Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/124/01. The contractual start date was in December 2011. The draft report began editorial review in February 2013 and was accepted for publication in June 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Boonacker et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and introduction
Otitis media (OM) continues to be one of the leading causes of medical consultations and the most frequent reason for antibiotic prescription and surgery in children in high-income countries. 1 The surgical procedures offered to children with recurrent or persistent OM are (1) insertion of grommets (ventilation tubes), (2) adenoidectomy and (3) a combination of the two. Two clinical conditions, although distinctly defined, are in fact closely related and can overlap. In young children, acute otitis media (AOM) is one of the most common causes of illness. AOM is defined as the presence of fluid in the middle ear with signs and symptoms of an acute infection. 2 Although many children have occasional AOM, an important group of children suffer from recurrent episodes, defined here as three or more AOM episodes in 6 months or four or more episodes in 1 year. These recurrent AOM episodes cause considerable distress to children and their parents, through frequent episodes of acute ear pain, fever and general illness. Children and parents experience sleepless nights and time is lost from nursery or school and from work. 3,4
Otitis media with effusion (OME, ‘glue ear’) is defined as the presence of fluid in the middle ear behind an intact tympanic membrane without signs and symptoms of an acute infection. It is most common in young children, with a bimodal peak around 2 and 5 years of age. In total, 80% of children will have had a least one episode of OME by the age of 10 years. The main symptom of OME is impaired hearing because the middle ear effusion causes a conductive hearing loss. 2
Children with persistent OME are prone to recurrent AOM episodes, and after an AOM episode all children suffer from OME for some time. As such, children with recurrent AOM not only suffer pain and discomfort during the acute episodes, but also experience OME-related hearing difficulties that may impact on their language, behaviour and progress at school. 5 It is known that the impact of recurrent AOM on a child’s quality of life is equivalent to that of chronic conditions such as asthma. 6,7
Although clear National Institute for Health and Care Excellence (NICE) guidance2 is available for the use of grommets in subgroups of children with persistent OME, it is not the case for the use of adenoidectomy, either in persistent OME or in recurrent AOM. NICE suggests that clinicians should consider the possible benefits of adenoidectomy in children selected for grommets for OME who also suffer from coexisting respiratory symptoms. However, NICE2 recognises a need for further studies documenting the effect of adenoidectomy, either alone or as an adjuvant to grommet insertion, in the management of recurrent or persistent OM in children. In particular, NICE identified a need for studies to identify any subgroups who might benefit more or less than others from surgical intervention. We know that adenoidectomy or adjuvant adenoidectomy is routinely performed in many countries for recurrent or persistent OM, but the concern of NICE2 reflects the knowledge that the practice is not backed by high-quality scientific evidence.
The adenoid is an aggregate of lymphoid tissue located in the nasopharynx. With an extensive system of folds and crypts on its surface, the adenoid traps viruses and bacteria that pass through the upper airways. As part of the immune system, the adenoid plays an important role in the body’s immune response to infectious microorganisms that pass through the upper airways. Although many of these microorganisms may simply be transient passengers, the adenoid may serve as a reservoir for a diverse microbial community, resulting in upper respiratory infections. Because the adenoid lies next to the orifices of the Eustachian tubes, it has long been recognised as an important factor in the pathogenesis of OM. Microorganisms may spread via the Eustachian tube to the middle ear and cause acute, recurrent or chronic infections. Adenoidectomy is thought to improve middle ear function by removing or reducing the reservoir of opportunistic pathogens. 8 A number of trials have studied the effect of adenoidectomy alone or of grommets with adjuvant adenoidectomy in children with OM. 9–25 Differences in the study design, population characteristics, outcomes measured and duration of follow-up, and particularly the use of small sample sizes, have made it difficult to come to any definite conclusions about the effects of adenoidectomy. It is possible – indeed likely – that both over- and undertreatment occurs. An individual patient data (IPD) meta-analysis, that is, a meta-analysis of the original individual data from previous trials, offers a unique opportunity to identify subgroups that may be more or less likely to benefit from adenoidectomy than others. Members of our group have successfully applied the IPD meta-analysis methodology to evaluate the effectiveness of antibiotics in children with acute OM and grommets in OME in specific subgroups. 26,27
Aims and objectives
In this IPD meta-analysis we therefore (1) developed a model to predict the risk of children referred for adenoidectomy having a prolonged duration of their otitis media. Then, (2a) having evaluated the overall effect of adenoidectomy, with or without grommets, on OM using these IPD, we (2b) identified those subgroups of children who benefit most, or who are most likely to benefit, from adenoidectomy, with or without grommets.
Chapter 2 Methods of research
Protocol and registration
The original protocol for this IPD meta-analysis was published on the website of the Health Technology Assessment programme of the National Institute for Health Research (see www.hta.ac.uk/2576). The protocol is included in this report (see Appendix 1). The study was also registered on 13 September 2011 in the PROSPERO register with the number CRD42011001549 (see www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42011001549).
As in any IPD meta-analysis, small but potentially important changes to intentions have to be made on the basis of the specific data that are made available to the research team. In this regard, discussion with the principal investigators and/or study representatives included in the IPD meta-analysis took place at two meetings (Amsterdam, the Netherlands, 21 May 2012, and Oxford, UK, 3 September 2012). This led to the decision to promote to the primary outcome a composite outcome measure that was already included in the protocol as a secondary outcome, because the primary outcome originally proposed could not be determined in a significant proportion of studies, severely limiting the value of any ensuing meta-analysis. Specifically, number of OM episodes was replaced by failure at 12 months. By using such a composite end point we were able to aggregate our data and generalise results. This was critical to accomplish our aim of establishing which children benefit more or less from adenoidectomy.
An overview of the adaptations to the study protocol and the reasons for them can be found in Appendix 2.
Selection of the trials and quality assessment
For this IPD meta-analysis the same search strategy was used as in our Cochrane review. 28 We searched the following databases from their inception: the Cochrane Ear, Nose and Throat Disorders Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 1, 2009), PubMed, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Latin American and Caribbean Health Sciences Literature (LILACS), KoreaMed, IndMED, PakMediNet, CAB Abstracts, Web of Science, BIOSIS Previews, China National Knowledge Infrastructure (CNKI), the metaRegister of Current Controlled Trials (mRCT), ClinicalTrials.gov, the International Clinical Trials Registry Platform (ICTRP), ClinicalStudyResults.org and Google. We used the following keywords with their synonyms: ‘adenoidectomy’ and ‘otitis’ (see Appendix 3 for the complete search strategy). We checked the bibliography of all relevant studies and reviews to identify supplemental studies. Unpublished studies were also eligible for inclusion. We imposed no language restriction on the searches. The date of the last search for the Cochrane review was 30 March 2009. Thereafter, we received weekly updates from PubMed and performed a full updated search on 7 June 2012 (including CENTRAL, The Cochrane Library Issue 5, 2012).
The final selection of eligible studies and the quality assessment were carried out by two of the authors of the Cochrane review (CB and MvdA) and disagreement was resolved by discussion.
For the quality assessment we used The Cochrane Collaboration quality assessment (risk of bias) tool,29 which requires a judgement on sequence generation, allocation concealment (whether or not assignment to the intervention or control group could be foreseen by the participants or the investigators), blinding, incomplete outcome data, selective outcome reporting and evaluation of other possible biases. The judgement on the risk of bias for this IPD meta-analysis may differ from the judgement in the Cochrane review because for this IPD meta-analysis we obtained the original raw data sets from the primary investigators of all of the included trials and we did not have to rely on the published information alone.
We checked and reanalysed all data sets to see if it was possible to recalculate the published results. When needed, we contacted the principal investigators to resolve any questions. The two data sets that were provided in Finnish16,17 were translated into English by one of the project members (CB). The dictionary developed during the translation of the first data set16 was approved by the principal investigator and was then used to translate the second dataset. 17 After checking the quality of the data sets we developed one overall data set by recoding the variables from the individual data sets to the set of variables used for the IPD meta-analysis. For season, we used country-specific seasons, that is, June could be either spring or summer, depending on the country in which the study was performed. We used date of surgery to calculate the season and winter/spring was used in the analyses as the measure for seasonality. For children in the non-surgical group we used the date of enrolment.
Types of studies and patients
Studies were eligible for inclusion in this IPD meta-analysis if they were a randomised controlled trial (RCT) in children up to 12 years of age diagnosed with OM (being recurrent AOM and/or persistent OME) in which adenoidectomy (with or without grommets) was compared with non-surgical treatment or grommets. Trials in which all children underwent adenoidectomy were therefore not eligible. We included trials in which the method of randomisation was not specified in detail, but we excluded quasi-randomised trials (e.g. allocation by date of birth or record number). Studies had to have a follow-up period of at least 1 year. Desirable time points for outcome assessment were 6, 12, 18 and 24 months.
Types of interventions
Interventions
Four interventions or intervention combinations were evaluated:
-
adenoidectomy alone
-
adenoidectomy with myringotomy
-
adenoidectomy with unilateral grommet
-
adenoidectomy with bilateral grommets.
However, these were grouped together in two intervention ‘bundles’:
-
adenoidectomy with unilateral or bilateral grommets
-
adenoidectomy with or without myringotomy.
Comparator
The comparators evaluated were:
-
unilateral or bilateral grommets
-
non-surgical treatment or myringotomy alone.
To evaluate the effects of adenoidectomy we compared the following interventions:
-
main comparison (Figure 1a):
-
adenoidectomy with or without grommets compared with non-surgical treatment or grommets only (this comparison was selected on the basis of prior knowledge of the available data sets included in our conventional meta-analysis and was chosen to maximise the statistical power to identify subgroups).
-
-
other comparisons (Figure 1b):
-
adenoidectomy with unilateral or bilateral grommets compared with non-surgical treatment
-
adenoidectomy with unilateral or bilateral grommets compared with unilateral or bilateral grommets
-
adenoidectomy without grommets compared with non-surgical treatment
-
adenoidectomy without grommets compared with unilateral or bilateral grommets.
-
FIGURE 1.
(a) Main comparison: adenoidectomy with or without grommets compared with non-surgical treatment or grommets only; and (b) other comparisons.
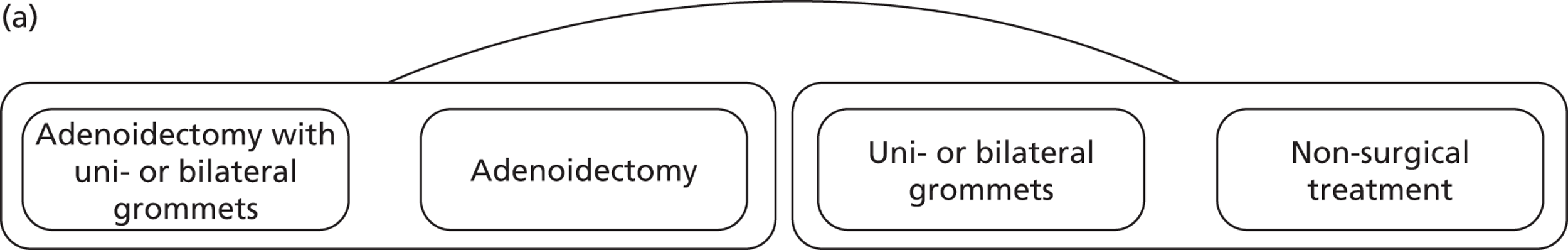
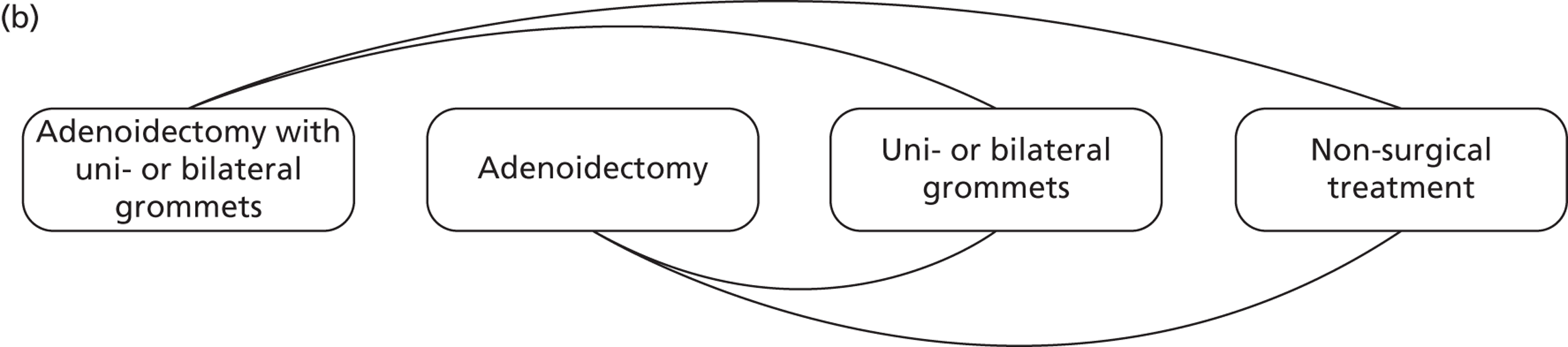
We did not compare the following interventions as these analyses do not fulfil the criteria for inclusion in this IPD meta-analysis:
-
adenoidectomy with unilateral or bilateral grommets compared with adenoidectomy without grommets
-
unilateral or bilateral grommets compared with non-surgical treatment.
Types of outcome measures
Primary outcome
The primary outcome is failure at 12 months, which was defined as one or more of the following components:
-
four or more AOM episodes (including episodes of otorrhoea) per year
-
presence of effusion for ≥ 50% of the time
-
need for additional surgery
-
hearing* improved by < 10 dB.
*Hearing was expressed as a mean air conduction hearing level measured by age-appropriate audiometry (if possible averaged over 500, 1000, 2000 and 4000 Hz). In all children the binaural average was taken. This includes trials that used unilateral grommets and randomised ears rather than children.
Not all components were recorded in each study and the number of children failing on each of these components by treatment group and study varied (see Appendix 10).
In the original protocol this composite outcome was included as a secondary outcome. The primary outcome was number of OM episodes. When we were analysing the available data sets we realised that data on the original primary outcome were incomplete as the definition and evaluation of outcomes varied considerably across the trials. We followed the recommended conventions for IPD meta-analysis and, rather than presenting an ‘empty’ review of little utility, we sought to develop – through consensus with clinicians, including the original triallists – a composite primary outcome that allowed inclusion of all of the trials, the aim being to make maximum use of the available information to develop a robust answer to the clinical questions posed. We emphasise to readers who may be unfamiliar with this specific type of secondary research that redefining a primary outcome in this way is not unusual and not the same as changing outcomes in primary research.
Secondary outcomes
-
All individual items of failure at 12 months:
-
four or more AOM episodes (including episodes of otorrhoea) per year
-
presence of effusion for ≥ 50% of the time (i.e. effusion for > 6 months)
-
need for additional surgery
-
hearing improved by < 10 dB.
-
-
Number of episodes of AOM during follow-up – in the first 6, 12, 18 and 24 months.
-
Time with effusion during follow-up measured in number of weeks – in the first 6, 12, 18 and 24 months.
-
Additional surgery during follow-up – in the first 6, 18 and 24 months.
-
Average hearing loss measured in dBHL (hearing level in decibels as assessed on an audiometer) – after 6, 12, 18 and 24 months’ follow-up.
-
Improvement in hearing level < 10 dB – after 6, 18 and 24 months’ follow-up.
-
Adverse effects and events (including morbidity of surgery).
Statistical analysis
1. Development of a model to predict the risk of children referred for adenoidectomy having a prolonged duration of their otitis media
To address this objective we examined the association between each potential predictor (subgrouping variable) and the primary outcome of failure at 12 months by univariate logistic regression analysis. The a priori-defined potential predictors were based on clinical reasoning, knowledge of the literature (including the analysed studies) and discussion with experts. They are:
-
age
-
sex
-
season (winter vs. summer)
-
day-care attendance
-
passive smoking
-
breastfeeding
-
pneumococcal vaccination status
-
atopy or allergy
-
number of previous episodes of upper respiratory tract infection
-
number of previous episodes of AOM
-
occurrence of one or more episodes of AOM in the previous year
-
age at onset
-
duration of symptoms
-
middle ear impedance (tympanometric findings)
-
baseline hearing level
-
indication for surgery (recurrent AOM or persistent OME)
-
presence of OME at study entry.
Only patients in the non-surgical group of the available RCTs were selected for the prognostic study, that is, children referred for adenoidectomy but randomised to the non-surgical group. This is appropriate because adenoidectomy with or without grommets might influence the course of the disease and might result in an invalid natural history model. Predictors that were univariately associated with the outcome (p < 0.10) were included in multivariate logistic regression analyses. The models were compacted by excluding predictors from the model with a p-value of > 0.05. The ability of the models to discriminate between children who will or will not experience failure at 12 months was estimated by the area under the receiver operating curve (ROC). The ROC area is a suitable parameter to summarise discriminative or predictive values and can range from 0.5 (no discrimination, like a coin flip) to 1.0 (perfect discrimination). In addition, we calculated the absolute risks of failure at 12 months using the predictors that were identified in the multivariate analyses and combinations of those predictors.
We also performed two sensitivity analyses. In the first we sought to determine whether the indication for adenoidectomy (recurrent AOM or persistent OME) resulted in different prognostic factors from those that were found for the total group. In the second sensitivity analysis we used an alternative set of definitions of failure at 12 months as the outcome.
2a. Evaluation of the overall effect of adenoidectomy, with or without grommets, on otitis media using these individual patient data
We quantified the overall effect of the interventions for all comparisons listed above (see also Figure 1a and b). We used Review Manager (RevMan) version 5.2 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) to create forest plots presenting both rate differences (RDs) and rate ratios (RRs) for the main effects. We also calculated RRs, RDs, number needed to treat (NNT) and 95% confidence intervals (CIs) for each of these using regression analysis.
We were unable to calculate adjusted RDs because of the calculation artefact, which occurs when there are zero events in a study (in this case that of Nguyen et al. 21). Therefore, we calculated unadjusted RDs with a binominal model using an identity link:
To adjust for a potential study effect we also performed a Poisson regression analysis with robust standard errors, which enabled us to calculate an adjusted RR:
For continuous outcomes we calculated adjusted mean differences:
2b. Identification of those subgroups of children who benefit most, or who are most likely to benefit, from adenoidectomy with or without grommets
To assess whether the effect of the intervention under study was modified by the identified prognostic factors, we performed an analysis using a binominal model with an identity link, including an interaction effect:
To adjust for study and the subgrouping variable(s) we also performed a Poisson regression analysis with robust standard errors including both interaction terms and potential confounders (i.e. study and age) to calculate adjusted RRs and their 95% CIs.
We used the Wald statistic from the adjusted Poisson regression to study the significance of the interaction term:
For continuous outcomes we calculated an adjusted mean difference:
We will present subgroup effects only for those variables that showed a significant interaction effect (p ≤ 0.1) and for subgroups with sufficient numbers (n > 15) to present stratified results. We used our clinical experience and reasoning and decided a priori to include age and indication as potential subgroup variables. Furthermore, we decided to examine subgroup effects in the two distinct populations defined by the indication for surgery – recurrent AOM compared with persistent OME.
As many of the subgroup comparisons are between studies rather than within study, we will not present forest plots for the subgrouping variables but tables with the RDs, RRs and adjusted RRs from the regression analyses.
We decided not to impute missing data because of the low number of missing data points within the included trials. Furthermore, we decided not to impute data across trials in situations in which a variable was completely missing from a trial. We found in an earlier study30 that imputation of missing data across trials might lead to bias, as association of covariates might differ across the included studies.
In addition to the intention-to-treat analysis, we also performed three sensitivity analyses to assess the robustness of our findings. In the first we performed a per-protocol analysis in which we excluded the children who did not follow the protocol to which they were randomised. The second was an as-treated analysis in which we analysed the children according to the treatment that they received. In the third we used an alternative set of definitions of failure at 12 months as the outcome. We performed all sensitivity analyses only for the primary outcome of the main comparison.
All analyses were performed with IBM SPSS Statistics version 20.0 (IBM Corp., Armonk, NY, USA) and Rothman’s Episheet (11 June 2008; Spreadsheets for the Analysis of Epidemiologic Data, see http://krothman.hostbyet2.com/Episheet.xls).
Medical ethical approval
The study team discussed the project with the Medical Ethical Committee of the University Medical Center Utrecht. The committee confirmed that ethical approval was not required as the study uses only anonymous data from previously performed studies for which both informed consent and ethical approval had already been obtained.
Chapter 3 Results
Searches
Our searches (2009 and 2012) retrieved a total of 503 articles (Figure 2). We first screened the articles by title/abstract and excluded five trials in which all children underwent adenoidectomy. 31–35 An additional 467 studies were excluded for other reasons, for example follow-up for <12 months. In total, 31 articles were eligible for further assessment. One study by Sagnelli et al. 36 could not be retrieved. We excluded three articles,37–39 describing two trials, because of inadequate randomisation or concealment of allocation. One article40 was excluded because it was found to report a non-RCT. Furthermore, we identified 11 of the publications9,41–50 as providing the same data as more recent articles included in this meta-analysis. Publications focused on adenotonsillectomy were also excluded except for those in which a separate analysis for adenoidectomy was undertaken. 19,22 No additional trials were identified from checking the bibliographies of the selected trials and reviews, nor by contacting the first or corresponding author of the eligible trials.
FIGURE 2.
Flow chart for adenoidectomy studies. n, number of publications (there could be more than one publication per study); k, number of studies.
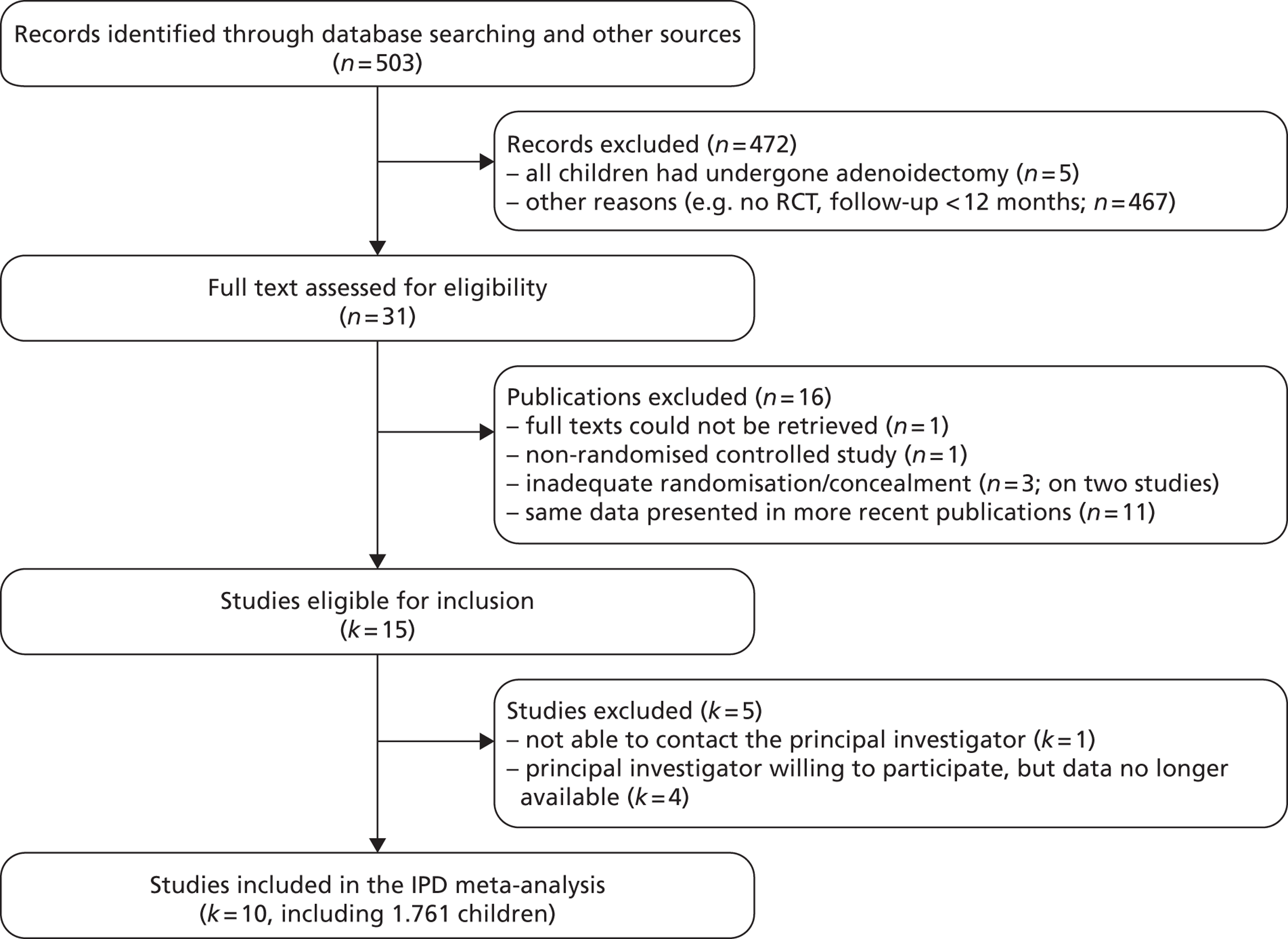
In total, 15 trials10–25,51,52 were eligible for inclusion in this IPD meta-analysis. We contacted the principal investigators of these trials to ask if they were willing and able to provide their raw data. We were unable to contact the principal investigator of one trial. 24 The data for four older trials (1978–99) were no longer available. 14,22,23,25 For one trial, additional follow-up data were available. 15,51 For another study, additional unpublished data were also provided. 20 Three older studies (1986–90) used a unilateral grommet,10,12,52 whereas the others used no grommets16 or bilateral grommets. 11,15,17,18,20,21
Characteristics of included studies
The characteristics of the included studies are shown in Table 1. The inclusion and exclusion criteria are shown in Appendix 5, the indication for surgery and the age ranges within studies are shown in Appendix 6 and the definitions of recurrent AOM and persistent OME used in the included studies are shown in Appendix 7.
| Study | No. of patients | Indication for surgery | Age (years) | Interventions | Randomisation strategy | Outcome measurements | Potential risk factors/subgroups | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Black 199052 | 149 | Persistent OME | 4–9 | (1) Unilateral grommet, (2) adenoidectomy + unilateral grommets Type of grommets: standard Shepard |
By ear | Audiometry, tympanometry, otoscopy | Age, sex, indication, season, number of previous episodes of URTI (including OM), number of previous episodes of AOM, duration of symptoms, middle ear impedance, OME present, baseline hearing level, type of grommets | 7 weeks, 6, 12 and 24 months |
| Casselbrant 200911 | 98 | Persistent OME | 2–3.9 | (1) Bilateral grommets, (2) adenoidectomy, (3) adenoidectomy + bilateral grommets Type of grommets: Teflon Armstrong |
By child | Audiometry, tympanometry, otoscopy | Age, sex, indication, siblings, season, day-care, smoking, atopy/allergy, number of previous episodes of URTI (including OM), number of previous episodes of AOM, duration of symptoms, age at onset, middle ear impedance, OME present, baseline hearing level, type of grommets | Monthly, up to 36 months; extra visits when sick |
| Dempster 199312 | 72 | Persistent OME | 3–12 | (1) Unilateral grommet, (2) adenoidectomy + unilateral grommet Type of grommet: Shah grommet |
By ear | Audiometry, tympanometry, otoscopy | Age, sex, indication, middle ear impedance, OME present, baseline hearing level, type of grommets | 6 and 12 months |
| Hammarén-Malmi 200515,51 | 217 | Both persistent OME and/or recurrent AOM | 1–4 | (1) Bilateral grommets, (2) adenoidectomy + bilateral grommets Type of grommets: Donaldson silicon |
By child | Otoscopy | Age, sex, indication, siblings, season, day-care, smoking, atopy/allergy, number of previous episodes of URTI (including OM), number of previous episodes of AOM, OME present, type of grommets | 12, 24 and 36 months |
| Koivunen 200416 | 180 | Recurrent AOM | 10 months to 2 years | (1) No surgery, (2) adenoidectomy | By child | Otoscopy | Age, sex, indication, day care, breastfeeding, smoking, PCV, atopy/allergy, number of previous episodes of URTI (including OM), number of previous episodes of AOM, age at onset, OME present | Up to 2 years with diaries |
| Kujala 201217 | 300 | Recurrent AOM | 10 months to 2 years | (1) No surgery, (2) bilateral grommets, (3) adenoidectomy + bilateral grommets Type of grommets: Donaldson silicone tubes |
By child | Otoscopy, tympanometry | Age, sex, indication, siblings, season, day-care, breastfeeding, smoking, atopy/allergy, number of previous episodes of URTI (including OM), number of previous episodes of AOM, age at onset, OME present, type of grommets | Every 4 months with diary and visits when sick |
| Mattila 200318 | 137 | Recurrent AOM | 1–2 | (1) Bilateral grommets, (2) adenoidectomy + bilateral grommets Type of grommets: Donaldson silicon |
By child | Otoscopy | Age, sex, indication, siblings, season, day-care, breastfeeding, smoking, PCV, OM, type of grommets | Up to the age of 24 months |
| Maw 1986,19 199310 | 228a | Persistent OME | 2–9 | (1) Unilateral grommet, (2) adenoidectomy + unilateral grommet, (3) adenotonsillectomy + unilateral grommeta Type of grommet: Xomed Shepard |
By ear | Audiometry, tympanometry, otoscopy | Age, sex, indication, siblings, season, breastfeeding, smoking, atopy/allergy, number of previous episodes of URTI (including OM), number of previous episodes of AOM, duration of symptoms, age at onset, OME present, baseline hearing level, type of grommets | 6, 12, 18 and 24 months, 3–10 years |
| MRC Multicentre Otitis Media Study Group 201220 | 376 | Persistent OME | 3.5–7 | (1) No surgery, (2) bilateral grommets, (3) adenoidectomy + bilateral grommets Type of grommets: Shepard |
By child | Audiometry, tympanometry, otoscopy, symptom scores, behaviour, child and parent quality of life | Age, sex, indication, siblings, season, day-care, breastfeeding, smoking, PCV, atopy/allergy, number of previous episodes of URTI (including OM), number of previous episodes of AOM, duration of symptoms, age at onset, middle ear impedance, OME present, baseline hearing level, type of grommets | 3, 6, 12, 18 and 24 months |
| Nguyen 200421 | 72 | Both persistent OME and/or recurrent AOM | 18 months to 18 years | (1) Bilateral grommets, (2) adenoidectomy + bilateral grommets Type of grommets: Reuter Bobbin without holes |
By child | Otoscopy | Age, sex, indication, OME present, type of grommets | 1 month, 6-week intervals up to 36 months |
We included raw data from 10 trials,10–12,15–21,51,52 including 1761 children. The trials were published between 1990 and 2012. Eight trials were undertaken in Europe (four in Finland15–18 and four in the UK10,12,20,52), one in the USA11 and one in Canada. 21
Specific differences between included studies
The 10 trials differed in a number of ways but the most important were in terms of indication for surgery, interventions studied and frequency of outcome assessment.
