Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/46/01. The contractual start date was in November 2012. The draft report began editorial review in April 2013 and was accepted for publication in September 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Leaviss et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Tobacco smoking is a major cause of a number of chronic diseases, including heart disease and cancers, and is attributed as the leading cause of preventable deaths worldwide. 1,2 Smoking-related illnesses include every type of cancer except for skin cancer; cardiovascular, respiratory and digestive problems;3 amputation due to peripheral vascular disease;4 diabetes; cataracts; and impotence and reproductive problems. 5
Despite these statistics, nearly one-fifth of adults in the UK are regular cigarette smokers. Although the rate of cigarette smoking has been falling slowly since the mid-1990s, in 2010 the proportion of males over 16 years who were smokers was reported to be 21%. 6 In 2006–7, smoking-related ill-health cost the NHS £3.3B. 7 Stopping smoking is known to reduce the risk of smoking-related disease, but it is challenging. Without smoking cessation aids, only between 2% and 5% of quit attempts are successful. 8,9 Smoking cessation strategies have varied success rates.
Available smoking cessation interventions
Broadly speaking, there are two types of smoking cessation intervention: (1) those designed to prompt quit attempts and (2) those designed to assist with quit attempts. The current review focuses on the latter type. A number of pharmacological interventions exist that aid smoking cessation in terms of assisting quit attempts. These include nicotine replacement therapy (NRT), typical antidepressant medications such as bupropion hydrochloride and nicotinic receptor partial agonists such as varenicline, cytisine and dianicline. Behavioural support interventions have also been developed to assist with quit attempts. Recent Cochrane reviews have demonstrated behavioural support (group, individual and telephone counselling), single-product nicotine replacement therapy and bupropion hydrochloride to demonstrate similar effectiveness as smoking cessation aids. 10–14 Greater effect sizes have been reported for nicotine partial agonists such as varenicline and cytisine. 15
This review assesses the clinical effectiveness and cost-effectiveness of two nicotinic receptor partial agonists: varenicline and cytisine. Nicotinic receptor partial agonists offer a pharmacological method to aid smoking cessation. Varenicline (Champix or Chantix; Pfizer, UK) and cytisine (Tabex; Sopharma, Bulgaria) are included in this class of drug. These drugs act by relieving craving and withdrawal symptoms, while also blocking the reinforcing effects of nicotine if a cigarette is smoked. 16 Cytisine is a naturally occurring product, extracted from the seeds of the plant Cytisus laborinum L. (golden rain acacia). It has been used as an aid to smoking cessation in former socialist economies for over 40 years, although West et al. 17 report that it has been withdrawn from many of these countries following their entry into the European Union. It is manufactured by the Bulgarian pharmaceutical company Sopharma. Varenicline is a synthetic product developed by Pfizer, with a similar structure to cytisine. Like cytisine, varenicline is a partial agonist of nicotinic acetylcholine receptors, with high affinity for α4β2 receptors, and is licensed for use as an aid to smoking cessation in the USA, Canada and across Europe including the UK. Although both varenicline and cytisine are used as aids to smoking cessation, Etter et al. 18 reported a dearth of scientific evidence on the properties of cytisine and its safety and efficacy profile. They provide an overview of the in vitro and in vivo profiles of both drugs. Cytisine is considerably less expensive than varenicline and, although costs vary between countries (Poland US$12 for a course, Russian Federation US$6 for a course), the cost of a course of cytisine is generally about 10–20% that of varenicline. 17 The standard dosage for varenicline according to the British National Formulary (BNF) is for adults over 18 years, the treatment to start with is usually 1–2 weeks before the target stop date (up to a maximum of 5 weeks before the target stop date). Initially, the dosage is 500 μg q.d. [quaque die (every day)] for 3 days, the dosage is increased to 500 μg b.i.d. [bis in die (twice a day)] for 4 days, followed by 1 mg b.i.d. for 11 weeks (the dose is reduced to 500 μg b.i.d. if it is not tolerated) and a 12-week course can be repeated in abstinent individuals to reduce the risk of relapse. 19 Although cytisine is not licensed for use in the UK or the USA, the usual starting dose is 1.5 mg six times daily. 20
Measurement of abstinence
Clinical trials of interventions for smoking cessation may use a range of outcome measurements to evaluate abstinence. The Russell Standard21 is a set of criteria widely used to define smoking abstinence. These guidelines were developed in response to results from smoking cessation trial data historically being reported in a number of different ways. The Russell Standard outlines criteria important to the measurement and reporting of outcome data in such trials. A full description of these criteria are found in West et al. 21 Regarding the measurement of abstinence, the criteria recommend a duration of 6 months or 12 months, either from a designated quit date or allowing for a predefined grace period. Shorter periods of abstinence are reported to be insufficient in their ability to accurately predict long-term cessation. Regarding the definition of abstinence, historically a number of methods of measuring abstinence have been used. These include continuous abstinence, defined as abstinence between quit day and follow-up; prolonged abstinence, defined as sustained abstinence after an initial grace period, or to a period of sustained abstinence between two follow-ups; point prevalence abstinence, defined as the prevalence of abstinence during a time window immediately preceding follow-up and repeated point prevalence abstinence, defined as point prevalence abstinence measured at two or more follow-ups between which smoking is allowed. 22 Abstinence is often biochemically verified by measurement of carbon monoxide (CO). However, CO is eliminated from the body in around 24 hours;15 therefore, abstinence cannot be verified for longer periods than this. The Russell Standard recommends that abstinence should be defined as ‘a self-report of smoking not more than five cigarettes from the start of the abstinence period, supported by a negative biochemical test at the final follow-up’. 21
Current service provision
Stop smoking clinics in the UK typically include the option to attend specialist one-to-one sessions with a trained stop smoking advisor, group sessions or drop-in sessions. 23 Clinics generally involve some form of assessment of current smoking behaviour and willingness to quit, including CO monitoring, prescription of some form of pharmacotherapy if desired (NRT, bupropion hydrochloride or varenicline) and behavioural support focused on managing withdrawal symptoms and preventing relapse (including preparing to quit, setting a quit date and making plans for situations where the client may be tempted to smoke). 24
Success rates for the NHS smoking cessation treatments
Eight hundred thousand smokers each year attempt cessation through the NHS stop smoking services. 25 In any given quit attempt 0.5% of smokers attempt cessation using varenicline with specialist individual behavioural support through NHS stop smoking clinics, 0.2% use varenicline with specialist group behavioural support, 0.1% use varenicline with specialist drop-in behavioural support and 2.8% obtain a prescription for varenicline in NHS settings (e.g. primary care, hospital). 23 The estimated 52-week continuous abstinence rates for NHS specialist individual behavioural support clinics are 15% when combined with NRT monotherapy, 20% with NRT combination therapy, 17% with bupropion hydrochloride and 24% with varenicline. The estimated 52-week continuous abstinence rates for NHS specialist group behavioural support clinics are 20% when combined with NRT monotherapy, 26% with NRT combination therapy, 23% with hydrochloride and 31% with varenicline. The estimated 52-week continuous abstinence rates for NHS specialist drop-in behavioural support clinics are 11% when combined with NRT monotherapy, 15% with NRT combination therapy, 13% with bupropion hydrochloride and 19% with varenicline. The estimated 52-week continuous abstinence rates for brief interventions in NHS settings (e.g. primary care, hospital) are 7% for NRT monotherapy, 10% for NRT combination therapy, 8% for bupropion hydrochloride and 12% for varenicline. 23
A recent Cochrane review of nicotinic receptor partial agonists as aids to smoking cessation showed a modest efficacy for cytisine over placebo in helping people to stop smoking, although the study reports low absolute quit rates for these trials. 15 The authors report a twofold increase in quit rates for varenicline over placebo. These analyses, comparing each drug with placebo, found no difference in their efficacy. The authors highlight that trials have now been conducted in real-world settings, for example in smokers with underlying diseases or medical conditions who might under ordinary circumstances be excluded from clinical trials, and report that the findings remain stable in these populations. A recent review of the efficacy and safety of cytisine found it to be an effective treatment for smoking cessation. 26 The review highlights the low cost of cytisine and suggests that licensing of this drug may therefore be warranted because of its potential public health benefit. No head-to-head trials between varenicline and cytisine were identified in either review and, to date, no indirect comparisons of the two drugs have been conducted in the absence of such trials.
Safety profile of varenicline
Concerns have been raised regarding the safety profile of varenicline. The US Food and Drug Administration (FDA) has issued a series of warnings, resulting from post-marketing reports of increased risk of suicidal behaviour or depression, serious adverse cardiac events and gastrointestinal complaints including a recently added warning highlighting a small increased risk of certain cardiovascular events in smokers with pre-existing cardiac conditions. A meta-analysis of adverse gastrointestinal events by Leung et al. 27 showed an increased risk after treatment with varenicline. In a review of 10 trials, Tonstad et al. 28 found no evidence of a link between varenicline and serious neuropsychiatric events. Reviews by Singh et al. 29 and Prochaska30 report conflicting findings, with Singh et al. showing an increased risk of serious cardiovascular events after treatment with varenicline and Prochaska finding no evidence of a link. Cahill et al. 15 found a lack of trial evidence indicating serious adverse events for varenicline. However, the studies do not rule out the possibility of a link, in light of the FDA warnings. Data extracted from randomised control trials (RCTs) may not provide a comprehensive account of all possible adverse events – participants may be excluded for having a history of a number of relevant medical conditions, for example depression or cardiovascular disease. In addition, the follow-up time period of trials may not be long enough to sufficiently capture all relevant adverse events.
This assessment aimed to review the efficacy of varenicline and cytisine as an aid to smoking cessation by updating the Cahill et al. 15 review and to conduct indirect comparisons where appropriate. A mathematical model compared the cost-effectiveness of cytisine with varenicline in the context of NHS stopping smoking services. Recommendations regarding the need for a head-to-head trial were made.
Chapter 2 Definition of the decision problem
This assessment addresses the question: what is the clinical effectiveness and cost-effectiveness of cytisine compared with varenicline for smoking cessation? Specifically, the assessment will (i) review evidence on the clinical effectiveness and safety of cytisine in smoking cessation compared with varenicline; (ii) develop an economic model to estimate the cost-effectiveness of cytisine in the context of NHS smoking cessation services and (iii) provide recommendations based on the value of information analyses whether or not a head-to-head trial of cytisine and varenicline would represent an effective use of resources.
Decision problem
Population: adult smokers.
Intervention and relevant comparators: cytisine, a nicotinic receptor partial agonist, used as an aid in the treatment of smoking cessation, and varenicline, in any formulation. In the likely absence of data from head-to-head studies of cytisine with varenicline, any comparators (e.g. placebo, NRT, bupropion) were considered that would allow an indirect comparison or network meta-analysis.
Outcomes: the primary outcome was smoking cessation, as defined by the study’s strictest reported definition of abstinence, at a minimum of 6 months’ follow-up, i.e. continuous abstinence rate (CAR) data were used in preference to point prevalence abstinence (PPA) data where both were reported. 22 Secondary outcomes were adverse events. The four most frequently reported adverse events, as reported in the Cahill15 review, were analysed and these were nausea, headache, insomnia and abnormal dreams. Serious adverse events (SAEs) were also analysed.
Overall aims and objectives of assessment
The overall aims and objectives of this assessment were to:
-
Update the Cahill et al. 15 search to identify additional clinical effectiveness and safety data for cytisine compared with varenicline in helping people to stop smoking.
-
In the absence of head-to-head trials, conduct indirect treatment comparisons for efficacy and adverse events for cytisine compared with varenicline.
-
Model the cost-effectiveness of cytisine and varenicline within the context of NHS smoking cessation services.
-
Make recommendations for commissioning a full head-to-head trial of varenicline compared with cytisine.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing clinical effectiveness
Identification of studies
A good-quality recent Cochrane review evaluating the clinical effectiveness and safety profile of both cytisine and varenicline was identified. 15 This Cochrane review will be referred to subsequently as Cahill et al. 15 The current review aimed to use the data from Cahill et al. 15 to inform clinical effectiveness and cost-effectiveness analyses. An update of this search was conducted, which aimed to identify any recent studies evaluating the clinical effectiveness of varenicline or cytisine. The aim of the current review was to compare the clinical effectiveness and cost-effectiveness of varenicline and cytisine, but in the likely absence of head-to-head trials between cytisine and varenicline, any comparators were considered that would enable an indirect comparison, for example placebo, NRT and bupropion.
The search was conducted in January 2013 and the search strategy from Cahill et al. 15 was rerun for trials and systematic reviews in the period December 2011 to January 2013. Although dianicline was included in the Cahill et al. 15 searches, development of the drug has been discontinued and, therefore, will not be included in the comparisons for this report. This term was therefore excluded from the search. Additionally, a search was run for the terms Champix or Chantix (brand names for varenicline) with no date restrictions, in order to identify earlier trials using brand rather than generic names, as these terms were not included in Cahill et al. 15
The search was also rerun with a cost-effectiveness filter with no date restriction for cost-effectiveness literature. The purpose of the cost-effectiveness search was to obtain data to inform the model and no systematic review of this literature was conducted. Cost-effectiveness methods and analyses are reported in Chapter 4.
Searches were conducted by an information specialist (AC). Examples of each of the search strategies in MEDLINE are provided in Appendix 1.
The following electronic databases were searched for published and unpublished research evidence, from December 2011 to January 2013 for the efficacy searches and from database inception for the cost-effectiveness searches:
-
MEDLINE (Ovid) 1950–
-
EMBASE (Ovid) 1980–
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL; EBSCOhost) 1982–
-
The Cochrane Library including the Cochrane Systematic Reviews Database, Cochrane Controlled Trials Register, Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA) database and NHS Economic Evaluation Database (NHS EED) 1991–
-
Biological Abstracts (via Web of Science) 1969–
-
Science Citation Index (via Web of Science) 1900–
-
Social Science Citation Index (via Web of Science) 1956–
-
EconLit 1961–
-
Conference Proceedings Citation Index–Science (CPCI–S) (via Web of Science) 1990–
-
UK Clinical Trials Research Network (UKCRN) and the National Research Register archive (NRR)
-
Current Controlled Trials 1898–
-
ClinicalTrials.gov 1998–.
All citations were imported into Reference Manager (Thomson ResearchSoft, San Francisco, CA, USA) software and duplicates deleted.
Inclusion and exclusion criteria
-
Study design: RCTs.
-
Intervention or comparators: cytisine, in any formulation; varenicline, in any formulation. In the likely absence of data from head-to-head studies of cytisine compared with varenicline, any other comparators (e.g. placebo, NRT, bupropion) were considered that would allow an indirect comparison.
-
Population: smokers.
-
The primary outcome was abstinence at a minimum 6 months’ follow-up.
Based on the above inclusion/exclusion criteria, study selection was conducted by two reviewers (JL and EEH). In the first instance titles and abstracts were examined for inclusion. Both reviewers independently screened all retrieved citations. Discrepancies between reviewers were discussed and any remaining disagreement resulted in retrieval of the full paper for further consideration. The full manuscripts of citations judged to be potentially relevant were retrieved and further assessed for inclusion. Any remaining discrepancies between reviewers at full-paper stage were discussed and, if no agreement could be reached, were resolved by referring to the review’s clinical experts. A table of studies excluded at full-paper stage with reasons for exclusion is presented in Appendix 3.
Data extraction
Data were extracted without blinding to either authors or journal. Data from studies included in the Cahill review15 were extracted directly from the review by one reviewer (JL or EEH). Data from studies included following the updated search were extracted by one reviewer (JL) and checked by a second (EEH). Any data for doses not reported in Cahill et al. 15 were extracted directly from the original papers. Efficacy data for newly included studies were calculated using the same method reported in Cahill et al. 15 – based on the numbers of people randomised to an intervention and excluding any deaths or untraceable moves in accordance with the Russell Standard. 31 Drop-outs or those patients lost to follow-up are treated as continuing smokers. It is beyond the scope of this short report to conduct a systematic review of adverse events for varenicline and cytisine, taking into account long-term observational studies. Therefore, this assessment will adopt the same approach to adverse events as the Cahill et al. 15 review, extracting this information from RCTs retrieved through an update of their efficacy search. Adverse events data were extracted on the basis of number of participants who had taken at least one dose of treatment. Data from the strictest reported measurement of smoking cessation were extracted for use in the analyses, i.e. 7-day PPA or CAR. Where studies reported both CAR and 7-day PPA, CAR data were extracted in preference to 7-day PPA22 and only data from studies measuring CAR were used in the network meta-analysis for efficacy. Data from both types of studies were used for adverse events analyses. Efficacy data from studies that reported only PPA were extracted with the purpose of conducting a sensitivity analysis for significant differences in results by method of outcome measurement. Adverse events data were extracted for the four most common adverse events, as identified in the Cahill review, and these were abnormal dreams, nausea, headache and insomnia. Data for SAEs were also extracted.
Critical appraisal strategy
Critical appraisals of the quality of studies, retrieved by the updated search, followed the same format as reported by Cahill,15 using the Cochrane risk of bias tool (Higgins et al). 32 Studies were critically appraised by JL or EEH and checked by the second reviewer. Discrepancies were resolved by discussion between reviewers. Quality assessments aimed to evaluate the risk of bias in the current evidence base for the clinical effectiveness of both varenicline and cytisine.
Methods of data synthesis
The continuous abstinence data and adverse events data including abnormal dreams, headache, insomnia, nausea and SAEs were synthesised using a network meta-analysis. The analysis combines evidence across studies in which there is at least one treatment in a study that is common to at least one other study.
A random (treatment) effects model was used to allow for heterogeneity in treatment effects between studies. The model assumed a fixed (i.e. unconstrained) baseline effect in each study so that treatment effects were estimated within study and combined across studies. All analyses were implemented in WinBUGS (MRC Biostatistics Unit, Cambridge, UK). 33
The continuous abstinence, abnormal dreams, headache, insomnia, nausea and SAEs data were modelled using a complementary log-log link function to allow for variation in duration of follow-up between studies (see Appendix 4). This assumes that the times to event follow an exponential distribution and, hence, that the treatment effect is constant over time. Although these are strong assumptions, they are expected to be better than assuming there is no effect of duration of follow-up.
Results of the network meta-analyses are reported in terms of the hazard ratios (HRs) and 95% credible intervals (CrIs) relative to the baseline intervention (i.e. placebo). The posterior medians of the between-study standard deviations (SDs) together with their 95% CrIs are also presented.
Convergence of the models to their posterior distributions was assessed using the Gelman–Rubin convergence statistic. 34 Convergence occurred after 50,000 iterations for all outcome measures except for SAEs, which converged after 60,000 iterations. There was some suggestion of moderate autocorrelation between successive iterations of the Markov chains; to compensate for this the Markov chains were thinned every five iterations for continuous abstinence, nausea and SAEs, and every 10 iterations for abnormal dreams, headache and insomnia. Parameter estimates were based on 10,000 iterations of the Markov chains for continuous abstinence and nausea; 5000 iterations of the Markov chains for abnormal dreams, headache and insomnia and 8000 iterations of the Markov chains for SAEs to ensure that the Monte Carlo error was < 5% of the posterior SD. Although fewer samples would have been sufficient for estimating parameters for continuous abstinence, 10,000 samples were taken for the purpose of the expected value of sample information (EVSI).
The total residual deviance was used to assess formally whether or not the statistical model provided a reasonable representation of the sample data. The total residual deviance is the mean of the deviance under the current model minus the deviance for the saturated model, so that each data point should contribute about one to the deviance. 35
To enable the estimation of intervention-specific CARs, as required for the economic model, a separate random-effects meta-analysis was conducted on the placebo intervention arms. Absolute estimates of CARs were generated for each intervention by projecting the estimates of treatment effect (i.e. the log-hazard ratio) from the network meta-analysis onto the baseline CAR.
Results
Updated search
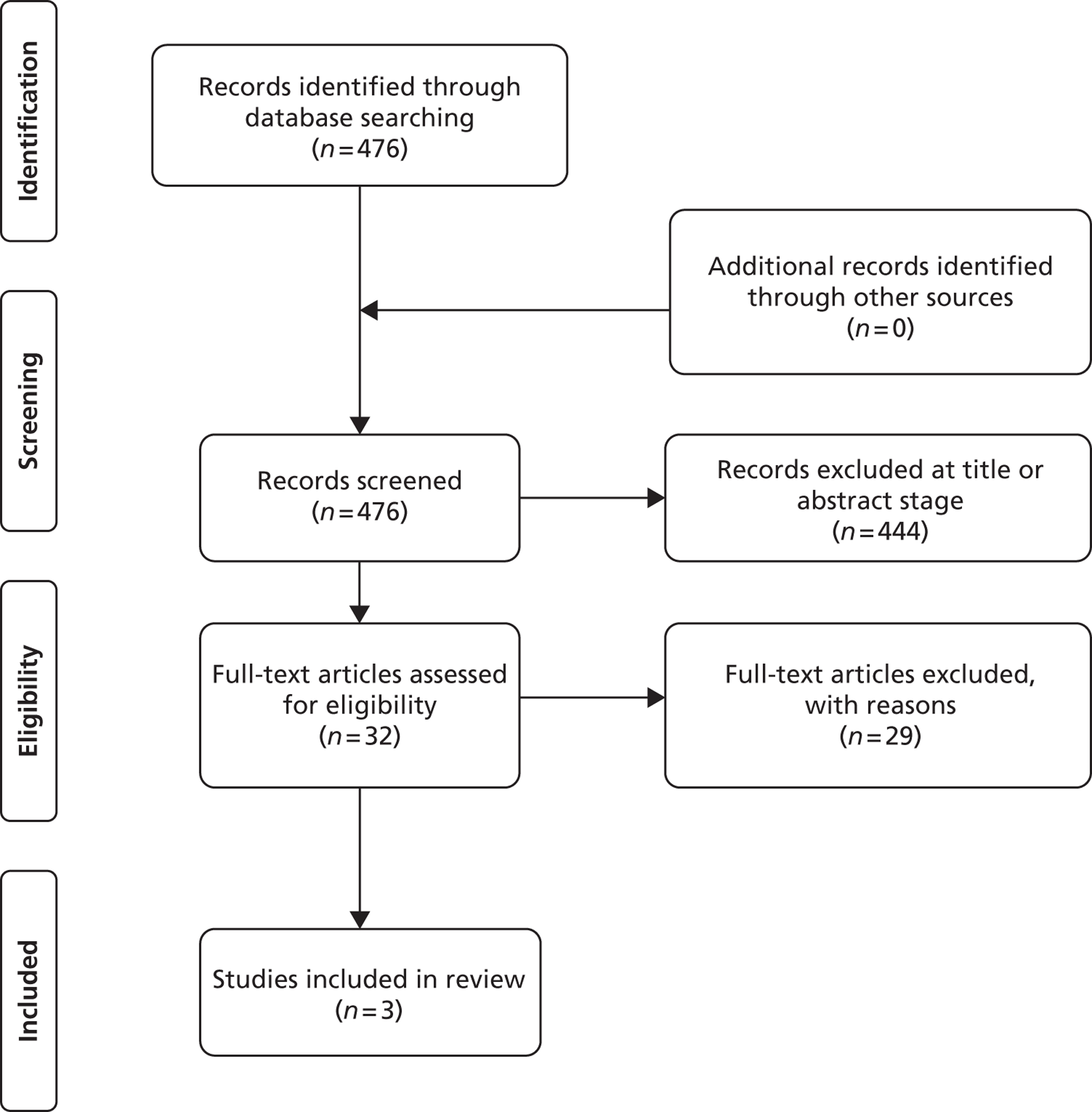
The updated bibliographic search for clinical effectiveness retrieved 476 papers. Figure 1 shows the results of this search. For the clinical effectiveness search, 32 full papers were retrieved after screening of titles and abstracts. After the reading of these full papers, a further 29 papers were excluded (reasons given in Appendix 3). Three papers were included from the updated search. 37–39 All three papers measured smoking cessation rates by PPA. The study reported in Cahill et al. 15 as Pfizer 201140 was identified in the updated search as now being a published paper. 41 There were no differences in reported data in the published paper. Data for efficacy and adverse events were extracted from all newly included studies.
FIGURE 1.
Study flow chart [adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6:e100009736].
Studies reported in the Cahill review
Cahill et al. 15 report 24 trials meeting their inclusion criteria. Their inclusion criteria considered any selective nicotinic receptor partial agonists, e.g. cytisine, varenicline, dianicline, or any other class of drug as they reach Phase III trial stage. Any comparators were considered, which included placebo, NRT, counselling and bupropion. RCTs of adult smokers were included. For outcomes, studies had to report a minimum of 6 months’ abstinence. Three of the reported trials evaluated cytisine, one trial evaluated dianicline and 20 trials evaluated varenicline. Four studies included in Cahill were not included in the current review for reasons outlined below.
Studies from Cahill not included in the current review
As the current review did not seek to evaluate dianicline as a result of its discontinuation, the trial evaluating dianicline reported in Cahill et al. 15 is not included here. Three studies of cytisine or varenicline that were included in the Cahill review (but not in their analyses) are not reported in the current review. Scharfenberg et al. 42 studied the efficacy of cytisine against placebo. In their review, Cahill et al. 15 reported the design and conduct of this early trial to be of indeterminate quality, using self-reported PPA and without biochemical verification. They therefore did not combine the results of this study with the two more recent cytisine studies. 17,43 Their sensitivity analysis combining these three trials indicated substantial heterogeneity between this older study and the newer ones. Therefore, this study has not been included in the current report. Two further studies from the Cahill review were not included in the current review. 44,45 Swan et al. 44 compared different counselling methods alongside varenicline treatment, with all groups receiving varenicline, and no non-treatment control groups. Tonstad45 studied varenicline as maintenance therapy, with both arms completing an initial course of varenicline before the comparison of varenicline and placebo for maintenance of the quit.
A summary of characteristics of all included studies is presented in Tables 1 and 2. A more detailed account of studies from the Cahill review is not provided in this update, but these characteristics are fully reported in Cahill et al. 15
| Study name | Country | n | Participants | Inclusion criteria | Intervention | Comparator | Smoking cessation (strictest definition) |
|---|---|---|---|---|---|---|---|
| Vinnikov 200843 | Kyrgyzstan | 197 | Cytisine mean age 38.3 years, placebo 39.4 years; cytisine percentage male 99%, placebo 95%; cytisine mean years smoking 19.8 years, placebo 21.9 years | Aged > 20 years, smoked ≥ 15 cigarettes per day during the year prior to inclusion into the trial, had claimed high motivation to quit smoking and readiness to do so immediately and had no previous experience of cytisine use | Cytisine (1.5-mg tablets): six times daily (one every 2 hours) on days 1–3; five times per day on days 4–12; four times per day on days 13–16; three tablets per day on days 17–20; two tablets per day on days 21–22; one tablet per day on days 23–25 | Placebo: tablets same regimen | CO-validated CAR. Day 5, week 8. Day 5, week 26 |
| West 201117 | Poland | 740 | Cytisine mean age: 49.5 years, placebo 43.5 years; cytisine percentage male 49.5%, placebo 43.5%; cytisine mean years smoking 28.1 years, placebo 28.6 years | Adults who smoked ≥ 10 cigarettes per day, willing to attempt to stop smoking permanently | Cytisine (1.5-mg tablets): six times daily (one every 2 hours) on days 1–3; five times per day on days 4–12; four times per day on days 13–16; three tablets per day on days 17–20; two tablets per day on days 21–25 | Placebo: tablets same regimen | CO-validated abstinence (fewer than five cigarettes during preceding 6 months) 12 months after end of treatment |
| Study name | Country | n | Participants | Inclusion criteria | Intervention | Comparator | Smoking cessation (strictest definition) |
|---|---|---|---|---|---|---|---|
| Aubin 200846 | Multinational (Belgium, France, the Netherlands, UK and the USA) | 757 (randomised), 746 (treated) | Varenicline: mean age 42.9 years, percentage male 48.4%, mean years smoking 25.9 years NRT: mean age 42.9 years, percentage male 50%, mean years smoking 25.2 years |
Smokers aged 18–75 years, smoking at least 15 cigarettes per day with no period of abstinence > 3 months in the previous year | Varenicline: 1 mg b.i.d. for 12 weeks, titrated during week 1 | Nicotine transdermal patch (21 mg weeks 2–6, 14 mg weeks 7–9, 7 mg weeks 10–11) | CO-confirmed CAR Varenicline: 9–12 weeks, 9–24 weeks, 9–52 weeks NRT: 8–11 weeks, 8–24 weeks, 8–52 weeks |
| Bolliger 201147 | Multinational (11 countries in Latin America, the Middle East and Africa) | 593 | Varenicline: mean age 43.1 years, percentage male 57.7%, mean years smoking 25.0 years Placebo: mean age 43.9 years, percentage male 65.7%, mean years smoking 26.8 years |
Smokers aged 18–75 years; motivated to stop smoking and had smoked a mean ≥ 10 cigarettes per day during the past 12 months; no cumulative period of abstinence > 3 months in the previous 12 months | Varenicline: 1 mg b.i.d. for 12 weeks, titrated during week 1 | Placebo: tablets same regimen | CO-validated CAR: 9–12 weeks, 9–24 weeks |
| de Dios 201238 | USA | 32 | Varenicline: mean age 45.7 years, percentage male 40% NRT: mean age 39.1 years, percentage male 54.5% Placebo: mean age 44.2 years, percentage male 45.5% |
Adult Latino light smokers ≤ 10 cigarettes per day for the past 3 months | Varenicline 1.0 mg b.i.d. for 12 weeks, titrated during week 1 | Nicotine patch for 12 weeks: 14 mg for 4 weeks, tapering to 7 mg for 8 weeks Placebo: matched to varenicline regimen |
CO-validated 7-day PPA: 2 weeks, 1 month, 2 months, 3 months, 4 months, 6 months |
| Gonzales 200648 | USA (multicentre) | 1025 | Varenicline: mean age 42.5 years, percentage male 50%, mean years smoked 24.3 years Bupropion: mean age 42.0 years, percentage male 58.4%, mean years smoked 24.1 years Placebo: mean age 42.6 years, percentage male 54.1%, mean years smoked 24.7 years |
Smokers aged 18–75 years, smoking 10 or more cigarettes per day, had < 3 months of smoking abstinence in the past year, motivated to stop smoking | Varenicline: 1 mg b.i.d. for 12 weeks, titrated week 1 | Bupropion: SR for 12 weeks, 150 mg b.i.d. through week 12, titrated days 1–3 Placebo: tablets same regimen |
CO-validated CAR: 9–12 weeks, 9–24 weeks, 9–52 weeks |
| Heydari 201239 | Iran | 272 | Mean age: counselling, 42.2 years, varenicline 43.5 years, NRT 41.8 years. Percentages for individual group sex unclear. Mean years smoked not reported. NB study includes smokers of < 10 cigarettes per day | Smokers who attended the clinic for help in quitting | Varenicline: 1.0 mg b.i.d. for 8 weeks, titrated during week 1, plus counselling | NRT: 15 mg daily nicotine patches for 8 weeks, plus counselling Counselling only |
CO-verified ‘smoke-free’: 1 month, 6 months, 12 months |
| Jorenby 200616 | USA (multicentre) | 1027 | Varenicline: mean age 44.6 years, percentage male 55.2%, mean years smoked 27.1 years Bupropion: mean age 42.9 years, percentage male 60.2%, mean years smoked 25.4 years Placebo: mean age 42.3 years, percentage male 58.1%, mean years smoked 24.4 years |
Smokers aged 18–75 years, smoking 10 or more cigarettes per day, had < 3 months of smoking abstinence in the past year | Varenicline: 1 mg b.i.d. for 12 weeks, titrated during week 1 | Bupropion: sustained release 150 mg b.i.d. through week 12, titrated to full strength during week 1 Placebo: tablets matching regimen |
CO-validated CAR: 9–12: weeks, 9–24 weeks, 9–52 weeks |
| Nakamura 200749 | Japan | 619 | Varenicline 0.25 mg (n = 128): mean age 40.2 years, percentage male 72.7%, mean years smoked 11.5 years Varenicline 0.5 mg (n = 128): mean age 39 years, percentage male 71.1%, mean years smoked 20.1 years Varenicline 1 mg (n = 130): mean age 40.1 years, percentage male 79.2%, mean years smoked 21.5 years Placebo (n = 129): mean age 39.9 years, percentage male 76%, mean years smoked 20.9 years |
Smokers aged between 20–75 years. Motivated to stop smoking, smoking more than 10 cigarettes per day for previous year without a period of abstinence > 90 days | Varenicline at 3 doses: 0.25 mg b.i.d., 0.5 mg b.i.d., 1 mg b.i.d. 12 weeks of treatment, titrated during week 1 | Placebo: tablets matching regimen | CO-validated CAR: 9–12 weeks, 9–24 weeks, 9–52 weeks |
| Niaura 200850 | USA | 320 | Varenicline (n = 157): mean age 41.5 years, percentage male 50.3%, mean years smoked 24.9 years Placebo (n = 155): mean age 42.1 years, percentage male 53.5%, mean years smoked 25.7 years |
Healthy adult cigarette smokers, motivated to quit, aged 18–65 years, smoked at least 10 cigarettes per day with no period of abstinence > 3 months in the past year | Varenicline: 1 week dose titration up to 0.5 mg b.i.d. Participants then chose dosing schedule, at least one 0.5-mg tablet daily, no more than two 0.5-mg tablets b.i.d. | Placebo: same regimen | CO-validated CAR: 4–7 weeks, 9–12 weeks, 9–52 weeks |
| Nides 200651 | USA | 638 | Varenicline 0.3 mg (n = 126): mean age 41.9 years, percentage male 50%, years smoked 24.6 years Varenicline 1.0 mg once per day (n = 126): mean age 42.9 years, percentage male 43.7%, mean years smoked 25.4 years Varenicline 1.0 mg b.i.d. (n = 125): mean age 41.9 years, percentage male 50.4%, mean years smoked 23.4 years Bupropion hydrochloride (n = 126): mean age 40.5 years, percentage male 45.2%, mean years smoked 23.4 years Placebo (n = 123): mean age 41.6 years, percentage male 52.0%, mean years smoked 23.9 years |
Male and female smokers aged 18–65 years who were in good general health, required to have smoked an average 10 cigarettes per day during the previous year without a period of abstinence > 3 months | Varenicline at one of three dose regimens: 0.3 mg q.d., 1.0 mg q.d., or 1.0 mg b.i.d. Subjects dosed for 6 weeks, then received blinded placebo for week 7 | Bupropion hydrochloride: dosed for 7 weeks, titrated days 1–3 to 150 mg b.i.d. through week 7 Placebo: at matching regimen |
CO-validated 4-week abstinence for any part of treatment Continuous quit rate: 4–7 weeks, 2–12 weeks, 4–24 weeks, 4–52 weeks |
| Oncken 200652 | USA | 647 | Varenicline 0.5 mg b.i.d. non-titrated: mean age 42.9 years, percentage male 45%, mean years smoked 26.0 years Varenicline 0.5 mg b.i.d. titrated: mean age 43.5 years, percentage male 53.1%, mean years smoked 25 years Varenicline 1.0 mg b.i.d. non-titrated: mean age 43.7 years, percentage male 48.8%, mean years smoked 25.7 years Varenicline 1.0 mg b.i.d. titrated: mean age 42.2 years, percentage male 48.5%, mean years smoked 24.0 years Placebo: mean age 43.0 years, percentage male 51.9%, mean years smoked 25.3 years |
Healthy cigarette smokers aged 18–65 years, who smoked at least 10 cigarettes per day | Varenicline: 0.5 mg b.i.d. non-titrated (12 weeks); 0.5 mg b.i.d. titrated (0.5 mg q.d. for 1 week, then b.i.d. through 12 weeks); 1.0 mg b.i.d. non-titrated (12 weeks); 1.0 mg b.i.d. titrated (0.5 mg once a day for 3 days, 0.5 mg b.i.d. for 4 days, then 1.0 mg b.i.d. through 12 weeks) | Placebo: tablets b.i.d. for 12 weeks | CO-validated continuous 4-week abstinence: 4–7 weeks, 9–12 weeks Continuous-verified abstinence: 2–12 weeks, 9–52 weeks |
| Pfizer 2011 40/Williams 201241 | USA and Canada | 128 | Varenicline: male 65/85; age 18–34 years, n = 33; age 35–44 years, n = 10; age 45–64 years, n = 42 Placebo: male 33/43; age 18–34 years, n = 11; age 35–44 years, n = 9; age 45–64 years, n = 23 |
Aged 18–75 years, male and female, have a diagnosis of schizophrenia or schizoaffective disorder and judged to be stable on psychiatric treatment. Current smokers, at least 15 cigarettes per day during the past year with no period of abstinence > 3 months in the past year. Motivated to stop smoking | Varenicline: 1.0 mg b.i.d. for 12 weeks, titrated during week 1 | Placebo: matching regimen | CO-validated PPA: 12 weeks, 24 weeks |
| Rennard 201253 | Multinational (14 countries) | 659 | Varenicline (n = 493): mean age 43.9 years, percentage male 60%, mean years smoked 26 years Placebo (n = 166): mean age 43.2 years, percentage male 59.6%, mean years smoked 24.6 |
Male and female smokers aged 18–75 years, who had smoked ≥ 10 cigarettes per day during the past year, with no period of abstinence > 3 months in the past year and who were motivated to stop smoking | Varenicline: 1.0 mg b.i.d. for 12 weeks, titrated during week 1 | Placebo: matching regimen | CO-validated CAR: 9–12 weeks, 9–24 weeks |
| Rigotti 201054 | Multinational (15 countries) | 714 | Varenicline: mean age 57 years, percentage male 75.2%, mean years smoked 40 years Placebo: mean age 55.9 years, percentage male 82.2%, mean years smoked 39 years |
Adults aged 35–75 years who had smoked > 10 cigarettes per day in the year prior to enrolment, wanted to stop smoking but had not tried to quit in the past 3 months. Had stable, documented CVD that had been diagnosed > 2 months. Eligible CVD diagnosis included history of myocardial infarction, coronary revascularisation, angina pectoris, peripheral arterial vascular disease, cerebrovascular disease | Varenicline 1.0 mg b.i.d. for 12 weeks, titrated during week 1 | Placebo: matching regimen | CO-validated CAR: 9–12 weeks, 9–24 weeks, 9–52 weeks |
| Smith 201355 | Australia | 392 | Aged 20–75 years with smoking-related illnesses | Aged between 20–75 years recruited from hospital wards with smoking-related illnesses | Varenicline: 1.0 mg b.i.d. for 12 weeks, titrated during week 1, plus Quit South Australia counselling | Quit South Australia counselling alone | CAR < 5 cigarettes total: 2 weeks–12 months CO validation only in subset of participants |
| Steinberg 201156 | USA | 79 | Mean age overall: 51 years. Mean years smoking not reported Varenicline: age distribution ≤ 40 years, 12%; 41–50 years, 22%; 51–59 years, 35%; ≥ 60 years, 30%. Percentage male 60% Placebo: age distribution ≤ 40 years, 15%; 41–50 years, 33%; 51–59 years, 36%; ≥ 60 years, 15%. Percentage male 59% |
Patients admitted to the hospital who smoked ≥ 10 cigarettes per day within the past month, were not being discharged into a setting of forced abstinence (e.g. institutionalised) | Varenicline: 1.0 mg b.i.d. for 12 weeks, titrated during week 1 | Placebo: matching regimen | 7-day PPA: 4 weeks, 26 weeks, 12 weeks |
| Tashkin 201157 | Multinational (four countries) | 504 | Varenicline: mean age 57.2 years, percentage male 62.5%, mean years smoking 40.4 years Placebo: mean age 57.1 years, percentage male 62.2%, mean years smoking 40.6 years |
Adults aged ≥ 35 years with a clinical diagnosis of mild to moderate COPD, motivated to stop smoking, smoking an average of ≥ 10 cigarettes per day over the past year with no period of abstinence > 3 months over that time | Varenicline: 1.0 mg b.i.d. for 12 weeks, titrated during week 1 | Placebo: matching regimen | CO-validated CAR: 9–12 weeks, 9–24 weeks, 9–52 weeks |
| Tsai 200758 | Republic of Korea and Taiwan, province of China | 250 | Varenicline: mean age 39.7 years, percentage male 84.9%, mean years smoking 20.2 years Placebo: mean age 40.9 years, percentage male 92.7%, mean years smoking 22.1 years |
Male and female smokers aged 18–75 years, who had smoked ≥ 10 cigarettes per day during the past year, with no period of abstinence > 3 months in the past year, and who were motivated to stop smoking | Varenicline 1.0 mg b.i.d. for 12 weeks, titrated during week 1 | Placebo: (no details reported) | CO-validated CAR: 9–12 weeks, 9–24 weeks |
| Tsukahara 201059 | Japan | 32 | Varenicline: mean age 45.4 years, percentage male 85.7%. mean years smoking 25.4 years Nicotine patch: mean age 46.8 years, percentage male 78.6%, mean years smoking 27.1 years |
Adult smokers who all wished to stop smoking immediately | Varenicline: 1.0 mg b.i.d. for 12 weeks, titrated during week 1 | Transdermal nicotine patch for 8 weeks, 52.5 mg for 4 weeks; 35 mg for 2 weeks; 17.5 mg for 2 weeks | CO-validated CAR: 9–12 weeks Self-reported CAR: 9–24 weeks |
| Wang 200960 | China, Singapore and Thailand | 333 | Varenicline: mean age 39.0 years, percentage male 96.4%, mean years smoking 20.5 years Placebo: mean age 38.5 years, percentage male 97.0%, mean years smoking 19.6 years |
Adults aged 18–75 years, smoked on average ≥ 10 cigarettes per day during the year prior to the screening visit with no period of abstinence > 3 months, motivated to stop smoking | Varenicline: 1.0 mg b.i.d. for 12 weeks, titrated during week 1 | Placebo: matching regimen | CO-validated CAR: 9–12 weeks, 9–24 weeks |
| Williams 200761 | USA and Australia | 377 | Varenicline: mean age 48.2 years, percentage male 50.6%, mean years smoking 30.7 years Placebo: mean age 46.6 years, percentage male 48.4%, mean years 29.9 years |
Adult smokers, aged 18–75 years, who had smoked an average of ≥ 10 cigarettes per day during the past year, with no period of abstinence > 3 months | Varenicline: 1.0 mg b.i.d. for 52 weeks, titrated during week 1 | Placebo: matching regimen | CO-validated 7-day PPA |
| Wong 201237 | Canada | 286 | Varenicline: mean age 51.9 years, percentage male 55% Placebo: mean age 53.3 years, percentage male 50.4% |
Adult patients 18 years or older who attended the preoperative clinic for surgery within 8–10 days, smoked a minimum of 10 cigarettes per day, with no period of abstinence > 3 months in the past year | Varenicline: 1.0 mg b.i.d. for 12 weeks, titrated during week 1 | Placebo: matching regimen | 7-day PPA CO-validated for some participants, but mailed in urinary cotinine validation for all: 3 months, 6 months, 12 months |
Description of studies from updated search
We found three additional studies that met our inclusion criteria, covering 590 participants. 37–39 All three studies were single-country studies, carried out in Canada, the USA, and Iran respectively. Wong et al. 37 conducted the study at two sites – both pre-operative clinics in hospitals in Canada. The Heydari et al. 39 study was set in tobacco cessation clinics in Iran and de Dios et al. 38 focused on Latino smokers in the USA. All studies evaluated varenicline 1.0 mg b.i.d. The duration of varenicline treatment for Wong et al. 37 and de Dios et al. 38 was 12 weeks, whereas for Heydari et al. 39 treatment duration was 8 weeks. Wong et al. 37 compared varenicline with placebo, while the remaining two studies had three arms: Heydari et al. 39 compared varenicline with nicotine patch or counselling and de Dios et al. 38 compared varenicline with nicotine patch or placebo. Wong et al. 37 only included adults who smoked more than 10 cigarettes per day, Heydari et al. 39 included smokers of both more or fewer than 10 cigarettes per day, and de Dios et al. 38 focused only on ‘light smokers’, i.e. those who smoked fewer than 10 cigarettes per day. Wong et al. 37 evaluated smoking cessation in a sample of patients who were due to undergo surgery.
Wong et al. 37 and de Dios et al. 38 measured smoking cessation using 7-day PPA. Heydari et al. 39 report their outcome measurement as being smoke free. An attempt to contact the authors to establish whether this was 7-day PPA or CAR failed and, therefore, we have made the conservative assumption that PPA was used. CO validation of smoking cessation outcomes was recorded in all three studies. Wong et al. 37 and Heydari et al. 39 followed up their participants for 12 months, while the longest follow-up for de Dios et al. 38 was 6 months. The target quit date for Heydari et al. 39 was day 14 of treatment, for Wong et al. 37 was 1 week after treatment began and in de Dios et al. 38 was not reported.
Risk of bias in included studies
A summary of the risk of bias judgements for all included studies is presented in Table 3. Support for judgements for quality assessments from Cahill et al. 15 is fully described in their review. Support for judgements of risk of bias for newly included studies is presented in Appendix 2. Both cytisine trials were judged to be of good quality. For varenicline trials, most studies were judged to be low risk for most of the risk of bias categories, although several studies were judged to have an unclear risk in one or more categories. For the newly included studies, Wong et al. 37 was assessed as low risk in all recorded categories of the Cochrane risk of bias tool. 32 De Dios et al. 38 was assessed as being unclear risk for both random sequence generation and allocation concealment as a result of unclear reporting, but was assessed as low risk for incomplete outcome data and selective reporting. Methods of randomisation, allocation concealment or blinding were not described in Heydari et al. ,39 and the study was therefore assessed as unclear risk for these categories. An assessment of low risk was given for incomplete outcome data as the authors report no dropouts from the study. Data on efficacy, the stated primary outcome, were reported and, therefore, the study was also assessed as low risk for this category.
| Study | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding (performance bias and detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) |
|---|---|---|---|---|---|
| aVinnikov 200843 | Low risk | Low risk | Low risk | Unclear risk | Low risk |
| aWest 201117 | Low risk | Low risk | Low risk | Low risk | Low risk |
| bBolliger 201147 | Low risk | Low risk | Low risk | Low risk | Low risk |
| bGonzales 200648 | Low risk | Low risk | Low risk | Low risk | Low risk |
| bJorenby 200616 | Low risk | Low risk | Unclear risk | Low risk | Low risk |
| bNakamura 200749 | Low risk | Low risk | Low risk | Unclear risk | High risk |
| bNiaura 200850 | Low risk | Low risk | Unclear risk | Low risk | Low risk |
| bNides 200651 | Low risk | Low risk | Unclear risk | Unclear risk | Low risk |
| bOncken 200652 | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| bPfizer 201140 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | NR |
| bRennard 201253 | Low risk | Low risk | Low risk | Low risk | Low risk |
| bRigotti 201054 | Low risk | Low risk | Low risk | Low risk | Low risk |
| bSteinberg 201156 | Low risk | Low risk | Low risk | Unclear risk | Low risk |
| bTashkin 201157 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk |
| bTsai 200758 | Low risk | Low risk | Low risk | Unclear risk | Unclear risk |
| bWang 200960 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk |
| bWilliams 200761 | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk |
| bSmith 201355 | Low risk | Low risk | Unclear risk | Unclear risk | NR |
| bAubin 200846 | Low risk | Low risk | High risk | Low risk | Low risk |
| bTsukahara 201059 | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk |
| cHeydari 201239 | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| cWong 201237 | Low risk | Low risk | Low risk | Low risk | Low risk |
| cde Dios 201238 | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk |
Summary of data used in the network meta-analyses (cytisine and varenicline compared with placebo)
A full description of the data used for each meta-analysis for all interventions and comparators can be found in Appendix 5. Tables 4 and 5 present a summary of the CAR and adverse events data used in the analyses of cytisine and varenicline compared with placebo.
| Study | CAR | Abnormal dreams | Headache | Insomnia | Nausea | SAEs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytisine | Placebo | Cytisine | Placebo | Cytisine | Placebo | Cytisine | Placebo | Cytisine | Placebo | Cytisine | Placebo | |
| Vinnikov 200843 | 9/100 | 1/97 | – | – | 1/86 | 1/85 | – | – | 2/85 | 1/86 | – | – |
| West 201117 | 31/370 | 9/370 | – | – | 7/370 | 8/370 | – | – | 10/370 | 14/370 | 4/370 | 3/370 |
| Study | CAR | Abnormal dreams | Headache | Insomnia | Nausea | SAEs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Varenicline | Placebo | Varenicline | Placebo | Varenicline | Placebo | Varenicline | Placebo | Varenicline | Placebo | Varenicline | Placebo | |
| Bolliger 201147 | 157/394 | 26/199 | – | – | 64/390 | 24/198 | 50/390 | 13/198 | 103/390 | 16/198 | 11/394 | 2/199 |
| Niaura 200850 | 35/160 | 12/160 | – | – | 25/157 | 20/155 | 34/157 | 17/155 | 21/157 | 8/155 | 3/160 | 0/160 |
| Pfizer 201140/Williams 201241 | – | – | 6/84 | 43/4 | 9/84 | 8/43 | 8/84 | 2/43 | 20/84 | 6/43 | 5/85 | 4/43 |
| Rennard 201253 | 171/493 | 21/166 | 61/486 | 5/165 | 55/486 | 20/165 | 43/486 | 6/165 | 142/486 | 15/165 | 6/493 | 1/166 |
| Rigotti 201054 | 68/355 | 26/359 | 28/353 | 6/350 | 45/353 | 39/350 | 42/353 | 23/350 | 104/353 | 30/350 | 23/353 | 21/354 |
| Steinberg 201156 | – | – | – | – | – | – | – | – | 10/38 | 2/37 | 6/40 | 5/39 |
| Tashkin 201157 | 47/250 | 14/254 | 27/248 | 7/251 | 20/248 | 20/251 | 24/248 | 15/251 | 67/248 | 20/251 | 12/248 | 15/253 |
| Tsai 200758 | 59/126 | 27/124 | 7/126 | 1/124 | – | – | 19/126 | 17/124 | 55/126 | 14/124 | 3/126 | 3/124 |
| Smith 201355 | 61/196 | 42/196 | 12/196 | 2/196 | 12/196 | 3/196 | 10/196 | 4/196 | 32/196 | 3/196 | 6/119 | 3/117 |
| Aubin 200846 | 98/378 | 75/379 | 44/376 | 31/370 | 72/376 | 36/376 | 80/376 | 71/370 | 140/376 | 36/370 | 2/376 | 8/370 |
| Wang 200960 | 63/165 | 42/168 | – | – | 9/165 | 7/168 | 10/165 | 5/168 | 48/165 | 20/168 | 0/165 | 2/168 |
| Tsukahara 201059 | – | – | – | – | – | – | 6/14 | 2/14 | 4/14 | 0/14 | – | – |
| Wong 201237 | – | – | 3/151 | 0/135 | 5/151 | 0/135 | – | – | 20/151 | 5/135 | – | – |
| Heydari 201239 | – | – | 3/89 | 0/91 | – | – | – | – | 8/89 | 0/91 | – | – |
| Gonzales 200648 | 77/352 | 29/344 | 36/349 | 19/344 | 54/349 | 42/344 | 49/349 | 44/344 | 98/349 | 29/344 | 4/349 | 9/344 |
| Jorenby 200616 | 79/344 | 35/341 | 45/343 | 12/340 | 44/343 | 43/340 | 49/343 | 42/340 | 101/343 | 33/340 | 8/344 | 6/341 |
| Oncken 200652 | 58/259 | 5/129 | 46/253 | 6/121 | 59/253 | 21/121 | 75/253 | 14/121 | 97/253 | 18/121 | – | – |
| Nakamura 200749 | 56/156 | 35/154 | – | – | 16/156 | 4/154 | – | – | 38/156 | 12/154 | 3/156 | 3/154 |
| Nides 200651 | 18/127 | 6/127 | 19/125 | 10/123 | 30/125 | 33/123 | 44/125 | 27/123 | 65/125 | 23/123 | 1/125 | 0/127 |
Assessment of clinical effectiveness
Results
Continuous abstinence
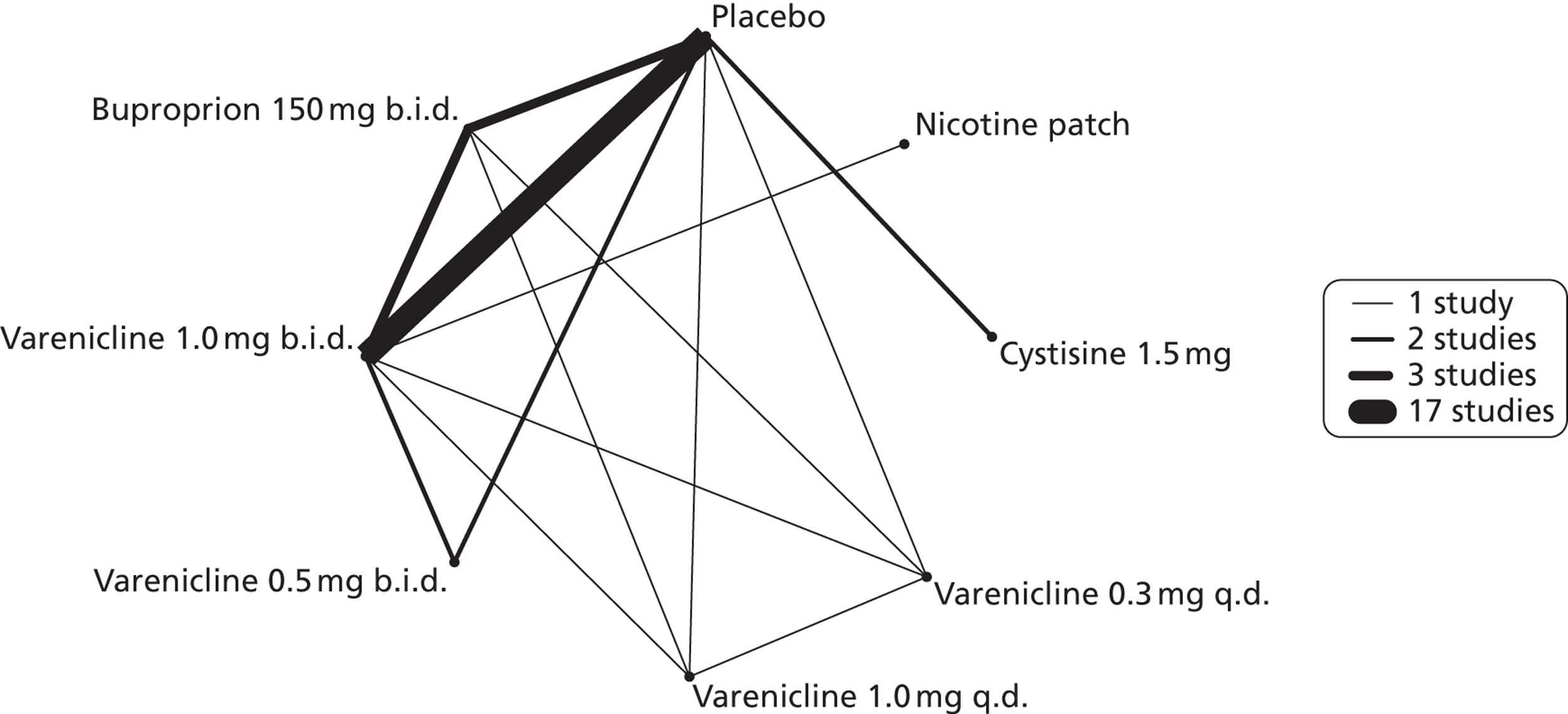
A network meta-analysis was used to compare the hazard of having continuous abstinence when treating with nicotine patch, cytisine 1.5 mg six times daily, varenicline 0.3 mg q.d., varenicline 1.0 mg q.d., varenicline 0.5 mg b.i.d., varenicline 1.0 mg b.i.d. and bupropion hydrochloride 150 mg b.i.d. compared with placebo. A total of 16 studies comparing pairs, triplets or quintuplets of interventions provided information at various study durations. 16,17,43,46–55,57,58,60
Figure 2 presents the network of evidence. A summary of all the trials (data) included in the network meta-analysis for continuous abstinence is provided (see Appendix 5, Table 38).
