50 | 609 | 1083 |
| 60 | 691 | 885 |
Chapter summary
The research and economic analyses presented here have provided an estimate of the resource use and cost associated with the EARS intervention, compared with brief advice, and have considered how to assess the cost-effectiveness of the EARS intervention alongside a future RCT. The exploratory analyses above, limited through uncertainty in the pilot RCT effectiveness data, have estimated cost-effectiveness of EARS versus control, and a wide range of sensitivity analyses are presented for consideration alongside the EARS pilot RCT and associated research.
The methods used to estimate the cost of the EARS intervention require some development prior to the conduct of a full RCT and economic evaluation; however, the routine recording of HT activity alongside the trial, together with a more comprehensive approach on work sampling methods, should provide a sufficient basis upon which to estimate the resource use associated with the intervention, where data are collected in a robust and comprehensive manner.
Future economic analyses, alongside a full RCT, will require strengthening through a more systematic search and review of the literature on cost-effectiveness in smoking cessation, this being outside the resources and remit of the present exploratory analyses. The modelling framework developed here is simple and parsimonious, and provides sufficient insight for the present exploratory analyses, but could be strengthened for future use through identification of data inputs using systematic search and review methods, and through investigation of some of the simplifying assumptions. However, given the expectation that EARS will be a relatively low-cost intervention, the framework set out here for modelling differences in life expectancy and QALYs over time, by smoking status, could be regarded as sufficient in scope to demonstrate cost-effectiveness, where the EARS intervention is expected to demonstrate a significant difference in proportion of quitters, at trial follow-up, at around 4% difference compared with control (see Table 58).
The smoking-cessation model set out here, for use in future CEAs, indicates that there are significant benefits associated with quitting smoking in terms of health benefits, although the current model does not include smoking-related morbidity and differences in costs associated with smoking cessation. The model does not consider the benefits associated with cutting down/reducing smoking, the benefits of increasing PA or the potential health risk associated with smoking cessation (e.g. weight gain). In addition, the model does not consider specific health outcomes related to reduced morbidity. It is also appropriate to acknowledge that the potential benefits from smoking cessation, at a wider societal perspective, have not been considered, such as increased productivity, potential reduction in absenteeism from the workplace, and the impacts on others, and as such the overall impact of EARS/smoking-cessation interventions are likely to be underestimated in the modelling framework (analytical perspective) used here. However, as demonstrated in other studies, this model framework shows the potential pay-offs from smoking cessation, and indicates that low-cost interventions (such as EARS) are likely to be cost-effective.
As with any model, or economic evaluation, there are a number of limitations inherent in the analyses and methods used. It has not been possible, within the current research, to develop a de novo model, and the model presented here adapts the methods previously presented by Coleman et al. 101 However, as with all modelling studies in this area, there are limitations with the methods and data available to model mortality by smoking status, and to consider the impact of smoking related comorbid conditions, as well as limitations in data and methods to consider wider societal impacts.
As discussed above, there are limitations in the data available to specifically consider ‘hard-to-reach’ smokers, and to specifically analyse costs and outcomes related to that target group, where there is no intention to make a quit attempt in the immediate future.
Chapter 7 Discussion and conclusions
Context
Few studies have evaluated the effectiveness and cost-effectiveness of behavioural support for smoking reduction among smokers who do not wish to quit in the immediate future, and none explicitly for disadvantaged adult smokers (see NICE Public Health Draft Guidance on ‘Tobacco: harm reduction approaches to smoking’: http://guidance.nice.org.uk/PHG/52). The few reported studies involving adults have shown no evidence for any effectiveness and vary in terms of counselling alone, or counselling in combination with pharmacological support. This study is therefore timely as interest grows in interventions for harm reduction, and ones that may reduce health inequalities.
This pilot RCT was not formally powered to detect plausible differences in outcomes or to undertake between-group (intervention vs. control) comparisons of outcomes at follow-up. However, to help inform the power of a future definitive trial we did estimate the intervention effect size (and its precision) for the primary outcome. The quantitative and qualitative findings provide some preliminary support for a behavioural approach to help disadvantaged smokers (who do not wish to quit in the immediate future) to reduce their smoking and, to some extent, use PA as an aid for smoking reduction.
There is little evidence that smoking reduction alone reduces health risk so our aim was to support smoking reduction, knowing that that those who smoke less are more likely to quit. Hence, our main research outcome was quitting, with secondary interest in smoking reduction, as well as changes in PA.
Intervention content, design, acceptance and feasibility
Following extensive background research (over many years) on the interactions between PA and smoking, we engaged with smokers, lifestyle advisors and service providers in disadvantaged communities, as well as with a range of academic expertise, to establish an appropriate intervention.
One of the primary aims of the EARS study was to develop an acceptable and feasible intervention for disadvantaged smokers who wish to reduce but not quit, using PA as an aid to smoking reduction. To avoid the potentially confounding effects of NRT, we asked participants to not use NRT during the intervention; those in the intervention arm would have used more NRT than the control arm if we had encouraged this and any effects on smoking may not have been attributable to PA. We found in the study that no more than two participants did use NRT during the intervention. We expected participants to use NRT after quitting and 11 of the 14 quitters did so.
Both quantitative and qualitative data indicated that the intervention was well received. A focus on reduction, rather than quitting, was a fundamentally important aspect of the intervention as identified by participants and HTs. Many would not have engaged with the study had it been introduced as a smoking-cessation programme. The number and timing of counselling sessions received, the way support was offered (by telephone or face to face), the location where sessions took place, the speed of progress in changing smoking and PA, and additional tailored support (e.g. subsidies for exercise facilities and use of local walking groups) all contributed to the delivery of a client-centred and empowering intervention.
The biggest challenge we faced was integrating the promotion of both PA and smoking reduction. Substantial effort was invested in how the participant information sheet presented the intervention content, how HTs introduced PA as a potentially facilitating behaviour for smoking reduction, and how they used behaviour change techniques to support both behaviours. Quantitative and qualitative information suggest that a number of intervention participants did increase PA (measured objectively and subjectively), at least during the intervention, but that not all participants used PA as an aid to smoking reduction. The intervention focused more on smoking-reduction strategies than increasing PA for many participants.
We have learned a lot about how the intervention was delivered and received from both quantitative and qualitative data. With further refinement to training and delivery, the acceptability and effectiveness could be further enhanced and fidelity improved. The following specific suggestions could be considered to improve the intervention delivery:
-
Train the EARs practitioners using case studies, and audio and role-play examples drawn from the present pilot study.
-
Use mobile telephone texts (that do not cost the smoker) and other technology to prompt smoking reduction (if acceptable to individual smokers) and use of PA.
-
Enhance the quality of tools that may support behaviour change (smoking reduction and PA) and intervention understanding (e.g. self-monitoring sheets, behavioural prompts, a case study and/or self-help guide to show how the intervention works and what behavioural options may be available) and ensure that these are available to smokers.
-
Consider further incentives and increasing uptake of subsidies to engage in PA that is sociable, fulfilling and self-determined.
-
Offer the use more sophisticated pedometers to assess and memorise step counts on a daily basis, between sessions, to enhance the utility of self-monitoring.
-
Consider allowing smokers to receive support for longer, especially if they are making progress with reduction.
Trial design and methods
The study was designed as a pilot RCT due to uncertainty about the recruitment of disadvantaged smokers, the acceptance of trial methods, and the likely effects on the primary outcomes (in order to adequately power the study). Given the paucity of research on behavioural support for smoking reduction and cessation induction we could only speculate on the proportion, in both arms of the trial, who would make a quit attempt and remain abstinent.
This study provides support for the trial design and methods being acceptable and feasible among disadvantaged smokers, as indicated by our ability to recruit 100 participants (with the resources available over 12 months) from our target groups, and retain over 60% of participants up to 16 weeks. Overall, the methods performed quite well for a pre-defined disadvantaged population. We have learned a lot about the optimal design and methods to conduct a fully powered trial, as well as the resources needed to do this in a timely manner, from both quantitative and qualitative data. We briefly consider some of these lessons in terms of personnel, location, recruitment, and outcomes and data collection procedures.
Discrepancies within this document
Readers may find different information reported with regard to the design and methods in different sections of this document. This is due to the sequential development from the initial proposal and protocol (see Appendix 2) through to the final analyses and reporting. Below are some examples:
-
Inclusion criteria by number of cigarettes smoked. The aim of the inclusion criteria (≥ 15 cigarettes per day) was to ensure that we had moderate to heavy smokers who have been reported to have a greater interest in reduction. We achieved this in terms of number of cigarettes smoked, but recruitment and assessment methods made the criteria hard to apply. From GP records patients were identified as smokers if they smoked at least 10 cigarettes per day. It seemed inappropriate to invite all those recorded as smokers into the trial and then exclude them when they showed interest in participating if they smoked just a few cigarettes fewer than 15 per day, especially when many (which turned out to be > 60%) smoked roll-ups. We adopted the conversion of 0.45 g per roll-up based on the most rigorous published approach although other conversion rates ranging from 0.38 to 0.8 g have been informally identified. We did seek to recruit those smoking ≥ 15 but given uncertainties about the accuracy of GP records and the conversion from grams/ounces to rolled number of cigarettes our researchers were trained to apply a more lenient inclusion criteria of ≥ 10 and that is what we reported. Some of the above only became apparent as the study progressed or at the end of the study. So in the context of a pilot study, in which we sought to test our methods, we have learned that in a future study aimed at supporting reduction we should aim to recruit those smoking ≥ 10, to match GP records and accommodate uncertainty about the precise number of cigarettes smoked. The lowering of the inclusion criteria on cigarettes smoked per day may have reduced the impact of the intervention (in terms of scope for reduction of cigarettes smoked) but also increased the generalisability to include a broader range of smokers.
-
Exclusion of people with mental illness or ongoing substance abuse. Within the context of a pilot study involving ‘hard-to-reach’ participants we needed to strike a balance between including as broad a range of participants across diverse definitions of ‘hard-to-reach’ as possible, while also safeguarding our research team. To achieve the latter, we put in place a number of checks, including the following: all participants had to be registered with and approved suitable for the study by the GP; our research team followed a lone worker policy which involved them meeting participants only in NHS facilities and, if working late, informing another member of the team prior to and after each session with a participant. We also wished to test the trial methods and intervention with smokers who were able to respond so while we had broad exclusion criteria to ensure the above, there were a small number of participants with mental health and substance abuse problems who were deemed suitable for the study by the GP. For example, one participant with serious mental illness was deemed suitable but then withdrew after the initial session due to emerging difficulties, as advised by a mental health care worker. All of the above issues were widely discussed among the research team, including four GPs.
-
Number of people to be recruited into the study. As a pilot study we estimated, to the best of our ability, what resources would be needed to recruit n = 120. We have learned that, despite our best efforts, the necessary resources were insufficient for the time scale and approaches set. One member of the research team (0.4 fte) took compassionate leave for 2 months, and another (0.5 fte) was on sick leave for 3 months during the final period of recruitment. We conducted an interim analysis (with the first 60 participants) of the implications of recruiting n = 100 versus seeking an extension to achieve our initial target of n = 120. We concluded that given the small numbers making a quit in the control arm, and the numbers quitting in the intervention arm, the study would provide the information we were seeking to gain within the aims of the study. The NHS Research Ethics Committee and National Institute for Health Research (NIHR) were informed and accepted this.
-
Recruitment of participants through the community to include mailed invitations from SSSs. As we described in the initial proposal/protocol and Chapter 2 (see Trial design and methods), we involved a wide range of potential community stakeholders (though smokers who want to reduce but not quit may not necessarily regard themselves as a clinical population like with other medical conditions) to inform how best to engage with participants. Distinct from the 50% recruited via primary care, we regarded the recruitment, by mailed invitation of those failing to quit (in the previous 2 years) with SSS support in Devonport and Stonehouse, as community recruits.
-
The capturing of the primary outcome. Within the context of this pilot trial we had intended that all those deciding to quit, in both arms of the trial, would contact the SSS to receive support (usual care) in the form of 6 or 7 weekly contacts during which CO would be monitored along with self-reported abstinence. This occurred for only two participants who decided to quit. We also offered to provide HT support while attending the SSS after quitting in the original protocol. A few participants chose this but not all. We also built into the study design an assessment (of self-reported abstinence and confirmed expired air CO) of those who we knew had set a quit date after 4 weeks. We did our best to ensure that all participants were contacted at 4 weeks after their quit date and that a measure of expired air CO and self-reported absence of smoking was obtained. For some participants this did not happen due to missed appointments until up to 8 weeks post quitting. All reported successful quitters did provide self-reported abstinence since the quit day (having smoked no more than five cigarettes since quitting) and expired air CO-confirmed abstinence between 4 and 8 weeks post quit.
Personnel
The EARS pilot trial and intervention was implemented through a dual HT/researcher role. In our initial proposal we argued that this could provide a more seamless interface between the disadvantaged smoker and the research team, compared with engaging with a practitioner to deliver the intervention and a researcher to collect data. A disadvantage was that the trial involved no blinding in the data collection at follow-up.
While interviews with participants suggested that they were generally in favour of this seamless approach, they had little or no experience of other research to make comparisons. We have identified a number of reasons why a future study should consider separating the roles of practitioner and researcher.
-
Capturing the primary outcome proved to be more complicated than initially envisaged (in part due to low uptake of SSSs by quitters) resulting in an overburdening of the HTs’ work capacity. A designated researcher role could focus more explicitly on following up and capturing the data outcomes without the time implications of having to deliver the intervention. This could potentially increase the standard and rate of data completion at follow-up.
-
The HTs found it very difficult to withhold any support from participants allocated to the control arm as they were trained in delivering the intervention, and withholding support and advice went against their instincts, something they reported as being morally challenging. A researcher, not trained in intervention delivery, would not face similar tensions.
-
The HT found the routine collection of data in some weeks, prior to proceeding with the intervention in a session, disruptive to establishing rapport. Thus a sole practitioner role may help the focus on intervention engagement.
-
It was a challenging dual role for our HTs and with this in mind they were appointed at a professional level more senior and experienced than the typical HT. If a future study was to evaluate the effectiveness of the intervention delivered by a typical HT then the roles would have to be separated, with designated researchers with different skill sets.
-
Our EARS training manual seeks to build on the skills and competencies of a HT. It may be that other lifestyle advisor roles (see Carr et al. 47) could also be adapted to deliver the intervention.
-
We did not fully utilise a trial administrator for arranging appointments and assisting with the monitoring of intervention sessions and assessments. A better use of such a resource could allow the HTs and a researcher to focus on their own roles.
We had planned to employ two HTs/researchers, each as 0.7 fte. Instead we had employed three, adding up to 1.4 fte. Shared supervision and team building took place for 2 hours on most weeks. This was very beneficial to the study as ideas and experiences were exchanged and during times of sick leave we could mostly provide some continuity of support for participants. We also implemented a flexible working policy and many support sessions with participants took place in the evening, using mobile telephones.
Location
We had originally envisaged conducting assessments and intervention sessions in a wide range of settings, such as in GP surgeries, SSS buildings, community centres, participants’ homes, and leisure facilities. We considered the implications of these settings on perceptions of the study and intervention, and the safety and risks posed to the research team. Eventually, following extensive consultation with local stakeholder groups and service users, a centralised NHS community minor injury and outpatient centre (with available clinical space) was selected to house the research team and conduct sessions.
Recruitment
Targets for recruiting subgroups of ‘hard-to-reach’ or disadvantaged smokers were set out in our research proposal, based on guidance from local GPs (also co-applicants) RA and RB, and their awareness of the demographics and smoking prevalence in Devonport and Stonehouse. These targets were at least met in this study in terms of employment and mental health status. The sample, overall, were heavy smokers but tended to be quite active, relative to the general population, due to manual employment and daily walking for transport. We aimed to explore the feasibility of recruitment and the resource implications. Once again, we learned a great deal about recruiting through mailed invitations, from both GP surgeries and the SSS. Between 5% and 11% of those invited can be recruited in this way, depending on the resources invested in reminders and reminder telephone calls. Finding available expertise to conduct searches of patient databases was challenging. There was variability in how surgeries wished to screen participants (i.e. everyone invited or only those responding to the invitation), which affected timescales relating to the mailing of invitations. We tried to reduce risk to both participant and HT through our screening procedures and this appeared to be effective as we encountered no adverse or serious adverse events or incidents that threatened the safety of our HTs.
Currently, the SSS only reaches a similar proportion (i.e. c. 5%) of smokers and new approaches are needed to increase the reach to more treatment-resistant smokers. Our blanket invitation to all smokers to join the trial included both those presently wishing to reduce (c. 60%) and those not interested in reducing (c. 40%). Therefore, in effect we recruited c. 15% of those wishing to reduce at the time of invitation, which increases the generalisability somewhat. The reported differences in smoking status in the present study, if confirmed in a larger study, would support this low-cost intervention as a way of providing support to the large numbers of smokers who do not wish to quit. Speculatively, similar trial methods in another study with fewer ‘hard-to-reach’ smokers would result in an even greater response rate. One may also speculate that outside the remit of a RCT the EARS intervention, if offered in primary care or the community, may appeal to a larger proportion of smokers.
Other approaches to community recruitment involved considerable effort and produced few recruits in return. This mirrored the experiences of SSS advisors who had previously sought to provide support for abrupt quit attempts in the same area of Plymouth.
A future study should consider the following:
-
A larger trial should recruit from a more ethnically diverse sample to enhance generalisability and explore subgroup effects.
-
Other outreach approaches to recruitment such as subcontracting personnel/agencies outside the research team, taking more time to develop relationships with community-based organisations and worksites, and the costs associated with such approaches.
Study retention
While the loss of 38% of participants to follow-up assessment is a potential threat to internal validity, our assumption that those who did not return for assessments remained smoking is based on previous research and widely accepted protocols (i.e. Russell Standard). The loss to follow-up was comparable in both arms of the trial but this does not confirm that the reasons for study attrition were the same. In reporting descriptive baseline data for those who completed the study and those who withdrew we tried to identify any clear baseline factors that may have threatened internal validity but none were evident given the small sample size.
In phase 1, during consultations with stakeholders, we did identify that participants would be more motivated to take part if there was a financial incentive. However, we chose not to adopt this approach due to budgetary, pragmatic and ethical issues that may arise. For example, some participants did not have a bank account and others feared losing benefit allowances by taking money. We also wanted to maximise external validity by working only with participants who were interested in the study and the possibility of receiving the intervention. As we note in the report, we did pay participants £10 for wearing and returning an accelerometer for 1 week, increasing to £30 later in the study, which did increase compliance to wearing. Without placing any emphasis on this when inviting participants into the study only a few participants anecdotally came for the first assessment to receive the money and did not return. A fear would be that a greater emphasis on incentives may confound intervention effects.
Outcomes and data collection
We originally expected to capture our primary outcome (4 weeks’ post-quit date expired air CO abstinence) with the help of a SSS advisor after referral of any participant who wished to quit. Only two of the participants who wished to quit used support from the SSS. This created a challenge for us to capture the most robust or conservative outcome measure possible. We identify the criteria used in Chapter 3 and Chapter 4 to provide the most conservative outcome. The number of successful quitters was also confirmed with data for 16-week point prevalence.
We report that in the intervention and control arms, respectively, 22% versus 6% had at least a 24-hour quit attempt, 14% versus 4% had expired air CO-confirmed abstinence at 4–8 weeks after quitting, and 10% versus 4% were abstinent at 16 weeks. Also, 31% versus 16%, and 39% versus 20% had reduced by at least 50% by weeks 8 and 16, respectively.
A future study should consider the following:
-
the measurement of variable quit dates and floating abstinence for trials involving behavioural support
-
use of differing process measures for those who quit and those who do not
-
consider a longer-term follow-up smoking outcome (e.g. 6 months post intervention).
Intervention adherence appeared to be associated with the main smoking outcomes, though no inferential statistics were conducted. A future study should consider the following:
-
consider ways to maximise adherence to the intervention
-
conduct sensitivity analysis to determine the effects of dose of intervention received on the main outcomes.
Strengths and limitations
Strengths
-
This is the first study to explore the effectiveness of a behavioural intervention to increase PA as an aid for smoking reduction and cessation induction.
-
The study provided valuable information to inform a future trial design and methods. Our scenario analysis conducted to determine a sample size for the present study closely resembled the findings in the study.
-
The mixed methods approach provided valuable information to further develop and deliver the intervention.
-
Targets were met for recruiting disadvantaged smokers.
-
The very detailed health economic analysis provides a solid basis for designing the economic analysis for a full-scale trial.
Limitations
-
The study involved 97% white British participants, which may limit the generalisability of the findings.
-
The expired air CO-confirmed 4 weeks’ post-quit outcome was not as robust as planned, though is supported by data collected at 16 weeks.
-
The researchers were not blinded to trial arm and this may have introduced some bias into the data collection procedures.
-
We describe the intervention as one delivered by a HT. Because of the dual role of HT and researcher we adopted for the study, our researchers were probably more skilled and experienced in behaviour change than many working as HTs. The findings (albeit not intended to inform practice and policy) may therefore not generalise to other HTs.
-
We did not systematically identify the extent to which the planned BCTs outlined in the protocol were delivered and during what phase of the intervention.
-
Cost-effectiveness analyses are exploratory, with uncertainty around effectiveness data inputs and other parameter inputs, and their policy relevance is limited.
Implications for health care
It is premature for any guidance to be derived from the present study for health professionals, policy makers and commissioners because the findings provide only preliminary support for the EARS intervention with a relatively short follow-up and sampled from a non-ethnically diverse population. The future of HTs may be uncertain, so any health professional could be involved in delivering the intervention with little impact on the apparent cost-effectiveness. The Health and Social Care Act of 2012132 creates uncertainty about where such an intervention would be delivered but if a definitive trial demonstrated the EARS intervention to be effective then a wide range of professionals could be trained to do so.
Implications for future research
A larger, fully powered trial with a longer follow-up is needed to confirm the effectiveness and cost-effectiveness of the EARS intervention. Minor refinement of the intervention is needed. There will be a need for further initial exploratory work (e.g. 4 months) on adapting the intervention for a more ethnically diverse sample. A larger study could help to add further information about the core effective components of the intervention, and any moderators and mediators of any effects. The framework set out here for future cost-effectiveness analyses appears sufficient where the EARS intervention is low cost and demonstrates modest effectiveness; however, further complexity in modelling could be useful to further demonstrate the broader potential benefits of the EARS intervention.
We are not aware of any research on weight gain associated with smoking reduction (i.e. not cessation). The present study suggests that this should be considered carefully in future research. If indeed reduction is associated with weight gain then this may provide a rationale for more support for smokers to further increase PA to minimise the risk of weight gain.
Future research should consider the reward of vouchers or other non-monetary incentives to increase study retention. In terms of staffing, a dedicated administrator should be used to arrange appointments and issue prior reminders, and thus add to the resources available to increase recruitment and retention.
Conclusions
Through a mixed methods approach, this study aimed to develop and evaluate the feasibility and acceptability of a novel PA and smoking-reduction counselling intervention (for disadvantaged smokers who do not wish to quit in the immediate future but do want to reduce their smoking) and the methods to inform the design of a fully powered definitive RCT.
Sufficient quantitative and qualitative data were gathered to indicate that the intervention was acceptable for participants and seemed to facilitate an increase in PA and reduction in smoking, as well as inducting more quit attempts and more short-term abstinence. Evaluation of the methods suggested that it is feasible to recruit, randomise and retain disadvantaged smokers in a trial with up to 16 weeks’ follow-up.
Important lessons were learned that may enhance the intervention delivery and the training of HTs. Some refinements of the trial methods could also help to enhance recruitment and retention of participants and improve the efficiency of the procedures, particularly in terms of staffing roles.
We estimated that the EARS intervention cost approximately £192 per participant. A full trial would provide greater confidence in modelling the costs and cost-effectiveness as a smoking-cessation induction intervention which could be delivered in disadvantaged communities to help address health inequalities.
Acknowledgements
We would like to thank the external members of the trial steering committee for their advice and support for the project: Linda Bauld, Marcus Munafo and Charlie Foster.
We are grateful to the participants and GPs who supported the study, giving so generously their time and sharing their experience with us; likewise, the practice managers and administrative staff at all of the collaborating practices and primary care trusts who provided valuable assistance to us throughout the study.
We would like to thank a number of people who helped towards the successful completion of the study, especially Mel Fairbairn, Maggie Kelly and Julie Lloyd who worked as the EARS HTs and researchers for the study; Marcela Haasova who helped with the baseline data analysis and Pippa Griew who supported in the preparation of the accelerometer data. We would also like to thank Naomi Southern who provided administrative support to the trial. We are grateful to the test subjects who provided invaluable feedback as smokers who did not wish to quit in the immediate future during the intervention development. We are also grateful for input from many community professionals and non-professionals. We thank the Plymouth Stop Smoking Service team, including Russell Moody and Mandy Luffman who advised on the intervention development, helped to recruit smokers, and received referred smokers who wished to quit. We thank the Plymouth NHS public health team, and community leaders and volunteers who advised on recruitment initiatives and intervention development. We thank John Thompson who supported practice managers and us with the searches of GP practice databases.
Ethical approval
Ethical approval for the study was granted by the NHS National Research Ethics Service Committee South West, UK.
Local Research Ethics Committee approval and the appropriate site-specific assessments were obtained from the Plymouth NHS Hospital Primary Care Trusts and all study staff held honorary contracts for working within the NHS trust.
The Trial was registered with the International Standard Randomised Controlled Trial Register – ISRCTN (16900744) and the National Research Register (2159).
Funding
This study was funded by the NIHR Health Technology Assessment Programme – project number 07/78/02.
The University of Exeter agreed to act as sponsor for the research and the study was adopted by the Primary Care Research Network (PCRN).
Contributions of authors
AH Taylor (as principal investigator) had overall responsibility for the study and TP Thompson was responsible for the day-to-day operationalisation and management of the study.
Initial drafting of the text was done by AH Taylor and TP Thompson, for all chapters except for Chapter 5 (Process and qualitative evaluation) which was written by TP Thompson, CJ Greaves and AH Taylor, and for Chapter 6 (Economic analysis), which was written by C Green and R Kandiyali.
AH Taylor and TP Thompson worked with FC Warren on data analysis and reporting for Chapter 3.
AH Taylor, TP Thompson, CJ Greaves and P Aveyard were responsible for developing the physical activity intervention and for its description in the report, as well as developing and applying the intervention fidelity checklist.
TP Thompson and CJ Greaves carried out the qualitative fieldwork, and performed the analysis, with the assistance of AH Taylor.
RS Taylor and FC Warren developed the statistical analysis plan with AH Taylor and TP Thompson.
R Kandiyali and C Green developed the economic analysis plan with TP Thompson and AH Taylor.
All co-applicants (AH Taylor, CJ Greaves, RS Taylor, C Green, P Aveyard, R Ayres, R Byng, JL Campbell, M Ussher, S Michie and R West) were involved in all stages of the work: the design, analysis, and commenting upon and drafting sections of the final report.
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Department of Health.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- McEwen A, Hajek P, McRobbie H, West R. Manual of Smoking Cessation: A Guide for Counsellors and Practitioners. Oxford: Blackwell Publishing; 2006.
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004;99:29-38. http://dx.doi.org/10.1111/j.1360-0443.2004.00540.x.
- Ferguson J, Bauld L, Chesterman J, Judge K. The English smoking treatment services: one-year outcomes. Addiction 2005;100:59-6. http://dx.doi.org/10.1111/j.1360-0443.2005.01028.x.
- Bauld L, Judge K, Platt S. Assessing the impact of smoking cessation services on reducing health inequalities in England: observational study. Tob Control 2007;16:400-4. http://dx.doi.org/10.1136/tc.2007.021626.
- Hiscock R, Bauld L, Amos A, Fidler JA, Munafo M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci 2012;1248:107-23. http://dx.doi.org/10.1111/j.1749-6632.2011.06202.x.
- Fidler JA, Shahab L, West O, Jarvis MJ, McEwen A, Stapleton JA, et al. The smoking toolkit study: a national study of smoking and smoking cessation in England. BMC Public Health 2001;11. http://dx.doi.org/10.1186/1471-2458-11-479.
- Bryant J, Bonevski B, Paul C, McElduff P, Attia J. A systematic review and meta-analysis of the effectiveness of behavioural smoking cessation interventions in selected disadvantaged groups. Addiction 2011;106:1568-85. http://dx.doi.org/10.1111/j.1360-0443.2011.03467.x.
- Michie S, Rumsey N, Fussell S, Hardeman W, Johnston M, Newman S, et al. Improving Health – Changing Behaviour: NHS Health Trainer Handbook. London: Department of Health and British Psychological Society; 2008.
- Michie S, Jochelson K, Markham WA, Bridle C. Low income groups and behaviour change interventions: a review of intervention content and effectiveness. J Epidemiol Community Health 2009;63:250-6. http://dx.doi.org/10.1136/jech.2008.078725.
- Beard E, Vangeli E, Michie S, West R. The use of nicotine replacement therapy for smoking reduction and temporary abstinence: an interview study. Nicotine Tob Res 2012;14:849-56. http://dx.doi.org/10.1093/ntr/ntr297.
- Black A, Beard E, Brown J, Fidler J, West R. Beliefs about the harms of long-term use of nicotine replacement therapy: perceptions of smokers in England. Addiction 2012;107:2037-42. http://dx.doi.org/10.1111/j.1360-0443.2012.03955.x.
- Beard E, McDermott M, McEwen A, West R. Beliefs of stop smoking practitioners in United kingdom on the use of nicotine replacement therapy for smoking reduction. Nicotine Tob Res 2012;14:639-47. http://dx.doi.org/10.1093/ntr/ntr260.
- Lindson N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database Syst Rev 2010;11. http://dx.doi.org/10.1002/14651858.CD008033.
- Shiffman S, Hughes JR, Ferguson SG, Pillitteri JL, Gitchell JG, Burton SL. Smokers’ interest in using nicotine replacement to aid smoking reduction. Nicotine Tob Res 2007;9:1177-82. http://dx.doi.org/10.1080/14622200701648441.
- Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technol Assess 2008;12.
- Vangeli E, Stapleton J, Smit ES, Borland R, West R. Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addiction 2011;106:2110-21. http://dx.doi.org/10.1111/j.1360-0443.2011.03565.x.
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol 2004;72:371-81. http://dx.doi.org/10.1037/0022-006X.72.3.371.
- Hughes JR, Carpenter MJ. The feasibility of smoking reduction: an update. Addiction 2005;100:1074-89. http://dx.doi.org/10.1111/j.1360-0443.2005.01174.x.
- Haasova M, Warren FC, Ussher M, Janse Van Rensburg K, Faulkner G, Cropley M, et al. The acute effects of physical activity on cigarette cravings: systematic review and meta-analysis with individual participant data (IPD). Addiction 2013;108:26-37. http://dx.doi.org/10.1111/j.1360-0443.2012.04034.x.
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction 2007;102:534-43. http://dx.doi.org/10.1111/j.1360-0443.2006.01739.x.
- Taylor A, Katomeri M. Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine Tob Res 2007;9:1183-90. http://dx.doi.org/10.1080/14622200701648896.
- Faulkner G, Arbour-Nicitopoulos KP, Hsin A. Cutting down one puff at a time: the acute effects of exercise on smoking behavior. J Smoking Cessation 2010;5:130-5. http://dx.doi.org/10.1375/jsc.5.2.130.
- Whiteley JA, Williams DM, Dunsiger S, Jennings EG, Ciccolo JT, Bock BC, et al. YMCA Commit to Quit: randomized trial outcomes. Am J Prev Med 2012;43:256-62.
- Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev 2012;1. http://dx.doi.org/10.1002/14651858.CD002295.pub2.
- Taylor AH, Ussher M, Ekkekakis P. Handbook on Physical Activity and Mental Health. New York, NY: Routledge; 2013.
- Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e4439.
- Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev 2012;1. http://dx.doi.org/10.1002/14651858.CD006219.pub3.
- Everson ES, Taylor AH, Ussher M, Faulkner G. A qualitative perspective on multiple health behaviour change: views of smoking cessation advisors who promote physical activity. J Smoking Cessation 2010;5:7-14. http://dx.doi.org/10.1375/jsc.5.1.7.
- Blundell JE, King NA. Exercise, appetite control, and energy balance. Nutrition 2000;16:519-22. http://dx.doi.org/10.1016/S0899-9007(00)00250-1.
- Taylor AH, Bouchard C, Katzmarzyk PT. Advances in Physical Activity and Obesity. Champaign, IL: Human Kinetics; 2010.
- Lycett D, Munafo M, Johnstone E, Murphy M, Aveyard P. Weight change over eight years in relation to alcohol consumption in a cohort of continuing smokers and quitters. Nicotine Tob Res 2011;13:1149-54. http://dx.doi.org/10.1093/ntr/ntr092.
- Aubin HJ, Rollema H, Svensson TH, Winterer G. Smoking, quitting, and psychiatric disease: a review. Neurosci Biobehav Rev 2012;36:271-84. http://dx.doi.org/10.1016/j.neubiorev.2011.06.007.
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, et al. Exercise for depression. Cochrane Database Syst Rev 2013;9.
- Taylor AH, Biddle S, Boutcher S, Fox K. Physical Activity and Psychological Well-being. London: Routledge; 2000.
- Van Rensburg KJ, Taylor A, Hodgson T. The effects of acute exercise on attentional bias towards smoking-related stimuli during temporary abstinence from smoking. Addiction 2009;104:1910-7. http://dx.doi.org/10.1111/j.1360-0443.2009.02692.x.
- Janse Van Rensburg K, Taylor A, Benattayallah A, Hodgson T. The effects of exercise on cigarette cravings and brain activation in response to smoking-related images. Psychopharmacology 2012;221:659-66. http://dx.doi.org/10.1007/s00213-011-2610-z.
- Verkooijen KT, Nielsen GA, Kremers SP. The association between leisure time physical activity and smoking in adolescence: an examination of potential mediating and moderating factors. Int J Behav Med 2008;15:157-63. http://dx.doi.org/10.1080/10705500801929833.
- Kaczynski AT, Manske SR, Mannell RC, Grewal K. Smoking and physical activity: a systematic review. Am J Health Behav 2008;32:93-110. http://dx.doi.org/10.5993/AJHB.32.1.9.
- Deruiter WK, Faulkner G, Cairney J, Veldhuizen S. Characteristics of physically active smokers and implications for harm reduction. Am J Public Health 2008;98:925-31. http://dx.doi.org/10.2105/AJPH.2007.120469.
- Taylor AH, Doust J, Webborn N. Randomised controlled trial to examine the effects of a GP exercise referral programme in Hailsham, East Sussex, on modifiable coronary heart disease risk factors. J Epidemiol Community Health 1998;52:595-601. http://dx.doi.org/10.1136/jech.52.9.595.
- Bull FC, Jamrozik K. Advice on exercise from a family physician can help sedentary patients to become active. Am J Prev Med 1998;15:85-94. http://dx.doi.org/10.1016/S0749-3797(98)00040-3.
- Cerin E, Leslie E. How socio-economic status contributes to participation in leisure-time physical activity. Soc Sci Med 2008;66:2596-609. http://dx.doi.org/10.1016/j.socscimed.2008.02.012.
- Cerin E, Leslie E, Owen N. Explaining socio-economic status differences in walking for transport: an ecological analysis of individual, social and environmental factors. Soc Sci Med 2009;68:1013-20. http://dx.doi.org/10.1016/j.socscimed.2009.01.008.
- Cleland CL, Tully MA, Kee F, Cupples ME. The effectiveness of physical activity interventions in socio-economically disadvantaged communities: a systematic review. Prev Med 2012;54:371-80. http://dx.doi.org/10.1016/j.ypmed.2012.04.004.
- Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-119.
- London: The Stationery Office; 2004.
- Carr SM, Lhussier M, Forster N, Geddes L, Deane K, Pennington M, et al. An evidence synthesis of qualitative and quantitative research on component intervention techniques, effectiveness, cost-effectiveness, equity and acceptability of different versions of health-related lifestyle advisor role in improving health. Health Technol Assess 2011;15.
- Taylor AH, Everson-Hock ES, Ussher M. Integrating the promotion of physical activity within a smoking cessation programme: findings from collaborative action research in UK Stop Smoking Services. BMC Health Serv Res 2010;10. http://dx.doi.org/10.1186/1472-6963-10-317.
- Koshy P, Mackenzie M, Leslie W, Lean M, Hankey C. Eating the elephant whole or in slices: views of participants in a smoking cessation intervention trial on multiple behaviour changes as sequential or concurrent tasks. BMC Public Health 2012;12. http://dx.doi.org/10.1186/1471-2458-12-500.
- Miller WR. Motivational Interviewing with problem drinkers. Behav Psychother 1983;11:147-72. http://dx.doi.org/10.1017/S0141347300006583.
- Deci EL, Ryan RM. Intrinsic motivation and self-determination in human behavior. New York, NY: Plenum Press; 1985.
- Deci EL, Ryan RM. The what and why of human goal pursuits: human needs and the self-determination of behavior. Psychol Inq 2000;11:227-68. http://dx.doi.org/10.1207/S15327965PLI1104_01.
- Williams GC, McGregor HA, Sharp D, Levesque C, Kouides RW, Ryan RM, et al. Testing a self-determination theory intervention for motivating tobacco cessation: supporting autonomy and competence in a clinical trial. Health Psychol 2006;25:91-101. http://dx.doi.org/10.1037/0278-6133.25.1.91.
- Vansteenkiste M, Sheldon KM. There’s nothing more practical than a good theory: integrating motivational interviewing and self-determination theory. Br J Clin Psychol 2006;45:63-82. http://dx.doi.org/10.1348/014466505X34192.
- Markland D, Ryan RM, Tobin VJ, Rollnick S. Motivational interviewing and self-determination theory. J Social Clin Psychol 2005;24:811-31. http://dx.doi.org/10.1521/jscp.2005.24.6.811.
- Miller WR, Rollnick S. Meeting in the middle: motivational interviewing and self-determination theory. Int J Behav Nutr Phys Act 2012;9. http://dx.doi.org/10.1186/1479-5868-9-25.
- Patrick H, Williams GC. Self-determination theory: its application to health behavior and complementarity with motivational interviewing. Int J Behav Nutr Phys Act 2012;9. http://dx.doi.org/10.1186/1479-5868-9-18.
- Boardman T, Catley D, Grobe JE, Little TD, Ahluwalia JS. Using motivational interviewing with smokers: do therapist behaviors relate to engagement and therapeutic alliance?. J Subst Abuse Treat 2006;31:329-39. http://dx.doi.org/10.1016/j.jsat.2006.05.006.
- Draycott S, Dabbs A. Cognitive dissonance. 2: a theoretical grounding of motivational interviewing. Br J Clin Psychol 1998;37:355-64. http://dx.doi.org/10.1111/j.2044-8260.1998.tb01391.x.
- Moyers TB, Martin T, Christopher PJ, Houck JM, Tonigan JS, Amrhein PC. Client language as a mediator of motivational interviewing efficacy: where is the evidence?. Alcohol Clin Exp Res 2007;31:40s-7s. http://dx.doi.org/10.1111/j.1530-0277.2007.00492.x.
- Smedslund G, Berg RC, Hammerstrom KT, Steiro A, Leiknes KA, Dahl HM, et al. Motivational interviewing for substance abuse. Cochrane Database Syst Rev 2011;5. http://dx.doi.org/10.1002/14651858.CD008063.
- Hettema JE, Hendricks PS. Motivational interviewing for smoking cessation: a meta-analytic review. J Consult Clin Psychol 2010;78:868-84. http://dx.doi.org/10.1037/a0021498.
- Hardcastle S, Taylor AH, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet, and CHD risk factors. Patient Educ Couns 2008;70:31-9. http://dx.doi.org/10.1016/j.pec.2007.09.014.
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986.
- Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality-social, clinical, and health psychology. Psychol Bull 1982;92:111-35. http://dx.doi.org/10.1037//0033-2909.92.1.111.
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390-5. http://dx.doi.org/10.1037//0022-006X.51.3.390.
- Everson-Hock ES, Taylor AH, Ussher M. Readiness to use physical activity as a smoking cessation aid: a multiple behaviour change application of the transtheoretical model among quitters attending Stop Smoking Clinics. Patient Educ Couns 2010;79:156-9. http://dx.doi.org/10.1016/j.pec.2009.09.016.
- Orbell S, Verplanken B. The automatic component of habit in health behavior: habit as cue-contingent automaticity. Health Psychol 2010;29:374-83. http://dx.doi.org/10.1037/a0019596.
- Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol 2006;14:12-9. http://dx.doi.org/10.1037/1064-1297.14.1.12.
