Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/117/02. The contractual start date was in November 2010. The draft report began editorial review in June 2012 and was accepted for publication in November 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Peter Bower has acted as a paid scientific consultant to the British Association of Counselling and Psychotherapy and Paul Stallard was a named co-applicant on two other research grants at the time this work was submitted: National Institute for Health Research Health Technology Assessment Grant 06/37/04 and National Institute for Health Research Public Health Grant 09/3000/03.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Bee et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and objectives
Serious parental mental illness poses a significant challenge to quality of life (QoL) in a substantial number of infants, children and adolescents. Research suggests that the children of parents with serious mental illness (SMI) are at increased risk of a range of emotional, social, behavioural and educational difficulties that arise from a complex interplay of genetic, environmental and psychosocial factors. 1 A recent shift in UK policy has placed greater emphasis on the well-being of these children, with the shared recommendation that their needs should be better addressed by health- and social-care services. 2 In 2010, the Health Technology Assessment (HTA) programme prioritised an evidence synthesis of the clinical effectiveness and cost-effectiveness of community-based interventions aimed at increasing or maintaining QoL in children and adolescents of parents with SMI. The results of this work are presented here.
The epidemiology of serious parental mental illness
A substantial proportion of children and adolescents experience SMI in family members. Many of these children remain invisible to services. A lack of recognition of the family circumstances of many service users,2 historically poor integration between adult and child mental health services3 and the inadequate identification of children caring for parents with mental illness4 have traditionally hampered the accurate quantification of point prevalence rates.
Conservative estimates suggest that, within the UK, approximately 175,000 children provide informal care for a parent or sibling,5 almost one-third (29%) of whom will care for a relative with a mental health difficulty. 6 However, such data pertain only to those children who are formally recognised as young carers and, as such, may substantially underestimate the true number of children affected by parental mental illness. Best estimates suggests that more than 4.2 million parents within the UK suffer from mental health problems. 7 Approximately half of all adult mental health service users will have children under the age of 18 years, and 1 in 10 will have a child under the age of 5 years. 8
The proportion of parents who experience SMI is less well defined. A recent systematic review has reported that at any one time in the UK, 9–10% of women and 5–6% of men will be parents with a mental health disorder, fewer than 0.5% of whom will be experiencing a psychotic disorder. 9 These data do not include adults with a personality disorder, for whom the UK prevalence rates are estimated at around 4%. 10 Other empirical work suggests that at least one-quarter of adults admitted to UK acute inpatient settings have dependent children and that between 50% and 66% of people with SMI will be living with children under the age of 18 years. 11
Current UK policy initiatives
Current guidance published by the Social Care Institute for Excellence (SCIE) recognises parents with mental health problems and their children as a high-risk group with multifaceted needs,2 which have historically been neglected by UK service provision. 12 Successive organisational evaluations acknowledge that parents with mental health problems are a group prone to exclusion from health- and social-care provision and in doing so emphasise that greater effort may be required to reach these vulnerable families. 2,13
In response, a recent and notable shift in UK health- and social-care policy has instigated new initiatives that place greater emphasis on the need to support parents in their parenting roles. 13,14 From a mental health perspective, national UK outcome strategies2,15–17 are explicit in targeting mental health across the lifespan and in steering services towards severing intergenerational cycles of mental health difficulties through the promotion of whole-family assessments and recovery plans. Similar advances are advocated by educational and social reform initiatives. These initiatives call for greater family-focused service provision, enhanced coordination between child and adult mental health services and increased intervention for troubled families. 6,14,18
The clinical and social consequences of serious parental mental illness
In any given population, SMI is likely to be associated with poorer mental and physical well-being, impaired functioning, lower economic productivity and marked decrements in an individual’s health-related QoL. 19–21 When parents experience SMI, this burden extends far beyond the individual concerned, with potential for multiple adverse outcomes in successive generations. 22
The burden that is placed on the children of parents with SMI is substantial. Evidence demonstrates that the children of mentally ill parents are at risk of poorer psychological and physical health23,24 increased behavioural and developmental difficulties,24–26 educational underachievement8,27 and lower competency than their peers. 26,28–30 These problems may be exacerbated if both parents suffer mental illness. 31
Children of parents with SMI may also experience greater exposure to parental substance misuse,1,32 domestic violence and child abuse. 1 A recent meta-analytic review has reported parental mental illness to be a key risk factor for child maltreatment,33 with parental depression, personality disorder and alcohol or substance misuse all implicated in the physical abuse and neglect of minors. In rare cases, parental mental health difficulties have also been associated with increased rates of all-cause and cause-specific mortality in children. 34 Large population studies have reported elevated risks for neonatal death, sudden infant death syndrome, accidental injury and child homicide. 34
The longer-term impact of serious parental mental illness has been demonstrated to extend into adulthood and includes a higher risk of social and occupational dysfunction,35,36 increased psychological and psychiatric morbidity,37 lower self-esteem and increased alcohol or substance misuse. 38–40
Potential mechanisms of effect
The mechanisms by which parental mental illness may impact on familial and child outcomes are multifarious, encompassing a broad range of temporal, genetic and psychosocial influences. 41 Nevertheless, the existing evidence is relatively consistent in suggesting that socioenvironmental factors, and particularly family context, may ultimately be more important in accounting for child outcomes than biological vulnerability. 42
Although not inevitable, the care-giving environments provided by parents with SMI have been associated with an increased risk of their children failing to meet developmental norms. 43 The cognitive and psychological impairments that accompany episodes of SMI can substantially affect a parent’s capacity to meet his or her child’s needs. 44 Adults with SMI have been reported to display less emotional availability, reduced parenting confidence and poorer quality stimulation for their children than their healthy counterparts. 26,45 The affective quality of parental–child interactions has in turn been associated with the socioemotional adjustment of children, including internalising and externalising behaviours, and the nature and quality of parent–child attachments. 46 Parenting resilience, especially that occurring early in life, is thus thought to play a key role in determining the developmental, psychosocial and clinical outcomes of children of parents with SMI. 47,48
Nonetheless, interrelationships between parental mental illness and child outcomes are complex and parents living with SMI are also likely to experience additional challenges to the provision of safe and stable family environments. 49 These challenges arise not only because of the difficulty of parenting while managing psychiatric symptoms, but also because of the threat of children being moved to out-of-home care. 50 Mothers with SMI are more likely to be involved with children’s social services and more likely to have children in care than mothers with more common mental health problems. 51 Parenting may be compromised by a need to overcome social isolation, social discrimination and other external stress factors which typically result in low social capital, poverty and health inequalities for mental health sufferers and their children. 52 Research suggests that families affected by parental mental illness are more likely to experience economic hardship, housing problems and relationship discord than families not affected by such illnesses. 42 A total of 140,000 families, approximately 2% of all UK families, are reported to suffer the combined effect of parental illness, low income, lower educational attainment and poor housing, and this group exists as one of the most vulnerable in society. 14,18
Ultimately, however, families are not homogeneous in social circumstance or demography and, thus, any intervention programmes developed for this population must also be capable of responding to a diversity of need. Key moderators of adverse outcomes in children include their age and developmental maturity at the onset of parental mental illness, the severity and duration of their parent’s symptoms, the strengths and resources of family members, their own resiliency and the degree of social exclusion or discrimination that they experience. 41,49 While impaired parenting during infancy may have a long-term impact on children’s social and cognitive development, for example, exposure to parental mental illness in later childhood may present a more immediate and self-acknowledged stressor, with a qualitatively very different effect. Recognition of this temporal influence highlights the importance of developing multiple evidence-based services capable of being delivered in a developmentally and age-appropriate manner.
Mapping interventions for families affected by serious parental mental illness
Developmental theorists conceptualise children and adolescents as active agents capable of both being influenced by, and exerting an influence upon, the social context in which they live. 53,54 Empirical research on parent–child interactions has also demonstrated a bidirectional relationship in which children and parents have been found to mutually influence each other’s behaviour. 55,56 QoL in children of parents with SMI thus attracts multiple influences and by implication multiple avenues of change.
A recent review of interventions for families affected by parental mental illness identifies a heterogeneous mix of interventions targeting children, parents and/or the parent–child dyad. 57 The format and content of these interventions varies. Direct interventions, by definition, establish the child as the major change agent and seek to improve child health or resiliency through either therapeutic or strength-based models of care. By virtue of their need for active child participation, these interventions typically target school-aged children or adolescents, with specific content and QoL outcomes dictated by the participants’ age, cognitive development and predominant life stressors. Examples from the literature include group-based psychoeducational programmes58,59 and psychotherapeutic techniques. 60,61
However, developmental immaturity often precludes direct intervention with infants under the age of 2 years. Therefore, in early childhood, parents will normally be considered the principal agent of change and interventions will aim to indirectly enhance child well-being through an improvement in parenting behaviour or enhanced parental health. Examples of these interventions include, but are not confined to, parenting education programmes,60,62,63 manualised parenting or behavioural skills programmes64,65 and parent-centred psychological therapies. Indirect interventions such as these may also be applied to the parents of older children.
In practice, direct and indirect interventions are not mutually exclusive and a limited number of hybrid interventions have also emerged. 66,67 These interventions seek to target both parents and children either simultaneously or separately and may be delivered to the families of both younger and older children.
Irrespective of their path of action, community-based interventions may be delivered to the individual, the individual family unit or operationalised within a wider group format intended to enhance interpersonal relationship building and peer support.
The economic benefits of intervention
The hidden nature of many children affected by parental mental illness and the historical disjuncture of adult and child services has made the true economic costs of these illnesses difficult to quantify. A rapid evidence assessment7 estimated in 2008 that for every pound invested in psychosocial interventions for young carers of parents with SMI, a conservative or ‘lower bound’ societal gain of almost seven times this amount may be achieved. This estimate takes into account the savings associated with supporting a young person’s caring activities alongside savings gained from reductions in the child truancy rate, teenage pregnancy rate and a reduced likelihood of a young person being taken into local authority care. Characteristically, the evidence on which this analysis was based was limited primarily to those in a recognised caring role and, as such, may not be representative of all children and adolescents of parents experiencing SMI. A separate, non-systematic synthesis of interventions for children of parents with SMI highlights a distinct paucity of published cost-effectiveness data, cost-effectiveness analyses and decision-modelling techniques. 52
The rationale for an evidence synthesis
As with any aspect of health delivery, the development of a clinically effective and cost-effective intervention programme for children of parents with SMI must be based upon the establishment of a secure evidence base. The measurement of children’s QoL has a central role in the evaluation of health-care interventions and in improving children’s experiences of health and social services. As yet, however, no comprehensive and rigorous review of the impact of community-based interventions on the QoL of children of parents with SMI exists. Previous reviews of parenting interventions and interventions aimed at the mother–child relationship have been published but these remain limited by a lack of systematic methodology, a neglect of grey literature and/or restrictions in the nature of the interventions, populations and outcomes studied. 68–70
In 2008, a SCIE-commissioned review57 evaluated the clinical effectiveness of interventions aimed at improving parenting skills and life outcomes for parents and families affected by mental illness. This study highlighted a lack of robust data resulting from a paucity of randomised controlled trials (RCTs), small sample sizes and a lack of consideration of attention control conditions. 57 However, the focus of this review was not directly orientated towards serious parental mental illness and it did not explicitly consider the effect of interventions on children’s and adolescents’ subjective QoL.
In 2012, a meta-analysis71 examined the clinical effectiveness of parent-based and parent–child dyadic interventions in enhancing the psychological well-being of children born to parents with mental illness. This study pooled data from 13 RCTs evaluating a range of cognitive, behavioural and psychoeducational approaches. Comparator conditions also varied and included treatment as usual, individual psychotherapy, and psychoeducational attention-control interventions. Pooling suggested that intervention had an overall positive effect on children’s internalising and externalising symptoms and significantly lowered the risk of children developing psychological disorders. The scope of this review extended to include children of parents with affective disorders and children of parents with alcohol dependence and substance misuse. The generalisability of its findings to children affected by SMI thus remains unclear.
To date, only two reviews32,52 have specifically focused on interventions for children of parents with SMI, only one of which adopts a systematic approach. 52 In 2006, Fraser et al. 52 conducted a systematic review of the literature and concluded that little evidence on the clinical effectiveness of interventions for children of parents with SMI could be found. Owing to a paucity of data, meta-analyses were not performed and no firm conclusions regarding the effectiveness of different intervention models could be made. Unfortunately, the authors of this synthesis did not fully report their review strategy and failed to specify a priori the criteria against which intervention and outcome eligibility judgements were made. Consequently, biases in the study findings cannot be ruled out.
Research aim and objectives
This review aimed to apply rigorous evidence synthesis techniques to provide a comprehensive and up to date summary of all available research evidence relating to the clinical effectiveness and cost-effectiveness of community-based interventions in maintaining or improving QoL in the children of parents with SMI. The objectives of this research were:
-
to provide a systematic and descriptive overview of all the evidence for community-based interventions for improving QoL in children and adolescents of parents with SMI, with specific reference to intervention format and content, participant characteristics, study validity and QoL outcomes measured
-
to examine the clinical effectiveness of community-based interventions in terms of their impact on a range of pre-determined outcomes, particularly those likely to be associated with QoL for children and adolescents of parents with SMI
-
to examine, when possible, potential associations between intervention effect and delivery including intervention format and content, prioritisation of child outcomes, child age group, parental mental health condition, family structure and residency
-
to explore all available data relating to the acceptability of community-based interventions intended to improve QoL for children and adolescents of parents with SMI, with specific reference to intervention uptake, adherence and patient satisfaction
-
to assess key factors influencing the acceptability of and barriers to the delivery and implementation of community-based interventions for improving QoL in children and adolescents of parents with SMI
-
to provide a systematic and descriptive overview of all the economic evidence for community-based interventions for improving QoL in children and adolescents of parents with SMI, with specific reference to intervention resources, cost burden, study validity, method of economic evaluation and economic outcomes measured
-
to examine the cost-effectiveness of community-based interventions in improving QoL for children and adolescents of parents with SMI using a decision-analytic model
-
to identify, from the perspective of the UK NHS and personal social services, research priorities and the potential value of future research into interventions for improved QoL in this population.
Chapter 2 Defining quality of life in the children of parents with serious mental illness
To ensure that the current synthesis delivered a comprehensive assessment of community-based interventions for improving or maintaining QoL in the children of parents with SMI, we first sought to define QoL in this population.
For ease of reading, the current chapter is divided into two parts. In the first we present a conceptual overview of QoL, the key similarities and differences between adult- and child-centred QoL concepts and current QoL models as applied to child populations. In the second, we consider the relevance of these models to the children of parents with SMI. A series of stakeholder consultation exercises were conducted as part of our review and the results of these are presented here. We conclude by presenting the outcome framework that was used to guide outcome extraction in this evidence synthesis.
Part 1: conceptualising quality of life
Quality of life is a complex concept and no widely accepted standard definition exists. Ultimately, interpretations will vary according to the priorities of different stakeholder groups. At a societal level, objective QoL indicators such as a community’s standard of living may be used to facilitate the distribution of public resources to the areas of greatest need. At a health policy level, standardised indicators such as quality-adjusted life-years (QALYs) are used to establish clinical and cost-effective services. At the individual level, QoL becomes much more synonymous with personal well-being.
Individual QoL constructs encompass both objective and subjective perspectives. 72 While objective perspectives focus on observable phenomena (e.g. a person’s physical health symptoms), subjective perspectives reflect people’s internal evaluations of their circumstances. Each type of measurement has its own strengths and weaknesses. Ultimately, objective measures may facilitate comparisons against population norms yet have relatively poor predictive validity for self-assessed QoL. Subjective measures are thus more generally accepted to reflect QoL constructs, despite being more easily influenced by respondent bias or adaptation to chronic life stressors. 73
Challenges to quality-of-life measurements
The inherent subjectivity of QoL belies some unique challenges to its measurement. At its most basic level, QoL can be conceptualised as comprising two key components: a cognitive component, typically expressed in terms of life satisfaction, and an affective component, typically expressed in terms of psychological health. 74 Different operational definitions, however, give rise to different assessment approaches. Distinction can be drawn between one-dimensional measures that quantify satisfaction with a single aspect of life and multidimensional models that consider satisfaction across a broader range of life domains. One-dimensional models often fail to reflect the full scope and complexity of QoL judgements and, as a consequence, lack sensitivity to change. Multidimensional models are therefore generally preferred. 73
The nature and number of life domains assessed by multidimensional QoL models are not fixed phenomena. Nevertheless, most generic models remain consistent in delineating five core life domains. These domains relate to (1) physical health, (2) emotional health, (3) material well-being, (4) environmental well-being and (5) social function. In addition, models that adopt a psychological or needs-based approach may also separately emphasise a unique contribution from self-actualisation and achievement. 75 These constructs overlap theoretically and empirically with measures of self-esteem and coping76–78 and in doing so introduce concepts of autonomy into subjective QoL assessments.
However, in certain contexts, narrower definitions may be applied, as is the case in health-related quality of life (HRQoL). HRQoL remains distinct from health status and is a particularly valuable tool in the assessment of behavioural and psychological interventions. HRQoL prioritises those domains that fall under the influence of health-care systems, policy makers and providers. 79 The World Health Organization’s (WHO) definition of health80 has been highly influential in determining the scope of these domains and focuses most attention towards the perceived quality of people’s physical, mental and social function. HRQoL thus remains distinct from broader QoL models in which material and environmental domains will typically be included. Greater emphasis is often placed on HRQoL in evaluative health research and health economic evaluations, for which the need to make resource allocation decisions between competing interventions for a disease, or between different categories of disease, has led to a policy preference for a common unit of outcome. 81–83 The National Institute for Health and Care Excellence (NICE), for example, requires outcomes to be measured in terms of QALYs, for which quality is determined using the European Quality of Life-5 Dimensions (EQ-5D) measure of HRQoL. 84 The EQ-5D is a generic, preference-based measure of HRQoL measured on five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), each rated on three levels (no problems, some problems, severe problems). Respondents are classified into one of 243 health states, each associated with a score that can be used to calculate QALYs. 85
Conceptualising children’s quality of life
Health-related QoL is an important outcome for both adult and child populations. However, compared with the exponential attention being directed towards adult QoL constructs, child-centred models remain in a relatively early stage of development. 74
UK policy perspectives on children’s quality of life
Several UK policy initiatives offer perspectives on children’s QoL. These policies include the Every Child Matters (ECM) agenda in England and Wales,86 the Children’s and Young People’s Strategy in Northern Ireland87 and the ‘Getting it Right for Every Child’ approach in Scotland. 17 Five broad QoL domains are shared between these initiatives and are termed within the ECM’s agenda as: (1) child health, (2) safety, (3) economic well-being, (4) enjoyment and achievement and (5) positive societal contribution. By placing equal emphasis on each of these domains, policy models uphold notions of children’s QoL as a multidimensional construct underpinned by various aspects of esteem, well-being and socialisation. However, a potential weakness to such models is their inevitable bias towards societal perspectives and thus to outcomes more readily quantified through objective means.
Research perspectives on children’s quality of life
Research instruments arguably offer a more direct approach to assessing children’s subjective QoL, although a systematic review in 2004 has highlighted marked inconsistency in the scope of children’s HRQoL measures. 88 While published scales remain relatively consistent in integrating physical, psychological and behavioural influences, the specific factors or items that make up these domains vary. 88 Early assessments of children’s HRQoL were developed purely from a biomedical perspective and, as such, remain largely disease specific. 76 Generic measures have developed from 1995 onwards with greater generalisability across clinical and non-clinical populations. 73,89
A review of generic child-centred measures reveals a wide array of factors that have previously contributed to assessments of children’s health-related QoL. 76 These include, but are not limited to, aspects of children’s physical appearance, peer relationships, recreational opportunities, family experience, cognitive functioning, academic performance, perceived autonomy and future life prospects. Consensus suggests that, at a minimum, peer relationships, family functioning and social interaction should be included in children and adolescents’ QoL models. 89 These factors display the greatest degree of coherence across published scales and underpin instruments developed from both child consultation and from expert opinion. 74
Challenges in measuring children’s quality of life
From a methodological perspective, notable challenges exist in the measurement of children’s HRQoL. 89 The WHO90 is clear in defining QoL as:
An individual’s perception of their position in life in the context of the culture and value systems in which they live, and in relation to their goals, expectations, standards and concerns.
This definition implies that QoL should, whenever possible, be measured directly from a person’s own perspective. Nevertheless, considerable debate surrounds the issue of children’s self-reported HRQoL. Best-estimates suggest that children can only reliably report concrete aspects of health, such as pain or medication use, from the age of approximately 5 years. 91–93 More complex, psychologically orientated constructs, such as the emotional impact of illness, may necessitate proxy measurement. The validity of these proxy measures is not well established. Limited evidence suggests that parental reports may be more accurate than those of health professionals,94 but empirical investigations of the level of agreement between parent and child appraisals yields mixed results. 89 Ultimately, greater agreement may be observed for ratings of children’s physical well-being than for assessments of emotional or social function. Further difficulties arise in establishing the levels of agreement between two parents,73,95 the potential for bias within parental ratings89 and the potential differences in the life priorities of parents and children. 96
Differences in life circumstances, intellectual development and peer group norms have all been implicated in influencing the manner in which children’s subjective QoL judgements are made. 89 Disparities in cognitive understanding, for example both between adults and children and between children of different ages, may manifest in very different appraisals of family experience. Likewise, differences in social maturity and autonomy may also influence the relative weighting that different children afford this domain. For the most part, however, family functioning is accepted as an extremely important influence on children’s psychosocial development and a central component in children’s QoL assessments. Empirical evidence has demonstrated associations between children’s familial experiences and their social cognitions, behaviours and relationships in external settings. 97–99 The inter-relationships between these variables challenge a clear distinction between children’s QoL outcomes and influences, and in doing so support the derivation of conceptual QoL models for use in specific populations.
Part 2: conceptualising quality of life in the children of parents with serious mental illness
A UK review of the cost-effectiveness of interventions for young carers has suggested that standard definitions of QoL may not fully capture the experiences of children with mentally ill parents. 7 Children living with serious parental mental illness are reported to encounter specific stressors related to disrupted life routines, family, academic and social dysfunction, poor mental health literacy and ineffectual coping. 100 Any consideration of QoL in this population group must thus also explicitly consider the scope and nature of the challenges encountered by this group.
Stakeholder consultation
In order to explore the clinical effectiveness and cost-effectiveness of community-based interventions in enhancing the QoL of children of parents with SMI, we first sought to develop a conceptual model of HRQoL in this population. It was established a priori that the primary outcomes for this review would comprise validated generic or population-specific QoL measures, including measures of life satisfaction and/or child-centred psychological health. Potential secondary outcomes pertaining to second-order QoL domains were identified from national policy agendas and child-centred, HRQoL models. Stakeholder consultation ultimately provided the mechanism by which to ensure that these secondary indicators remained cognisant of the potentially unique contexts in which the children of parents with SMI may live.
We acknowledged from the outset that the range of stakeholders consulted for this exercise was likely to hold a range of different views. Meaningful stakeholder engagement depends upon active efforts to identify and reflect the different perspectives of participant groups. Within the current review, three separate consultation exercises were undertaken. The first involved a mix of clinical academics (with backgrounds in mental health, child psychiatry and clinical psychology) in conjunction with professionals recruited from health- and social-care services, voluntary user-led organisations and national children’s trusts. The second and third consultations were undertaken with individuals with potentially lower influence yet higher stakes, in this case parents and the children of parents with SMI.
Stakeholder consultation took place early in the study to assist the research team in developing an outcome framework for evidence synthesis. Stakeholders also contributed to literature searching (see Chapter 3) and came together in a final meeting to assist in framing the presentation of our synthesis results.
Stakeholder participants
A favourable ethical review was obtained from the host institution’s Research Ethics Committee and the research panels of national voluntary user organisations as appropriate.
In total, 19 individuals participated in stakeholder consultation. Ethical requirements aimed at protecting participant anonymity demanded that each of the three stakeholder groups should be recruited from a different geographical area or via a distinct recruitment pathway. The first group comprised eight representatives recruited from clinical and academic settings or through direct correspondence with national user-led organisations, child-orientated charities and service initiatives. Organisational representation was present for Barnardo’s, Young Minds, the National Children’s Bureau, the National Society for the Protection and Care of Children (NSPCC) and the Fairbridge Trust. The second stakeholder group comprised five parents (four mothers and one father) who were independently recruited via advertisements placed on the website, e-mail bulletins and Twitter (Twitter, Inc., San Francisco, CA, USA) feeds of a large national mental health user and carer organisation (Rethink Mental Illness). Each parent had at least one child under the age of 18 years and suffered from a severe and enduring mental health difficulty, in this case personality disorder (n = 1), bipolar disorder (n = 2) and major depressive disorder (MDD) (n = 2). The final group consisted of six young people with current lived experience of parental mental illness. These children were recruited via a young carers’ service in the south-west of England and ranged in age from 13 to 18 years. Primary parental mental health diagnoses comprised bipolar disorder (n = 2), MDD (n = 2), schizophrenia (n = 1) and borderline personality disorder (n = 1). Owing to ethical and pragmatic constraints, the views of younger children could not be collected. Whenever possible, the interviewer sought to explore potential age-related variation in children’s life priorities.
Consultation methods
Stakeholders participated in focus groups or individual interviews according to availability and personal preference. In all cases, discussion centred around the participants’ general perceptions of QoL, their awareness of different QoL models, the perceived validity of these models to the children of parents with SMI and the key QoL domains that should be included in our evidence synthesis.
The data underwent an inductive thematic analysis for the purposes of informing a population-specific QoL model. All focus groups and interviews were recorded and transcribed verbatim. Data were analysed by author PBe using Microsoft Word 2007 (Microsoft Corporation, Redmond, WA, USA). Individual themes emerging from each stakeholder group were identified and combined into a smaller number of metathemes, each representing a key QoL subdomain. These subdomains were subsequently mapped against current QoL models used in policy and academic research to identify key similarities and differences in scope.
Consultation findings
Each stakeholder consultation exercise was inevitably influenced by the different perspectives and priorities of its participant group. Nonetheless, substantial overlap in QoL concepts emerged. Fifty-nine themes were initially identified from the data and grouped into 11 key metathemes (see Appendix 1). Mapping each metatheme against existing QoL concepts revealed a multidimensional model that endorsed to a greater or lesser degree the core domains of existing models (Figure 1). In total, five different domains were identified:
-
children’s emotional well-being
-
children’s social well-being
-
children’s economic well-being
-
children’s family contexts and experiences
-
children’s self-esteem and self-actualisation.
FIGURE 1.
Conceptual QoL map for children of parents with mental illness. Reproduced from Bee P, Berzins K, Calam R, Pryjmachuk S, Abel K. Defining quality of life in the children of parents with severe mental illness: a preliminary stakeholder-led model. PLOS ONE 2013;8:e73739. © 2013 Bee et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
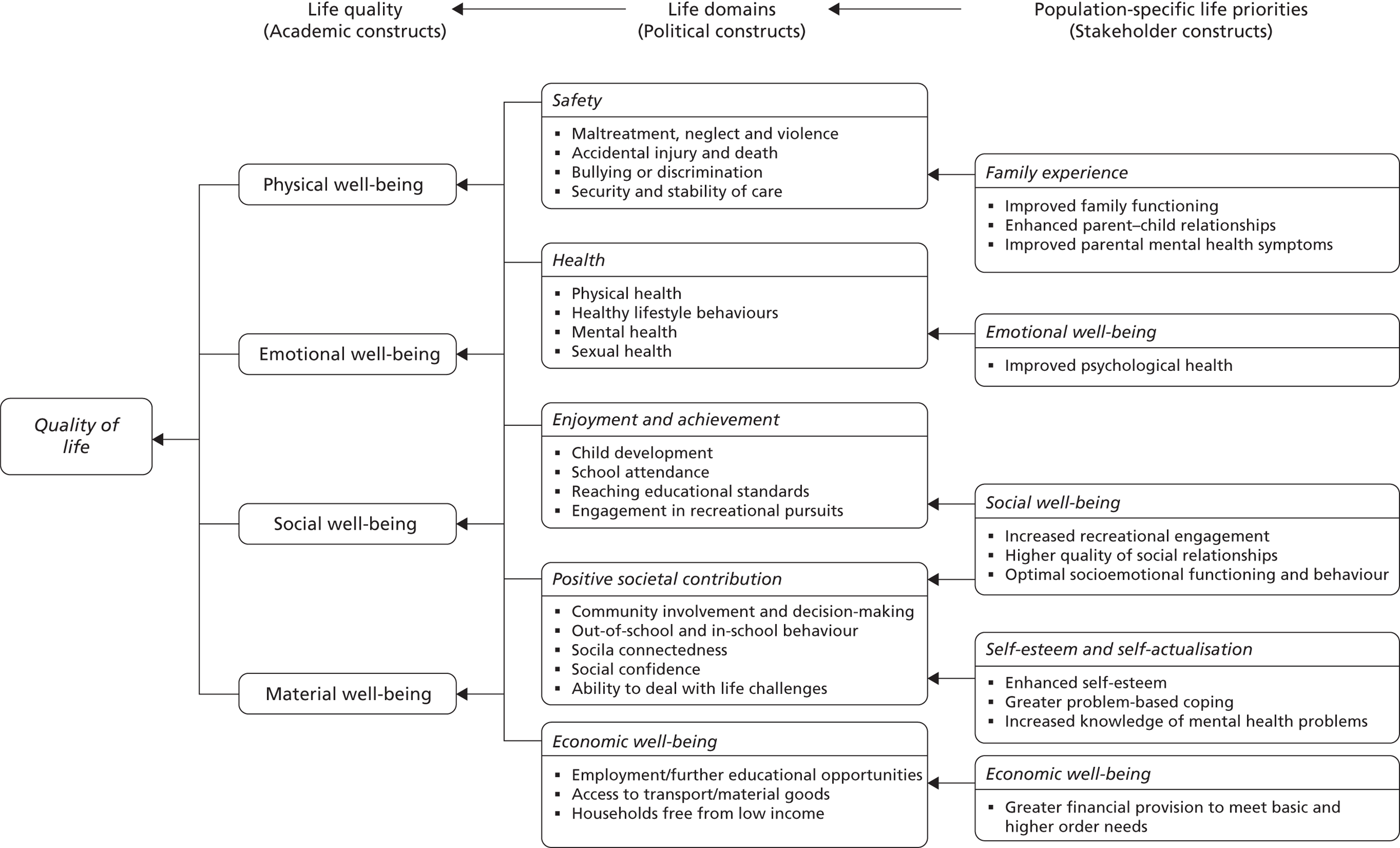
These five domains are discussed in further detail below.
Children’s emotional well-being
Children’s emotional well-being was endorsed by one broad metatheme related to children’s mental health. This metatheme was advocated by all three stakeholder groups. Both professionals and children focused heavily on children’s propensity to feel anxious or depressed about their parent’s mental health condition and highlighted the possibility of clinically significant symptoms of depression developing in children of parents with SMI.
I don’t know really it just . . . kind of affected me slightly mentally, having to deal with that, having to deal with what she’s like. Like, past like attempts of her trying to take too many pills, like, and sort of how to keep her calm. It’s hard. The doctor’s said I’m depressed.
Child stakeholder, 15 years old, mother with bipolar disorder
Parents expressed concern that their illness had led to mental health problems in their children, with genetic transmission, behavioural mimicking and increased psychosocial stress all being postulated as possible causes.
My son says, ‘I can accept it mum’, but, but, what also happens is, they adopt a different persona, it rubs off on him to a certain degree, and he becomes irritated as well.
Parent stakeholder, mother with depression
Children’s social well-being
Children’s social well-being was endorsed by three metathemes relating to: (1) children’s socioemotional functioning and behaviour, (2) social relationship quality and (3) recreational activity engagement. Children described feeling different to their peers and placed much emphasis on their need to access ‘normal’ recreational or social activities. Out-of-home activities were perceived by both parents and children to offer both respite from specific family stressors and more innate opportunities for general social and physical development.
Social, creative, miscellaneous type things, so my good day would be doing anything like that, playing the piano, going out with my friends, helping other people out, that would be part of my day.
Child stakeholder, 17 years old, mother with psychosis
Adequate social support delivered within the context of a high-quality social relationship was identified by all three stakeholder groups as a key aspect of children’s QoL and an important factor in enhancing children’s resilience to parental SMI. However, caring responsibilities and/or financial hardship were often found to prohibit opportunities for social networking and leisure pursuits. Parents described additional difficulties in fostering and nurturing their children’s independence and expressed concern that their own symptoms and behaviours had led to social withdrawal and behavioural dysfunction in their children.
If I’m punching the wall, say, or I scream or just get so angry, she curls over . . . she’s just not there, not there emotionally I mean.
Parent stakeholder, mother with bipolar disorder
Children’s economic well-being
Economic well-being was endorsed by one metatheme (economic resources) that encompassed a range of different yet inter-related needs. All three stakeholder groups upheld financial stability and economic resources as a central factor in determining children’s QoL, with multiple benefits emanating from a family’s capacity to meet children’s needs. Financial security was deemed vital both for the purposes of meeting basic family needs (e.g. food provision) and higher order needs including children’s engagement in recreational and social activity. Economic instability was identified by children as a key source of stigma and a frequent barrier to social integration with their peers.
And mum she just says that, ‘I don’t have any money at all,’ and so we literally have no food in our house, so I don’t really eat. My mum doesn’t have a fridge freezer, so we don’t have the normal things, things that everyone else would have . . .
Child stakeholder, 14 years old, mother with psychosis
Children’s family contexts and experiences
Children’s family contexts were endorsed by three metathemes. These metathemes related to: (1) parental mental health symptoms, (2) family functioning and conflict and (3) quality of the interaction occurring between children and their parents. Alleviating parental mental health symptoms was the main priority of all of the children we consulted. Across all three stakeholder groups, participants described a level of unpredictability in parents’ behaviour that impacted heavily on children’s own sense of security and emotional well-being. Both professional and child stakeholders described episodes of parental ill-health in which parenting may become more difficult and children’s needs may be less likely to be met.
She may not be able to depend on her mum as much as she used to and she’ll have to, kind of, grow up a bit more. When her mum’s ill, a lot really, sometimes she may have to put her mum in front of her, of what she wants and needs
Professional stakeholder
Psychiatric symptoms have been shown to account for most of the variance in the community functioning of mothers with mental illness101 and it is acknowledged that specific symptoms of SMI, such as delusional thoughts, may be focused on the child. 1 It is also accepted that children may experience intermittent or permanent separation from their parents as the result of a volitional or enforced hospital admission. Stakeholder discussions, however, also extended to encompass more routine aspects of domestic function. Adequate family functioning was consistently emphasised as a key contributor to children’s sense of belonging and a core factor influencing their QoL judgements. Children in particular described the enjoyment they derived from spending ‘ordinary’ time within their families and from engaging in warm and positive interactions with their parents.
My mum being happy, yes, seeing my mum have a smile on her face. Doing things together, even if it is going out, like walking down to the chip shop to go and get some chips, that would make me happy . . .
Child stakeholder, 13 years old, mother with personality disorder
Children’s esteem and self-actualisation
The final domain, children’s esteem and self-actualisation, was endorsed by three metathemes relating to: (1) children’s self-esteem, (2) children’s problem-based coping and (3) children’s levels of mental health literacy. Children described an inherent desire for greater autonomy both within their own lives and within the context of their parent’s care. Parents focused primarily on the need for children to develop their self-esteem while professionals emphasised the value of fostering children’s confidence, optimism and resiliency to parental mental illness.
It’s about accepting . . . not accepting it in a sort of negative way but appreciating just how well they’re doing to be coping with it, building up their own confidence about how much they can do.
Professional stakeholder
Studies specifically focusing on young carers report these children to have multiple responsibilities, including looking after other members of the family, mediating family conflict and seeking out help for the ‘looked-after’ person. 102 Such observations provide one explanation for why effective coping strategies, and particularly those based on problem-focused approaches, were endorsed by our stakeholders as a key mechanism through which children may be empowered to maintain their long-term emotional health. Low mental health literacy and poor understanding of parent’s symptoms and behaviours significantly reduced children’s abilities to cope with their parent’s mental illness, to the extent that greater communication between children, parents and health-care providers was advocated by all of our stakeholder participants.
I would like to know what to do, like for him, and how I can help, and to understand, understand what’s going on . . . because it’s just like really hard sometimes to know what to do.
Child stakeholder, 14 years, father with severe depression
Reflections on stakeholder perspectives
The contested nature of QoL suggests that greater emphasis should be placed on determining the relevance of generic QoL models to the children of parents with SMI. The current study drew on stakeholder perspectives to inform a conceptual model of QoL model in this population. A total of five key domains and 11 subdomains (metathemes) were identified from this consultation, all of which could be mapped to one or more components of existing QoL models.
Our stakeholder consultation identified some particular priorities specific to the children of parents with SMI. These included the alleviation of parental mental health symptoms, a pressing need for problem-based coping skills and increased mental health literacy. Similar requirements have been reported by other user consultation exercises1,57 and empirical work. 100 Population-specific QoL measures that take account of these issues may ultimately be more sensitive to changes and more effective at detecting treatment effects.
Notably, our stakeholder consultants failed to endorse three key QoL influences currently upheld by national child-centred policy initiatives. These components comprised children’s safety (defined in terms of child neglect, maltreatment or violence), children’s development and children’s physical and sexual health. Ultimately, the omission of these influences may reflect a bias towards healthy participants recruited from non-clinical settings. Alternatively, it may be that these factors do not sit well within children’s subjective QoL models. Self-perceived HRQoL remains somewhat distinct from physical health status and caution should always be taken when interpreting these outcomes as proxy indicators of children’s QoL.
Derivation of an outcome framework for evidence synthesis
For the purposes of this review, primary outcomes were established a priori to include validated measures of children’s QoL or mental health symptoms. In addition, 10 of the 11 subdomains that were identified by stakeholder consultation were retained as secondary outcome variables for our evidence synthesis. These individual subdomains can, at best, only be taken as proxy indicators of a multidimensional QoL construct.
Young people’s economic well-being, while pertinent to more generic QoL agendas, was judged to fall outside the auspices of HRQoL and was therefore omitted from our final outcome framework. Additionally, owing to their centrality within current UK child-orientated QoL models, outcomes related to children’s physical health, safety and cognitive development were also retained. The relative importance that stakeholders attributed to different secondary outcomes was considered during the data synthesis stage. The final framework guiding our evidence synthesis thus remained cognisant of a variety of research, policy and stakeholder perspectives. It was endorsed in its entirety by the advisory panel guiding this evidence synthesis and is delineated by source in Table 1.
| Review outcomes | Specified a priori | Conceptually supported | Politically supported | Stakeholder validated |
|---|---|---|---|---|
| Primary | ||||
| Validated measures of QoL/HRQoL | ✓ | |||
| Child-centred mental health symptoms | ✓ | ✓ | ✓ | ✓ |
| Secondary | ||||
| Children’s physical well-being | ||||
| Children’s physical health | ✓ | ✓ | ||
| Children’s safety, maltreatment and neglect | ✓ | |||
| Children’s social well-being | ||||
| Children’s social function and behaviour | ✓ | ✓ | ✓ | |
| Quality of children’s social relationships | ✓ | ✓ | ✓ | |
| Children’s recreational engagement | ✓ | ✓ | ✓ | |
| Children’s family contexts and experiences | ||||
| Parental mental health symptoms | ✓ | |||
| Family function and conflict | ✓ | ✓ | ||
| Quality of parent–child interactions | ✓ | ✓ | ||
| Children’s esteem and self-actualisation | ||||
| Children’s cognitive development | ✓ | ✓ | ||
| Children’s problem-focused coping | ✓ | ✓ | ✓ | |
| Children’s mental health literacy | ✓ | |||
| Children’s self-esteem | ✓ | ✓ | ✓ | |
Chapter 3 Review methods
We synthesised the available research literature regarding community-based interventions for children of parents with SMI. Our search was kept deliberately broad and targeted a range of study designs relevant to the aims set out in Chapter 1. The outcome extraction was guided by the QoL framework developed in Chapter 2. At all phases of the review, we adhered to guidelines outlined by the Centre for Reviews and Dissemination (CRD)103 and the Cochrane Collaboration. 82 One large search was undertaken across all phases of the review, with specific adaptations when necessary to reflect the different research objectives and research designs required to achieve them. A copy of the review protocol is provided in Appendix 2.
Search methods
Search term generation
Search terms relating to the key concepts of the review were identified by scanning the background literature, browsing the MEDLINE medical subject heading (MeSH) thesaurus and via discussion between the research team and an information officer from the Cochrane Collaboration Depression, Anxiety and Neurosis Review Group (CCDAN). The electronic search strategy was modified and refined several times before implementation. All searches took place between November 2010 and January 2011 (see Appendix 3 for the specific dates of individual searches) with an updating search being performed in May 2012. All databases were searched from inception. No language or design restrictions were applied.
In order to ensure comprehensive coverage of the evidence base, search terms were kept deliberately broad. It was acknowledged that children’s QoL outcomes had the potential to be both imprecise and poorly indexed and thus terms related to outcomes were not used to limit the search. Search terms were instead confined to population characteristics and broad intervention terms (e.g. program$, intervention$ or service$). Search terms relating to specific intervention or therapy models were not named in the search to avoid imposing unnecessary restrictions on the evidence that was retrieved. Full details of the search strategies and search terms used are reported in Appendix 3.
Search strategy
Changes to the search protocol
All searches were conducted as specified in the original review protocol with the exception of two electronic databases that were not searched as part of the final review (Social Work Abstracts and CommunityWise). These omissions were enforced owing to prolonged difficulties in obtaining access subscriptions. Reviews of the social-care literature are frequently limited by a distinct lack of empirical data, reflecting a preference for descriptive or theoretical study. 57 Coverage of the existing social-care evidence base was ensured through searches of two databases maintained by the SCIE. The potential impact of this protocol change was therefore judged to be minimal.
Searches of two further databases for economic evidence were not undertaken owing to lack of access subscriptions [The American Economic Association’s electronic bibliography (EconLit) and the Health Economic Evaluations Database (HEED)]. However, coverage of economic studies was ensured through searches of the NHS Economic Evaluation Database (NHS EED), the Paediatric Economic Database Evaluation (PEDE) database and the IDEAS database of economics and finance research, as well as via the searches undertaken to identify clinical effectiveness data.
Implementation of search strategies
In accordance with the review protocol, search strategies included electronic database searches, journal hand searches, reference list searches, targeted author searches, grey literature searches (including material generated by user-led organisations), research register searches, forward citation searching and stakeholder enquiry.
Electronic databases
To identify evidence relevant to the research question, electronic searches were undertaken on the following health, allied health and education databases:
-
MEDLINE (accessed via Ovid; www.ovid.com/)
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (accessed via Ovid; www.ovid.com/)
-
PsycINFO (accessed via Ovid; www.ovid.com/)
-
EMBASE (accessed via Ovid; www.ovid.com/)
-
CENTRAL (Cochrane Central Register of Controlled Trials) (accessed via The Cochrane Library; www.thecochranelibrary.com/)
-
CDSR (Cochrane Database of Systematic Reviews) and DARE (Database of Abstracts of Reviews of Effects) (accessed via The Cochrane Library; www.thecochranelibrary.com/)
-
ISI Web of Science including SSCI (Social Science Citation Index), AHCI (Arts and Humanities Citation Index) and SCIEXPANDED (Science Citation Index Expanded) (accessed via Web of Knowledge; www.wos.mimas.ac.uk/)
-
HSRProj (Health Services Research Projects in Progress) (accessed via www.nlm.nih.gov/hsrproj/)
-
HMIC (Health Management Information Consortium) (accessed via Ovid; www.ovid.com/)
-
ASSIA (Applied Social Sciences Index and Abstracts) (accessed via ProQuest; www.proquest.com)
-
Sciverse SCOPUS (accessed via www.scopus.com)
-
IBSS (International Bibliography of the Social Sciences) (accessed via ProQuest; www.proquest.com)
-
Social Services Abstracts (accessed via ProQuest; www.proquest.com)
-
Social Care Online (accessed via www.scie-socialcareonline.org.uk/)
-
ChildData (accessed via Athens; www.childdata.org.uk/)
-
ERIC (Education Resources Information Centre), AUEI (Australian Education Institute) and BRIE (British Education Institute) (accessed via ProQuest; www.proquest.com)
-
Dissertation Abstracts (accessed via Ovid; www.ovid.com/)
-
NCJRS (National Criminal Justice Reference Service) and Abstracts (accessed via ProQuest, www.proquest.com)
-
The publicly available parental mental health database created by SCIE in partnership with the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI) (accessed via www.eppi.ioe.ac.uk/webdatabases/).
In addition, the following databases were searched for economic studies:
-
NHS EED (accessed via CRD; www.york.ac.uk/inst/crd/crddatabases.htm)
-
PEDE (accessed via http://pede.ccb.sickkids.ca/pede/index.jsp)
-
IDEAS database of economic and finance research (accessed via http://ideas.repec.org/).
Hand searching
Nine psychiatry, psychology and child health journals were identified as being likely to contain relevant research evidence. These journals were hand-searched for the publication period 2010–11 and selected articles examined to establish relevance to this review. The journals that were searched comprised the American Journal of Psychiatry, the Journal of the American Academy of Child and Adolescent Psychiatry, the American Journal of Orthopsychiatry, Archives of General Psychiatry, Archives of Pediatrics and Adolescent Medicine, the British Journal of Psychiatry, the Journal of Clinical Psychology, Schizophrenia Bulletin and Psychological Medicine.
Reference lists
Additional studies were sought by examining the reference lists from the text of retrieved and eligible reports. Bibliographies of relevant retrieved reviews were also inspected to ensure that all potentially relevant studies had been identified. For studies that were published as abstracts, or when there was insufficient information to assess eligibility, full texts were obtained.
Targeted author searches and unpublished research
Brief targeted author searches were conducted following the identification of key researchers in the field. A list of key researchers was initially identified by the review team and subsequently augmented following abstract screening. Key researchers were contacted via email with a list of inclusion criteria for the review and a request for information regarding any studies that they felt may be relevant. A total of eight authors from the UK, the USA and Australia were contacted for further information and four authors responded providing further information. No studies were cited that had not already been retrieved by other means.
Ongoing research
The metaRegister of Controlled Trials (mRCT) (accessed via Current Controlled Trials; http://controlled-trials.com/) was examined for information on current or recent trials in the relevant area. Search terms for this register comprised ‘parent’, ‘mother’, ‘father’, ‘child’, ‘family’ and ‘mental health’. All references located by this method were cross-referenced with studies identified via other pathways to ensure comprehensive coverage. For trials that were identified but no publications existed, research teams were contacted directly to enquire about potentially eligible data. Nine teams were contacted for further information and seven authors responded providing further information.
Forward citation searching
Forward citation searching was undertaken for all trials eligible for inclusion in the review. This process was undertaken using the Web of Science (WoS) Institute of Scientific Information (ISI) citation database. Each study was entered separately and all citations to the paper since publication were identified. Titles and, when available, abstracts of the papers citing the eligible trials were downloaded and screened for eligibility according to the same criteria used for the primary searches (see below).
Theses
The Dissertations Abstracts International database was searched through PsycINFO using the comprehensive search strategy developed for the other electronic databases searches. Theses and dissertations were also identified through reference and bibliography lists.
Grey literature and material generated by user-led or voluntary sector enquiry
Grey literature, including conference abstracts, proceedings, policy documents and material generated by user-led enquiry, was identified via electronic databases, internet search engines and websites for relevant government departments and charities. These included, but were not limited to, the British National Bibliography for Report Literature, Google Scholar, Mental Health Foundation, Barnardo’s, Carers UK, ChildLine, Children’s Society, Depression Alliance, Mind, Anxiety UK, NSPCC, Princess Royal Trust for Carers, SANE, The Site, Turning Point and Young Minds. An exhaustive list of websites searched is provided in Appendix 3.
Stakeholder consultation
Requests for additional publications and potential references were lodged with the external advisory panel and with stakeholders during consultation exercises. A brief summary of the review was also posted on the website of the host institution with an email contact link through which people could submit additional references.
Study screening and selection
Eligibility criteria
All records retrieved from the searches were imported into a bibliographic referencing software program [Reference Manager 11.0 (Thomson Reuters, New York, NY, USA)] and duplicate references identified and removed. Two reviewers (JG, MC) independently screened titles and abstracts for eligibility using prespecified inclusion criteria (described below). Additional economic abstracts located through NHS EED, PEDE or IDEAS were independently screened for eligibility by two reviewers (SB and MC) using the same prespecified inclusion criteria.
When both reviewers agreed on exclusions, the reasons for exclusion were recorded. When both reviewers agreed on inclusion, or when there was ambiguity or disagreement, full text articles were retrieved. Two reviewers then independently assessed the full text of these articles against the predetermined inclusion criteria. Any remaining disagreements were resolved by consensus or discussion with a third party, if necessary. Our protocol originally specified that we would obtain a measure of inter-rater reliability regarding study eligibility judgements; however, given the stringent procedures that were put in place to resolve ambiguous cases, the meaningful contribution of this statistic remained unclear. Inter-rater reliability was therefore not calculated in this instance.
Study inclusion criteria
Studies were initially assessed for inclusion across all phases of the review according to a standard set of eligibility criteria summarised in Boxes 1 and 2, and described in full below. Additional inclusion and exclusion criteria were set for specific phases of the review and these are presented in the relevant chapters, when appropriate.
Child: Any individual aged 0 to < 18 years.
Parent: An umbrella term covering mothers, fathers, adoptive parents, legal guardians, foster parents or other adult assuming a primary care-giving role for a dependent child.
SMI: An umbrella term covering schizophrenia, psychosis, borderline personality disorder and personality disorder.
Severe affective mood disorders: An umbrella term comprising severe unipolar depression, severe postnatal depression and/or puerperal psychosis.
Community-based intervention: Any non-residential, psychological or psychosocial intervention involving professionals or paraprofessionals for the purposes of changing parents’ and/or children’s knowledge, attitudes, beliefs, emotions, skills or health behaviours.
QoL: A multidimensional construct arising from children’s mental and physical health, social well-being, family experiences, self-esteem and self-actualisation.
-
Population: Children aged 0 to < 18 years or their parents, one or more parents with SMI with or without substance misuse/other mental health comorbidity, > 50% sample participants experiencing parental SMI.
-
Intervention: Any health, social-care or educational intervention aimed at the young person, parent or family unit. Individual or group interventions delivered alone or in combination with pharmacology.
-
Comparator: Any active or inactive treatment, in which inactive treatment is defined as a waiting list delayed treatment or usual care management control.
-
Outcomes (clinical effectiveness/cost-effectiveness): Validated generic or population-specific measures of QoL such as children’s mental health, physical well-being, social well-being, family functioning and self-esteem or -actualisation.
-
Outcomes (acceptability): Intervention uptake, adherence or participant satisfaction.
-
Design (clinical effectiveness/cost-effectiveness): Priority given to randomised and quasi-RCTs or controlled observational studies (e.g. case–control studies). Uncontrolled studies retained and summarised.
-
Design (acceptability): Quantitative/qualitative data collected as either a stand-alone or mixed method study.
-
At-risk populations.
-
A population with < 50% or an unclear proportion of participants experiencing parental SMI.
-
Inpatient interventions, e.g. assisted accommodation, mother and baby residential units.
-
Pharmacological/physiological interventions without a psychological/social component.
-
Interventions aimed at health-care professionals.
-
Case studies, opinion papers, descriptive studies and editorials.
-
Non-English-language publications.
Participants
Study participants were children or adolescents aged 0 to < 18 years, or the parents of these children. For the purposes of our review, parents were defined as mothers, fathers, adoptive parents, legal guardians, foster parents or any other adults assuming a primary caring role for a dependent child, whether resident or non-resident. To be eligible for inclusion in the review, one or more parents had to have a serious mental health condition as defined by a current or lifetime clinical diagnosis or a comparable symptom profile. In accordance with the user perspective,104 serious mental health conditions were defined to include schizophrenia, psychosis, borderline personality disorder and personality disorder, with or without substance misuse or other mental health comorbidity. Severe affective mood disorders including severe unipolar depression, severe postnatal depression and puerperal psychosis were also included. Separate syntheses were conducted for parental SMI and severe parental depression. The method and rationale by which severe depression was defined in this review is described further in Chapter 5. Studies were eligible for inclusion in the review if the majority of participants (> 50%) in the sample fulfilled our criteria for SMI. Populations in which only a minority (< 50%), or an indeterminable proportion, of participants had SMI were excluded from the review. At-risk populations with no current or prior diagnoses were also excluded.
Interventions
Eligible interventions comprised any community-based (i.e. non-residential) psychological or psychosocial intervention that involved professionals or paraprofessionals and parents or children, for the purposes of changing knowledge, attitudes, beliefs, emotions, skills or behaviours concerning health and well-being. This included any health, social-care or educational intervention aimed at the young person, their parent or their family unit. Interventions that targeted children in the community were eligible for inclusion irrespective of their parents’ inpatient or outpatient status. Interventions in which both children and parents were required to be inpatients (e.g. mother and baby residential units, assisted accommodation) were excluded from the review.
Both individual and group interventions were included, whether delivered alone or in combination with pharmacological treatment. Prevention and treatment studies were both eligible for inclusion. Prevention studies were defined as those that recruited prospective parents with SMI in pregnancy and delivered all, or part, of the intervention in advance of parenthood. These studies were only eligible for inclusion if parents had a pre-existing and clinically diagnosed SMI, and eligible QoL outcomes were assessed in the postpartum period. Treatment studies both recruited parents and delivered their interventions post birth and up to 18 years of age.
Comparators
Comparisons of two or more active interventions or of an active treatment with a ‘no treatment’ comparator were included. The ‘no treatment’ category was defined to include waiting list controls, delayed treatment and usual care management. A previous review57 has reported difficulties in defining what may constitute standard care in this relatively disparate group and, therefore, no specific criteria were placed on this comparison. Restricting evidence to studies that adopt a strict ‘no treatment’ comparator raises the potential for bias due to possible placebo effects, i.e. the effect of a particular intervention cannot be differentiated from the non-specific effects of researcher or clinical attention. Marked differences in comparators were taken into account during data summary and analyses. Trials comparing pharmacological or physiological interventions without a psychological or social component were excluded from the review, as were trials assessing interventions aimed at health-care professionals.
Outcomes
Rigorous evidence syntheses demand the a priori selection of a manageable number of conceptually relevant outcomes. In the absence of a standard definition of QoL, we adopted a comprehensive and inclusive approach to the outcome framework guiding this review.
It was established a priori that our primary outcomes would comprise validated generic [e.g. Short Form questionnaire-36 items (SF-36)] or population-specific measures of QoL [e.g. Paediatric QoL Enjoyment and Satisfaction Questionnaire (PQ-LES-Q), KIDSCREEN-52]. Child-centred mental health symptoms were also specified a priori as a primary outcome.
Secondary outcomes comprised additional QoL indicators identified from conceptual models, empirical literature and UK policy. Stakeholder consultation provided the mechanism by which these generic QoL indicators were adapted to better reflect the specific goals and concerns of children whose parents experience SMI. The findings of this consultation have already been reported in Chapter 2. For the purposes of our evidence synthesis secondary outcomes comprised:
-
Children’s physical well-being, specifically children’s physical health, safety, maltreatment and neglect.
-
Children’s social well-being, specifically children’s socioemotional function and behaviour, social relationship quality and recreational engagement.
-
Children’s family experiences, specifically parental mental health symptoms, family function and the quality of parent–child interactions.
-
Children’s self-esteem and self-actualisation, specifically children’s cognitive development, problem-focused coping, mental health literacy and self-esteem.
Data on secondary outcomes were included however defined. For the purposes of synthesising evidence relating to intervention acceptability, additional outcomes comprised intervention uptake, adherence and participant satisfaction. Trials that reported no relevant parental or child outcomes were excluded from the review.
Study design
In synthesising evidence of clinical effectiveness and cost-effectiveness, priority was given to those designs in which a comparator or control group was present, i.e. RCTs, quasi-RCTs and controlled observational studies (e.g. case–control studies).
Discriminating between quasi-randomised and non-randomised studies is a difficult process, not least because there remains a lack of consensus over which types of studies are most relevant for systematic reviews. For the purposes of assessing clinical effectiveness, we used the Cochrane checklist for non-randomised studies82 and only included those studies that used randomised or quasi-randomised allocation methods potentially capable of creating similar groups, i.e. random-sequence generation, sequential assignment or matched pairs allocation. Differences in the risk of bias associated with these different methods were taken into account during data analysis. We excluded studies that reported non-randomised allocation methods founded on mechanisms highly likely to lead to important differences between groups, i.e. allocation by patient preference, treatment outcomes, service availability or time. Lower levels of evidence (i.e. non-randomised trials and uncontrolled studies) were retained and summarised either for the purposes of future research priority setting or to provide data that may be suitable for inclusion in an economic model (i.e. we included partial economic studies in the review to assess whether they contained resource use or cost data that may help populate an economic model).
Acceptability was assessed via quantitative and qualitative designs conducted either as stand-alone studies (i.e. a quantitative survey or qualitative investigation) or as part of a larger mixed-methods approach (e.g. a nested acceptability study).
Studies undertaken in any country were eligible for inclusion across all phases of the review and no restrictions were placed on date of publication. Case studies, opinion papers, descriptive studies, editorials and non-English-language publications were excluded.
Data extraction and quality assessment
Data extraction procedures
Data extraction and validity assessment of all studies included in our clinical effectiveness and acceptability syntheses was performed by one reviewer (KB) and independently verified by a second (SP). Study outcomes were extracted separately (by PBo) and independently verified (by PBe). Discrepancies were resolved by referral to the original studies and, if necessary, via arbitration from a third reviewer.
The data extraction process was guided by a prespecified data extraction sheet that detailed the study author, year of publication, study design and key features of the study sample, setting, and intervention and comparator conditions. When there were multiple publications for the same study, data were extracted from the most recent and complete publication. For cases in which the duplicate publications reported additional relevant data, these data were also extracted.
Data extraction from economic studies was performed by one reviewer (MC) and independently verified by a second (SB). Data extraction used a prespecified data extraction sheet designed for the purpose of this study. This included study author, year of publication, study design, setting, population, interventions, method of economic evaluation, economic perspective, costs and outcomes reported and quality criteria.
Methodological quality
Studies were assessed for methodological quality across all phases of the review. Evidence of clinical effectiveness was assessed for quality at the study level using the Cochrane Collaboration Risk of Bias Assessment Tool for RCTs82 or the Cochrane guidance for non-randomised designs. 82 Economic studies were assessed using a standard critical appraisal checklist for economic evaluations. 81
Qualitative studies eligible for inclusion in our acceptability synthesis were assessed for quality using the Critical Appraisal Skills Programme (CASP) tool for qualitative research105 and the principles of good practice for conducting social research with children. 106 Although all eligible studies were assessed for quality, no study was excluded on the basis of this quality appraisal. The relative impact of methodological flaws was summarised narratively or explored via a sensitivity analysis, when data allowed.
Methods of data synthesis
Across all phases of the review included studies were synthesised according to (1) the nature of the parents’ mental health disorder and (2) the level of evidence that was presented.
Classification by parental disorder
In the absence of an internationally agreed standard for SMI, irregularities in its scope and classification invariably exist. Nonetheless, both national user organisations104 and policy documents107 are consistent in the core components of their operational definitions. These definitions share common elements of diagnosis, disability, duration, safety and informal and formal care. According to these criteria, SMI is identifiable in people who (1) display florid symptoms and/or suffer from severe and enduring mental health difficulties, (2) experience occasional risk to their own safety or that of others, (3) undergo recurrent crises that lead to multiple hospital admissions and/or interventions and (4) suffer substantial disability or place significant burden on informal carers as a result. From a diagnostic perspective, such illnesses typically comprise non-organic psychoses (including schizophrenia and schizoaffective disorders), personality disorders and severe affective mood disorders, with or without concurrent substance misuse. Severe affective mood disorders can in turn be strictly defined to include bipolar disorder and puerperal psychosis.
However, broader definitions of SMI may also extend to include severe depression, largely represented within Diagnostic and Statistical Manual of Mental Disorders (DSM) taxonomies as MDD. According to DSM-Fourth Edition (DSM-IV) criteria,108 individuals meeting diagnostic criteria for MDD are required to display recurrent and clinically significant distress alongside substantial functional impairment, including the possibility of suicidal ideation or suicidal attempts. Although remaining explicitly distinct from bipolar and psychotic depressions, severe depression can thus be conceived to produce levels of individual and family burden equal to those of other SMIs. From a developmental perspective, the children of severely depressed adults may be exposed to comparable aberrations in their parent’s cognitive and psychosocial frameworks108 and similar disruptions in their care-giving environments to those experienced in other diagnoses.
For the purposes of the current research, an inclusive definition of SMI was adopted. Nonetheless, we remained sensitive to narrower interpretations of the term SMI and thus chose to synthesise our findings in two distinct groups, syntheses one and two.
Synthesis one described the existing evidence base for those interventions that target parental SMI, for which SMI was explicitly defined to incorporate schizophrenia, puerperal and non-puerperal psychosis, personality and borderline personality disorders and bipolar disorder. In line with our inclusion criteria, this synthesis was restricted to studies in which SMI was present in > 50% of participants.
Synthesis two focused on studies that evaluated interventions for severe parental depression. This synthesis included studies in which at least 50% of parents had a confirmed diagnosis of International Classification of Diseases, Tenth Edition (ICD-10)109 severe depression, DSM-Third (DSM-III) or Fourth (DSM-IV) Edition MDD, or who showed baseline symptoms commensurate with severe levels of depression inside or outside the postpartum period (see Chapter 5 for further details). It was acknowledged from the outset that this data set was likely to represent a somewhat different parental population to that described above, in terms of both epidemiology and UK service delivery models. Many studies that evaluate interventions for severe depression actively exclude participants with schizophrenia, psychosis, personality or bipolar disorders. The decision to include such studies in the current review was nonetheless advocated by stakeholder consultation and universally endorsed by the advisory panel guiding this review.
A third synthesis was planned for studies in which at least 50% of the sample suffered from either SMI or depression but not to the extent that either met criteria for one of the two syntheses defined above. No randomised or quasi-RCTs were identified that were eligible for this synthesis and, therefore, it did not take place.
Classification by level of evidence
Evidence of clinical effect was prioritised according to study design. The highest level of evidence comprised randomised or quasi-RCTs since these designs are generally considered more likely to minimise important differences between experimental groups. Non-randomised controlled trials (nRCTs) (i.e. studies allocated on the basis of service availability, treatment need or patient preference) remain much more susceptible to selection bias82 and for this reason were classified as secondary-level evidence. Adding non-randomised to randomised evidence may change an imprecise but unbiased estimate into a precise but biased estimate. 82 The lowest level of evidence pertained to uncontrolled designs.
For each of the syntheses described in the section above, highest-level evidence was analysed for each primary and secondary QoL outcome domain. Lower-level evidence was summarised for the purposes of future research priority setting.
Synthesis of clinical effectiveness data
For the purposes of this review, the unit of analysis was the comparison. Most two-arm trials provided a single comparison but the review included trials with multiple arms, which included both comparisons of ‘active’ treatment against ‘no treatment’ or ‘usual care’ and comparisons of different ‘active’ treatments.
We extracted outcome data for each relevant comparison into spreadsheets, categorising outcomes according to our QoL framework (see Chapter 2) and distinguishing between follow-up over three arbitrary durations: short term (1–6 months), medium term (7–12 months) and long term (> 12 months).
We extracted the majority of data into common formats [mean, standard deviation (SD) and sample size for continuous outcomes, numbers with ‘poor’ outcomes and sample size for dichotomous outcomes]. A small number of studies needed translation [e.g. 95% confidence interval (CI) to SDs] and we made imputations for a minority of outcomes (usually when sample sizes were not provided at follow-up, and we estimated a 75% follow-up rate from baseline to allow estimates of precision). Calculation of effect size (ES) was not possible for all comparisons or outcomes within comparisons. All coding was carried out by two raters who worked independently, with disagreements resolved by discussion.
Continuous measures were translated to a standardised mean difference ES (the mean of the intervention group minus the mean of the control group, divided by the pooled SD). We coded outcomes so that positive ESs always represented improvements for the intervention compared with control (e.g. reduced depressive symptoms). Outcomes reported as dichotomous variables (e.g. proportion with ‘good control’ or ‘remitted’) were translated to a standardised ES using the logit transformation. 110
When studies reported multiple comparisons that were eligible for the same meta-analysis (e.g. two types of intervention vs. control), both comparisons were included, but sample sizes in the control group were halved to avoid ‘double counting’ of participants in the control group and thus inappropriate precision in the relevant meta-analysis. We identified cluster trials and adjusted the effective sample size (and thus the precision) of these comparisons using methods recommended by the Effective Practice and Organisation of Care group of the Cochrane Collaboration82 and assuming an intraclass correlation of 0.02.
When sufficient data were reported for particular comparisons and when populations and interventions were considered sufficiently homogeneous, we pooled ESs. Owing to marked heterogeneity in interventions and outcomes, meta-analyses used random-effects modelling, with the I2 statistic used to quantify heterogeneity. 111 In instances where heterogeneity among populations and interventions made pooling inappropriate, we present a narrative synthesis of effectiveness.
Heterogeneity
Heterogeneity refers to differences in the estimated effects of different studies. For the purposes of this synthesis, distinctions were made between statistical heterogeneity (differences in reported effects), clinical heterogeneity (differences in participants or interventions) and methodological heterogeneity (differences in study design). When data could be pooled, statistical heterogeneity was examined via the I2 index calculated as part of the meta-analysis. This statistic assesses whether the variability that is observed in ESs is higher than that expected by chance. The analysis plan in the protocol proposed that clinical heterogeneity would be explored via an assessment of the relationships between treatment effectiveness and the following variables:
-
Therapeutic target (parental, individual or parent–child dyad/family based).
-
Intervention content and objectives (e.g. psychoeducational or psychotherapeutic, parenting or mental health perspectives).
-
User characteristics, specifically:
-
child age group (< 5 years, 5–11 years, 12–17 years)
-
parental mental health condition
-
family structure and child residency (colocated, forced or volitional separation, separation in crisis).
-
Sensitivity analyses
Exploration of methodological heterogeneity was undertaken, when possible, through sensitivity analyses. Sensitivity analyses enable the robustness of the review to be ascertained relative to key decisions undertaken during its execution. In the current synthesis, sensitivity analyses were defined a priori and were conducted to examine the impact of trial quality (defined in terms of overall risk of bias).
Synthesis of economic data
Planned economic synthesis included two components: first, a narrative synthesis of all full economic evaluations meeting the study inclusion criteria (a full economic evaluation is one that compares both the costs and consequences of two or more interventions); and, secondly, decision-analytic modelling to explore any interventions found to be associated with promising evidence of effectiveness in the clinical review, subject to the availability of adequate economic data. Decision analysis is used to compare the expected cost-effectiveness of identified intervention programmes and involves the construction of a logical model to represent long-term costs and outcomes in order to inform resource allocation decisions under conditions of uncertainty. 81,112 Resource allocation is explored by modelling existing data on costs and outcomes available from the literature or from expert opinion.
Value of information analysis
The purpose of synthesising cost-effectiveness data via decision-analytical techniques is to enable a decision regarding the most cost-effective intervention for a given monetary value assigned to a designated intervention outcome. However, it is recognised that there may be uncertainty in the effectiveness and cost parameters that feed into a cost-decision model. Planned synthesis, therefore, also included a value of information analysis (VOI). A VOI analysis quantifies the chance that a wrong decision has been made and the associated loss in monetary value from using a suboptimal intervention. VOI provides a formal assessment of the extent to which further primary research is warranted and may also indicate where additional research would be most valuable.
Synthesis of acceptability data
A parallel synthesis of acceptability data was undertaken according to recommended methods for the syntheses of qualitative and mixed-method evidence. 113,114 These methods necessitate that the identified studies are interrogated, reanalysed and combined in a logical format to produce an overarching view of the evidence. Synthesis may either be driven by the emerging data or by a predetermined theory. Our protocol proposed a textual narrative synthesis approach,115 in which studies are grouped together into theoretically important subgroups prior to data synthesis. The structure of this narrative was to be informed and framed by (1) previous work in the subject area,57 (2) knowledge and expertise within the research team and (3) consultation with our stakeholder advisory panel.
Overview of the evidence base
Figure 2 presents the flow of studies through the review. In total, 57 studies were identified as eligible for inclusion in one or more of the syntheses included in this report. Sixty-nine papers reporting on 52 of 57 eligible studies were identified from electronic bibliographic databases, one from reference/bibliography lists and four theses (identified from relevant databases). These studies were all published in English between 1982 and 2011. Please refer to Appendix 4 for a full list of the included studies and the study reference numbers that relate to these.
FIGURE 2.
Flow of studies through the evidence synthesis. a, Some studies contribute to more than one type of outcome data.
A total of 115 studies were identified but subsequently excluded from our synthesis. Of these, 35 were excluded because only a minority (< 50%) of participants experienced serious parental mental illness, 21 were excluded because they failed to meet our diagnostic or illness severity criteria and 31 did not provided sufficient information to allow us to determine the nature or severity of parental mental illnesses. A further 13 did not evaluate an eligible community-based intervention. Seven did not provide any eligible QoL outcome data and seven did not focus on children or the parents of children aged 0 to < 18 years. One final study, a clinical case series, was excluded on the basis of design. Please refer to Appendices 5 and 6 for a full list of the excluded studies and the study reference numbers that relate to these.
Twenty-nine of the 57 studies (51%) included in our synthesis reported on randomised or quasi-RCTs indexed as higher levels of research evidence. The vast majority of these trials (n = 26) focused on severe parental depression and were included in synthesis two (see Chapter 5).
Overview of the economic evidence base
The flow of studies described above includes the total number of studies meeting our inclusion criteria and providing data for one or more of our clinical effectiveness, cost-effectiveness or acceptability reviews. Ten papers reporting the results of an economic evaluation or partial economic evaluations containing cost or resource use data were located through the clinical review. A review of additional economic databases located a further 129 abstracts which were checked for inclusion. A total of 119 abstracts were excluded because they did not focus on the population of interest, either because the study participants were not children or adolescents or the parents of children aged 0 to < 18 years (n = 100), or because a minority of parents (< 50%) had SMI or severe unipolar depression (n = 19).
The remaining 10 papers plus the 10 located through the clinical review were reviewed in full. Of the 20 papers reviewed, a further 19 were excluded because they did not focus on children or adolescents aged 0 to < 18 years or the parents of these children (n = 7), the proportion of participants with a serious parental mental illness was zero, < 50% or unknown (n = 11), or because the study did not focus on community-based psychosocial interventions (n = 1). A full list of studies excluded from the economic review is provided in Appendix 4.
The one remaining paper was a cost-effectiveness analysis of psychiatric day hospital compared with routine primary care for the treatment of postnatal depression. 116 This analysis was carried out as part of a non-randomised prospective cohort study and is discussed further in Chapter 5.
Chapter 4 The clinical effectiveness and cost-effectiveness of community-based interventions to improve or maintain quality of life in the children of parents with serious mental illness
In phase 1 of the review, we synthesised all available evidence relating to the clinical effectiveness of community-based interventions for improving or maintaining QoL in children whose parents suffer from SMI. Serious parental mental illness was explicitly defined to include schizophrenia, schizoaffective disorder, puerperal and non-puerperal psychoses, personality and borderline personality disorders and/or bipolar disorder.
Methods of review
Studies were identified according to the search and review strategies outlined in Chapter 3.
Size of the evidence base
A total of 11 studies were eligible for inclusion in this phase of the review. Of these, only three were randomised or quasi-randomised trials with participants allocated either randomly or by sequential assignment. 60,61,117 A further four were non-randomised studies in which participants were allocated on the basis of service availability or treatment attendance. 58,59,118,119 The remaining studies (n = 4) were all uncontrolled designs. 62,120–122 One study122 used a non-randomised design but failed to compare post intervention outcomes between its experimental and comparator conditions. This study thus only offered pre–post comparison data and was classified as an uncontrolled design. Please refer to Appendix 4 for a full list of the included studies and the study reference numbers that relate to these.
Thirty-one studies were identified but subsequently excluded from synthesis. Of these, 13 were excluded because only a minority (< 50%) of participants experienced serious parental mental illness. Insufficient information was available to determine the nature and/or percentage distribution of SMI in another seven. Six studies did not report any eligible QoL outcome data, three did not evaluate community-based interventions, one did not focus on children or adolescents aged < 18 years and one was a case series not meeting our design inclusion criteria. Please refer to Appendix 5 for a full list of the references of excluded trials, together with the reasons for exclusion.
Economic evaluation
No studies reporting the results of an economic evaluation or providing cost or resource use data were located in this phase of the review. Eight studies (six with a clear focus on SMI and two with a focus on mental health more broadly) were identified but subsequently excluded. Of these, one was excluded because the proportion of participants with a serious parental mental illness was unknown, with no reported baseline symptoms to assess likely severity, and seven were excluded because they did not focus on children or adolescents aged 0 to < 18 years, or their parents. Please see Appendix 6 for a full list of the references of studies excluded from the economic evaluation.
