Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 05/506/03. The contractual start date was in February 2007. The draft report began editorial review in May 2012 and was accepted for publication in April 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
All authors have completed the unified competing interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare Medicines for Children Research Network funding (MD); involvement in the Health Technology Assessment programme SLEEPS (Safety profiLe Efficacy and Equivalence in Paediatric intensive care Sedation) trial and royalties for acting as an editor of handbook of PIC (KM); payment from Baxter for a single advisory meeting and from GlaxoSmithKline for pip contributions (MP); and a grant awarded from the neonatal and paediatric pharmacists group for an in vitro study of drug compatibility (JP).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Macrae et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Hyperglycaemia is a common element of the early phase of the neuroendocrine response to stress which is observed following the onset of illness or injury in both adults and children, and is sometimes referred to as the diabetes of critical illness,1–4 as a result of accelerated glucose production and acute development of relative insulin resistance.
Stress has long been recognised as a programmed, co-ordinated and adaptive process conferring survival advantage which may, if prolonged, lead to secondary harm. 5 Stress hyperglycaemia was therefore usually explained as being an adaptive response whose purpose could potentially be beneficial by maintaining intravascular volume or increasing energy substrate delivery to vital organs, and it was not usually treated unless glucose levels were grossly and persistently elevated. These assumptions around the lack of harm from or benefits of stress hyperglycaemia have increasingly been questioned in the light of reports from a wide range of illnesses and populations which have shown hyperglycaemia to be related to worse clinical outcomes.
Myocardial infarction
In a meta-analysis,6 patients with acute myocardial infarction, and without diabetes mellitus, who had glucose concentrations in the range 6.1–8.0 mmol/l or higher had a 3.9-fold [95% confidence interval (CI) 2.9- to 5.4-fold] higher risk of death than patients who had lower glucose concentrations. Glucose concentrations higher than values in the range of 8.0–10.0 mmol/l on admission were associated with increased risk of congestive heart failure or cardiogenic shock.
Stroke
Capes et al. 7 conducted a systematic review and meta-analysis of the literature relating glucose levels in the interval immediately post stroke to the subsequent course. A comprehensive literature search was carried out to identify cohort studies reporting mortality and/or functional recovery after stroke in relation to admission glucose level. In total, 32 studies were identified, and predefined outcomes could be analysed for 26 of these. After stroke, the unadjusted relative risk (RR) of in-hospital or 30-day mortality associated with an admission glucose level above the range of 6–8 mmol/l was 3.07 (95% CI 2.50 to 3.79) in non-diabetic patients and 1.30 (95% CI 0.49 to 3.43) in diabetic patients. Non-diabetic stroke survivors whose admission glucose level was above the range of 6.7–8 mmol/l also had a greater risk of poor functional recovery (RR 1.41; 95% CI 1.16 to 1.73).
Head injury and multisystem trauma
Hyperglycaemia has been shown to be an independent predictor of poor outcomes in adults with head injury8 and in cases of multiple trauma. 9
Pulmonary function
Hyperglycaemia has been shown to be associated with diminished pulmonary function in adults, even in the absence of diabetes mellitus,10 and a range of risk factors for lung injury. 11
Gastrointestinal effects
Hyperglycaemia has been shown to be associated with delayed gastric emptying,12 decreased small bowel motility and increased sensation and cerebral-evoked potentials in response to a range of gastrointestinal stimuli in adult volunteers. 13–16
Infections
The in vitro responsiveness of leucocytes stimulated by inflammatory mediators is inversely correlated with glycaemic control. 17 This reduction in polymorphonuclear leucocyte responsiveness may contribute to the compromised host defence associated with sustained hyperglycaemia,17 and, indeed, hyperglycaemia has been shown to be associated with an increased rate of serious infections after adult cardiac18 and vascular surgery. 19
These studies, which associate poorer outcomes with patients with the highest levels of stress glycaemia, raise the question of whether high blood glucose levels simply identify the more severely ill patients, in whom worse outcomes are inevitable, or whether specific homeostatic or allostatic glycaemic dysfunction influences outcomes independently. If the latter were true, then perhaps measures to prevent or limit stress-induced hyperglycaemia would improve clinical outcomes.
Does hyperglycaemia matter for adults in the critically ill setting?
Although the importance of good glycaemic control has long been established in minimising complications of chronic hyperglycaemia in patients with diabetes mellitus,20,21 and a number of mechanisms for glucotoxicity identified,22 in the era up to the year 2000, a permissive approach was typically adopted when managing non-diabetic patients in intensive care settings. A very reasonable question, however, is Could shorter-term hyperglycaemia in non-diabetic populations be associated with clinically important adverse outcomes? Early reports from adult populations started to explore the possible association between acute stress-induced hyperglycaemia and outcome in both diabetic and non-diabetic patients.
Furnary et al. 18 noted that hyperglycaemia is associated with higher sternal wound infection rates following adult cardiac surgery and questioned whether more aggressive control of glycaemia might lead to lower infection rates. In a prospective study of 2467 consecutive diabetic patients who underwent open-heart surgical procedures, patients were classified into two sequential groups. The control group included 968 patients treated with sliding-scale-guided intermittent subcutaneous insulin injections. The study group included 1499 patients treated with a continuous intravenous insulin infusion in an attempt to maintain a blood glucose level of < 11.1 mmol/l. Compared with subcutaneous insulin injections, continuous intravenous insulin infusion induced a significant reduction in perioperative blood glucose levels, which was associated with a significant reduction in the incidence of deep-sternal wound infection in the continuous intravenous insulin infusion group [0.8% (12 of 1499) vs. 2.0% (19 of 968) in the intermittent subcutaneous insulin injection group; p = 0.01]. The use of perioperative, continuous intravenous insulin infusion in diabetic patients undergoing open-heart surgical procedures appeared to significantly reduce the incidence of major infections.
Malmberg et al. 23 randomly allocated patients with diabetes mellitus and acute myocardial infarction to intensive insulin therapy (n = 306) or standard treatment (controls, n = 314). The mean (range) follow-up was 3.4 (1.6–5.6) years. There were 102 (33%) deaths in the treatment group compared with 138 (44%) deaths in the control group (RR 0.72; 95% CI 0.55 to 0.92; p = 0.011). The effect was most pronounced among a predefined group that included 272 patients who had not received insulin treatment previously and who were at a low cardiovascular risk (0.49; 0.30 to 0.80; p = 0.004). Intensive insulin therapy improved survival in diabetic patients with acute myocardial infarction. The effect seen at 1 year continued for at least 3.5 years, with an absolute reduction in mortality of 11%.
In 2001, Van den Berghe and colleagues from Leuven, Belgium,24 extended this approach to non-diabetic hyperglycaemic populations. They performed a single-centre randomised trial in adults undergoing intensive care following surgical procedures which showed that the use of insulin to tightly control blood glucose led to a reduction in mortality from 10.9% to 7.2%, and a significantly lower incidence of a range of important complications of critical illness including renal failure, infection, inflammation, anaemia and polyneuropathy and need for prolonged ventilatory support.
The same group undertook a similar trial in non-surgical, adult, critically ill patients25 and again found benefits from the control of blood glucose with intensive insulin therapy. Patients were randomly assigned to a regimen of strict normalisation of blood glucose (4.4–6.1 mmol/l) with use of insulin or conventional therapy whereby insulin was administered only when blood glucose levels exceeded 12 mmol/l, with the infusion tapered when the level fell below 10 mmol/l. In the intention-to-treat analysis of the 1200 patients, intensive care unit (ICU) and in-hospital mortality were not significantly altered by intensive insulin therapy; however, for those patients who stayed > 3 days in intensive care (an a priori subgroup), mortality was significantly reduced from 52.5% to 43% (p = 0.009). Morbidity was significantly reduced by intensive insulin therapy, with a lower incidence of renal injury and shorter length of mechanical ventilation and duration of hospital stay noted. For patients who stayed > 5 days in intensive care after trial entry, all morbidity end points were significantly improved in the intensive insulin therapy group.
Although the precise mechanisms by which different glucose control strategies might influence clinical outcomes had not been fully elucidated, the clinical effects of ‘tight glycaemic control’ (TGC) for adults in critical care appeared promising. As a result, TGC was widely adopted in adult critical care standards in the years following the publication of Van den Berghe et al. 2001 paper. 24
Stress hyperglycaemia in the critically ill child
Over 12,000 children are admitted to ICUs in England and Wales each year. 26 Hyperglycaemia occurs frequently during critical illness or after major surgery in children, with a reported incidence of up to 86%,3 but children in critical care may not respond to interventions in the same way as adults.
References to hyperglycaemia and its management in critically ill children were identified through searches in MEDLINE27 from 1990 to December 2006. Articles were also identified through searches of the authors’ own files. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the genesis of this research proposal. The search terms used were ‘glycaemia’, ‘control’, ‘insulin’, ‘critical illness’ and ‘intensive care’; the limits applied were ‘clinical trials’, ‘meta-analysis’, ‘randomised controlled trial’ and ‘humans’ and ‘age 0–18 years’. No randomised trials or meta-analyses of glycaemic control in childhood critical illness were identified.
The non-randomised studies identified included a number of reports of critically ill children receiving care in general,3,28,29 cardiac surgical,30,31 trauma9,32,33 and burns34 ICUs, all showing that high blood glucose levels occur frequently and that levels are significantly higher in children who die than in children who survive. As in adults, the occurrence of hyperglycaemia was associated with poorer outcomes including death, sepsis and longer length of intensive care stay for critically ill children.
Srinivasan et al. 3 studied the association of timing, duration and intensity of hyperglycaemia with mortality in critically ill children. The study had a retrospective, cohort design and included 152 critically ill children receiving vasoactive infusions or mechanical ventilation. A peak blood glucose of > 7 mmol/l occurred in 86% of patients. Non-survivors had a higher peak blood glucose [mean ± standard deviation (SD)] than survivors (17.3 ± 6.4 mmol/l vs. 11.4 ± 4.4 mmol/l, p < 0.001). Non-survivors had more intense hyperglycaemia during the first 48 hours in the paediatric intensive care unit (PICU) (7 ± 2.1 mmol/l) than survivors (6.4 ± 1.9 mmol/l, p < 0.05). Median blood glucose levels > 8.3 mmol/l were associated with a threefold increased risk of mortality compared with median levels of < 8.3 mmol/l. Univariate logistic regression analysis showed that peak blood glucose and the duration and intensity of hyperglycaemia were each associated with PICU mortality (p < 0.05). Multivariate modelling controlling for age and paediatric risk of mortality scores showed an independent association of peak blood glucose and duration of hyperglycaemia with PICU mortality (p < 0.05). This study demonstrated that hyperglycaemia is common among critically ill children. Peak blood glucose and duration of hyperglycaemia appear to be independently associated with mortality. The study was limited by its retrospective design, its single-centre location and the absence of cardiac surgical cases, a group which make up approximately 40% of paediatric intensive care (PIC) admissions in the UK.
Yates et al. 30 conducted a retrospective review of data from 184 children < 1 year of age who underwent major cardiac surgery over a 22-month period ending in August 2004. Factors analysed included peak glucose levels and duration of hyperglycaemia. The duration of hyperglycaemia was significantly longer in children who developed renal insufficiency, liver insufficiency and infection and those who required mechanical circulatory support or who died, and was associated with longer PICU and hospital lengths of stay (LOS).
Hall et al. 35 investigated the incidence of hyperglycaemia in infants with necrotising enterocolitis (NEC) and the relationship between glucose levels and outcome in these infants. Glucose measurements (n = 6508) in 95 neonates with confirmed NEC admitted to the surgical ICU were reviewed. Glucose levels ranged from 0.5 to 35.0 mmol/l; 69% of infants became hyperglycaemic (> 8 mmol/l) during their admission; and 32 infants died. The mortality rate tended to be higher in infants whose peak glucose concentration exceeded 11.9 mmol/l than in those with peak glucose concentrations of < 11.9 mmol/l, and the late (> 10 days after admission) mortality rate was significantly higher in the former infants (29% vs. 2%; p = 0.0009). Linear regression analysis indicated that peak glucose concentration was significantly related to LOS (p < 0.0001).
Branco et al. 29 showed an association between hyperglycaemia and increased mortality in children with septic shock. They prospectively studied children admitted to a regional PICU with septic shock refractory to fluid therapy over a period of 32 months. The peak glucose level in those with septic shock was 11.9 ± 5.4 mmol/l (mean ± SD), and the mortality rate was 49.1% (28/57). In non-survivors, the peak glucose level was 14.5 ± 6.1 mmol/l, which was higher (p < 0.01) than that found in survivors (9.3 ± 3.0 mmol/l). The RR of death in patients with peak glucose levels of ≥ 9.9 mmol/l was 2.59 (p = 0.012).
Faustino and Apkon28 demonstrated that hyperglycaemia occurs frequently among critically ill non-diabetic children and is associated with higher mortality and longer LOSs in PICUs. They performed a retrospective cohort study of 942 non-diabetic patients admitted to a PICU over a 3-year period. The prevalence of hyperglycaemia was based on initial PICU glucose measurement, peak value within 24 hours and peak value measured during PICU stay up to 10 days after the first measurement. Using three cut-off values (6.7, 8.3 and 11.1 mmol/l), the prevalence of hyperglycaemia was 16.7–75.0%. The RR for death increased for peak glucose within 24 hours of > 8.3 mmol/l (RR, 2.50; 95% CI 1.26 to 4.93) and peak glucose within 10 days of > 6.7 mmol/l (RR, 5.68; 95% CI 1.38 to 23.47).
Pham et al. 34 reviewed the records of children with ≥ 30% total body surface area burn injury admitted to a regional paediatric burn centre during two consecutive periods, during the first of which patients received ‘conventional insulin therapy’ (n = 31), and during the second of which they were managed with TGC (n = 33). Intensive insulin therapy was positively associated with survival and a reduced incidence of infections. The authors concluded that intensive insulin therapy to maintain normoglycaemia in severely burned children could be safely and effectively implemented in a paediatric burns unit and that this therapy seemed to lower infection rates and improve survival.
There was, therefore, mounting evidence to suggest that stress hyperglycaemia occurred in both neonates and children (as in adults). From adult studies, TGC appeared to offer the possibility of clinical benefits, particularly following surgery, but there was no convincing randomised controlled trial (RCT) evidence for children, whether or not admitted to PICUs following surgery. This was of particular importance as approximately one-third of admissions of children to UK PICUs are associated with surgery, in particular cardiac surgery.
Evidence on the cost-effectiveness of tight glycaemic control
The existing evidence on the clinical effectiveness of TGC is derived from studies in both critically ill adults and critically ill children. However, to inform whether or not the NHS should provide TGC rather than conventional management (CM) for critically ill children, it is important to consider whether or not the additional costs associated with implementing a TGC protocol are offset by subsequent reductions in resource use and improved health outcomes. Limited evidence suggests that any additional costs associated with implementing a TGC protocol may be relatively small. 36 A post-hoc analysis of the Van den Berge 2001 RCT24,37 for critically ill adults admitted for surgery reported that TGC can reduce ICU LOS, and hence hospital costs. 37 However, this study had several limitations. The study was not designed to measure costs; resource use after the initial hospital episode was not recorded; the study was undertaken in a single centre and lacked generalisability; and it is unclear whether the results apply to other patient groups (e.g. critically ill children, patients not admitted for surgery).
For critically ill children, any assessment of the effect of a TGC protocol compared with CM on resource use and costs is hindered by the lack of evidence from RCTs. The costs of each PICU bed-day are substantial (ranging from £1000 to £5000 per bed-day),38 so if TGC reduces PICU LOS then it would be anticipated to also reduce short-term costs (i.e. those incurred within 30 days of admission to the PICU). It is also plausible that TGC may have an effect on longer-term costs. A previous study reported that around 10% of PICU survivors had residual long-term disability (median follow-up of 3.5 years from initial admission). 39 Therefore, the long-term costs following PICU survival may be substantial, and may be increased if TGC increases PICU survival, or reduced if improved blood glucose control reduces morbidity. There is little available evidence on the net effect of TGC compared with CM on longer-term morbidity and hence costs, either in general or specifically for critically ill children.
The previous evidence, therefore, raises the hypotheses that TGC may have an impact on costs, both in the short term (e.g. 30 days post PICU admission) and in the longer term (e.g. 12 months post PICU admission). It would, therefore, seem important to consider the net effect of TGC on costs alongside any change in clinical outcomes. No previous study has considered the effect of TGC on health service costs for paediatric patients.
The Control of Hyperglycaemia in Paediatric intensive care (CHiP) trial, therefore, sought to address the question of whether or not a policy of strictly controlling blood glucose using insulin in children admitted to PIC reduces mortality and morbidity and is cost-effective.
Chapter 2 Methods
Study design
The study was an individually randomised controlled open trial with two parallel arms. The allocation ratio was 1 : 1.
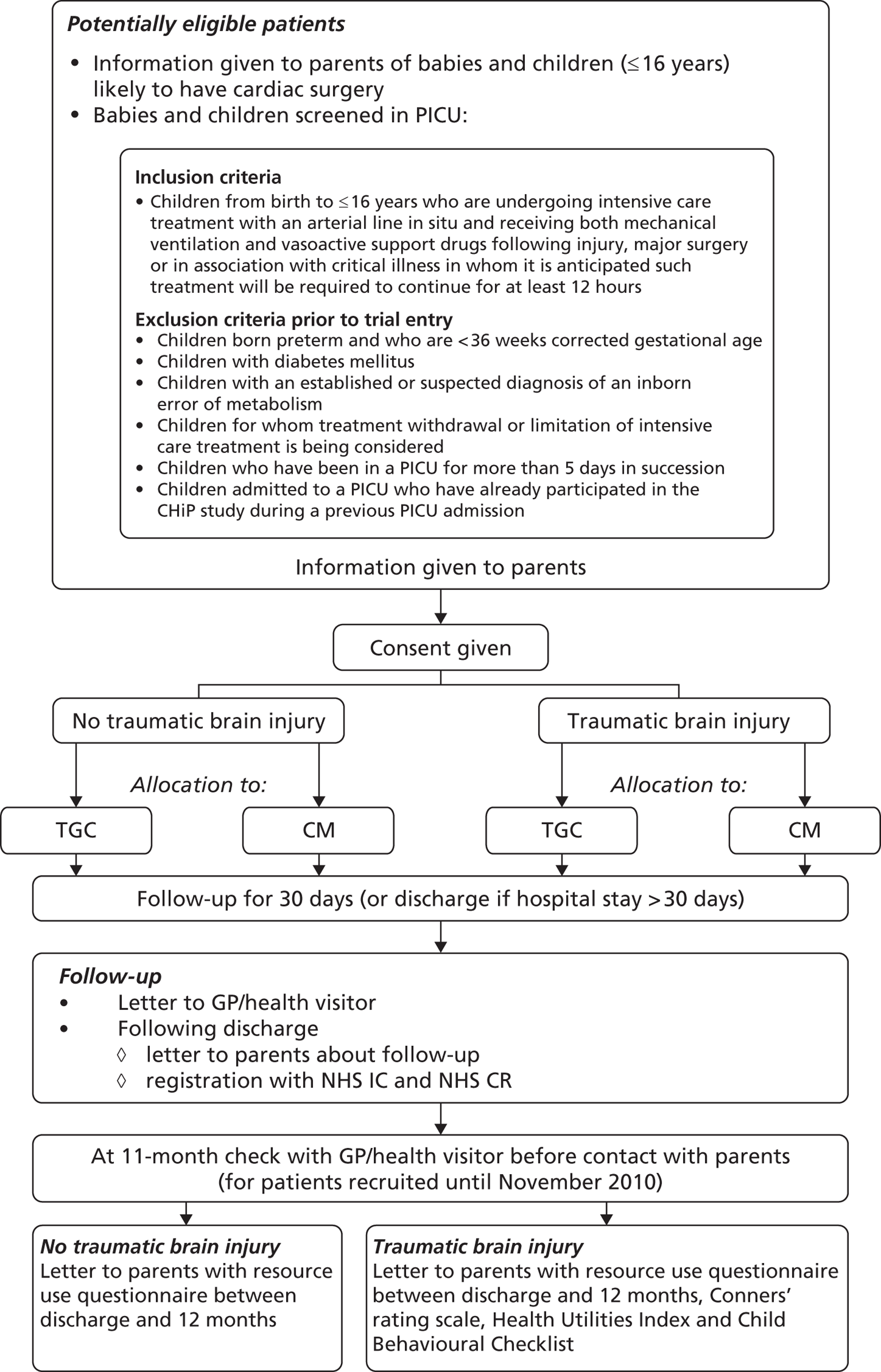
The planned flow of patients through the trial is summarised in Figure 1.
FIGURE 1.
Flow chart summarising the planned flow of patients through the trial. CR, Central Register; GP, general practitioner; IC, Information Centre.
Primary hypothesis
The primary hypothesis was that TGC will increase the numbers of days alive and free of mechanical ventilation at 30 days post randomisation (VFD-30) for children aged ≤ 16 years on ventilatory support and receiving vasoactive drugs.
Secondary hypotheses
The secondary hypotheses were as follows:
-
TGC will lead to improvement in a range of complications associated with intensive care treatment.
-
TGC will be cost-effective.
-
The clinical effectiveness of TGC will be similar whether children were admitted to PICU following cardiac surgery or for other reasons.
-
The cost-effectiveness of TGC will be similar whether children were admitted to PICU following cardiac surgery or for other reasons.
Participants
Inclusion criteria
Included were children ≥ 36 weeks corrected gestational age and ≤ 16 years admitted to PICU who had an arterial line in situ and who were also receiving both mechanical ventilation and vasoactive drugs [catecholamines or similar (dopamine, dobutamine, adrenaline, noradrenaline), phosphodiesterase type III inhibitors (milrinone, enoximone), other vasopressors (vasopressin, phenylephrine or similar)] following injury, following major surgery or in association with critical illness, and in whom it was anticipated such treatment would be required to continue for at least 12 hours.
Exclusion criteria prior to trial entry
-
Children born preterm (< 36 weeks corrected gestational age).
-
Children with diabetes mellitus.
-
Children with an established or suspected diagnosis of an inborn error of metabolism.
-
Children for whom treatment withdrawal or limitation of intensive care treatment was being considered.
-
Children who had been in a PICU for > 5 consecutive days.
-
Children admitted to PICU who had already participated in the CHiP study during a previous PICU admission.
Consent
All parents/guardians of children in PICUs who wished to enter their child into the trial were asked by the principal investigator (PI) or delegated investigator to give consent. The trial team recognised that parents were likely to be stressed and anxious, and often had limited time to consider trial entry, but it was considered medically inappropriate to delay the start of treatment. Parents of children listed for cardiac surgery were given information about the trial preoperatively by the PI or delegated investigator, and this afforded families some additional time to think about participation. Provisional consent was sought at this time, and confirmed later if the child was admitted to the PICU. In addition, when possible, older children were given information by the PI or delegated investigator and, if they wished to enter the trial, were asked to assent to their participation in the study. Information sheets and consent forms are shown in Appendix 1.
Patients not entered into the trial received standard care.
Ethical approval
The trial (protocol version 1) was approved by the Brighton East Research Ethics Committee (07/Q1907/24) in 2007. Subsequent amendments are detailed in Appendix 2. The final substantive version (protocol version 6, 23 August 2010) is shown in Appendix 3, and the published version is in Appendix 4.
Allocation of patients
After inclusion in the study, children were randomised to one of two arms:
-
group 1 – CM
-
group 2 – TGC.
To reduce the risk of selection bias at trial entry, allocation was administered through a central computerised 24-hour, 7-day-a-week randomisation service established at the London School of Hygiene and Tropical Medicine (LSHTM), with telephone backup if required. Minimisation was used, with the first child randomly allocated to a trial arm, and each subsequent child allocated randomly to a trial arm with a weighting in favour of the trial arm that minimises the imbalance on selected key prognostic factors.
The following factors were used:
-
centre
-
age ≤ 1 year compared with between 1 year and ≤ 16 years
-
admitted following cardiac surgery or not
-
for children who were admitted for cardiac surgery, risk-adjusted classification for congenital heart surgery (RACHS1)40 categories 1–4 compared with 5 or 6
-
for children who were not admitted for cardiac surgery, Paediatric Index of Mortality version 2 (PIM2) score at randomisation categorised by probabilities of death of < 5%, 5% to < 15% and ≥ 15%
-
accidental traumatic brain injury (TBI) or not.
Interventions
After inclusion in the study, children were randomised to one of two arms: arm 1 (CM) or arm 2 (TGC).
Arm 1: conventional management
Children in this arm were treated according to a standard approach to blood glucose management. Insulin was given by intravenous infusion in this group only if blood glucose levels exceeded 12 mmol/l on two blood samples taken at least 30 minutes apart and was discontinued once blood glucose fell to < 10 mmol/l.
The protocol for glucose control in this arm is shown in Appendix 3A.
Arm 2: tight glycaemic control
Children in this arm received insulin by intravenous infusion titrated to maintain a blood glucose level between the limits of 4 and 7.0 mmol/l.
The protocol for glucose control in this arm is shown in Appendix 3B.
The protocol for glucose control in arm 2 was carefully designed to achieve tight glucose control while minimising the risk of hypoglycaemia, the principal side effect of insulin therapy. Standard insulin solutions were used and changes in insulin infusion rates were guided by both the current glucose level and its rate of change from previous measurements. Blood glucose levels were routinely measured as in all ICUs using commercially available ‘point-of-care’ blood gas analysers, usually with extended biochemical panels, which utilise very small blood samples, producing results in approximately 1 minute. All of the hospitals in this study have laboratories registered with Clinical Pathology Accreditation (UK) Ltd. The NHS executive accreditation standards specify the requirement for the operation and management of chemical pathology, including the operation of a quality management system for all testing (see http://www.cpa-uk.co.uk). The CHiP protocol advised blood glucose testing using arterial rather than venous blood sampling.
Training in the use of the glucose control protocol was provided before the first patient was enrolled in each collaborating centre and for new staff throughout the trial. The clinical co-ordinating centre team liaised closely with local clinicians to ensure that glucose control algorithms were followed closely and safely.
Blinding
Following random allocation, care-givers and outcome assessors were no longer blind to allocation.
Outcome measures
Primary
Following the influential Acute Respiratory Distress Syndrome NETwork (ARDSNET) study,41 VFD-30 was chosen as the primary outcome measure. Death is obviously an important outcome. Mechanical ventilation can be seen as a measure of disease severity, defining the need for complex intensive care. The concept of ventilator-free days (VFDs) brings together these two outcomes. Schoenfeld et al. 42 define VFDs as follows: VFD = 0 if the child dies before 30 days; VFD = 30 – x if the child is successfully weaned from ventilator within 30 days (where x is the number of days on ventilator); or VFD = 0 if the child is ventilated for ≥ 30 days. This use of organ-failure-free days to determine patient-related morbidity surrogate end points in paediatric trials has been supported by influential paediatric triallists in the current low-mortality paediatric critical care environment. 43
Secondary
Clinical outcomes at discharge from paediatric intensive care unit or 30 days (if at paediatric intensive care unit ≥ 30 days)
-
Death within 30 days of trial entry (or before discharge from hospital if duration of hospital stay was ≥ 30 days).
-
Number of days in PICU.
-
Duration of mechanical ventilation.
-
Duration of vasoactive drug usage.
-
Need for renal replacement therapy (RRT).
-
Bloodstream infection (positive cultures associated with two or more features of systemic inflammation or any positive blood culture for fungi).
-
Use of antibiotics for > 10 days.
-
Number of red cell transfusions.
-
Number of hypoglycaemic episodes either moderate (blood glucose < 2.5 mmol/l) or severe (blood glucose < 2.0 mmol/l).
-
Occurrence of seizures (clinical seizures requiring anticonvulsant therapy).
-
Number of children readmitted within 30 days of trial entry.
Thirty-day economic outcomes
-
Hospital LOS within 30 days of trial entry.
-
Hospital costs within 30 days of trial entry.
Twelve-month end points (resource use; survival; attention and behaviour in traumatic brain injury patients; costs)
-
Number of days in PICU, and hospital LOS.
-
Death within 12 months of trial entry.
-
Assessment of attention and behaviour in patients with TBI as measured by the Health Utilities Index [HUI®, Health Utilities Inc. (HUInc), Dundas, ON, Canada; www.healthutilities.com], the King’s outcome scale for childhood head injury (KOSCHI),46 the Child Behavioural Checklist (CBCL) (ASEBA, University of Vermont, Burlington, VT, USA; www.ASEBA.org) and the Conners’ rating scales revised – short version (CRS-R:S). 47
-
Hospital and community health service costs within 12 months of trial.
Lifetime cost-effectiveness
-
Cost per life-year (based on 12-month costs and survival for all cases).
-
Cost per quality-adjusted life-year (QALY).
-
Incremental net benefits (INB).
-
Cost per disability-free survivor (based on 12-month cost and outcomes data for subgroup with TBI).
Follow-up at 12 months
Parents were informed about the follow-up study at trial entry and asked to give consent for their children to be included. The trial manager at the data co-ordinating centre (DCC) wrote to parents following discharge from hospital to remind them about the follow-up, ask them whether or not they wished to receive the trial results and ask them to keep the DCC informed about any change of address. A separate letter was sent to bereaved parents.
At hospital discharge, parents were given a sample copy of a questionnaire (see Appendix 8) about service use post discharge, and a letter explaining that they would be sent and asked to complete the same questionnaire at the 12-month follow-up. To help the parents record and later recall use of any NHS services, at hospital discharge parents were also given a diary (see Appendix 5). The purpose of this diary was to allow parents to prospectively note resource use and to help them to remember it when the time came to complete the questionnaire. They were not asked to return the diary. After 11 months, following checks with the patient’s general practitioner (GP) to find out whether or not the patient was still alive, and whether or not the GP judged it was appropriate for the parents to receive the service-use questionnaire, the trial manager sent the questionnaire to the parents of those patients who met the eligibility criteria. For those parents who did not respond within 4 weeks, a first reminder was issued by post, and, if there was still no response after a further 4 weeks, the parent was contacted by telephone. Follow-up ended when a postal questionnaire was returned either complete or blank, when a refusal was obtained or after both reminders had been issued.
Because of the slower than expected recruitment rates, the funder, the National Institute for Health Research’s Health Technology Assessment (HTA) programme, agreed a funding extension, but reduced the time period for which patients could be followed up, that is patients randomised after 30 October 2010 were ineligible for this 1-year follow-up. This also had implications for the analysis of total LOS and costs up to 12 months (see below).
Follow-up of traumatic brain injury subgroup
This subgroup is more likely to have longer-term morbidity. Although there were unlikely to be large numbers of such children in the trial, parents of children (aged ≥ 4 years) in this subgroup were asked to provide additional information at 12 months (for patients recruited until 2010), regarding overall health status, global neurological outcome, and attention and behavioural status. Further details are given in Appendix 6.