Indications
Interventions
For analysis we divided the trials into two groups based on the surgical interventions being ‘bundled’ together as described earlier:
Methodological quality of the included studies
The results of the risk of bias assessment are presented in Table 2. We classified the risk of selection bias because of inadequate sequence generation as low for eight10–12,15–17,20,52 out of 10 studies and unclear in two studies. 18,21 We classified the risk of selection bias because of inadequate allocation concealment as unclear in three studies11,18,21 and as low in the remaining seven studies. 10,12,15–17,20,52 The risk of performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment) was unclear in seven studies10,11,15–18,21 and six studies11,15–18,21 respectively. Incomplete outcome data may have caused attrition bias in five studies10,12,16,20,52 and is likely to have caused bias in two studies. 18,21 As the full data sets were provided, the risk of reporting bias because of selective reporting is low in all studies. We classified one study21 as having a high risk of other bias. In this study the randomisation did not result in two similar groups. Age and degree of adenoid hypertrophy differed between the two groups. Furthermore, the follow-up data were obtained by questionnaires completed retrospectively at 6-monthly intervals. This could have led to recall bias. The risk of other bias was classified as unclear for five studies10–12,18,52,53 and low for the remaining four studies. 15–17,20
| Study | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other bias | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Black 199052 | L | L | L | L | U | L | U | L |
| Casselbrant 200911 | L | U | U | U | L | L | U | L |
| Dempster 199312 | L | L | L | L | U | L | U | L |
| Hammarén-Malmi 200515,51 | L | L | U | U | L | L | L | L |
| Koivunen 200416 | L | L | U | U | U | L | L | L |
| Kujala 201217 | L | L | U | U | L | L | L | L |
| Mattila 200318 | U | U | U | U | H | L | U | M |
| Maw 1986,19 199310 | L | L | U | L | U | L | U | L |
| MRC Multicentre Otitis Media Study Group 201220 | L | L | L | L | U | L | L | L |
| Nguyen 200421 | U | U | U | U | H | L | H | M |
Overall we concluded that eight studies10–12,15–17,20,52 were at a low risk of bias and two18,21 were at a moderate risk of bias.
Note that the judgement of the risk of bias for selective reporting differs from the judgement in the Cochrane review28 because in this IPD meta-analysis the complete data sets were obtained and we did not have to rely on the publications alone, resulting in low risk for selective reporting in all studies. Also, the judgement on blinding is now divided into blinding of participants and personnel, and blinding of outcome assessment.
Characteristics of participants at baseline
All participants
The characteristics of all 1761 children are recorded in the final column of Table 3. Important characteristics to note are:
-
Age. The overall proportion of children aged < 2 years is 43% and most of these children had recurrent AOM. Some studies included no children aged < 2 years. Also, note that 42% of children were aged > 4 years.
-
Indication. Just over half of the children (54.3%) were offered surgery for persistent OME.
-
Pneumococcal vaccination. Most children (78.9%) were in the ‘not vaccinated’ group because the trials were performed in the era before the introduction of the vaccination.
-
Hearing loss. The average hearing loss was 31.9 dBHL (range 13.1–65 dBHL) but this average is based on 590 participants as hearing loss was not measured in all studies.
-
Type of grommets. No study included ‘long-term’ grommets.
| Characteristic | Adenoidectomy with or without unilateral or bilateral grommets (n = 767, k = 10), n (%) | Non-surgical treatment or unilateral or bilateral grommets only (n = 994, k = 10), n (%) | Total (n = 1761, k = 10), n (%) |
|---|---|---|---|
| Age (years), mean (SD), range | 3.6 (2.2), (0.7–9.7) | 3.3 (2.1), (0.5–9.8) | 3.4 (2.2), (0.5–9.8) |
| < 2 years | 302 (39.4) | 449 (45.2) | 751 (42.6) |
| < 4 years | 442 (57.6) | 575 (57.8) | 1017 (57.8) |
| Sex: male | 435 (56.7) | 550 (55.3) | 985 (55.9) |
| Indication | |||
| Recurrent AOM | 317 (41.3) | 466 (46.9) | 783 (44.5) |
| Persistent OME | 442 (57.6) | 514 (51.7) | 956 (54.3) |
| Both | 8 (1.0) | 12 (1.2) | 20 (1.1) |
| Grommets | |||
| No grommets | 95 (12.4) | 343 (34.5) | 438 (24.9) |
| Unilateral | 195 (25.4) | 195 (19.6) | 390 (22.1) |
| Bilateral | 477 (62.2) | 456 (45.9) | 933 (53.0) |
| Type of grommets | |||
| Shepard | 286 (42.6) | 286 (43.9) | 572 (43.2) |
| Shah | 37 (5.5) | 35 (5.4) | 72 (5.4) |
| Donaldsona | 284 (42.3) | 262 (40.2) | 546 (41.3) |
| Reuter Bobbin | 33 (4.9) | 37 (5.7) | 70 (5.3) |
| Armstrong | 32 (4.8) | 31 (4.8) | 63 (4.8) |
| Season at time of surgery | |||
| Winter/spring | 441 (61.1) | 548 (57.8) | 989 (56.2) |
| Siblings: yes | 446 (81.7) | 556 (79.3) | 1002 (80.4) |
| Passive smoking: yes | 379 (47.0) | 364 (45.7) | 643 (36.5) |
| Day-care attendance | |||
| Yes | 233 (53.1) | 310 (53.3) | 543 (53.2) |
| Not relevant (child too old) | 197 (31.0) | 271 (31.8) | 468 (31.4) |
| Pneumococcal vaccination | |||
| Yes | 47 (12.1) | 40 (5.0) | 87 (6.0) |
| Trial performed before vaccination was available | 533 (80.0) | 618 (77.9) | 1151 (78.9) |
| Number of AOM episodes in past | |||
| 0 | 125 (18.4) | 137 (15.2) | 262 (16.6) |
| 1–3 episodes | 193 (28.3) | 250 (27.7) | 443 (28.0) |
| ≥ 4 | 363 (53.3) | 514 (57.0) | 877 (55.4) |
| OME present at study entry | |||
| Yes – unilateral | 95 (14.1) | 63 (6.9) | 158 (10.0) |
| Yes – bilateral | 331 (49.3) | 440 (48.2) | 771 (48.6) |
| No | 246 (36.6) | 410 (44.9) | 656 (41.4) |
| Hearing loss (dB), mean (SD), range | 31.4 (7.3), (13.1–65.0) | 32.2 (6.7), (13.8–50.0) | 31.9 (6.9), (13.1–65.0) |
| < 25 dBHL | 45 (19.2) | 47 (13.2) | 92 (15.6) |
| ≥ 25 dBHL | 189 (80.8) | 309 (86.8) | 498 (84.4) |
Participants according to the groups used in the main comparison
Table 3 also presents the characteristics of the participants according to the groups used in the main comparison. No important differences were seen between the groups.
1. Development of a model to predict the risk of children referred for adenoidectomy having a prolonged duration of their otitis media
To determine the predictive factors for failure at 12 months in children referred for adenoidectomy but randomised to a non-surgical treatment we studied the following factors: age, sex, indication, season, day-care attendance, breastfeeding, passive smoking, atopy or allergy, number of previous AOM episodes (less than four vs. four or more), the occurrence of one or more episodes of AOM in the previous year, age at first occurrence of AOM, baseline hearing level, duration of symptoms, and OME at study entry. We could not include pneumococcal vaccination status as this was not reported in any of the studies included in this analysis.
Univariate analysis
The results of the univariate analysis are shown in Table 4. The results are in the form of odds ratios (ORs) and 95% CIs.
| Prognostic factor | Non-surgical groupa (n = 343, k = 3) | |
|---|---|---|
| OR (95% CI)b | p-valueb | |
| Indication: persistent OME | 13.68 (7.24 to 25.83) | < 0.0001 |
| Sex | 0.99 (0.65 to 1.52) | 0.973 |
| Age < 2 years | 12.61 (6.79 to 23.42) | < 0.0001 |
| Age < 4 years | 10.87 (5.76 to 20.51) | < 0.0001 |
| Season (winter/spring vs. summer/autumn) | 1.32 (0.85 to 20.06) | 0.212 |
| Day-care attendance | 2.12 (1.28 to 3.53) | 0.004 |
| Breastfeeding | 1.02 (0.66 to 1.57) | 0.941 |
| Passive smoking | 0.88 (0.57 to 1.37) | 0.574 |
| Atopy or allergy | 2.23 (0.88 to 5.67) | 0.091 |
| AOM in the past yes/no | 0.08 (0.02 to 0.26) | < 0.0001 |
| AOM in the past less than/more than four episodes | 0.18 (0.11 to 0.31) | < 0.0001 |
| URTI in the past less than/more than four episodes | 0.62 (0.16 to 2.50) | 0.623 |
| Age at first AOM (</> 1 year) | 0.39 (0.22 to 0.71) | 0.002 |
| Baseline hearing level (</> 25 dB) | 1.05 (0.12 to 9.15) | 0.963 |
| Duration of symptoms (</> 6 months) | 0.78 (0.09 to 6.60) | 0.816 |
| OME at study entry | 13.23 (7.19 to 24.35) | < 0.0001 |
Univariate predictors of failure at 12 months were:
-
indication
-
age
-
AOM in the past
-
day-care attendance
-
age at first AOM
-
OME at study entry.
Multivariate analysis
Table 5 shows the independent predictors of failing to improve at 12 months. The only remaining independent predictor of failure at 12 months (when those that are not statistically significant are removed) was indication (OR 19.8, 95% CI 9.7 to 40.6). The prognostic model had an area under the curve of 0.77 (95% CI 0.72 to 0.82).
| Model | No. of patients | Overall % of patients with outcome at 12 months correctly predicted | Area under the ROC curve (95% CI) | Remaining factor OR (95% CI) |
|---|---|---|---|---|
| Non-surgical group | 343 | 71.7 | 0.77 (0.72 to 0.82) | Indication: 19.8 (9.7 to 40.6) |
Absolute risks
The absolute risk for the whole group of failing to improve at 12 months was 56% (193 of the 343 children). The absolute risk of failing to improve was highest for children with the indication persistent OME (absolute risk 0.89, 95% CI 0.84 to 0.95, 36% of all children). The absolute risk for children with the indication recurrent AOM was 0.38 (95% CI 0.32 to 0.44, 64% of all children).
Sensitivity analyses
Sensitivity analyses using several alternative definitions of failure at 12 months gave similar results for both the univariate and the multivariate analyses.
2a. Evaluation of the overall effect of adenoidectomy, with or without grommets on otitis media using individual patient data
Main comparison: adenoidectomy with or without grommets compared with non-surgical treatment or grommets only
Primary outcome
Figure 3 provides an overview of the overall effect within each individual study.
FIGURE 3.
Overall failure at 12 months: effect of adenoidectomy with or without grommets compared with non-surgical treatment or grommets only by study. (a) RD; and (b) RR. Note that the overall pooled effect size is not adjusted for the study. Table 6 provides the calculated RDs, RRs and adjusted RRs which differ slightly from those in the forest plots as the underlying models differ.
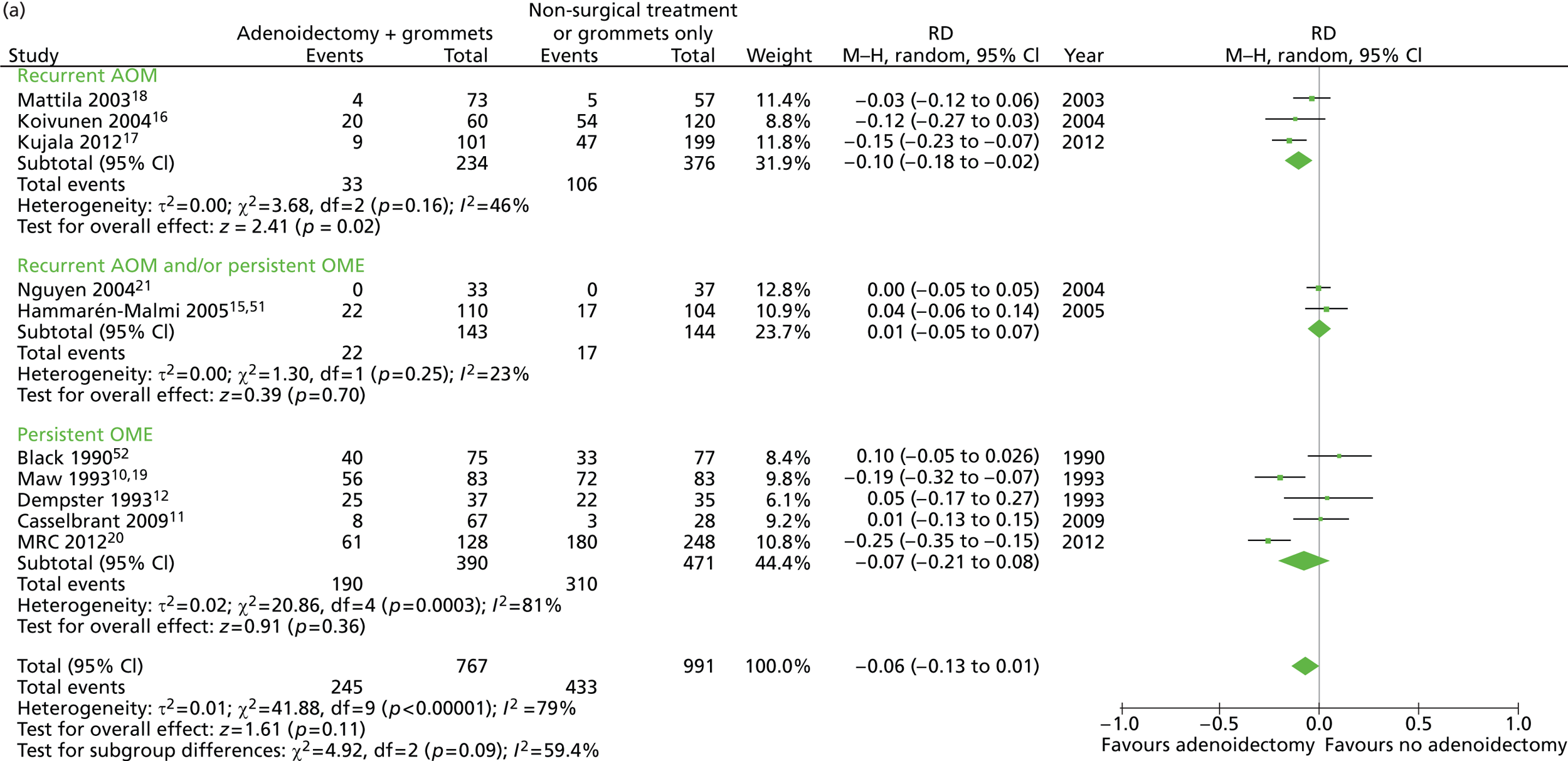
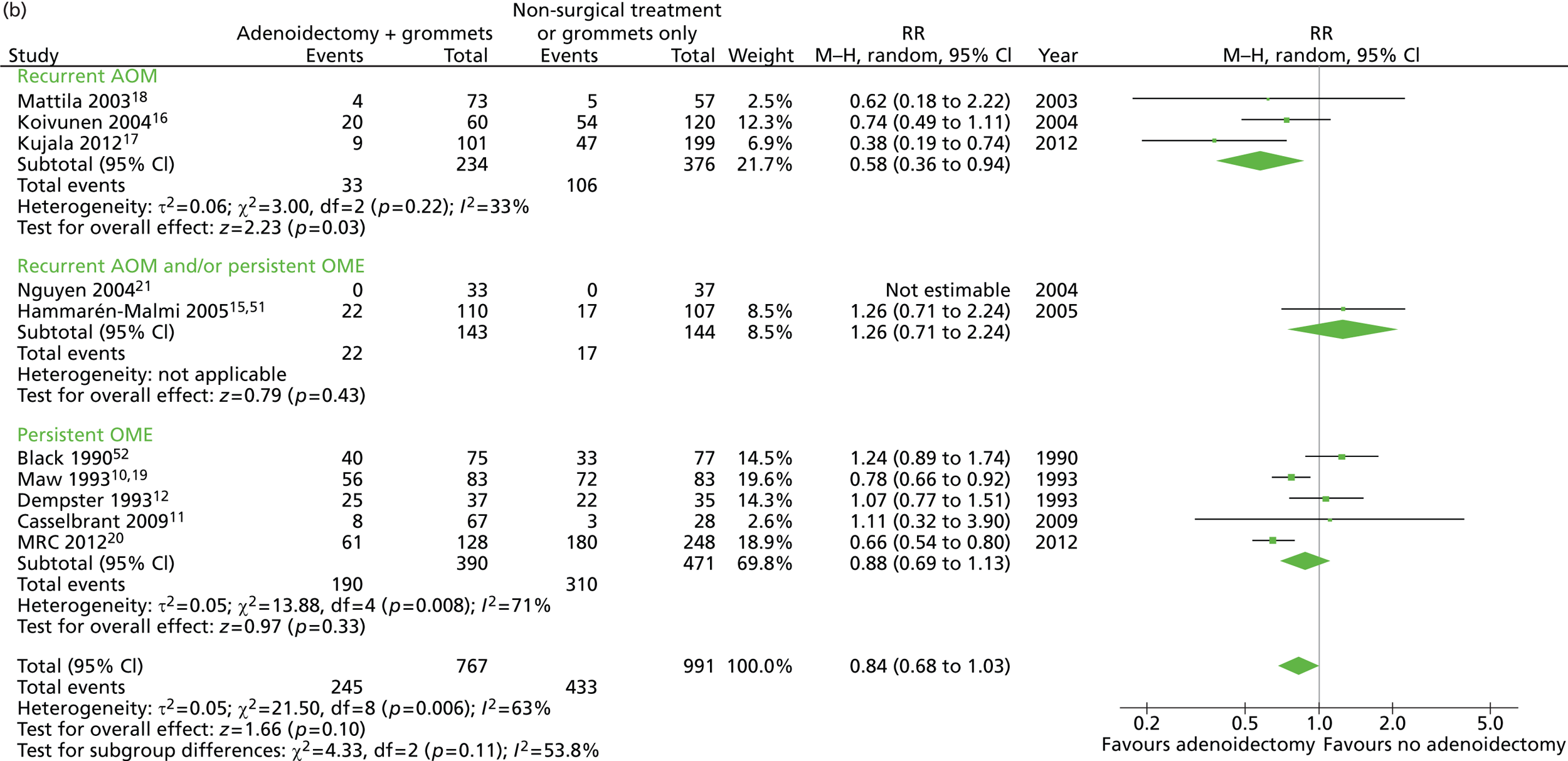
In total, the proportion of children who failed at 12 months in the adenoidectomy group (adenoidectomy with or without grommets) was 31.9% whereas the proportion of children who failed at 12 months in the no adenoidectomy (non-surgical or grommets alone) group was 44.7%. The RD for failure at 12 months was −12.7% (95% CI −17.3% to −8.2%), resulting in a NNT of eight children to prevent one failure. The RR was 0.72 (95% CI 0.63 to 0.81) and the adjusted RR (using a dummy for study) was 0.76 (95% CI 0.69 to 0.85) (Table 6).
| Outcome | Adenoidectomy with or without unilateral or bilateral grommets (n = 767, k = 10) | Non-surgical treatment or unilateral or bilateral grommets only (n = 994, k = 10) | RD (95% CI)a | NNTa | RR or mean difference (95% CI)a | Adjusted RR or mean difference (95% CI)a |
|---|---|---|---|---|---|---|
| Primary outcome, n/N (%) | ||||||
| Failure at 12 months | 245/767 (31.9) | 444/994 (44.7) | −12.7% (−17.2% to −8.2%) | 8 | 0.72 (0.63 to 0.81) | 0.76 (0.69 to 0.85) |
| Secondary outcome: individual items of failure at 12 months, n/N (%) | ||||||
| Four or more AOM episodes per year | 52/508 (10.2) | 111/737 (15.1) | −4.8% (−8.5% to −1.1%) | 21 | 0.65 (0.50 to 0.93) | 0.78 (0.58 to 1.04) |
| Presence of effusion for ≥ 50% of the time | 153/475 (32.2) | 254/652 (39.0) | −6.7% (−12.4% to −1.1%) | 15 | 0.83 (0.70 to 0.97) | 0.78 (0.68 to 0.89) |
| Surgery during follow-up | 38/764 (5.0) | 172/994 (17.3) | −12.3% (−15.1% to −9.5%) | 9 | 0.29 (0.21 to 0.40) | 0.30 (0.22 to 0.42) |
| Improvement in hearing < 10 dB | 55/197 (27.9) | 126/301 (41.9) | −13.9% (−22.3% to −5.6%) | 8 | 0.67 (0.51 to 0.87) | 0.67 (0.52 to 0.87) |
| Secondary outcome: number of AOM episodes during follow-up, mean (SD), range | ||||||
| In the first 6 months | 0.8 (1.3), 0–8.0 | 1.1 (1.6), 0–10.5 | – | – | −0.3 (−0.5 to −0.2) | −0.2 (−0.4 to −0.1) |
| In the first 12 months | 1.2 (1.7), 0–9.5 | 1.6 (2.1), 0–13.0 | – | – | −0.4 (−0.7 to −0.2) | −0.4 (−0.6 to −0.1) |
| In the first 18 months | 1.6 (2.2), 0–10.0 | 2.4 (2.9), 0–15.0 | – | – | −0.9 (−1.3 to −0.4) | −0.5 (−0.9 to −0.0) |
| In the first 24 months | 2.3 (2.6), 0–15.0 | 2.9 (3.2), 0–16.0 | – | – | −0.6 (−1.0 to −0.2) | −0.5 (−0.7 to 0.1) |
| Secondary outcome: time with effusion during follow-up (weeks), mean (SD), range | ||||||
| In the first 6 months | 10.0 (8.7), 0.0–26.0 | 12.6 (10.2), 0.0–26.0 | – | – | −2.6 (−3.8 to −1.4) | −2.6 (−3.4 to −1.8) |
| In the first 12 months | 16.4 (16.2), 0.0–52.0 | 20.1 (19.7), 0.0–52.0 | – | – | −3.8 (−5.9 to −1.6) | −4.5 (−6.0 to −2.9) |
| In the first 18 months | 25.3 (23.2), 0.0–78.0 | 33.2 (28.6), 0.0–78.0 | – | – | −7.9 (−11.4 to −4.4) | −7.9 (−10.6 to −5.3) |
| In the first 24 months | 31.9 (30.2), 0.0–104.0 | 43.0 (36.8), 0.0–104.0 | – | – | −11.1 (−15.7 to −6.6) | −11.2 (−14.7 to −7.6) |
| Secondary outcome: additional surgery during follow-up (yes), n/N (%) | ||||||
| In the first 6 months | 18/764 (2.4) | 93/994 (9.4) | −7.0% (−9.1% to −4.9%) | 15 | 0.25 (0.15 to 0.41) | 0.32 (0.20 to 0.52) |
| In the first 18 months | 50/480 (10.4) | 179/631 (28.4) | −18.0% (−22.4% to −13.5%) | 6 | 0.37 (0.28 to 0.49) | 0.36 (0.27 to 0.47) |
| In the first 24 months | 64/480 (13.3) | 211/631 (33.4) | −20.1% (−24.9% to −15.3%) | 5 | 0.40 (0.31 to 0.51) | 0.38 (0.30 to 0.48) |
| Secondary outcome: hearing loss (dB), mean (SD), range | ||||||
| After 6 months’ follow-up | 15.9 (8.3), 3.2–54.4 | 20.3 (9.3), −1.9–53.8 | – | – | −4.4 (−5.9 to −2.8) | −4.4 (−5.9 to −2.9) |
| After 12 months’ follow-up | 16.8 (8.5), 1.9–48.8 | 20.4 (9.6), 1.9–49.4 | – | – | −3.6 (−5.2 to −2.0) | −3.5 (−5.1 to −1.9) |
| After 18 months’ follow-up | 15.5 (7.0), 1.9–39.4 | 20.5 (10.0), 3.1–55.0 | – | – | −5.0 (−6.8 to −3.3) | −5.0 (−6.6 to −3.4) |
| After 24 months’ follow-up | 15.4 (7.8), 1.3–39.4 | 19.2 (8.8), 4.4–43.8 | – | – | −3.9 (−5.4 to −2.2) | −4.1 (−5.7 to −2.6) |
| Secondary outcome: improvement in hearing level, n/N (%) | ||||||
| < 10 dB after 6 months’ follow-up | 47/204 (23.0) | 115/305 (37.7) | −14.7% (−22.6% to −6.7%) | 7 | 0.61 (0.46 to 0.82) | 0.61 (0.46 to 0.81) |
| < 10 dB after 18 months’ follow-up | 37/160 (23.1) | 103/260 (39.6) | −16.5% (−25.3% to −7.7%) | 6 | 0.58 (0.42 to 0.80) | 0.57 (0.42 to 0.80) |
| < 10 dB after 24 months’ follow-up | 39/165 (23.6) | 96/275 (34.9) | −11.3% (−19.9% to −2.7%) | 9 | 0.68 (0.49 to 0.93) | 0.64 (0.47 to 0.89) |
In this analysis the proportion of children without grommets, with a unilateral grommet and with bilateral grommets differed between the groups. In the adenoidectomy group 12% did not have grommets, 25% had a unilateral grommet and 62% had bilateral grommets. In the no adenoidectomy group these percentages were 35%, 20% and 46% respectively (see Table 3). To study whether this imbalance influenced our results we performed sensitivity analyses by omitting one group at a time (i.e. no grommets, unilateral grommets or bilateral grommets). The results of the sensitivity analyses did not differ and therefore the imbalance did not affect the outcome.
Secondary outcomes
Data on all available secondary outcomes are also presented in Table 6.
For all secondary outcomes, results in children in the adenoidectomy group were statistically significantly better than results in those in the no adenoidectomy group. During follow-up, children in the adenoidectomy group had a lower number of AOM episodes, less time with effusion, less additional surgery, less hearing loss and improved hearing levels at 6, 12, 18 and 24 months’ follow-up. The clinical importance of these findings is not clear. The reductions in both time with effusion and number of AOM episodes are very modest. However, about one in five children avoid the need for additional surgery. The clinical importance of an average 4-dB improvement in hearing loss has been debated and we consider it further below.
Secondary comparisons
Adenoidectomy with unilateral or bilateral grommets compared with non-surgical treatment
Primary outcome
Figure 4 provides an overview of the overall effect within each individual study.
FIGURE 4.
Overall failure at 12 months: effect of adenoidectomy with unilateral or bilateral grommets compared with non-surgical treatment by study. (a) RD; and (b) RR. Note that the overall pooled effect size is not adjusted for the study. Table 7 provides the calculated RDs, RRs, and adjusted RRs which differ slightly from those in the forest plots as the underlying models differ and some indirect comparisons could be added to the regression analyses.