Approach to evidence synthesis
Our protocol stipulated that standardised ESs would be calculated for all RCTs providing sufficient data. However, owing to a paucity of eligible trials, data from non-randomised studies were also analysed. ESs were calculated for both designs but effects were not pooled across these two different levels of evidence. 82
Evidence from randomised controlled trials
Three randomised trials were eligible for inclusion in our synthesis, all of which were conducted in the USA and published between 1982 and 1984. 60,61,117 No contemporary evidence from the UK was identified. Tables 2–4 provide an overview of the study contexts, populations, types of interventions and outcome variables prioritised by these trials. Study quality indicators including randomisation procedures and methods of allocation concealment are presented in Table 5. The specific characteristics relevant to each trial are presented in Appendix 7 within the context of the data extraction sheet used to record individual study information for this review.
| Criterion | Characteristic | RCT | nRCT | Uncontrolled design | Study number(s)a |
|---|---|---|---|---|---|
| Country | USA | 3 | 1 | 1 | (60)–(62) (117) (118) |
| Australia | – | 3 | 1 | (58) (59) (119) (121) | |
| UK | – | – | 1 | (120) | |
| Canada | – | – | 1 | (122) | |
| Recruitment context | Mental health inpatient services | 1 | 1 | 1 | (61) (62) (118) |
| Mental health outpatient services | 3 | 1 | – | (60) (61) (117) (119) | |
| Mental health community services | 2 | 1 | 2 | (60)–(62) (118) (122) | |
| Intervention programme | – | 2 | 2 | (58) (59) (121) (122) | |
| Media adverts | 2 | – | – | (60) (61) | |
| General health services | – | – | 1 | (62) | |
| Voluntary sector | – | – | 1 | (120) | |
| Parent | All mothers | 2 | 1 | 1 | (60) (117) (118) (120) |
| All fathers | – | – | – | – | |
| Mixed (60–75% female) | – | 2 | 1 | (58) (119) (121) | |
| Not reported | 1 | 1 | 2 | (59) (61) (62) (122) | |
| Parental diagnosis | Psychosis/psychotic symptoms | 3 | 2 | – | (58) (60) (61) (117) (118) |
| Schizophrenia and related | – | 2 | 3 | (58) (59) (120)–(122) | |
| Bipolar disorder | – | 2 | 4 | (58) (59) (62) (120)–(122) | |
| Personality disorder and related | – | 3 | 2 | (58) (59) (119) (120) (122) | |
| % SMI | 100% | 3 | 3 | – | (58) (60) (61) (117)–(119) |
| ≥ 75–99% | – | – | 1 | (121) | |
| ≥ 50–74% | – | 1 | 3 | (59) (62) (120) (122) | |
| Other diagnoses in sample | MDD | – | – | 2 | (62) (122) |
| PND | – | – | 1 | (120) | |
| Depression | – | 2 | 1 | (58) (59) (120) | |
| Depression/anxiety | – | 1 | 1 | (59) (121) | |
| Depression/PTSD | – | 1 | – | (59) | |
| Exclusion criteria | In treatment | – | – | 1 | (62) |
| Substance misuse | – | 1 | 1 | (62) (118) | |
| Current crisis | – | – | 1 | (62) | |
| Parent/child learning difficulties | – | 1 | – | (118) | |
| Diagnosis of schizophrenia | – | – | 1 | (62) | |
| Not reported | 3 | 3 | 3 | (58)–(61) (117) (119)–(122) | |
| % BME parents | 50% BME | 1 | 1 | 1 | (60) (118) (122) |
| > 50% BME | – | – | 2 | (120) (121) | |
| Not reported | 2 | 3 | 1 | (58) (59) (61) (62) (117) (119) | |
| Child target age range | 0–5 years | 1 | 2 | 1 | (117)–(120) |
| 6–12 years | 3 | 2 | 3 | (59)–(62) (117) (119) (121) (122) | |
| 13–16 years | – | 2 | 2 | (58) (62) (119) (122) | |
| Child gender | < 75% female | 2 | 2 | 2 | (58)–(61) (121) (122) |
| Not reported | 1 | 2 | 2 | (61) (62) (118)–(120) |
| Criterion | Characteristic | RCT | nRCT | Uncontrolled design | Study number(s)a |
|---|---|---|---|---|---|
| Model | Psychoeducation | 1 | 3 | 2 | (58)–(60) (121) (122) |
| Psychotherapy | 3 | 1 | 1 | (60)–(62) (119) | |
| Extended care | 1 | 2 | 1 | (117) (118) (120) | |
| Objective | Parent well-being | – | 1 | – | (119) |
| Parenting relationship | 2 | – | 1 | (60) (62) | |
| Child well-being | 2 | 3 | 2 | (58)–(61) (121) (122) | |
| Hybrid/dual focus | 1 | 2 | 1 | (117) (118) (120) | |
| Target | Predominantly parent | 3 | 2 | 1 | (60) (117)–(120) |
| Predominantly child | 2 | 3 | 2 | (58)–(61) (121) (122) | |
| Parent–child or family | – | 1 | 1 | (62) (118) | |
| Setting | Home | 1 | 1 | – | (117) (118) |
| Community | – | 2 | 2 | (58) (118) (120) (121) | |
| Clinic | 4 | – | 1 | (60)–(62) | |
| Unclear/not reported | – | 3 | 1 | (59) (119) (122) | |
| Delivery | Face to face | 5 | 6 | 4 | (58)–(62) (117)–(122) |
| Individual | 1 | 3 | 2 | (62) (117)–(120) | |
| Group | 4 | 4 | 3 | (58)–(61) (118) (120)–(122) | |
| Personnel | MH nurse/clinician | 1 | 1 | 1 | (58) (117) (122) |
| General/unspecified nurse | – | 1 | – | (118) | |
| Unspecified clinician | – | – | 1 | (62) | |
| Social worker | 1 | 1 | – | (61) (118) | |
| Psychotherapist/counsellor | – | 2 | 1 | (118) (119) (121) | |
| Nursery nurse | – | 1 | – | (118) | |
| Not reported | 3 | 2 | 1 | (59) (60) (120) | |
| Monitoring | Training given | 2 | 2 | 2 | (61) (62) (117)–(119) (122) |
| Supervision received | – | 1 | 1 | (62) (119) | |
| Not possible/not reported | 3 | 4 | 2 | (58)–(60) (118) (120) (121) | |
| Session duration | Up to 1 hour | 4 | 1 | – | (60) (61) (119) |
| 1–2 hours | 1 | 1 | 1 | (59) (117) (122) | |
| > 2 hours to 1 day | – | 3 | 1 | (58) (59) (118) (121) | |
| Not reported/not applicable | – | 1 | 2 | (62) (118) (120) | |
| Session frequency | Two or more times a week | – | 3 | 1 | (59) (118) (119) (121) |
| Weekly | 5 | 1 | 1 | (60) (61) (117) (118) (122) | |
| Fortnightly | – | 1 | – | (58) | |
| Not reported/not applicable | – | 1 | 2 | (59) (62) (120) | |
| Total duration | Up to 8 weeks | – | 2 | 2 | (58) (59) (121) (122) |
| 9–16 weeks | 4 | 2 | – | (59)–(61) | |
| Up to 1 year | – | 3 | – | (118) (119) | |
| > 1 year | 1 | – | – | (117) | |
| Unclear/not reported | – | – | 2 | (62) (120) | |
| Total scheduled contact | 11–15 hours | 1 | – | 1 | (61) (122) |
| 16–20 hours | 3 | 1 | 1 | (58) (60) (121) | |
| > 26 hours | 1 | 3 | – | (59) (117)–(119) | |
| Not reported/not applicable | – | 2 | 2 | (59) (62) (118) (120) | |
| Comparator | Waiting list | – | 2 | – | (58) (119) |
| Standard care | 3 | – | – | (60) (61) (117) | |
| Active intervention | 1 | 2 | – | (59) (60) (118) |
| Criterion | Characteristic | RCT | nRCT | Study number(s)b |
|---|---|---|---|---|
| Primary outcomes | QoL | – | 1 | (58) |
| Emotional well-being | 1 | 1 | (58) (117) | |
| Secondary outcomes | Physical health | – | – | – |
| Safety | – | – | – | |
| Social function/behaviour | 3 | 2 | (58) (60) (61) (117) (118) | |
| Social relationship quality | – | 2 | (58) (59) | |
| Recreational engagement | – | 1 | (58) | |
| Family function | 1 | 1 | (117) (119) | |
| Parent–child relationship | 2 | 2 | (60) (117)–(119) | |
| Parent mental health symptoms | 2 | – | (60) (117) | |
| Cognitive function | 3 | 1 | (60) (61) (117) (118) | |
| Problem-based coping | 1 | 2 | (58) (59) (61) | |
| Mental health literacy | – | 1 | (58) | |
| Self-esteem | – | 2 | (58) (59) | |
| Outcome assessor | Child report | – | 2 | (58) (59) |
| Parent report | 2 | 2 | (60) (61) (117) (118) | |
| Observer report | 3 | 1 | (60) (61) (117) (119) | |
| Unclear/unspecified | 1 | – | (117) | |
| Data reporting | Continuous (mean SD) | 3 | 4 | (58)–(61) (117)–(119) |
| Dichotomous | – | 1 | (58) | |
| Insufficient data for standardised ES | 3 | 1 | (60) (61) (117) (118) | |
| Timing of follow-up assessment | 0–6 months | 2 | 2 | (58)–(61) |
| 7–12 months | – | 2 | (118) (119) | |
| > 12 months | 1 | 1 | (117) (118) |
| Criterion | Characteristic | RCT | nRCT | Study number(s)a |
|---|---|---|---|---|
| Allocation procedure | Randomised, method not stated | 2 | – | (61) (117) |
| Quasi-randomised, sequential | 1 | – | (60) | |
| Non-randomised, service availability | – | 3 | (58) (118) (119) | |
| Non-randomised, treatment preference | – | 1 | (59) | |
| Allocation concealment | Not reported | 3 | – | (60) (61) (117) |
| Not applicable | – | 4 | (58) (59) (118) (119) | |
| Unit of allocation | Parent | 1 | – | (117) |
| Child | 1 | – | (61) | |
| Parent/child | 1 | – | (60) | |
| Not applicable | – | 4 | (58) (59) (118) (119) | |
| Blinded participants/personnel | No | 3 | 4 | (58)–(61) (117)–(119) |
| Blinded outcome assessment | No | 3 | 4 | (58)–(61) (117)–(119) |
| Unclear | 3 | 1 | (60) (61) (117) (118) | |
| Primary outcome identification | No | 3 | 4 | (58)–(61) (117)–(119) |
| Selective outcome reporting | Yes | 2 | 1 | (60) (117) (118) |
| No | 1 | 3 | (58) (59) (61) (119) | |
| Sample size at baseline | 0–25 | 2 | – | (60) (61) |
| 26–50 | 2 | 2 | (58) (61) (117) (119) | |
| 50 + | – | 2 | (59) (118) | |
| Attrition rate post intervention | 0–10% | 1 | 2 | (58) (61) (119) |
| 11–20% | – | – | – | |
| > 20% | – | 1 | (59) | |
| Unclear/not reported | 2 | 1 | (60) (117) (118) | |
| Method of analysis | ITT/complete data set | – | 2 | (58) (119) |
| Incomplete data set | 1 | 1 | (59) (61) | |
| Unclear/not reported | 2 | 1 | (60) (117) (118) | |
| Overall risk of bias | High risk | 2 | 4 | (58)–(60) (117)–(119) |
| Unclear risk | 1 | – | (61) |
Parent and child populations
Consistent with our eligibility criteria, all included studies had samples in which more than 50% of parents met criteria for a SMI. All three trials recruited mothers, or the children of mothers, with non-puerperal psychosis or a comparable symptom profile. Clinical diagnoses were not confirmed as part of the research and maternal mental health co-morbidities were not reported. All mothers had children aged 12 years or below. Only one of the three trials reported parents’ ethnic status, with 45% of the sample being black or minority ethnic (BME). 60 Two trials reported children’s residential status; in both cases children were reported to co-reside with their parents. 61,117
Interventions and comparators
Substantial heterogeneity in intervention models, content and outcomes was observed. Overall, the three trials reported five different interventions. Two trials evaluated similar psychotherapeutic interventions delivered directly to children with the primary aim of enhancing their psychosocial well-being and/or resiliency to parental mental illness. 60,61 The remaining three interventions (reported across two studies) were consistent in targeting parents. 60,117 Each of these parent-based interventions encompassed a different psychotherapeutic, psychoeducational or extended model of care. Definitions of these different intervention models, as used in the current synthesis, are provided in Box 3. Two of the three parent-based interventions sought to indirectly influence children’s QoL by enhancing mothers’ parenting behaviours. 60 The third integrated interventions for mothers’ mental health symptoms alongside an intervention aimed at enhancing their parenting capacity. 117 One trial evaluated three different interventions60 and, therefore, the number of in-text study references may not total 100%.
Interventions that seek to improve mental well-being, self-acceptance and/or change behaviour through an examination of individual affect and/or the challenging of negative thoughts and beliefs. Examples include, but are not restricted to, CBTs, interpersonal therapies and supportive therapies. Psychotherapeutic interventions demand the maintenance of a therapeutic relationship and incorporate specific techniques such as problem identification, problem solving, goal setting and monitoring and/or the resolution of interpersonal conflict. With the exception of psychodynamic therapy, psychotherapeutic interventions typically follow a structured and time-limited format of between 10 and 20 weeks. Briefer programmes are also possible.
Psychoeducational interventionsInterventions that are aimed solely or predominantly at changing attitudes and/or resilience through an increased understanding of factual health information or subjective experience. Information may be delivered via didactic techniques or within the context of a group discussion facilitated by professional or lay leaders. The disseminated information is generic without any consideration of individual barriers or the generation of an individualised action plan. While behaviour change may be encouraged, its actual implementation will be at the discretion of the individual concerned.
Psychosocial interventionsInterventions that demand the participation of parents, children or family members for the purposes of improving mental well-being, self-acceptance and/or change behaviour via social integration, interaction or the provision of social support. These interventions typically comprise structured or unstructured peer and/or professional support.
Extended care interventionsInterventions that aim to provide both primary and secondary prevention through the adoption or inclusion of multiple strategies or interventions. Extended care interventions may be delivered across a range of home or community settings and include multiple foci relating to aspects of the child-parent relationship, parental rehabilitation and/or child well-being. Extended care interventions are typically less structured than other interventions and may be individually tailored. They are likely to include social activities, mental health rehabilitation and support and/or referral to external agencies as required. Care management strategies and advocacy are often included.
Parenting interventionsInterventions that target the parent, or parent–child dyad, with the specific aim of enhancing the quality of the interaction and/or relationships between them. Parenting interventions may consist of parenting-orientated psychoeducation, strategies to improve behavioural insight and/or the teaching of practical skills via direct observation and feedback techniques. More formal parenting-based therapies include, but are not limited to, psychotherapies based on behavioural therapy, attachment theory or social learning. Parenting interventions thus form a distinct subcategory of the four intervention models described above, classified according to the nature and scope of their intended approach.
All of the included trials compared one or more active interventions with a treatment as usual control. The first117 recruited formerly hospitalised women with psychosis from public and private psychiatric hospitals. Participation was limited to mothers of children aged < 6 years. The mothers were randomly allocated to a minimal standard care intervention or a grant-funded high-intensity home nurse visitation programme. At each visit, mental health nurses, trained specifically for the intervention, met mothers to discuss their parenting and illness experiences. Mothers were visited for approximately 1–1.5 hours a week over a 1- to 2-year period. Partners and children were not actively involved in the intervention.
The second included trial61 recruited the children of mothers who were judged to be suffering an ‘emotional disturbance of psychotic magnitude,’ defined as a T-score of 63 or more on the Global Severity Test of the Symptom Checklist-90. 123 Participants were recruited from a children’s community mental health project receiving referrals from adult secondary mental health services, community and social-care agencies and/or self-referrals following newspaper advertising. Participation was limited to children aged between 5 and 12 years, who were randomly allocated to either treatment as usual or a cognitive–behavioural therapy (CBT)-based problem-solving programme. The problem-solving programme emphasised inter- and intra-personal problem solving and was delivered outside the home by a doctoral student trained in counselling. Programme content was delivered directly to children in a face-to-face group format over 12 weekly 1-hour sessions. Parents were not involved in the intervention.
The third and final trial60 evaluated an identical child-centred CBT problem-solving intervention to that described above. This intervention was delivered and evaluated by an affiliated research team and recruited participants from the same community mental health project. Eligibility criteria once again restricted participation to mothers, or the children of mothers, achieving a T-score of 63 or more on the Global Severity Test of the Symptom Checklist-90. 123 Differences in outcome measures, study design and duration of the intervention justified the retention of the trials as two separate studies. Mothers and their children who were aged between 5 and 12 years were quasi-randomised by sequential assignment to a control group or one of three active interventions. These interventions comprised child-orientated cognitive–behavioural problem-solving, adult-orientated parent counselling based on social learning theory and adult-orientated parenting-based psychoeducation. All three were delivered outside the home in a face-to-face group format over 16 weekly 1-hour sessions. The personnel delivering the interventions were not reported and partners were not actively involved in the interventions.
Outcomes
Marked heterogeneity in trial outcomes and outcome measures was observed. Across the three randomised trials, primary and secondary QoL outcomes were measured using a total of nine validated and referenced instruments (included within Appendix 7, Table 26). Most trials used more than one outcome measure and, therefore, percentages in the tables do not always total 100%. One trial provided conceptually relevant secondary outcome data measured on non-specified scales. 117
Primary outcomes for the purposes of this evidence synthesis comprised validated measures of children’s QoL measures and/or mental health. No randomised trials were identified that measured children’s overall QoL. One trial117 reported carrying out children’s psychiatric evaluations and obtaining ratings of child affect. The measures used by this trial were not specified, however, and no outcome data were reported.
Secondary outcomes relevant to our QoL outcome framework were provided by all three trials. The most frequently measured outcomes related to children’s social function and behaviour and children’s cognitive function (see Table 4), thereby reflecting a tendency towards more developmentally focused and observer-rated outcomes. No RCTs measured outcomes related to children’s physical health and safety or subjective measures of children’s self-esteem, social relationship quality, recreational engagement or mental health literacy.
Short-term outcomes (defined as 0–6 months post randomisation) were reported by two of the three identified trials. 60,61 The exception117 reported a longer-term follow-up of between 12 and 24 months following participants’ discharge from a more prolonged intervention. All extracted outcomes were presented as continuous variables.
Methodological quality ratings
The Cochrane Risk of Bias Assessment tool82 was used to quality appraise the three included trials. No high-quality RCTs were identified. Overall risk of bias was judged to be high in two trials60,117 and unclear in the third. 61 An overview of key study quality indicators is presented in Table 5. Full quality appraisal tables are available in Appendix 7, Tables 23 and 24.
Selection biases were possible. Two of the three trials61,117 did not report details of their randomisation methods and thus it was difficult to judge whether or not the methods used to allocate participants and/or conceal the allocation sequence were appropriate. The third study60 was judged inadequate on the basis of a sequential approach to group allocation, which was not truly random.
Risks of performance and detection biases were also high. Commensurate with most trials of psychosocial interventions, behavioural changes associated with the interventions prevented participant or personnel blinding. Outcomes were measured predominantly by parental report, but also by independent assessment depending upon the nature of the QoL outcome being examined. Observer ratings were reported for measures of child affect, behaviour, cognitive development and parental mental health. Assessor blinding was not reported for these outcomes. No trials specified primary outcomes a priori and two trials60,117 failed to present complete outcome data, thereby raising the additional possibility of reporting biases.
Baseline sample sizes were notably small, varying from n = 1461 to n = 50. 117 Risks of attrition biases were judged to be low in one trial61 but unclear in the other two60,117 owing to inadequate reporting of the numbers and/or reasons for participant withdrawal. All three trials restricted their data analyses to intervention completers.
Unpublished data from included trials
Effect sizes could not be calculated for two trials60,117 across six outcomes owing to insufficient data. Attempts were made to obtain missing information but, owing to the date of trial publications, the study authors could not be traced.
Evidence of clinical effect from randomised controlled trials
Overall, the identified trials displayed marked clinical and methodological heterogeneity. Meta-analysis of the data was therefore judged to be inappropriate as pooled ESs would not be interpretable. In addition, there were insufficient comparisons to explore potential relationships between treatment effectiveness and the key contextual factors outlined in our protocol (i.e. therapeutic target, intervention content and user characteristics). Study results are thus presented in a narrative format, grouped by QoL outcome domains. When data allow, outcomes are displayed as standardised ESs in forest plots to facilitate inspection of the data and to aid an assessment of ESs across different studies on a common metric (Figure 3). However, study effects are not pooled and are not intended to provide an overall estimate of intervention effect.
FIGURE 3.
Secondary QoL outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: evidence from RCTs. (C), child target; EC, extended care; (P), parent target.
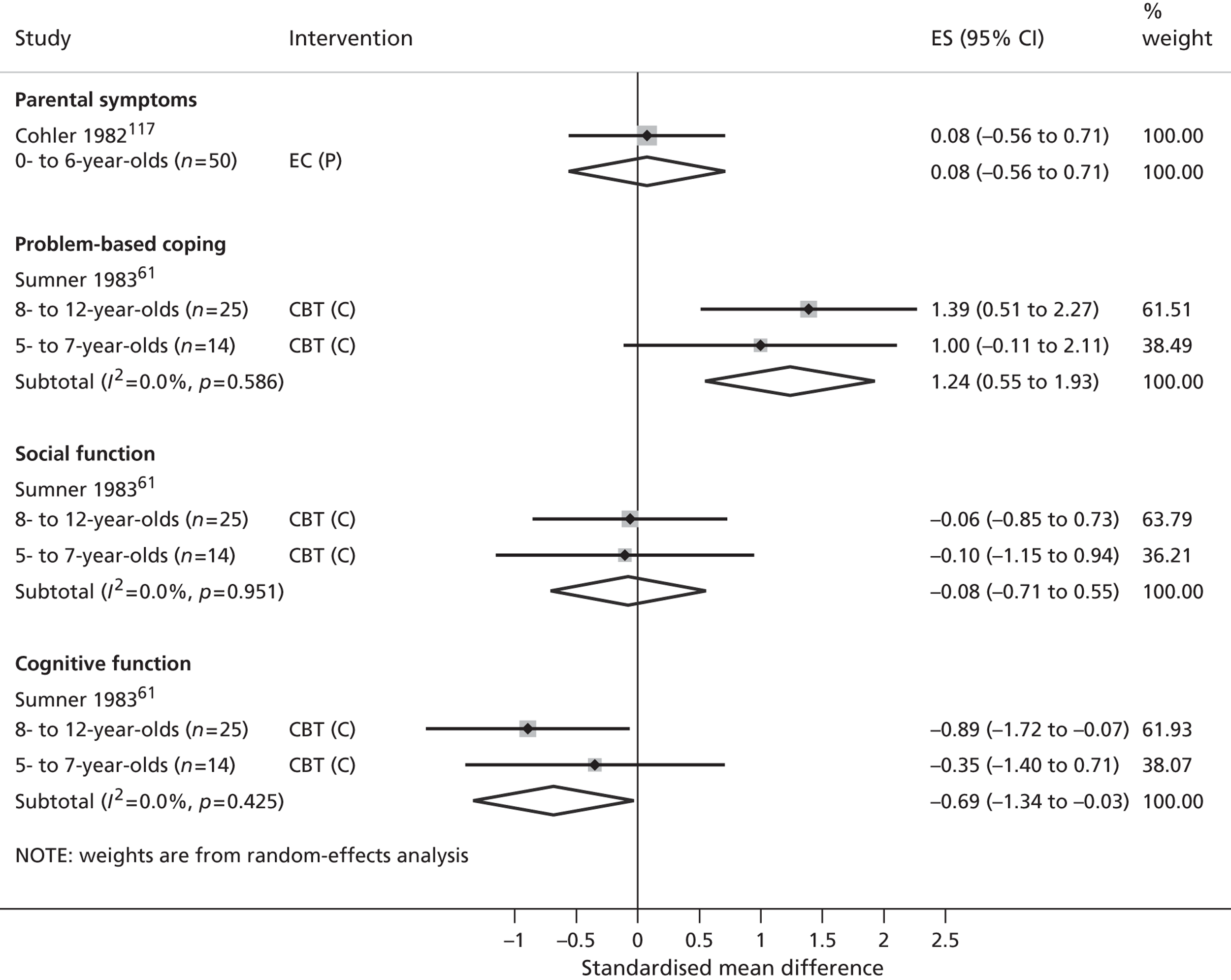
Primary outcomes
Children’s quality of life
No randomised trials measured validated QoL outcomes.
Children’s emotional well-being
One randomised trial, by Cohler and Grunebaum,117 reported measuring outcomes relevant to children’s mental health. This trial used a randomised design to compare a high-intensity home nurse visitation programme to minimal intensity standard care in 50 mothers of children aged < 6 years. Data for this outcome were not reported.
Secondary outcomes
Children’s physical well-being
Physical health
No randomised trials measured children’s physical health outcomes.
Safety
No randomised trials measured outcomes relevant to child safety.
Children’s social well-being
Social function and behaviour
All three randomised trials reported measuring outcomes relevant to children’s social function and behaviour, although only one reported these data. Sumner61 used a randomised design to compare child-orientated cognitive–behavioural problem solving with a treatment as usual control in 41 children aged 5–12 years. Outcomes were parental reports of children’s internalising and externalising behaviours at 3 months post randomisation. Data were reported and analysed separately for 5- to 7-year-olds (n = 14) and 8- to 12-year-olds (n = 25). Non-significant effects were observed in both age groups (5–7 years standardised ES –0.10, 95% CI –1.15 to 0.94; 8–12 years standardised ES –0.06, 95% CI –0.85 to 0.73).
Social relationship quality
No randomised trials measured outcomes relevant to the quality of children’s social relationships.
Recreational engagement
No randomised trials measured outcomes relevant to children’s recreational engagement.
Children’s family-based experiences
Family function
Two trials reported measuring family functioning outcomes. The first, conducted by Cohler and Grunebaum,117 used a randomised design to compare a high-intensity home nurse visitation programme to minimal intensity standard care in 50 mothers of children aged < 6 years. Outcomes were nurses’ independent assessments of family conflict resolution at 1–2 years post randomisation. No significant differences between the intervention and usual care were reported, although the authors failed to present any p-values or descriptive data to support this narrative.
The second, conducted by Lucas et al. 60 used a quasi-randomised design to compare child-orientated cognitive–behavioural problem, parent counselling, parenting education to a treatment as usual control. Outcomes were measured at 16 weeks post randomisation by maternal report on the family unit subscale of the social adjustment scale. The authors’ narrative reported parent counselling to reduce family difficulties in 5- to 7-year-olds (β = –0.586, p < 0.005) and parenting education to reduce family difficulties in 8- to 12-year-olds (β = –0.406, p < 0.05). Insufficient data were presented to enable the calculation of a standardised ES.
Parental mental health
The same two trials that measured family function also measured outcomes relevant to parental mental health. Cohler and Grunebaum117 reported on both nurses’ assessments of mothers’ mental health symptoms and the number of maternal rehospitalisations at 1–2 years post randomisation. The authors’ narrative reported home nurse visitation to be associated with a greater reduction in nurses’ symptom assessments (p < 0.02) than a treatment as usual control. However, insufficient data were presented to enable an independent calculation of a standardised ES. A non-significant effect of the intervention on maternal rehospitalisations was observed (standardised ES 0.08, 95% CI –0.56 to 0.71).
In the second trial by Lucas et al. ,60 group allocation to child-orientated cognitive–behavioural problem-solving, parent counselling, parent-based psychoeducation or treatment as usual was not reported to be a significant predictor of maternal self-reported mental health symptoms at short-term follow-up. Once again, however, the authors’ failed to present sufficient descriptive data to enable calculation of the standardised ES.
Children’s self-esteem and self-actualisation
Cognitive function
All three trials reported measuring outcomes relevant to children’s cognitive development, although only one presented such data. The trial by Sumner61 used a randomised design to compare child-orientated cognitive–behavioural problem solving with a ‘treatment as usual’ control. Outcomes were children’s intelligence quotient (IQ) scores measured at 3 months post randomisation. Data were reported separately for 5- to 7-year-olds (n = 14) and 8- to 12-year-olds (n = 25). A small, but non-significant, negative effect was observed in 5- to 7-year-olds (standardised ES –0.35, 95% CI –1.40 to 0.71) and a large, significant, negative effect in 8- to 12-year-olds (standardised ES –0.89, 95% CI –1.72 to –0.07).
Problem-based coping skills
Sumner61 also measured outcomes relative to children’s problem-solving abilities, specifically children’s performance levels on age-appropriate problem-solving measures at 3 months post randomisation. Data were reported separately for 5- to 7-year-olds (n = 14) and 8- to 12-year-olds (n = 25). A large significant effect was observed in 8- to 12-year-olds (standardised ES 1.39, 95% CI 0.51 to 2.27) and a large but non-significant effect was observed in 5- to 7-year-olds (standardised ES 1.00, 95% CI –0.11 to 2.11). Neither of the other two trials reported measuring this outcome.
Levels of mental health literacy
No randomised trials reported outcome relevant to children’s mental health literacy.
Self-esteem
No randomised trials reported outcomes relevant to children’s self-esteem.
Evidence from non-randomised controlled trials
Compared with the RCTs, the non-randomised trials typically provided more contemporary evidence. Four non-randomised trials were eligible for inclusion in this synthesis,58,59,118,119 three of which were Australian studies published within the last 4 years (i.e. 2008 or beyond). 58,59,119 Once again, no relevant UK studies were identified. The study contexts, populations, types of interventions and outcome variables prioritised by the non-randomised trials are summarised alongside the randomised trials in Tables 2–4. The specific characteristics relevant to each trial are presented in Appendix 7 within the context of the data extraction sheet used to record individual study information for this synthesis.
Parent and child populations
Across the four non-randomised trials, heterogeneity in parent populations was observed. The included studies all targeted parental psychosis and/or personality disorders, either alone118,119 or in combination with bipolar depression. 58,59 Other primary mental health diagnoses (e.g. affective disorders) were included in the sample of two trials58,59 and one trial reported co-morbid anxiety and depressive disorders. 58 Two of the four non-randomised trials recruited mothers and fathers with SMI,58,119 the remaining two studies focusing solely on mothers. 59,118
Variation in the child populations was observed. One trial recruited the mothers of preschool children aged < 5 years,118 one targeted primary school children aged 8–12 years,59 one targeted teenagers aged 12–16 years58 and one spanned a wide range of child ages broadly defined by the authors as ‘infancy to adolescence’. 119 Two of the four studies reported children’s residential status and in both cases the children co-resided with their parents. 118,119
Interventions and comparators
Additional heterogeneity in intervention models, content and the therapeutic target was observed. Overall, the four non-randomised trials reported on five different interventions spanning psychotherapeutic,119 psychoeducational58,59 and extended models of care. 118 Full definitions of these models are provided in Box 3. Two interventions comprised similar psychoeducational programmes delivered directly to children,58,59 one comprised a psychotherapeutic intervention aimed at enhancing parental well-being119 and the remaining two were classified as extended models of care aimed either at enhancing parenting and access to services for mothers and/or mothers and their children. 118 Two non-randomised trials59,118 reported on more than one intervention and, therefore, the number of in-text study references do not always total 100%.
Two trials compared an active intervention with a waiting list control. 58,119 The first119 recruited both mothers and fathers with borderline personality disorder from local hospital referrals. Their children ranged in age from infancy to adolescence. Participating parents were allocated on the basis of service availability to either a waiting list control or a psychotherapeutic intervention based on a conversational model. The intervention was delivered in an individual format outside the home by trainee psychotherapists qualified in medicine or psychiatry. Parents with mental illness participated in two 50-minute sessions per week over a 12-month period. Partners and children were not involved in the intervention.
The second58 recruited children aged 12–18 years directly from a programme initiative aimed specifically at the children of parents with SMI. Children were referred to the programme by their relatives or by external agencies including child and adolescent mental health services, adult mental health services, youth services and schools. Parental diagnoses comprised a mix of personality disorder, bipolar disorder and psychosis. Children were allocated to either the programme initiative or a waiting list control on the basis of service availability. The intervention programme adhered to a resilience framework and delivered psychoeducation in three fortnightly 2-hour sessions over a 6-week period. All classes were held in a group format outside the home facilitated by mental health clinicians. Parents were not actively involved in the intervention.
The remaining two non-randomised trials59,118 both compared two active interventions. One trial recruited children aged 8–12 years from a federally funded psychoeducational programme similar to that described above. 59 Eligible parental diagnoses included schizophrenia and schizoaffective disorder, personality disorder and bipolar disorder. Additional diagnoses in the study sample included anxiety and depressive disorders, which 26% of the sample suffered from. Children were allocated according to their attendance preferences to one of two identical interventions differing only in their delivery format. The first, an after-school programme, delivered group-based psychoeducation in 1- to 2-hour sessions held weekly or fortnightly over a maximum of one to two school terms. The second, a school holiday programme, delivered the same content over four consecutive days. Parents were not actively involved in either intervention.
The final trial118 compared two different models of extended care. Mothers with psychosis were recruited from inpatient psychiatric clinics and private mental health practices. Their children were all aged < 5 years. Women were randomised according to service availability to a home nursing intervention or an enhanced model of care. The home nursing intervention developed from a similar intervention that was evaluated by a randomised trial reported previously. 117 Mental health nurses visited women for 2 hours per week over a 12-month period to discuss parenting and illness experiences. Nurses also engaged in basic care management procedures, obtaining community service referrals for different family members as appropriate. In contrast, the enhanced care intervention was provided 4 days a week in a community setting. Psychologists and nursery nurses intervened with mothers and children both separately and together during the course of the 12-month programme. Intervention content included a broad mix of parental rehabilitation, social interaction, parenting therapy and child psychotherapy, when required. Partners were not explicitly involved in the intervention.
Outcomes
Comparable to the evidence provided by the RCTs, the identified non-randomised trials displayed marked heterogeneity in their outcomes and the outcome measures used. Primary and secondary QoL outcomes were measured using a total of fourteen validated and referenced instruments across all four non-randomised trials (see Appendix 7, Table 26). Most studies used more than one outcome measure and, therefore, percentages in the tables do not always total 100%. One study also provided conceptually relevant data measured on non-validated scales. 58
Primary outcomes for the purposes of this evidence synthesis comprised validated QoL measures and/or children’s mental health. Only one non-randomised trial58 measured these outcomes. Secondary outcomes relevant to specific QoL domains or subdomains were provided by all four non-randomised trials. In marked contrast to the more developmentally orientated outcomes prioritised by randomised trials, these outcomes targeted subjective QoL indicators reflecting children’s own appraisals of the quality of their social relationships, level of self-esteem and/or coping (see Table 4).
Short-term follow-up (defined as up to 6 months post randomisation) was provided by two trials58,59 and medium-term follow-up (defined as 7–12 months post randomisation) was provided by another two. 118,119 One non-randomised trial also provided a longer-term follow-up measured at 2 years post randomisation. 118 Relevant outcomes were presented as continuous and dichotomous data. Calculations of standardised ES proiritised continuous data when possible (see Appendix 7, Table 25).
Methodological quality ratings
The Cochrane Risk of Bias Assessment tool82 was used to quality appraise all non-randomised trials (see Appendix 7, Table 24). As anticipated, the risk of selection bias remained uniformly high (see Table 5). This risk arose directly from reliance on inadequate sequence generation procedures, specifically allocation by service availability58,118,119 or participant preference. 59
Risks of performance and detection biases were also high, not least because behavioural changes associated with the interventions prevented participant or personnel blinding. Outcomes were measured primarily by self-report,58,59,118,119 but also by independent assessment when appropriate. 118 In these instances, assessor blinding was not reported. No trials specified primary outcomes a priori and, therefore, risk of reporting biases remained unclear.
Owing to incomplete reporting of participant withdrawals, risks of attrition bias were also judged to be high or unclear in three of the four included trials. 58,59,118 Baseline sample sizes varied from n = 4458 to 6959 and sample attrition rates were relatively high, ranging from 0%119 to 49%. 118 Follow-up sample sizes were not reported by one trial;118 another two studies reported conducting their analysis on a complete data set, one of which reported no participant attrition119 and one of which imputed missing data by linear regression. 58
Unpublished data from included trials
Three of the four non-randomised studies reported sufficient data to enable an ES to be calculated (Figures 4 and 5). 58,59,119 ESs could not be calculated for one study across four outcomes because of insufficient data. 118 Attempts were made to obtain the missing information but study authors could not be contacted.
Evidence of clinical effect from non-randomised controlled trials
Primary outcomes
Children’s quality of life
Only one non-randomised trial incorporated a validated measure of children’s QoL. This trial, by Fraser and Pakenham,58 compared group-based psychoeducation to a waiting list control in a total of 40 children aged 12–18 years. Outcomes were children’s self-reports on the Life Satisfaction Scale at 4 weeks post randomisation. A small negative, but non-significant, effect was observed (standardised ES –0.18, 95% CI –0.82 to 0.46). No other non-randomised trials reported this outcome (see Figure 4).
Children’s mental health
Fraser and Pakenham58 also reported outcomes relevant to children’s mental health, and, in this case, children’s self-reports on the Child Depression Inventory. A small to medium, negative, non-significant effect was observed (standardised ES –0.35, 95% CI –1.00 to 0.29). No other non-randomised trials reported this outcome.
Secondary outcomes
Children’s physical well-being
Physical health
No non-randomised trials measured children’s physical health outcomes.
Safety
No non-randomised trials measured outcomes relevant to child safety.
Children’s social well-being
Social function and behaviour
Two non-randomised trials measured outcomes relevant to children’s social function and behaviour. The first, by Stott et al. ,118 used a non-randomised design to compare a home nurse visitation programme with a high-intensity extended care intervention in 83 mothers of children aged < 5 years. Outcomes were observer ratings on the Harvard Preschool Project Social Abilities Checklist measured at both 1 and 2 years post randomisation. The authors’ narrative reported no significant differences between these two interventions, although insufficient data were presented to enable the calculation of a standardised effect.
The second, conducted by Fraser and Pakenham,58 compared group-based psychoeducation with a waiting list control group in a total of 40 children aged 12–18 years. Outcomes were children’s self-reports on the prosocial behaviour subscale of the Strengths and Difficulties Questionnaire at 4 weeks post randomisation. A small to medium, non-significant effect was observed (standardised ES 0.32, 95% CI –0.32 to 0.96).
Social relationship quality
Two non-randomised trials reported outcomes relevant to children’s social relationship quality. Fraser and Pakenham58 compared group-based psychoeducation with a waiting list control group in a total of 40 children aged 12–18 years. Outcomes were children’s self-reports on the Social Connections Scale at 4 weeks post randomisation. A small to medium negative and non-significant effect was observed (standardised ES –0.30, 95% CI –1.87 to 1.27) (see Figure 4).
The second trial by Goodyear et al. 59 used a non-randomised design to compare two different formats of the same child-orientated psychoeducational programme in 65 children aged 8–12 years. Outcomes were children’s self-reports measured on the KIDS Connections Scale at approximately 4 weeks post intervention. A non-significant effect of school holiday programmes over after-school delivery was observed (standardised ES 0.03, 95% CI –0.51 to 0.46) (see Figure 5).
Recreational engagement
One non-randomised trial reported changes in children’s recreational engagement. As reported above, Fraser and Pakenham58 compared group-based psychoeducation with a waiting list control group in a total of 40 children aged 12–18 years. Outcomes were children’s self-reports on the Activity Subscale of the Young Caregiver of Parents Inventory (YCOPI) at 4 weeks post randomisation. A medium negative and non-significant effect was observed (standardised ES –0.47, 95% CI –1.11 to 0.18).
Children’s family-based experiences
Family function
Two non-randomised trials measured outcomes relevant to children’s family functioning. The first, conducted by Gerrull et al. 119 compared parent-centred psychotherapy based on a conversational model with a waiting list control in 32 parents of children aged from infancy to adolescence. Outcomes were maternal self-reports measured on the family unit subscale of the Social Adjustment Scale at 12 months. A medium but non-significant effect was observed (standardised ES 0.46, 95% CI –0.22 to 1.15) (see Figure 4).
The second trial by Goodyear et al. 59 was the only study to measure family functioning from a child’s perspective. As described previously, this trial used a non-randomised design to compare two different formats of the same child-orientated psychoeducational programme in 61 children aged 8–12 years. The number of participants in this study varies by outcome measure. Outcomes were children’s self-reports measured on the family subscale of the KIDS Problems scale. A small but non-significant effect in favour of school holiday programmes over after-school delivery was observed (standardised ES 0.29, 95% CI –0.21 to 0.80) (see Figure 5).
Parental mental health
No non-randomised trials reported this outcome.
Quality of parent–child interactions
Two non-randomised studies measured the quality of parent–child interactions. The first, by Stott et al. ,118 used a non-randomised design to compare a home-nurse visitation programme with a high-intensity extended care intervention in 83 mothers of children aged < 5 years. Outcomes were maternal self-reports on the maternal attitudes scale measured at 1 and 2 years post randomisation. The authors’ narrative reported no significant differences between the interventions, although insufficient data were presented to enable the calculation of a standardised ES.
The second study by Gerrull et al. ,119 compared parent-centred psychotherapy based on a conversational model with a waiting list control in 32 parents of children aged from infancy to adolescence. Outcomes were maternal self-reports on the child unit subscale of the Social Adjustment Scale at 12 months post randomisation. A medium to large, significant effect was observed (standardised ES 0.73, 95% CI 0.03 to 1.42).
Children’s self-esteem and -actualisation
Cognitive function
One non-randomised trial measured outcomes relevant to children’s cognitive function. As described above, Stott et al. 118 used a non-randomised design to compare a home-nurse visitation programme with a high-intensity extended care intervention in 83 mothers of children aged < 5 years. Outcomes were measured at 1 and 2 years post randomisation on the Bayley Scales of Infant Development Scale or Stanford–Binet Scale, as age-appropriate. The authors’ narrative reported no significant differences between the interventions, although insufficient data were presented to enable the calculation of a standardised effect.
Problem-based coping skills
Two non-randomised trials presented data relating to children’s problem-based coping skills. In the first, Fraser and Pakenham58 compared group-based psychoeducation to a waiting list control in a total of 40 children aged 12–18 years. Outcomes were children’s self-reports on the disengagement subscale of the Responses to Stress Questionnaire measured at 4 weeks post randomisation. A non-significant effect was observed (standardised ES –0.07, 95% CI –0.71 to 0.57) (see Figure 4).
In the second, Goodyear et al. 59 used a non-randomised design to compare two different formats of the same child-orientated psychoeducational programme in 64 children aged 8–12 years. Outcomes were children’s self-reports measured on the problem-focused subscale of the Children’s Coping Scale at 4 weeks post intervention. A small, non-significant effect in favour of school holiday programmes over after-school delivery was observed (standardised ES 0.24, 95% CI –0.26 to 0.73) (see Figure 5).
Levels of mental health literacy
Fraser and Pakenham58 also measured children’s levels of mental health literacy, although not from the child’s perspective. Outcomes were assessor ratings of children’s mental health knowledge at 4 weeks post randomisation. A medium to large and significant effect was observed (standardised ES 0.78 95% CI 0.11 to 1.44) (see Figure 4).
Self-esteem
Two non-randomised trials reported measuring children’s self-esteem, although only one used a validated outcome measure. Goodyear et al. 59 compared two different delivery formats of the same child-orientated psychoeducational programme in 69 children aged 8–12 years. Outcomes were children’s self-reports on the Rosenberg self-esteem scale at 4 weeks post intervention. A small to medium and non-significant effect in favour of school holiday programmes over after-school delivery was observed (standardised ES 0.38, 95% CI –0.10 to 0.85) (see Figure 5).
FIGURE 4.
Primary and secondary QoL outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: evidence from nRCTs. Fraser and Pakenham:58 12- to 18-year-olds; Gerrull et al. :119 infant to adolescent. (C), child target; Ed., psychoeducation; (P), parent target; PDT-IPT, psychodynamic and interpersonal therapy.
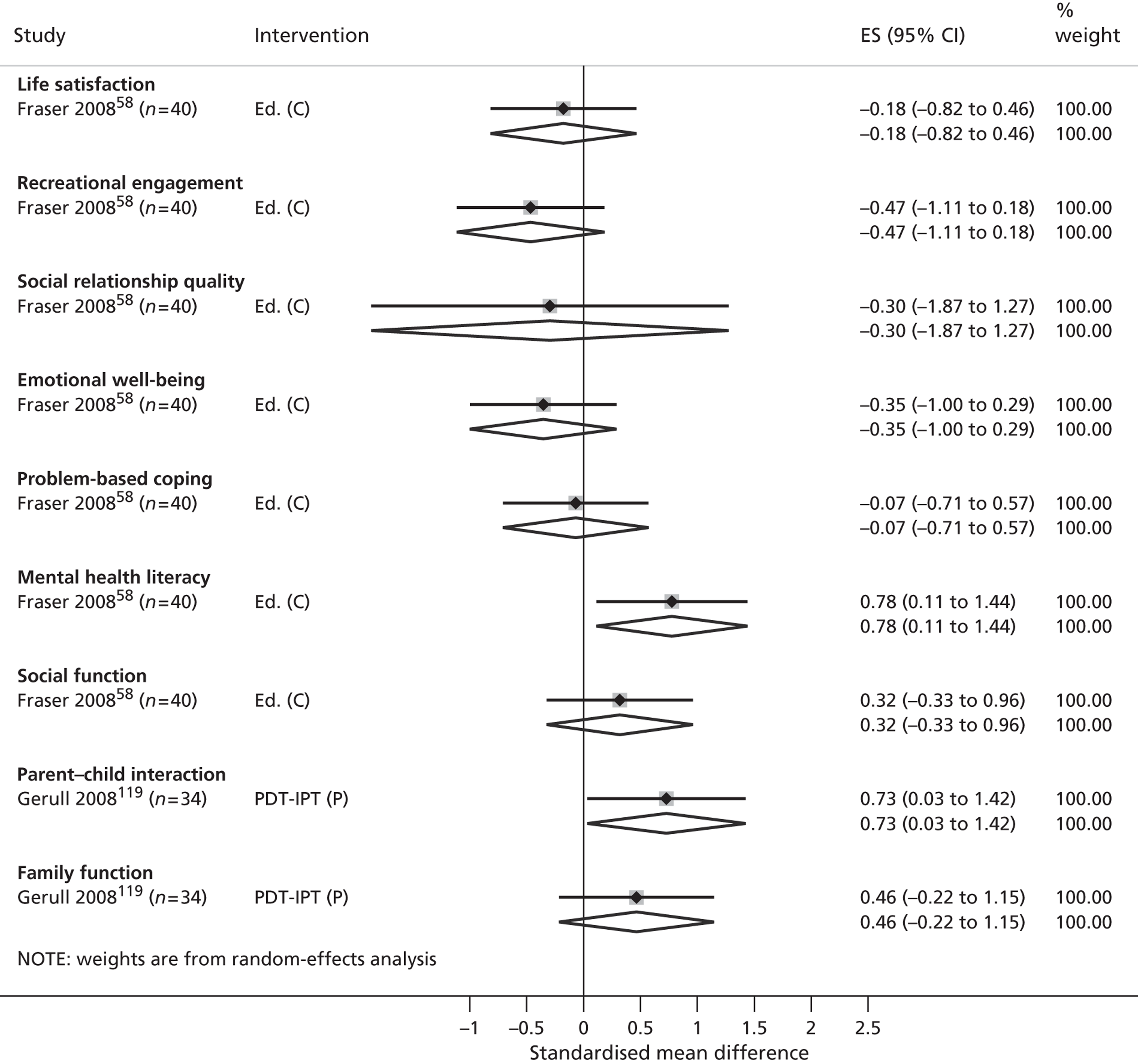
FIGURE 5.
Secondary QoL outcomes for a community-based child psychoeducation intervention vs. active comparison (school holiday vs. after-school delivery): evidence from nRCTs. Goodyear et al. :59 8- to 12-year-olds. (C), child target; Ed., psychoeducation.
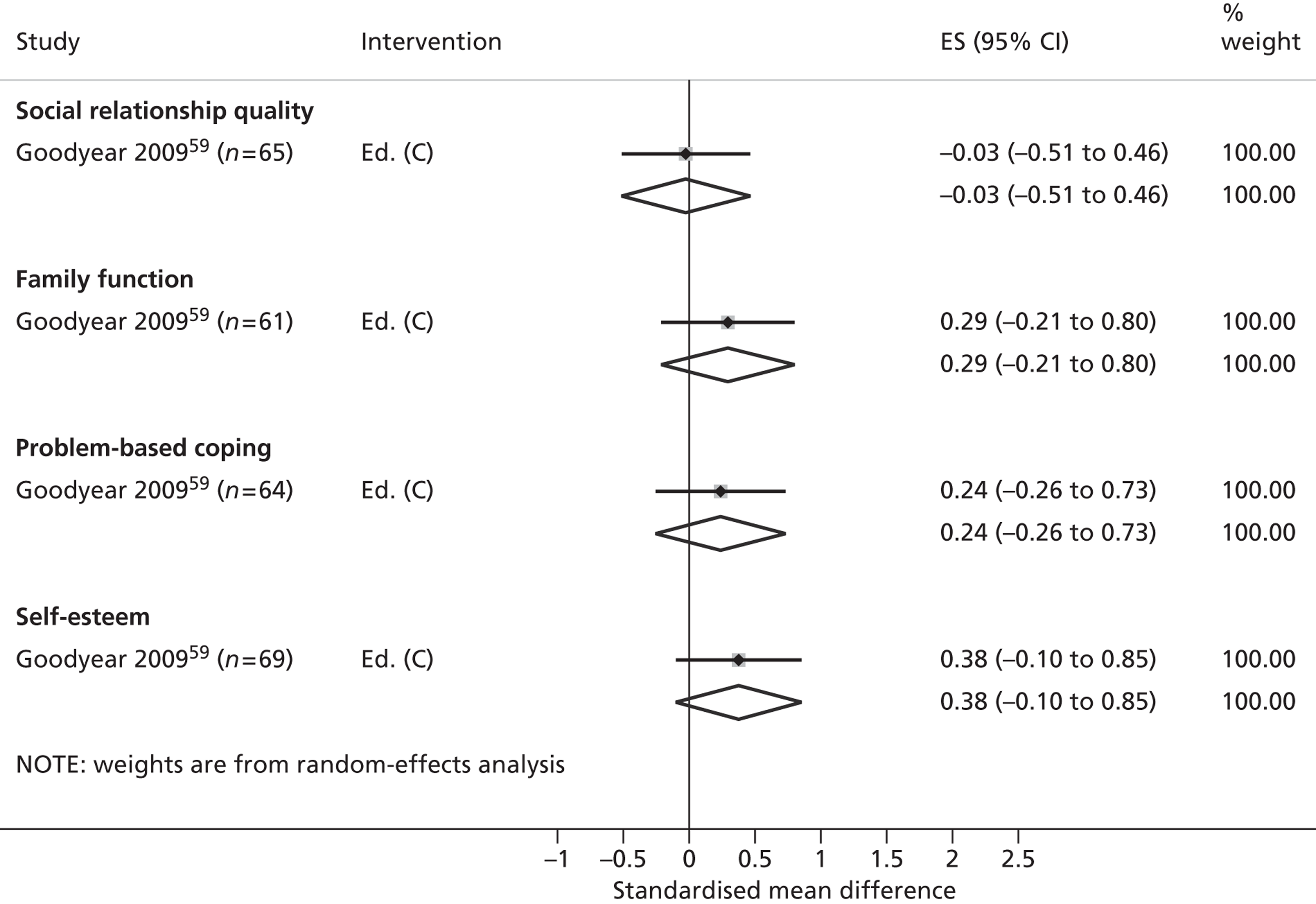
Evidence from uncontrolled studies
Four uncontrolled studies were eligible for inclusion in the synthesis. 62,120–122 The study contexts, populations, types of interventions and outcomes reported by these studies were extracted and summarised solely for the purposes of future research priority setting and are included in Tables 2 and 3. The specific characteristics relevant to each study are presented in Appendix 7 within the context of the data extraction sheet used to record individual study information. Publication dates for the four studies ranged from 1992 to 2006, with all but one study being conducted outside the UK. 120
The four studies employed a wide range of recruitment pathways. Recruitment contexts variously included adult psychiatric and general hospital referrals,62 prepaid health plans,62 voluntary sector agencies120,122 and child-centred coping initiatives. 121 All employed broad recruitment criteria that targeted participants experiencing a mix of parental mental health disorders. The overall proportion of the sample affected by serious parental mental illness ranged from 62% to 75% with a mean of 70%. Diagnoses included schizophrenia,121,122 bipolar disorder62,120–122 and personality disorder,120,122 recruited either in combination with MDD62,120,122 or other primary mental health diagnoses. 121 Alternative diagnoses, when reported, comprised major depression both inside and outside the postpartum period62,120–122 and non-specified anxiety disorders. 121,122
In sharp contrast to the RCTs that were identified, three of the four uncontrolled studies focused on older children or the parents of children aged > 7 years. 62,121,122 The majority of these studies spanned primary school and adolescent age ranges although only one was explicit in including children who resided separately from their parents. 122 Children’s residency arrangements remained unclear in the other three.
In accordance with the target age range of the participating children, all but one of the interventions120 actively involved the child. Two interventions directly targeted children and shared a similar group psychoeducational model aimed at enhancing children’s well-being and/or resiliency to parental mental illness. 121,122 One intervention delivered a brief family-based psychotherapy with the primary aim of enhancing parenting or family function. 62 The fourth focused on addressing parents’ immediate parenting, family and social needs. 120 Delivery models were most frequently face-to-face interventions delivered in a group format. Interventions were primarily delivered by mental health clinicians and all took place outside the home. Only two of the four studies provided full information regarding the length, duration or intensity of their interventions. 121,122 Both studies reported on group-based psychoeducational interventions for children, with the overall amount of face-to-face guidance totalling 13.5122 and 18 hours. 121
None of the four uncontrolled studies reported validated measures of children’s QoL or emotional health. However, in accordance with the observed predominance of child-orientated interventions, two of the four studies did assess secondary child-centred outcomes, specifically their mental health literacy,121 self-esteem121,122 and self-assessed coping. 122 The remaining studies assessed secondary parent outcomes, in this case parental mental health status120 and parenting behaviour. 62 Three of the four studies reported short-term data that was collected within the first 6 months of intervention commencement. 62,121,122 The fourth study failed to specify the timing of its outcome assessment. 120
Economic evidence
No full or partial economic studies were located in this phase of the review.
Discussion: implications for practice
As outlined in Chapter 1, current UK policy advocates greater service provision and support for families and children affected by parental mental illness. 2 Generic child-centred policies also aim to enhance the well-being of all UK children through the increased co-ordination and delivery of local authority services. 17,86,87
This synthesis defined serious parental mental illness to include schizophrenia, schizoaffective disorder, puerperal and non-puerperal psychoses, personality and borderline personality disorders and bipolar disorder. With regard to this population, national and international research effort is lacking. Following a systematic and comprehensive search, only seven controlled trials were identified, three of which followed a randomised or quasi-randomised design. Moreover, the few RCTs that were identified were all early studies from the USA, aimed at evaluating interventions for maternal psychosis. The generalisability of these data to the contemporary UK health-care system remains unclear. Interventions were heavily targeted towards group-based, parent-orientated models aimed at enhancing parenting behaviours in the mothers of children aged ≤ 12 years. Rigorous evidence of the effect of community-based interventions on QoL outcomes for older children and adolescents remains scarce.
Subjective child-centred QoL measures are strikingly absent from the existing evidence base. This omission is most likely an artefact of the observed predominance of interventions aimed at the parents of younger children and infants. Challenges in measuring subjective QoL outcomes in young children may make these outcome measures more difficult to ascertain. No validated measures of children’s overall QoL or emotional well-being were reported by any of the randomised trials identified for this review. Secondary outcomes, when available, were primarily restricted to observer ratings of children’s behaviour and cognitive function. Meaningful interpretation of these data is limited. High-quality trials are lacking and insufficient data are available to examine potential associations between clinical effect and user characteristics, or clinical effect and intervention format, content or target participants.
Economic evaluations are similarly absent. A lack of economic evidence combined with a heterogeneous mix of poor quality clinical studies and no clear evidence of promising effect currently makes decision modelling infeasible. VOI analysis is therefore also redundant.
Contemporary evidence of effect remains confined to a small number of non-randomised and uncontrolled studies that, by implication of their design, inevitably suffer a high risk of bias. Taken together, these studies begin to reflect a subtle shift in research effort away from parent-based interventions towards more child-centred initiatives aimed at school-aged children and adolescents. These interventions typically recruit children of parents with a broader mix of mental health diagnoses and are more likely to include the children of both mothers and fathers with SMI. Such initiatives typically place greater emphasis on improving or maintaining the subjective aspects of children’s well-being and, in doing so, are more likely to report the child-centred, esteem-based outcomes prioritised by our stakeholders. Nonetheless, a paucity of evidence prevents data pooling and the failure of these studies to randomise their participants prohibits any meaningful interpretation of intervention effects.
It is acknowledged that a number of studies (n = 13) were excluded from the current review because only a minority (< 50%) of participants experienced serious parental mental illness. A limited number of other studies (n = 7) were also excluded because the nature and/or distribution of parent’s mental illnesses could not be ascertained (see Appendix 5). It is possible that these studies may still contain relevant contextual information for those seeking to develop and evaluate interventions for this population. Three studies in particular, for example, reported a notable proportion of parents with SMI (46–48%) and in doing so only marginally missed our inclusion criteria. 124–126 All of these studies, however, were small-scale, uncontrolled evaluations with a high risk of bias.
On the basis of existing evidence, it is not yet possible to come to any firm conclusions regarding the clinical effectiveness or cost-effectiveness of community-based interventions for improving or maintaining QoL in children of parents with SMI. No clear recommendations for service delivery models can be made.
Chapter 5 The clinical effectiveness and cost-effectiveness of community-based interventions to improve or maintain quality of life in the children of parents with severe depression
In phase 2 of the review, we present a synthesis of all available evidence relating to the effectiveness of community-based interventions for improving QoL in children whose parents suffer from severe unipolar depression. Operational definitions of SMI remain inconsistent in their inclusion of this disorder. However, stakeholder consultation suggested that the children of severely depressed adults are likely to undergo qualitatively similar disruptions to their care-giving environments to the children of parents with other serious mental health difficulties. In light of the paucity of evidence described in Chapter 4, it is anticipated that this synthesis may hold value for future direction and practice.
Identifying and classifying severe depression
Definitions of SMI are consistent in encompassing severe affective mood disorders. Unipolar depression remains diagnostically distinct from bipolar depression, largely because of the lack of manic or hypomanic episodes. However, unipolar depression is not inevitably a milder disorder and different graduations of severity exist. For the purposes of the current review, it was necessary to distinguish between severe unipolar depression and more moderate disorders of lesser relevance to the commissioning brief.
Operationalising a definition of severe depression to capture the population of interest is challenging. A number of different depression rating scales and screening instruments have been validated for use in research and practice, many of which propose to discriminate between different severities of illness. While useful for monitoring changes, these scales are limited in their ability to diagnose the presence or absence of a depressive disorder. Limitations of such systems include their failure to take account of illness trajectory (i.e. prior or recurrent depressive episodes or to account for periods of partial remission) and context (i.e. alternative reasons for symptoms such as grief, stressful life events or physical illness) as well as inconsistencies in symptom count ranges, diagnostic cut-off scores and correlations between instruments. Symptom severity and degree of functional impairment correlate highly,127 but in individual cases this may not be the situation and some mildly symptomatic individuals may have marked functional impairment while some people who are severely symptomatic may, at least for a period of time, maintain good function.
Consideration of the severity and impact of a parental mood disorder thus demands more than a mere quantification of symptoms. UK NICE guidelines128 emphasise that for the term ‘severe’ to be of clinical value, it must imply a greater-than-usual degree of impairment and/or a greater intensity of treatment to achieve recovery. Both ICD-10109 and DSM-IV108 systems assign diagnoses according to the severity, duration and course of symptoms in conjunction with the degree of functional impairment present. However, while ICD-10 is explicit in discriminating between severe and mild to moderate levels of depression, DSM-IV criteria only distinguish between major and minor depressive disorders. 108 Additional DSM-IV diagnostic categories (such as psychotic depression) are available, but these rarely form distinct clinical categories or research classifications and are thus not normally operationalised within evidence syntheses. 128
Defining severe depression in the review
Consultation between the research team, review advisory panel and stakeholder groups indicated that any definition of severe depression adopted by the review would have to meet multiple criteria in order to qualify as a SMI. These criteria comprised high symptomatology, significant functional impairment, marked episodic duration and a chronic or recurrent course. In recognising that any single assessment of severity may be insufficient to fully capture all of these dimensions, we prioritised diagnostic classifications over symptom profiles. Eligible diagnoses were ICD-10 severe depression and DSM-III/IV MDD with or without postpartum onset. This approach aimed to minimise potential bias arising from the inclusion of participants experiencing severe yet non-recurrent depressive affect (e.g. grief responses) as well as bias from the exclusion of studies in which participants had a lifetime diagnosis of severe depression yet failed to display florid symptoms at the time of recruitment.
Studies were eligible for inclusion in the review if the majority of the sample (> 50%) had a primary diagnosis of ICD-10 severe or DSM-III/IV MDD according to the Research Diagnostic Criteria, the DSM-IIIR/IV,108 the ICD109 criteria or other validated diagnostic instruments. When eligible diagnoses were indicated but the proportion of participants remained unclear, depressive symptomatology measured through self-rated or clinician-rated validated instruments was also examined. Cut-off values for these instruments were established post hoc from the empirical literature and studies were only included if a clinical diagnosis was present and pooled mean baseline symptoms were equal or greater to these scores. Eight different symptom scales were shared across the studies retrieved by our searches. These scales and the cut-off criteria that were used to judge study eligibility in the current synthesis are displayed in Table 6. Further details of the rationale underpinning the selection of these criteria are provided in Appendix 8.
| Measure | Cut-off for severe depression | Study number(s) |
|---|---|---|
| BDI-I/BDI-II | ≥ 30/≥ 29 | (129) |
| 17-/25-item HRSD | ≥ 25/≥ 28 | (128) (130)–(132) |
| MADRS | ≥ 31 | (130) (132) (133) |
| CES-D | ≥ 27 | (134) (135) |
| PHQ-9 | ≥ 20 | (136) |
| EPDS | ≥ 20 | (137) (138) |
Methods of review
Studies were identified via the database searches and review strategies outlined in Chapter 3 and the population inclusion criteria specified above.
Size of the evidence base
In total, 41 studies, reported in 58 papers, were eligible for inclusion. Of these, 26 were randomised or quasi-randomised trials with participants allocated either randomly or by sequential or matched assignment. A further four were non-randomised studies in which participants were allocated on the basis of service availability or patient preference. The remaining studies (n = 11) were all uncontrolled designs.
A further 89 studies were identified but subsequently excluded, the majority of which failed to meet our criteria for severe parental depression. Twenty three reported fewer than half of their parent sample as having a clinical confirmed diagnosis of ICD-10 severe or DSM-III/IV major depression, 21 failed to confirm diagnoses and did not meet criteria for severe depressive symptoms at baseline and 31 failed to provide sufficient information to determine depression severity. A further 10 studies were excluded owing to a failure to meet our prespecified intervention inclusion criteria and two failed to provide any relevant outcome data. Two studies recruited depressed pregnant women but did not report any eligible postpartum outcomes. Please see Appendix 5 for a list of references of the studies that were excluded, together with their specific reasons for exclusion.
Economic evaluation
One study reporting the results of an economic evaluation or containing cost- or resource-use data was located in this phase of the review. 116 This study was a cost-effectiveness analysis of psychiatric day hospital compared with routine primary care for the treatment of postnatal depression, carried out as part of a non-randomised prospective cohort study. 139
Thirteen studies were identified but subsequently excluded on review of the papers (including two with a broad mental health focus also considered but excluded from the SMI review). One was excluded because there was no focus on children or adolescents aged 0–17 years or their parents, 11 were excluded because the proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity, and one was excluded because the study did not involve evaluation of a community-based psychosocial intervention. See Appendix 6 for a full list of the references of studies excluded from the economic evaluation.
Approach to evidence synthesis
As per protocol, ESs were calculated for all RCTs. The clinical and methodological characteristics of all eligible non-randomised and uncontrolled designs were summarised for the purposes of future research priority setting.
Evidence from randomised controlled trials
Twenty-six trials were included in this synthesis. Tables 7–9 summarise the study contexts, populations, types of interventions and outcome variables in these trials. Study quality indicators, including randomisation procedures and methods of allocation concealment, are presented in Table 10. The specific clinical and methodological characteristics relevant to each trial are presented in Appendix 9 within the context of the data extraction sheet used to record individual study information.
| Criterion | Characteristic | n (%) RCTs | Study number(s)a |
|---|---|---|---|
| Country | USA | 11 (42) | (64) (66) (67) (140)–(147) |
| Australia | 4 (15) | (65) (148)–(150) | |
| UK | 4 (15) | (151)–(154) | |
| Canada | 3 (12) | (63) (155) (156) | |
| France | 1 (4) | (157) | |
| Pakistan | 1 (4) | (158) | |
| Chile | 1 (4) | (138) | |
| Sweden | 1 (4) | (159) | |
| Recruitment context | Mental health inpatient services | 3 (12) | (63) (140) (141) |
| Mental health community services | 6 (23) | (63) (64) (142)–(144) (150) | |
| Media adverts | 7 (27) | (63) (64) (66) (143)–(145) (148) | |
| General health services (primary care) | 5 (19) | (64) (66) (67) (141) (158) | |
| Child health services | 4 (15) | (65) (147) (149) (159) | |
| Obstetrics/postnatal services | 7 (27) | (138) (145) (149) (151) (155)–(157) | |
| Epidemiological records | 4 (15) | (146) (152)–(154) | |
| Parent | All mothers | 21 (81) | (64) (66) (67) (138) (140) (144)–(159) |
| Mixed (> 50% female) | 4 (15) | (63) (141)–(143) | |
| Unclear/not reported | 1 (4) | (65) | |
| % severe depressionb diagnosis | 100% | 17 (65) | (64) (65)–(67) (138) (140) (143) (144) (146) (147) (150) (152) (155)–(159) |
| ≥ 75–99% | 5 (19) | (63) (141) (142) (145) (154) | |
| ≥ 50–74% | 2 (8) | (151) (153) | |
| Unclear, judged on symptom scores | 2 (8) | (148) (149) | |
| Severe mean baseline symptoms | Yes | 4 (15) | (140) (148)–(150) |
| No | 22 (85) | (63)–(67) (138) (141)–(147) (151)–(159) | |
| Other diagnoses in sample | Minor affective disorders | 7 (27) | (63) (141) (142) (145) (151) (153) (154) |
| Bipolar | 1 (4) | (141) | |
| Schizophrenia-affective disorder | 1 (4) | (141) | |
| Exclusion criteria | Domestic abuse | 2 (8) | (145) (147) |
| Group attendance barriers | 1 (4) | (63) | |
| Chronic depression/anxiety disorders | 2 (8) | (146) (151) | |
| Substance misuse | 8 (31) | (138) (141)–(143) (145) (147) (150) (151) | |
| Current crisis | 8 (31) | (138) (141) (142) (145) (147) (149) (150) (156) | |
| In current treatment | 4 (15) | (138) (145) (156) (157) | |
| Welfare dependent | 1 (4) | (64) | |
| Parent/child learning difficulties | 4 (15) | (65) (143) (146) (154) | |
| Parent child physical illness | 6 (23) | (145) (147) (151) (152) (156) (158) | |
| SMI | 12 (46) | (138) (141)–(143) (145)–(150) (154) (156) | |
| Local geography | 2 (8) | (151) (152) | |
| Language barriers | 5 (19) | (148) (149) (151) (154) (157) | |
| Not co-located | 2 (8) | (147) (154) | |
| % BME parents | 100% white Caucasian | 3 (12) | (67) (148) (157) |
| ≤ 50% BME | 12 (46) | (64) (66) (141)–(144) (146) (147) (149) (150) (155) (156) | |
| > 50% BME | 1 (4) | (145) | |
| Not reported | 10 (38) | (63) (65) (138) (140) (151)–(154) (158) (159) | |
| Child target age range | 0–2 years | 18 (69) | (64) (66) (67) (138) (144)–(146) (148)–(153) (155)–(159) |
| 3–4 years | 2 (8) | (65) (154) | |
| 5–12 years | 7 (27) | (63) (65) (140)–(143) (147) | |
| 13–17 years | 5 (19) | (63) (140) (141) (143) (147) | |
| Child gender | ≥ 50% female | 4 (15) | (64) (66) (67) (147) |
| < 50% female | 7 (27) | (65) (140) (142)–(144) (150) (155) | |
| Not reported | 15 (58) | (63) (138) (141) (145) (146) (148) (149) (151)–(154) (156)–(159) | |
| Comparator | Waiting list | 5 (19) | (66) (140) (142) (146) (148) |
| Standard care | 16 (62) | (63) (64) (67) (138) (144) (145) (147) (149) (150) (152)–(154) (156)–(159) | |
| Active intervention | 9 (35) | (65) (66) (141) (143) (149) (151) (152) (154) (155) |
| Criterion | Characteristic | n (%) RCTsa | Study number(s)b |
|---|---|---|---|
| Model | Psychoeducation | 6 (16) | (63) (141)–(143) (155) |
| Psychotherapy | 30 (79) | (64)–(67) (138) (140) (141) (143) (145)–(154) (156)–(159) | |
| Extended care | 1 (3) | (144) | |
| Psychosocial | 1 (3) | (154) | |
| Objective | Parent well-being | 23 (61) | (66) (138) (145)–(153) (155)–(159) |
| Parenting relationship | 9 (24) | (63)–(65) (141)–(143) (154) | |
| Child well-being | 1 (3) | (140) | |
| Hybrid/dual focus | 5 (13) | (65) (66) (67) (142) (143) | |
| Target | Predominantly parent | 31 (82) | (63)–(66) (138) (141) (144)–(159) |
| Predominantly child | 1 (3) | (140) | |
| Parent–child dyad or family | 6 (16) | (66) (67) (141)–(143) | |
| Setting | Home | 9 (24) | (64) (142) (144) (152) (153) (157) (158) |
| Community/clinic | 18 (47) | (66) (67) (138) (140) (145) (147)–(151) (154) (156) | |
| Mixed | 5 (13) | (65) (143) (159) | |
| Unclear/not reported | 6 (16) | (63) (141) (146) (155) | |
| Delivery | Face to face | 37 (97) | (63)–(66) (67) (138) (140) (141) (143) (144)–(159) |
| Non-face to face | 1 (3) | (142) | |
| Individual | 25 (66) | (64) (65) (141) (142) (144)–(147) (149) (151) (152) (153) (155)–(159) | |
| Group | 13 (34) | (63) (66) (67) (138) (140) (141) (143) (148) (150) (154) | |
| Personnel | Psychologist/psychology students | 20 (52) | (63) (65)–(67) (142) (143) (147)–(149) (151) (154) (156) (157) |
| Psychiatrist | 2 (5) | (142) (147) | |
| General/unspecified nurse | 6 (16) | (63) (138) (144) (147) (149) (159) | |
| Unspecified clinician/therapist | 3 (8) | (64) (141) (145) | |
| Midwives/health visitor/community health worker | 4 (11) | (138) (153) (154) (157) | |
| Social worker | 6 (16) | (63) (66) (140) (143) (147) | |
| Psychotherapist/counsellor | 4 (11) | (146) (152) | |
| Nursery nurse | 1 (3) | (154) | |
| Not reported | 3 (8) | (150) (155) | |
| Monitoring | Training given | 28 (74) | (63) (65)–(67) (138) (141) (143) (145) (146) (149)–(151)–(154) (156)–(159) |
| Supervision received | 24 (63) | (65)–(67) (138) (141) (143)–(147) (150)–(152) (154) (157)–(159) | |
| Not possible/not reported | 5 (13) | (140) (142) (148) (155) | |
| Session duration | Up to 1 hour | 14 (37) | (65) (138) (141) (142) (146) (151) (153) (156) (157) (159) |
| 1–2 hours | 11 (29) | (63) (65)–(67) (140) (148) (150) (154) | |
| Not reported/not applicable | 15 (39) | (64) (141) (143)–(145) (147) (149) (152) (155) (158) | |
| Session frequency | Weekly | 22 (58) | (63) (64) (66) (67) (138) (140) (146) (148) (149) (152) (153) (155)–(157) (159) |
| Variable | 5 (13) | (143)–(145) (158) | |
| Not reported/not applicable | 11 (29) | (65) (141) (142) (147) (150) (151) | |
| Total duration | Up to 8 weeks | 11 (29) | (63) (138) (140) (142) (145) (149) (150) (153) (157) (159) |
| 9–20 weeks | 19 (50) | (65)–(67) (146) (148) (151) (152) (154)–(156) | |
| 6 months to 1 year | 5 (13) | (64) (143) (144) (158) | |
| Unclear/not reported | 3 (8) | (141) (147) | |
| Total scheduled contact | 0–11 hours | 11 (29) | (138) (140)–(142) (151) (153) (157) (159) |
| 12–20 hours | 7 (18) | (63) (65) (66) (146) (148) (156) | |
| > 20 hours | 5 (13) | (66) (67) (150) (154) | |
| Not reported/not applicable | 15 (39) | (64) (141) (143) (145) (147) (149) (152) (155) (158) |
| Criterion | Characteristic | n (%) RCTs | Study number(s)a |
|---|---|---|---|
| Primary outcomes | QoL | – (–) | – |
| Emotional well-being | 7 (27) | (63) (66) (67) (140) (144) (147) (160) | |
| Secondary outcomes | Physical health | 1 (4) | (158) |
| Safety | – (–) | – | |
| Social function/behaviour | 12 (46) | (65)–(67) (140) (142) (144) (147) (148) (152) (154) (160) (161) | |
| Social relationship quality | 1 (4) | (63) | |
| Recreational engagement | 1 (4) | (63) | |
| Family function | 2 (8) | (63) (146) | |
| Parent–child relationship | 8 (31) | (63) (66) (67) (144) (150) (152) (158) (160) | |
| Parent mental health symptoms | 19 (73) | (63) (64) (66) (67) (138) (144)–(147) (149) (150) (152)–(154) (156)–(159) | |
| Cognitive function | 5 (7) | (64) (66) (67) (144) (152) | |
| Problem-based coping | – (–) | – | |
| Mental health literacy | 1 (4) | (142) | |
| Self-esteem | 2 (8) | (140) (141) | |
| Outcome assessor | Child report | 4 (15) | (140) (141) (143) (147) |
| Parent report | 25 (96) | (63)–(67) (138) (140)–(150) (152)–(155) (157)–(159) (161) | |
| Observer report | 10 (38) | (64)–(67) (143) (144) (146) (151) (152) (158) | |
| Data reporting | Continuous (i.e. mean, SD) | 23 (88) | (64) (66) (67) (140) (141) (143)–(145) (148) (149) (151) (152) (157) |
| Dichotomous (i.e. percentage) | 6 (23) | (63) (65) (138) (141) (142) (146) (147) (150) (152)–(156) (158) (159) | |
| Insufficient data for standardised ES | 8 (31) | (66) (141) (146)–(148) (151) (152) (156) | |
| Timing of follow-up assessment | 0–6 months | 23 (88) | (63) (65)–(67) (138) (140)–(143) (145)–(158) |
| 7–12 months | 6 (23) | (143) (147) (151) (152) (154) (162) | |
| > 12 months | 5 (7) | (64) (152) (155) (162) (163) |
| Criterion | Characteristic | n (%) RCTs | Study number(s)a |
|---|---|---|---|
| Allocation procedure | Random number generator/computer | 8 (31) | (63) (138) (143) (146) (150) (151) (153) (156) |
| Random number table | 2 (7) | (141) (158) | |
| Random coloured balls | 1 (4) | (152) | |
| Random method not stated | 11 (42) | (64) (65) (140) (142) (145) (147)–(149) (154) (155) (159) | |
| Quasi-randomised, sequential | 3 (12) | (66) (67) (157) | |
| Quasi-randomised, matched | 1 (4) | (144) | |
| Allocation concealment | Not reported/unclear | 16 (62) | (64) (65) (140)–(142) (145)–(148) (150)–(153) (155) (156) (159) |
| Not applicable (quasi-randomised) | 4 (15) | (66) (67) (144) (157) | |
| Sealed envelopes | 4 (15) | (63) (138) (143) (154) | |
| Independently hosted | 2 (7) | (149) (158) | |
| Unit of allocation | Parent/parent blocks | 15 (58) | (67) (138) (145)–(153) (155)–(157) (159) |
| Child | 1 (4) | (140) | |
| Mixed/family/family blocks | 9 (35) | (63)–(66) (141)–(144) (154) | |
| Health service clusters | 1 (4) | (158) | |
| Blinded participants/personnel | No/unclear | 26 (100) | (63)–(67) (138) (140)–(159) |
| Blinded outcome assessment | Yes | 5 (19) | (66) (143) (144) (151) (153) |
| No/unclear | 21 (81) | (63)–(65) (67) (138) (140)–(142) (145)–(150) (152) (154)–(159) | |
| Selective outcome reporting | No | 13 (50) | (64) (65) (142) (144)–(146) (150) (152) (154)–(158) |
| Yes/unclear | 13 (50) | (63) (66) (67) (138) (140) (141) (143) (147)–(149) (151) (153) (159) | |
| Sample size at baseline | < 50 | 9 (35) | (63) (65)–(67) (140) (148) (155) (156) (159) |
| 50–100 | 8 (31) | (142) (144) (145) (147) (149)–(151) (153) | |
| 100 + | 6 (23) | (64) (141)b (143) (146) (152) (154) | |
| 200 + | 3 (12) | (138) (157)b (158) | |
| Attrition rate post intervention | 0–10% | 10 (38) | (66) (67) (138) (140)–(143) (146) (152) (153) (155) (156) (158) (159) |
| 11–20% | 4 (15) | (65) (147) (154) | |
| > 20% | 12 (46) | (63)–(65) (144) (145) (147)–(151) (154) (157) | |
| Method of analysis | ITT/complete data set | 5 (19) | (66) (140) (151) (155) (159) |
| Incomplete data set | 21 (81) | (63)–(65) (67) (138) (141)–(150) (152) (153) (154) (156)–(158) | |
| Overall risk of bias | High risk | 4 (15) | (66) (67) (144) (157) |
| Unclear risk | 21 (81) | (63)–(65) (138) (141)–(143) (145)–(156) (159) | |
| Low risk | 1 (4) | (158) |
Overall, the included trials provided relatively contemporary research evidence. Publication dates for the 26 trials ranged from 1985 to 2011, with 16 being published within the last 10 years (2002 onwards). Two trials were conducted in developing countries,138,158 the remainder originated from a variety of different countries with potentially different health-care systems (see Table 7) and four trials were conducted within the UK. 151–154
Parent and child populations
Consistent with our eligibility criteria, all trials included in our synthesis had samples in which the majority of parents had current or lifetime experience of severe unipolar depression. Twenty-four trials reported > 50% of parents as having a confirmed clinical diagnosis of DSM-III/IV MDD; only two of these trials reported a symptom profile commensurate with severe depression at baseline. 140,150 Overall proportions of MDD within these trials ranged from 59% to 100%, with 17 (71%) reporting the entirety of their parental sample as suffering from major depression (see Table 7). The remaining two trials148,149 failed to specify the precise proportions of parental diagnoses but did nonetheless report pooled mean baseline symptoms commensurate with a severely depressed population [mean baseline Beck Depression Inventory (BDI) > 30, Edinburgh Postnatal Depression Scale (EPDS) > 20,148 mean BDI-II > 29]. 149 Owing to the small number of studies involved, potential differences in the effects reported by these trials could not be explored.