Survival up to 12 months
If parents gave their consent, all children who survived to hospital discharge were followed up for up to 12 months post randomisation to determine mortality using information from the participating PICUs, the children’s GPs or the NHS Information Centre and the NHS Central Register. The NHS number was used to ensure accurate linkage to national death registration using the ‘list cleaning’ service of the Medical Research Information Service at the NHS Information Centre for Health and Social Care.
Adverse events and safety reporting
The Royal Brompton and Harefield NHS Trust, as sponsor of this study, had the responsibility of ensuring arrangements were in place to record, notify, assess, report, analyse and manage adverse events in order to comply with Medicines for Human Use (Clinical Trials) Regulations 2004.
All sites involved in the study were expected to inform the chief investigator and lead research nurse of any serious adverse events (SAEs)/reactions within 24 hours so that appropriate safety reporting procedures could be followed by the sponsor.
It was therefore important that all site investigators involved in the study were aware of the reporting process and timelines. Details of the mandatory adverse event and safety reporting requirements are detailed in Appendix 3C.
Expected side effects
All adverse events judged by either the investigator or the sponsor as having a reasonable suspected causal relationship to insulin therapy qualified as adverse reactions. Whereas any suspected, unexpected, serious adverse reactions (SUSARs) involving insulin therapy were reported according to the timelines for SUSARs, expected side effects of insulin were reported in the annual safety report unless serious enough to warrant expedited reporting.
Hypoglycaemia is the principal side effect of insulin therapy. Moderate and severe hypoglycaemia were defined as a blood glucose < 2.5 mmol/l and < 2 mmol/l48 respectively. The insulin administration protocols aimed to achieve blood glucose control with the lowest possible incidence of hypoglycaemia and the avoidance of neuroglycopenia (hypoglycaemia associated with neurological symptoms and signs such as seizures and cerebral oedema). By definition, children in the TGC arm were at increased risk of hypoglycaemia because the target range in this arm of the study (i.e. blood glucose 4–7 mmol/l) was much closer to the trial’s predefined hypoglycaemic thresholds than the 10–12 mmol/l therapeutic window used as a target for the control of blood glucose in the CM arm of the study. The principal operating procedure used to avoid hypoglycaemia was blood glucose measurement every 30 minutes when insulin was first administered, and then every 45 minutes until blood glucose was controlled within the required range and stable glucose and insulin infusion rates were achieved, and then hourly once stabilised.
Insulin is reported to occasionally cause a rash which may be associated with itching.
Data collection
To minimise the data collection load for busy units, the trial collaborated with the Paediatric Intensive Care Audit Network (PICANet)49 to make best use of the established data collection infrastructure which exists in all PICUs in the UK. The PICANet data set included many of the items being used in the trial and these data were transmitted from the participating centres to the DCC electronically using strong encryption. The remaining short-term data items were collected locally by the research nurses, and those for the longer-term follow-up were collected separately by telephone and postal questionnaires. These data were used to report the mean number of inpatient days following readmissions after 30 days, and the mean outpatient and community service use at 12 months for all patients randomised. The main data collection forms, questionnaires and covering letters are shown in Appendix 7.
All case report form (CRF) data were double entered onto electronic database storage systems at the DCC.
Sample size
The primary outcome was VFD-30. A difference of 2 days in VFD-30 was considered clinically important. Information from PICANet from a sample of PICUs for 2003–4 provided estimates that the mean VFD-30 in cardiac patients is 26.7 days, with a SD of 4.2 days. The corresponding figures for non-cardiac patients were a mean of 22.7 days and a SD of 6.8 days. As the SD is estimated with error, to be conservative a SD nearer 5.5 days for the cardiac and 8 days for the non-cardiac patients was assumed. There were likely to be more non-cardiac than cardiac patients eligible for the trial. An overall SD across both cardiac and non-cardiac strata of 7 days was therefore assumed. Assuming this was the same in both trial arms, and taking a type I error of 1% (with a two-sided test), a total sample size of 750 patients would have 90% power to detect this difference. Although minimal loss to follow-up at 30 days could be assumed, there was the possibility of some non-compliance (some patients allocated to TGC not receiving this, and some allocated to CM being managed with TGC). The target size was therefore inflated to 1000 to take account of possible dilution of effect.
As information from PICANet indicated that there were differences in outcome between cardiac and non-cardiac patients, not merely in VFD-30 but also in 30-day mortality (3.4% vs. 20%) and mean duration of ventilation (3.7 vs. 8.0 days, survivors and non-survivors combined), the trial was powered to be able to detect whether or not any effect of tight glucose control differed between the cardiac and non-cardiac strata. To have 80% power for an interaction test to be able to detect a difference of 2 days in the effect of intervention between the strata at the 5% level of statistical significance, the sample size was increased to 1500. If the interaction test was positive, this size would allow assessment of the effect of TGC separately in the two strata.
Centres
The following PICUs in the UK planned to recruit patients into the CHiP trial: Birmingham Children’s Hospital; Bristol Royal Hospital for Children; Great Ormond Street Hospital; Leeds General Infirmary; University Hospitals of Leicester – Glenfield Hospital and Leicester Royal Infirmary; Royal Brompton and Harefield NHS Trust (Royal Brompton Hospital); Royal Liverpool Children’s NHS Trust; Royal Manchester Children’s Hospital; St George’s Hospital; St Mary’s Hospital; Sheffield Children’s NHS Foundation Trust; Southampton General Hospital; and University Hospital of North Staffordshire.
Recruitment rate
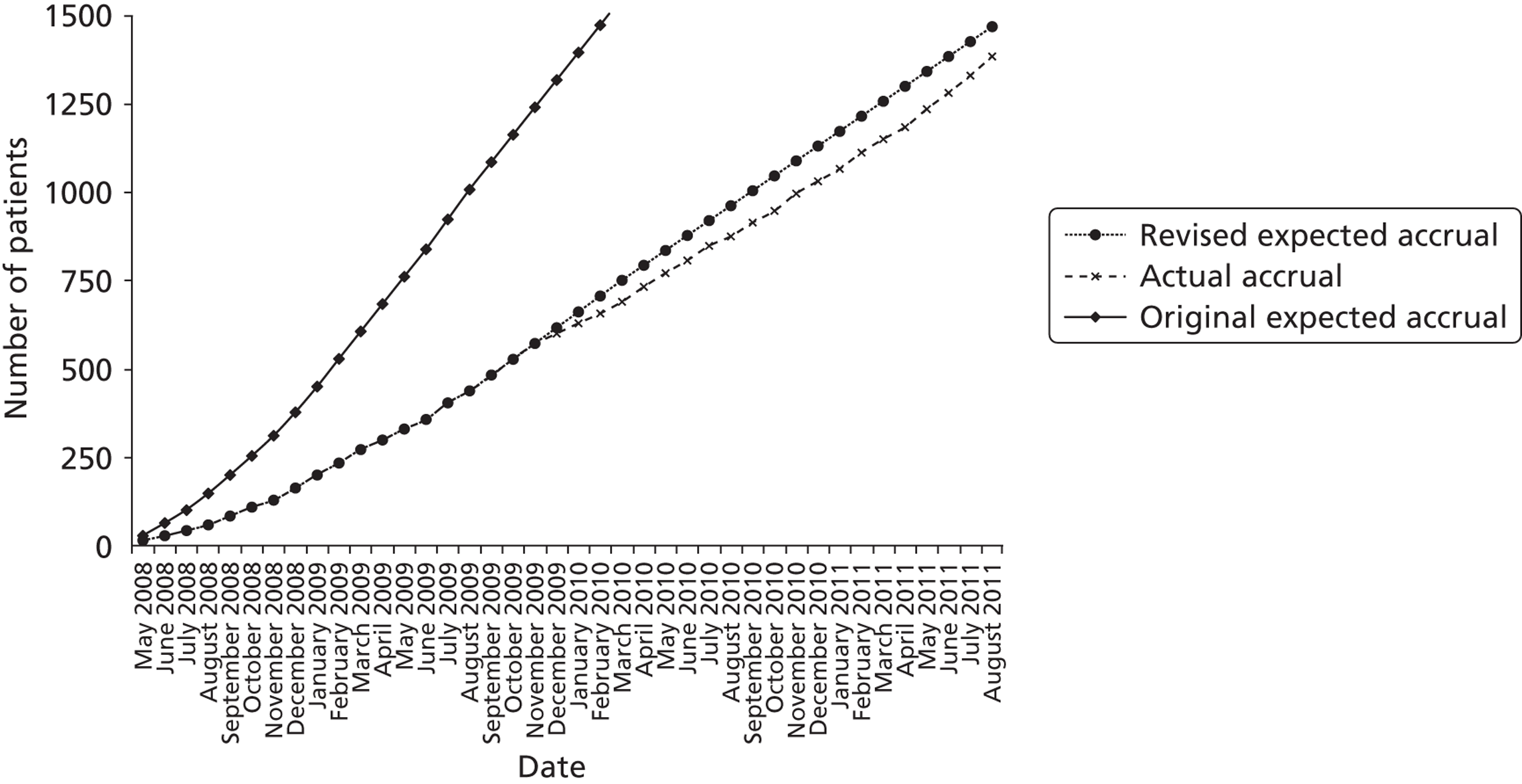
There were estimated to be approximately 1300 eligible cardiac and 1550 eligible non-cardiac patients per year in collaborating PICUs at the start of the trial. About half of those eligible were anticipated to be recruited into the trial, predicting that the overall total sample size of 1500 would be accrued by September 2011.
Type of analysis for clinical outcomes at discharge from paediatric intensive care unit or at 30 days
Primary analyses were by intention to treat. For the primary outcome, linear regression models were used to estimate a mean difference in VFD-30 between the two arms of the trial. For the secondary outcomes, appropriate generalised linear models were used to examine the effect of the intervention. Odds ratios and mean differences are reported with 95% CIs. Where there was evidence of non-normality in the continuous outcome measures, non-parametric bootstrapping, with 1000 samples, was used to estimate the effect of the intervention50 and bias-corrected CIs are reported.
Secondary analyses included the following prespecified subgroup analyses: cardiac surgical compared with non-cardiac cases, age (< 1 year or between 1 and ≤ 16 years), TBI or not, RACHS1 (cardiac cases) (groups 1–4 vs. 5 and 6), PIM2 risk of mortality (non-cardiac cases) (categorised by probabilities of death of < 5%, between 5% and < 15% and ≥ 15%), run-in cases (first 100 randomised) compared with non-run-in cases. Likelihood ratio tests for interactions were used to assess whether or not there was any difference in the effect of the intervention in the different subgroups. Where stratified results are presented, the effects in the different strata are estimated directly from the regression model with the interaction term included.
Frequency of analysis
An independent Data Monitoring and Ethics Committee (DMEC) planned to review, in strict confidence, data from the trial approximately half-way through the recruitment period. The chair of the DMEC could also request additional meetings/analyses. In the light of these data, and other evidence from relevant studies, the DMEC would inform the Trial Steering Committee (TSC) if in their view:
-
There was proof that the data indicated that any part of the protocol under investigation was either clearly indicated or clearly contraindicated for either all patients or a particular subgroup of patients, using the Peto and Haybittle rule. 51,52
-
It was evident that no clear outcome would be obtained with the current trial design.
-
They had a major ethical or safety concern.
Except for those who supplied the confidential information, everyone (including the TSC, funders, collaborators and administrative staff) remained ignorant of the results of the interim analysis
Economic evaluation
Overview
Cost–consequence analyses were undertaken to assess whether or not any additional costs of achieving TGC were justified by subsequent reductions in hospitalisation costs and/or by improvements in patient outcomes. The evaluations were conducted in two phases: in the first phase, all hospital costs at 30 days post randomisation were compared across randomised arms alongside 30-day clinical outcomes; and, in the second phase, cost and outcomes at 12 months post randomisation were compared between arms, and used to project relative cost-effectiveness over the lifetime. This aspect of the costing study took the health and personal services perspective recommended by the National Institute for Health and Care Excellence (NICE). 53
Measurement of resource use up to 30 days post randomisation
The trial CRFs recorded the number of inpatient days for the index hospital episode following randomisation, up to day 30. Within this index hospital episode, the CRFs recorded the number of PICU days spent on the unit where the patient was randomised, and any subsequent PICU bed-days following transfers to other hospitals. The CRFs also recorded the LOS on general medical (GM) wards, both within the acute hospital where the patient was randomised and following transfer to other hospitals. The number of day-case admissions was also noted. The total LOS for the initial hospital episode was calculated as the sum of the LOS at PICUs and GM wards up to a maximum of 30 days following randomisation. Any readmissions within 30 days to the PICU where the child was randomised were also recorded. The LOS following these readmissions was added to the total number of days for the initial episode, to give the total hospital LOS up to 30 days post randomisation.
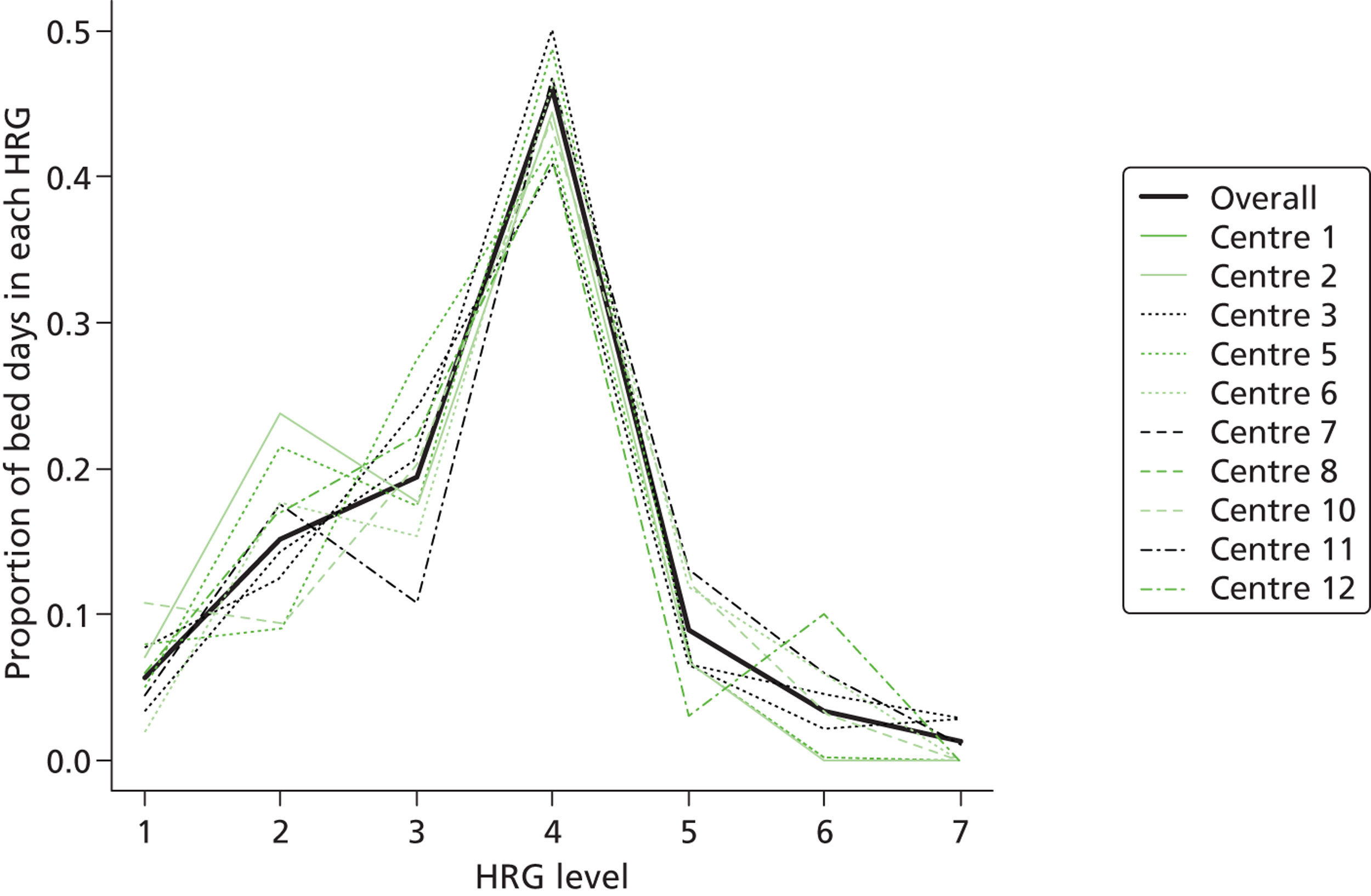
Data on the level of care for PICU bed-days were available through routine collection of the Paediatric Critical Care Minimum Data Set (PCCMDS)54 in 11 of the participating centres via the PICANet database. The PCCMDS consists of 32 items recorded for each PICU bed-day that can be used to define the level of care, that is the paediatric critical care health-care resource group (HRG). 55 The PCCMDS data items were extracted for each PICU bed-day after randomisation. Each PICU bed-day was then assigned to the appropriate HRG (HRG, version 4), using the HRG grouper. 55 [The HRG classification includes items for primary and secondary diagnosis, OPCS (Office of Population, Censuses and Surveys’ Classification of Surgical Operations and Procedures) codes for high-cost drugs, and fields for critical care activity.] Table 1 lists the HRG classifications for PIC with examples of procedures for each category. Figure 2 reports the distribution of PICU bed-days across HRG categories for the 8954 PICU bed-days that were grouped during the first 30 days, both overall and for each site. The most common HRG category was ‘intensive care basic enhanced’ [HRG level 4 (HRG4)]. For 1862 bed-days in the 11 sites that provided information, PCCMDS data were missing or incomplete, and, for three centres (254 bed-days), no PCCMDS information was available. All these bed-days were assigned to the modal HRG category (HRG4) (see Sensitivity analysis). For a total of 326 bed-days, the activity reported was categorised by the HRG grouper as ‘not critical care activity’, and designated as bed-days on GM wards.
| HRG Level | Critical care unit | Examples of procedures |
|---|---|---|
| HRG1 | HDU basic | ECG or CVP monitoring, oxygen therapy plus pulse oximetry |
| HRG2 | HDU advanced | Non-invasive ventilation, acute haemodialysis, vasoactive infusion (inotrope, vasodilator) |
| HRG3 | ICU basic | Invasive mechanical ventilation, or non-invasive ventilation + vasoactive infusion + haemofiltration |
| HRG4 | ICU basic enhanced | Invasive mechanical ventilation + vasoactive infusion, or advanced respiratory support |
| HRG5 | ICU advanced | Invasive mechanical ventilation or advanced respiratory support + haemofiltration |
| HRG6 | ICU advanced enhanced | Invasive mechanical ventilation or advanced respiratory support + burns > 79% BSA |
| HRG7 | ICU ECMO/ECLS | ECMO or ECLS, including VAD or aortic balloon pump |
FIGURE 2.
Distribution of paediatric critical care bed-days within 30 days post randomisation across HRG categorises for CHiP patients. Proportion of paediatric critical care bed-days within each HRG category, overall and by centre.
Unit costs
Unit costs were taken from the 2011 NHS Payments by Results (PbR) database, which includes reference costs returns from each NHS trust. 38 For all critical care admissions, each bed-day was costed with the corresponding unit cost per bed-day from the PbR database. 38 The unit cost of bed-days classified as ‘intensive care basic’ was taken as the average across all CHiP centres that returned reference costs for that HRG category (HRG3, Table 2) (see Sensitivity analysis). Few NHS trusts returned reference cost information for all seven paediatric critical care HRGs, so the relative unit costs for all HRGs apart from ‘intensive care basic’ (HRG3) were calculated by multiplying the unit costs for HRG 3 by the relative cost ratio from a previous detailed multicentre PICU costing study (see Sensitivity analysis). 56 This previous study assessed the relative staff input according to HRG category and reported the cost ratios listed (see Table 2). Table 2 reports the PICU costs taken for the base case and subsequent sensitivity analyses (SAs). The unit costs for GM bed-days and day-case admissions were taken from previous studies (Table 3). 58,59
| HRG | Cost ratios (NHS information centre) | PCC reference costs (n = 11 CHiP centres) | PCC reference cost for ICU basic weighted by cost ratios |
|---|---|---|---|
| HDU basic | 0.75 | 1112 | 1324 |
| HDU advanced | 0.91 | 1315 | 1607 |
| ICU basic | 1 | 1765 | 1765 |
| ICU basic enhanced | 1.22 | 2065 | 2154 |
| ICU advanced | 1.4 | 1998 | 2472 |
| ICU advanced enhanced | 2.12 | 3061 | 3743 |
| ICU ECMO/ECLS | 3.06 | 4026 | 5402 |
| Personal or social service | Unit costa |
|---|---|
| Hospital inpatient bed-day | 252 |
| Hospital outpatient visit | 147 |
| Day cases | 202 |
| GP contact | 47 |
| GP practice nurse contact | 11 |
| Health visitor contact | 11 |
| District nurse contact | 11 |
| Social worker contact | 9 |
| Speech and language therapist contact | 8 |
| Occupational therapist contact | 10 |
| Physiotherapist contact | 8 |
| Children’s disability team contact | 7 |
| Hospital discharge co-ordinator contact | 6 |
| Child psychologist contact | 15 |
| Dietitian contact | 8 |
| Mental health service contact | 27 |
| Specialist paediatric nurse contact | 14 |
| School nurse contact | 3 |
The base-case analysis assumed that all resource use required implementation of the TGC protocol or management of side effects and was recognised by the HRG categorisation, as including any additional resources could represent double-counting. A SA was also undertaken to investigate whether or not the results were robust to an alternative approach whereby the resources and unit costs of implementing the TGC protocol and managing hypoglycaemic events were considered as additional unit costs (see Sensitivity analysis). To inform these SAs, information was collected on the nurse time, clinical time, number of blood gas analyses and insulin required in managing a subsample of patients in either group during the first 48 hours in critical care after randomisation. Soluble insulin (Actrapid®, Novo Nordisk Limited) was assumed to be used over a 24-hour period, the unit costs of which were taken from the British National Formulary. 60 The additional resources required in managing patients who in the CRFs were recorded as having moderate or severe hypoglycaemic episodes were also considered. All unit costs were reported in 2010–11 prices.
Statistical analysis for the 30-day economic end points
All the economic analyses were based on the treatment arms as randomly allocated (‘intention to treat’). Mean differences between treatment arms in resource use (e.g. total LOS summed across the index hospital episode and readmissions to PICU within 30 days) were reported for the overall cohort, and separately for subgroups admitted following either cardiac surgery (cardiac surgery) or no cardiac surgery (non-cardiac). Incremental costs were estimated as the mean difference (95% CI) in total costs at 30 days using ordinary least squares (OLS) regression analysis, with randomised arm as the only independent variable. To test the hypothesis that incremental costs differed according to cardiac surgery status, the OLS regression was repeated, including independent effects for randomised arm, cardiac surgery status and an interaction term for randomised arm by cardiac status. A likelihood ratio test was then employed to compare the model fit to that from an OLS model that included randomised arm and cardiac surgery status as independent effects. Finally, the above OLS regression models that included the interaction terms for randomised arm by cardiac status were used to report the incremental costs for each subgroup (cardiac surgery, non-cardiac).
Resource-use measurement between 30 days and 12 months post randomisation
Index hospital episode and readmissions to the paediatric intensive care unit within 30 days post randomisation
Information on PICU days and hospital LOS for up to 12 months was collected from the CHiP trial CRFs. For CHiP patients whose index hospital episode exceeded 30 days, the CRFs collected information on their continuing hospital stay up to a maximum of 12 months. The CRFs also noted any readmissions that were within 30 days to the PICU where the patient was randomised. For both initial hospital episodes and readmissions, the CRFs recorded subsequent transfers to other hospitals. For initial hospital admissions and readmissions, the CRFs distinguished between the total LOS in PIC and those on GM wards, and day cases. These data were used to report the total days in PIC and on GM wards up to 12 months. The last time point at which 12-month follow-up data were available from the CRFs was 31 March 2012, so, for patients randomised after 1 April 2011, it was possible that an index admission or a readmission was censored before 12 months post randomisation.
Other hospital and community service use
The postal questionnaires were used to collect information on hospital and community service use, from discharge from the index hospital episode up to 12 months post randomisation. This questionnaire was based on one previously developed for neonatal intensive care,61 and modified to include those items of service use most relevant to patients discharged from PIC. The items for the questionnaires were further amended following comments from a panel of parents from the Medicines for Children Research Network (MCRN). The final questionnaire is appended61 (see Appendix 8). The items considered covered readmissions to PIC other than those collected on the CRFs, readmissions to GM wards, outpatient visits, and contacts with the GP, practice nurse, health visitor, social worker, speech and language therapist and child psychologist.
Unit costs and calculation of total 12-month costs per patient
For PICU bed-days from 30 days to 12 months post randomisation, PCCMDS information was not available for categorising each bed-day into the appropriate HRG4 category. Each PICU bed-day was, therefore, assumed to be in the modal HRG4 category (intensive care basic enhanced) and assigned the corresponding unit cost. GM bed-days and outpatient and community service use were valued with national unit costs (see Table 3). Total costs for each patient were then calculated by summing the costs of all hospital and community health services used.
Statistical analysis for the 12-month end points (resource use, survival, costs)
Mean differences in resource use (e.g. days in PICU, on GM wards and combined) between the randomised arms were reported. The proportion of patients still in hospital (index admission) was plotted for up to 12 months post randomisation. Each of these items was reported for the overall cohort and separately for those admitted for cardiac surgery or not.
The effect of TGC compared with CM on 12-month mortality and cost was then reported, overall and for the cardiac and non-cardiac stratum. For a subsample of patients, 12-month health and community cost data were censored. Other patients were judged ineligible or did not respond to the 12-month service-use questionnaire. The cost data that were either censored or missing at 12 months were addressed with multiple imputation (MI). 62–64 The imputation models included baseline covariates, the number of ventilated days, total LOS and costs at 30 days, and information on 12-month costs for those individuals for whom this end point was observed. Each imputation model assumed that the data were ‘missing at random’, that is conditional on the variables included in each imputation model. 62 MI was employed based on predictive mean matching,65 which offers relative advantages when dealing with data, such as costs that have irregular distributions. 66
Incremental costs were estimated as the mean difference (95% CI) in total costs at 12 months post randomisation with OLS regression analysis. Incremental costs were reported both overall and for the cardiac and non-cardiac strata.
As the missingness pattern may differ across treatment groups, separate imputation models were specified for each comparator. Five imputed data sets were generated for the imputation models (see Sensitivity analysis). After imputation, the analytical models were applied to estimate incremental costs overall, and by subgroup to each imputed data set. Each of the resultant estimates was combined with Rubin’s formulae,62 which recognise uncertainty both within and between imputations. All MI models were implemented in R with multivariate imputation by chained equations. 67
Sensitivity analysis
The base-case cost analysis made the following assumptions that a priori were judged potentially important: (1) all relevant resource use relating to implementing the TGC protocol and managing side effects was recognised by the paediatric critical care HRG categorisation; (2) PICU bed-days for which PCCMDS data were missing or incomplete were in the HRG category for ‘basic enhanced intensive care’ (HRG4); (3) the cost ratios from a previous PICU costing study reflected the relative costs; (4) the average unit costs for HRG4 were taken just from CHiP sites; (5) the regression models had residuals that were normally distributed.
The following separate, univariate, SAs tested whether or not the results were robust to the following alternative assumptions.
-
Inclusion of the costs of implementing the TGC protocol and managing hypoglycaemic episodes as specific additional items. The HRG categorisation could be insensitive to the resource use required to implement the TGC protocol, and for managing hypoglycaemic episodes. SAs were therefore conducted that considered any additional staff times, blood gas analyses and insulin required for:
-
implementing the TGC protocol compared with CM
-
as for i. but also including any further costs for managing the moderate or severe hypoglycaemic episodes recorded.
-
-
Reassignment of PICU bed-days without HRG classification to either:
-
‘ICU basic’ (HRG3); or
-
‘ICU advanced’ (HRG5).
-
The unit costs for each level of care in PICU were taken directly from PbR. Rather than using the cost ratios, unit costs from PbR were used for each HRG level.
-
PICU costs were taken as national averages: the unit costs of PIC were taken as averages from all centres that returned the relevant costs in PbR including non-CHiP sites.
-
Assume gamma rather than normal distributions for costs at 30 days and 12 months. The assumption that costs are normally distributed may not be plausible,68 so here costs were allowed to follow a gamma distribution. 69
-
-
The MI was rerun with 10 imputations. In some circumstances, five imputations may be insufficient to test the impact of increasing the number of imputations; the imputation models were rerun but with 10 imputations.
For each SA, the effect of TGC compared with CM on 12-month costs was reported, overall and for the cardiac and non-cardiac subgroups.
Lifetime cost-effectiveness analysis
The cost and outcome data collected at 1 year were used to project the impact of the intervention on longer-term costs and outcomes. Kaplan–Meier survival curves were plotted out to the maximum time of follow-up, overall and then separately for cardiac and non-cardiac cases. Alternative parametric functions were considered for extrapolating mortality up to 5 years, by fitting commonly recommended alternative distributions to the CHiP survival data, excluding the first 365 days post randomisation, as the event rate during this early period was anticipated to be atypical and not to provide an appropriate basis for extrapolation. The base-case analysis used the ‘most appropriate’ parametric survival curves judged according to which gave the best fit to the observed data and the most plausible extrapolation relative to the age- and gender-matched general population. 70 Survival extrapolations were considered for the first 5 years from randomisation, as this was anticipated to be the period over which the risk of death would be higher than that for the age- and gender-matched general population. After 5 years post randomisation, all-cause death rates were assumed to be that of the age- and gender-matched general population. The parametric extrapolations for years 1–5 were combined, applying all-cause death rates for years 6 onwards to report life expectancy for each CHiP patient observed to survive at 1 year. The projected life expectancy was used to report life-years following TGC compared with CM both overall and for the cardiac and non-cardiac strata.
Previous evidence suggests that a minority of PICU survivors may suffer from long-term disability and reductions in quality of life (QoL), and therefore QALYs were reported. QoL data were not collected for the patients who did not have TBI in the CHiP trial. Instead, information was used from a previous study that included a large sample of PICU patients with similar baseline characteristics to those of the patients of the CHiP study71 and reported QoL with the HUI questionnaire at 6 months after the index admission. The mean QoL from this previous study (0.73, on a scale anchored at 0, death, and 1, perfect health) was applied to weight each life-year of those CHiP patients predicted to be alive 12 months after randomisation.
