Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/39/01. The contractual start date was in October 2007. The draft report began editorial review in July 2012 and was accepted for publication in March 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
This study had an initial start up grant of £3000 from the Newborn Appeal charity. Drs Roberts, Martin, Green, Walkinshaw and Bricker are occasionally paid monies for expert witness reports. Dr Walkinshaw and Professor Shaw are occasionally paid for invited lectures. Dr Walkinshaw has received payment from Ferring Pharmaceutical for invited lectures and meetings.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Roberts et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and rationale
What are the risks of very early preterm prelabour rupture of membranes?
Preterm prelabour rupture of membranes (PPROM) is one of the major causes of perinatal mortality and morbidity because it causes preterm delivery in a third of cases in which it occurs. 1,2 Fetal survival is even more compromised when the amniotic membrane ruptures early in the second trimester.
There is a very high risk of delivery after very early PPROM. Moretti and Sibai3 reported a mean rupture to delivery interval of 13 days in pregnancies with PPROM between 16 and 26 weeks’ gestation, suggesting a high risk of delivery of previable fetuses and of infants at the extreme of viability. Forty-eight per cent of the pregnancies in their study delivered within 3 days of amniotic membrane rupture. The overall rate of preterm birth was 54%. Stillbirth after an infection, abruption or cord prolapse, prematurity and pulmonary hypoplasia are the major causes of perinatal mortality and morbidity in this group of babies.
The incidence of pulmonary hypoplasia in very early PPROM is reported to be as high as 62%. 4 Studies have suggested that oligohydramnios is the most important predictor of perinatal mortality in very early PPROM and that adequate residual amniotic fluid plays a critical role in determining the prevalence of pulmonary hypoplasia. 4–7 Oligohydramnios is also said to be associated with a higher risk of chorioamnionitis and neonatal infection. 5 Adequate amniotic fluid volumes, on the other hand, are said to be associated with better outcomes in pregnancies affected by very early PPROM. Locatelli et al. 8 found that pregnancies with a median residual amniotic fluid pocket persistently less than 2 cm were at highest risk of poor perinatal and long-term neurological outcome while pregnancies with a pocket greater than 2 cm had significantly better perinatal outcome (73–92% survival) and lower pulmonary hypoplasia rates. 8,9
What management options are available?
The management of cases with very early PPROM has changed over the years. Traditionally, termination of pregnancy was offered for these women because of the presumed risk of maternal sepsis and very poor fetal outcome. Expectant management (Exp) has, however, been shown to be relatively safe for mothers and results in the survival of a small proportion of infants.
Serial transabdominal amnioinfusion (AI) aiming to restore the amniotic fluid volume in pregnancies complicated by very early PPROM is an invasive procedure which has the potential to improve the perinatal outcome. 6 As discussed above, pregnancies with a median residual amniotic fluid pocket persistently less than 2 cm are at highest risk of poor perinatal and long-term neurological sequela. Those pregnancies that retain a pocket greater than 2 cm, either after AI or spontaneously, have significantly better perinatal outcome (73–92%) and lower pulmonary hypoplasia rates. 8 It has also been shown that women with persistent oligohydramnios after AI have a significantly shorter PPROM to delivery interval, lower neonatal survival (20%), higher rates of pulmonary hypoplasia (62%) and higher abnormal neurological outcomes (60%) than women in whom AI is successful (p < 0.01 for all cases). 7 AI is not, however, routinely used in the UK as it is an invasive procedure and its efficacy has not been evaluated fully in a well-conducted randomised controlled trial (RCT).
What is the evidence for management options in very early preterm prelabour rupture of membranes?
Most of the evidence on the management of very early PPROM is based on observational case–control or comparative studies. 3–11 The major risk of expectant is maternal infection leading to sepsis. High rates of postpartum morbidity10 and chorioamnionitis11 have been reported: 32% and 28%, respectively. The Royal College of Obstetricians and Gynaecologists (RCOG) guideline on PPROM12 does not give any specific guidance on the management of these pregnancies. It also does not support the practice of serial AI owing to lack of evidence.
To date, there have, to our knowledge, been no RCTs that have assessed the relative benefit of serial AI over expectant in pregnancies with PPROM between 16 and 26 weeks of pregnancy. Evidence from non-randomised cohorts is likely to be biased owing to selective reporting, and the comparisons are often based on historic cohorts and incomplete outcome data for a sample of pregnancies with PPROM not treated by AI. Long-term outcomes for surviving infants are rarely reported. Moreover, AI is an invasive intervention and, although, anecdotally, these studies suggest that it carries minimal risk to the mother and fetus,7 the evidence of harm is rarely systematically collected and reported.
Rationale for the trial
There is growing evidence to suggest that AI may have a role to play in improving the perinatal outcome in pregnancies with PPROM. A Cochrane review on AI for PPROM states: ‘These results are encouraging but are limited by the sparse data and unclear methodological robustness, therefore further evidence is required before AI for PPROM can be recommended for routine clinical practice’. 13 The National Institute for Health and Care Excellence (NICE) concluded, after review of existing literature, that more information from RCTs is required before AI can be considered routine therapy for very early PPROM.
Preterm birth represents a considerable burden to both patients and the NHS. The risk of neonatal death is high and surviving preterm babies are at risk of developing respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular haemorrhage (IVH), cerebral palsy, blindness and deafness, with huge impact on their families and society. The economic consequences of preterm birth are immense. A multilevel modelling of hospital service utilisation and cost profile of preterm birth using data from 117,212 children showed that the cumulative cost of hospital inpatient admissions averaged £17,819.94 for children born at less than 28 weeks’ gestation and £17,751.00 for children born at 28–31 weeks’ gestation. Evidence from observational studies suggests that most babies with very early PPROM are delivered before 31 weeks of pregnancy. If there was any chance that AI could improve outcomes for these babies, a well-designed trial would be required to determine that effect.
On the basis of this, we began a single-centre, investigator-led randomised trial in 2001. The trial was sponsored by the Liverpool Women’s NHS Foundation Trust and had North West Multiresearch Ethics Committee (MREC) approval. In response to the change in regulations for research trials in 2006, we applied to an open call for trial proposals by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme, which agreed to fund the long-term outcome phase of AI in preterm premature rupture of membranes (AMIPROM) pilot study – a pilot RCT on serial transabdominal AI versus expectant for very early PPROM – provided the trial was analysed as a pilot study and all outcomes were reported.
Specific objectives of the pilot study
-
To assess the feasibility of recruitment, the methods for conduct of the study and the retention through to long-term follow-up of participants in the study.
-
To perform an outcome assessment and to collect data to inform a larger, more definitive clinical trial if indicated.
Chapter 2 Methods
Trial design
The AMIPROM was a multicentre, two-armed, non-blinded pilot RCT with equal randomisation. Randomisation was stratified for pregnancies with PPROM prior to, and after, 20+0 weeks’ gestation. Participants were randomised in a 1 : 1 ratio to receive either:
-
expectant with weekly ultrasound assessments of the pregnancy, or
-
weekly AI if the deepest pool of amniotic fluid measured < 2 cm.
Approvals obtained
North West MREC approved the study in July 2002. Minor amendments to the protocol were made in October 2006. Substantial amendments were made in August 2007 and December 2008. The final protocol is in Appendix 1.
Clinical trial authorisations from the Medicines and Healthcare products Regulatory Agency were sought but not required as saline/Hartmann’s solution used to perform AI is not a medicinal product. The trial was registered with International Standard RCT number (ISCTRN; ISRCTN no. 8192589).
Trial sites
There were four recruiting sites:
-
Liverpool Women’s NHS Foundation Trust (∼8000 deliveries per annum)
-
St. Mary’s Hospital, Manchester (∼5500 deliveries per annum)
-
Birmingham Women’s NHS Foundation Trust (∼7000 deliveries per annum)
-
Wirral University Teaching Hospital (∼3700 deliveries per annum).
Participants were recruited from Birmingham Women’s NHS Foundation Trust in 2008 following HTA programme funding approval.
Participant eligibility
The participants were women with PPROM between 16+0 and 24+0 weeks’ gestation.
Inclusion criteria
-
Singleton pregnancy.
-
Rupture of amniotic membranes between 16 weeks’ gestation and 24 weeks’ gestation.
-
Rupture of amniotic membranes confirmed by the presence of amniotic fluid in the posterior fornix on speculum examination and/or severe oligohydramnios on ultrasound examination.
Exclusion criteria
-
There was an obstetric indication for immediate delivery (i.e. fetal bradycardia, abruption, cord prolapse, advanced labour > 5 cm).
-
Multiple pregnancy.
-
Fetal abnormality.
Participants were also not recruited if they were unable to give informed consent.
Recruitment to the trial
The principal investigators (PIs) in the pilot received ‘good clinical practice’ training as well as training in all aspects of the trial, including participant recruitment, eligibility criteria, trial protocol, adverse event reporting procedures and trial documentation. Each study site received a trial pack prior to commencement of recruitment.
Participants were identified by health-care professionals at the study site or one of the hospitals that referred patients to the study site. An appointment for further assessment and confirmation of PPROM at the local fetal medicine unit (FMU) was arranged. Participants were given an information leaflet by the health-care professional who first saw them. Following discussion of the trial at the FMU and confirmation of very early PPROM, consent was obtained.
Women were randomised only if the pregnancy was still ongoing 10 days after rupture because of the high risk of miscarriage in the first week after PPROM. This protocol change was implemented in 2002 following discussion at an international meeting of fetal medicine specialists. 14
Participants were given a minimum of 24 hours, but more commonly longer, to read the information sheet and consider participation. Consent was obtained only after further discussion of the study with the fetal medicine teams in the study sites.
Randomisation
A computer-generated random sequence using a 1 : 1 ratio was used. Randomisation was stratified for pregnancies in which the amniotic membrane ruptured between 16+0 and 19+6 weeks’ gestation and those in which rupture occurred between 20+0 and 23+6 weeks’ gestation to minimise the risk of random imbalance in gestational age distribution between randomised groups. The randomisation sequence was generated in blocks of four. The sequence was generated by the Division of Statistics and Operational Research, University of Liverpool. Owing to the nature of the intervention (multiple needle insertions during pregnancy), neither clinicians nor participants were blinded to the treatment allocation. Assessors of long-term outcomes were not blinded to the intervention because, although it is a source of bias that the participants were aware of which arm they were allocated to, it would simply not have been possible to prevent them discussing this with the long-term outcome assessors post delivery.
Participants who consented to take part in the study were assigned their trial arm by ringing the telephone randomisation service administered by the Liverpool Women’s Hospital Research and Development Office. None of the investigators had access to the randomisation sequence or knew the randomised treatment to be allocated next.
The flow of participants through the trial is presented in a Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 1).
FIGURE 1.
The CONSORT flow diagram. a, Four of the 26 women attended the study visits but had maintained a deepest pool of amniotic fluid > 2 cm throughout the duration of their participation so did not have any amnioinfusion fluid instilled at any time because they did not require it. They would have received amnioinfusion at a study visit had they required it. CGA, corrected gestational age; CRF, case report form; MDI, Mental Development Index; PDI, Psychomotor Development Index; TOP, termination of pregnancy.
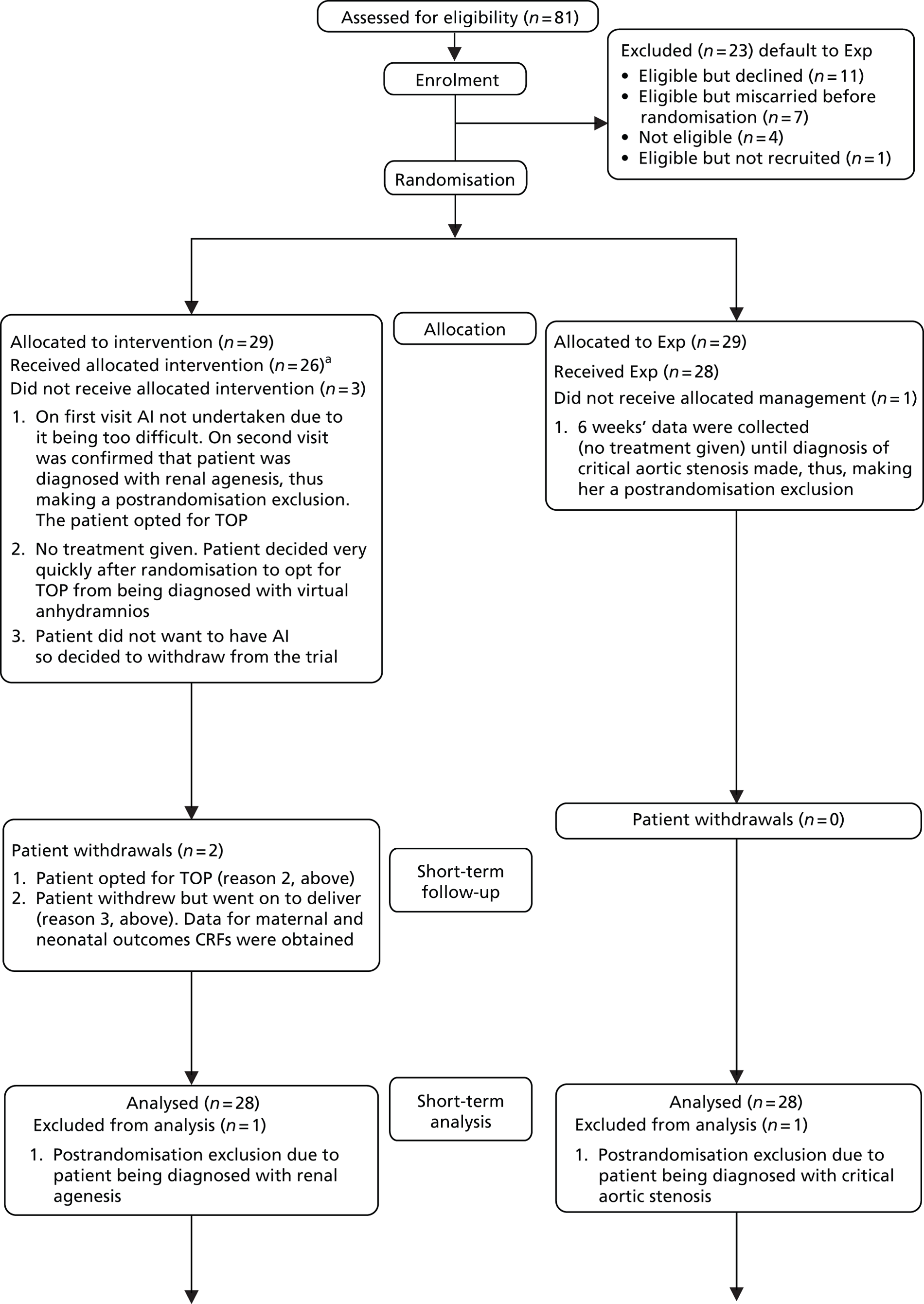
Eligible women who declined participation
The FMUs were asked to keep a log of patients who were eligible but opted not to participate in the trial, to generate an idea of potentially eligible participants who declined the study or miscarried. This was collected on A4 sheets of plain paper and kept in the trial folder in the FMUs (see Chapter 3).
Sample size
An initial presumptive sample size of 62 participants was calculated based on an audit performed at the Liverpool Women’s NHS Foundation Trust. The audit revealed a composite adverse outcome of 75% in pregnancies with very early PPROM, in which there was a mortality rate of 65% and approximately 25% respiratory morbidity in the survivors (overall composite outcome approximately 75%). A reduction in composite outcome by 50% was chosen as the target difference because the nature of the intervention is such (i.e. invasive and repeated) that only a large difference would justify its introduction into routine practice. To reduce the composite outcome by 50%, at a 5% significance level with 80% power, 31 participants were required in each group. This included an allowance of 10% loss to follow-up. However, review by referees for the HTA programme in 2007 required that the study be treated as a pilot study. The NIHR suggested that smaller differences in substantive outcomes (rather than composite) are of interest and that a much larger ‘definitive’ study should be considered to determine effectiveness (or lack of it) with much greater precision. The assumptions used for initial sample size calculations are therefore only indicative and were treated as such by the Data Monitoring Committee (DMC). The final sample size in this study was the number of participants recruited at the end of the period defined by the timelines for the grant, i.e. the grant was funded for recruitment until April 2009.
Interventions
Both trial arms
Rupture of amniotic membranes was confirmed by presence of amniotic fluid in the posterior fornix on speculum examination and/or severe oligohydramnios on ultrasound examination. A high vaginal swab (HVS) was taken on admission and oral erythromycin commenced for 10 days.
Once rupture of the amniotic membranes had been confirmed, women were referred to the first available FMU assessment to exclude fetal abnormality, confirm rupture of amniotic membranes using ultrasonography and discuss the study. Women in both groups were assessed weekly by ultrasound and the following measurements recorded: deepest pool of amniotic fluid, thoracic circumference, lung length and abdominal circumference. Maternal haemoglobin level, white cell count (WCC), platelet count, HVS, C-reactive protein (CRP) and temperature were also recorded at each visit if they had been measured.
Antenatal corticosteroids were administered at 26+0 weeks’ gestation as a matter of routine prophylaxis. Earlier antenatal corticosteroids (between 23+0 and 25+6 weeks’ gestation) were given at the clinician’s discretion. Hospital admission for rest was recommended between 26+0 and 30+0 weeks’ gestation, but not mandatory.
Induction of labour at 37 completed weeks’ gestation was advised unless there was an obstetric indication for earlier delivery, or delivery by caesarean section (elective or emergency).
Expectant management arm
Women were seen weekly and ultrasonography used to obtain the following measurements: deepest pool of amniotic fluid, thoracic circumference, lung length and abdominal circumference. Maternal haemoglobin level, WCC, platelet count, HVS, CRP and temperature were also recorded at each visit if they had been measured. Corticosteroid administration and admission was in accordance with the process described for both arms.