Only one trial included in the synthesis reported recruiting parents with SMI alongside severe depression. 141 Thirteen of the 26 trials (50%) explicitly excluded parents with psychosis, schizophrenia, personality disorders and/or bipolar disorders. 63,138,141–143,145–150,154,156 Three other trials excluded participants with substance abuse141,151 and/or a severe illness not specified as either mental or physical health. 158 Our a priori distinction between SMI and severe unipolar depression was thus reflected in the existing literature. The trials that focused on severe depression predominantly targeted female participants, with 21 out of the 26 trials (81%) recruiting only mothers (see Table 7). The ethnic status of participants was fully or partially reported in 16 trials (62%) and heavily focused on parents of European, Caucasian descent (see Table 7).
The vast majority of included trials aimed to ameliorate the effects of severe parental depression in early childhood. Eighteen of the 26 trials (69%) targeted mothers of infants aged < 2.5 years, 15 of which (58%) targeted mothers of children in the first year of life. Two studies recruited women diagnosed with MDD in the antenatal period. 145,158 Two studies evaluated interventions aimed at preschool or primary school-aged children (aged 2.5–9 years)65,154 and six studies evaluated interventions relevant to both primary school-aged children and beyond (6–18 years). 63,140,141–143,147 Two trials required children to have a clinically diagnosed conduct disorder65 or mental health difficulty,147 while six trials excluded children with developmental or congenital disabilities,65,152,154 behavioural disorders143 or serious mental health difficulties. 142,164 Only three trials reported on children’s residential status,143,147,154 although the implicit assumption in all trials was that children were co-residing with their parents.
Participants were recruited via a number of different pathways including newspaper advertising,63,64,66,143–145,148 hospital or health professional referrals63–67,138,140–145,147,149–151,155–159 and community health registers or birth records. 146,152–154 Clinical recruitment spanned both primary and secondary mental health and non-mental health services, including maternity, psychiatric and general hospitals, obstetrics and gynaecology clinics, postnatal or maternal and child health centres, adult community and outpatient mental health services, paediatric mental health clinics and non-specified health and welfare agencies (see Appendix 9). In one trial, child participants were also recruited directly from educational settings65 and two studies, both conducted by the same author, recruited parents from a specialist reproductive mental health programme. 155,156
Interventions and comparators
The 26 RCTs provided a total of 37 comparisons, of which 26 (70%) incorporated a waiting list (n = 7) or ‘treatment as usual’ control (n = 19).
Altogether, 38 active interventions were identified and classified as one of four main intervention models: psychotherapeutic (n = 30, 79%), psychoeducational (n = 6, 16%), psychosocial (n = 1, 3%) or extended care (n = 1, 3%). Some trials evaluated more than one intervention and, therefore, number in the tables may not always total 100%. Full definitions of the scope and nature of these different intervention models are provided in Box 3, Chapter 4. Both within and across model groupings, marked heterogeneity in intervention content, objectives, target populations and/or delivery formats was observed (see Table 8).
The vast majority of psychotherapeutic interventions (21 out of 30, 70%) were aimed primarily at reducing the severity of parents’ depressive symptoms. The nature of these interventions varied widely but most frequently spanned cognitive–behavioural138,148,149,151,152,156–158 and interpersonal approaches. 66,145–147,150 Psychodynamic152,157 and non-directive supportive therapies152,153,159 were reported less frequently. Four out of the 30 psychotherapeutic interventions (13%) targeted parenting skills or family function64,65,141,154 and four targeted parenting skills in combination with parental65–67 or child well-being. 143 Only one psychotherapeutic intervention (3%) was aimed solely at improving child well-being. 140 This intervention is described in further detail below.
Overall, 14 interventions (37%) sought to enhance some aspect of parenting or family function. These interventions included both psychotherapeutic models (n = 8) and other psychoeducational63,141–143 psychosocial154 or extended care approaches. 144 Eight were explicit in their theoretical underpinnings, with interventions variously incorporating principles of behavioural theory,65,143,154 attachment theory,64,66,67 social learning theory,63,66,67 psychodynamic theory,66,67 Soviet cognitive–linguistic theory,66,67 family systems theory63,66,67 and Sanders and Dadds’165 model of parent training. 65 Five interventions (including one extended care model) augmented parenting with other care components. 65–67,143,144 These included, but were not limited to, a CBT intervention aimed at ameliorating mothers’ depressive symptoms65 and cognitive–behavioural143 or developmental therapies66,67 aimed specifically at the child.
Overall, the vast majority of interventions (n = 31, 82%) were aimed predominantly, or solely, at the depressed parent. Comparatively fewer interventions identified children as potential agents of change. Fourteen interventions reported family participation or the participation of the parent–child dyad. 63–67,141–144,154 However, only six of these delivered an active and structured intervention directly to the child. 66,67,141–143 One trial intervened solely with children. 140 This trial evaluated a psychotherapeutic intervention based on CBT problem-solving techniques for children aged 8–13 years. In total, only 13 out of 38 interventions involved partners. 63,65–67,142,143,148,150,155
Delivery models were most frequently individual, face-to-face interventions (see Table 8). Commensurate with the heterogeneity that was observed in recruitment pathways, the interventions were delivered by a broad range of health- and social-care professionals including general practitioners (GPs), clinicians, social workers, clinical psychologists, psychiatrists, psychotherapists, midwives and community health workers (see Table 8). All but nine interventions took place either partially or fully outside the home. 64,142,144,152,153,157,158 When reported, intervention duration ranged from 50 minutes to 1 year, with a modal duration of 8 weeks. The total amount of guidance ranged from 50 minutes to 24 hours with a mean of 11.5 hours. Ten trials failed to provide sufficient information regarding the intensity and/or duration of their interventions. 64,141,143–145,147,149,152,158,166
Outcomes
Substantial heterogeneity in outcome measures was observed (see Appendix 9, Table 39). Primary outcomes for the purposes of this evidence synthesis comprised validated measures of children’s QoL and emotional well-being. No trials reported validated QoL measures. Measures of emotional well-being were reported by seven trials, although the precise nature of these outcomes varied according to the ages of the children involved. Trials conducted with school-age children were able to report validated measures of anxiety and depression. 63,140,147 In contrast, studies with infants remained dependant on observer or parent-rated measures of infant affect. 66,67,144,160 These latter outcomes were assessed via referenced or standardised methods with established psychometric properties and were therefore retained for subsequent synthesis.
Secondary outcomes were reported by all of the included trials (see Appendix 9). Commensurate with the predisposition towards parent-centred interventions, the most frequently assessed secondary outcome domains related to parents’ mental health symptoms (see Table 9) and notably fewer trials reported child-based outcomes. When these were reported, they focused most frequently on observer ratings of children’s behaviour and social function. Subjective, self-reported outcomes relating to children’s social relationship quality, recreational engagement or self-actualisation were rarely collected. Most trials reported more than one secondary outcome domain and thus percentages in the tables may not total 100%.
Marked heterogeneity in the measurement instruments used to quantify secondary outcomes was observed. Parental mental health symptoms were measured by a total of 16 different validated and referenced methods across the included trials, while parenting outcomes were assessed by 11 different specified and standardised means (see Appendix 9). Child behaviour and social functioning was assessed by eight different referenced and standardised means. Three trials provided conceptually relevant data measured on non-validated or non-specified scales. 141,142,158 Most trials used more than one outcome measure and, therefore, percentages in the table do not total 100%.
Extracted outcomes were presented as both continuous and dichotomous outcomes (see Table 9). Twenty-three trials provided short-term follow-up defined as up to 6 months post randomisation. In comparison, medium-term follow-ups of between 6 and 12 months’ duration were reported by only six trials,141,143,147,152,154,158 and longer-term follow-ups of > 12 months by five trials. 64,141,144,152,167 Two of the trials presenting long-term follow-ups remained clinically and methodologically distinct from those providing shorter-term outcomes. 64,144 One64 used a randomised design to evaluate a unique year-long psychotherapeutic intervention aimed at enhancing the mother–child relationship in families of children aged < 2 years and the other144 used a quasi-randomised design to compare an extended home care intervention with a treatment as usual control. This latter trial constituted the only study that contributed higher-level evidence relevant to this type of intervention model. Shorter-term outcomes were not reported by these trials.
Methodological quality ratings
The Cochrane Risk of Bias Assessment tool was used to quality appraise all RCTs. 82 Overall quality ratings were applied to the trials on the basis of their risk of selection bias and any additional biases arising from selective outcome reporting and/or sample attrition. Overall risk of bias was judged to be high in four trials66,67,144,157 and unclear in another 21 (see Table 10). Only one trial, conducted in a developing country, was judged to have a low risk of bias. 158 An overview of key study quality indicators is presented in Table 10 and full quality appraisal tables in Appendix 9.
Most variation occurred in terms of the trials’ randomisation and allocation procedures. Eleven out of the 26 trials (42%) reported adequate randomisation procedures, including random number or computer-generated sequences,63,138,141,143,146,150,151,153,156,158 or randomisation via the selection of concealed coloured balls. 152 Eleven trials did not provide any details of their randomisation methods and thus it was difficult to judge whether or not the methods used to allocate participants or conceal the allocation sequence were adequate (see Table 10). Three trials reported inadequate methods based on sequential approaches to group allocation66,67 or the matching of participants between groups. 144 A fourth trial reported alternate allocation to a preventative intervention or control group, with subsequent treatment comparisons only involving those who went on the develop depression. 157 In total, only six trials reported adequate allocation concealment. 63,138,143,149,154,158
Risks of performance and detection biases were also high. As with many trials of psychosocial interventions, the nature of the intervention prevented participant and personnel blinding. The majority of trials reported outcomes measured by parental, and to a lesser extent, observer report. Assessor blinding was only reported in five trials for a minority of outcomes related to parent mental health, parenting practices or infant affect. 67,143,144,151,153
Baseline sample sizes were typically small and ranged from 20148 to 903,158 with a median of 53 (see Table 10). Twenty-three trials (88%) reported sample attrition, with attrition rates for short-term outcomes (highest rate quoted when attrition varied by outcome) ranging from 0% to 81% with a median of 19%. The study reporting 81% attrition157 randomly allocated ‘at-risk’ participants to a preventative intervention prior to delivering treatment to a clinically depressed subsample. Post-intervention attrition for the remaining studies ranged from 0% to 48%. Reasons for attrition were inconsistently reported and bias from non-random dropouts was possible in 14 of the 26 trials (54%) (see Appendix 9, Table 35). One study that reported high levels of attrition at follow-up (43%) acknowledged this risk of bias and chose not to present their data. 63 In total, 21 of the trials (81%) analysed an incomplete data set post intervention and at follow-up.
Unpublished data from included trials
Effect sizes could not be calculated for eight studies across six outcome domains owing to insufficient data (see Appendix 9, Table 40). Study authors were contacted by email to request unreported information but no data could be obtained.
Evidence of clinical effect from randomised controlled trials
Meta-analysis was undertaken for two parent-centred outcomes (parental mental health symptoms and parenting behaviours) and two child-centred outcomes (child mental health and child behaviour and social function) for which sufficient data were available. With the exception of parental mental health symptoms, all meta-analyses were limited to short-term outcomes. A paucity of data prevented pooling of medium- and longer-term outcomes. These results are instead presented in a narrative format, grouped by QoL outcome domains.
Only one outcome (parental mental health symptoms) provided sufficient data to enable an exploration of heterogeneity. Our protocol identified a priori the characteristics that would be explored. These characteristics included the therapeutic target (i.e. parent, child, parent–child dyad or family based), the intervention content (i.e. psychoeducational/psychosocial, psychotherapeutic) and objectives (i.e. parenting, non-parenting perspectives), child age group (i.e. < 5 years, 5–11 years, 12–17 years), and parental mental health condition and child residency (i.e. colocated, forced or volitional separation, separation in crisis). However, the nature and availability of the data available meant that some of these characteristics were not applicable. Parental disorder was not relevant to this synthesis owing to its sole focus on severe unipolar depression and child residency was not reported in the majority of studies. In addition, child age ranges were found to span broad age bands that could not be easily divided according to those specified in our original protocol. Child age range was thus collapsed into two categories according to the distribution of the available data. Ultimately, formal examinations of heterogeneity were conducted on the following variables:
-
Child age, collapsed post hoc into infants (0–4 years) and children/adolescents (6–18 years). No studies included children aged between 4 and 6 years.
-
Intervention content, divided a priori into psychotherapeutic or non-psychotherapeutic (psychoeducational/psychosocial) approaches.
-
Intervention objectives divided a priori into parenting, non-parenting or combined perspectives.
-
Intervention target, collapsed into predominantly parent-orientated, child-orientated or dyadic/family approaches. Interventions were only classified as involving both parties if an active and structured child-centred intervention was reported. Informal play sessions facilitated by non-health professionals were not included.
Planned sensitivity analyses on trial quality were retained.
Comparisons of an active intervention versus a treatment as usual/waiting list control
Primary Outcomes
Children’s Quality of Life
Twenty-one trials compared an active intervention with a waiting list or treatment as usual control. No randomised trials measured validated QoL outcomes.
Children’s emotional well-being
Seven trials measured outcomes relevant to children’s emotional well-being. 63,66,67,140,144,147,160
Short-terms outcomes of up to 6 months post randomisation were measured by six trials63,66,67,140,144,160 but only reported by five. 63,66,67,140,160 These five trials provided a total of six comparisons and three comparisons were from quasi-randomised trials presenting a high risk of bias. 66,67
In total, the six comparisons reported on 213 participants using three different outcome measures. Four comparisons evaluated interventions for the mothers of children aged ≤ 2 years and reported observer ratings of infant affect. 66,67,160 Some trials report more than one comparison and, therefore, the number of references may be fewer than the number of comparisons being discussed. The remaining two comparisons evaluated interventions for children aged 6–18 years and measured symptoms of depression via validated self-report scales. 63,140 In total, five comparisons evaluated a therapeutic intervention;66,67,140,160 two of these targeted parents,66,160 two targeted parents and children66,67 and one targeted children. 140 One comparison evaluated parent-focused psychoeducation,63 three comparisons evaluated interventions with a parenting or family functioning component,63,66,67 two evaluated an intervention aimed at parental well-being66,160 and one evaluated an intervention aimed at child well-being. 140
Three comparisons reported standardised effects of at least small magnitude (standardised ES > 0.2), none of the standardised effects were statistically significant. 66,67,140 Two were from quasi-randomised trials presenting a high risk of bias. 66,67 A random-effects model was used to pool the data and the I2 index showed no more statistical heterogeneity than would be expected by chance (I2 = 0.0%, p = 0.842). The pooled ES was 0.06 (95% CI –0.20 to 0.33), suggesting no significant effect of intervention on children’s short-term mental health (Figure 6).
FIGURE 6.
Children’s short-term emotional well-being outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: evidence from RCTs. (C), child target; Ed., psychoeducation; IPT, interpersonal therapy; M-ITG, mother–infant therapy; (P), parent target; (PC), parent and child target. a, Quasi-randomised.
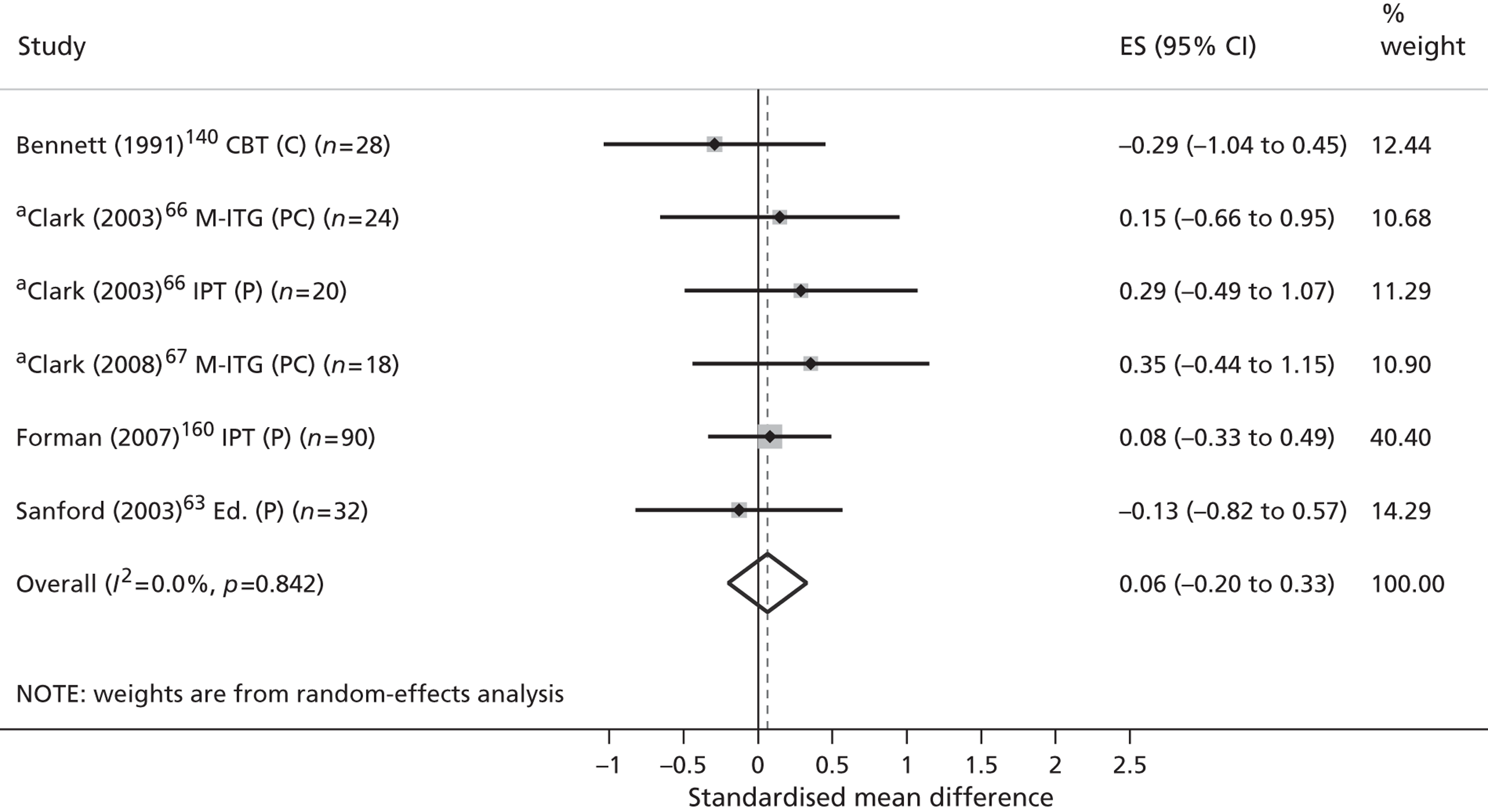
Dividing the trials according to their overall quality ratings suggested a trend in which the pooling of poorer quality trials66,67 demonstrated a small but non-significant effect (standardised ES 0.26, 95% CI –0.19 to 0.72). Pooling higher-quality trials 63,140,160 resulted in a non-significant effect (standardised ES –0.03, 95% CI –0.25 to 0.30). The limited number of comparisons contributing to this analysis, in conjunction with the heterogeneous mix of interventions, populations and outcomes included within it, means that these results should be interpreted with caution.
Medium-term child mental health outcomes were reported by one trial (n = 28). 147 A randomised design was used to compare treatment as usual to brief interpersonal psychotherapy aimed at the parents of children aged 6–18 years. The outcome was children’s self-reported depressive symptoms at 9 months. A large positive and significant effect was observed (standardised ES 1.62, 95% CI 0.75 to 2.49). No other evidence was available.
Long-term outcomes of > 12 months were reported by one trial (n = 98). 144 A quasi-randomised design was used to compare treatment as usual with an extended-care model incorporating home visitation, parenting education and care management for the parents of children aged 0–1 years. The outcome was observer ratings of infant emotion at approximately 16 months post randomisation. A small to medium negative and non-significant effect was observed (standardised ES –0.35, 95% CI –0.75 to 0.05). No other evidence was available.
Secondary outcomes
Children’s physical well-being
Only one trial measured outcomes relevant to children’s physical health. 158 This cluster randomised trial was conducted in a developing country and, therefore, caution must be taken in extrapolating findings to the UK context. For data completeness, ESs are reported here. The trial used a high-quality randomised design to compare treatment as usual with a CBT intervention for the parents of children aged up to 1 year. Outcomes were infant height (% stunted) and weight (% underweight) reported for 745 infants at 6- and 12-months follow-up. The study reported non-significant effects at 6-months for height (standardised ES 0.02, 95% CI –0.31 to 0.34) and weight (standardised ES 0.02, 95% CI –0.27 to 0.30). The 12-month effects were also non-significant for height (standardised ES 0.17, 95% CI –0.06 to 0.40) and weight (standardised ES 0.11, 95% CI –0.08 to 0.31). No other evidence was available.
No randomised trials measured outcomes relevant to child safety.
Children’s social well-being
Twelve trials measured outcomes relevant to children’s behaviour and social function. 65–67,140,142,144,147,148,152,154,160,161
Short-terms outcomes were measured by 10 trials,65–67,140,142,147,148,154,160,161 although only eight65–67,140,142,147,154,160 provided sufficient data to enable the calculation of a standardised effect. The authors’ narratives for the other two studies are provided in Appendix 9, Table 40. These eight trials provided a total of 10 comparisons. Three comparisons were from two quasi-randomised studies presenting a high risk of bias. 66,67
The 10 comparisons measured children’s functioning on a total of 397 participants, using five different outcome measures. Six comparisons evaluated interventions for the mothers of children aged between 0 and 4 years and reported observer ratings of infant behaviour. 66,67,154,160 Four comparisons evaluated interventions for children aged between 6 and 18 years and reported both observer and self-reported measures of behaviour or social function. 63,140,142,147 Seven comparisons evaluated a therapeutic intervention; four of these targeted parents,66,142,147,160 two targeted parents and children66,67 and one targeted children only. 140 Two comparisons evaluated psychoeducational interventions; one comparison targeted parents63 and the other involved both parents and children. 142 The final comparison comprised a predominantly parent-based psychosocial intervention. 154 Six comparisons evaluated interventions with a parenting or family functioning component,66,67,142,154 three evaluated an intervention aimed at parental well-being66,147,160 and one evaluated an intervention aimed at child well-being. 140
Five comparisons suggested efficacy in favour of intervention (standardised ES > 0.2),66,67,154,160 although only one was statistically significant. 160 Three were from quasi-randomised trials presenting a high risk of bias. 66,67 A random-effects model was used to pool the data and the I2 index showed no more statistical heterogeneity than would be expected by chance (I2 = 0.0%, p =0.609). The standardised ES was 0.23 (95% CI 0.00 to 0.46), suggesting a small but non-significant effect of community intervention on children’s short-term social and behavioural function (Figure 7).
FIGURE 7.
Children’s behaviour and social function outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: evidence from RCTs. (C), child target; Ed., psychoeducation; IPT, interpersonal therapy; M-ITG, mother–infant therapy; (P), parent target; (PC), parent and child target; Social, psychosocial. a, Quasi-randomised.
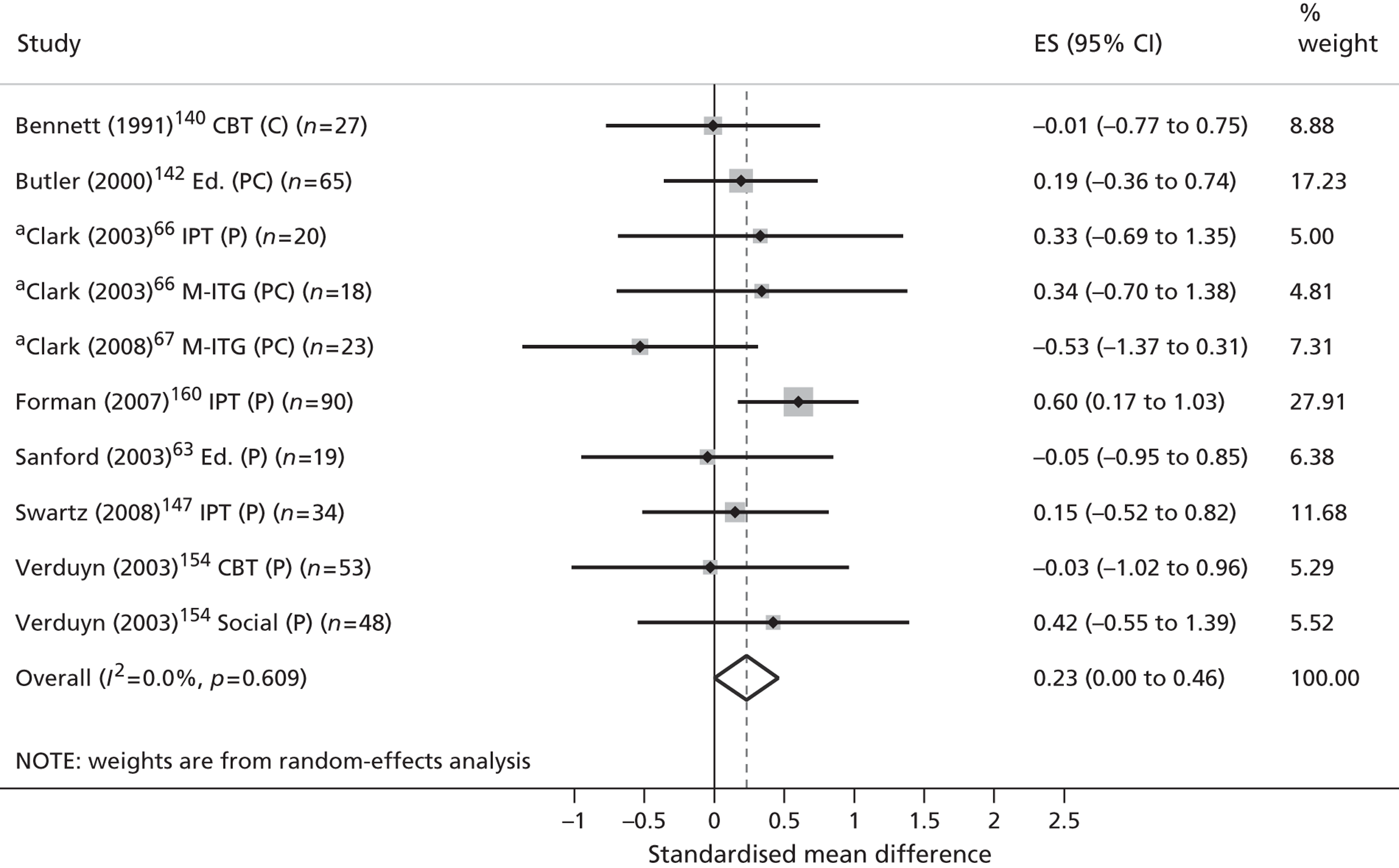
Dividing the trials according to their overall quality ratings suggested a trend in which the pooling of higher-quality trials resulted in a small, positive and significant effect (standardised ES 0.28, 95% CI 0.03 to 0.53). Pooling trials of poorer quality demonstrated no significant effect (standardised ES –0.02, 95% CI –0.62 to 0.57). The limited number of comparisons contributing to this analysis, in conjunction with the heterogeneous mix of interventions, populations and outcomes included, means that these results should be interpreted with caution. The small number of trials providing data for this outcome prevented any examination of clinical heterogeneity.
Medium-term outcomes of between 6 and 12 months post randomisation were measured by two trials. 147,154 These two trials provided a total of three comparisons. Owing to the small number of studies available, and heterogeneity within their interventions and populations, these results were not pooled.
One comparison147 used a randomised design to compare treatment as usual with brief interpersonal psychotherapy in 28 parents of children aged 6–18 years. The outcome was children’s self-reported socioemotional function at 9 months post randomisation. A large positive and significant effect was observed (standardised ES 1.06, 95% CI 0.25 to 1.86). The remaining two comparisons154 used a randomised design to compare treatment as usual with (1) CBT parenting-based therapy and (2) a psychosocial mother and toddler group in 86 mothers of children aged 0–2.5 years. The outcome was maternal reports of children’s behaviour at 12 months post randomisation. Small to medium negative and non-significant effects were observed for both the mother–toddler group (standardised ES –0.41, 95% CI –1.38 to 0.56) and the cognitive–behavioural parenting-based intervention (standardised ES –0.44, 95% CI –1.44 to 0.56).
Long-term outcomes of between 6 and 12 months post randomisation were measured by two trials,144,152 although only one144 presented sufficient data to enable the calculation of a standardised effect. The authors’ narrative pertaining to this study is provided in Appendix 9, Table 40. This comparison (n = 98) used a quasi-randomised design to compare treatment as usual with an extended care model incorporating home visitation, parenting education and care management for the parents of children aged 0–1 years. The outcome was observer ratings of infant behaviour at approximately 16 months post randomisation. A small positive, non-significant effect was observed (standardised ES 0.17, 95% CI –0.22 to 0.56). No other evidence was available.
One trial measured short-term outcomes relevant to children’s social relationship quality. 63 This comparison (n = 19) used a randomised design to compare treatment as usual with parent education aimed at the parents of children aged 6–13 years. The outcome was partner reports of children’s peer relationship quality at approximately 12 weeks post randomisation. A non-significant effect was observed (standardised ES 0.05, 95% CI –1.44 to 1.54). No other evidence was available.
The same trial reported above also measured short-term outcomes relevant to children’s recreational engagement. 63 The outcome was partner reports of children’s participation in recreational activities at approximately 12 weeks post randomisation. A small non-significant effect was observed (standardised ES 0.30, 95% CI –0.60 to 1.21). No other evidence was available.
Children’s family-based experiences
Two trials measured short-term outcomes relevant to family function,63,146 although only one63 provided sufficient data to enable a calculation of standardised effect. This comparison (n = 19) used a randomised study to compare treatment as usual with parent-education in the parents of children aged 6–13 years. The outcome was partner reports of family function at approximately 12 weeks post randomisation. A medium negative and non-significant effect was observed (standardised ES –0.47, 95% CI –1.55 to 0.68). No other evidence was available.
Parental mental health outcomes were the most common outcome measure, reported by a total of 19 trials. 63,64,66,67,138,144–150,152–154,156–159
Short-term outcomes of fewer than 6 months post randomisation were measured by 17 trials. 63,66,67,138,145–150,152–154,156–159 These trials provided a total of 22 comparisons. Four comparisons were from three quasi-randomised trials presenting a high risk of bias. 66,67,157
In total, the 22 comparisons reported on a total of 1855 patients using six different outcome measures. All but two trials63,147 evaluated interventions for the parents of infants aged 0–4 years. Twenty comparisons evaluated a therapeutic intervention, 18 of which targeted parents66,138,145–150,152–154,156–159 and two66,67 targeted parents and children. The remaining two comparisons comprised parent-orientated psychoeducation63 and a predominantly parent-based psychosocial intervention. 154 Five comparisons evaluated interventions aimed at enhancing parenting or family functioning, either alone63,154 or in combination with parent well-being. 66,67
All but one of the comparisons149 suggested efficacy in favour of intervention (standardised ES > 0.2) and nine were statistically significant. 67,145–148,150,153,157,158 Two extreme outcomes were observed,145,157 one of which was from a quasi-randomised study. 157 A random-effects model was used to pool the data and the I2 index showed marked levels of statistical heterogeneity according to standardised criteria (I2 = 67.8%, p = 0.000). The pooled ES was 0.73 (95% CI 0.51 to 0.94), suggesting a medium to large significant effect of community-based interventions on short-term parental mental health (Figure 8).
FIGURE 8.
Parents’ depressive symptom outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: evidence from RCTs. CBT-nurs., nurse delivered; CBT-PDT, mixed CBT/psychodynamic therapy; CBT psyc., psychologist delivered; Ed., psychoeducation; IPT, interpersonal therapy; M-ITG, mother–infant therapy; (P), parent target; (PC), parent and child target; PDT, psychodynamic therapy; Social, psychosocial; ST, supportive therapy. a, Quasi-randomised.
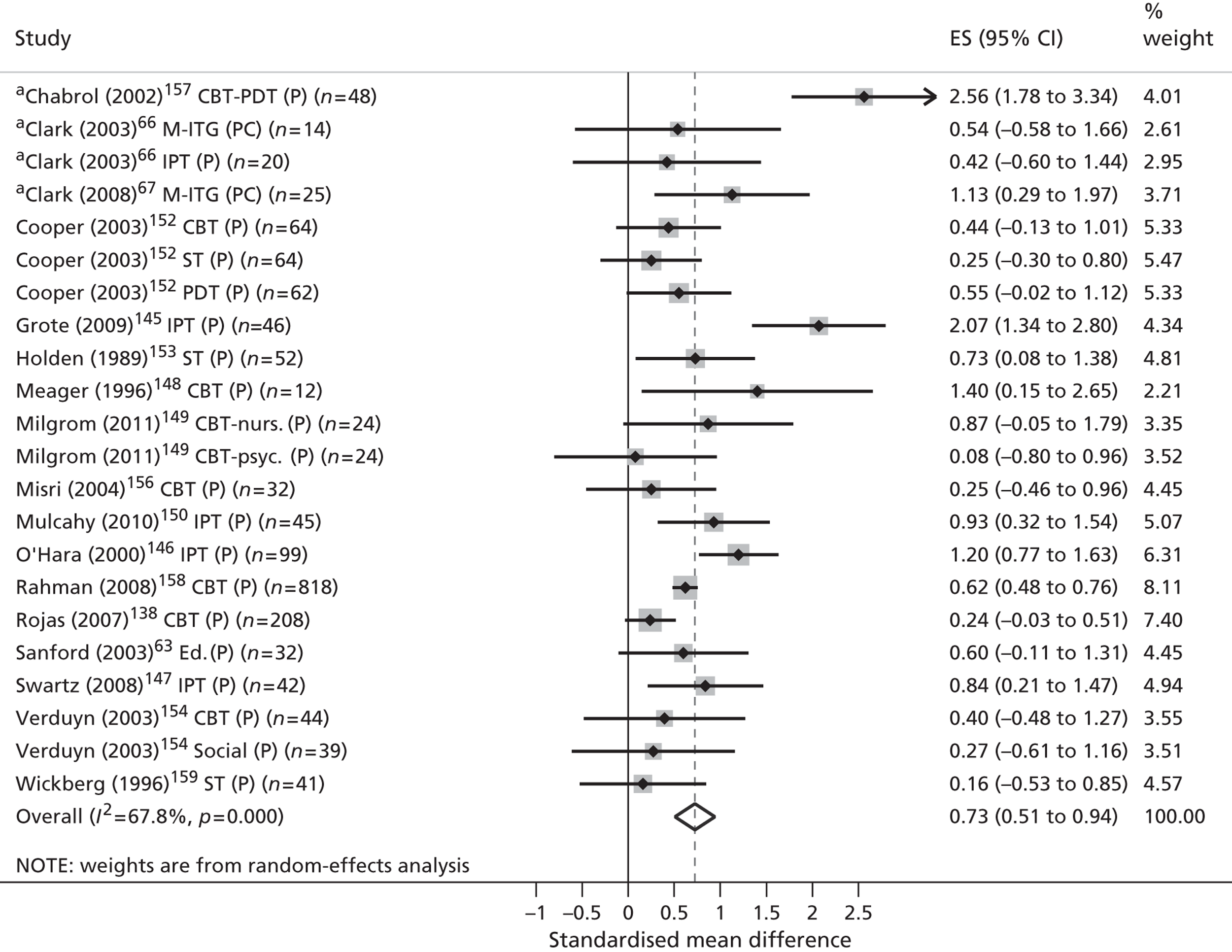
Dividing the trials according to their overall quality ratings (Figure 9) suggested a trend in which the pooling of poorer-quality trials produced a more pronounced effect (standardised ES 1.21, 95% CI 0.18 to 2.23). The pooling of higher-quality trials demonstrated a smaller but nonetheless medium to large significant effect (standardised ES 0.63, 95% CI 0.44 to 0.83).
FIGURE 9.
Parents’ depressive symptom outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: sensitivity analysis. CBT-nurs., nurse delivered; CBT-PDT, mixed CBT/psychodynamic therapy; CBT psyc., psychologist delivered; Ed., psychoeducation; IPT, interpersonal therapy; M-ITG, mother–infant therapy; (P), parent target; (PC), parent and child target; PDT, psychodynamic therapy; Social, psychosocial; ST, supportive therapy. a, Quasi-randomised.
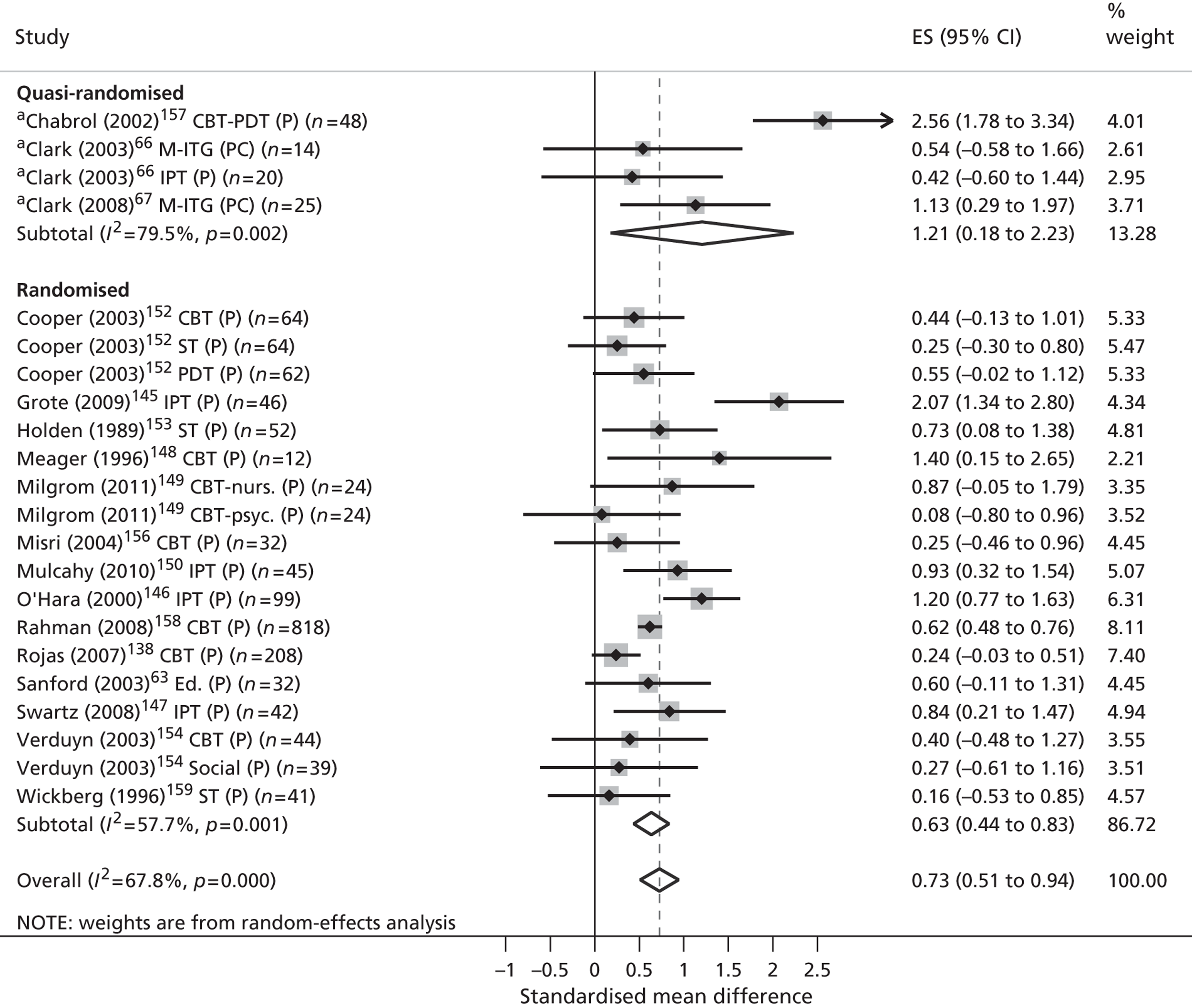
Examinations of heterogeneity were undertaken for this outcome. Figures 10–13 present pooled ESs for the comparisons divided by child age, intervention model, intervention objectives and target participants. However, it should be acknowledged that the meaningful interpretation of these data is limited by the small number of comparisons contributing data to some groups and by confounding variation in trial quality and the characteristics of the populations and interventions being compared. The results of these analyses are presented here but should be treated with the utmost caution.
FIGURE 10.
Parents’ depressive symptom outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: subgroup comparison on intervention model. CBT-nurs., nurse delivered; CBT-PDT, mixed CBT/psychodynamic therapy; CBT psyc., psychologist delivered; Ed., psychoeducation; IPT, interpersonal therapy; M-ITG, mother–infant therapy; (P), parent target; (PC), parent and child target; PDT, psychodynamic therapy; Social, psychosocial; ST, supportive therapy. a, Quasi-randomised.
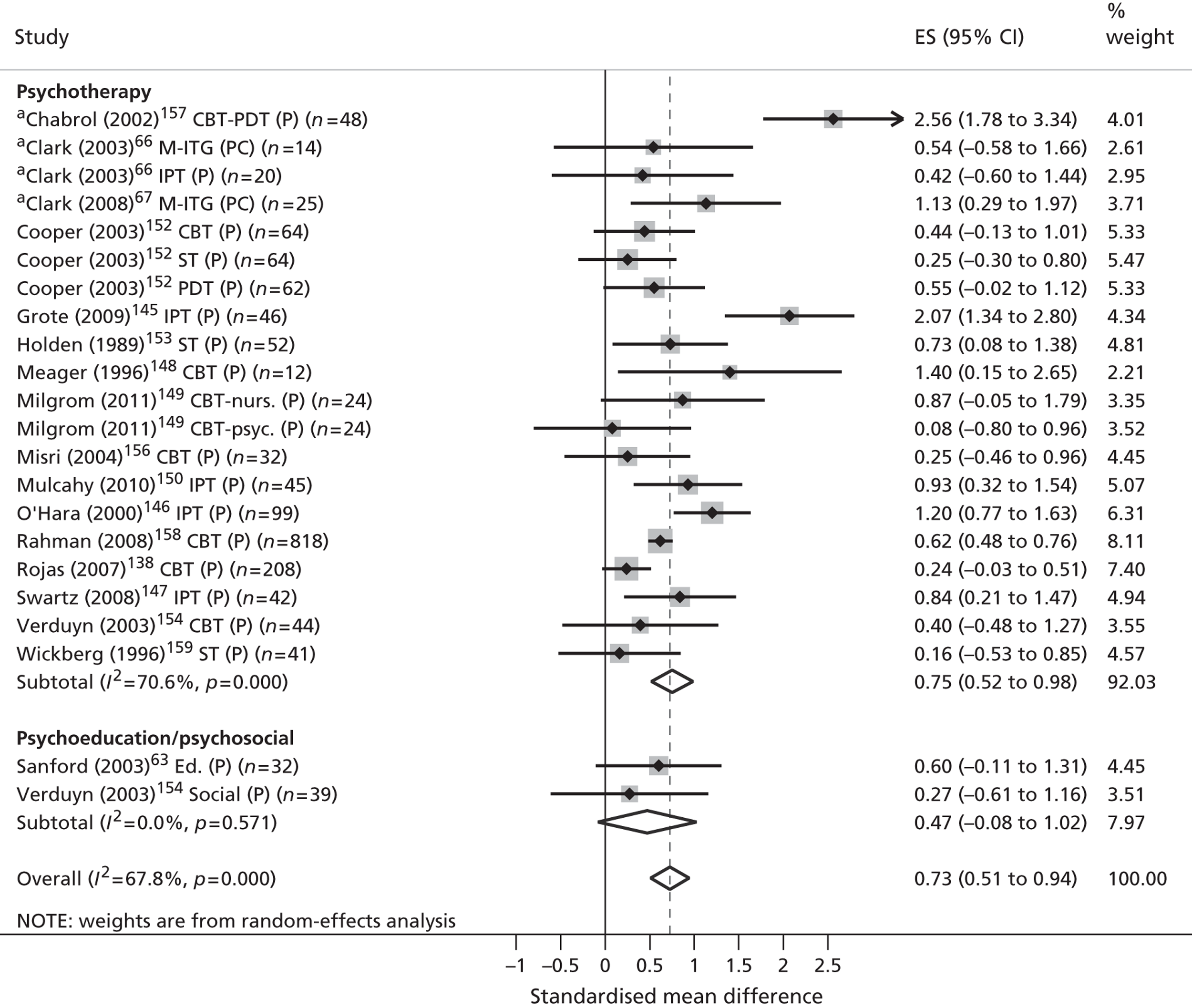
FIGURE 11.
Parents’ depressive symptom outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: subgroup analysis on child age. CBT-nurs., nurse delivered; CBT-PDT, mixed CBT/psychodynamic therapy; CBT psyc., psychologist delivered; Ed., psychoeducation; IPT, interpersonal therapy; M-ITG, mother–infant therapy; (P), parent target; (PC), parent and child target; PDT, psychodynamic therapy; Social, psychosocial; ST, supportive therapy. a, Quasi-randomised. Note: no studies were identified for children aged 5 years.
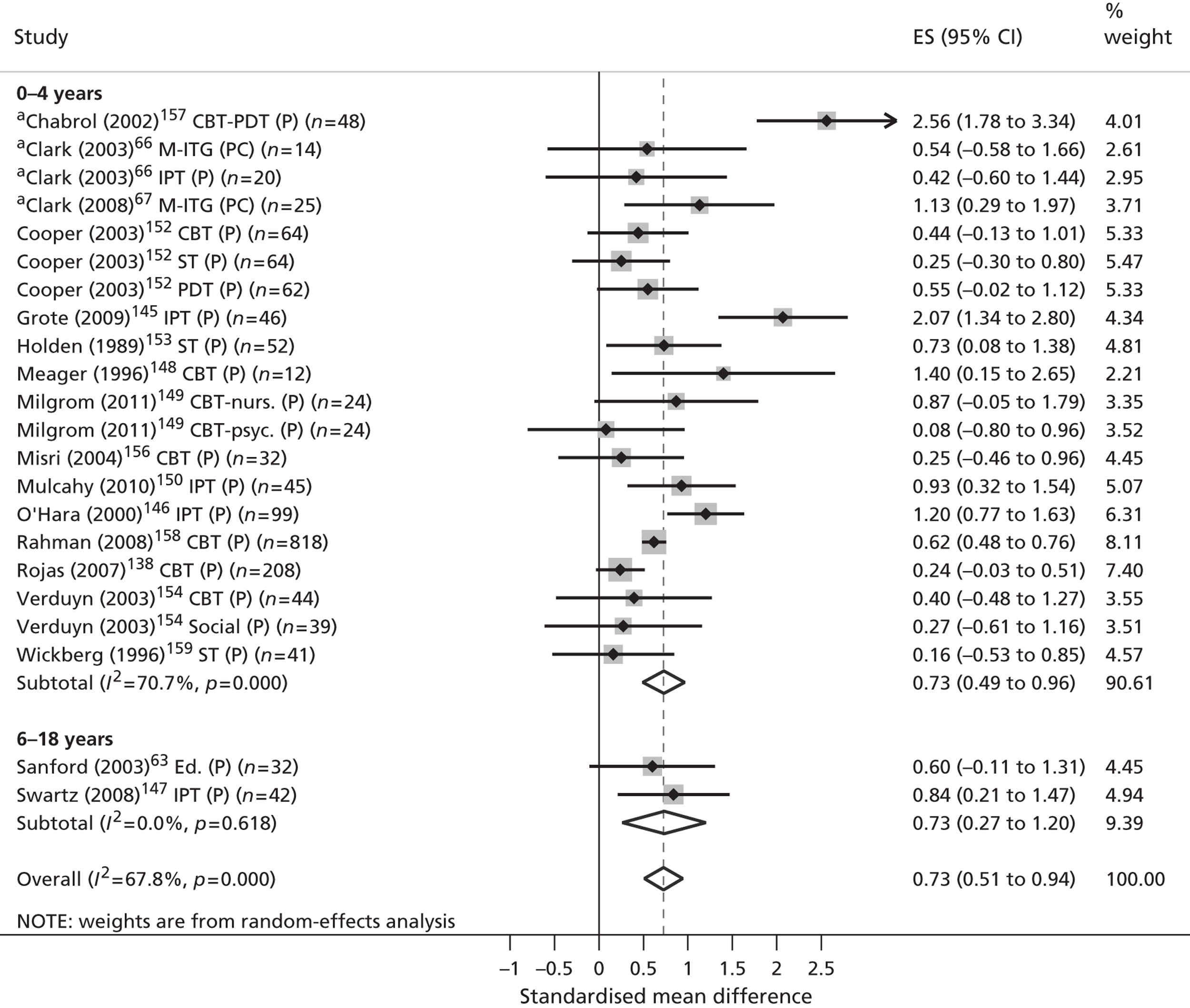
FIGURE 12.
Parents’ depressive symptom outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: subgroup analysis on intervention objective. CBT-nurs., nurse delivered; CBT-PDT, mixed CBT/psychodynamic therapy; CBT psyc., psychologist delivered; Ed., psychoeducation; IPT, interpersonal therapy; M-ITG, mother–infant therapy; (P), parent target; (PC), parent and child target; PDT, psychodynamic therapy; Social, psychosocial; ST, supportive therapy. a, Quasi-randomised.
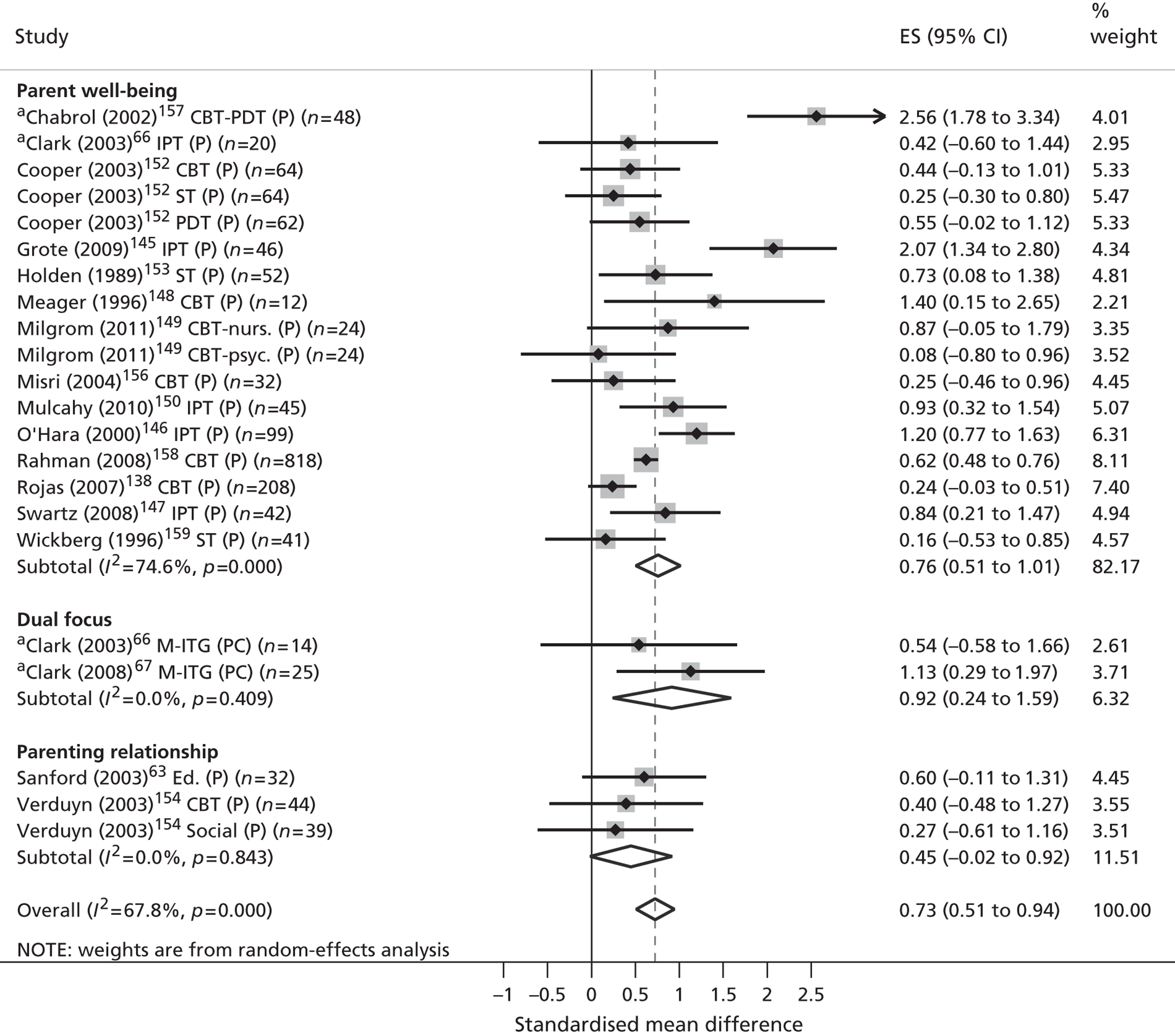
FIGURE 13.
Parents’ depressive symptom outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: subgroup analysis on intervention target. CBT-nurs., nurse delivered; CBT-PDT, mixed CBT/psychodynamic therapy; CBT psyc., psychologist delivered; Ed., psychoeducation; IPT, interpersonal therapy; M-ITG, mother–infant therapy; (P), parent target; (PC), parent and child target; PDT, psychodynamic therapy; Social, psychosocial; ST, supportive therapy. a, Quasi-randomised.
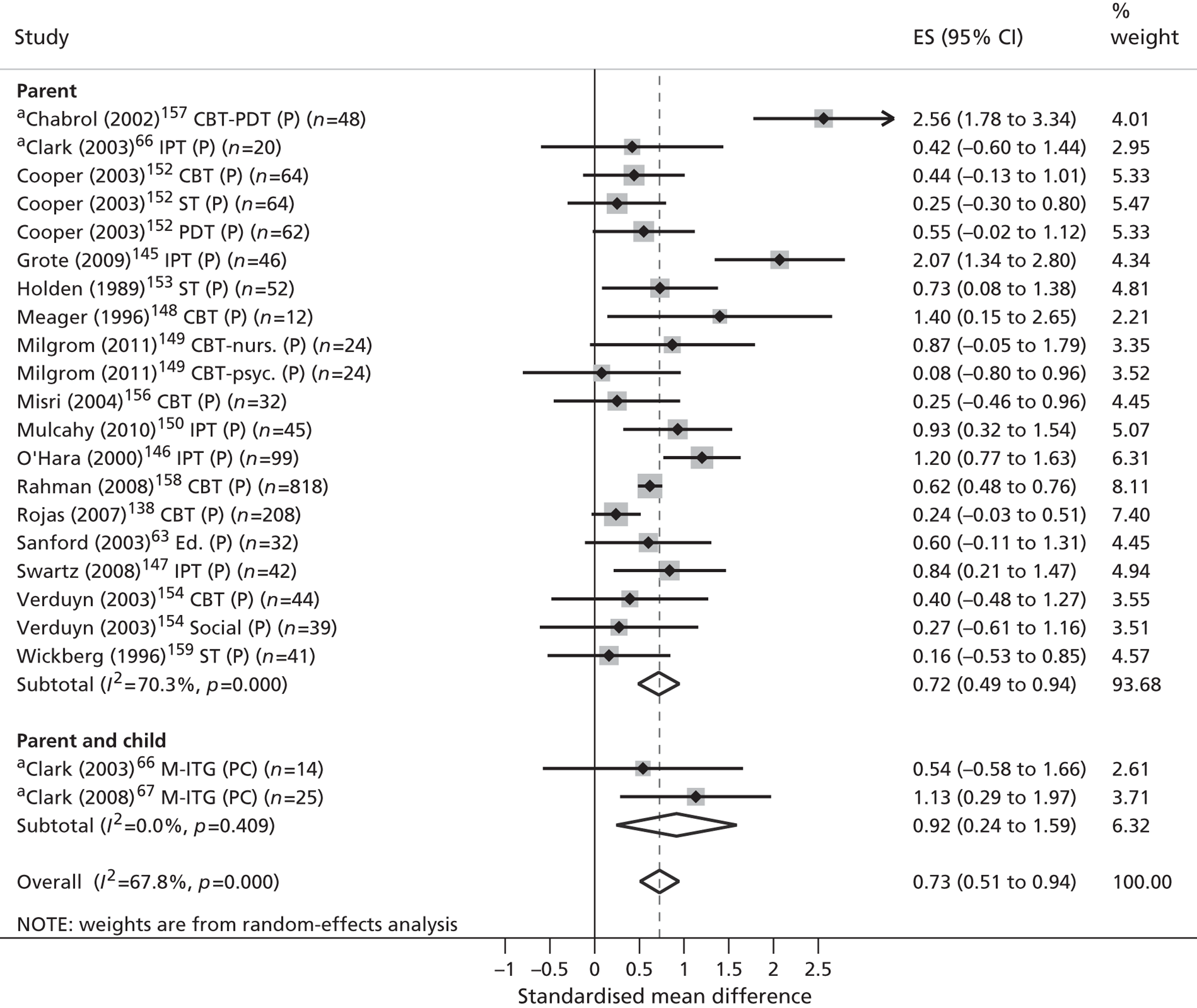
Dividing the trials according to intervention type resulted in a smaller effect for psychoeducational and psychosocial models (standardised ES 0.47, 95% CI –0.08 to 1.08) compared with psychotherapeutic interventions (standardised ES 0.75, 0.52 to 0.98 respectively). The pooled result for psychoeducational and psychosocial models was derived from a notably small number of comparisons (n = 2) and thus displayed substantially less precision in its estimate of effect.
Dividing the trials according to child age ranges revealed medium to large effects for both children aged 0–4 years (standardised ES 0.73, 95% CI 0.49 to 0.96) and children aged 6–18 years (standardised ES 0.73, 95% CI 0.27 to 1.20). Only two trials contributed data to the older age band and, therefore, the derived ES was an imprecise estimate.
Grouping the trials by intervention target resulted in a medium to large effect for parent-based interventions (standardised ES 0.72, 95% CI 0.49 to 0.94) and a large effect for dyadic interventions (standardised ES 0.92, 95% CI 0.24 to 1.59). This latter effect was derived from two quasi-randomised studies66,67 and was less precise in its estimate. A lack of data for child-based interventions prevented any direct comparisons with this group.
Pooling trials by intervention objectives revealed a medium to large effect for interventions targeting parental well-being (standardised ES 0.76, 95% CI 0.51 to 1.01) and a small to medium, non-significant effect for a small number of comparisons (n = 3) targeting the parent–child relationship (standardised ES 0.45, 95% CI –0.02 to 0.92). A pooled effect for dual focus interventions was obtained from two quasi-randomised comparisons. 66 This effect was large and significant but ultimately less precise in its estimate (standardised ES 0.92, 95% CI 0.24 to 1.59).
Medium-term parental mental health outcomes of 6–12 months post randomisation were measured by four trials. 147,152,154,158 These four trials provided a total of seven comparisons.
The seven comparisons reported parental mental health symptoms for a total of 1098 parents, using two different outcome measures. Six comparisons evaluated a therapeutic intervention,147,152,154,158 all of which targeted parents. One comparison evaluated a predominantly parent-based psychosocial intervention. 154 Two comparisons evaluated interventions aimed at enhancing parenting or family function. 154
Two comparisons suggested efficacy in favour of intervention (standardised ES > 0.2), both of which were statistically significant. 147,158 A random-effects model was used to pool the data and the I2 index showed marked levels of statistical heterogeneity according to standard criteria (I2 = 64.9%, p = 0.009). The overall effect was 0.34 (95% CI 0.00 to 0.68), suggesting a small to medium positive but non-significant effect of community intervention on parental depressive symptoms over the medium term. No quasi-randomised trials contributed data to this analysis and, therefore, sensitivity analyses were not warranted. Further explorations of heterogeneity were not conducted owing to the limited number of comparisons providing data for this outcome (Figure 14).
FIGURE 14.
Medium-term parents’ depressive symptom outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: evidence from RCTs. IPT, interpersonal therapy; (P), parent target; PDT, psychodynamic therapy; Social, psychosocial; ST, supportive therapy.
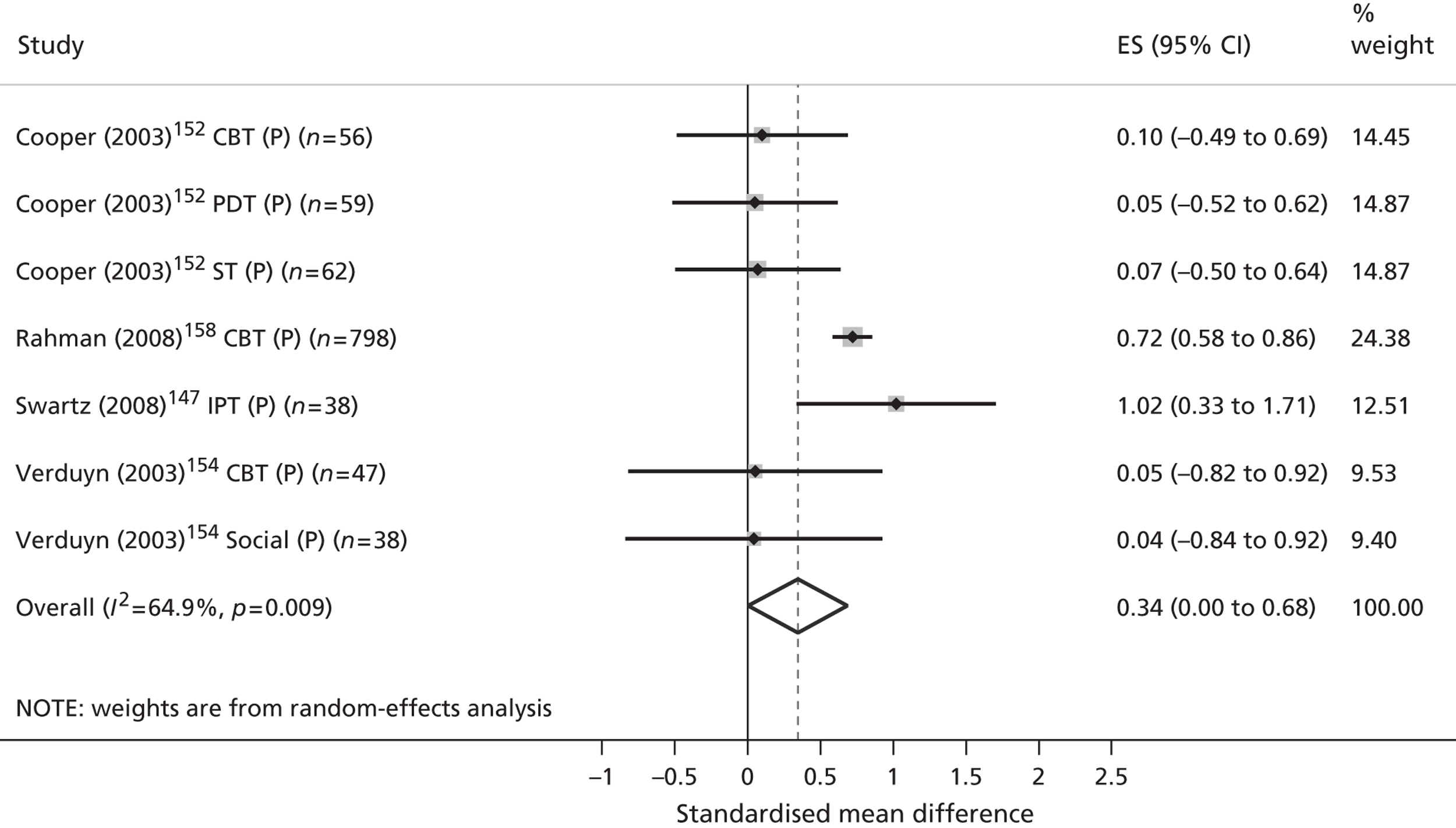
Long-term parental mental health outcomes of more than 12 months post randomisation were reported by three trials. 64,144,152 These three trials provided a total of five comparisons. One comparison was from a quasi-randomised trial presenting a high risk of bias144 and two comparisons were from trials not included in the meta-analyses for short- and medium-term outcomes;64,144 one of these comparisons targeted parental well-being. 144
The five comparisons reported parental mental health symptoms for a total of 373 parents, using two different outcome measures. All comparisons evaluated a therapeutic intervention, targeted at parents. Two comparisons evaluated interventions aimed at enhancing parenting or family functioning. 64,144
One of the five mean differences from individual trials suggested efficacy in favour of intervention (standardised ES > 0.2). This result was from a quasi-randomised trial presenting a high risk of bias. 144 A random-effects model was used to pool the data and the I2 index showed low levels of statistical heterogeneity according to standard criteria (I2 = 0.0%, p = 0.519). The overall effect was 0.17 (95% CI –0.04 to 0.39), suggesting a small positive but non-significant effect of community intervention on parental mental health symptoms (Figure 15). Sensitivity analyses that removed the poorer quality quasi-randomised trial144 reduced the pooled ES to 0.06 (95% CI –0.20 to 0.31). Further explorations of heterogeneity were not conducted owing to the small number of trials providing data for this outcome.
FIGURE 15.
Longer-term parents’ depressive symptom outcomes for all variants of community-based interventions vs. treatment as usual/waiting list control: evidence from RCTs. EC, extended care; M-TT, mother–toddler therapy; (P), parent target; (PC), parent and child target; PDT, psychodynamic therapy; ST, supportive therapy. a, Quasi-randomised.
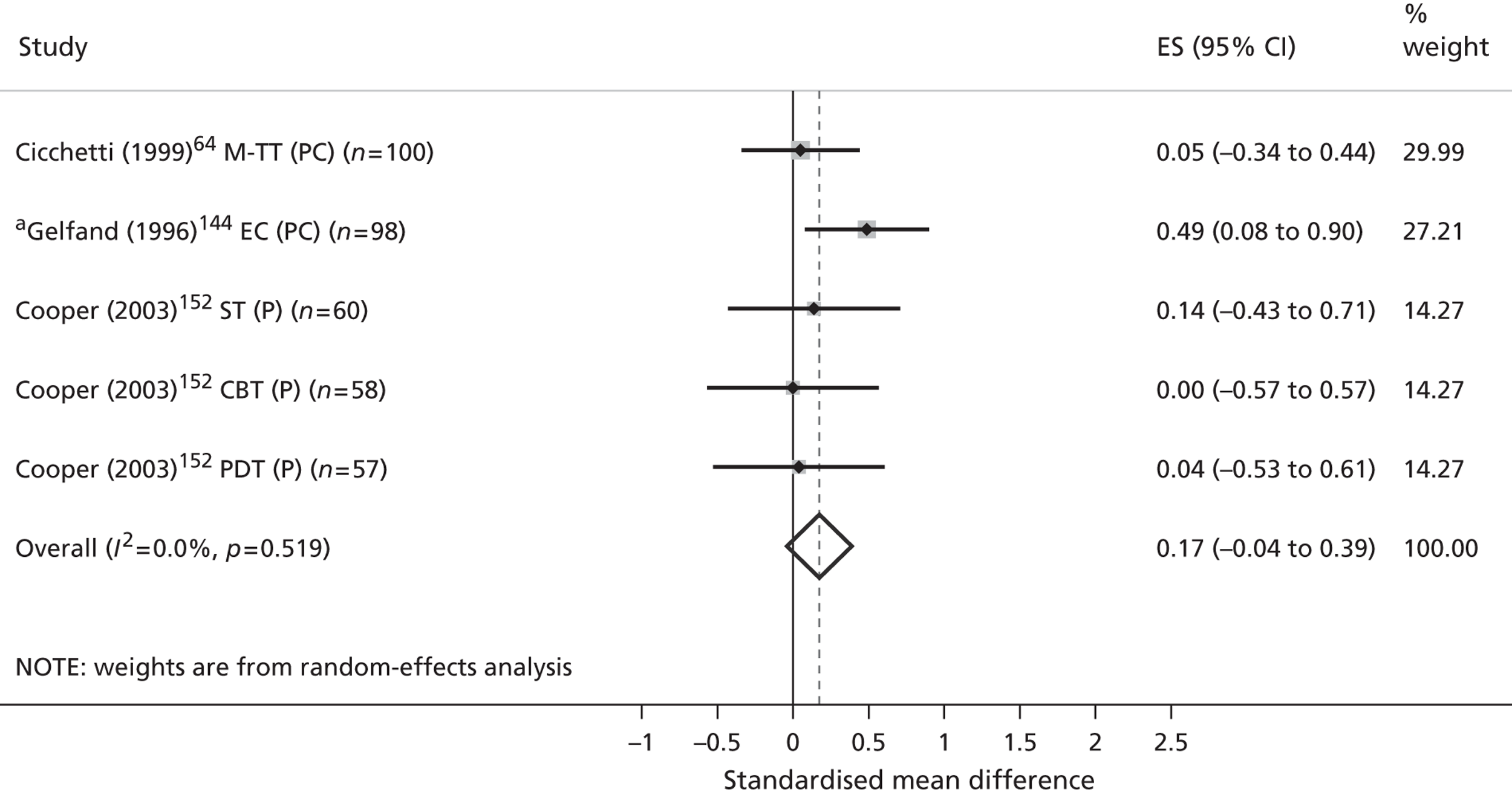
Quality of parent–child interactions
Eight trials measured outcomes relevant to the quality of parent–child interactions. 63,66,67,144,150,152,158,160
Short-term outcomes at less than 6 months post randomisation were reported by six trials. 63,66,67,150,152,160 These six trials provide a total of nine comparisons. Three comparisons were from two quasi-randomised trials presenting a high risk of bias. 66,67
In total, the nine comparisons reported on a total of 378 participants using four different outcome measures. Eight comparisons evaluated a therapeutic intervention; six of these comparisons targeted parents66,150,152,160 and two targeted parents and children only. 66,67 The remaining comparison comprised parent psychoeducation. 63 Three comparisons evaluated interventions aimed at enhancing parenting or family functioning. 63,66,67
All but one of the comparisons160 suggested efficacy in favour of intervention (standardised ES > 0.2) and four were statistically significant. 66,67,152 Three were from a quasi-randomised study presenting a high risk of bias. 66,67 A random-effects model was used to pool the data and the I2 index showed marked levels of heterogeneity according to standardised criteria (I2 = 50.8%, p = 0.039). The pooled ES was 0.67 (95% CI 0.32 to 1.02), suggesting a significant benefit of intervention on parenting behaviours (Figure 16). Dividing the trials according to their overall quality ratings suggested a trend in which the pooling of poorer quality trials revealed a more pronounced effect (standardised ES 1.26, 95% CI 0.77 to 1.79). Pooling trials of higher quality revealed a smaller but also significant effect (standardised ES 0.35, 95% CI 0.09 to 0.61). The small number of trials contributing data to this analysis combined with differences in intervention content, objective and target participants mean that these pooled results should be interpreted with caution. Further explorations of heterogeneity were not conducted because of the small number of trials providing data for this outcome.
FIGURE 16.
Short-term parenting behaviour for all variants of community-based interventions vs. treatment as usual/waiting list control: evidence from RCTs. Ed., psychoeducation; IPT, interpersonal therapy; M-ITG, mother–infant therapy; (P), parent target; (PC), parent and child target; PDT, psychodynamic therapy; ST, supportive therapy. a, Quasi-randomised.
