Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/01/13. The contractual start date was in October 2010. The draft report began editorial review in April 2012 and was accepted for publication in April 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jill Francis has a number of research projects funded by research councils and charities, and funds for travel for dissemination purposes are included in some research grants. Geoff Bellingan has received SuDDICU grant funding from University of Aberdeen for investigator travel to research meetings. He is the principal investigator in a HTA-funded trial of enteral vs. parental nutrition in intensive care units, and has spoken on the SuDDICU study at other related meetings, for example Scottish Intensive Care Society, and received travel support to attend these meetings. Martin Eccles’s institution received money for his efforts on this study. Peter Wilson received support for travel to meetings for the study from Aberdeen University. He has received payment for his work as a member of the Drug Safety Monitoring Board, Infection Control Services, for medico-legal work, and for Henry Smith professional lectures.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Francis et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Each year in the UK, 140,000 patients are admitted to intensive care and, of these, almost 60,000 will die within a year of admission. 1 Hospital-acquired infections (HAIs) are a major clinical problem for modern health services. Critically ill patients requiring intensive care unit (ICU) care are extremely susceptible to HAIs and these infections are associated with high additional mortality, prolonged hospital stays and large health-care resource utilisation. Between 20% and 50% of ICU patients suffer from such infections. Reducing the incidence and mortality from HAIs is currently the focus of many intensive care quality improvement programmes and government initiatives in the UK and worldwide. 2,3
One intervention that has gained much attention in reducing HAIs is selective decontamination of the digestive tract (SDD). SDD involves the application of topical non-absorbable antibiotics to the oropharynx and stomach and a short course of intravenous (i.v.) antibiotics. 4 The evidence base relating to SDD is reasonably strong, with the recent Cochrane review reporting a benefit in terms of reducing pneumonia rates. 4 A recent large cluster randomised study from the Netherlands enrolling an impressive 5939 patients demonstrated a 3.5% reduction in adjusted mortality with SDD, although the conclusion remains controversial and the authors concede that ‘since our study was performed in Dutch ICUs with a low prevalence of antibiotic resistance, our findings may not be applicable to settings with a markedly different bacterial ecology or different practices for preventing ventilator-associated pneumonia’. 5
In the Cochrane review, clinical heterogeneity is a problem potentially resulting from combining studies using both topical [which, when used on their own in the absence of systemic (i.v.) antibiotics, is called selective oral decontamination (SOD)] and topical plus systemic antimicrobials in the same analyses. Included studies suffered from several methodological flaws including lack of blinding, lack of data on compliance with intervention, mixing of studies of diverse patient groups, only including subgroups or no description of studies included. 4 The Cochrane review demonstrated that SDD was associated with reduction in pneumonia [odds ratio (OR) 0.32; 95% confidence interval (CI) 0.26 to 0.38] and death (OR 0.75; 95% CI 0.65 to 0.87). 4 Since the Cochrane review, additional primary research has been published, which also showed a mortality benefit (OR 0.63; 95% CI 0.46 to 0.87). 5,6 However, a degree of controversy exists, with Hurley et al. 7 challenging that, across these studies, the incidence of ventilator-associated pneumonia (VAP) at baseline was significantly lower in the ventilated control groups than in the SDD groups, which could falsely give the impression of benefit. 8 Nonetheless, if the documented mortality benefit could be realised in UK practice, then it could prevent as many as 2000–3000 avoidable deaths per annum.
Despite this evidence base, the UK ICU community has not widely adopted this intervention, with only 10–15 ICUs out of 240 reporting that they undertake SDD. 9,10 Existing practice surveys and our preliminary investigations as to why this strategy has not been fully adopted suggest three possibilities:9,10
-
Provision of prophylactic broad-spectrum antibiotics to critically ill patients may be counterintuitive to the principles of antibiotic stewardship whereby clinicians are encouraged to use antibiotics in a rational and sparing way to prevent the development of multiresistant organisms. 11–14
-
The current evidence base is inadequate in two ways. 9,10 First, there is a perception that the magnitude of the reported mortality benefit is not biologically plausible for such an intervention. Second, there is concern about the external validity and generalisability of the evidence. Most of the existing SDD trials have been conducted in countries where infections due to multiresistant Gram-positive organisms are uncommon and the incidence of multiresistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA), is low. Patterns of Gram-negative resistance are also different between the UK and the Netherlands, the country with the greatest evidence base for SDD. Hence, clinicians who take an evidence-based stance may come to radically different conclusions about SDD because they may doubt the validity or the applicability of the evidence that suggests clinical benefit.
-
It is a common perception that implementation is difficult in practice as SDD is time-consuming and difficult to administer, although this may be a secondary point compared with the reasons outlined above. 10
However, these simple surveys fail to fully dissect the complex issues related to SDD use in the UK or internationally.
Many clinicians argue that existing evidence should be replicated in health-care systems in which infections due to multiresistant organisms are common and the incidence of multiresistant organisms such as MRSA and Clostridium difficile is comparatively high, such as in the UK. Furthermore, they argue that no existing study has included parallel high-quality infection surveillance programmes to study the long-term effects of SDD on the microbial ecology of the ICU. This may be the single most important weakness of the current evidence base and has brought about appropriate caution with the use of SDD. There is also the potential that clinicians believe that with the widespread use of chlorhexidine, especially for ventilated patients, coupled with the broad adoption of care bundles aimed at preventing VAP, there now may be no need for SDD. Existing data on the ecological impact of SDD are indeed limited, with some studies suggesting an increase in the incidence of Gram-positive organisms such as S. aureus and others failing to show such effects. 5,15–17 In addition, it is possible that SDD is so counterintuitive to existing views on antibiotic use that clinicians will not change their practice regardless of the evidence base or that one clinician group may prevent others in favour of the intervention from implementing it. For example, Silvestri et al. argue that ‘the longstanding disagreement amongst opinion leaders, with the predominance of detractors on those who advocate SDD, is an important factor contributing to the confusion’. 18 Other writers have called for immediate implementation of SDD into routine practice. For example, Zandstra et al. argue that ‘withholding SDD is now ethically questionable given the vast body of evidence on the technique reducing severe infections and mortality, requiring less antibiotic use, and providing less resistance’. 19
In summary, despite the limited surveys undertaken to date, little systematic evidence is available about clinicians’ beliefs regarding the existing evidence base, perceived benefits and risks of SDD in clinical practice, factors that influence current practice and the likely barriers to implementation. In addition, it is unclear whether or not further high-level evidence of clinical effectiveness and ecological impact of SDD from within the UK is required before implementation would become acceptable and what sort of study would be feasible and acceptable to clinicians and triallists.
The multimethod exploratory study reported here attempted to address these issues. It investigated the perspectives of a wide range of stakeholders in multiple settings, using a mixed-methods approach that combined observational, interview and questionnaire data analysed both qualitatively and quantitatively. To facilitate a robust approach informed by previous research and focused on the views and actions of health-care professionals, we used the theoretical domains framework (TDF) of health professional behaviour change to inform our programme of research. 20 It has a good fit with the kinds of issues that health-care providers consider when making clinical decisions and has been used in > 20 studies of health-care professional behaviour. 21 This framework enables systematic identification of a wide range of potential barriers to changing clinical practice. The TDF is elaborated and exemplified in Chapter 3. The results of this research programme thus reflect a comprehensive, theoretically robust and multifaceted evidence base to inform a decision about the kind of research that is needed to address the SDD issue.
A recent National Institute for Health and Care Excellence (NICE) and National Patient Safety Agency (NPSA) pilot study on patient safety made a strong research recommendation that SDD be subject to study including investigation of barriers to implementation. 22 The current clinical focus on HAIs, the move to making HAIs a key target of patient safety initiatives, the political prioritisation of HAIs and increased interest in this subject from research funding bodies indicated that this research was timely. The study [known as the selective decontamination of the digestive tract in intensive care units (SuDDICU) study] was also formally adopted and financially supported by the Intensive Care Foundation as one of its new UK National research studies. This highlights the importance of this question to the UK ICU community.
The variable uptake in SDD is also apparent in other countries outside of the UK, for example in Canada, Australia and New Zealand, where no ICUs currently deliver SDD. Reflecting the international importance of the topic, partner teams in Canada and in Australia and New Zealand also acquired funding to undertake parallel investigations (using the SuDDICU protocol) into the reasons for low uptake in these countries. These partner projects were each designed to stand independently (and were funded independently), but add to the generalisability of the UK study findings.
Research objectives and research questions
The overall aim of the SuDDICU study was to identify the perceived risks, benefits and barriers to the use of SDD in UK ICUs to inform recommendations for further research.
The investigation involved four inter-related stages (expanded further in Chapters 2–6) and culminated in an assessment of the need for – and acceptability of – an effectiveness trial, an implementation trial or further exploratory observational research. Figure 1 provides a linear representation of the four-stage study design and the associated objectives and research questions. It was our intention that the evidence from this investigation would then form the basis on which to design a trial or other study and to specify the intervention to be evaluated or, for an implementation trial, to develop the intervention to be evaluated.
FIGURE 1.
Design of exploratory study showing links to research questions. RQ, research question.
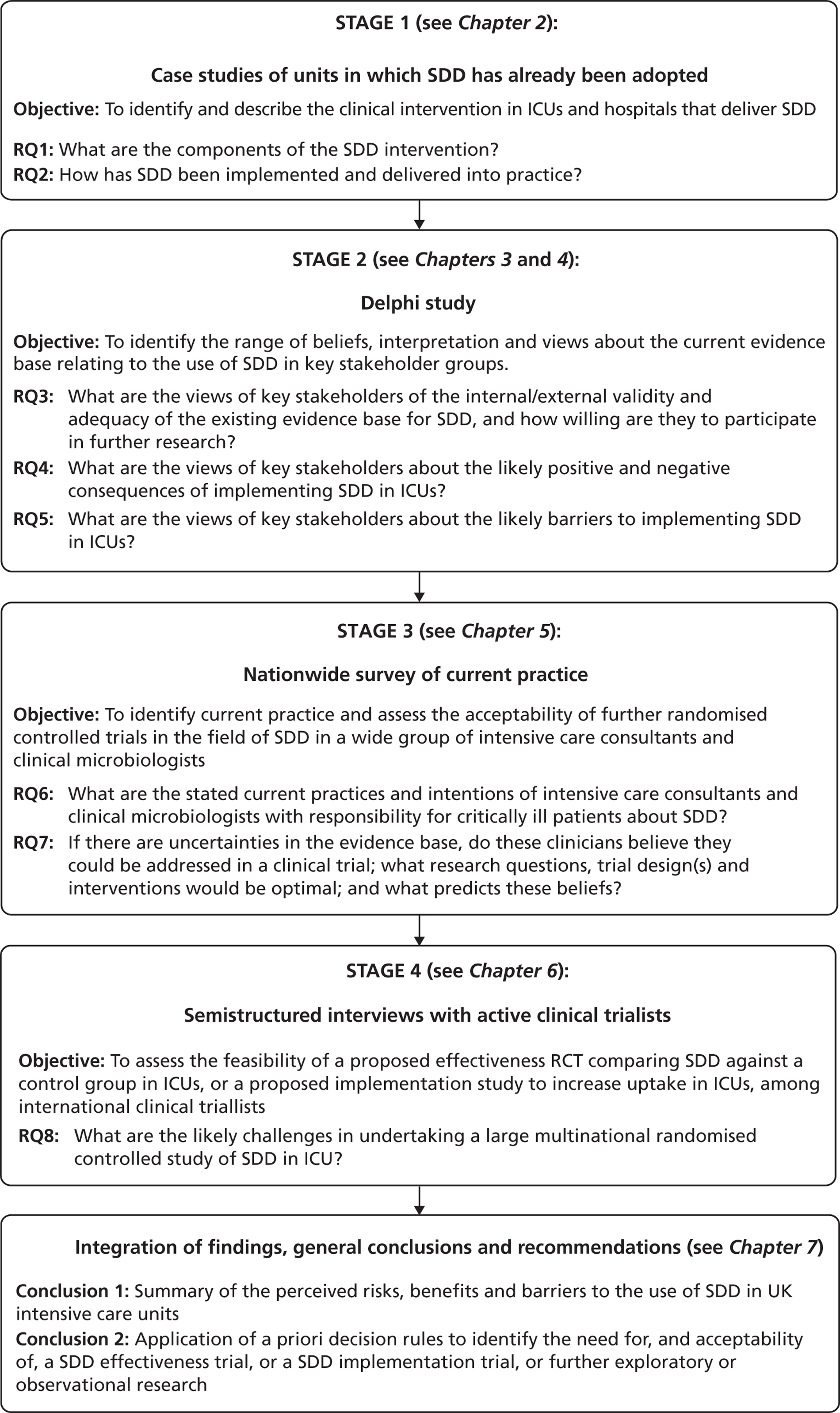
Reflecting the different stages of the research (as outlined in Figure 1), the results of the case studies are presented in Chapter 2, the results of the Delphi study are presented in Chapters 3 and 4, the results of the national survey are presented in Chapter 5 and the triallist interview data are presented in Chapter 6. In Chapter 7, the implications of the study as a whole are discussed, together with a summary of the implications for practice and for future research.
Chapter 2 Case studies to identify and precisely describe the clinical intervention in units and hospitals that deliver selective decontamination of the digestive tract
Background
Some of the uncertainty around the evidence relating to SDD may be associated with changes in the way SDD is specified in trial literature over time. A recent systematic Cochrane review noted that trials used different SDD protocols and investigators use different definitions for SDD. 4 Furthermore, some critics have proposed that SDD is difficult to deliver in practice. 10 Hence, before commencing a study to investigate clinicians’ views of SDD, it was important to be clear about its current specification and delivery in the UK practice setting. To this end, an observational study was conducted in two ICUs delivering SDD, to identify the similarities and differences in terms of the clinical and behavioural components (i.e. delivery features) of this intervention. Such specification would have implications for future research, but a more immediate objective was to ensure that all stages of this study were investigating SDD, based on an explicit and consistent definition. Thus, this study sought to address the following research questions:
Research question 1: What are the components of the SDD intervention?
Research question 2: How has SDD been implemented and delivered into practice?
At a more general level, health-care interventions are typically complex23 and involve two broad interacting categories of components: (1) clinical components, i.e. the clinical materials or equipment of the intervention and related features and (2) behavioural components, i.e. the actual behaviours required to deliver the intervention in practice. Health-care interventions are often specified clinically without explicitly addressing behavioural components. 24,25 Thus, interventions may be implemented differently across sites, potentially leading to variable effectiveness and resultant consequences for patient outcomes. The need to fully specify health-care interventions has been widely recognised, together with the need to report interventions in such a way as they could be directly replicated by others. 26
As described in Chapter 1, SDD is a complex intervention that has been shown to reduce HAI rates and mortality in critically ill patients. 4,5,27 SDD involves the application of antibiotics and antifungals to the mouth, throat and stomach combined with a short course of i.v. antibiotics. 28 Despite considerable evidence supporting the benefit of SDD,4,5,27 adoption internationally is low. 9,10 Among proposed reasons for this lack of adoption are controversies surrounding prophylactic use of antibiotics and associated risk of antibiotic resistance,29,30 and purported difficulty of SDD implementation and delivery. 31
In addition to the variation in the clinical components of SDD described in trials and used in clinical practice, behavioural components of SDD have not been systematically outlined in the empirical literature. A fully specified protocol describing both clinical and behavioural components of SDD implementation and delivery does not exist but could facilitate both widespread adoption and future implementation trials. Hence, this study sought to characterise the clinical and behavioural components of SDD as implemented in clinical practice.
Methods
Case study methodology32 was used in two UK ICUs routinely delivering SDD, with the ‘case’ (unit of analysis) consisting of an ICU. Data were collected from three sources: direct observation of SDD delivery at the bedside, face-to-face semistructured interviews with clinicians responsible for implementing and/or delivering SDD, and systematic assessment of written documentation (e.g. SDD protocols, training documents). The use of multiple data sources in case study research is considered to be one of its methodological strengths. 32 The chosen data sources were consistent with those commonly used in case studies and enabled triangulation for exploring the features of SDD delivery and implementation in context. 32
Sample
All UK ICUs delivering SDD, identified from a recent national SDD survey9 or known by the study investigators to deliver SDD, were deemed eligible for inclusion (15 ICUs). Two ICUs were purposively selected to represent different lengths of time since SDD adoption (one had adopted SDD < 5 years earlier and the other had adopted SDD > 5 years earlier) and different geographical locations (i.e. geographically dispersed ICUs to ensure different organisational profiles). Clinicians from different professions (i.e. intensive care consultants, clinical microbiologists, specialist clinical pharmacists and ICU nurses) have responsibility for the implementation and/or delivery of SDD. In each of the case study ICUs, all clinicians with potential involvement in the implementation and/or delivery of SDD were eligible for interview. From these, we recruited a purposive sample of clinicians from different professions. 33 Not all eligible clinicians were interviewed owing to lack of availability or time. Purposive sampling was appropriate for this small-scale exploratory qualitative study and the sample was not intended to be statistically representative. 33
Data collection
Direct observation offers the opportunity to record and analyse clinical behaviours and interactions as they occur in ‘real world’ contexts. 34 The use of direct observation as a data source allowed the process of SDD delivery to be ‘seen’ through the eyes of the researcher. Observations were conducted using an investigator-designed form to record all behaviours relating to ‘real time’ delivery of SDD. Additionally, the context (i.e. the physical environment where behaviours were performed), timing of procedures and physical presence of clinicians at time of delivery were recorded (see Appendix 1).
Semi-structured face-to-face clinician interviews were conducted in the study hospitals using a topic guide with prespecified prompts to ensure consistent coverage of key issues including behaviours relating to SDD implementation and SDD delivery as well as barriers and facilitators of described behaviours1 (see Appendix 1).
Finally, all written documentation relating to SDD implementation and delivery (e.g. SDD protocols, training documents) was provided by the participating ICUs for systematic analysis. This documentary information provided an unobtrusive and verifiable data source, which augmented and corroborated information from interviews and observations. The presence of relevant documents was established during the interviews. After the interviews, these documents were obtained.
Procedure
Data collection commenced with observation of SDD delivery performed by various ICU nurses to different patients at the bedside. A single researcher (SUD) visited the ICUs for 2 (case study 1) or 3 days (case study 2). Observation opportunities were identified by senior ICU nurses (i.e. they informed the researcher when and to whom SDD would be delivered). The number of patients eligible for, and receiving, SDD varied from day to day; therefore, it was not possible to prespecify the number of direct observations of SDD delivery that would occur during SUD’s visit. Semi-structured interviews were conducted in parallel with observations. Observed nurses were included in the interview sample to gain an in-depth understanding of observed behaviours. With participants’ permission, interviews were audio recorded, transcribed verbatim and anonymised. All observations and interviews were conducted by SUD. Written documentation from each ICU was examined (by SUD) following completion of all observations and interviews, in order to minimise researcher bias during these stages.
The written documentation was examined to identify clinical and behavioural components of SDD delivery. Clinical components were defined as the pharmaceutical regimens forming part of SDD including drug, dose, route, frequency and duration. Behavioural components were defined as any actions that were/would be directly observable. We recorded the behaviours involved in delivering clinical components and those not related specifically to drug administration.
Data management and analysis
Data from the three sources were analysed within case to describe the clinical and behavioural SDD components, and synthesised across case to identify emergent themes describing SDD implementation and delivery in context. The analytical process was guided by the study aims, which included identification of SDD clinical and behavioural components and exploration of SDD implementation and delivery.
The three data sources were analysed separately within ICUs and in reverse order to data collection. First, we systematically examined written documentation and extracted clinical and behavioural components of SDD delivery. Second, we performed content analysis35 of interview transcripts to identify additional behaviours involved in SDD delivery (i.e. those not specified in the documents). Third, direct observations provided contextual ‘real time’ data32 and identified new and corroborative evidence on SDD clinical and behavioural components (i.e. data triangulation from multiple sources). 32
To identify features of SDD implementation and delivery across ICUs, a thematic analysis of the interview data was conducted using a framework approach. 36 This approach involves five stages: (1) familiarisation with the raw data, (2) identification of emergent themes associated with SDD implementation and delivery (i.e. relating to the behaviours and clinician groups involved), (3) systematic coding/indexing of all data relevant to each theme within each transcript, (4) creating charts (Microsoft Word tables) that contain the coded data for each theme and distilled summaries of views and experiences, and (5) interpretation of the data (e.g. identifying associations between themes and providing explanations for the findings). 36 A single researcher (SUD) conducted the thematic analysis, a second researcher (EMD) independently coded randomly selected portions of the data set to identify clinical and behavioural components and three researchers (MEP, JJF, LR) provided critical comments on analyses drafts.
This study was classified as service evaluation by the Research Ethics Committee (10/MRE00/32) and, therefore, was deemed by them not to require ethical approval. All participants who were observed and interviewed were aware of the study purpose and provided verbal consent prior to data collection.
Results
Case 1 implemented SDD 3.5 years prior to this study in response to increased HAI rates. Collected data comprised four observations, eight interviews [intensive care consultants (n = 3), nurses (n = 3), clinical microbiologists (n = 1) and pharmacists (n = 1)] and three SDD documents (protocol, prescription chart, training slides). Case 2 implemented SDD as part of a clinical effectiveness trial 26 years prior to this study. Interview data identified that the rationale for continued use of SDD was its perceived effectiveness. Collected data comprised three observations, eight interviews [intensive care consultants (n = 3), nurses (n = 3) and pharmacists (n = 2)] and one document (protocol).
Selective decontamination of the digestive tract clinical and behavioural components
Protocols documenting the specific clinical behaviours required for drug preparation and administration in the two ICUs are detailed in Table 1, demonstrating the degree of clinical complexity and also the variation encountered in clinical aspects of SDD. The documents identified in the interviews and subsequently analysed listed nine different medications and a total of 13 different preparations as part of SDD in the two case studies (see Table 1). Several behaviours directly relevant for drug administration were identified in examined documentation.
| Drugs | Dose | Route | Frequency | Behaviours (what) | Directions (how) | Frequency/duration (when) |
|---|---|---|---|---|---|---|
| Case study 1 | ||||||
| Cefuroxime | 1.5 g (six doses) over 3–5 minutes | i.v. | 8-hourly | Prepare drug, administer drug | Dilute 1.5 g in 15 ml of water for injection. Administer intravenously over 3–5 minutes | Immediately after obtaining all admission surveillance and diagnostic microbiological samples and then at 8-hourly intervals |
| Ciprofloxacin (if allergic to cefuroxime) | 400 mg (four doses) over 60 minutes | i.v. | 12-hourly | Prepare drug, administer drug | Administer 400 mg intravenously over 60 minutes | Immediately after obtaining all admission surveillance and diagnostic microbiological samples and then at 12-hourly intervals |
| Nystatin | 100,000 units/ml | Oral and gastric tube | 8-hourly | Prepare drug, administer drug | Administer 5 ml topically to mouth and 5 ml via gastric tube. Use a new 30-ml bottle every 24 hours. If the tube in the stomach is draining freely into a drainage bag, flush tube with 20 ml sterile water and clamp for 30 minutes after administration of antibiotics/antifungals | Three times daily after oral hygiene regimen |
| Vancomycin | 500 mg | Oral and gastric tube | 6-hourlya | Prepare drug, administer drug | Reconstitute a 500-mg vial with 10 ml water for injections and administer 250 mg into the mouth and 250 mg via gastric tube | Four times daily after oral hygiene regimen |
| Colistin sulphate | 250,000 units/ml | Oral and gastric tube | 6-hourlya | Prepare drug, administer drug | Reconstitute a vial (licensed for injection) of 1,000,000 units with 0.9% sodium chloride (NaCl). Dilute the reconstituted vial to a total of 40 ml with NaCl 0.9%. This solution may be kept at the bed space for 24 hours. Administer 5 ml (125,000 units) of this solution into the mouth and 5 ml via gastric tube | Four times daily after oral hygiene regimen |
| Tobramycin | 80 mg | Oral and gastric tube | 6-hourlya | Prepare drug, administer drug | Dilute one ampoule of 80 mg (licensed for injection) in 10 ml NaCl 0.9%. Give 5 ml (40 mg) into mouth and 5 ml (40 mg) by gastric tube | Four times daily after oral hygiene regimen |
| Chlorhexidine gluconate; 4% liquid soap | 15 ml | Topical | 12-hourly | Administer body wash | Use 15 ml for body wash with water | Twice daily |
| Chlorhexidine gluconate; 0.2% mouthwash | 10 ml | Topical | 6-hourlya | Administer mouthwash | Not to be swallowed. Apply with pink sponge stick to teeth, gums, tongue and lining of the mouth as part of thorough mouth care | Twice daily before each application of topical antibiotics |
| Case study 2 | ||||||
| Cefotaxime | 1 g | i.v. | 8-hourly | Prepare drug, administer drug | Administer 1 g intravenously | Within 4 hours of admission. Administer first does prior to
intubation and other invasive ITU procedures Three times daily only for first 4 days of ITU admission |
| Tobramycin, colistin sulphate, amphotericin B, prepared by pharmacy manufacturing unit | 2% w/w of each constituent | Topical | 6-hourlya | Administer gel to oropharynx | Apply gel to palate and buccal surfaces | Within 4 hours of admission. Four times daily for duration of ITU admission until discharge |
| Tobramycin 27 mg/ml liquidb | 80 mg | Nasogastric tube | 6-hourlya | Administer solution/suspension | Deliver solution/suspension via nasogastric tube | Four times daily for duration of ITU admission |
| Colistimethate sodium (colistin) 50 mg/ml liquidb | 100 mg | Nasogastric tube | 6-hourlya | Administer solution/suspension | Deliver solution/suspension via nasogastric tube | Four times daily for duration of ITU admission |
| Amphotericin B 100 mg/ml liquidb | 500 mg | Nasogastric tube | 6-hourlya | Administer solution/suspension | Deliver solution/suspension via nasogastric tube | Four times daily for duration of ITU admission |
Aside from clinical and behavioural components directly relevant to SDD delivery, documents from both cases revealed several additional delivery behaviours performed by multiple clinicians in various clinical and environmental contexts (Table 2). To complement understanding of behavioural components that are important in SDD, but not specifically mentioned in the examined documentation, Table 3 outlines additional delivery behaviours identified through interviews and observations. Behaviours outlined in Tables 2 and 3 were performed by various clinician groups (e.g. nurses, physicians, pharmacists) in a variety of clinical and environmental contexts (e.g. bedside, ICU nursing stations, pharmacy).
| Behaviour | Professional group | Context | CS1 | CS2 |
|---|---|---|---|---|
| Clarifying SDD regimen (in ambiguous cases) | Nurse, intensivist, pharmacist, clinical microbiologist | ICU and bedside | ✓ | ✓ |
| Authorise SDD delivery | Intensivist, pharmacist | ICU (admission) and bedside | ✓ | |
| Prompt SDD authorisation | Nurse | ICU (admission) and bedside | ✓ | |
| Judging SDD delivery in unclear cases | Intensivist | ICU (admission) and bedside | ✓ | |
| Documenting SDD delivery | Nurse | ICU and bedside | ✓ | |
| Discarding of antibiotics (when out of date) | Nurse | Bedside | ✓ | |
| Storing reusable antibiotics | Nurse | ICU and bedside | ✓ | |
| Labelling leftover antibiotics/antifungals | Nurse | ICU and bedside | ✓ | |
| Check SDD is continued and operating | Intensivist, pharmacist | ICU, bedside | ✓ |
| Behavioural | Professional group | Context | CS1 | CS2 |
|---|---|---|---|---|
| Check patient eligibility for SDD | Intensivist, pharmacist | ICU (admission) and bedside | ✓a | ✓a |
| Review and optimise SDD delivery | Intensivist, pharmacist, clinical microbiologist | ICU, bedside | ✓a | ✓a |
| Attend ward rounds (at which SDD is discussed) | Intensivist, pharmacist, clinical microbiologist | ICU, bedside | ✓a | ✓a |
| Dispose of SDD waste | Nurse | Bedside | ✓a,b | ✓b |
| Order SDD drugs from pharmacy | Nurse | ICU | ✓a | ✓a |
| Reassure patient/patient visitors before/during SDD administration | Nurse | Bedside | ✓b | ✓a,b |
| Reposition patient for SDD administration | Nurse | Bedside | ✓b | ✓b |
| Decision to discontinue SDD drugs | Intensivist, pharmacist | ICU and bedside | ✓a | ✓a |
| Print SDD documentation | Ward clerk | ICU | ✓a | |
| Monitor for SDD drug reactions | Intensivist, pharmacist | Bedside | ✓a | |
| Check stock and supply SDD drugs | Pharmacy technician | ICU | ✓a | |
| Order SDD drugs from suppliers | Pharmacy technician | ICU | ✓a | |
| Describe SDD during shift communication | Nurse | ICU and bedside | ✓a | |
| Handling contraindications | Nurse | Bedside | ✓b | |
| Collecting SDD drugs | Nurse | ICU and bedside | ✓a,b | |
| Preparation of antibiotics | Pharmacist | Production unit2 | ✓a | |
| Order raw materials | Pharmacist | Analytic lab2 | ✓a | |
| Check of antibiotic quality | Pharmacist | Quality Assurance Department2 | ✓a | |
| Liaise with pharmacy production unit | Pharmacist | ICU | ✓a | |
| Check naso/orogastric aspirate | Nurse | Bedside | ✓a,b |
Participant interviews provided most data relating to behavioural components, 49 components were identified through interviews, 22 through documentation and 12 through observations. Each data source gave rise to unique behaviours not mentioned in other sources (28, seven and four unique behavioural components for interviews, documentation and observations, respectively), confirming the added value of analysing multiple information sources. The number of unique behavioural components was 29 (case study 1) and nine (case study 2). Twenty-six behavioural components were common across ICUs, being identified in at least one data source for each case.
Selective decontamination of the digestive tract implementation and delivery
Based on our analysis, SDD implementation and delivery was conceptualised as a complex procedure consisting of four overlapping processes, each involving specific behaviours: adoption, operationalisation, provision and surveillance. Adoption concerned the decision to introduce SDD; operationalisation referred to the processes required to introduce SDD into clinical practice; SDD provision included actions involved in delivery of the clinical components; and surveillance, mentioned in both case studies, provided the foundation for adoption, operationalisation and provision by checking that SDD was effective in preventing infection.
Adoption and operationalisation
For adoption, we identified that actions often occurred at the organisational and team level involving organisational and group processes as well as individual action. As the implementation process moved from adoption to operationalisation, more behaviours emerged that were performed by individual staff (see Tables 2 and 3). Although operationalisation was complete following SDD introduction, elements of operationalisation continued owing to clinician staff turnover (e.g. although SDD was a standard procedure within the ICUs, the low national baseline adoption meant that additional training for clinicians new to these ICUs and SDD delivery was required).
Provision of selective decontamination of the digestive tract
Three themes emerged from the interviews on SDD provision: complexity/difficulty, protocol adaptation in practice, and facilitators and barriers.
Complexity/difficulty
Reflecting the theme of complexity, one intensive care consultant and several nurses reported that SDD provision represented additional and time-consuming work that made it unpopular with staff. When examining the sequencing and flow of actions, we identified evidence of complexity – multiple clinicians were involved in managing various behaviours within multiple clinical and environmental contexts using a range of materials delivered in specific sequences in a continuing flow of action (see Box 1 for quotations: P, participant). However, most nurses and doctors refuted the idea that SDD was complex and time-consuming, stating that providing SDD was effortless (Box 1). Low complexity/difficulty of SDD for these staff was supported by observational data indicating that administration of clinical components took no longer than 5 minutes, often less, and was performed in a swift sequence of actions. However, it is important to note that these were highly practised actions and may require considerable skill development to achieve this high level of expertise.
Supporting difficulty of providing SDD:
. . . there is extra work, four times a
day . . .
P1
. . . it’s relatively unpopular with
most of the nursing staff [. . .] because they
see it as excess workload.
P10
. . . delivery [. . .] can be
difficult.
P5
It only takes five/ten minutes, although that is another
five/ten minutes added on to the other five/ten minutes for
everything else that you have to do.
P7
Not supporting difficulty of providing SDD:
. . . it’s a part of your routine
already so I don’t find it difficult, it’s just
finding ways of how to do it, I mean it’s not too
difficult.
P6
[SDD provision] is really straightforward.
P7
. . . very simple [. . .], a
fairly straight forward thing to do.
P3
. . . the main message to take across is that
it’s, it works well. It is very easy to do.
P13
I don’t find it difficult.
P14
It is not that hard. It is really
straightforward.
P15
Supporting complexity of providing SDD:
[Overall, SDD delivery] involves a large amount of
co-operation between the microbiologists, the nursing staff and
the medical staff to [. . .] maintain an
appropriate antibiotic policy; it also involves
[. . .] quite a lot of monitoring of what is
involved with the patients [. . .] so that we
can manage the infections appropriately [. . .]
it involves applying some paste and some nasogastric SDD, but
these are relatively minor parts of the whole. It is a system of
which that is part.
P11
Protocol adaptation in practice
Protocol adaptation in SDD delivery was noted in observational and interview data. Preparation of antibiotics/antifungals varied, suggesting some deviation from recommended practice. Further adaptation was evident in the provision of SDD oral components such as different ways of applying oral drug components and timing with other nursing interventions such as oral hygiene. Authorising SDD involved multiple staff and deviation from recommended practice was noted. Although documentation indicated that patients should be routinely commenced on SDD, this did not always occur, owing to more pressing clinical concerns. As a result, multiple layers of control to ensure protocol adherence were described (Box 2).
. . . although it says the dose is 500 mg I
have been taught, in order to better manage my time, that I use
[a] 1 g bottle instead and instead of reconstituting it with 10
ml I reconstitute it with 20 ml.
P5
‘I have different ways [. . .] because
there are a lot of antibiotics’ and he/she did
not ‘know if it’s a good thing to mix all 4
antibiotics in one go and put them orally in one go
also’ and that
‘. . . others might do it
differently’.
P14
. . . it sometimes slips off the main agenda
of the patient’s day . . .
P8
I would ensure that all the relevant people get
SDD.
P17
I just make sure it is being put on.
P11
. . . if they haven’t prescribed it,
I’ll ask them to prescribe.
P14
Facilitators and barriers
Facilitators and barriers to SDD delivery were evident across both cases (Box 3). One facilitating factor frequently reported was dovetailing of SDD with other established and routine procedures. Thus, intensive care consultants might include SDD delivery behaviours as part of the admission process, nurses might include SDD as part of oral hygiene or other activities and clinical microbiologists and pharmacists dovetailed SDD actions within ward rounds. Dovetailing was evident in multiple interviews and in documentary data on SDD provision for oral hygiene. Although barriers were commonly reported during interviews in response to specific prompts, these were often referred to as minor inconveniences, rather than significant obstacles to SDD delivery (see Box 3).
-
Policies and protocols, e.g. ‘We have an admission policy, so [patients] come in and we have a set of investigations and [. . .] they’ll get SDD and [. . .] that’s just part of the admission’ [P10].
-
Patient state, e.g. ‘patient is deeply sedated, it’s easier’ [P1].
-
Perceived effectiveness, e.g. ‘the fact that you have a very few incidents of pneumonia’ [P17].
-
Colleague support, e.g. ‘if you’re working side by side with a nurse, that nurse will help you’ [P5].
-
Dovetailing, e.g. ‘you just tag it on with your aspirating stomachs’ [P15].
-
Workload, e.g. ‘When it’s a really busy day then it gets a lot to do’ [P5].
-
Patient state, e.g. ‘if they’re intubated and they’re just maybe biting’ [P6].
-
Side effects, e.g. ‘patients tend to get more diarrhoea when they are [on] SDD’ [P1].
-
Staff changes, e.g. ‘losing a senior microbiologist was a stress, he was very supportive’ [P10].
-
Cost, e.g. ‘The main challenges are the cost. The drugs themselves cost a lot of money’ [P10].
-
Materials, e.g. ‘there’s been a few supply problems over the last couple of years. Sometimes [. . .] there can be national shortages which can be a bit of a problem’ [P16].
Infection surveillance
A fourth theme emerged from the documentary analysis. Surveillance was specified in the SDD protocol in one of the case study sites, but not in the other, in which it was part of the wider regimen to combat HAIs. Despite these differences, surveillance was integral to the provision of SDD and included the performance of multiple behaviours of various clinicians in several clinical and environmental contexts.
Discussion
In line with frameworks for intervention development23 and description,26 this study has specified (for the first time) the full clinical and behavioural components of SDD and has described how they impact on SDD implementation and delivery. There are several advantages of specifying an intervention behaviourally alongside clinical specifications. First, it demonstrates procedural complexity and the situations in which complexity may be experienced. This information has direct relevance to clinicians and hospital decision-makers considering implementation of particular health-care interventions. It also can inform the scale and content of implementation strategies to facilitate diffusion and adoption within specific contexts. 37 Second, behavioural specification identifies potential areas where behavioural variation in practice may occur and, thus, allows prior specification of acceptable limits of protocol adaptation. Third, it may identify training needs to facilitate adherence to an expected standard. Fourth, behaviour specification facilitates precision in protocols and training materials by describing who should do what and when and where this should occur.
Answers to research questions
We found variation in SDD clinical components, in terms of the drug regimen, mode of drug delivery and specification of components (i.e. surveillance) between the two study sites. This may be appropriate to make the intervention simple and feasible to deliver within a local context. Various behaviours related directly to drug provision as well as other aspects of the SDD intervention (e.g. authorisation of SDD delivery) were performed by multiple clinicians in differing contexts. In terms of clinical components, topical antibiotics/antifungals and i.v. antibiotics were identified as SDD components in documents, but surveillance, general hygiene and general infection control regimen were not. Such inconsistency is also identified in the literature. 18 Both ICUs administered i.v. as well as topical/oral components. Overall, SDD implementation and delivery comprised the interrelated phases of SDD adoption, operationalisation, provision and surveillance.
Additional behaviours to those specified in documentation were identified and these behaviours are essential for SDD delivery. SDD involved a range of health-care professionals performing various behaviours in differing contexts. These findings emerged in the interview and observational evidence but were not always clearly specified in the documentation. Ensuring that these additional behaviours are specified in protocols, guidelines and the academic literature should lead to improvements in implementation, delivery and reproducibility of SDD. 24,25
Various behaviours were identified for SDD implementation, many at the organisational and team levels and others at the individual level. Several features of operationalisation involved an ongoing process (e.g. nurse training for SDD provision) as a result of staff turnover. SDD could thus be construed as a simple and easy intervention from the individual behavioural perspective that becomes increasingly complex when focusing on the flow of actions required at an organisational level for its delivery in practice. Consequently, some of the barriers and facilitators to SDD provision tended to centre on the environmental context and resource issues, rather than specific attitudinal (e.g. beliefs about SDD effectiveness) or skills barriers.
Strengths and limitations
The current study is the first to systematically identify and specify a full range of SDD components throughout the steps of SDD implementation and delivery. A limitation is the potential lack of generalisability owing to the use of two cases only. Additional clinical and behavioural components, as well as alternative methods of SDD implementation and delivery, may be evident if investigating SDD practice in a larger number of ICUs. However, the study was exploratory in nature with the goal of providing information-rich case studies that facilitate in-depth understanding of SDD in practice rather than a comprehensive picture of SDD across all UK ICUs. We recruited one microbiologist only, limiting the perspective from this profession. Finally, clinicians in ICUs that did not deliver SDD may have different views about barriers to SDD implementation. This was investigated in subsequent stages of the study.
Conclusion
This study was the first to provide a formal specification of the full clinical and behavioural components of SDD. We described a wide range of behaviours involved in delivering SDD, several of which were not included in local SDD protocols. Significant protocol adaptations resulting from these behaviours were observed across sites, suggesting the need for routine behavioural specification in SDD delivery protocols. Such specification would greatly facilitate the subsequent detection of acceptable variations and those that may lead to significant differences in patient outcomes.
Key messages from the case studies are reported in Box 4. The findings of this study phase informed the next stages of the current study in the following way. At the start of interviews or questionnaires that sought clinicians’ views about SDD, we first defined the clinical components of the intervention. For brevity, we did not specify the behavioural components; however, such specifications would be an important aspect of future trial design.
-
Delivering selective decontamination of the digestive tract included more than the provision of clinical components and involved multiple behaviours performed by multiple clinical team members.
-
Not all behaviours relevant for SDD provision were specified in SDD documentation.
-
SDD implementation included the interrelated phases of deciding whether or not to implement SDD (adoption phase) and deciding how to implement SDD (operationalisation phase), with both phases involving organisation-, team- and individual-level behaviours.
-
There was some complexity in the interplay and flow of the clinical and behavioural components of SDD, involving multiple staff. However, provision of SDD was simple from the perspective of individual staff and delivery was regarded as straightforward.
-
Infection surveillance provided the foundation for SDD implementation and delivery, but may not be seen as part of the SDD regimen itself.
Chapter 3 Delphi study to identify stakeholder views about selective decontamination of the digestive tract: round 1 interviews
Background
The second stage in the study was an in-depth investigation, using Delphi methods, of the views of key stakeholders most likely to have decisional authority with respect to local SDD policy (i.e. those people most likely to be involved in the decision to adopt SDD). This stage of the study was conducted in collaboration with a multinational research team, with parallel studies being conducted in Canada, Australia and New Zealand [funded by the Canadian Institutes of Health Research, Intensive Care Foundation (Australia) and the Australian and New Zealand College of Anaesthetists Foundation].
The Delphi approach uses a structured, iterative process including anonymised feedback, in a series of sequential questionnaires or ‘rounds’. The Delphi approach has been widely applied in health-care research. 38–40 Although the approach was originally designed to achieve expert consensus,41 it has developed into a method that can also assess levels of agreement (or disagreement) within an expert group. 42,43 The objective of assessing consensus rather than achieving consensus was the goal of this study phase and influenced a number of the design features.
The Delphi study was thus designed to generate iterative evidence about consensus and stability of views about SDD and addressed the following research questions:
Research question 3: What are the views of key stakeholders of the internal/external validity and adequacy of the existing evidence base for SDD and how willing are they to participate in further research?
Research question 4:. What are the views of key stakeholders about the likely positive and negative consequences of implementing SDD in ICUs and what is the relative importance of these beliefs in influencing overall views about SDD?
Research question 5: What are the views of key stakeholders about the likely barriers to implementing SDD in ICUs?
The Delphi study comprised an initial exploratory round involving interviews (reported in this chapter), followed by two iterations (reported in Chapter 4) using items generated from interview data. Hence, the first Delphi round was used to generate round 2 items that represented the full range of views raised, so that all could then be considered by participants in later rounds. 1
Methods
The round 1 interviews were based on a topic guide (further details below) that was arranged in three sections:
-
Asking about, and establishing, a definition of SDD (based on findings from the case studies).
-
Asking about the likely consequences of delivering SDD and potential barriers to implementation. This section was based on the TDF (see Table 4). 20
-
Asking about participants’ willingness to participate in further research.
The TDF was developed to facilitate coverage of a full range of potential opinions about, and barriers to, the use of health-care procedures. 21 It was developed from 33 theories of individual and organisational behaviour to assist researchers to identify constructs likely to influence health professionals’ behaviour. 20 The framework proposes that determinants of health professionals’ behaviour cluster into 12 domains. Each domain includes constructs from a number of behavioural theories that are potentially overlapping. One domain of particular relevance to this study is labelled Beliefs about consequences, as it relates directly to research question 4. Table 4 presents the labels and descriptions of the 12 TDF domains. These descriptions were used to guide the data analysis.
| Domain label | Description |
|---|---|
| Behavioural regulation |
|
| Beliefs about capabilities |
|
| Beliefs about consequences |
|
| Emotion |
|
| Environmental context/resources |
|
| Knowledge |
|
| Memory, attention and decision processes |
|
| Motivation and goals |
|
| Nature of the behaviours |
|
| Professional role and identity |
|
| Skills |
|
| Social influences |
|
Sample
A Delphi process gauges views from a panel of experts. 44 Ideally, potential Delphi participants would thus be experts in delivering SDD, or experts in terms of their knowledge of the SDD evidence base. Because of the low SDD uptake in UK ICUs at the time of this study, restricting the study sample to those with direct experience of SDD delivery or with a special interest in SDD research could systematically bias findings in favour of SDD adoption and delivery. Therefore, we decided to define ‘expertise’ more broadly to include the four stakeholder groups likely to exert decisional authority with regard to an ICU’s SDD policy or to how such a policy would be implemented in practice. Hence, the participants for the Delphi study were intensive care consultants, ICU pharmacists, clinical microbiologists with ICU responsibility and an ICU leaders group (including medical leads, nurse managers and educators working in NHS hospitals throughout the UK).
There is a broad range of estimates of suitable sizes for a Delphi panel, but smaller sizes (such as 10 for each stakeholder group) have been deemed appropriate where panel members have similar training. 45 Our minimum target sample size was thus set at 40 (10 in each stakeholder group). We also sought participant representation from across the four UK home nations.
Three clinical members of the research team (GB, APRW, RS) compiled lists of their clinician group and ranked them according to predetermined diversity factors (location, ICU size, current SDD practice and academic affiliation). A list of ICU nurse managers/educators was compiled by three members of the research team (GB, BHC, MEP) and ranked for the above diversity factors. Study invitations were issued to individuals according to rankings and in order of approvals made by the research and development (R&D) offices of each participating hospital trust. Sample diversity was tracked during recruitment. Additional participants from stakeholder lists (those working in NHS Trusts for which we had R&D approval) were specifically targeted to maximise variation. To preserve a minimum sample size of 10 in each stakeholder group by Delphi round 3, we oversampled by one to three participants in each of the four groups.
Data collection
At the start of each interview, to establish a shared understanding of SDD components, participants were first asked what they understood ‘selective decontamination of the digestive tract’ to mean. Irrespective of their initial definition of SDD, participants were, for the remainder of the interview, asked to consider SDD as application of antibiotics comprising all the following: (1) oral administration, to the mouth and throat, (2) gastric application via a nasogastric tube or similar and (3) a short course of i.v. antibiotics. We then asked whether or not SDD was delivered in the participant’s ICU. Responses to this second question determined which of two topic guides was used for the remainder of the interview (‘ICU currently delivering SDD’ or ‘ICU not currently delivering SDD’). Both topic guides are presented in Appendix 2 and included questions about:
-
factors that might influence adoption of SDD, such as participants’ views about the likely positive and negative consequences of SDD and their knowledge of the evidence base
-
participants’ views on the need for further research to settle questions around harms/benefits of SDD, what type of research [an effectiveness study or an implementation study (i.e. a study to evaluate strategies to increase uptake of SDD)] would be most informative and whether or not further effectiveness research on SDD was ethical
-
whether participants would be willing to participate in an effectiveness study to evaluate SDD and/or an implementation study to assess strategies that aim to increase uptake.
Procedure
Potential participants from stakeholder groups were invited to take part by an investigator-signed e-mail invitation (GB, APRW or RS). Expressions of interest were followed up with a short telephone call or e-mail from the study co-ordinator to further describe the study, answer any questions and, if the participant agreed to take part, arrange a convenient time for a 30-minute telephone interview. After 1 week, non-responders were sent a reminder e-mail signed by the appropriate clinician researcher. No further contact with non-responders was attempted. Recruitment continued until target sample sizes were achieved for each stakeholder group.
At the start of each telephone interview, participants were reminded that the aim of the study was to identify their personal views and opinions on SDD (there were no right or wrong answers). Consent to audio record the interview was requested and received from all participants.
Data management and analysis
Recordings were transcribed verbatim, checked for accuracy and anonymised. The objective of the analysis was to develop questionnaire items for the second round of the Delphi study, the quantitative questionnaire round. Analysis of the transcripts proceeded through a number of stages. First, ‘specific beliefs’ were identified within the transcripts. A specific belief was defined as a statement for which the content may indicate a perceived influence on SDD adoption or delivery. Specific beliefs that expressed the same theme or were polar opposites of the same theme were grouped together and were considered as repeats of the same belief. Summary statements representing these beliefs were devised, and these became the basis of the round 2 questionnaire items. This analysis was performed using an iterative and parallel process with the SuDDICU Canada team (who had adopted an identical topic guide and sampling strategy). All summary statements identified in the analysis were discussed by an international working group of study investigators to identify appropriate wording for representing the beliefs in round 2 of the Delphi study. When it was justifiable from the interview data, identical wording of questionnaire items was agreed across nations. When specific beliefs emerged only in the UK study, these were included in the UK version of the round 2 materials.
The next stage in the analysis involved allocation of the specific beliefs to the prespecified TDF domains. This was carried out independently by two researchers (JJF and one research assistant) using the TDF as an analytic framework and content analysis methods previously employed by the research team in the context of intensive care. 46 When there was disagreement between coders, these were discussed with a third researcher (MEP). Agreement was achieved for 42 of the 46 UK items; the remaining four items, for which discrepancy in domain allocation persisted, were discussed with an expert group of 16 researchers who were familiar with the TDF (the Aberdeen Health Psychology Group) and the majority view was taken for domain allocation.
To address research question 4 we examined the Beliefs about Consequences domain in detail. A preliminary assessment was made of the perceived importance of each specific belief in this domain. In round 1, assessment of importance was based on evidence from cognitive psychology, which identifies that the ‘cognitive accessibility’ of a belief (i.e. the readiness with which it comes to mind) is an indicator of its importance to an individual. 47 In ‘social cognitive’ models of planned action,48,49 importance is assessed at the group level by identifying the ‘modal salience’ of the belief, i.e. how many times the same belief is elicited across the whole sample of study participants, relative to other beliefs. Hence, the frequency with which a belief was elicited in the interviews was taken as an indicator of its relative importance. It was recognised that this procedure is based on a range of assumptions and so this early indicator of importance was later compared with data from rounds 2 and 3 (reported in Chapter 4), in which participants were asked to report their ratings of the importance of each belief. Importance was also examined quantitatively in the national survey (see Chapter 5) so that these three methods for assessing the relative importance of consequences could be compared. This was done to ensure that robust evidence could be used to identify the most important outcome measures to include in a possible effectiveness trial of SDD.
Using similar assumptions, the importance of each theoretical domain was assessed, in round 1, by identifying the level of elaboration provided by participants in their responses to the interview questions. The relative importance of the domains would identify key barriers to implementation, which would provide an evidence base for the design of an intervention to increase uptake in a possible implementation trial of SDD. For each domain, we identified how many utterances were coded, how many specific beliefs were generated across the sample and the content of those beliefs. For example, if the belief was essentially that the issue was not important or not a problem, this content was taken into account.
The project was coordinated by the Health Services Research Unit, University of Aberdeen. Ethics approval for Delphi study was granted by NHS North of Scotland Ethics Service (10/S0801/69).
The project was subject to extreme delays and difficulties in obtaining research governance approvals for sites in England, Northern Ireland and Wales. These delays negatively impacted upon recruitment and hence may have compromised our attempt to sample for diversity. Despite repeated attempts to contact trusts, 15 NHS trusts had failed to issue a decision on research governance by the time data collection closed (6 months after submitting the documentation). Further details can be found in Appendix 3.
Results
Participant characteristics
Ninety-four health professionals were invited to take part. Eight participants were excluded as not meeting eligibility criteria (had left the hospital or their profession) and three e-mails were returned undelivered. Forty-seven participants consented to participate and were interviewed (57% consent rate). Consent rates were highest among pharmacists (92%) and lowest among clinical microbiologists (39%), shown in Table 5.
| Stakeholder group | Invitations | Participants | Consent rate |
|---|---|---|---|
| ICU physicians | 20 | 12 | 60% |
| ICU pharmacists | 12 | 11 | 92% |
| Medical microbiologists | 28 | 11 | 39% |
| ICU clinical leads/ICU nurse managers/educatorsa | 23 | 13 | 57% |
| Total | 83 | 47 | 57% |
The mean age of the 47 participants was 45.7 years and 31 (66%) were male. Participants were working in 25 different hospitals with ICU bed numbers ranging from 8 to 75. Five participants worked in ICUs that currently delivered SDD, another five had personal experience of SDD from previous positions and the remainder had no direct experience with SDD. Participants had a mean of 18.2 [standard deviation (SD) 7.2] years of ICU experience. Participants were recruited from all four home nations (30 participants from England, 12 from Scotland, two from Wales and one from Northern Ireland).
Importance analysis at the level of theoretical domains
Table 6 provides details of the number of times participants made comments that were coded into each theoretical domain and a brief description of the content of each domain, in so far as it indicates the perceived importance of the domain.
| Domains (ranked in terms of frequency in column 2) | Number of utterancesa (from 47 interviews) | Brief description of content | Number of items generated for round 2 (specific beliefs) |
|---|---|---|---|
| Beliefs about consequences | 470 | Highly elaborated by participants and discussed as being an important influence on the adoption of SDD | 18 |
| Memory, attention and decision processes | 154 | Decision-making processes relevant to adoption of SDD were discussed as an important influence | 3 |
| Knowledge | 154 | Participants reported variable knowledge of the evidence base for SDD and observed that this would need to be addressed before SDD could be adopted | 3 |
| Motivation and goals | 122 | A lack of motivation to adopt SDD was highlighted by participants as being an important barrier to adoption | 6 |
| Environmental context and resources | 90 | Discrepancies between the clinical contexts in which evidence has been gathered and participants’ own clinical contexts were reported to be an important influence on relevance of the evidence to the UK | 4 |
| Skills | 52 | Skill was discussed by participants but was not judged to be an important barrier to adopting SDD. Participants reported that ICU staff already have the skills necessary for delivering SDD | 1 |
| Nature of the behaviours | 31 | Complexity of, or experience with, SDD delivery behaviours were not judged to be an important barrier to adopting SDD | 3 |
| Beliefs about capabilities | 18 | Participants reported feeling confident in their ability to influence SDD adoption when this was in line with their professional responsibilities. This domain was, therefore, not considered an important barrier to adoption of SDD | 1 |
| Professional role and identity | 13 | Participants discussed the importance of professional obligations to reduce the use of antibiotics and how such directives impacted upon opinions about SDD adoption | 3 |
| Behavioural regulation | 15 | This domain was elaborated by participants but was mostly discussed in terms of recommended strategies for the hypothetical situation of adopting SDD | 3 |
| Social influences | 6 | Participants discussed the influence of other people, such as the perceived majority position among ICUs in the UK with respect to SDD adoption | 2 |
| Emotion | 1 | One person discussed emotion in the sense that it is difficult to have a dispassionate discussion with colleagues about SDD adoption | 0 |
As shown in Table 6, most utterances were coded under the Beliefs about consequences domain and these utterances were coded into 18 specific beliefs. However, not all domains showed a direct relationship between the level of elaboration in interviews and the number of specific beliefs that were identified. For example, although Memory, attention and decision processes was the second most elaborated domain, the participants’ responses could be distilled into three specific beliefs relating to the decision-making processes required for SDD adoption (see Table 9). Furthermore, Motivation and goals produced the second highest number of specific beliefs but, in terms of the number of utterances, was elaborated less than the Memory, attention and decision processes or Knowledge domains.
The following sections provide further detail about the specific beliefs that emerged in each of the theoretical domains.
Beliefs about consequences
Participants discussed 18 Beliefs about the consequences of SDD, both positive and negative, and noted that SDD could produce positive benefits such as reduced morbidity, rates of HAIs including VAP and ICU length of stay. The most frequently mentioned concern about the negative consequences of SDD adoption was the potential for increased antibiotic resistance. Participants also discussed their concerns about the potential for increased rates of C. difficile infections.
Non-nurse participants expressed concern that SDD delivery would increase nursing workload, specifically relating to administration of SDD components, taking cultures for microbiology and dealing with patient side-effects related to SDD such as diarrhoea. However, nurse participants raised none of these concerns. The only nurse to mention the impact of SDD delivery on nursing workload reported that it was not a barrier to implementation.
People who are wittering on about increased nursing time . . ., you cannot really use that as a great reason not to do something.
P41
Participants also stressed the importance of weighing risks and benefits associated with SDD. Specifically, they questioned whether the potential reductions in mortality and VAP were enough to balance the potential risk of increased antibiotic resistance and associated consequences. We noted considerable variation in viewpoints in this domain.
Financial consequences also were discussed both as potential cost-savings (due to reducing VAP and length of stay) but also the potential for increased costs in terms of SDD drugs and additional human resources required to deliver them.
Memory, attention and decision processes
Three beliefs relating to decision-making processes were identified that would be required prior to adoption of SDD: the need to review and appraise current evidence (n = 35), the need for consensus among colleagues (n = 32) and the role of senior ICU doctors (consultant level) as key decision-makers (n = 13).
Knowledge
Participants varied in their self-assessed levels of knowledge about SDD components and in their views of their own and others’ knowledge of the evidence base. Importantly (but not surprisingly), knowledge of the evidence base was linked by some to their rating of the importance of SDD:
I have never quite got round to looking at it and I have to be honest that . . . when I got the original email to say . . . there’s going to be a questionnaire on this, I thought . . . this is something I have been meaning to look into for a while so . . . having looked at the evidence now, it is very important.
P05
Some participants were very knowledgeable:
There have been some very, very good trials recently . . . I think there is certainly plenty of evidence there that some of us should be looking at and I think the big problem is . . . not everybody has fully appraised the papers.
P05
Other participants identified a limited knowledge of existing evidence, which was potentially an important factor in interpreting the study findings. Knowledge data are reported in Chapters 4 and 5.
Motivation and goals
Six beliefs were coded into this domain. These beliefs included the perception that SDD was not currently a ‘front-and-centre issue’ and, therefore, not a topic of discussion in their units or among colleagues. Two intensive care consultants and one microbiologist reported that VAP was already adequately addressed by other interventions and, therefore, there was no motivation to pursue other options to reduce HAIs such as SDD. Other clinical priorities were reported to be more important, such as the adequate implementation of existing VAP bundle procedures.