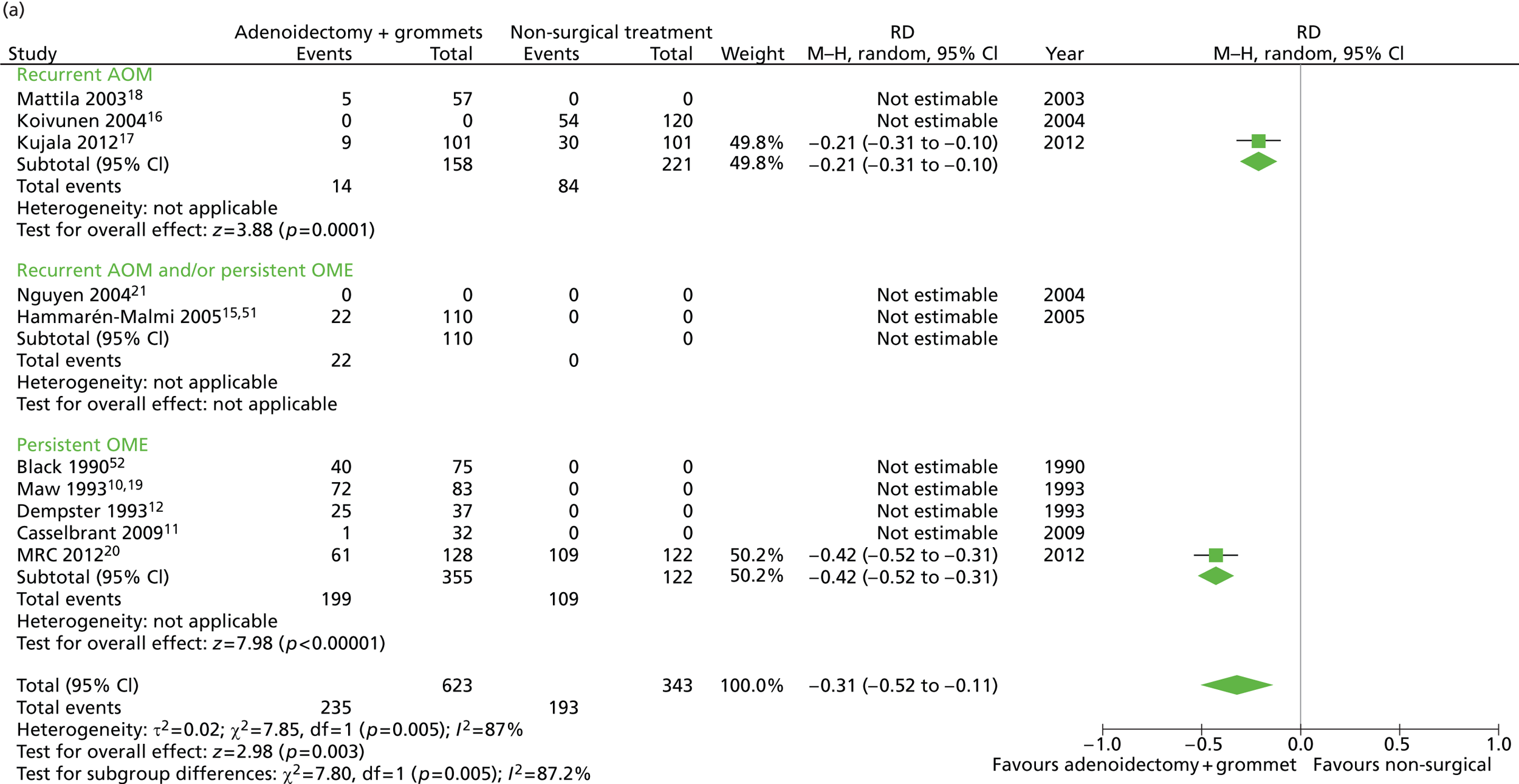
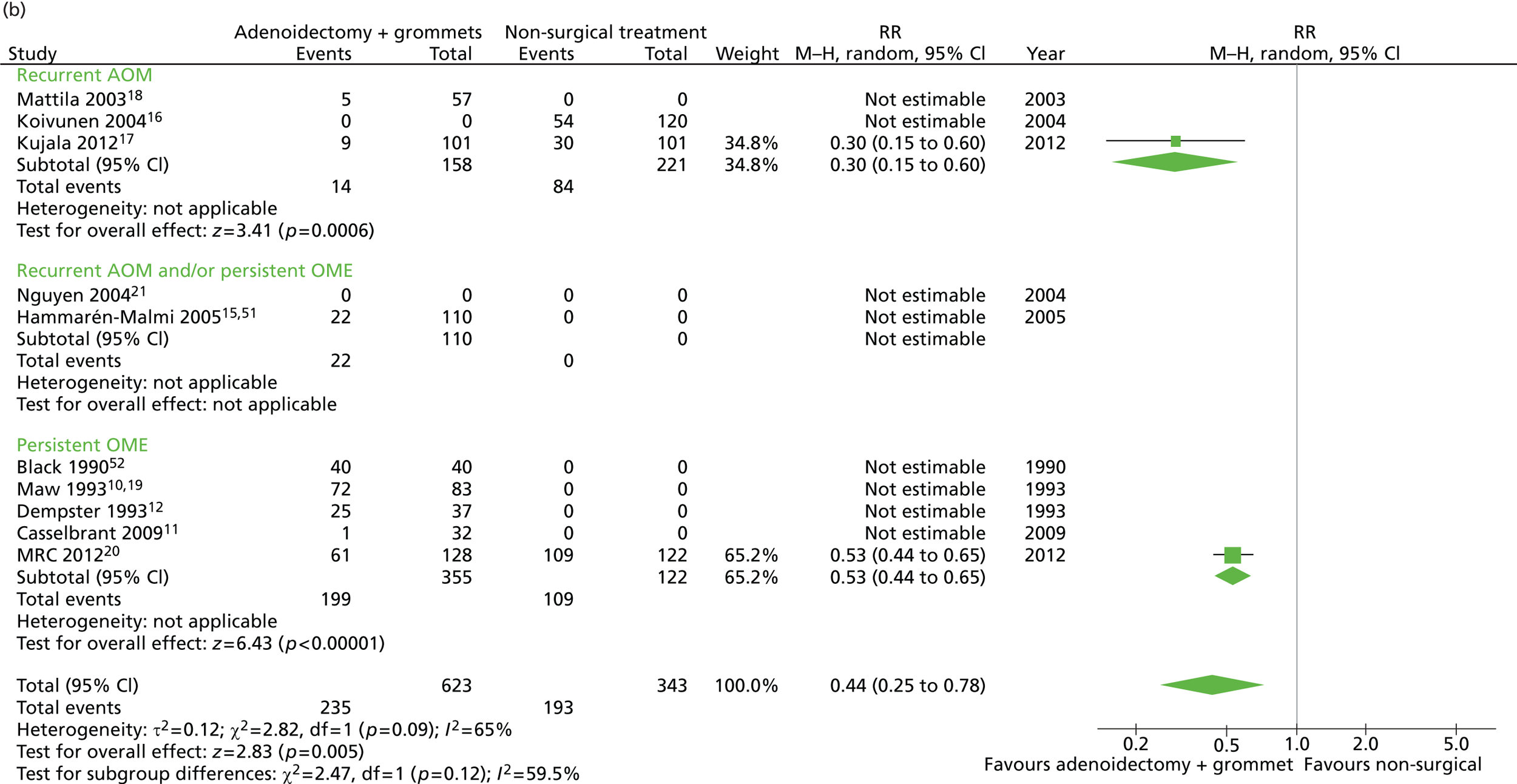
The proportion of children who failed at 12 months was 32.4% in the group in which all children had adenoidectomy with a grommet in one ear or both and 56.3% in the non-surgical group. The RD for failure at 12 months was −23.8% (95% CI −30.2% to −17.5%), resulting in a NNT of five children to prevent one failure. The RR was 0.58 (0.50 to 0.67) and the adjusted RR (using a dummy for study) was 0.48 (0.40 to 0.59) (Table 7).
| Outcome | Adenoidectomy with unilateral or bilateral grommets (n = 672, k = 9)a | Non-surgical treatment (n = 343, k = 3)b | RD (95% CI)c | NNTc | RR or mean difference (95% CI)c | Adjusted RR or mean difference (95% CI)c |
|---|---|---|---|---|---|---|
| Primary outcome, n/N (%) | ||||||
| Failure at 12 months | 218/672 (32.4) | 193/343 (56.3) | −23.8% (−30.2% to −17.5%) | 5 | 0.58 (0.50 to 0.67) | 0.48 (0.40 to 0.59) |
| Secondary outcome: individual items of failure at 12 months, n/N (%) | ||||||
| Four or more AOM episodes per year | 35/413 (8.5) | 77/333 (23.1) | −14.6% (−19.9% to −9.4%) | 7 | 0.37 (0.25 to 0.53) | 0.38 (0.21 to 0.68) |
| Presence of effusion for ≥ 50% of the time | 153/386 (39.6) | 83/253 (32.8) | 6.8% (−0.1% to 14.4%) | – | 1.21 (0.97 to 1.50) | 0.45 (0.34 to 0.60) |
| Surgery during follow-up | 27/669 (4.0) | 96/343 (28.0) | −24.0% (−28.9% to −19.0%) | 5 | 0.14 (0.10 to 0.22) | 0.12 (0.06 to 0.24) |
| Improvement in hearing < 10 dB | 55/197 (27.9) | 40/100 (40.0) | −12.1% (−23.5% to −0.6%) | 9 | 0.70 (0.50 to 0.97) | 0.68 (0.46 to 0.99) |
| Secondary outcome: number of AOM episodes during follow-up, mean (SD), range | ||||||
| In the first 6 months | 0.7 (1.2), 0–7.0 | 1.6 (1.9), 0–10.5 | – | – | −0.9 (−1.1 to −0.7) | −0.7 (−1.0 to −0.4) |
| In the first 12 months | 1.1 (1.6), 0–9.5 | 2.2 (2.5), 0–13.0 | – | – | −1.0 (−1.3 to −0.8) | −0.7 (−1.1 to −0.7) |
| In the first 18 months | 1.2 (2.0), 0–9.5 | 3.1 (3.2), 0–15.0 | – | – | −1.8 (−2.5 to −1.2) | −1.8 (−1.6 to −0.1) |
| In the first 24 months | 2.4 (2.6), 0–15.0 | 3.6 (3.6), 0–16.0 | – | – | −1.3 (−1.9 to −0.6) | −1.2 (−2.2 to −0.2) |
| Secondary outcome: time with effusion during follow-up (weeks), mean (SD), range | ||||||
| In the first 6 months | 12.3 (8.3), 0.0–26.0 | 10.8 (11.4), 0.0–26.0 | – | – | 1.5 (−0.1 to 3.1) | −10.1 (11.7 to −8.5) |
| In the first 12 months | 19.7 (16.2), 0.0–52.0 | 17.0 (20.4), 0.0–52.0 | – | – | 2.7 (−0.2 to 5.5) | −12.4 (−15.5 to −9.2) |
| In the first 18 months | 31.6 (22.4), 0.0–78.0 | 27.8 (29.5), 0.0–78.0 | – | – | 5.9 (1.4 to 10.3) | −17.6 (−23.4 to −11.9) |
| In the first 24 months | 39.5 (29.5), 0.0–104.0 | 33.0 (38.2), 0.0–104.0 | – | – | 6.6 (0.8 to 12.4) | −21.5 (−29.4 to −13.7) |
| Secondary outcome: additional surgery during follow-up (yes), n/N (%) | ||||||
| In the first 6 months | 12/669 (1.8) | 66/343 (19.2) | −17.4% (−21.7% to −13.2%) | 6 | 0.09 (0.05 to 0.17) | 0.15 (0.07 to 0.32) |
| In the first 18 months | 35/385 (9.1) | 97/242 (40.1) | −31.0% (−37.8% to −24.2%) | 4 | 0.23 (0.16 to 0.32) | 0.03 (0.08 to 0.13) |
| In the first 24 months | 48/385 (12.5) | 102/242 (42.1) | −29.7% (−36.7% to −22.6%) | 4 | 0.30 (0.22 to 0.40) | 0.05 (0.02 to 0.15) |
| Secondary outcome: hearing loss (dB), mean (SD), range | ||||||
| After 6 months’ follow-up | 15.9 (8.3), 3.1–54.4 | 23.1 (10.1), −1.9–53.8 | – | – | −7.2 (−9.3 to −5.1) | −7.8 (−10.2 to −5.3) |
| After 12 months’ follow-up | 16.8 (8.5), 1.9–48.8 | 20.5 (10.1), 4.4–45.0 | – | – | −3.6 (−5.8 to −1.4) | −3.4 (−6.0 to −0.8) |
| After 18 months’ follow-up | 15.5 (7.0), 1.9–39.4 | 19.7 (10.4), 4.4–55.0 | – | – | −4.2 (−6.3 to −2.1) | −4.0 (−6.5 to −1.5) |
| After 24 months’ follow-up | 15.4 (7.7), 1.3–39.3 | 18.2 (8.1), 5.6–41.3 | – | – | −2.9 (−4.8 to −9.3) | −3.5 (−5.6 to −1.4) |
| Secondary outcome: improvement in hearing level, n/N (%) | ||||||
| < 10 dB after 6 months’ follow-up | 47/204 (23.0) | 49/105 (46.7) | −23.6% (−34.8% to −12.5%) | 5 | 0.49 (0.36 to 0.68) | 0.46 (0.31 to 0.69) |
| < 10 dB after 18 months’ follow-up | 37/160 (23.1) | 30/98 (30.6) | −7.5% (−18.7% to 3.7%) | 14 | 0.76 (0.50 to 1.14) | 0.83 (0.53 to 1.29) |
| < 10 dB after 24 months’ follow-up | 39/165 (23.6) | 27/102 (26.5) | −2.8% (−13.6% to 7.9%) | 36 | 0.89 (0.58 to 1.37) | 0.80 (0.49 to 1.30) |
Secondary outcomes
Data on all available secondary outcomes are also presented in Table 7.
Adenoidectomy with unilateral or bilateral grommets compared with unilateral or bilateral grommets
Primary outcome
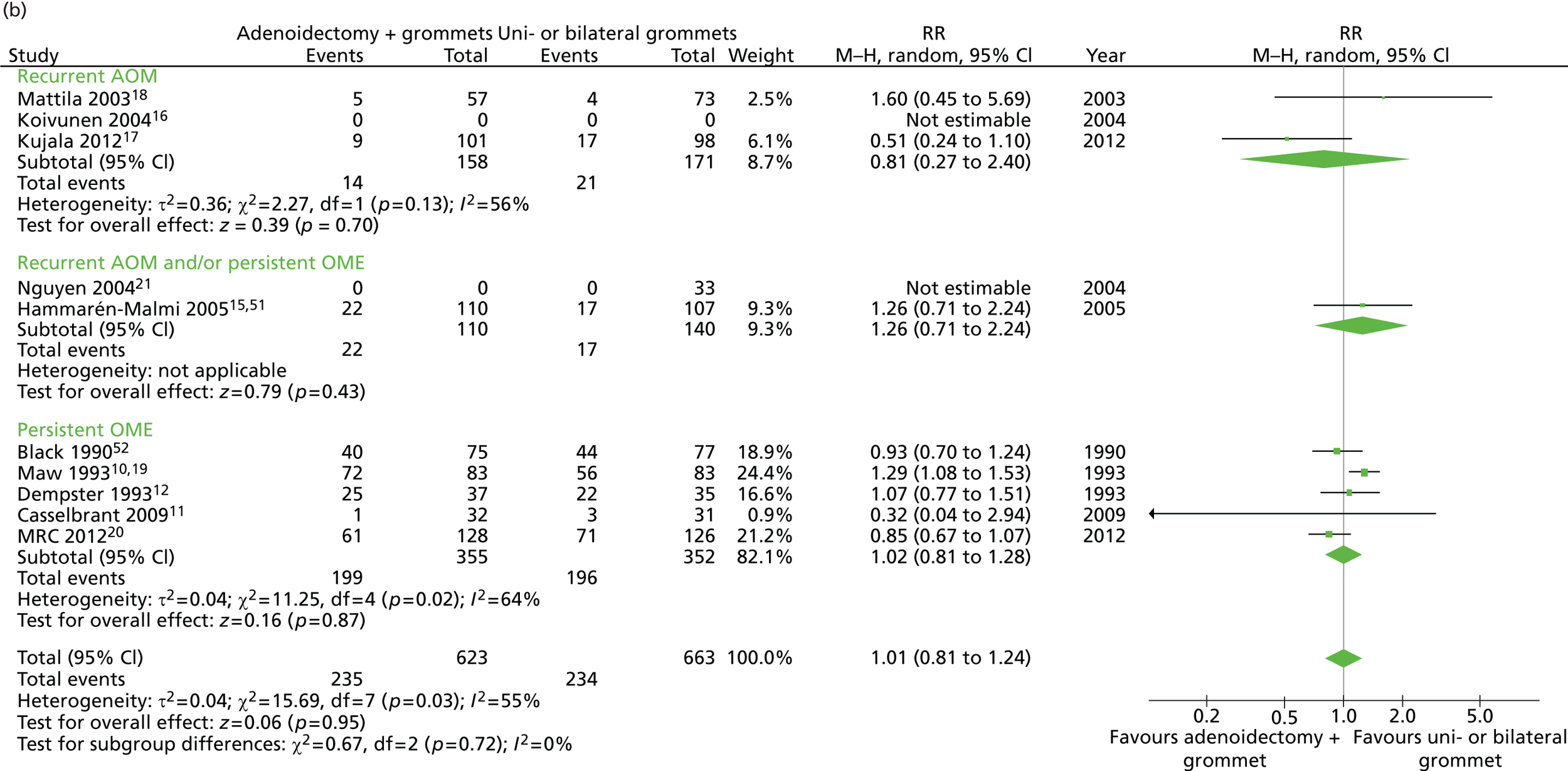
Figure 5 provides an overview of the overall effect within each individual study. The proportion of children who failed at 12 months was 32.4% in the group in which all children had adenoidectomy with a grommet in one or both ears and 38.6% in the group of children who had grommets only in one or both ears. The RD for failure at 12 months was −6.2% (95% CI −11.3% to −1.0%), resulting in a NNT of 17 children to prevent one failure. The RR was 0.84 (95% CI 0.73 to 0.97) and the adjusted RR (using a dummy for study) was 0.86 (95% CI 0.76 to 0.97) (Table 8).
FIGURE 5.
Overall failure at 12 months: effect of adenoidectomy with unilateral or bilateral grommets compared with unilateral or bilateral grommets by study. (a) RD; and (b) RR. Note that the overall pooled effect size is not adjusted for the study. Table 8 provides the calculated RDs, RRs, and adjusted RRs which differ slightly from those in the forest plots as the underlying models differ and some indirect comparisons could be added to the regression analyses.
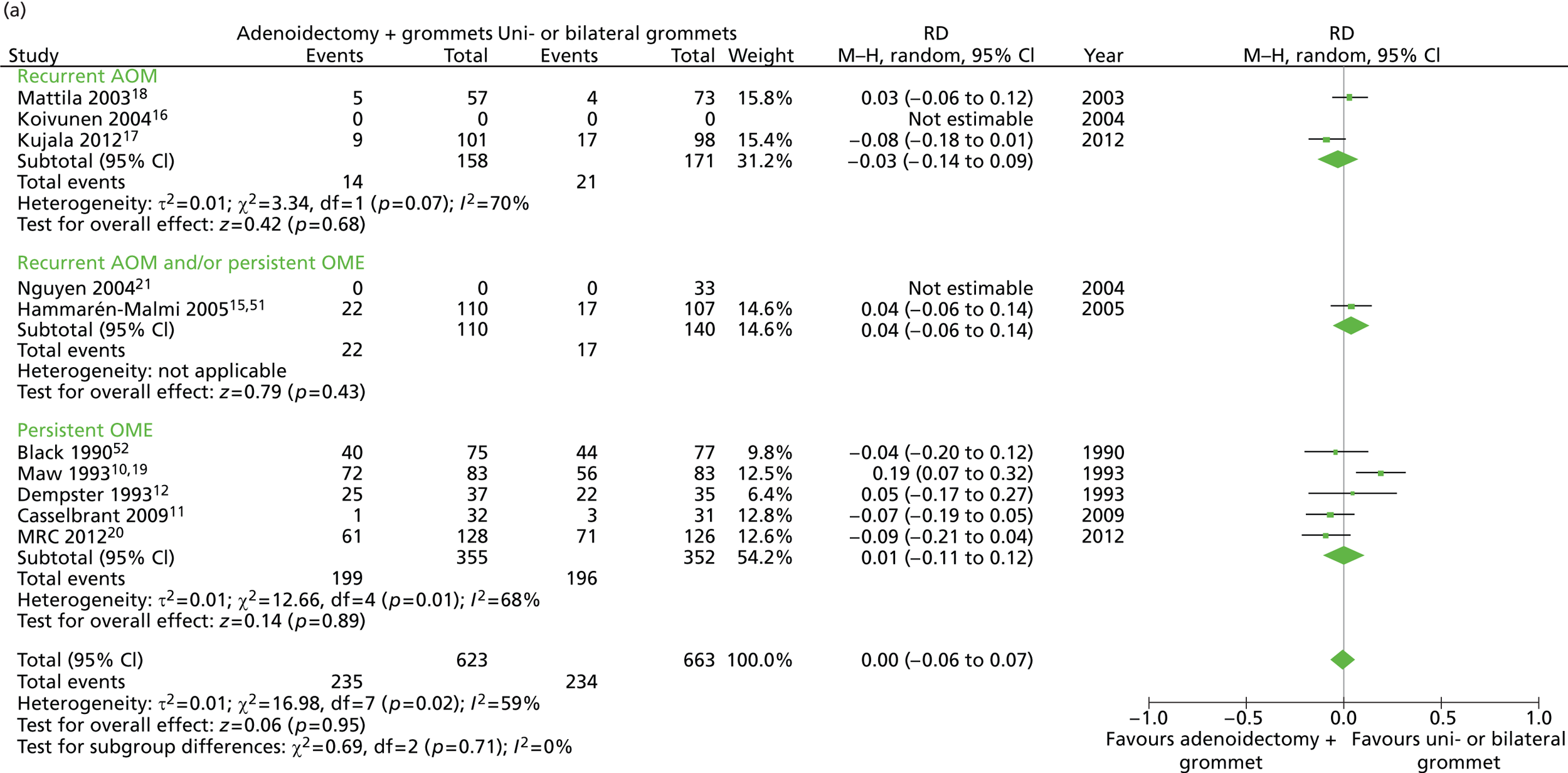
| Adenoidectomy with unilateral or bilateral grommets (n = 672, k = 9)a | Unilateral or bilateral grommets (n = 651, k = 9)b | RD (95% CI)c | NNTc | RR or mean difference (95% CI)c | Adjusted RR or mean difference (95% CI)c | |
|---|---|---|---|---|---|---|
| Primary outcome, n/N (%) | ||||||
| Failure at 12 months | 218/672 (32.4) | 251/651 (38.6) | −6.1% (−11.3% to −1.0%) | 17 | 0.84 (0.73 to 0.97) | 0.86 (0.76 to 0.97) |
| Secondary outcome: individual items of failure at 12 months, n/N (%) | ||||||
| Four or more AOM episodes per year | 35/413 (8.5) | 34/404 (8.4) | 0.1% (−3.8% to 3.9%) | – | 1.01 (0.64 to 1.58) | 0.97 (0.62 to 1.52) |
| Presence of effusion for ≥ 50% of the time | 153/386 (39.6) | 171/399 (42.9) | −3.2% (−10.1% to 3.7%) | 32 | 0.93 (0.78 to 1.09) | 0.88 (0.77 to 1.02) |
| Surgery during follow-up | 27/669 (4.0) | 76/651 (11.7) | −7.6% (−10.5% to −4.8%) | 13 | 0.35 (0.23 to 0.53) | 0.35 (0.23 to 0.53) |
| Improvement in hearing < 10 dB | 55/197 (27.9) | 86/201 (42.8) | −14.9% (−24.1% to −5.6%) | 7 | 0.65 (0.50 to 0.86) | 0.66 (0.50 to 0.87) |
| Secondary outcome: number of AOM episodes during follow-up, mean (SD), range | ||||||
| In the first 6 months | 0.7 (1.2), 0–7.0 | 0.7 (1.1), 0–6.0 | – | – | 0.0 (−0.1 to 0.2) | 0.0 (−0.1 to 0.2) |
| In the first 12 months | 1.1 (1.6), 0–9.5 | 1.2 (1.6), 0–10.0 | – | – | −0.1 (−0.3 to 0.2) | −0.1 (−0.3 to 0.1) |
| In the first 18 months | 1.2 (2.0), 0–9.5 | 1.4 (1.9), 0–8.5 | – | – | −0.2 (−0.6 to 0.3) | −0.2 (−0.6 to 0.3) |
| In the first 24 months | 2.4 (2.6), 0–15.0 | 2.2 (2.6), 0–12.0 | – | – | 0.2 (−0.3 to 0.7) | 0.1 (−0.4 to 0.6) |
| Secondary outcome: time with effusion during follow-up (weeks), mean (SD), range | ||||||
| In the first 6 months | 12.3 (8.3), 0.0 to 26.0 | 13.8 (9.2), 0.0 to 26.0 | – | – | −1.5 (−2.8 to −0.2) | −1.7 (−2.7 to −0.7) |
| In the first 12 months | 19.7 (16.2), 0.0 to 52.0 | 22.1 (18.9), 0.0 to 52.0 | – | – | −2.4 (−4.9 to 0.0) | −3.3 (−5.2 to −1.4) |
| In the first 18 months | 31.6 (22.4), 0.0 to 78.0 | 39.1 (26.4), 0.0 to 78.0 | – | – | −7.4 (−11.4 to −3.5) | −7.5 (−11.1 to −4.0) |
| In the first 24 months | 39.5 (29.5), 0.0 to 104.0 | 51.0 (33.6), 0.0 to 104.0 | – | – | −11.5 (−16.6 to −6.4) | −11.7 (−16.4 to −7.0) |
| Secondary outcome: additional surgery during follow-up (yes), n/N (%) | ||||||
| In the first 6 months | 12/669 (1.8) | 27/651 (4.1) | −2.4% (−4.2% to −0.5%) | 42 | 0.43 (0.22 to 0.85) | 0.44 (0.23 to 0.84) |
| In the first 18 months | 35/385 (9.1) | 82/389 (21.1) | −12.0% (−17.0% to −7.0%) | 9 | 0.43 (0.30 to 0.62) | 0.44 (0.31 to 0.62) |
| In the first 24 months | 48/385 (12.5) | 109/389 (28.0) | −15.6% (−21.1% to −10.0%) | 7 | 0.45 (0.33 to 0.61) | 0.45 (0.34 to 0.60) |
| Secondary outcome: hearing loss (dB), mean (SD), range | ||||||
| After 6 months’ follow-up | 15.9 (8.3), 3.1–54.4 | 18.9 (8.6), 5.0–47.5 | – | – | −2.9 (−4.6 to −1.3) | −2.9 (−4.5 to −1.3) |
| After 12 months’ follow-up | 16.8 (8.5), 1.9–48.8 | 20.4 (9.3), 1.9–49.4 | – | – | −3.6 (−5.3 to −1.9) | −3.6 (−5.3 to −1.9) |
| After 18 months’ follow-up | 15.5 (7.0), 1.9–39.4 | 21.0 (9.8), 3.1–52.0 | – | – | −5.5 (−7.3 to –3.7) | −5.5 (−7.3 to −3.7) |
| After 24 months’ follow-up | 15.4 (7.7), 1.3–39.3 | 19.8 (9.1), 4.4–43.8 | – | – | −4.4 (−6.2 to −2.6) | −4.3 (−6.1 to −2.6) |
| Secondary outcome: improvement in hearing level, n/N (%) | ||||||
| < 10 dB after 6 months’ follow-up | 47/204 (23.0) | 66/200 (33.0) | −10.0% (−18.7% to −1.3%) | 10 | 0.70 (0.51 to 0.96) | 0.71 (0.52 to 0.97) |
| < 10 dB after 18 months’ follow-up | 37/160 (23.1) | 73/162 (45.1) | −21.9% (−32.0% to −11.9%) | 5 | 0.51 (0.37 to 0.71) | 0.51 (0.37 to 0.71) |
| < 10 dB after 24 months’ follow-up | 39/165 (23.6) | 69/173 (39.9) | −16.2% (−26.0% to −6.5%) | 7 | 0.59 (0.43 to 0.82) | 0.60 (0.43 to 0.83) |
The proportions of unilateral and bilateral tubes were similar in both groups. In the adenoidectomy with grommets group 29% had a unilateral grommet and 71% had bilateral grommets whereas in the grommets only group the proportions were 30% and 70% respectively. It is therefore unlikely that the results are influenced by the proportion of unilateral or bilateral tubes within each group.
Secondary outcomes
Data on all available secondary outcomes are also presented in Table 8.