Amnioinfusion arm
Women who were randomised to the intervention arm received AI received AI of saline/Hartmann’s solution only if the deepest pool of amniotic fluid at the weekly ultrasound assessment was < 2 cm. The protocol did not specify a maximum pool depth of < 2 cm for inclusion to the study as we were keen to capture all women with PPROM at these gestations in case they went on to develop a pool of < 2 cm. Between 2002 and 2006, a small number of randomised women in the AI arm never developed a deepest pool of < 2 cm and, therefore, never required AI. Recruiters were advised that, from then on, they should randomise only at the visit in which the deepest pool measured < 2 cm between 16+0 and 24+0 weeks’ gestation. This was not considered a formal protocol amendment but was recommended.
Amnioinfusion were performed only by fetal medicine specialists who had expertise in invasive procedures. The protocol for the method of AI is given in Appendix 2. All AI were performed under ultrasound guidance. All study sites were given a copy of the protocol for AI to ensure consistency of the procedure.
The full calculated volume of Hartmann’s solution or normal saline for the pregnancy (10 ml per week of gestation) was always infused. This ensured an adequate amount of fluid replacement to account for immediate leakage through the rupture. AI was ceased if the specialist had concerns about continuing the procedure. Possible reasons for this would have been uncertainty about being in the right space or if uterine contractions began. Antibiotics were not given specifically for the AI procedure. All participants were treated with oral erythromycin for 10 days after diagnosis of PPROM. Tocolysis was not required for AI and the procedures were performed as outpatient procedures. Participants were admitted following the procedure if it was felt necessary to do so by the specialist who performed the procedure. The post AI deepest pool of amniotic fluid was measured after the full calculated volume for gestation was amnioinfused. Participants were seen weekly and the AI repeated if the deepest pool of amniotic fluid remained at < 2 cm.
Participant follow-up
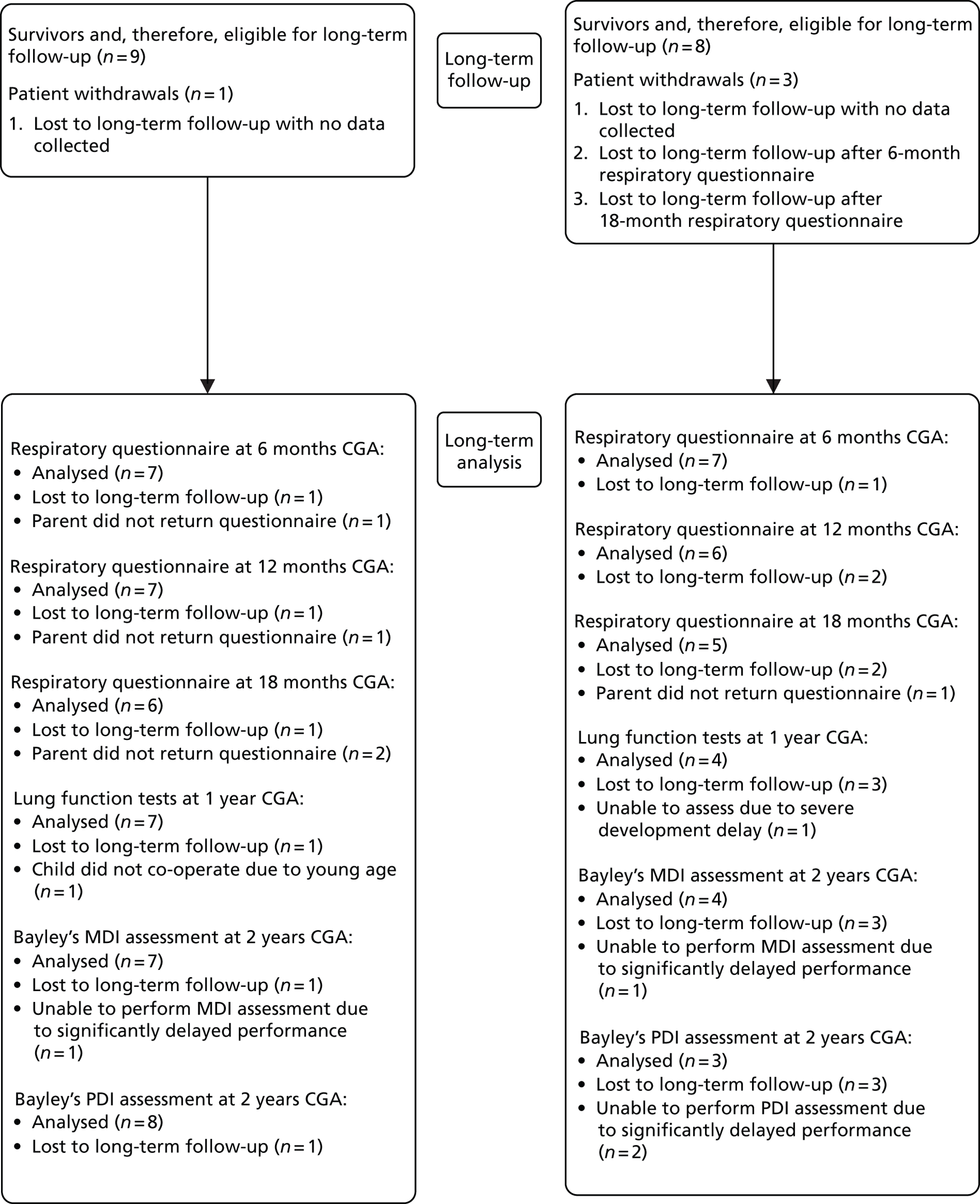
Figure 2 shows a summary of participant follow-up for the AMIPROM trial. Most participants were followed up in the FMUs, with a small proportion (four participants in Exp arm) followed up in their local units. This was mainly at the choice of the participant. Participants were sent paper respiratory questionnaires along with prepaid return envelopes by the trial co-ordinating centre at Liverpool Women’s NHS Foundation Trust. No incentives were given to increase the response rates to respiratory questionnaires. The Bayley’s assessments were performed in the homes of surviving children to increase response rate. The infant lung function tests were performed either at Leicester University Hospital or at Liverpool Women’s NHS Foundation Trust and participants were reimbursed for travel expenses to and from the Hospitals for the childhood follow-up part of the trial alone. Travel expenses were not reimbursed for weekly assessments at hospital or FMU as these were considered part of normal clinical care.
FIGURE 2.
Participant follow-up. TOP, termination of pregnancy.
Measurement of outcomes: short-term outcomes
Data were collected on five data sheets (see Appendix 1).
First visit post randomisation
Data sheet 1 was filled out by the specialist attending the participant on the day of randomisation. This was called the ‘first visit’ even though the participant may have attended the FMU previously for confirmation of the diagnosis and discussion about the study. Maternal parity, initial HVS, WCC, CRP and body temperature were recorded on data sheet 1, as well as whether the mother had a tender, irritable uterus or foul-smelling discharge. Other information recorded was the gestation at PPROM in weeks, the gestation at first AI in weeks, the deepest amniotic fluid pocket (before and after AI in the intervention arm), the thoracic circumference, lung length and abdominal circumference of the baby as measured using ultrasonography.
Subsequent visits
Measurements taken using ultrasonography of the baby’s thoracic circumference, the lung length, the abdominal circumference and the deepest amniotic fluid pocket (before and after AI in the AI arm) for subsequent visits were recorded on data sheet 2 by FMU staff.
Maternal outcomes
Maternal outcomes, including the result of maternal investigations, were recorded on data sheet 3. WCC and CRP measurements were performed weekly and HVS was performed at the discretion of the clinician attending the participant. HVS results were recorded whenever they were available and data sheet 3 was completed when the participant had delivered. Any missing data were reconciled by the chief investigator and trial administrator by contact with the PIs and examination of the hospital case notes.
The maternal and pregnancy outcomes recorded were antenatal corticosteroid prophylaxis, if the participant was given antibiotics, placental abruption, antepartum haemorrhage, chorioamnionitis, gestational age at delivery, mode of delivery, onset of labour, serious maternal sepsis requiring intensive therapy unit (ITU)/high-dependency unit (HDU) admission and maternal death.
Neonatal outcomes
Neonatal outcomes were recorded on data sheet 4. The neonatal outcomes recorded were gestational age at birth, birthweight, Apgar score at 5 minutes, cord blood gases, antepartum death, neonatal death, culture-positive sepsis, days on intermittent positive-pressure ventilation (IPPV), continuous positive airways pressure (CPAP) and high-frequency oscillatory ventilation (HFOV) (each analysed separately), pneumothorax requiring chest drain, discharge on home oxygen, O2 requirement at day 28, O2 requirement at week 36, necrotising enterocolitis (NEC) (including those who had surgery or were treated conservatively), treated seizures, treated retinopathy, IVH grade (0–3), periventricular leukomalacia (PVL), any shunting procedures and any fixed orthopaedic deformities.
The data sheet was completed when the baby was discharged home or after death. Any missing data were reconciled by the chief investigator and trial administrator by contact with the PIs and examination of the hospital case notes.
The data pack was returned to the trial co-ordination centre after the baby was discharged home or after death.
Measurement of outcomes: long-term outcomes
Respiratory questionnaires
Participants with surviving babies were sent a prepaid postal validated respiratory questionnaire at 6, 12 and 18 months after the birth of their baby. 15 These were sent out by the trial coordination centre at Liverpool Women’s NHS Foundation Trust and returned directly to the co-ordinating centre.
The respiratory questionnaire was designed to examine the frequency of mild respiratory symptoms such as wheezing in infants and preschool children. An abnormal score is described as one which falls within the confidence interval (CI) of children with asthma as defined by Powell et al. 15 We defined children with long-term mild respiratory symptoms as those at the 18-month questionnaire stage whose scores in any domain fell outside the 95% CI for asthma (Table 1).
| Score | 95% CI for children with a diagnosis of asthma |
|---|---|
| Daytime symptoms score | 21.7 to 43.5 |
| Night-time symptoms score | 6.4 to 10.8 |
| Impact on family score | 7.0 to 11.0 |
| Impact on child score | 5.5 to 9.2 |
Lung function tests
The protocol specified that surviving children had infant lung function tests performed when they were approaching 12 months’ gestational age. Lung function tests can be performed under sedation at this age. From the age of about 3 or 4 years, children can begin to do perform the blowing tests that older children can. Between these ages it is more difficult to perform these tests, for compliance reasons and, where possible, surviving children were invited to have the infant tests performed at Leicester Royal Infirmary. Where this was not possible, the simple blowing tests were performed at Liverpool Women’s NHS Foundation Trust.
The tests of lung function were chosen to detect small lung size. The most direct way of doing this is by whole-body plethysmography, which enables us to determine functional residual capacity (FRC). This test requires that the subject is enclosed within a Perspex chamber (that for older children or adults resembles a telephone kiosk) and breathes through an apparatus that measures the amount of air being breathed in or out. As the chest moves in and out, it causes small (but measurable) pressure changes in the Perspex chamber. Then, for a very short period of time, a shutter is transiently closed in the apparatus, so that the subject makes breathing efforts against this obstruction. This does not disturb the subject and, in the case of infant testing, does not last long enough to cause the sleeping infant to rouse. By measuring the pressure generated at the mouth when the shutter is closed, and relating this to the pressure changes in the chamber, it is possible to work out the size of the lungs. An alternative and indirect index of lung size is forced vital capacity (FVC), which is simply a measure of how much air can be breathed out between full inspiration to complete exhalation. The other measurements [forced expired volume in 1 second (FEV1) and maximum flow at FRC (VmaxFRC)] relate to airway function and give information relating to the dimensions and patency of the airways. Each measure of lung function was repeated at least three times to ensure reproducibility. For each test, predicted scores and z-values were calculated. A z-value < −2.00 is considered abnormal in any of the lung functions tested.
Neurological assessment
Developmental delay at 2 years corrected gestational age was assessed using the Bayley’s Scales of Infant Development-II (BSID-II). BSID-II is a standard series of measurements used primarily to assess the motor and cognitive development of infants and toddlers aged 0–3 years. This measure consists of a series of developmental play tasks. It takes between 45 and 60 minutes to administer, and raw scores of successfully completed items are converted to scale scores and to composite scores between 50 and 150 (mean score 100). These scores are used to determine the child’s performance compared with norms taken from typically developing children of their age (in months), e.g. going up the stairs unaided at 24 months.
The two scores reported in this trial are the Mental Development Index (MDI) and the Psychomotor Developmental Index (PDI). Their classifications are as follows:
-
A score of 50–69 suggests significantly delayed performance.
-
A score of 70–84 suggests mildly delayed performance.
-
A score of 85–114 is within normal limits.
-
A score of 115–150 suggests accelerated performance.
We defined major neurodevelopmental delay in any child as an MDI < 70 or a PDI < 70 or both MDI/PDI < 70. Mildly delayed performance was defined as a score of between 70 and 84 in any domain. 16
Neurodevelopmental assessments of the surviving children were performed in their own homes by a trained health professional. The protocol specified that the tests were to be performed at 24 months of age, corrected for prematurity. No monetary or other incentives were used to increase participation in the long-term outcome phase of the pilot. Participants were reimbursed their expenses for travelling to either Leicester or Liverpool for the infant lung function tests.
Trial completion
Recruitment and the final sample size was time limited as the study was funded until April 2009. The last woman was recruited to the study in April 2009. The last baby was assessed for long-term outcomes in July 2011.
Statistical analysis
Statistical analysis was performed by the Clinical Trials Research Centre, University of Liverpool. This pilot trial consists of both short-term outcomes of neonatal morbidity/mortality for the baby and maternal morbidities for the mother at birth and also various long-term developmental outcomes for the children assessed at 2 and 3 years corrected gestational age (CGA). The approach was first to write the short-term outcomes statistical analysis plan (SAP) (see Appendix 3) prior to completion of recruitment, then to perform the analyses once all the short-term outcome data had been received and then to present the results to the DMC. All outcomes were analysed using the intention-to-treat (ITT) principle. In the introduction of short-term outcomes SAP it stated that the DMC would give their recommendations to the Trial Steering Committee and they would decide whether to allow publication of the short-term outcome results. The short-term outcome results were presented to the DMC on 15 November 2011. The DMC agreed to unblinding of the short-term data to the trial team at this meeting so they could begin to write up the publication, but the publication should include the short-term and long-term outcome results. The DMC also requested that a per-protocol analysis be carried out on the short-term outcome data defined as mothers that had at least one AI or attended at least one hospital visit (Exp arm). The long-term outcomes SAP (see Appendix 4) was then written incorporating details of the per-protocol analysis that the DMC had requested. The statistical team made the decision not to do a per-protocol analysis for the long-term outcomes because so few participants were followed up as a result of all of the antenatal and neonatal deaths. Again, all outcomes were analysed using the ITT principle.
The statistical methods used are shown in Appendices 3 and 4. All of the statistical analyses for the trial results were carried out using SAS v.9.2 (SAS Institute Inc., Cary, NC, USA).
Missing data
Sensitivity analyses were performed to explore the effects of missing data on the long-term outcomes. These mostly considered the neonatal deaths and imputed on a worst-case scenario basis. Where other imputations were considered, these are described alongside the analyses.
Adverse events
All neonatal deaths were reported as adverse events on the Liverpool Women’s NHS Foundation Trust serious adverse event (SAE) reporting form (see Appendix 5). Suspected unexpected serious adverse reactions and all SAEs were reported to the PI or the Research and Development Department of the Liverpool Women’s NHS Foundation Trust.
Economic analysis
As this is a pilot study, no economic or cost-effectiveness analysis has been performed. It is envisaged that this will be performed if a larger, definitive trial is funded.
Chapter 3 Results (short-term outcomes)
Trial recruitment
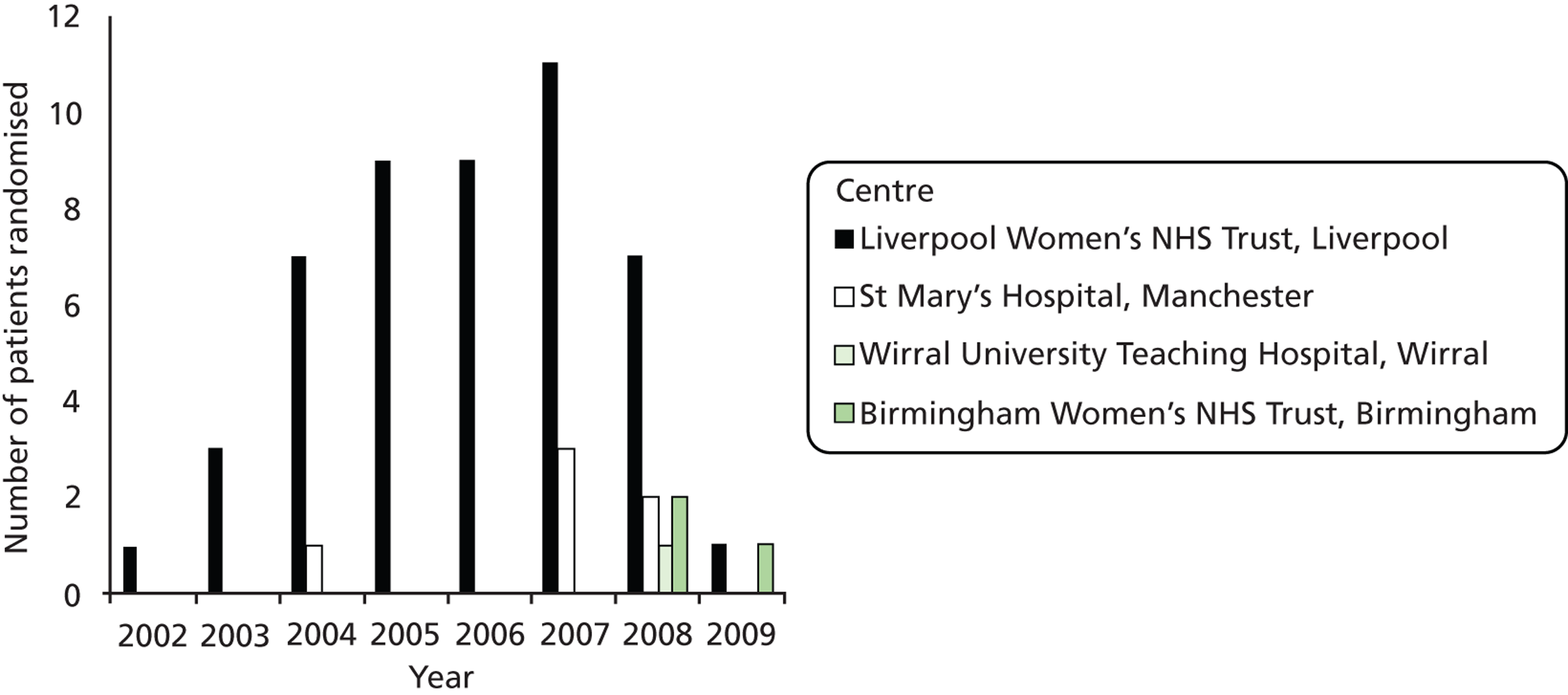
Recruitment began in September 2002 and ceased in April 2009. Centres were chosen for their ability to perform AI if required. There were initially five study sites proposed – Liverpool Women’s NHS Foundation Trust (chief investigator site and trial sponsor), St. Mary’s Hospital, Wirral University Teaching Hospital, Warrington Hospital and Queen Mother’s Glasgow. Owing to local research governance and funding issues, Queen Mother’s Glasgow was unable to formalise local ethics and recruit; therefore, it ceased to be a study site in 2006. Warrington Hospital preferred to refer to the tertiary referral unit rather than run the study locally and ceased to be a study site by 2006. Participants were recruited from Birmingham Women’s NHS Foundation Trust in 2008 following HTA programme funding approval.
