Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/01/25. The contractual start date was in September 2010. The draft report began editorial review in July 2012 and was accepted for publication in November 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors:
none
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Frampton et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Intravascular catheters are used for the administration of medication, fluids, blood products and parenteral nutrition, as well as for patient monitoring,1 but they are an important cause of bloodstream infections (BSIs). 2–4 The most frequently used type of intravascular catheter is a central venous catheter (CVC), also referred to as a central line. A CVC is defined as an intravascular device that terminates in one of the great veins, or in or near to the right atrium, and includes peripherally inserted central catheters (PICCs), haemodialysis catheters and parenteral nutrition catheters. Insertion sites for CVCs are usually the jugular, subclavian and femoral veins, although femoral insertion may be associated with higher risk of BSIs than insertions at other sites. 5 Intravascular catheters vary in the material from which they are made, the presence or absence of antimicrobial or anticoagulant coatings, the number of lumens present and whether they are tunnelled, and these variables may have an important bearing on the risk of BSI. 6
Four distinct pathways may be identified in the infection process of catheter-BSI. The two major pathways are the external and internal bacterial colonisation of the catheter surface, both eventually leading to catheter-tip colonisation, with the potential for subsequent bacteraemia. Additional pathways include microbial contamination of the infusate and direct mechanical introduction of pathogens into the bloodstream. 7 A wide variety of microorganisms may cause catheter-BSI, including, among others, Staphylococcus aureus, Staphylococcus epidermidis8 and enterococci. 9 Catheter-BSI result from inadequate hygiene and suboptimal catheter management procedures. These include inadequate hand hygiene by hospital staff; inadequate skin hygiene at the site of patients' catheter insertion; suboptimal location of catheters; and unnecessary placement of catheters. 6 Other risk factors are the patient's age and underlying disease,6 and the duration of catheterisation. 10
Bloodstream infections arising from the placement of vascular catheters (catheter-BSI) are a particular problem in critical care units owing to the high frequency of intravascular catheter placement and increased susceptibility to infections among critical care patients. 11 The latest (2011) point prevalence survey in England,12 showed that 64% of all patients with a BSI had a vascular access device in the 48 hours prior to onset of infection, and 59.3% of critical care patients received a CVC compared with 5.9% of other hospital patients. A 1-month audit in a hospital in England found that 65% of patients in the critical care unit required a CVC, whereas in surgical and renal wards the proportion ranged from 3.5% to 25%. 13
Prevalence of catheter-bloodstream infection
Prevalence of catheter-BSI is usually standardised to the number of CVC-days, and is typically expressed as the incidence density per 1000 CVC-days, although the definition of a device-day varies (multiple concurrent vascular catheters in the same patient are often not counted separately14). According to the 2011 point prevalence survey in England, intravascular catheter placement accounted for 29% of hospital-acquired BSI. 12 Unpublished data from presentations about the ‘Matching Michigan’ programme in England15–17 (described further below) reported an incidence density of catheter-BSI of 3.7 per 1000 CVC-days for a subset of 19 critical care units in northern England sampled in mid-2009. The most recent published incidence density data available for the UK are from the Intensive Care Unit Associated Infection (ICUAI) National Surveillance Programme (NSP), based on data from May 2009 until January 2010 for 19 critical care units in Scotland. The NSP reported a catheter-BSI incidence density of 0.7 per 1000 CVC-days. 18 A 2009 report of the European Centre for Disease Prevention and Control (ECDC) estimated prevalence of catheter-BSI to be 4.3 per 1000 CVC-days, based on aggregated data from 12 European countries, which included some data from England. 19
There is evidence that prevalence of BSI associated with vascular catheters has decreased as a result of national and local infection prevention programmes in several countries. For example, in the USA the Institute for Healthcare Improvement's ‘100,000 Lives’ Campaign (2005–6) and ‘5 Million Lives’ Campaign (2006–8) were among a number of programmes that recruited a large number of hospitals and promoted (among other objectives) strategies for the prevention of catheter-BSI. The most recent data available from the US Centers for Disease Control and Prevention (CDC)20 show that a 58% decrease in the prevalence of catheter-BSI occurred from 2001 to 2009. The point prevalence surveys conducted in England indicate a decline in the overall prevalence of BSI in recent years but the data are difficult to interpret owing to changes that occurred in the sampling methodology. 12
Definitions and diagnosis of catheter-bloodstream infection
Various definitions and terms are used, and sometimes confused, in the literature to describe a BSI that has developed as a consequence of an indwelling intravascular catheter. Laboratory-confirmed bloodstream infections (LCBSIs) which are proven microbiologically to result from vascular catheter use are generally referred to as catheter-related bloodstream infections (CRBSIs). In the absence of microbiological testing and after ruling out other possible sources, BSIs that appear to be linked to vascular catheter use are referred to as catheter-associated bloodstream infections (CABSIs). The gold standard for diagnosis of CRBSI is isolation of the same microorganism from a peripheral blood culture as that obtained from the tip of the removed catheter. 21 As many patients suspected of having a BSI will not have their catheter removed, and quantitative blood cultures are not universally performed, alternative definitions to CRBSI that do not require catheter removal are often used (i.e. CABSI), and these overestimate the true incidence of CRBSI. The most widely cited definitions of CABSI and CRBSI are the surveillance definitions of the US Centers for Disease Control and Prevention (CDC) National Nosocomial Infection Surveillance System (NNIS), which operated up to 2004, and the CDC National Healthcare Safety Network (NHSN) which replaced the NNIS in 2005. The latest CDC guidelines on defining and diagnosing CABSI and CRBSI, endorsed by the Infectious Diseases Society of America, were reported by Mermel and colleagues. 22 Recently, stringent definitions of CABSI and CRBSI, based on the CDC definitions, have been developed for use in the ‘Matching Michigan’ programme in England17 (see Table 1). As we describe below (see Current prevention of catheter-bloodstream infectionsin the NHS), Matching Michigan was a programme for prevention of catheter-BSI in critical care units in England that has direct relevance to NHS practice.
For the purposes of this report, we follow the definitions of CABSI, CRBSI and catheter-suspected BSIs, as defined in the Matching Michigan programme (Table 1), and these definitions are collectively referred to as catheter-BSI.
| Infection type | Definition |
|---|---|
| LCBSI | The patient has one or more recognised pathogens cultured from one blood culture If the microorganism is a common skin organism [i.e. diphtheroids (Corynebacterium spp.), Bacillus spp. (not B. anthracis), Propionibacterium spp., coagulase-negative staphylococci (excludes sensitive S. aureus), viridans-group streptococci, Aerococcus spp. or Micrococcus spp.], then . . . It must have been cultured from two or more blood cultures drawn on separate occasions, or from one blood culture in a patient in whom antimicrobial therapy has been started, and The patient has one of the following: fever of > 38 °C, chills or hypotension |
| CABSI | Criteria above must be met for LCBSI, and: The presence of one or more CVCs at the time of the blood culture, or up to 48 hours following removal of the CVC and: The signs and symptoms and positive laboratory results including the pathogen cultured from the blood are not primarily related to an infection at another site |
| CRBSI | Criteria above must be met for LCBSI, and: The presence of one or more CVCs at the time of the blood culture, or up to 48 hours following removal of the CVC, and One of the following: a positive semiquantitative (> 15 CFUs/catheter segment) or quantitative (> 103 CFU/ml or > 103 CFU/catheter segment) culture whereby the same organism (species and antibiogram) is isolated from blood sampled from the CVC or from the catheter tip, and peripheral blood; simultaneous quantitative blood cultures with a > 5 : 1 ratio of CVC vs. peripheral |
| Catheter-suspected BSI | Negative blood cultures in the presence of parenteral antimicrobials, and Clinical evidence of a systemic response to infection, and Clinical condition improves following removal of CVC, and No other likely source of infection |
Diagnosis of CRBSI is made in various ways, depending upon both local clinical practice and, for infection surveillance purposes, the definition of infection in use. The use of different definitions of infections can dramatically alter the reported infection rate unless they are aligned with clinical practice. For example, if clinical practice is not to send a CVC line tip to the laboratory for culture, or to draw only a single set of percutaneous cultures, then any definition requiring catheter-tip culture or more than one set of cultures will never be met, potentially giving an artificially low infection rate.
Impact of catheter-bloodstream infection on patients and health services
Catheter-BSI increase patients' discomfort and length of stay (LOS) in hospital23 and their risk of health complications and death. 24 Catheter-BSI can trigger a range of responses from systemic sepsis through to septic shock and multiple organ failure. Metastatic infection may lead to septic thrombosis, endocarditis and septic arthritis. 25 Further complications may include acute respiratory distress syndrome, disseminated intravascular coagulation and acute renal failure. 26
Robust data on mortality, quality of life (QoL) and long-term prognosis specifically related to catheter-BSI are not available for the UK. Recent estimates of the mortality rates of patients with catheter-BSI in critical care units in France, Germany and Italy ranged from 11% to 17.1%. 27 Estimates of the additional LOS per catheter-BSI episode in UK critical care units have ranged from 1.9 days27 to 11 days. 23 In 2006 the National Audit Office estimated the additional cost of a BSI to be £6209 per patient. 28 The most recent (2009) estimate of the financial impact for the NHS suggests that annual costs related to catheter-BSI in critical care units are £19.1–36.2M. 27
Educational interventions for preventing catheter-bloodstream infection
Educational interventions for preventing catheter-BSI have been trialled in critical care settings in many countries and vary considerably in their content and complexity. They range from the provision of simple fact sheets and posters29 to complex interventions comprising multiple behavioural components. 30 Educational approaches also include continuous quality improvement (CQI), which engages front-line staff in cycles of iterative problem solving, with decision-making based on real-time process measurements. 31 Interventions differ in the number and duration of education components, whether they are didactic or interactive, and whether infection surveillance feedback and performance feedback are also present. Interventions that contain several different elements which together aim to achieve a particular outcome are referred to as ‘multifaceted’, ‘multicomponent’ or ‘bundled’ interventions. 32 A care bundle is defined as a small set of practices that have been individually proven to improve patient outcomes and when implemented together are expected to result in better outcomes than when implemented individually. 33
Multifaceted educational interventions that have been developed for preventing catheter-BSI include the Michigan Keystone ICU project in the USA34 and the NHS ‘High Impact’ CVC care bundle. 28 These include, among others, specific components for ensuring appropriate staff behaviour for hand hygiene, patient skin hygiene, choice of catheter type and insertion site, and catheter ongoing care.
In general, educational interventions involve encounters between teachers and learners for one or more of the following purposes: to raise awareness; to enhance or improve knowledge; or to change behaviour. 35 Educational interventions for preventing catheter-BSI ideally should include behaviour modification components underpinned by relevant theory. 36
We defined educational interventions as those that contain any element of information provision intended to influence catheter-BSI outcomes (i.e. by changing health-care workers' behaviour). Included within this definition are checklists, and information feedback to health-care workers. We distinguish between infection surveillance feedback whereby staff are informed in real time of catheter-BSI incidence rates, and performance feedback whereby staff are informed of their compliance with evidence-based practices or progress with learning goals. According to our definition, educational interventions are not limited to purely educational practices but may also include non-educational activities, such as the provision of supplies. Such interventions may be described as providing ‘components beyond education’. 37
Current prevention of catheter-bloodstream infections in the NHS
Catheter-BSI are believed to be largely preventable following work in the UK that has successfully reduced the number of cases of methicillin-resistant S. aureus (MRSA) BSI. It has been proposed that the majority of catheter-BSI could be prevented using evidence-based educational interventions to ensure that doctors and nurses are committed to a culture of safety and follow best practice to achieve this. 26,38
Evidence-based practices that are recommended for prevention of catheter-BSI include selection of an appropriate catheter type; avoidance of the femoral insertion site; antimicrobial cleansing of the insertion site; use of maximal sterile barrier precautions and aseptic technique (gloves, mask, hat, patient drapes) during catheter insertion; and use of a sterile semipermeable transparent dressing to allow observation of the insertion site. 6,39
To address the prevention of catheter-BSI, the NHS has developed ‘Saving Lives’ tools,40 which include ‘high-impact’ care bundles for CVCs and peripheral intravenous cannula. 28 The High Impact No. 1 CVC bundle consists of actions for preventing catheter-BSI in relation to CVC insertion (Table 2) and CVC ongoing care (Table 3). These bundles are based on ‘epic2’ guidelines,39 which stress the importance of education of hospital staff for successful implementation of infection control programmes. Similar guidelines produced by the US CDC also strongly emphasise the need for education and training in evidence-based practices for preventing catheter-BSI. 6 However, in both the epic2 guidelines39 and US guidelines6 there is a lack of evidence on the types of educational interventions that are most appropriate and effective, and the guidelines do not make any recommendations that specifically relate to critical care settings. Following a recommendation in the Darzi Report,41 during 2009–11 the UK National Patient Safety Agency implemented an initiative known as ‘Matching Michigan’38,42 to prevent catheter-BSI. Matching Michigan was based on a regional-scale intervention that had successfully reduced catheter-BSI incidence density in the Keystone ICU project, conducted in 103 critical care units in Michigan, USA. 34 However, the original study in the USA34 was not randomised and did not assess the importance of the education strategy in the effectiveness of the overall care bundle.
| Catheter type | Single lumen unless indicated otherwise Consider antimicrobial impregnated catheter if duration of 1–3 weeks and risk of CRBSI high |
| Insertion site | Subclavian or internal jugular |
| Skin preparation | Preferable use 2% chlorhexidine gluconate in 70% isopropyl alcohol and allow to dry If patient has a sensitivity use a single-patient-use povidone–iodine application |
| Personal protective equipment | Gloves are single-use items and should be removed and discarded immediately after the care activity Eye/face protection is indicated if there is a risk of splashing with blood or body fluids |
| Hand hygiene | Decontaminate hands before and after each patient contact Use correct hand hygiene procedure |
| Aseptic technique | Gown, gloves and drapes, as indicated, should be used for the insertion of invasive devices |
| Dressing | Use a sterile, transparent, semipermeable dressing to allow observation of insertion site |
| Safe disposal of sharps | Sharps container should be available at point of use and should not be overfilled; do not disassemble needle and syringe; do not pass sharps from hand to hand |
| Documentation | Date of insertion should be recorded in notes |
| Hand hygiene | Decontaminate hands before and after each patient contact Use correct hand hygiene procedure |
| Catheter site inspection | Regular observation for signs of infection, at least daily |
| Dressing | An intact, dry, adherent transparent dressing should be present |
| Catheter access | Use aseptic technique and swab ports or hub with 2% chlorhexidine gluconate in 70% isopropyl alcohol prior to accessing the line for administering fluids or injections |
| Administration set replacement | Following administration of blood, blood products – immediately Following total parenteral nutrition – after 24 hours (72 hours if no lipid) With other fluid sets – after 72 hours |
The Matching Michigan programme in England recruited 240 adult and 40 paediatric critical care units, with 97% of acute health trusts in England participating. Technical components of the Matching Michigan programme were a data collection system for infection surveillance; CVC insertion checklist; CVC trolley inventory; catheter-BSI fact sheet; and Department of Health High Impact bundles. 28 Matching Michigan also included non-technical strategies, which were to develop a culture of safety; facilitate learning from incidents; foster teamwork and collaborations; and develop executive and clinical partnerships. The Matching Michigan programme ran for 2 years and ended in 2011. Preliminary results of audits from individual participating trusts are starting to appear at conferences15–17 and in online presentations, but, to date, a detailed formal analysis of findings from Matching Michigan has not been published.
Evidence from existing reviews
A number of narrative reviews have suggested that educational interventions including care bundles may be effective at preventing catheter-BSI in various health-care settings,7,43–46 but no systematic reviews have specifically investigated the effectiveness of educational interventions for preventing catheter-BSI in critical care units. The most relevant systematic reviews in related areas have investigated interventions for preventing catheter-BSI (not limited to educational interventions or critical care);47 bundled behavioural interventions to control health care-associated infections (not limited to education, catheter-BSI or critical care);30 interventions for preventing catheter-BSI in critical care (not limited to education or behavioural interventions);48 educational interventions for preventing health care-associated infections (not limited to educational or behavioural interventions, catheter-BSI or critical care);37 and features of educational interventions that impact on competence in aseptic insertion technique and maintenance of CVCs by health-care workers (not limited to catheter-BSI or critical care). 49 Some of these systematic reviews included primary research studies relevant to the scope of our current evidence synthesis but the most recent of these reviews49 did not include any studies published after August 2008.
None of the systematic reviews referred to above included economic analyses. Most of the available information on the economic impact of catheter-BSI in critical care is from work conducted in the USA. 50,51 A recent brief narrative review of epidemiological studies27 provides an insight into the economic burden of catheter-BSI in critical care in European countries including the UK but, owing to a shortage of information on costs, its findings are based on numerous assumptions and uncertainties.
Objectives
The overall aim of this evidence synthesis is to provide a rigorous evaluation of the clinical effectiveness and cost-effectiveness of educational interventions that are relevant to the NHS for preventing catheter-BSI in critical care units in England. The types of intervention that could be relevant appear diverse, but the quantity, quality and relevance of the primary clinical effectiveness and cost-effectiveness studies are unclear. To address these uncertainties the current project has the following specific objectives:
-
To create an evidence map summarising all potentially relevant primary research studies. This is necessary as a first step to clarify the quantity, quality and potential relevance to NHS policy and practice of the existing primary research studies.
-
To conduct a systematic review of a subset of studies in the evidence map considered most relevant to inform NHS policy and practice for prevention of catheter-BSI in critical care.
-
To conduct a systematic review of cost-effectiveness studies. This is necessary to clarify the quality and relevance of any existing studies of the cost-effectiveness of interventions for preventing catheter-BSI and may help to inform the structure of a decision-analytic economic model.
-
To develop a decision-analytic model to determine and compare cost-effectiveness of relevant groups of interventions and settings. This would utilise data on clinical effectiveness from Objective 2 and, if appropriate, relevant methodology and model parameters identified from Objective 3.
-
Based on the information provided from Objectives 1–4, to identify future research needs and consider the implications of implementing educational interventions for preventing catheter-BSI that are relevant to service users in the NHS.
Chapter 2 Methods for the mapping exercise and systematic review of clinical effectiveness
The a priori methods for systematically reviewing the evidence of clinical effectiveness and cost-effectiveness are described in the research protocol (see Appendix 1). The protocol was sent to our expert Advisory Group (AG) for comment. Minor amendments were made as appropriate, but none of the comments we received identified specific problems with the methods of the review. Methods outlined in our protocol are briefly summarised below.
Search strategy
A sensitive search strategy was developed and refined by an experienced information scientist (see Appendix 2).
Searches for clinical effectiveness literature were undertaken from inception of databases to January/February 2011. No trial or study filter and no language restrictions were included in the search strategy.
The strategies were applied during February 2011 to the following databases:
-
MEDLINE (Ovid; searched 1948 to 19 January 2011)
-
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations (searched to 1 February 2011)
-
EMBASE (Ovid; searched to 25 January 2011)
-
BIOSIS (searched 1969 to 2011)
-
Health Management Information Consortium (HMIC) (searched on 1 February 2011)
-
CINAHL EBSCOhost (searched on 2 February 2011)
-
Cochrane Central Register of Controlled Trials (CCRCT) (searched on 2 January 2011)
-
Cochrane Database of Systematic Reviews (CDSR) (searched on 2 January 2011)
-
Cochrane Database of Abstracts of Reviews of Effects (DARE) (searched on 3 February 2011)
-
Education Resources Information Center (ERIC) (searched from 1966)
-
Web of Science databases (searched to 1 February 2011):
-
Science Citation Index Expanded (SCIE) (searched from 1970)
-
Social Sciences Citation Index (SSCI) (searched from 1970)
-
Arts & Humanities Citation Index (A&HCI) (searched from 1975)
-
Conference Proceedings Citation Index – Science (CPCI-S) (searched from 1990)
-
Conference Proceedings Citation Index – Social Science & Humanities (CPCI-SSH) (searched from 1990).
-
In addition, we hand-searched reference lists of the papers retrieved and relevant systematic reviews for potential additional studies. Experts on the project AG were also asked to identify additional published and unpublished references. All search results were downloaded into a Reference Manager database version 12.0.3 (Thomson ResearchSoft, San Francisco, CA, USA). Searches were rerun using the same search strategies in March 2012 to identify any new primary research published during March 2011 to March 2012, which might impact on the findings of the report.
Inclusion criteria for descriptive mapping (stage 1)
The purpose of the mapping exercise was to facilitate a description of the evidence base so that a subset of policy-relevant studies from the map could be identified and subjected to a detailed systematic review. The following inclusion/exclusion criteria were applied to the references identified by the above search strategy to select studies eligible for inclusion in the evidence map:
-
Population Patients in critical care with any type of vascular catheter (including CVCs, arterial catheters, cannula). Critical care was defined as any critical or intensive care unit (ICU), including high-dependency units but excluded general or specialist (e.g. cardiac, neurological, surgical) non-critical units. Patients with urinary or other non-vascular catheters were only included if vascular catheters were also present. Studies solely on patients with urinary or other non-vascular catheters were excluded.
-
Design Interventional studies based on primary research.
-
Intervention(s) Educational interventions with an objective to reduce or prevent catheter-BSI. An educational intervention was defined as any intervention that aimed to prevent catheter-BSI and (1) included at least an element of factual information provision related to that aim; (2) was described by the authors as educational; or (3) was described by the authors as behavioural. Checklists were eligible as an educational tool. Interventions that did not target catheter-BSI (e.g. interventions for hand hygiene alone or for infection surveillance) were excluded; provision of factual information was also excluded if it was unrelated to prevention of catheter-BSI.
-
Outcomes Primary outcomes were BSIs, mortality or LOS associated with, related to, or suspected to result from intravascular catheter use. BSIs unrelated to vascular catheter use were excluded, as were non-vascular infections (e.g. urinary tract, organ space or skin). The following secondary outcomes were not used for study selection but were to be extracted from studies at the data collection stage if at least one primary outcome was reported: staff reaction to education; attitudes; knowledge; skills; compliance with interventions; and process evaluations (quantitative or qualitative descriptions of intervention processes, facilitators or barriers).
Study selection for descriptive mapping
Titles and abstracts of records identified by the bibliographic searches conducted in January/February 2011 were assessed independently by two reviewers for potential eligibility, using a pilot tested selection worksheet containing the above selection criteria (see Appendix 3). Any disagreements between the reviewers were resolved through discussion and in some cases with recourse to a third reviewer. Full-text papers were obtained for all titles and abstracts that met the selection criteria or were unclear. The full-text papers were then assessed independently by two reviewers using the same study selection worksheet. Any further disagreements between the reviewers were resolved through discussion and in some cases recourse to a third reviewer. Papers published in languages other than English were each assessed by at least one native or competent speaker of the publication language together with a member of the systematic review team.
In addition to the selection of primary research studies described above, any potentially relevant systematic reviews identified during title and abstract screening were obtained as full-text versions for detailed inspection.
Owing to lack of time, any new studies identified from the updated searches in March 2012 were not formally included in the evidence synthesis but their potential implications for interpretation of the evidence synthesis findings are considered in the discussion (see Chapter 7).
The process of descriptive mapping
As mentioned above, the purpose of the mapping exercise was to facilitate a description of the evidence base so that a subset of policy-relevant studies from the map could be identified and subjected to a detailed systematic review. This approach has been found to be useful in previously published systematic reviews. 52–54
Studies that met the selection criteria reported above were coded on the basis of their key characteristics using a classification instrument developed by the project team. The classification instrument (see Appendix 4) consisted of a standard list of keywords for capturing information on critical care specialties, vascular devices used, types of intervention used, study designs, outcomes and the educational strategies and topics covered. The classification instrument was provided in an Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) worksheet, together with instructions on how to interpret and apply the keywords, and was piloted by each member of the project team on three studies. Some amendments were made to both the keywords and the instructions to establish good inter-reviewer reliability within the team. Once finalised, the instrument was applied to the included studies by one or two reviewers depending on the publication language. Studies published in English were classified by one reviewer. Studies published in a language other than English were classified jointly by two reviewers, at least one of whom was a competent speaker of the publication language. A random sample of 40% of all the completed classifications was then checked independently by a further reviewer to ensure good inter-reviewer reliability.
Inclusion criteria for the systematic review (stage 2)
Once all of the studies had been classified, analysis was performed to construct the descriptive map (for results of the map, see Chapter 3).
The results of the descriptive map were presented to the AG in September 2011 for discussion. The group assisted us in prioritising a subset of studies for systematic review that most closely resemble current UK practice, and which are most likely to address current policy and practice needs for preventing catheter-BSI in critical care. Suggestions for systematic review study selection criteria were provided by seven members of the AG and 11 members of the project team, and these were discussed at a face-to-face meeting of the project team in October 2011.
Numerous potential selection criteria for the systematic review were discussed. These included limiting the systematic review to particular study designs; geographical regions; types of catheter; population age groups; types of educational approaches; or levels of study quality. A detailed description of the issues discussed is available from the authors upon request.
Based on the discussion, the inclusion criteria for the systematic review were set as follows:
-
Population Adults (i.e. excluding neonatal and paediatric critical care units)
-
Design Clearly reported as prospective
-
Outcomes Studies had to provide a definition of catheter-BSI (including any definitions given in the publication or accompanying supplementary material or hyperlinks).
Study selection for the systematic review
Once the criteria for the systematic review had been set, all of the studies classified in the map were rechecked by one reviewer to ensure that the keywords regarding the population, design and outcomes were accurate. Studies from the map which met the three inclusion criteria reported above for population, design and outcomes were then entered into the full systematic review.
Data extraction in the systematic review
A data extraction and quality assessment form was devised for the systematic review based on a standard template used by the project team for other systematic reviews. The template was adapted to take into account the reporting standards recommended by the TREND Statement for reporting non-randomised studies of behavioural interventions;55 a Best Evidence in Medical Education (BEME) Guide for systematically reviewing educational interventions;56 and the ORION Statement for reporting intervention studies of nosocomial infections. 57 The form was piloted on a subset of the studies to ensure good inter-reviewer reliability. Data from each study included in the systematic review were then extracted by one reviewer and were checked by a second reviewer. Any disagreements between reviewers were resolved by consensus or if necessary by arbitration by a third reviewer. The completed data extraction forms can be seen in Appendix 5.
Quality assessment
Using criteria specified a priori, we assessed two aspects of the quality of the included studies: (1) risk of bias, which identifies methodological deficiencies in studies that could lead to systematic errors in outcomes and (2) the reporting of the methods used to collect data, which was identified by the project AG as an important aspect of study quality that should be assessed.
Risk of bias
The concept of risk of bias indicates whether study outcomes are likely to be valid and, hence, whether they may be trusted in the data synthesis. 58 Project scoping searches indicated that before-and-after studies were likely to be the most frequent research approach used for evaluating the effectiveness of educational interventions for preventing catheter-BSI. Our quality assessment criteria therefore focused on before-and-after studies, with additional criteria provided for assessing the quality of randomised controlled trials (RCTs), where appropriate. Risk of bias criteria have been established by the Cochrane Collaboration for RCTs58 and could, in principle, be adapted or developed for assessing some aspects of bias in non-randomised studies. 59 Before-and-after studies without a concurrent control group may be particularly at risk of performance bias, as investigators may be unable to isolate effects of their intended changes in health-care practice (e.g. implementation of an intervention) from other changes that could influence patient outcomes (e.g. intrahospital, regional or national changes in health-care practices or policies). Before-and-after studies may also be at risk of selection bias if the population characteristics of the intervention and comparator (baseline) groups differ systematically, and attrition bias if availability of data differs between the intervention and comparator groups.
Specific criteria for assessing risk of bias have not been published for before-and-after studies. We developed assessment criteria for risk of selection bias, performance bias and attrition bias (Table 4), based on the general format of the Cochrane risk of bias tool, in which risk of bias is judged either as low, high or unclear. 58 Judgements of ‘unclear’ risk of bias were made if insufficient information was reported to assess a given risk of bias criterion. The risk of bias criteria were agreed by the review team and then applied independently by two reviewers to the studies included in the systematic review. The criteria were revised in light of disagreements between the reviewers' judgements. The final risk of bias criteria (see Table 4) are intended to assist interpretation of the current data synthesis [see Chapter 4, Synthesis of effectiveness (primary outcomes)] and may not be applicable outside this current systematic review. It should be recognised that assessment of risk of bias is an imperfect science, as subjectivity of interpretation and inter-reviewer disagreements occur even with the well-established Cochrane risk of bias criteria for RCTs. 60
| Type of bias | Criteria for judgement of ‘low’ | Criteria for judgement of ‘high’ |
|---|---|---|
| Selection bias risk: systematic differences between groups | Characteristics of the patient groups were similar in the baseline and intervention periods, or any differences would be unlikely to influence risk of catheter-BSI | Characteristics of the patient groups differed between the baseline and intervention periods to an extent that could influence risk of catheter-BSI |
| Performance bias risk: effects of other concurrent intervention(s) | Outcomes are interpretable in relation to a specified intervention | Outcomes could be explained by more than one intervention |
| Performance bias risk: other concurrent staff or policy changes | Baseline and intervention periods were similar in staff numbers and expertise; patient management policies (at hospital or critical care unit levels); and critical care unit infrastructure (e.g. bed numbers) | There were notable differences between the baseline and intervention periods in staff numbers or expertise; in patient management policies; and/or critical care unit infrastructure |
| Performance bias risk: outcome assessment not blinded | Staff involved in blood sampling and culturing were unaware they were in a research study | Staff involved in blood sampling and culturing were aware they were in a research study |
| Performance bias risk: outcome definition or measurement differences between groups | Methods for taking blood for cultures and/or criteria for diagnosing catheter-BSI did not differ between baseline and intervention periods | Methods for taking blood for cultures and/or criteria for diagnosing catheter-BSI were different in baseline and intervention periods |
| Attrition bias risk: imbalances between groups in missing data | Patient population clearly described and the numbers of patients who provided primary outcome data agree with the described population for baseline and intervention periods | Availability of primary outcome data differed between baseline and intervention periods; and/or reasons for missing data related to intervention effectiveness |
For RCTs, we applied the risk of bias criteria listed in Table 4 to before-and-after comparisons within each study arm so as to enable comparisons with the non-randomised studies; and we also assessed risks of selection bias according to the adequacy of randomisation and allocation concealment, according to the standard Cochrane risk of bias criteria for RCTs. 58
In addition to the risks of selection, performance and attrition bias reported above which we assessed using the criteria specified a priori (see Table 4), any other possible sources of bias noted by the reviewers in the primary studies were recorded at the data collection stage, in a free text field of the data extraction form.
Reporting of data collection in the primary studies
We recorded whether the methods of data collection were reported for clinical outcomes, infection surveillance and staff performance. Judgements were agreed by two reviewers and were recorded as yes, no, partly or unclear, together with an explanatory statement, in the data extraction form for each study. If data collection processes were reported, we also recorded whether they were shown to be validated and reliable.
Data synthesis
Studies were synthesised narratively following a structured approach similar to one proposed by Rodgers and colleagues. 61 In addition to the narrative synthesis, we explored the possibility of calculating pooled-effect estimates across independent studies in meta-analysis, taking into consideration methodological similarities and differences between the studies.
The primary review outcome was incidence density of catheter-BSI, expressed as the number of catheter-BSI per 1000 catheter-days. Effects were expressed as incidence density risk ratios (RRs) with 95% confidence intervals (CIs), for comparisons between intervention and baseline periods in the before-and-after studies. If a RR and CI were not reported for a primary study, we calculated these from catheter-BSI incidence data and the corresponding number of catheter-days, using the method of Kirkwood and Sterne. 62 In cases when catheter-BSI incidence and catheter-days were not reported, we sought these data from the study investigators. All secondary review outcomes (including compliance and process evaluations) were synthesised narratively. For all steps of the data synthesis, calculations and narrative syntheses were conducted by one reviewer and were then checked by a second reviewer.
Chapter 3 Results of the mapping exercise
Results of the literature search
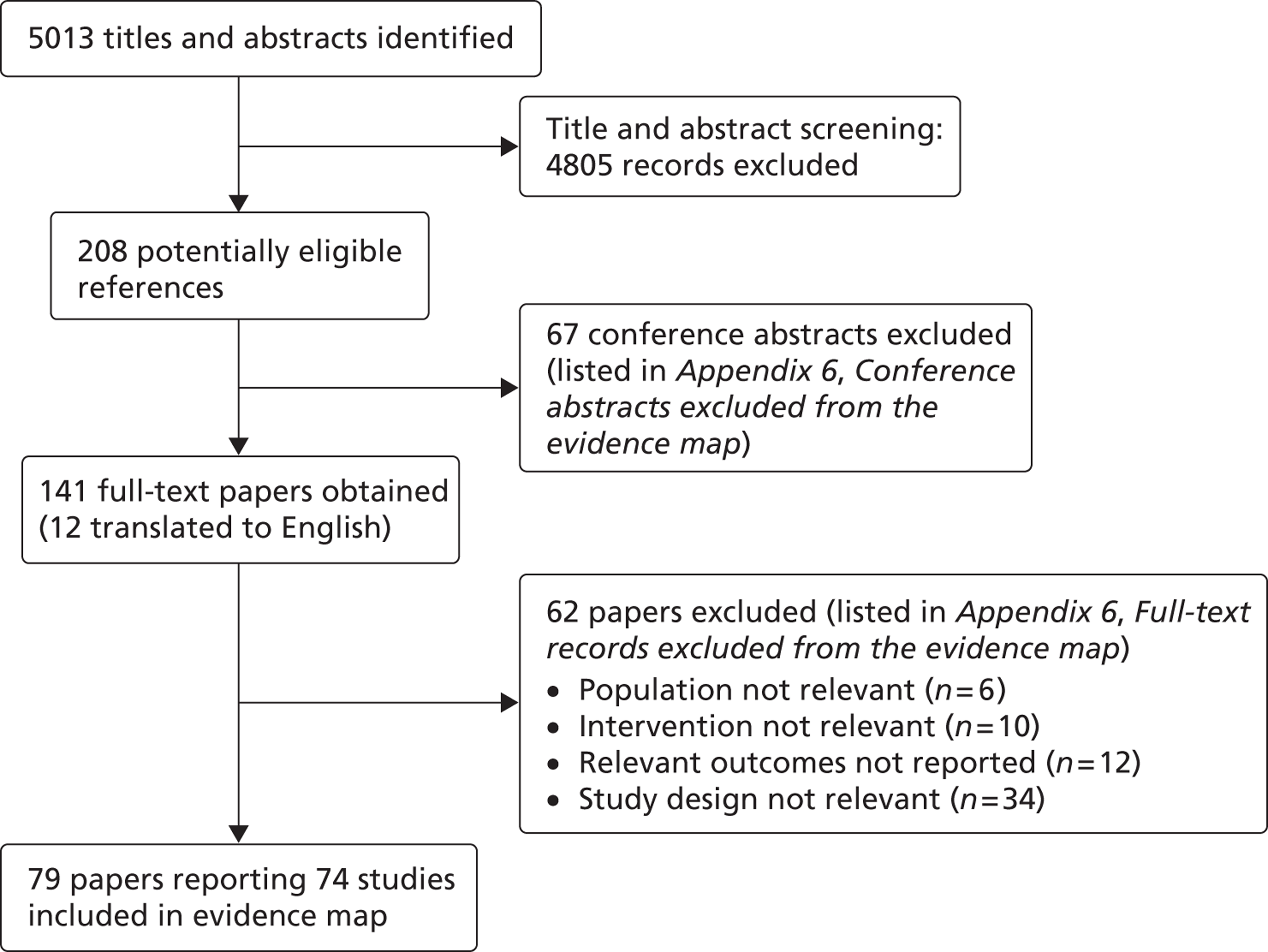
The process for identifying relevant references and selecting studies for the evidence map is shown in Figure 1. After excluding duplicates, we identified a total of 5013 potentially relevant references. Of these, we excluded 4805 references, as their titles and/or abstracts clearly did not meet the selection criteria. Reviewer agreement at the title and abstract screening step was 99% [Cohen's kappa (κ) = 0.90]. Sixty-seven of the records not initially excluded were conference abstracts. These were found to provide too little information to contribute to the keyword mapping exercise and were subsequently excluded. We retrieved the full-text publications for the remaining 141 records for inspection. These included 12 non-English-language publications, which we translated from Spanish,63–69 French,70–72 German73 and Swedish. 74 Following this selection process, 79 of the full-text records describing 74 primary research studies were included in the evidence map. 34,50,51,64,65,68–70,75–146
FIGURE 1.
Process for selecting studies in the evidence map
Characteristics of the studies
Geographical locations
Of the 74 primary research studies reporting educational interventions for preventing catheter-BSI which we included in the evidence map, most (65%) have been conducted in North America. Seven studies have been conducted in Spain (10%),64,65,68,69,121,122,127 five in Brazil (7%),108,109,112,118,132 two each in Argentina,129,130 France,70,117 Switzerland,94,144 and the UK,110,128 and one each in Australia,83 Belgium,111 Canada,119 Italy,120 South Korea142 and Mexico. 103 The UK studies were conducted in Northern Ireland128 and Scotland110 in paediatric and adult critical care units, respectively. No multinational studies were identified.
Spatial and temporal scales
Most (58) studies (78%) were conducted in single hospitals, with 40 of the studies (54%) conducted in single critical care units. Of the included studies, 11 were conducted in 10 or more critical care units. 34,68,82,83,85,91,107,115,133,140,141 The largest study conducted was the Michigan Keystone ICU project,34 which included 103 critical care units in 67 hospitals in Michigan, USA.
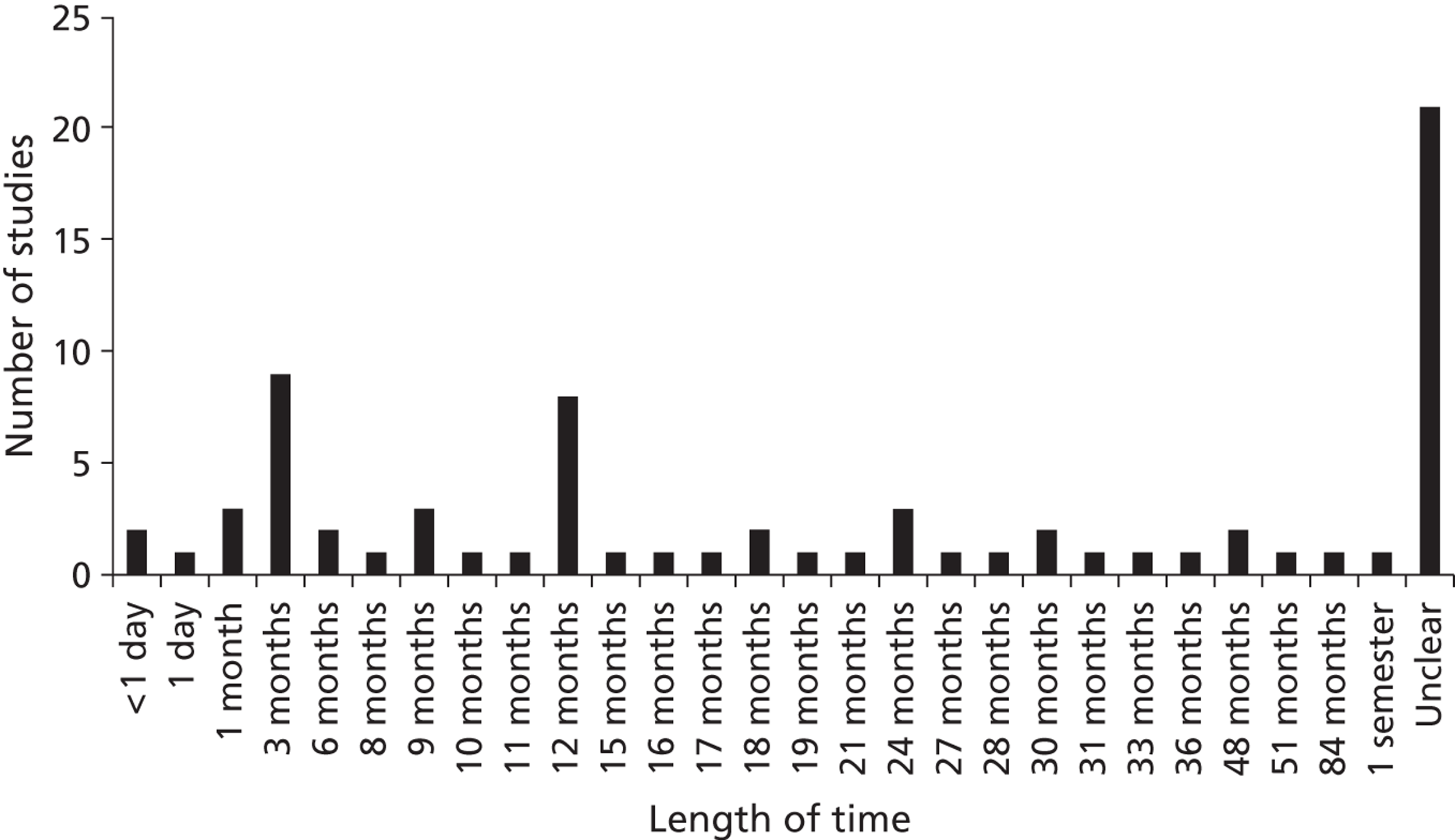
The duration of interventions ranged from < 1 day to 7 years. In approximately one-quarter of the studies the intervention duration was unclear owing to inadequate or ambiguous reporting (Figure 2).
FIGURE 2.
Duration of interventions
Study designs
The designs of the interventional studies are summarised in Table 5, based on a classification system proposed by the Cochrane Collaboration. 58,59 The order of study designs in Table 5 reflects a hierarchy of the reliability of evidence, with risk of bias likely to be lower in well-conducted RCTs than in cohort studies.
| Study design (Cochrane classification59) | No. (rounded %) of studies (n = 74) |
|---|---|
| Before-and-after study – prospective | 48 (65) |
| Design unclear | 9 (12) |
| Before-and-after study – historically controlled | 8 (11) |
| Controlled trial (including RCT, quasi-RCT, non-randomised) | 3 (4) |
| Interrupted time series (controlled or uncontrolled) | 2 (3) |
| Before-and-after study – unclear whether prospective | 4 (5) |
The most frequently used study design, in 48 studies (65%), was a prospective before-and-after study (see Table 5). Eight studies81,96,111,116,134,137,141,142 were historically controlled before-and-after studies. Three studies68,136,145 were controlled trials but only two of these were randomised. 136,145 Speroff and colleagues136 conducted a cluster RCT in the USA, in which 60 hospitals were randomised to either a virtual collaborative quality improvement (QI) programme or a toolkit-based approach for preventing catheter-BSI. Khouli and colleagues145 conducted a RCT in the USA in which medical residents were randomised in a simulation laboratory to simulation-based plus video training for CVC insertion or video training alone. Palomar-Martinez and colleagues68 conducted a study in Spain in which 17 critical care units received either an intervention based on the Michigan Keystone ICU project or served as controls. Two studies used an interrupted time series approach. 88,115 In the remaining 13 of the 74 studies (18%) the design was judged unclear, either because it was unclear whether pre-intervention groups were selected prospectively or retrospectively (four studies100,106,120,121), or because too little information about the study methods was reported to classify the design (nine studies78,80,82,90,101,102,105,113,125).
Critical care specialties
The majority of studies were in adult medical, surgical and cardiac critical care units (Table 6). The specialties were not described consistently in the studies and in some cases were unclear. Numbers in Table 6 do not sum to 74, as some studies covered multiple specialties.
| Specialty as described by the study authors | No. of studies |
|---|---|
| Medical | 20 |
| Medical–surgical | 18 |
| Surgical | 18 |
| Cardiac/coronary | 17 |
| Neonatal | 11 |
| Paediatric | 8 |
| Neurological/neurosurgical | 9 |
| Trauma | 6 |
| General or mixed | 4 |
| Burn | 1 |
| Other | 6 |
| Not reported or unclear | 7 |
Intravascular devices
The studies in general provided very little information about the vascular devices used. Sixty-nine studies (93%) referred to central lines or CVCs. Five studies65,94,105,117,131 stated that they included arterial catheters as well as CVCs, and one study51 specifically excluded arterial catheters. Many of the studies did not report the insertion site (64% of the studies), lumen material (95%), whether antimicrobial-impregnated catheters were used (74%) or the number of lumens used (91%) (Table 7). Comparisons between studies are problematic, as unreported differences in vascular devices could contribute to interstudy differences in catheter-BSI incidence rates. Only three studies83,90,131 reported catheter-BSI data separately for different types of vascular catheter.
| Device characteristics | No. (rounded %) of studies (n = 74) |
|---|---|
| Insertion site | |
| Fully reported | 15 (20) |
| Partly reported | 12 (16) |
| Not reported | 47 (64) |
| Lumen material | |
| Fully reported | 4 (5) |
| Partly reported | 0 (0) |
| Not reported | 70 (95) |
| Lumen coating/impregnation | |
| Fully reported | 13 (18) |
| Partly reported | 6 (8) |
| Not reported | 55 (74) |
| Lumen number | |
| Fully reported | 3 (4) |
| Partly reported | 4 (5) |
| Not reported | 67 (91) |
Description and definition of catheter-bloodstream infection
In 36 of the 74 studies (49%) the authors described BSIs as catheter-associated (CABSI) and in 31 of the studies (42%) the authors described BSIs as catheter-related (CRBSI). Three studies70,104,117,125 described BSIs in other ways and the remaining three studies80,86,118 provided no description for the infection data they presented. Twenty-eight of the 74 studies (38%) provided a definition of catheter-BSI within their report and 56 studies (76%) cited a reference to a definition. Most of the studies defined catheter-BSI according to criteria of the US CDC NNIS, which operated up to 2004, or the CDC NHSN, which replaced the NNIS in 2005. Although the CDC definitions have changed through time, studies continued to cite old versions. For example, at least seven studies published since 2005 referred to infection definitions published in 1988.
Aims of the studies
Most (57) of the 74 studies (77%) stated that their aim was specifically to prevent catheter-BSI in critical care. The remaining studies mostly aimed to prevent catheter-BSI together with ventilator-associated pneumonia (VAP),82,102,128,136 together with VAP and urinary tract infections (UTIs),86,104,114,117,130,137 or together with VAP and sepsis prevention. 91,101 Five studies89,93,94,118,120 each included prevention of catheter-BSI as part of a more general aim (Table 8).
| Aim | No. (rounded %) of studies (n = 74) |
|---|---|
| Specifically to prevent catheter-BSI | 57 (77) |
| Other aims | |
| Prevention of catheter-BSI and VAP | 4 (5) |
| Prevention of catheter-BSI and VAP and UTI | 6 (8) |
| Prevention of catheter-BSI and VAP and sepsis | 2 (3) |
| Prevention of MRSA, catheter-BSI, VAP and UTI | 1 (1) |
| Prevention of VAP, pressure ulcer, UTI, VAP, deep vein thrombosis | 1 (1) |
| Prevention of ICU-acquired infections including catheter-BSI | 1 (1) |
| Prevention of nosocomial infections including catheter-BSI | 1 (1) |
| Identification of risk factors for catheter-BSI | 1 (1) |
Types of educational intervention
Of the 74 studies, 25 (34%) provided information on development or testing of their interventions but only three77,102,119 (4%) reported that their interventions were based on educational theory.
The most frequently addressed clinical practices were hand hygiene (49 studies), catheter insertion site preparation (typically providing guidance on the use of chlorhexidine or povidone–iodine) (45 studies), use of full barrier precautions (43 studies), and catheter insertion site selection (typically discouraging use of the femoral site) (36 studies). The studies were variable in the extent to which they reported the clinical practices addressed by their interventions, and it was not always clear which clinical practices were included in education. For example, of the 49 studies with interventions that targeted hand hygiene behaviour, only 37 studies mentioned explicitly that intervention included education about hand hygiene (Table 9).
| Clinical practice | Included in intervention | Specified as target of education |
|---|---|---|
| Hand hygiene | 49 | 37 |
| Catheter insertion site preparation, management, and/or ongoing care | 45 | 29 |
| Barrier precautions – full | 43 | 28 |
| Catheter insertion site selection (e.g. avoidance of femoral site) | 36 | 24 |
| Checklist (principally about catheter insertion) | 35 | 5 |
| Dressing care (hygiene, removal, replacement) | 33 | 23 |
| Catheter need review (e.g. daily inspection; removal of unnecessary catheters) | 30 | 17 |
| Barrier precautions – specific (e.g. drapes, gloves, gown) | 27 | 20 |
| Unspecified aseptic or sterile technique | 22 | 19 |
| Catheter device ongoing management (e.g. flushing; hub care, including ‘scrub the hub’) | 20 | 19 |
| Dressing selection (e.g. antimicrobial biopatch) | 19 | 13 |
| Infection prevention and/or control (including evidence-based practice; guidelines) | 15 | 15 |
| Central line cart use | 15 | 1 |
| Catheter (re)placement procedure (e.g. use of ultrasound or radiography; avoidance of guidewires) | 10 | 8 |
| Epidemiology of BSIs | 8 | 8 |
| Catheter type selection (e.g. lumen number, chemical impregnation) | 5 | 4 |
| Documentation and auditing of processes | 5 | 3 |
| Team approach to catheter care | 5 | 4 |
| Administration of intravenous medication or infusate | 4 | 4 |
| Blood draw and/or culture technique | 4 | 4 |
| Measurement and/or definition of infection | 4 | 4 |
| Catheter-BSI risk factors | 2 | 2 |
| Responsibilities of health-care workers | 1 | 1 |
| Complications of vascular catheter insertion | 1 | 1 |
| Isolation and contact precautions | 1 | 1 |
| Educational topic(s) not reported or unclear (only a very general or vague description was provided) | 16 | 16 |
Twenty-two studies (30%) reported interventions that were purely educational and 52 studies (70%) reported that their interventions contained components beyond education (e.g. provision of antiseptic, or a catheter supplies cart).
The educational forum types (approaches to education delivery) are summarised in Table 10. Studies often gave rather general descriptions of educational approaches in which specific details were not reported. For this reason, the numbers of studies that used particular educational forum types may have been underestimated.
| Education forum | No. of studies |
|---|---|
| Checklist | 35 |
| Lecture(s), course(s) or workshop(s) | 29 (+ one unclear)a |
| Printed material (leaflet, pamphlet, magazine, book, course documents) | 27 |
| Discussion group(s) | 20 |
| Poster | 18 |
| Audiovisual (video or slide show) | 12 |
| Fact sheet | 11 |
| Electronic teaching materials (multimedia resources on CD, DVD, computer, internet) | 12 |
| Champion/opinion leader (= group based) | 11 (+ one unclear) |
| Practical demonstration of catheter technique or related activity | 10 |
| Face-to-face meetings | 9 |
| Self-study | 8 |
| Skills practice | 8 (+ one unclear) |
| Goal sheet | 7 |
| Virtual learning (computer-mediated instruction) | 6 |
| Supervision | 6 |
| Conference calls | 5 |
| Reminders | 4 |
| Simulation | 3 |
| Mentoring, shadowing or coaching | 3 |
| Information label | 2 |
| One or more component(s) of the educational forum unclear | 16 |
| Education materials not reported or unclear | 34 |
Thirty-one studies (42%) reported infection surveillance feedback and 36 studies (49%) reported performance feedback, but in nine studies79,80,85,115,118,119,125,128,142 it was unclear whether feedback approaches were used. In several studies, feedback approaches that were described as being part of an intervention also appear to have been used during the pre-intervention period. 50,87,136,138,139
Characteristics of the educational approaches are summarised in Table 11 and also may have been underestimated owing to incomplete reporting by the study authors. Active learning approaches that reinforced education delivery by repeated information provision, testing and assessment and/or feedback approaches were reported in 49 of the 74 studies (66%), but in 18 studies (24%) it was unclear whether active or passive (non-reinforced) educational approaches were used. It is difficult to draw firm conclusions about the use of didactic and interactive educational approaches because often teaching sessions were described too superficially for us to be sure of their approach. At least 31 of the studies (42%) used an interactive approach to learning which encourages self-discovery of information. Of the 74 studies, 21 studies (28%) made it clear that their educational sessions were mandatory, 46 studies (62%) conducted at least some of the training in-service, and group-based education appears to have been more frequently used than individual training (see Table 11).
| Education characteristics | No. (rounded %) of studies (n = 74) |
|---|---|
| Mode of learning | |
| Passive | 7 (9) |
| Active | 25 (34) |
| Both passive and active | 24 (32) |
| Unclear | 18 (24) |
| Mode of information presentation | |
| Didactic | 7 (9) |
| Interactive | 15 (20) |
| Both didactic and interactive | 16 (22) |
| Unclear | 36 (49) |
| Participation in educational sessions | |
| Mandatory | 21 (28) |
| Voluntary | 4 (5) |
| Unclear | 49 (66) |
| Location of education/training | |
| In service | 46 (62) |
| External, residential | 0 (0) |
| External, non-residential | 4 (5) |
| Unclear | 24 (32) |
| Contact type | |
| Individual based | 10 (14) |
| Group based | 38 (51) |
| Both individual and group based | 13 (18) |
| Unclear | 13 (18) |
Knowledge of the providers and recipients of the education (Table 12) and the intensity (concentration) of education (Table 13) is important for determining the resources that would be required if the intervention were to be replicated in another setting. Only 27 of the studies (36%) provided full information on who delivered the education, whereas 47 of the studies (64%) fully reported the intended recipients. In 42 of the studies (57%) it was unclear how many educational sessions were used and in 54 (73%) it was unclear how much time the educational sessions occupied.
| Category | No. (rounded %) of studies (n = 74) |
|---|---|
| Education providers | |
| Fully reported | 27 (36) |
| Partly reported | 12 (16) |
| Unclear | 35 (47) |
| Education recipients | |
| Fully reported | 47 (64) |
| Partly reported | 10 (14) |
| Unclear | 17 (23) |
| Educational sessions | No. (rounded %) of studies (n = 74) |
|---|---|
| Frequency | |
| Fully reported | 12 (16) |
| Partly reported | 20 (27) |
| Unclear | 42 (57) |
| Duration | |
| Fully reported | 6 (8) |
| Partly reported | 14 (19) |
| Unclear | 54 (73) |
| Both frequency and duration fully reported | 6 (8) |
| Frequency and duration both unclear | 33 (45) |
Concurrent changes in clinical practice, policy or infrastructure in addition to the intended intervention for preventing catheter-BSI were evident in 17 studies (23%). These included changes in the use of antimicrobial catheters, mechanical ventilation and/or UTI prevention bundles, hand hygiene programmes, staffing or critical care bed number. Where such changes occurred it would be difficult to isolate the effects of the intended intervention for prevention of catheter-BSI. Only 7 of the 74 studies (9%) explicitly stated that other concurrent interventions or changes in patient care did not occur during a study. 70,77,85,86,121,122,145
Mapping exercise: summary of results
Seventy-four studies were identified that have investigated the effectiveness of educational interventions for preventing catheter-BSI in critical care. The majority of research has involved uncontrolled before-and-after studies conducted in the USA, predominantly local-scale studies in single critical care units but also some regional-scale studies with up to 103 critical care units. Very limited information is available for the UK. Approximately half of the studies described catheter-BSI as CABSI and the other half as CRBSI, with few other descriptions reported. The most commonly targeted clinical practices were hand hygiene, preparation of the insertion site and the use of maximal sterile barrier precautions, with a wide variety of educational approaches used to address these. Interventions containing components beyond education were reported approximately twice as frequently as interventions consisting of education alone. Few studies reported the intensity of education they provided, which makes it difficult to determine resource requirements.
The evidence map highlights that secondary synthesis of educational interventions for preventing catheter-BSI is a complex area requiring appraisal of educational/behavioural strategies, complex interventions and non-randomised studies.
As mentioned earlier in Chapter 2 (see The process of descriptive mapping), the results of the mapping exercise were discussed with the project's AG to identify studies relevant to NHS practice and policy for prevention of catheter-BSI. Various different inclusion criteria for the systematic review were discussed, based on the findings of the map. These discussions led to the identification of the following inclusion criteria for the systematic review:
-
Population Adults (i.e. excluding neonatal and paediatric critical care units).
-
Design Clearly reported as prospective.
-
Outcomes Studies had to provide a definition of catheter-BSI (including any definitions given in the publication or accompanying supplementary material or hyperlinks).
Chapter 4 Results of the systematic review of clinical effectiveness
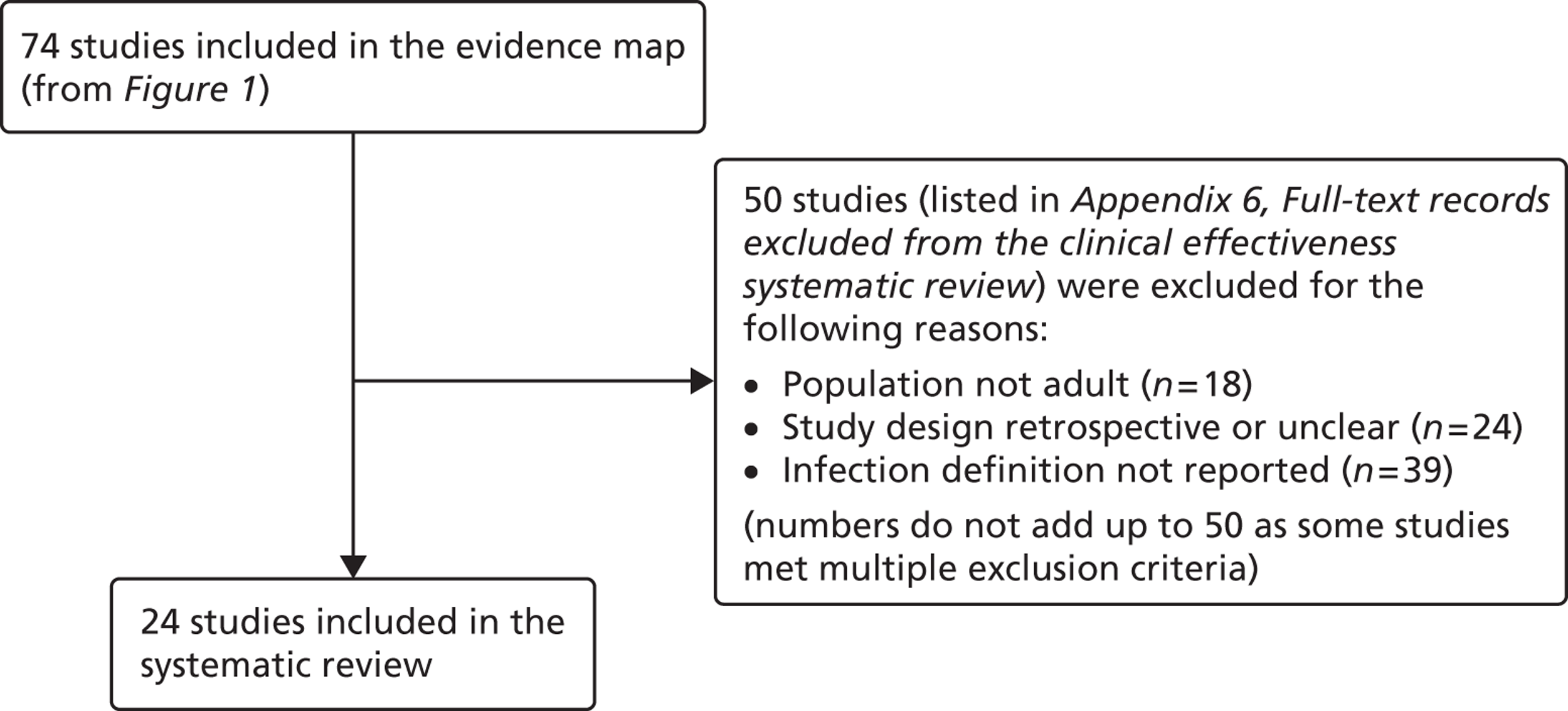
The inclusion criteria for the systematic review were chosen through discussion with the project's AG, to prioritise studies that were likely to be of most relevance to current NHS policy and practice for preventing catheter-BSI in critical care units in England. The agreed inclusion criteria specified that studies should have assessed interventions in adult critical care units; used and clearly reported a prospective study design; and provided a definition of catheter-BSI. Twenty-four34,50,51,68,83,87,93,94,97,98,103,108–110,117,122,126,129,130,135,136,138,139,144 of the 74 studies included in the evidence map met these criteria and were included in the systematic review (Figure 3). Below, we describe the key characteristics of these 24 studies, before going on to consider their methodological quality and the effectiveness of their educational interventions for the prevention of catheter-BSI.
FIGURE 3.
Process for selecting studies for inclusion in the systematic review
Characteristics of the included studies
The current systematic review focuses on studies of adult critical care units, with prospective designs, and which provided definitions of catheter-BSI, but in other respects the characteristics of the included studies are broadly representative of those included in the evidence map (Table 14). Half of the studies were conducted in the USA, and the majority used single-cohort before-and-after designs. One of the two UK studies in the evidence map was excluded from the systematic review because it was conducted in neonatal critical care units,128 and one of the two RCTs in the evidence map was excluded from the systematic review because it did not provide a definition of catheter-BSI. 145 The systematic review includes studies conducted at different spatial and temporal scales, and involving different types of education, either focusing on education alone, or including additional intervention strategies beyond education (see Table 14). Further details of the study characteristics are presented below.
| Study characteristic | No. (rounded %) of studies included in the evidence map (n = 74) | No. (rounded %) of studies included in the systematic review (n = 24) |
|---|---|---|
| Conducted in the USA | 48 (65) | 12 (50) |
| Conducted in the UK | 2 (3) | 1 (4) |
| Prospective before-and-after study | 40 (54) | 20 (83) |
| RCT | 2 (3) | 1 (4) |
| Design unclear | 13 (18) | 0 |
| Education alone | 22 (30) | 14 (58) |
| Components beyond education | 52 (70) | 10 (42) |
| Short term (≤ 12 months) | 32 (43) | 13 (54) |
| Long term (> 12 months) | 21 (28) | 11 (46) |
| Duration unclear | 21 (28) | 0 |
Study designs
The 24 studies included in the systematic review had the following designs. Most (20) were prospective before-and-after studies and these involved either single critical care units,50,51,87,93,94,97,108,110,138 multiple critical care units for which results were pooled across the units,34,83,98,126,129,130,135,139 or multiple critical care units for which results were presented separately for each unit. 103,109,122,144 One RCT136 was based on clusters, with hospitals randomised to interventions, but implementation was at the level of the critical care unit, comparing ‘Virtual Collaborative’ and ‘Toolkit’ CQI programmes in 60 critical care units. One non-randomised study68 compared a CQI intervention and control (no intervention) in 17 critical care units. The remaining two studies83,117 were single-cohort CQI programmes without a true baseline period in which data from early in the intervention period served as the baseline (Table 15).
| Study (publication year) | Country (no.: specialty of critical care units) [duration of intervention]. Intervention summary | Study design |
|---|---|---|
| Burrell;83 CEC146 (2010, 2011) | Australia (37: not reported; included paediatric units at two hospitals) [18 months]. CQI programme based on principles of the Michigan Keystone ICU intervention,34 including a ‘clinician bundle’ (hand hygiene, barrier precautions and sterile technique) and a patient bundle (skin preparation, patient draping and imaging catheter positioning during insertion) with performance feedback and infectionsurveillance feedback; aimed at ICU staff (staff grades not specified) | Single cohort without true baseline period |
| Coopersmith (2002)50 | USA (1: surgical/burn/trauma) [6 months]. Multimodal education on CVC care and unspecified topics including in-services, posters and fact sheets, self-study and performance feedback; aimed at critical care nurses and also non-critical care staff | Prospective before-and-after study |
| Coopersmith (2004)87 | USA (1: surgical) [15 months]. Multimodal education including lectures, self-study, practical demonstration, in-services, pictures of CVC maintenance, with broad topic coverage; aimed at nurses | Prospective before-and-after study |
| DuBose (2008)93 | USA (1: trauma) [3 months]. Daily quality rounds checklist with 2 out of 16 checklist items relevant to CRBSI prevention; aimed at ICU fellows, residents and medical students | Prospective before-and-after study |
| Eggimann (2000),94 (2005)95 | Switzerland (1: medical) [up to 6 years]. Education based on 30-minute slideshows, bedside in-services and practical demonstration; aimed at all critical care staff (physicians, nurses and nursing assistants) | Prospective before-and-after study |
| Galpern (2008)97 | USA (3: medical and surgical (mixed across three locations), one cardiac) [19 months]. Central line bundle including discussion sessions about CVC access and care, checklist, infection surveillance feedback, performance feedback and catheter cart; aimed at all ICU staff (physicians and nurses) | Prospective before-and-after study |
| Guerin (2010)98 | USA (2: medical, surgical) [1 year]. Post-insertion central line care bundle including 4-hour hands-on practical sessions on CVC access and care followed by competence evaluation; included performance feedback and provision of an intravenous therapy team; aimed at all critical care unit nursing staff | Prospective before-and-after study |
| Higuera (2005)103 | Mexico (2: medical surgical, neurosurgical) [9 months]. Process control intervention including 1-hour classes and provision of CDC infection control guidelines, with performance feedback and the provision of alcohol hand rub; aimed at ICU nurses, ancillary staff and physicians | Prospective before-and-after study |
| Lobo (2005)108 | Brazil (2: medical) [8 months + 12 months’ follow-up]. Multimodal education with infection surveillance feedback, including posters and fact sheets with emphasis on hand hygiene; aimed at all critical care staff | Prospective before-and-after study |
| Lobo (2010)109 | Brazil (2: medical) [9 months]. Two educational interventions with performance feedback and infection surveillance feedback: ICU A – monthly lectures and monthly questionnaire, ICU B – single lecture, unspecified duration; aimed at all critical care staff | Prospective before-and-after study |
| Longmate (2011)110 | Scotland (1: medical surgical) [up to 36 months]. CQI programme (including VAP prevention) incorporating checklist, infection surveillance feedback and performance feedback; aimed at critical care nurses and trainee doctors | Prospective before-and-after study |
| Misset (2004)117 | France (1: medical surgical) [unclear, ≈ 5–6 years]. CQI programme (including VAP and UTI prevention) based on infection surveillance feedback; aimed at all ICU staff (nurses and residents) | Single cohort without true baseline period |
| Palomar Martinez (2010)68 | Spain (17: not reported) [3 months]. Pilot study evaluating the feasibility of national implementation of a CQI intervention based on the Michigan Keystone ICU intervention34 | Non-randomised controlled trial, historical baseline |
| Perez Parra (2010)122 | Spain (3: medical, general post surgery, cardiac post surgery) [15–20 minutes + 9 months’ follow-up]. Short (15-minute) lecture on approaches for CVC care and maintenance; aimed at all critical care staff, with post-test 6 months after intervention | Prospective before-and-after study |
| Pronovost (2006),34 (2008),124 (2010)123 | USA (103: including medical, cardiac surgical, neurological surgical, cardiac medical, trauma and one paediatric) [up to 3 years]. CQI programme (Michigan Keystone ICU project) using a checklist, presentations and meetings, fact sheet, infection surveillance feedback and catheter supplies cart; aimed at ‘ICU colleagues’ (unspecified) | Prospective before-and-after study; some ICUs without true baseline period |
| Render (2006)126 | USA (8: medical) [unclear; data reported for 1 year]. Continuous QI programme based on work/learning/reporting cycles, including performance feedback and infection surveillance feedback; aimed at ICU nurses and physicians | Prospective before-and-after study |
| Rosenthal (2003)129 | Argentina (4: cardiac, medical-surgical) [8–10 months]. Unspecified education and performance feedback; aimed at health-care workers (unspecified). The education period (1–2 months) was followed by a performance feedback period (7–8 months) | Prospective before-and-after study |
| Rosenthal (2005)130 | Argentina (2: cardiac, medical surgical) [17 months]. Daily 1-hour educational classes and discussion groups for 1 week, followed by performance feedback during the remainder of the intervention period to enhance hand hygiene compliance; aimed at health-care workers (nurses, physicians and ancillary staff) | Prospective before-and-after study |
| Sherertz (2000)135 | USA (7: general medical and surgical + 1 step down unit) [3 days in each of 2 years]. Hands-on 1-day course on infection control practices and procedures including simulation and performance feedback; aimed at third year medical students and physicians completing their first postgraduate year. Course run on 3 days in June 1996 and 3 days in June 1997, with follow-up to December 1997 | Prospective before-and-after study |
| Speroff (2011)136 | USA (60: not reported, but included two paediatric per arm) [18 months]. Hospitals randomised to Virtual Collaborative and Toolkit CQI approaches (including VAP prevention), with access to interactive web seminars for both groups. Virtual collaborative: monthly educational and troubleshooting conference calls, individual coaching and electronic mailing list (designed to stimulate interaction among teams). Toolkit: based on evidence based guidelines, fact sheets, review of QI and teamwork methods, educational on-line tutorials and standardised data collection/charting tools. Included performance feedback | RCT |
| Wall (2005)138 | USA (1: medical) [2 years]. CQI programme involving real time feedback of infection rates and compliance with insertion practice, based on use of checklist, supervision of insertions, and web-based tutorial with competence assessment; aimed at ICU house staff (proceduralists) and nursing staff (as observers of procedures) | Prospective before-and-after study |
| Warren (2003)139 | USA (2: medical, surgical) [3 months + 10 months’ follow-up]. Multimodal education including lectures, self-study, bedside teaching, in-services, staff meetings, posters and fact sheets and performance feedback, with broad topic coverage; aimed at nurses and physicians | Prospective before-and-after study |
| Warren (2004)51 | USA (1: medical) [1 month + 23 months’ follow-up]. Multimodal education including lectures, self-study, bedside teaching, discussion groups, posters and fact sheets and performance feedback, with broad topic coverage; aimed at nurses and physicians | Prospective before-and-after study |
| Zingg (2009)144 | Switzerland (5: medical, trauma, cardiovascular, general surgery) [5 months]. Multimodal education including interactive training modules and video demonstrations focusing on hand hygiene and vascular catheter care; aimed at nurses and physicians | Prospective before-and-after study |
Populations
Characteristics of the critical care patient populations were generally reported superficially. Only 10 out of the 24 studies (42%)50,87,93,103,109,110,129,136,139,144 reported the age and/or sex of their patient population (Table 16). The youngest patients reported were on average in their early 40s, in the studies by DuBose and colleagues93 and Higuera and colleagues. 103 The oldest critical care population group reported was in the study by Rosenthal and colleagues,129 in which the mean age was close to 72 years. Where reported, the studies included mixed-sex populations, with the proportion of men ranging from 46% to 79%. Only one study mentioned the patients' ethnicity, stating that 94% of the population was Caucasian. 139 Comorbidities were reported inconsistently, with some studies providing considerable detail and others providing no information (see data extractions in Appendix 5). In most studies the population characteristics either did not differ between the intervention and comparator groups or were not reported. Where differences between groups were evident we considered the risk of bias (reported below).
| Study | Age (years) (mean unless stated) | Sex (% male) |
|---|---|---|
| Coopersmith (2002)50 | Not reported | Baseline 59.8; intervention 55.3 |
| Coopersmith (2004)87 | Baseline 54.5; intervention 57.4 | Baseline 49.4; intervention 56.9 |
| DuBose (2008)93 | Baseline 41.1; intervention range 40.3–41.6 | Baseline 73.0; intervention range 67.0–78.9 |
| Higuera (2005)103 | Baseline 44.32; intervention 45.91 | Baseline 45.5; intervention 48.2 |
| Lobo (2010)109 | ICU A: baseline 54; intervention 53 | ICU A: baseline 62; intervention 43 |
| ICU B: baseline 55; intervention 51 | ICU B: baseline 49; intervention 50 | |
| Longmate (2011)110 | Not reported | Baseline 56.1; intervention range 55.5–56.2 |
| Rosenthal (2003)129 | Baseline 71.98; intervention 71.91 | Baseline 48.8; intervention 53.6 |
| Speroff (2011)136 | Not reported | Baseline: virtual collaborative group 50.3; toolkit group 49.7 |
| Warren (2003)139 | Overall study period 67 | Overall study period: 52 |
| Zingg (2009)144 | Median: baseline 62; intervention 61 | Baseline 64; intervention 67 |
The critical care specialties most frequently reported were medical, surgical and cardiac. Three of the regional-scale studies33,83,136 were not conducted entirely in adult critical care units but we judged the studies to have met the population inclusion criterion for the systematic review because the proportion of non-adult critical units was small. Burrell and colleagues83 included two paediatric critical care units (95% were adult units), Pronovost and colleagues35 included one paediatric unit (99% were adult units) and Speroff and colleagues136 included two paediatric units in each study arm (93% were adult units). Palomar Martinez and colleagues68 appeared to focus on adult critical care but did not state this explicitly (the study authors were contacted but had not clarified this at the time of writing).
Vascular devices
All 24 studies included in the systematic review reported that they used CVCs, but it was not always clear whether other catheter types were also used. Misset and colleagues117 included arterial catheters, whereas Warren and colleagues51 specifically excluded these. Antimicrobial impregnated catheters were used routinely by DuBose and colleagues93 and Warren and colleagues139 but were not used by Sherertz and colleagues135 or Warren and colleagues. 51 Longmate and colleagues110 specified that their default catheters were non-impregnated and had four or five lumens, whereas Guerin and colleagues98 used antimicrobial-impregnated catheters unless patients were hypersensitive to them. Coopersmith and colleagues50,87 used four-lumen antimicrobial-impregnated catheters before their educational intervention but stated that their ‘accessibility was limited’ after the implementation of education (we discuss the implications of this for risk of bias below). The only other studies that reported lumen characteristics were by Wall and colleagues,138 who used triple-lumen catheters; Render and colleagues,126 who specified that 60% of the catheters were multilumen; and Misset and colleagues,117 who specified that single-lumen or multilumen catheters were used as clinically required.
Definitions of bloodstream infections
Of the 24 studies, 12 defined their infections as, or equivalent to, catheter-associated (CABSI), 11 defined their infections as, or equivalent to, catheter-related (CRBSI), and the remaining study117 gave an unclear definition (Table 17). Only eight studies provided definitions that agree with those of the Matching Michigan programme. In one of these studies, by Palomar Martinez and colleagues,68 definitions equivalent to CABSI and CRBSI were both accepted but were not separated when reporting the results (see Table 17). In support of their reported infection definitions, 28 of the studies also cited published references to definitions of CABSI or CRBSI. However, the most frequently-cited of these references, by Garner and colleagues,147 does not actually define CABSI or CRBSI.
| Study | Reported definition | Consistent with Matching Michigan definitions? |
|---|---|---|
| Burrell (2011)83,146 | CABSI | Yes – CABSI |
| Coopersmith (2002)50 | CRBSI | No |
| Coopersmith (2004)87 | CRBSI | No |
| DuBose (2008)93 | CRBSI | No |
| Eggimann (2000), 94, (2005)95 | CRBSI | No |
| Galpern (2008)97 | CABSI | No |
| Guerin (2010)98 | CABSI | Yes – CABSI |
| Higuera (2005)103 | CABSI | No |
| Lobo (2005)108 | CABSI | Yes – CABSI |
| Lobo (2010)109 | CABSI | No |
| Longmate (2011)110 | CRBSI | No |
| Misset (2004)117 | Unclear | No |
| Palomar Martinez (2010)68 | CRBSI | Yes – met either CRBSI or CABSI but not reported separately |
| Perez Parra (2010)122 | CABSI | No |
| Pronovost (2006),34, (2008),124 (2010)123 | CRBSI | No |
| Render (2006)126 | CRBSI | No |
| Rosenthal (2003)129 | CABSI | Yes – some met CABSI |
| Rosenthal (2005)130 | CABSI | Yes – CABSI |
| Sherertz (2000)135 | CRBSI | No |
| Speroff (2011)136 | CABSI | Yes – CABSI |
| Wall (2005)138 | CRBSI | No |
| Warren (2003)139 | CABSI | No |
| Warren (2004)51 | CABSI | Yes – CABSI |
| Zingg (2009)144 | CRBSI | No |
Types of educational intervention
The types of educational approach used in the 24 included studies are summarised in Table 15. As noted in the protocol (see Appendix 1), educational interventions were defined in a broad sense to capture any type of information provision relating to the prevention of catheter-BSI by critical care staff, including the use of checklists, performance feedback and information surveillance feedback. Fourteen of the studies (58%)50,51,87,93,94,108,109,122,129,130,135,138,139,144 used interventions that we classified as purely educational. These ranged from the provision of single lectures109,122 to multimodal combinations of bedside teaching,51,94,139 in-services 50,87,94,139 self-study,50,51,139 practical demonstrations,87,94,144 slide shows or videos,94,144 lectures,51,87,109,139 discussion groups or classes,51,130 supervision,138 simulations,135 and/or posters and fact sheets50,51,87,108,139 (see Table 15). In one study129 the educational approach was not reported and it is possible that other studies may not have fully reported all of the educational approaches that they used. These purely educational interventions were all implemented at a local scale, mostly in single critical care units.
A total of 1034,68,83,97,98,103,110,117,126,136 of the 24 studies (42%) were classified as having intervention components beyond education (a classification previously used by Safdar and Abad37 in a systematic review of interventions for preventing health care-associated infections). These studies included some of the educational approaches referred to above but, in addition, they used changes in equipment (e.g. the provision of a catheter supplies cart or alcohol for skin antisepsis) or infrastructure (e.g. the provision of a team). Five34,68,83,126,136 of these studies implemented their interventions at a regional scale, in 8,126 17,68 37,83 60136 or 10334 critical care units (see Table 15). These regional-scale studies included the Michigan ‘Keystone ICU’ project conducted in the USA;34,123,124 the ‘CLAB ICU’ project,83 which sought to replicate aspects of the Keystone ICU project in Australia; the ‘Bacteraemia Zero’ project,68 which sought to replicate aspects of the Keystone ICU project in Spain, and the RCT referred to above, which compared Virtual Collaborative and Toolkit approaches in the USA. 136 Four of these interventions could be described as CQI programmes34,83,126,136 in which iterative improvements in clinical practices became embedded over time, whereas the fifth68 was a short (3-month) pilot study of a CQI programme. A further two CQI programmes were implemented at a local scale in individual critical care units in France117 and Scotland. 110 The remaining three studies97,98,103 that implemented interventions with components beyond education at a local scale (in one to three critical care units) included a catheter insertion care bundle,97 a catheter ongoing care bundle,98 and an educational intervention with provision of alcohol hand rub. 103
The duration of interventions included in the systematic review ranged from a brief 15-minute lecture reported by Perez Parra and colleagues122 to up to 6 years in the case of a multimodal educational intervention reported by Eggimann and colleagues94,95 (see Table 15). With the exception of five studies,51,108,122,135,139 the studies monitored effects of the interventions on catheter-BSI incidence density only during the period of intervention implementation, without post-intervention follow-up. This reflects an intention in many of the studies that the interventions would become embedded into routine clinical practice.
Formal and informal education
Formal education implies that participants set aside some time for structured learning, for example to participate in classes, lectures, seminars, view slide shows, or take self-study modules. Informal education may involve passive information dissemination, for example in-service discussions during daily rounds, posters, newsletters, or supervision. The distinction is important from the perspective of resource provision since staff engaging in formal learning will be taken away from their critical care duties. Nearly all of the educational interventions contained formal education components (Table 18), implying that staff cover would need to be provided for those staff participating in an intervention. However, the concentration of the education (duration and frequency of sessions and whether they were periodically reinforced) was rarely reported (see data extraction forms in Appendix 5).
| Study | Type of education | Main formal education method used |
|---|---|---|
| Burrell (2011)83,146 | Formal + informal | Workshops |
| Coopersmith (2002)50 | Formal | 10-page self-study module |
| Coopersmith (2004)87 | Formal + informal | Lectures |
| DuBose (2008)93 | Unclear | – |
| Eggimann (2000),94 (2005)95 | Formal | Slide shows |
| Galpern (2008)97 | Informal | – |
| Guerin (2010)98 | Formal | 4-hour mandatory training |
| Higuera (2005)103 | Formal | 1-hour classes |
| Lobo (2005)108 | Formal | Monthly classes |
| Lobo (2010)109 | Formal | Lectures |
| Longmate (2011)110 | Formal | Self-guided education, including slide show |
| Misset (2004)117 | Unclear | – |
| Palomar Martinez (2010)68 | Formal + informal | ‘Kick-off’ meeting, online course |
| Perez Parra (2010)122 | Formal | 15-minute lecture |
| Pronovost (2006),34 (2008),124 (2010),123 | Formal + informal | PowerPoint (Microsoft Corporation, Redmond, WA, USA) presentation |
| Render (2006)126 | Formal + informal | 1.5-day ‘kick-off’ session of lectures/presentations – unclear if involved all staff |
| Rosenthal (2003)129 | Formal | Unclear, other than education and training provided |
| Rosenthal (2005)130 | Formal | 1-hour educational classes (repeated) |
| Sherertz (2000)135 | Formal | Classes on basic principles, plus skills station training |
| Speroff (2011)136 | Formal | Interactive web seminars |
| Wall (2005)138 | Formal | Web-based tutorial but not compulsory to complete |
| Warren (2003)139 | Formal | 45-minute lectures, and self-study module |
| Warren (2004)51 | Formal | 45-minute lectures, and self-study module |
| Zingg (2009)144 | Formal + informal | 45-minute classroom teaching sessions |
Educational theory
None of the 24 included studies specified an educational or behavioural theory. Pronovost and colleagues124 referred to the ‘4E’ framework proposed by Heifetz, in which the technical aspects (i.e. scientific evidence; definitions of measures standardised across hospitals) are classed as education and evaluation, whereas the adaptive elements (i.e. implementation of measures; interventions modified to fit the local context of a clinical area) are classed as engagement and execution. The authors stated only that the technical functions in the study were centralised.
Comparison of educational interventions with UK practice
The Matching Michigan programme is reflective of current NHS practice for preventing catheter-BSI (see Chapter 1) and educational interventions included in the systematic review shared some of the approaches used in Matching Michigan to varying degrees (Table 19). Of the 24 included studies, 11 studies34,68,83,93,97,108–110,126,136,138 used checklists to improve compliance with best practices for prevention of catheter-BSI. Three studies34,83,98 empowered their staff to halt CVC insertion procedures if protocols for infection prevention were not followed. Fourteen studies34,50,83,87,97,108–110,117,126,130,136,138,139 included infection surveillance feedback in their interventions, in which catheter-BSI incidence rates were reported to critical care staff at regular intervals, either at meetings or using posters or fact sheets. However, for six of these studies, infection surveillance feedback was already in place during the baseline period and would not explain any differences in catheter-BSI incidence densities observed between baseline and intervention periods. Fourteen studies involved interventions that provided some form of performance feedback to the critical care unit staff. The feedback primarily provided information on compliance with interventions or specific intervention components (e.g. hand hygiene110), but in some studies feedback was given on problems encountered109 or on staff competence assessed through tests of knowledge or skills, with staff required to repeat training if satisfactory scores were not achieved. 50,51,98 In three studies68,122,139 the critical care staff were given tests or questionnaires, but it is unclear whether the results were fed back to the staff.
| Study | Checklist | Staff empowerment | Infection surveillance feedback | Performance feedback |
|---|---|---|---|---|
| Burrell (2011)83 | • | • | • | • |
| Coopersmith (2002)50 | (•) | • | ||
| Coopersmith (2004)87 | (•) | |||
| DuBose (2008)93 | • | |||
| Eggimann (2000),94 | ||||
| Galpern (2008)97 | • | • | • | |
| Guerin (2010)98 | • | • | ||
| Higuera (2005)103 | • | |||
| Lobo (2005)108 | • | • | ||
| Lobo (2010)109 | • | • | • | |
| Longmate (2011)110 | • | • | • | |
| Misset (2004)117 | • | |||
| Palomar Martinez (2010)68 | • | ? | ? | |
| Perez Parra (2010)122 | ? | |||
| Pronovost (2006)34 | • | • | • | |
| Render (2006)126 | • | • | • | |
| Rosenthal (2003)129 | • | |||
| Rosenthal (2005)130 | (•) | • | ||
| Sherertz (2000)135 | ||||
| Speroff (2011)136 | • | (•) | • | |
| Wall (2005)138 | • | (•) | • | |
| Warren (2003)139 | (•) | ? | ||
| Warren (2004)51 | ? | • | ||
| Zingg (2009)144 | ||||
| Matching Michigan | • | • | • | • |
Clinical practices recommended in the Department of Health ‘High Impact Intervention No. 1’ for CVC insertion and maintenance28 (see Tables 2 and 3) and used in Matching Michigan were included to varying degrees in the primary studies (Table 20). Where studies had two interventions,109,136 the interventions within each study did not differ in the clinical practices addressed and have been summarised together in Table 20. The most frequently addressed of the recommended clinical practices were hand hygiene prior to patient contact, and the use of maximal sterile barrier precautions and antiseptic insertion site preparation. Only three studies93,129,139 did not address hand hygiene practices, although in five studies68,97,122,130,135 there was ambiguity as to whether hand hygiene was prior to or after contact with the patient (see Table 20). Generally, fewer studies addressed practices related to CVC ongoing care than practices related to CVC insertion. When interpreting Table 20, it is important to bear in mind that authors of the primary studies might not have reported all of the clinical practices that their interventions addressed, as studies were variable in the amount of detail they provided about their methods.
| Study | CVC insertion practices | CVC ongoing care practices | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hand hygiene | Maximal barrier precautions | Skin preparation | Insertion site selection | Dressing | Documentation (date of insertion) | Hand hygiene | Site inspection (daily) | Dressing | Catheter need review | Catheter access | Administration set replacement | No routine catheter replacement | |||
| Before contact | After contact | Before contact | After contact | ||||||||||||
| Burrell (2011)83 | • | • | • | • | • | • | • | • | • | • | • | ||||
| Coopersmith (2002)50 | • | ? | ? | ? | • | ||||||||||
| Coopersmith (2004)87 | • | ? | ? | ? | ? | ? | ? | ||||||||
| DuBose (2008)93 | • | ||||||||||||||
| Eggimann (2000)94 | • | • | • | • | • | • | • | • | • | • | • | • | |||
| Galpern (2008)97 | ○ | ○ | • | • | • | • | |||||||||
| Guerin (2010)98 | • | • | • | • | • | • | • | • | • | ||||||
| Higuera (2005)103 | • | • | • | • | • | ||||||||||
| Lobo (2005)108 | • | • | • | • | • | • | • | • | • | • | • | • | |||
| Lobo (2010)109 | • | • | • | ? | ? | ○ | • | • | ○ | • | • | ||||
| Longmate (2011)110 | • | • | • | • | • | • | ? | • | • | • | |||||
| Misset (2004)117 | • | • | • | • | • | • | • | ? | • | ||||||
| Palomar Martinez (2010)68 | ○ | ○ | • | • | • | ○ | ○ | • | |||||||
| Perez Parra (2010)122 | ○ | ○ | • | • | ○ | ○ | ? | • | |||||||
| Pronovost (2006)34 | • | • | • | • | • | • | • | • | |||||||
| Render (2006)126 | • | • | • | • | • | ||||||||||
| Rosenthal (2003)129 | • | • | • | ||||||||||||
| Rosenthal (2005)130 | ○ | ○ | |||||||||||||
| Sherertz (2000)135 | ○ | ○ | • | • | ○ | ○ | ○ | ○ | |||||||
| Speroff (2011)136 | • | • | • | • | • | • | • | ||||||||
| Wall (2005)138 | • | • | • | • | |||||||||||
| Warren (2003)139 | • | • | • | • | |||||||||||
| Warren (2004)51 | • | • | • | • | • | • | • | • | • | • | • | ||||
| Zingg (2009)144 | • | • | • | • | • | • | |||||||||
| Matching Michigan | • | • | • | • | • | • | • | • | • | ||||||
The most comprehensive interventions in terms of the number of evidence-based clinical practices they included were education-only local-scale interventions reported by Eggimann and colleagues,94 Lobo and colleagues108 and Warren and colleagues,51 and regional-scale interventions with components beyond education reported by Burrell and colleagues83 and Pronovost and colleagues. 34 Among these studies, the Burrell study (the CLAB ICU project)83 appears most relevant to current UK clinical practice, as it sought to replicate the Keystone ICU project at a regional scale.
Summary of study characteristics
Twenty-four studies were included in the systematic review. Most studies were conducted in medical, surgical and cardiac critical care units but the studies provided little information about their study populations and the vascular devices used. Where reported, patients' ages ranged from early 40s to early 70s. Half of the studies were conducted in the USA,34,50,51,87,93,97,98,126,135,136,138,139 with only one study conducted in the UK110 (a CQI programme in a single critical care unit in Scotland). The majority of studies used a single-cohort uncontrolled before-and-after design, with only one RCT included. The interventions evaluated in the studies were diverse in their educational approaches, ranging from single lectures in single critical care units to regional-scale CQI programmes in up to 103 critical care units. Fifty-eight per cent of the interventions reported were purely educational and 42% included components beyond education. Nearly all interventions included formal education approaches that would take staff away from bedside patient care. The interventions varied in the extent to which they addressed evidence-based clinical practices relevant to the NHS for preventing catheter-BSI. Most interventions focused on catheter insertion rather than catheter ongoing care, with hand hygiene, maximal barrier precautions and antiseptic preparation of the insertion site being the most frequently addressed practices. Different educational approaches, which were unique to individual studies, were used to address the same clinical practices. Although studies had to provide a definition of catheter-BSI to be included in the systematic review, only eight studies provided definitions consistent with those used in the NHS for the Matching Michigan programme. Two of the regional-scale interventions68,83 aimed to replicate the Keystone ICU project intervention in different countries, and, of these, the CLAB ICU project83 appears most relevant to NHS practice, although it was conducted in an Australian health-care setting.
Assessment of clinical effectiveness
Quality assessment: risk of bias
The included primary studies were difficult to assess for risk of bias because in most cases insufficient information was reported, resulting in a judgement of ‘unclear’ (Table 21). Methodological information supporting the judgements reached by the reviewers is given in the data extraction forms (see Appendix 5). When interpreting risk of bias it is important to bear in mind that, as judgements of bias are constrained by the availability of information, studies classified as having high or low risk of bias may not necessarily be at greater or lower risk of bias than those studies classified as unclear.
| Study | Selection bias risk (systematic differences between groups) | Performance bias risk (effects of other concurrent intervention/s) | Performance bias risk (other concurrent staff/policy changes) | Performance bias risk (outcome assessment not blinded) | Performance bias risk (outcome definition or measurement differences between groups) | Attrition bias risk (imbalances between groups in missing data) |
|---|---|---|---|---|---|---|
| Burrell (2011)83 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Coopersmith (2002)50 | Unclear | Unclear | High | High | High | Unclear |
| Coopersmith (2004)87 | Unclear | Unclear | High | Unclear | Unclear | Unclear |
| DuBose (2008)93 | Unclear | Unclear | Unclear | High | Low | Unclear |
| Eggimann (2000),94 (2005)95 | Low | Low | Low | Unclear | Unclear | Low |
| Galpern (2008)97 | Unclear | Low | Unclear | Unclear | Unclear | Unclear |
| Guerin (2010)98 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Higuera (2005)103 | Low | Unclear | Unclear | Unclear | Unclear | Unclear |
| Lobo (2005)108 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Lobo (2010)109 | High | High | Unclear | Unclear | Unclear | Unclear |
| Longmate (2011)110 | Unclear | Unclear | Unclear | High | Unclear | Unclear |
| Misset (2004)117 | Unclear | Unclear | Low | Unclear | Unclear | Unclear |
| Palomar Martinez (2010)68 | High | High | Unclear | Unclear | Unclear | Unclear |
| Perez Parra (2010)122 | Unclear | Low | Unclear | Unclear | Low | Unclear |
| Pronovost (2006),34 (2008),124 (2010)123 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Render (2006)126 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Rosenthal (2003)129 | Unclear | High | Unclear | Unclear | Unclear | Unclear |
| Rosenthal (2005)130 | Unclear | High | Unclear | Unclear | Unclear | Unclear |
| Sherertz (2000)135 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| aSperoff (2011) (RCT)136 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Wall (2005)138 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Warren (2003)139 | Low | Unclear | Unclear | Unclear | Unclear | Unclear |
| Warren (2004)51 | Unclear | Unclear | Unclear | High | Unclear | Unclear |
| Zingg (2009)144 | High | Unclear | Unclear | Unclear | Unclear | Low |
Studies judged to be at high risk of bias
Three studies68,109,144 were judged to be at high risk of selection bias, as the numbers of patient-days and CVC-days were consistently lower in the intervention period than in the pre-intervention period;109 baseline incidence of catheter-BSI was higher in control than in intervention critical care units;68 or the McCabe rapid fatality score, number of trauma patients and number of patients who had a CVC inserted were statistically significantly higher in the intervention than in the baseline period. 144
Four studies68,109,129,130 were judged to be at high risk of performance bias owing to a potential influence of other concurrent or overlapping interventions as the planned intervention overlapped with different hand hygiene interventions;129,130 staff in a critical care unit receiving one intervention moved to a different critical care unit receiving another intervention;109 or staff in control critical care units were able to attend training for the intervention. 68
Two studies50,87 were judged to be at high risk of performance bias owing to staff or policy changes because they reported changes in antibiotic prescribing patterns and accessibility of quadruple-lumen catheters,50 or reported that the number of ICU beds was higher in the intervention period (six beds; 33% increase). 87
Four studies50,51,93,110 were judged to be at high risk of performance bias because critical care staff assessing outcomes (the staff collecting and culturing blood samples and analysing the results) were not blinded. The remaining 19 studies did not report whether the staff were blinded but it seems unlikely that authors would have blinded study participants without reporting so.
One study50 was judged to be at high risk of performance bias, as the authors implied that there was a procedural difference in diagnosis and/or reporting of infections between the baseline and intervention periods (it was stated that treatment of catheter colonisation could have accounted for a large number of documented infections in the pre-intervention time period).
Studies judged to be at low risk of bias
Studies were judged to be at low risk of bias if their population characteristics did not appear to differ between baseline and intervention periods94,103,139 (low selection bias risk); they stated that no other concurrent interventions were conducted,94,97,122 or no changes in critical care structure or staffing occurred94,117 that could potentially affect catheter-BSI incidence; they stated that catheter-BSI definitions and diagnosis procedures did not change93,122 (low performance bias risk); or missing data were deducible and did not appear to differ between baseline and intervention periods94,144 (low attrition bias risk).
Risk of bias in randomised controlled trials
Only one RCT, by Speroff and colleagues,136 was included in the systematic review. In addition to the risk of bias criteria reported above for before-and-after studies, which we used to assess before-and-after comparisons within each of the RCT arms, we assessed the risk of selection bias, according to the adequacy of randomisation and allocation concealment, using the Cochrane Collaboration criteria for risk of bias in RCTs. 58 The RCT136 was judged to be at unclear risk of selection bias because insufficient information was provided about the methods of random sequence generation and allocation concealment.
Other bias risk
Information collected in the data extraction forms (see Appendix 5) suggests that studies may have been at risk of self-reporting bias (study outcomes were reported directly by the investigators without independent checking). For example, self-reporting of outcomes was routine110,136 or dependent upon staff availability;83 an audit tool and intervention were implemented by the same team that designed them;87 the study co-ordinator who was involved in delivering an intervention checked data sheets for missing items and errors;129 and it was assumed that nurses accurately captured information and completed a checklist for every insertion, without formal validity and reliability analyses. 138
In three studies110,117,136 the intervention as reported by the authors was not intended solely for the prevention of catheter-BSI: Longmate and colleagues110 reported an intervention for preventing CRBSI, VAP and MRSA; Misset and colleagues117 reported an intervention for preventing CRBSI, VAP and UTIs; and Speroff and colleagues136 reported two interventions, each for preventing both central line-associated bloodstream infection (CLABSI) and VAP. In these studies, clinical effectiveness must be evaluated for the intervention as a whole, as effects of the individual intervention components that target the different types of infections are not separable.
