Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/127/01. The contractual start date was in October 2011. The draft report began editorial review in April 2013 and was accepted for publication in August 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Robertson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
In this chapter we briefly discuss definitions, epidemiology and risks of obesity and possible benefits of reducing obesity in men. We show that men are under-represented in weight-loss programmes in developed countries and briefly discuss the growing literature on possible explanations. Evidence from qualitative and quantitative research is starting to accumulate on how men who are obese may be helped to lose weight, but there has been little systematic research to synthesise the evidence base. This project attempts to provide the current evidence base for engaging obese men with weight loss and provide pointers to designing successful services. The literature is still limited and we acknowledge that, although we would have liked to explore the effects of diversity, such as age, ethnic group, socioeconomic status, disability or sexual orientation, the evidence for these was sparse.
In this report we have tried to stick to accepted definitions of the words ‘sex’ and ‘gender’:1
The word ‘gender’ is used to define those characteristics of women and men that are socially constructed, while ‘sex’ refers to those that are biologically determined. People are born female or male but learn to be girls and boys who grow into women and men.
Definitions of obesity in men and women
A body mass index (BMI) of ≥ 30 kg/m2 [weight in kg/(height in m)2] is widely used to define obesity in both men and women, with a BMI of ≥ 25 kg/m2 and < 30 kg/m2 defining overweight. The term ‘morbid obesity’ is used to denote a BMI ≥ 40 kg/m2. BMI is widely used as an easy practical measure to classify the degree of obesity, predict the risk of obesity-related diseases and identify individuals or communities at risk. However, BMI does not distinguish between differences in body composition affected by sex, physique or ethnicity. For example, men will have a lower percentage of fat than women of an equivalent BMI. 2
Waist circumference is also used to assess increased body fat, particularly intra-abdominal fat. Unlike BMI, waist circumference cut-offs for risks of disease are sex specific. The National Institute for Health and Care Excellence (NICE)3 has advised that both BMI and waist circumference should be used to assess the risk of health problems (such as type 2 diabetes, coronary heart disease, osteoarthritis) in people with a BMI of < 35 kg/m2; above this BMI, risk will be high irrespective of waist circumference ( Table 1 ).
| BMI (kg/m2) | Waist circumference | ||
|---|---|---|---|
| Lowa | Highb | Very highc | |
| Normal: < 25 | No increased risk | No increased risk | Increased risk |
| Overweight: 25 to < 30 | No increased risk | Increased risk | High risk |
| Obese: 30 to < 35 | Increased risk | High risk | Very high risk |
Demographics of obesity in men and women
Based on BMI, more men than women are overweight or obese in the UK and this difference is projected to continue. In the Health Survey for England 2011,4 65% of men had a BMI of ≥ 25 kg/m2 whereas 58% of women fell into this category. As the prevalence of obesity continues to increase, it is likely that people who are overweight will become obese in the future. Thus, the Foresight report5 predicts that 36% of men and 28% of women will be obese by 2015 and 47% of men and 36% of women by 2025 in England. Figures from Wales6 (64% and 53%), Scotland7 (69.2% and 59.6%) and Northern Ireland8 (67% and 56%) show similar differences (men vs. women for overweight or obese respectively). In the UK, only in England do figures show that the prevalence of obesity in men is less than that in women,4 whereas in Scotland, Northern Ireland and Wales it is similar or higher in men. 6,7,8 However, morbid obesity tends to be less prevalent in men. 4,7 Worldwide, fewer men are obese than women but men have a higher BMI than women in high-income countries. 9
However, if waist circumference alone is used to define risks from obesity then women are more at risk, with 47% of women and 34% of men at risk in 2011 in England. 4 Using both BMI and waist circumference to define health risk, 18% of men had an increased risk, 15% had a high risk and 21% had a very high risk compared with 15%, 18% and 26% of women respectively. 4 Thus, measures of risk in men and women differ depending on the obesity measure used.
In England, the age-standardised prevalence of obesity and raised waist circumference for men and women was higher in households in lower quintiles than in households in higher quintiles of equivalised household income. 4 Some occupations, such as bus driving, may be at higher risk of obesity because of the work environment. 10 Work-related stress has different effects in men and women, increasing the risk of type 2 diabetes in obese women but not apparently for obese men. 11
Figures for different ethnic groups are not available from the recent Health Survey for England. 4 However, lower BMI and waist circumference cut-offs have been recommended for some ethnic groups, such as South Asian populations, as a measure of risk, particularly for type 2 diabetes, and have recently been recommended by NICE. 12 If existing BMI cut-offs are used, then data from the Health Survey for England from 200413 show lower prevalences of obesity in men from black African, Indian, Pakistani, Bangladeshi and Chinese groups.