Adenoidectomy without grommets compared with non-surgical treatment
Primary outcome
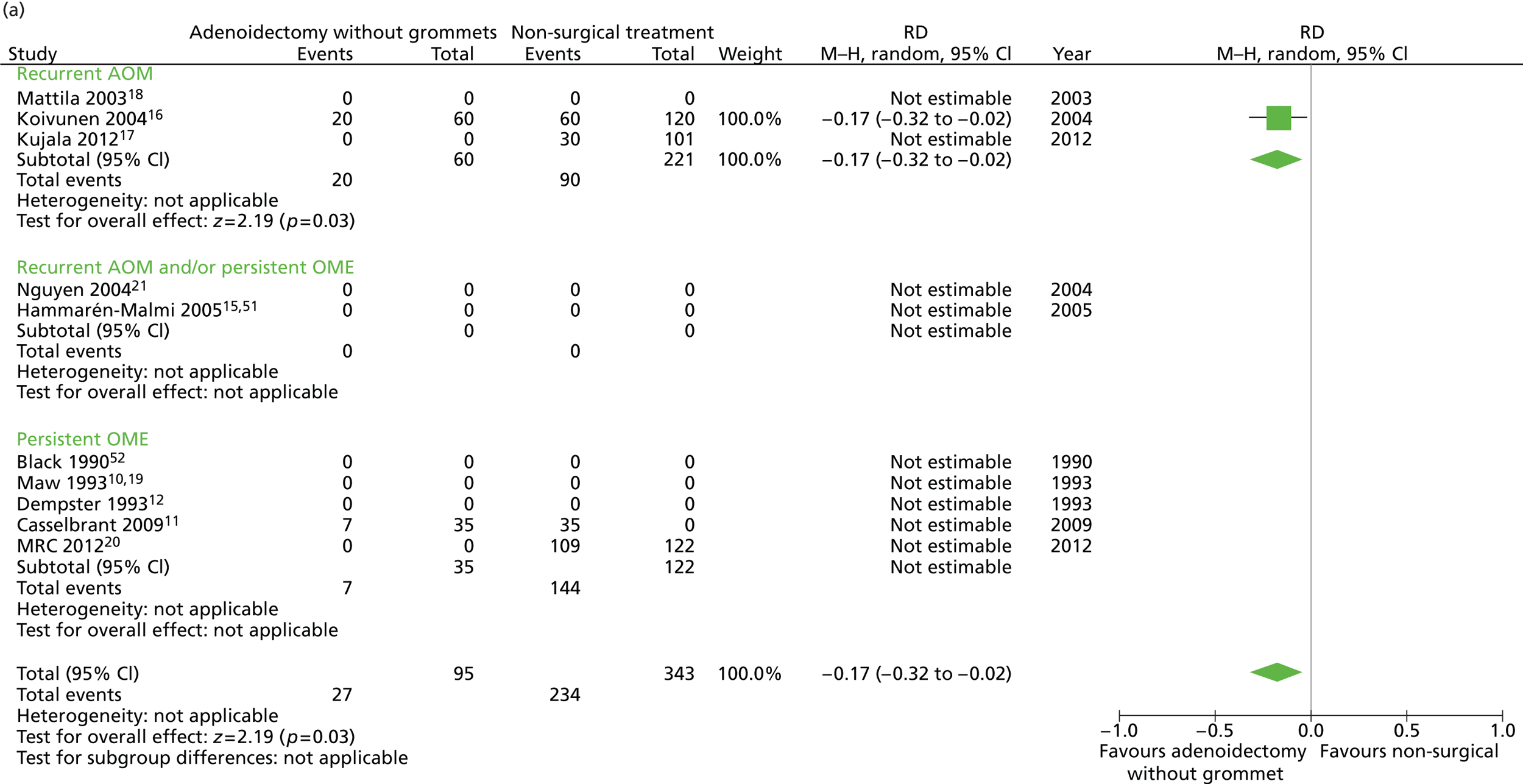
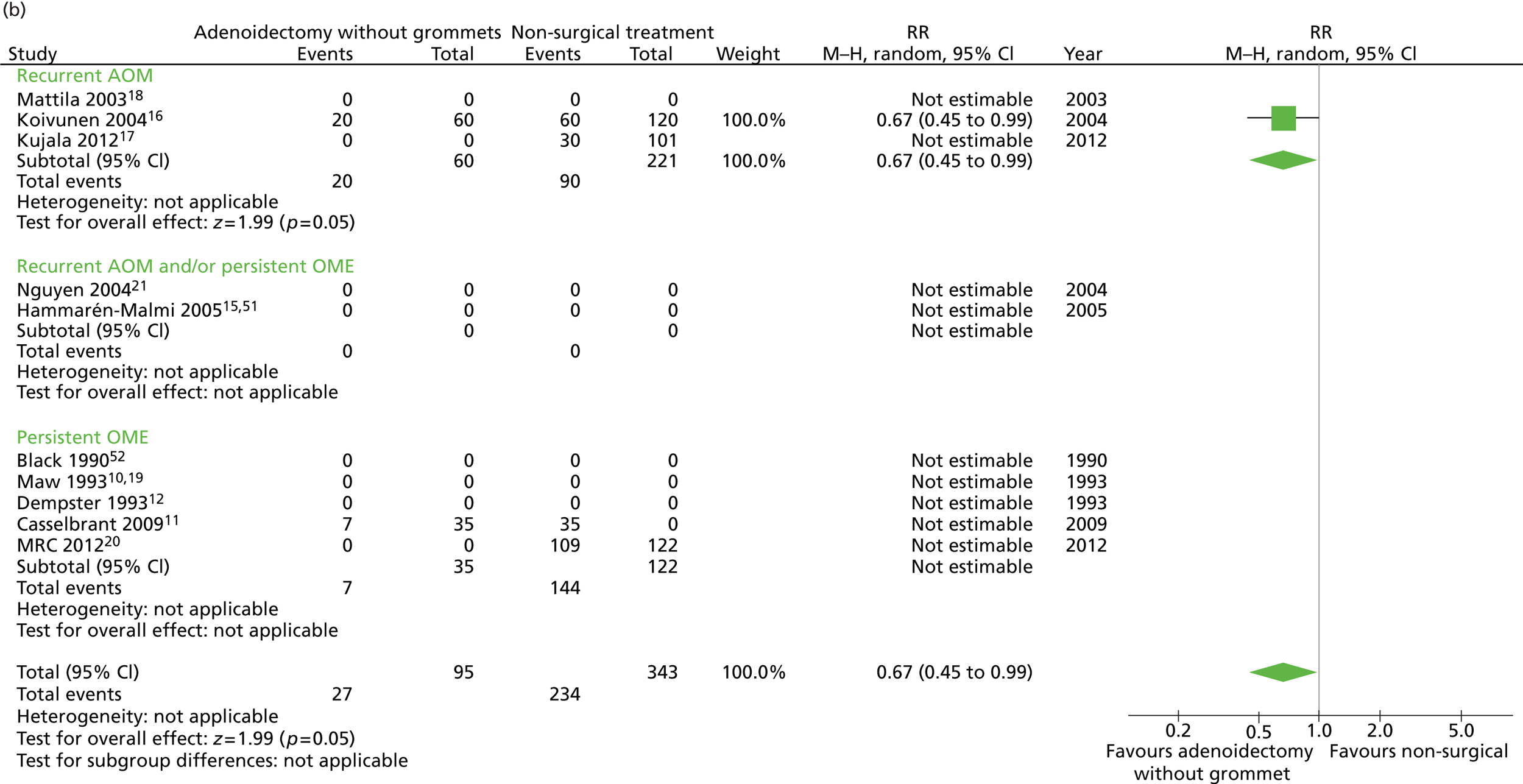
Figure 6 provides an overview of the overall effect within each individual study. The proportion of children who failed at 12 months was 28.4% in the group in which all children had adenoidectomy alone and 56.3% in the group of children who had non-surgical treatment. The RD for failure at 12 months was −27.8% (95% CI −38.3% to −17.4%), resulting in a NNT of four children to prevent one failure. The RR was 0.51 (95% CI 0.36 to 0.70) and the adjusted RR (using a dummy for study) was 0.74 (95% CI 0.49 to 1.12) (Table 9).
FIGURE 6.
Overall failure at 12 months: effect of adenoidectomy without grommets compared with non-surgical treatment by study. (a) RD; and (b) RR. Note that the overall pooled effect size is not adjusted for the study. Table 9 provides the calculated RDs, RRs, and adjusted RRs which differ slightly from those in the forest plots as the underlying models differ and some indirect comparisons could be added to the regression analyses.
| Adenoidectomy with or without myringotomy (n = 95, k = 2)a | Non-surgical treatment (n = 343, k = 3)b | RD (95% CI)c | NNTc | RR or mean difference (95% CI)c | Adjusted RR or mean difference (95% CI)c | |
|---|---|---|---|---|---|---|
| Primary outcome, n/N (%) | ||||||
| Failure at 12 months | 27/95 (28.4) | 193/343 (56.3) | −27.8% (−38.3% to −17.4%) | 4 | 0.51 (0.36 to 0.70) | 0.74 (0.49 to 1.12) |
| Secondary outcome: individual items of failure at 12 months, n/N (%) | ||||||
| Four or more AOM episodes per year | 17/95 (17.9) | 77/333 (23.1) | −5.2% (−14.2% to 3.7%) | 20 | 0.77 (0.48 to 1.24) | 0.78 (0.48 to 1.27) |
| Presence of effusion for ≥ 50% of the time | 0/89 (0.0) | 83/253 (32.8) | −32.8% (−38.3% to −27.0%) | 3 | NA | NA |
| Surgery during follow-up | 11/95 (11.6) | 96/343 (28.0) | −16.4% (−24.4% to −8.4%) | 6 | 0.41 (0.23 to 0.74) | 0.33 (0.14 to 0.82) |
| Secondary outcome: number of AOM episodes during follow-up, mean (SD), range | ||||||
| In the first 6 months | 1.1 (1.5), 0–8.0 | 1.6 (1.9), 0–10.5 | – | – | −0.6 (−1.0 to −0.2) | −0.6 (−1.2 to −0.1) |
| In the first 12 months | 1.5 (2.0), 0–9.0 | 2.2 (2.5), 0–13.0 | – | – | −0.7 (−1.2 to −0.2) | −0.8 (−1.6 to −0.1) |
| In the first 18 months | 2.0 (2.4), 0–10.0 | 3.1 (3.2), 0 –15.0 | – | – | −1.1 (−1.8 to −0.4) | −0.7 (−1.5 to 0.2) |
| In the first 24 months | 2.1 (2.6), 0–10.0 | 3.6 (3.6), 0–16.0 | – | – | −1.5 (−2.3 to −0.7) | −0.8 (−1.7 to 0.2) |
| Secondary outcome: time with effusion during follow-up (weeks), mean (SD), range | ||||||
| In the first 6 months | 1.2 (2.8), 0.0–17.5 | 10.8 (11.4), 0.0–26.0 | – | – | −9.6 (−12.0 to −7.2) | −0.1 (−1.1 to 0.8) |
| In the first 12 months | 2.0 (3.5), 0.0–17.5 | 17.0 (20.4), 0.0–52.0 | – | – | −15.0 (−19.3 to −10.8) | −0.4 (−1.4 to 0.7) |
| In the first 18 months | 2.6 (4.6), 0.0–26.0 | 27.8 (29.5), 0.0–78.0 | – | – | −23.1 (−29.6 to −16.7) | −0.3 (−1.8 to 1.2) |
| In the first 24 months | 3.3 (5.2), 0.0–26.0 | 33.0 (38.2), 0.0–104.0 | – | – | −29.7 (−38.2 to −21.2) | −0.8 (−2.5 to 0.9) |
| Secondary outcome: additional surgery during follow-up (yes), n/N (%) | ||||||
| In the first 6 months | 6/95 (6.3) | 66/343 (19.2) | −12.9% (−19.4% to −6.5%) | 8 | 0.33 (0.15 to 0.73) | 0.42 (0.15 to 1.18) |
| In the first 18 months | 15/95 (15.8) | 97/242 (40.1) | −24.3% (−33.9% to −14.7%) | 5 | 0.39 (0.24 to 0.64) | 0.36 (0.17 to 0.75) |
| In the first 24 months | 16/95 (16.8) | 102/242 (42.1) | −25.3% (−35.1% to −15.5%) | 4 | 0.40 (0.25 to 0.64) | 0.38 (0.19 to 0.76) |
Secondary outcomes
Data on all available secondary outcomes are also presented in Table 9.
A large difference was observed between groups during follow-up in the proportion of children with the presence of effusion for ≥ 50% of the time. However, this may be due to a measurement artefact because, as some studies had zero events, it was not possible to calculate an adjusted RR. This adjustment is particular important for these analyses as, in the studies contributing participants to the adenoidectomy only group,11,16 children were examined more frequently and more observations were made than in those studies contributing participants to the non-surgical group. 16,17,20
Adenoidectomy without grommets compared with unilateral or bilateral grommets
Primary outcome
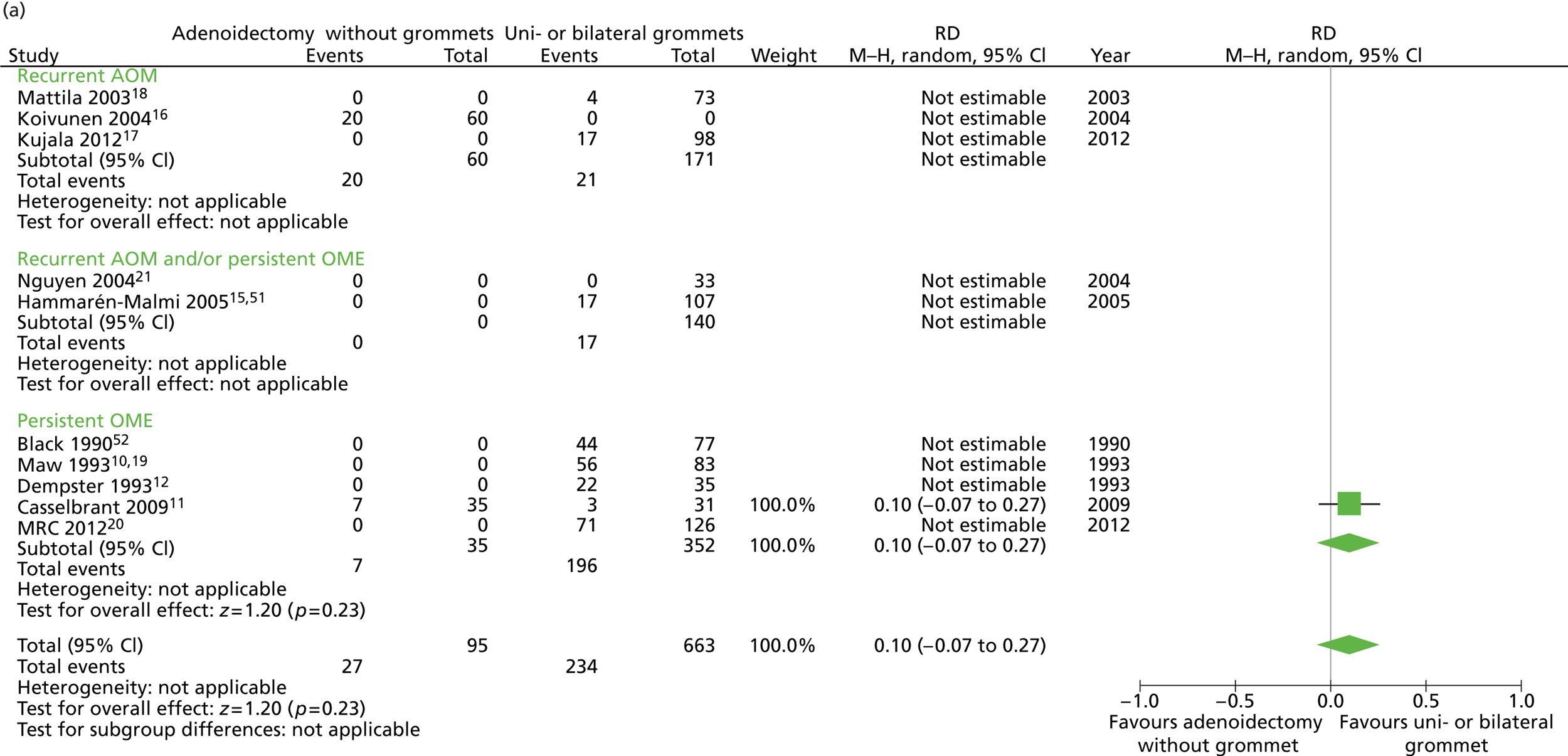
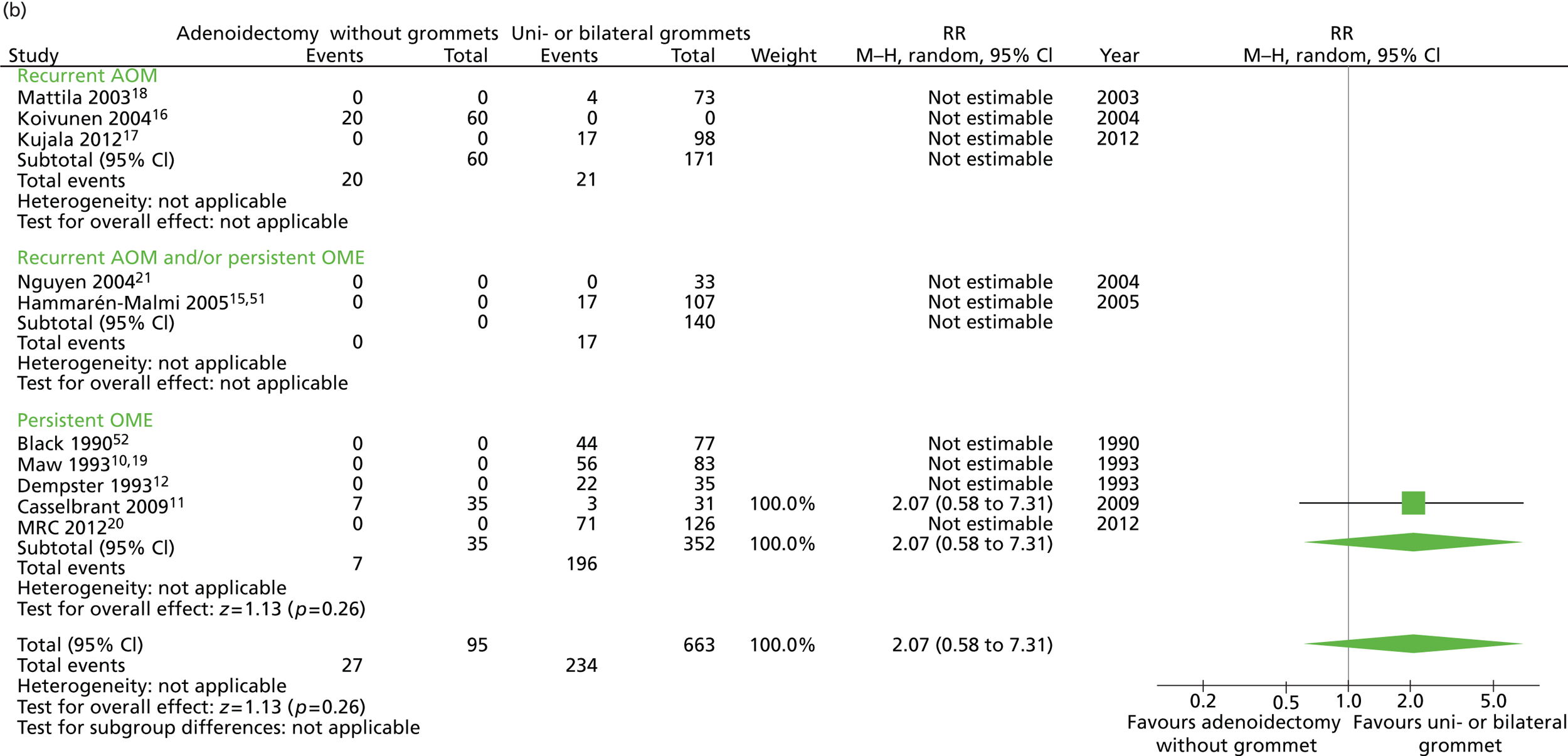
Figure 7 provides an overview of the overall effect within each individual study. The proportion of children who failed at 12 months was 28.4% in the group in which all children had adenoidectomy alone and 38.6% in the group who had grommets. The RD for failure at 12 months was −10.4% (95% CI −20.0% to −0.3%), resulting in a NNT of 10 children to prevent one failure. The RR was 0.74 (95% CI 0.53 to 1.03) and the adjusted RR (using a dummy for study) was 2.07 (95% CI 0.58 to 7.31) (Table 10).
FIGURE 7.
Overall failure at 12 months: effect of adenoidectomy without grommets compared with unilateral or bilateral grommets by study. (a) RD; and (b) RR. Note that the overall pooled effect size is not adjusted for the study. Table 10 provides the calculated RDs, RRs, and adjusted RRs which differ slightly from those in the forest plots as the underlying models differ and some indirect comparisons could be added to the regression analyses.
| Adenoidectomy with or without myringotomy (n = 95, k = 2)a | Unilateral or bilateral grommets (n = 651, k = 9)b | RD (95% CI)c | NNTc | RR or mean difference (95% CI)c | Adjusted RR or mean difference (95% CI)c | |
|---|---|---|---|---|---|---|
| Primary outcome, n/N (%) | ||||||
| Failure at 12 months | 27/95 (28.4) | 251/651 (38.6) | −10.1% (−20.0% to −0.3%) | 10 | 0.74 (0.53 to 1.03) | 2.07 (0.58 to 7.31) |
| Secondary outcome: individual items of failure at 12 months, n/N (%) | ||||||
| Four or more AOM episodes per year | 17/95 (17.9) | 34/404 (8.4) | 9.5% (1.3% to 17.7%) | – | 2.13 (1.24 to 3.6) | NA |
| Presence of effusion for ≥ 50% of the time | 0/89 (0.0) | 171/399 (42.9) | −42.9% (−47.7% to −38.0%) | 3 | NA | NA |
| Surgery during follow-up | 11/95 (11.6) | 76/651 (11.7) | −0.1% (−7.0% to 6.8%) | – | 0.99 (0.55 to 1.80) | 1.77 (0.48 to 6.49) |
| Secondary outcome: number of AOM episodes during follow-up, mean (SD), range | ||||||
| In the first 6 months | 1.1 (1.5), 0–8.0 | 0.7 (1.1), 0–6.0 | – | – | 0.3 (0.1 to 0.6) | 0.2 (−0.1 to 0.5) |
| In the first 12 months | 1.5 (2.0), 0–9.0 | 1.2 (1.6), 0–10.0 | – | – | 0.3 (−0.1 to 0.7) | 0.3 (−0.1 to 0.7) |
| In the first 18 months | 2.0 (2.4), 0–10.0 | 1.4 (1.9), 0–8.5 | – | – | 0.6 (0.0 to 1.1) | 0.2 (−0.3 to 0.7) |
| In the first 24 months | 2.1 (2.6), 0–10.0 | 2.2 (2.6), 0–12.0 | – | – | −0.1 (−0.7 to 0.5) | 0.1 (−0.5 to 0.6) |
| Secondary outcome: time with effusion during follow-up (weeks), mean (SD), range | ||||||
| In the first 6 months | 1.2 (2.8), 0.0–17.5 | 13.8 (9.2), 0.0–26.0 | – | – | −12.6 (−14.5 to −10.7) | 0.7 (0.1 to 1.2) |
| In the first 12 months | 2.0 (3.5), 0.0–17.5 | 22.1 (18.9), 0.0–52.0 | – | – | −20.2 (−24.1 to −16.2) | 1.5 (0.2 to 2.7) |
| In the first 18 months | 2.6 (4.6), 0.0–26.0 | 39.1 (26.4), 0.0–78.0 | – | – | −36.4 (−42.2 to −30.7) | 1.8 (0.2 to 3.4) |
| In the first 24 months | 3.3 (5.2), 0.0–26.0 | 51.0 (33.6), 0.0–104.0 | – | – | −47.8 (−55.2 to −40.3) | 1.4 (−1.0 to 3.8) |
| Secondary outcome: additional surgery during follow-up (yes), n/N (%) | ||||||
| In the first 6 months | 6/95 (6.3) | 27/651 (4.1) | 2.2% (−3.0% to 7.3%) | – | 1.52 (0.65 to 3.59) | NA |
| In the first 18 months | 15/95 (15.8) | 82/389 (21.1) | −5.3% (−13.7% to 3.1%) | 19 | 0.75 (0.45 to 1.24) | 1.42 (0.52 to 3.88) |
| In the first 24 months | 16/95 (16.8) | 109/389 (28.0) | −11.2% (−19.9% to −2.4%) | 9 | 0.60 (0.37 to 0.97) | 1.18 (0.46 to 3.03) |
Secondary outcomes
Data on all available secondary outcomes are also presented in Table 10. The same large difference between groups during follow-up in the proportion of children with effusion for ≥ 50% of the time was noted here, which again may be due to the measurement artefact described in the previous section.
Sensitivity analysis
A series of sensitivity analyses using alternative definitions of failure at 12 months showed similar results. A per-protocol analysis, in which we excluded children in the no adenoidectomy group who nevertheless underwent adenoidectomy, showed similar results to the intention-to-treat analysis. The as-treated analysis, in which children from the no adenoidectomy group who had their adenoid removed were analysed in the adenoidectomy group, was performed with two definitions of failure.
We had to use another definition of failure as ‘additional surgery’ was also part of our primary definition of failure, resulting in overlapping of the independent and dependent variables. With the main definition, that is, including ‘additional surgery’, no effect of adenoidectomy was found, which is probably because of the overlapping independent and dependent variables. When ‘additional surgery’ was omitted from the definition of failure (dependent variable), the significant adenoidectomy effect disappeared but the direction of the effects was similar. The results of the per-protocol and as-treated analyses for the overall effect are shown in Table 11.
| Analysis | Adenoidectomy with or without unilateral or bilateral grommets, n/N (%) | Non-surgical treatment or unilateral or bilateral grommets only, n/N (%) | RD (95% CI)a | NNTa | RR (95% CI)a | Adjusted RR (95% CI)a |
|---|---|---|---|---|---|---|
| Intention to treat | 245/767 (31.9) | 444/994 (44.7) | −12.7% (−17.2% to −8.2%) | 8 | 0.72 (0.63 to 0.81) | 0.76 (0.69 to 0.85) |
| Per protocol | 245/767 (31.9) | 355/905 (39.2) | −7.3% (−11.9% to −2.7%) | 10 | 0.81 (0.71 to 0.93) | 0.88 (0.78 to 0.98) |
| As treatedb | 334/856 (39.0) | 355/905 (39.2) | −0.2% (−4.8% to 4.4%) | – | 1.00 (0.89 to 1.12) | 1.08 (0.98 to 1.20) |
| As treatedc | 270/856 (31.5) | 324/905 (35.8) | −4.3% (−8.7% to 0.2%) | – | 0.88 (0.77 to 1.01) | 0.97 (0.87 to 1.08) |
2b. Identification of those subgroups of children who benefit most, or who are most likely to benefit, from adenoidectomy with or without grommets
The prognostic analyses revealed indication (recurrent AOM or persistent OME) as one potential subgrouping variable and, in addition, we had decided a priori to include age. We studied these factors individually and in combination.
All significant interaction effects (for both primary and secondary outcomes) for all comparisons are presented in Appendices 11–15. In the text and figures that follow we report only significant interaction effects for the analyses stratified on indication.
Subgroup analysis: main comparison
Adenoidectomy with or without grommets compared with non-surgical treatment or grommets only
Primary outcome
We found the anticipated differences in effects based on the indication for surgery (recurrent AOM compared with persistent OME) (Table 12).
| n (%) | Adenoidectomy with or without unilateral or bilateral grommetsa | Non-surgical treatment or unilateral or bilateral grommets onlyb | RD (95% CI)c | NNTc | RR or mean difference (95% CI)c | Adjusted RR or mean difference (95% CI)c | p-value for interactionc | |
|---|---|---|---|---|---|---|---|---|
| Indication: recurrent AOM | ||||||||
| Primary outcome: failure after 12 months, n/N (%) | ||||||||
| < 2 years | 719 (89.5) | 44/281 (15.7) | 120/438 (27.4) | −11.7% (−17.7% to −5.8%) | 9 | 0.57 (0.42 to 0.78) | 0.63 (0.47 to 0.85) | |
| ≥ 2 years | 84 (10.5) | 8/44 (18.2) | 1/40 (2.5) | 15.7% (3.3% to 28.1%) | – | 7.27 (0.95 to 55.6) | 4.96 (0.69 to 35.5) | 0.04 |
| Secondary outcome: four or more AOM episodes per year, n/N (%) | ||||||||
| < 2 years | 658 (91.0) | 32/252 (12.7) | 76/406 (18.7) | −6.0% (−11.6% to −0.4%) | 17 | 0.68 (0.46 to 0.99) | 0.71 (0.49 to 1.02) | |
| ≥ 2 years | 65 (9.0) | 7/33 (21.2) | 1/32 (3.1) | 18.1% (2.9% to 33.3%) | – | 6.79 (0.88 to 52.10) | 4.80 (0.64 to 35.7) | 0.05 |
| Secondary outcome: number of AOM episodes in the first 12 months of follow-up, mean (SD) | ||||||||
| < 2 years | 658 (91.0) | 1.5 (1.7) | 2.0 (2.1) | – | – | −0.5 (−0.8 to −0.2) | −0.5 (−0.8 to −0.2) | |
| ≥ 2 years | 65 (9.0) | 1.6 (2.0) | 0.8 (1.1) | – | – | 0.8 (0.0 to 1.6) | 0.6 (–0.1 to 1.4) | < 0.01 |
| Indication: persistent OME | ||||||||
| Primary outcome: failure after 12 months, n/N (%) | ||||||||
| < 4 years | 239 (24.5) | 30/128 (23.4) | 33/111 (29.7) | −6.9% (−17.5% to 4.9%) | 15 | 0.79 (0.52 to 1.20) | 0.98 (0.69 to 1.38) | |
| ≥ 4 years | 737 (75.5) | 163/322 (50.6) | 289/415 (69.6) | −19.0% (−26.1% to −12.0%) | 6 | 0.73 (0.64 to 0.82) | 0.77 (0.68 to 0.86) | 0.10 |
| Secondary outcome: surgery during the first 12 months of follow-up, n/N (%) | ||||||||
| < 4 years | 238 (24.5) | 13/127 (10.2) | 11/111 (9.9) | 0.3% (−7.3% to 8.0%) | – | 1.03 (0.48 to 2.21) | 1.03 (0.46 to 2.30) | |
| ≥ 4 years | 735 (75.5) | 7/320 (2.2) | 78/415 (18.8) | −16.6% (−20.7% to −12.5%) | 6 | 0.12 (0.05 to 0.25) | 0.13 (0.06 to 0.27) | < 0.01 |
| Secondary outcome: number of AOM episodes in the first 12 months of follow-up, mean (SD) | ||||||||
| < 4 years | 189 (35.5) | 0.6 (0.1) | 0.8 (0.1) | – | – | –0.2 (−0.5 to 0.2) | 0.1 (−0.1 to 0.4) | |
| ≥ 4 years | 343 (64.5) | 1.0 (0.2) | 1.5 (0.2) | – | – | −0.5 (−0.9 to −0.1) | −0.4 (−0.8 to 0.0) | 0.05 |
| Secondary outcome: time with effusion during the first 12 months of follow-up (weeks), mean (SD) | ||||||||
| < 4 years | 150 (18.1) | 11.1 (1.7) | 18.2 (2.4) | – | – | −7.1 (−12.9 to −1.4) | −3.2 (−6.4 to −0.1) | |
| ≥ 4 years | 681 (81.9) | 23.2 (0.9) | 30.1 (0.9) | – | – | −7.0 (−9.4 to −4.6) | −6.6 (−9.0 to −4.3) | 0.05 |
-
Indication: recurrent AOM – a significant interaction effect (p = 0.04) was found for age: < 2 years compared with ≥ 2 years:
-
< 2 years: the proportion who failed at 12 months was 15.7% in the adenoidectomy group (adenoidectomy with or without grommets) and 27.4% in the no adenoidectomy (non-surgical or grommets alone) group (unadjusted RD –11.7%, 95% CI −17.7% to −5.8%; unadjusted RR 0.57, 95% CI 0.42 to 0.78; adjusted RR 0.63, 95% CI 0.47 to 0.85)
-
≥ 2 years: the proportion who failed at 12 months was 18.5% in the adenoidectomy group (adenoidectomy with or without grommets) and 2.5% in the no adenoidectomy (non-surgical or grommets alone) group (unadjusted RD 15.7%, 95% CI 3.3% to 28.1%; unadjusted RR 7.27, 95% CI 0.95 to 55.6; adjusted RR 4.96, 95% CI 0.69 to 35.5).
-
-
Indication: persistent OME – a marginal significant interaction effect (p = 0.1) was found for age: < 4 years compared with ≥ 4 years:
-
< 4 years: the proportion who failed at 12 months was 23.4% in the adenoidectomy group (adenoidectomy with or without grommets) and 29.7% in the no adenoidectomy (non-surgical or grommets alone) group (unadjusted RD −6.9%, 95% CI −17.5% to 4.9%; unadjusted RR 0.79, 95% CI 0.52 to 1.20; adjusted RR 0.98, 95% CI 0.69 to 1.38)
-
≥ 4 years: the proportion who failed at 12 months was 50.6% in the adenoidectomy group (adenoidectomy with or without grommets) and 69.6% in the no adenoidectomy (non-surgical or grommets alone) group (unadjusted RD −19.0%, 95% CI −26.1% to −12.0%; unadjusted RR 0.73, 95% CI 0.64 to 0.82; adjusted RR 0.77, 95% CI 0.68 to 0.86).