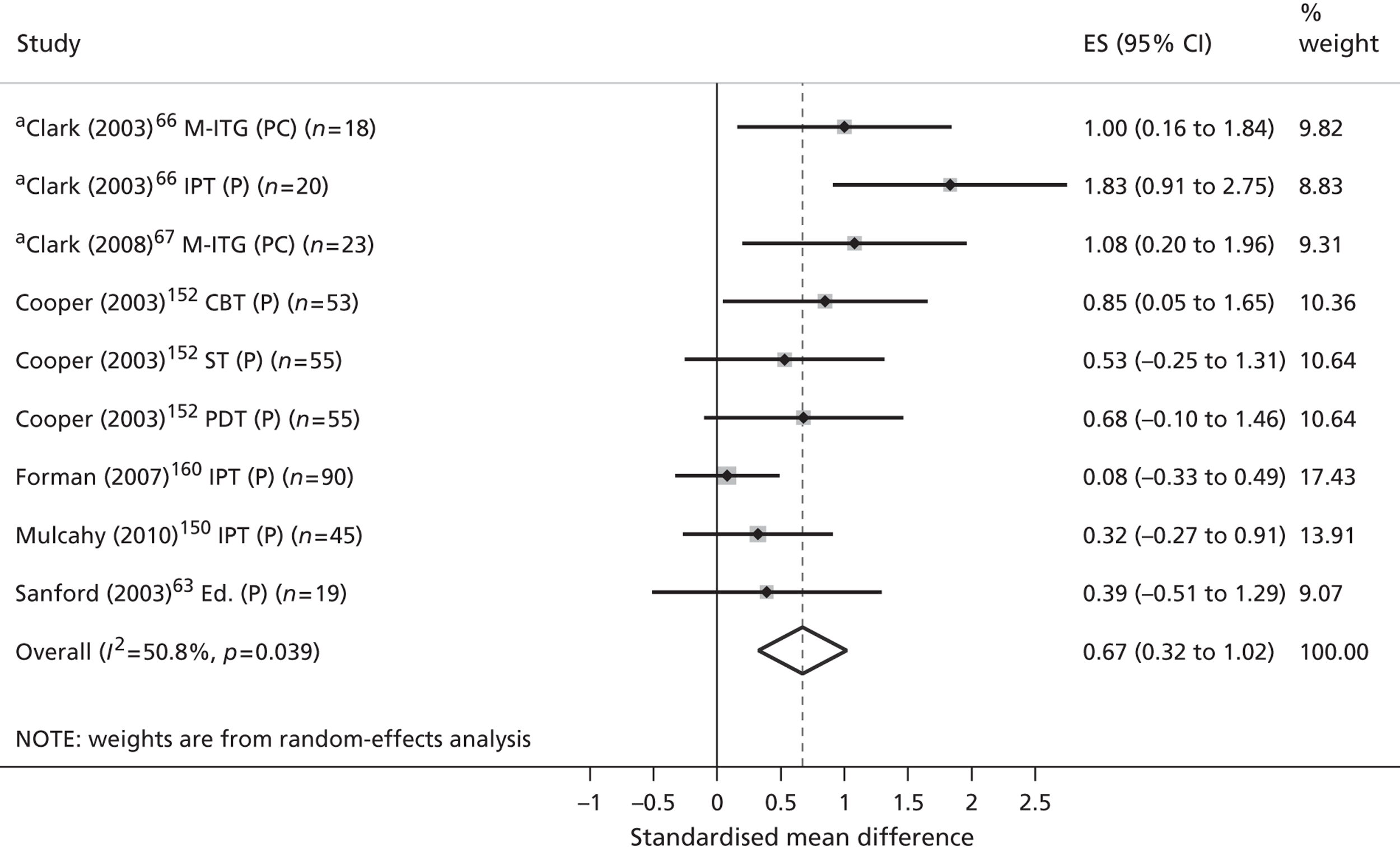
One comparison reported medium-term outcomes. 158 The comparison used a high-quality randomised design to compare treatment as usual with a CBT intervention in 705 parents of children aged up to 1 year. The outcome was parent reports of play frequency with their child. A medium positive and significant effect in favour of the intervention was observed (standardised ES 0.58, 95% CI 0.38 to 0.77). However, this comparison was conducted in a developing country and additional caution may be required when extrapolating outcomes to alternative contexts. No other evidence was available.
Long-term outcomes of more than 12 months were reported for one comparison (n = 98). 144 A quasi-randomised design was used to compare treatment as usual with an extended care model incorporating home visitation, parenting education and care management for the parents of children aged 0–1 year. The outcome was observer ratings of maternal responsiveness at approximately 16 months post randomisation. A small positive and non-significant effect was observed (standardised ES 0.27, 95% CI –0.13 to 0.67). No other evidence was available.
Children’s self-esteem and -actualisation
Five trials measured outcomes relevant to child development,64,66,67,144,152 although only three64,67,144 presented sufficient data to enable standardised ESs to be calculated. The authors’ narrative pertaining to the remaining two studies are provided in Appendix 9, Table 40. These three trials provided a total of three comparisons.
Short-term outcomes at up to 6 months post randomisation were reported for one comparison (n = 24). 67 This comparison used a quasi-randomised design to compare treatment as usual with a mother–infant therapy programme aimed at enhancing parental interaction between parents and children aged 0–2 years. The outcome was ratings of infant mental development at 12 weeks post randomisation. A non-significant effect was observed (standardised ES 0.08, 95% CI –0.45 to 0.6). No other evidence was available.
No trials provided medium-term outcomes relevant to children’s cognitive development.
Two trials reported long-term outcomes of more than 12 months post randomisation. 64,144 Owing to the small number of studies available and heterogeneity within their interventions and populations these results were not pooled.
The first comparison (n = 98) used a quasi-randomised design144 to compare treatment as usual with an extended care model incorporating home visitation, parenting education and care management for the parents of children aged < 1 year. The outcome was ratings of infant mental development at approximately 16 months post randomisation. A non-significant effect was observed (standardised ES 0.05, 95% CI –0.58 to 0.67). The second comparison (n = 97) used a randomised design64 to compare treatment as usual to a parent–child-focused parenting therapy programme for mothers and children aged 0–2 years. The outcome was ratings of infant mental development at approximately 16 months post randomisation. A non-significant effect was observed (standardised ES 0.03, 95% CI –0.63 to 0.69).
No randomised trials measured outcomes relevant to children’s coping skills.
One comparison reported short-term outcomes relating to children’s mental health literacy. 142 This comparison (n = 65) used a randomised trial to compare treatment as usual with psychoeducation for parents and children aged 7–12 years. The outcome was parental reports of child-centred discussions about depression at 6 weeks post randomisation. This study reported large significant effect (standardised ES 0.90, 95% CI 0.32, 1.48). No other evidence was available.
One comparison reported short-term outcomes relevant to children’s self-esteem. 140 This comparison (n = 37) used a randomised design to compare treatment as usual to a child-focused CBT problem-solving programme aimed at children aged 8–13 years. The outcome was validated child self-reports at 6 weeks post randomisation. A non-significant and negative effect was observed (standardised ES –0.14, 95% CI –1.43 to 1.18). No other evidence was available.
Consideration of other sources of bias
Funnel plots were conducted for one outcome (parental mental health symptoms) for which sufficient data were available. The purpose of a funnel plot is to map standardised ESs from individual studies against standard error, i.e. the underlying precision of the observed effect. A funnel plot is based on the premise that precision in the estimation of an ES will increase as sample size increases. Effect estimates from smaller studies with larger standard error should, therefore, scatter more widely at the bottom of the plot. Larger studies with smaller standard error should display a narrower spread. Bias is suggested by the emergence of a non-symmetrical plot.
A funnel plot was created for all trials that contributed outcome data to our comparison of all variants of community-based interventions versus a treatment as usual/waiting list control for short-term parental depressive symptoms (Figure 17). No evidence of additional bias was observed (Egger’s regression intercept 0.60, 95% CI –0.71 to 0.92, p = 0.35), although the low power of this test means that bias cannot definitively be ruled out.
FIGURE 17.
Funnel plot of standard error by standard difference in means for all variants of community-based interventions vs. a treatment as usual/waiting list control for short-term parental depressive symptoms.
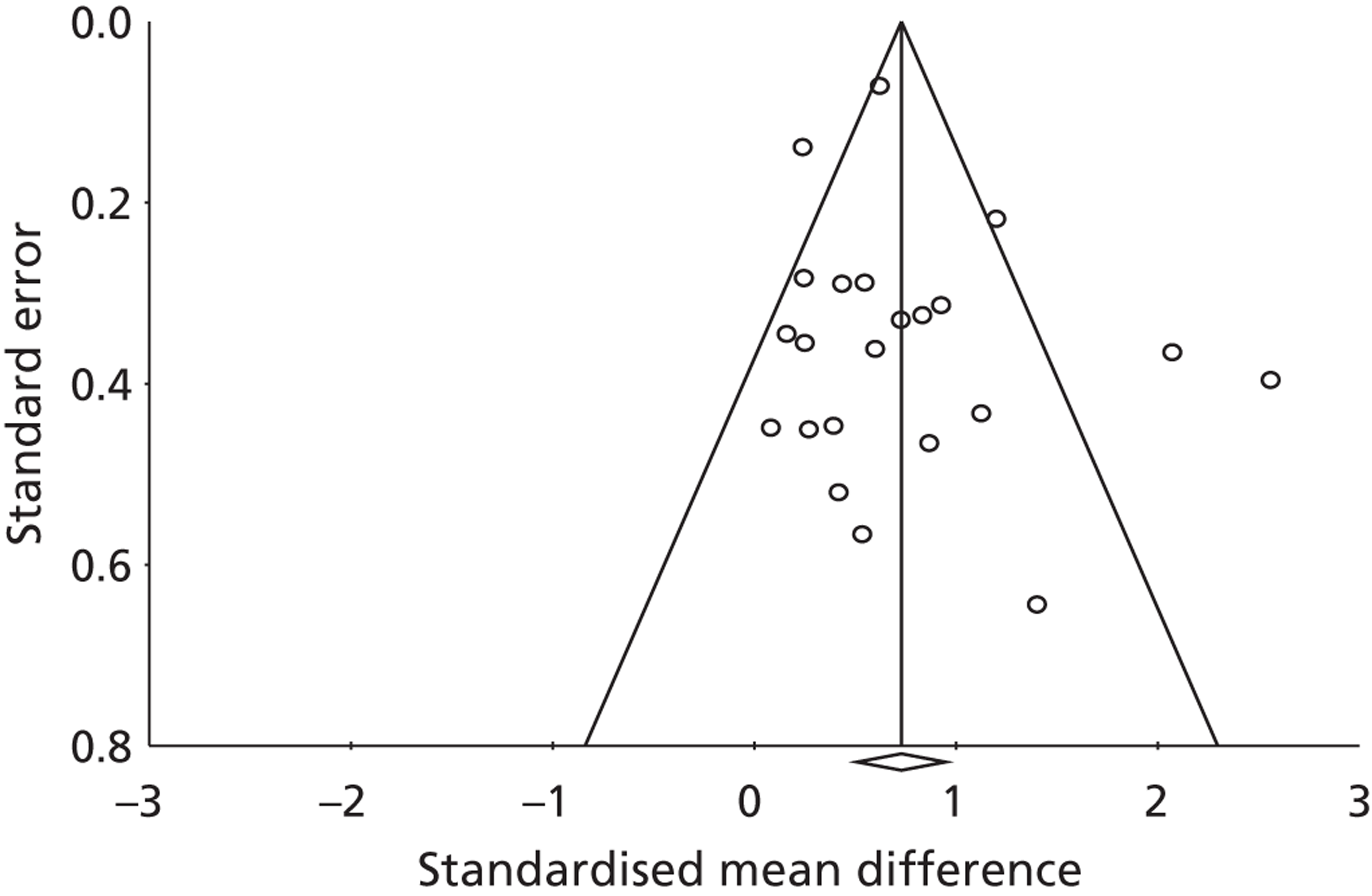
Tests for funnel plot asymmetry are only recommended for meta-analyses of ESs obtained from more than 10 studies. Insufficient data therefore prohibited any statistical exploration of bias for other outcomes reported in this synthesis.
Comparisons of two active interventions
Ten studies reported 37 comparisons between two active treatments. 65,66,143,149,152,154,155,162,168,169 Five studies reported within-model comparisons (e.g. psychotherapy model A vs. psychotherapy model B)65,66,149,152,155 and five reported across model comparisons (e.g. psychotherapy model A vs. psychoeducation model B). 143,154,162,168,169 Within-model comparisons comprised comparisons of two interventions differing in theoretical orientation,65,66,152 delivery mechanism149 or content. 155 Clinical and methodological heterogeneity across the comparisons prevented any meaningful pooling of these data. For data completion, details of each trial comparison, relevant QoL outcomes measured and corresponding standardised ESs are presented in Table 11. ESs could not be calculated for three trials66,152,169 across five outcomes owing to insufficient reporting of data. The authors’ narratives for these studies are presented in Appendix 9, Table 40.
| Outcome | Study reference number | Assessment timing | n | Intervention A | Intervention B | Standardised ES | SE |
|---|---|---|---|---|---|---|---|
| Child mental health: (0–6 months) | 143 | 6 months | 83 | 8 weeks’ parent–child CBT | 8 weeks’ parent–child psychoeducation | –0.12 | 0.22 |
| 66 | 12 weeks | 28 | 12 weeks’ mother–infant therapy | 12 weeks’ mother IPT | –0.12 | 0.40 | |
| Child mental health: (7–12 months) | 143 | 12 months | 83 | 8 weeks’ parent–child CBT | 8 weeks’ parent–child psychoeducation | 0.39 | 0.22 |
| Child social function: (0–6 months) | 143, 168 | 6 months | 83 | 8 weeks’ parent–child CBT | 8 weeks’ parent–child psychoeducation | –0.02 | 0.22 |
| 154 | 6 months | 67 | 16 weeks’ mother CBT | 16 weeks’ mother social group | –0.47 | 0.25 | |
| 66 | 12 weeks | 28 | 12 weeks’ mother–infant therapy | 12 weeks’ mother IPT | –0.56 | 0.40 | |
| 65 | 6 months | 33 | 12 sessions’ parent CBT | 12 sessions’ parent BT | –0.14 | 0.35 | |
| Child social function: (7–12 months) | 143, 168 | 12 months | 83 | 8 weeks’ parent–child CBT | 8 weeks’ parent–child psychoeducation | –0.53 | 0.22 |
| 154 | 12 months | 71 | 16 weeks’ mother CBT | 16 weeks’ mother social group | –0.11 | 0.25 | |
| Parent mental health: (0–6 months) | 143, 168 | 6 months | 83 | 8 weeks’ parent–child CBT | 8 weeks’ parent–child psychoeducation | –0.42 | 0.22 |
| 155 | 10 weeks | 29 | 6 weeks’ psychoeducation including partner | 6 weeks’ psychoeducation not including partner | 0.99 | 0.40 | |
| 65 | 6 months | 33 | 12 sessions’ parent CBT | 12 sessions’ parent BT | 0.44 | 0.35 | |
| 154 | 6 months | 66 | 16 weeks’ mother CBT | 16 weeks’ mother social group | –0.09 | 0.25 | |
| 149 | 8 weeks | 34 | 6 weeks’ nurse CBT counselling | 6 weeks’ psychologist CBT counselling | 0.57 | 0.35 | |
| 152 | 4 months | 89 | 10 weeks’ parent ST | 10 weeks’ parent CBT | 0.13 | 0.21 | |
| 152 | 4 months | 92 | 10 weeks’ parent ST | 10 weeks’ parent PDT | –0.20 | 0.21 | |
| 152 | 4 months | 87 | 10 weeks’ parent CBT | 10 weeks’ parent PDT | –0.07 | 0.22 | |
| 66 | 12 weeks | 28 | 12 weeks’ mother–infant therapy | 12 weeks’ mother IPT | 0.05 | 0.40 | |
| Parent mental health: (7–12 months) | 143, 168 | 12 months | 83 | 8 weeks’ parent–child CBT | 8 weeks’ parent–child psychoeducation | –0.30 | 0.22 |
| 154 | 12 months | 66 | 16 weeks’ mother CBT | 16 weeks’ mother social group | 0.04 | 0.25 | |
| 152 | 9 months | 89 | 10 weeks’ parent ST | 10 weeks’ parent CBT | –0.17 | 0.21 | |
| 152 | 9 months | 92 | 10 weeks’ parent ST | 10 weeks’ parent PDT | –0.02 | 0.21 | |
| 152 | 9 months | 87 | 10 weeks’ parent CBT | 10 weeks’ parent PDT | 0.16 | 0.22 | |
| Parent mental health: (≥ 13 months) | 152 | 5 years | 61 | 10 weeks’ parent ST | 10 weeks’ parent CBT | 0.00 | 0.25 |
| 152 | 5 years | 59 | 10 weeks’ parent ST | 10 weeks’ parent PDT | 0.02 | 0.24 | |
| 152 | 5 years | 66 | 10 weeks’ parent CBT | 10 weeks’ parent PDT | 0.02 | 0.26 | |
| Parent–child interaction: (0–6 months) | 168 | 6 months | 83 | 8 weeks’ parent–child CBT | 8 weeks’ parent–child psychoeducation | 0.21 | 0.22 |
| 65 | 6 months | 33 | 12 sessions’ parent CBT | 12 sessions’ parent BT | 0.31 | 0.35 | |
| 66 | 12 weeks | 28 | 12 weeks’ mother–infant therapy | 12 weeks’ mother IPT | –0.83 | 0.40 | |
| 162 | ∼10 weeks | 68 | 6–10 sessions’ parent–child therapy | 2 lectures’ psychoeducation | 1.36 | 0.27 | |
| 152 | 4 months | 85 | 10 weeks’ parent ST | 10 weeks’ parent CBT | 0.32 | 0.24 | |
| 152 | 4 months | 66 | 10 weeks’ parent ST | 10 weeks’ parent PDT | –0.97 | 0.38 | |
| 152 | 4 months | 64 | 10 weeks’ parent CBT | 10 weeks’ parent PDT | –1.29 | 0.38 | |
| Family function: (0–6 months) | 162 | ∼10 weeks | 66 | 6–10 sessions’ parent–child therapy | 2 lectures’ psychoeducation | 0.93 | 0.45 |
| Family function: (7–12 months) | 169 | ∼12 months | 36 | 6–10 sessions’ parent–child therapy | 2 lectures’ psychoeducation | 1.44 | 0.63 |
| Mental health knowledge: (0–6 months) | 162 | ∼10 weeks | 68 | 6–10 sessions’ parent–child therapy | 2 lectures’ psychoeducation | 0.43 | 0.27 |
| Child coping: (0–6 months) | 168 | 6 months | 83 | 8 weeks’ parent–child CBT | 8 weeks’ parent–child psychoeducation | 0.56 | 0.22 |
Evidence from non-randomised controlled trials
Four non-randomised trials were identified as eligible for inclusion in the present synthesis. 139,166,170,171 Trial participants were allocated to an intervention or treatment as usual control group on the basis of service availability139,166,171 or patient preference170 thereby introducing a non-random component into group assignment and a greater risk of bias in the observed effects. Full methodological quality ratings for the trials are provided in Appendix 9, Table 36.
As per our protocol, the context and methods of the included trials were summarised for the purposes of future research priority setting. The specific characteristics relevant to each trial are presented alongside their quality ratings in Appendix 9. These characteristics are presented within the context of the data extraction sheet that was used to record individual study information.
Publication dates for the four trials ranged from 1996 to 2010, with two trials being conducted outside the UK. 166,170 Commensurate with the inclusion criteria for this review, all non-randomised trials had samples in which the majority of trial participants met criteria for severe unipolar depression. All four trials reported 100% of their samples as having a primary diagnosis of MDD according to standard research or clinical diagnostic criteria. Only one trial, however, reported severe symptoms at baseline. 166 Two of the four trials were explicit in excluding parents with SMI, specifically psychosis, schizophrenia and bipolar disorder. 139,170
All identified non-randomised trials focused on severe depression in mothers of children aged < 2 years. Child residency was not explicitly reported by any of the trials, although the nature of the recruitment strategies and intervention procedures that were followed suggested that the majority of parents and children were colocated. Participants were predominantly recruited via adult community and outpatient mental health services139,166,170 maternity hospitals171 and community screening. 139 Two trials recruited mothers directly from services aimed specifically at depressed postpartum mothers. 139,170
Interventions were heavily orientated towards psychotherapy aimed directly at the depressed parent. The nature of these interventions varied and encompassed cognitive–behavioural,166,171 interpersonal170 and non-directive approaches. 139,171 One study evaluated therapy within the context of a broader, extended care intervention. 139 Commensurate with the nature of the populations sampled, none of the trials actively intervened directly with child participants.
Delivery models were most frequently face-to-face interventions139,166,170,171 delivered in an individual format;139,166,170,171 one intervention, however, also included group sessions. 139 Interventions were delivered by a broad range of health- and social-care professionals including psychotherapists,170 social workers,166 health visitors171 and multidisciplinary teams. 139 Two of the interventions took place inside the home. 166,171 When reported, intervention duration ranged from 8 to 45 sessions, with three166,170,171 of the four139,166,170,171 interventions reporting < 20 hours of total guidance. One trial failed to provide complete information regarding the intensity and/or duration of its intervention. 139
None of identified non-randomised trials provided validated measures of children’s QoL or emotional well-being. In accordance with the observed predominance of parent-centred interventions, the most frequently assessed secondary outcome domains were parental mental health symptoms (n = 4)139,166,170,171 and parent–child interactions (n = 3). 139,166,171 Only one trial reported child outcomes, in this case maternal reports of infant behaviour. 171 All four trials reported short-term outcomes and one trial also presented longer-term follow-up data collected 7 years post intervention. 139,172
Evidence from uncontrolled studies
Eleven uncontrolled studies were identified as eligible for inclusion in this synthesis. 173–183 As for the non-randomised trials, the context and methods of these studies were summarised for the purposes of future research priority setting. As before, the specific characteristics relevant to these studies are presented in Appendix 9 within the context of the data extraction sheet used to record individual study information.
Publication dates for the eligible uncontrolled studies ranged from 1988 to 2008, with eight being published within the last 10 years (i.e. post 2002). 173–180 All but two studies174,181 were conducted outside the UK, the majority originating from the USA and Australia. Commensurate with the inclusion criteria for this review, all uncontrolled studies had samples in which the majority of trial participants suffered from severe unipolar depression. Trials reported between 60% and 100% of their samples to have a primary diagnosis of MDD as confirmed according to standard research or clinical diagnostic criteria. Two studies175,180 also included a minority of participants (11–40%) with SMIs, in this case bipolar disorder, personality disorder or schizophrenia. However, the majority of studies (n = 6) explicitly excluded these diagnoses. 173,175–178,181,182 Other primary diagnoses present within the study samples included a minority of patients with dysthymia. 179,182
All but one180 of the identified uncontrolled studies focused on severe depression in women and all but four studies178–181 recruited mothers with MDD within the first 2 years of their child’s life. One study recruited children aged between 2 and 7 years181 and three studies recruited older children and adolescents aged between 6 and 18 years. 178–180 Child residency was inconsistently reported by the included studies, although one study was explicit in including a minority of children (12%) living separately from their depressed parent. 180
Consistent with the tendency towards parents of infants and younger children, study participants were most commonly recruited via obstetrics and gynaecology services,176,183 maternal and child health services,173,177 maternal mental health services175,181,182 and postnatal174 or community screening. 183 Two of the 11 studies recruited parent participants via child and adolescent mental health initiatives, and both studies intervened with the depressed mothers of depressed or suicidal children. 178,179 The final study recruited children directly from a psychoeducational programme specifically designed for the children of parents with mental illness. 180 These children were referred to the programme as a result of their parents’ engagement with adult mental health services.
With the exception of this final study, all included studies intervened at the level of the parent and all evaluated psychotherapeutic interventions aimed primarily at reducing mothers’ depressive symptoms. Six of these studies evaluated interpersonal therapy (IPT)176–179,182,183 and four evaluated CBT. 173–175,181 Maternal psychotherapy was delivered face-to-face in both individual176,178,179,181,183 and group formats. 173–175,177,182 Psychotherapy was delivered by a mix of health- and social-care professionals including social workers173,176 health visitors,174 psychiatric nurses,175 clinical psychologists,175 psychiatrists177 and psychotherapists. 179,182,183 Only two of the 11 interventions took place within the home. 173,181 When reported, therapy duration ranged from 4175 to 17173 weeks (median 11 weeks), with total scheduled guidance ranging from 7178 to 22177 hours (median 16 hours). Three studies failed to provide sufficient information regarding the intensity and/or duration of their intervention. 176,181,183 The only study to evaluate a psychoeducational intervention delivered a counsellor-facilitated group programme over a period of 3 consecutive days. 180
One study, conducted on children aged 6–18 years, reported validated outcomes relevant to children’s emotional health. 179 Commensurate with the observed predominance of parent-centred interventions, the most frequently assessed outcomes were secondary parent-based outcomes, in this case parental mental health symptoms (n = 10)173–179,181–183 and the quality of parent–child interactions (n = 3). 173,175,181 Three out of the 11 studies reported other secondary outcomes, specifically parent-rated child behaviour,181 self-reported social-function179 and self-reported mental health literacy and coping. 180 All 11 studies provided short-term follow-up data only.
Economic evidence
One economic study was located for our synthesis focused on severe parental depression. 116 This study was a cost-effectiveness analysis of a specialist psychiatric parent and baby day unit compared with routine primary care in the treatment of postnatal depression. The clinical study139 was included in this review as non-randomised evidence and summarised for the purposes of future research priority setting in Chapter 5, Evidence from non-randomised controlled trials and in Appendix 9. None of the other studies included in the clinical review were associated with economic evaluations.
Parent and child population
Participants (n = 60) were mothers of children aged between 6 weeks and 1 year with a diagnosis of major (93% of the sample) or minor depressive disorder according to the Research Diagnostic Criteria and a score above the threshold of 12 on the Edinburgh Postnatal Depression Scale (mean baseline score 19.24). Women were excluded if they had a puerperal psychosis, schizophrenia or a history of drug or alcohol abuse, or if they could not speak English.
Interventions and comparator
Thirty participants received routine primary care and 30 participants received the experimental intervention, a specialist psychiatric mother and baby day unit. The specialist day unit took a multidisciplinary approach to the treatment of postnatal depression and offered individual, high intensity, customised treatment, including individual, couple and family counselling, group therapy, creative therapy, hobbies and activities, stress management, assertiveness training, yoga and relaxation, and pharmacotherapy.
Outcomes
For the economic evaluation, effectiveness was measured in terms of illness recovery, defined as the failure to meet Research Diagnostic Criteria for major or minor depressive disorder. No generic, preference-based measure of QoL, the recommended approach to outcome measurement in health economic evaluations (as described in Chapter 2), was included in the study.
Methodological quality ratings
The economic evaluation was carried out as part of a naturalistic, prospective cohort study classified as non-randomised evidence. 139 Overall risk of bias was high owing to inadequate sequence generation (no random allocation), lack of allocation concealment and no blinding of participants. Lower risks of bias were observed for assessor blinding (assessors blind), attrition (no attrition) and outcome reporting (all outcomes reported).
Critical appraisal of the economic evaluation suggested moderate to low quality in terms of economic methods. While a well-defined question was posed comparing the specialist day unit to the next best alternative (routine primary care) in terms of both cost and consequences, no viewpoint was stated and effectiveness was established through a prospective cohort study with moderate risk of bias. Included costs were limited to health service costs and costs to the women (e.g. productivity, transport and child care costs) and effectiveness were measured in terms of recovery from depression. Social-care services were excluded, as were child-focused costs and effects. An incremental analysis was reported, in the form of an incremental cost-effectiveness ratio (ICER). However, interpretation was inappropriate, with the ICER being compared with the average cost-effectiveness ratio generated by routine primary care. Mean differences in costs and outcomes were not tested and univariate sensitivity analyses were not clearly justified. More sophisticated approaches to uncertainty were not taken (for example, probabilistic sensitivity analysis, cost-effectiveness acceptability curves) and the authors failed to compare their results with other studies. Results were not discussed with respect to generalisability, equity, affordability or implementation.
Results
Costs were significantly higher in the specialist day unit group compared with routine primary care (median £1351 vs. £231, p < 0.001). Recovery from depression at 6 months’ follow-up was evident for 21 of the 30 women in the specialist day unit and 7 of the 30 women receiving routine primary care. Outcomes were not tested for differences in the economic paper, but the data were available from the clinical paper139 that reported evidence of a significant difference between the two groups in favour of the specialist day unit at 3 months (χ2 = 11.34, p = 0.003) and at 6 months (χ2 = 17.89, p < 0.001). However, longer-term follow-up suggests these differences were not sustained, although only 23 of the original cohort of 60 women agreed to participate. 172
The economic data suggest that short-term improvements in outcome can be generated by the specialist day unit, but at a higher cost. The study offers no evidence to suggest that this additional cost may be considered worthwhile by society, so it is not possible to conclude that the specialist day unit is more cost-effective than routine primary care.
Decision modelling and value of information analysis
Despite some promising evidence of intervention effectiveness for selected secondary QoL outcomes in the main review (specifically parent mental health symptoms and positive parenting behaviour), a lack of economic data, a lack of high-quality studies and a substantial level of heterogeneity in the studies reviewed made decision modelling inappropriate. The ability to select studies suitable for synthesis using decision modelling techniques was particularly hindered by marked variation in the interventions reviewed, including heterogeneity in intervention content, objectives, target population, recruitment pathways, delivery format, intensity and duration (see Tables 6–9). Even in the largest intervention category (psychotherapeutic interventions, n = 30), the nature, content, intensity and duration of the interventions varied widely and spanned cognitive–behavioural, interpersonal, psychodynamic and non-directive (supportive) approaches. Clinical effectiveness also varied widely and no clear pattern favouring one particular psychotherapeutic intervention over another emerged. This heterogeneity in interventions, combined with an almost complete lack of any economic cost or resource-use data and a substantial lack of child focused measures of effect made meaningful decision modelling infeasible. Planned VOI analysis was, by implication, also prohibited by the quality of the existing data.
Discussion: implications for practice
This evidence synthesis focused on establishing the clinical effectiveness and cost-effectiveness of community-based interventions for improving or maintaining QoL in the children of parents with severe depression. Our searches revealed a much larger and more contemporary evidence base for this population than for children of parents with other SMIs (see Chapter 4). Nevertheless, evidence of the effect of interventions on children’s QoL remains sparse. This finding is in line with a previous review of economic evaluations in the field of child and adolescent mental health, which has suggested that economic evidence remains scarce and of poor quality. 184
The majority of trials included in our synthesis recruited and intervened directly with the mothers of infants in the first 2.5 years of life (69%). While the specific content of these interventions varied, the most common approaches comprised the higher intensity cognitive–behavioural and interpersonal therapies recommended by UK NICE guidelines for severe depression. 128 Comparatively fewer parenting- or child-orientated interventions were found.
Evidence of effect, therefore, remained heavily focused towards secondary parent-based outcomes, particularly self-reported maternal depressive symptoms and parent or observer reports of parental responsiveness. Pooling of short-term data suggested that severely depressed mothers who receive community-based intervention may exhibit significantly fewer depressive symptoms than mothers who receive treatment as usual. Provisional evidence also suggested that these positive effects may not be sustained over time. In order to confirm these findings, a greater number of methodologically rigorous trials with longer-term follow-up assessments are required.
Subgroup analyses suggested that psychoeducational and psychosocial interventions may ultimately have less effect on parents’ depressive symptoms than psychological interventions. Interventions focused on the parent–child relationship may also have less effect on mothers’ mental health than interventions aimed specifically at ameliorating depressive symptoms. However, caution should be expressed in overinterpreting these data owing to substantial limitations in the size and quality of the existing evidence.
Evidence-based UK recommendations for the treatment of severe depression in adults already exist. 128 However, evidence was only included in the current synthesis if trial participants were reported to be the parents of children aged < 18 years. The routine reporting of participants’ family circumstances and a greater integration of child-centred outcomes into intervention trials may be a useful first step in increasing the evidence base for the development of new community-based interventions capable of meeting both parents’ and children’s needs.
The primary focus of the current review is less about whether or not these interventions are effective in ameliorating parental mental health symptoms as whether or not they have a beneficial impact on child-centred outcomes, including parent–child interactional quality, family functioning and children’s subjective QoL. Pooling of data suggested that severely depressed mothers who receive any variant of community-based intervention may, in the short term, display significantly greater responsiveness to their children than parents who receive routine care. Insufficient data currently prevents any meaningful exploration of the association between intervention effect and intervention type. Longer-term follow-ups and rigorous head-to-head comparisons of different parent and family-based interventions are lacking. Further high-quality research is therefore needed to definitively assess the effects of different approaches, both on parenting-centred outcomes and more pertinently on children’s overall QoL.
Notably, no eligible studies identified in the current synthesis reported validated child-centred measures of QoL. Limited data permitted pooled analyses of the short-term effects of community-based intervention on children’s emotional well-being and social function. Tentative findings from these analyses suggested small or non-significant effects. However, an absence of evidence, a lack of high-quality trials, marked variation in the interventions evaluated and a notable paucity of child-centred outcome measures mean that these results must be treated judiciously. The generation of a much larger and more rigorous evidence base, with a greater focus on older children and adolescents, is urgently required.
Deficiencies in the existing evidence for clinical effect were further reflected in an overwhelming lack of meaningful opportunities for economic analysis. Only one economic evaluation was eligible for inclusion in the current synthesis and this was judged to be of moderate to low quality. Therefore, in summary, it is not yet possible to draw any firm conclusions regarding the clinical effectiveness and cost-effectiveness of community-based interventions aimed at improving or maintaining QoL in the children of parents with severe unipolar depression.
Chapter 6 The acceptability of community-based interventions to improve or maintain quality of life in the children of parents with serious mental illness
In phase 3 of the review, we systematically synthesised all quantitative and qualitative evidence relating to the acceptability of community-based interventions aimed at improving QoL for children of parents with SMI.
Methods of review
Studies were identified according to the search and review strategies outlined in Chapter 3.
Inclusion criteria
We included studies that adhered to our population and intervention inclusion criteria set out in Chapter 3. As per protocol, acceptability was defined in terms of participant uptake of, adherence to and satisfaction with community-based interventions aimed at improving or maintaining QoL in the children of parents with SMI. We included child-orientated, family-orientated and parent-orientated interventions in this synthesis. Studies examining the accessibility or acceptability of mental health services in general were excluded from the review. An existing review addresses barriers and facilitators to service use in families living with parental mental illness. 57
Primary research studies typically obtain data on intervention acceptability in one of four main ways:
-
by recording rates of intervention uptake (i.e. the number of participants receiving an allocated intervention)
-
by recording rates of intervention withdrawals (i.e. the number of participants discontinuing the allocated intervention)
-
by monitoring overall intervention adherence levels (i.e. the total or mean number of sessions attended)
-
by asking participants for their views on the interventions they have received.
For the purposes of our synthesis, we extracted data obtained by all four methods. Potential differences in the validity of these data are discussed at the end of this chapter.
In line with CONSORT principles,185 we distinguished between intervention withdrawals (i.e. those participants who discontinued treatment) and loss to follow-up (i.e. those participants not included in follow-up assessments), with only the former being extracted for this synthesis. Participant views included both quantitative and qualitative data. Quantitative data were defined to include data obtained directly from study participants via survey methods or satisfaction rating scales. Qualitative data were defined as data collected from participants’ semistructured or less structured methods, e.g. face-to-face or telephone interviews, open-ended questionnaires and focus groups.
Hierarchies of evidence for acceptability data are not well developed. 82,115,186 In the absence of any formal consensus, we extracted data from all eligible randomised trials, non-randomised studies, uncontrolled and qualitative designs. Case studies, opinion papers, descriptive studies, editorials and non-English-language publications were excluded from this synthesis.
Data management and extraction procedures
Data extraction and validity assessment were performed by one reviewer (KB) and independently verified by a second (SP). Discrepancies were resolved by referral to the original studies and if necessary via arbitration from a third reviewer (PBe).
Data extraction was guided by a data extraction sheet that detailed the study author, year of publication, key features of study design and quality, sample characteristics, and intervention settings and descriptions. When there were multiple publications for the same study, data were extracted from the most recent and/or complete publication. In cases in which duplicate publications reported additional data, these were also extracted.
Appraising the quality of acceptability data
There are various strategies and checklists available by which to appraise the quality of qualitative research. 186–190 In this review, qualitative evidence was assessed against the CASP tool for qualitative research105 and, when appropriate, the principles of good practice for conducting social research with children. 106 This included consideration of the study design, clarity of its sampling methods, the mode and timing of data collection, the age-appropriateness of the data collection methods (e.g. whether data collection methods were appropriate for helping children to express their views) and the type and rigour of the analysis.
For studies reporting quantitative satisfaction data, we chose to extract data that complemented the data extracted from our qualitative studies. We, therefore, considered the nature of the study design, the clarity of the sampling methods, the mode and timing of data collection, the age-appropriateness of the data collection methods and the type of data analysis conducted. Although all studies were assessed for quality, no study was excluded on the basis of this appraisal. This inclusive approach remained concordant with approaches taken by other acceptability syntheses115,186,191,192 and was driven by a lack of empirically tested methods for excluding qualitative studies on the basis of standardised quality criteria.
Approach to evidence synthesis
There is a growing recognition of the value of synthesising qualitative evidence to develop effective and acceptable interventions. However, methods for reviewing qualitative research are less well developed than they are for quantitative designs and remain the subject of methodological debate. 193–195 Criticism has previously been levelled at systematic reviews that seek to ‘decontextualise’ findings by integrating data obtained by markedly different methods or from different times or participant groups. 193,195 More recently, however, a case has emerged to support the role of qualitative syntheses in informing evidence-based health-care policy and practice115 and such data may ultimately be synthesised in a variety of ways.
For the purposes of this review, we adopted a ‘textual narrative’ approach. 196 This approach is a theory-driven approach that is particularly suited to the synthesis of mixed-method data. A textual narrative approach necessitates grouping studies together under prespecified areas of interest. From the outset, and before commencing data extraction, the research team developed a topic list to identify key areas of interest for this acceptability review. The list was developed from existing knowledge of the area and from previous work conducted in this field. Consultation with our review advisory panel determined our final scope and content of our topic list.
A recent SCIE review57 of the acceptability of services to support parents and the families of parents with mental health problems has delineated a typology of user and service variables most likely to impact on service and intervention acceptability. These topics formed the starting point for the list developed for this review. Broad topics that were identified by the research team as possible factors influencing parents’ or children’s views were:
-
user characteristics, e.g. sociodemographic factors, cultural or ethnic differences, mental health symptoms
-
attitudinal and social factors, e.g. custody fears, stigma, perceived need for support
-
family and life circumstance factors, e.g. conflicting demands or time pressures
-
delivery factors, e.g. intervention content, characteristics, delivery format or setting
-
personnel factors, e.g. staff expertise, staff accessibility and therapeutic alliance
-
access factors, e.g. transport, child care
-
outcome factors, e.g. lack of improvement
-
other factors.
Studies belonging to each of the subgroups delineated above were identified and synthesised narratively. Once we had extracted and synthesised all available quantitative and qualitative data, we sought, whenever possible, to integrate our findings. This was achieved by exploring the extent to which our identified qualitative themes mapped onto the quantitative satisfaction or adherence ratings reported by our included studies.
For the purposes of our synthesis, we estimated intervention adherence rates by aggregating data on intervention uptake and withdrawals as reported by the primary studies. Differences in reporting standards combined with a lack of data from high-quality randomised controlled designs made the pooling of these adherence rates infeasible. These data are instead presented in a narrative format, grouped by study population. Commensurate with the approach taken in previous chapters, studies pertaining to SMI were synthesised separately to data pertaining to severe depression. Within each of these syntheses, quantitative data relating to intervention uptake and adherence are summarised prior to presenting an in-depth synthesis of participant views. Study characteristics, quality ratings and findings are presented separately for parent and child participants.
Synthesis one: the acceptability of community-based interventions to improve or maintain quality of life in the children of parents with serious mental illness
In total, 10 studies were eligible for this synthesis. These studies are presented in Tables 12–15, which include information on study sample characteristics, intervention models and acceptability results. Five studies reported rates of intervention uptake60,118,197–199 and/or adherence and six studies contributed data to our synthesis of participant satisfaction. 58,62,117,118,120,121 One study provided both types of outcome and is therefore included in both sections. 118
| Study reference number | Design | Sample | Intervention | Target; objective | % uptake | % withdrawal | % retentiona | Definition of retention | Further information |
|---|---|---|---|---|---|---|---|---|---|
| 60 | RCT | 53 mothers with psychotic symptoms, children 5–12 years | 16 weeks’ group supportive therapy | P; parenting | 87% across groups | – | 87% across 3 groups | Attending 1+ sessions | – |
| 16 weeks’ group parenting psychoeducation | P; parenting | – | – | – | 1/9 (11%) withdrew prior to parent education but reapplied | ||||
| 16 weeks’ group CBT problem solving | C; child well-being | – | – | – | – | ||||
| 118 | nRCT | 83 mothers with psychosis, children aged < 5 years | 12 months’ home-nurse visitation | P; parenting, parent well-being | 76% | 31% | 53% | Completing intervention | Non-uptake owing to child custody losses; withdrawals owing to changed residence, rehospitalisations and child custody losses |
| 12 months’ multiagency programme | PC parenting, parent well-being | 80% | 38% | 49% | |||||
| 198 | Uncontrolled | 31 mothers with psychosis, preschool children | Minimum of 6 months’ parenting therapy | PC; parenting | – | 20% | 80% | Completing intervention and regular participation | Withdrawals owing to relinquished custody, minimal symptoms. irregular attendance not explained |
| 199 | Uncontrolled | 16 families with bipolar disorder, depression, schizophrenia, children 4–18 years | Eight sessions’ supportive parenting therapy | PC; parenting; child well-being | – | 19% | 81% | Attending 1+ sessions | Withdrawals owing to lack of readiness for programme |
| 197 | Uncontrolled | 11 families with schizophrenia, children 10–11 years | 12 sessions’ group supportive therapy | C; child well-being | 85% | – | 85% | Attending 1+ sessions | – |
| Study reference number | Intervention; population | Sample size; response rate | Sampling | Data collection methods; timing | Analysis method | Summary of qualitative findings | Quality overview |
|---|---|---|---|---|---|---|---|
| 58 | 3 × 6 hours group-based child psychoeducation, peer support; 44 children, mean age 13.0 years (SD 1.58), 61% female, living with parental Psy/Sz. BiP, PD | Unclear; unclear | Intervention completers | Three open-ended questions on written questionnaire; post
intervention Age-appropriate modifications not explicitly reported |
Summary overview | Aspects liked most: learning, having fun, doing activities and peer interactions. Aspect liked least: completing questionnaires. Most responses indicated there were no additional issues that participants wanted addressed | Limited method, data, data analysis |
| 121 | 3 × 6 hours group-based child psychoeducation, peer support; 9 children aged 7–12 years, 75% living with parental Sz, Sz-aff, BiP, 56% female, 77% Lebanese, Aboriginal or Cambodian | n = 9, 100% | Current participants | Three written open-ended questions, age-appropriate modifications not explicitly reported; post intervention | Summary overview | Things children liked most: activities, making friends. One child wanted a sibling with him/her. Parents were satisfied with information received prior to programme | Limited method, data, data analysis |
| Study reference number | Intervention; population | Sample size; response rate | Sampling | Data collection methods; timing | Analysis method | Qualitative findings | Quality overview |
|---|---|---|---|---|---|---|---|
| 118 | 1 year extended parent–child care, multiagency programme; 39 mothers with psychosis, children aged < 5 years, 45% non-white, whole sample mean age 28 years | Unclear; approximately 50% | Current and former participants, parents only | Three-point postal questionnaire; post intervention (current), unclear, (former participants) | Summary overview | Mother’s relationship with staff and choice of staff was a core ingredient in satisfaction | Some data in support of findings. Credibility not checked |
| 120 | Unlimited duration, extended parent care, multiagency programme; 13 mothers, 62% minimum Sz, BiP, PD, 61% BME, children aged nursery to school age | Unclear; unclear | Current participants | Unclear; unclear | Summary overview | Group meetings reduced isolation and stigma. Non-mental health setting perceived appropriate for children, as was approachability and accessibility of facilitators | Limited data presentation and analysis. Credibility not checked |
| Study reference number | Intervention; population | Sample size; response rate | Sampling | Data collection methods; timing | Analysis method | Qualitative findings | Quality overview |
|---|---|---|---|---|---|---|---|
| 117 | 1–2 year extended parent care, home-based nurse visitation; 50 mothers with psychosis, children aged < 6 years, mother’s age late 20s | Unclear; unclear | Intervention completers | Not reported; post intervention | Unclear | Greater change associated with greater satisfaction. Intervention participants reported greater sense of value | Limited/missing data analysis. Small survey sample |
| 118 | 1 year extended parent–child care, multiagency programme; 39 mothers with psychosis, children aged < 5 years, 45% non-white, whole sample mean age 28 years | Unclear; approximately 50% | Current and former participants, parents only | Three-point postal questionnaire; post intervention (current), unclear, (former participants). | Summary overview | Number of people rating helpful/extremely helpful: overall programme 13: caseworker 13, child’s nursery 12, mothers group 13, driver 7, lunch with staff 13, mothers time in nursery 11, camp 9, other agency groups 11, parenting video intervention 6, vocational training 12 | Small survey sample |
| 121 | 3-day group-based child psychoeducation, peer support; four parents of children aged 7–12 years, 75% Sz, Sz-aff, BiP | n = 3, 75% | Parents of current participants | Postal questionnaire with rating scales and open-ended questions; 2 weeks post intervention | Summary overview | All parent respondents would recommend the group to others | Limited data. Small survey sample |
| 62 | 8–10 sessions, clinician-facilitated parent–child psychotherapy; parents of children aged 8–14 years, 71% parental BiP | n = 7, 100% | Current participants | Seven-point rating scale from 1 (not at all) to 7 (extremely satisfied); post intervention | Summary overview, no formal method reported | Mean (SD) satisfaction scores: overall programme 5.29 (1.73), written material 5.36 (1.39), therapeutic alliance 5.4 (0.8) | Limited data. Small survey sample |
Intervention uptake and adherence
Five studies were identified that explicitly reported data on intervention uptake, withdrawals or adherence, as set out in our inclusion criteria. These studies are summarised in Table 12. Only two of the five studies that were identified were included within our clinical effectiveness and cost-effectiveness review. 60,118 The remaining three studies met the inclusion criteria for our acceptability synthesis but did not report clinical or economic data and, therefore, were excluded from our earlier syntheses. 197–199
Consistent with our eligibility criteria, all included studies had samples in which > 50% of parents met the criteria for a SMI. Four of the five studies evaluated interventions for parental psychosis or schizophrenia. 60,118,197,198 The fifth reported on a mix of parental diagnoses primarily comprising schizophrenia and bipolar disorder alongside a smaller minority of parents with MDD. 199 Two studies evaluated interventions for children or the parents of children aged < 5 years,118,198 two studies evaluated interventions for children or the parents of children aged between 5 and 12 years60,197 and one study evaluated interventions for children aged 4–18 years. 199 Heterogeneity in intervention models and content was observed (see Table 12). Of the five studies reporting relevant data, one was a randomised trial,60 one was a non-randomised trial118 and three were uncontrolled designs. 197–199 Only the non-randomised trial reported rates of intervention uptake and withdrawal by intervention group and, therefore, rigorous comparative data remain sparse.
Overall adherence rates estimated from these studies ranged from 49%118 to 87%,60 with a median of 81%; however, marked differences in the nature of the available data make the meaningful aggregation of these figures difficult. Studies reported different combinations of intervention uptake and withdrawal and used variable criteria against which intervention drop-outs were defined (see Table 12). One randomised trial aggregated data across three different intervention groups,60 thereby limiting the utility of the information provided.
When reported, intervention uptake rates ranged from 76%118 to 87%,60 with a median of 83%, and subsequent treatment withdrawals from 19%199 to 38%,118 with a median of 26%. One trial, conducted by Stott et al.,118 was notable in reporting a relatively lower rate of intervention adherence compared with other studies. This trial used a non-randomised design to compare a 12-month home nurse visitation programme for mothers with psychosis with a 12-month multiagency programme aimed at both mothers and their children. Participants were recruited from adult inpatient mental health services and the private practices of mental health professionals. Twenty-four per cent of mothers in the home-care intervention and 20% of families in the multiagency programme did not commence the intervention and an additional third in each group left prematurely (31% and 38%, respectively). The authors attributed this lack of adherence to the chaotic lifestyles exhibited by their participants and to a high rate of child custody losses. No reasons for study withdrawals were obtained directly from trial participants and, therefore, this finding must be treated judiciously. Only two other studies that evaluated parenting or family-based interventions198,199 provided full explanations for participant non-adherence. Compared with Stott et al. ,118 both of these studies reported a lower rate of intervention withdrawal (19–20%) and attributed their losses either to parents’ lack of readiness for participation,199 or to child custody losses and participant discharges on the basis of minimal symptoms. 198 The duration of these interventions was substantially shorter (≤ 6 months) than the intervention evaluated by Stott et al. 118
Only one study reported intervention uptake for a child-centred intervention. Finzi and Strange197 conducted a small-scale feasibility study of 12 sessions of group-based psychoeducational programme for children aged between 9 and 12 years. All had a parent with schizophrenia and were referred to the programme by GPs. Two out of 13 families (15%) refused to participate in the intervention project but reasons were not provided. It is thus difficult to ascertain whether these refusals were owing to the characteristics of the intervention itself or owing to broader issues related to research participation.
Children’s views of community-based interventions
Qualitative data
Two studies directly explored children’s views, both of which provided qualitative data. 58,121 Details of these studies are provided in Table 13. Both recruited the children of parents with a mix of mental health diagnoses, predominantly psychosis and bipolar disorder, and both evaluated a time-limited (maximum 18 hours) group-based psychoeducational intervention.
In both cases, convenience sampling was used to recruit the children who chose to participate in the intervention only. Sample size was reported by one study only and was small (n = 9, response rate 100%). 121 Both studies used open-ended written questionnaires although neither reported making age-specific modifications to their measures. The age of the children involved (7–18 years) potentially renders any such adaptation unnecessary. Qualitative data obtained from both studies suggested that the most popular aspects of the interventions related to children being able to learn and to have fun in a group setting. Peer–based interactions and opportunities to make friends were explicitly valued. 58,121 No in-depth qualitative studies of children’s views were found.
Parents’ views of community-based interventions
Five studies explored parents’ views of community-based intervention strategies aimed at improving or maintaining the QoL of children of parents with SMI and these studies are summarised in Tables 14 and 15. Two studies recruited mothers with psychosis or schizophrenia117,118 and the remaining three studies recruited parents, or the children of parents, with a mix of diagnoses, primarily schizophrenia and bipolar disorder. 62,120,121 Three studies evaluated an extended care intervention;117,118,120 two of these studies intervened with mothers of children aged < 6 years117,118 and the third targeted the mothers of children described as being of ‘nursery to school age’. 120 The extended care interventions that were evaluated included one home-nurse visitation programme for mothers117 delivered over 1–2 years and two multiagency programmes118,120 with a minimum duration of 1 year. The remaining studies evaluated a parent–child-based psychotherapeutic intervention62 delivered over 8–10 sessions and a 3-day group-based psychoeducation programme developed solely for use in children. 121
As before, sampling strategies were predominantly convenient, recruiting only those participants who completed the intervention. Sample sizes and response rates were inconsistently reported. Methods of collecting parents’ views were unclear in two studies. 117,120 The remaining three studies all used open-ended questionnaires. All data were collected from participants post intervention but one study also included data from former participants. 118 The delay in obtaining views from these individuals was not reported. No in-depth qualitative studies exploring parents’ views of community-based interventions were eligible for inclusion in this synthesis.
Qualitative data
Two of the five studies that were identified provided nested qualitative data; both studies explored the potential relationship between intervention acceptability and intervention personnel (see Table 14). Stott et al. 118 explored the views of an unspecified number of current and former participants in a long-term (1-year) multiagency programme of care and found that the nature and quality of mothers’ relationships with staff were a key ingredient in their satisfaction. Alder120 similarly explored the views of women participating in an unlimited multiagency intervention and reported mothers to attribute high value to the accessibility and perceived approachability of staff. In both instances, however, the number of women providing data and the method and timing of data collection were not clearly reported. The credibility of these findings thus remains unclear.
The study by Alder120 additionally examined the potential relationship between intervention acceptability and intervention delivery. Opportunities to meet in a small group were perceived favourably by the mothers in this study who felt that this delivery format was particularly advantageous in overcoming parents’ perceptions of social isolation and stigma. A non-mental health setting for group meetings was also perceived to be appropriate for interventions involving children. However, no data were collected from intervention drop-outs and, therefore, bias cannot be ruled out.
No qualitative data were identified that explored the potential influences of user characteristics, attitudinal factors, family circumstances, access factors or outcome factors on intervention acceptability for parents.
Quantitative data
Four of the five studies that explored parents’ views provided quantitative data relating to parents’ views. 62,117,118,121 Two of these studies assessed parents’ overall satisfaction with an intervention (see Table 15). Beardslee et al. 62 used a seven-point Likert scale to assess satisfaction with a brief (< 10 sessions) family-based psychotherapeutic intervention. Data were collected post intervention from seven parents, representing a 100% response rate. Moderate to high levels of satisfaction were reported for the overall intervention, as well as for the written materials used and the quality of the therapeutic alliance achieved. In a similarly small-scale study, Pitman and Matthey121 used a non-specified rating scale to survey four parents of children attending a 3-day group-based psychoeducation programme. Three parents responded, all of whom indicated that they were satisfied with the information they had received and all reported that they would recommend the intervention to others.
In contrast, Stott et al. 118 did not report overall satisfaction but instead rated parents’ satisfaction with different components of a 12-month multiagency intervention. An unspecified number of current and former participants were surveyed on a three-point Likert scale, for which response rates were reported to be approximately 50%. Moderate to high levels of satisfaction were observed for all components of the intervention, although relationships with staff and social group-based activities were valued the most. Notably, lower satisfaction ratings were observed for a video intervention aimed at enhancing parenting behaviours.
The fourth and final study providing quantitative data was unique in addressing the potential association between parents’ satisfaction and intervention outcome. 117 This study used a randomised controlled design to compare a 1- to 2-year high-intensity home-nurse visitation programme to minimal-intensity standard care in 50 mothers with psychosis, all with children aged < 6 years. The study reported that greater clinical change was associated with greater satisfaction with the home programme post intervention. However, the method by which satisfaction was measured remained unclear and descriptive data from the satisfaction measure were not presented. The validity of this finding is, therefore, difficult to establish.
Integrating quantitative and qualitative data
All five studies that provided data on parents’ views were limited in the scope of their discussion and in their consideration of the topics identified as potentially important by a previous review. 57 No in-depth qualitative studies exploring parents’ perspectives were found. The small amount of qualitative data that was available appeared to highlight a need for staff to establish open relationships with parents and for interventions to be designed in such a way that potential barriers arising from stigma and social isolation can be overcome. Only two of the four studies that collected quantitative data partially addressed these themes. 62,118 These studies similarly emphasised the importance of the staff–parent relationship despite the interventions under study being notably different to those evaluated by the qualitative studies. The available quantitative data, like the qualitative data, were limited in both size and quality. Further study in this area is required.
Synthesis two: the acceptability of community-based interventions to improve or maintain quality of life in the children of parents with severe depression
Thirty-seven studies were eligible for a synthesis of evidence relating to the acceptability of community-based interventions to improve or maintain QoL in children of parents with severe depression. Thirty-three studies reported rates of intervention uptake or adherence (see Appendix 9, Table 41) and 18 studies contributed to our synthesis of intervention satisfaction (Tables 16 and 17). Thirteen studies provided both types of outcome and are therefore included in both sections. 148,149,153,159,162,173–178,180,181
| Study | Intervention; population | Sample size; response rate | Sampling | Data collection methods; timing | Analysis method | Summary of qualitative findings | Quality overview |
|---|---|---|---|---|---|---|---|
| 180 | 3-day child psychoeducation, peer support; 25 children aged 8–16 years, mean age 10.8 (SD 2.0) years; 61% living with parental MDD | 25 children; 100% | All participants | Three written open-ended questions ongoing with intervention. Age-appropriate modifications not explicitly reported | Summary overview | The majority indicated there was not anything they would change. Some would have liked a longer programme. Children liked games and meeting new people | Limited method, data, data analysis |
| Study | Intervention; population | Sample size; response rate | Sampling | Data collection methods; timing | Analysis method | Summary of findings |
|---|---|---|---|---|---|---|
| 153 | Eight sessions of individual parents ST vs. TAU; 60 mothers, 68% MDD, mean age ST: 27.6 years, TAU: 24.6 years, children aged < 1 year | Unclear | All intervention participants | Tape-recorded interview; 3 months post intervention | Summary overview | Supportive relationship with health visitor appreciated. The importance of being given ‘permission to speak’ to overcome guilt and blame was valued |
| 148 | 10 sessions of group parent CBT vs. waiting list; 20 women, mean age CBT: 29.6 years, mean age waiting list: 27.9 years, 100% Australian, UK, Irish born, children aged < 1 year | n = 20; 100% | All participating in trial | Tape-recorded semi-structured interview; unclear | Summary overview | ‘Many’ women expressed a need for support services to extend beyond hospital admission |
| 159 | 6-week parent ST vs. TAU; 41 women, 100% MDD, mean age: ST: 21.2 years; TAU: 29.5 years, children aged < 1 year | n = 41; 100% | All participating in trial | Semi-structured interview; post intervention | Summary overview | The majority of ST parents valued freedom to talk to neutral staff. Staff showed genuine interest in women and helped to normalise experiences. Routine care perceived as limited and superficial in contact |
| 67 | 12-week mother–infant therapy vs. TAU; 32 women, 100% MDD, mean age therapy: 28.06 years (SD 4.40 years) TAU: 34.46 years, children aged < 2 years | n = 18; unclear | All intervention participants | CSQ; post intervention | Summary overview | Aspects most helpful: sharing feelings as parents, discussing experiences. 100% would recommend group to a friend |
| 181 | Eight sessions of parent CBT; 11 women, 100% MDD, children aged 2–7 years, mean 43.4 months (SD 15.3 months) | n = 10; 100% | All intervention participants | Face-to face interview; 1–4 weeks post intervention | Summary overview | Positive elements: self-monitoring tasks and ‘talking to someone’. One participant would have liked to work on couple relationship. The most critical comments related to the time-limited nature of therapy |
| 175 | Eight sessions of parent CBT; 45 women, 61% MDD, Mean age 32.0 years, children aged < 1 year | Unclear; unclear | All intervention participants | Open ended evaluation form; post intervention | Summary overview | Many wanted greater inclusion of partners. Some disliked the open format. Group work addressed social isolation, loneliness, stigma and normalised the experience |
| 180 | 3-day child psychoeducation, peer support; 61% MDD, children aged 8–16 years | n = 7; 28% | All parents of child participants | Postal rating scales and open-ended questions; 2 weeks post intervention | Summary overview | Parents wanted to share programme with other children in the family and have greater parental participation |
| 178 | 10-session parent IPT; 13 mothers 100% MDD, mean age 45.0 years, 77% white children aged 12–18 years | n = 11; 100% | All participants commencing intervention | CSQ, face-to face semi-structured exit interview, post-treatment | Summary overview | Helpful to talk to an unbiased, affirming professional. Majority felt treatment duration acceptable, some expressed a wish to continue work. Some thought it would be helpful to have at least one session with mother, child, and child and mother therapists. Mean CSQ post engagement 27.2 (SD 74.0), mean CSQ scores post therapy 29.6. Both indicated a high degree of satisfaction |
| 174 | 12-week parent CBT; eight women, 100% severe depression, children aged < 18 months | n = 8; 100% | All intervention participants | Anonymous open-ended questionnaires, post intervention | Summary overview | Every session acknowledged as helpful by one or more group member, ‘crooked thinking’ and self-esteem sessions most valuable. Group work and sharing experiences valued for normalising experiences and reducing isolation. Caring and supportive leadership and provision of a safe environment important |
| 200 | Up to 45 sessions extended parent care vs. TAU; 60 women, 93% MDD, mean age 27 years, children aged < 1 year | n = 60; 100% | All participating | 17-item satisfaction questionnaire using rating scales and open-ended questions, post intervention | Emergent themes, comparative analysis | Intervention staff perceived as helpful, time to talk to someone outside the family useful, peer and staff support reduced social isolation. 93% intervention, 43% TAU satisfied (p = 0.04). 90% intervention vs. 40% TAU would want the same treatment again. 93% intervention vs. 43% TAU would recommend to a friend |
| 201 | 1 year + extended care, family case management services; 9 parents, 55% MDD, aged 26–40 years, 33% African American, children aged 2–14 years | n = 9; 100% | Preselected participants | Face-to face, semi-structured Interviews, during ongoing intervention | Emergent themes with credibility checks | Unlimited access to staff and mutual respect afforded trust and engagement. Staff advocacy role and provision of concrete or material support valued. Parents felt perceived by staff as individuals, not patients. Strengths-based approaches positively influenced self-esteem |
| 142 | Parent–child video psychoeducation vs. waiting list; 76 families, children 7–12 years, 88% parental MDD, Mean child age 10.1 (SD 1.6) years, mean parent age 39.5 (5.6) years, 93% white | Unclear; unclear | All participating in intervention | Satisfaction ratings; post intervention | Descriptive overview | At least somewhat satisfied: 84% parent video, 89% child video, 86% parent manual. 95% said intervention provision improved satisfaction with health plan. Acute depression associated with less satisfaction |
| 173 | 17-week parent CBT; 6 mothers, 100% MDD, mean age 23 years, 46% Caucasian, children aged < 3 months | n = 26; 100% | All participating in intervention | Five-point rating scale; post intervention | Descriptive overview | Excellent therapist–home visitor collaboration (mean 4.50, SD 0.86) and appropriate confidentiality (mean 4.59, SD 0.59). (1 = strongly disagree, 5 = strongly agree) |
| 177 | 10 sessions of parent IPT; 18 women, 100% MDD, mean age 32 years, children aged < 1 year | n = 17; 100% | All completing intervention | Five-point Likert scale | Descriptive overview | All agreed therapy was an acceptable way to address problems (mean 4.4, SD 0.5), and would recommend therapy to others (mean 4.94, SD 0.24). (1 = strongly disagree, 5= strongly agree) |
| 149 | 6 sessions of CBT (psychologist) and 6 sessions of CBT (nurse) TAU; 68 women, mean age: TAU 30 (3.3) years, nurse 33.1 (4.4) years; psychologist 31.4 (5.6) years, 88% Australian born | n = 46; 68% | All completing intervention | Binary treatment sufficiency; post intervention | Descriptive overview | 89% nurse CBT, 86% psychologist CBT, 64% TAU indicated treatment sufficient |
| 176 | 8-week parent IPT; 12 mothers, 83% MDD, mean age 25 years, 75% African-American, children aged < 1 year | n = 12; 100% | All intervention participants | Four-item treatment satisfaction survey; ongoing with intervention | Descriptive overview | Mean satisfaction score across all sessions 4.8 ± 0.18 SD (maximum score of 5) |
| 202 | Parent–child CBT vs. parent psychoeducation; 37 families with children aged 8–14 years, 82% living with parents with MDD, 76% were mothers with MDD | n = 37; 100% | All parent trial participants | Seven-point Likert scale, post intervention and 10-month follow-up | Comparative analysis | Post intervention: overall satisfaction – CBT 6.1 (SD 1.2), lecture 3.8 (SD 1.7); satisfaction with written material: CBT 5.7 (SD 1.5), lecture 4.1 (SD 2.0). Follow-up: post intervention: overall satisfaction – CBT 6.0 (SD 1.1), lecture 3.8 (SD 1.6); satisfaction with written material: CBT 5.7 (SD 1.7) lecture 4.0 (SD 1.8) |
Intervention uptake and adherence
Thirty-three studies were identified that explicitly reported data on intervention uptake or adherence; these studies are summarised in Appendix 9. All of the studies that were identified were included within our clinical effectiveness review.
Heterogeneity in study populations, intervention models and content was observed. Twenty-seven studies evaluated psychotherapies aimed predominantly at parent well-being or parenting behaviours (see Appendix 9, Table 41), three evaluated parent-based psychoeducation,63,141,155 two evaluated psychotherapies aimed at both parents and children141,143 and one evaluated parent–child psychoeducation. 143 Only one evaluated an extended 12-month model of care involving both parents and children. 144 Some studies evaluated more than one intervention and, therefore, numbers may not total 100%. Only one study of a child-centred intervention was eligible for inclusion in this synthesis. 180 Of the 33 studies that reported relevant data, 20 were RCTs,63–65,138,141,143–146,148–153,155,157–159,176 another two were non-randomised trials allocated on service availability171 or patient preference170 and the remainder were all uncontrolled designs. 173–183
Overall adherence rates estimated from treatment uptake and withdrawal rates ranged from 50%182 to 100%,155 with a median of 81%, across all variants of community-based intervention. When reported, adherence rates for parent-based psychotherapies ranged from 50%182 to 98%,158 with a median of 80% (n = 29), and for parent-based psychoeducation ranged from 66%63 to 100%,155 with a median of 95% (n = 4). These rates compared favourably with adherence rates reported across treatment as usual conditions (range 7145–98%,158 median 85%, n = 7). As before, however, marked differences in the availability and the nature of the data reported make the meaningful interpretation of these figures difficult. Rates of intervention uptake, withdrawal and adherence were inconsistently reported and defined by according to different criteria by the authors of the primary studies. The authors’ definitions of intervention adherence were most often the number of participants completing the intervention, but also included the number attending one session,158,168 two or more sessions,63 attendance at all sessions,173,177,178,182 ‘regular’attendance64 or attendance at four or more sessions out of 10. 152 Five RCTs did not report intervention uptake or withdrawals, choosing instead to present the mean number of sessions attended. 138,143,149,153,157 A further two trials reported rates of treatment completion but failed to break these figures down across different intervention groups. 144,159
Three studies were notable in reporting estimated adherence rates of ≤ 60%. Two of these evaluated a 12-week parent-based IPT programme182,183 and one evaluated a 10 week parent-based CBT programme. 148 Meager and Milgrom148 recruited 20 depressed postpartum women via advertising in local hospitals and maternity and child health centres. Women were allocated by random assignment to 10 weekly, 1.5-hour sessions of group CBT or a waiting list control. Of the 10 women assigned to the intervention, one woman did not complete any sessions and a further three completed three sessions or fewer. Reasons for not completing the treatment programme were varied and included user factors (e.g. physical illness), family and life circumstance factors (e.g. family commitments) and access barriers (e.g. transport problems). The 10 mothers who were allocated to the waiting list control were also subsequently offered the intervention. Only three (30%) completed the programme. Two did not commence the intervention because they were no longer interested, one was in alternative therapy and one had been hospitalised in the intervening period. Three women withdrew from therapy because of work commitments or because of difficulties in organising attendance.
The two studies that evaluated 12-week IPT182,183 were both uncontrolled studies reporting adherence rates of between 50 and 58%. One failed to provide any reasons for the rate of adherence that was observed183 and in the second study, Klier et al. 182 recruited 34 postpartum women from a maternal mental health service, 22 of whom met study eligibility criteria. Seventeen Caucasian women aged between 27 and 41 years commenced the intervention. Five women (23%) did not attend owing to transportation difficulties, child care issues or a reluctance to be treated within the context of a research trial. A further six women terminated therapy early, two of whom were diagnosed with personality disorders and left after the first group session. The remainder withdrew because of work or school commitments.
Children’s views of community-based interventions
Qualitative data
Only one study included in our depression synthesis reported children’s views of intervention acceptability180 (see Table 16). Twenty-five children aged between 7 and 12 years participating in a 3-day group-based psychoeducational programme were asked to complete open-ended questionnaires both during and following the intervention. The response rate was 100%. The authors did not report making any age-specific modifications to their questionnaires and no formal qualitative analysis was undertaken. In line with our previous synthesis, responses suggested that the most popular aspects of the intervention related to children having fun with their peers. When asked what they would most like to change about the programme, an unspecified number of children expressed a preference for a longer intervention. No other data on children’s views were found.
Parents’ views of community-based interventions
In total, 18 studies explored parents’ views. Eleven of these evaluated parent-based psychotherapies,148,149,153,159,173–178,181 two evaluated parent–child psychotherapy,67,202 two evaluated parent or parent–child-based psychoeducation142,202 and two evaluated more complex, extended care interventions. 200,201 One study obtained parents’ views of a 3-day psychoeducational programme aimed predominantly at children. 180 Eleven of the 18 studies evaluated interventions aimed either directly or indirectly at children aged < 2.5 years. 67,148,149,153,159,173–177,200 A full summary of the characteristics of all of the included studies is provided in Table 17.
Once again, sampling strategies were predominantly convenience-based with all studies recruiting only those participants who remained in the interventions. Sample sizes and/or response rates were not provided by four studies. 67,142,153,175 When reported, sample sizes ranged from 7180 to 60200 participants, with a median of 19, and response rates from 28%177 to 100%. 148,159,173,174,176–178,181,200–202 Parents’ views were most commonly collected by binary or Likert-style rating scales, or open-ended questionnaires. Only six studies collected data by interview. 153,148,159,178,181,201 Qualitative data were analysed largely by thematic analysis and only one study reported taking steps to confirm the trustworthiness of their data. 201 Timing of data collection varied from intervention entry178 to 3 months post intervention. 153 Two studies collected satisfaction data during the intervention period,176,201 with one obtaining repeated ratings over multiple treatment sessions. 176 The potential for response bias in these studies is high.
Qualitative data
Eleven of the 18 studies included in this synthesis provided qualitative data pertaining to parents’ views. 67,148,153,159,174,175,178,180,181,200,201 These studies primarily focused on the potential relationship between intervention acceptability and intervention personnel. Nonetheless, many of the topics that parents discussed in relation to this topic were closely intertwined with attitudinal or social factors. Difficulties were encountered in separating the two for synthesis and, therefore, these data are presented together.
Seven of the studies emphasised the importance of establishing an emotionally supportive alliance between parents and staff, such that parents were afforded the necessary freedom to discuss their concerns. 153,159,174,178,181,200,201 Five of the seven studies focused on short-term psychotherapeutic interventions of up to 12 sessions,153,159,174,178,181 of which four highlighted the need for staff to facilitate the provision of a safe and non-judgemental environment in which mothers could share their feelings. 153,159,174,178 In two of these studies, discussion explicitly highlighted a perceived sense of culpability among mothers and a fear of how other people may react to their experiences. 153,159 Wickberg and Hwang159 interviewed 41 Swedish mothers at the end of a 6-week course of nurse-facilitated counselling. Respondents reported feeling free to talk about everything with their nurse, without any sense of blame or censure. Similarly, Holden et al. 153 examined the views of an unspecified number of women completing an 8-week programme of supportive therapy. Respondents reported feeling shame and guilt about the way they were feeling and highlighted specifically the value they placed on being given ‘permission’ to talk.
Two studies were notable in obtaining the views of women experiencing extended care interventions. Boath et al. 200 used a non-randomised design to compare a multicomponent programme, delivered over 45 sessions in a mother and baby day unit, with routine primary care. Open-ended questionnaire responses obtained from the 30 women who were allocated to the intervention once again identified the importance of approachable and communicative staff. Unbiased and affirming professionals who proactively and routinely enquired about mothers’ feelings were considered to be particularly valuable in overcoming the stigma experienced by these participants. Similarly, Hinden et al. 201 interviewed nine parents from eight families who had engaged in at least 12-months of an intensive case management service. Parents once again emphasised the saliency of accessible and collaborative staff, describing their appreciation of case managers who treated them ‘as people rather than patients.’ Trust and mutual respect between parents and staff was reported to be a cornerstone of this intervention and a mediating process from which all other positive outcomes emanated. However, in this instance, no data from parents were presented and, therefore, the credibility of these findings remains difficult to establish.
Ten of the 11 studies providing qualitative data separately focused on the potential relationship between intervention acceptability and intervention delivery. 67,148,159,174,175,178,180,181,200,201 Four of these studies addressed issues relating to short-term67,174,175 and longer-term200 group therapy, all of which were largely supportive of this delivery format. Parents were relatively consistent in perceiving group interventions to provide a route for much needed peer support and positive interpersonal relationships. In addition, four studies discussed the benefits of sharing parenting or illness concerns, particularly the role that group membership had played in overcoming stigma and normalising parents’ experiences. 67,159,174,175 However, two small-scale studies reported that a minority of parents were resistive to group-based interventions. Davies174 used open-ended questionnaires to collect the views of eight postpartum women at the end of a 12-week CBT programme. Although all participants reported finding small group work helpful, one woman who dropped out of treatment early expressed some discomfort in talking openly in a group forum, attributing her difficulties directly to the chronicity of her depressive symptoms. Griffiths and Barker-Collo175 also collected data from an unspecified number of women at the end of an 8-week CBT programme for postnatal depression for postnatal depression. A partners evening was included in the interventions and feedback suggested that this was an invaluable component of the group for the majority of women questioned. It was, however, recommended that partners be better prepared for this session, as some couples expressed dissatisfaction with the open format of the meeting.
Five studies also highlighted parents’ views of the availability of community-based support. 148,159,178,181,201 In four of these studies, discussion centred on a desire for a longer or more accessible models of care. 148,159,178,181 Meager and Milgrom148 interviewed 20 women at the start of a 10-session CBT programme and found that many expressed a preference for support services that extended beyond their previous hospital admissions. Similarly, Edwards181 interviewed 10 women completing a comparable programme and found the most critical and negative comments to be related to the time-limited nature of the intervention. Swartz et al. 178 interviewed 11 mothers of children aged 12–18 years at the end of nine sessions of IPT and found that the majority of women thought the duration of treatment was acceptable. However, some participants expressed a wish to continue working with their therapist beyond the end of the programme.
Only one study discussed parents’ views of a longer-term intervention. Hinden et al. 201 interviewed nine parents from eight families who had engaged in at least 12 months of an intensive case management service. Parents were positive in their appraisal of this intervention and identified the 24-hour, 7-days-a-week service model to be an advantageous and essential feature of the intervention. However, as discussed previously, a lack of supporting data serves to limit the credibility of this finding.
Five studies focused on the relationship between intervention acceptability and the scope of the support that was received. 175,178,180,181,201 Four of these studies evaluated short-term parent- or child-based interventions and all highlighted a preference among parents for greater couple- or family-focused participation. 175,178,180,181 Only one study, conducted by Hinden et al. ,201 evaluated an longer-term (12-month) intervention specifically designed to meet the needs of multiple family members. In line with the views obtained from other studies, parents were who had participated in this intervention expressed their appreciation for a service that was family centred and suggested that this comprehensive approach may have been instrumental in achieving positive parenting and child custody outcomes. Methodological weaknesses in this study have already been acknowledged.