Two sites were recruiting participants and submitting data by 2005 (Liverpool Women’s NHS Foundation Trust and St. Mary’s Hospital) and the other two were recruiting participants and submitting data by 2008 (Wirral University Teaching Hospital and Birmingham Women’s NHS Foundation Trust). The number of patients recruited per annum is shown in Table 2. The recruitment rate by each site is shown in Figure 3.
| Number randomised per annum by treatment arm | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 |
|---|---|---|---|---|---|---|---|---|
| Exp arm | 0 | 1 | 4 | 4 | 5 | 9 | 4 | 2 |
| AI arm | 1 | 2 | 4 | 5 | 4 | 5 | 8 | 0 |
| Overall | 1 | 3 | 8 | 9 | 9 | 14 | 12 | 2 |
FIGURE 3.
The number of patients randomised per centre, per annum.
In total, 81 women were screened as potential participants and 77 were eligible. The reasons why eligible participants did not enter the study are shown in Table 3 and Figure 1. Eleven women declined to participate in the study, seven miscarried in the 10 days after PPROM while considering the study and one decided too late (after 24 weeks) that she wanted to participate. This woman was not recruited, as she no longer met the criteria for inclusion to the study, i.e. between 16+0 and 24+0 weeks’ gestation.
| Reason for non-participation | Number of participants | Outcome of pregnancy |
|---|---|---|
| Eligible but declined | 11 | Termination of pregnancy (4) Miscarriage (2) Live birth with chronic lung disease (1) Neonatal death (3) No outcome data (1) |
| Eligible but miscarried before randomisation | 7 | Miscarriage (7) |
| Eligible but had exceeded 24 weeks’ gestation by the time decided to participate; too late to be randomised | 1 | Live birth (1) |
Baseline participant characteristics
Twenty-nine women were randomised to each group but one from each group was excluded post randomisation due to termination for fetal abnormality (renal agenesis in the AI arm and critical aortic stenosis in the Exp arm), leaving 28 in each arm for ITT analysis (see Figure 1).
The baseline characteristics are summarised by treatment arm in Table 4.
| Baseline characteristics | AI (participants randomised n = 28) | Exp (participants randomised n = 28) | Total (participants randomised n = 56) |
|---|---|---|---|
| Parity, n | (n = 26)a | (n = 28) | (n = 54) |
| 0 | 16 | 11 | 27 |
| 1 | 4 | 11 | 15 |
| 2 | 2 | 3 | 5 |
| 3 | 3 | 2 | 5 |
| 4 | 1 | 1 | 2 |
| HVS, n (%) | (n = 25)a [25 separate types]b | (n = 24)a [27 separate types]b | (n = 49)a [52 separate types]b |
| Bacterial vaginosis | 1 (4.0) | 2 (7.4) | 3 (5.8) |
| Coliform | 0 (0.0) | 1 (3.7) | 1 (1.9) |
| Enterococcus | 0 (0.0) | 1 (3.7) | 1 (1.9) |
| B Streptococcus | 0 (0.0) | 1 (3.7) | 1 (1.9) |
| Mixed anaerobes | 1 (4.0) | 1 (3.7) | 2 (3.8) |
| None | 1 (4.0) | 1 (3.7) | 2 (3.8) |
| Normal flora | 20 (80.0) | 16 (59.3) | 36 (69.1) |
| Staphylococcus aureus | 1 (4.0) | 0 (0.0) | 1 (1.9) |
| Streptococcus | 0 (0.0) | 2 (7.4) | 2 (3.8) |
| Yeast | 1 (4.0) | 2 (7.4) | 3 (5.8) |
| WCC (109/l) | (n = 26)a | (n = 26)a | (n = 52) |
| Mean (SD) | 10.74 (± 2.71) | 11.51 (± 2.28) | 11.13 (± 2.51) |
| Range | 5.8–18.6 | 7.1–17.6 | 5.8–18.6 |
| CRP (mg/l) | (n = 25)a | (n = 25)a | (n = 50)a |
| Median (IQR) | 5 (5–6) | 7 (5–16) | 6 (5–10) |
| Range | 2–22 | 3–44 | 2–44 |
| Temperature (°C) | (n = 23)a | (n = 19)a | (n = 42)a |
| Mean (SD) | 36.80 (± 0.34) | 36.93 (± 0.22) | 36.86 (± 0.29) |
| Range | 36.0–37.2 | 36.4–37.3 | 36.0–37.3 |
| Tender, irritable uterus, n (%) | (n = 26)a | (n = 28)a | (n = 54)a |
| Yes | 1 (3.8) | 0 (0) | 1 (1.9) |
| Foul-smelling discharge, n (%) | (n = 26)a | (n = 28)a | (n = 54)a |
| Yes | 0 (0) | 0 (0) | 0 (0) |
| Weeks’ gestation at PPROM | (n = 24)c | (n = 28) | (n = 52) |
| Mean (SD) | 19.21 (± 2.00) | 19.22 (± 2.21) | 19.22 (± 2.10) |
| Range | 16.0–22.6 | 15.1–23.3 | 15.1–23.3 |
| Weeks’ gestation at randomisation | (n = 28) | (n = 28) | (n = 56) |
| Mean (SD) | 21.36 (± 1.75) | 21.14 (± 2.00) | 21.25 (± 1.87) |
| Range | 17.7–25.4 | 7.4–24.7 | 17.4–25.4 |
| Maternal age at randomisation (years) | (n = 28) | (n = 28) | (n = 56) |
| Mean (SD) | 27.46 (± 5.88) | 28.30 (± 6.45) | 27.88 (± 6.13) |
| Range | 17.0–39.3 | 17.7–42.8 | 17.0–42.8 |
| Vaginal bleeding, n (%) | (n = 28) | (n = 28) | (n = 56) |
| Yes | 7 (25.0) | 11 (39.3) | 18 (32.1) |
| Thoracic circumference (mm) | (n = 23) | (n = 22) | (n = 45) |
| Mean (SD) | 146.84 (± 26.30) | 135.47 (± 25.88) | 141.28 (± 26.43) |
| Range | 105.0–238.2 | 89.5–202.2 | 89.5–238.2 |
| Abdominal circumference (mm) | (n = 24) | (n = 26) | (n = 50) |
| Mean (SD) | 166.43 (± 28.21) | 162.44 (± 24.11) | 164.35 (± 25.96) |
| Range | 105.0–218.0 | 117.8–198.0 | 105.0–218.0 |
| Lung length (mm) | (n = 21) | (n = 23) | (n = 44) |
| Mean (SD) | 23.34 (± 6.31) | 23.86 (± 5.30) | 23.61 (± 5.74) |
| Range | 15.0–45.0 | 12.0–34.6 | 12.0–45.0 |
Both arms are well balanced for possible confounders. There was no apparent difference in the mean WCC, temperature, weeks gestation at rupture of the amniotic membrane, weeks gestation at randomisation or maternal age at randomisation between arms. There was no apparent difference in the median CRP between the arms.
Antenatal course
The antenatal management of all participants in the trial followed the same pathway from diagnosis until randomisation to the trial. All women had a HVS taken and were given 250 mg oral erythromycin four times a day for 10 days following confirmation of rupture of amniotic membrane. As a result, the most commonly used antibiotic in the antenatal period was erythromycin.
Participants attended for their first fetal medicine assessment at the earliest convenient time, but were randomised to the study at least 10 days after the amniotic membrane ruptured. This criterion was adopted following discussions at an international fetal medicine meeting. 14 The international consensus at the time was that the risk of miscarriage in the first week after rupture was too high. In our cohort, seven of the 81 women (8.6%) miscarried before they could be randomised to the study (see Table 3).
Of the 29 women allocated to AI, 22 received the intervention, one had a termination of pregnancy, one declined AI after randomisation and four maintained a deepest pool level of approximately 2 cm throughout. No woman in the Exp arm received AI. One baby in each arm was found to have a fetal abnormality with an impact on neonatal survival (Figure 1).
Women were seen weekly for an ultrasonography assessment irrespective of the arm they were randomised to. The median number of antenatal visits prior to delivery was 5 (range 0–15) in the AI arm and 4.5 (range 1–14) in the Exp arm (Table 5). The median number of AI performed was 3 (range 0–12; Table 6).
| Number of visits | AI | Exp |
|---|---|---|
| n Median [Q1, Q3] Range |
(n = 28) 5 [2.5, 8.5] 0–15 |
(n = 28) 4.5 [2.0, 8.5] 1–14 |
| 0 | 2 | 0 |
| 1 | 2 | 5 |
| 2 | 3 | 5 |
| 3 | 2 | 3 |
| 4 | 3 | 1 |
| 5 | 4 | 2 |
| 6 | 1 | 4 |
| 7 | 3 | 0 |
| 8 | 1 | 1 |
| 9 | 3 | 2 |
| 10 | 0 | 2 |
| 11 | 2 | 2 |
| 12 | 1 | 0 |
| 13 | 0 | 0 |
| 14 | 0 | 1 |
| 15 | 1 | 0 |
| Number of AI | AI | Exp |
|---|---|---|
| n Median [Q1, Q3] Range |
(n = 28) 3 [1, 4] 0 to 12 |
(n = 28) 0 |
| 0 | 6a | 0 |
| 1 | 2 | 0 |
| 2 | 5 | 0 |
| 3 | 4 | 0 |
| 4 | 5 | 0 |
| 5 | 3 | 0 |
| 6 | 1 | 0 |
| 7 | 0 | 0 |
| 8 | 0 | 0 |
| 9 | 0 | 0 |
| 10 | 1 | 0 |
| 11 | 0 | 0 |
| 12 | 1 | 0 |
Table 7 shows that the volume of Hartmann’s solution infused (10 ml per week of gestation) was sufficient to produce an average amniotic fluid pocket difference of 2.66 cm, which is considered adequate to improve the risk of pulmonary hypoplasia. Three women had amniotic fluid pocket sizes of < 2 cm after AI because of amniotic fluid leakage as the procedure was taking place. For two of these women, AI improved the deepest pool from 0 to 1.9 cm and 1.0 cm, respectively. In one woman, there was no change in the deepest pool of amniotic fluid after AI.
| Women with at least one AI (n = 22) | n (%) |
| Fluid instilled on at least one occasion (n = 26a) | |
| Yes | 22 (84.62%) |
| Amniotic fluid pocket difference [after minus before (cm)] for those patients that had fluid instilled at visit (n = 78b) | |
| No. of visits | |
| Mean (SD) | 2.66 (1.33) |
| Range | 0.0–7.0 |
| Amniotic fluid pocket size at visit for patients with no fluid instilled (n = 65) | |
| No. of visits | |
| Mean (SD) | 2.73 (0.73) |
| Range | 1.2c–4.6 |
Not all participants in the AI arm required AI at every visit as it was performed only if the deepest pool of amniotic fluid was < 2 cm. Sixteen women had no fluid instilled on at least one visit and, for those visits in which no AI was performed, the mean pool depth was 2.73 cm.
The risks to the mother in the antenatal period are mainly of abruption, bleeding or infection. There was no difference in the arms for any of these outcomes (Tables 8 and 9).
| Maternal morbidity outcome in the antenatal period | AI (n = 28a) | Exp (n = 28) | RR (95% CI) (n = 56a) |
|---|---|---|---|
| Abruption of the placenta | (n = 27) | (n = 28) | – |
| n (%) | 4 (14.8) | 0 (0.0) | – |
| RR (95% CI) | – | – | 9.32 (0.53 to 165.26) |
| Antepartum haemorrhage | (n = 27) | (n = 28) | – |
| n (%) | 8 (29.6) | 7 (25.0) | – |
| RR (95% CI) | – | – | 1.19 (0.50 to 2.82) |
| Chorioamnionitis | (n = 27) | (n = 28) | – |
| n (%) | 4 (14.8) | 7 (25.0) | – |
| RR (95% CI) | – | – | 0.59 (0.20 to 1.80) |
| Required antibiotics antenatally | (n = 27) | (n = 28) | – |
| n (%) | 22 (81.5) | 22 (78.6) | – |
| RR (95% CI) | – | – | 1.04 (0.80 to 1.35) |
| Number of doses of steroids, n (%) | (n = 27) | (n = 28) | – |
| 0 | 8 (29.6) | 13 (46.4) | – |
| 1 | 3 (11.1) | 3 (10.7) | – |
| 2 | 15 (55.6) | 11 (39.3) | – |
| 3 | 0 (0.0) | 0 (0.0) | – |
| 4 | 1b (3.7) | 1b (3.6) | – |
| Chi-squared test for trend p-value | – | – | 0.25c |
| Maternal morbidity outcome | AI (n = 22a) | Exp (n = 25b) | RR (95% CI) (n = 47) |
|---|---|---|---|
| Abruption of the placenta | (n = 22) | (n = 25) | – |
| n (%) | 3 (13.6) | 0 (0.0) | – |
| RR (95% CI) | – | – | 7.91 (0.43 to 145.20) |
| Antepartum haemorrhage | (n = 22) | (n = 25) | – |
| n (%) | 7 (31.8) | 5 (20.0) | – |
| RR (95% CI) | – | – | 1.59 (0.59 to 4.30) |
| Chorioamnionitis | (n = 22) | (n = 25) | – |
| n (%) | 4 (18.2) | 6 (24.0) | – |
| RR (95% CI) | – | – | 0.76 (0.25 to 2.34) |
| Required antibiotics antenatally | (n = 22) | (n = 25) | – |
| n (%) | 18 (81.8) | 20 (80.0) | – |
| RR (95% CI) | – | – | 1.02 (0.77 to 1.35) |
| Number of doses of steroids, n (%) | (n = 22) | (n = 25) | |
| 0 | 8 (36.4) | 11 (44.0) | – |
| 1 | 3 (13.6) | 2 (8.0) | – |
| 2 | 11 (50.0) | 11 (44.0) | – |
| 3 | 0 (0.0) | 0 (0.0) | – |
| 4 | 0 (0.0) | 1c (4.0) | – |
| Chi-squared test for trend p-value | – | – | 0.96d |
The protocol required a single course (two doses) of antenatal corticosteroids to be given at 26+0 weeks’ gestation, or earlier if clinicians felt it was indicated. It is not routine practice to give an additional rescue course of steroids. One woman in the Exp arm was given a first course of corticosteroids before 26+0 weeks and a rescue course later in pregnancy (see Table 9). Those who did not receive any antenatal corticosteroids were women who delivered prior to achieving 26+0 weeks’ gestation.