The main reason is that we are on a very steep improvement curve for our intensive care unit, in terms of trying to improve the outcome of our patients and just haven’t got to SDD yet. We haven’t reached the level of sophistication by which we can look at interventions like SDD. We are still working on simpler things like sedation holding and breathing trials, you know, much simpler things and I think SDD will be on our list, I think we just haven’t got to it yet.
P19
Finally, four participants (two with previous experience of delivering SDD and two with no experience) reported that SDD was considered ‘old news’ and no longer a relevant clinical topic.
Environmental context and resources
Potential barriers to SDD were discussed by participants with respect to the domain Environmental context and resources. Previous trials, conducted outside the UK, were perceived to have limited generalisability to the UK ICU context owing to different patient characteristics, ICU ecology or microbial flora and standards of care.
It would be very helpful . . . if there is good research from the UK, because we use antibiotics differently, our ecology is different, our patients tend to be slightly different to other European ITUs [intensive therapy units].
P25
Professional role and identity
Three beliefs were identified within the Professional role and identity domain. Specifically, three participants reported that SDD conflicted with their professional obligations, most notably in reducing the administration of prophylactic antibiotics.
It is kind of bred into us, hammered into us publicly that we should limit and target the use of antibiotics as much as is possible.
P44
Participants reported that there were conflicting opinions among clinical microbiologists and intensive care consultants (on antibiotic delivery used within SDD) that could influence SDD adoption.
Behavioural regulation
Three beliefs coded into the Behavioural regulation domain were mentioned by five participants. They discussed the influence of national guidelines and regulatory requirements on local policy with respect to SDD adoption. There was a perception that endorsement of SDD from an authoritative body (e.g. mandate by NICE) would lead to the adoption of SDD into a unit’s routine clinical practice.
. . . if, certain NICE or a group like that . . . came forward and said this is an absolute . . . necessity for treatment in the ICU then I think it would happen in our unit.
P15
Social influences
Two beliefs were coded within the domain Social influence. Participants suggested that adoption would require a clinical champion or SDD expert to put SDD on the agenda, educate others and drive SDD forward. Two participants (a clinical lead and a microbiologist) also reported that their practice was influenced by the practice of other ICUs; more specifically, they felt reassured that their position on not delivering SDD was in line with the position of other ICUs.
Well I guess as the years go by and the lack of pressure to institute it, in other words, you know, lack of a persuasive argument that what we are doing leaves us as an outlier, then it becomes less important.
P31
Emotion
Finally, the domain Emotion was discussed by one participant, who described the emotion associated with colleagues’ certainty about the SDD issue:
. . . it is very difficult to have a dispassionate discussion with people about it [SDD]. They have already made up their minds.
P45
In the context of intensive care, three domains were similar conceptually, and all were viewed as not being barriers to adoption. These domains were Skills, Nature of the behaviour and Beliefs about capabilities. Skills was discussed by 34 participants, and the vast majority (n = 31) felt that delivering SDD was within the existing competencies of ICU nurses and, therefore, skills were not a barrier to SDD adoption.
I don’t think in the ICU there would be any reason to say that the nursing staff shouldn’t be able to give the drugs because they are doing that already.
P39
All participants (n = 47) made comments coded under the domain Nature of the behaviour, with most participants reporting that delivering SDD would not be a dramatic shift from current practice. This domain was not considered to be a barrier to SDD adoption and included in round 2.
I don’t think it would be that difficult [in comparison to what already doing].
P14
Participants’ Beliefs about capabilities (to influence adoption) were not considered to be a barrier to SDD adoption. Those key professionals who were responsible for instigating changes in policy (medical leads, intensivists and clinical microbiologists) reported that they felt they could influence the decision on whether or not to adopt SDD.
I would say that if I personally made it my crusade to do it [adopt SDD], we would do it.
P19
Nurses and pharmacists were less certain of their ability to influence SDD adoption, but most noted that they could suggest changes in policy to colleagues.
I can definitely suggest changes and I would have to get agreement with the consultants before we could institute a major change, but I could certainly discuss instituting major changes.
P24
In summary, when the theoretical domains were used as a framework for analysis, it was possible to identify beliefs about the likely positive and negatives consequences on SDD and other factors that are likely to be barriers to SDD adoption. Nine of the 12 domains were potentially important in this context; Beliefs about consequences, Memory, attention and decision processes, Knowledge, Motivation and goals, Environmental context and resources, Professional role and identity, Behavioural regulation, Social influence and Emotion.
Participants were also asked their views on the existing SDD evidence base and about potential future research directions. For discussion of data relating to these topics, see Views about the existing evidence base.
Views about the existing evidence base
There was considerable variation in participants’ reported views of the existing evidence base for SDD. Six participants reported that the evidence base for SDD was sufficient and three specifically stated that further effectiveness research would not be helpful in determining the next steps for SDD.
I think they have done enough research on it to show it is a good thing . . . the research has been done and it’s time to implement it.
P05
In contrast, 26 participants were not convinced by the existing evidence.
[Would further research settle some of the issues surrounding SDD?] I think so. I mean the problem with ITU is that we know that whatever is published one day, in about 6 months time or a year, there could be something different that will contradict it. But it [the research] has to be pretty definitive before, you know, we rush into anything.
P27
Most felt that further research was needed to address clinical uncertainties (see Table 9), in particular beliefs about the consequences of routine delivery of SDD.
The 26 participants who discussed further research needs mentioned the limitations of SDD studies reported to date, including the length of follow-up (as this was not sufficient to assess antibiotic resistance in the longer term) and the need for studies conducted in the context of patient populations with a resistance profile similar to that of the UK. Given the variation in self-assessed knowledge of the evidence, we investigated associations between knowledge and views about further research. Views about further research that are based on poor knowledge of the current evidence would clearly have a different status from views based on a sophisticated level of knowledge. Such associations were more effectively explored in the context of the quantitative Delphi rounds and are reported in Chapter 4.
Views about further research
Despite variation in participants’ views of the existing evidence base, most participants (n = 32) reported that they would be willing to facilitate recruitment of patients to a randomised controlled trial (RCT) to evaluate the effectiveness of SDD. The importance of obtaining agreement from colleagues to participate in any further research was raised by nine of these participants. Reasons for willingness to participate in effectiveness research included:
-
such a study would be important as it would help to resolve an unanswered question in critical care research
-
a general interest in research
-
a desire to improve patient care.
The case in favour of an effectiveness RCT is strengthened by the extent of elaboration of the Beliefs about consequences domain. Table 7 provides illustrative quotes that suggest an effectiveness RCT may be appropriate.
| Domain | Illustrative quotations from interview transcripts |
|---|---|
| Knowledge | I feel there has to be overwhelming evidence of the benefits in using it and also some kind of reassurance in the evidence that using the i.v. component wasn’t going to have a negative impact in terms of development of resistance. P17 |
| I think we would need conclusive evidence . . . that it was an effective treatment and that . . . there wasn’t a significant risk of the emergence of resistant organisms. P36 | |
| Beliefs about consequences | . . . in my opinion the jury is still out on the overall benefit of SDD and I think that we need to try and get data from [a] broad, multilocations study to see whether the overall benefit is worth pursuing. P03 |
| I don’t think that there is enough evidence in using the i.v. cephalosporins to suggest that it wouldn’t cause harm. P17 | |
| Environmental context and resources | . . . at the moment we are extrapolating data taken from different areas . . . although the majority of them do suggest a mortality benefit, the number needed to treat is quite variable. The actual range of antimicrobials is quite variable and the data on resistance, antimicrobial resistance, is quite variable in terms of the bugs . . . I think there’s still enough uncertainty there to actually say there is another study needed. P23 |
| There are a large number of multinational studies [which have] become biased by the use of non European or North American sites, where practice is very different and baseline infections are different and the microflora are potential[ly] different. P35 | |
| Memory, attention and decision processes | . . . the other thing that I am slightly worried about is the number you need to treat . . . and the selection of the patient population. Which one would get most benefit from SDD? It is not clear to me yet. P42 |
In contrast, five participants reported being unwilling to recruit patients to further effectiveness research as they perceived that enough research had been conducted (n = 3), they already delivered SDD and lacked equipoise (n = 3) and they wanted to implement SDD without further delay (n = 1). Contrasting views about the ethics of conducting further research were expressed: 27 participants reported that further research was ethical, 10 participants qualified this answer by stating that questions remained unanswered and five explicitly mentioned that equipoise existed. In contrast to this view, three participants reported that further SDD research would not necessarily be ethically sound because the question of effectiveness had already been answered in favour of delivering SDD.
When asked whether or not they would support an implementation study (i.e. a study to evaluate strategies to increase uptake of SDD), participants were hesitant. Seven participants felt that they would need more information about an implementation study before they could comment on participating in such a study and nine participants reported that there was insufficient evidence of effectiveness to proceed with such a study at this time. However, there was substantial evidence to suggest that the reasons for holding back on adoption of SDD were not strong. Table 8 presents quotes that suggest an implementation study may be appropriate or that propose specific implementation strategies.
| Theoretical domain | Illustrative quotations from interview transcripts |
|---|---|
| Beliefs about consequences | There is plenty of research to show what we should be doing, the big problem now is, is actually making sure it is adopted. P05 |
| I think the evidence is no better or no worse than a lot of things that we do regularly on intensive care. P09 | |
| Behavioural regulation | What would need to happen . . . it is going to take some form of national guideline . . . from an authoritative body . . . to state that this is a standard care that is expected. P31 |
| [We] would be looking for guidance from a higher source . . . some sort of national guidance. P01 | |
| [I]f . . . NICE or a group like that . . . said this is . . . a necessity for treatment in the ICU then I think it would happen in our unit. P15 | |
| There has never been a stimulus to start. P01 | |
| Motivation and goals | I think there are a lot of people who view it as one of those things that has been published that has got good results and questions asked why are we [not] doing it, but no-one is really getting round to doing it. P14 |
| Nobody has a strong feeling either way to be honest. It’s . . . not that anybody is violently opposed to it. It’s just that nobody’s violently [in favour] either . . . I think it is basically they have other interests that they concentrate on and SDD isn’t one of them. P15 | |
| Social influences | What is the main reason you would say that you don’t use it [SDD] now? I think basically there’s the individual who was enthusiastic about it stopped working at the trust and interest sort of waned as a result of it really. Basically we just stopped using it. P15 |
| We have never really had anyone that was interested in it, . . . As the years go by and the lack of pressure to institute it, . . . lack of a persuasive argument that what we are doing leaves us as an outlier, then it becomes less important. But that is describing human nature then rather than anything else. P31 |
In addition, there was considerable evidence that some participants were not aware of what is meant by ‘implementation study’. For example, when asked if they would be willing to participate in a study whose aim was to increase adoption of SDD based on current evidence, responses included:
It is not really a study is it? It is just a NICE guideline?
P19
I am not sure how that could be a study, it would be educational programme to raise awareness but I am not sure how in practice this would be a relevant study.
P44
In summary, participants reported a range of views on the existing evidence base and directions for future research. In general, they were willing to participate in effectiveness studies (although some voiced uncertainty about the ethics of conducting further effectiveness research in SDD). There was less support for participating in implementation studies, but also less clarity in understanding what an implementation study involves.
Findings from round 1 used to develop round 2 materials
In collaboration with the SuDDICU research teams in Canada and Australia/New Zealand, the results from the round 1 interviews were used to develop a set of 47 domains-based statements for inclusion in the instrument used for the quantitative rounds of the Delphi study (round 2 and round 3). The finalised items are displayed in Table 9. There were eight positively worded items (i.e. in favour of SDD), 31 negatively worded items (i.e. opposed to SDD) and eight neutrally worded items. Further detail on the methods used to decide item wording is provided in Chapter 4.
| Domain | Round 2 item | Supporting quotes from interview transcripts |
|---|---|---|
| Knowledge (three items) | I know to which patients I would administer SDD | I am not quite sure to be honest. That is one thing I haven’t gone into in great detail about, the exclusion criteria. P05 |
| I know the SDD evidence base well enough to have an informed opinion regarding its use | I think it is one of those things that, over the last couple of years, there has been more and more evidence published in the intensive care literature where it fairly consistently pointed to a good result in terms of outcomes in patients, and the reasons for not doing it in the UK are becoming less and less. P14 | |
| Research to date has not adequately addressed concerns about antibiotic resistance and SDD | The long term ecological consequences of using antibiotics in this way is . . . always going to be an uncertainty and it makes it very difficult for anyone reviewing the evidence to come down clearly with statements to the effect that this is something we ought to be doing . . . the evidence comes [from] short term studies and ecological changes take a long time to develop . . . like, global warming or changes in animal populations, these are things that don’t happen straight away; they take a long time to develop. P13 | |
| Motivation (six items) | SDD is not a topic of discussion among my colleagues | I think again, embarrassing and simplistic as it sounds, we’ve just never seriously discussed it. P02 |
| SDD is not on my unit’s list of clinical priorities | The main reason is that we are on a very steep improvement curve for our intensive care unit, in terms of trying to improve the outcome of our patients and just haven’t got to SDD yet. We haven’t reached the level of sophistication by which we can look at interventions like SDD. We are still working on simpler things like sedation holding and breathing trials, you know, much simpler things . . . I think SDD will be on our list, I think we just have not got to it yet. P19 | |
| We are addressing hospital-acquired infections using other strategies | Clearly the reduction of infection risk is a major priority in the ICU but . . . the other initiatives that are in place are, by definition, deemed more important because we are doing those . . . whereas we are not doing SDD. P31 | |
| We are addressing ventilator-associated pneumonia using other strategies | I do not think it [SDD] is hugely important right at the moment because I think there are a variety of other techniques and regimes and therapies that have been instigated in . . . intensive care in the last 2–3 years in an attempt to tackle ventilator associated pneumonia. P26 | |
| Our unit VAP rates are low | I think we have a pretty low rate of ventilator-associated pneumonia and other nosocomial infections. P27 | |
| SDD is outdated | It’s fallen out of fashion. P30 | |
| SDD is a hot topic again. P45 | ||
| Professional role and identity (three items) | There are conflicting opinions on antibiotic use among medical microbiologists/ID physicians and ICU physicians | Our main area of scepticism comes from our new consultant microbiologist whose background is from another intensive care unit and though he was hostile, he is now passively accepting of our continued use of SDD. P18 |
| Prophylactic antibiotic use in SDD is at odds with my professional training | It is kind of bred into us, hammered into us publicly that we should limit and target the use of antibiotics as much as is possible. P44 | |
| Prophylactic antibiotic use in SDD is at odds with my professional responsibilities | See quote above | |
| Social influence (two items) | I am reassured that our position on SDD adoption is in line with other hospitals’ | Well I guess as the years go by and the lack of pressure to institute it, . . . [a] lack of a persuasive argument that what we are doing leaves us as an outlier, then it becomes less important. P31 |
| SDD will not be adopted without a local champion | I don’t think it is going to push forward unless we get an individual in the unit who is an absolute, you know, you know, very, very keen on the idea and can persuade his colleagues. P15 | |
| Behavioural regulation (three items) | There are no national guidelines about SDD | I guess now it [implementing SDD] is going to take some form of national guideline. [Implies that there are no national guidelines currently.] P31 |
| The local decision to adopt SDD would be influenced by regulatory requirements | I think for starters they would be looking for guidance from a higher source, as it were. Some sort of national guidance that we would be following. P01 | |
| My hospital tries to reduce antibiotic use | . . . antibiotic prescribing . . . we try to limit as far as possible. P02 | |
| Beliefs about consequences (18 items) | There is no mortality benefit associated with SDD | It has got to show that it’s of benefit in reducing patient morbidity and mortality and that is where I think at the moment the case hasn’t been proven. P03 |
| The risks of SDD outweigh the benefits | It looks like the benefits will outweigh any potential risk. P05 | |
| SDD reduces VAP | It reduces the incidence of ventilator associated pneumonia, we believe. P18 | |
| SDD reduces length of stay | The benefit is hopefully reduced length of stay because you have got reduced infection rates. P14 | |
| Overall SDD benefits patients | I think it is beneficial to the patient. We don’t tend to see the more complicated infections. P06 | |
| SDD increases nursing workload | It would be extra nursing time as well, because most of these drugs are being given four times a day. So on top of all the other drugs that they are giving the patients, it is going to take a bit of extra time for them to prepare each of the agents of four lots of drugs, four times a day. P17 | |
| SDD increases pharmacy workload | (Item included to achieve balance across stakeholder groups) | |
| SDD increases microbiology workload | There is a cost, to our microbiology department. P18 | |
| SDD increases antibiotic resistance | I still have concerns about resistance but I know that there is a lot of evidence to suggest you don’t get that. P29 | |
| Units using SDD have better clinical outcomes | One of the big things putting me off is that there is one unit . . . that does use a lot of SDD and they also have very good audit data for outcomes and that unit is certainly no better than any other unit so it cannot be making that big a difference to outcomes. P01 | |
| SDD causes unpleasant side-effects for patients | It often causes diarrhoea. . .[staff] end up putting bowel management systems into the patients, which is a risk in itself. P21 | |
| SDD reduces hospital-acquired infections | It will lower your infection rates but I am not sure how solid that evidence is really. P09 | |
| I have concerns about the specific antimicrobials you need to use | One of the antibiotics most commonly recommended is cefotaxime for the intravenous component and we are actively trying to limit our use of cephalosporin because of concerns about Clostridium difficile infection. P02 | |
| I am opposed to the i.v. component of SDD | I think the concerns over using i.v. antibiotics. . . prior to any signs of infection, is a big concern in terms of development of resistance. P17 | |
| I am opposed to SDD | In fact some people are opposed to SDD drastically . . . But I personally want to move SDD [towards being adopted]. P25 | |
| SDD would increase ICU C. difficile infections | There are unanswered questions I think over C. diff. P07 | |
| Overall, SDD is cost-effective | The cost implication of course is going to be completely thrown out by the fact that if you are preventing infection then you are saving money. But you do have some initial outlay in the first place in order to provide the antibiotic cover. P10 | |
| Educating staff would be expensive | So that would all take time – to educate the staff about antibiotics, about why you are giving it, about the fact that everybody is covered in bacteria, including themselves. So that time is a huge issue. P45 | |
| Skills (one item) | The skills required to administer SDD fall within the competencies of our ICU nursing staff | I don’t think in the ICU there would be any reason to say that the nursing staff shouldn’t be able to give the drugs because they are doing that already. P39 |
| Nature of behaviours (three items) | SDD is straightforward to deliver | I don’t think it would be that difficult at all. P14 |
| The use of pastes may interfere with other treatments | [Interviewer: would SDD interrupt the feeding process?] No, we tend to feed for 24 hours and we give the antibiotics with the feed. P40 | |
| SDD would be a dramatic shift from our current practice | I don’t think it would be that difficult [in comparison to what already doing]. P14 | |
| Environmental context and resources (four items) | The SDD evidence base is not generalisable to my country | If the problem is that people say it is not done in the UK indeed our setting is indeed different to the Netherlands where the most of the studies is coming from then you could say the data from the Netherlands does not qualify. P44 |
| The SDD evidence base is generated in countries with different resistance profiles to my country | See quote above | |
| The SDD evidence base is not generalisable to my patient population | I think their feeling is that there hasn’t been enough research in their population group. P27 | |
| SDD drugs are expensive | It is quite expensive to do both in actual purchase costs for the materials and for the time involved for the nurses to administer it all. P01 | |
| Beliefs about capabilities (one item) | I could influence whether SDD is adopted in my hospital | I can definitely suggest changes and I would have to get agreement with the consultants before we could institute a major change, but I could certainly discuss instituting major changes. P24 |
| Memory, attention and decision processes (three items) | The decision to adopt SDD requires a review and appraisal of the current best evidence | We would need to review literature and determine whether there is sufficient evidence to support it. We’d also look at what sort of studies have been conducted whether randomised, blinded whether there was high levels of evidence. P30 |
| The decision to adopt SDD requires consensus between my colleagues | One tends to work as a group, so anything that we tend to treat, we treat as a package . . . a consensus discussion and a consensus opinion. P22 | |
| Part of the decision to adopt SDD requires agreement about which patients will receive it | You would need to identify which patients you were going to do it on or which patients you might wish to exclude. P02 | |
| Future research (four items) | I would support my ICU participating in a nationwide randomised control trial (RCT) | [Recruit patients to effectiveness RCT?] I would be willing to advocate that our unit take part in that – yes. [Why?] Because I think it is an unanswered question within the UK. P38 |
| Further SDD RCTs are ethical | I think it would only be unethical if we were already doing something that was proven to work and we were going to stop doing it to try this. Or we in some way thought we were going to run this against it and it was worse. I don’t think that’s the situation. P09 | |
| My concerns about antibiotic resistance limit my willingness to participate in future RCTs in SDD | I guess there’s some ethical dilemmas [about further research] . . . if you genuinely believe that [antibiotic] resistance. P38 | |
| I would support my centre being involved in a study to promote the adoption of SDD | If someone can present the evidence in a format that . . . satisfies some of the anxieties I, and my colleagues have, then that sort of a strategy may work as well. I mean a lot of people recognise that there is evidence to support the use of SDD, as gut decontamination, and the fact that we haven’t taken it up suggests that the message is not convincing people. So I think a strategy that improved communication, improved information dissemination, may be beneficial but . . . you have got a big mountain to climb here at the moment. P34 |
Discussion
The Delphi study recruited 47 participants from four clinical stakeholder groups, regarded as experts in critical care, to identify key issues to consider about the topics of SDD adoption and delivery, and further SDD research. An immediate objective was to generate items for inclusion in the materials used in later rounds of the Delphi study and eventually in a questionnaire for a national survey. Delphi approaches have previously been used for these purposes. For example, a Delphi technique has been used to identify potentially relevant determinants of innovation in health-care organisations50 and to develop a national survey about views on medical instrumentation. 51
Answers to research questions
Round 1 of the Delphi study provided preliminary answers to research questions 3, 4 and 5:
-
3. Stakeholders held mixed views of the internal/external validity and adequacy of the existing evidence base for SDD. They were willing to participate in further research but the most appropriate kind of research was not clearly informed by these interviews.
-
4. Stakeholders also held mixed views about the likely positive and negative consequences of implementing SDD in ICUs. The relative importance of these beliefs in influencing overall views about SDD was assessed using assumptions from cognitive psychology (but a range of further importance indices was examined in later stages of the study).
-
5. Stakeholders had a lot to say about the likely barriers to implementing SDD in ICUs. These tended to focus on Motivation and goals (simply not high on the priority list) or on Behavioural regulation (a national guideline would change practice) but there was also considerable doubt about the evidence base.
These preliminary results were used to generate questionnaire items for further consideration in later rounds of the Delphi study (see Chapter 4). All the beliefs generated in round 1 of the Delphi study (including minority beliefs) were to be taken forward for consideration by the panel in rounds 2 and 3. 1 In view of the importance of considering the full range of views about SDD, we used two approaches. First, we based the round 1 interviews on the TDF of clinical behaviour, as this framework has been shown to generate more beliefs (including lesser discussed issues such as emotion). 52 Second, we conducted a data saturation analysis, using previously published methods, to provide evidence that saturation had been reached.
Evidence of data saturation
Data saturation principles did not drive our sampling strategy as sample size was based on recommendations for Delphi studies. 53 Therefore, data saturation analysis was conducted after completion of data collection and content analysis. Published guidance for determining data saturation54 recommends a two-step process involving analysis of consecutive interviews to (1) plot all ‘new’ beliefs (i.e. not counting repeats of beliefs previously mentioned) in an ‘initial analysis sample’54 of 10 cases with adequate diversity and (2) declare saturation when there are three consecutive interviews with no new beliefs emerging. This is referred to as the ’10 + 3 rule’,54 according to which the smallest sample size for data saturation would be 13. Saturation analysis of our data illustrating these two steps is displayed in Figure 2. After the 13th interview, the 10 + 3 rule had not been met. After interview 22 (represented by the dotted vertical line), there were three consecutive interviews without new beliefs emerging (at interview 25, represented by the solid vertical line), whereas recruitment continued to interview 47. This indicates that the sampling process was more than adequate to achieve data saturation in terms of beliefs about SDD. However, Figure 2 also shows that new beliefs were elicited in interviews 40 and 45. These beliefs were included with all other beliefs in the materials for round 2 (see Chapter 4).
FIGURE 2.
Evidence of data saturation and cumulative frequency of new beliefs about SDD for Delphi round 1 analysis.
Details of findings
The aim of the round 1 interviews, described in this chapter, was to identify – in four groups of key clinical stakeholders – the perceived positive and negative consequences of SDD implementation in UK ICUs, likely barriers to adoption, stakeholders’ views on the existing evidence base related to SDD, and potential willingness to facilitate recruitment into future studies of either SDD effectiveness or SDD implementation. Nine domains of the TDF55 were potentially important in characterising perceptions regarding SDD adoption and delivery. These included Beliefs about consequences (which was the focus of research question 4) and eight possible types of barriers to SDD adoption and delivery: Memory, Attention and decision processes, Knowledge, Motivation and goals, Environmental context and resources, Professional role and identity, Behavioural regulation, Social influence and Emotion. This is a coherent finding in the context of health care delivered in ICUs. First, knowledge of the research evidence is fundamental to the provision of evidence-based care. Second, such evidence focuses on the consequences (benefits and risks) of providing, or not providing, certain interventions. Third, clinical decision making is a core aspect of practice and covers both decisions about individual patients but also (importantly for SDD) decisions about local policy. Hence, these three domains encapsulate the kind of thinking that is required for delivering high-quality care. Fourth, motivation is important in the context of intensive care as there are many potentially important interventions to consider and clinicians need to consider the relative importance of SDD in comparison with other urgent priorities. Environmental context and resources are potentially important for two reasons: if the settings in which evidence has been developed have poor environmental fit with the UK, in terms of background infection rates, the evidence may not be seen as applicable. In addition, the financial costs in terms of drug supplies and staff time should be weighed against the benefits and other priorities in the intensive care setting. Some interesting findings relating to Professional role and identity highlighted that the training and responsibilities of the different clinical professions may have a bearing on SDD policy. Beliefs relating to Behavioural regulation drew on participants’ hypotheses about what might work to change their own behaviour. Such hypotheses are opinion based and do not represent a high level of evidence; indeed, there is evidence to suggest systematic bias in people’s views about what will change their behaviour. 56 However, such hypotheses are, in principle, testable and could provide a list of strategies to consider for implementation. There was a clear view that Social influence could be important, as intensive care involves a collaborative approach between disciplines and policies are influenced by ICU practice more broadly. Emotion was potentially a factor determining whether or not people were prepared to engage in dispassionate discussion about SDD.
Domains that appeared to be unimportant were Skills, Nature of the behaviours and Beliefs about capabilities. In other words, the interviews identified beliefs that lack of appropriate skills would not be a barrier to SDD delivery; the demands of the behaviour itself, while complex, would again be unproblematic in so far as they reflect the kinds of demands that intensive care staff routinely manage. Consistent with this, participants were confident that staff would be capable to manage a SDD regimen if it were adopted into the ICU. Notwithstanding their apparent unimportance, beliefs within these domains were taken forward into round 2 for further consideration, as specified in the study protocol.
Even when there was clear evidence of the importance of certain issues, participants placed themselves differently along the continuum from negative to positive. In terms of Beliefs about consequences, divergent opinions were expressed about the effect of SDD on patient outcomes and impact on clinician workload. The need for informed decisions, as well as collegial and leadership support to implement SDD, was acknowledged. However, lack of relevance to current perceived priorities in delivery of ICU care was also noted, as well as potential differences in the UK ICU ecological environment that may influence SDD effectiveness and potential adverse consequences, such as increased antimicrobial resistance. In general, participants perceived that acquisition of new skills and behaviours was not a major barrier to SDD adoption. This is in contrast to a previous survey that identified perceived difficulty associated with the behaviours needed to deliver SDD. 31
Divergent opinions also were noted in terms of the adequacy of the existing evidence about SDD effectiveness. In particular, respondents reported that existing evidence conducted in countries such as the Netherlands may not be applicable to the UK context, owing to differences in the microbial ecological profile. Another key area in which there was perceived to be insufficient evidence was in the long-term effect of SDD.
Most respondents indicated they would support further research on the effectiveness of SDD. Fewer positive views were expressed for a trial of SDD implementation but what was also noted was a lack of awareness among some participants about what implementation research involves and what it might achieve. There were many instances in which participants’ views would be consistent with criteria to support an implementation study (see Table 8). The findings of this first Delphi round were then used to inform the content of the next two Delphi rounds designed to refine and confirm participant views on barriers to SDD implementation and the need for further research.
Strengths and limitations
It should be noted that the sample was mixed in terms of direct experience of delivering SDD in practice. Hence, some of the opinion-based data presented in this chapter may reflect erroneous beliefs. For example, the concerns of non-nurse participants about nursing workload were not shared by the nurse participants and the belief that people have already made up their minds about SDD was not consistent with other participants’ reports about their own change of mind (reported above under Knowledge and Behavioural regulation). Hence, it would not be appropriate to make recommendations for future research based solely on these opinion-based data. Therefore, later stages of this study tested the robustness of these views but could not fully offset the problem of using data involving opinions.
Box 5 presents the key findings that inform the need for, and acceptability of, a clinical effectiveness RCT or an implementation RCT. There were some initial indications that these participants consider the evidence base sufficiently uncertain to warrant further effectiveness research. However, there was also evidence that implementation would be appropriate. Four ideas to support implementation, based on four of the theoretical domains, emerged in the analysis. First, in the domain Beliefs about consequences, some participants felt that there was sufficient evidence in favour of SDD to warrant adoption now. Second, in the Motivation and goals domain, there was a view that SDD was simply not high enough on the unit’s priority list to be considered at this time. Third, in the Social influence domain, it was clear that the personal influence of key individuals (rather than problems with the evidence) could be sufficient to trigger adoption. Fourth, in the part of the interview that probed potential Behavioural regulation strategies, a recurring response was that SDD would be adopted (apparently quite straightforwardly) if adoption was mandated by regulatory bodies.
-
There was considerable uncertainty as to the adequacy of the existing evidence base for SDD and about the potential harm and benefits. Some of this uncertainty could be related to unfamiliarity with existing evidence.
-
The likely barriers to implementing SDD in ICUs include Motivation (simply not high on the priority list) or on Behavioural regulation (a national guideline would change practice). There was some evidence that an implementation study (to raise awareness or the raise priority of SDD) could be appropriate. There was little evidence that practical issues such as skills, capabilities or costs are significant barriers.
-
Further research was feasible and acceptable but there was uncertainty regarding the most appropriate kind of research that should follow this study.
Chapter 4 Delphi study to identify stakeholder views about selective decontamination of the digestive tract: quantitative rounds
Background
Following round 1 of the Delphi study (reported in Chapter 3), two further rounds were conducted with the same sample, comprising four key stakeholder groups (intensive care consultants, clinical microbiologists, pharmacists and ICU clinical leads or nurse managers). A fourth, international, round was also planned, involving all participating nations (Australia/New Zealand, Canada, UK) and in which the feedback would include a breakdown of responses by nation. 1 In line with Delphi methodology, the quantitative rounds were designed to generate iterative evidence. Whereas Delphi studies are often designed to achieve consensus (e.g. to agree recommendations for a clinical guideline), this study was designed to assess levels of consensus in the field about the evidence base relating to SDD, the likely consequences of delivering SDD and the feasibility of conducting further SDD research. Consistent with round 1 of the Delphi study, the rounds reported in this chapter focused on the following research questions:
Research question 3: What are the views of key stakeholders of the internal/external validity and adequacy of the existing evidence base for SDD and how willing are they to participate in further research?
Research question 4: What are the views of key stakeholders about the likely positive and negative consequences of implementing SDD in ICUs and what is the relative importance of these beliefs in influencing overall views about SDD?
Research question 5: What are the views of key stakeholders about the likely barriers to implementing SDD in ICUs?
Methods
Sample
All participants interviewed in round 1 were invited to participate in round 2. Only those who responded at round 2 were invited to participate in round 3.
Materials
An instrument was developed in questionnaire format. Item content was based on the findings from the first round of the Delphi study (see Table 9). The number of items included in round 2 relating to the Behavioural regulation domain was limited to ensure the resulting questionnaire did not suggest a bias towards adopting SDD. For example, beliefs reported in the Delphi interviews included hypothetical views about what would change practice and made the hypothetical assumption that SDD adoption is desirable.
No specific item relating to the emotion domain was taken into round 2 because participants were able to represent the extent of their emotional reaction in the questionnaire response scale (i.e. strongly against, neutral or strongly in favour). All other beliefs identified in the analysis were converted into questionnaire items. The content of these statements was decided using two principles:
-
The wording was chosen to reflect the language used in the interview data when possible.
-
The principles of questionnaire design and correct grammar were also observed (i.e. to assure brevity and maximise clarity and to avoid double negatives in the context of a ‘disagree to agree’ response format).
In round 2, participants were asked to consider 47 items based on the TDF20 and 10 questions relating to their views about further SDD research. For 46 of the 47 domains-based items, there were two questions: (1) ‘to what extent do you agree or disagree?’ (on a nine-point Likert scale, with 1 indicating strongly disagree and 9 indicating strongly agree) and (2) ‘how important is this issue in your overall opinion about the delivery of SDD to critically ill patients?’ (on a nine-point Likert scale, with 1 indicating not at all important and 9 indicating very important). 57 Importance ratings were not requested for the item relating to overall opinion on SDD (‘I am opposed to SDD’) because the importance of this item is reflected in the response format that assessed strength of agreement/disagreement.
The questionnaire was piloted with five clinicians who were not part of the Delphi study sample (one from the UK, one from Canada and three from Australia). The pilot process was also used to inform the wording of the participant information sheet. In round 3, participants were provided with feedback about the overall group responses to each question (in the form of a frequency histogram, Figure 3), they were given a reminder of their own previous response and were then asked to rate to the item again. Both rounds 2 and 3 were delivered online.
FIGURE 3.
Screenshot of a round 3 item.
Procedure
E-mails were sent to all 47 participants from round 1 with individual links to the electronic questionnaire. Responses were monitored and reminders sent to non-responders after a 2-week interval. After a further week, remaining non-responders were sent a second reminder (by telephone when possible, or by e-mail if unreachable by telephone).
Data management and analysis
Two participants responded late to round 2 (after feedback material had been produced). They were nevertheless included in the analysis reported in this chapter.
Ethics approval for the Delphi study was granted by NHS North of Scotland Ethics Service (10/S0801/69).
Results
Participant characteristics
Forty-four participants completed round 2 (94%) and 42 completed round 3 (95% of round 2 participants, 89% of total sample). The breakdown of participation by stakeholder group is given in Table 10. The table shows that the recruitment target of 10 participants (see Chapter 3, Methods) per professional group was met at the end of round 3. The participants who had experience of using SDD were spread across all professional groups (one, one, five and two of the intensivists, pharmacists, microbiologists and ICU leads, respectively).
| Delphi round | Intensivists | ICU pharmacists | Medical microbiologists | ICU clinical leads/ICU nurse managers or educators | Total |
|---|---|---|---|---|---|
| 2 | 11 | 11 | 11 | 6/5 (group total: 11) | 44 |
| 3 | 11 | 10 | 10 | 6/5 (group total: 11) | 42 |
Participants’ perception of their own knowledge was measured with the item ‘I know the SDD evidence base well enough to have an informed opinion of its use’. As shown in Figure 4, the participants generally tended to rate themselves as knowledgeable about SDD (modal score of 7 on a nine-point scale). However, 12 participants rated themselves below the mid-point of the scale. It was expected that these participants would be more likely to alter their responses in round 3 after viewing the round 2 feedback. This prediction was tested on the round 3 data, reported below (see Figure 7). Further participant characteristics are reported in Chapter 3.
FIGURE 4.
Round 3 responses to knowledge item ‘I know the SDD evidence base well enough to have an informed opinion of its use’.
The frequency distributions of all items at round 3 are presented in Appendix 4. The following sections report the remaining results of the quantitative Delphi rounds in four parts. First, we report the stability of responses between round 2 and round 3, addressing both individual-level and group-level stability. Second, we provide summary data relating to beliefs about the consequences of delivering SDD, addressing the question ’what is the perceived importance of these beliefs in influencing overall views about SDD?’ Third, we describe the range and level of participants’ agreement with each questionnaire item in terms of levels of consensus among the Delphi panel. Finally, we describe data relating to participants’ views about further SDD research.
Stability of ratings from round 2 to round 3
The Delphi literature provides a range of guidance about assessing stability. 58,59 Our innovative approach was to assess stability at the within-individual level (individual change scores) and at the within-group level (changes in group means) as follows. Individual-level change scores were calculated, such that a score of zero, signifying identical responses in round 2 and round 3, demonstrated high stability. As shown in Table 11, the percentage of individuals whose round 3 response differed by ≤ 1 scale point ranged from 100% (for the item ‘SDD reduces VAP’) to 76% (for the item ‘There are conflicting opinions on antibiotic use among clinical microbiologists/ID physicians and ICU physicians’). Histograms of these two items are given in Figure 5. There was thus high individual-level stability of responses from round 2 to round 3, with > 75% of change scores in the range −1 to 1 for all items.
| Item | Individual-level change | Group-level change | |
|---|---|---|---|
| % of participants with change score of 0 | % of participants with change score of +/–1 | Change in mean score, round 2 to round 3 | |
| Units using SDD have better clinical outcomes | 95 | 3 | 0.03 |
| SDD reduces VAP | 90 | 10 | 0.10 |
| The decision to adopt SDD requires a review and appraisal of the current best evidence | 88 | 10 | 0.14 |
| SDD would be a dramatic shift from our current practice | 86 | 10 | 0.10 |
| I would support my ICU participating in a nationwide randomised control trial (RCT) of SDD | 85 | 10 | 0.10 |
| I know the SDD evidence base well enough to have an informed opinion regarding its use | 85 | 13 | 0.20 |
| SDD reduces length of stay | 85 | 10 | 0.00 |
| I would support my centre being involved in a study to promote the adoption of SDD | 83 | 14 | 0.19 |
| SDD causes unpleasant side-effects for patients | 83 | 12 | –0.12 |
| SDD increases antibiotic resistance | 83 | 10 | –0.07 |
| There is no mortality benefit associated with SDD | 83 | 12 | –0.07 |
| SDD is outdated | 83 | 12 | –0.12 |
| The skills required to administer SDD fall within the competencies of our ICU nursing staff | 81 | 19 | 0.19 |
| The SDD evidence base is not generalisable to my patient population | 81 | 17 | 0.10 |
| SDD drugs are expensive | 81 | 14 | 0.07 |
| SDD increases nursing workload | 81 | 14 | 0.00 |
| I am opposed to the i.v. component of SDD | 81 | 10 | 0.12 |
| I would be more likely to participate in a RCT if patients in the control arm received VAP bundles as usual care (including chlorhexidine mouthwash/gel and head up positioning) | 81 | 10 | 0.33 |
| I could influence whether SDD is adopted in my hospital | 79 | 21 | 0.17 |
| Educating staff would be expensive | 79 | 12 | –0.19 |
| I am opposed to SDD | 78 | 22 | –0.12 |
| Part of the decision to adopt SDD requires agreement about which patients will receive it | 78 | 20 | 0.20 |
| We are addressing ventilator-associated pneumonia using other strategies | 78 | 20 | 0.20 |
| The decision to adopt SDD requires consensus between my colleagues | 78 | 20 | 0.10 |
| Prophylactic antibiotic use in SDD is at odds with my professional responsibilities | 78 | 17 | 0.05 |
| Prophylactic antibiotic use in SDD is at odds with my professional training | 78 | 15 | –0.05 |
| SDD reduces hospital-acquired infections | 78 | 23 | 0.03 |
| I would be more likely to participate in a RCT if it included pretrial, during-trial and post-trial monitoring of antibiotic resistance in all patients whether in the RCT or not | 77 | 18 | 0.41 |
| The SDD evidence base is not generalisable to my country | 76 | 19 | 0.17 |
| Overall, SDD is cost-effective | 76 | 17 | 0.02 |
| SDD increases pharmacy workload | 76 | 24 | 0.00 |
| I would be more likely to participate in a RCT if cost–benefit analysis was included | 76 | 22 | 0.12 |
| SDD is straightforward to deliver | 74 | 19 | 0.33 |
| I would be more likely to participate in a RCT if it included pretrial, during-trial and post-trial monitoring of antibiotic resistance in patients in the RCT | 74 | 17 | 0.38 |
| I have concerns about the specific antimicrobials you need to use | 74 | 17 | –0.02 |
| Further SDD RCTs are ethical | 73 | 22 | 0.32 |
| We are addressing hospital-acquired infections using other strategies | 73 | 20 | 0.34 |
| SDD will not be adopted without a local champion | 73 | 17 | 0.39 |
| The SDD evidence base has been generated in countries with different resistance profiles to my country | 73 | 20 | 0.28 |
| Overall, SDD benefits the patients to whom it is delivered | 71 | 24 | –0.05 |
| SDD would increase ICU C. difficile infections | 71 | 21 | 0.07 |
| I would be more likely to participate in a RCT if mortality is the end point | 71 | 19 | –0.02 |
| I know to which patients I would administer SDD | 71 | 22 | 0.32 |
| The local decision to adopt SDD would be influenced by regulatory requirements | 71 | 20 | 0.41 |
| There are no national guidelines about SDD | 71 | 15 | 0.44 |
| The use of pastes may interfere with other treatments | 69 | 19 | 0.14 |
| My hospital tries to reduce antibiotic use | 68 | 24 | 0.44 |
| SDD increases microbiology workload | 68 | 20 | 0.07 |
| I am reassured that our position on SDD adoption is in line with other hospitals | 68 | 17 | 0.12 |
| Our unit VAP rates are low | 67 | 26 | 0.02 |
| The risks of SDD outweigh the benefits | 67 | 21 | –0.07 |
| SDD is not on my units list of clinical priorities | 66 | 29 | 0.10 |
| There are conflicting opinions on antibiotic use among medical microbiologists/ID physicians and ICU physicians | 66 | 10 | 0.39 |
| My concerns about antibiotic resistance limit my willingness to participate in future RCTs of SDD | 64 | 21 | 0.00 |
| SDD is not a topic of discussion among my colleagues | 63 | 24 | 0.17 |
| Research to date has not adequately addressed concerns about antibiotic resistance and SDD | 63 | 23 | 0.58 |
| I would be more likely to participate in a RCT if patients in the control arm received selective oral decontamination as usual care (oral antibiotic pastes only) | 62 | 17 | –0.52 |
FIGURE 5.
Histograms of change scores (to indicate individual-level stability) from round 2 to round 3 for the items with (a) the most and (b) the least stability.
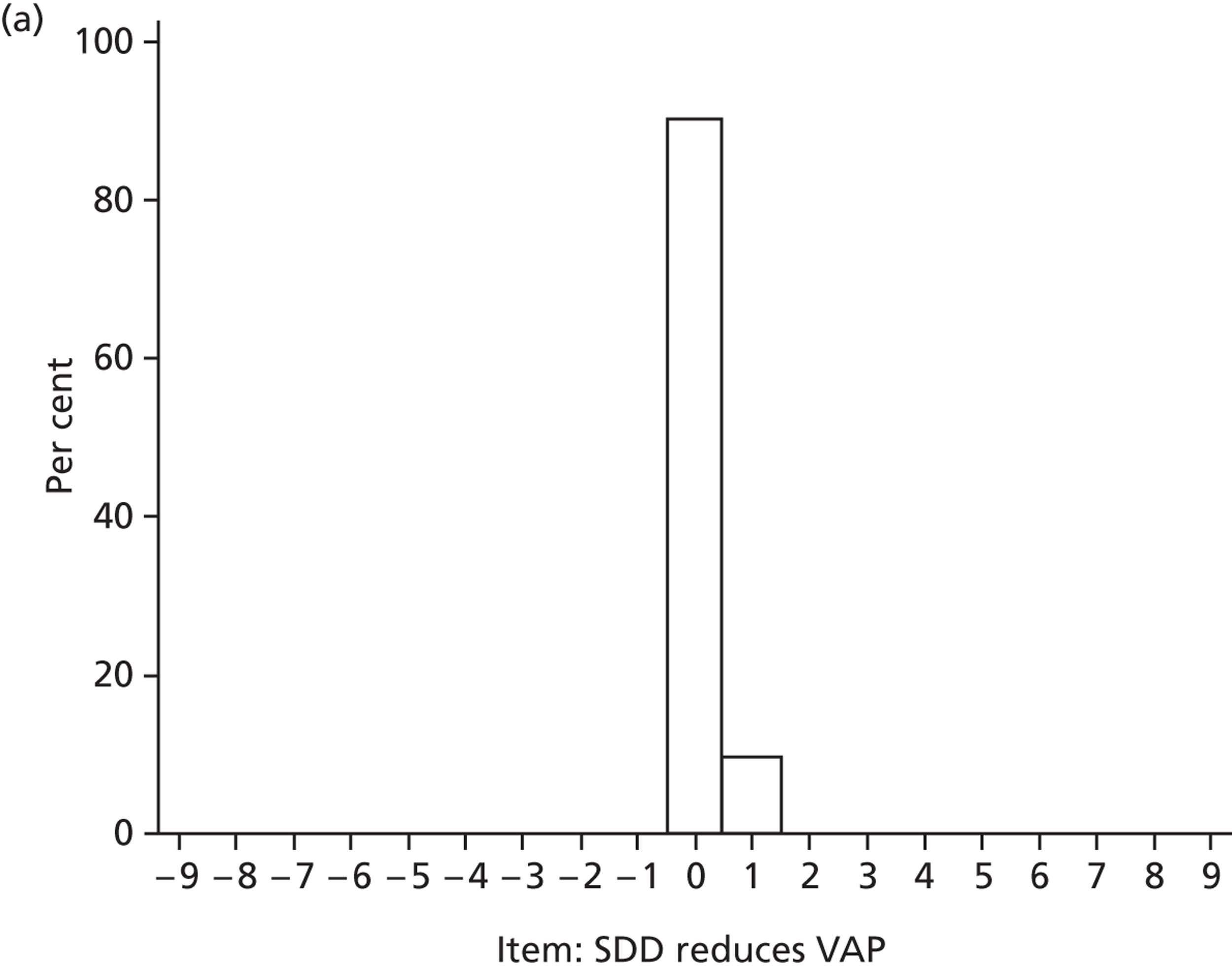
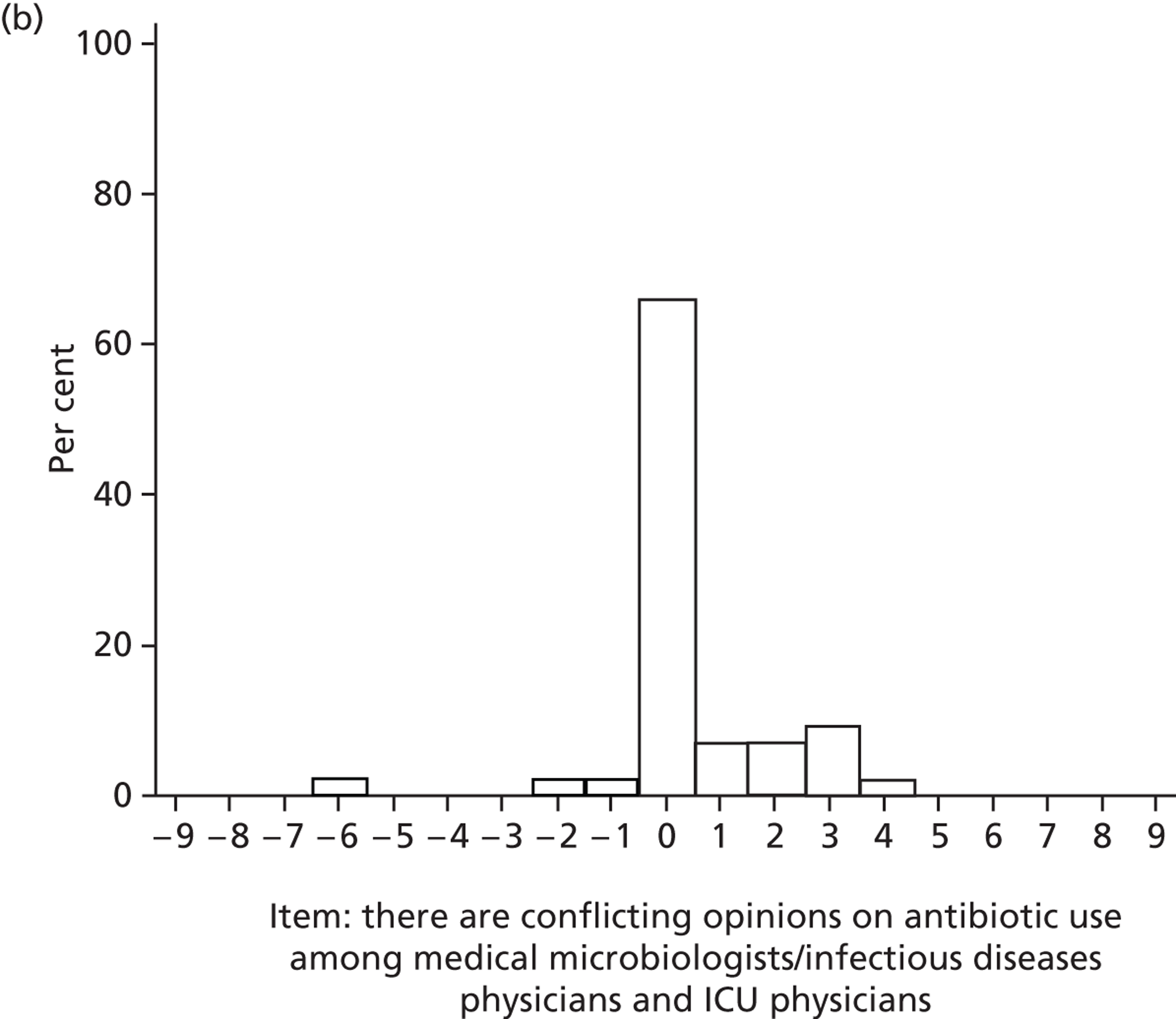
Group-level stability for each item was assessed by computing the change in arithmetic mean from round 2 to round 3. On the nine-point scale, a change of one scale point in the mean agreement level for any item was considered a potentially important change in views. Mean change scores are displayed in the last column of Table 11 (items presented in order of stability). These showed a high level of stability of views about SDD, with mean differences ranging from 0 to 0.52. In summary, at both the individual and group levels, the data appeared to be highly stable; therefore, the Delphi study did not proceed to a fourth round.
Group-level stability was also compared across stakeholder groups. Analysis of variance on change scores by group showed that there was a greater level of change from round 2 to round 3 in the clinical lead/nurse managers group than in the other stakeholder groups (Figure 6).
FIGURE 6.
Stability of responses from round 2 to round 3 for each stakeholder group. Note: the clinical lead group includes ICU clinical leads and ICU nurse managers or educators.
Participants who reported a low level of perceived knowledge (i.e. scoring below the mid-point for the item ‘I know the SDD evidence base well enough to have an informed opinion of its use’ at round 2) showed greater change in their responses from round 2 to round 3 (mean change 0.23, SD 0.35) than participants with a higher level of perceived knowledge (mean change 0.08, SD 0.18), as displayed in Figure 7.
FIGURE 7.
Self-reported knowledge of SDD evidence base and stability of responses from round 2 to round 3.
Importance ratings at round 3: beliefs about the consequences of selective decontamination of the digestive tract
The importance of each questionnaire item was assessed by calculating the mean rating of importance given by participants to the question ‘How important is this issue in your overall opinion about the delivery of SDD to critically ill patients?’. Table 12 presents the mean ratings of importance for each item assessing beliefs about consequences, presented in order of importance.
| Item stem | Mean rating |
|---|---|
| SDD increases antibiotic resistance | 7.62 |
| SDD would increase ICU C. difficile infections | 7.17 |
| The risks of SDD outweigh the benefits | 7.07 |
| SDD reduces VAP | 6.98 |
| SDD reduces hospital-acquired infections | 6.86 |
| There is no mortality benefit associated with SDD | 6.70 |
| Overall, SDD is cost-effective | 6.69 |
| Overall, SDD benefits the patients to whom it is delivered | 6.68 |
| Units using SDD have better clinical outcomes | 6.64 |
| I have concerns about the specific antimicrobials you need to use | 6.55 |
| I am opposed to the i.v. component of SDD | 6.52 |
| SDD reduces length of stay | 6.36 |
| SDD causes unpleasant side-effects for patients | 5.90 |
| SDD increases nursing workload | 5.86 |
| SDD drugs are expensive | 5.83 |
| SDD increases pharmacy workload | 5.24 |
| SDD increase microbiology workload | 5.05 |
| Educating staff would be expensive | 4.90 |
Importance ratings at round 3: domains level
Table 13 presents a range of indices that may indicate the relative importance of barriers to implementation for the other 11 theoretical domains on which this study was based.
| Domain | Mean importance (round 3) | Extent of elaboration (round 1) (number of utterances coded) | Rated as qualitatively important (round 1) (ratings based on content of interviews) | Number of items generated for round 2 |
|---|---|---|---|---|
| Memory, attention and decision processes | 7.88 | 154 | Yes | 4 |
| Knowledge | 7.48 | 154 | Yes | 3 |
| Behavioural regulation | 7.06 | 15 | Yes | 3 |
| Skills | 6.67 | 52 | No | 1 |
| Motivation and goals | 6.51 | 122 | Yes | 6 |
| Environmental context and resources | 6.44 | 90 | Yes | 4 |
| Nature of the behaviour | 6.13 | 31 | No | 3 |
| Social influences | 6.07 | 6 | Yes | 2 |
| Professional role and identity | 5.65 | 13 | Yes | 3 |
| Beliefs about capabilities | 5.31 | 18 | No | 1 |
| Emotion | N/A | 1 | No | 0 |
Table 13 shows that across different measures of importance, a number of other theoretical domains (in addition to Beliefs about consequences) included potentially important barriers to the implementation of SDD. The most elaborated domain in round 1, and the domain rated as most important in round 3, was Memory, attention and decision processes. However, as shown above, some less well-elaborated domains at round 1, for example, Behavioural regulation, were rated within the most important at round 3.