FIGURE 2.
Network diagram of different interventions for continuous abstinence. The nodes represent the interventions. Lines between nodes indicate when interventions have been compared. Different thicknesses of the lines represent the number of times that each pair of interventions has been compared.
The network meta-analysis model fitted the data well, with a total residual deviance close to the total number of data points included in the analysis. The total residual deviance was 40.99, which compared favourably with the 39 data points being analysed.
There was evidence of mild heterogeneity in treatment effects between studies (between-study heterogeneity SD 0.20, 95% CrI 0.02 to 0.45). All interventions apart from varenicline 0.3 mg q.d. and varenicline 1.0 mg q.d. were associated with a statistically significant effect on having continuous abstinence at a conventional 5% significance level relative to placebo. Cytisine 1.5 mg produced the greatest effect (HR 4.27, 95% CrI 2.05 to 10.05) relative to placebo (Table 6; see also Appendix 5, Figure 13). Cytisine 1.5 mg was the intervention with the highest probability of being the most effective intervention (p = 0.87) (Table 7).
| Intervention | Hazard ratio (95% CrI) |
|---|---|
| Nicotine patch | 1.89 (1.06 to 3.49) |
| Cytisine 1.5 mga | 4.27 (2.05 to 10.05) |
| Varenicline 0.3 mg q.d. | 1.58 (0.65 to 3.53) |
| Varenicline 1.0 mg q.d. | 1.08 (0.40 to 2.63) |
| Varenicline 0.5 mg b.i.d. | 2.16 (1.54 to 3.38) |
| Varenicline 1.0 mg b.i.d. | 2.58 (2.16 to 3.15) |
| Bupropion hydrochloride 150 mg b.i.d. | 1.59 (1.10 to 2.32) |
| Between-study SD | 0.20 (0.02 to 0.45) |
| Rank (b) | Treatment (j) | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Nicotine patch | Cytisine 1.5 mga | Varenicline 0.3 mg q.d. | Varenicline 1.0 mg q.d. | Varenicline 0.5 mg b.i.d. | Varenicline 1.0 mg b.i.d. | Bupropion hydrochloride 150 mg b.i.d. | |
| 1 | 0.00 | 0.02 | 0.87 | 0.02 | 0.00 | 0.03 | 0.06 | 0.00 |
| 2 | 0.00 | 0.09 | 0.07 | 0.08 | 0.02 | 0.14 | 0.61 | 0.00 |
| 3 | 0.00 | 0.17 | 0.04 | 0.09 | 0.03 | 0.37 | 0.27 | 0.03 |
| 4 | 0.00 | 0.30 | 0.02 | 0.14 | 0.05 | 0.28 | 0.05 | 0.16 |
| 5 | 0.00 | 0.22 | 0.01 | 0.18 | 0.08 | 0.12 | 0.01 | 0.38 |
| 6 | 0.09 | 0.14 | 0.00 | 0.24 | 0.15 | 0.05 | 0.00 | 0.33 |
| 7 | 0.42 | 0.06 | 0.00 | 0.15 | 0.26 | 0.01 | 0.00 | 0.10 |
| 8 | 0.48 | 0.01 | 0.00 | 0.09 | 0.41 | 0.00 | 0.00 | 0.00 |
Since repeated 7-day PPA may be used as a proxy for continuous abstinence,22 a sensitivity analysis was conducted including studies used measurement such as continuous abstinence or repeated 7-day PPA. Both the estimates and 95% CrIs of all treatment effects were similar to the results from the analysis including only studies that used continuous abstinence (see Appendix 5). However, the goodness of the model fit suggested that some studies, in particular those by Steinberg et al. ,56 Oncken et al. ,52 Heydari et al. 39 and de Dios et al. ,38 may come from a different model. The measurement used by Steinberg et al. ,56 Heydari et al. 39 and de Dios et al. 38 was the repeated 7-day PPA. Hence, the treatment effects were estimated using studies having continuous abstinence as the measurement and these were used in the economic model.
Abnormal dreams
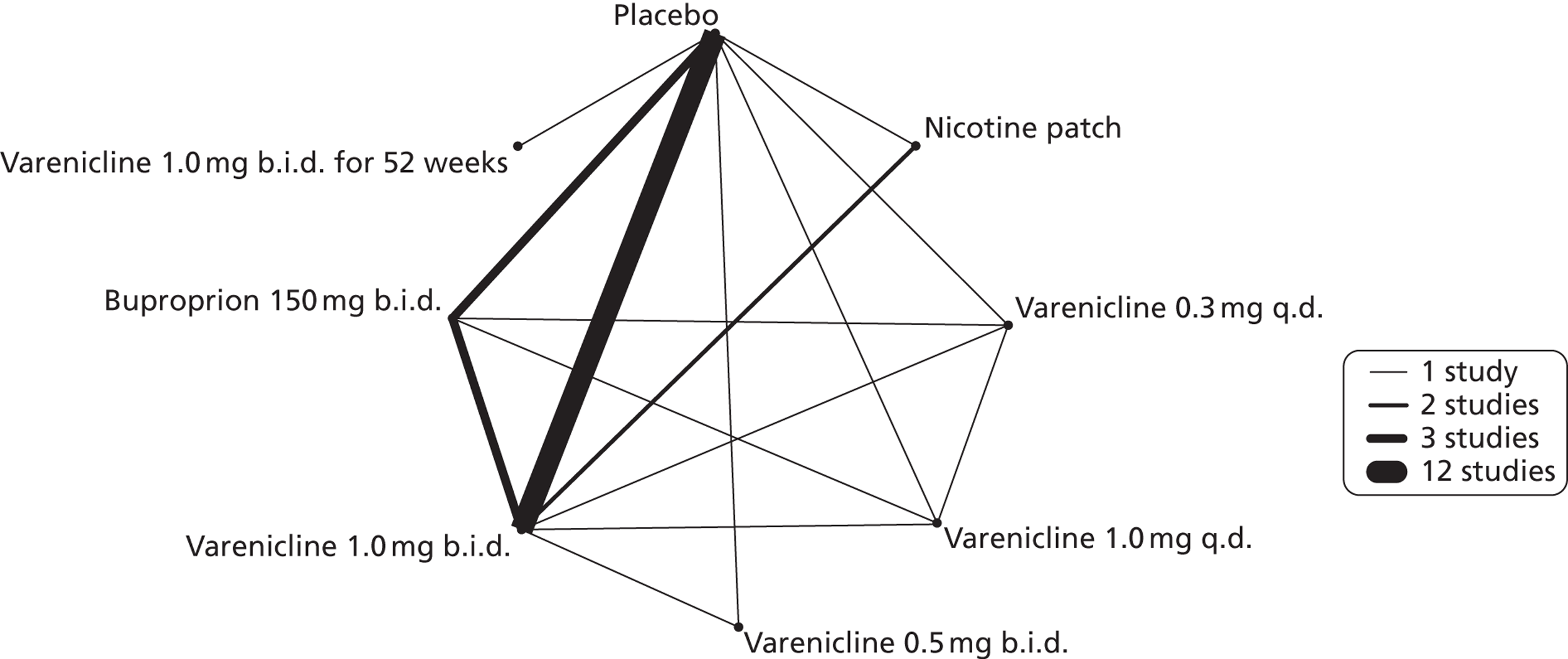
A network meta-analysis was used to compare the hazard of having abnormal dreams when treating with nicotine patch, varenicline 0.3 mg q.d., varenicline 1.0 mg q.d., varenicline 0.5 mg b.i.d., varenicline 1.0 mg b.i.d., bupropion hydrochloride 150 mg b.i.d. and varenicline 1.0 mg b.i.d. for 52 weeks or placebo. A total of 14 studies comparing pairs or triplets of interventions provided information at various study durations. 16,37,39,41,46,48,51–55,57,58,61 No data were available for cytisine for abnormal dreams.
Figure 3 presents the network of evidence. A summary of all the trials (data) included in the network meta-analysis for abnormal dreams is provided (see Appendix 5, Table 39).
FIGURE 3.
Network diagram of different interventions for abnormal dreams. The nodes represent the interventions. Lines between nodes indicate when interventions have been compared. Different thicknesses of the lines represent the number of times that each pair of interventions has been compared.
The network meta-analysis model fitted the data reasonably well, with a total residual deviance close to the total number of data points included in the analysis. The total residual deviance was 37.98, which was slightly less than the 39 data points being analysed.
There was evidence of mild heterogeneity in treatment effects between studies (between-study heterogeneity SD 0.24, 95% CrI 0.02 to 0.74). Varenicline 0.5 mg b.i.d., varenicline 1.0 mg b.i.d. and varenicline 1.0 mg b.i.d. for 52 weeks were associated with statistically significant effects of having abnormal dreams at a conventional 5% level relative to placebo. Varenicline 1.0 mg b.i.d. for 52 weeks produced the greatest effect (HR 3.64, 95% CrI 1.44 to 10.01) relative to placebo (Table 8; see also Appendix 5, Figure 14). Varenicline 1.0 mg b.i.d. for 52 weeks was the intervention with the highest probability of being the most likely intervention to be associated with abnormal dreams (p = 0.54) (Table 9).
| Intervention | Hazard ratio (95% CrI) |
|---|---|
| Nicotine patch | 2.01 (0.82 to 4.43) |
| Varenicline 0.3 mg q.d. | 1.21 (0.46 to 2.99) |
| Varenicline 1.0 mg q.d. | 1.75 (0.71 to 4.10) |
| Varenicline 0.5 mg b.i.d. | 2.56 (1.23 to 5.91) |
| Varenicline 1.0 mg b.i.d. | 3.29 (2.40 to 4.77) |
| Bupropion hydrochloride 150 mg b.i.d. | 1.58 (0.96 to 2.70) |
| Varenicline 1.0 mg b.i.d. for 52 weeks | 3.64 (1.44 to 10.01) |
| Between-study SD | 0.24 (0.02 to 0.74) |
| Rank (b) | Treatment (j) | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Nicotine patch | Varenicline 0.3 mg q.d. | Varenicline 1.0 mg q.d. | Varenicline 0.5 mg b.i.d. | Varenicline 1.0 mg b.i.d. | Bupropion hydrochloride 150 mg b.i.d. | Varenicline 1.0 mg b.i.d. for 52 weeks | |
| 1 | 0.00 | 0.03 | 0.00 | 0.03 | 0.12 | 0.28 | 0.00 | 0.54 |
| 2 | 0.00 | 0.08 | 0.01 | 0.05 | 0.19 | 0.51 | 0.01 | 0.16 |
| 3 | 0.00 | 0.18 | 0.03 | 0.11 | 0.32 | 0.18 | 0.04 | 0.13 |
| 4 | 0.00 | 0.28 | 0.06 | 0.19 | 0.22 | 0.03 | 0.13 | 0.08 |
| 5 | 0.01 | 0.20 | 0.11 | 0.23 | 0.09 | 0.00 | 0.31 | 0.05 |
| 6 | 0.08 | 0.12 | 0.18 | 0.20 | 0.04 | 0.00 | 0.35 | 0.02 |
| 7 | 0.32 | 0.08 | 0.29 | 0.13 | 0.02 | 0.00 | 0.15 | 0.01 |
| 8 | 0.58 | 0.03 | 0.31 | 0.06 | 0.00 | 0.00 | 0.01 | 0.00 |
Headache
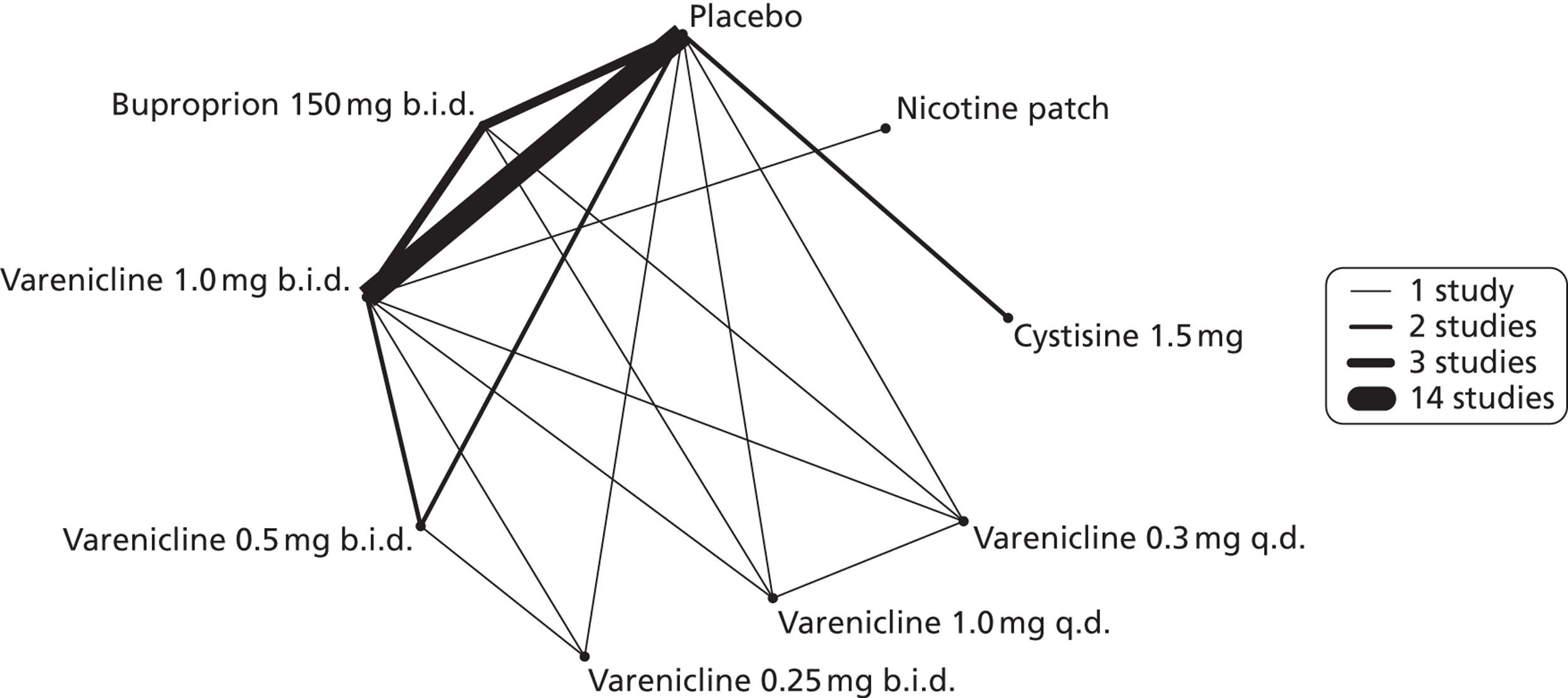
A network meta-analysis was used to compare the hazard of experiencing headaches when treating with nicotine patch, cytisine 1.5 mg, varenicline 0.3 mg q.d., varenicline 1.0 mg q.d., varenicline 0.25 mg b.i.d., varenicline 0.5 mg b.i.d., varenicline 1.0 mg b.i.d. and bupropion hydrochloride 150 mg b.i.d. compared with placebo. A total of 17 studies comparing pairs, triplets, quadruplets or quintuplets of interventions provided information at various study durations. 16,17,37,41,43,46–55,58,61
Figure 4 presents the network of evidence. A summary of all the trials (data) included in the network meta-analysis for headache is presented in Appendix 5, Table 40.
FIGURE 4.
Network diagram of different interventions for hazard of experiencing headaches. The nodes represent the interventions. Lines between nodes indicate when interventions have been compared. Different thicknesses of the lines represent the number of times that each pair of interventions has been compared.
The network meta-analysis model did not fit the data very well. The total residual deviance was 49.95, which was higher than would be expected given the 42 data points being analysed. In particular, the model did not fit the Smith et al. 55 and Nakamura et al. 49 studies particularly well. For Smith et al. 55 the model predicted six events in the placebo arm, which is twice as much as the number of events reported. For Nakamura et al. ,49 the model predicted nine events in the placebo arm, which is more than double the number of events reported in the study, which was only four. There was no obvious explanation in terms of the characteristics of the two studies, although we did not attempt a meta-regression because of limited available data.
There was evidence of mild heterogeneity in treatment effects between studies (between-study heterogeneity SD 0.19, 95% CrI 0.01 to 0.50). Varenicline 1.0 mg b.i.d. was the only intervention associated with a statistically significant increase in the hazard of experiencing headaches at a conventional 5% significance level relative to placebo (HR 1.23, 95% CrI 1.01 to 1.55). Varenicline 0.25 mg b.i.d. produced the greatest effect (HR 1.58, 95% CrI 0.78 to 3.47) relative to placebo (Table 10; see also Appendix 5, Figure 15), although the treatment effect was not statistically significant at a conventional 5% significance level. Varenicline 0.25 mg b.i.d. was the intervention with the highest probability of being associated with headaches (p = 0.46) (Table 11).
| Intervention | Hazard ratio (95% CrI) |
|---|---|
| Nicotine patch | 0.59 (0.30 to 1.16) |
| Cytisine 1.5 mga | 0.85 (0.31 to 2.54) |
| Varenicline 0.3 mg q.d. | 1.06 (0.61 to 1.92) |
| Varenicline 1.0 mg q.d. | 1.08 (0.61 to 1.90) |
| Varenicline 0.25 mg b.i.d. | 1.58 (0.78 to 3.47) |
| Varenicline 0.5 mg b.i.d. | 1.48 (0.97 to 2.50) |
| Varenicline 1.0 mg b.i.d. | 1.23 (1.01 to 1.55) |
| Bupropion hydrochloride 150 mg b.i.d. | 1.03 (0.76 to 1.48) |
| Between-study SD | 0.19 (0.01 to 0.50) |
| Rank (b) | Treatment (j) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | Nicotine patch | Cytisine 1.5 mg | Varenicline 0.3 mg q.d. | Varenicline 1.0 mg q.d. | Varenicline 0.25 mg b.i.d. | Varenicline 0.5 mg b.i.d. | Varenicline 1.0 mg b.i.d. | Bupropion hydrochloride 150 mg b.i.d. | |
| 1 | 0.00 | 0.00 | 0.10 | 0.06 | 0.05 | 0.46 | 0.30 | 0.02 | 0.01 |
| 2 | 0.00 | 0.00 | 0.06 | 0.09 | 0.10 | 0.21 | 0.37 | 0.12 | 0.03 |
| 3 | 0.01 | 0.00 | 0.06 | 0.11 | 0.12 | 0.10 | 0.16 | 0.36 | 0.07 |
| 4 | 0.09 | 0.01 | 0.06 | 0.14 | 0.14 | 0.06 | 0.08 | 0.29 | 0.14 |
| 5 | 0.21 | 0.02 | 0.06 | 0.13 | 0.14 | 0.05 | 0.04 | 0.14 | 0.21 |
| 6 | 0.30 | 0.02 | 0.06 | 0.14 | 0.13 | 0.04 | 0.02 | 0.05 | 0.23 |
| 7 | 0.27 | 0.05 | 0.08 | 0.16 | 0.17 | 0.04 | 0.01 | 0.01 | 0.20 |
| 8 | 0.10 | 0.24 | 0.26 | 0.14 | 0.12 | 0.03 | 0.01 | 0.00 | 0.10 |
| 9 | 0.01 | 0.66 | 0.25 | 0.03 | 0.03 | 0.01 | 0.00 | 0.00 | 0.01 |
Nicotine patch and cytisine 1.5 mg were less likely than placebo to be associated with headache, although the effects were not statistically significant at a conventional 5% significance level. The HR for nicotine patch compared with placebo was 0.59 (95% CrI 0.30 to 1.16) and for cytisine 1.5 mg compared with placebo was 0.85 (95% CrI 0.31 to 2.54).
Insomnia
A network meta-analysis was used to compare the hazard of experiencing insomnia when treating with nicotine patch, varenicline 0.3 mg q.d., varenicline 1.0 mg q.d., varenicline 0.5 mg b.i.d., varenicline 1.0 mg b.i.d., bupropion hydrochloride 150 mg b.i.d. and varenicline 1.0 mg b.i.d. for 52 weeks. A total of 16 studies comparing pairs, triplets or quintuplets of interventions provided information at various study durations. 16,41,46–48,50–55,57–61
Figure 5 presents the network of evidence. A summary of all the trials (data) included in the network meta-analysis for insomnia is presented in Appendix 5, Table 41.
FIGURE 5.
Network diagram of different interventions for insomnia. The nodes represent the interventions. Lines between nodes indicate when interventions have been compared. Different thicknesses of the lines represent the number of times that each pair of interventions has been compared.
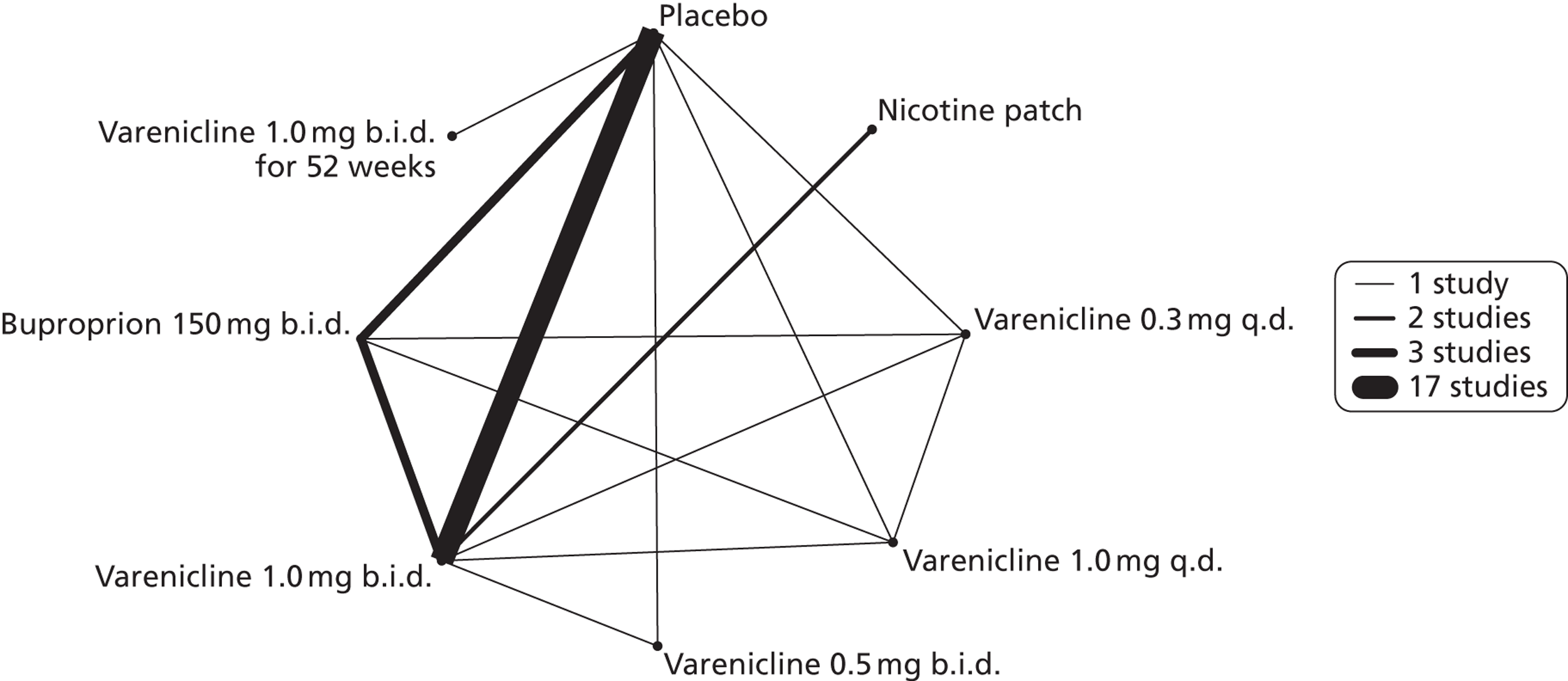
The network meta-analysis model fitted the data reasonably well, with a total residual deviance close to the total number of data points included in the analysis. The total residual deviance was 36.91, which was slightly less than the 38 data points being analysed.
There was evidence of mild heterogeneity in treatment effects between studies (between-study heterogeneity SD 0.15, 95% CrI 0.00 to 0.40). Varenicline 0.5 mg b.i.d., varenicline 1.0 mg b.i.d., bupropion hydrochloride 150 mg b.i.d. and varenicline 1.0 mg b.i.d. for 52 weeks were associated with a statistically significant increase in the hazard of experiencing insomnia at a conventional 5% significance level relative to placebo. Bupropion hydrochloride 150 mg b.i.d. produced the greatest effect (HR 2.27 95% CrI 1.70 to 3.05) relative to placebo (Table 12; see also Appendix 5, Figure 16). Bupropion hydrochloride 150 mg b.i.d. was the intervention with the highest probability of being associated with insomnia (p = 0.45) (Table 13).
| Intervention | Hazard ratio (95% CrI) |
|---|---|
| Nicotine patch | 1.36 (0.79 to 2.20) |
| Varenicline 0.3 mg q.d. | 0.84 (0.48 to 1.47) |
| Varenicline 1.0 mg q.d. | 1.22 (0.72 to 2.02) |
| Varenicline 0.5 mg b.i.d. | 1.76 (1.09 to 3.03) |
| Varenicline 1.0 mg b.i.d. | 1.68 (1.40 to 2.07) |
| Bupropion hydrochloride 150 mg b.i.d. | 2.27 (1.70 to 3.05) |
| Varenicline 1.0 mg b.i.d. for 52 weeks | 2.16 (1.03 to 4.79) |
| Between-study SD | 0.15 (0.00 to 0.40) |
| Rank (b) | Treatment (j) | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Nicotine patch | Varenicline 0.3 mg q.d. | Varenicline 1.0 mg q.d. | Varenicline 0.5 mg b.i.d. | Varenicline 1.0 mg b.i.d. | Bupropion hydrochloride 150 mg b.i.d. | Varenicline 1.0 mg b.i.d. for 52 weeks | |
| 1 | 0.00 | 0.01 | 0.00 | 0.00 | 0.10 | 0.00 | 0.45 | 0.43 |
| 2 | 0.00 | 0.04 | 0.00 | 0.02 | 0.20 | 0.09 | 0.44 | 0.21 |
| 3 | 0.00 | 0.08 | 0.00 | 0.04 | 0.29 | 0.37 | 0.08 | 0.12 |
| 4 | 0.00 | 0.14 | 0.01 | 0.09 | 0.21 | 0.43 | 0.02 | 0.10 |
| 5 | 0.03 | 0.37 | 0.04 | 0.24 | 0.14 | 0.10 | 0.00 | 0.08 |
| 6 | 0.24 | 0.21 | 0.09 | 0.36 | 0.05 | 0.01 | 0.00 | 0.03 |
| 7 | 0.53 | 0.09 | 0.17 | 0.18 | 0.01 | 0.00 | 0.00 | 0.01 |
| 8 | 0.19 | 0.05 | 0.68 | 0.07 | 0.00 | 0.00 | 0.00 | 0.01 |
Varenicline 0.3 mg q.d. was less likely than placebo to be associated with insomnia, although the effect was not statistically significant at a conventional 5% significance level (HR 0.84, 95% CrI 0.48 to 1.47).
Nausea
A network meta-analysis was used to compare the hazard of experiencing nausea when treating with nicotine patch, cytisine 1.5 mg, varenicline 0.3 mg q.d., varenicline 1.0 mg q.d., varenicline 0.25 mg b.i.d., varenicline 0.5 mg b.i.d., varenicline 1.0 mg b.i.d., bupropion hydrochloride 150 mg b.i.d. and varenicline 1.0 mg b.i.d. for 52 weeks. A total of 22 studies comparing pairs, triplets, quadruplets or quintuplets of interventions provided information at various study durations. 16,17,37,39,41,43,46–61
Figure 6 presents the network of evidence. A summary of all the trials (data) included in the network meta-analysis for nausea is presented in Appendix 5, Table 42.
FIGURE 6.
Network diagram of different interventions for hazard of experiencing nausea. The nodes represent the interventions. Lines between nodes indicate when interventions have been compared. Different thicknesses of the lines represent the number of times that each pair of interventions has been compared.
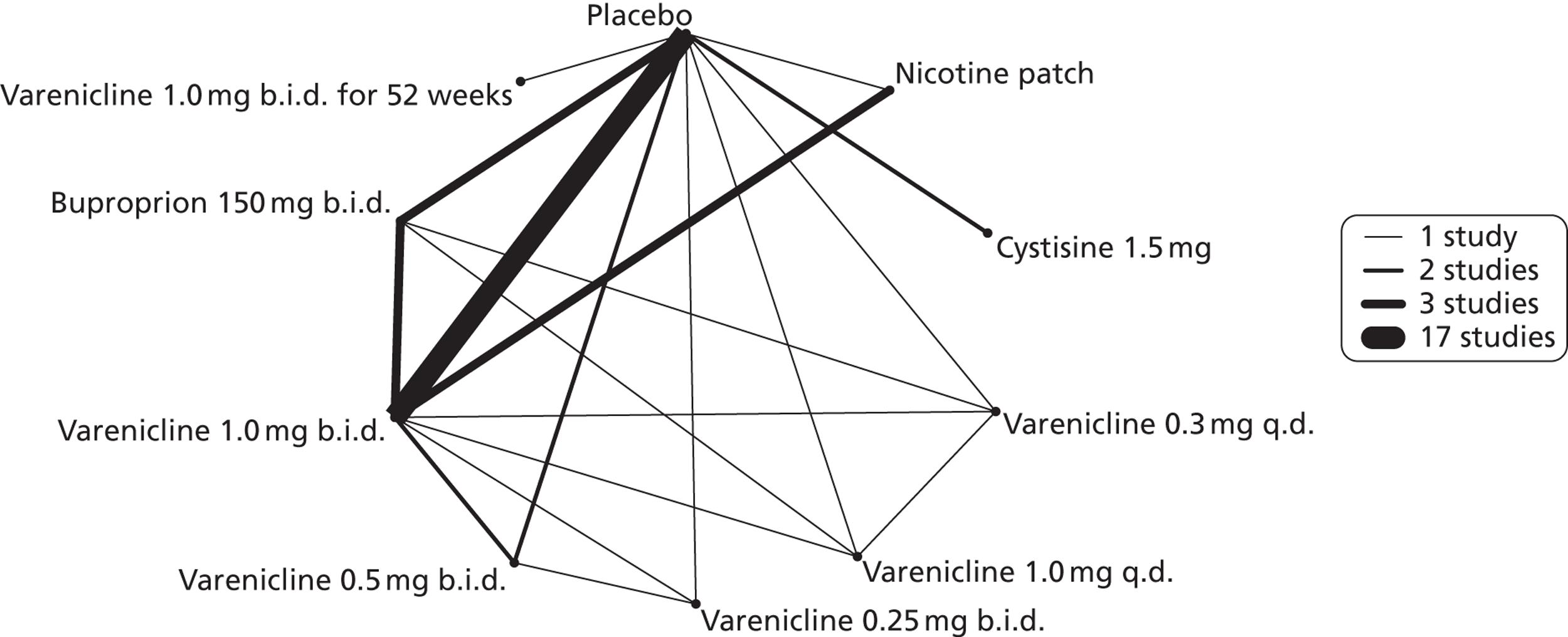
The network meta-analysis model fitted the data reasonably well, with a total residual deviance close to the total number of data points included in the analysis. The total residual deviance was 55.20, which compared favourably with the 53 non-zero data points being analysed.
There was evidence of mild heterogeneity in treatment effects between studies (between-study heterogeneity SD 0.08, 95% CrI 0.00 to 0.29). Varenicline 1.0 mg q.d., varenicline 0.5 mg b.i.d., varenicline 1.0 mg b.i.d., bupropion hydrochloride 150 mg b.i.d. and varenicline 1.0 mg b.i.d. for 52 weeks were more likely than placebo to be associated with nausea. Varenicline 1.0 mg q.d., varenicline 0.5 mg b.i.d., varenicline 1.0 mg b.i.d. and varenicline 1.0 mg b.i.d. for 52 weeks were associated with a statistically significant increase in the hazard of experiencing nausea at a conventional 5% significance level relative to placebo. Varenicline 1.0 mg b.i.d. for 52 weeks produced the greatest effect (HR 6.20, 95% CrI 3.30 to 13.61) relative to placebo (Table 14; see also Appendix 5, Figure 17). Varenicline 1.0 mg b.i.d. for 52 weeks was the intervention with the highest probability of being associated with nausea (p = 0.95) (Table 15).
| Intervention | Hazard ratio (95% CrI) |
|---|---|
| Nicotine patch | 0.73 (0.44 to 1.10) |
| Cytisine 1.5 mga | 0.78 (0.36 to 1.74) |
| Varenicline 0.3 mg q.d. | 0.92 (0.55 to 1.52) |
| Varenicline 1.0 mg q.d. | 2.26 (1.50 to 3.44) |
| Varenicline 0.25 mg b.i.d. | 0.98 (0.48 to 1.82) |
| Varenicline 0.5 mg b.i.d. | 1.48 (1.03 to 2.10) |
| Varenicline 1.0 mg b.i.d. | 3.63 (3.10 to 4.27) |
| Bupropion hydrochloride 150 mg b.i.d. | 1.12 (0.83 to 1.48) |
| Varenicline 1.0 mg b.i.d. for 52 weeks | 6.20 (3.30 to 13.61) |
| Between-study SD | 0.08 (0.00 to 0.29) |
| Rank (b) | Treatment (j) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Nicotine patch | Cytisine 1.5 mg | Varenicline 0.3 mg q.d. | Varenicline 1.0 mg q.d. | Varenicline 0.25 mg b.i.d. | Varenicline 0.5 mg b.i.d. | Varenicline 1.0 mg b.i.d. | Bupropion hydrochloride 150 mg b.i.d. | Varenicline 1.0 mg b.i.d. for 52 weeks | |
| 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.95 |
| 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.94 | 0.00 | 0.05 |
| 3 | 0.00 | 0.00 | 0.01 | 0.00 | 0.92 | 0.01 | 0.04 | 0.01 | 0.00 | 0.00 |
| 4 | 0.00 | 0.00 | 0.05 | 0.04 | 0.06 | 0.09 | 0.70 | 0.00 | 0.06 | 0.00 |
| 5 | 0.08 | 0.01 | 0.07 | 0.11 | 0.01 | 0.20 | 0.18 | 0.00 | 0.34 | 0.00 |
| 6 | 0.25 | 0.02 | 0.07 | 0.15 | 0.00 | 0.12 | 0.05 | 0.00 | 0.32 | 0.00 |
| 7 | 0.38 | 0.06 | 0.08 | 0.16 | 0.00 | 0.13 | 0.02 | 0.00 | 0.17 | 0.00 |
| 8 | 0.22 | 0.16 | 0.15 | 0.21 | 0.00 | 0.16 | 0.01 | 0.00 | 0.08 | 0.00 |
| 9 | 0.06 | 0.34 | 0.22 | 0.20 | 0.00 | 0.16 | 0.00 | 0.00 | 0.03 | 0.00 |
| 10 | 0.00 | 0.40 | 0.35 | 0.12 | 0.00 | 0.12 | 0.00 | 0.00 | 0.01 | 0.00 |
Nicotine patch, cytisine 1.5 mg, varenicline 0.3 mg q.d. and varenicline 0.25 mg b.i.d. were less likely than placebo to be associated with nausea, although the effects were not statistically significant at a conventional 5% significance level.
Serious adverse events
A network meta-analysis was used to compare the hazard of experiencing SAEs when treating with nicotine patch, cytisine 1.5 mg, varenicline 0.3 mg q.d., varenicline 1.0 mg q.d., varenicline 0.25 mg b.i.d., varenicline 0.5 mg b.i.d., varenicline 1.0 mg b.i.d., bupropion hydrochloride 150 mg b.i.d. and varenicline 1.0 mg b.i.d. for 52 weeks or placebo. A total of 18 studies comparing pairs, triplets, quadruplets or quintuplets of interventions provided information at various study durations. 16,17,41,46–58,60,61
Figure 7 presents the network of evidence. A summary of all the trials (data) included in the network meta-analysis for SAEs is presented in Appendix 5, Table 43.
FIGURE 7.
Network diagram of different interventions for risk of experiencing SAEs. The nodes represent the interventions. Lines between nodes indicate when interventions have been compared. Different thicknesses of the lines represent the number of times that each pair of interventions has been compared.
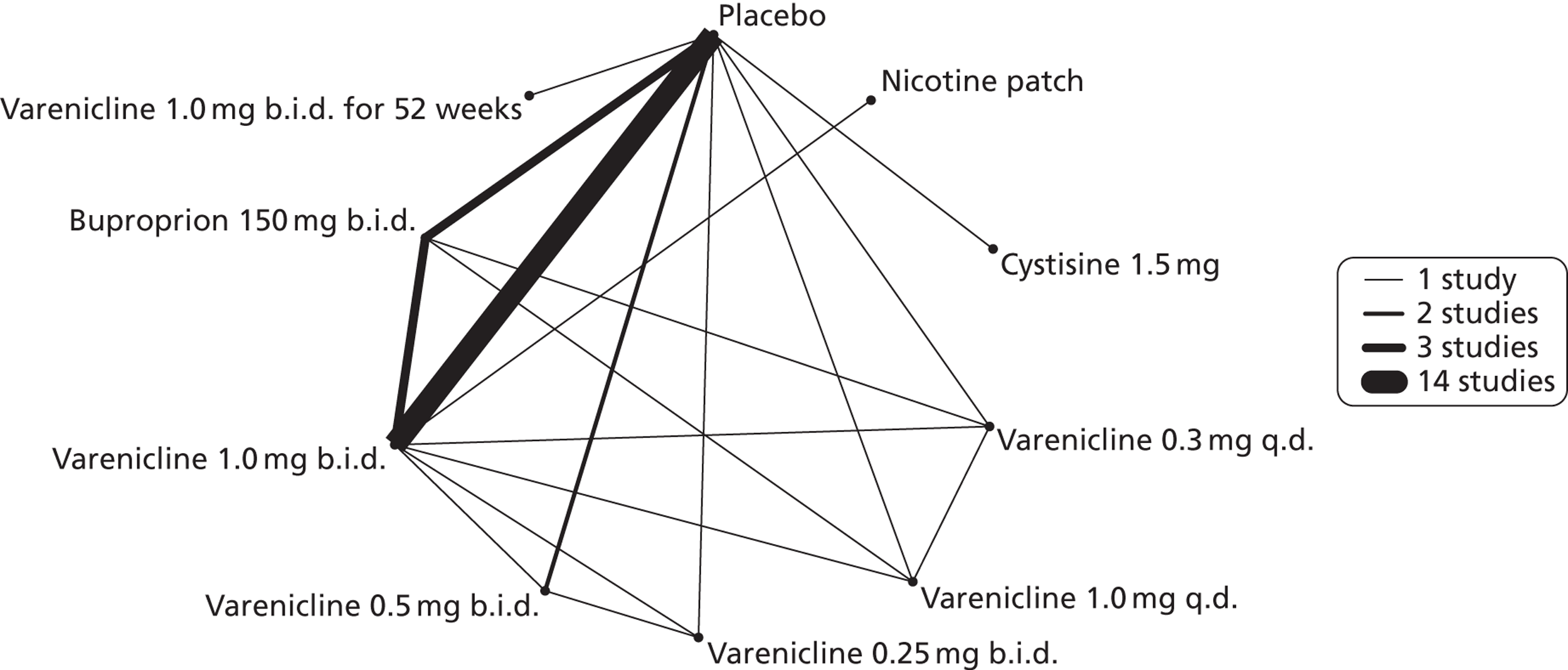
The network meta-analysis model fitted the data reasonably well, with a total residual deviance close to the total number of data points included in the analysis. The total residual deviance was 46.67, which compared favourably with the 43 data points being analysed.
There was evidence of mild heterogeneity in treatment effects between studies (between-study heterogeneity SD 0.25, 95% CrI 0.01 to 0.86). Nicotine patch, cytisine 1.5 mg, varenicline 0.25 mg b.i.d., varenicline 0.5 mg b.i.d., varenicline 1.0 mg b.i.d., bupropion hydrochloride 150 mg b.i.d. and varenicline 1.0 mg b.i.d. for 52 weeks were associated with a higher risk of experiencing SAEs relative to placebo.
Varenicline 0.3 mg q.d. and varenicline 1.0 mg q.d. weeks were associated with a lower risk of experiencing SAEs relative to placebo, although none of the treatment effects was statistically significant at a conventional 5% significance level.
Nicotine patch produced the greatest effect (HR 5.33, 95% CrI 0.98 to 45.21) relative to placebo (Table 16; see also Appendix 5, Figure 18). Nicotine patch was the intervention with the highest probability of being associated with SAEs (p = 0.62) (Table 17).
| Intervention | Hazard ratio (95% CrI) |
|---|---|
| Nicotine patch | 5.3 (0.98 to 45.21) |
| Cytisine 1.5 mga | 1.27 (0.24 to 8.58) |
| Varenicline 0.3 mg q.d. | 0.049 (0.00 to 1.34) |
| Varenicline 1.0 mg q.d. | 0.078 (0.00 to 1.32) |
| Varenicline 0.25 mg b.i.d. | 2.06 (0.46 to 7.83) |
| Varenicline 0.5 mg b.i.d. | 1.02 (0.31 to 3.46) |
| Varenicline 1.0 mg b.i.d. | 1.10 (0.74 to 1.65) |
| Bupropion hydrochloride 150 mg b.i.d. | 1.29 (0.61 to 2.78) |
| Varenicline 1.0 mg b.i.d. for 52 weeks | 2.61 (0.69 to 15.16) |
| Between-study SD | 0.25 (0.01 to 0.86) |
| Rank (b) | Treatment (j) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Nicotine patch | Cytisine 1.5 mga | Varenicline 0.3 mg q.d. | Varenicline 1.0 mg q.d. | Varenicline 0.25 mg b.i.d. | Varenicline 0.5 mg b.i.d. | Varenicline 1.0 mg b.i.d. | Bupropion hydrochloride 150 mg b.i.d. | Varenicline 1.0 mg b.i.d. for 52 weeks | |
| 1 | 0.00 | 0.62 | 0.06 | 0.00 | 0.00 | 0.10 | 0.01 | 0.00 | 0.01 | 0.20 |
| 2 | 0.00 | 0.19 | 0.14 | 0.00 | 0.00 | 0.23 | 0.04 | 0.00 | 0.05 | 0.33 |
| 3 | 0.01 | 0.10 | 0.14 | 0.01 | 0.01 | 0.26 | 0.08 | 0.04 | 0.14 | 0.20 |
| 4 | 0.05 | 0.04 | 0.12 | 0.01 | 0.01 | 0.15 | 0.14 | 0.14 | 0.22 | 0.11 |
| 5 | 0.15 | 0.02 | 0.09 | 0.01 | 0.01 | 0.07 | 0.13 | 0.25 | 0.22 | 0.06 |
| 6 | 0.29 | 0.01 | 0.06 | 0.01 | 0.01 | 0.06 | 0.11 | 0.28 | 0.13 | 0.03 |
| 7 | 0.33 | 0.01 | 0.09 | 0.01 | 0.01 | 0.06 | 0.16 | 0.19 | 0.11 | 0.03 |
| 8 | 0.15 | 0.01 | 0.23 | 0.04 | 0.03 | 0.06 | 0.26 | 0.09 | 0.10 | 0.03 |
| 9 | 0.01 | 0.00 | 0.05 | 0.36 | 0.48 | 0.01 | 0.06 | 0.01 | 0.01 | 0.00 |
| 10 | 0.00 | 0.00 | 0.01 | 0.55 | 0.44 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
Strengths and limitations
A strength of the network meta-analysis is that it enabled a comprehensive comparison of all interventions of interest taking into account all available information. Parameters were estimated using a Bayesian framework which allowed for uncertainty in the estimate of the between-study SD and the ability to make probabilistic statements about the rankings of the interventions.
The continuous abstinence and adverse events data were modelled using a complementary log-log link function to allow for variation in the duration of follow-up between studies. This model assumes that the times to event follow an exponential distribution and, hence, that the treatment effect is constant over time. While these are strong assumptions, they are expected to be better than assuming there is no effect of duration of follow-up on the observed event rates.
In practice, the exponential models fitted the data for each outcome measure reasonably well except for the headache data. There was no obvious reason for this in terms of study characteristics, although there were insufficient data to perform a meta-regression.
A sensitivity analysis of the continuous abstinence data, assuming that the repeated 7-day PPA measurement is equivalent to the continuous abstinence measurement, made negligible difference to the results and inferences. The results based on the analysis of the continuous abstinence data only were incorporated into the economic model because the goodness-of-fit of the model suggested that data from some of the studies using repeated 7-day PPA measurement may not belong to the same model.
The estimation of intervention-specific continuous abstinence rates as required for the economic model was calculated by projecting the estimates of treatment effect (i.e. the log-hazard ratio) from the network meta-analysis onto the absolute risk of continuous abstinence for the placebo group. The absolute risk of continuous abstinence for the placebo group was estimated from a separate random-effects meta-analysis of studies in which the control arm was the placebo intervention. The predictive distribution of the absolute risk in a new study was used to represent uncertainty to reflect heterogeneity between studies.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
The search strategy reported in Chapter 3, Methods for reviewing clinical effectiveness, did not identify any recent relevant economic evaluations that were not known to the authors at the time of the submission of the protocol. As such, as detailed in the protocol, the model structure was based on an existing and widely used model, the Benefits of Smoking Cessation on Outcomes (BENESCO) model. 62–72
Independent economic assessment
The economic analysis was focused on a population of smokers in England and Wales aged 18 years or over who are motivated to quit smoking, and explicitly evaluated the cost-effectiveness of a standard 25-day course of cytisine [six 1.5-mg tablets per day for 3 days (days 1–3), five tablets per day for 9 days (days 4–12), four tablets per day for 4 days (days 13–16), three tablets per day for 4 days (days 17–20), and two tablets per day for the final 5 days (days 21–25)]23 with a standard 12-week course of varenicline [500 μg q.d. for 3 days, increased to 500 μg b.i.d. for 4 days, then 1 mg b.i.d. for 11 weeks). 19,73
Methods
The conceptual model
The BENESCO model is a state transition model designed to capture important long-term outcomes of smoking cessation treatments. The BENESCO model has been used in numerous previous evaluations. 63–73
The model uses an annual cycle length and assumes that all smokers die at age 100 years, if death has not been simulated at an earlier age. A hypothetical cohort of 10,000 smokers enters the model, with each smoker assumed to make a single quit attempt, assisted by either varenicline or cytisine. The distribution of the cohort in terms of sex, age (three age categories are used – 18–34 years, 35–64 years and 65–100 years) and chronic smoking-related diseases [lung cancer, chronic obstructive pulmonary disease (COPD), coronary heart disease (CHD) and stroke] is assumed to be representative of smokers in England and Wales.
At the start of the model, every cohort member begins in the smoker state. At the end of the first year, a proportion of smokers successfully cease smoking and become quitters; this proportion is determined by the efficacy of the cessation aid treatment received. The model assumes that no further attempts to quit are made and that those who fail to quit remain smokers until death. However, there is a possibility that quitters may relapse and start smoking again in future years. Potential to relapse to smoking is incorporated into the model as a decreasing function of time since cessation and is independent of cessation treatment (varenicline or cytisine). For the four model cycles following cessation, cohort members are assigned recent quitter status and risk of relapse is highest. After four cycles without relapse, recent quitters attain long-run quitter status. The annual relapse rate is lower for long-run quitters than for recent quitters in the next five cycles and lower still in subsequent cycles, with this underlying relapse rate continuing for the duration of the model.
At the end of each year, the cohort is distributed into different smoking states (smoker, quitter, relapsed smoker) according to their current smoking state and relapse rates. Figure 8 details the possible transitions between smoking states.
FIGURE 8.
Transitions between smoking states.
Within these broad smoking states, cohort members are distributed between the following disease states: no current morbidity, lung cancer, COPD, CHD, stroke and asthma exacerbation. These health states were selected by the authors of the BENESCO model to correspond to the diseases accounting for the greatest morbidity, mortality and cost attributable to smoking. 65 The health states are mutually exclusive and death is an absorbing state. The probability of transition between disease states at the end of each cycle is dependent on current disease state, smoking status, age and sex, as these factors have been shown to be independent determinants of risk.
The model has categorised four of the health states as either acute (CHD and stroke) or chronic (COPD and lung cancer) conditions. Transitions within acute and chronic conditions are not allowed and, therefore, it is not possible for a cohort member to experience a CHD event following a stroke. Transitions from acute disease states to chronic disease states are possible, but not from chronic conditions to acute conditions. Asthma exacerbations were transient in nature and assumed to resolve within 1 year, and could only occur from the no current morbidity health state. Figure 9 illustrates possible transitions between health states in the model.
FIGURE 9.
Transitions between health states.
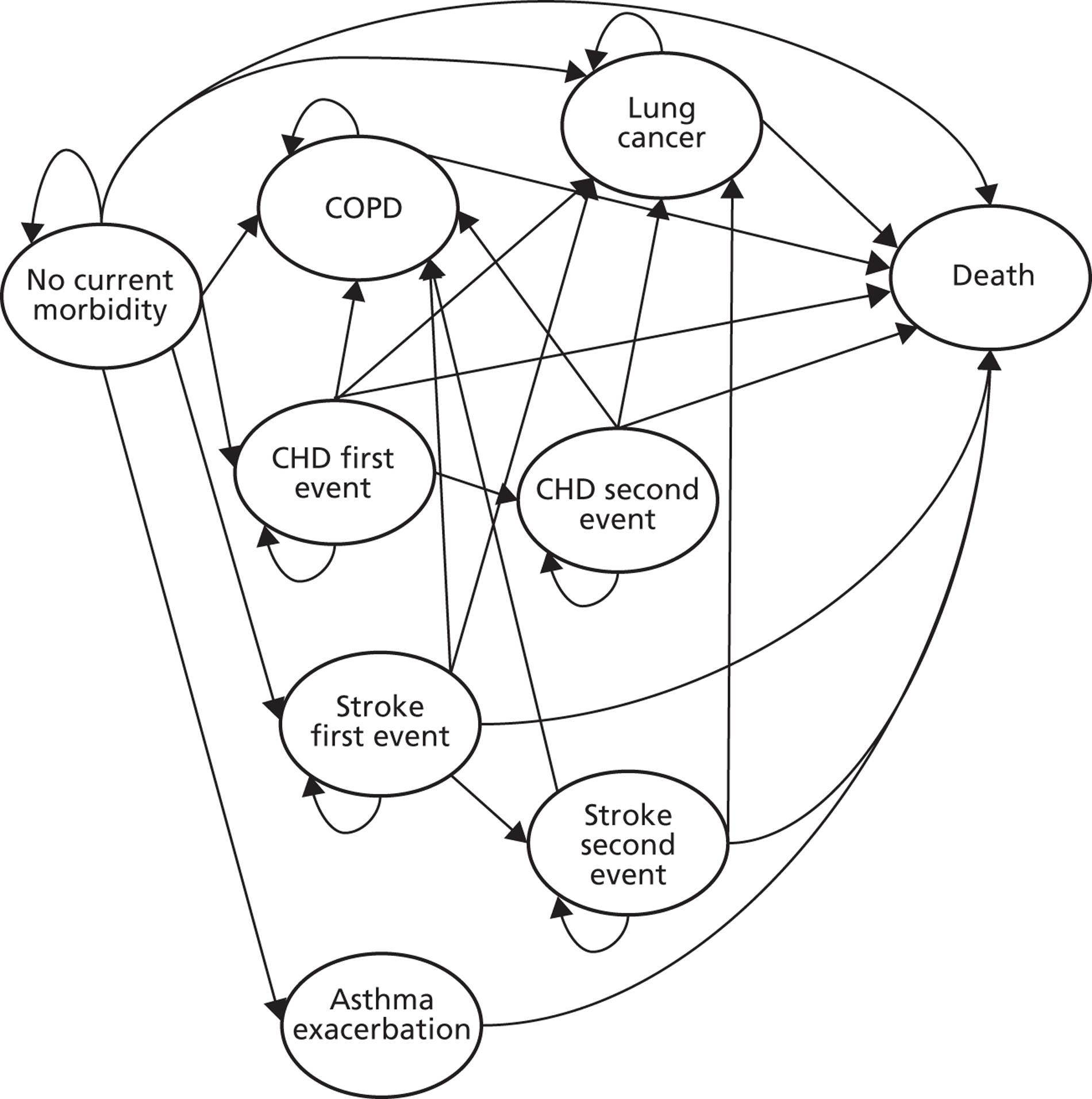
Each health state is associated with utility and cost values as detailed later. Therefore, cohort members accumulate costs and health outcomes each cycle until death. Adverse events are not considered within the BENESCO model framework.
Future costs and benefits were discounted at a rate of 3.5% per annum, and the perspective is that of the UK NHS for costs and health effects on the individual for outcomes, in line with National Institute for Health and Care Excellence (NICE) guidance. 74 Attention now turns to parameter values and distributions used in the probabilistic sensitivity analysis (PSA). As detailed in the protocol, many model inputs are derived from a previous manufacturer’s single technology appraisal (STA) report. 65 As this is a slight limitation, it is unlikely to affect the key conclusions from this report regarding the relative cost-effectiveness of varenicline and cytisine.