Labour and delivery
Women in the AI arm went into spontaneous preterm labour at a median gestation of 28.45 weeks ± 4.44 standard deviation (SD) and those in the Exp arm at 29.82 weeks 4.33 SD (Table 10). The default mode of delivery was vaginal unless there was a clinical indication to deliver by caesarean section. The pregnancy outcomes are shown in Tables 11 and 12. Of 39 pregnancies aiming for vaginal delivery at the onset of labour, 34 delivered vaginally. There were more caesarean sections in the AI arm than in the Exp arm, but this difference was not statistically significant.
| Neonatal morbidity outcome | AI | Exp | Mean difference |
|---|---|---|---|
| Fetal deaths omitted (n = 23) | Fetal deaths omitted (n = 17) | Fetal deaths omitted (n = 40) | |
| Gestational age at delivery (weeks) | (n = 23) | (n = 17) | – |
| Mean (SD) | 28.45 (4.44) | 29.82 (4.33) | – |
| Range | 19.4–37.6 | 24.9–38.1 | – |
| Mean difference (SD) | – | – | −1.36 (4.40) |
| 95% CI | – | – | −4.21 to 1.48 |
| Birthweight (kg) | (n = 23) | (n = 17) | – |
| Mean (SD) | 1.18 (0.62) | 1.46 (0.67) | – |
| Range | 0.2–3.0 | 0.7–3.1 | – |
| Mean difference (SD) | – | – | −0.28 (0.64) |
| 95% CI | – | – | −0.69 to 0.14 |
| Apgar score at 1 minute | (n = 21) | (n = 16) | – |
| Mean (SD) | 4.38 (2.78) | 5.25 (2.74) | – |
| Range | 1–10 | 0–9 | – |
| Mean difference (SD) | – | – | −0.87 (2.77) |
| 95% CI | – | – | −2.73 to 0.99 |
| Apgar score at 5 minutes | (n = 21) | (n = 16) | – |
| Mean (SD) | 6.86 (2.78) | 7.00 (2.31) | – |
| Range | 1–10 | 2–10 | – |
| Mean difference (SD) | – | – | −0.14 (2.59) |
| 95% CI | – | – | −1.89 to 1.60 |
| Cord pH | (n = 15) | (n = 8) | – |
| Mean (SD) | 7.26 (0.15) | 7.10 (0.46) | – |
| Range | 6.8–7.4 | 6.0–7.4 | – |
| Mean difference (SD) | – | – | 0.16 (0.29) |
| 95% CI | – | – | −0.10 to 0.43 |
| Base excess | (n = 12) | (n = 5) | – |
| Mean (SD) | 1.78 (8.42) | −1.18 (6.28) | – |
| Range | −8.5 to 18.8 | −9.3–6.8 | – |
| Mean difference (SD) | – | – | 2.96 (7.91) |
| 95% CI | – | – | −6.01 to 11.94 |
| Lactate | (n = 0) | (n = 1) | – |
| Mean (SD) | – | 4.8 | – |
| Range | – | – | – |
| Mean difference (SD) | – | – | – |
| 95% CI | – | – | – |
| Sex, n male (%) | (n = 26) | (n = 25) | – |
| ITT | 17 (65.4) | 15 (60.0) | – |
| Sex, n male (%) | (n = 21) | (n = 24) | – |
| Per protocol | 14 (66.7) | 15 (62.5) | – |
| Pregnancy outcome | AI (n = 28a) | Exp (n = 28) | Chi-squared test p-value (n = 56a) |
|---|---|---|---|
| Onset of labour, n (%) | (n = 28) | (n = 28) | – |
| Induced | 4 (14.2) | 5 (17.9) | – |
| Spontaneous | 12 (42.9) | 18 (64.2) | – |
| Caesarean section | 12 (42.9) | 5 (17.9) | – |
| Chi-squared test p-value | – | – | 0.12b |
| Mode of delivery, n (%) | (n = 28) | (n = 28) | – |
| Normal | 12 (42.9) | 20 (71.4) | – |
| Instrumental | 1 (3.5) | 1 (3.6) | – |
| Emergency LSCS | 12 (42.9) | 7 (25.0) | – |
| Elective LSCS | 3 (10.7) | 0 (0.0) | – |
| Chi-squared test p-value | – | – | 0.10b |
| Reason for delivery of fetus | (n = 27) | (n = 27) | – |
| APH | 2 (7.4) | 1 (3.7) | – |
| APH/abnormal cardiotocography | 1 (3.7) | 0 (0.0) | – |
| Placental abruption | 3 (11.1) | 0 (0.0) | – |
| Cord prolapse | 2 (7.4) | 2 (7.4) | – |
| Elective LSCS | 1 (3.7) | 1 (3.7) | – |
| Emergency caesarean section | 0 (0.0) | 2 (7.4) | – |
| Fetal death in utero | 1 (3.7) | 2 (7.4) | – |
| Fetal distress | 1 (3.7) | 1 (3.7) | – |
| Induction of labour | 2 (7.4) | 1 (3.7) | – |
| Spontaneous labour | 14 (51.9) | 11 (40.8) | – |
| Spontaneous miscarriage | 0 (0.0) | 6 (22.2) | – |
| Pregnancy outcome | AI (n = 22a) | Exp (n = 25b) | Chi-squared test p-value (n = 47) |
|---|---|---|---|
| Onset of labour, n (%) | (n = 22) | (n = 25) | – |
| Induced | 3 (13.6) | 4 (16.0) | – |
| Spontaneous | 9 (40.9) | 16 (64.0) | – |
| N/A (caesarean section) | 10 (45.5) | 5 (20.0) | – |
| Chi-squared test p-value | – | – | 0.17b |
| Mode of delivery, n (%) | (n = 22) | (n = 25) | – |
| Normal | 12 (54.5) | 18 (72.0) | – |
| Instrumental | 0 (0.0) | 0 (0.0) | – |
| Emergency LSCS | 9 (40.9) | 7 (28.0) | – |
| Elective LSCS | 1 (4.6) | 0 (0.0) | – |
| Chi-squared test p-value | – | – | 0.32c |
| Reason for delivery of fetus, n (%) | (n = 22) | (n = 25) | – |
| APH | 2 (9.0) | 1 (4.0) | – |
| APH/abnormal cardiotocography | 1 (4.6) | 0 (0.0) | – |
| Abruption | 3 (13.6) | 0 (0.0) | – |
| Cord prolapse | 2 (9.0) | 2 (8.0) | – |
| Elective LSCS | 0 (0.0) | 1 (4.0) | – |
| Emergency caesarean section | 0 (0.0) | 2 (8.0) | – |
| Fetal death in utero | 1 (4.6) | 2 (8.0) | – |
| Fetal distress | 1 (4.6) | 1 (4.0) | – |
| Induction of labour | 1 (4.6) | 1 (4.0) | – |
| Spontaneous labour | 11 (50.0) | 10 (40.0) | – |
| Spontaneous miscarriage | 0 (0.0) | 5 (20.0) | – |
Perinatal outcomes
Fourteen out of 81 women who could potentially have been recruited to the study had a miscarriage, giving an overall miscarriage rate of 17% (see Tables 3, 11 and 12 and Figure 1).
The overall perinatal survival in both arms was 17 out of 56 (30.4%) and the overall perinatal mortality was 39 out of 56 (69.6%) (Tables 13 and 14). Four antepartum deaths were secondary to cord prolapse, two in each arm. Neonatal deaths were attributable to extreme prematurity and/or small lungs and not oxygenating despite maximum ventilation. Further details about the perinatal deaths can be seen in Serious adverse events. All SAEs had a severity of ‘death’.
| Outcome | AI (n = 28a) | Exp (n = 28) | RR (95% CI) (n = 56a) |
|---|---|---|---|
| Fetal death, n | 5 | 11 | 0.4545 (0.1815 to 1.1386) |
| Neonatal and fetal death, n | 19 | 19 | 1.0000 (0.6973 to 1.4341) |
| Infant, neonatal and fetal death, n | 19 | 20 | 0.9500 (0.6720 to 1.3430) |
| Outcome | AI (n = 22a) | Exp (n = 25b) | RR (95% CI) (n = 47) |
|---|---|---|---|
| Fetal death, n | 4 | 9 | 0.5051 (0.1805 to 1.4133) |
| Neonatal and fetal death, n | 17 | 16 | 1.2074 (0.8330 to 1.7501) |
| Infant, neonatal and fetal death, n | 17 | 17 | 1.1364 (0.7995 to 1.6153) |
There was no significant difference in mean gestational age at delivery between the AI and Exp arms (28.45 weeks vs. 29.82 weeks; mean difference −1.36, 95% CI −4.21 to 1.48) or Apgar score at 5 minutes (6.86 vs. 7.00; mean difference SD −0.14, 95% CI −1.89 to 1.60). Birthweight in the Exp arm was, however, slightly higher (1.18 kg vs. 1.46 kg; mean difference SD −0.28, 95% CI −0.69 to 0.14) and cord pH was noted to be higher in the AI arm (7.26 vs. 7.10; mean difference SD 0.16, 95% CI −0.10 to 0.43) (see Table 10).
After removing fetal deaths, there were 23 patients in the AI arm and 17 in the Exp arm. Any neonatal morbidity outcome results with numbers lower than this are a result of missing patient data.
There was no difference between the arms in the overall risk of any serious neonatal morbidity by ITT [23/28 vs. 25/28; relative risk (RR) 0.92, 95% CI 0.74 to 1.14] (Tables 15 and 16), or in any morbidity at birth or some time after birth (Tables 17 and 18).
| Outcome | AI (n = 28) | Exp (n = 28) | RR (n = 56b) |
|---|---|---|---|
| Death or serious neonatal morbidity, n (%) | (n = 28) | (n = 28) | – |
| Yes | 23 (82.1) | 25 (89.3) | – |
| RR (95% CI) | – | – | 0.9200 (0.7419 to 1.1408) |
| Outcome | AI (n = 22b) | Exp (n = 25c) | RR (n = 47) |
|---|---|---|---|
| Death or serious neonatal morbidity, n (%) | (n = 22) | (n = 25) | – |
| Yes | 20 (90.9) | 22 (88.0) | – |
| RR (95% CI) | – | – | 1.0331 (0.8492 to 1.2567) |
| Neonatal morbidity outcome | AI | Exp | RR | |||
|---|---|---|---|---|---|---|
| All patients with data (n = 28a,b) | Fetal deaths omitted (n = 24a) | All patients with data (n = 28c) | Fetal deaths omitted (n = 17) | All patients with data (n = 56a) | Fetal deaths omitted (n = 41a) | |
| Culture-positive sepsis, n (%) | (n = 27) | (n = 23) | (n = 28) | (n = 17) | – | – |
| Yes | 5 (18.5) | 5 (21.7) | 7 (25.0) | 7 (41.2) | – | – |
| RR (95% CI) | – | – | – | – | 0.74 (0.27 to 2.05) | 0.53 (0.20 to 1.38) |
| Pneumothorax, n (%) | (n = 27) | (n = 23) | (n = 28) | (n = 17) | – | – |
| Yes | 3 (11.1) | 3 (13.0) | 3 (10.7) | 3 (17.7) | – | – |
| RR (95% CI) | – | – | – | – | 1.04 (0.23 to 4.70) | 0.74 (0.17 to 3.22) |
| NEC (operated), n (%) | (n = 27) | (n = 23) | (n = 28) | (n = 17) | – | – |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | – |
| RR (95% CI) | – | – | – | – | N/A as no events in either arm | N/A as no events in either arm |
| NEC (treated conservatively), n (%) | (n = 27) | (n = 23) | (n = 28) | (n = 17) | – | – |
| Yes | 2 (7.4) | 2 (8.7) | 1 (3.6) | 1 (5.9) | – | – |
| RR (95% CI) | – | – | – | – | 2.07 (0.20 to 21.56) | 1.48 (0.15 to 15.00) |
| Treated seizures, n (%) | (n = 27) | (n = 23) | (n = 28) | (n = 17) | – | – |
| Yes | 1 (3.7) | 1 (4.4) | 1 (3.6) | 1 (5.9) | – | – |
| RR (95% CI) | – | – | – | – | 1.04 (0.07 to 15.76) | 0.74 (0.05 to 11.00) |
| Treated retinopathy, n (%) | (n = 27) | (n = 23) | (n = 28) | (n = 17) | – | – |
| Yes | 1 (3.7) | 1 (4.4) | 0 (0.0) | 0 (0.0) | – | – |
| RR (95% CI) | – | – | – | – | 3.11 (0.13 to 73.11) | 2.25 (0.10 to 52.07) |
| PVL, n (%) | (n = 27) | (n = 23) | (n = 28) | (n = 17) | – | – |
| Yes | 0 (0.0) | 0 (0.0) | 1 (3.6) | 1 (5.9) | – | – |
| RR (95% CI) | – | – | – | – | 0.35 (0.01 to 8.12) | 0.25 (0.01 to 5.79) |
| Shunt, n (%) | (n = 27) | (n = 23) | (n = 28) | (n = 17) | – | – |
| Yes | 1 (3.7) | 1 (4.4) | 0 (0.0) | 0 (0.0) | – | – |
| RR (95% CI) | – | – | – | – | 3.11 (0.13 to 73.11) | 2.25 (0.10 to 52.07) |
| Home O2,d n (%) | (n = 27) | (n = 9) | (n = 28) | (n = 9) | – | – |
| Yes | 2 (7.4) | 2 (22.2) | 3 (10.7) | 3 (33.3) | – | – |
| RR (95% CI) | – | – | – | – | 0.69 (0.13 to 3.82) | 0.67 (0.14 to 3.09) |
| O2 requirement at day 28,d n (%) | (n = 27) | (n = 9) | (n = 28) | (n = 9) | – | – |
| Yes | 3 (11.1) | 3 (33.3) | 4 (14.3) | 3 (33.3) | – | – |
| RR (95% CI) | – | – | – | – | 0.78 (0.19 to 3.16) | 1.00 (0.27 to 3.69) |
| Neonatal morbidity outcome | AI | Exp | RR | |||
|---|---|---|---|---|---|---|
| All patients with data (n = 22a) | Fetal deaths omitted (n = 18) | All patients with data (n = 25b) | Fetal deaths omitted (n = 16) | All patients with data (n = 47) | Fetal deaths omitted (n = 34) | |
| Culture-positive sepsis, n (%) | (n = 22) | (n = 18) | (n = 25) | (n = 16) | – | – |
| Yes | 4 (18.2) | 4 (22.2) | 7 (28.0) | 7 (43.8) | – | – |
| RR (95% CI) | – | – | – | – | 0.65 (0.22 to 1.92) | 0.51 (0.18 to 1.42) |
| Pneumothorax, n (%) | (n = 22) | (n = 18) | (n = 25) | (n = 16) | – | – |
| Yes | 3 (13.6) | 3 (16.7) | 2 (8.0) | 2 (12.5) | – | – |
| RR (95% CI) | – | – | – | – | 1.70 (0.31 to 9.28) | 1.33 (0.25 to 7.00) |
| NEC (operated), n (%) | (n = 22) | (n = 18) | (n = 25) | (n = 16) | – | – |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | – |
| RR (95% CI) | – | – | – | – | N/A as no events in either arm | N/A as no events in either arm |
| NEC (treated conservatively), n (%) | (n = 22) | (n = 18) | (n = 25) | (n = 16) | – | – |
| Yes | 2 (9.1) | 2 (11.1) | 1 (4.0) | 1 (6.3) | – | – |
| RR (95% CI) | – | – | – | – | 2.27 (0.22 to 23.38) | 1.78 (0.18 to 17.80) |
| Treated seizures, n (%) | (n = 22) | (n = 18) | (n = 25) | (n = 16) | – | – |
| Yes | 0 (0.0) | 0 (0.0) | 1 (4.0) | 1 (6.3) | – | – |
| RR (95% CI) | – | – | – | – | 0.38 (0.02 to 8.80) | 0.30 (0.01 to 6.84) |
| Treated retinopathy, n (%) | (n = 22) | (n = 18) | (n = 25) | (n = 16) | – | – |
| Yes | 1 (4.6) | 1 (5.6) | 0 (0.0) | 0 (0.0) | – | – |
| RR (95% CI) | – | – | – | – | 3.39 (0.15 to 79.22) | 2.68 (0.12 to 61.58) |
| PVL, n (%) | (n = 22) | (n = 18) | (n = 25) | (n = 16) | – | – |
| Yes | 0 (0.0) | 0 (0.0) | 1 (4.0) | 1 (6.3) | – | – |
| RR (95% CI) | – | – | – | – | 0.38 (0.02 to 8.80) | 0.30 (0.01 to 6.84) |
| Shunt, n (%) | (n = 22) | (n = 18) | (n = 25) | (n = 16) | – | – |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | – |
| RR (95% CI) | – | – | – | – | N/A as no events in either arm | N/A as no events in either arm |
| Home O2,c n (%) | (n = 22) | (n = 5) | (n = 25) | (n = 9) | – | – |
| Yes | 2 (9.1) | 2 (40.0) | 3 (12.0) | 3 (33.3) | – | – |
| RR (95% CI) | – | – | – | – | 0.76 (0.14 to 4.13) | 1.20 (0.29 to 4.95) |
| O2 requirement at day 28,c n (%) | (n = 22) | (n = 5) | (n = 25) | (n = 9) | – | – |
| Yes | 3 (13.6) | 3 (60.0) | 4 (16.0) | 3 (33.3) | – | – |
| RR (95% CI) | – | – | – | – | 0.85 (0.21 to 3.40) | 1.80 (0.19 to 1.93) |
The data presented in Tables 15 and 16 are indicative of the overall morbidity and death in the cohort. Although outcomes such as culture-positive sepsis, pneumothorax, O2 requirement at day 28, NEC, seizures, retinopathy, PVL, shunt and IVH 3 or 4 have been described in the analysis of the short-term outcomes, the sequelae of these morbidities are assessed in terms of their impact on long-term outcomes, i.e. blindness, long-term respiratory morbidity as assessed by infant lung function tests and neurodevelopmental delay as assessed by BSID-II (see Chapter 4).
There was no difference between arms in O2 requirement at day 28 (Tables 17 and 18).