-
Secondary outcomes
The significant interaction effects for the secondary outcomes are reported in Table 12 and Appendix 15. This extends the analysis beyond that originally proposed but avoids the risk of missing a significant finding.
-
Indication: recurrent AOM – a significant interaction effect (p = 0.05) was found for age: < 2 years compared with ≥ 2 years:
-
< 2 years: the proportion of children with four or more AOM episodes per year was 12.7% in the adenoidectomy group (adenoidectomy with or without grommets) and 18.7% in the no adenoidectomy (non-surgical or grommets alone) group (unadjusted RD −6.0%, 95% CI −11.6% to −0.4%; unadjusted RR 0.68, 95% CI 0.46 to 0.99; adjusted RR 0.71, 95% CI 0.49 to 1.02)
-
≥ 2 years: the proportion of children with four or more AOM episodes per year was 21.2% in the adenoidectomy group (adenoidectomy with or without grommets) and 3.1% in the no adenoidectomy (non-surgical or grommets alone) group (unadjusted RD 18.1%, 95% CI 2.9% to 33.3%; unadjusted RR 6.79, 95% CI 0.88 to 52.10; adjusted RR 4.80, 95% CI 0.64 to 35.7).
-
-
Indication: recurrent AOM – a significant interaction effect (p = 0.01) was found for age: < 2 years compared with ≥ 2 years:
-
< 2 years: the number of AOM episodes in the first 12 months was 1.5 episodes [standard deviation (SD) 1.7 episodes] in the adenoidectomy group (adenoidectomy with or without grommets) and 2.0 episodes (SD 2.1 episodes) in the no adenoidectomy (non-surgical or grommets alone) group (adjusted mean difference −0.5 episodes, 95% CI −0.8 to −0.2 episodes)
-
≥ 2 years: the number of AOM episodes in the first 12 months was 1.6 episodes (SD 2.0 episodes) in the adenoidectomy group (adenoidectomy with or without grommets) and 0.8 episodes (SD 1.1 episodes) in the no adenoidectomy (non-surgical or grommets alone) group (adjusted mean difference 0.6 episodes, 95% CI −0.1 to 1.4 episodes).
-
-
Indication: persistent OME – a significant interaction effect (p = 0.05) was found for age: < 4 years compared with ≥ 4 years:
-
< 4 years: the number of AOM episodes in the first 12 months was 0.6 episodes (SD 0.1 episodes) in the adenoidectomy group (adenoidectomy with or without grommets) and 0.8 episodes (SD 0.1 episodes) in the no adenoidectomy (non-surgical or grommets alone) group (adjusted mean difference −0.1 episodes, 95% CI −0.1 to 0.4 episodes)
-
≥ 4 years: the number of AOM episodes in the first 12 months was 1.0 (SD 0.2 episodes) in the adenoidectomy group (adenoidectomy with or without grommets) and 1.5 episodes (SD 0.2 episodes) in the no adenoidectomy (non-surgical or grommets alone) group (adjusted mean difference −0.4 episodes, 95% CI −0.8 to 0.0 episodes).
-
-
Indication: persistent OME – a significant interaction effect (p < 0.01) was found for age: < 4 years compared with ≥ 4 years:
-
< 4 years: the proportion having additional surgery was 10.7% in the adenoidectomy group (adenoidectomy with or without grommets) and 9.9% in the no adenoidectomy (non-surgical or grommets alone) group (unadjusted RD 0.3%, 95% CI −7.3% to 8.0%; unadjusted RR 1.03, 95% CI 0.48 to 2.21; adjusted RR 1.03, 95% CI 0.46 to 2.30)
-
≥ 4 years: the proportion having additional surgery was 2.2% in the adenoidectomy group (adenoidectomy with or without grommets) and 18.8% in the no adenoidectomy (non-surgical or grommets alone) group (unadjusted RD −16.6%, 95% CI −20.7% to −12.5%; unadjusted RR 0.12, 95% CI 0.05 to 0.25; adjusted RR 0.13, 95% CI 0.06 to 0.27).
-
-
Indication: persistent OME – a significant interaction effect (p = 0.05) was found for age: < 4 years compared with ≥ 4 years:
-
< 4 years: the number of weeks with effusion in the first 12 months was 11.1 weeks (SD 1.7 weeks) in the adenoidectomy group (adenoidectomy with or without grommets) and 18.2 weeks (SD 2.4 weeks) in the no adenoidectomy (non-surgical or grommets alone) group (adjusted mean difference −3.2 weeks, 95% CI −6.4 to −0.1 weeks)
-
≥ 4 years: the number of weeks with effusion in the first 12 months was 23.2 weeks (SD 0.9 weeks) in the adenoidectomy group (adenoidectomy with or without grommets) and 30.1 weeks (SD 0.9 weeks) in the no adenoidectomy (non-surgical or grommets alone) group (adjusted mean difference −6.6 weeks, 95% CI −9.0 to −4.3 weeks).
-
Subgroup analysis: secondary comparisons
For most secondary outcomes the numbers per subgroup were < 15. The results are therefore inherently unstable and these analyses are not included.
Adenoidectomy with unilateral or bilateral grommets compared with non-surgical treatment
Primary outcome
No significant interactions were found for either indication.
Secondary outcomes
No significant interactions were found for either indication.
Adenoidectomy with unilateral or bilateral grommets compared with unilateral or bilateral grommets
Primary outcome
As above we found a difference in subgroup effects based on the indication for surgery (a history of recurrent AOM compared with a history of persistent OME). However, in this instance we found a difference only when the indication was recurrent AOM (Table 13).
| n (%) | Adenoidectomy with unilateral or bilateral grommets (n = 265) | Unilateral or bilateral grommets (n = 257) | RD (95% CI)a | NNTa | RR or mean difference (95% CI)a | Adjusted RR or mean difference (95% CI)a | p-value for interactiona | |
|---|---|---|---|---|---|---|---|---|
| Indication: recurrent AOM | ||||||||
| Primary outcome: failure after 12 months, n/N (%) | ||||||||
| < 2 years | 439 (84.1) | 24/221 (10.9) | 36/218 (16.5) | −5.7% (−12.1% to 0.8%) | 18 | 0.66 (0.41 to 1.06) | 0.69 (0.43 to 1.11) | |
| ≥ 2 years | 83 (15.9) | 8/44 (18.2) | 1/39 (2.6) | 15.6% (3.2% to 28.1%) | – | 7.09 (0.93 to 54.2) | 4.96 (0.69 to 35.52) | 0.06 |
| Secondary outcome: number of AOM episodes in the first 12 months of follow-up, mean (SD) | ||||||||
| < 2 years | 378 (85.5) | 1.3 (1.5) | 1.5 (1.7) | – | – | −0.2 (−0.5 to 0.7) | −0.3 (−0.6 to 0.5) | |
| ≥ 2 years | 64 (14.5) | 1.6 (2.0) | 0.8 (1.1) | – | – | 0.8 (−0.0 to 1.6) | 0.6 (−0.1 to 1.4) | 0.03 |
| Indication: persistent OME | ||||||||
| Secondary outcome: surgery during the first 12 months of follow-up, n/N (%) | ||||||||
| < 4 years | 192 (23.5) | 7/92 (7.6) | 8/100 (8.0) | −0.4% (−0.8% to 7.2%) | – | 0.95 (0.36 to 2.52) | 0.81 (0.31 to 2.15) | |
| ≥ 4 years | 624 (76.5) | 7/320 (2.2) | 38/304 (12.5) | −10.3% (−14.4% to −6.3%) | 10 | 0.18 (0.08 to 0.39) | 0.20 (0.09 to 0.44) | 0.06 |
-
Indication: recurrent AOM – a marginal significant interaction effect (p = 0.06) was found for age: < 2 years compared with ≥ 2 years:
-
< 2 years: the proportion who failed at 12 months was 10.9% in the adenoidectomy with grommets group and 16.5% in the grommets only group (unadjusted RD −5.7%, 95% CI −12.1% to 0.8%; unadjusted RR 0.66, 95% CI 0.41 to 1.06; adjusted RR 0.69, 95% CI 0.43 to 1.11)
-
≥ 2 years: the proportion who failed at 12 months was 18.2% in the adenoidectomy with grommets group and 2.6% in the grommets only group (unadjusted RD 15.6%, 95% CI 3.2% to 28.1%; unadjusted RR 7.09, 95% CI 0.93 to 54.2; adjusted RR 4.96, 95% CI 0.69 to 35.52).
-
Secondary outcomes
The significant interaction effects for the secondary outcomes are reported in Table 13 and Appendix 16. This extends the analysis beyond that originally proposed but avoids the risk of missing a significant finding.
-
Indication: recurrent AOM – a significant interaction effect (p = 0.03) was found for age: < 2 years compared with ≥ 2 years:
-
< 2 years: the number of AOM episodes in the first 12 months was 1.3 episodes (SD 1.5 episodes) in the adenoidectomy with grommets group and 1.5 episodes (SD 1.7 episodes) in the grommets only group (adjusted mean difference −0.2 episodes, 95% CI −0.5 to 0.7 episodes)
-
≥ 2 years: the number of AOM episodes in the first 12 months was 1.6 episodes (SD 2.0 episodes) in the adenoidectomy with grommets group and 0.8 episodes (SD 1.1 episodes) in the grommets only group (adjusted mean difference 0.8 episodes, 95% CI −0.0 to 1.6 episodes).
-
-
Indication: persistent OME – a marginally significant interaction effect (p = 0.06) was found for age: < 4 years compared with ≥ 4 years:
-
< 4 years: the proportion having additional surgery was 7.6% in the adenoidectomy with grommets group and 8.0% in the grommets only group (unadjusted RD −0.4%, 95% CI −0.8% to 0.7%; unadjusted RR 0.95, 95% CI 0.36 to 2.52; adjusted RR 0.81, 95% CI 0.31 to 2.15)
-
≥ 4 years: the proportion having additional surgery was 2.2% in the adenoidectomy with grommets group and 12.5% in the grommets only group (unadjusted RD −10.3%, 95% CI −14.4% to −6.3%; unadjusted RR 0.18, 95% CI 0.08 to 0.39; adjusted RR 0.20, 95% CI 0.09 to 0.44).
-
Adenoidectomy without grommets compared with non-surgical treatment
Primary outcome
No significant interactions were found for either indication.
Secondary outcomes
No significant interactions were found for either indication.
Adenoidectomy without grommets compared with unilateral or bilateral grommets
Primary outcome
No significant interactions were found for either indication.
Secondary outcomes
No significant interactions were found for either indication.
Sensitivity analysis
A series of sensitivity analyses using alternative definitions of failure at 12 months showed similar results. A per-protocol analysis, in which we excluded children in the no adenoidectomy group who nevertheless underwent adenoidectomy, showed similar results to the intention-to-treat analysis. In the as-treated analysis, in which children from the no adenoidectomy group who had their adenoid removed were analysed in the adenoidectomy group, the effect of adenoidectomy was no longer significant but the direction of the effects was similar when using a definition of failure that excluded additional surgery. The results of the per-protocol and as-treated analyses for the main comparison are shown in Appendix 15.
Adverse effects or events
As adverse effects and events are important secondary outcomes we report the available data here. We sought evidence of the specific complications of adenoidectomy: primary and secondary haemorrhage, hypernasal speech and the complications of general anaesthesia. Two studies11,20 reported data on postoperative complications and adverse effects, three12,16,17 reported that no surgery-related complications had occurred and five15,18,19,21,52 did not report on complications or adverse effects.
The adenoidectomy complication reported by Casselbrant et al. 11 was a single child in the adenoidectomy with grommets group (1/32, 3.1%) in whom difficulty during anaesthesia led to the child subsequently being treated with grommets only. In the MRC study20 one child (1/165, 0.6%) had to return to theatre because of postoperative haemorrhage.
Chapter 4 Discussion
Main findings
This IPD meta-analysis includes 10 trials involving 1761 children with OM (recurrent AOM or persistent OME). Nine out of the 10 studies made a comparison between adenoidectomy and grommets and grommets alone, that is, the direct comparisons from these nine studies provide the most robust evidence. Most of the included studies had a low risk of bias. Twelve months after surgery, children who have had their adenoid removed have a greater chance of clinical improvement. The size of that effect is, in general, small but persists for at least 2 years after surgery. The RD of 13% corresponds to a NNT of eight to prevent one failure; the adjusted RR was 0.76 (95% CI 0.69 to 0.85). Improvement was seen in outcomes that were not found to be significant in a previous non-IPD meta-analysis,28 for example lower number of AOM episodes, less time with effusion, less additional surgery, less hearing loss and improvement in hearing levels up to 2 years after surgery. Effect sizes are small but consistent, with NNTs ranging from 5 to 21.
To predict which children are likely to have a prolonged duration of their OM we looked specifically at those children who did not receive any surgery at all (adenoidectomy or grommets) at study entry and who are likely to best reflect the natural history of untreated disease (‘non-surgical children’). Of these children 56% failed to improve at 12 months. The only factor that predicted which children had a prolonged duration of their OM was the indication for surgery (recurrent AOM or persistent OME). In children with recurrent AOM as the indication for intervention 38% failed to improve, whereas in children with persistent OME as the indication for intervention 89% failed to improve.
In the subgroup analyses we found the anticipated differences in effects based on the indication for surgery (recurrent AOM compared with persistent OME). Two subgroups of children are most likely to benefit from adenoidectomy. These are (1) children with recurrent AOM aged < 2 years and (2) older children aged ≥ 4 years with persistent OME.
The proportion of children with recurrent AOM aged < 2 years who failed at 12 months was 16% in the adenoidectomy group and 27% in the group who did not have adenoidectomy. This RD of 12% corresponds to a NNT of nine to prevent one failure. The adjusted RR was 0.63 (95% CI 0.47 to 0.85). In contrast, in children aged ≥ 2 years, no benefit of adenoidectomy was seen.
The proportion of children with persistent OME aged ≥ 4 years who failed at 12 months was 51% in the adenoidectomy group and 70% in the group who did not have adenoidectomy. This RD of 19% corresponds to a NNT of six to prevent one failure. The adjusted RR was 0.77 (95% CI 0.68 to 0.86). In children aged < 4 years no significant benefit of adenoidectomy was seen.
These findings relate to the main comparison, that is, adenoidectomy or adjuvant adenoidectomy (adenoidectomy with grommets) compared with no surgery or grommets alone. When the subgroup analyses were repeated with our secondary comparisons, the statistical power to detect significant interactions was necessarily reduced. However, we still found a difference in subgroup effects based on indication for surgery and age when adenoidectomy with grommets was compared with grommets alone (the ‘adding adenoidectomy’ comparison, assessing the effect of so-called ‘adjuvant adenoidectomy’). Age was found to be of influence only in the recurrent AOM group.
In the development of the predictive model age was not identified as a factor predicting those children with a prolonged (12-month) duration of their OM. We had anticipated that it might be a factor and that there might be a detectable interaction between age and indication. Yet in the subgroup analysis, in children with recurrent AOM, age was a factor associated with failure at 12 months in young children aged < 2 years compared with older children. The reason for this is that almost all of the children in the trials that evaluate interventions for recurrent AOM are young – aged < 2 years. As one moves from analysing the whole group to subgroups, the recurrent AOM subgroup and the younger children (< 2 years) subgroup contain the same children. Identifying an interaction between indication and age was therefore impossible: there were insufficient data to detect an interaction even if one exists because there are not enough older children (aged ≥ 4 years) in the recurrent AOM group and also not enough younger children (aged < 2 years) in the persistent OME group.
These findings support the general understanding of at least two clinical entities within a spectrum of OM across the age range from birth to 12 years: recurrent AOM in younger children (aged < 2 years) and persistent OME in older children (aged ≥ 4 years). Children aged ≥ 4 years with recurrent AOM are rare within our IPD data set either because the trial entry criteria specifically excluded them (we know that this is true for some of our included trials – all of the trials that enrolled children with recurrent AOM only specifically excluded children aged > 2 years) or because this combination is a rare occurrence. We did in fact include trials in which children aged > 2 years with recurrent AOM could have been included, but they are present in very low numbers.
Strengths and limitations
The main strength of our study is that by reanalysing the original data from 10 trials of adenoidectomy we could include 1761 children in our IPD meta-analysis, which gave us the power to identify subgroups that could benefit most from adenoidectomy.
Some of our findings deserve further discussion:
-
We contacted the principal investigators of all 15 eligible RCTs to ask if they were willing and able to provide their raw data. We were unable to contact the principal investigator of one trial24 and the data for four older trials (1978–99)14,22,23,25 were no longer available. The main characteristics of these five trials are, however, much the same as those of the 10 included trials. Moreover, the overall results of our subset of 10 trials are very similar to the overall results reported by the Cochrane review28 that did include all of these trials. A funnel plot of the included studies (not shown) also indicates that publication bias is unlikely in this IPD meta-analysis.
-
Not all variables were measured in all eligible trials so we chose to use a composite outcome, failure at 12 months, defined as failing on at least one out of four components. Only one of the 10 included trials measured all four components; six trials measured three of the four components and the other three trials measured two of the four components (see also Appendix 10). Furthermore, we could not study all potential effect modifiers. The total number of baseline variables measured in the included trials varied between 9 and 39 per trial. Of the variables of interest, only three were measured in all 10 trials.
-
In our prognostic analyses we included only those children allocated to non-surgical treatment. This is appropriate because adenoidectomy with or without grommets might influence the course of the disease and might result in an invalid natural history model.
-
We did not study all potential subgroups. We selected previously established predictors of a prolonged course and some clinically relevant variables and performed stratified analyses only for those variables that showed a significant p-value (< 0.1) for interaction in the regression model. Did we therefore ‘miss’ a relevant subgroup? Our approach follows the recommendations for the study of subgroups. The strength of this approach is that our prognostic analyses revealed only a few relevant subgroups. By limiting the number of subgroup analyses we minimise the likelihood of false-positive findings (type I error) that could be caused by multiple testing. We were unable to study some clinically relevant subgroups that might benefit more from adenoidectomy, such as children with Down syndrome, because such children were excluded from the individual trials. The experience of many clinicians – that these and other subgroups of children benefit more from adenoidectomy than others – has not yet been evidenced in RCTs. Neither could we study the effect of adenoidectomy in children with differing baseline hearing levels as hearing was not assessed in all included studies. In the studies that did assess hearing at baseline, the effects of adenoidectomy did not differ between groups with different baseline hearing levels.
-
Although an overall measure of severity including both persistent AOM and recurrent OME is not available, frequency of AOM, duration of symptoms and degree of hearing loss are potential measures of severity. They were included in the prognostic analyses and were not found to be of influence when tested in the multivariate analyses.
-
Because of relatively wide variation in the specific surgical interventions across the 10 included trials, we had to make a fairly large number of comparisons, which renders interpretation of the results more difficult and introduces a degree of obfuscation. Although we focused on our main comparison, adenoidectomy with or without grommets compared with non-surgical treatment or grommets only, we also compared adenoidectomy with unilateral or bilateral grommets with non-surgical treatment; adenoidectomy with unilateral or bilateral grommets with unilateral or bilateral grommets; adenoidectomy without grommets with non-surgical treatment; and adenoidectomy without grommets with unilateral or bilateral grommets. Because of the smaller numbers in some of these secondary comparisons, the subgroup effects are inevitably less obvious. However, there were no inconsistencies in effect directions.
-
In all of our studies short-term tubes were used; therefore, ‘tube life’ is unlikely to have influenced the results.
-
In our pooled analysis of failure at 12 months we combined several outcomes, which were measured slightly differently across the 10 included trials. Sensitivity analysis using an alternative set of definitions of ‘failure’ showed similar results. Furthermore, the time points for follow-up assessments varied across the included trials (e.g. some took measurements every month, others every 3 or 6 months). As a result, a measurement artefact cannot be precluded. To evaluate this further we undertook a sensitivity analysis with the trials that assessed the participants most frequently and found that the results were consistent with the overall results.
-
We included trials that studied the effects of surgery in children with persistent OME as well as those studying the effects of surgery in children with recurrent AOM or combinations of persistent OME and recurrent AOM. We believe that these two clinical conditions, although distinctly defined, are in fact closely related as part of a spectrum of disease. Our analyses showed different effects based on the indication for surgery for both the main effect and the subgroup effects. This confirms that these two indications should be distinguished in both clinical and research settings, with implications for clinical practice and future research.
-
We chose a composite outcome as the included trials each used slightly different outcomes. It is known that combined outcomes can make a treatment seem more or less effective than it really is,54 that is, higher event rates and larger treatment effects associated with less important components may result in misleading impressions of the impact of treatment. We therefore performed sensitivity analyses using different composite outcomes, which all showed similar results. By using such a composite end point we were able to aggregate our data and generalise results. This was critical to accomplish our aim of establishing which children benefit more or less from adenoidectomy.
-
Some studies randomised ears rather than children, and it is self-evident that this applies only to the insertion of grommets (i.e. in some studies a grommet was inserted unilaterally). The focus of our IPD meta-analysis was the effect of adenoidectomy, and this intervention was always randomised to children. We compared children and those randomised to adenoidectomy with/without grommets with those randomised to non-surgical treatment or grommets only. Other comparisons included adenoidectomy with unilateral or bilateral grommets compared with adenoidectomy without grommets, and unilateral or bilateral grommets compared with non-surgical treatment. We pooled the studies that randomised ears with those that randomised children. Sensitivity analysis in which we excluded the (older) trials that randomised ears rather than children did not change our results.
-
We were unable to calculate adjusted RDs because of the calculation artefact that occurs when there are zero events in a study. 21 We decided to also present the unadjusted RDs as these are the easiest to understand and interpret. In most cases the adjusted RR and the raw RR did not differ much, suggesting that confounding is not a big issue.
-
Although informative, we did not compare (1) adenoidectomy plus unilateral or bilateral grommets with adenoidectomy without grommets or (2) unilateral or bilateral grommets with non-surgical treatment as these analyses do not fulfil the criteria for inclusion in this IPD meta-analysis.
-
Most data on recurrent AOM come from Finland and this may have influenced our results. Personal communication with the authors of these Finnish trials led us to realise that early surgery for recurrent AOM might be a cultural issue. Reasons for performing these trials included the high adenoidectomy rate in Finland compared with that in other Scandinavian countries, for example Norway. The authors also confirmed that the number of children undergoing adenoidectomy has decreased since the results of these trials were published. We are not aware of any societal or biological factors varying across Western countries that may cause the effects of treatment in Finland to differ from the effects of treatment in other countries.
-
In surgical trials such as those included in our IPD meta-analysis, only the children who are assigned to the control arm, who do not undergo the operation under study, can transfer, that is, move to a treatment arm because of persisting problems. Per-protocol analyses that exclude children who transfer to the other arm of the trial will therefore underestimate the effect of treatment. Conversely, analysing children on the basis of time spent in a treatment arm might overestimate or underestimate this effect.
-
Finally, nine out of the 10 included studies made a comparison between adenoidectomy with grommets and grommets alone. The comparisons from these nine studies therefore seem to provide the most robust evidence.
Clinical implications
The effect of adenoidectomy seems to vary with age both in children with recurrent AOM and in children with persistent OME. However, it may not be age per se that is relevant but rather the presence of differing pathophysiological mechanisms in different age groups that serves as the modifier, with age simply being a proxy for these. OM is known to result from an interplay between microbial load and the immune response. The Eustachian tube is the port of entry for middle ear pathogens from the nasopharynx, but it also plays an important part in clearing middle ear secretions. One responsible mechanism may relate to the relative immaturity of the immune response in younger children, particularly those children aged < 2 years. As the immune response improves with age, problems with infection may recede and those associated with a middle ear effusion, that is, hearing loss, become relatively more apparent. This coincides with a period of social and behavioural change in the child’s life. Starting school and becoming part of a peer group may be the factors that initiate concerns about performance and increase awareness of the child’s hearing. More insight into the pathophysiology of OM is needed to understand better the causal mechanisms of the subgroup effects. In the meantime, clinicians can still use the information about age and indication as part of the shared decision-making process when deciding with parents whether or not to operate on their child.
Like all health-care decisions, the decision whether or not to undertake adenoidectomy will be influenced by existing local practice. It is extremely unusual for adenoidectomy to be undertaken in the UK in children aged < 2 years for any indication other than obstructive sleep apnoea, when it may be performed with tonsillectomy. In other northern European countries adenoidectomy is common in young children, as the presence of such children in some of the included studies indicates.
In our analyses we found no evidence to support the suggestion by NICE that possible benefits of adenoidectomy should be considered in children selected for grommets for OME who also suffer from respiratory symptoms. The number of upper respiratory tract infections was not a predictor of failure at 12 months. However, this might be because upper respiratory tract infections were measured in only one trial, resulting in a relatively low number of children in whom upper respiratory tract infections could be studied as a predictor.
As with all interventions (and particularly in the case of surgical procedures), consideration must be given to the ratio between benefits and harms. In the children aged < 4 years a modest benefit may easily be outweighed by the potential risks and harms. Those same risks and harms are the reason why the overall small beneficial effect found for children of all ages should not be used as the rationale for treating all children.
The trials included in this IPD meta-analysis provide very limited information on the adverse effects of adenoidectomy, an understanding of which is clearly critical when undertaking a risk–benefit analysis before making a treatment decision.
Chapter 5 Conclusions
Children with OM who have their adenoid removed have a greater chance of clinical improvement; eight children need to receive adenoidectomy to prevent one failure. Adenoidectomy is most beneficial in children aged ≥ 4 years with persistent OME (six children needing adenoidectomy to prevent one failure). A smaller beneficial effect was found in children with recurrent AOM aged < 2 years (nine children needing adenoidectomy to prevent one failure). No beneficial effect was seen in children aged < 4 years with persistent OME or ≥ 2 with recurrent AOM.
The need to use a composite end point and the limited number of subgroup variables that could be studied are factors that reduce the robustness of these results; however, we do not believe that these factors reduce the validity of the conclusions.
As with all interventions (and in particular surgical procedures), consideration must be given to the balance between benefits and harms. Clinicians can discuss these issues with the parents of children with OM to allow them to make an informed treatment decision.
Future research is required in a number of key areas, including defining the best methods of selecting, developing and administering patient-reported outcome measures (PROMs) to assess the value of treatments for children with persistent OME and recurrent AOM and upper respiratory infections; investigating the clinical effectiveness and cost-effectiveness of hearing aids and the use of interventions to improve classroom acoustics for children with different degrees of persistence and severity of hearing loss associated with OME; and investigating why professionals’ and parents’/carers’ treatment preferences vary so much both nationally and internationally.
We do not understand why adenoidectomy works in different subgroups at different ages, nor its effects in special populations, such as children with Down syndrome. We also need further research on the impact and optimal management of otitis media in these special situations and others, such as in children with a cleft palate or developmental problems.
Acknowledgements
We are grateful and extend our thanks to all study representatives for providing their data, their willingness to answer questions and their input in the meetings that led to this final report. The study representatives are (in alphabetical order) NA Black, GG Browning, ML Casselbrant, MP Haggard, P Koivunen, JJ Manoukian, PS Mattila and AR Maw. All study representatives have been given the opportunity to read and comment on a draft version of this report.
The other authors of the original publications are also acknowledged for their work. These are (in alphabetical order) OP Alho, KH Al-Sebeih, R Bawden, CD Bluestone, JH Dempster, PA Fall, AP Freeland, SG Gatehouse, S Hammarén-Malmi, F Herod, E Herva, VP Joki-Erkkila, J Jokinen, T Kilpi, T Kontiokari, A Kristo, T Kujala, M Kurs-Lasky, J Luotonen, EM Mandel, MRC Multicentre Otitis Media Study Group, LH Nguyen, T Pokka, H Puhakka, R Raski, M Renko, HE Rockette, CF Sanderson, H Saxen, J Tarkkanen, M Uhari, MP Vessey and A Yoskovitch.
We would also like to thank Gemma Sandberg (Information Scientist/Trials Search Co-ordinator, Cochrane Ear, Nose and Throat Disorders Group) for conducting the literature search; Maaike van den Aardweg (PhD student and Registrar in Otorhinolaryngology) for selecting the eligible studies and assessing the study quality; and Samantha Faulkner (Assistant Managing Editor, Cochrane Ear, Nose and Throat Disorders Group) and Jenny Bellorini (Managing Editor, Cochrane Ear, Nose and Throat Disorders Group) for project management and administrative support.
This study was funded by the UK Department of Health through its Health Technology Assessment programme (project number 10/124/01). The opinions and conclusions expressed here are those of the authors and do not necessarily reflect those of the UK NHS or the Department of Health.
Contribution of authors
Contribution to the study
Dr Chantal Boonacker (Postdoctoral Researcher of Clinical Epidemiology) was the main researcher on the project, collected the data, performed the statistical analyses and interpreted the data.
Professor Maroeska Rovers (Professor of Evidence-based Surgery) developed the protocol, supervised the analyses and interpreted the data.
Emeritus Professor George Browning (Professor of Otorhinolaryngology) gave advice during the development of the protocol and interpreted the data.
Professor Arno Hoes (Professor of Clinical Epidemiology and General Practice and Chair of the Julius Centre for Health Sciences and Primary Care, University Medical Center Utrecht) developed the protocol and interpreted the data.
Professor Anne Schilder (Professor of Paediatric Otorhinolaryngology, Director of evidENT, University College London Ear Institute, and Joint Co-ordinating Editor of the Cochrane Ear, Nose and Throat Disorders Group) developed the protocol and interpreted the data.
Mr Martin Burton (Consultant Otolaryngologist, Joint Co-ordinating Editor of the Cochrane Ear, Nose and Throat Disorders Group and Director of the UK Cochrane Centre) was the lead investigator. He developed the protocol, was responsible for the overall management of the study and interpreted the data.
Contribution to the report
Dr Chantal Boonacker prepared the draft and finalised the report in close collaboration with Professors Maroeska Rovers and Anne Schilder.
Emeritus Professor George Browning and Professor Arno Hoes critically reviewed the report.
Mr Martin Burton proposed ideas, critically reviewed and redrafted the report and took overall responsibility for it.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Rovers MM, Schilder AG, Zielhuis GA, Rosenfeld RM. Otitis media. Lancet 2004;363:465-73. http://dx.doi.org/10.1016/S0140-6736(04)15495-0.
- Surgical Management of Otitis Media with Effusion in Children. London: RCOG Press; 2008.