Reporting of data collection in the primary studies
Of the 24 primary studies,34,50,51,68,83,87,93–95,97,103,108–110,117,122–124,126,129,130,135,136,138,139,144,146 none provided full details of their data collection processes. The most detailed descriptions of data collection were reported by Burrell and colleagues83 and Wall and colleagues,138 but even their descriptions were incomplete. Burrell and colleagues83,146 stated that data entry was manual, with an infection nurse checking data on each form. Collected data were received and collated by the Clinical Excellence Commission. Missing and invalid data were followed up and validity of reported central line-associated bacteraemia (CLAB) were confirmed with individual critical care units. However, many critical care units did not have microbiological support and reported CLAB through discussion with senior critical care staff, while improved understanding of surveillance definitions versus clinical definitions led to some CLAB cases being reclassified. Wall and colleagues138 reported that upon completing a checklist, a nurse detached the top page (with all items readable) and dropped it in a secure lockbox. The second page remained on the patient's chart with the sensitive items blacked out. The infection control practitioners collected the checklists daily and scanned the de-identified forms into a pre-established computerised database using scanning software which read pre-established fields on the checklist into a spreadsheet database. These data were stored on a secure computer at the Center for Clinical Improvement for future statistical analyses.
Only one94 of the 24 studies reported that their data collection approach was reliable (pre-tested and standardised in several pilot phases), for infection surveillance. One study98 mentioned that device-day data collected by critical care unit nursing staff were compared with data collected daily by the intravascular catheter management team to confirm the accuracy of data collection but did not provide any supporting data. Three studies110,136,137 stated explicitly that validity and/or reliability of the data collection method was not assessed. It seems improbable that validity and reliability of data collection would have been assessed in the remaining studies, as no mention was made in the publications.
Summary of quality assessment
Overall, the methodological quality of the 24 included primary studies was difficult to assess owing to limited or unclear reporting of study populations and research methods. Nine studies50,51,68,87,93,109,110,129,130 were judged to be at high risk of bias, but, as risk of bias could be assessed only for well-reported studies, the extent of risks of bias among the studies may have been underestimated. Several different types of data were collected in the primary studies, including infection surveillance information, results of tests and assessments, and information on patient outcomes. However, none of the included studies fully reported its data collection methods and the majority of studies did not report whether data collection approaches had been shown to be valid and/or reliable. In most cases the staff who were involved in data collection were not specified, and studies may have been at risk of self-reporting bias (we did not formally assess whether staff involved in data collection were independent, but the authors of five studies83,87,110,129,136 implied that they were not).
Synthesis of effectiveness (primary outcomes)
It was inappropriate to calculate pooled-effect estimates for study outcomes, as the primary studies included in the systematic review varied considerably in their temporal and spatial scales, objectives, and in the structure and content of their interventions. The data from the primary studies were also considered unsuitable for exploration using meta-regression to identify potential explanatory variables for intervention effects, as the data requirements for the conduct and clear interpretation of meta-regression as described by Thompson and Higgins148 would not be met. Instead, we present a structured narrative synthesis below.
It was not possible to identify studies that could be classed as ‘best’ or ‘worst’ in terms of methodological quality and risk of bias. We have therefore not used quality criteria to exclude any studies from the data synthesis. Instead, we summarise below the effectiveness for all studies, which provided relevant outcomes data. Issues of quality and bias identified above that could influence the interpretation of clinical effectiveness for specific studies are highlighted on a case-by-case basis.
Incidence density of catheter-bloodstream infection
In 10 studies, insufficient data were reported for the calculation of incidence density RRs with 95% CIs or there were ambiguities in the published data,83,93,94,97,117,126,130,135,136,138 and we attempted to contact the authors for clarification. Six authors responded with information, three did not respond and one responded stating that relevant data were not available. Data that were provided by the study authors in response to us contacting them are indicated in Appendix 7.
To enable comparisons of clinical effectiveness among the included interventions, incidence density RRs with their 95% CIs are displayed in forest plots, with the interventions grouped according to their spatial and temporal scales: regional-scale interventions (see Figure 4), local-scale interventions of duration ≤ 12 months (see Figure 5), and local-scale interventions of duration of > 12 months (see Figure 6). Within each forest plot, studies are ordered by effect size (larger effects of interventions relative to comparators are indicated by smaller incidence density RRs). As this is a narrative synthesis, pooled-effect estimates are not displayed in the forest plots. The amount of information available varied considerably among the studies, with some studies providing RRs for more than one intervention scenario or time period (multiple within-study effect estimates are not statistically independent but are included in the forest plots, as pooled-effect estimates are not calculated). The full data used in calculating RRs, including the baseline and intervention catheter-BSI incidence densities for each study, are given in Appendix 7.
Educational interventions were classified either as effective at reducing catheter-BSI incidence density, lacking convincing evidence for effectiveness, or ineffective according to the following criteria:
-
Effective The incidence density RR for intervention compared with comparator (baseline) was < 1.0 and the 95% CI of the RR did not include 1.0.
-
Ineffective The 95% CI of the incidence density RR for intervention compared with comparator (baseline) included 1.0.
-
Lack of convincing evidence for effectiveness Effectiveness was marginal, or serious limitations of the study methodology cast doubt on the reliability of the results (explanations are provided below on a case-by-case basis).
Regional-scale interventions
Five studies34,68,83,126,136 investigated regional-scale CQI interventions (Figure 4; for the full data see Appendix 7, Data for forest plot: regional-scale interventions). These studies included the Keystone ICU project,34,123,124 the ‘CLAB ICU’ project,83,146, which sought to replicate aspects of the Keystone ICU project in Australia, and the ‘Bacteraemia Zero’ project,68 which sought to replicate aspects of the Keystone ICU project in Spain. Three of the studies used uncontrolled before-and-after designs, whereas two (a RCT by Speroff and colleagues136 and the non-randomised controlled study by Palomar Martinez and colleagues68) included two parallel comparison groups of critical care units (virtual collaborative and toolkit groups136 or intervention and control groups68). The study by Palomar Martinez and colleagues68 reported historical baseline data on infection incidence for 3 years prior to the prospective intervention (2004–6), of which we report only the 2006 data, as these are most directly relevant for comparison with the study period (2007) (for full data see the data extraction form in Appendix 5). All of the regional-scale studies calculated the number of device-days based on presence/absence of vascular catheters, which does not take into account the number of concurrent catheters that a patient may have.
FIGURE 4.
Incidence density RRs (± 95% CI) for regional-scale interventions (multiple catheters per patient not counted separately in device-days). a, CI not calculable
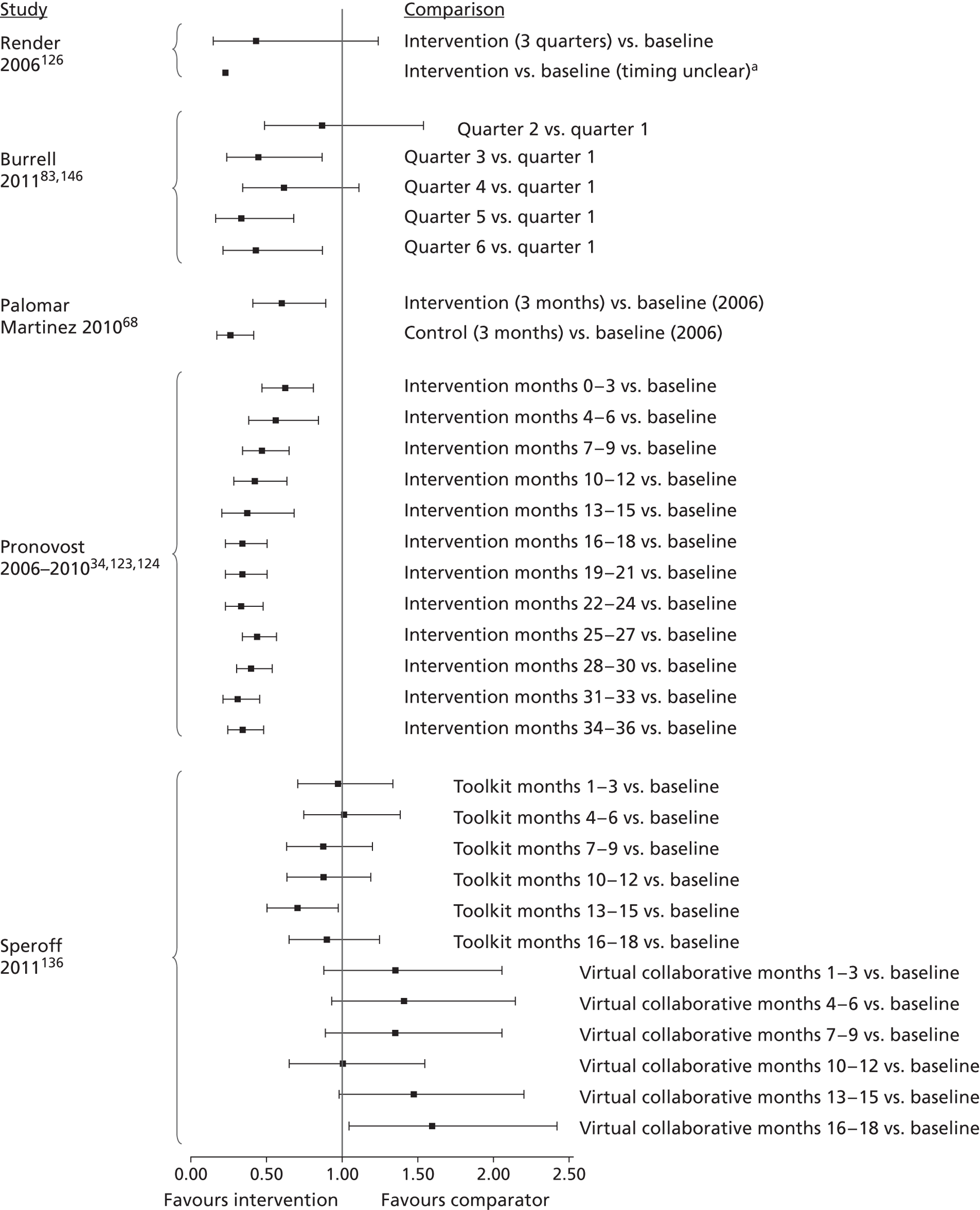
Clinically effective interventions (with unclear risk of bias)
Assuming that effects displayed in the forest plots reflect those of the planned interventions, the CLAB ICU project83,146 and Keystone ICU project34,123,124 interventions appear to have been effective in reducing catheter-BSI incidence densities relative to baseline, although both studies had unclear risk of bias (Box 1). The Keystone ICU project achieved a reduction in catheter-BSI incidence density after 3 months, which subsequently persisted through the 36-month intervention monitoring period. The CLAB ICU project achieved a reduction in catheter-BSI incidence density after 6 months, which was mostly sustained until the end of the 18-month intervention monitoring period (statistically significant in quarters 3, 5 and 6) (see Figure 4). These studies both have some relevance to clinical practices for prevention of catheter-BSI in critical care in the NHS and are considered in more detail below.
-
CQI programme conducted in 103 critical care units in the USA: the Keystone ICU project (duration of up to 36 months). Targeted clinicians' use of hand washing, full barrier precautions during CVC insertion, skin cleansing with chlorhexidine, avoiding the femoral site, and removing unnecessary catheters; included checklist, presentations and meetings, fact sheet, infection surveillance feedback and catheter supplies cart. 34,123,124
-
CQI programme conducted in 37 critical care units in Australia: the CLAB ICU project (duration of up to 18 months). Based on principles of the Michigan Keystone ICU project, including a ‘clinician bundle’ (hand hygiene, barrier precautions and sterile technique) and a patient bundle (skin preparation, patient draping and imaging catheter positioning during insertion); also included a checklist, infection surveillance feedback and catheter supplies cart. 83,146
Clinically ineffective interventions
The two interventions implemented in the RCT by Speroff and colleagues136 were clearly not effective at reducing catheter-BSI incidence density, as acknowledged by the study authors (Box 2). During the 18-month study period, catheter-BSI incidence density for the virtual collaborative intervention actually increased relative to baseline (see Figure 4).
Not effective: CQI programme conducted in 60 critical care units in the USA during 18 months. Hospitals were randomised to Virtual Collaborative and Toolkit CQI approaches (including VAP prevention), with access to interactive web seminars for both groups. Virtual collaborative: monthly educational and troubleshooting conference calls, individual coaching and electronic mailing list (designed to stimulate interaction among teams). Toolkit: based on evidence based guidelines, fact sheets, review of QI and teamwork methods, educational online tutorials and standardised data collection/charting tools136 (note that hospitals were randomised but implementation was at the level of critical care units).
Lack of convincing evidence for effectiveness: Continuous QI programme, duration approximately 1 year, in a single cohort of eight critical care units in the USA, based on work/learning/reporting cycles, including performance feedback and infection surveillance feedback. 126
Lack of convincing evidence for effectiveness: Three-month pilot study based on the Michigan Keystone ICU intervention, evaluating the feasibility of national implementation of a CQI intervention in 17 critical care units in Spain, with units allocated non-randomly to either intervention or control groups – the Bacteraemia Zero project68 (this study was judged to be at high risk of selection and performance bias).
Process evaluation conducted by Speroff and colleagues136 (discussed below: see Process evaluations, facilitators and barriers) indicated that adoption of intervention components was consistently higher in the virtual collaborative intervention arm than the toolkit approach arm, suggesting that failure of the interventions to reduce catheter-BSI incidence was not simply related to the extent of intervention implementation.
Interventions lacking convincing evidence for effectiveness
The two remaining regional-scale interventions, by Render and colleagues126 and Palomar Martinez and colleagues,68 appear at first sight to have been effective at reducing the incidence densities of catheter-BSI. However, upon closer inspection the findings of these studies are difficult to interpret.
Published data for the study by Render and colleagues126 indicate an incidence density RR below 1.0 but the publication did not provide a CI or sufficient data for us to calculate one. Further data obtained on request from the author (see Appendix 7, Data for forest plot: regional-scale interventions) enabled us to calculate an incidence density RR with a 95% CI, but the CI indicates lack of effectiveness (see Figure 4). On balance, the available information for the study by Render and colleagues126 does not provide convincing evidence that the intervention was effective at reducing the incidence density of catheter-BSI (see Box 2).
The results of the Bacteraemia Zero project reported by Palomar Martinez and colleagues68 are difficult to interpret because incidence density RRs were significantly lower than 1.0 both for the intervention and control groups of critical care units, with a larger effect (smaller RR) evident in the control group (see Figure 4). The Palomar Martinez study68 is notable in that we judged it to be at high risk of selection bias because the control group had higher baseline incidence density of catheter-BSI than the intervention group. We also judged this study to be at high risk of performance bias because staff in control critical care units were able to attend intervention group meetings at which intervention information and materials were disseminated. On balance, this 3-month pilot study by Palomar Martinez and colleagues68 does not provide convincing evidence of effectiveness of their CQI programme at reducing the incidence density of catheter-BSI.
Local-scale interventions of up to 12 months in duration
Twelve studies investigated local-scale interventions of up to 12 months' duration. Of these, 10 studies50,51,98,103,108,109,122,129,139,144 provided sufficient data for the calculation of incidence density RRs (Figure 5; for the full data, see Appendix 7, Data for forest plot: local-scale interventions of duration up to 12 months). These studies all calculated the number of device-days based on the presence/absence of vascular catheters, which does not distinguish multiple concurrent catheters in a patient. The educational interventions were diverse and included single lectures on practices related to CVC care;109,122 structured and interactive education modules focusing on hand hygiene and CVC care144 multimodal education of various types with performance feedback;50,51,103,129,139 multimodal education with infection surveillance feedback;108 and a post-insertion central line care bundle. 98 Three of these studies each involved a single critical care unit;50,51,108 three studies involved multiple critical care units and reported results pooled across the units;98,129,139 and four studies presented results separately for different critical care units. 103,109,122,144 Lobo and colleagues109 compared a single lecture in one critical care unit (‘ICU A’) with a tailored continuous educational intervention in another critical care unit (‘ICU B’) located at the same hospital, whereas Higuera and colleagues,103 Perez Parra and colleagues122 and Zingg and colleagues144 compared results from different critical care units which had received similar interventions (see Figure 5).
FIGURE 5.
Incidence density RRs (± 95% CI) for local-scale interventions of up to 12 months in duration (multiple catheters per patient not counted separately in device-days). a, Confidence interval not calculable
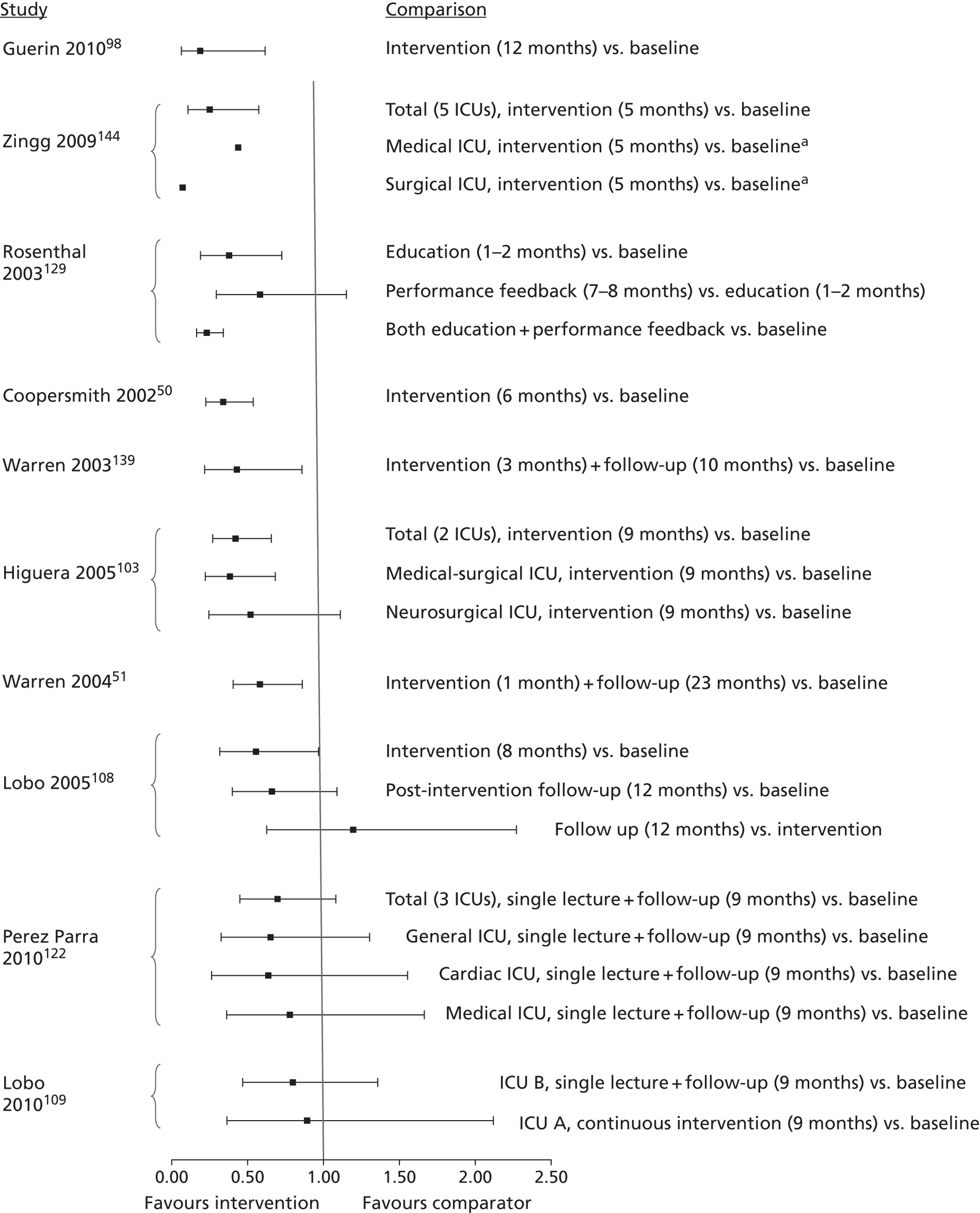
Clinically effective interventions (with unclear or high risk of bias)
Assuming that effects displayed in the forest plots reflect those of the planned interventions, seven of the interventions50,51,98,103,129,139,144 would appear to have been effective in reducing the incidence density of catheter-BSI (Box 3), as incidence density RRs were significantly below 1.0 (see Figure 5). The studies by Coopersmith and colleagues50 and Warren and colleagues51 were judged to be at high risk of performance bias because the authors stated that the staff assessing outcomes were not blinded to the intervention. However, it seems unlikely that staff would have been blinded in any of the other studies, although this was not reported. Two studies50,129 were judged to be at high risk of performance bias for other reasons. Effects of the educational intervention implemented by Coopersmith and colleagues50 appear to have been confounded with changes in antibiotic prescribing practice and availability of four-lumen catheters, whereas effects of the educational intervention implemented by Rosenthal and colleagues129 overlapped with a separate hand washing intervention. One study by Zingg and colleagues144 was judged to be at high risk of selection bias, as the study characteristics of the population differed between the baseline and intervention periods.
• Multimodal education including self-study, in-services and performance feedback in one critical care unit in the USA for 6 months, with broad topic coverage aimed at nurses; included infection surveillance feedback but this was also present in the baseline period. 50
• Multimodal education including self-study and performance feedback, with broad topic coverage aimed at nurses and physicians in two critical care units in one hospital in the USA for 3 months; included infection surveillance feedback but this was also present in the baseline period. 139
• Multimodal education including self-study and performance feedback, with broad topic coverage aimed at nurses and physicians in one critical care unit in the USA for 1 month; unclear whether included infection surveillance feedback. 51
• Multimodal education focusing on hand hygiene and vascular catheter care aimed at nurses and physicians in five critical care units in one hospital in Switzerland for 5 months144 (this study was judged to be at high risk of selection bias, as population characteristics differed between baseline and study periods).
• Unspecified education (1–2 months) followed by performance feedback (7–8 months) in four critical care units in two hospitals in Argentina129) (this study was judged to be at high risk of performance bias as the effects of the intervention appear to be confounded with those of a separate hand-washing intervention; also, as mentioned in the text, only the education phase can be reliably interpreted; this study also had relatively high baseline incidence density of catheter-BSI).
• Process control intervention in two critical care units in one hospital in Mexico for 9 months, including 1-hour classes and provision of CDC infection control guidelines, with performance feedback and the provision of alcohol hand rub103 (note that this study had relatively high baseline incidence density of catheter-BSI and effectiveness was not demonstrated in all participating critical care units).
• Post-insertion central line care bundle in two critical care units in one hospital in the USA for 12 months, including 4-hour hands-on practical sessions on CVC access and care followed by competence evaluation; included performance feedback and provision of an intravenous therapy team. 98
In two103,129 of the seven studies that appeared clinically effective, further caution on interpretation is required. In the study by Rosenthal and colleagues129 incidence density RRs were significantly < 1.0 for education or education and performance feedback periods together but not for the performance feedback period alone. These results are difficult to interpret because the education and performance feedback periods were sequential, and performance feedback may not have been independent of the effects of the preceding education. Interpretation should therefore be restricted to the education phase, which itself appeared to be clinically effective, although this was only monitored for 1–2 months (see Figure 5). In the study by Higuera and colleagues103 the intervention was effective when data for two critical care units were pooled but found to be effective in only one of the units when they were analysed separately (see Figure 5). It is notable that the medical–surgical critical care unit had a higher baseline incidence density of catheter-BSI per 1000 catheter-days (57.4) than the neurosurgical critical care unit (32.8). These baseline incidence densities and also the baseline catheter-BSI incidence density in the study by Rosenthal and colleagues128 (45.9 per 1000 catheter-days) are higher than typically found in European studies.
For these seven studies50,51,98,103,129,139,144 that appeared to be effective at reducing incidence of catheter-BSI (albeit with the caveats noted above), statistical significance indicated by the CIs of RRs in Figure 5 is consistent with the primary study publications, which, in all cases, claimed that effects of the interventions at reducing catheter-BSI incidence were statistically significant.
Two51,139 of the studies that appeared effective at reducing catheter-BSI incidence (see Box 3) reported post-intervention follow-up monitoring, which may provide an indication of the longer-term persistence or attenuation of intervention effectiveness. Warren and colleagues139 conducted 10 months of follow-up after a 3-month intervention; and Warren and colleagues51 conducted 23 months of follow-up after an intervention of about 1 month. Although the RRs based on the whole follow-up period appear encouraging, monthly data provided for the latter study51 (data extraction form – see Appendix 5) show that incidence densities varied considerably from month to month, and returned to baseline levels within the first 3 months of the 23-month follow-up period.
Clinically ineffective interventions
For two109,122 of the local-scale short-term studies, the 95% CIs for the RRs indicate that the reduction in catheter-BSI incidence density was not statistically significant (see Figure 5). In contrast, the primary publications for these studies both claimed statistically significant effects of the interventions. 109,122 Lobo and colleagues109 based their conclusion on incidence data rather than incidence density. Perez Parra and colleagues122 stated that they initially conducted a Wilcoxon rank sum test and then to control for (unspecified) confounding effects of external events and (unspecified) secular trends that occurred during the study period they used a Poisson regression approach. The statistical significance reported122 varied among the critical care units (overall: p = 0.3; general post surgical: p = 0.05; cardiac post surgical: p = 0.12; medical: p = 0.31) and it is not clear to which of the analytical approaches the p-values refer. On balance, we conclude that these two studies were not effective at reducing catheter-BSI incidence density (see Figure 5 and Box 4) and the claims of statistical significance in the primary publications do not provide sufficient grounds for us to alter our conclusion.
Not effective: Single lecture (15 minutes with 9 months of follow-up) on approaches for CVC care and maintenance, with performance feedback, aimed at all staff in three critical care units in one hospital in Spain. 122
Not effective: Single lecture (unspecified duration, with 9 months of follow-up) on CVC care aimed at all critical care staff in a single critical care (‘ICU B’) unit in Brazil109 (this study was judged to be at high risk of selection bias and performance bias).
Not effective: Continuous tailored education including lectures on CVC care and hand hygiene, posters, colourful labels, and infection surveillance feedback aimed at all critical care staff in a single critical care unit (‘ICU A’) in Brazil for 9 months109 (this study was judged to be at high risk of selection bias and performance bias).
Lack of convincing evidence for effectiveness: Multimodal education with emphasis on hand hygiene, aimed at all staff in two critical care units in one hospital in Brazil for 8 months, included infection surveillance feedback. 108
Interventions lacking convincing evidence for effectiveness
The intervention reported by Lobo and colleagues108 has a RR bordering on statistical non-significance (upper limit of the 95% CI 0.97) and a non-significant RR for the follow-up period suggesting that the intervention was only briefly effective (see Box 4) at reducing the incidence of catheter-BSI (see Figure 5). The publication for this study did not report statistical significance. 108
Local-scale interventions of more than 12 months in duration
Seven studies87,94,95,97,110,138 investigated local scale interventions of > 12 months in duration, of which five provided sufficient data for the calculation of incidence density RRs (Figure 6: for the full data see Appendix 7.3, Data for forest plot: local-scale interventions of duration of > 12 months). The studies were all conducted in single critical care units, apart from one which involved three units. 97 The interventions included multimodal education with slide shows and bedside in-services, repeated for up to 6 years;94,95 multimodal education with self-study, bedside in-services and performance feedback over 15 months;87 a central line bundle deployed over 19 months, including checklist, infection surveillance feedback, performance feedback and catheter supplies cart;97 a CQI programme implemented over 3 years, including checklist, infection surveillance feedback and performance feedback;110 and a CQI programme focused on real-time performance feedback over 2 years, including a checklist, supervision of insertions and web-based tutorial with competence assessment. 138
FIGURE 6.
Incidence density RRs (± 95% CI) for local-scale interventions of more than 12 months in duration (multiple catheters per patient not counted separately in device-days unless stated)
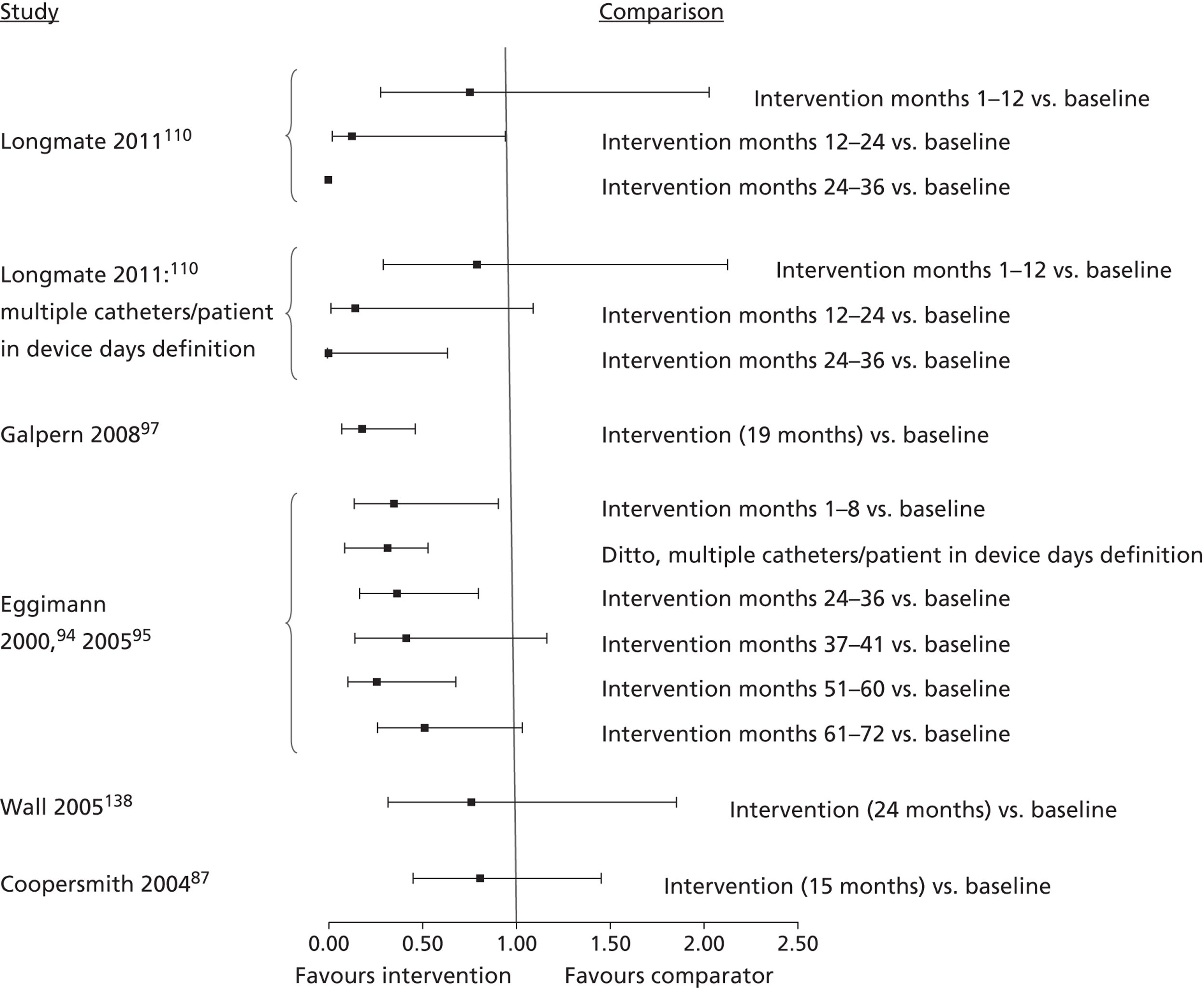
Three87,97,138 of these five studies calculated the number of device-days based on presence/absence of vascular catheters, which does not distinguish multiple concurrent catheters in a patient. Eggimann and colleagues94 and Longmate and colleagues110 calculated the number of device-days in two ways: based on the presence/absence of vascular catheters; and by counting each concurrent catheter as a separate device-day. In both studies the incidence density RRs were similar for both methods of calculating the device-days (see Figure 6).
Clinically effective interventions (with unclear risk of bias)
Assuming that effects displayed in the forest plots reflect those of the planned interventions, three of the interventions conducted by Eggimann and colleagues,94,95 Longmate and colleagues110 and Galpern and colleagues97 appear effective in reducing the incidence density of catheter-BSI (Box 5), with incidence density RRs significantly lower than 1.0 (see Figure 6). Exceptions were that the RRs were not significantly different from 1.0 on all of the monitoring dates reported by Eggimann and colleagues94,95 and Longmate and colleagues. 110 Data from the Eggimann study94,95 suggest that the intervention was effective for the initial 3 years of implementation but not consistently so thereafter. Data from the Longmate study110 suggest that the intervention did not become effective at reducing catheter-BSI until the third year of implementation (see Figure 6). The publications reporting these three studies94,95,97,110 either claimed statistically significant intervention effects97,110 or did not mention the statistical significance of effects. 94,95
• Multimodal education based on 30-minute slide shows and bedside in-services aimed at all critical care staff (physicians, nurses and nursing assistants) in one critical care unit in Switzerland for up to 6 years. 94,95
• Central line bundle including discussion sessions about CVC access and care, checklist, infection surveillance feedback, performance feedback and catheter cart; aimed at all staff (physicians and nurses) in three critical care units in the USA for 19 months. 97
• CQI programme (including VAP prevention) incorporating a CVC insertion bundle with checklist, infection surveillance feedback and performance feedback; aimed at ICU nurses and trainee doctors in one critical care unit in Scotland for up to 3 years. 110
The study by Longmate and colleagues110 was judged to be at high risk of performance bias, as the authors stated that the staff assessing outcomes were not blinded. However, as mentioned above, it is unlikely that staff would have been blinded in any of the other studies, although this was not reported. The other studies were judged to be mostly at unclear risk of bias, although (as mentioned in the section on quality assessment above) the Eggimann study94 was judged to be at low risk of bias for four of seven bias domains assessed (the other three were judged unclear).
Clinically ineffective interventions
Two87,138 of the five studies that implemented local-scale interventions of > 12 months' duration, by Coopersmith and colleagues87 and Wall and colleagues,138 were not effective at reducing catheter-BSI incidence density (Box 6), as the incidence density RRs were not significantly different from 1.0 (see Figure 6). Coopersmith and colleagues87 acknowledged that effects of their intervention were not statistically significant. Among possible reasons for the lack of success of the intervention, Coopersmith and colleagues87 suggested that that diffuseness of the educational message may have been a contributory factor and that ideally didactic education should be specifically targeted to support best clinical practices. Wall and colleagues138 did not report the statistical significance of the effectiveness of their real-time process feedback approach for reducing catheter-BSI. The number of infections appeared to decrease dramatically (from 25 per 24 months to 6 per 24 months),138 but there was also a marked decrease in the number of CVC-days during the study, which explains the lack of statistical significance when intervention effects are analysed in terms of the incidence density of catheter-BSI.
• Multimodal education on CVC care and unspecified topics, including self-study, in-services and performance feedback, with broad topic coverage aimed at nurses and other staff in one critical care unit in the USA for 15 months; included infection surveillance feedback but this was also present in the baseline period87 (this study was judged to be at high risk of performance bias).
• CQI programme involving real time feedback of infection rates and compliance with insertion practice, based on use of checklist, supervision of insertions, and web-based tutorial with competence assessment; aimed at ICU house staff (proceduralists) and nursing staff (as observers of procedures) in one critical care unit in the USA for 2 years; note that infection surveillance feedback was also present in the baseline period. 138
Overview of intervention effects on catheter-BSI incidence density
Assuming that the incidence density RRs reflect those of the planned interventions, then a range of different types of educational intervention would appear to have been effective at reducing catheter-BSI incidence in critical care units (Boxes 1, 3 and 5). A key proviso is that some studies were judged to be at high risk of specific types of bias, but risk of bias was generally unclear and it is not possible to objectively classify the studies on bias risk. The interventions that appeared effective, subject to the caveats above, include regional-scale CQI programmes34,83 or a local-scale CQI programme;110 local-scale multimodal education with or without performance feedback and infection surveillance feedback;50,51,94,103,129,139,144 a catheter insertion bundle;97 and a catheter ongoing care bundle. 98 However, single lectures on CVC care and infection prevention were not effective as a means of reducing catheter-BSI incidence densities. 109,122 There is no clear evidence to suggest that regional-scale studies were more effective than local-scale studies, or that short-term studies (up to 12 months' duration) had different effectiveness than long-term studies (exceeding 12 months' duration). All three groups of studies (regional scale, local scale short-term and local-scale long-term) contain examples of effective interventions (Boxes 1, 3 and 5) and ineffective interventions (Boxes 2, 4 and 6). The studies included in the systematic review provide no clear evidence that performance feedback and/or infection surveillance feedback were influential in achieving effectiveness. Among the 12 interventions classed as clinically effective, eight (67%) included performance feedback,50,51,83,97,98,103,110,129 four (33%) included infection surveillance feedback,34,83,97,110 three (25%) included both types of feedback83,97,110 and two (17%) did not include either type of feedback. 94,144 Among the eight interventions that were not classed as clinically effective, the respective proportions were similar: four (50%) included performance feedback,109,126,136,138 three (38%) included infection surveillance feedback,108,109,126 two (25%) included both types of feedback109,126 and one (13%) did not include either type of feedback. 122 Apart from single lectures being ineffective, there appears to be no clear evidence that interventions which included components beyond education (e.g. provision of antiseptic or a catheter supplies cart) were more or less effective than interventions that consisted of education alone.
Most studies did not separately count multiple vascular catheters per patient when calculating the number of device-days. In two studies that compared different approaches for calculating the number of device-days, the calculation approach used did not appear to influence the incidence density RR for catheter-BSI.
Mortality
Five studies94,103,110,139,144 reported mortality as an outcome but they did not specifically report mortality due to catheter-BSI or its sequelae.
Only one of these studies110 reported mortality specifically for critical care patients with CVCs (those patients in the critical care unit for > 2 days with a CVC for at least part of their critical care stay). Mortality rates significantly decreased during the study period, being 21.2% during the baseline period, and 20.9% and 16% during years 3 and 4 of the intervention, respectively (year 3 vs. 1, p = 0.328; year 4 vs. 1, p = 0.013).
Three94,103,144 of the five studies reported unadjusted mortality rates for critical care patients. Of these, two reported that there were no statistically significant differences between the baseline and intervention periods. 94,144 An exception was that mortality due to cardiac arrest was significantly more frequent during the intervention period of one study94 (p < 0.05). The third study103 reported statistically significantly lower rates of unadjusted mortality per 1000 critical care unit discharges during the intervention period than the baseline period (64/132 = 48.5% and 111/338 = 32.8%), respectively [RR = 0.68 (95% CI 0.50 to 0.91), p = 0.01].
The remaining study139 reported that mortality for an ambiguous population (‘in-hospital mortality rate for catheterised patients’) did not differ significantly between the baseline and intervention periods.
In summary, insufficient data are available for us to draw any firm conclusions about the effects of the educational interventions, or the effects of catheter-BSI, on rates of mortality among patients in critical care units.
Length of stay
Seven studies50,94,109,110,117,139,144 reported LOS. One of these studies110 explicitly stated that their LOS data were for critical care unit patients who had vascular catheters. One study144 compared LOS in patients with and without CRBSI. For the remaining five studies, LOS data appear to refer to all critical care unit patients, not limited to those with vascular catheters.
Longmate and colleagues110 reported both mean and median lengths of stay for patients who were in the critical care unit for > 2 days and had a CVC for at least part of their critical care stay. Mean (± SD) lengths of stay significantly increased [5.4 ± 3.9 days during year 1 (baseline period), 5.3 ± 3.7 days during intervention year 3, and 5.9 ± 3.7 days during intervention year 4] (differences from baseline, p > 0.05). The corresponding median [interquartile range (IQR)] lengths of stay decreased, and were, respectively, 9.7 (4–20) days, 8.9 (4–13) days and 6.0 (3–11) days (differences from baseline, p < 0.05).
Zingg and colleagues144 reported median overall LOS for patients in five critical care units, during a 4-month baseline and 5-month intervention period. LOS was statistically significantly longer during the intervention period. The median (IQR) LOS for baseline and intervention periods respectively were 3 (2–7) days and 4 (2–9) days – difference, p < 0.001. Corresponding mean LOS during these periods were 5.9 days and 7.5 days, respectively. Zingg and colleagues also reported that median (IQR) LOS was 15.5 (10–25) days in patients with CRBSI and 5 (3–12) days in patients without CRBSI (difference reported as 10.5 days).
In summary, two studies110,144 reported length of critical care unit stay in relevant populations of patients with CVCs. One study,144 a 9-month education intervention by Zingg and Colleagues, reported significantly longer duration of stay during the intervention period, whereas the other, a 4-year continuous QI programme by Longmate and colleagues,110 found significantly shorter duration of stay in the intervention period. In addition, Zingg and colleagues144 reported that the median LOS for critical care patients with CRBSI was 10.5 days longer than for critical care unit patients without CRBSI (reported as 15.5 and 5 days, respectively).
Synthesis of process (secondary outcomes)
Attitudes
One of the included studies by Sherertz and colleagues135 reported staff attitudes as a quantitative outcome. The proportions of postgraduate year 1 physicians-in-training who perceived a need for povidone–iodine, gloves, gowns and full sterile drapes increased significantly following a formal one-day education course (full data are in the data extraction form – see Appendix 5). However, incidence density RRs could not be calculated for this study135 so its clinical effectiveness at reducing catheter-BSI incidence density is unclear.
Four studies68,83,110,122 mentioned qualitative observations relating to the attitudes of critical care staff towards evidence-based infection prevention practices. Burrell and colleagues83 observed that some clinicians considered the incidence of CLAB in New South Wales to be low and doubted the value of the project, as existing Australian practice was felt to be equal to or better than the methods informing the project. Some clinicians doubted the evidence even with supportive CDC guidelines. Hat-wearing was a contentious element of the physician bundle: clinicians cited lack of evidence for hat use and four critical care units elected to omit their use as standard practice for CVC insertion. 83 The remaining studies provided only brief mention of staff attitudes. Longmate and colleagues110 reported that two consultant clinicians doubted the need for full aseptic technique for CVC insertion, although this was resolved after evidence sharing. Perez Parra and colleagues122 reported that staff incorrectly assumed that small drapes were sufficient for catheter-BSI prevention, although it is not clear whether they were referring to the baseline or intervention period. Palomar Martinez and colleagues68 reported that some practitioners expressed dissatisfaction with chlorhexidine for skin antisepsis and doubted its effectiveness.
Although limited in detail and lacking quantitative analysis, these observations of the attitudes of critical care staff highlight that staff may be liable to question the need for evidence-based infection prevention practices, even when presented with supporting evidence.
Knowledge
Two50,122 of the included studies indirectly reported improvements in knowledge, expressed as changes in the proportion of staff with correct test scores50 or the percentage of correctly answered questions. 122 In the latter study, the test questions most often answered incorrectly (fewer than 50% correct) were those about the need for full sterile barriers during CVC insertion and on the choice of antiseptic for skin disinfection. 122 These studies suggest that educational interventions can improve critical care staff knowledge of infection prevention practices but they did not report the types of knowledge gained through participation in the interventions.
Compliance
Compliance with evidence-based practices for preventing catheter-BSI was reported in 19 out of the 24 included studies.
Compliance with hand hygiene
Seven studies reported compliance with hand hygiene behaviour (Table 22). They all reported improvements in compliance relative to baseline, although these were not statistically significant in all cases. In most of the studies hand hygiene compliance rates did not reach 100% during educational interventions. In one study, the change was modest (final compliance 30%) and non-significant, possibly because hand hygiene was not a specific target of the education. 87
| Study | Baseline compliance (%) | Intervention compliance (%) | RR (95% CI); statistical significance | Comments |
|---|---|---|---|---|
| Coopersmith (2004)87 | 17 | 30 | p > 0.99 | |
| Higuera (2005)103 | 62 | 84.9 | RR = 1.37 (1.21 to 1.51); p = 0.0000 | Before patient contact |
| Lobo (2005)108 | 100 | 100 | Not reported | CVC insertion |
| 5 | 61 | RR = 12.78 (3.24 to 50.42); P < 0.001 | Before manipulation | |
| 38 | 43 | RR = 1.14 (0.69 to 1.90); p = 0.6 | After manipulation | |
| 45 | 68 | RR = 1.50 (0.95 to 2.37); p = 0.072 | Before dressing | |
| Lobo (2010)109 (data for interventions in two critical care units – ‘ICU A’ and ‘ICU B’) | 35 | 81 | RR = 2.32 (1.62 to 3.32); p= 0.370 | ICU A, before CVC handling |
| 19 | 84 | RR = 4.42 (2.63 to 7.43); p < 0.001 | ICU A, after CVC handling | |
| 15 | 48 | RR = 3.20 (1.33 to 7.72); p = 0.008 | ICU B, before CVC handling | |
| 9 | 55 | RR = 6.00 (1.95 to 18.44); p < 0.001 | ICU B, after CVC handling | |
| 28 | 98 | RR = 3.50 (2.24 to 5.47); p < 0.001 | ICU A, before CVC dressing | |
| 34 | 96 | RR = 2.82 (1.91 to 4.17); p < 0.001 | ICU A, after CVC dressing | |
| 6 | 76 | RR = 12.50 (3.22 to 48.56); p < 0.001 | ICU B, before CVC dressing | |
| 27 | 64 | RR = 2.33 (1.26 to 4.31); p < 0.006 | ICU B, after CVC dressing | |
| Rosenthal (2005)130 | 23.1 | 64.5 | RR = 2.79 (2.46 to 3.17); p < 0.0001 | |
| Wall (2005)138 | 73 | 94, 89 | Not reported | For last 2 quarters |
| Zingg (2009)144 | 59.1 | 65 | p = 0.466 | Overall compliance |
| 22.5 | 42.6 | p = 0.003 | Correct hand disinfection | |
| 26 | 45 | p = 0.007 | Before patient contact | |
| 21 | 56 | p < 0.001 | After patient contact |
Five103,108,109,130,144 of the studies that achieved statistically significant improvements in compliance had specified hand hygiene as a main108,109,130 or joint103,144 component of their education. High baseline variability in compliance rates is notable, both within and between the studies. For example, Lobo and colleagues108 found baseline compliance with hand hygiene at CVC insertion was already 100% but compliance with hand hygiene before line manipulation was only 5%. Overall, these findings suggest that hand hygiene behaviour in relation to the insertion and management of CVCs is complex and variable, and can differ considerably between the stages of intravascular catheter site preparation, insertion and ongoing management. Owing to the relatively small number of studies that reported hand hygiene compliance it is unclear whether the degree of compliance with hand hygiene practices had any bearing on the effectiveness of the interventions for preventing catheter-BSI.
Compliance with barrier precautions
None of the seven studies87,108,109,126,135,138 that measured compliance with barrier precautions (Table 23) had specified this as a target behaviour change, although most of the studies included an element of education about sterile barrier precautions in their interventions. Compliance with barrier precautions was highly variable at baseline, ranging from 0% to 100%. Final compliance with barrier precautions after study interventions ranged from 65% to 100%, indicating improvement, but this was reported to be statistically significant for only two out of eight comparisons.
| Study | Barrier type | Baseline compliance (%) | Intervention compliance (%) | RR (95% CI); statistical significance |
|---|---|---|---|---|
| Coopersmith (2004)87 | Maximal barriers | 50 | 80 | p = 0.29 |
| Lobo (2005)108 | Maximal barriers, CVC insertion | 91 | 100 | RR = 0.91 (0.80 to 1.04); p = 0.147 |
| Wall (2005)138 | Maximal barriers, for last quarter | 68 | 86 | Not reported |
| Lobo (2005)108 | Glove use at CVC manipulation | 40 | 98 | RR = 2.36 (1.64 to 3.40); p < 0.001 |
| Glove use at CVC dressing | 97 | 97 | RR = 1.00 (0.91 to 1.10); p = 1.0. | |
| Lobo (2010)109 | Glove use at CVC insertion and dressing | 100 | 100 | Not reported |
| Render (2006)126 | Large sterile drapes | 0 | 83 | Not reported |
| Sherertz (2000)135 | Sterile drapes | 44 | 65 | p < 0.001 |
The studies appeared to differ in their ability to detect statistically significant changes in compliance. A 30% increase in compliance with maximal sterile barrier use was not significant (p = 0.29) in one study,86 whereas a 21% increase in compliance with sterile drape use was significant (p < 0.001) in another study. 135 Wall and colleagues138 reported that a decline in compliance with maximal barrier precautions during the intervention period was caused by lack of use of patient drapes. Compliance with maximal barrier precautions improved after the team purchased new sterile kits pre-packaged with drapes, and confirmed providers had completed a tutorial. Wall and colleagues138 also mentioned that use of the femoral site, compared with other insertion sites, was associated with statistically significant lower compliance with hand washing, chlorhexidine skin antisepsis and maximal barrier precautions.
Compliance with dressing management
None of the six studies that measured compliance with dressing management (Table 24) explicitly specified this was a target behaviour, although dressing care was clearly stated as a topic in the educational interventions of the studies by Coopersmith and colleagues,87 Higuera and colleagues103 and Lobo and colleagues. 109 Compliance with various aspects of CVC dressing care ranged from 11% to 84% at baseline and improved to 21% to 97% after interventions, with the improvements all being reported to be statistically significant, although compliance with correct dating of CVC dressings reached only 21% after the intervention by Coopersmith and colleagues. 87 In the study by Rosenthal and colleagues,129 compliance with placing gauze dressings and with checking the condition of dressings both increased by a greater degree following performance feedback than following education alone. However, as these intervention components were implemented sequentially, the effects of performance feedback may not have been independent of the education.
| Study | Dressing action | Baseline compliance (%) | Intervention compliance (%) | RR (95% CI); statistical significance |
|---|---|---|---|---|
| Coopersmith (2004)87 | Dating dressing | 11 | 21 | p < 0.001 |
| Rosenthal (2003)129 | Placing gauze | 53.02 | 56.21 (96.53)a | RR = 1.06 (0.86 to 1.30); p = 0.64 RRb = 1.72 (1.40 to 2.10); p < 0.001 |
| Higuera (2005)103 | Placing gauze | 84.21 | 97.87 | RR = 1.16 (1.09 to 1.24); p = 0.0000 |
| Rosenthal (2003)129 | Checking condition | 48.70 | 43.19 (89.56)a | RR = 0.89 (0.67 to 1.17); p = 0.39 RRb = 2.07 (1.65 to 2.62); p < 0.001 |
| Lobo (2005)108 | Skin antisepsis | 26 | 58 | RR = 2.25 (1.15 to 4.39); p = 0.01 |
| Lobo (2010)109 | Skin antisepsis, ICU A | 54 | 100 | RR = 1.85 (1.43 to 2.39); p < 0.001 |
| Skin antisepsis, ICU B | 27 | 97 | RR = 3.56 (2.03 to 6.23); p < 0.001 |
Compliance with skin antisepsis prior to catheter insertion
Skin antisepsis prior to CVC insertion was a common element of the education in many of the studies included in the systematic review, but no studies specified explicitly that it was a target behaviour. Baseline rates of compliance with skin antisepsis ranged from 9% to 57% in the three studies that reported this outcome, and in all cases interventions resulted in improvements, with final compliance ranging from 82% to 100%. The change was statistically significant in one study108 but significance was not reported in the other two studies126,138 (see Table 25).
Compliance with hub disinfection and catheter set dating
Compliance rates for line protection and hub disinfection were reported in two studies108,109 and ranged from 33% to 69% at baseline and were improved by interventions, with final compliance in the range 82% to 98% (all improvements were reported statistically significant). However, there was a notable difference between two studies in compliance with the dating of intravenous administration sets, although in both cases increases in compliance occurred, which were statistically significant. Baseline compliance was only 0.57% and initially fell to 0% after education in the study by Rosenthal and colleagues129 but then reached 74% after a phase of performance feedback. In the study by Higuera and colleagues,103 compliance with set dating rose from 40.69% to 93.85% (see Table 25).
Compliance with overall bundles
Three studies83,98,110 reported compliance with overall care bundles (Table 25). These were ‘patient’ and ‘clinician’ bundles82 a CVC insertion bundle110 and a post-insertion care bundle. 98 Compliance improved in the studies by Burrell and colleagues83 and Longmate and colleagues110 but was reported to be statistically significant only in the former study. The intervention by Guerin and colleagues98 did not appear to affect compliance with the CVC post-insertion care bundle. It is notable that compliance with all four bundles was already high at baseline, ranging from 74% to 94%, and improvements appeared modest (increases of only 7% and 11% in the Burrell study83 despite being statistically significant, and approximately 20% in the Longmate study110).
| Study | Activity | Baseline compliance | Intervention compliance | RR (95% CI); statistical significance |
|---|---|---|---|---|
| Lobo (2005)108 | Skin antisepsis at CVC insertion | 9 | 100 | RR = 11.0 (3.24 to 50.42); p < 0.001 |
| Render (2006)126 | Skin antisepsis at CVC insertion | 42 | 82 | Not reported |
| Wall (2005)138 | Skin antisepsis at CVC insertion | 57 | 100 (last quarter) | Not reported |
| Rosenthal (2003)129 | Dating i.v. admin set | 0.57 | 0 (74)a | p = 0.32 p < 0.001b |
| Higuera (2005)103 | Dating i.v. admin set | 40.69 | 93.85 | RR = 2.34 (2.14 to 2.56); p = 0.0000 |
| Lobo (2005)108 | Line protection | 69 | 98 | RR = 1.38 (1.13 to 1.69); p < 0.001 |
| Hub disinfection | 33 | 93 | RR = 2.74 (1.78 to 4.22); p < 0.001 | |
| Lobo (2010)109 | Hub disinfection, CVC handling, ICU A | 68 | 97 | RR = 1.42 (1.19 to 1.69); p < 0.001 |
| Hub disinfection, CVC handling, ICU B | 44 | 82 | RR = 1.80 (1.20 to 2.70); p < 0.004 | |
| Burrell (2011)83 | Clinician bundle compliance | 74 | 81 | p = 0.0006; χ2 of slope = 11.71 |
| Patient bundle compliance | 81 | 92 | p = 0.001; χ2 of slope = 108.34 | |
| Guerin (2010)98 | CVC post-insertion bundle compliance | 94 | 93 | Not reported |
| Longmate (2011)110 | CVC insertion bundle compliance | 80 | 95–100 (approximate) | Not reported |
Burrell and colleagues83 demonstrated relationships between compliance with the two care bundles and the effectiveness of their CQI intervention at preventing catheter-BSI (central line-associated bacteraemia). Risk of catheter-BSI was reduced if insertion was conducted by physicians compliant in both bundles [RR = 0.5 (95% CI 0.4 to 0.8); p = 0.004], but risk was increased if insertion was conducted by physicians not compliant with the clinician bundle [RR = 1.62 (95% CI 1.1 to 2.4); p = 0.018. Most (94.0%) cases of non-compliance with the clinician bundle were due to failure to wear a hat, mask and eyewear.
Longmate and colleagues110 reported that all-or-nothing compliance with the CVC insertion bundle component of their CQI programme fluctuated between 80% and 100% during an 18-month period. The authors suggested that low initial compliance was attributed to a lack of checklist stickers early in the period; improved compliance later may have been due to introduction of a CVC insertion pack and the creation of subteam nurses to ‘own’ CVC processes, and also to greater scrutiny and follow up of episodes of incomplete compliance. 110
In addition to the studies listed in Table 25, Speroff and colleagues136 provided extensive data on the adoption of various components of flexible QI interventions in critical care units of hospitals that had been randomised to toolkit-based and virtual collaborative QI approaches (full data are given in the data extraction form: see Appendix 5). Adoption of intervention components was consistently higher in virtual collaborative hospitals than toolkit hospitals. However, as mentioned above, neither of these intervention approaches was successful in reducing catheter-BSI incidence density relative to baseline. Risk of catheter-BSI appeared higher under the virtual collaborative (see Figure 4), which had the more frequent adoption of intervention tools and strategies, suggesting that failure to reduce catheter-BSI incidence density was not simply related to the extent of intervention implementation.
Further data on compliance with evidence-based practices were reported by Coopersmith and colleagues,50 DuBose and colleagues,93 Lobo and colleagues,109 Palomar Martinez and colleagues,68 and Render and colleagues. 126 These data (see Appendix 5) are not discussed here as they are based on very small or unclear sample sizes, or it is unclear to which study periods they refer.
Summary of compliance
Overall, nearly all of the studies that reported compliance with evidence-based infection prevention practices reported improvements relative to the baseline period, although the uncontrolled studies cannot definitively exclude an influence of secular trends. Most of the information on compliance relates to hand hygiene, barrier precautions and CVC dressing care. The studies are difficult to compare, however, as in some studies small changes in compliance were reported to be statistically significant, whereas in other studies large changes were reported not statistically significant. Interventions targeting specific behaviours appear more likely to improve compliance but this is difficult to assess critically as target behaviours were not always clearly specified in the primary studies. Baseline compliance rates varied considerably between studies and for hand hygiene they varied markedly between the different stages of CVC site preparation, insertion and ongoing care. It is not clear from these data whether compliance with evidence-based practices was an important mediator of intervention effectiveness at preventing catheter-BSI, as baseline compliance rates were often already high. An exception is the study by Burrell and colleagues,83 which found that compliance with two care bundles significantly reduced the risk of catheter-BSI.
Other secondary outcomes
Our systematic review protocol (see Appendix 1) specified two secondary outcomes that we would assess if reported in the primary studies: (1) the reaction of critical care staff to education and (2) critical care staff practical skills in relation to infection prevention. However, none of the 24 studies included in the systematic review reported these outcomes.
Process evaluations, facilitators and barriers
In addition to the assessments of attitudes, knowledge and compliance reported above, six studies34,68,83,110,126,136 provided qualitative observations relevant to understanding intervention processes, facilitators and barriers, although none of these studies carried out a full process evaluation.
Three of the CQI studies83,110,136 identified a lack of adequate infrastructure to support data collection and dissemination as being a barrier to successful implementation of the interventions. Burrell and colleagues83 stated that reliable baseline data did not exist prior to the project owing to variable reporting mechanisms. Some critical care units were hesitant to accept previously reported rates, and inadequate staffing rates in some critical care units impacted on data capture rates. Difficulties were also encountered regarding data collection and associated information technology, such that the project team resorted to hard copy receipt of checklists. The lack of a continuous and sustainable data collection system involving cross-specialty collaboration was seen as a serious risk to sustainability of the CLAB ICU project principles. Speroff and colleagues136 commented that the lack of appropriate infrastructure to support data-driven QI was a significant barrier and that systematic standardised data collection was initially lacking in many of the study hospitals. Early effort was therefore needed to deploy a system-wide standardised infection control database registry. Longmate and colleagues110 reported that investigators were initially unable to reach agreement on a system – which had full support of both clinicians and data analysts – for collecting process measurements.
Three studies34,68,110 reported difficulties around the use of chlorhexidine for skin antisepsis. These related to difficulty in obtaining supplies (implying uneven compliance across critical care units)34,68 and the fact that chlorhexidine is colourless, making the skin area prepared with antiseptic difficult to see. 68,110 In one study110 it was agreed that povidone–iodine could be used to colour the skin, followed by chlorhexidine.
Two studies68,136 commented that their8 interventions provided insufficient time for some components to be implemented. Speroff and colleagues136 reported that implementation of checklists was slow, suggesting that beneficial translation of desired changes may take more than 18 months to achieve. Palomar Martinez and colleagues68 reported that none of the critical care units was able to implement catheter equipment carts within the 3-month duration of their pilot study.
Other aspects of intervention process evaluation were identified in specific studies:
Burrell and colleagues83 reported difficulty in ensuring application of, and adherence to, infection surveillance definitions. They also commented that the CLAB ICU project methodology was based on a single successful collaborative (the Keystone ICU project) without a detailed analysis of all available evidence, which allowed criticism of methodology to be an excuse for non-compliance.
Palomar Martinez and colleagues68 reported that critical care staff were reluctant to take a test as part of the training for the CQI intervention, as the test was not mandatory, results were not anonymous and there was no credit given for participation in the training.
Pronovost and colleagues34 reported that the Keystone ICU project intervention was modestly more effective in small hospitals, with an incidence rate ratio of 0.97 (95% CI 0.96 to 0.99, p < 0.001) for each 100-bed decrease in the size of the hospital. Although Pronovost and colleagues34 did not conduct a detailed process evaluation during the Keystone ICU project, Dixon-Woods and colleagues149 developed an ex-post theory of the processes that contributed to the project's success at preventing catheter-BSI. Although not noted by Dixon-Woods and colleagues,149 an important difference between the Keystone ICU project34 and other regional-scale CQI approaches that we reviewed68,83,110,136 could be that the Keystone ICU project was based on an established data collection system.
Render and colleagues126 provided a table of facilitators and barriers in their publication but it is unclear whether this was based on quantitative evidence. The study authors reported that after 6 months the project leader and project co-ordinator reviewed their detailed notes from the monthly reporting meetings to independently identify themes contributing to or delaying project success. The list of barriers and facilitators was then validated by the project leaders. However, the validation process was not explained.
Summary of the systematic review of clinical effectiveness
Twenty-four studies were included in the systematic review, half of which were conducted in the USA and most of which used a single-cohort uncontrolled before-and-after design. The interventions were diverse in their educational approaches, ranging from single lectures in single critical care units to regional-scale CQI programmes in up to 103 critical care units. Most interventions focused on catheter insertion rather than catheter ongoing care, with hand hygiene, maximal barrier precautions and antiseptic preparation of the insertion site being the most frequently addressed clinical practices. Different educational approaches, which were unique to individual studies, were used to address the same clinical practice. Nearly all interventions included formal education approaches that would take staff away from bedside patient care. Definitions of CABSI and CRBSI were used inconsistently and two-thirds of the studies provided infection definitions that did not agree with those used in the NHS for the Matching Michigan programme. Most studies did not separately count multiple vascular catheters per patient when calculating the number of device-days, but where different approaches for calculating the number of device-days were compared the method of calculation did not appear to influence the incidence density RRs for catheter-BSI.
Data synthesis was conducted narratively, as it was inappropriate to statistically pool effects from the different types of educational interventions, which varied in their spatial and temporal scales and intervention complexity. Some studies were identified to be at high risk of bias but, owing to deficiencies in the reporting of study methods, risk of bias was generally unclear and could not be used as a criterion for objectively excluding studies from the data synthesis. Assuming that the RRs for incidence density of catheter-BSI reflect those of the planned interventions, 12 studies reported interventions that appeared clinically effective, eight studies reported interventions judged not to be clinically effective, or not to have provided convincing evidence of effectiveness, and four studies provided insufficient data for effectiveness of their interventions to be assessed. The interventions that appeared effective (subject to caveats about possible risks of bias and establishing cause and effect in before-and-after studies ) included local- and regional-scale CQI programmes; local-scale multimodal education with or without performance feedback and infection surveillance feedback; a catheter insertion bundle; and a catheter ongoing care bundle. However, single lectures on CVC care and infection prevention in single critical care units were not effective. There was no evidence to suggest that the spatial or temporal scale, intervention complexity (education alone or with components beyond education) or the presence/absence of performance feedback and/or infection surveillance feedback had any appreciable influence on the incidence density of catheter-BSI. Too few studies provided information on mortality and LOS for effects of the educational interventions on these outcomes to be assessed.
Assessments of critical care staff attitudes, knowledge, compliance with clinical practices and other aspects of intervention processes identified several barriers or potential barriers to the successful implementation of educational interventions. Although limited in detail and lacking quantitative analysis, reports of the attitudes of critical care staff suggest that staff may be liable to question the need for evidence-based infection prevention practices, even when presented with supporting evidence. Most of the information on compliance with intervention practices was related to hand hygiene, barrier precautions and CVC dressing care. However, only one study had formally tested the influence of staff compliance on intervention effectiveness, finding that staff compliance with care bundles significantly reduced the risk of catheter-BSI. In several CQI programmes a lack of existing systems and infrastructure for data collection was reported to be a barrier to effective implementation of interventions. The availability of infrastructure at intervention inception seems to be a key difference between the Keystone ICU project which was based on an existing data collection system and the other CQI programmes, which appeared to have had to develop data collection systems for their interventions.
At the time of writing this report, the only published UK study110 to have implemented an educational intervention for adult critical care patients was conducted in Scotland by Longmate and colleagues (the results of Matching Michigan in England had not been published and so could not be included in our evidence map or systematic review). The CQI programme included some elements of the Keystone ICU project but it differed from the Matching Michigan programme, in that it aimed to prevent VAP as well as catheter-BSI; it was conducted in a single critical care unit; it took 3 years to achieve effectiveness and the last year of the intervention overlapped with the Scottish Patient Safety Programme. 110 The CLAB ICU project implemented by Burrell and colleagues83 in Australia appeared to be the most relevant of the clinically effective interventions to current NHS practice for prevention of catheter-BSI. CLAB ICU has similarities to Matching Michigan in England, as both programmes attempted to implement interventions based on the Keystone ICU project in new national settings. The CLAB ICU baseline incidence density of catheter-BSI was relatively low, consistent with the situation in English NHS trusts, and the starting infrastructure of the CLAB ICU study appears to have had some similarities with the English situation, as standard data collection strategies for infection surveillance appear not to have been in place at the start of CLAB ICU or Matching Michigan. An advantage of the CLAB ICU project, compared with most other studies included in the systematic review, is that the methods of the intervention were extensively reported. These are available in the published paper,83 project report146 and project website. Given the above considerations, the CLAB ICU project was used to provide some of the parameters used in the health economic model (see Chapter 6) to explore the cost-effectiveness of educational interventions for preventing catheter-BSI.
Chapter 5 Systematic review of cost-effectiveness studies
A systematic review of the literature was conducted to identify and assess the current evidence base for the cost-effectiveness of educational interventions for catheter-BSI and to inform the most appropriate approach for the de novo economic model (see Chapter 6).
Search strategy
A systematic literature search was undertaken to identify economic evaluations of educational interventions for preventing CRBSIs in critical care. Sensitive search strategies (shown in Appendix 2, Cost-effectiveness search strategy) were developed and tested by an experienced information scientist. These strategies were used to search the following electronic bibliographic databases:
-
MEDLINE (Ovid)
-
MEDLINE In-Process & Other Non-Indexed Citations
-
EMBASE (Ovid)
-
BIOSIS
-
Cochrane Central Register of Controlled Trials (CCRCT)
-
Cochrane Database of Systematic Reviews (CDSR)
-
Cochrane Database of Abstracts and Reviews of Effects
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCOhost
-
Web of Science databases:
-
Science Citation Index Expanded (SCIE), Social Sciences Citation Index (SSCI)
-
Arts & Humanities Citation Index (A&HCI)
-
Conference Proceedings Citation Index – Science (CPCI-S)
-
Conference Proceedings Citation Index – Social Science & Humanities (CPCI-SSH)
-
-
NHS Economic Evaluation Database [NHS EED via the Centre for Reviews and Dissemination (CRD)].
Searches were undertaken from inception of databases to February 2011 and updated in March 2012. Data from the studies that met the inclusion criteria were extracted and evaluated for quality and generalisability to the UK using a critical appraisal checklist. 150 The included studies were then described in more detail, including discussion of the main issues arising from each of the studies. The full data extraction forms for the studies are shown in Appendix 8.
Inclusion criteria
Titles and (where available) abstracts of references identified by the search strategy were assessed for eligibility against our inclusion criteria (Table 26) by two health economists. Conference abstracts were not eligible for inclusion unless sufficient detail was provided for critical appraisal. Articles published in languages other than English were eligible for inclusion. Full papers of those records that appeared relevant on title or abstract were retrieved and independently screened by two health economists. Any differences in judgement were resolved through discussion.
| Study characteristic | Inclusion criteria |
|---|---|
| Population | Patients in critical care with vascular catheter(s) |
| Intervention | Educational interventions with an objective to reduce or prevent CRBSIs |
| Outcomes | BSIs or mortality associated with, related to, or suspected to result from catheter use |
| Design | Full economic evaluations: cost-effectiveness, cost–utility, cost–benefit and cost–consequence |
Quantity and quality of published research
A total of 767 potentially relevant articles were identified in the cost-effectiveness searches. Through inspecting the titles and abstracts of these articles, 759 non-relevant studies were excluded. The full-text records of the remaining eight studies were retrieved, but only three met all the inclusion criteria,76,151,152 with the remaining five studies excluded because they were not full economic evaluations (Figure 7). A summary of the characteristics of the three included studies is given in Table 27 and the studies are described in more detail below.
FIGURE 7.
Identification of studies for inclusion in the systematic review of cost-effectiveness
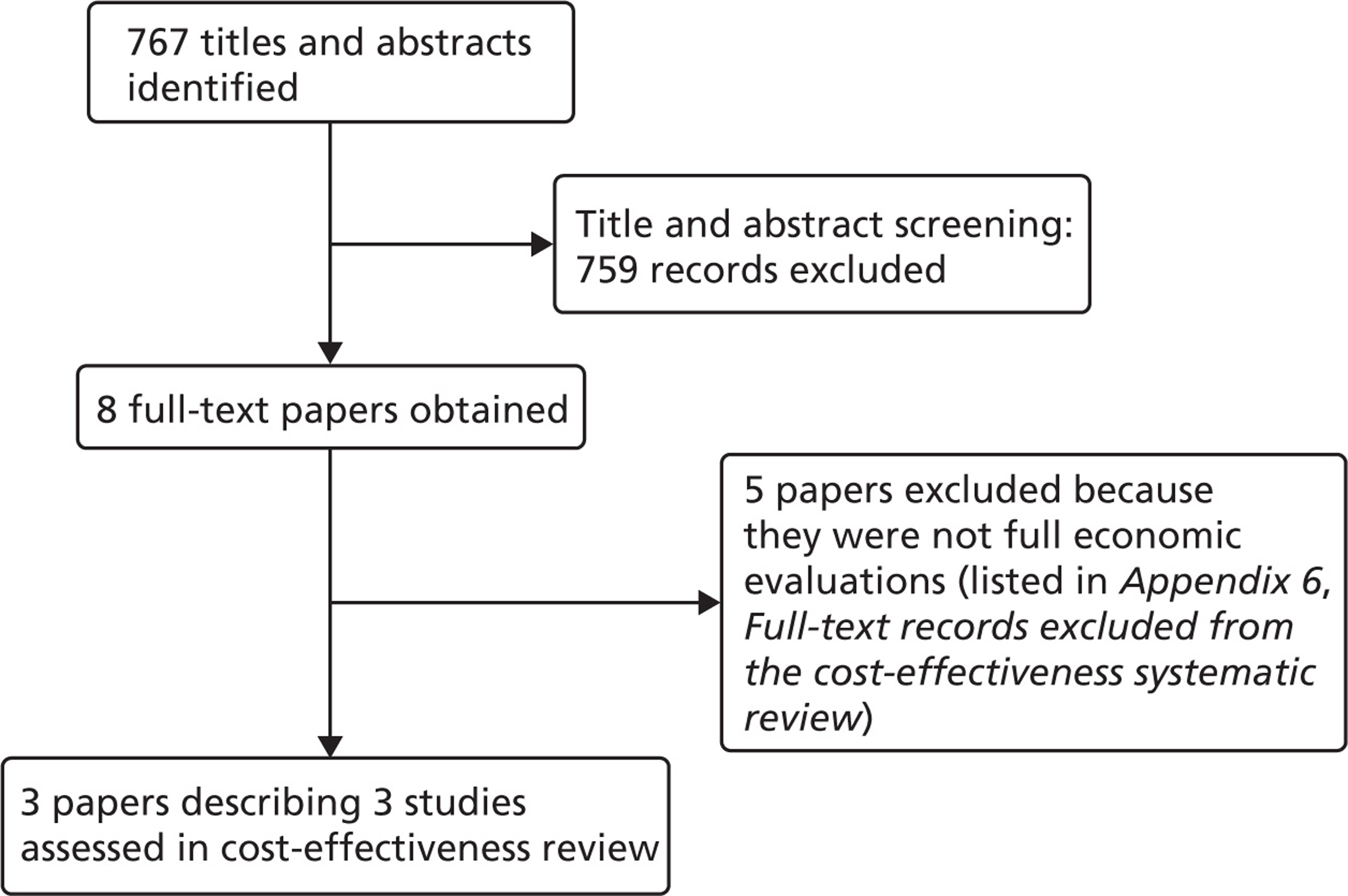
| Characteristics | Halton151 | Cohen76 | Bond and King152 |
|---|---|---|---|
| Publication year | 2010 | 2010 | 2011 |
| Country | Australia | USA | USA |
| Funding source | Queensland Health Quality and Safety programme and National Health and Medical Research Council | Not stated | None |
| Study type | Cost–utility analysis | Cost–consequence analysis | Cost-effectiveness analysis |
| Perspective | Australian health-care payer | United States health-care payer | Not stated |
| Study population | Adult critical care unit patients with a CVC | Adult patients with a CVC inserted in the MICU | Adult patients with a CVC |
| Intervention and comparator | Catheter care bundle vs. current practice | Simulated education training in catheter insertion vs. no educational intervention | CVC educational intervention vs. no educational intervention |
| Intervention effect | Reduction in the rate of CRBSI from 7.7 to 1.4 per 1000 line-days over an 18-month period, relative risk reduction of 0.34 (95% CI 0.23 to 0.50) | Prevented 9.95 cases of CRBSI among patients in MICU, with 14 additional hospital days (including 12 MICU days) gained | The number of CLAB was reduced from 4 to 2 per 600 CVCs inserted in a hypothetical year |
| Intervention cost | Unknown. A range of costs was used in the model | Total operating costs for training the medical residents, faculty and staff time, supplies and space rental were US$111,916 | The total cost per patient was US$546 with the education intervention vs. US$392 without the intervention |
| Currency base | A$ (2006) | US$ (2008) | US$ (year not stated) |
| Model type, health states | Markov model | Regression model | Decision-analytic model |
| Time horizon | Lifetime | 12 months | Hospital stay |
| Baseline case results | The base-case analysis shows that the care bundle is cost-effective up to an 18-month nationwide implementation cost of A$4,349,730 when compared with current practice | The intervention was highly cost-effective with overall cost savings | If the educational intervention is effective, a small increase in cost can reduce complications |
The cost-effectiveness studies were assessed using a critical appraisal checklist as shown in Table 28. The checklist assesses the studies for quality and their generalisability to the UK; it was adapted by the review authors from checklists originally put together by Phillips and colleagues,150 Drummond and colleagues153 and the National Institute for Health and Care Excellence (NICE) reference case requirements. 154 The definition of catheter-BSI provided was CRBSI in two of the studies76,151 CLAB in the third study. 152
| No. | Item | Halton 2010151 | Cohen 201076 | Bond and King152 |
|---|---|---|---|---|
| 1 | Is there a clear statement of decision problem? | Yes | Yes | Yes |
| 2 | Is the comparator routinely used in UK NHS? | Yes | Yes | Yes |
| 3 | Is the patient group in the study similar to those of interest in UK NHS? | Yes | Yes | Yes |
| 4 | Is the health-care system comparable that of the UK? | No | No | No |
| 5 | Is the setting comparable to that of the UK? | Yes | Yes | Yes |
| 6 | Is the perspective of the models clearly stated? | Yes | Yes | No |
| 7 | Is the study type appropriate? | Yes | Yes | Yes |
| 8 | Is the modelling methodology appropriate? | Yes | Yes | Yes |
| 9 | Is the model structure described and does it reflect the disease process? | Yes | Yes | Yes |
| 10 | Are the assumptions about the model structure listed and justified? | Unclear | Unclear | Yes |
| 11 | Are the data inputs for the model described and justified? | Yes | Yes | Unclear |
| 12 | Is the effectiveness of the intervention established based on systematic review? | Yes | No | No |
| 13 | Are health benefits measured in QALYs? | Yes | No | No |
| 14 | Are health benefits measured using a standardised questionnaire? | Yes | No | No |
| 15 | Are the resource costs described and justified? | Yes | Unclear | No |
| 16 | Have the costs and outcomes been discounted? | Yes | No | No |
| 17 | Has uncertainty been assessed? | Yes | No | Yes |
| 18 | Has the model been validated? | Yes | No | No |
The three studies76,151,152 specified the decision problem, the study rationale and justified the comparator. A detailed description was given of the patient groups and the health-care settings were similar to those in the UK. The health-care systems in all studies differ to that of the UK and therefore the practices may not be generalisable to the UK. The structure of the models used in the studies76,151,152 reflect the disease process and the modelling methodologies seem appropriate.
Cohen and colleagues76 and Bond and King152 did not base their effectiveness estimates on a systematic review, and did not use any instruments to measure health benefit. Halton and colleagues151 did both, using quality-adjusted life-years (QALYs) as a measure of health outcome. Data inputs for the model used in Cohen and colleagues76 and Halton and colleagues151 studies were adequately reported and justified, but not in the Bond and King152 study. Cohen and colleagues76 and Bond and King152 did not state the uncertainty surrounding the model and the included parameters, neither did they report any form of model validation, whereas Halton and colleagues151 reported both.
Description and results of the published economic evaluations
Full data extracted from the published economic evaluations are given in the data extraction forms (see Appendix 8).
Halton and colleagues
Halton and colleagues151 conducted an economic evaluation of the effectiveness of a CVC care bundle for preventing CRBSI relative to current practice, which was defined as a non-bundled approach to central line management with the use of uncoated catheters. The study setting was an adult ICU in Australia. The intervention ran over an 18-month period. The CVC care bundle encompassed five elements: optimal hand hygiene, chlorhexidine skin antisepsis, maximal barrier precautions for catheter insertion, choice of optimal insertion site and prompt catheter removal. The intervention also included a programme to educate staff about clinical leadership and the risk of infection.
The total costs of implementing the intervention were estimated in two parts: the costs directly associated with the components of the intervention and the costs related to the monitoring, education and leadership activities. A Markov model was constructed and was used to estimate the cost-effectiveness of the CVC care bundle for different combinations of antimicrobial and uncoated catheters. The analysis was conducted from the Australian health-care payer perspective with the costs reported in Australian dollars at 2006 prices. The study used various sources including the published literature, primary data from studies, national statistics, population surveys and health-care databases to derive costs and the associated health benefits. The economic outcomes from the model were summarised in terms of QALYs and total costs.
Modelling approach
The structure of the model was based on the patient clinical pathway. A Markov state–transition decision model, which had previously been developed to evaluate the cost-effectiveness of antimicrobial CVCs in an Australian setting, was adapted. 155 The Markov model consists of short- and long-term components: short term for the hospital stay and long term for the remainder of the patients' life after hospital discharge. The patients enter the model with the CVC in situ. The short-term Markov model consists of daily cycles whereby patients may develop CRBSI, remain as an ICU patient with the CVC or have their catheter removed. Patients who develop CRBSI have an increased risk of death in hospital (Figure 8).
The daily probabilities of catheter removal and CRBSI were estimated by fitting a Weibull distribution to data from an epidemiological study of CVCs. 156 In the long-term Markov model, the surviving cohort was followed for the remainder of their lifetimes. Utilities associated with different health states were assigned to cycles spent in the ICU and 6 months after discharge. Costs and QALYs were discounted using a rate of 3%.
Assumptions
Several assumptions were made in the model structure. It was assumed that the catheters were inserted or removed mainly within the ICUs and that no multiple catheterisations existed. ICUs were assumed to have an existing infection control procedure in place. The consequences of CRBSI were also assumed not to be dependent on age, disease severity or causative micro-organisms. As there was no information on reduction in QALYs among CRBSI survivors, the life expectancies among this group of patients were adjusted using Australian population QoL population norms. 157
Catheter colonisation was not included in the model, as the authors considered that this event does not carry health or economic consequences. It was assumed that preferences exist among the clinicians for the use of antimicrobial CVCs. This assumption led to comparisons between the current standard of practice using antimicrobial-impregnated catheters and adoption of the CVC care bundle.
Effectiveness of intervention
The study used estimates of the effectiveness of a catheter care bundle from the Keystone ICU project,34 which reported an 18-month intervention aimed at reducing the incidence of CRBSI in adult ICUs in Michigan, USA (as discussed in Chapter 4). The intervention focused on the clinicians' use of five evidence-based procedures as highlighted by the US CDC. The intervention included a programme to educate staff about clinical leadership and risk of infection. The intervention reduced the risk of infection over 18 months with a relative risk of 0.34 (95% CI 0.23 to 0.5).
Halton and colleagues151 estimated the effectiveness of each type of antimicrobial CVC from a systematic review of the literature conducted by Ramritu and colleagues. 158 The relative risks of contracting CRBSI with the use of a chlorhexidine/silver sulfadiazine (CH/SSD)-coated catheter, and minocycline and rifampicin (MR)-coated catheter were 0.66 and 0.39, respectively.
Estimation of quality-adjusted life-years
The health outcome in the model was expressed in QALYs. The utility weight estimates used in the model were taken from several sources. A utility value of 0.66 for the ICU stay was derived from a previous study that examined the changes in QoL before and after intensive care. 159 The study was a prospective cohort of 300 consecutive patients admitted to intensive care in a Scottish hospital. The SF-36 questionnaire was given to patients' relatives to assess the patients' health-related quality of life (HRQoL) before their current illness. The EQ-5D instrument was then used to elicit the patient's QoL over a 12-month period.
Age-related utility values were derived from a population-based survey. 157 The Australian Health Omnibus Survey was based on responses from a 3100 population using the Assessment of Quality of Life (AQoL) instrument. The data from the population based survey were stratified by age, gender and health status.
Estimation of costs
Halton and colleagues151 included costs associated with implementing the CVC care bundle and the costs of the monitoring, educational and leadership activities. The authors were not able to estimate the cost of the care bundle itself. The estimates of the resources associated with these activities were based on descriptions from the Keystone ICU project. 34 The overall resource use estimate was based on time spent on the activities directed towards the implementation of the intervention. Time spent on activities such as mentoring by key personnel, lectures for the clinical staff and preparation of care bundle components were among the key resource estimates. The overall time spent was expressed in days and man-hours to the nearest minute.
The additional costs for the CH/SSD and MR catheters were A$11.64 and A$59.36, respectively, relative to uncoated catheters. Cost estimates for CRBSI were categorised into costs of CRBSI diagnostics, treatment and hospitalisation. Diagnostic costs for CRBSI were A$101.70 (per patient with CRBSI), based on an estimate from the Australian health-system database, whereas the cost for treatment was A$591.30. CRBSI was associated with increased hospitalisation costs and longer inpatient stays. These costs were for ICU bed-days (A$3021 per day) and hospital bed-days (A$843 per day). There were an extra 2.41 ICU days and 7.54 hospital days associated with each case of CRBSI. Other sources of cost and resource use estimates included a prior economic evaluation,160 and costing study. 161
Cost-effectiveness results
As the cost of implementing a CVC care bundle in Australia was unknown, deterministic threshold analyses were conducted. The maximum cost for the care bundle was identified at which it would remain cost-effective (i.e. if the cost per QALY was less than the willingness-to-pay threshold of A$64,000). The baseline analyses show the benefits for the CVC care bundle or standard practice under different scenarios. The CVC care bundle was shown to be cost-effective up to a nationwide implementation cost of A$4,349,730 for an 18-month period (or A$94,559 per ICU) compared with current practice alone. For the strategy that includes CH/SSD and MR catheters, the CVC care bundle remains costs effective up to an implementation cost of A$2,287,400 and A$1,144,465, respectively, for the same period.
Sensitivity analyses
Sensitivity analyses were conducted to compare the CVC care bundle and different forms of vascular catheters used in current practice. The analyses included a pairwise comparison between current practice and the CVC care bundle; a three-way comparison between the CVC care bundle, the CH/SSD catheters and current practice; and a four-way comparison between the CVC care bundle, MR catheters, CH/SSD catheters and current practice.
Summary of key issues
-
The study estimated the cost-effectiveness of a CVC care bundle relative to current practice in an Australian setting.
-
The structure of the model, assumptions and methods used in estimating the utility weights incorporated into the model were clearly stated.
-
One key limitation is that the study was not able to estimate the cost of the CVC care bundle.
Cohen and colleagues
Cohen and colleagues76 conducted a retrospective analysis of the costs from a simulation-based educational intervention in CVC insertion as a means of reducing CRBSI among patients in a medical intensive care unit (MICU) in Chicago, USA. The study estimated the financial implication of the educational intervention by comparing the rates of CRBSI during the intervention period (December 2006 to November 2007) and the year before the intervention. The intervention included a 2-hour lecture, ultrasound training and organised practice with a CVC simulator, as well as feedback from an instructor. The education intervention involved emphasis on the core evidence-based protocol for reducing CRBSI, which includes hand washing, full sterile barrier technique, chlorhexidine skin preparation, avoidance of the femoral site and prompt CVC removal.
The cost-effectiveness model used regression analyses to estimate the costs from the non-randomised before-and-after study. The resources used and cost estimates for the intervention were derived from hospital cost accounting data. The costs included were the cost of the intervention and the associated costs for the hospital stay.
Modelling approach
Two statistical methods were used in the study: the propensity score matched case–control comparison method and the linear regression method. The trial data were analysed and regression models were used to derive estimates of cost and LOS for the intervention and control group, controlling for age, sex and Charlson score (an indicator of comorbidity). To give the intervention and the control group the same infection risk, a regression-based propensity score was used. Estimates of cost differences between the matched cases and controls were then derived.
Effectiveness of intervention
The effectiveness of the intervention was estimated retrospectively by comparing the CRBSI rates for the year before the intervention to the one after the simulation-based education. Using hospital accounting data, the incremental cost and LOS associated with MICU patients with a CVC and a CRBSI during the period were estimated.
A total of 477 patients who had a CVC inserted in the MICU during the period were included in the study. The baseline infection rate before the trial was 11 cases in 239 CVC patients, i.e. 4.2 per 100 MICU CVC admissions. After the medical residents had been fully trained, the infection rate was estimated to be 0.42 per 100 MICU CVC admissions. The study estimated that 9.95 CRBSI cases were prevented in the year after the intervention. Analysis of the data (for both statistical methods) gave an additional LOS for patients with CRBSI compared with those without CRBSI of 13.8–14.2 days for the hospital and 12.1–12.2 days for the MICU.
Estimation of quality adjusted life years
Utility weights were not reported in the model and the study in general, and so QALYs were not estimated. The health benefit from the study had been estimated in terms of the number of CRBSI cases averted.
Estimation of costs
The cost estimates in the study were sourced primarily from the hospital cost accounting data and include the costs of supplies, faculty and staff time and space rental (as outlined in the data extraction form: see Appendix 8).
All the reported costs were adjusted to 2008 US dollars. The cost to train 69 medical residents was US$111,916. The predicted annual cost to maintain the intervention was US$89,455. The additional cost estimates associated with CRBSI derived from the linear regression model and the risk-adjusted model were similar: US$82,005–82,730.
Cost-effectiveness results
The total annual estimated savings for the propensity score matched case–control comparison method and the linear regression method, respectively, were US$823,164 and US$815,950, 141 and 137 patient hospital days, and 120 and 121 MICU days. The net annual saving in monetary terms from the intervention was estimated to be US$700,000. Based on the results, a 7 : 1 rate of return on investment was achieved with the intervention. The study did not report any results of sensitivity analysis.
Summary of key issues
-
Data were from a before-and-after study and may not be a true reflection of the intervention effect.
-
The intervention was limited to a particular hospital setting and was conducted for a limited duration. This makes it unclear if the findings are generalisable to other hospitals or countries.
-
The study did not consider longer-term follow-up.
-
The health outcomes of the study were not presented in QALYs.
Bond and King
Bond and King152 conducted an economic evaluation of the theoretical impact of an educational intervention to improve the safety of CVC insertion. The study setting was a health-care system that included a tertiary care centre, a community hospital and an emergency department in the USA. The educational intervention consisted of a CVC education course. It was a day-long programme with brief introductory lectures followed by hands-on procedural simulation in CVC insertion, using training mannequins, appropriate sterile procedures, ultrasound imaging and feedback from instructors. In addition, doctors and nurses were taught the Institute for Healthcare Improvement (IHI) bundle of barrier precautions and new processes to encourage, ensure and track compliance.
A decision-analytic model was constructed and was used to estimate the cost-effectiveness of the education intervention. The authors did not state the perspective of the analysis, nor the base year for the costs. The study used various sources including data from published primary studies. The results from the model were presented in US dollars in terms of net monetary benefits of the education intervention compared with no education.
Modelling approach
A decision-analytic model was constructed in TreeAge software version 1.5 (TreeAge Software Inc., Williamstown, MA, USA) and describes the duration of the hospital stay. The model starts with the need for a CVC, with a focus on non-emergent cases, defined as those where there was sufficient time to follow sterile precautions in CVC insertion. One cohort of patients receive the education intervention and the other does not. In each cohort, a proportion receive the CVC in the internal jugular, subclavian and femoral veins. Patients then either have no complications or mechanical complications, such as iatrogenic pneumothorax or CLAB. Patients who suffer a CLAB or mechanical complication have corresponding higher costs and mortality than those without.
Effectiveness of intervention
The evaluation assumes that the educational intervention would reduce the rate of CLAB by 50% and reduce the rate of the mechanical complications by 25% in the base-case analysis. The study did not discuss the sources upon which these are based, nor the rationale behind their use. Patients with CLAB had an attributable mortality of 12%, compared with a baseline mortality risk of 10% for ICU patients, based on a review of studies.
Estimation of quality-adjusted life-years
Utility weights were not reported in the model and the study in general, and so QALYs were not estimated. The health benefit from the study had been estimated in terms of the number of CLAB cases averted.
Estimation of costs
The costs of the educational programme were presented but it is not clear how these were estimated or whether they were based upon an empirical study. The costs of the programme included the acquisition cost of mannequins and ultrasound technology, and the staff training time for the trainers and trainees. The cost of the programme was US$170,360 for the first year and an average of US$63,610 for the subsequent 4 years, for the health-care system described, in which 600 patients had CVCs inserted in each year.
The cost per CLAB case was US$16,350 based upon those reported by IHI; however, the full reference was not provided so we are unable to critique this cost. The mean excess cost of mechanical complications was US$17,312.
Cost-effectiveness results
The results were presented for the education intervention versus no intervention per individual with a CVC inserted in the ICU. The survival during the hospital stay was 89.9% for those in the education intervention cohort and 89.8% in the no-education intervention cohort. The costs with and without the education, based on the first year programme cost, were US$546 and US$392, respectively, i.e. an additional cost of US$154. For the health-care setting, the additional cost was US$92,400 to reduce the number of CLABs from 4.2 to 2.1. The study conducted a number of sensitivity analyses varying the programme cost, CLAB baseline rate and the intervention effectiveness. For the lower programme cost for years 2–5, the additional cost of the education intervention was US$44 per patient. Raising the CLAB rate to 5% gave a net monetary benefit of US$158 with an additional survival of 0.3%.
Summary of key issues
-
The study estimated the cost-effectiveness of a CVC education intervention relative to no education for a US health-care setting.
-
The model does not consider long-term follow-up beyond the hospital stay or include QALYs.
-
The derivation of some of the model parameter estimates is unclear, particularly the cost of the CLAB, and the effectiveness of the education intervention.
Published economic evaluations: summary of methods
-
A systematic review of cost-effectiveness studies identified three cost-effectiveness studies of educational interventions for preventing catheter-BSI in critical care. 76,151,152 Two studies used a decision modelling approach and the other was a trial-based economic analysis.
-
Halton and colleagues151 used a Markov decision-analytic approach to model Australian patients with CRBSI over their lifetime and the health benefits associated with a CVC care bundle. The model did not include the cost of the care bundle.
-
Cohen and colleagues76 used a trial-based cohort analysis to derive estimates of the costs and benefits associated with a simulation-based education intervention in a hospital in the USA. They used regression models to estimate the costs and health benefits for matched case and control groups with and without CRBSI. However, the intervention was limited to a particular hospital setting and for a short period of time.
-
Bond and colleagues152 used a Markov decision-analytic approach to evaluate effects of an educational intervention on CLAB among US patients. The model did not consider the long-term health benefit for the intervention.
-
None of the studies was considered appropriate to estimate the cost-effectiveness of educational interventions for preventing catheter-BSI in critical care units in the UK NHS.
Chapter 6 Economic evaluation
We developed a new model to estimate the costs, benefits and cost-effectiveness of implementing a CVC care bundle for preventing catheter-BSI in adult patients in critical care units in England, compared against current clinical practice. The CVC care bundle in this analysis was defined based upon the Matching Michigan programme in England and the original US Keystone ICU project approach. 34 It encompassed five elements: optimal hand hygiene, chlorhexidine skin antisepsis, maximal barrier precautions for catheter insertion, choice of optimal insertion site, and prompt catheter removal. Current clinical practice was defined as critical care that did not implement a CVC care bundle. The model was populated with clinical effectiveness data from the study most relevant to Matching Michigan identified in our systematic review of clinical effectiveness (see Chapter 4). HRQoL data were derived from the published literature and cost data derived from published studies (where available), and from national and local NHS unit costs.
The economic evaluation was from the perspective of the NHS and Personal Social Services, as only these direct costs were included. The model estimates the costs during hospital stay and the lifetime benefits for the intervention and its comparator. The benefits were discounted at 3.5%, as recommended by NICE. 154 The base price year for the costs was 2011. The outcome of the economic evaluation is reported as the incremental cost per QALY gained.
Methods for cost-effectiveness modelling
Description of the model structure
A decision-analytic model was designed to estimate the cost-effectiveness of a CVC care bundle for preventing catheter-BSI in critical care units compared with remaining with current clinical practice. A diagram of the model is shown below (Figure 9). The model follows cohorts of patients from their admission to the critical care unit. The cohorts of patients compared are those who receive the CVC care bundle and those who receive current clinical practice. The numbers of critical care patients infected with catheter-BSI depend upon the catheter-BSI incidence rate, the proportion of patients with a CVC, and the effectiveness of the intervention (CVC care bundle or current clinical practice) for preventing infections. Patients may die during their hospital stay and the risk of mortality is greater for those with catheter-BSI. Furthermore, LOS will be greater for patients with catheter-BSI. The model estimates the number of people who contract catheter-BSI, those who die in hospital and the total LOS for the two cohorts. The long-term survival of patients after discharge from the critical care unit is estimated using a simple Markov model with states for alive and dead. Long-term QALYs are estimated for these patients using general population age-related HRQoL utility values. The model calculates the costs associated with LOS and the treatment and diagnosis of the catheter-BSI infections. There are also costs of implementing the CVC care bundle.
FIGURE 9.