The incidence of IVH grades 2 and 3 (two from the AI arm vs. four from the Exp arm) and postural orthopaedic deformities (one from the AI arm vs. two from the Exp arm) were similar in both arms. The numbers are too small to conclude any significant differences. This would require a larger study. There were no incidences of fixed orthopaedic deformities (Tables 19 and 20). The number of days a patient spent on ventilation and the number of days that a patient required O2 are shown in Tables 21 and 22, respectively.
| Neonatal morbidity outcome | AI (n = 28) | Exp (n = 28) | Chi-squared test p-value (n = 56) |
|---|---|---|---|
| IVH grade, n (%) | (n = 27) | (n = 28) | – |
| No IVH | 25 (92.6) | 24 (85.7) | – |
| Grade 1 | 0 (0.0) | 0 (0.0) | – |
| Grade 2 | 1 (3.7) | 1 (3.6) | – |
| Grade 3 | 1 (3.7) | 3 (10.7) | – |
| Chi-squared test for trend p-value | – | – | 0.34a |
| Orthopaedic deformities, n (%) | (n = 27) | (n = 28) | – |
| None | 26 (96.3) | 26 (92.9) | – |
| Fixed | 0 (0.0) | 0 (0.0) | – |
| Postural | 1b (3.7) | 2c (7.1) | – |
| Chi-squared test p-value | – | – | 0.57a |
| Neonatal morbidity outcome | AI (n = 22a) | Exp (n = 25b) | Chi-squared test p-value (n = 47) |
|---|---|---|---|
| IVH grade, n (%) | (n = 22) | (n = 25) | – |
| No IVH | 21 (95.5) | 21 (84.0) | – |
| Grade 1 | 0 (0.0) | 0 (0.0) | – |
| Grade 2 | 0 (0.0) | 1 (4.0) | – |
| Grade 3 | 1 (4.5) | 3 (12.0) | – |
| Chi-squared test for trend p-value | – | – | 0.12a |
| Orthopaedic deformities, n (%) | (n = 22) | (n = 25) | – |
| None | 21 (95.5) | 23 (92.0) | – |
| Fixed | 0 (0.0) | 0 (0.0) | – |
| Postural | 1 (4.5) | 2 (8.0) | – |
| Chi-squared test p-value | – | – | 0.63c |
| Analysis | AI | Exp |
|---|---|---|
| Fetal deaths excluded, neonatal deaths with maximum observed value in trial imputed (n = 23) | Fetal deaths excluded, neonatal deaths with maximum observed value in trial imputed (n = 17) | |
| Number of neonatal deaths | 14 | 8 |
| Days IPPV, n (%) | (n = 23) | (n = 17) |
| Yes | 10 (43.5) | 10 (58.8) |
| Median (IQR) | 69 (3–69) | 5 (2–69) |
| Range | 0–69 | 0–69 |
| Days CPAP, n (%) | (n = 23) | (n = 17) |
| Yes | 7 (30.4) | 5 (29.4) |
| Median (IQR) | 35 (2–35) | 23 (1–35) |
| Range | 0–35 | 0–35 |
| Days HFOV, n (%) | (n = 23) | (n = 17) |
| Yes | 3 (13.0) | 2 (11.8) |
| Median (IQR) | 4 (0–4) | 2 (0–4) |
| Range | 0–4 | 0–4 |
| Outcome | AI | Exp |
|---|---|---|
| Fetal deaths excluded, neonatal deaths with maximum observed value in trial imputed (n = 23) | Fetal deaths excluded, neonatal deaths with maximum observed value in trial imputed (n = 17) | |
| Number of neonatal deaths | 14 | 8 |
| Days on O2, n (%) | (n = 23) | (n = 15a) |
| Median (IQR) | 28 (24–28) | 28 (5–28) |
| Range | 0–28 | 0–28 |
Postnatal maternal outcomes
Coamoxiclav, cephalosporins and metronidazole were most commonly used postnatally. One woman in the Exp arm had serious maternal sepsis requiring admission to ITU/HDU (Tables 23 and 24). There were no maternal deaths.
| Maternal morbidity outcome | AI (n = 28a) | Exp (n = 28) | RR (n = 56a) |
|---|---|---|---|
| Required antibiotics postnatally | (n = 27) | (n = 28) | – |
| n (%), yes | 6 (22.2) | 8 (28.6) | – |
| RR (95% CI) | – | – | 0.78 (0.31 to 1.95) |
| Serious maternal sepsis requiring ITU/HDU admission | |||
| ITT | (n = 27) | (n = 28) | – |
| n (%), yes | 0 (0.0) | 1 (3.6) | – |
| RR (95% CI) | – | – | 0.35 (0.01 to 8.12) |
| Maternal death | 0/28 | 0/28 | N/A |
| Maternal morbidity outcome | AI (n = 22a) | Exp (n = 25b) | RR (n = 47) |
|---|---|---|---|
| Required antibiotics postnatally, n (%) | (n = 22) | (n = 25) | – |
| Yes | 5 (22.7) | 6 (24.0) | – |
| RR (95% CI) | – | – | 0.95 (0.33 to 2.68) |
| Serious maternal sepsis requiring ITU/HDU admission, n (%) | (n = 22) | (n = 25) | – |
| Yes | 0 (0.0) | 1 (4.0) | – |
| RR (95% CI) | – | – | 0.38 (0.02 to 8.80) |
| Maternal death | 0/22 | 0/25 | N/A |
Serious adverse events
All SAEs had a severity of ‘death’ (Tables 25 and 26).
| Event | Description | AI (n) | Exp (n) |
|---|---|---|---|
| Antepartum death | Antepartum death – no additional information | 1 | 1 |
| Cord prolapse | 2 | 1 | |
| Cord prolapse, stillbirth | 0 | 1 | |
| Miscarriage | 0 | 5 | |
| Spontaneous miscarriage | 0 | 2 | |
| Stillbirth | 1 | 1 | |
| Termination of pregnancy | 1 | 0 | |
| Total | 5 | 11 | |
| Neonatal death | Cord prolapse, emergency caesarean section | 0 | 1 |
| Extreme prematurity, pulmonary hypoplasia, placental abruption | 1 | 0 | |
| Fetal abnormalities undiagnosed prior to birth | 1 | 0 | |
| Neonatal death – no additional information | 5 | 1 | |
| Preterm birth and extreme prematurity | 3 | 4 | |
| Preterm birth and extreme prematurity, pulmonary hypoplasia | 2 | 1 | |
| Pulmonary hypoplasia | 1 | 0 | |
| Pulmonary hypoplasia, pulmonary stenosis and small right ventricle | 0 | 1 | |
| Pulmonary hypoplasia, renal agenesis | 1 | 0 | |
| Total | 14 | 8 | |
| Infant death | Chronic lung disease | 0 | 1 |
| Total | 0 | 1 | |
| Total SAEs | 19 | 20 | |
| Event | Description | AI (n) | Exp (n) |
|---|---|---|---|
| Antepartum death | Antepartum death – no additional information | 1 | 1 |
| Cord prolapse | 2 | 1 | |
| Cord prolapse, stillbirth | 0 | 0 | |
| Miscarriage | 0 | 4 | |
| Spontaneous miscarriage | 0 | 2 | |
| Stillbirth | 1 | 1 | |
| Termination of pregnancy | 0 | 0 | |
| Total | 4 | 9 | |
| Neonatal death | Cord prolapse, emergency caesarean section | 0 | 1 |
| Extreme prematurity, pulmonary hypoplasia, placental abruption | 1 | 0 | |
| Fetal abnormalities undiagnosed prior to birth | 1 | 0 | |
| Neonatal death – no additional information | 4 | 1 | |
| Preterm birth and extreme prematurity | 3 | 3 | |
| Preterm birth and extreme prematurity, pulmonary hypoplasia | 2 | 1 | |
| Pulmonary hypoplasia | 1 | 0 | |
| Pulmonary hypoplasia, pulmonary stenosis and small right ventricle | 0 | 1 | |
| Pulmonary hypoplasia, renal agenesis | 1 | 0 | |
| Total | 13 | 7 | |
| Infant death | Chronic lung disease | 0 | 1 |
| Total | 0 | 1 | |
| Total SAEs | 17 | 17 | |
Chapter 4 Results (long-term outcomes)
There were nine survivors in the AI arm and eight in the Exp arm. The numbers are too small to make meaningful comparisons. This is, however, the first time that long-term follow-up of respiratory and neurodevelopmental outcomes has been performed in survivors of very early prelabour rupture of the amniotic membranes.
Respiratory questionnaires
Respiratory questionnaires were sent out three times in the period of long-term outcome analysis: at 6 months, 12 months and 18 months. Table 27 shows the questionnaire status at each of the time points.
| Questionnaire status | Questionnaire time point | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | 18 months | ||||
| AI survivors (n = 9) | Exp survivors (n =8) | AI survivors (n = 9) | Exp survivors (n = 8) | AI survivors (n = 9) | Exp survivors (n = 8) | |
| Questionnaires returned (and analysed) | 7 | 7 | 7 | 6 | 6 | 5 |
| Lost to follow-up | 1 | 1 | 1 | 2 | 1 | 2 |
| Parent did not return questionnaire | 1 | 0 | 1 | 0 | 2 | 1 |
The respiratory questionnaire scores at each time point are summarised in Table 28 and the outcomes of the latest returned questionnaires are shown in Table 29. At 18 months, two children in the Exp arm and two children in the AI arm had scores within the CIs for asthma defined by Powell et al. 15 Additionally, three children in the Exp arm (patient numbers 22, 28, 31) and three patients in the AI arm (patient numbers 8, 11, 16) did not have outcome data available at 18 months.
| Domain | Questionnaire time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | 18 months | |||||||
| AI | Exp | Difference in medians (95% CI) | AI | Exp | Difference in medians (95% CI) | AI | Exp | Difference in medians (95% CI) | |
| Overall total | |||||||||
| Complete case | |||||||||
| n = 7 | n = 7 | n = 7 | n = 6 | n = 6 | n = 5 | ||||
| Median (IQR) | 16 (14–32) | 13.5 (7–35) | 2 (−31 to 24) | 18 (3–28) | 15.5 (6–29) | 1.5 (−26 to 23) | 10.5 (4–40) | 13 (4–42) | 0 (−44 to 40) |
| Range | 4–59 | 0–63 | 0–50 | 0–62 | 0–60 | ||||
| Sensitivity analysis, neonatal | |||||||||
| n = 21 | n = 15 | n = 21 | n = 14 | n = 20 | n = 13 | ||||
| Median (IQR) | 84 (32–84) | 0 (0 to 6) | 84 (28–84) | 84 (16–84) | 0 (0 to 34) | 84 (51–84) | 84 (42–84) | 0 (0 to 20) | |
| Range | 4–84 | 0–84 | 0–84 | 0–84 | 0–84 | ||||
| Daytime symptoms | |||||||||
| Complete case | |||||||||
| n = 7c | n = 6a | n = 7 | n = 6 | n = 6 | n = 5 | ||||
| 10 (9–20) | 10 (5–18) | 1 (−17 to 15) | 14 (1–20) | 6 (3–22) | −0.5 (−17 to 17) | 6.5 (2–17) | 6 (4–21) | −3 (−29 to 17) | |
| 3–37 | 5–54 | 0–35 | 0–31 | 0–35 | 0–40 | ||||
| Sensitivity analysis, neonatal | |||||||||
| n = 21 | n = 14 | n = 21 | n = 14 | n = 20 | n = 13 | ||||
| Median (IQR) | 54 (20–54) | 54 (11–54) | 0 (0 to 4) | 54 (20–54) | 54 (8–54) | 0 (0–20) | 54 (26–54) | 54 (21–54) | 0 (0–11) |
| Rangea | 3–54 | 5–54 | 0–54 | 0–54 | 0–54 | 0–54 | |||
| Night-time symptoms | |||||||||
| Complete case | |||||||||
| n = 7 | n = 7 | n = 7 | n = 6a | n = 6 | n = 5 | ||||
| Median (IQR) | 2 (0–6) | 4 (1–10) | −2 (−10 to 1) | 2 (1–4) | 3 (0–5) | 0 (−4 to 4) | 4 (2–5) | 6 (0–7) | −0.5 (−6 to 5) |
| Range | 0–7 | 1–13 | 0–12 | 0–7 | 0–11 | 0–9 | |||
| Sensitivity analysis neonatal | |||||||||
| n = 21 | n = 15 | n = 21 | n = 14 | n = 20 | n = 13 | ||||
| Median (IQR) | 13 (6–13) | 13 (4–13) | 0 (0 to 1) | 13 (4–13) | 13 (5–13) | 0 (0 to 6) | 13 (8–13) | 13 (7–13) | |
| Range | 0–13 | 1–13 | 0–13 | 0–13 | 0–13 | 0–13 | 0 (0 to 4) | ||
| Effect on the child | |||||||||
| Complete case | |||||||||
| n = 7 | n = 7 | n = 7 | n = 6 | n = 6 | n = 5 | ||||
| Median (IQR) | 2 (0–3) | 0 (0–6) | 0 (−4 to 3) | 1 (0–3) | 2 (0–3) | 0 (−3 to 3) | 0 (0–8)b | 1 (0–5) | 0 (−5 to 8) |
| Range | 0–8 | 0–6 | 0–8 | 0–5 | 0–8 | 0–5 | |||
| Sensitivity analysis, neonatal | |||||||||
| n = 21 | n = 15 | n = 21 | n = 14 | n = 20 | n = 13 | ||||
| 8 (3–8) | 8 (0–8) | 0 (0 to 2) | 8 (3–8) | 8 (3–8) | 0 (0 to 3) | 8 (8–8) | 8 (5–8) | 0 (0 to 3) | |
| 0–8 | 0–8 | 0–8 | 0–8 | 0–8 | 0–8 | ||||
| Effect on the family | |||||||||
| Complete case | |||||||||
| n = 7 | n = 7 | n = 7 | n = 6 | n = 6 | n = 5 | ||||
| Median (IQR) | 2 (0–8) | 2 (0–6) | 0 (−5 to 5) | 1 (0–8) | 2.5 (1–3) | −0.5 (−3 to 7) | 0 (0–8)b | 0 (0–6) | 0 (−9 to 8) |
| Range | 0–10 | 0–11 | 0–11 | 0–9 | 0–10 | 0–9 | |||
| Sensitivity analysis, neonatal | |||||||||
| n = 21 | n = 15 | n = 21 | n = 14 | n = 20 | n = 13 | ||||
| Median (IQR) | 11 (8–11) | 11 (2–11) | 0 (0 to 2) | 11 (8–11) | 11 (3–11) | 0 (0 to 2) | 11 (9–11) | 11 (6–11) | 0 (0 to 2) |
| Range | 0–11 | 0–11 | 0–11 | 0–11 | 0–11 | 0–11 | |||
| Study arm | Patient number | Latest questionnaire available | Outcome |
|---|---|---|---|
| AI | 8 | 12 months | No indication of asthma |
| 10 | 18 months | Asthma | |
| 11 | No questionnaires returned | Missing data | |
| 16 | Lost to follow-up | Missing data | |
| 20 | 18 months | No indication of asthma | |
| 30 | 18 months | No indication of asthma | |
| 35 | 18 months | No indication of asthma | |
| 45 | 18 months | No indication of asthma | |
| 55 | 18 months | Asthma | |
| Exp | 5 | 18 months | No indication of asthma |
| 22 | 12 months | Asthma | |
| 25 | 18 months | Asthma | |
| 28 | 6 months | Asthma | |
| 31 | Lost to follow-up | Missing data | |
| 33 | 18 months | No indication of asthma | |
| 44 | 18 months | No indication of asthma | |
| 58 | 18 months | Asthma |
Complete-case analysis is defined as analysis of only those domain scores and overall scores that have no missing data owing to there being no validated methods available to handle missing data in this respiratory questionnaire.
-
Best case is sensitivity analysis assigning missing questions a score of 0.
-
Worst case is sensitivity analysis assigning missing questions a score of 4.
There was only one patient with missing answers to the questions in one of the sections in the ‘daytime symptoms’ domain so the best- and worst-case sensitivity analyses are only needed for ‘daytime symptoms’ and ‘overall total’ scores (Table 30).
| Domain | 6 months | ||
|---|---|---|---|
| AI | Exp | Difference in medians (95% CI) | |
| Overall total | |||
| Complete case | |||
| n = 7 | n = 6a | ||
| Median (IQR) | 16 (14–32) | 13.5 (7–35) | 2 (−31 to 24) |
| Range | 4–59 | 6–84 | |
| Best case (missing answers = 0) | |||
| n = 7 | |||
| Median (IQR) | 14 (7–41) | 1 (−27 to 18) | |
| Range | 6–84 | ||
| Worst case (missing answers = 4) | |||
| n = 7 | |||
| Median (IQR) | 14 (7–65) | 0 (−50 to 18) | |
| Range | 6–84 | ||
| Daytime symptoms | |||
| Complete case | |||
| n = 7 | n = 6a | ||
| Median (IQR) | 10 (9–20) | 10 (5–18) | 1 (−17 to 15) |
| Range | 3–37 | 5–54 | |
| Best case (missing answers = 0) | |||
| n = 7 | |||
| Median (IQR) | 11 (5–19) | 0 (−15 to 11) | |
| Range | 5–54 | ||
| Worst case (missing answers = 4) | |||
| n = 7 | |||
| Median (IQR) | 11 (5–43) | −1 (−33 to 10) | |
| Range | 5–54 | ||
The mean profile plots for overall total score of the respiratory questionnaires is shown in Figure 4.
FIGURE 4.
Mean profile plots for overall total score of the respiratory questionnaire.