- Lindson N, Aveyard P, Ingram JT, Inglis J, Beach J, West R, et al. Rapid reduction versus abrupt quitting for smokers who want to stop soon: a randomised controlled non-inferiority trial. Trials 2009;10. http://dx.doi.org/10.1186/1745-6215-10-69.
- Ussher M, Cropley M, Playle S, Mohidin R, West R. Effect of isometric exercise and body scanning on cigarette cravings and withdrawal symptoms. Addiction 2009;104:1251-7. http://dx.doi.org/10.1111/j.1360-0443.2009.02605.x.
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger A, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007;298:2296-304. http://dx.doi.org/10.1001/jama.298.19.2296.
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690-701. http://dx.doi.org/10.1037/a0016136.
- Michie S, Hyder N, Walia A, West R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict Behav 2011;36:315-9. http://dx.doi.org/10.1016/j.addbeh.2010.11.016.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479-98. http://dx.doi.org/10.1080/08870446.2010.540664.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. http://dx.doi.org/10.1136/bmj.321.7262.694.
- Murray RL, Bauld L, Hackshaw LE, McNeill A. Improving access to smoking cessation services for disadvantaged groups: a systematic review. J Public Health 2009;31:258-77. http://dx.doi.org/10.1093/pubmed/fdp008.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. http://dx.doi.org/10.1111/j.1360-0443.2004.00995.x.
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985;122:794-80.
- Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 1997;21:613-19. http://dx.doi.org/10.1111/j.1530-0277.1997.tb03811.x.
- West RJ, Russell MA. Pre-abstinence smoke intake and smoking motivation as predictors of severity of cigarette withdrawal symptoms. Psychopharmacology 1985;87:334-6. http://dx.doi.org/10.1007/BF00432717.
- West R, Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology 2004;177:195-9. http://dx.doi.org/10.1007/s00213-004-1923-6.
- Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes 2010;8. http://dx.doi.org/10.1186/1477-7525-8-13.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991;86:1119-27. http://dx.doi.org/10.1111/j.1360-0443.1991.tb01879.x.
- Fagerstrom K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res 2012;14:75-8.
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385-96. http://dx.doi.org/10.2307/2136404.
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav 2007;32:912-23. http://dx.doi.org/10.1016/j.addbeh.2006.06.028.
- Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c332.
- Laugesen M, Epton M, Frampton CM, Glover M, Lea RA. Hand-rolled cigarette smoking patterns compared with factory-made cigarette smoking in New Zealand men. BMC Public Health 2009;9. http://dx.doi.org/10.1186/1471-2458-9-194.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1655.
- Moyers TB, Martin T, Houck JM, Christopher PJ, Tonigan JS. From in-session behaviors to drinking outcomes: a causal chain for motivational interviewing. J Consult Clin Psychol 2009;77:1113-24. http://dx.doi.org/10.1037/a0017189.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. http://dx.doi.org/10.1037/0278-6133.23.5.443.
- Denford S, Campbell JL, Frost J, Greaves CJ. A qualitative exploration of processes of change in an asthma self-care intervention. Qual Health Res 2013;23:1419-29.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. http://dx.doi.org/10.1016/j.ijnurstu.2012.09.010.
- Dreyfus HL, Burke J. Competency Based Education and Training. London: Falmer Press; 1989.
- Ritchie JL, Spencer L, Bryman A, Burgess R. Analyzing Qualitative Data. London: Routledge; 1994.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU, University of Kent; 2011.
- Glick H. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Bolin K. Economic evaluation of smoking-cessation therapies: a critical and systematic review of simulation models. Pharmacoeconomics 2012;30:551-64. http://dx.doi.org/10.2165/11590120-000000000-00000.
- Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al. The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation. Health Technol Assess 2002;6.
- Coleman T, Agboola S, Leonardi-Bee J, Taylor M, McEwen A, McNeill A. Relapse prevention in UK Stop Smoking Services: current practice, systematic reviews of effectiveness and cost-effectiveness analysis. Health Technol Assess 2010;14.
- Howard P, Knight C, Boler A, Baker C. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO Simulation model: application to a population of US adult smokers. Pharmacoeconomics 2008;26:497-511. http://dx.doi.org/10.2165/00019053-200826060-00004.
- Ronckers ET, Groot W, Ament AJ. Systematic review of economic evaluations of smoking cessation: standardizing the cost-effectiveness. Med Decis Making 2005;25:437-48. http://dx.doi.org/10.1177/0272989X05278431.
- Feenstra TL, Hamberg-van Reenen HH, Hoogenveen RT, Rutten-van Molken MP. Cost-effectiveness of face-to-face smoking cessation interventions: a dynamic modeling study. Value Health 2005;8:178-90. http://dx.doi.org/10.1111/j.1524-4733.2005.04008.x.
- Hurley SF, Matthews JP. The Quit Benefits Model: a Markov model for assessing the health benefits and health care cost savings of quitting smoking. Cost Eff Resour Alloc 2007;5.
- Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling. JAMA 1996;275:1247-51. http://dx.doi.org/10.1001/jama.1996.03530400035035.
- Orme ME, Hogue SL, Kennedy LM, Paine AC, Godfrey C. Development of the health and economic consequences of smoking interactive model. Tob Control 2001;10:55-61. http://dx.doi.org/10.1136/tc.10.1.55.
- Godfrey C, Parrott S, Coleman T, Pound E. The cost-effectiveness of the English smoking treatment services: evidence from practice. Addiction 2005;100:70-83. http://dx.doi.org/10.1111/j.1360-0443.2005.01071.x.
- National Institute for Health and Care Excellence . PH1: Brief Interventions and Referral for Smoking Cessation 2006. www.nice.org.uk/guidance/PH1/guidance (accessed 8 November 2013).
- Bauld L, Boyd KA, Briggs AH, Chesterman J, Ferguson J, Judge K, et al. One-year outcomes and a cost-effectiveness analysis for smokers accessing group-based and pharmacy-led cessation services. Nicotine Tob Res 2011;13:135-45. http://dx.doi.org/10.1093/ntr/ntq222.
- Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ 1994;309:901-11. http://dx.doi.org/10.1136/bmj.309.6959.901.
- Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom. JAMA 2000;284:706-12. http://dx.doi.org/10.1001/jama.284.6.706.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.38142.554479.AE.
- Mahmoudi M, Coleman CI, Sobieraj DM. Systematic review of the cost-effectiveness of varenicline vs. bupropion for smoking cessation. Int J Clin Pract 2012;66:171-82. http://dx.doi.org/10.1111/j.1742-1241.2011.02877.x.
- Knight C, Howard P, Baker CL, Marton JP. The cost-effectiveness of an extended course (12+12 weeks) of varenicline compared with other available smoking cessation strategies in the United States: an extension and update to the BENESCO model. Value Health 2010;13:209-14. http://dx.doi.org/10.1111/j.1524-4733.2009.00672.x.
- Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2005;2. http://dx.doi.org/10.1002/14651858.CD001007.
- Yudkin P, Hey K, Roberts S, Welch S, Murphy M, Walton R. Abstinence from smoking eight years after participation in randomised controlled trial of nicotine patch. BMJ 2003;327:28-9. http://dx.doi.org/10.1136/bmj.327.7405.28.
- Curtis L. Unit Costs of Health and Social Care 2005. Canterbury: PSSRU, University of Kent; 2005.
- Health Survey for England 2004. London: The Stationery Office; 2006.
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583-637. http://dx.doi.org/10.1097/00005650-200006000-00004.
- Etter JF, Stapleton JA. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control 2006;15:280-5. http://dx.doi.org/10.1136/tc.2005.015487.
- Wetter DW, Cofta-Gunn L, Fouladi RT, Cinciripini PM, Sui D, Gritz ER. Late relapse/sustained abstinence among former smokers: a longitudinal study. Prev Med 2004;39:1156-63. http://dx.doi.org/10.1016/j.ypmed.2004.04.028.
- Krall EA, Garvey AJ, Garcia RI. Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine Tob Res 2002;4:95-100. http://dx.doi.org/10.1080/14622200110098428.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Kenfield SA, Wei EK, Rosner BA, Glynn RJ, Stampfer MJ, Colditz GA. Burden of smoking on cause-specific mortality: application to the Nurses’ Health Study. Tob Control 2010;19:248-54. http://dx.doi.org/10.1136/tc.2009.032839.
- Vogl M, Wenig CM, Leidl R, Pokhrel S. Smoking and health-related quality of life in English general population: implications for economic evaluations. BMC Public Health 2012;12. http://dx.doi.org/10.1186/1471-2458-12-203.
- Barendregt JJ, Bonneux L, van der Maas PJ. The health care costs of smoking. N Engl J Med 1997;337:1052-7. http://dx.doi.org/10.1056/NEJM199710093371506.
- Nyman JA. Should the consumption of survivors be included as a cost in cost-utility analysis?. Health Econ 2004;13:417-27. http://dx.doi.org/10.1002/hec.850.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Drummond MF. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Health and Social Care Act 2012. London: The Stationery Office; 2012.
Appendix 1 Exercise Assisted Reduction then Stop health trainer manual
Exercise Assisted Reduction then Stop health trainer manual (PDF download)
2 The EARS Project
EARS: Exercise Assisted Reduction then Stop smoking
2.1 Background and objectives
2.1.1 NHS stop smoking services
-
NHS Stop Smoking Services aims to help smokers to remain abstinent after an abrupt quit attempt and behavioural and pharmacological support increases success rates by four fold (compared with self-initiated attempts) but as few as 22% are still abstinent one year later.
-
No more than 5% of smokers receive NHS Stop Smoking Services when attempting to make an abrupt quit.
-
Little or no NHS support is currently available for the 60% of smokers who typically report that they would like to reduce the number of cigarettes smoked.
-
Almost half of adults in Devonport and Stonehouse, in Plymouth, smoke. This is double the prevalence across Plymouth as a whole.
-
New options are needed to help smokers who wish to reduce smoking but not quit . . .
-
Those who do reduce smoking are more likely to decide to quit, and remain abstinent.
2.1.2 Physical activity and smoking cessation
-
Physical activity can help to increase cessation rates among abrupt quitters (Ussher et al, 2008), though less is known about effects for ’hard-to-reach’ smokers.
-
Physical activity (structured exercise and short bouts of movement) reduces cravings and withdrawal symptoms, limits increases in cravings associated with smoking cues, and delays the time between smoking cigarettes.
-
Extensive pilot work has taken place to see how best to promote physical activity as an aid to making an abrupt quit attempt, in Plymouth and other NHS Stop Smoking Services (SSS), with input from advisors and smokers.
-
This involved a self-help guide, pedometers, and behaviour change strategies, such as setting goals and reviewing progress.
-
Physical activity has not previously been rigorously assessed as a strategy to help smokers to reduce, then possibly quit and remain abstinent.
2.1.3 Introduction to EARS
-
The Department of Health wish to determine if smokers who wish to cut down but not quit, can be supported in this adjustment with a Health Trainer, providing behavioural support.
-
The Health Trainer role will be to support both a reduction in smoking, using a variety of options, and to help smokers to increase their physical activity, again through a variety of personal choices.
-
Smokers who reach the point where they wish to quit will be offered the full range of NHS Stop Smoking Service professional support, with maintained support from the Health Trainer.
-
In Phase 1 of the study (from September 2010-March, 2011), we will identify the best way to engage with smokers who wish to reduce smoking but not quit, within Devonport and Stonehouse, through interviews and discussions with a variety of relevant stakeholder groups and individuals.
-
In Phase 2 of the study (from April, 2011-April, 2012), we aim to recruit 120 such smokers in these areas of Plymouth, equally from GP practices and non-primary care sites (e.g. community centres).
-
All will be asked to provide information about lifestyle, health and related thoughts initially and at several points in the study, over 4 months. Through a procedure called randomization volunteers will be allocated to one of two groups.
-
Sixty smokers will be encouraged to cut down as they normally would, and given information about NHS Stop Smoking Services, to access if they wish to quit.
-
Sixty smokers will be offered the weekly support of a Health Trainer, in person or by phone, to reduce smoking and increase physical activity. There will be a variety of options offered to support both smoking reduction and increasing physical activity.
-
2.1.4 How do smoking and physical activity link?
-
When smokers cut down they find it easier to breathe and this helps them to become more active, doing some of the things they haven’t enjoyed for a while.
-
Increasing physical activity can help in several ways:
-
It can reduce an urge to smoke as the period between cigarettes increases while reducing smoking.
-
It can reduce withdrawal symptoms such as stress and anxiety, low mood, irritability, restlessness, and hunger, in the absence of a cigarette.
-
It can serve as a distraction and become a new interest to replace the habitual need for a cigarette in certain situations.
-
If a quit attempt is made, it may be easier to become more physically active first, rather than trying to change two behaviours at the same time.
-
Doing some physical activity can remind a smoker just how breathless they have become, and this can prompt a desire to quit.
-
Fears about quitting, such as inability to cope with life’s demands, and weight gain, can be reduced as physical activity helps with both of these.
-
Becoming more physically active can lead to a shift in identity away from that of a smoker.
-
2.1.5 Will smokers be interested in EARS?
-
A survey of 178 smokers in a Plymouth GP practice found 62% were prepared to gradually cut down, of whom 70 (39% overall) were ’interested in taking part in a research study to see if physical activity is useful to reduce the amount you smoke.’ We added in the survey: ‘(The study would include support such as professional support, a self-help booklet, a free pedometer, and free access to an exercise facility).’
But a big part of this study is also to answer this question. How can we recruit people from different backgrounds with different needs, into such a study, and to engage in the intervention?
2.2 Summary of Research Protocol
EARS has been designed as a pilot, pragmatic, randomised controlled trial (RCT), to which participants who wish to reduce their smoking but not quit within the next month will be recruited and randomly assigned to one of two groups:
-
Brief Advice provided at baseline by the Health Trainer (HT) in the form of written and verbal information on the NHS SSS with information on the benefits of quitting and how to quit. Those expressing a desire to quit will be subsequently referred to the NHS SSS.
-
Health Trainer behavioural support in the form of written and verbal information on NHS SSS with information on the benefits of quitting and how to quit provided at baseline. Smokers will select one of 4 strategies for smoking reduction while also being encouraged to become more physically active through about 3 face-to-face and 5 telephone communications, over 8 weeks. Client-centred counselling, will focus on exploring beliefs about increasing physical activity and its use to reduce smoking, action planning (or SMART goal setting) and supporting behaviour change. The HT will seek to develop a supportive relationship, and provide guidance on using a free pedometer, an MP3 player (with an isometric exercise recording), self-monitoring and other self-regulating techniques, and signpost smokers to local exercise opportunities with subsidised access as required. Those expressing a desire to make a quit attempt will subsequently be referred to a professional NHS Stop Smoking Service advisor, with concurrent HT support over a further 6 weeks.
Standardised brief advice to be given to all participants after randomisation:
2.3 What changes are we looking for?
All individuals who receive the EARS intervention are hoped to achieve the following changes:
We want to see more:
-
Quit attempts (during period up to 12 weeks)
-
CO-confirmed abstinence (at 4 weeks post quit)
-
Smokers achieving a 50% reduction in smoking
We want to see more:
-
Moderate and/or vigorous minutes of physical activity (self-reported and from accelerometer recordings)
-
More favourable beliefs about the value of PA as an aid for smoking reduction
-
More positive beliefs about confidence to do PA
-
More positive beliefs about confidence and importance of quitting.
We want to see less:
-
Weekly Self-reported cravings and withdrawal symptoms
-
Cigarettes smoked and lower CO readings
We want to see:
-
At least 120 smokers recruited and randomised within the study
-
Maximise contacts with participants.
-
Evidence of fidelity to delivery of the intervention as per protocol (from sessional recordings, field notes, etc).
2.4 EARS and the role of the Health Trainer
What follows is a brief overview of the role of the EARS Health Trainers. A more detailed description and session-by-session breakdown is provided from Section 3 onwards.
From the participants’ perspective the intervention will last for up to 8 weeks + 6 weeks additional support during a quit attempt.
2.4.1 The Intervention
The initial session will be held at either a community venue, a GP practice, or other clinical setting. It is expected to last around 1 hour.
Working together the HT and participant will discuss feelings and attitudes towards smoking behaviour and physical activity. Being heavily client-centred, the HT and participant will agree on goals for the participant to work towards in terms of smoking reduction and physical activity. The goals should be tailored to the individual participant, with week-by-week short-term goals building in to a longer 8 week goal. General indicators would be a smoking reduction of 50% over 4 weeks with further reduction in the following 4 weeks, and an increase in PA to the maximum of the individual’s desire/capability. Over the next 6 weeks the HT will provide weekly phone calls to offer support and guidance for the participant in achieving their goals, and at 8 weeks the HT will meet the participant again for a final face-to-face session to review progress and discuss maintenance plans.
Whilst it is not within the aims of the HT to support a quit attempt, if at any time (up to 8 weeks from initial session) the participant desires to quit the HT will refer them to NHS SSS.
The HT will also make weekly support phone calls for 6 weeks during the quit attempt.
The aims of the intervention package are to:
-
Promote sustained increases in physical activity
-
Encourage sustained smoking reduction
-
Empower individuals to control cravings through PA
-
Provide information on local PA opportunities as necessary
-
Promote positive experiences and rewards from PA
-
Refer to appropriate services and provide support through any quit attempt
3 Core Knowledge and Skills
The concept of the Health Trainer (HT) was originally proposed in the 2004 Department of Health White Paper: Choosing Health: Making Healthy Choices Easier. HTs are traditionally people drawn from local communities and are trained to reach those who want to adopt healthier lifestyles but have little contact with services. HTs develop an understanding of the needs of people from deprived communities and apply basic behaviour change techniques. They typically embed themselves in communities in order to increase the reach of their service to the more ‘hard to reach’.
This section assumes the trainee has developed the core HT competencies with respect to:
-
Making relationships with communities.
-
Communicating with individuals about promoting their health and well-being.
-
Enabling individuals to change their behaviour to improve their own health and well-being.
-
Managing and organising own time and activities.
3.1 The role of the EARS Health Trainer
The role of the HT has been adopted in the EARS study because a client-centred intervention is planned for ‘hard to reach’ smokers.
Whilst the traditional health trainer assesses the client’s desire to address a particular behaviour from a choice of usually four behaviours (alcohol consumption, diet, smoking and physical activity) the EARS HTs will only focus on smoking reduction and cessation and physical activity, and their interaction. The EARS HTs will draw upon the skills and knowledge equivalent to the City and Guilds Level 3 HT qualification, but will adapt these skills in line with the EARS protocol and this training manual.
In summary, the present manual particularly builds on HT competency number 3 (see above), described in detail in the NHS Health Trainer Handbook, ‘Improving Health: Changing Behaviour.’ Department of Health & British Psychological Association, 2008. This competency (see p. 18 in this Handbook) is about enabling individuals to:
-
Identify how behaviour affects their health.
-
Develop a Personal Health Guide (action plan).
-
Change and maintain a health behaviour.
3.2 Smoking Addiction and Treatment
Smoking is the single biggest preventable cause of death in the world and the World Health Organisation predicts it will account for 8 million deaths per year globally by 2030. Half of those who smoke will die from, or succumb to disease directly resulting from, their smoking habits.
In the UK, the NHS spent £73 million on Stop Smoking Services in 2008/09, not including pharmacotherapy costs. The amount invested in services has risen steadily over the last decade, yet despite his fewer people successfully made a quit attempt in 2008/09 than in 2007/08.
Success rates among those who attempt to quit alone, without behavioural support or pharmacotherapies are extremely low – only around 3–5% will still be non smokers 12 months after quitting
The UK has also seen greater resources directed towards helping ‘hard to reach’ groups in an attempt to address health inequality. Yet, despite this, smoking prevalence is reducing at a slower rate among the social grades C2-E than social grades AB-C1 (1.3% and 2.3% between 2007-08, respectively).
New approaches are needed to increase the number of ‘hard to reach’ smokers making a quit attempt with the best available support and hence successfully quit. With no provision available for the 57-66% of smokers who wish to cut down, and the evidence that those who cut down are more likely to make a quit attempt, the EARS intervention aims to assess whether a smoking reduction programme is a successful way to engage with ‘hard to reach’ smokers, and subsequently increase the number of people making a quit attempt.
3.2.1 Nicotine
Nicotine is a highly addictive psychoactive stimulant. Cigarette smoking is a highly effective delivery method for nicotine, with a lag time of only 7-15 seconds from inhalation to reaching the brain (compare this to up to 20 minutes for nicotine gum).
Thus use of NRT in different forms does not have the same addictive properties (though may still have consequences for health) and is licensed to support smoking reduction and cessation. It does have side effects (see Appendix 7.5.11) and may not be suitable for everyone.
3.2.2 Mood and negative affect
As time increases between each cigarette, a smoker’s withdrawal symptoms and cravings will begin to rise. This leads to an increase in negative mood states such as low mood, irritability, anxiety, tension, hunger and stress. Drug seeking behaviour (needing to smoke a cigarette) in order to alleviate these negative feelings is common. In a sense, a smoker’s satisfaction from a cigarette comes from the alleviation of negative mood states – they smoke to feel normal (see Figure 1).
Fig. 1.
Graphical representation of smoking and mood
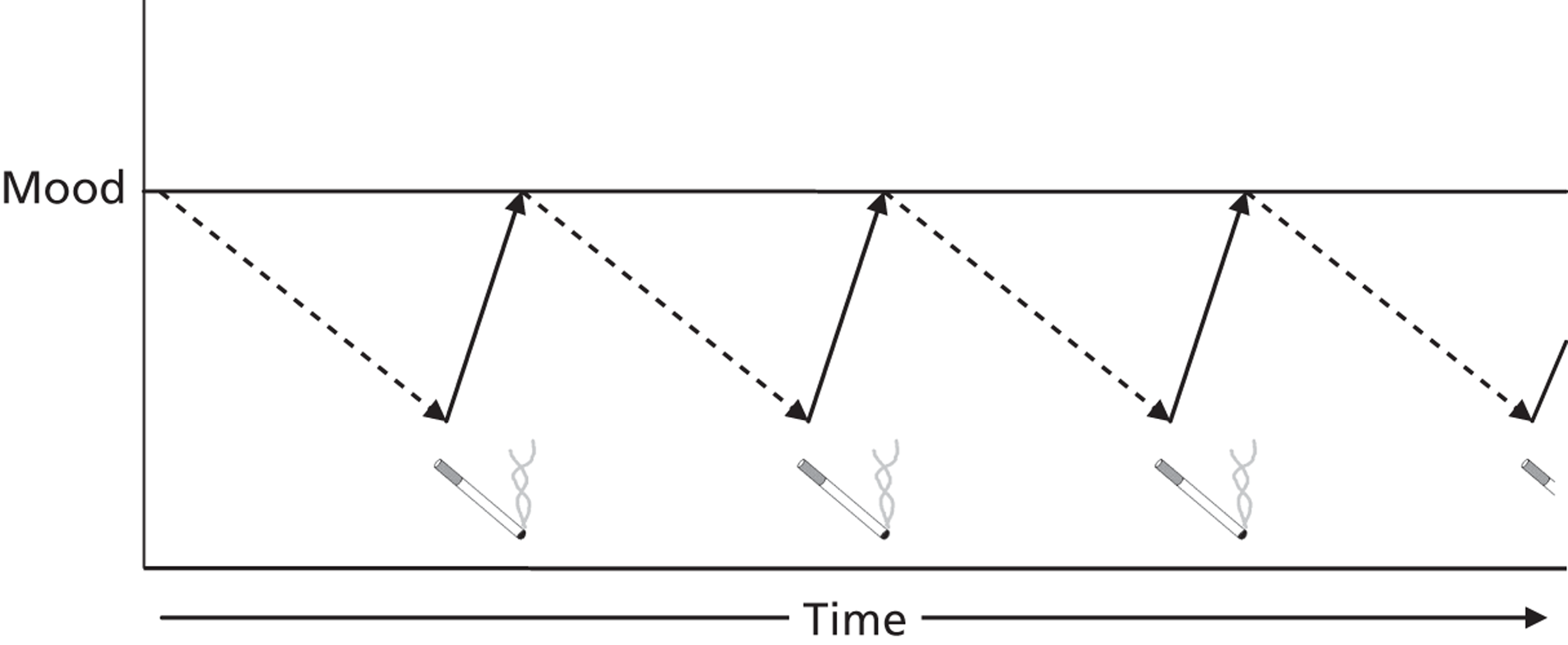
It is worth noting that reported reasons for smoking are often paradoxical in nature – people will smoke both for stimulation and for relaxation. Nicotine is a stimulant which can increase perceived alertness and concentration, and yet also relieve stress. Despite reports that smoking relieves stress, it has been shown that smokers generally exhibit higher stress levels than non-smokers. There is no evidence of a causal relationship (whether people with high stress levels tend to be drawn towards smoking or if smoking causes higher stress levels), but it has interesting implications for physical activity as discussed in Section 2.5.
3.2.3 Cue Reactivity
People often smoke as a result of being exposed to a certain situation or cue (such as having a drink in the pub or after a meal). The desire for a cigarette is stimulated by a learned response to a given stimulus. This form of classical conditioning where a conditioned response follows a conditioned stimulus is often developed over a long period of time and can be very hard to break. Psychological stress is often cited as a cue to smoking.
Cues provide opportunities for impulsive behaviour, which is not planned. So self-regulation and inhibiting a learned response (e.g. having a cigarette when offered one) is challenging. Learning ‘what-if’ strategies are often an important weapon to avoid lapses triggered by cues.
3.2.4 Reasons for smoking/Barriers to quitting
The reasons people smoke and the barriers which prevent people from quitting are often complex and numerous. Whilst EARS is primarily concerned with supporting people to cut down and not helping them to decide to quit, it is worth noting possible why people start and the barriers to them stopping as they can relate strongly to the role of physical activity.
| Reasons for smoking | Barriers to quitting |
|---|---|
| Boredom Smoking is part of a social activity Smoking is used as a coping strategy for when things get stressful or difficult Used as a weight management strategy Enjoyment Exposure to conditioned stimulus For stimulation For relaxation |
Lack of confidence to quit (previously failed quit attempts) Fear of withdrawal symptoms Motivation Desire Belief that smoking isn’t dangerous Peer pressure Social exposure Loss of smoking as a stress management tool Lack/cost/availability of support and NRT Powerful addiction to nicotine |
One important reason for sustaining smoking and for relapsing from a quit attempt is weight gain. Nicotine increases metabolism and suppresses appetite, so when someone stops smoking they will gain, on average, 7kg in 12 months. The weight gain is compounded by the potential replacement of the nicotine ‘hit’ with indulgent snacking and emotional eating. For many, even minimal weight gain is unacceptable, so strategies to prevent weight gain after smoking cessation are required. There is no evidence for what effects smoking reduction has on weight gain.
3.3 Abrupt quitting
At this time, the NHS Stop Smoking Service largely advocates abrupt approaches to quitting. With the support of a qualified stop smoking advisor, those wishing to quit will work to set a quit date, following which they will attempt to not smoke another cigarette. They will be offered the option of using a variety of pharmacotherapies (Nicotine Replacement Therapy (NRT), Bupropion, Varenicline etc) to help manage cravings and withdrawal symptoms. Even the most optimistic data suggests that with the best pharmacological and behavioural support, the chance of remaining abstinent 1-year post quit only rises to around 20% from around 3% unaided.
It is estimated the NHS SSS only engages with around 5% of smokers at any one time. The reach of the NHS could be limited by the fact it only engages with those who express a desire to quit abruptly, as well as a perception that smoking is not a clinical problem which required ‘treatment’.
3.4 Advanced reduction and cessation
3.4.1 Why quit?
Appendix 7.4.2 shows some of the advantages of quitting, over different time periods. The DoH HT Handbook also includes a Health Benefits card (see p. 17), which can be used to elicit smoker beliefs.
3.4.2 Why is it hard to quit?
There are many reasons, but the main ones are failure to cope with cravings and withdrawal symptoms (particularly during times of stress, or in the presence of smoking cues), difficulty in breaking a habit or conditioned responses in certain environments and situations, lack of confidence in avoiding smoking perhaps developed from previous failed attempts, being surrounded by others who offer limited support to quit, and weight gain. Alcohol consumption has also been linked to difficulties in avoiding smoking.
3.4.3 What support is available that is effective?
Appendices 7.5.11, 7.5.12 and 7.5.13 provide information on the use of NRT, Champix and Zyban which are the main pharmacotherapies with an evidence for effectiveness. These are most often prescribed by a clinical practitioner or fully trained Stop Smoking Service advisor. NRT is also available over the counter. Behavioural support is available through Stop Smoking Services and other trained professionals, which is also effective, and often delivered in conjunction with pharmacotherapies.
3.4.4 Building on the DoH Health Trainer Handbook
Smoking cessation is one behaviour that HTs are encouraged to support. However, without extensive training, if a client wishes to quit then an HT would normally refer a client to a professional with the skills to provide the support described above. The focus in the HT Handbook is on helping smokers to abruptly quit by setting a quit date and completely abstaining. There is little or no mention of smoking reduction in as a shift towards this approach is fairly recent, and is only recommended in conjunction with NRT. Since 60-70% of smokers want to cut down but not quit smoking reduction interventions are seen as a way of increasing the number of smokers who potentially get to the point, after reduction, of wanting to quit.
EARS aims to increase physical activity in the absence of NRT, but in conjunction with behavioural approaches to smoking reduction. In the following sections, different smoking reduction approaches are described, before we consider how physical activity can be promoted, and particularly in a way that could support smoking reduction, and cessation.
3.5 Behavioural approaches to smoking reduction
Smoking reduction has not been advocated as an appropriate technique for quitting as it has been widely believed that increasing the amount of time between cigarettes will only increase the reward and satisfaction obtained from the cigarette when it is smoked, thus increasing the value and desire of each cigarette.
Research in this area is still in its infancy, but a recent review by Aveyard et al (2010) has reported that there is no difference between abrupt quitting and cutting down to quit on long term cessation, but this is based on interventions involving Nicotine Assisted Reduction then Stop (NARS).
Several potential strategies for cutting down have been developed and proposed over recent years. Crucially they all hinge on breaking the conditioned responses to smoking stimulus. Unlike abrupt quitting, they aim to gradually breakdown learned routines and break habits which may increase confidence and desire to stop completely. The different strategies are presented in the following sections.
3.5.1 Hierarchical reduction
Certain cigarettes offer higher reward value than others and as such are harder to give up. The first cigarette in the morning (following overnight abstinence) is routinely reported to be the hardest to give up. It has even been suggested that the only question of importance in assessing a person’s level of smoking dependence is ‘how soon after waking do you smoke your first cigarette?’
Hierarchical reduction works by asking people to rank cigarettes in order of the easiest to the hardest to give up. Starting with the easiest, smokers plan which ones they will give up on a specified time scale. It may be one a day over a two week period or however the person feels best to progress, eliminating the easiest and eventually beginning on the harder cigarettes to give up, as confidence to go without a cigarette increases.
Fig. 2.
First illustration of hierarchical reduction method.
3.5.2 Smoke free periods
The Smoke free periods approach works by breaking an individual’s day up into blocks of specified time periods (e.g. 30 mins). Depending on their routine (work etc) there may be periods where they do not smoke anyway, and periods where they smoke more. Using chart, smokers then go on to block out certain times of the day where they will not smoke (perhaps increasing by one 30 minute smoke free period per day) until they have reached a certain goal.
Importantly with the smoke free periods approach, there is no specified number of cigarettes which are being cut out or smoked. They can smoke as much as they like, but ONLY in the periods not identified as smoke free. This approach aims to break the behavioural pattern of smoking which will result in a decreased desire for smoking and a natural reduction.
Fig. 3.
Second illustration of hierarchical reduction method.
3.5.3 Scheduled reduction
The aim of the scheduled reduction approach is to systematically reduce at a specified rate, breaking habit and routine gradually. It begins with identifying how many cigarettes a person smokes in a day, and calculating how much time between each cigarette is needed to space them evenly through the day. For example, a 40 a day smoker, who is awake for 16 hours a day, would need to smoke a cigarette every 24 minutes to get through 40 in one day. Targets are then set to gradually increase the time between each cigarette with a specific end goal in sight.
Important to this method is the necessity to smoke at every specified time point, whether it’s desired or not, which again helps to break the habit of smoking.
Fig. 4.
Illustration of scheduled reduction method.
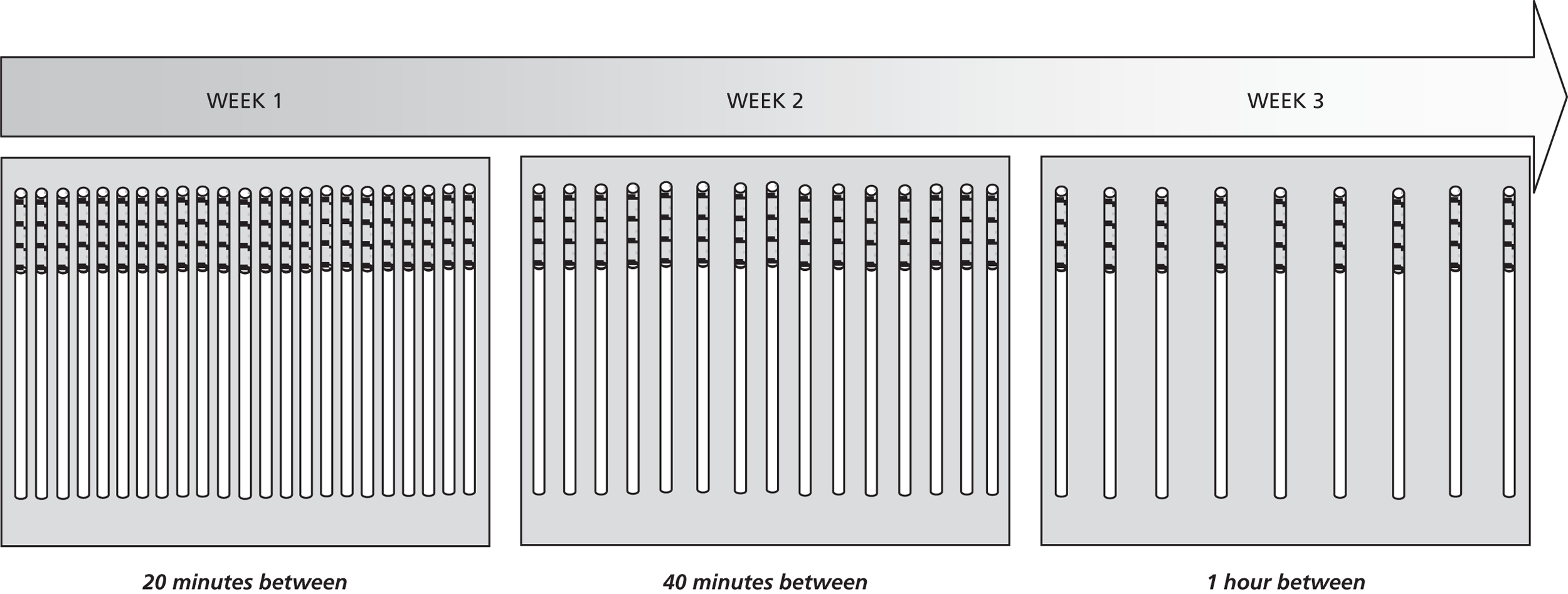
3.5.4 Planned reduction
Perhaps one of the simplest ways to plan reduction, this approach works by setting targets for how many cigarettes will be smoked each day. Then each day begins with that number in their pocket, and purchasing additional ones is to be avoided.
The rate at which they reduce is determined by them and ultimately how much they want to reduce by and over what period. This approach fits particularly well with goal setting and action planning processes described in the HT Handbook.
Fig. 5.
Illustration of planned reduction method.
All the above approaches have their pros and cons. The key will be to enable smokers within EARS to choose an approach to experiment with, and to help set a timescales for rate of reduction, as part of the action planning process. Our ideal would be to reduce by 50% over no more than 4 weeks, and then consider further reductions. But there will be considerable variation in participants’ responses and success.
3.6 Physical activity and health, optimal dose and promotion
If some of the benefits accruing from regular physical activity could be procured by any one medicine, then nothing in the world would be held in more esteem than that medicine.
Francis Fuller, 1705
Physical activity is widely accepted to benefit health both physically and mentally. Being regularly active decreases the risk of developing an extensive range of medical conditions such as: cardiovascular disease, diabetes, cancer, depression, anxiety, dementia, high blood pressure, osteoporosis, osteoarthritis, lower back pain and lowers the risk of falls among the elderly.
The Department of Health recommends that adults achieve at least 30 minutes of moderate intensity moderate-intensity physical activity on at least 5 days of the week, 3 × 20 mins of vigorous physical activity for cardiovascular health. Activity does not have to be continuous for 30 minutes but can be in shorter 10 minutes bouts throughout the day.
The dose for improving mental health is less clear, although 1 study suggested this same dose would be necessary to reduce depression. Short bouts of moderate physical activity can relieve stress and tension, whilst improving a sense of pleasure and activation. The figure below shows how regular short bouts throughout a day can help to elevate overall mood.
Importantly, by breaking physical activity into short bouts, it may become easier to meet the daily recommended dose in a sustainable way.
Fig. 6.
Graphical representation of physical activity and mood.
Using a Decision-Balance sheet, as shown in the DoH HT Handbook (p.26), it is easy to identify the pros and cons of becoming more active. But the commonly cited barriers shown below are largely a function of how we introduce or use the terms sport, exercise and physical activity. Short bouts of brisk walking do not have the same barriers as signing up for an exercise class or joining a sports club.
Commonly cited barriers to different types of physical activity.
-
I’ve never done it
-
I wasn’t good at sports at school
-
I would feel silly
-
Other people would make fun of me
-
It won’t help unless it hurts - ‘No pain, no gain’
-
It’s sweaty and uncomfortable
-
I’m too tired
-
I would rather do something else
-
It’s expensive
-
I think it will make me feel worse
-
I don’t have anyone to do it with
-
I don’t know where, when or how to start.
There are a number of factors which can influence how people relate to and perceive different types of physical activity. Typically activity is thought of in four or five different dimensions: Frequency, Intensity, Duration and Type. Timing is less commonly considered but may also be important in the context of using physical activity to specifically help cope with cigarette cravings.
Frequency: How often does the behaviour occur? Is it better to do some physical activity every day or just once in a longer block at weekends? For cardiovascular health, it does seem to be important to regularly exercise. Long periods of sedentary behaviour are increasingly being linked to increased risk of some health problems such as diabetes. The evidence is less clear for other conditions but we do know that even short bouts of activity can increase activation or energy levels, increase positive affect and reduce our natural psychological and physiological responses to stress or threatening situations. Therefore, repeated bouts may lead to an accumulated benefit over a period of time which one longer single session per week may not provide.
Intensity: How intense is the activity? How much effort or physical and mental discomfort does a person experience? The experience of how intense an exercise is can be highly individual, and may depend on several factors such as cardiovascular fitness, fatigue, previous experience, mood, and any existing physical disability.
| Intensity | What is it? | PROS | CONS |
|---|---|---|---|
| VIGOROUS E.g. Running, hard cycling, squash, aerobics, circuit training, hard manual work, team sports | An activity that leaves you feeling (extremely) out of breathe and unable to hold a conversation. Your heart rate will rise significantly and will often lead to high levels of perspiration. Breathing will become very rapid and heavy. | Evidence suggests it offers the most physical benefits for those who complete it. Can offer a greater sense of achievement and ‘feel good factor’ for the right person. |
Extremely off putting to most, especially people new to physical activity. Can cause delayed muscle pain (not necessarily serious, just uncomfortable) The risk of injury is greatly increased. Often needs specialised equipment and environments (cost) to do it, with high levels of supervision. For smokers it may exacerbate symptoms of breathlessness. |
| MODERATE E.g. Brisk walking (complete 1 mile in around 15 minutes), cycling, effortful housework, gardening, golf, tennis, dancing, tai chi. |
An activity which still allows you to hold a conversation, but you will still feel your heart rate rise, your skin warm and your breathing become slightly faster. | Is easily achievable for nearly everyone. Easily accessible and can be done without high levels of supervision. Still has significant benefits for health (national guidelines promote MODERATE activity) |
People may not think of moderate activity as having any benefits (too easy) Slightly increased risk of physical injury, but it is minimal |
| LOW E.g. slow walking/ strolling, easy housework, light gardening, yoga. |
An activity which is very easy to complete, only slightly raises heart rate and does not require faster breathing. | It can be a good starting point for increasing motivation and confidence to complete physical activity for those with little or no experience. Can increase confidence and self belief in moving onto moderate activity. Very small risk involved. Any energy expenditure is better than sitting. |
Conveys little or no health benefits. |
Duration: How long does the activity have to take place for? Is it in one long block or broken into smaller chunks?