The assumed characteristics of the initial cohort
The distribution of the cohort across sex and age categories at the start of the model was designed to reflect the distribution of smokers in the UK. Data on the demographics of the cohort and prevalence and incidence of diseases among smokers and non-smokers are assumed to be equal to those reported by Pfizer. 65 For convenience, these are reproduced in this report together with all-cause mortality risk along with the original source (Table 18). The proportion of male and female adults in each of the three age categories was determined from general population data. 75 Smoking prevalence data76 were applied to these data to calculate the distribution across age and sex groups for a representative sample of 10,000 UK smokers. Pfizer65 used interim life tables calculated by the UK Government Actuary’s Department for 2002–4, weighted by population size and averaged to fit the age categories in the model.
| Data | Original source | Males, 18–34 years | Males, 35–64 years | Males, 65 + years | Females, 18 –34 years | Females, 35–64 years | Females, 65 + years |
|---|---|---|---|---|---|---|---|
| General population (n) | Office for National Statistics 200675 – population trends | 6,727,400 | 11,843,600 | 4,040,000 | 6,660,700 | 12,140,100 | 5,189,300 |
| Smoking prevalence (% of population) | Office for National Statistics 200476 | 36.20% | 27.70% | 12.70% | 28.00% | 28.50% | 26.70% |
| Risk of all-cause mortality (annual probability of all-cause mortality) | Government Actuary’s Department 200677 | 0.09% | 0.47% | 4.88% | 0.04% | 0.30% | 3.87% |
The prevalence of smoking-related diseases in the cohort was estimated by Pfizer65 from data on the prevalence of each disease in the general UK population. Relative risks for the incidence of each disease in the model for smokers were taken from the literature and used to calculate the expected number of cases in the cohort of smokers. 78,79 These data are reproduced in Table 19.
| Data | Original source | Males, 18–34 years | Males, 35–64 years | Males, 65 + years | Females, 18–34 years | Females, 35–64 years | Females, 65 + years |
|---|---|---|---|---|---|---|---|
| COPD | Soriano 200080 | 0.00% | 1.00% | 3.00% | 0.00% | 1.00% | 2.00% |
| Lung cancer | Forman 200381 | 0.00% | 0.10% | 0.70% | 0.00% | 0.06% | 0.24% |
| History of CHD | Office for National Statistics 200582 (GHS) | 0.00% | 1.60% | 8.00% | 0.00% | 1.00% | 5.90% |
| History of stroke | Office for National Statistics 200582 | 0.00% | 0.50% | 3.00% | 0.00% | 0.30% | 2.00% |
| Asthma | Asthma UK 200483; Hoskins 200084 | 6.00% | 5.00% | 6.50% | 6.40% | 5.30% | 5.30% |
Transition probabilities
Annual incidence of disease was estimated by Pfizer,65 divided by age and sex categories, for smokers, recent quitters and long-run quitters. These values relied on estimates from the literature in the majority of cases,82,83,85–87 but for COPD there was a lack of available data and incidence was based on mortality data. 65 Office for National Statistics data were used to estimate stroke incidence and these data provided a split between first event and all events. 82 Tables 20–22 show estimates for smokers, recent and long-run quitters, respectively, along with original data sources. Relative risks for smokers, short-run and recent quitters were generated from the literature78,79 and used to generate absolute probabilities of incidence. As can be seen, the incidence of smoking-related diseases is at least as high in smokers compared with recent quitters and in recent quitters compared with long-run quitters.
| Data | Original source | Males, 18–34 years | Males, 35–64 years | Males, 65 + years | Females, 18–34 years | Females, 35–64 years | Females, 65 + years |
|---|---|---|---|---|---|---|---|
| COPD | Pfizer 200765 | 0.00% | 0.02% | 0.55% | 0.00% | 0.02% | 0.44% |
| Lung cancer | Office for National Statistics 200582 | 0.00% | 0.10% | 1.00% | 0.00% | 0.08% | 0.5% |
| CHD (first non-fatal) | British Heart Foundation 200685 | 0.00% | 0.10% | 1.00% | 0.00% | 0.05% | 0.86% |
| CHD (subsequent non-fatal) | Volmink 199886 | 0.00% | 0.19% | 1.74% | 0.00% | 0.05% | 1.18% |
| Stroke (first non-fatal) | Office for National Statistics 200187 | 0.00% | 0.26% | 0.92% | 0.00% | 0.20% | 0.74% |
| Stroke (subsequent non-fatal) | Office for National Statistics 200187 | 0.00% | 0.35% | 1.55% | 0.00% | 0.28% | 1.33% |
| Asthma exacerbation | Asthma UK 200483 | 0.08% | 0.05% | 0.07% | 0.08% | 0.05% | 0.06% |
| Data | Original source | Males, 18–34 years | Males, 35–64 years | Males, 65 + years | Females, 18–34 years | Females, 35–64 years | Females, 65 + years |
|---|---|---|---|---|---|---|---|
| COPD | Pfizer 200765 | 0.00% | 0.02% | 0.40% | 0.00% | 0.01% | 0.43% |
| Lung cancer | Office for National Statistics 200582 | 0.00% | 0.04% | 0.43% | 0.00% | 0.03% | 0.20% |
| CHD (first non-fatal) | British Heart Foundation 200685 | 0.00% | 0.08% | 0.81% | 0.00% | 0.02% | 0.71% |
| CHD (subsequent non-fatal) | Volmink 199886 | 0.00% | 0.12% | 1.39% | 0.00% | 0.02% | 0.97% |
| Stroke (first non-fatal) | Office for National Statistics 200187 | 0.00% | 0.11% | 0.61% | 0.00% | 0.08% | 0.55% |
| Stroke (subsequent non-fatal) | Office for National Statistics 200187 | 0.00% | 0.14% | 1.03% | 0.00% | 0.11% | 1.00% |
| Asthma exacerbation | Asthma UK 200483 | 0.05% | 0.05% | 0.06% | 0.06% | 0.05% | 0.06% |
| Data | Original source | Males, 18–34 years | Males, 35–64 years | Males, 65 + years | Females, 18–34 years | Females, 35–64 years | Females, 65 + years |
|---|---|---|---|---|---|---|---|
| COPD | Pfizer 200765 | 0.00% | 0.02% | 0.05% | 0.00% | 0.00% | 0.04% |
| Lung cancer | Office for National Statistics 200582 | 0.00% | 0.04% | 0.43% | 0.00% | 0.03% | 0.20% |
| CHD (first non-fatal) | British Heart Foundation 200685 | 0.00% | 0.05% | 0.68% | 0.00% | 0.01% | 0.50% |
| CHD (subsequent non-fatal) | Volmink 199886 | 0.00% | 0.07% | 1.16% | 0.00% | 0.02% | 0.69% |
| Stroke (first non-fatal) | Office for National Statistics 200187 | 0.00% | 0.11% | 0.61% | 0.00% | 0.05% | 0.46% |
| Stroke (subsequent non-fatal) | Office for National Statistics 200187 | 0.00% | 0.0014% | 0.010% | 0.00% | 0.0007% | 0.0083% |
| Asthma exacerbation | Asthma UK 200483 | 0.05% | 0.05% | 0.06% | 0.06% | 0.05% | 0.05% |
Annual mortality probability by condition was estimated by Pfizer65 for smokers, recent quitters and long-run quitters, by age- and sex-specific category. Mortality associated with asthma exacerbation was assumed to equal all-cause mortality (see Table 18). Mortality for chronic diseases, COPD and lung cancer is the probability of death from these diseases given the disease state is present. Mortality from acute events, CHD and stroke is the probability of a fatal event that differs by smoking status, age and sex. Tables 23–25 show disease-specific mortality estimates for smokers, recent quitters and long-run quitters, respectively, as reported by the manufacturer’s submission for the NICE varenicline STA,65 along with the original data sources. Relative risks of mortality for smokers, recent quitters and long-run quitters were generated from the literature. 78,79 The probability of smoking-related mortality is equivalent or lower for recent quitters compared with smokers, and for long-run quitters relative to recent quitters.
| Data | Original source | Males, 18–34 years | Males, 35–64 years | Males, 65 + years | Females, 18–34 years | Females, 35–64 years | Females, 65 + years |
|---|---|---|---|---|---|---|---|
| COPD | Office for National Statistics 200675 | 0.00% | 0.98% | 10.12% | 0.00% | 0.70% | 9.16% |
| Lung cancer | Office for National Statistics 200675 | 0.00% | 26.89% | 47.69% | 0.00% | 40.48% | 75.35% |
| CHD (first event fatal) | British Heart Foundation 200685 | 0.00% | 0.10% | 0.81% | 0.00% | 0.04% | 0.69% |
| CHD (subsequent event fatal) | Office for National Statistics 200675 | 0.00% | 0.15% | 1.39% | 0.00% | 0.04% | 0.94% |
| Stroke (first event fatal) | Office for National Statistics 200675 | 0.00% | 0.02% | 0.30% | 0.00% | 0.02% | 0.38% |
| Stroke (subsequent event fatal) | Office for National Statistics 200675 | 0.00% | 0.03% | 0.50% | 0.00% | 0.03% | 0.56% |
| Data | Original source | Males, 18–34 years | Males, 35–64 years | Males, 65 + years | Females, 18–34 years | Females, 35–64 years | Females, 65 + years |
|---|---|---|---|---|---|---|---|
| COPD | Office for National Statistics 200675 | 0.00% | 0.98% | 10.12% | 0.00% | 0.70% | 9.16% |
| Lung cancer | Office for National Statistics 200675 | 0.00% | 26.89% | 47.69% | 0.00% | 40.48% | 75.35% |
| CHD (first event fatal) | British Heart Foundation 200685 | 0.00% | 0.06% | 0.65% | 0.00% | 0.02% | 0.56% |
| CHD (subsequent event fatal) | Office for National Statistics 200675 | 0.00% | 0.09% | 1.12% | 0.00% | 0.02% | 0.78% |
| Stroke (first event fatal) | Office for National Statistics 200675 | 0.00% | 0.01% | 0.20% | 0.00% | 0.01% | 0.28% |
| Stroke (subsequent event fatal) | Office for National Statistics 200675 | 0.00% | 0.01% | 0.33% | 0.00% | 0.01% | 0.42% |
| Data | Original source | Males, 18–34 years | Males, 35–64 years | Males, 65 + years | Females, 18–34 years | Females, 35–64 years | Females, 65 + years |
|---|---|---|---|---|---|---|---|
| COPD | Office for National Statistics 200675 | 0.00% | 0.98% | 10.12% | 0.00% | 0.70% | 9.16% |
| Lung cancer | Office for National Statistics 200675 | 0.00% | 26.89% | 47.69% | 0.00% | 40.48% | 75.35% |
| CHD (first event fatal) | British Heart Foundation 200685 | 0.00% | 0.04% | 0.54% | 0.00% | 0.01% | 0.40% |
| CHD (subsequent event fatal) | Office for National Statistics 200675 | 0.00% | 0.06% | 0.93% | 0.00% | 0.01% | 0.55% |
| Stroke (first event fatal) | Office for National Statistics 200675 | 0.00% | 0.01% | 0.20% | 0.00% | 0.01% | 0.24% |
| Stroke (subsequent event fatal) | Office for National Statistics 200675 | 0.00% | 0.01% | 0.33% | 0.00% | 0.01% | 0.35% |
Relapse rates
In the previous manufacturer’s submission for the NICE varenicline STA,65 the annual probability of relapse to smoking for the first 5 years following cessation was calculated from a longitudinal US 4-year follow-up study of a health improvement initiative in the workplace (n = 1143). 88 The probability used was criticised by the evidence review group, as it was incorrectly derived from baseline length of abstinence data. 89 Although it was possible to estimate annual probability of relapse from this study, using follow-up data for the subsample of participants who had been abstinent for 1–2 years at baseline, this subsample comprises only 79 participants.
A more recent study has used British Household Panel Survey data to analyse relapse to smoking (n = 1578). 90 The article shows numbers of previous smokers relapsing who reported cessation for a minimum of 1 year up to 10 years. These data were used to calculate the annual relapse probability for short-run quitters (< 5 years since quit) and a proportion of long-run quitters (> 5 years but < 10 years post-quit). Data on annual relapse probability 10 or more years post cessation are scarce and, in the absence of more robust data, the same data (as used by Pfizer65 ) were employed here. 91
Table 26 shows the probabilities of relapse that were used in the model. The probability of relapse in the first 10 years post 1 year of cessation is higher than estimates used in some previous models,62,63,65,66,69 but is in line with other research which suggests that around half of those abstinent at 1 year will relapse to smoking in the next 7 years. 92,93 The annual probability of relapse after 10 years of abstinence was assumed to be 1% in the STA submission and several other applications of the BENESCO model,62,66,69 all of which based their estimate on a longitudinal study. 91 The authors of this longitudinal study report that ‘the (annual) rate of smoking relapse . . . fell to less than 1% after 10 years of abstinence’. Using the data reported by Krall et al. ,91 the annual probability of relapse is much lower than 1%. Uncertainty around relapse rates is modelled in this current report as a beta distribution, using event data from the original studies. 90,91
| Data | Original source | Mean probability (3 SF) | 95% CI (3 SF) | Distribution over relapse category time perioda |
|---|---|---|---|---|
| Annual relapse probability, > 1 and < 5 years post cessation (time period 4 years) | Hawkins 201090 | 0.129 | 0.117 to 0.141 | β(395–535) |
| Annual relapse probability, ≥ 5 and < 10 years post cessation (time period 5 years) | Hawkins 201090 | 0.0331 | 0.0230 to 0.0452 | β(33–180) |
| Annual relapse probability, > 10 years post cessation (time period 26 years) | Krall 200291 | 0.00112 | 0.000402 to 0.00153 | β(9–390) |
Costs
Costs included in the model were costs relevant to disease states and intervention costs. The mean costs for COPD, CHD and asthma are those reported in Hind et al. 89 The source for COPD cost is the average direct cost of treatment, weighted by severity, taken from a study estimating burden of disease in the UK. 94 The annual cost of lung cancer was taken from a NICE rapid review,95 sourced from a UK epidemiology study. 96 The annual patient cost for CHD is an estimate of the aggregate cost of CHD to the NHS,97 divided by estimated prevalence. The cost of asthma exacerbations represented a mixture of the estimated cost of an accident and emergency (A&E) attendance and NHS reference cost of inpatient attendance, with the ratio of A&E to inpatient admissions estimate taken from Hoskins et al. 84 Costs for stroke were taken from a recent National Institute for Health Research (NIHR) commissioned technology assessment report,98 and incorporates the one-off and ongoing costs of stroke in addition to the reported difference in costs and prevalence of dependent and independent patient states following a stroke incident. 99 All costs have been adjusted for inflation to 2010/11 prices. 100
Uncertainty around cost estimates were incorporated into the probabilistic analysis. In the absence of data, the standard errors for COPD, lung cancer, CHD and asthma exacerbation were assumed to be 10% of the mean estimate. These data were assumed to follow a gamma distribution, as is common practice for cost data. 101 Confidence intervals around costs following stroke events were reported in Simpson et al. 98 and informed the uncertainty around mean costs for stroke, which was assumed to fit a normal distribution. Table 27 reports the source, summary estimates and distributions used for the disease state costs employed in the model.
| Data | Original source | Mean cost (£) | 95% CrI | Distributiona |
|---|---|---|---|---|
| COPD | Britton 200394 | 971.31 | 780.93 to 1161.69 | Gamma (100, 9.71) |
| Lung cancer | Sanderson 200496 | 6524.02 | 5245.31 to 7802.72 | Gamma (100, 65.24) |
| CHD (non-fatal event) | McMurray 199397 | 1162.50 | 934.45 to 1390.05 | Gamma (100, 11.62) |
| Stroke (non- fatal event) | Simpson 201198 | 5484.31 | 4996.99 to 5970.85 | 0.741 × [normal(576.51,15.74) + normal(3398.40,175.83)] + 0.259 × [normal(3010.17,66.21) + normal(6792.55,345.70)] |
| Asthma exacerbation | Hoskins 200084 | 1162.25 | 846.73 to 1259.56 | Gamma (100, 10.53) |
Intervention costs comprised the cost of the drug regimen. Costs of brief counselling and support of a health professional are also likely to occur but were not likely to differ between drug treatments, thus not impacting relative cost–utility, and were not included in the economic analysis. For the comparator intervention, standard treatment with varenicline, BNF data on dosage and pricing are used. 19 The cost of treatment is the cost of a starter pack covering the first 2 weeks of tapered treatment (£27.30) plus the cost of 10 weeks at full dose (5 × £27.30), £163.80 in total. The cost of cytisine treatment within a UK setting is not determined. The manufacturers of cytisine were contacted by the research team, but no reply was received. In the absence of firm evidence, it is strongly suspected that a course of cytisine will be significantly cheaper than a standard course of varenicline. 23,102 A previous model of the costs and effects of cytisine for smoking cessation assumed treatment costs to be US$10 per smoker. 102 It is possible to buy Tabex (active ingredient cytisine) online in the UK for £16.79 for 100 1.5-mg tablets,103 which represents approximately a standard course, and this cost is used in the model. Table 28 shows the treatment costs used in the model.
| Data | Original source | Total cost (£) |
|---|---|---|
| Cytisine treatment cost | Assumption | 16.79 |
| Varenicline treatment cost | BNF 201219 | 163.80 |
Utilities associated with health states
Baseline utility for smokers with no current comorbidity was taken from the general population utility profile estimated by Ara and Brazier using Health Survey for England (HSE) data. 104 These data are a function of age and sex. Disease-specific utility values for smoking-related diseases are the same as reported by the manufacturer submission team. 65 For lung cancer utility,105 asthma exacerbation utility106 and a second non-fatal stroke event utility,107 a utility multiplier associated with the disease was estimated by comparing the reported utility value with the expected value for a person of the same age within the general population, assuming that age-specific values from the UK were applicable for all populations. The average ages of the samples from which utility values were drawn were 62 years, 49 years and 65 years for lung cancer, asthma exacerbations and a second non-fatal stroke respectively. The mean ages of the population for which the utilities were provided for a first non-fatal stroke event,108 COPD109 and following any CHD event110 were not reported. For these disease states, an average age of 60 years is assumed with the sensitivity of the results to this assumption is explored by altering baseline utility estimates for these diseases to correspond to ages 50 and 70 years respectively.
Disease state utility was determined using a multiplicative approach, i.e. baseline utility is multiplied by an estimate of the impact of the disease. Thus, a male aged 40 years with lung cancer would have an estimated utility of 0.44 (0.88 × 0.50). Table 29 displays the mean utility values for health states in the model.
| Health state | Utility source | Mean age (years) | Mean utility |
|---|---|---|---|
| NCM, males, 18–34 years | Ara 2010104 | 26.5 | 0.94 |
| NCM, males, 35–64 years | Ara 2010104 | 49 | 0.88 |
| NCM, males, 65–100 years | Ara 2010104 | 82.5 | 0.72 |
| NCM, females, 18–34 years | Ara 2010104 | 26.5 | 0.92 |
| NCM, females, 35–64 years | Ara 2010104 | 49 | 0.86 |
| NCM, females, 65–100 years | Ara 2010104 | 82.5 | 0.70 |
| Lung cancer | Trippoli 2001105 | 62 | 0.50 |
| COPD | Spencer 2005109 | 60 | 0.63 |
| CHD | Hay 2005110 | 60 | 0.63 |
| Stroke (first event) | Tengs 2003108 | 60 | 0.62 |
| Stroke (second event) | Gage 1998107 | 65 | 0.12 |
| Asthma exacerbation | Szende 2004106 | 49 | 0.45 |
Uncertainty around utility estimates is explored in the probabilistic analysis. Normally distributed error terms from ordinary least squares regressions used to predict baseline utility by Ara and Brazier104 represent uncertainty around utility inputs and are used to explore uncertainty in model outputs as part of the PSA. Uncertainty in the values reported for each health state was not considered and, therefore, the true uncertainty will be underestimated.
Intervention effectiveness
The absolute probabilities of cessation at 1 year for interventions were generated by combining the results of the network meta-analysis with an estimate of the placebo response, as described in Chapter 3. The median and mean probability of 1-year continuous abstinence for cytisine and varenicline and 95% CrIs are shown in Table 30. The absolute quit rates for cytisine appear low (see Table 4). However, when the rates of abstinence shown in Table 5 are analysed, a quit rate of 33% for varenicline is reasonable. The greater relative effectiveness for cytisine against placebo compared with varenicline against placebo results in the 45% quit rate. The wide CrIs are reflective of uncertainty around the baseline (placebo) effect. There is much less uncertainty about the treatment effects and the order of the clinical effectiveness of the two treatment comparators. The probability that cytisine 1.5 mg was the most effective treatment of the eight compared in the meta-analysis was 0.86, as shown in Table 3 (see Chapter 3). When only cytisine 1.5 mg and varenicline 1 mg b.i.d. are compared, the probability that cytisine is the most clinically effective treatment is estimated to be 0.90. The 95% CrI around the difference between clinical effectiveness of the interventions (probability of quit with cytisine minus probability of quit with varenicline) includes zero (95% CrI –0.048 to 0.389).
| 1-year continuous abstinence probability | Median | Mean | 95% CrI |
|---|---|---|---|
| Cytisine | 0.394 | 0.449 | 0.040 to 0.998 |
| Varenicline | 0.257 | 0.330 | 0.026 to 0.958 |
Discussion of key assumptions
The modelling approach involves several assumptions, as noted throughout Chapter 4, Independent economic assessment. A key assumption implicit in the model is that cohort members can only quit after treatment for smoking cessation, within the first model cycle, and at no other point until death. In reality, smokers who are willing to quit but fail during one attempt will have a probability of successfully quitting at a later stage in their lives. This assumption is likely to favour interventions with greater efficacy. If the 1-year probability of cessation is significantly higher for one treatment than another, that treatment will have greater health outcomes across the cohort over the lifetime horizon. This assumption is a feature of all previous applications of the BENESCO model. 62–72
The economic model has relied, in part, on input data from a previous manufacturer’s submission for the NICE varenicline STA,65 as set out in the protocol. It is not known if these inputs are the best available as (i) at least 5 years have elapsed since these data were identified and (ii) identification of input studies was not always clearly reported. The majority of cost, utility and relapse data were from the UK, but a proportion of these data were from non-UK studies. 78,79,91 The model assumes transferability of these data to a UK NHS setting. Additionally, the model assumes treatments are not associated with adverse events. This assumption is justified on the basis of the finding of no significant difference in SAEs between the two treatments (see Chapter 3), but may favour the varenicline treatment strategy.
Analysis of uncertainty
The uncertainty around key parameter estimates was modelled by the use of probability distributions which allowed PSA to be undertaken. Ten thousand draws from distributions of treatment effectiveness, health state utility, disease costs and relapse probabilities were used as model inputs. Furthermore, univariate sensitivity analysis was performed to ascertain the key drivers of model outputs.
Value of information analyses was undertaken to establish whether or not a direct head-to-head trial of cytisine compared with varenicline might represent a cost-effective use of resources. The methodological plan was to undertake the analysis in three stages. The first stage involved the calculation of the expected value of perfect information (EVPI). 111 If the value produced appeared to be greater than the cost for which a RCT comparing the efficacies of the two interventions could be undertaken, then the second stage would be performed.
The second stage would estimate the expected value of partial perfect information (EVPPI)112 jointly on the efficacies of varenicline and cytisine. If the value produced appeared to be above the cost for which a RCT comparing the efficacies of the two interventions could be undertaken, then the third stage would be performed.
The third stage involves the calculation of the EVSI. 113 This value explicitly evaluates the potential inaccuracy associated with trials of smaller sizes, contrasting with EVPPI, which assumes that the information is perfect and, thus, in essence, is derived from a trial of infinite size.
Results
Mean costs and mean treatment effects associated with each treatment
The results of the PSA are presented as the primary results of interest, as, unlike deterministic estimates, they take into account the distributions of input parameters and interaction between parameters and, therefore, are the more accurate estimates. Table 31 shows the primary results of the PSA analysis: per smoker total discounted costs, life-years (LYs) and quality-adjusted life-years (QALYs) for the two treatments. Cytisine is expected to be less costly and more effective than varenicline and, so, can be said to dominate varenicline based on the expected values.
| Treatment | Costs | LYs | QALYs | |||
|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | Total | Incremental | |
| Cytisine | £4973 | –£251 | 17.53 | 0.03 | 14.38 | 0.03 |
| Varenicline | £5225 | – | 17.50 | – | 14.35 | – |
Figure 10 presents the cost-effectiveness acceptability curve114 for the two treatments. At any threshold of willingness to pay, up to £100,000 per QALY gained, cytisine was the optimal intervention in over 90% of the simulations within the PSA. This reflects the higher costs associated with varenicline treatment. As the willingness to pay increases, the probability that cytisine is preferable falls and the likelihood that varenicline is optimal rises. Given that cytisine was estimated to be the more effective treatment in 90% of simulations, the value for cytisine on the cost-effectiveness acceptability curve will asymptote at 90%.
FIGURE 10.
Cost-effectiveness acceptability curves for cytisine and varenicline.
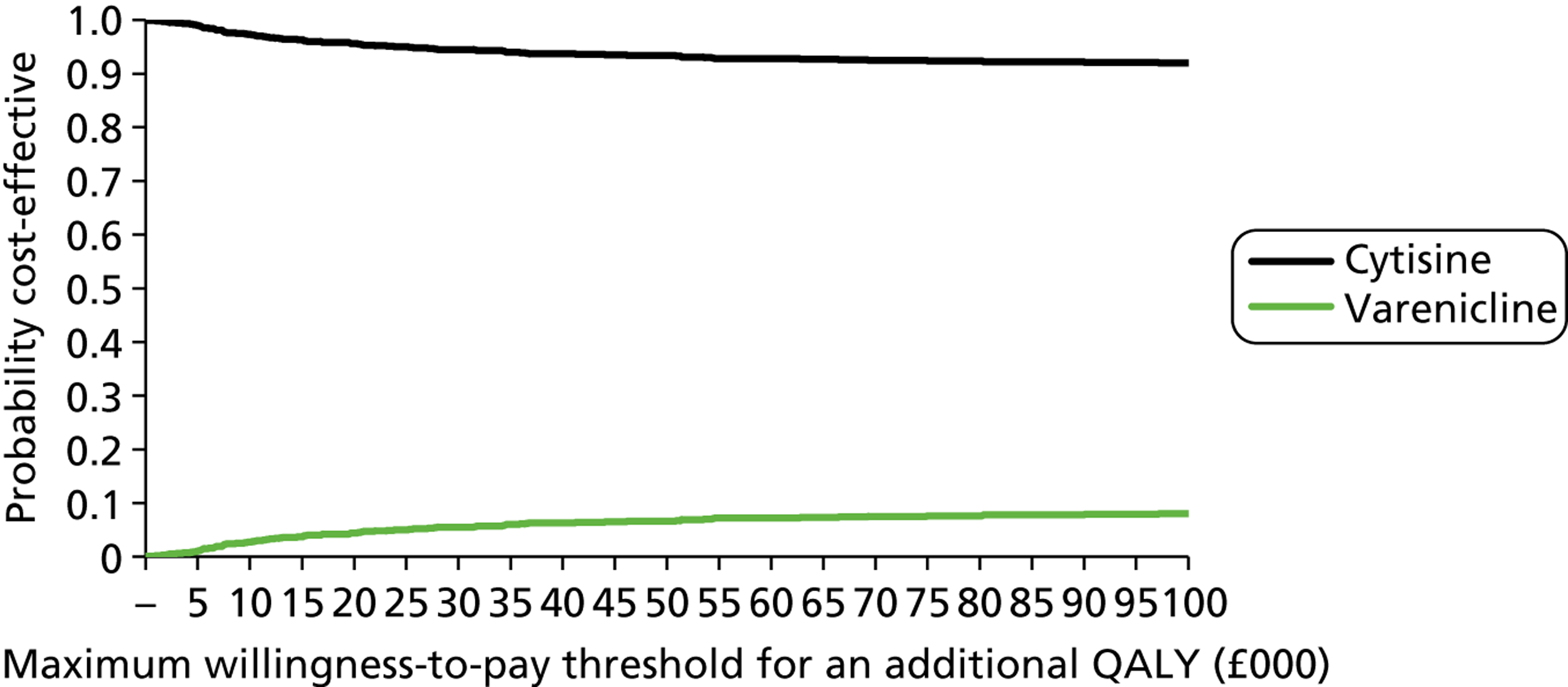
Univariate sensitivity analyses
Table 32 details the results from the univariate sensitivity analyses. In all the analyses, bar one, the conclusion that cytisine dominates varenicline is upheld. The exception was in altering the relative efficacies of varenicline and cytisine. This analysis was operationalised by ranking the output from the network meta-analysis based on the value in the differences of the absolute quit rates between cytisine and varenicline and using the 2.5th and 97.5th percentile. When using the value most favourable to varenicline, an additional 0.01 QALYs at an additional cost of £87, resulting in a cost per QALY gained of just under £6700, would be typically seen as cost-effective under typical NICE thresholds. 74
| Variable | Sensitivity analysis | Treatment | Costs | LYs | QALYs | |||
|---|---|---|---|---|---|---|---|---|
| Total | Incremental | Total | Incremental | Total | Incremental | |||
| Baseline | – | Cytisine | £4972 | –£256 | 17.53 | 0.04 | 14.38 | 0.03 |
| Varenicline | £5228 | – | 17.49 | – | 14.35 | – | ||
| COPD utility | Assumed age of study sample 50 years | Cytisine | £4972 | –£256 | 17.53 | 0.04 | 14.39 | 0.03 |
| Varenicline | £5224 | – | 17.49 | – | 14.36 | – | ||
| Assumed age of study sample 70 years | Cytisine | £4972 | –£256 | 17.53 | 0.04 | 14.37 | 0.03 | |
| Varenicline | £5228 | – | 17.49 | – | 14.33 | – | ||
| CHD utility | Assumed age of study sample 50 years | Cytisine | £4972 | –£256 | 17.53 | 0.04 | 14.40 | 0.03 |
| Varenicline | £5228 | – | 17.49 | – | 14.37 | – | ||
| Assumed age of study sample 70 years | Cytisine | £4972 | –£256 | 17.53 | 0.04 | 14.36 | 0.03 | |
| Varenicline | £5228 | – | 17.49 | – | 14.32 | – | ||
| Stroke first event utility | Assumed age of study sample 50 years | Cytisine | £4972 | –£256 | 17.53 | 0.04 | 14.40 | 0.03 |
| Varenicline | £5228 | – | 17.49 | – | 14.36 | – | ||
| Assumed age of study sample 70 years | Cytisine | £4972 | –£256 | 17.53 | 0.04 | 14.36 | 0.03 | |
| Varenicline | £5228 | – | 17.49 | – | 14.30 | – | ||
| Cytisine cost | Double | Cytisine | £4989 | –£239 | 17.53 | 0.04 | 14.38 | 0.03 |
| Varenicline | £5228 | – | 17.49 | – | 14.35 | – | ||
| COPD cost | Upper 95% CI value | Cytisine | £5053 | –£240 | 17.53 | 0.04 | 14.38 | 0.03 |
| Varenicline | £5293 | – | 17.49 | – | 14.35 | – | ||
| Lower 95% CI value | Cytisine | £4925 | –£238 | 17.53 | 0.04 | 14.38 | 0.03 | |
| Varenicline | £5163 | – | 17.49 | – | 14.35 | – | ||
| Lung cancer cost | Upper 95% CI value | Cytisine | £5078 | –£242 | 17.53 | 0.04 | 14.38 | 0.03 |
| Varenicline | £5321 | – | 17.49 | – | 14.35 | – | ||
| Lower 95% CI value | Cytisine | £4899 | –£236 | 17.53 | 0.04 | 14.38 | 0.03 | |
| Varenicline | £5135 | – | 17.49 | – | 14.35 | – | ||
| CHD event cost | Upper 95% CI value | Cytisine | £5142 | –£240 | 17.53 | 0.04 | 14.38 | 0.03 |
| Varenicline | £5382 | – | 17.49 | – | 14.35 | – | ||
| Lower 95% CI value | Cytisine | £4836 | –£238 | 17.53 | 0.04 | 14.38 | 0.03 | |
| Varenicline | £5074 | – | 17.49 | – | 14.35 | – | ||
| Stroke event cost | Upper 95% CI value | Cytisine | £5289 | –£246 | 17.53 | 0.04 | 14.38 | 0.03 |
| Varenicline | £5535 | – | 17.49 | – | 14.35 | – | ||
| Lower 95% CI value | Cytisine | £4688 | –£231 | 17.53 | 0.04 | 14.38 | 0.03 | |
| Varenicline | £4920 | – | 17.49 | – | 14.35 | – | ||
| Asthma event cost | Upper 95% CI value | Cytisine | £4991 | –£239 | 17.53 | 0.04 | 14.38 | 0.03 |
| Varenicline | £5230 | – | 17.49 | – | 14.35 | – | ||
| Lower 95% CI value | Cytisine | £4987 | –£239 | 17.53 | 0.04 | 14.38 | 0.03 | |
| Varenicline | £5226 | – | 17.49 | – | 14.35 | – | ||
| Relapse probability 1–4 years | Upper 95% CI value | Cytisine | £5008 | –£234 | 17.52 | 0.03 | 14.37 | 0.03 |
| Varenicline | £5242 | – | 17.49 | – | 14.34 | – | ||
| Lower 95% CI value | Cytisine | £4970 | –£244 | 17.54 | 0.04 | 14.38 | 0.03 | |
| Varenicline | £5214 | – | 17.50 | – | 14.35 | – | ||
| Relapse probability 5–9 years | Upper 95% CI value | Cytisine | £5005 | –£234 | 17.52 | 0.03 | 14.37 | 0.03 |
| Varenicline | £5240 | – | 17.49 | – | 14.34 | – | ||
| Lower 95% CI value | Cytisine | £4975 | –£243 | 17.53 | 0.04 | 14.38 | 0.03 | |
| Varenicline | £5218 | – | 17.50 | – | 14.35 | – | ||
| Relapse probability 10 + years | Upper 95% CI value | Cytisine | £4991 | –£239 | 17.53 | 0.03 | 14.38 | 0.03 |
| Varenicline | £5229 | – | 17.49 | – | 14.35 | – | ||
| Lower 95% CI value | Cytisine | £4988 | –£240 | 17.53 | 0.04 | 14.38 | 0.03 | |
| Varenicline | £5227 | – | 17.49 | – | 14.35 | – | ||
| Difference between treatment effectiveness (cytisine minus varenicline) | Upper 95% CrI value | Cytisine | £4760 | –£487 | 17.60 | 0.12 | 14.45 | 0.11 |
| Varenicline | £5246 | – | 17.49 | – | 14.34 | – | ||
| Lower 95% CrI value | Cytisine | £5223 | –£87 | 17.45 | –0.01 | 14.31 | –0.01 | |
| Varenicline | £5309 | – | 17.47 | – | 14.32 | – | ||
The assumed treatment cost for cytisine is lower than that for varenicline, but the cytisine cost estimate if adopted for use within the NHS is uncertain. In a threshold analysis it was estimated that the price of the cytisine regimen would have to rise to over £250 (from an estimate of £16.79, a greater than 14-fold rise) for the total expected lifetime cost with cytisine treatment to equal the total expected lifetime cost with varenicline treatment.
Calculation of the expected value of perfect information
Expected value of perfect information is defined as the value of eliminating all uncertainty around the adoption decision. The value is determined by both (i) the probability that a wrong adoption decision will be made and (ii) the costs of forgoing the optimal treatment strategy.
In order to calculate the EVPI, an estimate of the number of people affected using more accurate information was required. A recent Office for National Statistics report estimated that 21% of the UK adult population smoke, around 10 million people, and the same report found that 63% of smokers want to quit smoking. 115 If even half of those with a desire to quit attempt assisted cessation, while the choice between cytisine and varenicline is relevant, the adoption decision will affect more than 3 million UK smokers. Elsewhere, it has been estimated that 800,000 smokers currently access stop smoking services in England each year,116 supporting the notion that 3 million smokers could be affected in England and Wales.
Analysis of US data from the 2003 Tobacco Use Cessation Supplement to the Current Population Survey found that, of those attempting to quit (43.5% of all smokers), one-third (32.2%) used medication. 117 This figure was lower in the UK at the turn of the century, but increasing as NRT and bupropion hydrochloride became available on prescription. 118 However, a study into the reasons smokers shy away from medications suggests that perceived clinical effectiveness has lessened use of smoking cessation drugs in the past. 119 The high efficacy of the dopamine inhibitors cytisine and varenicline, in comparison with NRT, will probably attenuate this effect. Ease of access has also been cited as a factor. 119 This all suggests that with a focus on implementation in UK stop smoking services, to overcome these barriers, the proportion of quit attempts assisted by medication could rise significantly in the next 10 years. The figure of 3 million affected smokers is considered reasonable, but the EVPI was also calculated with the assumption of 1 million smokers affected.
The incremental net benefit (INB) of cytisine compared with varenicline was calculated per smoker for each of the PSA runs for willingness-to-pay thresholds for an additional QALY of £20,000 and £30,000. In over 90% of PSA runs the INB was positive, indicating that varenicline was not cost-effective. However, in the remainder of the PSA runs the value was negative, indicating that varenicline was cost-effective. The maximum INB was calculated as the sum of all positive INB, divided by the number of PSA runs (10,000). The expected INB was calculated as the sum of all INB, divided by the number of PSA runs. The EVPI was calculated as the difference between maximum INB and expected INB. This value was £12 per smoker assuming a willingness to pay of £20,000 per QALY gained and £21 per smoker assuming a willingness to pay of £30,000 per QALY.
Although these are small EVPI values per person, the value becomes much greater when multiplied by 3 million to represent the likely population affected by the decision, resulting in EVPI values of £35M and £63M at a willingness-to-pay level of £20,000 and £30,000 per QALY respectively. Even with a conservative value of only 1 million smokers affected by the decision and with willingness to pay £20,000 for an additional QALY, the EVPI was over £11M.
The EVPI is far greater than the potential cost of a head-to-head trial comparing cytisine and varenicline, and, so, according to the protocol, the second stage of the value of information analysis calculating the EVPPI was necessary.
Expected value of partial perfect information analyses
Rather than use a traditional two-loop procedure for calculating the EVPPI112 a novel method was employed that allows the computational time to be markedly reduced. This method has been shown to replicate the EVPPI in examples where an analytical solution existed; a manuscript describing the methodology is currently under consideration for publication in the peer-reviewed journal Medical Decision Making.
The results of key parameters are shown in Table 33 assuming a willingness to pay of £20,000 per QALY. The EVPPI on the HR of varenicline compared with cytisine, which would be the information garnered from the direct head-to-head trial of the two interventions, was estimated to be £33.6M. The EVPPI of conducting a trial of each intervention against placebo was additionally estimated showing that the bulk of the uncertainty existed regarding cytisine efficacy rather than varenicline efficacy (EVPPIs of £25.3M and £0.4M respectively). Table 34 replicates this analysis assuming a threshold of £30,000 per QALY.
| Parameter | EVPPI (£M) |
|---|---|
| HR of cytisine vs. varenicline | 33.6 |
| HR of cytisine vs. placebo | 25.3 |
| HR of varenicline vs. placebo | 0.4 |
| Parameter | EVPPI (£M) |
|---|---|
| HR of cytisine vs. varenicline | 58.6 |
| HR of cytisine vs. placebo | 46.7 |
| HR of varenicline vs. placebo | 0.7 |
The EVPPI associated with the HR of cytisine and varenicline were large, in excess of £33M. This was deemed sufficient to fund a trial and, therefore, EVSI analyses were conducted.
Expected value of sample information analyses
Similarly to EVPPI, a novel method was undertaken that allowed the computational time required to be markedly reduced. Within this method the posterior expectation for the INB conditional on each new simulated trial data were approximated using approximate Bayesian computation. The method produced results comparable to those present by Ades et al. 113 in the pivotal methodological paper. A manuscript is current being prepared for submission to a peer-reviewed journal.
Within the EVSI calculation it was assumed that 3 million people would benefit from the increased information regarding the relative efficacies of cytisine and varenicline. The EVSI for a year-long trial that directly compares varenicline 1.0 mg b.i.d. with cytisine 1.5 mg was estimated using trial sample sizes ranging from 50 to 20,000 participants per arm, and at willingness-to-pay values of £20,000 and £30,000. The net value of the RCT was estimated assuming that to enrol a person in a RCT was £1000, as previously used by Stevenson et al. 120 This paper stated that ‘although in reality there will be fixed costs and some form of economies of scale to be exploited, this value appears a reasonable approximation to the costs of successfully funded bids in the United Kingdom’.
A graphical depiction of the results of the EVSI analyses are shown in Figure 11 (when a threshold of £20,000 per QALY is used) and Figure 12 (when a value of £30,000 per QALY is used).
FIGURE 11.
Results from the EVSI analyses assuming a willingness to pay of £20,000 per QALY.
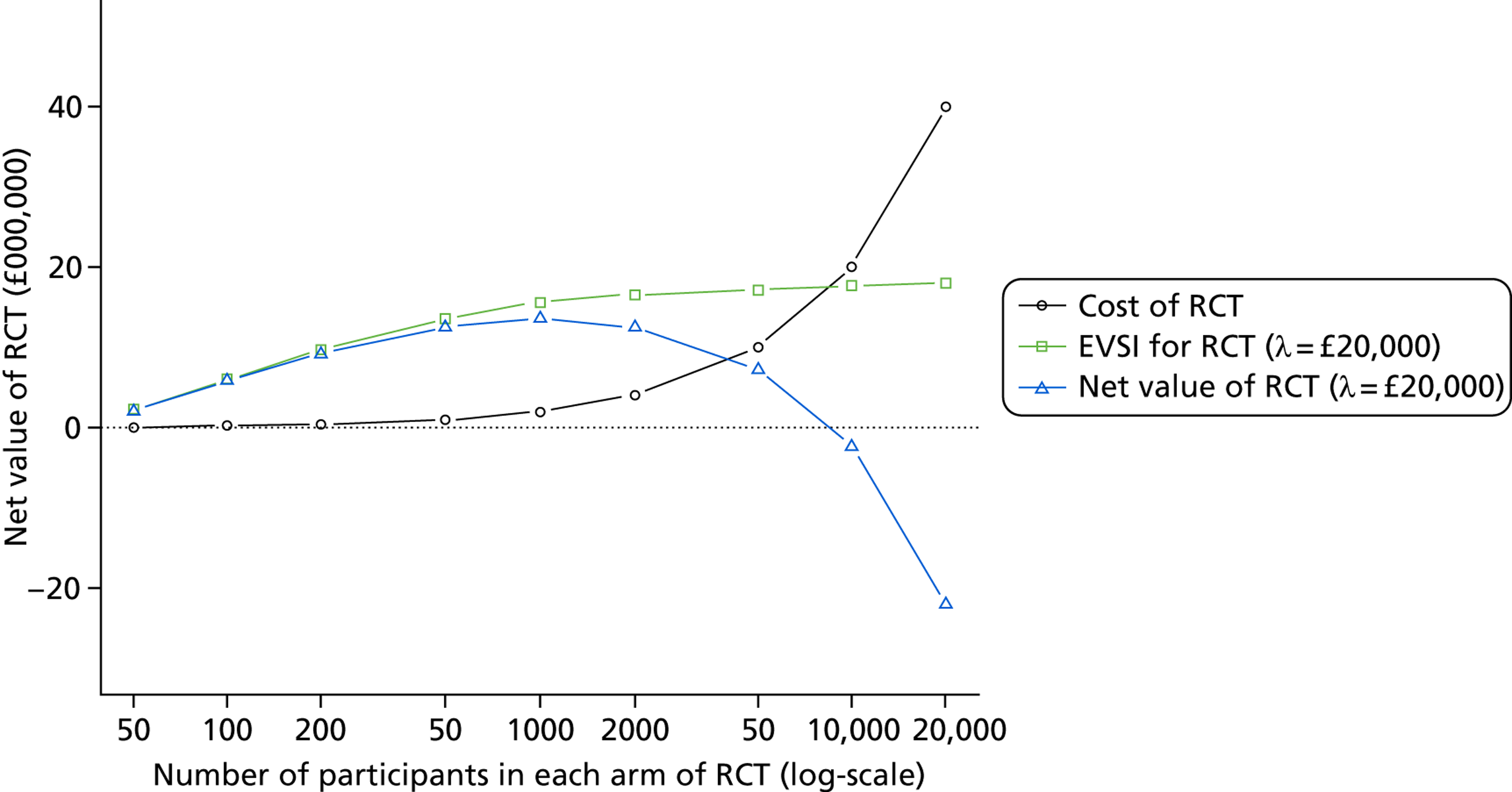
FIGURE 12.
Results from the EVSI analyses assuming a willingness to pay of £30,000 per QALY.
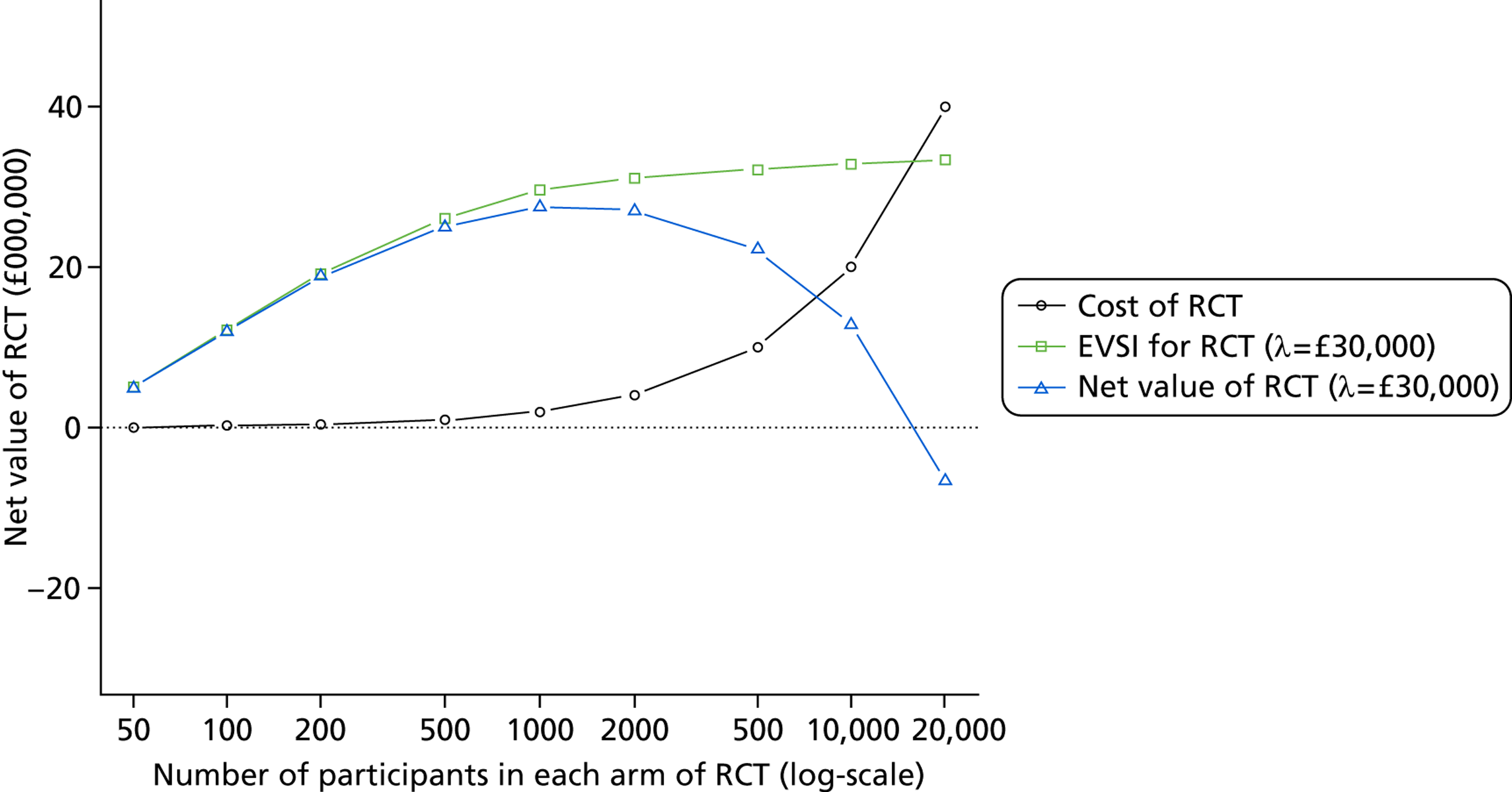
In both analyses, conducting a RCT of varenicline compared with cytisine with 1000 smokers per arm appeared optimal, although the results were comparable to trials of 500 or 2000 smokers per arm. Sensitivity analyses (not shown) were conducted reducing the cost per person in the RCT to £500 to acknowledge the relatively small duration of the trial. Assuming a willingness to pay of £20,000 per QALY, the conclusions did not alter; at £30,000 per QALY a trial of 2000 in each arm was estimated to be slightly preferable to 1000 in each arm, although the results were very similar for these trial sizes.
Discussion on modelling aspects
Probabilistic sensitivity analysis outputs from the economic model suggest that cytisine for smoking cessation will produce greater mean LYs and QALYs, and lower mean lifetime costs than varenicline, which was previously considered to be the most cost-effective smoking cessation treatment strategy. At a willingness-to-pay threshold of £20,000 for an additional QALY, the probability that cytisine treatment is preferable to varenicline treatment is 0.95, and this probability does not fall below 0.9. Despite this, the value of further information on the relative effectiveness of the two strategies is high because of the very large numbers of smokers treated.
A key driver of the dominance of cytisine treatment over varenicline treatment in the economic analysis is the relative effectiveness of cytisine compared with varenicline, as shown in the univariate sensitivity analysis. In summary, the treatment which generates the greatest number of quitters will have the best long-term health outcomes as efficacious treatment also has the impact of reducing costs associated with longer-term conditions associated with smoking. If treatment costs were equal for varenicline and cytisine, the probability that cytisine is the optimal choice is 0.9 (at any willingness-to-pay value), reflecting the 0.9 probability that cytisine has the greater 1-year continuous cessation probability.
It was not possible to validate the economic model outputs against results in the STA report, as the number in the simulated cohort in the latter was not reported and, therefore, per smoker values are unknown. 89 Other previous applications of the BENESCO model have used non-UK populations and parameter inputs, making comparison of total LYs and QALYs difficult. 62–64,66–72,89 However, results across these studies and here have been similar, in that the intervention with the greatest clinical effectiveness (short-term cessation probability) has consistently had the greatest cost–utility.
The key limitation of the model structure used is the imposed assumption of no underlying quit rate, among failed quitters or relapsed smokers, which is likely to favour treatments with higher effectiveness. Other UK studies have modelled the cost–utility of competing smoking cessation strategies and incorporated an underlying quit rate. 92,95,121 In each of these studies, unlike here, the most efficacious strategy had the highest treatment cost, but like here, the strategy with greatest short-term clinical effectiveness was the optimal strategy, at a willingness-to-pay threshold of £20,000 per QALY gained. In these models the annual probabilities of relapse to smoking, smoking-related disease incidence and death have been assumed to be constant92,95,121 compared with the decreases related to time since cessation in the present model. Assuming a sharp fall in probabilities linked to unfavourable health outcomes, rather than a decline over time, is less realistic, but further biases results towards those with higher clinical effectiveness if the full benefits of smoking cessation are assumed instantly obtainable.
It is difficult to incorporate both an underlying quit rate and transition probabilities that vary with time since quit into a state transition model structure, without incorporating numerous tunnel states. Individual person-level models may be a better avenue for accurately quantifying the cost–utility of smoking cessation strategies in future. At least two such models have been built to date. 122,123 Given the resources provided for this project it was agreed in the protocol that construction of a more accurate model than the BENESCO model was not feasible.
The transition probabilities and some parameter inputs in the model were taken from the manufacturer’s submission for the NICE varenicline STA,65 and it is not known whether or not these data are the best currently available. From the results of the deterministic sensitivity analysis, model outputs are robust to parameter inputs other than relative effectiveness of the two treatments. Uncertainty around the probabilities of transition to disease states has not been explored, but if the relative risks of smoking-related disease incidence and mortality can be assumed to decrease after smoking cessation, cytisine for smoking cessation will represent a better use of the health-care budget than varenicline using average values given current information.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness findings
The systematic review of clinical effectiveness included 23 studies, comprising a total of 10,610 participants. 16,17,37–39,41,46–61 The review was an update of a previous Cochrane review by Cahill et al. 15 and the updated search added three new trials to the previous review. All of these trials were of varenicline. No new trials of cytisine were identified, so that only two high-quality RCTs of cytisine with outcome data after a minimum follow-up of 6 months have been conducted to date. 17,43
A network meta-analysis was used to allow a comprehensive synthesis and comparison between smoking cessation treatments including cytisine, varenicline, nicotine patch and bupropion hydrochloride. A random (treatment)-effects model was used incorporating a log-log link function to allow for variation in the duration of follow-up between studies.
Results showed that cytisine 1.5 mg produced the greatest effect on CAR relative to placebo (HR 4.21, 95% CrI 2.11 to 9.84). Cytisine 1.5 mg was the intervention with the highest probability of being the most effective intervention (probability = 0.86). As point prevalence abstinence is often used in smoking cessation trials as a proxy for continuous abstinence, a sensitivity analysis was conducted including studies using both continuous abstinence rates and 7-day point prevalence abstinence. The results of this analysis were similar to those using only CAR data. However, the goodness of fit of the model suggested that the data from some of the trials using point prevalence as an outcome measure may arise from a different model. Consequently, only treatment effects estimated from data obtained from the CAR studies were included in the economic model.