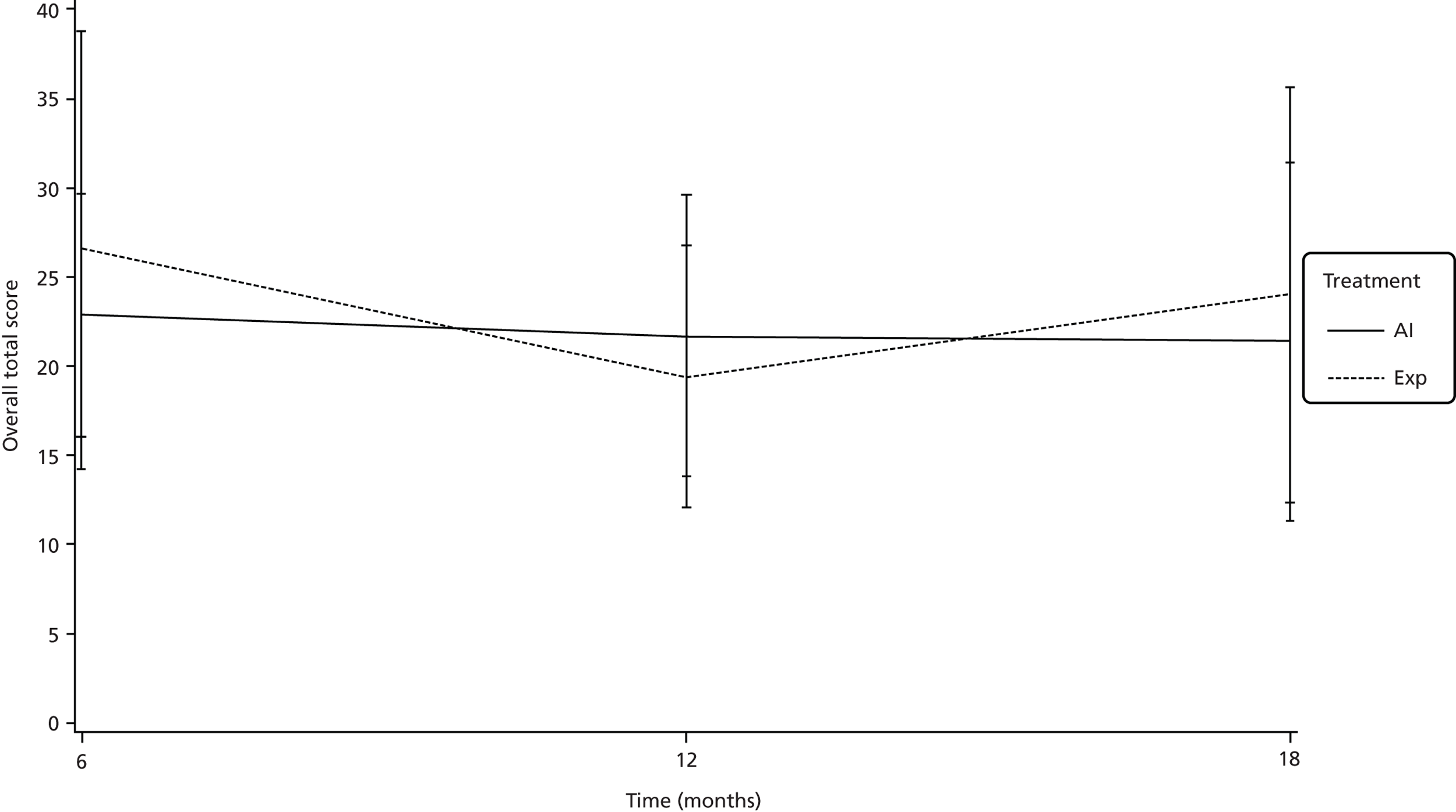
Table 31 shows descriptive statistics regarding asthma diagnosis, medications for asthma, and hospital and general practitioner visits for chest symptoms. Numbers were too small to perform meaningful statistical analyses.
| Domain | Questionnaire time point | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | 18 months | ||||
| AI (n = 7) | Exp (n = 7) | AI (n = 7) | Exp (n = 6) | AI (n = 6) | Exp (n = 5) | |
| Inhalers taken as treatment for chest symptoms,a n (%) | 2/7 (28.6) | 3/7 (42.9) | 3/7 (42.9) | 3/6 (50.0) | 2/6 (33.3) | 2/5 (40.0) |
| Medicines taken as treatment for chest symptoms,b n (%) | 4/7 (57.1) | 3/7 (42.9) | 2/7 (28.6) | 1/6 (16.7) | 2/6 (33.3) | 2/5 (40.0) |
| Child had visited or had a visit from a general practitioner for chest problems, n (%) | 5/7 (71.4) | 3/7 (50.0) | 5/7 (71.4) | 2/6 (33.3) | 3/6 (50.0) | 2/5 (40.0) |
| Child had attended hospital clinics for chest problems, n (%) | 2/7 (28.6) | 0/7 (0.0) | 1/7 (13.3) | 3/6 (50.0) | 2/6 (33.3) | 1/5 (20.0) |
| Has child been diagnosed with asthma by a doctor, n (%) | 1/7 (14.3) | 1/7 (14.3) | 1/7 (14.3) | 0/6 (0.0) | 1/6 (16.7) | 0/5 (0.0) |
Lung function tests
The purpose of the lung function tests was primarily to determine whether there was evidence of small lungs in either patient arm. The individual lung function test results are shown in Table 32. Data presented in Table 32 are for means of at least three recorded values, unless otherwise indicated.
| Study number | Treatment | Reason tests not performed | Age at tests (years) | Age category | FRCP (l) | FRCP predicted (l) | FRCP z-value | FVC (l) | FVC predicted (l) | FVC z-value | FEV1 (l) | FEV1 predicted (l) | FEV1 z-value | Vmax FRC (ml/s) | Vmax FRC predicted (ml/s) | Vmax FRC z-value | Comment on test | Comment on result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | AI | – | 3.50 | Preschool | 0.88 | – | – | 0.98 | 0.83 | 1.08 | 0.83 | 0.81 | 0.16 | – | – | – | Plethysmography done with child sitting on mother’s knee. All measurements somewhat variable, as expected in a young child, but child did very well | Spirometry indicates normal forced expiratory volumes and the shape of the flow-volume curve was normal |
| 10 | AI | – | 3.08 | Preschool | 1.07 | – | – | 0.85 | 0.73 | 0.95 | 0.76 | 0.71 | 0.41 | – | – | – | Child did very well | Flow-volume loop showed no evidence of gross abnormality |
| 11 | AI | – | 2.89 | Preschool | 0.83a | – | – | 0.34 | 0.61 | −2.58 | – | – | – | – | – | – | Child did well for his age. FRCp is based on a single value so should be viewed with extreme caution | Predicted values are scarce for small preschool children so measured values should be interpreted with caution |
| 16 | AI | Lost to long-term follow-up | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| 20 | AI | – | 4.18 | Preschool | – | – | 0.89 | 1.00 | −0.69 | 0.84 | 0.96 | –0.80 | Excellent co-operation | Normal spirometry | ||||
| 30 | AI | – | 1.42 | Infant | 0.25 | 0.26 | −0.18 | – | – | – | – | – | – | 117.00 | 315.00 | −2.31 | Very good. Settled well. No problems | Resting lung volume is normal but maximum expiratory flow is somewhat reduced |
| 35 | AI | – | 1.06 | Infant | 0.18 | 0.27 | −1.16 | – | – | – | – | – | – | 64.00 | 253.00 | −2.63 | Straightforward, no problems. Child noted to be a little snuffly, either was just starting a cold or was teething | |
| 45 | AI | – | 2.26 | Preschool | – | – | 0.68 | 0.62 | 0.61 | 0.67 | 0.60 | 0.68 | Did well for his age | Normal spirometry | ||||
| 55 | AI | – | 2.44 | Preschool | – | – | – | – | – | – | – | – | – | – | – | Not really old enough to have the co-operation for spirometry | Co-operation not good enough for results to be reliable. Cautious report sent to medical staff caring for him | |
| 5 | Exp | – | 3.95 | Preschool | 1.37a | – | – | 0.61 | 0.90 | –2.01 | 0.44 | 0.87 | −3.20 | – | – | – | Data shown are baseline. Child responded to bronchodilator, so that FEV1 increased to 0.64 l (z-value −1.70) and FVC increased to 0.87 (z-value −0.22) | |
| 22 | Exp | – | 1.67 | Infant | 0.18 | 0.21 | −0.49 | – | – | – | – | – | – | 74.00 | 158.00 | −1.31 | Uneventful. Child slept well. No problems, no alarms | All normal for body size |
| 25 | Exp | – | 1.02 | Infant | 0.18 | 0.22 | −0.60 | – | – | – | – | – | – | 74.00 | 269.00 | −2.86 | Child had only a short sleep after sedation. Child woke up so was given a second dose of chloral hydrate, after which child slept well and measurements were completed without any problems. No desaturations or alarms | Resting lung volume is normal but maximum expiratory flow is somewhat reduced |
| 28 | Exp | Lost to long-term follow-up | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 31 | Exp | Lost to long-term follow-up | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 33 | Exp | Lost to long-term follow-up | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| 44 | Exp | 2.45 | Preschool | – | – | – | 0.71 | 0.65 | 0.54 | – | – | – | – | – | – | Not really old enough to have the co-operation for spirometry | – | |
| 58 | Exp | Unable to assess owing to severe developmental delay | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Age categories:
-
Age < 2 years CGA: infant-style testing, studied supine while sleeping.
-
Age ≥ 2 years CGA: preschool testing, requiring the child to breathe or blow through a mouthpiece; getting good results is dependent on child co-operation.
One child in each arm had a z-value below −2.00 for FVC and the two values were −2.01 (number 5) and −2.58 (number 11). Two children in each arm had evidence of reduced maximum expiratory flow, whether shown by VmaxFRC or FEV1 (study numbers 30, 35, 22, 5). The child in study number 5 had initial tests that indicated a reduced FEV1 and an FVC just below the lower limit of prediction. His tests were repeated following bronchodilator and both indices improved to well within normal values. The finding of reduction in maximum expiratory flow in some children in this study is consistent with other reports of lung function in children born preterm. 17
There was one child in the AI arm and four children in the Exp arm that were lost to follow-up or were not able to provide test results. These children may have experienced reduced lung capacity; hence, for sensitivity will be included with those children showing a z-value below −2.0.
The difference in medians in z-values for infant lung function tests could not be performed, as there was too little data to do so. However, when sensitivity analyses were performed for missing data, there was no difference between arms for any of the functions (Table 33).
| AI | Exp | Difference in medians (95% CI) | |
|---|---|---|---|
| FRCP | |||
| Complete case (nAI = 2, nexp = 2) | |||
| n = 2 | n = 2 | N/Aa | |
| Median (IQR) | −0.67 (−1.16 to −0.18) | −0.54 (−0.60 to −0.49) | |
| Range | −1.16 to −0.18 | −0.60 to −0.49 | |
| Sensitivity analysis neonatal (maximumb) (nAI = 16, nexp = 11c) | |||
| n = 16 | n = 11c | ||
| Median (IQR) | −0.18 (−0.18 to −0.18) | −0.18 (−0.18 to −0.18) | 0 (0 to 0) |
| Range | −1.16 to −0.18 | −0.60 to −0.18 | |
| Sensitivity analysis neonatal (minimum) (nAI = 16, nexp = 11c) | |||
| n = 16 | n = 11c | ||
| Median (IQR) | −1.16 (−1.16 to −1.16) | −1.16 (−1.16 to −1.16) | 0 (0 to 0) |
| Range | −1.16 to −0.18 | −1.16 to −0.49 | |
| FVC | |||
| Complete case (nAI = 5, nexp = 2) | |||
| Median (IQR) | n = 5 | n = 2 | N/Aa |
| Range | 0.61 (−0.69 to 0.95) | −0.74 (−2.01 to 0.54) | 0 (0 to 0) |
| −2.58 to 1.08 | −2.01 to 0.54 | ||
| Sensitivity analysis neonatal (maximum) (nAI = 19, nexp = 10) | |||
| n = 19 | n = 10 | ||
| Median (IQR) | 1.08 (1.08 to 1.08) | 1.08 (1.08 to 1.08) | 0 (0 to 0) |
| Range | −2.58 to 1.08 | −2.01 to 1.08 | |
| Sensitivity analysis neonatal (minimum) (nAI = 19, nexp = 10) | |||
| n = 19 | n = 10 | ||
| Median (IQR) | −2.58 (−2.58 to −2.58) | −2.58 (−2.58 to −2.58) | |
| Range | −2.58 to 1.08 | −2.58 to 0.54 | |
| FEV1 | |||
| Complete case (nAI = 4, nexp = 1) | |||
| n = 4 | n = 1 | N/Aa | |
| Median (IQR) | 0.29 (−0.32 to 0.55) | −3.20 | |
| Range | −0.80 to 0.68 | ||
| Sensitivity analysis neonatal (maximum) (nAI = 18, nexp = 9) | |||
| Median (IQR) | n = 18 | n = 9 | |
| Range | 0.68 (0.68 to 0.68) | 0.68 (0.68 to 0.68) | 0 (0 to 0) |
| −0.80 to 0.68 | −3.20 to 0.68 | ||
| Sensitivity analysis neonatal (minimum) (nAI = 18, nexp = 9) | |||
| Median (IQR) | n = 18 | n = 9 | |
| Range | −3.20 (−3.20 to −3.20) | −3.20 (−3.20 to −3.20) | 0 (0 to 0) |
| −3.20 to 0.68 | −3.20 to −3.20 | ||
| VmaxFRC | |||
| Complete case (nAI = 2, nexp = 2) | |||
| n = 2 | n = 2 | N/Aa | |
| Median (IQR) | −2.47 (−2.63 to −2.31) | −2.09 (−2.86 to −1.31) | |
| Range | −2.63 to −2.31 | −2.86 to −1.31 | |
| Sensitivity analysis neonatal (maximumb) (nAI = 16, nexp = 11c) | |||
| n = 16 | n = 11c | ||
| −1.31 (−1.31 to −1.31) | −1.31 (−1.31 to −1.31) | 0 (0 to 0) | |
| −2.63 to −1.31 | −2.86 to −1.31 | ||
| Sensitivity analysis neonatal (minimum) (nAI = 16, nexp = 11c) | |||
| n = 16 | n = 11c | ||
| Median (IQR) | −2.86 (−2.86 to −2.86) | −2.86 (−2.86 to −2.86) | 0 (0 to 0) |
| Range | −2.86 to −2.31 | −2.86 to −1.31 | |
Analysis of lung function z-values was performed using complete-case analysis, i.e. only surviving patients who had lung function assessments were analysed. Sensitivity analysis neonatal (maximum) is defined as the sensitivity analysis that assigned the neonatal deaths the largest observed positive z-value for each test. In addition, any patients in whom lung function tests could not be performed and assessed because of severe developmental delay were included in the infant analyses (FRCP and VmaxFRC) and handled the same way as the neonatal deaths, as per the SAP. Sensitivity analysis neonatal (minimum) is defined as the sensitivity analysis that assigned the neonatal deaths the smallest observed negative z-value for each test.
Neurodevelopment
The Bayley assessments were carried out between the ages of 2 years 3 months and 3 years 3 months. The assessments were performed at the home of the child by a trained nurse. At the protocol stage, a trained nurse was not identified, so this explains why Bayley assessment was delayed in some of the earlier children. Other delays were due to parents and trained nurse finding it difficult to agreee a convenient time to meet.
A sensitivity analysis was performed to account for the children in whom a Bayley assessment for either MDI, PDI or both was not possible owing to a significantly delayed performance. These are assigned a score of 50 (i.e. the worst possible score) for the score analysis and classified as ‘significantly delayed performance’ for the classification summary. The results are shown in Table 34 (Sens. 1 is the sensitivity analysis including the imputations described).
| AI | Exp | |
|---|---|---|
| MDI classification | ||
| Complete case,a n (%) | ||
| n = 7 | n = 4 | |
| Significantly delayed performance | 1 (14.3) | 1 (25.0) |
| Mildly delayed performance | 1 (14.3) | 2 (50.0) |
| Within normal limits | 5 (71.4) | 1 (25.0) |
| Accelerated performance | 0 (0.0) | 0 (0.0) |
| Sensitivity analysis including the imputations described, n (%) | ||
| n = 8 | n = 5 | |
| Significantly delayed performance | 2 (25.0) | 2 (40.0) |
| Mildly delayed performance | 1 (12.5) | 2 (40.0) |
| Within normal limits | 5 (62.5) | 1 (20.0) |
| Accelerated performance | 0 (0.0) | 0 (0.0) |
| PDI classification | ||
| Complete case, n (%) | ||
| n = 8 | n = 3 | |
| Significantly delayed performance | 1 (12.5) | 0 (0.0) |
| Mildly delayed performance | 4 (50.0) | 1 (33.3) |
| Within normal limits | 3 (37.5) | 2 (66.7) |
| Accelerated performance | 0 (0.0) | 0 (0.0) |
| Sensitivity analysis including the imputations described, n (%) | ||
| n = 8 | n = 5 | |
| Significantly delayed performance | 1 (12.5) | 2 (40.0) |
| Mildly delayed performance | 4 (50.0) | 1 (20.0) |
| Within normal limits | 3 (37.5) | 2 (40.0) |
| Accelerated performance | 0 (0.0) | 0 (0.0) |
Overall, both Bayley’s scores were within the normal range in only 31% of surviving children (4 out of 13) (Figure 5). Three out of eight children (37.5%) in the AI arm had normal scores for both PDI and MDI, compared with 1 out of 5 in the Exp arm (20%). Only one child, overall, had both MDI and PDI assessed as severely delayed. This child was in the Exp arm and did not have a test result for either domain as the assessor was unable to perform the tests owing to significantly delayed performance; these results were assumed to be in the severely delayed category. The average deepest pool of amniotic fluid in this pregnancy was 1.4 cm. The amniotic membrane ruptured at 23 weeks and delivery was at 31 weeks’ gestation. Three children in the AI arm (37.5%) and three in the Exp arm (60%) had significant delay in either PDI or MDI scores, including one child in the AI arm and two children in the Exp arm who did not have a test result as the assessor was unable to perform the tests owing to significantly delayed performance.
FIGURE 5.
Neurodevelopmental outcome of followed-up survivors by study arm (includes children who were too delayed to be scored).