To project costs attributable to the initial critical care admission for years 1–5, the inpatient, outpatient and community service costs reported from the service-use survey at 1 year were assumed to be maintained until the end of year 2. Previous studies have suggested that, between 3 and 4 years after the initial PICU admission, around 10% of survivors have relatively severe disability. Those predicted to survive in years 3–5 were, therefore, assumed to have incurred 10% of the mean costs reported at 12 months. Those patients who were observed or predicted to die before 12 months were assigned zero QALYs.
Lifetime incremental costs per life-year, and per QALY gained, were reported. INBs were calculated by valuing each QALY at the £20,000 per QALY threshold recommended by NICE. 53 All future costs and life-years were discounted at the recommended rate of 3.5%. 53 All incremental cost-effectiveness results are reported overall, and then for the cardiac and non-cardiac strata.
Sensitivity analysis on lifetime cost-effectiveness analysis
The following further SAs were run to test the assumptions made in the lifetime analyses:
-
An alternative parametric extrapolation was used to project survival from year 1 to 5.
-
Excess mortality compared with the age- and gender-matched general population was assumed to remain for 10 rather than 5 years post randomisation, that is the parametric extrapolation was applied for years 1–10, and then applied for all-cause death rates.
-
It was assumed that trial patients were not subject to any excess mortality other than that of the age- and gender-matched general population.
-
Rather than assuming the mean QoL (0.73) from a previous study, the values given by the lower and upper 95% CIs around that mean (0.71 to 0.75) were assumed.
-
Alternative assumptions were made about the duration and magnitude of the costs:
-
For all patients who survived beyond 1 year, it was assumed that costs were maintained until the end of year 3 (rather than 2).
-
10% of costs were assumed to be maintained over years 3–10 rather than years 3–5.
-
The costs for years 2–5 were assumed to be 50% not 10% of those at 12 months.
-
i. to iii. above were combined.
-
For each of these SAs, the lifetime INBs of TGC compared with CM overall and for the cardiac and non-cardiac strata were reported.
Ancillary studies
In addition to the main study, the grant holders welcomed more detailed or complementary studies, provided that proposals were discussed in advance with the TSC and appropriate additional research ethics approval was sought. These will not be discussed further in this monograph.
Publication policy
To safeguard the integrity of the trial, data from this study were not presented in public or submitted for publication without requesting comments and receiving agreement from the TSC. The primary results of the trial will be published by the group as a whole in collaboration with local investigators and local investigators will be acknowledged. The success of the trial was dependent on the collaboration of many people. The results were, therefore, presented first to the trial local investigators. A summary of the results of the trial will be sent to parents of participating children on request and also made available on the trial website.
Organisation
A TSC (see Appendix 9) and a DMEC were established (see Appendix 10). Day-to-day management of the trial was overseen by a Trial Management Group (TMG) (see Appendix 11). Each participating centre identified a paediatric intensivist as a PI (see Appendix 12). Each participating centre was allocated funding (from the core trial grant, from the MCRN and/or from local Comprehensive Local Research Networks) for research nursing time, and employed or reallocated a research nurse to support all aspects of the trial at the local centre.
Confidentiality
Patients were identified by their trial number to ensure confidentiality. However, as the patients in the trial were contacted about the study results (and patients recruited until November 2010 were followed up for 12 months following randomisation), it was essential that the team at the DCC had the names and addresses of the trial participants recorded on the data collection forms in addition to the allocated trial number. Stringent precautions were taken to ensure confidentiality of names and addresses at the DCC.
The chief investigator and local investigators ensured conservation of records in areas to which access is restricted.
Audit
To ensure that the trial was conducted according to the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and Good Clinical Practice (GCP) guidelines, site audits were carried out on a random basis. The local investigator was required to demonstrate knowledge of the trial protocol and procedures and ICH and GCP. The accessibility of the site file to trial staff and its contents were checked to ensure all trial records were being properly maintained. Adherence to local requirements for consent was examined.
If the site had full compliance, the site visit form was signed by the lead research nurse. In the event of non-compliance, the DCC and/or the lead research nurse addressed the specific issues to ensure that relevant training and instruction were given.
The CHiP trial also passed an inspection by the Medicines and Healthcare products Regulatory Agency (MHRA) (August 2009).
Termination of the study
At the termination of planned recruitment, the DCC contacted all sites by telephone, email or fax in order to terminate all patient recruitment as quickly as possible. After all recruited patients had been followed until 30 days post randomisation (or hospital discharge if stay > 30 days), a declaration of the end of trial form was sent to EudraCT and the Multicentre Research Ethics Committee (MREC). The following documents will be archived in each site file and kept for at least 5 years: original consent forms, data forms, trial-related documents and correspondence. At the end of the analysis and reporting phase, the trial master files at the clinical co-ordinating centre and DCC will be archived for 15 years.
Funding
The costs for the study itself were covered by a grant from the HTA programme. Clinical costs were met by the NHS under existing contracts.
Indemnity
If there is negligent harm during the clinical trial, when the NHS body owes a duty of care to the person harmed, NHS indemnity covers NHS staff, medical academic staff with honorary contracts and those conducting the trial. NHS indemnity does not offer no-fault compensation.
Chapter 3 Results
Recruitment
Trial recruitment began on 4 May 2008. As indicated in Chapter 2, recruitment was slower than expected. This was mainly a result of delays in trial initiation at some sites, clinical constraints and a ‘research learning curve’ in many of the participating units which had no previous experience of recruiting critically ill children to clinical trials. These delays necessitated an application to the HTA programme for an extension to the trial. The HTA programme granted funding to allow recruitment to be extended to allow the trial to achieve sufficient power (1500 children) to identify whether or not there was a differential effect for the primary end point (VFD-30) in the two strata (cardiac and non-cardiac).
The DMEC confidentially reviewed unblinded interim analyses on two occasions. In addition, they met to discuss SAEs and recruitment rates on three further occasions.
Recruitment closed on 31 August 2011, as agreed in the HTA funding. A total of 19,924 children were screened from 13 sites. Of these, 1384 were recruited and randomised (701 to TGC and 683 to CM). The reasons for non-recruitment are shown in Table 4. Of the 1384, 15 were subsequently found to be ineligible (Table 5), leaving 1369 eligible children (694 to TGC and 675 to CM) randomised into the trial – 91% of the original target of 1500. The flow of patients is shown in Figure 3 and cumulative recruitment in Figure 4. Recruitment by site is shown in Table 6.
| Reason not recruited | All screened, non-recruited patients N = 18,540 | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n/N (%) | |||||||||||||||||||||||||||||||||||||||
| Inclusion criteria | > 16 years | 211 | 1.14 | |||||||||||||||||||||||||||||||||||||
| Mechanical ventilator and vasoactive drugs not likely to be continued for > 12 hours | 2285 | 12.32 | ||||||||||||||||||||||||||||||||||||||
| Arterial line not in situ | 1576 | 8.50 | ||||||||||||||||||||||||||||||||||||||
| Not ventilated | 2637 | 14.22 | ||||||||||||||||||||||||||||||||||||||
| No inotropes | 9601 | 51.79 | ||||||||||||||||||||||||||||||||||||||
| Exclusion criteria | < 36 weeks corrected gestational age | 565 | 3.05 | |||||||||||||||||||||||||||||||||||||
| Diabetes mellitus | 56 | 0.30 | ||||||||||||||||||||||||||||||||||||||
| Error of metabolism | 268 | 1.45 | ||||||||||||||||||||||||||||||||||||||
| Treatment withdrawal/limitation | 312 | 1.68 | ||||||||||||||||||||||||||||||||||||||
| > 5 days on PICU | 135 | 0.73 | ||||||||||||||||||||||||||||||||||||||
| Already participated in CHiP | 262 | 1.41 | ||||||||||||||||||||||||||||||||||||||
| Other | Refused consent | 1116 | 6.02 | |||||||||||||||||||||||||||||||||||||
| Patient died | 143 | 0.77 | ||||||||||||||||||||||||||||||||||||||
| Not asked within time frame | 531 | 2.86 | ||||||||||||||||||||||||||||||||||||||
| Other (further details in box below) | 1573 | 8.48 | ||||||||||||||||||||||||||||||||||||||
| Text responses for category
‘other’ Research nurse on leave/ill/unavailable (weekend) 322 ECMO/transplant 134 Language difficulties 127 In another trial/approached for another trial 116 Legal/social issues 109 No decision within time frame 97 PI – clinical decision 95 Parents not available/too upset 89 Transferred to another hospital 48 Non-TBI site 33 Other 403 ECMO, extracorporeal membrane oxygenation. |
Research nurse on leave/ill/unavailable (weekend) | 322 | ECMO/transplant | 134 | Language difficulties | 127 | In another trial/approached for another trial | 116 | Legal/social issues | 109 | No decision within time frame | 97 | PI – clinical decision | 95 | Parents not available/too upset | 89 | Transferred to another hospital | 48 | Non-TBI site | 33 | Other | 403 | ECMO, extracorporeal membrane oxygenation. | |||||||||||||||||
| Research nurse on leave/ill/unavailable (weekend) | 322 | |||||||||||||||||||||||||||||||||||||||
| ECMO/transplant | 134 | |||||||||||||||||||||||||||||||||||||||
| Language difficulties | 127 | |||||||||||||||||||||||||||||||||||||||
| In another trial/approached for another trial | 116 | |||||||||||||||||||||||||||||||||||||||
| Legal/social issues | 109 | |||||||||||||||||||||||||||||||||||||||
| No decision within time frame | 97 | |||||||||||||||||||||||||||||||||||||||
| PI – clinical decision | 95 | |||||||||||||||||||||||||||||||||||||||
| Parents not available/too upset | 89 | |||||||||||||||||||||||||||||||||||||||
| Transferred to another hospital | 48 | |||||||||||||||||||||||||||||||||||||||
| Non-TBI site | 33 | |||||||||||||||||||||||||||||||||||||||
| Other | 403 | |||||||||||||||||||||||||||||||||||||||
| ECMO, extracorporeal membrane oxygenation. | ||||||||||||||||||||||||||||||||||||||||
| TGC arm | CM arm |
|---|---|
| During a monitoring visit it was found that this patient had not fully consented | During the final data analysis the trial statistician found that this patient actually met one of the exclusion criteria, i.e. he or she was in PICU for > 5 days before he or she was randomised |
| In response to a query it was found that this patient was recruited in error. He or she did not meet one of the eligibility criteria (taking vasoactive drugs) | During the final data analysis the trial statistician found that this patient actually met one of the exclusion criteria, i.e. he or she was in PICU for > 5 days before he or she was randomised |
| Patient randomised incorrectly, possibly met one of the exclusion criteria,as when randomised had suspected metabolic illness; therefore treatment was stopped early | During the final data analysis the trial statistician found that this patient actually met one of the exclusion criteria, i.e. he or she was in PICU for > 5 days before he or she was randomised |
| Parents had forgotten that their child had previously been in the trial; therefore he or she was not eligible for the trial | During the final data analysis the trial statistician found that this patient actually met one of the exclusion criteria, i.e. he or she was in PICU for > 5 days before he or she was randomised |
| Patient randomised in error: met the exclusion criterion of having an inborn error of metabolism (Refsum’s disease, which is a rare disorder of lipid metabolism) | Patient randomised in error: met the exclusion criterion of being in PICU for > 5 days but site did not realise until after randomised |
| During the final data analysis the trial statistician found that this patient actually met one of the exclusion criteria, i.e. he or she was in PICU for > 5 days before he or she was randomised | Patient randomised in error: met the exclusion criterion of being in PICU for > 5 days but site did not realise until after randomised |
| Patient randomised in error: did not meet the inclusion criterion of being on inotropes at randomisation | Patient randomised in error: met the exclusion criterion of having an inborn error of metabolism (Barth syndrome) |
| Patient randomised in error: did not meet the inclusion criterion of being on inotropes at randomisation |
FIGURE 3.
A flow chart showing the flow of patients. Note that the information on vital status up to 12 months was available from the Office for National Statistics, aside from for 17 non-UK nationals.
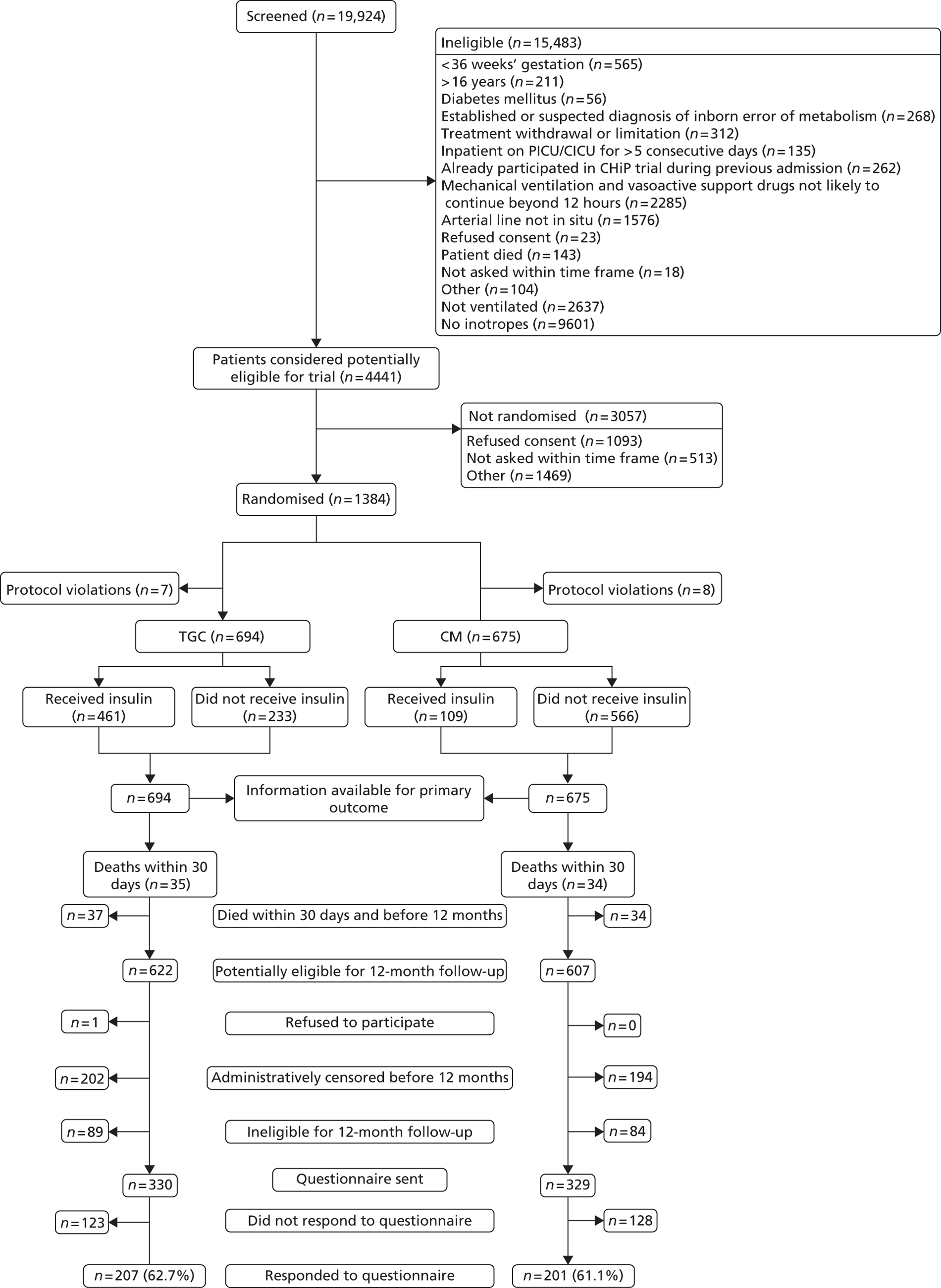
FIGURE 4.
Cumulative recruitment – actual accrual vs. revised expected.
| Site | Screened (N) | Randomised [n (%)] |
|---|---|---|
| 1. Birmingham Children's Hospital | 3490 | 241 (6.9) |
| 2. Bristol Royal Hospital for Children | 1525 | 148 (9.7) |
| 3. Great Ormond Street Hospital | 3878 | 210 (5.4) |
| 4. Leeds General Infirmary | 278 | 3 (1.1) |
| 5. Royal Brompton Hospital | 1695 | 158 (9.3) |
| 6. Royal Liverpool Children's NHS Trust | 2145 | 164 (7.6) |
| 7. Royal Manchester Children's Hospital | 1806 | 75 (4.2) |
| 8. St Mary’s Hospital | 935 | 60 (6.4) |
| 9. Sheffield Children's NHS Foundation Trust | 320 | 22 (6.9) |
| 10. Southampton General Hospital | 2416 | 219 (9.1) |
| 11. University Hospital of North Staffordshire | 419 | 15 (3.6) |
| 12. University Hospitals of Leicester | 946 | 63 (6.7) |
| 14. St George's Hospital | 71 | 6 (8.5) |
| Total | 19,924 | 1384 |
Comparability at baseline
The characteristics of the children at baseline are shown in Table 7. The randomised groups were broadly comparable at trial entry. Sixty-two per cent were randomised within 1 day of admission to PICU. In terms of the prespecified stratifying factors, two-thirds were aged < 1 year, and 60% of the children were in the cardiac surgery stratum. Seven per cent of children in the cardiac surgery stratum were considered to be undergoing surgical procedures associated with a high risk of mortality (RACHS1 score 5 or 6), and 19% of children in the non-cardiac group had a PIM2 score indicative of a ≥ 15% risk of PICU mortality.
| Characteristic | TGC (N = 694) | CM (N = 675) |
|---|---|---|
| Centrea | ||
| 1 | 122 | 119 |
| 2 | 71 | 75 |
| 3 | 101 | 106 |
| 4 | 1 | 2 |
| 5 | 78 | 76 |
| 6 | 82 | 80 |
| 7 | 41 | 34 |
| 8 | 31 | 28 |
| 9 | 10 | 12 |
| 10 | 111 | 106 |
| 11 | 9 | 6 |
| 12 | 34 | 29 |
| 14 | 3 | 2 |
| Sex | ||
| Male [n (%)] | 389 (56.05) | 363 (53.78) |
| Age (years)a | ||
| 0 to < 1 [n (%)] | 432 (62.24) | 421 (62.36) |
| 1 to < 16 [n (%)] | 262 (37.75) | 254 (37.63) |
| Median (IQR) | 0.49 (0.07–2.72) | 0.53 (0.08–2.73) |
| Weight (kg) [mean (SD)] | 12.13 (15.11) | 11.31 (12.88) |
| Height (cm) [mean (SD)] | 76.24 (32.67) | 76.09 (31.75) |
| Not measured | 65 | 63 |
| Waist circumference (cm) [mean (SD)] | 44.88 (14.30) | 44.13 (12.82) |
| Not measured | 140 | 150 |
| Trial entry following cardiac surgery [n (%)]a | 421 (60.66) | 416 (61.63) |
| Other trial entry [n (%)]a | 273 (39.34) | 259 (38.37) |
| Undergoing cardiopulmonary bypass | N = 421b | N = 416b |
| n (%) | 396 (94.06) | 392 (94.23) |
| RACHS1 scorea,c | N = 421b | N = 416b |
| 1–4 [n (%)] | 388 (92.16) | 393 (94.47) |
| 5–6 [n (%)] | 33 (7.84) | 23 (5.53) |
| Predicted risk of mortality (PIM2)a,c | N = 273b | N = 259b |
| < 5% [n (%)] | 74 (27.11) | 67 (25.87) |
| 5–15% [n (%)] | 144 (52.75) | 144 (55.60) |
| ≥ 15% [n (%)] | 55 (20.15) | 48 (18.53) |
| Time from admission to trial entry (days) [median (IQR)] | 1 (0–2) | 1 (0–1) |
| Inotrope score [mean (SD)] | 15.00 (21.58) | 16.22 (21.07) |
| Blood glucose (mmol/l) [mean (SD)] | 7.10 (2.76) | 7.02 (2.86) |
| Not measured | 1 | |
| Plasma creatinine (µmol/l) [mean (SD)] | 50.69 (44.33) | 54.54 (40.58) |
| Not measured | 69 | 52 |
| PELOD scorec [mean (SD)] | 7.46 (7.04) | 7.70 (6.58) |
Actual management
Table 8 describes the observed management of blood glucose after randomisation, and shows a clear difference between the two arms of the study. In the TGC arm, 461 of the children (66%) received insulin compared with 109 of 675 (16%) in the CM arm. Children in the TGC arm received more insulin, and continued on insulin for longer. Figure 5 shows the mean daily blood glucose level by arm. There was a clear separation between the two randomised arms, with children in the TGC arm having a significantly lower blood glucose profile than those in the CM arm.
| Actual management | TGC (N = 694) | CM (N = 675) |
|---|---|---|
| Insulin administered (yes) [n (%)] | 461 (66.43) | 109 (16.15) |
| Total insulin given (IU/kg body weight) [mean (SD)] | 2.56 (6.18) (N = 461) | 1.29 (2.85) (N = 109) |
| Number of days on insulin [mean (SD)] | 3.24 (3.32) | 1.71 (1.19) |
| Time from randomisation to starting insulin (hours) [median (IQR)] | 3.37 (0.78–17.93) | 3.1 (0.09–42.18) |
FIGURE 5.
Mean glucose level.
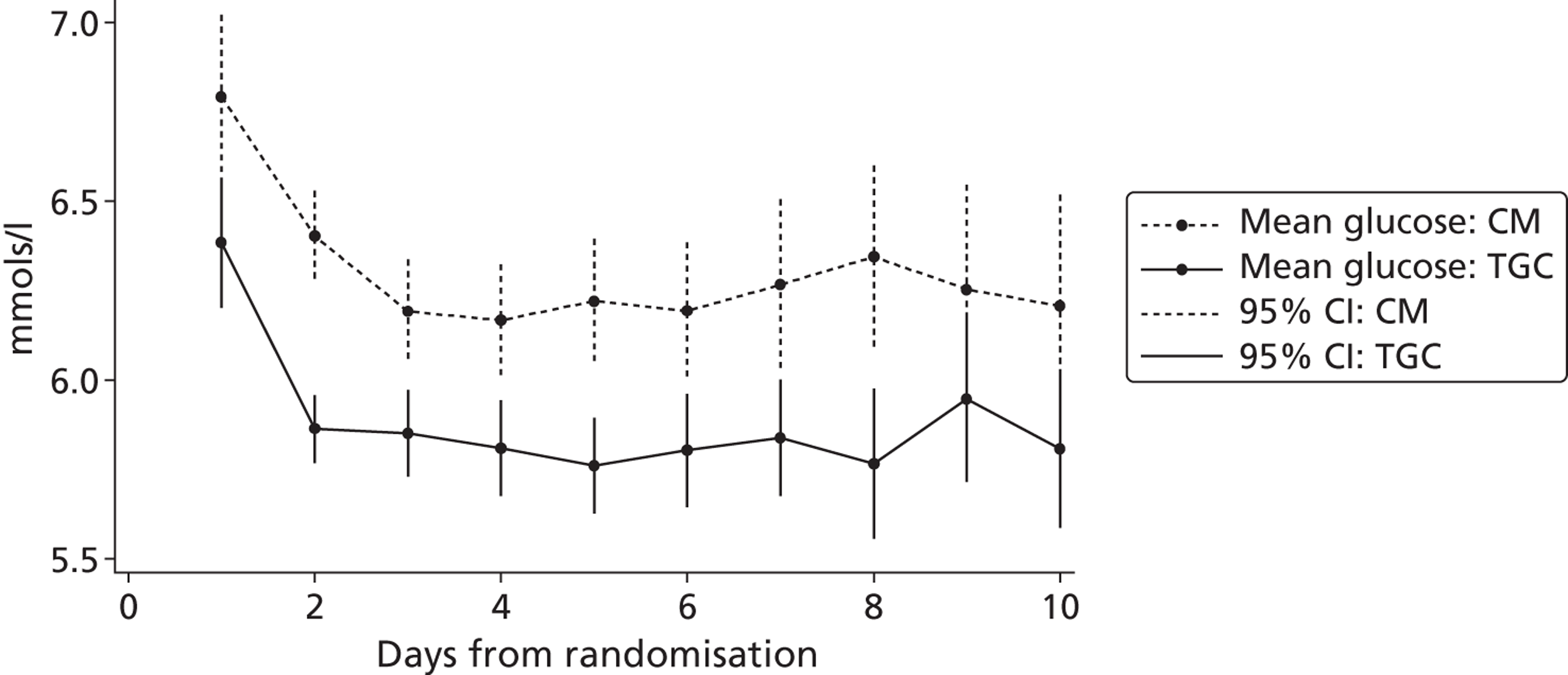
Thirty-day clinical outcomes
Primary outcome
Results for the primary outcome are shown in Table 9. The mean number of VFD-30 from randomisation was 23 in both trial arms (mean difference 0.36; 95% CI –0.42 to 1.14).
| Outcome | TGC (n = 694) [mean (standard error)] | CM (n = 675) [mean (standard error)] | Mean difference/odds ratio (95% CI) |
|---|---|---|---|
| VFD-30 | 23.61 (0.27) | 23.24 (0.29) | 0.36 (–0.42 to 1.14) |
Secondary outcomes
The secondary outcomes up to 30 days are shown in Table 10, and the duration of ventilation in Figure 6 and of vasoactive drug use in Figure 7. In general, the secondary outcomes are similar between the arms over the 30-day period, although less RRT was undertaken in the TGC arm (odds ratio 0.63; 95% CI 0.45 to 0.89). Additionally, mean caloric intake (Figure 8) was similar between the two groups.
| Outcome | TGC (N = 694) | CM (N = 675) | Mean difference/odds ratio (95% CI) |
|---|---|---|---|
| Death within 30 days of trial entry [n (%)] | 35 (5.04)a | 34 (5.04)a | 1.00 (0.62 to 1.63) |
| Number of days in PICU [mean (SE)] | 6.50 (0.21) | 6.96 (0.24) | –0.47 (–1.12 to 0.15) |
| Number of days in hospital [mean (SE)] | 16.40 (0.34) | 16.73 (0.36) | –0.33 (–1.24 to 0.62) |
| Duration of mechanical ventilation (days) [mean (SE)] | 5.30 (0.19) | 5.61 (0.21) | –0.31 (–0.87 to 0.25) |
| PELOD score [mean (SE)] | 9.79 (0.19) | 9.79 (0.21) | –0.31 (–0.87 to 0.25) |
| Duration of vasoactive drug use (days) [median (IQR)] | 3 (2 to 6) | 4 (2 to 6) | –0.20 (–0.64 to 0.25) |
| RRT [n (%)] | 62 (8.93) | 91 (13.48) | 0.63 (0.45 to 0.89) |
| Bloodstream infection [n (%)] | 38 (5.48) | 43 (6.37) | 0.85 (0.54 to 1.34) |
| Use of antibiotics > 10 days [n (%)] | 62 (8.93) | 74 (10.96) | 0.80 (0.56 to 1.14) |
| Number of red blood cell transfusions [mean (SE)] | 1.00 (0.11) | 1.12 (0.11) | –0.11 (–0.43 to 0.18) |
| Number of patients who experienced at least one hypoglycaemic episode (moderate or severe) [n (%)] | 110 (15.85) | 25 (3.70) | 4.90 (3.13 to 7.67) |
| Number of moderate hypoglycaemic episodes (< 2.0–2.5 mmol/l) | |||
| Number of episodes | 127 | 30 | |
| Number of patients who experienced at least one episodeb [n (%)] | 87 (12.54) | 21 (3.11) | 4.46 (2.73 to 7.28) |
| Mean number of episodes per patient (SE) | 0.18 (0.03) | 0.04 (0.01) | |
| Number of severe hypoglycaemic episodes (< 2.0 mmol/l) | |||
| Number of episodes | 70 | 11 | |
| Number of patients who experienced at least one episodeb [n (%)] | 51 (7.35) | 10 (1.48) | 5.27 (2.65 to 10.48) |
| Mean number of episodes per patient (SE) | 0.1 (0.02) | 0.02 (0.005) | |
| Seizures given pharmacological treatment [n (%)] | 23 (3.31) | 15 (2.22) | 1.15 (0.77 to 2.98) |
FIGURE 6.
Proportion of patients on mechanical ventilation.
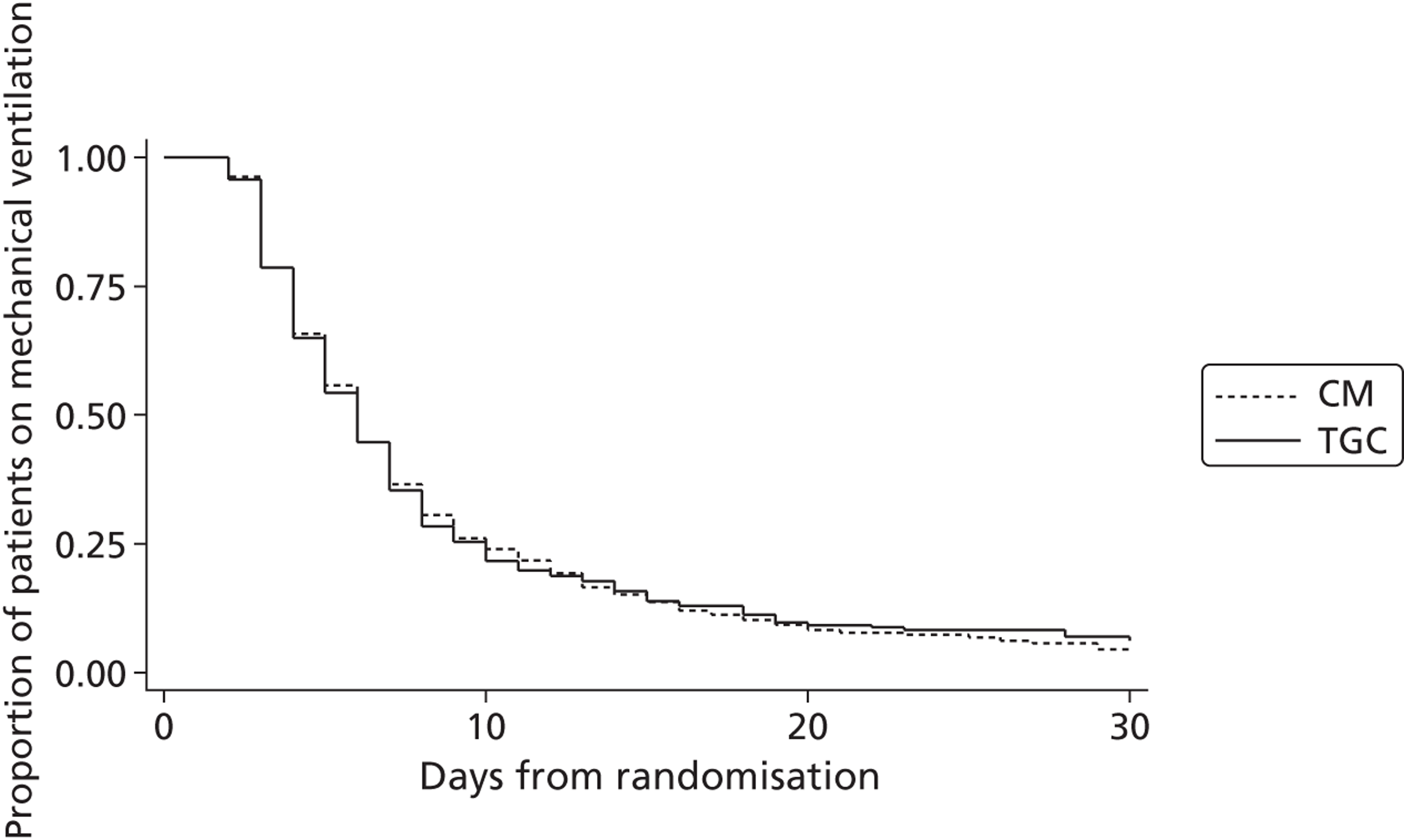
FIGURE 7.