-
Presenting people with the task of walking or cycling continuously for an hour or even 30 minutes can seem daunting and lowers motivation and confidence. Breaking activity up can make it seem more achievable and easier to fit into people’s lives.
-
The national guidelines suggest 30 minutes of daily moderate activity can be achieved in blocks of 10 minutes. Therefore, someone could walk briskly to work or shops in the morning, take a short 10 minute walk at lunch time and walk home again and they would meet the minimal recommended guidelines for physical activity. This can often be perceived to be far more achievable than one longer walk.
Timing: What time of the day, or maybe week, are people completing physical activity? This is perhaps one of the less considered aspects of physical activity, but remains important none the less.
-
It could be that a person aims to complete a walk first thing in the morning, but in reality they are pushed for time in the morning and simply cannot sustain it. Or, perhaps more detrimentally, the idea of going for a walk in the morning becomes a burden and adds pressure to them at a time when they feel they simply cannot fit it in, resulting in feelings of guilt for having not done it.
-
It could also be that exercising vigorously or in a way that is unfamiliar in the evening can result in disturbed sleep as a result of a raised body temperature and hormonal responses.
-
A person may also gain enjoyment from completing the same activity at different times of day. For example, they may enjoy walking the dog early in the morning compared to late at night because of the different environments (light vs dark) and feelings of safety. They may also want to do it after a busy day to ‘unwind’.
Mode: Physical activity takes many different forms and can serve many different purposes. It is important to know what type of physical activity a person believes they may enjoy/have enjoyed in the past. Running can be completely off putting for one individual, but potentially rewarding and enjoyable for another.
-
Promoting an activity which a person does not enjoy will likely limit adoption and maintenance of that activity.
-
It is important to consider that although an individual may not enjoy an activity of a certain intensity (such as jogging), they may however enjoy an alternative activity of a similar intensity but different mode (eg cycling).
-
Certain modes of activity can also have time implications. For example, arranging a game of badminton can require travelling time, perhaps a minimum court booking of an hour, and may depend on facility opening times. Compare this with taking a walk, which needs little preparation and planning and can be completed at most times of the day.
-
Different modes of activity can also have different cost implications. Cost is often a large barrier to the adoption and continued participation in certain activities. The cost of going for a walk is minimal compared to going for a cycle if the person does not own a bike.
There are some other important psychosocial factors surrounding physical activity which can strongly influence its successful adoption and enhance the positive experience of being physically active:
Environment: It is entirely plausible that the location in which an activity takes place will influence how an individual experiences that activity. Walking on a treadmill in a gym will provide an entirely different experience to walking through a country park, despite being the same physical activity. The experience of one exercise or yoga class may be entirely different to another class with a different instructor elsewhere.
It is also important to consider how an environment has the potential to damage an individual’s confidence and motivation. For example, attending a heavily strength and weights orientated gym can be a highly off putting experience for a beginner or somebody with low physical self-esteem. A bad experience of a physical activity environment can make it highly unlikely for the behaviour to re occur.
Social Support: Can be considered in two forms – in terms who the activity is done with and support from others for completing the activity.
Going for a walk with a friend can enhance the enjoyment of the activity, and agreeing to attend a new exercise class with a friend will enhance the motivation and confidence for continued attendance. Whilst it can often be reported that completing physical activity alone can offer enjoyment (a chance to ‘get away from it all’, to ‘clear your head’), completing it with others can go some way to fulfilling the psychological need to feel connected with others.
Support from significant others (friends, family, partners, etc) in relation to completing an activity is also important in adopting and maintaining new behaviours. It is important to explore ways that support can be found and elicited from those close to the people around them. For example, ask how a partner feels about them trying to become more active. If the people close to them understand their reasons for adopting new behaviours it is less likely they will be a negative influence or inadvertently create additional barriers. A person’s confidence is also likely to grow if the people around them are supportive and encouraging of them trying new behaviours.
One of the most successful and sustainable forms of physical activity was called ‘Mums on the Run.’ Given the barriers for exercise and needs for companionship for parents of pre-school children a group of up to 10 met weekly at a different person’s dwelling for coffee. Half the group went out for a jog/walk, leaving the others to enjoy a chat and look after the children. They switched roles after 30 mins and everyone’s needs were met at no cost.
3.7 Advanced Physical Activity Promotion
3.7.1 Why increase physical activity?
The general health benefits of increasing PA are shown on a Health Benefits card in the DoH HT handbook (p. 18). Clients may identify with these and other potential benefits when they see this list. These potential benefits may also be elicited using the Decision-Balance sheet. Smokers are often aware of the link between smoking and weight management. One of the particularly relevant benefits of increasing PA may be to prevent weight gain once smoking is reduced or stopped.
3.7.2 Barriers to increasing physical activity?
It is not difficult to elicit a client’s perceived barriers to doing more PA in general. A more fruitful approach is to identify specific forms of PA and then seek to elicit perceived barriers to that dose and type of PA, which may be preferred. Negotiation with a client should go back and forth until ultimately goals are set which are specific attainable and realistic. This is another way of saying, that the barriers are not insurmountable: They can be overcome. Setting goals which are unrealistic is inviting failure.
3.7.3 What support is available that can help a client to increase PA?
There are three ways of looking at supporting increases in PA:
-
Working with a client to change cognitions (such as benefits and barriers, and self-efficacy/confidence).
-
Build an empathetic relationship with the client, and encourage them to seek and gain support from others to achieve PA goals.
-
Direct clients to sources of information and opportunities for PA. Also, help a client to develop behavioural skills (e.g. self-monitoring using a pedometer) to enable them to achieve goals. To remove financial barriers to doing PA, participants in the EARS intervention will be offered incentives (e.g. free access to gyms).
3.7.4 Building on the DoH Health Trainer Handbook
The approaches for supporting clients to increase physical activity in the HT Handbook are largely sufficient for setting and evaluating goals, avoiding relapse and resetting goals over time. The HT may be able to extend initial discussions by asking clients to think about benefits and perceptions associated with different doses (frequency, intensity, duration, type, and timing). This may help to identify a client’s preference for types of PA.
The HT Handbook does not consider when best to do PA. If the value of PA for regulating mood is recognised then a client may be helped to identify when it may be most valuable to engage in PA. The HT Handbook also does not consider how to support an increase in PA while reducing or stopping smoking at the same time. There is a view held by some that it may overload clients if too many behaviour changes are tackled at the same time, though others have suggested this does not have to be the case.
3.8 Physical Activity and Smoking Behaviour
3.8.1 Chronic physical activity and smoking cessation
Cross sectional data reveals that those who are more active are less likely to smoke, and smokers are typically less active as shown below. Does this mean that by increasing PA there will be a tendency to reduce or stop smoking? And do smokers become more active when they reduce or stop smoking?
Several well conducted trials have considered what happens when a smoker who quits increases physical activity, with encouraging results. In one study, vigorous intensity structured (gym-based) exercise on three days a week over 15 weeks increased the number of female quitters at 12 months, relative to controls. But smokers may be more interested in moderate rather than vigorous activity.
Fig. 7.
Graphical representation of physical activity levels and the chance of being a smoker
3.8.2 How can physical activity help with smoking reduction and cessation?
Physical activity could influence smoking behaviour through either EXPLICIT or IMPLICIT processes. Smokers have told us that they deliberately use exercise such as going for a walk after a meal, to distract them from smoking when they would otherwise have smoked. Others have said that they were afraid of gaining weight so started doing more physical activity. Using physical activity as a method of directly compensating for the negative effects of smoking and quitting would be explicit.
In contrast, general increases in physical activity that may have indirect effects on smoking behaviour would be implicit. The table below lists some examples.
| EXPLICIT processes | IMPLICIT processes |
|---|---|
|
|
These processes all have implications for promoting PA to help smokers to reduce and quit smoking. Given that coping with cravings and withdrawal symptoms is one of the main reasons why smokers find it difficult to cut down or quit, using PA as a coping strategy may be important. Just generally increasing PA may also have valuable indirect benefits.
3.8.3 Acute physical activity management of cravings and withdrawal symptoms
Studies have consistently shown that during temporary smoking abstinence, when cravings are high, a short bout of PA (e.g. a brisk 15 min walk or 5 mins of seated isometric exercise) reduces cravings and withdrawal symptoms. The effects last beyond the exercise, for at least as long as the exercise itself.
When smoking cues are introduced, PA has been shown to limit the increases in cravings. Also, a session of PA delays ad libitum smoking. It would therefore appear appropriate for smokers to explicitly use short bouts of PA to aid smoking reduction and quitting.
If smoking is based on the need to relieve negative mood states then a single bout of physical activity can help control withdrawal symptoms and relieve cravings, as shown in the Figure below. Repeated exposure to physical activity, with enhanced mood, may help to increase a belief in the value of exercise for managing cravings and withdrawal symptoms.
Fig. 8.
Graphical representation of negative mood states and smoking withdrawal symptoms
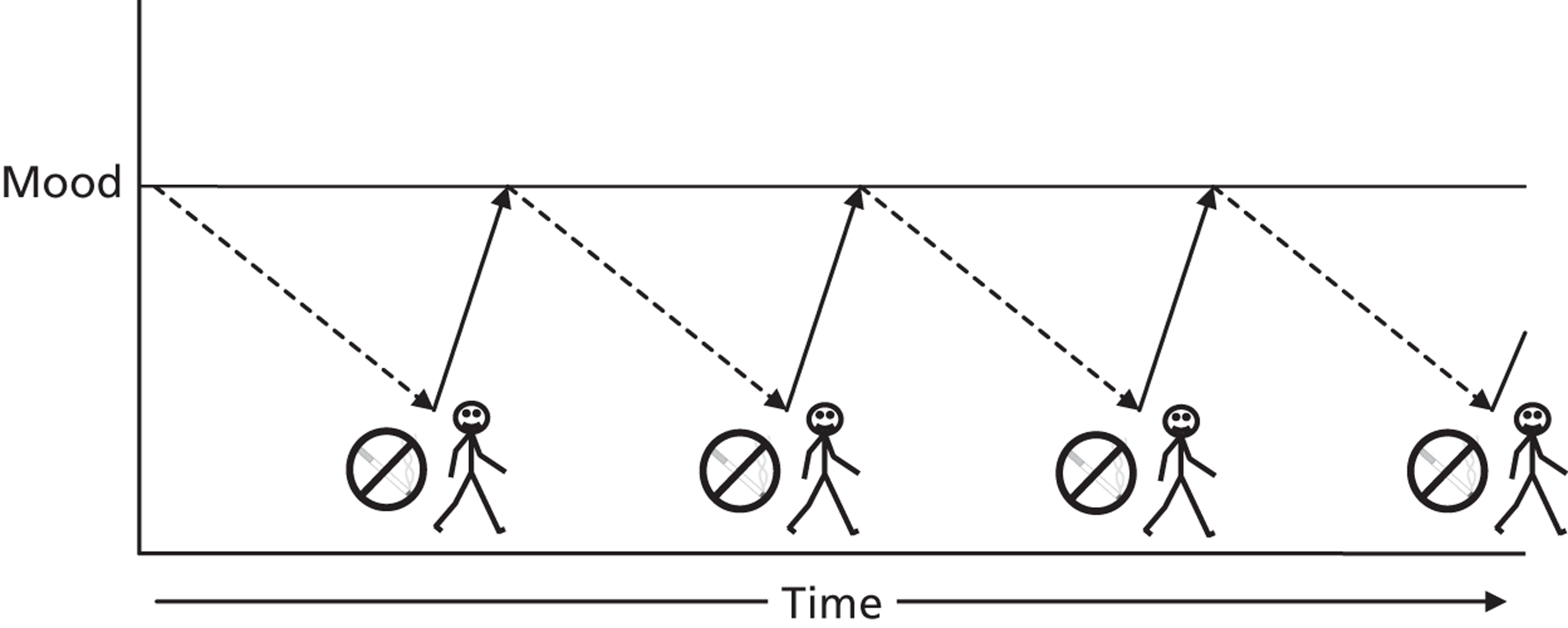
3.8.4 Physical activity and weight gain
After smoking cessation, smokers (and particularly women) experience an average of 5-7kg weight gain within a year of quitting. Fear of this weight gain prevents many people from quitting. The effects on weight gain from smoking reduction are not known. Weight gain is a result of a slower metabolic rate without nicotine in the body and also emotional eating.
Increasing PA while cutting down (and quitting) may reduce weight gain not only by increased energy expenditure but also through improved control of energy intake (particularly via emotional eating).
3.9 Advanced elements of supporting behaviour change
As a HT working within EARS there is an opportunity to develop a more advanced understanding of how best to support behaviour change. In any attempt to support behaviour change there is a chance, indeed a high possibility of several things:
-
That clients may fear or experience failure to achieve goals.
-
That clients feel they are changing for someone else and not because they really want to.
-
That they are doing something which is not in line with what others feels they should do.
Such emotional responses minimise the chance of sustained engagement in an intervention, like the one planned in EARS, and also successful changes in behaviour.
In contrast, HT support that limits failure, encourages ownership and control of the behavioural change, and provides or facilitates opportunities for social support, may be more likely to result in sustained engagement in the intervention, and hence successful changes in behaviour.
Here we consider the value and process of promoting self-determined behaviour. Self Determination Theory (SDT) predicts that real shifts in behaviour result from satisfying three essential psychological needs (called the 3 Cs), which are having a sense of:
-
Competence: When an individual feels capable to affect a desired behavioural outcome.
-
Control: When an individual feels to have a sense of personal choice in deciding what to do.
-
Companionship: When an individual feels secure within an environment while also fulfilling a need to feel connected to others.
Goal setting is a core part of the role of the HT. It is very easy to see how goals could be set that undermine all these needs. A HT could also communicate in a way that undermines these needs.
So there are two key elements for developing advanced behaviour change skills:
-
Negotiate with clients to ensure the actions planned will satisfy these core needs (i.e. 3 Cs).
-
Communicate with clients in a way that will satisfy these core needs (i.e. 3 Cs).
3.9.1 Promoting physical activity to satisfy the 3 Cs
Supporting a client’s increase in physical activity provides an opportunity for that individual to satisfy the three ‘C’s as follows:
COMPETENCE – By setting and achieving realistic goals an individual can build a sense of competence. [Equally, inappropriate goals can undermine a sense of competence, and clients with initially low self-efficacy may be quick to say, ‘I told you so’ when they experience failure.] Goals that are measurable provide an opportunity to gain a sense of achievement. Help the individual to identify these achievements and link them to the client’s efforts. Short-term goals can help to build into long-term achievements and again, with reflection, provide a sense of achievement.
CONTROL – Through a client-centred approach, the client is involved in the goal setting process, and encouraged to link effort and success. Achievements linked to the role of the HT rather than the individual does not enhance a sense of control. Giving advice and information, when the individual could find this out for themselves can also undermine a sense of ownership and control or autonomy. The client should choose what activity to do, when, and where to do it.
COMPANIONSHIP – Quality PA experiences often involve other people, and the connection felt with others can be a strong motivator for that behaviour. The individual can also feel companionship in the environment they are in – a sense of belonging where they feel secure and competent.
Physical activity experiences which provide the individual with the satisfaction of these three Cs will see higher adherence rates. Activities which meet these needs for each individual will be vary greatly as different people put different values on different experiences. There is no ‘one size fits all’ activity, and tailoring action plans is crucial in developing an intrinsic motivation and sustained change.
3.10 Communicating with patients
Overall Aim: To maximise sustained behaviour change with a variable amount of the intervention.
Objectives
-
Achieve flexibility in the programme based on each individual’s readiness to be introduced to and try participating in physical activity (PA) and reducing smoking – negotiate the type, intensity, duration, and frequency of activity the patient believes they can achieve.
-
Highlight and enable patients to access physical activity opportunities that minimise barriers, provide rewarding experiences, and result in sustainable physical activity.
-
Use compiled regional current information on physical activity opportunities matched to the patients’ preferences and motivation/readiness to change.
-
Recognise the boundaries of the facilitator and be aware of risk assessment in case of the need of referral back to the GP (and informing trial coordinator).
Outcomes
-
Enable patients to access physical activity opportunities that minimise barriers
-
Provide rewarding experiences through negotiation & reinforcing positive events
-
Achieve sustainable increases in physical activity (however subtle)
-
Acquire self-regulatory skills in managing smoking cravings and withdrawal through the use of physical activity
Key principles
The following are key principles to follow when working with patients:
Allow choice
-
Ensure that the patient understands the approach/model and acknowledge this approach makes sense to the patient
-
Be flexible in assisting patients to decide upon activity
-
Be aware that not all patients will embrace physical activity after initial session
-
Be aware that any activity will be beneficial regardless of smoking habit and the patient may not achieve the government recommended levels of activity by the end of the contact time. But the physical activity patterns will have been established.
Develop rapport
-
Listen to the patient. Make sure you have understood what they have said. Ask questions if you are not sure.
-
Don’t be judgemental. Respect their point of view. Do not disagree with a patient.
-
Summarise what the patient has said. Don’t assume you have understood what they have said. Make sure you repeat back what they have told you.
Avoid disagreement, lecturing or nagging
-
Ask questions rather than give instructions
-
It is a collaboration, make sure you work with your patient
-
Never disagree or argue with a patient
-
Don’t nag, ask how you can help them achieve their plans
-
Ask what stopped them achieving things this week
Make sure the patient understands the rationale
-
Refer back to the patient’s list of problems
-
Make the link between their desire to cut down and the benefits of physical activity
-
Repeat the rationale
Many of the above principles and techniques for developing rapport with clients are common sense and come naturally to a good communicator. They also overlap with an approach that you may have heard about called Motivational Interviewing (MI). It also links well the 3 Cs from Self-Determination Theory. The key thing is that in EARS the HT should adopt a client-centred communication style, which is compatible with the techniques and approaches described in the HT Handbook.
There may be times when clients request information and direction but, while this may have short term effects, it may not help sustainable changes in health behaviour.
The EARS intervention is not, however, about the effects of MI on smoking reduction and cessation, but we will borrow principles and techniques commonly used in MI.
The following 4 pages highlight some of the key aspects of MI.
3.10.1 Motivational interviewing
Definition of brief motivational interviewing: a directive, client-centred negotiating style for helping patients explore and resolve ambivalence about exercise (and other health behaviours)
(Rollnick, 1992)
Motivational interviewing (MI) is a philosophical approach to behaviour change based around the idea that motivation to change behaviour will be enhanced, negotiated and directed by the interpersonal interaction between the patient and facilitator or professional. It is important to understand the philosophy behind motivational interviewing in order to correctly use techniques and work through ambivalence with patients. As a patient centred approach, MI assists patients in articulating their concerns and arguments about behaviour change. MI is a flexible approach, with a number of strategies to choose from to match the level of readiness to change within each individual.
The goal of motivational interviewing is to help patients with their ambivalence towards changing behaviour through a series of techniques.
Ambivalence: Conflict between two different actions both having perceived costs and benefits. The main concept used is decision balance, weighing up the pros and cons of remaining inactive as compared to the pros and cons of being active.
Readiness to change: Determining where the patient is on a continuum of motivation and being ready to change their behaviour is also crucial for the facilitator to interpret. The readiness to change is an important factor to address in order to negotiate the patient through from not being prepared to change to already changing stage. Key questions to ask regarding this are ‘How important is it to you to change?’ and ‘How confident are you in making that change?’ These two questions will provide indication of the levels of readiness to change and are also extremely useful tools for you to use as the facilitator to encourage discussion around ambivalence.
Key principles
Roll with resistance – As facilitator it can be useful to offer new perspectives, but it is important not to impose them on the patient.
Express empathy – the key is to actively listen to the patient’s point of view and accept it even if you don’t approve of it.
Avoid argument – remember not to ‘label’ the patient as it encourages defensiveness and resistance from the patient.
Develop discrepancy – negotiate with patient to consider the consequences of their health behaviour and develop an awareness of the importance of the consequences.
Support self-efficacy – Assist patient through determining their own choices and understanding their own capabilities, pushing the boundaries progressively but only with their permission.
Skills you need as facilitator
-
Asking open ended questions
-
Use reflective listening
-
Summarising/paraphrasing
-
R: Roll with resistance
-
E: Express empathy
-
A: Avoid argument
-
D: Develop discrepancy
-
S: Support self-efficacy
Patients resistant to change: Why?
There are three main reasons why patients may be resistant to behaviour change. The first reason is that they may feel like they are having their control taken away from them. A good way to deal with this is to emphasise personal choice and control.
A second reason may be that you as the facilitator have misjudged or misinterpreted the patient’s readiness to change, how important and/or how confident they are in changing. By revisiting these issues, the facilitator will have an opportunity to make a clearer judgment regarding these points.
The third reason may be that you as the facilitator have been a bit too confrontational, confronting force with force. This may occur when discussion around issues that the facilitator may consider straightforward in one instance turns out not to be so straightforward in the patient’s view. To manage this, it’s best to back off and essentially ‘come alongside’ the patient, not agreeing with them but changing tack and emphasising their own control and choice in the matter and negotiating the idea of change back into the discussion.
Menu of strategies
-
Opening strategy: Lifestyle, stresses, health
-
A typical day
-
Assess motivation and confidence
-
Good things and less good things about behaviour
-
Providing information
-
Future and present
-
Exploring concerns
-
Helping with decision making
-
Modified barrier approach: reasons why do you want to and reasons why not
-
Explore reasons
-
Emphasise personal control and choice
-
Re-assess readiness, importance and confidence
-
-
Social support – social benefits of exercise, group exercise
3.10.2 Counselling techniques
Breakdown tasks
People often tend to be discouraged by large tasks and any difficulties or problems seem overwhelming. The main strategy to prevent this is to break down large tasks into smaller tasks that are easier.
For some people, it might be important to suggest doing a limited number of these tasks during a week. For example, agree to perform steps 1–4 above in the first week.
-
Find information on local classes
-
Identify sessions which could be attended
-
Speak to friend/s about attending with them (if appropriate)
-
Contact class to book/check availability
-
Obtain suitable footwear/clothing
-
Arrange transport to and from class
-
Agree a date for the first class
-
Attend class
-
Identify times in the week when a walk could take place
-
Locate possible walking routes
-
If transport is needed (rural walks) obtain information on travel options
-
Speak to friend/s about walking together (if appropriate)
-
Find suitable footwear and clothing
-
Find information on walking groups (if appropriate)
-
Plan a date for the first walk
-
Go for walk
Agree achievable goals
The goals for activity need to be agreed with the patient. It is a collaborative activity. People often set unrealistic goals that are too ambitious. If someone has not been exercising for some time they might set a target more appropriate for when they were more active in the past. Therefore make sure that you agree a realistic goal, particularly one that is easy to achieve. If people fail to achieve their goals then it can be discouraging.
Be aware that sometimes, people might achieve the goal but still come back and describe it as a disaster. This is because they have added on extra aims that you were not aware of at the time. For example, they might say ‘I went for a run around the park but had to stop twice’. The original agreed task was to run around the park but on return they have added an extra goal, to carry out the run without stopping. Remind the patient of the original aim and suggest that you include the additional aims in next week’s tasks.
Treat the activity as an experiment
Make sure you have elicited expectations about the activity that has been agreed. This is important to ensure that you agree with the patient what to achieve.
There are two aspects to the possible psychological benefit from exercise:
-
Enjoyment
-
Sense of achievement
If you treat the exercise as an experiment, you could suggest that the patient rates their expected enjoyment and sense of achievement before they carry out the agreed task. Then complete the same ratings after the task. Quite often, the patient either enjoys or has a greater sense of achievement than he or she expected. However, this is an experiment and everyone is different. It might also help them to choose the kind of things that they get the most benefit from.
4 The Intervention
Whilst the message being portrayed by the intervention is not one of smoking cessation but rather reduction, the main desired outcomes from the intervention are concerned with quitting and remaining abstinent. It is important to remember this and quitting should not be discussed with the participants unless they express a desire to do so (i.e. it is on their agenda, not the HT’s agenda). The focus should always remain on reducing smoking behaviour and increasing physical activity.
4.1 Multiple behaviour change outline
Smokers who quit are generally advised not to change PA and diet at the same time by the Stop Smoking Service advisors. However, simultaneous multiple behaviour changes at the time of quitting does appear to be possible for some people, especially when PA is considered in terms of short bouts of daily activity, rather than structured, facility-based exercise on 2-3 occasions per week.
The goal of the EARS intervention is to support multiple behaviour changes in a way that limits mental overload, but uses PA to facilitate smoking reduction. The Figure below shows clearly the ideal scenario, and captures the dual aims of EARS.
Fig. 9.
Graphical representation of ideal simultaneous multiple behaviour change
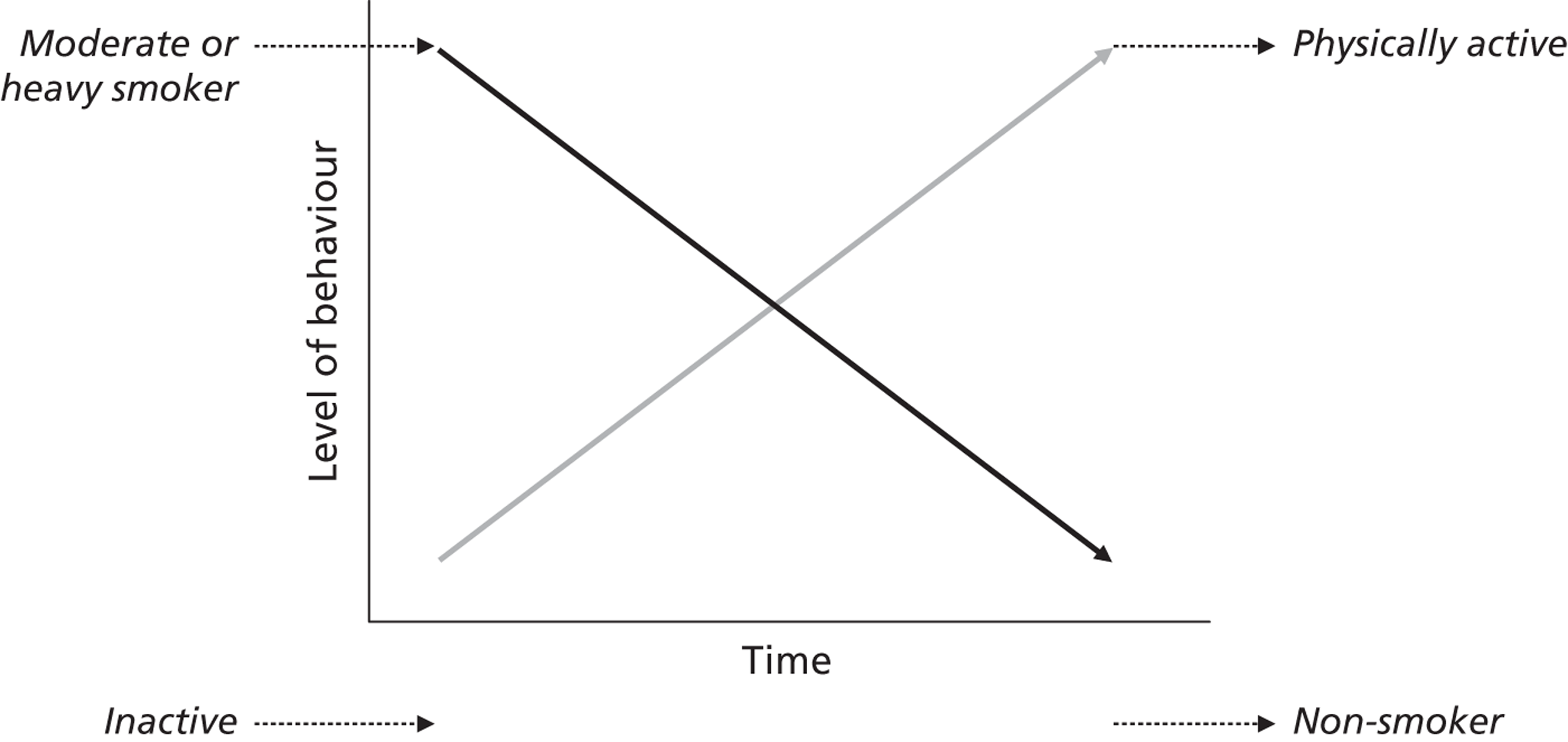
It is important that the participants appreciate how physical activity can impact on smoking; some clients will already accept this based on past experiences. Others will need more persuasion and experimentation. But it is a key component of EARS.
This Figure is available as a tool for generating initial discussions with smokers, alongside a Decision Balance sheet for the advantages and disadvantages of PA. Table 3 on p.32 also highlights how PA may explicitly and implicitly support smoking reduction and cessation, and these could be used as a tool to prompt clients.
4.2 Structure of the intervention
The EARS intervention sits inside a black box if you like, as shown in the Figure below. Ideally, we have inactive smokers coming in and active non-smokers going out, based on the efforts of the HT in what is a complex intervention.
Fig. 10.
Graphical representation of the EARS intervention as a black box

We would like to be able to describe it in a way that others could reproduce in future health services. But we accept that this may not be easy.
To be a truly client-centred intervention we need flexibility in how much support each smoker receives and when; it will not be a ‘one size fits all’ approach.
The study aims to determine what and how much support smokers want.
Nevertheless, we had to set a target for what support to offer and how to structure it, for the purposes of resource/staffing allocation. The EARS intervention will initially aim to consist of 2 face-to-face and 6 telephone communications, over 8 weeks, after baseline assessment and randomisation. We expect that many smokers will not want weekly contacts for 8 weeks. There will therefore be capacity to offer more to some people as required.
If at any point smokers express a desire to quit within those 8 weeks, they will be referred to a NHS SSS advisor for up to 6 weeks of support for quitting, using the usual pharmacotherapy and behavioural support. Six concurrent sessions will be delivered by the HT during this quit attempt to support the maintenance of PA?
4.3 Progression
Behaviour change is rarely a linear process, as the Figure on p. 11 of the HT Handbook shows. HT will help smokers to prepare for setbacks. Clients will increase PA and reduce smoking in a variable way, and could decide to quit at any point, if at all, within the initial 8 weeks of support. For one person, a 30 min walk on 5 days a week could be a great achievement that is worked towards over the 8 weeks, whereas others may accumulate shorter bouts within days. Reducing from 40 to 10 a day will require different progression compared with reducing from 20 to 10 a day.
Our early experiences of delivering the EARs intervention suggest that many smokers want to focus on smoking reduction initially, and already have ideas of which cigarettes to eliminate first. It then becomes a challenge to enhance any beliefs that physical activity maybe useful, as the remaining cigarettes pose a greater challenge to eliminate. HTs should not forget the focus of EARS on increasing physical activity.
Fig. 11.
Graphical representation of the EARS trial process in detail
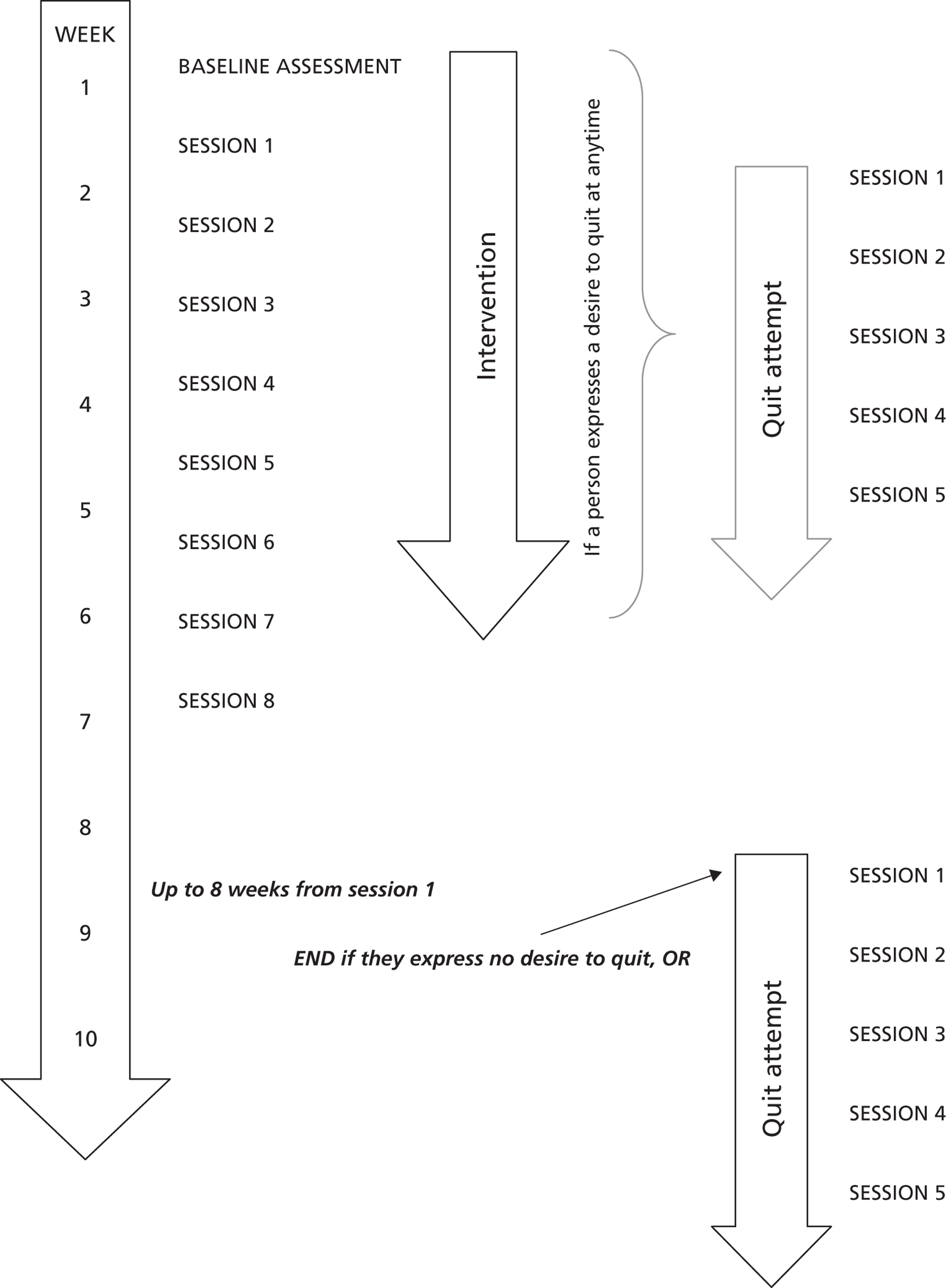
4.3.1 Face-to-face meetings
Face-to-face meetings will take place in variety of locations acceptable to the participants. This could be in a community location, or in a GP practice or similar. The location will be negotiated with the participant. For the initial settings a community location or GP practice is preferable, and thereafter at the patient’s home if the HT feels comfortable. It is the responsibility of the HT to book/arrange locations for meeting the participant. For any lone visits the LONE WORKER POLICY must be adhered to. See section 8.1.
The sessions can take place out of normal office hours in order to maximise participant attendance and retention, to be negotiated with the participant.
4.3.2 Telephone sessions
Each Health Trainer will be supplied with a mobile phone. This must be used for each contact made with the patient. The number should not be withheld and should be easily identifiable for the participant.
Again, the timing of the sessions should be flexible to suit the participant’s needs.
Telephone sessions must be completed in a confidential manner where no one can over hear your conversation. If the participant has others around them that is their choice, check they are happy to continue, but the HT must be in a private space.
4.3.3 Keeping in contact
ALL contacts and attempted contacts with participants must be recorded. This is to be able to calculate how much of the planned intervention has been delivered and hence how much has it cost.
Each participant may have a different preference for ways of keeping contact. Email, text messaging, postal letters and telephone calls are all acceptable.
Every effort should be made to ensure successful contact – this could include postal reminders of appointments, text messages before calling or calling in the morning of an appointment as a gentle reminder. It is a frustrating waste of your time and project time if somebody does not keep their appointment, but it will happen, so do not be disheartened and continue to make attempts to rearrange the appointment in line with the operating procedures.
5 Recruitment/Referral Procedures
An important part of the trial is to examine whether hard to reach smokers can be recruited into a smoking reduction trial such as EARS. The outlined recruitment strategy is to aim for 60 participants recruited through primary care (GP Practice lists) and 60 recruited through community-based approaches (NHS SSS lists, outreach work etc).
Of the 120 participants recruited and randomised, over 12 months from March/April 2011, we would like approximately 75% (n=80) of the sample to be unemployed, receiving benefits, or in social class C2-E; 30% (n=36) from single parent families; 20% (n=24) with mental health problems, with some overlap between sub-groups. As the study progresses we will get a feel for whether we are meeting these targets, and hence if specific strategies need to be adopted to achieve them.
5.1 Primary Care Referral
Two GP practices have agreed to take part in the study:
Marlborough Surgery, 1 Marlborough St., Devonport
PL11 4AE
Adelaide Surgery, 20 Adelaide St, Stonehouse
PL1 3JF
With support from the Primary Care Research Network (PCRN) the practice lists will be systematically searched. GP practice lists contain information on smoking status: It is this information which will be used to identify potential participants who will be sent an invitation letter. To prevent the possibility of having too high an influx into the trial, this will be done in batches of 50 letters per practice every week. Depending on response/uptake rate this may change.
The study is adopting an ‘opt out’ approach in order to not exclude those with low literacy levels. After the letter is sent there are five possible next steps:
-
The participant contacts the HTs expressing an interest in taking part and is recruited.
-
The participant contacts the HTs expressing no desire to take part.
-
There is no contact from the participant within one week, then the HT makes contact by telephone and they are screened and if suitable recruited into the trial.
-
There is no contact from the participant within one week and the HT makes contact with the participant and they decline to take part.
-
There is no contact from the participant within one week and the HT fails to make contact with the participant.
5.2 Community Recruitment
Community recruitment approaches will be explored and developed throughout the trial. They will need to be well documented as reporting on effective community approaches is of great interest in a study of this nature. It is likely to consist of a very diverse range of approaches, including:
-
NHS SSS Lists
NHS SSS possess lists of all those who have attempted to make an abrupt quit attempt using their service but have failed in the past. Invitation letters will be sent to those who meet the inclusion criteria, and the same 5 possible next steps as for the primary care referral will follow.
-
Community Centres
-
Community Events
-
Outreach work
-
Voluntary groups
-
‘Health Champion’ referral
The community recruitment approaches will be established in an exploratory way. Detailed records of the success of different type of contacts and approaches will be maintained.