- Gates GA, Klein JO, Lim DJ, Mogi G, Ogra PL, Pararella MM, et al. Recent advances in otitis media. 1. Definitions, terminology, and classification of otitis media. Ann Otol Rhinol Laryngol Suppl 2002;188:8-18.
- Marcy M, Takata G, Chan LS, Shekelle P, Mason W, Wachsman L, et al. Management of acute otitis media. Evid Rep Technol Assess 2000;15:1-4. http://dx.doi.org/10.1097/01.inf.0000076542.92305.8b.
- Paradise JL, Haggard MP, Lous J, Roberts JE, Schilder AG. Developmental implications of early-life otitis media. Int J Pediatr Otorhinolaryngol 1995;32:S37-44. http://dx.doi.org/10.1016/0165-5876(94)01140-S.
- Brouwer CN, Maille AR, Rovers MM, Grobbee DE, Sanders EA, Schilder AG. Health-related quality of life in children with otitis media. Int J Pediatr Otorhinolaryngol 2005;69:1031-41. http://dx.doi.org/10.1016/j.ijporl.2005.03.013.
- Brouwer CN, Rovers MM, Maille AR, Veenhoven RH, Grobbee DE, Sanders EA, et al. The impact of recurrent acute otitis media on the quality of life of children and their caregivers. Clin Otolaryngol 2005;30:258-65. http://dx.doi.org/10.1111/j.1365-2273.2005.00995.x.
- Ren T, Glatt DU, Nguyen TN, Allen EK, Early SV, Sale M, et al. 16S rRNA survey revealed complex bacterial communities and evidence of bacterial interference on human adenoids. Environ Microbiol 2013;15:525-47. http://dx.doi.org/10.1111/1462-2920.12000.
- Black N, Crowther J, Freeland A. The effectiveness of adenoidectomy in the treatment of glue ear: a randomized controlled trial. Clin Otolaryngol Allied Sci 1986;11:149-55. http://dx.doi.org/10.1111/j.1365-2273.1986.tb00121.x.
- Maw R, Bawden R. Spontaneous resolution of severe chronic glue ear in children and the effect of adenoidectomy, tonsillectomy, and insertion of ventilation tubes (grommets). BMJ 1993;306:756-60. http://dx.doi.org/10.1136/bmj.306.6880.756.
- Casselbrant ML, Mandel EM, Rockette HE, Kurs-Lasky M, Fall PA, Bluestone CD. Adenoidectomy for otitis media with effusion in 2–3-year-old children. Int J Pediatr Otorhinolaryngol 2009;73:1718-24. http://dx.doi.org/10.1016/j.ijporl.2009.09.007.
- Dempster JH, Browning GG, Gatehouse SG. A randomized study of the surgical management of children with persistent otitis media with effusion associated with a hearing impairment. J Laryngol Otol 1993;107:284-9. http://dx.doi.org/10.1017/S0022215100122844.
- Fiellau-Nikolajsen M, Falbe-Hansen J, Knudstrup P. Adenoidectomy for middle ear disorders: a randomized controlled trial. Clin Otolaryngol Allied Sci 1980;5:323-7. http://dx.doi.org/10.1111/j.1365-2273.1980.tb00898.x.
- Gates GA, Avery CA, Prihoda TJ, Cooper JC. Effectiveness of adenoidectomy and tympanostomy tubes in the treatment of chronic otitis media with effusion. N Engl J Med 1987;317:1444-51. http://dx.doi.org/10.1056/NEJM198712033172305.
- Hammarén-Malmi S, Saxen H, Tarkkanen J, Mattila PS. Adenoidectomy does not significantly reduce the incidence of otitis media in conjunction with the insertion of tympanostomy tubes in children who are younger than 4 years: a randomized trial. Pediatrics 2005;116:185-9. http://dx.doi.org/10.1542/peds.2004-2253.
- Koivunen P, Uhari M, Luotonen J, Kristo A, Raski R, Pokka T, et al. Adenoidectomy versus chemoprophylaxis and placebo for recurrent acute otitis media in children aged under 2 years: randomised controlled trial. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.37972.678345.0D.
- Kujala T, Alho OP, Luotonen J, Kristo A, Uhari M, Renko M, et al. Tympanostomy with and without adenoidectomy for the prevention of recurrences of acute otitis media: a randomized controlled trial. Pediatr Infect Dis J 2012;31:565-9. http://dx.doi.org/10.1097/INF.0b013e318255ddde.
- Mattila PS, Joki-Erkkila VP, Kilpi T, Jokinen J, Herva E, Puhakka H. Prevention of otitis media by adenoidectomy in children younger than 2 years. Arch Otolaryngol Head Neck Surg 2003;129:163-8. http://dx.doi.org/10.1001/archotol.129.2.163.
- Maw AR, Herod F. Otoscopic, impedance, and audiometric findings in glue ear treated by adenoidectomy and tonsillectomy. A prospective randomised study. Lancet 1986;1:1399-402. http://dx.doi.org/10.1016/S0140-6736(86)91552-7.
- MRC Multicentre Otitis Media Study Group . Adjuvant adenoidectomy in persistent bilateral otitis media with effusion: hearing and revision surgery outcomes through 2 years in the TARGET randomised trial. Clin Otolaryngol 2012;37:107-16.
- Nguyen LH, Manoukian JJ, Yoskovitch A, Al-Sebeih KH. Adenoidectomy: selection criteria for surgical cases of otitis media. Laryngoscope 2004;114:863-6. http://dx.doi.org/10.1097/00005537-200405000-00014.
- Paradise JL, Bluestone CD, Colborn DK, Bernard BS, Smith CG, Rockette HE, et al. Adenoidectomy and adenotonsillectomy for recurrent acute otitis media: parallel randomized clinical trials in children not previously treated with tympanostomy tubes. JAMA 1999;282:945-53. http://dx.doi.org/10.1001/jama.282.10.945.
- Paradise JL, Bluestone CD, Rogers KD, Taylor FH, Colborn DK, Bachman RZ, et al. Efficacy of adenoidectomy for recurrent otitis media in children previously treated with tympanostomy-tube placement. Results of parallel randomized and nonrandomized trials. JAMA 1990;263:2066-73. http://dx.doi.org/10.1001/jama.263.15.2066.
- Roydhouse N. Adenoidectomy for otitis media with mucoid effusion. Ann Otol Rhinol Laryngol Suppl 1980;89:312-15.
- Rynnel-Dagöö B, Ahlbom A, Schiratzki H. Effects of adenoidectomy: a controlled two-year follow-up. Ann Otol Rhinol Laryngol 1978;87:272-8.
- Rovers MM, Black N, Browning GG, Maw R, Zielhuis GA, Haggard MP. Grommets in otitis media with effusion: an individual patient data meta-analysis. Arch Dis Child 2005;90:480-5. http://dx.doi.org/10.1136/adc.2004.059444.
- Rovers MM, Glasziou P, Appelman CL, Burke P, McCormick DP, Damoiseaux RA, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet 2006;368:1429-35. http://dx.doi.org/10.1016/S0140-6736(06)69606-2.
- van den Aardweg MT, Schilder AG, Herkert E, Boonacker CW, Rovers MM. Adenoidectomy for otitis media in children. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.CD007810.pub2.
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions 5.1.0 (updated March 2011). The Cochrane Collaboration n.d. www cochrane-handbook.org (accessed March 2011).
- Koopman L, van der Heijden GJ, Grobbee DE, Rovers MM. Comparison of methods of handling missing data in individual patient data meta-analyses: an empirical example on antibiotics in children with acute otitis media. Am J Epidemiol 2008;167:540-5. http://dx.doi.org/10.1093/aje/kwm341.
- Brown MJ, Richards SH, Ambegaokar AG. Grommets and glue ear: a five-year follow up of a controlled trial. J R Soc Med 1978;71:353-6.
- Popova D, Varbanova S, Popov TM. Comparison between myringotomy and tympanostomy tubes in combination with adenoidectomy in 3–7-year-old children with otitis media with effusion. Int J Pediatr Otorhinolaryngol 2010;74:777-80. http://dx.doi.org/10.1016/j.ijporl.2010.03.054.
- Richards SH, Shaw JD, Kilby D, Campbell H. Grommets and glue ears: a clinical trial. J Laryngol Otol 1971;85:17-22. http://dx.doi.org/10.1017/S0022215100073096.
- To SS, Pahor AL, Robin PE. A prospective trial of unilateral grommets for bilateral secretory otitis media in children. Clin Otolaryngol Allied Sci 1984;9:115-17. http://dx.doi.org/10.1111/j.1365-2273.1984.tb01483.x.
- Vlastos IM, Houlakis M, Kandiloros D, Manolopoulos L, Ferekidis E, Yiotakis I. Adenoidectomy plus tympanostomy tube insertion versus adenoidectomy plus myringotomy in children with obstructive sleep apnoea syndrome. J Laryngol Otol 2010;16:1-5. http://dx.doi.org/10.1017/S0022215110002549.
- Sagnelli M, Marzullo C, Pollastrini L, Marullo MN. Secretory otitis media: current aspects and therapeutic role of adenoidectomy. Medicina 1990;10:16-22.
- Bulman CH, Brook SJ, Berry MG. A prospective randomized trial of adenoidectomy vs grommet insertion in the treatment of glue ear. Clin Otolaryngol Allied Sci 1984;9:67-75. http://dx.doi.org/10.1111/j.1365-2273.1984.tb01476.x.
- Widemar L, Rynnel-Dagöö B, Schiratzki H, Svensson C. The effect of adenoidectomy on secretory otitis media. Acta Otolaryngol Suppl 1982;386:132-3. http://dx.doi.org/10.3109/00016488209108495.
- Widemar L, Svensson C, Rynnel-Dagöö B, Schiratzki H. The effect of adenoidectomy on secretory otitis media: a 2-year controlled prospective study. Clin Otolaryngol Allied Sci 1985;10:345-50. http://dx.doi.org/10.1111/j.1365-2273.1985.tb00267.x.
- Marshak G, Neriah ZB. Adenoidectomy versus tympanostomy in chronic secretory otitis media. Ann Otol Rhinol Laryngol Suppl 1980;89:316-18.
- Fiellau-Nikolajsen M, Hojslet P, Felding J. Adenoidectomy for Eustachian tube dysfunction: long-term results from a randomized controlled trial. Acta Otolaryngol Suppl 1982;386:129-31. http://dx.doi.org/10.3109/00016488209108494.
- Gates GA, Avery CA, Prihoda TJ. Effect of adenoidectomy upon children with chronic otitis media with effusion. Laryngoscope 1988;98:58-63. http://dx.doi.org/10.1288/00005537-198801000-00013.
- Gates GA, Avery CA, Cooper JC, Prihoda TJ. Chronic secretory otitis media: effects of surgical management. Ann Otol Rhinol Laryngol Suppl 1989;138:2-32.
- Maw AR. Chronic otitis media with effusion (glue ear) and adenotonsillectomy: prospective randomised controlled study. Br Med J 1983;287:1586-8. http://dx.doi.org/10.1136/bmj.287.6405.1586.
- Maw AR. The long term effect of adenoidectomy on established otitis media with effusion in children. Auris Nasus Larynx 1985;12:S234-6.
- Maw AR. Factors affecting adenoidectomy for otitis media with effusion (glue ear). J R Soc Med 1985;78:1014-18.
- Maw AR. The effectiveness of adenoidectomy in the treatment of glue ear: a randomized controlled trial. Clin Otolaryngol Allied Sci 1987;12:155-6.
- Maw AR, Parker A. Surgery of the tonsils and adenoids in relation to secretory otitis media in children. Acta Otolaryngol Suppl 1988;454:202-7. http://dx.doi.org/10.3109/00016488809125027.
- Maw AR, Bawden R. The long term outcome of secretory otitis media in children and the effects of surgical treatment: a ten year study. Acta Otorhinolaryngol Belg 1994;48:317-24.
- Maw AR, Bawden R. Does adenoidectomy have an adjuvant effect on ventilation tube insertion and thus reduce the need for re-treatment?. Clin Otolaryngol Allied Sci 1994;19:340-3. http://dx.doi.org/10.1111/j.1365-2273.1994.tb01242.x.
- Mattila PS, Hammarén-Malmi S, Pelkonen AS, Malmberg LP, Makela MJ, Saxen H, et al. Effect of adenoidectomy on respiratory function: a randomised prospective study. Arch Dis Child 2009;94:366-70. http://dx.doi.org/10.1136/adc.2008.145664.
- Black NA, Sanderson CF, Freeland AP, Vessey MP. A randomised controlled trial of surgery for glue ear. BMJ 1990;300:1551-6. http://dx.doi.org/10.1136/bmj.300.6739.1551.
- Mattila PS. Adenoidectomy and tympanostomy tubes in the management of otitis media. Curr Allergy Asthma Rep 2006;6:321-6. http://dx.doi.org/10.1007/s11882-006-0067-7.
- Cordoba G, Schwartz L, Woloshin S, Bae H, Gotzsche PC. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c3920.
- Medical Research Council Multicentre Otitis Media Study Group . Surgery for persistent otitis media with effusion: generalizability of results from the UK trial (TARGET). Trial of Alternative Regimens in Glue Ear Treatment. Clin Otolaryngol Allied Sci 2001;26:417-24.
Appendix 1 Protocol
Which children with otitis media with effusion benefit most from adenoidectomy with or without grommets (ventilation tubes)? An individual patient data meta-analysis
Authors: Maroeska Rovers, Martin Burton, Arno Hoes, Anne Schilder
Summary
Current evidence suggests that overall effects of adenoidectomy are limited. Nevertheless, in day-to-day practice, both general practitioners and ENT-surgeons believe that certain subgroups of patients may benefit from adenoidectomy. So far, identifying these subgroups has been problematic, as the individual trials have been too small to allow valid and reliable subgroup analyses to be performed. Consequently it is likely that both over- and under-treatment occurs. The proposed Individual Patient Data (IPD) meta-analysis will include the individual data of about 2000 children aged 10 months to 12 years who participated in 9 to 12 randomised controlled trials into the effectiveness of adenoidectomy.
We aim to:
-
predict which children referred for adenoidectomy have a higher risk for a prolonged course of their upper respiratory tract infections, and
-
identify subgroups of children that most likely benefit from adenoidectomy.
The current proposal will lead to a better diagnostic and treatment protocol for children with otitis media for whom the general practitioner considers referral for adenoidectomy.
Background
Adenoidectomy is one of the most frequently performed surgical procedures in children in high-income countries. Annual adenoidectomy rates differ, however, between countries. When considering rates per 10,000 children per year, they vary, being 127 in Belgium, 101 in the Netherlands, 39 in England and 24 and 17 in the United States and Canada, respectively. 1
Indications for adenoidectomy include recurrent episodes of acute otitis media (AOM) and persistent otitis media with effusion (OME). 2 The operation involves removing the adenoid – a nasopharyngeal reservoir of potential respiratory pathogens and a potential cause of obstruction of the nasal airway. Removal is thought to improve Eustachian tube function.
We recently performed a Cochrane review, which showed a significant benefit of adenoidectomy as far as the resolution of middle ear effusion in children with OME is concerned. 3 However, the benefit to hearing is small and the effects on changes in the tympanic membrane are unknown. The risks of operating should therefore be weighed against these potential benefits. Furthermore, no benefit of adenoidectomy was found on AOM. However, in day-to-day general practice about one in ten children with otitis media is referred to an ENT-surgeon and in some countries the vast majority of these children are selected for surgery. This confirms that both general practitioners and ENT-surgeons believe that certain subgroups of patients may benefit from adenoidectomy. The identification of these subgroups has, however, been problematic, as the individual trials performed so far were too small to perform valid and reliable subgroup analyses. Consequently it is likely that both over- and under-treatment occurs. An IPD meta-analysis, i.e. a meta-analysis on the individual original data of previously performed trials, offers the unique opportunity to identify subgroups more likely to benefit from an intervention. 4,5 Since IPD meta-analyses include more detailed data, they have much more statistical power to carry out informative subgroup analyses. Furthermore, by using individual patient data the flexibility of subgroup analyses is enhanced. Consequently, the estimated subgroup effects may be less influenced by misclassification and (ecological) bias. IPD meta-analysis therefore allows a more thorough assessment as to whether differences in treatment effects across subgroups are spurious or not. 6 An earlier IPD meta-analysis from our research group in children with acute otitis media showed that such an approach is capable of identifying subgroups of children that are most likely to benefit from antibiotic therapy. 7,8
Objective
In this IPD meta-analysis with the individual data of at least nine (but probably 12) randomized controlled trials into the effectiveness of adenoidectomy including more than 2000 children aged 10 months to 12 years, we aim to:
-
predict which children referred for adenoidectomy have a higher risk for a prolonged course of their upper respiratory tract infection
-
identify subgroups of children that benefit most or are most likely to benefit from adenoidectomy.
Methods
Design
We will perform an individual patient data meta-analysis with individual data of trials performed so far on the effectiveness of adenoidectomy in children. Our previous IPD meta-analysis on the effect of tympanostomy tubes in children9 will give us access to the data of four trials of the effects of the combination of adenoidectomy and tympanostomy tubes, versus those of insertion of tympanostomy tubes alone or watchful waiting. 10–13 To study the effect of adenoidectomy in all clinically relevant subgroups, we have also contacted the primary investigators (PIs) of all other existing adenoidectomy trials,14–26 and asked them to provide the raw data of their trials. Of the 17 trials performed up to now, 4 were performed before 1990 and the PIs have indicated that their data are no longer available. Of the remaining 13 trials, the PIs of 9 have already agreed to participate in our IPD meta-analysis, which underpins the international support of the current proposal. Three other PIs have shown interest, but are awaiting approval by their IRB. The PI of the 13th trials has declined to collaborate in our IPD meta-analysis.
Study population
In total data of at least 2000 children aged 10 months to 12 years selected for adenoidectomy are available. Such a new dataset will offer the unique opportunity to identify which children benefit most from adenoidectomy. In 5 of the 12 eligible studies adenoidectomy (with or without myringotomy) is compared with non-surgical treatment; in 3 trials adenoidectomy with tympanostomy tubes is compared with adenoidectomy alone; and in 4 trials adenoidectomy with bilateral insertion of tympanostomy tubes is compared with bilateral tympanostomy tubes alone. Follow-up varies from 6 to 36 months.
Subgroups
Clinically relevant subgroups that will be studied include: age (≤ 2 years vs. > 2 years), sex, season (winter vs. summer), attending day-care, passive smoking, being breast fed, vaccine status, atopy, number of previous episodes of upper respiratory tract infections (including otitis media), duration of symptoms, middle ear impedance and baseline hearing level. Age, sex, season, previous episodes of otitis and impedance are measured in all nine trials, whose principal investigators have already agreed to participate. Information on attending a day-care centre, passive smoking, atopy, being breastfed, and baseline hearing level are available in 5 trials. Vaccination status is measured in 3 trials, whereas 4 other trials were performed before the pneumococcal vaccine was available.
The subgrouping variables have been assessed in similar ways and can therefore be pooled over the trials.
As the pathogenesis of otitis media is known to be multifactorial, children with more than one predisposing factor may have more persistent or severe disease and hence might benefit more from treatment with adenoidectomy than children with only one such factor. To study this possibility, we will also study the combinations of predictors. Furthermore, separate analyses will be performed for the groups that also received tympanostomy tubes and those that did not.
Outcomes
The primary outcome is number of otitis episodes in the first 12 months after adenoidectomy. The definitions of acute otitis media (AOM) and otitis media with effusion (OME) proposed by the American Association of Family Physicians (AAFP) and the American Academy of Pediatrics (AAP) will be used.
For AOM this is: acute onset of signs and symptoms, the presence of middle ear effusion (bulging of the tympanic membrane, or limited or absent mobility of the tympanic membrane, or air–fluid level behind the tympanic membrane, or otorrhoea), and signs and symptoms of middle ear inflammation (distinct erythema of the tympanic membrane or distinct otalgia).
For OME this is: presence of fluid in the middle ear, without signs and symptoms of acute ear infection as diagnosed by (pneumatic) otoscopy or tympanometry. Effusion should be measured with tympanometry, mean hearing loss with audiometry.
The number of episodes in the first 12 months was chosen as our primary outcomes since it has been measured in all available trials. We also believe this is an important outcome both from a patient and a societal point of view. The high rate of spontaneous recovery in the short term also mandates a follow-up period of this length.
Secondary outcomes that will be studied are:
-
treatment failures after 3, 6, and 12 months
-
acute otitis media
-
number of otitis episodes per year
-
number of days per episode and per year
-
proportion of children with recurrent episodes.
-
-
otitis media with effusion
-
number of episodes per year
-
number of days per episode and per year
-
proportion of children with recurrent episodes
-
prevalence at 3, 6, 12 and 24 months.
-
-
mean hearing loss at 6, 12, and 24 months
-
adverse effects.
Treatment failure will be defined as a composite outcome consisting of 4 independent components:
-
no improvement in number of OM episodes per person month
-
no improvement in prevalence of OME at follow-up visits
-
no improvement in mean hearing level at follow-up visits
-
crossing over from watchful waiting to surgical treatment arm.
We did not choose this outcome as our primary outcome since not all components are measured in all trials.
The hearing loss is defined as the main air conduction which should be measured with audiometry.
Adverse events include:
-
changes to the ears (including tympanic membrane: atrophy, tympanosclerosis, retraction of the pars tensa and pars flaccida and cholesteatoma) and
-
postoperative bleeding and velo-pharyngeal insufficiency.
Statistical analysis
To minimise bias and to increase statistical efficiency, we will impute the missing data per trial using the linear regression method (MVA analyses) available in SPSS (SPSS for Windows, version 15.0, SPSS Inc.). Such imputation is based on the correlation between each variable with missing values and all other variables as estimated from the set of complete subjects. We will impute missing values only within trials.
To assess the first research question, i.e. to predict which children referred for adenoidectomy have a higher risk for a prolonged course of their upper respiratory symptoms, the association between each subgrouping variable and each of the outcomes will be examined by univariate logistic regression analyses. Predictors that are univariately associated with the outcome (p-value > 0.10) will be included in multivariate logistic regression analyses. The model will be reduced by excluding predictors from the model with a p-value of > 0.05. The predictive accuracy of the models will be estimated by their reliability/callibrations (goodness of fit) using Hosmer and Lemeshow tests. The model’s ability to discriminate between children that will or not develop the outcome will be estimated by the area under the receiver-operating curve (ROC) of the model. The ROC area is a suitable parameter to summarise the discriminative or predictive value and can range from 0.5 (no discrimination, like a coin flip) to 1.0 (perfect discrimination). In addition, we will calculate the absolute risks of a prolonged course across combinations of independent predictors.
To answer the second research question, first the effect of adenoidectomy will be quantified by calculating relative risks (RR), rate differences (RD), mean differences, numbers needed to treat (NNT) and their 95% confidence intervals (CI). To assess whether the effect of adenoidectomy is modified by the identified prognostic factors, a random effect logistic and linear regression analysis will be performed. In this saturated model adenoidectomy (yes vs. no), the potential effect modifier, a dummy for the particular study, and an interaction term (adenoidectomy × potential effect modifier) will be included as independent variables, and the outcomes as dependent variables. Finally, sensitivity analyses, for example, including only those trials that measured the outcomes at the same moment, will be performed to assess the robustness of the findings. All analyses will be performed according to the intention-to-treat principle.
Time schedule
The research will start in 2011. A time line is provided below.
The proposal will be undertaken in Utrecht by a PhD student (Chantal Boonacker) under primary supervision of Professor Maroeska Rovers (clinical-epidemiologist, Utrecht) and Dr. M. Burton (ENT-surgeon, Oxford) and supported by a systematic reviewer based in Oxford. Professor Arno Hoes (clinical-epidemiologist, Utrecht), Professor Anne Schilder (Paediatric ENT-surgeon and clinical trialist, Utrecht and London) and Professor George Browning (ENT-surgeon with special expertise in otitis media trials) will be available for further support. Project management and administrative support will be provided by staff from the Cochrane ENT Disorders Group in Oxford.
The PIs of the original trials will also be involved by means of regularly updates. The commitment and enthusiasm of several has been demonstrated by their already having provided their raw data. A formal meeting will be held in Oxford (September 2012) to discuss the results and funding for the senior PIs to travel to this meeting (several from the USA) has been included in the budget. It seems likely that new research questions will emerge from this work and an important element of this project is to continue a process of international engagement and dialogue with the world’s most experienced otitis media triallists to ensure that the results of this review are used to best effect.
If statistical questions or problems arise, which Maroeska Rovers cannot solve, she will consult her colleagues from the Cochrane IPD meta-analysis methods group, i.e. Mike Clarke, Jayne Thierney or Lesley Clarke. For more details see the table at the following page.
| Finalising data-collection of all trials | ||||||||||||
| Data management, i.e. recoding, imputations, merge data, etc. | ||||||||||||
| Statistical analysis I (prognosis) | ||||||||||||
| Statistical analysis II (subgroups) | ||||||||||||
| Collaborators meeting | ||||||||||||
| Writing paper on prognostic factors | ||||||||||||
| Writing paper on subgroups | ||||||||||||
| TIME PERIOD | March 11 | April | May | June | July | August | September | October | November | December | January | February |
References
- Schilder AG, Lok W, Rovers MM. International perspectives on management of acute otitis media: a qualitative review. Int J Pediatr Otorhinolaryngol 2004;68:29-36.
- van den Aardweg MT, Rovers MM, Kraal A, Schilder AG. Current indications for adenoidectomy in a sample of children in the Netherlands. B-ENT 2010;6:15-8.
- van den Aardweg MT, Schilder AG, Herkert E, Boonacker CW, Rovers MM. Adenoidectomy for otitis media in children. Cochrane Database Syst Rev 2010;1.
- Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340.
- Stewart LA, Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof 2002;25:76-97.
- Thompson SG, Higgins JP. Treating individuals 4: can meta-analysis help target interventions at individuals most likely to benefit?. Lancet 2005;365:341-6.
- Rovers MM, Glasziou P, Appelman CL, Burke P, McCormick DP, Damoiseaux RA, et al. Predictors of pain and/or fever at 3 to 7 days for children with acute otitis media not treated initially with antibiotics: a meta-analysis of individual patient data. Pediatrics 2007;119:579-8.
- Rovers MM, Glasziou P, Appelman C, Burke P, McCormick DP, Damoiseaux RAMJ, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet 2006;368:1429-35.
- Rovers MM, Black N, Browning GG, Maw R, Zielhuis GA, Haggard MP. Grommets in otitis media with effusion: an individual patient data meta-analysis. Arch Dis Child 2005;90:480-5.
- Maw AR, Herod F. Otoscopic, impedance, and audiometric findings in glue ear treated by adenoidectomy and tonsillectomy. A prospective randomised study. Lancet 1986;1:1399-402.
- Black NA, Sanderson CF, Freeland AP, Vessey MP. A randomised controlled trial of surgery for glue ear. BMJ 1990;300:1551-6.
- Dempster JH, Browning GG, Gatehouse SG. A randomized study of the surgical management of children with persistent otitis media with effusion associated with a hearing impairment. J Laryngol Otol 1993;107:284-9.
- MRC Multicentre Otitis Media Study Group . An extension of the Jerger classification of tympanograms for ventilation tube patency – specification and evaluation of equivalent ear-canal volume criteria. Ear Hear n.d.:894-906.
- Nguyen LH, Manoukian JJ, Yoskovitch A, Al-Sebeih KH. Adenoidectomy: selection criteria for surgical cases of otitis media. Laryngoscope 2004;114:863-6.
- Hammaren-Malmi S, Saxen H, Tarkkanen J, Mattila PS. Adenoidectomy does not significantly reduce the incidence of otitis media in conjunction with the insertion of tympanostomy tubes in children who are younger than 4 years: a randomized trial. Pediatrics 2005;116:185-9.
- Koivunen P, Uhari M, Luotonen J, Kristo A, Raski R, Pokka T, et al. Adenoidectomy versus chemoprophylaxis and placebo for recurrent acute otitis media in children aged under 2 years: randomised controlled trial. BMJ 2004;328.
- Paradise JL, Bluestone CD, Colborn DK, Bernard BS, Smith CG, Rockette HE, et al. Adenoidectomy and adenotonsillectomy for recurrent acute otitis media: parallel randomized clinical trials in children not previously treated with tympanostomy tubes. JAMA 1999;282:945-53.
- Paradise JL, Bluestone CD, Colborn DK, . Adenoidectomy and adenotonsillectomy for recurrent acute otitis media: parallel randomized clinical trials in children not previously treated with tympanostomy tubes. JAMA 1999;282:945-53.
- Gates GA, Avery CA, Prihoda TJ, Cooper JC. Effectiveness of adenoidectomy and tympanostomy tubes in the treatment of chronic otitis media with effusion. N Engl J Med 1987;317:1444-51.
- Fiellau-Nikolajsen M, Falbe-Hansen J, Knudstrup P. Adenoidectomy for middle ear disorders: a randomized controlled trial. Clin Otolaryngol 1980;5:323-7.
- Matilla PS, Joki-Erkkila VP, Kilpi T, Jokinen J, Herva E, Puhakka H. Prevention of otitis media by adenoidectomy in children younger than 2 years. Arch Otolaryngol Head Neck Surg 2003;129:163-8.
- Roydhouse N. Adenoidectomy for otitis media with mucoid effusion. Ann Otol Rhinol Laryngol Suppl 1980;89:312-5.
- Rynnel-Dagoo B, Ahlbom A, Schiratzki H. Effects of adenoidectomy: a controlled two-year follow-up. Ann Otol Rhinol Laryngol 1978;87:272-8.
- Vlastos IM, Houlakis M, Kandiloros D, Manolopoulos L, Ferekidis E, Yiotakis I. Adenoidectomy plus tympanostomy tube insertion versus adenoidectomy plus myringotomy in children with obstructive sleep apnoea syndrome. J Laryngol Otol 2010;16:1-5.
- Casselbrant ML, Mandel EM, Rockette HE, Kurs-Lasky M, Fall PA, Bluestone CD. Adenoidectomy for otitis media with effusion in 2–3-year-old children. Int J Pediatr Otorhinolaryngol 2009;73:1718-24.
- Popova D, Varbanova S, Popov TM. Comparison between myringotomy and tympanostomy tubes in combination with adenoidectomy in 3–7-year-old children with otitis media with effusion. Int J Pediatr Otorhinolaryngol 2010;74:777-80.