Proportion of patients receiving vasoactive drugs.
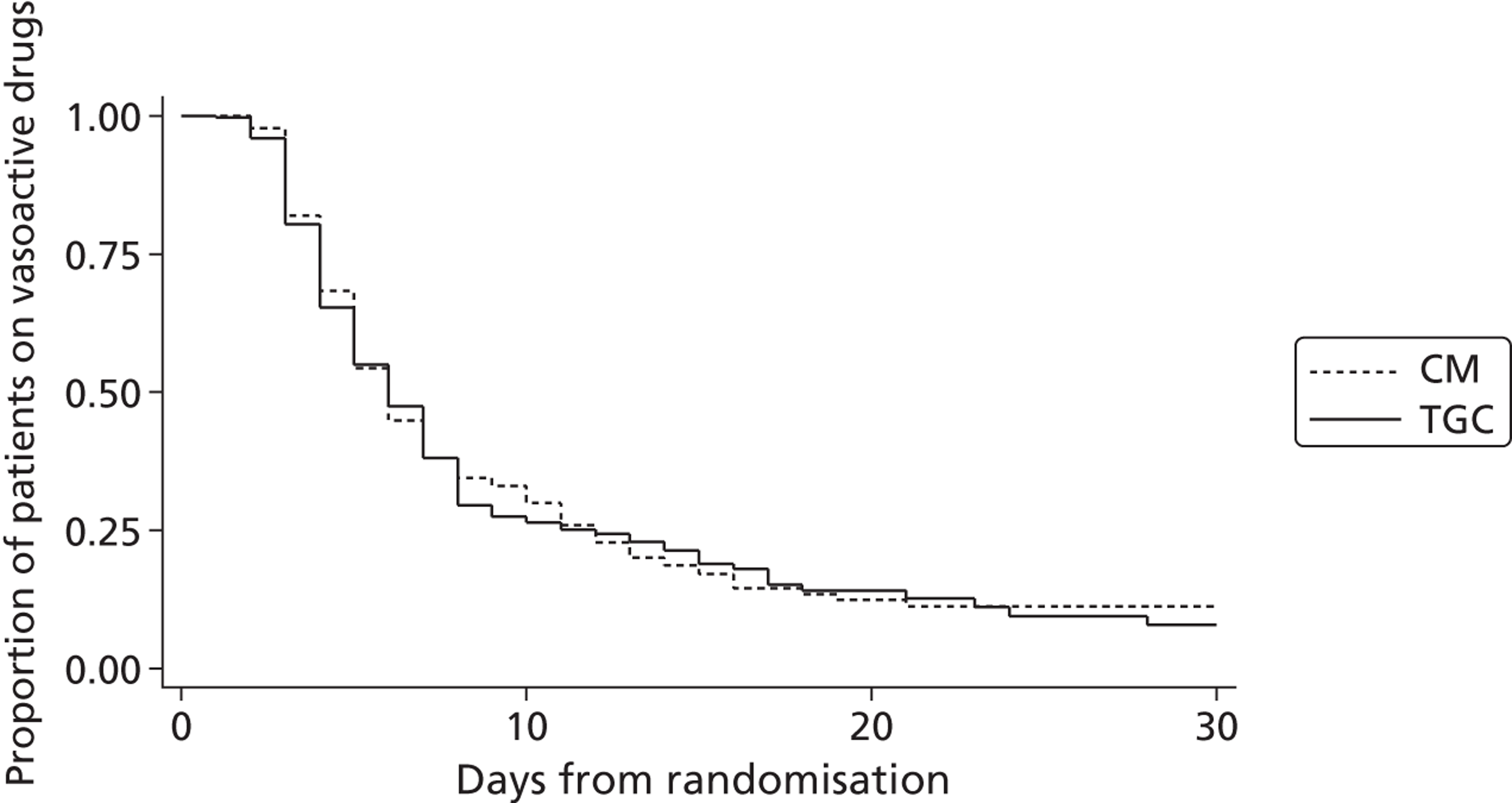
FIGURE 8.
Mean caloric intake.
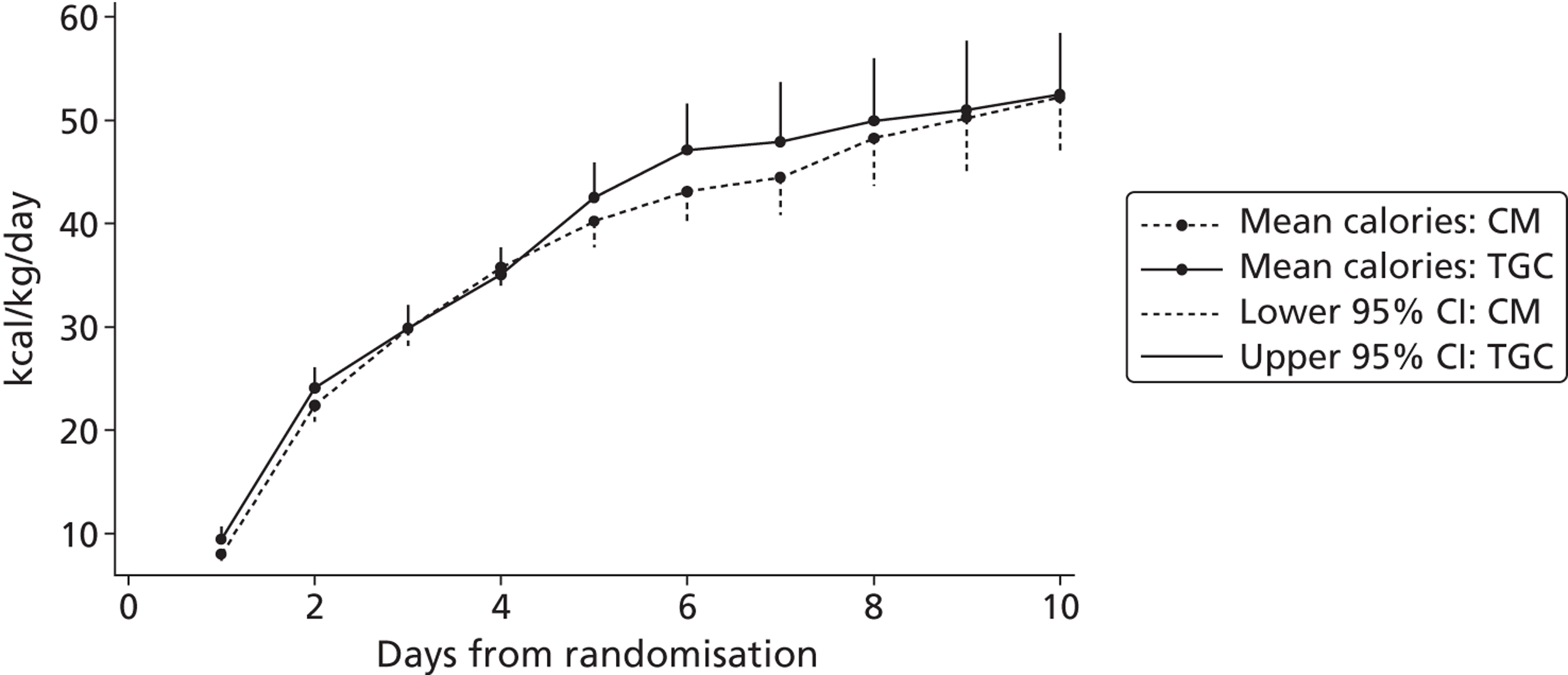
In terms of adverse effects, there were 135 patients whose blood glucose level was below the threshold that defined moderate hypoglycaemia; 61 of these had one or more episodes that were considered severe. Hypoglycaemia occurred in 33 (4.1%) patients not given insulin, but was more commonly observed in patients who received insulin [102 (17.9%)].
Hypoglycaemia occurred in a greater proportion of patients in the TGC arm than in the CM arm of the study (moderate, 12.5% vs. 3.1%, p < 0.001; severe, 7.3% vs. 1.5%, p < 0.001). Of the patients who experienced any hypoglycaemic episode, 11.1% died as opposed to 4.4% of those who did not experience any hypoglycaemic episode (p = 0.001).
Stratified analyses
Table 11a–e shows the primary outcome for the main prespecified stratification factors. None of the interaction tests between the intervention and prespecified subgroups was statistically significant, suggesting that there is no difference in the effect of TGC on VFD-30 in the different strata [p = 0.63 (cardiac vs. non-cardiac); p = 0.28 (age < 1 vs. ≥ 1 year); p = 0.09 (RACHS1 1–4 vs. 5–6); p = 0.88 (PIM2 < 5% vs. 5–15% vs. ≥ 15%) and p = 0.66 (run-in cases vs. non-run-in cases)]. One of the prespecified stratified analyses (TBI or not) was not included, as only 13 TBI patients were followed up at 1 year.
| TGC (N = 694) | CM (N = 675) | Mean difference (95% CI) | |
|---|---|---|---|
| Cardiac | |||
| n | 421 | 416 | |
| Mean (SE) | 25.05 (0.27) | 24.80 (0.31) | 0.25 (–0.71 to 1.22) |
| Non-cardiac | |||
| n | 273 | 259 | |
| Mean (SE) | 21.37 (0.52) | 20.74 (0.54) | 0.63 (–0.58 to 1.84) |
| TGC (N = 694) | CM (N = 675) | Mean difference (95% CI) | |
|---|---|---|---|
| Age < 1 year | |||
| n | 432 | 421 | |
| Mean (SE) | 23.59 (0.35) | 23.56 (0.36) | 0.03 (–0.96 to 1.02) |
| Age ≥ 1 year | |||
| n | 262 | 254 | |
| Mean (SE) | 23.63 (0.44) | 22.72 (0.49) | 0.91 (–0.36 to 2.18) |
| Operative complexity | TGC (N = 421) | CM (N = 416) | Mean difference (95% CI) |
|---|---|---|---|
| RACHS 1–4 | |||
| n | 388 | 393 | |
| Mean (SE) | 25.29 (0.27) | 25.13 (0.29) | 0.17 (–0.66 to 0.99) |
| RACHS 5 or 6 | |||
| n | 33 | 23 | |
| Mean (SE) | 22.21 (1.32) | 19.26 (2.27) | 2.95 (–0.15 to 6.06) |
| Predicted risk of mortality | TGC (N = 272) | CM (N = 261) | Mean difference (95% CI) |
|---|---|---|---|
| PIM2 < 5% | |||
| n | 74 | 67 | |
| Mean (SE) | 24.22 (0.70) | 22.82 (0.87) | 1.40 (–1.42 to 4.42) |
| PIM2 5–15% | |||
| n | 144 | 144 | |
| Mean (SE) | 21.22 (0.72) | 20.59 (0.70) | 0.63 (–1.33 to 2.58) |
| PIM2 ≥ 15% | |||
| n | 55 | 48 | |
| Mean (SE) | 17.95 (1.39) | 18.29 (1.50) | –0.35 (–3.63 to 2.94) |
| TGC (N = 694) | CM (N = 675) | Mean difference (95% CI) | |
|---|---|---|---|
| Run-in – first 100 cases | |||
| n | 47 | 53 | |
| Mean (SE) | 23.62 (1.04) | 22.64 (1.05) | 0.98 (–1.91 to 3.86) |
| Non-run-in | |||
| n | 647 | 254 | |
| Mean (SE) | 23.61 (0.28) | 23.30 (0.30) | 0.31 (–0.50 to 1.12) |
In both cardiac and non-cardiac strata, hypoglycaemia occurred in a greater proportion of patients in the TGC arm than in the CM arm of the trial (moderate: cardiac 10.9% vs. 1.4%, p < 0.001; non-cardiac 15.4% vs. 5.8% p < 0.001; severe: cardiac 5.5% vs. 0.5%, p < 0.001; non-cardiac 10.3% vs. 3.1%, p = 0.001). Cardiac cases receiving insulin were not at a greater risk of hypoglycaemia than non-cardiac cases (16.4% vs. 20.3%).
Thirty-day economic outcomes
For the index hospital episode, the mean PICU bed-days, LOS on GM wards and total LOS for the index hospital episode were similar between arms (Table 12). The mean total number of hospital days up to day 30, including both the initial episode and readmissions to the initial PICU before day 30, were similar between arms (see Table 12). For the stratum admitted for cardiac surgery, the mean total LOS was again comparable between arms (Table 13). As regards the non-cardiac stratum, for the initial hospital episode, the mean numbers of PICU days, LOS on GM wards and total LOS were lower for the TGC than the CM arm (Table 14).
| TGC (N = 694) | CM (N = 675) | |
|---|---|---|
| Index hospital episode | ||
| Mean (SD) PICU days | 6.50 (5.50) | 6.96 (6.12) |
| Mean (SD) days on general wards | 9.91 (7.43) | 9.77 (7.52) |
| Mean (SD) total days | 16.40 (8.84) | 16.73 (9.26) |
| Readmission | ||
| n (%) | 39 (5.62) | 37 (5.48) |
| Mean (SD) total days | 0.21 (1.02) | 0.25 (1.27) |
| Mean (SD) total hospital daysa | 16.62 (8.81) | 16.98 (9.25) |
| TGC (N = 421) | CM (N = 416) | |
|---|---|---|
| Index hospital episode | ||
| Mean (SD) PICU days | 5.69 (4.79) | 5.89 (5.37) |
| Mean (SD) days on general wards | 9.26 (6.83) | 8.32 (6.34) |
| Mean (SD) total days | 14.96 (8.29) | 14.22 (8.19) |
| Readmission | ||
| n (%) | 35 (8.31) | 26 (6.25) |
| Mean (SD) total days | 0.31 (1.20) | 0.32 (1.49) |
| Mean (SD) total hospital daysa | 15.27 (8.29) | 14.54 (8.25) |
| TGC (N = 273) | CM (N = 259) | |
|---|---|---|
| Index hospital episode | ||
| Mean (SD) PICU days | 7.74 (6.25) | 8.68 (6.83) |
| Mean (SD) days on general wards | 10.90 (8.18) | 12.10 (8.62) |
| Mean (SD) total days | 18.63 (9.22) | 20.78 (9.46) |
| Readmission | ||
| n (%) | 4 (1.47) | 11 (4.25) |
| Mean (SD) total days | 0.07 (0.64) | 0.13 (0.80) |
| Mean (SD) total hospital daysa | 18.70 (9.20) | 20.91 (9.43) |
Tables 15–17 report that the mean numbers of PICU bed-days, by HRG level, were similar between arms.
| HRG level | TGC (n = 694) | CM (n = 675) |
|---|---|---|
| 1 | 0.31 (0.80) | 0.26 (0.63) |
| 2 | 0.74 (1.32) | 0.90 (1.83) |
| 3 | 0.93 (2.08) | 1.10 (2.44) |
| 4 | 2.59 (3.13) | 2.48 (3.14) |
| 5 | 0.52 (2.20) | 0.48 (1.44) |
| 6 | 0.18 (0.63) | 0.22 (0.74) |
| 7 | 0.11 (1.49) | 0.05 (0.50) |
| HRG level | TGC (n = 421) | CM (n = 416) |
|---|---|---|
| 1 | 0.17 (0.48) | 0.19 (0.55) |
| 2 | 0.81 (1.26) | 0.96 (1.77) |
| 3 | 0.57 (1.46) | 0.60 (1.57) |
| 4 | 2.44 (2.99) | 2.42 (2.79) |
| 5 | 0.42 (1.62) | 0.37 (1.21) |
| 6 | 0.14 (0.65) | 0.11 (0.39) |
| 7 | 0.09 (1.40) | 0.03 (0.35) |
| HRG level | TGC (n = 273) | CM (n = 259) |
|---|---|---|
| 1 | 0.53 (1.07) | 0.37 (0.73) |
| 2 | 0.63 (1.40) | 0.80 (1.90) |
| 3 | 1.48 (2.69) | 1.91 (3.26) |
| 4 | 2.80 (3.33) | 2.61 (3.65) |
| 5 | 0.68 (2.88) | 0.65 (1.74) |
| 6 | 0.25 (0.59) | 0.40 (1.10) |
| 7 | 0.14 (1.62) | 0.08 (0.66) |
Overall, the mean total costs at 30 days post randomisation were similar between arms (Table 18). For the cardiac subgroup, the mean total costs per patient were £16,228 (TGC) and £17,005 (CM) (Table 19). For the non-cardiac subgroup, the TGC arm had lower mean costs than the CM group, with an incremental cost of –£2319 (95% CI –£4702 to £124) (Table 20). Including the treatment by cardiac interaction term led to a statistically significant improvement in model fit (p < 0.001).
| TGC (n = 694) [mean (SD)] | CM (n = 675) [mean (SD)] | Incremental [mean (95% CI)] | |
|---|---|---|---|
| Index hospital episode | |||
| PICU costs | 13,607 (13,579) | 14,446 (12,938) | |
| GM costs | 2497 (1872) | 2463 (1895) | |
| Total costs | 16,104 (13,499) | 16,908 (12,923) | |
| Readmission | |||
| PICU costs | 58 (658) | 26 (499) | |
| GM costs | 54 (240) | 64 (316) | |
| Total costs | 112 (750) | 90 (612) | |
| Total costs (index admission and readmissions) | 16,228 (13,504) | 17,005 (12,913) | –776 (–2183 to 632) |
| TGC (n = 421) [mean (SD)] | CM (n = 416) [mean (SD)] | Incremental [mean (95% CI)] | |
|---|---|---|---|
| Index hospital admission | |||
| PICU costs | 11,956 (12,379) | 12,119 (11,200) | |
| GM costs | 2336 (1722) | 2098 (1597) | |
| Total costs | 14,291 (12,413) | 14,216 (11,272) | |
| Readmissions | |||
| PICU costs | 80 (771) | 42 (635) | |
| Other ward costs | 76 (288) | 82 (367) | |
| Total costs | 156 (873) | 124 (761) | |
| Total costs (index admission and readmissions) | 14,465 (12,437) | 14,350 (11,285) | 114 (–1496 to 1725) |
| TGC (n = 273) [mean (SD)] | CM (n = 259) [mean (SD)] | Incremental [mean (95% CI)] | |
|---|---|---|---|
| Index hospital admission | |||
| PICU costs | 16,153 (14,915) | 18,184 (14,587) | |
| GM costs | 2746 (2060) | 3048 (2172) | |
| Total costs | 18,899 (14,609) | 21,232 (14,195) | |
| Readmission | |||
| PICU costs | 26 (427) | 0 (0) | |
| GM costs | 19 (129) | 36 (207) | |
| Total costs | 45 (501) | 36 (207) | |
| Total costs (index admission and readmissions) | 18,949 (14,614) | 21,268 (14,183) | –2319 (–4762 to 124) |
Twelve-month results
Index hospital episode and readmissions to paediatric intensive care unit within 30 days post randomisation
Table 21 reports the mean total number of hospital days up to 12 months post randomisation, including the initial hospital episode and any readmissions to PICU within 30 days. A lower proportion of patients in the TGC than in CM arm had an index hospital admission or relevant readmission that continued beyond day 30. Between 30 days and 12 months post randomisation, the mean number of days in PICU, on GM wards and in total, was lower for the TGC than the CM arm (see Table 21).
| TGC (N = 694) | CM (N = 675) | |
|---|---|---|
| Mean (SD) total days up to 30 days post randomisation | 16.62 (8.81) | 16.98 (9.25) |
| 30 days to 1 year | ||
| n (%) continuing admission | 130 (18.73) | 152 (22.52) |
| Mean (SD) PICU days | 1.98 (11.86) | 2.79 (15.19) |
| Mean (SD) days on general wards | 5.67 (25.89) | 9.75 (36.22) |
| Mean (SD) total days | 7.64 (31.18) | 12.54 (41.47) |
| Mean (SD) total hospital daysa | 24.26 (35.40) | 29.52 (46.18) |
Four patients were still in hospital at the date of administrative censoring, with LOS ranging from 119 to 359 days. Each of these patients was assumed to have the mean total LOS taken across the whole sample still in hospital at the respective time point. (For example, for the patient censored at a LOS of 119 days, the assumed LOS was 228 days, according to the mean across the 46 patients still in hospital 118 days post randomisation.) One patient withdrew consent for participation in the study, after 8 days in hospital, and was assumed to have a total hospital LOS of 60 days, the mean across the whole sample of patients who were still in hospital after day 8.
For the cardiac stratum, the mean total LOS at 12 months was similar between arms (Table 22). For the non-cardiac subgroup, the TGC arm had a lower proportion of patients who had a hospital admission that continued beyond 30 days post randomisation, and on average reported fewer days on PICUs, and on GM wards (Table 23), than the CM arm. For the non-cardiac stratum, the mean total LOS for the initial episode and readmissions to PICU within 30 days was 31.0 days for the TGC arm compared with 44.5 days for the CM arm (see Table 23).
| TGC (N = 421) | CM (N = 416) | |
|---|---|---|
| Mean (SD) days at 30 days post randomisation | 15.27 (8.29) | 14.54 (8.25) |
| 30 days to 1 year | ||
| n (%) continuing admission | 59 (14.01) | 52 (12.50) |
| Mean (SD) PICU days | 1.39 (8.81) | 1.26 (6.70) |
| Mean (SD) days on general wards | 3.25 (18.54) | 4.40 (26.48) |
| Mean (SD) total days | 4.64 (22.17) | 5.65 (29.30) |
| Mean (SD) total hospital daysa | 19.90 (26.40) | 20.19 (33.19) |
| TGC (N = 273) | CM (N = 259) | |
|---|---|---|
| Mean (SD) days at 30 days post randomisation | 18.70 (9.20) | 20.91 (9.43) |
| 30 days to 1 year | ||
| n (%) continuing admission | 71 (26.01) | 100 (38.61) |
| Mean (SD) PICU days | 2.88 (15.40) | 5.25 (22.82) |
| Mean (SD) days on general wards | 9.40 (33.97) | 18.35 (46.66) |
| Mean (SD) total days | 12.27 (41.03) | 23.60 (53.98) |
| Mean (SD) total hospital daysa | 30.98 (45.18) | 44.51 (58.59) |
Figure 9 plots the proportion of patients over time who were still in hospital following the index admission. For the overall sample, and the cardiac patients, the proportion still in hospital was similar between arms at each time point. For the non-cardiac patients, a higher proportion of the CM than the TGC arm were still in hospital 60 and 90 days post randomisation.
FIGURE 9.
Proportion of patients remaining in hospital (index admission) up to 180 days post randomisation. Proportion of patients remaining in hospital: (a) whole study cohort; (b) cardiac surgery subgroup; and (c) non-cardiac surgery group.
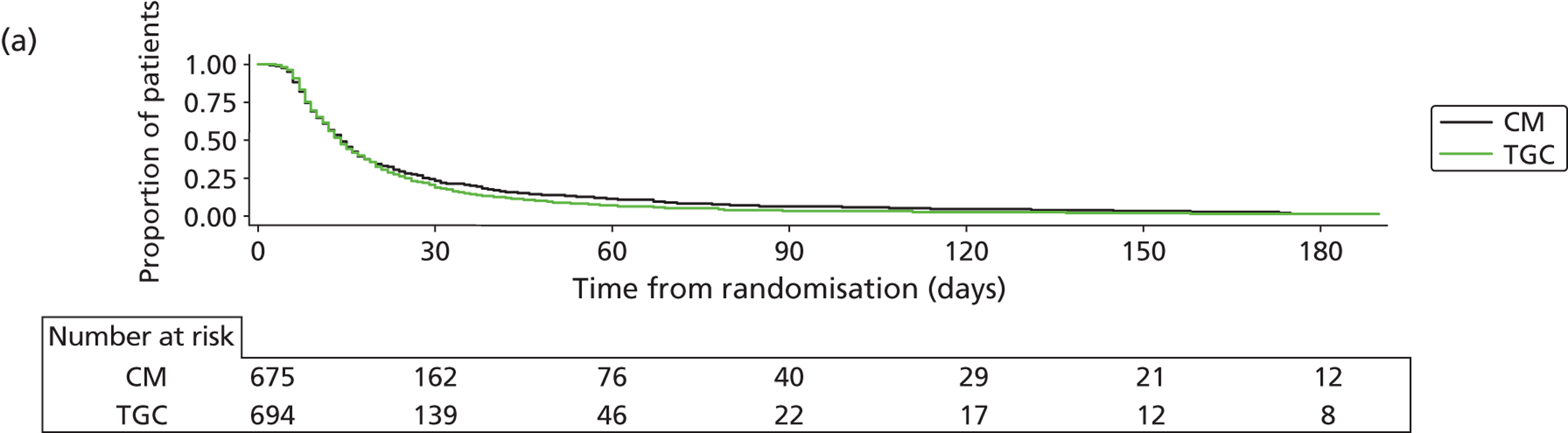
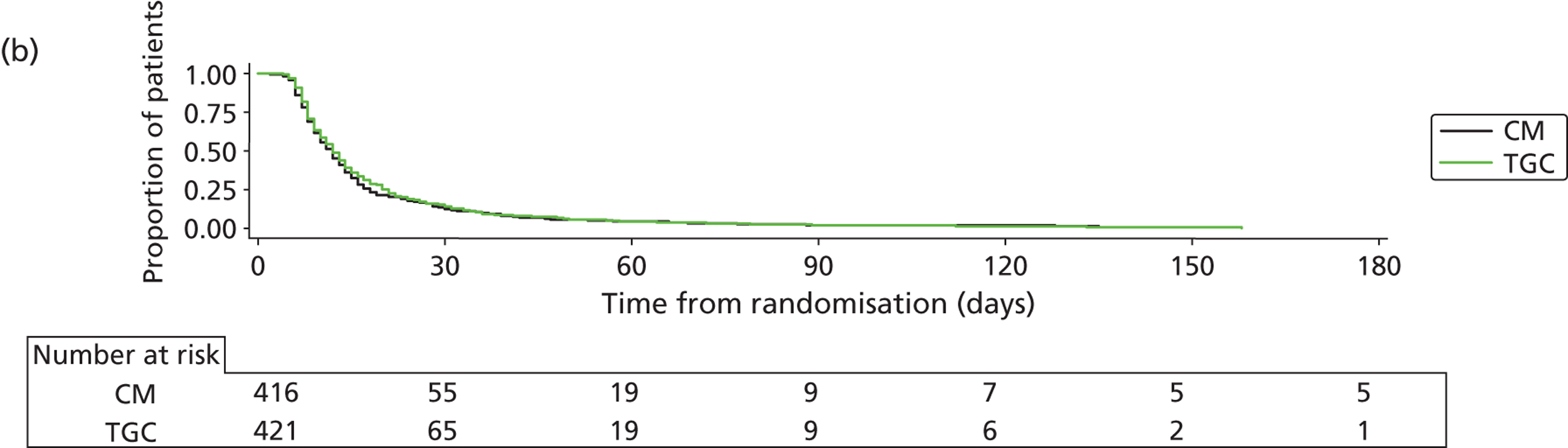
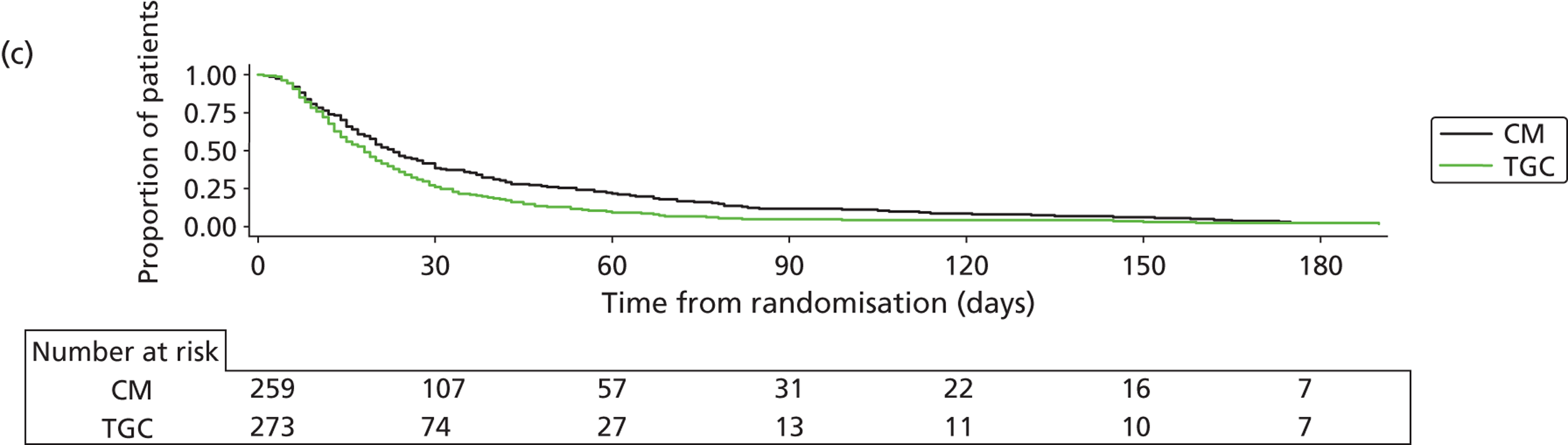
Mortality
Mortality at 12 months was similar between the randomised groups (Table 24). The CIs around each of the odds ratios all encompassed 1.
| Deaths within 12 months by group [n (%)] | Odds ratio (95% CI) | ||
|---|---|---|---|
| TGC | CM | ||
| Overall (TGC, N = 694; CM, N = 675) | 73 (10.52) | 71 (10.52) | 1.00 (0.71 to 1.41) |
| Cardiac (TGC, N = 421; CM, N = 416) | 31 (7.36) | 30 (7.21) | 1.02 (0.61 to 1.72) |
| Non-cardiac (TGC, N = 273; CM, N = 259) | 42 (15.38) | 41 (15.83) | 0.97 (0.61 to 1.54) |
Assessment of attention and behaviour in patients with traumatic brain injury
No differences were found between the two arms of the trial in attention and behaviour measures for those patients with TBI (Table 25).
| Measure | TGC (N = 6) | CM (N = 7) |
|---|---|---|
| KOSCHIa | ||
| 1–4A [n (%)] | 2 (33.3) | 2 (28.7) |
| 4B–5B [n (%)] | 4 (66.7) | 5 (71.4) |
| HUIa [mean (SE)] | 0.74 (0.15) | 0.97 (0.03) |
| Behaviour – CBCL total scoreb [mean (SD)] | 62.8 (5.66) | 55.6 (4.22) |
| CRS-R:S total scoreb [mean (SE)] | 51.2 (5.53) | 54.4 (5.40) |
Other hospital and community service use (after discharge from index hospital episode but excluding any readmissions to the initial paediatric intensive care unit within 30 days)
Figure 10 shows the flow of patients from randomisation to response to the service-use questionnaire. In the overall sample, a total of 397 patients (203 in the TGC arm, 194 in the CM arm) were randomised after 30 October 2010 and could not be followed up for 1 year; that is, for the purposes of collecting information on service use, these patients were administratively censored. Patients were also ineligible for the service-use questionnaire if their GP did not confirm that it was appropriate to contact them to administer the questionnaire. Of the eligible patients, the response rate to the service-use questionnaire was 63% in the TGC arm and 61% in the CM arm. For those who responded to the questionnaire, the mean LOS following hospital readmissions after 30 days post randomisation, and the mean number of contacts with hospital and personal social services, was similar between the randomised arms (Tables 26–28). The mean total costs of hospital and community health services were also similar between the randomised arms (Tables 29–31).