Cost-effectiveness model for the CVC care bundle to prevent catheter-BSI
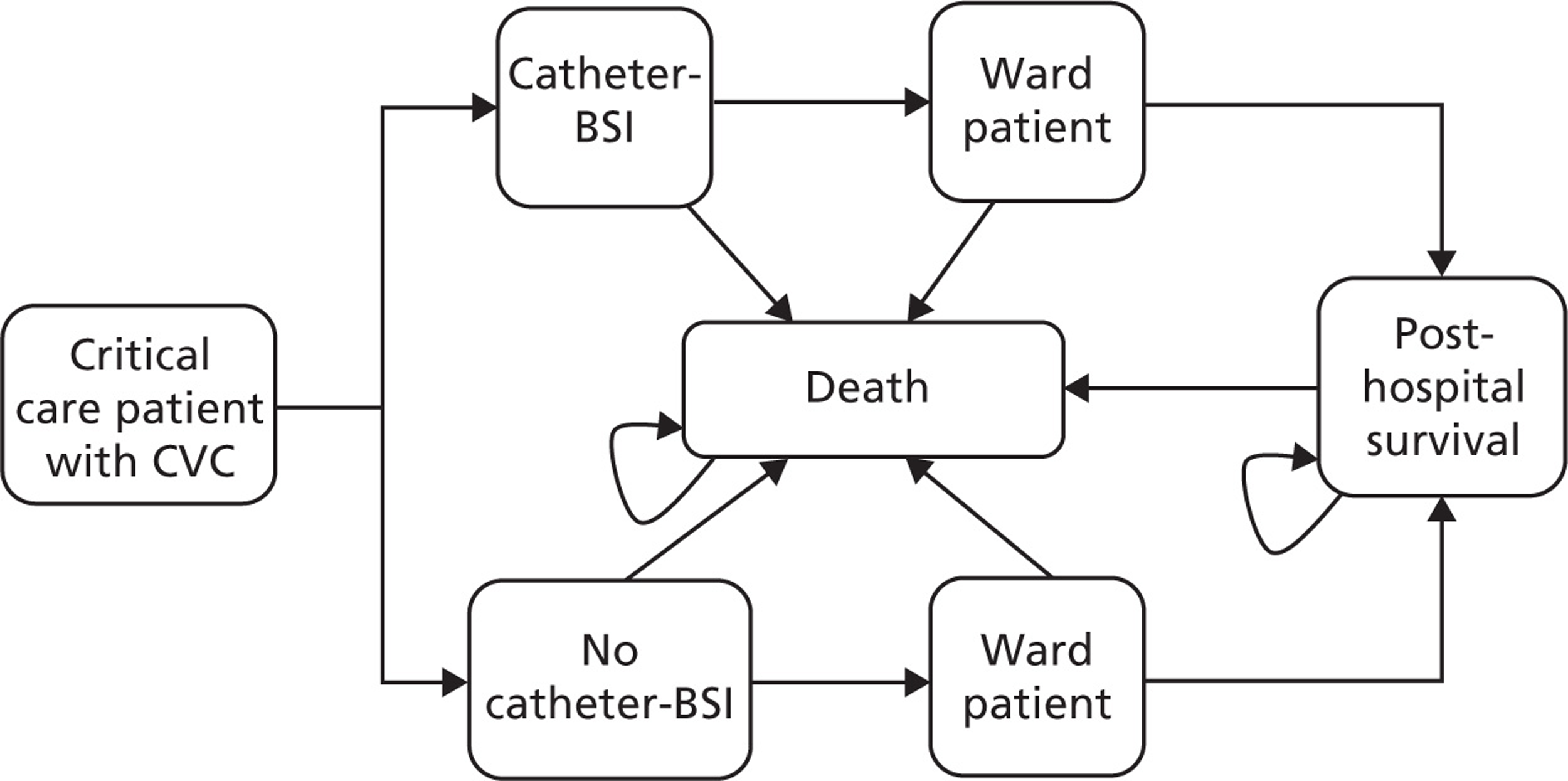
In the model, the total costs and discounted QALYs are calculated for both cohorts and thus the cost-effectiveness of the CVC care bundle is calculated:
The model is based on the following assumptions:
-
We assumed for the purposes of the model that CABSI and CRBSI are synonymous, and are collectively referred to as catheter-BSI. It is not possible to distinguish between the surveillance definition of catheter-associated BSI and the clinical definition of catheter-related BSI. Although in theory CABSI overestimates the true incidence of CRBSI (for definitions see Chapter 1), our review of clinical effectiveness (see Chapter 4) and another systematic review21 found that the definitions are used interchangeably and inconsistently in primary research studies.
-
We assumed that there is no difference in mortality during the hospital stay following critical care discharge for patients who had catheter-BSI in the critical care unit compared with those who did not. We were unable to find any data for the mortality during the hospital stay following critical care discharge for these two groups.
-
We assumed that there was no difference in mortality after hospital discharge for those who had catheter-BSI in the critical care unit compared with those who did not.
The model does not consider the HRQoL of patients in critical care units, because the time spent in critical care is very small compared with the lifetime horizon, and therefore any QALY gains during this period are insignificant. In addition, non-fatal adverse events associated with catheter-BSI were included within the model as a cost, but not as a utility decrement.
The economic evaluation does not include non-tangible benefits or disbenefits associated with the intervention, such as changes to staff morale and public confidence in the health-care system.
Evaluation of uncertainty
There are uncertainties in the evaluation of the cost-effectiveness of the CVC care bundle. These reflect a lack of information about the content of interventions, their costs and resource use, and the extent to which they were implemented in primary research studies, as well as a general lack of information about the effects of catheter-BSI on patient survival and HRQoL. Uncertainty was evaluated using deterministic and probabilistic sensitivity analyses (PSAs). One-way deterministic sensitivity analyses (described below) were conducted to evaluate the influence of individual parameters on the model results and to test the robustness of the cost-effectiveness results to variations in the structural assumptions and parameter inputs.
Multiparameter uncertainty in the model was addressed using PSA. 162 In the PSA, probability distributions were assigned to point estimates of all parameters used in the base-case analysis. The model was run for 1000 iterations, with a different set of parameter values for each iteration, by sampling parameter values at random from their probability distributions. The uncertainty surrounding the cost-effectiveness of the CVC care bundle is represented according to the range of cost-effectiveness results. The parameters included in the PSA, the distribution used for sampling each parameter, and the upper and lower limits assumed for each variable are reported in Appendix 9.
Model validation
The economic model was validated by checking the model structure, calculations and data inputs for technical correctness by an independent health economist. The structure was reviewed by two clinical experts for its appropriateness for the disease process and the treatments considered. The robustness of the model to changes in input values was tested using sensitivity analyses to ensure that any changes to the input values produced changes to the results of the expected direction and magnitude. Finally, the model results were compared with those from previous published cost-effectiveness analyses.
Data sources
Catheter-bloodstream infection epidemiology
Catheter-bloodstream infection incidence rate
Catheter-bloodstream infection incidence data were collected from critical care units in England during the Matching Michigan programme. Matching Michigan recruited 97% of the acute health-care trusts in England, representing 216 critical care units. The main elements of the scheme involved defining and measuring infections and then reporting results on a monthly basis. 163 Although full data from the Matching Michigan programme were not available at the time of writing this report, the incidence per 1000 catheter-days was reported as 3.7 at the start of the collecting period (May 2009). For the purposes of our analyses, we have used the incidence rate from before the introduction of the Matching Michigan intervention (Table 29) to reflect the baseline incidence density of catheter-BSI associated with current clinical practice.
| Parameter name | Base case | Higher estimate | Lower estimate | Source |
|---|---|---|---|---|
| Catheter-BSI epidemiology | ||||
| Catheter-BSI incidence rate, per 1000 catheter-days for current clinical practice | 3.7 | 5.0 | 1.3 | NPSA163 |
| Critical care mortality, no catheter-BSI | 0.169 | 0.203 | 0.135 | ICNARC 2011164 |
| RR for critical care mortality due to catheter-BSI | 3.25 | 3.6 | 2.7 | Lambert et al.165 |
| Proportion of patients with a CVC | 0.71 | 0.96 | 0.49 | ICCTG166 |
| Costs | ||||
| Ward bed-day, £ | 246 | 295.2 | 196.8 | HRG 2010/11167 |
| Critical care bed-day, £ | 1440 | 1171 | 1657 | HRG 2010/11167 |
| Catheter-BSI diagnosis and treatment costs, £ | 518 | 622 | 415 | Halton et al.151 |
| CVC care bundle (per critical care patient), £ | 15.48 | 20.13 | 10.84 | Various |
| Clinical effectiveness | ||||
| Bundle effectiveness, RR | 0.4 | 0.67 | 0.22 | Burrell et al.83 |
| Additional critical care LOS for catheter-BSI, days | 1.5 | 2.5 | 0.0 | Lambert et al.165 |
| Additional ward LOS for catheter-BSI, days | 5.13 | 8.68 | 1.58 | Warren et al.168 |
| Other parameters | ||||
| Starting age, years | 60 | 70 | 50 | ICNARC169 |
| Intercept | Age coefficient | |||
| HRQoL utility coefficients | 1.0604 | 0.0043 | Ward et al.168 | |
Within the model, the incidence density per 1000 catheter-days was converted to the percentage of critical care patients who contracted catheter-BSI by assuming a mean stay in critical care with a CVC of 5 days. 164
The proportion of patients with a central venous catheter
No estimate was found of the proportion of patients in critical care requiring a CVC in England (or anywhere else in the UK). However, a pilot surveillance study166 reported infection control data for nine hospitals in Irish critical care units between November 2010 and January 2011. The characteristics of the Irish units were assumed to be similar to those in the UK. The proportion of patients with a CVC was defined as the percentage of patients in the critical care unit who had one or more CVC inserted. Nationally, it was 71% and ranged between individual units from 49% to 96%.
Clinical effectiveness
Central venous care bundle effectiveness
At the time of constructing the model, full results of the Matching Michigan programme had not been published. An estimate of the effectiveness of the CVC care bundle was derived from our systematic review of clinical effectiveness (see Chapter 4). In this review the most relevant studies in terms of geographical scale for providing clinical effectiveness data were the five regional-scale projects34,68,83,126,136 as they most closely resemble the regional multicentre approach used in Matching Michigan in England. We considered the ‘CLAB ICU’ study by Burrell and colleagues in Australia83 to be the most appropriate for the following reasons: (1) it appeared to closely reflect the strategic approach used in Matching Michigan in England (provision of a CVC insertion kit, checklist, infection surveillance feedback and staff empowerment among other components); (2) sufficient details of the intervention were reported for the intervention to be classified by the reviewers as reproducible based on the published information; (3) costs of the intervention were reported; (4) the intervention was, like Matching Michigan, specifically intended to replicate the original US Keystone ICU project approach34 in a new national setting. The study by Burrell and colleagues83 is reported in more detail in Chapter 4. Burrell and colleagues83 used a definition of CABSI during the hospital stay, rather than CRBSI, as used with other model parameters. However, as noted above, we assume that CABSI and CRBSI are synonymous and are collectively referred to as catheter-BSI.
Burrell and colleagues83 reported a 60% reduction in the number of catheter-BSI in critical care, i.e. a relative risk of 0.4 compared with baseline clinical practice.
Our model compares a CVC care bundle against remaining with current clinical practice, where those elements from the CVC care bundle have not been implemented on a co-ordinated, national or regional basis. However, it may be the case that some hospitals (or individual critical care units) would have incorporated some elements of the CVC care bundle into current clinical practice, prior to implementation of a co-ordinated, national or regional programme. The baseline catheter-BSI incidence data used in the model, based on the rate reported in acute health-care trusts in England prior to the implementation of Matching Michigan, should reflect such partial implementation. It should also be noted that there may be variation in the degree to which aspects of the bundle are implemented between different hospitals. Similar variability would likely have been present in the regional-scale CLAB ICU project83 that provides an estimate of clinical effectiveness of the CVC care bundle.
Increased length of stay due to infection
Length of stay was reported in seven studies50,94,109,110,117,139,144 in our clinical effectiveness review (see Chapter 4). Only one of these studies, by Zingg and colleagues,144 reported LOS data separately for patients with and without catheter-BSI, and we have therefore additionally searched the literature for further supporting information on the additional LOS due to catheter-BSI.
Estimates for increased LOS due to catheter-BSI vary widely in the literature. However, some of these studies170,171 have overestimated the additional LOS due to the acquired infection by ignoring the timing of the infection. This leads to ‘time-dependent bias’ in multiplicative hazard ratios. A more appropriate method to estimate LOS due to an infection is to use a multistate or longitudinal model that accounts for the time of the infection. 170–172
Barnett and colleagues170 demonstrated the effect of time-dependent bias on LOS. They used a multistate model that accounted for the time of infection and compared that to a commonly used model that ignores the time of infection (a generalised linear model assuming a gamma distribution). They applied the two methods to a large prospective cohort of hospital admissions from Argentina and validated their results using a simulation study. The additional critical care LOS due to nosocomial infection was 11.23 days when ignoring time dependence and only 1.35 days after accounting for the time of infection. The simulation results showed that ignoring time dependence consistently overestimated the additional LOS.
We found four studies165,168,170,173 that used the appropriate methodology to estimate the additional LOS attributable to BSIs in the critical care unit. From these, we considered a study by Lambert and colleagues165 to be most relevant and appropriate as it was a large European study. Lambert and colleagues165 analysed data for 119,699 patients collected prospectively from 537 critical care units in 10 participating countries during 2005–8, using a standard European protocol for the surveillance of infections [Hospitals In Europe Link for Infection Control through Surveillance (HELICS)-ICU]. 174 They assessed the excess mortality and critical care LOS associated with BSI (and pneumonia). They focused on the most frequent causative microorganisms for BSI and pneumonia (Acinetobacter baumannii, Escherichia coli, Pseudomonas aeruginosa and S. aureus). The risk of death in the critical care unit was modelled with a time-dependent regression model assuming proportional hazards, taking into account the indirect effect on mortality of (a potentially) extended stay due to the infection. The study165 reported analyses separately for infections caused by pathogens with and without antimicrobial resistance (sensitive or resistant). The time adjusted excess LOS for all four microorganisms varied from 1.1 days to 2.5 days for sensitive and resistant pathogens, respectively. The excess LOS for all patients was not reported and so we estimated the pooled population excess LOS using the proportions of patients with sensitive (71%) and resistant (29%) microorganisms, to give an estimate of 1.5 days.
We found one study168 that used the appropriate methodology to estimate the additional LOS attributable to catheter-BSI during hospital stay, after discharge from the critical care unit. Warren and colleagues168 prospectively collected data on all patients admitted to the medical–surgical critical care units of the Missouri Baptist Medical Centre in St Louis during 1998–2000. They analysed data for patients who had required a CVC during their critical care stay. Two multiple regression models were created to evaluate hospital LOS and critical care LOS due to catheter-BSI using the backward stepwise method. Compared with non-infected patients, those patients with catheter-BSI had longer critical care and total hospital LOS. The unadjusted mean difference in overall LOS was 21.8 days for the critical care unit and 51.7 days for hospital stay. After controlling for confounding factors, the attributable critical care LOS due to catheter-BSI was 2.41 days and the attributable hospital LOS was 7.54 days (95% CI 3.99 to11.09). Warren and colleagues168 used a definition of CABSI, rather than CRBSI but, as noted above, it is not possible to isolate the effect of using different definitions and therefore catheter-BSI used in the model does not distinguish between CABSI and CRBSI.
Critical care unit mortality
General population data for patients in UK critical care units are collated by the Intensive Care National Audit & Research Centre (ICNARC). 163 The latest data available were for the period for April 2009 to March 2010, from 188 NHS critical care units with 96,810 patients admitted. The mean patient age was 60.5 years and the mortality rate reported for critical care was 16.9%. Mean LOS in the unit was 5 days. The mean mortality rate during the hospital stay (outside the critical care unit) was 8.3%.
As with estimates for LOS, estimates for the attributable mortality for catheter-BSI vary widely according to the methodology adopted. Mortality was reported in five studies94,103,110,139,144 in our clinical effectiveness review (see Chapter 4); however, none of the studies reported mortality data separately for patients with and without catheter-BSI. As with LOS, we considered the study by Lambert and colleagues165 to be the most appropriate. Their findings suggest that BSI caused by all four microorganisms treble the risk of mortality for patients in the critical care unit. The fully adjusted hazard ratios for critical care deaths were 3.1 and 3.6 for sensitive and resistant microorganisms, respectively. We used the same method as for LOS to estimate an overall hazard ratio for critical care mortality of 3.25.
We found no evidence for any differences between mortality risks for patients with catheter-BSI during their hospital stay, outside the critical care unit, and therefore assumed there was no additional mortality risk during this period.
Estimation of costs
The costs included in the model were critical care unit and ward bed-day costs, catheter-BSI diagnostic and treatment costs and the cost of the CVC care bundle. The base year for the analysis was 2011; where necessary, costs were inflated to that year using the inflation indices from the Unit Costs of Health and Social Care. 175
Critical care unit and ward bed-days
Critical care unit and ward bed-day costs were taken from Healthcare Resource Group (HRG) costs. 176 The cost of a critical care bed-day was estimated based upon the cost for the number of organs supported (zero to six). We assumed that for our cohort of patients there would be the equivalent of an average of three organs supported (HRG code XC03Z), and we tested this assumption in the sensitivity analysis. The HRG bed-day cost for patients after they leave the critical care unit is dependent upon the reason for their critical care admission. We used the median cost for all non-elective inpatient (long-stay) excess bed-days reported.
Catheter-bloodstream infection diagnostic and treatment costs
Halton and colleagues151 estimated the consumable costs associated with catheter-BSI in Australia and these prices reflected the cost to Queensland Health decision-makers. These costs included the price of the catheters, catheter-BSI diagnosis (one catheter tip culture and two blood cultures) and catheter-BSI treatment. Treatment costs were a weighted average of the costs of standard regimens for causative organisms observed within the surveillance system: i.e. 2 weeks of vancomycin, 10 days of ticarcillin and clavulanate or 4 weeks of fluconazole. We converted these costs to UK pound sterling (£) and inflated them to our analysis base year (exchange rate £1 = A$1.477).
Central venous catheter care bundle cost
The cost of the CVC care bundle used in the model refers to the additional costs – above those of current clinical practice – of implementing the bundle. For the purposes of the analysis the costs of current clinical practice are assumed to be zero. The cost of the CVC care bundle consists of the national programme and local implementation costs. The programme grant for implementing Matching Michigan in England was £1,750,000 for a 2-year period. The programme grant costs covered the costs of the support for central training days and web-based data collection tools, but did not cover any payments to local health professionals. Thus, the annual cost would be £875,000 per annum. The local training costs were calculated based on clinical advice we received about the implementation of Matching Michigan in one local centre. In this centre, one Band 6 nurse trained and monitored ICU staff in three critical care units and this took 20% whole time equivalent. Using the assumptions above, the CVC care bundle costs were estimated as £15.48 per patient attending critical care (see Table 29) and these are shown in Appendix 10. The CLAB ICU project,83 which provides our estimate of clinical effectiveness, included 37 critical care units at a total cost of A$508,831. Assuming critical care patient admissions were similar to those in the UK, this would equate to an average cost per critical care patient of about £15, which is consistent with the cost used for the CVC care bundle in our analysis.
Estimation of health-related quality of life and long-term survival
The long-term survival of patients after discharge from the critical care unit was estimated based on England and Wales population mortality rates177 using a simple Markov model with states for alive and dead. Survival of patients after discharge from critical care is lower than for the general population as reported by Williams and Dobb. 178 We multiplied the general population mortality rates by the relative risks of mortality reported in their study:178 2.9 in the first year and 1.5 thereafter. Long-term QALYs were estimated using general population utility values stratified for age as derived in a previous Health Technology Assessment (HTA) report for statins169 (utility = 1.0604 – 0.0043x, where x is the person's age). We used the mean age of a critical care patient of 60 years, according to the ICNARC data. 164 The calculated mean discounted life expectancy for a typical patient aged 60 years from hospital discharge was 11.8 years, and the mean discounted QALY was 9.1.
Results of the modelling
This section reports the cost-effectiveness results for cohorts of 100 adult patients aged 60 years admitted to the critical care unit. The analysis evaluates a CVC care bundle compared with remaining with current practice, where current practice relates to the period before the introduction of the Matching Michigan programme. Results are presented for costs and QALYs for the CVC care bundle cohort and the current clinical practice cohort with undiscounted costs and health outcomes discounted at 3.5%.
The base-case results show that there are 0.79 fewer catheter-BSI in the CVC care bundle cohort than in the current clinical practice cohort (Table 30), a corresponding 0.3 fewer deaths during critical care, which leads to an increased survival of 3.55 years and 2.72 QALYs. The bundle dominates current practice, i.e. it is more effective and less costly. The cost savings are largely as a result of the savings from reduced LOS in the critical care unit. The incremental cost per QALY gained was –£573 (Table 31). The incremental cost per catheter-BSI averted was –£1976.
| Outcome | Current practice | CVC care bundle | Difference |
|---|---|---|---|
| Patients with catheter-BSI in critical care (per 100 adult critical care patients) | 1.31 | 0.53 | 0.79 |
| Total mortality, critical care unit (per 100 adult critical care patients) | 17.40 | 17.10 | 0.30 |
| Total survivors, hospital discharge (per 100 adult critical care patients) | 74.30 | 74.60 | 0.30 |
| Additional critical care LOS for catheter-BSI (days per 100 adult critical care patients) | 1.97 | 0.79 | –1.18 |
| Additional ward LOS for catheter-BSI (days per 100 adult critical care patients) | 6.74 | 2.70 | –4.04 |
| Discounted life-years | 879 | 883 | 3.55 |
| Discounted QALYs | 674 | 677 | 2.72 |
| Extra inpatient bed-day cost, £ | 4494 | 1798 | –2697 |
| Cost diagnosis + treatment catheter-BSI, £ | 681 | 272 | –408 |
| Intervention cost, £ | 0 | 1548 | 1548 |
| Total cost, £ | 5175 | 3618 | –1557 |
| Strategy | Cost, £ | Life-years | QALYs | ICER (£/QALY) |
|---|---|---|---|---|
| Current clinical practice | 5175 | 879.3 | 674.0 | – |
| CVC care bundle | 3618 | 882.8 | 676.7 | – |
| Difference | –1557 | 3.55 | 2.72 | –573 |
Sensitivity analysis
Deterministic sensitivity analysis
One-way deterministic sensitivity analyses were performed, in which model parameters were systematically and independently varied, using realistic minimum and maximum values. The sensitivity analyses investigated the effect of uncertainty around the model assumptions, structure and parameter values on the cost-effectiveness results to highlight the most influential parameters. The effects of uncertainty in multiple parameters were addressed using PSA (reported below). Where possible, the parameters were varied according to the ranges of their CIs, based on published estimates. Where these data were not available an alternative suitable range was chosen, based upon expert opinion. The same ranges were used in the deterministic analyses and PSA (see Appendix 9).
Tables 32–34 show the results of the deterministic sensitivity analyses for the incremental cost-effectiveness ratios (ICERs), incremental costs and incremental QALYs. As the ICERs are negative in the base case, some of the results appear counterintuitive owing to the nature of the cost-effectiveness ratio, and care is needed in their interpretation. As an example to illustrate the nature of a negative ICER, readers may consider two results from hypothetical cost-effectiveness analyses comparing two treatments: (1) a saving of £1000 and a gain of 0.5 QALYs (ICER = –£2000/QALY) and (2) the same saving and a gain of 1 QALY (ICER = –£1000/QALY). The negative ICERs would appear to suggest that (1) is more cost-effective than (2). However, (1) is actually less cost-effective than (2).
| Parameter | Baseline | Upper value | Lower value | Upper value ICER (£/QALY) | Lower value ICER (£/QALY) | Range (£) |
|---|---|---|---|---|---|---|
| Catheter-BSI incidence rate | 3.7 | 5 | 1.3 | –721 | 479 | 1200 |
| Additional critical care LOS for catheter-BSI | 1.5 | 2.5 | 0.001 | –990 | 53 | 1043 |
| CVC care bundle effectiveness | 0.4 | 0.67 | 0.22 | –107 | –704 | 597 |
| Additional ward LOS for catheter-BSI | 5.13 | 8.68 | 1.58 | –826 | –320 | 506 |
| Proportion of patients with CVC | 0.71 | 0.96 | 0.49 | –721 | –317 | 404 |
| Cost of CVC care bundle (per critical care patient), £ | 15.48 | 20.13 | 10.84 | –402 | –744 | 342 |
| RR for critical care mortality due to catheter-BSI | 3.25 | 3.6 | 2.7 | –496 | –758 | 262 |
| Critical care mortality, no catheter-BSI | 0.169 | 0.2028 | 0.1352 | –477 | –716 | 239 |
| Critical care unit bed-day cost, £ | 1440 | 1171 | 1657 | –456 | –667 | 212 |
| Ward bed-day cost, £ | 246 | 295 | 197 | –646 | –500 | 146 |
| Catheter-BSI diagnosis and treatment cost, £ | 518 | 622 | 415 | –603 | –543 | 60 |
| Parameter | Baseline | Upper value | Lower value | Upper value incremental cost, £ | Lower value incremental cost, £ | Range |
|---|---|---|---|---|---|---|
| Catheter-BSI incidence rate | 3.7 | 5 | 1.3 | –2648 | £457 | 3105 |
| Additional critical care LOS for catheter-BSI | 1.5 | 2.5 | 0.001 | –2692 | £144 | 2836 |
| CVC care bundle effectiveness | 0.4 | 0.67 | 0.22 | –160 | –2488 | 2329 |
| Proportion of patients with CVC | 0.71 | 0.96 | 0.49 | –2650 | –595 | 2056 |
| Additional ward LOS for catheter-BSI | 5.13 | 8.68 | 1.58 | –2245 | –869 | 1376 |
| Cost of CVC care bundle (per critical care patient), £ | 15.48 | 20.13 | 10.84 | –1092 | –2021 | 929 |
| Critical care bed-day cost, £ | 1440 | 1171 | 1657 | –1239 | –1814 | 575 |
| Ward bed-day cost, £ | 246 | 295 | 197 | –1756 | –1358 | 398 |
| Catheter-BSI diagnosis and treatment cost, £ | 518 | 622 | 415 | –1639 | –1475 | 163 |
| Parameter | Baseline | Upper value | Lower value | Upper value QALYs | Lower value QALYs | Range |
|---|---|---|---|---|---|---|
| Catheter-BSI incidence rate | 3.7 | 5 | 1.3 | 3.67 | 0.96 | 2.72 |
| CVC care bundle effectiveness | 0.4 | 0.67 | 0.22 | 1.50 | 3.53 | 2.04 |
| Proportion of patients with CVC | 0.71 | 0.96 | 0.49 | 3.68 | 1.88 | 1.80 |
| Critical care mortality, no catheter-BSI | 0.169 | 0.203 | 0.135 | 3.26 | 2.17 | 1.09 |
| RR for critical care mortality due to catheter-BSI | 3.25 | 3.6 | 2.7 | 3.67 | 0.96 | 2.72 |
The cost-effectiveness results are robust to changes in all parameters in the deterministic sensitivity analysis. The cost-effectiveness estimates for the CVC care bundle vary from –£990 to £479 per QALY gained for all analyses (see Table 32). With the exception of catheter-BSI incidence rate and additional critical care LOS for patients with catheter-BSI, the model results are cost saving for all parameter values, and the results are most sensitive to changes in these two parameters. For changes to the values of the catheter-BSI incidence rate and the additional critical care LOS for patients with catheter-BSI, the cost-effectiveness estimates vary between –£721 and £479 and between –£990 and £53, respectively.
The most influential parameters on the incremental cost are the catheter-BSI incidence rate, the additional critical care LOS for patients with catheter-BSI and the CVC care bundle effectiveness (see Table 33). The incremental cost varies between –£2692 and £457 for all analyses. The most influential parameters on the incremental QALYs are the catheter-BSI incidence rate and the CVC care bundle effectiveness (see Table 34). The incremental QALYs vary between 0.96 and 3.68 for all analyses.
Probabilistic sensitivity analyses
In the PSA, all parameters were sampled probabilistically from an appropriate distribution162 using similar ranges as used in the deterministic sensitivity analyses (see Appendix 9). The parameters sampled were: catheter-BSI incidence rate, critical care mortality, catheter-BSI mortality risk, catheter utilisation, critical care unit and ward bed-day costs, treatment costs for catheter-BSI, critical care and ward LOS and CVC care bundle effectiveness and cost.
One thousand simulations were run. The PSA results are presented in Table 35 and show similar results to the deterministic analyses (see Tables 32–34) with an ICER of –£488 per QALY gained. The variability of the results is explored in more detail in Table 36, by showing the IQRs for model outputs. The CVC care bundle cost varies between £1377 and £1702, and this is offset by the savings from the inpatient bed-day cost (–£3647 to –£1553). The scatterplots and histogram for cost and health outcomes for the PSA are shown in Figures 10 and 11. The majority (83%) of the results show that the bundle is cost saving compared with current clinical practice. The cost-effectiveness of the bundle was < £5000 per QALY gained for all simulation results.
| Strategy | Cost, £ | Life-years | QALYs | ICER (£/QALY) | ||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Current clinical practice | 4903 | 3351–6831 | 878.9 | 865–894 | 673.7 | 663–685 | – | – |
| CVC care bundle | 3489 | 2817–4462 | 882.7 | 868–897 | 676.6 | 666–688 | – | – |
| Difference | –1414 | –2582 to –293 | 3.78 | 2.43–4.58 | 2.90 | 1.83–3.51 | –488 | –827 to –131 |
| Outcome | Median | IQR |
|---|---|---|
| Patient with catheter-BSI in critical care (cases per 100 adult critical care patients) | 0.76 | 0.54–1.02 |
| Total mortality, critical care (cases per 100 adult critical care patients) | 0.28 | 0.20–0.39 |
| Additional critical care LOS for catheter-BSI (days per 100 adult critical care patients) | –0.93 | –1.60 to –0.49 |
| Additional ward LOS for catheter-BSI (days per 100 adult critical care patients) | –3.98 | –5.81 to –2.63 |
| Inpatient bed-day cost, £ | –2358 | –3647 to –1553 |
| Intervention cost, £ | 1542 | 1377–1702 |
FIGURE 10.
Histogram of ICERs for CVC care bundle compared with current clinical practice from the PSA simulation runs
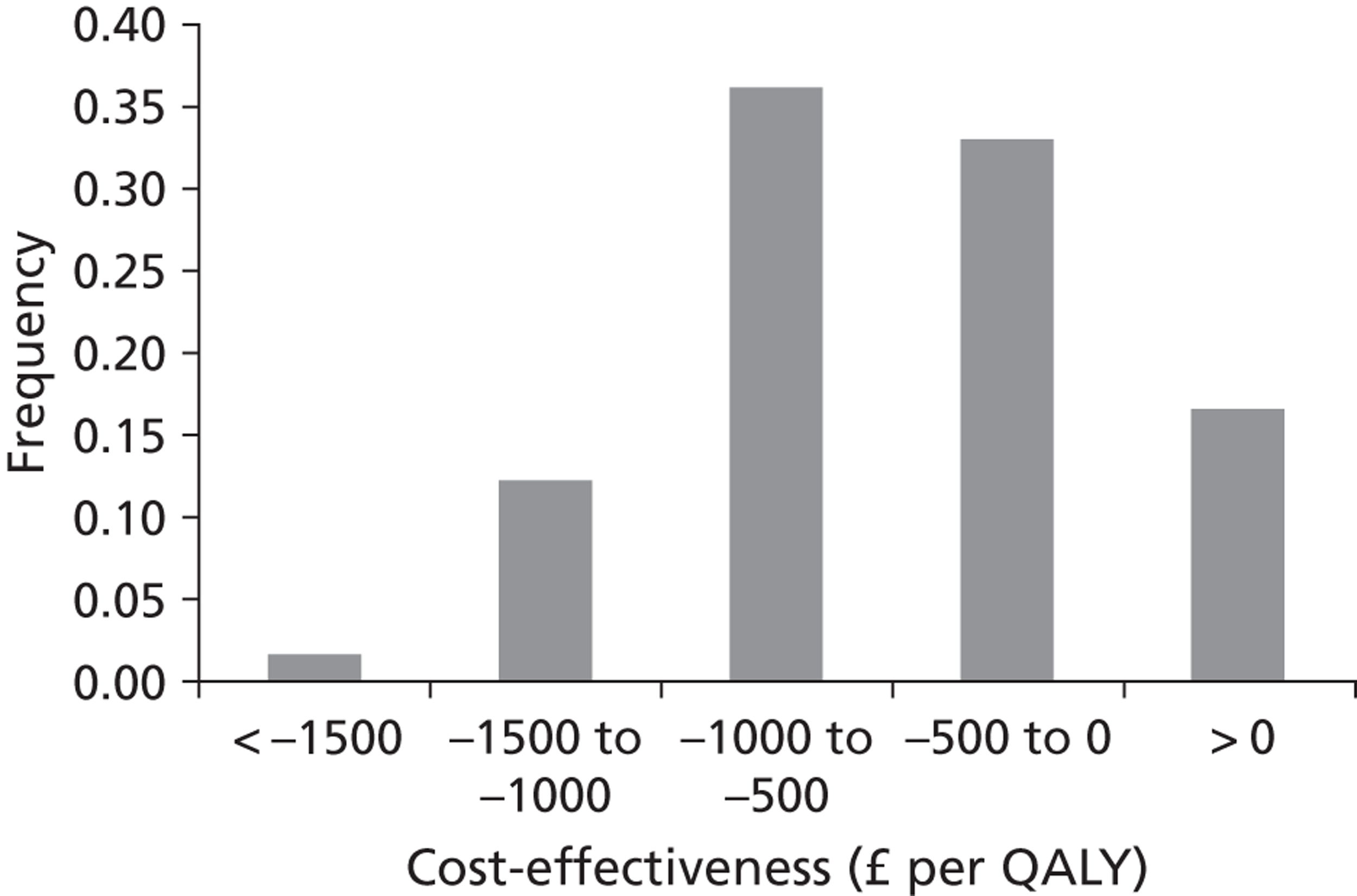
FIGURE 11.
Scatterplot for PSA
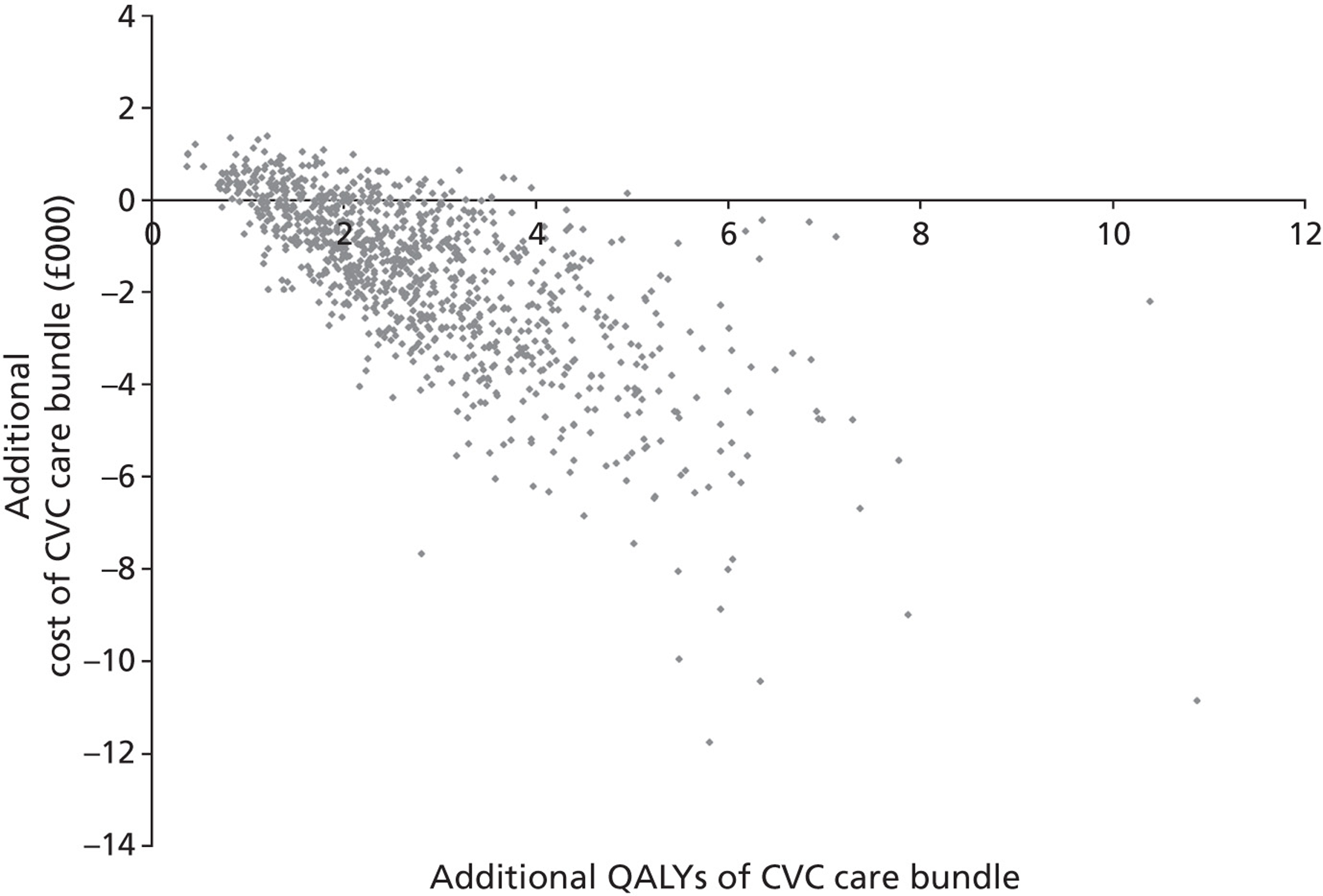
Scenario analyses
In addition to the sensitivity analyses, additional scenario analyses were undertaken to investigate the uncertainty in the model results for simultaneous changes to more than one parameter.
Effect of changing the age of patients in critical care
A scenario analysis was conducted for alternative mean starting ages for patients in the critical care unit, using the analysis described above (see the section ‘Estimation of HRQoL and long-term survival’). The calculated mean discounted life expectancy from hospital discharge for a typical patient of age 50 and 70 years was 15.8 and 7.6 years respectively and the corresponding mean discounted QALY was 12.6 and 5.6.
For a cohort of patients with mean age of 50 years, the cost-effectiveness is slightly improved at –£413 per QALY gained. At mean age of 70 years, the cost-effectiveness is slightly worse at –£926 per QALY gained.
Threshold analysis
Table 37 shows the results of a threshold analysis in which all parameters were varied until the CVC care bundle became more costly than the current clinical practice. For this scenario, the CVC care bundle would only be more expensive for any feasible changes to four of the parameters, and would remain cost saving for the other parameters. The results show that the bundle would no longer be cost saving if the bundle cost per patient was > £30, the bundle effectiveness incidence density RR was > 0.7, the catheter-BSI incidence density of < 1.8 per 1000 catheter-days, or the proportion of critical care patients with a CVC of < 0.35.
| Parameter | Value at which CVC care bundle becomes more expensive than current practice |
|---|---|
| Catheter-BSI incidence, per 1000 catheter-days | < 1.8 |
| Proportion of patients with CVC | < 0.35 |
| CVC care bundle cost, per patient, £ | > 30 |
| CVC care bundle effectiveness | > 0.7 |
Two-way sensitivity analysis comparing central venous catheter care bundle cost versus effectiveness
Figure 12 shows a two-way sensitivity analysis of the effect on the model results of simultaneously varying both bundle cost and bundle effectiveness. These results show that, even when using extreme values for the parameters, for example with a bundle effectiveness of 0.7 and bundle cost of £85 per critical care patient, the bundle (although no longer cost saving) remains cost-effective with an ICER of < £5000 per QALY gained.
FIGURE 12.
Two-way sensitivity analysis comparing CVC care bundle cost vs. effectiveness
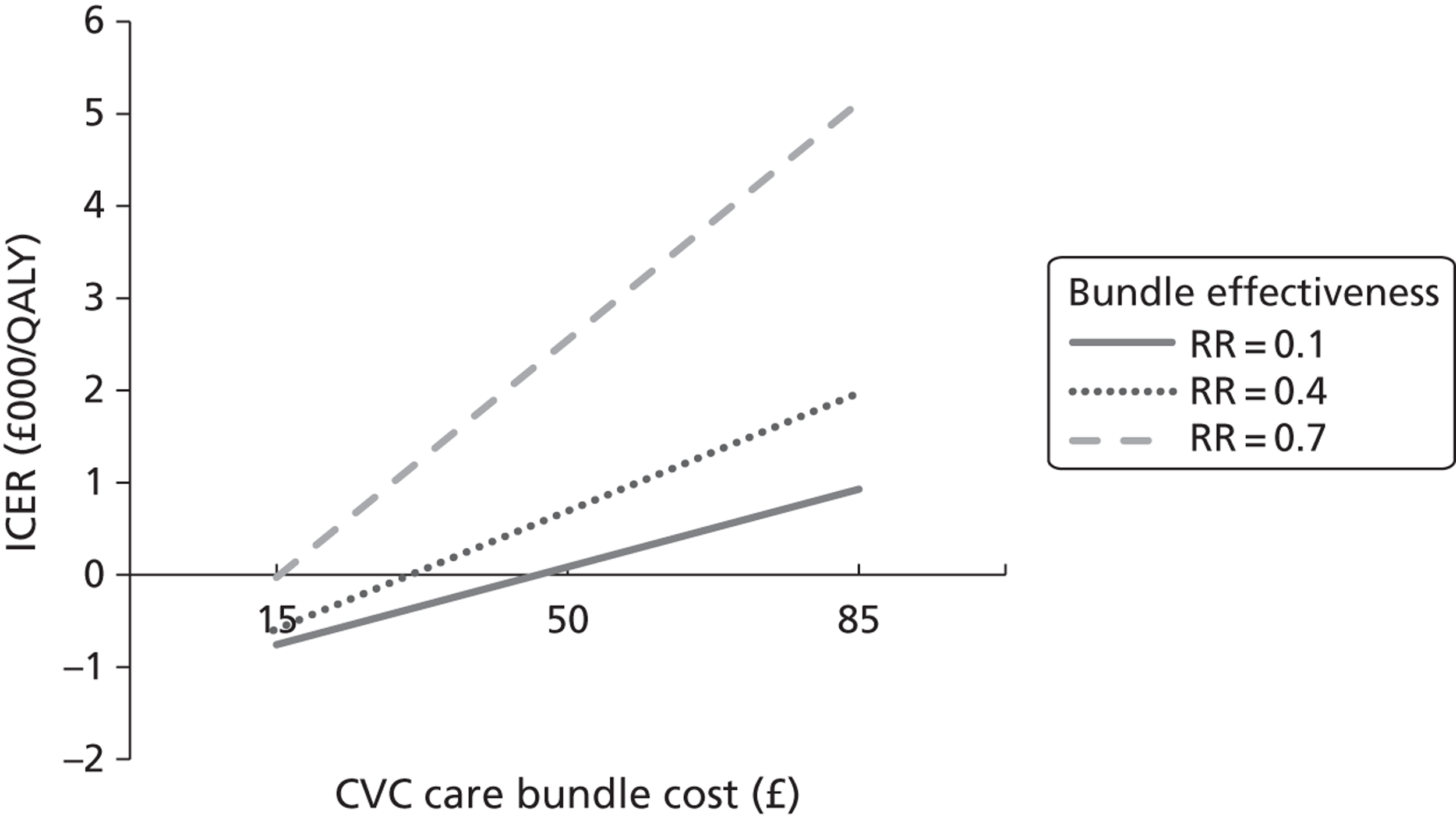
Two-way sensitivity analysis comparing catheter-bloodstream incidence versus additional critical care length of stay
Figure 13 shows a two-way sensitivity analysis of the effect of simultaneously changing both catheter-BSI incidence and additional critical care LOS for catheter-BSI on the model results. These results show that even when using extreme values for the parameters, for example with an incidence density of 1.0 catheter-BSI per 1000 catheter-days and no additional critical care LOS for catheter-BSI, the CVC care bundle is no longer cost-saving, but remains cost-effective with an ICER of < £2000 per QALY gained.
FIGURE 13.
Two-way sensitivity analysis comparing CRBSI incidence vs. critical care additional LOS
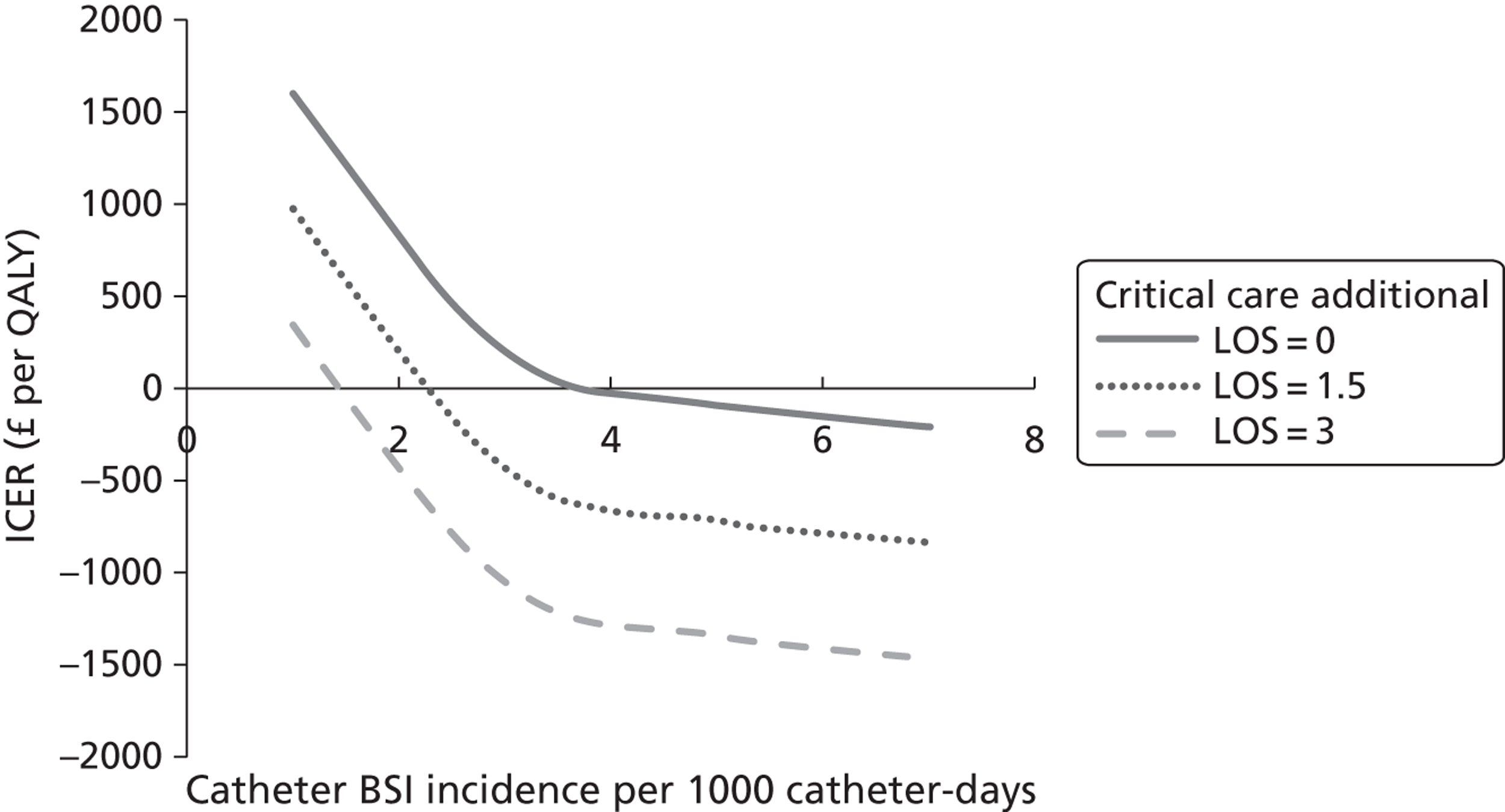
Two-way sensitivity analysis comparing catheter-bloodstream infection incidence versus bundle effectiveness
Figure 14 shows a two-way sensitivity analysis of the effect of simultaneously changing both catheter-BSI incidence and CVC bundle effectiveness on the model results. These results show that, even when using extreme values for the parameters, for example with a bundle effectiveness of 0.7 and incidence density of 1.0 catheter-BSI per 1000 catheter-days, the bundle, although no longer cost saving, remains cost-effective with an ICER of about £3000 per QALY gained.
FIGURE 14.
Two-way sensitivity analysis comparing catheter-BSI incidence vs. bundle effectiveness
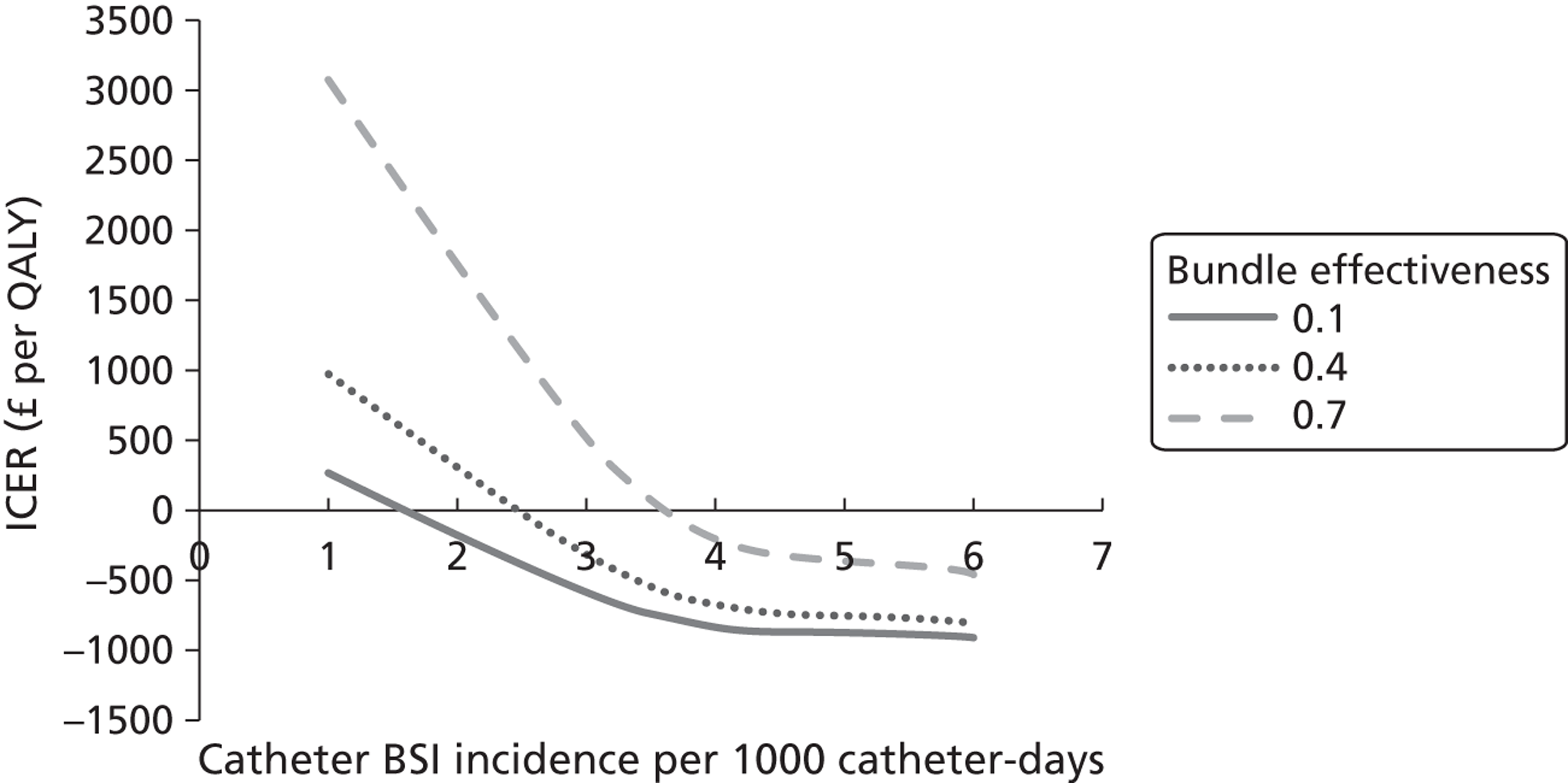
Estimating the cost of national implementation
The national costs and benefits of implementing the CVC care bundle were estimated using the model based on the total annual number of admissions to critical care units during 1 year (2009/10) (ICNARC). In that year, there were 96,810 admissions to 188 NHS adult general critical care units in England, Wales and Northern Ireland. Note that this may slightly underestimate the total number of admissions, as not all critical care units participated in the data collection. The results are shown in Table 38.
| Outcome | Current clinical practice | CVC care bundle | Difference | Difference, 25% percentile | Difference, 75% percentile |
|---|---|---|---|---|---|
| No. of patients | 90000 | 90000 | |||
| Health outcomes | |||||
| Catheter-BSI cases | 1182 | 473 | 709 | 482 | 914 |
| Total critical care mortality | 15,660 | 15,390 | 270 | 184 | 348 |
| Additional critical care LOS due to catheter-BSI, days | 1773 | 709 | –1064 | –1439 | –439 |
| Additional ward LOS due to catheter-BSI, days | 6064 | 2426 | –3639 | –5232 | –2365 |
| Discounted life-years | 879,665 | 882,856 | 3192 | 2183 | 4118 |
| Discounted QALYs | 674,267 | 676,713 | 2447 | 1673 | 3156 |
| Costs | |||||
| Inpatient bed-day cost, £ | 4,045,018 | 1,618,007 | –2,427,011 | –3,282,553 | –1,397,455 |
| Intervention cost, £ | 0 | 1,393,409 | 1,393,409 | 1,239,459 | 1,532,181 |
| Total cost (£) | 4,657,753 | 3,256,510 | –1,401,243 | –2,323,724 | –263,726 |
For England, with 90,000 critical care patients per year, the model estimates that implementing the CVC care bundle would reduce the number of catheter-BSI infections by > 700 (IQR 482–914) and save 270 (IQR 184–348) lives per year. The yearly additional cost to implement the intervention used in England would be £1.4M (IQR £1.2M–£1.5M). However, if the intervention was implemented, not only would the cost of implementation be recouped, but also there would be a net saving from implementing the intervention of £1.5M, largely as a result of the savings in costs related to reduced LOS (£2.4M, IQR £1.4M–£3.3M). The CVC care bundle remains cost saving up to an annual implementation cost of £2.7M (equivalent to £30 per critical care patient).
Summary of cost-effectiveness
We developed a decision-analytic model to assess the cost-effectiveness of a CVC care bundle compared with current clinical practice. The model followed cohorts of patients from their admission to critical care and estimated the associated costs, mortality and lifetime QALYs. The results from the model showed that the CVC care bundle saved 0.8 catheter-BSI and 0.3 lives per 100 patients admitted to critical care compared with current clinical practice, and led to an increased survival of 3.55 years and 2.72 QALYs. The CVC care bundle is more effective and less costly than current clinical practice, largely as a result of the saving from the reduced length of critical care stay.
The effects of a range of parameter values in the economic model were evaluated in sensitivity analyses. The model results were found to be robust to changes in the parameter values. The model results are most sensitive to changes in catheter-BSI incidence and the additional critical care LOS for catheter-BSI.
There is uncertainty in the model-based analysis relating to variation in implementation of the CVC care bundle. Some of the interventions included in the bundle (to be implemented on a regional or national basis) may already be partially implemented (by individual hospitals or ICUs). The effect of this would, all other things being equal, be to lower the baseline incidence of catheter-BSI (prior to regional or national implementation of the CVC care bundle). In addition, there is likely to be variation in the success or completeness of implementing the CVC care bundle which would be expected – all other things being equal – to result in a lower reduction in catheter-BSI following implementation. We conducted a two-way sensitivity analysis on these variables, which indicated that implementing the CVC care bundle was less cost-effective at lower catheter-BSI incidence density. However, even at the least favourable values tested (a relative risk of catheter-BSI with the CVC care bundle = 0.7 and baseline incidence density of 1.0 per 1000 catheter-days) the ICER remained below the threshold conventionally considered as cost-effective.
The PSA estimated the probability that the CVC care bundle is cost-effective at different willingness-to-pay thresholds. The majority (83%) of the results show that the CVC care bundle would be cost saving compared with current clinical practice.
Chapter 7 Discussion
Discussion of the systematic review of clinical effectiveness
A wide range of educational approaches has been used in interventions for preventing catheter-BSI in critical care units. Results of updated literature searches (discussed further below) suggest that this is an active area of primary research. Many of the interventions appear to have potential relevance to NHS practice, as they addressed clinical practices for infection prevention recommended in the ‘epic2’ national evidence-based guidance for NHS hospitals in England39 to varying degrees. The intervention that has attracted the most attention and publicity is the Keystone ICU project, which achieved a reduction in catheter-BSI incidence density in 103 critical care units in the USA during 3 years of implementation. 34,123,124 Owing to the apparent success of the Keystone ICU project, there have been several attempts to replicate it in other countries, including the Matching Michigan programme in England, the CLAB ICU project in Australia83 and the Bacteraemia Zero project in Spain. 68 Given that the Bacteraemia Zero project68 did not provide convincing evidence of effectiveness, and the Matching Michigan programme results had not been published, we considered the CLAB ICU project to be most relevant to NHS practice. As noted in Chapter 4, one of the interventions included in the systematic review was conducted in the UK (in Scotland) but this had limitations including delayed effectiveness at reducing catheter-BSI and overlap with a national patient safety programme, and the intervention appeared less relevant than the Australian CLAB ICU project to NHS practice.
Study designs
The only studies with concurrent control groups68,109,136 included in the systematic review did not demonstrate clinical effectiveness of their interventions. The entire evidence base for clinical effectiveness thus comes from single-cohort studies that would be classed as low quality according to the criteria for grading quality of evidence and strength of recommendations (GRADE). 181
A problem with these poor-quality studies is that they cannot usefully inform infection prevention guidelines such as ‘epic2’, which need to be based on reliable evidence. More rigorous study designs are needed to enable the determination of cause–effect relationships. In the Bacteraemia Zero project, for example, the intervention was no more effective than the control (i.e. no-intervention group), but within the intervention group of critical care units catheter-BSI incidence density was significantly lower during the intervention period than at baseline. This example illustrates how potentially misleading single-cohort studies can be for deducing effectiveness, as they do not control for secular trends. It is important that the study design employed is appropriate for the research question and capable of determining causality. Although not always feasible, RCTs may be appropriate for, and have been used in, evaluations of the effectiveness of educational interventions. 180 As there are often two or three levels of infrastructural organisation, i.e. critical care units grouped within hospitals, and hospitals grouped within regions, a cluster RCT design may be appropriate. Scales and colleagues181 provided an example of the utility of a cluster RCT approach for evaluating the effectiveness of a QI improvement programme on adoption of evidence-based practices in critical care units. An alternative approach to the use of concurrent control groups is to use an interrupted time series (ITS) design. An ITS design requires sufficiently fine resolution of temporal monitoring of outcomes (e.g. weekly or monthly) to enable detection of any changes in outcomes when an intervention is implemented, against any background secular trends. However, there is no clear consensus on how many temporal outcome assessments would be needed for a study to be classified as ITS. Only two88,115 of the 74 studies included in the evidence map (none of which was included in the systematic review) employed an ITS approach (a further study127 claimed to use an ITS approach but was reported as a single-cohort before-and-after study design).
Study reporting
Studies that have been conducted need to be more clearly and fully reported. Guidance on the reporting of observational studies of educational interventions is available from several sources, including the STROBE checklist (strengthening the reporting of observational studies in epidemiology),182 the TREND checklist (improving the reporting quality of nonrandomised evaluations in behavioural and public health interventions)55 and the ORION statement (reporting intervention studies of nosocomial infections). 57 Item 7 in the STROBE checklist encourages study authors to report all potential confounders and effect modifiers. This item should be particularly emphasised by peer reviewed journals as, in this review, a failure of authors to disclose this information was a major reason why many primary studies were judged to be at unclear risk of performance bias. Another key deficiency in the primary studies was the failure to report whether valid and reliable data collection methods had been used. A potential problem with these checklists and statements is that when reporting non-randomised educational interventions for preventing catheter-BSI it may not be obvious which checklist(s) the authors should use. Our assessment of study quality suggested non-compliance of authors with the checklists and statements is frequent.
Populations
The eligibility criteria for the systematic review developed in consultation with the project AG focused on adult critical care patients, who make up the majority of the critical care population and were identified in the evidence map as having the most primary evidence. Neonatal critical care units were not specified in the systematic review eligibility criteria as they are very different to those of adult specialties and experience higher rates of BSI-related mortality. 24 In practice it was not feasible for us to completely exclude children from the systematic review since some adult critical care specialties may include a small proportion of children, but the studies rarely reported this level of detail about their populations. Three of the regional-scale interventions34,83,136 were not conducted entirely in adult critical care units but we judged them to have met the population inclusion criterion for the systematic review because the proportion of paediatric critical units they included was small (in all cases < 9%), whereas a fourth regional-scale intervention did not explicitly state the population but we assumed it to be primarily adult. 68 If we had strictly limited the systematic review to entirely adult critical care patients then we would have excluded several studies of relevance to NHS practice, notably the Keystone ICU project,34 CLAB ICU project83 and Bacteraemia Zero project. 68
Primary outcomes
All 24 of the studies included in the systematic review reported the incidence of catheter-BSI but 10 studies did not report sufficient data for us to calculate the incidence density RR and its 95% CI, and we therefore had to contact the study authors for this information. The incidence density expressed per 1000 catheter-days is a common metric used to compare infection prevalence, but studies reporting catheter-BSI incidence density typically follow the NNIS and CDC approach,22 which does not count separately any individual catheters present in the same patient during a 24-hour period. A more precise definition employed by HELICS174 defines a catheter day as a patient having a single catheter for a whole or part 24-hour period, whereas two catheters for a part or whole 24-hour period would be defined as two device-days. Only two of the studies included in the systematic review94,110 used both definitions of catheter-days, enabling comparisons. The limited data available from these two studies suggest that the definition of catheter-days did not have an appreciable impact on incidence density RRs.
The temporal and spatial resolution of outcome assessments varied considerably among the studies, illustrating why standardisation to catheter-days is necessary to enable comparisons. Most studies reported incidence density RRs for the cumulative number of catheter-BSI and catheter-days registered during a single intervention period compared with a single baseline period. Where additional finer-resolution (e.g. monthly) data were also provided,50,51,103,109,122,139 (data in Appendix 5) these illustrated considerable short-term temporal variability in catheter-BSI incidence density that was not reflected in overall estimates for intervention and baseline periods. Studies which included multiple critical care units usually provided pooled estimates of catheter-BSI incidence density for all the units combined. Where catheter-BSI incidence density was also reported separately for different critical care units103,122,144 considerable spatial variability in catheter-BSI incidence density was evident, which would be obscured when pooling incidence densities across units. We recommend that primary studies should always provide estimates of the temporal and spatial variability of their data when reporting outcomes.
Relatively limited data were provided about mortality and LOS in the primary studies. It would be helpful in future studies for records to be kept of the mortality and LOS for critical care patients with and without catheter-BSI, to clarify the burden of catheter-BSI in settings relevant to the NHS.
Secondary outcomes and process evaluations
The Medical Research Council (MRC) guidance on developing and evaluating complex interventions183 stresses the importance of conducting process evaluations to help in understanding the reasons why complex interventions may or may not be effective. Assessments of attitudes, knowledge, compliance and other aspects of intervention processes identified several barriers or potential barriers to the successful implementation of the educational interventions included in the systematic review. Staff attitudes towards the need for evidence-based infection-prevention practices appear to be a barrier, as several studies reported staff resistance to the use of sterile drapes, gloves, masks and hats, with some staff questioning evidence-based guidance. In the CQI programmes a notable issue was a lack of existing systems and infrastructure for data collection. This seems to be an important difference between the Keystone ICU project, which was based on an existing data collection system, and the other CQI programmes that appeared to have had to develop data collection systems for their interventions. These observations of process improve understanding of potential facilitators and barriers to the successful implementation of educational interventions for preventing catheter-BSI but they were primarily based on ad hoc observations reported by the study authors, and it may be that other important facilitators or barriers were identified but not reported.
Educational approaches
Although many of the interventions included in the systematic review addressed similar sets of clinical practices, these were often addressed using very different, and often unclear, educational approaches. In many cases educational interventions were described superficially and it was difficult to tell whether all of the educational approaches used had been fully reported. Ideally, educational interventions should be reported in sufficient detail, and the resource requirements needed to implement the education elucidated, so they could be replicated. The information needed would include, for example, the total staff time involved and the cost of all materials, preferably itemised to allow interpretation of how costs might vary if intervention concentration (i.e. frequency and duration of sessions) were varied. The types of education should be clearly reported, as formal educational approaches that take staff away from bedside patient care will have associated opportunity costs. An example of good reporting of the education frequency/duration and staffing requirements was provided by Zingg and colleagues. 144 Educational interventions tend to evolve once they are implemented,149,184 so it is important that any differences between the intended and actual implementation are reported. Where possible, primary outcomes research studies should include an integral cost-effectiveness evaluation.
Instead of each intervention using its own unvalidated educational approach, as was the case in the studies we reviewed, there is a good case to be made for educational approaches to be developed in a more co-ordinated way so that they integrally support the national guidance on care bundles and can be deployed in a more consistent and similar manner. The educational interventions which we reviewed do not draw upon pedagogical, theoretical or conceptual frameworks that would enable understanding of why a particular approach works, or does not work. Consequently there are no generalisable lessons to inform the ‘epic2’ guidelines on which types of educational approach should be used in support of infection prevention practices. Unless some action is taken to effect a change, it seems likely that the current primary research activity in this area will continue to develop further ad hoc and unvalidated educational approaches. A necessary first step to address this issue would be to determine a responsible organisation for considering the harmonisation of educational approaches (e.g. an evidence-based-practice guidelines development group). Involvement of educationalists in the design of interventions would help to ensure that the interventions are well supported by relevant theory and are reliable and generalisable.
Information feedback approaches
Infection surveillance feedback, in which results of infection surveillance are provided to health-care workers to inform their clinical practices, is thought to be an effective strategy for reducing nosocomial infection incidence. 185 Our clinical effectiveness findings suggested that infection surveillance feedback and/or performance feedback were not essential for effecting reductions in catheter-BSI incidence density. However, the feedback approaches varied considerably among the primary studies, with some studies providing active feedback (e.g. at meetings), whereas others provided passive feedback (e.g. in wall charts) and none of the studies clearly reported the data collection methods used or whether they were valid and reliable. It may be that the interventions included in our systematic review were too heterogeneous in their intervention characteristics for any underlying influences of feedback approaches on catheter-BSI to have been detectable. For some studies, it became apparent only when the publications were scrutinised in detail that infection surveillance feedback was already practised in the pre-intervention period, and would therefore not explain any observed changes in catheter-BSI incidence when interventions were implemented.
Definitions of catheter-bloodstream infection
During the preparation of this report we identified citations to more than 15 different published sources of CDC definitions in support of CABSI or CRBSI. The most frequently cited publications containing CDC definitions were by Garner and colleagues,147 NNIS186 and Horan and colleagues. 187 However, although two of these references define LCBSI,147,187 none of them actually defines CABSI or CRBSI. A systematic review on 191 studies of patients with cancer published by Tomlinson and colleagues in 201121 found that CABSI and CRBSI are defined inconsistently in primary research studies. Our findings (see Chapter 4) provide further evidence that CABSI and CRBSI are inconsistently defined in primary research. For the NHS, the definitions of CABSI and CRBSI that were used during Matching Michigan (see Table 1) appear appropriate and could be formally recommended for wider use.
Influence of updated literature searches on the clinical effectiveness results
Bibliographic searches, which were rerun in March 2012 using the original search strategy, identified 933 potentially relevant new titles and abstracts published between February 2011 and March 2012, of which 19 full-text publications met the criteria for inclusion in the evidence map (see Appendix 6, Clinical effectiveness full-text records identified in search updates) and 12 potentially relevant new conference abstracts were identified (see Appendix 6, Clinical effectiveness conference abstracts identified in search updates).
Of the 19 new full-text publications, 18 reported 18 new primary research studies and one publication188 reported on an existing study already in the evidence map. 83 The new studies published during February 2011 to March 2012 would increase the size of the evidence map from 74 studies to 92 studies (i.e. a 24% increase), indicating that the evaluation of educational interventions for preventing catheter-BSI is an active area of research. This appears to be particularly the case for paediatric and neonatal critical care (60% increase and 36% increase, respectively).
Among the 18 new studies identified that would be eligible for inclusion in the evidence map, three studies would appear to also meet the inclusion criteria for the systematic review, although this was not assessed formally. Render and colleagues189 evaluated a regional-scale CQI programme conducted in 174 critical care units in 123 hospitals in the USA, which was based on a centralised infrastructure to support the co-ordinated implementation of care bundles containing infection prevention learning modules and five evidence-based practices (hand hygiene, maximal barrier precautions, skin antisepsis, avoidance of femoral insertions, removal of unnecessary CVCs). Kim and colleagues190 evaluated a catheter care bundle in six critical care units in a hospital in the USA, including checklist, supplies cart, catheter need review, avoidance of the femoral site, staff empowerment to halt incorrect procedures, staff education, and infection surveillance and performance feedback. Seddon and colleagues191 evaluated a CQI programme in a single critical care unit in New Zealand based on a care bundle, insertion and maintenance checklists and performance feedback but we noted that this intervention coincided with a major expansion of the critical care unit, which could be a confounding factor.
These three newly published studies all used before-and-after designs and claimed statistically significant reductions in the incidence density of catheter-BSI, although we did not check their calculations or methodological quality. The Render study189 is notable in being the largest study we have come across in terms of the number of critical care units involved (174 units were included). The Seddon study191 is notable in being the only study conducted in New Zealand. If these studies were, upon more rigorous scrutiny, shown to be clinically effective at preventing catheter-BSI then their findings would be broadly consistent with those of our systematic review which demonstrated both local-scale and regional-scale CQI programmes including CVC care bundles appear effective at preventing catheter-BSI. However, as with all the before-and-after studies, a key assumption is that the observed effects were a result of the planned interventions.
Searches of databases of ongoing research did not identify any other relevant primary research studies taking place up to May 2012.
In summary, the updated searches indicate that educational interventions for preventing catheter-BSI is an active area of research, but did not identify any new evidence that would appear to change the conclusions of our systematic review.
Findings from other systematic reviews
Systematic reviews of interventions for preventing health care-associated infections identified before this project commenced are summarised in Chapter 1. The most directly relevant and recent of these were published in 2008 by Safdar and Abad37 and in 2010 by Cherry and colleagues. 49
Safdar and Abad37 conducted a systematic review of educational interventions for preventing health-care associated infections, not limited to catheter-BSI or critical care. Of 26 primary studies they included, 10 were also included in our evidence map. The main conclusion from their systematic review, which was limited to English-language publications, was that implementation of educational interventions may reduce health care-associated infections considerably but the before-and-after design of studies precluded drawing firm conclusions. Safdar and Abad37 recommended cluster randomised trials using validated educational interventions and costing methods should be developed to determine the independent effect of education on reducing healthcare-associated infections and the cost-savings that may be realised with this approach.
Cherry and colleagues49 aimed to determine individual features of educational interventions that impact on competence in aseptic insertion technique and maintenance of CVCs by health-care workers. Their review was not limited to catheter-BSI or critical care and it is unclear whether they applied publication language restrictions. Of 47 primary studies included, 28 were also included in our evidence map. Their main conclusions were that educational interventions appear to have the most profound and prolonged effect when used in conjunction with audit, feedback, and availability of new clinical supplies consistent with the content of the education provided, and if baseline compliance to best practice is low. Cherry and colleagues49 did not include informal learning and they did not explicitly report data to support their conclusions concerning audit and feedback.
Our searches did not find any more recent relevant systematic reviews than those by Safdar and Abad37 and Cherry and colleagues. 49 These reviews are now relatively old, with searches conducted up to November 2006 and August 2008, respectively. Our current evidence synthesis is more rigorous than these preceding systematic reviews in that we were not limited to English-language publications and we formally assessed study quality using risk of bias criteria. In contrast, Cherry and colleagues49 used a composite quality score expressed as a percentage which is difficult to interpret. The quality score focused on the quality of education rather than other aspects of methodological quality and some studies that were also included in our systematic review50,108 received a maximum quality score (100%) from Cherry and colleagues49 despite having methodological limitations (see Chapter 4).
Discussion of the economic evaluation
Our systematic review of cost-effectiveness studies identified only three economic evaluations of educational interventions to prevent CRBSIs. One of the studies did not include the cost associated with the care bundle in the analysis; another study used a trial-based cohort analysis to derive estimates of the costs and benefits associated with a simulation-based education intervention in a hospital in the USA, whereas the third study did not consider long-term health benefits beyond the hospital stay. It was not possible to conclude from any study what the likely cost-effectiveness of the intervention would be in a UK setting. Literature searches updated in March 2012 did not identify any further relevant studies.
The model developed in this study allows us to estimate the cost-effectiveness of a CVC care bundle versus current clinical practice to prevent catheter-BSI. The CVC care bundle was found to be more effective and cheaper than current practice, i.e. a dominant strategy. The model results were robust to changes in the model parameters. The model results were most sensitive to the catheter-BSI incidence rate and the additional length of critical care stay associated with catheter-BSI. The PSA showed that the probability that the CVC care bundle dominated current practice was 0.85.
For the purposes of the model, we assumed that CABSI and CRBSI were synonymous and were collectively referred to as catheter-BSI. Although in theory CABSI overestimates the true incidence of CRBSI, our review of clinical effectiveness and another systematic review21 found that the definitions are used interchangeably and inconsistently in primary research studies.
Experience from a clinical member of the review team (TC) working in English health-care trusts suggested that implementation of evidence-based practices would vary between hospitals, and may even vary between critical care units within the same hospital. There is uncertainty about the uptake of evidence-based practices before Matching Michigan was introduced, although many English health-care trusts would likely have followed ‘epic2’ guidelines39 and the Department of Health ‘High Impact Intervention’28 for best practice in prevention of catheter-BSI. It is possible that current clinical practice in some critical care units already incorporated some elements of the CVC care bundle. This variation was not reported in primary studies and cannot be directly quantified. However, there is likely to have been similar variability in implementing evidence-based practices among the critical care units in the CLAB ICU study by Burrell and colleagues83 that we have chosen for providing an estimate of the clinical effectiveness of the CVC care bundle.
Much of the primary evidence relating to educational interventions for preventing catheter-BSI is from studies conducted in the USA, whilst the study used for the clinical effectiveness estimate for the economic model was conducted in Australia. Critical care in the USA is believed to be significantly different from practice in the UK with regard to bed number, staffing, resource and case mix. 192 The UK has the lowest number of critical care beds per capita in the developed world whereas the USA has the highest. Differences also exist for general health care and critical care expenditure. 192,193 Australian critical care is believed to lie somewhere between these two outlier nations.
Despite being populated with some data from outside the UK, there are two aspects of the model that provide reassurance that the model outputs are relevant to critical care units in English NHS trusts. First, the clinical effectiveness estimate from the Australian CLAB ICU study is similar to clinical effectiveness estimates from other primary studies conducted in different countries, suggesting that clinical effectiveness may not be strongly dependent on the geographical variations in critical care infrastructure and case mix noted above. Second, as the model outputs are robust to sensitivity analyses it seems likely that variation that would result from geographical differences in critical care delivery approaches will already have been captured.
As the model was restricted to adult critical care, it is unclear whether the current results are generalisable to paediatric critical care or especially to neonatal critical care which has different risk factors for and prognosis of catheter-BSI. In principle, the model structure is likely to also be applicable to neonatal and paediatric populations and could be rerun using parameter estimates from studies conducted specifically on these populations. A question relevant to the primary evidence is whether effective educational interventions for preventing catheter-BSI in paediatric and neonatal populations are any different to those used in adult populations. Results of updated clinical effectiveness literature searches (described above) suggest that an increasing proportion of the primary research is being conducted in paediatric and neonatal critical care units and that at least 16 studies in paediatric and 15 studies in neonatal critical care units have been published that could be assessed.
In the NHS, although national patient safety initiatives are intended to be implemented throughout England, variations in infrastructure and resource availability (e.g. staffing) mean that in practice local interventions are often implemented in a small scale way, frequently without good baseline data collection. Many of the different educational interventions reported in Chapter 4 may therefore reflect actual practice. The model results indicate that a range of educational intervention types for preventing catheter-BSI could be cost-saving, meaning that the NHS could consider implementing smaller-scale interventions that appear effective, as listed in Boxes 3 and 5 in Chapter 4.
At present there is no information available on how the individual components of a care bundle contribute to the overall cost-effectiveness of the bundle. An assumption is made that all components are necessary for clinical effectiveness and, hence, cost-effectiveness, and care bundles are intended to be implemented on an all-or-nothing basis. 33
Strengths, limitations and uncertainties of this report
Systematic review of clinical effectiveness
One of the strengths of this review is its adherence to rigorous systematic review methods. We conducted exhaustive searches that were not limited to English-language publications, applied explicit inclusion criteria to the search results, critically appraised the included studies, and used transparent methods to synthesise study findings. A further strength is our inclusion of an initial descriptive mapping stage followed by a systematic review of a subset of studies. This process facilitated the involvement of clinical experts in the design of the review. The review team included clinical experts in infection prevention, critical care and medical education and was supported by an AG that included clinical experts directly involved in implementing NHS policies for the prevention of catheter-BSI.
Despite its strengths the review had limitations. It was preferable for the subset of the studies in the systematic review to be homogeneous in terms of the intervention characteristics to ensure that their aggregation statistically would be meaningful and appropriate (i.e. comparing like with like) but, in accordance with the systematic review eligibility criteria developed in consultation with clinical experts, a wide variety of intervention types was included. Although this diversity of interventional approaches does appear relevant to NHS practice, it was not appropriate to pool outcomes for different interventions in a quantitative meta-analysis and so we instead conducted a narrative synthesis. The systematic review was restricted to studies that defined catheter-BSI so that we could investigate any impact of differing infection definitions on study outcomes. However, upon scrutiny of the studies we found that definitions of CABSI and CRBSI were applied inconsistently and generally did not agree with the definitions used in NHS practice. Therefore, we could not explore the potential influence of different infection definitions on effectiveness outcomes.
There are some uncertainties in our systematic review of clinical effectiveness. All studies that appeared to demonstrate clinical effectiveness of their educational interventions were single-cohort studies without concurrent control groups. The extent to which secular trends might have played a part in determining the observed changes in catheter-BSI incidence densities is unclear. The systematic review focused on adult critical care specialties that represent the majority of critical care specialties in the NHS, but it is not known whether similar findings would have been obtained for studies in paediatric and neonatal critical care units (it was not feasible to conduct a systematic review on all specialties in this project owing to the review team resources that would be required). Other uncertainties concerned poor reporting in the primary study publications: intervention details, especially the concentration of education and resources needed to implement education were often unclear or not reported, and nearly all of the studies were judged to be at unclear risk of bias owing to inadequate reporting of key methodological information.
Although it would have been preferable to have included the Matching Michigan programme in our evidence map and systematic review of clinical effectiveness (subject to meeting the inclusion criteria), this was not feasible as results from Matching Michigan were not available at the time of our analyses. However, where possible we have used interim information from Matching Michigan to inform our economic evaluation.
Economic evaluation
Our economic evaluation is the only published example of an assessment of the cost-effectiveness of educational interventions for prevention of catheter-BSI in the UK. The model specifically addresses clinical practices in critical care units in NHS trusts in England but the results are probably generalisable to the wider UK. The economic evaluation was informed by systematic reviews of effectiveness and cost-effectiveness, and systematic searches for input parameter data. The model was developed following a structured and objective process in accordance with standard NICE practice, and the model structure and data inputs are clearly presented in this report to facilitate replication and testing of our model assumptions.
Despite these strengths, the economic evaluation has some limitations. Owing to lack of data, we had to assume that mortality rates during the hospital stay following critical care discharge, and after hospital discharge, do not differ between patients who have catheter-BSI in the critical care unit and those who do not. We also had to assume that CABSI and CRBSI are synonymous, as the systematic review of clinical effectiveness had indicated that these definitions were not meaningfully separable in the primary research literature. The model does not consider the HRQoL of patients in critical care units because the time spent in critical care is very small compared with the lifetime horizon, and therefore any QALY gains during this period are insignificant. In addition, non-fatal adverse events associated with catheter-BSI were included within the model as a cost but not as a utility decrement. The economic evaluation does not include non-tangible benefits or disbenefits associated with the intervention, such as changes to staff morale and public confidence in the health-care system.
There are uncertainties around the cost of the CVC care bundle intervention. The implementation of the intervention is likely to vary widely in practice between critical care units. Different critical care units are also likely to vary in the extent to which they would have already implemented components of the CVC care bundle at baseline. Some model input parameters could not be sourced from the UK and clinical effectiveness estimates were obtained from a relevant Australian study. Where possible we conducted sensitivity analyses to illustrate the influence of these uncertainties on the cost-effectiveness estimates produced by the model.
Chapter 8 Conclusions
The evaluation of educational interventions for preventing catheter-BSI is an active area of primary research, but there are concerns about the reliability of the evidence, which comes predominantly from uncontrolled before-and-after studies and may not convincingly distinguish intervention effectiveness from background secular trends. Very limited primary research has been conducted in the UK and at the time this report was prepared none had been published from England.
Our economic evaluation suggests that an educational intervention based on a CVC care bundle implemented in critical care units in England would be more effective and less costly than current clinical practice, even after allowing for heterogeneity of baseline clinical practices and heterogeneity of implementation. The model results are robust to variations in cost of the CVC care bundle and clinical effectiveness, indicating that a variety of other educational interventions could potentially be cost-saving if implemented at local or regional scales. More robust primary studies of clinical effectiveness are needed, however, to clarify cause and effect to ensure that model input parameters for clinical effectiveness truly reflect intervention impacts rather than secular trends.
There is general agreement about the types of evidence-based practices that should be employed for preventing catheter-BSI, as reflected in the UK ‘epic2’ guidelines (and also the US IHI guidelines). These guidelines emphasise the need for education to support the evidence-based clinical practices for infection prevention but cannot make recommendations about which educational practices are appropriate as the primary research studies are of insufficient quality to be informative. Primary study investigators should be informed of the limitations of uncontrolled cohort studies and encouraged to use more robust methods that can identify causality. These could include RCTs and ITS designs.
The same core sets of clinical practices for infection prevention in relation to CVC insertion and ongoing care are currently being addressed by a wide variety of different educational strategies. The educational approaches do not draw upon pedagogic, theoretical or conceptual frameworks and, consequently, do not provide generalisable lessons to inform the ‘epic2’ (or IHI) guidelines. Harmonisation of education, using validated approaches (that could link to national curricula) would improve the relevance of primary research for informing national guidelines, and improve the comparability and analysis of the primary research studies. It seems appropriate that the evaluation of whether and how to standardise educational approaches could be considered by a relevant evidence-based-practice guideline development group.
Despite the existence of several checklists and statements relevant to the reporting of educational interventions for infection prevention (e.g. STROBE, TREND, ORION), the standard of reporting primary studies appears very poor. Consideration should be given to why these statements/checklists may not have been effective at improving reporting standards, and whether the compliance of study investigators with the reporting standards could be improved.
Recommendations for practice
NHS organisations should carefully consider whether existing practice for preventing catheter-BSI may be improved by implementing educational interventions in critical care units either at local or regional scales. Although it is not possible to be specific about which type of intervention may be most appropriate, economic evaluation suggests that a variety of approaches could be cost-effective or cost-saving. As potential cost savings could be achieved, implementation of educational interventions may be compatible with organisational cost reduction plans.
Consideration should be given to the need to adopt standard definitions of CABSI and CRBSI, and apply and report these consistently. Definitions used by the NHS during the Matching Michigan programme (and presented in Table 1 of this report) may be appropriate.
When clinical practice is delivered within a research setting, for example if interventions are intended to be implemented into practice while their effectiveness is monitored, consideration should be given to ensuring that the research design is appropriate for cause–effect relationships to be determined (as discussed in this report). To assist future economic evaluations, the resources required to implement and sustain an intervention should be clearly reported.
Coordinated collection of surveillance data on catheter-BSI, mortality and LOS in critical care units would be helpful to inform future economic evaluations, particularly to assist in establishing the extent to which infection with catheter-BSI influences these outcomes.
Recommendations for research
To ensure that future primary studies of the effectiveness of educational interventions for prevention of catheter-BSI provide useful evidence to inform national guidelines, researchers should give careful consideration to the choice of study design. Uncontrolled single-cohort study designs are unlikely to provide information of sufficient quality to inform national guidelines, unless a detailed ITS analysis can be undertaken to exclude any influences of secular trends on study outcomes.
It is important that the study design is appropriate for the research question and can identify causality. Where feasible, the preferred research design may be a RCT.
When developing educational interventions for prevention of catheter-BSI, consideration should be given to the MRC guidance on the development and evaluation of complex interventions. To ensure generalisability, educational interventions should be supported by educational and behavioural theory, and educationalists should be involved in their design. Outcome evaluations should be accompanied by process evaluations and have an integral cost-effectiveness evaluation.
Development of educational interventions for preventing catheter-BSI (and other infections) is likely to benefit from being co-ordinated at a national level, to ensure that valid and reliable approaches are employed that yield findings which are generalisable and inform national guidelines.
Research commissioners, journal editors and other relevant research stakeholders should encourage researchers to clearly report research studies of educational interventions to provide greater confidence about the validity and generalisability of the results and to fully identify the risks of bias and confounding. Consideration should be given to whether current reporting guidelines (e.g. the STROBE, TREND and ORION checklists/statements) are being adequately followed, and whether steps could be taken to improve authors' compliance with recommended reporting standards.
Updates to this review may help to clarify the extent of the growing evidence base and to ensure that the quality controls recommended above, if implemented, are effective. Given that there is current research interest in the effectiveness of educational interventions for preventing catheter-BSI in paediatric and neonatal populations, a more detailed review of the evidence for these populations would be appropriate.
Acknowledgements
We thank the AG for their input throughout the course of the project and for commenting on a draft of this report.
Members of the AG were:
Julian Bion, Professor and Dean, Faculty of Intensive Care Medicine, University of Birmingham; clinical lead for Matching Michigan in England.
Andrew Jackson, Consultant Nurse IV Therapy & Care; Infection Prevention Society (IPS) representative.
Annette Richardson, Nurse Consultant Critical Care, The Newcastle upon Tyne Hospitals NHS Foundation Trust; British Association of Critical Care Nurses (BACCN) National Board.
Trudie Roberts, Professor and Director, Leeds Institute of Medical Education; Association for the Study of Medical Education (ASME) representative.
Katie Scales, Consultant Nurse in Critical care; Member of the Steering Committee of the RCN IV forum; National Infusion and Vascular Access Society (NIVAS) representative.
Barry Williams, Chair of CritPal (Critical Care Patient Liaison Committee of the Intensive Care Society); member of Critical Care Stakeholders' Forum and Intercollegiate Board for Training in Intensive Care Medicine.
Duncan Wyncoll, Consultant Intensivist, St Thomas Hospital, London, Intensive Care Society (ICS) representative.
We also thank the following people:
Jackie Bryant, at Southampton Health Technology Assessments Centre (SHTAC), for internal editing of a draft version of this report.
Olatunde Aremu, formerly at SHTAC, for contributing to writing the protocol, screening studies for inclusion in the descriptive map, applying mapping keywords and assisting with the translation of non-English-language research publications.
Diana Mendes and Jo Picot, at SHTAC, for assisting with the translation of non-English-language research publications.
Hanne Bruhn and Yvonne Hopf, at the Centre for Primary Care, University of Aberdeen, for assisting with the translation of non-English-language research publications.
Angela Vincent, at SHTAC, for assistance with project administration.
Contributions of authors
Geoff K Frampton (Research Fellow, SHTAC) was the Principal Investigator and chaired the project meetings and AG. He scoped the research question, prepared the draft protocol and contributed to the writing of the protocol; screening studies for inclusion in the descriptive map; piloting and applying mapping keywords; extracting data from studies included in the systematic review of clinical effectiveness; assessing the quality of the included studies; conducting the analysis of the descriptive map; conducting the synthesis of outcome data in the systematic review of effectiveness; and writing the final report.
Petra Harris (Research Fellow, SHTAC) contributed to discussions at project meetings; writing of the protocol; screening studies for inclusion in the descriptive map; piloting and applying mapping keywords; extracting data from studies included in the systematic review of clinical effectiveness; assessing the quality of the included studies; conducting the analysis of the descriptive map; conducting the synthesis of outcome data in the systematic review of effectiveness; and writing the final report.
Keith Cooper (Senior Research Fellow, Health Economics, SHTAC) contributed to discussions at project meetings; piloting and applying mapping keywords; screening studies for inclusion in the systematic review of cost-effectiveness; extracting data from studies included in the systematic review of cost-effectiveness; assessing the quality of the included studies; developing the economic model and conducting the cost-effectiveness analysis; and writing the final report.
Tracey Cooper (Director of Infection Prevention & Control, South London Healthcare NHS Trust) contributed to discussions at project meetings; writing of the protocol; piloting and applying mapping keywords; and writing the final report.
Jennifer Cleland (Professor of Medical Education, University of Aberdeen) contributed to discussions at project meetings; writing of the protocol; piloting and applying mapping keywords; and writing the final report.
Jeremy Jones (Principal Research Fellow, Health Economics, SHTAC) contributed to discussions at project meetings; piloting and applying mapping keywords; screening studies for inclusion in the systematic review of cost-effectiveness; extracting data from studies included in the systematic review of cost-effectiveness; assessing the quality of the included studies; developing the economic model and conducting the cost-effectiveness analysis; and writing the final report.
Jonathan Shepherd (Principal Research Fellow, SHTAC) contributed to discussions at project meetings; writing of the protocol; piloting and applying mapping keywords; and writing the final report.
Andrew Clegg (Professor of Health Services Research & Director of SHTAC) contributed to discussions at project meetings; writing of the protocol; piloting and applying mapping keywords; and writing the final report.
Nicholas Graves (Professor of Health Economics, Queensland University of Technology) contributed to discussions at project meetings; piloting and applying mapping keywords; commenting on and reviewing a draft version of the economic model; and writing the final report.
Karen Welch (Information Specialist, SHTAC) conducted the literature searches for the systematic reviews of clinical effectiveness and cost-effectiveness, and commented on a draft version of the final report.
Brian H Cuthbertson (Professor of Critical Care, Sunnybrook Health Sciences) contributed to the writing of the protocol; piloting and applying mapping keywords; and writing the final report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Lorente L, Henry C, Martin MM, Jimenez A, Mora ML. Central venous catheter-related infection in a prospective and observational study of 2,595 catheters. Crit Care 2005;9:R631-5.
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo Clin Proc 2006;81:1159-71. http://dx.doi.org/10.4065/81.9.1159.
- The Third Prevalence Survey of Healthcare Associated Infections in Acute Hospitals in England 2006. London: Department of Health; 2007.
- Penne K. Using evidence in central catheter care. Semin Oncol Nurs 2002;18:66-70. http://dx.doi.org/10.1053/sonu.2002.30047.
- Timsit J-F, Bruneel F, Cheval C, Mamzer M-F, Garrouste-Org, Wolff M, et al. Use of tunneled femoral catheters to prevent catheter-related infection: a randomized, controlled trial. Ann Intern Med 1999;130:729-35. http://dx.doi.org/10.7326/0003-4819-130-9-199905040-00004.
- O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011;52:e162-93. http://dx.doi.org/10.1093/cid/cir257.
- Eggimann P, Pittet D. Overview of catheter-related infections with special emphasis on prevention based on educational programs. Clin Microbiol Infect 2002;8:295-309. http://dx.doi.org/10.1046/j.1469-0691.2002.00467.x.
- Osma S, Kahveci SF, Kaya FN, Akalin H, Ozakin C, Yilmaz E, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect 2006;62:156-62. http://dx.doi.org/10.1016/j.jhin.2005.06.030.
- Pearson ML, Hierholzer WJ, Garner JS, Mayhall CG, Adams A, Craven DE, et al. Guideline for prevention of intravascular device-related infections. Am J Infect Control 1996;24:262-93. http://dx.doi.org/10.1086/647338.
- van der Kooi TI, de Boer AS, Mannien J, Wille JC, Beaumont MT, Mooi BW, et al. Incidence and risk factors of device-associated infections and associated mortality at the intensive care in the Dutch surveillance system. Intensive Care Med 2007;33:271-8.
- Johnston B, Conly J. What do central venous catheter-associated bloodstream infections have to do with bundles?. Can J Infect Dis Med Microbiol 2005;16:215-18.
- English National Point Prevalence Survey on Healthcare-associated Infections and Antimicrobial Use, 2011: preliminary data. London: HPA; 2012.
- Shapey IM, Foster MA, Whitehouse T, Jumaa P, Bion JF. Central venous catheter-related bloodstream infections: improving post-insertion catheter care. J Hosp Infect 2009;71:117-22. http://dx.doi.org/10.1016/j.jhin.2008.09.016.
- Aslakson RA, Romig M, Galvagno SM, Colantuoni E, Cosgrove SE, Perl TM, et al. Effect of accounting for multiple concurrent catheters on central line-associated bloodstream infection rates: practical data supporting a theoretical concern. Infect Control Hosp Epidemiol 2011;32:121-4. http://dx.doi.org/10.1086/657941.
- Plimmer SE, Miles A, Harding R. Implementation of a multidisciplinary framework for prevention of central venous catheter blood stream infections in a rural district general ICU. Intensive Care Med 2011;37.
- Kulkarni S, Nair P, Sule A. Trends in the central line related infections in winter months in a tertiary neurosurgical intensive care unit in the north west of England in the last 2 years in comparison with the national statistics and with the ongoing Matching Michigan trial: a systematic statistical review and analysis. Intensive Care Med 2011.
- Abrusci T, Bion J. Validating the diagnosis of central venous catheter bloodstream infections (CVC-BSIs) in ICUs in England. Intensive Care Med 2011.
- Intensive Care Unit Associated Infection National Surveillance Programme. Glasgow: Health Protection Scotland; 2011.
- Annual Epidemiological Report on Communicable Diseases in Europe. Stockholm: European Centre for Disease Prevention and Control; 2009.
- Centers for Disease Control and Prevention (CDC) . Vital signs: central line-associated blood stream infections – United States, 2001, 2008, and 2009. MMWR – Morb Mort Weekly Rep 2011;60:243-8.
- Tomlinson D, Mermel LA, Ethier MC, Matlow A, Gillmeister B, Sung L. Defining bloodstream infections related to central venous catheters in patients with cancer: a systematic review. Clin Infect Dis 2011;53:697-710. http://dx.doi.org/10.1093/cid/cir523.
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009;49:1-45. http://dx.doi.org/10.1086/599376.
- Plowman R, Graves N, Griffin M, Roberts JA, Swan AV, Cookson B, et al. The Socio-economic Burden of Hospital Acquired Infection. London: School of Hygiene and Tropical Medicine, Public Health Laboratory Service; 1999.
- Armenian SH, Singh J, Arrieta AC. Risk factors for mortality resulting from bloodstream infections in a pediatric intensive care unit. Pediatr Infect Dis J 2005;24:309-14. http://dx.doi.org/10.1097/01.inf.0000157086.97503.bd.
- Fletcher S. Catheter-related bloodstream infection. Contin Educ Anaesth Crit Care Pain 2005;5:49-51. http://dx.doi.org/10.1093/bjaceaccp/mki011.
- Wenzel RP, Edmond MB. Team-based prevention of catheter-related infections. N Engl J Med 2006;355:2781-3. http://dx.doi.org/10.1056/NEJMe068230.
- Tacconelli E, Smith G, Hieke K, Lafuma A, Bastide P. Epidemiology, medical outcomes and costs of catheter-related bloodstream infections in intensive care units of four European countries: literature- and registry-based estimates. J Hosp Infect 2009;72:97-103. http://dx.doi.org/10.1016/j.jhin.2008.12.012.
- Department of Health . High Impact Intervention No. 1 Central Venous Catheter Care Bundle n.d. www.gosh.nhs.uk/EasySiteWeb/GatewayLink.aspx?alId=80528 (accessed May 2012).
- Atherton SL, Tjoelker RC. Evidence based fact sheet: an effective method for implementing change. Am J Infect Control 2006;34. http://dx.doi.org/10.1016/j.ajic.2006.05.107.
- Aboelela SW, Stone PW, Larson EL. Effectiveness of bundled behavioural interventions to control healthcare-associated infections: a systematic review of the literature. J Hosp Infect 2007;66:101-8. http://dx.doi.org/10.1016/j.jhin.2006.10.019.
- Rogers S, Brennan S. Continuous quality improvement: effects on professional practice and patient outcomes. Cochrane Database Syst Rev 2001;4. http://dx.doi.org/10.1002/14651858.CD003319.
- Conn VS, Rantz MJ, Wipke-Tevis DD, Maas ML. Designing effective nursing interventions. Res Nurs Health 2001;24:433-42. http://dx.doi.org/10.1002/nur.1043.
- Marwick C, Davey P. Care bundles: The holy grail of infectious risk management in hospital?. Curr Opin Infect Dis 2009;22:364-9. http://dx.doi.org/10.1097/QCO.0b013e32832e0736.
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006;355:2725-32. http://dx.doi.org/10.1056/NEJMoa061115.
- Griesbach D, Taylor A. Educational Interventions to Prevent Hepatitis C: A Review of the Literature and Expert Opinion 2009.
- Reed D, Price EG, Windish DM, Wright SM, Gozu A, Hsu EB, et al. Challenges in systematic reviews of educational intervention studies. Ann Intern Med 2005;142:1080-9. http://dx.doi.org/10.7326/0003-4819-142-12_Part_2-200506211-00008.
- Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: a systematic review. Crit Care Med 2008;36:933-40. http://dx.doi.org/10.1097/CCM.0B013E318165FAF3.
- Richardson A. Minimising central venous catheter-associated bloodstream infections – ‘Matching Michigan’ in England. Nurs Crit Care 2009;14:105-6.
- Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones SR, et al. epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2007;65:1-64. http://dx.doi.org/10.1016/S0195-6701(07)60002-4.
- Department of Health . Clean, Safe Care: Reducing Infections and Saving Lives n.d. http://antibiotic-action.com/wp-content/uploads/2011/07/DH-Clean-safe-care-v2007.pdf (accessed May 2012).
- High Quality Care for All. Department of Health; 2008.
- National Patient Safety Agency . Minimising Central Venous Catheter Associated Bloodstream Infections: Matching Michigan n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_085825 (accessed May 2012).
- Adams-Chapman I, Stoll BJ. Prevention of nosocomial infections in the neonatal intensive care unit. Curr Opin Pediatr 2002;14:157-64. http://dx.doi.org/10.1097/00008480-200204000-00003.
- Chittick P, Sherertz RJ. Recognition and prevention of nosocomial vascular device and related bloodstream infections in the intensive care unit. Crit Care Med 2010;38:363-72. http://dx.doi.org/10.1097/CCM.0b013e3181e6cdca.
- Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care 2010;14. http://dx.doi.org/10.1186/cc8853.
- Semelsberger CF. Educational interventions to reduce the rate of central catheter-related bloodstream infections in the NICU: a review of the research literature. Neonatal Netw 2009;28:391-5. http://dx.doi.org/10.1891/0730-0832.28.6.391.
- Gastmeier P, Geffers C. Prevention of catheter-related bloodstream infections: analysis of studies published between 2002 and 2005. J Hosp Infect 2006;64:326-35. http://dx.doi.org/10.1016/j.jhin.2006.07.005.
- Ramritu P, Halton K, Cook D, Whitby M, Graves N. Catheter-related bloodstream infections in intensive care units: a systematic review with meta-analysis. J Adv Nurs 2008;62:3-21. http://dx.doi.org/10.1111/j.1365-2648.2007.04564.x.
- Cherry MG, Brown JM, Neal T, Shaw NB. What features of educational interventions lead to competence in aseptic insertion and maintenance of CV catheters in acute care? BEME Guide No. 15. Med Teach 2010;32:198-21. http://dx.doi.org/10.3109/01421591003596600.
- Coopersmith CM, Rebmann TL, Zack JE, Ward MR, Corcoran RM, Schallom ME, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med 2002;30:59-64. http://dx.doi.org/10.1097/00003246-200201000-00009.
- Warren DK, Zack JE, Mayfield JL, Chen A, Prentice D, Fraser VJ, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest 2004;126:1612-18. http://dx.doi.org/10.1378/chest.126.5.1612.
- Shepherd J, Kavanagh J, Picot J, Cooper K, Harden A, Barnett-Page E, et al. The effectiveness and cost-effectiveness of behavioural interventions for the prevention of sexually transmitted infections in young people aged 13–19: a systematic review and economic evaluation. Health Technol Assess 2010;14.
- Shepherd J, Harden A, Rees R, Brunton G, Garcia J, Oliver S, et al. Young people and healthy eating: a systematic review of research on barriers and facilitators. Health Educ Res 2006;21:239-57. http://dx.doi.org/10.1093/her/cyh060.
- Rees R, Kavanagh J, Harden A, Shepherd J, Brunton G, Oliver S, et al. Young people and physical activity: a systematic review matching their views to effective interventions. Health Educ Res 2006;21:806-25. http://dx.doi.org/10.1093/her/cyl120.
- Des Jarlais DC, Lyles C, Crepaz N. The TREND Group . Improving the reporting quality of nonrandomised evaluations of behavioural and public health interventions: the TREND statement. Am J Public Health 2004;94:361-6.
- Hammick M, Dornan T, Steinert Y. Conducting a best evidence systematic review. Part 1. From idea to data coding. BEME Guide No 13. Med Teach 2010;32:3-15. http://dx.doi.org/10.3109/01421590903414245.
- Stone SP, Cooper BS, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al. The ORION Statement: guidelines for transparent reporting of Outbreak Reports and Intervention studies of Nosocomial infection. J Antimicrob Chemother 2007;59:833-40. http://dx.doi.org/10.1093/jac/dkm055.
- Higgins JPT, Altman DG, Gøetzsche P, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Reeves CB, Deeks JJ, Higgins JPT, Wells GA, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration; 2008.
- Armijo-Olivo S, Stiles CR, Hagen NA, Biondo BD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration risk of bias tool and the Effective Public Health Practice Project quality assessment tool: methodological research. J Eval Clin Pract 2010;18:12-8. http://dx.doi.org/10.1111/j.1365-2753.2010.01516.x.
- Rodgers M, Sowden A, Petticrew M, Arai L, Roberts H, Britten N, et al. Testing methodological guidance on the conduct of narrative synthesis in systematic reviews. Evaluation 2009;15:47-71. http://dx.doi.org/10.1177/1356389008097871.
- Kirkwood BR, Sterne JAC. Essential Medical Statistics. Oxford: Blackwell Science; 2003.
- Agra Varela Y, Sierra E, Drake M, Terol E. The Zero Bacteremia Project. Reduction of bacteremia caused by central venous catheters at ICUs in Spain. Rev Enferm 2009;32:15-8.
- Anguera Saperas L, Aragonés Mestre M, March JM, Rius Ferrus LI, Uruén Pueyo S, Diaz Santos E, et al. New strategy of actuation in central venous catheter and its influence in infection. Enferm Intensiva 2004;15:11-6.
- Esteve F, Pujol M, Ariza J, Gudiol F, Verdaguer R, Cisnal M, et al. Impact of a prevention program for catheter-related bloodstream infection in the intensive care unit of a tertiary hospital. Enferm Infecc Microbiol Clin 2009;27:561-5.
- Lisboa T, Rello J. Prevention of nosocomial infections: strategies to improve the safety of the patients in the Intensive Care Unit. Med Intensiva 2008;32:248-52.
- Palomar M, Rodriguez P, Nieto M, Sancho S. Prevention of nosocomial infection in critical patients. Med Intensiva 2010;34:523-33.
- Palomar Martinez M, Lerma FA, Badia MAR, Gil CL, Pueyo MJL, Tobajas CD, et al. Prevention of bacteriema related with ICU catheters by multifactorial intervention: a report of the pilot study. Med Intensiva 2010;34:581-9.
- Urrea Ayala M, Rozas QL. Catheter-associated bloodstream infections: Implementation of a new consensus protocol. An Pediatr 2009;71:20-4.
- Leboucher B, Leblanc M, Berlie I, Savagner C, Lemarie C, Le Bouedec S. Effectiveness of an informative report on the prevention of nosocomial bloodstream infections in a neonatal intensive care unit. Arch Pediatrie 2006;13:436-41.
- Riel-Roberge G. Central catheterization. Reduction of bacteremia. Sensitivity of intensive care nursing personnel. Perspect Infirm 2010;7.
- Lolom I, Deblangy C, Capelle A, Guerinot W, Bouvert E, Barry B, et al. Effect of a long-term quality improvement program on the risk of infection related to peripheral venous catheters. Presse Med 2009;38:34-42.
- Schindler B. Prevention of catheter-related infections in the intensive care unit. Krankenhauspharmazie 2007;28.
- Agvald-Ohman C, Hanberger H, Struwe J, Walther SM. The “push” and “pull” method to reduce nosocomial infections at ICU. A pilot project with an active follow up. Lakartidningen 2010;107:28-31.
- Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med 2009;169:1420-3. http://dx.doi.org/10.1001/archinternmed.2009.215.
- Cohen ER, Feinglass J, Barsuk JH, Barnard C, O’Donnell A, McGaghie WC, et al. Cost savings from reduced catheter-related bloodstream infection after simulation-based education for residents in a medical intensive care unit. Simul Healthc 2010;5:98-102. http://dx.doi.org/10.1097/SIH.0b013e3181bc8304.
- Berenholtz SM, Pronovost PJ, Lipsett PA, Hobson D, Earsing K, Parley JE, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med 2004;32:2014-20. http://dx.doi.org/10.1097/01.CCM.0000142399.70913.2F.
- Bhutta A, Gilliam C, Honeycutt M, Schexnayder S, Green J, Moss M, et al. Reduction of bloodstream infections associated with catheters in paediatric intensive care unit: stepwise approach. BMJ 2007;334:362-5. http://dx.doi.org/10.1136/bmj.39064.457025.DE.
- Berriel-Cass D, Adkins FW, Jones P, Fakih MG. Eliminating nosocomial infections at Ascension Health. Jt Comm J Qual Pt Saf 2006;32:612-20.
- Bishop-Kurylo D. The clinical experience of continuous quality improvement in the neonatal intensive care unit. J Perinat Neonat Nurs 1998;12:51-7. http://dx.doi.org/10.1097/00005237-199806000-00008.
- Bizzarro MJ, Sabo B, Noonan M, Bonfiglio M-P, Northrup V, Diefenbach K. A quality improvement initiative to reduce central line-associated bloodstream infections in a neonatal intensive care unit. Infect Control Hosp Epidemiol 2010;31:241-8. http://dx.doi.org/10.1086/650448.
- Bonello RS, Fletcher CE, Becker WK, Clutter KL, Arjes SL, Cook JJ, et al. An intensive care unit quality improvement collaborative in nine Department of Veterans Affairs hospitals: reducing ventilator-associated pneumonia and catheter-related bloodstream infection rates. Jt Comm J Qual Pt Saf 2008;34:639-45.
- Burrell AR, McLaws ML, Murgo M, Calabria E, Pantle AC, Herkes R. Aseptic insertion of central venous lines to reduce bacteraemia. Med J Aus 2011;194:583-7.
- Buttes P, Lattus J, Stout C, Thomas LA. Drive down infection rates. Nurs Crit Care 2006;1:41-5. http://dx.doi.org/10.1097/01244666-200605000-00008.
- Centers for Disease Control and Prevention (CDC) . Reduction in central line-associated bloodstream infections among patients in intensive care units: Pennsylvania, April 2001-March 2005. Morb Mort Weekly Rep 2005;54:1013-16.
- Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs 2010;17:163-6. http://dx.doi.org/10.1097/JTN.0b013e3181fb38a6.
- Coopersmith CM, Zack JE, Ward MR, Sona CS, Schallom ME, Everett SJ, et al. the impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg 2004;139:131-6. http://dx.doi.org/10.1001/archsurg.139.2.131.
- Costello JM, Morrow DF, Graham DA, Potter-Bynoe G, Sandora TJ, Laussen PC. Systematic intervention to reduce central line-associated bloodstream infection rates in a pediatric cardiac intensive care unit. Pediatrics 2008;121:915-23. http://dx.doi.org/10.1542/peds.2007-1577.
- Curchoe RM, Powers J, El-Daher N. Weekly transparent dressing changes linked to increased bacteremia rates. Infect Control Hosp Epidemiol 2002;23:730-2. http://dx.doi.org/10.1086/502002.
- Curry S, Honeycutt M, Goins G, Gilliam C. Catheter-associated bloodstream infections in the NICU: getting to zero. Neonatal Netw 2009;28:151-5. http://dx.doi.org/10.1891/0730-0832.28.3.151.
- DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care 2010;19:555-61. http://dx.doi.org/10.1136/qshc.2009.038265.
- Duane TM, Brown H, Borchers CT, Wolfe LG, Malhotra AK, Aboutanos MB, et al. A central venous line protocol decreases bloodstream infections and length of stay in a trauma intensive care unit population. Am Surg 2009;75:1166-70.
- DuBose JJ, Inaba K, Shiflett A, Trankiem C, Teixeira PGR, Salim A, et al. Measurable outcomes of quality improvement in the trauma intensive care unit: The impact of a daily quality rounding checklist. J Trauma 2008;64:22-7. http://dx.doi.org/10.1097/TA.0b013e318161b0c8.
- Eggimann P, Harbarth S, Constantin M-N, Touveneau S, Chevrolet J-C, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet 2000;355:1864-8. http://dx.doi.org/10.1016/S0140-6736(00)02291-1.
- Eggimann P, Hugonnet S, Sax H, Harbarth S, Chevrolet J-C, Pittet D. Long-term reduction of vascular access-associated bloodstream infection. Ann Intern Med 2005;142:875-6. http://dx.doi.org/10.7326/0003-4819-142-10-200505170-00025.
- Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate six sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg 2005;201:349-58. http://dx.doi.org/10.1016/j.jamcollsurg.2005.04.027.
- Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery 2008;144:492-5. http://dx.doi.org/10.1016/j.surg.2008.06.004.
- Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control 2010;38:430-3. http://dx.doi.org/10.1016/j.ajic.2010.03.007.
- Harnage S. Innovative bundle wipes out catheter-related bloodstream infections. Nursing 2008;38:17-8. http://dx.doi.org/10.1097/01.NURSE.0000337217.01808.5a.
- Harnage SA. Achieving zero catheter related blood stream infections: 15 months success in a community based medical center. JAVA 2007;12:218-24. http://dx.doi.org/10.2309/java.12-4-8.
- Harrigan S, Hurst D, Lee C, Christie V, Wolfe RB, Morrical D, et al. Developing and implementing quality initiatives in the ICU: strategies and outcomes. Crit Care Nurs Clin N Am 2006;18:469-79. http://dx.doi.org/10.1016/j.ccell.2006.09.004.
- Hatler CW, Mast D, Corderella J, Mitchell G, Howard K, Aragon J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care 2006;15:549-54.
- Higuera F, Rosenthal VD, Duarte P, Ruiz J, Franco G, Safdar N. The effect of process control on the incidence of central venous catheter-associated bloodstream infections and mortality in intensive care units in Mexico. Crit Care Med 2005;33:2022-7. http://dx.doi.org/10.1097/01.CCM.0000178190.89663.E5.
- Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care 2006;15:235-9. http://dx.doi.org/10.1136/qshc.2005.016576.
- Joy-Joseph L, Colin JM, Rosenstein CR, Chinye-Onyejuruwa F. An evidence-based approach for managing catheter-associated bloodstream infection in a level II neonatal intensive care unit. J Nurs Care Qual 2010;25:100-4. http://dx.doi.org/10.1097/NCQ.0b013e3181d151b8.
- Koll B, Nolan KM, Schall MW. Spreading Improvement Across Your Health Care Organization. Oakbrook Terrace, IL: Joint Commission Resources; 2007.
- Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Pt Saf 2008;34:713-23.
- Lobo RD, Levin AS, Brasileiro Gomes LM, Cursino R, Park M, Figueiredo VB, et al. Impact of an educational program and policy changes on decreasing catheter-associated bloodstream infections in a medical intensive care unit in Brazil. Am J Infect Control 2005;33:83-7. http://dx.doi.org/10.1016/j.ajic.2004.05.003.
- Lobo RD, Levin AS, Oliveira MS, Gomes LMB, Gobara S, Park M, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: Continuous tailored education versus one basic lecture. Am J Infect Control 2010;38:440-8. http://dx.doi.org/10.1016/j.ajic.2009.09.013.
- Longmate AG, Ellis KS, Boyle L, Maher S, Cairns CJS, Lloyd SM, et al. Elimination of central-venous-catheter-related bloodstream infectons from the intensive care unit. BMJ Qual Saf 2011;20:174-80.
- Maas A, Flament P, Pardou A, Deplano A, Dramaix M, Struelens MJ. Central venous catheter-related bacteraemia in critically ill neonates: Risk factors and impact of a prevention programme. J Hosp Infect 1998;40:211-24. http://dx.doi.org/10.1016/S0195-6701(98)90139-6.
- Marra AR, Cal RGR, Durao MS, Correa L, Guastelli LR, Moura J, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control 2010;38:434-9. http://dx.doi.org/10.1016/j.ajic.2009.11.012.
- McKee C, Berkowitz I, Cosgrove SE, Bradley K, Beers C, Perl TM, et al. Reduction of catheter-associated bloodstream infections in pediatric patients: experimentation and reality. Pediatr Crit Care Med 2008;9:40-6. http://dx.doi.org/10.1097/01.PCC.0000299821.46193.A3.
- Miller RS, Norris PR, Jenkins JM, Talbot TR, Starmer JM, Hutchison SA, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma 2010;68:23-31. http://dx.doi.org/10.1097/TA.0b013e3181c82678.
- Miller MR, Griswold M, Harris JM, Yenokyan G, Charles HW, Moss M, et al. Decreasing PICU catheter-associated bloodstream infections: NACHRI's quality transformation efforts. Pediatrics 2010;125:206-13. http://dx.doi.org/10.1542/peds.2009-1382.
- Miller-Hoover S. Pediatric central line: bundle implementation and outcomes. J Infusion Nurs 2011;34:36-48.
- Misset B, Timsit J-F, Dumay M-F, Garrouste M, Chalfine A, Flouriot I, et al. A continuous quality-improvement program reduces nosocomial infection rates in the ICU. Intensive Care Med 2004;30:395-400. http://dx.doi.org/10.1007/s00134-003-2096-1.
- Moreira M, Freitas MR, Martias ST, Castelo A, Medeires EAS. Efficacy of a program of prevention and control for methicillin-resistant Staphylococcus aureus infections in an intensive-care unit. Braz J Infect Dis 2007;11:57-62. http://dx.doi.org/10.1590/S1413-86702007000100015.
- Northway T, Mawdsley C. The Canadian ICU Collaborative: sustaining the gains: a lesson in humility. Dynamics 2009;20:23-7.
- Orsi GB, Raponi M, Franchi C, Rocco M, Mancini C, Venditti M. Surveillance and infection control in an intensive care unit. Infect Control Hosp Epidemiol 2005;26:321-5. http://dx.doi.org/10.1086/502547.
- Peredo R, Sabatier C, Villagra A, Gonzalez J, Hernandez C, Perez F, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis 2010;29:1173-7. http://dx.doi.org/10.1007/s10096-010-0971-6.
- Perez Parra A, Menarguez MC, Granda MJP, Tomey MJ, Padilla B, Bouza E. A Simple Educational Intervention to Decrease Incidence of Central Line-Associated Bloodstream Infection (CLABSI) in Intensive Care Units with Low Baseline Incidence of CLABSI. Infect Control Hosp Epidemiol 2010;31:964-7. http://dx.doi.org/10.1086/655841.
- Pronovost PJ, Goeschel CA, Colantuoni E, Watson S, Lubomski LH, Berenholtz SM, et al. Sustaining reductions in catheter-related bloodstream infections in Michigan intensive care units: observational study. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c309.
- Pronovost P. Interventions to decrease catheter-related bloodstream infections in the ICU: The Keystone Intensive Care Unit Project. Am J Infect Control 2008;36:e1-5. http://dx.doi.org/10.1016/j.ajic.2008.10.008.
- Racco M, Horn K. Central catheter infections: use of a multidisciplinary team to find simple solutions. Crit Care Nurs 2007;27:78-9.
- Render ML, Brungs S, Kotagal U, Nicholson M, Burns P, Ellis D, et al. Evidence-based practice to reduce central line infections. Jt Comm J Qual Pt Saf 2006;32:253-60.
- Rey C, Alvarez F, De-La-Rua V, Concha A, Medina A, Díaz J-J, et al. Intervention to reduce catheter-related bloodstream infections in a pediatric intensive care unit. Intensive Care Med 2011;37:678-85. http://dx.doi.org/10.1007/s00134-010-2116-x.
- Rogers E, Alderdice F, McCall E, Jenkins J, Craig S. Reducing nosocomial infections in neonatal intensive care. J Matern Fetal Neonatal Med 2010;23:1039-46. http://dx.doi.org/10.3109/14767050903387029.
- Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control 2003;31:405-9. http://dx.doi.org/10.1067/mic.2003.52.
- Rosenthal VD, Guzman S, Safdar N. Reduction in nosocomial infection with improved hand hygiene in intensive care units of a tertiary care hospital in Argentina. Am J Infect Control 2005;33:392-7. http://dx.doi.org/10.1016/j.ajic.2004.08.009.
- Sannoh S, Clones B, Munoz J, Montecalvo M, Parvez B. A multimodal approach to central venous catheter hub care can decrease catheter-related bloodstream infection. Am J Infect Control 2010;38:424-9. http://dx.doi.org/10.1016/j.ajic.2009.07.014.
- Santana SL, Furtado GHC, Wey SB, Medeiros EAS. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol 2008;29:1171-3. http://dx.doi.org/10.1086/591862.
- Schulman J, Stricof R, Stevens TP, Horgan M, Gase K, Holzman IR, et al. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics 2011;127:436-44. http://dx.doi.org/10.1542/peds.2010-2873.
- Shannon RP, Frndak D, Grunden N, Lloyd JC, Herbert C, Patel B, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Pt Saf 2006;32:479-87.
- Sherertz RJ, Ely EW, Westbrook DM, Gledhill KS, Streed SA, Kiger B, et al. Education of physicians-in-training can decrease the risk for vascular catheter infection. Ann Intern Med 2000;132:641-8. http://dx.doi.org/10.7326/0003-4819-132-8-200004180-00007.
- Speroff T, Ely EW, Greevy R, Weinger MB, Talbot TR, Wall RJ, et al. Quality improvement projects targeting health care-associated infections: comparing virtual collaborative and toolkit approaches. J Hosp Med 2011;6:271-8. http://dx.doi.org/10.1002/jhm.873.
- Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of ‘bundles’ in a closed-model medical intensive care unit. J Crit Care 2010;25:e11-18. http://dx.doi.org/10.1016/j.jcrc.2009.06.016.
- Wall RJ, Ely EW, Elasy TA, Dittus RS, Foss J, Wilkerson KS, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care 2005;14:295-302. http://dx.doi.org/10.1136/qshc.2004.013516.
- Warren DK, Zack JE, Cox MJ, Cohen MM, Fraser VJ. An educational intervention to prevent catheter-associated bloodstream infections in a nonteaching, community medical center. Crit Care Med 2003;31:1959-63. http://dx.doi.org/10.1097/01.CCM.0000069513.15417.1C.
- Warren DK, Cosgrove SE, Diekema DJ, Zuccotti G, Climo MW, Bolon MK, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol 2006;27:662-9. http://dx.doi.org/10.1086/506184.
- Wirtschafter DD, Pettit J, Kurtin P, Dalsey M, Chance K, Morrow HW, et al. A statewide quality improvement collaborative to reduce neonatal central line-associated blood stream infections. J Perinatol 2010;30:170-81. http://dx.doi.org/10.1038/jp.2009.172.
- Yoo S, Ha M, Choi D, Pai H. Effectiveness of surveillance of central catheter-related bloodstream infection in an ICU in Korea. Infect Control Hosp Epidemiol 2001;22:433-6. http://dx.doi.org/10.1086/501930.
- Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control 2008;36:e1-2. http://dx.doi.org/10.1016/j.ajic.2008.10.014.
- Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med 2009;37:2167-73. http://dx.doi.org/10.1097/CCM.0b013e3181a02d8f.
- Khouli H, Jahnes K, Shapiro J, Rose K, Mathew J, Gohil A, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest 2011;139:80-7. http://dx.doi.org/10.1378/chest.10-0979.
- Central Line Associated Bacteraemia in NSW Intensive Care Units (CLAB ICU) Final Report. Sydney, Australia: Clinical Excellence Commission; 2010.
- Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988;16:128-40. http://dx.doi.org/10.1016/0196-6553(88)90053-3.
- Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted?. Stat Med 2002;21:1559-73. http://dx.doi.org/10.1002/sim.1187.
- Dixon-Woods M, Bosk CL, Aveling EL, Goeschel CA, Pronovost PJ. Explaining Michigan: developing an ex post theory of a quality improvement program. Millbank Q 2011;89:167-205. http://dx.doi.org/10.1111/j.1468-0009.2011.00625.x.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.2165/00019053-200624040-00006.
- Halton KA, Cook D, Paterson DL, Safdar N, Graves N. Cost-effectiveness of a central venous catheter care bundle. PLoS ONE 2010;5:1-11. http://dx.doi.org/10.1371/journal.pone.0012815.
- Bond WF, King AE. Modeling for the decision process to implement an educational intervention: an example of a central venous catheter insertion course. J Patient Saf 2011;7:85-91. http://dx.doi.org/10.1097/PTS.0b013e31821b3ab6.
- Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed May 2010).
- Halton KA, Cook DA, Whitby M, Paterson DL, Graves N. Cost effectiveness of antimicrobial catheters in the intensive care unit: addressing uncertainty in the decision. Crit Care 2009;13. http://dx.doi.org/10.1186/cc7744.
- McLaws ML, Berry G. Nonuniform risk of bloodstream infection with increasing central venous catheter-days. Infect Control Hosp Epidemiol 2005;26:715-19. http://dx.doi.org/10.1086/502608.
- Hawthorne G, Osborne R. Population norms and meaningful differences for the Assessment of Quality of Life (AQoL) measure. Aus N Z J Public Health 2005;29:136-42. http://dx.doi.org/10.1111/j.1467-842X.2005.tb00063.x.
- Ramritu P, Halton K, Collignon P, Cook D, Fraenkel D, Battistutta D, et al. A systematic review comparing the relative effectiveness of antimicrobial-coated catheters in intensive care units. Am J Infect Control 2008;36:104-17. http://dx.doi.org/10.1016/j.ajic.2007.02.012.
- Cuthbertson BH, Scott J, Strachan M, Kilonzo M, Vale L. Quality of life before and after intensive care. Anaesthesia 2005;60:332-9. http://dx.doi.org/10.1111/j.1365-2044.2004.04109.x.
- Rechner IJ, Lipman J. The costs of caring for patients in a tertiary referral Australian Intensive Care Unit. Anaesth Intensive Care 2005;33:477-82.
- Graves N, Birrell FA, Whitby M. Modeling the economic losses from pressure ulcers among hospitalized patients in Australia. Wound Repair Regen 2005;13:462-7. http://dx.doi.org/10.1111/j.1067-1927.2005.00066.x.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Richardson A, Bion J. Matching Michigan in England 2010.
- Intensive Care National Audit & Research Centre (ICNARC) . CMP Case Mix and Outcome Summary Statistics n.d. www.icnarc.org (accessed May 2012).
- Lambert ML, Suetens C, Savey A, Palomar M, Hiesmayr M, Morales I, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis 2011;11:30-8. http://dx.doi.org/10.1016/S1473-3099(10)70258-9.
- Irish Critical Care Trials Group (ICCTG), Intensive Care Society of Ireland (ICSI), Health Protection Surveillance Centre (HPSC), Health Service Executive Critical Care Program (HSE-CCP) . Catheter-Related Infection (CRI) in Irish Intensive Care Units – A Pilot Surveillance Study n.d. www.hpsc.ie/hpsc/ (accessed May 2012).
- Department of Health . NHS Reference Costs 2010 11 n.d. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_095859 (accessed May 2012).
- Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, Fraser VJ. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit Care Med 2006;34:2084-9. http://dx.doi.org/10.1097/01.CCM.0000227648.15804.2D.
- Ward S, Lloyd JM, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11.
- Barnett AG, Graves N, Rosenthal VD, Salomao R, Rangel-Frausto MS. Excess length of stay due to central line-associated bloodstream infection in intensive care units in Argentina, Brazil, and Mexico. Infect Control Hosp Epidemiol 2010;31:1106-14. http://dx.doi.org/10.1086/656593.
- De Angelis G, Murthy A, Beyersmann J, Harbarth S. Estimating the impact of healthcare-associated infections on length of stay and costs. Clin Microbiol Infect 2010;16:1729-35. http://dx.doi.org/10.1111/j.1469-0691.2010.03332.x.
- Wolkewitz M, Beyersmann J, Gastmeier P, Schumacher M. Modeling the effect of time-dependent exposure on intensive care unit mortality. Intensive Care Med 2009;35:826-32. http://dx.doi.org/10.1007/s00134-009-1423-6.
- Barnett AG, Beyersmann J, Allignol A, Rosenthal VD, Graves N, Wolkewitz M. The time-dependent bias and its effect on extra length of stay due to nosocomial infection. Value Health 2010;14:381-6.
- European Centre for Disease Control . Hospital in Europe Link for Infection Control Through Surveillnace (HELICS): Surveillance of Nosocomial Infections in Intensive Care Units Protocol 6.1 n.d. http://helics univ-lyon1 fr/home htm (accessed May 2012).
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit; 2011.
- Department of Health . NHS Reference Costs 2009–10 2011. www.dh.gov.uk/ (accessed May 2012).
- Office of National Statistics . Death Registrations by Single Year of Age, United Kingdom 2010 2011. www.ons.gov.uk (accessed May 2012).
- Williams TA, Dobb GJ, Finn JC, Knuiman MW, Geelhoed E, Lee KY, et al. Determinants of long-term survival after intensive care. Crit Care Med 2012;36:1523-30.
- GRADE Working Group . Grading quality of evidence and strength of recommendations. BMJ 2004;328.
- Cook DA. Randomized controlled trials and meta-analysis in medical education: what role do they play?. Med Teach 2012;34:468-73. http://dx.doi.org/10.3109/0142159X.2012.671978.
- Scales DC, Dainty K, Hales B, Pinto R, Fowler RA, Adhikari NK, et al. A multifaceted intervention for quality improvement in a network of intensive care units: a cluster randomized trial. JAMA 2011;305:363-72. http://dx.doi.org/10.1001/jama.2010.2000.
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLOS Med 2012;4.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1655.
- Cohen DJ, Crabtree BF, Etz RS, Balasubramanian BA, Donahue KE, Leviton LC, et al. Fidelity versus flexibility. Translating evidence-based research into practice. Am J Prev Med 2008;35:S381-9.
- Haley RW, Culver DH, White JW, Morgan WM, Emori TG, Munn VP, et al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol 1985;121:182-205.
- National Nosocomial Infections Surveillance (NNIS) . Am J Infect Control 1998;26:522-33.
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. http://dx.doi.org/10.1016/j.ajic.2008.03.002.
- McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet?. Crit Care Med 2012;40:388-93. http://dx.doi.org/10.1097/CCM.0b013e318232e4f3.
- Render ML, Hasselbeck R, Freyberg RW, Hofer TP, Sales AE, Almenoff PL, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf 2011;20:725-32. http://dx.doi.org/10.1136/bmjqs.2010.048462.
- Kim JS, Holtom P, Vigen C. Reduction of catheter-related bloodstream infections through the use of a central venous line bundle: epidemiologic and economic consequences. Am J Infect Control 2011;39:640-6. http://dx.doi.org/10.1016/j.ajic.2010.11.005.
- Seddon ME, Hocking CJ, Mead P, Simpson C. Aiming for zero: decreasing central line associated bacteraemia in the intensive care unit. NZ Med J 2011;124:9-21.
- Wunsch H, Angus DC, Harrison DA, Collange O, Fowler R, Hoste EA, et al. Variation in critical care services across North America and Western Europe. Crit Care Med 2008;36:2787-93. http://dx.doi.org/10.1097/CCM.0b013e318186aec8.
- Adhikari NKJ, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet 2010;376:1339-46. http://dx.doi.org/10.1016/S0140-6736(10)60446-1.
- Drennan K, Hicks P, Hart GK. Intensive Care Resources and Activity: Australia and New Zealand 2007/2008. Victoria, Australia: Australian and New Zealand Intensive Care Society (ANZICS); 2010.
- Drennan K, Hicks P, Hart GK. Intensive Care Safety and Quality Survey: Australia and New Zealand 2007/2008. Victoria, Australia: Australian and New Zealand Intensive Care Society (ANZICS); 2010.
- O’Grady, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections. Morb Mortal Wkly Rep 2002;51:1-26.
- Pearson ML. Guideline for prevention of intravascular device-related infections. Hospital infection control practices advisory committee. Infect Control Hosp Epidemiol 1996;17:438-73. http://dx.doi.org/10.1086/647338.
- The National Healthcare Safety Network (NHSN) Manual. Atlanta, GA: CDC; 2008.
- Centers for Disease Control and Prevention . CDC definitions for nosocomial infections. Am J Infect Control 1989;17:42-3.
- Render M, Nicholson M. Collaborative cuts ICU infection rate in half. Hosp Peer Rev 2006;31:33-4.
- Elder NC, Brungs SM, Nagy M, Kudel I, Render ML. Intensive care unit nurses' perceptions of safety after a highly specific safety intervention. Qual Saf Health Care 2008;17:25-30. http://dx.doi.org/10.1136/qshc.2006.021949.
- Raad II, Bodey GP. Infectious complications of indwelling vascular catheters. Clin Infect Dis 1992;15:197-208. http://dx.doi.org/10.1093/clinids/15.2.197.
- Talbot TR, Tejedor SC, Greevy RA, Burgess H, Williams MV, Deshpande JK, et al. Survey of infection control programs in a large national healthcare system. Infect Control Hosp Epidemiol 2007;28:1401-3. http://dx.doi.org/10.1086/523867.
- Berg DE, Hershow RC, Ramirez CA, Weinstein RA. Control of nosocomial infections in an intensive care unit in Guatemala City. Clin Infect Dis 1995;21:588-93. http://dx.doi.org/10.1093/clinids/21.3.588.
- Bezzio S, Scolfaro C, Broglia R, Calabrese R, Mignone F, Abruzzese PA, et al. prospective incidence study of bloodstream infection in infants and children with central venous catheters after cardiac surgery in Italy. Infect Control Hosp Epidemiol 2009;30:698-701. http://dx.doi.org/10.1086/598242.
- Bijma R, Girbes AR, Kleijer DJ, Zwaveling JH. Preventing central venous catheter-related infection in a surgical intensive-care unit. Infect Control Hosp Epidemiol 1999;20:618-20. http://dx.doi.org/10.1086/501682.
- Chien LY, MacNab Y, Aziz K, Newman C, McMillan DD, Lee SK, et al. Central venous catheter related nosocomial bloodstream infections in the neonatal intensive care unit. Pediatr Res 2001;49.
- Clancy CM. Reducing central line-related blood stream infections. AORN Journal 2009;89:1123-5. http://dx.doi.org/10.1016/j.aorn.2009.05.009.
- Collignon PJ, Sorrell TC, Uther JB. Prevention of sepsis associated with the insertion of intravenous cannulae. The experience in a coronary care unit. Med J Aus 1985;142:346-8.
- Cooley K, Grady S. Minimizing catheter-related bloodstream infections: one unit's approach. Adv Neonatal Care 2009;9:209-26.
- DuBose J, Teixeira PGR, Inaba K, Lam L, Talving P, Putty B, et al. Measurable outcomes of quality improvement using a daily quality rounds checklist: One-year analysis in a trauma intensive care unit with sustained ventilator-associated pneumonia reduction. J Trauma – Injury Infect Crit Care 2010;69:855-60. http://dx.doi.org/10.1097/TA.0b013e3181c4526f.
- Eggimann P, Pittet D, O’Grady NP, Pittet D. Catheter-Related Infections in the Critically Ill. Dordrecht: Kluwer Academic Publishers; 2004.
- Gnass SA, Barbosa L, Bilicich D, Angeloro P, Treiyer W, Grenovero S, et al. Prevention of central venous catheter-related bloodstream infections using non-technologic strategies. Infect Control Hosp Epidemiol 2004;25:657-77.
- Goeschel CA, Holzmueller CG, Cosgrove SE, Ristaino P, Pronovost PJ. Infection prevention checklist to improve culture and reduce central line-associated bloodstream infections. Joint Commission J Qual Patient Saf 2010;36:571-5.
- Goeschel CA. Nursing leadership at the crossroads: evidence-based practice ‘Matching Michigan-minimizing catheter related blood stream infections’. Nurs Crit Care 2011;16:36-43. http://dx.doi.org/10.1111/j.1478-5153.2010.00400.x.
- Gurskis V, Asembergiene J, Kevalas R, Miciuleviciene J, Pavilonis A, Valinteliene R, et al. Reduction of nosocomial infections and mortality attributable to nosocomial infections in pediatric intensive care units in Lithuania. Medicina-Lithuania 2009;45:203-13.
- Harnage SA. Frontline fame: A peripherally inserted central line catheter (PICC) team ends CRBSIs. RN 2008;71:34-6.
- Jeffries HE, Mason W, Brewer M, Oakes KL, Munoz EI, Gornick W, et al. Prevention of central venous catheter-associated bloodstream infections in pediatric intensive care units: a performance improvement collaborative. Infect Control Hosp Epidemiol 2009;30:645-51. http://dx.doi.org/10.1086/598341.
- Joshi M, Kazandjian V, Martin P, Minogue W, Christian D, Silverman N, et al. A comprehensive grassroots model for statewide safety improvement. Jt Comm J Qual Patient Saf 2005;31:671-7.
- Kilbride HW, Powers R, Wirtschafter DD, Sheehan MB, Charsha DS, LaCorte M, et al. Evaluation and development of potentially better practices to prevent neonatal nosocomial bacteremia. Pediatrics 2003;111:e504-18.
- Kilbride HW, Wirtschafter DD, Powers RJ, Sheehan MB. Implementation of evidence-based potentially better practices to decrease nosocomial infections. Pediatrics 2003;111:e519-33.
- Lindsey H, Chu J, Price C. Informing practice: An intervention reduces catheter-related bloodstream infections. Am J Nurs 2007;107.
- Meier PA, Fredrickson M, Catney M, Nettleman MD. Impact of a dedicated intravenous therapy team on nosocomial bloodstream infection rates. Am J Infect Control 1998;26:388-92. http://dx.doi.org/10.1016/S0196-6553(98)70033-1.
- Moureau N. Legally speaking – the impact of competency, training, and quality assessment. JAVA: J Assoc Vasc Access 2005;10:106-7. http://dx.doi.org/10.2309/java.10-3-4.
- Moureau NL. Reducing the cost of catheter-related bloodstream infections. Nursing 2009;39:14-5. http://dx.doi.org/10.1097/01.NURSE.0000357259.58840.eb.
- Nelson SL, Schouten JM, Valentine LO, Hicks GM. Reducing vascular access complications: An evidence-based approach. J Vasc Access 2005;6:167-70.
- Northway T, Mawdsley C. The Canadian ICU Collaborative: on being a nurse champion. Influencing support and change. Dynamics (Pembroke, Ont.) 2007;18:25-7.
- O’Grady NP, O’Grady NP, Pittet D. Catheter-Related Infections in the Critically Ill. New York, NY: Springer Verlag; 2004.
- Papadimos TJ, Hensely SJ, Duggan JM, Hofmann JP, Khuder SA, Borst MJ, et al. Intensivist supervision of resident-placed central venous catheters decreases the incidence of catheter-related blood stream infections. Patient Saf Surg [Electronic Resource] 2008;2. http://dx.doi.org/10.1186/1754-9493-2-11.
- Plouffe JA. Spacelabs Innovative Project Award winner–2007. Solar system of safety. Dynamics (Pembroke, Ont.) 2010;21:20-1.
- Pronovost P, Goeschel C. Improving ICU care: it takes a team. Healthc Exec 2005;20:14-20.
- Rizzo M. Striving to eliminate catheter-related bloodstream infections: A literature review of evidence-based strategies. Semin Anesth Periop Med Pain 2005;24:214-25. http://dx.doi.org/10.1053/j.sane.2005.10.001.
- Rodriguez-Paz JM, Pronovost P. Prevention of catheter-related bloodstream infections. Adv Surg 2008;42:229-48. http://dx.doi.org/10.1016/j.yasu.2008.03.012.
- Rosenthal VD. Device-associated nosocomial infections in limited-resources countries: findings of the International Nosocomial Infection Control Consortium (INICC). Am J Infect Control 2008;36:S171.e7-12. http://dx.doi.org/10.1016/j.ajic.2008.10.009.
- Rosenthal VD, Maki DG, Rodrigues C, Varez-Moreno C, Leblebicioglu H, Sobreyra-Oropeza M, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol 2010;31:1264-72. http://dx.doi.org/10.1086/657140.
- Schelonka RL, Scruggs S, Nichols K, Dimmitt RA, Carlo WA. Sustained reductions in neonatal nosocomial infection rates following a comprehensive infection control intervention. J Perinatol 2006;26:176-9. http://dx.doi.org/10.1038/sj.jp.7211411.
- Schuerer DJE, Zack JE, Thomas J, Borecki IB, Sona CS, Schallom ME, et al. Effect of chlorhexidine/silver sulfadiazine-impregnated central venous catheters in an intensive care unit with a low blood stream infection rate after implementation of an educational program: A before–after trial. Surg Infect 2007;8:445-54. http://dx.doi.org/10.1089/sur.2006.073.
- Schulman J, Stricof RL, Stevens TP, Holzman IR, Shields EP, Angert RM, et al. Development of a statewide collaborative to decrease NICU central line-associated bloodstream infections. J Perinatol 2009;29:591-9. http://dx.doi.org/10.1038/jp.2009.18.
- Seguin P, Laviolle B, Isslame S, Coue A, Malledant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med 2010;36:1202-6. http://dx.doi.org/10.1007/s00134-010-1829-1.
- Sherertz RJ, O’Grady NP, Pittet D. Catheter-Related Infections in the Critically Ill. New York, NY: Springer Verlag; 2004.
- Sherman RA, Flynn NM, Bradford M, Ryder MA, Cohen SH. Multilumen catheter sepsis and an educational program to combat it. Am J Infect Control 1988;16:31A-34A. http://dx.doi.org/10.1016/0196-6553(88)90037-5.
- Smith A. The effect of a nursing staff education program on compliance with central line care policy in the cardiac intensive care unit. Pediatr Nurs 2006;32.
- Smith M. A care bundle for management of central venous catheters. Paediatr Nurs 2007;19:39-44. http://dx.doi.org/10.7748/paed2007.05.19.4.39.c7792.
- Stewart DB. Eradicating catheter-associated bloodstream: infections in the PICU. Crit Care Nurse 2008;28:71-2.
- Tsuchida T, Makimoto K, Toki M, Sakai K, Onaka E, Otani Y. The effectiveness of a nurse-initiated intervention to reduce catheter-associated bloodstream infections in an urban acute hospital: An intervention study with before and after comparison. Int J Nurs Stud 2007;44:1324-33. http://dx.doi.org/10.1016/j.ijnurstu.2006.07.008.
- Vandijck DM, Labeau SO, Brusselaers N, De Wandel D, Vogelaers DP, Blot SI. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med 2009;37:2998-9. http://dx.doi.org/10.1097/CCM.0b013e3181b77da9.
- Verdier R, Parer S, Jean-Pierre H, Dujols P, Picot M-C. Impact of an infection control program in an intensive care unit in France. Infect Control Hosp Epidemiol 2006;27:60-6. http://dx.doi.org/10.1086/499150.
- Warye K, Granato J. Target: zero hospital-acquired infections. Healthc Financ Manage 2009;63:86-91.
- Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf 2009;35:593-7.
- Weber DJ, Brown VM, Sickbert-Bennett EE, Rutala WA. Sustained and prolonged reduction in central line-associated bloodstream infections as a result of multiple interventions. Infect Control Hosp Epidemiol 2010;31:875-7. http://dx.doi.org/10.1086/655438.
- Yilmaz G, Caylan R, Aydin K, Topbas M, Koksal I. Effect of education on the rate of and the understanding of risk factors for intravascular catheter-related infections. Infect Control Hosp Epidemiol 2007;28:689-94. http://dx.doi.org/10.1086/517976.
- Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter-associated bloodstream infections: A systems-based intervention. Am J Infect Control 2006;34:503-6. http://dx.doi.org/10.1016/j.ajic.2006.03.011.
- Zack JE. Designing a getting-to-zero programme that works for you: a focus on process improvement. Infection Control Today 2009:42-6.
Appendix 1 Protocol for Technology Assessment Report 9/01/25
COMMISSIONED BY THE NIHR HTA PROGRAMME MARCH 2011
1. Title of the project
A systematic review and economic evaluation of the effectiveness and generality of educational interventions for preventing CRBSIs in critical care
2. Protocol version: 2
This version of the protocol was amended on 14th April 2011. The inclusion criteria for participants in Table 1 were slightly modified and the updated criteria were applied to all titles and abstracts.
3. Plain English summary
Catheters are very important for the treatment of patients in critical care but provide a route of entry for bacteria and other micro-organisms into the bloodstream, and are frequently associated with serious infections. These infections increase patients' discomfort, length of stay in hospital, the cost of their treatment and risk of death.
Education is important for ensuring that hospital staff understand how to maintain hygiene and follow practices that reduce the risk of infections. Education may be part of a ‘care bundle’, alongside other activities designed to reduce infections. Types of education are very diverse, ranging from simple leaflets or posters to seminars and group discussions and complex strategies designed to encourage staff to follow more hygienic procedures. Although some of these education strategies can prevent infections and potentially save lives, the effectiveness of most has not been evaluated in detail, especially whether infection prevention can be maintained in the longer term, and whether education carried out in one critical care setting is applicable to other settings.
This project (an evidence synthesis) will rigorously and systematically assess the evidence to determine which types of educational intervention can help prevent infections in critical care patients who have a vascular catheter, whether they can maintain long-term prevention of infections, and whether they are cost-effective. To address the difficulty of evaluating complex educational strategies, the project will employ an evidence mapping technique that can help to visualise the different parts of complex strategies and enable them to be assessed and compared. A decrease in the frequency of CRBSIs will be the key measure of the effectiveness of education. Where available, information will also be collected on the extent to which education strategies are followed and implemented by nurses and doctors.
This project will help the NHS to implement educational procedures for reducing infections that are the most effective and the best value for money. The project is particularly relevant to a strategy that was implemented during 2009–2011 in critical care units in some NHS trusts in England. The strategy, known as ‘Matching Michigan’, was originally developed in the USA and has not previously been evaluated to see if it has similar findings in the UK. The project will link to the Matching Michigan team in England to ensure that the assessment of cost-effectiveness is directly relevant to NHS trusts in England. The findings of this project could assist future planning of infection prevention strategies related to Matching Michigan.
4. Decision problem
The aim of this health technology assessment project is to assess the clinical effectiveness and cost-effectiveness of different educational schemes for preventing catheter-related bloodstream infections (CRBSI) in patients in critical care. Initial scoping searches for this project suggest that research on educational interventions for preventing CRBSI appears to be mostly from studies that may not have optimal study designs and may not be representative of critical care settings and practices in the UK. Uncertainty remains about the extent of the evidence and effectiveness of interventions. There is therefore a need to systematically synthesise all relevant evidence about these educational interventions to clarify their effectiveness, strengths and limitations, and their relevance to the NHS. Results of this evidence synthesis will help to inform future research and policy for implementing educational infection prevention schemes.
5. Background
5.1 Catheter-related bloodstream infections (CRBSI) in critical care
Intravascular catheter placement is an important cause of bloodstream infections (BSI)1,2, and is the commonest source of hospital-acquired bacteraemia in hospitals in England3. CRBSIs (CRBSI) are a particular problem in critical care due to the high frequency of intravascular catheter placement and increased susceptibility to infections among critical care patients. CRBSI are associated with morbidity and, especially in paediatric critical care, also mortality4. Estimates of the additional length of stay per CRBSI episode in UK critical care units have ranged from 1.9 days5 to 11 days6. Owing to a lag in the publication of infection rates, there is uncertainty as to whether these published data are representative of current rates of CRBSI in UK critical care units. 1
(1 Recent unpublished data from UK critical care units suggest that CRBSI rates may in some cases be much lower than those reported in the published literature (Dr D. Wyncoll, personal communication). In the current project, as indicated in section 8.2, the most relevant data to UK critical care units will be used to evaluate cost-effectiveness of educational interventions.)
CRBSI result from inadequate hygiene and suboptimal catheter management procedures. These include among others inadequate hand hygiene of hospital staff, inadequate skin hygiene at the site of patients' catheter insertion, suboptimal location of catheters, and unnecessary placement of catheters. CRBSI are believed to be largely preventable following work in the UK that has successfully reduced the number of cases of MRSA BSI. It has been proposed that the majority of CRBSI could be prevented using evidence-based educational interventions to ensure that doctors and nurses are committed to a culture of safety and follow best practice to achieve this7,8.
5.1.1 Definitions of CRBSI
Various definitions and terms are used, and sometimes confused, in the literature to describe a bloodstream infection that has developed as a consequence of an indwelling intravascular catheter. To define CRBSI (sometimes also referred to as CRBI), both a percutaneously drawn blood culture, and a catheter tip culture (or blood drawn through the catheter itself) should quantitatively or semiquantitatively confirm the same organism up to 48 hours after removal of the catheter, together with clinical manifestations of systemic infection (e.g., fever, chills, hypotension)9,10.
Catheter-associated BSI (CABSI), sometimes also referred to as CABI or central line associated bacteraemia (CLAB), are defined as all BSI in patients with central venous catheters (CVC) after excluding other sites of infection by medical review9,10. CLAB means a bloodstream infection with no other apparent focus of infection where a central line (i.e. CVC) has been in situ within 48 hours of the event.
According to these strict definitions, CABSI overestimates the true incidence of CRBSI. However, these definitions are not always rigidly adhered to.
These various definitions make direct comparison of rates of infection difficult and at times misleading, and care will be taken when reviewing studies that report rates of infection based upon the different definitions. The Matching Michigan project provides clear quantitative criteria for defining CABSI, CRBSI, and catheter-suspected BSI; these may assist classification of infections in the current work.
5.1.2 Diagnosis of CRBSI
Diagnosis of CRBSI is made in various ways, depending upon both local clinical practice and, for infection surveillance purposes, the definition of infection in use. Diagnostic criteria for surveillance purposes are rigorously applied and take account of multiple factors including:
-
The number of blood culture specimens performed, and whether these cultures are percutaneously-drawn, or drawn via the CVC
-
Whether the CVC has been removed, and if so whether culture of the line tip demonstrates significant quantities of the same micro-organism as is detected in percutaneously drawn blood
-
Identification of a known pathogen in a single blood culture, or a common skin organism identified in two or more sets of blood cultures
Presentation of identified signs of systemic infection in a patient, linked to one or more positive blood cultures
Use of different definitions of infections can dramatically alter the reported infection rate unless they are aligned with clinical practice. For example, if clinical practice is not to send a CVC line tip to the laboratory for culture, or to draw only a single set of percutaneous cultures, then any definition requiring catheter-tip culture or more than one set of cultures will never be met, potentially giving an artificially low infection rate. However, provided that an infection definition is applied consistently over time, then the impact of interventions aimed at improving practice and reducing infection rates should still be reliably demonstrated. Care will be taken when reviewing studies to ensure that infection definitions have been applied consistently.
5.1.3 Impact of CRBSI on patients and health services
CRBSI increases patients' discomfort and length of stay in hospital6 and their risk of health complications and death4. Complications include acute respiratory distress syndrome, disseminated intravascular coagulation, acute renal failure, and shock7. However, data on mortality, quality of life and long-term prognosis specifically related to CRBSI are not available for the UK. Recent estimates of the mortality rates of patients with CRBSI in critical care units in France, Germany and Italy ranged from 11% to 17.1%5. The most recent (2009) estimate of the financial impact for the NHS suggests that annual costs related to CRBSI in intensive care units are £19.1 to £36.2 million5.
5.2 Educational interventions for preventing CRBSI
5.2.1 Definition of educational interventions
In general, educational interventions involve the communication of information to a specific target group for one or more of the following purposes: to raise awareness; to enhance or improve knowledge; or to change behaviour11. Educational interventions for preventing CRBSI ideally should include behaviour modification components underpinned by relevant theory12. For the purposes of this project our working definition of an educational intervention is any intervention that aims to prevent CRBSI and: (a) includes at least an element of factual information provision related to that aim; (b) is described by the authors as educational; or (c) is described by the authors as behavioural. Project scoping searches indicated that behaviour-modifying interventions to prevent CRBSI are often called ‘educational’ rather than ‘behavioural’ interventions, and behaviour modification components of interventions are not always mentioned in the titles and abstracts of studies. We define educational interventions broadly in this project to ensure that relevant behavioural interventions are not missed at the study selection step.
5.2.2 Types of intervention
Educational interventions for preventing CRBSI have been trialled in critical care settings in many countries and vary considerably in their content and complexity. They range from the provision of simple fact sheets and posters13 to complex interventions comprising multiple behavioural components14. Interventions differ in the number and duration of education components, whether they are didactic or interactive, and whether surveillance and performance feedback are also present. Interventions that contain several different elements which together aim to achieve a particular outcome are referred to as ‘multi-faceted’, ‘multi-component’, or ‘bundled’ interventions15. Multi-faceted educational interventions that have been developed for preventing CRBSI include the Michigan project in the USA16 and the NHS Central Venous Catheter Care Bundle17. These include, among others, specific components for ensuring staff hand hygiene, patient skin hygiene, appropriate choice of catheter type and insertion site, and appropriate ongoing catheter care.
5.2.3 Current usage in the NHS
To address the prevention of CRBSI, the NHS has recently developed ‘Saving Lives’ tools18 which include the ‘High-Impact’ care bundles for central venous catheters and peripheral intravenous cannula17. These bundles are based on ‘EPIC-2’ guidelines19, which stress the importance of education of hospital staff for successful implementation of infection control programmes. However, in the EPIC-2 guidelines there is a lack of evidence on the types of educational interventions that are most appropriate and effective, and the guidelines do not make any recommendations that specifically relate to critical care settings. EPIC-2 guidelines are also inconsistent with US guidelines9 in interpreting the quality of evidence. Following a recommendation in the Darzi Report20, during 2009–2011 the UK National Patient Safety Agency implemented an initiative known as ‘Matching Michigan’8,21 to reduce CRBSI, based on a care bundle that has successfully reduced CRBSI in over 100 intensive care units (ICU) in the Michigan study in the USA16. However, the original study in the USA was not randomised and did not assess the importance of the education strategy in the effectiveness of the overall care bundle16. Guidance is needed from the wider literature on how to implement educational strategies to optimise the clinical effectiveness and cost-effectiveness of this and other related bundled interventions, but the evidence to support such guidance has not been critically synthesised.
6. Planned investigation
6.1 Existing research
6.1.1 Clinical effectiveness of educational interventions
Studies have suggested that the introduction of interventions involving staff education alone or in combination with performance feedback can reduce the frequency of CRBSI in ICU by 40% to 89%16,22–29. Various multi-faceted interventions involving staff education alongside other strategies have also been shown to reduce the frequency of CRBSI in ICU30–32. However, most of the evidence has not been critically appraised and appears to be mainly from non-randomised studies of relatively short duration. These may give an over optimistic picture of infection control, as they do not consider longer-term attenuation of the effectiveness of interventions. Some multi-component bundled interventions involving staff education in critical care may provide sustained (3-year) reductions in infections33, whereas other bundled interventions appear to have had no effect34. Although prevention of infections in some critical care units may be enhanced using staff interventions with education reinforcement, surveillance, performance feedback and process control23, the cost-effectiveness and wider generalisability of these is unclear. Strategies that combine both education and behaviour change stimuli would be expected to have greater impact, by providing a paradigm in which education includes components to target change in the knowledge, beliefs and skills which influence practice35,36. However, health agencies also have to consider how to avoid overwhelming staff with new initiatives and deal with competing demands for safer care with higher throughput9, particularly as increased staff workload negatively affects the care of critically ill patients37. The most complex interventions might not therefore necessarily be the most clinically and cost-effective38.
6.1.2 Cost-effectiveness of educational interventions
Based on the estimated annual costs of CRBSI to the NHS above5, the potential cost reduction to the NHS that could be made by preventing CRBSI would clearly be substantial. The costs of implementing educational interventions to achieve this however are rather unclear. The Michigan intervention16 could prevent up to 15 deaths and save around $2 million annually in one intensive care unit (ICU) based on rates of CRBSI in the USA39 (which might not be representative of current rates of CRBSI in the UK – section 6.1), but there are many uncertainties about how to transfer this type of intervention to UK practice. For example, it is unclear whether interventions tested in ICU in specific localities are generalisable to different geographic regions and healthcare systems, and whether education reinforcement works in situations of high staff turnover and staff shortage as often occur in the UK. The purported simplicity and cost of some interventions is also questionable, for example the Michigan intervention was described as simple and inexpensive but appears to require the delivery of at least 16 lectures by trained staff39, the overall cost of which has not been explored.
6.1.3 Evidence scoping
Scoping searches for this proposal (which are likely to underestimate the true extent of the evidence) identified more than 20 prospective cohort studies of potentially relevant educational interventions (some of which are cited above) but no randomised controlled trials (RCTs). Eight potentially relevant narrative reviews were identified in scoping, but no systematic reviews have directly assessed the clinical effectiveness of educational strategies for preventing CRBSI in critical care. The most relevant systematic reviews in related areas have investigated: the effectiveness of bundled behavioural interventions to control healthcare associated infections (not limited to education, CRBSI or critical care)14; the effectiveness of interventions for preventing CRBSI in critical care (not limited to education or behavioural interventions)40; and educational interventions for preventing healthcare associated infections (not limited to educational or behavioural interventions, CRBSI, or critical care)41.
None of these systematic reviews included economic analyses. Most of the available information on the economic impact of CRBSI in critical care is from work conducted in the USA22,42. A recent brief narrative review of epidemiological studies, referred to above5, provides an insight into the economic burden of CRBSI in critical care in European countries including the UK but, due to a shortage of information on costs, its findings are based on numerous assumptions and uncertainties.
The scoping search highlights the need for an evidence synthesis assessing both the clinical and cost-effectiveness of educational interventions for preventing CRBSI in critical care, to assist decision making in the NHS.
6.2 Research objectives
The aim of this project is to conduct an evidence synthesis of the clinical and cost-effectiveness of educational interventions aimed at hospital staff in critical care (doctors and nurses) for preventing CRBSI. An economic model will be devised by adapting an existing cost-effectiveness model or constructing a new one using the best available evidence to determine cost-effectiveness in a UK critical care setting. The project aims also to provide recommendations that will be sufficiently specific to be of use to those implementing infection-prevention strategies in the NHS and for further research.
The main objectives will be as follows:
-
To systematically review: (a) the clinical effectiveness; and (b) the cost-effectiveness of educational interventions for the prevention of CRBSI.
-
To use an evidence mapping approach to describe the scope of the clinical effectiveness evidence base in terms of the different types of educational interventions, critical care settings, study designs and their theoretical basis, types and duration of education reinforcement, and outcomes reported including evidence of sustainability of effect. The evidence map would: (a) provide an overview, classification and characterisation of relevant educational interventions to enable complex interventions to be visualised and, where appropriate, compared; (b) provide a classification and report of other key study attributes, for example illustrating how CRBSI and CABSI are defined and applied in the studies; and (c) use recognised criteria to screen studies in terms of their relevance to the NHS.
-
To apply the evidence mapping exercise results to prioritise a subset of studies of highest relevance for detailed appraisal in the systematic review of clinical effectiveness.
-
To develop a decision-analytic model to determine and compare cost-effectiveness of relevant groups of interventions and settings identified through evidence mapping, either by adapting an existing economic model or constructing a model for the UK de novo.
-
To identify future research needs and make specific recommendations about the implementation of educational interventions for preventing CRBSI that are relevant to service users in the NHS.
7. Research methods
The project will involve a systematic review of the clinical effectiveness (section 8.1) and a systematic review and economic evaluation of the cost-effectiveness (section 8.2) of educational interventions for preventing CRBSI in critical care (Figure 1). The purpose of the cost-effectiveness systematic review will be twofold: to assess whether an appropriate economic evaluation has been undertaken and, if not, to provide evidence to develop and populate a de novo economic evaluation.
FIGURE 1.
Overview of the project approach.
7.1 Systematic review of clinical effectiveness
7.1.1 Literature search
Literature will be identified from several sources including:
-
General health and biomedical databases including BIOSIS, the British Nursing Index, CINAHL, EMBASE, MEDLINE, the Science Citation Index, and the Social Sciences Citation Index (also others if considered relevant);
-
Specialist electronic databases (e.g. The Cochrane Library; Database of Abstracts and Reviews of Effectiveness);
-
Unpublished literature and conference proceedings;
-
Contact with individuals with expertise in the field;
-
Checking of reference lists;
-
Research in progress databases (e.g. the UK Clinical Research Network website, Current Controlled Trials, and Clinical trials.gov);
-
Relevant websites identified by the project team and Advisory Group.
All databases will be searched from their inception to the current date. Hand searching will focus on key journals and meeting abstracts published in the past two years, with the key journals identified in consultation with experts and from analyses of search results. Based on the scoping searches, we do not envisage that many non-English-language studies will be found (studies conducted in other countries were usually reported in English). We will include relevant non-English language as well as English-language studies in the project, irrespective of their geographical location. The search strategy will be developed and applied by an experienced information specialist to ensure that as many relevant foreign-language studies as possible are identified. Where required, translation will be done by native speakers of the language within the project team's research institutions. If an excessive number of foreign-language studies requires translation, we will contact the HTA programme to advise of the situation, in case provision of additional resources is considered appropriate.
A comprehensive database of relevant published and unpublished articles will be constructed using Reference Manager bibliographic software.
7.1.2 Inclusion criteria and search strategy
Inclusion criteria for the systematic review of clinical effectiveness will be based on the PICOD scheme (Population, Intervention, Outcome, Comparator, Design) and are shown in Table 1. Inclusion criteria for the systematic review of cost-effectiveness are reported below (section 8.2). Note that although the target for educational interventions is critical care staff (doctors and nurses), it is the patients that are the relevant population for inclusion in evidence synthesis. This is because the relevant primary outcomes (CRBSI) are reported for patients.
| Participants (P) | Patients in any critical care units who receive vascular catheters of any type whilst in critical care (including tunnelled and non-tunnelled catheters, subcutaneous catheter ports, peripherally inserted central catheters and cannula) for any medical purposes. |
| Intervention (I) | Any educational interventions for preventing CRBSI in critical care as defined in section 6.2.1. Studies that do not explicitly state an aim to prevent infections, but which report educational interventions that could prevent CRBSI in critical care, will be included if they meet the other inclusion criteria and report relevant outcomes. |
| Comparator (C) | Relevant comparators are: usual care (no active intervention) or any educational intervention that differs from the primary intervention in one or more educational components. |
| Outcomes (O) | Primary outcomes will be used for study selection decisions. Primary outcomes: (1) The frequency of CRBSIs, expressed as infection rates per device-days (usually expressed as BSI per 1000 catheter-days), per hospital days, as a proportion of the study population, or relative to a comparator. Any related infection definitions will be accepted for inclusion screening (e.g. CRBSI, CABSI) as the accuracy and appropriateness of these will be scrutinised at the evidence mapping step. (2) Mortality due to CRBSI. Secondary outcomes: These will be assessed if relevant primary outcomes are reported, and may include: knowledge; attitudes; behaviour; and compliance of critical care staff. Process evaluations: These will be assessed if relevant primary outcomes are reported. |
| Design (D) | Interventional studies only. Randomised controlled trials, non-randomised or quasi-randomised controlled clinical trials, prospective cohort studies, retrospective cohort studies, controlled before-and-after studies, and interrupted time series studies will be included if they evaluate a relevant intervention. Case–control studies, case series, cross-sectional studies, and descriptive studies will be excluded. Where there is evidence from different types of study design for a specific intervention, only those studies with the most rigorous designs will be included and data extracted. |
Care will be taken to ensure that the search strategy can adequately capture educational interventions, given that these may be very diverse, inconsistently or poorly reported, or that education may make up a relatively small component of multi-faceted interventions. The search strategy will also be developed to capture the different possible variants, acronyms, synonyms and definitions of CRBSIs (including CRBSI, CABSI), taking into consideration that these might not have been used consistently and correctly in the literature. A search strategy for studies of cost-effectiveness will also be developed, following standard procedures (section 8.2).
Understanding how and why interventions work is an integral part of the appraisal of complex interventions43. Process evaluations and secondary outcomes (e.g. knowledge, behaviour, attitudes or compliance of staff) may help to explain intervention mechanisms. Process evaluations and secondary outcomes will be included provided that relevant primary (infection) outcomes are also reported (Table 1). If reported in sufficient detail, process evaluations will be assessed following a systematic approach, to be agreed by the project team (e.g. an approach employed in a recent synthesis of evidence on sexually transmitted infections44 may be suitable).
The study designs which will be included are not limited to controlled trials (Table 1). This is because in a scoping exercise much of the evidence found was from cohort studies. For the systematic review of cost-effectiveness, studies will only be included if they report the results of full economic evaluations [cost-effectiveness, cost–utility, cost–benefit or cost–consequence analyses].
7.1.3 Study selection
Studies will be selected for inclusion through a two-stage process using the pre-defined and explicit criteria outlined in Table 1. The full literature search results will be screened by two reviewers to identify all citations that may meet the inclusion criteria. Full manuscripts of all selected citations will be retrieved and assessed by two reviewers against the inclusion criteria. Studies published as abstracts or conference presentations will only be included if sufficient details are presented to allow an appraisal of the methodology and the assessment of results to be undertaken. To ensure that studies are screened consistently, an inclusion decision checklist will be developed and used for each manuscript assessed. Any disagreements over study inclusion will be resolved by consensus or if necessary by arbitration by a third reviewer.
7.1.4 Evidence mapping
All studies that meet the inclusion criteria will be entered into a mapping exercise in order to clarify the structure of educational interventions and identify those that are potentially of most relevance to the NHS.
The mapping exercise is summarised in Figure 2 and will follow principles developed by the Global Evidence Mapping Initiative45 to classify and summarise the evidence base as well as guidance from the Medical Research Council43 and the National Institute for Health and Clinical Excellence (NICE)46 on the reporting and evaluation of complex interventions. The key objectives of this step will be to: (a) provide an overview, classification and characterisation of relevant educational interventions to enable complex interventions to be visualised and, where appropriate, compared; (b) provide a classification and report of other key study attributes, for example illustrating how CRBSI and CABSI are defined and applied in the studies, and whether studies included process evaluations, information on potential facilitators or barriers to implementation, or other secondary outcomes; and (c) identify studies that are of most relevance to the NHS which should be prioritised for full evidence synthesis. The mapping exercise will be conducted as follows (Figure 2):
-
With assistance from the Project Advisory Group (section 10), the characteristics of studies to be included in the descriptive map will be determined and made into a list;
-
In a pilot exercise involving two researchers, keywords will be developed that reliably and reproducibly describe each of the study characteristics in the list;
-
Each included study will be mapped by one researcher and the agreed keywords relevant to describe the characteristics of each study will be entered into a Microsoft Excel or Access database such that study interventions and keywords can be cross-tabulated;
-
Keyword assignments and database entries for each study will be checked by a second researcher;
-
Entries in the database will be used to concisely summarise the structure and composition of the study interventions using numerical, graphical and/or narrative methods where appropriate.
FIGURE 2.
Overview of the evidence mapping procedure for studies of clinical effectiveness.
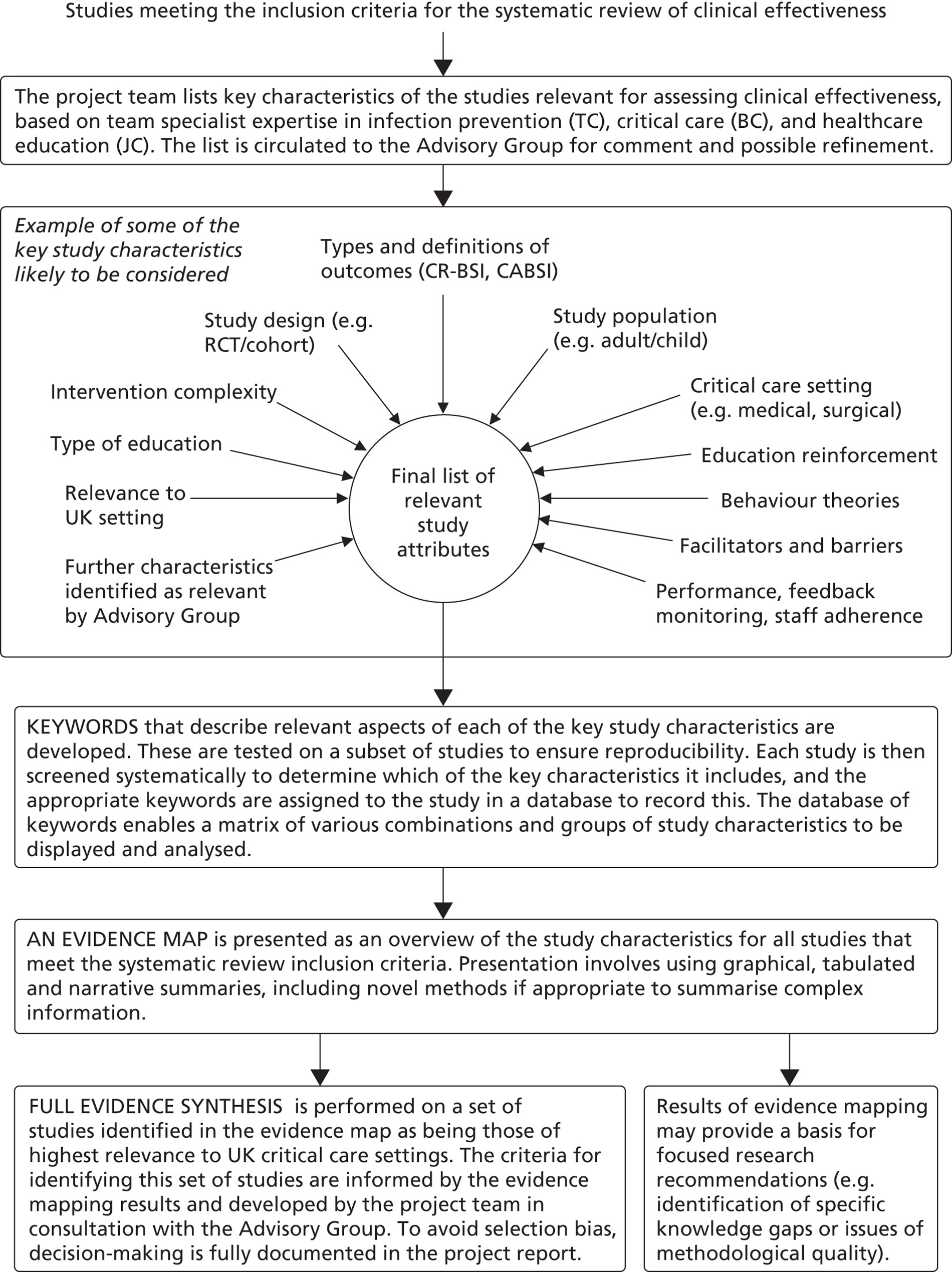
Depending upon the overall quality and quantity of evidence available, the evidence mapping exercise could include a preliminary appraisal of methodological quality to help decide which studies are prioritised for detailed full evidence synthesis (e.g. based on study design and sample size). A thorough appraisal of methodological quality, using risk of bias criteria, will be applied later to those studies that are identified as being of most relevance to the NHS and which proceed for full data extraction (section 8.1.5).
Depending upon the overall quality and quantity of evidence available, the evidence mapping exercise could include a preliminary appraisal of methodological quality to help decide which studies are prioritised for detailed full evidence synthesis (e.g. based on study design and sample size). A thorough appraisal of methodological quality, using risk of bias criteria, will be applied later to those studies that are identified as being of most relevance to the NHS and which proceed for full data extraction (section 8.1.5).
7.1.5 Data extraction and quality assessment
The extraction of studies' findings will be conducted by one reviewer and independently checked by a second reviewer using a pre-designed and piloted data extraction form to avoid any errors. The data extraction form will be based on the PICOD scheme to clearly record and report all relevant aspects of the populations (P), interventions (I), comparators (C) outcomes (O), as well as methodological aspects of the study designs (D). Any disagreements between reviewers will be resolved by consensus or if necessary by arbitration involving a third reviewer. This process will be applied to those studies identified in the mapping exercise (section 8.1.4) as being of highest relevance in the context of current practice in the NHS. The methodological quality of these included studies, including their internal and external validity, will be appraised using established criteria for studies of clinical effectiveness47 and recognised quality assessment approaches for studies of cost-effectiveness and economic models (section 8.2). Missing information will be obtained from investigators of the primary studies if possible, so as to maximise the information about the educational interventions that can be extracted from each study.
7.1.6 Data synthesis
Studies will be synthesised through a narrative review with tabulation of results of included studies. If feasible, the results from individual studies will be synthesised through meta-analysis using established methods47, with causes of heterogeneity of results examined.
7.2 Economic evaluation
The cost-effectiveness of educational interventions in preventing CRBSI in critical care will be assessed in two stages: a systematic review of cost-effectiveness and development of a decision-analytic economic model (Figure 1).
7.2.1 Systematic review of cost-effectiveness
Searches of general health and biomedical databases (as listed in section 8.1.1), specialist electronic databases (e.g. the NHS Economic Evaluation Database; The Cochrane Library), and unpublished literature and conference proceedings will be carried out to identify relevant studies. The systematic review will focus on economic evaluations of educational interventions to prevent CRBSI. To inform development of our economic evaluation we will also include any studies that report model-based economic evaluations of other (non-educational) interventions, if they were published since a 2007 review of the economics of preventing CRBSI48. Experts will be contacted to ask if they know of any relevant published or unpublished studies that we have not identified. Studies will be included in the systematic review if they are full economic evaluations (cost utility or cost-effectiveness studies) that report both measures of costs and consequences, and include outcomes expressed as CRBSI cases avoided, or life years or quality-adjusted life years gained. The methodological quality of included cost-effectiveness studies will be appraised using accepted criteria for appraising economic evaluations49,50. Studies will be synthesised through a narrative review that includes: a clear explanation of the assessment process; a detailed critical appraisal of study methods; tabulation of the results of the included studies; a summary indicating which data are used in the economic model; and an explanation of any knowledge gaps and assumptions.
7.2.2 Decision-analytic model
Evidence from both the systematic review of cost-effectiveness and the systematic review of clinical effectiveness (section 8.1) will be used to develop the economic model. Existing economic models of interventions to prevent CRBSI identified in the systematic review of economic evaluations will be assessed for their relevance and quality. If these are not suitable, a de novo decision-analytic model will be developed. Development of model structure will be informed by previously published models (such as that developed by one of the applicants51) and validated through discussion with clinical and methodological advisors. Accepted guidelines for good practice in decision-analytic modelling52 and the general principles outlined in the NICE ‘reference case’53 will be followed. Clinical effectiveness parameters in the model will be taken from the systematic review of clinical effectiveness. Additional targeted literature searches will be required to populate other parameters in the model, such as the baseline risk of CRBSI. Expert opinion will be used where suitable data to populate the model cannot be identified from the literature. Where expert opinion has been used, this will be clearly identified in the report of the model.
The model will provide a cost-consequences analysis, reporting the costs of alternative educational interventions (broken down by key components, such as staff training, administration, consumables etc. where possible) and their consequences in terms of patient outcomes, principally any effect on the risk of CRBSI. The outcome of the model will be presented as the incremental cost per CRBSI avoided. We will consider the feasibility of developing also a cost-utility analysis model incorporating final outcomes (life expectancy or quality-adjusted life expectancy – i.e. QALYs). This will require estimating excess mortality attributable to CRBSI in patients admitted to ICU and the impact of such infections on patients' quality of life. The model will adopt a UK NHS and Personal Social Services perspective.
The resources necessary for providing the educational interventions will be estimated from studies included in the systematic review of effectiveness (section 8.1), and from discussion with expert advisors. The costing will concentrate on costing studies that were conducted in health systems with similar institutional arrangements to the NHS, and those including educational interventions that are similar to those being introduced in the NHS (for example, ‘Matching Michigan’). Unit costs will be developed based on published evidence, official sources such as NHS Reference Costs54 and Unit Costs of Health and Social Care55, and from the Costing Unit at Southampton General Hospital. Costs will be inflated to current prices as necessary. If no published data on resource use are available, estimates will be based on information from expert advisors.
Uncertainty relating to key parameters will be explored using deterministic and, where appropriate, probabilistic sensitivity analyses. If it is feasible to develop a full cost-utility model, probabilistic sensitivity analyses will be conducted and the results expressed using cost-effectiveness acceptability curves (CEACs). The key variables to be explored will include: effectiveness of educational interventions, baseline risk of CRBSI, cost and duration of CRBSI, mortality attributable to CRBSI, and QALYs.
The model will be developed using standard software including Excel and TreeAge Pro to ensure transparency and would be flexible in terms of permitting different estimates to be used for key input parameters. Any structural assumptions underlying the model would be transparently reported. The model could therefore be updated in response to new information about critical care (intensive care) practices. We propose to consult the project's Advisory Group, which will include clinicians working in critical care, to identify which of the possible changes in critical care practices are likely to be most relevant. This will ensure that appropriate, modifiable, input parameters and structural assumptions are included in the model.
8. Project timetable and milestones
The project will take 18 months, commencing 4th January 2011. Twelve milestones and three proposed research publications arising from the project are detailed in the full project proposal (see also Fig 1). Interim reports will be prepared and submitted at dates to be confirmed by the HTA programme. A final project report will be completed and disseminated by 30 June 2012.
9. Advisory Group
Julian Bion – Professor of Critical Care; clinical lead of Matching Michigan project
Andrew Jackson – Consultant Nurse IV Therapy & Care; Infection Prevention Society
Annette Richardson – Nurse Consultant Critical Care; British Association of Critical Care Nurses
Trudie Roberts – Professor of Medical Education; Association for the Study of Medical Education
Katie Scales – Consultant Nurse Critical care; National Infusion and Vascular Access Society
Barry Williams – Critical Care Patient Liaison Committee (CritPal)
Duncan Wyncoll – Consultant Intensivist; Intensive Care Society
10. References
- Penne K. Using evidence in central catheter care. Semin Oncol Nurs 2002;18:66-70.
- The third prevalence survey of healthcare associated infections in acute hospitals in England 2006. England: Department of Health; 2007.
- Nosocomial Infection National Surveillance Service (NINSS) . Surveillance of Hospital-Acquired Bacteraemia in English Hospitals 1997–2002 2003. URL: www.library.nhs.uk/HEALTHMANAGEMENT/ViewResource.aspx?resID=264523.
- Armenian SH, Arrieta AC, Singh J. Risk factors for mortality resulting from bloodstream infections in a pediatric intensive care unit. Pediatr Infect Dis 2005;24:309-14.
- Tacconelli E, Smith G, Hieke K, Lafuma A, Bastide P. Epidemiology, medical outcomes and costs of catheter-related bloodstream infections in intensive care units of four European countries: literature and registry-based estimates. J Hosp Infect 2009;72:97-103.
- Plowman R, Graves N, Griffin M, Roberts JA, Swan AV, Cookson B, et al. The socio-economic burden of hospital acquired infection. London: School of Hygiene and Tropical Medicine: Public Health Laboratory Service; 1999.
- Wenzel RP, Edmond MB. Team-based prevention of catheter-related infections. N Engl J Med 2006;355:2781-3.
- Richardson A. Minimising central venous catheter-associated bloodstream infections –‘Matching Michigan’ in England. Nurs Crit Care 2009;14:105-6.
- O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections. Morb Mortal Wkly Rep 2002;51:1-26.
- Mermel LA, Farr BM, Sherertz RJ, Raad II, O’Grady N, Harris JS, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis 2001;32:1249-72.
- Griesbach D. Educational interventions to prevent hepatitis C: a review of the literature and expert opinion. NHS Health Scotland; 2009.
- Reed D, Price EG, Windish DM, Wright SM, Gozu A, Hsu EB, et al. Challenges in systematic reviews of educational intervention studies. Ann Intern Med 2005;142:1080-90.
- Atherton SL, Tjoelker RC. Evidence based fact sheet: An effective method for implementing change. Am J Infect Control 2006;34.
- Aboelela SW, Stone PW, Larson EL. Effectiveness of bundled behavioural interventions to control healthcare-associated infections: a systematic review of the literature. J Hosp Infect 2007;66:101-8.
- Conn VS, Rantz MJ, Wipke-Tevis DD, Maas ML. Designing effective nursing interventions. Res Nurs Health 2001;24:433-42.
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006;355:2725-32.
- Department of Health . High Impact Intervention No. 1 Central Venous Catheter Care Bundle 2008. URL: www.clean-safe-care.nhs.uk/toolfiles/14_SL_HII_1_v2.pdf.
- Department of Health . Clean, Safe Care: Reducing Infections and Saving Lives 2008. URL: www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_081650.
- Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones SRLJ, et al. epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2007;65S:1-64.
- Department of Health . High Quality Care for All 2008. URL: www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_085825.
- National Patient Safety Agency . Minimising Central Venous Catheter Associated Bloodstream Infections: Matching Michigan 2009. URL: www.npsa.nhs.uk/nrls/improvingpatientsafety/anaesthesia-and-surgery/matching-michigan/.
- Warren DK, Zack JE, Mayfield JL, Chen A, Prentice D, Fraser VJ, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest 2004;126:1612-18.
- Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control 2003;31:405-9.
- Yilmaz G, Caylan R, Aydin K, Topbas M, Koksal I. Effect of education on the rate of and the understanding of risk factors for intravascular catheter-related infections. Infect Control Hosp Epidemiol 2007;28:689-94.
- Salomao R, Blecher S, Da Silva M, Villins M, Da Silva EH. Education and performance feedback effect on rates of central vascular catheter-associated bloodstream infections in adult intensive care units in one hospital in Sao Paulo, Brazil. Am J Infect Control 2005;33.
- Gómez WV, Vergara GR, Pertuz AM, Rosenthal VD. Education and performance feedback effect on rates of central vascular catheter-associated bloodstream infections in newborn intensive care units in a private hospital in Colombia. Am J Infect Control 2005;33:E76-7.
- Ozgultekin A, Rosenthal VD, Turan G, Akgun N. Education and performance feedback effect on rates of central vascular catheter -associated bloodstream infections in adult intensive care units of one Turkish hospital. Am J Infect Control 2006;34.
- Higuera F, Rosenthal VD, Duarte P, Ruiz J, Franco G, Safdar N. The effect of process control on the incidence of central venous catheter-associated bloodstream infections and mortality in intensive care units in Mexico. Crit Care Med 2005;33:2022-7.
- Maas A, Flament P, Pardou A, Deplano A, Dramaix M, Struelens MJ. Central venous catheter-related bacteraemia in critically ill neonates: risk factors and impact of a prevention programme. J Hosp Infect 1998;40:211-24.
- Eggimann P, Harbarth S, Constantin M-N, Touveneau S, Chevrolet J-C, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet 2000;355:1864-8.
- Karali V, Stefanopoulou P, Bitzani M, Ambatzidou F, Vassiliadou G, Riggos D. Effects of an education: prevention strategy on decreasing catheter-related infections in intensive care. Critical Care 2003.
- Lobo RD, Levin AS, Brasileiro Gomes LM, Cursino R, Park M, Figueiredo VB, et al. Impact of an educational program and policy changes on decreasing catheter-associated bloodstream infections in a medical intensive care unit in Brazil. Am J Infect Control 2005;33:83-7.
- Schelonka RL, Scruggs S, Nichols K, Dimmitt RA, Carlo WA. Sustained reductions in neonatal nosocomial infection rates following a comprehensive infection control intervention. J Perinatol 2009;26:176-9.
- Cohran J, Larson E, Roach H, Blane C, Pierce P. Effect of intravascular surveillance and education program on rates of nosocomial bloodstream infections. Heart Lung 1996;25:161-4.
- Bonetti D, Eccles M, Johnston M, Steen M, Grimshaw J, Baker R, et al. Guiding the design and selection of interventions to influence the implementation of evidence-based practice: An experimental simulation of a complex intervention trial. Soc Sci Med 2005;60:2135-47.
- The Improved Clinical Effectiveness through Behavioural Research Group . Designing theoretically-informed implementation interventions. Implement Sci 2006;1.
- Hugonnet S, Chevrolet J-C, Pittet D. The effect of workload on infection risk in critically ill patients. Crit Care Med 2007;35:76-81.
- Collins LM, Murphy SA, Nair VN, Strecher VJ. A strategy for optimizing and evaluating behavioural interventions. Ann Behav Med 2005;30:65-73.
- Berenholtz SM, Pronovost PJ, Lipsett PA, Hobson D, Earsing K, Farley JE, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med 2004;32:2014-20.
- Ramritu P, Halton K, Cook D, Whitby M, Graves N. Catheter-related bloodstream infections in intensive care units: a systematic review with meta-analysis. J Adv Nurs 2008;62:3-21.
- Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: A systematic review. Crit Care Med 2008;36:933-40.
- Coopersmith CM, Rebmann TL, Zack JE, Ward MR, Corcoran RM, Schallom ME, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med 2002;30:59-64.
- Medical Research Council . Developing and Evaluating Complex Interventions: New Guidance 2008. URL: www.mrc.ac.uk/complexinterventionsguidance.
- Shepherd J, Kavanagh J, Picot J, Cooper K, Harden A, Barnett-Page E, et al. The effectiveness and cost-effectiveness of behavioural interventions for the prevention of sexually transmitted infections in young people aged 13–19: a systematic review and economic evaluation. Health Technol Assess 2010;14:1-230.
- Global Evidence Mapping (GEM) Initiative n.d. URL: www.evidencemap.org/.
- National Institute for Health and Clinical Excellence . Behaviour Change at Population, Community and Individual Levels. n.d. URL: www.nice.org.uk/PH006 (accessed October 2007).
- Higgins JPT, Green S. The Cochrane Handbook for Systematic Reviews of Interventions n.d. URL: www.cochrane-handbook.org/.
- Halton K, Graves N. Economics of preventing catheter-related bloodstream infection?. Emerg Infect Dis 2007;13:815-23.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- Halton KA, Cook DA, Whitby M, Paterson DL, Graves N. Cost effectiveness of antimicrobial catheters in the intensive care unit: addressing uncertainty in the decision. Crit Care 2009;13.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2008. URL: www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf.
- Department of Health . NHS Reference Costs 2008 09 2009. URL: www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_095859.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: University of Kent; 2008.