Agreement ratings at round 3: consensus of opinions about selective decontamination of the digestive tract
The Delphi literature operationalises consensus in various ways. In this study, we were interested not only in the proportion of participants who agreed with each item, but also in the proportion of participants who were uncertain about their agreement with the items. Hence, levels of consensus for the questions ‘To what extent do you agree or disagree …?, were assessed by noting the highest percentage of participants whose scores fell within any three-point band on the nine-point scale. 53 Table 14 displays a summary of the item content, grouped by the mid-point of the most populous three-point band and by consensus level. As shown in the table, there was high consensus (> 90% of sample within a three-point band) around strong agreement (mid-point of 8) for a subset of items. There was also consensus around uncertainty (mid-points around 4, 5 and 6) for different kinds of items.
| Consensus | Number of items | Theoretical domains | Item |
|---|---|---|---|
| Mid-point of most populous three-point band: 8 | |||
| > 90% | 8 | Memory, attention and decision processes | The decision to adopt SDD requires consensus between my colleagues |
| Part of the decision to adopt SDD requires agreement about which patients will receive it | |||
| Motivation | We are addressing hospital-acquired infections using other strategies | ||
| We are addressing ventilator-associated pneumonia using other strategies | |||
| Behavioural regulation | My hospital tries to reduce antibiotic use | ||
| Skills | The skills required to administer SDD fall within the competencies of our ICU nursing staff | ||
| Researcha | I would be more likely to participate in a RCT if patients in the control arm received VAP bundles as usual care (including chlorhexidine mouthwash/gel and head up positioning) | ||
| I would be more likely to participate in a RCT if cost–benefit analysis was included | |||
| 75–90% | 6 | Researcha | Further SDD RCTs are ethical |
| I would be more likely to participate in a RCT if it included pretrial, during-trial and post-trial monitoring of antibiotic resistance in all patients whether in the RCT or not | |||
| I would be more likely to participate in a RCT if it included pretrial, during-trial and post-trial monitoring of antibiotic resistance in patients in the RCT | |||
| I would support my ICU participating in a nationwide RCT of SDD | |||
| Memory, attention and decision processes | The decision to adopt SDD requires a review and appraisal of the current best evidence | ||
| Social influence | SDD will not be adopted without a local champion | ||
| 50–74% | 6 | Behavioural regulation | There are no national guidelines about SDD |
| The local decision to adopt SDD would be influenced by regulatory requirements | |||
| Motivation | SDD is not on my Units list of clinical priorities | ||
| SDD is not a topic of discussion among my colleagues | |||
| Nature of the behaviour | SDD would be a dramatic shift from our current practice | ||
| Researcha | I would support my centre being involved in a study to promote the adoption of SDD | ||
| Mid-point of most populous three-point band: 7 | |||
| > 90% | 0 | N/A | |
| 75–90% | 3 | Beliefs about capabilities | I could influence whether SDD is adopted in my hospital |
| Beliefs about consequences | SDD increases nursing workload | ||
| Knowledge | Research to date has not adequately addressed concerns about antibiotic resistance and SDD | ||
| 50–74% | 5 | Environmental context and resources | The SDD evidence base has been generated in countries with different resistance profiles to my country |
| Knowledge | I know to which patients I would administer SDD | ||
| Beliefs about consequences | SDD would increase ICU C. difficile infections | ||
| Nature of the behaviour | SDD is straightforward to deliver | ||
| Professional role and identity | There are conflicting opinions on antibiotic use among medical microbiologists/ID physicians and ICU physicians | ||
| Mid-point of most populous three-point band: 6 | |||
| > 90% | 0 | N/A | |
| 75–90% | 3 | Beliefs about consequences | Overall, SDD benefits the patients to whom it is delivered |
| SDD reduces hospital-acquired infections | |||
| SDD increases pharmacy workload | |||
| 50–74% | 12 | Beliefs about consequences | SDD reduces VAP |
| SDD increases antibiotic resistance | |||
| SDD increases microbiology workload | |||
| I am opposed to the i.v. component of SDD | |||
| I have concerns about the specific antimicrobials you need to use | |||
| Environmental context and resources | SDD drugs are expensive | ||
| The SDD evidence base is not generalisable to my country | |||
| Nature of the behaviour | The use of pastes may interfere with other treatments | ||
| Knowledge | I know the SDD evidence base well enough to have an informed opinion regarding its use | ||
| Motivation | Our unit VAP rates are low | ||
| Social | I am reassured that our position on SDD adoption is in line with other hospitals | ||
| Researcha | I would be more likely to participate in a nationwide RCT of SDD if mortality is the primary end point | ||
| Mid-point of most populous three-point band: 5 | |||
| > 90% | 0 | N/A | |
| 75–90% | 2 | Beliefs about consequences | Overall, SDD is cost-effective |
| SDD reduces length of stay | |||
| 50–74% | 1 | Beliefs about consequences | SDD causes unpleasant side effects for patients |
| Mid-point of most populous three-point band: 4 | |||
| > 90% | 0 | N/A | |
| 75–90% | 2 | Beliefs about consequences | Units using SDD have better clinical outcomes |
| The risks of SDD outweigh the benefits | |||
| 50–74% | 3 | Beliefs about consequences | There is no mortality benefit associated with SDD |
| Environmental context and resources | The SDD evidence base is not generalisable to my patient population | ||
| Motivation | SDD is outdated | ||
| Mid-point of most populous three-point band: 3 | |||
| > 90% | 0 | N/A | |
| 75–90% | 0 | N/A | |
| 50–74% | 1 | Beliefs about consequences | Educating staff would be expensive |
| Mid-point of most populous three-point band: 2 | |||
| > 90% | 0 | N/A | |
| 75–90% | 0 | N/A | |
| 50–74% | 3 | Professional role and identity | Prophylactic antibiotic use in SDD is at odds with my professional responsibilities |
| Beliefs about consequences | I am opposed to SDD | ||
| Researcha | I would be more likely to participate in a nationwide RCT of SDD if patients in the control arm received selective oral decontamination as usual care (oral antibiotic pastes only) | ||
| No consensus | |||
| (< 50%) | 2 | Professional role and identity | Prophylactic antibiotic use in SDD is at odds with my professional training |
| Researcha | My concerns about antibiotic resistance limit my willingness to participate in future RCTs of SDD | ||
Beliefs about consequences items (n = 18) made up 38% of the domains-based items, but a striking feature of Table 14 is that all but one of these items had either a low level of consensus (e.g. 50–75% consensus for SDD increases C. difficile) and/or consensus around the middle of the scale (e.g. SDD reduces VAP, concerns about specific antimicrobials, cost-effectiveness, length of stay, mortality benefit). In general, the Beliefs about consequences items that were rated as more important (see Table 12) had lower agreement scores (e.g. mortality benefit was important, nursing workload was less important).
Another interesting finding is that no-one reported strong opposition to SDD although scores ranged from 1 to 7 (where 1 means not opposed to SDD and 9 means opposed to SDD).
To examine potential differences between professional groups, we closely inspected four key beliefs about the consequences of SDD (‘There is no mortality benefit’, ‘The risks outweigh the benefits’, ‘SDD reduces VAP’ and ‘SDD increases antibiotic resistance’). We selected these beliefs to illustrate patterns of group responses because they were rated as important and had a range of levels of consensus, but the modal values were towards the centre of the response scale (reflecting uncertainty). Responses of the mean scores for the four professional groups showed that the most negative views were reported by pharmacists and clinical leads for ‘There is no mortality benefit’, by microbiologists for ‘The risks outweigh the benefits’, by pharmacists for ‘SDD reduces VAP’ and by intensivists for ‘SDD increases antibiotic resistance’. In other words, there was no consistent pattern of unfavourable views based on professional group. Furthermore, based on these items, the responses of participants who were current SDD users were only slightly more positive (in the order of 0.5 of a scale point) than the responses of participants who were not current SDD users. Owing to small group sizes, it was not appropriate to test the significance of these differences.
Non-normal distributions
Where consensus was ≤ 60% of responses occurring within a three-point band, the statistical description no longer portrayed the nature of the distribution of responses. For over half of these items (n = 9), there was evidence suggesting bimodality as shown in Figure 8.
FIGURE 8.
Non-normal distributions for items with consensus at ≤ 60% of responses occurring within a three-point band. A notable pattern was that in seven of these nine items, at least 20% of the participants registered a score of 5, reflecting uncertainty. (a) ‘SDD is straightforward to deliver’; (b) ‘I am reassured that our position on SDD adoption is in line with other hospitals’; (c) ‘I would be more likely to participate in a RCT if mortality is the end point’; (d) ‘I am opposed to the i.v. component of SDD’; (e) ‘SDD is outdated ’; (f) ‘The SDD evidence base is not generalisable to my patient population’; (g) ‘I am opposed to SDD’; (h) ‘Prophylactic antibiotic use in SDD is at odds with my professional training’; and (i) ‘My concerns about antibiotic resistance limit my willingness to participate in future RCTs of SDD’.
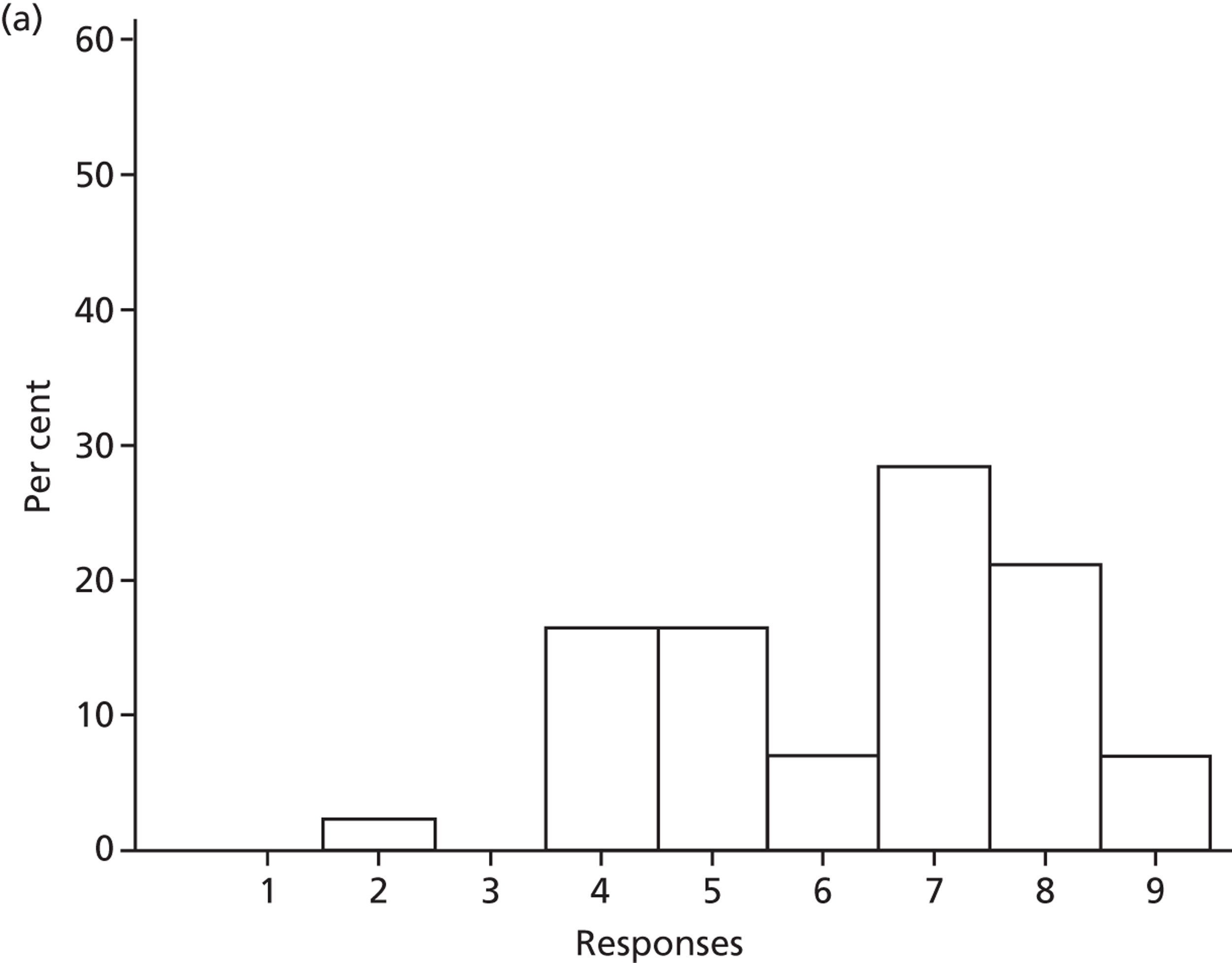
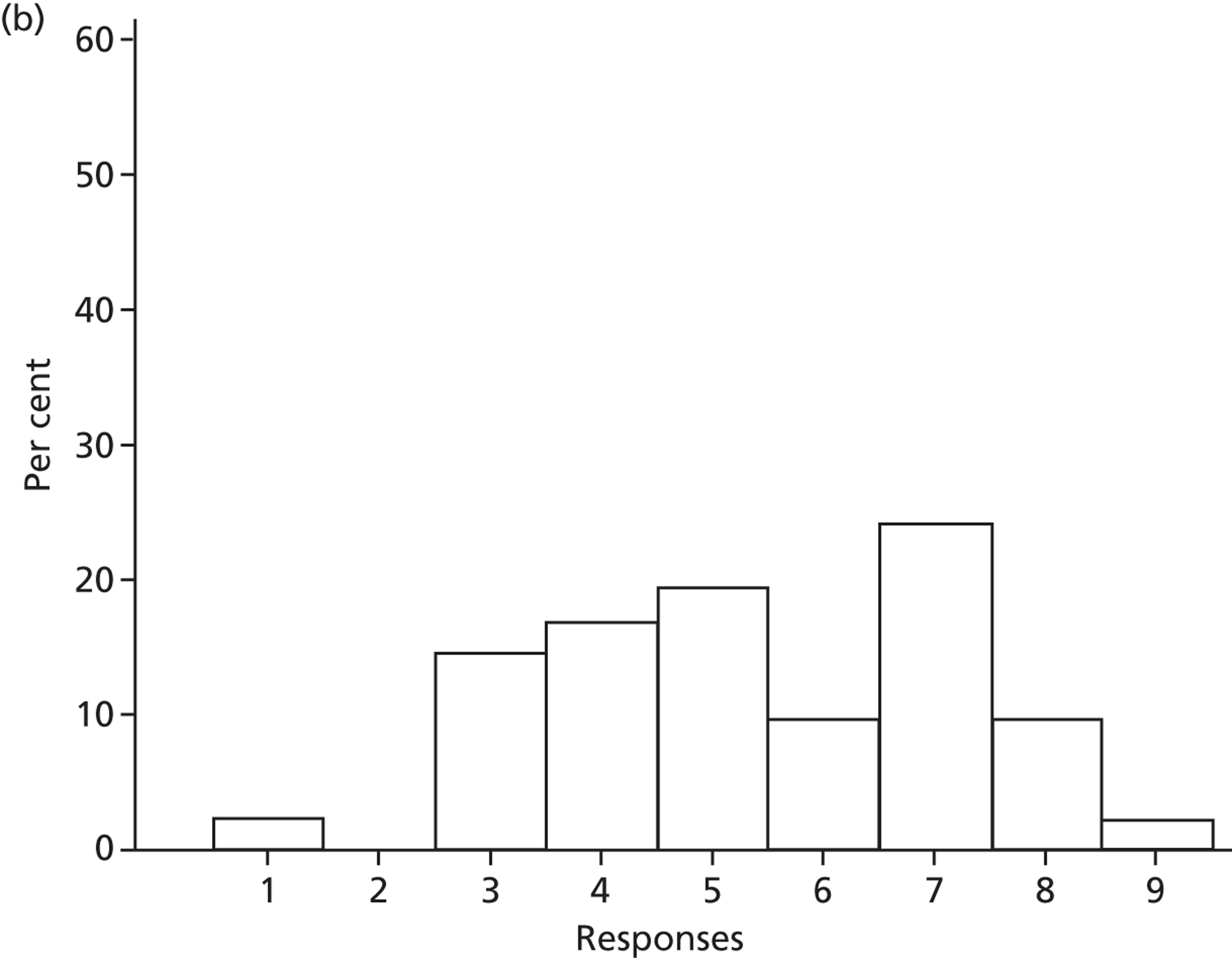
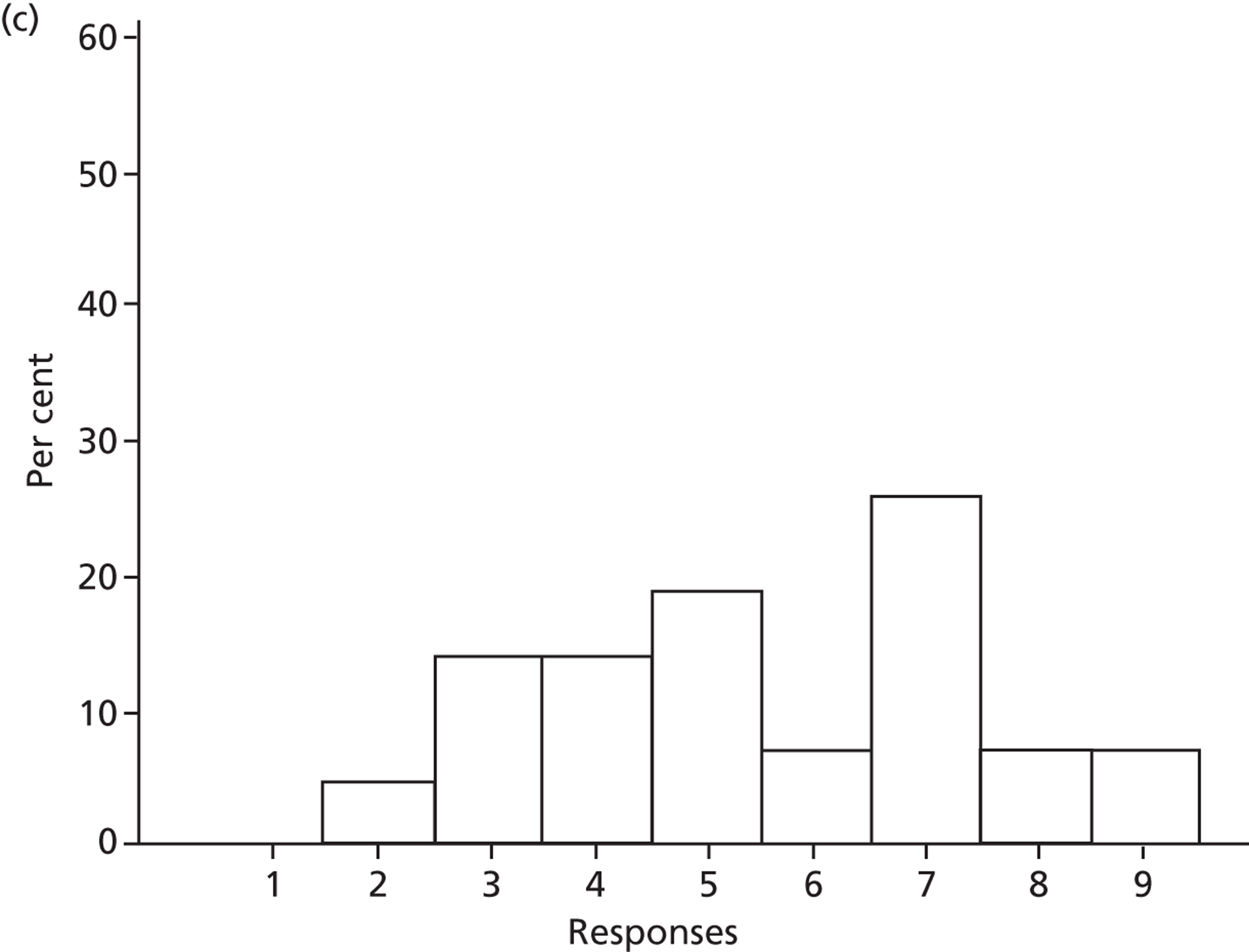
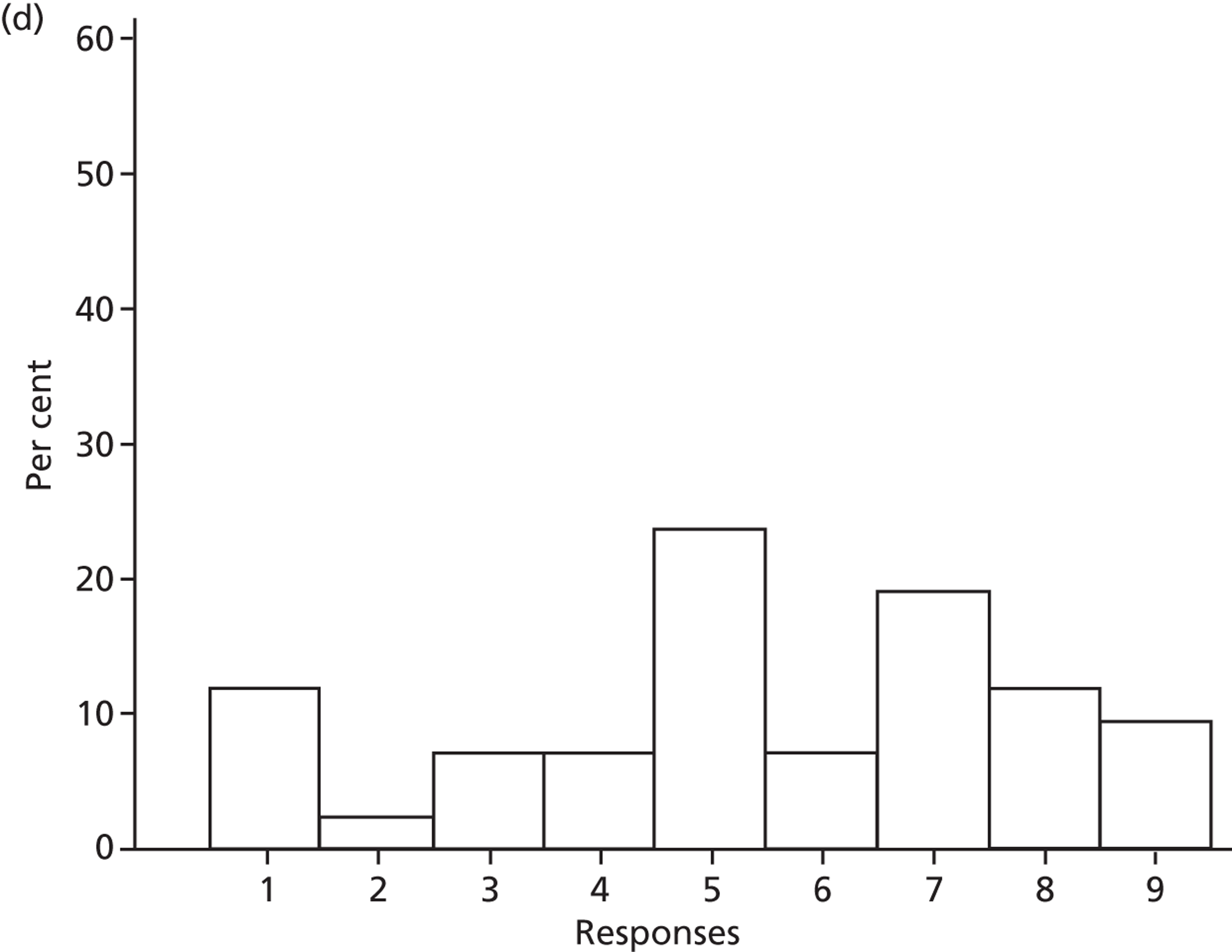
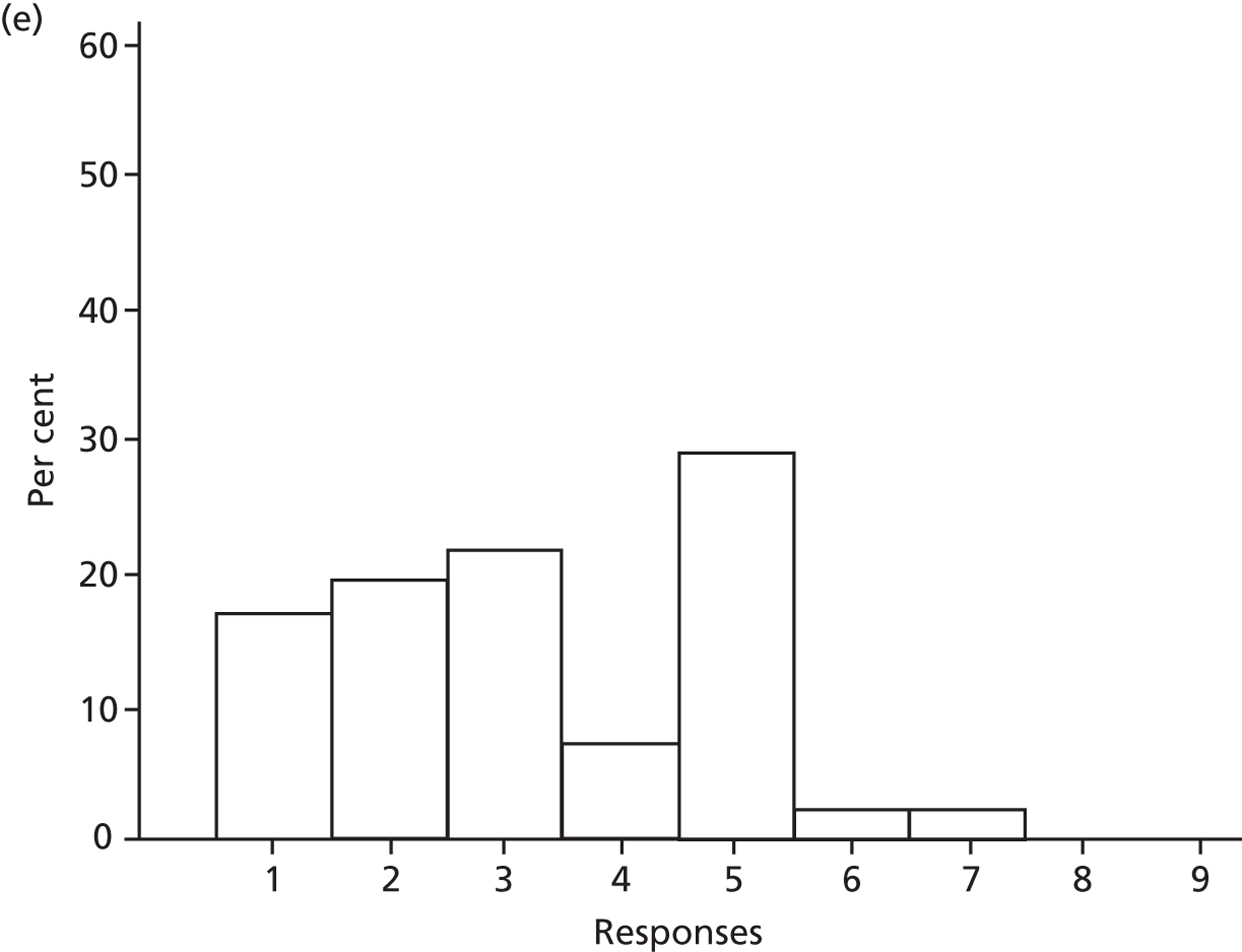
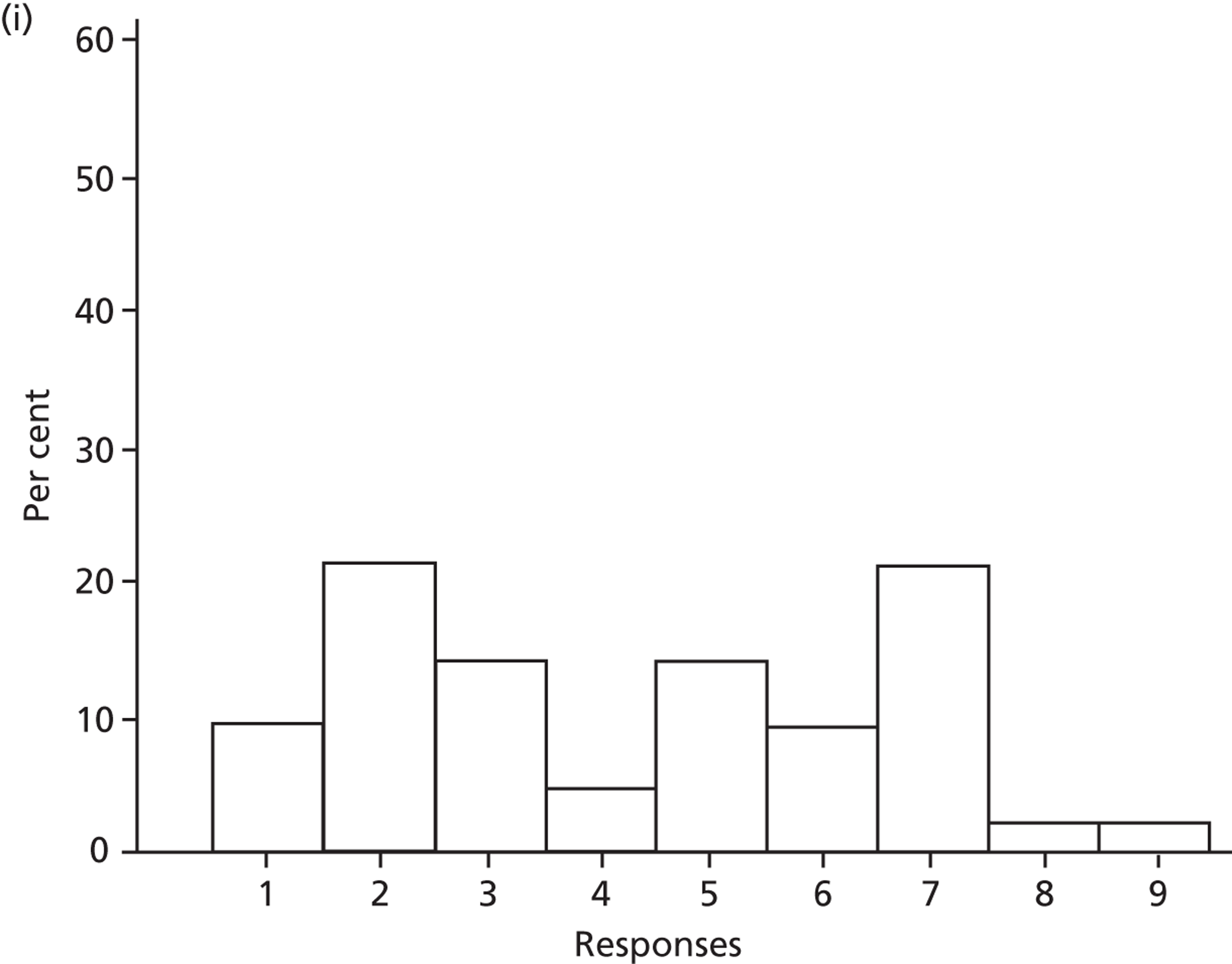
Discussion
This Delphi study was designed to address three research questions that could inform further SDD research. The Delphi participants, consisting of experts from four key stakeholder groups, (1) provided their views about the validity and adequacy of the SDD evidence base, (2) rated their agreement with, and importance of, a range of potential positive and negative consequences of SDD and (3) rated the likely barriers to implementing SDD in ICUs. This discussion draws on the results from round 2 and round 3 of the study to answer each research question in turn. We then comment on the strengths and limitations of this Delphi study, identify the methodological developments exemplified and explain how this stage of the SuDDICU study was used to inform the next stage.
Answers to research questions
-
The validity and adequacy of the existing SDD evidence base was perceived to be limited. There was consensus around moderate agreement (7 on the nine-point scale) with the item ‘Research to date has not addressed concerns of antibiotic resistance and SDD’ and consensus around slight agreement (6 on a nine-point scale) with the items ‘The SDD evidence base is not generalisable to my patient population’ and ‘The SDD evidence base is not generalisable to my country’. There was consensus around the mid-point of the scale (reflecting uncertainty or equipoise) for the item ‘SDD evidence from countries with different resistance profiles’. Taken together, these findings suggest that the Delphi panel was not persuaded of either the adequacy or the external validity of the evidence base.
-
A striking aspect of the results was the high level of consensus around uncertainty (or equipoise) for many of the items from the domain Beliefs about consequences. For the nine items rated as most important in driving participants’ overall opinion about SDD (i.e. the top 50% of items from Table 12), the majority of agreement responses occurred within a three-point range with a mid-point of 4, 5 or 6. The only exception to this was the item ‘SDD would increase ICU C. difficile infections’, for which the majority of responses occurred within a three-point range with a mid-point of 7. This pattern of findings was similar across the four professional groups and across users and non-users of SDD, and thus suggests a need for further evidence about the consequences of SDD.
-
In contrast to the Beliefs about the consequences of SDD, the theoretical domains relating to the likely barriers to implementing SDD in ICUs showed a different pattern of responses. Greater than 90% consensus around the three-point range 7 to 9 (reflecting strong agreement) was evident for items from the two domains that had the highest importance ratings (> 7). These items focused on perceived barriers to the decision processes that would be required in order to adopt SDD (‘Requires agreement among colleagues and agreement about which patients would receive SDD’) and aspects of motivation (‘VAP and HAI are already being addressed using other strategies’). The other important barrier (i.e. other domain with a mean importance rating > 7) was Knowledge. This is discussed in Strengths and limitations. For items with levels of consensus below 60% (within a three-point range), true consensus was not met as there was evidence of bimodality.
-
Specific trial design and feasibility issues were also explored. The majority of participants would support their ICU’s involvement in a nationwide RCT to evaluate the effectiveness of SDD and this finding was assessed further in the national survey (see Chapter 5). There was a high level of consensus (> 90%) in favour of participating in such a trial if the control arm received VAP bundles and if a cost–benefit analysis was included. There was also consensus (> 85%) that such a trial should include pretrial, during-trial and post-trial monitoring of antibiotic resistance. Trial design features and their associated challenges were also discussed in the interviews with international clinical triallists and are reported in Chapter 6.
Strengths and limitations
This study benefited from the inclusion of four key clinical groups that could have an influence on SDD policy in ICUs or on the way in which SDD is delivered in practice. There was a high retention rate (85%) of participants across the three Delphi rounds. There was evidence of high stability of responses between rounds 2 and 3, although less knowledgeable participants shifted significantly more than the rest of the sample after viewing the group-level feedback from round 2. This stability of opinion suggested that it was appropriate to interpret the round 3 results and that proceeding to a fourth round was unlikely to produce new findings. As for round 1 of the Delphi study, rounds 2 and 3 gathered opinion-based data that have clear limitations. One belief that emerged in the Delphi study, and for which there was low consensus, reflected the theoretical domain Professional role and identity and was worded in rounds 2 and 3 as ‘There are conflicting opinions on antibiotic use among Medical Microbiologists/ID Physicians and ICU physicians’. The national survey provided the opportunity to test the accuracy of this belief systematically as we sampled these two professions (intensive care consultants and clinical microbiologists) for the survey stage of the study (reported in Chapter 5).
Self-rated knowledge of the field was generally high, although a third of the participants rated their knowledge of the evidence base as uncertain or low. This variation in knowledge of the evidence base is a potential limitation of the study as it makes uncertainty difficult to interpret. There has been longstanding debate in the literature regarding the meaning of the ‘neutral’ response in Likert-type response scales (in this case, the score of 5 in a 1–9 scale). 60 When investigating views that may be based on knowledge of evidence, interpretation is rendered even more uncertain. Box 6 presents three different meanings of a neutral score in this context.
-
Clinical equipoise (I am familiar with the evidence base and I believe the evidence is evenly balanced).
-
Uncertainty (I am familiar with the evidence base and I believe there are gaps in valid evidence).
-
Self-rated ignorance (I am unfamiliar with the evidence base and so I do not know).
This point is important because it would be unethical to randomise patients to an effective treatment in a new clinical trial of SDD if neutral scores on key items were interpreted as equipoise or uncertainty when actually they could reflect ignorance. To check this possibility, we inspected cross-tabulations between knowledge scores and scores on all the items relating to Beliefs about the consequences of SDD. If neutral scores on the consequences items were over-represented by participants whose self-assessed knowledge of the SDD evidence base was at or below the mid-point, this would suggest that a modal score of 5 largely reflected ignorance. These cross-tabulations showed that the mid-point on the Beliefs about consequences items was endorsed by a large proportion of participants whose self-assessed knowledge of the evidence base was high. The one exception was that participants with higher knowledge scores tended to agree more strongly that ‘SDD reduces VAP’. Overall, this suggests that endorsement of the mid-point signified options 1 or 2 from Box 6: uncertainty arising from evenly balanced evidence or gaps in the evidence base.
Further methodological strengths
As noted in Chapter 3, Delphi methodology is often associated with achieving consensus whereas, in this study, we aimed to assess consensus, identifying the most important views and barriers to implementing SDD and, in particular, assessing the views of the four stakeholder groups about the current evidence base for SDD. This objective (rather than any imperative to achieve consensus) informed the methodological approach in this study.
Indices of group stability may mask individual instability if different subgroups change their views in opposite directions. In this study there was evidence of stability at both the individual and group levels. To our knowledge, this is the first study to assess stability of views at both these levels. The observed stability enhances our confidence that the identified views will be relevant over time, unless the profile of evidence or the knowledge of the evidence changes.
It is also rare in Delphi studies to identify consensus around uncertainty, where this exists, and to contrast this with consensus around agreement. Given the overarching objective of the study (to identify the most appropriate SDD research agenda), consensus around uncertainty was of great importance. With regard to this, the findings were critically appraised to identify whether uncertainty was likely to have arisen from inadequacies of the evidence base or inadequacies in individuals’ knowledge of the evidence. For the first time in a Delphi study in this field, we explored, in detail, the distributions of responses beyond a simple cut-off criterion for consensus by identifying different levels of consensus (> 90%, 75–90%, 50–74%). Below 60% consensus levels, there was evidence of bimodality. In other words, instead of sample statistics describing the views of one group (spread around a modal score), the distributions of scores suggested that there were two or more subgroups each with their own (different) modal score. Importantly, the distribution of responses for the item ‘I am opposed to SDD’ was spread around the two modes 1 (i.e. not opposed) and 5 (i.e. uncertain). It is possible that this distribution reflected subgroups representing professional groupings with different views of SDD, or groups of participants with either in-depth knowledge or poorer knowledge of the SDD evidence base. The sample size for the Delphi study was not sufficient to address this question. Factors associated with opposition to, or support of, SDD were explored further with the larger sample in the national survey, reported in Chapter 5.
A further strength of this study was its grounding in a theoretical framework that enabled us to distinguish between factors related to the clinical evidence (i.e. Beliefs about consequences of SDD) and potential barriers related to more practical issues to do with professional roles, resources and the management of change. Box 7 presents the key findings from the Delphi study that inform the need for, and acceptability of, a clinical effectiveness RCT or an implementation RCT.
-
There was consensus about uncertainty with regard to the clinical consequences of SDD. This finding would appear to support the need for an effectiveness RCT.
-
Furthermore, this uncertainty was not associated with poor knowledge of the evidence base in SDD. These conclusions are tentative and were explored further in the national survey (reported in Chapter 5).
-
Further effectiveness or implementation RCTs appeared to be acceptable, with most individuals in the Delphi study being willing to participate in further randomised studies.
-
There was strong support for specific design features of an effectiveness RCT, including VAP bundles delivered to patients in the control arm, cost–benefit analysis and pretrial, during-trial and post-trial monitoring of antibiotic resistance.
Chapter 5 A UK-wide survey of consultants in intensive care medicine and consultant clinical microbiologists
Background
The aim of this stage of the research was to identify current SDD practice and assess the acceptability and feasibility of further RCTs, or other research designs on SDD, among a wide group of consultants in intensive care medicine and consultant clinical microbiologists. This study was a large-scale online questionnaire survey of all consultant members of the UK Intensive Care Society (ICS) and of clinical microbiologists involved in intensive care in the UK [accessed through their membership of either the Healthcare Infection Society (HIS) or the British Society for Antimicrobial Chemotherapy (BSAC)]. The study was designed to complement and build on the results of the Delphi study (reported in Chapters 3 and 4) by identifying the willingness (intentions) of intensive care consultants and clinical microbiologists to participate in further SDD research and the predictors of these intentions. The survey was designed to answer the following research questions:
Research question 6: What are the stated current practices and intentions about SDD of intensive care consultants and clinical microbiologists with responsibility for critically ill patients about SDD?
Research question 7: If there are uncertainties in the evidence base, do these clinicians believe they could be addressed in a clinical trial; what research questions, trial design(s) and interventions would be optimal and what predicts these beliefs?
Methods
Sample
(a) Intensive care consultants
The membership list of the ICS (the primary society for intensive care professionals in the UK) was used to approach intensive care consultants. The ICS database contains contact details for 2908 intensive care consultants in the UK. The Intensive Care National Audit and Research Centre (ICNARC) database was also used as a means of contacting intensive care consultants.
(b) Clinical microbiologists involved in intensive care
Membership lists of the HIS and the BSAC were used to identify potential consultant clinical microbiologist participants involved in intensive care. The HIS database contains contact details for 629 clinical microbiologists worldwide and the BSAC contains contact details for 866, with considerable overlap in membership between the two societies. Both societies assisted with the recruitment procedure to maximise the possibility that all clinical microbiologists working in intensive care in the UK (estimated at 20% of total membership) were given the opportunity to participate. Hence, we estimated that around 250 microbiologists would be eligible to participate.
Materials
The development and validation of the questionnaire consisted of two steps:
-
Item generation. We included items relating to the issues identified in the Delphi study as important. This included overall views about SDD, intention (willingness) to participate in a randomised trial and the factors likely to influence these intentions (e.g. beliefs about the consequences of SDD, views about the ethics of further SDD effectiveness research). The questionnaire commenced with preliminary questions to check participant eligibility: (1) ‘Have you answered this questionnaire before?’ and (2) ‘Do you have clinical involvement in the care of patients in intensive care?’. The questionnaire was required to be relatively brief to maximise response rates and comprised 23 items. The first question asked about current SDD practice in the participants’ units. Further items assessed participants’ knowledge (item 2) and views about SDD (items 3 and 4). Beliefs about consequences was by far the most elaborated domain in the Delphi study; therefore, the seven items with the highest importance ratings from this domain at round 3 of the Delphi were included (items 5–11). Items 12–17 were included to address research question 7. Response options followed guidance for questionnaire design (seven options for a well-educated sample from 1, strongly disagree, to 7, strongly agree). 61 Finally, six items (items 18–23) were included to assess demographics characteristics in order to describe the sample and assess the representativeness of the responder group. Participants were also provided with the space to include free-format comments at the mid- and end point of the questionnaire. The comments data were used to check the content validity (coverage) of the questionnaire as it was assumed that participants would comment on any important issues that had been omitted from the questionnaire. The final version of the questionnaire is presented in Appendix 5.
-
Pretesting. The draft questionnaire (and cover letter) was pilot tested to assess wording, acceptability and length, using personal interviews with four clinical collaborators (these individuals were not from the sampling frame so that data from all intensive care consultants and microbiologists could be used in the analysis). Each question was evaluated by the research team in the light of the pilot test findings and reworded if necessary to enhance meaning and acceptability. 62
Procedure
An e-mail invitation, containing a link to the online questionnaire, was sent to all members of the ICS on 1 December 2011 by the society, on behalf of the study team. Two reminder e-mails were sent to all ICS members 3 weeks after the first posting and 3 weeks after the Christmas and New Year holiday period. Personalised e-mails were additionally sent to consultant members of the ICNARC case mix programme on 14 February 2012 by the Director of ICNARC (KMR; one of the SuDDICU study investigators) requesting their support for the survey and sending an electronic copy of the questionnaire and a link to the online questionnaire.
E-mail invitations were sent to the clinical microbiologists by the HIS on 1 December 2011. The second society (BSAC) sent the invitation to its members on 10 February 2012. We relied on the professional societies to send reminders to their members if they deemed this appropriate based on other communications being sent at the same time.
The questionnaire instrument was programmed so that clicking on the submit button resulted in upload of data into an electronic database. Questionnaires were anonymous and so it was not possible to target reminders to non-responders. The national survey was advertised at a national conference63 in order to generate publicity and interest from key stakeholder groups and maximise response rates.
Data management and analysis
Analysis included simple descriptive statistical testing and statistical prediction techniques. First, responses to each question were summarised using frequency distributions. Second, multiple regression techniques (including linear regression and, when appropriate, logistic regression) were used to identify the predictors (i.e. theoretical constructs and characteristics of responders) with intention to participate in the SDD research. The differences between intensive care consultants and microbiologists were explored.
The comments provided by participants were sorted into three categories: new information (reported in the results section), elaboration of the reasons for giving certain responses and comments about the procedure and quality of the questionnaire.
Ethics approval for the national survey was granted by the Research Ethics Board of the College of Life Sciences and Medicine, University of Aberdeen (CERB/2011/8/633).
Results
Response rates
Invitations were sent to 2908 ICS members (including 1685 intensive care consultants), 528 members of HIS (excluding 101 invitations that were not delivered), 866 BSAC members and 500 consultants registered with ICNARC. As mentioned previously, there is likely to be considerable overlap across each of these membership lists. A recruitment flow chart is displayed in Figure 9.
FIGURE 9.
National survey recruitment flow chart.
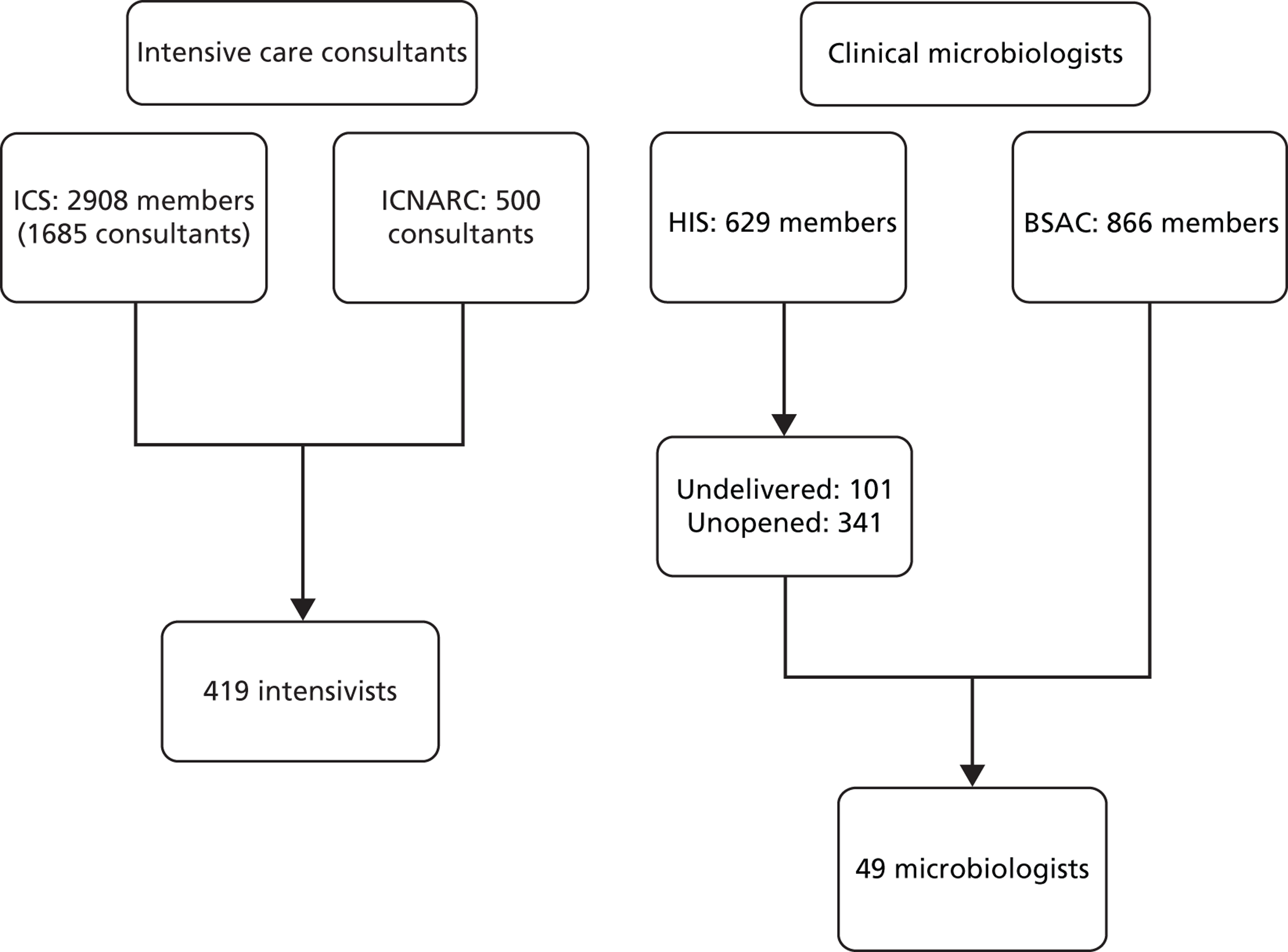
Responses were received from 468 UK clinicians and the breakdown by professional group is shown in Table 15. Clinicians were likely to be a member of more than one professional society; therefore, only an estimate of the response rate could be generated. Furthermore, it was unclear how many e-mail addresses would have been out of date or how many e-mail messages would have reached spam folders rather than inboxes of eligible clinicians. We provide a worst-case response rate in Table 15.
| Professional group | Participants | Estimated denominator | Estimated minimum response rates |
|---|---|---|---|
| Consultants in intensive care medicine | 419 | 1685a | 25% |
| Consultant clinical microbiologists | 49 | 250b | 20% |
| Total | 468 | 1935 | 24% |
Participant characteristics
Four hundred and nineteen participants were consultants in intensive care medicine and 49 were consultant clinical microbiologists. The sample as a whole reported a high level of experience in clinical practice (mean 19 years, SD 8 years). Participants also provided descriptive details about the hospital(s) they worked in, shown in Table 16. For comparative purposes, data from the ICNARC database and UK ICUs are presented alongside the participant characteristics of this sample. As shown in Table 16, the SuDDICU sample had greater representation from larger units and from units affiliated with universities.
| Hospital characteristics | SuDDICU sample (%) | ICNARC data, n (%) |
|---|---|---|
| University affiliation | ||
| Full university affiliation | 52 | 75 (30) |
| Partial university affiliation | 28 | 42 (17) |
| No university affiliation | 19 | 134 (53) |
| Private | 2 | N/A |
| Number of beds in the ICU | ||
| < 10 | 26 | 130 (52) |
| 10–20 | 46 | 107 (43) |
| > 20 | 27 | 14 (6) |
Participants were asked to report their hospital’s current policy with respect to SDD delivery. Six options were offered:
-
not delivering SDD and have not considered this issue
-
not delivering SDD after careful consideration
-
not delivering SDD but issue currently being considered
-
delivered SDD in the past but reversed policy and now do not deliver
-
full SDD sometimes delivered but not protocolised, full SDD formally adopted
-
full SDD formally adopted, protocolised and routinely delivered to specific patient subgroups.
Responses are displayed in Figure 10.
FIGURE 10.
SDD policy in participants’ hospitals.
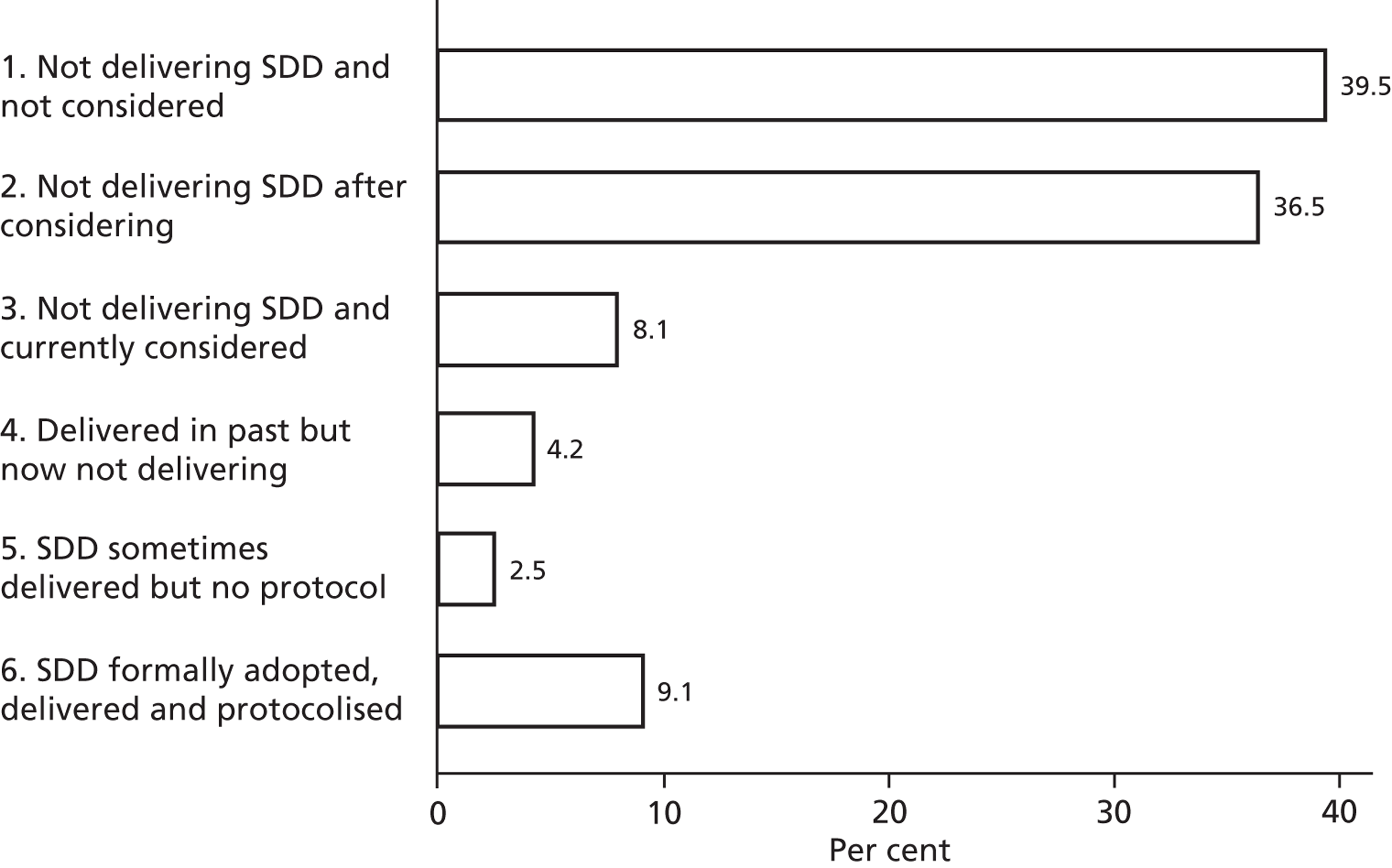
Participants’ self-reported knowledge of the SDD evidence base was assessed with the item ‘I know the SDD evidence base well enough to have an informed opinion regarding its use’. The mean response (1, strongly disagree, to 7, strongly agree) to this item was 4.7 (SD 1.4). Intensive care consultants’ responses to this item had greater spread than the microbiologists’ responses, but the modal score was 5 (signifying slight agreement), as displayed in Figure 11.
FIGURE 11.
Self-reported knowledge of the SDD evidence base (1 = strongly disagree, 7 = strongly agree). Responses to the item, ‘I know the SDD evidence base well enough to have an informed opinion of its use’. (a) Intensivists; and (b) microbiologists.
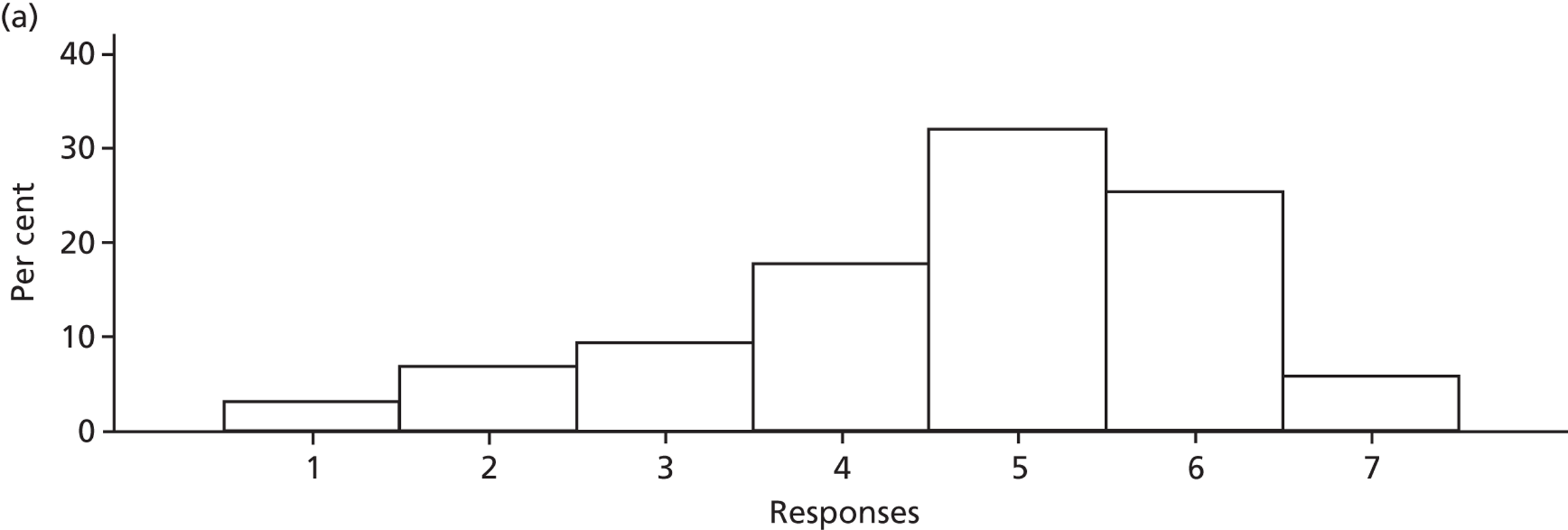
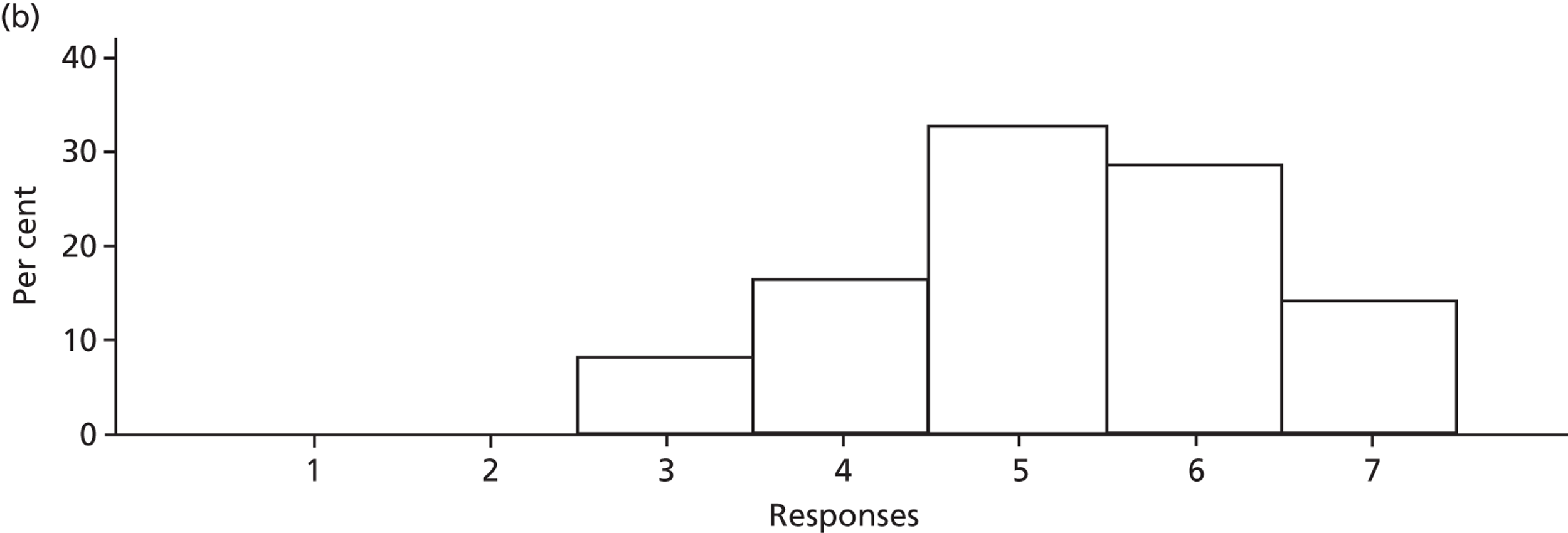
Free-text comments on content validity of the questionnaire
Four participants made comments on the content validity of the questionnaire. One of these suggested that there should have been clearer differentiation between SDD and selective digestive decontamination. The other three suggested that the questionnaire was insufficiently disease specific and that some questions should have been asked with respect to specific disease categories or patient subpopulations (e.g. trauma patients). A total of 80 participants left a comment and the vast majority of these elaborated on their response to particular items. This information will be considered in the design of any future SDD research, but is not reported further in this chapter.