FIGURE 10.
Flow chart for 12-month follow-up for service-use questionnaire.
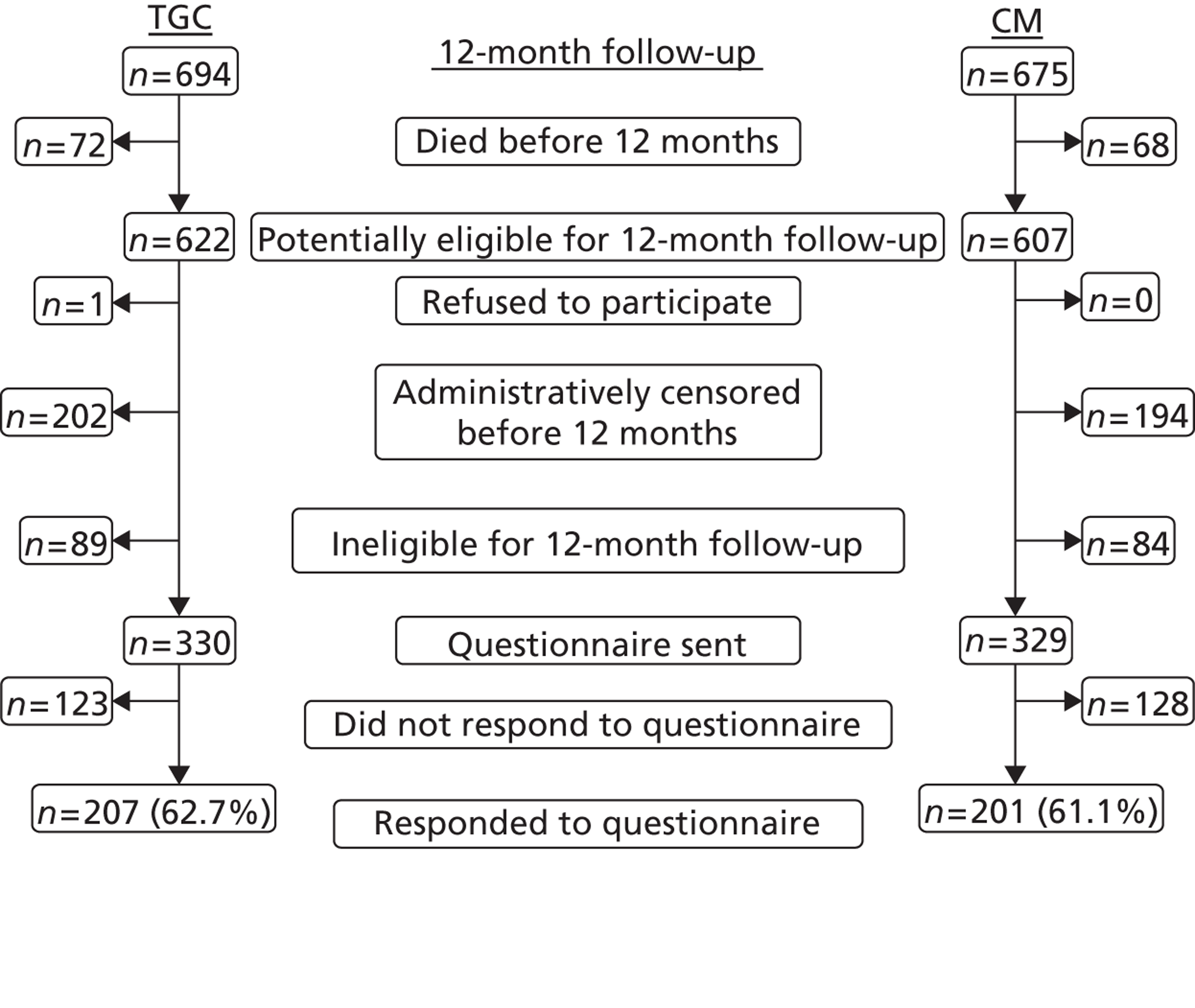
| Service | TGC (n = 207) [mean (SD)] | CM (n = 201) [mean (SD)] |
|---|---|---|
| Inpatient hospital days following readmissiona | 6.12 (1.01) | 6.63 (1.29) |
| Hospital outpatient visits | 6.81 (0.46) | 6.93 (0.45) |
| GP contacts | 1.23 (0.12) | 1.08 (0.99) |
| Practice nurse contacts | 1.76 (0.21) | 2.14 (0.29) |
| Health visitor contacts | 5.85 (0.74) | 6.87 (0.69) |
| Social worker contacts | 0.10 (0.02) | 0.08 (0.02) |
| Speech and language therapist contacts | 2.18 (0.38) | 1.88 (0.32) |
| Occupational therapist contacts | 7.83 (1.80) | 11.94 (2.30) |
| Other health service contacts | 9.52 (1.13) | 10.04 (1.11) |
| Service | TGC (n = 127) [mean (SD)] | CM (n = 121) [mean (SD)] |
|---|---|---|
| Inpatient hospital days following readmissiona | 7.10 (1.50) | 8.25 (2.03) |
| Hospital outpatient visits | 6.81 (0.59) | 6.64 (0.55) |
| GP contacts | 1.37 (0.17) | 1.19 (0.13) |
| Practice nurse contacts | 1.88 (0.25) | 2.11 (0.05) |
| Health visitor contacts | 6.24 (0.78) | 8.24 (0.98) |
| Social worker contacts | 0.05 (0.02) | 0.03 (0.01) |
| Speech and language therapist contacts | 2.32 (0.71) | 1.77 (0.50) |
| Occupational therapist contacts | 5.84 (1.99) | 7.20 (2.26) |
| Other health service contacts | 8.86 (1.31) | 9.06 (1.44) |
| Service | TGC (n = 79) [mean (SD)] | CM (n = 80) [mean (SD)] |
|---|---|---|
| Inpatient hospital daysa | 4.54 (1.00) | 4.15 (1.00) |
| Hospital outpatient visits | 6.82 (0.75) | 7.36 (0.75) |
| GP contacts | 1.02 (0.19) | 0.91 (0.13) |
| Practice nurse contacts | 1.58 (0.39) | 2.18 (0.53) |
| Health visitor contacts | 5.22 (1.47) | 4.78 (0.86) |
| Social worker contacts | 0.17 (0.04) | 0.17 (0.04) |
| Speech and language therapist contacts | 2.32 (0.71) | 1.77 (0.50) |
| Occupational therapist contacts | 11.04 (3.45) | 11.17 (4.58) |
| Other health service contacts | 10.60 (2.08) | 10.63 (1.97) |
| Service | TGC (n = 207) [mean (SD)] | CM (n = 201) [mean (SD)] |
|---|---|---|
| Inpatient hospital daysa | 1367 (243) | 1402 (264) |
| Hospital outpatient visits | 1019 (66) | 1002 (68) |
| GP contacts | 51 (5) | 58 (6) |
| Practice nurse contacts | 13 (3) | 10 (4) |
| Health visitor contacts | 67 (8) | 78 (8) |
| Social worker contacts | 3 (1) | 3 (1) |
| Speech and language therapist contacts | 18 (3) | 15 (3) |
| Other health service contacts | 115 (15) | 119 (15) |
| Service | TGC (n = 127) [mean (SD)] | CM (n = 121) [mean (SD)] |
|---|---|---|
| Inpatient hospital daysa | 1576 (358) | 1732 (414) |
| Hospital outpatient visits | 976 (82) | 1001 (87) |
| GP contacts | 56 (6) | 65 (8) |
| Practice nurse contacts | 12 (5) | 5 (3) |
| Health visitor contacts | 71 (9) | 94 (11) |
| Social worker contacts | 2 (1) | 1 (1) |
| Speech and language therapist contacts | 17 (16) | 16 (3) |
| Other health service contacts | 107 (19) | 104 (19) |
| Service | TGC (n = 79) [mean (SD)] | CM (n = 80) [mean (SD)] |
|---|---|---|
| Hospital inpatient daysa | 1029 (256) | 900 (211) |
| Hospital outpatient visits | 1082 (111) | 1004 (111) |
| GP contacts | 48 (7) | 48 (9) |
| Practice nurse contacts | 16 (7) | 17 (8) |
| Health visitor contacts | 59 (17) | 54 (10) |
| Social worker contacts | 4 (11) | 5 (11) |
| Speech and language therapist contacts | 19 (6) | 14 (4) |
| Other health service contacts | 127 (25) | 141 (26) |
Twelve-month total costs
Tables 32–34 report the total costs at 12 months across all the resource-use items recorded. The values presented are the results after using MI to handle missing values for health and community service costs at 12 months. Table 32 reports that, overall, the mean total costs were lower in the TGC than in the CM group, but with 95% CIs that encompass zero. For the cardiac surgery stratum, the mean total costs were similar between the groups (see Table 33), but, for non-cardiac patients, the mean costs were lower in the TGC than in the CM group, with an incremental cost of –£9865 (95% CI –£18,558 to –£1172) (see Table 34).
| TGC (n = 694) [mean (SD)] | CM (n = 675) [mean (SD)] | Incremental [mean (95% CI)] | |
|---|---|---|---|
| Hospital costs at 30 days | 16,228 (13,504) | 17,005 (12,913) | |
| Hospital costs between 30 days and 12 monthsa | 5683 (27,978) | 8463 (35,366) | |
| Other hospital and community health service costs at 12 monthsb | 2388 (3659) | 2452 (4010) | |
| Grand total costs up to 1 year | 24,300 (34,503) | 27,920 (42,775) | –3620 (–7743 to 502) |
| TGC (n = 421) [mean (SD)] | CM (n = 416) [mean (SD)] | Incremental [mean (95% CI)] | |
|---|---|---|---|
| Hospital costs at 30 days | 14,465 (12,437) | 14,350 (11,285) | |
| Hospital costs between 30 days and 12 monthsa | 3811 (20,497) | 3815 (17,720) | |
| Other hospital and community health service costs at 12 monthsb | 2652 (3890) | 2630 (4319) | |
| Grand total costs up to 1 year | 20,929 (27,385) | 20,796 (26,520) | 133 (–3568 to 3833) |
| TGC (n = 273) [mean (SD)] | CM (n = 259) [mean (SD)] | Incremental [mean (95% CI)] | |
|---|---|---|---|
| Hospital costs at 30 days | 18,949 (14,614) | 21,268 (14,183) | |
| Hospital costs between 30 days and 12 monthsa | 8569 (36,495) | 15,927 (51,688) | |
| Other hospital and community health service costs at 12 monthsb | 1979 (3056) | 2167 (3437) | |
| Grand total costs up to 1 year | 29,498 (4267) | 39,363 (58,551) | –9865 (–18,558 to –1172) |
Figures 11–13 report SAs that investigate whether or not the base-case results are robust to alternative assumptions. The results show that the incremental costs under these alternative scenarios are similar to the base case. For example, in the SAs that include additional costs of staff time and tests associated with monitoring TGC, and further costs for managing hypoglycaemic episodes, the mean incremental costs of TGC overall and for the non-cardiac subgroup are similar to the base case (see Figures 11–13). Moreover, when alternative approaches were taken to unit costing, this had little impact on the results.
FIGURE 11.
Sensitivity analysis reporting mean total costs at 12 months post randomisation according to alternative assumptions: whole study cohort.
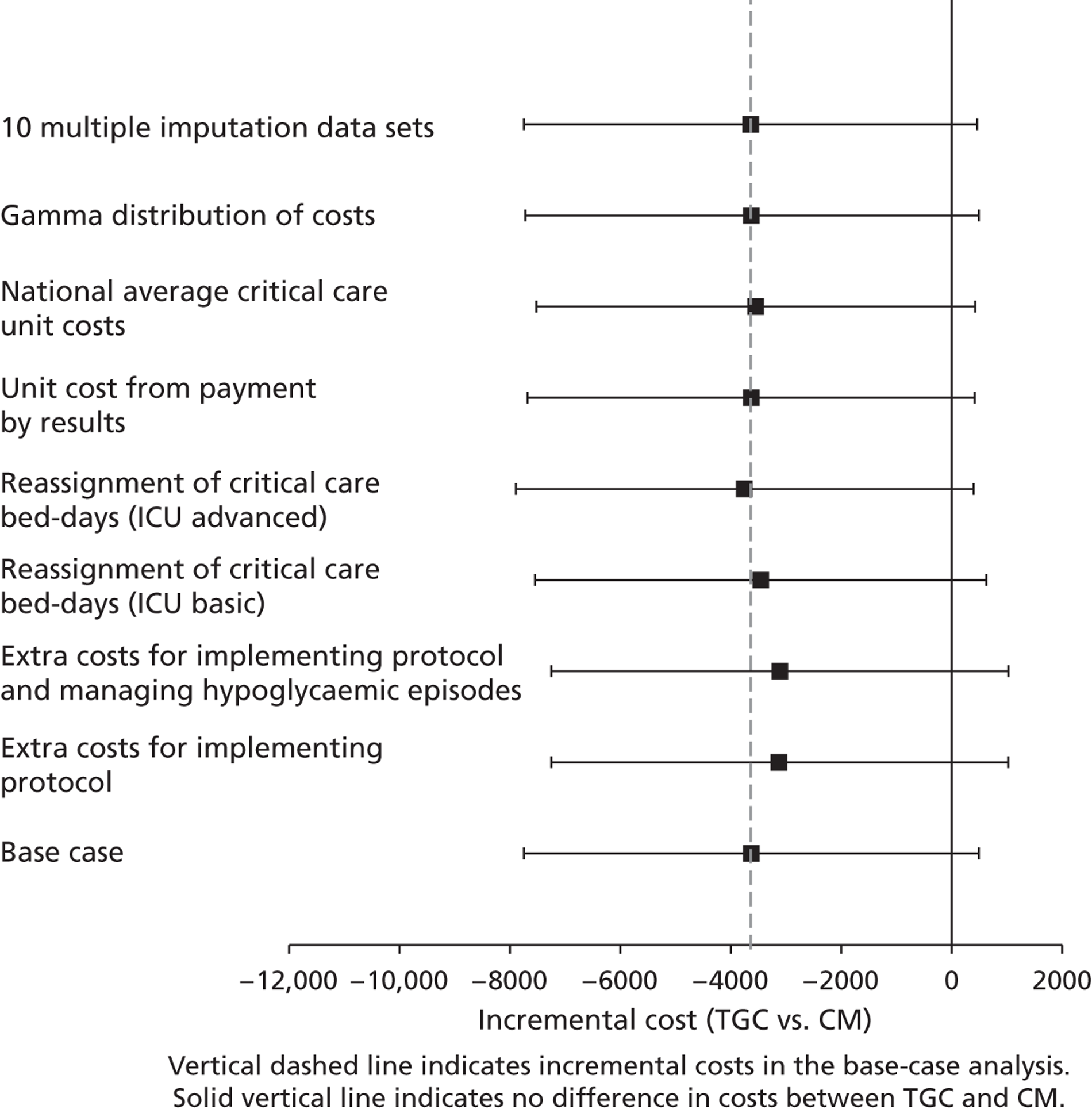
FIGURE 12.
Sensitivity analysis reporting mean total costs at 12 months post randomisation according to alternative assumptions: cardiac surgery subgroup.
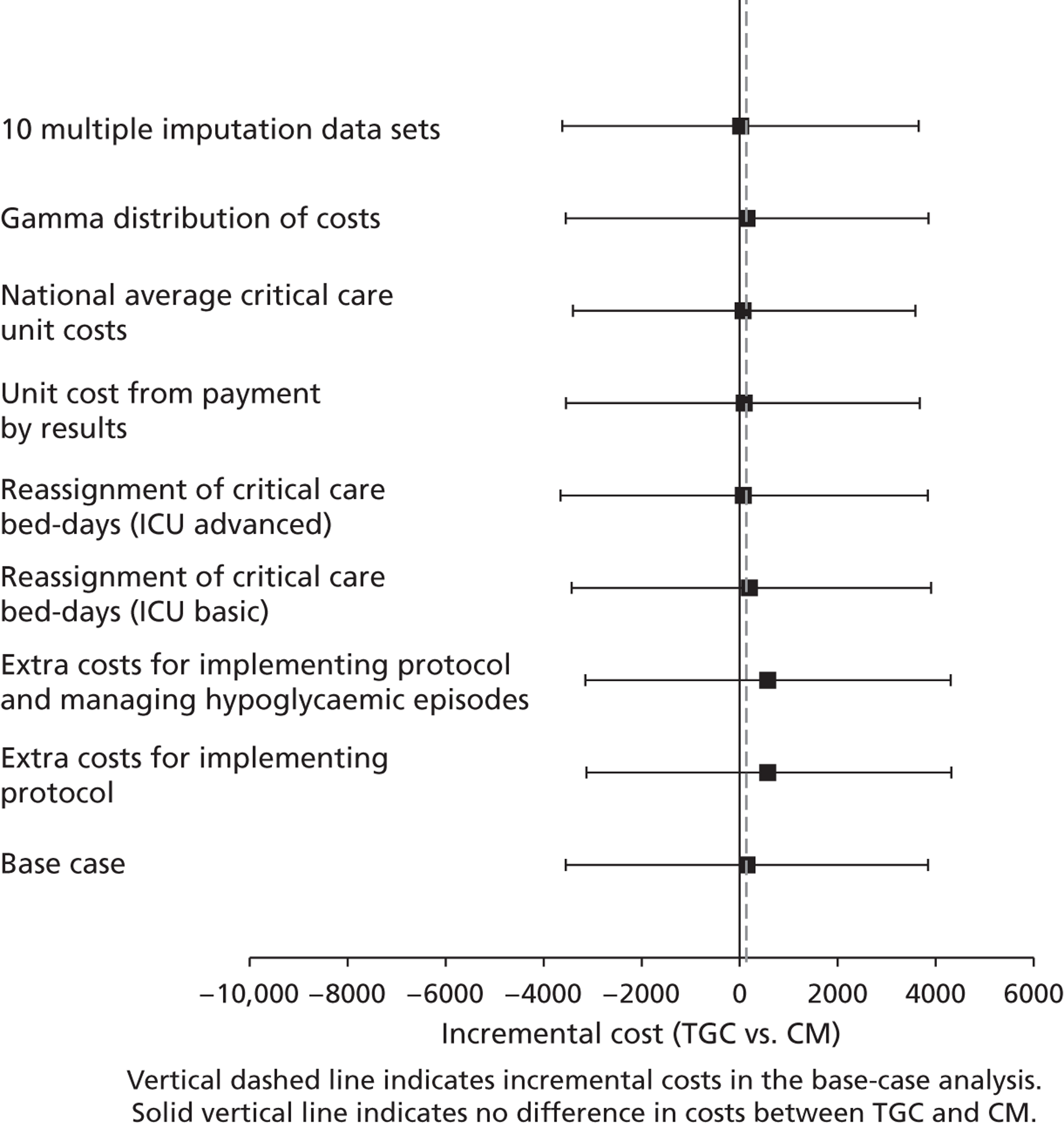
FIGURE 13.
Sensitivity analysis reporting mean total costs at 12 months post randomisation according to alternative assumptions: non-cardiac surgery subgroup.
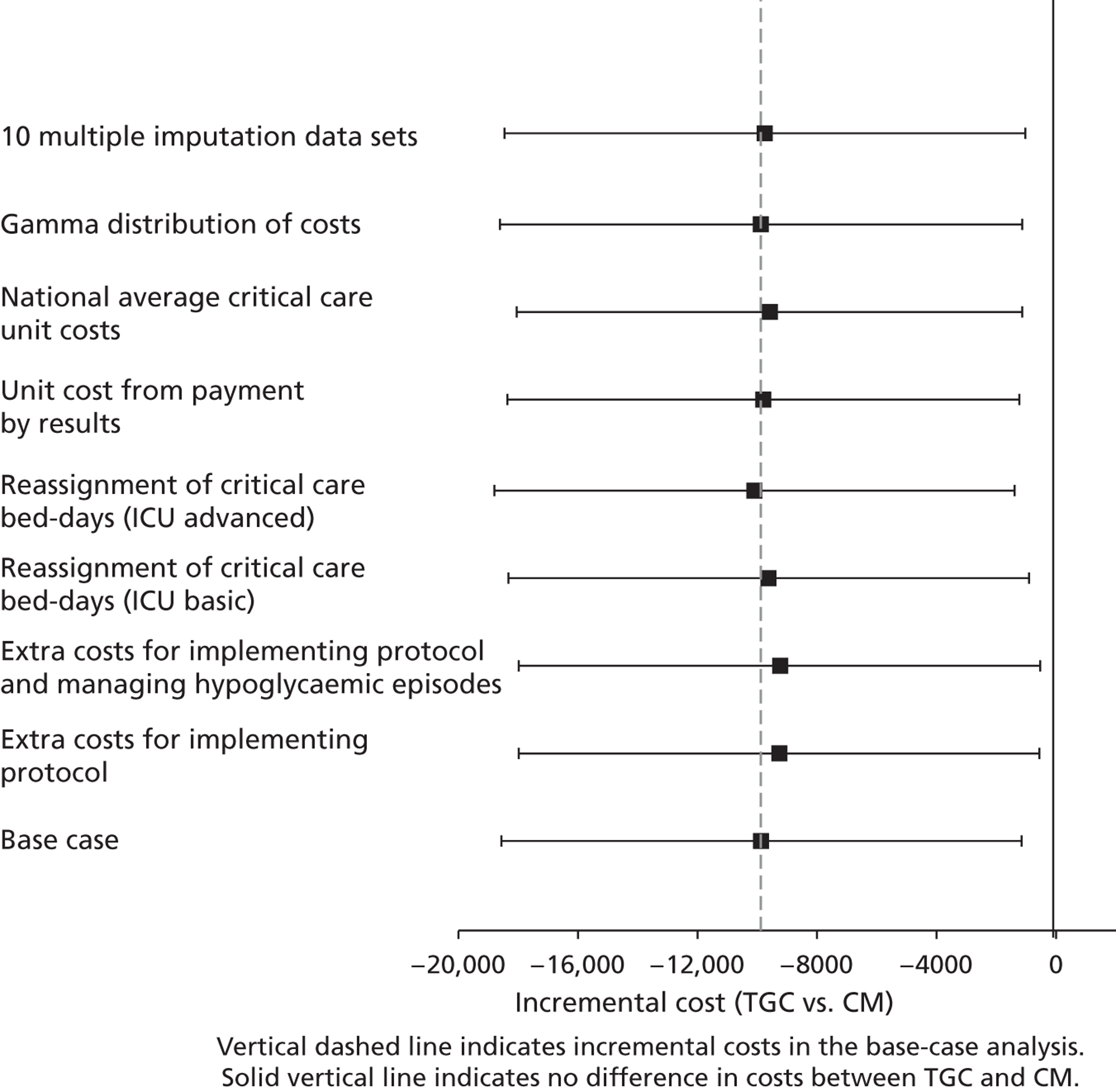
Lifetime cost-effectiveness results
The Kaplan–Meier survival curves show that when the time horizon was extended beyond 1 year, for those for whom survival data were available, the probability of survival remained similar between arms (Figure 14).
FIGURE 14.
Kaplan–Meier survival curves. Overall cohort, TGC vs. CM.
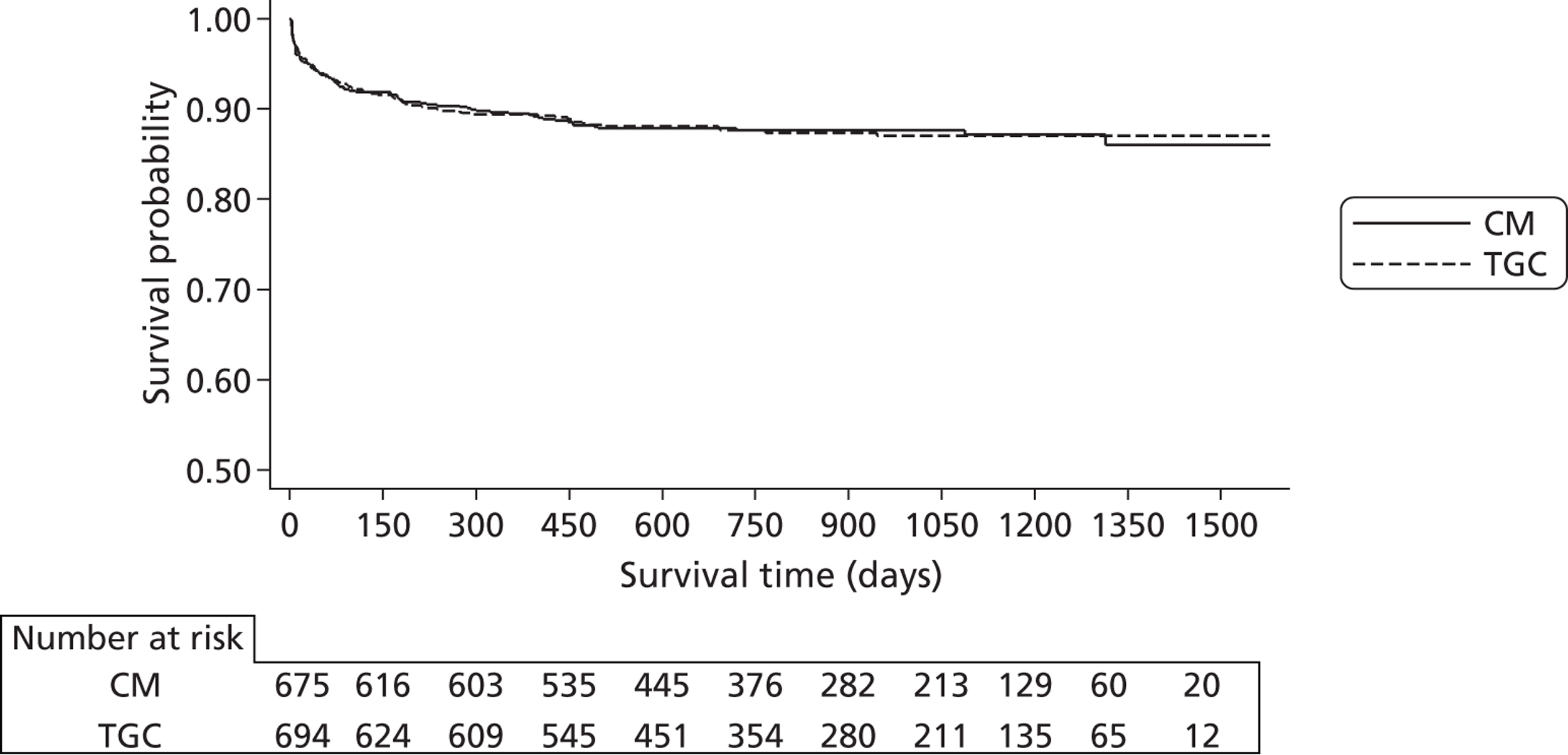
Figure 15 considers alternative parametric extrapolations for both treatment arms combined, using the observed survival data after day 30. Of the alternative survival functions, the Gompertz function appears to fit the observed data best in that it reports the lowest Akaike and Bayesian information criterion (Table 35). The Gompertz function also offers the most plausible projections of future survival (Table 36), in that the levels of excess death compared with those for the age- and gender-matched general population remain constant over time from 2 years post randomisation onwards.
FIGURE 15.
Comparison of alternative parametric extrapolations for survival from 12 months to 5 years post randomisation, across both randomised arms.