Previous recent systematic reviews have reported both cytisine and varenicline to be clinically effective aids to smoking cessation. Cahill et al. 15 reported both varenicline and cytisine to be clinically effective treatments for smoking cessation. Cytisine was reported to have modest clinical efficacy, although the authors noted low absolute quit rates. Cytisine has been licensed as a treatment for smoking cessation in a number of Eastern European countries for several decades. Despite this, only two trials of good quality with a minimum of 6 months’ follow-up that have evaluated the clinical efficacy of cytisine compared with placebo met the inclusion criteria for both the Cahill review15 and the current review. The Hajek review26 of cytisine identified more trials; however, these did not fit the inclusion criteria for the Cahill review15 or this current review. The most common reason for this was that the length of follow-up was too short. However, their analysis of including only the high-quality trials still showed cytisine to be a clinically effective smoking cessation aid. For varenicline, the Cahill review15 reported that participants treated with the standard dose of varenicline had a twofold increased chance of quitting than placebo. The authors note that varenicline has been shown to be clinically effective in trials in real-world settings and for participants who may ordinarily be excluded from clinical trials. These participants include patients awaiting surgery, or patients hospitalised with medical or psychiatric conditions. The trials identified in the updated search support this assertion, with varenicline showing clinical efficacy for pre-operative smokers37 and for light smokers in a Latino community setting. 38
Adverse events findings
Data for the four most common adverse events (abnormal dreams, headache, insomnia and nausea), as identified in the Cahill review,15 and SAEs were analysed. Standard-dose varenicline treatment was associated with significantly higher rates of headaches, insomnia and nausea than placebo; there was no significant difference in the rates of abnormal dreams. There were no significant differences in rates of headaches or nausea between cytisine and placebo; data were not identified for abnormal dreams or insomnia. However, these results must be interpreted with caution, as they are a result of data from RCTs only. Most of the trials reported in this review applied criteria that excluded individuals with underlying medical conditions such as a history of depression or cardiovascular illness. Such individuals may be more likely to develop SAEs than those with no history of these medical conditions. In addition, the follow-up period of many trials may not be sufficiently long to capture all relevant adverse events. Etter124 notes with caution the toxicity of the seeds of C. laborinum, from which cytisine is derived. The lethal dose in humans is not currently known; however, there is no current evidence to suggest that poisoning can occur from use of cytisine used for smoking cessation. A lack of systematic reviews of adverse events that include cohort studies means that, as is emphasised by Cahill et al.,15 conclusions regarding the safety profile of both varenicline and cytisine are currently limited.
Nevertheless, the current review and network meta-analysis found that there were no differences in SAEs, and that differences in mild, transient adverse events were not clinically significant. Overall, the safety evidence in the current review was weak and a full safety review was not undertaken. A full safety review of cohort studies for both varenicline and cytisine is needed. For example, a large cohort study found no evidence of an increased risk of self-harm, depression and suicidal thoughts relative to NRT or bupropion hydrochloride. 125
A number of systematic reviews and meta-analyses report on specific adverse events of varenicline. Previous reviews have reported mixed results when considering the association of varenicline with adverse events (e.g. Singh et al. ,29 found an increased risk of cardiovascular events, while Prochaska et al. 126 found no such link). Leung et al. 27 found an association between varenicline and an increased risk of gastrointestinal events. Tonstad et al. 28 found no significant association between varenicline and serious neuropsychiatric events; however, an association has been suggested for those taking varenicline who are currently experiencing depression. The FDA’s recently added warning to the product label of Chantix highlighted a small increased risk of certain cardiovascular events in patients with pre-existing cardiac conditions. This warning, coupled with its existing warnings, supports Cahill et al. ’s conclusion that a link between varenicline and certain adverse events cannot be ruled out. Concerns about the safety of varenicline have been raised, resulting in a series of warnings from the FDA. These have arisen through post-marketing reports of an increased risk of suicidal behaviour, serious cardiac events and gastrointestinal complaints. A full and detailed account of the current available evidence is presented in the review by Cahill et al. 15
Previous reviews have reported slightly more frequent adverse events among those taking cytisine than those taking placebo, which include weight gain, nausea and digestive problems, tachycardia and changes in blood pressure. 124 A review by Hajek et al. 26 found that many of the cytisine trials provided minimal support to participants, for example the drug was distributed by post. They highlight that more intensive support during attempted smoking cessation increases quit rates, and that this may be more relevant for participants taking cytisine, as its dosing regimen is complex. As both studies of cytisine identified in this review used the same standard dosing schedule, it has not been possible to identify advantages or disadvantages of different doses, in terms of both efficacy and adherence. In the event that cytisine were to be licensed in the UK, practitioners should consider the potential risk of adverse events when making treatment decisions for individual patients.
Cost-effectiveness findings
Probabilistic sensitivity analyses showed cytisine to produce greater expected mean LYs and QALYs, and lower mean expected lifetime costs than varenicline, and is therefore expected to dominate varenicline. The economic analysis is driven by the relative clinical effectiveness of cytisine and varenicline. The treatment that generates the greatest number of quitters will have the best long-term health outcomes, as smoking cessation produces reduced costs associated with longer-term conditions associated with smoking. Based on the currently available data there is a greater probability that cytisine is more efficacious than varenicline. However, this conclusion is uncertain, and owing to the very large numbers of smokers treated (around 800,000 receiving NHS treatment for smoking cessation each year), the value of further information on the relative effectiveness of the two treatments is high.
The EVPI was calculated as £12 per smoker assuming a willingness to pay of £20,000 per QALY gained and £21 per smoker assuming a willingness to pay of £20,000 per QALY gained. Although these are small EVPI values per person, the number of people affected by the decision is large – with a likely population of 3 million affected. The current economic analysis suggests EVPI values of £35M and £63M at a willingness-to-pay levels per QALY of £20,000 and £30,000 respectively. With a more conservative value of 1 million smokers affected, the values are in the region of £15M. As the EVPI is greater than the potential cost of a head-to-head trial, the second stage of the value of information analysis was necessary, i.e. calculating the EVPPI. The EVPPI of the HR of smoking cessation of cytisine compared with varenicline remained high (in excess of £33M) and, therefore, formal EVSI analyses were undertaken. This indicated that a direct head-to-head trial of cytisine and varenicline, with 1000 patients in each arm appeared an appropriate use of resources.
Recommendations for future research
It is recommended that a head-to-head trial of varenicline and cytisine is undertaken, with 1000 patients in each arm being an appropriate number.
Strengths and limitations of the review
A strength of this review was the quality of the trials included. No high-risk trials were included, with most trials judged to be at low risk of bias. Strict inclusion criteria of the trials meant that many trials excluded participants who may be more at risk of adverse events, for example those with underlying medical conditions. For this reason, the adverse events analyses may not give a comprehensive picture of adverse events associated with each treatment.
Use of a network meta-analysis allowed a comprehensive comparison of all interventions of interest, including a number of different dosing schedules. Only two studies matching the inclusion criteria that evaluated cytisine were identified,17,43 compared with 21 studies of varenicline. 16,37–39,41,46–61 The varenicline data include studies of its clinical effectiveness in a number of real-world settings, which allows their results to be generalised to wider populations. Cytisine has yet to be studied in subpopulations.
A strength of the economic modelling is that EVSI analyses were undertaken to quantify whether or not a RCT is justified and, if so, an appropriate number of smokers to recruit. The model constructed was based on the BENESCO model and the limitations of this model, primarily no underlying quit rate, are applicable here. Furthermore, the transition probabilities and some parameter inputs were taken from a manufacturer’s submission to NICE65 and it is not known whether or not these data are the best available. However, such limitations are unlikely to significantly bias the comparison of varenicline and cytisine.
It was not possible to obtain a cost for cytisine direct from the manufacturer and costs may vary between countries. The cost of cytisine may increase if the manufacturer is required to incur costs associated with fulfilling UK licensing requirements, although this does not change the fundamental conclusion that a head-to-head trial of cytisine compared with varenicline is needed.
A potential limitation is that the review and economic evaluation contained only two trials that examined cytisine. In addition, the safety evidence in the current review was weak and a full safety review was not undertaken. The dearth of robust evidence concerning cytisine further highlights the importance of a high-quality head-to-head trial.
Chapter 6 Conclusions
Clinical effectiveness
The current review evaluated two nicotinic receptor partial agonists, varenicline and cytisine, and supported previous findings that both drugs are effective for smoking cessation when compared with placebo. Cytisine was estimated to be more clinically effective than varenicline and also more cost-effective; however, it is yet to be licensed for use in the UK and its safety profile has yet to be adequately evaluated.
Cost-effectiveness
Given current evidence cytisine appears more clinically effective and cost-effective than varenicline based on expected costs and QALY values. However, there is uncertainty in this decision and formal EVSI analyses were undertaken that indicate that a RCT of varenicline compared with cytisine recruiting 1000 smokers per arm would be an efficient use of resources.
Suggested research priorities
A head-to-head trial comparing varenicline with cytisine is recommended, with 1000 smokers per arm being an appropriate number. Concerns about the potential toxicity of C. laborinum support the need for observational databases with a long duration follow-up so that all potential adverse events are captured.
A review of cohort studies evaluating varenicline or cytisine in the long-term is recommended in order to provide a more accurate estimate of the occurrence of adverse events for smokers taking these drugs as an aid for cessation in real-world settings.
Implications for service provision
As cytisine is currently not licensed in the UK, there are no implications at present for service provision.
Acknowledgements
Thank you to Professor Peter Hajek, Dr Michael Ussher and Dr Sally Hope for their expert clinical advice during the project and for their comments on the first draft of the report. Thank you to Dr Chris Carroll, Dr Paul Tappenden and Dr Eva Kaltenhaler for their comments on the first draft of the report.
Professor Peter Hajek has received research funding from and provided consultancy to manufacturers of stop-smoking medications. He has no links with manufacturers of cytisine tablets. Neither Dr Michael Ussher nor Dr Sally Hope has any competing interests.
Thank you to Andrea Shippam and Gill Rooney for providing administrative support and preparing and formatting the report.
Contributions of authors
Joanna Leaviss and Emma Everson-Hock carried out the systematic review and quality assessment of the studies.
William Sullivan and Matt Stevenson constructed the mathematical model.
Shijie Ren and John W Stevens provided statistical support and undertook the network meta-analyses.
Mark Strong undertook the value of information analyses and Anna Cantrell carried out the searches.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Peto R. Smoking and death: the past 40 years and the next 40. BMJ 1994;309:937-9. http://dx.doi.org/10.1136/bmj.309.6959.937.
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 2004;291:1238-45. http://dx.doi.org/10.1001/jama.291.10.1238.
- Nicotine Addiction in Britain: a Report of the Tobacco Advisory Group of the Royal College of Physicians. London: Royal College of Physicians; 2000.
- Mukherjee D, Yadav JS. Update on peripheral vascular diseases: From smoking cessation to stenting. Cleve Clin J Med 2001;68:723-33. http://dx.doi.org/10.3949/ccjm.68.8.723.
- Musk AW, de Klerk NH. History of tobacco and health. Respirology 2003;8:286-90.
- General Household Survey 2004. London: National Statistics; 2013.
- Scarborough P, Bhatnagar P, Wickramasinghe KK, Allender S, Foster C, Rayner M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: an update to 2006–07 NHS costs. J Public Health 2011;33:527-35.
- Hughes J, Stead L, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2004;4. http://dx.doi.org/10.1002/14651858.CD000031.
- West R. Addressing regulatory barriers to licensing nicotine products for smoking reduction. Addiction 2000;95:S29-34. http://dx.doi.org/10.1046/j.1360-0443.95.1s1.4.x.
- Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2013;8. http://dx.doi.org/10.1002/14651858.CD002850.
- Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2005;2. http://dx.doi.org/10.1002/14651858.CD001007.
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2005;2. http://dx.doi.org/10.1002/14651858.CD001292.
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.CD000146.pub3.
- Hughes JR, Stead, LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007;1. http://dx.doi.org/10.1002/14651858.CD000031.
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2012;4. http://dx.doi.org/10.1002/14651858.CD006103.pub2.
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006;296:56-63. http://dx.doi.org/10.1001/jama.296.1.56.
- West R, Zatonski W, Cedzynska M, Lewandowska D, Pazik J, Aveyard P, et al. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med 2011;365:1193-200. http://dx.doi.org/10.1056/NEJMoa1102035.
- Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM. Cytisine for smoking cessation: a research agenda. Drug Alcohol Depend 2008;92:3-8. http://dx.doi.org/10.1016/j.drugalcdep.2007.06.017.
- BNF 2013. www.medicinescomplete.com/mc/bnf/current/PHP3209-champix.htm#PHP3209-champix (accessed 18 April 2013).
- Zatonski W, Cedzynska M, Tutka P, West R. An uncontrolled trial of cytisine (Tabex) for smoking cessation. Tob Control 2006;15:481-4. http://dx.doi.org/10.1136/tc.2006.016097.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. http://dx.doi.org/10.1111/j.1360-0443.2004.00995.x.
- Hughes JR. Motivating and helping smokers to stop smoking. J Gen Intern Med 2003;18:1053-7. http://dx.doi.org/10.1111/j.1525-1497.2003.20640.x.
- West R, Owen L. Estimates of 52-Week Continuous Abstinence Rates Following Selected Smoking Cessation Interventions in England. Version 2 2012. www.smokinginengland.info/reports (accessed 4 March 2014).
- McEwen A, Hajek P, McRobbie H, West R. Manual of Smoking Cessation. Oxford: Blackwell Publishing; 2006.
- West R, May S, West M, Croghan E, McEwan A. Performance of English stop smoking services in first 10 years: analysis of service monitoring data. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4921.
- Hajek P, McRobbie H, Myers K. Efficacy of cytisine in helping smokers quit: systematic review and meta-analysis. Thorax 2013;68:1037-42. http://dx.doi.org/10.1136/thoraxjnl-2012-203035.
- Leung LK, Patafio FM, Rosser WW. Gastrointestinal adverse effects of varenicline at maintenance dose: a meta-analysis. BMC Clin Pharmacol 2011;11. http://dx.doi.org/10.1186/1472-6904-11-15.
- Tonstad S, Davie, S, Flammer M, Russ C, Hughes J. Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: a pooled analysis. Drug Safe 2010;33:289-301. http://dx.doi.org/10.2165/11319180-000000000-00000.
- Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ 2011;183:1359-66. http://dx.doi.org/10.1503/cmaj.110218.
- Prochaska JJ. Review: Varenicline for tobacco cessation does not increase CV serious adverse events. Ann Intern Med 2012;157.
- West CH, Weiss JM. A selective test for antidepressant treatments using rats bred for stress-induced reduction of motor activity in the swim test. Psychopharmacology 2005;182:9-23. http://dx.doi.org/10.1007/s00213-005-0048-x.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Lunn D, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37.
- Brooks S, Gelman A. Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-45.
- McCullagh P. Generalized Linear Models. London: Chapman & Hall/CRC; 1989.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group (2009) . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000097.
- Wong J, Abrishami A, Yang Y, Zaki A, Friedman Z, Selby P, et al. A perioperative smoking cessation intervention with varenicline: a double-blind, randomized, placebo-controlled trial. Anesthesiology 2012;117:755-64. http://dx.doi.org/10.1097/ALN.0b013e3182698b42.
- de Dios MA, Anderson BJ, Stanton C, Audet DA, Stein M. Project Impact: A pharmacotherapy pilot trial investigating the abstinence and treatment adherence of Latino light smokers. J Subst Abuse Treat 2012;43:322-30. http://dx.doi.org/10.1016/j.jsat.2012.01.004.
- Heydari G, Talischi F, Tafti SF, Masjedi MR. Quitting smoking with varenicline: parallel, randomised efficacy trial in Iran. Int J Tuberc Lung Dis 2012;16:268-72. http://dx.doi.org/10.5588/ijtld.11.0183.
- Pfizer Inc . Smoking Cessation Study for Patients With Schizophrenia or Schizoaffective Disorder. 2011. http://clinicaltrials.gov/ct2/show/NCT00644969?term=NCT00644969&rank=1.
- Williams JM, Anthenelli RM, Morris CD, Treadow J, Thompson JR, Yunis C, et al. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 2012;73:654-60. http://dx.doi.org/10.4088/JCP.11m07522.
- Scharfenberg G, Benndorf S, Kempe G. Cytisine (Tabex) as a pharmaceutical aid in stopping smoking. Dtsch Gesundheitsw 1971;26:463-5.
- Vinnikov D, Brimkulov N, Burjubaeva A. A double-blind, randomised, placebo-controlled trial of cytisine for smoking cessation in medium-dependent workers. J Smok Cessat 2008;3:57-62. http://dx.doi.org/10.1375/jsc.3.1.57.
- Swan G, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, et al. Behavioural Counselling and varenicline tretament for smoking cessation. Am J Prev Med 2010;38:482-90.
- Tonstad S. Smoking cessation efficacy and safety of varenicline, an alpha4beta2 nicotinic receptor partial agonist. J Cardiovasc Nurs 2006;21:433-6. http://dx.doi.org/10.1097/00005082-200611000-00004.
- Aubin H-J, Bobak A, Britton JR, Oncken C, Billing CB, Gong J, et al. Varenicline compared with transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax 2008;63:717-24.
- Bolliger CT, Issa JS, Posadas VR, Safwat T, Abreu P, Correia EA, et al. Effects of varenicline in adult smokers: a multinational, 24-week, randomized, double-blind, placebo-controlled study. Clin Ther 2011;33:465-77. http://dx.doi.org/10.1016/j.clinthera.2011.04.013.
- Gonzales D, Rennard S, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release buprion and placebo for smoking cessation. A randomized trial. JAMA 2006;296:47-55.
- Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves K. Efficacy and tolerability of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40 week follow-up for smoking cessation in Japanese smokers. Clin Ther 2007;29:1040-56.
- Niaura R, Taylor Hays J, Jorenby DE, Leone FT, Pappas JE, Reeves KR, et al. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomised controlled trial. Curr Med Res Opin 2008;24:1931-41.
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R. Smoking cessation with varenicline, a selective α4β2 nicotinic receptor partial agonist. Arch Intern Med 2006;166:1561-77. http://dx.doi.org/10.1001/archinte.166.15.1561.
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med 2006;166:1571-7. http://dx.doi.org/10.1001/archinte.166.15.1571.
- Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, et al. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res 2012;14:343-50. http://dx.doi.org/10.1093/ntr/ntr220.
- Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: A randomized trial. Circulation 2010;121:221-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.869008.
- Smith B, Carson K, Brinn M, Labiszewski N, Peters M, Fitridge R, et al. Smoking Termination Opportunity for inPatients (STOP): superiority of a course of varenicline tartrate plus counselling over counselling alone for smoking cessation: a 12-month randomised controlled trial. Thorax 2013;68:485-6. http://dx.doi.org/10.1136/thoraxjnl-2012-202484.
- Steinberg MB, Randall J, Greenhaus S, Schmelzer AC, Richardson DL, Carson JL. Tobacco dependence treatment for hospitalized smokers: a randomized, controlled, pilot trial using varenicline. Addict Behav 2011;36:1127-32. http://dx.doi.org/10.1016/j.addbeh.2011.07.002.
- Tashkin DP, Rennard S, Hays J, Ma W, Lawrence D, Lee TC. Effects of varenicline on smoking cessation in patients with mild to moderate COPD. Chest 2011;139:591-9. http://dx.doi.org/10.1378/chest.10-0865.
- Tsai S-T, Cho H-J, Cheng H-S, Kim C-H, Hsueh, K-C, Billing CB, et al. A randomized, placebo-controlled trial of varenicline, a selective α4β2, nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther 2007;29:1027-39. http://dx.doi.org/10.1016/j.clinthera.2007.06.011.
- Tsukahara H, Noda K, Saku K. A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers. Circ J 2010;74:771-8. http://dx.doi.org/10.1253/circj.CJ-09-0803.
- Wang C, Xiao D, Chan KPW, Pothirat C, Garza D, Davies S. Varenicline for smoking cessation: A placebo-controlled, randomized study. Respirology 2009;14:384-92. http://dx.doi.org/10.1111/j.1440-1843.2008.01476.x.
- Williams KE, Reeves KR, Billing CB, Pennignton AM, Gong J. A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Curr Med Res Opin 2007;23:793-801. http://dx.doi.org/10.1185/030079907X182185.
- Annemans L, Nackaerts K, Bartsch, P, Prignot J, Marbaix S. Cost-effectiveness of varenicline in Belgium, compared with bupropion, nicotine replacement therapy, brief counselling and unaided smoking cessation: a BENESCO Markov cost-effectiveness analysis. Clin Drug Investig 2009;29:655-65. http://dx.doi.org/10.2165/11317730-000000000-00000.
- Hoogendoorn M, Welsing P, Rutten-van Molken MP. Cost-effectiveness of varenicline compared with bupropion, NRT, and nortriptyline for smoking cessation in the Netherlands. Curr Med Res Opin 2008;24:51-6. http://dx.doi.org/10.1185/030079907X242917.
- Linden K, Jormanainen V, Linna M, Sintonen H, Wilson K, Kotomaki T. Cost-effectiveness of varenicline compared with bupropion and unaided cessation for smoking cessation in a cohort of Finnish adult smokers. Curr Med Res Opin 2010;26:549-60.
- Manufacturers Submission for NICE STA of Varenicline for Smoking Cessation. Tadworth, Surrey: Pfizer Ltd; 2007.
- Bae JY, Kim CH, Lee EK. Evaluation of cost-utility of varenicline compared with existing smoking cessation therapies in South Korea. Value Health 2009;12:S70-3. http://dx.doi.org/10.1111/j.1524-4733.2009.00631.x.
- Bolin K, Mork AC, Wilson K. Smoking-cessation therapy using varenicline: the cost-utility of an additional 12-week course of varenicline for the maintenance of smoking abstinence. J Eval Clin Pract 2009;15:478-85. http://dx.doi.org/10.1111/j.1365-2753.2008.01045.x.
- Bolin K, Wilson K, Benhaddi H, de NE, Marbaix S, Mork AC, et al. Cost-effectiveness of varenicline compared with nicotine patches for smoking cessation – results from four European countries. Eur J Public Health 2009;19:650-4. http://dx.doi.org/10.1093/eurpub/ckp075.
- Bolin K, Mork AC, Willers S, Lindgren B. Varenicline as compared with bupropion in smoking-cessation therapy--cost-utility results for Sweden 2003. Respir Med 2008;102:699-710. http://dx.doi.org/10.1016/j.rmed.2007.12.018.
- Howard P, Knight C, Boler A, Baker C. Pharmacoeconomics 2008;26:497-511.
- Knight C, Howard P, Baker, CL, Marton JP. The cost-effectiveness of an extended course (12+12 weeks) of varenicline compared with other available smoking cessation strategies in the United States: an extension and update to the BENESCO model. Value Health 2010;13:209-14. http://dx.doi.org/10.1111/j.1524-4733.2009.00672.x.
- Vemer P, Rutten-van Molken MP. Crossing borders: factors affecting differences in cost-effectiveness of smoking cessation interventions between European countries. Value Health 2010;13:230-41. http://dx.doi.org/10.1111/j.1524-4733.2009.00612.x.
- EPAR Summary for the Public, Champix (Varenicline). London: European Medicines Agency; 2012.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Population Trends. Newport: ONS; 2006.
- General Household Survey, 2004 Report – Smoking and Drinking. Newport: ONS; 2004.
- Government Actuary’s Department . Historic Interim Life Tables; Interim Life Tables 2002-2004 2013. www.gad.gov.uk/Demography%20Data/Life%20Tables/historic_interim_life_tables.html (accessed 1 March 2013).
- Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom. JAMA 2000;284:706-12. http://dx.doi.org/10.1001/jama.284.6.706.
- Cassino C, Ito K, Bader I, Ciotoli C, Thurston G, Reibman J. Cigarette smoking and ozone-associated emergency department use for asthma by adults in New York City. Am J Respir Crit Care Med 1999;159:1773-9. http://dx.doi.org/10.1164/ajrccm.159.6.9809042.
- Soriano JB, Maier WC, Egger P, Visick G, Thakrar B, Sykes J, et al. Recent trends in physician diagnosed COPD in women and men in the UK. Thorax 2000;55:789-94. http://dx.doi.org/10.1136/thorax.55.9.789.
- Forman D, Stockton D, Moller H, Quinn M, Babb P, De AR, et al. Cancer prevalence in the UK: results from the EUROPREVAL study. Ann Oncol 2003;14:648-54. http://dx.doi.org/10.1093/annonc/mdg169.
- Registrations of Cancer Diagnosed in 2003, England. Newport: ONS; 2005.
- Asthma UK. Where Do We Stand?: Asthma in the UK Today. London: Asthma UK; 2004.
- Hoskins G, McCowan C, Neville RG, Thomas GE, Smith B, Silverman S. Risk factors and costs associated with an asthma attack. Thorax 2000;55:19-24. http://dx.doi.org/10.1136/thorax.55.1.19.
- Coronary Heart Disease Statistics fact sheet. London: British Heart Foundation; 2006.
- Volmink JA, Newton JN, Hicks NR, Sleight P, Fowler GH, Neil HA. Coronary event and case fatality rates in an English population: results of the Oxford myocardial infarction incidence study. The Oxford Myocardial Infarction Incidence Study Group. Heart 1998;80:40-4.
- Health Statistics Quarterly, No.12. London: The Stationery Office; 2001.
- Wetter DW, Cofta-Gunn L, Fouladi RT, Cinciripini PM, Sui D, Gritz ER. Late relapse/sustained abstinence among former smokers: a longitudinal study. Prev Med 2004;39:1156-63. http://dx.doi.org/10.1016/j.ypmed.2004.04.028.
- Hind D, Tappenden P, Peters J, Kenjegalieva K. Varenicline in the management of smoking cessation: a single technology appraisal. Health Technol Assess 2009;13.
- Hawkins J, Hollingworth W, Campbell R. Long-term smoking relapse: a study using the british household panel survey. Nicotine Tob Res 2010;12:1228-35. http://dx.doi.org/10.1093/ntr/ntq175.
- Krall EA, Garvey AJ, Garcia RI. Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine Tob Res 2002;4:95-100. http://dx.doi.org/10.1080/14622200110098428.
- Godfrey C, Parrott S, Coleman T, Pound E. The cost-effectiveness of the English smoking treatment services: evidence from practice. Addiction 2005;100:70-83. http://dx.doi.org/10.1111/j.1360-0443.2005.01071.x.
- Yudkin P, Hey K, Roberts S, Welch S, Murphy M, Walton R. Abstinence from smoking eight years after participation in randomised controlled trial of nicotine patch. BMJ 2003;327:28-9. http://dx.doi.org/10.1136/bmj.327.7405.28.
- Britton M. The burden of COPD in the U.K.: results from the Confronting COPD survey. Respir Med 2003;97:S71-9. http://dx.doi.org/10.1016/S0954-6111(03)80027-6.
- Flack S, Taylor M, Trueman P. Cost-effectiveness of Interventions for Smoking Cessation. York: York Health Economics Consortium, University of York; 2007.
- Sanderson H, Spiro S, Stevens A, Raftery J, Mant J, Simpson S. Health Care Needs Assessment: the Epidemiologically Based Needs Assessment Reviews. Abingdon: Radcliffe Publishing; 2004.
- McMurray J, Hart W, Rhodes G. An evaluation of the cost of heart failure to the National Health Service in the UK. J Med Econ 1993;6:99-110.
- Simpson EL, Stevenson MD, Scope A, Poku A, Minton J, Evans P. Echocardiography in Newly Diagnosed Atrial Fibrillation Patients: A Systematic Review and Economic Evaluation. Sheffield: School of Health and Related Research (ScHARR) Technology Assessment Group, The University of Sheffield; 2011.
- Rivero-Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo-Fernandez R. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making 2010;30:341-54. http://dx.doi.org/10.1177/0272989X09349961.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit, The University of Kent; 2012.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation (Handbooks for Health Economic Evaluation). Oxford: Oxford University Press; 2006.
- Stapleton JA, West RA. Direct Method and ICER Tables for the Estimation of the Cost-Effectiveness of Smoking Cessation Interventions in General Populations: Application to a New Cytisine Trial and Other Examples. Oxford: Oxford University Press; 2012.
- Tabex Tablets 2013. www.tabextablets.co.uk/ (accessed 18 March 2013).
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Trippoli S, Vaiani M, Lucioni C, Messori A. Quality of life and utility in patients with non-small cell lung cancer. Quality-of-life Study Group of the Master 2 Project in Pharmacoeconomics. Pharmacoeconomics 2001;19:855-63.
- Szende A, Svensson K, Stahl E, Meszaros A, Berta GY. Psychometric and utility-based measures of health status of asthmatic patients with different disease control level. Pharmacoeconomics 2004;22:537-47. http://dx.doi.org/10.2165/00019053-200422080-00005.
- Gage BF, Cardinalli AB, Owens DK. Cost-effectiveness of preference-based antithrombotic therapy for patients with nonvalvular atrial fibrillation. Stroke 1998;29:1083-91. http://dx.doi.org/10.1161/01.STR.29.6.1083.
- Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics 2003;21:191-200. http://dx.doi.org/10.2165/00019053-200321030-00004.
- Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost-effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics 2005;23:619-37. http://dx.doi.org/10.2165/00019053-200523060-00008.
- Hay JW, Sterling KL. Cost-effectiveness of treating low HDL-cholesterol in the primary prevention of coronary heart disease. Pharmacoeconomics 2005;23:133-41. http://dx.doi.org/10.2165/00019053-200523020-00005.
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24. http://dx.doi.org/10.1002/(SICI)1099-1050(199611)5:6%3C513::AID-HEC237%3E3.0.CO;2-9.
- Felli JC, Hazen GB. Sensitivity analysis and the expected value of perfect information. Med Decis Making 1998;18:95-109. http://dx.doi.org/10.1177/0272989X9801800117.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27. http://dx.doi.org/10.1177/0272989X04263162.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Robinson S, Harris H. Smoking and Drinking Among Adults 2009, A Report on the 2009 General Lifestyle Survey. Newport: Office for National Statistics; 2011.
- Brown J, Michie S, West R. The case of Stop Smoking Services in England. Br J Psychiatry 2013;202:74-6.
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med 2008;34:102-11. http://dx.doi.org/10.1016/j.amepre.2007.09.033.
- West R, DiMarino ME, Gitchell J, McNeill A. Impact of UK policy initiatives on use of medicines to aid smoking cessation. Tob Control 2005;14:166-71. http://dx.doi.org/10.1136/tc.2004.008649.
- Vogt F, Hall S, Marteau TM. Understanding why smokers do not want to use nicotine dependence medications to stop smoking: qualitative and quantitative studies. Nicotine Tob Res 2008;10:1405-13. http://dx.doi.org/10.1080/14622200802239280.
- Stevenson MD, Oakley J, Lloyd Jones M, Brennan A, Compston J, McCloskey E, et al. The cost-effectiveness of an RCT to establish whether 5 or 10 years of bisphosphonate treatment is the better duration for women with a prior fracture. Med Decis Making 2009;29:678-89. http://dx.doi.org/10.1177/0272989X09336077.
- Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al. The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation. Health Technol Assess 2002;6.
- Heitjan DF, Asch DA, Ray R, Rukstalis M, Patterson F, Lerman C. Cost-effectiveness of pharmacogenetic testing to tailor smoking-cessation treatment. Pharmacogenomics J 2008;8:391-9. http://dx.doi.org/10.1038/sj.tpj.6500492.
- Xenakis JG, Kinter ET, Ishak KJ, Ward AJ, Marton JP, Willke RJ, et al. A discrete-event simulation of smoking-cessation strategies based on varenicline pivotal trial data. Pharmacoeconomics 2011;29:497-510. http://dx.doi.org/10.2165/11589230-000000000-00000.
- Etter JF. Cytisine for smoking cessation: a literature review and a meta-analysis. Arch Intern Med 2006;166:1553-9. http://dx.doi.org/10.1001/archinte.166.15.1553.
- Gunnell D, Irvine D, Wise L, Davies C, Martin RM. Varenicline and suicidal behaviour: a cohort study based on data from the General Practice Research Database. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3805.
- Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2856.
Appendix 1 Literature search strategies
MEDLINE search strategies
Cahill search
-
(‘cytisine’ or ‘tabex’ or ‘varenicline’ or ‘nicotine receptor partial agonist’).tw.
Champix or Chantix search
-
(Champix or Chantix).tw.
Clinical effectiveness
To find papers on the clinical effectiveness of cytisine or varenicline the above searches were combined with filters designed to retrieve RCTs and systematic reviews.
Randomised controlled trials filter
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized controlled trials/
-
random allocation/
-
double blind method/
-
single blind method/
-
clinical trial.pt.
-
exp Clinical Trial/
-
(clin$ adj25 trial$).ti,ab.
-
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
-
placebos/
-
placebos.ti,ab.
-
random.ti,ab.
-
research design/
-
or/1-14
Systematic reviews filter
-
Meta analysis/
-
Meta analys$.tw.
-
Metaanaly$.tw.
-
exp Literature review/
-
(systematic adj (review or overview)).tw.
-
or/1-5
-
Commentary.pt.
-
Letter.pt.
-
Editorial.pt.
-
Animals/
-
or/7-10
-
6 not 11
Cost-effectiveness
The searches were also combined with an economics filter to find papers on the cost-effectiveness of cystisine and varenicline.
MEDLINE economics filter
-
Economics/
-
“costs and cost analysis”/
-
Cost allocation/
-
Cost-benefit analysis/
-
Cost control/
-
cost savings/
-
Cost of illness/
-
Cost sharing/
-
“deductibles and coinsurance”/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
exp economics, hospital/
-
exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
exp "fees and charges"/
-
exp budgets/
-
(low adj cost).mp.
-
(high adj cost).mp.
-
(health?care adj cost$).mp.
-
(fiscal or funding or financial or finance).tw.
-
(cost adj estimate$).mp.
-
(cost adj variable).mp.
-
(unit adj cost$).mp.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
or/1-31
Appendix 2 Quality assessment
Tables 35–37 detail the quality assessment of newly included studies using the Cochrane risk of bias tool. 32
| Risk of bias | Author’s judgment | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Low risk | A computer-generated stratified randomisation with blocks of 40 based on smokers stage of change |
| Allocation concealment (selection bias) | Low risk | Patient’s assignments were placed in sequentially numbered, opaque sealed envelopes kept by independent researcher |
| Blinding (performance bias and detection bias) | Low risk | Patients, health-care personnel and research staff blinded to the randomisation throughout the study |
| Incomplete outcome data (attrition bias) | Low risk | Intention-to-treat analysis conducted |
| Selective reporting (reporting bias) | Low risk | Primary outcomes (efficacy) reported |
| Risk of bias | Author’s judgement | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | Primary outcomes (efficacy) reported |
| Risk of bias | Author’s judgement | Support for judgement |
|---|---|---|
| Random sequence generation (selection bias) | Unclear risk | Randomised trial, but method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Study personnel and participants were blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | Observations missing at follow-up were treated as smokers |
| Selective reporting (reporting bias) | Low risk | Primary outcomes (efficacy) reported |
Appendix 3 Table of excluded studies with rationale
| Paper | Reasons for exclusion |
|---|---|
| Brown University Varenicline effects in heavy-drinking smokers. Brown Univ Psychopharmacol Update 2011;22:4 | Commentary/summary |
| Allan GM, Ivers N, Els C. Pharmacotherapy for smoking. Can Fam Physician 2011;57:47 | Commentary/summary |
| Ashare RL, McKee, SA. Effects of varenicline and bupropion on cognitive processes among nicotine-deprived smokers. Exp Clin Psychopharmacol 2012;20:63–70 | Not smoking cessation |
| Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, Zbikowski SM, et al. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine Tob Res 2011;13:361–8 | Data are from Swan44 |
| Christalla P, Dewenter M, El-Armouche A. Effectiveness and safety of varenicline for smoking cessation. Dtsch Med Wochenschr 2012;137:940–4 | No data |
| Cui Q, Robinson L, Elston D, Smaill F, Cohen J, Quan C, et al. Safety and tolerability of varenicline tartrate Champix®/Chantix® for smoking cessation in HIV-infected subjects: a pilot open-label study. AIDS Patient Care STDS 2012;26:12–19 | Not a RCT |
| Dutra SJ, Stoeckel LE, Carlini SV, Pizzagalli, DA, Evins AE. Varenicline as a smoking cessation aid in schizophrenia patients: Effects on smoking behavior and reward sensitivity. Biol Psychiatry 2011;69(Suppl. 9):280S | Not a RCT |
| Ferketich AK, Otterson GA, King M, Hall N, Browning KK, Wewers ME. A pilot test of a combined tobacco dependence treatment and lung cancer screening program. Lung Cancer 2012;76:211–15 | No comparison of drugs |
| Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology 2011;215:655–63 | < 6 months’ follow–up of abstinence |
| Garrison GD. Varenicline for 4 weeks prior to target quit date reduces prequit date smoking and increases 12-week abstinence. Evid Based Med 2012;17 | Comment/summary of Hajek |
| Grassi MC, Enea D, Ferketich AK, Lu B, Pasquariello S, Nencini P. Effectiveness of varenicline for smoking cessation: A 1-year follow-up study. J Subst Abuse Treat 2011;41:64–70 | Not a RCT – participants chose whether or not to have varenicline |
| Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med 2011;171:770–7 | Studies different varenicline preloading. < 6 months’ follow-up of abstinence |
| Hawk J, Ashare RL, Lohnes SF, Schlienz NJ, Rhodes JD, Tiffany ST, et al. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence. Clin Pharmacol Ther 2012;91:172–80 | < 6 months’ follow-up data |
| Hays JT, Croghan IT, Baker CL, Cappelleri JC, Bushmakin, AG. Changes in health-related quality of life with smoking cessation treatment. Eur J Public Health 2012;22:224–9 | Data are from Jorenby16 and Gonzales48 |
| Javitz HS, Swan GE, Lerman C. The dynamics of the urge-to-smoke following smoking cessation via pharmacotherapy. Addiction 2011;106:1835–45 | Data are from Swan44 |
| Jimenez Ruiz, CA Pinedo AR, Guerrero AC, Uibarri MM, Fernandez MC, Gonzalez GL. Characteristics of COPD smokers and effectiveness and safety of smoking cessation medications. Nicotine Tob Res 2012;14:1035–9 | Not a RCT |
| King DP, Paciga S, Pickering, E, Benowitz NL, Bierut LJ, Conti DV, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology 2012;37:641–50 | Data is from Gonzales,48 Jorenby16 and Oncken52 |
| Kotseva K, Jennings C, De Bacquer D, Hoes A, De Velasco J, Brusaferro S, et al. Euroaction Plus: A Randomised Controlled Trial on Preventive Cardiology Programme Plus Intensive Smoking Cessation with Varenicline for Vascular and High CVD Risk Smokers and Their Partners-Principal Results. Heart 2012;98:A80–1 | Unclear whether or not the goal was smoking cessation. Varenicline was optional |
| Moon KT. Does adjusting varenicline dosing enhance smoking cessation rates? Am Fam Physician 2012;85 | No new study/commentary |
| Nollen NL, Cox LS, Nazir N, Ellerbeck EF, Owen A, Pankey S, et al. A pilot clinical trial of varenicline for smoking cessation in black smokers. Nicotine Tob Res 2011;13:868–73 | All groups varenicline plus different methods of counselling. < 6 months’ follow-up of abstinence |
| Pachas GN, Cather C, Pratt SI, Hoeppner B, Nino J, Carlini SV, et al. Varenicline for smoking cessation in schizophrenia: safety and effectiveness in a 12-week open-label trial. J Dual Diagn 2012;8:117–25 | Not a RCT |
| Selby P, Brosky G, Oh PI, Raymond V, Ranger S. How pragmatic or explanatory is the randomized, controlled trial? The application and enhancement of the PRECIS tool to the evaluation of a smoking cessation trial. BMC Med Res Methodol 2012;12:101 | No quit data |
| Shim JC, Jung D, Oh M, Kong B, Ha T, Cho D, et al. Varenicline treatment for smoking cessation in people with schizophrenia: A randomized double-blind placebo-controlled trial. Schizophr Bull 2011;37:320–1 | Not smoking cessation |
| Sofuoglu M, Duffey D, Mooney ME. Varenicline increases smoking abstinence at 6 months to a year compared with placebo or bupropion; nausea is the most commonly reported adverse effect. Evid Based Med 2011;16:113–14 | Commentary agree |
| Solano RS, Vaquero LP, Solano Garcia-Tenorio R, Lopez RT, Jimenez Ruiz, CA, de Granda Orive JI Treatment of smoking habit in chronic obstructive pulmonary disease. Revista De Patologia Respiratoria 2012;15:123–8 | Paper unavailable |
| Weiner E, Buchholz A, Coffay A, Liu F, McMahon RP, Buchanan RW, et al. Varenicline for smoking cessation in people with schizophrenia: a double blind randomized pilot study. Schizophr Res 2011;129:94–5 | Letter with new data, but reported < 6 months’ follow-up of abstinence |
| Williams JM, Anthenelli RM, Morris CD, Treadow J, Thompson JR, Yunis C, et al. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 2012;73:654–60 | Pfizer study |
| Wilson K, Hettle R, Marbaix S, Diaz CS, Ines M, Santoni L, et al. An economic evaluation based on a randomized placebo-controlled trial of varenicline in smokers with cardiovascular disease: results for Belgium, Spain, Portugal, and Italy. Eur J Prev Cardiol 2012;19:1173–83 | Economic model – not a RCT |
| Zhou W, Wei X, Ke H. Psychiatric adverse reactions in a prospective, randomized clinical trial of varenicline for smoking cessation in patients with COPD. Respirology 2011;16:11 | < 6 months’ follow-up |
Appendix 4 Statistical methods used to analyse continuous abstinence and adverse event data
The analyses assumed that the studies are exchangeable in the sense that the investigators would be willing to assign each of the smokers in the studies to any of the interventions. A random-effects network meta-analysis was conducted with the reference treatment being defined as placebo.
The studies presented data in terms of the number of smokers who had an event (e.g. quit smoking when the outcome measure is continuous abstinence). Define rik as the number of events out of the total number of smokers in each arm for arm k of study i with study duration fi. We assume that the data follow a binomial likelihood such that:
where pik represents the probability of an event in arm k of study i after follow-up time fi.
To account for different study durations, it was assumed that the time until an event occurs in arm k of study i,Tik, is from an exponential distribution such that:
Therefore, the probability that there are no events by time fi in arm k of study i (i.e. the survivor function of an exponential distribution) is:
Hence, for each study i, pik, the probability of an event in arm k of study i after study duration time fi, can be written as:
which is time dependent. Since pik is a non-linear function of log(λik), the complementary log-log link function was used to model pik.
where δi,bk are the treatment effects of interest which are the log-hazard ratios relative to the baseline intervention in each study and μi are the study-specific baseline effects in a study i.
We treat μi as nuisance parameters with fixed (but known) study effects and given them weak prior distribution such that:
We assume a random treatment effects model in which δik are assumed to come from a common population distribution such that:
The treatment effects d12, . . . , d1k were also given a weak prior distribution N(0,100). The model is completed by giving the between-study standard deviation a uniform prior distribution.
In the case of SAEs, there were several trials with low or zero observed events. Posterior distributions based on a N(0,100) prior distribution for population log-hazard ratios included implausible values for varenicline 0.3 mg q.d. and varenicline 1.0 mg q.d. Hence, a more informative N(0,10) prior distribution was used for the log-hazard ratio for these two treatments.
Appendix 5 Data used in analyses
| Author, year | Study duration (years) | Treatments | Number of events | Number of patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | ||
| Vinnikov 200843 | 0.50 | 1 | 3 | – | – | – | 1 | 9 | – | – | – | 97 | 100 | – | – | – |
| West 201117 | 1.00 | 1 | 3 | – | – | – | 9 | 31 | – | – | – | 370 | 370 | – | – | – |
| Bolliger 201147 | 0.46 | 1 | 7 | – | – | – | 26 | 157 | – | – | – | 199 | 394 | – | – | – |
| Niaura 200850 | 1.00 | 1 | 7 | – | – | – | 12 | 35 | – | – | – | 160 | 160 | – | – | – |
| Rennard 201253 | 0.46 | 1 | 7 | – | – | – | 21 | 171 | – | – | – | 166 | 493 | – | – | – |
| Rigotti 201054 | 1.00 | 1 | 7 | – | – | – | 26 | 68 | – | – | – | 359 | 355 | – | – | – |
| Tashkin 201157 | 1.00 | 1 | 7 | – | – | – | 14 | 47 | – | – | – | 254 | 250 | – | – | – |
| Tsai 200758 | 0.46 | 1 | 7 | – | – | – | 27 | 59 | – | – | – | 124 | 126 | – | – | – |
| Smith 201355 | 1.00 | 1 | 7 | – | – | – | 42 | 61 | – | – | – | 196 | 196 | – | – | – |
| Aubin 200846 | 1.00 | 2 | 7 | – | – | – | 75 | 98 | – | – | – | 379 | 378 | – | – | – |
| Wang 200960 | 0.46 | 1 | 7 | – | – | – | 42 | 63 | – | – | – | 168 | 165 | – | – | – |
| Gonzales 200648 | 1.00 | 1 | 7 | 8 | – | – | 29 | 77 | 53 | – | – | 344 | 352 | 329 | – | – |
| Jorenby 200616 | 1.00 | 1 | 7 | 8 | – | – | 35 | 79 | 50 | – | – | 341 | 344 | 342 | – | – |
| Oncken 200652 | 1.00 | 1 | 6 | 7 | – | – | 5 | 48 | 58 | – | – | 129 | 259 | 259 | – | – |
| Nakamura 200749 | 1.00 | 1 | 6 | 7 | – | – | 35 | 51 | 56 | – | – | 154 | 156 | 156 | – | – |
| Nides 200651 | 1.00 | 1 | 4 | 5 | 7 | 8 | 6 | 10 | 7 | 18 | 8 | 127 | 128 | 128 | 127 | 128 |
| Author, year | Study duration (weeks) | Treatments | Number of events | Number of patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | ||
| Pfizer 201140/Williams 201241 | 24 | 1 | 6 | – | – | – | 4 | 6 | – | – | – | 43 | 84 | – | – | – |
| Rennard 201253 | 24 | 1 | 6 | – | – | – | 5 | 61 | – | – | – | 165 | 486 | – | – | – |
| Rigotti 201054 | 52 | 1 | 6 | – | – | – | 6 | 28 | – | – | – | 350 | 353 | – | – | – |
| Tashkin 201157 | 52 | 1 | 6 | – | – | – | 7 | 27 | – | – | – | 251 | 248 | – | – | – |
| Tsai 200758 | 24 | 1 | 6 | – | – | – | 1 | 7 | – | – | – | 124 | 126 | – | – | – |
| Williams 200761 | 52 | 1 | 8 | – | – | – | 9 | 57 | – | – | – | 126 | 251 | – | – | – |
| Smith 201355 | 52 | 1 | 6 | – | – | – | 2 | 12 | – | – | – | 196 | 196 | – | – | – |
| Aubin 200846 | 52 | 2 | 6 | – | – | – | 31 | 44 | – | – | – | 370 | 376 | – | – | – |
| Wong 201237 | 52 | 1 | 6 | – | – | – | 0 | 3 | – | – | – | 135 | 151 | – | – | – |
| Heydari 201239 | 52 | 1 | 2 | 6 | – | – | 0 | 0 | 3 | – | – | 91 | 92 | 89 | – | – |
| Gonzales 200648 | 52 | 1 | 6 | 7 | – | – | 19 | 36 | 18 | – | – | 344 | 349 | 329 | – | – |
| Jorenby 200616 | 52 | 1 | 6 | 7 | – | – | 12 | 45 | 20 | – | – | 340 | 343 | 340 | – | – |
| Oncken 200652 | 52 | 1 | 5 | 6 | – | – | 6 | 36 | 46 | – | – | 121 | 253 | 253 | – | – |
| Nides 200651 | 52 | 1 | 3 | 4 | 6 | 7 | 10 | 10 | 14 | 19 | 15 | 123 | 126 | 126 | 125 | 126 |
| Author, year | Study duration (weeks) | Treatments | Number of events | Number of patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | ||
| Vinnikov 200843 | 26 | 1 | 3 | – | – | – | 1 | 1 | – | – | – | 86 | 85 | – | – | – |
| West 201117 | 52 | 1 | 3 | – | – | – | 8 | 7 | – | – | – | 370 | 370 | – | – | – |
| Bolliger 201147 | 24 | 1 | 8 | – | – | – | 24 | 64 | – | – | – | 198 | 390 | – | – | – |
| Niaura 200850 | 52 | 1 | 8 | – | – | – | 20 | 25 | – | – | – | 155 | 157 | – | – | – |
| Pfizer 201140/Williams 201241 | 24 | 1 | 8 | – | – | – | 8 | 9 | – | – | – | 43 | 84 | – | – | – |
| Rennard 201253 | 24 | 1 | 8 | – | – | – | 20 | 55 | – | – | – | 165 | 486 | – | – | – |
| Rigotti 201054 | 52 | 1 | 8 | – | – | – | 39 | 45 | – | – | – | 350 | 353 | – | – | – |
| Tashkin 201157 | 52 | 1 | 8 | – | – | – | 20 | 20 | – | – | – | 251 | 248 | – | – | – |
| Wang 200960 | 24 | 1 | 8 | – | – | – | 7 | 9 | – | – | – | 168 | 165 | – | – | – |
| Smith 201355 | 52 | 1 | 8 | – | – | – | 3 | 12 | – | – | – | 196 | 196 | – | – | – |
| Aubin 200846 | 52 | 2 | 8 | – | – | – | 36 | 72 | – | – | – | 370 | 376 | – | – | – |
| Wong 201237 | 52 | 1 | 8 | – | – | – | 0 | 5 | – | – | – | 135 | 151 | – | – | – |
| Gonzales 200648 | 52 | 1 | 8 | 9 | – | – | 42 | 54 | 47 | – | – | 344 | 349 | 329 | – | – |
| Jorenby 200616 | 52 | 1 | 8 | 9 | – | – | 43 | 44 | 27 | – | – | 340 | 343 | 340 | – | – |
| Oncken 200652 | 52 | 1 | 7 | 8 | – | – | 21 | 59 | 59 | – | – | 121 | 253 | 253 | – | – |
| Nakamura 200749 | 52 | 1 | 6 | 7 | 8 | – | 4 | 16 | 18 | 16 | – | 154 | 153 | 155 | 156 | – |
| Nides 200651 | 52 | 1 | 4 | 5 | 8 | 9 | 33 | 34 | 34 | 30 | 38 | 123 | 126 | 126 | 125 | 126 |
| Author, year | Study duration (weeks) | Treatments | Number of events | Number of patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | ||
| Bolliger 201147 | 24 | 1 | 6 | – | – | – | 13 | 50 | – | – | – | 198 | 390 | – | – | – |
| Niaura 200850 | 52 | 1 | 6 | – | – | – | 17 | 34 | – | – | – | 155 | 157 | – | – | – |
| Pfizer 201140/Williams 201241 | 24 | 1 | 6 | – | – | – | 2 | 8 | – | – | – | 43 | 84 | – | – | – |
| Rennard 201253 | 24 | 1 | 6 | – | – | – | 6 | 43 | – | – | – | 165 | 486 | – | – | – |
| Rigotti 201054 | 52 | 1 | 6 | – | – | – | 23 | 42 | – | – | – | 350 | 353 | – | – | – |
| Tashkin 201157 | 52 | 1 | 6 | – | – | – | 15 | 24 | – | – | – | 251 | 248 | – | – | – |
| Tsai 200758 | 24 | 1 | 6 | – | – | – | 17 | 19 | – | – | – | 124 | 126 | – | – | – |
| Wang 200960 | 24 | 1 | 6 | – | – | – | 5 | 10 | – | – | – | 168 | 165 | – | – | – |
| Williams 200761 | 52 | 1 | 8 | – | – | – | 12 | 48 | – | – | – | 126 | 251 | – | – | – |
| Smith 201355 | 52 | 1 | 6 | – | – | – | 4 | 10 | – | – | – | 196 | 196 | – | – | – |
| Aubin 200846 | 52 | 2 | 6 | – | – | – | 71 | 80 | – | – | – | 370 | 376 | – | – | – |
| Tsukahara 201059 | 24 | 2 | 6 | – | – | – | 2 | 6 | – | – | – | 14 | 14 | – | – | – |
| Gonzales 200648 | 52 | 1 | 6 | 7 | – | – | 44 | 49 | 72 | – | – | 344 | 349 | 329 | – | – |
| Jorenby 200616 | 52 | 1 | 6 | 7 | – | – | 42 | 49 | 72 | – | – | 340 | 343 | 340 | – | – |
| Oncken 200652 | 52 | 1 | 5 | 6 | – | – | 14 | 69 | 75 | – | – | 121 | 253 | 253 | – | – |
| Nides 200651 | 52 | 1 | 3 | 4 | 6 | 7 | 27 | 25 | 34 | 44 | 57 | 123 | 126 | 126 | 125 | 126 |
| Author, year | Study duration (weeks) | Treatments | Number of events | Number of patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | ||
| Vinnikov 200843 | 26 | 1 | 3 | – | – | – | 1 | 2 | – | – | – | 86 | 85 | – | – | – |
| West 201117 | 52 | 1 | 3 | – | – | – | 14 | 10 | – | – | – | 370 | 370 | – | – | – |
| Bolliger 201147 | 24 | 1 | 8 | – | – | – | 16 | 103 | – | – | – | 198 | 390 | – | – | – |
| Niaura 200850 | 52 | 1 | 8 | – | – | – | 8 | 21 | – | – | – | 155 | 157 | – | – | – |
| Pfizer 201140/Williams 201241 | 24 | 1 | 8 | – | – | – | 6 | 20 | – | – | – | 43 | 84 | – | – | – |
| Rennard 201253 | 24 | 1 | 8 | – | – | – | 15 | 142 | – | – | – | 165 | 486 | – | – | – |
| Rigotti 201054 | 52 | 1 | 8 | – | – | – | 30 | 104 | – | – | – | 350 | 353 | – | – | – |
| Steinberg 201156 | 24 | 1 | 8 | – | – | – | 2 | 10 | – | – | – | 37 | 38 | – | – | – |
| Tashkin 201157 | 52 | 1 | 8 | – | – | – | 20 | 67 | – | – | – | 251 | 248 | – | – | – |
| Tsai 200758 | 24 | 1 | 8 | – | – | – | 14 | 55 | – | – | – | 124 | 126 | – | – | – |
| Wang 200960 | 24 | 1 | 8 | – | – | – | 20 | 48 | – | – | – | 168 | 165 | – | – | – |
| Williams 200761 | 52 | 1 | 10 | – | – | – | 10 | 101 | – | – | – | 126 | 251 | – | – | – |
| Smith 201355 | 52 | 1 | 8 | – | – | – | 3 | 32 | – | – | – | 196 | 196 | – | – | – |
| Aubin 200846 | 52 | 2 | 8 | – | – | – | 36 | 140 | – | – | – | 370 | 376 | – | – | – |
| Tsukahara 201059 | 24 | 2 | 8 | – | – | – | 0 | 4 | – | – | – | 14 | 14 | – | – | – |
| Wong 201237 | 52 | 1 | 8 | – | – | – | 5 | 20 | – | – | – | 135 | 151 | – | – | – |
| Gonzales 200648 | 52 | 1 | 8 | 9 | – | – | 29 | 98 | 41 | – | – | 344 | 349 | 329 | – | – |
| Jorenby 200616 | 52 | 1 | 8 | 9 | – | – | 33 | 101 | 25 | – | – | 340 | 343 | 340 | – | – |
| Oncken 200652 | 52 | 1 | 7 | 8 | – | – | 18 | 49 | 97 | – | – | 121 | 253 | 253 | – | – |
| Heydari 201239 | 52 | 1 | 8 | 2 | – | – | 0 | 8 | 0 | – | – | 91 | 89 | 92 | – | – |
| Nakamura 200749 | 52 | 1 | 6 | 7 | 8 | – | 12 | 11 | 15 | 38 | – | 154 | 153 | 155 | 156 | – |
| Nides 200651 | 52 | 1 | 4 | 5 | 8 | 9 | 23 | 22 | 47 | 65 | 27 | 123 | 126 | 126 | 125 | 126 |
| Author, year | Study duration (weeks) | Treatments | Number of events | Number of patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | ||
| West 201117 | 52 | 1 | 3 | – | – | – | 3 | 4 | – | – | – | 370 | 370 | – | – | – |
| Bolliger 201147 | 24 | 1 | 8 | – | – | – | 2 | 11 | – | – | – | 199 | 394 | – | – | – |
| Niaura 200850 | 52 | 1 | 8 | – | – | – | 0 | 3 | – | – | – | 160 | 160 | – | – | – |
| Oncken 200652 | 52 | 1 | 7 | – | – | – | 2 | 6 | – | – | – | 129 | 253 | – | – | – |
| Pfizer 201140/Williams 201241 | 24 | 1 | 8 | – | – | – | 4 | 5 | – | – | – | 43 | 85 | – | – | – |
| Rennard 201253 | 24 | 1 | 8 | – | – | – | 1 | 6 | – | – | – | 166 | 493 | – | – | – |
| Rigotti 201054 | 52 | 1 | 8 | – | – | – | 21 | 23 | – | – | – | 354 | 353 | – | – | – |
| Steinberg 201156 | 24 | 1 | 8 | – | – | – | 5 | 6 | – | – | – | 39 | 40 | – | – | – |
| Tashkin 201157 | 52 | 1 | 8 | – | – | – | 15 | 12 | – | – | – | 253 | 248 | – | – | – |
| Tsai 200758 | 24 | 1 | 8 | – | – | – | 3 | 3 | – | – | – | 124 | 126 | – | – | – |
| Wang 200960 | 24 | 1 | 8 | – | – | – | 2 | 0 | – | – | – | 168 | 165 | – | – | – |
| Williams 200761 | 52 | 1 | 10 | – | – | – | 3 | 15 | – | – | – | 126 | 251 | – | – | – |
| Smith 201355 | 52 | 1 | 8 | – | – | – | 3 | 6 | – | – | – | 117 | 119 | – | – | – |
| Aubin 200846 | 52 | 2 | 8 | – | – | – | 8 | 2 | – | – | – | 370 | 376 | – | – | – |
| Gonzales 200648 | 52 | 1 | 8 | 9 | – | – | 9 | 4 | 3 | – | – | 344 | 349 | 329 | – | – |
| Jorenby 200616 | 52 | 1 | 8 | 9 | – | – | 6 | 8 | 9 | – | – | 341 | 344 | 340 | – | – |
| Nakamura 200749 | 52 | 1 | 6 | 7 | 8 | – | 3 | 5 | 2 | 3 | – | 154 | 153 | 155 | 156 | – |
| Nides 200651 | 52 | 1 | 4 | 5 | 8 | 9 | 0 | 0 | 0 | 1 | 4 | 127 | 126 | 126 | 125 | 126 |
FIGURE 13.