Appendix 2 Adaptation of the study protocol
As anticipated for any IPD meta-analysis, most adaptations were made after data collection and following discussion with the principal investigators and/or study representatives of those studies included in the meta-analysis. Two face-to-face meetings were held (Amsterdam, the Netherlands, 21 May 2012, and Oxford, UK, 3 September 2012).
Methods
Design and study population
In the protocol, studies that compared adenoidectomy with grommets compared with adenoidectomy without grommets were mentioned. However, these studies do not fulfil the inclusion criteria as we sought studies in which one comparator was either non-surgical treatment or grommets only. The studies of Vlastos35 (who had agreed to participate and provide data) and Popova32 (who declined to participate) were therefore excluded.
In the protocol it was mentioned that the follow-up of the trials that had been undertaken varied from six to 36 months. However, to be eligible for inclusion in this IPD meta-analysis, studies needed to have followed up participants for at least 12 months since a six-month follow-up period was deemed to be too short. This resulted in one study, mentioned in the protocol (Fiellau-Nikolajsen,13 not being eligible. However, this study would not have been included even had studies with six months follow-up been eligible, since we were unable to contact the principal investigator, making individual patient data unavailable.
All of these factors affected the number of trials and children that we had thought we might be able to include. On the other hand, one new trial was published and included, resulting in 15 eligible studies.
Subgroups
Based on the suggestions of the PIs, several clinically relevant subgroups were added to the list of prognostic factors defined a priori. The final list includes (additions underlined):
-
age (≤ 2 years versus > 2 years and ≤ 4 years versus > 4 years)
-
sex
-
indication
-
season (winter vs. summer)
-
day-care attendance
-
passive smoking
-
breastfeeding
-
PCV vaccine status
-
atopy
-
allergy
-
number of previous episodes of upper respiratory tract infections (including otitis media)
-
number of previous episodes of otitis media
-
duration of symptoms
-
age at onset
-
middle ear impedance/OME present at study entry
-
baseline hearing level.
In practice, not all of the desired factors could be included in the univariate analyse. The reasons included low numbers or different definitions across studies.
The comparisons of the interventions were worked out in detail:
-
adenoidectomy with or without grommets compared with non-surgical treatment or grommets only (main comparison)
-
adenoidectomy with uni- or bilateral grommets compared with non-surgical treatment
-
adenoidectomy with uni- or bilateral grommets compared with uni- or bilateral grommets
-
adenoidectomy without grommets compared with non-surgical treatment
-
adenoidectomy without grommets compared with uni- or bilateral grommets.
We did not compare the following interventions since these analyses do not fulfil the criteria for inclusion in this IPD meta-analysis:
-
adenoidectomy with uni- or bilateral grommets compared with adenoidectomy without grommets
-
uni- or bilateral grommets compared with non-surgical treatment.
Outcomes
The most notable adaptation was the decision to change the primary outcome from ‘number of otitis media episodes’ to ‘failure at 12 months’ – a composite endpoint which was already included in the protocol as a secondary outcome. When our study was underway and we were analysing the available datasets, we realised that data on the original desired primary outcome would not be available, because the original trialists did not evaluate outcomes in the same way. As is usual in many IPD meta-analyses, rather than presenting an ‘empty’ review of little utility we sought – through consensus with clinicians, including the original trialists – to develop a composite primary outcome that allowed inclusion of all of the trials. The aim of this is, of course, to make maximum use of the available information to develop a robust answer to the clinical questions posed. We emphasise to those who may be unfamiliar with this specific form of secondary research that re-defining a primary outcome in this way is significantly different from changing outcomes in primary research.
The composite primary outcome used was ‘failure at 12 months’, defined as one or more of the following components:
-
four or more AOM episodes (including episodes of otorrhoea) per year
-
presence of effusion for ≥ 50% of time
-
need for additional surgery
-
hearinga improved by less than 10 dB.
aHearing was expressed as a mean air conduction hearing level measured by age-appropriate audiometry (if possible averaged over 500, 1000, 2000 and 4000 Hz). In all children the binaural average was taken. This includes trials that used unilateral grommets and randomised ears rather than children.
The choice of this primary outcome also had consequences for the secondary outcomes. Based on the availability of data, the time points for the secondary outcomes were changed from 3, 6, 12 and 24 months to 6, 12, 18 and 24 months.
Secondary outcomes
-
Each of the individual items used as part of the definition of ‘failure at 12 months’. That is:
-
four or more AOM episodes/year
-
presence of effusion for ≥ 50% of time
-
need for additional surgery
-
hearing improved by less than 10 dB.
-
-
Number of episodes of AOM during follow-up:
-
in the first 6, 12, 18 and 24 months.
-
-
Time with effusion during follow-up measured in number of weeks:
-
in the first 6, 12, 18 and 24 months.
-
-
Additional surgery during follow-up:
-
in the first 6, 18 and 24 months.
-
-
Average hearing loss measured in dBHL:
-
after 6, 12, 18 and 24 months follow-up.
-
-
Improvement in hearing level < 10 dB:
-
after 6, 18 and 24 months follow-up.
-
-
Adverse effects and events (including morbidity of surgery).
Statistical analysis
For Research Question 2a: to evaluate the overall effect of adenoidectomy, with or without grommets on otitis media using these individual patient data
We quantified the overall effect of the interventions for all comparisons listed above (see also Figure 1a and b). We used Review Manager 5 to create forest plots presenting both RDs and RRs for the main effects. We also calculated relative risks (RR), rate differences (RD), numbers needed to treat (NNT) and the 95% confidence intervals (CI) for each of these using regression analyses.
We were unable to calculate adjusted RDs due to the calculation artefact which occurs when there are zero events in a study (in this case that of Nguyen et al. 21). Therefore, we calculated unadjusted RDs with a binominal model using an identity link:
To adjust for a potential study effect we also performed a Poisson regression analysis with robust standard errors, which enabled us to calculate an adjusted RR:
For continuous outcomes we calculated an adjusted mean difference:
For Research Question 2b. To identify those subgroups of children who benefit most, or are most likely to benefit, from adenoidectomy with or without grommets.
To assess whether the effect of the intervention under study was modified by the identified prognostic factors, we performed an analysis using a binominal model with an identity link, including an interaction effect.
To adjust for study and the subgrouping variable(s) we also performed a Poisson regression analysis with robust standard errors including both interaction terms and potential confounders (i.e. study and age) to calculate adjusted RR and their 95% confidence intervals.
We used the Wald statistic from the adjusted Poisson regression to study the significance of the interaction term.
For continuous outcomes we calculated an adjusted mean difference:
We will only present subgroup effects for those variables that showed a significant interaction effect (P ≤ 0.1) and for subgroups with sufficient numbers (> 15) to present stratified results. We used our clinical experience and reasoning and decided a priori to include age and indication as potential subgroup variables. Furthermore we decided to examine subgroup effects in the two distinct populations defined by the indication for surgery – recurrent AOM versus persistent OME.
Since many of the subgroup comparisons are between studies rather than within study, we will not present forest plots for the subgrouping variables, but tables with the RD, RR and adjusted RRs from the regression analyses.
We decided not to impute missing data due to the low number of missing data points within the included trials. Furthermore, we decided not to impute data across trials in situations where a variable was completely missing from a trial. We found in an earlier study that imputation of missing data across trials might lead to bias, as association of covariates might differ across the included studies. 30
In addition to the intention-to-treat analysis, we also performed three sensitivity analyses to assess the robustness of our findings. In the first we performed a per protocol analysis in which we excluded the children who did not follow the protocol to which they were randomised. The second was an as treated analysis in which we analysed the children according to the treatment they received. In the third we used an alternative set of definitions of failure at 12 months as the outcome. We performed all sensitivity analyses only for the primary outcome of the main comparison.
All analyses were performed with SPSS, version 20 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) and Rothman’s Episheet (11 June 2008).
Time schedule
The research did not start in April 2011 as suggested by the timeline, but in December 2011. The formal meeting was indeed held in September 2012 in Oxford, United Kingdom and was preceded by an informal meeting during the ESPO conference in Amsterdam, the Netherlands.
Appendix 3 Search strategies
Date: 30 March 2009, updated on 7 June 2012.
Cochrane Central Register of Controlled Trials
| #1 | MeSH descriptor Adenoidectomy explode all trees |
| #2 | MeSH descriptor Adenoids explode all trees with qualifier: SU |
| #3 | adenoidectom* or adenotonsillectom* or adenotonsilectom* or adeno NEXT tonsillectomy* or adeno NEXT tonsilectom* |
| #4 | (#1 OR #2 OR #3) |
| #5 | MeSH descriptor Adenoids explode all trees |
| #6 | adenoid* or adenotonsil* |
| #7 | (#5 OR #6) |
| #8 | MeSH descriptor Surgical Procedures, Operative explode all trees |
| #9 | (surg*:ti or operat*:ti or excis*:ti or extract*: ti or remov*:ti or dissect*:ti or ablat*: ti or coblat*:ti or laser*:ti) |
| #10 | (#8 OR #9) |
| #11 | (#7 AND #10) |
| #12 | (#4 OR #11) |
| #13 | (nose OR nasal) NEAR (symptom* OR discharg* OR secret* OR obstruct*) |
| #14 | rhinorrhea OR rhinorrhoea |
| #15 | MeSH descriptor Nasal Obstruction explode all trees |
| #16 | airway* AND obstruct* |
| #17 | breath* AND impair* |
| #18 | MeSH descriptor Otitis Media explode all trees |
| #19 | middle NEXT ear NEXT (infect* OR inflam* OR disease*) |
| #20 | otitis OR aom OR ome |
| #21 | glue AND ear |
| #22 | (#13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) |
| #23 | (#12 AND #22) |
PubMed
| #1 | “Adenoidectomy”[Mesh] |
| #2 | “Adenoids/surgery”[Mesh] |
| #3 | adenoidectom* [tiab] OR adenotonsillectom* [tiab] OR adenotonsilectom* [tiab]OR “adeno tonsillectomy” [tiab]OR “adeno tonsilectom” [tiab] |
| #4 | #1 OR #2 OR #3 |
| #5 | “Adenoids”/[Mesh] |
| #6 | adenoid* [tiab] OR adenotonsil* [tiab] |
| #7 | #5 OR #6 |
| #8 | “Surgical Procedures, Operative”[Mesh] |
| #9 | “surgery”[Subheading] |
| #10 | surg* [tiab] OR operat* [tiab] OR excis* [tiab] OR extract* [tiab] OR remov* [tiab] OR dissect* [tiab] OR ablat* [tiab] OR coblat* [tiab] OR laser* [tiab] |
| #11 | #8 OR #9 OR #10 |
| #12 | #7 AND #11 |
| #13 | #4 OR #12 |
| #14 | (nose [tiab] OR nasal [tiab]) AND (symptom* [tiab] OR discharg* [tiab] OR secret* [tiab] OR obstruct* [tiab]) |
| #15 | rhinorrhea [tiab] OR rhinorrhoea [tiab] |
| #16 | “Nasal Obstruction”[Mesh] |
| #17 | airway* [tiab] AND obstruct* [tiab] |
| #18 | breath* [tiab] AND impair* [tiab] |
| #19 | “Otitis Media”[Mesh] |
| #20 | middle [tiab] AND ear [tiab] AND (infect* [tiab] OR inflam* [tiab] OR disease* [tiab]) |
| #21 | otitis [tiab] OR aom [tiab] OR ome [tiab] |
| #22 | glue [tiab] AND ear [tiab] |
| #23 | #14 OR #15 OR #16 |
EMBASE (Ovid)
| 1 | adenoidectomy/ |
| 2 | (adenoidectom* or adenotonsillectom* or adenotonsilectom* or “adeno tonsillectomy*” or “adeno tonsilectom*”).tw. |
| 3 | 1 or 2 |
| 4 | *Adenoid/ |
| 5 | (adenoid* or adenotonsil*).ti. |
| 6 | 4 or 5 |
| 7 | (surg* or operat* or excis* or extract* or remov* or dissect* or ablat* or coblat* or laser*).ti. |
| 8 | exp *Surgery/ |
| 9 | 8 or 7 |
| 10 | 6 and 9 |
| 11 | 3 or 10 |
| 12 | nose obstruction/or rhinorrhea/ |
| 13 | *airway obstruction/or *upper respiratory tract obstruction/ |
| 14 | ((nose or nasal) and (symptom* or discharg* or obstruct* or secret*)).tw. |
| 15 | (rhinorrhea or rhinorrhoea).tw. |
| 16 | (airway* and obstruct*).tw. |
| 17 | (breath* and impair*).tw. |
| 18 | exp Middle Ear Disease/ |
| 19 | (middle and ear and (infect* or inflamm* or disease*)).tw. |
| 20 | (otitis or aom or raom or ome).tw. |
| 21 | (glue and ear).tw. |
| 22 | 21 or 17 or 12 or 20 or 15 or 14 or 18 or 13 or 16 or 19 |
| 23 | 22 and 11 |
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost)
| S1 | (MH “Adenoidectomy”) |
| S2 | (MH “Adenoids/SU”) |
| S3 | adenoidectom* or adenotonsillectom* or adenotonsilectom* or “adeno tonsillectomy*” or “adeno tonsilectom*” |
| S4 | (MM “Adenoids”) |
| S5 | TI adenoid* or adenotonsil* |
| S6 | TI surg* or operat* or excis* or extract* or remov* or dissect* or ablat* or cobalt* or laser* |
| S7 | (MH “Surgery, Operative”) |
| S8 | S6 or S7 |
| S9 | S4 or S5 |
| S10 | S8 and S9 |
| S11 | S1 or S2 or S3 or S10 |
Web of Science
| #1 | TS=(adenoidectom* or adenotonsillectom* or adenotonsilectom* or “adeno tonsillectomy*” or “adeno tonsilectom*”) |
| #2 | TI=(adenoid* or adenotonsil*) |
| #3 | TI=(surg* or operat* or excis* or extract* or remov* or dissect* or ablat* or coblat* or laser*) |
| #4 | #2 AND #3 |
| #5 | #1 OR #4 |
| #6 | TS=((nose or nasal) and (symptom* or discharg* or obstruct* or secret*)) |
| #7 | TS=(rhinorrhea or rhinorrhoea) |
| #8 | TS=(airway* and obstruct*) |
| #9 | TS=(breath* and impair*) |
| #10 | TS=(middle and ear and (infect* or inflamm* or disease*)) |
| #11 | TS=(otitis or aom or raom or ome) |
| #12 | TS=(glue and ear) |
| #13 | #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 |
| #14 | #5 AND #13 |
Bioscience Information Service previews/CAB Abstracts (Ovid)
| 1 | (adenoidectom* or adenotonsillectom* or adenotonsilectom* or “adeno tonsillectomy*” or “adeno tonsilectom*”).tw. |
| 2 | (adenoid* or adenotonsil*).ti. |
| 3 | (surg* or operat* or excis* or extract* or remov* or dissect* or ablat* or coblat* or laser*).ti. |
| 4 | ((nose or nasal) and (symptom* or discharg* or obstruct* or secret*)).tw. |
| 5 | (rhinorrhea or rhinorrhoea).tw. |
| 6 | (airway* and obstruct*).tw. |
| 7 | (breath* and impair*).tw. |
| 8 | (middle and ear and (infect* or inflamm* or disease*)).tw. |
| 9 | (otitis or aom or raom or ome).tw. |
| 10 | (glue and ear).tw. |
| 11 | 3 and 2 |
| 12 | 11 or 1 |
| 13 | 8 or 6 or 4 or 7 or 10 or 9 or 5 |
| 14 | 13 and 12 |
Appendix 4 Study representatives for the included studies (ordered alphabetically by first author)
| No. | Study | Representative |
|---|---|---|
| 1 | Black NA, Sanderson CF, Freeland AP, Vessey MP. A randomised controlled trial of surgery for glue ear. BMJ 1990;300:1551–6 | NA Black |
| 2 | Casselbrant ML, Mandel EM, Rockette HE, Kurs-Lasky M, Fall PA, Bluestone CD. Adenoidectomy for otitis media in 2–3-year old children. Int J Pediatr Otorhinolaryngol 2009;73;1718–24 | ML Casselbrant |
| 3 | Dempster JH, Browning GG, Gatehouse SG. A randomized study of the surgical management of children with persistent otitis media with effusion associated with a hearing impairment. J Laryngol Otol 1993;107:284–9 | GG Browning |
| 4 | Hammarén-Malmi S, Saxen H, Tarkkanen J, Mattila PS. Adenoidectomy does not significantly reduce the incidence of otitis media in conjunction with the insertion of tympanostomy tubes in children who are younger than 4 years: a randomized trial. Pediatrics 2005;116:185–9 | PS Mattila |
| 5 | Koivunen P, Uhari M, Luotonen J, Kristo A, Raski R, Pokka T, et al. Adenoidectomy versus chemoprophylaxis and placebo for recurrent acute otitis media in children aged under 2 years: randomised controlled trial. BMJ 2004;328:487 | P Koivunen |
| 6 | Kujala T, Alho OP, Luotonen J, Kristo A, Uhari M, Renko M, et al. Tympanostomy with and without adenoidectomy for prevention of acute otitis media: a randomized controlled trial. Pediatric Infect Dis J 2012;31:565–9 | P Koivunen |
| 7 | Mattila PS, Joki-Erkkila VP, Kilpi T, Jokinen J, Herva E, Puhakka H. Prevention of otitis media by adenoidectomy in children younger than 2 years. Arch Otolaryngol Head Neck Surg 2003;129:163–8 | PS Mattila |
| 8 | Maw AR, Herod F. Otoscopic, impedance, and audiometric findings in glue ear treated by adenoidectomy and tonsillectomy. A prospective randomised study. Lancet 1986;1:1399–402Maw R, Bawden R. Spontaneous resolution of severe chronic glue ear in children and the effect of adenoidectomy, tonsillectomy, and insertion of ventilation tubes (grommets). BMJ 1993;306:756–60 | AR Maw |
| 9 | MRC Multicentre Otitis Media Study Group. Adjuvant adenoidectomy in persistent bilateral otitis media with effusion: hearing and revision surgery outcomes through 2 years in the TARGET randomised trial. Clin Otolaryngol 2012;37:107–16 | MP Haggard/GG Browning |
| 10 | Nguyen LH, Manoukian JJ, Yoskovitch A, Al Sebeih KH. Adenoidectomy: selection criteria for surgical cases of otitis media. Laryngoscope 2004;114:863–6 | JJ Manoukian |
Appendix 5 Inclusion and exclusion criteria for the 10 included studies
| Study | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Black 199052 | Age 4–9 years; operation indication for glue ear (secretory OM) based on the clinical judgement of the otolaryngologist | Previous operations on tonsils, adenoids or ears; cleft palate or any sensorineural deafness; conditions other than glue ear, such as gross nasal obstruction |
| Casselbrant 200911 | Age 2–3.9 years; history of bilateral middle ear effusion (MEE) for at least 3 months, unilateral for ≥ 6 months or unilateral for 3 months after extrusion of one grommet with the other still in place and patent, and who had completed a 10-day course of a broad-spectrum antimicrobial agent within the past month | Previous tonsillectomy and/or adenoidectomy; previous ear surgery other than tympanocentesis or myringotomy with or without tube insertion; history of seizure disorder, diabetes mellitus, asthma requiring daily medication or any health condition that would make entry potentially disadvantageous to the child; medical conditions with a predisposition for MEE, such as cleft palate, Down syndrome, congenital malformations of the ear; cholesteatoma or chronic mastoiditis; severe retraction pockets; acute or chronic diffuse external OM; perforation of the tympanic membrane; intracranial or intratemporal complications of MEE; upper respiratory tract obstruction attributable to tonsil or adenoid enlargement or both with cor pulmonale, sleep apnoea or severe dysphagia; conductive hearing loss attributable to destructive changes in the middle ear; sensorineural hearing loss; distance from hospital that would make follow-up difficult |
| Dempster 199312 | Age 3–12 years; otoscopic evidence of bilateral OME with a pure-tone air conduction threshold average over 0.5, 1 and 2 kHz of > 25 dBHL, and with an air-bone gap over 0.5, 1 and 2 kHz of > 25 dB, and a type B tympanogram | Previous adenoidectomy or aural surgery or additional symptoms requiring surgical intervention, e.g. recurrent sore throat; cleft palate |
| Hammarén-Malmi 200515,51 | Age 1–4 years; recurrent AOM (more than three episodes of AOM during the preceding 6 months or more than five episodes of AOM during the preceding 12 months) or a suspicion of chronic OME as judged by examination with a pneumatic otoscope | Previous adenotonsillar surgery or placement of tympanostomy tubes; children with asthma, cleft palate or diabetes or children who were judged to require prompt removal of adenoids because of obstructive symptoms resulting in continuous mouth breathing or sleep apnoea, were excluded |
| Koivunen 200416 | Age 10 months to 2 years; three or more episodes of AOM in the last 6 months | Previously performed adenoidectomy or tympanostomy tube placement; cranial anomalies; documented immunological disorders and ongoing antimicrobial chemoprophylaxis |
| Kujala 201217 | Age 10 months to 2 years; at least three AOM episodes during the past 6 months and residence within 25 miles of the hospital | Chronic OME; a prior adenoidectomy or tympanostomy tubes; cranial anomalies; documented immunological disorders or ongoing anti-microbial prophylaxis for a disease other than AOM |
| Mattila 200318 | Age 1–2 years; three to five events of AOM during the last 6 months or four to six events of AOM during the last year | Nothing known about exclusion criteria |
| Maw 1986,19 199310 | Age 2–9 years; persistent subjective hearing difficulty; pneumatic otoscopic confirmation of bilateral effusions; symmetrical audiometric hearing loss > 25 dB at one or more frequencies; impedance measurements not showing a peak A-type curve | Resolution of effusions in one or both ears during 3 months’ preoperative follow-up; upper airway obstruction from gross adenoidal hyperplasia; parents’ refusal of randomisation; reappraisal of audiometric data, either because the loss was asymmetrical or because of superadded sensorineural loss; loss to preoperative follow-up; the child was found to be ineligible for inclusion because at the moment of operation he or she did not have bilateral effusion |
| MRC Multicentre Otitis Media Study Group 201220 | Age 3.5–7 years; on two qualifying visits, 3 months apart, a bilateral B + B or B + C2 tympanogram combination (modified Jerger) and better-ear hearing loss ≥ 20 dBHL averaged across 0.5, 1, 2 and 4 kHz and an air-bone gap > 10 dB; non-independence of these markers entails that the conjunction is not greatly more stringent than the 20-dBHL component alone | Previous ear surgery; craniofacial structural abnormalities; severe systemic disease (e.g. diabetes) and non-OME ear disease (e.g. perforation). Optional exclusion: hearing loss ≤ 40 dBHL in the better-hearing ear55 |
| Nguyen 200421 | Age 18 months to 18 years; following indications for grommet insertion as the first surgical treatment of OM: (1) recurrent OM with more than three episodes during the preceding 6 months or more than four during the preceding 12 months, (2) OME persisting for > 3 months or producing a conducting hearing loss > 30 dB with a type B tympanogram or (3) both | Previous grommet insertion; Down syndrome; craniofacial anomalies such as cleft palate; immune deficiency; bleeding disorders; ciliary dyskinesia; follow-up period of < 6 months |
Appendix 6 Indication for surgery sorted by age range
| Study | Indication for surgery | Age range | |
|---|---|---|---|
| Eligible for inclusion | Empirical data | ||
| Koivunen 200416 | Recurrent AOM | 10 months to 2 years | 0.8–2.0 years |
| Kujala 201217 | Recurrent AOM | 10 months to 2 years | 0.5–2.0 years |
| Mattila 200318 | Recurrent AOM | 1–2 years | 1.0–2.0 years |
| Hammarén-Malmi 200515,51 | Both recurrent AOM and/or persistent OME | 1–4 years | 1.0–4.0 years |
| Nguyen 200421 | Both recurrent AOM and/or persistent OME | 18 months to 18 years | 1.5–9.6 years |
| Casselbrant 200911 | Persistent OME | 2–3.9 years | 2.0–4.0 years |
| Maw 1986,19 199310 | Persistent OME | 2–9 years | 2.5–8.7 years |
| Dempster 199312 | Persistent OME | 3–12 years | 4.0–9.0 years |
| MRC Multicentre Otitis Media Study Group 201220 | Persistent OME | 3.5–7 years | 3.5–7.0 years |
| Black 199052 | Persistent OME | 4–9 years | 3.4–9.8 years |
Appendix 7 Definitions of recurrent acute otitis media and persistent otitis media with effusion as used in the included studies
| Study | Recurrent AOM | Persistent OME | ||||||
|---|---|---|---|---|---|---|---|---|
| Three or more episodes in past 6 months | Four or more episodes in past 12 months | Five or more episodes in past 12 months | Indication for surgery based on clinical judgement | Otoscopy | Hearing loss | Tympanogram | History of symptoms | |
| Recurrent AOM | ||||||||
| Koivunen 200416 | ✗ | NA | NA | NA | NA | NA | ||
| Kujala 201217 | ✗ | NA | NA | NA | NA | NA | ||
| Mattila 200318 | ✗ | ✗ | NA | NA | NA | NA | NA | |
| Recurrent AOM and/or persistent OME | ||||||||
| Hammarén-Malmi 200515,51 | ✗ | ✗ | ✗ | Not indicated | ||||
| Nguyen 200421 | ✗ | ✗ | ≥ 30 dB | B | 3+ months | |||
| Persistent OME | ||||||||
| Black 199052 | NA | NA | NA | ✗ | Not indicated | |||
| Casselbrant 200911 | NA | NA | NA | Bilateral > 3 months or unilateral > 6 months or unilateral > 3 after extrusion of one grommet with the other still in place and patent | ||||
| Dempster 199312 | NA | NA | NA | ✗ | Pure-tone air conduction threshold average over 0.5, 1 and 2 kHz of > 25 dBHL, and with an air-bone gap over 0.5, 1 and 2 kHz of > 25 dB | B | Not indicated | |
| Maw 1986,19 199310 | NA | NA | NA | ✗ | Symmetrical audiometric hearing loss in excess of 25 dB at one or more frequencies | Not showing a peak A-type curve | Not indicated | |
| MRC Multicentre Otitis Media Study Group 201220 | NA | NA | NA | ≥ 20 dBHL averaged across 0.5, 1, 2 and 4 kHz and an air-bone gap > 10 dB | Bilateral B + B or B + C2 combination (modified Jerger) | 3+ months | ||
Appendix 8 Characteristics of the five eligible but unavailable studies
| Study | No. of patients | Indication for surgery | Age (years) | Interventions | Randomisation strategy | Outcome measurements | Potential risk factors/subgroups | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Gates 198714 | 491 | Persistent OME | 4–8 | (1) no surgery, (2) bilateral grommets, (3) adenoidectomy (4) adenoidectomy + bilateral grommets | By child | Tympanometry, audiometry | Age, sex, indication, race/ethnic group, SES, allergy, middle ear impedance/OME present, age at onset, adenoid size, siblings, family history | 12 and 24 months |