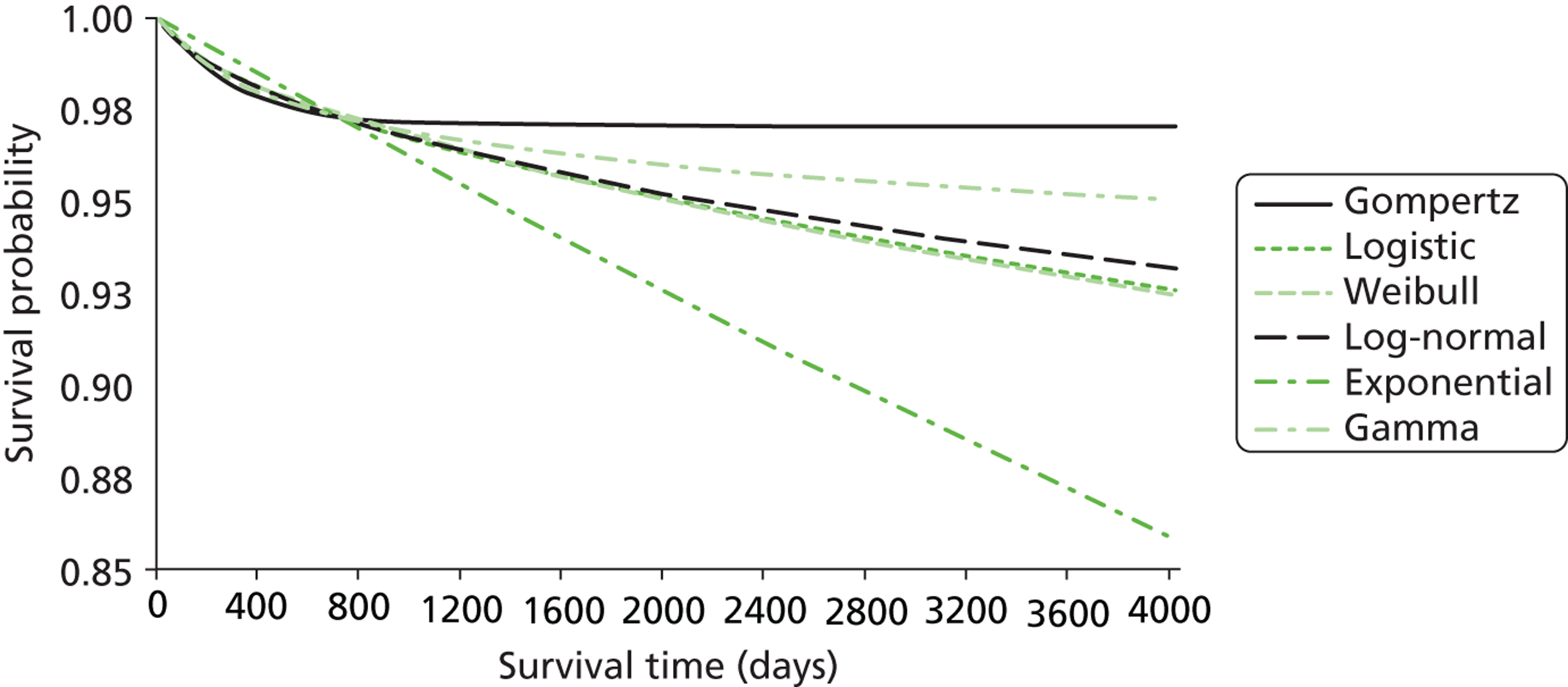
| Distribution | AIC | BIC |
|---|---|---|
| Gompertz | 305.9559 | 326.3428 |
| Gamma | 302.0634 | 327.547 |
| Log-normal | 306.6391 | 327.026 |
| Logistic | 310.0006 | 330.3875 |
| Weibull | 310.2893 | 330.6762 |
| Exponential | 315.8474 | 331.1376 |
| Year | Gompertz | Logistic | Weibull | Log-normal | Exponential | Gamma |
|---|---|---|---|---|---|---|
| 1 | 6.51 | 5.60 | 5.57 | 5.65 | 3.55 | 6.05 |
| 2 | 4.86 | 4.49 | 4.46 | 4.58 | 4.25 | 4.72 |
| 3 | 5.28 | 5.85 | 5.82 | 5.90 | 6.67 | 5.67 |
| 4 | 5.31 | 6.96 | 6.95 | 6.91 | 8.97 | 6.28 |
| 5 | 5.23 | 7.89 | 7.89 | 7.73 | 11.12 | 6.70 |
Tables 37–39 present the resultant life-years, QALYs, lifetime costs and INBs according to the base-case assumptions. Overall, at a threshold of £20,000 per QALY, the INBs are positive, but with wide 95% CIs that include zero. For cardiac patients, the INBs are close to zero with wide CIs. For non-cardiac patients, the INBs are positive but with 95% CIs that include zero.
| TGC (n = 694) [mean (SD)] | CM (n = 675) [mean (SD)] | Incremental [mean (95% CI)] | |
|---|---|---|---|
| Lifetime costs | 27,330 (34,262) | 30,951 (42,600) | –3620 (–7723 to 482) |
| Life-years | 24.99 (8.58) | 25.01 (8.58) | –0.02 (–0.93 to 0.89) |
| QALY | 18.25 (6.26) | 18.26 (6.27) | –0.01 (–0.68 to 0.65) |
| INBs | 3346 (–11,203 to 17,894) |
| TGC (n = 421) [mean (SD)] | CM (n = 416) [mean (SD)] | Incremental [mean (95% CI)] | |
|---|---|---|---|
| Lifetime costs | 24,066 (27,139) | 23,939 (26,304) | 128 (–3542 to 3797) |
| Life-years | 25.97 (7.33) | 26.03 (7.26) | –0.05 (–1.04 to 0.94) |
| QALY | 18.96 (5.35) | 19.00 (5.30) | –0.04 (–0.76 to 0.68) |
| INBs | –919 (–16,661 to 14,823) |
| TGC (n = 273) [mean (SD)] | CM (n = 259) [mean (SD)] | Incremental [mean (95% CI)] | |
|---|---|---|---|
| Lifetime costs | 32,364 (42,515) | 42,214 (58,432) | –9850 (–18,521 to –1180) |
| Life-years | 23.48 (10.03) | 23.38 (10.15) | 0.10 (–1.62 to 1.82) |
| QALY | 17.14 (7.32) | 17.07 (7.41) | 0.07 (–1.18 to 1.33) |
| INBs | 11,322 (–15,791 to 38,615) |
The cost-effectiveness acceptability curves consider alternative thresholds of willingness to pay for a QALY gain, and show that, overall and for the cardiac surgery stratum, it is highly uncertain whether or not TGC is cost-effective (Figures 16 and 17). For the non-cardiac stratum, the probability that TGC is cost-effective is relatively high. For example, at ceiling ratios of £10,000 to £30,000 per QALY, the probability that TGC is cost-effective ranges from 90% to 70% (Figure 18).
FIGURE 16.
Probability that TGC vs. CM is cost-effective at alternative levels of willingness to pay for a life-year gained: whole study cohort.
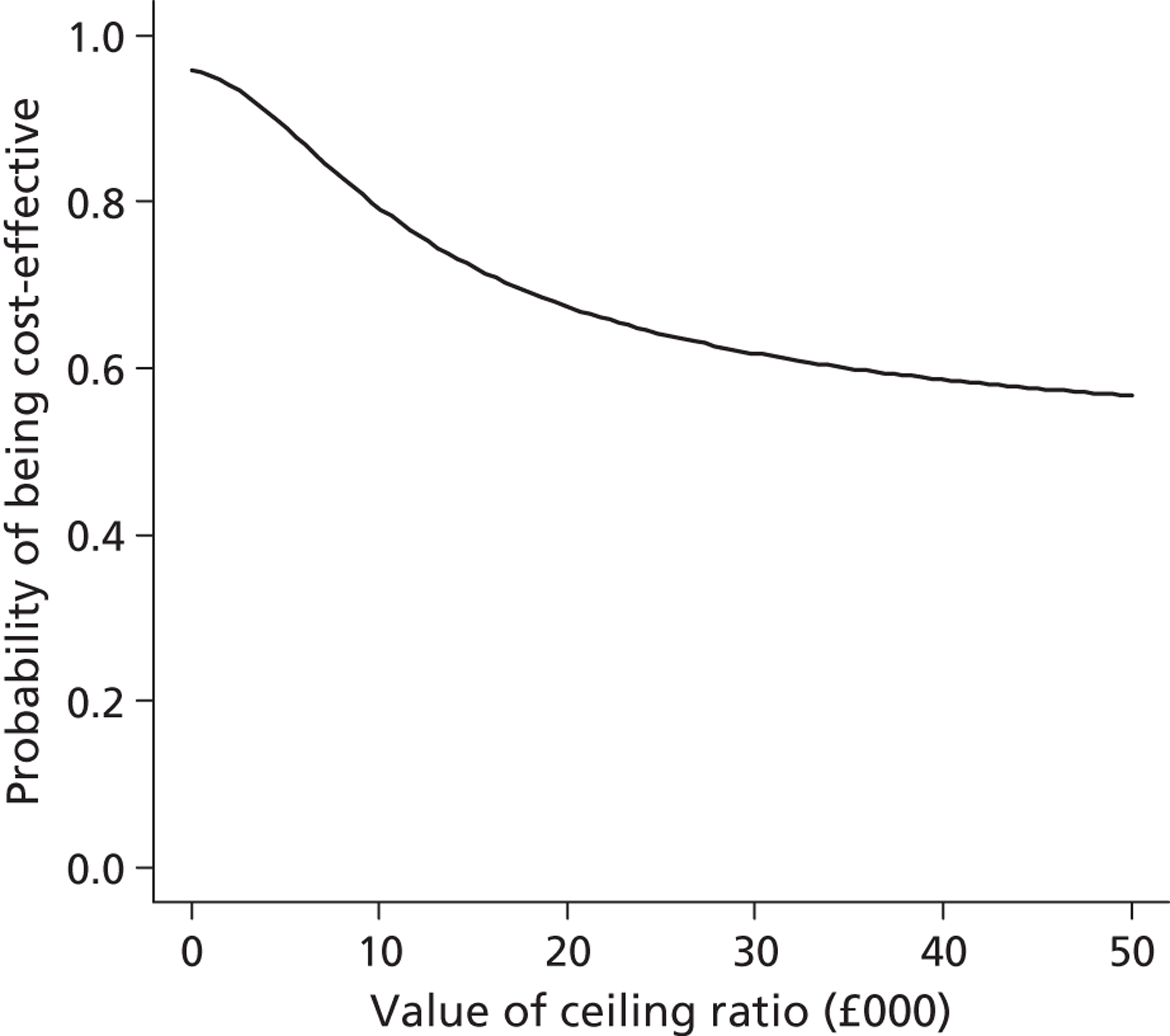
FIGURE 17.
Probability that TGC vs. CM is cost-effective at alternative levels of willingness to pay for a life-year gained: cardiac surgery subgroup.
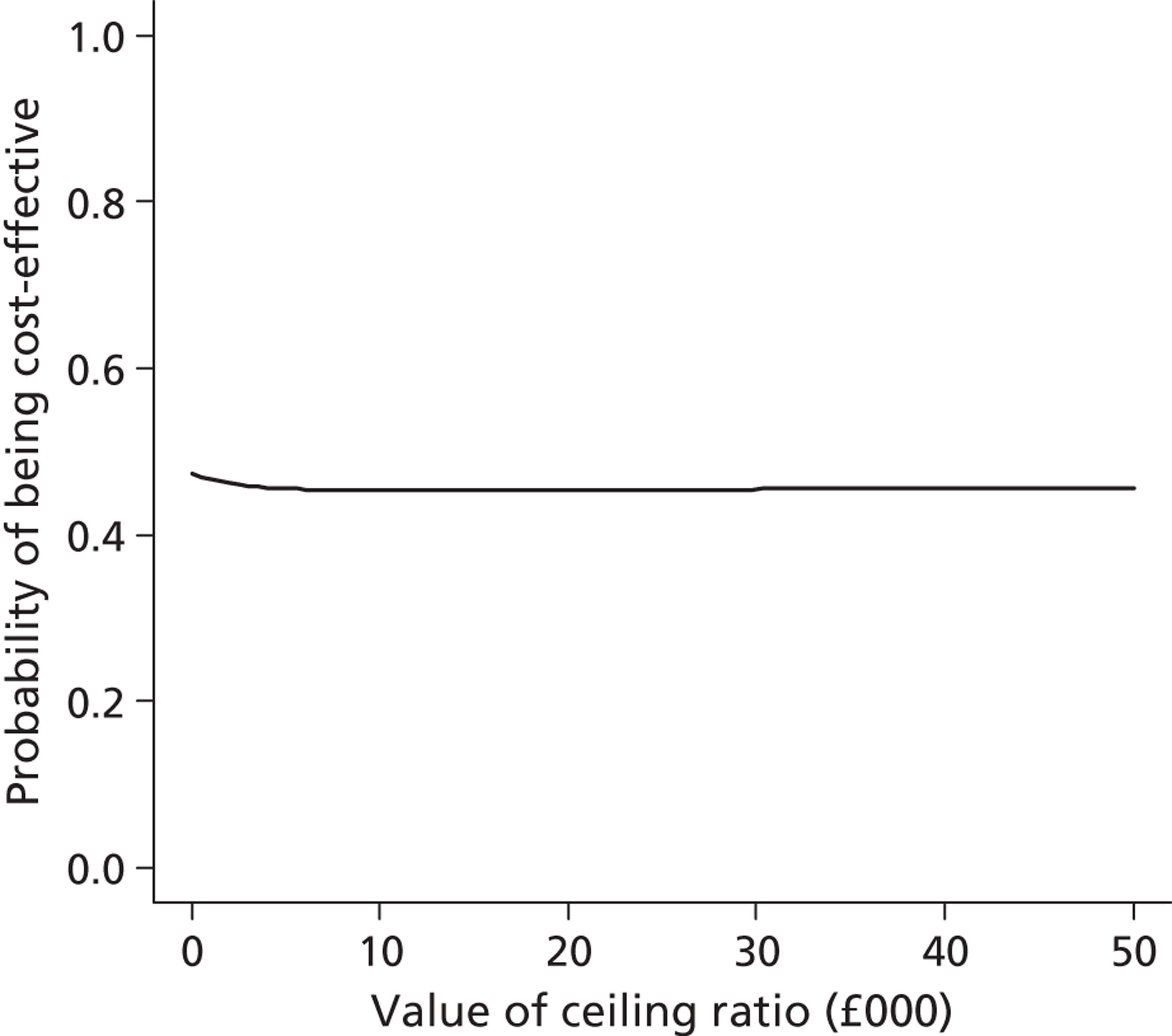
FIGURE 18.
Probability that TGC vs. CM is cost-effective at alternative levels of willingness to pay for a life-year gained: non-cardiac surgery subgroup.
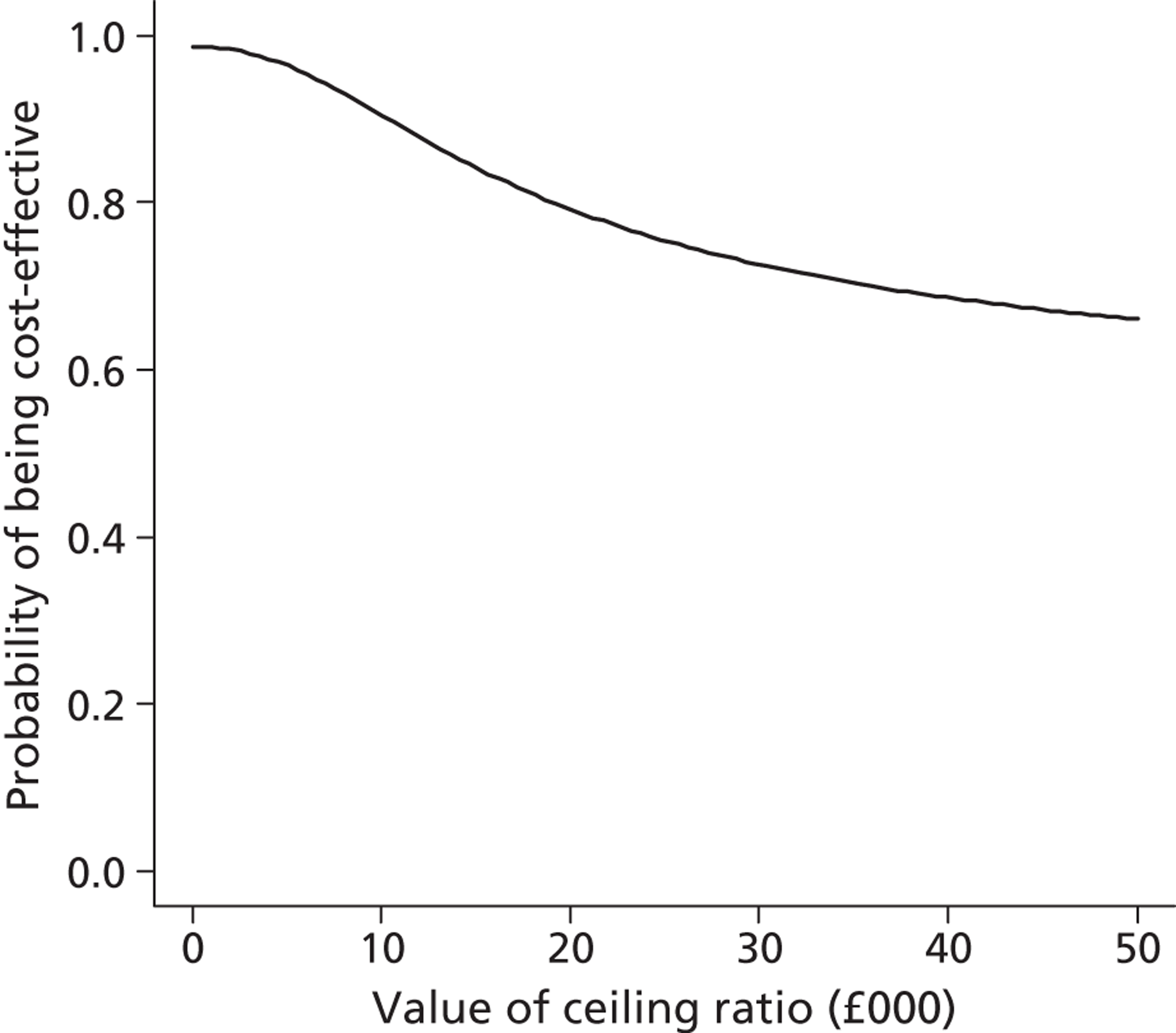
The SA on the lifetime results suggests that these findings are robust to alternative assumptions about the extrapolation of long-term survival, QoL for PICU survivors or long-term costs (Figures 19–21).
FIGURE 19.
Sensitivity analysis reporting lifetime INBs (£) according to alternative assumptions: whole study cohort.
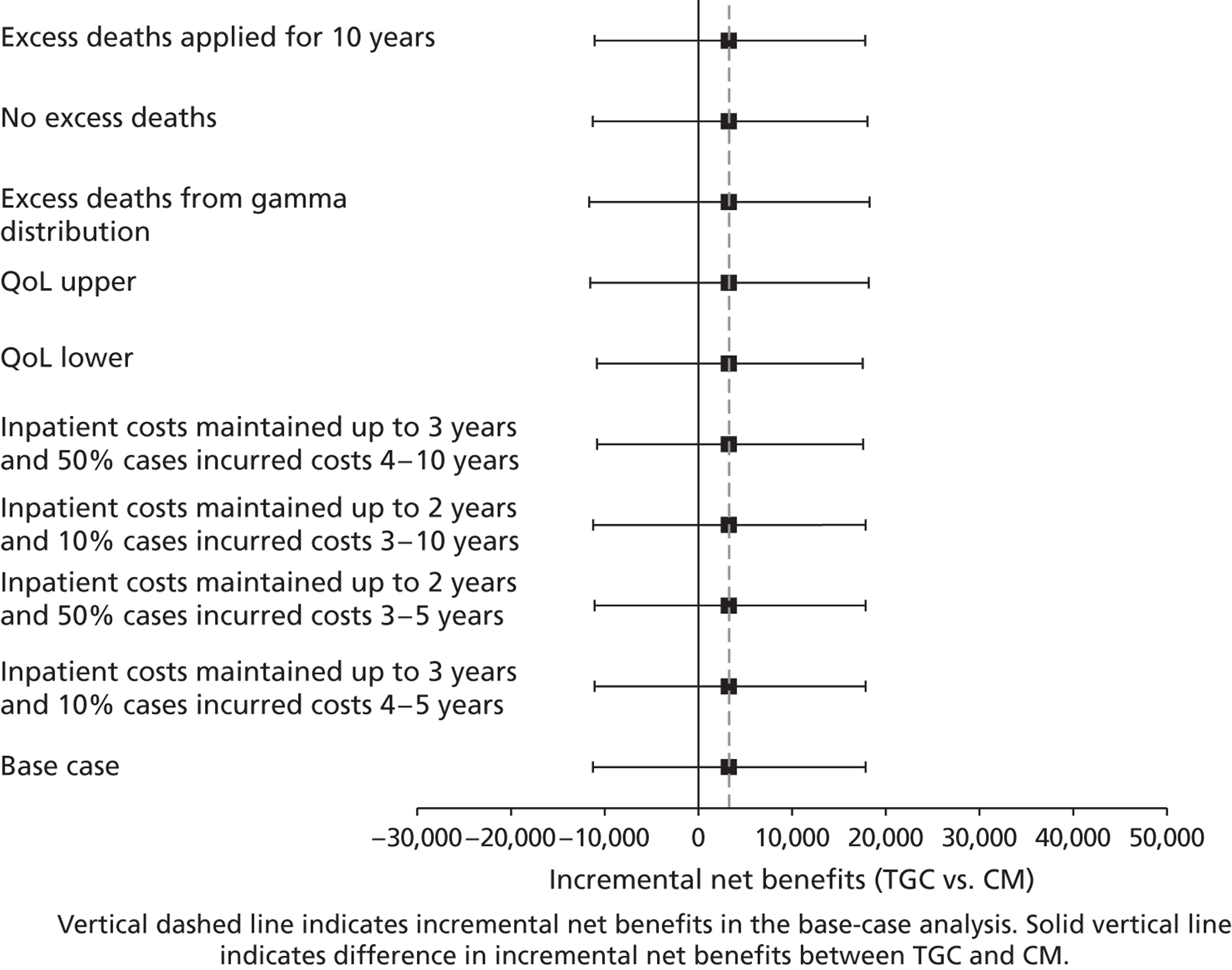
FIGURE 20.
Sensitivity analysis reporting lifetime INBs (£) according to alternative assumptions: cardiac surgery subgroup.
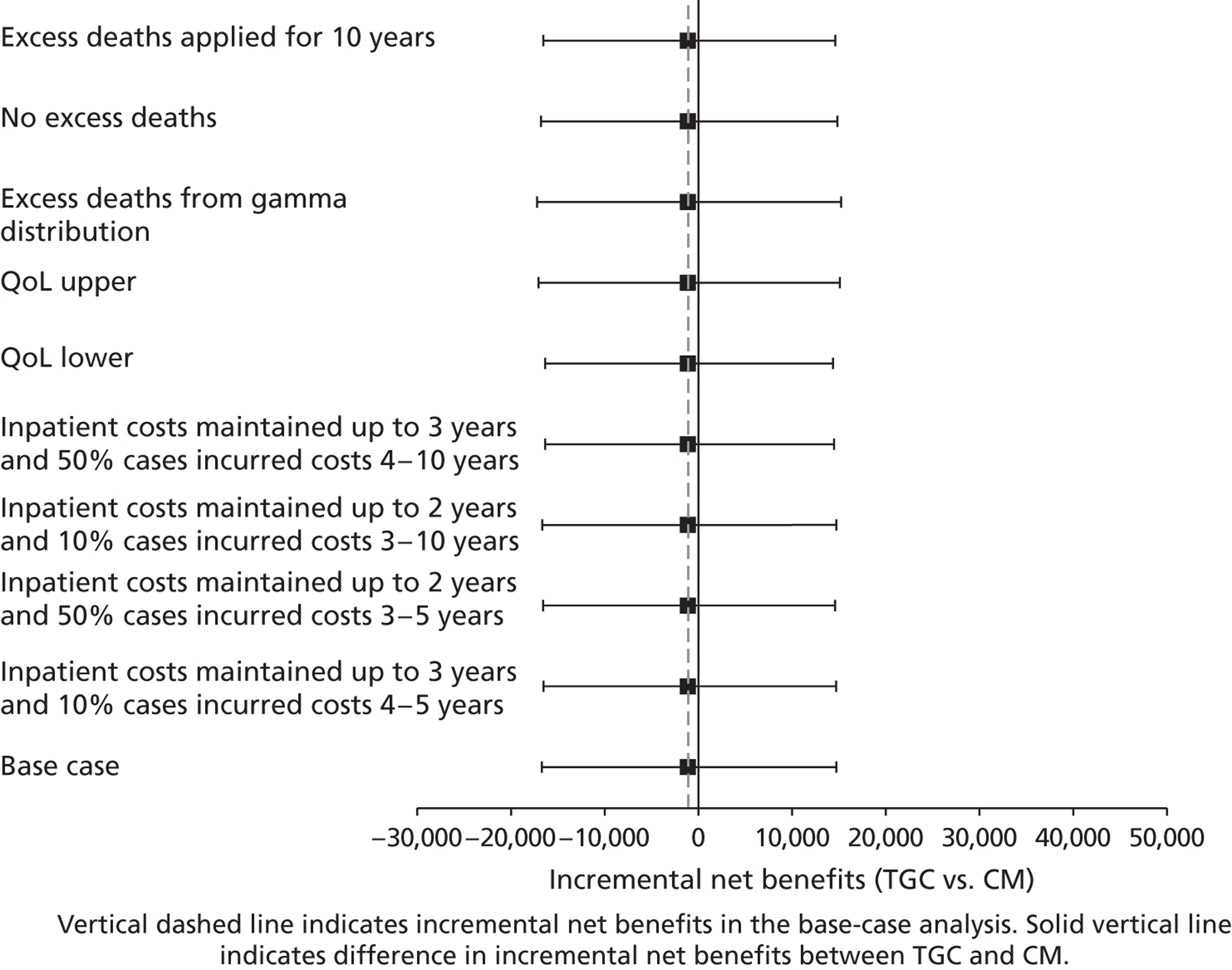
FIGURE 21.
Sensitivity analysis reporting lifetime INBs (£) according to alternative assumptions: non-cardiac surgery subgroup.
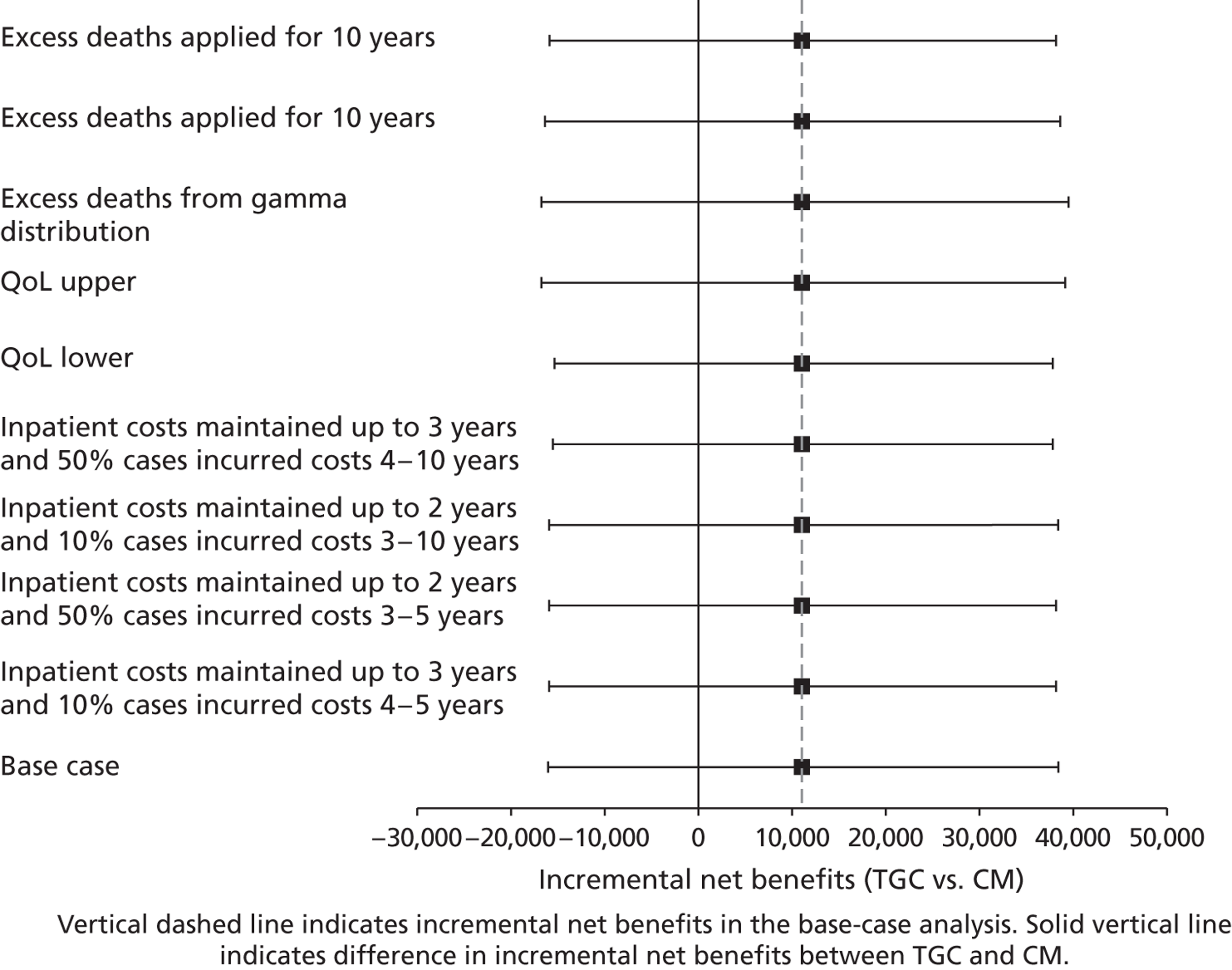
Chapter 4 Discussion
The aims of the CHiP trial were to evaluate the clinical effectiveness, safety and cost-effectiveness of a policy of TGC compared with CM for an overall population of children in PICUs and for subgroups admitted to PICU following cardiac surgery or for other reasons. The results suggest that, overall, TGC has no effects on major clinical outcomes such as death and/or ventilator days, whether at 30 days or at 1 year. However, the secondary outcomes reveal a complex relationship of gains and potential harm from the intervention. TGC results in a slight reduction in the proportion of patients receiving RRT, but hypoglycaemia occurs in a greater proportion of patients in the TGC arm than in the CM arm of the study (severe hypoglycaemia 7.3% vs. 1.5%). TGC reduces mean hospital LOS and total costs at 12 months after PICU admission. The lower costs reflect the reductions in PICU days and total hospital LOS for the subgroup not admitted for cardiac surgery. For children admitted for cardiac surgery, the LOS and costs are similar between the randomised arms.
It is interesting to speculate why there might be a differential effect of TGC on non-cardiac surgery cases as opposed to cardiac surgery cases. Hyperglycaemia is known to be associated with the activation of alternative metabolic pathways for glucose, some of which have the potential to generate reactive metabolites or trigger inflammatory pathways. Insulin, in contrast, can promote an anti-inflammatory milieu. Cardiopulmonary bypass has often been proposed as a proxy model for inflammation, in that it is known to be associated with the activation of major inflammatory pathways. Recent developments in clinical strategies are thought to have improved outcomes in children undergoing cardiopulmonary bypass. For instance, corticosteroids, modified ultrafiltration, low-prime-volume circuits and newer circuit components have been shown to, or are assumed to, favourably modify these inflammatory processes. It may be, therefore, that cardiac surgery in children is no longer associated with ‘sufficient’ inflammatory pathway activation for TGC to result in significant clinical benefit. In contrast, in the non-cardiac surgery cases, stress hyperglycaemia from sepsis and a variety of other medical conditions may, in fact, be truly detrimental,29 and hence control of the derangement is important for survival and limiting renal injury. Given that these problems may take days to manifest, it is entirely possible that the effects may be more identifiable at later time points, for example at 60 or even 90 days.