Views about selective decontamination of the digestive tract
There was a bimodal distribution of responses in both professional groups for the item ‘I am opposed to SDD’, as shown in Figure 12. The clinical microbiologist participants tended to be less favourable towards SDD (i.e. towards the right of the scale; modal score of 6) than the intensive care consultant participants (primary mode of 4), but there was a secondary mode of 2 signifying ‘not opposed’ in both groups.
FIGURE 12.
Opposition to SDD by professional group (1 = strongly disagree, 7 = strongly agree). Response to the item ‘I am opposed to SDD’. (a) Intensivists; and (b) microbiologists.
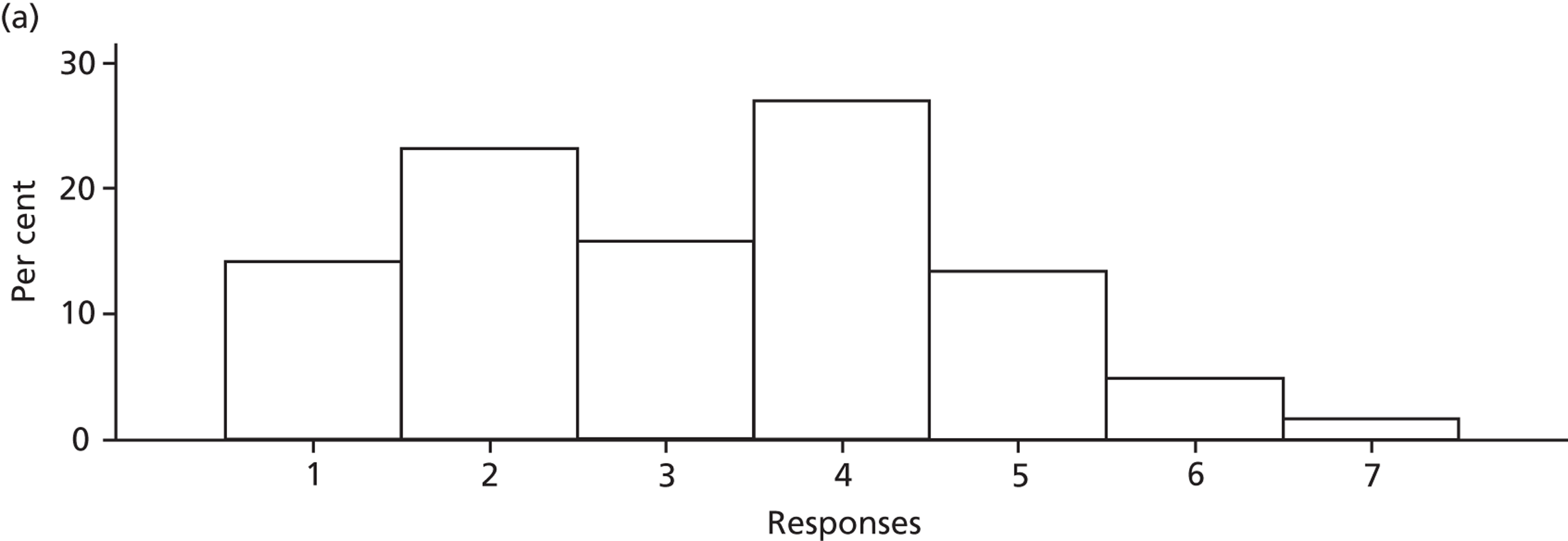
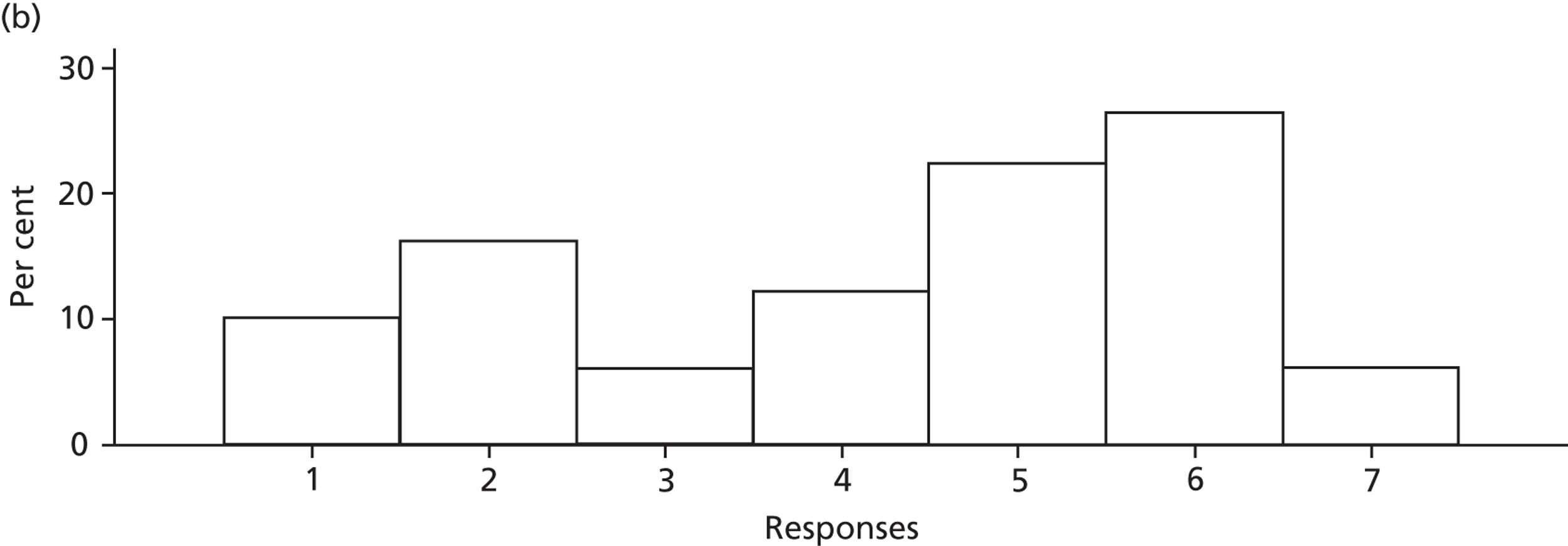
The association between current SDD delivery and views about SDD is presented in Figure 13.
FIGURE 13.
Box and whisker plot of opposition to SDD by SDD delivery group (larger circles represent mean score).
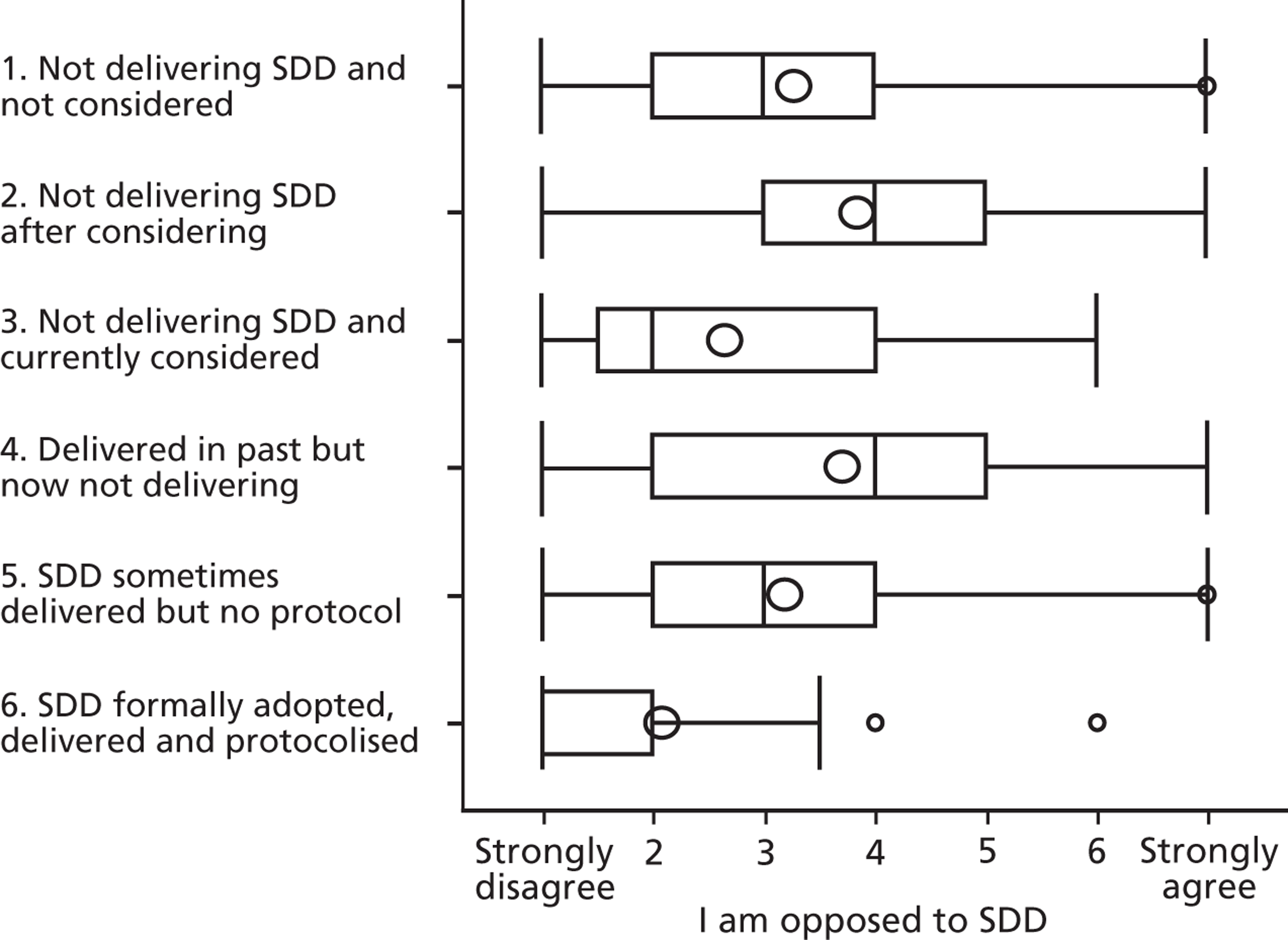
A one-way analysis of variance on mean score of opposition indicated that there was a difference in mean scores between the groups. Post hoc pairwise comparisons revealed that group 6, the participants working in hospitals where SDD was formally adopted, protocolised and routinely delivered, scored significantly lower on opposition to SDD compared with groups 1, 2 and 4. These results were confirmed using non-parametric tests (Kruskal–Wallis test of medians).
Opposition to SDD was significantly correlated with all seven items relating to the beliefs about the consequences of SDD and with the item ‘I am opposed to the i.v. component of SDD’, as displayed in Table 17. There was no significant association between knowledge of the SDD evidence base and opposition to SDD.
| Item | Correlation coefficient (for the relationship with the question ‘I am opposed to SDD’) | 95% CI | p-value |
|---|---|---|---|
| I am opposed to the i.v. component of SDD | +0.60 | 0.54 to 0.66 | < 0.001 |
| SDD increases antibiotic resistance | +0.61 | 0.55 to 0.66 | < 0.001 |
| Overall, SDD benefits the patients to whom it is delivered | –0.59 | –0.64 to –0.52 | < 0.001 |
| The risks of SDD outweigh the benefits | +0.23 | 0.14 to 0.31 | < 0.001 |
| SDD reduces HAIs | –0.51 | –0.57 to –0.44 | < 0.001 |
| SDD increases C. difficile infections | +0.44 | 0.35 to 0.50 | < 0.001 |
| SDD reduces ventilator-associated pneumonia | –0.43 | –0.49 to –0.34 | < 0.001 |
| SDD reduces mortality | –0.49 | –0.55 to –0.41 | < 0.001 |
| I know the SDD evidence base well enough to have an informed opinion of regarding its use | –0.01 | –0.09 to 0.09 | 0.82 |
Regression analysis was performed to investigate multiple predictors of opposition to SDD. Nine item variables and five dummy variables (to represent current practice) were entered into the analysis. As shown in Table 18, there were eight significant predictors: current practice (currently delivering full protocolised SDD), opposition to the i.v. component of SDD, self-assessed knowledge of the evidence base and five specific beliefs about the consequences of delivering SDD. The five specific beliefs were SDD increases antibiotic resistance, SDD benefits patients, SDD reduces HAIs, SDD reduces VAP and risks of SDD outweigh benefits.
| Item | Coefficient | 95% CI | p-value |
|---|---|---|---|
| Not delivering SDD and not considered | –0.05 | –0.26 to 0.17 | 0.666 |
| Not delivering SDD after considering | [Reference category] | ||
| Not delivering SDD and currently considered | –0.13 | –0.49 to 0.23 | 0.479 |
| Delivered in past but now not delivering | 0.24 | –0.21 to 0.70 | 0.297 |
| SDD sometimes delivered but no protocol | 0.25 | –0.36 to 0.85 | 0.424 |
| SDD formally adopted delivered and protocolised | –0.67 | –1.02 to –0.31 | < 0.001 |
| I know the SDD evidence base well enough to have an informed opinion regarding its use | 0.08 | 0.01 to 0.15 | 0.026 |
| I am opposed to the i.v. component of SDD | 0.33 | 0.27 to 0.40 | < 0.001 |
| SDD increases antibiotic resistance | 0.22 | 0.13 to 0.31 | < 0.001 |
| Overall, SDD benefits the patients to whom it is delivered | –0.24 | –0.35 to –0.13 | < 0.001 |
| The risks of SDD outweigh the benefits | 0.10 | 0.03 to 0.17 | 0.005 |
| SDD reduces HAIs | –0.15 | –0.24 to –0.05 | 0.003 |
| SDD increases C. difficile infections | 0.08 | –0.02 to 0.17 | 0.114 |
| SDD reduces ventilator-associated pneumonia | –0.12 | –0.22 to –0.01 | 0.028 |
| SDD reduces mortality | –0.03 | –0.14 to 0.08 | 0.616 |
Acceptability of further selective decontamination of the digestive tract research
Most participants (85%) reported that the current uncertainties in the SDD evidence base should be addressed in a new study. Participants’ views about whether or not it is ethically acceptable to conduct further RCTs evaluating effectiveness are displayed in Figure 14. The mean score was 5.4 (SD 1.5) on the scale range of 1–7, indicating that participants tended to agree that further effectiveness RCTs were ethically acceptable.
FIGURE 14.
Views about ethical acceptability of further effectiveness RCTs. (1 = strongly disagree, 7 = strongly agree) Responses to the item ‘It is ethically acceptable to conduct further RCTs evaluating the effectiveness of SDD’. (a) Intensivists; and (b) microbiologists.
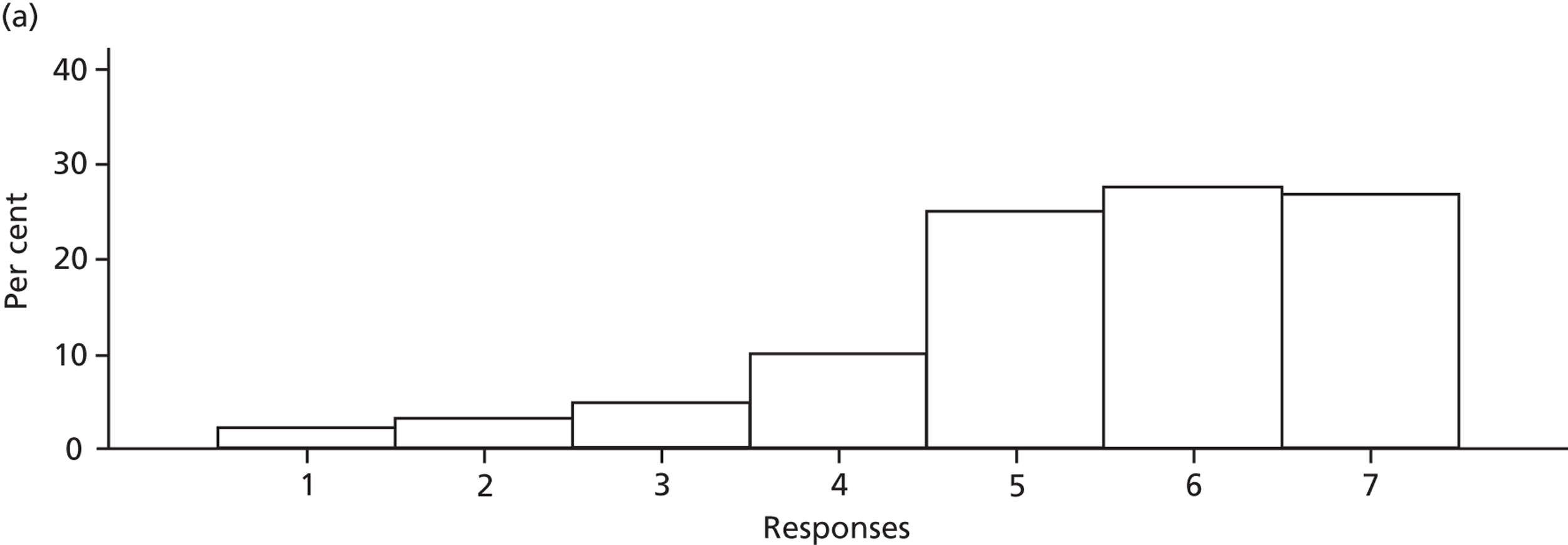
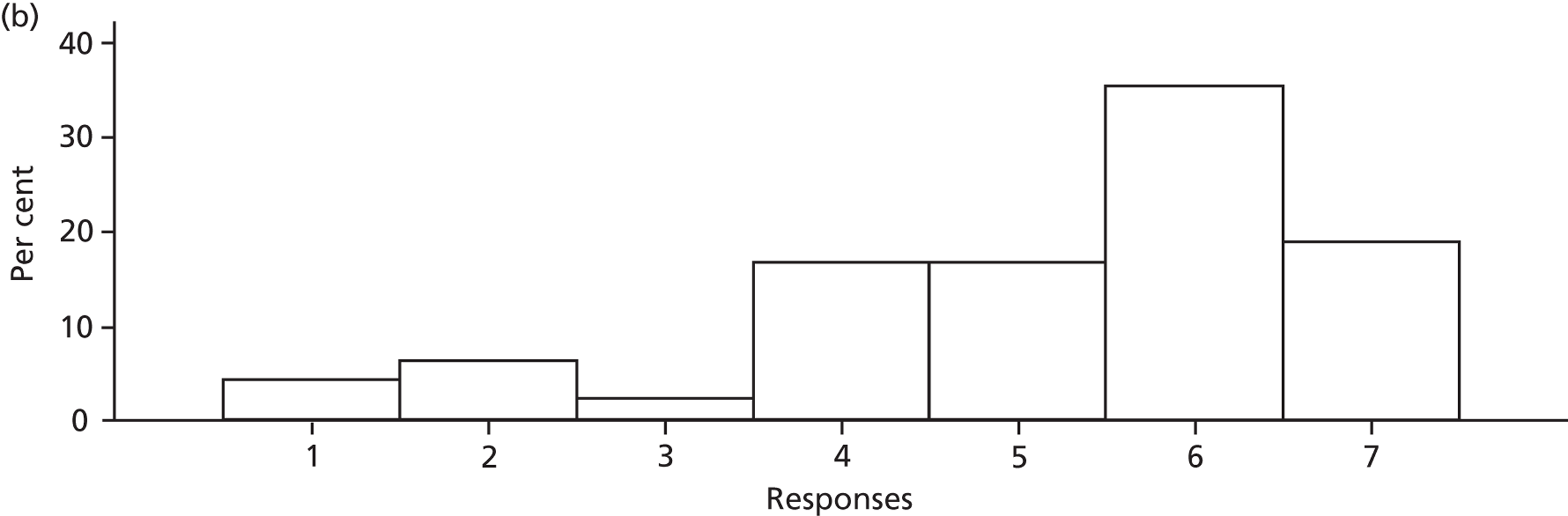
Ethical acceptability of further effectiveness research was significantly correlated with four items relating to the beliefs about the consequences of SDD, as displayed in Table 19.
| Item | Correlation coefficient (for the relationship with the question ‘Further SDD effectiveness research is ethical’) | 95% CI | p-value |
|---|---|---|---|
| SDD increases antibiotic resistance | –0.05 | –0.14 to 0.04 | 0.31 |
| Overall, SDD benefits the patients to whom it is delivered | +0.21 | 0.12 to 0.30 | < 0.001 |
| The risks of SDD outweigh the benefits | +0.01 | –0.08 to 0.10 | 0.79 |
| SDD reduces HAIs | +0.19 | 0.10 to 0.27 | < 0.001 |
| SDD increases C. difficile infections | –0.08 | –0.17 to 0.01 | 0.09 |
| SDD reduces ventilator-associated pneumonia | +0.22 | 0.13 to 0.30 | < 0.001 |
| SDD reduces mortality | +0.13 | 0.04 to 0.22 | 0.004 |
Regression analysis was performed to investigate multiple predictors of views about the ethical acceptability of further SDD effectiveness research. Seven variables were entered into the analysis. As shown in Table 20, there were two significant predictors: ‘Overall, SDD benefits the patients to whom it is delivered’ and ‘SDD reduces VAP’.
| Item | Coefficient | 95% CI | p-value |
|---|---|---|---|
| SDD increases antibiotic resistance | 0.09 | –0.03 to 0.21 | 0.15 |
| Overall, SDD benefits the patients to whom it is delivered | 0.17 | 0.01 to 0.33 | 0.04 |
| The risks of SDD outweigh the benefits | 0.07 | –0.04 to 0.17 | 0.21 |
| SDD reduces HAIs | 0.10 | –0.04 to 0.24 | 0.15 |
| SDD increases C. difficile infections | –0.07 | –0.21 to 0.07 | 0.32 |
| SDD reduces ventilator-associated pneumonia | 0.18 | 0.02 to 0.33 | 0.02 |
| SDD reduces mortality | –0.08 | –0.24 to 0.08 | 0.33 |
It could be argued that instead of a linear relationship between ethical acceptability and beliefs about consequences, there should be an inverted-U relationship between these factors. That is, if the evidence base clearly shows benefit or harm, then further effectiveness research will be uninformative, and uncertainty (i.e. the neutral response on the Likert scale) should be associated with the highest levels of ethical acceptability. To identify whether or not there was a signal in the data to support this possibility, we inspected the scatterplots for two key beliefs about the consequences of SDD: increase in antibiotic resistance and reduction in mortality. These scatterplots, displayed in Figure 15, indicate a weak signal for a curvilinear relationship as proposed. However, the trend was not strong enough to test for an inverted U–shaped relationship in a multivariate model.
FIGURE 15.
Scatterplots indicating a curvilinear relationship between ethical acceptability of further SDD effectiveness research (on y-axis) and (a) ‘SDD increases antibiotic resistance’ and (b) ‘SDD reduces mortality’ (on x-axis). (1 = strongly disagree, 7 = strongly agree.)
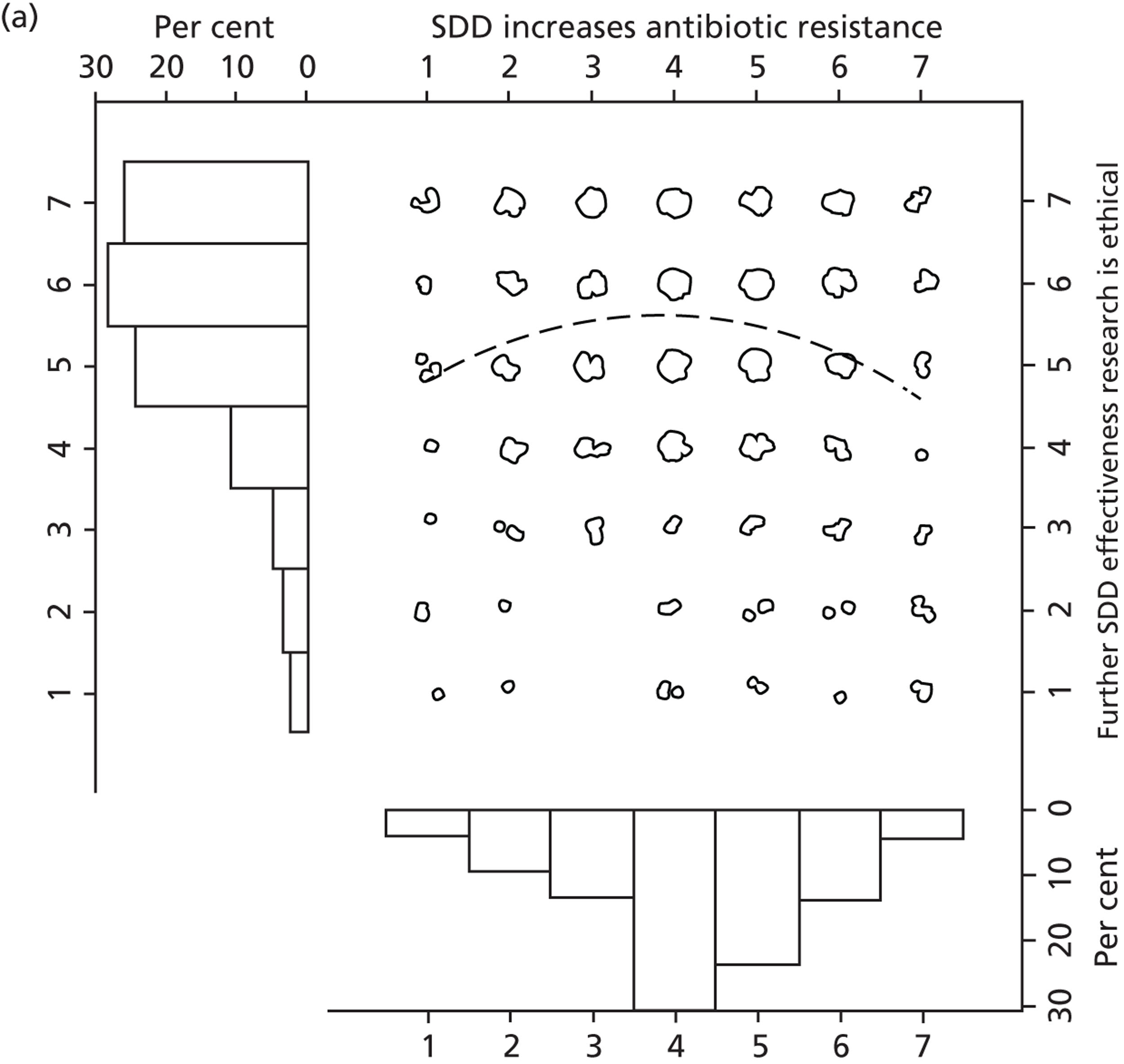
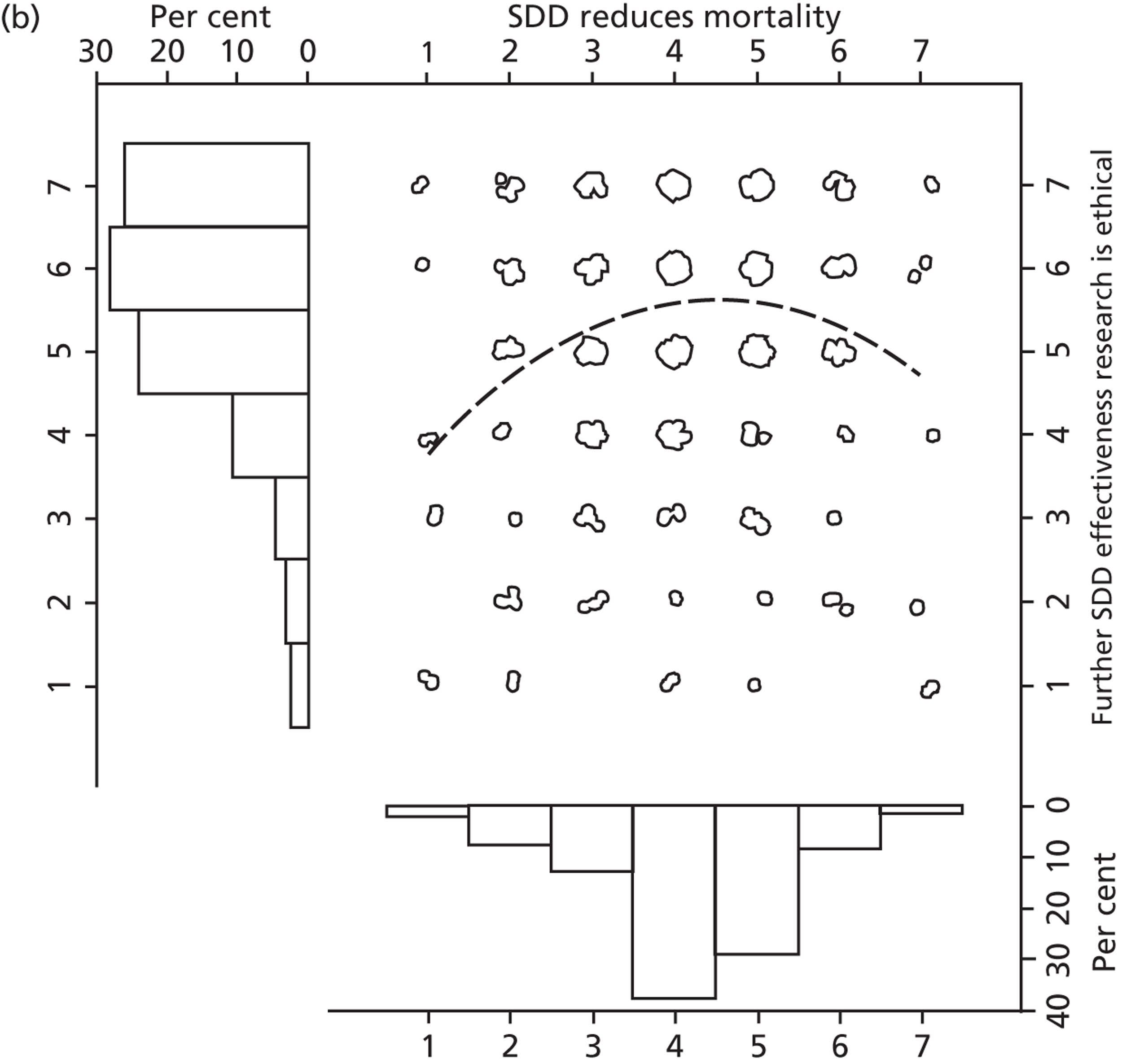
The multimethod nature of this study afforded the opportunity to examine the question of the relative importance of different beliefs about the consequences of delivering SDD, using a multimeasure approach to check whether or not three indices of importance converged. The three indices of importance were:
-
the frequency with which each belief was elicited in the Delphi round 1 interviews (as discussed in Chapter 3)
-
importance ratings (possible range 1–9) made by the Delphi participants in round 3 (reported in Chapter 4)
-
the size of the standardised regression coefficients when the beliefs were used to predict scores representing opposition to SDD (in the national survey, reported in Table 18).
The three indicators of importance for the Beliefs about consequences items in the national survey are presented in Table 21 in order of apparent importance from round 1 data. The table shows that, on all three measures, the potential effect of SDD on antibiotic resistance was the most important clinical consequence of SDD. Furthermore, the rank orders of the importance indicators based on round 1 data and regression coefficients were consistent (except for one item with changed wording for the national survey). The least convergent index of importance was the mean rating of importance at round 3.
| Item | Extent of elaboration (number of utterances round 1 interviews) | Mean ratings of importance (round 3) | Standardised regression coefficients to predict opposition to SDD |
|---|---|---|---|
| SDD increases antibiotic resistance | 92 | 7.6 | 0.20 |
| Overall, SDD benefits the patients to whom it is delivered | 60 | 6.7 | –0.19 |
| SDD reduces hospital-acquired infections | 37 | 6.9 | –0.12 |
| SDD reduces VAP | 29 | 7.0 | –0.09 |
| There is no mortality benefit associated with SDDa | 23 | 6.7 | –0.02 |
| The risks of SDD outweigh the benefits | 22 | 7.1 | 0.08 |
| SDD would increase ICU C. difficile infections | 7 | 7.2 | 0.06 |
Feasibility of further selective decontamination of the digestive tract research
Participants were asked to state whether they would be prepared for their patients or centre to be randomised in an effectiveness RCT and the majority of participants indicated that they would (78.4%), as displayed in Table 22. Furthermore, almost all participants (94.2%) reported that they would go along with an effectiveness RCT if their colleagues supported it. Almost two-thirds of participants (63.5%) were willing to support an implementation study. Only 14.3% of participants were unwilling to participate in either an effectiveness RCT or an implementation study. The majority of participants (58.1%) indicated they were willing to participate in either type of study design. A lower proportion of microbiologists than intensive care consultants were prepared to participate in an effectiveness RCT (42.9%) or an implementation study (34.7%).
| Willing to participate | Total sample frequency (n = 468) (%) | Intensivists frequency (n = 419) (%) | Medical microbiologists frequency (n = 49) (%) |
|---|---|---|---|
| Effectiveness RCT | 367 (78.4) | 346 (82.6) | 21 (42.9) |
| Implementation study | 297 (63.5) | 280 (66.8) | 17 (34.7) |
Multiple regression analyses were conducted to examine the predictors of participants’ willingness to participate in (1) an effectiveness RCT and (2) an implementation study. The results for the first regression analysis are displayed in Table 23.
| Item | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| I am opposed to SDD | 0.74 | 0.61 to 0.91 | 0.004 |
| I am opposed to the i.v. component of SDD | 1.02 | 0.84 to 1.24 | 0.872 |
| It is ethically acceptable to conduct further RCTs evaluating the effectiveness of SDD | 1.45 | 1.21 to 1.73 | < 0.001 |
| Current uncertainties in the evidence base should be addressed in a new study | 2.33 | 1.21 to 4.51 | 0.012 |
Table 23 shows that willingness to participate in an effectiveness trial was predicted by participants’ responses to the items asking whether or not it is ethically acceptable to conduct further RCTs evaluating the effectiveness of SDD and participants’ overall opposition to SDD. However, the most powerful predictor of this item was whether or not a new study should address current uncertainties in the evidence base.
The results of the regression analysis of willingness to participate in an implementation study are displayed in Table 24. Willingness to participate in an implementation study was predicted by overall opposition to SDD and responses to the item ‘Current uncertainties in the evidence base should be addressed in a new study’.
| Item | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| I am opposed to SDD | 0.51 | 0.42 to 0.62 | < 0.001 |
| I am opposed to the i.v. component of SDD | 1.13 | 0.95 to 1.34 | 0.176 |
| It is ethically acceptable to conduct further RCTs evaluating the effectiveness of SDD | 1.01 | 0.85 to 1.19 | 0.925 |
| Current uncertainties in the evidence base should be addressed in a new study | 3.23 | 1.64 to 6.36 | 0.001 |
Participants were further asked to specify the components of a control group for a possible future effectiveness RCT of SDD. Responses are summarised in Table 25. The greatest proportion of participants (90.4%) stated a desire for a control group that delivered VAP bundles. Slightly fewer participants preferred the control group to include chlorhexidine mouthwash (85.7%) and a smaller proportion of participants felt that the control group should include a ‘standard practice’ intervention that would reflect variations in current practice. In terms of outcome measures, the majority of participants (91.9%) agreed that an effectiveness RCT should include a measure of antibiotic resistance (details are presented in Table 25).
| Item | Intensivists,n (%) | Clinical microbiologists, n (%) | Total sample, n (%) |
|---|---|---|---|
| An effectiveness RCT of SDD should include the following components | |||
| A control group that receives chlorhexidine mouth wash | 366 (87.4) | 35 (71.4) | 401 (85.7) |
| A control group that receives ‘VAP bundles’ | 382 (91.2) | 41 (83.7) | 423 (90.4) |
| A standard practice control group that reflects variations in current practice | 227 (54.2) | 36 (73.5) | 263 (56.2) |
| A measure of antibiotic resistance as an outcome | 389 (92.8) | 41 (83.7) | 430 (91.9) |
Discussion
The national survey recruited 468 intensive care consultants and clinical microbiologists to identify current SDD practice, current uncertainties in the evidence base and the feasibility of further SDD research.
Answers to research questions
In terms of current SDD practice, around 10% of the sample reported currently delivering SDD whereas around 40% have not yet considered SDD.
The distribution of scores reflecting opposition to SDD was bimodal and this bimodality was evident among both intensive care consultants and clinical microbiologists. In other words, both professional groups included a substantial proportion (approximately 20%) who were not opposed to SDD. The other, primary, mode was at the mid-point of the scale (reflecting uncertainty) for intensive care consultants and at the ‘opposed’ end of the scale for microbiologists. Opposition to SDD was significantly predicted by all the beliefs about consequences items in the questionnaire but was not predicted by self-assessed knowledge of the SDD evidence base.
A large majority of the participating clinicians reported that uncertainties should be addressed in a new study, that further SDD research would be ethically acceptable and that they would be prepared to participate in further SDD research. Hence, further research appears to be appropriate, acceptable and (from the perspective of recruiting ICUs for a trial) feasible.
As would be expected, there was a strong association between current provision of SDD (with full protocol) and support for SDD (see Table 18). In terms of participants’ beliefs, the strength of belief about whether or not SDD increases antibiotic resistance was the strongest predictor of two key opinions: opposition to SDD and the belief that further SDD research would be ethically acceptable. The strength of belief that current uncertainties in the evidence base should be addressed in a new study was the strongest predictor of two intentions: willingness to participate in future effectiveness research and willingness to participate in future implementation research. There was strong support for the following trial design features: antibiotic resistance to be measured as an outcome and control group to receive VAP bundles or chlorhexidine mouthwash.
Some differences were evident between intensive care consultants and clinical microbiologists, a finding that is not surprising as many microbiologists see their professional role as centred around antimicrobial stewardship. Hence, the profession repeatedly advances the rationalisation of antibiotic use as a Department of Health priority. 64 However, the current study suggests that not all clinical microbiologists are certain of the evidence base to support this view in the context of SDD.
Strengths and limitations
The response rate was difficult to estimate but was probably in excess of 25%. Thus, although the results may not have been based on a representative sample, they do indicate a substantial mass of support among the intensive care community for further SDD research. Indeed, the number of intensive care consultants and clinical microbiologists who would be prepared to participate in an effectiveness trial or an implementation trial involving SDD is far in excess of the number that would be required to mount such a trial. Furthermore, this study reflects the largest number of clinical microbiologists’ views on SDD recorded to date. However, the response rate remains a substantial limitation to the generalisability of these findings. A further limitation is that only two of the four stakeholder groups from the Delphi study were included in the survey study. However, these two groups (intensive care consultants and clinical microbiologists) were the two groups that Delphi participants identified as potentially holding conflicting views. Although there were some differences between these two groups, there was also considerable overlap in terms of their support of, or opposition to, delivering SDD, as described in Figure 12.
A further limitation may be that the participant group possibly did not fully grasp what is meant by ‘implementation trial’. It is puzzling that so many participants (51%) reported being prepared to participate in both an effectiveness trial and an implementation trial. On the face of it, an effectiveness trial should be considered only when there are uncertainties in the evidence base, whereas an implementation trial should be considered only when the evidence shows that an intervention has clear benefit, but has not yet been adopted. The implementation trial then evaluates the extent to which an intervention succeeds in changing practice. Although a naive view is that implementation of evidence-based interventions into routine practice is a simple matter of publishing a guideline, there is ample evidence that such passive strategies are often insufficient to improve patient care. 65
This survey had a number of methodological strengths. First, the questionnaire items were generated from theory-based interviews with four stakeholder groups (reported in Chapter 3) and were subsequently selected for inclusion on the basis of an iterative process of prioritisation (reported in Chapter 4). The importance of a range of clinical outcomes of SDD was assessed using multiple methods. An extremely robust finding across indices of importance was that antibiotic resistance is the most important issue. Hence, careful attention should be given to measuring this in any further SDD research. Importance ratings did not converge with the other indices of importance. This has implications for Delphi methodology, as ratings of importance are key to the assessment of clinical consensus using this approach. 53
A further strength is that we identified that further trials are feasible (i.e. a sufficiently large number of clinicians would be prepared to participate) and also ethically acceptable. Ethical acceptability could arguably be a function of uncertainty about the evidence base (rather than being a function of certainty that the intervention is effective). There was a small signal in the data to suggest this may be the way in which clinicians think about ethical acceptability and such thinking could be investigated in more detail in future research. Box 8 presents the key findings from the national survey that inform the need for, and acceptability of, a clinical effectiveness RCT or an implementation RCT.
-
The national survey replicated the major findings of the Delphi study in that clinicians are uncertain about the evidence relating to clinical outcomes of SDD, including potential harms, and that more research is needed.
-
Further SDD research was feasible, with 85% of intensive care consultants and clinical microbiologists in the sample being prepared to participate in future SDD research.
-
Further effectiveness or implementation RCTs were regarded as ethically acceptable.
-
Monitoring of antibiotic resistance in the context of a trial would be essential. Importance was assessed in three ways and this was the most important consequence of SDD, according to all three measures.
-
Despite reporting uncertainty about the effectiveness the SDD evidence base, the majority of participants were willing to participate in implementation research to increase uptake of SDD. This suggests that clinicians may not be aware of what implementation research involves and when it is appropriate.
Chapter 6 Feasibility of possible future randomised trials of selective decontamination of the digestive tract: interview study with international triallists
Background
Chapters 4 and 5 (Delphi study) reported stakeholders’ beliefs about SDD and about the importance of further research in SDD. However, while further research might be desirable, it remains unclear what challenges or barriers may be encountered should further SDD research be proposed. Therefore, the aim of the study reported in this chapter was to investigate potential feasibility issues associated with possible future RCTs involving SDD from the perspective of experienced international triallists (expert triallists with experience of the design and conduct of trials in intensive care and/or the design and conduct of implementation trials). Implementation trials are randomised studies designed to evaluate interventions that aim to increase the uptake of evidence-based health care. Such trials are designed to address quality gaps, rather than evidence gaps, and they address the well-known lag between evidence of effectiveness and care quality. There is substantial evidence that dissemination alone (of evidence or even of guidelines) is often insufficient to achieve changes in clinical practice. 65
Hence, this study focused on the challenges involved in both the design and delivery of (1) an effectiveness trial and (2) an implementation trial of SDD in intensive care. We sought to identify possible problems with feasibility and acceptability (including ethical issues), views about trial design and beliefs about practical barriers to recruitment and intervention delivery. We also sought comment on potential ways to overcome these problems and recommendations about trial design (including eligibility criteria and the nature of the control condition). The overarching research question guiding this study was:
Research question 8: What are the likely challenges in undertaking a large multinational randomised controlled study of SDD in ICUs?
Methods
Sample
A list of expert international triallists with known expertise in intensive care trials and/or implementation trials was developed by members of the international research team (MKC, BHC, GB, GSM and one Australian collaborator). As this study sought the views of experts with in-depth experience in their field, the target sample size was relatively small, being set at 10. This number was likely to provide a suitable balance between the expected homogeneity of the sample and the study’s objective of eliciting views from those who might favour a clinical trial and those who might argue for an implementation trial. To ensure that 10 interviews were available for analysis, we identified an initial list of 20 expert triallists to approach. Potential participants from the UK, North America, Europe and Australia were identified from their public profile and the research team’s knowledge of the field, and were invited to take part if they had expertise in (1) randomised clinical trials of intensive care interventions or (2) implementation trials. 66
Data collection
Semistructured one-to-one telephone interviews were conducted using a topic guide developed from expert experience and informed by the identification (from earlier phases of the study) of clinicians’ interest in further SDD research. Telephone interviews were considered a more efficient use of resources (i.e. time, cost, effort) than face-to-face interviews for generating data on this international sample. 67 Topic guide prompts included questions about participants’ preference for an effectiveness or implementation trial and aspects of trial design (e.g. individual vs. cluster randomisation), specification of the components of the SDD intervention and of control group care, outcome measurement, recruitment and ethical considerations. The topic guide for these interviews is included in Appendix 6.
Procedure
The UK clinical lead (GB) sent a personal e-mail to each potential participant informing them that they would be invited to take part in the study and would receive an information sheet and consent form, by e-mail, from the project manager (EMD). Those expressing an interest in taking part were contacted to set up mutually convenient times for a 30-minute recorded telephone interview. A senior triallist (GB) conducted all interviews by telephone and began by giving a summary of the SuDDICU study. Participants were asked to provide their general views of conducting further research about SDD in ICUs and any issues they foresaw arising in undertaking such research. Participants were then asked to consider two possible trial types: a RCT to evaluate the effectiveness of SDD or a RCT to evaluate a behaviour change intervention (aimed at health-care professionals and ICUs) to increase uptake of SDD (i.e. an implementation trial). They were asked to indicate whether or not they had an initial preference for discussing one particular type of trial (effectiveness vs. implementation). The interviewer returned to the other option if time allowed. Prompts were used to elicit trial design features and associated challenges and barriers. Triallists were encouraged to talk freely and prompts were used only if the participants did not cover specific areas relating to the research question. While triallists’ preferences for effectiveness or implementation trials and for specific trial design features (e.g. cluster randomisation) were explored during interview, these were not the primary focus of this stage of the research. Preferences were elicited to provide the context to ask about challenges and barriers to undertaking a large multinational RCT of SDD in ICUs. Interviews were audio recorded and transcribed verbatim.
Sample characteristics were not requested during interview, as participants’ eligibility for this study was based on information available from participants’ public profiles (e.g. the country in which they work and their clinical and research expertise) rather than on demographic information (e.g. age).
Data management and analysis
Interview transcripts were analysed thematically using a framework approach. 36 The technique involves a systematic process of inductive qualitative data analysis. Familiarisation with the content of each transcript was followed by the creation of ‘charts’ (i.e. Word document tables) to summarise issues identified as relating to potential challenges and barriers to undertaking further research in the field of SDD. The issues identified were grouped into themes, discussed within the research team, refined and checked against the charts to ensure that all relevant issues were accommodated.
One researcher conducted the initial coding of the first six transcripts, working systematically through the transcripts as they became available and highlighting all text referring to (1) trial design/conduct or (2) trial challenges/barriers. In order to retain the context of each quotation, highlighted sections were extensive and inclusive of all text relevant to a particular design feature or challenge. Highlighted text from each transcript was then copied into Word document tables (‘Charting’). 36 A second researcher (MEP) reviewed the coding/highlighting on two transcripts to check that no relevant text had been excluded. MEP conducted the initial coding of the remaining transcripts and undertook the second phase of analysis: identifying initial themes and using the themes to group together quotations from different participants. The headings and content of the tables produced during the charting phase were refined in numerous versions until all relevant data were tabulated without overlap or duplication. Data were then systematically examined and emerging typologies discussed with other members of the research team (GB, JJF, EMD) to ensure clinical sensibility and methodological rigour. In this study (and in the rest of the SUDDICU study) the terms ‘acceptability’ and ‘feasibility’ were defined, respectively, as a willingness to participate in further SDD randomised trials and an ability to participate or to conduct such trials. 1
Ethics approval for this study was obtained from the University of Aberdeen College of Life Sciences and Medicine Ethics Review Board (Ref: CERB/2011/3/610).
Results
Participant characteristics
Twenty-two international triallists were identified as meeting the eligibility criteria and were invited to participate. Sixteen triallists were recruited and 13 were interviewed. However, the audio-recording equipment failed during three interviews, with a resultant loss of data from these participants (T2, T4 and T8). The 10 interviews that generated completed transcripts were included in the analysis and form the data presented below. The interviewer’s notes from the other three interviews are presented at the end of the results section (see Findings based on notes from the three interviews that were not transcribed).
Of the 10 triallist interviews available for analysis, four triallists were from Canada, three from Australia, two from the USA and one was from Europe. All 10 triallists had expertise in randomised clinical trials of intensive care interventions and three of them also had expertise in leading implementation trials.
Context for discussing challenges: effectiveness or implementation trial?
First, we focus on participants’ views about the current evidence base in SDD and, in the light of these, their views about appropriate implementation strategies.
Of the 10 triallists whose interviews were analysed, nine reported being persuaded by the evidence in favour of SDD. These triallists offered their opinions about the interventions that would be effective in changing the behaviour of the relevant clinicians, or suggested research designs to evaluate the effectiveness of behaviour change interventions. These views are presented in Table 26. This table presents multiple rows for some participants, as this helps to retain the context of the linked quotations.
| Participant ID | Quotes suggesting that participants are convinced by the current evidence base for SDD | Quotes suggesting that participants are uncertain about the balance between potential benefits harms of SDD | Quotes proposing how to change SDD practice |
|---|---|---|---|
| 1 | I think that if you look at the Cochrane analysis . . . I think you have to accept the data, however you may be unhappy about it, that it works. | . . . [uncertain about] the effect that widespread use of this would have on the microbial ecology of ICU populations around the globe. | I think that in order to . . . to change behaviour of entire intensive care units . . . you’re still going to have to convince people. So in an ideal world I would personally vote that a large multi centre trial be instituted. |
| 1 | [I’m] actually reasonably convinced by the literature . . . | I’m convinced it does work but . . . I’m not convinced what happens to the microbiology. I think that’s still an open question so do a big study and find out. | but [to] really change people’s behaviour I think that it [a large trial] is going to be necessary so it’s unfortunate but that’s how I feel about it. |
| 1 | I’ve been a big fan of the idea since it was proposed 30 years ago, . . . I really like the idea of colonisation resistance and we definitely can show this in animals, there’s no doubt we do this all the time on our rat experiments and it’s very, very clear that certain antibiotics really do disrupt colonisation resistance; colonisation resistance is a real thing . . . | – | I wish that we could convince our colleagues of this and think we won’t be able to do it unless somebody gets the courage to do it in a big way. |
| 3 | I’ve always been a little bit of a fan of it [SDD] and always wondered whether our reasons are scientific for not adopting it. | – | . . . before going straight to a behavioural interventional trial I would focus on the psyche of key opinions, which is exactly what you are doing. |
| 5 | It is pretty clear there are signals for pneumonia prevention and if you put all the trials together there is also a mortality advantage. So I think the data are quite compelling . . . | . . . but obviously you’ve heard the many reasons why people are not convinced and most of the concern is about emerging resistance which has not necessarily been documented at all, or not done well. | So I don’t think another big trial, unless it is very large and very generalisable, is too likely to change practice. |
| 5 | The VAP signal is already strong . . . | . . . the VAP rates are going down because we have . . . more ancillary VAP prevention strateg[ies], [so] we need to have a plausible control event rate. | . . . should [include] economic evaluation, given, effectiveness is not sometimes enough to change practice unless the associated economic evaluation suggests the benefit. |
| 6 | [Name] brought up SDD at the [clinical] meeting . . . and said ‘Why are we not recommending this, it has got better evidence than everything else’, and there [were] a lot of people around the table who started shuffling and looking at their feet and then the chair decided to have a vote . . . There was just strong opposition to it, which was irrational . . . | People just are not convinced by the evidence. | I am not convinced that a kind of behaviour change-type research or programme that is not tied to very convincing evidence would change people’s practice or behaviour. |
| 6 | – | [In cluster trials] there is a large number of people within the clusters that are allocated to SDD who actually do not get it because they are not going to be in the ICU long enough or they are intubated or whatever, and if they come down from a ward, you don’t know what they are bringing with them . . . | . . . so I’ve erred towards thinking, if you want to convince people, what would convince people and make them change their mind would actually be a conventional individual patient randomised trial |
| 7 | – | – | We [should] ask people whether this [a trial] would make them change practice. The trial design that is voted by the majority as the trial design that would make them change practice would have to be the one that you would want to do . . . But then the trial design and statistical analysis are of such . . . complexity and such a proneness to error that no-one will react to it. They will say, that is interesting; god knows what it means, goodbye. |
| 7 | Why [are] people not doing this therapy when they are doing other therapies that have a lesser evidence base behind them? | – | What I think will change practice is only a double-blind placebo controlled traditional individual randomisation based trial of the order of 8 to 10,000 people. |
| 9 | [referring to] the [country name] propaganda [that] it only works in Holland. I’m not sure about that. The data . . . is valid. It’s not only Holland data, I think there’s some other data as well . . . some German data. | I think the [country name] don’t want it and that’s always a blocker because they will always talk against it. | I think you’ve got to do the trial and . . . that you want to test whether SDD works. The two big questions in my mind [are] a) are you going to do clustering randomisation and b) how you’re going to involve SOD? |
| 10 | I can remember the systematic reviews of nationalities published in the BMJ in the mid-90s saying it appeared to be an effective intervention so I was quite surprised when . . . 10 to 15 years later . . . it was still not being implemented in ICU settings. | . . . my sense of the field is that the concern is more about harms. . . . I feel it’s quite legitimate for doctors in ICU settings to be cautious about new technology if they believe there are harms associated with it, particularly if also they’re getting a lot of push back from their infection control people. | And on that basis I would be reasonably comfortable to suggest that . . . the right way forward would be to do a definitive . . . randomised control trial, . . . well it would have to be a cluster randomised control trial. |
| 10 | I think [it’s] quite unusual to have so much evidence and still to feel that there are unresolved issues. | It’s not clear how informed everyone [is about] the evidence around SDD, . . . the benefits and harms . . . But my sense is there a group of highly informed clinicians who just worry about the likely harms and that for me would probably [be] justification for some form of further clinical trial rather than implementation trial. | – |
| 11 | This intervention is not highly toxic, it’s not difficult to implement and the . . . potential adverse effects are minimal. It’s the perceptions that clinicians have to the contrary that make the implementation of this very challenging, and the theoretical aspects of what everybody’s worried about. | – | [In an implementation trial] I would assume that you’re going to . . . run the study long enough to be able to measure behaviour change, but also to determine whether your resistance profiles change at each hospital. |
| 11 | [Interviewer summarising and reflecting participant’s earlier responses] We’ve avoided looking at an effectiveness trial because you think the data is probably there, it’s more people’s attitude of mind or . . . institution; attitude of other people around. | the tendency sometimes is . . . as you’re looking at trying to tease out what the barriers are [you include collaborators], and then [once you get to the intervention study] you . . . go, great, I’ve got that, I don’t need these people any more. | As you develop your intervention, it will be important to continue to have collaboration from the ICU clinic physician, nurse, nurse educators potentially, Pharmacists, Medical Microbiologists and your behaviour change experts in a collaborative manner. |
| 2 | [Interviewer responding to previous utterance] That’s right. It’s the right of the individual versus the rights of society. | I think that is probably a problem with the uptake . . . resistance is seen at a societal or population level. | I would probably go for a cluster trial where you are evaluating both the effectiveness as well as the uptake and translational aspects of your intervention, in both arms, . . . not the uptake in the control arms but . . . attitudes and beliefs. |
From Table 26, six triallists were persuaded by the evidence of effectiveness but also noted widespread uncertainty with respect to potential harms of SDD, at least at the population level. Four of these participants recommended an effectiveness trial that focused on microbial ecology and one also noted the importance of an economic evaluation. Two participants recommended an implementation trial with clinical measures as secondary outcomes and one recommended a behavioural intervention trial based on the findings from the current series of studies.
Analysis of challenges and barriers
This section focuses on the reported challenges and barriers to further SDD research and to the design and conduct issues associated with a possible future effectiveness RCT comparing SDD against a control group (as this design was prioritised in nine out of 10 interviews analysed). Potential challenges and barriers relevant to different stages in the design of future SDD research, and/or any subsequent RCT, were identified from the data. These were conceptualised into four levels depicting the hierarchy of potential challenges (i.e. from the broad challenge of conducting any further SDD research to practical barriers associated with specific aspects of trial conduct) (Figure 16).
FIGURE 16.
Hierarchy of challenges to further SDD randomised trials.
While triallists tended to describe a range of potential challenges, there were few suggestions of ways in which such challenges could be overcome. The findings presented below provide a descriptive account of triallists’ views.
Challenges to the acceptability and feasibility of further selective decontamination of the digestive tract research in intensive care units
The first level of challenges related to the acceptability of SDD as an intervention and to the acceptability of any further SDD research. These challenges were seen to represent potential barriers that would need to be overcome before proceeding with any effectiveness RCT (of any design).
It was suggested that gaining sufficient international support for further SDD research could be a challenge (T, triallist participant code):
I am not sure how many people in the world there are that are willing to do that [support further SDD research], which have such a strong belief in this intervention.
T7
Achieving widespread multidisciplinary endorsement and support for further SDD research in ICU was identified as a potential challenge.
. . . not only have you got to overcome the ICU, the surgeons and everyone else, but you’ve also got all these different views in terms of infectious diseases and microbiology about what we should and shouldn’t be doing.
T6
However, such endorsement and support was noted to be a prerequisite for conducting further research to evaluate either the effectiveness of SDD in ICU, or a behavioural intervention to increase SDD uptake.
I think it is getting ICUs that want to play ball and where it goes beyond more than just verbal commitment, that there is commitment from the entire team and the hospital, I think is paramount.
T12
Another suggested barrier to further SDD research focused on perceptions of tension between the individual and societal consequences of delivering SDD in ICU (i.e. the potential benefit to individual patients receiving SDD vs. the concerns about the societal/population impact of antibiotic resistance resulting from the routine delivery of SDD).