The study by Hinden et al. 201 was one of only two studies174,201 that provided qualitative data relating to intervention content. The nine parents’ interviewed in this study were reported to value several unique aspects of the case management model. Particular appreciation was expressed for the greater consistency that case managers introduced into parents’ care and for the advocacy that occurred between parents and other service providers. Other aspects of the programme that were valued include the provision of financial and material support and the adoption of a strengths-based approach that increased self-esteem and self-efficacy among parents. In a similarly small-scale study, Davies174 interviewed eight parents who had completed a 12-week CBT programme. All eight respondents found at least one session helpful, with sessions on ‘crooked thinking’ and self-esteem being the most highly valued.
No qualitative data were identified that explored the potential influence of user characteristics, family circumstances, access factors or outcome factors on intervention acceptability.
Quantitative data
Eight of the 18 studies that explored parents’ views provided quantitative data, all of which reported parents’ levels of overall satisfaction with an intervention, or with particular components incorporated within it. 142,149,173,176–178,200,202 The majority of these studies evaluated psychotherapeutic interventions aimed primarily at reducing parental mental health symptoms (see Table 17).
Three studies sought to compare parents’ overall levels of satisfaction across two or more treatment conditions. 149,200,202 All of these studies were judged to be at risk of bias. Milgrom et al. 149 used a randomised design to allocate 68 depressed postnatal women to usual care or six sessions of CBT delivered either by a nurse or a psychologist. Forty-six women (68%) responded to a binary question regarding treatment sufficiency. The majority of women in all groups indicated that treatment was sufficient (64%, 88% and 86% for the TAU, nurse-delivered and psychologist-delivered trial intervention arms, respectively), with a trend towards higher satisfaction in the intervention groups. Failure to provide reasons for sample attrition and an incomplete response to the satisfaction questionnaires raises doubt as to the validity of these findings.
Beardslee et al. 202 used a seven-point Likert scale to assess parents’ satisfaction with a brief, time-limited family-based psychotherapy programme compared with parent-based psychoeducation. Compared with 32 parents randomised to the educational intervention, the 34 parents receiving psychotherapy reported a significantly higher level of overall satisfaction and a significantly higher level of satisfaction with the written materials that were presented. Once again, data were only reported for those families who had participated in the intervention and provided data at both baseline and follow-up assessment. The potential for attrition bias thus remains high.
In a non-randomised design, Boath et al. 200 asked 60 depressed postnatal women to complete a users’ view questionnaire and found that 28 out of 30 women receiving a prolonged 45-session multicomponent intervention felt satisfied with their care. Twenty-seven also indicated that they would like the same treatment again. This compared with only 13 out of 30 women who felt satisfied with routine care (p = 0.04) and 12 who would want the same treatment again (p < 0.001). Participants in this study were allocated on the basis of service availability, which raises the potential for selection bias. Satisfaction ratings may also have been influenced by differences in participants’ knowledge of alternative treatments at each site.
Four uncontrolled studies also reported relevant quantitative data,159,176–178 across which parents’ satisfaction ratings remained consistently high. Grote et al. 176 asked 12 women, mostly African-Americans, to complete a four-item treatment satisfaction survey at the end of each therapy and maintenance session in an 8-week IPT programme. Average treatment satisfaction across all sessions was reported to be high (mean 4.8, SD 1.8, maximum possible score was 5). Similarly Swartz et al. 178 collected satisfaction data from 11 depressed mothers seeking treatment for mental health problems in their children. Satisfaction was measured for all treatment attendees on the client satisfaction questionnaire (CSQ) both after the first IPT engagement session and post intervention. The mean CSQ score was 27.2 (SD 4.0) following engagement and 29.6 (SD 3.7) post intervention, indicating a high degree of overall satisfaction. Reay et al. 177 surveyed 17 women completing 10 sessions of IPT and found that all participants reported therapy to be an acceptable way to address their problems. Likewise, Wickberg and Hwang159 interviewed all 41 women who had participated in their 6-week supportive therapy programme post intervention. All women responded and indicated that they would recommend the group to a friend.
Only one study considered the impact of mental health on parents’ satisfaction with an intervention. Butler et al. 142 surveyed parents at the end of a brief parent–child-based psychoeducational intervention. The overall proportion of parents responding remained unclear, although 95% reported that provision of the intervention would improve satisfaction with their health plan and the majority (84–86%) were at least somewhat satisfied with the materials presented. The authors reported that the presence of acute depressive symptoms in parents was associated with a lower rate of satisfaction.
A final study conducted by Ammerman et al. 173 provided the only quantitative data regarding professional relationships. Twenty-six women participated in a 17-week home-based CBT programme and all responded on a five-point Likert scale post intervention. Mothers reported that there was excellent collaboration between therapists and routine home visitors and that an appropriate level of confidentiality had been maintained. The quality of the alliance between mothers and the therapists was not assessed.
Integrating qualitative and quantitative data
Overall, a notable number of studies provided data for the synthesis of parents’ views, although very few high-quality in-depth studies were found. Key topics emerging from the available qualitative data highlighted the significance of establishing high-quality relationships between staff and parents, and the importance of delivering interventions in such a way that stigma and social isolation could be reduced.
Quantitative studies rarely sought to examine parents’ views on these potentially more nebulous issues. Only one quantitative study asked parents to rate their satisfaction with intervention personnel, quantified in terms of their satisfaction with the therapeutic alliance. 173 The vast majority of quantitative studies remained focused on overall satisfaction or on satisfaction with particular aspects of an intervention programme. No large-scale satisfaction surveys were found. The available quantitative data, like the qualitative data, thus remain limited in both number and quality. Further study is needed to address these important evidence gaps.
Discussion: implications for practice
This third evidence synthesis focused on establishing the acceptability of community-based interventions for improving or maintaining QoL in the children of parents with SMI. We identified studies via a systematic and comprehensive literature search, independently selected those that were eligible and extracted synthesised all relevant data using a textual narrative approach. Commensurate with the findings of our clinical effectiveness and cost-effectiveness reviews, a paucity of high-quality evidence was found. The majority of data that currently exist are quantitative in nature and pertain primarily to parents with severe depression. In-depth qualitative studies of the views of parents with SMIs, and more pertinently the views of their children, are strikingly sparse.
Despite the paucity of research, some conclusions with potentially important clinical implications can be drawn from the findings of this review. Preliminary data suggest that families adherence to community-based interventions may be relatively high. Adherence rates estimated across the different populations and intervention models remained reasonably consistent, with median rates of 80–95% (i.e. non-adherence rates of 5–20%). These rates compare favourably with those reported for other community-based services for adults with SMI. A meta-analysis of non-adherence to medication and scheduled appointments in community psychiatric services among people with psychosis has reported a mean rate of 25.78%, i.e. an adherence rate of approximately 74%. 203 This may imply that families would be keen to engage in interventions were they to be offered. Nonetheless, there exist a number of limitations that serve to temper the validity of these findings and the generalisability of this review.
First, rates of intervention uptake and adherence were inconsistently reported across the studies included in our syntheses and a lack of data from high-quality RCTs made meta-analysis inappropriate. The difficulties that were encountered in aggregating and interpreting these data highlight a need for a more consistent approach to the definition and presentation of these issues. Greater adoption of reporting standards, including those advocated by the CONSORT group,185 is required if such data are to play a meaningful role in rigorous evidence syntheses and in the development of future evidence-based services.
Second, measures of intervention uptake and adherence are in themselves insufficient measures of intervention acceptability. This is because participation in an intervention may remain somewhat independent from participant satisfaction, particularly when the availability of alternative services or treatment options is limited. Much more emphasis needs to be placed on data collected directly from intervention participants. As evidenced by the current synthesis, such data may be either quantitative or qualitative.
The overall pattern of findings from the quantitative satisfaction data included in this synthesis is that parents generally hold community-based interventions in high regard. However, additional limitations in the nature of these data limit the utility of these results. These limitations include the recruitment of small study samples, inconsistent reporting of response rates and likely attrition biases arising from samples that include only intervention completers. Moreover, satisfaction as a service outcome is notoriously difficult to measure. Patient satisfaction surveys have long been acknowledged to generate high levels of satisfaction with low response variability. 204 Greater emphasis should thus be placed on acceptability data obtained via more qualitative approaches.
Qualitative data identified for the current syntheses were consistent in highlighting the importance of developing intervention models and delivery mechanisms that are capable of transcending the potentially high levels of social isolation and stigma faced by families living with SMI. Both children and parents placed considerable value on peer support and normalising activities, with relationships between parents and staff appearing particularly influential in determining intervention acceptability. The omission of these factors from the majority of surveys further emphasises the inherent limitations in the existing quantitative data.
Our qualitative findings should also be treated with caution. Although preliminary findings have identified specific areas with high face validity for the development of intervention programmes, no rigorous qualitative studies were found. Therefore, further research in this area is required. These studies should seek to verify the credibility of their findings by paying adequate attention to the sampling and data collection methods that are used and by employing formal and methodologically appropriate qualitative analytical strategies. On the basis of current evidence, it is not yet possible to draw any firm conclusions regarding the acceptability of community-based interventions aimed at improving or maintaining QoL in the children of parents with SMI.
Chapter 7 Discussion
Improving the lives of children born to a parent with SMI is an increasingly urgent political and public health concern. 2 The key challenge for services is in knowing when, and how best, to intervene. With an emphasis on evidence-based NHS practice, there is a pressing need to demonstrate the clinical effectiveness and cost-effectiveness of interventions for these populations. This HTA sought to identify how future UK resources might best be distributed to improve the QoL of children through evidence-based health- and social-care development. Its primary aim was to conduct a comprehensive and rigorous synthesis of all available evidence to establish the clinical effectiveness, cost-effectiveness and acceptability of community-based interventions aimed at improving or maintaining the QoL of children of parents with SMI. Findings from this research are discussed below within the context of the objectives outlined in Chapter 1.
Objective 1: overview of the evidence
We conducted two parallel syntheses. These were constructed to accommodate two distinct definitions of SMI. First, we identified and synthesised all randomised trials in which serious parental mental illness encompassed schizophrenia or related disorders, puerperal or non-puerperal psychosis, bipolar disorder and personality and borderline personality disorders. In this synthesis, we excluded studies in which < 50% of participants experienced these illnesses as a primary diagnosis on the basis that any observed effects would be difficult to generalise to our population of interest. A second synthesis was undertaken on studies in which the majority of the population (≥ 50%) had a primary diagnosis of major unipolar depressive disorder.
The a priori distinction between SMIs and more severe unipolar depressions was reflected in much of the identified research literature. Only one trial that recruited parents with MDD also recruited participants with bipolar or schizoaffective disorder. 141 Half of all trials that recruited depressed parents explicitly excluded schizophrenia, psychosis, bipolar and borderline personality disorders. No randomised trials were identified that could only meet our 50% sample criterion by mixing the two populations.
Across both our primary (SMI) synthesis and our secondary (depression) synthesis, 57 studies fulfilled our eligibility criteria58–67,117–122,138–159,166,170,171,173–183,197–201 (see Figure 2). A substantial proportion of these (n = 28) were classified as non-randomised or uncontrolled studies. 58,59,62,118–122,139,166,170,171,173–183,197–201 In total, 29 RCTs60,61,63–67,117,138,140–159 were included; 26 (90%)63–67,138,140–159 intervened exclusively or predominantly with families living with parental severe unipolar depression. Only three randomised trials60,61,117 provided evidence of effects in families experiencing other SMIs and all were published over 25 years ago, between 1982 and 1984, in the USA.
More contemporary literature published in the last 10 years (i.e. post 2002) showed a striking predominance of trials of interventions for parents with severe unipolar depression. Eighteen64,66,67,138,144–146,148–153,155–159 of the 2663–67,138,140–159 (69%) RCTs included in the depression synthesis were for maternal depression occurring in the first 2.5 years of a child’s life. Two key factors may help to explain this focus. First, early interventions aimed at enhancing parenting and/or child development have the potential to confer significant long-term personal, societal and economic benefits. 205,206 Second, depression is far more common over a woman’s lifetime than other serious maternal mental illness and especially around childbirth;207 however, perinatal depression is more likely to be time limited and to resolve with short-term intervention. 208 It is unclear how generalisable the findings from this synthesis are to other populations and a clear need remains for research aimed at children of parents with other serious and enduring mental illnesses. In summary, there is a striking lack of high-quality evidence for the clinical effectiveness and cost-effectiveness and acceptability of community-based interventions to improve or maintain QoL in children with parents suffering from SMI.
User characteristics
Out of the 29 trials included across our two syntheses (SMI = 3, severe depression = 26), the vast majority (n = 24,60,61,64–67,117,138,142,144–146,148–159 83%) evaluated interventions for children, or the parents of children, aged ≤ 12 years. Eighteen64,66,67,138,144–146,148–153,155–159 out of 2960,61,63–67,117,138,140–159 (62%) targeted parents of children in the first 2.5 years of life. Only five trials were identified that evaluated interventions relevant to adolescents aged ≥ 13 years,63,140,141,143,147 none of which recruited parents or the children of parents meeting our definition for SMI. All were included in our secondary synthesis for severe parental depression.
We had originally intended to stratify children by age into infants (aged 0–2 years), preschool children (aged 3 to < 5 years), primary school children (aged 5–11 years) and adolescents (aged 12 to < 18 years); such an age-focused stratagem was rarely adopted by the trials published in the literature. All five randomised trials that recruited adolescents also recruited younger children aged ≥ 6 years to the same intervention. 63,140,141,143,147 We included only two trials that recruited the parents of preschool children (aged 3 to < 5 years);65,154 one of which also included parents of primary school children aged up to 9 years. 65 Therefore, further consideration should be given to development of evidence-based interventions which establish the most feasible interventions and outcome measures for children of different ages and developmental stages.
The relevance of fathers and partners in children’s outcomes is increasingly being recognised. 209 All but six61,63,65,141–143 of the 29 trials in the current syntheses (79%) focused solely on maternal mental illness, with the proportion of women in the remaining trials ranging from 70% to 91%. Two trials61,65 (7%) failed to report these data. Relatively few interventions (13 out of 43, 30%) explicitly included partners. 63,65–67,142,143,148,150,155 Evidence suggests that successful parenting outcomes for mothers with SMI may be likely when mothers have adequate support from their partners, especially if these partners are mentally well. 210 Future studies thus need to consider the potential importance of fathers’ and partners’ roles in the lives of children with mentally ill parents and to take account of this factor when designing interventions and measuring outcomes.
Many of the studies included in this evidence synthesis failed to report whether or not children were living with their parents at the time of the intervention, or provide any details of the child’s family context. This is such a fundamental issue that further consideration needs to be given to the possible influence of different residency arrangements on intervention content and outcomes, and to the potential differences between children’s and parents’ needs. Information on household composition and the parenting and care context, including the presence of extended family support and close social networks, is essential in understanding the experience and outcomes for family members living with serious parental mental illness.
Poor reporting of sociodemographic characteristics, coupled with differences in the context and setting of the included trials, challenges any clear assertions about participants’ educational or socioeconomic status. All three trials with SMI parents recruited participants of low to moderate socioeconomic status, while best estimates suggest a greater mix of household incomes among the trials for severe parental depression. The ethnic status of parents was either fully or partially reported in 1760,64,66,67,142–151,155–157 of the 2960,61,63–67,117,138,140–159 trials (59%) and heavily focused on parents of European, Caucasian descent. In the UK, BME adults tend to have a greater number of children211 and BME communities show higher incidence of SMI. 212 Furthermore, BME families, especially those of Caribbean origin, tend to encounter greater barriers to service use. 213–216 More recent NICE guidance for the treatment of schizophrenia and related psychoses recognises the need for further research to determine risk and solutions for minority populations. 217 Our acceptability synthesis failed to identify any studies addressing ethnicity or cultural factors.
Intervention models
Four key intervention models were identified in both our syntheses (SMI and major unipolar depression) and comprised (1) psychotherapy, (2) psychoeducation, (3) psychosocial and (4) more complex, extended models of care (see Chapter 4 for model definitions). Psychotherapy was by far the most frequently adopted model, trialled in 33 out of a total of 43 (77%) interventions;60,61,64–67,138,140,141,143,145–154,156–159 fewer psychoeducational (n = 7),60,63,141–143,155 psychosocial (n = 1)154 and extended care interventions (n = 2)117,144 were identified. Considerable heterogeneity was observed in intervention content, objectives, delivery formats and target populations.
In our primary SMI synthesis, one trial evaluated a 2-year extended-care home nurse visitation programme in formerly hospitalised mothers with psychosis. 117 A second study evaluated 16 weeks of parenting therapy based on a social learning model and a parenting-based psychoeducation programme for mothers with psychotic symptoms. 60 Two trials evaluated a child-centred CBT programme. 60,61
Our primary synthesis also considered non-randomised and uncontrolled designs. These poorer-quality studies suggest a more recent and tentative shift away from parent-orientated models to direct intervention strategies specifically aimed at improving children’s QoL. Key examples include two recent, non-randomised trials of innovative child-orientated interventions published under the auspices of the Australian COPMI (Children of Parents with Mental Illness) initiative. 58,59 Although small in number, these trials represent a notable proportion of the existing evidence base for older children and adolescents. Ultimately, the utility of their findings is constrained by participant allocation methods based on service availability or attendance preference. The potential for bias in the selection of these groups is significant, and further high-quality studies are required.
In our second depression-focused synthesis, the majority of interventions were psychotherapeutic interventions (n = 30,64–67,138,140,141,143,145–154,156–159 79%). Almost all (n = 21)64–66,138,141,145–154,156–159 were parent-orientated therapies aimed directly at alleviating parents’ depressive symptoms, most commonly using the cognitive–behavioural (n = 12)65,138,140,143,148,149,151,152,154,156–158 and interpersonal approaches (n = 5)66,145–147,150 recommended by NICE. 207 Psychodynamic and non-directive, supportive therapies were evaluated less frequently (n = 2152,157 and n = 3,152,153,159 respectively). One trial included in these numbers evaluated a parent-oriented psychotherapeutic intervention combining both CBT and psychodynamic perspectives. 157
Parent-orientated psychotherapies were most frequently delivered as individualised, face-to-face interventions (n = 17,64,65,145–147,149,151–153,156– 159 81%), although a small number of group-based therapies (n = 4,66,138,148,150 19%) were also observed. Limited data and additional heterogeneity between the model groupings prevented a direct comparison of this delivery effect. All but nine interventions took place either fully or partially outside the home. 64,142,144,152,153,157,158 Thirteen65,66,138,146,148,151,153,156,157,159 out of the 2164–66,138,141,145–154,156–159 parent-based psychotherapies provided full details of their session length, frequency and overall duration. In five instances, CBT or IPT was delivered via ≥ 12 hours of scheduled contact, commensurate with NICE guidance that recommends higher intensity psychological interventions for severe depression. 66,146,148,150,156 Briefer interventions, demanding < 6.5 hours of therapist time, were reported in eight instances. 138,151,153,157,159 Parent-orientated psychotherapies were delivered by a broad range of health- and social-care professionals including GPs, social workers, clinical or counselling psychologists, masters and doctoral-level psychotherapists, midwives, health visitors and community health workers. Five non-UK trials also reported interventions facilitated by PhD or masters-level psychology students or trainees. 65–67,143,147 Previous reviews have questioned the extent to which these may generalise to UK health services and settings. 218
Across both our primary SMI synthesis and our secondary depression synthesis, only 1760,63–67,117,141–144,154 interventions (40%) were identified that sought to enhance parenting or family function and few (n = 8)63–67,143,154,164 were explicit in their theoretical underpinnings. Marked heterogeneity in parenting models was once again observed. When reported, parenting interventions incorporated principles drawn from behavioural theory, attachment theory, social learning theory, psychodynamic theory, Soviet cognitive–linguistic theory, family systems theory and Sanders and Dadds (1993)165 model of parent training. Six studies augmented a parenting intervention with additional care components. 65–67,117,143,144 Five of these studies also focused on the mother’s symptoms and illness experiences65–67,117,144 and one actively and simultaneously intervened with the child. 143
Child-centred interventions
One of the most striking findings of this review is the overwhelming absence of child-centred interventions. In total, across both our SMI- and depression-focused syntheses, only 9 of the 43 interventions (21%) evaluated delivered an active and structured intervention directly to children. Of the nine interventions, only three (7%) intervened solely with the child,60,61,140 and two of these stemmed from the same research team. 60,61
Measured quality-of-life outcomes
Unlike previous reviews, this evidence synthesis aimed not only to provide a descriptive overview of community-based interventions for families affected by SMI, but also to do so with specific reference to child-centred QoL outcomes. Our primary outcomes for this evidence synthesis were established a priori and comprised validated measures of children’s QoL and emotional well-being. A key finding of this review is that very few trials have measured children’s QoL. If we consider evidence from trials recruiting parents, or the children of parents with SMI, only one small, non-randomised trial reported these outcomes. 58 Broadening our focus to interventions for severely depressed parents or their children located seven randomised trials that reported outcomes relevant to children’s emotional well-being. 63,66,67,140,144,147,160 Four of these were confined to observer ratings of infant affect,66,67,144,160 leaving only three trials that reported validated mental health outcomes in older children and adolescents. 63,140,147
Key QoL outcomes are difficult to define. 74 Our secondary outcomes were derived from existing literature and UK child-centred policy and the syntheses considered a broad spectrum of child-centred outcomes as suggested by the England and Wales multidimensional ECM agenda. 86 This agenda highlights specific QoL domains relevant to child health (e.g. physical and emotional health), safety (e.g. accidents, injury and maltreatment, stability of care), enjoyment and achievement (e.g. cognitive development, school and recreational engagement), making a positive societal contribution (e.g. social behaviour, self-esteem and coping) and economic well-being (e.g. access to material resources or income).
Through stakeholder consultation, we adapted and refined these five QoL domains to reflect better the needs and life priorities of children of parents with SMI. This innovative conceptual QoL model, grounded in lived experience, is illustrated in and summarised in terms of the available evidence in Table 18. The trials in our primary SMI synthesis most frequently measured children’s behaviour and cognitive function (n = 3, 100%). 60,61,117 The trials in our secondary SMI synthesis (which was focused on severe parental depression) most frequently assessed maternal mental health symptoms (n = 19,63,64,66,67,138,144–150,152–154,156–159 88%). Only one randomised trial for parental depression reported outcomes quantifying children’s social relationship quality and recreational engagement levels,63 and mental health literacy. 142 Two trials for parental depression measured children’s self-esteem,140,141 although only one reported data140 and one randomised trial for serious parental mental illness reported on children’s problem-based coping skills. 61
| Outcome level | Outcome variable | SMI | Severe depression | Total RCTs | % RCTs |
|---|---|---|---|---|---|
| Primary outcomes | QoL | – | – | – | – |
| Emotional well-being | 1 | 7 | 8 | 28 | |
| Secondary outcomes | Physical health | – | 1 | 1 | 3 |
| Safety | – | – | – | – | |
| Social function/behaviour | 3 | 12 | 15 | 52 | |
| Social relationship quality | – | 1 | 1 | 3 | |
| Recreational engagement | – | 1 | 1 | 3 | |
| Family function | 1 | 2 | 3 | 10 | |
| Parent–child relationship | 2 | 8 | 10 | 34 | |
| Parent mental health symptoms | 2 | 19 | 21 | 72 | |
| Cognitive function | 3 | 5 | 8 | 28 | |
| Problem-based coping | 1 | – | 1 | 3 | |
| Mental health literacy | – | 1 | 1 | 3 | |
| Self-esteem | – | 2 | 2 | 7 |
Outcomes pertaining to child safety were absent from the included literature and only one randomised trial conducted in a developing country considered the impact of community-based interventions on children’s physical health status. 158 It may be that a relatively low incidence of reported child maltreatment cases and a comparatively higher level of physical health within developed countries make these measures potentially less accessible and less sensitive as short-term outcome data.
The emphasis on research- and parent-centred outcomes in part reflects the predisposition of evidence towards the effects of early interventions on parental depressive symptoms and parenting in the first 2 years of life. Measurable and subjective QoL constructs may not emerge until school age and few HRQoL measures are available for children under the age of 4 years. 89 In preschool and infant children, family context, parenting and parent–child relationship qualities arguably remain the only indicators of children’s QoL currently available. Our stakeholder consultation highlighted the potential importance of these outcomes to children of all ages.
Family and parental experiences are generally accepted to remain an important component of children’s life experiences and a key contributor to children’s QoL judgements, particularly when parents suffer from SMI. 7,100 Interventions that target maternal mental health or family function and monitor treatment effects in terms of parental mental health symptoms, parenting behaviours or child-centred psychopathological outcomes are thus likely to be relevant to children’s QoL, particularly when mentally ill parents and children live together. The challenge that remains is in establishing how, and to what extent, these outcomes translate into children’s own self-appraised QoL. Only one non-randomised trial included in our syntheses sought to measure family functioning from the child’s perspective.
Quality of life is a multidimensional construct, and no single secondary outcome is likely to represent it completely. However, our stakeholder panels suggested the importance of using measures of children’s coping skills, mental health literacy and self-esteem; such measures were more commonly reported in non-randomised and uncontrolled studies than in RCTs and, therefore, risk of bias was high. Our review found that studies using group psychoeducation programmes or group psychotherapy models in primary school-aged children and adolescents appeared to fit well with these more child-centred objectives and specifically with the goals identified by our stakeholder participants. Therefore, these interventions may exist as potential areas for further research and service development aimed at this population.
Child-centred QoL data have previously been collected in studies of vulnerable children from multiple high-risk families. Approximately 2% of UK families are reported to suffer the combined effect of parental illness, low income, lower educational attainment and poor housing, and this group is one of the most vulnerable in society. 14,18 However, many multirisk families are characterised and defined by social deprivation indices rather than by mental illness; therefore, substantial numbers of children experiencing parental ill health are missed. Although valuable lessons may be learned from the child-centred QoL data collected from these samples, the specific needs of the two groups are likely to differ. Children living in economically deprived families will not necessarily be acting as informal carers and will not routinely experience chaotic behaviour or repeated hospitals admissions of their parents.
Lack of follow-up data
The current synthesis has identified a lack of follow-up in existing RCTs. Across both our primary SMI and secondary depression syntheses, almost all included trials (n = 26, 90%)60,61,63,65–67,138,140–143,145–159 conducted outcome assessments post intervention or within 6 months of participant randomisation. Overall, only six trials (21%) reported medium-term follow-up data of up to 1 year post randomisation141,143,147,152,154,158 and six trials (21%) collected longer-term data after 1 year. 64,117,141,144,152,167 The longest follow-up reported 5 years post randomisation and this was for a trial included in our depression synthesis. 152 A lack of long-term follow-up is not unusual in trials of psychological or behavioural interventions. For families living with SMI, such data may have particular implications, not least because of the potential long-term effects of enduring parental mental illness on children and the associated economic impact that these may have. 2,219
Study validity
All but one of the trials included across our syntheses were judged to have unclear or high risk of bias (i.e. they were poorer quality trials). The one exception was a trial of a community-based intervention for severely depressed mothers in Pakistan. 158 The generalisability of these findings to UK families and the UK health context is not clear.
The very nature of non-pharmacological interventions poses some specific challenges to their evaluation. Gold standard RCTs seek to eliminate bias through the random allocation of participants and the blinding of intervention facilitators, participants and outcome assessors. However, while allocation concealment is feasible in trials of behavioural interventions, blinding of intervention personnel and participants is difficult. Independent and blinded outcome assessments are possible, but when the primary outcome is inherently subjective (as is the case in QoL) the most valid measures may ultimately be those that rely on self-report. In such situations, the difficulties in obtaining blinded outcome assessments from trial participants remain. These challenges had to be taken into account when judging trial quality in the current review.
Despite the difficulties highlighted above, it is always possible to conduct well-designed trials using random-sequence generation with adequate allocation concealment. Empirical evidence suggests that differences in the methods of randomisation and allocation concealment can influence the ESs reported by different trials. 82 Allocation concealment is potentially more important than the method by which allocation sequences are generated, since any effort to allocate participants randomly will be undermined if trial recruitment and assignment methods remain open to manipulation.
In our primary SMI synthesis, risks of selection bias from inadequate randomisation and concealment were high. Randomisation methods were not reported by two of the three trials included in this synthesis61,117 and the third used a sequential approach that could not be considered truly random. 60 Allocation concealment was not reported by any of the three trials. In our secondary depression synthesis, four out of the 26 trials reported inadequate randomisation,66,67,144,157 and another 11 failed to report this information (see Appendix 9, Table 37). Only six trials reported adequate methods of allocation concealment. 63,138,143,149,154,158 The potential for investigator bias in the allocation of trial participants cannot, therefore, be ruled out.
In total, seven comparisons from six randomised trials employed a waiting list comparator arm. 66,67,140,142,146,148 While often used in trials of behavioural interventions, the validity of this approach is difficult to establish. The primary purpose of a non-active comparison condition (i.e. a waiting list control) is to enable the calculation of intervention effect, independently of any effect arising from natural recovery. However, when clinical patients are allocated to a waiting list control, this can in itself influence the size of the observed effect simply by implying that some form of treatment is required. Consequently, waiting list controls may display more negative outcomes than would have occurred naturally and bias may be observed in favour of the intervention. Conversely, waiting list controls may display more positive outcomes than would naturally occur in instances when participants view trial participation as finite and therapeutic intervention impending. Therefore, the true effect of the waiting list comparator is difficult to establish in our identified trials.
A further 19 comparisons were included which used routine care or ‘treatment as usual’ as the comparator. Naturalistic treatments that occur simultaneously with either the comparator or intervention arm of a trial may also introduce bias into observed effects, particularly if these treatments differ substantially between the two groups. Such comparisons are not uncommon in health-care trials for which ethical and practical constraints may limit the withdrawal of routine care.
The majority of studies were underpowered to test reported differences. In our primary SMI synthesis, sample sizes ranged between 4161 and 51,60 with a median of 27. In our depression synthesis, sample size ranged from 20148 to 903158 with a median of 53. Other risks of bias were also identified. No trials in our primary synthesis specified their primary outcomes a priori and two out of three failed to present complete outcome data raising the possibility of reporting bias. Risk of attrition bias was judged to be low in one of the trials61 but unclear in the other two owing to inadequate reporting of the level or reasons for participant withdrawal. 60,117
Similar limitations were identified in the trials focused on severe depression. Attrition rates for these trials ranged from 0%140 to 81%. 157 Poor or absent reporting of the reasons for participant attrition means that the possibility of systematic differences between those providing and not providing data cannot be ruled out. Furthermore, a notable number of trials included within our syntheses exacerbated these constraints by inadequately reporting their outcome data. Out of a total of 2960,61,63–67,117,138,140–159 trials eligible for inclusion in one or other of our syntheses, 10 (34%)60,66,117,141,146–148,151,152,156 provided insufficient data for one or more outcomes to enable the calculation of a standardised effect.
Objectives 2 and 3: evidence of effect and associations with intervention characteristics
Current evidence is limited in both size and quality. Our findings are in line with older and non-systematic reviews52,57 that noted a striking absence of high-quality evidence relating to the clinical effectiveness of interventions and services for families of parents with SMI. Only three randomised or quasi-randomised trials were identified that met the criteria for inclusion in our primary synthesis and none of these presented validated measures of children’s QoL. 60,61,117 We also considered poorer-quality evidence from non-randomised trials, although we did not pool data across the different study designs. Only four heterogeneous non-randomised trials were found. 58,59,118,119 On the basis of this evidence, it not possible to draw any meaningful conclusions of clinical effect.
There were sufficient data from the depression trials for four secondary outcomes to be combined to produce pooled summary estimates of effect, although differences between populations, interventions and outcome measures mean these results must remain preliminary. Substantial variation existed between trials in terms of intervention models that were assessed. This heterogeneity limits the value of subgroup analyses by making it difficult to distinguish between effects stemming from the content of the intervention, the target of the intervention (i.e. parent, family or child) or the format in which the intervention was delivered.
Owing to limitations in the available data, potential associations between user characteristics and effect, and intervention characteristics and effect, could not be fully explored. Evidence comparing two active intervention models was also limited by marked variation in the comparisons reported and the small number of trials that were identified. No trials compared group to individual delivery formats for the same intervention.
Child-centred outcomes
Data pooling was not possible in our primary SMI synthesis. In our secondary depression synthesis, five trials contributed data to a meta-analysis comparing the effect of any variant of community-based psychosocial intervention to a waiting list/treatment as usual control on children’s short-term emotional health. 63,66,67,140,160 Pooled ESs suggested no significant short-term improvement in children’s emotional health. A trend towards larger effects in poorer quality trials was observed. The small number of trials contributing to this analysis prevented any further subgroup analysis, which, in turn, limits the meaningful interpretation of the findings. Follow-up data for longer-term outcomes (> 6 months) were sparse and the pooling of medium- and long-term follow-up data did not occur. In the absence of high-quality research evidence, it is difficult to recommend any specific community-based interventions for improving the mental health of children born to parents with SMI.
Eight depression trials contributed data to a meta-analysis comparing the effect of any variant of community-based intervention to a waiting list/treatment as usual control on children’s social function and behaviour. 63,66,67,140,142,147,154,160 Pooling these data produced non-significant results. Sensitivity analysis suggested that higher-quality trials may be associated with statistically significant effects, although overall ESs remained small. Once again, insufficient evidence is available on which to base firm conclusions.
Outcomes relevant to children’s physical health, safety, social relationship quality, recreational engagement, self-esteem, cognitive functioning, problem-based coping and mental health literacy were too limited to draw conclusions. The absence of these specific data from the existing evidence base and the overall dearth of evidence examining QoL in the children of parents with SMI suggests highlights an urgent need for services and research to include a wider range of child-centred QoL outcomes in the development, evaluation and implementation of new interventions and care pathways.
Parent-centred outcomes
The pooling of parent-centred outcome data was not possible in our primary SMI synthesis, owing to an overwhelming lack of data, exacerbated by the selective reporting of some outcomes. A lack of rigorous evidence severely limits the capacity to draw conclusions and indicates a pressing need for further work.
Parental mental health symptoms were the most commonly reported secondary outcome in our depression synthesis. Meta-analysis of these data suggested a significant medium to large effect of community-based interventions, and particularly psychotherapeutic interventions, on parents’ short-term mental health. Preliminary evidence suggests however that these effects may diminish over time. In order to confirm these findings, a greater number of trials with longer-term follow-up are required.
Six trials contributed data to a meta-analysis comparing the effect of any variant of community-based psychosocial intervention to a waiting list/treatment as usual control on parents’ responsiveness to their children. 63,66,67,150,152,160 Pooling these data produced a medium to large result, suggesting a significant short-term improvement in parenting behaviours post intervention. Confirmation of these findings once again demands a greater number of high-quality trials.
In summary, our synthesis suggests that there is insufficient evidence of an effect of community-based interventions on children’s QoL. Although positive effects were observed for short-term, parent-centred outcomes, there is currently no evidence that these effects are maintained long term. Evidence that community-based interventions improve relevant child-centred outcomes is particularly sparse. An overwhelming lack of interventions aimed directly at older children and adolescents, who are best able to provide reliable and direct reports of their experience, has limited the number of studies providing subjective, child-centred measures.
Objectives 4 and 5: intervention acceptability and facilitators and barriers to implementation
Commensurate with the findings of our clinical effectiveness and cost-effectiveness reviews, a paucity of high-quality acceptability data were found. The majority of data that exist are quantitative in nature and pertain primarily to parents with severe depression. Quantitative data show low response variability, measure participant satisfaction only with those aspects of an intervention deemed to be of interest a priori and may be influenced by a lack of knowledge of, or access to, alternative treatments. In-depth qualitative studies of the views of parents with SMI, and, more importantly, of the views of their children, are lacking.
The overall pattern of findings from the quantitative data included in this synthesis is that parents with SMI generally hold community-based interventions in high regard. Whole-family assessments delivered via adult mental health services may thus hold promise as a potentially viable means of accessing and engaging these vulnerable families and children. Estimates of rates of intervention uptake and adherence compare favourably to those of other community-based services aimed at adults with SMI, although the inconsistent reporting of these data highlights a need for more rigorous evaluation. Satisfaction data also demonstrate positive results, although common weaknesses in these data include a predominance of small study samples, a poor reporting of participant response rates, a risk of social desirability bias and likely attrition biases emanating from a focus on intervention completers.
Limited qualitative data suggest that the acceptability of community-based interventions for parents with severe depression may be enhanced by including group-based activities and/or normalising components aimed at reducing parents’ sense of social isolation and stigma. The generalisability of these findings to parents with SMI is as yet unclear. In our primary synthesis, for which rates of attrition were high and reasons for attrition identified, chaotic family life and loss of children to custody were identified as prominent causes. In such instances, intervention may be of even greater importance to support a continuing parent–child relationship. Assumptions should not be made about the relevance or outcome of interventions when children are not living with their parents.
Current UK policy continues to advocate greater support for families and children affected by mental illness, including working directly with children. As it is recognised that different families will have different and multifaceted needs, then it is likely that a multiagency approach will be required. This necessitates child and adult mental health services being able to work seamlessly together with other agencies, specifically statutory education and social-care services and a growing number of third-sector services working in this area. Extended services in the NHS context, outreach services linking the NHS to the community and community-based services involving other agency organisations may all have a role. However, the fear of child custody losses as a barrier to accessing support is an important finding and those working with such families need to remain sensitive to this concern. Our synthesis has suggested that the establishment of a trusting and non-judgemental relationship, in which intervention providers view participants as parents rather than patients, may be a key contributor to adult-orientated interventions. It is important, therefore, that this finding is given adequate priority in future service planning and staff training initiatives. Ultimately, those contexts that are most embedded within family routines, such as schools, community centres and patients’ own homes, may offer the most viable locations in which to deliver interventions to children and families affected by SMI. At present, however, rigorous research evidence on this issue is lacking and qualitative assessments of child-focused interventions are particularly sparse. In summary, there currently exists insufficient evidence on which to base any firm conclusions regarding the acceptability of community-based interventions aimed at improving or maintaining QoL in the children of parents with SMI.
Objectives 6, 7 and 8: cost-effectiveness of community-based interventions and value of future research
To date, studies have failed to consider the economic implications or cost-effectiveness of community-based interventions for improving or maintaining QoL in the parents of children with SMI. With respect to our primary SMI synthesis, no studies were identified that included a consideration of the costs or the consequences of the interventions under evaluation. In our secondary depression synthesis, only one non-randomised evaluation was located. While this economic evaluation was clearly unique, it was not without limitations, including a high risk of bias and a narrow assessment of costs and effects. Furthermore, the study focused on the costs and benefits from the perspective of the mother, so cannot be used to directly support resource allocation decisions to improve the QoL of children.
As a result of the absence of economic resource use and cost data, it is not possible to estimate the cost burden associated with children and adolescents of parents with SMI or severe unipolar depression. In addition, the absence of clear evidence to support the clinical effectiveness of specific interventions, combined with an absence of economic data, rendered economic modelling of cost-effectiveness (and any subsequent VOI) impossible. Therefore, it is not possible to come to any conclusions regarding the cost-effectiveness of alternative interventions to support these young people.
The lack of a cost-effectiveness model meant that it was not possible to quantify the value of collecting further information formally. However, conservative estimates suggest that between approximately 50% and 66% of people with SMI will be living with children under the age of 18 years11 and that around 175,000 children provide informal care for a parent or sibling,5 almost one-third (29%) of whom will care for a relative with a mental health difficulty. 6 It is likely then that there is potential value in developing interventions for this population and in the collection of good-quality evidence on their costs and effects. A cost-effectiveness model requires long-term outcomes, which, in this context, may include time in social care, mental health problems and other morbidity in childhood and adulthood. Therefore, as well as undertaking short-term trials to quantify the effect of interventions on child-centred QoL, cohort studies would likely also be of value to help map between these shorter-term measures and longer-term health and social-care outcomes.
Strengths and limitations in evidence synthesis
This is the largest and most comprehensive review of evidence for the clinical effectiveness, cost-effectiveness and acceptability of interventions for parents with mental illness. It is the first and only review focused on children and children’s QoL in families with SMI. It is strengthened by a number of methodological features.
First, stakeholders, including service users, contributed to the development of a QoL framework for the population under review, from which we identified our primary and secondary outcomes. We also gained stakeholder comment on the draft report and include their conclusions and recommendations in our final report.
Second, systematic searches were undertaken using 19 electronic health, allied health and educational databases from database inception to May 2012. These searches were supplemented with reference checking, hand searching, targeted author searches, forward citation searching, grey literature searches and searches on theses and ongoing research. All databases were searched from their inception until November–January 2011. Search terms were deliberately kept broad and a substantial number of potentially relevant articles were located. A total of 34,659 articles were initially identified, of which 51 were selected for full assessment following a rigorous, gold standard systematic review approach. 82 It is possible that some articles of relevance were not found or were mistakenly excluded but the breadth of these searches and the fact that two reviewers independently judged study inclusion substantially lessens this possibility.
It is possible that new literature will have emerged while preparing this HTA report for publication. To assess the likely magnitude of literature published during this time, we undertook an updating search on the MEDLINE database in May 2012, using identical search terms to those used in the original searches. After deduplicating and excluding studies included in the previous searches, 124 studies were retrieved. After screening titles and abstracts, five new studies appeared to be potentially eligible for inclusion. On closer inspection, two of the five studies had already been included in our synthesis and two did not meet our inclusion criteria. Hence, only one additional study would have been eligible for inclusion. 220 This study reported longer-term follow-up outcomes for an RCT already included in our secondary depression synthesis. 143 The effect of a family group cognitive–behavioural intervention was compared with a parenting-based psychoeducational intervention on mental health outcomes in children aged 9–15 years with parents with a history of MDD. Children in the intervention group reported significantly lower levels of anxiety and depression and internalising symptoms at 18 months and significantly lower self-reports of externalising symptoms at 18 and 24 months (odds ratio = 2.91). Given the small number of studies reporting this type of comparison, it would not have been possible to include these data in a meta-analysis.
Nonetheless, there exist a number of limitations in the validity of our findings and in the generalisability of this review. The lack of high-quality, randomised evidence and the very small number of studies meeting our inclusion criteria meant it was not possible to undertake meta-analysis for any outcomes in our primary synthesis. A narrative synthesis was instead presented. In our secondary depression focused synthesis, sufficient data were available to meta-analyse two child-centred and two parent-centred secondary outcomes identified by our stakeholder panel. Too few studies were available to report medium- and long-term follow-up effect or to fully consider the associations between different intervention characteristics and intervention effect.
Inclusion of poorer-quality trials increased the risk of confounding. We used the Cochrane checklist for non-randomised studies and only included randomised or quasi-randomised trials potentially capable of creating similar groups. Four trials in our primary synthesis and four trials in our depression review were quasi-randomised studies. Sensitivity analyses suggested these trials demonstrated systematic differences in their effects. Possible bias from selective outcome reporting must also be acknowledged. Ten trials provided insufficient data to contribute to one or more of the meta-analyses undertaken. It is unclear how inclusion of these trials may have influenced the pooled-effects since many provided little or no narrative of their findings. The possibility of publication bias could only be formally explored for one secondary outcome, which, in this case, was parents’ depressive symptoms. Funnel plots drawn from the data showed no evidence of bias, although the relatively low power of the associated regression test suggests that such bias cannot be definitively ruled out.
We adopted a systematic and rigorous approach to study eligibility judgements. A number of UK studies have reported child-specific QoL outcomes within the last decade. These data typically apply to interventions delivered in the social-care sector, such as Homestart or Surestart. 221,222 Such studies target high-risk or multirisk families, in which risk is defined predominantly in terms of broad social deprivation indicators rather than parental mental illness. The needs of children within such families may be qualitatively very different from those in our syntheses since the majority of participants within such studies are not seriously mentally ill. However, we acknowledge that some evidence relevant to our target population will have been excluded through these studies failing to meet our inclusion criteria.
In the absence of an internationally agreed standard for SMI, irregularities in its scope and classification invariably exist. The current synthesis operationalised a working definition of SMI that prioritised those disorders most commonly indexed. For the purpose of our primary synthesis, trial eligibility criteria required > 50% of parent participants (or the parents of child participants) to have a primary diagnosis of schizophrenia or related disorder, puerperal or non-puerperal psychosis, personality or borderline personality disorder and/or bipolar disorder, either alone or alongside with secondary mental health diagnoses. The 50% cut-off was an arbitrary but necessary criteria but consensus on how to deal with mixed populations in evidence syntheses is lacking.
Our second synthesis targeted populations in which ≥ 50% of parents experienced severe unipolar depression. This synthesis was undertaken in response to broader definitions of SMI that encompass this disorder and guidance received from our advisory panel. However, within this synthesis, some unique challenges were presented. Diagnostic measures of MDD such as the DSM, ICD and RDC were considered the gold standard for the classification of severe mood disorders and all but two trials met these criteria at the time of recruitment. 148,149 The remaining two studies did not confirm diagnoses but were included on the basis that they met our criteria for substantially elevated symptoms at baseline.
Prior evidence syntheses have acknowledged that studies rarely employ diagnostic specifiers beyond those distinguishing between major and minor depressive disorders and that as such, greater specificity of target populations is not normally possible. Nonetheless, the extent to which MDD acts as a SMI in this context is debatable.
The parents recruited to the trials included in our syntheses showed considerable variation in baseline symptoms, ranging from mild to severe on validated self-reported depression scales. Many patients with chronic and severe depression experienced fluctuations in symptom severity and this range of symptoms may ultimately reflect the variation that health-care professionals are likely to encounter clinically in service users. Service users may choose to access services for themselves and their children both during, and outside, severe symptomatic episodes. A clinical diagnosis of severe unipolar depression may not equate to the severity of symptoms and functional impairment experienced in serious illnesses such as psychosis or bipolar disorder. Further consideration should be given to the optimal method of identifying families and children affected by serious parental mental illness and to the possibility that functional outcomes, rather than diagnostic indicators, may be more appropriate markers of illness presence and severity.
Finally, our synthesis remained heavily focused on evidence of effects from interventions for depressed mothers with infant children. The generalisability of our findings to families living with other SMI, to older children and adolescents and to families in which fathers have SMI, is limited.
Chapter 8 Conclusions
The primary aim of this systematic review was to provide a full evidence synthesis of the clinical effectiveness, cost-effectiveness and acceptability of community-based interventions for improving or enhancing QoL in children of parents with SMI. The development of successful care pathways capable of delivering evidence-based health and welfare services to families in these circumstances demands clear evidence of intervention effect alongside the successful integration of child and adult services including both statutory and non-statutory agencies. At present, clinical services have little choice but to rely on ‘best guess’ approaches, without clear evidence to guide them towards the most effective options.
Recommendations for practice
This review has confirmed that the existing evidence base for community-based interventions to enhance the QoL in children of parents with SMI remains severely underdeveloped. In its current form, it cannot provide an adequate rationale on which to base UK service development or delivery.
The overwhelming lack of contemporary data on clinical effectiveness, cost-effectiveness and acceptability of interventions for families affected by serious parental mental illness (data that would most closely relate to current practice and to the real life experiences of UK children today) must be of particular concern to the UK NHS Commissioning Board. Our primary evidence synthesis only identified three randomised trials eligible for inclusion, all of which were conducted in the USA more than two decades ago. Service configuration models and the interface between adult psychiatric services, children’s services and social care are likely to vary considerably between countries and changes in service provision across time limit the applicability of this older literature. Equally, children’s QoL judgements are likely to be influenced by the cultural and temporal contexts in which they occur. Therefore, doubt exists as to the generalisability of this limited evidence to contemporary UK health- and social-care settings.
Despite many of the data in this review being relatively old, current consensus within the broader child mental health arena is that parenting interventions (particularly the Triple P and Webster–Stratton models) and cognitive–behavioural therapies are two of the most effective approaches for a range of childhood mental health concerns (anxiety, depression and serious behavioural disorders in particular). Indeed, the first wave of the recently announced Improving Access for Psychological Therapies (IAPT) Children and Young People’s programme has focused entirely on these two approaches. Later waves of the programme will also include systemic family and interpersonal therapies. Unlike IAPT services for working age adults who have successfully established separate community and primary care-based services, those trained in children’s IAPT are expected to be integrated into secondary care. A possible benefit of this configuration is that adult mental health services may be able to liaise directly with child and adolescent mental health services to obtain support for the children of parents with SMI in the future. The embedding of children’s IAPT into secondary care may also mean that such services are limited to those in the greatest or most urgent need of support. Therefore, further developmental work is likely to be required before effective evidence-based services can be routinely provided to all children of parents with SMI.
Recommendations for research
Our findings have clear implications for future research. The new NHS Commissioning Board intends the development of evidence-based services to deliver care quality improvements against clear agreed standards. 223 Without such evidence, service providers will fail to deliver adequate care improvements within services and funding will only follow services that can demonstrate adequate standards of care delivery, improved quality of care for clients and value for money. The design of studies that focus on user-centred values such as QoL are an important aspect of gathering evidence for this new commissioning agenda.
Appropriate research methodology
Future studies must include designs with properly framed a priori research questions and adequate methods and power to deliver answers. Trials must follow appropriate randomisation and allocation procedures, with formal monitoring of intervention uptake and adherence rates. Appropriately validated, child-centred and age-appropriate primary outcomes measures for QoL should be employed. Trials must ensure full reporting of primary outcome data and incorporate longer as well as shorter-term outcomes. The need to nest qualitative studies to provide in-depth information about the intervention (e.g. acceptability, usefulness, content) within these trials and to collect high-quality cost data cannot be overemphasised.
At present, the scale of the gaps in the evidence indicate a clear need for the identification and large-scale evaluation of existing interventions that may offer promising effects. A substantial programme of pilot work, consistent with the philosophy of the Medical Research Council framework for RCT development, is advocated. 224,225
The need for developmental and modelling work
Rigorous evidence is required to underpin the development of acceptable and feasible community-based interventions for improving or maintaining QoL in the children of parents with SMI. A recurrent theme in this report is the need for intervention design and outcome measurement that is child-centred. The current synthesis has found a striking lack of evidence for family- and child-based interventions and, therefore, feasible, acceptable and innovative interventions that meet the needs of both children and parents may have to be developed. Particular focus is needed on the optimal content, structure and delivery mechanisms of these interventions and the likely commonalities and differences between community-based interventions for different age groups. Such work may successfully be achieved via a programme of rigorous developmental work that includes a large-scale stakeholder survey, face-to-face stakeholder consultation events and an in-depth exploration of relevant issues using standard qualitative approaches. This work must give due consideration to the likely skill mix required to deliver the interventions and the potential facilitators and constraints present in the health-care systems that will host them.
Additional effort may usefully be spent conducting a scoping review of third-sector services. The present synthesis undertook a systematic and comprehensive search of material generated by user-led and voluntary sector enquiry and identified no RCTs from these sources. However, it is possible that third-sector services represent an important and neglected route into child- and family-centred interventions, as well as a potentially important means by which to engage these service users. Our acceptability review suggested that many parents feel stigmatised by SMI, while children may place high significance on peer support and respite. Templates for interventions that may usefully address these concerns may be found in third-sector services in which the drive for the routine integration of structured and evidence-based services maybe less pressing. Our acceptability review also suggested that parents’ fear being judged by health services and professionals. It is likely that substantial preparatory work would be required to recruit families and to encourage them to share their perspectives. Engaging users outside statutory services may help to minimise inequalities in the research relationship.
Greater evidence is also required to underpin the identification of valid QoL measures for the children of parents with SMI. This could successfully be achieved by undertaking a comprehensive and systematic review of children’s needs and experiences and by conducting a series of qualitative studies aimed at delineating the QoL priorities of different groups. Due consideration should be given to determining the relevance of existing QoL measures to the children of parents with SMI and to establishing potential differences in the QoL priorities of children of different ages. When necessary, a programme of work should be undertaken to develop and validate new age-appropriate measures of HRQoL for these populations. Our stakeholder consultation identified some key challenges and needs among children of parents with SMI that are supported by the findings of other empirical work. Population specific measures that take account of these issues may well be more sensitive to changes and more effective at detecting treatment effects. We recommend that any QoL measures that are newly developed be done so with the meaningful involvement of their target participants.
The need for exploratory trials
At an intermediary stage, a series of exploratory trials may be advocated. These pilots should evaluate the effects of the most feasible and acceptable interventions emerging from the modelling work described above. Two tentative observations can be made:
First, in order to directly improve children’s QoL, direct consideration should be given to interventions (or components of interventions) aimed at family functioning and the parent–child relationship, two components that were identified as key outcome domains by our stakeholder panel. Rigorous evidence of the clinical effectiveness of parenting-based interventions in families experiencing SMI is lacking, although feasibility and acceptability data have been collected using video-guidance techniques in new SMI mothers to improve maternal sensitivity to infants and mother–infant interactions. 70,226 Manualised parenting interventions with proven efficacy in multirisk families exist as other potential candidates for modification and piloting in this group. Pilot trials should recruit families with clinically diagnosed serious parental mental illness and draw comparisons between the effectiveness and acceptability of these interventions compared with usual care in different settings. Outcomes should include family-based, as well as parenting-based measures, reported from the child’s perspective whenever possible. Specific attention may need to be given to intervention uptake and adherence and the feasibility of delivering and evaluating these interventions in a hard-to-access group.
Second, and most importantly, consideration should be given to interventions aimed specifically at the children of parents with SMI. As part of the current work, stakeholder consultations identified a range of service outcomes relevant to this target population. These included aspects of family functioning and parental well-being but also extended to include children’s social relationship quality, recreational engagement, self-esteem, problem-based coping skills and mental health literacy. It is debatable whether or not these outcomes could be improved by parenting interventions alone. Further research aimed at developing truly child-centred health- and social-care services thus needs to consider ways of addressing these potentially important components of QoL. A key empirical question is whether or not children’s QoL can be improved independently of parenting behaviour or parental psychopathology and which combination of parent, child- and family-based interventions will lead to the greatest effect.
A key aim of this work should be the delivery of a manualised or sufficiently structured intervention to permit its routine integration into UK clinical practice. It is acknowledged that, while the children of parents with SMI remain at significantly greater risk of psychiatric mental health difficulties, they will not invariably have clinically significant mental health difficulties of their own. The limited data synthesised within our acceptability review suggests that psychoeducational and psychosocial approaches may ultimately constitute feasible and acceptable services for these individuals. Such interventions have increasingly been adopted by non-UK services. The Australian COPMI model is noteworthy in targeting multiple outcomes prioritised by our stakeholder group. Strengths-based programmes such as these aim to enhance a child’s resilience and self-esteem by combining psychoeducation, social support and recreational respite activities. However, these services have not been developed within a research framework and current evidence of their effect remains poor. To date, evaluations of COPMI models have been non-randomised or uncontrolled in their design. Similarly, studies provide no information about the likely costs and benefits of these interventions or the maintenance of their effects over time. The short- and longer-term value of these approaches currently remains unclear.
New UK research should focus on whether or not, and how, group-based psychoeducational programmes deliver benefit to children compared with standard care across settings. They should also consider the relative acceptability and effectiveness of child-orientated psychoeducation and child-orientated psychotherapies on children’s overall QoL. We recognise that placing children at the centre of care planning may be challenging in a context in which child and adult mental health and social-care services are managed separately. The delivery mechanisms and care pathways needed to support these child-focused interventions, and the likely feasibility of studies designed to evaluate them, will have to take these operational considerations into account. Higher-quality evidence from user-centred research may ultimately facilitate partnership working in this highly complex and sensitive area.
Future research priorities
In summary, there is currently a dearth of evidence regarding the clinical effectiveness and cost-effectiveness of community-based interventions to improve or maintain QoL in children of parents with SMI. Before undertaking a large-scale and potentially cost-intensive trial, further research is required:
-
First, evidence is required to underpin the identification and development of feasible health- and social-care interventions for children and families experiencing serious parental mental illness. This may be achieved via a large-scale audit and scoping review designed to map current service provision across statutory and non-statutory services. Professional stakeholder consultation events designed to ascertain the likely facilitators and constraints in the host health-care system are also recommended.
-
Second, evidence is required to underpin the acceptability of any promising or newly developed interventions. This may be achieved most easily via child-centred developmental work undertaken with children, parents and families with lived experience of SMI. Qualitative work, including individual interviews and focus groups, is needed to ascertain the optimal content, format and delivery mechanism of any new interventions required.
-
Third, effort is required to ascertain the likely uptake and optimal recruitment methods for any future evaluative research study. This evidence may be most easily achieved via the two studies described above, with additional data collection relevant to the processes and contexts through which children and families may most successfully be accessed.
-
Fourth, evidence is required to underpin the relevance of existing QoL measures to the children of parents with SMI. This would be achieved most successfully by extending the developmental work undertaken with stakeholders as part of this review. This would include a comprehensive and systematic review of children’s needs and experiences as well as a series of qualitative studies aimed at delineating the QoL priorities of children of different ages and experiences of different parental mental health disorders.
-
Subsequently, an exploratory randomised trial may be conducted. Manualised parenting interventions with proven efficacy in multirisk families and group-based psychoeducational programmes exist as potential candidates for modification and piloting in this group. Trials should seek to compare these interventions with routine care, with additional training on intervention content and delivery being provided for health-care professionals or paraprofessionals. Short-term outcomes should include family-based as well as parenting-based measures, reported whenever possible from the child’s perspective.
-
Only then should interventions with promising effects be subjected to a large-scale rigorous RCT designed to assess both clinical effectiveness and cost-effectiveness. Appropriately validated, primary outcomes should be collected over the longer as well as shorter term and nested qualitative studies designed to examine user and staff perspectives should be included. Opportunities to extend data collection via a longitudinal cohort study should be considered.
Acknowledgements
The authors would like to thank Jo Abbott, information officer from the CCDAN, for her work in developing the electronic search strategies used in this review and Nicky Welton, who consulted on the feasibility of a VOI. We would also like to thank the individuals who responded to requests for further data, copies of articles or points of clarification, particularly E Pitman, R Clark, D Chicchetti, L Sheeber, R Boyd, A Reupert, E Fudge, F Judd, B Gladstone, M Nolan, K Smith, S Einfeld, J Gibb, C McMahon and R Ammerman.
We would also like to thank Kathyrn Berzins and Maria Cary, who worked as research associates on the project, Anja Wittkowski for her time in translating non-English-language publications for reference checking and Judith Gellatly for her time in tracing articles. We would also like to thank Professor Karina Lovell for her involvement in reviewing the draft report.
Finally we would like to thank members of our review advisory panel (Professor Simon Gilbody, Dr Dean McMillan, Professor Eric Taylor, Dr Jo Alridge, Dr Anja Wittkowski, Professor Gillian Haddock, Professor Steve Jones, Professor Tony Morrison, Professor Jane Barlow, Mr Ian Shelmit, Professor Bryony Beresford) and all of the individuals who participated in our stakeholder consultation events and/or subsequent correspondence, specifically Bath Young Carers, Barnardo’s, Young Minds, National Children’s Bureau, NSPCC, The Fairbridge Trust, Bolton and ReThink Parent Members.
This report was commissioned by the NHS R&D Health Technology Assessment programme. The views expressed in this report are those of the authors and not necessarily those of the funding body.
Contributions of authors
All authors were involved in the conception and design of the study, the acquisition of data or analysis, data interpretation, drafting and/or revising the report and final approval of the version to be published. Individual contributions were as follows:
Penny Bee was the principal investigator and was involved in the conception and design of the study, day-to-day supervision of the project, study selection, data extraction, conducting the review of clinical effectiveness studies, quality assessment, data analysis, developing the stakeholder consultation, drafting the full report and making revisions to the report.
Peter Bower was involved in the conception and design of the study, study selection, data outcome extraction, analysis of clinical effectiveness data, drafting and making revisions to the full report.
Sarah Byford was involved in the conception and design of the study, particularly the economic component, providing day-to-day supervision of the economic analysis, drafting and revising the economic synthesis and commenting on the full report.
Rachel Churchill was involved in the conception and design of the study, interpretation of findings and commenting on the full report.
Rachel Calam was involved in the conception and design of the study, data extraction, quality assessment and making revisions to the report.
Paul Stallard was involved in the conception and design of the study, developing and implementing the stakeholder consultation, data extraction, interpretation of findings and commenting on the report.
Steven Pryjmachuk was involved in data extraction, quality assessment and making revisions to the report.
Kathryn Berzins was involved in the day-to-day supervision of the project, study selection, data extraction, quality assessment, developing the stakeholder consultation and drafting the full report.
Maria Cary was involved in the day-to-day supervision of the project, study selection, data extraction and quality assessment, particularly the economic component.
Ming Wan was involved in study selection and drafting and making revisions to the report.
Kathryn Abel was involved in the conception and design of the study, study selection, data extraction and drafting and making revisions to the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publication
Bee P, Berzins K, Calam R, Pryjmachuk S, Abel K. Defining quality of life in the children of parents with severe mental illness: a preliminary stakeholder-led model. PLOS ONE 2013;8:e73739. http://dx.doi.org/10.1371/journal.pone.0073739
References
- Royal College of Psychiatrists . CR164 Parents As Patients: Supporting the Needs of Patients Who Are Parents and Their Children 2010. www.rcpsych.ac.uk/publications/collegereports/cr/cr164.aspx (accessed 26 April 2012).
- Diggins M. Think Child, Think Parent, Think Family: a Guide to Parental Mental Health and Child Welfare. London: Social Care Institute for Excellence; 2011.
- Stanley N. Working on the interface: identifying professional responses to families with mental health and child-care needs. Health Soc Care Community 2003;11:208-18. http://dx.doi.org/10.1046/j.1365-2524.2003.00428.x.
- Hidden Lives: Unidentified Young Carers In The UK. Essex: Barnardo’s; 2006.
- UK Census 2001. London: Office for National Statistics; 2003.
- Dearden C, Becker S. Young Carers in the UK: the 2004 report. London: Carers UK; 2004.
- Crossroads Caring for Carers and The Princess Royal Trust for Carers . Economic Evaluation of Young Carers’ Interventions 2008. http://static.carers.org/files/finalfinal3-4040.pdf (accessed November 2013).
- Seeman MV, Gopfert M, Gopfert M, Webster J, Seeman MV. Parental Psychiatric Disorder: Distressed Parents and their Families. New York, NY: Cambridge University Press; 2004.
- Parker G, Beresford B, Clarke S, Gridley K, Pitman R, Spiers G, et al. Research Reviews on Prevalence, Detection and Interventions in Parental Mental Health and Child Welfare: Summary Report. York: Social Policy Research Unit; 2008.
- Coid J, Yang M, Tyrer P, Roberts A, Ullrich S. Prevalence and correlates of personality disorder in Great Britain. Br J Psychiatry 2006;188:423-31. http://dx.doi.org/10.1192/bjp.188.5.423.
- Gopfert M, Webster J, Seeman MV. Parental Psychiatric Disorder: Distressed Parents and Their Families. Cambridge: Cambridge University Press; 1996.
- Mental Health and Social Exclusion. Social Exclusion Unit Report R2089. London: Office of the Deputy Prime Minister; 2004.
- Families Matter: Supporting Families in Northern Ireland: Regional Family and Parenting Strategy. Belfast: Department of Health, Social Services and Public Safety; 2009.
- Reaching Out: Think Family. London: Cabinet Office HMSO; 2007.
- HM Government . No Health Without Mental Health: A Cross-Government Mental Health Outcomes Strategy For People Of All Ages 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123766 (accessed 4 June 2012).
- Welsh Government . Mental Health (Wales) Measure 2010 Guidance n.d. http://wales.gov.uk/topics/health/publications/health/guidance/mhmeasure/?lang=en (accessed 4 June 2012).
- Towards A Mentally Flourishing Scotland: Policy and Action Plan 2009–2011. Edinburgh: Scottish Government; 2009.
- Families At Risk Review: Background Analysis. London: HMSO; 2008.
- Atkinson M, Zibin S, Chuang H. Characterizing quality of life among patients with chronic mental illness: A critical examination of the self-report methodology. Am J Psychiatry 1997;154:99-105.
- Becker M. Quality-of-life instruments for severe chronic mental illness. Implications for pharmacotherapy. Pharmacoeconomics 1995;7:229-37. http://dx.doi.org/10.2165/00019053-199507030-00006.
- Massion AO, Warshaw MG, Keller MB. Quality of life and psychiatric morbidity in panic disorder and generalized anxiety disorder. Am J Psychiatry 1993;150:600-7.
- Berg-Nielsen TS, Vikan A, Dahl AA. Parenting related to child and parental psychopathology: a descriptive review of the literature. Clin Child Psychol Psychiatry 2002;7:529-52. http://dx.doi.org/10.1177/1359104502007004006.
- Reupert A, Maybery D. Families affected by parental mental illness: a multiperspective account of issues and interventions. Am J Orthopsychiatry 2007;77:362-9. http://dx.doi.org/10.1037/0002-9432.77.3.362.
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev 2011;14:1-27. http://dx.doi.org/10.1007/s10567-010-0080-1.
- Beardselee WR, Versage EM, Giadstone TRG. Children of affectively ill parents: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 1998;37:1134-41. http://dx.doi.org/10.1097/00004583-199811000-00012.
- Oyserman D, Mowbray CT, Meares PA, Firminger KB. Parenting among mothers with a serious mental illness. Am J Orthopsychiatry 2000;70:296-315. http://dx.doi.org/10.1037/h0087733.
- Fernbacher S, Goodyear M, Farhall J. Taking a closer look: a cross-sector audit of families where a parent has a mental illness. Adv Mental Health 2009;8:242-9. http://dx.doi.org/10.5172/jamh.8.3.242.
- Larsson B, Knutsson-Medin L, Sundelin C, Trost von Werder A. Social competence and emotional/behavioural problems in children of psychiatric inpatients. Eur Child Adolesc Psychiatry 2000;9:122-8. http://dx.doi.org/10.1007/PL00010708.
- Somers V. Schizophrenia: the impact of parental illness on children. Br J Soc Work 2007;37:1319-34. http://dx.doi.org/10.1093/bjsw/bcl083.
- Bella T, Goldstein T, Axelson D, Obreja M, Monk K, Hickey MB, et al. Psychosocial functioning in offspring of parents with bipolar disorder. J Affect Disord 2011;133:204-11. http://dx.doi.org/10.1016/j.jad.2011.03.022.
- Kahn RS, Brandt D, Whitaker RC. Combined effect of mothers’ and fathers’ mental health symptoms on children’s behavioral and emotional well-being. Arch Pediatr Adolesc Med 2004;158:721-9. http://dx.doi.org/10.1001/archpedi.158.8.721.
- Mattejat F, Remschmidt H. The children of mentally ill parents. Dtsch Arztebl Int 2008;105:413-18.
- Stith SM, Liu T, Davies LC, Boykin EL, Alder MC, Harris JM, et al. Risk factors in child maltreatment: a meta-analytic review of the literature. Aggress Violent Behav 2009;14:13-29.
- Webb R, Abel K, Pickles A, Appleby L. Mortality in offspring of parents with psychotic disorders: a critical review and meta-analysis. Am J Psychiatry 2005;162:1045-56. http://dx.doi.org/10.1176/appi.ajp.162.6.1045.
- Weissman MM, Warner V, Wickramaratne P, Moreau D, Olfson M. Offspring of depressed parents: 10 years later. Arch Gen Psychiatry 1997;54:932-40. http://dx.doi.org/10.1001/archpsyc.1997.01830220054009.
- Terzian ACC, Andreoli SB, de Oliveira LM, de Jesus Mari J, McGrath J. A cross-sectional study to investigate current social adjustment of offspring of patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 2007;257:230-6. http://dx.doi.org/10.1007/s00406-007-0714-6.
- Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. Am J Psychiatry 2006;163:1001-8. http://dx.doi.org/10.1176/appi.ajp.163.6.1001.
- Östman M, Hansson L. Children in families with a severely mentally ill member. Prevalence and needs for support. Soc Psychiatry Psychiatr Epidemiol 2002;37:243-8.
- Jacob T, Windle M. Young adult children of alcoholic, depressed and nondistressed parents. J Stud Alcohol Drugs 2000;61:836-44.
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med 1997;27:1101-19. http://dx.doi.org/10.1017/S0033291797005588.
- Elgar FJ, McGrath PJ, Waschbusch DA, Stewart SH, Curtis LJ. Mutual influences on maternal depression and child adjustment problems. Clin Psychol Rev 2004;24:441-59. http://dx.doi.org/10.1016/j.cpr.2004.02.002.
- Maybery D, Ling L, Szakacs E, Reupert A. Children of a parent with a mental illness: perspectives on need. Adv Mental Health 2005;4:78-8. http://dx.doi.org/10.5172/jamh.4.2.78.
- Henriksson KM, McNeil TF. Health and development in the first 4 years of life in offspring of women with schizophrenia and affective psychoses: Well-Baby Clinic information. Schizophr Res 2004;70:39-48. http://dx.doi.org/10.1016/j.schres.2003.11.003.
- Phelan R, Lee L, Howe D, Walter G. Parenting and mental illness: a pilot group programme for parents. Australas Psychiatry 2006;14:399-402. http://dx.doi.org/10.1111/j.1440-1665.2006.02312.x.
- Wan MW, Warburton AL, Appleby L, Abel KM. Mother and baby unit admissions: feasibility study examining child outcomes 4–6 years on. Aust NZ J Psychiatry 2007;41:150-6.
- O’Connell ME, Boat T, Warner KE. National Research Council (US) and Institute of Medicine (US) Committee on the Prevention of Mental Disorders and Substance Abuse Among Children, Youth, and Young Adults: Research Advances and Promising Interventions. Washington DC: National Academies Press (US); 2009.
- Schiffman J, LaBrie J, Carter J, Cannon T, Schulsinger F, Parnas J, et al. Perception of parent–child relationships in high-risk families, and adult schizophrenia outcome of offspring. J Psychiatr Res 2002;36:41-7. http://dx.doi.org/10.1016/S0022-3956(01)00046-2.
- Wynne LC, Tienari P, Nieminen P, Sorri A, Lahti I, Moring J, et al. I. Genotype–environment interaction in the schizophrenia spectrum: genetic liability and global family ratings in the Finnish adoption study. Fam Process 2006;45:419-34. http://dx.doi.org/10.1111/j.1545-5300.2006.00180.x.
- Nicholson J, Biebel K, Hinden BR, Henry AD, Stier L. Critical Issues For Parents With Mental Illness and Their Families. Rockville, MD: Centre for Mental Health Services of the Substance Abuse & Mental Health Services Administration; 2001.
- Hinden BR, Biebel K, Nicholson J, Henry A, Katz-Leavy J. A survey of programs for parents with mental illness and their families: identifying common elements to build the evidence base. J Behav Health Serv Res 2006;33:21-38. http://dx.doi.org/10.1007/s11414-005-9007-x.
- Park J, Solomon P, Mandell D. Involvement in the child welfare system among mothers with serious mental illness. Psychiatr Serv 2006;57:493-7. http://dx.doi.org/10.1176/appi.ps.57.4.493.
- Fraser C, James EL, Anderson K, Lloyd D, Judd F. Intervention programs for children of parents with a mental illness: a critical review. Int J Ment Health Promotion 2006;8:9-20. http://dx.doi.org/10.1080/14623730.2006.9721897.
- Cox MJ, Paley B. Families as systems. Annu Rev Psychol 1997;48:243-67. http://dx.doi.org/10.1146/annurev.psych.48.1.243.
- Bronfenbrenner U. The Ecology Of Human Development: Experiments By Nature and Design. Cambridge, MA: Harvard University Press; 1979.
- Cook WL. Interpersonal influence in family systems: a social relations model analysis. Child Dev 2001;72:1179-97.
- Cummings EM, Davies PT. Maternal depression and child development. J Child Psychol Psychiatry 1994;35:73-122. http://dx.doi.org/10.1111/j.1469-7610.1994.tb01133.x.
- Beresford B, Clarke S, Gridley K, Parker G, Pitman R, Spiers G, et al. Technical Report for SCIE Research Review on Access, Acceptability and Outcomes of Services/Interventions to Support Parents with Mental Health Problems and Their Families. York: Social Policy Research Unit, University of York; 2008.
- Fraser E, Pakenham KI. Evaluation of a resilience-based intervention for children of parents with mental illness. Australas Psychiatry 2008;42:1041-50. http://dx.doi.org/10.1080/00048670802512065.
- Goodyear M, Cuff R, Maybery D, Reupert A. CHAMPS: a peer support program for children of parents with a mental illness. Adv Mental Health 2009;8:296-304. http://dx.doi.org/10.5172/jamh.8.3.296.
- Lucas LE, Montgomery SH, Richardson DA, Rivers PA. Impact project: reducing the risk of mental illness to children of distressed mothers. New Dir Ment Health Serv 1984:79-94. http://dx.doi.org/10.1002/yd.23319842406.
- Sumner GS. The Design and Implementation of a Cognitive Behavioural Problem-Solving Training Program for Children of Severely Disturbed Parents 1983.
- Beardslee WR, Hoke L, Wheelock I, Rothberg PC, Vandevelde P, Swatling S. Initial findings on preventive intervention for families with parental-affective disorders. Am J Psychiatry 1992;149:1335-40.
- Sanford M, Byrne C, Williams S, Atley S, Ridley T, Miller J, et al. A pilot study of a parent-education group for families affected by depression. Can J Psychiatry 2003;48:78-86.
- Cicchetti D, Toth SL, Rogosch FA. The efficacy of toddler-parent psychotherapy to increase attachment security in offspring of depressed mothers. Attach Hum Dev 1999;1:34-66. http://dx.doi.org/10.1080/14616739900134021.
- Sanders MR, McFarland M. Treatment of depressed mothers with disruptive children: a controlled evaluation of cognitive behavioral family intervention. Behav Ther 2000;31:89-112. http://dx.doi.org/10.1016/S0005-7894(00)80006-4.
- Clark R, Tluczek A, Wenzel A. Psychotherapy for postpartum depression: a preliminary report. Am J Orthopsychiatry 2003;73:441-54. http://dx.doi.org/10.1037/0002-9432.73.4.441.
- Clark R, Tluczek A, Brown R. A mother–infant therapy group model for postpartum depression. Infant Ment Health J 2008;29:514-36. http://dx.doi.org/10.1002/imhj.20189.
- Barlow J, Coren E. Parent-training programmes for improving maternal psychosocial health. Cochrane Database Syst Rev 2004;1. http://dx.doi.org/10.1002/14651858.CD002020.
- Coren E, Barlow J. Individual and group-based parenting programmes for improving psychosocial outcomes for teenage parents and their children. Cochrane Database Syst Rev 2011;3. http://dx.doi.org/10.1002/14651858.CD002964.
- Wan MW, Moulton S, Abel KM. A review of mother–child relational interventions and their usefulness for mothers with schizophrenia. Arch Womens Ment Health 2008;11:171-9. http://dx.doi.org/10.1007/s00737-008-0010-0.