The finding that TGC leads to an average reduction in 12-month costs of approximately £10,000 for the non-cardiac surgery subgroup is robust to alternative stand points (such as the inclusion of any additional costs from monitoring the increased number of hypoglycaemic episodes in the TGC arm). The lifetime cost-effectiveness analysis suggests that it is highly uncertain that TGC is cost-effective in the cardiac surgery subgroup, whereas, for children admitted for other reasons, TGC appears relatively cost-effective. Therefore, for the patients not admitted for cardiac surgery, the findings from this study suggest that a TGC protocol would lead to earlier discharge from PICUs and hospital, leading to possible cost savings.
The potential NHS cost savings from a TGC policy can be estimated by combining the CHiP findings with projections of the annual incidence of eligible cases using the trial screening logs, or the PICANet49 database. The trial screening logs suggest that approximately 1300 admissions per year from the 13 CHiP centres would be eligible for TGC in routine practice, approximately 500 of which (around 40%) would be admitted for reasons other than cardiac surgery. If implementing TGC rather than CM for this subgroup does reduce average costs per patient by £10,000, then TGC could yield annual cost savings of around 5 million pounds in the CHiP centres alone. This approach underestimates total cost reductions, as not all potentially eligible cases were screened in CHiP centres, and some PICUs in England and Wales did not participate in the study.
PICANet provides an alternative source for calculating the annual incidence of eligible cases; it includes all PICUs in England and Wales and avoids undercounting the incidence of eligible cases from incomplete screening. We applied the main CHiP eligibility criteria to PICANet data from 2004–11. This includes all children who stayed for > 2 days in PIC in England and Wales, who received inotropes and mechanical ventilation and who were not admitted following cardiac surgery. The result was approximately 1500 admissions per year. To allow for the other CHiP eligibility criteria, which could not be applied to PICANet data, we reduced the anticipated number of admissions by 20% to 1200 per year, which gives annual cost savings to the NHS in England and Wales of approximately £12M. This approximation recognises that most, but not all, of the inclusion criteria required for children to be eligible for TGC could be applied to the PICANet data. More fundamentally, it should be recognised that introducing a policy of TGC would not realise full financial cost savings. Instead, PICU and hospital beds would be ‘released’ if patients were discharged earlier and other patients were allowed to use these resources.
Limitations
The primary end point in the study was selected based on the best evidence at the time and took into account usual practice in reporting short-term outcomes in studies conducted on critically ill patients. Our results, however, indicate that for non-cardiac surgical cases, it may be better to assess VFDs or QoL at a later time point, for example day 60, in future studies.
The cost-effectiveness analysis has some limitations. First, the level of activity was measured only within PICUs and only for up to day 30, after which general PICU costs were assumed. But analysis using different assumptions made little difference to the results. Second, there were some missing data on follow-up costs and at 12 months from administrative censoring owing to study funding, but also non-response to questionnaire. This was handled using MI, which assumes that data are missing conditional on baseline factors and other end point and process measures that are observed; therefore, if missingness is driven by unobserved prognostic factors, this could have led to biased estimates. However, for questionnaire responders, the follow-up costs were similar between the groups. Third, like previous RCTs in PIC, this study did not measure QoL. Instead, the cost-effectiveness analysis used QoL data from a previous study that included children admitted to UK PICUs who were at least 6-months-old. 71 It is unclear whether or not the QoL values from this sample of older children (median age of approximately 5-years-old) apply directly to the population represented by CHiP patients. However, the results of the SA suggest that the lifetime cost-effectiveness results were robust to the QoL value assumed for PICU survivors. Four, the cost-effectiveness analysis required that survival data from the CHiP trial were extrapolated over the lifetime. The analysis took a standard approach and applied the parametric survival function that was judged most plausible. There was no evidence from the survival data available from CHiP of any differences in survival up to 24 months, and the SA again shows results were robust to alternative assumptions.
Strengths
The CHiP trial addressed both clinical and economic questions, with follow-up at 12 months. The trial was the largest RCT in PIC, and was rigorously designed and conducted. Randomisation reduced the potential for selection bias at trial entry, and there was no loss to follow-up for the primary outcome. Although the local clinicians could not be blinded to allocation post randomisation, the use of a hard outcome (mortality) as an integral component of the 30-day primary outcome ensured the risk of biased outcome assessment was low, and it is not plausible that LOS would be influenced by allocation across the range of PICUs involved. The reasonably small number of secondary outcomes reduced the risks of problems associated with multiple testing.
There was careful preparation and training for the introduction of the clinical management protocol with a run-in period and minor adjustments to address any concerns about risks of hypoglycaemia. The delivery of TGC may be further improved by the use of continuous glucose monitoring systems,72 including those with paediatric decision support.
The biggest drivers of cost differences between the arms were PICU and total hospital LOS. For the vast majority of patients (99%), these resource-use data were measured until they were discharge from their index hospital admission or following readmission to the PICU within 30 days, and up to a maximum of 12 months from randomisation. The study employed careful resource-use and cost measurement, estimated from large sample patient-level data drawing on the PCCMDS, which provided reasonable inference on costs. State-of-the-art approaches to handling missing 12-month cost data were used. The study also recorded use of other hospital and community health services and found that reductions in hospital LOS for the TGC arm were not offset by increased use of other services.
The CHiP trial was conducted in a large proportion of the PICUs in England, increasing the generalisability of the clinical and cost-effectiveness results to other UK PICUs, and other settings with similar populations and systems of care.
As the largest PICU trial to date, CHiP had sufficient power to reliably assess whether the balance of costs and benefits differed depending on if the admission was following cardiac surgery or not and to provide reasonable inference on costs.
The results of the economic evaluation were subject to extensive SAs and were robust to alternative assumptions, including, for example, the approaches taken to unit costing or the projection of life expectancy from the trial data.
The CHiP trial results in context
In considering how the CHiP trial results compare with other research, we have drawn on the trials of TGC with adults in critical care, with preterm neonates in neonatal ICUs (NICU) and with children in PICUs, using studies published prior to CHiP’s initiation and reviewed in Chapter 1, and later relevant studies in different populations of critically ill patients. The particular emphases here are the size and type of effect of the intervention, the rate of hypoglycaemia and the differential effect in medical and surgical populations. There are some distinctions in these classifications between the CHiP trial and the adult studies. About a third of the patients in the Leuven surgical trial24 underwent surgery that was not cardiovascular, and, in the CHiP trial, general surgery was incorporated in the non-cardiac surgery group. The CHiP trial stratification of cardiac surgical and non-cardiac surgical does, however, allow direct comparison with the Leuven paediatric study73 and the ongoing US paediatric cardiac surgical study. 74
Adults
The CHiP trial findings differ from those of the initial adult study from the Leuven group. 24 In patients randomised to receive TGC in an adult surgical intensive care setting, Van den Berghe et al. 24 reported substantial reductions in mortality, other important complications of intensive care, and length of PICU and hospital stay. By contrast, the CHiP trial found no difference in mortality or VFD-30, and only minimal differences in secondary outcomes in the cardiac surgical subgroup. For the non-cardiac surgical subgroup from the CHiP trial, however, findings follow those from the Leuven25 study in critically ill, adult patients not admitted for surgery. Neither study found benefits for TGC on major clinical end points, but both studies found that TGC reduced hospital LOS, and reduced hospital costs.
Since these studies, several more trials and two meta-analyses have been published. The largest trial, the Normoglycaemia in Intensive Care Evaluation – Survival Using Glucose Algorithm Regulation (NICE SUGAR),75 was an international RCT that enrolled 6104 adults undergoing intensive care to undergo TGC (target blood glucose range 4.5–6.0 mmol/l) or conventional blood glucose control (target ≤ 10.0 mmol/l). Contrary to the Leuven studies, the NICE SUGAR investigators found that TGC increased mortality. A total of 829 patients in the TGC group (27.5%) died compared with 751 (24.9%) in the conventional-control group (odds ratio for intensive control 1.14, 95% CI 1.02 to 1.28, p = 0.02). Treatment effects did not differ significantly between surgical and non-surgical groups. There was no significant difference between the treatment groups in the median number of days in ICU, but severe hypoglycaemia (blood glucose level ≤ 2.2 mmol/l) was reported in 206 of 3016 patients (6.8%) in the intensive-control group and 15 of 3014 (0.5%) in the conventional-control group (odds ratio 14.7, 95% CI 9.0 to 25.9, p < 0.001). No long-term sequelae of severe hypoglycaemia were reported.
In 2008, Wiener et al. 76 reported a meta-analysis of TGC in critically ill adults. In total, the authors identified 8432 patients in 34 randomised trials (23 full publications, 9 abstracts, 2 unpublished studies) and did not find a survival benefit of TGC, but showed that TGC was associated with an increased risk of hypoglycaemia (glucose ≤ 2.2 mmol/l; 13.7% vs. 2.5%; RR, 5.13; 95% CI 4.09 to 6.43). This increased risk was fairly consistent across different ICU settings (surgical, medical and mixed). Most trials reported that very few of the hypoglycaemic events were associated with overt symptoms, but some studies found that patients who experienced hypoglycaemia had a higher risk of death.
A later meta-analysis,77 which contained 26 adult studies including the NICE SUGAR trial, also concluded that TGC conferred no overall benefit among critically ill patients. A subanalysis of five trials that included only surgical patients suggested possible benefits from TGC, although the predominant influence on this subgroup was the original Leuven study,24 which contributed 765 of the 1037 patients in the TGC group and 783 of 935 patients in the control group. The differences between the two meta-analyses were likely to be a result of their inclusion criteria, with the positive results for surgical patients in the Griesdale meta-analysis reflecting publication bias from excluding unpublished trials with negative results.
A major impetus for the CHiP trial was the concern that the results of trials in adult ICUs may not be easily transferable to children. In 2008 and 2009, two trials did report results for children.
Preterm neonates
The NIRTURE (neonatal insulin therapy in Europe) study78 enrolled 389 very low-birthweight neonates from eight NICUs in the UK and mainland Europe. The trial sought to determine whether or not early insulin replacement (continuous infusion of insulin at a dose of 0.05 IU/kg/hour with 20% dextrose support) on days 1–7 reduced hyperglycaemia and affected outcomes in preterm newborns compared with standard neonatal care, the primary outcome being death at the expected date of delivery (EDD).
The early insulin group had significantly more carbohydrate infused and less weight loss in the first week than infants in the control group. However, more infants in the early insulin group had episodes of hypoglycaemia (< 2.6 mmol/l) than in the control group (29% vs. 17%; odds ratio 2.21; 95% CI 1.34 to 3.65; p < 0.005). In prespecified subgroup analyses, the increase in hypoglycaemia was significant only in the infants with a birth weight of > 1 kg (34% vs. 12% in the control group; odds ratio 3.96; 95% CI 1.85 to 8.47; p < 0.001). There was no increase in hypoglycaemia in infants with a birth weight of < 1 kg (26% in the early insulin group vs. 23% in the control group; odds ratio 1.17; 95% CI 0.60 to 2.28; p = 0.7). Clinicians reported episodes of hypoglycaemia in 17 infants in the early insulin group (8.8%) (including two who had protocol violations and four who were withdrawn from the study) and in three in the control group (1.6%). Episodes of hypoglycaemia were not associated with clinical alterations in physiology.
The trial was stopped early by the TSC on the advice of the independent Data Monitoring Committee for a combination of futility in terms of the primary mortality outcome, and concerns about potential harm in terms of an excess of ventricular haemorrhage and parenchymal lesions on cerebral ultrasound scans. There was no statistically significant difference in the primary outcome of mortality at EDD (14% vs. 9%; odds ratio 1.64; 95% CI 0.87 to 3.03; p = 0.2), or duration of neonatal intensive care, but mortality at 28 days was increased in the TGC group (12% vs. 6%; odds ratio 2.22; 95% CI 1.04 to 4.76; p = 0.04).
The authors speculated that the results might be due to a smaller difference in the levels of glucose control than seen in their pilot study, or too short a period (7 days) of insulin replacement. The design of this study was fundamentally different from other TGC studies in that a constant dose of insulin was infused, and blood glucose levels normalised by increasing the amount of glucose infused. This different methodology and the obvious developmental differences between the preterm infants included in this study and term or older infants and children in the CHiP trial make direct comparisons difficult to interpret.
Children
We are aware of only one published RCT that has investigated the effects of TGC in critically ill infants and children. This single-centre trial73 evaluated whether or not targeting age-adjusted normoglycaemia (TGC) would improve outcomes. This trial enrolled 700 children, 317 of whom were < 1 year of age, with 75% admitted for cardiac surgery. Patients were randomly assigned to normoglycaemia/TGC, defined as 2.8–4.4 mmol/l in infants and 3.9–5.6 mmol/l in children, with insulin infusion throughout their PICU stay. Primary end points were duration of PICU stay and inflammation, C-reactive protein.
Hypoglycaemia (blood glucose ≤ 2.2 mmol/l) occurred in 87 (25%) patients in the intensively targeted blood glucose group (p < 0.0001) compared with five (1%) patients in the conventional group; hypoglycaemia, defined as blood glucose < 1.7 mmol/l, arose in 17 (5%) patients in the intensive insulin group (15 infants and 2 children) and in 3 (1%) in the conventional group (two infants and one child) (p = 0.001). Hypoglycaemia (≤ 2.2 mmol/l) occurred at a median of day 2 (interquartile range 1–5) in the intensive insulin group compared with day 1 (1–3) in the conventional group (p = 0.29). Hypoglycaemia (≤ 2.2 mmol/l) on more than two occasions occurred in 18 patients (5%) treated with intensive insulin compared with none in the conventional group.
The study found that TGC reduced the duration of PICU stay from a mean of 6.15 days (95% CI 5.25 days to 7.05 days) in the control group to 5.51 days (95% CI 4.65 days to 6.37 days; p = 0.017). The number of patients whose stay in the ICU was extended (> median) was 132 (38%) in the intensively targeted blood glucose group compared with 165 (47%) in the conventional group (p = 0.013). TGC resulted in a greater reduction in C-reactive protein at day 5 compared with baseline [–9.75 mg/l (95% CI –19.93 mg/l to 0.43 mg/l) vs. 8.97 mg/l (95% CI –0.9 mg/l to 18.84 mg/l), p = 0.007], indicating an attenuated inflammatory response.
As in the CHiP trial, Vlasselaers et al. 73 noted a lower requirement for RRT in those children managed by TGC (0.6% vs. 1.7%) than in their control group. This difference did not reach statistical significance, perhaps owing to small numbers requiring dialysis, but a reduction in RRT was shown in the meta-analysis by Wiener et al. 76 (RR 0.64, 95% CI 0.45 to 0.92) when analysing their data using a fixed-effects model.
There were 20 deaths in the conventional group (5.7%), compared with nine deaths in the TGC group (2.6%, p = 0.38), but, given the substantially lower mortality rates in PIC than usually experienced in adult populations, the study was not powered for a mortality end point.
During the course of the CHiP trial, the results of these trials and meta-analyses were noted by the CHiP TSC and DMEC. The continuing uncertainty with regards to the clinical effectiveness and safety of TGC were noted, together with the absence of evidence on its long-term clinical effectiveness and cost-effectiveness.
Although the Leuven paediatric trial is the most similar to the CHiP trial, the two trials differ in a number of important respects. First, the CHiP trial is twice as large, giving more power to determine whether or not TGC led to different outcomes in the cardiac surgical and non-cardiac surgical subpopulations. Three-quarters of the Leuven trial population underwent cardiac surgery, but the two strata are not reported separately. Second, the CHiP trial is also a test of the potential diffusibility of TGC across a range of PICUs, contrasting with the Leuven paediatric study, which was a single-centre trial from an ‘early adopting’ setting. Third, the hypoglycaemia rates were lower in the CHiP trial, possibly as a result of the choice of lower glucose control ranges for TGC in the Leuven paediatric study. Finally, the CHiP trial measured the impact of TGC over a longer follow-up period and included a rigorous economic evaluation. 73
Vlasselaers and colleagues are conducting a longer-term follow-up of the children enrolled in their study (D Vlasselaers, Catholic University of Leuven, 2011, personal communication). In addition, other groups are planning or are recruiting to74 similar clinical trials, the findings of which will add to those of the Leuven and CHiP trials, and further inform clinicians about the risks and benefits of TGC.
Chapter 5 Conclusions
Implications for health care
For the cardiac surgery subgroup, average costs at 12 months post randomisation were similar between arms and TGC was unlikely to be cost-effective. For children admitted to PICUs following cardiac surgery, our study suggests that PICUs should not adopt TGC and should continue with CM.
For children admitted to PICUs for other reasons, although TGC did not improve short-term clinical outcomes compared with CM, children in this subgroup were discharged earlier from hospital. This clinical benefit (earlier discharge) was associated with substantially reduced NHS costs. These average reductions in NHS costs were not offset by increased use of community health services in the TGC versus CM arm.
This is the first study to find that TGC may be cost-effective in children in PIC who were not admitted for cardiac surgery. Before a policy of TGC can be recommended for this important subgroup of NHS patients (around 1200 patients per year), careful consideration should be given to the balance of risk and benefit of this intervention: between the small increased risk of hypoglycaemia and the potential reduction in length of hospital stay and associated cost savings.
Recommendations for further research
The findings of the CHiP trial raise the following important questions:
-
Does the excess rate of moderate and severe hypoglycaemia during TGC for children admitted to PICUs for reasons other than cardiac surgery have an impact on long-term neurodevelopmental outcomes?
-
Before a policy of TGC can be recommended for children admitted to PICUs for reasons other than cardiac surgery, further information is needed about the long-term implications of the small increased risk of hypoglycaemia. Over 100 children in the CHiP trial experienced at least one episode of moderate or severe hypoglycaemia. In the first instance, we will undertake a post-hoc analysis of the data along the lines of the recent analysis of hypoglycaemia complicating TGC in adults that was published in the New England Journal of Medicine. One of the CHiP investigators has been in contact with the lead investigator of NICE SUGAR and plans are being made for the post-hoc data evaluation. Subsequently, a neurodevelopmental follow-up study of these children would inform clinicians of the long-term risk of hypoglycaemia in this population.
-
-
Can we improve the delivery of TGC to minimise the risk of hypoglycaemia?
-
If a policy of TGC is to be recommended for children admitted to PICUs for reasons other than cardiac surgery, research is needed to further refine clinical algorithms for the delivery of TGC, and to assess whether or not delivery of TGC may be further improved by the use of continuous glucose monitoring systems,72 including those with paediatric decision support.
-
-
Does TGC in critically ill children protect the kidneys from injury?
-
One hypothesis raised by the CHiP trial is that TGC can reduce acute kidney injury (AKI). AKI is a common complication of critical illness, which in its most severe form requires RRT. Children requiring RRT have a prolonged stay in PICU and increased mortality (≈ 40%). Children in the TGC arm in CHiP had a lower incidence of RRT than those in the CM arm. However, RRT represents the most severe end of the spectrum of AKI and is a rare outcome compared with lesser degrees of AKI. An add-on study in CHiP patients could provide more precise measures of AKI, through estimation of daily glomerular filtration rate from sequential plasma creatinine values obtained as part of routine clinical care. Creatinine values would be retrieved from laboratory databases and statistical analysis would be undertaken to investigate this research question.
-
-
Do the findings from CHiP apply to routine clinical practice?
-
The CHiP RCT had a pragmatic design, included the majority of English PICUs and had broad patient eligibility criteria. However, as with any RCT, the findings might not apply directly to routine clinical practice. Inevitably, the CHiP centres did not screen all potential patients, and eligible patients were excluded for various reasons, for example because of refused consent. Further, the CM delivered in the CHiP study may differ from that provided routinely. These potential concerns illustrate the general challenge that RCT findings might not apply directly to routine clinical practice. To address these issues, further research is required that develops an approach for maximising external validity. One possible approach to extending the CHiP findings would be to carefully assess the external validity of the study findings using PICANet data. This further research could examine whether or not the patients’ baseline characteristics, resource use, costs and outcomes following CM in CHiP differed from those observed in routine clinical practice. This setting, with a large RCT nested within an observational data set, would provide an opportunity for considering whether or not results from pragmatic trials such as CHiP have external validity.
-
-
What can be learnt from triallists, clinicians, parents and older children about their experiences of participating in CHiP, to aid the design and conduct of future PICU trials?
-
As more PICU RCTs assessing treatments in the NHS are funded, it is important for their success to learn from the experiences of participants in existing trials. A substantial body of research on participants’ experiences of NICU trials aided development of CHiP trial procedures, but there is currently no equivalent literature from PICUs. As the largest UK PICU trial to date, CHiP offers a timely opportunity for such work. Qualitative research exploring clinician and family experiences of implementing the CHiP protocol would inform the conduct of future PICU trials.
-
Acknowledgements
We would like to thank all the parents and guardians of participating children.
Trial Steering Committee
P Barnes, S Edwards, D Field, J Hooper, M Preece (chairperson), T Quick, C Snowdon, L Tume, D Vlasselaers and P Williamson.
Project management group
E Allen, H Betts, D Elbourne, R Grieve, C Guerriero, M Loverage, D Macrae, K Morris, J Pappachan, R Parslow, D Piercy, Z Sadique, L Van Dyck, N Smith-Wilson and R Tasker.
Trial co-ordinating centre
E Allen, L Brooks, K Diallo, D Elbourne, C Frost, A Gadhiya, P Henley, E Morris, D Piercy, P Ramos, J Sturgess and A Truesdale.
Clinical coordination centre (Royal Brompton Hospital)
S Bacon, H Betts and D Macrae.
Economic evaluation
R Grieve, C Guerriero and Z Sadique.
Data Monitoring Committee
D Dunger, D Harrison, D Hatch, G Peek and J Smith.
Information technology services
M Bennett, T Flemming and J Strachan.
Paediatric Intensive Care Audit Network
T Flemming and R Parslow.
Design, branding and case report form development
S Moncrieff.
Official scoring
A Kirby (Clinical Psychologist).
Recruiting centres: collaborating doctors and nurses [the principal investigator(s) and lead research nurse(s) are listed first and italicised]
Alder Hey Hospital
P Baines, C Morlidge, M Christopherson, R Guhadasan, F Haigh, D Hawcutt, H Hill, P Holmes, M Horan, R Jennings, S Kerr, F Potter, J Ratcliffe, E Scott, A Selby, C Sellers, N Shetty, D Sidaras, E Simpson, S Siner and K Thorburn.
Birmingham Children’s Hospital
K Morris, S Laker, E Benson, H Duncan, K Ewing, J Faulkner, N Holdback, L Hydes, J Martin, J Menzies, S Sebastian, M Smith, J Spry and H Winmill.
Bristol Royal Hospital for Children
M Schindler, N Robinson, M Allen, P Davis, S Dean, N Fineman, J Fraser, S Goodwin, D Grant, I Jenkins, S Marriage, J Talmud, P Weir, M White, A Wolf and I Zafurallah.
Glenfield Hospital and Leicester Royal Infirmary
M Duthie, C Brunskill, R Patel, P Barry, S Pooboni, R Ramaiah, A Vora, C Westrope and J Whitelaw.
Great Ormond Street Hospital for Children
M Peters, M Broadhead, S Riordan, T Blatcher, J Brierley, L Durkan, A Jones, U Krukenburg, P Lister, Q Mok, A Petros, C Pierce and S Sharma.
Leeds General Infirmary
M Darowski, P Atwal and L Cooper.
Royal Brompton Hospital
D Macrae, S Bacon, T Adamovic, M Burmester, A Desai, A Furck, S Gala, E Harrison, A Lammers, J LaRovere, A Mittal, M Montgomery, J Pallawela, N Pathan, W Rodrigues, T Samad and P Toohey.
Royal Manchester Children’s Hospital
P-M Fortune, M MacDonald, C Rishton, R Barber, M Gnanalingham, K Hawkins, S Playfor, M Samuels, D Stewart and R Yates.
St George’s Hospital
M Gray, E Wall and S Smith.
St Mary’s Hospital
D Inwald, A Abdulla, A Brewer, M Cooper, P Habibi, C de Munter, S Nadel, S Qureshi, P Ramnarayan, A Smale and M Wolverson.
Sheffield Children’s
A Mayer, K Wall, C Bevan, L Fernando, S Hancock and J Perring.
Southampton General Hospital
J Pappachan, H Gale, L Grace, P Hyde, G Jones, I Macintosh, J McCorkell, R Mitchell, K Morton, K Ramakrishnan, S Rees, V Stanley, K Sykes and P Wilson.
University Hospital of North Staffordshire
P McMaster, P Ramesh, S Lownds, J Alexander, K Beaumond, M Bebbington, S Dodd, S Lightfoot, E Newman, P Percival, T Proctor, K Robinson, H Shepley, C Sidley and T Wilson.
National Institute for Health Research Health Technology Assessment
R Davies, L Dodd and C Gregory.
Medicines for Children Research Network
V Poustie, R Smyth and W Van’t Hoff.
Research and development (Royal Brompton Hospital)
W Butcher, A Cooper, L Henderson and J Fatukasi.
The UK Paediatric Intensive Care Society Study Group
Publication
Macrae D, Grieve R, Allen E, Sadique Z, Morris K, Pappachan J, et al. A randomized trial of hyperglycemic control in pediatric intensive care. N Engl J Med 2014;370:107–18.
Contributions of authors
Duncan Macrae (Consultant in Paediatric Intensive Care) was the chief investigator. He was involved in the design and conduct of research and was a member of the trial development group, the project management group and the TSC. He was also involved in the development and refinement of the trial protocol, the conduct of the research interpretation and the reporting of results, and he revised the manuscript for important intellectual content.
Richard Grieve (Reader in Health Economics) led the health economic evaluation and was involved in the design and conduct of research, the trial development group, the project management group and the TSC, the statistical analysis, and the interpretation and reporting of results. He wrote the economic evaluation sections of the report and revised the manuscript for important intellectual content.
Elizabeth Allen (Senior Lecturer in Medical Statistics) was the lead statistician and was involved in the design and conduct of research, the Data Monitoring Committee, the project management team, the interpretation and reporting of results, and the draft and revision of the manuscript for important intellectual content.
Zia Sadique (Research Fellow in Health Economics) undertook the majority of the health economics analysis and was involved in the design and conduct of research, the project management team, the interpretation and reporting of results, and revision of the manuscript for important intellectual content.
Helen Betts (Lead Research Nurse, London) was involved in the set up, conduct and acquisition of data of this research and in the preparation of the manuscript.
Kevin Morris (Consultant Paediatric Intensivist) was a member of the trial development group, the TMG and the TSC. He was the PI for Birmingham Children’s Hospital. He was involved in the development and refinement of the trial protocol, the conduct of the research, acquisition of data, the interpretation and reporting of results, and revision of the manuscript for important intellectual content. In addition, he was chief investigator for an add-on study to CHiP investigating the mechanism of hyperglycaemia in critically ill children.
Vithayathil John Pappachan (Consultant Paediatric Intensivist) was the PI for University Hospitals Southampton NHS Foundation Trust and was involved in the conduct of research, trial design and management acquisition of data, the interpretation and reporting of result and manuscript preparation.
Roger Parslow (Senior Lecturer at the University of Leeds) was the PI for PICANet and was a member of the TMG involved in the conduct of research facilitating the acquisition of data, the interpretation and reporting of results and revision of the manuscript for important intellectual content.
Robert C Tasker (Professor of Neurology and Anaesthesia) was part of the senior management structure for the trial: he was involved with the conception and design of the trial, its conduct of research, and interpretation and reporting of results. He helped draft and revise the manuscript for important intellectual content.
Paul Baines (Consultant Paediatric Intensivist) was the PI at Alder Hey Hospital and was involved in the conduct of research, acquisition of data, and interpretation and reporting of results.
Michael Broadhead (Consultant Cardiac Intensivist) was the PI at Great Ormond Street Hospital for Children and was involved in the conduct of research, patient consent, data review and reporting.
Mark L Duthie (Consultant Paediatric Intensivist) was the PI for University Hospitals of Leicester and was involved in conduct of the trial, recruitment of subjects, reporting and interpretation of acquired data.
Peter-Marc Fortune (Consultant Paediatric Intensivist) was the PI for Royal Manchester Children’s Hospital and was involved with the drafting of the trial protocols, conduct of the research and acquisition of data.
David Inwald (Senior Lecturer and Honorary Consultant Paediatric Intensivist) was the PI for Imperial College Healthcare NHS Trust and was involved in the conduct of research, acquisition of data, and interpretation and reporting of results.
Paddy McMaster (Consultant Paediatrician) was the PI for the University Hospital of North Staffordshire and was involved in the conduct of research, acquisition of data and interpretation and reporting of results.
Mark J Peters (Senior Lecturer in Paediatric Intensive Care) was the PI for Great Ormond Street Hospital for Children paediatric and NICUs, and was involved in the design and conduct of research, acquisition of data, and interpretation and reporting of results.
Margrid Schindler (Consultant Paediatric Intensivist) was the PI for Bristol Royal Hospital for Children and was involved in the conduct of the research, acquisition of data and reporting of results.
Carla Guerriero (Research Fellow in Health Economics) helped with the design and analysis of the health economic evaluation and was involved in the project management team and in the interpretation and reporting of results.
Deborah Piercy (Trial Manager) was the trial manager (until 2009) and was involved in the set up, running and management of the trial.
Zdenek Slavik (Consultant Paediatric Cardiologist/Intensivist) originally suggested carrying out a prospective study involving TGC in paediatric patients requiring intensive care treatment, and was involved in the initial discussion of its merits and initial planning stages of the trial.
Claire Snowdon (Qualitative Researcher) was a member of the TSC, was involved in the design of the trial communication strategies with parents, advised on the conduct of research and the acquisition of data in her role as TSC member and contributed to the reporting of results.
Laura Van Dyck (Trial Manager) was involved in the day-to-day running of the trial, in particular the data management, and was a member of the project management team.
Diana Elbourne (Professor of Healthcare Evaluation) was the lead investigator for trial design and management, and was involved in the design and conduct of the research, the trial development group, the project management group and the TSC, the statistical analysis, the interpretation and reporting of results, and revision of the manuscript for important intellectual content.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin 2001;17:107-24. http://dx.doi.org/10.1016/S0749-0704(05)70154-8.
- Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med 1987;317:403-8. http://dx.doi.org/10.1056/NEJM198708133170702.
- Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med 2004;5:329-36. http://dx.doi.org/10.1097/01.PCC.0000128607.68261.7C.
- Yung M, Wilkins B, Norton L, Slater A. Group ftPS . Glucose control, organ failure, and mortality in pediatric intensive care. Pediatr Crit Care Med 2008;9:147-52. http://dx.doi.org/10.1097/PCC.0b013e3181668c22.
- Selye H. The Stress of Life. New York, NY: McGraw-Hill; 1956.