| Paradise 199023 | 99 | Both persistent OME and/or recurrent AOM | 1–15 | (1) no surgery, (2) adenoidectomy | By child | Tympanometry, otoscopy | Age, sex, indication, race/ethnic group, age at onset, middle ear impedance/OME present, baseline hearing, nasal obstruction, siblings, SES | 12, 24 and 36 months |
| Paradise 199922 | 304 | Both persistent OME and/or recurrent AOM | 3–15 | (1) no surgery, (2) adenoidectomy, (3) adenotonsillectomya | By child | Tympanometry, otoscopy | Age, sex, indication, race/ethnic group, age at onset, middle ear impedance/OME present, baseline hearing, nasal obstruction, siblings, SES | 12, 24 and 36 months |
| Roydhouse 198024 | 169 | Persistent OME | 2–14 | (1) no surgery, (2) bilateral grommets, (3) adenoidectomy + bilateral grommets | By child | Tympanometry, otoscopy | Age, sex, indication, race/ethnic group | 12, 24 and 36 months |
| Rynnel-Daggöö 198725 | 105 | Both persistent OME and/or recurrent AOM | < 12 | (1) no surgery, (2) adenoidectomy | By child | Tympanometry | Age, sex, indication, day care, URTI/AOM in the past, allergy, baseline hearing, middle ear impedance/OME present | 12 and 24 months |
Appendix 9 Inclusion and exclusion criteria of the five eligible but unavailable studies
| Study | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Gates 198714 | Age 4–8 years; bilateral chronic effusion diagnosed by an otoscopist | History of previous tonsil or adenoid surgery; placement of grommets (within 2 years); cleft palate; any severe chronic illness; advanced or irreversible structural damage of the tympanum (such as cholesteatoma, perforation, atelectasis) |
| Paradise 199023 | Age 1–15 years; (1) a history of persistent and/or recurrent OM; (2) thereafter received tympanostomy tube placement in one or both ears on one or more occasions; and (3) developed, after extrusion and within the year that preceded enrolment, one or more additional, well-documented episodes of either suppurative (A)OM or non-suppurative (A)OM | Overt or submucous palatal clefts |
| Paradise 199922 | Age 3–15 years; (1) at least three episodes of AOM during the preceding 6 months, or at least four episodes during the preceding 12 months including at least one episode during the preceding 6 months, with at least one of the episodes having been documented with a recorded description of symptoms and tympanic membrane findings or confirmed by tympanometry or myringotomy; or (2) middle ear effusion in one or both ears extending over at least 180 days during the preceding year and documented by at least two clinical observations at least 6 months apart, the most recent by a study team clinician and/or confirmed by tympanometry | Overt or submucous palatal clefts or previous grommet placement |
| Roydhouse 198024 | Age 2–14 years; OME diagnosed on clinical grounds and with impedance audiometry | A primary bias towards recurrent tonsillitis |
| Rynnel-Daggöö 198725 | Age < 12 years; recurrent serous/purulent OM; frequent upper airway infections and nasal obstruction | Severe nasal obstruction; previous operation performed; refused operation by parents; recurring adenoids; diabetes; or administrative mishaps |
Appendix 10 Number and percentage of children failing according to each component of the composite end point
| Recurrent AOM, n/N (%) | Persistent OME, n/N (%) | Improvement in hearing level < 10 dB, n/N (%) | Additional surgery, n/N (%) | Composite end point, n/N (%) | |
|---|---|---|---|---|---|
| Adenoidectomy with unilateral or bilateral grommets | |||||
| Black 199052 | – | 39/75 (52.0) | – | 1/75 (1.3) | 40/75 (53.3) |
| Casselbrant 200911 | 0/31 (0.0) | 0/24 (0.0) | – | 1/32 (3.1) | 1/32 (3.1) |
| Dempster 199312 | – | 24/37 (64.9) | 7/37 (18.9) | NA | 25/37 (67.6) |
| Hammarén-Malmi 200515,51 | 17/102 (16.7) | – | – | 10/110 (9.1) | 22/110 (20.0) |
| Kujala 201217 | 4/101 (4.0) | 0/40 (0.0) | – | 6/101 (5.9) | 9/101 (8.9) |
| Mattila 200318 | 4/45 (8.9) | – | – | 0/73 (0.0) | 4/73 (5.5) |
| Maw 1986,19 199310 | – | 52/83 (62.7) | 18/49 (36.7) | 7/80 (8.8) | 56/83 (67.5) |
| MRC Multicentre Otitis Media Study Group 201220 | 10/117 (8.5) | 38/114 (33.3) | 30/111 (27.0) | 2/128 (1.6) | 61/128 (47.7) |
| Nguyen 200421 | 0/17 (0.0) | 0/13 (0.0) | – | 0/33 (0.0) | 0/33 (0.0) |
| Adenoidectomy alone | |||||
| Casselbrant 200911 | 1/35 (2.9) | 0/29 (0.0) | – | 6/35 (17.1) | 7/35 (20.0) |
| Koivunen 200416 | 16/60 (26.7) | 0/60 (0.0) | – | 5/60 (8.3) | 20/60 (33.3) |
| Unilateral or bilateral grommets | |||||
| Black 199052 | – | 39/74 (52.7) | – | 7/77 (9.1) | 44/77 (57.1) |
| Casselbrant 200911 | 0/31 (0.0) | 0/27 (0.0) | – | 3/31 (9.7) | 3/31 (9.7) |
| Dempster 199312 | – | 21/35 (60.0) | 11/35 (31.4) | NA | 22/35 (62.9) |
| Hammarén-Malmi 200515,51 | 7/96 (7.3) | – | – | 13/107 (12.1) | 17/107 (15.9) |
| Kujala 201217 | 9/98 (9.2) | 0/48 (0.0) | – | 13/98 (13.3) | 17/98 (17.3) |
| Mattila 200318 | 3/32 (9.4) | – | – | 5/57 (8.8) | 5/57 (8.8) |
| Maw 1986,19 199310 | – | 67/83 (80.7) | 28/56 (50.0) | 31/83 (37.3) | 72/83 (86.7) |
| MRC Multicentre Otitis Media Study Group 201220 | 15/119 (12.6) | 44/113 (38.9) | 47/110 (42.7) | 4/126 (3.2) | 71/126 (56.3) |
| Nguyen 200421 | 0/28 (0.0) | 0/19 (0.0) | – | 0/37 (0.0) | 0/37 (0.0) |
| Non-surgical | |||||
| Koivunen 200416 | 41/120 (34.2) | 0/120 (0.0) | – | 30/120 (25.0) | 54/120 (45.0) |
| Kujala 201217 | 17/101 (16.8) | 0/21 (0.0) | – | 23/101 (22.8) | 30/101 (29.7) |
| MRC Multicentre Otitis Media Study Group 201220 | 19/112 (17.0) | 83/112 (74.1) | 40/100 (40.0) | 43/122 (35.2) | 109/122 (89.3) |
Appendix 11 Significant interaction effects for adenoidectomy with or without grommets compared with non-surgical treatment or grommets only
| n (%) | Adenoidectomy with or without unilateral or bilateral grommets (n = 767) | Non-surgical treatment or unilateral or bilateral grommets only (n = 994) | RD (95% CI)a | NNTa | RR or mean difference (95% CI)a | Adjusted RR or mean difference (95% CI)a | p-value for interactiona | |
|---|---|---|---|---|---|---|---|---|
| Secondary outcome: presence of effusion for ≥ 50% of the time, n/N (%) | ||||||||
| < 2 years | 291 (25.8) | 0/99 (0.0) | 0/192 (0.0) | – | – | – | – | |
| ≥ 2 years | 836 (74.2) | 153/376 (40.7) | 254/460 (55.2) | −14.5% (−21.3% to −7.8%) | 7 | 0.74 (0.64 to 0.85) | 0.78 (0.68 to 0.89) | 0.07 |
| Secondary outcome: surgery during the first 12 months of follow-up, n/N (%) | ||||||||
| < 4 years | 1016 (57.8) | 31/441 (7.0) | 94/575 (16.3) | −9.3% (−13.2% to −5.5%) | 11 | 0.43 (0.29 to 0.63) | 0.45 (0.31 to 0.66) | |
| ≥ 4 years | 742 (42.2) | 7/323 (2.2) | 78/419 (18.6) | −16.4% (−20.5% to −12.4%) | 6 | 0.12 (0.05 to 0.25) | 0.13 (0.06 to 0.27) | < 0.01 |
| Secondary outcome: time with effusion during the first 12 months of follow-up (weeks), mean (SD) | ||||||||
| Indication recurrent AOM | 303 (26.9) | 0.7 (2.7) | 1.0 (2.6) | – | – | −0.3 (−0.9 to 0.4) | −0.2 (−0.8 to 0.4) | |
| Indication persistent OME | 824 (73.1) | 20.8 (15.8) | 28.5 (18.0) | – | – | −7.7 (−10.0 to −5.4) | −6.0 (−8.0 to −4.0) | < 0.01 |
| < 2 years | 291 (25.8) | 0.75 (2.8) | 1.1 (2.6) | – | – | −0.3 (−0.9 to 0.4) | −0.2 (−0.9 to 0.4) | |
| ≥ 2 years | 836 (74.2) | 20.5 (15.8) | 28.2 (18.1) | – | – | −7.6 (−10.0 to −5.3) | −5.9 (−7.9 to −3.8) | < 0.01 |
| < 4 years | 446 (39.6) | 5.3 (11.6) | 5.6 (13.0) | – | – | −0.2 (−2.6 to 2.1) | −1.2 (−2.3 to −0.1) | |
| ≥ 4 years | 681 (60.4) | 23.2 (14.9) | 30.1 (17.0) | – | – | −7.0 (−9.4 to −4.5) | −6.6 (−9.0 to −4.3) | < 0.01 |
Appendix 12 Significant interaction effects for adenoidectomy with unilateral or bilateral grommets compared with non-surgical treatment
| n (%) | Adenoidectomy with unilateral or bilateral grommets (n = 672)a | Non-surgical treatment (n = 343)b | RD (95% CI)c | NNTc | RR or mean difference (95% CI)c | Adjusted RR or mean difference (95% CI)c | p-value for interactionc | |
|---|---|---|---|---|---|---|---|---|
| Primary outcome: failure after 12 months, n/N (%) | ||||||||
| < 2 years | 462 (45.5) | 84/220 (38.2) | 27/242 (11.2) | −27.0% (−34.6% to −19.5%) | 4 | 0.29 (0.20 to 0.43) | 0.28 (0.13 to 0.56) | |
| ≥ 2 years | 553 (54.5) | 191/430 (44.4) | 109/123 (88.6) | −44.2% (−51.5% to −36.9%) | 3 | 0.50 (0.43 to 0.57) | 0.53 (0.44 to 0.65) | 0.09 |
| Secondary outcome: surgery during the first 12 months of follow-up, n/N (%) | ||||||||
| Indication recurrent AOM | 486 (48.0) | 13/265 (4.9) | 53/221 (24.0) | −19.1% (−25.3% to −12.9%) | 6 | 0.21 (0.12 to 0.37) | 0.26 (0.11 to 0.61) | |
| Indication persistent OME | 526 (52.0) | 14/404 (3.5) | 43/122 (35.2) | −31.8% (−40.4% to −23.1%) | 4 | 0.10 (0.06 to 0.17) | 0.04 (0.01 to 0.18) | 0.03 |
| < 2 years | 462 (45.7) | 13/242 (5.4) | 53/220 (24.1) | −18.7% (−25.0% to −12.4%) | 6 | 0.22 (0.13 to 0.40) | 0.23 (0.09 to 0.57) | |
| ≥ 2 years | 550 (54.3) | 14/427 (3.3) | 43/123 (35.0) | −31.7% (−40.3% to −23.1%) | 4 | 0.09 (0.05 to 0.17) | 0.04 (0.01 to 0.18) | 0.02 |
| < 4 years | 578 (57.1) | 20/346 (5.8) | 56/232 (24.1) | −18.4% (−24.4% to −12.3%) | 6 | 0.24 (0.15 to 0.39) | 0.25 (0.11 to 0.57) | |
| ≥ 4 years | 434 (42.9) | 7/323 (2.2) | 40/111 (36.0) | −33.9% (−42.9% to −24.8%) | 3 | 0.06 (0.03 to 0.13) | 0.05 (0.01 to 0.18) | < 0.01 |
| Secondary outcome: presence of effusion for ≥ 50% of the time, n/N (%) | ||||||||
| < 2 years | 179 (28.0) | 0/39 (0.0) | 0/140 (0.0) | – | – | – | – | |
| ≥ 2 years | 460 (72.0) | 153/374 (44.1) | 83/113 (73.5) | −29.4% (−39.0% to −19.7%) | 4 | 0.60 (0.51 to 0.71) | 0.45 (0.34 to 0.60) | 0.01 |
| Secondary outcome: time with effusion during the first 12 months of follow-up (weeks), mean (SD) | ||||||||
| Indication recurrent AOM | 186 (29.1) | 0.4 (0.5) | 1.2 (3.0) | – | – | −0.9 (−1.8 to 0.0) | 0.1 (−0.1 to 0.2) | |
| Indication persistent OME | 453 (70.9) | 22.2 (15.6) | 36.8 (15.0) | – | – | −14.6 (−17.9 to −11.3) | −15.4 (−19.2 to −11.6) | < 0.01 |
| < 2 years | 179 (28.0) | 0.3 (0.3) | 1.3 (3.0) | – | – | −1.0 (−2.0 to −0.1) | 0.1 (−0.1 to 0.2) | |
| ≥ 2 years | 460 (72.0) | 21.9 (15.7) | 36.5 (15.3) | – | – | −14.6 (−17.9 to −11.3) | −15.4 (−19.2 to −11.6) | < 0.01 |
| < 4 years | 244 (38.2) | 8.6 (15.3) | 4.5 (12.4) | – | – | 4.1 (0.6 to 7.6) | −4.5 (−9.2 to 0.1) | |
| ≥ 4 years | 395 (61.8) | 23.2 (14.9) | 35.8 (14.9) | – | – | −12.7 (−16.0 to −9.3) | −14.4 (−18.3 to −10.5) | 0.01 |
Appendix 13 Significant interaction effects for adenoidectomy with unilateral or bilateral grommets compared with unilateral or bilateral grommets
| n (%) | Adenoidectomy with unilateral or bilateral grommets (n = 672)a | Unilateral or bilateral grommets (n = 651)a | RD (95% CI)b | NNTb | RR or mean difference (95% CI)b | Adjusted RR or mean difference (95% CI)b | p-value for interactionb | |
|---|---|---|---|---|---|---|---|---|
| Secondary outcome: surgery during the first 12 months of follow-up, n/N (%) | ||||||||
| < 4 years | 689 (52.2) | 20/346 (5.8) | 38/343 (11.1) | −5.3% (−9.4 to −1.2) | 19 | 0.52 (0.31 to 0.88) | 0.49 (0.29 to 0.83) | |
| ≥ 4 years | 631 (47.8) | 7/323 (2.2) | 38/308 (12.3) | −10.2% (−14.2 to −6.2) | 10 | 0.18 (0.08 to 0.39) | 0.20 (0.09 to 0.44) | 0.07 |
| Secondary outcome: time with effusion during the first 12 months of follow-up (weeks), mean (SD) | ||||||||
| Indication recurrent AOM | 102 (13.0) | 0.4 (0.5) | 0.4 (0.9) | – | – | 0.0 (−0.3 to 0.3) | 0.0 (−0.2 to 0.2) | |
| Indication persistent OME | 683 (87.0) | 22.2 (15.6) | 25.8 (18.1) | – | – | −3.5 (−6.0 to −1.0) | −3.8 (−6.0 to −1.7) | < 0.01 |
| < 2 years | 91 (11.6) | 0.3 (0.3) | 0.4 (1.0) | – | – | −0.1 (−0.5 to 0.2) | −0.0 (−0.1 to 0.1) | |
| ≥ 2 years | 694 (88.4) | 21.9 (15.7) | 25.4 (18.1) | – | – | −3.5 (−6.1 to −1.0) | −3.8 (−5.9 to −1.6) | 0.02 |
| < 4 years | 205 (26.1) | 8.6 (15.3) | 7.0 (13.8) | – | – | 1.6 (−2.4 to 5.6) | −1.5 (−3.3 to 0.3) | |
| ≥ 4 years | 580 (73.9) | 23.2 (14.9) | 28.1 (17.3) | – | – | −4.9 (−7.6 to −2.3) | −4.4 (−6.8 to −1.9) | < 0.01 |
Appendix 14 Significant interaction effects for adenoidectomy without grommets compared with non-surgical treatment
| n (%) | Adenoidectomy without grommets (n = 95)a | Non-surgical (n = 343)b | RD (95% CI)c | NNTc | RR or mean difference (95% CI)c | Adjusted RR or mean difference (95% CI)c | p-value for interactionc | |
|---|---|---|---|---|---|---|---|---|
| Primary outcome: failure after 12 months, n/N (%) | ||||||||
| Indication recurrent AOM | 281 (64.2) | 20/60 (33.3) | 84/221 (38.0) | −4.7% (−18.2% to 8.9%) | 22 | 0.88 (0.59 to 1.30) | 0.75 (0.49 to 1.12) | |
| Indication persistent OME | 157 (35.8) | 7/35 (20.0) | 109/122 (89.3) | −69.3% (−83.7% to −55.0%) | 2 | 0.22 (0.12 to 0.44) | 0.22 (0.12 to 0.44) | < 0.01 |
| < 2 years | 280 (63.9) | 20/60 (33.3) | 84/220 (38.2) | −4.9% (−18.4% to 8.7%) | 21 | 0.87 (0.59 to 1.30) | 0.74 (0.49 to 1.11) | |
| ≥ 2 years | 158 (36.1) | 7/35 (20.0) | 109/123 (88.6) | −68.8% (−83.0% to −54.2%) | 2 | 0.23 (0.12 to 0.44) | 0.22 (0.12 to 0.44) | < 0.01 |
| Secondary outcome: presence of effusion for ≥ 50% of the time, n/N (%) | ||||||||
| Indication recurrent AOM | 201 (58.8) | 0/60 (0.0) | 0/141 (0.0) | NA | – | – | – | |
| Indication persistent OME | 141 (41.2) | 0/29 (0.0) | 83/112 (74.1) | −74.1% (−82.2% to −66.0%) | 2 | – | – | < 0.01 |
| < 2 years | 200 (58.5) | 0/60 (0.0) | 0/140 (0.0) | NA | – | – | – | |
| ≥ 2 years | 142 (41.5) | 0/29 (0.0) | 83/113 (73.5) | −73.5% (−81.6% to −65.3%) | 2 | – | – | < 0.01 |
| Secondary outcome: time with effusion during the first 12 months of follow-up (weeks), mean (SD) | ||||||||
| Indication recurrent AOM | 201 (58.8) | 1.1 (3.5) | 1.3 (3.0) | – | – | −0.2 (−1.1 to 0.8) | −0.4 (−1.4 to 0.6) | |
| Indication persistent OME | 141 (41.2) | 3.8 (2.6) | 36.8 (15.1) | – | – | −33.0 (−38.6 to −27.5) | −33.0 (−35.9 to −30.1) | < 0.01 |
| < 2 years | 200 (58.5) | 1.1 (3.5) | 1.3 (3.0) | – | – | −0.2 (−0.9 to 0.4) | −0.4 (−1.4 to 0.6) | |
| ≥ 2 years | 142 (41.5) | 3.8 (2.7) | 36.5 (15.3) | – | – | −32.7 (−35.7 to −29.7) | −33.0 (−35.9 to −30.1) | < 0.01 |
Appendix 15 Significant interaction effects for adenoidectomy without grommets compared with grommets only
| n (%) | Adenoidectomy without grommets (n = 95)a | Unilateral or bilateral grommets (n = 651)b | RD (95% CI)c | NNTc | RR or mean difference (95% CI)c | Adjusted RR or mean difference (95% CI)c | p-value for interactionc | |
|---|---|---|---|---|---|---|---|---|
| Primary outcome: failure after 12 months, n/N (%) | ||||||||
| Indication recurrent AOM | 317 (42.6) | 20/60 (33.3) | 37/257 (14.4) | 18.9% (6.3% to 31.6%) | – | 2.32 (1.45 to 2.69) | 1.78 (1.00 to 3.17) | |
| Indication persistent OME | 427 (57.4) | 7/35 (20.0) | 231/392 (54.3) | −34.3% (−48.5% to −20.2%) | 3 | 0.37 (0.19 to 0.72) | 2.07 (0.58 to 7.31) | 0.02 |
| < 2 years | 289 (38.7) | 20/60 (33.3) | 37/229 (16.2) | 17.2% (4.3% to 30.0%) | – | 2.06 (1.30 to 3.28) | 1.48 (0.85 to 2.59) | |
| ≥ 2 years | 457 (61.2) | 7/35 (20.0) | 214/422 (50.7) | −30.7% (−44.8% to −16.6%) | 4 | 0.39 (0.20 to 0.77) | 2.07 (0.58 to 7.31) | < 0.01 |
| Secondary outcome: four or more AOM episodes per year, n/N (%) | ||||||||
| < 2 years | 255 (51.1) | 16/60 (26.7) | 18/195 (9.2) | 17.4% (5.5% to 29.3%) | – | 2.88 (1.57 to 5.31) | ||
| ≥ 2 years | 244 (48.9) | 1/35 (2.9) | 16/209 (7.7) | −4.8% (−11.4% to 1.8%) | 21 | 0.37 (0.05 to 2.72) | < 0.01 | |
| Secondary outcome: presence of effusion for ≥ 50% of the time, n/N (%) | ||||||||
| Indication recurrent AOM | 117 (24.0) | 0/60 (0.0) | 0/57 (0.0) | – | – | – | – | |
| Indication persistent OME | 371 (76.0) | 0/29 (0.0) | 171/342 (50.0) | −50.0% (−55.3% to −44.7%) | 2 | – | – | < 0.01 |
| < 2 years | 112 (23.0) | 0/60 (0.0) | 0/52 (0.0) | – | – | – | – | |
| ≥ 2 years | 376 (77.0) | 0/29 (0.0) | 171/347 (49.3) | −49.3% (−54.5% to −44.0%) | – | – | – | < 0.01 |
| Secondary outcome: number of AOM episodes in the first 12 months of follow-up, mean (SD) | ||||||||
| Indication recurrent AOM | 227 (50.6) | 2.1 (2.1) | 1.4 (1.0) | – | – | 0.7 (0.2 to 1.2) | 0.5 (−0.2 to 1.1) | |
| Indication persistent OME | 222 (49.4) | 0.5 (1.2) | 1.0 (1.6) | – | – | −0.52 (−1.1 to 0.0) | −0.3 (−0.1 to 0.7) | 0.01 |
| < 2 years | 255 (51.1) | 2.1 (2.1) | 1.5 (1.6) | – | – | 0.6 (0.1 to 1.1) | 0.3 (−0.4 to 0.9) | |
| ≥ 2 years | 244 (48.9) | 0.5 (1.2) | 0.9 (1.5) | – | – | −0.5 (−1.0 to 0.0) | −0.3 (−0.1 to 0.7) | 0.06 |
| Secondary outcome: time with effusion during the first 12 months of follow-up (weeks), mean (SD) | ||||||||
| Indication recurrent AOM | 117 (24.0) | 1.1 (3.5) | 0.4 (0.9) | – | – | 0.7 (−0.3 to 1.6) | 0.0 (−1.5 to 1.6) | |
| Indication persistent OME | 371 (76.0) | 3.8 (2.6) | 25.8 (18.1) | – | – | −22.0 (−28.6 to −15.4) | 1.5 (0.2 to 2.7) | < 0.01 |
| < 2 years | 112 (23.0) | 1.1 (3.5) | 0.4 (1.0) | – | – | 0.7 (−0.3 to 1.7) | −1.0 (−3.2 to 1.2) | |
| ≥ 2 years | 376 (77.0) | 3.8 (2.6) | 25.4 (18.2) | – | – | −21.6 (−28.3 to −15.0) | 1.5 (0.2 to 2.7) | < 0.01 |
Appendix 16 Per-protocol and as-treated analysis for the subgroup analysis of the main comparison between adenoidectomy with or without grommets and non-surgical treatment or grommets only
| n (%) | Adenoidectomy with or without unilateral or bilateral grommets, n/N (%) | Non-surgical treatment or unilateral or bilateral grommets only, n/N (%) | RD or mean difference (95% CI)a | NNTa | RR (95% CI)a | Adjusted RR (95% CI)a | p-value for interactiona | |
|---|---|---|---|---|---|---|---|---|
| Indication recurrent AOM | ||||||||
| Intention to treat | ||||||||
| < 2 years | 719 (89.5) | 44/281 (15.7) | 120/438 (27.4) | −11.7% (−17.7% to −5.8%) | 9 | 0.57 (0.42 to 0.78) | 0.63 (0.47 to 0.85) | |
| ≥ 2 years | 84 (10.5) | 8/44 (18.5) | 1/40 (2.5) | 15.7% (3.3% to 28.1%) | – | 7.27 (0.95 to 55.6) | 4.96 (0.69 to 35.5) | 0.04 |
| Per protocol | ||||||||
| < 2 years | 660 (88.9) | 44/281 (15.7) | 61/379 (16.1) | −0.4% (−6.1% to 5.2%) | – | 0.97 (0.68 to 1.39) | 1.05 (0.75 to 1.47) | |
| ≥ 2 years | 83 (11.1) | 8/44 (18.5) | 0/39 (0.0) | 18.2% (6.8% to 29.6%) | – | – | – | NA |
| As treated (using a definition of failure of four or more episodes of AOM per year, effusion for ≥ 50% of the time, additional surgery, improvement in hearing level < 10 dB) | ||||||||
| < 2 years | 719 (89.5) | 103/340 (30.3) | 61/379 (16.1) | 14.2% (8.1% to 20.3%) | – | 1.88 (1.42 to 2.49) | 1.94 (1.48 to 2.53) | |
| ≥ 2 years | 84 (10.5) | 9/45 (20.0) | 0/39 (0.0) | 20.0% (8.3% to 31.7%) | – | – | – | NA |
| As treated (using a definition of failure of four or more episodes of AOM per year, effusion for ≥ 50% of the time, improvement in hearing level < 10 dB) | ||||||||
| < 2 years | 719 (89.5) | 60/340 (17.6) | 48/379 (12.7) | 5.0% (−0.3% to 10.2%) | – | 1.39 (0.98 to 1.98) | 1.39 (1.00 to 1.94) | |
| ≥ 2 years | 84 (10.5) | 8/45 (17.8) | 0/39 (0.0) | 17.8% (6.6% to 29.0%) | – | – | – | NA |
| Indication persistent OME | ||||||||
| Intention to treat | ||||||||
| < 4 years | 239 (24.5) | 30/128 (23.4) | 33/111 (29.7) | −6.9% (−17.5% to 4.9%) | 15 | 0.79 (0.52 to 1.20) | 0.98 (0.69 to 1.38) | |
| ≥ 4 years | 737 (75.5) | 163/322 (50.6) | 289/415 (69.6) | −19.0% (−26.1% to −12.0%) | 6 | 0.73 (0.64 to 0.82) | 0.77 (0.68 to 0.86) | 0.10 |
| Per protocol | ||||||||
| < 4 years | 231(24.4) | 30/128 (23.4) | 25/103 (24.3) | −0.8% (−11.9% to 10.2%) | – | 0.97 (0.61 to 1.53) | 1.20 (0.85 to 1.66) | |
| ≥ 4 years | 717 (75.6) | 163/322 (50.6) | 269/395 (68.1) | −17.5% (−24.6% to −10.3%) | 6 | 0.74 (0.65 to 0.84) | 0.78 (0.69 to 0.88) | 0.01 |
| As treated (using a definition of failure of four or more episodes of AOM per year, effusion for ≥ 50% of the time, additional surgery, improvement in hearing level < 10 dB) | ||||||||
| < 4 years | 239 (24.5) | 38/136 (27.9) | 25/103 (24.3) | 3.7% (−7.5% to 14.9%) | – | 1.15 (0.75 to 1.78) | 1.31 (0.98 to 1.75) | |
| ≥ 4 years | 737 (75.5) | 183/342 (53.5) | 269/395 (68.1) | −14.6% (−21.6% to −7.6%) | 7 | 0.79 (0.70 to 0.89) | 0.83 (0.74 to 0.93) | < 0.01 |
| As treated (using a definition of failure of four or more episodes of AOM per year, effusion for ≥ 50% of the time, improvement in hearing level < 10 dB) | ||||||||
| < 4 years | 239 (24.5) | 27/136 (19.9) | 23/103 (22.3) | −2.5% (−13.0% to 8.0%) | – | 0.89 (0.54 to 1.46) | 1.08 (0.81 to 1.42) | |
| ≥ 4 years | 737 (75.5) | 175/342 (51.2) | 253/395 (64.1) | −12.9% (−20.0% to −5.8%) | 8 | 0.80 (0.70 to 0.91) | 0.84 (0.74 to 0.94) | 0.06 |
Appendix 17 Recommendations for further research
Introduction
As requested in the brief, we have summarised the outstanding research questions based on the specific project and its position in the range of systematic reviews that it complements, covering grommets and adenoidectomy in children with OME (‘glue ear’), and recurrent upper respiratory symptoms.
Several specific recommendations are made and these follow the generic answers to the questions posed.
Briefly explain why this research or evidence is important to the NHS or for wider public health
Upper respiratory tract infections are the most common infectious diseases in children. It is one of the most common diagnoses in primary care. Although many infections are self-limiting, some require treatment.
What is the patient/population group likely to benefit from this research and why/how will they benefit?
Children with recurrent upper respiratory tract symptoms – especially nasal obstruction and discharge and symptoms of recurrent AOM and chronic OME – are often considered for surgical intervention (grommets, adenoidectomy and tonsillectomy). Alternatively they are treated non-surgically or are simply ‘actively monitored’ with a period of ‘watchful waiting’.
In what setting will the outcome of the research be delivered (e.g. primary care, the community)?
The optimal management of the population comprising children with recurrent upper respiratory tract symptoms needs to be considered on a population basis rather than in terms of distinct strategies in primary or secondary care. We know that expectations set in primary care can have profound effects on management choices in secondary care. Shared decision-making processes, based on outcomes important to patients, must be supported by relevant evidence in all health-care settings.
Important research questions
Question 1: what are the best methods of selecting, developing and administering patient-reported outcome measures to assess the value of treatments for children with persistent otitis media with effusion and recurrent acute otitis media and upper respiratory tract infections?
Notes
This requires:
-
A systematic literature review to inform the selection of PROMs in children with ear, nose and throat (ENT) infections.
-
An evaluation of the role of PROMs in stimulating shared decision-making.
-
The design of a ‘PROM-related intervention’ that might be used with the parents and carers of children with ENT infections who are faced with making decisions about the alternative options of surgical and non-surgical management. This PROM-related intervention should be shaped by a scoping exercise to look at which questionnaire(s) to use, how and when to administer and score them and who to administer them to, how to report and present the results to children and parents/carers and how to respond to issues raised.
-
An evaluation of the effectiveness of the use of PROMs in this way in children with ENT infections in a cluster randomised trial.
Question 2: what is the clinical effectiveness and cost-effectiveness of hearing aids (air or bone conduction) for children with different degrees of persistence and severity of hearing loss associated with otitis media with effusion?
Question 3: why do treatment preferences (both professionals’ and parents’/carers’ preferences) vary so much?
Notes
Further studies are required of the practice variation seen in the management of (recurrent) AOM, (persistent) OME and (recurrent) upper respiratory tract infections. Research should include the linking of existing data sets [including Flu Watch (see www.fluwatch.co.uk), the Clinical Practice Research Datalink (CPRD) (see www.cprd.com) and Hospital Episode Statistics (HES) (see www.hscic.gov.uk/hes)] and qualitative studies.
Question 4: why does adenoidectomy work in different subgroups at different ages?
Notes
Age may be a proxy measure for some maturational physiological changes that could be better understood.
Further studies are required into the aetiology and prognostics of (persistent) OME and (recurrent) AOM and upper respiratory tract infections attributable (rightly or wrongly) to adenoid hypertrophy or infection. Research enquiries should include risk factors such as reflux and the microbiome of the upper airways.
Question 5: special populations – children with Down syndrome, cleft palate or developmental problems
Notes
All of the existing systematic reviews (and, as far as we are aware, all RCTs) exclude children with these special conditions. The question therefore remains: what are the most clinically effective and cost-effective treatment strategies for children with recurrent AOM and persistent OME who also have Down syndrome, cleft palate or developmental problems?
Glossary
- Acute otitis media
- An acute infection of the middle ear that can be viral and/or bacterial in origin and which may result in the formation of pus and lead to an acute perforation of the tympanic membrane.
- Otitis media with effusion (‘glue ear’)
- The presence of fluid in the middle ear behind an intact tympanic membrane without signs and/or symptoms of an acute infection.
List of abbreviations
- AOM
- acute otitis media
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- dBHL
- hearing level in decibels as assessed on an audiometer
- ENT
- ear, nose and throat
- IPD
- individual patient data
- NICE
- National Institute for Health and Care Excellence
- NNT
- number needed to treat
- OM
- otitis media
- OME
- otitis media with effusion
- OR
- odds ratio
- PI
- primary investigator
- PROM
- patient-reported outcome measure
- RCT
- randomised controlled trial
- RD
- rate difference
- ROC
- receiver operating curve
- RR
- rate ratio
- SD
- standard deviation