Continuous abstinence: forest plots of study-specific treatment effects and population mean treatment effects. a See Table 1 for regime details.
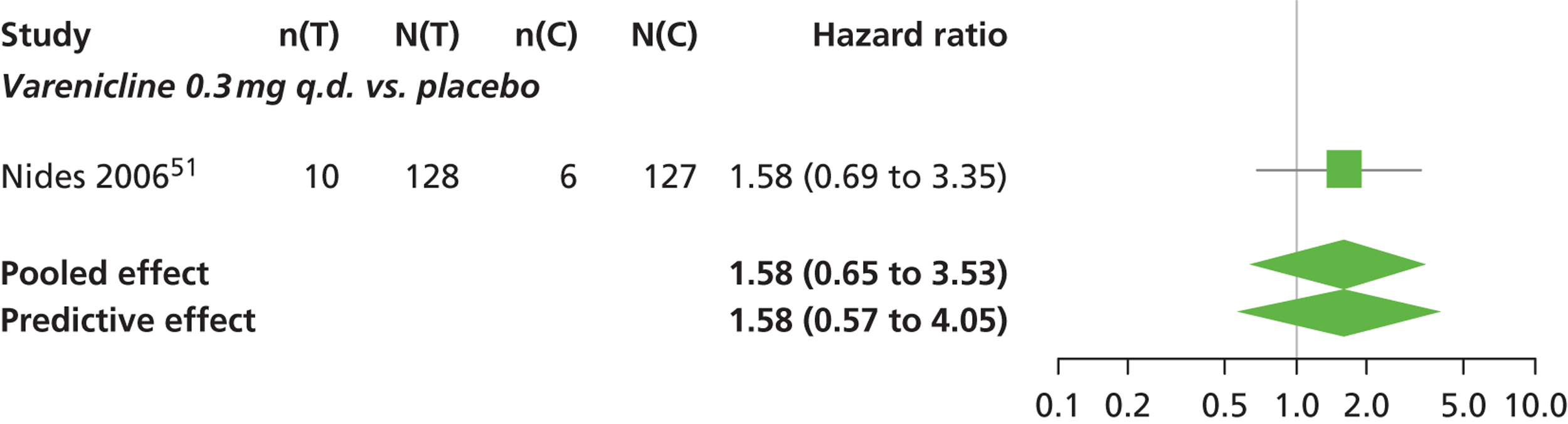
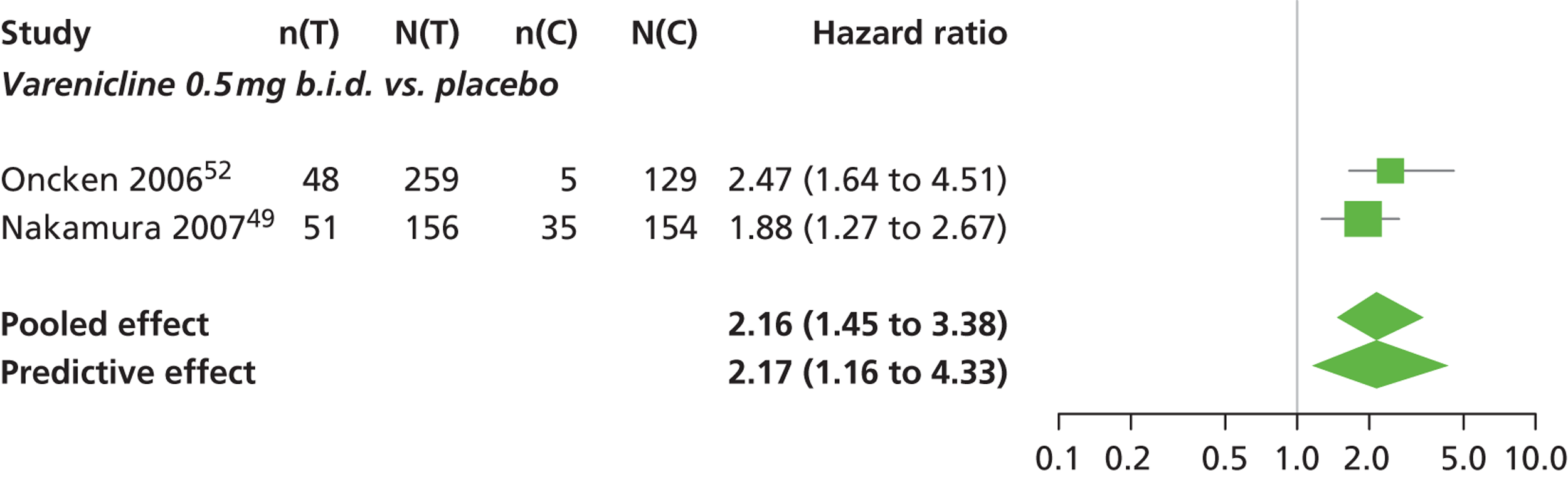
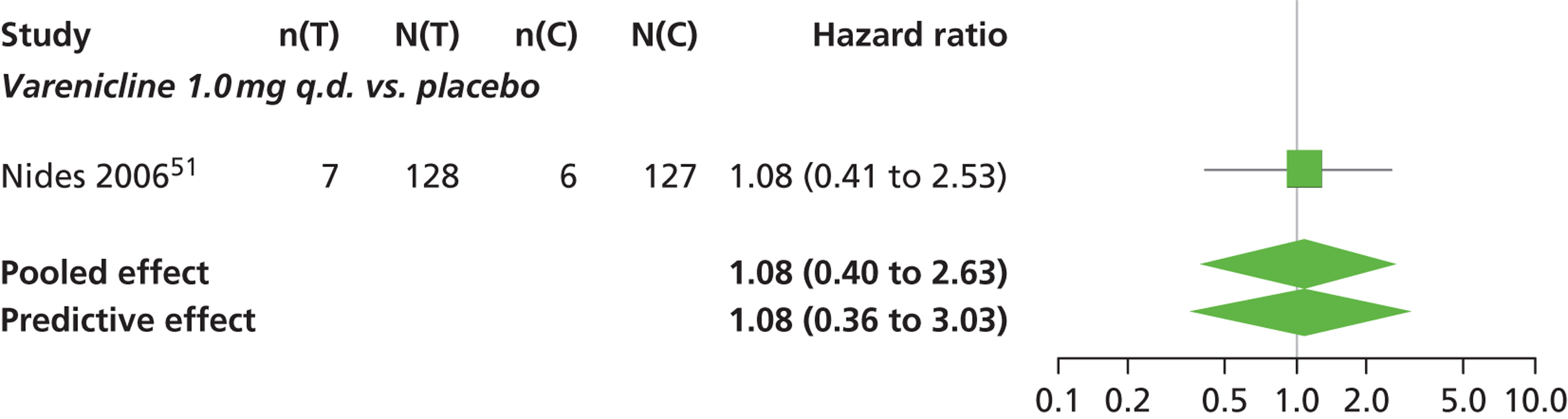
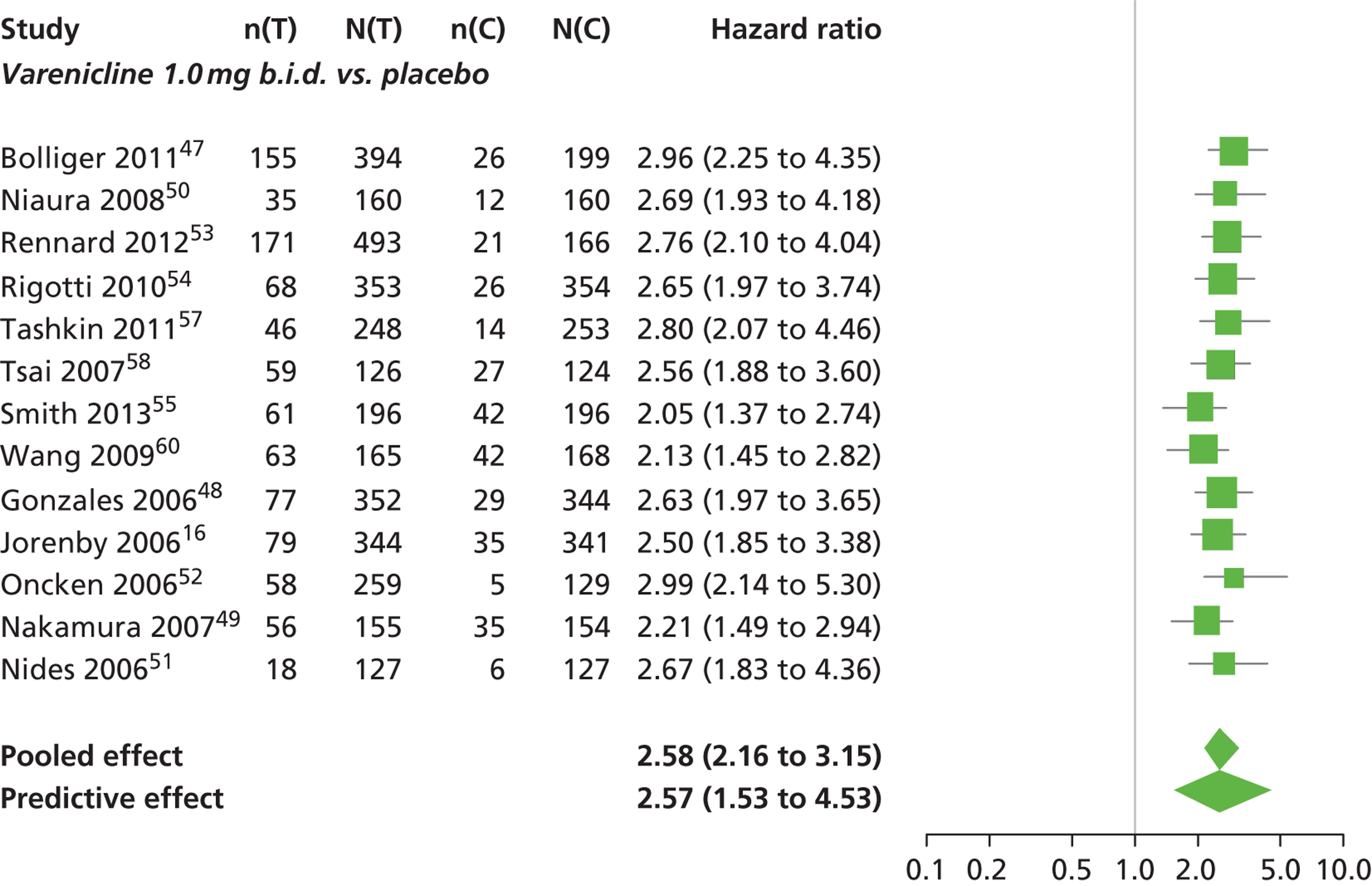
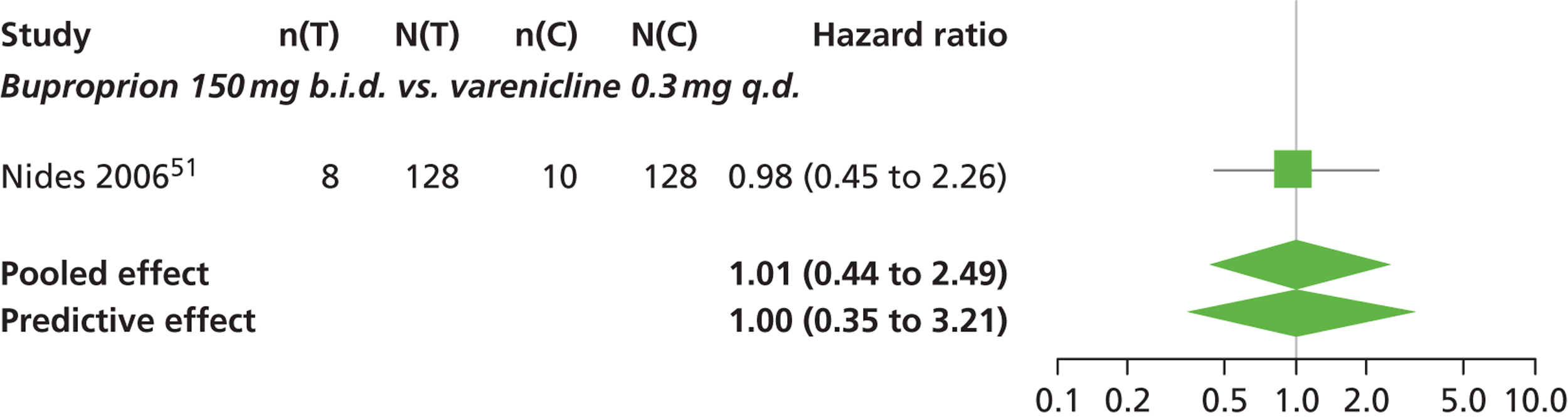
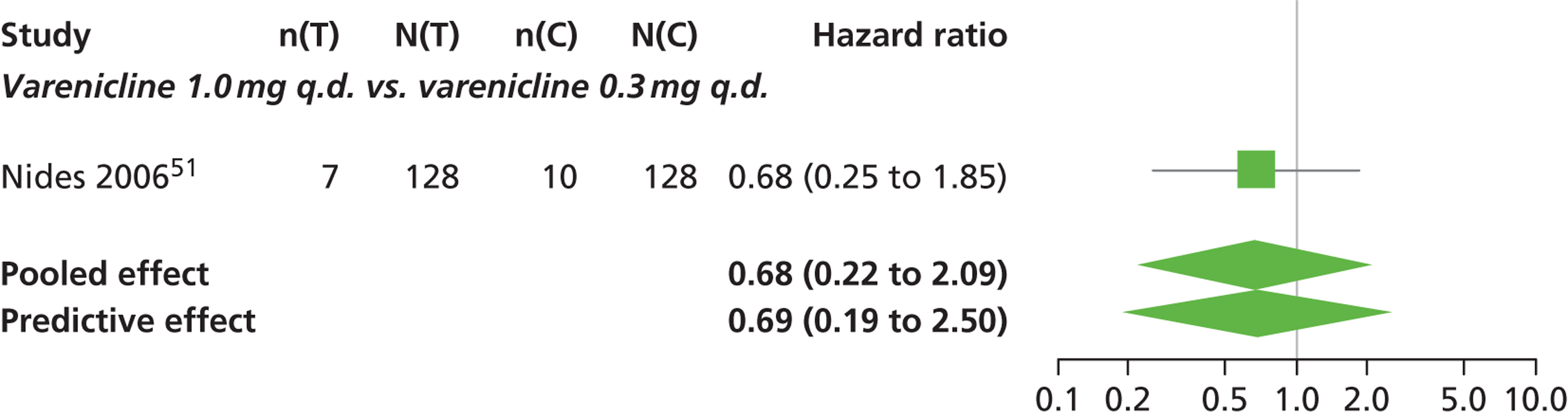
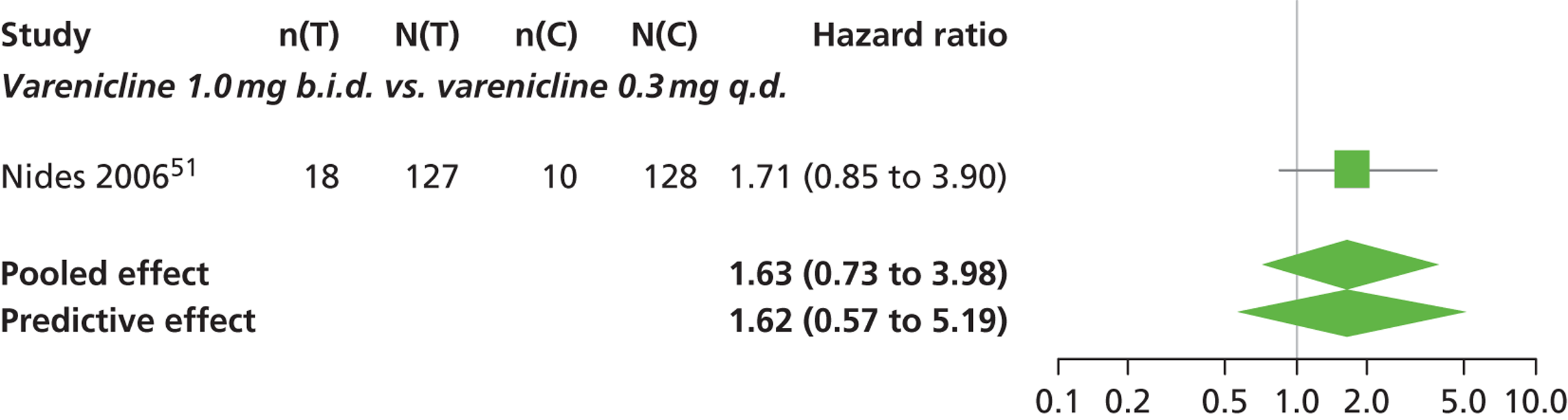
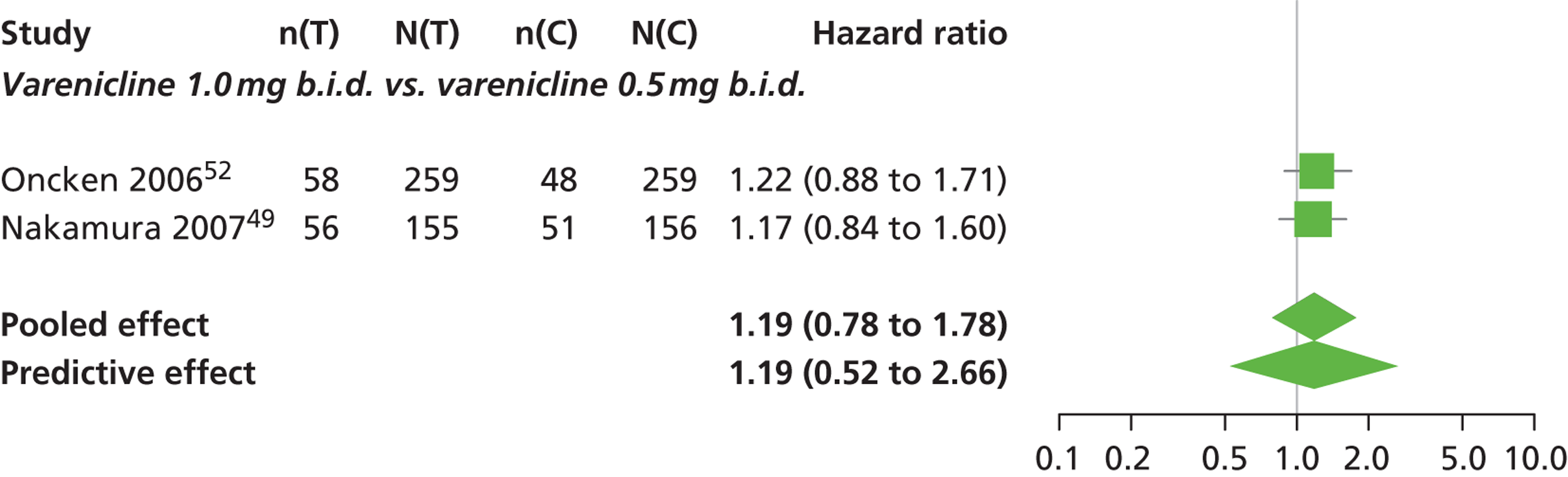
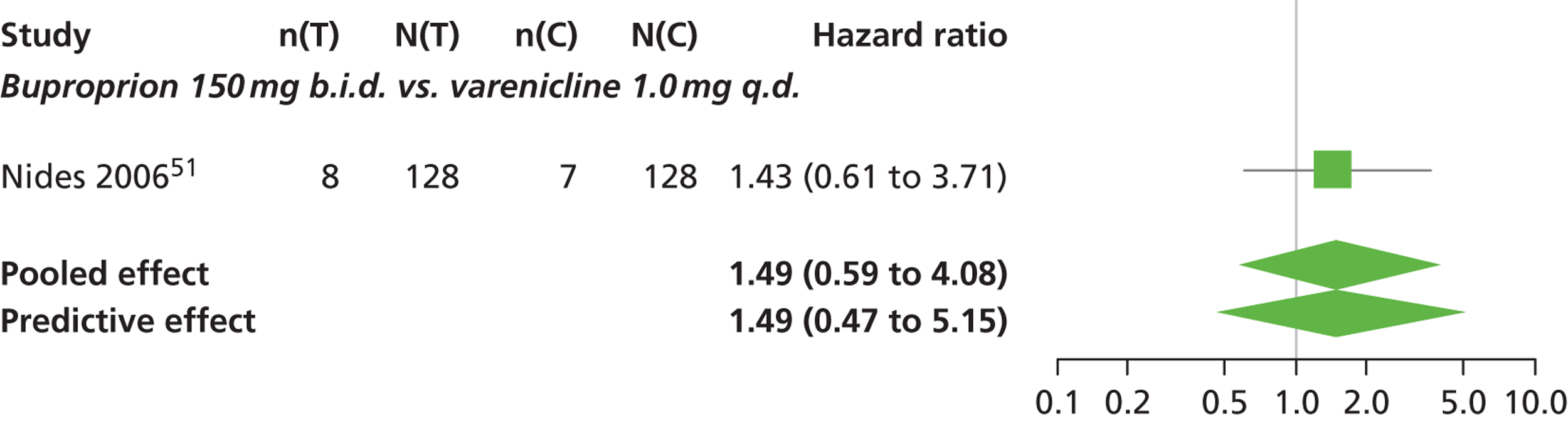
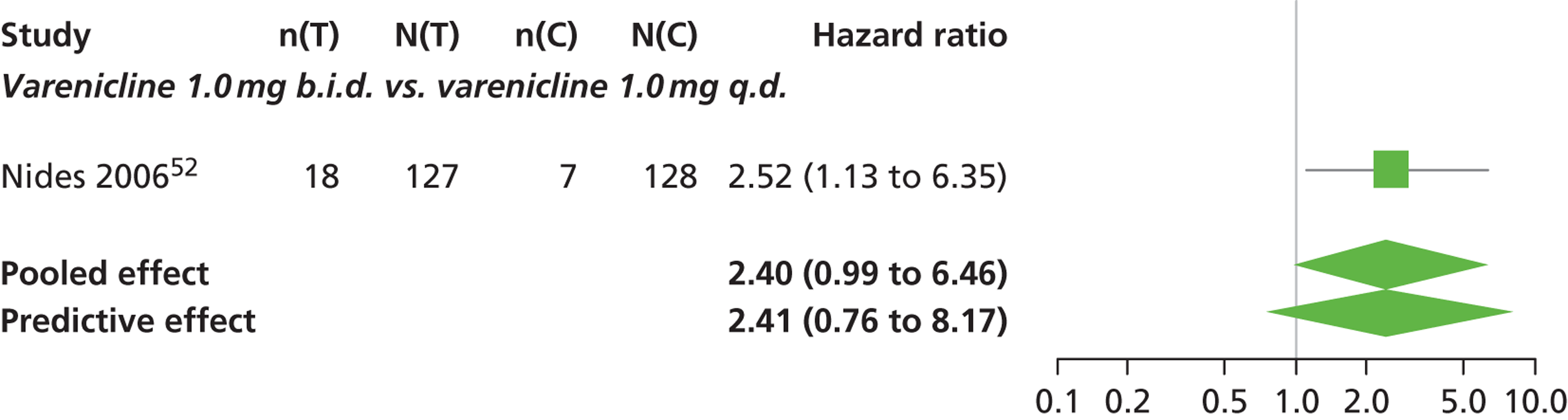
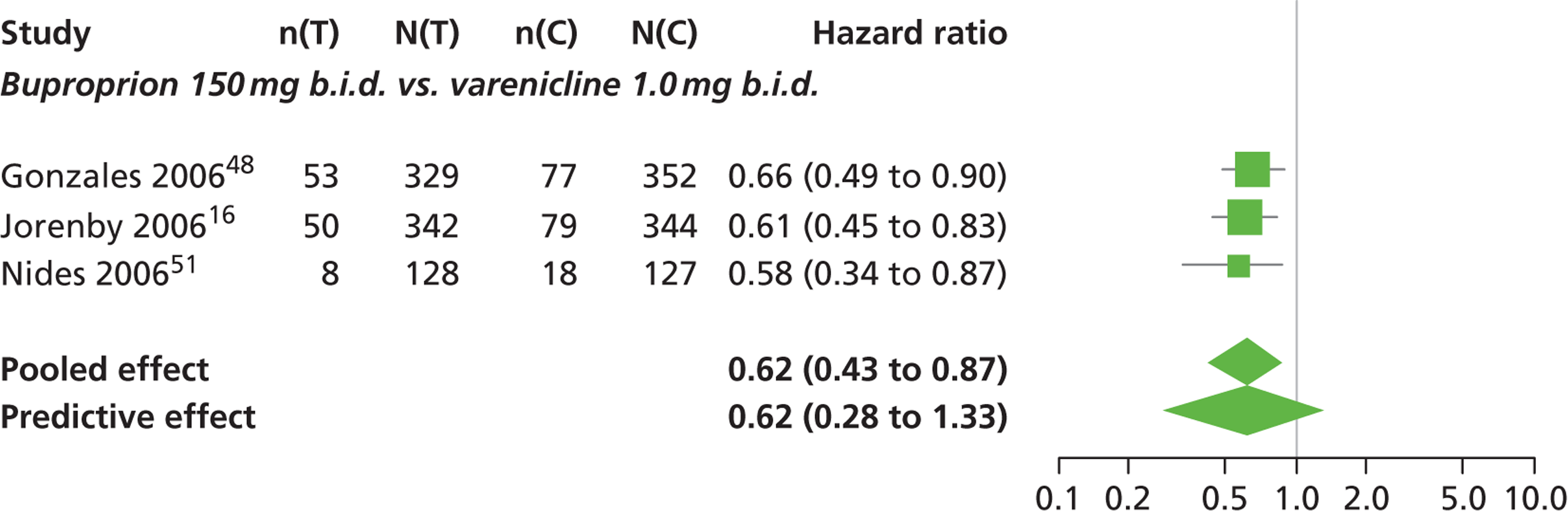
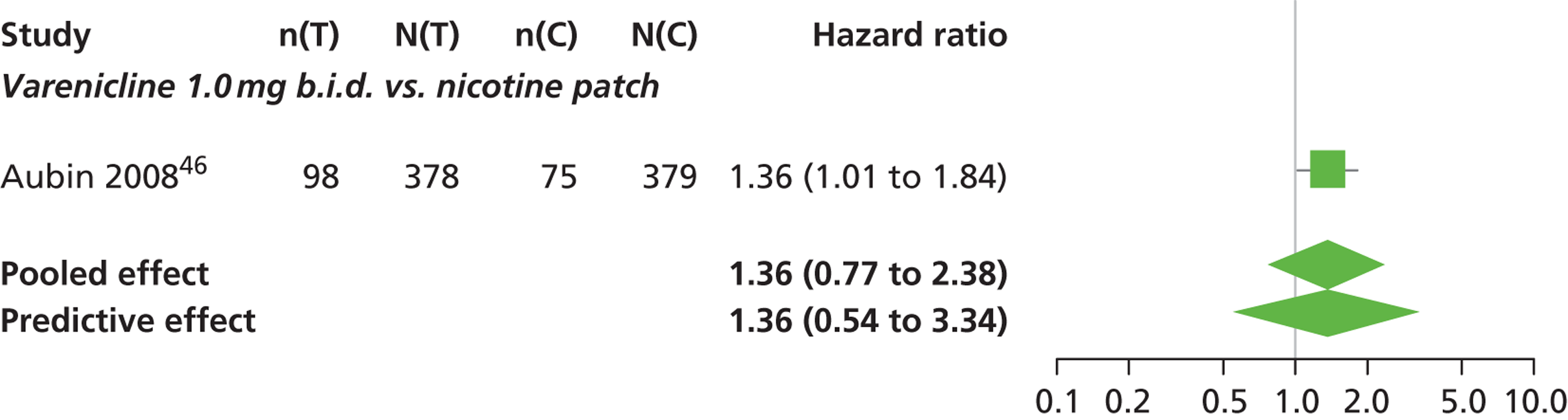
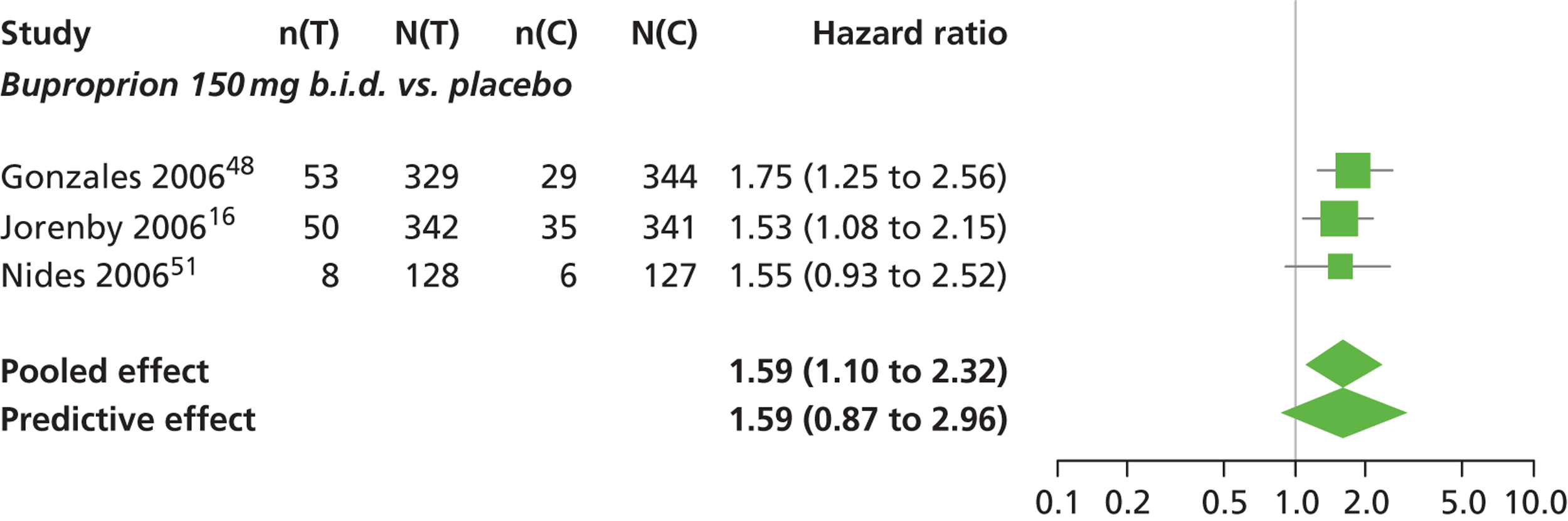
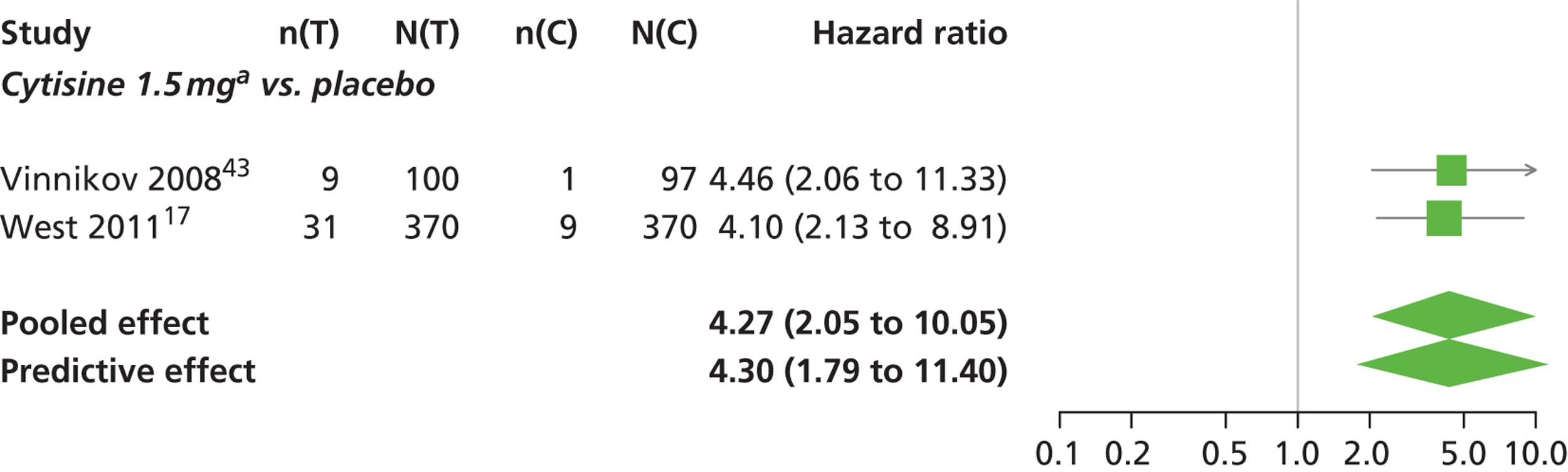
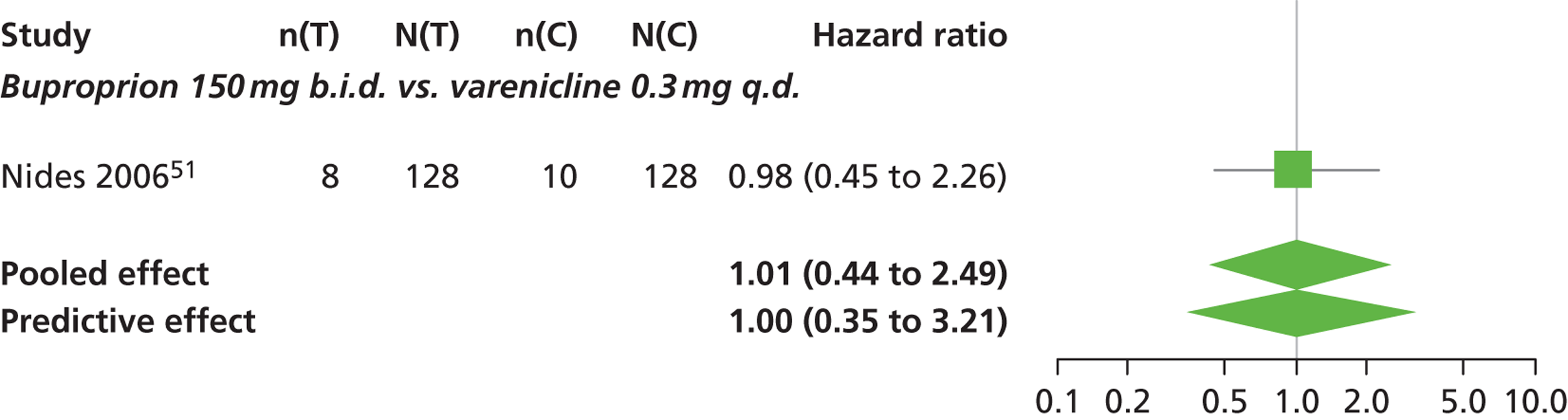
FIGURE 14.
Abnormal dreams: forest plots of study-specific treatment effects and population mean treatment effects, where median is used as the best estimate.
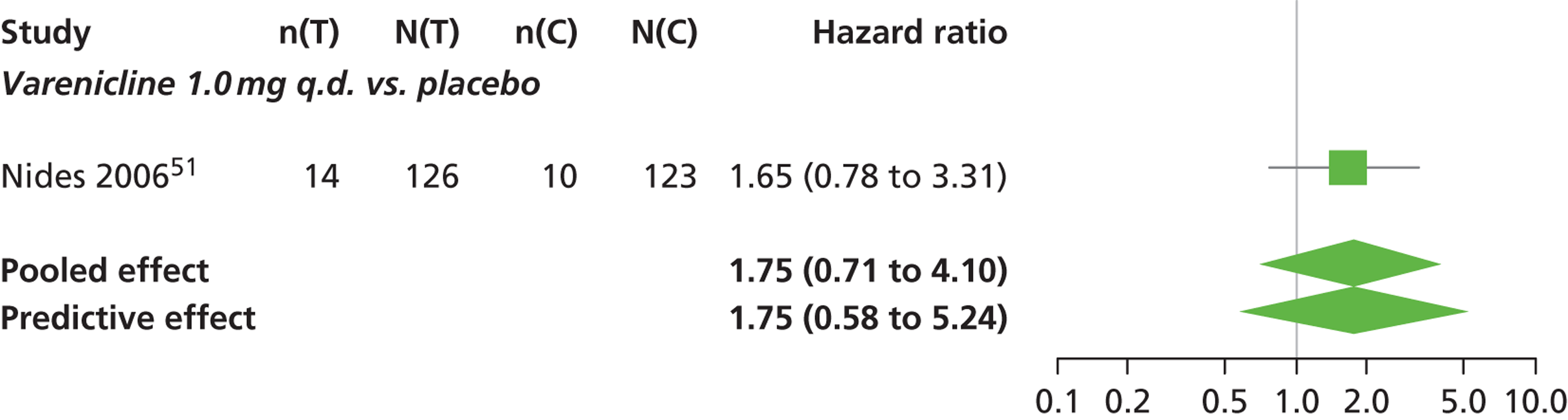
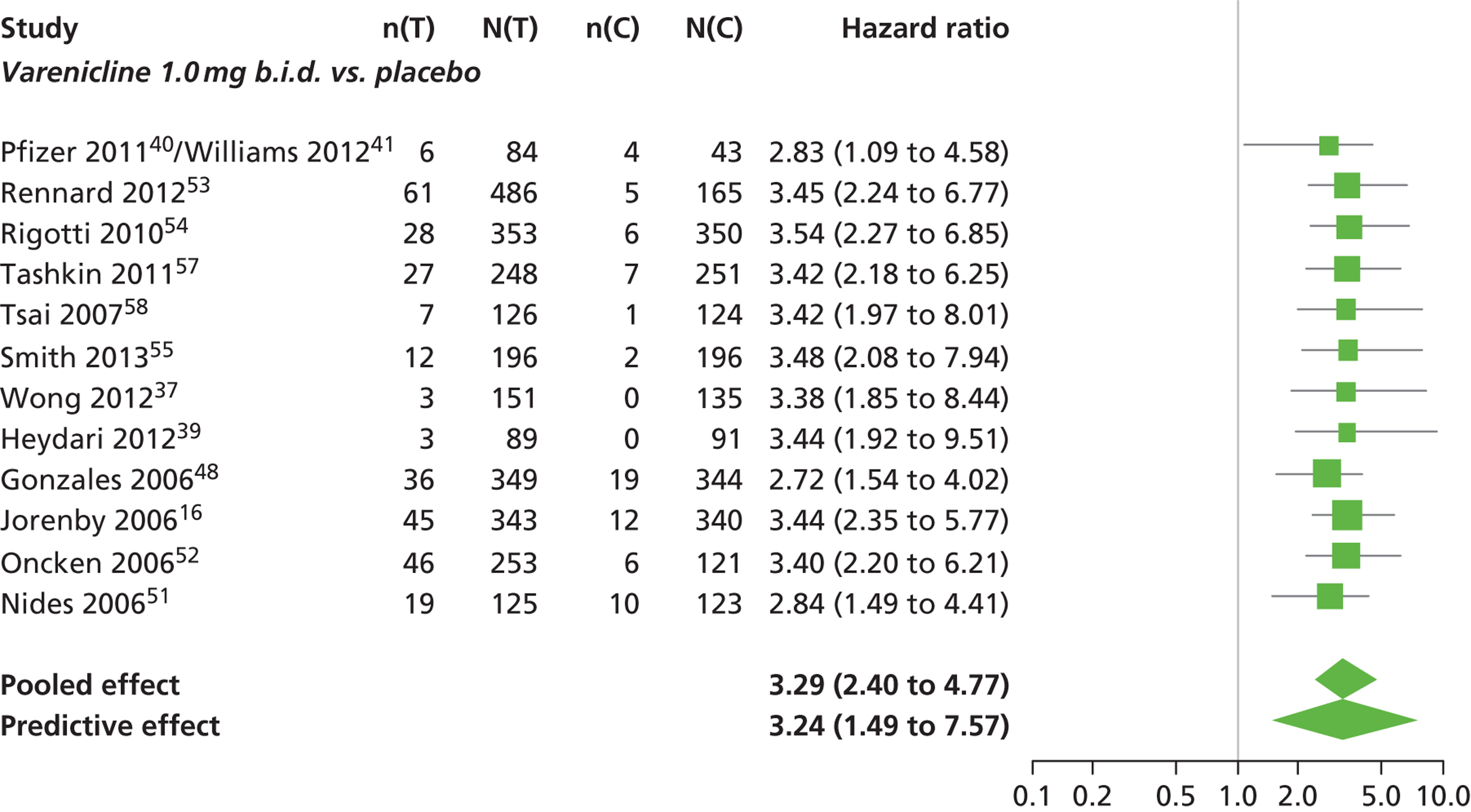
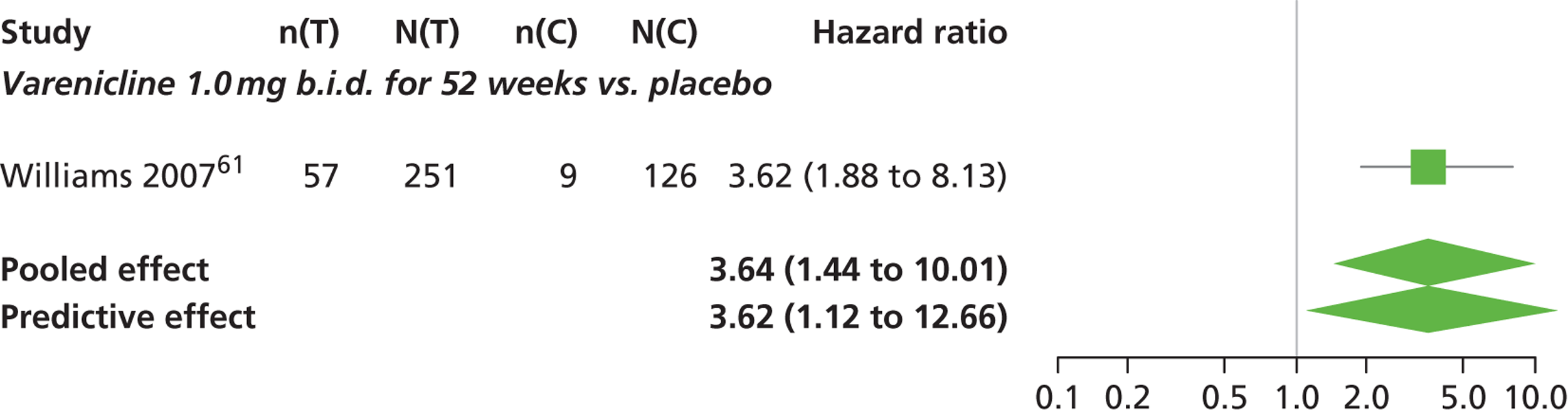
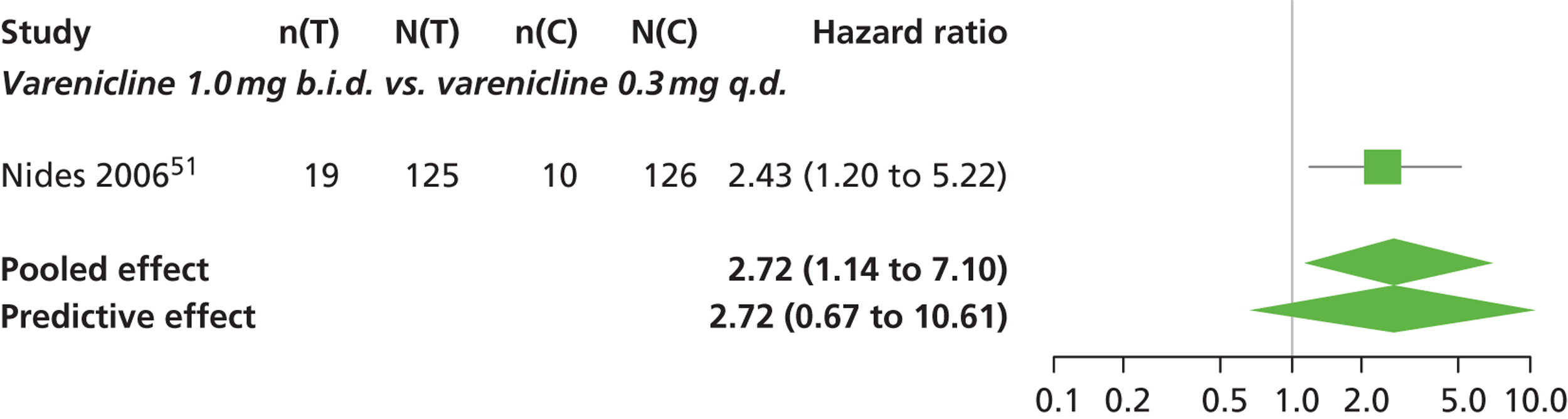
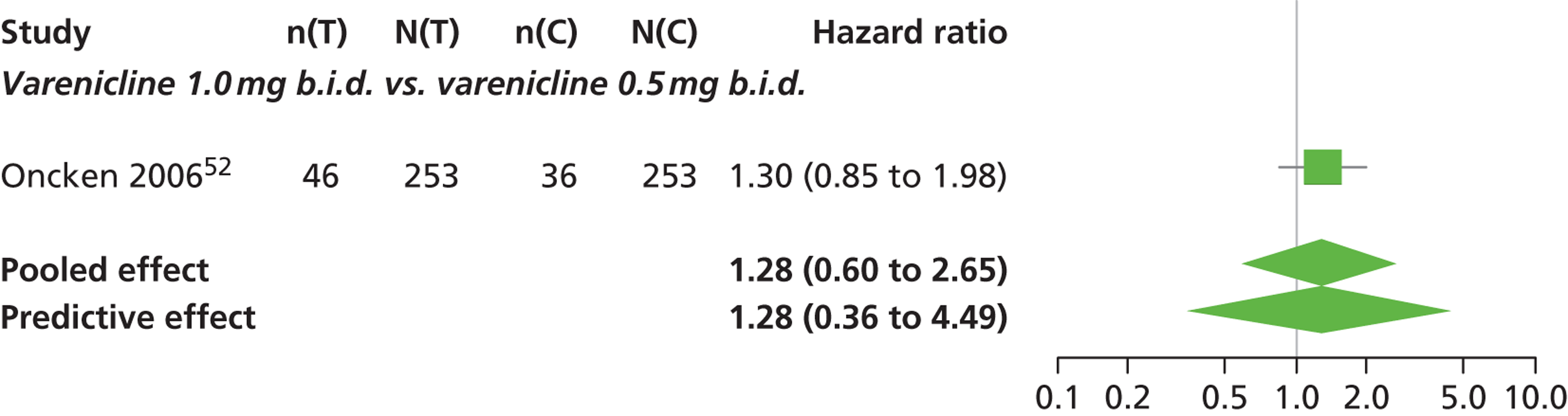
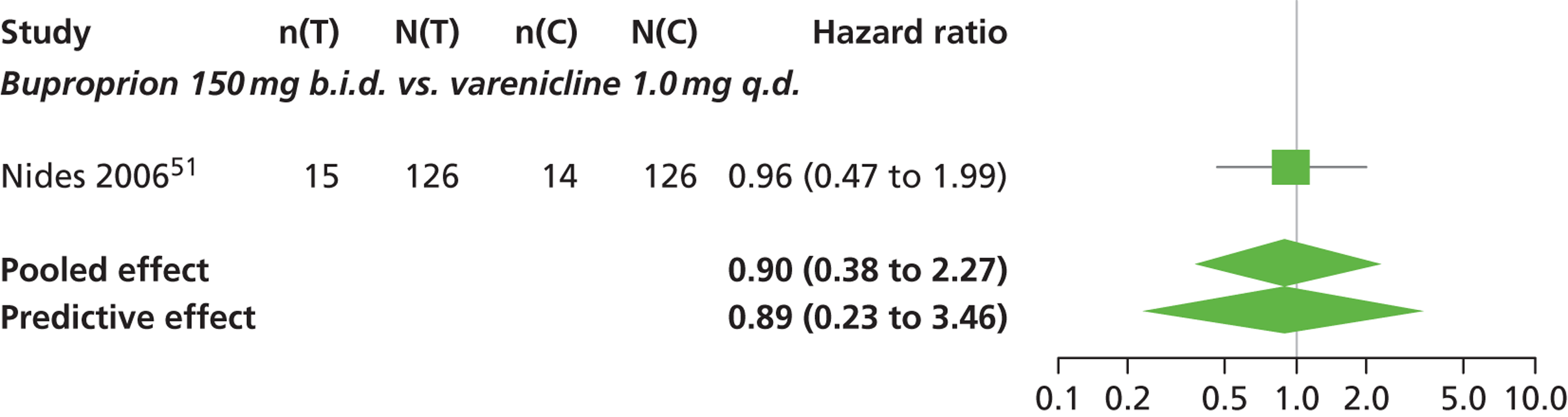
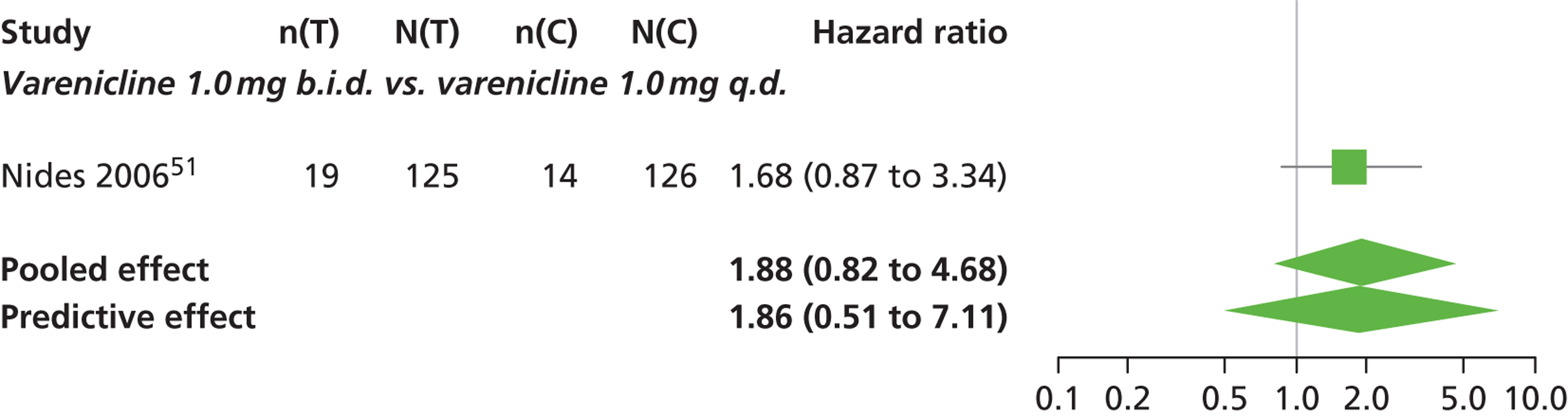
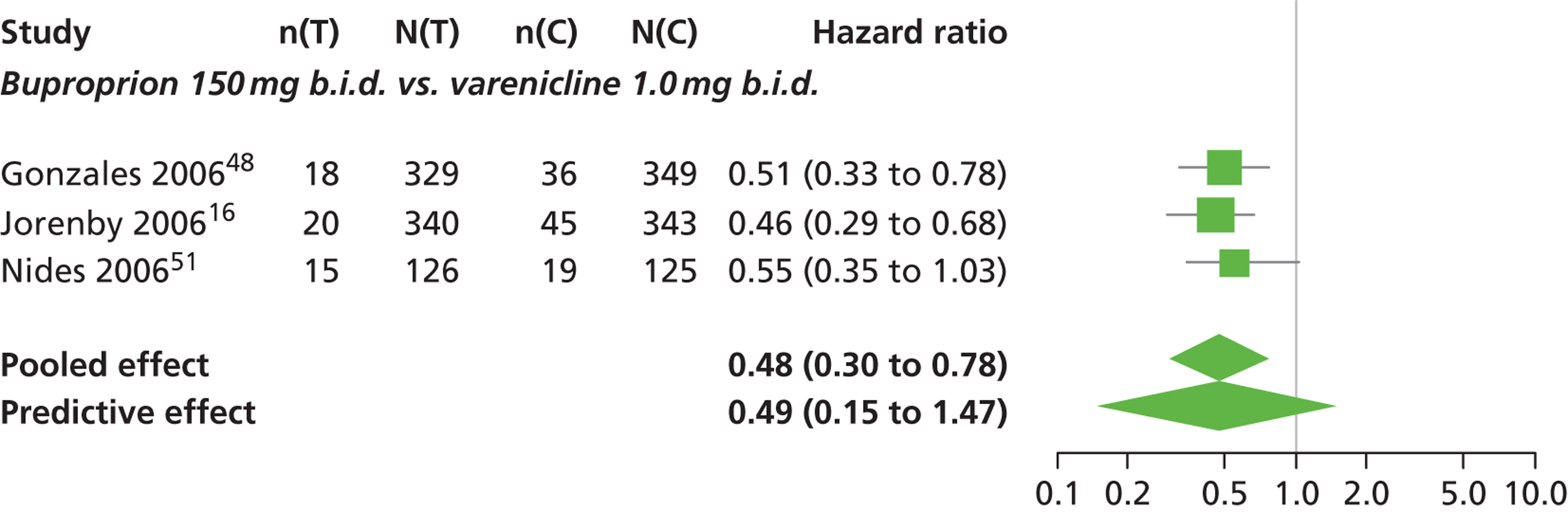
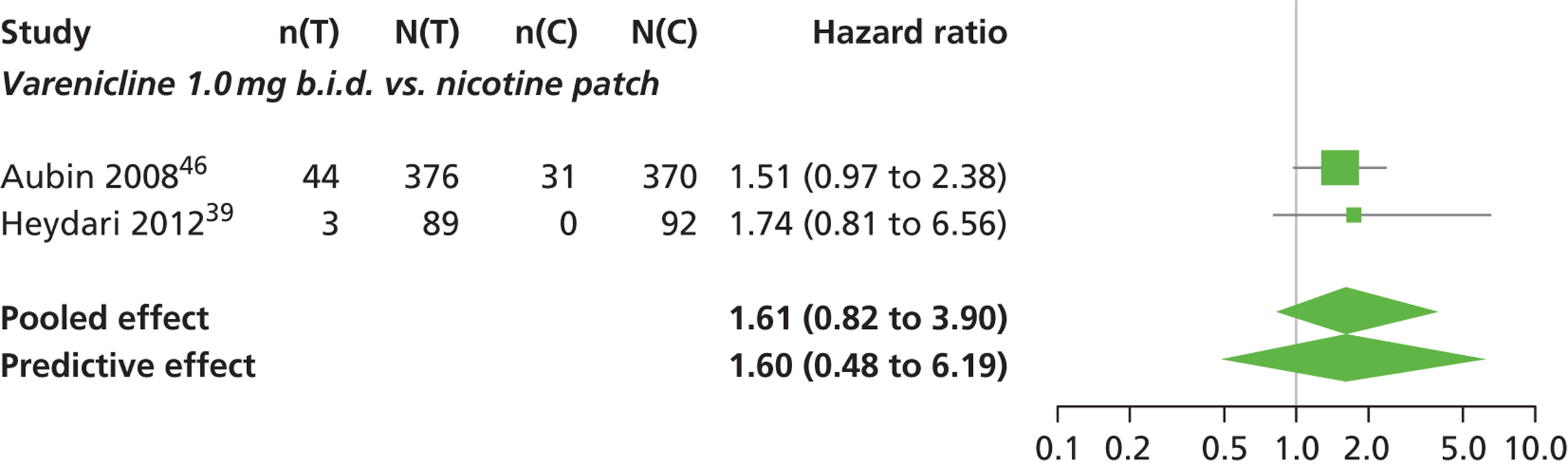
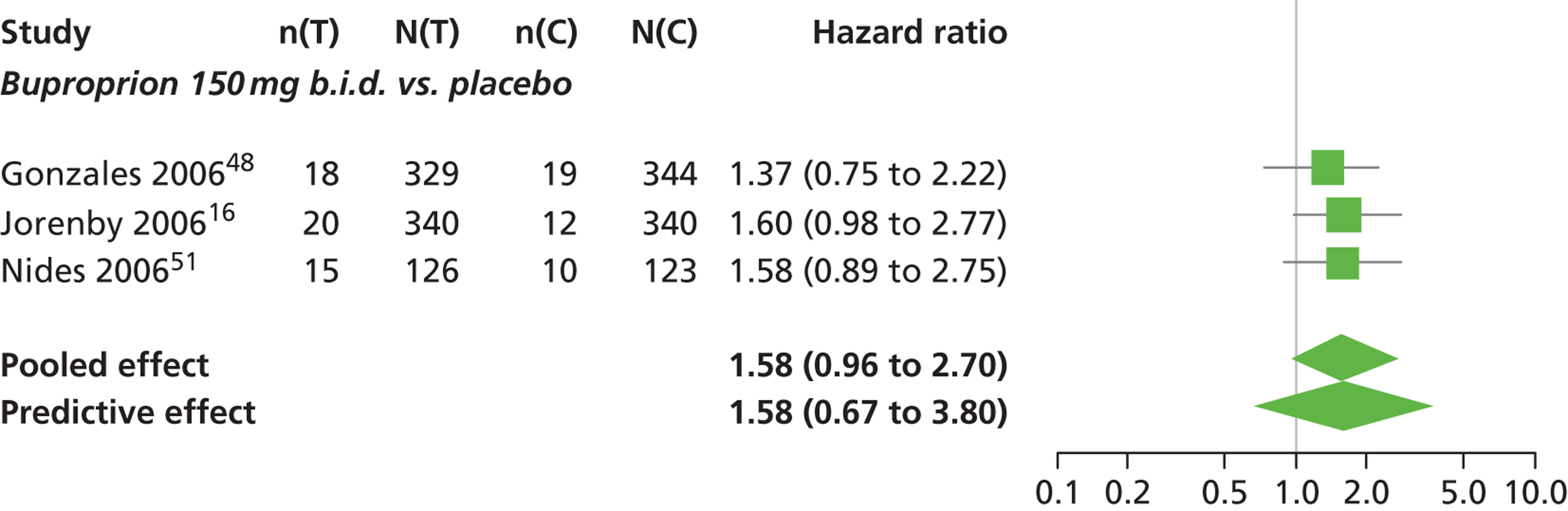
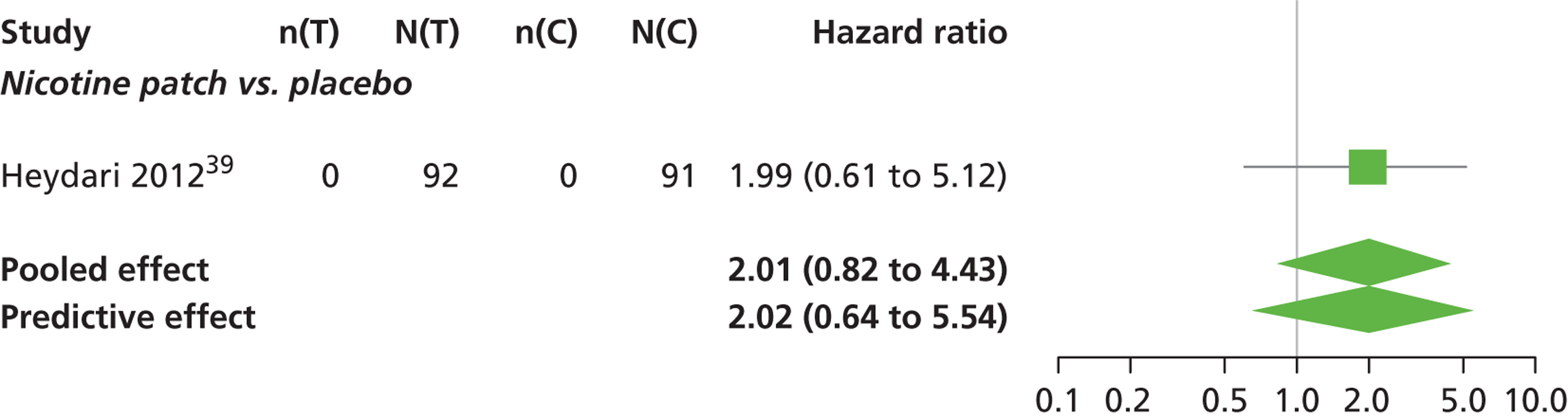
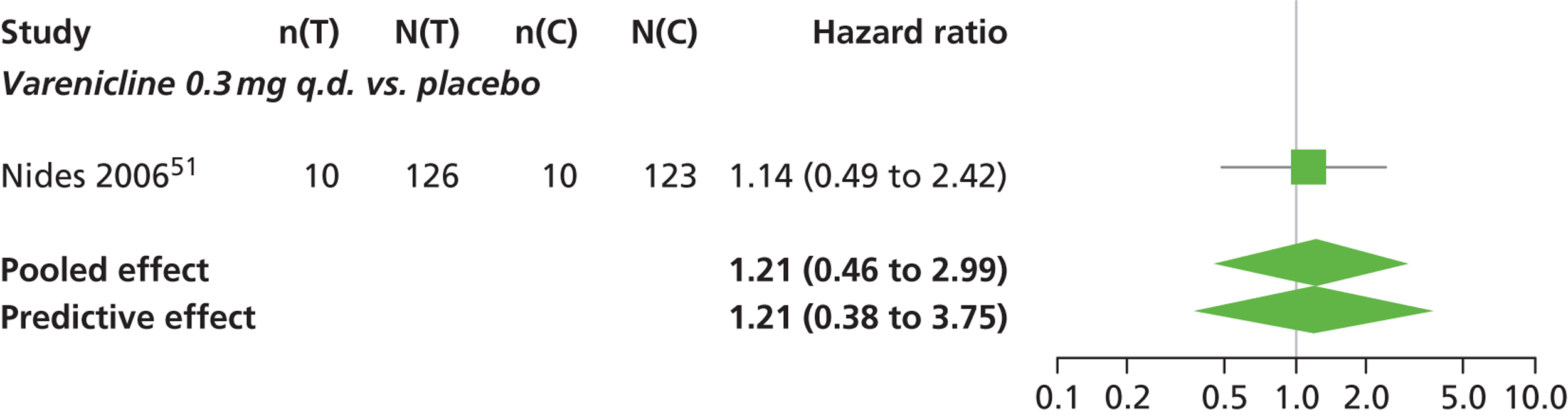
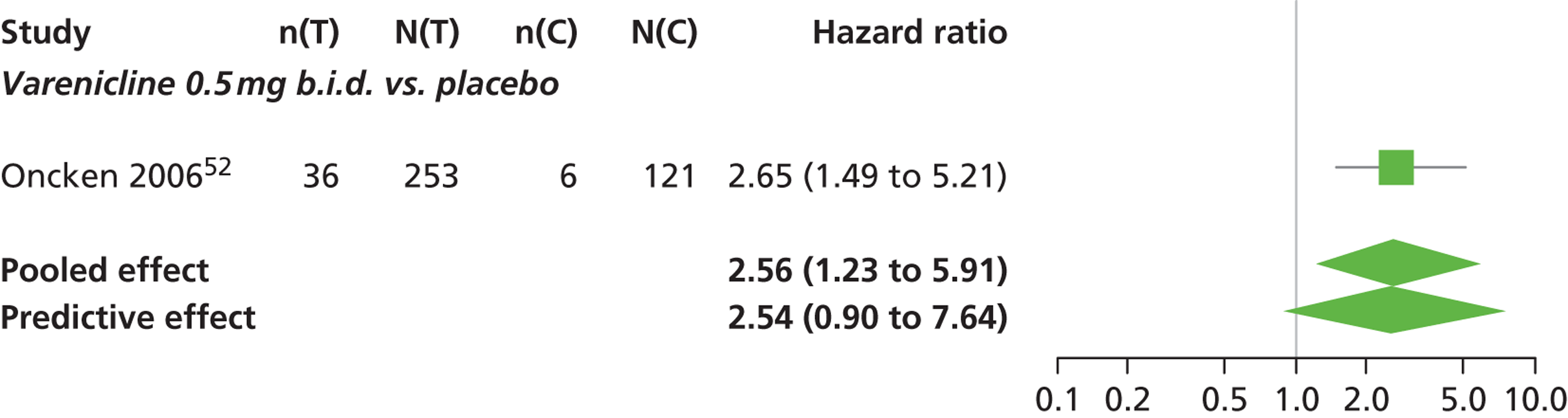
FIGURE 15.