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000;355:773-8. http://dx.doi.org/10.1016/S0140-6736(99)08415-9.
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001;32:2426-32. http://dx.doi.org/10.1161/hs1001.096194.
- Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery 2000;46. http://dx.doi.org/10.1097/00006123-200002000-00015.
- Laird AM, Miller PR, Kilgo PD, Meredith JW, Chang MC. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma Acute Care Surg 2004;56:1058-62. http://dx.doi.org/10.1097/01.TA.0000123267.39011.9F.
- Lange P, Groth S, Kastrup J, Mortensen J, Appleyard M, Nyboe J, et al. Diabetes mellitus, plasma glucose and lung function in a cross-sectional population study. Eur Respir J 1989;2:14-9.
- Ardigo D, Valtuena S, Zavaroni I, Baroni MC, Delsignore R. Pulmonary complications in diabetes mellitus: the role of glycemic control. Curr Drug Targets Inflamm Allergy 2004;3:455-8. http://dx.doi.org/10.2174/1568010042634488.
- van Petersen AS, Vu MK, Lam WF, Lamers, CB, Ringers J, Masclee AA. Effects of hyperglycaemia and hyperinsulinaemia on proximal gastric motor and sensory function in humans. Clin Sci 2000;99:37-46. http://dx.doi.org/10.1042/CS19990341.
- Russo A, Fraser R, Horowitz M. The effect of acute hyperglycaemia on small intestinal motility in normal subjects. Diabetologia 1996;39:984-9. http://dx.doi.org/10.1007/BF00403919.
- Russo A, Smout AJ, Kositchaiwat C, Rayner C, Sattawatthamrong Y, Semmler J, et al. The effect of hyperglycaemia on cerebral potentials evoked by rapid rectal distension in healthy humans. Eur J Clin Invest 1999;29:512-18. http://dx.doi.org/10.1046/j.1365-2362.1999.00487.x.
- Russo A, Sun WM, Sattawatthamrong Y, Fraser R, Horowitz M, Andrews JM, et al. Acute hyperglycaemia affects anorectal motor and sensory function in normal subjects. Gut 1997;41:494-9. http://dx.doi.org/10.1136/gut.41.4.494.
- Rayner CK, Smout AJ, Sun WM, Russo A, Semmler J, Sattawatthamrong Y, et al. Effects of hyperglycemia on cortical response to esophageal distension in normal subjects. Dig Dis Sci 1999;44:279-85.
- McManus LM, Bloodworth RC, Prihoda TJ, Blodgett JL, Pinckard RN. Agonist-dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia. J Leukoc Biol 2001;70:395-404.
- Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract 2004;10:21-33. http://dx.doi.org/10.4158/EP.10.S2.21.
- Vriesendorp TM, Morelis QJ, Devries JH, Legemate DA, Hoekstra JB. Early post-operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study. Eur J Vasc Endovasc Surg 2004;28:520-5. http://dx.doi.org/10.1016/j.ejvs.2004.08.006.
- UKPDS Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854-65.
- The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86.
- Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 2002;288:2579-88. http://dx.doi.org/10.1001/jama.288.20.2579.
- Malmberg K, Rydén L, Efendic S, Herlitz J, Nicol P, Waldenstrom A, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995;26:57-65. http://dx.doi.org/10.1016/0735-1097(95)00126-K.
- Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359-67. http://dx.doi.org/10.1056/NEJMoa011300.
- Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449-61. http://dx.doi.org/10.1056/NEJMoa052521.
- Draper E, Hobson R, Lamming C, McShane P, Norman L, Parslow R, et al. Annual Report of the Paediatric Intensive Care Audit Network 2008–2010. UK: Universities of Leeds and Leicester; 2011.
- PubMed.gov . Information NCfB n.d. www.ncbi.nlm.nih.gov/pubmed/ (accessed April 2012).
- Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr 2005;146:30-4. http://dx.doi.org/10.1016/j.jpeds.2004.08.076.
- Branco RG, Garcia PC, Piva JP, Casartelli CH, Seibel V, Tasker RC. Glucose level and risk of mortality in pediatric septic shock. Pediatr Crit Care Med 2005;6:470-2. http://dx.doi.org/10.1097/01.PCC.0000161284.96739.3A.
- Yates AR, Dyke PC, 2nd, Taeed R, Hoffman TM, Hayes J, Feltes TF, et al. Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patient. Pediatr Crit Care Med 2006;7:351-5. http://dx.doi.org/10.1097/01.PCC.0000227755.96700.98.
- Landers C, Douglas W. Hyperglycemia in pediatric post-operative cardiac patients: 22. Pediatr Crit Care Med 2005;6. http://dx.doi.org/10.1097/00130478-200501000-00074.
- Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J Trauma Acute Care Surg 2005;58:47-50. http://dx.doi.org/10.1097/01.TA.0000135158.42242.B1.
- Cochran A, Scaife ER, Hansen KW, Downey EC. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma Acute Care Surg 2003;55:1035-8. http://dx.doi.org/10.1097/01.TA.0000031175.96507.48.
- Pham TN, Warren AJ, Phan HH, Molitor F, Greenhalgh DG, Palmieri TL. Impact of tight glycemic control in severely burned children. J Trauma 2005;59:1148-54. http://dx.doi.org/10.1097/01.ta.0000188933.16637.68.
- Hall NJ, Peters M, Eaton S, Pierro A. Hyperglycemia is associated with increased morbidity and mortality rates in neonates with necrotizing enterocolitis. J Pediatr Surg 2004;39:898-901. http://dx.doi.org/10.1016/j.jpedsurg.2004.02.005.
- Hilleman DE. Cost considerations with tight glycemic control in the acute care setting. Semin Thoracic Cardiovasc Surg 2006;18:359-65. http://dx.doi.org/10.1053/j.semtcvs.2006.12.004.
- Van den Berghe G, Wouters PJ, Kesteloot K, Hilleman DE. Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients. Crit Care Med 2006;34. http://dx.doi.org/10.1097/01.CCM.0000201408.15502.24.
- NHS Reference Costs 2010–2011. London: Department of Health; 2011.
- Taylor A, Butt W, Ciardulli M. The functional outcome and quality of life of children after admission to an intensive care unit. Intensive Care Med 2003;29:795-800.
- Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 2002;123:110-18. http://dx.doi.org/10.1067/mtc.2002.119064.
- The Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8.
- Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002;30:1772-7. http://dx.doi.org/10.1097/00003246-200208000-00016.
- Curley MA, Zimmerman JJ. Alternative outcome measures for pediatric clinical sepsis trials. Pediatr Crit Care Med 2005;6:S150-6. http://dx.doi.org/10.1097/01.PCC.0000161582.63265.B6.
- Leteurtre S, Martinot A, Duhamel A, Gauvin F, Grandbastien B, Nam TV, et al. Development of a pediatric multiple organ dysfunction score: use of two strategies. Med Decis Making 1999;19:399-410. http://dx.doi.org/10.1177/0272989X9901900408.
- Lacroix J, Cotting J. Severity of illness and organ dysfunction scoring in children. Pediatr Crit Care Med 2005;6:S126-34. http://dx.doi.org/10.1097/01.PCC.0000161287.61028.D4.
- Crouchman M, Rossiter L, Colaco T, Forsyth R. A practical outcome scale for paediatric head injury. Arch Dis Child 2001;84:120-4. http://dx.doi.org/10.1136/adc.84.2.120.
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised Conners’ parent rating scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998;26:257-68.
- Cornblath M, Schwartz R, Aynsley-Green A, Lloyd JK. Hypoglycemia in infancy: the need for a rational definition. A Ciba Foundation discussion meeting. Pediatrics 1990;85:834-7.
- Paediatric Intensive Care Audit Network. Universities of Leeds and Leicester; n.d.
- Mooney CZ, Duval RD. Bootstrapping: A Nonparametric Approach to Statistical Inference. Newbury Park, CA: Sage; 1993.
- Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol 1971;44:793-7. http://dx.doi.org/10.1259/0007-1285-44-526-793.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 1977;35:1-39. http://dx.doi.org/10.1038/bjc.1977.1.
- Guide to the Methods of Technology Appraisal. London: Institute for Health and Care Excellence; 2008.
- Paediatric Critical Care Minimum Data Set. London: NHS Information Standards Board; 2006.
- HRG4 2011/12 Road Test Grouper. The Health and Social Care Information Centre; 2011.
- Reference Cost Guidance, 2007. University of Sheffield and University of Leicester; 2007.
- Curtis L. Unit Costs of Health and Social Care. Canterbury, UK: Personal Social Services Research Unit; 2011.
- Rowan KA, Ball C, Bray K, Baker-McLearn D, Carmel S, Daly K, et al. Evaluation of Outreach Services in Critical Care: Project SDO/74/2004. London: Service Delivery and Organisation (now HSDR); 2007.
- Suri R, Grieve R, Normand C, Metcalfe C, Thompson S, Wallis C, et al. Effects of hypertonic saline, alternate day and daily rhDNase on healthcare use, costs and outcomes in children with cystic fibrosis. Thorax 2002;57:841-6. http://dx.doi.org/10.1136/thorax.57.10.841.
- bnf.org . Society BMAatRP n.d. www.bnf.org/bnf/ (accessed April 2012).
- Field DE, Elbourne D, Truesdale A, Grieve R, Hardy P, Fenton AC, et al. Neonatal ventilation with inhaled nitric oxide versus ventilatory support without inhaled nitric oxide for preterm infants with severe respiratory failure: the INNOVO multicentre randomised controlled trial (ISRCTN 17821339). Pediatrics 2005;115:926-36. http://dx.doi.org/10.1542/peds.2004-1209.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 1987.
- Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b2393.
- Kenward MG, Carpenter J. Multiple imputation: current perspectives. Stat Methods Med Res 2007;16:199-218. http://dx.doi.org/10.1177/0962280206075304.
- Little R. Missing data adjustments in large surveys. J Business Econom Stat 1988;6:287-301. http://dx.doi.org/10.2307/1391878.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99.
- van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw 2011;30:377-99.
- Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed?. BMJ 2000;320:1197-200. http://dx.doi.org/10.1136/bmj.320.7243.1197.
- Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy 2004;9:197-204. http://dx.doi.org/10.1258/1355819042250249.
- Interim Life Tables, 2008–2010. Newport: Office for National Statistics; 2011.
- Jones SR, Stevens K, Colwell K, Ratcliffe B, Jane R, Holland P, et al. Outcome at 6 months after admission for pediatric intensive care: a report of a national study of pediatric intensive care units in the United Kingdom. Pediatrics 2006;118:2101-8. http://dx.doi.org/10.1542/peds.2006-1455.
- Bridges BC, Preissig CM, Maher KO, Rigby MR. Continuous glucose monitors prove highly accurate in critically ill children. Crit Care 2010;14. http://dx.doi.org/10.1186/cc9280.
- Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet 2009;373:547-56. http://dx.doi.org/10.1016/S0140-6736(09)60044-1.
- National Heart, Lung and Blood Institute . SPECS: Safe Pediatric Euglycemia in Cardiac Surgery n.d. http://clinicaltrials.gov/ct2/show/NCT00443599 (accessed April 2012).
- The NICE-SUGAR Study Investigators . Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283-97.
- Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults. JAMA 2008;300:933-44. http://dx.doi.org/10.1001/jama.300.8.933.
- Griesdale DEG, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. Can Med Assoc J 2009;180:821-7. http://dx.doi.org/10.1503/cmaj.090206.
- Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, van Weissenbruch M, et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med 2008;359:1873-84. http://dx.doi.org/10.1056/NEJMoa0803725.
Appendix 1 Consent/assent forms and patient information leaflets
Appendix 1a
Appendix 1b
Appendix 1c
Appendix 1d
Appendix 1e
Appendix 1f
Appendix 2 CHiP: summary of protocol amendments
Amendment 1 (protocol version 2, 20 November 2007)
-
Changes to the Patient Information Sheets and Consent Forms for parents to include why their child had been chosen the this study, that blood taken specifically for this study would be painless and taken only from a line that already been inserted, and clarity on where and who was signing the consent form.
-
Changes to the protocol to more clearly define how the insulin infusions should be prepared and managed.
Amendment 2 (protocol version 3, 13 February 2008)
-
Changes to the protocol to clarify the dosing in the control group.
Amendment 3 (16 October 2008)
-
Poster to be displayed in intensive care units.
Amendment 4 (17 March 2009)
-
Addition of the following two sites:
-
Leicester Royal Infirmary and Glenfield Hospital (in Leicester).
-
University Hospital of North Staffordshire (in Stoke on Trent).
-
Amendment 5 (5 February 2009)
-
Letter to be sent to parents at discharge.
-
Diary to be given to parents at discharge.
-
Letter to be sent to parents at 12 months.
-
Questionnaire to be sent to parents at 12 months.
Amendment 6 (protocol version 4, 15 May 2009)
-
Changes to the protocol to make minor alterations to the insulin control guidelines for the tight group.
Amendment 7 (not applicable)
-
Not applicable, due to misnumbering by ethics (incorrectly number amendment 6 as amendment 7, and then unable to correct the numbering in their database).
Amendment 8 (28 June 2009)
-
Follow-up questionnaires to be sent at 1 year to TBI patients (Conners’ Parent Rating Scale - Revised, Health Utilities Index-2, and Child Behaviour Checklist).
Amendment 9 (18 November 2009)
-
Letter to parents of children who have died.
Amendment 10 (protocol version 5, 5 March 2010)
-
Changes in the protocol, patient information sheets and GP and parent letters to reflect changes in the TBI follow-up (all questionnaires now to be sent to them, rather than one to be completed over the telephone).
-
Changes in the protocol to add the publication policy.
-
Letter for parents of children who are inpatients for over 1 year.
Amendment 11 (protocol version 6, 23 August 2010)
-
Changes in the protocol, patient information sheets, consent forms, GP and parent letters due to the 1 year follow-up questionnaires no longer being sent out to parents (due to reasons of cost).
-
Changes in patient information sheets and consent forms to reflect the change in name of the NHS information centre.
-
Changes in the protocol, patient information sheets and consent forms to make it clearer that their patient data (including personal details) would be sent to the London School of Hygiene and Tropical Medicine.
-
Changes in the protocol to update the study background section to include more recent studies and literature.
Amendment 12 (23 August 2010)
-
Addition of the following site:
-
St George’s Healthcare NHS Trust (in London).
-
Amendment 13 (14 December 2010)
-
Change in PI at University Hospitals of North Staffordshire NHS Trust.
Appendix 3 Protocol version 6 (August 2010)
Appendix 4 Published protocol
Appendix 5 Diary on use of health services
Appendix 6 Follow-up: traumatic brain injury subgroup
The subgroup of children with TBI is more likely to have longer-term morbidity and parents of children (aged ≥ 4 years) in this subgroup will be asked to provide additional information at 12 months (for patients recruited until September 2010). We will specifically include assessments of attention and behaviour as patients with TBI are commonly left with deficits in these areas.
Definition of traumatic brain injury
Accidental trauma to the head resulting in need for intubation and mechanical ventilation.
Population
Seven hundred and fifty ICU admissions per year in UK. Estimate of 150 recruited to the trial.
Outcomes assessment
This will comprise four components:
Overall health status: measured by the Health Utilities Index (HUI-3) [HUI-3. Copyright 2002 Health Utilities Inc. (HUInc), 88 Sydenham Street, Dundas ON, Canada L9H 2V3. www.healthutilities.com].
Global neurological outcome: measured by the Kings Outcome Scale for Childhood Head Injury (KOSCHI). 46
Attention and behavioural assessment: measured by the CBCL (CBCL. Copyright 2000 Achenbach T and Rescorla L. ASEBA, University of Vermont, 1 South Prospect Street, Burlington, VT 05401-3456. www.ASEBA.org).
The CRS-R:S. 47
The HUI, CBCL and CRS-R:S are written questionnaires that will be posted out to the families. They take approximately 30 minutes to complete.
Health Utilities Index is a multi-attribute health status classification system. Seven attributes (sensation, mobility, emotion, cognition, self-care, pain, fertility) are categorised according to one of 4 or 5 levels. In this population fertility will be excluded. The algorithm (from death to perfect health scale) provides a single numerical value.
KOSCHI is a 5-point categorical scale, ranging from death to normal neurological function, and is similar in structure to the Glasgow Outcome Scale, which is widely used in adult studies. In addition the KOSCHI is further subdivided into two subcategories at points 4 and 5 on the scale (moderate outcome and good outcome). Patient outcomes will be dichotomised between patients in categories 1, 2, 3 and 4A, and those in 4B, 5A and 5B.
Child behaviour checklist (CBCL/4–18), problem scales
The CBCL is based on parent’s report and assesses problematic child behaviour that is summarised in internalising behaviour (anxious/depressed, withdrawn/depressed, somatic complaints), externalising behaviour (rule-breaking, aggressive) and other (social problems, thought problems, attention problems).
In reference to 1991 normative data:
| T-score (whole) | Guideline | T-score (individual scale) | Guideline |
|---|---|---|---|
| < 60 | Normal | < 65 | Normal |
| 60–63 | Borderline | 65–69 | Borderline |
| > 63 | Clinical | > 69 | Clinical |
Patient outcome can be summarised according to placement within one of the three groups, or according to the T-score.
Conners’ rating scales revised – short version
The CRS-R:S assesses symptoms of attention-deficit/hyperactivity disorder and related problem behaviour in children and adolescents based on parent’s report.
In reference to 1993 normative data:
| T-score | Guideline |
|---|---|
| ≥ 70 | Markedly atypical (significant problem) |
| 66–69 | Moderately atypical (significant problem) |
| 61–65 | Mildly atypical (possible significant problem) |
| 56–60 | Slightly atypical (borderline) |
| 45–55 | Average (no concern) |
| ≤ 44 | Good |
Patient outcome can be summarised according to placement within one of the three groups (marked + moderate, mild + slight, average + good), or according to the T-score.
Appendix 7 Post-discharge letters to patients and general practitioners, follow-up forms and case report form
Appendix 7a
Appendix 7b
Appendix 7c
Appendix 7d
Appendix 7e
Appendix 7f
Appendix 7g
Appendix 7h
Appendix 8 Questionnaire on use of health services
Appendix 9 Trial Steering Committee: terms of reference and membership
The responsibilities of the TSC were to approve the main study protocol and any amendments, monitor and supervise the trial towards its interim and overall objectives, review relevant information from other sources, consider the recommendations of the DMEC, and resolve problems brought by the trial co-ordinating centres. The TSC therefore provided overall supervision for CHiP on behalf of the HTA and the Royal Brompton and Harefield NHS Trust (sponsor) to ensure that the trial was conducted to the rigorous standards set out in the MRC Guidelines for GCP. Face-to-face meetings were held at regular intervals determined by need and not less than once a year. Routine business was conducted by telephone, email and post.
Terms of reference
-
The TSC should approve the protocol and trial documentation in a timely manner.
-
In particular the TSC should concentrate on progress of the trial, adherence to the protocol, patient safety and consideration of new information of relevance to the research question.
-
The safety and well-being of the trial participants are the most important consideration and should prevail over the interests of science and society.
-
The TSC should provide advice, through its chair, to the chief investigator, the trial sponsor, the trial funder, on all appropriate aspects of the trial. Specifically, the TSC will:
-
Monitor recruitment rates and encourage the TMG to develop strategies to deal with any recruitment problems.
-
Monitor completion of data sheets and comment on strategies from TMG to encourage satisfactory completion in the future.
-
Monitor follow-up rates and review strategies from TMG to deal with problems including sites that deviate from the protocol.
-
Approve any amendments to the protocol, where appropriate.
-
Approve any proposals by the TMG concerning any change to the design of the trial, including additional sub-studies.
-
Oversee the timely reporting of trial results.
-
Approve and comment on the statistical analysis plan.
-
Approve and comment on the publication policy.
-
Approve and comment on the main trial manuscript.
-
Approve and comment on any abstracts and presentations of any results during the running of the trial.
-
Approve external or early internal requests for release of data or subsets of data or samples including clinical data and stored biological samples.
-
Receive reports from the DMEC.
-
The TSC will make decisions as to the future continuation (or otherwise) of the trial.
-
-
Membership of the TSC should be limited and include an independent chair, at least two other independent members, two collaborators and two members of the public. The investigators and the trial project staff are ex officio.
-
Representatives of the trial sponsor and the HTA should be invited to all TSC meetings.
-
Responsibility for calling and organising the TSC meetings lies with the chief investigator. The TSC should meet at least annually, although there may be periods when more frequent meetings are necessary.
-
There may be occasions when the trial sponsor or the HTA will wish to organise and administer these meetings in exceptional circumstances.
-
The TSC will provide evidence to support any requests for extensions, including that all practicable steps have been taken to achieve targets.
-
The TSC will maintain confidentiality of all trial information that is not already in the public domain.
Membership
Professor Michael Preece (chairperson) Consultant Paediatrician, Great Ormond Street Children’s Hospital
Mrs Pamela Barnes Lay member
Ms Sian Edwards Paediatric Pharmacist, Royal Brompton Hospital
Professor David Field Neonatologist, Leicester Royal Infirmary and the University of Leicester
Dr James Hooper Consultant Clinical Biochemist, Royal Brompton Hospital
Mrs Tara Quick Lay member, parent
Dr Claire Snowdon Lecturer, Medical Statistics Department, LSHTM, and Centre for Family Research, University of Cambridge (until 2011)
Ms Lyvonne Tume Research Nurse, Royal Liverpool Children’s Hospital
Dr Dirk Vlasselaers Consultant Paediatric Intensivist, Leuven, Belgium
Professor Paula Williamson Professor of Medical Statistics, University of Liverpool
In attendance
Mr Michael Loveridge (till March 2008) Royal Brompton Hospital (Trial sponsor)
HTA representative
Trial Management Group (see below)
Dr Duncan Macrae (Chief Investigator) Director of Paediatric Intensive Care, Royal Brompton Hospital
Dr Elizabeth Allen Senior Lecturer, Medical Statistics Department, LSHTM
Miss Helen Betts Lead Study Nurse, Royal Brompton Hospital
Professor Diana Elbourne Professor of Healthcare Evaluation, Medical Statistics Department, LSHTM
Dr Richard Grieve Senior Lecturer in Health Economics, Health Services Research Department, LSHTM
Dr Kevin Morris Consultant in Paediatric Intensive Care, Birmingham Children’s Hospital
Dr Roger Parslow Senior research fellow, University of Leeds
Dr Robert Tasker Professor of Neurology and Anesthesia (Pediatric), Harvard Medical School and Children’s Hospital Boston, Boston, MA, USA
Mrs Ann Truesdale (till 2008) Trials Advisor, Medical Statistics Department, LSHTM
Miss Laura Van Dyck (from August 2009) Study Manager, Medical Statistics Department, LSHTM
Appendix 10 Data Monitoring and Ethics Committee
Terms of reference
To safeguard the interests of trial participants, monitor the main outcome measures including safety and efficacy, and monitor the overall conduct of the CHiP study.
The DMEC should receive and review information on the progress and accruing data of CHiP and provide advice on the conduct of the trial to the Trial Steering Committee (TSC).
The DMEC should inform the Chair of the TSC if, in their view the results are likely to convince a broad range of clinicians, including those supporting the trial and the general clinical community, that, on balance, one trial arm is clearly indicated or contraindicated for all participants or a particular category of participants, and there was a reasonable expectation that this new evidence would materially influence patient management.
Interim review of the trial’s progress including updated figures on recruitment, data quality, adherence to protocol, follow-up, and main outcomes and safety data. Specifically, these roles include to:
-
monitor evidence for treatment differences in the main efficacy outcome measures
-
monitor evidence for treatment harm (e.g. toxicity, SAEs and SARs, treatment related deaths)
-
assess the impact and relevance of external evidence
-
decide whether to recommend that the trial continues to recruit participants or if recruitment should be terminated either for everyone or for some treatment groups and/or some participant subgroups
-
decide whether or not trial follow-up should be stopped earlier
-
assess data quality, including completeness (and by so doing encourage collection of high quality data)
-
maintain confidentiality of all trial information that is not in the public domain
-
monitor recruitment figures and losses to follow-up
-
monitor compliance with the protocol by participants and investigators
-
consider the ethical implications of any recommendations made by the DMEC
-
monitor planned sample size assumptions, preferably with regards to
-
(i) a priori assumptions about the control arm outcome and/or
-
(ii) emerging differences in clinically relevant subgroups, rather than on emerging, unblinded differences between treatment groups, overall
-
-
-
suggest additional data analyses if necessary
-
advise on protocol modifications proposed by investigators or HTA (e.g. to inclusion criteria, trial endpoints, or sample size)
-
monitor continuing appropriateness of patient information
-
monitor compliance with previous DMEC recommendations.
Membership
Professor David Dunger (chairperson) Department of Paediatrics, University of Cambridge
Dr David Harrison Statistician, Intensive Care Audit and Research Network (ICNARC), Professor David Hatch Emeritus Professor of Paediatric Anaesthesia and Intensive Care, Great Ormond Street Hospital
Mr Giles Peek (till Sept. 2009) Consultant Cardiac Surgeon, Glenfield Hospital, Leicester
Dr Jon Smith (from Sept. 2009) Consultant Cardiothoracic Anaesthetist, Newcastle General Hospital
Appendix 11 Trial Management Group
A Trial Management Group was established and was responsible for the day-to-day management of the trial. The group comprised the grant holders and project staff from the clinical co-ordinating centre at the Royal Brompton Hospital NHS Trust and the data co-ordinating centre at the LSHTM. The group met regularly in person and by telephone.
The responsibilities of the TMG were:
-
to establish and monitor recruitment of participating centres
-
to distribute and supply of data collection forms and other appropriate documentation for the trial
-
data collection and management
-
data entry and cleaning
-
data analysis
-
organising and servicing the DMEC
-
assure data security and quality and observe data protection laws
-
ensure trial is conducted in accordance with ICH GCP.
Data co-ordinating centre responsibilities
-
To ensure that all members of the study team are able by knowledge, training and experience to undertake the roles assigned to them and to comply with requirements as specified by the host organisation.
-
To provide overall efficient day-to-day management of the trial ensuring compliance with GCP.
-
To ensure each centre is put on-line with the randomisation service after Local Research Ethics Committee (LREC), research and development (R&D) approval and the signed local collaborator agreement have been received from the sponsor.
-
To provide site folders and relevant documentation to each centre.
-
To contribute to the development of the protocol, and all study documentation including data sheets.
-
To design, produce and regularly update all trial materials and arrange printing and supply of documentation.
-
To monitor recruitment and advise on remedial action if targets are not being met.
-
To set up and maintain the website.
-
To service the Project management Committee, Steering Committee and any other relevant advisory groups.
-
To use all reasonable efforts to ensure that the data collected and reported are accurate, complete and identifiable at source; and that record keeping and data transfer procedures adhere to the Data Protection Act 1998.
-
To undertake the interim and final analyses and report regularly to the DMEC in a timely way at their request.
-
To supply documentation and reports as deemed necessary by the sponsors to fulfil their obligations.
-
To co-ordinate the preparation and publication of data, reports and information, ensuring that these meet legislative, contractual and ethical requirements.
-
To co-operate with audits or inspections undertaken by the host institution, the sponsors and regulatory authorities including the MHRA as required.
-
To assist investigations into any alleged research misconduct undertaken by or on behalf of the co-sponsors.
-
To ensure safe storage of data, including trial site file, data sheets and other records for a period of 15 years after the conclusion of the trial.
-
To inform the chief investigator of any changes in the trial protocol that affect the conduct of the trial.
Appendix 12 Principal investigator’s responsibilities
Each participating centre identified a paediatric intensivist as a PI. Each participating centre was allocated funding for research nursing time and expected to employ or second a Research Nurse to support all aspects of the trial at the local centre.
The responsibility of the PI will be to:
-
ensure local research ethics and R&D approval is obtained
-
discuss the trial with medical, and nursing staff who see eligible patients and ensure that they are updated on the current state of knowledge, the trial and its procedures
-
provide clinical support for the trial research nurse ensuring that relevant staff are trained in the trial procedures
-
ensure that potentially eligible patients are considered for the trial
-
report promptly to the clinical co-ordinating centre any problems in meeting recruitment targets so that support can be provided
-
maintain good contact with the paediatric cardiac unit to ensure that potentially eligible patients are given information about the trial
-
ensure that mechanisms for consent and recruitment are in place
-
ensure that data collection forms are completed and returned to the data co-ordinating centre promptly and to deal with any queries
-
inform and advise the relevant co-ordinating centre promptly
-
facilitate other aspects of co-ordination as relevant
-
make data available for verification, audit and inspection purposes as necessary
-
respond to requests for data from the Economics team
-
ensure that the confidentiality of all information about trial participants is respected by all persons and that records are kept in areas to which access is restricted
-
ensure the trial is conducted in accordance with ICH GCP
-
allow access to source data for audit and verification
-
ensure that adverse events are reported in line with statutory guidelines.
List of abbreviations
- AKI
- acute kidney injury
- CBCL
- Child Behavioural Checklist
- CHiP
- Control of Hyperglycaemia in Paediatric intensive care
- CI
- confidence interval
- CM
- conventional management
- CRF
- case report form
- CRS-R:S
- Conners’ Rating Scales revised – short version
- DCC
- data co-ordinating centre
- DMEC
- Data Monitoring and Ethics Committee
- EDD
- expected date of delivery
- GCP
- good clinical practice
- GM
- general medical
- GP
- general practitioner
- HRG
- health-care resource group
- HTA
- Health Technology Assessment
- HUI
- Health Utilities Index®
- ICH
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- ICU
- intensive care unit
- INB
- incremental net benefit
- LOS
- length of stay
- LSHTM
- London School of Hygiene and Tropical Medicine
- MCRN
- Medicines for Children Research Network
- MI
- multiple imputation
- MREC
- Multicentre Research Ethics Committee
- NEC
- necrotising enterocolitis
- NICE
- National Institute for Health and Care Excellence
- NICU
- neonatal intensive care unit
- OLS
- ordinary least squares
- PbR
- NHS Payments by Results database
- PCCMDS
- Paediatric Critical Care Minimum Data Set
- PI
- principal investigator
- PIC
- paediatric intensive care
- PICANet
- Paediatric Intensive Care Audit Network
- PICU
- paediatric intensive care unit
- PIM2
- Paediatric Index of Mortality version 2
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RACHS1
- Risk-adjusted Classification for Congenital Heart Surgery 1
- RCT
- randomised controlled trial
- RR
- relative risk
- RRT
- renal replacement therapy
- SA
- sensitivity analysis
- SAE
- serious adverse event
- SD
- standard deviation
- SUSAR
- suspected, unexpected, serious adverse reaction
- TBI
- traumatic brain injury
- TGC
- tight glycaemic control
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VFD
- ventilator-free day
- VFD-30
- ventilator-free days 30 days post randomisation