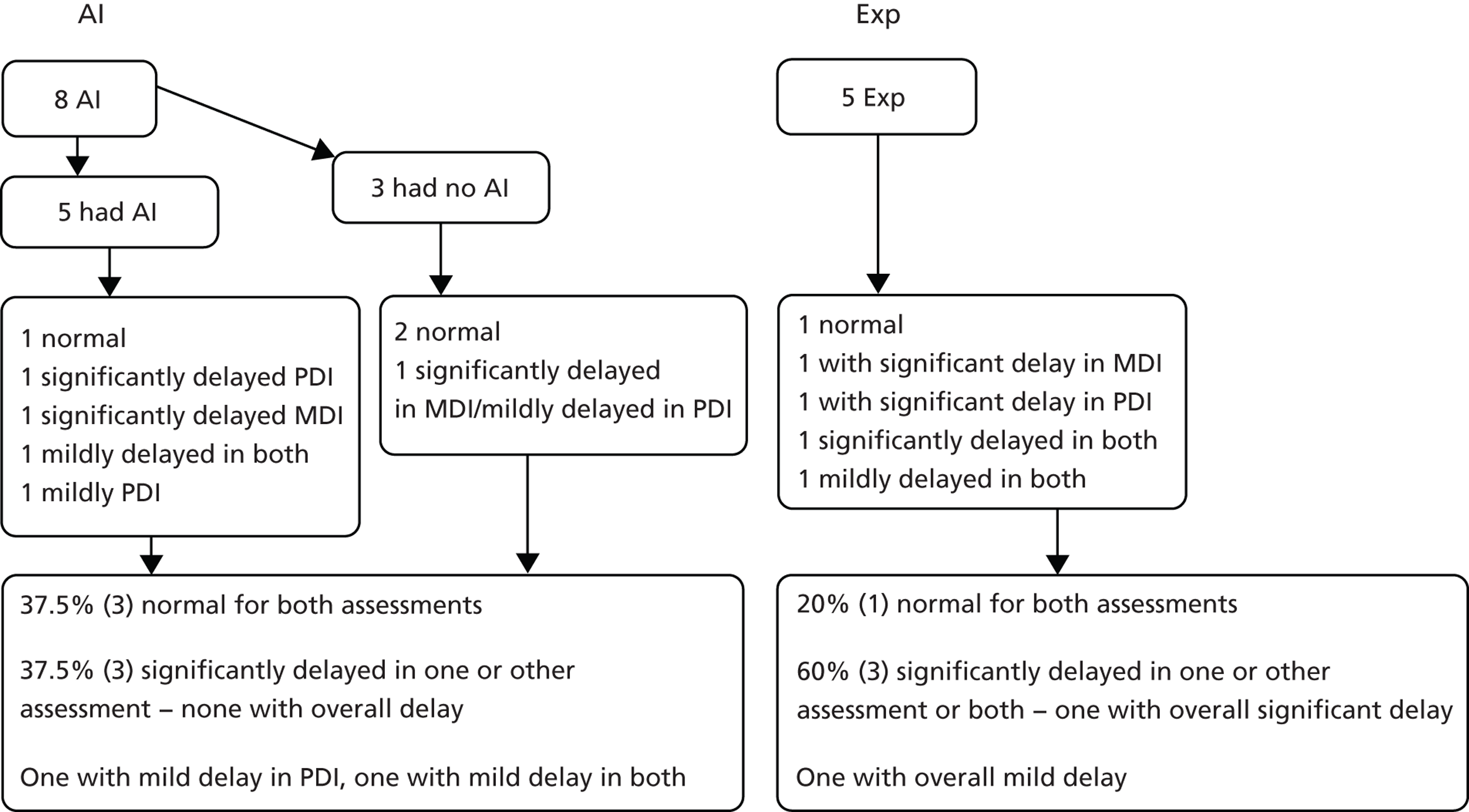
Complete-case analysis: only surviving patients who have Bayley’s data are analysed
Sens. 1 is defined as sensitivity analysis that includes three additional patients in whom a Bayley’s assessment for either MDI, PDI or both was unable to be carried out due to the children having a significantly delayed performance. These are assigned a score of 50 (i.e. the worst possible score) for the score analysis and classified as ‘significantly delayed performance’ for the classification summary.
The number of surviving children in each arm with normal MDI and PDI and cross-tabulation of all other Bayley’s assessments is shown in Table 35.
| PDI | |||||
|---|---|---|---|---|---|
| Significantly delayed performance | Mildly delayed performance | Within normal limits | Unable to do due to significantly delayed performance | ||
| AI | |||||
| MDI | Significantly delayed performance | – | 1 | – | – |
| Mildly delayed performance | – | 1 | – | – | |
| Within normal limits | 1 | 1 | 3 | – | |
| Unable to do due to significantly delayed performance | – | 1 | – | – | |
| Exp | |||||
| MDI | Significantly delayed performance | – | – | 1 | – |
| Mildly delayed performance | – | 1 | – | 1 | |
| Within normal limits | – | 1 | – | ||
| Unable to do due to significantly delayed performance | – | – | – | 1 | |
Orthopaedic follow-up
Three babies had postural orthopaedic problems identified in the neonatal period; in two the problems resolved spontaneously and one surviving child required only referral for orthopaedic follow-up. This child was in the Exp arm. The child had bilateral contractures in the right knee and elbow but surgery was not required as all resolved by 9.5 months. This is patient number 2 in the third footnote of the neonatal morbidity outcomes (see Table 19). The numbers of survivors are too small to draw any conclusions, but the rate of orthopaedic deformity appears low.
Chapter 5 Exploratory summary analysis of the long-term outcome data
All pre-specified outcomes analysed as per the SAP (Appendices 3 and 4) have been presented in Chapters 3 and 4. The initial focus of this study was on short-term outcomes and, while these are clearly of interest, particularly because of their impact on the utilisation of health-care resources (e.g. neonatal intensive care unit), in this chapter we present additional, post-hoc analysis that focuses on a clinically most important outcome in this cohort – a healthy survivor. We have opted to do this to summarise the long-term outcome results from this pilot study in a clinically meaningful way. In the context of this study, being healthy is defined as being alive with the absence of serious respiratory and neurological problems at the end of a follow-up period (27–39 months). For the purpose of this post-hoc analysis we needed to define clinically meaningful definitions of respiratory and neurological disability.
-
Respiratory disability. Abnormal respiratory function has been defined as a z-value < −2.00 on any of the whole body plethysmography parameters (FRC, FVC), FEV1 and VmaxFRC. Although the respiratory questionnaires are validated, the authors acknowledge that more work, in terms of sensitivity/specificity analyses to define cut off points, is required. The results from the questionnaire are, therefore, not currently decisive enough to be used in the definition of respiratory disability in the long term.
-
Neurological disability. We defined major neurodevelopmental delay in any child with an MDI < 70 or a PDI < 70 or both MDI/PDI < 70. Mildly delayed performance was defined as a score of between 70 and 84 in any domain. Adopting this post-hoc definition of healthy survivors, and assuming that all babies lost to follow-up were unhealthy, there were 4 out of 56 (7.1%) healthy survivors in the whole cohort, 4 out of 28 (14.3%) in the AI arm and 0 out of 28 (0.0%) in the Exp arm (RR 9.0; 95% CI 0.51 to 159.70) (Figure 6).
FIGURE 6.
Long-term healthy survivors by cohort.
One of the babies with an abnormal z-value also had a PDI score < 70. This baby is, therefore, included in the babies with significant disability.
The long-term outcomes by arm are shown in Figure 7. There were 4 out of 28 healthy survivors in the AI arm, compared with 0 out of 28 in the Exp arm (Table 36). The frequency of respiratory and neurological morbidity in each arm is shown in Table 37.
FIGURE 7.
Long-term healthy survivors by arm.
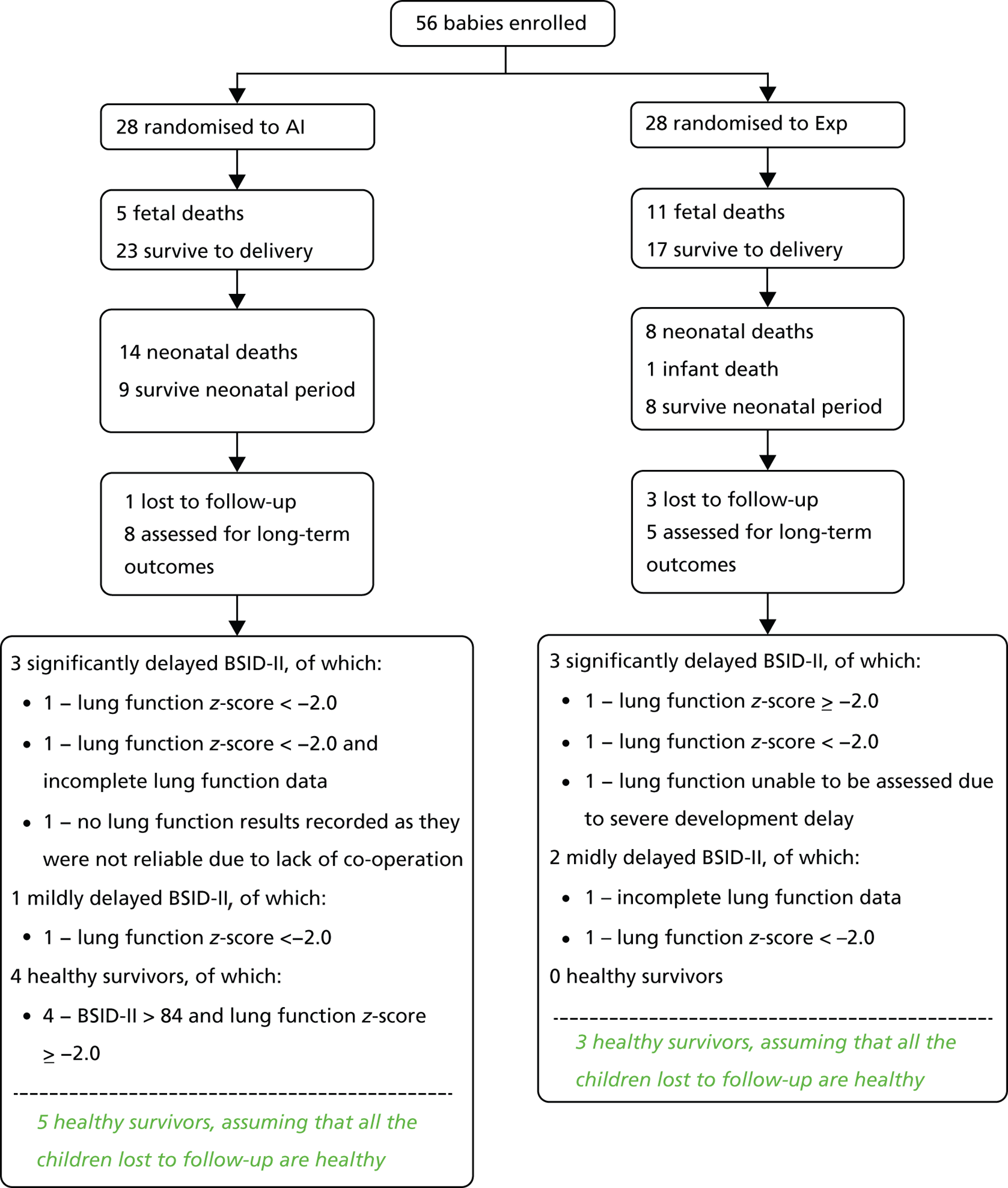
| Analysis | AI | Exp | Relative risk (95% CI) |
|---|---|---|---|
| Observed (ITT) | 4/28 (14.3%) | 0/28 (0.0%) | 9.0 (0.51 to 159.70) |
| If all lost to follow-up were healthy | 5/28 (17.9%) | 3/28 (10.7%) | 1.27 (0.44 to 6.31) |
| Analysis | AI | Exp | RR (95% CI) |
|---|---|---|---|
| Severe respiratory morbiditya | |||
| Observed (ITT) | 3b | 2b | RR1:c 4.00 (0.48
to 33.58) RR2:d 1.71 (0.31 to 9.61) |
| If all lost to follow-up and those that did not have a test done had respiratory morbidity | 5 | 7 | |
| Severe neurological morbiditye | |||
| Observed (ITT) | 3 | 3 | RR2:d 2.50 (0.53
to 11.82) RR2:d 2.14 (0.40 to 11.35) |
| If all lost to follow-up had severe neuromorbidity | 4 | 6 | |
Chapter 6 Discussion
Here we report the results of a pilot RCT designed to assess the effect on the pregnancy, maternal and perinatal outcomes of women with very early PPROM (16+0 to 24+0 weeks’ gestation) treated with serial AI when compared with expectant and whether such a trial is feasible. The study was conducted because of increasing reports of this intervention, which had not been evaluated in an RCT. The intervention is very invasive and has the potential to increase maternal morbidity, although there were no reports of this in the observational studies in the literature. We were motivated to conduct this study to determine whether such a study was feasible, to inform and help design a definitive trial on the subject and also because NICE18 concluded that more information from RCTs is required before AI can be considered routine therapy for very early PPROM.
This discussion summarises the key findings, compares the findings with the results of published studies, considers the strengths and limitations of the present study and the lessons learnt and summarises the clinical and research implications of the work.
Key findings
This pilot study demonstrates that, with appropriate funding, it is possible to recruit to such a study. During the study period, very early PPROM was a rare event, and, with no external funding, most of the recruitment relied on the main recruiting centre. NIHR funding had a significant impact on the enthusiasm of other large tertiary FMUs to recruit to the study. The key factor was the adoption on the NIHR portfolio, which allowed access to comprehensive local research network (CLRN) research staff that facilitated identification of potential participants and recruitment/consenting. They were also instrumental in improving completeness of follow-up. The HTA programme funding gave the pilot study more weight, attracted a large tertiary centre to the trial and allowed the other centres to maintain involvement in the study.
Seventy-five per cent of eligible women participated in the study; therefore, acceptance rate for the study was high. There were very few postrandomisation exclusions, and these were mainly due to fetal abnormalities that are difficult to detect on ultrasound when there is no residual amniotic fluid in the amniotic sac. Retention of participants throughout the study period was high. Long-term follow-up of the surviving infants was feasible, although the loss rate was around 38%, and this is an area that will require more input in a larger study.
The overall perinatal mortality rate was higher than expected, at 67.9%, and the proportion of healthy survivors was much lower than anticipated, 7%. We found no statistically significant difference in any of the outcomes between the two arms, although it must be noted that patient numbers were small and it is, therefore, not appropriate to draw too many conclusions from the statistical testing.
The assessment of long-term respiratory morbidity was performed using two modalities: respiratory questionnaires and infant lung function tests. The respiratory questionnaires, although validated, had cut-off points that were not defined enough to be used in the identification of long-term respiratory morbidity. Lung function tests had clearer defined cut-offs and were, therefore, found to be the most useful tests for long-term respiratory morbidity.
Overall, only 7.1% of babies [4/28 (14.3%) in the AI arm and 0/28 (0.0%) in the Exp arm] were known to be alive without respiratory or neurological disability at 2 years of age. The findings from this pilot study suggest that the clinically meaningful outcome of a healthy survivor (alive without defined respiratory or neurological disability at 2 years of age) should be the outcome on which to base a larger, and more definitive, study.
Comparison with other studies
The AMIPROM pilot included a very strict definition for very early PPROM i.e. rupture of amniotic membranes between 16+0 and 24+0 weeks of pregnancy. This is the first randomised study to use these inclusion criteria. Only one other randomised trial19 which included pregnancies with rupture of amniotic membranes at < 27 weeks’ gestation has been performed. In order to put our results in the wider context of other available evidence, we have performed a systematic review of published studies with data on singleton pregnancies with PPROM at < 28+0 weeks’ gestation, treated with serial non-continuous transabdominal AI. The full results will be published in a separate publication. In brief, we have searched MEDLINE from 1985 to date, using the medical subject heading terms AI, preterm premature rupture of amniotic membranes, rupture of amniotic membranes, preterm premature rupture of fetal amniotic membranes, rupture of fetal amniotic membranes, PROM. No language restrictions were employed. The results were pooled using StatsDirect Version 2.7.8 (StatsDirect Ltd, Cheshire, UK) and previously described methodology. 20 In the presence of significant heterogeneity we have used random effects to pool the results.
Data from seven eligible studies (including AMIPROM pilot) were analysed. 4,9,19,21–23 Our pilot suggests that perinatal mortality in infants treated with AI is likely to be higher than previously reported (Figure 8). This is most likely due to the inclusion of the clinically relevant group of pregnancies between 16+0 and 24+0 weeks’ gestation in the AMIPROM study. Pregnancies with PPROM after 24 weeks’ gestation would be expected to do better as the critical time for lung development and the need for adequate volumes of amniotic fluid is between 16+0 and 24+0 weeks’ gestation.
FIGURE 8.
Pooled perinatal mortality in pregnancies treated with AI (AMIPROM compared with other studies), heterogeneity I 2 = 46.2%.
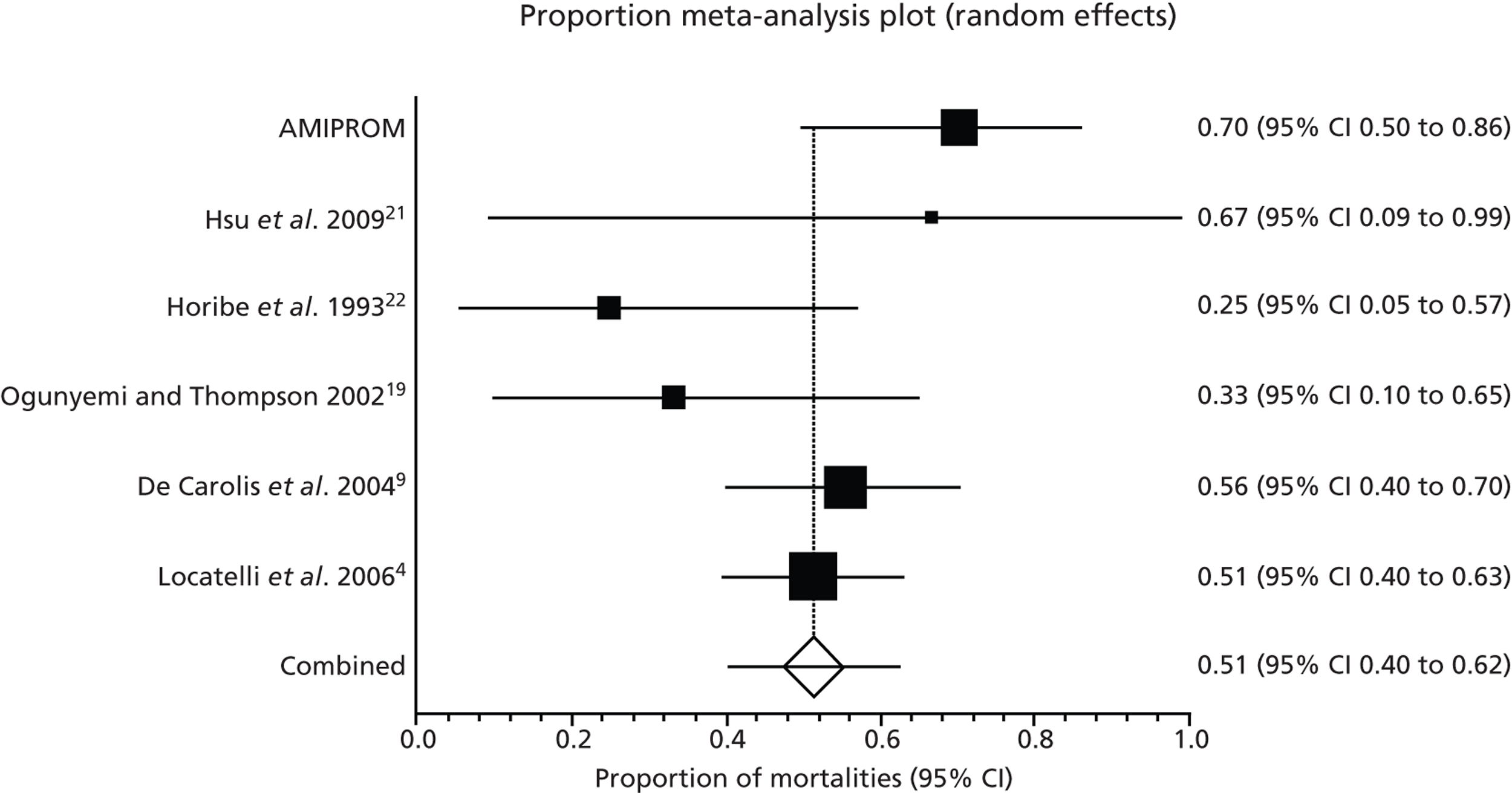
The pooled respiratory morbidity in AMIPROM, when compared with the other studies, is not significantly different (Figure 9). Pooled neurodisability at any time as defined by authors is shown in Figure 10.