Appendix 2 Search strategies
Clinical effectiveness search strategy
| Database, date searched, host, years, keywords | Search strategy | Results |
|---|---|---|
| MEDLINE (Ovid) 1948–2010 Searched 19 January 2011 |
|
2383 Updated 13 March 2012: 215 |
| Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations Searched 1 February 2011 |
Adapted from MEDLINE strategy | 92 Updated 13 March 2012: 71 |
| EMBASE (Ovid) Searched 25 January 2011 |
|
2944 Updated 13 March 2012: 659 |
| Web of Science Timespan = all years Searched on 1 February 2011 Databases: Science Citation Index Expanded (SCI-E): 1970–present Social Sciences Citation Index (SSCI): 1970–present Arts & Humanities Citation Index (A&HCI): 1975–present Conference Proceedings Citation Index- Science (CPCI-S): 1990–present Conference Proceedings Citation Index-Social Science & Humanities (CPCI-SSH): 1990–present |
Databases=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH # 1 83,956 TS=(“intensive care” or “critical care” or “intensive therapy unit” or ITU or ICU or CCU or CICU or CITU) # 2 24,167 TS=(catheter* SAME (venous or intravenous or arterial or vascular or intravascular or central or indwelling or peripheral or peripherally)) # 3 1511 TS=(tunnel* SAME (venous or intravenous or arterial or vascular or intravascular or central or indwelling or peripheral or peripherally)) # 4 7870 TS=(device* SAME (venous or intravenous or arterial or vascular or intravascular or central or indwelling or peripheral or peripherally)) # 5 1193 TS=(“central line”) # 6 19,168 TS=(CVC or PICC or JICC or SICC or SBCC or PVC or IVI) # 7 1434 TS=(“Porta-cath” or Portacath or Hickman* or Broviac or Venflon or Groshong) #8 22,666 TS=(“implantable port” or “access port” or cannula*) # 9 5752 TS=(infection* SAME (“bloodstream” or “blood stream” or “blood-stream”)) # 10 > 100,000 TS=(sepsis or asepsis or septic* or bacteremia or bacteraemia) # 11 1873 TS=(healthcare associated infection*) # 12 5279 TS=(“CR-BSI” or “CR-BSIs” or CRBSI or CRBSIs or CABI or CABIs or CABSI or CABSIs or CLABSI or CLABSIs or CRI or CRIs or BSI or BSIs or “AC-CRI “ or “AC-CRIs”) # 13 72,693 (#2 or #3 or #4 or #5 or #6 or #7 or #8) # 14 > 100,000 (#9 or #10 or #11 or #12 or #13) # 15 3003 (#1 and #13 and #14) # 16 464 (#1 and #12) # 17 3178 (#15 or #16) # 18 >100,000 TS=(educat*) # 19 137 (#17 and #18) # 20 > 100,000 TS=(train* or teach* or program* or feedback or lean* or instruct*) # 21 > 100,000 TS=(strateg* or component* or initiative*) #22 > 100,000 TS=(booklet* or workbook* or checklist* or library or libraries or questionnaire* or pamphlet* or poster* or pictorial* or verbal* or video* or audiovisual* or podcast* or telemedicine or teleconferenc* or website*) # 23 9557 TS=(“scrub the hub” or “Matching Michigan” or “Michigan project” or “Michigan Intervention” or “NHS Venous Catheter Care” or EPIC or “EPIC-2” or “saving lives”) # 24 > 100,000 (#20 or #21 or #22 or #23) # 25 807 (#17 and #24) # 26 838 (#19 or #25) |
838 Updated13 Marc h 2012: 138 |
| BIOSIS 1969–2011 Searched 1 February 2011 |
Same strategy as Web of Science | 449 Updated 13 March 2012: 11 BIOSIS Previews 82 BIOSIS Citation Index Total N = 93 |
| HMIC Searched 1 February 2011 |
As MEDLINE search – HMIC download filter would not work easily – only 37 imported but remainder checked and were duplicates so 37 were downloaded | 43 (37 downloaded) DATABASE NO LONGER SUBSCRIBED TO SO NO UPDATE WAS FEASIBLE |
| The Cochrane Library Searched 2 January 2011 |
#1 Medical subject heading (MeSH) descriptor Intensive Care explode all trees (1050) #2 MeSH descriptor Critical Care explode all trees (1704) #3 MeSH descriptor Intensive Care Units explode all trees (2344) #4 (“acute care” or “critical care” or “critically ill” or “critical illness”) (9593) #5 “high dependency care” or “high dependency unit” (51) #6 high next dependency next unit* (41) #7 “intensive care” (11,969) #8 (ITU or ICU or CCU or CICU or CITU) (2595) #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) (19,362) #10 MeSH descriptor Catheterization, Central Venous explode all trees (667) #11 central near catheter* (1867) #12 central near cannul* (182) #13 central near3 line (298) #14 central near3 device (198) #15 central near3 device (198) #16 (catheter NEAR (venous or intravenous or arterial or vascular or intravascular or indwelling or peripheral or peripherally)) (2518) #17 (catheterization NEAR (venous or intravenous or arterial or vascular or intravascular or indwelling or peripheral or peripherally)) (1494) #18 (catheterisation NEAR (venous or intravenous or arterial or vascular or intravascular or indwelling or peripheral or peripherally)) (1494) #19 (tunnel near (venous or intravenous or arterial or vascular or intravascular or central or indwelling or peripheral or peripherally)) (262) #20 (device near (venous or intravenous or arterial or vascular or intravascular or central or indwelling or peripheral or peripherally)) (1098) #21 (CVC or PICC or JICC or SICC or SBCC or PVC or IVI or CVL or PCVC or CVAD or TCVC) (475) #22 umbilical near catheter* (94) #23 umbilical near cannul* (6) #24 umbilical near3 line* (8) #25 (Portacath or Hickman* or Broviac or Venflon or Groshong) (195) #26 port next cath* (9) #27 porta next cath* (2) #28 (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27) (5519) #29 blood next stream next infection (50) #30 bloodstream near infection* (273) #31 blood stream near infection* (56) #32 bacteria* near infection* (5699) #33 MeSH descriptor Infection explode all trees (15,511) #34 MeSH descriptor Communicable Diseases explode all trees (126) #35 MeSH descriptor Bacterial Infections and Mycoses explode all trees (24,790) #36 MeSH descriptor Infectious Disease Transmission, Professional-to-Patient explode all trees (29) #37 MeSH descriptor Bacteremia explode all trees (670) #38 (sepsis or asepsis or septic* or bacteremia* or bacteraemia* or bacteria* or endotoxin*) (24,032) #39 blood near poison* (177) #40 culture near3 (blood or positive) (2428) #41 “healthcare associated” next infection* (22) #42 (HAI or HAIs or “HAI's” or HCAI or HCAIs or “HCAI's”) (471) #43 (MRSA or MSSA) (259) #44 infection*:ti,ab (28,643) #45 (#29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44) (52,153) #46 MeSH descriptor Catheter-Related Infections, this term only 49 #47 catheter* near3 infect* (719) #48 catheter* near3 microb* (138) #49 (catheter* and blood and infection*):ti,ab (208) #50 (“CR-BSI” or “CR-BSIs” or CRBSI or CRBSIs or CABI or CABIs or CABSI or CABSIs or CLABSI or CLABSIs or CRI or CRIs or BSI or BSIs or “AC-CRI “ or “AC-CRIs”) (316) #51 (#46 OR #47 OR #48 OR #49 OR #50) (1110) #52 MeSH descriptor Education explode all trees (15,114) #53 MeSH descriptor Inservice Training explode all trees (441) #54 MeSH descriptor Training Support, this term only (14) #55 (awareness or bundle* or collaborat* or campaign* or communicat*):ti,ab (9811) #56 (feedback or “feed back” or “feeding back” or course* or instruct* or inform* or impart* or knowledge or learn* or “e-learn” or “e-learning” or lecture*) (111,227) #57 (module* or modular or message* or session* or curriculum* or evaluat* or seminar* or test* or teach* or taught or train* or simulat* or refresh* or tool* or meeting* or presentation* or skill* or drill* or workshop* or outreach) (335,667) #58 (booklet* or workbook* or checklist* or library or libraries or questionnaire* or sheet* or pamphlet* or poster* or pictorial* or verbal* or video* or audiovisual* or podcast or telemedicine or teleconferenc* or symposia or symposium) (61,099) #59 change next management (35) #60 ((change or changing) near (process or procedure)) (1161) #61 early next warning next system* (23) #62 (“scrub the hub” or “Matching Michigan” or “Michigan project” or “Michigan Intervention” or “NHS Venous Catheter Care” or EPIC or “EPIC-2” or “saving lives”) (124) #63 educat* (30,296) #64 (chang* near (hygien* or handwash* or hand wash* or disinfect* or sterilisation or sterilization)) (85) #65 (alter* near (hygien* or handwash* or hand wash* or disinfect* or sterilisation or sterilization)) (53) #66 (management near (contaminat* or hygien* or handwash* or hand wash* or disinfect* or sterilisation or sterilization)) (45) #67 (precaution* near (hygien* or handwash* or hand wash* or disinfect* or sterilisation or sterilization)) (13) #68 prevent* near infect* (10,190) #69 (quality near (strateg* or intervention* or management or initiative*)) (4354) #70 (risk near (management or prevention or intervention*)) (8647) #71 (precaution near infect*) (25) #72 (#52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71) (387,381) #73 (#9 AND #28 AND #45 AND #72) (325) #74 (#9 AND #51 AND 72) (48) #75 (#73 OR #74) (340) |
CDSR 71 CENTRAL 187 DARE 30 Updated 13 March 2012: CDSR 63 CENTRAL 5 DARE 0 |
| CINAHL EBSCOhost Searched 2 February 2011 |
S1 (MH “Intensive Care Units”) OR (MH “Intensive Care Units, Pediatric”) OR (MH “Intensive Care, Neonatal”) OR (MH “Neonatal Intensive Care Nursing”) OR (MH “Critical Care”) OR (MH “Critical Care Nursing”) OR (MH “Intensive Care Units, Neonatal”) (33,939) S2 TX (ITU or ICU or CCU or CICU or CITU) (9272) S3 S1 or S2 (38,088) S4 (MH “Central Venous Catheters”) OR (MH “Catheters, Vascular”) OR (MH “Catheters+”) OR (MH “Catheters and Tubes”) OR (MH “Peripherally Inserted Central Catheters”) OR (MH “Catheterization”) OR (MH “Catheter Care”) OR “catheter*” (21,661) S5 TX (CVC or PICC or JICC or SICC or SBCC or PVC or IVI or CVL or PCVC or CVAD or TCVC or Portacath or Hickman* or Broviac or Venflon or Groshong) (1654) S6 TX (central n3 cannul* or central n3 line* or central n3 device (1166) S7 TX (Portacath or “Port-A-Cath” or Hickman or Broviac or Venflon or Groshong) (540) S8 S4 or S5 or S6 or S7 (22,929) S9 (MH “Infection”) OR “infection” OR (MH “Bacterial Infections) (64,326) S10 (MH “Bacteremia”) (2044) S11 TX (bacteremia or bacteraemia or sepsis or asepsis or septic* or “blood poison*”) (11,692) S12 (MH “Endotoxins”) OR “endotoxin” OR (MH “Endotoxemia”) (840) S13 (MH “Toxemia”) OR “toxemia” (51) S14 TX bloodstream infection* (1127 S15 TX blood-stream infection* (98) S16 (MH “Bacterial Colonization”) OR “colonization” (3085) S17 S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 (73,507) S18 (MH “Catheter-Related Infections”) (2447) S19 TX (catheter related bloodstream infection*) (298) S20 TX (CRBSI or CLABSI or CABSI or BSI or CRI) (1812) S21 S18 or S19 or S20 (4115) S22 (MH “Education, Clinical”) OR (MH “Education, Competency-Based”) OR (MH “Education, Allied Health”) OR “education*” (306,068) S23 (MH “Hygiene/ED”) OR (MH “Handwashing/ED”) (250) S24 TX (“scrub the hub” or “Matching Michigan” or “Michigan project” or “Michigan Intervention” or “NHS Venous Catheter Care” or EPIC or “EPIC-2” or “saving lives”) (650) S25 (learn* or teach* or train* or feedback) (170,039) S26 S22 or S23 or S24 or S25 (395,945) S27 S3 and S8 and S17 and S26 (199) S28 S3 and S21 and S26 (150) S29 S27 or S28 (209) |
209 Updated 13 March 2012: 78 |
| CRD Searched 3 February 2011 |
# 1 ( “intensive care” OR “critical care” OR “critical illness” OR “critically ill” ) (2133) # 2 MeSH Critical Care EXPLODE 1 2 (484) # 3 MeSH Intensive Care EXPLODE 1 2 (313) # 4 MeSH Intensive Care Units, Neonatal EXPLODE 1 (107) # 5 MeSH Intensive Care Units, Pediatric EXPLODE 1 (157) # 6 ( ITU OR ICU OR CCU OR CICU OR CITU ) (464) # 7 #1 or #2 or #3 or #4 or #5 or #6 (2435) # 8 MeSH Catheterization, Central Venous EXPLODE 1 (154) # 9 MeSH Catheterization, Peripheral EXPLODE 1 (68) # 10 MeSH Catheters, Indwelling EXPLODE 1 (123) # 11 ( CVC OR PICC OR JICC OR SICC OR SBCC OR PVC OR IVI OR CVL OR PCVC OR CVAD OR TCVC ) (56) # 12 ( catheter* OR cannul* ) (1084) # 13 #8 or #9 or #10 or #11 or #12 (1162) # 14 MeSH Bacterial Infections EXPLODE 1 (1999) # 15 MeSH Bacteremia EXPLODE 1 2 3 (147) # 16 MeSH Sepsis EXPLODE 1 2 (390) # 17 MeSH Asepsis EXPLODE 1 (3) # 18 MeSH Fungemia EXPLODE 1 2 3 (20) # 19 ( infection* OR acinetobacter* OR asepsis OR bacter?emia* OR bacteria* OR candida OR coloni?ation OR contaminat* OR cfu OR colony OR colonies OR corynebacterium OR escherichia OR enterococc* OR enterobacter* OR fungi OR fungus OR fungal OR fung?emia OR klebsiella OR met?icillin OR microorganism* OR micro-organism* OR microbial* OR microbe* OR microbiologic* OR mycology OR mycological OR organism* OR nosocomial* OR pathogen* OR sepsis OR septic OR septic?emia AND septicemia OR staphylococc* OR streptococc* ) (6148) # 20 ( HAI OR HAIs OR “HAI’s” OR HCAI OR HCAIs OR “HCAI’s” ) (15) # 21 ( MRSA OR MSSA ) (85) # 22 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 (6993) # 23 ( “CR-BSI” OR “CR-BSIs” OR CRBSI OR CRBSIs OR CABI OR CABIs OR CABSI OR CABSIs OR CLABSI OR CLABSIs OR CRI OR CRIs OR BSI OR BSIs OR “AC-CRI “ OR “AC-CRIs” ) (77) # 24 catheter AND related AND infection* (170) # 25 ( line AND ( infection* OR sepsis OR bacter?emia ) ) (292) # 26 ( catheter* AND ( sepsis OR septic OR bacter?emia* ) ) (56) # 27 #23 or #24 or #25 or #26 (518) # 28 ( educat* OR train* OR teach* OR feedback OR bundle OR course* OR learn* OR lecture* OR workshop* ) (7927) # 29 ( component* OR “multi-component” OR “multi-faceted” OR “multi-modal” OR initiative* OR intervention* OR program* OR strateg* ) (24,642) # 30 ( booklet* OR checklist* OR library OR libraries OR literature OR questionnaire* OR sheet* OR pamphlet* OR poster* OR pictorial* OR verbal* OR video* OR audiovisual* OR podcast OR telemedicine OR teleconferenc* OR workbook ) (20,644) # 31 ( “scrub the hub” OR “Matching Michigan” OR “Michigan project” OR “Michigan Intervention” OR “NHS Venous Catheter Care” OR EPIC OR “EPIC-2” OR “saving lives” ) (20) # 32 #25 or #26 or #27 or #28 or #29 or #30 or #31 (33,207) # 33 7 AND 13 AND 22 AND 32 (82) # 34 7 AND 27 AND 32 (187) # 35 #33 or #34 (237) # 36 #2 OR #3 OR #4 OR #5 (620) # 37 #13 AND #22 AND #32 AND #36 (17) # 38 #27 AND #32 AND #36 (18) # 39 #37 OR #38 (23) 40 ( “intensive care” OR “critical care” ) (2038) # 41 27 AND 32 AND 40 (125) # 42 catheter* AND educat* (42) # 43 ( catheter* AND educat* AND intensive AND unit* ) (12) # 44 ( catheter* AND educat* AND critical AND unit* ) (7) # 45 #43 or #44 (17) |
Results cross checked manually against database – nothing new to add Updated 13 March 2012: 0 relevant to education Taking education out of the equation 3 |
Cost-effectiveness search strategy
| Database | Search strategy | Results |
|---|---|---|
| MEDLINE Ovid Serached 22 February 2011 |
|
266 Updated 14 March 2012: 43 |
| MEDLINE in Process & Other Non-Indexed Citations Ovid Searched 22 February 2011 |
As per MEDLINE | 9 Updated 14 March 2012: 38 |
| EMBASE Ovid Searched 22 February 2011 |
|
305 Updated 20 March 2012: 116 |
| CINAHL EBSCOhost Searched 22 February 2011 |
Following COST FILTER Added on to clinical search: S30 (MH “Economics+”) (341,991) S31 (MH “Financial Management+”) (26,259) S32 (MH “Financial Support+”) (217674) S33 (MH “Financing, Organized+”) (67782) S34 (MH “Business+”) (50121) S35 S31 or S32 or S33 or S34 (337744) S36 S30 NOT S35 (35930) S37 (MH “Health Resource Allocation”) (4520) S38 (MH “Health Resource Utilization”) (6625) S39 S37 or S38 (10,914) S40 S36 or S39 (43,151) S41 TI (cost or costs or economic* or pharmacoeconomic* or price* or pricing) OR AB (cost or costs or economic* or pharmacoeconomic* or price* or pricing*) (64,812) S42 S40 or S41 (93,923) S43 S29 and S42 (41) |
41 Updated 20 February 2012: 24 |
| Web of Science Searched 23 March 2011 |
Following COST FILTER added on to clinical search: # 27 > 100,000 TI=(cost* or economic* or pharmacoeconomic*) # 28 > 100,000 TS=(“cost effective*” or “cost benefit*” or “cost saving*” or “cost analys*” or “cost utili*” or “cost mimimi*” or “cost consequence*” or “cost comparison*” or “cost identificat*”) # 29 15,887 TS=(“health economic*” or “healthcare cost*” or “health care cost*” or “economic evaluation*”) # 30 32,009 TS=(economical) # 31 > 100,000 (#27 or #28 or #29 or #30) #32 45 (#26 and #31) |
45 Updated 21 March 2012: 12 |
| BIOSIS Searched 23 February 2011 |
Same as Web of Science Strategy | 6 Updated 21 March 2012: 17 |
| The Cochrane Library Searched 23 February 2011 |
#1 MeSH descriptor Intensive Care explode all trees #2 MeSH descriptor Critical Care explode all trees #3 MeSH descriptor Intensive Care Units explode all trees #4 (“acute care” or “critical care” or “critically ill” or “critical illness”) #5 “high dependency care” or “high dependency unit” #6 high next dependency next unit* #7 “intensive care” #8 (ITU or ICU or CCU or CICU or CITU) #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 MeSH descriptor Catheterization, Central Venous explode all trees #11 central near catheter* #12 central near cannul* #13 central near3 line #14 central near3 device #15 central near3 device #16 (catheter NEAR (venous or intravenous or arterial or vascular or intravascular or indwelling or peripheral or peripherally)) #17 (catheterization NEAR (venous or intravenous or arterial or vascular or intravascular or indwelling or peripheral or peripherally)) #18 (catheterisation NEAR (venous or intravenous or arterial or vascular or intravascular or indwelling or peripheral or peripherally)) #19 (tunnel near (venous or intravenous or arterial or vascular or intravascular or central or indwelling or peripheral or peripherally)) #20 (device near (venous or intravenous or arterial or vascular or intravascular or central or indwelling or peripheral or peripherally)) #21 (CVC or PICC or JICC or SICC or SBCC or PVC or IVI or CVL or PCVC or CVAD or TCVC) #22 umbilical near catheter* #23 umbilical near cannul* #24 umbilical near3 line* #25 (Portacath or Hickman* or Broviac or Venflon or Groshong) #26 port next cath* #27 porta next cath* #28 (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27) #29 blood next stream next infection #30 bloodstream near infection* #31 blood stream near infection* #32 bacteria* near infection* #33 MeSH descriptor Infection explode all trees #34 MeSH descriptor Communicable Diseases explode all trees #35 MeSH descriptor Bacterial Infections and Mycoses explode all trees #36 MeSH descriptor Infectious Disease Transmission, Professional-to-Patient explode all trees #37 MeSH descriptor Bacteremia explode all trees #38 (sepsis or asepsis or septic* or bacteremia* or bacteraemia* or bacteria* or endotoxin*) #39 blood near poison* #40 culture near3 (blood or positive) #41 “healthcare associated” next infection* #42 (HAI or HAIs or “HAI's” or HCAI or HCAIs or “HCAI's”) #43 (MRSA or MSSA) #44 infection*:ti,ab #45 (#29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44) #46 MeSH descriptor Catheter-Related Infections, this term only #47 catheter* near3 infect* #48 catheter* near3 microb* #49 (catheter* and blood and infection*):ti,ab #50 (“CR-BSI” or “CR-BSIs” or CRBSI or CRBSIs or CABI or CABIs or CABSI or CABSIs or CLABSI or CLABSIs or CRI or CRIs or BSI or BSIs or “AC-CRI “ or “AC-CRIs”) #51 (#46 OR #47 OR #48 OR #49 OR #50) #52 MeSH descriptor Education explode all trees #53 MeSH descriptor Inservice Training explode all trees #54 MeSH descriptor Training Support, this term only #55 (awareness or bundle* or collaborat* or campaign* or communicat*):ti,ab #56 (feedback or “feed back” or “feeding back” or course* or instruct* or inform* or impart* or knowledge or learn* or “e-learn” or “e-learning” or lecture*) #57 (module* or modular or message* or session* or curriculum* or evaluat* or seminar* or test* or teach* or taught or train* or simulat* or refresh* or tool* or meeting* or presentation* or skill* or drill* or workshop* or outreach) #58 (booklet* or workbook* or checklist* or library or libraries or questionnaire* or sheet* or pamphlet* or poster* or pictorial* or verbal* or video* or audiovisual* or podcast or telemedicine or teleconferenc* or symposia or symposium) #59 change next management #60 ((change or changing) near (process or procedure)) #61 early next warning next system* #62 (“scrub the hub” or “Matching Michigan” or “Michigan project” or “Michigan Intervention” or “NHS Venous Catheter Care” or EPIC or “EPIC-2” or “saving lives”) #63 educat* #64 (chang* near (hygien* or handwash* or hand wash* or disinfect* or sterilisation or sterilization)) #65 (alter* near (hygien* or handwash* or hand wash* or disinfect* or sterilisation or sterilization)) #66 (management near (contaminat* or hygien* or handwash* or hand wash* or disinfect* or sterilisation or sterilization)) #67 (precaution* near (hygien* or handwash* or hand wash* or disinfect* or sterilisation or sterilization)) #68 prevent* near infect* #69 (quality near (strateg* or intervention* or management or initiative*)) #70 (risk near (management or prevention or intervention*)) #71 (precaution near infect*) #72 (#52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71) #73 (#9 AND #28 AND #45 AND #72) #74 (#9 AND #51 AND 72) #75 (#73 OR #74) #76 (“cost effective*” or “cost benefit*” or “cost saving*” or “cost analys*” or “cost utili*” or “cost mimimi*” or “cost consequence*” or “cost comparison*” or “cost identificat*”):ti,ab,kw #77 (cost* or economic* or pharmacoeconomic*):ti #78 (“health economic*” or “healthcare cost*” or “health care cost*” or “economic evaluation*”):ti,ab,kw #79 (economical):ti,ab #80 MeSH descriptor Costs and Cost Analysis explode all trees #81 MeSH descriptor Cost-Benefit Analysis explode all trees #82 MeSH descriptor Models, Economic, this term only #83 MeSH descriptor Economics, Hospital explode all trees #84 MeSH descriptor Economics, Medical explode all trees #85 MeSH descriptor Economics, Nursing explode all trees #86 MeSH descriptor Health Care Costs explode all trees #87 (#76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86) #88 (#75 AND #87) |
50 in total 11 CENTRAL 4 DARE 35 NHSEED 2 CDSR not downloaded not relevant) Updated 21 March 2012: 1 Cochrane HTA 4 Cochrane CDSR |
| CRD Searched 23 February 2011 |
# 1 ( “intensive care” OR “critical care” OR “critical illness” OR “critically ill” ) # 2 MeSH Critical Care EXPLODE 1 2 # 3 MeSH Intensive Care EXPLODE 1 2 # 4 MeSH Intensive Care Units, Neonatal EXPLODE 1 # 5 MeSH Intensive Care Units, Pediatric EXPLODE 1 # 6 ( ITU OR ICU OR CCU OR CICU OR CITU ) # 7 #1 or #2 or #3 or #4 or #5 or #6 # 8 MeSH Catheterization, Central Venous EXPLODE 1 154 # 9 MeSH Catheterization, Peripheral EXPLODE 1 # 10 MeSH Catheters, Indwelling EXPLODE 1 # 11 ( CVC OR PICC OR JICC OR SICC OR SBCC OR PVC OR IVI OR CVL OR PCVC OR CVAD OR TCVC ) # 12 ( catheter* OR cannul* ) # 13 #8 or #9 or #10 or #11 or #12 # 14 MeSH Bacterial Infections EXPLODE 1 # 15 MeSH Bacteremia EXPLODE 1 2 3 # 16 MeSH Sepsis EXPLODE 1 2 # 17 MeSH Asepsis EXPLODE 1 3 # 18 MeSH Fungemia EXPLODE 1 2 3 # 19 ( infection* OR acinetobacter* OR asepsis OR bacter?emia* OR bacteria* OR candida OR coloni?ation OR contaminat* OR cfu OR colony OR colonies OR corynebacterium OR escherichia OR enterococc* OR enterobacter* OR fungi OR fungus OR fungal OR fung?emia OR klebsiella OR met?icillin OR microorganism* OR micro-organism* OR microbial* OR microbe* OR microbiologic* OR mycology OR mycological OR organism* OR nosocomial* OR pathogen* OR sepsis OR septic OR septic?emia AND septicemia OR staphylococc* OR streptococc* ) # 20 ( HAI OR HAIs OR “HAI's” OR HCAI OR HCAIs OR “HCAI's” ) # 21 ( MRSA OR MSSA ) # 22 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 # 23 ( “CR-BSI” OR “CR-BSIs” OR CRBSI OR CRBSIs OR CABI OR CABIs OR CABSI OR CABSIs OR CLABSI OR CLABSIs OR CRI OR CRIs OR BSI OR BSIs OR “AC-CRI “ OR “AC-CRIs” ) # 24 ( catheter AND related AND infection* ) # 25 ( line AND ( infection* OR sepsis OR bacter?emia ) ) # 26 ( catheter* AND ( sepsis OR septic OR bacter?emia* ) ) # 27 #23 or #24 or #25 or #26 # 28 ( educat* OR train* OR teach* OR feedback OR bundle OR course* OR learn* OR lecture* OR workshop* ) # 29 ( component* OR “multi-component” OR “multi-faceted” OR “multi-modal” OR initiative* OR intervention* OR program* OR strateg* ) # 30 ( booklet* OR checklist* OR library OR libraries OR literature OR questionnaire* OR sheet* OR pamphlet* OR poster* OR pictorial* OR verbal* OR video* OR audiovisual* OR podcast OR telemedicine OR teleconferenc* OR workbook ) # 31 ( “scrub the hub” OR “Matching Michigan” OR “Michigan project” OR “Michigan Intervention” OR “NHS Venous Catheter Care” OR EPIC OR “EPIC-2” OR “saving lives” ) # 32 #25 or #26 or #27 or #28 or #29 or #30 or #31 # 33 7 AND 13 AND 22 AND 32 # 34 7 AND 27 AND 32 # 35 #33 or #34 # 36 #2 OR #3 OR #4 OR #5 # 37 #13 AND #22 AND #32 # 38 #27 AND #32 AND #36 # 39 #37 OR #38 # 40 #13 AND #22 AND #32 AND #36 # 41 #38 or #40 # 42 ( catheter* AND educat* AND intensive AND unit* ) # 43 ( catheter* AND educat* AND critical AND unit* ) # 44 #41 or #42 or #43 |
38 Updated 21 March 2012: 36 |
Appendix 3 Study inclusion worksheet for titles and abstracts
Selection criteria worksheet to be used on titles and abstracts
Educational interventions for preventing catheter-related bloodstream infections in critical care
| Lead author name and ref. ID number: | |||
|---|---|---|---|
| Population Patients in critical carea with vascular catheter(s)b | Yes | Unclear | No |
| ↓ | ↓ | → | |
| Next question | Next question | EXCLUDE | |
| Design Interventional study, primary research | Yes | Unclear | No |
| ↓ | ↓ | → | |
| Next question | Next question | EXCLUDE | |
| Intervention Educational interventionsc with an objective to reduce or prevent CRBSIs | Yes | Unclear | No |
| ↓ | ↓ | → | |
| Next question | Next question | EXCLUDE | |
| Outcomes BSIs, or mortality associated with, related to, or suspected to result from catheter use.d | Yes | Unclear | No |
| ↓ | ↓ | → | |
| Next question | Next question | EXCLUDE | |
| Final decision | INCLUDE | UNCLEAR (discuss) | EXCLUDE |
Appendix 4 Keyword tool for evidence map study classification
1. Study identification
| Reference ID: | Reviewer: |
| Lead author: | |
| Publication year: | |
| Intervention implementation date(s): | |
| Country (if USA, also the state): Recording the state here (USA studies only) helps us to identify linked studies. Write ‘multistate’ if more than one, or ‘state not reported’ if appropriate |
|
| No. of critical care units: | |
| No. of centres (specify if not hospitals): ‘Centres’ here refers to the hospitals in which critical care units are sited (not the departments within hospitals, or clusters of hospitals within administrative regions). Please write the type of unit here if not a hospital. |
|
| If one centre only, name of centre: This information helps us to identify linked studies (it will not be systematically collected for evidence mapping). |
|
2. Critical care specialties
| Cardiac | If ‘Other’ is selected please write the type(s) of critical care here (this will be used to update the list) | |
| Medical | ||
| Neonatal | ||
| Neurological | ||
| Paediatric | ||
| Surgical | ||
| Trauma | ||
| Mixed | ||
| Other | ||
| Unclear |
3. Vascular devices
| Device type(s) reported | Device type refers to what type of catheter it is (e.g. CVC, PICC) | |
| Insertion site(s) reported | Insertion site refers to the specific blood vessel in which the catheter is inserted | |
| Lumen material reportedsww | Lumen material refers to what the catheter tubing is made of | |
| Lumen coating reported | Lumen coating refers to any chemical coating or impregnation of the catheter, e.g. catheters with antimicrobial or anticoagulant surfaces | |
| Lumen no. and use reported | The number of catheter lumens can affect risk of CRBSI (unused lumens can act as a conduit for microorganisms). Select this option only if the use of all reported lumens is explained | |
| Device changes reported | Changing catheters can increase risk of infections and other complications (e.g. pneumothorax). Select this option if any information about device changes (e.g. number, frequency, interval or reasons for changes) is reported | |
| Device purpose(s) stated | Device purpose refers to what the catheter(s) lumen(s) are used for (e.g. drug provision, nutrition provision, haemodynamic monitoring or haemodialysis) | |
| None of the above reported | This is a required response if none of the other options applies, to help ensure that you have systematically checked the paper and not inadvertently missed information |
4. Device placement
| Precritical care | Patients arrive in critical care with vascular catheters already in situ | |
| In critical care unit | Vascular catheters are placed while patients are in the critical care unit. Select both options if catheters are placed both before and during critical care stay | |
| Unclear |
5. Concurrent invasive devices
| Urinary catheter | |
| Other non-vascular | |
| None | |
| Unclear |
6. Population information
| Sample size: patients | This refers to the number of patients for whom relevant outcome data are presented. It may or may not reflect the number of critical care beds | |
| Sample size: critical care staff | This refers to the number of critical care staff who receive the relevant intervention(s) (not necessarily the same as the number of critical care staff) | |
| Attrition: patients | ||
| Attrition: critical care staff | ||
| None of the above reported | This is a required response if none of the other options applies, to help ensure that you have systematically checked the paper and not inadvertently missed information |
7. Length of follow-up (months)
| Insert a number, or state if follow-up timing is not reported or unclear. |
8. Type of intervention
-
A ‘bundle’ is defined as two or more interventions that are implemented together and aim to influence the same outcome(s).
-
An ‘initiative’ refers to two or more interventions delivered together or in a linked way such that the structure and process may appear rather complex and does not clearly fit the description of a bundle. Initiatives may include additional components in support of intervention implementation (e.g. provider collaboration strategies).
| Education alone | Select this option if the intervention is purely educational | |
| Single intervention with education component(s) or support | Select this option if there is a single intervention that contains a mix of educational and non-educational components, or a single non-educational intervention supported by education provided separately (i.e. education that supports the intervention but is not part of it) | |
| Bundle or initiative with discrete education component(s) | Select this option if a relevant bundle or initiative to prevent BSIs is reported and:
|
|
| Bundle or initiative with unspecified/unclear education component(s) | Select this option if a relevant bundle or initiative to prevent BSIs is reported and education is provided to support the bundle or initiative, but it is unclear to which part(s) of the bundle or initiative the education relates | |
| Unclear | Select this option if none of the above options can be ascertained (e.g. owing to inadequate or ambiguous reporting) |
9. Aim of intervention
| Specifically to prevent CRBSI | |
| Other |
10. Study design
Select one from ‘a’ to ‘j’; select ‘k’ if appropriate.
This classification is consistent with chapter 13 of the Cochrane Handbook. 58
| a. Controlled trial (including RCT, quasi-RCT, non-randomised) | A study with two or more parallel groups recruited simultaneously and matched for population characteristics, at least one of which receives a relevant intervention and at least one of which acts as a relevant comparator (e.g. no-intervention control group). As we expect to find relatively few controlled trials, this option covers both randomised and non-randomised designs | |
| b. ITS (controlled or uncontrolled) | A study that uses observations at multiple time points before and after an intervention (the ‘interruption’). The design attempts to detect whether the intervention has had an effect significantly greater than any underlying trend over time. There is no clear consensus on how many measurements are required in order to classify the design as an ITS (as the number and spacing of measurements is determined by the hypothesis of individual studies). Only select this option if the study authors call the design an ITS | |
| c. Controlled cohort before-and-after study (CchBA) – prospective | A study with two or more separately-recruited concurrent groups (cohorts), at least one of which receives a relevant intervention, and at least one of which acts as a relevant comparator (e.g. no-intervention control group). A ‘prospective’ controlled cohort study recruits participants before any intervention and follows them into the future | |
| d. Controlled cohort before-and-after study (CchBA) – retrospective | A study with two or more separately-recruited concurrent groups (cohorts), at least one of which receives a relevant intervention and at least one of which acts as a relevant comparator (e.g. no-intervention control group). A ‘retrospective’ controlled cohort study identifies subjects from past records describing the interventions received and follows them from the time of those records | |
| e. Controlled cohort before-and-after study (CchBA) – unclear | A controlled cohort before-and-after study for which it is unclear whether the study was done prospectively or retrospectively | |
| f. Cohort before-and-after study (ChBA) – prospective | A study on a single group, which is monitored before and after a relevant intervention is implemented. A ‘prospective’ cohort study recruits participants before any intervention and follows them into the future | |
| g. Cohort before-and-after study (ChBA) – retrospective | A study on a single group, which is monitored before and after a relevant intervention is implemented. A ‘retrospective’ cohort study identifies subjects from past records describing the interventions received and follows them from the time of those records | |
| h. Cohort before-and-after study (ChBA) – unclear | A cohort before-and-after study for which it is unclear whether the study was done prospectively or retrospectively | |
| i. Historically controlled study | A study that compares a group of participants who received an intervention with a similar group from the past who did not | |
| j. Design unclear | ||
| k. Incremental or phased implementation of intervention(s) | This option identifies studies in which parts of an intervention are implemented at different times, or different interventions are introduced in a staggered or sequential fashion. It can apply to different study designs. Please select the study design above |
11. Concurrent interventions
| Yes | Concurrent interventions are interventions that overlap in time with the educational intervention of interest. Examples include simultaneous or overlapping implementation of interventions for CRBSI, VAP, pressure ulcers, sepsis, or glycaemic control. This option identifies studies in which confounding of interventions might be a problem | |
| No | ||
| Unclear |
12. Intervention appraisal
| Evidence base specified | We are seeking any justification given in the paper for why this intervention is being applied in this population and setting. A statement merely that the intervention is ‘evidence-based’ would not be acceptable as a justification unless supported by relevant citations | |
| Educational theory specified | ||
| Previous development and/or testing reported | This refers only to previous primary research that has evaluated this intervention or its precursors. If only secondary research is available to justify the use of this intervention, select ‘Evidence base specified’ instead | |
| Facilitators reported | Facilitators are variables measured in the study that could contribute to the intervention working as (or better than) intended (i.e. at preventing CRBSI). Examples could include positive attitudes, good compliance, possession of relevant clinical skills, or high intervention implementation fidelity | |
| Barriers reported | Barriers are variables measured in the study that could contribute to the intervention not working as intended (i.e. not preventing CRBSI). Examples could include negative attitudes, poor compliance, lack of relevant clinical skills, high staff turnover, or low intervention implementation fidelity | |
| Other process evaluation | A process evaluation seeks to establish why an intervention works (or does not). By definition, analysis of facilitators and barriers is part of process evaluation. ‘Other process evaluation’ refers to any aspects of evaluating why the intervention works (or does not) that do not clearly fit under the headings of facilitators or barriers | |
| None of the above reported | This is a required response if none of the other options apply, to help ensure that you have systematically checked the paper and not inadvertently missed information |
13. Education messages (targets)
| Antimicrobial use | ||
| Barrier precautions – full | ||
| Barrier precautions – specific (e.g. drapes, gloves, gown) | ||
| Catheter type selection (e.g. lumen number, chemical impregnation) | ||
| Catheter placement procedure (e.g. use of ultrasound or X-ray; avoidance of guidewires) | ||
| Catheter insertion site selection (e.g. avoidance of femoral site) | ||
| Catheter insertion site preparation (e.g. chlorhexidine) | ||
| Catheter ongoing management (e.g. flushing; hub care, including ‘scrub the hub’) | ||
| Catheter need review (e.g. daily inspection; removal of unnecessary catheters) | ||
| Checklist use [i.e. education on how to use checklist(s)] | ||
| Central line cart use | ||
| Documentation and auditing of processes | ||
| Dressing selection (e.g. antimicrobial bioptach) | ||
| Dressing care (hygiene, removal, replacement) | ||
| Hand hygiene | ||
| Infection prevention and/or control (including evidence-based practice; guidelines) | ||
| Micro-organism epidemiology | ||
| Nurse empowerment (in critical care or infection prevention) | ||
| Team approach to catheter care | ||
| Other | ||
| Unclear |
14. Type(s) of education
| Checklist with factual information | ||
| CD or DVD | ||
| Device training | ||
| Discussion group | ||
| Fact sheet | ||
| Goal sheet | ||
| In-service training | ||
| Lecture | ||
| Mentor led | ||
| Peer led | ||
| Poster | ||
| Practical demonstration | ||
| Residential course | ||
| Skills practice | ||
| Self-study module | ||
| Simulation based | ||
| Slide show | ||
| Supervision | ||
| Website | ||
| Workshop | ||
| Written material | ||
| Unclear component(s) |
15. Education delivery
| Providers specified | The provider is the person or technology (e.g. computer) delivering the intervention (i.e. the communicator of the information provision or practical skills training) | |
| Recipients specified | The recipient is the staff member (or group) within the critical care unit to whom the educational intervention is directed | |
| Neither of the above reported | This is a required response if none of the other options applies, to help ensure that you have systematically checked the paper and not inadvertently missed information |
16. Education contextual support
| Performance feedback (including assessment/testing) | Performance feedback refers to the provision of feedback to the person or group receiving the educational intervention on how well they are progressing with the intended learning outcomes. This includes tests or assessments (e.g. of knowledge, behaviour or skills) | |
| Infection surveillance feedback | Infection surveillance feedback is any process whereby real-time or recent information on CRBSI infection rates is provided in support of an educational intervention. The information may be made available to intervention providers and/or recipients |
17. Duration of education
| Fully reported | Select this option if all educational components associated with the intervention(s) are reported sufficiently well that the total amount of time needed to implement the education can be ascertained (e.g. total number of hours required for lectures, tests and practical skills training sessions) | |
| Partially reported | Select this option if there are several educational components associated with the intervention(s) but the duration is only reported for some of them | |
| Unclear | Select this option if the duration is not reported for any educational components associated with the intervention(s), or is reported but unclear |
18. Bloodstream infections outcomes reported
| Catheter related (CRBSI) | Select this option if catheter- or central line-related BSIs are reported, irrespective of how they are defined | |
| Catheter associated (CABSI, CLAB, CLABSI) | Select this option if catheter- or central line-associated BSIs are reported, irrespective of how they are defined | |
| Catheter suspected BSI | Select this option if catheter- or central line-associated BSIs are reported, irrespective of how they are defined | |
| Other or unclear | Select this option if outcomes do not match any of the above categories |
19. Bloodstream infections outcome units
| Rate/1000 device-days | ||
| Rate/no. of admissions | ||
| Rate/no. of patient-days | ||
| Relative risk reduction | ||
| Absolute risk reduction | ||
| Number needed to treat | ||
| Infections prevented | ||
| Percentage change infection rate | ||
| Incidence rate ratio |
20. Secondary outcomes
| Attitudes | ||
| Compliance | ||
| Knowledge | ||
| Skills |
21. Other outcomes
| Mortality | Select this option if any type of mortality is reported (e.g. catheter related, BSI related, all cause) | |
| Adverse events | Select this option if adverse events due to catheter use and/or BSIs are reported | |
| LOS | Select this option if any LOS is reported (critical care or total hospital stay) |
Appendix 5 Data extraction forms for clinical effectiveness studies
BURRELL (2011)83
Methods
Study characteristics
| Lead author, publication year(s) and reference ID(s) | Burrell (2011);83 CEC (2010)146 |
| Summary of approach | A state-wide collaborative QI programme (CLAB ICU project) in Australia based on the principles of the Michigan Keystone ICU project, with top-down and bottom-up drivers of change |
| Location | Australia: New South Wales |
| Language | English |
| Critical care specialty | Not specified other than ICUs were primarily adult (2/37 units were paediatric) |
| No. of critical care units | 37 ICUs (10 tertiary, 12 metropolitan, 13 rural, 2 paediatric) |
| No. of hospitals | Not reported |
| Study design | Variant of a cohort before-and-after study in which true pre-intervention data were not collected but instead the first 12 months of intervention were considered to be the baseline (‘run-in’ period) |
| Study time periods | Pre-intervention: No data reported Total duration of intervention: 18 months (July 2007 to December 2008)
Uptake of the project was staggered, with a small (unspecified) percentage of units late to start146 |
| Funding source | New South Wales Department of Health provided funding for the CEC to implement and support the project |
| Conflicts of interest | Stated that none were identified |
Population and setting
| Critical care unit characteristics |
|---|
| Not reported. General information on the characteristics of most of the participating ICUs including staffing and bed number is available in cited surveys194,195 but the survey data cover the whole study period, not discriminating the ‘run-in’ and ‘analysis’ periods. From the surveys it appears that in addition to the two paediatric intensive care units (PICUs), adult units in New South Wales also included some paediatric patients.194 Adult ICUs in New South Wales also appear to have included some high-dependency beds.194 |
Data are from the subset of checklists that reported insertion site (10,850 of 11,575 checklists; 93.7%).
| Population and setting characteristics | Run-in period (months 1–12) (24 critical care units in month 1) | Analysis period (months 13–18) (up to 34 critical care units) | Difference between analysis and run-in periods |
|---|---|---|---|
| Patient population characteristics | Not reported | Not reported | Not reported |
| Device characteristics | |||
| Insertion site – jugular | 1119 (33.0%) | 2452 (32.9%) | Overall effect of insertion site p = 0.998 (ridit analysis) |
| Insertion site – subclavian | 999 (29.4%) | 2082 (27.9%) | |
| Insertion site – femoral | 772 (22.8%) | 1875 (25.1%) | |
| Insertion site – cubital fossa | 400 (11.8%) | 809 (10.8%) | |
| Insertion site – other/unknown | 103 (3.0%) | 239 (3.2%) | |
| Total available data for insertion site (93.7% of the checklists that were returned) | 3393 (100%) | 7457 (100%) | |
| Insertion site antisepsis used | Not reported. A project update in an ICCMU newsletter (February 2008) noted that for one-third of central lines in the project database the line coating was not specified | ||
| Dressing type and duration/frequency | Not reported | ||
Intervention characteristics
| Objective | To achieve a measurable reduction of CLAB in New South Wales ICUs. Initially defined by health service agreements as a 20% reduction by January 2008 and a further 80% by January 2010146 |
| Main focus of education | Catheter insertion. Focusing on the preparation of the clinician (hand hygiene, barrier precautions and sterile technique) and patient [skin preparation, patient draping and catheter positioning during insertion (imaging)]. Referred to as a clinician bundle and a patient bundle, respectively |
| Trainers (providers) | CEC and ICCMU promoted the intervention to ICU clinicians. Project governance was provided by a steering committee, with stakeholder representation. The steering committee members and their contributions to meetings during 2007–9 are listed in the final project report.146 ICUs developed improvement teams with physician and nursing representation from existing staff |
| Training of trainers | Not reported |
| Learners (recipients) | ICU staff (‘proceduralists’) who were permitted or being trained to insert CVLs (staff grades not specified) |
| Target behaviour change | Sterile catheter insertion procedure |
| Development and testing | A multidisciplinary expert group was convened to develop a guideline for CVL insertion based on existing guidelines. A checklist including the ‘patient bundle’ and the ‘clinician bundle’ was developed to support the compliance with the guideline and to collect data. The intervention was based in principle on the Michigan Keystone ICU project intervention. As a QI initiative, some aspects of development and testing ran concurrently with implementation |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Principal focus on catheter insertion guidelines supported by an insertion checklist and awareness campaign. The guideline, checklist, training framework, posters, newsletters and data collection document are available at: www.cec.health.nsw.gov.au/programs/clab-icu#resources2 (accessed October 2012) Evidence-based percutaneous catheter insertion guideline: Emphasised importance of hand hygiene, skin preparation and full barrier precautions (displayed prominently in some ICUs) Checklist: Developed and revised based on feedback from learning sessions. To facilitate data collection and ensure compliance with the guideline. Observer empowered to stop procedure if a significant breach of aseptic technique by the proceduralist. Check items were: staff competency; hat, mask and protective eyewear, hand hygiene, sterile gloves and gown, skin preparation with alcoholic chlorhexidine; full patient draping with sterile sheets; maintenance of sterile technique throughout procedure; documentation of guidewire removal; securing and dressing catheter; confirmation of catheter vascular position; complications. Education and training workshops: Two large initial workshops in June 2007 (introduction and forging links) and November 2007 (feedback and sharing initial successes). Included open discussion of barriers to implementation and suggestions for improvement. Subsequently, smaller workshops were held to develop standard education and training approaches and an e-learning package. Some ICUs had weekly or biweekly multidisciplinary meetings to review CLABs (e.g. as reported in November 2008 ICCMU newsletter) Training framework (developed and improved during October 2007 to August 2008): Defining the minimum knowledge and practical skills required for CVC insertion, with catheter insertion competence assessed under supervision and documentation of feedback and learning needs in a logbook. Supported by a 1-page training support tool listing 56 competence/knowledge criteria Posters of three types: (1) explained the definition of CLAB; (2) explained the process for confirming CLAB including need for multidisciplinary team consultation; (3) bullet point reminders of appropriate site selection; hand hygiene; aseptic technique; maximal barrier precautions; securing and dressing of catheter; and daily assessment of need for catheter. Data collection document: Provided an explanation of CLAB definitions; emphasised that collection and submission of CLAB data are a multidisciplinary activity involving intensive care, microbiology and infection control practitioners Unstructured teleconferences: Monthly for first year then bimonthly, held by the project co-ordinator Electronic updates: Articles in bimonthly newsletters of the ICCMU, Listserv circulars and ad hoc e-mails from the project team to participating sites |
| Infection surveillance feedback approach | Checklists were submitted by ICUs to the CEC project co-ordinators who prepared and disseminated surveillance reports to ICUs as they were received. The project team conducted data integrity checks and followed up missing or improbable data, and confirmed the validity of reported CLABs with individual ICUs. Outliers were identified based on CLAB numbers or incidence rates, with identified ICUs subjected to additional analyses to provide insight into causes and to promote improvement |
| Performance feedback approach | Monthly reports of compliance with the patient bundle and clinician bundle were provided to ICUs, starting in October 2007. Reports were also issued monthly to area health service clinical governance units and quarterly to New South Wales Health. Checklist submission was used as a proxy for participation. Validation and reliability of the reporting process not stated Within the training framework undertaken by new or unqualified staff, proceduralists were supervised before performing independent CVL insertions. The supervisor recorded feedback and learning needs in a log book |
| Concentration of education | Owing to the complex nature of the collaborative intervention, in which the methods adopted varied among the participating ICUs and were continuously implemented, the precise frequency and duration of all education activities cannot be established. However, sufficiently detailed information is given (if the online supporting documents are consulted) to allow numerous aspects of the programme to be repeated or adapted (including the guideline, checklist, posters and training framework). The frequency and type of meetings required is largely discernible from the information given |
| Non-educational intervention components | Standard sterile equipment pack made available for use across all New South Wales adult ICUs; staff empowerment to halt procedure |
| Costs reported | Yes. Detailed costs of the intervention for 2008 are given in an appendix to the project report |
Outcome characteristics
| Catheter-BSI definition Reference cited: O'Grady et al.196 |
Based on New South Wales Department of Health 2005 and CDC surveillance definitions for CLAB (reference cited), except that only CLABs occurring in patients in the critical care unit or within 24 hours of transfer out were recorded
|
| Outcomes reported | Not referred to as primary or secondary, but stated that the main outcomes were compliance with aseptic CVL insertion and rates of CLAB CLAB incidence: per 1000 central line-days; % change No. of catheters used Catheter dwell time Compliance with clinician bundle Compliance with patient bundle Adverse events (retained and broken guidewires) (data not extracted by reviewers) |
Results data
Primary outcomes
Data are from the subset of checklists that reported central venous line (CVL) type (10,890 of 11,575 checklists; 94.1%). Date of discharge from ICU was used as a proxy for date of CVL removal if a CVL was still present at discharge. Simultaneous CVLs in the same patient were counted as a single device.
| Outcome | Baseline (run-in period) (months 1–12) (24 critical care units in month 1) | Intervention (analysis period) (months 13–18) (up to 34 critical care units) | Difference between baseline (run-in) and intervention (analysis) periods |
|---|---|---|---|
| Device utilisation | |||
| CVLs used | |||
| Centrally inserted | 2432 | 5289 | Statistical comparison not reported |
| Peripherally inserted | 475 | 992 | |
| Dialysis | 397 | 865 | |
| Other and unspecified | 43 | 82 | |
| Total | 3347 | 7228 | |
| Total (range) line duration, days* | |||
| Centrally inserted | 13,174 (1–58) | 29,331 (1–104) | Statistical comparison not reported |
| Peripherally inserted | 1352 (1–37) | 3365 (1–242) | |
| Dialysis | 2215 (1–130) | 4434 (1–52) | |
| Other and unspecified | 210 (1–25) | 290 (1–21) | |
| Total | 16,951 (1–130) | 37,420 (1–248) | |
| *Includes only inserted CVLs for which data on CVL type and line-days were available | |||
| Median (IQR) in situ period, days | |||
| Centrally inserted | 8 (8) | 8 (8) | Statistical comparison not reported |
| Peripherally inserted | 6 (10) | 8 (15) | |
| Dialysis | 9 (9) | 8 (8) | |
| Other and unspecified | 8 (10) | 6 (10) | |
| Total | 8 (8) | 8 (8) | |
| Device-days (not reported; e-mailed by author) | First quarter 9308 | Second quarter 7684 | |
| Third quarter 9634 | |||
| Fourth quarter 9725 | |||
| Fifth quarter 9589 | |||
| Sixth quarter 8773 | |||
| No. of devices/patient | Not reported | Not reported | Not reported |
| CLAB incidence rate | Not reported | Not reported | Not reported |
| CLAB incidence per 1000 catheter-days:(a) reported; (b) e-mailed by the author | (a) First quarter 3.0 (95% CI 2.0 to 4.3)a | (a) Sixth quarter 1.2 (95% CI 0.6 to 2.2)a | (a) 60% reduction; p = 0.0006; χ2 of slope = 11.71 |
| (b) First quarter 3.0 | (b) Second quarter 2.6 | ||
| (b) Third quarter 1.3 | |||
| (b) Fourth quarter 1.9 | |||
| (b) Fifth quarter 1.0 | |||
| (b) Sixth quarter 1.3 | |||
| CLAB incidence per 1000 patient-days | Not reported | Not reported | Not reported |
| LOS | Not reported | Not reported | Not reported |
| Mortality | Not reported | Not reported | Estimated only, not measured |
Secondary outcomes
| Outcome | Baseline (run-in period) (months 1–12) (24 critical care units in month 1) | Intervention (analysis period) (months 13–18) (up to 34 critical care units) | Difference between baseline (run-in) and intervention (analysis) periods |
|---|---|---|---|
| Reaction to education | Not a study outcome | ||
| Attitudes | Not a study outcome | ||
| Compliance with clinician bundle | 74% (reported for a quarter but unclear which one) | 81% (for last quarter of project) | 7% improvement; p < 0.0001; χ2 of slope = 118.83 |
| Compliance with patient bundle | 81% (reported for a quarter but unclear which one) | 92% (for last quarter of project) | 11% improvement; p < 0.001; χ2 of slope = 108.34 |
| Compliance with both bundles | Not reported | Not reported | 1.4 times more likely in analysis period; p = 0.0001; χ2 of slope = 14.325 |
| Knowledge | Not a study outcome | ||
| Skills | Not a study outcome | ||
| Process evaluation Internal evaluation revealed:83
Attendance at teleconferences by expert group and month was recorded and reported146 The following potential or actual barriers to implementation were reported, but not quantified:146
|
|||
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NOT REPORTED |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? NOT REPORTED. No state-wide changes in related health policy or practice concurrent with the intervention were mentioned by the authors but it was not explicitly stated that such changes did not occur. However, although most ICUs formed improvement teams, there was variable engagement of key personnel |
| Was the effect of educational practice separable from effects of non-educational practice? NO. Although the intervention was primarily based on educational approaches, other non-education components were included, notably a standard sterile equipment pack made for use across all New South Wales adult ICUs Were the intervention component(s) implemented as planned? PARTIALLY. Reported all-or-nothing compliance with patient and clinician bundles improved from baseline and reached 81% and 92%, respectively. However, difficulties were encountered regarding data collection and associated information technology such that the project team resorted to hard copy receipt of checklists in October 2007 and manual data entry in August 2008 |
|
| Missing data | Were there systematic differences between the baseline and intervention groups in attrition or exclusions (includes withdrawal due to mortality; missing outcome data)? UNCLEAR. Device utilisation data differed between the two study periods but it is unclear whether this reflects real differences in device utilisation and/or differences in checklist returns |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? UNCLEAR. This intervention encouraged standardisation of how outcomes were defined, detected and reported so it is difficult to identify possible bias in outcome measurement |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | Self-reporting bias: some checklists were filled in by the proceduralist, as assistance was not always available. Authors stated that as this was a QI initiative and not a study, these factors could not be controlled. Data on CVL type and line-days not available for all CVLs – unclear whether this is a possible source of bias as reasons for data non-availability not given |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? YES |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. Stated that data entry was manual, with an infection nurse checking data on each form. Collected data were received and collated by the CEC | |
| If YES or PARTIALLY, was the data collection process shown to be valid? PARTIALLY. Authors state that missing and invalid data were followed up and validity of reported CLAB were confirmed with individual ICUs. However, a quality test to review data capture was not completed by all sites and at least one tertiary unit had low data capture | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NO. Many ICUs did not have microbiological support and reported CLAB with discussion with senior ICU staff. Improved understanding of surveillance definitions vs. clinical definitions led to some CLABs cases to be reclassified | |
| Statistical tests described? PARTIALLY. Not all outcome analyses (e.g. ridit) were reported in the methods section | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? PARTIALLY. Some aspects of catheter insertion procedure were complied with more extensively than others. Changing sterile hat-wearing behaviour seemed to be a particular obstacle |
Additional comments
-
The project involved collaboration between the New South Wales Department of Health, the NSW Clinical Excellence Commission (CEC), ICCMU and individual ICUs.
-
Following the project, an independent external project evaluation was conducted by the Centre for Clinical Governance Research University of New South Wales. This led to seven key recommendations for the operation of similar collaborative projects.
-
The data reported above are from the primary research publication,83 final project report146 and extensive online material associated with the project: a catheter insertion guideline, checklist, training framework, posters, data collection document, and newsletters, available at: www.cec.health.nsw.gov.au/programs/clab-icu#resources2 (accessed 9 October 2013).
COOPERSMITH (2002)50
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Coopersmith (2002)50 |
| Summary of approach | Single unit, education initiative to decrease the primary BSI rate, based on a self-study module aimed primarily at full-time ICU nursing staff (to provide continuity given the rotation of residents and fellows in the ICU) |
| Location | USA, Missouri |
| Language | English |
| Critical care specialty | Surgical/burn/trauma Intensive Care Unit (ICU) |
| No. of critical care units | 1 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Barnes-Jewish Hospital, St Louis, MO, USA |
| Study design | Single cohort before-and-after study |
| Study time periods | Baseline: January 1998 to June 1999 (18 months) Intervention: July to December 1999 (6 months) Follow-up: none (only intervention monitored) |
| Funding source | Stated that work was supported in part by funding from the Centers for Disease Control and Prevention Cooperative Agreement, the BJC Hospital Epidemiology and Infection Control Consortium and the National Institutes of Health |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics University-affiliated teaching hospital with 18 ICU beds, with an average of 1400 patients admitted per year Mean LOS: 3.8 days Nurse to patient ratio 2 : 1, but with very high acuity levels may be 1 : 1 (at the discretion of the charge nurse) Clinical practice: nursing staff have primary responsibility for catheter maintenance and function as bedside assistants when physicians place CVCs. CVCs placed throughout the length of the study were inserted by residents from the departments of surgery (PGY-1, PGY-2), anaesthesiology (PGY-2, PGY-3) and emergency medicine (PGY-2) as part of their 4 - to 6-week rotation through the ICU. CVC placement was supervised by surgery, anaesthesiology or pulmonary critical care fellows Use of antibiotics and antiseptics: Not reported Stated that nursing and technician staffing patterns were similar throughout the length of the study |
|||
| Patient population characteristics (not stated whether mean or median) | Baseline | Intervention | Difference between baseline and intervention |
|---|---|---|---|
| Patient census per month | 121.6 | 115.6 | Statistical significance of acuity score not mentioned; stated all other p-values were not statistically significant |
| Bed occupancy, % | 85.8 | 83.7 | |
| Ventilated patients per month | 68.3 | 66.3 | |
| Length of mechanical ventilation, days | 2.5 | 2.8 | |
| Patient acuity score (average of 36-indicator Medicus system) | 2.9 | 2.9 | |
| Male, % | 59.8 | 55.3 | |
| Weight, kg | 78.0 | 80.2 | |
| CVCs placed per month | 40.2 | 40.4 | |
| Device characteristics | Devices reported as CVC; PICCs were excluded from analysis. Chlorhexidine and silver sulfadiazine-impregnated catheters were inserted when patients were clinically judged to need four CVC lumens for access purposes (around 1–2%). Quadruple-lumen, antibiotic-impregnated catheters were used pre and post interventions but accessibility was ‘specifically limited’ after the implementation of the education | ||
| Insertion site antisepsis used | Not reported | ||
| Dressing type and duration/frequency | Not reported | ||
Intervention characteristics
| Objective | To determine whether an education initiative aimed at improving CVC insertion and care in a surgical/burn/trauma ICU can decrease the rate of primary BSIs |
| Main focus of education | Detailed module covering CVC insertion and maintenance, and prevention of primary BSI; aimed primarily at nurses |
| Trainers (providers) | Not applicable for self-study module; providers of in-service and lectures not reported |
| Training of trainers | Not reported |
| Learners (recipients) | All registered nurses in the ICU [the self-study module and tests were mandatory – but note that compliance (reported below) was not 100%]. All new nurses hired after 1/7/1999 were required to complete the study module and post test but no critical care fellow hired after this date took part in the programme; no residents placing CVCs participated in the full education module. |
| Target behaviour change | Multiple hygienic practices associated with catheter insertion and maintenance |
| Development and testing | Comparison of hospital policy with CDC recommendations on insertion and care of CVCs by a multidisciplinary task force (a physician and infection control practitioners) consisting of representatives from nine hospitals in the Barnes-Jewish-Christian Health System (13 acute-care hospital located in the St Louis area). Nurses in the ICU completed a 17-question survey about their own CVC care practice and a 13-question observation survey on physician practice that they witnessed during CVC insertion. Based on the information obtained, the task force designed an education module to improve practices related to CVC insertion and care. No pilot testing of the module was reported |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Ten-page self-study module: topics included epidemiology and scope of the problem, risk factors, aetiology, definition and methods to decrease risk. Risk factors specifically addressed included length of both hospitalisation and catheterisation, colonisation of insertion site and hub, and anatomical location of CVC placement. Specific risk reduction strategies addressed included hand washing and aseptic technique; methods for detecting potential clinical signs and symptoms of local infection; technique for sending catheter tip culture; routine catheter site care; replacing administration sets and fluids; cleaning and changing injection ports and Luer-lock caps; how to handle parenteral fluids and multidose vials; and procedure for drawing blood cultures. Guidelines covered in the module: included changing injection caps and intravenous tubing for fluids and medications every 72 hours (or immediately if blood accumulated around the cap or its integrity was compromised); replacing transparent line dressings every 7 days or gauze dressings (used solely if bleeding or oozing at the insertion site) every 48 hours; and immediate replacement of dressings that were soiled or no longer occlusive Staff-wide in-service training: implied that staff listened to this; content not reported Six fact sheets and one poster from the study module were displayed in the ICU Lectures: Given to a subset of attending physicians, fellows and a single group of residents rotating through the ICU (content, providers, duration and frequency not reported) |
| Infection surveillance feedback approach | Conducted during both baseline and intervention periods: Monthly updates on the ICU's infection rate and comparisons to NNIS data were presented at staff meetings. |
| Performance feedback approach | Mandatory 20-question examination on knowledge of catheter-related bacteraemia before and after the self-study module and in-service training. Details of the examination topics were provided in a table (data not extracted by reviewers). Nurses who scored < 80% in the post-test were required to repeat the module |
| Concentration of education | Total time for two tests, reading the self-study module and listening to the in-service was around 1 hour (variations between staff mentioned but no further details reported). Staff scoring below 80% had to repeat the self-study module (time taken unclear). Apart for monthly BSI rate updates in the ICU, paper states that no formal programme aimed at reinforcing the material in the education module was required |
| Non-educational intervention components | Quadruple-lumen, antibiotic-impregnated catheters were used pre and post interventions, but accessibility was ‘specifically limited’ after the implementation of the education |
| Costs reported | No. Only a crude estimate of cost saving reported |
Outcome characteristics
| Catheter-BSI definition Reference cited: Pearson197 |
Infections were classified as primary or secondary based upon CDC NNIS definitions (reference cited) Primary BSI (bacteraemia) was defined as (1) recognised pathogen isolated from blood culture not related to infection at another site or (2) fever of > 38.5 °C, chills or hypotension, and either of the following: (a) common skin contaminant isolated from two blood cultures drawn on separate occasions, within 24 hours, unrelated to infection at another site, or (b) common skin contaminant isolated from a blood culture from a patient with an intravascular device and the physician institutes appropriate antimicrobial therapy Secondary bacteraemia was defined as BSI, which develops as a result of a documented infection with the same microorganism at another body site |
| Outcomes reported | Not stated whether primary or secondary: BSIs per 1000 catheter-days; % decrease of BSIs; primary BSIs in 6-month intervals pre and post intervention; total number of catheter-days; number of isolates and % decrease of total isolates; bacteraemia; acuity; compliance; average % correct on pre-intervention test; improvement in test scores; average increase in pre to post-test scores; identical average scores pre and post-test, average decrease scores from pre to post-test Monthly rate per 1000 CVC-days of BSIs from January 1998 to December 2000 displayed in graph format (not data extracted) |
Results data
Primary outcomes
| Outcome | Baseline 2188 patients; 18 months | Intervention 2095 patients; 6 months | Difference between baseline and intervention (statistics not reported unless stated) |
|---|---|---|---|
| Device duration | Not reported | Not reported | |
| Total device utilisation, days | 6874 | 7044 | |
| No. of devices/patient | Not reported | Not reported | |
| Total no. of primary BSI | 74 | 26 | |
| BSI incidence per 1000 catheter-days | 10.8 | 3.7 | 66% decrease; p < 0.0001 |
| BSI incidence per 1000 patient-days | Not reported | Not reported | |
| LOS, days | 3.7 | 4.0 | |
| Mortality | Not reported | Not reported |
Outcomes data for 6-month periods
| Reporting perioda | Infections per 1000 CVC-days (no. of primary BSI) | |
|---|---|---|
| Baseline | Intervention | |
| January to June 1998 | 11.6 (25) (for 2150 CVC-days) | |
| July to December 1998 | 11.9 (28) (for 2355 CVC-days) | |
| January to June 1999 | 8.9 (21) (for 2369 CVC-days) | |
| July to December 1999 | 5.1 (12) (for 2358 CVC-days) | |
| January to June 2000 | 2.4 (6) (for 2455 CVC-days) | |
| July to December 2000 | 3.6 (8) (for 2231 CVC-days) | |
Secondary outcomes
| Reaction to education | Not a study outcome | ||
| Attitudes | Not a study outcome | ||
| Compliance | Overall 52/66 staff (78.8%) completed the entire education module, comprising 49/56 full-time nurses (87.5%); 1/6 attending physicians (17%); and 2/4 critical care fellows (50%); 5 (9%) nurses read the study module and took only the post test 2 (3.5%) nurses read the study module but did not take any tests |
||
| Knowledge, test scores | Baseline | Intervention | Difference between baseline and intervention |
| Average score, % correct ± SD | 78.3% ± 12.9% | 89.9% ± 8.3% | p < 0.0001 (95% CI 7.97 to 15.30) |
| No. (%) with higher score on post test | 36 (69.2%) | Not reported | |
| Average test score increase, % ± SD | 74.0% ± 11.7% to 91.7% ± 6.9% | p < 0.0001 (95% CI 13.8 to 21.5) | |
| No. (%) with identical baseline and post-intervention scores (average score 87% correct) | 12 (23.1%) | Not applicable | |
| No. (%) with decrease on % correct post intervention | 4 (7.7%) | Change in average correct score from 92.5% to 85% | |
| Average score for five nurses who took module and post test only | 86% | Not reported | |
| Skills | Not a study outcome | ||
| Process evaluation | Compliance assessed (data above). No other process evaluation reported | ||
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? UNCLEAR. Patient age was not reported |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? YES. Authors stated that decreases in catheter-related fungaemia from 9 to 0 cases may be a reflection of changes in antibiotic prescribing patterns. The accessibility of quadruple-lumen antibiotic-coated catheters changed during the study; it was ‘specifically limited’ after the implementation of the education, with the authors stating they could not rule this out as a confounding influence |
| Was the effect of educational practice separable from effects of non-educational practice? YES (educational intervention), assuming that the potential confounders noted above would be of negligible importance | |
| Were the intervention component(s) implemented as planned? PARTIALLY. Education was mandatory but compliance was not 100% | |
| Missing data | Were there systematic differences between the baseline and intervention groups in attrition or exclusions (includes withdrawal due to mortality; missing outcome data)? NOT REPORTED |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? YES. The authors stated: Because one definition of infection was ‘common skin contaminant isolated from blood culture from patient with intravascular device and physician institutes appropriate antimicrobial therapy,’ treatment of catheter colonisation could have accounted for a large number of documented infections in the pre-intervention time period. Although the meaning is unclear, it implies a procedural difference occurred between the baseline and intervention periods |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? ICU staff not blinded, no further details reported | |
| Other possible sources of bias | Authors stated that treatment of catheter colonisation could have accounted for a large number of documented infections in the pre-intervention period |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. The content of the self-study module and tests was reasonably well described but did not precisely list the test questions asked. The in-service training and lectures were not described at all. The reported completion time of one hour on average for two 20-question tests, a 10-page self-study module and unspecified in-service training suggests that only superficial coverage of the content could have been achieved |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT APPLICABLE | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT APPLICABLE | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? NOT REPORTED. The authors stated that improvement in test scores led to a change in behavioural pattern but this was based on an assumption, not on measured changes in bedside practice |
Additional comments
-
Monthly infection incidence data per 1000 catheter-days were reported in figure 1 of the primary study (full data not extracted by reviewers). These data indicate high month-to-month variability in incidence rates. During July, August and September 1998 baseline incidence rates were, respectively, about 5, 24 and 12 per 1000 catheter-days (data estimated from graph). During July and August 1999, incidence rates (around 9 per 1000 catheter-days) exceeded some of the monthly incidence rates in the baseline period.
-
No information on what proportion of patients in either time period were completely monitored with regard to BSIs.
-
Unclear whether BSI monitoring was based on blood culture monitoring in response to clinical signs or whether there was routine monitoring of factors, such as catheter tip removal.
-
Unclear how long after the education module the post test was, and unclear how long the knowledge gained persisted.
-
The target nurses were not involved in the development of the intervention that was aimed primarily at them.
-
Rationale of intervention was that educating nurses may have had a knock-on effect on practitioners who inserted CVCs but this was not tested.
-
Very limited, almost no, information on the use of antibiotics and antiseptics.
-
Unclear whether staff were advised of the existence of the study.
-
During development of the intervention the views of physicians who insert CVCs were not included.
-
Coopersmith (2004)87 provides additional follow-up data and suggests that compliance with evidence-based practices was poor following the initial intervention.
COOPERSMITH (2004)87
Methods
Study characteristics
| Lead author, publication year(s) and reference ID(s) | Coopersmith (2004)87 |
| Summary of approach | Single unit behavioural intervention to improve compliance with all facets of best practice of CVC insertions and maintenance (followed, but independent of, a previous intervention reported by Coopersmith (2002)50) |
| Location | USA, Missouri |
| Language | English |
| Critical care specialty | SICU |
| No. of critical care units | 1 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Barnes-Jewish Hospital, St Louis |
| Study design | Single cohort before-and-after study |
| Study time periods | Baseline: July 1999 to June 2001 (24 months)* Pre-intervention audit: November 2000 to January 2001 Intervention: July 2001 to September 2002 (15 months) Post-intervention audit: November 2001 to February 2002 Follow-up: None (continuous intervention implementation) [*Previous education intervention implemented July 1999 and monitored up to December 1999; reported by Coopersmith (2002)50] |
| Funding source | Funding received from the Centers for Disease Control and Prevention Cooperative Agreement, the BJC Hospital Epidemiology and Infection Control Consortium and the National Institutes of Health |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics Eighteen beds (staffed by 69 nurses during previous educational programme implementation), with around 1400 patients admitted per year. Extended to 24 beds by the end of the study (exact time of change not reported), with 12 attending physicians (final number of nursing staff not reported) Average LOS: 4.3 days Clinical practice based on an educational programme aimed at preventing CRBSIs: reported by Coopersmith et al.50 (Note that there was relatively poor compliance with this – details below) During the whole study period, CVC maintenance and site care were performed by registered nurses only. The CVCs were placed by residents from the departments of surgery (PGY-1, PGY-2), anaesthesiology (PGY-2, PGY-3) and emergency medicine (PGY-2) as part of their 4- to 6-week rotation through the ICU and a full-time nurse practitioner. CVC placement was supervised by surgery, anaesthesiology or pulmonary/critical care fellows or by ICU attending staff. CVCs were not routinely changed over guidewires at any time during the study |
|||
| Baseline | Intervention | ||
| Patient population characteristics | Male, % | 49.4 | 56.9 |
| Mean age, years | 54.5 | 57.4 | |
| Contact isolation, % | 25.2 | 25.0 | |
| Device characteristics (% of CVCs) | (171 CVCs in 99 patients) | (138 CVCs in 72 patients) | |
| Insertion site | Subclavian | 53 | 53 |
| Internal jugular | 41 | 41 | |
| Femoral | 6 | 6 | |
| Location of catheter insertion | SICU | 73 | 84 |
| Operating room | 21 | 10 | |
| Emergency department | 1 | 2 | |
| Other (hospital ward, outside hospital) | 5 | 4 | |
| Insertion site antisepsis used: | (171 CVCs in 99 patients) | (138 CVCs in 72 patients) | |
| Antibiotic ointment (% of CVCs) | 3 | 4 | |
| Dressing type (% of CVCs): | (171 CVCs in 99 patients) | (138 CVCs in 72 patients) | |
| Transparent | 93 | 98 | |
| Gauze | 3 | 1 | |
| Both | 4 | 1 | |
Intervention characteristics
| Objective | To improve compliance with evidence-based guidelines for CVC insertion and maintenance |
| Main focus of education | Specified behaviours associated with CVC insertion and maintenance (hand hygiene; barrier precautions; antibiotic ointment and dressing use; dating dressings; avoiding stopcocks) |
| Trainers (providers) | One or two named study authors gave lectures and hands-on demonstrations |
| Training of trainers | Not reported |
| Learners (recipients) | Nurses received lectures and hands-on demonstrations as part of their annual skills sessions. The entire resident staff of surgery and emergency medicine departments received lectures. Monthly lectures were given to all residents rotating through the ICU |
| Target behaviour change | Compliance with best practice for CVC maintenance for nursing staff and CVC insertion for physicians including preferred insertion site and no stopcocks, hand hygiene, maximal sterile barrier, type of catheter site dressing, and avoidance of antibiotic ointment |
| Development and testing | Audit tool: Development of flow charts of evidence-based best practice, detailing maintenance and insertion of CVC by a multidisciplinary team of physicians (an infection control specialist, nurses, a pharmacist and a QI specialist). Team used literature-based determination of risk factors involved in catheter infections to develop an audit tool and behaviours on the audit sheet were marked as ‘yes’ or ‘no’ (e.g. did the person inserting the CVC use appropriate hand hygiene before insertion?) Behavioural intervention: Stated only that this was based on the results of the bedside audits |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Referred to as a behavioural intervention; stressed compliance with all facets of best practice of CVC insertions and maintenance Pictures: Placed at end of every patient’s bed, throughout the ICU, and in the manual each resident received when they rotated through the ICU. These demonstrated each step of CVC insertion (aimed at physicians) and maintenance (aimed at nursing staff) Lectures: Topics not reported Hands-on demonstrations: Topics not reported |
| Infection surveillance feedback approach | During both the baseline and intervention periods: BSI rates and comparison with national rates using NNIS data were presented on a monthly basis at multidisciplinary staff meetings |
| Performance feedback approach | Not included in intervention. Audits of compliance informed the development of the intervention but audit results were not reported to have been directly fed back to the ICU teams |
| Concentration of education | Lectures and hands-on demonstrations were given to nurses as part of their annual skills sessions. Lectures of unspecified frequency were given to the entire resident staff in the departments of surgery and emergency medicine and monthly lectures were given to all residents rotating through the ICU. Frequency and duration of sessions not reported. Reinforcement of sessions (e.g. whether annual repetition) not reported |
| Non-educational intervention components | None – intervention purely educational |
| Costs reported | Not reported |
Outcome characteristics
| Catheter-BSI definition Reference cited: Pearson197 |
BSIs were classified as primary or secondary on the basis of NNIS definitions (reference cited). CRBSI defined as: a microorganism isolated from a blood culture not related to distant infection, or:
|
| Outcomes reported | Primary outcome: Compliance with practices known to decrease CRBSI (appropriate hand hygiene, sterile gown, mask, sterile gloves, large sterile drape, absence of antibiotic ointment, catheter sutured in, transparent dressing appropriately placed) Secondary outcome: CRBSI rate for all ICU patients |
Results data
Primary outcomes
| Outcome | Baseline (24 months), 2716 patients, 9353 catheter-days | Intervention (15 months), 1773 patients, 6152 catheter-days | Difference between baseline and intervention | |
|---|---|---|---|---|
| Device duration (days), % | < 7 days | 62.9 | 57.8 | |
| 7–10 days | 29.4 | 35.9 | ||
| > 10 days | 7.7 | 6.3 | ||
| Days in place, mean | 6.0 | 6.3 | ||
| Total device utilisation, CVC-days | 9353 (n = 2716) | 6152 (n = 1773) | ||
| No. of devices/patient | Not reported | Not reported | ||
| CRBSI incidence rate | 32 | 17 | ||
| Incidence per 1000 catheter-days | 3.4 | 2.8 | p = 0.40 | |
| Incidence per 1000 patient-days | Not reported | Not reported | ||
| LOS | Not reported | Not reported | ||
| Mortality | Not reported | Not reported | ||
Additional primary outcomes data
| Device duration (% of CVCs) –data from randomly selected subsets of patients | Baseline (3 months), 99 patients, 171 CVCs | Intervention (4 months), 72 patients, 138 CVCs | Difference between baseline and intervention (not reported unless indicated) |
|---|---|---|---|
| < 7 days | 62.9% | 57.8% | Statistics not reported |
| 7–10 days | 29.4% | 35.9% | |
| > 10 days | 7.7% | 6.3% | |
| Mean duration, days | 6.0 | 6.3 |
Secondary outcomes
| Compliance with target behaviour (number (%) of CVCs) – data from randomly selected subsets of CVCs | Baseline (3 months) six CVCs, six patients | Intervention (4 months) 10 CVCs, 10 patients | Difference between baseline and intervention |
|---|---|---|---|
| Appropriate hand hygiene | 1 (17%) | 3 (30%) | Statistics not reported |
| Sterile gown | 6 (100%) | 10 (100%) | |
| Mask | 6 (100%) | 10 (100%) | |
| Sterile gloves | 6 (100%) | 10 (100%) | |
| Large sterile drape | 3 (50%) | 8 (80%) | |
| Absence of antibiotic ointment | 6 (100%) | 10 (100%) | |
| Catheter sutured in | 5 (83%) | 10 (100%) | |
| Transparent dressing appropriately placed | 6 (100%) | 10 (100%) | |
| Compliance with target behaviour associated with CVC insertion (% of CVCs) – data from randomly selected subsets of patients | Baseline (3 months), 99 patients, 171 CVCs | Intervention (4 months), 72 patients, 138 CVCs | Difference between baseline and intervention |
| Properly dating the CVC dressing | 11% | 21% | p < 0.001 |
| Stopcock use (avoidance of) | 70% | 24% | p < 0.001 |
| Appropriate hand hygiene use | 17% | 30% | p > 0.99 |
| Maximal sterile barrier precautions | 50% | 80% | p = 0.29 |
| Reaction to education | Not a study outcome | ||
| Attitudes | Not a study outcome | ||
| Knowledge | Not a study outcome | ||
| Skills | Not a study outcome | ||
| Process evaluation | No other process evaluation apart from the random audits, which suggested lack of compliance with target behaviours may have been a barrier (data above) | ||
Critical appraisal
Potential for bias
| Selection bias | Were there systematic differences between the baseline and intervention groups? UNCLEAR. Authors stated that patient demographics were similar throughout the course of the study but limited data were reported, with no indication of patients’ health status. Random subsets of patients and CVC insertions were used for reporting outcomes in the baseline and intervention groups but no information was provided on how random selection was achieved |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the estimate for the overall intervention? YES. There were six additional ICU beds in the intervention period (33% increase) but staff numbers and staff to patient ratios were not reported. The number CVCs inserted in the ICU increased by 9%, whereas those inserted in the operating room decreased by 11% |
| Was the effect of educational practice separable from effects of non-educational practice? YES. The intervention comprised purely educational components. However, the reduction in infection rates was not statistically significant | |
| Were the intervention component(s) implemented as planned? UNCLEAR. Stated in methods section that lectures and hands-on demonstrations were given to nurses as part of their annual skills sessions by two of the authors and also to all staff in departments of emergency medicine and surgery, and monthly lectures were given to all residents rotating through the ICU. However, no details of attendance for lectures or hands-on demonstrations were reported | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? UNCLEAR. For CRBSI data it appears that all patients in the ICU were monitored. However, for behavioural outcomes subgroups of patients and CVCs were randomly selected but no details were provided on how random selection was carried out |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? NOT REPORTED |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NOT REPORTED | |
| Other possible sources of bias | Were any other sources of bias present? YES. The authors reported a potential investigator bias, as the audit tool was administered by members of the team that designed the intervention and audit tool. Authors stated that knowledge of observations alone could alter staff behaviours. Intervention took place 24 months after a previous educational programme targeting CRBSIs |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. Pictures of the CVC insertion process were mentioned but no details of the content of lectures and hands-on demonstrations were reported so the mechanisms for eliciting the target behaviour changes are unclear |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? NOT REPORTED. The authors stated only that the audit tool was administered by members of the team who designed the intervention and the audit tool. | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT APPLICABLE | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT APPLICABLE | |
| Statistical tests described? YES | |
| Educational significance or effect size assessed? NOT REPORTED | |
| Target behaviour change achieved? PARTIALLY. Compliance with all target behaviours increased following implementation of the intervention but for hand hygiene and dating of dressings the improvements were small, and post-intervention compliance was only 30% and 21%, respectively |
Additional comments
-
Authors commented that the diffuseness of the behavioural intervention message may have reduced the impact on CRBSI rates.
-
Monthly infection incidence data reported in a graph – illustrates high temporal variability in BSI incidence, with incidence rates in some months post intervention implementation being higher than some baseline rates.
-
Unclear whether staff were advised of the existence of the study
DUBOSE (2008)93
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | DuBose (2008)93 |
| Summary of approach | Single-unit daily quality rounds checklist to increase compliance with prophylactic measures for prevention of VAP, deep venous thrombosis or pulmonary embolism, central line infection and other ICU complications |
| Location | USA: California |
| Language | English |
| Critical care specialty | Trauma |
| No. of critical care units | 1 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Division of Trauma Surgery and Surgical Critical Care, University of Southern California, Los Angeles |
| Study design | Single cohort before-and-after study |
| Study time periods | Baseline: 1 month (date not reported) Intervention: 3 months (date not reported) Follow-up: None (monitoring only during intervention) |
| Funding source | Not reported |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics (stated only that unit was a high-volume level I trauma centre) | Baseline (1 month) | Intervention month 1 | Intervention month 2 | Intervention month 3 | |
|---|---|---|---|---|---|
| Patient population characteristics | ICU patient-days surveyed | 244 | 185 | 188 | 193 |
| Mean age, years | 41.1 | 41.0 | 41.6 | 40.3 | |
| Gender, % male | 73.0 | 76.8 | 67.0 | 78.9 | |
| Mean injury severity score | 17.3 | 20.9 | 15.0 | 16.1 | |
| Device characteristics | CVCs routinely used were antimicrobial-coated (ARROWgard Blue PLUS Multi-lumen, antimicrobial surface-coated using chlorhexidine, chlorhexidine acetate, and silver sulfadiazine) and placed directly through an introducer (ARROW percutaneous sheath introducer kit 8.5 Fr). All routine catheters were placed with full barrier precautions, and catheters placed in emergency situations where the use of full barrier precautions was not documented were removed within 24 hours | ||||
| Insertion site antisepsis used | Not reported | ||||
| Dressing type and duration/frequency | Not reported | ||||
Intervention characteristics
| Objective | To increase compliance with measures that aim to reduce ICU complications and identify areas for improvement in quality of care |
| Main focus of education | General: to reduce ICU complications. Prevention of CRBSI was a relatively minor part (2/16 checklist items were relevant). |
| Trainers (providers) | Not explicitly stated; appears to have involved the multidisciplinary team noted below under development and testing and/or the trauma and surgical critical care QI committee. Stated surgical critical care fellows acted as ‘champions’ of daily prophylaxis |
| Training of trainers | Not reported |
| Learners (recipients) | Checklists were completed by ICU fellows and their team of residents and medical students on the daily rounding basis |
| Target behaviour change | General: Sixteen different activities for reducing complications in the ICU |
| Development and testing | A multidisciplinary team of care providers that included intensivists, trauma surgeons, nursing staff and a biostatistician conducted a comprehensive review of best-practices data to identify items to be included in the checklist |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Daily quality rounds checklist. Listed 16 evidence-based measures for preventing ICU complications. Of these, two were relevant to prevention of central line infections: checking the central line day and checking the continued need for invasive devices Monthly review of deficiencies by a multidisciplinary team of intensivists, trauma surgeons, nursing staff and a biostatistician resulted in the highlighting of those measures most clearly requiring more focused effort. Improvements were then designed and implemented following discussion Training on new protocol strategies and improvement of existing approaches (no details given) |
| Infection surveillance feedback | Not included |
| Performance feedback | Unclear. Some feedback of process was mentioned for insulin management; results appear to have been fed back monthly to the trauma and surgical critical care QI committee – unclear whether and how disseminated to ICU staff. Paper checklists were completed by an ICU fellow and manually entered into an Excel database |
| Concentration of education | The initial time required for completion of the checklist and institution of corrective changes averaged approximately 1 hour per day. With familiarity, after the first few weeks of use, the time decreased to 20–30 minutes per day. This equated to approximately two additional minutes per patient |
| Non-educational intervention components | None. However, the majority of checklist topics were not directly relevant to catheter BSIs. |
| Costs reported | No, but authors stated that the cost was quite minimal: the completion of the checklist represented little additional burden on the ICU fellow and did not significantly alter nursing workloads |
Outcome characteristics
| Catheter-BSI definition | Central-line related infection: Positive blood cultures with a recognised pathogen without evidence of alternative septic source must be documented, and the catheter must have been in place for > 48 hours |
| Outcomes reported | Not stated whether primary or secondary: |
Results data
Primary outcomes
| Outcome | Baseline (3 months; dates not reported) | Intervention (3 months; dates not reported) | Difference between baseline and intervention | |
|---|---|---|---|---|
| CVC duration | % lines > 24 hours | 89.4 | 88.4 (86.2 = result for first month of intervention) | Statistical test of difference not reported |
| % lines > 48 hours | 74.1 | 68.4 (64.1 = result for first month of intervention) | ||
| % lines > 72 hours | 62.4 | 52.8 (49.1 = result for first month of intervention) | ||
| Total device utilisation | Not reported | Not reported | Not reported | |
| No. of devices/patient | Not reported | Not reported | Not reported | |
| Mean monthly central line-associated BSI [central line-related infection] incidence rate per 1000 device-days a | 8.9b 11.3b |
5.8 | Statistical test of difference not reported | |
| Incidence per 1000 patient-days | Not reported | Not reported | Not reported | |
| LOS | Not reported | Not reported | Not reported | |
| Mortality | Not reported | Not reported | Not reported | |
Secondary outcomes
| Reaction to education | Not a study outcome |
| Attitudes | Not a study outcome |
| Compliance | Compliance with daily CVC site evaluation according to a previously established quality assurance pathway exceeded 95% [not stated which time period(s) this referred to] Compliance was reported in more detail for VAP prevention (data not extracted by reviewers) |
| Knowledge | Not a study outcome |
| Skills | Not a study outcome |
| Process evaluation | Not a study outcome |
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? UNCLEAR. Reported that demographic characteristics ‘remained constant’ between groups but limited data given (the maximum difference in mean age between the baseline and intervention periods was 1.3 years; the maximum difference in injury severity score was 4.8; and the maximum difference in the proportion male was 11.9%) |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? NOT REPORTED |
| Was the effect of educational practice separable from effects of non-educational practice? YES. Only a checklist (i.e. educational) was used (although only 2 of the 16 checklist items concerned catheter BSIs) | |
| Were the intervention component(s) implemented as planned? NOT REPORTED. Compliance with the checklist was not reported | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? UNCLEAR. The number of checklists completed and entered into the database was not reported |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? NO. States that a standard definition of infections was used throughout the study and all potential nosocomial infections, central line-related infections and VAP were diagnosed by the hospital epidemiology service |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? YES. The checklist was used by an ICU fellow not directly involved in patient care for 1 month during the baseline period to establish pre-intervention compliance. During this period only, nursing and clinical staff were blinded to the use of the checklist | |
| Other possible sources of bias | No other sources of bias were reported by the authors or identified by the reviewers based on the information presented |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? YES. The intervention consisted principally of a checklist that could be reproduced or adapted |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? NOT REPORTED. Stated only that compliance of the nursing staff in the completion of specified measures was ensured by the monitoring of a unit nurse manager through a previously established quality assurance pathway (details not reported) | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? NO | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? UNCLEAR. Some aspects of practice for reducing complications in the ICU were complied with more extensively than others. Primarily reported compliance data for a VAP intervention (data not extracted by reviewers). Compliance with daily CVC site evaluation (assessed according to a previously established quality assurance pathway) was reported to have only exceeded 95%, but it is not clear by how much compliance had increased or to which time period(s) of the study this refers |
EGGIMANN (2000–2005)94,95
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Eggimann (2000);94 Eggimann (2005)95 |
| Summary of approach | Single unit educational intervention consisting primarily of short slide shows and bedside education |
| Location | Switzerland |
| Language | English |
| Critical care specialty | Medical |
| No. of critical care units | 1 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | University of Geneva Hospital; Geneva |
| Study design | Single cohort before-and-after study |
| Study time periods | Baseline: October 1995 to February 1997 (16 months) Intervention: March 1997 to October 1997 (8 months)94 Intervention continuation: March 1997 to 2002 (month unspecified) (total duration approximately 6 years)95 Follow-up: None (continuous monitoring of intervention) |
| Funding source | Lead author was supported in part by a grant from G and L Hirsch, Geneva, Switzerland during the preparation of the paper. Co-author (Harbarth) was supported by a grant from the Max-Kade Foundation, New York, USA during the preparation of the paper. The intervention continuation was supported in part by a grant from the Swiss National Science Foundation |
| Conflicts of interest | Stated in the intervention continuation report95 that no potential financial conflicts of interest were disclosed |
Population and setting
| Critical care unit characteristics | 18 beds in a MICU at a 1500-bed primary care and tertiary care centre |
| Average of 1400 patients per year admitted to the ICU | |
| Mean LOS 4 days (further data in Results section) | |
| All vascular lines were inserted by advanced internal medicine residents or fellows in the MICU; there was no change in the physicians' profile between the study periods | |
| Device insertion and management were based on institutional written guidelines promulgated by the nursing department | |
| Material preparation: Based on physicians' individual preferences | |
| Patient positioning: According to nursing habits acquired elsewhere | |
| Skin preparation: Hair shaving | |
| Barrier precautions: Sterile gloves, small fenestrated sheets, paper mask | |
| Insertion technique: Various techniques; no specific training | |
| Device replacement: Every 24 hours for all administration sets and devices. | |
| Device removal: Peripheral line after 3–5 days; no specific recommendation for central lines | |
| Hand hygiene: With surgical soap in sink before and after each patient care, or hand disinfection |
| Patient population characteristics: all adult patients admitted to the MICU for more than 48 hours | Baseline: October 1995 to February 1997 (16 months), 2104 patients | Intervention: April to November 1997 (8 months), 1050 patients | Difference between baseline and intervention: total patient-days for baseline and intervention = 13,200 |
|---|---|---|---|
| No. of patients | |||
| Unstable angina | 554 (26%) | 296 (28%) | Difference in number of patients not statistically significant |
| Myocardial infarction | 406 (19%) | 218 (21%) | |
| Cardiac monitoring | 222 (11%) | 116 (11%) | |
| Cardiac arrest | 48 (2.3%) | 32 (3.0%) | |
| Hypertensive crisis | 76 (3.6%) | 25 (2.3%) | |
| Acute heart failure | 55 (2.6%) | 41 (3.9%) | |
| Acute respiratory insufficiency | 122 (5.8%) | 74 (7.1%) | |
| Asthma | 54 (2.6%) | 19 (1.8%) | |
| COPD | 54 (2.6%) | 15 (1.4%) | |
| ARDS | 20 (1.0%) | 9 (0.9%) | |
| Neurological – intoxication | 106 (5.0%) | 55 (5.2%) | |
| Neurological – miscellaneous | 105 (5.0%) | 42 (4.0%) | |
| Infections | 98 (4.7%) | 32 (3.0%) | |
| Other disorders | 184 (8.7%) | 76 (7.2%) | |
| Total patients | 2104 | 1050 | |
| Age, years, mean (SD) | |||
| Unstable angina | 66 (12) | 66 (12) | Differences in age not statistically significant except *p < 0.05 |
| Myocardial infarction | 63 (13) | 67 (13)* | |
| Cardiac monitoring | 68 (14) | 67 (13) | |
| Cardiac arrest | 68 (14) | 65 (15) | |
| Hypertensive crisis | 57 (21) | 58 (17) | |
| Acute heart failure | 70 (10) | 73 (8) | |
| Acute respiratory insufficiency | 63 (14) | 67 (13) | |
| Asthma | 45 (18) | 36 (14) | |
| COPD | 66 (13) | 66 (10)49 (14) | |
| ARDS | 42 (17) | 42 (20) | |
| Neurological – intoxication | 38 (16) | 56 (19) | |
| Neurological – miscellaneous | 58 (17) | 57 (16) | |
| Infections | 54 (18) | 57 (17) | |
| Other disorders | 56 (18) | 63 (16)* | |
| Total patients | 62 (16) | 66 (12) | |
| No. mechanically ventilated | |||
| Unstable angina | 5 (0.9%) | 3 (0.9%) | Differences in number mechanically ventilated not statistically significant except *p < 0.05 |
| Myocardial infarction | 54 (13%) | 15 (7.0%)* | |
| Cardiac monitoring | 7 (3.2%) | 1 (0.8%) | |
| Cardiac arrest | 43 (90%) | 30 (94%) | |
| Hypertensive crisis | 9 (12%) | 3 (12%) | |
| Acute heart failure | 26 (47%) | 16 (39%) | |
| Acute respiratory insufficiency | 59 (48%) | 28 (38%) | |
| Asthma | 12 (22%) | 4 (21%) | |
| COPD | 9 (17%) | 2 (13%) | |
| ARDS | 2 (100%) | 9 (100%) | |
| Neurological – intoxication | 35 (33%) | 17 (31%) | |
| Neurological – miscellaneous | 68 (65%) | 25 (60%) | |
| Infections | 59 (60%) | 20 (63%) | |
| Other disorders | 33 (18%) | 24 (32%)* | |
| Total patients | 439 (21%) | 194 (19%) | |
| Nursing workload: mean (SD; range) monthly Research in Nursing system scores: | 188 (66; 152–210) | 191 (61; 178–207) | |
| Device characteristics | A total of 3154 patients in the study (baseline and intervention combined) had at least one intravenous device inserted, 966 (31%) were exposed to arterial lines and 1121 (35%) to CVCs, with similar proportions in the baseline and intervention periods | ||
| Insertion site antisepsis used | Povidone–iodine or chlorhexidine gluconate | ||
| Dressing type and duration/frequency | Several types according to individual non-standardised criteria. Transparent occlusive dressings or preprepared devices for peripheral lines. Dressing replacement every 24 hours | ||
Intervention characteristics
| Objective | To decrease rates of vascular-access infections using a multimodal, multidisciplinary prevention strategy and to assess the impact of the strategy on the incidence of ICU-acquired infections |
| Main focus of education | Prevention of ICU acquired infections, including those associated vascular catheter insertion, maintenance and use |
| Trainers (providers) | Five named study authors carried out the educational intervention |
| Training of trainers | Not reported directly but the identity of the trainers was reported so their expertise could be checked (three authors wrote and five authors reviewed the guidelines) |
| Learners (recipients) | All ICU staff received the slide shows and practical demonstrations: 21 fellows or residents, 82 nurses and 15 nursing assistants |
| Target behaviour change | Behaviours associated with the insertion, maintenance and use of vascular catheters |
| Development and testing | Not reported |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | 30-minute slide shows complemented by individual bedside teaching.* Topics covered were:
|
| Infection surveillance feedback approach | Not included in intervention |
| Performance feedback approach | Not included in intervention |
| Concentration of education | Education comprised 30-minute slide show sessions and unspecified bedside teaching sessions given to all staff. Frequency of slide shows unclear; not stated whether sessions were repeated annually or at other intervals for staff who had already been trained |
| Non-educational intervention components | None |
| Costs reported | No |
Outcome characteristics
| Catheter-BSI definition | The infection was regarded as ICU acquired if it occurred within 48 hours of discharge from the ICU. Primary BSIs were defined as bacteraemia (or fungaemia) for which there was no documented distal source, and included infections resulting from insertion of an intravenous or arterial line. The infection was categorised either as microbiologically documented or as clinical sepsis. CRBSI were those for which the same organism had been isolated from a quantitative culture of the distal catheter segment, and from the blood of a patient with clinical symptoms and no other apparent source of infection. In the absence of catheter culture, defervescence after removal of an implicated catheter from a patient with a BSI was regarded as indirect evidence of infection. Catheter exit site infection, catheter colonisation and clinical sepsis were also defined Infections were defined according to Garner et al.147 |
| Outcomes reported | Not stated whether primary or secondary:
|
Results data
Primary outcomes
| Outcome | Baseline: October 1995 to February 1997 (16 months), 2104 patients | Intervention: April to November 1997 (8 months), 1050 patients (longer-term data also reported94) | Difference between baseline and intervention: relative risk (95% CI) | |
|---|---|---|---|---|
| Device duration, days, median | 8 | 7 | ||
| Exposure to CVCs, days, median | 4 | 4 | ||
| Total device utilisation | Not reported | Not reported | ||
| No. of devices/patient | Not reported | Not reported | ||
| BSI per 1000 CVC-days, mean (95% CI) | (a) Initial data from Eggimann (2000)94 | (a) 6.6 | (a) 2.3 | (a) Not reported |
| (b) Longer-term data from Eggimann (2005)95 | (b) 6.3 (4.0 to 9.4) | (b) Annual rates:1997: 2.9 (0.8 to 7.3)1998: Not reported1999: 2.4 (1.0 to 4.7)2000: 2.7 (0.9 to 6.3)2001: 1.7 (0.5 to 3.9)2002: 3.4 (1.7 to 6.1) | (b) Average rate reduction over the study period 56% (range 46% to 63%); no statistical tests of differences reported | |
| [Note: Data for calculating CIs of risk ratios (presented in the main report) were not reported by Eggimann et al.95 but were obtained by contacting the study authors (see Appendix 7)] | ||||
| BSI incidence per 1000 patient-days | Not reported | Not reported | ||
| LOS, days, mean (SD) | ||||
| Unstable angina | 2.6 (2.7) | 2.7 (1.3) | Differences not statistically significant | |
| Myocardial infarction | 3.8 (3.6) | 3.6 (2.5) | ||
| Cardiac monitoring | 2.1 (1.7) | 2.3 (1.6) | ||
| Cardiac arrest | 6.0 (5.6) | 6.0 (4.8) | ||
| Hypertensive crisis | 6.1 (14.0) | 2.8 (1.2) | ||
| Acute heart failure | 5.4 (5.3) | 5.2 (4.4) | ||
| Acute respiratory insufficiency | 9.0 (8.2) | 8.2 (8.5) | ||
| Asthma | 3.9 (3.3) | 3.5 (2.0) | ||
| COPD | 7.6 (6.0) | 6.1 (2.9) | ||
| ARDS | 13.6 (12.0) | 13.8 (9.7) | ||
| Neurological – intoxication | 3.5 (7.1) | 2.6 (1.4) | ||
| Neurological – miscellaneous | 5.8 (8.2) | 5.0 (3.6) | ||
| Infections | 5.3 (4.4) | 8.3 (8.6) | ||
| Other disorders | 4.0 (6.4) | 3.8 (3.4) | ||
| Total patients | 4.1 (5.8) | 3.9 (4.2) | ||
| Mortality (no. of ICU deaths) | ||||
| Unstable angina | 2 (0.4%) | 1 (0.3%) | Differences not statistically significant except *p < 0.05 | |
| Myocardial infarction | 31 (7.6%) | 9 (4.1%) | ||
| Cardiac monitoring | 4 (1.8%) | 0 | ||
| Cardiac arrest | 15 (31%) | 19 (59%)* | ||
| Hypertensive crisis | 7 (9.2%) | 3 (12%) | ||
| Acute heart failure | 2 (3.6%) | 2 (4.9%) | ||
| Acute respiratory insufficiency | 19 (16%) | 10 (14%) | ||
| Asthma | 0 | 0 | ||
| COPD | 3 (5.6%) | 0 | ||
| ARDS | 13 (65%) | 5 (56%) | ||
| Neurological – intoxication | 2 (1.9%) | 0 | ||
| Neurological – miscellaneous | 16 (15%) | 6 (14%) | ||
| Infections | 33 (34%) | 11 (34%) | ||
| Other disorders | 12 (6.5%) | 7 (9.2%) | ||
| Total patients | 159 (7.6%) | 71 (6.8%) | ||
Secondary outcomes
| Reaction to education | Not a study outcome |
| Attitudes | Not a study outcome |
| Compliance | Not a study outcome |
| Knowledge | Not a study outcome |
| Skills | Not a study outcome |
| Process evaluation | Not a study outcome |
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NO. The groups were similar with respect to number of patients per month, age, number mechanically ventilated, LOS, infection risk factors and mortality |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? NO. The authors stated that there were no changes in the physicians’ profile or in nursing workload. A hospital-wide campaign to improve compliance with hand hygiene practices was introduced before the start of the baseline period. Monitoring infection incidence in a SICU at the same hospital revealed no changes over time in infection incidence rates, suggesting that those observed in the study ICU did not result from external factors |
| Was the effect of educational practice separable from effects of non-educational practice? YES. The intervention was purely educational | |
| Were the intervention component(s) implemented as planned? NOT REPORTED | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? NO. The number of patients in the baseline and intervention periods did not differ noticeably (quantitative data reported). Infection surveillance involved all patients (dedicated chart for each patient). Mortality rates were slightly but not statistically significantly lower in the intervention period. Data on the number of devices per patient were not separable by baseline and intervention period |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? UNCLEAR. The annual incidence rates reported by Eggiman (2005)95 do not specify which months were used in the measurement ranges: it is unclear whether the 1997 data were adjusted to account for 1997 comprising only 9 months of intervention (since the first 3 months were part of the baseline period). |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | No other sources of bias were reported by the authors or identified by the reviewers based on the information presented |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. The educational characteristics of the bedside education sessions were not reported. The extent of education reinforcement needed (how often staff were re-educated) to achieve the reported changes in infection rates was not reported. The main components of the slide show were reported but a copy of the slide show was not provided |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. Surveillance of nosocomial infections was conducted by two named infection control nurses who visited the ICU daily and completed a dedicated surveillance chart for each patient. Surveillance was continued until 5 days after discharge to detect incubating infections attributable to ICU stay. All surveillance records were prospectively reviewed and validated by two named infection control physicians | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? YES. Surveillance methods were pretested and standardised in several pilot phases. Interobserver variability was assessed during three separate periods when the two observers worked simultaneously. Inter-rater reliability was high for all infections (ĸ = 0.89; range 0.78–1.0) | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? UNCLEAR. Information on compliance or other aspects of process was not reported |
GALPERN (2008)97
Methods
Study characteristics
| Lead author, publication year(s) and reference ID(s) | Galpern (2008)97 |
| Summary of approach | Single unit central line bundle implementation (proposed by Greater New York Hospital Association) to decrease CLABSIs |
| Location (country, state/region) | USA, New York |
| Language | English |
| Critical care specialty | Mentions SICU, but in discussion of question and answer section states that it included a MICU and a CCU. |
| No. of critical care units | 3 (medical–surgical, mixed across three locations; and cardiac) (not reported in the publication – information provided by the author) |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Methodist Hospital, Brooklyn, New York |
| Study design | Single cohort before-and-after study |
| Study time periods | Baseline: February to June 2005 (5 months) Intervention: July 2005 to February 2006 (19 months) Infection surveillance: 1 February 2005 to 31 April 2007 (26 months) Follow-up: Unclear (not explicitly stated whether results are for intervention period only (0 months follow up) or for full infection monitoring period (14 months’ follow-up) |
| Funding source | None reported |
| Conflicts of interest | None reported |
Population and setting
| Critical care unit characteristics | Ranged from 26 to 30 ICU beds The ICUs were in a 628-bed community teaching hospital. Stated that there were no changes in materials during the time of the study (catheter kits, drapes, gowns, gloves and caps were all kept the same) |
| Patient population characteristics | Not reported |
| Device characteristics | CVCs; avoidance of femoral site; device duration reported. Ultrasonography was used intermittently in the placement of the central lines. Central lines were placed by critical care physicians without assistance, unless requested |
| Insertion site antisepsis used | Chlorhexidine (ChloraPrep) |
| Dressing type and duration/frequency | Not reported, but no antibiotic patches were used owing to lack of level 1 evidence |
Intervention characteristics
| Objective | To determine whether using central line bundles would decrease the incidence of CLABSI |
| Main focus of education | Catheter insertion |
| Trainers (providers) | Team to implement bundle: head of SICU as team leader, ICU nurse managers and two infection-control nurses |
| Training of trainers | Not reported |
| Learners (recipients) | All ICU staff (physicians and nurses) |
| Target behaviour change | Nurse to assist with central line insertion, compliance with hand washing, use of full-barrier precautions, appropriate skin preparation, checking and restocking of central cart, avoidance of femoral lines, and early removal of central lines |
| Development and testing | None reported, but mentions that the bundle protocol was based on the latest evidence-based techniques |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Education of resident physicians and nurses on BSI control practices. Included: Discussion of: proper hand washing, use of full barrier precautions during central line insertion, appropriate skin preparation with chlorhexidine (ChloraPrep), avoiding the femoral site if possible, and early removal of all central lines (justification for continued line use noted on a chart). Checklist completed by nurses to ensure compliance with the evidence-based guidelines |
| Infection surveillance feedback approach | The data (number of critical-care beds used, number of catheters placed, number of days catheters left in place, and number of line-associated infections) were collected by a trained, hospital-based infection-control practitioner. The data were reported to the directors of the surgical and MICUs, which allowed for real-time feedback to the staff on how the intervention was proceeding. Validation and reliability of process was not reported |
| Process audit feedback approach | Monthly feedback (number of critical care beds used, number of catheters placed, number of days catheters left in place, and number of line-associated infections) to directors of SICU, CCU and MICU, allowing for real-time staff feedback on intervention process |
| Performance feedback approach | Not included in intervention |
| Concentration of education | Not reported |
| Non-educational intervention components | Catheter cart provided Personnel change: A policy was instituted that required nurses to assist in central line insertion |
| Costs reported | Cost reported as being limited to central line cart cost (reported as around US$500 in the discussion of question and answer section) |
Outcome characteristics
| Catheter-BSI definition | NNIS criteria: presence of a recognised pathogen cultured from one or more blood cultures and the organism cultured from the blood not to be related to infection at another site plus presence of a temperature greater > 38 °C, chills or hypotension, along with signs and symptoms of an infection not related to another site and presence of a common skin contaminant cultured from two or more blood samples on separate occasions, or common skin contaminant cultured from at least one blood sample in a patient with an intravascular catheter, or a positive antigen test on diagnostic phlebotomy |
| Outcomes reported | Not stated whether primary or secondary: Central line-associated BSI per 1000 catheter-days No. of central lines No. of central line days Development of a line infection |
Results data
Primary outcomes
| Outcome | Baseline (5 months) | Intervention (19 months) | Difference between baseline and intervention |
|---|---|---|---|
| Device duration (days), average | 8.5 ± 1.3 | 6.8 ± 0.97 | p = 0.17 |
| Total device utilisation | Not reported | Not reported | (Total for both baseline and intervention: 1395 CVCs; 9938 central line-days) |
| No. of devices/patient | Not reported | Not reported | |
| CABSI incidence rate | Not reported | Not reported | |
| Incidence per 1000 catheter-days – average a | 5.0 ± 4.3 | 0.90 ± 1.3 | p < 0.001 |
| Incidence per 1000 patient-days | Not reported | Not reported | |
| LOS | Not reported | Not reported | |
| Mortality | Not reported | Not reported |
Secondary outcomes
| Reaction to education | Not a study outcome |
| Attitudes | Not a study outcome |
| Compliance | Not a study outcome |
| Knowledge | Not a study outcome |
| Skills | Not a study outcome |
| Process evaluation | Stated only that ongoing monitoring kept ICU teams committed to the new protocols (no details given) |
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NOT REPORTED |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? NO. Authors stated that there were no changes in materials and that catheter kits, drapes, gowns, gloves and caps were all kept the same during the study period). However, no information was provided on staffing, infrastructure or policy changes, except for a specific staff role change that occurred within the intervention bundle (policy required nurses to assist critical care physicians, who previously did all insertions) |
| Was the effect of educational practice separable from effects of non-educational practice? NO. Education was part of a bundle: catheter carts were provided and staff changes also occurred | |
| Were the intervention component(s) implemented as planned? NOT REPORTED | |
| Missing data | Were there systematic differences between study groups in attrition or exclusions (includes withdrawal due to mortality; missing outcome data)? NOT REPORTED |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? NOT REPORTED |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | Were any other sources of bias present? NO. No other sources of bias were reported by the authors or identified by the reviewers based on the information presented. Authors state that bias of under-reporting infections was controlled by having each patient checked by a qualified trained infection-control nurse on a daily basis |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. The duration and number of educational sessions required was not reported, although an overview of the content was given. The checklist was briefly described but was not presented so could not be duplicated or adapted |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. Stated only that data were collected on a monthly basis, which included the number of critical care beds in use at the time, the number of catheters placed, the number of days the catheters were left in place expressed as catheter-days, and the number of line-associated infections. The data were then entered into a spreadsheet and descriptive analysis was performed | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? NO | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? NOT REPORTED |
GUERIN (2010)98
Methods
Study characteristics
| Lead author, publication year(s) and reference ID(s) | Guerin (2010)98 |
| Summary of approach | Local addition of a CVC post-insertion care bundle to an existing nationwide CVC insertion bundle as the latter did not prevent CLABSI, despite high compliance |
| Location (country, state/region) | USA, Colorado |
| Language | English |
| Critical care specialty | MICU and SICU |
| No. of critical care units | 2 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Department of Veterans Affairs Medical Center, Denver |
| Study design | Single cohort before-and-after study (two ICUs treated as a single group) |
| Study time periods | Baseline: 1 October 2006 to 30 September 2008 (24 months) Intervention: 1 October 2008 to 30 September 2009 (12 months) Follow-up: None (only intervention monitored) [Note: A nationwide CVC insertion bundle was implemented in April 2006 (i.e. during the baseline period)] |
| Funding source | None reported |
| Conflicts of interest | Stated none |
Population and setting
| Critical care unit characteristics | MICU = 10 beds; SICU = 13 beds Physician staff members were postgraduate residents 1 : 2 nurse to patient ratio University-affiliated acute care teaching hospital From April 2006 onwards: Nationwide CVC insertion bundle: hand hygiene: sterile gloves and gown for all in the room prior to procedure; cap and mask for physician inserting the catheter; use of a 2% chlorhexidine gluconate in 70% ethanol scrub for the insertion site; head-to-toe sterile drape of the patient during insertion; time out before performing the procedure; avoidance of the femoral insertion site. Intravenous tubing for parenteral nutrition solutions changed daily, tubing for other intravenous solutions changed every 72 hours Baseline period (October 2006 onwards): standard infection control practices as per facility’s infection control manual and annual online review training for all nursing staff (required pass rate 80%). Implementation of insertion bundle Training in proper application of the chlorhexidine gluconate-impregnated sponge provided by the manufacturer’s representative educator Staff empowerment: Nurses monitored compliance of insertion bundle and were empowered to stop procedures breaching sterile technique. Four trained nurses inserted polyurethane peripheral CVCs (PICCs) |
| Patient population characteristics | Not reported |
| Device characteristics | 96% PICCs (Bard, Murray Hill, NJ, USA) coated in chlorhexidine gluconate and silver sulfadiazine. In patients allergic to coatings, uncoated PICCs (4%) were used – silicone Groshong catheters (Bard). No other concurrent invasive devices were used Insertion sites: subclavian, internal jugular and femoral |
| Insertion site antisepsis used | Chlorhexidine gluconate-impregnated sponge |
| Dressing type and duration/frequency | Transparent dressing applied weekly or more frequently if wet or soiled, with a new chlorhexidine gluconate-impregnated sponge applied at each dressing change |
Intervention characteristics
| Objective | Prevention of post-insertion CLABSI |
| Main focus of education | Post-insertion care |
| Trainers (providers) | Line care bundle was implemented by an intravenous champion in each unit (no details reported). Providers of hands-on training not reported |
| Training of trainers | Not reported |
| Learners (recipients) | All nursing staff |
| Target behaviour change | Hand hygiene, insertion and maintenance of PICCs |
| Development and testing | Stated that the line care bundle was developed by the nursing staff, and that the interventions were developed by frontline nursing staff. A 6-month pilot phase enabled staff to gain experience with device-day monitoring and data collection |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Hands-on training and competency evaluation: for all nursing staff based on accessing and caring for all intravenous catheters. Insertion site competence covered wearing a mask and sterile gloves for a central line dressing change, scrubbing the site with 2% chlorhexidine gluconate in alcohol for 30 seconds and applying the chlorhexidine gluconate-impregnated sponge properly. Hub care competence included scrubbing the catheter hub for 15 seconds with an alcohol pad at each access and replacing the hub every 72 hours |
| Infection surveillance feedback approach | Infection surveillance reported but not feedback – except to infection prevention and control (not ICU staff) |
| Performance feedback approach | Competence evaluation following the hands-on training in which each nurse was required to demonstrate competence in catheter insertion site and hub care |
| Concentration of education | Four-hour hands-on mandatory training for all nursing staff followed by a competency evaluation (duration not specified). Not stated whether reinforced or re-assessed annually |
| Other intervention components beyond education |
|
| Device insertion procedure | Continued baseline infection control practices (see pre-intervention baseline control characteristics, clinical practice protocols) |
| Costs reported | Not reported |
Outcome characteristics
| Catheter-BSI definition Reference cited: Centers for Disease Control and Prevention198 |
Criterion 1: Patient has a recognised pathogen cultured from one or more blood cultures and the organism cultured from blood is not related to an infection at another site Criterion 2: Patient has at least one of fever (> 38 °C), chills, or hypotension; positive laboratory results are not related to an infection at another site; and common skin contaminant [i.e. diphtheroids (Corynebacterium spp.), Bacillus (not B. anthracis) spp., Propionibacterium spp., coagulase-negative staphylococci (including S. epidermidis), viridans-group streptococci, Aerococcus spp., Micrococcus spp.] is cultured from two or more blood cultures drawn on separate occasions |
| Outcomes reported | Not stated whether primary or secondary:
|
Results data
Primary outcomes
| Outcome | Baseline | Intervention | Difference between baseline and intervention: relative risk (RR) (95% CI); p-value. Except where indicated, statistical comparisons were not reported |
|---|---|---|---|
| Catheter dwell time a (no. of days between catheter insertion and onset of CLABSI), days | Mean = 14.5 Median = 12 Range = 0–47b IQR = 6–24 |
Mean = 15 Median = 7 Range = 7–33 IQR not reported |
|
| Total device utilisation, catheter-days | 4415 | 2825 | |
| Total no. of patient-days | 11,434 | 5937 | |
| Catheter utilisation proportion: no. of catheter-days divided by no. of patient-days | 0.39 | 0.48 | p < 0.0001 |
| No. of devices/patient | Not reported | Not reported | |
| No. of CLABSI | 25 | 3 | |
| CLABSI incidence per 1000 catheter-days | 5.7 | 1.1 | RR = 0.19 (0.06 to 0.63); p = 0.004 |
| Adjusted RRc = 0.23 (0.07 –0.77); p = 0.017 | |||
| Incidence per 1000 patient-days | Not reported | Not reported | |
| LOS | Not reported | Not reported | |
| Mortality | Not reported | Not reported |
Secondary outcomes
| Reaction to education | Not a study outcome | |
| Attitudes | Not a study outcome | |
| Compliance: CVC insertion bundle | Pre-intervention period: 94% | During intervention period: 93% |
| Knowledge | Not a study outcome | |
| Skills | Not reported | |
| Process evaluation | Brief narrative comment only. The authors stated that their study of local compliance with recommended post-insertion care techniques revealed opportunities for improvement that could possibly lead to reductions in CLABSI. However, the only quantitative compliance data reported were for the CVC insertion bundle, not the post-insertion care bundle | |
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NOT REPORTED |
| Were any systematic differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT REPORTED | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? NOT REPORTED |
| Was the effect of educational practice separable from effects of non-educational practice? NO, part of a bundle | |
| Were the intervention component(s) implemented as planned? UNCLEAR. The hands-on training and competency evaluation were compulsory. Teaching was given to all ICU nurses and other staff, but implementation of teaching was not reported. Stated that compliance with the CVC insertion bundle was 94% during the pre-intervention period and 93% during the intervention period | |
| Missing data | Were there systematic differences between the baseline and intervention groups in attrition or exclusions (includes withdrawal due to mortality; missing outcome data)? NOT REPORTED |
| Outcome measurement | Were there systematic differences between the study groups in how outcomes were determined (including differences in how outcomes were defined)? NOT REPORTED |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | Were any other sources of bias present? NO. No other sources of bias were reported by the authors or identified by the reviewers based on the information presented |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. No documentation of the bundle protocol or training sessions was provided. The principles and components of the line care bundle are reasonably clear but it would be difficult to replicate the hands-on training session owing to lack of information on the practical skills components (i.e. which of the line care topics had ‘hands-on’ activities) |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. Surveillance was conducted by four certified infection preventionists. They reviewed the medical record of every patient who had a positive blood culture, using a standard data collection form. Each case was then reviewed by the hospital epidemiologist to ensure that it met the case definition. Device-day data collected by ICU nursing staff were compared with data collected daily by the intravenous catheter management team to confirm the accuracy of data collection | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED, but stated that it was piloted | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? UNCLEAR. Stated that device-day data collected by ICU nursing staff were compared with data collected daily by the intravenous catheter management team to confirm the accuracy of data collection, but no data were reported | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? NOT REPORTED. No information on compliance with the post-insertion care bundle was reported |
HIGUERA (2005)103
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Higuera (2005)103 |
| Summary of approach | Single hospital infection control programme comprising 1-hour classes and feedback of compliance with catheter care and hand hygiene, conducted in two ICUs. Focused on CVC-associated BSI but also covered VAP and UTIs Part of an international multicentre project of nosocomial infection surveillance and control called the International Infection Control Consortium (49 ICUs of 39 hospitals in 28 cities of 12 countries) |
| Location | Mexico |
| Language | English |
| Critical care specialties | Medical SICU, neuroSICU |
| No. of critical care units | 2 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | General Hospital, Mexico City |
| Study design | Before-and-after study in two ICUs that were treated mainly as a single cohort; limited outcomes data reported for the ICUs separately |
| Study time periods | Baseline: June to August 2002 (3 months) Intervention: September 2002 to May 2003 (9 months) Follow-up: none (continuous monitoring of intervention) |
| Funding source | Supported by a grant from Baxter Health Care International |
| Conflicts of interest | Not reported |
Population and setting
All adult patients admitted to the study ICUs who had a CVC in place for at least 24 hours; patients had undergone neurosurgical, general and orthopaedic surgery or had severe medical illness. Full barrier precautions were used occasionally as resources permitted.