. . . the key aspects to me are the resistances seen at the population level whereas the preventing the infection with SDD is more at the individual than population level . . .
T12
These findings suggest that the acceptability of SDD as an intervention may not be related to the perceived effectiveness of SDD in individual patients, but to beliefs that these benefits are outweighed by the potential harms of SDD at a population/societal level.
Challenges to the acceptability and feasibility of conducting a definitive effectiveness randomised controlled trial comparing selective decontamination of the digestive tract with a control group
It was suggested that even if clinicians were convinced of the benefit of SDD as an intervention, and that support for further SDD research in ICUs could be achieved, there were acceptability and feasibility issues that present further barriers/challenges.
Triallists reported that, were a definitive effectiveness RCT to be planned, it would have to be large.
. . . it would be a trial of that order, 6000 or 7000 people, ideally executed across the world.
T7
The size and international nature of such a trial was suggested by participant T7 to present acceptability and feasibility issues, for example the level of personal commitment required from a chief investigator to run such a trial and the challenge of gaining international funding:
I hope you have sold your children to medical science because you are not going to have time to do anything else and just huge personal burdens. It is a big deal; I would not take it on; it would have to be someone younger with more energy.
T7
Barriers relating to specific design features of an effectiveness randomised controlled trial comparing selective decontamination of the digestive tract with a control group
In addition to the general challenges of conducting a definitive multinational effectiveness RCT, triallists identified potential barriers relating to the specific design features of such a trial (e.g. unit of randomisation).
Triallists’ views on the specific design features of a definitive effectiveness RCT varied, as did their views on the barriers related to specific features. The majority of discussion focused on triallists’ perceptions of the challenges associated with the choice of clinical intervention and comparison/control, when the intervention would be started, outcome measures, unit of randomisation and level of consent.
Specification of the clinical intervention and control
The lack of a formal and universally accepted SDD regimen was suggested by triallists to pose potential acceptability issues for a trial.
The discussion of what you use as antibiotics in such a protocol . . . would you use some sort of so-called ‘old fashioned’ antibiotic like cefotaxime or do we now need to go to something else . . .
T6
One triallist stressed the importance of transparency in the intervention development phase and consultation with stakeholders to address this issue and optimise acceptability of both the trial and the intervention.
I think in developing what the intervention is, the different aspects of the intervention for SDD, it obviously has to be built from consensus . . . amongst intensivists in terms of their buy-in, so going beyond the current group of co-investigators and . . . testing it on people who are outside that group would be really important . . . documenting . . . the process of determining what the . . . intervention is and even publishing that would be important too.
T12
Establishing current international standard care for ICU infection prevention and choosing an appropriate comparison/control for a SDD effectiveness trial were also identified as problematic. It was suggested that the widespread use of oral antiseptics (e.g. chlorhexidine) and/or the use of other procedures associated with SOD or ventilator bundles (e.g. semi-recumbent positioning, subglottic suctioning) present a challenge to determining the components of the intervention and control arms in a definitive SDD effectiveness trial.
If people are using chlorhexidine universally and I cannot really see why not except for the usual, that it takes forever to get people to change behaviour, given the low cost, given the benefit of chlorhexidine and its availability, then both groups should have chlorhexidine . . . So I think we need to be very, very careful about how we handle oral antiseptics when we’re considering an SDD trial.
T5
If you/I are going to develop a trial, I think that there is now SOD and this is complicating everything . . . how you’re going to involve SOD? In other words, are we going to have three arms or are you going to leave out SOD . . . you have to address the problem of SOD.
T9
. . . the subglottic suctioning and . . . the positioning of the paste and all that kind of stuff [is] now considered basically standard care in most places . . . I think it might be difficult to randomise people to doing that or not doing that.
T1
One suggestion to overcome the variation in usual care was to ignore it and accept a lack of standardisation in the care received by patients in the trial control group (i.e. assume a completely pragmatic design).
I would do absolutely nothing, I would just say this is a large multicentre individual randomisation base pragmatic trial . . . Let’s not create a world where we know everything about a place that doesn’t exist. Let’s keep the world dirty, messy, chaotic, random, insane, etc., as it is, and within that world, let’s just change one thing.
T7
The potential benefit of conducting a double-blinded trial using placebo oral pastes, mouthwashes and i.v. components was mentioned during the interviews. However, the associated ethical implications and practical challenges were not discussed, although one triallist reported experience of using placebo oral pastes during a trial to treat, rather than prevent, infections in an ICU context.
We just did an intervention of SDD. We tried breaking an outbreak. We got a neutral paste. That’s how we blinded it. In the placebo arm we used a neutral paste.
T9
Outcome measures
Triallists suggested a number of outcome measures that could be used to evaluate the effectiveness of SDD. The most commonly discussed measures were mortality, VAP and antibiotic resistance patterns. However, triallists reported potential challenges with the use of each of these measures. Mortality (timescale unspecified by participants) was proposed by some triallists to be the optimal primary outcome measure as it is straightforward to measure and a large, multinational SDD effectiveness trial that demonstrated a significant effect on mortality would be persuasive and conclusive.
. . . giving clarity around mortality outcome would be crucial. So international, multinational, large, focused on mortality.
T5
Nevertheless, it was suggested by others that the low incidence of mortality limits its suitability as a primary outcome measure to evaluate the effectiveness of SDD.
So it [SDD] might actually be an impossibly high hurdle to change mortality . . . if you enrolled a thousand patients . . . cut 100 VAPs to 80 VAPs and so you’ve changed 20 VAPs, of those 20 VAPs, there might only have been one extra death in the 20 VAPs, so you’ve enrolled a thousand patients and [avoided] one extra death.
T3
The main challenge identified with the use of VAP as an outcome measure related to difficulties with its definition and diagnosis.
I think the major problems are diagnosing a pneumonia you know, which is always been a vexatious issue and so I think because it’s a relatively loose diagnosis, that’s one issue.
T13
It was also suggested that, as with mortality, the low incidence of VAP in ICUs was a potential barrier to its acceptability as an outcome measure and to the feasibility of its use in a trial to evaluate the effectiveness of SDD (particularly in the context of the widespread use of SOD).
. . . we don’t have much in the way of Gram-negative VAP that . . . we’re aware of. So whether or not we need a different approach for more of a Gram-positive VAP, but again, we don’t seem to be having much of that at the moment. So my worry is that it’s a relatively low-grade problem and you’re having to treat everyone or everyone who’s at high risk for a low-grade problem.
T13
Bacteraemia was suggested by some triallists as a viable alternative outcome measure to VAP.
If you really wanted to know something, I would do some hard stuff like bacteraemia. It would have to be logistically simple, objective, easily ascertained, easily monitored, identified without question by somebody else, like bacteraemia would be and that is the end of that.
T7
It was acknowledged by a number of triallists that the inclusion of some measure of antibiotic resistance would be worthwhile to address the current lack of evidence of the effect of SDD on rates and patterns of antibiotic resistant pathogens. However, it was suggested that such an outcome measure would be costly and would impact on power calculations and other aspects of the trial design such as the unit of randomisation.
. . . we cycled antibiotics and we looked at a resistance and it is hard. It’s very hard. You need a hell of a lot of events to . . . get decent numbers out of it. I think that’s just unachievable . . . you know, you’re looking at thousands and thousands of patients and that’s why I said microbiology . . . to me is hideous. No way are you going to overcome that.
T9
. . . it [incidence of multidrug resistant pathogens] would be very difficult . . . what kind of power calculations can you do if you wanted to use incidence of development of multi germ resistance pathogens . . . because you really don’t know what’s going to happen and there’s such a big variation. I think it . . . would be difficult . . . as your primary influence even though it’s really critical issue.
T1
Unit of randomisation
Triallists reported advantages and disadvantages associated with using cluster randomisation or individual patient randomisation in a SDD effectiveness RCT. Two triallists suggested the unit of randomisation and choice of outcome measure are inextricably linked.
. . . you can’t really do an ICU population level outcome if you are randomising at the patient level.
T3
The problem is if you randomising individual patients, you’re ignoring the possibility of a horizontal spread . . . and therefore you’ve got to cluster randomise.
T9
Reasons given in favour of randomisation at the individual patient level, or against cluster randomisation, were:
-
1. Individual randomisation is more feasible for a worldwide study.
I would think that the cluster randomisation as a worldwide phenomenon for SDD is going to be very difficult so you are going to have to look at individual randomisation.
T9
-
2. Differing baselines in ICUs.
A cluster RCT is a scary high-risk position because if there’s a difference in baseline severity of illness within the ICU then you’re in trouble.
T3
-
3. Recruitment challenges to cluster randomisation resulting from lack of willingness of a sufficiently large number of units.
A cluster randomised trial requires dozens and dozens of units agreeing to be allocated to one or the other . . . there may not be sufficient of units who may say yes to agreeing to participate for months or years in a randomised trial whereby either one get it or not. It is hard. So the logistics of doing a cluster randomisation are enormous . . . because this is so loaded and everybody has opinions.
T5
Level of consent
The unit of randomisation and level of consent were sometimes linked by triallists when expressing their views on the potential challenges associated with gaining consent at the individual patient level.
. . . the cluster trial would be a much more efficient design because you could make much more use of all patients. I think at, an individual level, you know that is not always the case because, of those eligible, very few end up being consented or being enrolled and then having full consent . . . the consent not withdrawn I guess I should say in this case. So I think again, . . . from the public health perspective, using a cluster trial is not only efficient, it is just the right thing to do and I am fairly adamant about that.
T12
There were also suggestions that the use of individual patient consent in a cluster-randomised trial was impractical and could potentially affect recruitment.
. . . if you do it by individual form of consent obviously some people will say ‘no’ and then you’ll have a hard time doing your analysis because some people would have been put into it.
T1
Barriers resulting from international differences in models of consent and research governance procedures were also mentioned during interview.
I would imagine there would be some countries if not regions . . . whereby an approach to units being randomised might be more palatable when alternate models of consent have already been endorsed, such as waived or deferred consent, [as in studies in] some parts of Europe and parts of Australia.
T5
It was suggested that it may be difficult to get approval in the USA for a trial that used unit level consent.
On the downside would be the individual consent . . . very hard in the US to configure an alternate consent model other than the standard which would be first person consent which we can’t do much on the ICU or a priori substitute who can legally consent. I think it’s hard for Americans to plot anything other than that model.
T5
In contrast, research governance procedures in Australia were reported to allow delayed consent (i.e. gaining consent from a patient or relative after enrolment into the trial) in order to aid trial recruitment. However, it was suggested that such a model may not be acceptable elsewhere in the world.
Well this is a study that you probably would allow delayed consent for here in Australia because it so much facilitates patient recruitment . . . In our experience delayed consent confirmation takes about 20 minutes and prospective consent takes about 60 and 70 minutes and again, if you are running a trial of 20 people, that’s okay. If you are running a trial for 10,000 people, that is 10,000 hours of work that you have to add and that’s really hard, and your consent rate will decrease and your recruitment rate will also decrease and then people will develop trial fatigue.
T7
Practical barriers to trial conduct
In addition to the potential challenges associated with conducting further SDD research and the design of a trial to evaluate the effectiveness of SDD, the triallists suggested that there may be a fourth level of challenge associated with practical barriers to the conduct of such a trial. These potential barriers were also examined in earlier stages of this study. The following results should be seen in the light of other study findings, which are integrated in Chapter 7.
Three triallists stated that, in their experience, nurses find administering some SDD components (e.g. oral pastes) unpleasant and/or burdensome.
. . . some of those oral based solutions are gooey, sticky mess that frankly nurses find a pain . . . the chlorhexidine products I think are nicer to use. So there’s a practical barrier around making sure that is not [difficult] to use.
T3
We pretty much used an oral paste as well as GI and the nurses hated it. We put it in several times a day and everyone hated it, paste around the mouth and in the gut. The nurses hated doing it. So to get it implemented, to get compliance is going to be hard . . .
T9
There were also perceptions of increased nursing and/or pharmacy workload associated with the preparation or delivery of SDD components.
. . . it’s a lot of nurse time with all the pastes and all of that . . . [named hospital] make up their own drug but I don’t think it’s generally like off the shelf available; you’ve got to make up the paste which is a lot of work.
T13
The challenges of maintaining intervention fidelity and preventing trial fatigue were also briefly discussed and ideas to overcome such issues were proposed.
In terms of the implementation and compliance from nursing staff, that is an education process . . . you’ve got to have your investigators and research nurses running an education programme for the bedside nurses and providing them with feedback and lunches and breakfast meetings where you tell them all about the trial and how it is going.
T6
Findings based on notes from the three interviews that were not transcribed
The interviewer’s notes from three triallist interviews for which recording failed and hence were not transcribed are summarised below.
Interview triallist 2
This triallist supported a further compelling interventional trial in SDD that would need mortality as an outcome, would need to track any microbiological changes arising as a consequence and would demonstrate any economic impact. It would need to be a cluster randomised trial to accommodate any benefit from unit-wide microbiological flora changes and would need to be an intercontinental study. Barriers included the sheer scale required for this venture.
Interview triallist 4
This triallist felt SDD was problematic to investigate given uncertainties with current evidence, problems with when to start SDD (day 0 or day 3 and, thus, variability in who gets the intervention), problems with VAP as an end point and difficulties in ethics in that different countries may or may not allow cluster randomised studies. Overall they were supportive of an intervention study but believed better data were needed to design the ideal trial.
Interview triallist 8
Triallist 8 posed several important challenges to address before progressing to a further intervention trial (which is what they felt was needed). These included how to blind such a trial, something that this participant felt was vital, how to identify good leaders for the trial and which countries this should be conducted in for credibility. The issue of different microbiological resistance patterns in different countries was also relevant to this credibility. They believed more evidence was required to understand exactly the groups to receive the treatment and how to deal with chlorhexidine in the treatment (and control) groups. They also wanted clarification of the place of SOD in such a study.
It can be seen that these three interviews broadly included themes elicited in the 10 previously reported interviews and that the hierarchy described was also applied within these interviews.
Summary of results
In summary, triallists were interviewed from across a wide spectrum both geographically and from their area of specialist expertise. The majority spent most time discussing an effectiveness trial. This was the case for the intensive care clinical triallists, while those with implementation trial expertise discussed both types of trial. The hierarchy outlined in Figure 17 holds as a framework for summarising the responses of all the triallists.
FIGURE 17.
Summary of hierarchy challenges of conducting further SDD randomised trials, as reported by triallists.

Discussion
Major challenges in future studies
There were differing views from triallists on the need for further research on SDD. Not all the triallists were convinced that further research was justified or feasible although the majority felt that it was warranted and important. The challenges identified by triallists are summarised in Figure 17. There was no consensus amongst the triallists on approaches to dealing with the challenges presented in this hierarchy.
Triallists from both an effectiveness and an implementation background highlighted the complexities of further research. The triallists focused on approaches to an effectiveness study and few views emerged on the appropriateness of, or approaches to, an implementation study. This may be partly due to the lack of clarity about the core components of an implementation trial.
All triallists proposed that an effectiveness trial would be large and demanding and that this could well lead to burnout for the chief investigator and team. They also identified the need for multidisciplinary support to conduct and complete an effectiveness study. All agreed with the requirement for any study to be multinational, although there was not clarity as to which countries could, or should, be included.
Triallists also identified key stakeholders such as clinical microbiologist and infectious disease clinicians as potential facilitators or barriers to the successful conduct and completion of any study. Nurses and pharmacists delivering SDD were suggested as potentially raising sustainability challenges owing to workload acceptability, although this was discussed more as anecdote than fact.
Study design issues
There was a range of views on the primary end points because of perceived problems with the size and cost of the study and the ability to identify funding. There was concern about using VAP as an end point owing to difficulties in establishing an agreed and standardised diagnosis. Many triallists identified antibiotic resistance as an important end point; however, most preferred this as a secondary end point. The nature of the control group was frequently discussed. Triallists acknowledged the widespread use of chlorhexidine and VAP bundles and there were varied views on whether or not there should be clear specification of the control group through to a more pragmatic control group without specification.
There was no consensus on the ideal unit of randomisation. The potential benefits of unit level randomisation were discussed with regard to both individual benefit and harm. The issue of unit of randomisation also brought into focus the question of patient consent. It was clear that different countries would approach this in different ways from an ethical perspective.
The impact of SDD on the local microbiological flora was widely discussed. Most saw this as a parallel issue to the primary end point, yet an important one, and most felt this should be measured to convince the community that resistance patterns were not adversely affected.
A editorial on SDD in 2012 also plays out the conflicts. 68 This editorial discussed study design, outcome measures, the impact of antibiotic resistance, the role of SOD and chlorhexidine and the place for a cost–benefit analysis amongst other issues. This article concluded that a cluster-randomised design should be the basis of future effectiveness studies with unambiguous end points such as mortality. It also identified that this would be a very large study with a requirement for detailed monitoring of resistance patterns and recognised the need to undertake the study in a range of settings with different microbiological ecology. 68 The current findings are thus consistent with recently published opinion.
Strengths and limitations
In drawing these findings together, one should of course distinguish between the participants’ views that are based on their experience and expertise as triallists and their personal views derived from other forms of evidence. Specifically, data from other stages of this study (e.g. whether or not nurses find it burdensome to deliver SDD) or from the published literature (e.g. techniques that are effective in changing professional behaviour) can inform some of the opinion-based evidence presented in this chapter. There was an interesting pattern of findings with respect to triallists’ beliefs about what will change behaviour (see Table 26). Although people may believe that more evidence will change clinical behaviour, the weight of evidence in the field of implementation research is that this not an effective approach. There is also a potential ethical question of randomising patients to a control group merely to persuade clinicians of what existing evidence already shows. Hence, a limitation of this study is that the participants may have gone beyond their level of expertise in presenting their own hypotheses about barriers to SDD delivery or about how best to change clinicians’ behaviour.
A further limitation of the study was that we did not succeed in engaging sufficient numbers of European triallists who have led SDD effectiveness trials. Their specific expertise would have been invaluable. Apart from this, the geographical spread of the sample across three continents and four health systems was a strength of this study.
Box 9 presents the key findings from the triallist interview study that inform the need for, and acceptability of, a clinical effectiveness RCT or an implementation RCT. The majority of triallists either accepted that further SDD effectiveness research should be carried out or were actively interested in participating. At least two were not convinced and a third felt that the scale of the venture was probably too great. However, this is set against the persisting issue of concern about resistance and the potential for antimicrobial resistance to lead to harm for an individual patient, even if the ICU population overall benefits. However, this study was designed to address the research question concerning challenges to conducting a trial, so we did not discuss non-trial designs in these interviews (e.g. studies of microbial resistance patterns). Hence, these findings should not be taken as an indication that a randomised trial is the only appropriate kind of study design for further SDD research.
-
Most triallists reported there was a place for a further effectiveness trial.
-
Numerous important challenges to the ideal design of an effectiveness trial were identified at multiple levels.
-
One triallist clearly felt further study not warranted from a futility point of view.
-
An implementation study was not dismissed, but to define the potential nature of this would need more specific interrogation.
-
We did not discuss non-trial designs in these interviews.
Chapter 7 General discussion and synthesis
The results of the four stages of research presented in this monograph were designed to lead to an evidence-based decision about whether to proceed to (1) a trial to evaluate the effectiveness of SDD, (2) an implementation trial (development and evaluation of an intervention to increase uptake of SDD) or (3) further exploratory research. There was also the possibility of a lack of belief in the relevance, topicality, appropriateness or clinical interest in this intervention, or a lack of equipoise owing to a belief of proven or potential harm being associated with the intervention, which would make further studies inappropriate and unethical.
Methods of synthesis
In collaboration with the leads of parallel studies in Australia/New Zealand, Canada and the UK, it was identified a priori that an international project steering group would make an assessment of whether or not a future trial is necessary, justifiable, acceptable and feasible based on a set of decision rules. The decision rules, as proposed in the study protocol, were to be based on the study findings and were as follows:
Decision rule 1: If support for SDD is low or variable and predicted by scores for specific beliefs about the consequences (benefits and harms) of implementing SDD, it may be judged appropriate to proceed to a clinical effectiveness trial. Such a pattern of results would suggest that dissatisfaction with the current evidence base explains the lack of uptake.
Decision rule 2: If support for SDD is low or variable and predicted by scores relating to social influence (e.g. pressure from colleagues in other disciplines), this would suggest that an implementation intervention could be effective if delivered by an identified opinion leader, clinical lead or local ‘champion’ through team meetings. We would probably proceed to an implementation trial to evaluate such an intervention.
Decision rule 3: If support for SDD is low or variable and predicted by beliefs relating to lack of capacity to implement SDD (e.g. resource issues), this would suggest that an implementation intervention could be effective if it focuses on barrier identification and generation of strategies to overcome barriers, known as ‘coping planning’,55 we would probably proceed to an implementation trial to evaluate such an intervention.
Decision rule 4: By contrast, if support for SDD is high (i.e. if there are ceiling effects and restricted variance), then the current low level of implementation will be attributable to an intention–behaviour gap, suggesting that external barriers prevent clinical staff from translating their intentions into action. This pattern of results would suggest that an implementation trial to test strategies for facilitating uptake may be appropriate.
Findings about acceptability and feasibility of a clinical trial would also inform the decision of how to proceed. Specifically:
Decision rule 5: If willingness (intention) to participate in an effectiveness trial is low, we are unlikely to proceed to such a trial.
Decision rule 6: If willingness (intention) to participate in an effectiveness trial is high, this would indicate that an effectiveness trial is sufficiently acceptable to proceed.
We also took into account scores for the factors that were perceived to make participation more likely.
Decision rule 7: If either of the stakeholder groups (intensive care consultants or microbiologists) seems to be uniformly of the opinion that there are no design features that would make a trial acceptable, and/or if a third (or more) of the members of the two groups deem any such trial unacceptable, an effectiveness trial would be unlikely to be pursued.
Decision rule 8: Depending on the views expressed about the existing evidence base (i.e. if the intervention is viewed as potentially beneficial) we would consider the role of an implementation trial to change clinical practice. If appropriate, the change techniques that would form the components of such an intervention would be selected using methods previously shown to effectively change behaviour in other settings. 69
In addition, we used the results of the investigation of two ICUs for which SDD had been implemented, consensus data from the Delphi study, results of the national survey and findings from the interviews with triallists to inform the:
-
behavioural (practical, organisational and management) issues that would need to be addressed in order to mount a trial
-
ethical issues relating to informed acceptance of trial entry among eligible patients
-
trial design issues including measurement of outcome and process variables.
Evidence for relevance, topicality, appropriateness and clinical interest in selective decontamination of the digestive tract
In the context of a TDF of clinical behaviour change, we asked specific questions in the Delphi study relating to the domain Motivation and goals. This domain focuses on the relevance, topicality, appropriateness and clinical interest in SDD. There was low-level consensus (50–75%) for the relevant items, tending towards agreement with the statements, ‘SDD is not on my Unit’s list of priorities’ and ‘SDD is not a topic of discussion among my colleagues’, but tending towards disagreement (with bimodality) for the item, ‘SDD is outdated’. Overall, there was a range of levels of engagement with the issue of SDD.
To assess the feasibility and acceptability of further effectiveness research, we also asked three key questions, in both the Delphi study and the national survey, about:
-
support for, or opposition to, SDD
-
whether or not further SDD RCTs are ethical
-
willingness to participate in a national RCT of SDD.
Responses to the question I am opposed to selective decontamination of the digestive tract
Findings from the Delphi study (see Chapter 2) and the national survey (see Chapter 5) showed remarkable similarity, with bimodal distributions in which the modes were (1) the neutral score (indicating uncertainty) and (2) disagreement (indicating that a substantial portion of each sample was not opposed to SDD). In the survey, there was evidence of bimodality in both groups (intensive care consultants and clinical microbiologists). Clinical microbiologists were split between being opposed and not opposed (modes of 6 and 2 on the seven-point scale), whereas intensive care consultants were split between being neutral and not opposed (modes of 4 and 2 on the seven-point scale) (see Figure 12).
The evidence from the Delphi and national survey (see Chapters 3, 4 and 5) confirm the perception that clinicians reported dealing with HAIs such as VAP using strategies other than SDD. This may reduce the relevance of SDD since prevention of HAIs such as VAP may be seen as the key mode of action and, therefore, efficacy of SDD could be seen as a low priority. There is further evidence that SDD was not a high clinical priority or topic of discussion amongst clinicians. However, when asked if participants were ‘opposed to SDD’, they had notably mixed responses (indicated by a bimodal distribution of scores) and participants did not think prophylactic antibiotic usage in SDD was against their training or responsibilities. Further evidence on relevance is presented in Whether or not further selective decontamination of the digestive tract randomised controlled trials are ethical: clinical equipoise and uncertainty.
Whether or not further selective decontamination of the digestive tract randomised controlled trials are ethical: clinical equipoise and uncertainty
In the Delphi study, responses to questions about the consequences of SDD indicated that there was consensus around equipoise and/or uncertainty. Specifically, there were a high number of responses around the mid-point of the response scales. Similar distributions were evident in the national survey data. In addition, we asked questions that related directly to clinical equipoise and uncertainty regarding the evidence base for SDD in both study stages. Further information on equipoise and uncertainty can also be gleaned from the data in Willingness to participate in further research in selective decontamination of the digestive tract.
In the Delphi study and national survey, there was a clear finding that further RCTs are ethical and that current uncertainties in the evidence base should be addressed in a new study. There was also a message that the existing evidence base may not be generalisable to the participants’ practice. These observations strongly suggest the existence of a state of clinical equipoise for individuals within the clinical community. This statement is supported by uncertainty in responses to beliefs about the potential benefits of SDD such as effects on mortality, HAIs, VAP or cost-effectiveness. There was also uncertainty in responses about beliefs on the potential harms related to SDD such as effects on antibiotics resistance and, more generally, whether or not the harm outweighs the benefits.
Willingness to participate in further research in selective decontamination of the digestive tract
Evidence of willingness to participate in further research came from the Delphi study and the national survey.
The evidence is interesting – there seemed to be clear support for participation in further research in the field of SDD evidenced by participants’ agreement with the statement, ‘current uncertainties in the evidence base should be addressed in a new study’. There are three main research designs that could be utilised to undertake this further research: efficacy/effectiveness RCTs, implementation studies or prospective non-randomised study designs. The significant majority of clinicians stated they would be prepared for their patients to be randomised to either SDD or control in an effectiveness study. Despite the importance attached to the issue of antibiotic resistance, the response to the question suggesting concerns with antibiotic resistance would limit participation was answered in a neutral fashion. This is balanced by the clear signal for any future effectiveness study to include a pretrial, during-trial and post-trial monitoring of antibiotic resistance and this statement was strongly supported by participants.
For the question most directly related to support for an implementation study in the Delphi, ‘I would support my centre being involved in a study to promote the adoption of SDD’, there seemed to be moderately strong support. This is a little hard to explain since it would be expected that clinicians would favour this option only if they believed that the SDD literature contained compelling evidence for efficacy or effectiveness for SDD and that respondents did not have equipoise owing to this strong supportive evidence. This does not seem to be the case (see Whether or not further selective decontamination of the digestive tract randomised controlled trials are ethical: clinical equipoise and uncertainty). However, two of the triallists interviewed suggested that an implementation study could be combined with the collection of data regarding effectiveness, thereby addressing the objectives of both an effectiveness and an implementation trial. Furthermore, when asked about knowledge of the evidence base, a substantial number of respondents admitted they did not know the evidence base well enough to make an informed opinion regarding the use of SDD.
Qualitative evidence from the interviews with clinical triallists showed that 9 out of 10 of these triallists (including triallists with significant experience in implementation research) focused primarily on an effectiveness study throughout interview, despite eight of the nine reporting being individually persuaded by the evidence of SDD’s effectiveness. However, the triallists did express doubts about the potential harms, a finding that converged with both the Delphi study and the survey. Only one triallist focused solely on an implementation study.
Finally, the question of performing further non-randomised studies was not raised by participants in any phase of the research. There could be an argument for performing matched or comparative cohort studies to try to answer some of the remaining questions regarding SDD. However, it could be argued that with the high degree of remaining uncertainty and equipoise about both the benefits and potential harms associated with SDD, a randomised trial design is the most efficient and ethical research method with the lowest risk to patients if harms are realised.
Design features of an effectiveness randomised controlled trial
Interviews with clinical triallists were the main method used to identify trial design and conduct issues. However, some information was also obtained from the Delphi study and the national survey.
Delphi participants generally agreed with the statement ‘I would be more likely to participate in an RCT if mortality is the end point’, but national survey respondents less so. This end point was also supported in the triallist interviews. A full cost–benefit analysis was also identified as an integral part of any future study in the Delphi study but was not a strongly stated issue from the clinical triallists. Delphi participants would strongly favour a chlorhexidine and/or a VAP bundle (including chlorhexidine mouthwash and semi-recumbent positioning) for the control arm of a future study. Delphi participants identified a strong desire for future efficacy/effectiveness studies to include a pretrial, during-trial and post-trial monitoring of antibiotic resistance. This was also raised in the triallist interviews.
Challenges of an effectiveness randomised controlled trial
Data on the challenges for an effectiveness RCT come from the case studies (see Chapter 2), the Delphi study (see Chapters 3 and 4) and the interviews with triallists (see Chapter 6).
In the case studies, there was marked variation in the clinical as well as behavioural specification of the intervention, and this is also noted from the available literature on SDD. Clearly, the accurate clinical and behavioural specification of the intervention will be crucial to the acceptability and success of any future study and this needs careful consideration. The lack of a clear specification of the intervention was also identified as a barrier by the triallists in the interview study.
The Delphi study raised some significant concerns with the conduct of an effectiveness RCT including major issues such as culture change, requirement for local champions and possible conflicting opinions between clinical microbiologists and intensive care consultants, although our results suggest this is possibly less marked than perhaps suspected (see Chapters 4 and 5). These issues may present major challenges to any future study.
There are also concerns about workload for nurses, pharmacists and clinical microbiologists. In the case studies reported in Chapter 2, it was actually doctors who identified the increase in nursing workload more than nurses themselves. Clearly, appropriate support for staff time and intervention development and delivery costs will have to be built into any future RCT. Encouragingly, participants also identify some facilitators such as SDD being straightforward to deliver and that educating staff would not be expensive.
The interviews with triallists also gave valuable information on challenges of conducting such a study, and these are discussed in length in Chapter 6. The major challenges identified included the acceptability and support for SDD as an intervention, the difficulty in balancing the tensions between the individual and societal consequences related to SDD, the international and multicentre nature as well as the very large size of any proposed future study. These are major barriers and threats to the feasibility of such a study.
There was also much discussion about cluster versus individual randomisation and their relative merits within an effectiveness trial. Furthermore, triallists identified the interaction between trial design and trial feasibility, choice of outcomes, level of consent and trial costs. The control group for such a study was also seen as crucial to the success and acceptability of the trial result. All of these issues would need careful consideration in the development of any future proposed trial.
Application of decision rules
We synthesised the findings from the multifaceted study described in this report by applying the decision rules to address the question of what, if any, further SDD research is appropriate and feasible?
-
Decision rule 1. Results of the Delphi study and national survey indicate that this criterion was met, although the issues related to difficulty in participating are identified in decision rule 6.
-
Decision rule 2. Results of the Delphi study and national survey indicate that this criterion was not met.
-
Decision rule 3. Results of the Delphi study and national survey indicate that this criterion was not met.
-
Decision rule 4. As support for SDD was mixed (see Figure 12), the data did not meet this criterion.
-
Decision rule 5. This criterion was not met, as willingness to participate in a trial was high.
-
Decision rule 6. This criterion was met and has to be considered with decision rule 1.
-
Decision rule 7. These criteria were not met. A further trial was seen as acceptable by the majority, although some participants were firmly of the view that the evidence base was clear enough to proceed with implementation.
-
Decision rule 8. This criterion was not met, in general. However, as noted above, in the Delphi study, the national survey and the triallist interviews, some participants felt that it would now be appropriate to implement SDD or to conduct an implementation trial.
We identified the behavioural (practical, organisational and management) issues that would need to be addressed and fully considered in any future study design. We also identified that there were ethical issues relating to informed consent among eligible patients and further detailed ethical study would have to be undertaken if a future proposed trial is planned.
Strengths and limitations
The conclusions from application of the decision rules should be viewed with caution owing to the low response rate in the UK-wide survey. It is possible that willingness data were biased as physicians who are more interested in participating in SDD research may have self-selected into this sample. Nonetheless, it is clear that sufficient numbers of physicians expressed interest for an effectiveness trial to appear feasible in the UK. The possibility of non-trial designs did not emerge in the interview stages of the study and such designs may require further consideration.
In general, the multilens, multimethod approach adopted by the SuDDICU feasibility study facilitated an investigation that was both in depth and broad based. Clinical, practical and methodological issues were examined using a robust theoretical structure; yet the exploration also allowed new issues to be elicited and taken forward for detailed investigation. In the Delphi study, findings from the qualitative (see Chapter 3) and quantitative (see Chapter 4) phases were congruent and these were also confirmed in the nationwide survey (see Chapter 5). Across the facets of the study, a clear picture emerged of the strengths and weaknesses in the SDD evidence base. The study thus identified consistent patterns of uncertainty about the evidence that should be addressed in future SDD research.
However, these findings are set in a rapidly changing context. First, it is evident that other strategies to address infection are currently being used in ICUs. Second, and perhaps related to the first, rates of VAP may be reducing in some ICUs. Hence, the findings reported in this study may be time sensitive and it may be that the methods of investigation illustrated here are of more lasting significance than the specific clinical findings. Nonetheless, it is clear, based on the level of consensus around uncertainty about the evidence for and against SDD, that further research is warranted at this time.
Conclusions
Having considered all the data from the four substudies, presented in this report in Chapters 2–6, and the application of the a priori decision rules, we draw a number of conclusions that have implications for health care and for further research.
Implications for health care
-
There was a striking level of uncertainty about the effects of SDD on clinical outcomes that are regarded as important. This uncertainty suggests that there is considerable potential for improvement in prevention of HAIs in critically ill patients but further evidence is required to clarify the balance between potential individual-level benefits (e.g. infections, mortality) and potential society-level harms (e.g. antibiotic resistance) related to SDD.
-
There was significant confusion apparent in clinicians’ understanding and perceptions of the components that constitute SDD and related interventions, e.g. SOD. The importance of detailed guidance on what constitutes different interventions was clear.
-
For those units considering the adoption of SDD, it was apparent from our research that the delivery of SDD is feasible and can be adopted into unit practices. However, a detailed specification of the proposed clinical and behavioural components of the intervention should be developed.
-
This study highlighted that the introduction of SDD, whether into routine practice or within a research context, requires consensus across a range of different stakeholders (including ICU colleagues, clinical microbiologists and medical directors/those with decisional authority within units). Our study also highlighted that medical microbiologists appear to be more opposed to SDD than intensivists. Representatives of these stakeholder groups should be engaged early in any discussions around the use/introduction of SDD.
-
A substantial minority of participants reported that SDD would be adopted (apparently quite straightforwardly) if adoption was mandated by regulatory bodies.
-
There was acknowledgement that the infections that SDD was developed to target (such as VAP) are currently less of a problem owing to other current prophylaxis and treatment regimens.
Recommendations for research
Further SDD research was viewed as important, acceptable and feasible to the key stakeholder groups who participated in this study. However, further effectiveness research would need to be on a scale that raises challenges for trial design and trial conduct. Research priorities, in rank order, are as follows:
-
A study within UK ICU is required to model resistance patterns as a function of SDD use.
-
Further large-scale effectiveness trials of SDD in intensive care practice are required to answer the remaining uncertainties, especially those issues relating to antimicrobial resistance.
-
There is a general willingness to participate in a future effectiveness RCT of SDD; however, support for further research is time-sensitive (owing to the changing context) and is not unanimous. Future research needs to address the substantial barriers to acceptance and participation in any future trial. These barriers should be addressed with reference to the study findings, for example (1) clinicians with lower self-assessed knowledge of the SDD evidence base shifted their opinions following feedback about others’ views, suggesting a role for discussion among clinical colleagues; (2) concerns about antibiotic resistance and other potential harms were of paramount importance, suggesting the importance of emphasising that a UK trial would assess antibiotic resistance patterns; (3) consensus between ICU colleagues was seen as important, suggesting that consensus building and development are key to acceptance and participation; and (4) a substantial proportion of clinicians would be prepared to participate in a trial of SDD if their colleagues were in favour, suggesting that the presence of a SDD champion in an ICU could influence participation.
-
Future trials should include (1) a primary mortality outcome, (2) pretrial, during-trial and post-trial monitoring of antimicrobial resistance, (3) a control group that includes chlorhexidine and/or VAP bundles, (4) a cost–benefit analysis, and (5) a qualitative study to investigate the fidelity of the SDD intervention as delivered.
-
Groups proposing to undertake such a trial need to overcome the following challenges: (1) gaining sufficient acceptance of the trial, (2) gaining adequate participation in a trial, (3) the clear specification of the trial intervention, (4) major methodological issues relating to trial design and conduct, (5) clarification of the acceptability (to ethics committees) of cluster-level consent in the case of a cluster RCT and (6) major funding issues.
-
At this time, there is a much lower level of interest in adoption of SDD, or studies designed to encourage implementation of SDD, into practice.
Acknowledgements
The authors wish to thank the members of the Project Steering Committee (who also served a Data Monitoring Committee function): Tim Walsh (Chairperson, Professor and Consultant in Anaesthetics and Intensive Care), Robbie Foy (independent member, Professor of Primary Care) and Barry Williams (independent member, PPI representative).
The authors also wish to thank the following researchers for their assistance with qualitative analysis: Denise Bolsover, Laura Todd, Elizabeth Wells and Andrea Marshall; Sean Wang and Mark Forrest for their excellent programming support and Karen McLeod for excellent secretarial support. The authors also thank the many health-care staff working with patients in ICUs across the UK, who volunteered to provide data for this study. The authors also acknowledge the assistance of Sue Rust, the ICS, the HIS, the BSAC, the ICNARC and the United Kingdom Clinical Pharmacists Association Critical Care Group for aiding recruitment.
Contributions of authors
Jill J Francis (Professor of Health Psychology; implementation researcher) was the co-chief investigator and international methods lead, contributed to the development of the study protocol, led the preparation of the report and was responsible overall for the conduct of the study including the maintenance of parallel timelines across the studies internationally.
Eilidh M Duncan (Research Fellow; health psychology researcher) was responsible for the day-to-day management of the study, monitored data collection and assisted in drafting and preparing the report.
Maria E Prior (Research Fellow; health services researcher) advised on the analysis of the case studies data, led the qualitative analyses relating to the Delphi study interviews and the triallist interviews, and assisted in drafting and preparing the report.
Graeme S MacLennan (Senior Statistician) contributed to the grant application and the study design and conducted the statistical analyses.
Stephan U Dombrowski (Research Fellow; health psychology researcher) collected and analysed the data for the case studies and led on the writing of this part of the report.
Geoff Bellingan (Director; intensive care consultant) was the co-chief investigator, conducted the interviews with triallists and led on the writing of this part of the report.
Marion K Campbell (Director; Health Services Research triallist and methodologist) was chairperson of the International Project Steering Group, contributed to the development of the study design, commented on all aspects of the conduct of the study and contributed to the preparation of the report.
Martin P Eccles (Professor of Clinical Effectiveness; implementation triallist) advised on all aspects of the study relating to implementation issues and trial design, and contributed to the preparation of the report.
Louise Rose (Assistant Professor of Nursing; mixed-methods researcher) commented on all aspects of the study and contributed to the preparation of the report.
Kathy M Rowan (Director and Professor, ICNARC) commented on all aspects of the study and contributed to the preparation of the report.
Rob Shulman (ICU pharmacist) commented on aspects of the study from a pharmacy perspective and contributed to the preparation of the report.
A Peter R Wilson (Clinical microbiologist) commented on aspects of the study from a microbiology perspective and contributed to the preparation of the report. Dr Wilson was part funded by the University College London Hospital/University College London Comprehensive Biomedical Centre with funding from the Department of Health’s NIHR Biomedical Research Centres.
Brian H Cuthbertson (Chief; intensive care consultant) was the international clinical lead and conceived the study, led the development of the study protocol, was responsible overall for the conduct of the study internationally and contributed to the preparation of the report, leading the writing of the final chapter.
The UK ICS adopted this issue as a clinical priority and provided financial support for developing the funding application for this study. The authors thank the ICS for this support. The Health Services Research Unit is funded by the Chief Scientist Office of the Scottish Government Health Directorates. The views expressed are those of the authors alone.
Members of the SuDDICU study groups from the other participating nations are listed in the box below.
SuDDICU UK study group. Jill J Francis, International methods lead, Professor of Health Psychology, Health Services Research Unit, University of Aberdeen, Aberdeen, UK; Eilidh M Duncan, Research Fellow in Health Psychology, Health Services Research Unit, University of Aberdeen, Aberdeen, UK; Maria E Prior, Research Fellow, Health Services Research Unit, University of Aberdeen, Aberdeen, UK; Geoff Bellingan, UK clinical lead, Director, ICU, University College Hospital, London, UK; Marion K Campbell, Director, Health Services Research Unit, University of Aberdeen, Aberdeen, UK; Martin P Eccles, Professor of Clinical Effectiveness, University of Newcastle, Newcastle, UK; Marie Johnston, Professor of Health Psychology, Institute of Applied Health Sciences, University of Aberdeen, Aberdeen, UK; Graeme S MacLennan, Senior Statistician, Health Services Research Unit, University of Aberdeen, Aberdeen, UK; Craig Ramsay, Director, Health Care Assessment Programme, Health Services Research Unit, University of Aberdeen, Aberdeen, UK; Louise Rose, Assistant Professor of Nursing, University of Toronto, Toronto, ON, Canada; Kathy M Rowan, Director and Professor, ICNARC, London, UK; Rob Shulman, ICU Pharmacist, University College Hospital, London, UK; A Peter R Wilson, Medical Microbiologist, University College Hospital, London, UK; Stephan U Dombrowski, Research Associate, Health Services Research Unit, University of Aberdeen, Aberdeen, UK; Brian H Cuthbertson, International lead, Chief, Department of Critical Care Medicine, Sunnybrook Health Sciences Centre, Toronto, ON, Canada.
SuDDICU Canada study group. Brian H Cuthbertson, Canadian lead, Chief, Department of Critical Care Medicine, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; Jill J Francis, International methods lead, Professor of Health Psychology, Health Services Research Unit, University of Aberdeen, Aberdeen, UK; Karen Burns, Clinician Scientist and Attending Physician, St Michaels Hospital, Toronto, ON, Canada; Deborah Cook, Canada Research Chair in Critical Care, McMaster University, Hamilton, ON, Canada; Peter Dodek, Professor, Centre for Health Evaluation and Outcome Sciences, St. Paul’s Hospital, Vancouver, BC, Canada; Niall Ferguson, Associate Professor and Attending Physician, University Health Network, Toronto, ON, Canada; Richard Hall, Professor of Anaesthesiology and Pharmacology Associate Professor of Surgery Dalhousie University and The Queen Elizabeth II Health Sciences Centre, Halifax, NS, Canada; Lynn Johnston, Professor of Medicine, Chief of Infectious Diseases, Dalhousie University, Halifax, NS, Canada; Salmann Kanji, ICU Pharmacist, Critical Care Unit, The Ottawa Hospital, Ottawa, ON, Canada; John Marshall, Professor of Surgery, University of Toronto and Attending Surgeon, St Michaels Hospital, Toronto, ON, Canada; Lauralyn McIntyre, Associate Professor, Clinical Epidemiology, Ottawa Health Research Institute, Ottawa, ON, Canada; John Muscedere, Associate Professor of Medicine, Queen’s University, Intensivist Kingston General Hospital, Kingston, ON, Canada; Joe Pagliarello, University of Ottawa, Ottawa, ON, Canada; Louise Rose, Assistant Professor of Nursing, University of Toronto, Toronto, ON, Canada; Laura Todd, Research Assistant, Department of Family and Community Medicine, University of Toronto, Toronto, ON, Canada. Fiona Webster, Knowledge Translation Scientist, Department of Family and Community Medicine, University of Toronto, Toronto, ON, Canada; Elisabeth Wells, Research Fellow, Department of Critical Care Medicine, Sunnybrook Health Sciences Centre, Toronto, ON, Canada.
SuDDICU Australia and New Zealand Study Group. Ian Seppelt, Australia/New Zealand lead, Nepean Hospital, Penrith, NSW, Australia; Simon Finfer, Professor of Intensive Care and Staff Specialist, Royal North Shore Hospital and the George Institute, Sydney, NSW, Australia; Jeff Lipman, Professor and Head ICU, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia; John Myburgh, Professor, ICU, St George Hospital Sydney and the George Institute, Sydney, NSW, Australia; David Paterson, Professor Infectious Diseases and Microbiology, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia; Brian H Cuthbertson, International lead, Chief, Department of Critical Care Medicine, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; Jill J Francis, International methods lead, Professor of Health Psychology, Health Services Research Unit, University of Aberdeen, Aberdeen, UK.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Cuthbertson BH, Campbell MK, MacLennan G, Duncan EM, Marshall AP, Wells EC, et al. Clinical stakeholders’ opinions on the use of selective decontamination of the digestive tract in critically ill patients in intensive care units: an international Delphi study. Crit Care 2013;17:R266.
Cuthbertson BH, Francis J, Campbell M, Seppelt I, Macintyre L, Grimshaw J. A study of the perceived risks, benefits and barriers to the use of SDD in adult critical care units (the SuDDICU study). Trials 2010;11:117.
Duncan EM, Cuthbertson BH, Prior ME, Marshall AP, Wells EC, Todd LE, et al. The views of health care professionals about selective decontamination of the digestive tract: an international, theoretically-informed interview study. J Crit Care 2014; in press.
Dombrowski SU, Prior ME, Duncan E, Cuthbertson BH, Bellingan G, Campbell MK, et al. Clinical components and associated behavioural aspects of a complex healthcare intervention: multi-methods study of selective decontamination of the digestive tract in critical care. Aust Crit Care 2013;26:173–9.
Francis JJ, Duncan EM, Prior ME, MacLennan G, Marshall AP, Wells EC, et al. Comparison of four methods for assessing the importance of attitudinal beliefs: an international Delphi study in intensive care settings [published online ahead of print 23 September 2013]. Br J Health Psych 2013. doi:10.1111/bjhp.12066
Marshall AP, Weisbrodt L, Rose L, Duncan E, Prior M, Todd L, et al. Implementing selective digestive tract decontamination in the intensive care unit: a qualitative analysis of nurse-identified considerations. Heart Lung 2014;43:13–18.
References
- Cuthbertson BH, Francis J, Campbell MK, MacIntyre L, Seppelt I, Grimshaw J. A study of the perceived risks, benefits and barriers to the use of SDD in adult critical care units (The SuDDICU study). Trials 2010;11. http://dx.doi.org/10.1186%2F1745-6215-11-117.
- Healthcare Improvement Scotland . The Scottish Patient Safety Programme 2010. www.patientsafetyalliance.scot.nhs.uk/programme (accessed April 2012).
- Institute of Healthcare Improvement . Institute of Healthcare Improvement. 2012. www.ihi.org/explore/HAI/Pages/default.aspx (accessed December 2013).
- Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev 2009;4. http://dx.doi.org/10.1002%2F14651858.CD000022.pub2.
- de Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 2009;360:20-31. http://dx.doi.org/10.1056%2FNEJMoa0800394.
- Silvestri L, van Saene HK, Milanese M, Gregori D, Gullo A. Selective decontamination of the digestive tract reduces bacterial bloodstream infection and mortality in critically ill patients. Systematic review of randomized, controlled trials. J Hosp Infect 2007;65:187-203. http://dx.doi.org/10.1016%2Fj.jhin.2006.10.014.
- Hurley JC. Prophylaxis with enteral antibiotics in ventilated patients: selective decontamination or selective cross-infection?. Antimicrob Agents Chemother 1995;39:941-7. http://dx.doi.org/10.1128%2FAAC.39.4.941.
- Hurley JC. Paradoxical ventilator associated pneumonia incidences among selective digestive decontamination studies versus other studies of mechanically ventilated patients: benchmarking the evidence base. Crit Care 2011;15. http://dx.doi.org/10.1186%2Fcc9406.
- Bastin AJ, Ryanna KB. Use of selective decontamination of the digestive tract in United Kingdom intensive care units. Anaesthesia 2009;64:46-9. http://dx.doi.org/10.1111%2Fj.1365-2044.2008.05676.x.
- Shah R, Louw J, Veenith T. National survey on current practice of use of selective digestive decontamination in the United Kingdom. Crit Care 2008;12. http://dx.doi.org/10.1186%2Fcc6228.
- Wunderink RG. Welkommen to our world: emergence of antibiotic resistance with selective decontamination of the digestive tract. Am J Respir Crit Care Med 2010;181:426-7. http://dx.doi.org/10.1164%2Frccm.200912-1821ED.
- Ochoa-Ardila ME, García-Cañas A, Gómez-Mediavilla K, González-Torralba A, Alía I, García-Hierro P, et al. Long-term use of selective decontamination of the digestive tract does not increase antibiotic resistance: a 5-year prospective cohort study. Intensive Care Med 2011;37:1458-65. http://dx.doi.org/10.1007%2Fs00134-011-2307-0.
- Oostdijk EAN, Bonten M. Intestinal colonization with Gram-negative bacteria and subsequent ICU-acquired bacteraemia during selective digestive decontamination. Clin Microbiol Infect 2010;16.
- Oostdijk EAN, De Smet AMGA, Blok HEM, Thieme Groen ES, Van Asselt GJ, Benus RFJ, et al. Ecological effects of selective decontamination on resistant gram-negative bacterial colonization. Am J Respir Crit Care Med 2010;181:452-7. http://dx.doi.org/10.1164%2Frccm.200908-1210OC.
- Rocha LA, Martin MJ, Pita S, Paz J, Seco C, Margusino L, et al. Prevention of nosocomial infection in critically ill patients by selective decontamination of the digestive tract. A randomized, double blind, placebo-controlled study. Intensive Care Med 1992;18:398-404. http://dx.doi.org/10.1007%2FBF01694341.
- Ulrich C, Harinck-de Weerd JE, Bakker NC, Jacz K, Doornbos L, de Ridder VA. Selective decontamination of the digestive tract with norfloxacin in the prevention of ICU-acquired infections: a prospective randomized study. Intensive Care Med 1989;15:424-31. http://dx.doi.org/10.1007%2FBF00255597.
- Winter R, Humphreys H, Pick A, MacGowan AP, Willatts SM, Speller DCE. A controlled trial of selective decontamination of the digestive tract in intensive care and its effect on nosocomial infection. J Antimicrob Chemother 1992;30:73-87. http://dx.doi.org/10.1093%2Fjac%2F30.1.73.
- Silvestri L, Petros AJ, De La Cal MA, Visintin S. Selective digestive decontamination. Why are intensivists more ‘resistant’ than microorganisms?. Minerva Anestesiol 2011;77:212-19.
- Zandstra DF, Petros AJ, Taylor N, Silvestri L, de la Cal MA, van Saene HKF. Withholding selective decontamination of the digestive tract from critically ill patients must now surely be ethically questionable given the vast evidence base. Crit Care 2010;14. http://dx.doi.org/10.1186%2Fcc9255.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. http://dx.doi.org/10.1136%2Fqshc.2004.011155.
- Francis JJ, O’Connor D, Curran J. Theories of behaviour change synthesised into a set of theoretical groupings: introducing a thematic series on the Theoretical Domains Framework. Implement Sci 2012;7. http://dx.doi.org/10.1186%2F1748-5908-7-35.
- PSG002: Technical Patient Safety Solutions for Ventilator-Associated Pneumonia in Adults: Guidance. London: National Institute for Health and Care Excellence; 2009.
- Craig P, Dieppe P, Macintyre S, Mitchie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:979-83. http://dx.doi.org/10.1136%2Fbmj.a1655.
- Michie S, Johnston M. Changing clinical behaviour by making guidelines specific. BMJ 2004;328:343-5. http://dx.doi.org/10.1136%2Fbmj.328.7435.343.
- Michie S, Lester K. Words matter: Increasing the implementation of clinical guidelines. Qual Saf Health Care 2005;14:367-70. http://dx.doi.org/10.1136%2Fqshc.2005.014100.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. http://dx.doi.org/10.7326%2F0003-4819-148-4-200802190-00008.
- Silvestri L, van Saene HK, Zandstra DF, Marshall JC, Gregori D, Gullo A. Impact of selective decontamination of the digestive tract on multiple organ dysfunction syndrome: systematic review of randomized controlled trials. Crit Care Med 2010;38:1370-6.
- Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med 1984;10:185-92. http://dx.doi.org/10.1007%2FBF00259435.
- Shibli AB, Milbrandt EB, Baldisseri M. Dirty mouth? Should you clean it out? Decontamination for the prevention of pneumonia and mortality in the ICU. Crit Care 2010;14. http://dx.doi.org/10.1186%2Fcc9048.
- Zandstra DF, Petros AJ, Silvestri L, De La Cal MA, Taylor N, Damjanovic V, et al. Selective decontamination of the digestive tract: selectivity is not required. Intensive Care Med 2010;36:1783-4. http://dx.doi.org/10.1007%2Fs00134-010-1945-y.
- Jongerden IP, de Smet AM, Kluytmans JA, te Velde LF, Dennesen PJ, Wesselink RM, et al. Physicians’ and nurses’ opinions on selective decontamination of the digestive tract and selective oropharyngeal decontamination: a survey. Crit Care 2010;14. http://dx.doi.org/10.1186%2Fcc9180.
- Yin RK. Case Study Research, Design and Methods. London: Sage Publications Ltd; 2003.
- Ritchie J, Lewis J, Elam G, Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage Publications Ltd; 2003.
- Ritchie J, Ritchie J, Lewis J. Qualitative Research Practice. London: Sage Publications Ltd; 2003.
- Krippendorff K. Content Analysis: An Introduction to its Methodology. Thousand Oaks, CA: Sage Publications Ltd; 2004.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analysing Qualitative Data. London: Routledge; 1994.
- Rogers EM. Diffusion of Innovations. New York, NY: Free Press; 1983.
- Jackson T, van Teijlingen E, Bruce J. Light retrograde massage for the treatment of post-stroke upper limb oedema: clinical consequences using the Delphi technique. Br J Occup Ther 2012;75:549-54.
- Kennie-Kaulbach N, Farrell B, Ward N, Johnston S, Gubbels A, Eguale L, et al. Pharmacist provision of primary health care: a modified Delphi validation of pharmacists’ competencies. BMC Fam Pract 2012;13. http://dx.doi.org/10.1186%2F1471-2296-13-27.
- Karamitri I, Bellali T, Galanis P, Kaitelidou D. The accessibility of vulnerable groups to health services in Greece: a Delphi study on the perceptions of health professionals. Int J Health Plan Manage 2013;28:35-47. http://dx.doi.org/10.1002%2Fhpm.2115.
- Dalkey NC. The Delphi Method: An Experimental Study of Group Opinion. Santa Monica, CA: RAND Corporation; 1969.
- Park RE, Fink A, Brook RH, Chassin MR, Kahn KL, Merrick NJ, et al. Physician ratings of appropriate indications for three procedures: theoretical indications vs indications used in practice. Am J Public Health 1989;79:445-7. http://dx.doi.org/10.2105%2FAJPH.79.4.445.
- van Teijlingen E, Pitchford E, Bishop C, Russell E. Delphi method and nominal group techniques in family planning and reproductive health research. J Fam Plan Reprod Health Care 2006;32:249-52. http://dx.doi.org/10.1783%2F147118906778586598.
- Dalkey NC, Helmer O. An experimental application of the Delphi method to the use of experts. Manage Sci 1963;9:458-67. http://dx.doi.org/10.1287%2Fmnsc.9.3.458.
- Akins RB, Tolson H, Cole BR. Stability of response characteristics of a Delphi panel: application of bootstrap data expansion. BMC Med Res Method 2005;5.
- Francis JJ, Stockton C, Eccles MP, Johnston M, Cuthbertson BH, Grimshaw JM, et al. Evidence-based selection of theories for designing behaviour change interventions: using methods based on theoretical construct domains to understand clinicians’ blood transfusion behaviour. Br J Health Psy 2009;14:625-46. http://dx.doi.org/10.1348%2F135910708X397025.
- Higgins ET, King G, Cantor N, Kihlstrom J. Cognition, Social Interaction, and Personality. Hillsdale, NJ: Erlbaum; 1981.
- Fishbein M, Fishbein M. Readings in Attitude Theory and Measurement. New York, NY: Wiley; 1967.
- Ajzen I. The theory of planned behavior. Organ Behav Hum Dec 1991;50:179-211. http://dx.doi.org/10.1016%2F0749-5978%2891%2990020-T.
- Fleuren M, Wiefferink K, Paulussen T. Determinants of innovation within healthcare organizations: literature review and Delphi study. Int J Quality Health Care 2004;16:107-23.
- Winters JMW, Story MF, Barnekow K, Premo B, Kailes JI, Schwier E, et al. Problems with medical instrumentation experienced by patients with disabilities. Med Syst Rehab 2004;5:1778-82. http://dx.doi.org/10.1177%2F154193120404801532.
- Dyson J, Lawton R, Jackson C, Cheater F. Does the use of a theoretical approach tell us more about hand hygiene behaviour? The barriers and levers to hand hygiene. J Infect Prevent 2011;12:17-24. http://dx.doi.org/10.1177%2F1757177410384300.