FIGURE 9.
Pooled respiratory morbidity in pregnancies treated with AI (AMIPROM compared with other studies), heterogeneity I 2 = 0%.
FIGURE 10.
Pooled neurodisability at any age (as defined by authors) in pregnancies treated with AI (AMIPROM compared with other studies), heterogeneity I 2 = 25.8%.
Strengths and limitations of current study
This is the first study to evaluate outcomes in PPROM pregnancies between 16+0 and 24+0 weeks’ gestation. It is also the first study to evaluate long-term respiratory and neurodevelopmental outcomes in this group of babies at high risk of neonatal mortality and morbidity.
Data on post-mortems were not specifically collected and were not pre-specified in the SAP. This is a potential source of bias. A postmortem was carried out on only one of the neonatal deaths; in addition, a limited postmortem was carried out on one neoonatal death and one antenatal death. All these babies were normal. All fetal abnormalities detected antenatally and postnatally are accounted for either as postrandomisation exclusions or SAE (see Tables 25 and 26). The authors are sure, therefore, that any potential confounders to outcomes from undetected fetal abnormalities have been accounted for. Collecting data from post-mortem exminations is something to consider in a future study.
The limitations of this study are that, as a pilot, it does not have adequate power to evaluate the effectiveness and safety of serial AI. The lack of clear methods for assessment of asthma using respiratory questionnaires was identified. More work needs to be done in this area to reach a consensus on what constitute clinically significant respiratory morbidity in very young children. Even with this caveat, the study indicates that the overall chance of healthy survival at age two is small in this group of infants.
Lessons learnt about conduct of the study
-
Traditionally, women with very early PPROM are not referred to tertiary FMUs for assessment. To maximise recruitment, we realised that all clinicians in local referring units had to be informed about the study. We found that this was best done by presenting at their local obstetrics and gynaecology study/audit days to get the largest audience of clinicians. This allowed for question and answer sessions and more detail around the study to be explored. We also found that the junior doctors rotating to different units were particularly useful in informing local clinicians about the study. NIHR funding contributed significantly towards the recruitment of one large FMU to the study and facilitated the recruitment of five additional participants to the study. In a future study, CLRN nurses and research staff would be crucial in improving recruitment. Their impact in this study came late (as the study was mainly funded for the long-term outcome phase) but we have survey evidence to suggest that more units would be interested in participating, if assured the support of the CLRN.
-
Women were informed about the study as soon as the diagnosis of very early PPROM was made. They were then seen at the next FMU to discuss the study in detail. Clinical staff in all emergency attendance areas were informed about the study. The use of posters in the emergency areas was particularly useful as reminders.
-
Randomisation occurred only if participants were still pregnant 10 days after rupture of amniotic membranes. This is crucial to avoid attrition from the high likelihood of miscarriage within the first week after rupture has occurred.
-
Registration of the study on the ISRCTN and the NIHR website meant that clinicians out of the area and, in some instances, patients were aware of the study and approached the PI directly.
-
Retention of participants from randomisation to delivery was excellent. The losses to follow-up tended to be those participants with social issues or those in the experimental arm who were managed in local units. As NIHR funding was granted towards the end of the recruitment phase of the study, it was not possible to assess the impact of funding on retention in long-term follow-up. In a larger study, funding for a research programme manager would be imperative to improve this area. NIHR funding would be required for this.
-
Parts of the protocol, such as admission from 26 to 30 weeks’ gestation and steroid administration at 26+0 weeks’ gestation as routine, will need to be discussed in a larger study. This is not currently routine care, but was done to standardise care in both arms and to reduce bias in the analysis of outcomes.
-
As this was a pilot study, all outcomes and results were collected. Longitudinal data on blood tests and ultrasound measurements were collected but no differences between arms were found. In a future study, it may be necessary only to compare the differences in these data at inclusion to the study.
-
Data were also collected on respiratory questionnaires for long-term respiratory outcomes. In analysis, it became clear that this method although validated, requires more work in terms of sensitivity/specificity analyses to define cut off points. The results from the questionnaire are therefore not currently decisive enough to be used in the definition of respiratory disability in the long term. They will therefore not be used in a larger study.
-
Bayley’s scores were obtained in all surviving children. Since AMIPROM, other fetal medicine studies, such as the Trial of Umbilical and Foetal Flow in Europe,24 have used questionnaire-based screening tools, reserving Bayley’s assessment for those children for whom the questionnaire suggests it is required. The use of this methodology will significantly reduce the reliance on assessors and improve long-term follow-up in a future study.
Generalisability of the findings
The AMIPROM study recruited participants across four large tertiary referral units offering fetal medicine expertise. AI were performed in FMUs with specialists trained in invasive procedures. Expectant, which is the mainstay of management of this condition currently, was shown to be feasible in all hospital settings with some recourse to specialist outpatient care.
The findings from this study should generate enough reasons for equipoise to allow clinicians in all hospital settings to refer eligible women for participation in a larger, more definitive, study.
Chapter 7 Conclusion
Implications for health care
The findings from this pilot study do not suggest that clinicians should alter the current practice of expectant management rupture of amniotic membranes between 16+0 and 24+0 weeks’ gestation.
Implications for research
A larger, definitive, study with full health economic analysis and patient perspective assessment is required to show whether AI can improve the healthy survivor rate (Table 38).
| Anticipated incidence of healthy outcome in Exp arm | Sample size per arm | Total sample size allowing for 10% loss to follow-up |
|---|---|---|
| 0.01 | 58 | 128 |
| 0.02 | 65 | 144 |
| 0.03 | 71 | 158 |
| 0.04 | 76 | 168 |
| 0.05 | 82 | 182 |
| 0.06 | 88 | 194 |
| 0.07 | 94 | 208 |
| 0.08 | 99 | 218 |
| 0.09 | 105 | 232 |
| 0.10 | 110 | 242 |
The pilot study allowed the assessment of factors critical for the success of future trials, namely:
-
Timely identification of eligible women across the whole footprint (District General Hospitals and Tertiary FMUs) and clinical staff involvement. It is important that a network is set up to identify eligible women in local areas.
-
NIHR support is critical to improving recruitment and retention and to allowing units to access the infrastructure of the CLRN.
-
Publication of the study protocol on the ISRCTN allows access to lay personnel as well as health professionals.
-
Counselling by specialists is the key to prevent interventions that are not evidence-based, to avoid misinformation and to allow time to consider the full impact of the condition and the study.
-
The timing and eligibility criteria for randomisation are important to avoid high loss rate from the study.
-
The study population and the comparisons were feasible and adequate.
-
Long-term respiratory outcomes need to be based on infant lung function tests alone.
To explore a definitive study, indicative samples size calculations were performed based on the assumption that healthy survival rate in the definitive study would range between 0.1% and 15.0%, in keeping with our pilot data and other similar cohorts.
The feasibility of the definitive study of this magnitude has been discussed at the RCOG British Maternal Fetal Medicine Society Fetal Medicine Clinical Scientific Group in which considerable interest has been expressed by 12 FMU centres nationally. Our pilot suggests that even with full NIHR support, the definitive study would have to include international centres in other to be achieve even the minimum sample size in a reasonable time frame (2–3 years).
Acknowledgements
The authors wish to thank Louise Hardman, trial manager, for retrieving the data and overall trial co-ordination. This trial would not have been possible without her hard work and unstinting support.
Trial Steering Committee chairperson Professor Jim Thornton.
ISDMC chairpersons Professor Kate Costeloe, until 2009, and Professor Andrew Shennan, from 2009.
Liverpool Women’s NHS Foundation Trust Research and Development Department for sponsoring the study and support throughout the trial.
Contributions of authors
Devender Roberts formulated the research idea, designed the study, randomised to the study, performed the intervention, trial management, conducted the study, analysed the data and prepared the HTA report.
Sarah Vause, William Martin and Pauline Green were PIs in their centres, randomised participants to the study, performed the intervention and contributed to the final version of the HTA report.
Stephen Walkinshaw and Leanne Bricker assisted with initial study design, randomised participants to the study, performed the intervention and contributed to the final version of the HTA report.
Caroline Beardsmore advised on, performed and analysed infant lung function tests and contributed to the final version of the HTA report.
Ben NJ Shaw advised on neonatal outcome data and contributed to the final version of the HTA report.
Andrew McKay, Gaynor Skotny and Paula Williamson advised on statistical performance of the study, collated and analysed the data and contributed to the final version of the HTA report.
Zarko Alfirevic conducted trial management, assisted with initial study design, they randomised participants to the study, performed the intervention and is main co-author of the final version of the HTA report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Romero R, Quintero R, Oryazun E, Wu YK, Sabo V, Mazor M, et al. Intra-amniotic infection and the onset of labour in preterm premature rupture of membranes. Am J Obstet Gynecol 1988;159:661-6.
- Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic glucose, white blood cell count, interleukin-6 and Gram-stain in the detection of microbial invasion in patients with preterm rupture of membranes. Am J Obstet Gynecol 1993;169:839-51. http://dx.doi.org/10.1016/0002-9378(93)90014-A.
- Moretti M, Sibai BM. Maternal and perinatal outcome of expectant management of premature rupture of membranes in the midtrimester. Am J Obstet Gynecol 1988;159:390-6. http://dx.doi.org/10.1016/S0002-9378(88)80092-9.
- Locatelli A, Ghidini A, Verderio M, Andreani M, Strobelt N, Pezullo J, et al. Predictors of perinatal survival in a cohort of pregnancies with severe oligohydramnios due to preterm rupture of membranes at <26 weeks managed with serial amnioinfusions. Eur J Obstet Gynecol Reprod Biol 2006;128:97-102.
- Vergani P, Locatelli A, Verderio M, Assi F. Preterm rupture of the membranes at <26 weeks’ gestation: role of amnioinfusion in the management of oligohydramnios. Acta Bio-Medica De L Ateneo Parmense 2004;75:62-6.
- Hadi HA, Hodson CA, Strickland D. Preterm rupture of the membranes between 20 and 25 weeks’ gestation: role of amniotic fluid in perinatal outcome. Am J Obstet Gynecol 1994;170:1139-44.
- Vintzielos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Degree of oligohydramnios and pregnancy outcome in patients with preterm rupture of membranes. Obstet Gynecol 1985;66:162-7.
- Locatelli A, Vergani P, Di Pirro G, Doria V, Biffi A, Ghidini A. Role of amnioinfusion in the management of preterm rupture of the membranes at < 26 weeks’ gestation. Am J Obstet Gynecol 2000;183:878-82.
- De Carolis MP, Romagnoli C, De Santis M, Piersigilli F, Vento G, Caruso A. Is there significant improvement in neonatal outcome after treating pPROM mothers with amnio-infusion?. Biol Neonate 2004;86:222-9. http://dx.doi.org/10.1159/000079657.
- Grisaru-Granovsky S, Eitan R, Kaplan M, Samueloff A. Expectant management of midtrimester premature rupture of membranes: a plea for limits. J Perinatol 2003;23:235-9. http://dx.doi.org/10.1038/sj.jp.7210880.
- Farooqi A, Holmgren PA, Engberg S, Serenius F. Survival and 2-year outcome with expectant management of second trimester rupture of membranes. Obstet Gynecol 1998;92:895-901. http://dx.doi.org/10.1016/S0029-7844(98)00287-7.
- Royal College of Obstetricians and Gynaecologists Greentop . Guideline No. 44. Preterm Prelabour Rupture of Membranes 2006. http://www.rcog.org.uk/files/rcog-corp/GTG44PPROM28022011.pdf (accessed March 2013).
- Hofmeyr GJ, Essifie-Appiah G, Lawrie TA. Amnioinfusion for preterm premature rupture of membranes. Cochrane Database Syst Rev 1998;1. http://dx.doi.org/10.1002/14651858.CD000942.pub2.
- London: King’s College; 2002.
- Powell CVE, McNamara P, Solis A, Shaw NJ. A parent completed questionnaire to describe the patterns of wheezing and other respiratory symptoms in infants and preschool children. Arch Dis Child 2002;87:376-9. http://dx.doi.org/10.1136/adc.87.5.376.
- The Magpie Trial Follow Up Study Management Group . The Magpie Trial Follow Up Study: outcome after discharge from hospital for women and children recruited to a trial comparing magnesium sulphate with placebo for pre-eclampsia (study protocol). BMC Pregnancy Childbirth 2004;4.
- Friedrich L, Stein RT, Pitrez PM, Corso AL, Jones MH. Reduced lung function in healthy preterm infants in the first months of life. Am J Respir Crit Care Med 2006;173:442-7. http://dx.doi.org/10.1164/rccm.200503-444OC.
- NICE . Therapeutic Amnionfusion for Oligohydramnios During Pregnancy (Excluding Labour) 2006. http://publications.nice.org.uk/therapeutic-amnioinfusion-for-oligohydramnios-during-pregnancy-excluding-labour-ipg192 (accessed 3 October 2013).
- Ogunyemi D, Thompson W. A case controlled study of serial transabdominal amnioinfusions in the management of second trimester oligohydramnios due to premature rupture of membranes. Eur J Obstet Gynecol Reprod Biol 2002;102:167-72. http://dx.doi.org/10.1016/S0301-2115(01)00612-1.
- Mujenovic F, Alfirevic Z. Procedure-related complications of amniocentesis and chorionic villous sampling. A systematic review. Obstet Gynecol 2007;110:687-94.
- Hsu TY, Hsu JJ, Fu HC, Ou CY, Tsai CC, Cheng BH, et al. The changes in Doppler indices of fetal ductus venosus and umbilical artery after amnioinfusion for women with preterm premature rupture of membranes before 26 weeks’ gestation. Taiwan J Obstet Gynecol 2009;48:268-72. http://dx.doi.org/10.1016/S1028-4559(09)60302-8.
- Horibe N, Ishikawa K, Kosaki H, Hashiba Y, Kuno N, Ito H, et al. The amnioinfusion therapy of saline solution for premature rupture of the membranes before 27 weeks gestational age. Nihon Sanka Fujinka Gakkai Zasshi 1993;45:1023-9.
- Tranquilli AL, Giannubilo SR, Bezzecheri V, Scagnoli C. Transabdominal amnioinfusion in preterm premature rupture of membranes: a randomised controlled trial. BJOG 2005;112:759-63. http://dx.doi.org/10.1111/j.1471-0528.2005.00544.x.
- ISRCTN Register . Trial of Umbilical and Foetal Flow in Europe: TRUFFLE Study n.d. http://www.controlled-trials.com/ISRCTN56204499 (accessed 3 October 2013).
Appendix 1 AMIPROM trial protocol
Appendix 2 Method for amnioinfusion
Appendix 3 Statistical analysis plan for short-term outcomes
Appendix 4 Statistical analysis plan for long-term outcomes
Appendix 5 Serious adverse event form
Glossary
- Abruption
- Premature separation of the placenta from the uterine wall.
- Amnioinfusion
- Returning fluid into the amniotic cavity under ultrasound control.
- Bradycardia
- Fetal heart rate below 110 beats per minute.
- Oligohydramnios
- Reduced amniotic fluid around the fetus.
- Perinatal mortality
- Death before, and up to 28 days after, birth.
- Pulmonary hypoplasia
- Small, underdeveloped lungs.
- Second trimester
- Weeks 13–28 of pregnancy.
- Very early preterm prelabour rupture of membranes (PPROM)
- Rupture of amniotic membranes between 16 and 24 weeks of pregnancy.
List of abbreviations
- AI
- amnioinfusion
- AMIPROM
- amnioinfusion in preterm premature rupture of membranes pilot study
- BSID-II
- Bayley’s Scale of Infant Development-II
- CGA
- corrected gestational age
- CI
- confidence interval
- CLRN
- comprehensive local research network
- CONSORT
- Consolidated Standards of Reporting Trials
- CPAP
- continuous positive airways pressure
- CRP
- C-reactive protein
- DMC
- Data Monitoring Committee
- Exp
- expectant management
- FEV1
- forced expired volume in 1 second
- FMU
- fetal medicine unit
- FRC
- functional residual capacity
- FVC
- forced vital capacity
- HDU
- high-dependency unit
- HFOV
- high-frequency oscillatory ventilation
- HTA
- Health Technology Assessment
- HVS
- high vaginal swab
- IPPV
- intermittent positive-pressure ventilation
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- ITU
- intensive therapy unit
- IVH
- intraventricular haemorrhage
- MDI
- Mental Development Index
- MREC
- Multiresearch Ethics Committee
- NEC
- necrotising enterocolitis
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PDI
- Psychomotor Development Index
- PI
- principal investigator
- PPROM
- preterm prelabour rupture of membranes
- PVL
- periventricular leukomalacia
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- VmaxFRC
- maximum flow at FRC
- WCC
- white cell count