| Critical care unit characteristics: two level 3 ICUs in a 1000-bed public university hospital with six ICUs | Baseline: June to August 2003 (3 months) | Intervention: September 2002 to May 2003 (9 months) | Difference between baseline and intervention: relative risk (RR) (95% CI); p-value |
|---|---|---|---|
| Mean patients per month | 44.0 | 37.5 | Statistics not reported except: |
| No. of beds per ICU | 12 | 12 | Bed occupancy RR = 0.84 (0.69 to 1.02); p = 0.08 |
| Total available ICU bed-days | 2160 | 6480 | Nurse to patient ratio RR = 1.00 (0.91 to 1.00);a p = 1.00 |
| Actual bed occupancy, % | 45.9 | 40.1 | |
| Nurses per ICU per work shift | 6 | 6 | |
| Total available nurse days | 540 | 1620 | |
| Nurse–patient ratio | 0.54 | 0.61 | |
| Patient characteristics | |||
| Total no. of patients | 132 | 338 | |
| In medical-surgical ICUb | 52 | 170 | |
| In neuroSICUb | 80 | 168 | |
| Males, n (%) | 60 (45.5) | 163 (48.2) | p = 0.588 |
| Mean ± SD age, years | 44.32 ± 18.3 | 45.91 ± 17.88 | p = 0.422 |
| Diabetes, n (%) | 26 (19.7) | 72 (21.3) | p = 0.700 |
| Cancer, n (%) | 1 (0.8) | 7 (2.1) | p = 0.322 |
| Hypertension,c n (%) | 22 (16.7) | 75 (22.2) | p = 0.183 |
| Cardiac failure, n (%) | 3 (2.3) | 16 (4.7) | p = 0.223 |
| Chronic obstructive pulmonary disease, n (%) | 3 (2.3) | 16 (4.7) | p = 0.223 |
| Smoker, n (%) | 17 (12.9) | 55 (16.3) | p = 0.358 |
| Alcoholism, n (%) | 24 (18.2) | 65 (19.2) | p = 0.794 |
| Renal impairment, n (%) | 3 (2.3) | 19 (5.6) | p = 0.722 |
| Device characteristics | A semirigid plastic open infusion system was used instead of a collapsible flexible closed infusion system |
| Insertion site antisepsis used | Povidone–iodine |
| Dressing type and duration/frequency | If a dressing was used at all, it would be a gauze dressing; no transparent dressings were used |
Intervention characteristics
| Objective | To ascertain the effect of an infection control programme including process control on ICU rates of intravascular device-associated bloodstream infection (BSI) |
| Main focus of education | Insertion site dressing, intravenous administration set dating, and hand hygiene |
| Trainers (providers) | An infection control nurse presented the educational classes |
| Training of trainers | The project co-ordinator trained the data collectors at each ICU before the start of the study |
| Learners (recipients) | Health-care workers in the ICU (nurses, ancillary staff and physicians) |
| Target behaviour change | Insertion site dressing selection and placement, and hand hygiene before patient contact |
| Development and testing | Not reported |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | One-hour classes on epidemiology of nosocomial infections, hand hygiene, disinfection, prevention of intravascular device-associated BSIs, prevention of VAP, and prevention of UTIs Infection control guidelines: as published by the CDC. Not reported whether these were disseminated separately or included within the educational classes |
| Infection surveillance feedback approach | Not included in the intervention (active surveillance for infections took place during both the baseline and intervention periods but feedback to ICU staff was not reported) |
| Performance feedback approach | Compliance with hand hygiene and catheter care were assessed using a standardised form. Placement of gauze on intravascular device insertion sites, marking the date on the intravascular administration set, condition of the gauze dressing, and hand hygiene with alcohol hand rub or povidone–iodine soap before patient contact were assessed on a standard form by local researchers who observed health-care worker behaviours in the ICUs 5 days a week. The gauze dressing was inspected, and the presence or absence of blood, moisture, and gross soilage, and the appearance of the insertion site were noted. Feedback was provided monthly by a chart with columns representing each month showing rates of compliance with hand hygiene, gauze on CVC insertion sites, dates on intravenous administration sets, and maintenance of gauze dressings on catheter sites. The charts were posted on the walls of the ICU in a visible place in front of the health-care workers (nurses, ancillary staff and physicians). Validity and reliability of the data collection approach not reported |
| Concentration of education | Classes lasted 1 hour. Number of classes not reported. Classes were given to all the work shifts, with extra classes for new nurses (only few) and new medical residents. Not reported whether staff were re-educated annually |
| Non-educational intervention components | Alcohol hand rub or hand washing with povidone–iodine soap was started during the intervention period; previously regular non-antiseptic soap had been used |
| Costs reported | No |
Outcome characteristics
| Catheter-BSI definition Reference cited: Garner et al.147 |
Criterion 1: Laboratory-confirmed BSI: The patient had a recognised pathogen cultured from one or more percutaneous blood specimens after 48 hours of vascular catheterisation, and the pathogen isolated from the blood was not related to an infection at another site; for common skin commensals (e.g. diphtheroids, Bacillus spp., Propionibacterium spp., coagulase-negative staphylococci or micrococci), the organism was cultured from two or more blood specimens obtained on separate occasions Criterion 2: The patient had fever (temperature > 38 °C), hypotension, (systolic blood pressure < 90 mmHg), and/or oliguria (< 20 ml/hour) with no other recognised cause but blood specimens were not obtained for culture or organisms were not recovered from blood cultures; however, there was not apparent infection at another site, and the physician instituted treatment for sepsis Decisions to remove catheters and obtain blood specimens for culture were made independently by the patient’s attending physicians. CVCs were removed aseptically and the last 5 cm of the catheter tip was cultured using a semiquantitative method. All cultures were inoculated within 8 hours of catheter removal. For blood cultures, two blood samples (5–10 ml) were obtained from two separate veins within an interval of 15–20 minutes, inoculated in a 50-ml bottle, and sent to the microbiological laboratory |
| Outcomes reported | Primary: Rate of intravascular device-associated BSI Secondary: Hand hygiene Catheter care compliance: dressing use and placement; dating of the catheter administration set Hand hygiene compliance Mortality rate |
Results data
Primary outcomes
| Outcome | Baseline: June to August 2002 (3 months) | Intervention: September 2002 to May 2003 (9 months) | Difference between baseline and intervention: relative risk (RR) (95% CI); p-value | |
|---|---|---|---|---|
| Device duration | Not reported | Not reported | Not reported | |
| Device utilisation, CVC-days | 605 | 2824 | Not reported | |
| No. of devices/patient | Not reported | Not reported | Not reported | |
| No. of CVC-associated BSI | 28 | 55 | Not reported | |
| CVC-associated BSI incidence/1000 CVC-days | Total | 46.3 | 19.5 | RR = 0.42 (0.27 to 0.66); p = 0.0001 |
| Medical-surgical ICU | 57.4 | 22.1 | RR = 0.38 (0.22 to 0.68); p = 0.000 | |
| Neurosurgical ICU | 32.8 | 17.1 | RR = 0.52 (0.24 to 1.11); p = 0.08 | |
| CVC-associated BSI incidence/1000 patient-days | Not reported | Not reported | Not reported | |
| LOS | Not reported | Not reported | Not reported | |
| Mortality | Total deaths | 64/132 | 111/338 | RR = 0.68 (0.50 to 0.91);a p = 0.01 |
| Unadjusted mortality per 100 discharges | 48.5% | 32.8% | ||
Additional data on central venous catheter-associated BSI incidence/1000 central venous catheter-daysa
| Month | Medical-surgical ICU | Neurosurgical ICU |
|---|---|---|
| June 2003 | 35.7 | 13.1 |
| July 2003 | 67.7 | 36.6 |
| August 2003 | 43.2 | 31.3 |
| September 2003 | 40.8 | 16.9 |
| October 2003 | 15.5 | 24.2 |
| November 2003 | 16.5 | 19 |
| December 2003 | 15.9 | 6 |
| January 2004 | 46.2 | 16.4 |
| February 2004 | 14.9 | 18.2 |
| March 2004 | 15.3 | 9.4 |
| April 2004 | 18.3 | 32.3 |
| May 2004 | 21.9 | 13.9 |
Secondary outcomes
| Outcome | Baseline: June to August 2002 (3 months) | Intervention: September 2002 to May 2003 (9 months) | Difference between baseline and intervention: relative risk (RR) (95% CI); p-value |
|---|---|---|---|
| Compliance | |||
| Proper catheter care | |||
| No. of observations | 1413 | 2912 | |
| Gauze presence on insertion site | 86.69% | 99.24% | RR = 1.14 (1.07 to 1.22); p = 0.0000 |
| Proper gauze placement at insertion site | 84.21% | 97.87% | RR = 1.16 (1.09 to 1.24); p = 0.0000 |
| Dating of intravenous administration set | 40.69% | 93.85% | RR = 2.34 (2.14 to 2.56); p = 0.0000 |
| Hand hygiene before patient contact | |||
| No. of observations | 584 | 584 | |
| Hand hygiene practised | 62% | 62% | RR = 1.37 (1.21 to 1.51); p = 0.0000 |
| Reaction to education | Not a study outcome | ||
| Attitudes | Not a study outcome | ||
| Knowledge | Not a study outcome | ||
| Skills | Not a study outcome | ||
| Process evaluation | Not reported other than compliance with catheter care and hand hygiene was assessed and reported back to ICU staff (as summarised above) | ||
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NO. Patients were similar with regard to sex, age, major disease, smoking and alcoholism. Available beds and nurse to patient ratios were also similar in the baseline and intervention periods. Bed occupancy and mean number of patients per month were slightly lower (more favourable) in the intervention period but the differences were not statistically significant |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? UNCLEAR. The authors stated that a programme to improve hand hygiene was already in place in the neurosurgical ICU but the implementation date was not reported |
| Was the effect of educational practice separable from effects of non-educational practice? YES. The intervention was primarily educational (including information provision for compliance). Alcohol hand rub or hand washing with povidone iodine soap was started during the intervention period but although not an educational activity in itself this was a target of the education; no information was reported to suggest that use of the soap was due to non-educational factors (such as changes in availability) | |
| Were the intervention component(s) implemented as planned? UNCLEAR. Compliance with the educational classes themselves (e.g. whether participation was mandatory for all ICU staff) was not reported and it is unclear whether dissemination of the CDC infection control guidelines was included in the classes or separate (and if the latter, how implementation was achieved). However, compliance with all target behaviours statistically significantly improved (did not reach 100%) | |
| Missing data | Were there systematic differences between the baseline and intervention groups in attrition or exclusions (includes withdrawal due to mortality; missing outcome data)? UNCLEAR. The authors report two different sets of data on the number of patients recruited. Tables 1–4 each agree that the number was 132 in the baseline period and 338 in the intervention period. The text states that the numbers for baseline and intervention periods were, respectively, 173 and 297 (but overall total of 470 patients is the same). Mortality rate was significantly lower in the intervention period; it is unclear whether this was a consequence of the intervention (not discussed by the authors) |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? UNCLEAR. The definition of infections used appears to be that used within the International Infection Control Consortium; no changes in definition or microbiological culture technique during the study were reported. However, no information about staff diagnosing infections was given |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | The authors stated that study centre data collection sheets were checked for potential errors and missing items by the project co-ordinator to confirm each diagnosis of intravascular device-associated BSI, but no checking of compliance data was reported |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? PARTIALLY. The process feedback approach is reported well enough to be repeated in principle. However, the educational classes are reported superficially and the number of classes required to achieve the observed intervention effect is not clear. The dissemination approach for CDC guidelines is also not clear (whether standalone or included in classes) |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. A medical doctor at each ICU collected infection data prospectively from patient charts. Compliance data were collected by unspecified local researchers who observed health-care worker behaviours in the ICUs 5 days a week. Not reported how the data were stored after collection | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED. However, the authors stated that checks for errors and missing data were conducted for infection surveillance (see above) | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? YES. Compliance with the target behaviours (insertion site dressing selection and use; hand hygiene before patient contact) was increased by the intervention (compliance was high but did not reach 100%). |
LOBO (2005)108
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Lobo et al. (2005)108 |
| Summary of approach | Single unit educational programme targeted to specific points observed during CVC care practices on decreasing CVC-blood stream infections (BSI) in a MICU |
| Location | Brazil |
| Language | English |
| Critical care specialty | MICU |
| No. of critical care units | 1 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Hospital das Clinicas of University of São Paulo |
| Study design | Cohort before-and-after study |
| Study time periods | Baseline: January 2001 to April 2002 (Phase 1: described as 1-year pre-intervention period and 3 months observation; described as 16 months in Results section) Intervention: May 2002 to December 2002 (Phase 2: 8 months, including an observation period) Follow-up: CVC-BSI were also evaluated during the following year after the educational intervention (Phase 3) (Note: monthly surveillance feedback and prevention guide that was given to all medical residents were continued during follow-up) |
| Funding source | None reported |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics: The nursing staff was responsible for the CVC dressing and line care; the medical residents were responsible for CVC insertion and replacement; and CVC manipulation was done by both. ICU staff: five physicians, 36 medical residents, 23 assistant nurses and 10 nurses | |
| Patient population characteristics | All adult patients admitted to the ICU with a CVC for > 24 hours |
| Device characteristics | Device reported as CVC, no other details reported |
| Insertion site antisepsis used | Alcohol (chlorhexidine was not available at the hospital) |
| Dressing type and duration/frequency | Not reported |
Intervention characteristics
| Objective | Determine the impact of an educational programme targeted to specific points observed during CVC care practices on decreasing CVC-BSI in a MICU |
| Main focus of education | Hand washing, skin preparation of CVC insertion, pathogenesis of CVC infection and standardisation of CVC manipulation |
| Trainers (providers) | Not reported (the infection control committee made the posters and set up the hand washing campaign) |
| Training of trainers | Not reported |
| Learners (recipients) | The entire unit staff was involved in monthly classes and discussion of infection rates |
| Target behaviour change | Hand hygiene, sterile catheter insertion procedure and CVC maintenance |
| Development and testing | The educational programme was developed by a multidisciplinary task force to highlight correct practices for CVC insertion, manipulation, and care. The multidisciplinary task force included three infection control nurses, one physician, and the entire staff of the unit. The educational programme was based on observations by the infection control staff of CVC care practices in the ICU |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted The programme consisted of a pre-test and monthly classes, posters, discussions with staff, and a new observation period (Phase 2). The programme was applied for the same people during the study period |
Pre-test: included 10 questions concerning hand washing, information about when the health-care professional should wash their hands (before and after contact with the patient, before and after CVC insertion, manipulation and dressing) and how (with alcohol povidone–iodine or chlorhexidine), and that the use of glove does not exclude hand washing. The pre-test also covered CVC insertion (skin preparation, CVC site, technique, and the use of maximum barriers), CVC dressing (type, replacement and skin preparation), CVC manipulation (disinfection), hub and line care (replacement) and CVC replacement Observation Phase 1: 3 months Observation checklist: included hand washing, CVC insertion (skin preparation, site, technique, and use of maximum barrier), CVC dressing (hand washing; type, frequency of replacement, skin care), and CVC manipulation (hand washing, hub disinfecting and line care) Intervention: Classes/discussion: monthly classes and discussion with staff on infection rates Hand washing campaign: use of chlorhexidine and posters with hand washing classes (hand-washing product remained the same pre and post intervention and was not alcohol based). Colourful stamps: with tips to remind the unit staff of the importance of hand washing and CVC care were placed on the CVC insertion site or on the CVC lines Posters: concerning hand hygiene and pathogenesis of CVC infection CVC-BSI prevention guide: the catheter care policy included hand washing with chlorhexidine before and after any patient care and recommendations concerning CVC insertion (skin preparation with povidone–iodine and skin antisepsis with 70% alcohol povidone–iodine and use of maximum barriers; sterile gown and gloves, large sheets, cap and surgical mask, dressing (skin care with alcohol povidone–iodine and dry gauze, replacement every 24 hours and when wet), manipulation (hub disinfection with 70% alcohol), line care (replacement every 72 hours for administration sets, every 24 hours for lipid emulsion, and immediately removed after use for blood products), and CVC replacement (no routine replacement except when clinically indicated, e.g. if unexplained fever). Observation Phase 2: checklist (not explicitly stated, but presumed to be the same as pre-intervention) on hand washing, CVC insertion (skin preparation, site, technique and use of maximum barriers), CVC dressing (hand washing; type, frequency of replacement, skin care) and CVC manipulation (hand washing, hub disinfecting and line care) |
| Infection surveillance feedback approach | Monthly feedback on the CVC-BSI rate during the first year after the end of the intervention was given to the unit and the CVC-BSI prevention guide was given to all medical residents. Unclear if feedback was practiced prior to intervention. Validation and reliability of the reporting process not stated |
| Performance feedback approach | Not reported |
| Concentration of education | Stated monthly classes, no other details reported |
| Non-educational intervention components | None (intervention purely educational) |
| Costs reported | Not reported (stated campaign was done with own resources) |
Outcome characteristics
| Catheter-BSI definition Reference cited: Garner et al.147 |
All patients with CVC for > 24 hours admitted to ICU were included in study. Laboratory-confirmed CVC-BSI was defined according to the Centers for Disease Control and Prevention (CDC). The infection was regarded as ICU acquired if it occurred during ICU stay or within 48 hours of discharge from the MICU. CVC-BSI representing primary bloodstream infection was defined by one of the following clinical signs or symptoms with no other recognised cause: recognised pathogen isolated from blood culture not related to infection at another site; common skin contaminant isolated, such as coagulase-negative Staphylococcus from two or more blood cultures drawn on separate occasions, within 24 hours, unrelated to infection at another site; and patients had at least one of the following signs or symptoms – fever of > 37.8 °C, chills or hypotension |
| Outcomes reported | Primary outcome: rate of CVC-BSI Secondary outcomes: compliance with the CVC-BSI prevention guide |
Results data
Primary outcomes
| Outcome | Baseline (16 months), n = 316 | Intervention (8 months), n = 190 | Follow-up (12 months), n = 266 | Difference between baseline, intervention and follow-up periods |
|---|---|---|---|---|
| n = no. of patients | ||||
| CVC-days | Not reported | Not reported | Not reported | Not reported |
| Total device utilisation, days | 2450 | 1481 | 1701 | Not reported |
| No. of devices/patient | Not reported | Not reported | Not reported | Not reported |
| CLAB incidence rate | 48 | 16 | 22 | Not reported |
| CLAB incidence per 1000 catheter-days | 20 | 11 | 12 | Baseline to post intervention: 40% reduction (no p-value reported)a |
| CLAB incidence per 1000 patient-days | Not reported | Not reported | Not reported | Not reported |
| LOS | Not reported | Not reported | Not reported | Not reported |
| Mortality | Not reported | Not reported | Not reported | Not reported |
Secondary outcomes
| Outcome | Baseline (16 months) | Intervention (8 months) | Difference in compliance between baseline and intervention [relative risk (RR) (95% CI); p-value]a |
|---|---|---|---|
| Reaction to education | Not a study outcome | ||
| Attitudes | Not a study outcome | ||
| Compliance, n (%) | |||
| CVC insertion (medical residents) | n = 22 | n = 22 | |
| Hand washing before (chlorhexidine) | 22 (100) | 22 (100) | |
| Maximal barrier (gloves, gown, mask) | 20 (91) | 22 (100) | 0.91 (0.80 to 1.04a); p = 0.147 |
| Skin antisepsis/alcohol-based povidone–iodine | 2 (10) | 22 (100) | 11.0 (2.93 to 41.2a); p < 0.001 |
| CVC manipulation (nurses and medical residents) | n = 42 | n = 46 | |
| Hand washing before (chlorhexidine) | 2 (5) | 28 (55) | 12.78 (3.24 to 50.42); p < 0.001 |
| Glove use | 17 (40) | 45 (98)b | 2.36 (1.64 to 3.40a); p < 0.001 |
| Line protection | 29 (69)b | 45 (98)b | 1.38 (1.13 to 1.69a); p < 0.001 |
| Hub disinfection | 14 (34) | 43 (90) | 2.74 (1.78 to 4.22a); p < 0.001 |
| Hand washing after (chlorhexidine) | 16 (38) | 20 (43) | 1.14 (0.69 to 1.90a); p = 0.6079 |
| CVC dressing (nurses) | n = 31 | n = 31 | |
| Hand washing before (chlorhexidine) | 14 (45) | 21 (68) | 1.50 (0.95 to 2.37); p = 0.072 |
| Glove use | 30 (97) | 30 (97) | 1.00 (0.91 to 1.10); p = 1.00 |
| Alcohol povidone–iodine antisepsis | 1 (3) | 31 (100) | 31.00 (4.51 to 213.18); p ≤ 0.001 |
| Hand washing after (chlorhexidine) | 8 (26) | 18 (58)b | 2.25 (1.15 to 4.39); p = 0.010 |
| Knowledge | Not a study outcome | ||
| Skills | Not a study outcome | ||
| Process evaluation | Not reported other than compliance (above) | ||
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NOT REPORTED |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? NOT REPORTED. However, authors stated that criteria to consider a common skin contaminant as a cause of CVC-BSI were rigid and that the pre- and post-intervention programme was applied to the same people during the study period |
| Was the effect of educational practice separable from effects of non-educational practice? YES. Education-only intervention | |
| Were the intervention component(s) implemented as planned? UNCLEAR. Stated in methods section that the entire unit staff was involved in the monthly classes and discussion of infection rates, but implementation was not reported in the results. All staff took part in a pre-test but post-testing was not reported. Authors stated they noticed a profound involvement of the unit staff during the educational period but did not give details | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? UNCLEAR. The combined number of nurses and medical residents differed from pre- (n = 42) to post-intervention (n = 46) for observations on CVC manipulation data. However, the number of medical residents was the same both pre- and post-intervention for observations on CVC insertion, as was the number of nurses' observations on CVC dressing |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? NOT REPORTED |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? UNCLEAR. Blinding of assessors reported. Stated that the observation was blinded to following the CVC dressing, manipulation, and care. However, also stated that at times the infection staff was contacted to follow the CVC insertion (mainly during the night) | |
| Other possible sources of bias | No other sources of bias were reported by the authors or identified by the reviewers based on the information presented |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. Details about the structure, content and time resources required were not reported |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. Surveillance of nosocomial infections was conducted by 2 infection control nurses, who visited the medical unit daily | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? PARTLY. Compliance with hand hygiene did not improve significantly but other behaviours did. Compliance statistically significantly increased in four out of five target behaviour components for CVC manipulation, in one of three target behaviour components for CVC insertion, and one out of four target behaviour components for CVC dressing. Compliance with all target behaviour components increased only for CVC manipulation, but was inconsistent for CVC insertion and CVC dressing |
Additional comments
-
Device-days not defined (i.e. unclear whether accounts for multiple simultaneous CVCs in a patient).
-
Not reported whether participants were aware they were in a study.
-
Pre-test and the pre-intervention observation period showed that compliance with the guideline was low, with major problems concerning skin preparation during CVC insertion, disinfection of CVC during manipulation, and non-use of an alcohol-based product during the CVC dressing. Knowledge of CVC insertion procedures and line care were reasonable, but low compliance with disinfection of the CVC hub during manipulation and use of an alcohol-based product during preparation of the CVC dressing because only saline solution was used (data not extracted).
-
Authors stated that the distribution of pathogens was different comparing the pre- and post-intervention period. S. aureus was the most common pathogen in phase 1 and decreased significantly during the study period (p = 0.02).
-
Also stated that the adhesion to the overall catheter care policy improved significantly in the post-intervention period (p = 0.01).
LOBO (2010)109
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Lobo et al. (2010)109 |
| Summary of approach | Single centre evaluation of the impact of two models of educational intervention (tailored continuous intervention vs. a single lecture) on the rate of CVC-associated bloodstream infections (CVC-BSI) in 2 MICUs |
| Location | Brazil |
| Language | English |
| Critical care specialty | MICU |
| No. of critical care units | 2 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Hospital das Clinicas, São Paulo, Brazil |
| Study design | Cohort before-and-after study comprising two separate cohorts with different interventions |
| Study time periods | Baseline (surveillance): January 2005 to December 2005 (12 months) Pre-intervention (knowledge assessment and observation of CVC care): January 2006 to September 2006 (9 months) Intervention (differed in the two ICUs): October 2006 to June 2007 (9 months) Follow-up: None (tailored continuous educational programme) but stated that continued educational intervention resulted in no CVC-BSIs in 2008 |
| Funding source | Main author received a grant from CAPES. (Not defined by the authors; assumed to refer to the CAPES Foundation – a Brazilian government agency awarding scholarship grants to graduate students at universities and research centres) |
| Conflicts of interest | None stated |
Population and setting
| Critical care unit characteristics | 1000-bed tertiary care teaching hospital attached to the University of São Paulo. Medical residents rotated each 40 days | ||
|---|---|---|---|
| ICU A | ICU B (control) | ||
| Beds | 5 | 8 | |
| Physician–bed ratio | 1 : 5 | 1 : 8 | |
| Medical resident–bed ratio | 1 : 1 | 7 : 8 | |
| Registered nurse–bed ratio | 1 : 5 | 3 : 8 | |
| Assistant nurse–bed ratio | 2 : 5 | 4 : 8 | |
| Sinks–bed ratio | 1 : 3 | 1 : 3 | |
| Alcohol-based gel dispensers–bed ratio | 1 : 2 | 1 : 2 | |
| Patient population characteristics | ICU A | ||
|---|---|---|---|
| Pre-intervention: n = 141 | Intervention: n = 41 | Difference between pre-intervention and intervention | |
| Patient-days | 1699 | 1341 | Not reported |
| CVC days | 940 | 843 | Not reported |
| Age, years: median (range) | 51 (8–87) | 50 (13–86) | p = 0.832 |
| Age, years: mean (SD) | 54 (± 21) | 53 (± 20) | |
| Male: n, (%) | 87 (62) | 18 (43) | p = 0.042 |
| Underlying diseases, n | p = 0.890 | ||
| Cancer | 12 | 2 | |
| Cardiovascular disease | 6 | 7 | |
| Surgical condition | 8 | 5 | |
| Diabetes mellitus | 0 | 0 | |
| Haematological disease | 0 | 0 | |
| Infectious disease | 22 | 5 | |
| Neurological disease | 0 | 0 | |
| Other diseases | 46 | 12 | |
| Pneumonia | 23 | 5 | |
| Renal failure | 11 | 0 | |
| Respiratory failure | 13 | 5 | |
| Septic shock | 0 | 0 | |
| Patient population characteristics | ICU B | ||
|---|---|---|---|
| Pre-intervention, n = 378 | Intervention, n = 262 | Difference between pre-intervention and intervention | |
| Patient-days | 3704 | 2523 | Not reported |
| CVC-days | 2175 | 1694 | Not reported |
| Age, years median (range) | 53 (11–97) | 50 (14–93) | p = 0.045 |
| Age, years mean (± SD) | 55 (± 20) | 51 (± 19) | |
| Male, n (%) | 6 (10) | 6 (14) | p = 0.815 |
| Underlying diseases, n | p = 0.386 | ||
| Cancer | 22 | 14 | |
| Cardiovascular disease | 50 | 26 | |
| Surgical condition | 20 | 13 | |
| Diabetes mellitus | 18 | 20 | |
| Haematological disease | 16 | 4 | |
| Infectious disease | 36 | 27 | |
| Neurological disease | 47 | 37 | |
| Other diseases | 69 | 40 | |
| Pneumonia | 34 | 23 | |
| Renal failure | 14 | 9 | |
| Respiratory failure | 36 | 30 | |
| Septic shock | 16 | 19 | |
| Device characteristics | Device only described as CVC, no further details reported | ||
| Insertion site antisepsis used | Standardised chlorhexidine gluconate 0.5% alcoholic – remained unchanged during the study | ||
| Dressing type and duration/frequency | Not reported | ||
Intervention characteristics
| Objective | Evaluate the impact of two models of educational intervention on the rate of CVC-BSI |
| Main focus of education | Hand washing, CVC care and maintenance |
| Trainers (providers) | Not reported |
| Training of trainers | Not reported |
| Learners (recipients) | All staff and medical residents in both ICUs |
| Target behaviour change | Adherence to CVC practices |
| Development and testing | The programme in ICU A was based on problems found during an observation phase |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Questionnaire pre-intervention period (ICU A and B): 11-question questionnaire designed to evaluate health-care workers' knowledge of the Nosocomial Infection Control Committee's recommendations regarding CVC care (adapted from CDC guidelines; reference cited) was applied in both ICUs. The questionnaire covered the following issues:
|
| Second observation period (checklist): started 1 month after the start of the intervention period in ICU A and 1 month after the lecture in ICU B ICU A: Questionnaire (same as at pre-intervention): administered on a monthly basis as an educational strategy to medical residents Monthly lectures: CVC care for all new medical residents Lectures to small groups: simple messages, focusing on problems identified in the observation phase Lectures on hand hygiene practices: intervention lectures included hand hygiene before and after any CVC care; use of alcohol-based gel or hand hygiene with soap for soiled hands; replacement of intravenous tubing (including add-on devices) no more frequently than at 72-hour intervals; replacement of tubing used to administer blood, blood products or lipid emulsions within 24 hours of initiating infusion; replacement of the extension tubing used for intermittent infusions at every catheter change; use of alcoholic chlorhexidine gluconate 0.5% solution for skin antisepsis during catheter dressing; replacement of gauze dressing every 24 hours or when damp, loose or visibly soiled; replacement of transparent dressings every 7 days or when damp, loose or visibly soiled; protection of the catheter with impermeable material during showering; disinfection of hub and line with 70% alcohol; and protection of line and sets during catheter handling Posters: displayed in the unit with stimulating messages in visible places and containing a step-by-step description of hand hygiene Colourful labels with tips: reminder to the health-care worker of the importance of CVC care ICU B: Lecture: one lecture on CVC care given to all staff and medical residents who rotated in the unit during this period (immediately after the pre-intervention period) Observation checklist: included CVC insertion (hand hygiene, skin antisepsis, and use of maximal barrier precautions); CVC dressing (hand hygiene, glove use, skin antisepsis); CVC handling (hand hygiene, glove use, hub disinfection) |
|
| Infection surveillance feedback approach | Surveillance was done by three infection control nurses who visited the ICUs daily. Intervention period: monthly updates of CVC-BSI rates posted in multiple locations in ICU A only. Validation and reliability of the reporting process not stated |
| Performance feedback approach | Intervention period: Feedback to the entire staff of ICU A regarding problems noted during the observation phase. No further details reported. Validation and reliability of the reporting process not stated |
| Concentration of education | Monthly lectures and monthly questionnaire in ICU A; single lecture in ICU B. Lengths of lectures not reported |
| Non-educational intervention components | None (intervention purely educational) |
| Costs reported | Not reported |
Outcome characteristics
| Catheter-BSI definition Reference cited: Garner et al.147 |
Diagnosis of infection was based on CDC criteria. CVC-BSI was defined as primary BSI in the presence of a CVC. Only laboratory-documented infections were included. Each patient was followed up until 48 hours after discharge from the ICU |
| Outcomes reported | Not stated whether primary or secondary:
|
Results data
Primary outcomes
| Outcome | ICU A | Difference between baseline, pre-intervention and intervention | ||
|---|---|---|---|---|
| Baseline (12 months) | Pre-intervention (9 months) | Intervention (9 months) | ||
| Device duration | Not reported | Not reported | Not reported | Not reported |
| CVC-days | Not reported | 940 | 843 | Not reported |
| Device utilisation rate a | 0.72 | 0.55 | Not reported | |
| Month 1: 0.53 | Not reported | |||
| Month 2: 0.65 | Not reported | |||
| Month 3: 0.75 | Not reported | |||
| Month 4: 0.64 | Not reported | |||
| Month 5: 0.62 | Not reported | |||
| Month 6: 0.59 | Not reported | |||
| Month 7: 0.7 | Not reported | |||
| Month 8: Not reported | Not reported | |||
| Month 9: Not reported | Not reported | |||
| Patient-days | 1699 | 1341 | Not reported | Not reported |
| No. of devices/patient | Not reported | Not reported | Not reported | Not reported |
| CLAB incidence per 1000 catheter-days | Not reported | 12 | 10.6 | p = 0.03 (pre-intervention vs. intervention) (chi-squared test for trend) |
| Month 1: 0 | Not reported | |||
| Month 2: 10.3 | Not reported | |||
| Month 3: 8.8 | Not reported | |||
| Month 4: 0 | Not reported | |||
| Month 5: 0 | Not reported | |||
| Month 6: 8.8 | Not reported | |||
| Month 7: 0 | Not reported | |||
| Month 8: 0 | Not reported | |||
| Month 9: 0 | Not reported | |||
| CLAB incidence per 1000 patient-days | Not reported | Not reported | Not reported | Not reported |
| LOS, days: median (range), mean (SD) | Not reported | 7 (1–32), 5 (7) | 9 (1–57), 4 (11) | p = 0.386 |
| Mortality | Not reported | Not reported | Not reported | Not reported |
| Primary outcomes | ICU B | Difference between baseline, pre-intervention and intervention | ||
|---|---|---|---|---|
| Baseline (12 months) | Pre-intervention (9 months) | Intervention (9 months) | ||
| Device duration | Not reported | Not reported | Not reported | Not reported |
| CVC-days | Not reported | 2175 | 1694 | Not reported |
| Device utilisation rate a | 0.61 | 0.59 | Month 1: 0.67b | Not reported |
| Month 2: 0.68 | Not reported | |||
| Month 3: 0.74 | Not reported | |||
| Month 4: 0.7 | Not reported | |||
| Month 5: 0.69 | Not reported | |||
| Month 6: 0.7 | Not reported | |||
| Month 7: 0.69 | Not reported | |||
| Month 8: 0.63 | Not reported | |||
| Month 9: 0.64 | Not reported | |||
| Patient-days | 3704 | 2523 | Not reported | Not reported |
| No. of devices/patient | Not reported | Not reported | Not reported | Not reported |
| CLAB incidence rate | Not reported | Not reported | Not reported | Not reported |
| CLAB incidence per 1000 catheter-days | Not reported | 16.2 | Overall: 12.9 | Not reported by month p = 0.41 (pre-intervention vs. intervention) (chi-squared test for trend) |
| Month 1: 0 | ||||
| Month 2: 0 | ||||
| Month 3: 7.41 | ||||
| Month 4: 11.43 | ||||
| Month 5: 0 | ||||
| Month 6: 5.59 | ||||
| Month 7: 4.24 | ||||
| Month 8: 3.44 | ||||
| Month 9: 13.7 | ||||
| CLAB incidence per 1000 patient-days | Not reported | Not reported | Not reported | Not reported |
| LOS, days: median (range), mean (SD) | Not reported | 10 (1–99), 6 (10) | 11 (1–112), 6 (14) | p = 0.402 |
| Mortality | Not reported | Not reported | Not reported | Not reported |
Secondary outcomes
| Reaction to education | Not a study outcome | ||
| Attitudes | Not a study outcome | ||
| Compliance (ICU A) ( n = no. of observations) | Pre-intervention (9 months), n (%) | Intervention (9 months), n (%) | Difference in compliance between baseline and intervention: relative risk (RR) (95% CI); p -value) |
| CVC insertion | n = 12 | n = 12 | |
| Hand hygiene before procedure | 12 (100) | 12 (100) | |
| Maximal barrier precautions | 12 (100) | 12 (100) | |
| Skin antisepsis | 11 (100) | 12 (100) | |
| Hand hygiene after procedure | 7 (58) | 10 (83) | 1.43 (0.83 to 2.45); p = 0.370 |
| CVC handling | n = 63 | n = 63 | |
| Hand hygiene before procedure | 22 (35) | 51 (81) | 2.32 (1.62 to 3.32); p < 0.001 |
| Washing with chlorhexidine soap | 18 (29) | 26 (41) | Not reported |
| Alcohol-based gel | 4 (6) | 25 (40) | Not reported |
| Use of gloves | 63 (100) | 63 (100) | |
| Disinfection of hub | 43 (68) | 61 (97) | 1.42 (1.19 to 1.69); p < 0.001 |
| Hand hygiene after procedure | 12 (19) | 53 (84) | 4.42 (2.63 to 7.43), p < 0.001 |
| Washing with chlorhexidine soap | 6 (9) | 27 (43) | Not reported |
| Alcohol-based gel | 6 (9) | 26 (41) | Not reported |
| CVC dressing | n = 50 | n = 50 | |
| Hand hygiene before procedure | 14 (28) | 49 (98) | 3.50 (2.24 to 5.47); p < 0.001 |
| Washing with chlorhexidine soap | 12 (24) | 8 (16) | Not reported |
| Alcohol-based gel | 2 (4) | 41 (82) | Not reported |
| Use of gloves | 50 (100) | 50 (100) | Not reported |
| Skin antisepsis | 27 (54) | 50 (100) | 1.85 (1.43 to 2.39); p < 0.001 |
| Hand hygiene after procedure | 17 (34) | 48 (96) | 2.82 (1.91 to 4.17); p < 0.001 |
| Washing with chlorhexidine soap | 10 (20) | 22 (44) | Not reported |
| Alcohol-based gel | 7 (14) | 26 (52) | Not reported |
| Compliance (ICU B) ( n = no. of observations) | Pre-intervention, n (%) (9 months) | Intervention, n (%) (9 months) | Difference in compliance between pre-intervention and intervention: relative risk (RR) (95% CI); p -value |
| CVC insertion | n = 12 | n = 12 | |
| Hand hygiene before procedure | 12 (100) | 12 (100) | |
| Maximal barrier precautions | 12 (100) | 12 (100) | |
| Skin antisepsis | 12 (100) | 12 (100) | |
| Hand hygiene after procedure | 8 (67) | 8 (67) | |
| CVC handling | n = 34 | n = 34 | |
| Hand hygiene before procedure | 5 (15) | 16 (48) | 3.20 (1.33 to 7.72); p = 0.008 |
| Washing with chlorhexidine soap | 3 (9) | 15 (45) | Not reported |
| Alcohol-based gel | 2 (6) | 1 (3) | Not reported |
| Use of gloves | 34 (100) | 34 (100) | |
| Disinfection of hub | 15 (44)a | 27 (82) | 1.80 (1.20 to 2.70; p < 0.004 |
| Hand hygiene after procedure | 3 (9) | 18 (54) | 6.00 (1.95 to 18.44), p < 0.001 |
| Washing with chlorhexidine soap | 3 (9) | 13 (39) | Not reported |
| Alcohol-based gel | 0 | 5 (15) | Not reported |
| CVC dressing | n = 33 | n = 33 | |
| Hand hygiene before procedure | 2 (6) | 25 (76) | 12.50 (3.22 to 48.56); p < 0.001 |
| Washing with chlorhexidine soap | 2 (6) | 23(70) | Not reported |
| Alcohol-based gel | 0 | 2 (6) | Not reported |
| Use of gloves | 33 (100) | 33 (100) | |
| Skin antisepsis | 9 (27) | 32 (97) | 3.56 (2.03 to 6.23); p < 0.001 |
| Hand hygiene after procedure | 9 (27) | 21 (64) | 2.33 (1.26 to 4.31); p < 0.006 |
| Washing with chlorhexidine soap | 9 (27) | 18 (55) | Not reported |
| Alcohol-based gel | 0 | 3 (9) | Not reported |
| Knowledge | Not a study outcome | ||
| Skills | Not a study outcome | ||
| Process evaluation | See above compliance with CVC practices | ||
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? YES. There was no statistical comparison between ICU A and ICU B. Reported data that showed minor differences between the study periods in age, gender, LOS and frequency of underlying diseases. However, in both ICUs the number of patient-days and CVC-days were consistently lower in the intervention period than in the pre-intervention period |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups or stratification by propensity scores)? NOT REPORTED | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? YES. Authors report that both ICUs were located within the same building, that the chief nurse of ICU A was transferred to ICU B during the study, and that nurses and physicians of all hospital units attended regular general meetings during which the success in ICU A was reported. This could have affected health-care workers' behaviour and had an impact on other units as well, possibly contributing to the initial decreased CVC-BSI rate in ICU B |
| Was the effect of educational practice separable from effects of non-educational practice? YES (education alone) | |
| Were the intervention component(s) implemented as planned? NOT REPORTED. Stated that all staff in both ICUs responded to the questionnaire. However, the percentage of staff observed is unclear and there were a higher number of observations for CVC handling and dressing in ICU A, which had much lower patient numbers than ICU B during the intervention period (n = 41 vs. n = 262, respectively) | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? UNCLEAR. Patient numbers differed considerably between ICUs, as well as between baseline and intervention |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? UNCLEAR. Authors implied (not explicitly stated) that the definition of infections and the approach for assessing outcomes would have been the same in both study periods. However, no information was given on the staff diagnosing infections |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | Stated that the high compliance with the CVC insertion recommendations observed in the study was likely related to a previous intervention performed in the hospital; however, these recommendations were not stressed in the present educational intervention. CVC-BSI rates already improved in the pre-intervention period prior to the start of the intervention in both ICUs |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? PARTIALLY. Good description of the content of the intervention, but no breakdown of the content of each lecture or information about lecture duration |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. Three infection control nurses visited the ICUs daily and each patient was followed until 48 hours after discharge from ICU. However, does not state whether manual or electronic record management was used | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? PARTIALLY. Adherence to CVC practice was not 100% in all areas |
Additional comments
-
Not reported whether the participants knew that they were being observed.
-
Device-days not defined (i.e. unclear whether captures multiple simultaneous CVCs/patients).
-
Authors were contacted for CVC utilisation rate definition (defined as: number of central line-days/number of patient-days).
LONGMATE (2011)110
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Longmate (2011)110 |
| Summary of approach | Single ICU, 4-year QI intervention based on gradual and iterative implementation of bundles, including infection surveillance feedback and performance feedback |
| Location | Scotland |
| Language | English |
| Critical care specialty | MICU and SICU |
| No. of critical care units | 1 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Stirling Royal Infirmary, Stirling |
| Study design | Single-cohort before-and-after study with phased implementation of interventions |
| Study time periods | Baseline 1 September 2005 to 31 December 2006: Infection surveillance and interventions aimed at improving hand hygiene practices January 2007: CRBSI bundles and education implemented March 2007 to August 2009: interventions to reduce VAP March 2008: Scottish Patient Safety Programme launched in the ICU [stated this was for the last 16 months of the study (online Appendix 6) or last 18 months (primary publication)] November to December 2008: centralisation of CVC insertion pack March 2009: Three-nurse team assembled to support CVC bundles August 2009: End of monitoring Follow-up: None (continuous monitoring of QI programme) |
| Funding source | Stated that Health Protection Scotland provided funding for a nurse salary for the second year of the project |
| Conflicts of interest | Two authors had completed or were taking a SPSP Fellowship; one author was Nursing Lead for the Critical Care work stream of the SPSP at Stirling Royal Infirmary |
Population and setting
Critical care unit characteristics
Nine-bed ICU in a general hospital. Full aseptic technique was not consistently performed at catheter insertion. For established CVCs there was variation in hand hygiene practices, and techniques for commencing injections and infusions. No daily prompt and no consistent approach was used for CVC removal (common practice was routine replacement after 7 days, sometimes over guidewire)
CVC defined as any i.v. catheter ending at or near the heart. Every patient admitted for more than 48 hours who during at least part of their admission had a CVC was assessed daily until discharge for CRBSI occurrence
| Baseline (year 1) (September 2005 to August 2006)a | Intervention (January 2007 to 31 August 2009)b | Difference between baseline and intervention | |
|---|---|---|---|
| Patient population characteristics (all ICU patients) | |||
| No. of patients admitted | 439 | Not tested statistically | |
| September 2007 to August 2008 (year 3) | 465 | ||
| September 2008 to August 2009 (year 4) | 358 | ||
| Patients ventilated, % | 69 | Not tested statistically | |
| September 2007 to August 2008 (year 3) | 74 | ||
| September 2008 to August 2009 (year 4) | 73 | ||
| Mean (SD) APACHE II score | 19.8 (8.4) | ||
| September 2007 to August 2008 (year 3) | 21.0 (7.3) | Year 3 vs. 1: p = 0.89 | |
| September 2008 to August 2009 (year 4) | 18.7 (8.7) | Year 4 vs. 1: p = 0.38 | |
| Median LOS, days | 2.2 | ||
| September 2007 to August 2008 (year 3) | 2.5 | Year 3 vs. 1: p = 0.18 | |
| September 2008 to August 2009 (year 4) | 2.3 | Year 4 vs. 1: p = 0.72 | |
| ICU mortality rate, % | 20 | ||
| September 2007 to August 2008 (year 3) | 22 | Year 3 vs. 1: p = 0.48 | |
| September 2008 to August 2009 (year 4) | 22.6 | Year 4 vs. 1: p = 0.38 | |
| Male gender, n (%) for subgroup with CVC and ICU stay > 2 days | 143/255 (56.1) | Not tested statistically | |
| September 2007 to August 2008 (year 3) | 127/235 (54.0) | ||
| September 2008 to August 2009 (year 4) | 125/225 (55.5) | ||
| Device characteristics | |||
| Total no. of CVCs | 414 | ||
| September 2007 to August 2008 (year 3) | 317 | Year 3 vs. 1: p < 0.01 | |
| September 2008 to August 2009 (year 4) | 249 | Year 4 vs. 1: p < 0.01 | |
| Insertion site, n (%) | |||
| Internal jugular | 332 (80.2) | ||
| September 2007 to August 2008 (year 3) | 283 (89.3) | Year 3 vs. 1: p < 0.01 | |
| September 2008 to August 2009 (year 4) | 227 (91.1) | Year 4 vs. 1: p < 0.01 | |
| Subclavian | 63 (15.2) | ||
| September 2007 to August 2008 (year 3) | 24 (7.6) | Year 3 vs. 1: p < 0.01 | |
| September 2008 to August 2009 (year 4) | 15 (6.02) | Year 4 vs. 1: p < 0.01 | |
| Femoral | 19 (4.6) | ||
| September 2007 to August 2008 (year 3) | 10 (3.1) | Year 3 vs. 1: p > 0.33 | |
| September 2008 to August 2009 (year 4) | 7 (2.81) | Year 4 vs. 1: p < 0.01 | |
| Insertion site antisepsis used | Default catheters were non-impregnated, four- or five-lumen CVCs. Chlorhexidine antisepsis was not consistently performed at catheter insertion (online appendix 6) | ||
| Dressing type and duration/frequency | IV 3000 semipermeable transparent dressing (Smith and Nephew) (online appendix 6) | ||
Intervention characteristics
| Objective | To reduce CRBSI in a UK ICU setting |
| Main focus of education | Catheter insertion and maintenance |
| Trainers (providers) | Group comprising three consultant clinicians, an infection surveillance nurse and two ICU charge nurses whose stated aim was to reduce CRBSI in the ICU. Two consultants had responsibility for training doctors in anaesthesia and intensive care |
| Training of trainers | Not reported |
| Learners (recipients) | ICU nurses and trainee doctors |
| Target behaviour change | Multiple behaviours associated with catheter insertion and maintenance practices |
| Development and testing | Not reported other than some changes were made as a result of discussions |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted (part from online appendix 6) | Self-adhesive insertion checklist: affixed to patients’ notes. Highlighted aseptic technique, maximal barrier precautions, chlorhexidine skin antisepsis, preferred insertion site, and whether staff had completed CVC education package Self-guided education programme including slide show: Information on the insertion and maintenance bundles, and on pathogenesis, recognition and consequences of CRBSI. Completed by nurses and trainee doctors, followed by a brief test involving simple questions, with a record kept of which staff completed the test. Slide show and test mandatory for nurses from late 2008 Regular updates, presentations and tutorials: no details given Supervision: trainee doctors had to perform three supervised CVC insertions before working alone Quarterly compulsory group education (9–18 per group): Part of ongoing nurse development programmes: covered rates of infections and preventative behaviours (insertion and maintenance bundles) Transient information provision: discussion and visual displays Lasting information provision: statistical process control charts displayed in the ICU (variables not stated, other than infection incidence) Promotion of staff engagement: Initial daytime presence of an infection surveillance and QI nurse to prompt staff to follow bundles; checklist stickers made widely available in the ICU. Interventions publicised through presentations at hospital department meetings and a regional ICU meeting; open discussion and debate about the evidence base and bundles, with some changes made where necessary. Regular meetings with local infection control team and representatives of Health Protection Scotland |
| Infection surveillance feedback approach | Infection incidence per 1000 device-days and 1000 patient-days was displayed on statistical process control charts. Data were checked against ICU admission records and Ward Watcher database to ensure every patient was captured. Although data in the study were reported monthly and quarterly, it is unclear how often the charts in the ICU were updated. Validity and reliability of data collection not reported |
| Performance feedback approach (from online Appendix 6) | Hand washing behaviours were audited prior to handling CVCs and results were reported to staff. Details, validity, and reliability of method not stated |
| Concentration of education | Other than daily checklists and quarterly group sessions, frequency and duration educational approaches not reported |
| Non-educational intervention components (online Appendix 6) | Self-contained CVC insertion pack (introduced November 2008) Other interventions:Interventions to reduce VAP/MRSA (March 2007–August 2009)Scottish Patient Safety Programme (introduced March 2008)Nurse team to support bundles (introduced March 2009) |
| Costs reported | Not reported |
Outcome characteristics
| Catheter-BSI definition (from online appendix 2) Reference cited: Hospital In Europe Link for Infection Control through Surveillance (HELICS);174 supported by flow chart to help classify infection as BSI-A or BSI-B: online appendix 3); supported by flow chart to help diagnose catheter-related infection as CRI 1, CRI 2 or CRI 3: online appendix 4); definitions reported for CRI 1 and CRI 2 (local or general CVC-related infection without a positive blood culture – data not extracted) |
HELICS catheter-related infection (CRI 3) definition (equivalent to CRBSI)
HELICS BSI classification BSI-A: 1 positive blood culture for a recognised pathogen or Patient has at least one of the following signs or symptoms: fever (> 38 °C), chills, or hypotension and two positive blood cultures for a common skin contaminant (from two separate blood cultures drawn within 48 hours) (skin contaminants = coagulase-negative staphylococci, Micrococcus spp., Propionibacterium acnes, Bacillus spp., Corynebacterium spp.) BSI-B: Patient has at least one of the following signs or symptoms: fever (> 38 °C), chills, or hypotension and either One positive blood culture with a skin contaminant in patient with an intravascular line in place and in whom the physician instituted appropriate antimicrobial therapy or Positive blood antigen test (e.g. Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis or group B Streptococcus) CVCs removed had their tips routinely sent for Maki roll testing (method reported in online appendix 5). |
| Outcomes reported | Not stated whether primary or secondary:
|
Results data
Primary outcomes
| Outcome | Baseline (year 1) (September 2005 to August 2006)a | Intervention (January 2007 to 31 August 2009)b | Difference between baseline and intervention |
|---|---|---|---|
| n = no. of patients who had a CVC during at least part of ICU stay and ICU stay of > 2 days | |||
| Mean (SD) CVC duration, days | 4.94 (4.11) | ||
| September 2007 to August 2008 (year 3) | 6.11 (3.6) | Year 3 vs. 1: p < 0.001 | |
| September 2008 to August 2009 (year 4) | 9.6 (8.46) | Year 4 vs. 1: p < 0.001 | |
| Total device utilisation, catheter-days – HELICS definition c | 2660 (n = 255) | Not reported | |
| September 2006 – August 2007 (year 2) | 2613 (n = 257) | ||
| September 2007 to August 2008 (year 3) | 2155 (n = 235) | ||
| September 2008 to August 2009 (year 4) | 2138 (n = 225) | ||
| Patient-days – HELICS definition d | 1918 (n = 255) | Not reported | |
| September 2006 to August 2007 (year 2) | 1990 (n = 257) | ||
| September 2007 to August 2008 (year 3) | 1800 (n = 235) | ||
| September 2008 to August 2009 (year 4) | 1562 (n = 225) | ||
| No. of devices/patient | Not reported | Not reported | Not reported |
| CRBSI incidence | 9 (n = 255) | Not reported | |
| September 2006 to August 2007 (year 2) | 7 (n = 257) | ||
| September 2007 to August 2008 (year 3) | 1 (n = 235) | ||
| September 2008 to August 2009 (year 4) | 0 (n = 225) | ||
| CRBSI incidence per 1000 catheter-days: HELICS definition c | 3.38 (n = 255) | Incidence rate ratio (95% CI): Year 3 vs. 1: 0.137 (0.003 to 0.990); p = 0.0134 Year 4 vs. 1: 0 (0.0 to 0.63); p = 0.0025 |
|
| September 2006 to August 2007 (year 2) | 2.68 (n = 257) | ||
| September 2007 to August 2008 (year 3) | 0.46 (n = 235) | ||
| September 2008 to August 2009 (year 4) | 0.00 (n = 225) | ||
| CRBSI incidence per 1000 patient-days: HELICS definition e | 4.69 (n = 255) | Not reported | |
| September 2006 to August 2007 (year 2) | 3.52 (n = 257) | ||
| September 2007 to August 2008 (year 3) | 0.56 (n = 235) | ||
| September 2008 to August 2009 (year 4) | 0.00 (n = 225) | ||
| Median (Q1, Q3) LOS, days | 9.7 (4, 20) | ||
| September 2007 to August 2008 (year 3) | 8.9 (4, 13) | Year 3 vs. 1: p = 0.64 | |
| September 2008 to August 2009 (year 4) | 6.0 (3, 11) | Year 4 vs. 1: p = 0.63 | |
| Mean (SD) LOS, days | 5.4 (3.9) | ||
| September 2007 to August 2008 (year 3) | 5.3 (3.7) | Year 3 vs. 1: p = 0.65 | |
| September 2008 to August 2009 (year 4) | 5.9 (3.7) | Year 4 vs. 1: p = 0.13 | |
| Mortality, n (%) | 54 (21.2) | ||
| September 2007 to August 2008 (year 3) | 49 (20.9)f | Year 3 vs. 1: p = 0.328 | |
| September 2008 to August 2009 (year 4) | 36 (16) | Year 4 vs. 1: p = 0.013 |
Secondary outcomes
| Reaction to education | Not a study outcome |
| Attitudes | Not a study outcome |
| Compliance | Compliance with CVC insertion bundle reported (chart only) from March 2008 onwards – the time of implementation of Scottish Patient Safety Programme (data not extracted) |
| Knowledge | Not a study outcome |
| Skills | Not a study outcome |
| Process evaluation | Barriers identified (online appendix 6) (no quantitative data reported):
|
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? UNCLEAR. Gender mix, overall morbidity score, LOS and mortality were broadly similar for years 1, 3 and 4. However, no patient characteristics were reported for year 2. Age not reported |
| If YES, were the differences between the groups adjusted for in statistical analyses? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? UNCLEAR. Organisational changes took place during the intervention but were not fully described. The Scottish Patient Safety Programme (a government-supported national initiative) was applied in the ICU during the last 16 months (according to appendix 6) or 18 months (according to main text) of the study, although this was stated as being part of the QI process. Authors stated that this had significant positive implications for leadership, administrative support, prioritisation and infrastructure but details were not reported |
| Was the effect of educational practice separable from effects of non-educational practice? NO. Multicomponent interventions not limited to education | |
| Were the intervention component(s) implemented as planned? PARTIALLY. Compliance (‘reliability’) reported only for the insertion bundle: data showed compliance reached 100% in some but not all months from March 2008 onwards | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? UNCLEAR. Two data sets are reported for the number of patients with CVCs. The data appear to show that the number of patients who had a CVC was smaller than the ‘study group’ number who had a CVC for at least part of a > 2-day stay in ICU |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined? NOT REPORTED. The HELICS definition and classification of infections was used throughout the study but no information was given on the staff involved in diagnosing infections |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO. Stated that the data collection and interventions were non-blinded | |
| Other possible sources of bias | Insertion bundle compliance (‘reliability’) was self-reported by clinicians |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. A complex intervention comprising many components, not all of which were clearly reported |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. Limited information provided. No information on validity and reliability given | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NO. Authors stated that they did not seek to further enhance validity of the process measurement | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NO |
| Target behaviour change achieved? NOT REPORTED |
MISSET (2004)117
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Misset et al. (2004)117 |
| Summary of approach | Single unit continuous quality-improvement programme to reduce nosocomial infection (NI) rates (VAP, UTI and CVC related) |
| Location | France |
| Language | English |
| Critical care specialty | Medical–surgical |
| No. of critical care units | 1 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Saint Joseph Hospital, Paris |
| Study design | Variation of single-cohort before-and-after study, with trend analysis instead of baseline data |
| Study time periods | Baseline: noneIntervention: 1995–2000Follow-up: none (continuous quality-improvement programme) |
| Funding source | None reported |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics 10-bed ICU in a 450-bed tertiary care centre hospital Infection control throughout the hospital was co-ordinated by an NI Control Committee and an Operative Infection Control Unit New nurses received a 2-month training course, during which specific protocols regarding NI prevention were discussed Nurse to patient ratio: 1 : 2 Nursing assistant to patient ratio: 1 : 10 Universal measures for preventing person-to-person transmission: Hand-washing before and after each patient contact Wearing gloves for handling secretions or contaminated objects Wearing a gown when soiling was anticipated and/or When the patient had multiple-drug-resistant bacteria and Geographical isolation of all patients CVCs and arterial catheters were not routinely placed and CVCs were removed when infections was suspected. CVCs inserted before ICU admission were routinely removed or replaced within 48 hours after ICU admission |
||
| Patient population characteristics during the 5-year study period (starting/baseline data not reported)a | Total patients= 1764 (n = no. of patients) | |
|---|---|---|
| Age (years), mean (± SD) | 61 (± 18) | |
| Length of ICU stay, mean days (± SD) | 9.7 (± 16.1) | |
| SAPS IIb score, mean (± SD) | 37 (± 21) | |
| Omega score per ICU stay,c mean (± SD) | 138 (± 256) | |
| In-ICU mortality, % | 21 | |
| Surgical reasons for admission, n | n = 529 | |
| Scheduled postoperative surveillance, n | n = 104 | |
| Postoperative complications, n | n = 396 | |
| Trauma, n | n = 29 | |
| Medial reasons for admission, n | n = 1235 | |
| Infection as the main diagnosis, n | n = 553 | |
|
73 | |
|
12 | |
|
6 | |
|
6 | |
|
3 | |
| Non-infectious disease as the main diagnosis, n | n = 682 | |
| Device characteristics during the 5-year study period (starting/baseline data not reported) | The site of CVC insertion was at the physician’s discretion. Use of single or multiple-lumen catheters as clinically required and use of haemodialysis catheters for fluids or parenteral nutrition only when there was no alternative. CVCs and arterial catheters were not routinely placed and CVCs were removed when infection was suspected. CVCs inserted before ICU admission were routinely removed or replaced within 48 hours after ICU admission | |
| Device characteristics during the 5-year study period (starting/baseline data not reported)a | Total patients= 1764 (n = no. of patients) | |
| CVC (≥ 1), n (%) | 765 (43) | |
| CVC used for dialysis, n | 203 | |
| Arterial catheter (≥ 1), n (%) | 261 (15) | |
| Other devices: | ||
| Mechanical ventilation, n (%) | 976 (55) | |
| Mechanical ventilation > 48 hours, n (%) | 682 (39) | |
| Urinary tract catheter, n (%) | 960 (55) | |
| Invasive procedure utilisation rate over the 5-year study period d | Procedure utilisation rate, all patients (%) | Duration of procedure use per patient, mean days (± SD) |
| Mechanical ventilation | 0.74 | 13.0 (± 18.8) |
| Urinary tract catheter | 0.73 | 13.1 (± 17.9) |
| CVC | 0.61 | 13.8 (± 18.3) |
| Per CVC | 6.2 (± 4.4) | |
| Arterial catheter | 0.05 | 3.5 (± 3.2) |
| Per arterial catheter | 3.1 (± 2.3) | |
| Insertion site antisepsis used | Skin disinfection with 10% povidone–iodine solution | |
| Dressing type and duration/frequency | Replacement of catheter site dressings every 48 hours or at longer intervals when clinically indicated | |
Intervention characteristics
| Objective | Maximise compliance of physicians and nurses with infection control practices and assess its long-term impact on the rates of VAP, UTI and vascular catheter-related infection in critically ill patients |
| Main focus of education | Simultaneous education and training for vascular catheter, VAP and UTI infection prevention and catheter maintenance |
| Trainers (providers) | Head nurse |
| Training of trainers | Not reported |
| Learners (recipients) | All staff (nurses and residents) |
| Target behaviour change | Compliance with infection control practices |
| Development and testing | Infection control practices were written by the ICU staff together with the Infection Control Unit and were derived from CDC recommendations |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Guidelines or their modifications were explained and given to all the staff during a yearly unit meeting and to the new nurses and residents by the head nurse. They were continuously available in a specific written form located in the unit Infection prevention and catheter maintenance, including daily examination of catheter sites, skin disinfection with 10% povidone–iodine, use of surgical drapes and of a gown for the operator, use of single- or multiple-lumen catheters as clinically required, use of haemodialysis catheters for fluids or parenteral nutrition only when there was no alternative, replacement of catheter site dressings every 48 hours or at longer intervals when clinically indicated. Measures for preventing person-to-person transmission included hand washing before and after each patient contact, wearing gloves for handling secretions or contaminated objects, and wearing a gown when soiling was anticipated and/or when the patient had multiple-drug resistant bacteria, and geographical isolation of all patients. The site of the CVC insertion was at the physician’s discretion. The programme was updated regularly according to infection and colonisation rates and reports in the literature (no further details reported) |
| Infection surveillance feedback approach | All the patients referred to the ICU were included in the nosocomial infection surveillance programme. Microbiological specimens were collected when the attending physician suspected infection based on systemic signs (unexplained fever, chills, hypotension) and/or local signs (pus or pain at a vascular catheter insertion site), consisting of the catheter tip for vascular catheter colonisation (details for VAP/UTI procedure not data extracted). All CVCs and arterial catheters were cultured at removal, regardless of whether or not infection was suspected. ICU-acquired infection was defined as infection documented after at least 48 hours in the ICU Monitoring of trends in vascular catheter colonisation and related bacteraemia rates: programme on microbial resistance containment (policy for containing antibiotic resistance included collection of microbiological specimens from suspected infection sites if at all possible and starting broad-spectrum antibiotic therapy early, then changing to narrow-spectrum therapy as soon as the organism was identified and its antimicrobial susceptibility profile established. Selective digestive decontamination was used in 15 patients (0.8%) as an adjunctive technique to control outbreaks of multiple-drug-resistant bacteria). Descriptive statistics of nosocomial infection rates were communicated to the ICU staff every 6 months (no further details reported). Validity and reliability of the assessment approach were not reported. |
| Performance feedback approach | Not included in the study |
| Concentration of education | Not reported, other than yearly unit meetings |
| Non-educational intervention components | Catheter tunnelling was required for internal jugular and femoral sites from 1996 and 1999, respectively; use of povidone–iodine |
| Costs reported | Not reported |
Outcome characteristics
| Catheter-BSI definition Reference cited: Centers for Disease Control and Prevention199 |
All patients in ICU were included. All CVCs and arterial catheters were cultured at removal, regardless of whether infection was suspected. ICU-acquired infection was defined as infection documented after at least 48 hours in the ICU Microbiological specimens were collected as recommended by the CDC. For CVCs and arterial catheters. Colonisation was assessed by quantitatively culturing CVC/arterial catheter tips. CVC and arterial catheter infection was considered when bacteraemia due to a micro-organism simultaneously colonising the catheter (at least two positive blood cultures in the case of Staphylococcus spp. other than S. aureus) was present. Thresholds above which cultures were considered positive were 103 CFU/ml for CVC or arterial catheter colonisation |
| Outcomes reported | Not stated whether primary or secondary:
|
Results data
Primary outcomes
| Outcome | 1995–6 | 1996–7 | 1997–8 | 1998–9 | 1999–2000 | Changes in incidence rates over the 5-year study period (chi-squared test for tendency) | |
|---|---|---|---|---|---|---|---|
| Device duration | Not reported | Not reported | |||||
| Total device utilisation a | CVC | 0.65 | 0.76 | 0.56 | 0.58 | 0.57 | 5-year rate not significant (no p-value reported) |
| Arterial catheter | 0.07 | 0.07 | 0.05 | 0.05 | 0.04 | ||
| CVC-BSI/CVC patients | 7/117 | 6/154 | 6/178 | 2/147 | 0/153 | p = 0.001 | |
| CLAB incidence rate | 7 | 6 | 6 | 2 | 0 | Not reported | |
| CVC-BSI incidence per 1000 CVC-days | 3.5 | 2.4 | 2.9 | 0.9 | 0.0 | Not reported | |
| Mean length of ICU stay per year a | 9.52 | 10.67 | 9.52 | 9.14 | 8.76 | Not reported | |
| Mortality | Not reported other than 5-year rate | Not reported | |||||
Secondary outcomes
| Reaction to education | Not a study outcome |
| Attitudes | Not a study outcome |
| Compliance: | Compliance with hand washing guidelines was only assessed in the first year of the project |
| Knowledge | Not a study outcome |
| Skills | Not a study outcome |
| Process evaluation | Not a study outcome |
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NOT REPORTED. However, states that there were no significant changes in disease severity (SAPS II score), mean length of ICU stay, and treatment intensity (Omega score) (graph provided), regardless of invasive device utilisation rates (no p-value reported) |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? NOT REPORTED. However, stated that there were no changes in the structure or staffing of the unit during the study |
| Was the effect of educational practice separable from effects of non-educational practice? NO | |
| Were the intervention component(s) implemented as planned? NOT REPORTED. However, states that 6% of items in an audit were considered insufficiently respected and the results allowed the continuous reinforcement of the observance of procedures of hand washing and surfaces cleaning and implementing formation sessions about cross-transmission | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? NOT REPORTED |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? NOT REPORTED |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | No other sources of bias were reported by the authors or identified by the reviewers based on the information presented |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. No details of education and training provided |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. Nurses collected the specimens and documented the dates of insertion and removal of devices. The physicians collected all other data. Format of data collection (e.g. electronic, hard copy) and nature of achiving not reported | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? UNCLEAR. Compliance assessment was made by the Infection Control Unit only twice during the first year of study and hand washing compliance was assessed only during the first year, but compliance data were not reported |
Additional comments
-
Assessment of the quality of hand washing and invasive devices management was made each time a patient was colonised with MRSA among the nurses and physicians in charge of the colonised patient (136 audits assessing 26 items). No further details reported.
-
Hand washing compliance was assessed during the first year of the study only, with a global score built from the quality of washing, rinsing, soap or antiseptic utilisation, drying and duration. The results were based on 46 samples of hands before and after washing from 12 nurses and 8 physicians/residents [first 6 months of study: mean score 88 (± 5%); second 6 months of study: mean score 93 (± 4%)].
-
CVC colonisation data reported (not extracted).
-
Data were compared between first 2.5 years and last 2.5 years but these periods appear arbitrary (no justification was provided in relation to the timing of intervention components).
PALOMAR MARTINEZ (2010)68
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Palomar Martinez (2010)68 |
| Summary of approach | A multicentre regional-scale pilot study to test the feasibility of applying the Michigan approach for preventing CRBSI in Spanish critical care units. Catheter maintenance practice was included in addition to the five-catheter insertion bundle components used in the Michigan study |
| Location | Spain (three separate regions: Castilla-León, Cataluña, Andalucía) |
| Language | Spanish |
| Critical care specialty | Not reported (list of participating hospitals included cardiac surgery and general ICUs but specialties not mentioned for most hospitals) |
| No. of critical care units | 17 (nine intervention, eight control) in study period; varied in baseline period |
| No. of hospitals | 16 |
| Hospital name (unless multicentre); city | Hospital names are listed in the publication |
| Study design | Non-randomised parallel group study with nine intervention and nine control ICUs, distributed evenly among three separate autonomous regions of Spain |
| Study time periods | Baseline: 2004, 2005, 2006 (3 years, with separate historical data for each) (note: no baseline data immediately preceding the intervention, i.e. during January to September 2007) Intervention: 1 October to 31 December 2007 (3 months) Follow-up: None reported |
| Funding source | Spanish Ministry of Health and Social Policy |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics | Not reported |
| Patient population characteristics | Not reported |
| Device characteristics | Described only as CVCs; stated that the use of catheters impregnated with antiseptic or antibiotics was not monitored |
| Insertion site antisepsis used | Not reported |
| Dressing type and duration/frequency | Not reported |
Intervention characteristics
| Objective | To assess the applicability on a national level in Spain of the interventions proposed by Pronovost and colleagues in Michigan, USA, for the prevention of central vascular catheter-related bacteraemia in patients admitted to the ICU |
| Main focus of education | Catheter insertion and maintenance |
| Trainers (providers) | Not explicitly reported. Authors stated that a responsible physician and a nurse were identified (in each intervention ICU) to ensure compliance with the intervention |
| Training of trainers | Not reported |
| Learners (recipients) | ICU doctors and nurses |
| Target behaviour change | Not stated explicitly but compliance was assessed for skin preparation with chlorhexidine, hand hygiene, preparation and maintenance of the sterile field, and the use of barrier precautions (gloves, masks, gowns) |
| Development and testing | A pilot study – no prior development or testing reported other than that the study was based on the Michigan intervention in the USA |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Meetings: Briefing meeting: Held in September, 2007, attended by two representatives (physician and nurse) from each participating ICU. At this meeting, materials to be used were distributed, including an electronic version of the programme/intervention. To assess compliance with the recommendations and the degree of acceptance of the project, a meeting was held at the end of the study period, in which each of the leaders explained their experiences and reported especially on the worst aspects of compliance Daily goals sheet (no details provided) Catheter insertion checklist (no details provided) Safety rounds (no details provided) Online 2-hour course: For all ICU staff, synthesising the key points of catheter-related infections, particularly clinical impact and prevention measures Poster(s): Covered the five proposed procedures to reduce bloodstream infections (hand hygiene, maximum use of aseptic barriers during insertion, asepsis of the skin at the insertion site with chlorhexidine, avoiding the femoral access route and removing all unnecessary CVCs). Posted in all intervention ICUs in joint meetings between the medical and nursing staff Teams: Trained in each hospital or working group to ensure the implementation of interventions and safety of hospitalised patients (no further details given) Supporting document(s): Available in graphical format recalling the points of intervention (no details provided) |
| Infection surveillance feedback approach | Stated that to be eligible in the project the ICUs should have experience in nosocomial infection surveillance, although not itself part of the intervention |
| Performance feedback approach | Not explicitly stated as being part of the intervention; unclear whether performance feedback might have occurred in specific ICUs. A standardised questionnaire [the Spanish version of the Hospital Survey on Patient Safety of the Agency for Healthcare Research and Quality (AHRQ)] was used to assess the safety culture, but it was not stated whether the results were known by ICU staff |
| Concentration of education | Unclear whether all the meetings that took place are described. The main training component appeared to be an online study module with 2-hour duration |
| Non-educational intervention components | Chlorhexidine skin antisepsis used. Note that, although a catheter insertion cart was also planned, this was not actually implemented |
| Costs reported | No |
Outcome characteristics
| CVC-related bacteraemia infection definition | Infections meeting the following definitions were accepted as CVC-related bacteraemia: CVC-related bacteraemia (after withdrawal of the CVC)
|
| Outcomes reported | Catheter-related bacteraemia incidence and incidence density per 1000 CVC-days Secondary bacteraemia incidence and incidence density per 1000 patient-days No. of CVC-days No. of patient-days Compliance with evidence-based practices (most data reported for only six ICUs that submitted data) |
Results data
Primary outcomes
| Outcome | Intervention ICUs | Control ICUs | Difference between baseline and post intervention |
|---|---|---|---|
| n = no. of ICUs | |||
| Device duration | Not reported | Not reported | Not reported |
| Total device utilisation, CVC-days | |||
| (a) Baseline: 2004 | (a) 6434 (n = 5) | (a) 2600 (n = 5) | Statistical significance not reported |
| (b) Baseline: 2005 | (b) 7960 (n = 7) | (b) 3260 (n = 6) | |
| (c) Baseline: 2006 | (c) 9164 (n = 8) | (c) 4644 (n = 8) | |
| (d) Intervention (2007; 3 months) | (d) 11,432 (n = 9) | (d) 8453 (n = 8) | |
| No. of devices/patient | Not reported | Not reported | Not reported |
| CVC-related bacteraemia incidence | |||
| (a) Baseline: 2004 | (a) 39 (n = 5) | (a) 32 (n = 5) | Statistical significance not reported |
| (b) Baseline: 2005 | (b) 60 (n = 7) | (b) 47 (n = 6) | |
| (c) Baseline: 2006 | (c) 59 (n = 8) | (c) 59 (n = 8) | |
| (d) Intervention (2007; 3 months) | (d) 44 (n = 9) | (d) 28 (n = 8) | |
| CVC-related bacteraemia incidence density per 1000 catheter-days | |||
| All three regions* | |||
| (a) Baseline: 2004 | (a) 6.06 (n = 5) | (a) 12.31 (n = 5) | Differences among all four study dates:Intervention: p < 0.0032Control: p < 0.0001 |
| (b) Baseline: 2005 | (b) 7.54 (n = 7) | (b) 14.4 (n = 6) | |
| (c) Baseline: 2006 | (c) 6.44 (n = 8) | (c) 12.7 (n = 8) | |
| (d) Intervention (2007; 3 months) | (d) 3.85 (n = 9) | (d) 3.31 (n = 8) | |
| *Data were also reported separately for Castilla-León, Andalucía and Cataluña but the numbers of ICUs contributing data for these regions in each year were not specified (data not extracted by reviewers) | |||
| CVC-related bacteraemia incidence per 1000 patient-days | Not reported | Not reported | Not reported |
| LOS | Not reported | Not reported | Not reported |
| Mortality | Not reported | Not reported | Not reported |
Secondary outcomes
| Reaction to education | Not a study outcome |
| Attitudes | Not a study outcome |
| Compliance | No compliance data were reported for the baseline years. For the study period, compliance with insertion checklists was reported narratively to be around 90%. No hospitals created a catheter insertion cart (i.e. 0% compliance). Removal of unnecessary catheters was not quantified. Femoral insertions were 18% of the total CVC insertions. Further data on compliance with the following practices were reported only for six ICUs that submitted data: skin preparation with chlorhexidine, hand hygiene, preparation and maintenance of the sterile field, and the use of barrier precautions (gloves, masks, gowns). Compliance was high in all six ICUs except for chlorhexidine use in one ICU (approximately 17%) owing to lack of availability. The above data are assumed to be for intervention ICUs, although this was not stated explicitly |
| Knowledge | Not a study outcome |
| Skills | Not a study outcome |
| Process evaluation | Formal process evaluation was not reported, although the following potential barriers and other factors were mentioned narratively Variable implementation:
|
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? YES. Stated that the improvement (in CVC-bacteraemia rates) was more pronounced in those units that had the highest starting rates, which generally occurred more in the control ICUs |
| If YES, were the differences between the groups adjusted for in statistical analyses? NO | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? UNCLEAR. Limited detail reported. Stated that the use of antiseptic or antibiotic impregnated catheters was not monitored |
| Was the effect of educational practice separable from effects of non-educational practice? UNCLEAR. No information provided about other practices such as use of antibiotics or impregnated catheters | |
| Were the intervention component(s) implemented as planned? NO. None of the hospitals implemented a catheter insertion cart. Chlorhexidine was difficult to obtain in one ICU | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? NOT REPORTED. No information given on the patient population |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? UNCLEAR. Standard infection definitions appear to have been used, although it was not stated explicitly that these were always applied in all ICUs in all project years |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | The authors acknowledged that participants in control interventions were able to attend the initial intervention briefing meetings and would have been aware of the published Michigan study so may have independently implemented some aspects of clinical practice that formed the basis of the intervention. This could lead to performance bias |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? PARTIALLY. The objectives and the evidence-based practices included in the intervention are clear but details of how they were implemented are lacking, so the general principles could be reproduced but not the specific approaches used |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT APPLICABLE | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT APPLICABLE | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NO |
| Target behaviour change achieved? UNCLEAR. Formal behaviour change targets were not explicitly specified. Compliance with some evidence-based practices was high but the data appear to be only from intervention ICUs (no baseline or control ICU data appear to have been presented for compliance) |
PEREZ PARRA (2010)122
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Perez Parra (2010)122 |
| Summary of approach | Single hospital intervention based on short (15-minute) lecture on evidence-based approaches for catheter insertion and maintenance, with pre- and post-tests in three ICUs (no reinforcement of education) |
| Location | Spain |
| Language | English |
| Critical care specialty | Medical, general post-surgery, cardiac post-surgery |
| No. of critical care units | 3 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Hospital General Universitario Gregorio Maragñón, Madrid |
| Study design | Cohort before-and-after study, with outcomes data for three ICUs grouped as a single cohort and also presented as three separate cohorts |
| Study time periods | Baseline: February to October 2006 (9 months) Intervention: November 2006 (15–20 minutes) Follow-up: December 2006 to August 2007 (9 months) Post-intervention test: May 2007 (6 months after intervention) |
| Funding source | La Investigación Biomédica del Hospital General Universitario Gregorio Maragñón and Fondo de Investigación Sanitaria (funding to lead author) |
| Conflicts of interest | Stated no conflicts of interest |
Population and setting
| Critical care unit characteristics | Three critical care units with a joint total of 60 beds in a tertiary university hospital. CVCs were routinely inserted by physicians and managed by nurses Mean (95% CI) duration of work experience for the ICU staff:
|
| Patient population characteristics | Not reported |
| Device characteristics | Described only as CVCs; stated that the type of CVC did not change during the study |
| Insertion site antisepsis used | Not reported, but stated that there were no changes in antiseptics during the study |
| Dressing type and duration/frequency | Not reported, but stated there were no changes in supplies used in CVC insertion and care during the study |
Intervention characteristics
| Objective | To analyse the effect of a single evidence-based educational intervention on the incidence of CLABSI in ICUs with acceptable baseline incidences and to assess the knowledge standards for CLABSI prevention among health-care workers in a large teaching hospital |
| Main focus of education | Catheter insertion and maintenance |
| Trainers (providers) | Not stated explicitly but the study authors implied that they gave the lectures |
| Training of trainers | Not reported |
| Learners (recipients) | The same lecture was given to all ICU workers (physicians, residents, nurses and students) on all shifts |
| Target behaviour change | Compliance with IDSA-CDC guidelines for preventing intravascular catheter-related infections (10-point list as below) |
| Development and testing | Not reported |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Short lecture (15 minutes): On the 10 main points of the IDSA-CDC guidelines for prevention of intravascular catheter-related infections:
|
| Infection surveillance feedback approach | Not included in intervention |
| Performance feedback approach | Multiple-choice questionnaire conducted a few minutes before and 6 months after the lecture. Covered the 10 points specified in the lecture (see above). Validity and reliability of questionnaire not reported |
| Concentration of education | Each questionnaire test took 15–20 minutes to complete. Total time to complete a 15-minute lecture and two tests would be 45–55 minutes. In total, 30 lectures were given to cover all shifts in the three ICUs. Six months between education and post-education test |
| Non-educational intervention components | None (intervention purely educational) |
| Costs reported | No |
Outcome characteristics
| Catheter-BSI definition Reference cited: O'Grady NP et al.196 |
Followed definitions and recommendations of the IDSA-CDC guidelines (reference cited). A CVC-related bloodstream infection was considered to be ICU-related if it occurred at least 48 hours after admission to or up to 48 hours after discharge from the ICU. Catheter tips were cultured using the roll plate method (references and equipment for catheter tip and blood cultures cited) |
| Outcomes reported | Not stated whether primary or secondary: CLABSI incidence Device utilisation (catheter-days) Knowledge |
Results data
Primary outcomes
| Outcome | Baseline (9 months) | Post intervention (9 months) | Difference between baseline and post intervention |
|---|---|---|---|
| Device duration | Not reported | Not reported | Not reported |
| Total device utilisation, catheter-days | |||
| All three ICUs | 10,661 | 11,582 | Not reported |
| General post-surgery ICU | 3,403 | 4,064 | |
| Cardiac post-surgery ICU | 2,842 | 2,981 | |
| MICU | 4,416 | 4,537 | |
| No. of devices/patient | Not reported | Not reported | Not reported |
| CLABSI incidence | |||
| All three ICUs | 45 | 34 | Relative riska = 0.69 (95%CI 0.44 to 1.08); p = 0.11 |
| General post-surgery ICU | 18 | 14 | |
| Cardiac post-surgery ICU | 12 | 8 | |
| MICU | 15 | 12 | |
| CLABSI incidence per 1000 catheter-days | |||
| All three ICUs | 4.22 | 2.94 | 30.3%;bp = 0.03 |
| General post-surgery ICU | 5.3 | 3.4 | 35.8%; p = 0.05 |
| Cardiac post-surgery ICU | 4.2 | 2.7 | 35.7%; p = 0.12 |
| MICU | 3.4 | 2.6 | 23.5%; p = 0.31 |
| CLABSI incidence per 1000 patient-days | Not reported | Not reported | Not reported |
| LOS | Not reported | Not reported | Not reported |
| Mortality | Not reported | Not reported | Not reported |
| Additional monthly CLABSI data extracted from graph (figure 1)122 using Engauge software | Baseline | Post intervention |
|---|---|---|
| January 2006 | 2.5 | |
| February 2006 | 5.9 | |
| March 2006 | 4.8 | |
| April 2006 | 3.8 | |
| May 2006 | 4.4 | |
| June 2006 | 4.6 | |
| July 2006 | 4.2 | |
| August 2006 | 3.3 | |
| September 2006 | 4.4 | |
| October 2006 | 3.2 | |
| November 2006 | 3.0 | |
| December 2006 | 2.9 | |
| January 2007 | 3.5 | |
| February 2007 | 3.8 | |
| March 2007 | 0 | |
| April 2007 | 3.6 | |
| May 2007 | 1.7 | |
| June 2007 | 3.6 | |
| July 2007 | 4.2 | |
| August 2007 | 2.7 | |
| September 2007 | 3.1 | |
| October 2007 | 2.3 | |
| November 2007 | 0.9 | |
| December 2007 | 2.9 | |
| January 2008 | 5.7 | |
| February 2008 | 2.2 | |
| March 2008 | 1.8 | |
| April 2008 | 2.5 | |
| May 2008 | 0.9 | |
| June 2008 | 6.9 | |
| July 2008 | 2.5 | |
| August 2008 | 4.6 | |
| September 2008 | 5.6 | |
| October 2008 | 2.3 | |
| November 2008 | 3.8 | |
| December 2008 | 2.5 |
Secondary outcomes
| Reaction to education | Not a study outcome |
| Attitudes | Not a study outcome |
| Compliance | Number receiving the pre-test and intervention: Nurses: 125 (including 22 students) Physicians: 30 (including 10 residents) Total health-care workers: 155 No. completing the post-intervention test (% of those who received the intervention):
|
| Knowledge | Mean (95% CI) percentage of correctly answered questions among all health-care workers who took the intervention: Baseline: 59.7% (39.9% to 78.4%) Post intervention: 73.4% (58.1% to 88.6%) Difference: 13.7%; p = 0.01 |
| Skills | Not a study outcome |
| Process evaluation | The authors noted that the test questions that received the greatest number of incorrect responses (< 50% correct responses) were those on the use of full sterile barriers during CVC insertion and on the choice of antiseptic for skin disinfection. Small drapes were incorrectly assumed sufficient for CLABSI prevention and the 2002 IDSA-CDC guidelines indicated (incorrectly) that tincture of iodine could still be used, although Chlorhexidine was the current recommendation |
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NOT REPORTED. Authors stated that there were no changes in hospital policy on prevention of CLABSI or in the availability of supplies used (type of CVC, connectors, antiseptics or other supplies used in CVC insertion and care) but no data reported; no information at all provided on the patient population |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? NO. The authors stated no other interventions potentially affecting the incidence of CLABSI were performed. However, they also stated that to control for the confounding effects of secular trends and external events that occurred during the study period a Poisson regression technique was used. The potential confounding variables in question were not reported |
| Was the effect of educational practice separable from effects of non-educational practice? YES (education alone) | |
| Were the intervention component(s) implemented as planned? UNCLEAR. Not reported what proportion of the ICU staff participated in the intervention. Of those that participated, approximately half completed the post test | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? NOT REPORTED. No information given on the patient population |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? NO. The authors stated that there were no changes in CLABSI diagnosis procedures in the microbiology laboratory and the staff members responsible for data collection did not change during the study period |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | No other sources of bias were reported by the authors or identified by the reviewers based on the information presented |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? YES. A short 15-minute lecture and tests, for which the component 10 topics were specified in a table; the number of lectures required was also reported |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT APPLICABLE | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT APPLICABLE | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NO |
| Target behaviour change achieved? NOT REPORTED |
Additional comments
-
The proportion of the patients monitored for CLABSI was not reported.