- Jones J, Hunter D, Pope C, Mays N. Qualitative Research in Health Care. Boston, MA: Blackwell Publishing; 2000.
- Francis JJ, Johnston M, Robertson C, Glidewell L, Entwistle V, Eccles ME, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health 2010;25:1229-45. http://dx.doi.org/10.1080%2F08870440903194015.
- Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol 2008;57:660-80. http://dx.doi.org/10.1111%2Fj.1464-0597.2008.00341.x.
- Weiner B. An attributional theory of achievement motivation and emotion. Psychol Rev 1985;92:548-73. http://dx.doi.org/10.1037%2F%2F0033-295X.92.4.548.
- Campbell SM, Shield T, Rogers A, Gask L. How do stakeholder groups vary in a Delphi technique about primary mental health care and what factors influence their ratings?. Qual Saf Health Care 2004;13:428-34. http://dx.doi.org/10.1136%2Fqhc.13.6.428.
- Holey EA, Feeley JL, Dixon J, Whittaker VJ. An exploration of the use of simple statistics to measure consensus and stability in Delphi studies. BMC Med Res Methodol 2007;7. http://dx.doi.org/10.1186%2F1471-2288-7-52.
- von der Gracht HA. Consensus measurement in Delphi studies: review and implications for future quality assurance. Technol Forecast Soc Change 2012;79:1525-36.
- Komorita SS. Attitude content, intensity and the neutral point on a Likert scale. J Soc Psychol 1963;61:327-34. http://dx.doi.org/10.1080%2F00224545.1963.9919489.
- Francis JJ, Eccles MP, Johnston M, Walker A, Grimshaw J, Foy R, et al. Constructing Questionnaires Based on the Theory of Planned Behaviour. A Manual for Health Services Researchers. University of Newcastle upon Tyne: Centre for Health Services Research; 2004.
- Burns KEA, Duffett M, Kho ME, Meade MO, Adhikari NKJ, Sinuff T, et al. A guide for the design and conduct of self-administered surveys of clinicians. Can Med Assoc J 2008;179:245-52. http://dx.doi.org/10.1503%2Fcmaj.080372.
- Intensive Care Society 2011. www.ics.ac.uk/ics-homepage/events/ics-past-meetings-events/state-of-the-art-meeting-2011/ (accessed 11 December 2013).
- Department of Health Advisory Committee on Antimicrobial Resistance and Healthcare Associated Infection (ARHAI) . Antimicrobial Stewardship: ‘Start Smart – Then Focus’. 2011. www.gov.uk/government/uploads/system/uploads/attachment_data/file/215308/dh_131181.pdf (accessed December 2013).
- Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8.
- Eccles MP, Mittman B. Welcome to Implementation Science. Implement Sci 2006;1.
- Carr ECJ, Worth A. The use of the telephone interview for research. Nursing Times Res 2001;6:511-24. http://dx.doi.org/10.1177%2F136140960100600107.
- Oostdijk EAN, Wittekamp HJ, Brun-Buisson C, Bonten MJM. Selective decontamination in European intensive care patients. Intensive Care Med 2012;38:533-8. http://dx.doi.org/10.1007%2Fs00134-012-2488-1.
- Davidson KW, Goldstein M, Kaplan RM, Kaufmann PG, Knatterud GL, Orleans CT, et al. Evidence-based behavioral medicine: what is it and how do we achieve it?. Ann Behav Med 2003;26:161-71. http://dx.doi.org/10.1207%2FS15324796ABM2603_01.
Appendix 1 Materials for case studies
Appendix 2 Materials for Delphi study
Appendix 3 Regulatory governance report
Report of the delays caused by local governance approval process
The study was submitted for R&D approval on 2 December 2010 to the Scottish central co-ordinating centre [NHS Research Scotland Permissions Coordinating Centre (NRSPCC)]. Local approvals for Scottish sites followed in December and January (Forth Valley 22 December 2010, Greater Glasgow and Clyde 23 December 2010, Fife 6 January 2011, Lothian 13 January 2011 and Grampian 27 January 2011). However, the local approvals process in England and Wales involved extreme delays. The extreme delay in receiving approvals had a negative impact upon recruitment by both delaying targets and potentially reducing the diversity of the sample (as, to limit damage to the study timeline, we were forced to send recruitment invitations based on where we had received approvals rather than to purposively sample based on diversity factors). An outline of the problems experienced in obtaining approvals.
We received notification that the project had been validated by the co-ordinating centre for England and Wales (NIHR Co-ordinated System for gaining NHS Permission) on 20 December 2010 and that study wide checks were completed on 31 January 2011. The lead Comprehensive Local Research Network for the study (Essex and Hertfordshire) informed us that the target time for processing approvals is 30 days. However, of the 47 NHS trusts we were targeting in England and Wales, approvals were still outstanding for 15 trusts when recruitment closed 6 months after the application was submitted. For at least one R&D office (Pennine Acute Hospitals NHS Trust), the delay in approving the study can be attributed to miscommunication between R&D offices regarding the use of a generic site-specific information (SSI) form for this study. The Pennine office informed us that they had been waiting to receive notification of a SSI before beginning the approvals process (despite this having been made available to all R&D departments via the NIHR Co-ordinated System for gaining NHS Permission and lead Comprehensive Local Research Network when approval was first sought).
The Welsh approvals process was hampered by miscommunication between the Scottish central coordinating centre and the offices in Wales. NRSPCC submitted the application to the Welsh Research Management office without being aware that the study was a secondary care study and could not be managed by the Welsh central system (which is exclusively for primary care studies). The Welsh office sent no reply to inform the NRSPCC of this mistake and nor did they contact the study team. When NRSPCC became aware of the mistake, they negotiated a rapid review of the study application and permission was received from one Welsh NHS trust (Cardiff and Vale NHS Trust) and two participants were recruited from Wales before recruitment had to close.
The Northern Ireland approvals process for the study was also problematic. The Southern Health and Social Care Trust at first agreed to review the study and produce a global governance report, which would cover all NHS trusts in Northern Ireland. However, 4 weeks after making our application, we received notification from the Research Manager for the Southern Health and Social Care Trust that they were no longer willing to process the study through the research governance approval system because the view received from the relevant staff was that they did not wish to participate because the nosocomial infection rate was already extremely small and the treatment was perceived to be very expensive. In other words, key staff were not in favour of SDD and decided to pre-empt the study findings, thereby potentially biasing the results of this study by excluding these important views. It appears that the judgement of this R&D office was given without consideration to the peer review performed by HTA and the ethics committee. Despite this setback, the study was approved by Western Health and Social Care Trust on 19 May 2011 and one participant was recruited from Northern Ireland.
Appendix 4 Detailed data for Delphi study
FIGURE 18.
Consensus of 90% or higher around mid-value of 8.
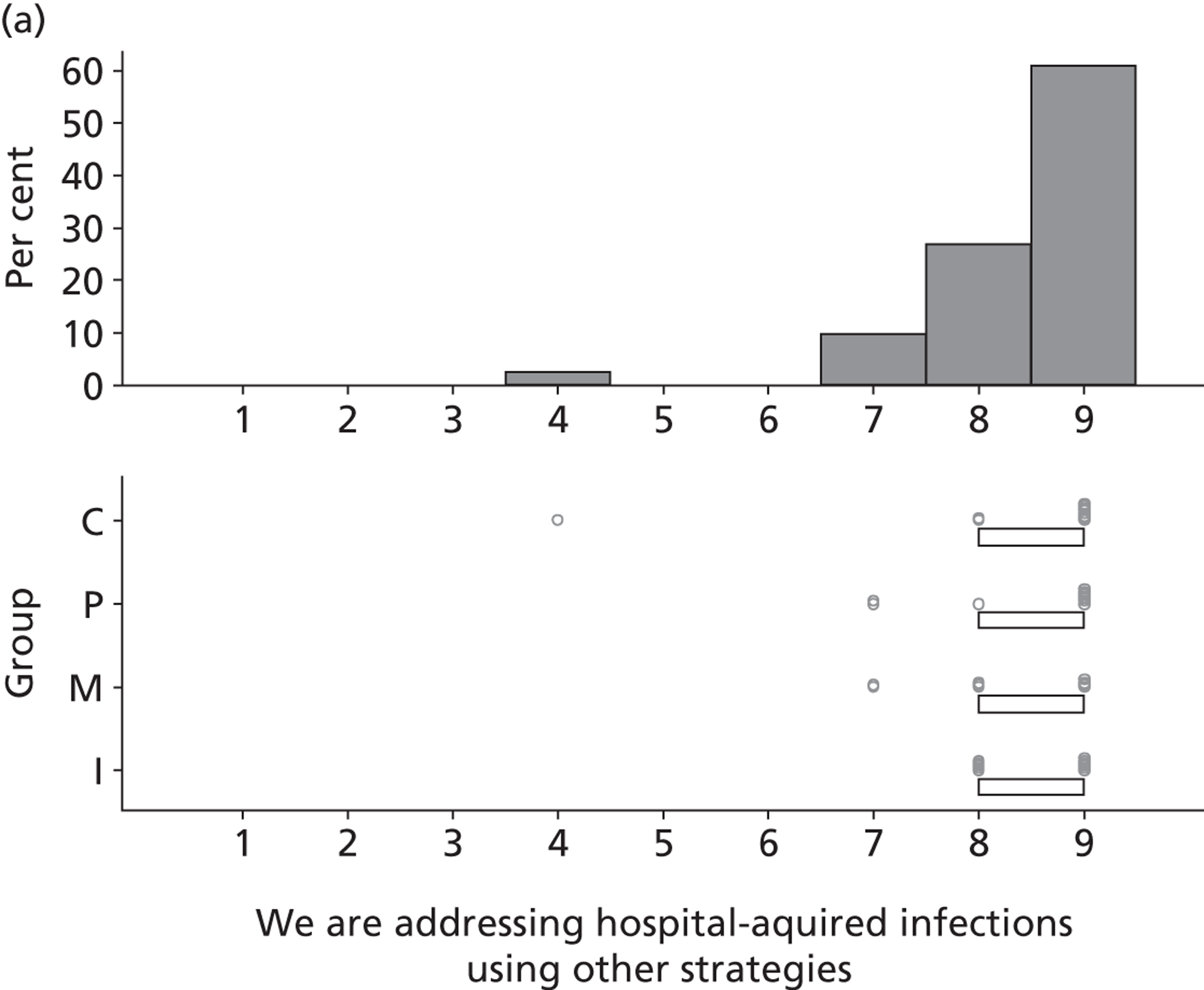
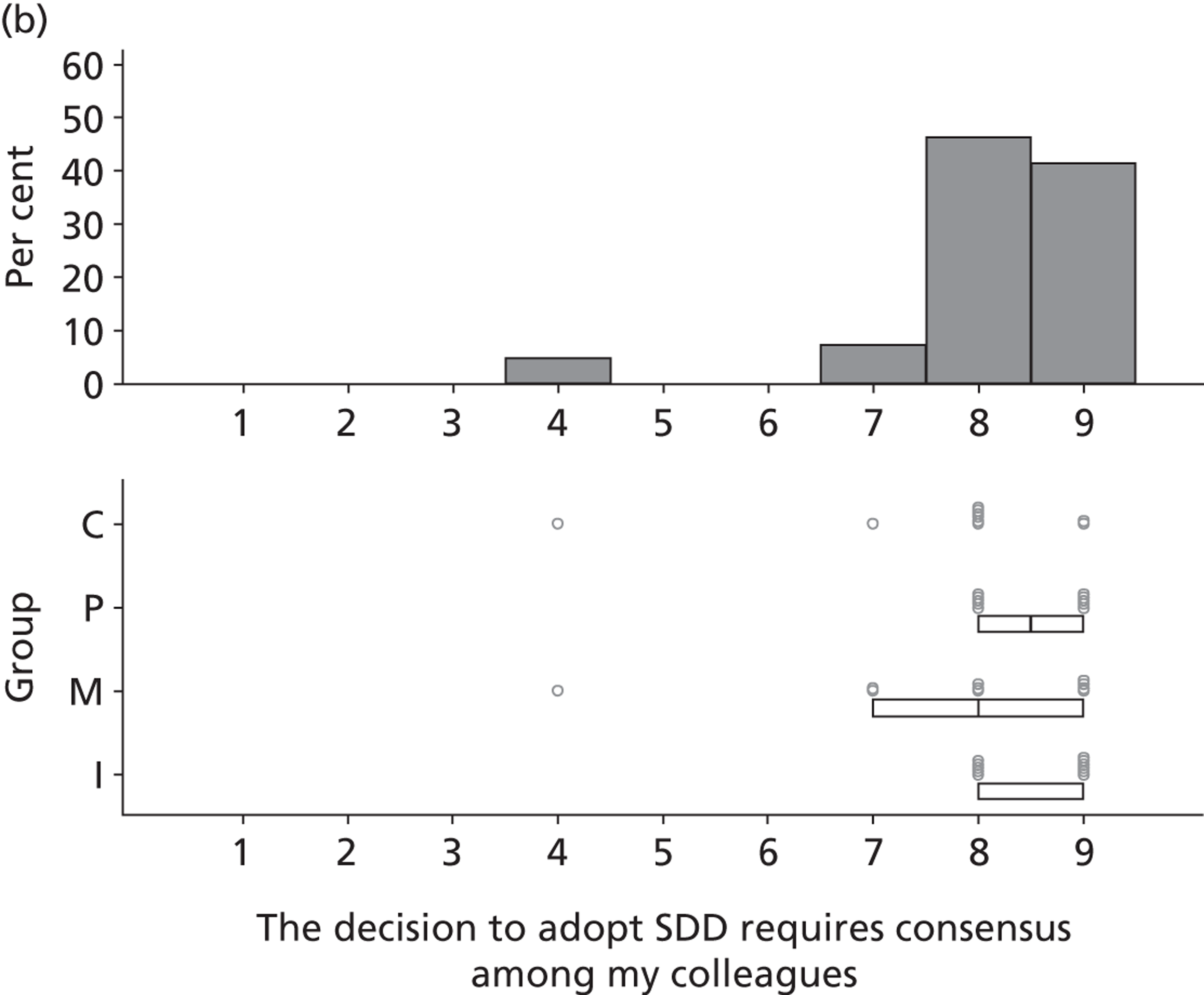
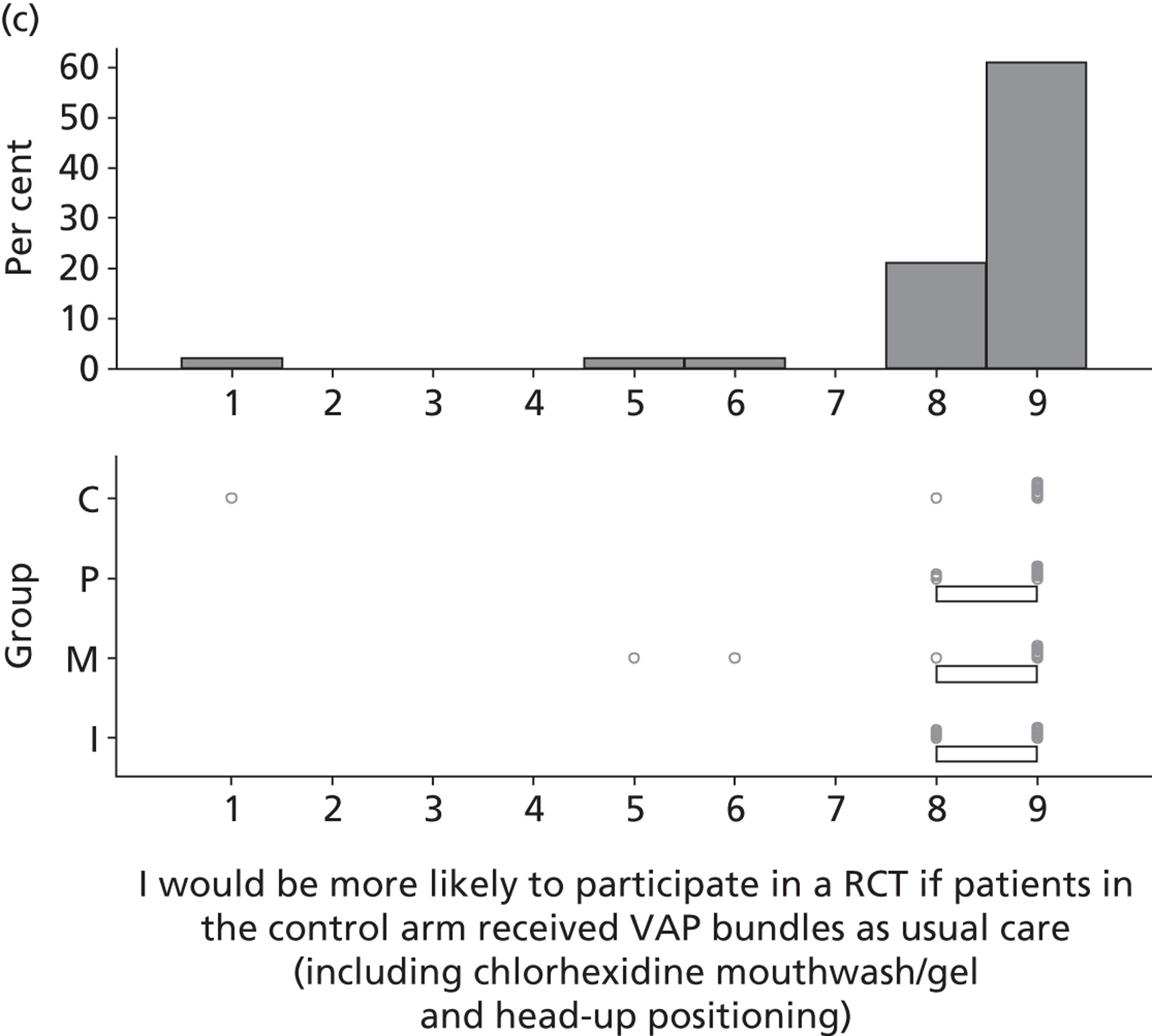
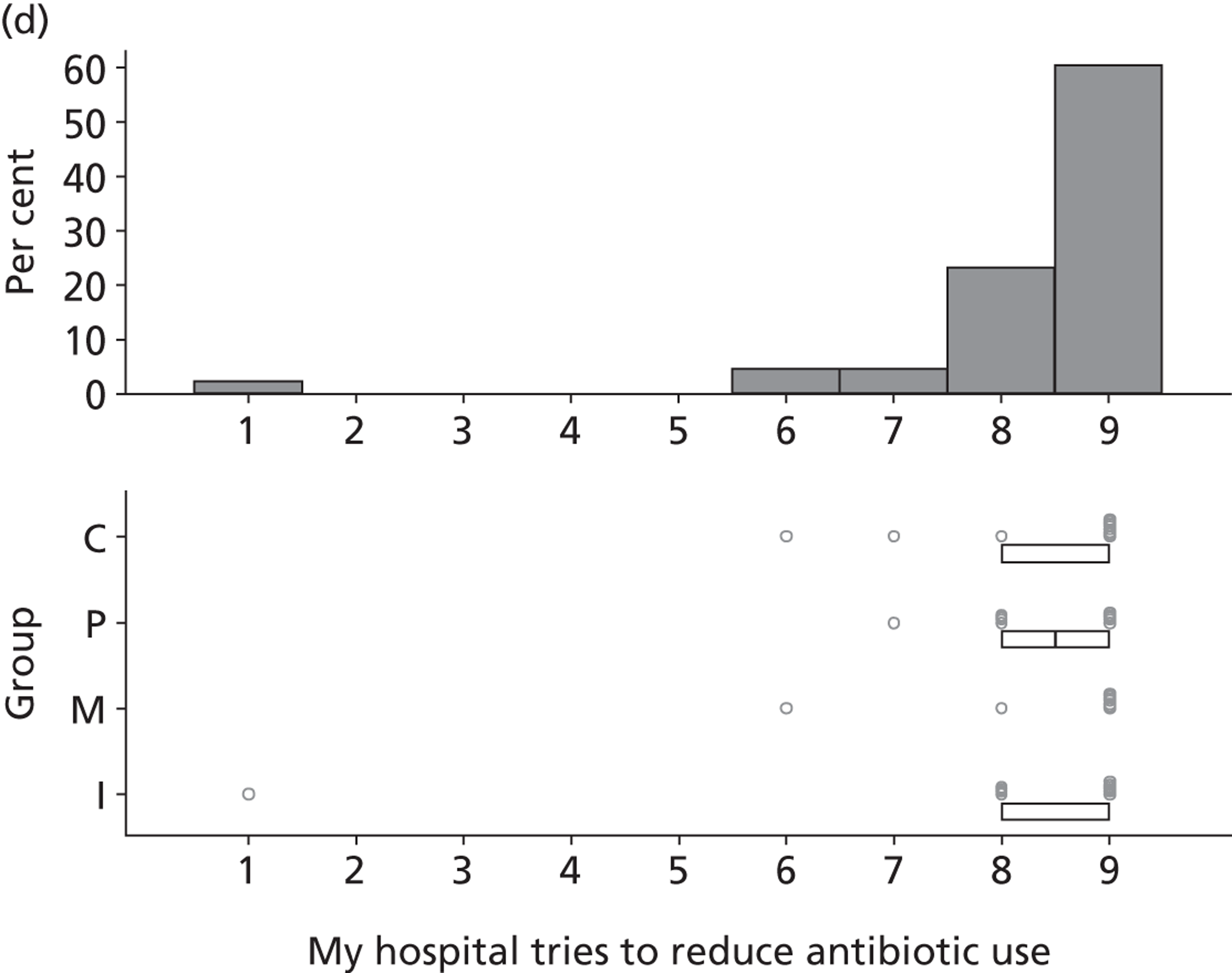
FIGURE 19.
75–90% consensus around mid-value of 8.
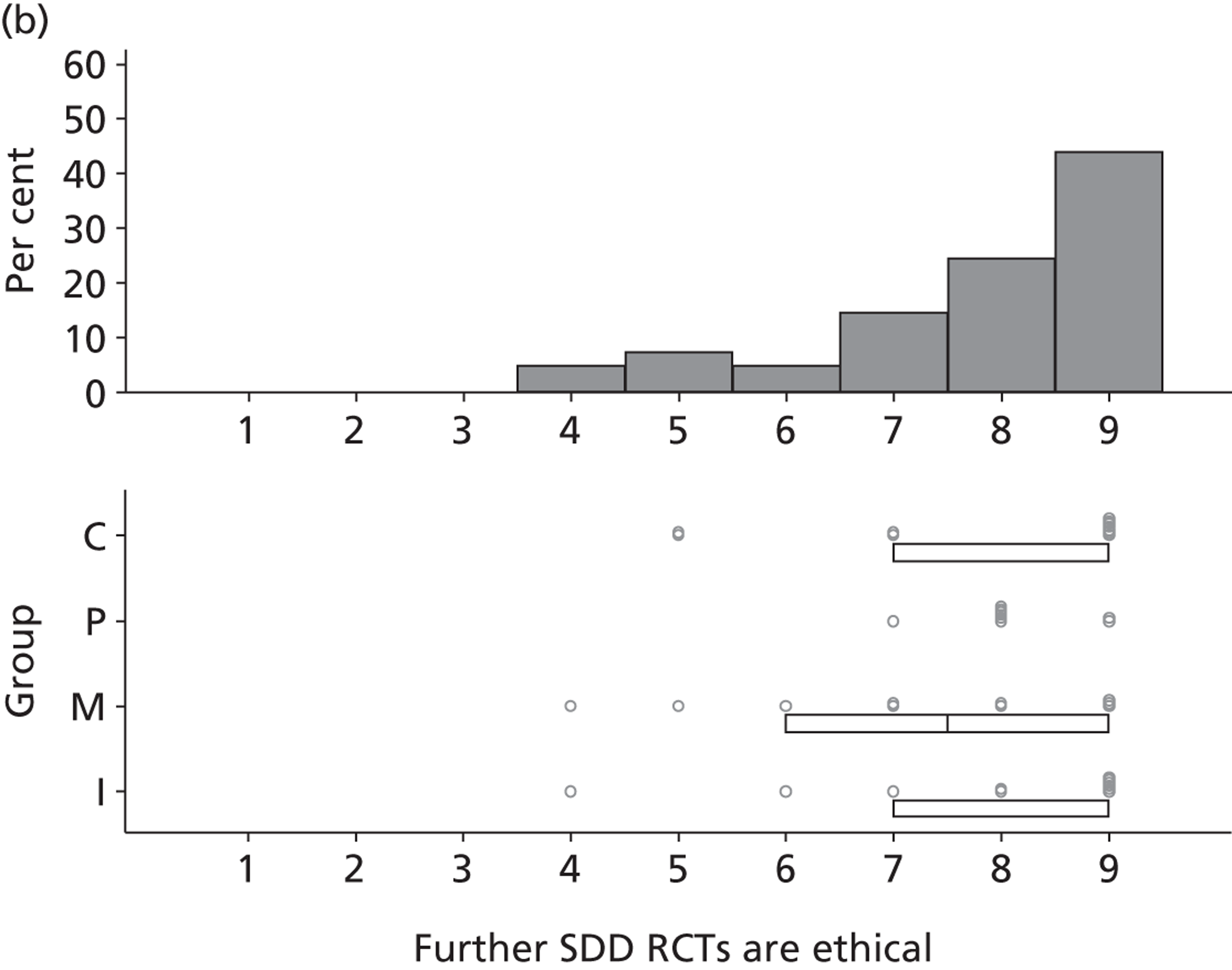
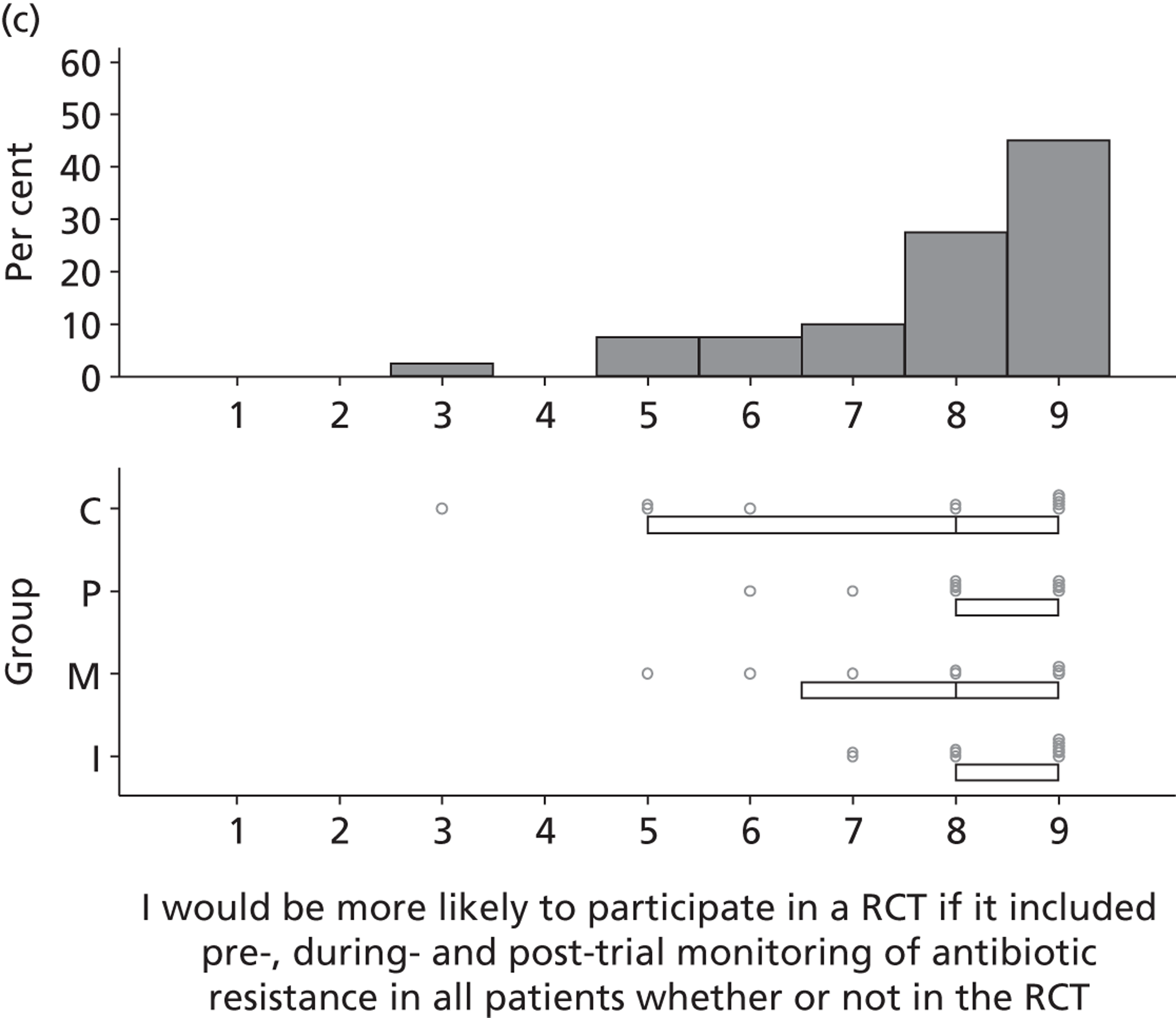
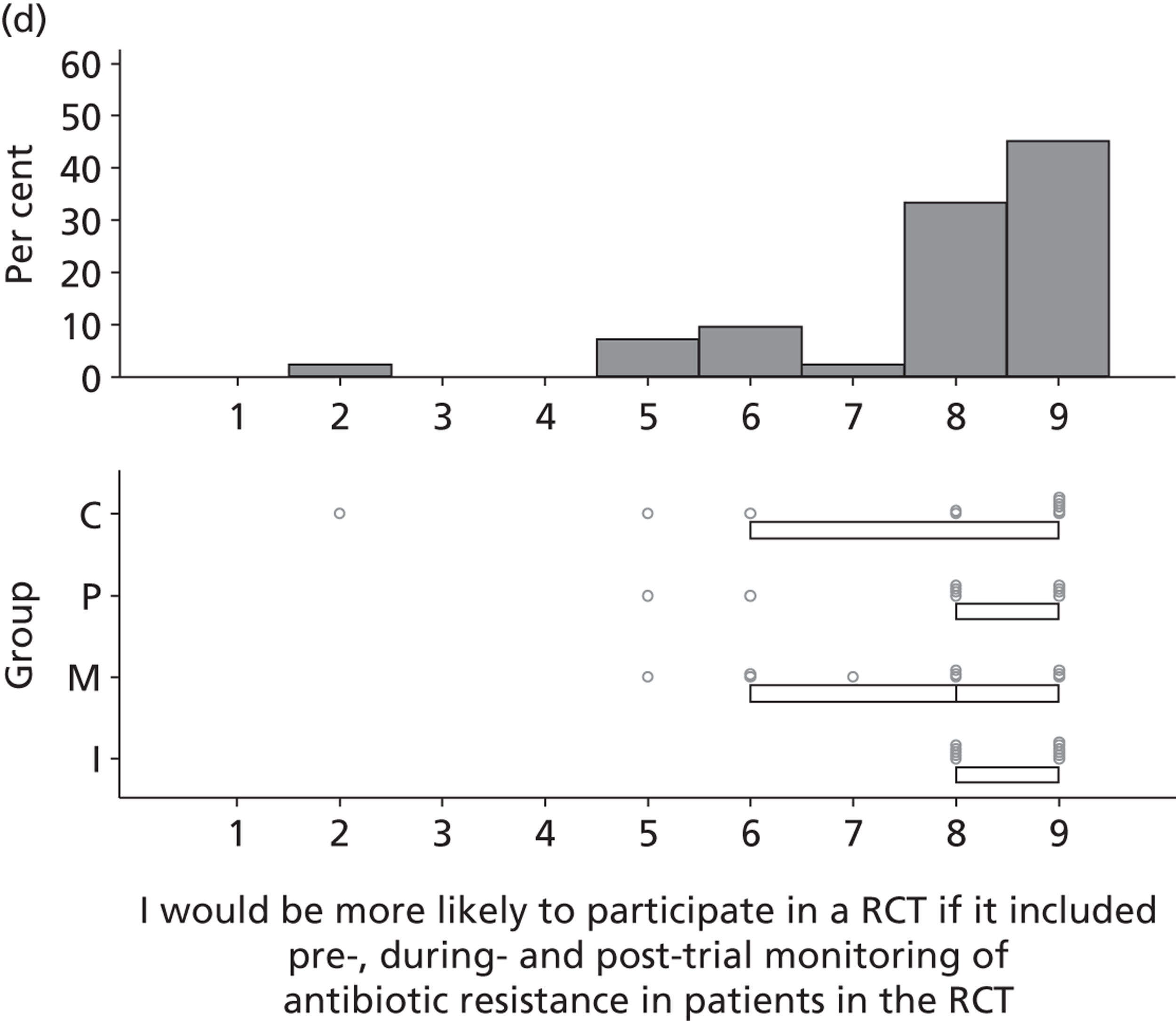
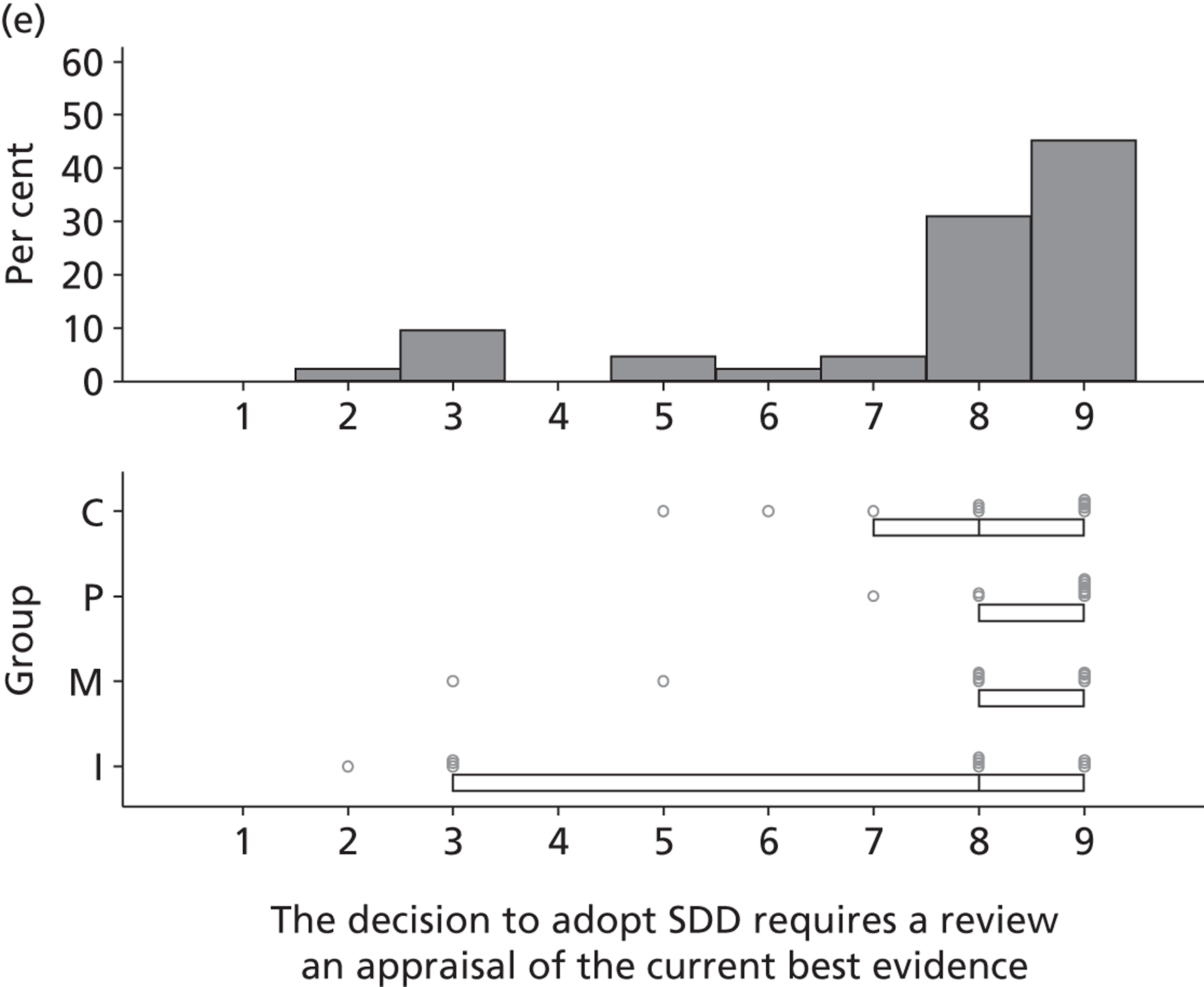
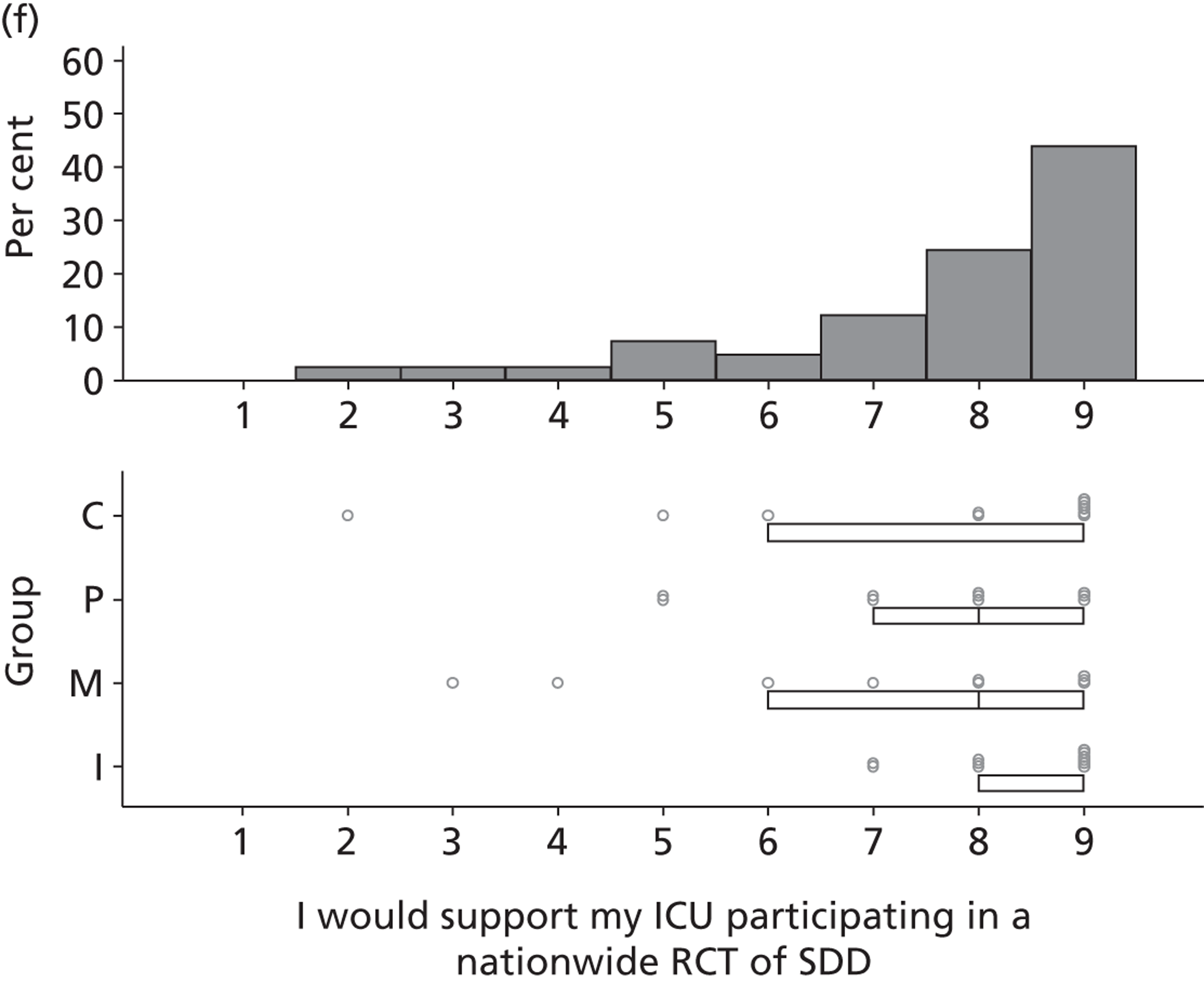
FIGURE 20.
75–90% consensus around mid-value of 7.
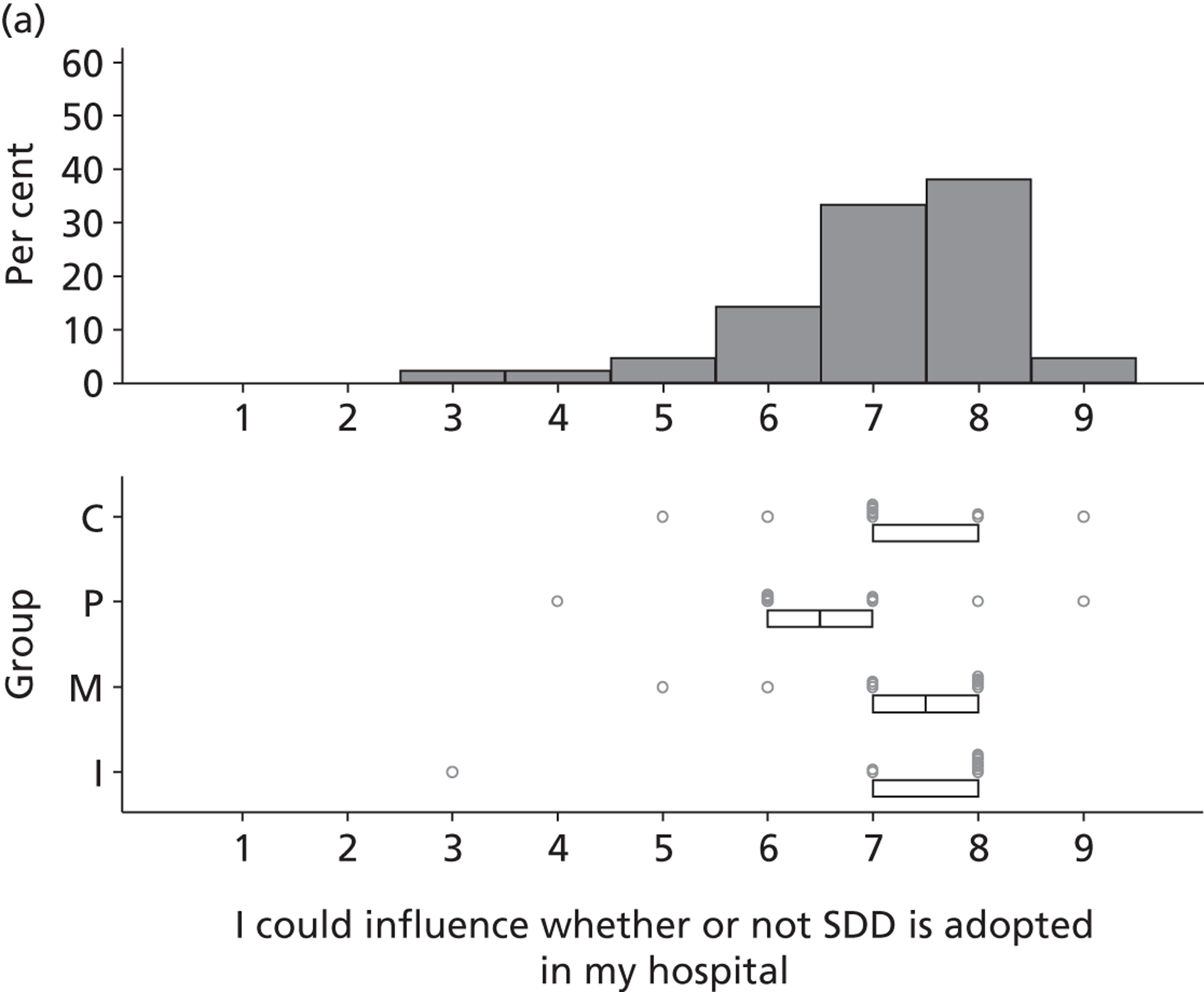
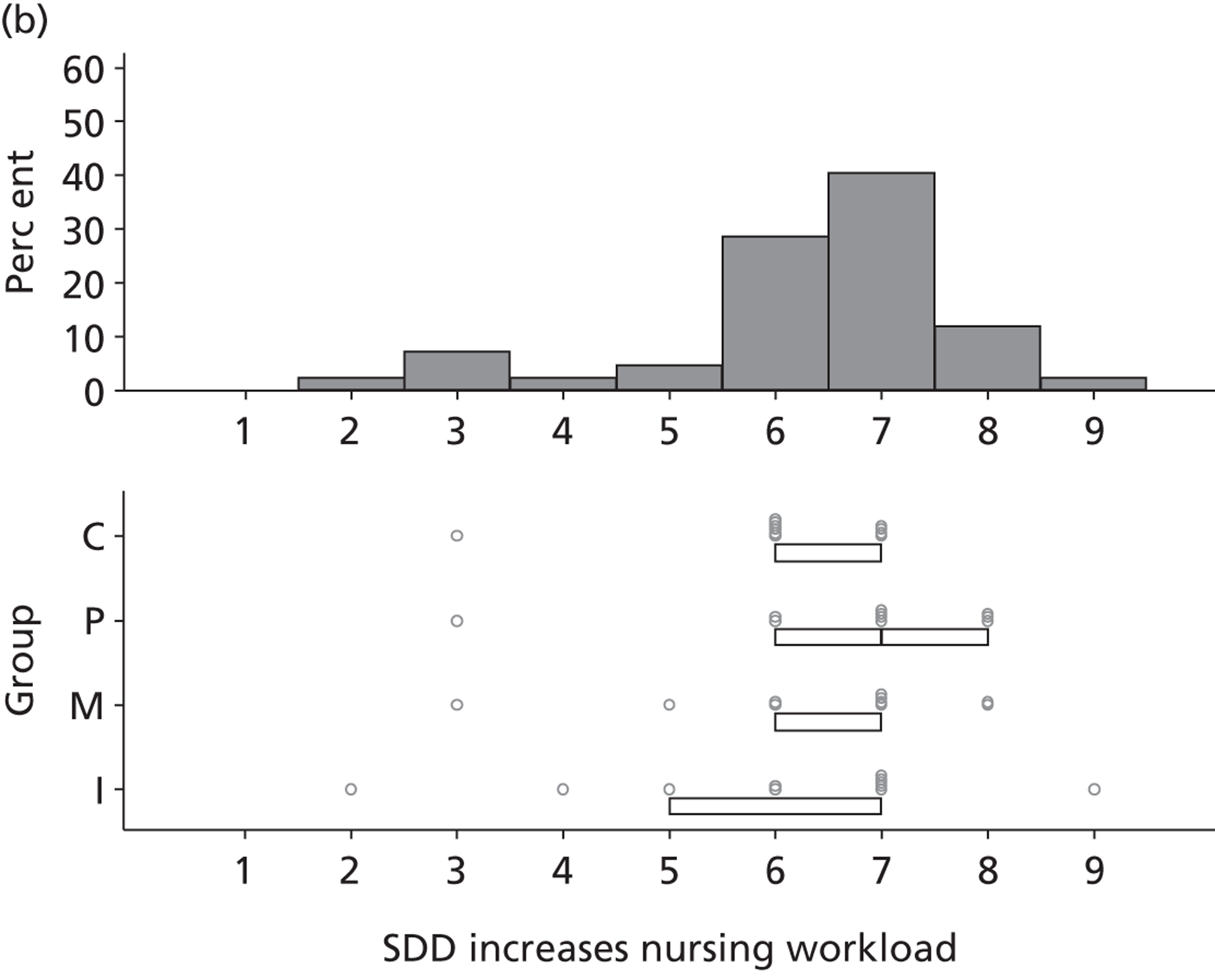
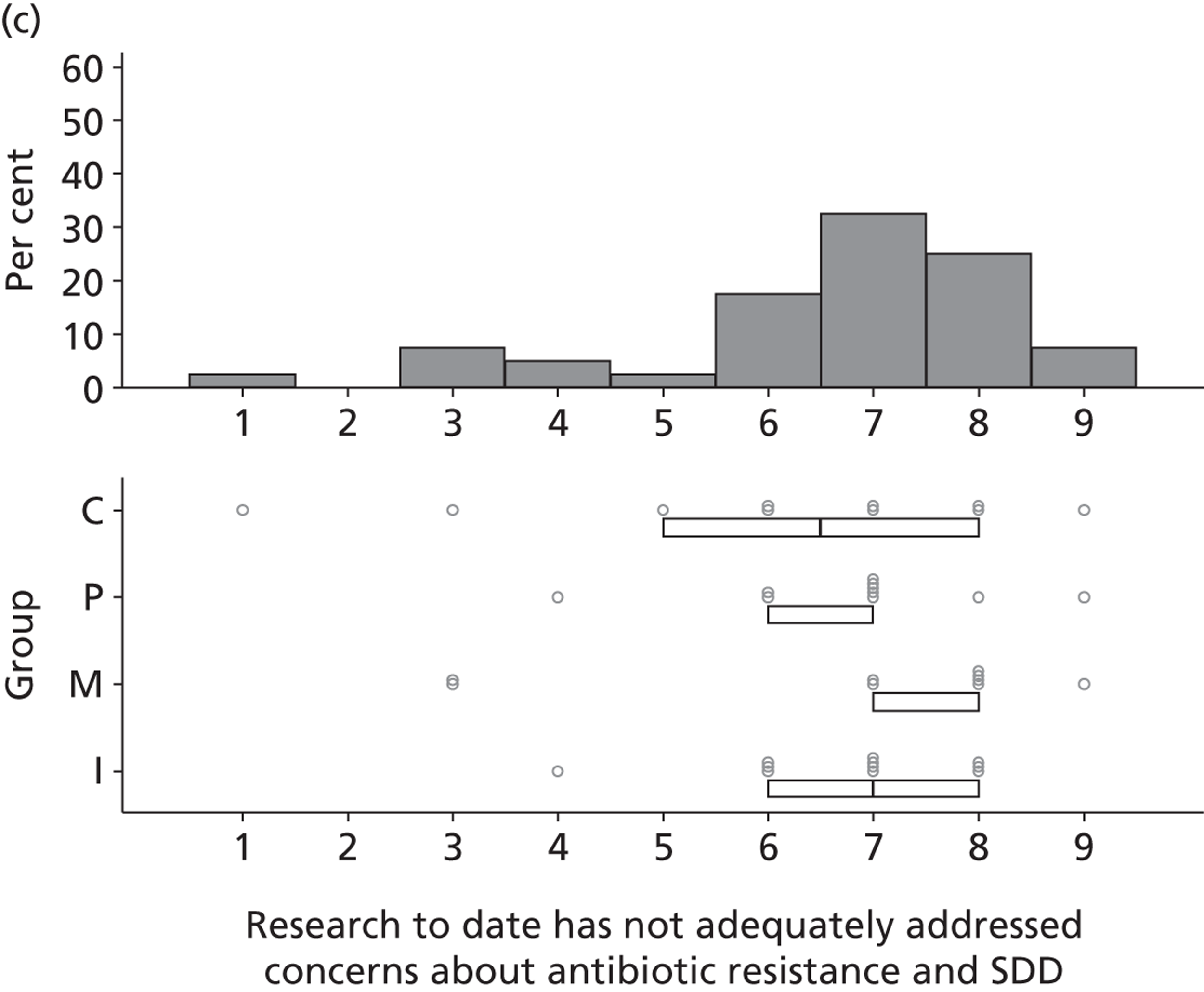
FIGURE 21.
75–90% consensus around mid-value of 6.
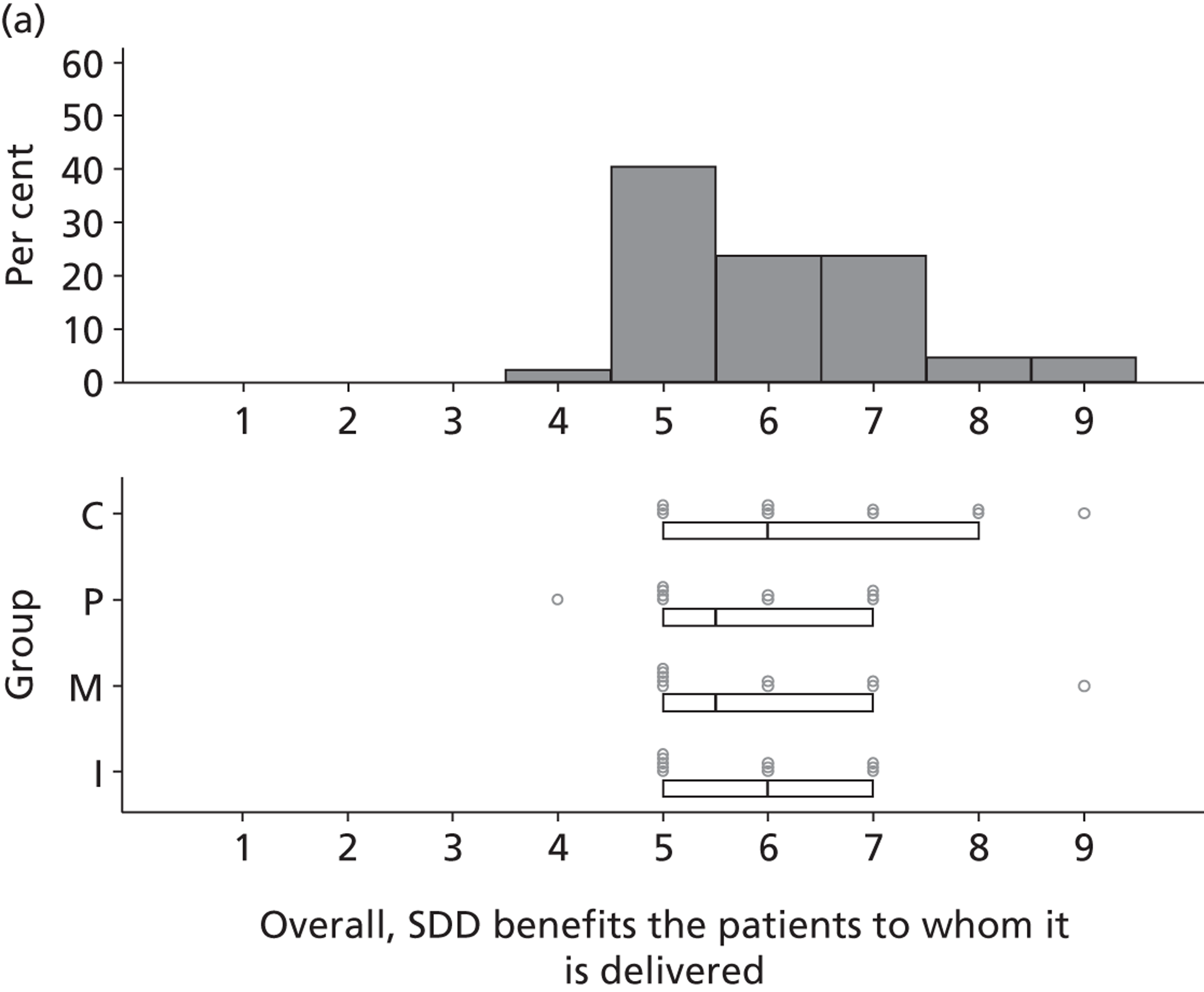
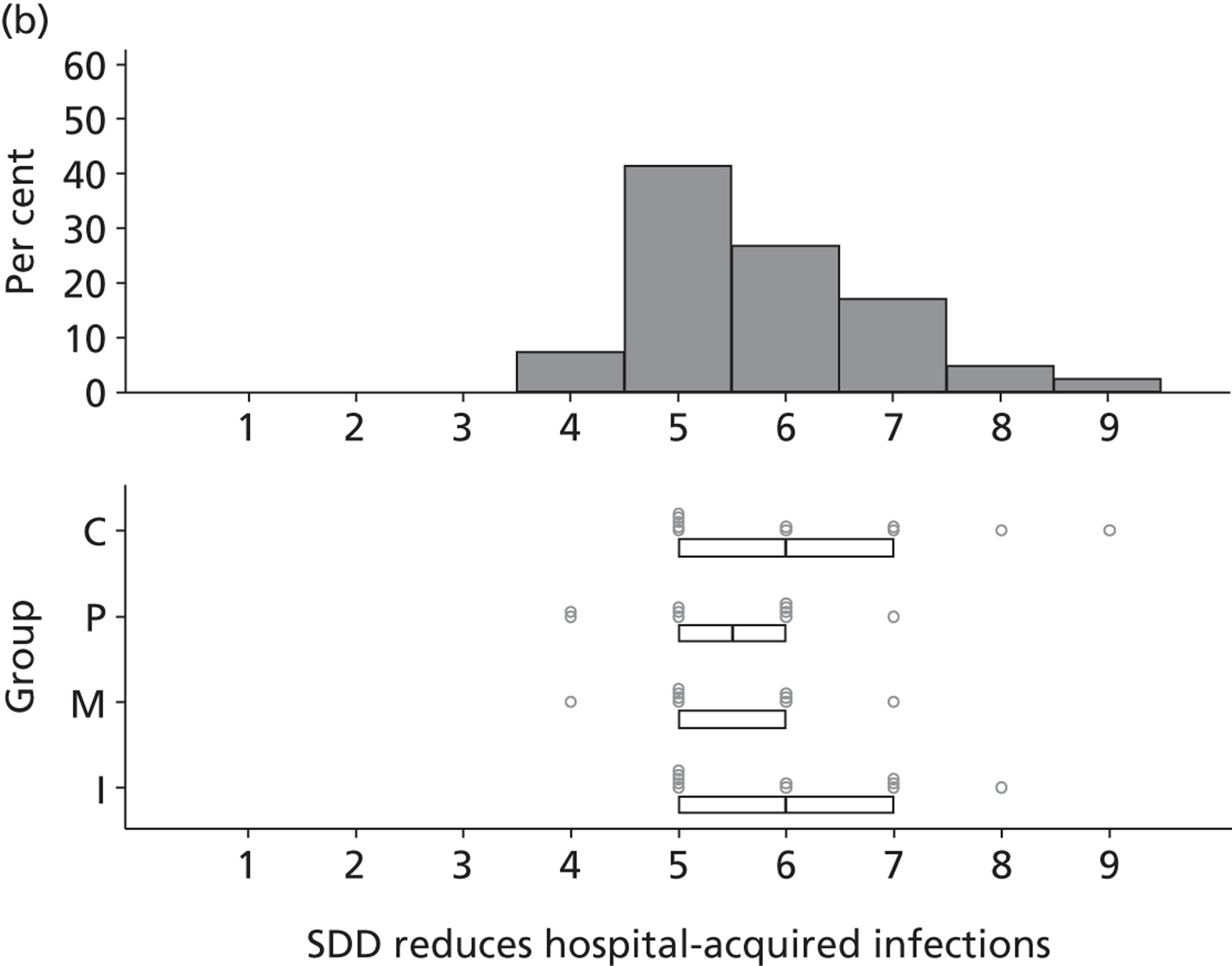
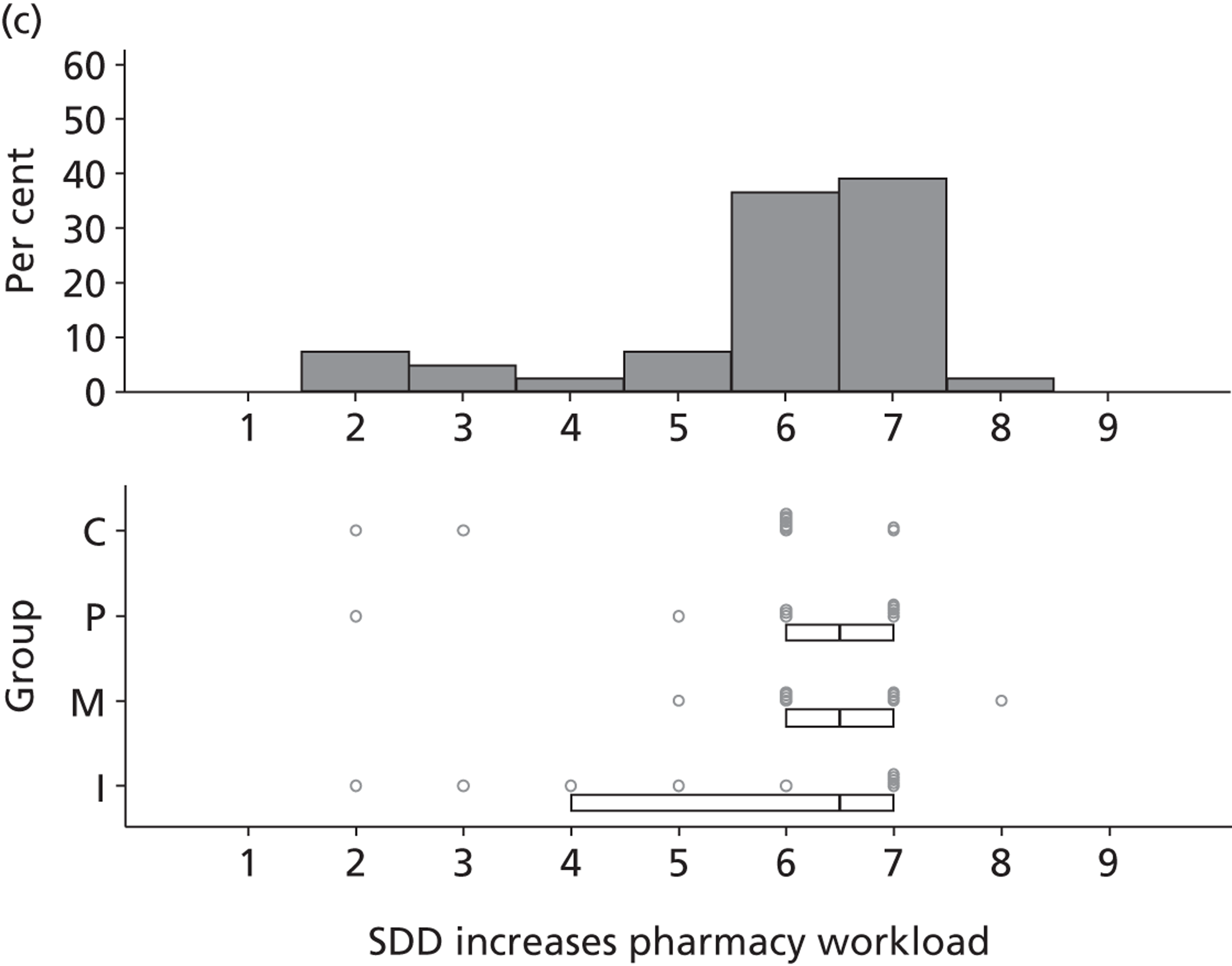
FIGURE 22.