Headache: forest plots of study-specific treatment effects and population mean treatment effects, where median is used as the best estimate. a See Table 1 for regime details.
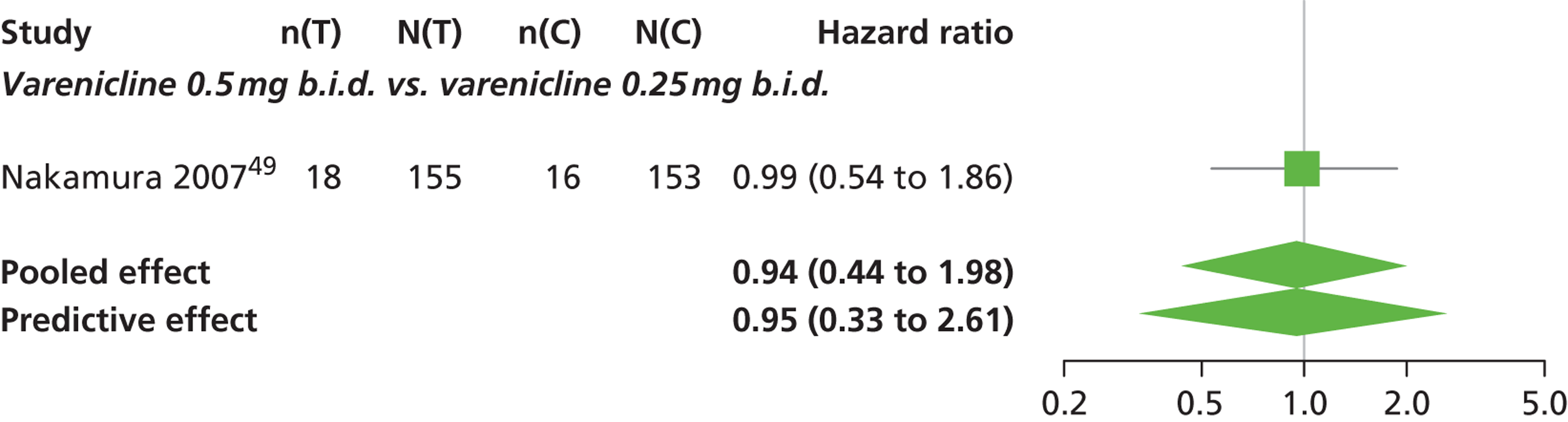
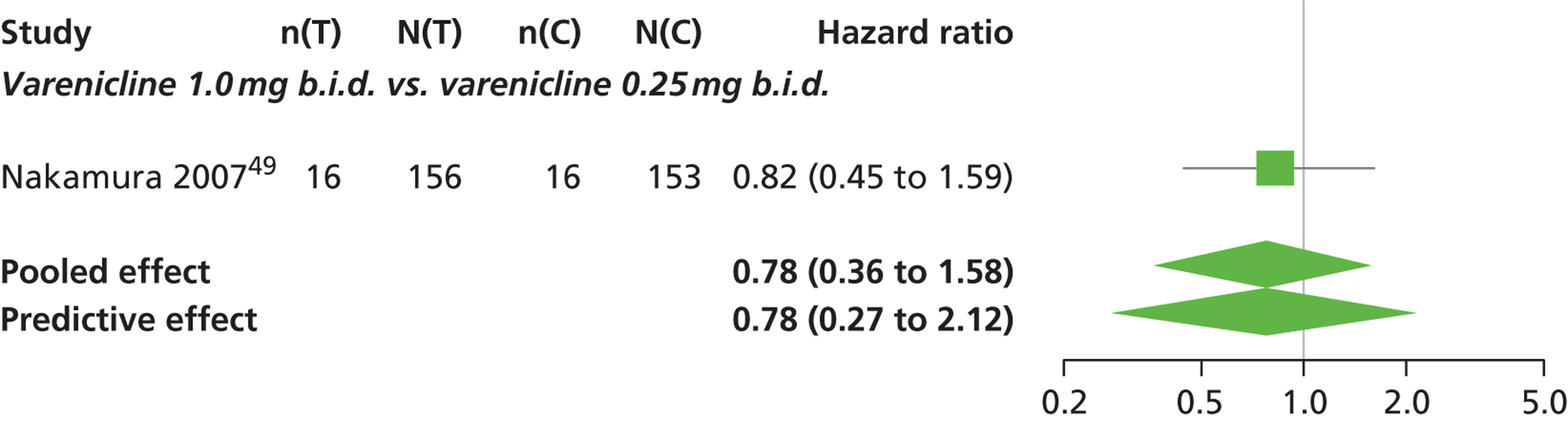
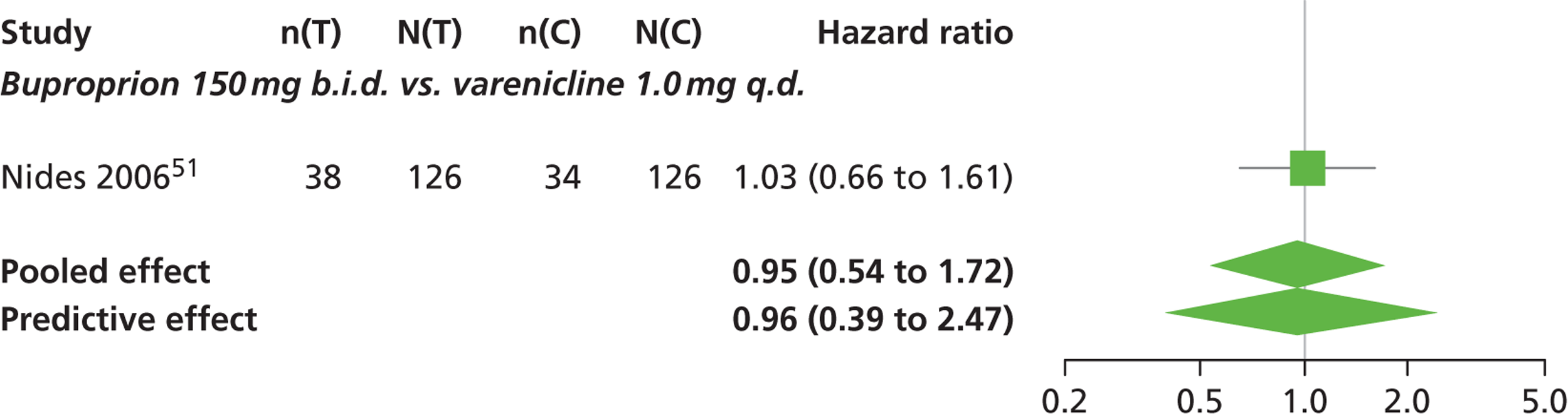
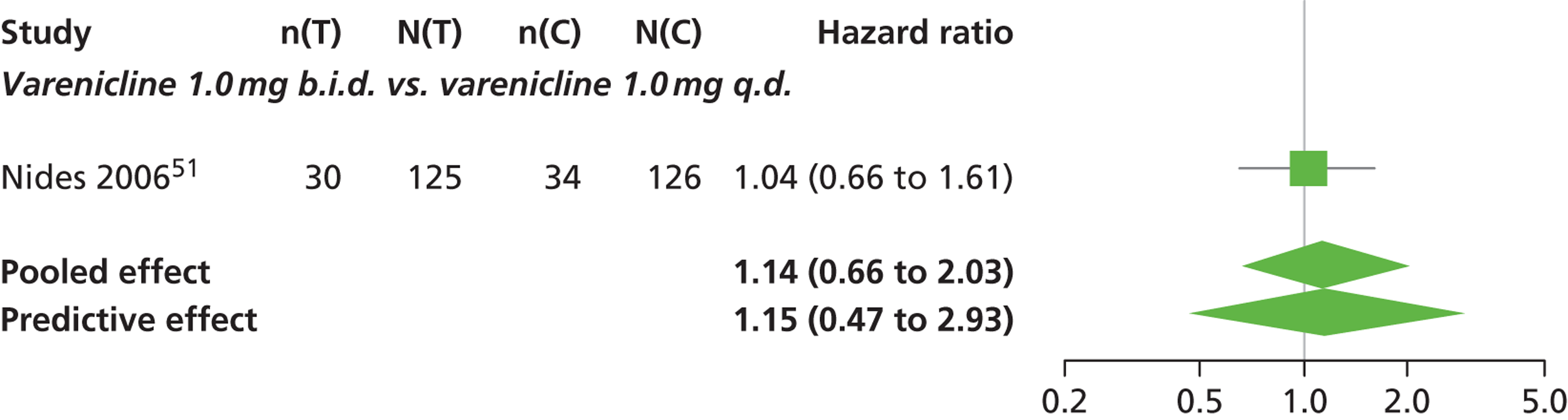
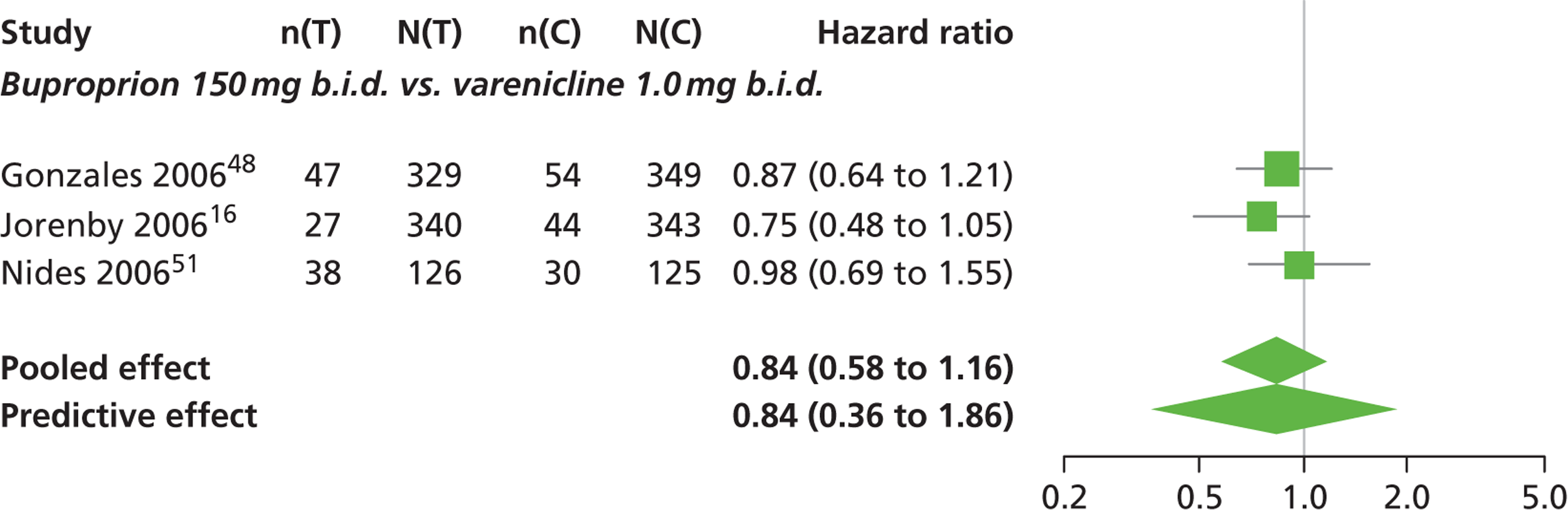
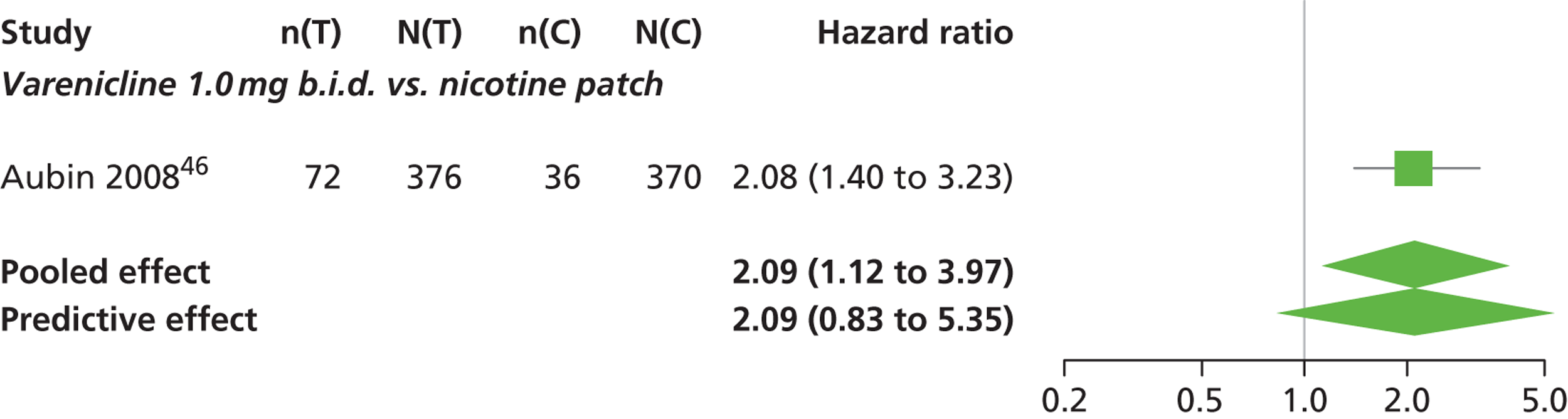
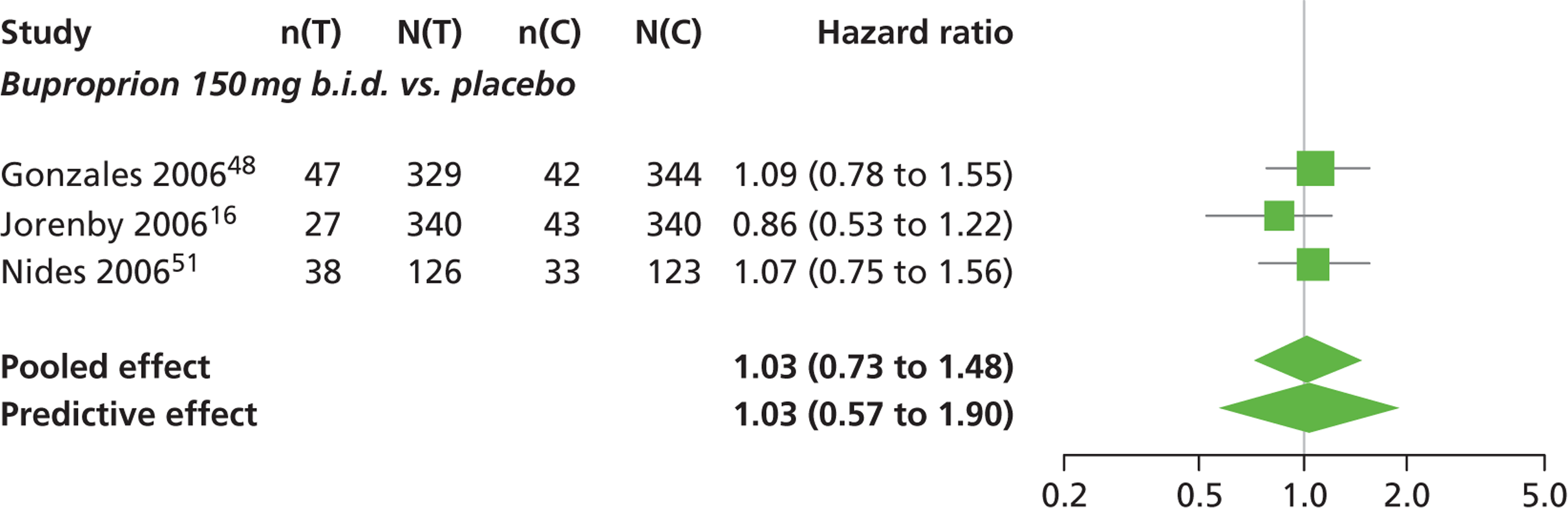
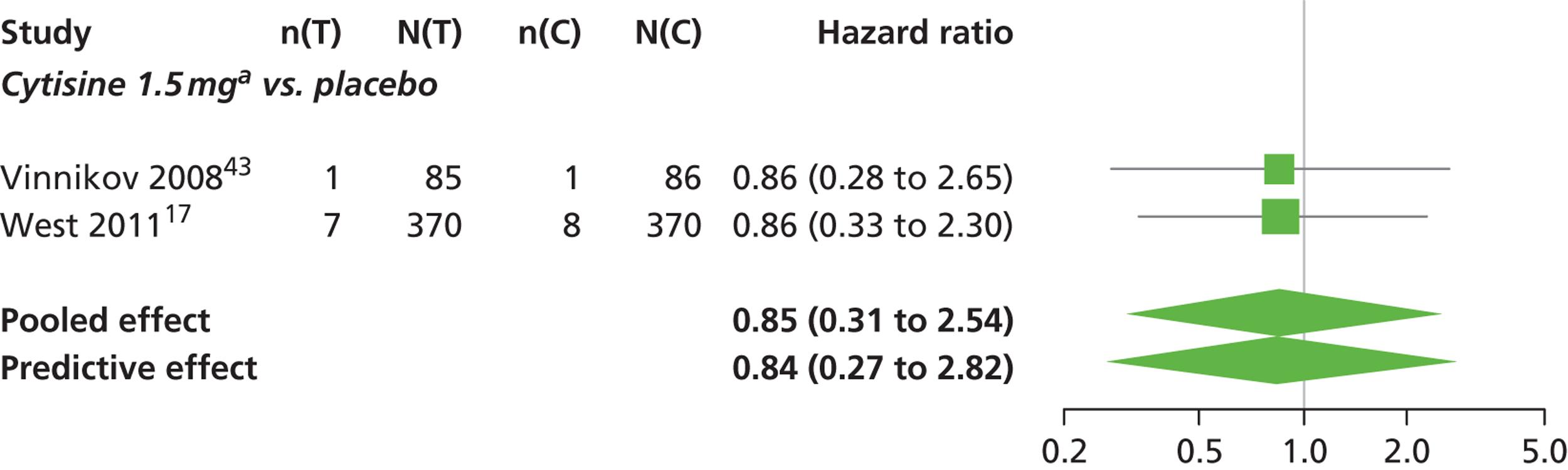
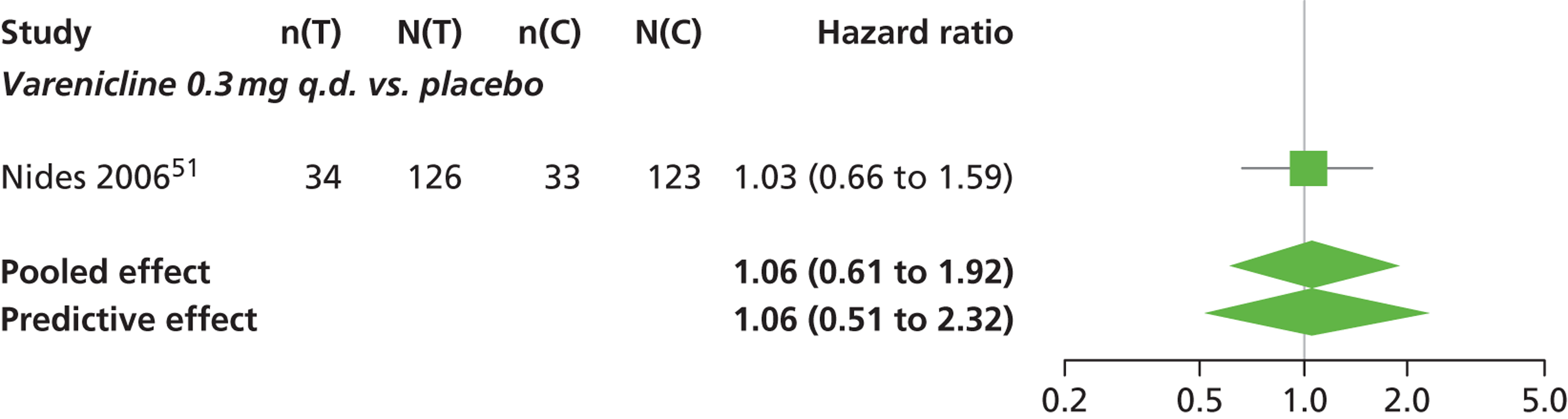
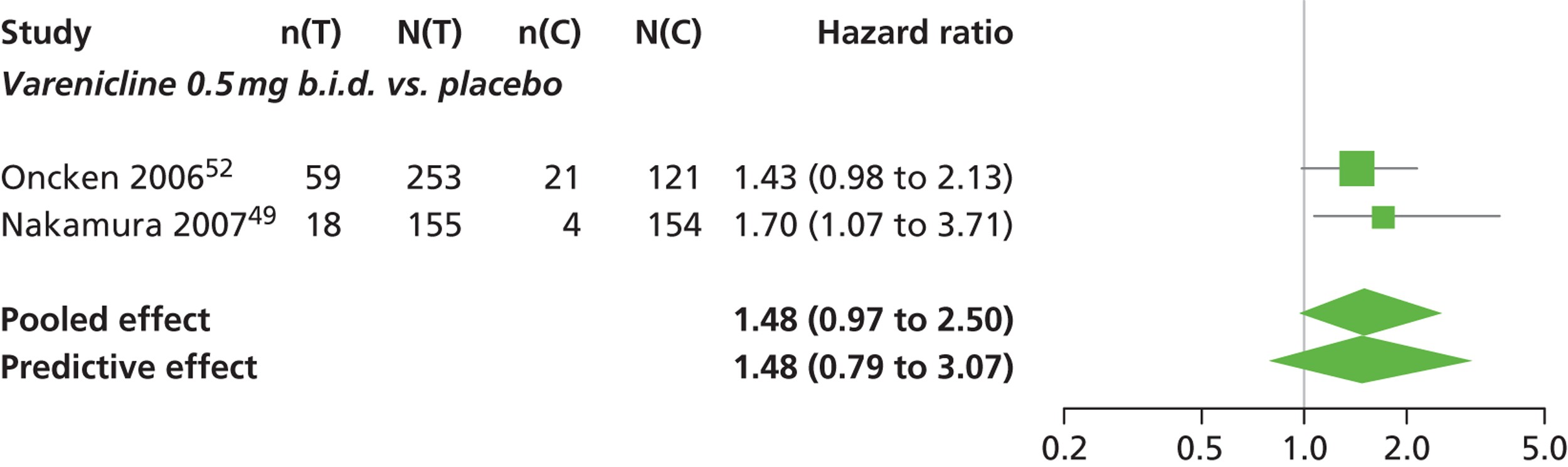
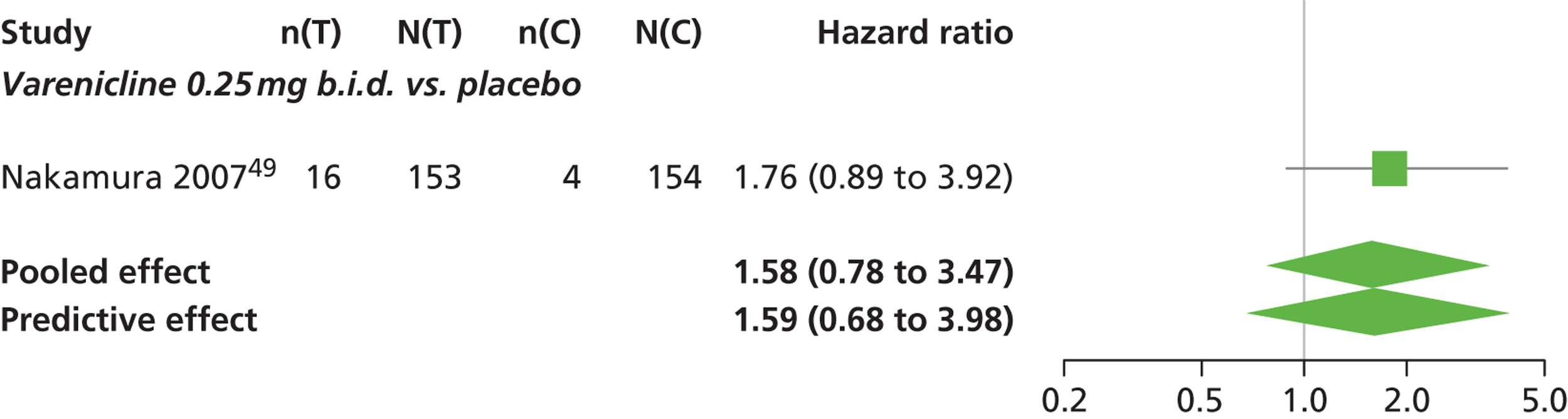
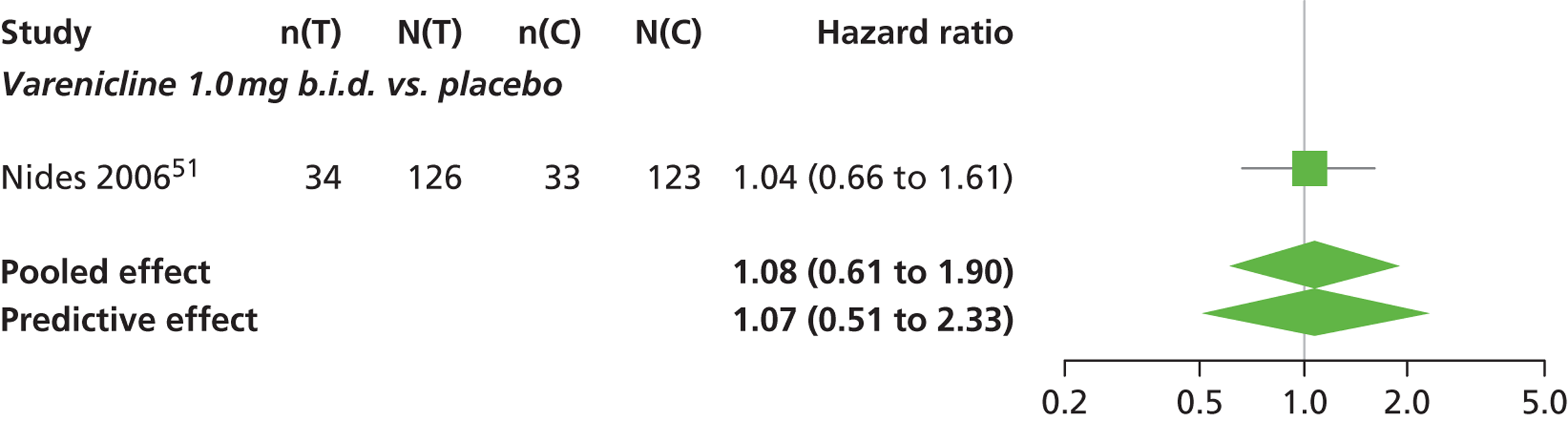
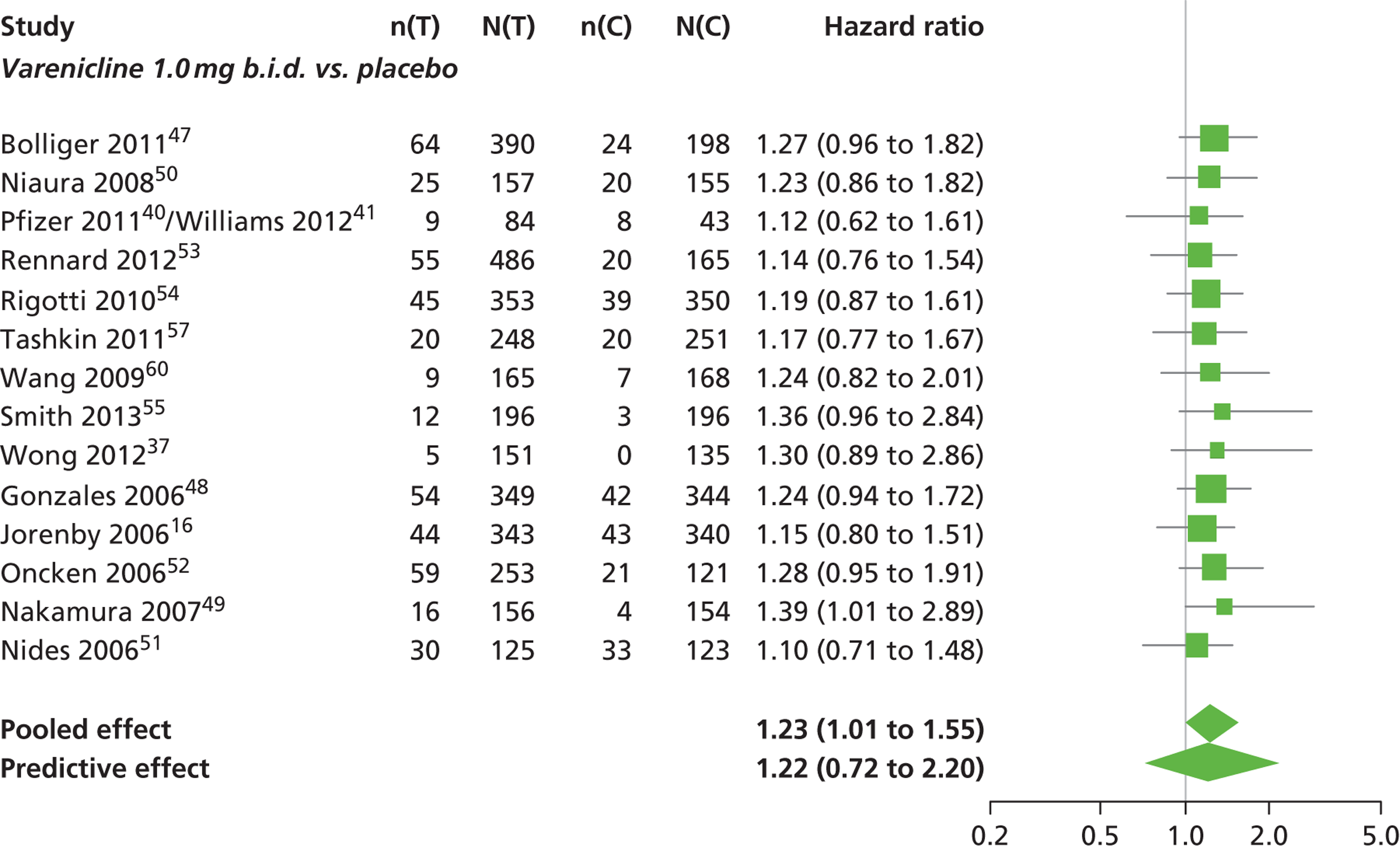
FIGURE 16.
Insomnia: forest plots of study-specific treatment effects and population mean treatment effects, where median is used as the best estimate.