-
Not reported whether the participants knew they were involved in a research study.
PRONOVOST (2006–10)34,123,124
Methods
Study characteristics
| Lead author, publication year(s) and reference ID(s) | Pronovost 2006,34 2008,124 2010123 (data are from Pronovost 200634 unless specified) |
| Summary of approach | State-wide safety initiative regarding patients in ICUs known as the MHA Keystone Center for Patient safety and Quality Keystone ICU project; set predominantly in Michigan |
| Location | USA, Michigan (plus five out-of-state hospitals) |
| Language | English |
| Critical care specialty | ICUs for adults: included medical, surgical, cardiac medical, cardiac surgical, neurological, surgical trauma and one paediatric unit |
| No. of critical care units | 103 (originally 108: two merged, four reported no data) participated in monitoring up to 18 months.34 Ninety participated in monitoring up to 36 months; of which 43 reported data continuously for 0–36 months123 |
| No. of hospitals | Sixty-seven participated in monitoring up to 18 months (34 hospitals in Michigan did not participate).34 Sixty-one participated in monitoring up to 36 months123 |
| Hospital name (unless multicentre); city | Ninety-three hospital units listed in an appendix to the paper (unclear how these relate to the number of ICUs included in the different study periods) |
| Study design | Cohort before-and-after study |
| Study time periods | Baseline: Not reported (varied by ICU) aImplementation period: The first 3 months of intervention123 Intervention period: March 2004 to September 2005 (18 months) (starting time varied by ICU: 40/103 ICUs started intervention immediately, i.e. had no baseline period) Sustainability period: 19 to 36 months after implementation (continuation of same intervention but with slightly different eligibility criteria: participation fee)123 Follow-up: None (continuous monitoring of ongoing intervention) Note: Staggered implementation: Two cultural interventions, followed by two interventions targeting patients’ safety (in any order), were each implemented at 3-month intervals |
| Funding source | Funded predominantly by the AHRQ and the MHA, plus congressional funding to develop and maintain the NNIS and a staff of hospital-based infection control practitioners. Stated there was no influence of the sponsors on study design, conduct, interpretation or publication |
| Conflicts of interest | Reported in detail |
Population and setting in the baseline (control) group
| Critical care unit characteristics | 1625 beds in total (103 ICUs); 15.8 beds/ICU on average; represented 85% of all ICU beds in Michigan 52% of the hospitals were teaching facilities |
| Patient population characteristics | Not reported |
| Device characteristics | Central catheters (defined) including PICCs (no further details reported). Average duration of catheter use in individual patients was not monitored |
| Insertion site antisepsis used | 13/93 hospitals (19%) included chlorhexidine in the central-line kits used in the ICUs |
| Dressing type and duration/frequency | Not reported |
Intervention characteristics
| Objective | Reduction of CRBSIs |
| Main focus of education | Catheter insertion |
| Trainers (providers) | Team leaders: At least one physician and one nurse designated per ICU, dedicating 20% of their effort to project activities (at a minimum, a team consisted of senior executive, the ICU director and nurse manager, plus a designated physician and nurse team leader) |
| Training of trainers | Instructed in the science of safety and in the interventions then disseminated this information among their colleagues. Training received through conference calls every other week, coaching by research staff and state-wide meetings twice a year. Team leaders were partnered with their local hospital-based infection-control practitioners to assist implementation of the intervention and to obtain data on CRBSI |
| Learners (recipients) | ICU colleagues (no further details) |
| Target behaviour change | Hand washing, use of full-barrier precautions during insertion of CVC, skin cleaning with chlorhexidine, avoidance of femoral site and removal of unnecessary catheters |
| Development and testing | Based on evidence-based procedures recommended by the CDC and identified as having the greatest effect on the rate of CRBSI and the lowest barriers to implementation (reference cited). No other details of development/testing reported. Conceptual model for large-scale knowledge translation123 |
| Educational or behavioural theory | Not reported. However, the authors refer to the ‘4E’ framework proposed by Heifetz for distinguishing technical and adaptive aspects of the intervention; technical aspects are education and evaluation; adaptive aspects are engagement and execution |
| Educational strategies and topics targeted | Education was one of five interventions to reduce CRBSI by targeting awareness of evidence-based infection control practices (hand hygiene, chlorhexidine skin preparation, full-barrier precautions during CVC insertion, subclavian vein placement as the preferred site, and CVC maintenance). Stated that clinicians were educated about practices to control infection and harm resulting from CRBSI – cited Berenholtz et al.77 but no other details given. Strategies included: Checklist: To monitor adherence to infection control practice/CVC maintenance. Details not reported. Staff were empowered to halt breaches of evidence-based practice Discussion: about removal of catheters at daily rounds One-page fact sheet: Available in an online appendix.34 Lists evidence sources for evidence-based practices (hand hygiene, skin preparation with chlorhexidine, maximal barrier precautions, and avoidance of the femoral site). Provided to participants to share with their staff Daily goals sheet: to improve clinician-to-clinician communication within the ICU. No other details reported (classified by the authors as separate from the educational intervention) PowerPoint presentation: Provided to participants to share with their staff. Content not reported Also stated that participants were provided with articles – but no details of which ones and whether reading was voluntary or mandatory and how encouraged or enforced |
| Infection surveillance feedback approach | Number and rates of CRBSI were provided to staff at monthly and quarterly meetings respectively. Infection rates were collected and reported according to the guidelines of the NNIS by trained hospital-based infection-control practitioners who were independent of the ICU staff implementing the intervention. Validity and reliability not reported. Stated that infection control staff at the hospitals adjudicated cultures before submitting data for the study but no details reported |
| Performance feedback approach | Not reported |
| Concentration of education | Not reported |
| Non-educational intervention components | Catheter insertion cart; chlorhexidine for skin antisepsis |
| Costs reported | No. The authors stated that the intervention was implemented without the use of expensive technology or additional ICU staffing. Funding was from the AHRQ and MHA and staffing was provided by the participating hospitals. However, resources were insufficient to enable evaluation of compliance with the study intervention. After the first 18 months of intervention ICUs were required to pay a MHA fee for continued participation (amount not specified)124 |
Outcome characteristics
| Catheter-BSI definition Defined according to the National Nosocomial Infections Surveillance System |
Presence of a recognised pathogen cultured from one or more blood cultures, and organism cultured from blood not related to infection at another site or Presence of one or more of the following: fever (temperature > 38 °C), chills, hypotension, and: Signs and symptoms and positive results not related to infection at another site, and: Presence of one or more of the following: Common skin contaminant (e.g. diphtheroids, Bacillus spp., Propionibacterium spp., coagulase-negative staphylococci or micrococci) cultured from two or more blood samples drawn on separate occasions Common skin contaminant cultured from at least one blood culture in a sample from a patient with an intravascular catheter Positive antigen test on blood (e.g. Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis or group B streptococcus) |
| Outcomes reported | Not stated whether primary or secondary:
|
Results data
Primary outcomes
The 0- to 18-month data are from Pronovost et al. ,34 and 19- to 36-month data are from Pronovost et al. ,124 except where stated otherwise.
| Outcome | Baseline | Intervention: (a) implementation period; (b) months 0–18; (c) months 19–36; and (d) individual quarters |
Difference between baseline and intervention | |
|---|---|---|---|---|
| n = no. of ICUs | ||||
| Device duration (months) | Not reported | Not reported | Not reported | |
| 551 (220–1091) (n = 55) | (a): 57,033 (n = 96) | Not reported | ||
| (b): 300,175 (n = 103) | ||||
| (c): 300,310 (n = 90) | ||||
| (d) | ||||
| 0–3 (n = 95) | 436 (246–771) [4779a]* | |||
| 4–6 (n = 95) | 460 (228–743) [4757) | |||
| 7–9 (n = 96) | 467 (252–725) | |||
| 10–12 (n = 95) | 431 (249–743)55,70 | |||
| 13–15 (n = 95) | 404 (158–695) | |||
| 16–18 (n = 95) | 367 (177–682) | |||
| 19–21 (n = 89) | 399 (230–680) | |||
| 22–24 (n = 89) | 450 (254–817) | |||
| 25–27 (n = 88) | 481 (266–769) | |||
| 28–30 (n = 90) | 479 (253–846) | |||
| 31–33 (n = 88) | 495 (265–779) | |||
| 34–36 (n = 85) | 456 (235–787) | |||
| *Total device-days, median (IQR) [monthly mean]; n = no. of ICUs. | ||||
| No. of devices/patient | Not reported | Not reported | Not reported | |
| 2 (1–3) | (a): 1 (0–2)* | Not reported | ||
| (b), (c): not reported | ||||
| (d) | ||||
| 0– 3 (n = 95) | 0 (0–2) | |||
| 4–6 (n = 95) | 0 (0–1) | |||
| 7–9 (n = 96) | 0 (0–1) | |||
| 10–12 (n = 95) | 0 (0–1) | |||
| 13–15 (n = 95) | 0 (0–1) | |||
| 16–18 (n = 95) | 0 (0–1) | |||
| 19–21 (n = 89) | 0 (0–1) | |||
| 22–24 (n = 89) | 0 (0–1) | |||
| 25–27 (n = 88) | 0 (0–1) | |||
| 28–30 (n = 90) | 0 (0–1) | |||
| 31–33 (n = 88) | 0 (0–1) | |||
| 34–36 (n = 85) | 0 (0–1) | |||
| *No. of CRBSI, median (IQR) | ||||
| CRBSI incidence per 1000 catheter-days | 2.7 (0.6–4.8); 7.7 ± 28.9 (n = 55) | (a): 1.6 (0–4.4); 2.8 ± 4.0* | ||
| (b), (c): Not reported | ||||
| (d) | ||||
| 0–3 (n = 96) | 0 (0–3.0); 2.3 ± 4.0 | p ≤ 0.002 | ||
| 4–6 (n = 96) | 0 (0–2.7); 1.8 ± 3.2 | p ≤ 0.002 | ||
| 7–9 (n = 95) | 0 (0–2.1b); 1.4 ± 2.8 | p ≤ 0.002 | ||
| 10–12 (n = 90) | 0 (0–1.9b); 1.2 ± 1.9 | p ≤ 0.002 | ||
| 13–15 (n = 85) | 0 (0–1.6b); 1.5 ± 4.0 | p ≤ 0.002 | ||
| 16–18 (n = 70) | 0 (0–2.4); 1.3 ± 2.4 | p ≤ 0.002 | ||
| 19–21 (n = 89) | 0 (0–1.4); 1.8 ± 5.2 | Not reported | ||
| 22–24 (n = 89) | 0 (0–1.6); 1.4 ± 3.5 | Not reported | ||
| 25–27 (n = 88) | 0 (0–2.1); 1.6 ± 3.9 | Not reported | ||
| 28–30 (n = 90) | 0 (0–1.6); 1.3 ± 3.7 | Not reported | ||
| 31–33 (n = 88) | 0 (0–1.1); 0.9 ± 1.9 | Not reported | ||
| 34–36 (n = 85) | 0 (0–1.2); 1.1 ± 2.7 | Not reported | ||
| *Median (IQR); mean ± SD | ||||
| CRBSI incidence per 1000 patient-days | Not reported | Not reported | ||
| 1.00* | (a): 0.81 (0.61 to 1.08)* | |||
| (b), (c): Not reported | ||||
| (d) | ||||
| 0–3 months | 0.62 (0.47 to 0.81b) | p = 0.001 | ||
| 4–6 months | 0.56 (0.38 to 0.84b) | p = 0.005 | ||
| 7–9 months | 0.47 (0.34 to 0.65b) | p < 0.001 | ||
| 10–12 months | 0.42 (0.28 to 0.63b) | p < 0.001 | ||
| 13–15 months | 0.37 (0.20 to 0.68b) | p = 0.001 | ||
| 16–18 months | 0.34 (0.23 to 0.50b) | p < 0.001 | ||
| 19–21 (n = 89) | 0.34 (0.23 to 0.50) | Not reported | ||
| 22–24 (n = 89) | 0.33 (0.23 to 0.48) | Not reported | ||
| 25–27 (n = 88) | 0.44 (0.34 to 0.57) | Not reported | ||
| 28–30 (n = 90) | 0.40 (0.30 to 0.53) | Not reported | ||
| 31–33 (n = 88) | 0.31 (0.21 to 0.45) | Not reported | ||
| 34–36 (n = 85) | 0.34 (0.24 to 0.48) | Not reported | ||
| *Incidence rate ratio (95% CI)c | ||||
| Mean per quarter change in rate of CRBSI (95% CI) | ||||
| All ICUs (n not reported): | 0–18 months | 12% (9% to 15%) | Not reported | |
| 19–36 months | –1% (–9% to 7%) | |||
| ICUs with continuous data (n = 43) | 0–18 months | 13% (9% to 16%) | ||
| 19–36 months | –1% (–4% to 5%) | |||
| LOS | Not reported | Not reported | Not reported | |
| Mortality | Not reported | Not reported | Not reported | |
Secondary outcomes
| Reaction to education | Not assessed as an outcome |
| Attitudes | Not assessed as an outcome |
| Compliance | Not assessed as an outcome |
| Knowledge | Not assessed as an outcome |
| Skills | Not assessed as an outcome |
| Process Evaluation | Effectiveness of intervention was found to be modestly higher in small hospitals, with an incidence rate ratio of 0.97 (95% CI 0.96 to 0.99; p < 0.001) for each 100-bed decrease in the size of the hospital, but it is unclear if the study was powered for this type of analysis. The stocking of chlorhexidine was also monitored and, 6 weeks following a letter to hospital CEOs to provide it, 56/67 (84%) stocked chlorhexidine, 46/67 (69%) stocked the agent in the ICU and 43/67 (64%) stocked it in central-line carts (1/67; 19% at baseline) |
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NOT REPORTED. No information was presented on patient demographics or health status other than that patients were mostly adult |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? UNCLEAR. Analyses were conducted to account for nested clustering within the data; analyses also adjusted for hospitals’ teaching status and bed size. However, no population variables were included in the analyses. A sensitivity analysis of results was conducted, which included only ICUs with continuous data, including baseline data. During months 19–36 after intervention implementation (the ‘sustainability period’), 13 hospitals dropped out because they chose not to pay the MHA fee for continued participation).124 These 13 hospitals were more likely to be a teaching hospital (93% vs. 65%), p = 0.04, but they did not vary in median number of hospital beds (383 vs. 338) or in CRBSI incidence rates during the first and final quarters of the preceding 18-month intervention period123 | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? NOT REPORTED, but ICUs could implement two interventions in any order. Authors stated that according to their knowledge, no other infection-reducing practices were implemented during the study. They also stated that they did not evaluate the use of new technologies such as impregnated dressings or catheters and chlorhexidine baths, on rates of infection124 |
| Was the effect of educational practice separable from effects of non-educational practice? NO. Change in supplies (central line cart and chlorhexidine). Also introduced new QI initiative unrelated to CRBSI,124 including an intervention to reduce the incidence of VAP | |
| Were the intervention component(s) implemented as planned? NOT REPORTED | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? UNCLEAR. Only 53 ICUs reported data continuously from baseline to 18 months124 and 43 reported data continuously to 36 months.125 Sensitivity analysis (up to 18 months) showed little change in the association between the intervention and outcomes when only ICUs for which complete data (including baseline data) were available were included. However, data availability from 19–36 months may be related to hospital teaching status (see above). Stated124 that missing data were very high and lowered to about 12% by sending letters to CEOs of the hospitals informing them of the per cent of missing data for their hospital; CEOs were requested to submit the required data or advised they would have to be dropped from the collaborative. Also stated123 that roughly 5% of data were lost when the 13 units left the collaboration, and roughly 5% of CRBSI were missing for the remaining units |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? UNCLEAR. Three ICUs changed the CRBSI definition used from their own to that of the NNIS but these were not included in a sensitivity analysis. Authors stated that medians were used to summarise the data as the data were non-normal.34 However, means were also reported123,124 and in some cases were markedly different to median values – but no guidance on interpretation was given |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NOT REPORTED | |
| Other possible sources of bias | Were any other sources of bias present? UNCLEAR. Despite conducting a sensitivity analysis, authors refer to the possibility of measurement bias, which can if present exaggerate results |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? PARTIALLY. The principles of the intervention are described well enough to be replicated, but not the specific content. A copy of the fact sheet was provided in a supplementary appendix. However, this was a very small component of the overall intervention and its effectiveness in isolation is not known. An underlying principle of the intervention is that it should be adaptable according to local circumstances: an independent post hoc analysis149 revealed that the intervention evolved beyond what was initially stated in the protocol |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. Stated only that throughout the study, data on the number of CRBSIs and catheter-days were collected monthly from a trained, hospital-based infection-control practitioner. Potential underreporting of catheter-related bloodstream infections and the lack of baseline data from ICUs that immediately implemented the intervention when the project was launched could have created a measurement bias that exaggerated the results | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? YES | |
| Educational significance or effect size assessed? NO | |
| Target behaviour change achieved? NOT REPORTED (behavioural outcomes not reported) |
Additional comments
-
A programme evaluation of the Michigan study by Dixon-Woods and colleagues149 highlighted the fact that the intervention evolved over time. Later innovations included a networking section at residential project workshops, as well as project tokens, all not part of the original protocol.
-
The authors stated that random assignment of the intervention and the time of implementation was not feasible because all of the ICU teams wanted to implement the intervention and to decide for themselves when to do so. Some ICUs immediately implemented the intervention without collecting any baseline data but were included in sensitivity analyses.
-
The authors stated that they did not monitor catheter duration to simplify data collection, and they did not collect data on who inserted the central catheters.
-
Not reported why 67 hospitals participated, whereas 34 hospitals in Michigan did not (stated that hospitals were not asked to provide reasons). Mean number of beds per participating ICU was 1625/103 = 15.8; mean number of beds per non-participating ICU was 268/34 = 7.9. Suggests study specifically excluded smaller ICUs but reason not given. The authors included hospital bed numbers as a variable and found the intervention appeared to be more effective in smaller hospitals but this analysis appears not to consider the size of the ICUs so its interpretation is unclear.
-
Statistical analyses accounted for clustering of infections/ICUs/hospitals but did not account for any population variables.
-
Stated that inclusion of clustering effects in the statistical analysis (CRBSIs within ICUs, ICUs within hospitals, and hospitals within geographical regions) did not change the results.
-
The authors noted that similar large decreases in infection rates were not observed outside Michigan during the study period; however, CRBSI data were not collected from the non-participating ICUs.
-
Not reported whether participating staff were aware that they were part of a study.
-
Stated125 that Rhode Island implemented a similar programme, with every hospital in the state participating and reported similar results as Michigan.
-
The AHRQ has awarded a grant to the Health Research and Educational Trust and Quality and Safety Research Group (QSRG) researchers at Johns Hopkins to replicate the Michigan study in 10 states and the QSRG has received ‘philanthropic support’ to implement the collaborative in another 20 states. 124
-
A graph123 shows that in some cases the 3-monthly infection incidence per 1000 catheter-days in 50 randomly selected ICUs equalled or exceeded that in the baseline and implementation phases, but the graph does not distinguish the individual ICU data within this subgroup of 50. Not reported whether the graph presents mean, median or total values
RENDER (2006)126, 200
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Render (2006);126,200, Elder (2008)201 |
| Summary of approach | Regional collaborative based on work/learning/reporting cycles, including performance feedback and infection surveillance feedback |
| Location | USA |
| Language | English |
| Critical care specialty | Not reported, but stated that intervention was in a MICU if more than one ICU was present at a given hospital |
| No. of critical care units | 8 |
| No. of hospitals | 4 |
| Hospital name (unless multicentre); city | Multicentre |
| Study design | Single cohort before-and-after study (results from eight critical care units pooled). Hospitals were randomised to CRBSI prevention in critical care units or surgical site prevention in operating rooms – only results for four hospitals that were randomised to CRBSI prevention are reported |
| Study time periods | Baseline: 2003 (months not specified) Intervention: Not explicitly stated. intervention period appears to be January 2004 to December 2006 (data reported for 2004) Follow-up: None (continuous monitoring of ongoing QI intervention during 2004) |
| Funding source | 50% funded by the health-care systems to which the hospitals belonged. No other funding sources reported |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics | Eight units in four hospitals (two urban referral and two suburban; one affiliated with a national health-care organisation) Total critical care unit beds = 104 Total central line days per year = 7714 Total patients in the four hospitals in first year = 686 No other information on critical care units reported (Also included a control group: five hospitals with 13 ICUs, no data reported) |
| Patient population characteristics | Not reported; author contacted by reviewer and confirmed that patients were adult (author's reply would indicate that the included children's hospital is part of the control group) |
| Device characteristics | Central lines, defined as any intravenous catheter whose distal end was in a central veinDuring first year:
|
| Insertion site antisepsis used | Not stated explicitly; implied that betadine was used |
| Dressing type and duration/frequency | Not reported |
Intervention characteristics
| Objective | To implement evidence-based patient safety practices that reduce CRBSI (chlorhexidine and maximal sterile barriers) in a regional group of hospitals, and train hospital staff in methods to successfully create and sustain change. Four hospitals that started the project set a goal to reduce their CRBSI rates by 50% |
| Main focus of education | Catheter insertion, focusing on use of maximal sterile barriers and skin antisepsis |
| Trainers (providers) | Experts in organisational change (two named co-authors) facilitated practice change strategies. At project initiation each hospital's CEO and other hospital leaders identified the project leader and team members. Project leaders, often the infection control professionals, led the team, which consisted of the nurse manager of the intervention unit, two or more staff nurses, a physician champion, and the supply manager. Teams implemented the evidence based practices using a collaborative approach. Stated that all team members were invited but the number participating varied from only the team leader (the infection control practitioner) to eight or more team members |
| Training of trainers | Not reported. However, stated that the leadership role of the infection control practitioner proved important because he/she already knew and believed the literature, had previously collaborated outside the confines of their own hospital, and understood the data collection issues. Stated200 that the infection control practitioners who were the project leaders had previous experience working together |
| Learners (recipients) | ICU nurses and physicians |
| Target behaviour change | Not explicitly stated but focused on use of skin antisepsis and large drapes during catheter insertion |
| Development and testing | Stated that the project was developed by a patient safety researcher (the lead author). No other information provided |
| Educational or behavioural theory | None reported |
| Educational strategies and topics targeted | Joint 1.5-day kick-off session: National experts presented evidence for practices; experts in organisational change presented the methods for change and mentored teams' implementation planning. Teams were requested to provide: a ‘SMART’ aim (specific, measurable, actionable, reliable, and time driven); a 90-day goal, with communication strategy, measurement, and tests of change; and a 3-day itemised action plan addressing recruitment of additional team members, the first test of change, and roles Work/learning/reporting cycles: Organised by project leaders at each site. Involved at minimum one (initially very small) test of change each month Monthly small group meetings (< 10 people) between project leaders and leadership: to present experience in a standard format (6 presentation slides) to share effective strategies, solve joint problems together, reinforce the change theory (not specified) and methodology, and develop consistent data collection strategies and definitions Checklist (provided in paper): Completed by ICU nurses as the physician prepared the catheter insertion. Nurses recorded binary choices (yes/no) for hand washing; chlorhexidine use; bed-sized sterile drape; and use of cap, mask, sterile gown, and sterile gloves during insertion; and the catheter date and insertion site. Stated200 that personnel were encouraged to use the checklist to identify the earliest possible moment when the central line could be removed; however, line need review was not a specific item on the checklist Written policies: approved by the clinical executive board to match the best practices to codify practice changes Stated200 that preferred insertion site was head or neck, with femoral lines to be removed within 48 hours, and all clinical lines were prepped with chlorhexidine rather than betadine. However, the specific educational approaches used to convey these policies were not reported |
| Infection surveillance feedback approach | Each patient record was reviewed by the infection control nurses and compliance and infection rates were reported to the ICU clinical collaborative committee and posted in the unit monthly.200 Validity and reliability of data collection and reporting methods not reported |
| Performance feedback approach | Unit level: Team leaders entered information from checklists in a secure de-identified web-based database. Each project leader reported processes and outcomes to the unit staff, usually posting the monthly project meeting slides on a bulletin board throughout the unit. Results also reported through existing hospital committees' structure (e.g. infection control and critical care committees) and to the wider hospital through its newsletter Community level: Project leadership reported outcomes to Greater Cincinnati Health Council (GCHC) infection control and patient safety committees. Twice yearly, hospital CEOs were informed of results by project leadership. Validity and reliability of data collection and reporting methods not reported. Stated that to ascertain capture of all central lines, nurse managers each month sampled the lines in place in the ICU against the forms (not described) |
| Concentration of education | Not reported, other than kick-off meeting had duration of 1.5 days, checklists were used daily, and feedback was monthly. Unclear whether newly recruited staff received the intervention. Stated in a commentary paper200 that data continued to be posted quarterly but unclear when the switch from monthly to quarterly reporting occurred |
| Non-educational intervention components | In collaboration with two manufacturers, team leaders modified pre-packaged insertion trays, removing the betadine (in most units) and small drape and developed an accessory pack that bundled the large drape, sterile gown, cap and mask. The insertion tray, accessory pack and sterile gloves were kept on a central line cart |
| Costs reported | Not reported; stated that the drapes and new kits added some cost to the procedure, which had to be justified to leadership200 |
Outcome characteristics
| CLBSI infection definition Reference cited: Raad and Bodey201 |
A hospital-acquired infection is one that occurs 48 hours after admission to the hospital and < 72 hours following discharge A CRBSI is defined as a positive blood culture with clinical or microbiological evidence that strongly implicates the catheter as the source of infection (reference cited, left). It is distinguished from local infection (evidence of purulence at the site of insertion) and catheter colonisation (growth of greater than 15 CFUs of an organism from the tip or subcutaneous portion of the catheter using the semiquantitative roll-plate culture technique) (reference cited) |
| Outcomes reported | Not stated whether primary or secondary:
|
Results data
Primary outcomes
| Outcome | Baseline (2003) | Intervention (2004) | Difference between baseline and intervention | |
|---|---|---|---|---|
| For eight critical care units | ||||
| Mean (± SD) line duration, days | 7.0 (± 6.2) | Not reported | Not reported | |
| Total device utilisation | Not reported | Not reported | Not reported | |
| No. of devices/patient (not reported; data e-mailed by the author) | 7593 (in three quarters of 2003) | 7830 (in three quarters of 2004) | Not reported | |
| CRBSI incidence rate (not reported; data e-mailed by the author) | 11 (in three quarters of 2003) | 5 (in three quarters of 2004) | Not reported | |
| CRBSI incidence per 1000 line-days a | (a) reported (period unclear) | (a) 1.7 | (a) 0.4 | (a) p < 0.05 |
| (b) calculated from data e-mailed by the author (three-quarter period) | (b) 1.4 | (b) 0.6 | ||
| CRBSI incidence per 1000 patient-days | Not reported | Not reported | Not reported | |
| LOS | Not reported | Not reported | Not reported | |
| Mortality | Not reported | Not reported | Not reported | |
Secondary outcomes
| Reaction to education | Not a study outcome | |
| Attitudes | Not a study outcome | |
| Compliance | The data presented indicate that an improvement in compliance occurred both for chlorhexidine and large drape use, but it is not clear from the information provided how the data relate to the timing of intervention implementation. Stated that four of the health-care systems implemented chlorhexidine and maximal sterile barriers in the first year and timing of antibiotics in the operating room or consistent use of maximal sterile barriers during the second year200 Adherence to evidence-based practices was reported to have increased from 30% to 95% at the project midpoint (three quarters of 2004) |
|
| Project year a | Adherence to chlorhexidine, % | Adherence to large drape, % |
| Month 1 | 42 | 0 |
| Month 2 | 45 | 39 |
| Month 3 | 49 | 47 |
| Month 4 | 72 | 67 |
| Month 5 | 86 | 82 |
| Month 6 | 90 | 82 |
| Month 7 | 72 | 95 |
| Month 8 | 73 | 62 |
| Month 9 | 73 | 87 |
| Month 10 | 92 | 68 |
| Month 11 | 94 | 83 |
| Month 12 | 82 | 83 |
| Knowledge | Not a study outcome | |
| Skills | Not a study outcome | |
| Process evaluation | A table (table 2) of facilitators and barriers is provided, but it is unclear whether this is based on quantitative evidence. Authors reported that after 6 months, the project leader and project co-ordinator (named authors) reviewed their detailed notes from the monthly reporting meetings to independently identify themes contributing to or delaying project success. The list of barriers and facilitators was then validated by the project leaders. However, the validation process was not explained. Some aspects of the reported information are unclear – for example, building on a prior pilot was rated as a facilitator but no prior pilot was reported elsewhere in the publications | |
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? UNCLEAR. Population characteristics not reported |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? NOT REPORTED. Not mentioned whether any changes in staffing occurred or whether any interventions were introduced in parallel at any of the ICUs. Stated that all commercially available central line trays included a small drape and both chlorhexidine and betadine, allowing practitioners to easily avoid practice change (meaning unclear) |
| Was the effect of educational practice separable from effects of non-educational practice? NO. In addition to educational activities, the insertion site antisepsis was changed from betadine to chlorhexidine and catheter insertion kits were re-organised, which may have influenced infection incidence | |
| Were the intervention component(s) implemented as planned? NOT REPORTED | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? NOT REPORTED |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? UNCLEAR. Stated that hospitals were already collecting CRBSI data using the CDC definitions at the project's inception. However, also stated that collection of line days was more accurate after intervention |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | Stated in a subsequent publication201 that the four participating hospitals were more willing than others to participate in the study – suggesting that the management at these ICUs may possess different qualities than non-participating ICUs |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. The general intervention approach is described and the key topics are reported but it is unclear which parts of the approach were used to support each topic. The checklist is reproducible but is a rudimentary list mainly of binary (yes/no) responses that would be difficult to implement effectively without additional instructions |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? YES. Line days were stratified by ICU | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? PARTIALLY. Improvements in adherence to chlorhexidine and large drapes were reported but remained below 100% for both |
Additional comments
-
Stated that infection rates were zero in three of four hospitals for four quarters.
-
Lead author was contacted to clarify whether the population included children. She confirmed that the population was entirely adult.
-
A line-day was not defined – it is unclear whether the data reflect all the vascular catheters used or the number of days on which patients had at least one vascular catheter.
-
Not reported whether participants were aware they were being studied.
ROSENTHAL (2003)129
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Rosenthal (2003)129 |
| Summary of approach | Infection control intervention in four ICUs, comprising education followed by performance feedback; the performance feedback focused on compliance with the management of catheter dressings and dating of intravenous tubing sets |
| Location | Argentina |
| Language | English |
| Critical care specialty | Cardiac, medical/surgical |
| No. of critical care units | 4 |
| No. of hospitals | 2 |
| Hospital name (unless multicentre); city | Bernal Medical Center and Colegiales Medical Center, Buenos Aires |
| Study design | Single cohort before-and-after study, with data from four ICUs pooled into single cohort |
| Study time periods Note that a hand washing programme (not described) was implemented concurrently during the same time periods as the education and performance feedback intervention at Bernal Medical Center (total duration 12 months) but implementation times varied or differed in all phases at Colegiales Medical Center (total duration 11 months) |
Phase 1, baseline (infection surveillance) Bernal Medical Center: April to May 1999 (2 months) Colegiales Medical Center: September to November 2000 (3 months) Intervention: Phase 2, education:a Bernal Medical Center: June to July 1999 (2 months) Colegiales Medical Center: December 2000 (1 month) Phase 3, performance feedback Bernal Medical Center: August 1999 to March 2001 (8 months) Colegiales Medical Center: January to July 2001 (7 months) Follow-up: None (monitoring only during intervention implementation phases) |
| Funding source | Supported in part by a grant from Baxter Healthcare |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics | Four level III cardiac and MICUs/SICUs in two hospitals Bernal Medical Center: Private hospital, 150 beds 1 medical–surgical ICU (17 beds) 1 coronary ICU (15 beds) Colegiales Medical Center: Private hospital, 180 beds 1 medical–surgical ICU (10 beds) 1 coronary ICU (10 beds) Each hospital had an infection control team that comprised a medical doctor (with formal education and medical background in internal medicine, infectious diseases, and hospital epidemiology) and an infection control nurse |
||
| Patient population characteristicsa All admitted adult patients with a CVC in place for at least 24 hours (840 patients) were included |
Baseline (2 or 3 months) (172 patientsb) |
Intervention (8 or 10 months) (668 patientsb) |
Difference between baseline and intervention |
| Male sex, n (%) | 84 (48.8) | 358 (53.6) | p = 0.265 |
| Mean ± SD age, years | 71.98 ± 13.45 | 71.91 ± 12.19 | p = 0.944 |
| Diabetes, n (%) | 32 (18.6) | 103 (15.4) | p = 0.314 |
| Cancer, n (%) | 9 (4.8) | 33 (4.9) | p = 0.925 |
| HIV, n (%) | 1 (0.6) | 0 (0) | p = 0.205 |
| Device characteristics | Not reported | Not reported | Not reported |
| Insertion site antisepsis used | Not reported | Not reported | Not reported |
| Dressing type and duration/frequency | Not reported | Not reported | Not reported |
Intervention characteristics
| Objective | To ascertain the effect of an infection control programme, using education and performance feedback, on intravascular device-associated BSI |
| Main focus of education | Unclear – appears to be catheter care in general (guidelines cited but unclear whether education was based on part or all of the r) – but management and care of dressings and dating of intravascular administration sets were the areas for which compliance was assessed |
| Trainers (providers) | Not reported |
| Training of trainers | The principal investigator (lead author) trained the data collectors at each centre before in initiation of the trial, but the training of education providers was not reported |
| Learners (recipients) | Not specified other than health-care workers from the ICUs |
| Target behaviour change | Not explicitly stated in education, but target behaviours for which compliance was assessed were: placement of gauze on intravascular device insertion sites; marking of the date on the intravascular administration set; and checking/ensuring appropriate condition of the gauze dressing |
| Development and testing | Not reported |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Health-care workers from the ICUs underwent education and training for CVC care based on infection control practices published by the CDC and Hospital Infection Control Practices Advisory Committee (reference cited). This occurred during 1 month (Bernal Medical Center) or 3 months (Colegiales Medical Center). However, table 1 shows the duration of this phase as two months for Bernal Medical Center and 1 month for Colegiales Medical Center |
| Infection surveillance feedback approach | Not included in the intervention |
| Performance feedback approach | A research nurse observed health-care worker behaviour in the ICUs twice a week, and assessed and entered the following into a standard form: placement of gauze on intravascular device insertion sites; marking of the date on the intravascular administration set; and condition of the gauze dressing. Performance feedback was provided at monthly infection control meetings using bar charts documenting rates of compliance with hand washing, gauze on CVC insertion sites, dates on intravascular administration sets, and maintaining the condition of catheter gauze dressings. Also, a formal report of compliance rates was provided to administrators in each ICU. Validity and reliability of the method not reported |
| Concentration of education | Not reported – no information was given on the number and duration of sessions, other than that performance feedback occurred monthly |
| Non-educational intervention components | A study to improve health-care worker compliance with hand washing was undertaken at each institution and overlapped in time with the current trial. However, no details were reported |
| Costs reported | Not reported |
Outcome characteristics
| Catheter-BSI definition Reference cited: Garner et al.147 |
There were two criteria for ‘laboratory-confirmed BSI’:
Blood and catheter tip culture techniques were briefly described (references cited) |
| Outcomes reported | Primary outcome: The combined rate of intravascular device-related BSI in Phases 2 and 3 of the study (education and performance feedback) compared with the rate in Phase 1 (baseline infection surveillance) Secondary outcomes: Rate of intravascular device-related BSI in phase 2 vs. Phase 1 Rate of intravascular device-related BSI in phase 3 vs. Phase 2 |
Results data
Primary outcomes
| Outcome | Phase 1 (baseline) | Phase 2 (education) | Phase 3 (performance feedback) | Phase 2 + Phase 3 (overall intervention) | Difference (relative risk (RR) (95% CI); p-value) |
|---|---|---|---|---|---|
| Device duration | Not reported | Not reported | Not reported | Not reported | Not reported |
| Total device utilisation, intravascular device-days | 1219 | 586 | 4140 | 4726 | Not reported |
| No. of devices/patient | Not reported | Not reported | Not reported | Not reported | Not reported |
| Intravascular device-related BSI incidence rate | 56 | 10 | 41 | 51 | Not reported |
| Intravascular device-related BSI incidence per 1000 catheter-days | 45.94 | 17.06 | 9.90 | 11.10 | Phase 2 vs. 1: RR = 0.37 (0.19 to 0.73), p < 0.001 |
| Phase 3 vs. 2: RR = 0.58 (0.29 to 1.18), p = 0.11 | |||||
| Phase 2 + 3 vs. 1: RR = 0.25 (0.17 to 0.36), p < 0.001 | |||||
| Intravascular device-related BSI incidence per 1000 patient-days | Not reported | Not reported | Not reported | Not reported | Not reported |
| LOS | Not reported | Not reported | Not reported | Not reported | Not reported |
| Mortality | Not reported | Not reported | Not reported | Not reported | Not reported |
Secondary outcomes
| Compliance (n = no. of observations) | Baseline (Phase 1) (n = 347) | Education (Phase 2) (n = 169) | Performance feedback (Phase 3) (n = 5165) | Difference (relative risk (RR) (95% CI); p-value) |
|---|---|---|---|---|
| Presence of gauze on intravascular device site | 53.02% (184/347) | 56.21% (95/169) | 96.53% (4986/5165) | Phase 2 vs. Phase 1: RR = 1.06 (0.86 to 1.30); p = 0.64 |
| Phase 3 vs. Phase 2: RR = 1.72 (1.40 to 2.10); p < 0.001 | ||||
| Date on intravascular administration set | 0.57% (2/347) | 0% (0/169) | 74.00% (3839/5165) | Phase 2 vs. Phase 1: p = 0.32 |
| Phase 3 vs. Phase 2: p < 0.001 | ||||
| Good gauze condition | 48.70% (169/347) | 43.19% (73/169) | 89.56% (4626/5165) | Phase 2 vs. Phase 1: RR = 0.89 (0.67 to 1.17); p = 0.39 |
| Phase 3 vs. Phase 2: RR = 2.07 (1.65 to 2.62); p < 0.001 | ||||
| Outcome | ||||
| Reaction to education | Not a study outcome | |||
| Attitudes | Not a study outcome | |||
| Knowledge | Not a study outcome | |||
| Skills | Not a study outcome | |||
| Process evaluation | Not included in the study | |||
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? UNCLEAR. Patients were similar in the baseline and intervention periods for gender, age, and proportion with diabetes, cancer or HIV. However, no other aspects of patients' health status were reported |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? YES. Stated that a similar study to improve health-care worker compliance with hand washing was undertaken at each institution resulting in overlap with the current trial. However, no information on the hand washing programme was reported |
| Was the effect of educational practice separable from effects of non-educational practice? YES. Education-only intervention with effects of education per se and performance feedback separable | |
| Were the intervention component(s) implemented as planned? NOT REPORTED. The educational approach was not described | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? NOT REPORTED. The sample sizes for the different study periods were not explicitly stated |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? NOT REPORTED |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | YES. Study centre data collection sheets were checked for potential errors and missing items by one person – the study co-ordinator – to confirm each diagnosis of intravascular device-associated BSI. The study co-ordinator might have a vested interest in a successful outcome The authors mentioned that the parallel implementation of a hand washing programme probably had an impact on the results of this study. They stated that in particular, it is possible that the earlier implementation of a hand washing programme at both institutions enhanced the impact of the educational phase of this study, potentially reducing the overall impact of performance feedback. However, this potential effect is speculative |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. The educational approach was not reported. |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. An infection control nurse at each study centre extracted data prospectively from charts. Study centre data collection sheets were checked for potential errors and missing items by the study co-ordinator to confirm each diagnosis of intravascular device-associated BSI | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NO |
| Target behaviour change achieved? UNCLEAR. The target behaviours addressed by the education were not reported. Compliance with three reported behaviours ranged from 0% to 56.21% after education alone and from 74.00 to 96.53% after both education and performance feedback |
Additional comments
-
The authors commented that most health-care institutions in Latin America lack the resources to prevent intravascular device-related BSI and many hospitals lack basic infection control programmes. In this context the high baseline incidence is notable.
-
It was not reported whether the participants were aware they were being studied.
ROSENTHAL (2005)130
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Rosenthal (2005)130 |
| Summary of approach | Education and performance feedback intervention to improve hand hygiene practices in two ICUs |
| Location | Argentina |
| Language | English |
| Critical care specialty | Coronary, medical surgical |
| No. of critical care units | 2 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Colegiales Medical Center, Buenos Aires |
| Study design | Single cohort before-and-after study, which pooled data from two ICUs |
| Study time periods | Baseline (Phase 1): September to December 2000 (4 months) Intervention (Phase 2): January 2001 to May 2002 (17 months) Follow-up: None. The specified follow-up period from January 2001 to May 2002 included the implementation of education in January 2001 and implementation of performance feedback in May 2001 (although education sessions appear to have been 1 week long, performance feedback appears to have been continuous during the monitoring period – but precise details of timing were not reported) |
| Funding source | Stated no external funding was provided; the human resources for the intervention were those of the infection control programme |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics | Private hospital, 180 beds: 1 medical–surgical ICU (12 beds) 1 coronary ICU (12 beds) Infection control team composed of a medical doctor (with formal education and medical background in internal medicine, infectious diseases and hospital epidemiology), an infection control nurse, and personnel support Stated that soap and antibiotic use were not changed No other information reported |
| Patient population characteristics | Not reported |
| Device characteristics | Mentioned only that devices were centrally inserted and peripherally inserted CVCs |
| Insertion site antisepsis used | Not reported other than that soap and antibiotic use were not changed |
| Dressing type and duration/frequency | Not reported |
Intervention characteristics
| Objective | To enhance compliance with hand hygiene by implementing education, training and performance feedback |
| Main focus of education | Hand hygiene |
| Trainers (providers) | Education providers were not reported. One nurse was trained to detect hand washing compliance and record it on a form designed for the study. A trained infection control nurse identified nosocomial infections. Senior hospital management provided full administrative support for the study |
| Training of trainers | Not reported |
| Learners (recipients) | Health-care workers in the ICU (nurses, physicians, ancillary staff) |
| Target behaviour change | Hand hygiene |
| Development and testing | Not reported |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Focused education of all health-care workers: One-hour group session educational classes: For all shifts every day for 1 week. All health-care workers were given a comprehensive infection control manual, and the Association for Professionals in Infection Control (APIC) hand hygiene guideline was used as an educational tool to reinforce classroom teaching. Attendance was voluntary and monitored; each health-care worker could attend the course as many times as desired. Theoretical and practical indications for the use of hand hygiene were reviewed. The guidelines were also posted at a strategic location in each ICU (display format and location not specified) Post tests: To evaluate retention of educational material – were given 1 month later Routine infection control review classes: Held by the primary investigator to provide an opportunity for infection control questions and sharing of infection and compliance surveillance data |
| Infection surveillance feedback approach | Not part of the intervention: standardised NNIS data collection methods (reference cited) were used during baseline as well as intervention periods. Surveillance data were shared via: routine infection control review classes; reports to the ICU manager and administrator; and feedback data posted in the ICUs (no details of format given). Stated that feedback was ‘frequent’ but the timing was not specified. Validity and reliability of the data collection were not reported |
| Performance feedback approach | Pre- and post-intervention data on compliance of health-care workers with hand hygiene before contact with patients were collected in the ICUs by a trained infection control practitioner, who covertly observed the hand washing techniques of the health-care workers (physicians, nursing staff, and technicians) at random times twice a week, including all shifts, for 30-minute intervals. Data were recorded on a specially designed form and shared via routine infection control review classes; graphic presentations of hand hygiene rates in the form of bar charts were displayed in monthly meetings; reports to the ICU manager and administrator; and feedback data in the form of bar charts were posted in the ICUs. Validity and reliability of the data collection method were not reported. Health-care workers were informed that their hand hygiene practices were being monitored but were not aware of precisely when these observations were being made |
| Concentration of education | Timing of group sessions was one hour per day for 1 week for all shifts. Timing of infection review classes not reported. Performance feedback on compliance was monthly. Timing and duration of post tests not reported |
| Non-educational intervention components | Interventions for reducing CVC-associated BSI and for reducing urinary catheter-associated UTIs overlapped with the current intervention by 7 of 21 months and 17 of 21 months, respectively |
| Costs reported | Not reported, but described as ‘relatively low-cost approach’ |
Outcome characteristics
| Catheter-BSI definition Reference cited: Garner et al.147 |
Laboratory-confirmed CABSI A patient with a CVC has a recognised pathogen cultured from one or more percutaneous blood cultures, after 48 hours of vascular catheterisation, and the pathogen cultured from the blood is not related to an infection at another site and patient has at least one of the following signs or symptoms: fever (≥ 38 °C), chills or hypotension. With common skin commensals (e.g. diphtheroids, Bacillus spp., Propionibacterium spp., coagulase-negative staphylococci or micrococci), the organism is cultured from two or more blood cultures drawn on separate occasions |
| Outcomes reported | Not stated explicitly as primary or secondary Focus of a priori hypothesis:
|
Results data
Primary outcomes
| Outcome | Baseline (4 months; 2000) (2187 bed-days) | Intervention (17 months; 2001–2) (7409 bed-days) | Difference in compliance between baseline and intervention |
|---|---|---|---|
| Device duration | Not reported | Not reported | Statistical comparison (relative risk) reported only for the total nosocomial infections per 1000 bed-days; number of catheters not reported |
| Total device utilisation | Not reported | Not reported | |
| No. of devices/patient | Not reported | Not reported | |
| Central catheter-associated laboratory-confirmed BSI incidence rate | 15 (3.75 per montha) | 12 (0.71 per montha) | |
| Peripheral catheter-associated laboratory-confirmed BSI incidence rate | 3 (0.75 per montha) | 4 (0.24 per montha) | |
| Total CABSI incidence a | 18 (4.5 per month) | 16 (0.94 per month) | |
| CABSI incidence per 1000 catheter-days | Not reported | Not reported | |
| CABSI incidence per 1000 patient-days | Not reported | Not reported | |
| LOS | Not reported | Not reported | |
| Mortality | Not reported | Not reported |
Secondary outcomes
| Compliance with hand hygiene | Baseline (4 months; 2000) (n = 1160) | Intervention (17 months; 2001–2) (n = 3187) |
Difference in compliance between baseline and intervention [relative risk (RR) (95% CI); p-value] |
|---|---|---|---|
| Total observed hand washing episodes, number (%) | 268 (23.1) | 2056 (64.5) | RR = 2.79 (2.46 to 3.17); p < 0.0001 |
| Observed hand washing episodes by post-intervention study period (baseline = period 1),a number (%) | 1. 268 (23.1) | ||
| 2. January to June 2001 | 724 (53.5) (n = 1353)b | Period 2 vs. period 1: RR = 2.32 (2.01 to 2.66); p = 0.0001 | |
| 3. July to December 2001 | 828 (72.1) (n = 1148)b | Period 3 vs. period 2: RR = 1.35 (1.22 to 1.49); p = 0.0001 | |
| 4. January to May 2002 | 518 (73.8) (n = 701)b | Period 4 vs. period 3: RR = 1.02 (0.92 to 1.14); p = 0.665 | |
| n, number of opportunities for hand hygiene. | |||
| Further information on compliance. Compliance was assessed and compared for different subgroups of health-care workers (nurses, physicians, ancillary staff), men and women, different types of procedures (superficial, invasive), times of shift work (morning, afternoon, night), and the different ICUs (medical surgical, coronary). Data on per cent adherence to hand hygiene are presented but it is unclear to which study periods they refer (in table 3 the data for each subgroup sum to 2564 (in one case 2563) opportunities for hand hygiene, which is less than the 3187 post-intervention opportunities reported in table 2). Compliance data ranged from a minimum of 30.8% adherence (physicians subgroup) to a maximum of 66.0% adherence (night period subgroup). It is unclear if the study was powered for this type of comparison (statistics were reported only for comparisons within subgroups (e.g. men vs. women), not for pre- versus post-intervention implementation) | |||
| Reaction to education | Not a study outcome | ||
| Attitudes | Not a study outcome | ||
| Knowledge | Not a study outcome | ||
| Skills | Not a study outcome | ||
| Process evaluation | Not reported other than compliance as detailed above | ||
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NOT REPORTED – no data reported on the population and only limited information given on the critical care characteristics and devices |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? YES. Reported that interventions for reducing CVC-associated BSI and for reducing urinary catheter-associated UTIs overlapped with the current intervention by 7 of 21 months and 17 of 21 months, respectively |
| Was the effect of educational practice separable from effects of non-educational practice? UNCLEAR. Possible confounding owing to two other overlapping interventions | |
| Were the intervention component(s) implemented as planned? UNCLEAR. Stated that attendance at education sessions while voluntary was monitored, but no attendance data were reported. Post-evaluation test results also not reported (education was aimed at enhancing compliance with hand hygiene, which statically significantly increased but did not reach 100%) | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? UNCLEAR. There are discrepancies in both the data for the number of hand washing opportunities and the number of observed hand washing episodes. Numbers of patients and catheters were not reported |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? NOT REPORTED |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? UNCLEAR. Observations of hand washing were done covertly but blinding of data collection for infection surveillance was not reported | |
| Other possible sources of bias | The authors acknowledged that the Hawthorne effect must be taken into account – i.e. observed effects may have resulted from the ICU staff being observed (staff were aware they were being monitored but not precisely when) |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? PARTIALLY. The nature of the education sessions was poorly described. However, the educational material is available as published guidelines which could be reproduced, disseminated and discussed |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. Compliance data were collected by a trained infection control practitioner and data were recorded on a specially designed form (no further details provided) | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NO |
| Target behaviour change achieved? PARTIALLY. The authors hypothesised that they could increase hand hygiene compliance to 70% with a reduction in nosocomial infection of 30%. Compliance with hand hygiene did not reach this target when the overall 17 months of intervention were taken together but did reach the target for arbitrary time periods covering the last 11 months of the intervention period (possibly selective definition of monitoring periods?). Compliance with hand hygiene was significantly increased from 23.1% to 64.5%. Nosocomial infection reductions exceeded the target reduction but this outcome does not capture device-related infections |
Additional comments
This study primarily focused on hand hygiene compliance, and catheter-associated infection incidence was not a main outcome.
SHERERTZ (2000)135
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Sherertz (2000)135 |
| Summary of approach | Single hospital, multiple-unit, 1-day hands-on course on infection control practices and procedures given in June 1996 and June 1997 for third-year medical students and physicians completing their first postgraduate year |
| Location | USA, North Carolina |
| Language | English |
| Critical care specialty | General MICUs/SICUs and associated step-down unit |
| No. of critical care units | Six ICUs and one step-down unit (data pooled) |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Wake Forest University Baptist Medical Center, Winston-Salem |
| Study design | Single-cohort before-and-after study |
| Study time periods | Baseline: July 1995 to June 1996 Intervention, course 1: 3 days in June 1996 Intervention, course 2: 3 days in June 1997 Follow-up: June 1997 to December 1997 |
| Funding source | Not reported |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics | CVC and arterial catheter insertions were performed by physicians in training The hospital's infection control policy on vascular catheters did not change substantially during the study period, with the exception of the educational intervention Stated that the seven study units did not differ between the baseline and post-intervention periods in number of admissions or severity of illness (data not shown) No other information provided |
| Patient population characteristics | None reported |
| Device characteristics | Included CVCs, PICCs and arterial catheters. Antibiotic-coated catheters were not used. No other details reported |
| Insertion site antisepsis used | Povidone–iodine; avoidance of antibiotic ointment |
| Dressing type and duration/frequency | Clear plastic dressing |
Intervention characteristics
| Objective | To improve standardisation of infection control practices and techniques during invasive procedures |
| Main focus of education | CVC insertion, specifically the use of full sterile drapes and other barrier precautions |
| Trainers (providers) | Infection control practitioners and a hospital epidemiologist taught 1 hour of basic infection control principles One to three faculty members oversaw training at hands-on rotation stations. Staff teaching the hands-on sessions were: Oncology catheter care nurses: Blood draws through vascular lines Respiratory therapists: Arterial puncture for analysis of blood gas Critical care medicine faculty and fellows and trauma faculty: Insertion of arterial catheters and CVCs Nurse instructors: Urinary catheter insertion and peripheral venous catheter insertion (PICC) Oncologist: Lumbar puncture Faculty from the School of Medical Technology: Phlebotomy |
| Training of trainers | Not reported |
| Learners (recipients) | Third-year medical students and physicians completing their PGY-1 |
| Target behaviour change | Principal focus was to determine whether the intervention could increase the use of full-size sterile drapes for CVC insertion |
| Development and testing | Not reported. Informal surveys indicated physicians in training at the study hospital had poor compliance with maximal barrier precautions when inserting CVCs, despite conventional bedside and didactic instruction by critical care medicine faculty over 2 years. Unpublished observations also suggested CVC insertion practices varied widely |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | 1-hour session on basic infection control principles: Content included hand washing, isolation, appropriate use of barrier garments, handling of patients with resistant organisms and varicella 1-hour session on Occupational Safety and Health Administration (OSHA) considerations for blood and body fluids and tuberculosis – taught on a different day 7 × 1-hour rotation stations (7–16 participants per group): Each had 5–15 minutes of didactic instruction (in year 2 this was done mostly by videotape), followed by hands-on skills practice overseen by one to three faculty members. The hands-on sessions were delivered by various trainers (see above): (1) blood draws through vascular lines; (2) arterial puncture for obtaining arterial blood gas; (3) insertion of arterial catheters and CVCs; (4) urinary catheter insertion; (5) lumbar puncture; (6) PICC insertion; and (7) phlebotomy Simulation with mannequins was used in all hands-on sessions for skills practice. Phlebotomy skills were practised on fellow students. PICCs were started first on a mannequin and later on a fellow student A member of the infection control department reviewed the content of each didactic session to ensure consistency with existing infection control policies. To ensure appropriate content delivery, the course director observed each rotation station instructor for an entire session Courses on vascular catheters covered: use of povidone–iodine for skin preparation; avoidance of antibiotic ointment at the insertion site; use of clear plastic dressings; regular changes of intravenous tubing every 3 days; and instruction not to adhere to fixed schedules for changing CVCs |
| Infection surveillance feedback approach | Not included in the intervention |
| Performance feedback approach | Not included in the intervention |
| Concentration of education | A 1-day course run on 3 days in June in 1996 and on 3 days in June 1997 (different participants each year). In each year the course was for medical students (1 day) and for two groups of PGY-1 physicians (1 day each, with about 50 physicians per group). The physician education was part of the orientation for new interns. The course comprised 7 × 1-hour hands-on sessions, with 15-minute morning and afternoon breaks and a 1-hour lunch break. Two 1-hour sessions on basic infection control principles and OSHA were also reported but it is unclear how they relate to the 1-day course (stated that the OSHA session was on a different day). Authors mention when considering costs that there were eight hands-on stations, not seven (see below) No. of participants was:
|
| Non-educational intervention components | None: educational intervention only |
| Costs reported | During year 1, supplies cost approximately US$25,000, largely because of the mannequins and CVC kits. Year 2 supplies cost approximately US$12,000. Almost all physicians teaching the course were fellows; most other faculty were nurses or had salaries equivalent to those of nurses. Using a yearly salary plus benefits (US$50,000) as an average cost for the participating faculty, it was estimated that each day of participation time cost approximately US$200 (excluding faculty preparation time or lost opportunity). Assuming eight stations with two faculty each for three different course days, the total cost for faculty time was approximately US$9600 per year (total for 2 years US$19,200). The 2021 full-size drapes used cost US$9.28 each (total US$18,755). In the baseline year the sterile sheets cost approximately US$874 (US$1.00 per reprocessed drape for 874 drapes). The overall course cost was therefore US$74,081 [= US$25,000 + US$12,000 + US$19,200 + (US$18,755 to US$874)] |
Outcome characteristics
| Catheter-BSI definition Reference cited: Garner et al.147 |
Primary bloodstream infections: a pathogen is isolated from one or more blood cultures and is not related to an infection at another site, unless that site is a catheter Catheter-related infections were defined as meeting definition 3 of the CDC Cardiovascular System Infection Criteria for arterial or venous infection (reference, left). Fulfilment of this definition required the presence of fever (temperature > 38 °C), pain, erythema or heat at the catheter site plus the presence of a negative blood culture or absence of any blood cultures and the presence of a positive roll-plate culture of the catheter (method reported). Blood cultures (method reported) were predominantly drawn through only a peripheral vein, or as paired cultures through a peripheral vein and through a catheter |
| Outcomes reported | Not stated whether primary or secondary:
|
Results data
Primary outcomes
| Outcome | Baseline (12 months) | Post–intervention (18 months) | Difference between baseline and post intervention |
|---|---|---|---|
| Device duration | Not reported | Not reported | Not reported |
| Total device utilisation | Not reported | Not reported | Not reported |
| Total no. of CVCs inserted | 2009 | 3090 | |
| CRBSI incidence rate (CVCs and arterial catheters): a | |||
| July to December 1995 | 19 | Not tested statistically | |
| January to June 1996 | 13 | ||
| July to December 1996 | 14 | ||
| January to June 1997 | 8 | ||
| July to December 1997 | 8 | ||
| CRBSI incidence per 1000 catheter-days | Not reported | Not reported | Not reported |
| CRBSI incidence per 1000 patient-days: b | Baseline vs. time after first course: | ||
| July to December 1995 | 1.4 (3.0) | CRBSI not tested statistically | |
| January to June 1996 | 0.9 (3.5) | ||
| July to December 1996 | 1.0 (2.4) | Total number of infections (CRBSI + primary BSI); p = 0.01 | |
| January to June 1997 | 0.6 (2.6) | ||
| July to December 1997 | 1.3 (1.6) | ||
| LOS | Not reported | Not reported | Not reported |
| Mortality | Not reported | Not reported | Not reported |
Secondary outcomes
| Outcome [in relation to first (June 1996) training course only] | Immediately before course (baseline) | Immediately after course | Six months after course | Difference relative to baseline |
|---|---|---|---|---|
| Reaction to education | Not assessed as an outcome | |||
| Attitudes: % of physicians ( n = 109) who perceived a need for | ||||
| Full drapes | 33 | 99 | 73 | p < 0.01a,b |
| Towels | 88 | 25 | 53 | p < 0.01a,b |
| Povidone–iodine | 97 | 99 | 96 | Not significant |
| Gowns | 80 | 98 | 82 | Not significant |
| Gloves | 96 | 99 | 98 | Not significant |
| Masks | 82 | 98 | 91 | p < 0.01a |
| Compliance: % of CVC insertions for which sterile drapes used | 44 | 65 | p < 0.001 | |
| Knowledge | Not assessed as an outcome | |||
| Skills | Not assessed as an outcome | |||
| Process evaluation | At the end of each 1-day course participants were asked to rate various factors, including each instructor on a scale of 1 (poor) to 5 (excellent). In both years, all mean evaluation scores for course instructors ranged from 4.4 to 4.8 (no further data provided) | |||
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NOT REPORTED |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? UNCLEAR. Stated that hospital’s infection control policy on vascular catheters did not change substantially during the study period, and the seven study units did not differ between the baseline and post-intervention periods in number of admissions or severity of illness. However, no quantitative data were provided and no information at all was given about the patient population. |
| Was the effect of educational practice separable from effects of non-educational practice? YES. Education-only intervention (although catheter kits and drapes were provided, these were standard hospital practice) | |
| Were the intervention component(s) implemented as planned? PARTIALLY. The number of staff who took the intervention was reported for each year, but it was not reported whether all staff in the ICU took all components of the intervention | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? UNCLEAR. For attitudes, the same sample size was reported for pre-intervention and post-intervention results. For other outcomes, it was not indicated or discernible whether there were any missing data |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? UNCLEAR. The definition and microbiological culture approach for infections were the same pre-and post-intervention, but no information was provided on the staff diagnosing infections. Information on sterile drape and CVC use was provided by the purchasing department, but the person(s) responsible for transcribing and analysing this information were not reported |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | The authors acknowledged that other unmeasured effects may have affected outcomes, and because faculty participated in the course, they may have changed their approach to care of patients and subsequent supervision of physicians-in-training. Only processes that are routinely performed by physicians were monitored (i.e. excluding arterial punctures, urinary catheter insertions, blood draws through lines, peripheral line insertions, and phlebotomy) |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? YES. The numbers, duration, content, and cost of sessions were reported, although it is unclear whether the training course comprised 7- or 8-hour-long sessions and whether a session on OSHA was included |
| Justification given for sample size? NO | |
| Data collection process reported? NOT REPORTED. Methods for assessing outcomes were reported but no information was provided on who collected the outcomes data or how the data were managed | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT APPLICABLE | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT APPLICABLE | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NO. Significance discussed but no effect size provided. |
| Target behaviour change achieved? PARTIALLY. Reported briefly the proportion of CVC insertions for which sterile drapes were used immediately before and in the 6 months after an education course. There was a statistically significant increase in compliance with the use of drapes; before and after the education course compliance was 44% and 65% respectively (p < 0.001) |
Additional comments
-
Stated that use of CVCs in the seven study units was high (central-line days/patient-days × 100 = 73%). Because of this, the authors concluded that patient-days could serve as a surrogate of device-days (even though the latter would probably be more accurate under other circumstances).
-
Authors refer to catheter related infections inconsistently in the text, in some cases to describe the total number of (catheter related + primary BSI) infections. The definition of ‘catheter related’ infections given is consistent with catheter-associated infections (blood cultures negative or not done).
-
Surveys (data reported but not extracted) showed that most physicians had little experience performing procedures during medical school (1 of 3 PGY-1 physicians had never inserted a CVC during medical school).
SPEROFF (2011)136
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Speroff (2011)136 |
| Summary of approach | Multicentre, cluster RCT in ≥ 60 ICUs (number not stated) comparing virtual collaborative and toolkit QI approaches for preventing CLABSI and VAP |
| Location | USA, multistate (primarily southern states) |
| Language | English |
| Critical care specialty | Not reported (multiple ICUs) |
| No. of critical care units | Not reported (≥ 60 assuming at least one per hospital) |
| No. of hospitals | 60 in total (29 and 31 per group) |
| Hospital name (unless multicentre); city | Multicentre (hospital names not reported) |
| Study design | Cluster RCT (Clinicaltrials.gov registration number NCT 00975923) |
| Study time periods | Baseline surveys: July to November 2005 Randomised December 2005 Intervention: January 2006 to September 2007 (18 months) Follow-up: September 2007 to January 2008; outcomes survey January to April 2008 (data not reported) Note: Reported that study was 18 months with follow-up thereafter: unclear whether any embedding of QI practices continued into the follow-up period |
| Funding source | Supported by a grant from the Partnerships in Implementing Patient Safety (PIPS) from the Agency for Healthcare Research and Quality (AHRQ) |
| Conflicts of interest | Stated no disclosure to report: AHRQ provided funding only |
Population and setting
Median hospital size = 117 beds; median ICU size = 16 beds; medical centres: rural (11%), inner city (28%) and suburban (61%). At baseline 45% of the facilities reported having a CLABSI programme and 62% a VAP programme (not stated whether facilities refer to hospitals or ICUs; number of ICUs per hospital not reported).
| Baseline data Critical care unit characteristics |
Group 1: Toolkit (29 hospitals) | Group 2: Virtual collaborative (31 hospitals) | Difference Group 1 vs. Group 2 |
|---|---|---|---|
| Median (IQR) ICU patient volume per year | 578 (244–1077) | 568 (294–904) | p = 0.93 |
| Median (IQR) ICU LOS, days | 4228 (1645–6725)a | 3882 (1758–5718)a | p = 0.95 |
| Mean (SD)% ICU mortality rateb | 7.1 (3.6) | 5.7 (3.1) | p = 0.13 |
| Mean (SD)% patients admitted to ICU from emergency departmentb | 67 (20) | 71 (15) | p = 0.27 |
| Mean (SD)% Medicare/Medicaidb | 68.5 (10.1) | 68.6 (9.5) | p = 0.95 |
| % hospitalist ICU management | 40 | 47 | p = 0.61 |
| Patient population characteristics | |||
| Mean (SD)% femaleb | 50.3 (7.7) | 49.7 (5.7) | p = 0.79 |
| Age, ethnicity, health status, socioeconomic status, oral or i.v. antimicrobial use | Not reported | Not reported | Not reported |
| Device characteristics | None reported | None reported | Not reported |
| Insertion site antisepsis used | Not reported | Not reported | Not reported |
| Dressing type and duration/frequency | Not reported | Not reported | Not reported |
Intervention characteristics
| Objective | To determine if a QI virtual collaborative intervention would perform better than a toolkit-only approach at preventing CLABSI and VAP |
| Main focus of education | Not explicitly stated, but the key interventions for preventing CLABSI were routine hand hygiene, site selection, barrier precautions, chlorhexidine skin preparation, and catheter need review. Education also covered epidemiology and prevention of infections, teamwork and QI approaches. Education also included in intervention for preventing VAP |
| Trainers (providers) | Not reported; appears to be ICU nurse and quality managers |
| Training of trainers | Not reported; appears to be coordinated by the Hospital Corporation of America (HCA) corporate office of Quality, Safety, and Performance Improvement |
| Learners (recipients) | ICU teams; no details reported |
| Target behaviour change | Not explicitly stated: interventions address multiple possible behaviours associated with prevention of CLABSI and VAP |
| Development and testing | Drew upon existing research in QI approaches; intervention components were iteratively developed by teams based on plan-do-study-act cycles |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted For the hospitals owned and operated by the HCA203, stated that 130/133 infection control programmes (97.7%) conducted surveillance in accordance with the CDC definitions for BSI |
Both groups: Both groups were offered interactive web seminars: five were on clinical subject matter, and five were on patient safety, charting use of statistical process control and QI methods (total number of seminars not stated) Group 1 (toolkit) Hospitals received a toolkit containing a set of evidence based guidelines and fact sheets for preventing CLABSI and VAP, a review of QI and teamwork methods, and standardised data collection and charting tools. HCA website provided nurse and quality managers with access to all educational seminars, clinical and QI tools. Education was provided to standardise outcome measurement (online tutorial, Talbot TR, November 2005). Other aspects of QI for preventing CLABSI and VAP were left to the discretion of the ICU to develop and implement Group 2 (virtual collaborative) Teams attended web seminars and teleconferences for reporting back to the larger group. Supported by monthly educational and troubleshooting conference calls, individual coaching co-ordinated by the HCA corporate office of quality, safety and performance improvement, and an e-mail Listserv designed to stimulate interaction among teams Intervention components reportedly used in both interventions included BSI checklists; daily catheter review; seminars; continuing education classes for BSI; written education; BSI surveillance guide; QI efforts to prevent CLABSI; and statistical process control |
| Infection surveillance feedback approach | Self-reported. Stated that data collection and surveillance methods varied across hospitals (reference cited203). Survey203 indicated that hospital acquired infection (HAI) reports were routinely provided to ICU staff and other stakeholders during the baseline period (September 2005). Web application data registry created and infection data reporting by the infection control personnel mandated from first quarter of 2006. To verify electronic data and correct missing information, infection control personnel were requested to complete a retrospective data collection sheet providing quarterly reports from January 2005 to December 2007 (i.e. part baseline, part intervention period). Validity and reliability of data collection not reported. Stated ‘Nearly all ICPs provided HAI reports to senior hospital leaders, nurse management of the ICU, and institutional committees. Feedback of data was less frequent for front-line health-care workers, such as nurses, physicians, respiratory therapists, and support staff |
| Performance feedback approach | Self-reported. Referred to as provider performance feedback for BSI and VAP but method of feedback not reported. Stated that validity of data collection method was not assessed |
| Concentration of education | Not reported and not discernible owing to the large-scale nature of the intervention with multiple educational activities, site-to-site variation and iterative implementation. |
| Non-educational intervention components | Infrastructural changes (team development); IHI care bundles for CLABSI and VAP (not described but reference cited); Concurrent intervention to prevent VAP |
| Costs reported | Not reported |
Outcome characteristics
| Catheter-BSI definition From online HCA webcast at Vanderbilt Medical School (TR Talbot, 2005) Stated that although most hospitals defined CLABSI using CDC definitions, data collection and surveillance methods varied across hospitals (reference cited203) |
CDC NNIS laboratory-confirmed CVC-related bloodstream infection. Must meet at least one of the following criteria: Criterion 1: Patient with CVC has a recognised pathogen cultured from one or more blood cultures and organism cultured from blood is not related to an infection at another site Criterion 2: Patient with CVC has at least one of the following signs or symptoms: fever, chills, or hypotension, and at least one of the following: (a) common skin contaminant (e.g. coagulase-negative staphylococci) is cultured from two or more blood cultures drawn on separate occasions; (b) common skin contaminant is cultured from at least one blood culture from a patient with CVC, and the physician institutes appropriate antimicrobial therapy; and signs and symptoms and positive laboratory results are not related to an infection at another site |
| Outcomes reported | Not stated whether primary or secondary (study power based on hospital-acquired infections, not specifically mentioned whether CLABSI or VAP): CLABSI per 1000 catheter-days; VAP per 1000 ventilator days (data not extracted); Process implementation |
Results data
Primary outcomes
| Outcome | Group 1: Toolkit (29 hospitals) | Group 2: Virtual collaborative (31 hospitals) | Difference between Groups 1 and 2 |
|---|---|---|---|
| Hospital-wide outcomes data (data pooled per hospital; not reported for separate ICUs) | |||
| Device duration | Not reported | Not reported | Not reported |
| Device-days (not reported; e-mailed by the author) | |||
| Baseline | 26,599 | 22,172 | Not reported |
| 3 months | 25,257 | 22,202 | |
| 6 months | 24,618 | 22,951 | |
| 9 months | 27,821 | 23,268 | |
| 12 months | 29,066 | 26,211 | |
| 15 months | 29,818 | 25,646 | |
| 18 months | 24,245 | 19,276 | |
| No. of devices/patient | Not reported | Not reported | Not reported |
| CLAB incidence rate (not reported; e-mailed by the author) | |||
| Baseline | 81 | 37 | Not reported |
| 3 months | 72 | 52 | |
| 6 months | 76 | 54 | |
| 9 months | 72 | 53 | |
| 12 months | 75 | 45 | |
| 15 months | 62 | 65 | |
| 18 months | 66 | 53 | |
| Median (IQR) CLABSI incidence per 1000 catheter-days per hospital | |||
| Baseline | 2.42 (0.65–6.80) | 1.18 (0.00–3.83) 2.24 (0.54–4.69) | Baseline: p = 0.24 Baseline vs. 12 months: p = 0.13 Baseline vs. 18 months: p = 0.95 Overall trend: p = 0.71a |
| 3 months | 2.47 (1.48–5.35) | 2.28 (0.00–3.73) | |
| 6 months | 2.54 (0.00–4.98) | 1.76 (0.00–3.74) | |
| 9 months | 1.23 (0.00–3.93) | 1.18 (0.00–2.71) | |
| 12 months | 1.17 (0.00–3.61) | 2.04 (0.00–4.91) | |
| 15 months | 1.77 (0.00–3.30) | 2.76 (0.00–4.67) | |
| 18 months | 1.16 (0.00–5.46) | 1.18 (0.00–3.83) 2.24 (0.54–4.69) | |
| CLABSI incidence per 1000 catheter-days per hospital calculated by reviewers from authors' device-days and incidence data b | |||
| Baseline | 3.0 | 1.7 | |
| 3 months | 2.9 | 2.3 | |
| 6 months | 3.1 | 2.4 | |
| 9 months | 2.6 | 2.3 | |
| 12 months | 2.6 | 1.7 | |
| 15 months | 2.1 | 2.5 | |
| 18 months | 2.7 | 2.7 | |
| CLAB incidence per 1000 patient-days | Not reported | Not reported | Not reported |
| LOS | Not reported | Not reported | Not reported |
| Mortality | Not reported | Not reported | Not reported |
Secondary outcomes
| Reaction to education | Not a study outcome | ||
| Attitudes | Not a study outcome | ||
| Compliance | Not a study outcome | ||
| Knowledge | Not a study outcome | ||
| Skills | Not a study outcome | ||
| Process evaluation | Group 1: Toolkit (29 hospitals) | Group 2: Virtual collaborative (31 hospitals) | Difference between Groups 1 and 2 |
|---|---|---|---|
| No. (%) of hospitals responding to survey | 19 (66) | 27 (87) | |
| No. of ICUs responding to survey | 25 | 36 | |
| Clinical tool use a | 49% | 61% | p = 0.23 |
| BSI surveillance guide | 13/25 (52%) | 22/36 (61%) | p = 0.60 |
| BSI checklist | 16/25 (64%) | 31/36 (86%) | p = 0.06 |
| Data tool use a | 30% | 56% | p = 0.004 |
| QI implementation tools | 6/25 (24%) | 19/36 (53%) | p = 0.03 |
| BSI statistical process control | 5/25 (20%) | 23/36 (64%) | p = 0.001 |
| All tools used | |||
| Median no. of tools downloaded | 7 | 10 | p = 0.051 |
| Strategy use a | 54% | 69% | p = 0.017 |
| Protocols for BSI | 19/25 (76%) | 24/36 (67%) | p = 0.57 |
| Computer documentation for BSI | 13/25 (52%) | 24/36 (67%) | p = 0.29 |
| Increased staffing | 0/25 (0%) | 3/36 (8%) | p = 0.26 |
| Written education for BSI | 19/25 (76%) | 31/36 (86%) | p = 0.33 |
| Continuing education classes for BSI | 16/25 (64%) | 28/36 (78%) | p = 0.26 |
| QI teams | 14/25 (56%) | 27/36 (75%) | p = 0.16 |
| Provider performance feedback for BSI | 11/25 (44%) | 23/36 (64%) | p = 0.18 |
| Implementation | |||
| QI efforts to prevent CLABSI | 88% | 97% | p = 0.99 |
| Implemented hand hygiene b | 93% | 100% | Not reported |
| Implemented site selection b | 72% | 86% | Not reported |
| Implemented barrier precautions b | 97% | 100% | Not reported |
| Implemented chlorhexidine b | 100% | 100% | Not reported |
| Implemented daily assessmentb,c | 68% | 92% | Not reported |
| Implemented daily catheter reviewc | Less often (no data) | More often (no data) | p = 0.04 |
| Implemented all components of BSI interventions | 64% | 100% | p = 0.13 |
| Implemented BSI checklist | 15/25 (60%) | 28/36 (78%) | p = 0.16 |
| Participation in education | |||
| Participated in seminars | 39% | 57% | p = 0.014 |
| Attended the clinical topics | 56% | 64% | p = 0.37 |
| Participated in data and method topics | 22% | 50% | p < 0.001 |
| Other | |||
| Hospitals found seminars useful to QI efforts | 30% | 49% | p = 0.017 |
Critical appraisal
Potential for bias
| Group selection a | Were there systematic differences between the study groups? UNCLEAR. Very limited demographic information reported; unable to rule out selective presentation of data, as no justification provided for the focus on those variables presented | |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE (note that part of analysis included adjustment for covariates, but the covariates were not specified) | ||
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? UNCLEAR. Limited information on staffing, infrastructure and care policy reported (staffing of infection control programmes varied among hospitals203). The study authors noted that the study was implemented during the IHI's 100,000 Lives Campaign, which may have influenced regional infection rates (baseline incidence rate was noted to be low) | |
| Was the effect of educational practice separable from effects of non-educational practice? NO. Although many aspects of the interventions were educational, there were other non-educational components, e.g. provision of QI teams | ||
| Were the intervention component(s) implemented as planned? PARTIALLY. The intervention components varied across hospitals but implementation was reported only for hospitals who responded to surveys (see Process Evaluation section for details) | ||
| Missing data a | Were there systematic differences between the study groups in data availability? NOT REPORTED. The number of hospitals was specified for each study group but no indication was given of whether any hospitals did not provide follow-up data. The number of ICUs was given only for those reporting follow-up data; the number of ICUs in each study group was not stated | |
| Outcome measurement a | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? UNCLEAR. Stated that data collection and surveillance methods varied across hospitals in the baseline period. Education aimed to improve standardisation of outcome definitions but it appears that same education may have been common to both study groups | |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | ||
| Other possible sources of bias |
|
|
| Risk of selection bias in randomised studies | Risk of bias: reviewer judgement (low/high/unclear) | Support for judgement |
|---|---|---|
| Random sequence generation | UNCLEAR | No information given. Stated that hospitals willing to participate were matched on geographic location and ICU volume and then randomised but method of achieving this randomisation approach not stated |
| Allocation concealment | UNCLEAR | No information given |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. A large-scale study that did not mandate fixed specific intervention components, with each centre free to tailor its use of tools and change ideas. Many education components appear to have been common to both study groups, but this was not clearly reported, hence although numerous online supporting resources were provided, it is difficult to establish which are relevant to each study group. Although implementation was at the level of ICUs, the number of ICUs in the study, the number per hospital, and their specialties were not reported |
| Justification given for sample size? YES. Power of 82–91% for testing group differences calculated a priori with one-tailed = 0.05 and group size of 30, assuming a 50% decrease in hospital-associated infection rates for the collaborative group vs. a 10–15% decrease for the toolkit group. | |
| Data collection process reported? PARTIALLY. Limited information given on infection surveillance feedback; no information given on performance feedback methods | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NO. Stated that data were not verified by independent assessment | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NO. Stated that data were not verified by independent assessment | |
| Statistical tests described? PARTIALLY. Stated that trend analysis was adjusted for clustering of ICUs within hospitals and adjusted for covariates but no intraclass correlation coefficient to account for clustering was reported. The covariates were not specified (limited baseline variables reported). Not stated whether analysis was by intention to treat (missing data not reported) | |
| Results | Educational significance or effect size assessed? NO |
| Target behaviour change achieved? NOT REPORTED. Implementation of infection-prevention practices is reported through a post-study survey but unclear whether these practices had changed relative to baseline |
Additional comments
-
Medical centres included were those of the HCA, a network of hospitals located primarily in the southern USA. Stated that to minimise contamination bias between study groups within the same facility, the unit of randomisation was the hospital and implementation was at the level of the ICU.
-
Stated that outcomes were reported hospital-wide, but appears to present data from ICUs.
-
A related paper by Talbot and colleagues203 (cited) indicated that nearly half of 126 hospitals in the HCA system surveyed from September 2005 reported difficulty in obtaining denominator data needed to determine BSI rates, with nearly one-quarter of the 126 hospitals unable to provide BSI rates for some months. Unclear whether these figures are representative for the 60 hospitals included in the present study.
-
Speroff and colleagues136 stated that data collection and surveillance methods varied across hospitals, citing evidence from Talbot and colleagues203 but unclear how representative the latter data from 162 hospitals are for the 60 hospitals included in the present study.
-
Stated that the 60 participating sites did not differ from 113 non-participating sites (data not shown).
-
The study population consisted of hospitals that were part of a larger health-care system owned by a specific corporation. This may influence generalisability.
-
Correspondence with the main author confirmed that, while the majority of ICUs were adult based, there were two paediatric ICUs per study arm.
WALL (2005)138
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Wall (2005)138 |
| Summary of approach | Single-unit, continuous QI programme involving real-time feedback of CRBSI rates and compliance with insertion practice, based on data collected by nurses using a standard checklist |
| Location | USA: Tennessee |
| Language | English |
| Critical care specialty | Adult MICU |
| No. of critical care units | 1 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Vanderbilt University Medical Center, Nashville |
| Study design | Single cohort before-and-after study |
| Study time periods | Baseline: January 2000 to October 2002 (CRBSI data reported for November 2000 to October 2002) (2 years) Intervention: November 2002 to October 2004 (2 years) Follow-up: None (continuous QI programme) |
| Funding source | Lead author (Wall) was supported by the Office of Academic affiliations, Department of Veterans Affairs, VA National Quality Scholars Program, and with resources at the VA Tennessee Valley Healthcare System, Nashville, TN. Co-author (Ely) received the Paul Beeson Faculty Scholar Award from the Alliance for Aging Research and received a K23 from the National Institute of Health |
| Conflicts of interest | Stated no competing interests declared |
Population and setting
| Critical care unit characteristics | 14 MICU beds in a 640-bed tertiary teaching hospital Approximately 600 MICU admissions per year Most CVCs in the medical MICU were inserted by trainees (house staff). Details of the staff who inserted catheters were reported only for the intervention period: Medical student = 19 (3%) Intern (postgraduate year 1) = 357 (57%) Resident (postgraduate year 2) = 176 (28%) Fellow/attending = 78 (12%) Total insertions = 630 (100%) |
| Patient population characteristics | Patients with conditions including acute respiratory distress syndrome, asthma, chronic obstructive pulmonary disease, respiratory failure, pneumonia, sepsis, poisoning, drug overdose and gastrointestinal bleeding. Also critically ill solid organ and bone marrow transplant patients |
| Device characteristics | Only devices placed by MICU staff were included (the authors implied that dialysis catheters were excluded but the numbers given below for the 630 catheters inserted by MICU staff during the intervention period include dialysis catheters). Catheter types and insertion sites were reported only for the intervention period:Catheter type (total = 630):
|
| Insertion site antisepsis used | Not reported |
| Dressing type and duration/frequency | Not reported |
Intervention characteristics
| Objective | The primary goal was to show that real-time measurement of CVC care was feasible in the MICU and that process measurements would guide continuous QI and thereby lead to a reduced CRBSI rate |
| Main focus of education | Catheter insertion. Real-time feedback of compliance with insertion practices and data on infections based on checklists completed by nurses |
| Trainers (providers) | Voluntary interdisciplinary team |
| Training of trainers | Not reported |
| Learners (recipients) | MICU house staff (proceduralists) and MICU nursing staff (as observers of procedures) |
| Target behaviour change | Sterile catheter insertion procedure |
| Development and testing | A voluntary interdisciplinary team of leaders (nurse manager, MICU director), front-line staff (MICU nurses and physicians), infectious diseases experts (chief hospital epidemiologist, infection control practitioners) and improvement experts developed a system for measuring the process of CVC care in real time based on review of the published literature and observation of MICU practice to identify risk factors during catheter insertion and maintenance Priority areas for measurement established by the multidisciplinary team included provider education, trainee supervision, insertion site, hand hygiene, skin antisepsis, and use of maximal sterile barriers. Nursing checklist for CVC insertion was pilot tested for 1 week with two MICU nurses and revised before being implemented across the MICU |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Nursing checklist for CVC insertion. The checklist recorded through check boxes whether providers used hand hygiene; used barrier precautions (gloves, gown, mask, patient drape; using all four together being defined as maximal barrier precautions); maintained a sterile field; used chlorhexidine skin antisepsis; and were properly supervised. ‘Supervision’ was defined as the proceduralist having successfully completed five CVC insertions or if another provider with experience of at least five CVC insertions supervised them during the procedure. Nurses obtained this information for the checklist but had the option of recording ‘didn’t ask’. Providers were not obliged to follow infection reducing steps if a life-threatening situation mandated immediate CVC insertion Web-based tutorial. This discussed catheter-related infections, described the checklist and explained the evidence base. House staff were requested by letter to complete the tutorial before their rotation. Nursing staff were requested by letter to complete the tutorial as part of their annual competencies curriculum. The website recorded whether each provider completed a self-assessment examination. Reminder letters were re-sent after 3 months if necessary, and MICU leadership personally approached non-compliers |
| Infection surveillance feedback approach | Pre-intervention: Monthly infection incidence reports were provided by infection control practitioners to the MICU staff Intervention period: To better account for the rarity of infections, infection surveillance data were fed back to MICUs as the time interval between infections rather than as incidence rates. This approach followed the principles of statistical process control, with g-charts displaying the time interval between infections. Formal validity and reliability analyses for data capture using the checklist were not undertaken |
| Performance feedback approach | Completed checklists were dropped in a secure lock box then collected daily by infection control practitioners who scanned the de-identified information into a spreadsheet database using automatic scanning software. Data were held on a central computer at the Centre for Clinical Improvement. Process measurements obtained from the checklists were ‘bundled’ with the monthly infection surveillance reports and fed back to front-line MICU staff (feedback presentation method not reported). Formal validity and reliability analyses for data capture using the checklist were not undertaken. Competence in completing the online tutorial (knowledge) was assessed but the method of feeding back the results to staff (e.g. identifying learning goals) was not reported |
| Concentration of education | Checklist was applied daily and could be completed in 1 minute Feedback on infections and compliance with insertion practice was reported as in ‘real time’ but the way MICU staff accessed the information was not reported. The time required for training and assessment was not reported |
| Non-educational intervention components | None (all components including checklist, infection surveillance feedback and performance feedback are considered educational for the purposes of this systematic review) |
| Costs reported | No |
Outcome characteristics
| Catheter-BSI definition Reference cited: O’Grady et al.196 |
NNIS definition (reference cited). Laboratory-confirmed bacteraemia (or fungaemia) is attributed to a patient with a CVC if they have been in the MICU for at least 48 hours, provided that the infection is not related to another distal source. Patients with a CVC who develop a BSI within 48 hours of MICU discharge also have CR-BSI |
| Outcomes reported | Not referred to as primary or secondary: CRBSI rate and the time between CRBSI events Compliance with CVC insertion practices |
Results data
Primary outcomes
| Outcome | Baseline: 24 months (November 2000 to October 2002) | Intervention: 24 months (November 2002 to October 2004) | Difference between baseline and intervention |
|---|---|---|---|
| Device duration | Not reported | Not reported | |
| Total device-days (not reported; e-mailed by the author) | 3571 during 24 months | 1132 during 24 months | |
| No. of devices/patient | Not reported | Not reported | |
| CRBSI number | 25 during 24 months | 6 during 24 months | 76% reduction |
| CRBSI incidence per 1000 catheter-days (a) reported and also confirmed by the author; (b) not reported; e-mailed by the author | (a) 7.0 during 24 months | (a) 3.8 during final 6 months(b) 5.3 during 24 months | No statistical comparison reported |
| CRBSI incidence per 1000 patient-days | Not reported | Not reported | |
| LOS | Not reported | Not reported | |
| Mortality | Not reported | Not reported |
Secondary outcomes
| Reaction to education | Not a study outcome | ||||
| Attitudes | Not a study outcome | ||||
| Knowledge | Not a study outcome | ||||
| Skills | Not a study outcome | ||||
| Compliance: Percentage of insertions compliant. Data extracted by reviewer from line graph using Engauge software | |||||
| Month of intervention | Hand hygiene | Chlorhexidine skin preparation | Maximal barriers | Guidewire exchange | |
|---|---|---|---|---|---|
| 1–3 | 73 | 57 | 68 | 18 | |
| 4–6 | 87 | 76 | 49 | 13 | |
| 7–9 | 91 | 88 | 62 | 15 | |
| 10–12 | 92 | 95 | 70 | 16 | |
| 13–15 | 95 | 91 | 75 | 18 | |
| 16–18 | 91 | 96 | 69 | 25 | |
| 19–21 | 94 | 96 | 70 | 13 | |
| 22–24 | 89 | 100 | 86 | 20 | |
| Process evaluation The checklist generated real-time measurements for the CVC insertion process. These data were used for cycles of continuous QI in which the reasons for certain provider behaviours were explored A decline in adherence to maximal barrier precautions during December 2002 to February 2003 was found to have been caused by lack of use of patient drapes (data presented but not extracted by reviewers). Adherence to maximal barrier precautions improved after the team purchased new sterile kits pre-packaged with drapes, and confirmed providers had completed the tutorial Use of the femoral insertion site compared with other insertion sites was associated with statistically significantly lower adherence to hand washing, chlorhexidine skin preparation and maximal sterile barriers (data presented but not extracted by reviewers) |
|||||
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? UNCLEAR. The authors stated that mortality rates, admission diagnoses, ventilator days, and lengths of stay were similar for patients admitted during the pre-intervention and intervention periods. However, other patient characteristics (e.g. age, gender, health acuity) were not reported |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? UNCLEAR. The authors acknowledged that it is possible that some undetected case mix variable or change in catheter duration may have contributed to the decreased CRBSI rate and that while the checklist specifically targeted catheter insertion, unmeasured improvements during catheter maintenance may also have contributed to the reduced CRBSI rate |
| Was the effect of educational practice separable from effects of non-educational practice? YES. All components of this intervention (checklist, infection surveillance feedback and process feedback) are classed as educational | |
| Were the intervention component(s) implemented as planned? PARTIALLY. Stated in the methods section that the checklist was introduced to all nursing staff and implemented throughout the entire MICU. Letters were sent requesting ICU staff to complete the tutorial. If individuals still did not complete the tutorial, the MICU leadership approached them privately. Stated that a total of 630 CVCs were inserted using the new checklist | |
| Missing data | Were there systematic differences between the baseline and intervention groups in attrition or exclusions (includes withdrawal due to mortality; missing outcome data)? UNCLEAR. The authors stated that admissions, admission diagnoses, and mortality did not differ between the baseline and intervention groups; however, no quantitative data were reported |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? UNCLEAR. The MICU used a standard definition of CRBSI which appears to have applied both in the baseline and intervention periods. However, no information was given on the staff diagnosing infections |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | No other sources of bias were reported by the authors or identified by the reviewers based on the information presented |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. Although the principles and practices are mostly well described, only a summary of the checklist content is reported. The training module is general and covers topics relevant to infection prevention beyond those described by the authors; the scope of the knowledge assessment based on the tutorial is not reported |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? PARTIALLY. Stated that upon completing the checklist, a nurse detached the top page (with all items readable) and dropped it in a secure lockbox. The second page remained on the patient's chart with the sensitive items blacked out. The infection control practitioners collected the checklists daily and scanned the de-identified forms into a pre-established computerised database using scanning software which scanned pre-established fields on the checklist and imported the information into a spreadsheet database. These data were stored on a secure computer at the Center for Clinical Improvement for future statistical analyses | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NO. Formal validity and reliability analyses for data capture using the checklist were not undertaken | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NO. Formal validity and reliability analyses for data capture using the checklist were not undertaken | |
| Statistical tests described? YES. Statistical process control approach | |
| Results | Educational significance or effect size assessed? NOT REPORTED |
| Target behaviour change achieved? PARTIALLY. Some aspects of catheter insertion procedure were complied with more extensively than others. Failure to use patient drapes was identified as a problem and steps were taken to improve compliance |
Additional comments
The data reported above are from the primary research publication138 and web-based tutorial available at www.mc.vanderbilt.edu/cvctutorial.