75–90% consensus around mid-value of 5.
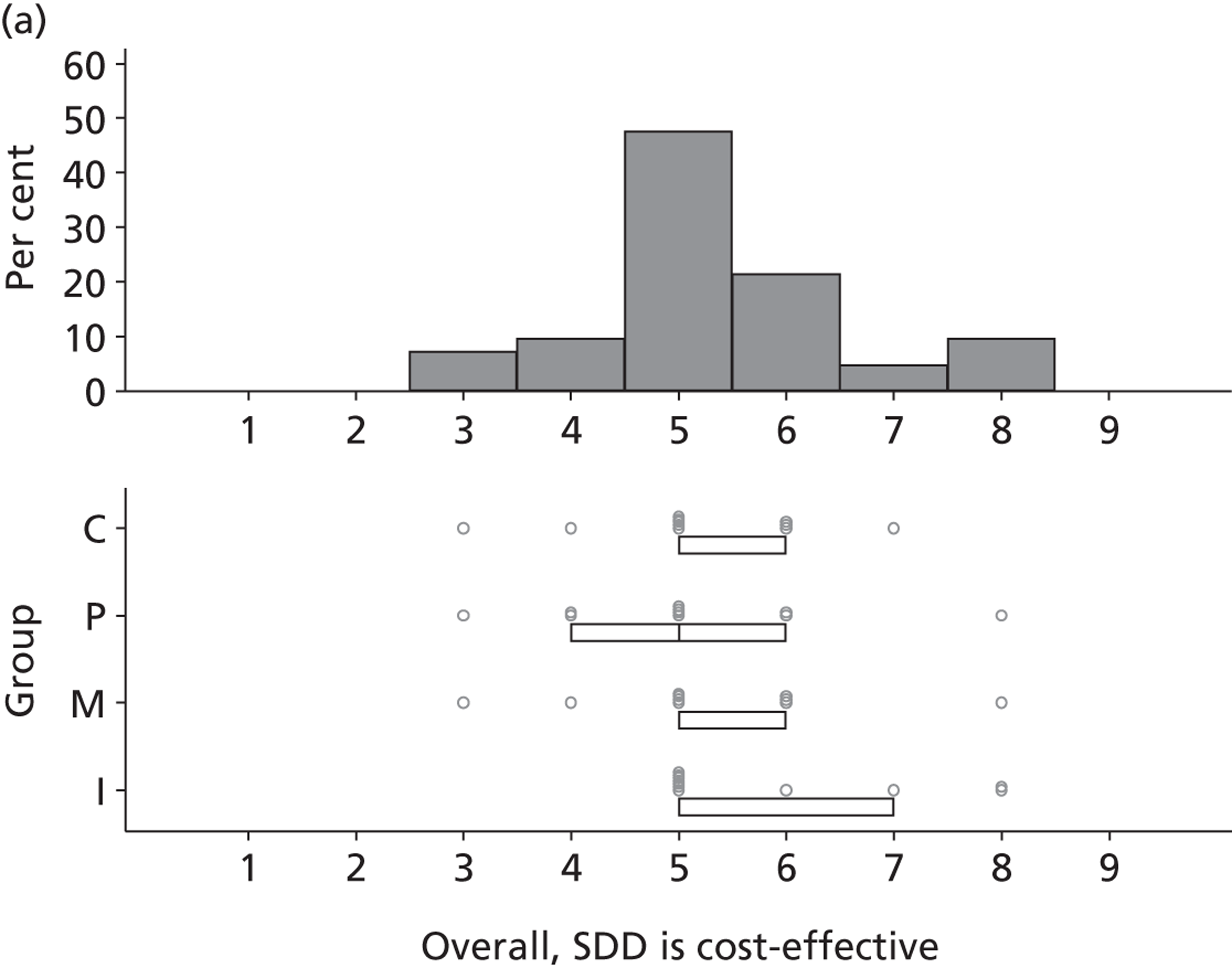
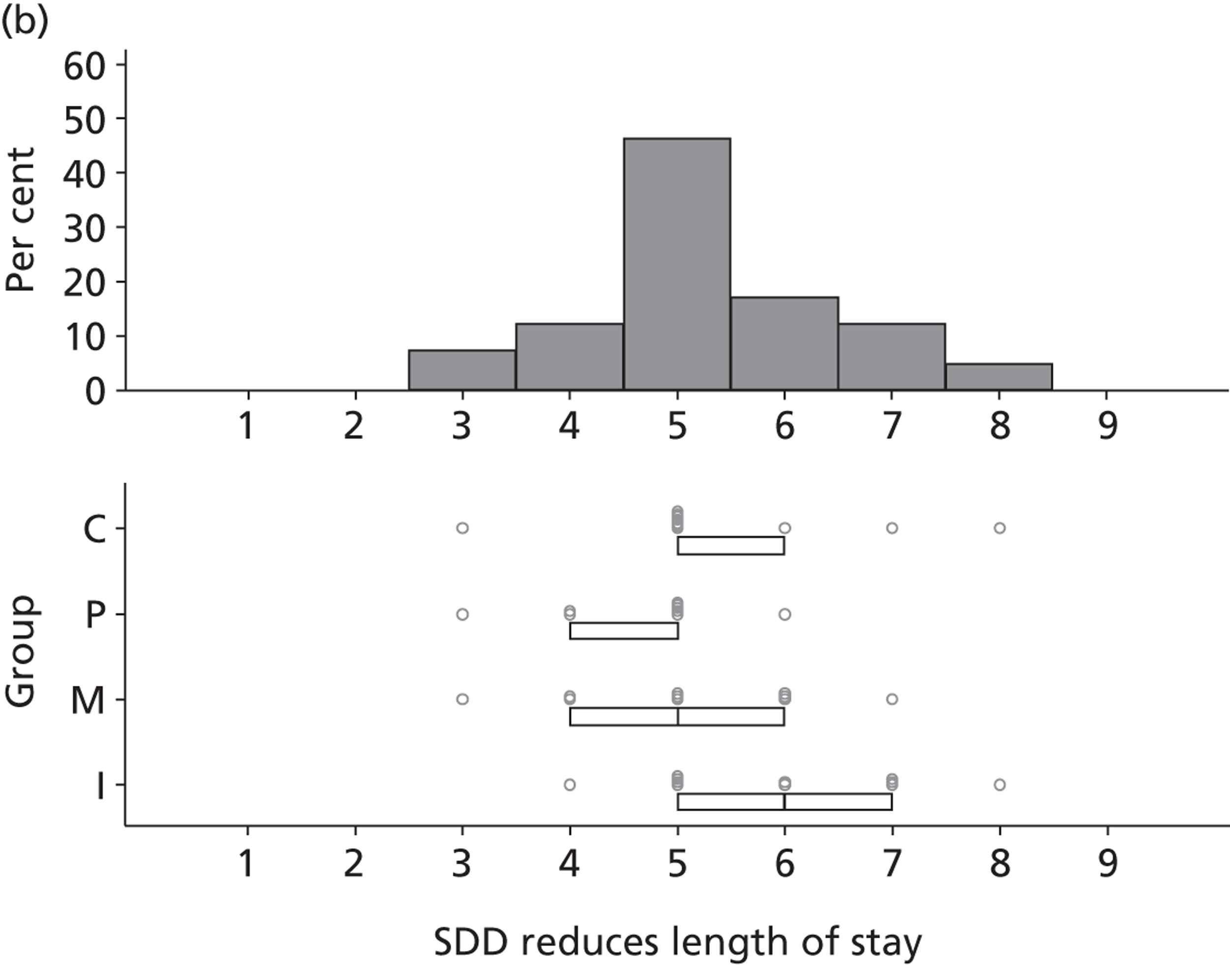
FIGURE 23.
75–90% consensus around mid-value of 4.
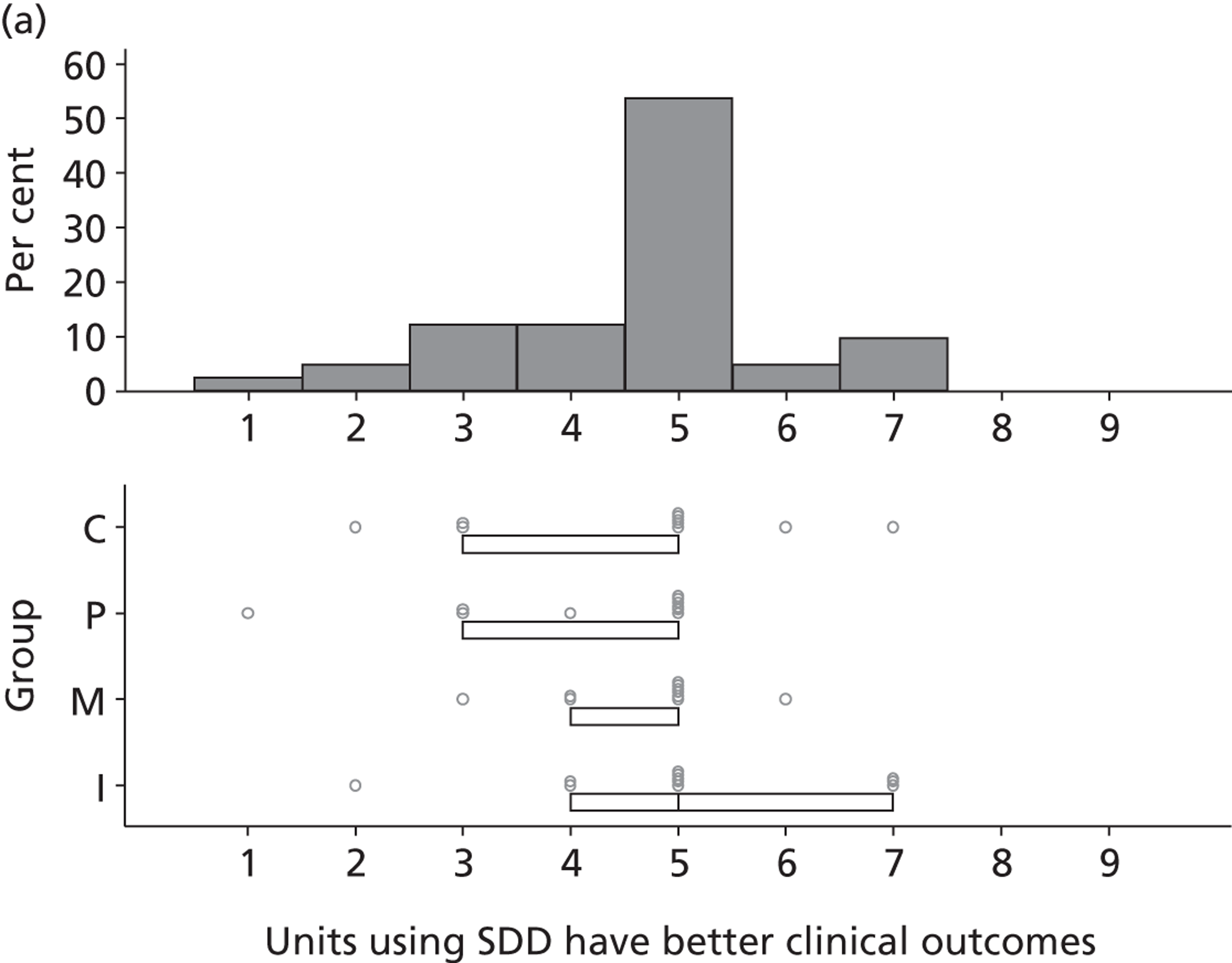
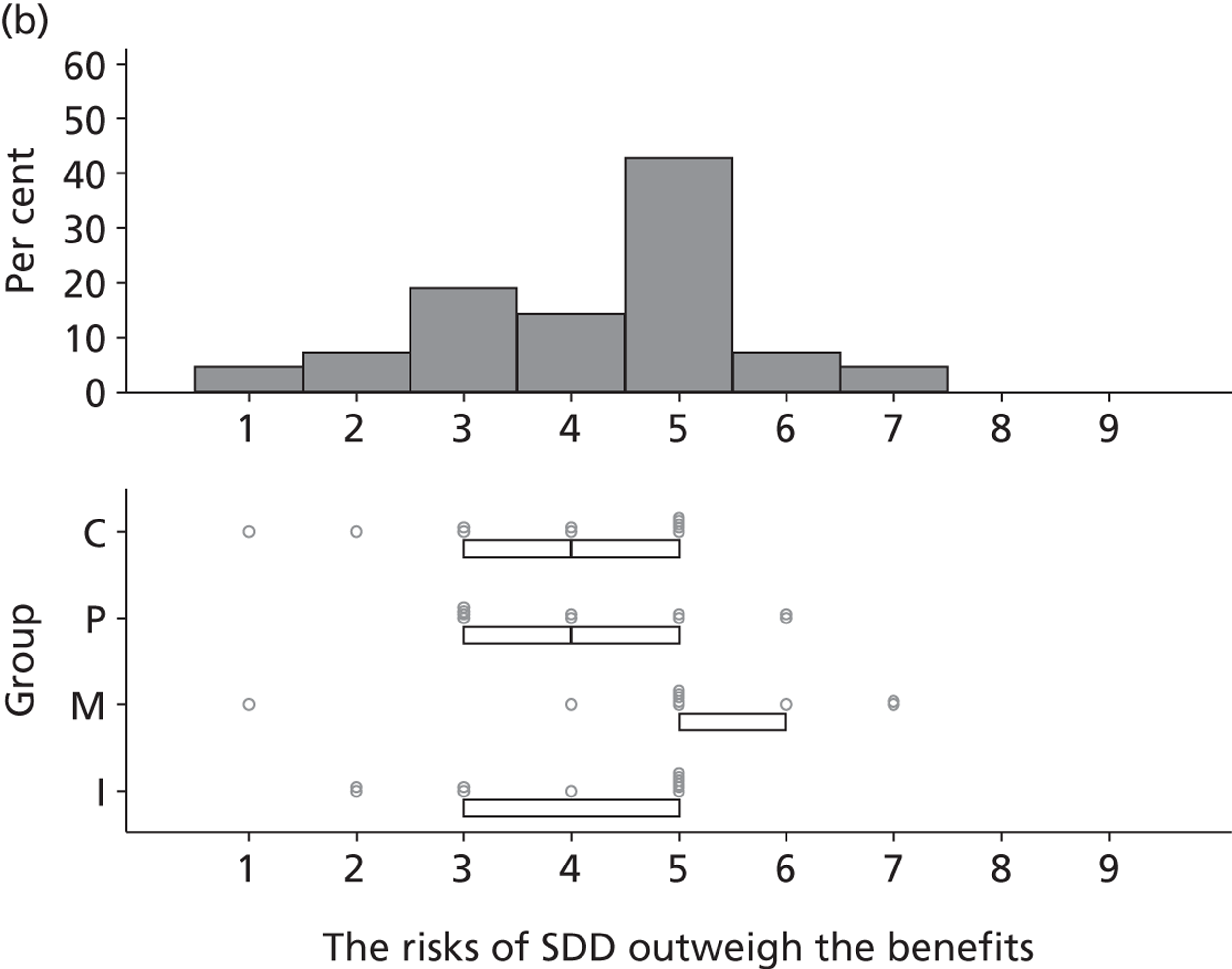
FIGURE 24.
50–75% consensus around mid-value of 8.
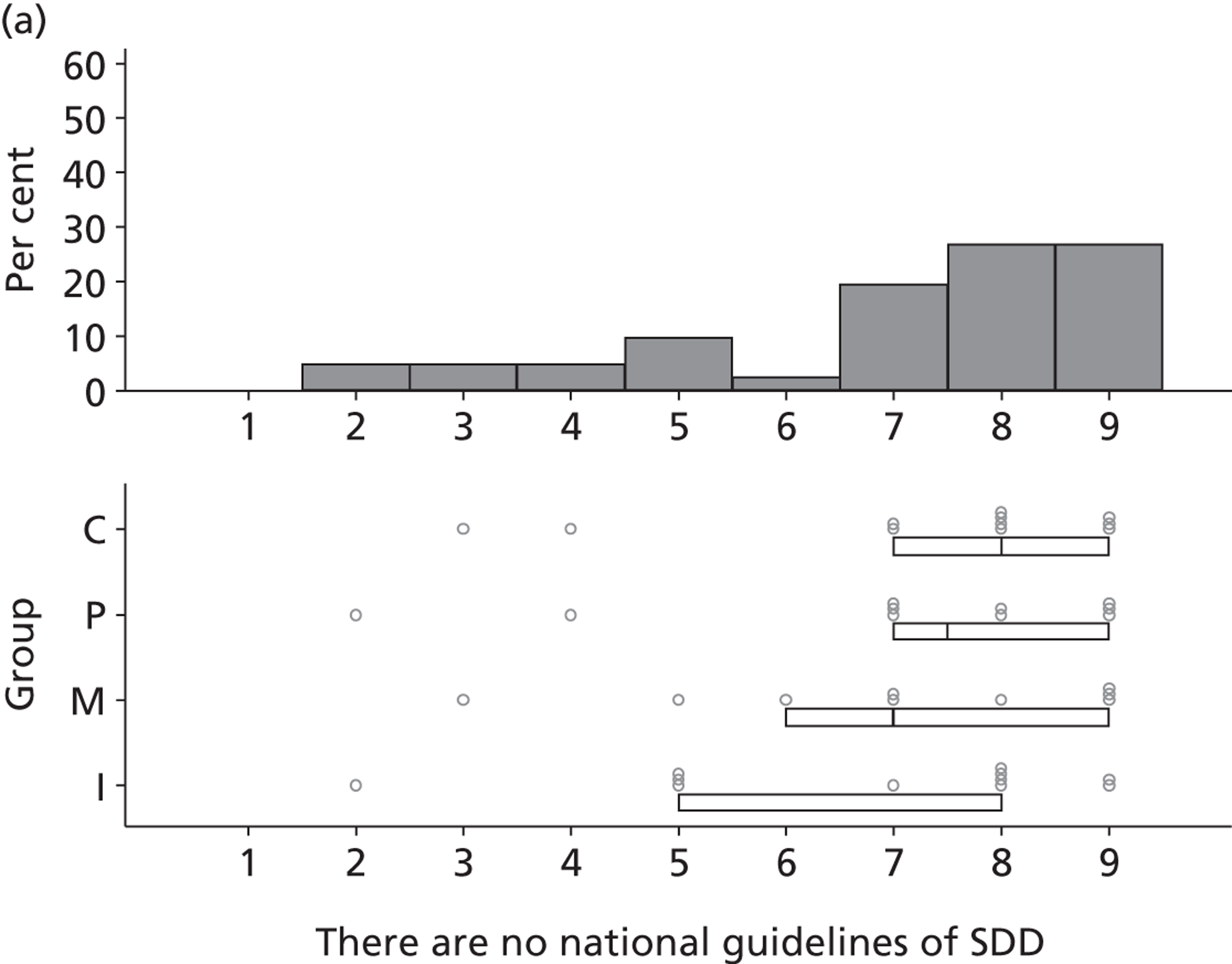
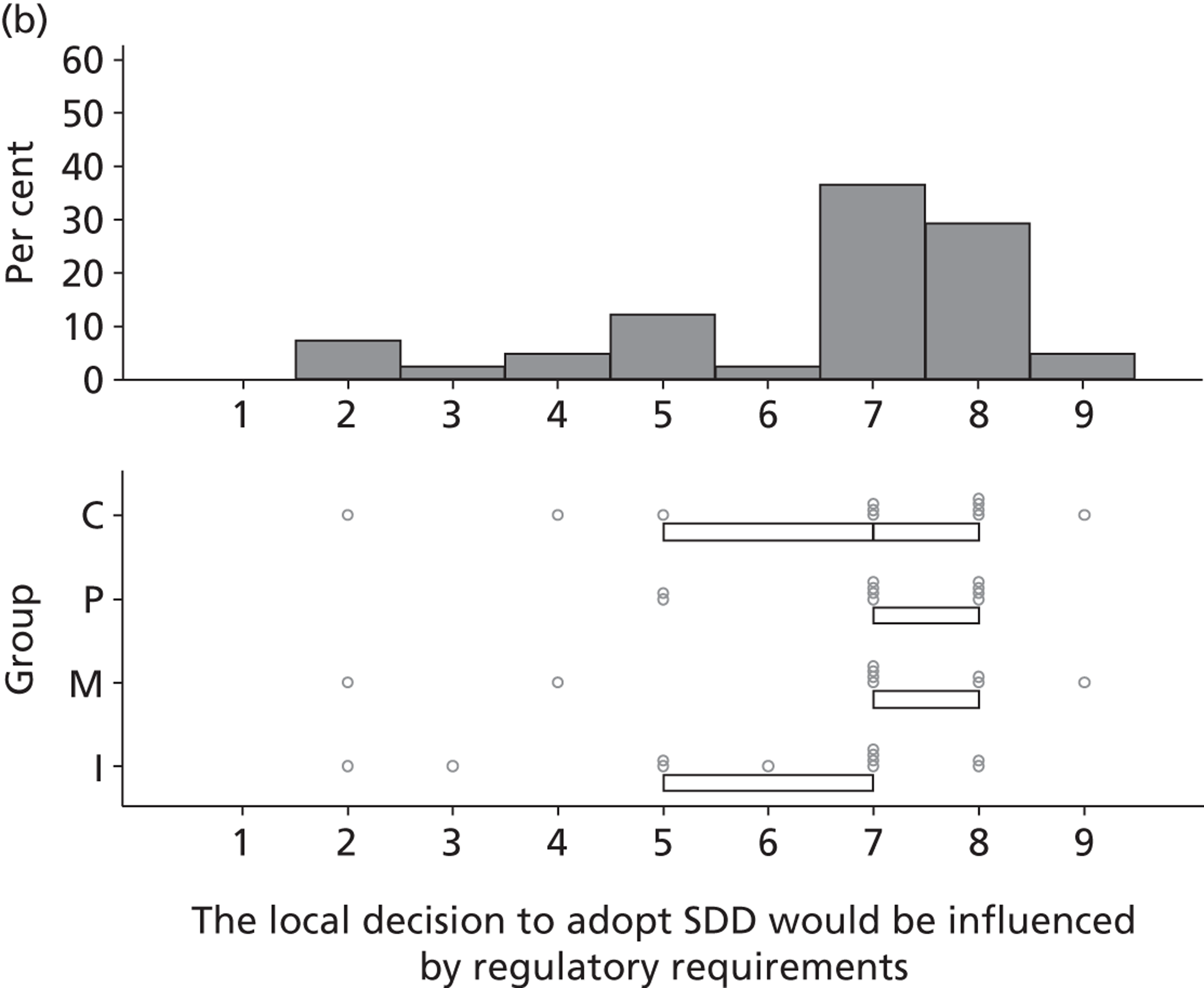
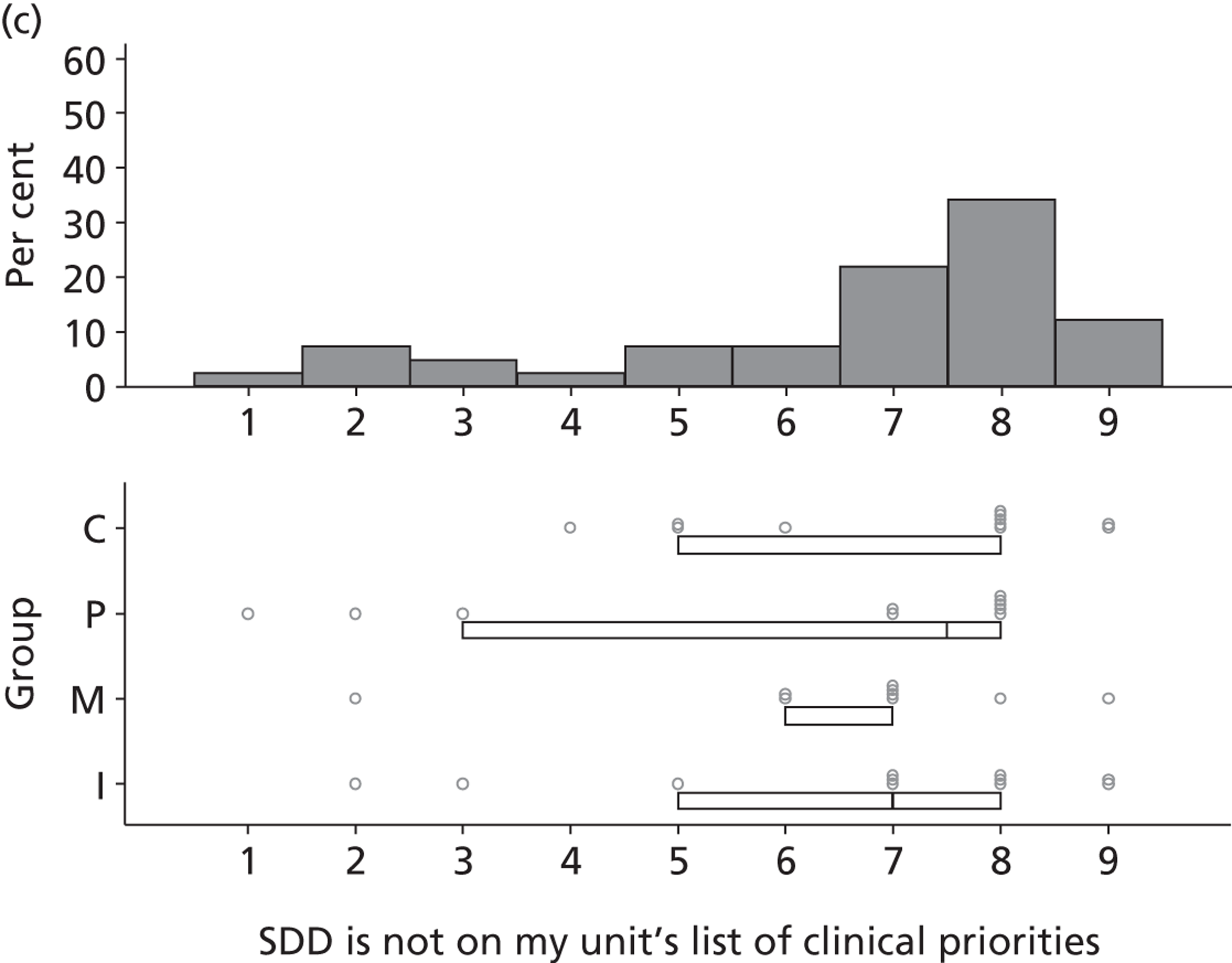
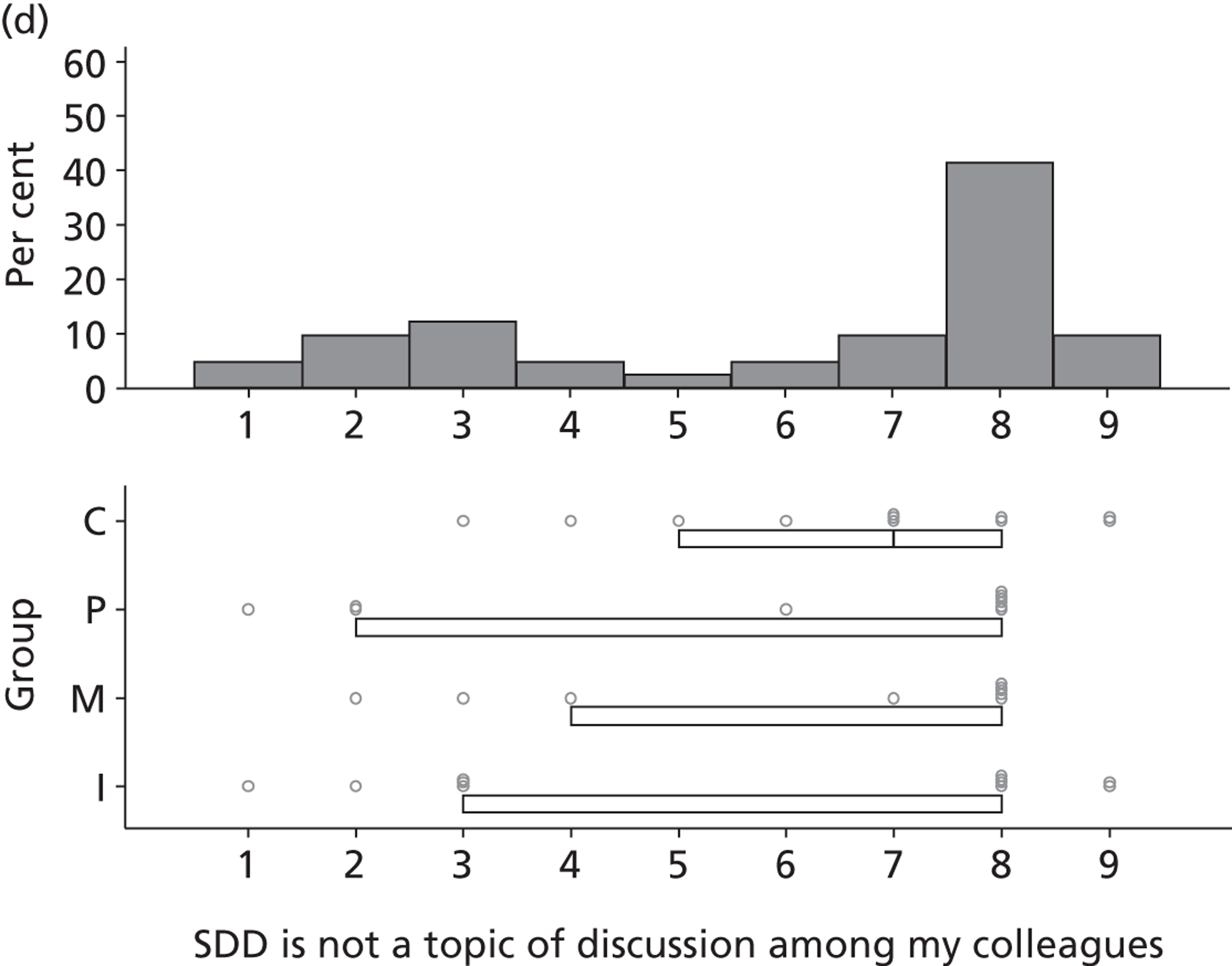
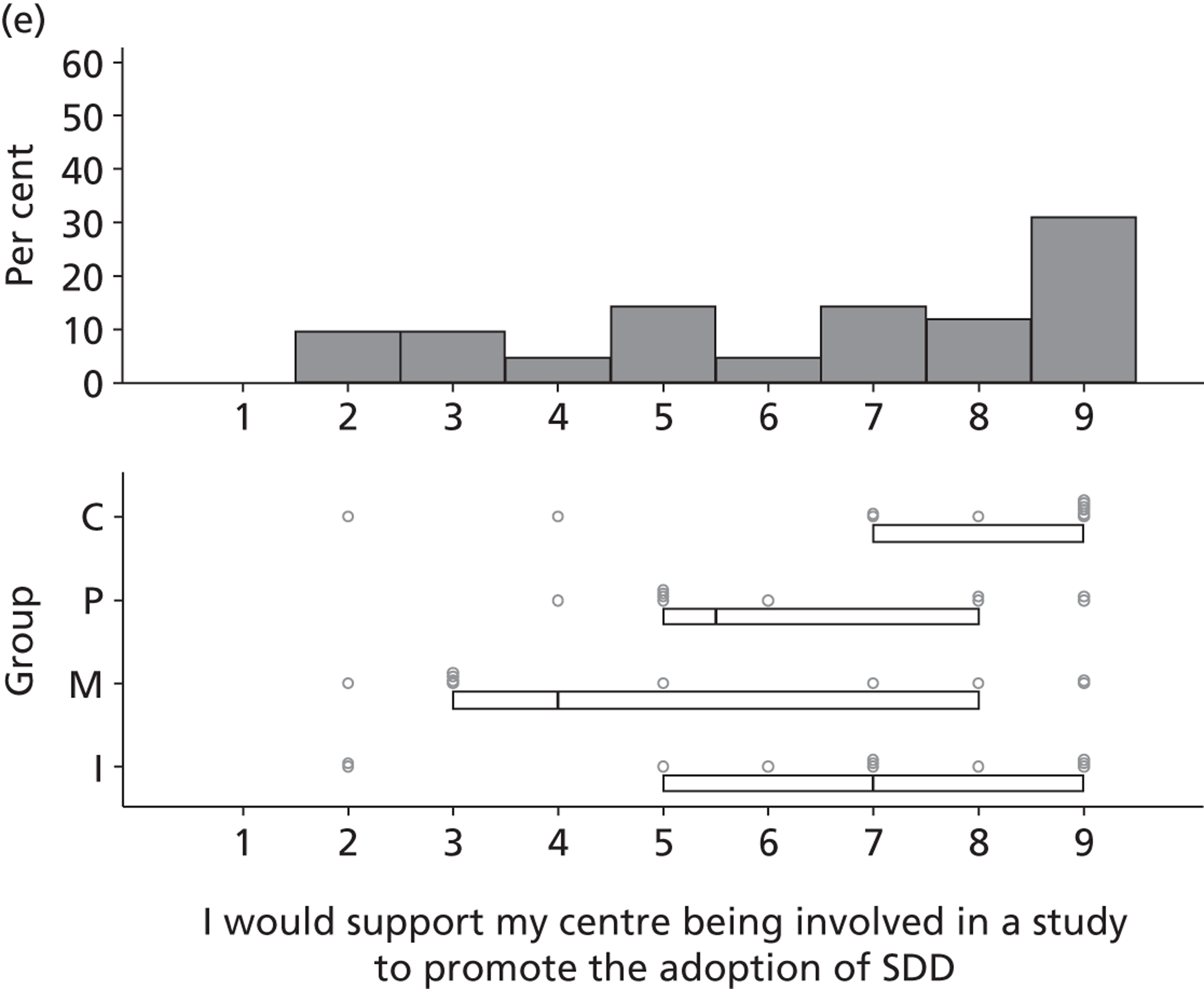
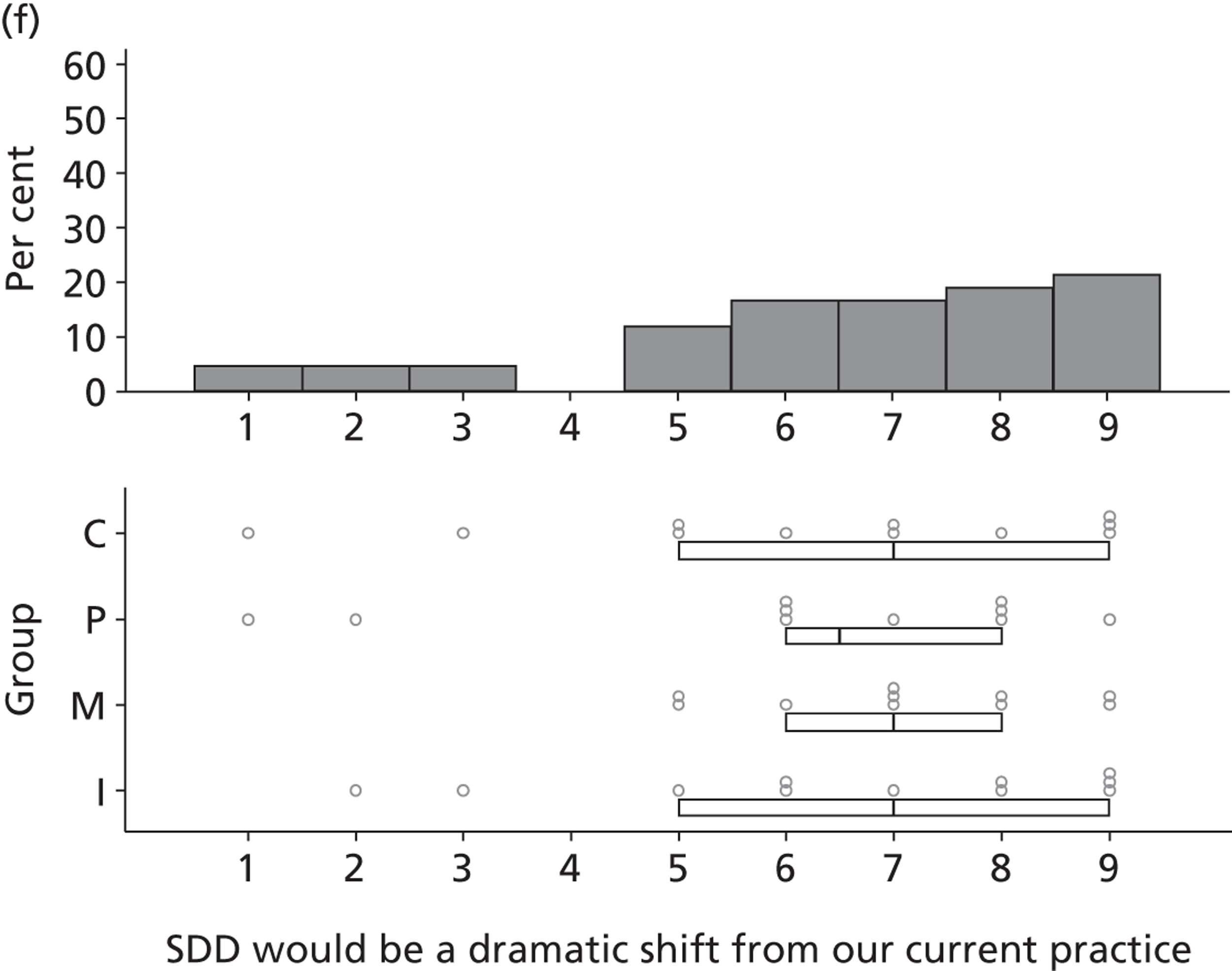
FIGURE 25.
50–75% consensus around mid-value of 7. ID, infectious diseases.
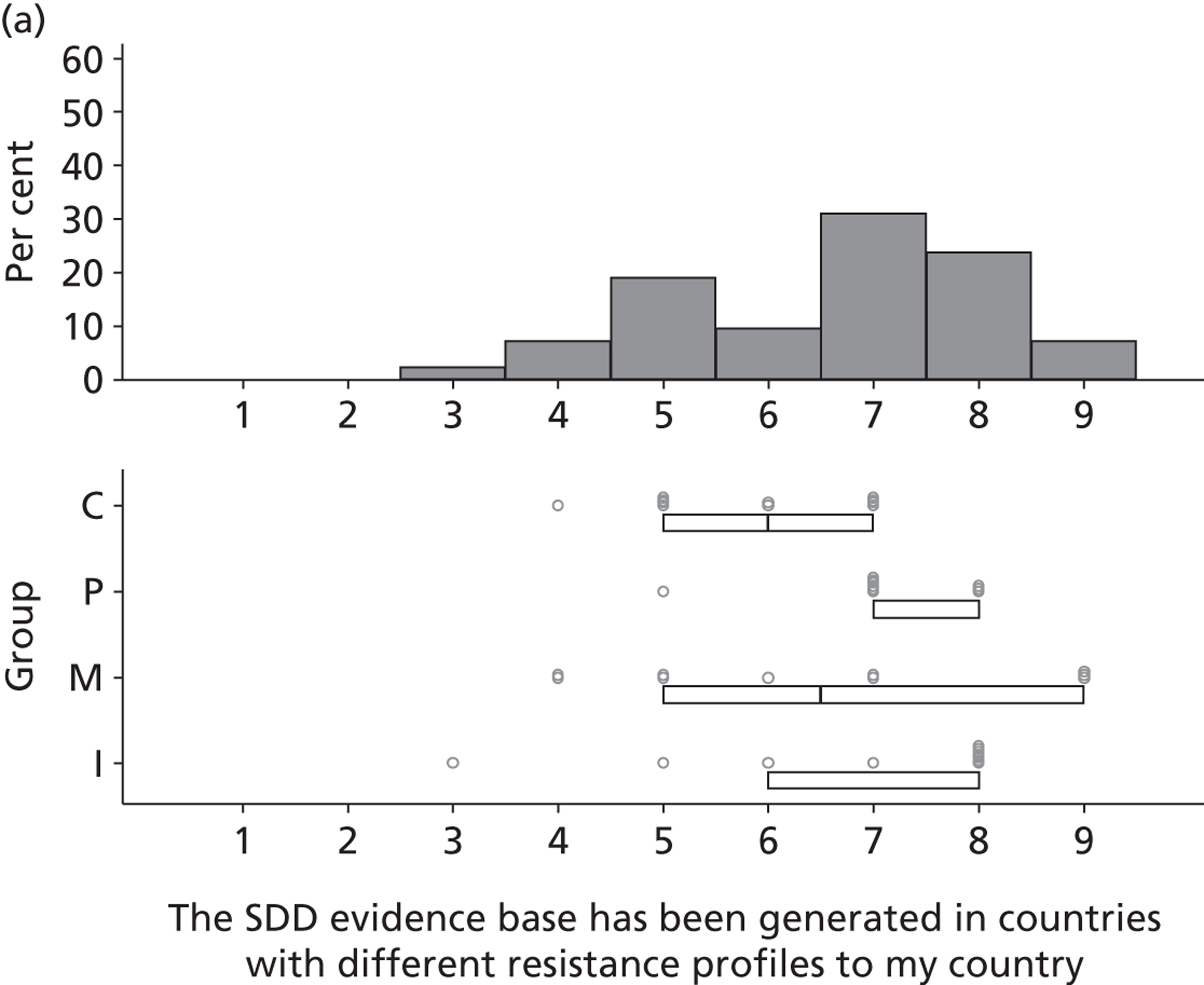
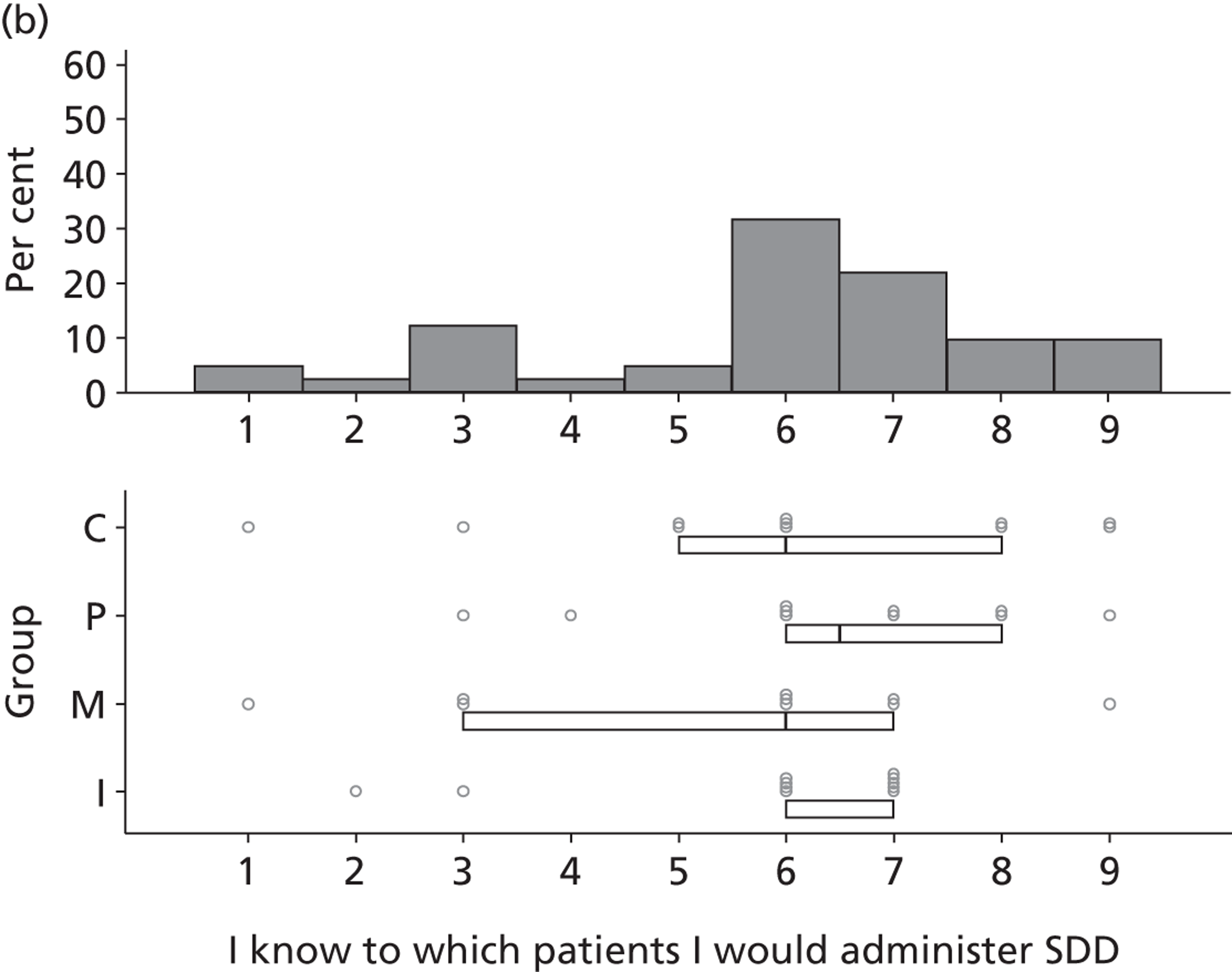
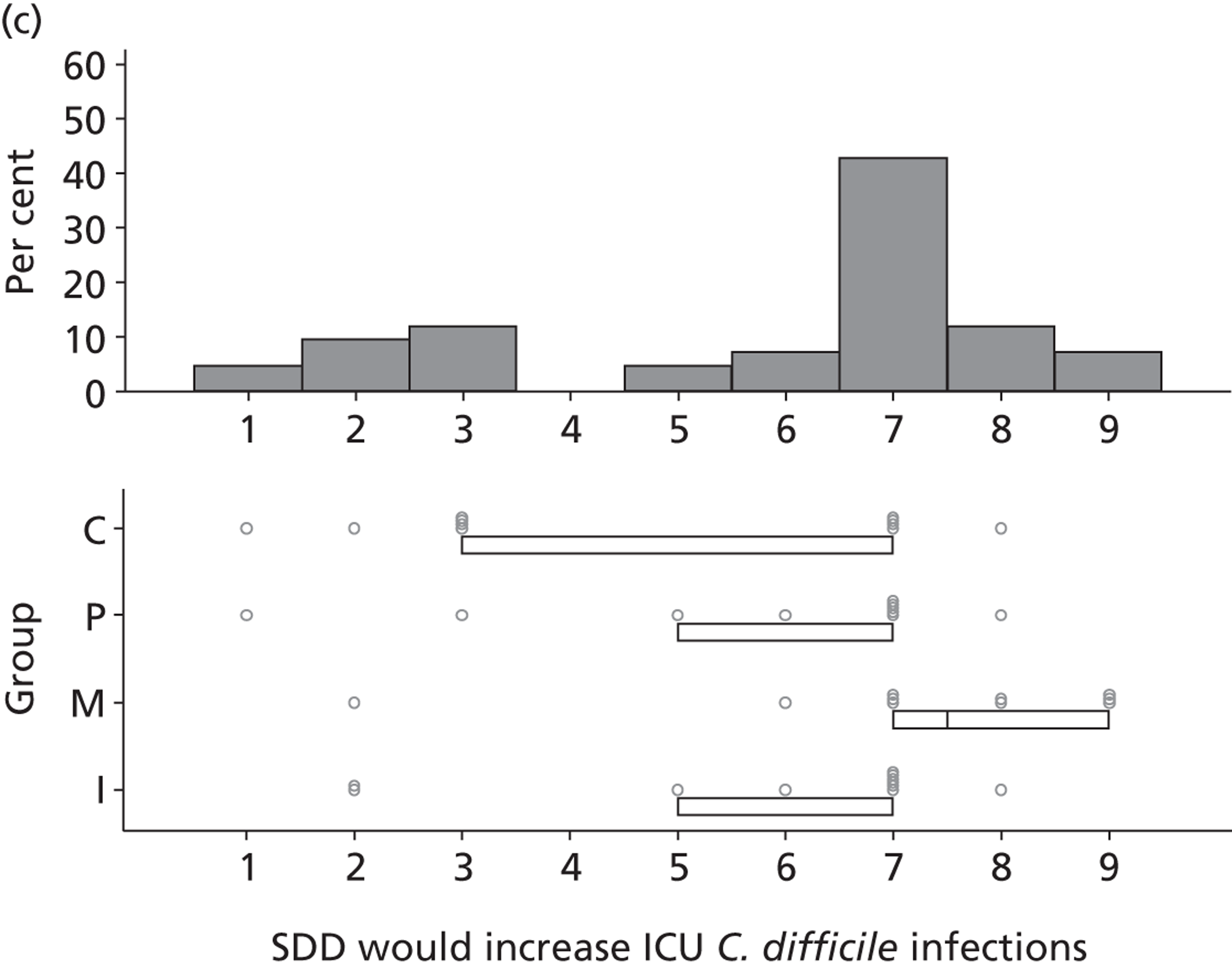
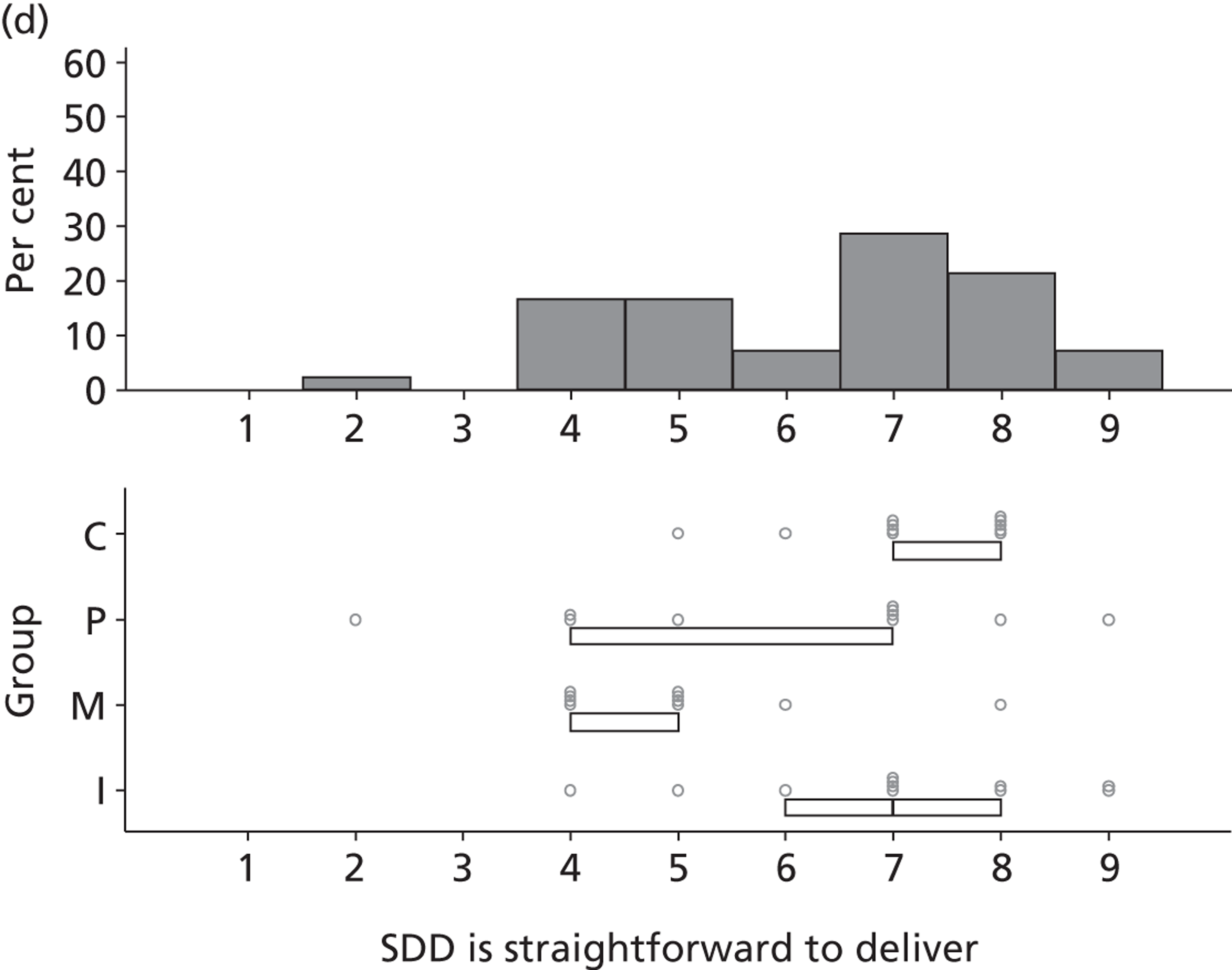
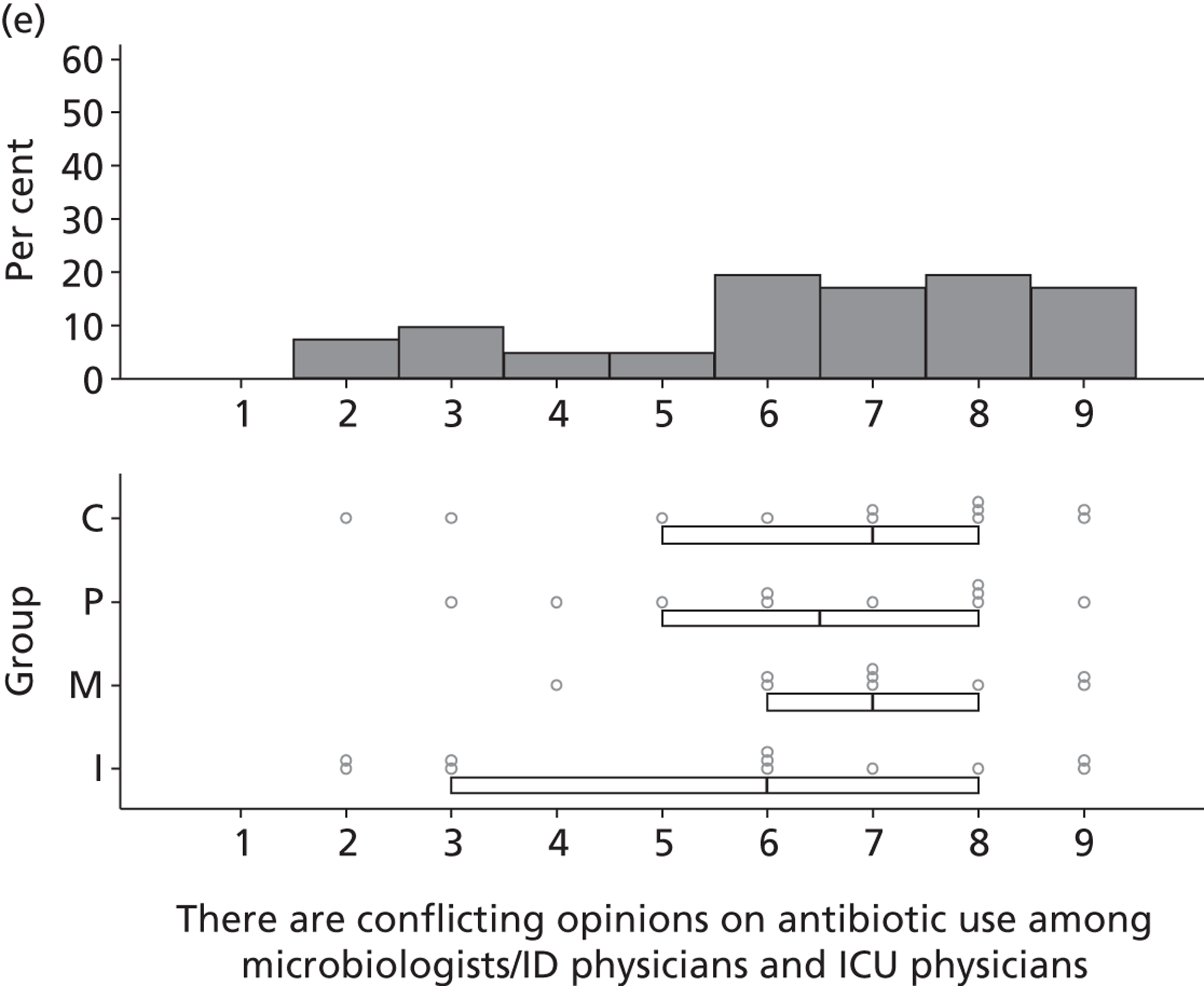
FIGURE 26.