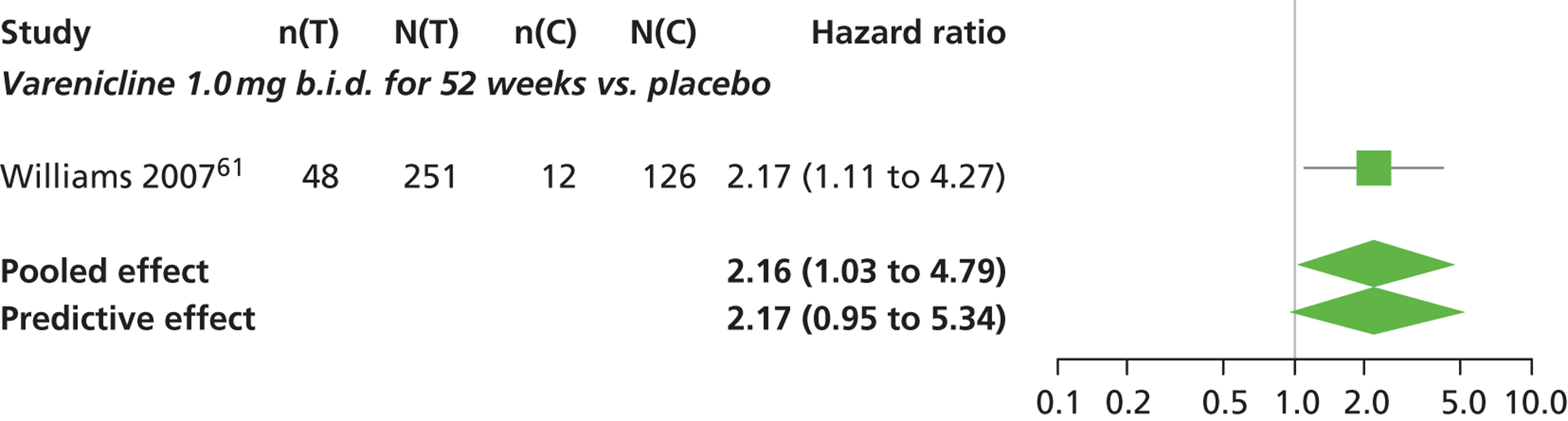
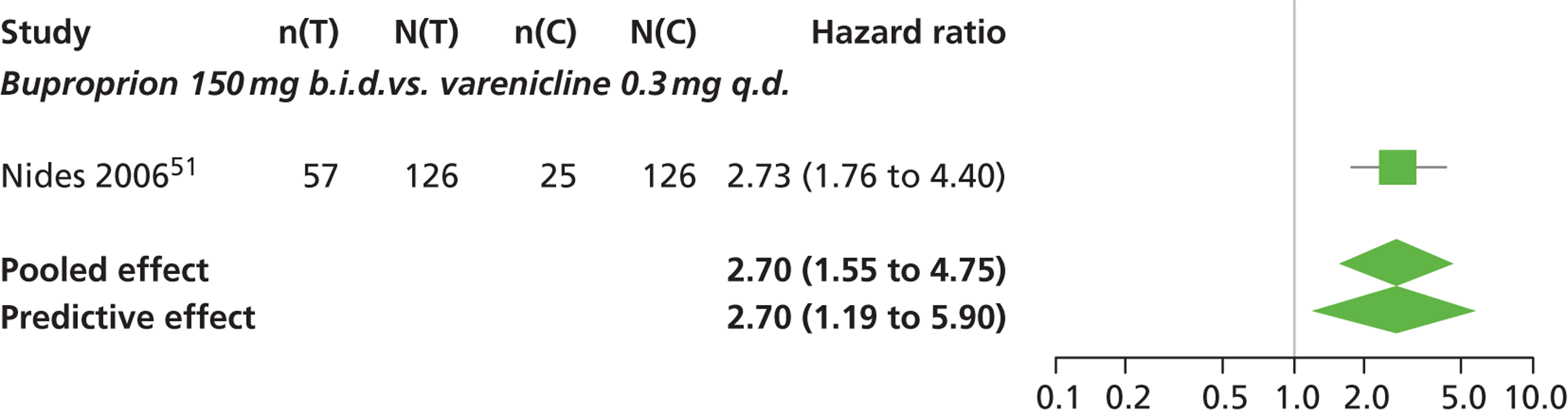
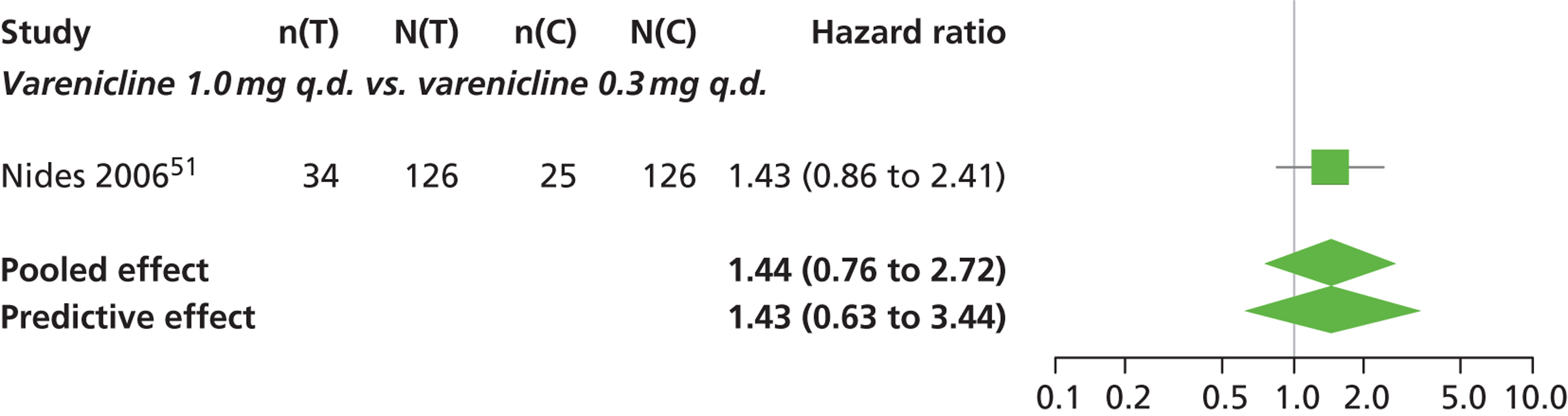
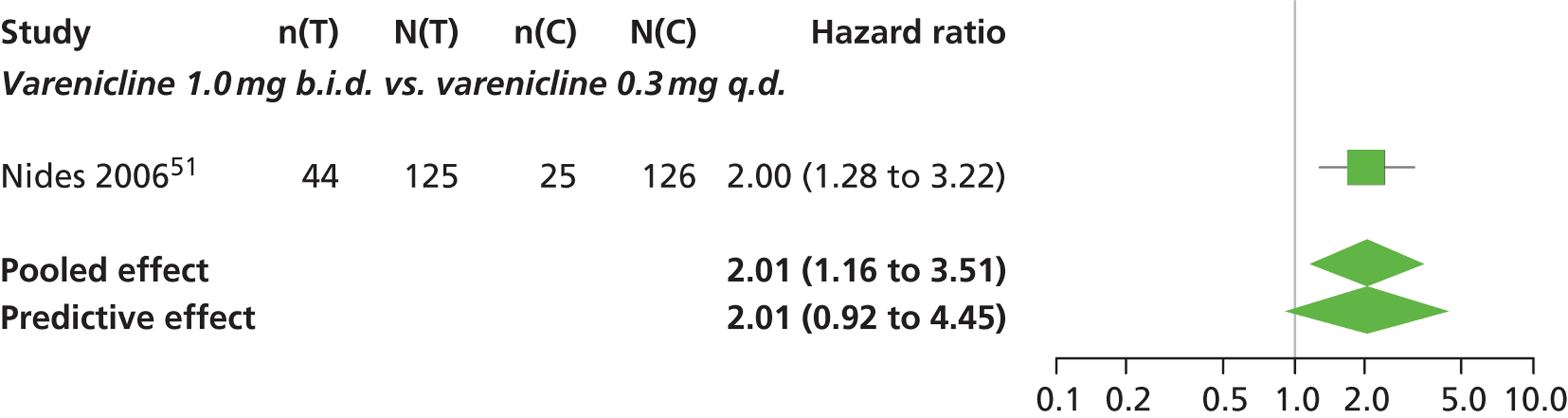
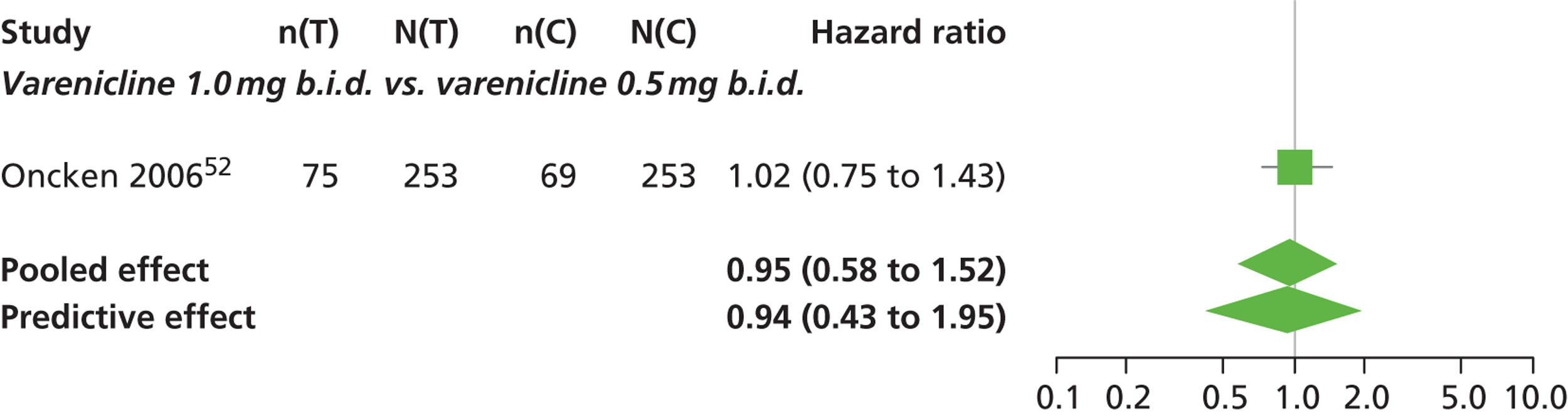
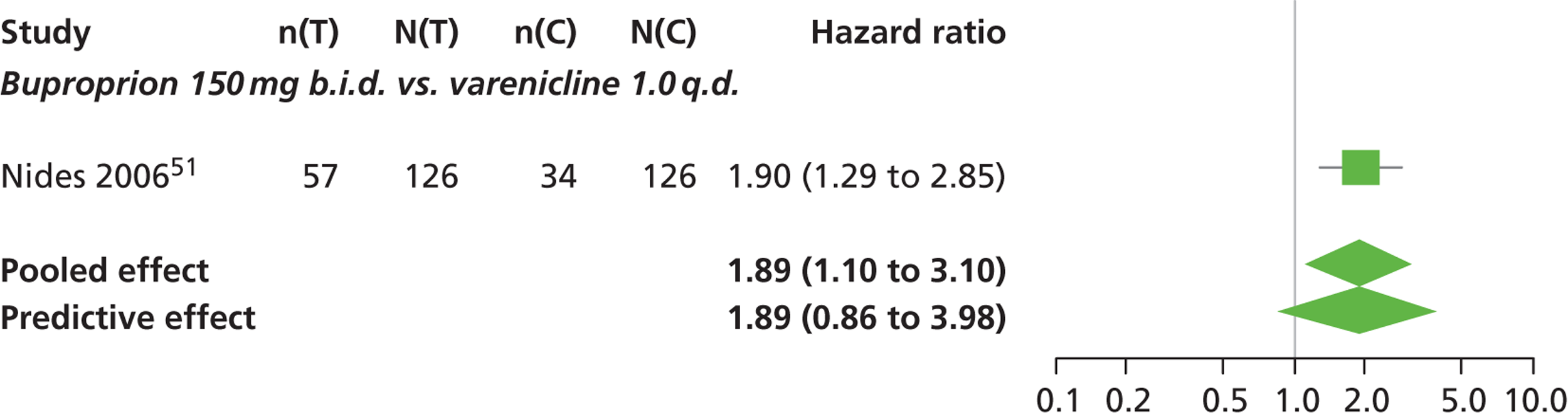
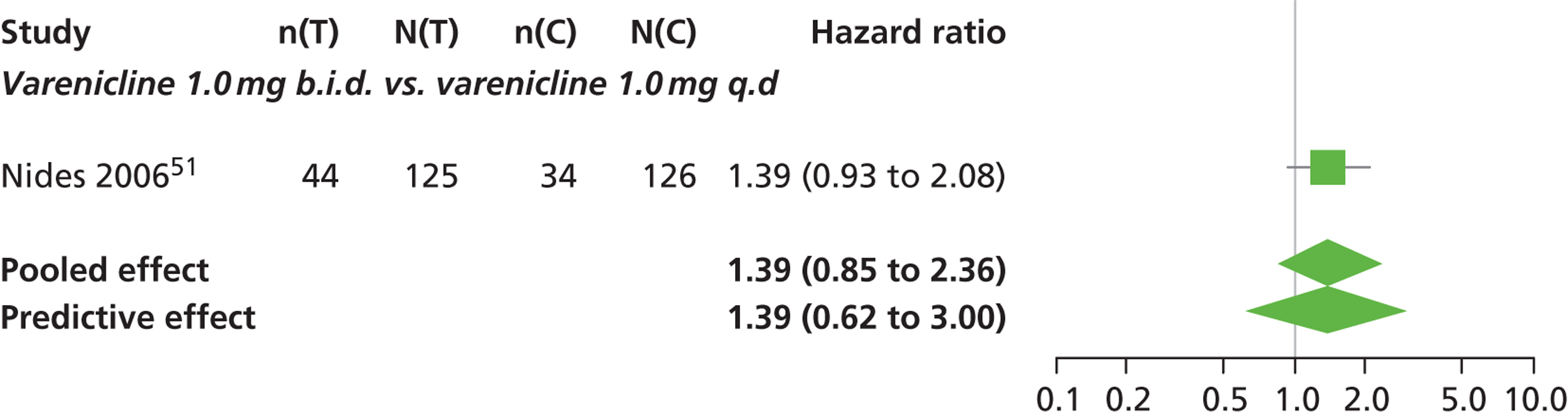
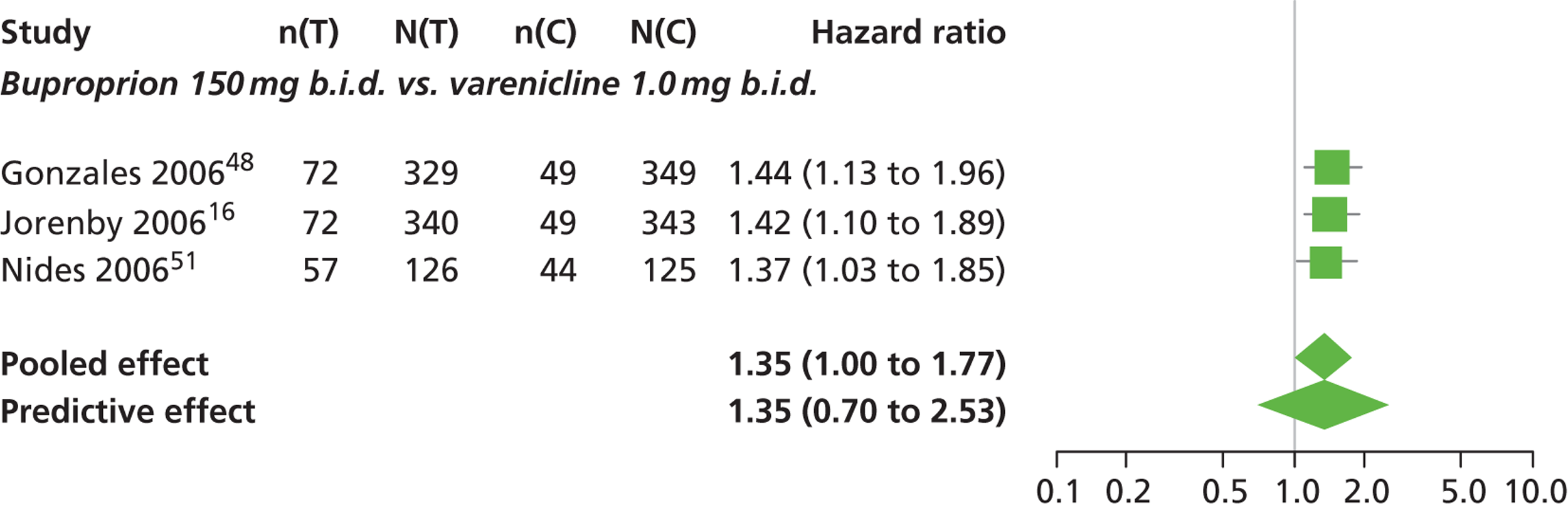
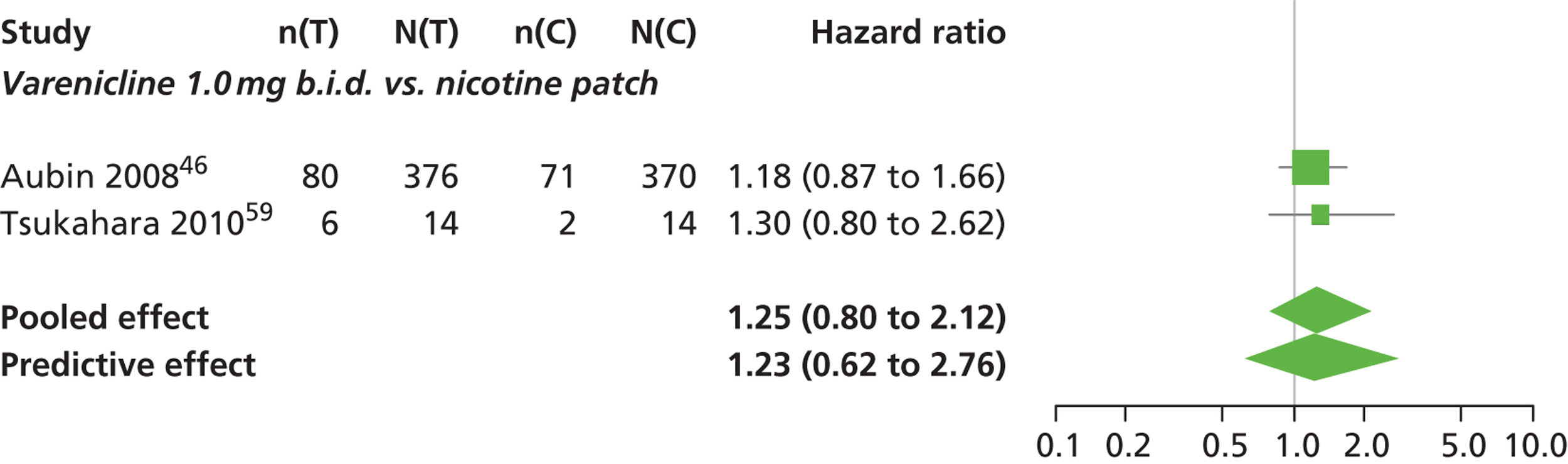
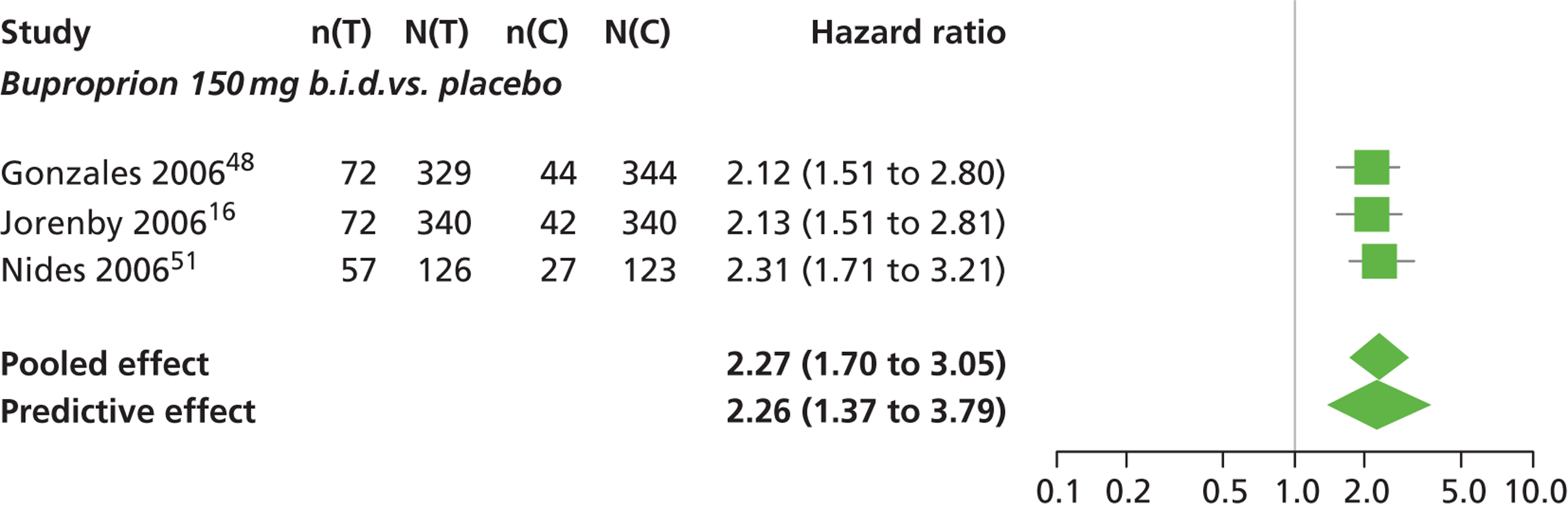
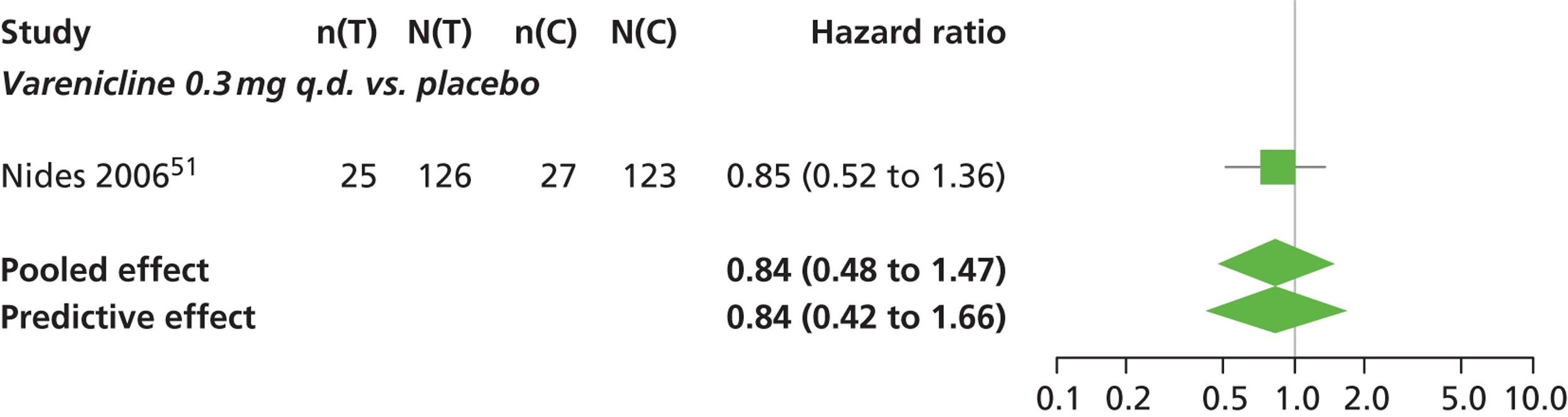
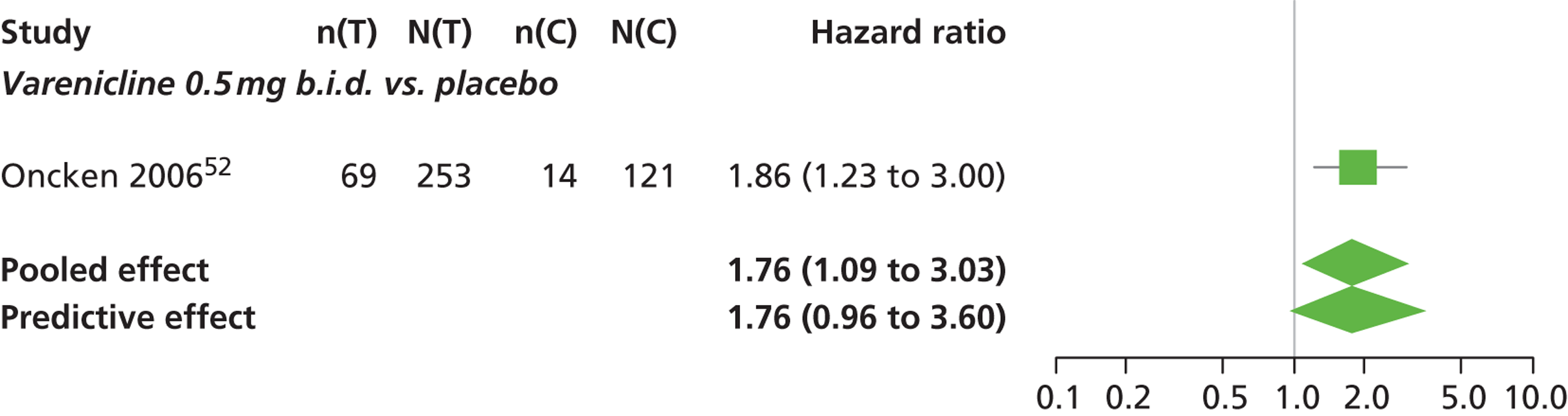
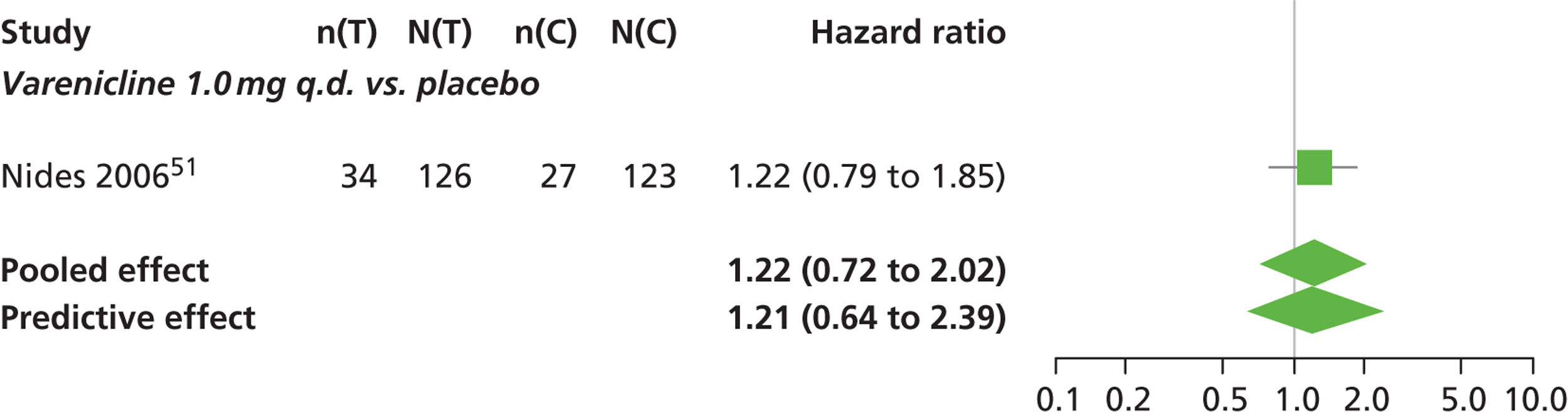
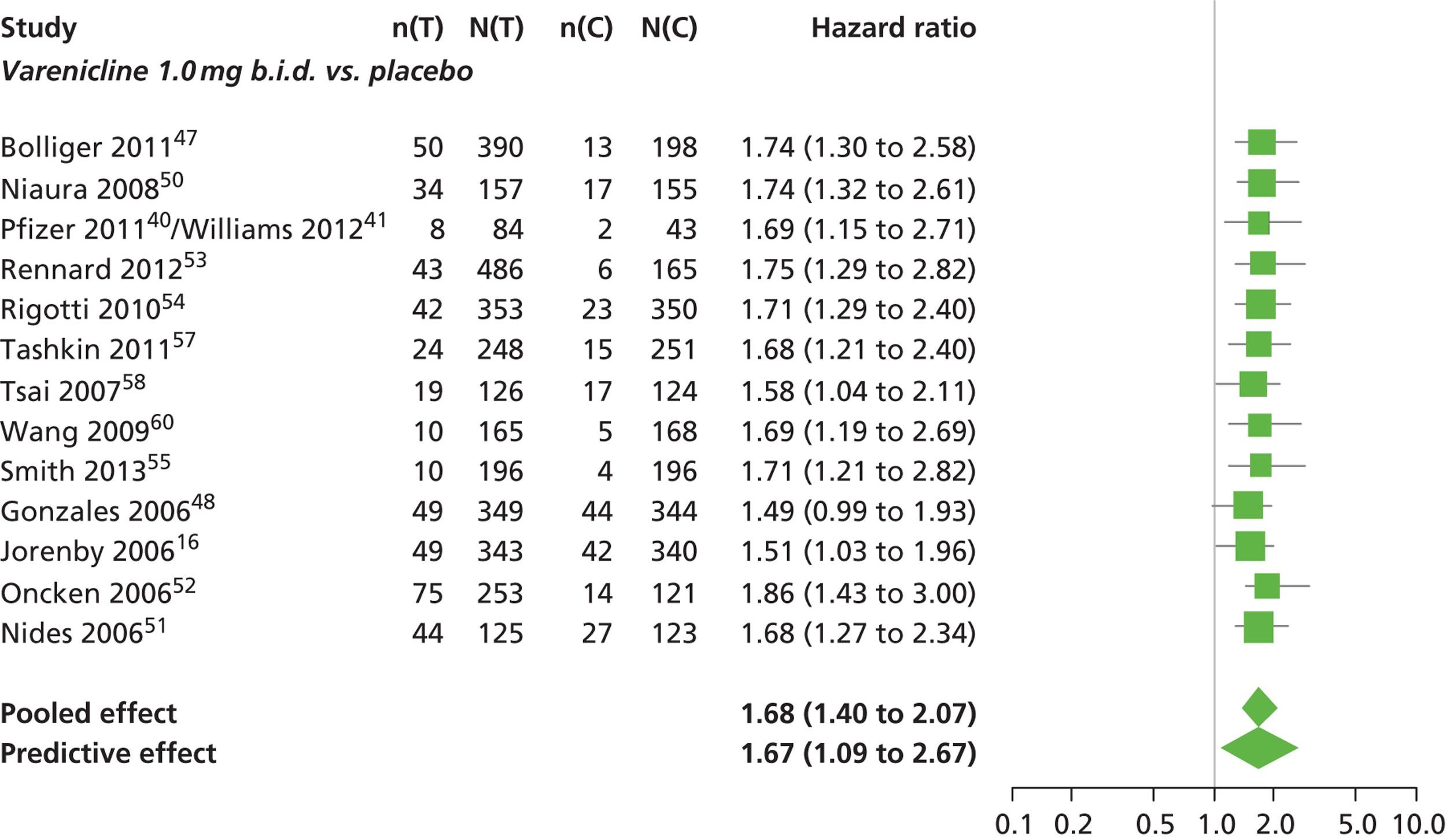
FIGURE 17.
Nausea: forest plots of study-specific treatment effects and population mean treatment effects, where median is used as the best estimate. a See Table 1 for regime details.
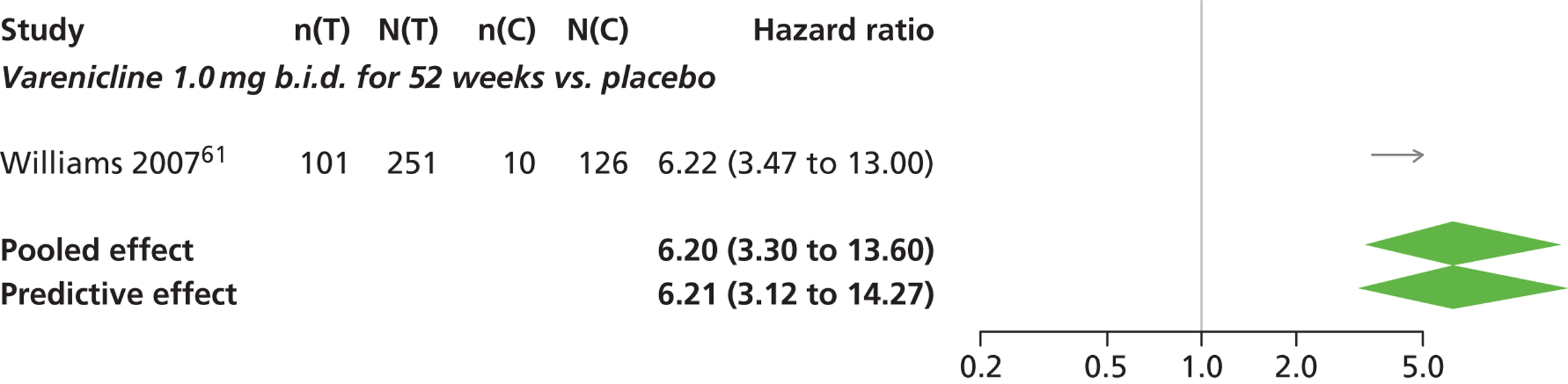
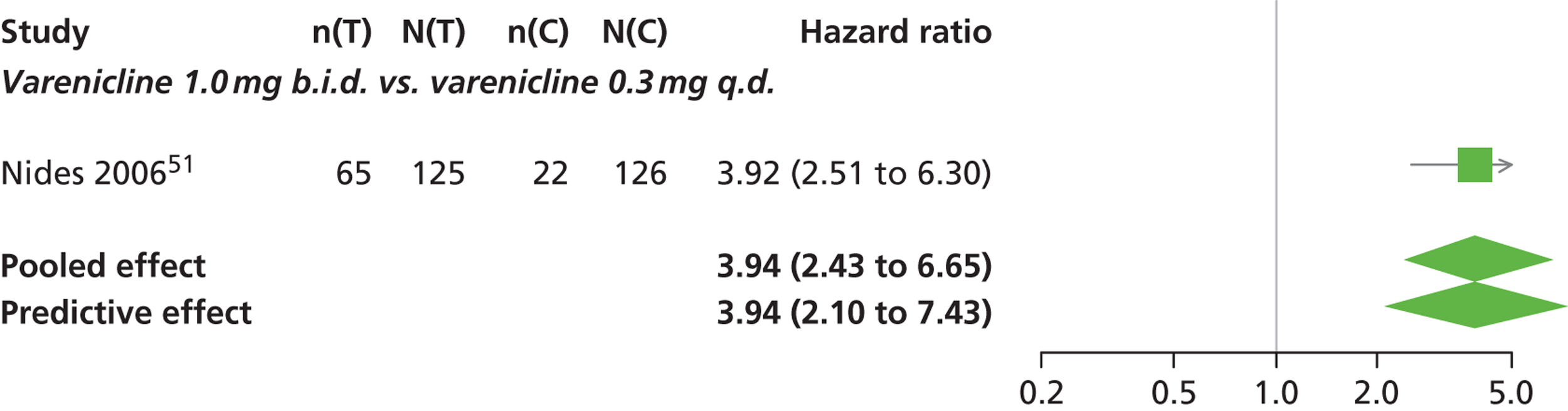
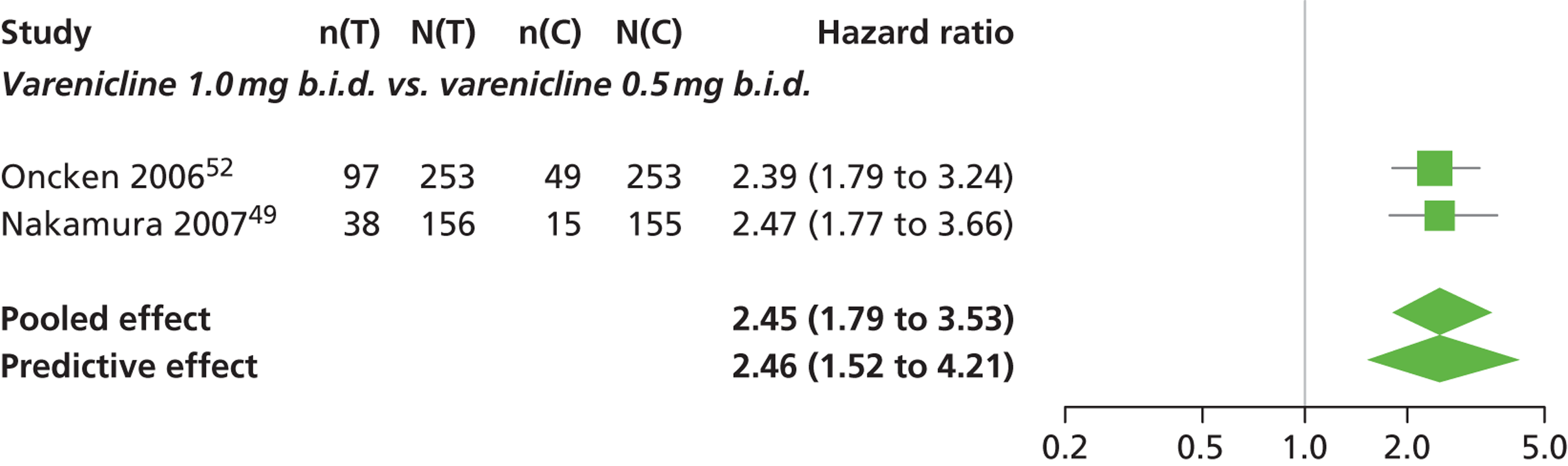
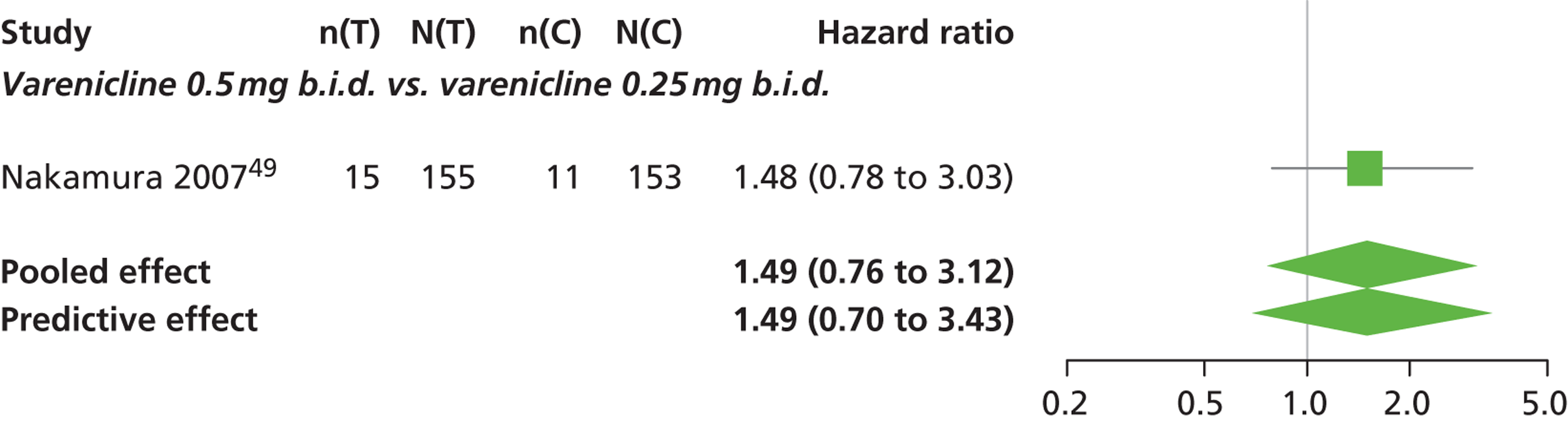
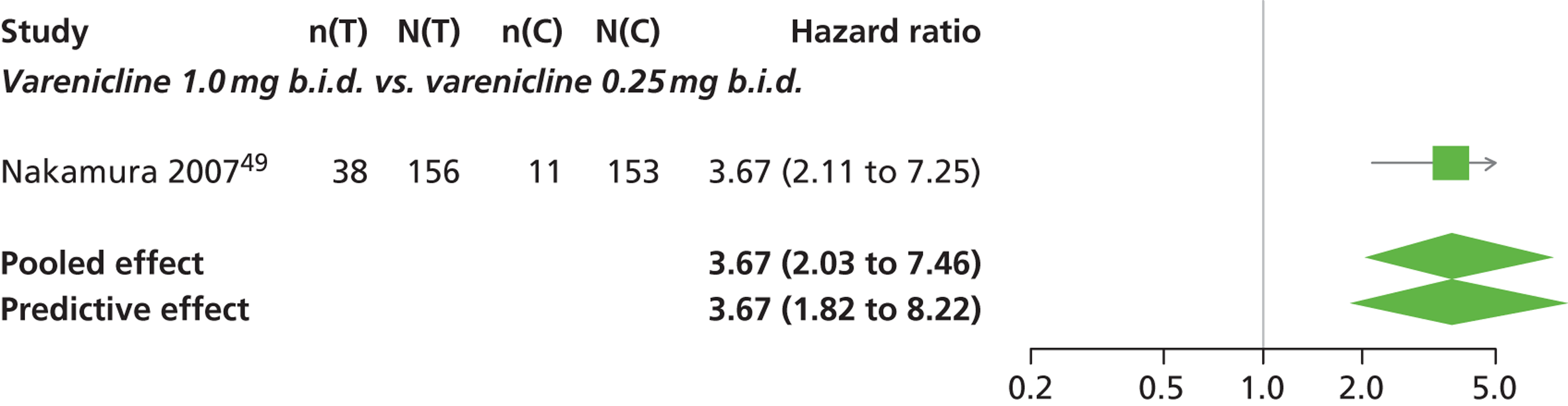
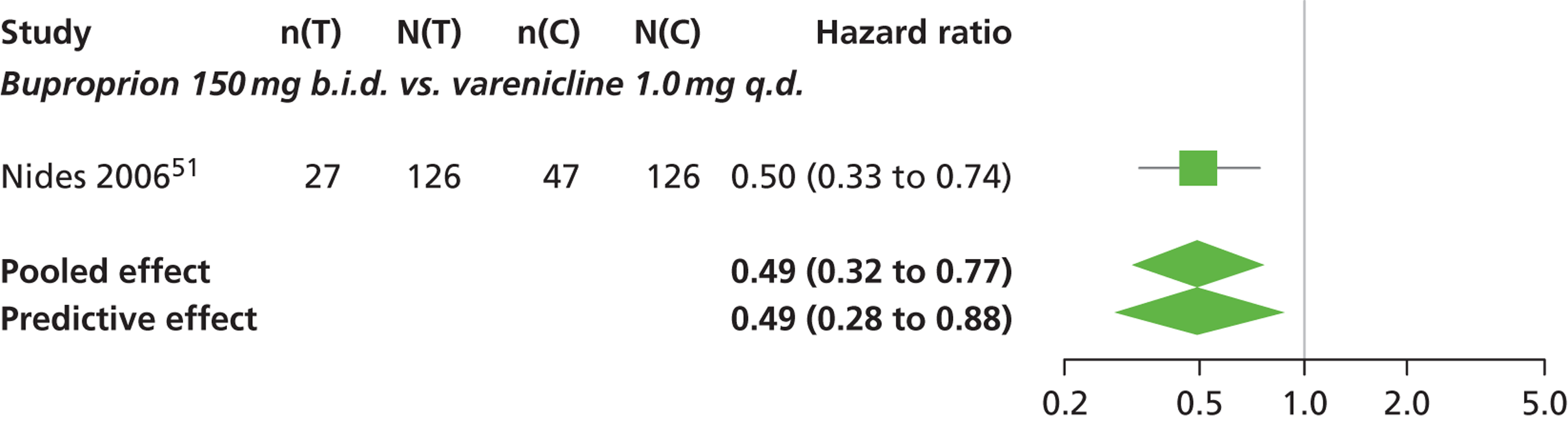
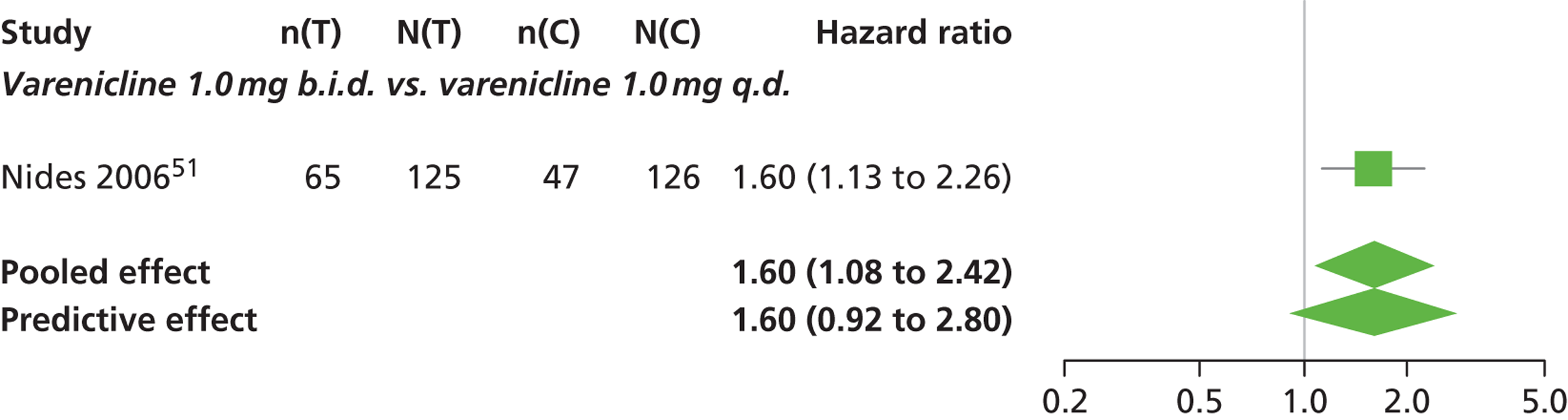
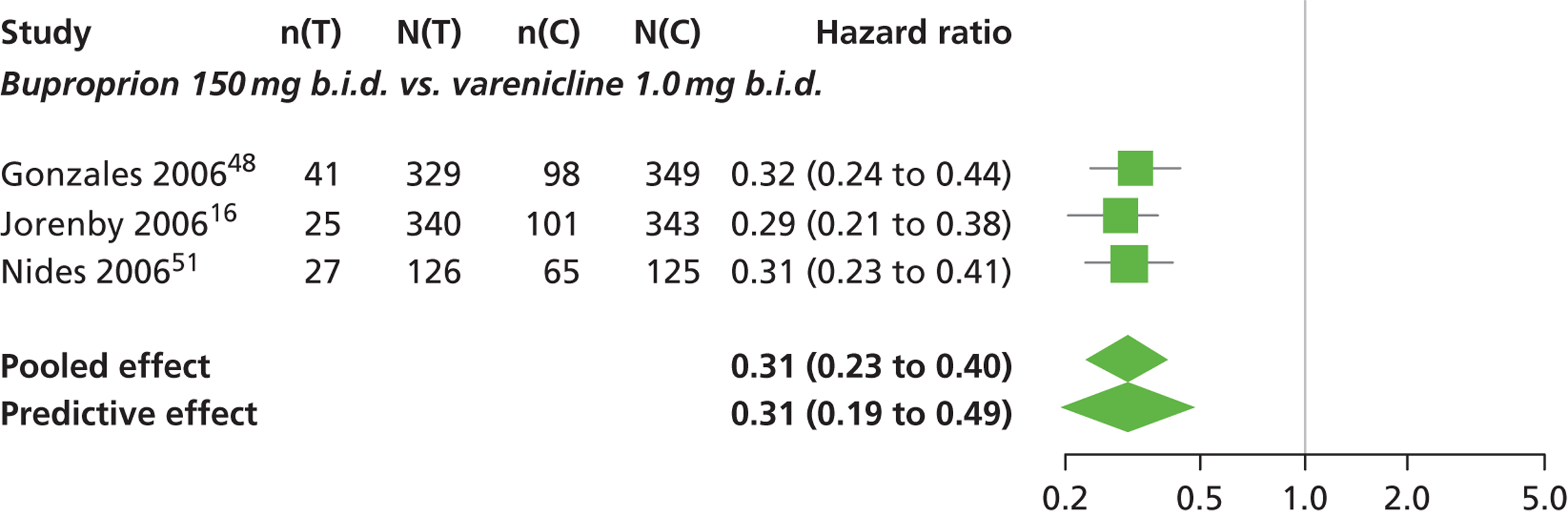
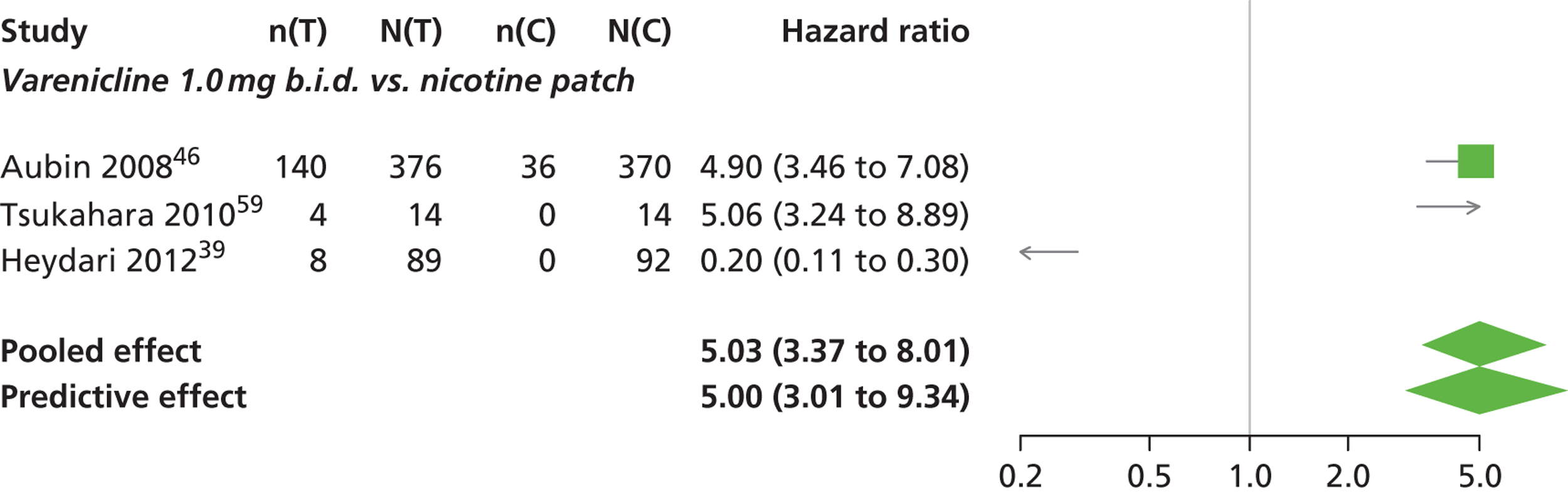
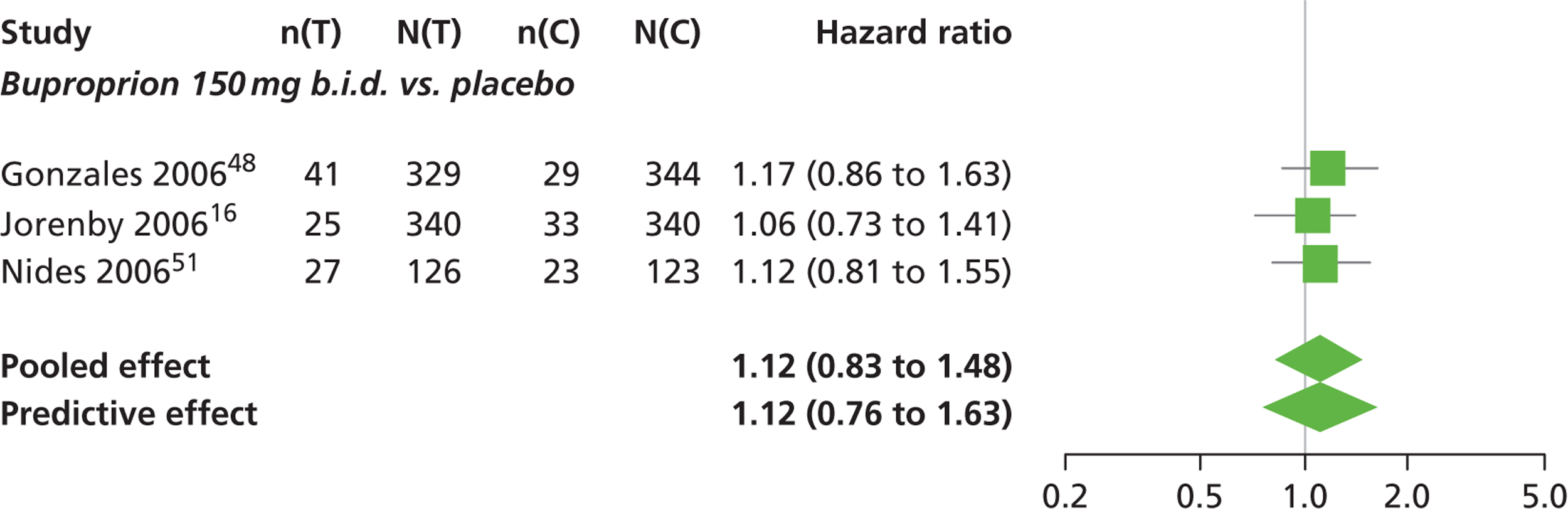
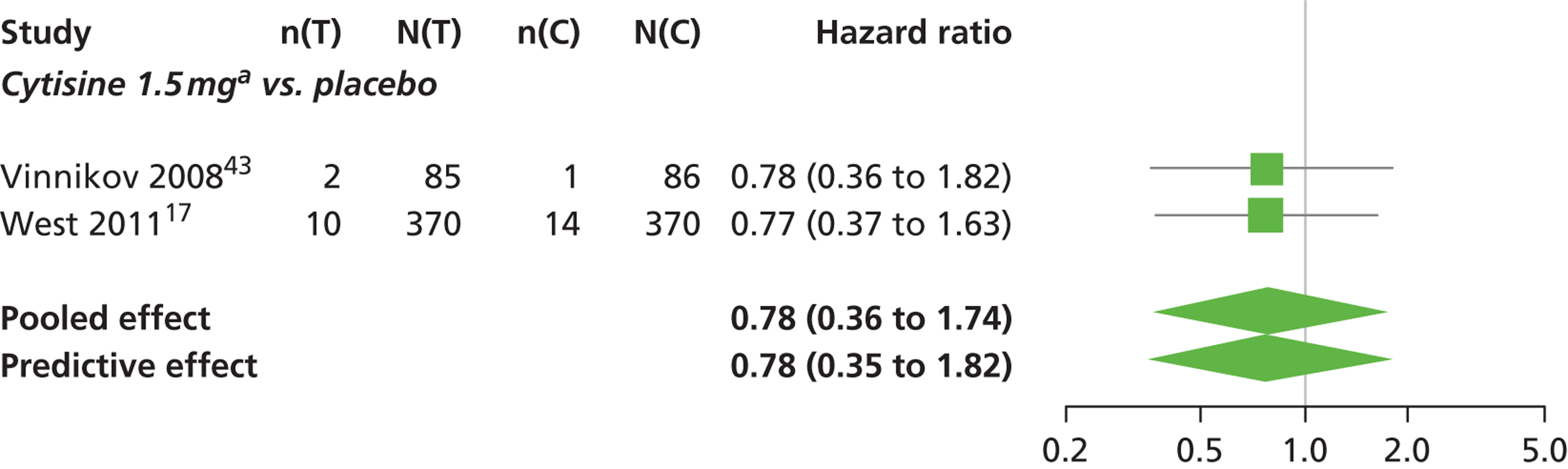
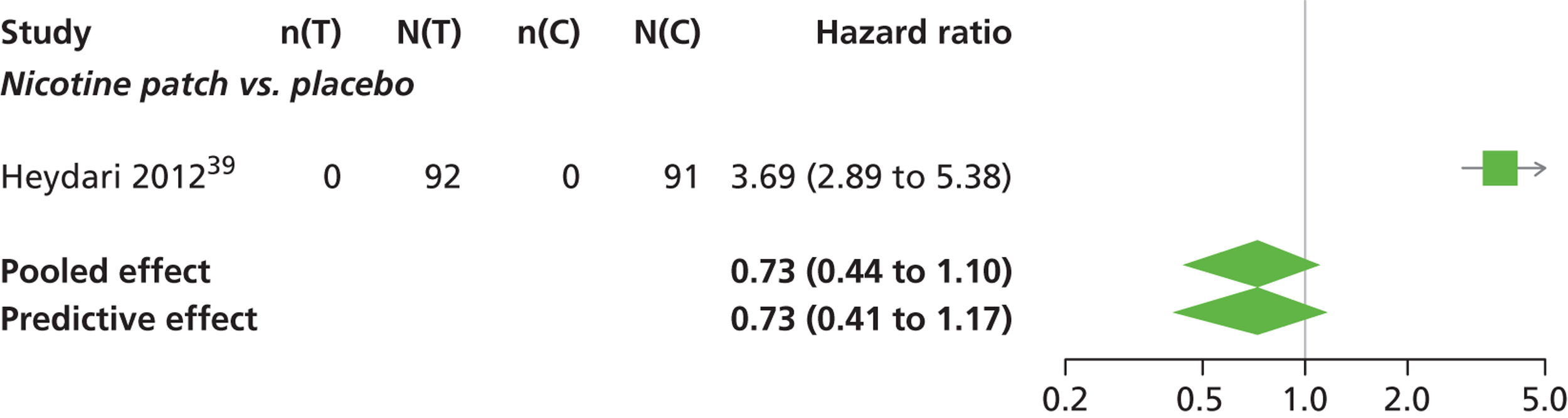
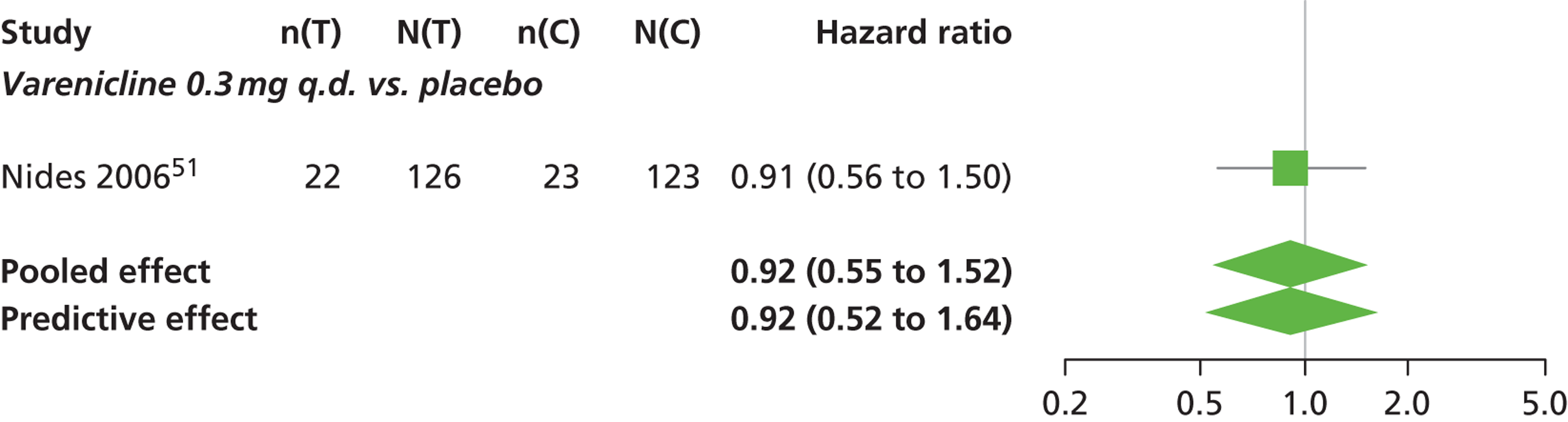
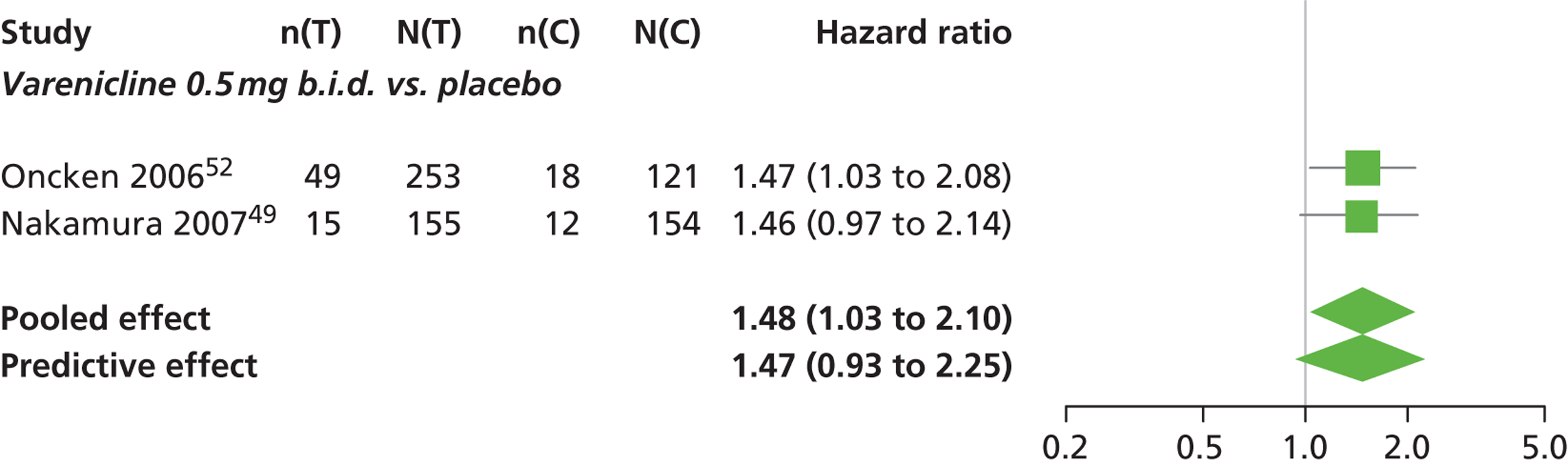
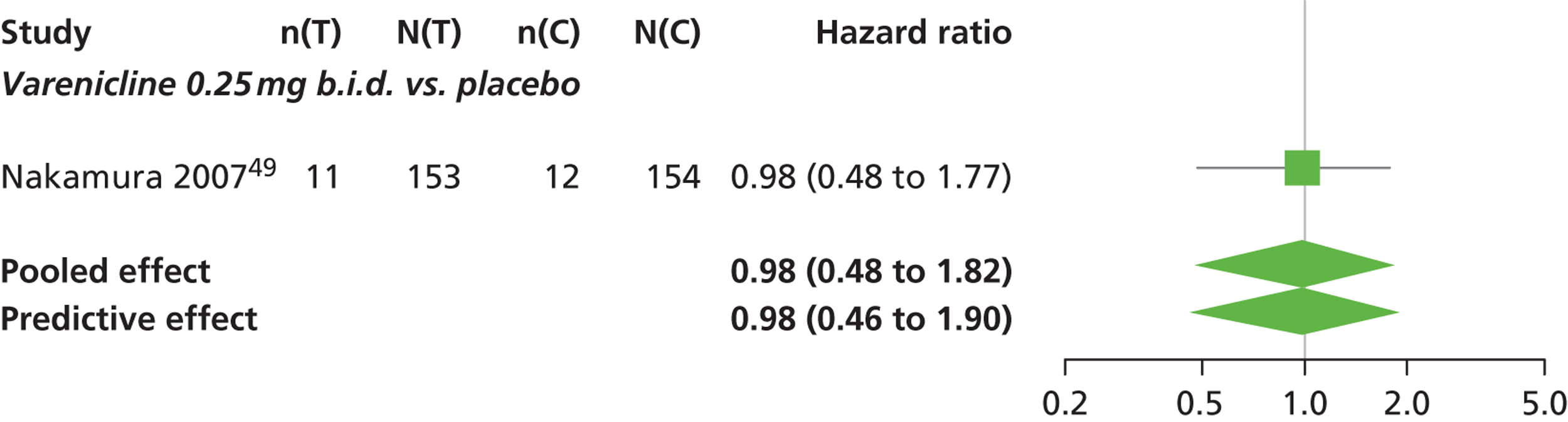
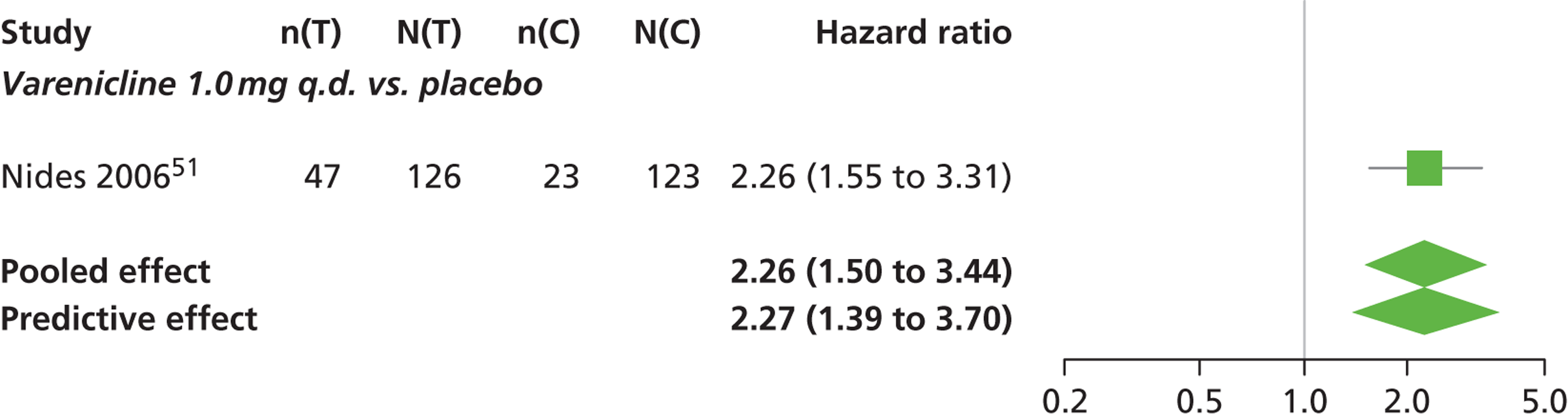
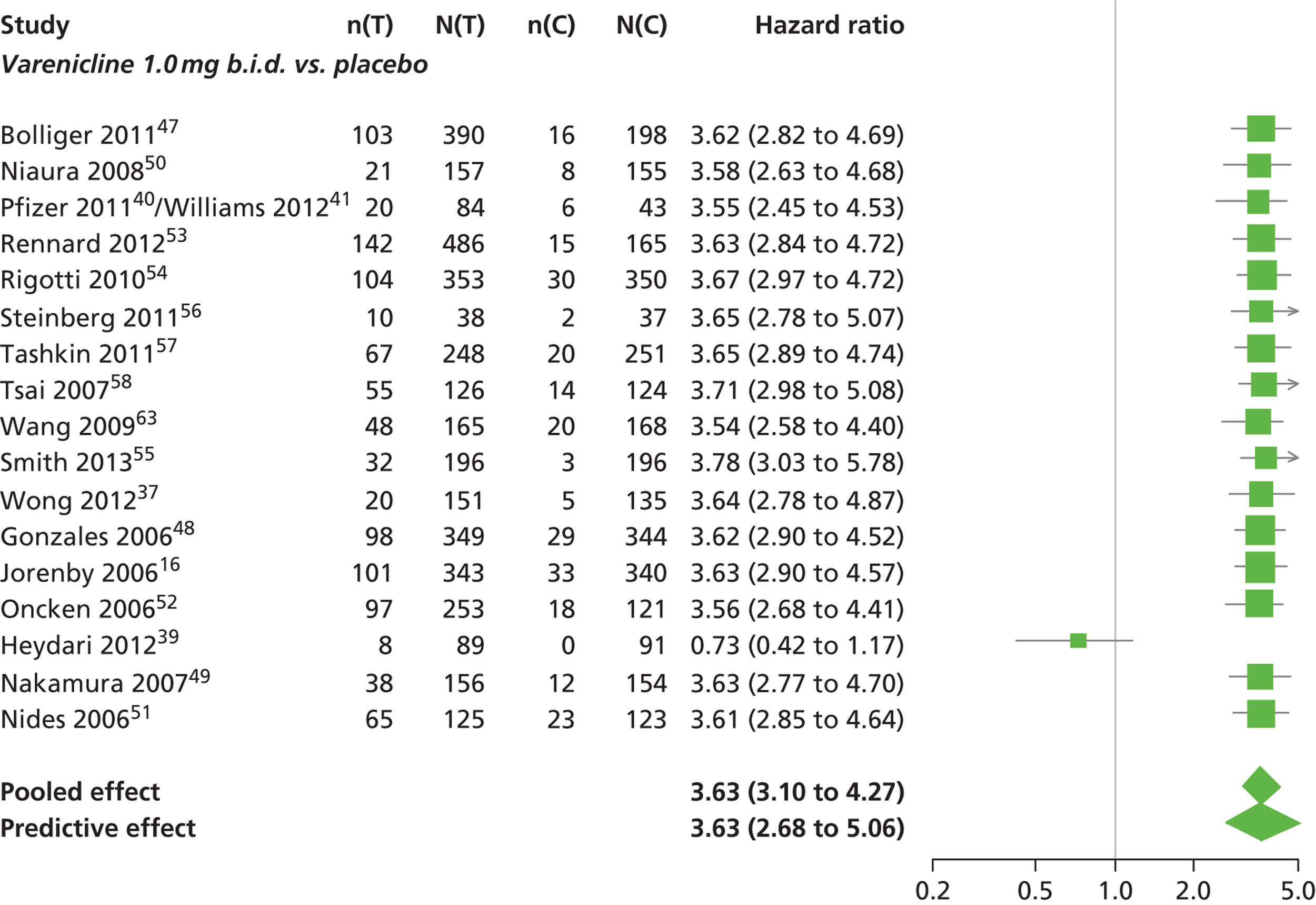
FIGURE 18.