- Siegenthaler E, Munder T, Egger M. Effect of preventive interventions in mentally ill parents on the mental health of the offspring: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 2012;51:8-17. http://dx.doi.org/10.1016/j.jaac.2011.10.018.
- Lawton MP, Birren JE, Lubben JE, Rowe JC, Deutchman DE. The Concept and Measurement of Quality of Life in the Frail Elderly. San Diego, CA: Academic Press; 1991.
- Eiser C, Morse R. Assessment NCC for HT, NHSR. Quality-of-life measures in chronic diseases of childhood. Health Technol Assess 2001;5.
- Dannerbeck A, Casas F, Sadurni M, Coenders G. Social Indicators Research Series. Quality-of-Life Research on Children and Adolescents 2004;23.
- Bowling A, Gabriel Z. An integrational model of quality of life in older age. A comparison of analytic and lay models of quality of life. Social Indicators Res 2004;69:1-36.
- Harding L. Children’s quality of life assessments: a review of generic and health related quality of life measures completed by children and adolescents. Clin Psychol Psychother 2001;8:79-96. http://dx.doi.org/10.1002/cpp.275.
- Huebner ES, Valois RF, Suldo SM, Smith LC, McKnight CG, Seligson JL, et al. Perceived quality of life: a neglected component of adolescent health assessment and intervention. J Adolesc Health 2004;34:270-8. http://dx.doi.org/10.1016/j.jadohealth.2003.07.007.
- Brissette I, Scheier MF, Carver CS. The role of optimism in social network development, coping, and psychological adjustment during a life transition. J Pers Soc Psychol 2002;82:102-11. http://dx.doi.org/10.1037//0022-3514.82.1.102.
- Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. JAMA 1995;273:59-65. http://dx.doi.org/10.1001/jama.1995.03520250075037.
- Constitution of the WHO. Geneva: World Health Organization; 1946.
- Drummond MF, Sculpher MJ, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2011. www.cochrane-handbook.org (accessed 19 November 2013).
- Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. JAMA 1996;276:1339-41. http://dx.doi.org/10.1001/jama.1996.03540160061034.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy n.d.;16:199-208.
- Department of Children, Schools and Families . Every Child Matters Outcomes Framework 2008. http://cw.routledge.com/textbooks/9780415485586/data/EveryChildMatters-OutcomesFramework.pdf (accessed 25 April 2012).
- Macdonald G, Livingstone N, Davidson G, Sloan S, Fargas M, McSherry D. Improving the Mental Health of Northern Ireland’s Children and Young People. Queens University, Belfast: Institute of Child Care Research; 2011.
- Rajmil L, Herdman M, Fernandez de Sanmamed MJ, Detmar S, Bruil J, Ravens-Sieberer U, et al. Generic health-related quality of life instruments in children and adolescents: a qualitative analysis of content. J Adolesc Health 2004;34:37-45. http://dx.doi.org/10.1016/S1054-139X(03)00249-0.
- Matza LS, Swensen AR, Flood EM, Secnik K, Leidy NK. Assessment of health-related quality of life in children: a review of conceptual, methodological, and regulatory issues. Value Health 2004;7:79-92. http://dx.doi.org/10.1111/j.1524-4733.2004.71273.x.
- The WHOQOL group . The World Health Organization Quality of Life Assessment (WHOQOL): Position Paper From the World Health Organization. Soc Sci Med 1995;41:1403-9.
- Juniper EF. Health-related quality of life in asthma. Curr Opin Pulm Med 1999;5:105-10. http://dx.doi.org/10.1097/00063198-199903000-00005.
- Connolly MA, Johnson JA. Measuring quality of life in paediatric patients. Pharmacoeconomics 1999;16:605-25. http://dx.doi.org/10.2165/00019053-199916060-00002.
- Annett RD. Assessment of health status and quality of life outcomes for children with asthma. J Allergy Clin Immunol 2001;107:S473-S81. http://dx.doi.org/10.1067/mai.2001.114949.
- Barr R, Pai M, Weitzman S, Feeny D, Furlong W, Rosenbaum P, et al. A multiattribute approach to health-status measurement and clinical management illustrated by an application to brain tumors in childhood. Int J Oncol 1994;4:639-48.
- Landgraf JM, Abetz LN. Measuring health outcomes in pediatric populations: issues in psychometrics and application. Qual Life Pharmacoeconomics Clin Trials 1996;2:793-802.
- Eiser C, Mohay H, Morse R. The measurement of quality of life in young children. Child Care Health Dev 2000;26:401-14. http://dx.doi.org/10.1046/j.1365-2214.2000.00154.x.
- Parke RD, Ladd GW. Family–Peer Relationships: Modes of Linkage. Hillsdale, NJ: L. Erlbaum Associates; 1992.
- Matza LS, Kupersmidt JB, Goodman WB, Patterson MM, Meadors PL, Robertson J, et al. Maternal Disciplinary Style and Children'S Cognitions about 2002.
- Ladd GW, Le Sier KD. Parents and Children’s Peer Relationships. Handbook of Parenting. Mahwah, NJ: L. Erlbaum Associates; 2002.
- Gladstone BM, Boydell KM, Seeman MV, McKeever PD. Children’s experiences of parental mental illness: a literature review. Early Interv Psychiatr 2011;5:271-89. http://dx.doi.org/10.1111/j.1751-7893.2011.00287.x.
- Bybee D, Mowbray CT, Oyserman D, Lewandowski L. Variability in community functioning of mothers with serious mental illness. J Behav Health Serv Res 2003;30:269-89. http://dx.doi.org/10.1097/00075484-200307000-00002.
- Grant G, Repper J, Nolan M. Young people supporting parents with mental health problems: experiences of assessment and support. Health Soc Care Community 2008;16:271-81. http://dx.doi.org/10.1111/j.1365-2524.2008.00766.x.
- Undertaking Systematic Reviews of Research on Effectiveness: CRD's Guidance for Those Carrying Out or Commissioning Reviews. York: CRD Report 4 (2nd edn), University of York; 2001.
- Rethink Policy Statement 43: Rethink’s Understanding of Severe Mental Illness. London: Rethink; 2008.
- CASP UK . CASP UK: Making Sense of Evidence n.d. www.casp-uk.net/wp-content/uploads/2011/11/CASP-Qualitative-Research-Checklist-31.05.13.pdf (accessed 19 November 2013).
- Alderson P. Listening to Children: Children, Social Research And Ethics. Essex: Barnardo’s; 1995.
- Building Bridges: A Guide to Arrangements for Inter-Agency Working for the Care and Protection of Severely Mentally Ill People. London: Department of Health; 1995.
- Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. Arlington, VA: American Psychiatric Press Inc.; 2000.
- ICD-10: The ICD-10 Classification of Mental and Behavioural Disorders?: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992.
- Lipsey MW, Wilson DB. The efficacy of psychological, educational, and behavioral treatment: Confirmation from meta-analysis. Am Psychol 1993;48:1181-209. http://dx.doi.org/10.1037//0003-066X.48.12.1181.
- Higgins J, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. http://dx.doi.org/10.1136/bmj.327.7414.557.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Petticrew M, Roberts H. Systematic Reviews in the Social Sciences: A Practical Guide. Oxford: Blackwell Publishing; 2006.
- Pope C, Mays N, Popay J. Synthesizing Qualitative and Quantitative Health Evidence: A Guide to Methods. Berkshire: Open University Press; 2007.
- Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme. Lancaster: Institute of Health Research, University of Lancaster; 2006.
- Boath E, Major K, Cox J. When the cradle falls II: the cost-effectiveness of treating postnatal depression in a psychiatric day hospital compared with routine primary care. J Affect Disord 2003;74:159-66. http://dx.doi.org/10.1016/S0165-0327(02)00007-1.
- Cohler BJ, Grunebaum H. Children of parents hospitalized for mental illness: the evaluation of an intervention program for mentally ill mothers of young children (Part 2). J Children Contemp Soc 1982;15:57-66.
- Stott FM, Musick JS, Cohler BJ, Spencer KK, Goldman J, Clark R, et al. Intervention for the severely disturbed mother. New Direct Mental Health Serv 1984;24:7-32. http://dx.doi.org/10.1002/yd.23319842403.
- Gerull F, Meares R, Stevenson J, Korner A, Newman L. The beneficial effect on family life in treating borderline personality. Psychiatry 2008;7:59-70. http://dx.doi.org/10.1521/psyc.2008.71.1.59.
- Alder S. Reaching out to women. Ment Health Today 2005:26-8.
- Pitman E, Matthey S. Evaluation of the SMILES Program for Children with Mentally Ill Parents: Bankstown, Sydney. Sydney: South West Sydney Area Health Service; 2002.
- Richter GA. Fostering Resilience: Evaluating the Effectiveness of Kids in Control 2006.
- Derogatis LR. SCL-90: Administration, Scoring & Procedures Manual for the R(evised) Version and Other Instruments of the Psychopathology Rating Scale Series. Baltimore, MD: Clinical Psychometric Research; 1977.
- Croake JW, Kelly FD. Adlerian Family Therapy with schizophrenic and depressed patients. Individ Psychol J Adlerian Theory Res Pract 1985;41.
- Hanrahan P, McCoy ML, Cloninger L, Dincin J, Zeitz MA, Simpatico TA, et al. The mothers’ project for homeless mothers with mental illnesses and their children: a pilot study. Psychiatr Rehabil J 2005;28:291-4. http://dx.doi.org/10.2975/28.2005.291.294.
- Riebschleger J, Tableman B, Rudder D, Onaga E, Whalen P. Early outcomes of a pilot psychoeducation group intervention for children of a parent with a psychiatric illness. Psychiatr Rehabil J 2009;33:133-41. http://dx.doi.org/10.2975/33.2.2009.133.141.
- Zimmerman M, McGlinchey JB, Posternak MA, Friedman M, Boerescu D, Attiullah N. Remission in depressed outpatients: more than just symptom resolution?. J Psychiatr Res 2008;42:797-801. http://dx.doi.org/10.1016/j.jpsychires.2007.09.004.
- The NICE Guideline on the Treatment and Management of Depression in Adults. London: NICE; 2010.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-71. http://dx.doi.org/10.1001/archpsyc.1961.01710120031004.
- Montgomery SA, Lecrubier Y. Is severe depression a separate indication?. Eur Neuropsychopharmacol 1999;9:259-64. http://dx.doi.org/10.1016/S0924-977X(98)00048-0.
- Kriston L, von Wolff A. Not as golden as standards should be: interpretation of the Hamilton Rating Scale for Depression. J Affect Disord 2011;128:175-7. http://dx.doi.org/10.1016/j.jad.2010.07.011.
- Müller MJ, Szegedi A, Wetzel H, Benkert O. Moderate and severe depression. Gradations for the Montgomery–Åsberg Depression Rating Scale. J Affect Disord 2000;60:137-40.
- Müller M. Differentiating moderate and severe depression using the Montgomery–Åsberg depression rating scale (MADRS). J Affect Disord 2003;77:255-60. http://dx.doi.org/10.1016/S0165-0327(02)00120-9.
- Zich JM, Attkisson CC, Greenfield TK. Screening for depression in primary care clinics: the CES-D and the BDI. Int J Psychiatry Med 1990;20:259-77. http://dx.doi.org/10.2190/LYKR-7VHP-YJEM-MKM2.
- Ensel WM, Lin N, Dean A, Ensel W. Social Support, Life Events, and Depression. Orlando, FL: Academic Press; 1986.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Int Med 2001;16:606-13. http://dx.doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Sharp DJ, Chew-Graham CA, Tylee A, Lewis G, Howard L, Anderson I, et al. A pragmatic randomised controlled trial to compare antidepressants with a community-based psychosocial intervention for the treatment of women with postnatal depression: the RESPOND trial. Health Technol Assess 2010;14.
- Rojas G, Fritsch R, Solis J, Jadresic E, Castillo C, González M, et al. Treatment of postnatal depression in low-income mothers in primary-care clinics in Santiago, Chile: a randomised controlled trial. Lancet 2007;370:1629-37. http://dx.doi.org/10.1016/S0140-6736(07)61685-7.
- Boath E, Cox J, Lewis M, Jones P, Pryce A. When the cradle falls: the treatment of postnatal depression in a psychiatric day hospital compared with routine primary care. J Affect Disord 1999;53:143-51. http://dx.doi.org/10.1016/S0165-0327(98)00101-3.
- Bennett RB. An intervention program for children of recently hospitalized, depressed mothers 1991.
- Beardslee WR, Wright EJ, Gladstone TRG, Forbes P. Long-term effects from a randomized trial of two public health preventive interventions for parental depression. J Fam Psychol 2007;21:703-13. http://dx.doi.org/10.1037/0893-3200.21.4.703.
- Butler SF, Budman SH, Beardslee W. Risk reduction in children from families with parental depression: a videotape psychoeducation program. National Academies of Practice Forum: Issues in Interdisciplinary Care 2000;2:267-76.
- Compas BE, Forehand R, Keller G, Champion JE, Rakow A, Reeslund KL, et al. Randomized controlled trial of a family cognitive–behavioral preventive intervention for children of depressed parents. J Consult Clin Psychol 2009;77:1007-20. http://dx.doi.org/10.1037/a0016930.
- Gelfand DM, Teti DM, Seiner SA, Jameson PB. Helping mothers fight depression: evaluation of a home-based intervention program for depressed mothers and their infants. J Clin Child Psychol 1996;25:406-22. http://dx.doi.org/10.1207/s15374424jccp2504_6.
- Grote NK, Swartz HA, Geibel SL, Zuckoff A, Houck PR, Frank E. A randomized controlled trial of culturally relevant, brief interpersonal psychotherapy for perinatal depression. Psychiatr Serv 2009;60:313-21. http://dx.doi.org/10.1176/appi.ps.60.3.313.
- O’Hara MW, Stuart S, Gorman LL, Wenzel A. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry 2000;57:1039-45. http://dx.doi.org/10.1001/archpsyc.57.11.1039.
- Swartz HA, Frank E, Zuckoff A, Cyranowski JM, Houck PR, Cheng Y, et al. Brief interpersonal psychotherapy for depressed mothers whose children are receiving psychiatric treatment. Am J Pychiatry 2008;165:1155-62. http://dx.doi.org/10.1176/appi.ajp.2008.07081339.
- Meager I, Milgrom J. Group treatment for postpartum depression: a pilot study. Australas Psychiatry 1996;30:852-60. http://dx.doi.org/10.3109/00048679609065055.
- Milgrom J, Holt C, Gemmill A, Ericksen J, Leigh B, Buist A, et al. Treating postnatal depressive symptoms in primary care: a randomised controlled trial of GP management, with and without adjunctive counselling. BMC Psychiatry 2011;11. http://dx.doi.org/10.1186/1471-244X-11-95.
- Mulcahy R, Reay RE, Wilkinson RB, Owen C. A randomised control trial for the effectiveness of group interpersonal psychotherapy for postnatal depression. Arch Womens Ment Health 2010;13:125-39. http://dx.doi.org/10.1007/s00737-009-0101-6.
- Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive–behavioural counselling in the treatment of postnatal depression. BMJ 1997;314:932-6. http://dx.doi.org/10.1136/bmj.314.7085.932.
- Cooper PJ, Murray L, Wilson A, Romaniuk H. Controlled trial of the short- and long-term effect of psychological treatment of post-partum depression. 1. Impact on maternal mood. Br J Psychiatry 2003;182:412-19.
- Holden JM, Sagovsky R, Cox JL. Counselling in a general practice setting: controlled study of health visitor intervention in treatment of postnatal depression. BMJ 1989;298:223-6. http://dx.doi.org/10.1136/bmj.298.6668.223.
- Verduyn C, Barrowclough C, Roberts J, Tarrier N, Harrington R. Maternal depression and child behaviour problems. Randomised placebo-controlled trial of a cognitive–behavioural group intervention. Br J Psychiatry 2003;183:342-8. http://dx.doi.org/10.1192/bjp.183.4.342.
- Misri S, Kostaras X, Fox D, Kostaras D. The impact of partner support in the treatment of postpartum depression. Can J Psychiatry 2000;45:554-8.
- Misri S, Reebye P, Corral M, Mills L. The use of paroxetine and cognitive–behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry 2004;65:1236-41. http://dx.doi.org/10.4088/JCP.v65n0913.
- Chabrol H, Teissedre F, Saint-Jean M, Teisseyre N, Roge B, Mullet E, et al. Prevention and treatment of post-partum depression: a controlled randomized study on women at risk. Psychol Med 2002;32:1039-47. http://dx.doi.org/10.1017/S0033291702006062.
- Rahman A, Malik A, Sikander S, Roberts C, Creed F. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet 2008;372:902-9. http://dx.doi.org/10.1016/S0140-6736(08)61400-2.
- Wickberg B, Hwang CP. Counselling of postnatal depression: a controlled study on a population based Swedish sample. J Affect Disord 1996;39:209-16. http://dx.doi.org/10.1016/0165-0327(96)00034-1.
- Forman DR, O’Hara MW, Stuart S, Gorman LL, Larsen KE, Coy KC. Effective treatment for postpartum depression is not sufficient to improve the developing mother–child relationship. Dev Psychopathol 2007;19:585-602. http://dx.doi.org/10.1017/S0954579407070289.
- Misri S, Reebye P, Milis L, Shah S. The impact of treatment intervention on parenting stress in postpartum depressed mothers: a prospective study. Am J Orthopsychiatry 2006;76:115-19. http://dx.doi.org/10.1037/0002-9432.76.1.115.
- Beardslee WR, Versage EM, Wright EJ, Salt P, Rothberg PC, Drezner K, et al. Examination of preventive interventions for families with depression: evidence of change. Dev Psychopathol 1997;9:109-30. http://dx.doi.org/10.1017/S0954579497001090.
- Murray L, Cooper PJ, Wilson A, Romaniuk H. Controlled trial of the short-and long-term effect of psychological treatment of post-partum depression. 2. Impact on the mother–child relationship and child outcome. Br J Psychiatry 2003;182:420-7. http://dx.doi.org/10.1192/bjp.182.5.420.
- Beardslee WR, Keller MB, Lavori PW, Staley J, Sacks N. The impact of parental affective disorder on depression in offspring: a longitudinal follow-up in a nonreferred sample. J Am Acad Child Adolesc Psychiatry 1993;32:723-30. http://dx.doi.org/10.1097/00004583-199307000-00004.
- Sanders MR, Dadds MR. Behavioral Family Intervention. Boston, MA: Allyn & Bacon; 1993.
- Ammerman RT, Putman FW, Stevens J, Bosse NR, Short JA, Bodley AL, et al. An open trial of in-home CBT for depressed mothers in home visitation. Matern Child Health J 2010;15:1333-41. http://dx.doi.org/10.1007/s10995-010-0691-7.
- Nylen KJ, O’Hara MW, Brock R, Moel J, Gorman L, Stuart S. Predictors of the longitudinal course of postpartum depression following interpersonal psychotherapy. J Consult Clin Psychol 2010;78:757-63. http://dx.doi.org/10.1037/a0020623.
- Compas BE, Champion JE, Forehand R, Cole DA, Reeslund KL, Fear J, et al. Coping and parenting: mediators of 12-month outcomes of a family group cognitive–behavioral preventive intervention with families of depressed parents. J Consult Clin Psychol 2010;78:623-34. http://dx.doi.org/10.1037/a0020459.
- Beardslee WR, Wright EJ, Salt P, Drezner K, Gladstone TRG, Versage EM, et al. Examination of children’s responses to two preventive intervention strategies over time. J Am Acad Child Adolesc Psychiatry 1997;36:196-204. http://dx.doi.org/10.1097/00004583-199702000-00010.
- Pearlstein TB, Zlotnick C, Battle CL, Stuart S, O’Hara MW, Price AB, et al. Patient choice of treatment for postpartum depression: a pilot study. Arch Womens Ment Health 2006;9:303-8. http://dx.doi.org/10.1007/s00737-006-0145-9.
- Seeley S, Murray L, Cooper PJ. The outcome for mothers and babies of health visitor intervention. Health Visitor 1996;69:135-8.
- O’Brien LM, Boath E, Hodgson R, Cox JL. The long-term follow-up of women treated for postnatal depression at a specialist day hospital compared to routine primary care. Int J Psychiatry Clin Pract 2002;6:199-203. http://dx.doi.org/10.1080/136515002761580992.
- Ammerman RT, Putnam FW, Stevens J, Holleb LJ, Novak AL, Van Ginkel JB. In-home cognitive behavior therapy for depression: an adapted treatment for first-time mothers in home visitation. Best Pract Ment Health 2005;1:1-14.
- Davies S. A first-stage evaluation of a group programme for PND. Community Pract 2004;77:426-33.
- Griffiths P, Barker-Collo S. Study of a group treatment program for postnatal adjustment difficulties. Arch Womens Ment Health 2008;11:33-41. http://dx.doi.org/10.1007/s00737-008-0220-5.
- Grote NK, Bledsoe SE, Swartz HA, Frank E. Feasibility of providing culturally relevant, brief interpersonal psychotherapy for antenatal depression in an obstetrics clinic: a pilot study. Research on Social Work Practice 2004;14:397-40. http://dx.doi.org/10.1177/1049731504265835.
- Reay R, Fisher Y, Robertson M, Adams E, Owen C. Group interpersonal psychotherapy for postnatal depression: a pilot study. Arch Womens Ment Health 2006;9:31-9. http://dx.doi.org/10.1007/s00737-005-0104-x.
- Swartz HA, Zuckoff A, Frank E, Spielvogle HN, Shear MK, Fleming MA, et al. An open-label trial of enhanced brief interpersonal psychotherapy in depressed mothers whose children are receiving psychiatric treatment. Depress Anxiety 2006;23:398-404. http://dx.doi.org/10.1002/da.20212.
- Verdeli H, Ferro T, Wickramaratne P, Greenwald S, Blanco C, Weissman MM. Treatment of depressed mothers of depressed children: pilot study of feasibility. Depress Anxiety 2004;19:51-8. http://dx.doi.org/10.1002/da.10139.
- Pitman E, Matthey S. The SMILES program: A group program for children with mentally ill parents or siblings. Am J Orthopsychiatry 2004;74:383-8. http://dx.doi.org/10.1037/0002-9432.74.3.383.
- Edwards M. Reported and Observed Disruptive Behaviours in Children of Depressed Mothers Prior to and Following Cognitive–Behaviour Therapy for Maternal Depression 1998.
- Klier CM, Muzik M, Rosenblum KL, Lenz G. Interpersonal psychotherapy adapted for the group setting in the treatment of postpartum depression. J Psychother Pract Res 2001;10:124-31.
- Stuart S, O’Hara MW. Treatment of postpartum depression with interpersonal psychotherapy. Arch Gen Psychiatry 1995;52:75-6.
- Romeo R, Byford S, Knapp M. Economic evaluations of child and adolescent mental health interventions: a systematic review. J Child Psychol Psychiatry 2005;46:919-30. http://dx.doi.org/10.1111/j.1469-7610.2005.00407.x.
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663-94. http://dx.doi.org/10.7326/0003-4819-134-8-200104170-00012.
- Thomas J, Harden A, Oakley A, Oliver S, Sutcliffe K, Rees R, et al. Integrating qualitative research with trials in systematic reviews. BMJ 2004;328:1010-12. http://dx.doi.org/10.1136/bmj.328.7446.1010.
- Boulton M, Fitzpatrick R, Swinburn C. Qualitative research in health care: II. A structured review and evaluation of studies. J Eval Clin Pract 1996;2:171-9. http://dx.doi.org/10.1111/j.1365-2753.1996.tb00041.x.
- Mays N, Pope C. Qualitative research: rigour and qualitative research. BMJ 1995;311. http://dx.doi.org/10.1136/bmj.311.6997.109.
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess 1998;2.
- Spencer L, Ritchie J, Lewis J, Dillon L. Quality in Qualitative Evaluation: a Framework for Assessing Research Evidence. London: Government Chief Social Researcher’s Office; n.d.
- Noyes J, Popay J. Directly observed therapy and tuberculosis: how can a systematic review of qualitative research contribute to improving services? A qualitative meta-synthesis. J Adv Nurs 2007;57:227-43. http://dx.doi.org/10.1111/j.1365-2648.2006.04092.x.
- Hewitt C, Gilbody S, Brealey S, Paulden M, Palmer S, Mann R, et al. Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis. Health Technol Assess 2009;13.
- Campbell R, Pound P, Pope C, Britten N, Pill R, Morgan M, et al. Evaluating meta-ethnography: a synthesis of qualitative research on lay experiences of diabetes and diabetes care. Soc Sci Med 2003;56:671-84. http://dx.doi.org/10.1016/S0277-9536(02)00064-3.
- Dixon-Woods M, Bonas S, Booth A, Jones DR, Miller T, Sutton AJ, et al. How can systematic reviews incorporate qualitative research? A critical perspective. Qualitative Research 2006;6:27-44.
- Sandelowski M, Barroso J. Handbook for Synthesizing Qualitative Research. New York, NY: Springer Publishing Company; 2007.
- Lucas P, Baird J, Arai L, Law C, Roberts H. Worked examples of alternative methods for the synthesis of qualitative and quantitative research in systematic reviews. BMC Med Res Methodol 2007;7.
- Finzi R, Strange D. Short term group intervention as a means of improving the adjustment of children of mentally ill parents. Social Work With Groups 2007;20:69-80. http://dx.doi.org/10.1300/J009v20n04_06.
- Waldo MC, Roath M, Levine W, Freedman R. A model program to teach parenting skills to schizophrenic mothers. Psychiatric Serv 1987;38:1110-12.
- Cowling V, Garrett M. Child and family inclusive practice: a pilot program in a community adult mental health service. Australas Psychiatry 2009;17:279-82. http://dx.doi.org/10.1080/10398560902840232.
- Boath E, Bradley E, Anthony P. Users’ views of two alternative approaches to the treatment of postnatal depression. J Reprod Infant Psychol 2004;22:13-24. http://dx.doi.org/10.1080/02646830310001643085.
- Hinden BR, Hinden BR, Biebel K, Nicholson J, Mehnert L. The invisible children’s project. J Behav Health Serv Res 2005;32:393-408. http://dx.doi.org/10.1097/00075484-200510000-00006.
- Beardslee WR, Salt P, Versage EM, Gladstone TR, Wright EJ, Rothberg PC. Sustained change in parents receiving preventive interventions for families with depression. Am J Psychiatry 1997;154:510-5.
- Nose M, Barbui C, Tansella M. How often do patients with psychosis fail to adhere to treatment programmes? A systematic review. Psychol Med 2003;33:1149-60.
- Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al. The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature. Health Technol Assess 2002;6.
- Allen G. Early Intervention: Smart Investment, Massive Savings. London: Cabinet Office; 2011.
- London: C4EO; 2011.
- National Institute for Health and Care Excellence (NICE) . Antenatal and Postnatal Mental Health (APMH) n.d. http://guidance.nice.org.uk/CG45/NICEGuidance/pdf/English (accessed 10 June 2012).
- Cooper PJ, Murray L. Course and recurrence of postnatal depression. Evidence for the specificity of the diagnostic concept. Br J Psychiatry 1995;166:191-5. http://dx.doi.org/10.1192/bjp.166.2.191.
- Ramchandani P, Stein A, Evans J, O’Connor TG. The ALSPAC team . Paternal depression in the postnatal period and child development: a prospective population study. Lancet 2005;365:2201-5. http://dx.doi.org/10.1016/S0140-6736(05)66778-5.
- Abel KM, Webb RT, Salmon MP, Wan MW, Appleby L. Prevalence and predictors of parenting outcomes in a cohort of mothers with schizophrenia admitted for joint mother and baby psychiatric care in England. J Clin Psychiatry 2005;66:781-9. http://dx.doi.org/10.4088/JCP.v66n0618.
- Office for National Statistics . Birth Statistics, England and Wales (Series FM1) 2009. www.ons.gov.uk/ons/rel/vsob1/birth-statistics–england-and-wales–series-fm1-/no-–37-–2008/index.html (accessed 13 June 2012).
- Fearon P, Kirkbride JB, Morgan C, Dazzan P, Morgan K, Lloyd T, et al. Incidence of schizophrenia and other psychoses in ethnic minority groups: results from the MRC AESOP Study. Psychol Med 2006;36:1541-50. http://dx.doi.org/10.1017/S0033291706008774.
- Morgan C, Abdul-Al R, Lappin JM, Jones P, Fearon P, Leese M, et al. Clinical and social determinants of duration of untreated psychosis in the AESOP first-episode psychosis study. Br J Psychiatry 2006;189:446-52. http://dx.doi.org/10.1192/bjp.bp.106.021303.
- Bhugra D, Leff J, Mallett R, Der G, Corridan B, Rudge S. Incidence and outcome of schizophrenia in whites, African-Caribbeans and Asians in London. Psychol Med 1997;27:791-8. http://dx.doi.org/10.1017/S0033291797005369.
- Bhui K, Stansfeld S, Hull S, Priebe S, Mole F, Feder G. Ethnic variations in pathways to and use of specialist mental health services in the UK. Br J Psychiatry 2003;182:105-16. http://dx.doi.org/10.1192/bjp.182.2.105.
- Count Me In 2010. The National Mental Health and Learning Disability Ethnicity Census. London: Department of Health; 2010.
- Schizophrenia: Core Interventions in the Treatment and Management of Schizophrenia in Adults in Primary and Secondary Care. Leicester and London: The British Psychological Society and the Royal College of Psychiatrists; 2010.
- Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al. A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression. Health Technol Assess 2001;5.
- Farahati F, Marcotte DE, Wilcox-Gök V. The effects of parents’ psychiatric disorders on children’s high school dropout. Economics of Education Review 2003;22:167-78.
- Compas BE, Forehand R, Thigpen JC, Keller G, Hardcastle EJ, Cole DA, et al. Family group cognitive–behavioral preventive intervention for families of depressed parents: 18-and 24-month outcomes. J Consult Clin Psychol 2011;79:488-99. http://dx.doi.org/10.1037/a0024254.
- The Impact of Sure Start Local Programmes on Five Year Olds and Their Families. London: Department of Families and Education; 2010.
- Belsky J, Leyland A, Barnes J, Melhuish E. Sure start in England. Lancet 2009;373. http://dx.doi.org/10.1016/S0140-6736(09)60132-X.
- Department of Health . NHS Commisioning Board n.d. www.commissioningboard.nhs.uk/ (accessed 10 June 2012).
- A Framework for the Development and Evaluation of RCTs for Complex Interventions to Improve Health. London: Medical Research Council; 2000.
- Development and Evaluating Complex Interventions: New Guidance. London: Research Council; 2008.
- Pawlby S, Marks M, Clarke R, Best E, Weir D, O’Keane V. Mother–infant interaction in postpartum women with severe mental illness, before and after treatment. Arch Womens Ment Health 2005;8.
- Beardslee WR, Wright E, Rothberg PC, Salt P, Versage E. Response of families to two preventive intervention strategies: long-term differences in behavior and attitude change. J Am Acad Child Adolesc Psychiatry 1996;35:774-82. http://dx.doi.org/10.1097/00004583-199606000-00017.
- Beardslee WR, Gladstone TRG, Wright EJ, Cooper AB. A family-based approach to the prevention of depressive symptoms in children at risk: evidence of parental and child change. Pediatrics 2003;112:e119-31. http://dx.doi.org/10.1542/peds.112.2.e119.
- Cicchetti D, Rogosch FA, Toth SL. The efficacy of toddler–parent psychotherapy for fostering cognitive development in offspring of depressed mothers. J Abnorm Child Psychol 2000;28:135-48.
- Toth SL, Rogosch FA, Manly JT, Cicchetti D. The efficacy of toddler–parent psychotherapy to reorganize attachment in the young offspring of mothers with major depressive disorder: a randomized preventive trial. J Consult Clin Psychol 2006;74:1006-16. http://dx.doi.org/10.1037/0022-006X.74.6.1006.
- Pagnini D. Longitudinal Follow-up Report. Sydney: Carers NSW, Inc.; 2007.
- Pagnini D. Carers NSW Carers Mental Health Project Stage One Final Evaluation Report. Sydney: Carers NSW, Inc.; 2004.
- Pagnini D. Carers Mental Health Project Stage 2: Action Evaluation Report, July 2004–January 2005. Sydney: Carers NSW, Inc.; 2005.
- Austin MP, Dudley M, Launders C, Dixon C, Macartney-Bourne F. Description and evaluation of a domiciliary perinatal mental health service focussing on early intervention. Arch Womens Ment Health 1999;2:169-73. http://dx.doi.org/10.1007/s007370050045.
- Arkansas Center for Addictions Research, Education and Services (Arkansas CARES) . Integrated services for mothers with dual diagnoses and their children. Psychiatric Serv 2002;53:1311-13.
- Brunette MF, Richardson F, White L, Bemis G, Eelkema RE. Integrated family treatment for parents with severe psychiatric disabilities. Psychiatric Rehabil J 2004;28:177-80. http://dx.doi.org/10.2975/28.2004.177.180.
- Coster D, Shaw C. Evaluation of the CAPE Project. London: National Children’s Bureau; 2008.
- The Emerson-Davis Family Development Center . Supportive residential services to reunite homeless mentally ill single parents with their children: The Emerson-Davis Family Development Center in Brooklyn, New York City. Psychiatric Serv 2000;51:1433-5.
- Free K, Alechina J, Zahn-Waxler C. Affective language between depressed mothers and their children: the potential impact of psychotherapy. J Am Acad Child Adolesc Psychiatry 1996;35:783-90.
- Gladstone BMC. ‘All in the Same Boat’: An Analysis of a Support Group for Children of Parents With Mental Illnesses 2010.
- Hamill K. Children’s Perceptions Of A Psycho-Educational Program About Parental Mental Illness. 2008.
- Hargreaves J, Bond L, Brien M, Forer D, Davies L. The PATS peer support program?: prevention/early intervention for adolescents who have a parent with mental illness. Youth Studies Australia 2008;27:43-51.
- Hayman FM. Kids with confidence: a program for adolescents living in families affected by mental illness. Aust J Rural Health 2009;17:268-72. http://dx.doi.org/10.1111/j.1440-1584.2009.01090.x.
- Kahana B, Anthony EJ. Combining research and service – multidisciplinary intervention program with children of psychotic parents. Am J Orthopsychiatr 1972;42:333-4.
- Kersting A, Fisch S, Arolt V. Outpatient psychotherapy for mothers – a new treatment. Arch Womens Ment Health 2003;6:65-9. http://dx.doi.org/10.1007/s00737-002-0157-z.
- Kersting A, Kuschel S, Reutemann M, Ohrmann P, Arolt V. Outpatient psychotherapy for mothers – first empirical results. Psychiatry 2003;66:335-45. http://dx.doi.org/10.1521/psyc.66.4.335.25442.
- Moukaddem S, Fitzgerald M, Barry M. Evaluation of a child and family centre. Child Psychol Psychiatry Rev 1998;3:161-8. http://dx.doi.org/10.1017/S1360641798001713.
- Nicholson J, Albert K, Gershenson B, Williams V, Biebel K. Family options for parents with mental illnesses: a developmental, mixed methods pilot study. Psychiatric Rehabil J 2009;33:106-14. http://dx.doi.org/10.2975/33.2.2009.106.114.
- Nielsen N. Evaluation of family therapy. Nordic J Psychiatry 2006;60:137-43. http://dx.doi.org/10.1080/08039480600583712.
- Orel NA, Groves PA, Shannon L. Positive connections: a programme for children who have a parent with a mental illness. Child Fam Social Work 2003;8:113-22. http://dx.doi.org/10.1046/j.1365-2206.2003.00273.x.
- Place M, Reynolds J, Cousins A, O’Neill S. Developing a resilience package for vulnerable children. Child Adolesc Ment Health 2002;7:162-7. http://dx.doi.org/10.1111/1475-3588.00029.
- Brownrigg A, Soulsby A, Place M. Helping vulnerable children to become more resilient. Int J Child Fam Welfare 2004;7:14-25.
- Solantaus T, Paavonen EJ, Toikka S, Punamäki RL. Preventive interventions in families with parental depression: children’s psychosocial symptoms and prosocial behaviour. Eur Child Adolesc Psychiatry 2010;19:883-92. http://dx.doi.org/10.1007/s00787-010-0135-3.
- Tamminen T, Kaukonen P. Family ward: a new therapeutic approach. Keio J Med 1999;48:132-9. http://dx.doi.org/10.2302/kjm.48.132.
- Tustin D. Adelaide: Australian Infant, Child, Adolescent and Family Mental Health Association Ltd; 2002.
- van der Zanden RAP, Speetjens PAM, Arntz KSE, Onrust SA. Online group course for parents with mental illness: development and pilot study. J Med Internet Res 2010;12. http://dx.doi.org/10.2196/jmir.1394.
- Wadsby M, Sydsjo G, Svedin CG. Evaluation of a short-term intervention program for psychosocial-risk mothers and their infants. Nordic J Psychiatry 1998;52:501-11.
- Wasylenki D, Gehrs M, Goering P, Toner B. A home-based program for the treatment of acute psychosis. Community Ment Health J 1997;33:151-62.
- Alder E, Truman J. Counselling for postnatal depression in the voluntary sector. Psychol Psychother 2002;75:207-20. http://dx.doi.org/10.1348/147608302169652.
- Ammerman RT, Putman FW, Chard KM, Stevens J, VanGinkel JB. PTSD in depressed mothers in home visitation. Psycholog Trauma Theory Res Pract Policy 2011;4:186-95. http://dx.doi.org/10.1037/a0023062.
- Armstrong K, Edwards H. The effects of exercise and social support on mothers reporting depressive symptoms: a pilot randomized controlled trial. Int J Ment Health Nurs 2003;12:130-8. http://dx.doi.org/10.1046/j.1440-0979.2003.00229.x.
- Armstrong K, Edwards H. The effectiveness of a pram-walking exercise programme in reducing depressive symptomatology for postnatal women. Int J Nurs Pract 2004;10:177-94. http://dx.doi.org/10.1111/j.1440-172X.2004.00478.x.
- Baggett KM, Davis B, Feil EG, Sheeber LL, Landry SH, Carta JJ, et al. Technologies for expanding the reach of evidence-based interventions: preliminary results for promoting social-emotional development in early childhood. Topics Early Child Spec Edu 2010;29:226-38. http://dx.doi.org/10.1177/0271121409354782.
- Barnes J, Senior R, MacPherson K. The utility of volunteer home-visiting support to prevent maternal depression in the first year of life. Child Care Health Dev 2009;35:807-16. http://dx.doi.org/10.1111/j.1365-2214.2009.01007.x.
- Buultjens M, Robinson P, Liamputtong P. A holistic programme for mothers with postnatal depression: pilot study. J Adv Nurs 2008;63:181-8. http://dx.doi.org/10.1111/j.1365-2648.2008.04692.x.
- Chazan-Cohen R, Ayoub C, Pan BA, Roggman L, Raikes H, McKelvey L, et al. It takes time: impacts of early head start that lead to reductions in maternal depression two years later. Infant Ment Health J 2007;28:151-70. http://dx.doi.org/10.1002/imhj.20127.
- Chien WT, Chan S, Morrissey J, Thompson D. Effectiveness of a mutual support group for families of patients with schizophrenia. J Adv Nurs 2005;51:595-608. http://dx.doi.org/10.1111/j.1365-2648.2005.03545.x.
- Cho HJ, Kwon JH, Lee JJ. Antenatal cognitive–behavioral therapy for prevention of postpartum depression: a pilot study. Yonsei Med J 2008;49:553-62. http://dx.doi.org/10.3349/ymj.2008.49.4.553.
- Clarke GN, Hornbrook M, Lynch F, Polen M, Gale J, Beardslee W, et al. A randomized trial of a group cognitive intervention for preventing depression in adolescent offspring of depressed parents. Arch Gen Psychiatry 2001;58:1127-34. http://dx.doi.org/10.1001/archpsyc.58.12.1127.
- Clarke GN, Hornbrook M, Lynch F, Polen M, Gale J, O’Connor E, et al. Group cognitive–behavioral treatment for depressed adolescent offspring of depressed parents in a health maintenance organization. J Am Acad Child Adolesc Psychiatry 2002;41:305-13. http://dx.doi.org/10.1097/00004583-200203000-00010.
- Clarke-Akalanne E, Myers P. Supporting new mothers with postnatal depression: an evaluation. J Comm Nurs 2002;16.
- Clulow C, Donaghy M. Developing the couple perspective in parenting support: evaluation of a service initiative for vulnerable families. J Fam Therapy 2010;32:142-68. http://dx.doi.org/10.1111/j.1467-6427.2010.00491.x.
- Cooper PJ, Tomlinson M, Swartz L, Landman M, Molteno C, Stein A, et al. Improving quality of mother–infant relationship and infant attachment in socioeconomically deprived community in South Africa: randomised controlled trial. BMJ 2009;14. http://dx.doi.org/10.1136/bmj.b974.
- Cowell JM, McNaughton D, Ailey S, Gross D, Fogg L. Clinical trial outcomes of the Mexican American Problem Solving Program (MAPS). Hispanic Health Care Int 2009;7:178-89. http://dx.doi.org/10.1891/1540-4153.7.4.178.
- Daley AJ, Winter H, Grimmett C, McGuinness M, McManus R, MacArthur C. Feasibility of an exercise intervention for women with postnatal depression: a pilot randomised controlled trial. Br J Gen Pract 2008;58:178-83. http://dx.doi.org/10.3399/bjgp08X277195.
- Field T, Diego M, Hernandez-Reif M, Deeds O, Figueiredo B. Pregnancy massage reduces prematurity, low birthweight and postpartum depression. Infant Behav Dev 2009;32:454-60. http://dx.doi.org/10.1016/j.infbeh.2009.07.001.
- Fisher JRW, Wynter KH, Rowe HJ. Innovative psycho-educational program to prevent common postpartum mental disorders in primiparous women: a before and after controlled study. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-432.
- Foster CE, Webster MC, Weissman MM, Pilowsky DJ, Wickramaratne PJ, Talati A, et al. Remission of maternal depression: relations to family functioning and youth internalizing and externalizing symptoms. J Clin Child Adolesc Psychol 2008;37:714-24. http://dx.doi.org/10.1080/15374410802359726.
- Garber J, Clarke GN, Weersing VR, Beardslee WR, Brent DA, Gladstone TR, et al. Prevention of depression in at-risk adolescents: a randomized controlled trial. JAMA 2009;301:2215-24. http://dx.doi.org/10.1001/jama.2009.788.
- Gjerdingen D, Crow S, McGovern P, Miner M, Center B. Stepped care treatment of postpartum depression: impact on treatment, health, and work outcomes. J Am Board Fam Med 2009;22:473-82. http://dx.doi.org/10.3122/jabfm.2009.05.080192.
- Goodman SH, Broth MR, Hall CM, Stowe ZN. Treatment of postpartum depression in mothers: secondary benefits to the infants. Infant Ment Health J 2008;29:492-513. http://dx.doi.org/10.1002/imhj.20188.
- Hayes L, Matthews J, Copley A, Welsh D. A randomized controlled trial of a mother–infant or toddler parenting program: demonstrating effectiveness in practice. J Pediatric Psychol 2008;33:473-86. http://dx.doi.org/10.1093/jpepsy/jsm085.
- Highet N, Drummond P. A comparative evaluation of community treatments for post-partum depression: implications for treatment and management practices. Aust NZ J Psychiatry 2004;38:212-18. http://dx.doi.org/10.1111/j.1440-1614.2004.01342.x.
- Ho LKL. The Treatment Effectiveness of Parent–Child Interaction Therapy With Depressed Mother-Child Dyads. Stockton, CA: University of the Pacific; 2005.
- Honey KL, Bennett P, Morgan M. A brief psycho-educational group intervention for postnatal depression. Br J Clin Psychol 2002;41:405-9. http://dx.doi.org/10.1348/014466502760387515.
- Kersten-Alvarez LE, Hosman CMH, Riksen-Walraven JM, van Doesum KTM, Hoefnagels C. Long-term effects of a home-visiting intervention for depressed mothers and their infants. J Child Psychol Psychiatry 2010;51:1160-70. http://dx.doi.org/10.1111/j.1469-7610.2010.02268.x.
- Khazan IZ. Expectations-Based Intervention as a Tool to Reduce Severity of Postpartum Depression and Improve Coparenting. Worcester, MA: Clark University; 2006.
- Kurzweil S. Relational-developmental therapy group for postnatal depression. Int J Group Psychother 2008;58:17-34. http://dx.doi.org/10.1521/ijgp.2008.58.1.17.
- Logsdon MC, Foltz MP, Stein B, Usui W, Josephson A. Adapting and testing telephone-based depression care management intervention for adolescent mothers. Arch Womens Ment Health 2010;13:307-17.
- Marks MN, Siddle K, Warwick C. Can we prevent postnatal depression? A randomized controlled trial to assess the effect of continuity of midwifery care on rates of postnatal depression in high-risk women. J Matern Fetal Neonatal Med 2003;13:119-27.
- McCarthy M, McMahon C. Acceptance and experience of treatment for postnatal depression in a community mental health setting. Health Care Women Int 2008;29:618-37. http://dx.doi.org/10.1080/07399330802089172.
- Milgrom J, Negri LM, Gemmill AW, McNeil M, Martin PR. A randomized controlled trial of psychological interventions for postnatal depression. Br J Clin Psychol 2005;44:529-42. http://dx.doi.org/10.1348/014466505X34200.
- Morrell CJ, Slade P, Warner R, Paley G, Dixon S, Walters SJ, et al. Clinical effectiveness of health visitor training in psychologically informed approaches for depression in postnatal women: pragmatic cluster randomised trial in primary care. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.a3045.
- Morris JB. Group psychotherapy for prolonged postnatal depression. Br J Med Psychol 1987;60:279-81. http://dx.doi.org/10.1111/j.2044-8341.1987.tb02742.x.
- Morse C, Durkin S, Buist A, Milgrom J. Improving the postnatal outcomes of new mothers. J Advanc Nurs 2004;45:465-74. http://dx.doi.org/10.1046/j.1365-2648.2003.02927.x.
- Munoz RF, Le HN, Ippen CG, Diaz MA, Urizar GGJ, Soto J, et al. Prevention of postpartum depression in low-income women: development of the mamas y bebes/mothers and babies course. Cogn Behav Pract 2007;14:70-83.
- Nicol AR, Stretch DD, Davison I, Fundudis T. Controlled comparison of three interventions for mother and toddler problems: preliminary communication. J Royal Soc Med 1984;77:488-91.
- Okano T, Nagata S, Hasegawa M, Nomura J, Kumar R. Effectiveness of antenatal education about postnatal depression: a comparison of two groups of Japanese mothers. J Ment Health 1998;7:191-8.
- Orhon FS, Soykan A, Ulukol B. Patient compliance to psychiatric interventions and course of postpartum mood disorders. Int J Psychiatry Med 2007;37:445-57. http://dx.doi.org/10.2190/PM.37.4.g.
- Paris R, Bolton RE, Spielman E. Evaluating a home-based dyadic intervention: changes in postpartum depression, maternal perceptions, and mother–infant interactions. Infant Ment Health J 2011;32:319-38. http://dx.doi.org/10.1002/imhj.20299.
- Parry BL, Curran ML, Stuenkel CA, Yokimozo M, Tam L, Powell KA, et al. Can critically timed sleep deprivation be useful in pregnancy and postpartum depressions?. J Affect Disord 2000;60:201-12. http://dx.doi.org/10.1016/S0165-0327(99)00179-2.
- Phillips J, Sharpe L, Nemeth D. Maternal psychopathology and outcomes of a residential mother–infant intervention for unsettled infant behaviour. Aust NZ J Psychiatry 2010;44:280-9. http://dx.doi.org/10.3109/00048670903487225.
- Pihkala H, Johansson EE. Longing and fearing for dialogue with children – depressed parents’ way into Beardslee’s preventive family intervention. Nordic J Psychiatry 2008;62:399-404. http://dx.doi.org/10.1080/08039480801984800.
- Prendergast J, Austin MP. Early childhood nurse-delivered cognitive behavioural counselling for post-natal depression. Australas Psychiatry 2001;9:255-9. http://dx.doi.org/10.1046/j.1440-1665.2001.00330.x.
- Puckering C, Rogers J, Mills M, Cox A, Raff MMG. Process and evaluation of a group intervention for mothers with parenting difficulties. Child Abuse Rev 1994;3:299-310. http://dx.doi.org/10.1002/car.2380030409.
- Puckering C, McIntosh E, Hickey A, Longford J. Mellow babies: a group intervention for infants and mothers experiencing postnatal depression 2010;25:28-40.
- Riley AW, Valdez CR, Barrueco S, Mills C, Beardslee W, Sandler I, et al. Development of a family-based program to reduce risk and promote resilience among families affected by maternal depression: theoretical basis and program description. Clin Child Fam Psychol Rev 2008;11:12-29. http://dx.doi.org/10.1007/s10567-008-0030-3.
- Selkirk R, McLaren S, Ollerenshaw A, McLachlan AJ, Moten J. The longitudinal effects of midwife-led postnatal debriefing on the psychological health of mothers. J Reprod Infant Psychol 2006;24:133-47. http://dx.doi.org/10.1080/02646830600643916.
- Sheeber LB, Seeley JR, Feil EG, Davis B, Sorensen ED, Lewinsohn PM. Mom-net: development and pilot evaluation of an internet-facilitated intervention for maternal depression. J Consult Clin Psychol 2012;80:739-49.
- Slade A, Sadler L, De Dios-Kenn C, Webb D, Currier-Ezepchick J, Mayes L. Minding the baby: a reflective parenting program. Psychoanalytic Stud Child 2005;60:74-100.
- Spinelli MG. Interpersonal psychotherapy for depressed antepartum women: a pilot study. Am J Psychiatry 1997;154:1028-30.
- Spinelli MG, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry 2003;160:555-62. http://dx.doi.org/10.1176/appi.ajp.160.3.555.
- Stavros HJ. Reducing the Effects of Maternal Depression on the Family: a Study of the Effectiveness of a Short-Term Preventive Intervention Program. New Orleans, LA: Tulane University; 2002.
- Steinberg SI, Bellavance F. Characteristics and treatment of women with antenatal and postpartum depression. Int J Psychiatry Med 1999;29:209-33.
- Tamaki A. Effectiveness of home visits by mental health nurses for Japanese women with post-partum depression. Int J Ment Health Nurs 2008;17:419-27. http://dx.doi.org/10.1111/j.1447-0349.2008.00568.x.
- Tezel A, Gozum S. Comparison of effects of nursing care to problem solving training on levels of depressive symptoms in post partum women. Patient Educ Couns 2006;63:64-73. http://dx.doi.org/10.1016/j.pec.2005.08.011.
- Tischier V. A family support service for homeless children and parents?: users’ perspectives and characteristics. Health Soc Care Comm 2004;12:327-35.
- Turner KM, Chew-Graham C, Folkes L, Sharp D. Women’s experiences of health visitor delivered listening visits as a treatment for postnatal depression: a qualitative study. Patient Educ Couns 2010;78:234-9. http://dx.doi.org/10.1016/j.pec.2009.05.022.
- Ugarriza DN, Schmidt L. Telecare for women with postpartum depression. J Psychosoc Nurs Ment Health Serv 2006;44:37-4.
- Valdez CR, Mills CL, Barrueco S, Leis J, Riley AW. A pilot study of a family-focused intervention for children and families affected by maternal depression. J Fam Ther 2011;33:3-19. http://dx.doi.org/10.1111/j.1467-6427.2010.00529.x.
- van-Doesum KT, Riksen-Walraven JM, Hosman CM, Hoefnagels C. A randomized controlled trial of a home-visiting intervention aimed at preventing relationship problems in depressed mothers and their infants. Child Dev 2008;79:547-61. http://dx.doi.org/10.1111/j.1467-8624.2008.01142.x.
- Vorhies V, Glover CM, Davis K, Hardin T, Krzyzanowski A, Harris M, et al. Improving outcomes for pregnant and parenting foster care youth with severe mental illness: an evaluation of a transitional living program. Psychiatric Rehabil J 2009;33:115-24. http://dx.doi.org/10.2975/33.2.2009.115.124.
- Wan MW, Sharp DJ, Howard LM, Abel KM. Attitudes and adjustment to the parental role in mothers following treatment for postnatal depression. J Affect Disord 2011;131:284-92. http://dx.doi.org/10.1016/j.jad.2011.01.009.
- Wood A, Middleton SG, Leonard D. ‘When It’s More Than the Blues’: a collaborative response to postpartum depression. Public Health Nurs 2010;27:248-54. http://dx.doi.org/10.1111/j.1525-1446.2010.00850.x.
- Zlotnick C, Miller IW, Pearlstein T, Howard M, Sweeney P. A preventive intervention for pregnant women on public assistance at risk for postpartum depression. Am J Psychiatry 2006;163:1443-5. http://dx.doi.org/10.1176/appi.ajp.163.8.1443.
- Andrews G, Sanderson K, Corry J, Issakidis C, Lapsley H. Cost-effectiveness of current and optimal treatment for schizophrenia. Br J Psychiatry 2003;183:427-35. http://dx.doi.org/10.1192/bjp.183.5.427.
- Andrews G. It would be cost-effective to treat more people with mental disorders. Aust NZ J Psychiatry 2006;40:613-15. http://dx.doi.org/10.1111/j.1440-1614.2006.01859.x.
- Appleby L, Hirst E, Marshall S, Keeling F, Brind J, Butterworth T, et al. The treatment of postnatal depression by health visitors: impact of brief training on skills and clinical practice. J Affect Disord 2003;77:261-6. http://dx.doi.org/10.1016/S0165-0327(02)00145-3.
- Breitborde N, Woods S, Srihari V. Multifamily psychoeducation for first-episode psychosis: a cost-effectiveness analysis. Psychiatric Serv 2009;60:1477-83. http://dx.doi.org/10.1176/appi.ps.60.11.1477.
- Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L. Evidence-based, cost-effective interventions: how many newborn babies can we save?. Lancet 2005;365:977-88. http://dx.doi.org/10.1016/S0140-6736(05)71088-6.
- Darmstadt GL, Walker N, Lawn JE, Bhutta ZA, Haws RA, Cousens S. Saving newborn lives in Asia and Africa: cost and impact of phased scale-up of interventions within the continuum of care. Health Policy Plan 2008;23:101-17. http://dx.doi.org/10.1093/heapol/czn001.
- Dixon L, Lyles A, Scott J, Lehman A, Postrado L, Goldman H, et al. Services to families of adults with schizophrenia: from treatment recommendations to dissemination. Psychiatric Serv 1999;50:233-8.
- Fuggle P, Haydon K. Estimating resources for setting up an HV service for postnatal depression. Br J Comm Nurs 2000;5:348-51.
- Haddock G, Barrowclough C, Tarrier N, Moring J, O’Brien R, Schofield N, et al. Cognitive–behavioural therapy and motivational intervention for schizophrenia and substance misuse 18-month outcomes of a randomised controlled trial. Br J Psychiatry 2003;183:418-26. http://dx.doi.org/10.1192/bjp.183.5.418.
- Higgins P, Murray ML. Perinatal outcomes and level of prenatal care: a study of the Southwestern United States. J Perinatal Education 1996;5:37-46.
- Lieu TA, Wikler C, Capra AM, Martin KE, Escobar GJ, Braveman PA. Clinical outcomes and maternal perceptions of an updated model of perinatal care. Pediatrics 1998;102:1437-44. http://dx.doi.org/10.1542/peds.102.6.1437.
- Lin HC, Lin YJ, Hsiao FH, Li CY. Prenatal care visits and associated costs for treatment-seeking women with depressive disorders. Psychiatric Serv 2009;60:1261-4. http://dx.doi.org/10.1176/appi.ps.60.9.1261.
- Mihalopoulos C, Magnus A, Carter R, Vos T. Assessing cost-effectiveness in mental health: family interventions for schizophrenia and related conditions. Aust NZ 2004;38:511-19. http://dx.doi.org/10.1111/j.1440-1614.2004.01404.x.
- Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A. Costs and effectiveness of community postnatal support workers: randomised controlled trial. BMJ 2000;321:593-8. http://dx.doi.org/10.1136/bmj.321.7261.593.
- Petrou S, Cooper P, Murray L, Davidson LL. Economic costs of post-natal depression in a high-risk British cohort. Br 2002;181:505-12. http://dx.doi.org/10.1192/bjp.181.6.505.
- Petrou S, Cooper P, Murray L, Davidson LL. Cost-effectiveness of a preventive counseling and support package for postnatal depression. Int J Technol Assess Health Care 2006;22:443-53. http://dx.doi.org/10.1017/S0266462306051361.
- Stevenson MD, Sutcliffe PA. The cost-effectiveness of group cognitive behavioral therapy compared with routine primary care for women with postnatal depression in the UK. Value Health 2010;13:580-4. http://dx.doi.org/10.1111/j.1524-4733.2010.00720.x.
- The Campbell and Cochrane Economics Methods Group (CCEMG) and the Evidence for Policy and Practice Information and Coordinating Centre (EPPI-Centre) . CCEMG – EPPI-Centre Cost Converter n.d. http://eppi.ioe.ac.uk/costconversion/default.aspx (accessed 19 November 2013).
Appendix 1 Initial themes from stakeholder consultation
| Metatheme | Child participants | Parent participants | Professional participants |
|---|---|---|---|
| Child mental health | High anxiety regarding parents’ health and well-being. Daily stressors related to lack of resources, family conflict. Children engaged with primary care and in receipt of anti-depressant medication | Concern that illness leads to common mental health problems in children. Concern that SMI will be inherited. Desire for children to be emotionally healthy and resilient to SMI | Children fear they may develop a mental health problem. Children frequently experience anxiety regarding parents’ and/or own health |
| Child social functioning/behaviour | Could feel isolated because of differences between themselves and their peer group | Concern that their illness had caused behavioural or cognitive problems in the child | Social withdrawal and distancing in children possible. Erratic or poor behaviour may develop |
| Social relationship quality | Friends important for ‘normal’ interaction/distraction | Concern that children did not bring friends home. Friends important for ‘normal’ development and emotional health | Children may be protected by the availability of social support. Presence of a supportive adult was important |
| Opportunities for recreational engagement | Used hobbies and social activities as a mechanism for reducing stress. Independently getting out of the house was often important. Friends and time to socialise was important to well-being | Concern they did not take their child to recreational activities. Children’s need for independence was recognised but it can be hard to allow this. Support from wider family helped | Children may be protected by the availability of social support. Presence of a supportive adult was important |
| Child self-esteem | Children express a need for autonomy | Children need to have a strong character to cope. Encouragement of hobbies and interests to facilitate children’s self-esteem | Need to build children’s confidence, aspirations and inner strengths |
| Child coping/problem solving | Children want practical solutions to help | Want child to develop practical and effective skills to be mentally healthy in the future | A need to services to foster empowerment, resilience and advocacy in children |
| Child mental health literacy | Confusion and disorientation about parents’ behaviour adds to stressors. Information about a parent’s condition and care very important. Lack of information available | Found it useful to be able to explain mental illness in an age-appropriate manner | Children often want knowledge about mental health problems and services to retain control |
| Family functioning and conflict | Were concerned for the well-being/stability of the family unit. Enjoyed spending quality time with their families | Had experienced conflict with family members | Important for services to understand the needs of the whole family as well as the needs of each individual |
| Parental mental health symptoms | Unpredictability of a parent’s responses difficult to manage. Concern regarding parents’ safety, well-being and future life | Hospitalisation had led to unwanted separation from the child | Child’s basic needs may not always be met. Deficits exacerbated in times of crisis. Child may have responsibility in terms of looking after themselves, siblings or parent |
| Quality of parent-child interaction | Strong desire for warmth and responsiveness from parents. May feel like a target for their parents’ hostile behaviour | Awareness that their behaviour can be erratic, particularly with regard to boundaries and anger. Guilt over perceived inadequate parenting and lack of quality time together | Inconsistency of parental behaviour may cause the child to feel responsible. Parents may be emotionally unavailable |
| Economic resources | Wanted more money to alleviate everyday financial pressures and to enable the family to go on outings. Financial hardship led to lack of food, hunger. Found that low family income differentiated them from their peers | Perceived a lack of material possessions owing to reduced income. Experienced a lack of or erratic provision of activities for the child owing to financial hardship | Financial stability important for the whole family |
Appendix 2 Study protocol
1. Background
Improving the lives of children born to parents with serious mental illness (SMI) is an urgent political and public health concern. A substantial proportion of children and adolescents experience SMI in family members. Approximately 50% of adults seen in mental health services are parents with dependent children,8 with variation depending upon the country, clinical setting and population studied. 52 The burden placed on these young people is substantial. Research shows that serious parental mental illness is associated with increased risk of adverse outcomes in children. Short-term outcomes include poorer psychological and physical ill health, behavioural and developmental difficulties, educational underachievement and lower social competency. 23,25–27 Longer-term outcomes extend into adulthood and may include social or occupational dysfunction, lower self-esteem, increased psychiatric morbidity and alcohol or substance misuse. 38–40
2. Review Objectives
The aim of this project is to apply rigorous evidence synthesis techniques to evaluate current evidence of the effectiveness and cost-effectiveness of community-based intervention in maintaining or improving quality of life children of parents with Serious Mental Illness (SMI). The objectives of the work are to:
-
Provide a descriptive overview of all the evidence for community-based interventions for improving quality of life in children and adolescents of parents with serious mental illness, with specific reference to intervention format and content, participant characteristics, costs and QoL outcomes measured.
-
Examine the clinical effectiveness of community-based interventions in terms of their impact on a range of outcomes, particularly those likely to be associated with quality of life for children and adolescents of parents with serious mental illness.
-
Examine, wherever possible, potential associations between intervention effect and delivery including intervention format and content, prioritisation of child outcomes, child age group, parental mental health condition, family structure and residency.
-
Examine the acceptability of community-based interventions intended to improve quality of life for children and adolescents of parents with serious mental illness in terms of uptake, adherence and patient satisfaction.
-
Estimate the cost-effectiveness of community-based interventions in improving quality of life for children and adolescents of parents with serious mental illness.
-
Assess key factors influencing the acceptability of and barriers to the delivery and implementation of community-based interventions for improving quality of life in children and adolescents of parents with serious mental illness.
-
Identify, from the perspective of the UK NHS and personal social services, research priorities and the potential value of future research into interventions for improved quality of life in this population.
3. Criteria for considering studies for this review
-
Population: Children or adolescents aged 0-17 years with a parent (or adult assuming a parental role) with a serious mental health condition. In accordance with the user perspective,104 serious mental health conditions will include clinically diagnosed (ICD-10 or DSM-IV) schizophrenia, psychosis, severe affective mood disorders, borderline personality disorder and personality disorder, with or without substance misuse co-morbidity. Severe postnatal depression and puerperal psychosis will be included.
-
Interventions: Any community-based, health, social care or educational intervention (e.g. psychoeducation, parental skills programs, dyadic therapies, individual, group or family-based therapies) aimed at the young person, their parent or family unit.
-
Comparators: All controlled studies meeting eligibility criteria, irrespective of their control condition. Comparisons of two or more active interventions or of an active treatment with a ‘no treatment’ comparator will be included.
-
Outcomes (Effectiveness): Primary outcomes will comprise validated generic (e.g.SF-36) or population-specific quality of life measures (e.g. PQ-LES-Q, PANOC-YC20, KIDSCREEN), and/or child-centred mental health symptoms (e.g. anxiety, depression). Secondary outcomes will comprise additional QoL indicators relevant to the UK’s Every Child Matters agenda86 and defined by stakeholder consultation, early QoL mediators (e.g. parenting skills) and parental mental health symptoms.
-
Outcomes (Acceptability): For the purposes of this review, acceptability will be defined and sub-divided into intervention uptake, adherence and participant satisfaction.
-
Design (Clinical Effectiveness): Priority will be given to those designs in which a comparator or control group is present, i.e. randomised controlled trials, quasi-experimental controlled studies and controlled observational studies (cohort studies and case control studies). Other designs will only be included where no other evidence exists to address the review objectives and will be summarized for the purposes of informing future research priority-setting.
-
Design (Acceptability): Acceptability will be assessed via studies that (1) ask participants and parents for their views using qualitative or quantitative methods; and/or (2) quantitatively record non-participation, withdrawal and adherence rates in studies examining effectiveness. Qualitative research will be defined as those studies that collect data using specific qualitative techniques such as unstructured interviews, semi-structured interviews or focus groups, either as a stand-alone methodology or as discrete part of a larger mixed-method study.
-
Design (Cost-Effectiveness): Synthesis of the cost-effectiveness evidence will, prioritise randomised controlled trials, quasi-experimental controlled studies and controlled observational studies (cohort studies and case control studies). Other designs (i.e. uncontrolled costing studies) may be included for the purpose of populating the decision model.
Exclusion criteria will include case studies, opinion papers, descriptive studies, editorials, non-English-language publications, evaluations of physical or pharmacological interventions without any psychosocial component.
4. Search strategy
4.1 Electronic Searches
An extensive search will be undertaken for qualitative and quantitative literature. To identify evidence relevant to the clinical effectiveness and/or acceptability of interventions, searches will be undertaken on the following electronic databases:
-
Health and allied health literature: MEDLINE, CINAHL PSYCInfo, EMBASE, HSRProj, HMIC
-
Social work: ASSIA, CSA, SCOPUS, IBSS, Social Services Abstracts Social Work Abstracts, Social Care Online, Childata, CommunityWISE
-
Education: ERIC AUEI, BRIE
-
Other evidence-based research: CENTRAL, DARE, CDSR ISI WoS including SSCI, AHCI, SCIEXPANDED, ReFeR, metaRegister of Controlled Trials, National Criminal Justice Reference Service Abstracts. The publically available SCIE database developed for the SCIE ‘scoping exercise’ into interventions to support parents with SMI and their families will also be searched. 58
Economic evidence (resource use, cost and cost-effectiveness data) will be located through the clinical effectiveness and acceptability search strategy described above, plus additional relevant economic databases: the NHS Economic Evaluation database (NHS EED), the Paediatric Economic Evaluation database (PEDE), the Health Economic Evaluations database (HEED), the American Economic Association’s electronic bibliography (EconLit) and the IDEAS economics database.
4.2 Searching other resources
-
Material generated by user-led or voluntary sector enquiry will be identified via electronic databases for grey literature, internet search engines and websites for relevant UK government departments and charities, specifically British National Bibliography for Report Literature, GOOGLE Scholar, Mental Health Foundation, Barnardos, Carers UK, Childline, Children’s Society, Depression Alliance, MIND, AnxietyUK, NSPCC, Princess Royal Trust for Carers, SANE, The Site, Turning Point, Young Minds.
-
Additional studies will be identified by scanning the bibliographies of recent reviews and newly retrieved articles, by brief targeted author searches and forward citation searching and via requests to members of the review’s advisory panel.
-
Authors of ongoing and recently completed research projects will be contacted directly to enquire whether or not the research has been completed and if there are any subsequent publications (e.g. SCIE parental mental health and child welfare network).
4.3 Search Limits
All databases will be searched from their inception to the date of the search. Searches will be limited to English-language publications. No other geographical restrictions will be applied. The relevance of international literature and initiatives to the UK health system will be considered against the stated inclusion criteria, in conjunction with stakeholder consultation regarding the acceptability and feasibility of future service implementation.
5. Review Strategy
5.1 Study Selection
All potentially eligible records will be imported into a bibliographic referencing software program (Endnote version 9) and duplicate references identified and deleted. Two reviewers will independently screen titles and abstracts for relevance, using the inclusion criteria set out above. A measure of inter-rater reliability will be obtained. Where both reviewers agree on exclusions, titles and abstracts will be discarded and the reasons for exclusion will be recorded. Where both reviewers agree on inclusion, or where there is disagreement, the full text article will be retrieved. Two reviewers will independently assess the full text against the inclusion criteria, with any remaining disagreements being resolved through discussion with the project team.
5.2 Data Management & Extraction
Where there is insufficient information to assess eligibility or extract the relevant data, study authors will be contacted directly. Data extraction and validity assessment will be performed by one reviewer and independently verified by a second. Discrepancies will be resolved by referral to the original studies and if necessary through arbitration by a third reviewer. Data extraction will be guided by a pre-specified data extraction sheet detailing key features of the study sample, setting, methods, intervention, control if appropriate), results and conclusions.
5.3 Quality assessment
Studies will be assessed for methodological quality across all phases of the review. Evidence of clinical effectiveness will be assessed for quality according to the Cochrane Collaboration Risk of Bias Assessment Tool for randomised controlled trials,82 and/or Cochrane guidance for non-randomised designs. 82 Economic studies will be assessed using a standard critical appraisal checklist for economic evaluations. Qualitative acceptability evidence will be assessed for quality against the CASP tool for qualitative research105 and the principles of good practice for conducting social research with children. 106 All eligible studies will be assessed for quality although no study will be excluded from the acceptability study on the grounds of evidence strength. The relative impact of methodological flaws on the findings will be summarised narratively and, where data allow, investigated using a sensitivity analysis.
6. Data Synthesis
6.1 Clinical Effectiveness
Where the availability of evidence allows, data will be pooled. Potential associations between treatment effectiveness, target (parental, individual, parent–child dyad, family–based), content (e.g. psychoeducational, psychotherapeutic perspectives), and user characteristics, including child age group (< 5, 5–11, 12–17 years); parental mental health condition and residency (co-located, forced or volitional separation, transient separation at times of crisis) will be explored. In the event that study heterogeneity makes the pooling of data difficult, a narrative synthesis of effectiveness will be undertaken. Publication bias and small study effects will be investigated where possible. If insufficient data are available to assess these potential biases using standard methods (e.g. funnel plots), the likelihood of publication bias will be summarized narratively.
6.2 Acceptability
A parallel synthesis of acceptability data will be undertaken according to recommended methods shown to be successful in the synthesis of qualitative and mixed-method evidence. 113,114 A narrative synthesis approach115 will be adopted in which studies will be grouped together into theoretically important subgroups; prior to conducting a subgroup synthesis. The structure of this narrative will be informed and framed by i) previous work in the subject area, ii) content and qualitative research expertise available within the research team and iii) and consultation with a stakeholder advisory panel.
6.3 Cost-effectiveness
A decision-analytic model will be constructed to compare the expected cost-effectiveness of identified intervention programmes. The model will be populated by data from the systematic review. Where gaps in the literature exist, consultation with experts will be undertaken. Decision analyses will be carried out using TreeAge Pro software. It is anticipated that a simple decision tree structure will be sufficient for the proposed work, although Markov modeling will be considered if a more sophisticated approach is deemed appropriate. Uncertainty will be characterized by assigning distributions to each model input and applying Monte Carlo simulation techniques. Probabilistic sensitivity analysis will be used to explore the impact of uncertainty on key model parameters. If the format of the cost-effectiveness analysis is appropriate, then we will calculate VOI to assess the value in eliminating uncertainty in all parameters (EVPI). If the format of the evidence structure and cost-effectiveness analysis allows, we will also assess the value of eliminating uncertainty in subsets of parameters (EVPPI).
The primary cost perspective of the analysis will focus on health and personal social services. Additional perspectives (e.g. education, the young person and their family) will be explored if data are available. Dependent on data availability, outcomes will focus on the primary clinical effectiveness measures (validated generic or population-specific quality of life measures and/or child-centred mental health symptoms).
Appendix 3 Search strategies and dates
MEDLINE
Searched 15 November 2010.
Updated 30 May 2012.
Search terms
-
exp Mental Disorders/
-
((mental$ or psychiatric or psychologic$) adj3 (illness$ or condition$ or disabil$ or disorder$ or disease$ or impair$ or problem$)).tw.
-
(nervous breakdown$ or depression or depressive$ or affective disorder$ or mood disorder$ or anxiety disorder$ or phobia or phobic or panic disorder$ or PTSD or stress disorder$ or OCD or compulsive disorder$).tw.
-
seasonal affective disorder$.tw.
-
(bipolar or mania or dissociative disorder$ or neurosis or personality disorder$ or psychosis or paranoi$ or schizophren$ or schizoaffective disorder$ or psychotic).tw.
-
2 or 3 or 4 or 5
-
adolescent/ or exp child/ or infant/
-
(child$ or adolescen$ or teen$ or juvenile$ or boy$ or girl$ or baby or babies or infant$ or toddler$ or preschool$ or pre-school$ or schoolchild$ or youth$ or family or families or young person$ or young people).tw.
-
Family/
-
7 or 8 or 9
-
exp Parents/
-
(parent$ or mother$ or father$ or step-parent$ or step-father$ or step-mother$ or maternal or paternal or (primary adj3 carer)).tw.
-
11 or 12
-
((severe postpartum or severe post-partum or severe post partum or severe postnatal$ or severe post-natal$ or severe post natal$) adj (depress$)).tw.
-
((postpartum or post-partum or post partum or postnatal$ or post-natal$ or post natal) adj (psycho$)).tw.
-
((postpartum or post-partum or post partum or postnatal$ or post-natal$ or post natal) adj4 (major depress$)).tw.
-
(puerperal$ adj psycho$).tw.
-
6 or 14 or 15 or 16 or 17
-
((parent$ or mother$ or father$ or step-parent$ or step-father$ or step-mother$ or maternal or paternal or (primary adj3 carer)) adj5 (((mental$ or psychiatric or psychologic$) adj3 (illness$ or condition$ or disabil$ or disorder$ or disease$ or impair$ or problem$)) or (nervous breakdown$ or depression or depressive$ or affective disorder$ or mood disorder$ or anxiety disorder$ or phobia or phobic or panic disorder$ or PTSD or stress disorder$ or OCD or compulsive disorder$) or seasonal affective disorder$ or (bipolar or mania or dissociative disorder$ or neurosis or personality disorder$ or psychosis or paranoi$ or schizophren$ or schizoaffective disorder$ or psychotic) or ((severe postpartum or severe post-partum or severe post partum or severe postnatal$ or severe post-natal$ or severe post natal$) adj (depress$)) or ((postpartum or post-partum or post partum or postnatal$ or post-natal$ or post natal) adj (psycho$)) or (puerperal$ adj psycho$) or ((postpartum or post-partum or post partum or postnatal$ or post-natal$ or post natal) adj4 (major depress$)))).tw.
-
1 and 11
-
19 or 20
-
10 and 21
-
(therapy or therapies or program$ or intervention$ or service$ or training).tw.
-
22 and 23
Cumulative Index to Nursing and Allied Health Literature
Searched 18 January 2011.