50–75% consensus around mid-value of 6.
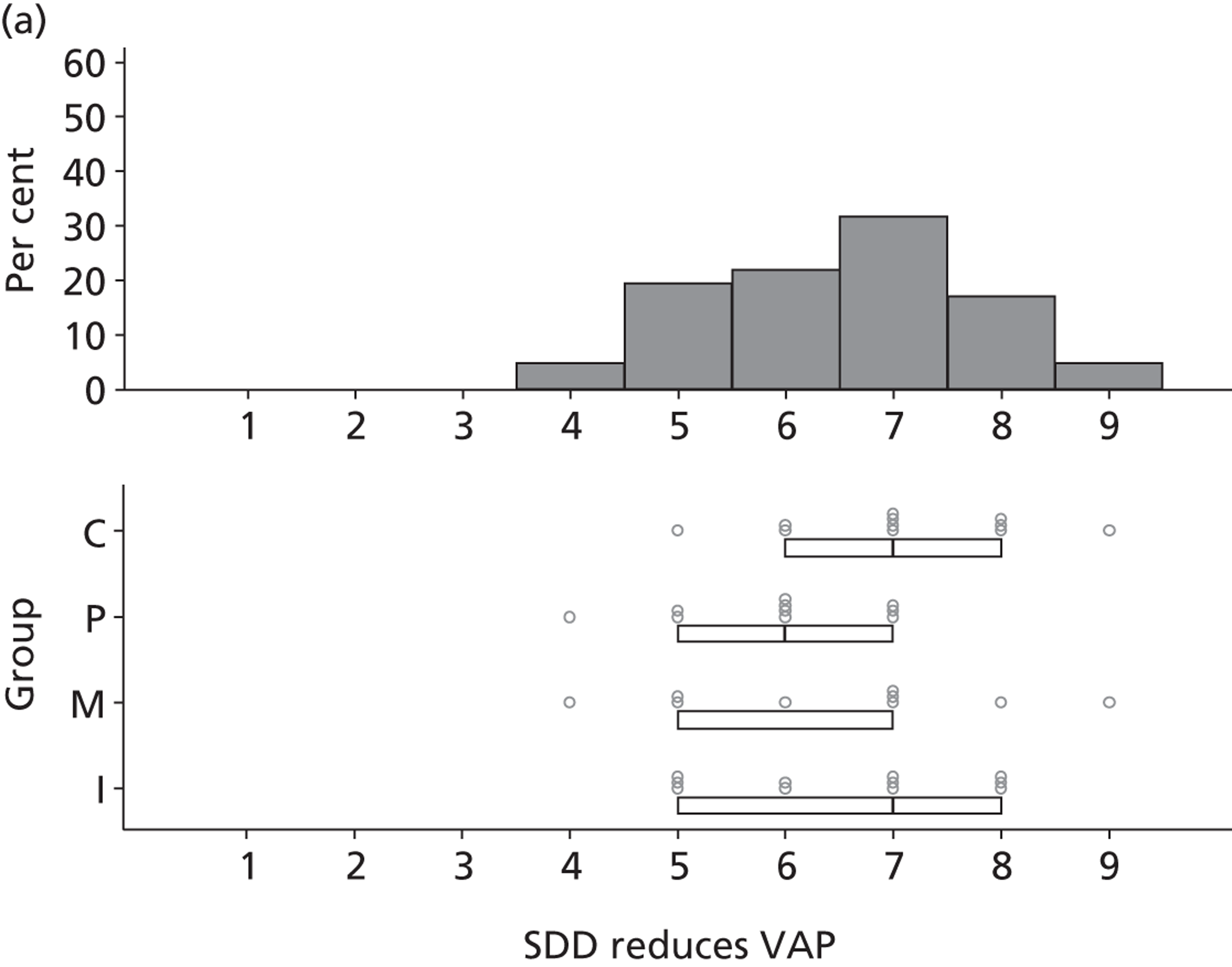
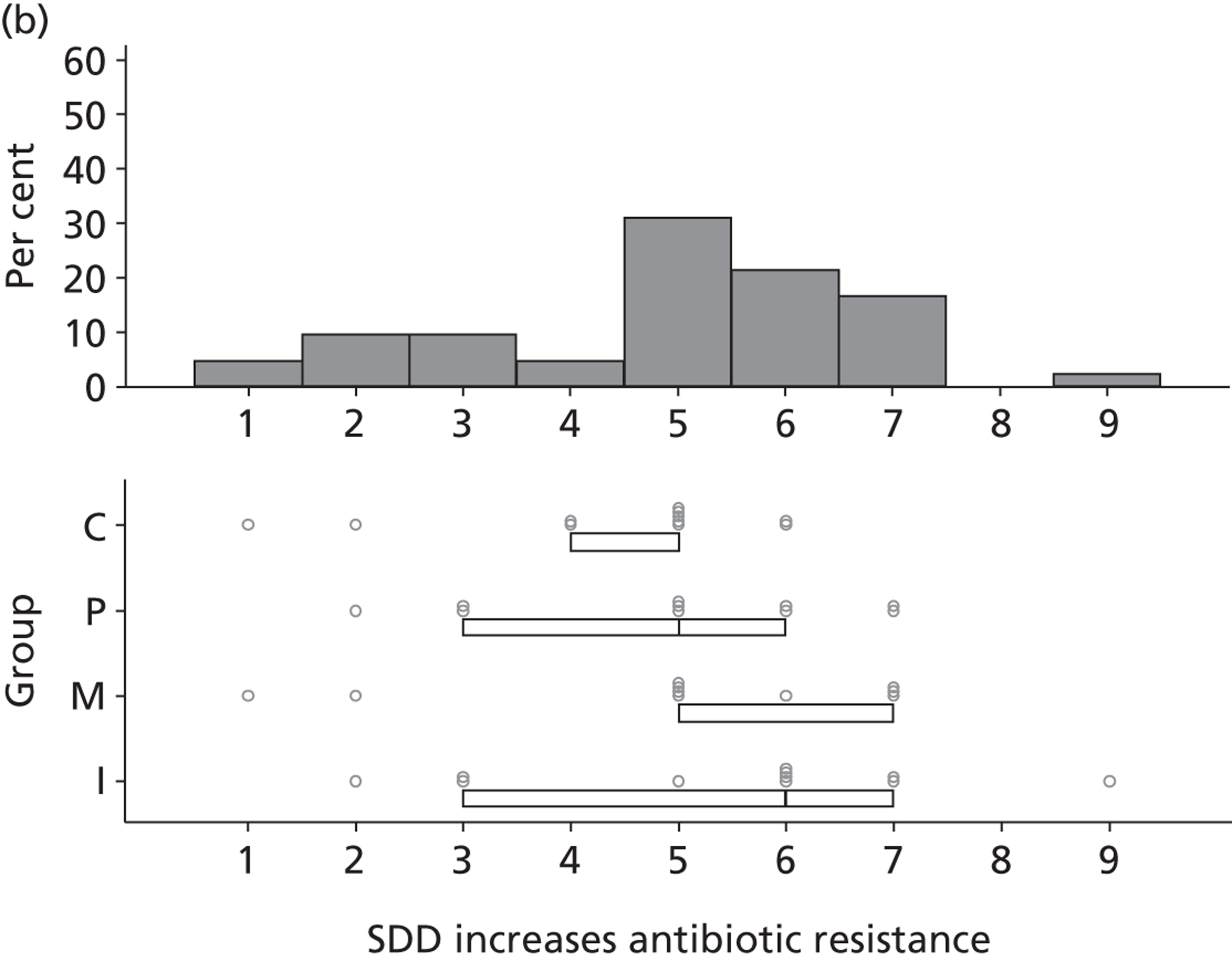
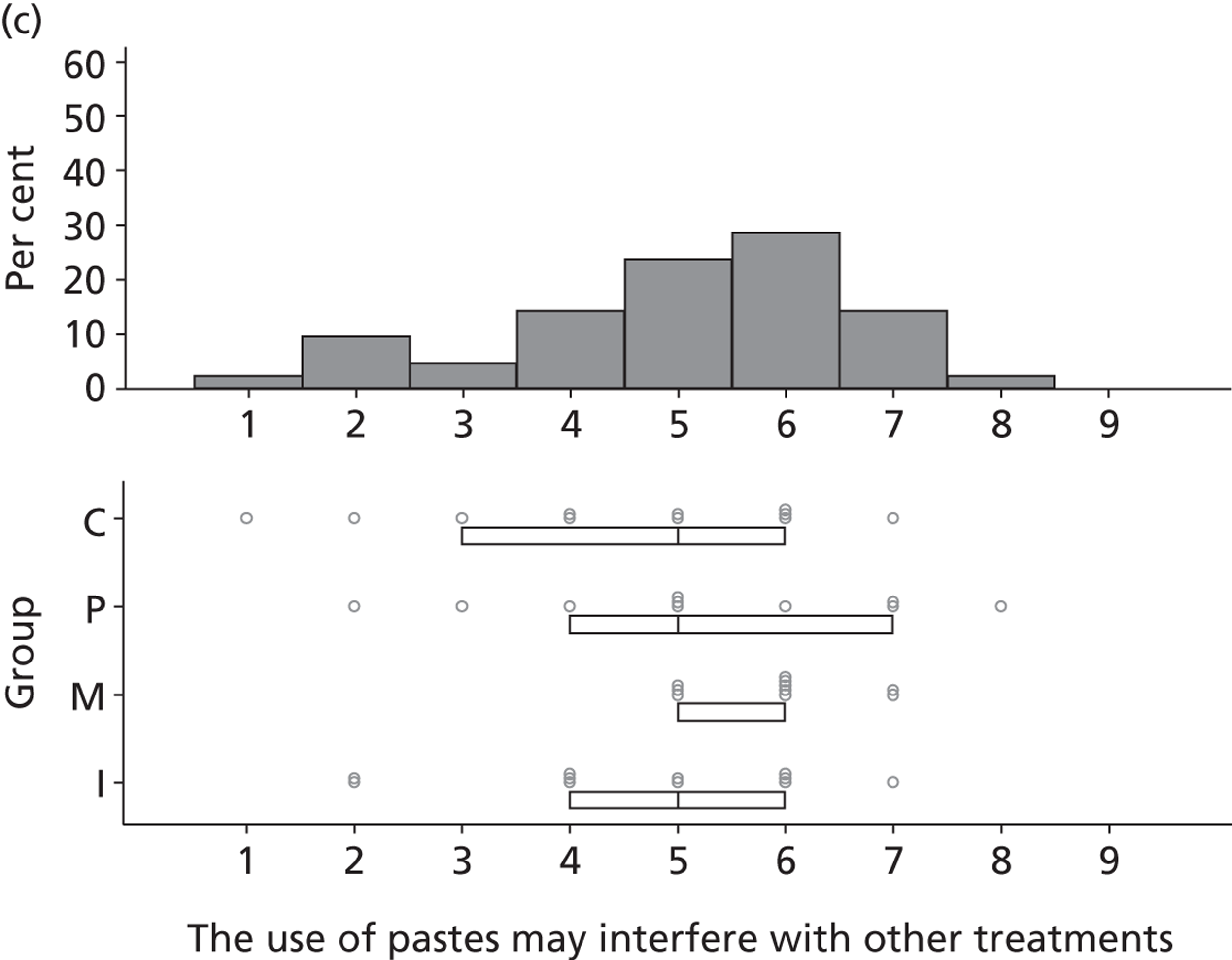
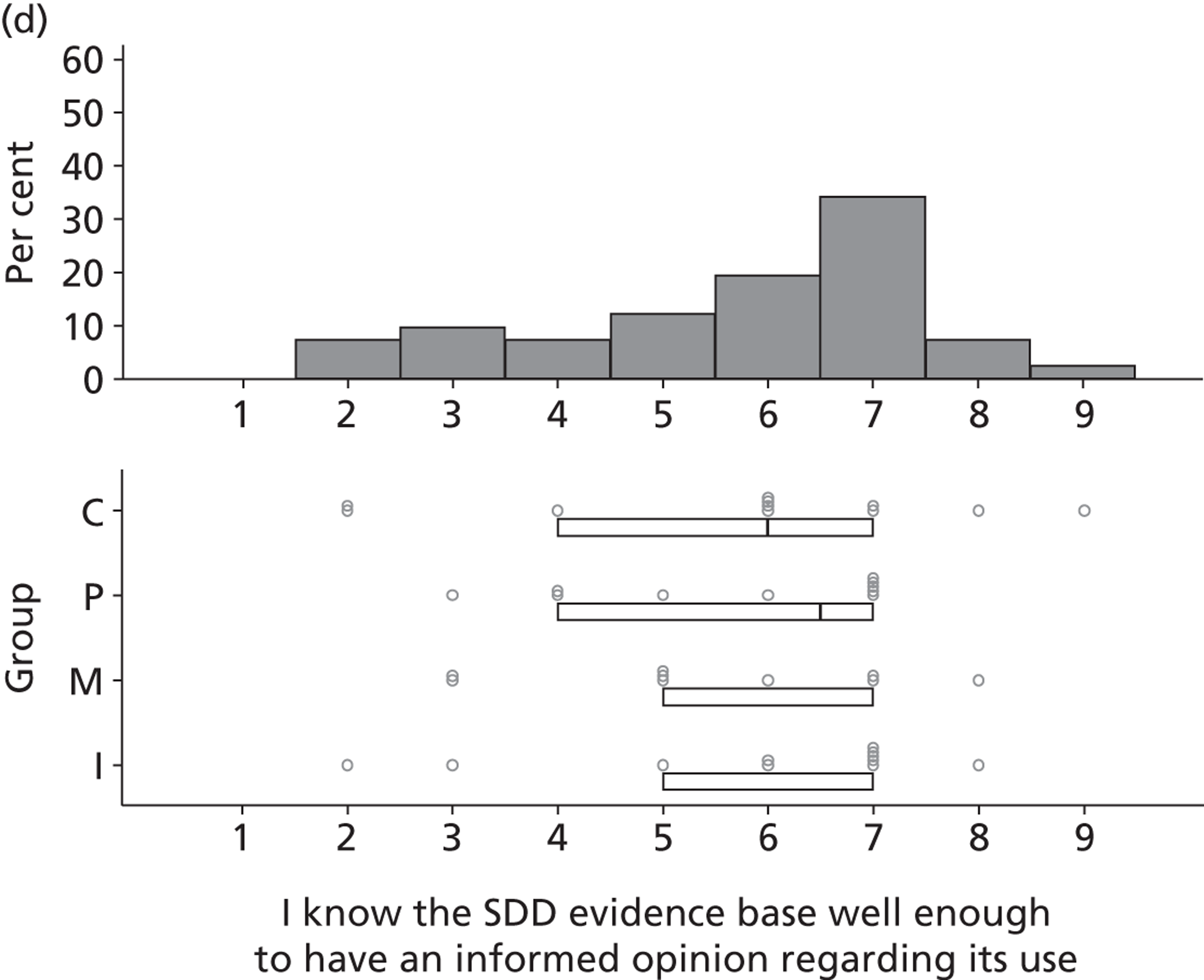
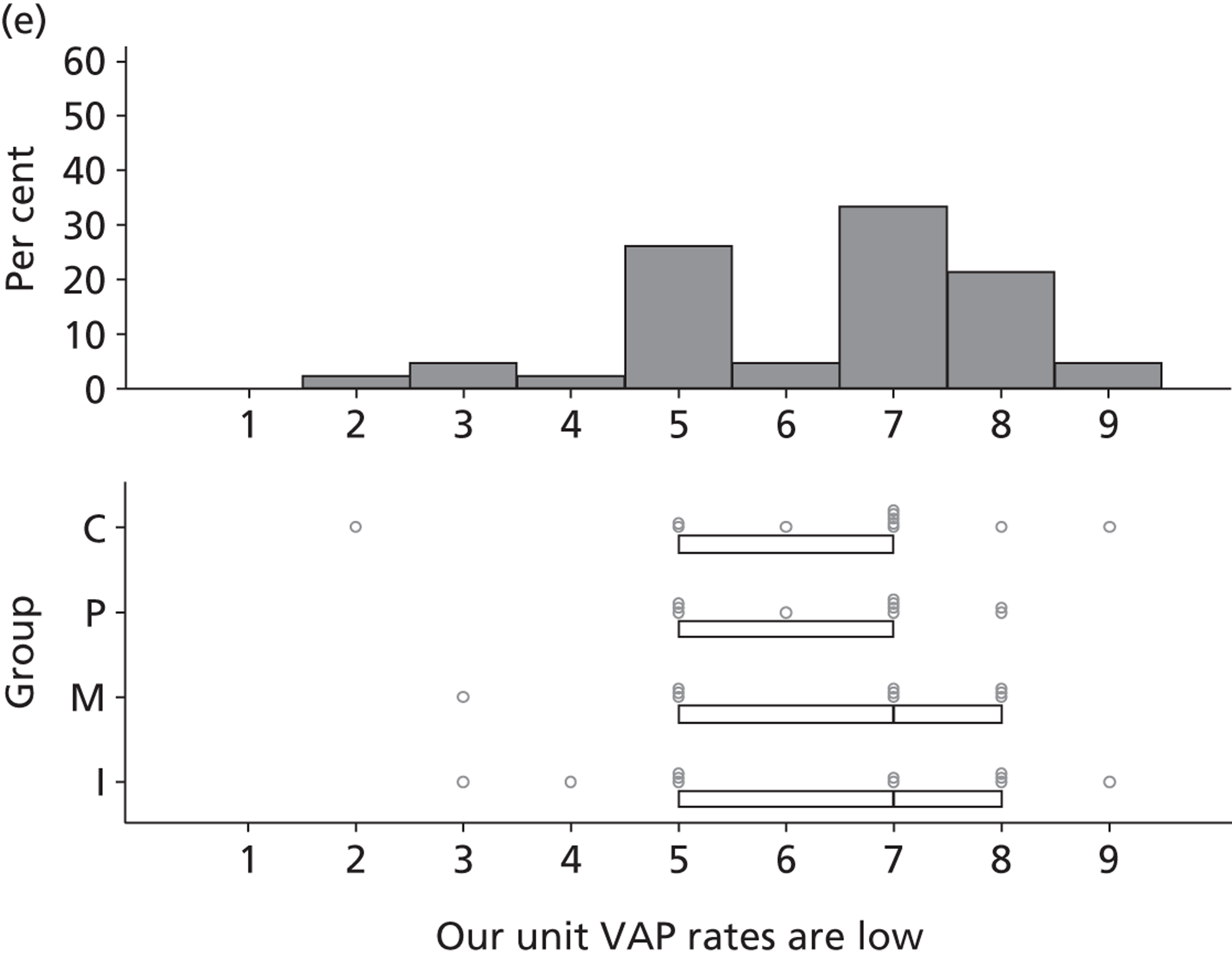
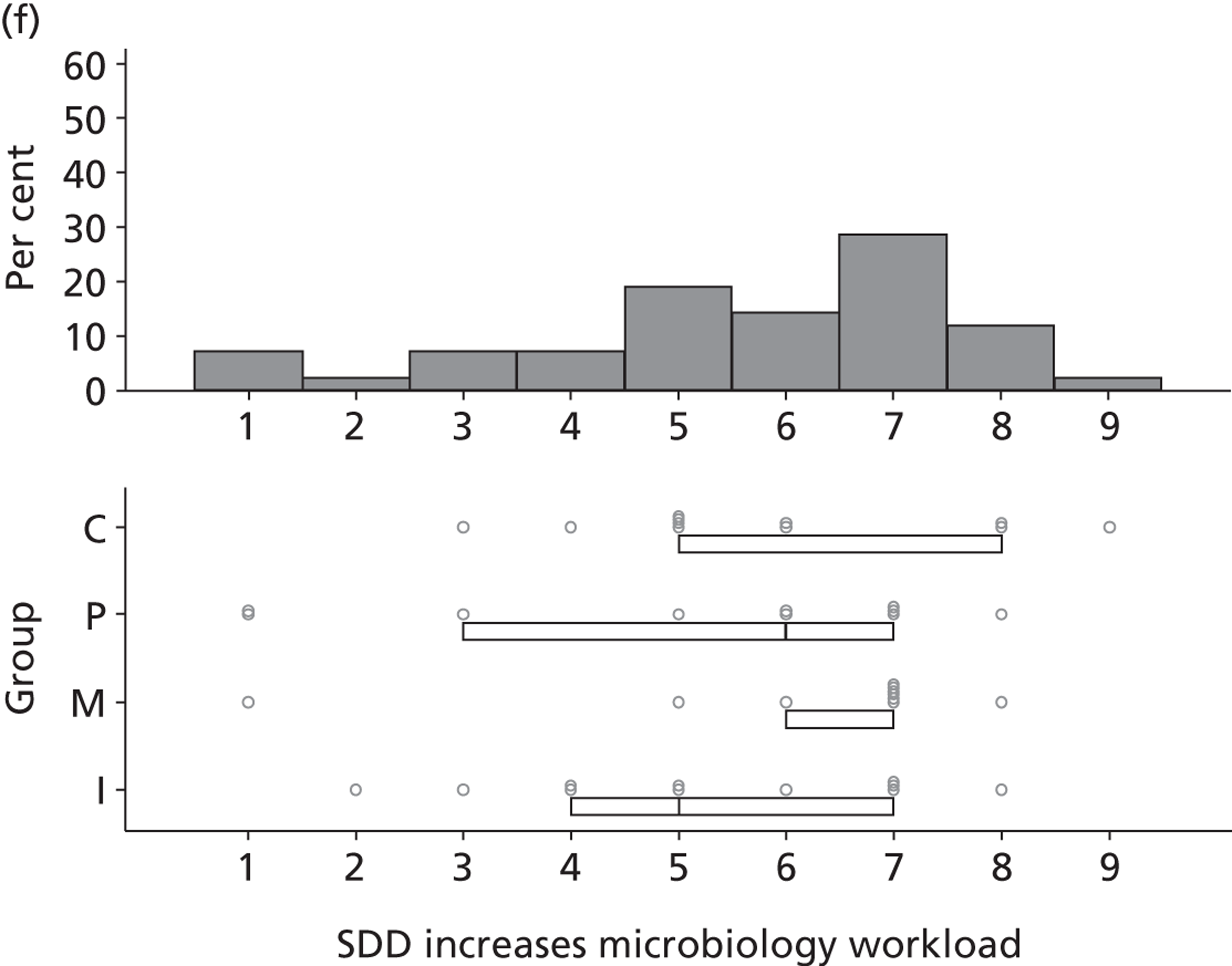
FIGURE 27.
50–75% consensus around mid-value of 2, 3, 4 and 5.
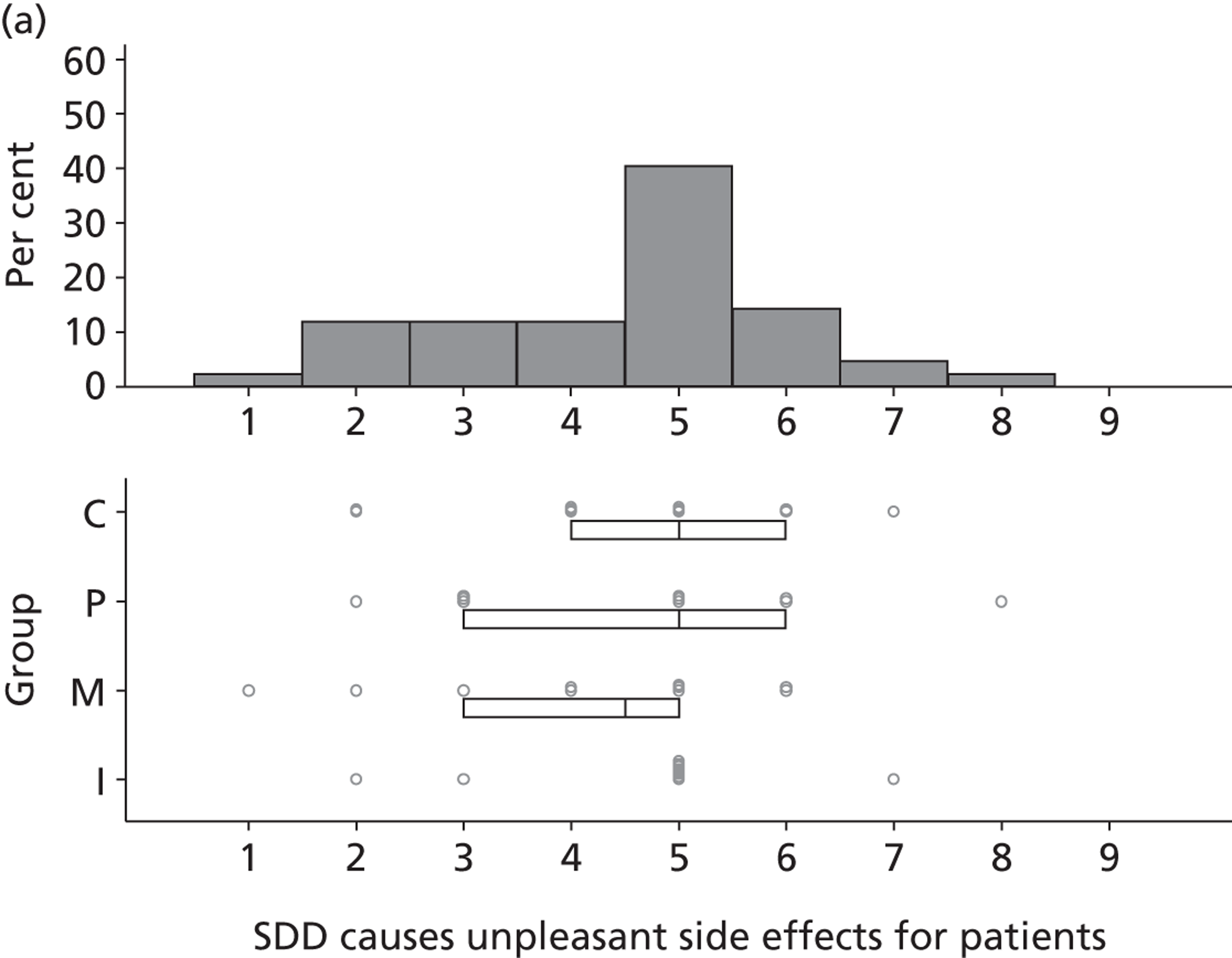
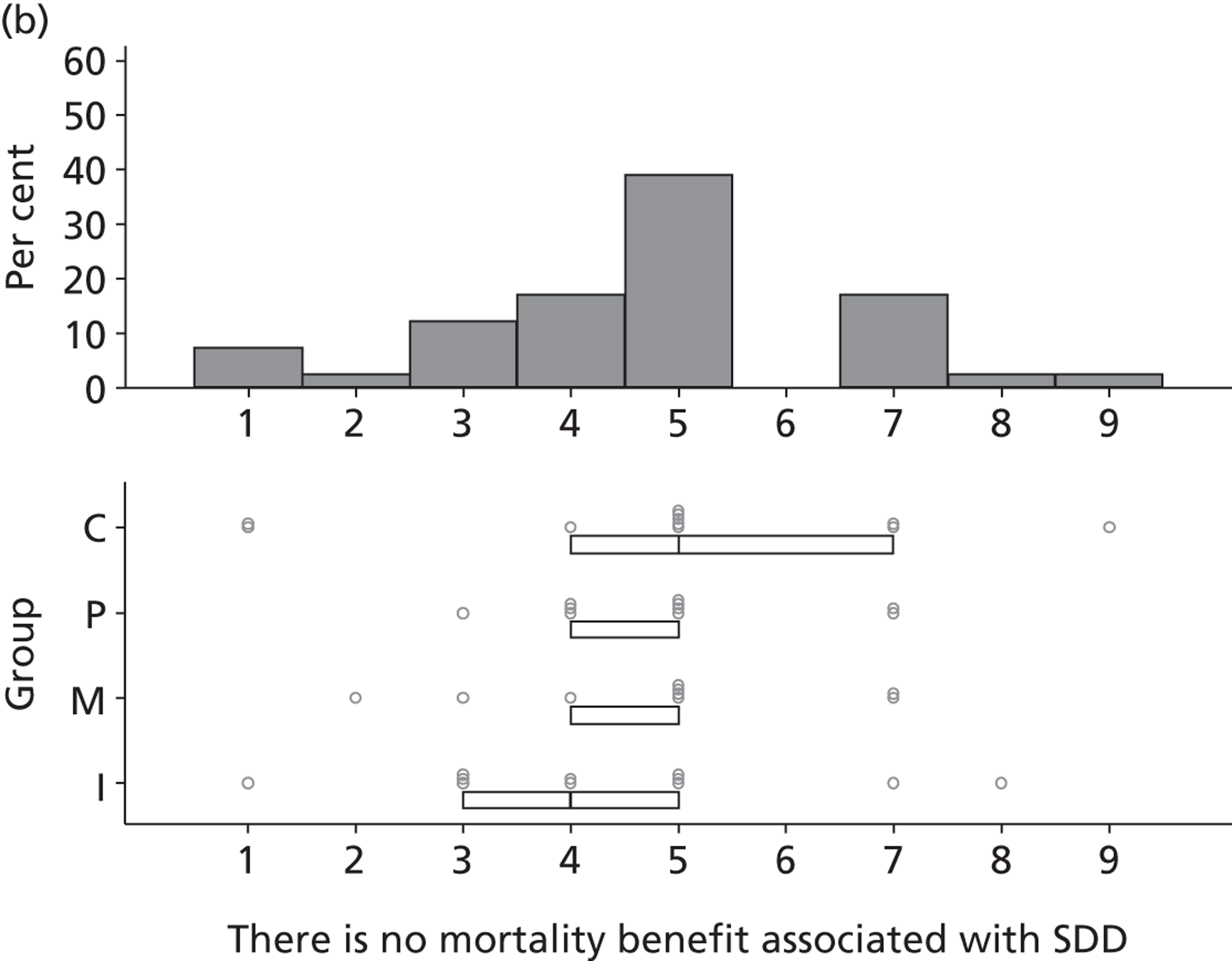
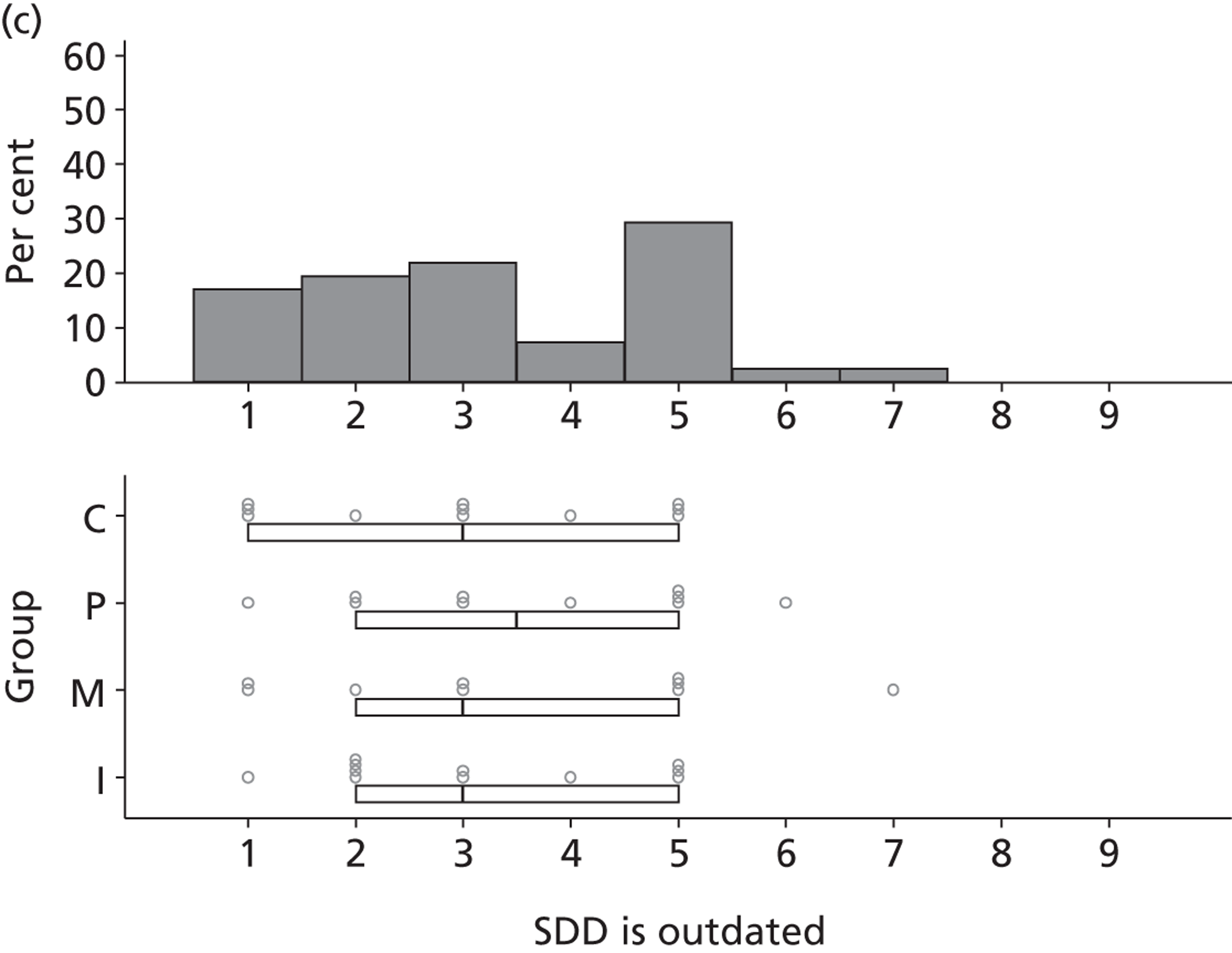
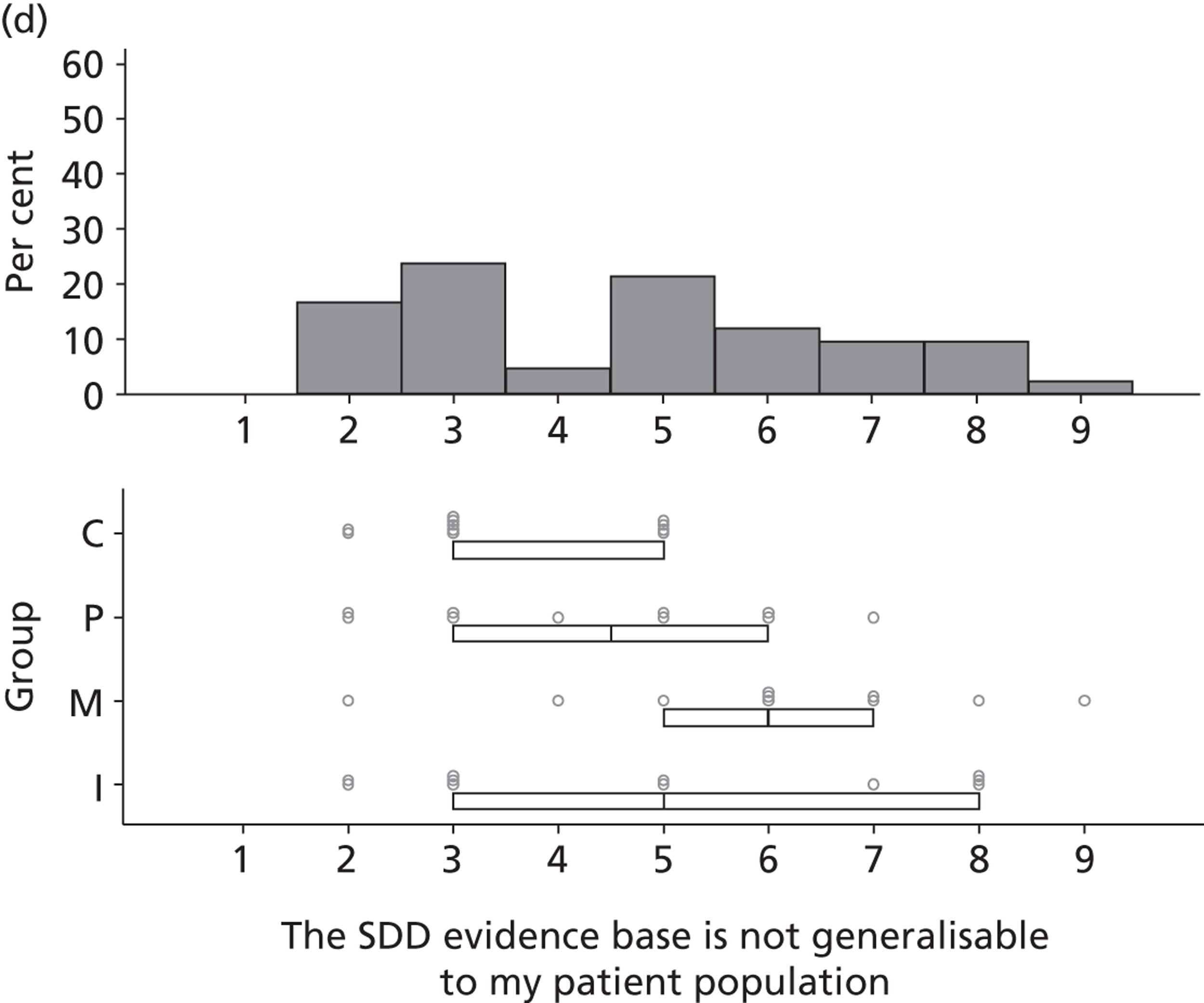
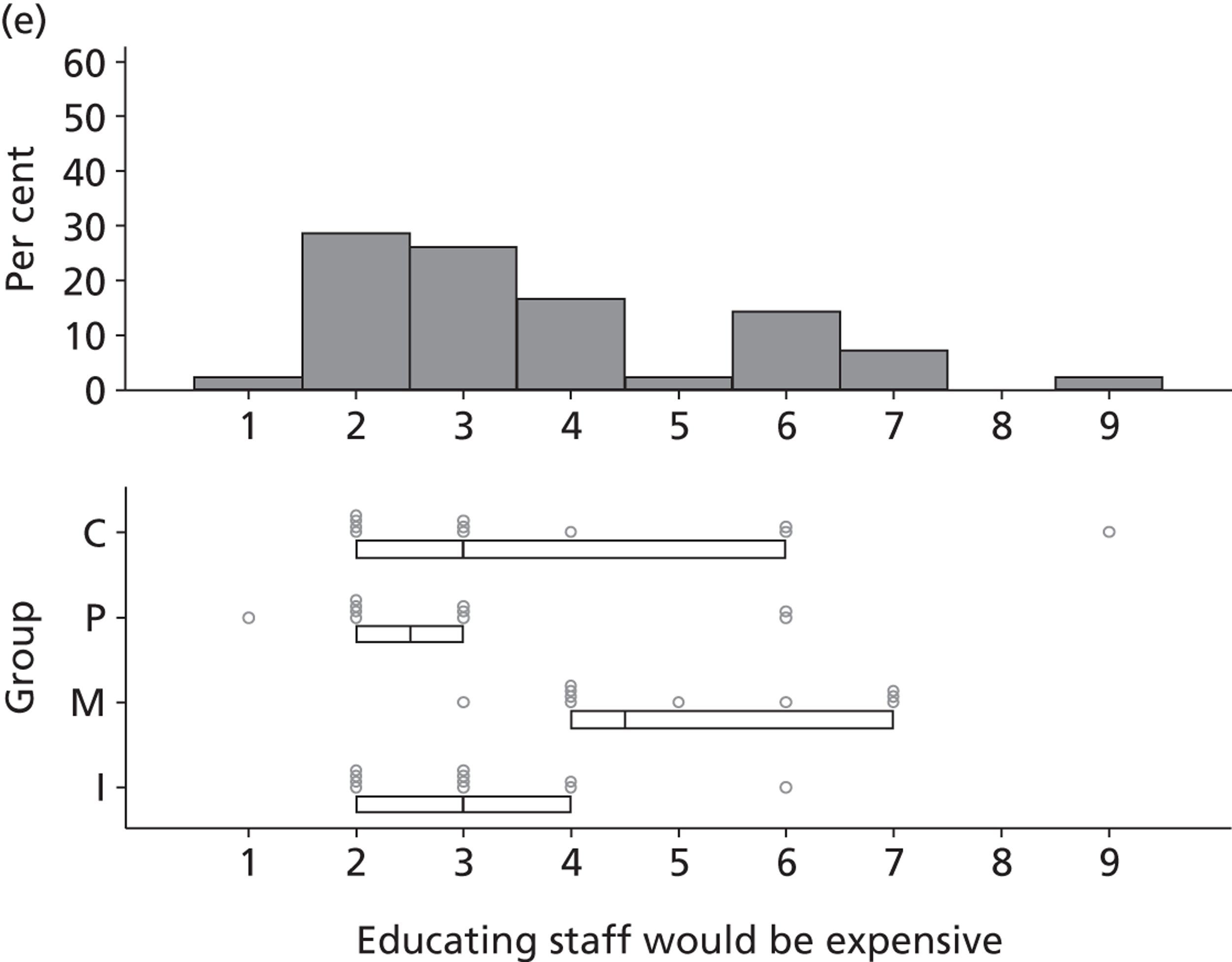
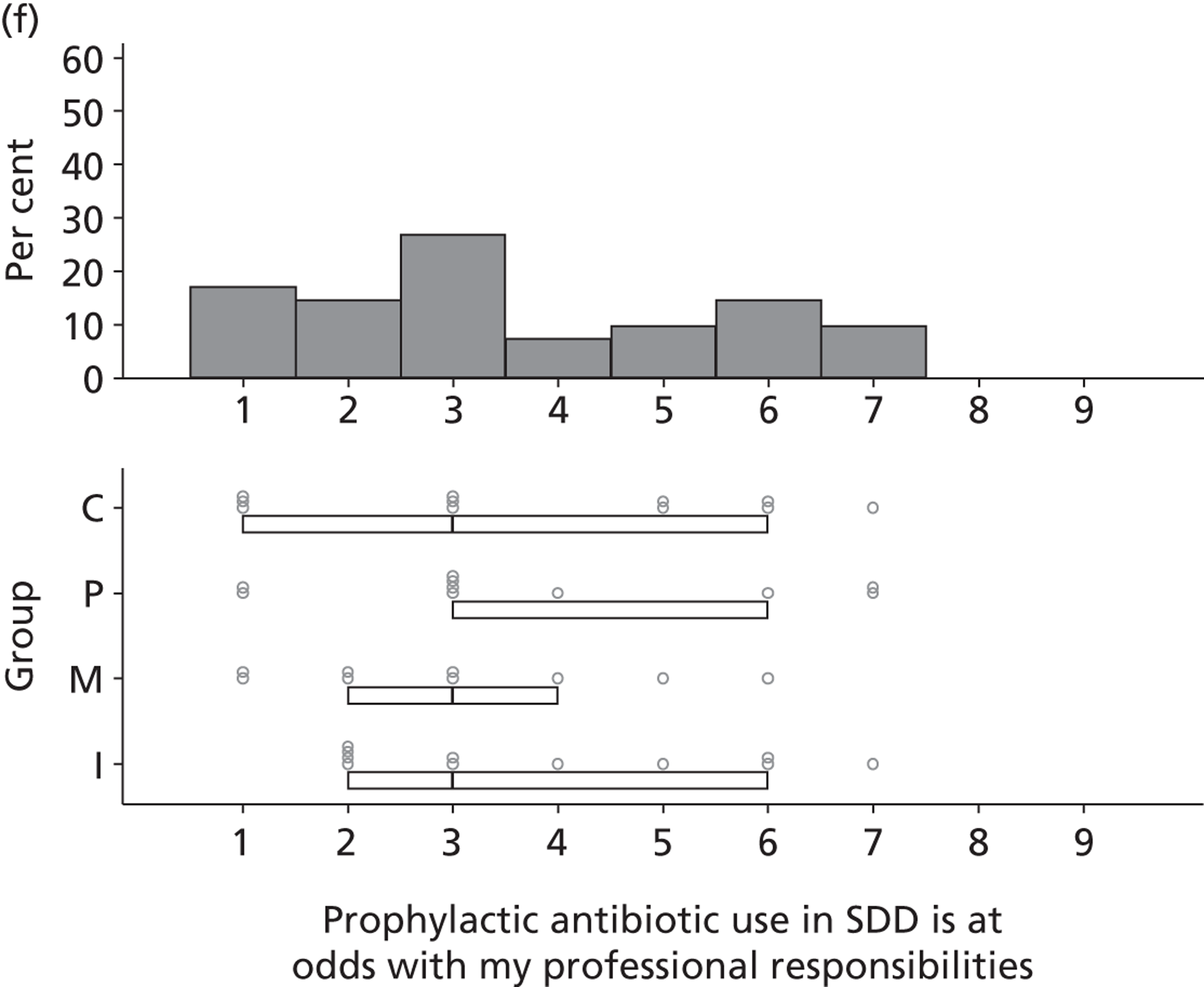
FIGURE 28.
Lack of consensus.
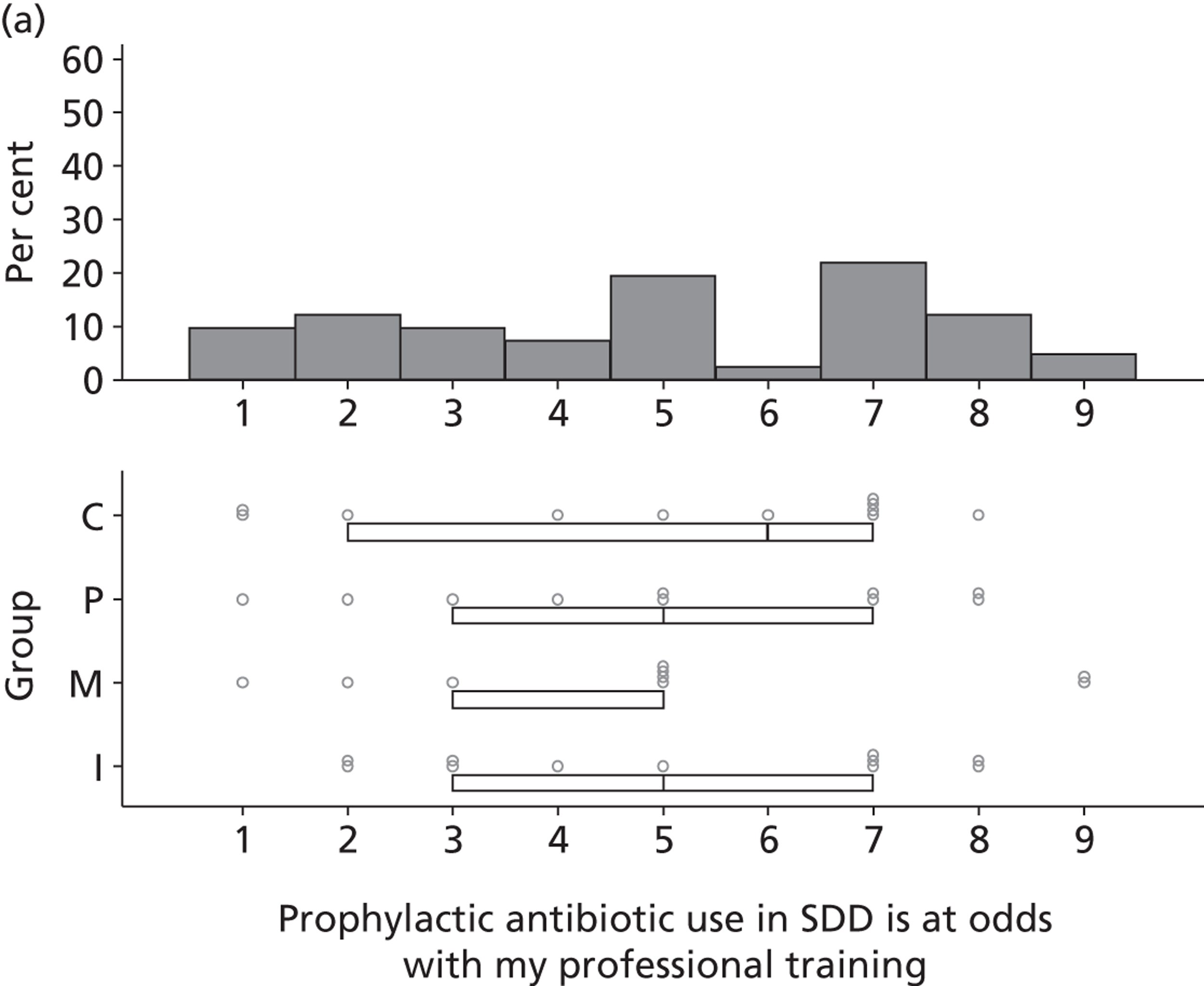
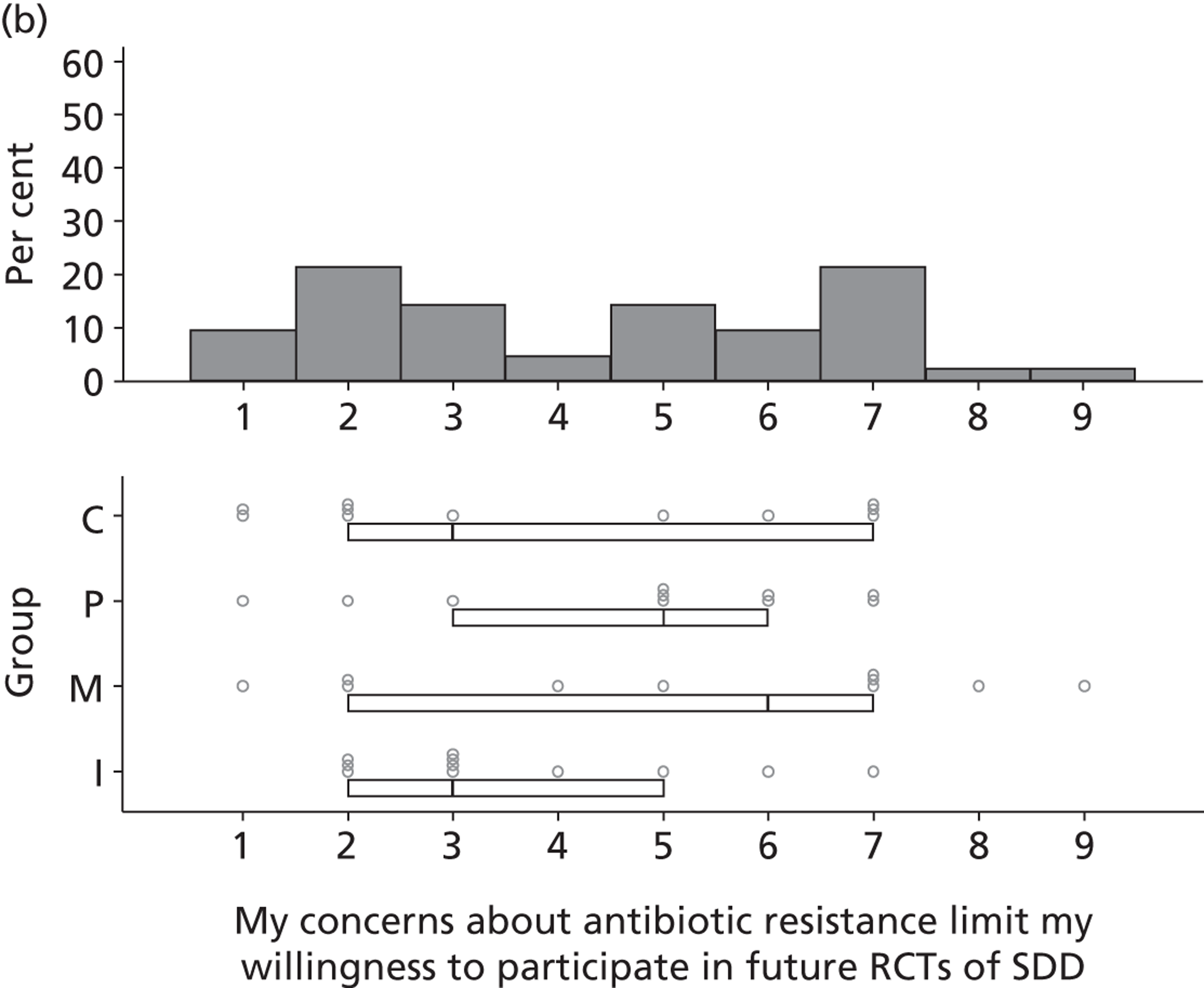
Appendix 5 Materials for national survey
Appendix 6 Materials for triallist interview study
List of abbreviations
- BSAC
- British Society for Antimicrobial Chemotherapy
- CI
- confidence interval
- HAI
- hospital-acquired infection
- HIS
- Healthcare Infection Society
- ICNARC
- Intensive Care National Audit and Research Centre
- ICS
- Intensive Care Society
- ICU
- intensive care unit
- i.v.
- intravenous
- MRSA
- methicillin-resistant Staphylococcus aureus
- NICE
- National Institute for Health and Care Excellence
- NPSA
- National Patient Safety Agency
- NRSPCC
- NHS Research Scotland Permission Coordinating Centre
- OR
- odds ratio
- P
- participant (when citing participant codes following quotations)
- R&D
- research and development
- RCT
- randomised controlled trial
- SD
- standard deviation
- SDD
- selective decontamination of the digestive tract
- SOD
- selective oral decontamination
- SSI
- site-specific information
- SuDDICU
- selective decontamination of the digestive tract in intensive care units
- T
- triallist participant (when citing participant codes following quotations)
- TDF
- theoretical domains framework
- VAP
- ventilator-associated pneumonia