Serious adverse events: forest plots of study-specific treatment effects and population mean treatment effects, where median is used as the best estimate. a See Table 1 for regime details.
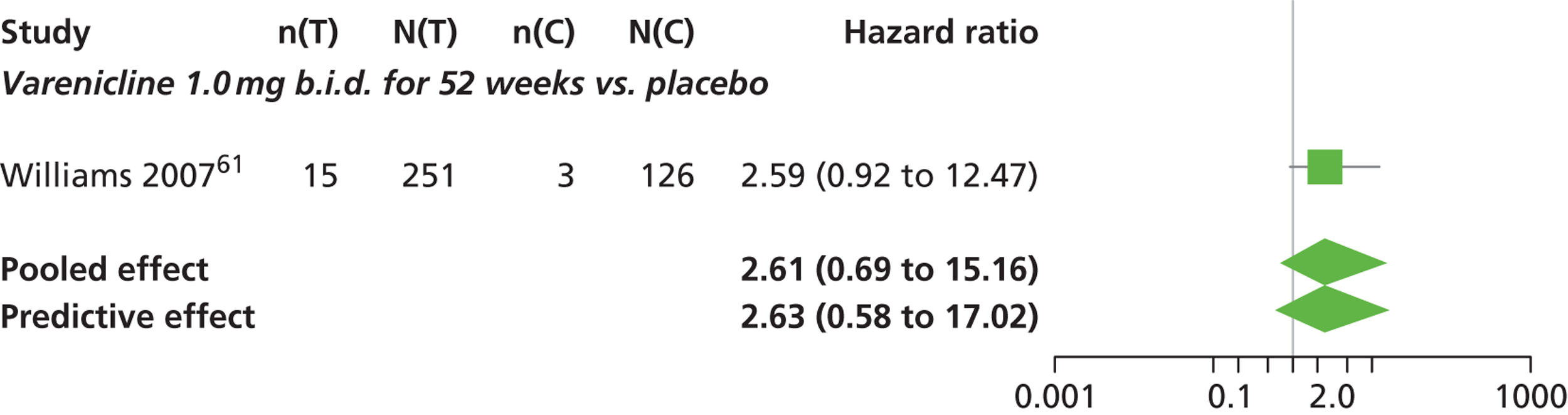
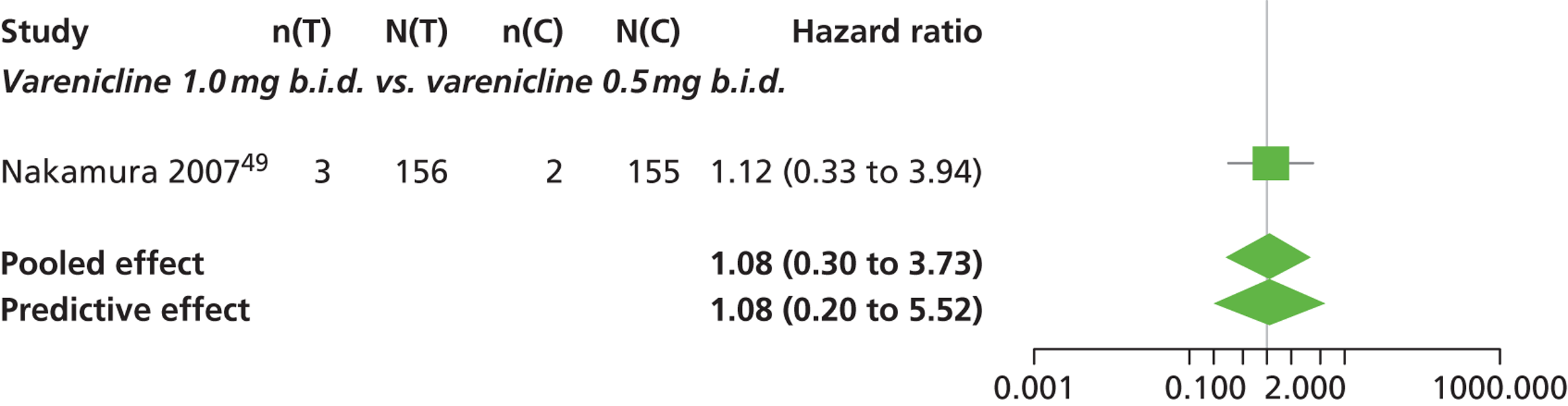
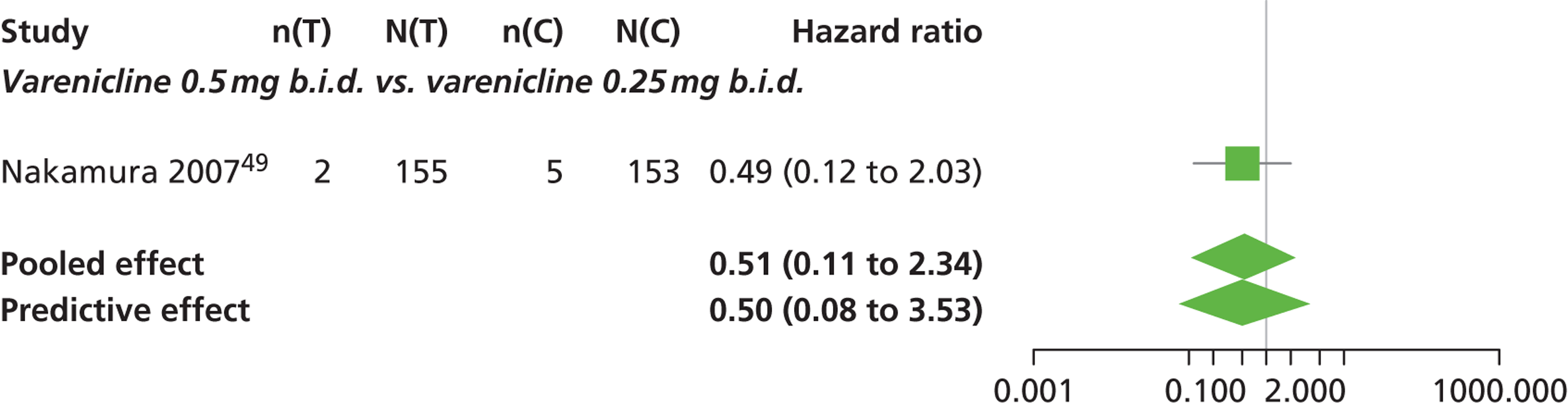
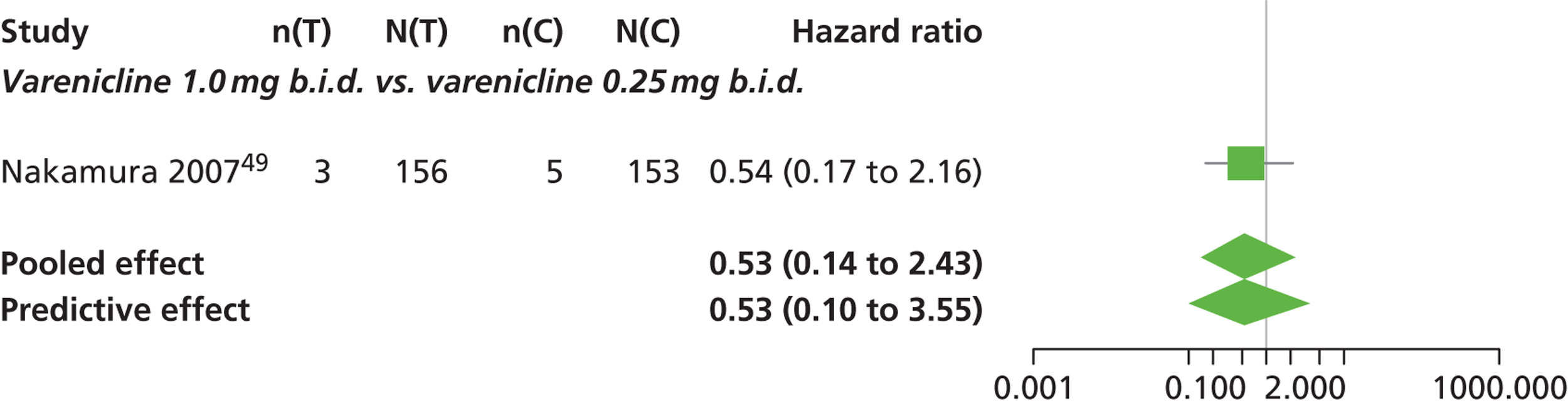

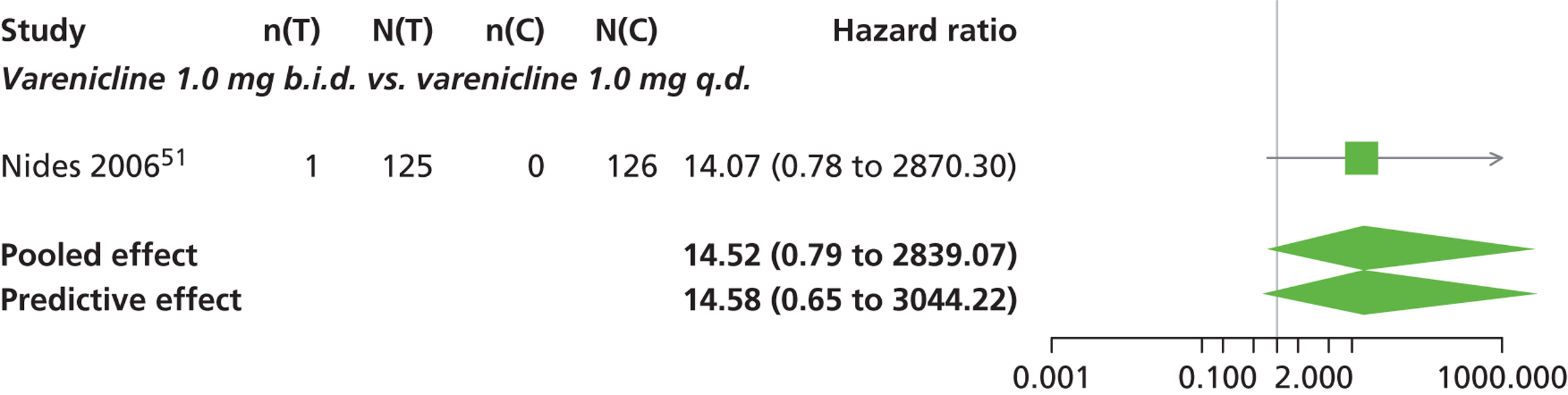
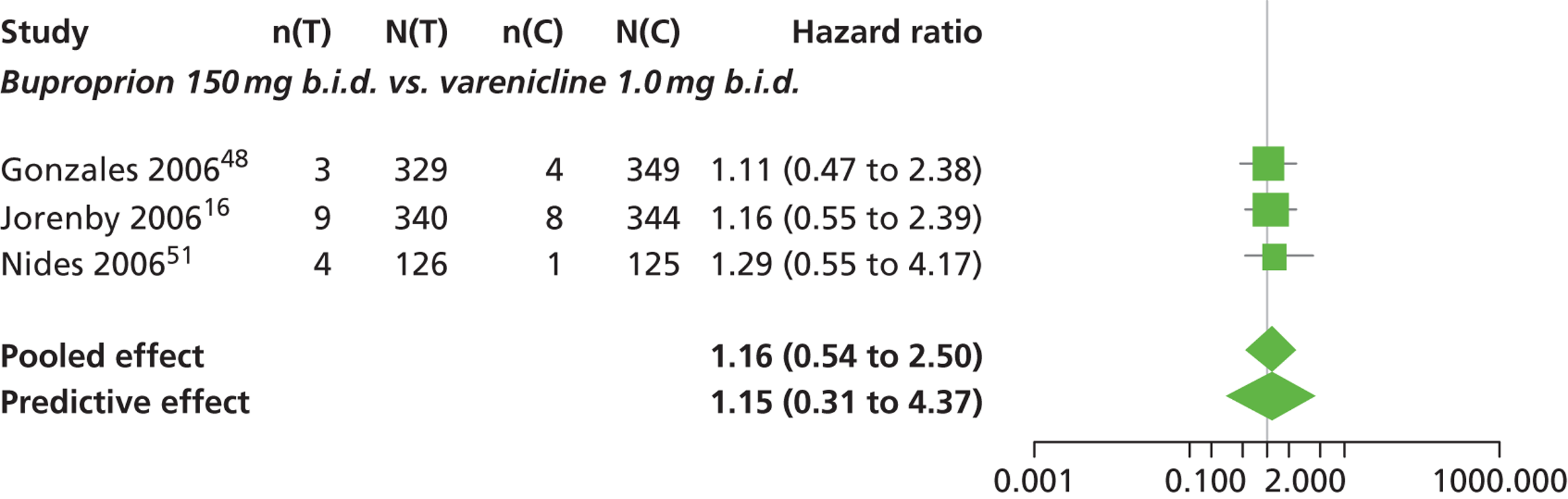
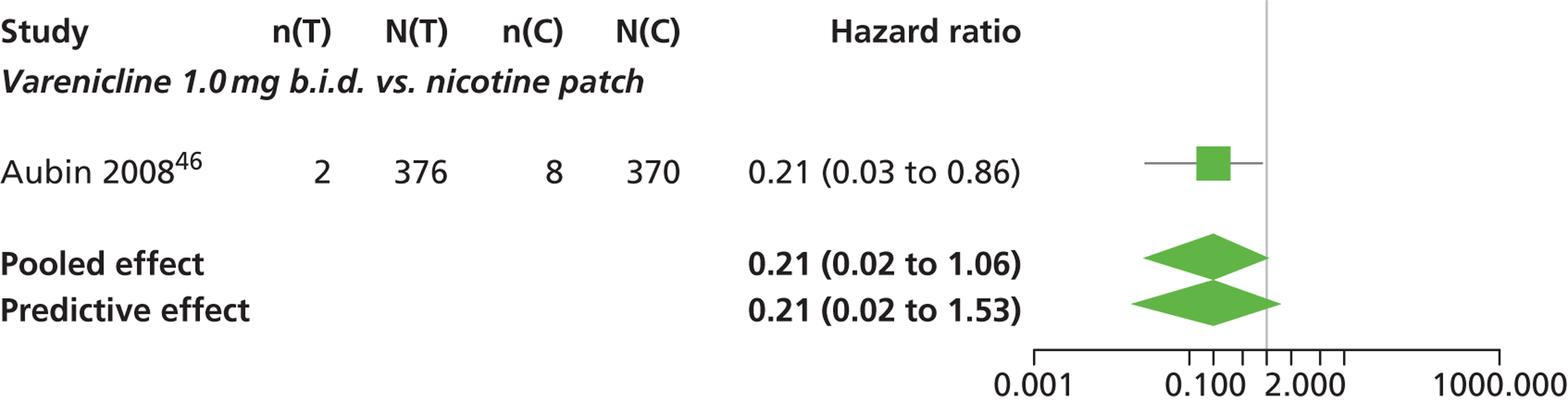
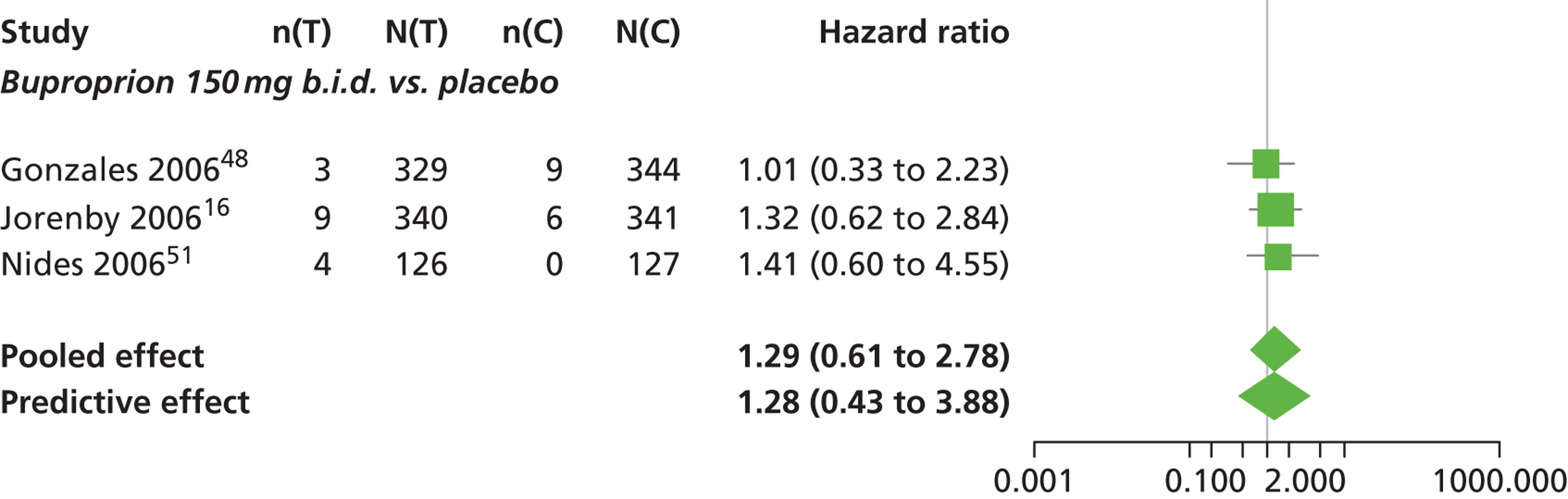
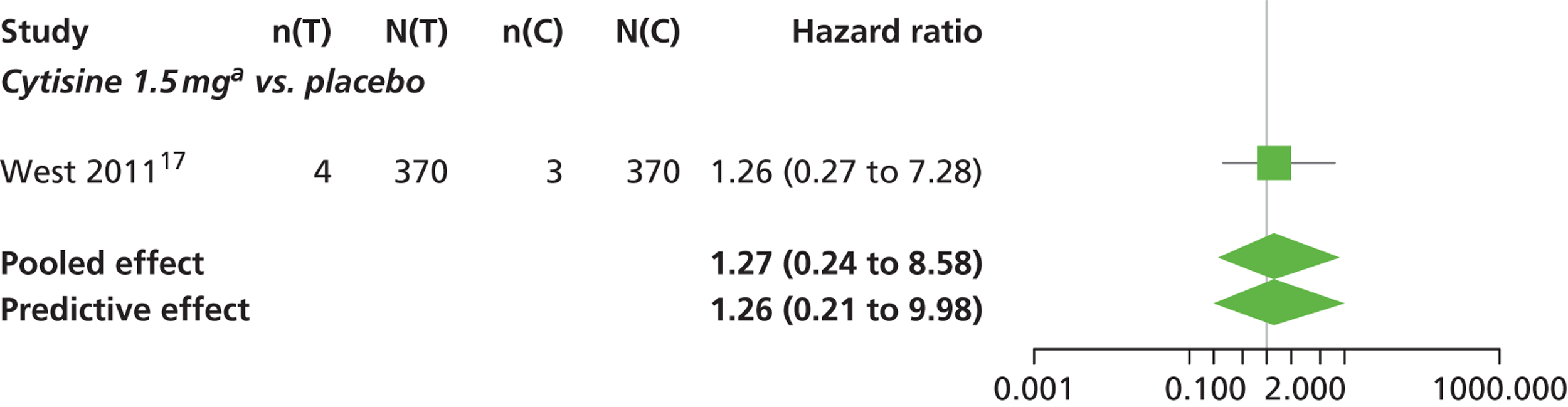
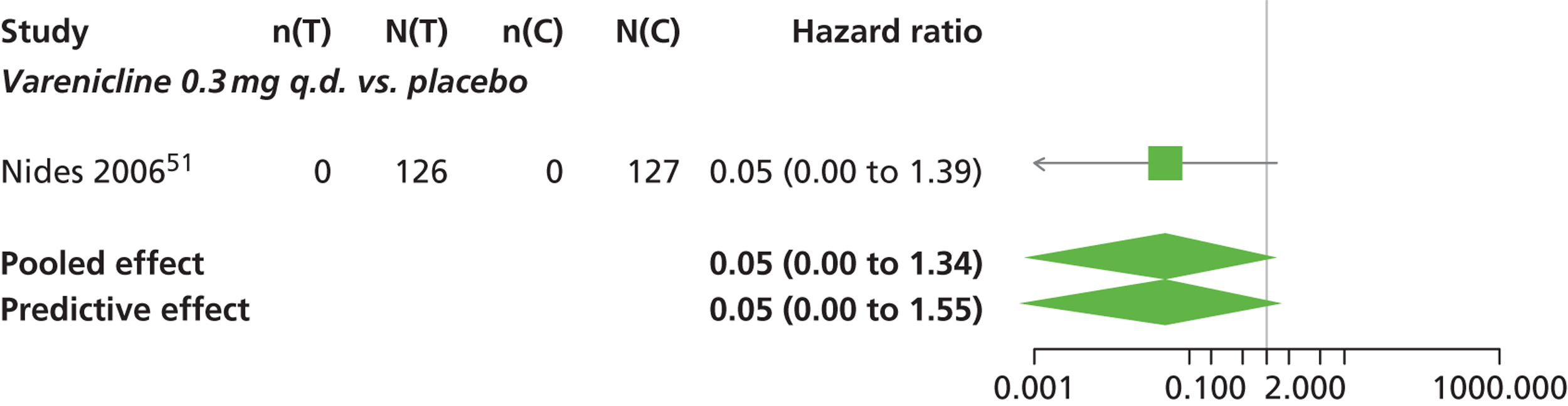
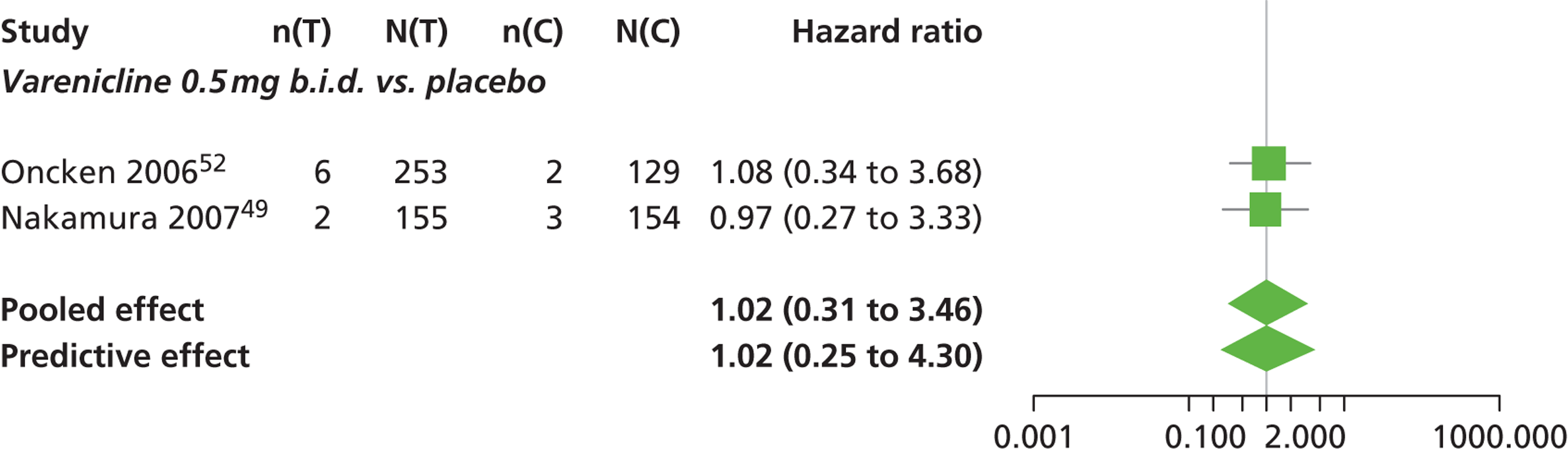
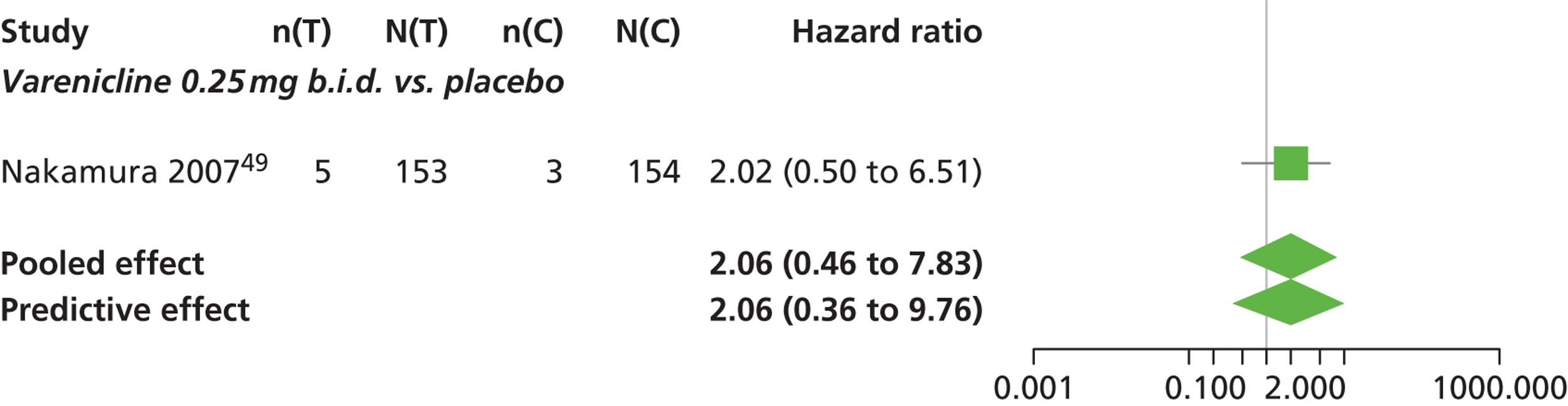
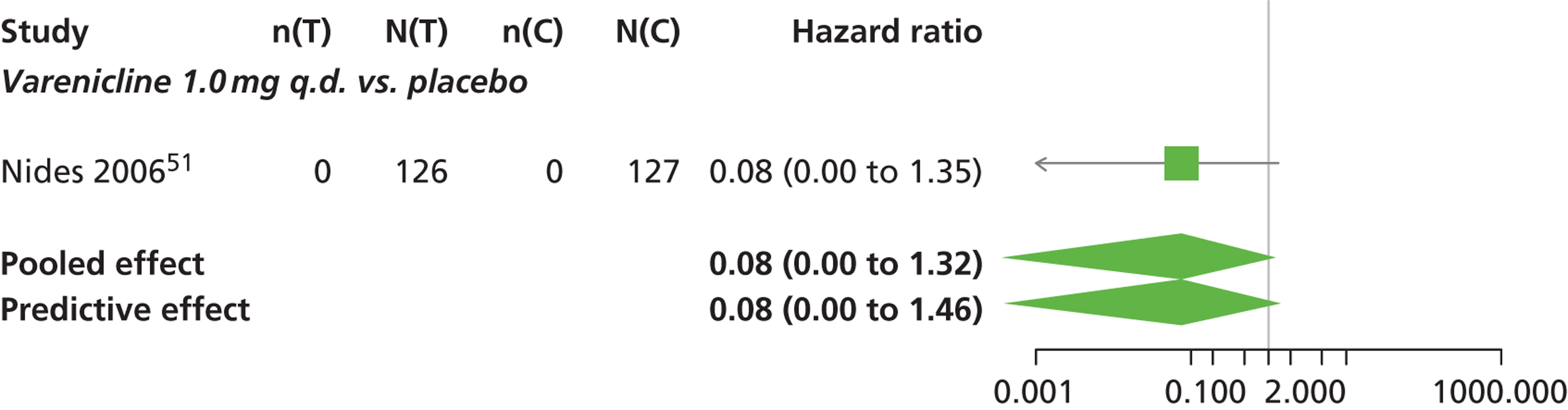
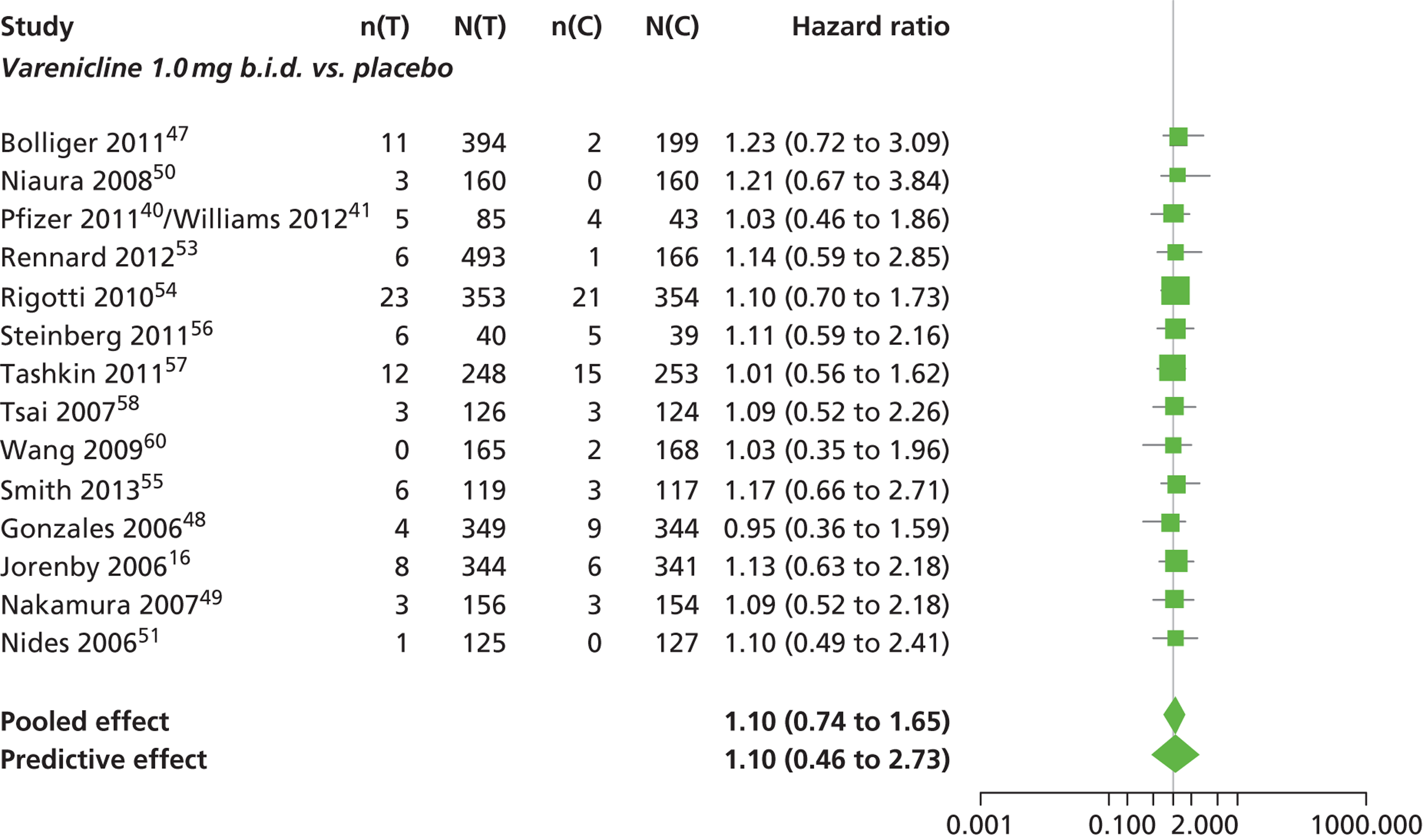
Appendix 6 Results of the sensitivity analysis of the efficacy data using studies measured either continuous abstinence or repeated 7-day point prevalence abstinence
A sensitivity analysis was carried out including all studies measured using either continuous abstinence or repeated 7-day point prevalence abstinence. A total of 21 studies comparing pairs, triplets or quintuplets of interventions provided information at various study durations. The varenicline 0.25 mg b.i.d. arm in study Nakamura et al. 49 was removed from the data because the number of events was not reported for this particular arm.
A summary of all the trials (data) included in the network meta-analysis for continuous abstinence including repeated 7-day point prevalence abstinence is presented in Table 44. Figure 19 presents the network of evidence.
| Author, year | Study duration (years) | Treatments | Number of events | Number of patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | ||
| Vinnikov 200843 | 0.50 | 1 | 3 | – | – | – | 1 | 9 | – | – | – | 97 | 100 | – | – | – |
| West 201117 | 1.00 | 1 | 3 | – | – | – | 9 | 31 | – | – | – | 370 | 370 | – | – | – |
| Bolliger 201147 | 0.46 | 1 | 7 | – | – | – | 26 | 157 | – | – | – | 199 | 394 | – | – | – |
| Niaura 200850 | 1.00 | 1 | 7 | – | – | – | 12 | 35 | – | – | – | 160 | 160 | – | – | – |
| Rennard 201253 | 0.46 | 1 | 7 | – | – | – | 21 | 171 | – | – | – | 166 | 493 | – | – | – |
| Rigotti 201054 | 1.00 | 1 | 7 | – | – | – | 26 | 68 | – | – | – | 359 | 355 | – | – | – |
| Tashkin 201157 | 1.00 | 1 | 7 | – | – | – | 14 | 47 | – | – | – | 254 | 250 | – | – | – |
| Tsai 200758 | 0.46 | 1 | 7 | – | – | – | 27 | 59 | – | – | – | 124 | 126 | – | – | – |
| Smith 201355 | 1.00 | 1 | 7 | – | – | – | 42 | 61 | – | – | – | 196 | 196 | – | – | – |
| Aubin 200846 | 1.00 | 2 | 7 | – | – | – | 75 | 98 | – | – | – | 379 | 378 | – | – | – |
| Steinberg 201162 | 0.46 | 1 | 7 | – | – | – | 12 | 9 | – | – | – | 39 | 40 | – | – | – |
| Wang 200960 | 0.46 | 1 | 7 | – | – | – | 42 | 63 | – | – | – | 168 | 165 | – | – | – |
| Wong 201237 | 1.00 | 1 | 7 | – | – | – | 34 | 55 | – | – | – | 135 | 151 | – | – | – |
| Pfizer 201140/Williams 201241 | 0.46 | 1 | 7 | – | – | – | 1 | 10 | – | – | – | 43 | 85 | – | – | – |
| Gonzales 200648 | 1.00 | 1 | 7 | 8 | – | – | 29 | 77 | 53 | – | – | 344 | 352 | 329 | – | – |
| Jorenby 200616 | 1.00 | 1 | 7 | 8 | – | – | 35 | 79 | 50 | – | – | 341 | 344 | 342 | – | – |
| Oncken 200652 | 1.00 | 1 | 6 | 7 | – | – | 5 | 48 | 58 | – | – | 129 | 259 | 259 | – | – |
| Heydari 201239 | 1.00 | 1 | 2 | 7 | – | – | 6 | 23 | 29 | – | – | 91 | 92 | 89 | – | – |
| de Dios 201238 | 0.46 | 1 | 2 | 7 | – | – | 0 | 0 | 3 | – | – | 11 | 11 | 10 | – | – |
| Nakamura 200749 | 1.00 | 1 | 6 | 7 | – | – | 35 | 51 | 56 | – | – | 154 | 156 | 156 | – | – |
| Nides 200651 | 1.00 | 1 | 4 | 5 | 7 | 8 | 6 | 10 | 7 | 18 | 8 | 127 | 128 | 128 | 127 | 128 |
FIGURE 19.
Network diagram of different interventions compared with placebo for continuous abstinence including repeated 7-day point prevalence abstinence. The nodes represent the interventions. Lines between nodes indicate when interventions have been compared. Different thicknesses of the lines represent the number of times that each pair of interventions has been compared.
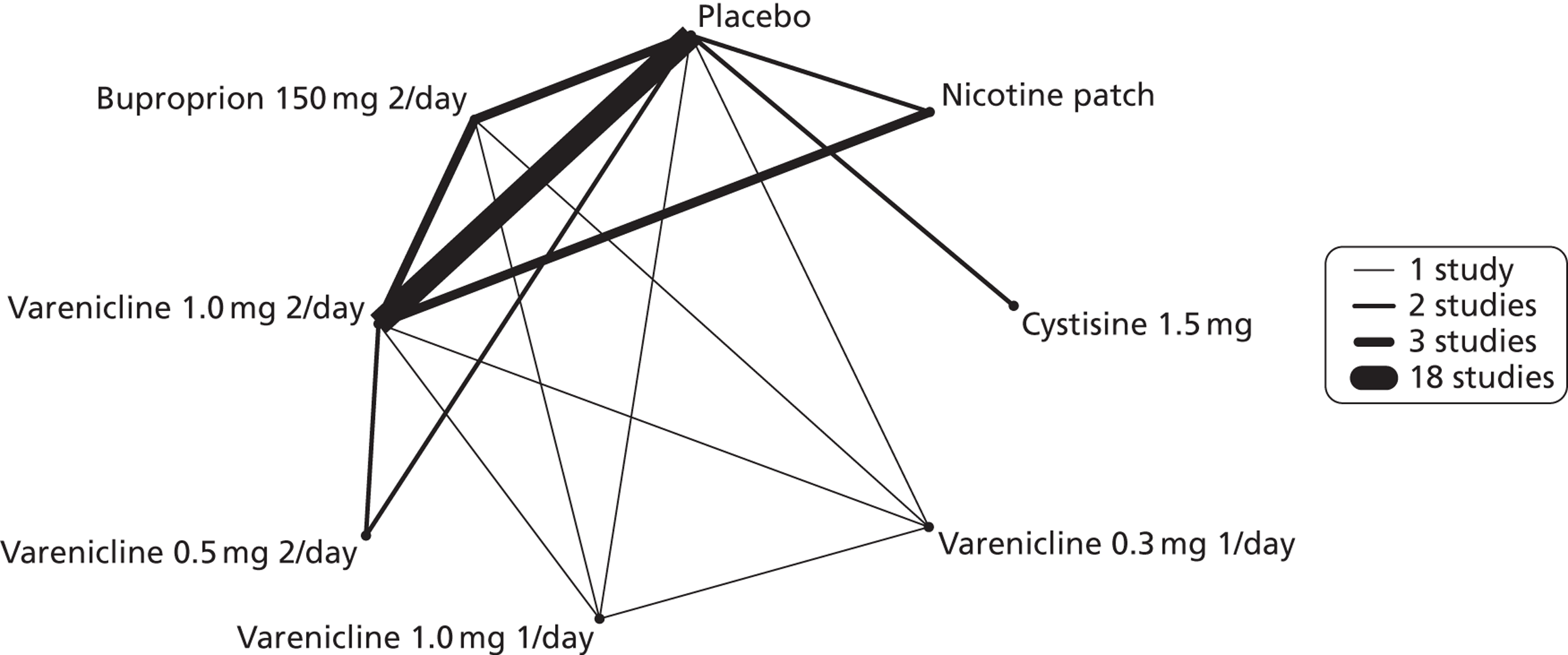
The network meta-analysis model fitted the data not very well, with a total residual deviance not very close to the total number of data points included in the analysis. The total residual deviance was 58.72, which compared favourably with the 51 data points being analysed.
There was evidence of mild heterogeneity in treatment effects between studies, 0.28 with 95% CrI (0.098 to 0.53). All interventions apart from varenicline 0.3 mg q.d. and 1.0 mg q.d. were associated with a statistically significant effect on continuous abstinence at a conventional 5% level relative to placebo. Cytisine 1.5 mg produced the greatest effect [4.35 with 95% CrI (1.91 to 11.75) relative to placebo] (see Table 39). Cytisine 1.5 mg was the intervention with the highest probability of being the most effective intervention (probability = 0.84) (see Table 40).
| Intervention | Hazard ratio (95% CrI) |
|---|---|
| Nicotine patch | 1.94 (1.14 to 3.40) |
| Cytisine 1.5 mga | 4.35 (1.91 to 11.75) |
| Varenicline 0.3 mg q.d. | 1.58 (0.62 to 3.89) |
| Varenicline 1.0 mg q.d. | 1.10 (0.37 to 2.86) |
| Varenicline 0.5 mg b.i.d. | 2.17 (1.35 to 3.61) |
| Varenicline 1.0 mg b.i.d. | 2.54 (2.09 to 3.14) |
| Bupropion hydrochloride 150 mg b.i.d. | 1.58 (1.02 to 2.38) |
| Between-study standard deviation | 0.28 (0.098 to 0.53) |
| Rank b | Treatment j | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Nicotine patch | Cytisine 1.5 mg | Varenicline 0.3 mg q.d. | Varenicline 1.0 mg q.d. | Varenicline 0.5 mg b.i.d. | Varenicline 1.0 mg b.i.d. | Bupropion hydrochloride 150 mg b.i.d. | |
| 1 | 0.00 | 0.03 | 0.84 | 0.03 | 0.01 | 0.03 | 0.06 | 0.00 |
| 2 | 0.00 | 0.08 | 0.07 | 0.09 | 0.02 | 0.19 | 0.53 | 0.00 |
| 3 | 0.00 | 0.18 | 0.04 | 0.09 | 0.03 | 0.30 | 0.32 | 0.04 |
| 4 | 0.00 | 0.31 | 0.02 | 0.14 | 0.05 | 0.26 | 0.07 | 0.15 |
| 5 | 0.01 | 0.21 | 0.02 | 0.17 | 0.10 | 0.13 | 0.01 | 0.36 |
| 6 | 0.10 | 0.14 | 0.00 | 0.22 | 0.15 | 0.07 | 0.00 | 0.32 |
| 7 | 0.40 | 0.05 | 0.00 | 0.16 | 0.26 | 0.01 | 0.00 | 0.12 |
| 8 | 0.49 | 0.00 | 0.00 | 0.10 | 0.39 | 0.00 | 0.00 | 0.01 |
| Study | Serious adverse events |
|---|---|
| Aubin 200846 | Varenicline: seven nausea; five headache; and five insomnia NRT: one insomnia |
| Bolliger 201147 | Varenicline: abortion; hypersensitivity; overdose; bronchitis and asthma; nasal septum deviation; suicidal ideation and depressed mood; suicidal ideation; tachycardia, bradycardia, and dyspnoea; panic attack; injury; and appendicitis Placebo: thyroid neoplasm; peritonitis, appendicitis and diverticulitis |
| de Dios 201238 | Not reported |
| Gonzales 200648 | Varenicline: abdominal pain, atrial fibrillation, pneumonia and possible stroke (one attributed to study drug) Bupropion: cholecystitis and septic shock, headache and grand mal seizure (one attributed to study drug) Placebo: lung cancer, acute myocardial infarction, schizophrenia (acute exacerbation), chest pain, urinary tract infection, atrial fibrillation and chest pain (under arms) |
| Heydari 201239 | Not reported |
| Jorenby 200616 | Varenicline, during treatment: cancer (lung or brain); acute coronary syndrome; chest pain; dehydration, periorbital cellulitis; acute psychosis, emotional lability; worsening vertigo, elevated blood pressure, chest pain (judged to be related to study medication) Varenicline, during follow-up: right-arm staphylococcal cellulitis and acute psychosis (same participant as in the treatment phase) Bupropion, during treatment: ectopic pregnancy, angiooedema (judged to be related to study medication), gun shot wound to left shoulder, postoperative bleeding, right leg pain below knee and breast cancer (female) Bupropion, during follow-up: occlusion coronary artery, a fatal motorcycle accident and a miscarriage Specific SAEs not reported for placebo |
| Nakamura 200749 | Varenicline (considered treatment related): one case of cholecystitis in the varenicline 0.25 mg b.i.d. group, which resolved after laparoscopic cholecystectomy, and one case of angina pectoris in the 1 mg b.i.d. group, which resolved after discontinuation of treatment Placebo: nature of SAEs not specified for placebo group |
| Niaura 200850 | Varenicline: myocardial infarction, ventricular fibrillation and spontaneous abortion (not considered related to treatment) Placebo: none reported |
| Nides 200651 | Varenicline: transient ischemic attacks in a subject with mild stenosis of the ipsilateral common carotid artery (considered possibly related to the study drug Bupropion: persistent intermittent bloody diarrhoea, syncope, and convulsion (two subjects) (considered possibly related to the study drug) Placebo: nature of SAEs not specified for placebo group |
| Oncken 200652 | Varenicline, within 30 days of last study medication dose: one subject in the 0.5 mg b.i.d. non-titrated group had a syncopal episode; four SAEs occurred in the 0.5 mg b.i.d. titrated group (one duodenal ulcer, one right ear cholesteatoma, one unstable angina, and one seizure following a car crash) and four SAEs occurred in the 1.0 mg b.i.d. group (one paroxysmal supraventricular tachycardia, one aseptic meningitis, one multiple sclerosis and one carcinoid colon cancer) Varenicline, more than 30 days after last study medication dose: diabetes mellitus in the 0.5 mg b.i.d. titrated group and cholelithiasis in the 1 mg b.i.d. non-titrated group Placebo: one syncope and one suicide attempt |
| Pfizer 201140/Williams 201241 | Varenicline: one chest pain; one convulsion; one depression; one psychiatric symptom; one suicidal ideation; one suicide attempt; and one asthma Placebo: one hyperglycaemia; one breast cancer; one aggression; and one suicidal ideation |
| Rennard 201253 | Varenicline: two intervertebral disc protrusion; one caratoid artery stenosis; one syncope; one peripheral arterial occlusive disease and one ureteric calculus with obstruction Placebo: one suicidal ideation |
| Rigotti 201054 | Nature of SAEs not specified |
| Smith 201355 | Varenicline: six deaths all non-related to study medication (patients had co-existing morbidities). One atrial fibrillation; four depressive episodes; and one aggression Counselling: seven deaths, all patients had underlying comorbidities |
| Steinberg 201156 | Varenicline: 15 SAEs defined as requiring or prolonging hospitalisation, but not further defined Placebo: 13 SAEs defined as requiring or prolonging hospitalisation, but not further defined |
| Tashkin 201157 | Nature of SAEs not specified |
| Tsai 200758 | Varenicline: one unstable angina, one peritonitis, one acute pyelonephritis Placebo: three traffic accidents |
| Tsukahara 201059 | Nature of SAEs not specified |
| Vinnikov 200843 | None reported |
| Wang 200960 | Placebo: one ulcer, one other not specified. |
| West 201117 | Not listed |
| Williams 200761 | Nature of all SAEs not specified |
| Wong 201237 | No severe adverse events reported |
Glossary
All definitions are taken from Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2012;4:CD006103.
- Abstinence
- A period of being quit, i.e. stopping the use of cigarettes or other tobacco products. May be defined in various ways – see also point prevalence abstinence, prolonged abstinence and continuous/sustained abstinence.
- Biochemical verification
- Also called biochemical validation or biochemical confirmation. Biochemical verification is procedure for checking a tobacco user’s report that he or she has not smoked or used tobacco. It can be measured by testing levels of nicotine or cotinine or other chemicals in blood, urine or saliva, or by measuring levels of carbon monoxide in exhaled breath or in blood.
- Bupropion
- A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation. The trade names are Zyban® (GlaxoSmithKline, UK) and Wellbutrin XL® (GlaxoSmithKline, UK) when prescribed as an antidepressant.
- Carbon monoxide
- A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence.
- Cessation
- Also called quitting. The goal of treatment to help people achieve abstinence from smoking or other tobacco use, also used to describe the process of changing the behaviour.
- Continuous abstinence
- Also called sustained abstinence. A measure of cessation often used in clinical trials involving avoidance of all tobacco use since the quit day until the time the assessment is made. The definition occasionally allows for lapses. This is the most rigorous measure of abstinence.
- Efficacy
- Also called treatment effect or effect size. The difference in outcome between the experimental and control groups.
- Nicotine
- An alkaloid derived from tobacco, responsible for the psychoactive and additive effects of smoking.
- Nicotine replacement therapy
- A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco free. The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges.
- Pharmacotherapy
- A treatment using pharmaceutical drugs, e.g. nicotine replacement therapy, bupropion.
- Point prevalence abstinence
- A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long-term quitters. Cf. prolonged abstinence, continuous abstinence.
- Prolonged abstinence
- A measure of cessation which typically allows a grace period following the quit date (usually of about 2 weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging.
- Titration
- A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects.
List of abbreviations
- A&E
- accident and emergency
- BENESCO
- Benefits of Smoking Cessation on Outcomes
- b.i.d.
- bis in die (twice a day)
- BNF
- British National Formulary
- CAR
- continuous abstinence rate
- CHD
- coronary heart disease
- CO
- carbon monoxide
- COPD
- chronic obstructive pulmonary disease
- CrI
- credible interval
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- EVSI
- expected value of sample information
- FDA
- US Food and Drug Administration
- HR
- hazard ratio
- INB
- incremental net benefit
- LY
- life-year
- NICE
- National Institute for Health and Care Excellence
- NRT
- nicotine replacement therapy
- PPA
- point prevalence abstinence
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- q.d.
- quaque die (every day)
- RCT
- randomised control trial
- SAE
- serious adverse event
- SD
- standard deviation
- STA
- single technology appraisal