6 Session by session
Overview of each session:
| Session | Aim | Content (may be transient across sessions depending on individual progress) |
|---|---|---|
| 1 (face-to-face) | Introduction and assessment. Build rapport with patient. Explore patient beliefs about PA and smoking reduction. Enhance intrinsic motivation. Planning and goal setting. Enhance confidence for change. |
Discuss collaborative approach and explore nature of the patient’s smoking and physical activity habits. Build belief in the value of and importance of cutting down. Explore pros and cons of change. Present 4 possible approaches to cutting down and ask participant to identify which is most appropriate to them. Explore PA history, interests, pros and cons of different forms of PA. Explore beliefs in PA as an aid to cutting down (explicitly – past experience?, and implicit effects). Develop intrinsic motivation for PA and cutting down. Present treatment structure, flexibility of sessions, organization of sessions. Explore tasks for next session, self-monitoring with weekly worksheet. This content can extend into and be repeated in subsequent sessions depending upon patient’s readiness to change. |
| 2 (phone call) | Building commitment to increase PA and reduce smoking. Planning and goal setting. Provide feedback. |
Review progress (tasks, new tasks, changes and barriers). Work through pros and cons of increasing and maintaining PA. Encourage self-monitoring with a weekly worksheet to identify PA level, and strengthen perceived links between PA and smoking levels. Support client in planning and goal setting for PA and continued reduction. Signpost client to PA opportunities if needed. Encourage a different reduction approach if the first approach has been unsuccessful. Revise strategy and plans if necessary (phone vs in person session). This content can extend into and be repeated in subsequent sessions depending upon patient’s readiness to change. |
| 3 (phone call) | Discussion of progress, outcomes and barriers. Support psychological needs associated with PA (the three Cs). Encourage belief in value of PA as a tool for coping with reduction. Provide feedback. |
Assess progress with goals set at last session Facilitating PA experience – explore ways to build competence, autonomy and relatedness in an enjoyable way Discuss completion of goals, what they found easy or difficult and why (autonomy) Discussion of ‘barriers and facilitators’ Discuss revision of goals, plan new goals (competence) Discuss how to progress with new goals to increase interaction with significant others (relatedness) |
| 4 (phone call) | Revise progress, discuss medium/long term goals. Continue to support psychological needs Encourage belief in value of PA. |
Review previous goals, reflect on achievements and plan new goals – building a sense of competence Explore ways to build a sense of control (self-regulation) over PA behaviour and mood. Explore ways to build relatedness or companionship through PA |
| 5 (phone call) | Discuss progress/changes in well-being. Barrier management and continuing activity. Establish/maintain (or progress to) PA and reduction targets. |
Promote self-regulatory skills and ownership of PA decisions/choices and reflect on progress on reduction Encourage quality social opportunities (companionship) through PA participation. Maintain use of goal setting (worksheets if used) Discuss potential strategies to help maintain activity. |
| 6 (phone call) | Review maintenance strategies. Reinforcing activity and revision. |
Highlight patients’ control over PA choices and effects on mood and smoking behaviour. Reinforce any changes in self-confidence related to PA and smoking reduction. Review progress and explore how to manage relapse. Encourage reflection on situations which illicit undesirable behaviour (increases smoking) and explore ways to deal with these |
| 7 (phone call) | Review maintenance strategies. Reflect on achievements. Consider long term maintenance/goals. |
Reinforce positive changes in behaviour to this point Reflect on the benefits gained from changing behaviour Emphasise distance travelled and achievements made, no matter how small Begin to discuss potential strategies for long term maintenance (eg identifying relapse) |
| 8 (face-to-face) | Final discussions. Exit strategy. |
Discuss triggers/cues to changes in PA and smoking behaviour Explore management and modification to strategies Consider positive experiences and how to re-engage in PA if relapse or smoking increases. |
| The sessions during a quit attempt may begin at any time up to session 8 or after session 8 if they express a desire to quit during that session. Although the main outcome measure (expired air CO) is taken 4 weeks post quit, 6 weeks of support will be provided to allow for lag time between expressing a desire to quit and accessing NHS SSS support and then setting a quit date. There is an element of uncertainty in these timings and flexibility is essential. | ||
| START OF QUIT ATTEMPT 1 (face-to-face) | Goal setting. Support psychological and behavioural needs. Coping with cravings |
Discuss explicit use of PA as a coping strategy for cravings Build link between inactivity and elevated cravings (weekly worksheets on cravings and PA) Revise activity goals in line with quit attempt |
| QUIT ATTEMPT 2 (face-to-face) | Review of progress. Revision of goals. Building three ‘c’s of activity |
Review progress of goals – what was easy what was hard. Explore situations and behaviours which elevated cravings and strategies to cope with these in future Reflect on nature and quality of PA (is it satisfying the three ‘c’s of Control, Competence, and Companionship?) |
| QUIT ATTEMPT 3 (face-to-face) | Review of progress. Revision of goals. Building three ‘c’s of activity Overcoming barriers |
Review progress of goals – what was easy what was hard. Explore situations and behaviours which elevated cravings and strategies to cope with these in future Reflect on nature and quality of PA (is it satisfying the three ‘c’s of Control, Competence, and Companionship?) |
| QUIT ATTEMPT 4 (face-to-face) | Review of progress. Revision of goals. Building three ‘c’s of activity Overcoming barriers. Identifying smoking cues. |
Review progress of goals – what was easy what was hard. Explore situations and behaviours which elevated cravings and strategies to cope with these in future Reflect on nature and quality of PA (is it satisfying the three ‘c’s of Control, Competence, and Companionship?) |
| QUIT ATTEMPT 5 (face-to-face) | Review of progress. Revision of goals. Building three ‘c’s of activity Overcoming barriers. Identifying smoking cues. Relapse prevention planning |
Review progress of goals – what was easy what was hard. Explore situations and behaviours which elevated cravings and strategies to cope with these in future Reflect on nature and quality of PA (is it satisfying the three ‘c’s of Control, Competence, and Companionship?) |
| QUIT ATTEMPT 6 (face-to-face) | Review of progress. Revision of goals. Building three ‘c’s of activity Overcoming barriers. Identifying smoking cues. Relapse prevention planning |
Review progress of goals – what was easy what was hard. Explore situations and behaviours which elevated cravings and strategies to cope with these in future Reflect on nature and quality of PA (is it satisfying the three ‘c’s of Control, Competence, and Companionship?) |
7 Appendices
7.1 Reflective checklists
7.1.1 Session 1 checklist
7.1.2 Session 2-8 checklist
7.2 Problem-solving worksheets
7.2.1 Physical activity worksheet
7.2.2 Cutting down on smoking worksheet
7.2.3 Coping strategies worksheet
7.2.4 Cravings worksheet
7.2.5 Typical day & reasons for smoking worksheet
7.3 Self-monitoring worksheets
7.3.1 Physical activity diary
7.3.2 Smoking diary
7.4 Information sheets
7.4.1 Benefits of reducing smoking
7.4.2 Benefits of quitting
7.4.3 Smoking and physical activity
7.4.4 Physical activity
7.4.5 How active are you?
7.5.6 How much does smoking cost you?
7.5.7 Barriers to cutting down
7.5.8 Barriers to exercise
7.5.9 Coping strategies for different situations
7.5.10 Working out cigarette equivalents
7.5.11 NRT Guide
7.5.12 Champix guide
7.5.13 Zyban Guide
Appendix 2 Exercise Assisted Reduction then Stop study protocol
An exploratory trial to evaluate the effects of a physical activity intervention as a smoking cessation induction and cessation aid among the ‘hard to reach’
EARS
(Exercise Assisted Reduction then Stop smoking)
Study Protocol
HTA no: 07/78/02
ISRCTN no: 13837944
UKCRN Study ID: 8937
LREC no: 10/H0106/59
List of Abbreviations
CICODMECDRCPESFTNDGPHTHTAmCEQMIMPSSNARSNHSNRTPAPAR-QPCRNPCTPIPMQALYRCTSPSSSSSTSC
Confidence Interval
Carbon Monoxide
Data Monitoring and Ethics Committee
Devonport Regeneration Community Partnership
Effect Size
Fagerström Test for Nicotine Dependence
General Practitioner
Health Trainer
Health Technology Assessment
Modified Cigarette Evaluation Questionnaire
Motivational Interviewing
Mood and Physical Symptoms Scale
Nicotine Assisted Reduction then Stop
National Health Service
Nicotine Replacement Therapy
Physical Activity
Physical Activity Readiness Questionnaire
Primary Care Research Network
Primary Care Trust
Principal Investigator
Project Manager
Quality Adjusted Life Years
Randomised Controlled Trial
Statistical Package for the Social Sciences
Stop Smoking Services
Trial Steering Committee
3 Summary of study
| Title: | An exploratory trial to evaluate the effects of a physical activity intervention as a smoking cessation induction and cessation aid among the ‘hard to reach’. |
| Short Title: | Exercise Assisted Reduction then Stop (trial acronym: EARS) |
| Objectives: | Primary Objective: To develop a multi-component PA intervention aimed at helping smokers (not intending to quit in the next month), among ‘hard to reach’ groups, to cut down. Secondary Objectives: (i) To assess via interview the acceptability of such a PA intervention as an aid to cutting down, among ’hard to reach’ smokers. (ii) To assess via interview the acceptability of recruitment, assessment and randomisation procedures within a pilot pragmatic randomised controlled trial to compare the effects of a PA intervention versus brief advice (usual care) on quitting, among ’hard to reach’ smokers. (iii) To obtain an estimate of the intervention (PA v brief advice) effect size, relative risk and its precision to inform sample size calculations for a fully powered trial, from a pilot randomised trial to assess carbon monoxide confirmed abstinence at 4 weeks post-quit date. (iv) To assess process measures at 4, 8 and 16 weeks post-baseline including: self-reported cigarettes smoked; number of quit attempts; self-reported quality of life; mood & physical symptoms; cravings; PA by self-report and accelerometer (in a sub-sample); pharmacological and behavioural support used; and weight. (v) To estimate the resource use and costs associated with delivery of the intervention, and to pilot methods for determining future cost-effectiveness analyses. |
| Design: | A randomised Controlled Trial involving 120 heavy smokers (>15 cigarettes per day) from ‘hard to reach’ groups who wish to cut down the number of cigarettes they smoke but do not wish to quit within the next month. Randomised equally to brief advice or PA intervention. Week 1: Screening/Baseline Assessment: demographics, height and weight, expired CO measurement, randomisation, questionnaires, accelerometer (1 week, sub sample), Week 4: Questionnaires Week 8 (or start of quit attempt): Questionnaires, height and weight, expired CO measurement, accelerometer (1 week, sub sample) Week 16 (or 4 weeks post quit): Follow Up Assessment: Questionnaires, height and weight, expired CO measurement, accelerometer (1 week, sub sample) |
| Treatment Schedule: | Brief Advice: Written and verbal information on NHS Stop Smoking Service (SSS) with information on the benefits of quitting and how to quit provided at baseline. Those expressing a desire to make a quit attempt will subsequently be referred to NHS SSS. PA Intervention: Written and verbal information on NHS SSS with information on the benefits of quitting and how to quit provided at baseline. Smokers will select one of three strategies for smoking reduction and receive weekly support to attain this. Face to face physical activity support sessions will be conducted at weeks 1, 4, and 8 along with supportive phone calls in each intermediate week. The communications will involve tailored physical activity counselling, guidance on using a free pedometer to achieve SMART goals, and signposting to local exercise opportunities with subsidised access as required, with the aim of increasing the amount of regular physical activity completed by each participant for both implicit and explicit purposes as an aid to quit. Those expressing a desire to make a quit attempt will subsequently be referred to NHS SSS. |
| Treatment Groups: | (i) Brief Advice; (ii) Brief advice + PA Intervention |
4 Main research question
What is the effect of a physical activity intervention designed to aid ‘hard to reach’ smokers wishing to cut down, but not quit, on smoking reduction and cessation, when compared to brief advice?
5 Plain English Summary
5.1 Background
NHS smoking cessation treatment aims to help people to remain abstinent after an abrupt quit attempt but even with the best available behavioural and pharmacological support as few as 22% are abstinent at 12 months. The addition of physical activity to usual care has been shown, among sufficiently large studies, to increase quit rates[1].
For an abrupt quit attempt recruitment into an NHS SSS is difficult among ’hard to reach’ smokers. However, surveys suggest that between 57-66% of smokers would like to cut down but are not yet ready to quit. Nicotine Assisted Reduction then Stop (NARS) studies have shown increased quit attempts and cessation rates[2], but NICE guidelines do not recommend NRT products for smoking reduction and only one study (pre-post one group design) has suggested that physical activity may help in cessation induction[3]. Exercise Assisted Reduction then Stop (EARS) could be effective for several reasons: (1) Increasing physical activity before tackling a quit attempt may be easier; (2) physical activity related breathlessness may prompt a desire to quit; (3) it builds confidence to use physical activity to cope with cravings, stress and low mood, before and after quitting; and (4) it may attenuate the stronger reinforcement value from each cigarette, observed when smokers try to cut down. We therefore wish to examine if physical activity v brief advice enhances smoking reduction attempts and successful quitting for 4 weeks, among ’hard-to-reach’ smokers from lower-socio-economic groups, building on the experiences of a wide range of professionals and residents in Plymouth.
5.2 Intervention design
In Phase 1 we will establish appropriate ways to increase physical activity while reducing smoking among ’hard-to-reach’ smokers, using an individually tailored counselling intervention, which can be described in a manual and replicated elsewhere. From our extensive previous quantitative and qualitative information on client preference and needs and professional views, and new information from interviews in the present study with smokers and community action-type leaders (e.g. health promotion specialists) we will develop and pilot the EARS intervention targeted at ’hard to reach’ heavy smokers (>15 cigarettes per day) wishing to cut down but not yet quit.
5.3 Methods
Phase 1 will explore the utility of various approaches (e.g. GP invitation, community advertising) to recruit ’hard to reach’ smokers, and pilot the intervention and assessment procedures. Phase 2 will aim to provide information from which to plan a larger trial. In an exploratory trial involving 120 smokers (wishing to cut down) will be randomly allocated equally to: (i) brief advice on cutting down; (ii) physical activity intervention + brief advice; with support from an NHS SSS for those wishing to quit, with on-going support for physical activity, for those in (ii). After baseline screening and assessments those in (ii) will receive the EARS intervention package (Health Trainer counselling, use of pedometers and guidance into free physical activity options). Smokers wishing to quit in both conditions will be offered support by the Plymouth NHS SSS for up to 6 weeks. Those in (ii) will receive additional parallel weekly Health Trainer support to remain physically active. The smoking status of all participants will be assessed, using expired carbon monoxide measures, and self-reported continuous abstinence, at 8 and 16 weeks after baseline, and at 4 weeks after any quit attempt. This will provide information to estimate expected effects at longer follow-up points. Secondary outcomes at 8 and 16 weeks (and at 4 weeks after any quit attempt) will include: number of quit attempts; self-reported quality of life; withdrawal symptoms, cravings, readiness to quit, confidence to quit and stay quit, use of NHS SSS after quitting and pharmacological support; physical activity and weight. All assessments will be conducted by a Health Trainer/researcher (in a GP surgery or community setting). Taped interviews will be conducted with GPs, stop smoking advisors and smokers to establish information on feasibility and acceptability of the recruitment, randomisation, intervention and assessment procedures.
Smokers will be recruited with the support of the Primary Care Research Network, community advertising and community outreach workers. Trial management will be with the support of the Peninsula Medical School’s accredited Clinical Trials Unit, and follow Good Clinical Practice. A 0.7 Project Manager (for 27 months) will co-ordinate the study, lead Phase 1, support recruitment in Phase 2, and help with data analysis and report writing. Two 0.7 FTE Health Trainers/researchers (for 19 months) will recruit, and do baseline screening, randomisation, all assessments and provide the PA intervention.
6 Background and rationale
6.1 Reducing heath inequalities
NHS priorities for helping people to quit smoking focus on identifying a quit date and abrupt cessation, with pharmacological and behavioural support[4]. After one year, only about 4% of those who attempt to quit alone succeed[5], whereas that figure is almost doubled (7%) with NHS support in primary care and almost quadrupled (15%) with the support of NHS specialist stop smoking services[6]. In recent years greater resources have been directed towards helping ‘hard to reach’ groups to quit in an attempt to address health inequalities[7]. These groups include the unemployed, those in social class grade C2-E (among whom smoking prevalence rates reduced only 1.3% compared with 2.3% for grade AB-C1, from 2007-8[8]), people with mental health problems, ethnic minorities, and young single parents. However, it is challenging to recruit such smokers into NHS services that focus on abrupt cessation[9]. New approaches are needed to increase the number of ‘hard to reach’ smokers who make a quit attempt with NHS support and hence successfully quit, such as locating services in community settings with most need[4], developing roles for NHS outreach workers (e.g. Health Trainers)[10], and developing complex behaviour change interventions that target multiple behaviours among ‘hard to reach’ groups[11].
6.2 Abrupt cessation versus Cut down then quit
Abrupt cessation is the preferred NHS approach because it is believed that if smokers cut down prior to quitting they may gain greater reward from each cigarette and hence find quitting even more difficult[4]. Yet, in the English Smoking Toolkit Study, 57% of current smokers reported they were in the process of cutting down, of whom a quarter were using NRT, with no difference across social class[5]. This suggests that the majority of smokers are using other cognitive and/or behavioural approaches to cut down. In a US survey interest in reduction was highest among those who were less interested in quitting and heavier smokers [12]. There is also evidence from epidemiological studies that cutting down is associated with an increased probability of trying to quit. Recent evidence suggests that smokers who do not intend to quit in the next month, but cut down with the use of nicotine replacement therapy (NRT), are more likely to make a quit attempt and be abstinent at follow-up[2]. However, there is a lack of research on behavioural support programmes for those wishing to cut down, to inform NHS policy and practice.
6.3 Why reduction programmes may work
There are several reasons why a reduction programme may work, namely:
(1) Increasing the length of time between cigarettes may reflect steps in moving from the identity of a heavy or moderate smoker to that of a light then non-smoker. Identity shifts are important in smoking cessation. (2) Increasingly longer periods between smoking a cigarette may progressively raise confidence to abstain, which may generate intentions to actually quit and reduce the risk of relapse. (3) With fewer and fewer cigarettes the association between environmental cues and conditioned response (to smoke) weakens, which will lead to low urges to smoke when abstinent. (4) A lower drug intake might reduce drug dependence thus increasing the ability to abstain completely. Nicotine assisted reduction then stop (NARS) programmes aim to facilitate these changes by providing a dose of nicotine to relieve cravings and withdrawal symptoms. Other behavioural strategies for reducing cravings and withdrawal symptoms may also be efficacious but little is known about their effectiveness.
6.4 PA as an aid to smoking reduction and cessation
Theoretically, increasing PA may help reduction in several ways:
-
Systematic reviews of 20[1] and 14[13] studies showed that during temporary smoking abstinence, a short bout of PA (e.g. a brisk 15 min walk or 5 mins of seated isometric exercise) reduces cravings and withdrawal symptoms. PA also appears to reduce reactivity to smoking cues, which have been shown to predict lapses and relapse during a quit attempt,[14] and delays ad libitum smoking[14–17]. It would therefore appear appropriate for smokers to explicitly use short bouts of PA to aid smoking reduction and quitting.
-
PA enhances mental health which has been associated with reduced smoking. Also, doing more PA may cause a shift from the identity of a smoker to that of an exerciser; consequently increasing PA may have implicit positive effects on smoking habits.
-
Increasing PA while cutting down (then quitting) may reduce weight gain. In prospective population surveys and trials weight gain and fear of weight gain is associated with quitting smoking, especially among women and initially heavier smokers[18–20], with an average of 7kg gained within a year of quitting[21]. Increasing PA has been suggested as a useful strategy to prevent weight gain[22], not only by increased energy expenditure but also through self-regulation of energy intake, particularly emotional snacking in response to withdrawal symptoms such as depression and anxiety[23,24]. The effects of exercise on preventing weight gain are likely dependent on the exercise dose[25].
6.5 Chronic effects of exercise as an aid to smoking cessation
Four adequately powered RCTs have assessed physical activity as an aid for smoking cessation after an abrupt quit attempt[1] with encouraging results. One study[22] showed that vigorous intensity structured (gym-based) exercise on three days a week over 15 weeks plus behavioural support produced significantly higher cessation rates at 12 months, relative to controls, among female smokers (Odds Ratio = 2.4, 95% CIs = 1.0 to 5.6). However, smokers may be more interested in moderate rather than vigorous activity. Three studies have investigated the effects of promoting moderate intensity exercise. One study showed that four sessions of supervised exercise produced higher abstinence rates, versus controls, at the end of 12 weeks of treatment (EOT) (OR = 3.2, 95%,CIs = 1.6 to 6.6), but not at 6 or 12 months[26]. Another study found that supervised weekly exercise for 8 weeks significantly increased abstinence rates at 3 months (OR = 2.8, 95%, CIs = 1.3 to 6.8), but not at 12 months, among women smokers[27]. However, women achieving at least 110 minutes/week of activity were significantly more likely to achieve cessation at 12 months. A final study, involving 3 of the researchers, found that, although there were some increases in unsupervised activity levels, physical activity counselling alone (7 sessions) did not increase abstinence rates at EOT or 12 months[28,29]. The latter study was the only one to focus on unstructured PA, but this was equivalent to only 5-10 mins per week of cognitive–behavioural physical activity counselling, incorporating decision balance sheets, goal-setting, relapse prevention planning and self-monitoring, embedded within standard smoking cessation counselling, and advice to use 15 mg 16 h nicotine patches after quitting, throughout the treatment program. The target was to accumulate 30 mins of moderate intensity PA per day on at least 5 days per week and among those remaining in the study at 4 weeks after quitting (i.e. were abstinent at the end of the intervention) those in the PA group had increased time doing moderate and vigorous intensity exercise by an average of 120 mins compared with 24 mins in the control group.
There is wide variation in the timing of the start of the exercise programme in the studies reviewed that focused on smoking cessation[1]. For those beginning exercise either on or after the quit date[26, 30–32] success rates may have been hampered by the demand to cope simultaneously with two major changes in health behaviour[31, 33, 34]. Furthermore, where the exercise programme started after a period of smoking abstinence the potential for exercise to moderate withdrawal symptoms during this period was lost[35,36]. Increasing PA prior to any quit attempt (planned or otherwise) may address concerns about simultaneous health behaviour changes. Indeed NHS guidance for stop smoking advisors is to avoid changing diet and exercise during a quit attempt[4], and as a result advisors generally spend little time promoting PA[37], although recent evidence suggests that simultaneous health behaviour changes may not be detrimental[38]. However, a sample of advisors who did advocate simultaneous health behaviour changes had developed integrated strategies for using one behaviour change to positively support a quit attempt (Taylor et al, in press).
6.6 Trials involving PA promotion among smokers
Taylor et al[39,40] reported no effect of increasing PA on self-reported smoking but did find that smokers in a GP exercise referral scheme were more likely to increase their readiness to quit smoking. A similar finding was reported by Hardcastle et al[41] (and personal communication) in response to a PA and dietary motivational interviewing intervention, in which participants with multiple coronary heart disease risk factors (e.g. mean body mass index was 34, some smokers) were offered up to 5 sessions of lifestyle counselling. The findings of these two studies are limited by the numbers of smokers involved but support the idea that physical activity interventions are acceptable to many and may implicitly change thoughts about smoking.
6.7 Interest in using PA as an aid to quitting
109 Canadian community dwelling people with mental health problems, who were receiving smoking cessation treatment, completed a survey assessing perceived interest in physical activity and a 24-item decisional balance questionnaire reflecting potential advantages and disadvantages of becoming more physically active[42]. In the only literature to have considered the use of physical activity specifically among such smokers, 63% reported being interested in assistance in becoming more active and there were generally positive beliefs about the benefits.
Patten and colleagues[43,44] reported on two studies involving exercise targeted at smokers with a history of alcoholism. While the studies were not strongly designed to determine the effects of exercise, they did demonstrate that the participants were willing to increase their physical activity. In one study[43], behavioural counselling plus exercise was as effective as standard treatment, or behavioural counselling plus nicotine gum in reducing cigarettes smoked, prior to quitting.
In a survey of 181 smokers attending an NHS Stop Smoking Clinic in England and Scotland, 22% of quitters reported currently using PA to control their smoking, and 34% of those who had made a previous quit attempt reported having used PA as an aid[45]. Those more ready to use PA as a cessation aid and more physically active, held more positive beliefs regarding confidence to do more PA and expected value of doing more PA to aid quitting. The survey involved participants attending stop smoking services but little is known about the acceptability of PA as an aid to cutting down. In an audit of 178 smokers in a Plymouth GP practice, in a mainly deprived area, 39% were prepared to both gradually cut down over 8 weeks and were interested in taking part in research involving exercise as an aid to cutting down.
Several cycles of collaborative action research with advisors in Plymouth and South Birmingham helped us to develop and pilot our ‘Walk-2-Quit’ intervention, embedded within NHS SSS. In brief this involved providing all clients with a Self-Help Guide (SHG) and a pedometer prior to quitting. A variety of cognitive-behaviour approaches were used to increase client’s beliefs in the value of PA as an aid, and in their own confidence to use PA as it may best help them with quitting smoking. In a group setting, it was not possible to stage-match the intervention on an individual basis but the Transtheoretical Model[31,46] provided the framework in which to direct focus towards changing cognitions about PA as an aid. In a later section our proposed intervention will refer to and build on this extensive feasibility work (developed for the context of supporting abrupt quitters).
Following pre-clinical research that supported the efficacy of using isometric exercise for reducing cravings after temporary abstinence[47] Al-Chalabi and colleagues[48] examined the acceptability and feasibility of using MP3 files with guided isometric exercises (e.g. placing the palms of the hands together and pushing) to manage urges to smoke within a pilot randomised trial. While the study was not designed to detect differences in quit rates, about 80% of quitters reported using the isometric exercises each week, intended to continue doing so and would recommend these techniques to others trying to stop.
6.8 Other smoking reduction techniques with behavioural support
There are several techniques which have been proposed for smoking reduction which are still under evaluation. These include:
-
Setting a smoking time at equally spaced intervals throughout the day depending on the number of cigarettes smoked (e.g. a 16 a day smoker smokes on the hour every hour during a 16 hour day).
-
Identify half hour blocks where smoking occurs each day and attempt to reduce the blocks over time.
-
Rank the least to most important cigarettes of the day and aim to cut them down in order, although this can be problematic as smokers can easily identify the least and most important cigarettes but not the ones in the middle.
-
Schedule a per cent reduction in cigarettes each week.
-
We plan to offer smokers specific choices (1 or 2 from above) to take place as a reduction strategy alongside increasing PA.
6.9 Summary
Much of the above research has taken place in the context of helping smokers following an abrupt quit attempt. However, given the potential mechanisms for how physical activity may be beneficial and the need to consider behavioural approaches to helping smokers to cut down, and possibly quit, the proposed study seeks to provide the first scientific study on using PA for smoking reduction, then as an aid for quitting if a quit attempt is made. Given that physically active smokers are more likely to have attempted cessation in the past year, than inactive smokers[49], it seems justified to explore if helping smokers to become physically active will lead to more quit attempts and successful quitting. In the context of health behaviour change for ‘hard to reach’ smokers support to overcome environmental, financial, social, cognitive and emotional barriers to increase PA and reduce smoking will be needed.
7 Aims and objectives
We aim to perform an RCT of the effects of behavioural counselling to reduce smoking and increase PA to make it easier for ‘hard to reach’ smokers to cut down then quit, compared to brief advice on SSS and the benefits of quitting.
7.1 Main outcome measure
-
Confirmed expired CO concentration (Bedfont Smokerlyzer) abstinence at 4 weeks post-quit (if a quit attempt is made).
7.2 Other outcome measures
7.3 Process measures
7.4 Baseline measures and demographics
-
Age
-
Gender
-
Marital status
-
Highest education qualification
-
Ethnicity/race
-
Occupational status
-
Co-habitation with other smokers
-
Cigarettes smoked per day
-
Type of cigarette smoked
-
Quit attempts within the previous 8 months
-
Fagerström Test for Nicotine Dependence (FTND)
-
Self-reports of smoking and expired CO levels
7.5 Other measures
-
Reasons for withdrawing from the study
-
Reasons for missing pre and post quit support sessions
-
Adverse or serious adverse events
-
Number of pre- and post-quit sessions completed with the HT (and if a quit attempt is made with a SSS advisor) in the intervention arm
-
Alcohol consumption
8 Ethical approval
R & D approval to undertake the study will be obtained from Plymouth Teaching Primary Care Trust.
9 Population
9.1 Proposed sample size
Given the lack of research involving behavioural smoking reduction interventions in the ‘hard to reach’ population, the effect size of our intervention is uncertain (see above). We will therefore use the pilot trial in order to provide a quantitative estimate of the intervention impact (relative to control) in order to inform the sample size estimation for a definitive trial. Using data from a recent HTA meta-analysis of trials of smoking cessation[2] we have undertaken a scenario analysis in order to examine the impact on our estimation of effect size precision given differing pilot trial sample sizes and plausible effect sizes (see table). In the case of recruiting 120 participants (60 in each arm) if 5% are successfully quit after 4 weeks of making an attempt, in the control group, then we would need an effect size of 4 for the confidence interval to not include 1.0. With a smaller ES in the pilot RCT, clearly, for the CI to not include 1.0, the N would have to be considerably larger.
| Control quit rate* | Sample size^ (control : Rx) | Effect size Relative risk |
Precision of effect size estimate (95% CI)** |
|---|---|---|---|
| 5% | 60 (1 : 1) 60 (1 : 1) |
2.00 4.00 |
0.19 to 20.89 0.47 to 33.72 |
| 5% | 120 (1 : 1) 120 (1 : 1) |
2.00 4.00 |
0.52 to 7.63 1.18 to 13.46 |
| 5% | 160 (1 : 1) 160 (1 : 1) |
2.00 4.00 |
0.63 to 6.38 1.28 to 11.41 |
We believe this sample size provides an acceptable level of precision of effect size estimation i.e. upper estimate that is within the range of relative risk reported in the HTA report. Although a larger sample size (160) would offer greater precision, we believe that would not be feasible within the constraints of the time and resources sought for this pilot study.
9.2 Recruitment of ‘hard to reach’ smokers
In searching through the literature we have identified several ways of defining ‘hard to reach’ but broadly it refers to sections of the community that are difficult to involve in public participation. Such sections may include: (1) Minority groups; (2) Those ‘Slipping through the net‘; (3) Those who are ‘service resistant.’
Local data confirm that the proposed geographical location for the research has a higher prevalence of people who are unemployed, in social class C2-E, single parents, and have mental health problems, than in most other areas in the South West and the UK as we previously described. Residents also have a higher prevalence of smoking, lower use of NHS Stop Smoking Services, and lower levels of PA. We therefore implicitly expect our recruitment to be targeted at ‘hard to reach’ smokers, but will also explicitly aim to recruit smokers who have these characteristics. Further liaison with the local community in Plymouth will determine if the following recruitment targets would be realistic: 75% (n = 80) of the sample to be unemployed, receiving benefits, or in social class C2-E; 30% (n = 36) from single parent families; 20% (n = 24) with mental health problems, with some overlap between sub-groups. As noted above we will also target diverse ethnic minority groups.
A range of strategies will be explored in Phase 1 of the study, particularly through initiating a community advisory group (including smokers and community workers), to inform Phase 2. There is a wealth of experience for the best ways to try to reach the most difficult to engage with and promote health in the proposed areas of Plymouth, and we will also draw on other evidence from evaluations involving interventions delivered by HTs across the UK. Broadly, the strategies will include:
-
Networking and making links with other professionals who have offered services to ‘hard to reach’ groups.
-
Specifically targeting members of hard-to-reach groups and formally inviting them to participate in consultations (e.g. through GP lists).
-
Outreach work in community venues where hard-to-reach groups could be identified, approached and consulted (e.g. job centres).
Regarding the targeting of ethnic minorities, we accept that this will be more challenging than in other UK locations, due to the low proportion of minorities in Plymouth, but we will involve such groups in the advisory group to ensure as heterogeneous a sample in the pilot trial as possible.
In many ways, our study implicitly focuses on ‘hard to reach’ smokers simply because we intend to recruit smokers who are not wishing to quit but who wish to cut down and are typically heavier smokers. NHS Stop Smoking Services have traditionally focused on those wishing to make an abrupt quit attempt, who may be more successful and hence help to meet challenging targets for quit rates. The proposed work will target those for whom NHS support is not currently available.
9.2.1 The context
Recruitment will take place in and around Devonport and Stonehouse, the two most deprived neighbourhoods in Plymouth. In 2004, Devonport’s health deprivation score (within the Index of Multiple Deprivation) was 1.59; above the New Deals for Communities (NDC) average of 1.23[56], with a population of about 7000. In Devonport 49% smoke (MORI Household Survey, 2006) compared with 27% across Plymouth, and 23% nationally. In 2004, targets were set within the scope of the Devonport Regeneration Community Project (DRCP) to reduce smoking prevalence to 39% by 2010. Limited numbers accessing NHS Stop Smoking Services were thought to be due to delivery in clinical settings (e.g. Nuffield Clinic, Lipson Rd) and inadequate tailoring of services for local residents. In 2007-8, only 79 patients from the two Devonport GP practices (Marlborough St Surgery and Cumberland Centre) were recorded as having set a quit date by a specialist stop smoking advisor, of whom 43% were still quit after 4 weeks, verified by standard CO monitoring. In response, as part of the DRCP in July 2008, a level 3 specialist stop smoking advisor and registered nurse were appointed, and they have developed new outreach and community development approaches to service recruitment (e.g. cold approaches outside a supermarket with follow-up calls to respondents about NHS services, visits to the Salvation Army centre, home visits, providing drop-in opportunities within non-clinical community facilities, attending local events). By the end of 2010, the Brickfields Healthy Living Centre (HLC) is expected to be completed in Devonport, to complement similar facilities (e.g. Jan Cutting HLC) with access to exercise classes, catering, health promotion and community development initiatives in neighbouring areas. This information gives an indication of the prevalence and scope for conducting the proposed study among ‘hard to reach’ smokers in Plymouth. There is strong interest from the Public Health team (K Elliston, S. Thomas, personal communication), local GPs (R. Jones, R. Ayres, R. Byng personal communication) and NHS SSS (R. Moody – Manager of Services, M. Cheshire, personal communication) in the proposed study, as a novel approach to possibly increasing quit rates among ‘hard to reach’ groups.
9.2.2 Describing the study and intervention to potential participants and its impact on recruitment
An audit in 2008 of 178 smokers in a GP practice (in a mainly deprived area of Plymouth), revealed that 39% were not interested in quitting but were prepared to gradually cut down over 8 weeks, and were also interested in joining a study ‘to see if physical activity is useful for helping you to reduce the amount you smoke. [The study would include support such as professional support, a self-help booklet, a free pedometer, and free access to an exercise facility]’. Initially we will begin by describing the intervention as one involving behavioural support for reducing smoking, with additional support to increase PA to be delievered by a person (Health Trainer) with local knowledge of opportunities for engaging in PA or exercise. The initially planned Patient Information Sheet is shown in Appendix X.
Recruitment will be through both Primary Care (1) and community-based (2) approaches (with approximately equal numbers from each) as follows:
-
The HTs, with Service Support from the Primary Care Research Network (PCRN), will identify heavy smokers on GP practice lists (though not necessarily attending GP surgeries on a regular basis) in Devonport, Stonehouse and adjacent neighbourhoods. Smokers will be contacted by a letter signed by the patient’s GP, inviting them to take part in the study, and a brief Information Flyer. They will be asked to return a reply slip to the GP practice or phone (to a mobile) the HT directly indicating interest in the study. The HT will contact the participant, confirm eligibility for the study, arrange a time to meet them at the GP surgery if suitable, and also mail a more detailed Patient Information Sheet. At the meeting, the study will be further explained and Informed Consent provided if appropriate. This may limit recruitment based on literacy, and we will take advice from our user advisory group on refining such an invitation.
-
The HTs will circulate a brief Information Flyer and posters, about the study to appropriate NHS staff (e.g. health visitors, specialist and non-specialist stop smoking advisors, public health workers) working outside primary care. Posters and public adverts will be circulated throughout the respective neighbourhoods (e.g. pubs, media, community centres), guided by consultation with community leaders (e.g. MIND centre and other charity managers) and smokers or recent quitters identified as possible champions for promoting PA and healthy living. There may be scope to identify people with specific skills and attributes who are able to engage in recruitment activities (e.g. giving out flyers in pubs and job seeking centres). Interviews in Phase 1 will inform the selection of such approaches. The PI will work closely with Dr Richard Ayes (Medical Advisor to the Devonport Community Regeneration scheme, and lead for health inequalities in Plymouth Teaching PCT), to establish a working group with relevant community stakeholders, to specifically identify recruitment strategies, and scope for intervention delivery by an HT.
A recent evaluation of the first HT service in the UK[57], specifically seeking to determine if the most disadvantaged groups were being seen, revealed that 83% were referred by a GP or practice nurse, 11% were self-referrals, 1% by a pharmacist, and 5% from ‘other’ sources including dietitian, midwife, GP receptionist, attendance at a health promotion event or other health promoting service. A high proportion (53% vs 24% nationally) of those seen by the HT in the central London service, were smokers, which probably reflected the gatekeepers’ priorities for referral. Socio-economic characteristics of the sample indicated that recruitment had successfully reached a considerably higher proportion of people with income from benefits, living in rented accommodation, and with no qualifications, compared with London generally, and nationally. In contrast to this London HT service, we will seek to recruit 50% of the sample from the community.
9.2.3 Recruitment Rate (Phase 2)
We will recruit 3 smokers per week (12 per month for 10 months) which will result in a maximum number of 48 participants in the trial (after 16 weeks) at any one time, 24 of whom will be in the PA arm. Not all patients will be in contact with the HT at any one time, through participant choice, so we expect that this workload will be sufficient for 1.4 full-time equivalent Health Trainers. Recruitment will take from 2/2011 - 12/2011 (10 months). During the middle of the trial the Research Fellow will also be available to conduct some assessments.
9.3 Inclusion criteria
-
Written informed consent
-
People who are currently smoking at least 15 cigarettes a day and have done for a minimum of 3 years;
-
are at least 18 years of age;
-
and are not motivated to quit smoking in the next month but do wish to cut down the number of cigarettes they do smoke
9.4 Exclusion criteria
-
Are contra-indicated for moderate physical activity;
-
have an injury or illness that might be exacerbated by exercise;
-
are pregnant;
-
or wish to use Nicotine Replacement Therapy (NRT) as an aid for smoking reduction
10 Informed consent
Patients will receive their information sheet at least seven days prior to the screening assessment in order to allow sufficient time for consideration of participation.
At the screening visit the details and implications will be explained and the opportunity will be given for the participant to ask questions. Written informed consent will be obtained by the Researcher/Health Trainer prior to screening or any study procedures being undertaken. Participants will also be asked to agree to their GP being informed of their involvement in the study.
11 Study procedure
See Appendix II.
11.1 Study week (1): Screening/Baseline assessment and randomisation
Suitable participants will be assessed by the Researcher/HT at an agreeable location (e.g. GP practice, community centre):
-
Expired CO (p.p.m.)
-
Weight and height (BMI)
-
Accelerometer use (1 week, in sub sample)
-
Questionnaires:
-
Smoking History
-
PAR-Q
-
Demographics
-
Self-reported quit attempts in past 8 months lasting at least 24 hours, use of cessation aids and length of prolonged abstinence
-
Self-reported cigarettes, pipes, and cigars currently smoked in the past week
-
Current readiness to quit smoking
-
Cigarettes smoked in past week
-
FTND
-
Urge to smoke (single item)
-
Mood and Physical Symptoms Scale (MPSS) (7 items)
-
Perceived Stress Scale (PSS)
-
Confidence to quit, cut down, and importance of quitting
-
Cigarette Evaluation Questionnaire (mCEQ)
-
Self-reported Physical activity (PA)
-
Alcohol consumption
-
SF36
-
Self-reported use of NRT products or smoking related aids
-
11.2 Study week (2) and (3): Behavioural intervention arm
-
Pedometer step count (diary)
-
Adverse or Serious Adverse events
-
Questionnaires:
-
Confidence to quit, cut down, and importance of quitting
-
mCEQ
-
Self-reported PA
-
PSS
-
MPSS (7 items)
-
Urge to smoke (single item)
-
Self-reported use of NRT products or smoking related aids
-
Cigarettes smoked in past week
-
Prolonged abstinence
-
Self-reported cigarettes, pipes, and cigars smoked
-
11.3 Study week (4): Phone call measure at 4 weeks post-baseline
-
Adverse or serious adverse events
-
Questionnaires:
-
Confidence to quit, cut down, and importance of quitting
-
mCEQ
-
Self-reported PA
-
PSS
-
MPSS (7 items)
-
Urge to smoke (single item)
-
Self-reported use of NRT products or smoking related aids
-
Cigarettes smoked in past week
-
Prolonged abstinence
-
Readiness to quit smoking
-
Self-reported cigarettes, pipes, and cigars smoked
-
11.4 Study week (5), (6) and (7): Behavioural intervention arm
-
Pedometer step count (diary)
-
Adverse or Serious Adverse events
-
Questionnaires:
-
Confidence to quit, cut down, and importance of quitting
-
mCEQ
-
Self-reported PA
-
PSS
-
MPSS (7 items)
-
Urge to smoke (single item)
-
Self-reported use of NRT products or smoking related aids
-
Cigarettes smoked in past week
-
Prolonged abstinence
-
Self-reported cigarettes, pipes, and cigars smoked
-
11.5 Study week (8) or start of quit attempt
To be conducted by the Researcher/HT in person at an agreeable location:
-
Expired CO (p.p.m.)
-
Weight and height (BMI)
-
Accelerometer use (1 week, in sub sample)
-
Adverse or serious adverse events
-
Questionnaires:
-
Self-reported cigarettes, pipes, and cigars smoked
-
Prolonged abstinence
-
Readiness to quit smoking
-
Cigarettes smoked in past week
-
FTND
-
Urge to smoke (single item)
-
MPSS (7 items)
-
PSS
-
Confidence to quit, cut down, and importance of quitting
-
mCEQ
-
Self-reported PA
-
Alcohol consumption
-
SF36
-
Self-reported use of NRT products or smoking related aids
-
11.6 Weekly (6 weeks) during quit attempt with NHS SSS support: Behavioural intervention arm
To be conducted by the Researcher/HT in person at an agreeable location:
-
Expired CO (p.p.m.)