WARREN (2003)139
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Warren (2003)139 |
| Summary of approach | Ten-page self-study module, lectures and posters on the prevention of CABSIs given in two ICUs during 3 months in a non-teaching hospital |
| Location | USA, Missouri |
| Language | English |
| Critical care specialty | Medical and SICU |
| No. of critical care units | 2 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Missouri Baptist Medical Center, St Louis |
| Study design | Single-cohort before-and-after study combining data from two ICUs |
| Study time periods | Baseline: 1 March 1998 to 30 June 1999 (16 months) Intervention: 1 July to 30 September 1999 (3 months) Follow-up: 1 October 1999 to 31 July 2000 (10 months) Data on anatomical insertion site of CVCs were collected from 1 August 1998 |
| Funding source | Supported in part by a CDC Cooperative Agreement and the National Foundation for Infectious Diseases (NFID) postdoctoral fellowship in Nosocomial Infection Research and Training (to lead author) |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics: 500-bed non-teaching private community hospital affiliated to a 13-hospital integrated health-care system Each ICU: 10 beds (total 20 beds); combined admission approximately 150 patients per month No medical students or house staff; only registered nurses provided patient care Four hospital-employed, board-certified critical care physicians staffed both ICUs and were responsible for central catheter insertion and critical care management |
|||
| Patient population characteristics: 1215 of 3943 of patients admitted to the ICU during the study (30%) had a CVC | Baseline (16 months) | Post intervention (10 months) | Difference between baseline and post intervention |
| Total no. of patients | 674 | 541 | Not reported |
| No. (%) in MICU | 310 (46) | 239 (44) | p = 0.56 |
| No. (%) in SICU | 364 (54) | 302 (56%) | Not reported |
| Male gender, n (%) | 351 (52) | 280 (52) | p = 0.91 |
| Median age, years | 71 | 71 | p = 0.62 |
| Caucasian, n (%) | 630 (94) | 499 (92) | p = 0.65 |
| Congestive heart failure, n (%) | 280 (42) | 248 (46) | p = 0.13 |
| COPD, n (%) | 226 (34) | 180 (33) | p = 0.92 |
| Malignancy, n (%) | 113 (17) | 106 (20) | p = 0.20 |
| Diabetes mellitus, n (%) | 200 (30) | 170 (31) | p = 0.51 |
| HIV infection, n (%) | 1 (0.1) | 0 (0) | p = 1.0 |
| Mean APACHE II score | 25.2 | 25.1 | p = 0.86 |
| Haemodialysis, n (%) | 91 (14) | 69 (13) | p = 0.70 |
| Corticosteroid use, n (%) | 231 (34) | 197 (36) | p = 0.44 |
| Immunocompromised, n (%) | 84 (13) | 51 (9) | p = 0.09 |
| Mechanically ventilated, n (%) | 389 (58) | 305 (56) | p = 0.64 |
| Mean no. of ventilator days | 9.1 | 9.0 | p = 0.28 |
| Tracheostomy, n (%) | 49 (7) | 45 (8) | p = 0.50 |
| Re-intubation, n (%) | 70 (10)a | 64 (12) | p = 0.95 |
| Device characteristics | Antimicrobial (chlorhexidine and silver sulfadiazine)-coated CVCs were used routinely during the entire study period CVC supplies were kept in a supply room within the unit; no changes in the accessibility of supplies were made during the intervention Insertion site data were available for 651 of the patients (54%) who had 941 non-tunnelled CVCs: Proportion inserted in subclavian vein: Baseline: 124 (25%) of 487 CVCs (in 318 patients) Intervention: 188 (41%) of 454 CVCs (in 333 patients) Difference: p < 0.001 |
||
| Insertion site antisepsis used | Not reported | ||
| Dressing type and duration/frequency | Not reported | ||
Intervention characteristics
| Critical care unit characteristics: 500-bed non-teaching private community hospital affiliated to a 13-hospital integrated health-care system Each ICU: 10 beds (total 20 beds); combined admission approximately 150 patients per month No medical students or house staff; only registered nurses provided patient care Four hospital-employed, board-certified critical care physicians staffed both ICUs and were responsible for central catheter insertion and critical care management |
|||
| Patient population characteristics: 1215 of 3943 of patients admitted to the ICU during the study (30%) had a CVC | Baseline (16 months) | Post intervention (10 months) | Difference between baseline and post intervention |
| Total no. of patients | 674 | 541 | Not reported |
| No. (%) in MICU | 310 (46) | 239 (44) | p = 0.56 |
| No. (%) in SICU | 364 (54) | 302 (56%) | Not reported |
| Male gender, n (%) | 351 (52) | 280 (52) | p = 0.91 |
| Median age, years | 71 | 71 | p = 0.62 |
| Caucasian, n (%) | 630 (94) | 499 (92) | p = 0.65 |
| Congestive heart failure, n (%) | 280 (42) | 248 (46) | p = 0.13 |
| COPD, n (%) | 226 (34) | 180 (33) | p = 0.92 |
| Malignancy, n (%) | 113 (17) | 106 (20) | p = 0.20 |
| Diabetes mellitus, n (%) | 200 (30) | 170 (31) | p = 0.51 |
| HIV infection, n (%) | 1 (0.1) | 0 (0) | p = 1.0 |
| Mean APACHE II score | 25.2 | 25.1 | p = 0.86 |
| Haemodialysis, n (%) | 91 (14) | 69 (13) | p = 0.70 |
| Corticosteroid use, n (%) | 231 (34) | 197 (36) | p = 0.44 |
| Immunocompromised, n (%) | 84 (13) | 51 (9) | p = 0.09 |
| Mechanically ventilated, n (%) | 389 (58) | 305 (56) | p = 0.64 |
| Mean no. of ventilator days | 9.1 | 9.0 | p = 0.28 |
| Tracheostomy, n (%) | 49 (7) | 45 (8) | p = 0.50 |
| Re-intubation, n (%) | 70 (10)a | 64 (12) | p = 0.95 |
| Device characteristics | Antimicrobial (chlorhexidine and silver sulfadiazine)-coated CVCs were used routinely during the entire study period. CVC supplies were kept in a supply room within the unit; no changes in the accessibility of supplies were made during the intervention. Insertion site data were available for 651 of the patients (54%) who had 941 non-tunnelled CVCs: Proportion inserted in subclavian vein: Baseline: 124 (25%) of 487 CVCs (in 318 patients) Intervention: 188 (41%) of 454 CVCs (in 333 patients) Difference: p < 0.001 |
||
| Insertion site antisepsis used | Not reported | ||
| Dressing type and duration/frequency | Not reported | ||
Outcome characteristics
| Catheter-BSI definition Reference cited: National Nosocomial Infections Surveillance186 |
CABSI was defined by using National Nosocomial Infections Surveillance (NNIS) system criteria. CABSI defined as concordant growth between cultures obtained from the catheter tip; hub, infusate, or insertion site exudate and percutaneously drawn blood cultures; or a recognised pathogen isolated from blood culture that is not related to infection at another site. A CABSI was considered ICU related if it occurred > 24 hours after admission to the ICU. For low-virulence organisms, two or more positive blood cultures obtained on separate occasions had to be noted for the isolate to be considered a true pathogen, and at least one of the blood cultures had to be from a peripheral venepuncture |
| Outcomes reported | Not stated whether primary or secondary: CABSI incidence and incidence density Time to infection LOS Mortality Completion of pre- and post-intervention tests |
Results data
Primary outcomes
| Outcome | Baseline (16 months, 674 patients) | Post intervention (10 months, 541 patients) | Difference between baseline and post intervention | |
|---|---|---|---|---|
| Device duration | Not reported | Not reported | Not reported | |
| Total device utilisation, catheter-days | Total | 6110 | 5210 | p = 0.46 |
| Mean per patient | 9.1 | 9.6 | ||
| No. of devices/patient | Not reported | Not reported | Not reported | |
| CABSI incidence, n (% of patients) | ||||
| (a) Overall | 30 (4) | 11 (2) | p = 0.02 | |
| (b) For specific post-intervention time periods | ||||
| 1 July to 30 September 1999 | 1 | |||
| 1 October to 31 December 1999 | 4 | |||
| 1 January to 31 March 2000 | 4 | |||
| 1 April to 31 July 2000 | 2 | |||
| CABSI incidence per 1000 catheter-days | 0 | |||
| (a) Overall | 4.9 | 2.1 | Relative risk = 0.43 (95% CI 0.22 to 0.84) (57% decrease) | |
| (b) For specific post-intervention time periods | ||||
| 1 July to 30 September 1999 | 0.9 | |||
| 1 October to 31 December 1999 | 3.1 | |||
| 1 January to 31 March 2000 | 2.7 | |||
| 1 April to 31 July 2000 | 1.6 | |||
| CABSI incidence per 1000 patient-days | Not reported | Not reported | Not reported | |
| Median time to infection among patients with CABSI, days | 9 | 6 | p = 0.70 | |
| Mean LOS, days | ICU | 7.8 | 8.0 | p = 0.09 |
| Hospital | 19.0 | 19.3 | p = 0.37 | |
| Mortality, n (%) | 182 (27) | 128 (24) | p = 0.18 | |
Secondary outcomes
| Reaction to education | Not a study outcome |
| Attitudes | Not a study outcome |
| Compliance with pre-and post-tests: | |
| Total no. of nurses staffing both ICUs | 110 |
| Were distributed the pre-test, n (%) | 71 (65%) |
| Completed the pre-test, n (%) | 64 (58%) |
| Completed the post-test, n (%) | 103 (94%) |
| Total no. of physicians staffing both ICUs | 4 |
| Completed the pre-test, n (%) | 4 (100) |
| Completed the post-test, n (%) | 4 (100) |
| Knowledge | Not a study outcome |
| Skills | Not a study outcome |
| Process evaluation | Not included in the study, other than compliance with tests noted above |
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NO. Data showed that the study periods were similar in terms of patients’ gender, age, ethnicity, major disease, APACHE score, frequency of haemodialysis, immune incompetence, corticosteroid use, mechanical ventilation, ventilator days, tracheostomy and re-intubation |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT APPLICABLE | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? UNCLEAR. Stated that antimicrobial catheters and CVC supplies did not differ between the baseline and intervention periods and that no formal education programme was in place before or after the adoption of the antimicrobial catheters hospital-wide in the early 1990s. However, data on markers of changes in processes of care (i.e. systematic observations of CVC insertion and care techniques, and appearances of insertion site dressing) were not collected, apart from the insertion site |
| Was the effect of educational practice separable from effects of non-educational practice? YES. Education-only intervention | |
| Were the intervention component(s) implemented as planned? PARTIALLY. Exposure to education was not explicitly reported and it is unclear whether test participation is reflective of education exposure. However, post-tests described as mandatory were completed by 94% of nurses and 100% of physicians, exposing nearly all the staff to part of the intervention | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? UNCLEAR. For most outcomes, data were for all patients who had a CVC. However, data for insertion site were reported to be available for 381 patients (47%) in the baseline period and 333 patients (62%) in the intervention period (reason for the missing data not reported) |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? NOT REPORTED |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | The authors acknowledged that staff behaviour might have changed as a result of observation alone, independent of the intervention; this can neither be ruled out nor confirmed |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? NO. The educational topics were described superficially and it was not reported how the topics were allocated among the educational strategies. The test questions were not reported |
| Justification given for sample size? NO. Authors commented that the study was not powered to determine the impact of the intervention on lengths of stay, but they did not comment on whether it was powered to detect differences in infection incidence rates | |
| Data collection process reported? PARTIALLY. Stated only that trained data collectors, including infection control and research nurses, collected data prospectively on all admissions to both ICUs | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? YES. Data were initially analysed separately for each ICU and found to be homogeneous (Breslow–Day test), so were pooled in the final analysis | |
| Results | Educational significance or effect size assessed? NO |
| Target behaviour change achieved? NOT REPORTED |
Additional comments
-
Stated that the intervention was similar to that reported by Coopersmith (2002). 50
-
In this hospital a dedicated team of intensivists performed catheter insertion in the ICUs. This may not reflect practice in other hospitals.
WARREN (2004)51
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Warren (2004)51 |
| Summary of approach | 10-page self-study module on the prevention of CABSIs, lectures and posters and an awareness campaign given during 1 month in one ICU in a teaching hospital |
| Location | USA, Missouri |
| Language | English |
| Critical care specialty | MICU |
| No. of critical care units | 1 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | Barnes-Jewish Hospital, St Louis |
| Study design | Single-cohort before-and-after study |
| Study time periods | Baseline: January 2000 to December 2001 (24 months) Intervention: January 2002 Follow-up: January 2002 to December 2003 (23 months) Authors reported data for 2002 and 2003 (24 months) as post intervention, although this period included the intervention itself (January 2002). Newly hired nurses received education but it was not reported when or how many nurses were hired during the study period |
| Funding source | Work supported by funding from a Centers for Disease Control and Prevention Cooperative Agreement and the Barnes-Jewish Hospital Foundation |
| Conflicts of interest | Not reported |
Population and setting
| Critical care unit characteristics | ICU had 19 beds; located in a 1400-bed university-affiliated teaching hospital Patient care was provided by a multidisciplinary team directed by attending physicians who were board certified in critical care medicine Nurse to patient ratio 1 : 2. CVCs were usually inserted by resident physicians (i.e. physicians in training) Stated that patient care policies and protocols in the MICU remained unchanged during the study period, except for a new policy for prevention of VAP (introduced October 2000 during the baseline period) |
| Patient population characteristics | No details of the patients were provided. All patients admitted to the MICU during the study period were prospectively followed up by members of the hospital infection control team and surveyed for occurrence of CVC-associated bloodstream infection |
| Device characteristics | Included CVCs, dialysis catheters, pulmonary artery catheters but excluded arterial catheters All were standard catheters without antimicrobial or antiseptic coatings Mean ± SD monthly % of CVCs placed in the femoral vein: Baseline: 26.3 ± 5.8% Post-intervention: 20.4 ± 6.6% Difference: p = 0.002 |
| Insertion site antisepsis used | Not reported |
| Dressing type and duration/frequency | Not reported |
Intervention characteristics
| Objective | To determine whether an educational programme could decrease the rate of catheter-associated bloodstream infection in the MICU of a teaching hospital |
| Main focus of education | Covered catheter insertion and maintenance and the collection of blood cultures, also infection epidemiology |
| Trainers (providers) | Not explicitly reported; probably provided by registered infection control nurses (as in Warren 2003138) who were among the study authors and contributed to programme development |
| Training of trainers | Development and testing (see below) involved information exchange among infection control professionals |
| Learners (recipients) | All nurses in the ICU received the intervention by the end of January 2002. Newly-hired nurses were required to complete the education module as part of their job orientation. Physicians (interns, residents, fellows, attending physicians) completed the module during the first 3 days of their ICU rotation |
| Target behaviour change | Education appears to target multiple behaviours, including: aseptic technique; use of maximal barrier precautions during CVC insertion; selection of the subclavian vein as an insertion site; routine CVC site care; proper technique for obtaining blood cultures; and changing intravenous tubing and administration sets |
| Development and testing | The education programme was developed by a multidisciplinary task force in 1998 by infection control practitioners representing nine hospitals in the Barnes-Jewish-Christian Health System (Coopersmith (2002)50 cited). The local implementation plan was developed during monthly meetings of the MICU infection control community from July to December 2001. The committee comprised two ICU infection control nurses, two medical directors of the hospital infection control group, two physicians from the ICU, the unit clinical nurse specialist (all named authors), and members of the nursing staff. Objectives of the meetings were: educate the ICU leadership on the problem of CABSI; review in detail the optimal practices for catheter insertion and maintenance in the ICU; describe components of the education programme and their local implementation; foster team building; develop a strategy for educating resident and attending physicians; and have a feedback mechanism for reporting problems during implementation. Additional meetings were held by members of the ICU infection control committee to revise CVC insertion and maintenance policies and procedures. Flow charts for all aspects of CVC care were developed and approved by the committee |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Approach was similar to that of Warren (2003)139 10-page self-study module: Based on guidelines of the Hospital Infection Control Practices Advisory Committee as updated in 2002 (reference cited). Five major sections: (1) Goal of the Self-study Module; (2) Risk Factors; (3) Causes of CABSI; (4) Definition of CABSI; and (5) How to Decrease CABSI Risk In-services at scheduled staff meetings: No details provided (mentioned only in the abstract) 45-minute lectures: No details provided Group discussions: No details provided, but implied that group discussions occurred between taking the self-study module and post-test Posters and fact sheets: Distributed at each patient computer terminal located directly outside of the patient room (unclear whether these were the same posters and fact sheets referred to in the promotional campaign) Promotional campaign: Involved regular administration of lapel buttons to a staff member promoting the education programme, fact sheets and posters displayed throughout the ICU describing the programme, and photographic guidelines at each bedside computer station illustrating correct CVC insertion and maintenance, including dressing of the insertion site Topics in the education module and tests: The epidemiology of CABSI; aseptic technique and the use of maximal barrier precautions during CVC insertion; preference for the subclavian vein as an insertion site; routine CVC site care; proper technique for obtaining blood cultures; and guidelines for changing intravenous tubing and administration sets. Specific topics listed were: (1) hand hygiene before and after patient contact; (2) sterile gloves when changing dressing; (3) avoidance of femoral insertion site where possible; (4) wear sterile gown, gloves, mask and cap when inserting a catheter; (5) remove hair around insertion site only with scissors or clippers; (6) use appropriate insertion site antiseptic; (7) use full sterile drape; (8) use sterile technique to apply transparent dressing; (9) do not use antimicrobial ointment at insertion site except for dialysis catheters; (10) avoid catheter changes over a guidewire; (11) change dressing only when necessary; and (12) follow hospital protocol for changing i.v. fluid administration and cleaning of injection ports |
| Infection surveillance feedback approach | A monthly update of the CABSI was posted in the ICU in multiple locations for feedback to ICU staff. Not reported whether this system was already in place before the educational intervention |
| Performance feedback approach | Tests were based on the same guidelines as used in the self-study module The same 20-question test was conducted before and after implementation of the self-study module and group discussion. Score required to pass the test was 85%; if necessary the self-study module and post-test were repeated until a pass score was achieved Validity and reliability of the assessment approach were not reported |
| Concentration of education | Not reported other than that the lecture was 45 minutes. More detailed information on the time and staff resources required for a similar intervention were reported by Warren (2003)139 but unclear whether they are applicable to this intervention |
| Non-educational intervention components | A new policy for prevention of VAP was introduced in October 2000 (during the baseline period) and was in place for > 1 year before the educational intervention started |
| Costs reported | Not reported (crude estimate of cost savings presented based on assumed infection incidence) |
Outcome characteristics
| Catheter-BSI definition Reference cited: National Nosocomial Infections Surveillance186 |
Bloodstream infections were classified as primary or secondary according to CDC NNIS surveillance definitions. Primary BSI (bacteraemia) was defined employing either of the following two criteria:
|
| Outcomes reported | Specified as the main outcome measure: CABSI incidence and incidence per 1000 catheter-days |
Results data
Primary outcomes
| Outcome | Baseline (24 months) | Post intervention (23 months) | Difference between baseline and post intervention – only reported for incidence per 1000 CVC-days |
|---|---|---|---|
| Device duration | Not reported | Not reported | |
| Total device utilisation, catheter-days | 7876a | 7455 | |
| No. of devices/patient | Not reported | Not reported | |
| CABSI incidence rate, n | 74 | 41 | |
| CABSI incidence per 1000 catheter-days | 9.4 | 5.5 | 3.9 (95% CI 1.2 to 6.6); 41.5% decrease; p = 0.019 (risk ratio not reported) |
| CABSI incidence per 1000 patient-days | Not reported | Not reported | |
| LOS | Not reported | Not reported | |
| Mortality | Not reported | Not reported |
Additional CVC incidence density data per month (data extracted from graph by reviewer using Engauge software)
| Monthly CVC rate per 1000 CVC-days | Baseline | Post intervention | ||
|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | |
| January | 5.3 | 7.5 | 0 | 0 |
| February | 18.5 | 18.9 | 3.9 | 0 |
| March | 7.8 | 12.1 | 18.6 | 3.4 |
| April | 14.2 | 15.6 | 4.3 | 3.3 |
| May | 11.6 | 2.8 | 8.4 | 9.6 |
| June | 6.4 | 0 | 13.3 | 0 |
| July | 12.6 | 6.8 | 14.3 | 5.7 |
| August | 12.7 | 16.6 | 0 | 7.8 |
| September | 15.4 | 0 | 9.0 | 5.3 |
| Octobera | 6.0 | 12.6 | 3.3 | 5.0 |
| November | 6.9 | 0 | 5.9 | 10.6 |
| December | 5.7 | 6.6 | 0 | 0 |
Secondary outcomes
| Reaction to education | Not a study outcome |
| Attitudes | Not a study outcome |
| Compliance | Not a study outcome |
| Knowledge | Not a study outcome |
| Skills | Not a study outcome |
| Process evaluation | Not included in the study other than recording the frequency of insertions in the femoral vein (noted above) |
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? NOT REPORTED. No data were given on the patient population |
| If YES, were the differences between the groups adjusted for in statistical analyses (e.g. as covariates or subgroups, or stratification by propensity scores)? NOT REPORTED | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? UNCLEAR. Stated that patient care policies and protocols in the MICU remained unchanged during the study period, except for a new policy for prevention of VAP (introduced October 2000 – during the baseline period). However, data on markers of changes in processes of care (i.e. systematic observations of CVC insertion and care techniques, device duration, and appearances of insertion site dressing) were not collected, apart from the insertion site |
| Was the effect of educational practice separable from effects of non-educational practice? YES. Education-only intervention | |
| Were the intervention component(s) implemented as planned? NOT REPORTED | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? NOT REPORTED. The number of patients in the ICU and the proportion of those that had a CVC were not specified |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? NOT REPORTED |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO. The authors stated that the ICU staff was not blinded to either the presence of or the recipients of the education intervention | |
| Other possible sources of bias | The authors acknowledged that other sources of potential bias may have influenced their results. These would include unrecognised differences in ascertainment or reporting of CABSI between the two study periods |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? PARTIALLY. The self-study module was described well enough for the main educational topics to be reproduced in a similar strategy. However, the test questions were not reported and the nature of other educational elements (lectures, in-service and group discussions) was not reported |
| Justification given for sample size? NOT REPORTED | |
| Data collection process reported? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT APPLICABLE | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT APPLICABLE | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NO |
| Target behaviour change achieved? NOT REPORTED |
Additional comments
-
Unclear whether infection surveillance feedback approach changed during the study period.
-
The majority of CVCs in this ICU were placed by physicians in training.
-
Related studies: Coopersmith (2002)50 study was in the SICU of the same hospital; Warren (2003)139 applied a nearly identical programme within two ICUs of a community hospital.
-
Stated that the 20-question post test at the end of the module was to reinforce the topic and group discussion; the authors planned to reinforce the education by repeating the programme at 2-year intervals.
ZINGG (2009)144
Methods
Study characteristics
| Lead author, publication year(s) and reference(s) | Zingg (2009)144 |
| Summary of approach | Educational programme targeting hand hygiene and catheter care in five ICUs at a single hospital |
| Location | Switzerland |
| Language | English |
| Critical care specialty | Medical, cardiovascular, trauma, general surgery and neurosurgery ICUs |
| No. of critical care units | 5 |
| No. of hospitals | 1 |
| Hospital name (unless multicentre); city | University Hospital, Zurich |
| Study design | Single-cohort before-and-after study that combined data from five ICUs into a single cohort (data also presented also separately for two of the five ICUs) |
| Study time periods | Baseline: September to December 2003 (4 months) Intervention: March to July 2004 (5 months) Follow-up: None (monitoring during intervention only) |
| Funding source | Not reported |
| Conflicts of interest | Authors disclosed no potential conflicts of interest |
Population and setting
| Critical care unit characteristics Total for the five ICUs: Beds = 52, sited in a 960-bed tertiary referral centre Nursing staff = 395 (head nurses, teaching nurses, assistant nurses, trainee nurses) Medical staff = 34 Internal guidelines recommended the use of maximal barrier precautions – but not stated whether the guidelines applied equally to baseline and intervention periods |
|||
| Patient population characteristics: all adult patients hospitalised in any of the ICUs and with one or more CVCs in place were eligible for study entry; there were no exclusion criteria | Baseline (4 months) | Intervention (5 months) | Difference between baseline and intervention |
| Total no. of patients | 499 | 500 | |
| Cumulative no. of ICU-days | 2944 | 3705 | Not reported |
| Median (IQR) age, years | 62 (49–71) | 61 (48–73) | p = 0.75 |
| Male sex, n (%) | 320 (64) | 334 (67) | p = 0.35 |
| McCabe fatality score < 6 months, n (%) | 187 (37) | 223 (45) | p = 0.01 |
| Median (IQR) SAPSa II score | 23 (16–31) | 24 (18–34) | p = 0.11 |
| Charlson score > 3, n (%) | 67 (13) | 63 (13) | p = 0.73 |
| Cardiovascular surgery, n | 208 | 196 | p = 0.42 |
| Diabetes, n | 71 | 60 | p = 0.30 |
| Trauma, n (%) | 10 (2) | 55 (11) | p < 0.001 |
| Stay on MICU, n (%) | 62 (12) | 74 (15) | p = 0.26 |
| Intubation, n (%) | 130 (26) | 131 (26) | p = 0.96 |
| Non-CRBSI nosocomial infections, n (%) | 129 (26) | 131 (26) | p = 0.90 |
| Device characteristics | Baseline (4 months) | Intervention (5 months) | Difference between baseline and intervention |
|---|---|---|---|
| Insertion vein | |||
| Subclavian | 494 | 477 | p = 0.10 |
| Jugular | 370 | 398 | p = 0.56 |
| Femoral | 110 | 139 | p = 0.10 |
| (1 missing data) | |||
| Catheter type | |||
| CVC | 626 | 673 | p = 0.34 |
| Pulmonary CVC | 211 | 175 | p = 0.01 |
| Other | 137 | 167 | p = 0.14 |
| Lumens | |||
| Single lumen | 100 | 121 | p = 0.21 |
| Multi (> 1) lumen | 868 | 878 | p = 0.21 |
| (6 missing data) | (16 missing data) | ||
| Insertion venue: | |||
| Emergency room | 39 | 31 | p = 0.25 |
| Operating room | 644 | 636 | p = 0.11 |
| ICU | 290 | 347 | p = 0.03 |
| (1 missing data) | (1 missing data) | ||
| Insertion site antisepsis used | Povidone–iodine (three SICUs) and octenidine (medical and neurosurgical ICUs) | Same as baseline practice | Quantitative data not reported |
| Dressing type and duration/frequency | Not reported | Not reported | Not reported |
Intervention characteristics
| Objective | To study the impact of a teaching intervention on the rate of central venous CRBSIs in intensive care patients |
| Main focus of education | Hand hygiene, dressing of the insertion site, manipulation of tubing and stopcocks, and aseptic preparation of infusates; explicitly stated that CVC insertion was not the focus of the intervention and was not observed |
| Trainers (providers) | Two infection control nurses were responsible for teaching phases 1 and 2 (details of phases below). Two additional infection control nurses from the infection control unit participated in the bedside teaching sessions of phase 3. An infection control physician and an infection control nurse conducted teaching phase 4. (Note that the authors described teaching phases and content modules but mixed up the terminology – the above interpretation provided by the reviewers) |
| Training of trainers | Not stated explicitly but appears that phase 1 (training of nurses) was conducted by two infection control nurses. |
| Learners (recipients) | Nurses and physicians in the ICU (different emphasis of education for each group) |
| Target behaviour change | Hand hygiene, dressing of the insertion site, manipulation of tubing and stopcocks, and aseptic preparation of infusates |
| Development and testing | Not reported, except that teaching phase 1 was very helpful to build trust and learn from nurses’ (i.e. the learners’) experiences, and that procedure proposals were adapted to everyday situations which changed the modules significantly |
| Educational or behavioural theory | Not reported |
| Educational strategies and topics targeted | Four sequential teaching phases within each of which four sequential content modules were provided (in the order below) Teaching phases:
|
| Infection surveillance feedback approach | Not included in the intervention |
| Performance feedback approach | Not included in the intervention |
| Concentration of education | The intervention comprised 47 hours of teaching by two infection control nurses (total 94 staff working hours), divided as follows:
|
| Non-educational intervention components | None (intervention purely educational) |
| Costs reported | Not reported |
Outcome characteristics
| Catheter-BSI definition Reference cited: Garner et al.147 |
Primary bloodstream infection was defined as bacteraemia (or fungaemia) without any other documented source. For coagulase-negative staphylococci, two positive blood cultures or a complete antibiotic therapy adjusted for susceptibility testing was required. All patients were monitored for the development of CRBSI until 48 hours after ICU discharge. CRBSI was considered as ICU acquired if diagnosed ≥ 48 hours after admission or within 48 hours after discharge from the ICU. Patients with CRBSI > 48 hours after discharge were excluded from the risk analysis |
| Outcomes reported | Primary (study powered to detect 2% change): CRBSI incidence and incidence density Secondary: Compliance with hand hygiene Other outcomes: Device duration Time to CRBSI LOS Mortality |
Results data
Primary outcomes
| Baseline (4 months) (974 CVCs) (499 patients) (2944 ICU-days) | Intervention (5 months) (1015 CVCs) (500 patients) (3705 ICU-days) | Difference between baseline and intervention | |
|---|---|---|---|
| Median (range) device duration, catheter-days | 5 (1–39) | 6 (1–44) | p < 0.001 |
| Total device utilisation, catheter-days | 6200 | 7279 | Not reported |
| No. of devices/patient | Not reported | Not reported | Not reported |
| Median (IQR) and [mean] catheter-days per patient | 5 (3–8) [6.4] | 6 (3–9) [7.2] | p < 0.001 |
| CRBSI incidence rate | 24 | 7 | p < 0.001 |
| CRBSI incidence per 1000 catheter-days | |||
| Overall (5 ICUs) | 3.9 | 1.0 | p < 0.001 |
| MICU | 9.0a | 3.9a | Not reported |
| SICU | 3.0a | 0.2a | Not reported |
| Other 3 ICUs | Not reported | Not reported | Not reported |
| Median (IQR) time to CRBSI, days | 6.5 (3–19) | 9 (7–16) | p = 0.02 |
| No. of blood cultures obtained per 1000 catheter-days | 190 | 225 | Not reported |
| CRBSI incidence per 1000 patient-days | Not reported | Not reported | Not reported |
| Median (IQR) and [mean] length of ICU stay, daysb | 3 (2–7) [5.9] | 4 (2–9) [7.5] | p < 0.001 |
| Mortality | 44 | 44 | p = 0.99 |
Secondary outcomes
| Reaction to education | Not a study outcome |
| Attitudes | Not a study outcome |
| Compliance | Overall compliance with hand hygiene:
|
| Knowledge | Not a study outcome |
| Skills | Not a study outcome |
| Process evaluation | Not included in the study, apart from the observations on compliance noted above |
Critical appraisal
Potential for bias
| Group selection | Were there systematic differences between the baseline and intervention groups? YES. The number of ICU-days, McCabe rapid fatality score, number of trauma patients and number who had a CVC inserted in the ICU were statistically significantly higher in the intervention than the baseline period. LOS in the MICU was also significantly longer in the intervention period, by around 10 days. The converse applied to the number of patients with pulmonary CVCs. Significantly fewer patients had pulmonary CVCs in the intervention period. The data suggest that patients in the intervention period were sicker than in the baseline period but data for non-CRBSI infections and mortality were identical for both periods |
| If YES, were the differences between the groups adjusted for in statistical analyses? NO | |
| Intervention administration | Were any confounding variables identified that could influence the effect estimate for the overall intervention? NOT REPORTED. The staffing and staff to patient ratios were not reported separately for baseline and intervention periods. It was not reported whether any care policies other than those in the intervention changed during the study period |
| Was the effect of educational practice separable from effects of non-educational practice? YES. Education-only intervention | |
| Were the intervention component(s) implemented as planned? UNCLEAR All of the teaching models appear to have been implemented, but it is unclear if participation was mandatory or how many staff took part in all or some of the modules (compliance for hand hygiene statistically significantly increased, but did not reach 100%) | |
| Missing data | Were there systematic differences between the baseline and intervention groups in data availability? NO. Missing data were reported and appear to represent a small proportion of the total data for the outcomes in question (insertion vein, lumen number, and insertion venue). Specified that the population was all patients in the ICUs who had a CVC |
| Outcome measurement | Were there systematic differences between the baseline and intervention groups in how outcomes were determined (including differences in how outcomes were defined)? NOT REPORTED |
| Was any blinding of personnel and/or outcome assessors reported (including assessors of blood cultures)? NO | |
| Other possible sources of bias | No other sources of bias were reported by the authors or identified by the reviewers based on the information presented |
Other critical appraisal criteria
| Methods | Intervention described in sufficient detail to be replicated? YES. The structure, content and time resources required were reported in detail |
| Justification given for sample size? YES. Stated that a minimum of 870 catheters was needed to prove a significant CRBSI reduction from 3% (baseline) to 1% (intervention) (80% power, 0.05 significance level) | |
| Data collection process reported? PARTIALLY. CVC surveillance was conducted by a trained infection control nurse who visited all ICUs daily and recorded relevant information in a surveillance protocol for each patient. All protocols were reviewed and checked for completeness and plausibility by two study physicians | |
| If YES or PARTIALLY, was the data collection process shown to be valid? NOT REPORTED | |
| If YES or PARTIALLY, was the data collection process shown to be reliable? NOT REPORTED | |
| Statistical tests described? YES | |
| Results | Educational significance or effect size assessed? NO |
| Target behaviour change achieved? PARTIALLY. Compliance was only reported for one of the behaviour changes (hand hygiene); compliance with hand hygiene was not 100%, although there were improvements |
Additional comments
-
Not reported whether the participants were aware that they were being studied.
Appendix 6 Lists of records not included in the data syntheses
Conference abstracts (n = 67) excluded from the evidence map
These abstracts either met the inclusion criteria or were of unclear relevance at the title and abstract screening step but were excluded as they provided insufficient information for inclusion in the keyword mapping exercise.
Abrusci TA, Bion JF, Richardson A. Central venous catheter blood-stream infections (CVC-BSIs) in ICUs in England: Phase 1 pilot study. Intensive Care Med 2010:S90.
Adams T, Williams M, Brown V, Troxler H, Wood S, Tate A, et al. Using lean/Six sigma quality improvement methodologies to reduce central line-associated bloodstream infections (CLABSI) in a pediatric hospital. Am J Infect Control 2010:38(5):E112.
Almeida M, Ferreira A, Reis P, Alves V, Dias C, Granja C. Reducing catheter-related bloodstream infections (CRBSI) in the ICU with an evidence-based intervention. Intensive Care Med 2009:S271.
Alvarez C, Pisapia J, Rosello C, Lira M, Curone M, Vidiella G. Implementation of a central line bundle to reduce central line associated bacteremia at the intensive care unit. Int J Infect Dis 2010; Conference (ICID):ASM.
Andion E, Bologna R, Battistezza J, Carbonaro M, Sasbon J, Weller G, et al. Control program for central line associated bloodstream infections in two pediatric intensive care units. Infect Control Hosp Epidemiol 2000;21:93.
Armstrong P, Alfieri N, Clowser M, Steinberg R, Spornitz M, Runge W, et al. Central line-associated (CLA) surveillance and continuing quality improvement in an intensive care unit (ICU). J Hosp Infect 1998;40(Suppl. 1):45.
Atherton SL, Tjoelker RC. Evidence based fact sheet: An effective method for implementing change. Am J Infect Control 2006;34:E51.
Balkhy HH, Alsaif S, El-Saed A, Dichinee R, Memish Z. Approaching zero rates bloodstream infections in a tertiary care neonatal intensive care unit: a multifaceted approach. Clin Microbiol Infect 2009; 15(Suppl. S4):S322.
Beckett P, Jerrett H, Pain T, Hermon A, Szakmany T. Effect of care bundle implementation on catheter related bloodstream infection on the intensive care unit. Intensive Care Med 2010:S126.
Benjamin-Phillips S, Nelson E. Keeping the bugs out: strategies to reduce central line bloodstream infections. J Pediatr Nurs 2007;22:143.
Bhattacharyya M, Bhakta A, Todi S. Impact of quality improvement process on healthcare-associated infection in the ICU in a tertiary care hospital in India. Crit Care 2010:S154.
Brennan PJ, Hoegg C, Samel C, Skalina D, Barbagallo S, Shulkin D. Performance improvement in a medical intensive care unit (MICU) resulting from device based surveillance (DBS) from central venous catheter related bloodstream infections (CVC-BSI). Infect Control Hosp Epidemiol 1997;18:20.
Cherry-Bukowiec JR, Denchev KL, Dickinson S, Chenoweth C, Zalewski C, Meldrum C, et al. Prevention of catheter-related BSIs: back to basics? Surg Infect 2009:10(2).
Conceicao F, Wey S, Amaral J, Medeiros E. Blood stream infection associated with central venous catheter in an intensive care unit. Abst Intersci Conf Antimicrob Agents Chemother 1997;37.
Eggimann P, Hugonnet S, Harbarth S, Chraiti M, Touveneau S, Chevrolet J, et al. Reduction of bloodstream infection 2 years following a global prevention strategy targeted at vascular access in ICU. Abstr Intersci Conf Antimicrob Agents Chemother 2001;41.
Eggimann P, Hugonnet S, Harbarth S, Sax H, Chevrolet J, Pittet D. Long-term reduction of vascular access-associated bloodstream infection (BSI) 6 years after a global prevention strategy. Abstr Intersci Conf Antimicrob Agents and Chemother 2004;44.
Ellis D, Brungs S, Burns P, Render M, Nicholson M. Implementing evidence-based practices to reduce catheter-related bloodstream infections in the intensive care unit. Am J Infect Control 2005:33:e61–e62.
Elsayed A, Mahanes D, Nathan B, Gress D. Prevention of catheter-related blood stream infection in the neurointensive care unit. Neurocrit Care 2010;S140.
Fauerbach LL, Gross MA, Ruse C, Kelly R. The quest for the irreducible minimum: 8 years of performance improvement in preventing central line-associated infections in a surgical intensive care unit. Am J Infect Control 2005;33:e60–1.
Flinchum A, Harris C, Swiderski P, Conigliaro J, Chang P. A novel five-tier approach to reduce central line-associated blood stream infections in an academic medical center. Am J Infect Control 2010;38:E24–5.
Fontcuberta A, Chacon E, Valente A, Alcaraz D, Pedragosa R, Turegano C, et al. Do nurse consultant teams prevent mechanical ventilation associated pneumonia and catheter-related bloodstream infection in the ICU? The Sabadell experience. Intensive Care Med 2010:S330.
Frankel H, Rabinovici R, Crede W, Roumanis S, Topal J, Devlin M, et al. The use of corporate Six Sigma performance improvement strategies to reduce the incidence of catheter-related bloodstream infections (CR-BSI) in a surgical intensive care unit (SICU) of a tertiary referral university hospital. Crit Care Medicine 2003;31:436.
Gómez WV, Vergara GR, Pertuz AM, Rosenthal VD. Education and performance feedback effect on rates of central vascular catheter-associated bloodstream infections in newborn intensive care units in a private hospital in Colombia. Am J Infect Control 2005;33:E76–7.
Gowell J, Marrorana K, Gregory ML, Natale K, Nickerson C, Carter J, et al. Re-education initiative to reduce central line associated blood stream infections in the neonatal intensive care unit. Crit Care Med 2009;37(12)(Suppl.):A279.
Gross I. Effect of an intervention on rate of central vascular catheter-related bloodstream infection, in intensive care units at Hadassah Medical Center. Sourced from Israel Medical Convention website. Date unknown.
Hansen S, Schwab F, Schneider S, Sohr D, Geffers C, Gastmeier P. Significant decrease of CVC-associated BSI rates in 38 German ICUs. Int J Med Microbiol 2008;298(Suppl. 45):35–6.
Honeycutt M, Curry S, Goins G, Fugitt D, Marotti T, Gilliam C. Implementing a catheter-associated blood stream infection prevention bundle in the neonatal intensive care unit – Will it reduce methicillin resistant staphylococcus aureus? Am J Infect Control 2009;37(5):E146.
Hujcs M, Eckhradt D, Danielle M. Clinical nurse champions improve patient outcome: sustaining catheter-related BSI reduction in neurocritical care. Crit Care Nurse 2009;29:e5–6.
Karali V, Stefanopoulou P, Bitzani M, Ambatzidou F, Vassiliadou G, Riggos D. Effects of an education: prevention strategy on decreasing catheter-related infections in intensive care. Crit Care 2003;7(Suppl. 2)S60.
Kawagoe JY, Dal Forno CB, Dornaus MF, Cunha LB, Santos MFC, Martins RAL, et al. Cultural and clinical changes in the NICU: Impact of a multi-faceted program on reduction of CVC associated BSI. Am J Infect Control 2009;37(5):E123.
Khouli HI, Jahnes K, Mathew J, Gohil A, Shapiro J, Rose K, et al. Medical residents performance in maximum barrier precautions during central venous catheter placement: Effect of simulation-based training. Chest 2009;136(4 meeting abstracts):12S.
Koch D, Sykora C, Ferrara L, Griesbaum R, Cruz O. An effective intervention program that resulted in a sustained significant reduction in catheter-associated bloodstream infections in three intensive care units (ICUs). Am J Infect Control 2005;33(5):E26.
Kovari F. The elimination of central line related bloodstream infection (CRBSI) on the intensive care unit. Intensive Care Med 2009:S269.
Kurachek S, Rusakov A, Thornton A, Kuelbs M, Sturtevant B, Finkelstein M. Reducing central line entries (CLEs): an adjunctive maneuver to reduce catheter associated blood stream infections (CABSIS). Crit Care Med 2009;37(12)(Suppl.):A322.
Loh-Trivedi M, Croley W, Goudzwaard C. Impact of multidisciplinary team training in a surgical intensive care unit. 9th Critical Care and Emergency Medicine Meeting of the Greek Armed Forces Medical Corps, Athens, 27–28 May 2011.
Longo A, Hensley B, Schilling S. Striving for excellence in central venous catheter care: Applying a blended learning approach to nursing staff education on CVC bundle care practices aimed at reducing catheter-associated blood stream infection. J Pediatr Nurs 2008;23:e18–e19.
Madrid PA, Berkowitz K, Farber M, Weldon S, Bachmeier L. Nosocomial infections? Get a CAT! Crit Care Nurse 2009;29:e18.
Magerl MA, Madalone J, Gwardschaladse C, Longo K, Hemmer D, Haas J, et al. A regional trauma intensive care unit (ICU) thirty-one month experience in decreasing central venous catheter-bloodstream infections (CVC-BSI). Am J Infect Control 2009;37(5):E39.
McCalla S, Downing-Janos T, Ellsworth K, Allinger P, Kirkpatrick K, Zaicek S. Central line-associated blood-stream infections in the ICU: the struggle to achieve and the effort to maintain zero. Am J Infect Control 2009;37(5):E41.
Melamed R, Zmora E, Peled N, Schlaeffer P, Gilad J, Eskira S, et al. Control of bloodstream infection (BSI) in a neonatal intensive-care unit (NICU). Abstr Intersci Conf Antimicrob Agents Chemother 2002;42.
Miller M, Brilli R, Moss M. Elimination of Pediatric CA-BSI. URL: http://aap.confex.com/aap/2009/webprogram/Paper5966.html 2009 (accessed May 2012).
Nair P, Pabs-Garnon E, Whitehead CF. Survey of central line related sepsis in a neurointensive care unit. Intensive Care Med 2010:S128.
Oriza N, Kuyumjian G, Imperial-Perez F, Rizzi-Wagner L. The impact of evidenced-based practices in reducing catheter-related blood stream infections in a pediatric CTICU. Crit Care Nurse 2009;29:e24.
Orsman PJ. Multi-faceted interventions to prevent bloodstream MRSA infections. Am J Infect Control 2009;37(5):E51.
Ozgultekin A, Rosenthal VD, Turan G, Akgun N. Education and performance feedback effect on rates of central vascular catheter -associated bloodstream infections in adult intensive care units of one Turkish hospital. Am J Infect Control 2006;34:E23.
Palomar M, Lopez Pueyo MJ, Alvarez LF, Olaechea P, Insausti J, Otal JJ. Impact of a safety program in rates of ICU-acquired infections. Intensive Care Med 2010:S129.
Peace D. Targeting zero: A systematic approach to elimination of catheter related bloodstream infections in a pediatric healthcare system. Am J Infect Control 2010;38(5):E37.
Peredo R, Sabatier C, Villagr A, Suarez D, Gonzlez J, Hernandez C, et al. Reduction of the catheter-related bloodstream infections in critically ill patients. Crit Care 2009:S77.
Perez Parra A, Menarguez M, Perez-Granda M, Tomey M, Padilla B, Bouza E. Simple Educative Interventions Could Significantly Reduce Catheter-Related Bloodstream Infections (CR-BSI) in ICUs With Already Low Baseline Rates of CR-BSI. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC)/IDSA 46th Annual Meeting (ICAAC) 2008.
Reddy A, Cobb S, Hanane T, Kus S, Strauser C, Guzman J. A multidisciplinary approach to central line placement and care can reduce rates of CLABSI. Crit Care Med 2010:A164.
Rhoton B, Annibale DJ, Southgate WM, Salgado C, Chase KE, Beardsley WE, et al. NICU Journey to zero central line-associated bloodstream infections: Incremental interventions lead to sustainable outcomes. Am J Infect Control 2010;38(5):E114.
Richtmann R, Costa M, Takagi N, Kusano E, Costa H. Bloodstream infection (BSI) in neonatal intensive care unit (NICU): What's going on in a Brazilian Hospital. J Hosp Infect 1998;40(Suppl. A).
Rorke J, Higgins RD. Quality improvement intervention utilizing percutaneous central catheters in a neonatal intensive care unit. Pediatr Res 1998;43(4 Part 2).
Rosenthal V, Higuera F, Franco G, Duarte P, Ruiz J. Education and performance feedback effect on rates of central vascular catheter-associated bloodstream infections in intensive care units in Mexico. Abstr Intersci Conf Antimicrob Agents Chemother 2003;43.
Rosenthal V, Maki D. The Impact of Education and Feedback of Outcome and Process Surveillance on Rates of CLABSI in 86 ICUs of 15 Developed Countries. Abstr Intersci Conf Antimicrob Agents Chemother 2009:Abstract K-299/317.
Rouge A, Espinola I, Paixo CT, Prado L, Nascimento GKS, Senna KM, et al. Effects of the implementation of the vascular catheter bundle in a cardiac surgery postoperative unit in Rio de Janeiro, Brazil, years 2007–2009. Intensive Care Med 2010:S249.
Royer TI. Maintaining a zero central line-associated bloodstream infection rate for 17 months across a large and diverse adult patient population: What gets the credit? Am J Infect Control 2010;38:E31–2.
Ryan A, Sample G, Verstraete R, Donegan N, Madden AM. An innovative contest highlights CLABSI prevention. Crit Care Med 2009;37(12)(suppl.):A357.
Salomao R, Blecher S, Da Silva M, Villins M, Da Silva EH. Education and performance feedback effect on rates of central vascular catheter-associated bloodstream infections in adult intensive care units in one hospital in Sao Paulo, Brazil. Am J Infect Control 2005;33:E58.
Sessler CN, Seago B, Gray ND, Grossman CE, Miller KB, Brant SE, et al. Central venous catheterization education and task simulation training: large scale implementation and reduced rates of bloodstream infection. Chest 2009;136(4 meeting abstracts):12S.
Sircar M, Gupta R, Kumar A, Gupta A, Vohra H, Chavhan N, et al. Impact of short term focused education intervention on ICU nurses' awareness of infection control measures, ICU hand washing practices and nosocomial infections. Intensive Care Med 2009:S206.
Smith RL, Rivera K, Snedeker L, Wolfgang J, Rose L, Hardenstine H. Reduction in central line-related bloodstream infection (CLBSI) as a result of multiple process improvement changes. Am J Infect Control 2010;38:E35.
Trenado J, Riera M, Jane R, Valls J, Freixas N, Nava JM. Results of a program to reduce catheter-related bloodstream infection in the ICU: two years' follow-up. Intensive Care Med 2010:S127.
Vos P, Verhoeven T, Speelberg B. An education-based intervention reduces catheter-related bloodstream infection in the intensive care unit and changes puncture site preference. Intensive Care Med 2009;S262.
Wagner JM. Impact of a dedicated IV team. Crit Care Nurse 2009;29:e12–13.
Zack JE, Osmon S, Chen A, Prentice D, Fraser VJ, Kollef MH. The effect of an education program on the incidence of catheter-associated bloodstream infection in a medical intensive care unit. Chest 2004;126(Suppl. 4S).
Zastrow R, Husch M, Rivera S, Grisco P, Shields K, Foster W. A comprehensive, ongoing program to eliminate central line associated bloodstream infections in two intensive care units. Am J Infect Control 2010;38:E53–4.
Full-text records (n = 62) excluded from the evidence map
Note that for most records only the most apparent exclusion criteria agreed by the reviewers are listed. Records may have also failed to meet other criteria that are not listed below.
| Reference | Reason for exclusion | |||
|---|---|---|---|---|
| Population criteria not met | Design criteria not met | Intervention criteria not met | Relevant outcomes not reported | |
| Agra Varela (2009)63 | ✗ | |||
| Agvald-Ohman (2010)74 | ✗ | |||
| Berg (1995)204 | ✗ | |||
| Bezzio (2009)205 | ✗ | ✗ | ✗ | |
| Bijma (1999)206 | ✗ | |||
| Chien (2001)207 | ✗ | |||
| Clancy (2009)208 | ✗ | |||
| Collignon (1985)209 | ✗ | |||
| Cooley (2009)210 | ✗ | |||
| DuBose (2010)211 | ✗ | |||
| Eggimann (2004)212 | ✗ | |||
| Elder (2008)201 | ✗ | |||
| Gnass (2004)213 | ✗ | |||
| Goeschel (2010)214 | ✗ | |||
| Goeschel (2011)215 | ✗ | |||
| Gurskis (2009)216 | ✗ | |||
| Halton (2010)151 | ✗ | |||
| Harnage (2008)217 | ✗ | |||
| Jeffries (2009)218 | ✗ | |||
| Joshi (2005)219 | ✗ | |||
| Kilbride (2003)220 | ✗ | |||
| Kilbride (2003)221 | ✗ | |||
| Lindsey (2007)222 | ✗ | |||
| Lisboa (2008)66 | ✗ | |||
| Lolom (2009)72 | ✗ | |||
| Meier (1998)223 | ✗ | ✗ | ||
| Moureau (2005)224 | ✗ | |||
| Moureau (2009)225 | ✗ | ✗ | ||
| Nelson (2005)226 | ✗ | |||
| Northway (2005)227 | ✗ | |||
| O'Grady (2007)228 | ✗ | |||
| Palomar (2010)67 | ✗ | |||
| Papadimos (2008)229 | ✗ | |||
| Penne (2002)4 | ✗ | |||
| Plouffe (2010)230 | ✗ | |||
| Pronovost (2005)231 | ✗ | |||
| Render (2006)200 | ✗ | |||
| Riel-Roberge (2010)71 | ✗ | |||
| Rizzo (2005)232 | ✗ | |||
| Rodriguez-Paz (2008)233 | ✗ | |||
| Rosenthal (2008)234 | ✗ | |||
| Rosenthal (2010)235 | ✗ | |||
| Scales (2011)181 | ✗ | |||
| Schelonka (2006)236 | ✗ | |||
| Schindler (2007)73 | ✗ | |||
| Schuerer (2007)237 | ✗ | |||
| Schulman (2009)238 | ✗ | |||
| Seguin (2010)239 | ✗ | |||
| Sherertz (2004)240 | ✗ | |||
| Sherman (1988)241 | ✗ | |||
| Smith (2006)242 | ✗ | |||
| Smith (2007)243 | ✗ | |||
| Stewart (2008)244 | ✗ | |||
| Tsuchida (2007)245 | ✗ | |||
| Vandijck (2009)246 | ✗ | |||
| Verdier (2006)247 | ✗ | |||
| Warye (2009)248 | ✗ | ✗ | ||
| Watson (2009)249 | ✗ | |||
| Weber (2010)250 | ✗ | |||
| Yilmaz (2007)251 | ✗ | |||
| Young (2006)252 | ✗ | |||
| Zack (2009)253 | ✗ | |||
Studies (n = 50) excluded from the clinical effectiveness systematic review (references listed below table)
| Study | Reason for exclusion from systematic review | ||
|---|---|---|---|
| Population not adult | Design unclear or not prospective | Catheter-BSI definition not reported | |
| Anguera Saperas64 | ✗ | ||
| Barsuk75/Cohen76 | ✗ | ||
| Berenholtz77 | ✗ | ||
| Berriel-Cass79 | ✗ | ||
| Bhutta78 | ✗ | ✗ | |
| Bishop-Kurylo80 | ✗ | ✗ | ✗ |
| Bizzarro81 | ✗ | ✗ | |
| Bonello82 | ✗ | ✗ | |
| Buttes84 | ✗ | ||
| CDC85 | ✗ | ||
| Chua86 | ✗ | ||
| Costello88 | ✗ | ||
| Curchoe89 | ✗ | ||
| Curry90 | ✗ | ✗ | ✗ |
| DePalo91 | ✗ | ||
| Duane92 | ✗ | ||
| Esteve65 | ✗ | ||
| Frankel96 | ✗ | ||
| Harnage100 | ✗ | ||
| Harrigan101 | ✗ | ||
| Hatler102 | ✗ | ✗ | |
| Jain104 | ✗ | ||
| Joy-Joseph105 | ✗ | ✗ | ✗ |
| Khouli145 | ✗ | ||
| Koll106 | ✗ | ✗ | |
| Koll107 | ✗ | ||
| Leboucher70 | ✗ | ✗ | |
| Maas111 | ✗ | ✗ | |
| Marra112 | ✗ | ||
| McKee113 | ✗ | ✗ | ✗ |
| Miller114 | ✗ | ||
| Miller115 | ✗ | ✗ | |
| Miller-Hoover116 | ✗ | ✗ | ✗ |
| Moreira118 | ✗ | ||
| Northway119 | ✗ | ✗ | |
| Orsi120 | ✗ | ||
| Peredo121 | ✗ | ||
| Racco125 | ✗ | ✗ | |
| Rey127 | ✗ | ||
| Rogers128 | ✗ | ||
| Sannoh131 | ✗ | ||
| Santana132 | ✗ | ||
| Schulman133 | ✗ | ||
| Shannon134 | ✗ | ✗ | |
| Urrea Ayala69 | ✗ | ✗ | |
| Venkatram | ✗ | ✗ | |
| Warren140 | ✗ | ||
| Wirtschafter141 | ✗ | ✗ | ✗ |
| Yoo142 | ✗ | ||
| Zack143 | ✗ | ||
Excluded studies from the systematic review of cost-effectiveness
| Study | Reason for exclusion |
|---|---|
| Moureau NL. Reducing the cost of catheter-related bloodstream infections. Nursing 2009;39:14–15 | Not cost-effectiveness study (review) |
| Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter-associated bloodstream infections: a systems-based intervention. Am J Infect Control 2006;34:503–6 | Not economic analysis (not full-cost study) |
| Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, Fraser VJ. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a non-teaching hospital. Crit Care Med 2006;34:2084–9 | Not economic analysis (not full-cost study) |
| Coopersmith CM, Rebmann TL, Zack JE, Ward MR, Corcoran RM, Schallom ME et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med 2002;30:59–64 | Not economic analysis (not full-cost study) |
| Kim JS, Holtom P, Vigen C. Reduction of catheter-related bloodstream infections through the use of a central venous line bundle: epidemiologic and economic consequences. Am J Infect Control 2011;39:640–6 | Not economic analysis (not full-cost study) |
Clinical effectiveness full-text records (n = 19) identified in search updates (March 2012) (excluded from data synthesis but discussed in report)
Abramczyk ML, Carvalho WB, Medeiros EA. Preventing catheter-associated infections in the Pediatric Intensive Care Unit: impact of an educational program surveying policies for insertion and care of central venous catheters in a Brazilian teaching hospital. Braz J Infect Dis 2011;15:573–7.
Espiau M, Pujol M, Campins-Marti M, Planes AM, Pena Y, Balcells J, et al. [Incidence of central line-associated bloodstream infection in an intensive care unit.] An Pediatr 2011;75:188–93.
Esteban E, Ferrer R, Jordan I, Urrea M, Palomeque A. Educational intervention to reduce nosocomial infection rates in the pediatric intensive care unit. Intensive Care Med 2011;S175.
Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol 2011;32:619–22.
Harris BD, Hanson C, Christy C, Adams T, Banks A, Willis TS, et al. Strict hand hygiene and other practices shortened stays and cut costs and mortality in a pediatric intensive care unit. Health Affairs 2011;30:1751–61.
Jacob J, Sims D, Van de Rostyne C, Schmidt G, O'Leary K. Toward the elimination of catheter-related bloodstream infections in a newborn intensive care unit (NICU). Jt Comm J Qual Patient Saf 2011;37:211–16.
Kim JS, Holtom P, Vigen C. Reduction of catheter-related bloodstream infections through the use of a central venous line bundle: epidemiologic and economic consequences. Am J Infect Control 2011;39(8):640–6.
Kime T, Mohsini K, Nwankwo MU, Turner B. Central line ‘attention’ is their best prevention. Adv Neonatal Care 2011;11:242–8.
McLaughlin GE, Nares MA, Smith LJ, Feinroth CA. Preventing central-line-associated bloodstream infections in pediatric specialized care units: a case study of successful quality improvement. Progress Pediatr Cardiol 2012;33:47–52.
McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med 2012;40:388–93.
Miller MR, Niedner MF, Huskins WC, Colantuoni E, Yenokyan G, Moss M, et al. Reducing PICU central line-associated bloodstream infections: 3-year results. Pediatrics 2011;128:e1077–83.
Ong A, Dysert K, Herbert C, Laux L, Granato J, Crawford J, et al. Trends in central line-associated bloodstream infections in a trauma-surgical intensive care unit. Arch Surg 2011;146:302–7.
Payne NR, Barry J, Berg W, Brasel DE, Hagen EA, Matthews D, et al. Sustained reduction in neonatal nosocomial infections through quality improvement efforts. Pediatrics 2012;129:e165–73.
Render ML, Hasselbeck R, Freyberg RW, Hofer TP, Sales AE, Almenoff PL, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf 2011;20:725–32.
Resende DS, do OJ, de Brito DD, Abdallah VO, Gontijo Filho PP. Reduction of catheter-associated bloodstream infections through procedures in newborn babies admitted in a university hospital intensive care unit in Brazil. Revista Da Sociedade Brasileira de Medicina Tropical 2011;44:731–4.
Seddon ME, Hocking CJ, Mead P, Simpson C. Aiming for zero: decreasing central line associated bacteraemia in the intensive care unit. N Z Med J 2011;124:9–21.
Shannon RP. Eliminating hospital acquired infections: is it possible? Is it sustainable? Is it worth it? Trans Am Clin Climatol Assoc 2011;122:103–14.
Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf 2011;37:509–14.
Weiss CH, Moazed F, Mcevoy CA, Singer BD, Szleifer I, Amaral LAN,et al. Prompting Physicians to Address a Daily Checklist and Process of Care and Clinical Outcomes A Single-Site Study. Am J Respir Crit Care Med 2011;184:680–6.
Clinical effectiveness conference abstracts (n = 12) identified in search updates (March 2012) (excluded from data synthesis)
Caswell H. Utilizing a proactive approach to preventing device associated infections in a new pediatric medical ICU. Pediatr Crit Care Med 2011;12(3).
Eggimann P, Joseph C, Thevenin M-J, Bellini C, Voirol P, Revelly J-P. Reduction of primary bacteremia following the introduction of chlorhexidine-impregnated sponges combined with transparent dressings on central venous catheters in a mixed ICU. J Vasc Access 2011;12(1).
Fleming A, Harris B. Strive toward elimination of catheter-related blood stream infections. Pediatr Crit Care Med 2011;12(3).
Guner R, Hasanoglu I, Keske S, Tasyaran MA. Educational approach to reduce catheter-related bloodstream infections and catheter-related urinary tract infection rates in intensive care unit. Clin Microbiol Infect 2011;17(Suppl. 4):S775.
Kaye G, Mateo C. Multifaceted initiatives to eliminate central catheter-associated blood stream infections in the intensive care unit. Crit Care Nurse 2011;31(2):E35.
Kellie S, Scott M, Saad M, Cavallazzi R, Wiemken T. Procedural and educational interventions reduce ventilator associated pneumonia (VAP) and central line associated bloodstream infections (CLABSI). Chest 2011;140(4).
Palomar M, Lopez Pueyo M, Alvarez Lerma F, Olaechea P, Otal J, Insausti J. ICU-Acquired Infections. Do We Need Bundles? Abstr Intersci Conf Antimicrob Agents Chemother 2010;50.
Perez AJ, Monge FJC, Vivas AM. Effect of a program to improve prevention measures of catheter-related bloodstream infections in a medical ICU. Intensive Care Med 2011;37(1)(Suppl.):S172.
Ritter G. A central venous catheter line protocol by the continuum of care decreases complications in hospitalized patients: two steps beyond a checklist. Chest 2011;140(4).
Rooney KD, Sundaram R, Gibson L, Price RJ. Safety programme reduces ICU mortality. Crit Care 2011;15:S173–4.
Szakmany T, Pain T, Beckett P, Jerrett H, Hermon A. Eliminating catheter related bloodstream infection on the intensive care unit: Not a Myth! Intensive Care Med 2011;37(1)(Suppl.):S16.
Zack JE, Spence D, Lamb B, Martin RS, Striker DA, Krettek JE, et al. Creating a culture of zero tolerance for central line associated bacteraemia. The National Database of Nursing Quality Indicators 2010.
Appendix 7 Data for clinical effectiveness forest plots
Data for forest plot: regional-scale interventions (see Figure 4)
| Study | Comparison | Intervention incidence | Intervention device-days | Baseline incidence | Baseline device-days | Intervention incidence density | Baseline incidence density | RR | SE of log RR | 95% confidence limits of RR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||||
| Burrell 201183 | INT months 4–6 vs. INT months 1–3 | (20) | (7684) | (28) | (9308) | [2.6] | [3.0] | [0.87] | [0.293] | [0.49] | [1.54] |
| INT months 7–9 vs. INT months 1–3 | (13) | (9634) | (28) | (9308) | [1.3] | [3.0] | [0.45] | [0.336] | [0.23] | [0.87] | |
| INT months 10–12 vs. INT months 1–3 | (18) | (9725) | (28) | (9308) | [1.9] | [3.0] | [0.63] | [0.302] | [0.34] | [1.11] | |
| INT months 13–15 vs. INT months 1–3 | (10) | (9589) | (28) | (9308) | [1.0] | [3.0] | [0.33] | [0.368] | [0.17] | [0.71] | |
| INT months 16–18 vs. INT months 1–3 | (11) | (8773) | (28) | (9308) | [1.3] | [3.0] | [0.43] | [0.356] | [0.21] | [0.84] | |
| Palomar Martinez 201068 | INT (3 months) vs. B (2006 data) | 44 | 11432 | 59 | 9164 | 3.85 | 6.44 | 0.60 | 0.199 | 0.41 | 0.89 |
| Control (3 months) vs. B (2006 data) | 28 | 8453 | 59 | 4644 | 3.31 | 12.70 | 0.26 | 0.229 | 0.17 | 0.41 | |
| Pronovost 2006, 2008, 2010 34,123,124 | INT months 0–3 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.62 | Not reported | 0.47 | 0.81 | |||||
| INT months 4–6 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.56 | Not reported | 0.38 | 0.84 | ||||||
| INT months 7–9 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.47 | Not reported | 0.34 | 0.65 | ||||||
| INT months 10–12 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.42 | Not reported | 0.28 | 0.63 | ||||||
| INT months 13–15 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.37 | Not reported | 0.20 | 0.68 | ||||||
| INT months 16–18 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.34 | Not reported | 0.23 | 0.50 | ||||||
| INT months 19–21 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.34 | Not reported | 0.23 | 0.50 | ||||||
| INT months 22–24 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.33 | Not reported | 0.23 | 0.48 | ||||||
| INT months 25–27 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.44 | Not reported | 0.34 | 0.57 | ||||||
| INT months 28–30 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.40 | Not reported | 0.30 | 0.53 | ||||||
| INT months 31–33 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.31 | Not reported | 0.21 | 0.45 | ||||||
| INT months 34–36 vs. B | Reported as summary statistics and ranges (see data extraction form) | 0.34 | Not reported | 0.24 | 0.48 | ||||||
| Render 2006126 | INT vs. B (timing unclear) | Not reported | Not reported | Not reported | Not reported | 0.4 | 1.7 | 0.24 | Not reported (not calculable) | ||
| INT (9 months) vs. B | (5) | (7830) | (11) | (7593) | [0.6] | [1.4] | [0.44] | [0.539] | [0.15] | [1.27] | |
| Speroff 2011136 | Toolkit months 1–3 vs. B | (72) | (25257) | (81) | (26599) | [2.9] | [3.0] | [0.97] | [0.162] | [0.68] | [1.29] |
| Toolkit months 4–6 vs. B | (76) | (24618) | (81) | (26599) | [3.1] | [3.0] | [1.03] | [0.160] | [0.74] | [1.39] | |
| Toolkit months 7–9 vs. B | (72) | (27821) | (81) | (26599) | [2.6] | [3.0] | [0.87] | [0.162] | [0.62] | [1.17] | |
| Toolkit months 10–12 vs. B | (75) | (29066) | (81) | (26599) | [2.6] | [3.0] | [0.87] | [0.160] | [0.62] | [1.16] | |
| Toolkit months 13–15 vs. B | (62) | (29818) | (81) | (26599) | [2.1] | [3.0] | [0.7] | [0.169] | [0.49] | [0.95] | |
| Toolkit months 16–18 vs. B | (66) | (24245) | (81) | (26599) | [2.7] | [3.0] | [0.9] | [0.166] | [0.65] | [1.24] | |
| Virtual collab. months 1–3 vs. B | (52) | (22202) | (37) | (22172) | [2.3] | [1.7] | [1.35] | [0.215] | [0.92] | [2.14] | |
| Virtual collab. months 4–6 vs. B | (54) | (22951) | (37) | (22172) | [2.4] | [1.7] | [1.41] | [0.213] | [0.93] | [2.14] | |
| Virtual collab. months 7–9 vs. B | (53) | (23268) | (37) | (22172) | [2.3] | [1.7] | [1.36] | [0.214] | [0.90] | [2.08] | |
| Virtual collab. months 10–12 vs. B | (45) | (26211) | (37) | (22172) | [1.7] | [1.7] | [1.0] | [0.222] | [0.67] | [1.59] | |
| Virtual collab. months 13–15 vs. B | (65) | (25646) | (37) | (22172) | [2.5] | [1.7] | [1.47] | [0.206] | [1.01] | [2.27] | |
| Virtual collab. months 16–18 vs. B | (53) | (19276) | (37) | (22172) | [2.7] | [1.7] | [1.59] | [0.214] | [1.08] | [2.51] | |
Data for forest plot: local-scale interventions of duration up to 12 months (see Figure 5)
| Study | Comparison | Intervention incidence | Intervention device-days | Baseline incidence | Baseline device-days | Intervention incidence density | Baseline incidence density | RR | SE of log risk ratio | 95% confidence limits of RR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||||
| Coopersmith 200250 | INT (6 months) vs. B | 26 | 7044 | 74 | 6874 | 3.7 | 10.8 | [0.34] | [0.228] | [0.22] | [0.54] |
| DuBose 200893 | INT (1 month) vs. B | Not reported | Not reported | Not reported | Not reported | 5.8 | Unclear | Not reported (not calculable) |
Not reported (not calculable) |
||
| Guerin 201098 | INT (12 months) vs. B | 3 | 2825 | 25 | 4415 | 1.1 | 5.7 | 0.19 | 0.611 | 0.06 | 0.63 |
| Higuera 2005103 | Total, INT (9 months) vs. B | 55 | 2824 | 28 | 605 | 19.5 | 46.3 | 0.42 | 0.232 | 0.27 | 0.66 |
| Medical-surgical ICU, INT vs. B | Not reported | Not reported | Not reported | Not reported | 22.1 | 57.4 | 0.38 | Not reported | 0.22 | 0.68 | |
| Neurosurgical ICU, INT vs. B | Not reported | Not reported | Not reported | Not reported | 17.1 | 32.8 | 0.52 | Not reported | 0.24 | 1.11 | |
| Lobo 2005108 | INT (8 months) vs. B | 16 | 1481 | 48 | 2450 | 10.8 | 19.6 | [0.55] | [0.289] | [0.31] | [0.97] |
| F (12 months) vs. B | 22 | 1701 | 48 | 2450 | 12.9 | 19.6 | [0.66] | [0.257] | [0.40] | [1.09] | |
| F vs. INT | 22 | 1701 | 16 | 1481 | 12.9 | 10.8 | [1.19] | [0.329] | [0.63] | [2.28] | |
| Lobo 2010109 | ICU ‘A,’ INT (9 months) vs. B | 8.94 | 843 | 11.28 | 940 | 10.6 | 12.0 | [0.88] | [0.448] | [0.37] | [2.13] |
| ICU ‘B’, INT (9 months) vs. B | 21.85 | 1694 | 35.24 | 2175 | 12.9 | 16.2 | [0.80] | [0.272] | [0.47] | [1.36] | |
| Perez Parra 2010122 | Total (three ICUs), INT (9 months) vs. B | 34 | 11582 | 45 | 10661 | 2.9 | 4.2 | 0.70 | 0.227 | 0.45 | 1.09 |
| General ICU, INT (9 months) vs. B | 14 | 4064 | 18 | 3403 | 3.4 | 5.3 | [0.65] | [0.356] | [0.32] | [1.31] | |
| Cardiac ICU, INT (9 months) vs. B | 8 | 2981 | 12 | 2842 | 2.7 | 4.2 | [0.64] | [0.456] | [0.26] | [1.55] | |
| MICU, INT (9 months) vs. B | 12 | 4537 | 15 | 4416 | 2.6 | 3.4 | [0.76] | [0.387] | [0.36] | [1.66] | |
| Rosenthal 2003129 | Education (1–2 months) vs. B | 10 | 586 | 56 | 1219 | 17.1 | 45.9 | 0.37 | 0.343 | 0.19 | 0.73 |
| PF (7–8 months) vs. education | 41 | 4140 | 10 | 586 | 9.9 | 17.1 | 0.58 | 0.353 | 0.29 | 1.18 | |
| PF + education (8–10 months) vs. B | 51 | 4726 | 56 | 1219 | 10.8 | 45.9 | 0.24 | 0.194 | 0.17 | 0.36 | |
| Sherertz 2000135 | F (18 months) vs. B (12 months) | 30 | Not reporteda | 32 | Not reporteda | Not reported | Not reported | Not reported (not calculable) |
Not reported (not calculable) |
||
| Warren 2003139 | INT (10 months) vs. B | 11 | 5210 | 30 | 6110 | 2.1 | 4.9 | 0.43 | 0.352 | 0.22 | 0.84 |
| Warren 200451 | INT (1 month) + F (23 months) vs. B | 41 | 7455 | 74 | 7879 | 5.5 | 9.4 | [0.59] | [0.195] | [0.40] | [0.86] |
| Zingg 2009144 | Total (5 ICUs), INT (5 months) vs. B | 7 | 7279 | 24 | 6200 | 1.0 | 3.9 | [0.26] | [0.430] | [0.11] | [0.58] |
| MICU, INT (5 months) vs. B | Not reported | Not reported | 24 | 6200 | 3.9 | 9 | [0.43] | Not reported (not calculable) |
Not reported (not calculable) |
||
| SICU, INT (5 months) vs. B | Not reported | Not reported | 24 | 6200 | 0.2 | 3 | [0.07] | Not reported (not calculable) |
Not reported (not calculable) |
||
Data for forest plot: local-scale interventions of duration of > 12 months (see Figure 6)
| Study | Comparison | Intervention incidence | Intervention device-days | Baseline incidence | Baseline device-days | Intervention incidence density | Baseline incidence density | RR | SE of log risk ratio | 95% confidence limits of RR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||||
| Coopersmith 200487 | INT (15 months) vs. B | 17 | 6152 | 32 | 9353 | 2.8 | 3.4 | [0.82] | [0.300] | [0.45] | [1.45] |
| Eggimann 2000;94 200595 | INT months 1–8 vs. B | (5) | (2174) | (28) | (4243) | (2.3) | (6.6) | [0.35] | [0.486] | [0.13] | [0.90] |
| INT months 1–8 vs. Ba | Not reported | Not reported | Not reported | Not reported | 0.8 | 2.4 | 0.31 | Not reported | 0.09 | 0.53 | |
| INT months 24–36 vs. B | (8) | (3333) | (28) | (4243) | (2.4) | (6.6) | [0.36] | [0.401] | [0.17] | [0.80] | |
| INT months 37–41 vs. B | (4) | (1481) | (28) | (4243) | (2.7) | (6.6) | [0.41] | [0.535] | [0.14] | [1.17] | |
| INT months 51–60 vs. B | (5) | (2941) | (28) | (4243) | (1.7) | (6.6) | [0.26] | [0.486] | [0.10] | [0.67] | |
| INT months 61–72 vs. B | (11) | (3235) | (28) | (4243) | (3.4) | (6.6) | [0.52] | [0.356] | [0.26] | [1.03] | |
| Galpern 200897 | INT (19 months) vs. B | (7) | (7345) | (12) | (2593) | [1.0] | [4.6] | [0.22] | [0.476] | [0.08] | [0.52] |
| Longmate 2011110 | INT months 1–12 vs. B | 7 | 1981 | 9 | 1918 | 3.5 | 4.7 | [0.74] | [0.504] | [0.28] | [2.02] |
| INT months 12–24 vs. B | 1 | 1786 | 9 | 1918 | 0.6 | 4.7 | [0.1] | [1.054] | [0.02] | [0.94] | |
| INT months 24–36 vs. B | 0 | Not reported | 9 | 1918 | 0.0 | 4.7 | 0.00 | Not reported (not calculable) |
Not reported (not calculable) |
||
| INT months 1–12 vs. Ba | 7 | 2613 | 9 | 2660 | 2.7 | 3.4 | 0.79 | 0.504 | 0.29 | 2.13 | |
| INT months 12–24 vs. Ba | 1 | 2155 | 9 | 2660 | 0.5 | 3.4 | 0.14 | 1.054 | 0.02 | 1.08 | |
| INT months 24–36 vs. Ba | 0 | 2138 | 9 | 2660 | 0.0 | 3.4 | 0.00 | Not reported | 0.00 | 0.63 | |
| Misset 2004117 | INT years 2–5 vs. INT year 1 | 14 | Not reported | 7 | Not reported | 0–2.9 | 3.5 | Not reported (not calculable) |
Not reported (not calculable) |
||
| Rosenthal 2005130 | INT (17 months) vs. B (4 months) | [18] | Not reported | [16] | Not reported | Not reported | Not reported | Not reported (not calculable) |
Not reported (not calculable) |
||
| Wall 2005138 | INT (24 months) vs. B | 6 | [1132] | 25 | [3571] | (5.3) | 7.0 | [0.76] | [0.455] | [0.31] | [1.85] |
| INT (last 6 months) vs. B | Not reported | Not reported | 25 | [3571] | (3.8) | 7.0 | [0.54] | Not reported (not calculable) |
Not reported (not calculable) |
||
Appendix 8 Data extraction forms for cost-effectiveness analyses
Cohen and colleagues
This record was compiled by the Southampton Health Technology Assessments Centre (SHTAC) following the format used by the NHS CRD Economic Evaluation Database.
Study characteristics
Reference
Cohen et al. (2010). 76
Health technology
Simulation-based education for prevention of CRBSIs in an ICU.
Interventions and comparators
Simulation-based education training in CVC insertion versus no training.
Was a no-treatment/supportive care strategy included?
Yes.
Describe interventions/strategies
Simulation-based education with training in CVC insertion. The training consisted of two 2-hour educational sessions of a lecture, ultrasound training, and deliberate practice with the CVC simulator and instructor feedback. The CVC simulator features a realistic tissue with ultrasound compatibility, an arterial tube, and self-sealing veins and skins.
Research question
To estimate cost savings related to a reduction in CRBSI after simulation training.
Study type
Cost–consequence analysis.
Study population
A total of 477 patients had a CVC inserted in the MICU during the study period.
Institutional setting
Intensive Care Unit.
Country/currency
United States of America; US dollars 2008.
Funding source
Not stated.
Analytical perspective
United States health-care payer.
Effectiveness
Before simulation-based intervention, the average infection rate was 4.2/100 (11 infections in 239 CVC patients). After the intervention, the infection rate was reduced to 0.42/100 admission (one infection in 238 CVC patients). Thus, preventing an estimated 9.95 CRBSI cases.
Intervention costs
Intervention costs stated in the analysis includes hospital costs, obtained from the hospital finance department. The stated costs included both direct and indirect costs estimates and are listed as follows.
| Item | Units | Cost (US$)/unit | Total cost (US$) |
|---|---|---|---|
| Ultrasounda | 1 | 19,475.07 | 19,475.07 |
| Central line simulatora | 1 | 1353.40 | 1353.40 |
| CVC kits | 210 | 35.73 | 7429.80 |
| Simulator supplies | 16 | 439.35 | 6960.00 |
| Ultrasound cover probes | 90 | 14.10 | 1256.40 |
| Sterile gowns | 150 | 2.98 | 442.50 |
| Sterile drapes | 15 | 50.08 | 743.70 |
| Supply carta | 1 | 1633.20 | 1633.20 |
| Supplies total | 39,294.07 | ||
| Other expenses | Hour | Cost/hour | Total cost |
| Simulator facility rental | 330 | 45.00 | 14,850.00 |
| Salary support | |||
| Instructor | 50,500.00 | ||
| Research assistant | 7272.00 | ||
| Total costs | 111,916.07 |
Indirect costs
Were indirect costs included?
No.
Health-state valuations/utilities
None included.
List the utility values used in the evaluation
None.
Modelling
The study developed a trial-based economic model based on a non-randomised trial for the intervention. Two statistical methods were used: the propensity score match case–control comparison method and linear regression models. The trial data were analysed and regression models were used to derive estimates of cost and LOS for the intervention and control group, controlling for age, sex and Charlson score (an indicator of the severity of the disease). In order to give the intervention and the control group the same infection risk, a regression-based propensity score was used. Estimates of cost differences between the matched cases and controls were then derived.
Extract transition probabilities for the model and show sources
The model does not use transition probabilities but reported propensity score quartiles based on predicted probabilities of infection in relation to LOS in the hospital and MICU.
What is the model time horizon?
12 months.
What, if any, discount rates have been applied in the model? Same rate for costs and outcomes?
Not applicable.
Results/analysis
What measure(s) of benefit were reported in the evaluation?
Average LOS:
| Quartile | No. non –CRBSI patients | Hospital MICU | |||||
|---|---|---|---|---|---|---|---|
| Non-CRBSI patients | CRBSI patients | Difference | Non-CRBSI patients | CRBSI patients | Difference | ||
| 1.2–1.4 | 42 | 12.98 | 25.67 | 12.69 | 6.39 | 23.00 | 16.61 |
| 1.5–3.4 | 267 | 16.18 | 38.67 | 22.49 | 7.69 | 27.33 | 19.64 |
| 3.5–4.2 | 39 | 18.21 | 34.00 | 15.79 | 8.79 | 13.33 | 4.54 |
| > 4.2 | 49 | 16.33 | 22.00 | 5.67 | 8.43 | 16.00 | 7.57 |
| Average total | 397 | 15.93 | 30.09 | 14.16 | 7.83 | 19.92 | 12.09 |
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
| Quartile | No. of non-CRBSI patients | Non-CRBSI patients (US$) | CRBSI patients (US$) | Difference (US$) |
|---|---|---|---|---|
| 1.2–1.4 | 42 | 51,829 | 152,678 | 100,849 |
| 1.5–3.4 | 267 | 58,335 | 155,878 | 97,543 |
| 3.5–4.2 | 39 | 63,057 | 108,581 | 45,524 |
| > 4.2 | 49 | 57,465 | 144,468 | 87,003 |
| Average total | 397 | 57,671 | 140,401 | 82,730 |
Synthesis of costs and benefits: are the costs and outcomes reported together? If so, provide a summary of the results
9.95 CRBSI prevented, total annual savings were US$704,034 and US$711,248 and 137 patients hospital days and 120 and 121 MICU days.
Give results of any statistical analysis of the results of the evaluation
Not applicable.
Was any sensitivity analysis performed?
No.
What scenarios were tested in the sensitivity analysis?
None.
Give a summary of the results of the sensitivity analysis
None.
Conclusions/implications
Give a brief summary of the author's conclusions from their analysis
The results of the analyses show that simulation-based educational intervention is highly cost-effective.
Southampton Health Technology Assessments Centre Commentary
Selection of comparators
Appropriate.
Validity of estimate of measure of benefit
Appropriate.
Validity of estimate of costs
Acceptable.
Halton and colleagues
This record was compiled by SHTAC following the format used by the NHS CRD Economic Evaluation Database.
Study characteristics
Reference
Halton et al. (2010). 151
Health technology
Central venous catheter care bundle.
Interventions and comparators
A bundle approach to CVC care compared with non-bundled approach.
Was a no-treatment/supportive care strategy included?
Yes.
Describe interventions/strategies
Intervention: optimal hand hygiene, chlorhexidine skin antiseptic, maximal barrier precautions for catheter insertion, choice of optimal insertion site and prompt catheter removal.
Research question
To estimate cost-effectiveness of catheter care bundle in the prevention of CRBSIs in intensive care.
Study type
Cost-effectiveness and cost–utility.
Study population
Patients aged 50–80+ years in ICUs.
Institutional setting
ICU.
Country/currency
2006 Australian dollars (A$).
Funding source
Medical Research Council.
Analytical perspective
Australian health-care payer perspective.
Effectiveness
The effectiveness data were derived from the study by Pronovost et al. 34 The bundle was comprised five elements. The intervention reduced the rate of CRBSI from 7.7 to 1.4 per 1000 line-days over a 18-month period, a reduction in the relative risk of 0.34 (95% CI 0.23 to 0.5). Effect estimates for each type of commercially available antimicrobial central venous catheter, relative to uncoated catheters, were taken from the results of a meta-analysis: chlorhexidine/silver sulfadiazine (CH/SSD)-coated catheters (RR = 0.66) and minocycline and rifampicin (MR)-coated catheters (RR = 0.39).
Intervention costs
The costs for the intervention were unknown.
List the direct intervention costs and other direct costs used in the evaluation.
| Parameters | Estimate | Source | Ref. | Level of evidence |
|---|---|---|---|---|
| ICU bed-days (2006 A$) | 3021 | Costing study | 161 | 4 |
| Hospital bed-day (2006 A$) | 843 | Prior economic evaluation | 162 | 3 |
| Diagnostics CRBSI (2006 A$) | 101.7 | Health system database | – | 1 |
| Treatment CRBSI (2006 A$) | 591.3 |
Were the methods for deriving these data adequately described?
Yes.
Indirect costs
No.
Health state valuations/utilities
Preference-based utility weights were taken from a study with participant demographics similar to the modelled cohort. These weights were assigned to patients in ICU and 6 months post discharge. Australian population QoL norms were used for long-term survival.
Were the methods for deriving these data adequately described?
Yes
List the utility values used in the evaluation:
The utilities are as follows:
| Parameters | Age, years | Estimate |
|---|---|---|
| ICU | 0.66 | |
| Population norms | 50–59 | 0.80 |
| 60–69 | 0.79 | |
| 70–79 | 0.75 | |
| 80+ | 0.66 |
Modelling
A Markov state transition model was used. The model was adapted from a previously reported model. 155 Patients were assumed to receive a CVC on entry to the ICU. Over subsequent cycles the catheter was either removed as no longer necessary, or retained owing to the patient developing CRBSI. The model consists of six health states as follows: ICU patient with CVC; ICU patient recently infected with CRBSI; ICU patient without CRBSI and with CVC being removed; hospital ward patient with previous history of CRBSI; remaining ward patient without CRBSI; and recently discharged patient with or without previous history of CRBSI.
What was the purpose of the model?
To evaluate the cost-effectiveness of CVC care bundle.
What are the main components of the model (e.g. health states within a Markov model)?
The main components are as follows:
Patients were assumed to receive a CVC on entry to ICU, and over subsequent daily cycles either retained their catheter, had it removed, or developed a CRBSI. Patient faced an underlying risk of mortality while in the ICU and a further risk if they developed CRBSI. The surviving cohorts were modelled for the remainder of their life time in monthly cycles. The estimated risk of mortality due to hospital acquired CRBSI was 1.06.
Extract transition probabilities for [natural history/disease progression] model and show sources (or refer to table in text).
| Parameters | Estimates | Sources | |
|---|---|---|---|
| Daily probabilities of CRBSI | Day 1–5 | 0.4% | Table 1 |
| Day 6–15 | 0.9% | ||
| Day 16–30 | 2% | ||
| Daily probability of catheter removal | Not stated (varied) | ||
| Baseline mortality (probabilities) | |||
| ICU | 0.098 | ||
| Hospital | 0.069 | ||
| Annual post discharge | Year 1 | 0.050 | |
| Year 2–3 | 0.027 | ||
| Year 4–5 | 0.028 | ||
| Year 6–10 | 0.037 | ||
| Year 11–15 | 0.042 | ||
| Underlying annual mortality | 45–64 years | 0.004 | |
| 65–84 years | 0.030 | ||
| 85+ years | 0.140 |
What is the model time horizon?
The patients were followed for the remaining part of their lifetime in monthly cycles.
What, if any, discount rates have been applied in the model?
Three per cent applied to both costs and outcomes
Results/analysis
What measure(s) of benefit were reported in the evaluation?
The measure of benefit reported was QALYs and number of bed-days gained for the catheter care bundle relative to current practice.
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
Not applicable.
Synthesis of costs and benefits
As the cost of implementing a bundle in Australia was unknown, deterministic threshold analyses were conducted. The maximum cost for the bundle was identified at which it would remain cost-effective (i.e. if the cost per QALY was less than A$64,000) assuming it would reduce the risk of CRBSI by 0.34. The baseline analyses show the benefits for the bundle or standard practice under different scenarios. The bundle at an implementation cost of A$4,349,730 was shown to be cost-effective for the whole 18-month period compared with current practice alone. For the strategy that includes CH/SSD catheters, the implementation cost over the same period was estimated to be A$2,287,400. MR catheter would result in a total implementation cost of A$1,144,465.
Halton and colleagues151 also estimated the maximum cost for the bundle at which it would remain cost-effective assuming it would effectively eliminate infection (RR CRBSI = 0.001). According to this assumption, the maximum nationwide implementation cost would be below A$6.6M if the A-CVCs are not considered a good alternative.
Give results of any statistical analysis of the results of the evaluation.
Not applicable.
Was any sensitivity analysis performed? If yes, what type(s)?
Yes, deterministic (one-way).
What scenarios were tested in the sensitivity analysis?
Threshold analyses were used to determine the effectiveness and implementation cost of different types of bundle that would be more cost-effective relative to the current practice. The analyses were undertaken in three different ways; where three separate comparisons were made one after the other. An initial comparison was between the current practice and the bundle. A subsequent comparison includes a three-way comparison; where current practice was compared with the bundle and then with CH/SSD catheters. The final stage of the analysis, involved a four-way comparison of current practice against the bundle, CH/SSD catheters and MR catheters.
Give a summary of the results of the sensitivity analysis.
See above.
Conclusions/implications
Give a brief summary of the author's conclusions from their analysis
Implementation of catheter care bundles is cost-effective in the intensive care setting, if the decision-makers are willing to spend more on infection intervention control.
Southampton Health Technology Assessments Centre commentary
Selection of comparators
Comparator selection is adequate.
Validity of estimate of measure of benefit
Valid.
Validity of estimate of costs
Reasonable but no costs estimated for the intervention.
Bond and King
This record was compiled by SHTAC following the format used by the NHS CRD Economic Evaluation Database.
Study characteristics
Reference
Bond and King (2011). 152
Health technology
Educational intervention to improve the safety of CVC insertion.
Interventions and comparators
Educational intervention vs. no educational intervention.
Was a no treatment/supportive care strategy included?
Yes.
Describe interventions/strategies
The educational intervention consists of a CVC education course. It is a day-long programme with brief introductory lectures followed by hands-on procedural simulation in CVC insertion, using training mannequins, appropriate sterile procedures, ultrasound imaging and receiving feedback from instructors. In addition, residents and nurses are taught the Institute for Healthcare bundle of barrier precautions and new processes to encourage, ensure and track compliance.
Research question
To model the cost and mortality outcomes of CVC placement with respect to an educational intervention that attempts to reduce both infectious and mechanical complications.
Study type
Cost-effectiveness analysis.
Study population
Baseline cohort was 600 patients who had CVCs placed outside the operating room setting in the hospital setting each year. The proposed setting was described as a health-care system that includes a tertiary care center, a community hospital, and a free-standing emergency department. There are three emergency departments and 12 ICUs in the system.
Institutional setting
ICU.
Country/currency
United States of America; US dollars. Cost year not stated.
Funding source
None.
Analytical perspective
Not stated.
Effectiveness
The evaluation assumes that the educational intervention would reduce the rate of the infectious complication CLAB by 50% and reduce the rate of the mechanical complications by 25%. The study does not discuss the sources that these are based upon nor the rationale behind their use.
Intervention costs
The intervention costs are detailed although it is not stated where these costs were collected.
| Item | No. item/hours per year | Cost per item/hour | Programme Year 1 | Programme Year 2–5 |
|---|---|---|---|---|
| Acquisition of training mannequins | 5 | US$2000 | US$10,000 | US$0 |
| Acquisition of ultrasound technology | 3 | US$18,000 | US$54,000 | US$0 |
| Trainer time | 10 | US$150 | US$1500 | US$1500 |
| Trainee time (nurses) | 1900 | US$25 | US$47,500 | US$4750 |
| Trainee time EM residents (14/year) | 112 | US$20 | US$2240 | US$2240 |
| Transitional interns (12/year) | 96 | US$20 | US$1920 | US$1920 |
| Trainee time IM residents (16/year) | 128 | US$20 | US$2560 | US$2560 |
| Trainee time surgery residents (4/year) | 32 | US$20 | US$640 | US$640 |
| Chart review 0.5 FTE | 2000 | US$30,000 | US$30,000 | |
| Data analysis | 40 | US$100 | US$4000 | US$4000 |
| Programme oversight | 400 | US$40 | US$16,000 | US$16,000 |
| Total per programme year | US$170,360 | US$63,610a |
The cost per CLAB case was US$16,350 based upon those reported by Institute of Healthcare (full reference not supplied). The mean excess cost of mechanical complication was US$17,312.
Indirect costs
None included.
Health-state valuations/utilities
None included.
List the utility values used in the evaluation
None.
Modelling
A decision-analytic model was compiled in TreeAge. The model began with the need for a CVC, with a focus on non-emergent cases, defines as those where there was sufficient time to follow sterile precautions in CVC insertion. One cohort of patients received the education intervention and the other did not. In each cohort, a proportion received the CVC in the internal jugular, subclavian and femoral. Patients then either had no complications, mechanical complication or CLAB. Patients who suffered a CLAB or a mechanical complication had corresponding higher costs and mortality than those without.
Extract transition probabilities for the model and show sources:
-
Location of CVC: internal jugular 0.35; subclavian 0.6, femoral 0.05.
-
Probability of CLAB line infection 0.7%.
-
Baseline mortality risk for an ICU patient: 10.
-
Mortality risk for patients with CLAB: 12.
-
Excess mortality of mechanical complications associated with iatrogenic pneumothorax: 7.
What is the model time horizon?
The duration of the hospital stay.
What, if any, discount rates have been applied in the model?
None applied.
Results/analysis
What measure(s) of benefit were reported in the evaluation?
Survival with education (per patient) 0.899; survival without education 0.898.
Provide a summary of the costs estimated for each intervention/strategy assessed in the evaluation
Cost with education (per patient) US$546; cost without education US$392. Additional cost of education intervention US$154. Using programme cost of US$170,360 per year.
Synthesis of costs and benefits: are the costs and outcomes reported together? If so, provide a summary of the results
Yearly annual additional cost of US$92,400 to a large health-care system is the cost to reduce the number of CLAB infections from 4 to 2. (No discussion in the text on the reduction in mortality.)
Give results of any statistical analysis of the results of the evaluation
Not applicable.
Was any sensitivity analysis performed? If yes, what type(s)? [i.e. deterministic (one-way, two-way, etc.) or probabilistic]
One-way sensitivity analyses.
What scenarios were tested in the sensitivity analysis?
The programme cost, CLAB baseline rate, intervention effectiveness on CLAB rate were varied.
Give a summary of the results of the sensitivity analysis
For lower programme cost of US$63,610 per year, additional cost of education intervention (per patient) US$44*. Raising CLAB infection rate to 5%* gives a net monetary benefit of US$158 (i.e. cost saving) with an additional survival of 0.003.
(*Note that neither of the sensitivity analyses had a reduction in mechanical complications).
Conclusions/implications
Give a brief summary of the author's conclusions from their analysis
If the educational intervention is effective, a small increase in costs can reduce complications.
Southampton Health Technology Assessments Centre commentary
Selection of comparators
Appropriate.
Validity of estimate of measure of benefit
Appropriate, although rationale for selection not given.
Validity of estimate of costs
Uncertain. Derivation of cost associated with CLAB is unclear.
Appendix 9 The model parameters and their distributions included in the probabilistic sensitivity analysis
| Name | Base case | Upper value | Lower value | SE | Distribution | Alpha | Beta |
|---|---|---|---|---|---|---|---|
| Catheter-BSI incidence rate, per 1000 catheter-days | 3.7 | 5.0 | 1.3 | 1.22 | Log-normal | 1.6 | 0.3 |
| Critical care mortality, no catheter-BSI | 0.169 | 0.203 | 0.135 | 0.02 | Beta | 79.6 | 391.6 |
| RR for critical care mortality due to catheter-BSI | 3.25 | 3.6 | 2.7 | 0.28 | Log-normal | 1.3 | 1.0 |
| Catheter utilisation | 0.71 | 0.96 | 0.49 | 0.13 | Beta | 8.3 | 3.4 |
| Costs | |||||||
| Ward bed-day, £ | 246 | 295.2 | 196.8 | 25.10 | Gamma | 96.0 | 2.6 |
| Critical care bed-day, £ | 1440 | 1171 | 1657 | –110.93 | Gamma | 168.5 | 8.5 |
| Treatment for catheter-BSI | 518 | 622 | 415 | 52.89 | Gamma | 96.0 | 5.4 |
| Standard care (per patient in critical care) | 0 | ||||||
| Bundle (per patient in critical care) | 15.48 | 20.13 | 10.84 | 2.37 | Gamma | 42.7 | 0.4 |
| Clinical effectiveness | |||||||
| Bundle effectiveness | 0.4 | 0.67 | 0.22 | 0.10 | Log-normal | –0.5 | –1.6 |
| Additional LOS for catheter-BSI, critical care | 1.5 | 2.5 | 0.0 | 0.30 | Triangle | 2.0 | 0.5 |
| Additional LOS for catheter-BSI, ward | 5.13 | 8.68 | 1.58 | 1.81 | Log-normal | 2.2 | 0.5 |
Appendix 10 Calculating the cost of the central venous catheter care bundle
The cost of the bundle consists of the national programme and local implementation costs. The programme grant for implementing Matching Michigan in England was £1,750,000 for a 2-year period. Thus the annual cost would be £875,000 per annum.
The local training costs were calculated based on advice on implementation of Matching Michigan in one local centre. In this centre, one Band 6 nurse trained and monitored critical care staff in three critical care units and this took 20% whole time equivalent (WTE).
Using the assumptions above, the costs were estimated per patient attending ICU and these are shown in the table below.
| Parameter | Value | Source |
|---|---|---|
| National ICU data | ||
| No. of patients attending critical care per year in England | 89,618 | ICNARC163 |
| No. of critical care units | 188 | ICNARC163 |
| Average no. of patients per critical care unit | 477 | ICNARC163 |
| National programme costs | ||
| National programme costs/year | £875,000 | Matching Michigan162 |
| National programme costs per patient in critical care | £9.76 | Matching Michigan162 |
| Local unit costs | ||
| Annual salary of a band 6 nurse | £40,917 | Agenda for Changea |
| Proportion of time allocated to staff training | 20% | Assumption |
| No. of critical care units covered | 3 | Assumption |
| No. of patients admitted to critical care | 1431 | ICNARC |
| Local training costs per patient in critical care (expert opinion, Matching Michigan163) | £5.72 | |
| Total cost of bundle | £15.48 | |
Glossary
- Catheter-associated bloodstream infection
- A primary laboratory-confirmed bloodstream infection in a patient with a central line at the time of (or within 48 hours prior to) the onset of symptoms, and which is not related to an infection from another site. Synonyms include central line-associated bacteraemia and central line-associated bloodstream infection.
- Catheter-bloodstream infection (catheter-BSI)
- An umbrella term referring to catheter-associated bloodstream infections, catheter-related bloodstream infections or catheter-suspected bloodstream infections and their synonyms.
- Catheter-related bloodstream infection
- Various definitions and terms are used, and sometimes confused, in the literature to describe a bloodstream infection that has developed as a consequence of an indwelling intravascular catheter. Bloodstream infections that are proven microbiologically to result from vascular catheter use are generally referred to as catheter-related bloodstream infections. For the purposes of this report, a definition as used in the Matching Michigan programme in England is provided (see Table 1).
- Critical care unit
- A specially equipped hospital area designed for the specialised care of patients whose conditions are life-threatening and who require comprehensive care and constant monitoring. For the purposes of this report, synonymous with intensive care unit, including high-dependency units, but excluding step-down units.
- Device-days
- The total number of days that patients have one or more vascular devices in a given period. Definitions vary depending on whether multiple vascular devices per patient are counted separately.
- Incidence density
- The standardised incidence of infection. For the purposes of this report, standardised to the number of vascular device-days and expressed per 1000 device-days.
- Incidence density risk ratio
- The ratio of incidence densities in study and comparator groups. Values of < 1 favour the study group; values of > 1 favour the comparator group.
- Intensive care unit
- See Critical care unit.
- Matching Michigan
- An infection prevention strategy adapted from the US Keystone ICU ('Michigan')project intervention and implemented in English NHS trusts in 2009.
- ORION
- Guidelines for the transparent reporting of nosocomial infections, including a 22-item checklist and summary table.
- STROBE
- Guidelines for the reporting of observational studies in epidemiology, including a 22-item checklist and supporting information with examples.
- TREND
- Guidelines for the reporting of non-randomised evaluations of behavioural and public health interventions, including a 22-item checklist.
List of abbreviations
- AG
- Advisory Group
- BEME
- Best Evidence in Medical Education
- BSI
- bloodstream infection
- CABSI
- catheter-associated bloodstream infection
- CDC
- Centers for Disease Control and Prevention
- CEO
- chief executive officer
- CFU
- colony-forming unit
- CH/SSD
- chlorhexidine/silver sulfadiazine
- CI
- confidence interval
- CLAB
- central line-associated bacteraemia (synonymous with CABSI)
- CLABSI
- central line-associated bloodstream infection (synonymous with CABSI)
- CQI
- continuous quality improvement
- CRBSI
- catheter-related bloodstream infection
- CVC
- central venous catheter
- CVL
- central venous line
- ECDC
- European Centre for Disease Prevention and Control
- HELICS
- Hospitals In Europe Link for Infection Control through Surveillance
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- ICUAI
- Intensive Care Unit-Associated Infection
- IHI
- Institute for Healthcare Improvement
- IQR
- interquartile range
- ITS
- interrupted time series
- LCBSI
- laboratory-confirmed bloodstream infection
- LOS
- length of stay
- MeSH
- medical subject heading
- MHA
- Michigan Health and Hospital Association
- MICU
- medical intensive care unit
- MR
- minocycline and rifampicin
- MRC
- Medical Research Council
- MRSA
- methicillin-resistant Staphylococcus aureus
- NHSN
- National Healthcare Safety Network (US CDC)
- NICE
- National Institute for Health and Care Excellence
- NNIS
- Nosocomial Infection Surveillance System (US CDC)
- NSP
- National Surveillance Programme
- PICC
- peripherally inserted central catheter
- PICU
- paediatric intensive care unit
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QI
- quality improvement
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- risk ratio
- SHTAC
- Southampton Health Technology Assessments Centre
- SICU
- surgical intensive care unit
- UTI
- urinary tract infection
- VAP
- ventilator-associated pneumonia