Search terms
S50 S44 and S47 and S48 and S49
Search modes: Boolean/phrase
Interface: EBSCOhost
S49 therapy or therapies of program* or intervention* or service* or training
Search modes: Boolean/phrase
Interface: EBSCOhost
S48 S26 or S47
Search modes: Boolean/phrase
Interface: EBSCOhost
S47 S37 or S45 or S46
Search modes: Boolean/phrase
Interface: EBSCOhost
S46 (puerperal* psycho*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S45 ((postpartum psycho*) or (post-partum psycho*) or (post partum psycho*)) or ((postnatal* psycho*) or (post-natal* psycho*) or (post natal* psycho*))
Search modes: Boolean/phrase
Interface: EBSCOhost
S44 S42 or S43
Search modes: Boolean/phrase
Interface: EBSCOhost
S43 (parent* or mother* or father* or step-parent* or step-father* or step-mother* or maternal or paternal or (primary carer))
Search modes: Boolean/phrase
Interface: EBSCOhost
S42 (MH "Parents+")
Search modes: Boolean/phrase
Interface: EBSCOhost
S41 S38 or S39 or S40
Search modes: Boolean/phrase
Interface: EBSCOhost
S40 (MH "Family")
Search modes: Boolean/phrase
Interface: EBSCOhost
S39 (child* or adolescen* or teen* or juvenile* or boy* or girl* or baby or babies or infant* or toddler* or preschool* or pre-school* or schoolchild* or youth* or family or families or young person* or young people)
Search modes: Boolean/phrase
Interface: EBSCOhost
S38 (MH "Adolescence") OR (MH "Infant") OR (MH "Child+")
Search modes: Boolean/phrase
Interface: EBSCOhost
S37 S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36
Search modes: Boolean/phrase
Interface: EBSCOhost
S36 (bipolar or mania or (dissociative disorder*) or neurosis or (personality disorder*) or psychosis or paranoi* or schizophren* or (schizoaffective disorder*) or psychotic)
Search modes: Boolean/phrase
Interface: EBSCOhost
S35 (seasonal affective disorder*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S34 (nervous breakdown* or depression or depressive* or affective disorder* or mood disorder* or anxiety disorder* or phobia or phobic or panic disoder* or PTSD or stress disorder* or OCD or compulsive disorder*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S33 (mental* problem*) or (psychiatric problem*) or (psychologic* problem*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S32 (mental* impair*) or (psychiatric impair*) or (psychologic* impair*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S31 (mental* disease*) or (psychiatric disease*) or (psychologic* disease*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S30 (mental* disorder*) or (psychiatric disorder*) or (psychologic* disorder*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S29 (mental* disabil*) or (psychiatric disabil*) or (psychologic* disabil*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S28 (mental* condition*) or (psychiatric condition*) or (psychologic* condition*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S27 (mental* illness*) or (psychiatric illness*) or (psychologic* illness*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S26 (MH "Mental Disorders+")
Search modes: Boolean/phrase
Interface: EBSCOhost
S25 S19 and S22 and S23 and S24
Search modes: Boolean/phrase
Interface: EBSCOhost
S24 therapy or therapies of program* or intervention* or service* or training
Search modes: Boolean/phrase
Interface: EBSCOhost
S23 S1 or S22
Search modes: Boolean/phrase
Interface: EBSCOhost
S22 S12 or S20 or S21
Search modes: Boolean/phrase
Interface: EBSCOhost
S21 (puerperal* psycho*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S20 ((postpartum psycho*) or (post-partum psycho*) or (post partum psycho*)) or ((postnatal* psycho*) or (post-natal* psycho*) or (post natal* psycho*))
Search modes: Boolean/phrase
Interface: EBSCOhost
S19 S17 or S18
Search modes: Boolean/phrase
Interface: EBSCOhost
S18 (parent* or mother* or father* or step-parent* or step-father* or step-mother* or maternal or paternal or (primary carer))
Search modes: Boolean/Phrase
Interface: EBSCOhost
S17 (MH "Parents+")
Search modes: Boolean/phrase
Interface: EBSCOhost
S16 S13 or S14 or S15
Search modes: Boolean/phrase
Interface: EBSCOhost
S15 (MH "Family")
Search modes: Boolean/phrase
Interface: EBSCOhost
S14 (child* or adolescen* or teen* or juvenile* or boy* or girl* or baby or babies or infant* or toddler* or preschool* or pre-school* or schoolchild* or youth* or family or families or young person* or young people)
Search modes: Boolean/phrase
Interface: EBSCOhost
S13 (MH "Adolescence") OR (MH "Infant") OR (MH "Child+")
Search modes: Boolean/phrase
Interface: EBSCOhost
S12 S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11
Search modes: Boolean/phrase
Interface: EBSCOhost
S11 (bipolar or mania or (dissociative disorder*) or neurosis or (personality disorder*) or psychosis or paranoi* or schizophren* or (schizoaffective disorder*) or psychotic)
Search modes: Boolean/phrase
Interface: EBSCOhost
S10 (seasonal affective disorder*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S9 (nervous breakdown* or depression or depressive* or affective disorder* or mood disorder* or anxiety disorder* or phobia or phobic or panic disoder* or PTSD or stress disorder* or OCD or compulsive disorder*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S8 (mental* problem*) or (psychiatric problem*) or (psychologic* problem*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S7 (mental* impair*) or (psychiatric impair*) or (psychologic* impair*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S6 (mental* disease*) or (psychiatric disease*) or (psychologic* disease*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S5 (mental* disorder*) or (psychiatric disorder*) or (psychologic* disorder*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S4 (mental* disabil*) or (psychiatric disabil*) or (psychologic* disabil*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S3 (mental* condition*) or (psychiatric condition*) or (psychologic* condition*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S2 (mental* illness*) or (psychiatric illness*) or (psychologic* illness*)
Search modes: Boolean/phrase
Interface: EBSCOhost
S1 (MH "Mental Disorders+")
Search modes: Boolean/phrase
Interface: EBSCOhost
The Cochrane Library databases
Searched 12 December 2010.
Search terms
#1 MeSH descriptor Mental Disorders explode all trees 35549 edit delete
#2 (mental* or psychiatric or psychologic*) near/3 (illness or condition* or disabil* or disorder* or disease* or impair* or problem*) 8626 edit delete
#3 ((nervous breakdown*) or depression or depressive* or (affective disorder*) or (mood disorder*) or (anxiety disorder*) or phobia or phobic or (panic disorder*) or PTSD or (stress disorder*) or OCD or (compulsive disorder*)) 34551 edit delete
#4 (seasonal affective disorder*) 265 edit delete
#5 (bipolar or mania or (dissociative disorder*) or neurosis or (personality disorder*) or psychosis or paranoi* or schizophren* or (schizoaffective disorder$) or psychotic) 18990 edit delete
#6 (#2 OR #3 OR #4 OR #5) 52472 edit delete
#7 MeSH descriptor Infant explode all trees 11386 edit delete
#8 MeSH descriptor Adolescent explode all trees 67473 edit delete
#9 MeSH descriptor Child explode all trees 13 edit delete
#10 MeSH descriptor Family, this term only 834 edit delete
#11 (child* or adolescen* or teen* or juvenile* or boy* or girl* or baby or babies or infant* or toddler* or preschool* or (pre next school*) or schoolchild* or youth* or family or families or (young person*) or (young people)) 143669 edit delete
#12 (#7 OR #8 OR #9 OR #10 OR #11) 143669 edit delete
#13 MeSH descriptor Parents explode all trees 1898 edit delete
#14 (parent* or mother* or father* or step-parent* or step-father* or step-mother* or maternal or paternal or (primary near/3 carer*)) 25781 edit delete
#15 puerperal* next psycho* 7 edit delete
#16 ((severe postpartum or (severe post next partum) or severe postnatal or (severe post next natal)) near/1 (depress*)) 428 edit delete
#17 ((postpartum or (post next partum) or postnatal or (post next natal)) near/1 (psycho*)) 81
#18 ((postpartum or post next partum or post partum or postnatal* or post next natal* or post natal) near/4 (major depress*))
#19 (#6 OR #15 OR #16 OR #17 OR #18) 52495 edit delete
#20 (#19 AND #14) 2386 edit delete
#21 (#1 AND #13) 513 edit delete
#22 (#20 OR #21) 2664 edit delete
#23 (therapy or therapies or program* or intervention* or service* or training) 447017 edit delete
#24 (#12 AND #22 AND #23) 2186 edit delete
Education Resources Information Centre, British Education Index and the Australian Education Index
Searched 18 January 2011.
Search terms
-
parent$ OR mother$ OR father$ OR step-parent$ OR step-father$ OR step-mother$ OR maternal OR paternal OR primary ADJ carer$
-
bipolar OR mania OR dissociative ADJ disorder$ OR neurosis OR personality ADJ disorder$ OR psychosis OR paranoi$ OR schizophren$ OR schizoaffective ADJ disorder$ OR psychotic OR seasonal ADJ affective ADJ disorder
-
nervous ADJ breakdown$ OR depression OR depressive$ OR affective ADJ disorder$ OR mood ADJ disorder$ OR anxiety ADJ disorder$ OR phobia OR phobic OR panic ADJ disorder$ OR PTSD OR stress ADJ disorder$ OR OCD OR compulsive ADJ disorder$
-
severe ADJ ((postpartum OR post-partum OR post ADJ partum OR postnatal OR post-natal OR post ADJ natal) ADJ (depress$ OR psycho$))
-
(major ADJ depress$) NEAR (postpartum OR post-partum OR post ADJ partum OR postnatal OR post-natal OR post ADJ natal)
-
puerperal ADJ psycho$
-
1 NEAR (2 OR 3 OR 4 OR 5 OR 6)
-
(mental$ OR psychiatric OR psychologic$) NEAR (illness$ OR condition$ OR disabil$ OR disorder$ OR disease$ OR impair$ OR problem$)
-
1 NEAR 8
-
7 OR 9
-
child$ OR adolescen$ OR teen$ OR juvenile$ OR boy$ OR girl$ OR baby OR babies OR infant$ OR toddler$ OR preschool$ OR pre-school$ OR schoolchild$ OR youth$ OR family OR families OR young ADJ person$ OR young ADJ people
-
therapy OR therapies OR program$ OR intervention$ OR service$ OR training
-
10 and 11 and 12
Applied Social Sciences Index and Abstracts, and Sociological Abstracts
Searched 15 January 2011.
Search terms
((parent* or mother* or father* or step-parent* or step-father* or step-mother* or maternal or paternal or (primary within 3 carer)) within 5 (((mental* or psychiatric or psychologic*) within 3 (illness* or condition* or disabil* or disorder* or disease* or impair* or problem*)) or (nervous breakdown* or depression or depressive* or affective disorder* or mood disorder* or anxiety disorder* or phobia or phobic or panic disorder* or PTSD or stress disorder* or OCD or compulsive disorder*) or seasonal affective disorder* or (bipolar or mania or dissociative disorder* or neurosis or personality disorder* or psychosis or paranoi* or schizophren* or schizoaffective disorder* or psychotic) or ((severe postpartum or severe post-partum or severe post partum or severe postnatal* or severe post-natal* or severe post natal*) within 1 (depress*)) or ((postpartum or post-partum or post partum or postnatal* or post-natal* or post natal) within 1 (psycho*)) or (puerperal* within 1 psycho*) or ((postpartum or post-partum or post partum or postnatal* or post-natal* or post natal) within 4 (major depress*)))) and(child* or adolescen* or teen* or juvenile* or boy* or girl* or baby or babies or infant* or toddler* or preschool* or pre-school* or schoolchild* or youth* or family or families or (young person*) or (young people)) and(therapy or therapies or program* or intervention* or education* or service* or support or training or management or care)
Scopus
Searched 31 January 2011.
Search terms
parent* OR mother* OR father* OR step-parent* OR step-father* OR step-father* OR maternal OR (primary w/3 carer) AND (mental* w/3 illness*) OR (mental* w/3 condition*) OR (mental* w/3 disabil*) OR (mental* w/3 disorder*) OR (mental* w/3 disease*) OR (mental* w/3 impair*) OR (mental* w/3 problem*) OR (psychiatric w/3 illness*) OR (psychiatric w/3 condition*) OR (psychiatric w/3 disabil*) OR (psychiatric w/3 disorder*) OR (psychiatric w/3 disease*) OR (psychiatric w/3 impair*) OR (psychiatric w/3 problem*) OR (psychologic* w/3 illness*) OR (psychologic* w/3 condition*) OR (psychologic* w/3 disabil*) OR (psychologic* w/3 disorder*) OR (psychologic* w/3 disease*) OR (psychologic* w/3 impair*) OR (psychologic* w/3 problem*) OR nervous breakdown* OR depression OR depressive OR affective disorder* OR mood disorder* OR anxiety disorder* OR phobia OR phobic OR panic disorder* OR ptsd OR stress disorder* OR ocd OR compulsive disorder* OR seasonal affective disorder* OR bipolar OR mania OR dissociative disorder* OR neurosis OR personality disorder* OR psychosis OR paranoi* OR schizophren* OR schizoaffective disorder* OR psychotic OR puerperal* psycho* OR severe postpartum depress* OR severe post-partum depress* OR severe post partum depress* OR severe postnatal depress* OR severe post-natal depress* OR severe post natal depress* OR postpartum psycho* or post-partum psycho* or postnatal psycho* or post-natal psycho* OR (postpartum w/4 major depress*)OR (post-partum w/4 major depress*)OR (post partum w/4 major depress*) OR (postnatal w/4 major depress*) OR (post-natal w/4 major depress*) OR (post natal w/4 major depress*)AND (child* OR adolescen* OR teen* OR juvenile* OR boy* OR girl* OR baby OR babies OR infant* OR toddler* OR preschool* OR pre-school* OR schoolchild* OR youth* OR family OR families OR young person* OR young people) AND (therapy OR therapies OR program* OR intervention* OR service* OR training)
ISI Web of Science (Science Citation Index and the Social Science Citation Index)
Searched 31 January 2011.
Search terms
#11 #10 AND #9 AND #8
#10 Topic=((therapy or therapies or program* or intervention* or service* or training))
#9 Topic=((child* or adolescen* or teen* or juvenile* or boy* or girl* or baby or babies or infant* or toddler* or preschool* or pre-school* or schoolchild* or youth* or family or families or young person* or young people))
#8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#7 Topic=((parent* or mother* or father* or step-parent* or step-mother* or step-father* or primary carer) same (psychologic* illness* or psychologic* condition* or psychologic* disabil* or psychologic* disorder* or psychologic* disease* or psychologic* impair* or psychologic* problem*))
#6 Topic=((parent* or mother* or father* or step-parent* or step-mother* or step-father* or primary carer) same (psychiatric illness* or psychiatric condition* or psychiatric disabil* or psychiatric disorder* or psychiatric disease* or psychiatric impair* or psychiatric problem*))
#5 Topic=((parent* or mother* or father* or step-parent* or step-mother* or step-father* or primary carer) same (mental* illness* or mental* condition* or mental* disabil* or mental* disorder* or mental* disease* or mental* impair* or mental*problem*))
#4 Topic=((parent* or mother* or father* or step-parent* or step-mother* or step-father* or primary carer) same ((postpartum or post-partum or postnatal or post-natal or post natal) same (major depress*)))
#3 Topic=((parent* or mother* or father* or step-parent* or step-mother* or step-father* or primary carer) same (severe postpartum depress* or severe post-partum depress* or severe post partum depress* or postpartum psycho* or post-partum psycho* or postnatal psycho* or post-natal psycho* or puerperal* psycho*))
#2 Topic=((parent* or mother* or father* or step-parent* or step-mother* or step-father* or primary carer) same (nervous breakdown or depression or depressive or affective disorder* or mood disorder* or anxiety disorder* or phobia or phobic or panic disorder* or PTSD or stress disorder* or OCD or compulsive disorder* or seasonal affective disorder*))
#1 Topic=((parent* or mother* or father* or step-parent* or step-mother* or step-father* or primary carer) same (bipolar or mania or dissociative disorder* or neurosis or personality disorder* or psychosis or paranoi* or schizophren* or schizoaffective disorder* or psychotic))
Website searches
Searched December 2010 to January 2011.
A.I.C.A.F.M.N.A.
ADOLEC
Anglicare
Anxiety UK
Auseinet
Barnardos
BBC Health
British National Bibliography for Report Literature
British Psychological Society Conference abstracts
C4EO
Care Services Improvement Partnership
Carers UK
Childline
Children’s Society
COPMI
Department for Education
Department of Health
Depression Alliance
Family Action
Family matters
Google Scholar
King’s Fund
Mental Health Foundation
Mind
National Academy of Parenting Research
National Children’s Bureau
National Guidelines Clearing House (at NIH)
NSPCC
Parental Mental Illness.org
Parental MH & Child Welfare Network
Parenting well
Princess Royal Trust for Carers (and professional website)
Re-think
Royal College of Psychiatrists
Sainsbury Centre for Mental Health
SANE
SCIE
The Joseph Rowntree Trust
The Leverhulme Trust
The Site
The World Health Organization
Turning Point
UNICEF
Young Carers
Young Mind
Appendix 4 List of included studies
Indented references represent multiple publications of the first study.
Synthesis one Chapter 4 (serious mental illness): clinical effectiveness
Randomised controlled trial
Cohler BJ, Grunebaum H. Children of parents hospitalized for mental illness: the evaluation of an intervention program for mentally ill mothers of young children (Part 2). Journal of Children in Contemporary Society 1982;15:57–66. 117
Lucas LE, Montgomery SH, Richardson DA, Rivers PA. Impact project: reducing the risk of mental illness to children of distressed mothers. New Dir Ment Health Serv 1984;79–94. 60
Sumner GS. The Design and Implementation of a Cognitive Behavioural Problem-Solving Training Program for Children of Severely Disturbed Parents. PhD thesis. FL: Florida State University; 1983. 61
Non-randomised controlled trial
Fraser E, Pakenham KI. Evaluation of a resilience-based intervention for children of parents with mental illness. Australas Psychiatry 2008;42:1041–50. 58
Gerull F, Meares R, Stevenson J, Korner A, Newman L. The beneficial effect on family life in treating borderline personality. Psychiatry 2008;7:59–70. 119
Goodyear M, Cuff R, Maybery D, Reupert A. CHAMPS: a peer support program for children of parents with a mental illness. Adv Mental Health 2009;8:296–304. 59
Stott FM, Musick JS, Cohler BJ, Spencer KK, Goldman J, Clark R, et al. Intervention for the severely disturbed mother. New Direct Mental Health Serv 1984;24:7–32. 118
Uncontrolled studies
Alder S. Reaching out to women. Ment Health Today. 2005;26–8. 120
Beardslee WR, Hoke L, Wheelock I, Rothberg PC, Vandevelde P, Swatling S. Initial findings on preventive intervention for families with parental-affective disorders. Am J Psychiatry 1992;149:1335–40. 62
Pitman E, Matthey S. Evaluation of the SMILES Program for Children with Mentally Ill Parents: Bankstown, Sydney. Sydney: South West Sydney Area Health Service; 2002. 121
Richter GA. Fostering Resilience: Evaluating the Effectiveness of Kids in Control. Dissertation. Canada: Trinity Western University; 2006. 122
Synthesis two Chapter 5 (severe depression): clinical effectiveness
Randomised controlled trial
Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive–behavioural counselling in the treatment of postnatal depression. BMJ 1997;314:932–6. 151
Beardslee WR, Wright EJ, Gladstone TRG, Forbes P. Long-term effects from a randomized trial of two public health preventive interventions for parental depression. J Fam Psychol 2007;21:703–13. 141
Beardslee WR, Keller MB, Lavori PW, Staley J, Sacks N. The impact of parental affective disorder on depression in offspring: a longitudinal follow-up in a nonreferred sample. J Am Acad Child Adolesc Psychiatry 1993;32:723–30. 164
Beardslee WR, Versage EM, Wright EJ, Salt P, Rothberg PC, Drezner K, et al. Examination of preventive interventions for families with depression: evidence of change. Dev Psychopathol 1997;9:109–30. 162
Beardslee WR, Salt P, Versage EM, Gladstone TR, Wright EJ, Rothberg PC. Sustained change in parents receiving preventive interventions for families with depression. Am J Psychiatry 1997;154:510–15. 202
Beardslee WR, Wright E, Rothberg PC, Salt P, Versage E. Response of families to two preventive intervention strategies: long-term differences in behavior and attitude change. J Am Acad Child Adolesc Psychiatry 1996;35:774–82. 227
Beardslee WR, Wright EJ, Salt P, Drezner K, Gladstone TRG, Versage EM, et al. Examination of children’s responses to two preventive intervention strategies over time. J Am Acad Child Adolesc Psychiatry 1997;36:196–204. 169
Beardslee WR, Gladstone TRG, Wright EJ, Cooper AB. A family-based approach to the prevention of depressive symptoms in children at risk: evidence of parental and child change. Pediatrics 2003;112:e119–31. 228
Bennett RB. An intervention program for children of recently hospitalized, depressed mothers. 1991. 140
Butler SF, Budman SH, Beardslee W. Risk reduction in children from families with parental depression: a videotape psychoeducation program. National Academies of Practice Forum: Issues in Interdisciplinary Care 2000;2:267–76. 142
Chabrol H, Teissedre F, Saint-Jean M, Teisseyre N, Roge B, Mullet E, et al. Prevention and treatment of post-partum depression: a controlled randomized study on women at risk. Psychol Med 2002;32:1039–47. 157
Clark R, Tluczek A, Wenzel A. Psychotherapy for postpartum depression: a preliminary report. Am J Orthopsychiatry 2003;73:441–54. 66
Clark R, Tluczek A, Brown R. A mother–infant therapy group model for postpartum depression. Infant Ment Health J 2008;29:514–36. 67
Cicchetti D, Toth SL, Rogosch FA. The efficacy of toddler-parent psychotherapy to increase attachment security in offspring of depressed mothers. Attach Hum Dev 1999;1:34–66. 64
Cicchetti D, Rogosch FA, Toth SL. The efficacy of toddler-parent psychotherapy for fostering cognitive development in offspring of depressed mothers. J Abnorm Child Psychol 2000;28:135–48. 229
Toth SL, Rogosch FA, Manly JT, Cicchetti D. The efficacy of toddler-parent psychotherapy to reorganize attachment in the young offspring of mothers with major depressive disorder: a randomized preventive trial. J Consult Clin Psychol 2006;74:1006–16. 230
Compas BE, Forehand R, Keller G, Champion JE, Rakow A, Reeslund KL, et al. Randomized controlled trial of a family cognitive–behavioral preventive intervention for children of depressed parents. J Consult Clin Psychol 2009;77:1007–20. 143
Compas BE, Champion JE, Forehand R, Cole DA, Reeslund KL, Fear J, et al. Coping and parenting: mediators of 12-month outcomes of a family group cognitive–behavioral preventive intervention with families of depressed parents. J Consult Clin Psychol 2010;78:623–34. 168
Cooper PJ, Murray L, Wilson A, Romaniuk H. Controlled trial of the short- and long-term effect of psychological treatment of post-partum depression. 1. Impact on maternal mood. Br J Psychiatry 2003;182:412–9. 152
Murray L, Cooper PJ, Wilson A, Romaniuk H. Controlled trial of the short-and long-term effect of psychological treatment of post-partum depression. 2. Impact on the mother–child relationship and child outcome. Br J Psychiatry 2003;182:420–7. 163
Gelfand DM, Teti DM, Seiner SA, Jameson PB. Helping mothers fight depression: Evaluation of a home-based intervention program for depressed mothers and their infants. J Clin Child Psychol 1996;25:406–22. 144
Grote NK, Swartz HA, Geibel SL, Zuckoff A, Houck PR, Frank E. A randomized controlled trial of culturally relevant, brief interpersonal psychotherapy for perinatal depression. Psychiatr Serv 2009;60:313–21. 145
Holden JM, Sagovsky R, Cox JL. Counselling in a general practice setting: controlled study of health visitor intervention in treatment of postnatal depression. BMJ 1989;298:223–6. 153
Meager I, Milgrom J. Group treatment for postpartum depression: a pilot study. Australas Psychiatry 1996;30:852–60. 148
Milgrom J, Holt C, Gemmill A, Ericksen J, Leigh B, Buist A, et al. Treating postnatal depressive symptoms in primary care: a randomised controlled trial of GP management, with and without adjunctive counselling. BMC Psychiatry 2011;11:95. 149
Misri S, Kostaras X, Fox D, Kostaras D. The impact of partner support in the treatment of postpartum depression. Can JPsychiatry 2000;45:554–8. 155
Misri S, Reebye P, Corral M, Mills L. The use of paroxetine and cognitive–behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry 2004; 65:1236–41. 156
Misri S, Reebye P, Milis L, Shah S. The impact of treatment intervention on parenting stress in postpartum depressed mothers: a prospective study. Am J Orthopsychiatry 2006;76:115–19. 161
Mulcahy R, Reay RE, Wilkinson RB, Owen C. A randomised control trial for the effectiveness of group interpersonal psychotherapy for postnatal depression. Arch Womens Ment Health 2010;13:125–39. 150
O’Hara MW, Stuart S, Gorman LL, Wenzel A. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry 2000;57:1039–45. 146
Nylen KJ, O’Hara MW, Brock R, Moel J, Gorman L, Stuart S. Predictors of the longitudinal course of postpartum depression following interpersonal psychotherapy. J Consult Clin Psychol 2010;78:757–63. 167
Forman DR, O’Hara MW, Stuart S, Gorman LL, Larsen KE, Coy KC. Effective treatment for postpartum depression is not sufficient to improve the developing mother–child relationship. Dev Psychopathol 2007;19:585–602. 160
Rahman A, Malik A, Sikander S, Roberts C, Creed F. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet 2008;372:902–9. 158
Rojas G, Fritsch R, Solis J, Jadresic E, Castillo C, González M, et al. Treatment of postnatal depression in low-income mothers in primary-care clinics in Santiago, Chile: a randomised controlled trial. Lancet 2007;370:1629–37. 138
Sanders MR, McFarland M. Treatment of depressed mothers with disruptive children: a controlled evaluation of cognitive behavioral family intervention. Behav Ther 2000;31:89–112. 67
Sanford M, Byrne C, Williams S, Atley S, Ridley T, Miller J, et al. A pilot study of a parent-education group for families affected by depression. Can J Psychiatry 2003;48:78–86. 63
Swartz HA, Frank E, Zuckoff A, Cyranowski JM, Houck PR, Cheng Y, et al. Brief interpersonal psychotherapy for depressed mothers whose children are receiving psychiatric treatment. Am J Pychiatry 2008;165:1155–62. 147
Verduyn C, Barrowclough C, Roberts J, Tarrier N, Harrington R. Maternal depression and child behaviour problems Randomised placebo-controlled trial of a cognitive–behavioural group intervention. Br J Psychiatry 2003;183:342–8. 154
Wickberg B, Hwang CP. Counselling of postnatal depression: a controlled study on a population based Swedish sample. J Affect Disord 1996;39:209–16. 159
Non-randomised controlled trials
Ammerman RT, Putman FW, Stevens J, Bosse NR, Short JA, Bodley AL, et al. An open trial of in-home CBT for depressed mothers in home visitation. Matern Child Health J 2010;15:1333–41. 166
Boath E, Cox J, Lewis M, Jones P, Pryce A. When the cradle falls: the treatment of postnatal depression in a psychiatric day hospital compared with routine primary care. J Affect Disord 1999;53:143–51. 139
O’Brien LM, Boath E, Hodgson R, Cox JL. The long-term follow-up of women treated for postnatal depression at a specialist day hospital compared to routine primary care. Int J Psychiatry Clin Pract 2002;6:199–203. 172
Pearlstein TB, Zlotnick C, Battle CL, Stuart S, O’Hara MW, Price AB, et al. Patient choice of treatment for postpartum depression: a pilot study. Arch Womens Ment Health 2006;9:303–8. 170
Seeley S, Murray L, Cooper PJ. The outcome for mothers and babies of health visitor intervention. Health Visitor 1996;69:135–8. 171
Uncontrolled studies
Ammerman RT, Putnam FW, Stevens J, Holleb LJ, Novak AL, Van Ginkel JB. In-home cognitive behavior therapy for depression: an adapted treatment for first-time mothers in home visitation. Best Pract Ment Health 2005;1:1–14. 173
Davies S. A first-stage evaluation of a group programme for PND. Community Pract 2004;77:426–33. 174
Edwards M. Reported and Observed Disruptive Behaviours in Children of Depressed Mothers Prior to and Following Cognitive–Behaviour Therapy for Maternal Depression. PhD thesis. Bangor: University of Wales; 1998. 181
Griffiths P, Barker-Collo S. Study of a group treatment program for postnatal adjustment difficulties. Arch Womens Ment Health 2008;11:33–41. 175
Grote NK, Bledsoe SE, Swartz HA, Frank E. Feasibility of providing culturally relevant, brief interpersonal psychotherapy for antenatal depression in an obstetrics clinic: a pilot study. Research on Social Work Practice 2004;14:397–407. 176
Klier CM, Muzik M, Rosenblum KL, Lenz G. Interpersonal psychotherapy adapted for the group setting in the treatment of postpartum depression. J Psychother Pract Res 2001;10:124–31. 182
Pitman E, Matthey S. The SMILES program: a group program for children with mentally ill parents or siblings. Am J Orthopsychiatry 2004;74:383–8. 180
Pagnini D. Carers NSW Carers Mental Health Project (2001–2004) Longitudinal Follow-up Report. Sydney: Carers NSW, Inc.; 2007. 231
Pagnini D. Carers NSW Carers Mental Health Project Stage One Final Evaluation Report August 2001 – June 2004. Sydney: Carers NSW, Inc.; 2004. 232
Pagnini D. Carers Mental Health Project Stage 2: Action Evaluation Report July 2004 – January 2005. Sydney: Carers NSW, Inc.; 2005. 233
Reay R, Fisher Y, Robertson M, Adams E, Owen C. Group interpersonal psychotherapy for postnatal depression: a pilot study. Arch Womens Ment Health 2006;9:31–9. 177
Stuart S, O’Hara MW. Treatment of postpartum depression with interpersonal psychotherapy. Arch Gen Psychiatry 1995;52:75–6. 183
Swartz HA, Zuckoff A, Frank E, Spielvogle HN, Shear MK, Fleming MA, et al. An open-label trial of enhanced brief interpersonal psychotherapy in depressed mothers whose children are receiving psychiatric treatment. Depress Anxiety 2006;23:398–404. 178
Verdeli H, Ferro T, Wickramaratne P, Greenwald S, Blanco C, Weissman MM. Treatment of depressed mothers of depressed children: pilot study of feasibility. Depress Anxiety 2004;19:51–8. 179
Synthesis two Chapter 5 (severe depression): cost-effectiveness
Boath E, Major K, Cox J. When the cradle falls II: the cost-effectiveness of treating postnatal depression in a psychiatric day hospital compared with routine primary care. J Affect Disord 2003;74:159–66. 116
Synthesis 3 Chapter 6 (serious mental illness): acceptability
Lucas LE, Montgomery SH, Richardson DA, Rivers PA. Impact project: reducing the risk of mental illness to children of distressed mothers. New Dir Ment Health Serv 1984; 79–94. 60
Stott FM, Musick JS, Cohler BJ, Spencer KK, Goldman J, Clark R, et al. Intervention for the severely disturbed mother. New Directions for Mental Health Services 1984;24:7–32. 118
Finzi R, Strange D. Short term group intervention as a means of improving the adjustment of children of mentally ill parents. Social Work With Groups 2007;20:69–80197
Waldo MC, Roath M, Levine W, Freedman R. A model program to teach parenting skills to schizophrenic mothers. Psychiatric Serv 1987;38:1110–12. 198
Cowling V, Garrett M. Child and family inclusive practice: a pilot program in a community adult mental health service. Australas Psychiatry 2009;17:279–82. 199
Fraser E, Pakenham KI. Evaluation of a resilience-based intervention for children of parents with mental illness. Australas Psychiatry 2008;42:1041–50. 58
Cohler BJ, Grunebaum H. Children of parents hospitalized for mental illness: the evaluation of an intervention program for mentally ill mothers of young children (Part 2). Journal of Children in Contemporary Society 1982;15:57–66. 117
Alder S. Reaching out to women. Ment Health Today. 2005; 26–8. 120
Beardslee WR, Hoke L, Wheelock I, Rothberg PC, Vandevelde P, Swatling S. Initial findings on preventive intervention for families with parental affective-disorders. Am J Psychiatry 1992;149:1335–40. 62
Pitman E, Matthey S. Evaluation of the SMILES program for children with mentally ill parents: Bankstown, Sydney. Sydney: South West Sydney Area Health Service; 2002. 121
Synthesis 3 Chapter 6 (severe depression): acceptability
Ammerman RT, Putnam FW, Stevens J, Holleb LJ, Novak AL, Van Ginkel JB. In-home cognitive behavior therapy for depression: an adapted treatment for first-time mothers in home visitation. Best Pract Ment Health 2005;1:1–14. 173
Beardslee WR, Wright EJ, Gladstone TRG, Forbes P. Long-term effects from a randomized trial of two public health preventive interventions for parental depression. J Fam Psychol 2007;21:703–13. 141
Beardslee WR, Versage EM, Wright EJ, Salt P, Rothberg PC, Drezner K, et al. Examination of preventive interventions for families with depression: Evidence of change. Dev Psychopathol 1997;9:109–30. 162
Beardslee WR, Salt P, Versage EM, Gladstone TR, Wright EJ, Rothberg PC. Sustained change in parents receiving preventive interventions for families with depression. Am J Psychiatry 1997;154:510–15. 202
Beardslee WR, Wright E, Rothberg PC, Salt P, Versage E. Response of families to two preventive intervention strategies: Long-term differences in behavior and attitude change. J Am Acad Child Adolesc Psychiatry 1996;35:774–82. 227
Beardslee WR, Wright EJ, Salt P, Drezner K, Gladstone TRG, Versage EM, et al. Examination of children’s responses to two preventive intervention strategies over time. J Am Acad Child Adolesc Psychiatry 1997;36:196–204. 169
Beardslee WR, Gladstone TRG, Wright EJ, Cooper AB. A family-based approach to the prevention of depressive symptoms in children at risk: evidence of parental and child change. Pediatrics 2003;112:e119–31. 228
Davies S. A first-stage evaluation of a group programme for PND. Community Pract 2004;77:426–33. 174
Edwards M. Reported and Observed Disruptive Behaviours in Children of Depressed Mothers Prior to and Following Cognitive–Behaviour Therapy for Maternal Depression. PhD thesis. Bangor: University of Wales; 1998. 181
Griffiths P, Barker-Collo S. Study of a group treatment program for postnatal adjustment difficulties. Arch Womens Ment Health 2008;11:33–41. 175
Grote NK, Bledsoe SE, Swartz HA, Frank E. Feasibility of providing culturally relevant, brief interpersonal psychotherapy for antenatal depression in an obstetrics clinic: a pilot study. Research on Social Work Practice 2004;14:397–407. 176
Holden JM, Sagovsky R, Cox JL. Counselling in a general practice setting: controlled study of health visitor intervention in treatment of postnatal depression. BMJ 1989;298:223–6. 153
Meager I, Milgrom J. Group treatment for postpartum depression: a pilot study. Australas Psychiatry 1996;30:852–60. 148
Milgrom J, Holt C, Gemmill A, Ericksen J, Leigh B, Buist A, et al. Treating postnatal depressive symptoms in primary care: a randomised controlled trial of GP management, with and without adjunctive counselling. BMC Psychiatry 2011;11:95. 149
Pitman E, Matthey S. The SMILES program: a group program for children with mentally ill parents or siblings. Am J Orthopsychiatry 2004;74:383–8. 180
Reay R, Fisher Y, Robertson M, Adams E, Owen C. Group interpersonal psychotherapy for postnatal depression: a pilot study. Arch Womens Ment Health 2006;9:31–9. 177
Swartz HA, Zuckoff A, Frank E, Spielvogle HN, Shear MK, Fleming MA, et al. An open-label trial of enhanced brief interpersonal psychotherapy in depressed mothers whose children are receiving psychiatric treatment. Depress Anxiety 2006;23:398–404. 178
Wickberg B, Hwang CP. Counselling of postnatal depression: a controlled study on a population based Swedish sample. J Affect Disord 1996;39:209–16. 159
Sanford M, Byrne C, Williams S, Atley S, Ridley T, Miller J, et al. A pilot study of a parent-education group for families affected by depression. Can J Psychiatry 2003;48:78–86. 63
Misri S, Kostaras X, Fox D, Kostaras D. The impact of partner support in the treatment of postpartum depression. Can JPsychiatry 2000;45:554–8. 155
Gelfand DM, Teti DM, Seiner SA, Jameson PB. Helping mothers fight depression: Evaluation of a home-based intervention program for depressed mothers and their infants. J Clin Child Psychol 1996;25:406–22. 144
Seeley S, Murray L, Cooper PJ. The outcome for mothers and babies of health visitor intervention. Health Visitor 1996;69:135–8. 171
Pearlstein TB, Zlotnick C, Battle CL, Stuart S, O’Hara MW, Price AB, et al. Patient choice of treatment for postpartum depression: a pilot study. Arch Womens Ment Health 2006;9:303–8. 170
Compas BE, Forehand R, Keller G, Champion JE, Rakow A, Reeslund KL, et al. Randomized controlled trial of a family cognitive–behavioral preventive intervention for children of depressed parents. J Consult Clin Psychol 2009;77:1007–20. 143
Klier CM, Muzik M, Rosenblum KL, Lenz G. Interpersonal psychotherapy adapted for the group setting in the treatment of postpartum depression. J Psychother Pract Res 2001;10:124–31. 182
Rahman A, Malik A, Sikander S, Roberts C, Creed F. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet 2008;372:902–9. 158
Cooper PJ, Murray L, Wilson A, Romaniuk H. Controlled trial of the short- and long-term effect of psychological treatment of post-partum depression. 1. Impact on maternal mood. Br J Psychiatry 2003;182:412–9. 152
Stuart S, O’Hara MW. Treatment of postpartum depression with interpersonal psychotherapy. Arch Gen Psychiatry 1995;52:75–6. 183
Boath E, Bradley E, Anthony P. Users’ views of two alternative approaches to the treatment of postnatal depression. J Reprod Infant Psychol 2004;22:13–24. 200
Hinden BR, Hinden BR, Biebel K, Nicholson J, Mehnert L. The invisible children’s project. J Behav Health Serv Res 2005;32:393–408. 201
Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive–behavioural counselling in the treatment of postnatal depression. BMJ 1997;314:932–6. 151
Chabrol H, Teissedre F, Saint-Jean M, Teisseyre N, Roge B, Mullet E, et al. Prevention and treatment of post-partum depression: a controlled randomized study on women at risk. Psychol Med 2002;32:1039–47. 157
Cicchetti D, Toth SL, Rogosch FA. The efficacy of toddler-parent psychotherapy to increase attachment security in offspring of depressed mothers. Attach Hum Dev 1999;1:34–66. 64
Cicchetti D, Rogosch FA, Toth SL. The efficacy of toddler-parent psychotherapy for fostering cognitive development in offspring of depressed mothers. J Abnorm Child Psychol 2000;28:135–48. 229
Grote NK, Swartz HA, Geibel SL, Zuckoff A, Houck PR, Frank E. A randomized controlled trial of culturally relevant, brief interpersonal psychotherapy for perinatal depression. Psychiatr Serv 2009;60:313–21. 145
Misri S, Reebye P, Corral M, Mills L. The use of paroxetine and cognitive–behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry 2004; 65:1236–41. 156
Misri S, Reebye P, Milis L, Shah S. The impact of treatment intervention on parenting stress in postpartum depressed mothers: a prospective study. Am J Orthopsychiatry 2006;76:115–19. 161
Mulcahy R, Reay RE, Wilkinson RB, Owen C. A randomised control trial for the effectiveness of group interpersonal psychotherapy for postnatal depression. Arch Womens Ment Health 2010;13:125–39. 150
O’Hara MW, Stuart S, Gorman LL, Wenzel A. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry 2000;57:1039–45. 146
Nylen KJ, O’Hara MW, Brock R, Moel J, Gorman L, Stuart S. Predictors of the longitudinal course of postpartum depression following interpersonal psychotherapy. J Consult Clin Psychol 2010;78:757–63. 167
Forman DR, O’Hara MW, Stuart S, Gorman LL, Larsen KE, Coy KC. Effective treatment for postpartum depression is not sufficient to improve the developing mother–child relationship. Dev Psychopathol 2007;19:585–602. 160
Rojas G, Fritsch R, Solis J, Jadresic E, Castillo C, González M, et al. Treatment of postnatal depression in low-income mothers in primary-care clinics in Santiago, Chile: a randomised controlled trial. Lancet 2007;370:1629–37. 138
Sanders MR, McFarland M. Treatment of depressed mothers with disruptive children: a controlled evaluation of cognitive behavioral family intervention. Behav Ther 2000;31:89–112. 65
Verdeli H, Ferro T, Wickramaratne P, Greenwald S, Blanco C, Weissman MM. Treatment of depressed mothers of depressed children: pilot study of feasibility. Depress Anxiety 2004;19:51–8. 179
Appendix 5 List of excluded studies: clinical effectiveness
Synthesis one, Chapter 4 (serious mental illness)
| Austin et al. 1999234 | < 50% study sample with eligible SMI |
| Arkansas Centre 2002235 | Residential intervention |
| Brunette et al. 2004236 | Percentage study sample with SMI unclear, symptoms unclear |
| Croake et al. 1985124 | < 50% study sample with eligible SMI |
| Coster et al. 2008237 | Case studies |
| Emerson-Davies et al. 2000238 | Inpatient intervention |
| Finzi et al. 2007197 | No eligible effectiveness outcomes |
| Free et al. 1996239 | No defined intervention |
| Gladstone et al. 2010240 | No eligible outcomes |
| Hamill et al. 2008241 | Percentage study sample with SMI unclear, symptoms unclear |
| Hanrahan et al. 2005125 | < 50% study sample with eligible SMI |
| Hargreaves et al. 2005242 | Percentage study sample with SMI unclear, symptoms unclear |
| Hayman et al. 2005243 | < 50% study sample with eligible SMI |
| Kahana et al. 1972244 | No eligible outcomes |
| Kersting et al. 2003245 | < 50% study sample with eligible SMI |
| Kersting et al. 2003246 | < 50% study sample with eligible SMI |
| Moukaddem et al. 1998247 | Percentage study sample with SMI unclear, symptoms unclear |
| Nicholson et al. 2009248 | < 50% study sample with eligible SMI |
| Nielsen et al. 2006249 | < 50% study sample with eligible SMI |
| Orel et al. 2003250 | Percentage study sample with SMI unclear, symptoms unclear |
| Phelan et al. 200644 | < 50% study sample with eligible SMI |
| Pitman et al. 2004,180 Pagnini 2004,232 Pagnini 2005,233 Pagnini 2007231 | < 50% study sample with eligible SMI (Pagnini 2004232 includes 56% SMI but only 75% parents) |
| Place et al. 2002;251 Brownrigg et al. 2004252 | < 50% study sample with eligible SMI |
| Riebschleger et al. 2009126 | < 50% study sample with eligible SMI |
| Solantuas et al. 2010253 | < 50% study sample with eligible SMI |
| Tamminen et al. 1999254 | No eligible outcomes |
| Tustin et al. 2002255 | No useable outcomes |
| van der Zanden et al. 2010256 | Percentage study sample with SMI unclear (minimum of 44%) |
| Wadsby et al. 1998257 | Percentage study sample with SMI unclear, symptoms unclear |
| Waldo et al. 1987198 | No useable effectiveness outcomes |
| Wasylenki et al. 1997258 | Not focused on parents with SMI |
Synthesis two, Chapter 5 (severe depression)
| Austin et al. 1999234 | < 50% study sample with eligible diagnosis |
| Alder et al. 2002259 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Ammerman et al. 2011260 | No eligible psychosocial intervention |
| Armstrong et al. 2003261 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Armstrong et al. 2004262 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Baggett et al. 2010263 | < 50% study sample with eligible diagnosis |
| Barnes et al. 2009264 | < 50% study sample with eligible diagnosis |
| Brunette et al. 2004236 | Diagnosis and/or baseline symptoms unclear |
| Buultjens et al. 2008265 | Diagnosis and/or baseline symptoms unclear |
| Chazen-Cohen et al. 2007266 | Diagnosis and/or baseline symptoms unclear |
| Chien et al. 2005267 | Diagnosis and/or baseline symptoms unclear |
| Cho et al. 2008268 | < 50% study sample with eligible diagnosis |
| Clarke et al. 2001269 | Diagnosis and/or baseline symptoms unclear |
| Clarke et al. 2002270 | Diagnosis and/or baseline symptoms unclear |
| Clarke-Akalanne et al. 2002271 | Diagnosis and/or baseline symptoms unclear |
| Clulow et al. 2010272 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Cooper et al. 2009273 | < 50% study sample with eligible diagnosis |
| Cowell et al. 2009274 | Diagnosis and/or baseline symptoms unclear |
| Croake et al. 1985124 | Diagnosis and/or baseline symptoms unclear |
| Daley et al. 2008275 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Field et al. 2009276 | No eligible community-based psychosocial intervention |
| Fisher et al. 2010277 | < 50% study sample with eligible diagnosis |
| Foster et al. 2008278 | No eligible community-based psychosocial intervention |
| Garber et al. 2009279 | < 50% study sample with eligible diagnosis |
| Gjerdingen et al. 2009280 | < 50% study sample with eligible diagnosis |
| Gladstone et al. 2010240 | No eligible outcomes |
| Goodman et al. 2008281 | No eligible community-based psychosocial intervention |
| Hamill et al. 2008241 | Diagnosis and/or baseline symptoms unclear |
| Hanrahan et al. 2005125 | < 50% study sample with eligible diagnosis |
| Hargreaves et al. 2008242 | Diagnosis and/or baseline symptoms unclear |
| Hayes et al. 2008282 | Diagnosis and/or baseline symptoms unclear |
| Hayman et al. 2005243 | Diagnosis and/or baseline symptoms unclear |
| Highet et al. 2004283 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Ho et al. 2004284 | Diagnosis and/or baseline symptoms unclear |
| Honey et al. 2002285 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Kersten-Alvarez et al. 2010286 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Kersting et al. 2003245 | < 50% study sample with eligible diagnosis |
| Kersting et al. 2003246 | < 50% study sample with eligible diagnosis |
| Khazan et al. 2006287 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Kurzweil et al. 2008288 | < 50% study sample with eligible diagnosis |
| Logsdon et al. 2010289 | < 50% study sample with eligible diagnosis |
| Marks et al. 2003290 | No eligible community-based psychosocial intervention |
| McCarthy et al. 2008291 | No eligible community-based psychosocial intervention |
| Milgrom et al. 2005292 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Morrell et al. 2009293 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Morris et al. 1987294 | Diagnosis and/or baseline symptoms unclear |
| Morse et al. 2004295 | No eligible community-based psychosocial intervention |
| Moukaddem et al. 1998247 | Diagnosis and/or baseline symptoms unclear |
| Munoz et al. 2007296 | Diagnosis and/or baseline symptoms unclear |
| Nicholson et al. 2009248 | < 50% study sample with eligible diagnosis |
| Nicol et al. 1984297 | No eligible outcomes |
| Nielsen et al. 2006249 | < 50% study sample with eligible diagnosis |
| Okano et al. 1998298 | No eligible community-based psychosocial intervention |
| Orel et al. 2003250 | Diagnosis and/or baseline symptoms unclear |
| Orhon et al. 2007299 | < 50% study sample with eligible diagnosis |
| Paris et al. 2011300 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Parry et al. 2000301 | No eligible community-based psychosocial intervention |
| Phelan et al. 200644 | Diagnosis and/or baseline symptoms unclear |
| Phillips et al. 2010302 | < 50% study sample with eligible diagnosis |
| Pinkhala et al. 2008303 | Diagnosis and/or baseline symptoms unclear |
| Place et al. 2002;251 Brownrigg et al. 2004252 | Diagnosis and/or baseline symptoms unclear |
| Prendergast et al. 2001304 | < 50% study sample with eligible diagnosis |
| Puckering et al. 1994305 | Diagnosis and/or baseline symptoms unclear |
| Puckering et al. 2010306 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Riebschleger et al. 2009126 | Diagnosis and/or baseline symptoms unclear |
| Riley et al. 2008307 | Diagnosis and/or baseline symptoms unclear |
| Selkirk et al. 2006308 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Sharp et al. 2010137 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Sheeber et al. 2011309 | < 50% study sample with eligible diagnosis |
| Slade et al. 2005310 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Solantuas et al. 2010253 | Includes any ICD-10 mood disorder, baseline symptoms low |
| Spinelli et al. 1997311 | Antenatal intervention, no eligible postpartum outcomes |
| Spinelli et al. 2003312 | Antenatal intervention, no eligible postpartum outcomes |
| Stavros et al. 2002313 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Steinberg et al. 1999314 | < 50% study sample with eligible diagnosis |
| Tamaki et al. 2008315 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Tezel et al. 2006316 | < 50% study sample with eligible diagnosis |
| Tischler et al. 2004317 | Diagnosis and/or baseline symptoms unclear |
| Turner et al. 2010318 | Diagnosis and/or baseline symptoms unclear |
| Ugarriza et al. 2006319 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Valdez et al. 2011320 | Diagnosis and/or baseline symptoms unclear |
| van der Zanden et al. 2010256 | Diagnosis and/or baseline symptoms unclear |
| van Doesum et al. 2008321 | Diagnosis and/or baseline symptoms unclear |
| Vorhies et al. 2009322 | No eligible community-based psychosocial intervention |
| Wadsby et al. 1998257 | Diagnosis and/or baseline symptoms unclear |
| Wan et al. 2011323 | Diagnosis unclear, symptoms do meet severity inclusion criteria |
| Wood et al. 2010324 | < 50% study sample with eligible diagnosis |
| Zlotnik et al. 2006325 | < 50% study sample with eligible diagnosis |
Appendix 6 List of excluded economic studies
| Study | Reason for exclusion |
|---|---|
| Andrews et al. 2003326 | No focus on children or adolescents aged 0–17 years or their parents |
| Andrews et al. 2006327 | No focus on children or adolescents aged 0–17 years or their parents |
| Appleby et al. 2003328 | The proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity |
| Breitborde et al. 2009329 | No focus on children or adolescents aged 0–17 years or their parents |
| Crossroads et al. 20087 | The proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity |
| Darmstadt et al. 2005330 | The proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity |
| Darmstadt et al. 2008331 | The proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity |
| Dixon et al. 1999332 | No focus on children or adolescents aged 0–17 years or their parents |
| Fuggle et al. 2000333 | The proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity |
| Haddock et al. 2003334 | No focus on children or adolescents aged 0–17 years or their parents |
| Higgins et al. 1996335 | The proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity |
| Lieu et al. 1998336 | The proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity |
| Lin et al. 2009337 | Did not involve evaluation of a community-based psychosocial intervention |
| Mihalopoulos et al. 2004338 | No focus on children or adolescents aged 0–17 years or their parents |
| Morrell et al. 2000339 | The proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity |
| Petrou et al. 2002340 | The proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity |
| Petrou et al. 2006341 | The proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity |
| Stevenson et al. 2010342 | The proportion of participants with a serious parental mental illness was zero, < 50% or unknown, with no reported baseline symptoms to assess likely severity |
| Wasylenki et al. 1997258 | No focus on children or adolescents aged 0–17 years or their parents |
Excluded economic study reference list
Andrews G, Sanderson K, Corry J, Issakidis C, Lapsley H. Cost-effectiveness of current and optimal treatment for schizophrenia. Br J Psychiatry 2003;183:427–35. 326
Andrews G. It would be cost-effective to treat more people with mental disorders. Aust NZJ Psychiatry 2006;40:613–15. 327
Appleby L, Hirst E, Marshall S, Keeling F, Brind J, Butterworth T, et al. The treatment of postnatal depression by health visitors: impact of brief training on skills and clinical practice. J Affect Disord 2003;77:261–6. 328
Breitborde N, Woods S, Srihari V. Multifamily psychoeducation for first-episode psychosis: a cost-effectiveness analysis. Psychiatric Serv 2009;60:1477–83. 329
Crossroads Caring for Carers and The Princess Royal Trust for Carers. Economic Evaluation of Young Carers’ Interventions. 2008. URL: http://static.carers.org/files/finalfinal3-4040.pdf (accessed November 2013). 7
Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet 2005;365:977–88. 330
Darmstadt GL, Walker N, Lawn JE, Bhutta ZA, Haws RA, Cousens S. Saving newborn lives in Asia and Africa: cost and impact of phased scale-up of interventions within the continuum of care. Health Policy Plan 2008;23:101–17. 331
Dixon L, Lyles A, Scott J, Lehman A, Postrado L, Goldman H, et al. Services to families of adults with schizophrenia: from treatment recommendations to dissemination. Psychiatric Serv 1999;50:233–8. 332
Fuggle P, Haydon K. Estimating resources for setting up an HV service for postnatal depression. Br J Comm Nurs 2000;5:348–51. 333
Haddock G, Barrowclough C, Tarrier N, Moring J, O’Brien R, Schofield N, et al. Cognitive–behavioural therapy and motivational intervention for schizophrenia and substance misuse 18-month outcomes of a randomised controlled trial. Br J Psychiatry 2003;183:418–26. 334
Higgins P, Murray ML. Perinatal outcomes and level of prenatal care: a study of the Southwestern United States. J Perinatal Education 1996;5:37–46. 335
Lieu TA, Wikler C, Capra AM, Martin KE, Escobar GJ, Braveman PA. Clinical outcomes and maternal perceptions of an updated model of perinatal care. Pediatrics 1998;102:1437–44. 336
Lin HC, Lin YJ, Hsiao FH, Li CY. Prenatal care visits and associated costs for treatment-seeking women with depressive disorders. Psychiatric Serv 2009;60:1261–4. 337
Mihalopoulos C, Magnus A, Carter R, Vos T. Assessing cost-effectiveness in mental health: family interventions for schizophrenia and related conditions. Aust NZJ Psychiatry 2004;38:511–19. 338
Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A. Costs and effectiveness of community postnatal support workers: randomised controlled trial. BMJ 2000;321:593–8. 339
Petrou S, Cooper P, Murray L, Davidson LL. Economic costs of post-natal depression in a high-risk British cohort. BrJ Psychiatry 2002;181:505–12. 340
Petrou S, Cooper P, Murray L, Davidson LL. Cost-effectiveness of a preventive counseling and support package for postnatal depression. Int J Technol Assess Health Care 2006;22:443–53. 341
Stevenson MD, Sutcliffe PA. The cost-effectiveness of group cognitive behavioral therapy compared with routine primary care for women with postnatal depression in the UK. Value Health 2010;13:580–4. 342
Wasylenki D, Gehrs M, Goering P, Toner B. A home-based program for the treatment of acute psychosis. Community Ment Health J 1997;33:151–65. 258
Appendix 7 Serious mental illness study characteristics
| Study | Design | Number randomised; number at baseline | Country | % SMI; primary diagnosis | Non-SMI primary diagnosis | SMI history; MH comorbidity | Recruitment context | Inclusion criteria | Target child age range (years) | Child mean (SD) age; % female | Child residency % colocated | Parent mean (SD) age; % female | Parental education; standardised ES | Parent % BME status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 117 | RCT | 50; 50a | USA | 100% Psy. | – | Unclear; unclear | Public and private psychiatric hospitals | Formerly hospitalised psychotic mothers, child < 6 years | < 6 | Unclear; unclear | 100% | Late 20s; 100% | Unclear; lower middle class | Unclear |
| 60 | RCT | 53; 51b | USA | 100% Psy. symptoms | – | Unclear; unclear | Mental health agency, hospital referrals, media adverts, outpatient recruiting booth | Global Severity Index T-score > 63; child aged 5–12 years | 5–12 | Unclear; 34% | Unclear | 32.49 (unclear); 100% | High school; 67% Hollingshead IV-Vc | 45% black |
| 61 | RCT | 41; 41d | USA | 100% Psy. symptoms | – | Unclear; unclear | Hospital mental health services, agency referrals media adverts | Global Severity Index score > 63; legal custody of child aged 5–12 years enrolled in school; competent; local resident | 5–12 | Unclear; 55% | 100% | Unclear; unclear | Unclear; predominantly low | Unclear |
| 58 | nRCT | 44; 44 | Australia | 100% Psy/Sz. BiP, PD | Depression | Unclear; 25% | Child/adolescent intervention programme | 12–18 years; parent with mental illness | 12–16 | 13.0 (1.58); 61% | Unclear | Unclear; 70% | Unclear; unclear | Unclear |
| 119 | nRCT | 45; 45 | Australia | 100% BPD | – | Unclear; unclear | Hospital outpatient MH services | Parent living with children; in receipt of intervention service | Infant to adolescent | Unclear; unclear | 100% | Intervention: 26.9 (5.4); control: 27.7 (5.1); 60% | Not completed high school; 68% unemployed | Unclear |
| 59 | nRCT | 129; 69 | Australia | 74% Sz, Sz-aff, BiP, BPD | Depression; depression-anxiety, depression-PTSD | Unclear; unclear | Child programme initiative | 12–18 years; parent with mental illness; providing pre–post data | 8–12 | 9.3 (unclear); 71% | Unclear | Unclear; unclear | Unclear; unclear | Unclear |
| 118 | nRCT | 83; 65 | USA | 100% Psy. | – | Unclear; unclear | Inpatient clinics, community private practice | 18+; youngest child < 5 years; diagnosis of psychosis, no mental retardation, drug/alcoholism history | < 5 | Unclear; unclear | 100% | 28.0 (unclear); 100% | High school; typically Hollingshead IV-V | 45% non-white (multiagency group only) |
| Study | Design | n | Country | % SMI; primary diagnosis | Non-SMI primary diagnoses | SMI history; MH comorbidity | Recruitment context | Inclusion criteria | Exclusion criteria | Target child age range (years) | Child mean (SD) age; % female | Child residency % colocated | Parent mean (SD) age; % female | Parental education; standardised ES | Parent % BME status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 120 | Uncontrolled | 13 | UK | 62% minimum Sz, BiP, PD | Depression PND | Unclear; unclear | Voluntary sector | Severe enduring illness; dependent children; in receipt of intervention | Unclear | Nursery-school age | Unclear; unclear | Unclear | Unclear; 100% | Unclear; unclear | 61% BME |
| 62 | Uncontrolled | 7 | USA | 71% BiP | MDD | Mean age: 10.2 (SD 15.3) years; unclear | Prepaid health plan, general/psychiatric hospitals | History of affective disorder; one or more children aged 8–14 years, presence of treating professional | Sz; current crisis, substance misuse | 8–14 | 10.9 (2.0); unclear | Unclear | Unclear; unclear | All Hollingshead I-II | Unclear |
| 121 | Uncontrolled | 9 | Australia | 75% Sz, Sz-aff, BiP | Depression-anxiety | 1–2 years; unclear | Child intervention programme | 6–18 years; parent or sibling with schizophrenia, bipolar disorder, depression | Unclear | 7–12 | Unclear; 56% | Unclear | Unclear; 75% | Unclear; unclear | 77% Lebanese, Aboriginal, Cambodian |
| 122 | Uncontrolled | 33 | Canada | 72% BiP, Sz, PD | MDD | Unclear; 54.5% | Child/adolescent intervention programme, referred by mental health workers, social workers, school counsellors | Eligible for child coping initiative; aged 8–13 years; at least one parent with mental illness | Unclear | 7–14 | 9.73 (2.02); 33% | 57.6% | Unclear; unclear | Unclear; unclear | 15% Asian-Canadian |
| Study | Design | Comparator | Intervention model(s) | Primary objective(s) | Content description(s) | Target | Setting | Delivery; format | Personnel | Intervention monitoring | Session length; frequency | Total duration; total scheduled contact |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 117 | RCT | TAU | Extended care | Parent well-being, parenting | Home-nurse visitation, parenting observation/discussion, community referrals | Parent | Home | Face to face; individual | MH nurse | Specialist training | 1–1.5 hours; weekly | 1–2 years; approximately 104 hours |
| 60 | RCT | TAU | Psychotherapy 1 | Child well-being | CBT problem solving, supportive therapy | Child | Clinic | Face to face; group | Unclear | Unclear | 1 hour; weekly | 16 weeks; 16 hours |
| Psychotherapy 2 | Parenting | Supportive therapy with parenting focus based on social learning theory | Parent | Clinic | Face to face; group | Unclear | Unclear | 1 hour; weekly | 16 weeks; 16 hours | |||
| Psychoeducation | Parenting | Generic parenting education | Parent | Clinic | Face to face; group | Unclear | Unclear | 1 hour; weekly | 16 weeks; 16 hours | |||
| 61 | RCT | TAU | Psychotherapy | Child well-being | CBT problem solving | Child | Clinic | Face to face; group | Social worker/mixed | Specialist training | 1 hour; weekly | 12 weeks; 12 hours |
| 58 | nRCT | Waiting list | Psychoeducation | Child well-being | Psychoeducation, peer support | Child | Community | Face to face; group | MH clinician | Unclear | 6 hours; fortnightly | 6 weeks; 18 hours |
| 119 | nRCT | Waiting list | Psychotherapy | Parent well-being | Conversational model PDT/IPT | Parent | Unclear | Face to face; individual | Trainee psychotherapist qualified in medicine or psychiatry | Specialist training; supervision with audiotapes | 50 minutes; twice weekly | 1 year; approximately 86 hours |
| 59 | nRCT | Active | Psychoeducation 1 | Child well-being | Psychoeducation, peer support | Child | Unclear | Face to face; group | Unclear | Unclear | 1 day; daily | 4 days; approximately 24 hours |
| Psychoeducation 2 | Child well-being | Psychoeducation, peer support | Child | Unclear | Face to face; group | Unclear | Unclear | 2 hours; unclear | One school term approximately 12 weeks, unclear | |||
| 118 | nRCT | Active | Extended care 1 | Parent well-being, parenting | Home-nurse visitation, parenting role-modelling, community referrals | Parent | Home | Face to face; individual | Nurse/social worker | Specialist training | Unclear; weekly | 1 year; unclear |
| Extended care 2 | Parent well-being, child well-being, parenting | Social/occupational rehabilitation, MH care, parenting, community agency referrals, nursery | Parent–child | Community | Face to face; individual and group | Therapists; nursery nurse | Unclear | Up to 1 day; 4 days per week | 1 year; approximately 208 hours |
| Study | Design | Comparator | Intervention model(s) | Primary objective(s) | Content description(s) | Target | Setting | Delivery; format | Personnel | Intervention monitoring | Session length; frequency | Total duration; total scheduled contact |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 120 | Uncontrolled | – | Extended care | Parenting and child well-being | Social/occupational rehabilitation, one-to-one meetings, events | Parent | Community | Face to face; individual and group | Unclear | Unclear | Unlimited; unlimited | Unclear; unclear |
| 62 | Uncontrolled | – | Psychotherapy | Parenting | Clinician-faciliated psychoeducation, individualised family plan | Parent(s)–child(ren) | Clinic | Face to face; individual family | Clinician | Specialist training, ongoing supervision | Unclear; unclear | 8–10 sessions; unclear |
| 121 | Uncontrolled | – | Psychoeducation | Child well-being | Psychoeducation, peer support | Child | Community | Face to face; group | Counsellors | Delivered by programme developer | 6 hours; daily | 3 days; 18 hours |
| 122 | Uncontrolled | – | Psychoeducation | Child well-being | Psychoeducation, peer support | Child | Unclear | Face to face; group | Masters level (various backgrounds), MH workers | Training, manualised intervention | 90 minutes; weekly | 8 weeks plus follow-up session; approximately 13.5 hours |
| Study | Design | n | Method; unit of allocation | Allocation concealment | Participant; personnel blinding | Outcome type(s) | Assessor blinding; inter-rater reliability | A priori primary outcome | All outcomes reported | Attrition post intervention; last follow-up | Reasons for attrition | Power calculation | Method of analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 117 | RCT | 50a | Unclear states random; parent | Not reported | No; no | Parent report Nurse reported Non-specified | – no; NA, – | No | No | Unclear; NA | Unclear | No | Completers only |
| 60 | RCT | 53b | Sequential; parent–child | Not reported | No; no | Parent report Teacher-report Non-specified | – no; NA, – | No | No | 25%; NA | 11 dropped out, 1 lost custody, 2 moved away; 7 did not attend; 2 missing data | No | Completers only |
| 61 | RCT | 41c | Unclear states random; child | Not reported | No; no | Self-report Researcher-assessed Non-specified | – no; NA – | No | Assume so, no protocol | 5% | 2 moved away | No | Completers only |
| 58 | nRCT | 44 | Service availability; child | – | No; no | Child-report | – | No | Yes | 11% | 5 no reasons given | No | ITT with imputed data |
| 119 | nRCT | 45 | Service availability; parent | – | No; no | Parent-report | – | No | Yes | 0% | NA | No | Complete data set |
| 59 | nRCT | 129 | Treatment attendance; child | – | No; no | Self-report; psychologist assessed | –, no; NA, – | No | No | 47% | Sample limited to pre–post data; reasons for attrition not given | No | Completers only |
| 118 | nRCT | 83 | Random limited by service availability; parent | Not reported | No; no | Parent-report; non-specified | – | No | No | 49%; unclear | 23 lost custody, moved away or were hospitalised; 18 missing data | No | Completers only |
| Study | Random-sequence generation (selection bias) | Allocation concealment (selection bias) | Participant/personnel blinding (performance bias) | Assessor blinding: patient reported outcomes (detection bias) | Assessor blinding: observer reported outcomes (detection bias) | Selective reporting (reporting bias) | Incomplete post intervention outcome data (attrition bias) | Overall risk of biasa |
|---|---|---|---|---|---|---|---|---|
| 117 | ? | ? | + | + | + | + | ? | + |
| 60 | + | ? | + | + | + | + | ? | + |
| 61 | ? | ? | + | + | + | ? | – | ? |
| 58 | + | + | + | + | + | – | + | + |
| 119 | + | + | + | + | + | – | – | + |
| 59 | + | + | + | + | + | – | + | + |
| 118 | + | + | + | + | + | + | ? | + |
| Study | Design | Number of trial arms | Number of eligible arms | Arms excluded from analysis | Intervention(s)/comparator(s) | Follow-up (post allocation) | Relevant continuous outcomes | Dichotomous data used for standardised ES | Full/partial outcome reporting | Summary of findingsa/authors’ narrative |
|---|---|---|---|---|---|---|---|---|---|---|
| 117 | RCT | 3 | 2 | Healthy controls | Parent home, nurse visitation: 1–2 years, weekly home visits, parenting observation, illness experience, community MH referrals | 12–24 months | Child affect ratings, child psychiatric evaluation, child behaviour and social functioning rating, parent hospitalisations, parent MMPI, nurse parental symptom ratings, nurse reports of family conflict, maternal social role performance (child interaction), child IQ | – | No data for child affect, social function, nurse reports of family function or parents’ symptoms, maternal social role performance, child IQ | Number of parent rehospitalisations (standardised ES 0.08, 95% CI −0.56 to 0.71). Authors report no significant differences in family conflict resolution, greater reduction in nurses’ parental symptoms assessments (p < 0.02) |
| TAU: minimal home treatment | ||||||||||
| 60 | RCT | 4 | 4 | – | Child CBT: 16 weekly group sessions of CBT/problem-solving | 16 weeks | Child SAS; CPQ; DESBRS; parent SCL-90; parent SAS; child IQ | – | No data for child SAS, CPQ, DESBRS, parent SAS, child IQ | Supportive therapy associated with lower family conflict in 5- to 7-year-olds (β = −0.59, p < 0.005), parenting education associated with lower family conflict in 8- to 12-year-olds (β = −0.41, p < 0.05). Group allocation not a significant predictor of parental mental health |
| Parent ST: 16 weeks’ supportive group therapy with parenting focus based on social learning theory | ||||||||||
| Parent Education: generic parenting advice | ||||||||||
| TAU: no details given | ||||||||||
| 61 | RCT | 2 | 2 | – | Child CBT: 12 weekly group sessions’ CBT/problem-solving | 12 weeks | CPQ; DESBRS; Child IQ; MEPS; SPSSA | – | Full | CPQ 5–7 years (standardised ES −0.10, 95% CI −1.15 to 0.94); 8–12 years (standardised ES −0.06, 95% CI −0.85 to 0.73); IQ 5–7 years (standardised ES −0.35, 95% CI −1.40 to 0.71); 8–12 years (standardised ES −0.89, 95% CI −1.72 to −0.07); SPSSA 5–7 years (standardised ES 0.89, 95% CI −0.11 to 2.11); MEPS 8–12 years (standardised ES 1.00, 95% CI 0.51 to 2.27) |
| TAU: no details given | ||||||||||
| 58 | nRCT | 2 | 2 | – | Child psychoeducation: 3 days’ group-based education, peer support, respite | 4 weeks | SLS; CDI; SDQ; SCS; YCOPI; RS-FV; mental health literacy rating; self-esteem rating | – | No comparison data for self-esteem outcomes | SLS (standardised ES −0.18, 95% CI −0.82 to 0.46); CDI (standardised ES −0.35, 95% CI −1.00 to 0.29); SDQ prosocial (standardised ES 0.32, 95% CI −0.33 to 0.96); SCS (standardised ES −0.30, 95% CI −1.87 to 1.27); YCOPI activity (standardised ES −0.47, 95% CI −1.11 to 0.18); RS-FV (standardised ES 0.07, 95% CI −0.71 to 0.57); mental health literacy (standardised ES 0.78, 95% CI 0.11 to 1.44) |
| Waiting list: programme waiting list | ||||||||||
| 119 | nRCT | 2 | 2 | – | Parent therapy: 1 year of individual therapy based on the conversational model (IPT/PD) | 12 months | SAS (family, child subscales) | – | Full | SAS family (standardised ES 0.46, 95% CI –0.22 to 1.15); SAS child (0.73, 95% CI 0.03 to 1.42) |
| Waiting list: therapy waiting list, with treatment as usual by referring clinician | ||||||||||
| 59 | nRCT | 2 | 2 | – | Child psychoeducation:b after-school programme offering one term of group-based education, peer support, respite | 4-weeks post completion | KIDS problems; KIDS connections; KIDS coping; RSES | – | Full | KIDS connections (standardised ES 0.03, 95% CI −0.51 to 0.46); KIDS problems (standardised ES 0.29, 95% CI −0.21 to 0.80); KIDS coping (standardised ES 0.24, 95% CI −0.26 to 0.73); RSES (standardised ES 0.38, 95% CI −0.10 to 0.85) |
| Child psychoeducation: school holiday programme offering 4 days’ group-based education, peer support, respite | ||||||||||
| 118 | nRCT | 2 | 2 | – | Parent home-nurse visitation: 1–2 years of weekly home visits, parenting observation, illness experience, community MH referrals | 12 months, 24 months | HPPSAC; MAS; B/SB; | – | HPPSAC; MAS; B/SB | Authors report no significant differences on HPPSAC, MAS; B/SB |
| Parent–child multiagency care: social/occupational rehabilitation, parenting, MH care, referrals, developmental nursery, social events |
| Primary outcomes | Instrument used | RCT | nRCT | Usual care | Total | Study number |
|---|---|---|---|---|---|---|
| Quality of life | Satisfaction with life scale | – | 1 | – | 1 | (58) |
| Emotional well-being | Children's depression inventory | – | 1 | – | 1 | (58) |
| Child affect rating, unspecified | 1 | – | – | 1 | (117) | |
| Child psychiatric evaluation, unspecified | 1 | – | – | 1 | (117) | |
| Secondary outcomes | ||||||
| Physical health | None | – | – | – | – | – |
| Safety | None | – | – | – | – | – |
| Social function/behaviour | Strengths and difficulties questionnaire | – | 1 | – | 1 | (58) |
| Harvard preschool project social abilities checklist | 2 | 1 | – | 1 | (118) | |
| Conners parent questionnaire | 2 | – | – | – | (60) (61) | |
| Devereux elementary school behaviour rating scale | 1 | – | – | – | (60) (61) | |
| Social adjustment scale | 1 | – | – | – | (60) | |
| Child behaviour rating, unspecified | 1 | – | – | – | (117) | |
| Child social functioning rating, unspecified | – | – | – | – | (117) | |
| Social relationship quality | Social connectedness scale | – | 1 | – | 1 | (58) |
| KIDS connections scale | – | 1 | – | 1 | (59) | |
| KIDS problems scale | – | 1 | – | 1 | (59) | |
| Recreational engagement | YCOPI activity restriction subscale | – | 1 | – | 1 | (58) |
| Parental mental health symptoms | Number and length of hospitalisations | 1 | – | 1 | 1 | (117) (120) |
| MMPI subscales | 1 | – | 1 | 1 | (117) | |
| Nurse symptom report | 1 | – | – | 1 | (117) | |
| SCL-90 Global Severity Index | 1 | – | – | 1 | (60) | |
| Self-reported improvement | – | – | – | – | (120) | |
| Family function/conflict | Nurse report of resolution of family conflicts | 1 | – | – | 1 | (117) |
| Social adjustment scale, family unit subscale | – | 1 | – | 1 | (119) | |
| Parent–child interaction | Social adjustment scale, child subscale | – | 1 | – | 1 | (119) |
| Maternal attitudes scale | 1 | 1 | 1 | 1 | (118) | |
| Social adjustment scale, parent subscale | 1 | – | – | 1 | (60) | |