-
Adverse or serious adverse events
-
Pedometer step count (diary)
-
Questionnaires:
-
Self-reported PA
-
Self-reported use of NRT products or smoking related aids
-
Urge to smoke (single item)
-
MPSS (7 items)
-
Perceived stress
-
Prolonged abstinence
-
11.7 Study week (16) or 4 weeks post-quit if same: All participants
-
Expired CO (p.p.m.)
-
Weight and height (BMI)
-
Accelerometer use (1 week, in sub sample)
-
Adverse or serious adverse events
-
Questionnaires:
-
Self-reported cigarettes, pipes, and cigars smoked
-
Prolonged abstinence
-
Readiness to quit smoking
-
Cigarettes smoked in past week
-
FTND
-
Urge to smoke (single item)
-
MPSS (7 items)
-
PSS
-
Self-reported PA
-
Alcohol consumption
-
SF36
-
Self-reported use of NRT products or smoking related aids
-
12 Randomisation
Randomisation will occur following a successful screening assessment where participants will be allocated a study number and randomised equally into either (i) brief advice on cutting down; or (ii) to receive the behavioural intervention. Randomisation will be completed by random number generation using a computer held in Vesey Building, Salmon Pool, Lane, PCMD, Exeter. The randomisation code will be kept in a sealed envelope inside a locked cabinet inside the Principal Investigator’s office at the School of Sport and Health Sciences at The University of Exeter.
13 The EARS intervention
13.1 Phase 1: Developing and piloting the intervention
Professionals from public health, primary care, The Jan Cutting Healthy Living Centre, and NHS Stop Smoking Services working in the targeted areas of Plymouth have been consulted on the proposed study and several ideas have already emerged for developing an appropriate intervention with specific groups. The Plymouth YMCA is also keen to be involved, given the acceptability of recruiting smokers into a YMCA exercise programme in a deprived community in the US[3]. A co-applicant, Dr Richard Ayres is Medical Advisor for DRCP, and lead in Plymouth Teaching PCT for Health Inequalities has also provided valuable information. We will establish a working group, prior to the project formally beginning to identify appropriate stakeholders from the respective neighbourhoods, as well as identifying potentially suitable candidates for the HT/researcher role.
The intervention will be delivered by a Health Trainer (HT). The 2004 Department of Health White Paper Choosing Health: Making healthy choices easier proposed the development of a new role for improving health and reducing health inequalities – accredited HT. HTs are drawn from local communities and are trained to reach those who want to adopt healthier lifestyles (e.g. reducing or quitting smoking, increasing PA), but who have little contact with services[10,11]. HTs develop an understanding of the needs of people in deprived communities, while applying basic health behaviour change strategies. Co-applicant SM helped to develop the competences and the national training programme for HTs and is involved in their evaluation. Our intervention manual (and the subsequent intervention to be tested in the exploratory trial) will describe approaches that build on the competences that an HT would be expected to have.
13.2 What is the best approach to changing PA and smoking?
Research on multiple health behaviour change has tended to focus on how best to change several individual behaviours. In the present context there may be two types of processes involved in how increases in physical activity influences smoking, namely implicit and explicit ones. Implicit processes may be involved particularly if the focus is on increasing PA, rather than smoking reduction, with an expectation that PA will, through previously identified mechanisms, influence smoking behaviour. For example, increasing PA may enhance mood and reduce stress, which reduces the urge to smoke, or additionally becoming involved in exercise initiates an identity change (perhaps through new non-smoking social networks). There is considerable research on the effectiveness of interventions for increasing PA in general (e.g. [58]) though relatively little has focused on ‘hard to reach’ groups[11]. Explicit processes may be involve if the focus is on how best to cut down smoking, or support a quit attempt, specifically using PA. For example, exercise sessions (e.g. seated isometric exercise, aerobic exercise) could be recommended as a coping strategy for managing cravings and withdrawal symptoms or weight management. In the former, the focus is mostly on a single behaviour and in the latter there is a need to think about increasing PA while also managing a change in smoking (i.e. multiple behaviour changes). It may be that both processes play a part, but each has implications for designing an intervention, which may be influenced by the target group, and the individual’s ability to take on simultaneous multiple behaviour changes. In Phase 1 we will interview smokers from different ‘hard to reach’ groups about how PA could be used to reduce smoking, and their preferences for just focusing on increasing PA, with the possibility that smoking will decrease, or focusing on how best to cut down, explicitly using PA as an aid to reduce cravings and increasing the duration between cigarettes (as employed with nicotine assisted reduction to stop programmes).
The PA and abrupt smoking cessation trials, reviewed above (see[1]), largely focused on the effects of structured exercise in addition to smoking cessation advice. Less is known about promoting PA for smoking reduction, and in developing and then trying our intervention, prior to the pilot RCT, we will monitor how smokers view the utility of PA and exercise as they attempt to cut down. In particular, we will seek to identify if PA has utility to help smokers to achieve SMART goal oriented behaviour (e.g. cutting down by 50% over 2-4 weeks) as described in the HT training manual[10], and at a later stage, staying quit on a weekly basis, and if increases in PA increases confidence to cut down (and make a quit attempt). As ideas emerge from target groups, academic literature will also be reviewed to consider best practice for health promotion activity among ‘hard to reach’ groups. For example, voucher-based reinforcement therapy has been shown to be an effective treatment across a wide range of substance use disorders, including smoking cessation[59,60]. The intervention will eventually be defined in a manual with flexibility to tailor the intervention to participant needs and the local environment/facilities. The table below provides a guide to how the intervention may build on the typical approaches used by a HT, with an integrated approach to promoting PA as an aid to smoking reduction and quitting. We also identify specific process and outcome variables that may indicate how the intervention is working. HTs are trained to adopt a motivational interviewing (MI) counselling style (i.e. client-focused, non-judgemental) which also draws on psychological theories (e.g. Self-determination Theory; suggesting individual’s motivation is driven by basic needs to feel competent, in control and for companionship or relatedness) to help promote health behaviour change[61].
| Intervention component | Aim | Content | Process and outcome evaluation |
|---|---|---|---|
| Use MI principles and strategies in counselling sessions. | Develop rapport with smoker, building trust, and shared respect. | Effective communication skills. Exhibit empathy, listen, reflect, summarise. | Participant feedback on HT-led support. |
| Explore initial beliefs about cutting down, and quitting (e.g. pros & cons, confidence, triggers for smoking). | Identify readiness to quit, and ambivalence towards quitting. Increase self-awareness and build confidence to cut down. | Smoker identifies why it is important to cut down and quit, and identifies the challenges. The role of PA as an aid may emerge. | A shift towards stronger intentions to not only cut down but also quit over the early sessions. Smoker self-monitors own smoking behaviour. |
| Introduce PA as a healthy behaviour and aid to cutting down and quitting. | Increase beliefs in pros of PA alone and as an aid to cutting down & quitting. | Introduce PA as a behaviour that may regulate smoking. Explore beliefs about PA. | Participant increases beliefs in PA as a coping strategy and aid to cutting down. |
| Set goals to reduce smoking and increase PA. | Develop strategies to reduce smoking and increase PA. | Set SMART goals with smoker to reduce smoking and increase PA. Signpost to PA/exercise opportunities & remove barriers to do PA. | Goals identified and action plans developed. Self-monitoring used (e.g. smoking and PA diary kept). Rewards and reinforcement contingencies established. |
| Review and reflect on behaviour change. | Build confidence and perceptions of control, & ability to self-regulate. | Smoker reflects on PA successes and sets new targets; perhaps to quit. | Participant increases beliefs in confidence to cut down, and quit. |
| Maintenance and relapse prevention. Using NHS Stop Smoking Services. | To strengthen self-regulatory skills. To identify identity shifts. | Smoker reflects on own skills and strategies for avoiding relapse, including use of PA. Ready to quit? | Participant seeks support for a quit attempt, possibly using NHS specialist support. |
| On-going phone support provided for quit attempt in addition to NHS support. | To reinforce participants shift to non-smoker and more physically active. | Reflect on achievements and explore future needs for support to maintain PA. | Successful quit attempt and increase in PA. |
13.3 Phase 2: Exploratory trial
13.3.1 Standard treatment: Brief advice for quitting
After completing baseline assessments and random assignment, all control participants will receive written and verbal information on what the NHS Stop Smoking Services offer, including some brief information on the benefits of quitting smoking and how to quit in line with NICE guidelines. No information will be given on how to cut down the number of cigarettes smoked. If smokers wish to quit at any time they will be encouraged to contact the trial staff who will arrange for a member of the NHS Stop Smoking Services to contact them for individual or group standard 6/7 week (or matched to the participants needs) behavioural and pharmacological support for a quit attempt.
13.3.2 Behavioural intervention
Phase 1 will help us to develop a participant-centred framework. As shown in the table above, we envisage that it will involve multiple components that aim to address real and perceived environmental (e.g. nowhere to do PA), social (e.g. no one to do it with), financial (e.g. too costly), cognitive (e.g. not confident to do PA), and emotional (e.g. feel too tired) barriers to increase PA and reduce smoking, as considered above.
After completing baseline assessments and random assignment (by the HT/researcher), all PA participants will be offered a 45 min face to face PA support session in week 1, 4 and 8, and a supportive phone call in each intermediate week. The communications will involve tailored PA counselling, guidance on using a free Digi-Walker SW-200 pedometer to achieve SMART goals, and signposting to local exercise opportunities with subsidised access as required. The counselling will follow principles described in the HT manual[10], adapted in response to findings from Phase 1. If a smoker wishes to quit at any time they will be offered a meeting with a specialist NHS SSS advisor for individual or group standard 6/7 week (or matched to the participants needs) behavioural and pharmacological support for a quit attempt. At the same time, those attempting to quit will receive parallel weekly telephone support to maintain (or increase) physically activity.
Behaviour change strategies will focus on self-regulation and may involve the following: (1) A choice of alternatives for reducing cigarettes smoked as discussed previously (see 6.8). (2) Identification of situations, cues and moods/affect that elicit urges to smoke; (3) Increase perceived expectancy that PA may help to regulate mood and affect (instead of a cigarette); (4) Raise awareness of PA or sedentary patterns (what, where, when, who with) overall and in relation to smoking through self-monitoring (using a pedometer and daily diary); (5) Build confidence to increase PA primarily as a lifestyle behaviour (i.e. brief bouts of 10 mins) but not exclusively (i.e. can include structured PA), using standard cognitive-behavioural techniques; including goal setting, and self-monitoring (daily diary and pedometer). After a baseline period of pedometer wearing for 2 weeks, smokers will be encouraged to increase pedometer step counts by 10% from one week to the next (or at a self-determined rate) until achieving 10,000 steps per day or the participant’s target. At the same time they will be encouraged to monitor mood/affect and smoking patterns. Counselling sessions will seek to reinforce the smoker’s beliefs in the value of PA as an effective self-control strategy for reducing cigarette cravings and withdrawal. Up to £100 vouchers per smoker will be offered to support involvement in PA and minimise barriers to access. MP3 players (c. £15) will be provided to those interested, with a recording of a guided seated isometric exercise programme. Research suggests that such a programme can reduce momentary urges to smoke.
Financial subsidy will be available to support involvement in structured, supervised moderate-vigorous intensity exercise in gyms/leisure centres for example. This intensity of exercise may well reduce cigarette smoking and lead to increases in quit attempts for several reasons, such as: it may raise awareness of symptoms of breathlessness associated with smoking which may trigger a stronger motivation to quit; it places smokers in environments with fewer smokers, which reinforces reduced smoking and a non-smoking identity; and there may be a greater sense of achievement from doing more strenuous exercise. However, heavier smokers are more likely to do less moderate-vigorous physical activity and our experiences suggest that most smokers prefer walking. In contrast, moderate intensity exercise is more pleasurable[62], creates fewer perceived barriers (e.g. cost, convenience and time, and social) and hence is likely to be more sustainable, and has similar effects in reducing cigarette cravings and withdrawal symptoms to more vigorous exercise[63,64]. It is also used by about 35% of quitters as a smoking cessation aid[45] and promoted by over half of NHS SSS advisors[37].
14 Withdrawal from the study
Participation in the study is entirely voluntary and participants will be free to withdraw at any time.
15 Ethical Considerations
15.1 Risks to Subjects
Moderate intensity physical activity is safe and is recommended for most adults. It is anticipated that most smokers will increase walking and walking has no contraindications for most. Other physical activities will also be offered in the community and participants will be advised on the suitability of these (see below).
15.2 Adequacy of protections against risks
During the screening process those smokers who are contraindicated for moderate intensity physical activity or who have injury or illness which might be aggravated by exercise, will be required to gain approval from their GP before engaging in the study. Vigorous intensity activity can acutely and transiently increase the risk of sudden cardiac death and acute myocardial infarction in susceptible persons,[96] so the focus of all recommendations for increasing PA will be on moderate intensity PA. Participants will be given clear guidance on exercising at this intensity (i.e. something that increases the heart and breathing rate but not to the point of breathlessness or unable to maintain a conversation). Participants will be advised to seek approval from their GP prior to engaging in any vigorous intensity PA, regardless of age and gender. The smokers will be monitored for contraindications to exercise, for adverse events (see section (v) ‘Data and safety monitoring plan’ below) including physical symptoms (e.g. chest pain, extreme breathlessness), or change in health status at each counselling session and follow-up appointment. If there is any further doubt about the involvement of a participant in the study the Trial Manager will liaise with the participant’s GP.
15.3 Potential benefits of the proposed research to the participants and others
The smokers participating in the study may have a greater chance of stopping smoking and remaining abstinent, relative to those who try to stop without behavioural support. Though we do not plan to test the hypothesis in this pilot study, we may expect that those in the exercise condition will have an enhanced opportunity of stopping smoking. Those who increase and maintain regular physical activity during and following the study will receive many general health benefits, including a reduced risk of developing cardiovascular disease, stroke, hypertension, obesity and some cancers, even if they continue smoking[65].
15.4 Importance of the knowledge to be gained
Little is known about if and how behavioural support can help smokers to cut down, and if cutting down then leads to more quit attempts and continuous abstinence. The planned study seeks to inform the feasibility and acceptability of a behavioural intervention to be tested against brief advice (usual care) in a future large scale trial. This pilot trial will allow us to estimate if a full trial is justified, from both an effectiveness and cost-effectiveness perspective. If such a physical activity intervention was shown in a full trial to be effective and cost-effective for increasing quit attempts and smoking cessation it would offer important evidence for the design of behavioural interventions which are not currently available in the NHS. Smokers are typically less active than the general population[66] and evidence from interventions that help change multiple health behaviours are urgently required. Weight gain is common among quitters [67,68], but nothing is known about the effects of smoking reduction on weight gain or weight concern. The proposed study may provide unique information on changes in a variety of psychological variables (e.g. cravings and withdrawal symptoms) and weight gain and weight concerns among those who cut down and quit.
16 Adverse Events
It is anticipated that there will be few, if any, adverse events in response to the behavioural intervention. Any adverse events (AEs) will be monitored by the researchers and practitioners. If necessary these events will be discussed with one of the GPs (Dr Jones, Dr Ayres, Dr Aveyard, Dr Byng or Prof Campbell) on the research team. Where necessary AE Reports will be produced and will be forwarded to the Data Monitoring and Ethics Committee (DMEC) or to the TSC if a DMEC is not considered necessary for the trial. This will include, reviews of the research protocol and any recommendations for changes to the safety monitoring procedures. In addition, any AEs which could possibly be related to the study will be followed up.
17 Data Management
17.1 Data Security and Confidentiality
Data management will follow study specific data management standard operating procedures, and will operate in accordance with the Data Protection Act (1998).
All computers holding study data will be password protected. Files containing study data and information will be accessible to only the study team, and all files containing personally identifiable data will also be password protected. Backup copies of the study data will be kept in a locked filing cabinet.
The data will be entered on to an Access database by the research staff, and will be later transferred to SPSS for analysis. The ACCESS database will be held on a secure internet server. The participants will be assigned a study number and will be identified on the database only by this number and will not be identified by name. Access to the database will be password protected and will be permitted only by the researchers, the PI and the trial statistician (Prof Rod Taylor). Only these individuals will have the information linking the study numbers to the participants’ names. The clinical record forms and a copy of the database on CD will be stored in a locked filing cabinet in the Department of Primary Care, PCMD, Plymouth. Only the researchers, the PI and the trial statistician will have access to these records.
17.2 Long Term Storage of Data
Data will be stored in the Principal Investigator’s office in the School of Sport and Health Sciences at the University of Exeter, St Lukes Campus, Magdalen Road, Exeter.
17.3 Data Analysis
Analysis will be undertaken to provide an estimate of the intervention (vs control) effect size and its precision based on continuous abstinence at 4 weeks post quit. Given the pilot nature of the trial, it is anticipated that hypothesis testing will not be undertaken on the primary outcome of interest. We will however, explore differences in other measures at the respective follow-up assessment, after determining if groups were comparable at baseline. For example, we will compare the groups on the number of quit attempts and readiness to quit 8 weeks post-baseline, number achieving at least a 50% reduction in cigarettes, and importance of, and confidence for, quitting. We will explore if FTND, perceived stress and strength of cravings and withdrawal symptoms at baseline predict outcomes, and whether amount of physical activity is associated with changes in outcomes.
The data analysis will be completed by the Principal Investigator (Prof Adrian Taylor) in the School of Sport and Health Sciences in The University of Exeter and by the Trial Statistician (Prof Rod Taylor) in the Clinical Trials Unit, PCMD, at The University of Exeter.
18 Cost effectiveness
The research will identify the key areas of resource use and costs (e.g. Health Trainer time, subsidised access to exercise, recruitment of those ‘hard to reach’) associated with the delivery of the intervention (and control, where appropriate), and will test/develop methods for the collection of data in a future trial (e.g. through use of ‘work-sampling’ methods, person-level recording of resource use via either routinely collected data, and/or self report methods). Identification of resource use, and methods for measurement of resource use, will include areas covered by NHS stop smoking services.
Any future economic evaluation alongside a RCT is expected to involve presentation of costs and benefits (e.g. in the form of a cost-consequences analysis), and will also involve modelling of longer term policy-relevant outcomes (i.e. impact on life-expectancy), from trial outcomes, to estimate cost per life-year and cost per QALY gained, and these requirements will set the context for pilot research proposed here. Research proposed here will include a general literature review of the data required for cost-effectiveness analyses, and evidence synthesis will be used to inform exploratory modelling of cost-effectiveness analyses (e.g. building on the work of Wang[2]), with simulation modelling and the related approach of ‘value of information analysis’[69] used where possible to investigate areas of uncertainty. It is anticipated that the pilot research will offer a good indication of the expected costs of delivering the intervention, but we acknowledge that any economic modelling will involve assumptions, and ‘what if’ analyses, in a number of areas (including measure of effectiveness). Therefore, research will assess both the issue of potential value in terms of commissioning future research (e.g. RCT)[70], and the issues of design (framework for CEA) for future research.
Current estimates are that it costs approximately £250 per smoker (for recruitment, and behavioural and pharmacological NHS support) who is still abstinent 4 weeks after quitting. Our cost effectiveness work will provide estimates of whether the intervention would cost more than this, and lead to discussion about the merits of proceeding to a full trial. Also, data from the SF36 may allow us to estimate the cost of improvements in quality of life.
19 Qualitative aspects
In the Table below we identify a framework for conducting the proposed qualitative work. After training, semi-structured interviews (focus groups and individual) led by the PM will be digitally recorded, transcribed and anonymised (with the consent of participants). Interview schedules (topic guides) will be developed with reference to existing literature on assessing the feasibility and acceptability of trial methods and /or behavioural interventions[71] and on developing behavioural interventions[72,73].
| Phase: (1) Pre-trial | ||
|---|---|---|
| Key issues | Who | Content/focus |
| Recruitment | Focus groups with professionals, community workers (and volunteers) and smokers. Individual interviews with HTs working outside Plymouth. | Defining ‘hard to reach’; how to identify and recruit them (in primary care and the community); snowball sampling (who, where, when). |
| Study design & methods | Focus groups with professionals, community workers (and volunteers) and smokers. | Assessments (adapting to sample); How best to conduct assessments in PA and control group (where, when, who), including self-report, accelerometers and CO monitoring. |
| Intervention development | Focus groups with professionals, community workers (and volunteers) and smokers from different ‘hard to reach’ groups. | General behavioural strategies used by smokers for smoking reduction. Selecting core behavioural change components (initial and progressive support), implicit (focus on just increasing PA) v explicit (focus on increasing PA as a coping behaviour) PA promotion, PA opportunities and support in community, links to NHS SSS, integrated post-quit support. Training HTs and treatment fidelity. |
| Intervention pre-pilot trial. | Recipients of the intervention and HTs. | Smokers: Acceptability of intervention. What worked and didn’t? Perceived value of intervention for cutting down (and quitting). Suggestions for additional support. HTs: Discussion of sample of video replays of sessions with smokers with PM and PI, with focus on fidelity (counselling style, content, motivational strategies). |
| Phase: (2) Pilot trial | ||
| Recruitment | During and after pilot RCT. HTs and others in a position to recruit (e.g. practice nurses, Stop Smoking Services). Decliners from different ‘hard to reach’ groups. | Feasibility and engagement: Which groups were harder to reach and why? What worked and didn’t to recruit the most resistant? How did primary care v community recruitment compare? |
| Intervention | HTs and smokers from different ‘hard to reach’ groups. Also, sample smokers who: were able to cut down but not quit; cut down then quit; didn’t cut down or quit; didn’t cut down but attempted to quit. | Acceptability of intervention. What worked and didn’t. Perceived value of intervention for cutting down (and quitting). Suggestions for additional support. |
For most qualitative issues, the data will be subject to thematic analysis using constant comparison techniques to extract concepts and themes[74] (and using NVivo to manage the data). The transcripts relating to smoker experiences of the intervention will also be analysed to produce individual narratives, allowing an increased insight into the processes of recruitment/engagement and the processes of supporting behaviour change[75]. These will inform modification of the recruitment techniques and the intervention/behaviour change process model to tailor the intervention better to the needs of hard-to-reach participants[76]. Second coding of a sample of the transcripts and discussion of the emerging coding framework, as well as techniques such as negative case-finding and hypothesis testing will be used to increase the depth of analysis and enhance the likely objectivity of interpretation[77]. Interviews will be conducted in community locations acceptable to those involved.
20 Quality control
A quality control plan will be implemented as follows:
20.1 Phase 1
Interviews will be digitally recorded and transcripts produced and stored securely as below. Any samples of quotes will be linked to the securely stored transcripts, using a securely stored coding system. Transcripts will be subject to thematic analysis and verified by the PI.
20.2 Phase 2
20.2.1 Sampling and Recruitment
20% of screening forms will be checked by the project manager. If it is found that a smoker has failed to meet the inclusion criteria and has still been recruited, the smoker will be immediately withdrawn from the study, but will continue to be offered smoking reduction support. If more than a 1% error rate is detected all the screening forms will be checked.
20.2.2 Intervention and Delivery
Audio recordings will be made of a sample of intervention sessions to check for treatment fidelity.
20.2.3 Data Collection
10% of all clinical record forms (CRFs) will be checked by the project manager. If any baseline data on demographics or smoking characteristics are missing the smokers will be contacted and the data will be entered.
20.2.4 Data Entry
10% of all data entered will be checked against the hard data (in the CRFs) by a second researcher. If the error rate is greater than 1% all the data will be checked against the hard data. 100% of the data for self-reports of continuous smoking abstinence at 4 weeks post-quit (or 16 weeks post-baseline) will be checked against the hard data.
20.2.5 Expired CO
Using the manufacturers’ recommendations, the performance of the CO measure for expired air will be regularly checked and calibrated accordingly.
21 Trial management and supervision
21.1 Trial Steering Committee (TSC)
A TSC will be established to provide overall supervision of the trial. This committee will include key personal and independent members who are experts on exercise or smoking cessation, as well as at least one smoker or ex-smoker who would fall into our categories of ‘hard to reach’ smokers. The steering committee will convene during preparation for the trial to contribute to and approve the final study protocol and every six months during the first year of the study and yearly thereafter, or as deemed necessary. The committee’s responsibilities will include monitoring: recruitment rates, adherence to the interventions and retention in the study, as well as monitoring research developments which may impact on the merit of the scientific output from the study.
21.2 Data monitoring and Ethics Committee
Given the lack of safety concerns and the lack of stopping rules for the trial, we do not anticipate a DMEC will be necessary but this will be to the discretion of the TSC.
21.3 Project management
Bi-weekly progress meetings will be held involving AT (PI), the PM, the 2 HT/researchers, and RA, in Plymouth. Quarterly meetings will be held, in addition to Phase 1 interviews/focus groups, involving the above plus RT, CG, & AR, as appropriate, plus representatives from Public Health network in Plymouth and local community groups (e.g. Devonport Regeneration Community Partnership). The expertise of other co applicants (see below) will be drawn upon at appropriate times during the project.
21.4 Project Manager (PM)
The PM will work in collaboration with the PI to finalise the protocol and assessments, manage ethical approval processes, approval to manage the day to day running of Phase 1 and 2, manage the HT/researchers, maintain the central database and coordinate management meetings. RA will provide local support for the PM and HT/researcher staff, and RB will also serve as a conduit for working with primary care in Plymouth. The PM, HTs/researchers, and will be housed in a PCMD office in Plymouth, but it is envisaged that the HTs will spend a considerable amount of time engaging with participants and other relevant personnel in and about the Devonport, Stonehouse and adjacent neighbourhoods.
22 Expertise
AT will lead the study as PI (0.2 FTE). He has been PI for 3 trials (including ‘Walk to Quit’, an RCT of a GP exercise referral scheme, and HEALTH – a COPD and exercise RCT), and co-applicant on 3 other funded RCTs involving PA promotion. He also has experience in conducting collaborative action research, and qualitative studies. Design and data analysis support will be provided by RT, in the PCMD’s Clinical Trials Unit (CTU), and has extensive experience in trial methodology and statistics, and works with AT & JC on 2 RCTs. CG is a Senior Lecturer in Health Economics and has expertise in cost-effectiveness analysis. JC has experience with both exercise and primary care trials, including TREAD (HTA 03/45/07), GETuP (RDA 02/06), HEALTH (IPCRG funded) all with AT. RJ has experience of conducting trials and is clinical lead for Plymouth Respiratory Care services. MU has experience as PI for 3 trials on exercise and smoking cessation, including an HTA trial, LEAP, currently underway with pregnant smokers. PNA & RW have experience with smoking cessation trials, and have worked with MU and AT on previous trials. PNA has co-authored a recent HTA review on Cut Down Then Quit trials. AR has extensive expertise in measuring PA with accelerometers. SM has extensive expertise in designing and evaluating complex behavioural interventions, particularly among the ‘hard to reach.’ On-going trials include the evaluation of promoting walking in primary care, and the effects of a bespoke smoking cessation intervention for people with severe mental illness. She is on Department of Health committees for developing and evaluation of the Health Trainer professional, and advising on complex behavioural interventions. We do not envisage any difficulty in recruiting suitable personnel for the roles of project manager and Health Trainer/researcher. RA has worked closely in and with the neighbourhoods where the study will take place in his capacity as part-time GP, Medical Advisor for Devonport Community Regeneration Project and as lead for Plymouth Teaching PCT for Health Inequalities. RB is a clinical academic with a specialism in mental health and is based in Primary Care, PCMD, Plymouth.
23 Dissemination
The results will be published in peer review journals and presented to at relevant conferences, nationally and internationally. The production of a manual for the physical activity intervention will enable us to provide specific guidance on the training that would be needed and the nature of the intervention for a larger scale trial. Findings on acceptability and feasibility of the trial procedures will inform the design of a future larger scale trial. The findings will also be disseminated locally (via workshops and conferences) among health professionals and those working for community agencies, in a professional and voluntary capacity. An HTA report will also be produced, and published within the HTA series of peer review publications.
24 Costing
TOTAL (£)
STAFF COSTS 231,559
NON-STAFF COSTS
Travel/Subsistence 14,540
Equipment 5,660
Consumables 13,100
Consultancy 4,000
Indirect Costs 251,257
RESEARCH GRANT TOTAL (80%) 416,093
NHS Costs 19,443
RESEARCH GRANT INC. NHS COSTS £435,536
25 References
- Ussher M.H., Taylor A., Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev 2008.
- Wang D., . ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technol Assess 2008;12:iii-iv.
- Whiteley J.A., . Commit to Quit in the YMCAs: translating an evidence-based quit smoking program for women into a community setting. Nicotine Tob Res 2007;9:1227-35.
- McEwan A.H.P., McRobbie H., West R. Manual of Smoking Cessation: A guide for counsellors and practitioners. Oxford: Blackwell Publishing; 2006.
- Hughes J.R., Keely J., Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004;99:29-38.
- Ferguson J., . The English smoking treatment services: one-year outcomes. Addiction 2005;100:59-6.
- Bauld L., Judge K., Platt S. Assessing the impact of smoking cessation services on reducing health inequalities in England: observational study. Tob Control 2007;16:400-4.
- West R. Smoking Toolkit Study 2008. URL: http://www.smokinginengland.info/.
- Pound E., . Targeting smokers in priority groups: the influence of government targets and policy statements. Addiction 2005;100:28-35.
- Michie S., Rumsey N., Fussell S., Hardeman W., Johnston M., Newman S., et al. Improving Health - Changing Behaviour: NHS Health Trainer Handbook. London: Department of Health and British Psychological Society; 2008.
- Michie S., Jochelson K., Markham W. A., Bridle C. Low Income Groups and Behaviour Change Interventions: An Analysis of Techniques in Effective and Ineffective Interventions 2008.
- Shiffman S., . Smokers’ interest in using nicotine replacement to aid smoking reduction. Nicotine Tob Res 2007;9:1177-82.
- Taylor A.H., Ussher M.H., Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction 2007;102:534-43.
- Taylor A., Katomeri M. Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine Tob Res 2007;9:1183-90.
- Reeser K.A. The Effects of Repeated Aerobic and Non-Aerobic Exercise on Cigarette Smoking 1983.
- Thayer R., Peters D., Takahaski P., Birkhead-Flight A. Mod and behaviour (smoking and sugar snacking) following moderate exercise: A partial test of self-regulation theory. Pers Ind Diff 1993:97-104.
- Katomeri M., Taylor A., Hoopeler H., . Abstracts Eur Coll of Sp Sci Conf. Lausanne, Switzerland; 2006.
- Pisinger C., Jorgensen T. Waist circumference and weight following smoking cessation in a general population: the Inter99 study. Prev Med 2007;44:290-5.
- Filozof C., Fernandez Pinilla M.C., Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev 2004;5:95-103.
- Meyers A.W., . Are weight concerns predictive of smoking cessation? A prospective analysis. J Consult Clin Psychol 1997;65:448-52.
- Parsons A.C., . Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev 2009.
- Marcus B.H., . The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Arch Intern Med 1999;159:1229-34.
- Blundell J.E, King N.A. Exercise, appetite control, and energy balance. Nutrition 2000;16:519-22.
- Taylor A.H., Bouchard C., Katzmarzyk. Advances in Physical Activity and Obesity. Champaign, IL: Human Kinetics; n.d.
- Kawachi I., . Can physical activity minimize weight gain in women after smoking cessation?. Am J Public Health 1996;86:999-1004.
- Martin J.E., . Prospective evaluation of three smoking interventions in 205 recovering alcoholics: one-year results of Project SCRAP-Tobacco. J Consult Clin Psychol 1997;65:190-4.
- Marcus B.H., . The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine Tob Res 2005;7:871-80.
- Ussher M., . Efficacy of exercise counselling as an aid for smoking cessation: a randomized controlled trial. Addiction 2003;98:523-32.
- Ussher M., . Randomized controlled trial of physical activity counseling as an aid to smoking cessation: 12 month follow-up. Addict Behav 2007;32:3060-4.
- Hill J.S. Effect of a program of aerobic exercise on the smoking behaviour of a group of adult volunteers. Can J Public Health 1985;76:183-6.
- King T.K., . Cognitive-behavioral mediators of changing multiple behaviors: smoking and a sedentary lifestyle. Prev Med 1996;25:684-91.
- Russell P.O., . The effects of physical activity as maintenance for smoking cessation. Addict Behav 1988;13:215-8.
- Emmons K.M., . Mechanisms in multiple risk factor interventions: smoking, physical activity, and dietary fat intake among manufacturing workers. Working Well Research Group. Prev Med 1994;23:481-9.
- Patten C.A., . Behavioral treatment for smokers with a history of alcoholism: predictors of successful outcome. J Consult Clin Psychol 2001;69:796-801.
- Bock B.C., . Exercise effects on withdrawal and mood among women attempting smoking cessation. Addict Behav 1999;24:399-410.
- Grove J.R, . Effects of exercise on selected correlates of smoking withdrawal. Int J of Sp Psych 1993;24:481-9.
- Everson E.S., Taylor A. H., Ussher M. Determinants of physical activity promotion by smoking cessation advisors as an aid for quitting: Support for the Transtheoretical Model. Patient Education and Counselling n.d.
- Hyman D.J., . Simultaneous vs sequential counseling for multiple behavior change. Arch Intern Med 2007;167:1152-8.
- Taylor A.H. Evaluating GP Exercise Referral Schemes: Findings from an RCT 1996.
- Taylor A.H., Doust J., Webborn N. Randomised controlled trial to examine the effects of a GP exercise referral programme in Hailsham, East Sussex, on modifiable coronary heart disease risk factors. J Epidemiol Community Health 1998;52:595-601.
- Hardcastle S., . A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns 2008;70:31-9.
- Faulkner G., . The acceptability of physical activity programming within a smoking cessation service for individuals with severe mental illness. Patient Educ Couns 2007;66:123-6.
- Patten C.A., . Effect of three smoking cessation treatments on nicotine withdrawal in 141 abstinent alcoholic smokers. Addict Behav 2000;25:301-6.
- Patten C.A., . Exercise interventions for smokers with a history of alcoholism: exercise adherence rates and effect of depression on adherence. Addict Behav 2003;28:657-67.
- Everson E.S., Taylor A. H., Ussher M. Readiness to use physical activity as a smoking cessation aid: A multiple behaviour change application of the Transtheoretical Model among quitters attending Stop Smoking Clinics. Patient Educ Couns n.d.
- Prochaska J.O., DiClemente C.C. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390-5.
- Ussher M., . Acute effect of isometric exercise on desire to smoke and tobacco withdrawal symptoms. Hum Psychopharmacol 2006;21:39-46.
- Al-Chalabi L., . A pilot randomised controlled trial of the feasibility of using body scan and isometric exercises for reducing urge to smoke in a smoking cessation clinic. BMC Public Health 2008;8.
- Deruiter W.K., . Characteristics of physically active smokers and implications for harm reduction. Am J Public Health 2008;98:925-31.
- Aveyard P., . Assessing the outcomes of prolonged cessation-induction and aid-to-cessation trials: floating prolonged abstinence. Nicotine Tob Res 2009;11:475-80.
- West R., . Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100.
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385-96.
- West R., Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology (Berl) 2004;177:195-9.
- West R.J., Hajek P., Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol Med 1989;19:981-5.
- Cappelleri J.C., . Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav 2007;32:912-23.
- Entilla E., Wright G. University of Oxford; 2004.
- Wilkinson D., Sniehotta F., Michie S. Do Health Trainers reach disadvantaged groups? Baseline data from the first pilot NHS Health Trainer Scheme in England n.d.
- Hillsdon M., Foster C., Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev 2005.
- Lussier J.P., . A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006;101:192-203.
- Heil S.H., . Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction 2008;103:1009-18.
- Vansteenkiste M, Sheldon K.M. There’s nothing more practical than a good theory: integrating motivational interviewing and self-determination theory. Br J Clin Psychol 2006;45:63-82.
- Ekkekakis P., . Walking in (affective) circles: can short walks enhance affect?. J Behav Med 2000;23:245-75.
- Everson E.S., Daley A.J., Ussher M. Moderate and vigorous intensity exercise acutely reduce cravings and withdrawal symptoms in abstaining young adult smokers. Mental Health &Amp; Physical Activity 2008;1:26-31.
- Scerbo F., Faulkner G., Taylor A. H., Thomas S. The impact acute vigorous physical activity on cravings and salivary cortisol concentration among abstinent smokers experiencing withdrawal. J of Sports Science n.d.
- deRuiter W, Faulkner G. Tobacco harm reduction strategies: the case for physical activity. Nicotine Tob Res 2006;8:157-68.
- Kaczynski A.T., . Smoking and physical activity: a systematic review. Am J Health Behav 2008;32:93-110.
- US Surgeon General’s Report: Treating Tobacco Use and Dependence n.d.
- Pisinger C., Jorgensen T. Weight concerns and smoking in a general population: the Inter99 study. Prev Med 2007;44:283-9.
- Claxton K.P., Sculpher M.J. Using value of information analysis to prioritise health research: some lessons from recent UK experience. Pharmacoeconomics 2006;24:1055-68.
- Eldridge S., . Why modelling a complex intervention is an important precursor to trial design: lessons from studying an intervention to reduce falls-related injuries in older people. J Health Serv Res Policy 2005;10:133-42.
- Donovan J., . Prostate Testing for Cancer and Treatment (ProtecT) feasibility study. Health Technol Assess 2003;7:1-88.
- Bartholomew L.K., Parcel G. S., Kok G., Gottlieb N. Intervention Mapping: a Process for Designing Theory – and Evidence-Based Health Education Programs. Mountain View: Mayfield; 2001.
- Hardeman W., . A causal modelling approach to the development of theory-based behaviour change programmes for trial evaluation. Health Educ Res 2005;20:676-87.
- Bryman J. Social Research Methods. Oxford: Oxford University Press; 2001.
- Smith J.A., Jarman M., Osborn M., Murray M., Chamberlain K. Qualitative Health Psychology: Theories and Methods. Sage: London; 1999.
- Wormald H., . Participants’ perceptions of a lifestyle approach to promoting physical activity: targeting deprived communities in Kingston-upon-Hull. BMC Public Health 2006;6.
- Murphy E., . Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess 1998;2:iii-ix.