| Mothers social role performance interview | – | – | – | 1 | (117) | |
| General parent interview | – | – | – | – | (62) | |
| Cognitive development | Bayley Scales/Stanford–Binet | – | 1 | – | 1 | (118) |
| Quick test IQ | 2 | – | – | 2 | (60) (61) | |
| Child intelligence test, unspecified | 1 | – | – | 1 | (117) | |
| Problem-focused coping | Responses to stress, family version | – | 1 | – | 1 | (58) |
| KIDS coping scale | 1 | 1 | 1 | 2 | (59) (122) | |
| Means-ends problem solving | 1 | – | – | 1 | (61) | |
| Social problem-solving situation analysis | – | – | – | 1 | (61) | |
| Mental health literacy | Study-specific Likert scale | – | 1 | 1 | 2 | (58) (121) |
| Other study-specific scale | – | – | 1 | 1 | (122) | |
| Self-esteem | Study-specific Likert scale | – | 1 | 1 | 2 | (58) (121) |
| Rosenberg self-esteem scale | – | 1 | 1 | 1 | (59) | |
| Coopersmith self-esteem scale | – | – | – | 1 | (122) | |
Appendix 8 Classification criteria for severe depression
| Measure | Cut point for severe depression | Rationale | Reference source |
|---|---|---|---|
| BDI-I/BDI-II | ≥ 30/≥ 29 | Published scale with validated cut-off for severe depression129 | (129) |
| 17-item/25-item HRSD | ≥ 25/≥ 28 | Considerable variation in cut-off scores exists, ranging from ≥ 19 to 29.131 UK NICE guidelines recommend ≥ 23 for severe depression but do not stipulate scale versions or provide any empirical source for their recommendations.128 A direct comparison of the 17-item HRSD with Clinical Global Impression Scores suggests a cut point of ≥ 25 adequately differentiates severe from moderate depression.132 Expert and clinical trial consensus suggest cut points ≥ 25 and ≥ 28 for the 17- and 25-item scale, respectively130 | (128) (130)–(132) |
| MADRS | ≥ 31 | A depression screening measure with arbitrary cut offs ranging from 28 to 35.130,133 In the absence of a validated cut point for severe depression, the selected cut-offs were based on expert opinion and consensus from clinical trials.130 Empirical comparisons drawn between MADRS and Clinical Global Impression Scores suggest scores of ≥ 31 adequately differentiate severe from moderate depression. The same cut-off point corresponds to a HRSD 17-item score of ≥ 25132 | (130) (132) (133) |
| CES-D | ≥ 27 | A screening measure developed from five validated depression scales including the BDI. Scores range from 0 to 60, with higher scores indicating more symptoms of depression. Scores of ≥ 16 are accepted as an indicator of clinical depression.134,135 Scores of ≥ 27 are reported to be a more stringent indicator of major depression in medical patients134 | (134) (135) |
| PHQ-9 | ≥ 20 | Published scale with validated cut-off for severe major depression | (136) |
| EPDS | ≥ 20 | Screening measure without a validated cut-off for severe depression. Cut-off scores vary between international studies, with developed settings tending to report higher thresholds than developing countries.138 Scores of ≥ 20 correspond to the 75th quartile of a large UK sample with postnatal depression, 22% of whom were diagnosed as severely depressed by ICD-10 criteria137 | (137) (138) |
Appendix 9 Severe depression study characteristics
| Study | Design, country | n | % severe depression | Recruitment context | Inclusion criteriaa | Exclusion criteria | Diagnosis and method | Severe at baselinea | Other primary diagnoses | MDD history and/or comorbidity |
|---|---|---|---|---|---|---|---|---|---|---|
| 151 | RCT, UK | 87 | 59 | Maternity hospitals | Scoring > 12 CIS-R, RDC major/minor depression disorder | Chronic depression > 2 years, current drug/alcohol abuse, severe illness, breast feeding, non-fluent English, living outside district | MDD RDC, CIS-R | No | Minor depression | 15% history depression |
| 141, 162, 169, 202, 227, 228 | RCT, USA | 105 (37b) | 82 | Prepaid health plan, psychiatric hospital, general medical hospital | Dual- and single-parent families; ≥ 1 child 8–15 years, > 1 parent with mood disorder in last 18 months | Serious current substance abuse, Sz, severe marital/life crisis, current marital/family therapy more than twice monthly. Children with current/past MDD | MDD by RDC, SADS-L | Unclear | BiP, Sz-aff, minor affective disorders | 79% history of mood disorder, most frequently MDD, 54% history of non-affective disorders; substance abuse. 52% men and 76% women MH comorbidities |
| 140 | RCT, USA | 37 | 100 | Hospital admissions | Children 8–13 years, mother hospitalised for depression | Unclear | MDD by DSM-IV | Yes, BDI > 30 | – | Unclear |
| 142 | RCT, USA | 76 | 88 | MH outpatients | > 1 parent with depression, treatment visit in last year | Alcohol/drug misuse, Sz, organic brain damage, active crisis, suicidal, in need of treatment, severely mentally ill children | MDD by SADS-L | No | Minor/intermittent depression | GAD 25.5%, panic 14.5%, phobia 21.1%; substance abuse 60.5% |
| 157 | RCT, France | 258 (60)c | 100c | Obstetric clinics | > 8 EPDS, MDD diagnosis (treatment only) | Current treatment with psychiatrist/psychologist, poor French language skills | MDD by DSM-IV, mini-neuropsychiatric interview | No | – | – |
| 64, 229, 230 | RCT, USA | 131 (70)d | 100 | Community health services | Moderate-high socioeconomic status, DSM-IV MDD post birth, at least high school education, not reliant on public assistance, child approximately 18 months old | Low income | MDD by DSM-III-R | No | – | 12.4% exclusively depression in postpartum; ‘high’ comorbidity rate including anxiety, PTSD, OCD, bulimia, substance use |
| 66 | RCT, USA | 39 | 100 | Health-care providers, media adverts | DSM-IV MDD in postpartum period | – | MDD by DSM-IV | No | – | – |
| 67 | RCT, USA | 32 | 100 | Primary and secondary care services | DSM-IV MDD in postpartum period | – | MDD by DSM-IV interview | No | – | – |
| 143, 168 | RCT, USA | 111 | 100 | Community/MH service advertising | Parent legal guardian, child aged 9–15 years | History of bipolar I, Sz, Sz-aff; mental retardation, conduct disorder, substance/alcohol abuse, children with autism spectrum disorders | MDD by DSM-IV SCID | No | – | Anxiety disorders |
| 152, 163 | RCT, UK | 193 | 100 | Hospital birth records | Primiparous, living within 15-mile radius of maternity hospital, English as first language | Delivery < 36 weeks, gross congenital abnormality, multiple birth, planning to leave area | MDD by DSM-III-R SCID | No data | – | Unclear |
| 144 | RCT, USA | 73 (38)d | 100 | Community mental health agency referrals, advertising | Unclear | Unclear | MDD by DSM-III/III-R; RDC-SADS-L | No | – | 85% ≥ 1 previous episode; mean episode length 9.43 (unclear SD) months |
| 145 | RCT, USA | 53 | 85 | Clinic referrals, research registries, clinic flyers | ≥ 18 years, 10–32 weeks gestation, >12 EPDS, English speaking, telephone access, local area | Substance abuse in last 6 months, suicidal, BiP, Psy., organic mental disorder, unstable medical condition confounding mood assessment, severe intimate partner violence, in receipt of depression treatment | MDD by DSM-IV SCID, Depression Status Inventory | No | Dysthymia minor depression | Median 2.5 prior depression episodes, dysthymia, panic, PTSD, social phobia |
| 153 | RCT, UK | 55 | 68 | Postnatal screening | RDC Depression | Unclear | MDD by RDC interview | No | Minor depression | 42% previous depression |
| 148 | RCT, Australia | 20 | Mean score only | Local hospital, maternal–child health centre advertising | Depression within 6 months postpartum, ≥ 12 EPDS, ≥ 15 BDI | Major psychiatric disorder, insufficient English | MDD, symptoms only | Yes, BDI > 30, EPDS > 20 | – | At least 6 months duration |
| 149 | RCT, Australia | 68 | Mean score only | Maternal–child health centres | > 13 EPDS, infant aged: 6 weeks–4 months | Insufficient English, psychotic symptoms, immediate crisis management | MDD, symptoms only | Yes, BDI-II > 29 | – | Unclear |
| 155 | RCT, Canada | 29 | 100 | Reproductive mental health hospital department | DSM-IV MDD, EPDS > 12 | Unclear | MDD by DSM-IV mini-neuropsychiatric interview | No | – | Eating disorder, anxiety, OCD |
| 156, 161 | RCT, Canada | 35 | 100 | Outpatient referrals to a reproductive mental health program | ≥ 18 HRSD, ≥ 20 HAM-A, ≥ 12 EPDS; 18–40 years old, able to understand English, healthy delivery 37–42 weeks, minimum birth weight of child 2.5 kg, non-smokers, willing to use contraception | Psy., suicidal, substance misuse, receiving psychotherapy or psychotropic medications, unable to be prescribed paroxetine | MDD by DSM-IV | No | – | Anxiety, OCD |
| 150 | RCT, Australia | 57 | 100 | Health professional referrals | DSM-IV criteria MDD, infant 0–12 months | Severe PD, acute Psy., suicidal, significant substance abuse, child abuse or neglect | MDD by DSM-IV, MCMI-III | Borderline BDI-II 28.8 | – | Previous depression 78.3%, mean 16.17 months (24.40) |
| 146, 160, 167 | RCT, USA | 120 (56)d | 100 | Birth records | ≥ 18 years old, married/cohabiting for 6 months, DSM-IV MDE, ≥ 12 on 17-item HRSD | Lifetime history of BiP, Sz, organic brain syndrome, mental retardation, antisocial PD; current alcohol/substance abuse, panic, somatisation disorder, ≥ 3 schizotypal features, Psy., depression, serious eating disorders, OCD | MDD by SCID | No | – | 3 patients chronic depression (episode length > 2.5 years), mean episode length for remaining subjects 7.0 (unclear SD) months |
| 158 | RCT, Pakistan | 903 | 100 | Rural basic health units | Women aged 16–45 years, married, third trimester of pregnancy | Diagnosed medical condition requiring inpatient or outpatient treatment, pregnancy-related illness | MDD by DSM-IV SCID | No | – | Unclear |
| 138 | RCT, Chile | 230 | 100 | Postnatal clinics | Child < 1 year, 10 + EPDS | In receipt of treatment for depression during current postnatal period, pregnant, psychotic symptoms, suicidal risk, history of mania, alcohol/drug abuse | MDD by DSM-IV mini-neuropsychiatric interview | No | – | 35% previous depression |
| 65 | RCT, Australia | 47 | 100 | Health/welfare agencies, preschools, elementary schools | DSM-IV MDD with ≥ 1 child aged 3–9 years meeting DSM-IV conduct disorder/oppositional-defiant disorder, child with no evidence of developmental disability | Child had no evidence of developmental disability | MDD by DSM-IV | No | – | Unclear |
| 63 | RCT, Canada | 44 | 80 | Hospital and community health services | MDD according to referring physician, currently under medical care for depression, child aged 6–13 years | Major psychoses, unable to participate in groups | MDD by DSM-IV CIDI | No | Depression not meeting CIDI MDD criteria | Lifetime MDD episodes: Intervention – 7.8 (5.1), Control – 7.6 (SD 7.6) |
| 147 | RCT, USA | 65 | 100 | Paediatric mental health clinics | 18 – 65 years, DSM-IV MDD, HRSD 17-item ≥ 15, biological/adoptive custodial parent of child age 6–18 years receiving psychiatric treatment for internalising/externalizing disorder | Not living with child, risk of child abuse/neglect, substance abuse in preceding 6 months, suicidal, Psy., BPD, PD, unstable medical condition confounding mood assessment, receiving individual psychotherapy | MDD by DSM-IV SCID | No | – | Duration of current episode: Intervention – 52 (range: 7–184), Control – 37 (range: 4–361). Lifetime/current comorbid anxiety disorder |
| 154 | RCT, UK | 119 | 88 | Community, child health register | BDI > 14, child BSQ score > 7 | Child not living with parent, first language not English, major psychiatric disorder other than depression child with developmental disability | MDD by SCID DSM-IV | No | Dysthymic disorder | Mean number of previous episodes: CBT: 1.9 (SD 1.3) mother and toddler group: 2.4 (SD 1.3) TAU: 2.2 (SD 1.3), current episode length: 29.7 (SD 27.4) months; CBT, 39.8 (SD 48.2) months; mother and toddler group, 42.1 (SD 37.9) months; TAU; 32–39% comorbid diagnoses |
| 159 | RCT, Sweden | 41 | 100 | Child health clinics | DSM-III-R MDD, MADRS > 10 | Unclear | MDD by DSM-III-R | No | – | 37% received counselling for previous depression |
| Study | Target child age range | Child mean (SD) age | Child percentage female | Child percentage colocated | Parent mean (SD) age | Parent% female | Parent education | Parent standardised ES | Parent % BME status |
|---|---|---|---|---|---|---|---|---|---|
| 151 | < 1 year | Unclear | Unclear | Unclear | 25.0 (?) years | 100 | Predominantly higher education | 76% unemployed | Unclear |
| 141, 162, 169, 202, 227, 228 | 8–15 years | Range: 8–14 years | Unclear | Unclear | Unclear | 70 | Unclear | ‘Largely’ middle class | ‘Largely’ Caucasian |
| 140 | 8–13 years | Unclear | 35 | Unclear | Unclear | 100 | Unclear | Unclear | Unclear |
| 142 | 7–12 years | 10.1 (1.6) years | 40 | Unclear | 39.5 (5.6) years | 77 | Unclear | 38% household income of $40,001–65000 (£34,589–56,191)a | 93% white |
| 157 | < 1 year | Unclear | Unclear | Unclear | Intervention: 30.5 (4.3) years; control: 30.5 (?) years (treatment phase) | 100 | Unclear | 25% unemployed | 100% Caucasian |
| 64, 229, 230 | 0–2 years | 20.47 (2.49) months | 50 | Unclear | 31.62 (4.51) years | 100 | Predominantly higher education | 74% Hollingshead's class IV and V | 92% Caucasian |
| 66 | 0–2 years | Mother–infant therapy group: 6.3 (6) months, IPT: 8.2 (7.7) months, waiting list control: 12.8 (7.5) months | 54 | Unclear | Mother–infant therapy group: 27.6 (5.0) years, IPT: 32.4 (4) years, waiting list control: 34.6 (6.8) years | 100 | Predominantly higher education | Mean household income of $32,481 (£26,451.78) | 98% Caucasian |
| 67 | 0–2 years | 7.86 (6.75) months | 63 | Unclear | Intervention: 28.06 (4.40) years; control: 34.46 (6.33) years | 100 | High school | Mean household income of $32,506 (£22,923.88) | 100% white |
| 143, 168 | 9–15 years | Girls: 11.5 (2) years, boys: 11.3 (2) years | 45 | 100 | Mothers: 41.2(6.8) years; fathers: 48.3 (8.2) years | 86 | Predominantly higher education | Median household income of $40,000 (£31,676.30) | 86% Euro-American |
| 152, 163 | < 1 year | Unclear | Unclear | Unclear | Counselling: 28.4 (5.3) years, CBT: 27.9 (5.4) years; PD: 28.1 (5.6) years | 100 | High school | 21% high social disadvantage | Unclear |
| 144 | Pregnancy to < 1 year | Intervention: 6.3 (2.91) months; control: 5.86 (2.8) months | 42 | Unclear | 30.27 (5.42) years | 100 | Predominantly higher education | ‘Most’ middle or lower middle class | 95% Caucasian |
| 145 | < 1 year | Unclear | Unclear | Unclear | 24.3 (5.3) years | 100 | Predominantly higher education | 58% household income of < $10,000 (£6991.17) | 57% African American |
| 153 | < 1 year | 13 (?) weeks | Unclear | Unclear | Intervention: 27.6 (?) years; control: 24.6 (?) years | 100 | Unclear | 48% social class IV or V | Unclear |
| 148 | < 1 year | 10.6 (?) months | Unclear | Unclear | 29.6 (?) years | 100 | Unclear | 83% employed | 100% Australian, Ireland and UK |
| 149 | < 1 year | GP: 17.03 (9.22) weeks; nurse: 14.84 (11.44) weeks; psychologist: 20.68 (9.15) weeks | Unclear | Unclear | GP: 30.0 (3.3) years; nurse: 33.1 (4.4) years; psychologist: 31.4 (5.6) years | 100 | Predominantly higher education | 35% income of between $40,000 and $80,000 (£28,010.61 and £42,015.92) | 88% born in Australia |
| 155 | < 1 year | Control: 5.4 (3.2) months; intervention: 4.9 (3.11) months | 48 | Unclear | Control: 33.5 (4.4) years; intervention: 32.9 (7.2) years | 100 | Predominantly higher education | 45% no paid employment | 92% Caucasian |
| 156, 161 | < 1 year | 5.27 (2.68) months | Unclear | Unclear | CBT and paroxetine: 30.81 (3.31) years; CBT: 29.52 (5.85) years | 100 | High school | 61% no paid employment | 62% white |
| 150 | < 1 year | 6.65 (3.43) months | 48 | Unclear | 32.41 (3.47) years | 100 | Predominantly higher education | Unclear | 86% born in Australia |
| 146, 160, 167 | < 1 year | Unclear | Unclear | Unclear | 29.4 (4.9) years | 100 | Predominantly higher education | 63.3% working | ‘Mostly white’ |
| 158 | Pregnancy to < 1 year | Unclear | Unclear | Unclear | 26.5 (5.2) years | 100 | Primary school or below | 37% in category 3 (Likert scale: 1 = rich 5 = poor) | Unclear |
| 138 | < 1 year | Intervention: 5.4 (2.9) months; control: 6.2 (6.5) months | Unclear | Unclear | Intervention: 26.7(6.4) years; control: 26.6 (7.4) years | 100 | High school | All low income | Unclear |
| 65 | 3–9 years | 4.39 (?) years | 26 | Unclear | Behavioural family intervention: 33.39 (5.29) years; cognitive–behavioural family intervention: 32.29 (5.35) years | Unclear | Unclear | Mean 1.6 on sociodemographic disadvantage Index | Unclear |
| 63 | 6–13 years | Intervention: 10.6 (2.5) years; control: 9.7 (2.7) years | Unclear | Unclear | Intervention: 41.3 (6.0) years; control: 40.8 (6.8) years | 91 | Predominantly higher education | 50% middle class | Unclear |
| 147 | 6–18 years | Intervention: 13.7 (3.5) years; control: 13.9 (3.3) years | 76 | 100 | Intervention: 41.6 (8.7) years; control: 44.2 (7.6) years | 100 | High school | 48% household income between $15,000 and $50,000 (£10,582.91 and £35,276.37) | 80% Caucasian |
| 154 | 2.5–4 years | CBT: 38.1 (6.4) months; group: 36.4 (4.7) months; control: 36.6 (5.9) months | Unclear | 100 | CBT: 28.7 (5.02) years; group: 30.8 (5.53) years, control: 30.1 (6.39) years | 100 | High school | 83% not working | Unclear |
| 159 | < 1 year | Unclear | Unclear | Unclear | Intervention: 21.2 (?)years; control: 29.5 (?)years | 100 | Unclear | Unclear | Unclear |
| Study | Design, country | n | Percentage severe depression | Recruitment context | Inclusion criteriaa | Exclusion criteria | Diagnosis and method | Severe at baselinea | Other primary diagnoses | MDD history and/or comorbidity |
|---|---|---|---|---|---|---|---|---|---|---|
| 166 | nRCT, USA | 118 | 100 | Home visiting programme | First time mothers having ≥ 1 risk characteristics: unmarried, low income or inadequate prenatal care, > 20 BDI-II | Mental retardation, neurological disease, substance dependence, receiving psychotherapy, suicidal/homicidal | MDD by DSM | Borderline BDI-II 28.9 | – | – |
| 139, 172 | nRCT, UK | 60 | 93 | Day hospital referrals, community screening | Infant aged 6 weeks–1 year, EPDS > 12, RDC minor/major depression | Puerperal Psy., Sz, drug, alcohol abuse, non-English speaker | MDD by RDC via CIS | No | Minor depressive disorder | Previous psychiatric history 33%, previous PND 2% |
| 170 | nRCT, USA | 23 | 100 | Outpatient mental health services | MDD by clinical interview, BDI > 25, HRSD-17 > 14, age 18–50 years, delivery of a healthy infant in last 6 months, use of birth control | Current anxiety disorder, current/past BiP, Psy., substance abuse, anorexia/bulimia nervosa in past year, suicidal or homicidal risk, current treatment with adequate dose of antidepressant or psychotropic medication, current psychotherapy, significant medical problems suggesting contraindication to Sertraline | MDD by DSM clinical interview | No | – | 17% comorbid axis I disorder, 30% previous MDD, 9% previous PND |
| 171 | nRCT, UK | 40 | 100 | Maternity hospitals | DSM-III-R MDD | Unclear | MDD by DSM-III-R | No data | Unclear | Unclear |
| Study | Target child age range | Child mean (SD) age | Child percentage female | Child percentage colocated | Parent mean (SD) age | Parent % female | Parent education | Parent standardised ES | Parent % BME status |
|---|---|---|---|---|---|---|---|---|---|
| 166 | 0–2 years | Unclear | Unclear | Unclear | Intervention: 22.6 (5.0) years; control: 20.2 (4.2) years | 100 | Predominantly high school | 90% ‘low income’ | 57% Caucasian |
| 139, 172 | < 1 year | Intervention: 10.4 (10) weeks; control: 12 (12) weeks | Unclear | Unclear | Intervention: 28.4 (4.5) years; control: 26.9 (5) years | 100 | Unclear | 65% class III | Unclear |
| 170 | < 1 year | 8.52 (5.6) weeks | Unclear | Unclear | 28.5 (6.01) years | 100 | Predominantly high school | Unclear | 78% white |
| 171 | < 1 year | Unclear | Unclear | Unclear | Unclear | 100 | Unclear | Unclear | Unclear |
| Study | Design, country | n | Percentage severe depression | Recruitment context | Inclusion criteriaa | Exclusion criteria | Diagnosis and method | Severe at baselinea | Other primary diagnoses | MDD history and/or comorbidity |
|---|---|---|---|---|---|---|---|---|---|---|
| 173 | Uncontrolled, USA | 26 | 100 | Community- based home visiting programme | DSM-IV MDD diagnosis, BDI > 20 | Recent medication, primary disorder other than MDD | MDD by PRIME-MD DSM-IV | Yes, BDI-II > 29 | – | 62% comorbid including mood disorder, anxiety disorder, alcohol misuse, eating disorder, somatoform disorder |
| 174 | Uncontrolled, UK | 8 | 100 | Health visitor postnatal screening | Health visitor referral, infant aged < 18 months | Unclear | MDD by DSM-IV | No | – | Unclear |
| 181 | Uncontrolled, UK | 11 | 100 | Community mental health team | GP depression referral, BDI-II > 13, child aged 2–7 years, ECBI > 127 or ECBI problem > 11 | History of SMI, primary anxiety diagnosis | MDD by DSM-IV | No | – | 67% past history depression, mean episode length 6.4 months (3.59 months) |
| 175 | Uncontrolled, New Zealand | 45 | 61/55b | Maternal mental health service | Psychologically stable, able to cope cognitively, able to benefit from group therapy, baby old enough to leave in nurse respite care. | Psychotic, suicidal, manic, socially phobic | MDD by DSM-II-R | No | BiP, BPD, anxiety disorders, substance abuse | Unclear |
| 176 | Uncontrolled, USA | 12 | 83 | Obstetric/gynaecology clinic | ≥ 18 years, 12–28 weeks’ gestation, EPDS > 10 | Current treatment for Depression comorbid psychotic disorder, organic mental disorder, substance abuse in the past 6 months, suicidal, concurrent medical condition compounding symptoms, aggressive partner relationship | MDD by DSM-IV SCID | No | – | Current: GAD, panic, phobia; lifetime: substance misuse, depression disorder, dysthymic disorder, panic |
| 182 | Uncontrolled, Australia | 17 | 88 | Maternal mental health services | Depression disorder within 6 months childbirth, HRSD-21 > 13 | Substance dependence, suicidal, Psy. | MDD by DSM-IV SCID | No | Dysthymia | Dysthymia |
| 180, 231–233 | Uncontrolled, Australia/Canada | 25 | 64 | Chid coping initiative | Aged 8–16 years with parent/sibling with mental health problem | Unclear | NA: child initiative | No data | BiP, Sz | Agoraphobia, anxiety, PTSD |
| 177 | Uncontrolled, Australia | 18 | 100 | Community services, maternal–child health services | Infant 0–12 months, DSM-IV MDD, EPDS > 12, health professional involvement | Severe personality disorder, acute psychosis, significant substance abuse, child abuse or neglect | MDD by DSM-IV | No | – | 67% past history depression, mean episode length 6.4 months (3.59 months) |
| 183 | Uncontrolled, USA | 9 | 100 | Obstetrician referrals, community screening | 2–6 months postpartum meeting DSM-II-R MDD | Unclear | MDD by DSM-II-R | No | - | Unclear |
| 178 | Uncontrolled, USA | 13 | 100 | Clinic specialising in the treatment of suicidal adolescents | Child 6–18 years with DSM-IV unipolar depression in treatment, IQ > 75 percentile, not participating in research | No child custody, not living with child, at risk for child abuse/neglect, substance abuse in past 6 months, currently suicidal, psychotic disorder, current individual psychotherapy | MDD by DSM-IV SCID | No | – | Current: GAD, panic, phobia; lifetime: substance misuse, depression disorder, dysthymic disorder, panic |
| 179 | Uncontrolled, USA | 12 | 93 | Treatment services for childhood depression | Biological mothers ≥ 18 years meeting DSM-IV depression, residing with child for > 1 year, fluent in English or Spanish | Substance misuse, suicide risk, current depression treatment, medical condition causing depression | MDD by DSM-IV SCID | No | Dysthymia | Dysthymia |
| Study | Target child age range | Child mean (SD) age | Child percentage female | Child percentage co-located | Parent mean age (SD) | Parent percentage female | Parent education; | Parent standardised ES | Parent % BME status |
|---|---|---|---|---|---|---|---|---|---|
| 173 | 0–3 months | Unclear | Unclear | Unclear | 23.0 years | 100 | Unclear | 46% household income of between $9,001 and $20,000 (£6,897.80 and £15,326.74) | 46% Caucasian |
| 174 | < 1.5 years | Range: 4–18 months | Unclear | Unclear | Unclear | 100 | Unclear | Unclear | Unclear |
| 181 | 2–7 years | 43.4 (15.3) months | Unclear | Unclear | 29.6 (4.65) years | 100 | Did not complete high school | 45% experiencing multistressed environments | Unclear |
| 175 | < 1 year | Unclear | Unclear | Unclear | 32.4 (?) years | 100 | Unclear | Unclear | 87% European ethnicity |
| 176 | < 1 year | Parents recruited at mean 19.5 (8.2) weeks’ gestation | Unclear | Unclear | 25.3 (6.5) years | 100 | Predominantly higher education | 58% household income of < $10,000 (< £6991.17) | 75% African-American |
| 182 | < 1 year | 19.0 (12.9) weeks | Unclear | Unclear | 32.0 (range 27–41) years | 100 | Unclear | 88% employed | 100% Caucasian |
| 180, 231, 232, 233 | 8–16 years | 10.8 (2.0) years | 66 | Unclear | Unclear | 72 | Unclear | Unclear | Unclear |
| 177 | < 1 year | 6.2 (2.2) months | Unclear | Unclear | 31.8 (6.2) years | 100 | Unclear | 31.6% employed | Unclear |
| 183 | < 1 year | Range: 2–6 months | Unclear | Unclear | Unclear | 100 | Predominantly higher education | 100% working at least part time | Unclear |
| 178 | 12–18 years | Unclear | Unclear | 100 | 45.3 (8.9) years | 100 | Predominantly higher education | Unclear | 77% white |
| 179 | 6–18 years | 14.1 years | 67 | 100 | 42.0 (6.1) years | 100 | Predominantly high school | 60% household income < $10,000 (< £7919.08) | 67% Hispanic |
| Study | Comparison | Intervention model | Intervention objective | Intervention content | Target | Setting | Personnel | Delivery | Format | Session number; timing | Session length | Total scheduled contact | Training; supervision | Total duration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 151 | Active | Psychotherapy | Parent well-being | Fluoxetine and six CBT counselling sessions | Parent | Community | Psychologist with no clinical training | Face to face | Individual | Six sessions over 12 weeks | 1 hour then 30 minutes | 3.5 hours | Yes; yes | 12 weeks |
| 151 | – | Psychotherapy | Parent well-being | Fluoxetine and one CBT counselling session | Parent | Community | Psychologist with no clinical training | Face to face | Individual | One session | 1 hour | 1 hour | Yes; yes | 12 weeks |
| 151 | – | Psychotherapy | Parent well-being | Placebo and six CBT counselling sessions | Parent | Community | Psychologist with no clinical training | Face to face | Individual | Six sessions over 12 weeks | 1 hour then 30 minutes | 3.5 hours | Yes; yes | 12 weeks |
| 151 | – | Psychotherapy | Parent well-being | Placebo and one CBT counselling session | Parent | Community | Psychologist with no clinical training | Face to face | Individual | One session | 1 hour | 3.5 hours | Yes; yes | 12 weeks |
| 141, 162, 169, 202, 227, 228 | Active | Psychotherapy | Parenting | Psychoeducation and individualised family plan | Parent and child | Unclear | Clinician | Face to face and telephone support | Individual family | 6–10 sessions, frequency unclear | Unclear | Unclear | Yes; yes, manualised | 6–10 weeks |
| 141, 162, 169, 202, 227, 228 | – | Psychoeducation | Parenting | Lecture | Parent | Unclear | Unclear | Face to face | Group | Two lectures | 1 hour | 2 hours | Manualised | Unclear |
| 140 | Waiting list | Psychotherapy | Child well-being | CBT problem solving, peer support | Child | Community | Social work backgrounds | Face to face | Group | Six weekly sessions | 90 minutes | 9 hours | Unclear; unclear | 6 weeks |
| 142 | Waiting list | Psychoeducation | Parenting | Videotaped psychoeducation | Parent and child | Home | Created by psychologists/psychiatrists | Video | Individual | Unclear | 50 minutes | 50 minutes | NA; unclear | 50 minutes |
| 157 | TAU | Psychotherapy | Parent well-being | Mixed CBT PD | Parent | Home | Psychology masters students | Face to face | Individual | 5–8 weekly sessions | 1 hour | Approximately 6.5 hours | Yes; yes, manualised | 5–8 weeks |
| 64, 229, 230 | TAU | Psychotherapy | Parenting | Mother toddler psychotherapy (attachment theory) | Parent (child play only) | Home | PhD or masters-level students | Face to face | Individual | Mean of 45 weekly sessions | Unclear | Unclear | Yes; yes | Average of 45 weeks |
| 66 | Waiting list, active | Psychotherapy | Parent well-being | IPT | Parent | Community | Psychologists, social workers, psychology interns, post doc fellows with 2 years’ clinical experience | Face to face | Group | 12 weekly sessions and initial evaluation | 1.5–2 hours | 18 hours and 1.5 evaluation | Yes; unclear, manualised | 12 weeks |
| 66 | Waiting list | Psychotherapy | Parenting and parent well-being | Mother–infant therapy | Parent and child | Community | Psychologists/psychiatry residents, psychology interns, child development trainees | Face to face | Group | 12 weekly sessions | 2 hours | 24 hours | Yes; yes | 12 weeks |
| 67 | TAU | Psychotherapy | Parenting and parent well-being | Mother–infant therapy | Parent and child | Community | Psychologists/psychiatry residents, psychology interns, child development trainees | Face to face | Group | 12 weekly sessions | 2 hours | 24 hours | Yes; yes | 12 weeks |
| 143, 168 | Active | Psychoeducation | Parenting | Written materials | Parent and child | Home and community | Clinical social workers, clinical psychology students | Face to face | Group | Eight weekly and four monthly sessions | Unclear | Unclear | Yes; yes | 6 months |
| 143, 168 | – | Psychotherapy | Parenting and child well-being | Family CBT | Parent and child | Home and community | Clinical social workers, clinical psychology students | Face to face | Group | Eight weekly and four monthly sessions | Unclear | Unclear | Yes; yes | 6 months |
| 152, 163 | TAU, active | Psychotherapy | Parent well-being | CBT | Parent | Home | CBT specialists and non-specialists | Face to face | Individual | 10 weekly sessions | Unclear | Unclear | Yes; yes | 10 weeks |
| – | Psychotherapy | Parent well-being | Psychodynamic therapy | Parent | Home | CBT specialists and non-specialists | Face to face | Individual | 10 weekly sessions | Unclear | Unclear | Yes; yes | 10 weeks | |
| – | Psychotherapy | Parent well-being | Non-directive counselling (ST) | Parent | Home | CBT specialists and non-specialists | Face to face | Individual | 10 weekly sessions | Unclear | Unclear | Yes; yes | 10 weeks | |
| 144 | TAU | Extended care | Parenting and parent well-being | Home visiting, parenting, signposting, referrals, advocacy | Parent (child play only) | Home | Public health nurses | Face to face | Individual | 25–29 sessions at 1–3 week intervals | Unclear | Unclear | Unclear; yes | 1 year |
| 145 | TAU | Psychotherapy | Parent well-being | Brief IPT | Parent | Clinic | Doctoral/masters-level clinicians | Face to face or telephone | Individual | Eight weekly then bi-weekly/monthly maintenance | Unclear | Unclear | Yes; yes, manualised | 8 weeks and ad hoc follow up |
| 153 | TAU | Psychotherapy | Parent well-being | Home counselling | Parent | Home | Health visitor | Face to face | Individual | Eight weekly | 30 minutes | 4 hours | Yes; unclear | 8 weeks |
| 148 | Waiting list | Psychotherapy | Parent well-being | CBT | Parent | Clinic | Clinical psychologist | Face to face | Group | 10 weekly sessions | 1.5 hours | 15 hours | Unclear; unclear | 10 weeks |
| 149 | TAU, active | Psychotherapy | Parent well-being | CBT, nurse delivered | Parent | Clinic | Nurses | Face to face | Individual | Six weekly | Unclear | Unclear | Yes; unclear | 6 weeks |
| 149 | – | Psychotherapy | Parent well-being | CBT, psychologist delivered | Parent | Clinic | Psychologist | Face to face | Individual | Six weekly | Unclear | Unclear | Yes; unclear | 6 weeks |
| 155 | Active | Psychoeducation | Parent well-being | Psychoeducation with partner involvement | Parent | Unclear | Unclear | Face to face | Individual | Six weekly then monthly follow-ups | Unclear | Unclear | Unclear; unclear | 10 weeks |
| 155 | – | Psychoeducation | Parent well-being | Psychoeducation without partner involvement | Parent | Unclear | Unclear | Face to face | Individual | Six weekly then 1-month follow-up | Unclear | Unclear | Unclear; unclear | 10 weeks |
| 156, 161 | TAU | Psychotherapy | Parent well-being | CBT + paroxetine | Parent | Clinic | Psychologist | Face to face | Individual | 12 weekly | 1 hour | 12 hours | Yes; unclear, manualised | 12 weeks |
| 150 | TAU | Psychotherapy | Parent well-being | IPT | Parent | Community | Unclear | Face to face | Group | Unclear | 2 hours | 22 hours | Yes; yes | 8 weeks |
| 146, 160, 167 | Waiting list | Psychotherapy | Parent well-being | IPT | Parent | Unclear | Psychotherapists with clinical/counselling psychology degrees | Face to face | Individual | 12 weekly | 1 hour | 12 hours | Yes; yes | 12 weeks |
| 158 | TAU | Psychotherapy | Parent well-being | CBT techniques | Parent | Home | Community health workers | Face to face | Individual | Seven weekly and monthly thereafter | Unclear | Unclear | Yes; yes, manualised | 11 months |
| 138 | TAU | Psychotherapy | Parent well-being | Brief CBT, education | Parent | Clinic | Midwives or nurses | Face to face | Group | Eight weekly | 50 minutes | 6 hours 40 minutes | Yes; yes | 8 weeks |
| 65 | Active | Psychotherapy | Parenting | BT | Parent | Clinic and home | Trainee in clinical psychology | Face to face | Individual family | Timing unclear, eight clinic and four home visits | Clinic: 90 minutes, home visit: 40 minutes | 14.66 hours | Yes; yes, manualised | 3–5 months |
| 65 | – | Psychotherapy | Parenting and parent well-being | CBT | Parent (child play only) | Clinic and home | Trainee in clinical psychology | Face to face | Individual family | Timing unclear, eight clinic and four home visits | Clinic: 90 minutes, home visit: 40 minutes | 16 hours | Yes; yes, manualised | 3–5 months |
| 63 | TAU | Psychoeducation | Parenting | Family psychoeducation | Parent (child play only) | Unclear | Nurses, social worker, psychology degrees | Face to face | Group | Eight weekly | 2 hours | 16 hours | Yes; manualised | 8 weeks |
| 147 | TAU | Psychotherapy | Parent well-being | Brief IPT | Parent | Clinic | Masters/doctoral degrees in social work, nursing, psychology, or medicine (psychiatry) | Face to face | Individual | Nine sessions | Unclear | Unclear | Unclear; yes | Unclear |
| 154 | TAU, active | Psychotherapy | Parenting | CBT | Parent (child play only) | Community | Clinical psychologist and nursery nurse | Face to face | Group | 16 weekly | 90 minutes | 24 hours | Yes; yes | 16 weeks |
| 154 | – | Psychosocial | Parenting | Mother and toddler group | Parent (child play only) | Community | Health visitor, psychologist | Face to face | Group | 16 weekly | 90 minutes | 24 hours | Unclear; unclear | 16 weeks |
| 159 | TAU | Psychotherapy | Parent well-being | ST counselling | Parent | Clinic and home | Nurses | Face to face | Individual | Six weekly | 1 hour | 6 hours | Yes; yes | 6 weeks |
| Study | Intervention model | Intervention objective | Intervention content | Target | Setting | Personnel | Delivery | Format | Session number, timing | Session length | Total scheduled contact | Training | Supervision | Total duration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 166 | Psychotherapy vs. TAU | Parent well-being | CBT, home visiting | Parent | Home | Masters-level social worker | Face to face | Individual | 15 weekly and booster 1 month from completion | 60 minutes | 16 hours | Unclear but manualised | Yes | 15 weeks |
| 139, 172 | Extended care vs. TAU | Parent well-being | Counselling, group, creative therapy | Parent | Community | MDT | Face to face | Both individual and group | 2–45 sessions | 1 hour | Not scheduled | Unclear | Unclear | Unclear |
| 170 | Psychotherapy vs. TAU | Parent well-being | IPT | Parent | Unclear | Psychotherapist | Face to face | Individual | 12 sessions | 50 minutes | 10 hours | Yes | Yes | Unclear |
| 171 | Psychotherapy vs. TAU | Parent well-being | Counselling, CBT techniques | Parent | Home | Health visitor | Face to face | Individual | Eight weekly sessions, maximum | 1 hour | 8 hours | Unclear | Unclear | 8 weeks |
| Study | Intervention model | Intervention objective | Intervention content | Target | Setting | Personnel | Delivery | Format | Session number, timing | Session length | Total scheduled contact | Training | Supervision | Total duration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 173 | Psychotherapy | Parent well-being | CBT | Parent | Home | Masters-level social worker | Face to face | Group | 17 weekly plus 1 month booster | 1 hour | 18 hours | Yes | Unclear | 17 weeks plus booster |
| 174 | Psychotherapy | Parent well-being | CBT | Parent | Community | Health visitor | Face to face | Group | 12 weekly and 6 week reunion | 90 minutes | 18 hours | Yes | Unclear | 12 weeks |
| 181 | Psychotherapy | Parent well-being | CBT | Parent | Clinic/home | Unclear | Face to face | Individual | Eight sessions | 1 hour | 8 hours | Unclear | Unclear | Unclear |
| 175 | Psychotherapy | Parent well-being | CBT | Parent | Community | Clinical psychologist, psychiatric nurses both CBT trained | Face to face | Group | Four sessions, twice weekly | 2 hours | 16 hours | Yes | Unclear | 4 weeks |
| 176 | Psychotherapy | Parent well-being | Brief IPT | Parent | Clinic | Therapists, masters-level social worker, PhD psychologist | Face to face/telephone | Individual | Eight weekly and six optional follow-up sessions | Unclear | Unclear | Yes | Yes | 8 weeks plus maintenance |
| 182 | Psychotherapy | Parent well-being | IPT | Parent | Unclear | Certified IPT therapist/cotherapist | Face to face | Group | Weekly sessions | 60 or 90 minutes | 16.5 hours | Yes | Unclear | 12 weeks |
| 177 | Psychotherapy | Parent well-being | IPT | Parent | Community | Psychiatrist, postgraduate clinical psychologist and occupational therapist | Face to face | Group | Two individual sessions, eight group sessions, 2-hour partners evening and follow-up | 2 hours | 22 hours | Yes | Yes | 11 weeks plus 6-week follow-up |
| 178 | Psychotherapy | Parent well-being | IPT | Parent | Clinic | Unclear | Face to face | Individual | Nine sessions and one engagement session | 45 minutes | 6.75 hours | Yes | Yes | 14 weeks |
| 179 | Psychotherapy | Parent well-being | IPT | Parent | Clinic | IPT therapist | Face to face | Individual | 12 weekly sessions | 45 minutes | 9 hours | Unclear | Unclear | 9 hours |
| 183 | Psychotherapy | Parent well-being | IPT | Parent | Clinic | Psychotherapist | Face to face | Individual | 12 sessions | Unclear | Unclear | Unclear | Unclear | Unclear |
| 180, 231–233 | Psychoeducation | Child well-being | Psychoeducation, peer support | Child | Community | Programme developer and co-facilitators | Face to face | Group | Three daily sessions | 6 hours | 18 hours | Unclear | Unclear | 3 days |
| Study | n | Unit of randomisation | Method of allocation | Method of allocation concealment | Participant; personnel blinding | Assessor blinding; inter-rater reliability | A priori primary outcome | All outcomes reported; missing data | Attrition post intervention; final follow-up % | Attrition reasons reported | Power calculation | Method of analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 151 | 87 | Parent | Random number generator, i.e. computer | Unclear | No; no | Blinded for all relevant outcomes; unclear | Yes | Partial reporting on some outcomes | 30; NA | Missing reasons | No | ITT |
| 141, 162, 169, 202, 227, 228 | 105 | Family | Random numbers table | Unclear | No; unclear | No; no | Yes | Partial reporting on some outcomes | 8; 13 | Missing reasons | No | Incomplete data set |
| 140 | 37 | Child | Unclear/not reported | Unclear | No; no | No; no | Yes | Partial reporting on some outcomes | 0; NA | NA | No | Complete data set |
| 142 | 76 | Families | Unclear/not reported | Unclear | No; none involved | No; no | Yes | Yes | 14; NA | Missing reasons | No | Incomplete data set |
| 157 | 258 | Parent | Alternately allocated then treatment need | Unclear | No; no | No; no | Yes | Yes | 81; NA | Yes: treatment on subsample | No | Incomplete data set |
| 64, 229, 230 | 188 | Parent–child dyad | Randomised blocks, method unclear | Unclear | No; unclear | Unclear; unclear | Yes | Yes | 23; NA | Missing reasons | No | Incomplete data set |
| 66 | 39 | Parent–child dyad | Sequential allocation matched on demographics | NA | No; no | Blinded only for parenting practice; unclear | Yes | Partial reporting on some outcomes | 10; NA | Missing reasons | No | Complete data set |
| 67 | 32 | Blocks of parents | Sequential allocation matched on demographics | NA | No; no | Unclear; unclear | Yes | Partial reporting on some outcomes | 12; NA | No loss | No | Incomplete data set |
| 143, 168 | 111 | Blocks of families | Random number generator, i.e. computer | Sealed envelopes | No; no | Blinded only for parenting practice; > 80% IRR | Yes | Partial reporting on some outcomes | 14; NA | Missing reasons | No | Incomplete data set |
| 152, 163 | 193 | Parent | Random selection from four coloured balls | Unclear | No; no | Unclear; unclear | Yes | Yes | 5; 9 | Unequal loss distribution, missing reasons | Yes | Incomplete data set (ITT primary outcome only) |
| 144 | 73 | Parent–child dyad | Matched groups | NA | No; no | Blinded only for parenting practices and infant behaviour 85% IRR | Yes | Yes | 25; NA | Missing reasons | No | Incomplete data set |
| 145 | 53 | Blocks of parents | Unclear/not reported | Unclear | No; unclear | Unclear; unclear | Yes | Yes | 30; NA | Missing reasons | No | Incomplete data set |
| 153 | 55 | Parent | Random number generator, i.e. computer | Unclear | No; no | Blinded for all relevant outcomes; unclear | Yes | Partial reporting on some outcomes | 10; NA | NA | No | Incomplete data set |
| 148 | 20 | Parent | Unclear/not reported | Unclear | No; unclear | Unclear; unclear | Yes | Partial reporting on some outcomes | 40; NA | Equally distributed with non-treatment related reasons | No | Incomplete data set |
| 149 | 68 | Parent | Coded, pre-generated schedule, method unclear | Independently hosted | No; no | No; no | Yes | Partial reporting on some outcomes | 28; NA | Missing reasons | No | Incomplete data set |
| 155 | 29 | Parent | Unclear/not reported | Unclear | No; unclear | Unclear; unclear | Yes | Yes | 0; NA | Missing reasons | No | Complete data set |
| 156, 161 | 35 | Parent | Random number generator, i.e. computer | Unclear | No; no | No; no | Yes | Yes | 20; NA | Small numbers, potentially treatment related | No | Incomplete data set |
| 150 | 57 | Parent | Random number generator, i.e. computer | Unclear | No; unclear | No; no | Yes | Yes | 21; NA | Reasons provided | Post hoc only | Incomplete data set |
| 146, 160, 167 | 120 | Parent | Random number generator, i.e. computer | Unclear | No; no | No; no | Yes | Yes | 17; NA | Missing reasons | No | Incomplete data set |
| 158 | 903 | Union council clusters | Random numbers table | Independently hosted | No; no | No; no | Yes | Yes | 9; 22 | Non-treatment related | No | Incomplete data set |
| 138 | 230 | Parent | Random number generator, i.e. computer | Sealed envelopes | No; no | No; no | Yes | Partial reporting on some outcomes | 10; NA | Missing reasons | No | Incomplete data set |
| 65 | 47 | Family | Unclear/not reported | Unclear | No; unclear | Unclear; unclear | Yes | Yes | 23; NA | Missing reasons | No | Incomplete data set |
| 63 | 44 | Family | Random number generator, i.e. computer | Sealed envelopes | No; no | Unclear; unclear | Yes | Missing outcomes; insufficient standardisedES data | 27; NA | Missing reasons | Post hoc only | Incomplete data set |
| 147 | 65 | Parent | Unclear/not reported | Unclear | No; unclear | No; no | Yes | One missing outcome; insufficient standardisedES data | 48; 57 | Missing reasons | No | Incomplete data set |
| 154 | 119 | Child | Pre-genrated schedule, method unclear | Sealed envelopes | No; unclear | No; no | Yes | Yes | 31; NA | Missing reasons | No | Incomplete data set |
| 159 | 41 | Parent | Unclear/not reported | Unclear | No; unclear | No; no | Yes | Partial reporting on some outcomes | 0; NA | NA | No | Complete data set |
| Study | n | Method; unit of allocation | Allocation concealment | Participant; personnel blinding | Outcome type(s) | Assessor blinding; inter-rater reliability | A priori primary outcome | All outcomes reported | Attrition post intervention; last follow-up | Reasons for attrition | Power calculation | Method of analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 170 | 23 | Patient preference; parent | NA | No; no | Parent report; clinician report | NA; unclear | No | Assume so, no protocol | None; NA | NA | No | Whole sample |
| 171 | 40 | Service availability; parent | NA | No; no | Parent report; clinician report | NA; no | No | No | Unclear; NA | NA | No | Unclear |
| 166 | 118 | Service availability; parent | NA | No; no | Self-report | NA | No | Assume so, no protocol | 46%; NA | 54 did not complete treatment | No | Completers only |
| 139 | 60 | Service availability; parent | NA | No; no | Self-report; clinician report | Yes, NA | No | Assume so, no protocol Yes | None; NA | NA | Yes | Whole sample |
| Study | Random-sequence generation (selection bias) | Allocation concealment (selection bias) | Participant/personnel blinding (performance bias) | Assessor blinding: patient reported outcomes (detection bias) | Assessor blinding: observer reported outcomes (detection bias) | Selective reporting (reporting bias) | Incomplete postintervention outcome data (attrition bias) | Overall risk of biasa |
|---|---|---|---|---|---|---|---|---|
| 151 | – | ? | + | + | – | ? | – | ? |
| 141, 162, 169, 202, 227, 228 | – | ? | + | + | + | ? | ? | ? |
| 140 | ? | ? | + | + | + | ? | – | ? |
| 142 | ? | ? | + | + | + | – | – | ? |
| 157 | + | ? | + | + | + | – | – | + |
| 64, 229, 230 | ? | ? | + | + | ? | – | ? | ? |
| 66 | + | + | + | + | + | ? | ? | + |
| 67 | + | + | + | + | + | ? | – | + |
| 143, 168 | – | – | + | + | – | ? | ? | ? |
| 152, 163 | – | ? | + | + | ? | – | ? | ? |
| 144 | + | + | + | + | + | – | ? | + |
| 145 | ? | ? | + | + | ? | – | ? | ? |
| 153 | – | ? | + | + | ? | ? | – | ? |
| 148 | ? | ? | + | + | ? | ? | – | ? |
| 149 | ? | – | + | + | + | ? | ? | ? |
| 155 | ? | ? | + | + | ? | – | – | ? |
| 156, 161 | – | ? | + | + | + | – | – | ? |
| 150 | – | ? | + | + | + | – | – | ? |
| 146, 160, 167 | – | ? | + | + | + | – | ? | ? |
| 158 | – | – | + | + | + | – | – | - |
| 138 | – | – | + | + | + | ? | ? | ? |
| 65 | ? | ? | + | + | ? | – | ? | ? |
| 63 | – | – | + | + | ? | ? | ? | ? |
| 147 | ? | ? | + | + | + | ? | ? | ? |
| 154 | ? | – | + | + | + | – | ? | ? |
| 159 | ? | ? | + | + | + | ? | – | ? |
| Study | Random-sequence generation (selection bias) | Allocation concealment (selection bias) | Participant/personnel blinding (performance bias) | Assessor blinding: patient reported outcomes (detection bias) | Assessor blinding: observer reported outcomes (detection bias) | Selective reporting (reporting bias) | Incomplete postintervention outcome data (attrition bias) | Overall risk of biasa |
|---|---|---|---|---|---|---|---|---|
| 170 | + | + | + | + | ? | ? | – | + |
| 171 | + | + | + | + | + | – | ? | + |
| 166 | + | + | + | + | – | ? | – | + |
| 139 | + | + | + | + | – | ? | – | + |
| Outcome | Measure |
|---|---|
| Children’s emotional well-being | Diagnostic interview schedules (e.g. Kiddie-SADS-E-R) |
| CDI (self-report) | |
| CDI (parent/proband informant version) | |
| Child CES-D | |
| YSR/CBCL (anxiety/depression) | |
| Parenting Stress Inventory (child mood) | |
| Observer-rated infant emotion | |
| Mother-reported infant behaviour questionnaire (emotionality) | |
| Child social functioning/behaviour | Parenting Stress Inventory (child domain) |
| CBCL/YSR | |
| Parental Daily Report Scale | |
| Family observation system | |
| Infant Behaviour Record (social responsibility) | |
| Behavioural Screening Questionnaire | |
| Rutter A2 scale | |
| Teacher Preschool Behaviour Checklist | |
| Parent-reported functioning problems, non-specified | |
| Columbia Impairment Scale | |
| Recreational engagement | Health behaviours questionnaire and ACTIVITY Scale |
| Social relationship quality | Peer relationship scale |
| Child physical health | Stunting |
| Height/weight | |
| Diarrhoea episodes | |
| Children’s cognitive development | Bayley Scales of Infant Development Mental Development Index |
| Wechsler Preschool and Primary Scales of Intelligence Full Scale IQ | |
| McCarthy Scales of Children’s Abilities | |
| Self-esteem | Coopersmith Self-Esteem Inventory |
| Children’s reported self-worth | |
| Mental health literary | Parental report |
| Coping | Responses to Stress Questionnaire, parental depression version |
| Parental mental health | Diagnostic interview schedules (e.g. SCID, mini-neuropsychiatric interview, Goldberg, SADS-L) |
| EPDS | |
| HRSD | |
| BDI/BDI-II | |
| CES-D | |
| MADRS | |
| POMS (Depression) | |
| Kellner Symptom Questionnaire | |
| General Health Questionnaire | |
| Automatic Thoughts Questionnaire | |
| SF-36 mental health | |
| CIS-R | |
| Parenting Stress Inventory (depression) | |
| Child-parent interaction | Maternal Attachment Inventory |
| Social Adjustment Scale | |
| Parental bonding instrument | |
| Caldwell HOME Inventory (independent ratings) | |
| Parenting Stress Inventory (parent attachment) | |
| Positive Parenting Practices Scale | |
| Maternal reported child relationship problems | |
| Parent reported child play frequency | |
| PCERA (independent home observation) | |
| Family Observation Schedule Independent home ratings | |
| Iowa Family Interaction Rating Scales | |
| Other independent home rating | |
| Parent interview, non-specified | |
| Family Functioning | SAS-SR |
| Family Conflict Scale (proband/partner report) | |
| Family Assessment Device (proband/partner report) | |
| Family Relationship Inventory (parent/child report) | |
| Parent interview, non-specified | |
| Assessor-rated, non-specified |
| Study | Number of trial arms | Number of eligible arms | Intervention(s)/comparator(s) | Follow-up timing (post allocation) | Continuous outcome(s) selected for analysis | Dichotomous outcome(s) selected for analysis | Incomplete outcome reporting for standardised ES | Authors narrative for which standardised ES not calculable |
|---|---|---|---|---|---|---|---|---|
| 151 | 4 | 4 | One session of parent CBT + placebo vs. six sessions’ parent CBT + placebo vs. one session of parent CBT + fluoxetine vs. six sessions’ parent CBT + fluoxetine | 12 weeks | Observer rated; parent HRSD | – | Yes, missing SDs | ‘Highly significant’ improvements seen in all four treatment groups, six sessions CBT superior to one session on the HRSD. Women who received one CBT session with placebo had smaller changes than other groups, but interaction between medication and therapy not significant |
| 141, 162, 169, 202, 227, 228 | 2 | 2 | Six to 10 sessions’ parent–child psychotherapy, two lectures’ parent psychoeducation | Approximately 10 weeks, approximately 12 months, approximately 36 months | Child-report: YSR, family relationship inventory, child illness understanding, global self-worth | Child report: Kiddie-SADS-E-R; parent-report: focus on children, family closeness, parent SADS-L | Multiple missing data for YSR, global self-worth parent SADS-L | Standardised ES possible for family relationship inventory, family closeness (parent report) and child focus (parent-report) and child illness understanding. authors report internalising symptoms to decrease for both groups, with no significant group difference. No significant differences in children’s reported self-worth at 12-month follow-up |
| 140 | 2 | 2 | 6 weeks’ child CBT vs. waiting list | 3 months | Child-report: CDI, Coopersmith self-esteem; parent-report: CBCL | – | – | Standardised ES in meta-analyses |
| 142 | 2 | 2 | 50 minutes’ parent–child psychoeducation vs. waiting list | 6 weeks | – | Parent report: child functioning, child depression knowledge | – | Standardised ES in meta-analysis |
| 157 | 2 | 2 | 5–8 weeks’ parent CBT/PD vs. TAU | 10–12 weeks | Parent HRSD | – | – | Standardised ES in meta-analysis |
| 64, 229, 230 | 3 | 2a | 45 weeks’ parent psychotherapy vs. TAU | Approximately 16 months | Parent BDI, observer-rated: Wechsler Preschool/Primary Full Scale IQ | – | – | Standardised ES in meta-analysis |
| 66 | 3 | 3 | 12 weeks’ parent IPT vs. 12 weeks’ parent–child mother–infant therapy vs. TAU | 12 weeks | Parent BDI, observer rated: PCERA infant affect, dysregulation, maternal affect, Bayley Mental Scale of Infant Development | – | Multiple missing data for Bayley Scale | Standardised ES possible for BDI, PCERA. Authors report no significant group differences on Bayley Scales |
| 67 | 2 | 2 | 12 weeks’ parent–child mother–infant therapy vs. TAU | 12 weeks | Parent BDI, observer rated: PCERA infant affect, dysregulation, maternal affect, Bayley Mental Scale of Infant Development (observer-rated) | – | – | Standardised ES in meta-analysis |
| 143, 168 | 2 | 2 | 8 weeks’ parent–child CBT vs. 8 weeks’ parent–child psycho-education | 6 months, 12 months | Child report: CES-D, YSR/CBCL; observer rated: positive parenting, parent-report: BDI-II, child coping | – | – | Standardised ES reported |
| 152, 163 | 4 | 4 | 10 weeks’ parent CBT vs. 10 weeks’ parent ST; 10 weeks’ parent PD vs. TAU | 4.5 months, 9 months; 18 months, 5 years | Parent report: EPDS, Behavioural Screening Questionnaire; observer rated: maternal sensitivity, McCarthy Scales of Children’s Abilities, preschool behaviour checklist | Parent report: parent–child relationship problems | Multiple missing data for maternal sensitivity; medians for PBCL, BSQ, McCarthy scales | Estimate of effect for observed maternal sensitivity at 4 months –0.23 (SE 0.15). No significant differences between groups in 5-year PBCL scores (p = 0.99) or McCarthy scores. A generalised linear model suggested no significant treatment effects after controlling for the level of social adversity |
| 144 | 3 | 2a | 1 year of extended care home visitation vs. TAU | 16.7 months | Parent BDI, observer-rated: infant behaviour record emotional tone, social responsibility, maternal behaviour; Bayley Mental Scale of Infant development | – | – | Standardised ES in meta-analysis |
| 145 | 2 | 2 | 8 weeks’ parent IPT vs. TAU | 6 months | Parent EPDS | – | – | Standardised ES in meta-analysis |
| 153 | 2 | 2 | 8 weeks’ parent home counselling vs. TAU | 13 weeks | – | Parent Goldberg’s standard psychiatric interview | – | Standardised ES in meta-analysis |
| 148 | 2 | 2 | 10 weeks’ parent CBT vs. waiting list | 10 weeks | Parent report: BDI, PSI; child domain | – | Missing SD for PSI child domain | PSI child domain showed significant deterioration for waiting list control group over time (p < 0.05). Treatment and control group mean scores at baseline were 131.20 (n = 10) and 130.10 (n = 10), respectively, and at post intervention, 127.40 (n = 6) and 146.00 (n = 6), respectively |
| 149 | 3 | 3 | 6 weeks’ parent nurse-delivered CBT vs. 6 weeks’ parent psychologist-delivered CBT vs. TAU | 8 weeks | Parent BDI-II | – | – | Standardised ES in meta-analysis |
| 155 | 2 | 2 | 6 weeks’ parent psychoeducation with partner support vs. 6 weeks’ parent psychoeducation without partner support | 10 weeks | Parent EPDS | – | – | Standardised ES reported |
| 156, 161 | 2 | 2 | 12 weeks’ parent CBT + paroxetine vs. TAU (paroxetine monotherapy) | 12 weeks | Parent-report EPDS; PSI child domain | – | Multiple data missing for PSI child domain | Significant decrease in PSI child domain (p < 0.05) pre to post-treatment. Between group differences not reported |
| 150 | 2 | 2 | 12 weeks’ parent IPT vs. TAU | 3 months | Parent BDI-II, Maternal Attachment Inventory | – | – | Standardised ES in meta-analysis |
| 146, 160, 167 | 3 | 2a | 8 weeks’ parent IPT vs. TAU | 12 weeks, 18 months | Parent-report: BDI SAS; observer rated: PSI (child domain), infant emotion, maternal responsiveness | – | SAS missing subscale data | Subscales showed significant group assessment occasion effects in favour of IPT, including ‘relationship with children older than 2 years’ (p = 0.05) and ‘relationship with immediate family’ (p = 0.002) |
| 158 | 2 | 2 | 11 months’ parent CBT vs. TAU | 6 months, 12 months | Parent HRSD | Observer-rated: stunted, underweight; parent report: parent–child play frequency | – | Standardised ES in meta-analysis |
| 138 | 2 | 2 | 8 weeks’ parent CBT vs. TAU | 3 months | Parent EPDS | Standardised ES in meta-analysis | ||
| 65 | 2 | 2 | 12 sessions’ parent BT vs. 14 sessions’ parent CBT | 6 months | Parent BDI; observer rated: home observations negative child behaviour | – | – | Standardised ES in meta-analysis |
| 63 | 2 | 2 | 8 weeks’ parent psychoeducation vs. TAU | 8 weeks | Parent/partner report: parent CES-D, child school functioning, CDI; positive parenting practices, peer relationship scale, Health Behaviours Questionnaire and Activities Scale, family assessment device | – | – | Standardised ES in meta-analysis |
| 147 | 2 | 2 | 9 sessions’ parent IPT vs. TAU | 3 months, 9 months | Parent report: BDI, HRSD, CBCL; child report: Columbia Impairment Scale, CDI | – | Multiple missing data for 3 month CDI | No significant differences between groups on child symptoms and functioning at 3-month follow-up. Controlling for baseline at 9 months, CDI and Columbia Impairment scores significantly better in IPT group |
| 154 | 3 | 3 | 16 weeks’ parent CBT vs. 16 weeks’ parent social group vs. TAU | 6 months, 12 months | Parent BDI, HRSD, CBCL | – | – | Standardised ES in meta-analysis |
| 159 | 2 | 2 | 6 weeks’ parent ST vs. TAU | 6 weeks | – | Parent MADRS | – | Standardised ES in meta-analysis |
| Study | Design, n, % MDD | Intervention(s), comparator(s) | Intervention target, objective | Target child age range | Percentage uptake | Percentage subsequent withdrawal | Percentage overall retention a | Retention definition | Mean adherence | Additional information |
|---|---|---|---|---|---|---|---|---|---|---|
| 151 | RCT, 87, 59 | One session of CBT + fluoxetine | Parent, parent well-being | < 1 year | 86% | 16% | 73% | Completing intervention | – | Reasons for non-retention: 54% none given, 19% disliked drug, 15% side effects, 12% lack of improvement. Dropouts were younger (p = 0.04), more likely to have unemployed partner (p = 0.05) and planned pregnancy (p = 0.03). No significant differences in baseline psychiatric morbidity, employment, obstetric complications, parity, family or depression history |
| Six sessions’ CBT + fluoxetine | Parent, parent well-being | < 1 year | 81% | 24% | 62% | |||||
| One session of CBT + placebo | Parent, parent well-being | < 1 year | 87% | 15% | 74% | Completing intervention | – | |||
| Six sessions’ CBT + placebo | Parent, parent well-being | < 1 year | 71% | 0% | 71% | |||||
| 141, 162, 169, 202, 227, 228 | RCT, 105, 82 | Six to 10 sessions’ family therapy | Parent–child, parenting | 8–15 years | – | – | 93% | Attending all sessions | – | – |
| Two lectures | Parent, parenting | 91% | ||||||||
| 157 | RCT, 60, 100 | Five to eight sessions’ mixed CBT/PD | Parent, parent well-being | < 1 year | – | – | – | – | Intervention visits 6.1 (SD 1.6), maximum 8 | No significant differences between drop-outs and completers on any demographic or clinical variable |
| TAU | – | |||||||||
| 64, 229, 230 | RCT, 131, 100 | Approximately 45 sessions’ mother–toddler psychotherapy | Parent, parenting | 0–2 years | 92% uptake | 13% | 80% | Completing intervention | – | – |
| TAU | 13% withdrawal | Regular participation | ||||||||
| 143, 168 | RCT, 111,100 | Eight sessions’ CBT | Parent–child, parenting/child well-being | 9–15 years | – | – | – | – | CBT sessions 7.9; CBT sessions attended by ≥ 1 session attenders 10.5, maximum 12 | – |
| Written psychoeducation | Parent–child, parenting | |||||||||
| 152, 163 | RCT, 193, 100 | 10 sessions’ individual ST | Parent, parent well-being | < 1 year | 100% | 13% | 86% | Completing > 4 sessions | Not reported | No significant difference between completers and non-completers on maternal mood pre therapy, education, orientation to motherhood, social adversity. Non-completers significantly younger (p < 0.004) and more likely single/separated (p < 0.05) |
| 10 sessions’ individual CBT | Parent, parent well-being | 98% | 2% | 95% | ||||||
| 10 sessions’ individual PD | Parent, parent well-being | 96% | 17% | 80% | ||||||
| TAU | – | 100% | 8% | 85% | ||||||
| 144 | RCT, 73, 100 | 25–29 sessions’ home visiting | Parent, parenting/parent well-being | < 1 year | – | – | 74% across groups | Completing intervention | – | – |
| TAU | – | |||||||||
| 145 | RCT, 53, 85 | Eight sessions’ individual IPT | Parent, parent well-being | < 1 year | – | – | 68% | Completing intervention | – | 68% IPT averaged six maintenance sessions (range 2–10), 50% averaged 2–3 adjunct telephone sessions (range 1–6) |
| TAU | – | % | ||||||||
| 153 | RCT, 55, 68 | Eight sessions’ individual ST | Parent, parent well-being | < 1 year | – | – | – | – | – | Mean session attendance 8.8 out of an intended eight-weekly counselling visits |
| TAU | – | |||||||||
| 148 | RCT, 20, symptom based | 10 sessions’ group CBT | Parent, parent well-being | < 1 year | 90% | 33% | 60% | Completing intervention | – | Reasons for non-retention: 25% physical illness, 25% need to support husband, 25% difficulty attending, 25% distance to travel. Reasons for non attendance: 29% not interested, 14% private therapy, 14% hospitalised, 14% return to work, 14% job change, 14% difficulty attending |
| Waiting list | – | 60%a | 50%a | 30% | ||||||
| 149 | RCT, 68, symptom based | Six sessions’ CBT (psychologist) | Parent, parent well-being | < 1 year | – | – | – | – | – | CBT attendance 4.6 sessions (nurse facilitated), 4.0 sessions (psychologist facilitated); 71% GP appointments kept |
| Six sessions’ CBT (nurse) | Parent, parent well-being | |||||||||
| TAU | – | |||||||||
| 155 | RCT, 29, 100 | Seven sessions’ psychoeducation (partner involvement) | Parent, parent well-being | < 1 year | 100% | 0% | 100% | Completing intervention | – | – |
| Seven sessions’ psychoeducation (no partner involvement) | Parent, parent well-being | |||||||||
| 176, 161 | RCT, 35, 100 | 12 sessions’ CBT, paroxetine | Parent, parent well-being | < 1 year | – | 11% | 89% | Completing intervention | – | 5% CBT + paroxetine group non-compliant with medication, 5% non-compliance with CBT protocol; 7% TAU suicidal ideation. No significant differences between completers and dropouts on age, marital status, employment status, education level, ethnicity, or number of children |
| TAU, paroxetine only | – | 7% | 93% | |||||||
| 150 | RCT, 57, 100 | 8 weeks’ group IPT | Parent, parent well-being | < 1 year | 82% | 4% | 79% | Completing intervention | – | Reasons for non-retention: IPT – relocation interstate, preference for individual therapy, disclosed domestic violence, improved symptoms. TAU – dissatisfaction with treatment as usual |
| TAU | – | 96% | 3% | 93% | ||||||
| 146, 160, 167 | RCT, 120, 100 | 12 sessions’ individual IPT | Parent, parent well-being | < 1 year | – | – | 80% | Completing intervention | – | Withdrawals not significantly different between groups (p = 0.47). No significant differences between dropouts and completers on any demographic variables |
| TAU | – | 85% | ||||||||
| 158 | RCT, 903, 100 | Seven sessions’ individual CBT | Parent, parent well-being | < 1 year | 98% | – | 98% | Attending ≥ 1 session | – | – |
| TAU | – | |||||||||
| 138 | RCT, 230, 100 | Eight sessions’ group CBT | Parent, parent well-being | < 1 year | – | – | – | – | Intervention sessions 2.7 (3.1), maximum 8 | Attendance not associated with EPDS scores (p = 0.47). Women taking medication attended more sessions than those discontinuing but differences not significant (p = 0.89) |
| TAU | – | |||||||||
| 65 | RCT, 47, 100 | 12 sessions’ family BT | Parent, parenting | 3–9 years | – | 21% | 79% | Completing intervention | – | Dropout rates not significant different between groups. No significant differences between completers and non-completers on any measures |
| 14 sessions’ family CBT | Parent, parenting/parent well-being | 13% | 87% | |||||||
| 63 | RCT, 44, 80 | Family psychoeducation | Parent, parenting | 6–13 years | – | 33% | 66% | Completing ≥ 2 sessions | – | Reasons for non-retention: 29% inconvenient timing/location, 29% attending groups elsewhere, 14% did not feel like attending, 14% no reason given and 14% excessive symptoms |
| TAU | – | – | ||||||||
| 159 | RCT, 41, 100 | 6 weeks’ ST | Parent, parent well-being | < 1 year | – | 15% across groups | 85% across groups | Completing intervention | – | Reasons for non-retention: 14% dropouts moved away, 14% denied participation by spouse, 14% no reason, 57% needed more specialised treatment |
| TAU | ||||||||||
| 170 | nRCT, 23, 100 | 12 sessions’ IPT | Parent, parent well-being | < 1 year | – | 18% | 82% | Completing intervention | – | Allocated on preference: 48% IPT, 9% medication, 44% IPT + medication. Women selecting medication alone cited time constraints prohibiting therapy participation. Non-significant trend for breastfeeding women to opt for non-medication (66.7%) and women with no depression history to choose medication (85.7%). 60% dropouts non-compliant with IPT protocol, 20% switched to couple counselling, 20% treatment no longer necessary. Non-completers significantly higher baseline mean BDI scores. No significant differences on HRSD or EPDS scores |
| 12 sessions’ IPT + Sertraline | Parent, parent well-being | 30% | 70% | |||||||
| TAU, Sertraline alone | – | 0% | 100% | |||||||
| 171 | nRCT, 40, 100 | Eight sessions’ CBT techniques | Parent, parent well-being | < 1 year | 95% | – | 95% | Attending ≥ 1 sessions | – | – |
| TAU | – | |||||||||
| 174 | Uncontrolled, 8, 100 | 12 sessions’ group CBT | Parent, parent well-being | < 1.5 years | – | 12% | 88% | Completing intervention | – | – |
| 181 | Uncontrolled, 11, 100. | Eight sessions’ individual CBT | Parent, parent well-being | 2–7 years | – | 9% | 91% | Completing intervention | – | One withdrew owing to physical health problems |
| 175 | Uncontrolled, 45, 61. | Eight sessions’ group CBT | Parent, parent well-being | < 1 year | – | 15% | 85% | Completing intervention | – | Dropouts owing to significant relapse (e.g. manic episode, psychotic episode), mother/child physical health. Dropouts did not differ significantly from completers in age, ethnicity or time postpartum |
| 176 | Uncontrolled, 12, 83 | Eight sessions’ individual IPT | Parent, parent well-being | < 1 year | – | 17% | 83% | Completing intervention | 66% received a mean of four to six follow-up sessions | One out of two dropouts did not consider symptoms to warrant treatment (diagnosed with minor depression), one out of two no explanation |
| 182 | Uncontrolled, 17, 88 | 12 sessions’ group IPT | Parent, parent well-being | < 1 year | – | 50% | 50% | Completing intervention | – | 22% chose not to participate owing to transportation or child-care difficulties, or reluctance to be treated in the context of a trial. Other reasons unknown |
| 180, 231–233 | Uncontrolled, 25, 64 | 3 days’ group psychoeducation, peer support | Child, child well-being | 8–16 years | – | 32% | 68% | Completing intervention | 32% only completed 2 days | Reasons for non-attendance: illness, previous commitments, parental illness requiring children to cover homecare |
| 177 | Uncontrolled, 18, 100 | 10 sessions’ group + individual IPT, supplementary partner sessions | Parent, parent well-being | < 1 year | – | 5% | 95% | Completing intervention | 95% completed all sessions | One dropped out owing to an ongoing court hearing |
| 183 | Uncontrolled, 9, 100 | 12 sessions’ individuals IPT | Parent, psychotherapy: parent well-being | < 1 year | 75% | 22% | 58% | Completing intervention | – | – |
| 178 | Uncontrolled, 13, 100 | Nine sessions’ individual IPT + engagement session | Parent, psychotherapy: parent well-being | 12–18 years | 85% | 10% | 77% | Completing intervention | All who participated in engagement session attended ≥ 1 session, mean 7.9 sessions. 10 out of 11 completed full course | – |
| 179 | Uncontrolled, 12, 93. | 12 sessions’ individual IPT | Parent, psychotherapy: parent well-being | 6–18 years | NR | 25% | 75% | Completing intervention | Sessions attended by treatment completers 11.1 (SD 2.7). | Reasons for non-retention: 33% lack of time, 33% child in foster care, 33% felt no need to continue |
| 173 | Uncontrolled, 26, 100 | 17 weekly sessions’ CBT | Parent, psychotherapy: parent well-being | < 1 year | – | – | 85% | Attending all sessions | – | Reasons for non-retention: 75% moving out of the area and 25% discontinuing owing to rapid improvement |
Appendix 10 Data extraction sheet (economic evaluation)
| Study characteristics | First author |
| Year | |
| Clinical paper published and included in the clinical review: yes/no | |
| Disorder | |
| Interventions | |
| Comparator | |
| Country | |
| Study design | |
| Patient group | |
| Sample size | |
| Age group | |
| Outcome-primary (economic evaluation’) | |
| Outcome-secondary (economic evaluation’) | |
| Costs collected (list) | |
| Source of unit costs | |
| Method of economic data collection | |
| Perspective | |
| Method of economic evaluation | |
| Cost year | |
| Time horizon | |
| Clinical results | |
| Cost results | |
| Cost-effectiveness results | |
| Conclusions according to authors | |
| Quality | Question |
| Alternatives | |
| Effectiveness | |
| Identification of costs and consequences | |
| Measurement of costs and consequences | |
| Valuation of costs and consequences | |
| Discounting | |
| Incremental analysis | |
| Uncertainty | |
| Results |
List of abbreviations
- AHCI
- Arts and Humanities Citation Index
- ASSIA
- Applied Social Sciences Index and Abstracts
- AUEI
- Australian Education Institute
- BDI
- Beck Depression Inventory
- BME
- black or minority ethnic
- BRIE
- British Education Institute
- CASP
- Critical Appraisal Skills Programme
- CBT
- cognitive–behavioural therapy
- CCDAN
- Cochrane Collaboration Depression, Anxiety and Neurosis Review Group
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CRD
- Centre for Reviews and Dissemination
- CSQ
- client satisfaction questionnaire
- DARE
- Database of Abstracts of Reviews of Effects
- DSM
- Diagnostic and Statistical Manual of Mental Disorders
- ECM
- Every Child Matters
- EPDS
- Edinburgh Postnatal Depression Scale
- EPPI
- Evidence for Policy and Practice Information and Co-ordinating Centre
- EQ-5D
- European Quality of Life-5 Dimensions
- ERIC
- Education Resources Information Centre
- ES
- effect size
- GP
- general practitioner
- HEED
- Health Economic Evaluations Database
- HMIC
- Health Management Information Consortium
- HRQoL
- health-related quality of life
- HSRProj
- Health Services Research Projects in Progress
- IAPT
- Improving Access for Psychological Therapies
- IBSS
- International Bibliography of the Social Sciences
- ICD
- International Classification of Diseases
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- IPT
- interpersonal therapy
- MDD
- major depressive disorder
- MeSH
- medical subject heading
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- nRCT
- non-randomised controlled trial
- NSPCC
- National Society for the Protection and Care of Children
- PEDE
- Paediatric Economic Database Evaluation
- PQ-LES-Q
- Paediatric Quality of Life Enjoyment and Satisfaction Questionnaire
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SCIE
- Social Care Institute for Excellence
- SCIEXPANDED
- Science Citation Index Expanded
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SMI
- serious mental illness
- SSCI
- Social Science Citation Index
- VOI
- value of information analysis
- WHO
- World Health Organization
- WoS
- Web of Science