26 Minor amendments approved by NHS Ethics (REC)
| Final Schedule of assessments (using Case Report Forms) | 4/5/2011 |
| Final Baseline Case Report Form | 4/5/2011 |
| Letter of invitation to participant (from GP surgeries) | 4/5/2011 |
| Participant Information Sheet (revised into folded format) | 4/5/2011 |
| Participant reply sheet | 4/5/2011 |
| Follow-up phone call script | 21/7/2011 |
| Phone answer phone script | 21/7/2011 |
| Revised sample size: reduced from 120 to 100 | 24/4/2012 |
27 List of appendices
I EARS Consort Diagram
II Measures to be Taken and Schedule of Assessments
III GANTT Chart
IV Recruitment Flow Chart
V Draft Recruitment Poster
VI Phase 1: Letter of Invitation to Participant
VII Phase 1: Participant Information Sheet
VIII Phase 1: Informed Consent
IX Phase 2: Letter of Invitation to Participant from GP
X Phase 2: Participant Information Sheet
XI Phase 2: Informed Consent
Appendix I: EARS CONSORT diagram
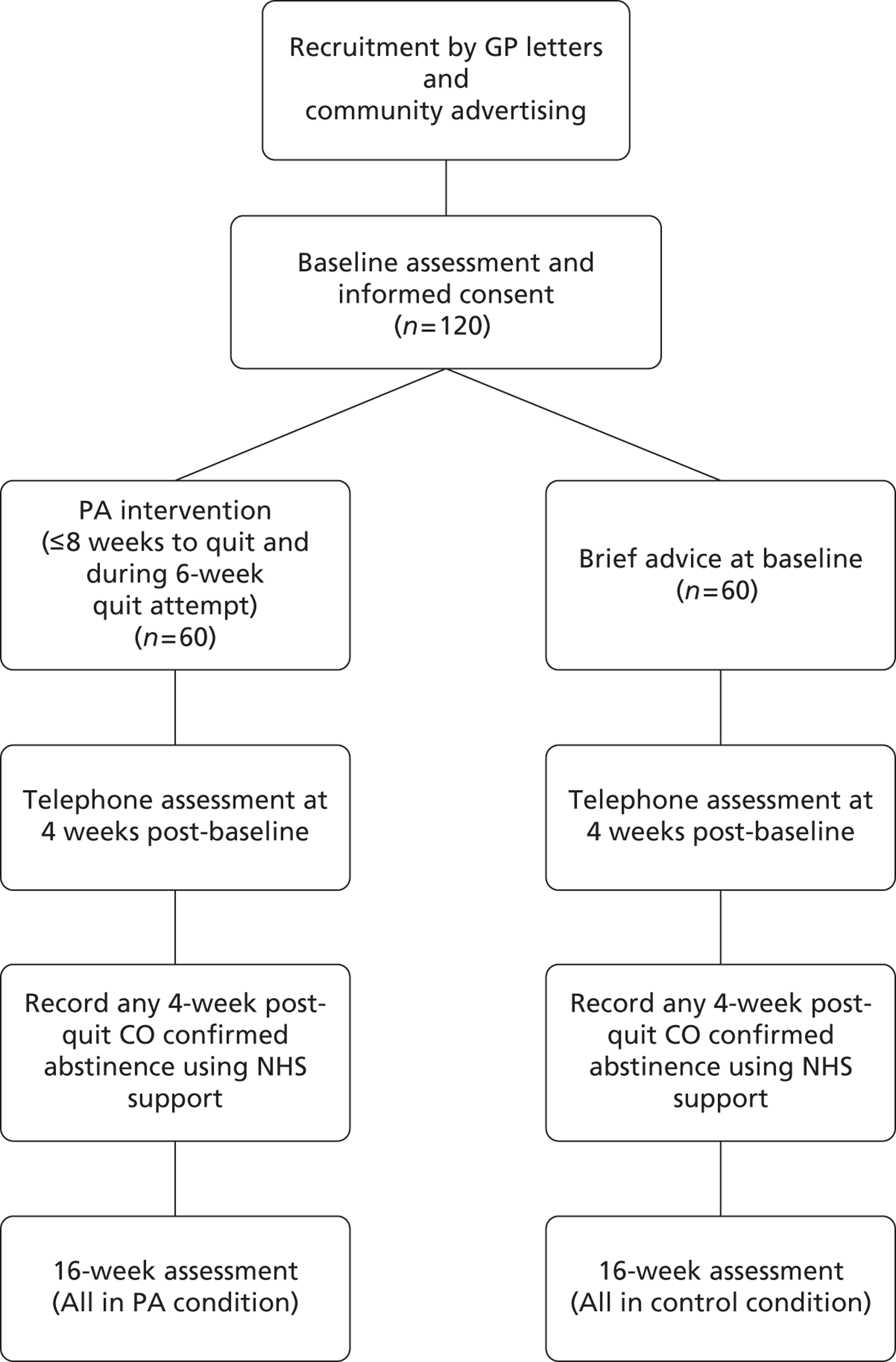
Appendix II: Measures to be taken and schedule of assessments
| Measure | Wk 1 | Week 2-3 in PA arm | Phone call measures at 4 weeks post-baseline | Week 5-7 in PA arm | At start of quit attempt or 8 wks post-baseline | Weekly, 6 weeks during quit attempt (with NHS support) | Week 16 post-baseline (or at 4 wks post-quit if same) |
|---|---|---|---|---|---|---|---|
| Demographics | ✗ | ||||||
| Smoking history | ✗ | ||||||
| Self-reported quit attempts in past 8 months lasting ≥ 24 hrs | ✗ | ||||||
| Self-reported cigarettes (pipes, cigars) smoked | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Prolonged abstinence | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Readiness to quit smoking | ✗ | ✗ | ✗ | ✗ | |||
| Expired CO | ✗ | ✗ | ✗ | ✗ (primary) | |||
| FTND | ✗ | ✗ | ✗ | ||||
| Urge to smoke (2 items) + other MPSS items | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| PSS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Confidence to quit, cut down & importance of quitting | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| mCEQ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Self-reported physical activity (PA) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Pedometer step counts (diary) | ✗ | ✗ | ✗ | ||||
| Accelerometer (1 week) | ✗ | ✗ | ✗ | ||||
| Weight & height (BMI) | ✗ | ✗ | ✗ | ||||
| Alcohol consumption | ✗ | ✗ | ✗ | ||||
| SF36/EQ5-D | ✗ | ✗ | ✗ | ||||
| Self-reported use of NRT products or smoking-related aids | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Adverse or serious adverse events | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
Appendix III: GANTT chart – Project milestones and timetable
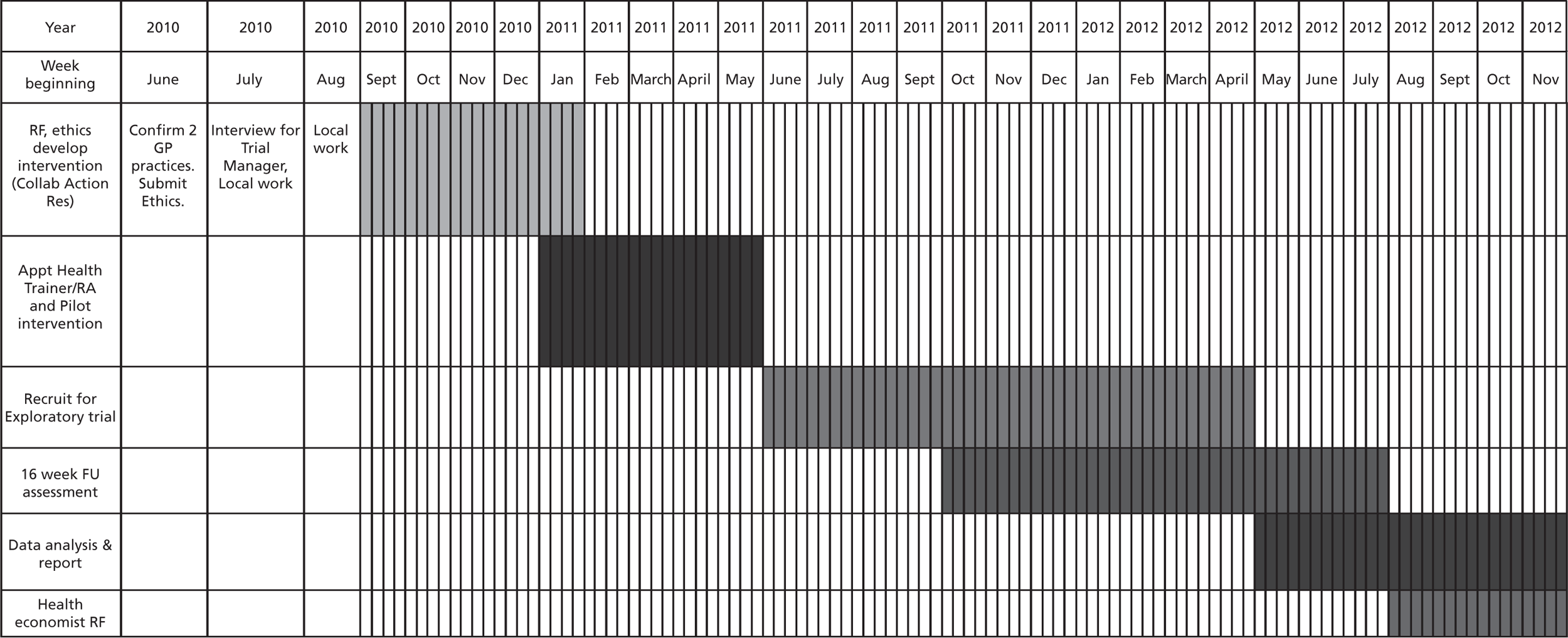
Appendix IV: Recruitment flowchart
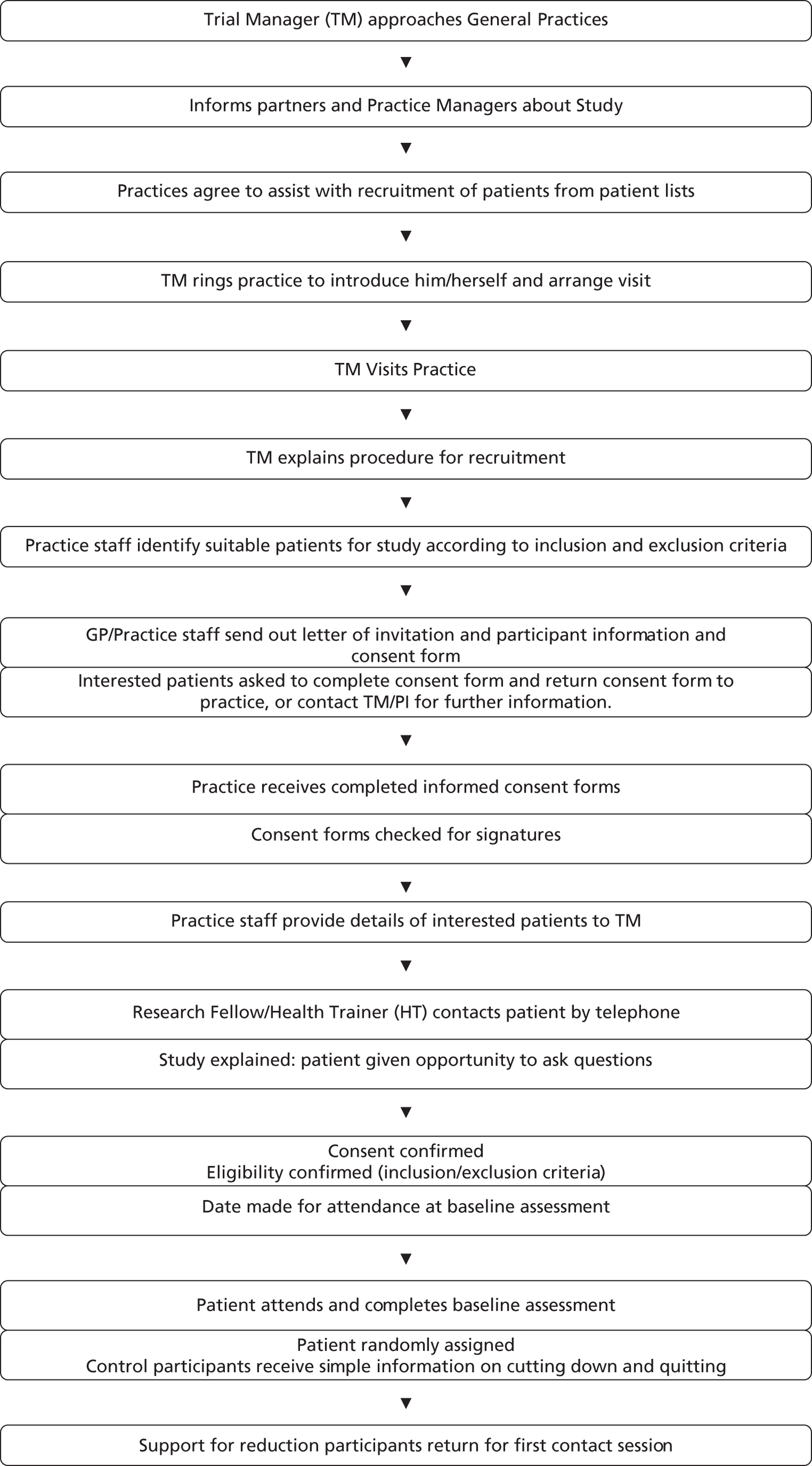
Appendix V: Draft recruitment poster
A study of the effects of local support for smokers who wish to cut down but not quit.
Are you a moderate or heavy smoker?
Do you wish to cut down but not make an abrupt quit attempt?
Many smokers want to cut down the number of cigarettes they smoke but few are ready to make an abrupt quit attempt. Little is known about the best way to cut down, but those who do try often don’t succeed. Some smokers have given us some ideas and we want to see if they work.
So who are we?
We are researchers at the Peninsula College of Medicine and Dentistry and funded by the government.
Is this a genuine study?
We have NHS ethical approval and will follow the strictest guidelines on conducting research with the general public.
What’s in it for you?
Cutting down (and quitting) can really help your health.
We may be able to help you cut down by increasing physical activity with free pedometers and access to exercise facilities if you want.
Where can I found out more about taking part?
Please read the enclosed Participant Information Sheet before you decide whether or not you are interested in taking part. If you would like more information, please contact the study team on one of the following telephone numbers or by e-mail:
Appendix VI: Phase 1: Invitation to participant
Date
Ref: A study of the effects of local support for smokers who wish to cut down but not quit.
I am writing to ask if you would be interested in taking part in a research study run by researchers in the Peninsula College of Medicine and Dentistry and the University of Exeter, here in Plymouth. This study is funded by the National Institute of Health Research.
The overall study involves two parts:
Phase 1, preliminary work to establish how best to conduct the research.
Phase 2, a larger trial to determine the effects of local support for smokers who wish to cut down but not quit
In this Phase 1, we are interested in your views concerning how moderate to heavy smokers who wish to cut down but not make an abrupt quit attempt (in the next 4 weeks) can be recruited into a study and would engage in a local intervention to provide support to cut down.
It is important that we capture the views of a range of people, including smokers, professionals who help people to quit and others who work alongside smokers. The best way to do this is to conduct individual and group-based interviews.
Many smokers want to cut down the number of cigarettes they smoke but few are ready to make an abrupt quit attempt. However, little is known about the best way to cut down, but those who do try often don’t succeed. Some smokers and people who help people to quit have already given us some ideas and we want to explore them a bit more before finally offering this as part of a larger randomised trial involving 120 smokers, 60 of whom will be offered the support.
Participation in the study is entirely voluntary, and if you decide not to participate this will not affect your treatment in any way. Any information that you provide will be treated with confidentiality and will not be disclosed to anyone without your prior permission. Any published information will be fully anonymised.
Please read the enclosed Participant Information Sheet before you decide whether or not you are interested in taking part. If you would like more information, please contact the study team on one of the following telephone numbers or by e-mail.
Project Manager:
Tom Thompson
Peninsula College of Medicine and Dentistry:
Telephone number: 01752 437 300
E-mail: T.P.Thompson@exeter.ac.uk
Or:
The Principal Investigator:
Professor: Adrian Taylor:
School of Sports and Health Sciences (University of Exeter):
Telephone number: 01392-264747.
E-mail: A.H.Taylor@ex.ac.uk
Thank you,
Yours faithfully <GP Name>
Appendix VII: Phase 1: Participant information sheet
A study of the effects of local support for smokers who wish to cut down but not quit.
Before you decide whether or not to take part it is important for you to understand why the research is being done and what it involves. Please read the following information carefully, and take time to decide. Please ask if you would like more information.
What is the purpose of this study?
In this Phase 1, we are interested in your views concerning how moderate to heavy smokers who wish to cut down but not make an abrupt quit attempt (in the next 4 weeks) can be recruited into a study and would engage in a local intervention to provide support to cut down. It is important that we capture the views of a range of people, including smokers, professionals who help people to quit and others who work alongside smokers. The best way to do this is to conduct individual and group-based interviews.
What will happen if I want to take part?
You will be invited to attend 30-60 min individual or group-based interviews/discussion about how to recruit smokers into such as study, and about how best to design the intervention or support package to help smokers cut down, and possibly quit. This will take place in a convenient location. Any reasonable expenses incurred will be reimbursed.
What are the possible benefits of taking part?
As a smoker you may gain an insight into how best to cut down or quit smoking, as one of the best ways to improve your health. As a non-smoker you can help us to improve the services offered to smokers in helping them to reduce and/or quit.
Do I have to take part in this research?
No, it is entirely voluntary. If you do not, you will still be entitled to the same NHS support and treatment.
Will any information I provide be confidential?
Yes. Information relating to your participation will be kept confidential and will not be disclosed without your prior permission. Confidential information will be stored safely in a locked cabinet. The study will be written up in scientific journals in such a way that none of the people taking part can be identified.
Where can I get more information about this study?
By contacting:
Project Manager: Tom Thompson
Peninsula College of Medicine and Dentistry:
Telephone number: 01752 437 300
E-mail: T.P.Thompson@exeter.ac.uk
Or:
The Principal Investigator: Professor: Adrian Taylor:
School of Sports and Health Sciences (University of Exeter):
Telephone number: 01392-264747.
E-mail: A.H.Taylor@ex.ac.uk
Appendix VIII: Phase 1: Participant informed consent form
Appendix VIII: Phase 1: Participant informed consent form (PDF download)
Appendix IX: Phase 2: Letter of invitation to participant from GP
<GP address>
Date
Dear <Patient Name>
Ref: A study of the effects of local support for smokers who wish to cut down but not quit.
I am writing to ask if you would be interested in taking part in a research study run by the Peninsula College of Medicine and Dentistry and the School of Sports and Health Sciences at the University of Exeter. This study is funded by the National Institute of Health Research.
You are being invited to take part in this study because you are a moderate or heavy smoker according to our records held in your GP practice. We wish to recruit smokers who wish to cut down but not make an abrupt quit attempt (in the next 4 weeks). Please read on before deciding whether this is something you are interested in or not.
Many smokers want to cut down the number of cigarettes they smoke but few are ready to make an abrupt quit attempt. However, little is known about the best way to cut down, but those who do try often don’t succeed. Some smokers have given us some ideas and we want to try them out to see if they work.
We are interested in whether providing some simple support in your local community can make a difference to successfully helping smokers, who want to cut down but not quit, to reduce their smoking. That support will be for setting plans to reduce smoking and also increase physical activity which may help with withdrawal symptoms. We plan to remove as many barriers to becoming more physical activity as possible, as part of the support you may receive.
Participation in the study is entirely voluntary, and if you decide not to participate this will not affect your treatment in any way. Any information that you provide will be treated with confidentiality and will not be disclosed to anyone without your prior permission. Any published information will be fully anonymised.
Please read the enclosed Participant Information Sheet before you decide whether or not you are interested in taking part. If you would like more information, please contact the study team on one of the following telephone numbers or by e-mail.
Project Manager:
Tom Thompson
Peninsula College of Medicine and Dentistry:
Telephone number: 01752 437 300
E-mail: T.P.Thompson@exeter.ac.uk
Or:
The Principal Investigator:
Professor: Adrian Taylor:
Sports and Health Sciences (University of Exeter):
Telephone number: 01392-264747.
E-mail: A.H.Taylor@ex.ac.uk
Thank you,
Yours faithfully <GP Name>
Appendix X: Phase 2: Participant information sheet
A study of the effects of local support for smokers who wish to cut down but not quit
You are being invited to take part in a research study which is being funded by the National Institute of Health Research. Before you decide whether or not to take part it is important for you to understand why the research is being done and what it involves. Please read the following information carefully, and take time to decide. Please ask if you would like more information.
What is the purpose of this study?
Many smokers want to cut down the number of cigarettes they smoke but few are ready to make an abrupt quit attempt. Those that do, with NHS Stop Smoking Service support, are four times more likely to successfully quit. However, little is known about the best way to cut down, but those who do try often don’t succeed.
The purpose of this study is to investigate whether providing some simple support in your local community can make a difference to successfully helping smokers, who want to cut down but not quit in the next 4 weeks, to reduce their smoking. The effects of different approaches, supported by a local Health Trainer, will be compared with normal guidance. One hundred and twenty moderate to heavy smokers will take part, half will be allocated to receive support from a community health trainer and half will continue with normal care. You have been asked to take part because you are currently a moderate to heavy smoker, and you may wish to reduce your smoking but not quit just now.
What will happen if I want to take part?
You will be invited to attend an assessment to check your suitability to join the study, and complete some simple questions about your smoking and other behaviours that have been linked to health, including alcohol consumption and physical activity. A computer will be used to randomly (by chance) allocate various study numbers to receive either the support for reduction programme or normal care.
If you are allocated to the support for reduction programme, you will be able to receive three face to face sessions with a Health Trainer for up to 45 mins, in week 1, 4 and 8, plus 15 min phone calls in weeks 2, 3, 5, 6 and 7. The phone support will be extended for a further 6 weeks if you choose to quit. If you do choose to quit you will be given information on how to seek support to quit from the National Health Service (NHS) Stop Smoking Services.
The support from a Health Trainer will include help with deciding how best to reduce your smoking, from several alternatives from which you can choose, plus support to increase your physical activity or exercise. The latter may include setting targets, using a free pedometer, and having subsidised access to exercise facilities and opportunities.
If you are allocated to the normal care condition you will be offered advice on the benefits of cutting down and quitting and information on how to seek support to quit from the NHS Stop Smoking Services. At the end of the study (after 4 months) you will be given further material that may help you cut down or quit if you wish.
All participants will attend assessments at a convenient local place in weeks 8 and 16 weeks, and be asked similar questions on the phone in week 4, after the initial assessment. Each assessment session will last about 20-25 mins,
What are the possible benefits of taking part?
Reducing smoking and increasing physical activity are two of the most important things that you can do to improve your health and quality of life. The support you may receive could help you to do both. Less heavy smokers are more likely to have the confidence to quit smoking, and give up for good. The information you provide may help to design better support to help you and others to cut down in the future and possibly quitting. Cutting down does not offer the same protection for your future health as quitting.
Will taking part in this research affect my treatment?
No, you will be entitled to the same NHS support and treatment whether you take part in this study or not.
Are there any side effects?
As you may well have experienced, reducing or stopping smoking may cause some moderate or intense withdrawal symptoms. Failure to cope with these often leads to a return to previous smoking levels. The study aims to provide support with strategies to deal with these withdrawal symptoms. One approach is to use physical activity to reduce withdrawal symptoms, such as low mood and irritability. Moderate intensity physical activity will be recommended, which has few if any side effects. You will be given guidance on how best to avoid stiffness after exercise. You will be advised to check with your doctor if you wish to engage in vigorous exercise which may be associated with greater risk of health problems, particularly if you have existing poor health and have been inactive for some time.
What will happen if I do not want to take part in the study?
Taking part is entirely voluntary. If you do not wish to take part, this decision will not affect your medical care in any way. Even if you are recruited into the study you are free to withdraw at any time without explanation. However it would be helpful for the study team to know why you do not wish to continue. Your withdrawal will not affect the progress of the study.
Under what circumstances will the study be stopped?
If the Principal Investigator considers it necessary he will stop the study in the interest of the safety and well being of the participants.
Will any information I provide be confidential?
Yes. Information relating to your participation will be kept confidential and will not be disclosed without your prior permission. Confidential information will be stored safely in a locked cabinet. The study will be written up in scientific journals in such a way that none of the people taking part can be identified.
What should I do if something goes wrong during the study?
If you feel unusually unwell you should contact your GP in the first instance. If you have a medical emergency at any time you should ring 999 and request an ambulance.
Problems relating to your participation in the study should be reported to the person who conducts your initial assessment as part of the study. Details of how they can be contacted will be provided at the beginning of the study.
If you have received an invitation to join the study from your GP, does your GP receive any financial reward for your participation in the study?
A small payment will be paid to the practice in respect of the administrative costs involved in assisting with the study. This is the normal procedure for research with NHS patients.
Where can I get more information about this study?
By contacting:
Project Manager:
Tom Thompson
Peninsula College of Medicine and Dentistry:
Telephone number: 01752 437 300
E-mail: T.P.Thompson@exeter.ac.uk
Or:
The Principal Investigator:
Professor: Adrian Taylor:
School of Sports and Health Sciences (University of Exeter):
Telephone number: 01392-264747.
E-mail: A.H.Taylor@ex.ac.uk
Appendix XI: Phase 2: Participant informed consent form
Appendix XI: Phase 2: Participant informed consent form (PDF download)
Appendix 3 Recruitment materials
Community recruitment poster
Candidate identification process
GP recruitment method summary
Three stages of filtering were applied to identify candidates to be approached for recruitment to the study. Great care was taken to protect sensitive patient information.
-
An initial potential candidate list was generated using MIQUEST searches (filter #1).
-
MIQUEST (Morbidy Import QUEry SynTax) is a standard query engine that runs on the majority of GP IT systems. It can be used to run similar queries across a group of practices.
-
A set of MIQUEST queries were assembled and tested.
-
Identify all patients who may be potential members of the study group
-
Smokers
-
Aged 18-69 years
-
-
-
Identify all patients who are in potential exclusion groups.
-
Coronary heart disease
-
Stroke
-
Exercise intolerant/refused
-
Stopped smoking
-
NRT therapy
-
-
Merge a and b (set a NOT set b) to produce a list of candidates (group c) and exclude anyone clearly not eligible.
-
Generate a report on group c, including health data on mental health and cardiovascular disease that could be used to filter for eligibility.
-
-
The candidate list (group c) was pseudo-anonymised (personal information was removed and replaced with a coded identifier) and this coded list was supplied to the research team.
-
The research team scrutinised the coded list to select potential candidates based on health data (filter #2). Selected coded patient ID’s were passed back.
-
The research analyst decoded the anonymous ID list supplied by the research team and passed it back to the practice as a set of names and addresses.
-
The practice scrutinised the list to remove any further names based on specific knowledge of the patients (filter #3).
-
The practice wrote to patients to invite them to participate in the study.
-
All data was destroyed as soon as it was no longer required.
Since the list of names and addresses of selected candidates contained no data that could be linked to the initial data set (health data and coded identifiers only), no identifiable health information about individuals was disclosed to the research team. The only definite knowledge the research team had on individuals was that they met the eligibility criteria for the study.
*QRY_WDATE,20110428,28/04/2011
*QRY_SDATE,20110428,28/04/2011
*QRY_TITLE,EARS,SUBSET : All eligible smokers
*QRY_ORDER,001
*QRY_MEDIA,D,Disk
*QRY_AGREE,LOCAL,
*ENQ_IDENT,LOCAL,
*QRY_SETID,RA5B,RA 5 BYTE
*ENQ_RSPID,L83624,Marlborough Street Surgery
*QRY_CODES,0,9999R2,Read version 2
DEFINE AGE AS @YEARS("28/04/2011",DATE_OF_BIRTH)
# Select all eligible smokers at practice
SUBSET EARS1
FROM JOURNALS (LATEST FOR PATIENT)
WHERE CODE IN ("1374","1375","1376")
AND AGE IN ("18"-"69")
*QRY_WDATE,20110428,28/04/2011
*QRY_SDATE,20110428,28/04/2011
*QRY_TITLE,EARS,SUBSET : Definite exclusions
*QRY_ORDER,002
*QRY_MEDIA,D,Disk
*QRY_AGREE,LOCAL,
*ENQ_IDENT,LOCAL,
*QRY_SETID,RA5B,RA 5 BYTE
*ENQ_RSPID,L83624,Marlborough Street Surgery
*QRY_CODES,0,9999R2,Read version 2
DEFINE AGE AS @YEARS("11/10/2006",DATE_OF_BIRTH)
# Select all definite exclusions at practice
SUBSET EARS2
FROM JOURNALS (ONE FOR PATIENT)
WHERE CODE IN ("137J","137K","137L","8B2B","8B3Y","8B3f","8I3S","33BE","G3%","G6%","Gyu%")
AND AGE IN ("18"-"69")
*QRY_WDATE,20110428,28/04/2011
*QRY_SDATE,20110428,28/04/2011
*QRY_TITLE,EARS,SUBSET : Potentialy eligible
*QRY_ORDER,003
*QRY_MEDIA,D,Disk
*QRY_AGREE,LOCAL,
*ENQ_IDENT,LOCAL,
*QRY_SETID,RA5B,RA 5 BYTE
*ENQ_RSPID,L83624,Marlborough Street Surgery
*QRY_CODES,0,9999R2,Read version 2
# Select all potentially eligible patients
FOR EARS1 NOT EARS2
SUBSET EARS3
FROM PATIENTs
*QRY_WDATE,20110428,28/04/2011
*QRY_SDATE,20110428,28/04/2011
*QRY_TITLE,EARS,REPORT : Eligibility Reference
*QRY_ORDER,004
*QRY_MEDIA,D,Disk
*QRY_AGREE,LOCAL,
*ENQ_IDENT,LOCAL,
*QRY_SETID,RA5B,RA 5 BYTE
*ENQ_RSPID,L83624,Marlborough Street Surgery
*QRY_CODES,0,9999R2,Read version 2
# Report all potentially eligible patients
DEFINE AGE AS @YEARS("28/04/2011",DATE_OF_BIRTH)
FOR EARS3
REPORT
# Patient summary data
PRINT SURNAME,FORENAME,SEX,AGE,ADDRESS_1,ADDRESS_2,ADDRESS_3,ADDRESS_4,POSTCODE,
FROM PATIENTS
# Smoking Data
PRINT CODE,RUBRIC,DATE,VALUE1
FROM JOURNALS (LATEST FOR PATIENT)
WHERE CODE IN("137%")
# Potentially relevant exclusion data
PRINT CODE,RUBRIC,DATE
FROM JOURNALS (ALL FOR PATIENT)
WHERE CODE IN("G%","E%","H%","9h%")
Appendix 4 Participant information sheets
GP leaflet
Community leaflet
Intervention leaflet
Appendix 5 Feedback from the health trainers
Feedback on training procedures in the EARS study
The table below shows the training plan for HTs. A selection of the interviews with HTs early and later during intervention delivery is shown after the table.
| Session (AM = 9–12) (PM = 1–4) | Topic | Aims | Content |
|---|---|---|---|
| AM | EARS COMPETENCIES | Introduce advanced behaviour change skills and logistics | Intro to EARS Manual. Participant progression and keeping in touch |
| PM | Build advanced PA behaviour change skills | Multifaceted role for PA (counselling, support and signposting). Using pedometers and MP3 players | |
| AM | Build advanced PA behaviour change skills | Building three Cs for PA (competence, control and companionship) | |
| PM | Smoking reduction with exercise | Smoking reduction and physical activity: targeting implicit and explicit processes | |
| AM | Build advanced Smoking reduction skills | Smoking-reduction strategies | |
| PM | SMOKING CESSATION | Identifying professional support for quitting. Smoking data collection | Referral to NHS SSS or other options. CO, self-report. Participant (subsidising exercise, travel, etc.) |
| AM | Other data collection | Video role-play | |
| PM | Review counselling skills | Review role-plays |
The training aimed to check the HT’s existing skills and knowledge and build on these to allow them:
-
to deliver the research process including recruitment, baseline assessment and outcomes measurement;
-
to deliver the EARS intervention.
Key: HT1 = health trainer 1; HT2 = health trainer 2; HT3 = health trainer 3; CG = interviewer. ((. . .)) = transcriber’s notes; ((. . . )) = summary.
Training and partnership working was generally good:
. . . I think for, for me being involved in the writing of stuff you know, and the set up right at the beginning, very beginning, it just means that we have a, we’re involved from the very beginning and we feel part of the project.
However, it seemed unstructured:
. . . One minute we’re trying to do things to help develop the project and then another minute we’re having sort of training. It just felt like, perhaps it just felt a bit too messy for me . . .
. . . I think the training was a bit ‘bitty’, it was a little bit all over the place. I mean the practice participants were good, that was good. But I think it was just, it’s actually it’s doing it and then sort of, because you do come across different scenarios and different barriers and what have you, and sort of knowing how to deal with those or the best way to sort of deal with those really . . .
What worked well?
Practice sessions, reflecting on practice (recorded consultations) with a health psychologist, the intervention manual:
. . . I enjoyed those [practice sessions], I thought they were useful and I thought it was good to have those . . .
. . . The sessions with Colin [Greaves] were quite good as well, particularly the talking about using physical activity to deal with cravings, you know, that side of it . . .
Need for more interactive practice:
. . . I think we should have done it on the third day and on the fourth day. Yes, there should have been more of it throughout the thing. We could have done it with each other as well as the practice participants, but it felt there wasn’t enough emphasis on that side of it . . .
. . . I wonder if it could have been a bit more interactive, because I can remember some of those days we were sat, the five of us in a room for a whole day together. It could feel very dry and heavy . . . Set us a task, like, ‘Go off, read it and you’ve each got to come back, present a bit about the things.’ Something like that . . .
However, in contrast, one HT would have preferred a more tutorial style and less interactive practice:
. . . No, I did find those quite useful. Would I have used so many, for as long? I’m not sure . . .
Need more on techniques beyond getting motivated:
What didn’t work well?
Early recording was challenging/would be better post learning:
Gap between end of training and starting with clients:
. . . And then I sort of came in and we started, so that was okayOK. But I guess, I just think we just all wanting to get started at that point really. It just seemed to be quite a long time before we actually started getting participants in . . .
Practice effects
At the early stages, some of the HTs were struggling with the new concepts introduced:
. . . I mean for me, it’s a very, very different way of delivery from what I’m used to because of course NHS courses are taught a different way of delivery.
. . . You know, the more you do it, the easier it gets.
However, confidence built up through practice:
. . . Yeah, yeah, I definitely think I improved over the time. Yeah.
Other training ideas:
. . . Just put the cogs right in the correct order . . .
. . . Definitely include more in the motivational stuff. Yeah, and give backgrounds of, you know, why it’s done, and what you can gain from doing it that way or rather than from doing it that way. ((explaining purpose/contrasting communication styles))
Feedback on recruitment procedures in the EARS study
Key: HT1 = health trainer 1; HT2 = health trainer 2; HT3 = health trainer 3; CG = interviewer. ((. . .)) = transcriber’s notes; ((. . . )) = summary.
Recruitment strategies
Recruiting through GPs was relatively easy:
. . . I’ve got a very good relationship with them and they are very open . . .
. . . Yeah, it’s been smooth running, you know I’ve been able to phone them up and say, ‘I need this consent,’ you know, ‘Can I have a room for the interview?’ . . .
However, there were some challenges: identifying the right contact and some problems with checking exclusion criteria:
Recruiting in the community was much harder:
. . . And then the community recruitment, you know, it seemed like we put a lot of effort into going to groups, standing outside the job centre, giving our flyers and things to various people, engaging with community leaders, for very, very little result; well, I had two people that came through that route . . . so most of our community recruitment has come through the Stop Smoking Service . . .
. . . There was a lot of effort. Yeah, it was an awful lot of effort, going along to Mother-Toddlers groups and, actually I think that we were eleven hours there and actually we didn’t get one . . .
But engaging key contacts was helpful:
It may also take more time to build relationships with the community
. . . Because I feel like we, I mean we could have started trying to recruit from the community much earlier, you know, I think that if we were serious about actually trying to hit a target from the community in terms of the research project we should have started trying to do it earlier. Because if you look at the GP surgery, say for every hundred letters we sent out we had about four responses. In terms of the community I think the returns were much lower, so you had to work that much harder . . .
What attracts participants?
Reasons for non-engagement: Already quit; other priorities; illness.
Reasons for dropout
Making contact by phone: generally this was comfortable to do, but with mixed views from different HTs. We need to avoid hassling people.
Most people are very polite, um, they’ll either just say, ‘No thanks, I’m not interested,’ or they’ll give me a bit of their life history and then say, ‘No thanks,’ ((laughs)) or, um, but I haven’t had anybody being rude really . . .
. . . I kind of feel like after I have called them or left a message and rung them again, I kind of feel that, ‘Oh, do I have to ring them again?’
And it is time-consuming:
Control group allocation often led to disappointment:
. . . I know a lot of them were disappointed. They were disappointed that they were in the control group. They’d come into the study thinking they were going to get this help and ((silence)) . . .
Other ideas on recruitment:
-
Could offer them intervention at the end of the study, possibly in groups.
-
Need a strategy for what to say if someone else answers the phone:
The most uncomfortable thing is when somebody else answers the phone and then you think, God, they’ll want to know who I am and I say I’m ringing from the GP surgery and I want to say, there is nothing to worry about, it’s not important. That kind of gets a bit of an uncomfortable thing.
HT1
-
Talking about PA during recruitment stage might be counter-productive as it ‘sort of confuses people’ (HT2).
-
Possible ‘near-patient’ delivery:
Yeah. I think that could work more, and you might have one or two people who think, ‘Yeah, we’re happy to talk to the smoking lady.’ You know, “If we can leave Johnny singing ‘Wheels on the Bus’ and we can escape for five minutes to talk about our smoking, that might be quite nice.
HT1
The interviewer asked about problems with access or non-attendance or workload (for participants after being enrolled), but no major problems were reported.
Feedback on intervention delivery procedures in the EARS study
Key: HT1 = health trainer 1; HT2 = health trainer 2; HT3 = health trainer 3; CG = interviewer. ((. . .)) = transcriber’s notes; ((. . .)) = summary.
Intervention techniques/delivery issues
The health trainers reported no problems in using most of the intended intervention techniques, including exploring a typical day; encouraging self-monitoring (which was seen as particularly useful); problem-solving; empathy-building/person-centred counselling; exploring importance (including pros and cons and using 1 to 10 scales); exploring confidence; using motivational interviewing techniques (including affirmation and reflective listening); reviewing progress; assessing existing smoking; offering alternative strategies for smoking reduction; setting realistic/SMART goals (usually verbally, or with the HT writing them down) and making coping plans.
What worked well? Regular contact, self-monitoring, MI techniques, pedometers, behavioural strategies for quitting.
What works well: self-monitoring; PA as a distraction (but not as a way to reduce cravings).
Introducing PA:
Making the link to PA through experiences:
Exploring the general health benefits of PA, enjoyment of PA and perception about what PA entails was also sometimes useful:
It can be quite hard to make the link to PA: some ‘got it’ but some found it more difficult: Need for individual tailoring on this/not pushing it too hard - not all are interested in increasing PA:
. . . But she [participant] does realise when she goes for a walk she doesn’t have that cigarette because she can’t walk and smoke at the same time.
. . . It’s that, you know, ‘I can go to the gym, have a really good workout, I go outside and the first thing I do is light up.
And for individual tailoring more generally:
. . . I mean everybody’s very, very different aren’t they? Sort of receptive to different things, and it’s trying to sort of gauge that, you can’t sort of just go by set criteria and just sort of like, ‘This is what you’re doing, this is cessation,’ and what have you.
. . . So I feel like I tried to be creative with the different problems that were presenting themselves with people . . .
. . . I didn’t feel constrained by it [the session plan], no. I think that it felt safe to have that as an agenda because you knew, ‘OK, I’m supposed to go in and do this,’ but it also . . . I suppose I felt I had the confidence to abandon if I didn’t feel it was working . . .
. . . I might think, ‘Oh,’ you know, ‘They talked about X, Y or Z, I’ll try and pick up on that next time,’ but it doesn’t always happen that way. Obviously the sessions are quite effected by the person and what’s, you know, going on for them . . .
Tailoring for the ‘hardest-to-reach’ sectors of the study population:
The link may be subconscious though:
There were mixed views about delivering the process measures alongside intervention interviews (NB: this was noted as being a potentially difficult process when reviewing the consultation recordings):
. . . In terms of exploring confidence to change, because it’s a question within the template, I’m focusing on getting, doing the questions rather than possible sticking with this question and really exploring confidence to change with them . . .
. . . I think there are problems with delivering the intervention which are because it’s part of a research project, which actually if you were just delivering the intervention wouldn’t kind of exist. Because there wouldn’t be the pressures that there are to recruit or to record information; there wouldn’t be perhaps that tension between the health trainer and the researcher role and things. So, if there was to be a bigger trial I think it would be important to have a different person doing the first session, and then somebody else who delivers the intervention . . .
Timescale: sometimes not enough/could use some flexibility:
. . . ((She said that weekly sessions good, but suggested spreading out the later ones to every two weeks to allow more time for learning from experience.)) I think it might be better if the support was for a longer period, maybe tailing it off but just for a longer period . . .
. . . So, I think . . . I think at the end of the eight weeks if we could go possibly every two weeks, every three weeks and carry on ‘til week sixteen, but not necessarily engage it, I think that might . . . it’s just having that extra support there . . .
. . . I think the only change that I would like to see is that we can use our own initiative and be more flexible with the sixteen weeks. Because, you know, we can see that quit, that reduction and possibly a quit attempt to be two, three weeks down the line, but we haven’t got that flexibility . . .
Implementing social support can be problematic as not everyone has any support, so it can be provocative:
Also not high on the agenda:
But was acknowledged as important in the end-of-intervention interview in one case:
Making action plans specific was sometimes difficult, although this was recognised as being important:
. . . I mean I think it’s helpful, yeah. The more specific people are. The more specific I’ve pushed them to be as well, I guess. You know, the more . . . ((pause)) Well, the more you know what’s going on . . .
Using problem-solving techniques versus solution-focused approach:
Managing expectations about the PA component at the start of the study:
Identity change:
HTs enjoyed the experience /being part of the study:
Some of the HTs struggled initially with the idea of exchanging information and had the idea that they weren’t allowed to offer information . . .
. . . but this was improved by the end of the intervention (following further supervision/feedback):
Other ideas for improvement
Training ideas:
-
How to rein people in if they go off track: ‘Maybe need to encourage participants to take a longer term to judging the benefits of PA. Not focusing on how they feel immediately afterwards – usually the first thing they want to do is have a cigarette! – maybe monitor both over a few weeks . . . ’ (HT2).
-
How to respond if the patient asks for advice or wants to be told what to do (HT2). We need to address the misconception that if you are using motivational interviewing you cannot offer (or exchange) information.
-
How to avoid or minimise dependence somehow: ‘I guess one problem that did, I think, occur is that some participants sort of become . . . I don’t know if they’re doing it because they’re trying to please you as well, that’s sort of a danger, and become reliant, in a way reliant on you . . . ’ (HT2).
-
How to deal with people who are already active: ‘I think all of us had some people that were like, especially if they have a sort of manual jobs, building and things like that, would consider themselves as being physically active’ (HT2). ‘So many people that we’ve recruited do physical jobs as well . . . that felt quite unsatisfactory really, all that whole physical activity side of it really . . . ’ (HT1).
-
How to deal with cannabis use as a related behaviour: ‘I think we did have a few problems when I think people were coming and they were actually smoking cannabis as well. That was an issue with a few participants . . . ’ (HT2).
-
How to deal with passive resistance/participants who are evasive: ‘I suppose the other difficult client I’ve had this last bit is one that’s just been very evasive and very vague, and she seems to be saying something different every time, and I don’t think I’ve been successful in working with her. I probably needed to challenge her more . . . There’s a big challenge in that sense, and also with the other guy. I sort of said to him, you know, ‘This is week four, how have you found it, what have you got out of it?’ and got him to reflect on it and then re-engage . . . ’ (HT1).
Regarding the delivery of the intervention, the HTs noted that text reminders were useful to remind people about appointments. Being flexible to the individual’s needs or preferences is another important element that we should emphasise. Suggestions were made regarding further supervision: ‘I think the quality of the intervention would have been improved if we’d had better supervision . . . ’
There was a further suggestion that some type of debriefing supervision might be useful, where the HTs could discuss difficult cases or their own feelings about certain difficult clients:
. . . The sort of thing that really I need to sort of discuss with somebody. What I would think is that I would need one-to-one every couple of weeks, and in that time I should be able to talk through, you know, who my clients are and what I’ve done with them, what problems I’ve got with them, what I think I’ve done well with them; and get some feedback and support around any clients that I’m struggling with. So that could be that the person I’m having supervision with might have listened to the tapes as well, and might have something to kind of say about me, what they hear me doing . . .
There was also a suggestion from one HT that at the baseline interviews, participants often wanted to talk about their experiences with smoking cessation and it was difficult not to get drawn into intervention. This might result in some contamination of the control group (albeit quite low level) if the data collection is not separated from the intervention. There was a tension between the need to build empathy at this stage and the need not to engage therapeutically.
Appendix 6 Exercise Assisted Reduction then Stop process fidelity scales
The rating scale
The present six-point scale (i.e. a 0-6 Likert scale) extends from 0 [where the HT did not deliver the intervention element appropriately – either they didn’t do it well or didn’t do it sufficiently (low fidelity)] to 6 where there is the element is delivered appropriately (high fidelity). Thus, the scale assesses a composite of adherence to the intended intervention method and skill of the HT. To aid with the rating of items, an outline of the key features of each item is provided at the top of each section. A description of the various rating criteria is given in Figure 1. The examples are intended to be used as useful guidelines only, providing illustrative anchor points, rather than prescriptive scoring criteria.
Adjusting for the presence of participant difficulties
Adjustments may be needed when participant difficulties are evident (e.g. excessive avoidance or resistance). In such circumstances, the rater needs to assess the HT’s therapeutic skills in the application of the methods. Even though the HT may not facilitate change, credit should be given for demonstrating appropriate skilful interaction.
Competence level* Scoring Examples* The scale incorporates the Dreyfus system (Dreyfus, 1989) for denoting competence. Please note that the ’top marks (i.e. near the ‘expert’ end of the continuum) are reserved for those HTs demonstrating highly effective skills, particularly in the face of difficulties (i.e. smokers with high resistance to change; high levels of emotional expression; and complex situational barriers). Please note that there are 5 competence levels but six potential scores.

When rating the item, you should first identify whether some of the ‘Key Features’ are present. If the HT includes most of the key features and uses them appropriately (i.e. misses few relevant opportunities to use them and delivers them well), the HT should be rated very highly. It is important to remember that the scoring profile for this scale should approximate to a normal distribution (i.e. mid-point 3), with relatively few scoring at the extremes.
Dreyfus, H. L. (1989). The Dreyfus model of skill acquisition. In J. Burke (ed.) Competency based education and training. London: Falmer Press.
ITEM 1: Active participant involvement
Key features: The HT should encourage the smoker to be actively involved in the consultation. The idea is to maximise the smoker’s autonomy as the main agent of change, developing intrinsic rather than extrinsic motivation, and encouraging her /him to be the person coming up with ideas for improving the situation. However, the smoker should not be allowed to ramble in an unstructured way and the consultation should be guided. A collaborative /shared decision-making style is appropriate and the HT may share his /her own expertise and ideas, using techniques such as elicit-provide-elicit (below). Overall, the smoker should be increasingly empowered to take control of her/his smoking and related physical activity behaviour. Interactions should be encouraging, respectful and non-judgemental (the opposite of a didactic, telling or persuading style of interaction). The smoker should ideally talk for at least half of the time. The interaction should also be individually tailored to the participant’s specific information needs, beliefs, motivations and barriers. The HT should engender a clear sense of warmth, genuineness and empathy (within professional boundaries).
Intervention techniques: OARS (Open questions, Affirmation, Reflective listening, Summaries). Reflective listening may include simple reflections of content but may also be more sophisticated (e.g. amplified reflection; reflection with a twist) and used to direct the conversation or highlight key strengths or barriers. The Ask-Tell-Discuss (elicit-provide-elicit) technique should be used to exchange information (e.g. to address misconceptions, or offer helpful new information). The above empathy-building techniques and Individual tailoring should be used throughout the consultations - from the initial consultation through action-planning through to review /maintenance sessions.
Mark with an ’X’ on the vertical line, using whole and half numbers, the level to which you think the HT has delivered this intervention process
0 Absence of active participant involvement techniques. A highly didactic /practitioner-led or ‘lecturing’ style of interaction, which may increase or sustain client’s resistance
1 Minimal participant involvement or use of active participant involvement techniques. The practitioner dominates the discussion
2 Appropriate use of participant involvement techniques, but not frequent enough. The practitioner sometimes dominates the discussion
3 Appropriate and frequent use of participant involvement techniques. Teamwork evident, but some difficulties in content or method of delivery
4 Appropriate and frequent use of participant involvement techniques. Minor problems evident (e.g. some reflection opportunities missed)
5 Highly appropriate and regular use of participant involvement techniques, facilitating shared understanding and decision making. Minimal problems
6 Excellent/expert use of participant involvement techniques throughout all consultations. A clear sense of collaborative alliance is developed.
ITEM 2: Motivation-building for cutting down/quitting
Key features: The HT should work with the smoker to explore initial beliefs about cutting down, and quitting (importance and confidence, triggers for smoking). The smoker’s motivation and confidence for cutting down is built up/enhanced through the exchange of information and techniques to assess and enhance motivation – i.e. to enhance the perceived benefits (importance) of cutting down /quitting and confidence (self-efficacy) to take the actions needed.
Intervention techniques: OARS (Open questions, Affirmation, Reflective listening, Summaries) should be used specifically to explore current and past smoking behaviour, the pros and cons of cutting down and to develop discrepancies between current behaviour and desired behaviour (or outcomes). The decisional balance technique or 0–10 questions may be used to explore importance and confidence. Information should be exchanged on the pros and cons of cutting down and this and other techniques (exploring possible futures; discussing past quitting attempts) should be used to explore barriers and possible solutions to increase confidence about cutting down/quitting. Motivation-building should ideally happen around the start of the intervention process, although it can be further explored and reinforced at later (action-planning, review and maintenance) stages. Establishing self-rewards or incentives (e.g. saving money in a jar, planning rewards) may be part of the process for maintaining motivation.
Mark with an ’X’ on the vertical line, using whole and half numbers, the level to which you think the HT has delivered this intervention process. NB: achieving a strong motivation is not necessary to score highly here – the aim is to explore motivation sufficiently to allow the client to be able to make an informed choice (which may be not to make any changes at this point in time)
0 Absence (or very poor delivery) of motivation-building techniques. Motivation to cut down or quit smoking is assumed or not discussed
1 Minimal use of (or poor delivery of) motivation-building techniques. Minimal exploration of either reasons for change or confidence about making changes.
2 Some use of motivation-building techniques, but the exploration of motivation to cut down or quit is not of sufficient depth or detail
3 Appropriate use of motivation-building techniques. However, some difficulties evident (e.g. moving on to change talk before motivation is fully established)
4 Appropriate and frequent use of motivation-building techniques relating to cutting down or quitting smoking. Minor problems evident (e.g. some inconsistencies)
5 Highly appropriate and sufficient use of motivation-building techniques, facilitating a clear understanding of reasons for change and confidence issues. Minimal problems
6 Excellent/expert use of motivation-building techniques, facilitating a clear understanding of reasons for change and confidence issues. No real problems
ITEM 3: Motivation-building for physical activity
Key features: The HT should work with the smoker to introduce PA as an aid to cutting down and quitting. They should explore initial beliefs about increasing physical activity (importance and confidence). The smoker’s motivation and confidence for introducing new physical activity behaviours should be built up through the exchange of information and techniques to assess and enhance motivation – i.e. to enhance the smoker’s perceived benefits and usefulness (importance) of physical activity and confidence (self-efficacy) to take the actions needed.
Intervention techniques: OARS (Open questions, Affirmation, Reflective listening, Summaries) should be used specifically to explore current and past physical activity behaviour, the pros and cons of increasing PA and to develop discrepancies between current behaviour and desired behaviour (or outcomes). The decisional balance technique or 0–10 questions may be used to explore importance and confidence. Information should be exchanged on the pros and cons of physical activity and this and other techniques (exploring possible futures; discussing past quitting attempts) should be used to explore barriers and possible solutions to adopting PA strategies /increasing PA. Motivation-building should ideally happen around the start of the intervention process, although it can be further explored and reinforced at later (action-planning, review and maintenance) stages.
Mark with an ’X’ on the vertical line, using whole and half numbers, the level to which you think the HT has delivered this intervention process. NB: achieving a strong motivation or any changes is not necessary to score highly – the aim is to explore motivation sufficiently to allow the client to be able to make an informed choice about whether to change or not.
0 Absence (or very poor delivery) of motivation-building techniques. Motivation to adopt physical activity strategies is assumed or not discussed
1 Minimal use of (or poor delivery of) motivation-building techniques. Minimal exploration of either reasons for change or confidence about making changes.
2 Some use of motivation-building techniques, but the exploration of motivation for physical activity is not of sufficient depth or detail
3 Appropriate use of motivation-building techniques. However, some difficulties evident (e.g. moving on to change talk before motivation is fully established)
4 Appropriate and frequent use of motivation-building techniques relating to physical activity. Minor problems evident (e.g. some inconsistencies)
5 Highly appropriate and sufficient use of motivation-building techniques, facilitating a clear understanding of reasons for change and confidence issues. Minimal problems
6 Excellent/expert use of motivation-building techniques, facilitating a clear understanding of reasons for change and confidence issues. No real problems
ITEM 4: Set goals and discuss strategies to reduce smoking
Key features: The HT should work with the smoker to discuss a range of strategies for reducing the amount of cigarettes smoked. They should agree a verbal plan of action, seeking to make this as specific as possible. They should discuss the use of self-monitoring to keep track of progress.
Intervention techniques: Goal-setting (with gradual /graded progression), Action Planning, Self-Monitoring, Deconditioning strategies. Any or all of the four distinct EARS strategies for cutting down (based on breaking the conditioned/automated link between smoking and reward and replacing this with consciously mediated strategies) may be presented and discussed. The action plan should normally be made verbally, but the HT should seek to make this as specific as possible in terms of ‘What, Where, When and Who with’ and making the goal as SMART (Specific, Measurable, Achievable, Relevant and Time-related) as possible. The HT should introduce and discuss with the smoker the usefulness of self-monitoring of behaviours (number of cigarettes smoked, pattern of use). A specific plan for self-monitoring should be included in the action plan. The HT may also encourage self-monitoring of the contexts (social or environmental or emotional circumstances) in which problems/relapses might occur. Pre-empting and thinking of solutions for possible problems (making a coping plan) is also appropriate here and may involve the use of other recognised behaviour change techniques (e.g. engaging social support, stress-management).
Mark with an ’X’ on the vertical line, using whole and half numbers, the level to which you think the HT has delivered this intervention process
0 Absence (or very poor delivery) of action-planning techniques or discussion of smoking-reduction strategies
1 Minimal use (or poor delivery) of action-planning techniques or discussion of smoking-reduction strategies
2 Some use of action-planning techniques or discussion of smoking-reduction strategies, but not in sufficient depth or detail
3 Appropriate use of action-planning techniques and discussion of smoking-reduction strategies. However, some difficulties evident (e.g. not setting up self-monitoring; plan generated more by the HT than by the smoker)
4 Appropriate use of action-planning techniques and discussion of strategies. Minor problems evident (e.g. the plan is a bit less specific than it could be)
5 Highly appropriate and sufficient use of action-planning techniques and discussion of smoking-reduction strategies. Minimal problems
6 Excellent/expert use of action-planning techniques and discussion of smoking-reduction strategies. No real problems
ITEM 5: Set goals and discuss strategies to Set goals to increase Physical Activity
Key features: The HT should work with the smoker to discuss ideas for introducing new physical activities that might help to reduce smoking. They should agree a verbal plan of action, seeking to make this as specific as possible. They should discuss the use of self-monitoring to keep track of progress, including offering a pedometer as a means of monitoring walking activity if appropriate.
Intervention techniques: Goal-setting (with gradual/graded progression), Action Planning, Self-Monitoring. Ideas for introducing relevant physical activities should be discussed. The action plan should normally be made verbally, but the HT should seek to make this as specific as possible in terms of ‘What, Where, When and Who with’ and making the goal as SMART (Specific, Measurable, Achievable, Relevant and Time-related) as possible. The HT should introduce and discuss with the smoker the usefulness of self-monitoring of behaviours (using memory, a diary and/or a pedometer). A specific plan for self-monitoring should be included in the action plan. Pre-empting and thinking of solutions for possible problems (making a coping plan) is also appropriate here and may involve the use of other recognised behaviour change techniques (e.g. establishing prompts or cues to do physical activity).
Mark with an ’X’ on the vertical line, using whole and half numbers, the level to which you think the HT has delivered this intervention process
0 Absence (or very poor delivery) of action-planning techniques in relation to physical activity
1 Minimal use (or poor delivery) of action-planning techniques
2 Some use of action-planning techniques relating to physical activity, but not in sufficient depth or detail
3 Appropriate use of action-planning techniques. However, some difficulties evident (e.g. no self-monitoring; plan generated more by the HT than by the smoker)
4 Appropriate use of action-planning techniques relating to physical activity. Minor problems evident (e.g. the plan is a bit less specific than it could be)
5 Highly appropriate and sufficient use of action-planning techniques. Minimal problems
6 Excellent/expert use of action-planning techniques relating to physical activity. No real problems
ITEM 6: Review efforts to cut down smoking/problem-solving
Key features: The HT should work with the smoker to reflect on progress with smoking reduction. The HT should affirm/reinforce any successes. The smoker and HT should discuss any setbacks (reframing to normalise them, identifying barriers and exploring ways to overcome them). The HT and smoker should then set new targets (possibly including making an attempt to quit).
Intervention techniques: Use of OARS (Open questions, Affirmation, Reflective listening, Summaries) specifically to reinforce successes, to discuss setbacks, to identify barriers (including social or environmental contexts which increase cravings) and explore ways to overcome them (problem-solving). Reframing should be used to normalise setbacks. Goals/action plans should then be reviewed. There may also be some reflection on, and reinforcement of the smoker’s skills in avoiding or managing relapse (building skills and self-efficacy). Problem-solving may involve the use of other recognised behaviour change techniques (e.g. engaging social support, stress-management).
Mark with an ’X’ on the vertical line, using whole and half numbers, the level to which you think the HT has delivered this intervention process
0 Absence (or very poor delivery) of progress review or problem-solving techniques in relation to smoking reduction
1 Minimal use (or poor delivery) of progress review or problem-solving techniques
2 Some use of progress review and problem-solving techniques in relation to smoking reduction, but lacking sufficient depth or detail
3 Appropriate use of progress review and problem-solving techniques. However, some difficulties evident (e.g. not reinforcing successes, providing rather than eliciting possible solutions to problems)
4 Appropriate and frequent use of progress review and problem-solving techniques in relation to smoking reduction. Minor problems evident
5 Highly appropriate and sufficient use of progress review and problem-solving techniques, facilitating a clear understanding of the current situation and how to move forward. Minimal problems
6 Excellent/expert use of progress review and problem-solving techniques in relation to smoking reduction, facilitating a clear understanding of the current situation and how to move forward. No real problems
ITEM 7: Review efforts to INCREASE physical activity /problem-solving
Key features: The HT should work with the smoker to reflect on progress with introducing relevant physical activities. The HT should affirm/reinforce any successes. The smoker and HT should discuss any setbacks (reframing to normalise them, identifying barriers and exploring ways to overcome them). The HT and smoker should then revise the smokers PA-related goals.
Intervention techniques: Use of OARS (Open questions, Affirmation, Reflective listening, Summaries) specifically to reinforce successes, to discuss setbacks, to identify barriers and explore ways to overcome them (problem-solving). Reframing should be used to normalise setbacks. Goals/action plans should then be reviewed. There may also be some reflection on, and reinforcement of the smoker’s skills in avoiding or managing relapse (building skills and self-efficacy).
Mark with an ’X’ on the vertical line, using whole and half numbers, the level to which you think the HT has delivered this intervention process
0 Absence (or very poor delivery) of progress review or problem-solving techniques in relation to the physical activity component of the intervention
1 Minimal use (or poor delivery) of progress review or problem-solving techniques
2 Some use of progress review and problem-solving techniques in relation to physical activity, but lacking sufficient depth or detail
3 Appropriate use of progress review and problem-solving techniques. However, some difficulties evident (e.g. not reinforcing successes, providing rather than eliciting possible solutions to problems)
4 Appropriate and frequent use of progress review and problem-solving techniques in relation to physical activity. Minor problems evident
5 Highly appropriate and sufficient use of progress review and problem-solving techniques, facilitating a clear understanding of the current situation and how to move forward. Minimal problems
6 Excellent/expert use of progress review and problem-solving techniques in relation to physical activity, facilitating a clear understanding of the current situation and how to move forward. No real problems
ITEM 8: Integration of concepts: Building an association between PA and smoking reduction
Key features: The HT should work with the smoker specifically to help her/him gain an appreciation of the relationship between physical activity and smoking. A clear rationale should be presented for how PA might be relevant to reducing smoking (e.g. as a distraction, as a way to reduce withdrawal symptoms such as stress or cravings, as a way to prevent weight gain when reducing smoking). However, both explicit processes (explanations) and implicit processes (learning from experience, disrupting usual patterns of smoking behaviour; reductions in withdrawal symptoms that the smoker is not consciously aware of) should be facilitated by the HT.
Intervention techniques: Explicit integration techniques might include (a) developing (ideally using the Ask-Tell-Discuss information-exchange technique) an appropriate conceptualisation or rationale for increasing PA as an aid to reducing smoking (b) setting up an experiment (to do some extra PA) and encouraging self-monitoring of links between physical activity and cigarette cravings, as well as on cigarette use. Implicit techniques might include (a) setting up an experiment to see if it helps reduce smoking, with monitoring only of outcomes (cigarette use) and without trying to make a conscious link between PA and strength of cravings. Review of experiences with using PA and its impact on cravings or smoking behaviour may also be used in later sessions.
Mark with an ’X’ on the vertical line, using whole and half numbers, the level to which you think the HT has delivered this intervention process
The absence (or very poor delivery) of techniques to link PA to cravings or amount smoked
1 Minimal use (or poor delivery) of techniques to link PA to cravings or amount smoked. No clear rationale linking PA to smoking reduction is understood by the client
2 Some use of techniques to link PA to cravings or amount of cigarettes smoked, but not of sufficient depth or detail. Only a limited rationale linking PA to smoking reduction is understood by the client.
3 Appropriate use of techniques to link PA to cravings or amount of cigarettes smoked. The rationale is at least partly understood by the client. Some difficulties evident (e.g. not addressing misconceptions, not using Ask-Tell-Discuss)
4 Appropriate use of techniques to link PA to cravings or amount of cigarettes smoked. The rationale is understood by the client. Minor problems evident (e.g. minor inconsistencies)
5 Highly appropriate use of techniques to link PA to cravings or amount of cigarettes smoked. The rationale is well developed and understood. Minimal problems
6 Excellent/expert use of techniques to link PA to cravings or amount of cigarettes smoked. The rationale is well developed and understood. No real problems
ITEM 9: Identify and reinforce any identity shifts towards being a more ‘healthy person’ or ‘healthy living’
Key features: The HT should pick up on any opportunity to reflect or reinforce statements that the smoker makes relating to becoming or wanting to become a more healthy person in general.
Intervention techniques: Open questions, Affirmation, Reflective listening. Reflective listening may include simple reflections of content but may also be more sophisticated (e.g. amplified reflection; reflection with a twist) and used to direct the conversation or highlight key changes in thinking that may generalise to a change in the client’s self concept or identity, particularly with regard to being a healthy person or living a healthy lifestyle.
Mark with an ’X’ on the vertical line, using whole and half numbers, the level to which you think the HT has delivered this intervention process. It is recognised that there may only be a few, if any opportunities to deliver this aspect of the intervention. Hence, we expect scores to be relatively low for this item.
0 Absence (or very poor delivery) of identity-building interactions
1 Minimal (or poorly delivered) identity-building interaction
2 Some identity-building interaction
3 Several examples of identity-building interaction. However, some difficulties evident (e.g. missed opportunities, talking at odds with the participant)
4 Appropriate use of identity-building interactions, taking almost all opportunities. Minor problems evident
5 Highly appropriate and sufficient use of identity-building interactions. Minimal problems
6 Excellent/expert use of identity-building interactions. No real problems
ITEM 10: Engaging social support and managing social influences on smoking reduction
Key features: The HT should encourage the smoker to engage social support (to assist on making or carrying out plans) or manage social influences on smoking behaviour. Social support can be informational (helping to make plans, providing ideas), emotional (not putting pressure on the person to smoke/accepting their decision to cut down or quit), or practical (e.g. helping to monitor progress).
Intervention techniques: Open questions, Affirmation, Reflective listening and Summaries may be used to explore social influences and to identify possible problems and solutions relating to social influences.
Mark with an ’X’ on the vertical line, using whole and half numbers, the level to which you think the HT has delivered this intervention process
0 Absence (or very poor delivery) of interactions around engaging social support or managing social influences on smoking behaviour
1 Minimal (or poorly delivered) interaction around engaging social support or managing social influences
2 Some interaction around engaging social support or managing social influences on smoking behaviour, but not in sufficient depth or detail
3 Several examples of interaction around engaging social support or managing social influences. However, some difficulties evident (e.g. missed opportunities, talking at odds with the participant)
4 Appropriate use of interactions to engage social support or manage social influences on smoking behaviour, taking almost all opportunities. Minor problems evident
5 Highly appropriate and sufficient use of interactions to engage social support or manage social influences. Minimal problems
6 Excellent/expert use of interactions to engage social support or manage social influences on smoking behaviour. No real problems
ITEM 11: Engaging social support and managing social influences on physical activity
Key features: The HT should encourage the smoker to engage social support (to assist on making or carrying out plans) or manage social influences on physical activity. Social support can be informational (helping to make plans, providing ideas), emotional (not putting pressure on the person to smoke/accepting their decision to cut down or quit), or practical (e.g. helping to monitor progress).
Intervention techniques: Open questions, Affirmation, Reflective listening and Summaries may be used to explore social influences and to identify possible problems and solutions relating to social influences.
Mark with an ’X’ on the vertical line, using whole and half numbers, the level to which you think the HT has delivered this intervention process
0 Absence (or very poor delivery) of interactions around engaging social support or managing social influences on physical activity
1 Minimal (or poorly delivered) interactions around engaging social support or managing social influences
2 Some interaction around engaging social support or managing social influences on physical activity, but not in sufficient depth or detail
3 Several examples of interaction around engaging social support or managing social influences. However, some difficulties evident (e.g. missed opportunities, talking at odds with the participant)
4 Appropriate use of interactions to engage social support or manage social influences on physical activity, taking almost all opportunities. Minor problems evident
5 Highly appropriate and sufficient use of interactions to engage social support or manage social influences. Minimal problems
6 Excellent/expert use of interactions to engage social support or manage social influences on physical activity. No real problems
ITEM 12: REFERRAL TO SMOKING CESSATION SERVICES
Was the issue of making an attempt to stop smoking raised and the response appropriately addressed (i.e. if desired, to make a referral to NHS SSS)?
Yes □ No □
Appendix 7 Suspected adverse event form
Appendix 8 Session notes forms
Session 1 notes form
Session 2–8 notes form
Appendix 9 Cost-effectiveness data collection materials
Exercise Assisted Reduction then Stop work sampling form
Exercise Assisted Reduction then Stop work sampling form (PDF download)
Estimating the resource use and cost for the Exercise Assisted Reduction then Stop Intervention
Training and set-up costs
Experience in the pilot trial indicates that training for HTs in EARS intervention delivery requires 30 hours of training time (5 × 6-hour sessions), with an expectation that one lead trainer undertakes training for three trainees. Table 1 sets out the cost estimates for training activity based on these assumptions.
| Item | Resource use | Unit cost | 1-year costs |
|---|---|---|---|
| HT training | Three HTs × 30 hours | £27 per hour | £2430.00 |
| Lead training | 1 trainer × 37 hours | £38 per hour | £1406.00 |
| Venue opportunity cost | 5 days | £100 | £500.00 |
| Materials | £200.00 | ||
| Travelb | 50 miles per day (trainer) | £0.21 per mile | £52.50 |
| Subtotal (based on three HTs) | £4588.50 | ||
| Training cost per HT | £1529.50 | ||
| Total training attributable to participant based on three HTs each working 42 weeks per year | |||
| Training/participant (assumed caseload = 250 per HT) | £6.12 c | ||
Health trainer contact time with participants (electronic data)
Analysis of within-trial data (electronic data reportage by HTs) provided the following summary statistics:
-
EARS participants n = 49
-
Missing (no contact time) n = 0
-
Using n = 49:
-
mean number of intervention sessions attended = 4.10 (SD 2.69)
-
mean number of DNA events = 0.65 (SD 1.13) (conservative)
-
mean number of contacts with EARS (intervention + continued support for quitters) = 4.26.
-
| Mean (minutes) | SD | Comment | |
|---|---|---|---|
| Face to face | 86.6 | 73.4 | |
| Telephone contact | 35.5 | 52.1 | |
| Face-to-face contact delivery time | 122.1 | – | |
| E-mails | 0.2 | 1.5 | Based on 10-minute e-mail |
| Texts | 2.7 | 3.5 | Based on 2-minute text |
| Voicemail | 1.9 | 2.4 | |
| Letters | 1.8 | 5.9 | Based on 10-minute letter |
| Non-intervention telephone calls | 8.0 | 8.9 | |
| Total participant level contact time (excluding supervision) | 136.7 | ||
| Supervision (per participant) | 9.80 | – | Assumption based on 1-hour supervision per HT per week and intervention turnover of cases every 2 months; supervision time 60 minutes × 8 weeks = 480 minutes for 49 participants in the trial (estimate per participant = 9.8 minutes) |
| Total contact time | 146.50 | – |
Health trainer non-contact time
Electronic records
The data/information we have from HT electronic record suggest only a small proportion of non-face-to-face activities delivering EARS (37% of HT time), the majority of which was captured in identification and recruitment of smokers for the EARS service. It is apparent that electronic methods of data reportage failed to fully capture time spent in non-contact activities.
Work sampling records
Work sampling of two HTs was carried out over a 2-week period. Data collection in the pilot indicated that some developments were needed to the data collection form and also, given that data were collected from only two HTs, it is difficult to draw firm conclusions from the pilot study. However, the method was tested out, it was acceptable to HTs, and the pilot study indicates that it is an important source of future data (within cost estimates). In theory this approach is able to provide data that will allow an approximation to be made over the ratio of contact/non-contact HT time in EARS delivery. In the pilot data, we found the ratio difficult to determine as feedback from HTs on work sampling indicated that HTs used research-related data collection questions to launch into intervention delivery. Adjusting for this bias by excluding all data collection activities suggests a considerable and substantial proportion of non-contact activities for the two HTs sampled (57% of total HT time).
Identification of ‘hard-to-reach’ smokers and recruitment
In the pilot trial, a number of different modes of identification of participants for EARS were explored. In addition, some identification work (screening and searching for ‘hard-to-reach’ participants) was taken by the principal investigator and trial manager, whereas in practice this work is likely to be done by a HT. Within the trial, the most efficient models of recruitment were from GPs and previous treatment failures from SSS (in the region of 1–1.5 hours of recruitment activity per participant randomised). We therefore assumed that a mean of 1.25 hours of recruitment-based activity might be required. This may be conservative as anecdotal evidence suggests that recruitment into a trial is much more difficult than recruitment into a service.
Supervision time
Current costs assume 1 hour’s supervision per HT per week and intervention turnover of cases every 2 months. The assumption is based on experiences in the trial with 49 EARS participants.
Exercise aids
Data estimated within trial (electronic reporting) showed the per-participant costs to be in the region of £19. The data/information we have from the trial suggested that only a low proportion of participants had financial subsidies. Further details are provided in Table 3. Accelerometers were used in the trial to measure exercise outcomes, but unit cost of intervention was assumed to be for a cheaper pedometer.
| Exercise aid | Cost (£) | SD |
|---|---|---|
| Cost of subsidies | 7.17 | 18.82 |
| Cost of pedometers | 10 | – |
| Cost of MP3s | 1.25 | – |
| Cost of exercise bands | 0.65 | – |
| Total cost of exercise aids | 19.07 |
Sensitivity analysis to explore inclusion of indirect costs associated with intervention
Effect of implementation on Stop Smoking services
It is possible that the EARS intervention could increase the number of participants wishing to make a quit attempt via SSS over and above control. The sample size of the pilot trial made it difficult to make inference on this, but a full RCT may be more informative. In the pilot trial, 2/49 participants in the EARS active arm were referred to SSS compared with 0/50 in control. The analysis of intervention cost of EARS at present makes no projection about how services could be integrated. Assuming a scenario where 50% (rounded up) of the 11 making a quit attempt in EARS seek help through SSS, using a cost of SSS per participant at £350 – as reported by Carr et al. 1 – we explored including this data (along with pharmacotherapies) with the components of the total service cost for EARS in a sensitivity analysis presented in Table 4. The figures overestimate the cost of SSS within the trial period.
We restrict our base-case analysis of costs to those incurred as a direct result of the intervention on the basis that it is questionable as to whether or not there would be additional indirect intervention costs over time with EARS relative to comparator. Smoking is a chronic condition, and many smokers typically receive multiple interventions before achieving long-term quit.
Effect of implementation on pharmacotherapy usage
As with smoking services, it is plausible that the EARS intervention could result in additional usage of pharmacotherapy compared with alternatives (NRT, buproprion and/or varenicline) to support a quit attempt. It is also possible that an EARS-type intervention could result in lower usage of pharmacotherapy if exercise alone proves effective at minimising cravings.
In a sensitivity analysis, we assumed that an additional 50% of the 11 out of 12 making a quit attempt made pharmacotherapy-assisted quit attempts on prescription. One course of pharmacotherapy equates to £62 per participant; this assumption is based on cost of GP advice plus prescription by Wang et al. ,2 inflated from 2006 prices using the PSSRU Hospital and Community Pay and Prices index. 3
Results
Including additional pharmacotherapy and SSS resource cost increases the total per-participant cost of the EARS service by £50.45, that is to say a total of £242.62.
| Other items | Cost | 1-year costs |
|---|---|---|
| Additional referrals to SSS | 6 × £350 | £2100.00 |
| Additional pharmacotherapy prescription | 6 × £62 | £372.00 |
| Subtotal | £2472.00 | |
| Total indirect costs per participant based on three HTs each working 42 weeks per year | ||
| Indirect costs per participant (caseload based on trial intervention, n = 49) | £50.45 | |
Effect of implementation on other services
For pragmatic reasons this is assumed to be zero cost.
Economic evaluation search strategy used to identify cost-effectiveness analysis studies in smoking cessation
The following electronic databases were searched from their inception up to August 2012: MEDLINE, EMBASE (both via Ovid) and Cochrane Reviews and Dissemination (CRD). The search terms used included smoking, tobacco and terms relating to types of economic evaluation.
Search strategy (MEDLINE)
cost benefit analysis/
cost effectiveness analysis/
cost minimization analysis/
cost utility analysis/
(technology assessment).tw.
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
or/1-6
(nicotine$ or smok$ or tobacco).ti.
and/ 8-9
Search strategy (EMBASE)
cost benefit analysis/
cost effectiveness analysis/
cost minimization analysis/
cost utility analysis/
(technology assessment).tw.
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
or/1-6
(nicotine$ or smok$ or tobacco).ti.
and/ 8-9
Search strategy (CRD)
We searched for economic models and economic evaluations performed by a search of all publications using the terms smoking, tobacco and nicotine.
Evidence review summary table
| Wang et al.2 (UK) | |
|---|---|
| (a) Model structure | Aim: to assess the cost-effectiveness of making NRT available in the context of a CDTQ programme for a suitable population of smokers Model: decision-analytic model (structure reported – decision tree), parameters estimated from published literature Time horizon: lifetime Viewpoint: UK NHS/Personal Social Services Viewpoint justification: none reported Alternatives: No NRT. In the full model, for attempts made with NRT the attempts were categorised as either ‘abrupt’ or CDTQ. Within each of these possibilities, the form by which smokers CDTQ or quit modelled are OTC NRT, prescription NRT or a smokers’ clinic. The authors also show a schematic for a mixed analysis. The reporting does not lend itself to a clear distinction between the approaches Study population: current smokers (previous quit attempts or exposure to other interventions in the past not dealt with. Future quit attempts – assumption that risk of relapse is independent of current quit attempt) Evaluation method: cost-effectiveness analysis |
| (b) Data inputs – effectiveness data | Effectiveness data: model refers to meta-analyses conducted elsewhere for probability of successful quit attempt (at 6 months, 12 months and lifetime) Method used to estimate effectiveness: sustained abstinence measures from meta-analysis of individual patient data at 6 months. Evidence synthesis (meta-analysis or other method, NR, for abrupt quitters) of relapse rates from 12 studies for the period between 6 and 12 months using: 12 months’ sustained abstinence = 6 months’ sustained abstinence × risk of relapse Lifetime abstinence estimated by assumption that 30% relapse after 12 months, i.e. 70% of 12-month quitters succeed Valuation of health benefits: CQG (authors estimate the QALY gain using data from Doll et al., they do not estimate aggregate/total QALYs as these data apparently unavailable) Indirect benefits: NR |
| (b) Data inputs – resource use | Resource use: therapy and clinicians’ time, OTC treatments resulted in zero cost to NHS as defrayed to patient Unit cost data: reported in appendix Resources reported separately from cost: yes Currency (exchange rate): £ Price year (adjustment for inflation): 2008 |
| (c) Consideration of uncertainty/consistency | Discount rate: 3.5% costs and QALYs Uncertainty: simple deterministic analysis, main focus is on the CDTQ quit rate vs. the abrupt quit rate. Authors refer presentation of ICERs by subgroup sensitivity analysis The authors also perform a sort of threshold analysis to examine the trade-off (to examine possible effect of smokers who would otherwise have made an abrupt quit now cutting down vs. those making no attempt) Authors’ conclusion: CDTQ delivers ICERs within margins considered cost-effective. CDTQ is less effective and more costly than abrupt quitting but may address a different population |
| (d) Presentation of CEA findings | Outcome of model: (as identified by authors) expected lifetime QALYs. In reality, the model only estimates the CQG associated with a quit attempt. The summary outcome measure is the ICER and is therefore an incremental analysis (CQG) Probability of lifetime quit rate: values reported for different options within the range of 0.0137–0.1119 (abrupt quit) Total cost: (£0–153.79) ICER: range of ICERs presented for different service strategies |
| NICE guidance PH14 | |
| (a) Model structure | Aim: cost-effectiveness of brief intervention and referral (multiple scenarios) GP and nurse delivered and referral to specialist SSS for smoking cessation Model: decision tree, projection of final outcomes Time horizon: lifetime (analysis). Decision tree corresponds to 12 months Viewpoint: NHS and Personal and Social Services Viewpoint justification: NICE guidance Alternatives: various. Brief opportunistic advice from GP alone (with adjunct NRT, telephone helpline, self-help material). Nurse-led brief interventions (30 minutes) in primary or hospital setting Study population: UK smokers Evaluation method: CUA |
| (b) Data inputs – effectiveness data | Probability data: 12-month quit rate Method used to estimate comparative effectiveness: ORs applied to 12-month quit rates for different types of brief intervention Primary outcome measure: 12 month quit rate (in some instances calculated from ORs) obtained from evidence synthesis of trial data. Other outcome measures: adjustment for risk of longer-term risk of relapse from Yudkin et al.5 Detailed methods not provided Valuation of health benefits: secondary EQ-5D data from an unpublished survey of 15,000 current and ex-smokers Indirect benefits: not considered |
| (b) Data inputs – resource use | Resource use: GP/nurse time, nurse training in brief advice, cost of self-help materials, provision of telephone helpline service Unit cost data: staff costs from Curtis and Netten,6 NRT costs from BNF 2005, other overheads (telephone calls, literature) estimated, source not reported Cost savings in terms of smoking-related diseases per long-term quitter estimated from published source Godfrey7 Resources reported separately from cost: no; however, itemised resource use presented alongside total costs Currency (exchange rate): £ Price year (adjustment for inflation): 2005 |
| (c) Consideration of uncertainty/consistency | Key parameters varied: effectiveness and background quit rates, length of intervention. Discussion of other factors: socio-economic deprivation, tobacco dependence, extending model to include previous smokers not responding, alternative treatment settings Discount rate: 3.5% Uncertainty: deterministic – series of univariate scenario analyses. Threshold analysis to explore minimum effect size required to remain cost-effectiveness |
| (d) Presentation of CEA findings | Outcome: Cost per QALY (£) Probability of lifetime quit rate: assumes maximal period of follow-up by Yudkin5 at 8 years reflects lifetime rate Total cost: not presented ICER: Dependent on age and sex the ICERs relating to a brief (5-minute) intervention range from £636 to £1677 Authors’ conclusion: cost-effective results can be generated in all settings and age-group for all forms of brief advice. ICERs tend to increase with cohort age |
| Bauld et al.8 | |
| (a) Model structure | Aim: cost-effective analysis of smoking-cessation programs Model: decision tree (52-week model) with a Markov model (lifetime model) Time horizon: lifetime Viewpoint: NHS health service perspective (NHS Scotland) Viewpoint justification: not explicitly discussed Alternatives: both a pharmacy and a group-based SSS were compared against self-quit attempts. The authors suggest that direct comparison of pharmacy and group-based services would be inappropriate as they attract different ‘types’ of smoker Study population: smokers using NHS smoking cessation services in Glasgow Evaluation method: CEA |
| (b) Data inputs – effectiveness data | Method used to estimate effectiveness: verified 52-week abstinence rates to determine the numbers of current and ex-smokers in each cohort. Relapse rates applied for 8 years post quit. Risk of smoking related mortality applied 12 years post quit. Primary outcome measure: 52-week abstinence as verified by CO monitor Other effectiveness data considered: relapse rates and risk of smoking related death in ex-smokers. The methods for calculating smoking attributable mortality appear to be based on methods based on Thun et al.,9 and are outlined in a report commissioned by NHS Health Scotland Valuation of health benefits: secondary sources. Health state utility values from Tengs and Wallace10 – 0.8 smoker, 0.87 ex-smoker Indirect benefits: not considered |
| (b) Data inputs – Resource use | Resource use: all relevant health service costs associated with NRT, professional time, overheads and materials Unit cost data: BNF, PSSRU and within study Resources reported separately from cost: in summary form Currency (exchange rate): £ Price year (adjustment for inflation): 2007 |
| (c) Consideration of uncertainty/consistency | Key parameters varied in sensitivity analysis: scenario analyses based on (i) incorporating the future cost of smoking-related disease, (ii) not discounting QALYS, (iii) including self-reported quit, (iv) including a small cost (to the NHS) based on self-quit attempts Probability data: 12-month quit rates from within-trial analysis Discount rate: 3.5% QALYs only Uncertainty: scenario analyses, one of which includes future health costs of smokers |
| (d) Presentation of CEA findings | Outcomes: cost per client, cost per 52-week quitter, cost per QALY Probability of lifetime quit rate: applied by calculating year-on-year transitions (time dependent probabilities) Total cost: pharmacy cost per client £79.00. Group cost £368.00 ICER: (vs. self-quit) pharmacy service cost per 52-week quitter = £7800, group-based service cost per 52-week quitter = £9200 Authors’ conclusion: both pharmacy and group based services represent cost-effective interventions to help smokers to quit. Observed quit rates were lower than in some other studies; while this is commonly true in observational study designs, the lower effectiveness rates may also reflect a greater resistance to smoking cessation in Glasgow |
| Howard,11 BENESCO model | |
| (a) Model structure | Aim: to assess the cost-effectiveness of varenicline vs. alternative smoking-cessation strategies Model: Markov Time horizon: 20-year and lifetime Viewpoint: US health care Viewpoint justification: US budget holders Time horizon: 20-year and lifetime Alternatives: Buproprion, NRT, unaided quitting Study population: overall US population (of smokers) Evaluation method: CEA, CUA, budget impact |
| (b) Data inputs – effectiveness data | Method used to estimate effectiveness: continuous abstinence rates, hazard ratios (published literature Thun et al.9) (used to calculate number of smoking related diseases and deaths) Valuation of health benefits: QALYs from published literature (Fiscella and Franks12) Indirect benefits: not included |
| (b) Data inputs – resource use | Resource use: cost of treatments Unit cost data: drugs, dispensing fees, physician fees and treatment costs Resources reported separately from cost: yes Currency (exchange rate): $ Price year (adjustment for inflation): 2005 (inflation index detail not provided) |
| (c) Consideration of uncertainty/consistency | Key parameters: costs, efficacy rates, relapse rates, disease incidence, prevalence and mortality rates Time horizon: 2, 5,10, 20 years and lifetime Discount rate: 3% per annum Uncertainty: scenario analysis, probabilistic sensitivity analysis (details of distributions provided) |
| (d) Presentation of CEA findings | Outcomes: incremental cost and incremental QALYs, presented in disaggregated form for the entire modelled cohort (just under 12 million smokers). No summary statistic presented cost per QALY due to dominance of modelled intervention Probability of lifetime quit rate: Relapse rates assumed to be higher in first 5 years Total cost: for the US health system and a cohort of 11.9 million smokers, the total costs for varenicline were $328,541M over a lifetime compared with $330,958 for bupropion, $332,662 for NRT and $333,283 for unaided cessation ICER: varenicline dominated all alternatives in model base case Authors’ conclusion: varenicline is very likely cost-effective (based on probabilistic output presented in cost-effectiveness acceptability curves) Comments: population-based US model with a focus on pharmacological aids to smoking cessation |
| Hurley,13 Quit Benefits Model | |
| (a) Model structure | Aim: to create a tool to evaluate tobacco control programs when the number of quitters is known Model: Markov – model included four smoking associated diseases: MI, stroke, lung cancer, COPD Time horizon: 10 years (after quitting smoking) Viewpoint: health care system (primary care) Viewpoint justification: Australian policy-makers and health program funders Alternatives: N/A Study population: simulated quitters (Australians aged between 15 and 74) Evaluation method: CEA, CUA |
| (b) Data inputs – effectiveness data | Method used to estimate effectiveness: relative risks from observational studies (Australian) Valuation of health benefits: reduction in smoking related disease and death, life-years saved, QALYs Utilities of less than one for smoking related sequelae only. Utilities from CEA registry Indirect costs/benefits: not included |
| (b) Data inputs – resource use | Resource use: cost of treating smoking-related diseases Unit cost data: no, total cost of smoking-related diseases reported for direct health care costs only. Some information about different cost categories Resources reported separately from cost: no Currency (exchange rate): $AUS Price year (adjustment for inflation): 2001 (no inflation adjustments) |
| (c) Consideration of uncertainty/consistency | Key parameters: relative risks Probability data: some modelling of source data to derive transition probabilities (i.e. using exponential models in the case of smoking-related diseases. Model and assumptions well documented Time horizon: 10 years (after quitting smoking) Discount rate: 0, 3% and 5% Uncertainty: univariate and multivariate sensitivity analysis of all model inputs (+/–) 10% |
| (d) Presentation of CEA findings | Outcomes: life-years saved, QALYs, costs Probability of lifetime quit rate: N/A Total cost: average saving AUS $373,000 per 1000 quitters (men and women combined) ICER: not presented in a summary outcome measure as quitters had lower costs and better outcomes than non-quitters Authors’ conclusion: QBM highlights the clinical and economic benefits of a tobacco control programme showing the impact on the individual Comments: this model was implicitly compared with no intervention, i.e. illustrates the burden of disease and how it might be reduced by an effective tobacco control programme |
| Woolacott et al.14 | |
| (a) Model structure | Aim: to estimate the cost-effectiveness of NRT and/or bupropion sustained release for smoking cessation Model: decision-analytic (appears to be decision tree) Time horizon: unclear; not lifetime Viewpoint: UK NHS Viewpoint justification: not provided (HTA report) Alternatives: brief advice alone and in combination with either buproprion-sustained release or NRT. Counselling (individual or group) Study population: primary care Evaluation method: CEA, CUA |
| (b) Data inputs – effectiveness data | Effectiveness data: from published studies Method used to estimate effectiveness: continuous quit rate at 12 months (control/treated) Valuation of health benefits: life-years saved (from published review). QALYS (from Fiscella and Franks12) Indirect costs/benefits: not included |
| (b) Data inputs – resource use | Resource use: resource use relating to intervention only Unit cost data: yes, but not all intervention costs have sources which are referenced Resources reported separately from cost: yes Currency (exchange rate): £ Price year (adjustment for inflation): not reported – 2002? |
| (c) Consideration of uncertainty/consistency | Key parameters: continuous quit rate p.a. (advice) = 3% Continuous quit rate (counselling) p.a. = 9% Spontaneous cessation p.a. = 1% Probability data: for buproprion/NRT derived from meta-analysed or indirect comparisons of ORs to calculate annual probabilities for decision tree nodes Time horizon: unclear; not lifetime Discount rate: not applied Uncertainty: univariate and multivariate sensitivity analysis of all model inputs (+/–) 10% |
| (d) Presentation of CEA findings | Outcomes: ICER/life-years saved Probability of lifetime quit rate: 40% risk of relapse (in what period?) Total cost: £67–202M (NHS England and Wales) ICER: £1459–1777 when compared with advice alone Authors’ conclusion: NRT/buproprion-sustained release are cost-effective Comments: this model demonstrates the budget impact on a hypothetical NHS population for England and Wales |
| Godfrey et al.7 | |
| (a) Model structure | Aim: to assess the cost-effectiveness of English smoking treatment services Model: not presented formally as a model, but analysis is conformant with decision-analytic modelling. Epidemiological data was combined with observational/survey data to form the basis of CEA. Regression-based techniques used to account for within-service variations Time horizon: lifetime (not explicitly stated) Viewpoint: UK NHS (perspective of the unit of the smoking service, not the individual smoker) Viewpoint justification: to establish if actual practice is cost-effective Alternatives: as this was an evaluation of treatment services, this reflected variation in actual practices (e.g. different service configurations, staffing, interventions) Study population: English NHS smoking treatment services Evaluation method: CEA and regression based analysis of determinants of service-level total and incremental costs (per life-years) |
| (b) Data inputs – effectiveness data | Effectiveness data: from published studies Method used to estimate effectiveness: epidemiological model Valuation of health benefits: Doll et al.15 as basis for estimating life-years saved Indirect benefits: not included |
| (b) Data inputs – Resource use | Unit cost data: yes, relying on published sources (Curtis3) Resources reported separately from cost: yes Currency (exchange rate): £ Price year (adjustment for inflation): 2000–1 Costs of smoking-related diseases: included in sensitivity analysis |
| (c) Consideration of uncertainty/consistency | Key parameters: background quit rate, relapse rate Discount rate: varied at 0%, 3.5% (base case), 6% Uncertainty: series of univariate analyses (varying background cessation, relapse after 4 weeks, life-years gained from smoking cessation. A worst- and best-case scenario analysis was additionally performed |
| (d) Presentation of CEA findings | Outcomes: cost per life-year saved, cost per person setting a quit data Probability of lifetime quit rate: 65% of those quitting at 4 weeks relapse at 12 months. 54% relapse between 1 and 7 years (from Yudkin et al.5). Total cost per person setting a quit date: mean cost of £123.40 Summary measure of cost-effectiveness: average cost per life-year gained per smoker is £684 (£483 if future savings in smoker’s health care costs are considered) Authors’ conclusion: Comments: this model demonstrates the cost-effectiveness of English smoking services. As such, it presents evidence of cost-effective actual practice of English smoking services. As such, it presents evidence of cost-effective actual practice. The use of observational data from 10 years ago might render some of the studies estimates slightly out of date, not least because it was conducted when SSS had only recently been implemented. The model compares results with other studies |
| Coleman16 | |
| (a) Model structure | Aim: to derive cost-effectiveness of interventions (NRT, buproprion, varenicline) for preventing relapse in recently abstinent smokers Model: cohort simulation Time horizon: lifetime Viewpoint: UK NHS Viewpoint justification: HTA standard Alternatives: no intervention Study population: England and Wales (hypothetical) smokers Evaluation method: CUA |
| (b) Data inputs – effectiveness data | Effectiveness data: from published studies Method used to estimate effectiveness: Indirect comparisons of interventions Valuation of health benefits: Doll17 – mortality rates used to determine odds ratios and smoking attributable deaths. Tengs and Wallace for the majority of comorbid states.10 Tilman and Silcock18 for former and current smokers Indirect benefits: not included |
| (b) Data inputs – resource use | Unit cost data: partial, use of published sources Resources reported separately from cost: partial (intervention cost) Currency (exchange rate): £ Price year (adjustment for inflation): Not stated, but unit costs for therapies sourced from 2008 formulary prices Costs of smoking related diseases: included |
| (c) Consideration of uncertainty/consistency | Key parameters: background quit rate = 2%, relapse rate Discount rate: 3.5% (benefits, as costs are incurred immediately) Uncertainty: deterministic – changes in intervention cost, effectiveness and background quit rates |
| (d) Presentation of CEA findings | Outcomes: cost per QALY Probability of lifetime quit rate: N/A Total cost per person setting a quit date: N/A Summary measure of cost-effectiveness: no synthesis of dominant interventions. Very cost-effective at commonly applied UK NHS threshold Authors conclusion: interventions appear cost-effective and similar in magnitude to other studies Comments: paper discusses limitations: lack of data on comorbidities and smoking status; only single interventions considered in projected ICERs; efficacy from RCTs may be better than routine clinical care; paper makes distinction that 1 QALY today (after application of a 3.5% discount rate) is worth 0.25 QALYs in 40 years’ time – i.e. health gains are reduced fourfold |
| Feenstra19 | |
| (a) Model structure | Aim: estimate CEA of five face-to-face smoking-cessation interventions Model: dynamic simulation model Time horizon: 75 years Viewpoint: Dutch (third-party payer) health care Viewpoint justification: to support Dutch smoking-cessation guidelines Alternatives: face-to-face smoking-cessation interventions in community and primary care Study population: Dutch population (smokers, non-smokers, former smokers) Evaluation method: CEA, CUA |
| (b) Data inputs – effectiveness data | Method used to estimate effectiveness: detailed and complex demographic and epidemiologic data. In terms of smoking cessation rates, 12-month continuous abstinence rates (dynamic) converted to risk ratios for current and former smokers Valuation of health benefits: Dutch quality of life weights from published sources for smoking-related diseases Indirect benefits: not considered |
| (b) Data inputs – resource use | Unit cost data: Dutch sources Resources reported separately from cost: yes, intervention Currency (exchange rate): € Price year (adjustment for inflation): 2000 |
| (c) Consideration of uncertainty/consistency | Key parameters varied in sensitivity analysis: national population statistics and disease incidence Discount rate: 4% Uncertainty: univariate analyses including varying the: discount rate, time horizon, resource usage and cessation rate |
| (d) Presentation of CEA findings | Outcomes: Probability of lifetime quit rate: NR as this was a dynamic model Total cost: costs presented are for the entire population over time, so difficult to interpret. Costs per life-year gained are presented and in the region of 1400–6800 dependent on intervention and period of implementation ICER: €1100–4900 for telephone and intensive counselling Authors’ conclusion: all interventions considered were cost-effective compared with current practice and minimal GP counselling was cost saving |
| Fiscella and Franks12 (US) | |
| (a) Model structure | Aim: to assess the cost-effectiveness of nicotine patch Model: decision-analytic model (calculated analytically) Time horizon: lifetime Viewpoint: US health care payer Viewpoint justification: to inform payer decision re reimbursement Alternatives: physician counselling alone Study population: primary care population of smokers Evaluation method: cost-effectiveness analysis |
| (b) Data inputs – effectiveness data | Effectiveness data: quit rate from brief counselling. Quit odds rate from within trial for nicotine patch Method used to estimate effectiveness: quit odds ratio for the relative success of NRT active/placebo (from within trial). Acceptance rate of the patch was considered Lifetime abstinence estimated by assumption (35%) Valuation of health benefits: years of healthy life measure (YOLS) Indirect benefits: discussed, not included in analysis |
| (b) Data inputs – resource use | Resource use: therapy and clinicians’ time, OTC treatments resulted in zero cost to NHS as defrayed to patient Unit cost data: yes Resources reported separately from cost: yes Currency (exchange rate): US$ Price year (adjustment for inflation): 1995 US$ |
| (c) Consideration of uncertainty/consistency | Discount rate: 3.5% costs and QALYs Uncertainty: series of one-way sensitivity analyses. PSA using the normal distribution as candidate for 10,000 trials |
| (d) Presentation of CEA findings | Outcome of model: cost per quitter/cost per QALYS Probability of lifetime quit rate: 35% Total cost: $7332 per additional quitter (base case) ICER: cost-effective at $2422–14,112 per QALY from (PSA) |
| HECOS/Orme et al.20 | |
| (a) Model structure | Aim: to estimate the health and economic outcomes associated with smoking and the benefits of smoking cessation Model: cohort-level Markov (complex) developed analytically via application of difference equations Time horizon: lifetime Viewpoint: UK NHS Viewpoint justification: authors were tasked with creating a tool for use by health care payers, government policy makers and health-care organisations Alternatives: pharmacological, GP advice, group therapy Study population: hypothetical UK population Evaluation method: cost-effectiveness analysis |
| (b) Data inputs – effectiveness data | Effectiveness data: populated with UK data. Details restricted to online appendices Method used to estimate effectiveness: construction of an epidemiological model. Reporting not transparent due to complexity of model and aim of paper Lifetime abstinence estimated by assumption that a fixed percentage relapse per year Valuation of health benefits: via consideration of change in health status to morbid states and death. Life-years not adjusted for quality Indirect benefits: included |
| (b) Data inputs – Resource use | Unit cost data: yes Resource use: Not itemised, total cost only presented by cessation strategy Resources reported separately from cost: no – relied on secondary source Currency (exchange rate): £ Price year (adjustment for inflation): 1999 |
| (c) Consideration of uncertainty/consistency | Discount rate (varied): 6% (0–10%) Uncertainty: extensive deterministic sensitivity analysis around base-case assumptions of the model |
| (d) Presentation of CEA findings | Outcome of model: cost per life-year saved/cost per death averted Probability of lifetime quit rate: 30% Total cost: intervention costs reported by strategy per participant (secondary source) ICER: £1200 per life-year saved. £22,000 per death averted |
Data used in probabilistic analysis
| Parameters | Point estimates (base case) | SEa | Candidate distribution |
|---|---|---|---|
| Quit rate control (proportion starting as ex-smoker) | 0.04 | 0.20 | Beta |
| Quit rate comparator (proportion starting as ex-smoker) | 0.14 | 0.05 | Beta |
| Intervention cost for EARS (total) | £192 | 19.61 | Gamma |
| Smoking-related disease (cost estimate) | £27,120 | £2767.40 | Gamma |
| Relapse rate in year 1– (post intervention) | 0.28 | 0.03 | Beta |
| Hazard rate (annual) based on Yudkin et al. | 0.0874 | 0.0089 | Beta |
| Health state values/utilities (by age and sex cohort) | See table 10 in chapter 4 | Beta | |
| Mortality rates (by age and sex cohort) | See table 2 below | Beta | |
| Adjusted mortality rates (SE) | ||||
|---|---|---|---|---|
| Age (years) | Current | Former smoker by age stopped | ||
| < 10 years | < 20 years | ≥ 20 years | ||
| Males | ||||
| 35–44 | 0.001302 (0.0434) | 0.000964 (0.0001) | – | – |
| 45–54 | 0.002479 (0.0001) | 0.001575 (0.0002) | – | – |
| 55–64 | 0.006684 (0.0003) | 0.005123 (0.0005) | 0.002811 (0.0003) | – |
| 65–74 | 0.018818 (0.0007) | 0.013510 (0.0015) | 0.011766 (0.0013) | 0.008425 (0.0010) |
| 75–84 | 0.045752 (0.0056) | 0.032571 (0.0040) | 0.032202 (0.0039) | 0.028202 (0.0034) |
| Females | ||||
| 35–44 | 0.000987 (0.0434) | 0.000731 (0.0047) | – | – |
| 45–54 | 0.002460 (0.0001) | 0.001563 (0.0001) | – | – |
| 55–64 | 0.006878 (0.0003) | 0.005271 (0.0002) | 0.002892 (0.0003) | – |
| 65–74 | 0.021015 (0.0007) | 0.015087 (0.005) | 0.013139 (0.0012) | 0.009409 (0.0009) |
| 75–84 | 0.054728 (0.0019) | 0.038961 (0.0014) | 0.038520 (0.0033) | 0.033735 (0.0029) |
Other results/cost-effectiveness analyses/analyses
FIGURE 1.
Cost-effectiveness plane for 60-year-old male, base-case CEA. Scatterplot of costs and effects.
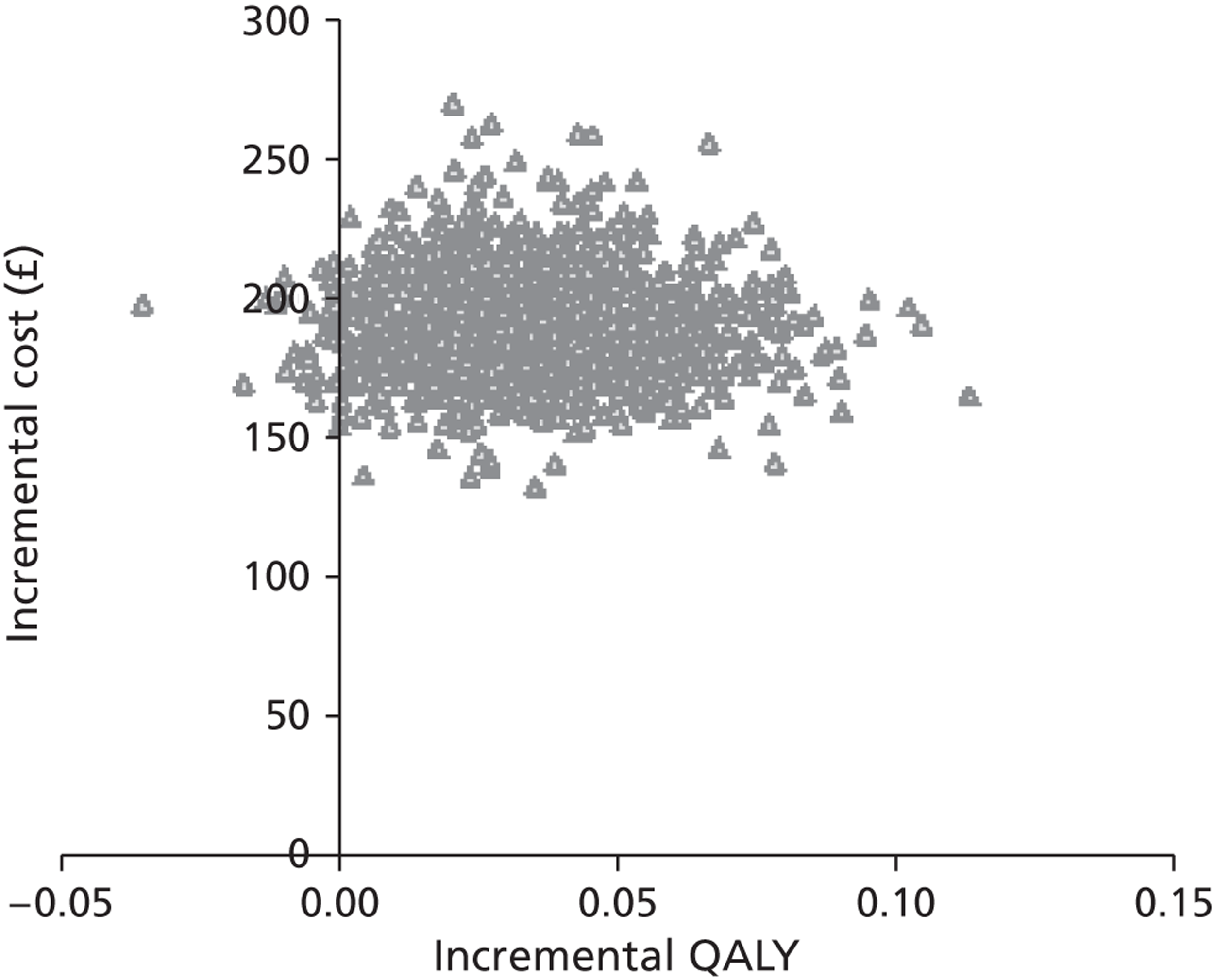
FIGURE 2.
Cost-effectiveness acceptability curve for 60-year-old male, base-case CEA.
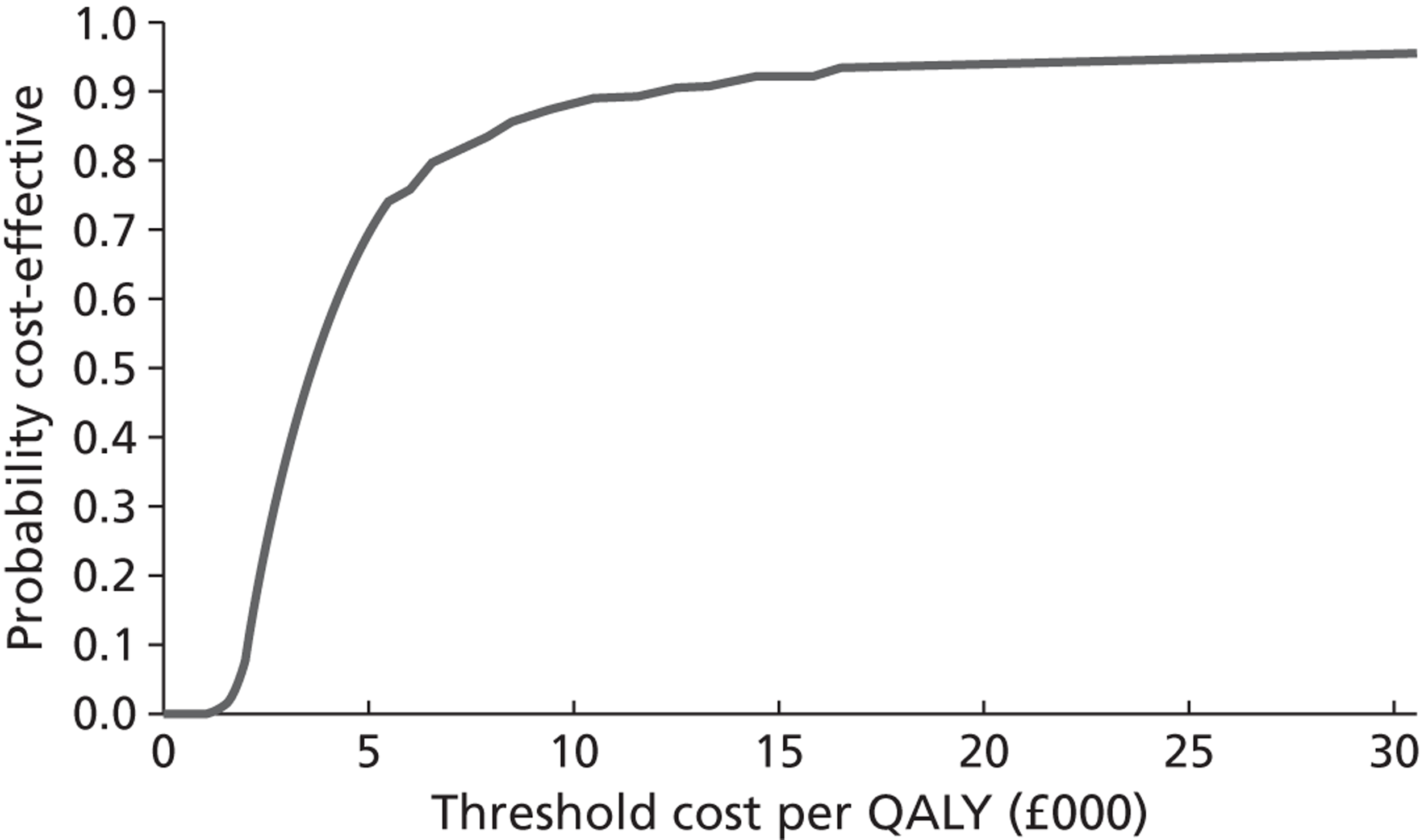
FIGURE 3.
Cost-effectiveness acceptability curve for 40-year-old female, base-case CEA.
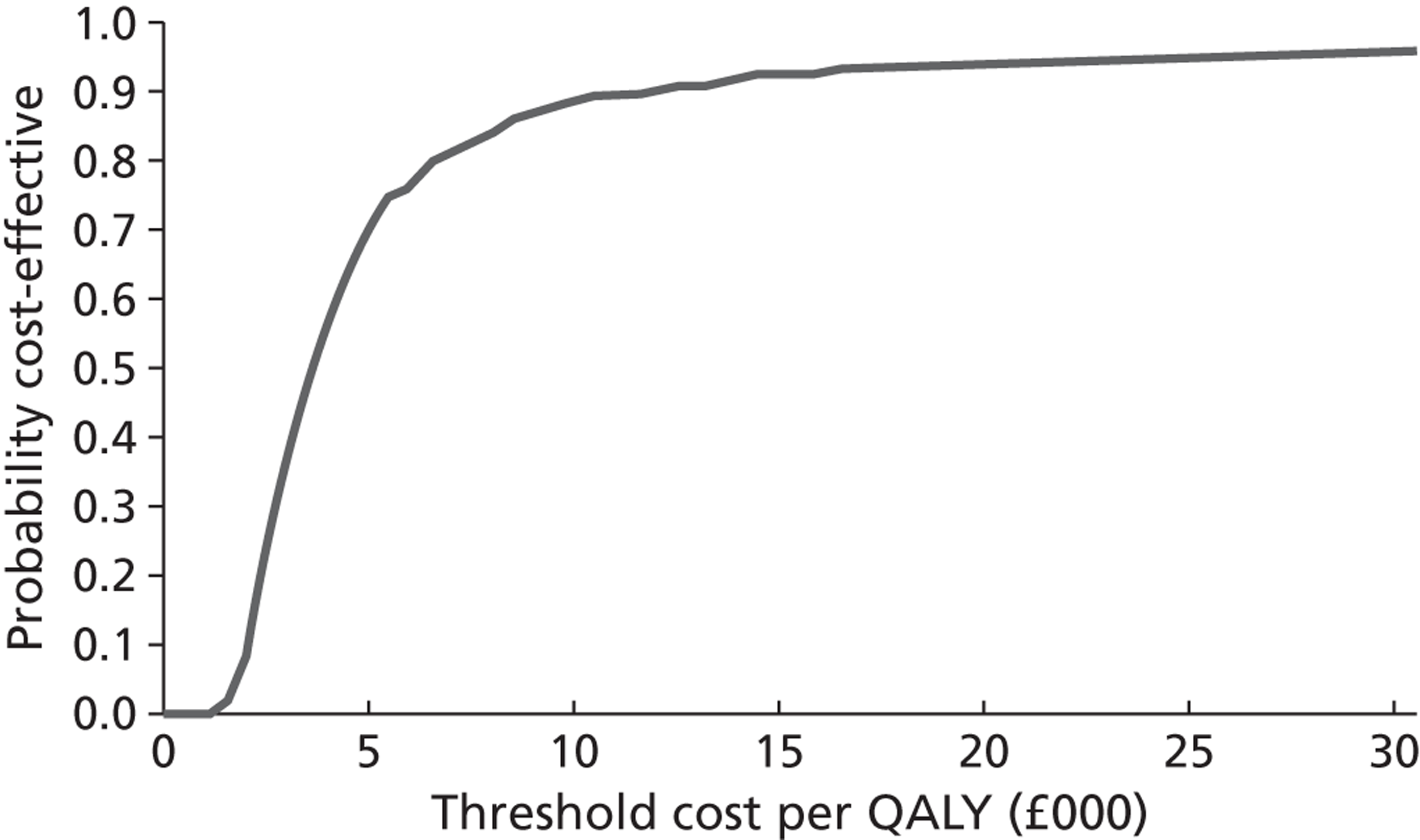
FIGURE 4.
Cost-effectiveness acceptability curve for 50-year-old female, base-case CEA.
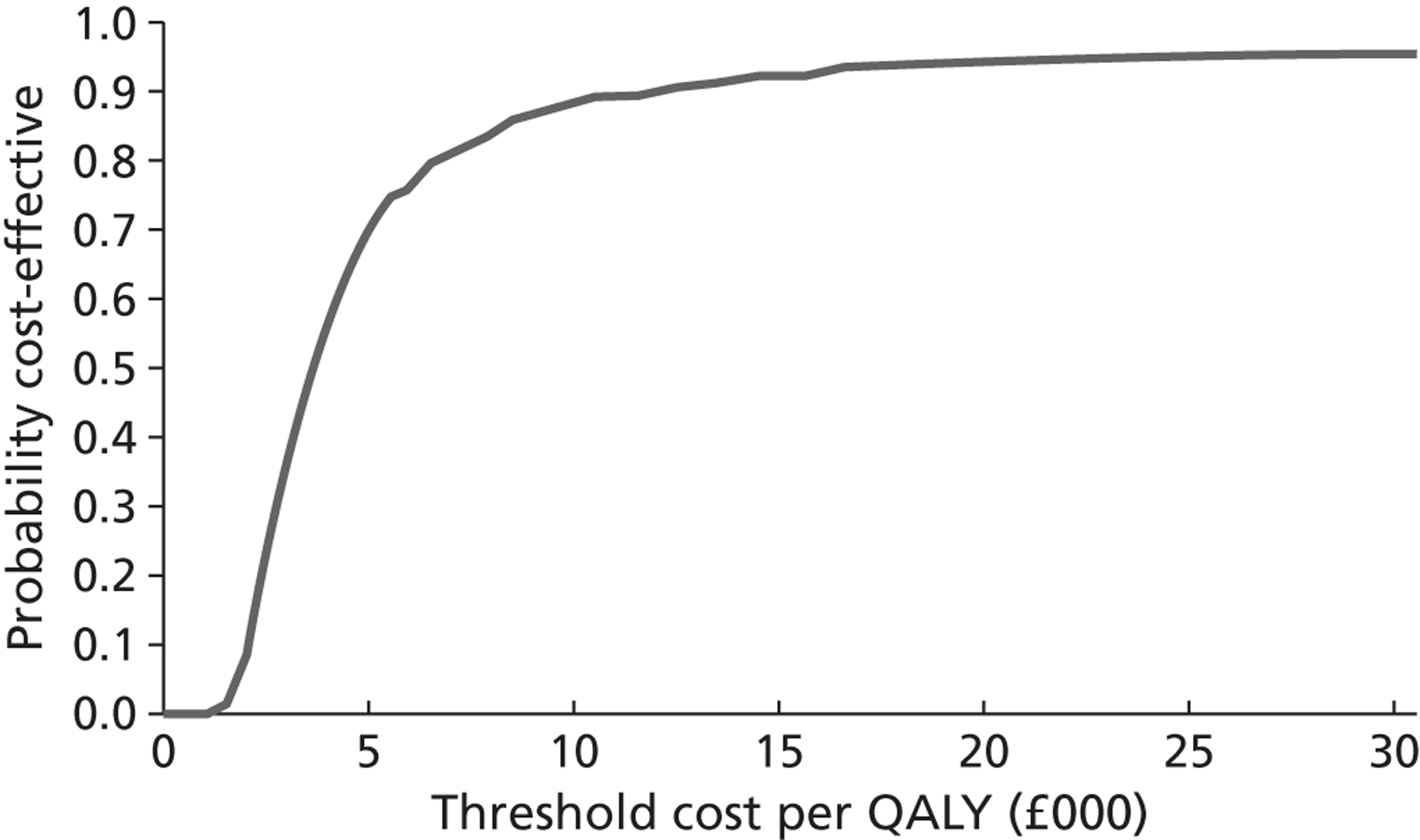
FIGURE 5.
Cost-effectiveness acceptability curve for 60-year-old female, base-case CEA.
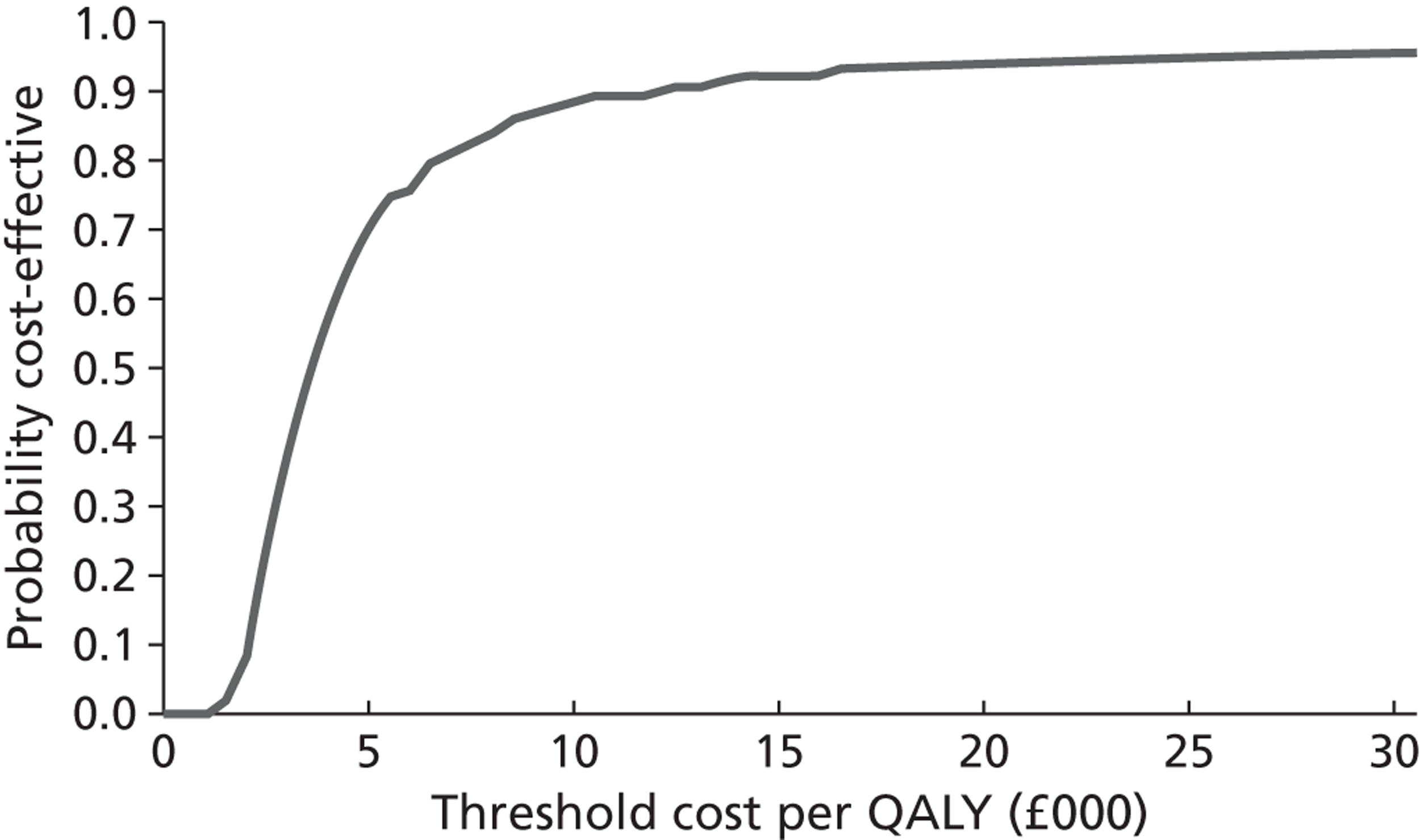
References
- Carr SM, Lhussier M, Forster N, Geddes L, Deane K, Pennington M, et al. An evidence synthesis of qualitative and quantitative research on component intervention techniques, effectiveness, cost-effectiveness, equity and acceptability of different versions of health-related lifestyle advisor role in improving health. Health Technol Assess 2011;15.
- Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technol Assess 2008;12.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU, University of Kent; 2011.
- NICE . Brief Interventions and Referral for Smoking Cessation: Economic Modelling Report 2008.
- Yudkin P, Hey K, Roberts S, Welch S, Murphy M, Walton R. Abstinence from smoking eight years after participation in randomised controlled trial of nicotine patch. BMJ 2003;327:28-9.
- Curtis L, Netten A. Unit Costs of Health and Social Care. Canterbury: PSSRU, University of Kent; 2005.
- Godfrey C, Parrott S, Coleman T, Pound E. The cost-effectiveness of the English smoking treatment services: evidence from practice. Addiction 2005;100:70-83.
- Bauld L, Boyd KA, Briggs AH, Chesterman J, Ferguson J, Judge K, et al. One-year outcomes and a cost-effectiveness analysis for smokers accessing group-based and pharmacy-led cessation services. Nicotine Tob Res 2011;13:135-45.
- Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom. JAMA 2000;284:706-12.
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583-637.
- Howard P, Knight C, Boler A, Baker C. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO Simulation model: application to a population of US adult smokers. Pharmacoeconomics 2008;26:497-511.
- Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling. JAMA 1996;275:1247-51.
- Hurley SF, Matthews JP. The Quit Benefits Model: a Markov model for assessing the health benefits and health care cost savings of quitting smoking. Cost Eff Resour Alloc 2007;5.
- Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al. The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a sytmeatic review and economic evaluation. Health Technol Assess 2002;6.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328.
- Coleman T, Agboola S, Leonardi-Bee J, Taylor M, McEwen A, McNeill A. Relapse prevention in UK Stop Smoking Services: current practice, systematic reviews of effectiveness and cost-effectiveness analysis. Health Technol Assess 2010;14.
- Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ 1994;309:901-11.
- Tillmann M, Silcock J. A comparison of smokers’ and ex-smokers’ health-related quality of life. J Public Health Med 1997;19:268-73.
- Feenstra TL, Hamberg-van Reenen HH, Hoogenveen RT, Rutten-van Molken MP. Cost-effectiveness of face-to-face smoking cessation interventions: a dynamic modeling study. Value Health 2005;8:178-90.
- Orme ME, Hogue SL, Kennedy LM, Paine AC, Godfrey C. Development of the health and economic consequences of smoking interactive model. Tob Control 2001;10:55-61.
- Vogl M, Wenig CM, Leidl R, Pokhrel S. Smoking and health-related quality of life in English general population: implications for economic evaluations. BMC Public Health 2012;12.
List of abbreviations
- BCT
- behaviour change technique
- BENESCO
- Benefits of Smoking Cessation on Outcomes
- BMI
- body mass index
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CHD
- coronary heart disease
- CI
- confidence interval
- CO
- carbon monoxide
- COPD
- chronic obstructive pulmonary disease
- EARS
- Exercise Assisted Reduction then Stop
- EQ-5D
- European Quality of Life-5 Dimensions
- fte
- full-time equivalent
- FTND
- Fagerström Test for Nicotine Dependence
- GP
- general practitioner
- HECOS
- Health and Economic Consequences of Smoking
- HT
- health trainer
- HTA
- Health Technology Assessment
- IAPT
- Improving Access to Psychological Therapies
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ISRCTN
- International Standard Randomised Controlled Trial Register
- mCEQ
- modified Cigarette Evaluation Questionnaire
- MI
- motivational interviewing
- MPSS
- Mood and Physical Symptoms Scale
- MVPA
- moderate and vigorous physical activity
- NICE
- National Institute of Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRT
- nicotine replacement therapy
- OR
- odds ratio
- PA
- physical activity
- p.p.m.
- parts per million
- PSS
- Perceived Stress Scale
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SD
- standard deviation
- SDT
- self-determination theory
- SMART
- Specific, Measurable, Attainable, Realistic, Time-referenced
- SSS
- Stop Smoking Service
- WHO
- World Health Organization