In England the prevalence of overweight and obese individuals using BMI increases with age in men and women, with 29% of men and 32% of women aged ≥ 75 years being obese. 4
Risks of obesity in men and women
Collaborative analyses from 57 prospective studies, mainly from Western Europe and North America, with a mean recruitment age of 46 years, have found that mortality in both men and women is lowest for a baseline BMI of 22.5–25 kg/m2. 14 Each additional 5 kg/m2 was approximately associated with 30% higher overall mortality, 40% higher vascular mortality, 60–120% higher diabetic, renal and hepatic mortality, 20% higher respiratory disease mortality and 10% higher cancer mortality. Median survival was reduced by 2–4 years for a BMI of 30–35 kg/m2 and by 8–10 years for a BMI of 40–45 kg/m2. However, others have found that all-cause mortality does not appear to increase relative to normal weight until BMI is ≥ 35 kg/m2. 15
Pischon and colleagues16 found that waist circumference or waist-to-hip ratio enhanced the ability of BMI to predict risk of death in men and women in nine countries in Europe. However, the Emerging Risk Factors Collaboration17 found little difference in the ability of BMI, waist circumference and waist-to-hip ratio to predict cardiovascular disease in men and women in developed countries, but BMI had greater reproducibility. Similarly, there was little difference in the ability of BMI or waist circumference to predict the risk of developing type 2 diabetes in men, which is so strongly associated with obesity. 18 However, Cameron and colleagues19 considered that including both waist and hip circumference together, rather than as a ratio, may improve risk prediction models for mortality and type 2 diabetes.
Positive associations have been found between increasing BMI and subsequent risk of death from liver, kidney, prostate, breast, endometrial and large bowel cancer. 14 Others have found strong associations between obesity in men and subsequent oesophageal, thyroid, colon and renal cancer. 20
Obesity is a risk factor for a very wide range of diseases impacting on health and quality of life. Men with a BMI ≥ 30 kg/m2 and a waist circumference ≥ 102 cm have an increased risk of at least one symptom of impaired physical, psychological or sexual function, and these symptoms are also more likely in men with a raised waist circumference but a BMI of < 30 kg/m2. 21 Men who are overweight or obese in midlife also have a higher risk of frailty in old age. 22
Costs of obesity
Although the Foresight report5 predicted that future costs to the NHS of elevated BMI could be £6.4B per year by 2015 and £9.7B per year by 2050, no breakdown by sex was given, despite there being clear differences for the risk of diseases related to obesity, such as coronary heart disease and type 2 diabetes.
Benefits of weight loss in men and women
Although there are many diseases associated with obesity, it has been difficult to demonstrate that prevention or treatment of obesity reduces the risk of disease long term, despite beneficial changes in cardiovascular risk factors in randomised controlled trials (RCTs) of lifestyle interventions. 23 The evidence for a reduction in mortality from long-term weight loss from cohort studies and randomised trials is strongest for both overweight or obese men and overweight or obese women with diabetes. 24 There is some evidence that intentional weight loss may reduce mortality in women, but benefits in men are not clear. 24 Maintaining or increasing physical activity seems to be particularly beneficial to survival. 25
Randomised trials of lifestyle interventions for weight loss have confirmed the long-term prevention of type 2 diabetes in men and women. 26 – 28 Randomised trials of weight-loss interventions in men and women have also shown significant reductions in blood pressure or cardiovascular events. 23
Under-representation of men in weight-loss programmes
Men are under-represented in randomised trials of weight-loss interventions and in health services and commercial programmes for weight loss. In a systematic review, Pagoto and colleagues29 found that only 27% of participants in randomised trials were men, although the percentage was higher in interventions for obesity with related comorbidities (36% men). There was also a trend towards lower participation by men in group formats (24%) compared with individual counselling (29%) or mail/e-mail/internet formats (34%); however, the male/female mix of the groups was not specified. In another systematic review, Moroshko and colleagues30 did not find sex to be a predictor of dropout in weight-loss interventions.
Services for the treatment of adults with obesity in the UK have consistently shown an under-representation of men. In the Counterweight programme in 65 general practices in seven UK regions, only 23% of participants were men. 31 Men made up only 27.6% of referrals to the NHS Glasgow and Clyde Weight Management Service and, once referred, women were slightly more likely to opt in (73.6% vs. 69.4%), but there was no significant difference in completion rates by sex. 32
Commercial programmes in the UK, such as Weight Watchers,33 Slimming World34 and LighterLife,35 and some NHS organisations have only recently started to evaluate men-only weight-loss groups. When services were not sex specific, men made up only 10.7% of 34,271 adults in a slimming on referral scheme between Slimming World and 77 primary care trusts or NHS trusts,36 and 10.5% of 29,326 adults referred from NHS primary care to Weight Watchers. 37 Thus, UK figures suggest that men may be even less likely to attend commercial weight-loss programmes than programmes provided by the NHS.
Two systematic reviews have examined the qualitative evidence on people’s views and experiences of weight management. 38,39 Most of the evidence came from studies with women or studies with groups of men and women in which the majority of participants were women. The authors did not specifically examine the evidence from male participants in these studies, or men compared with women, so it is unclear whether or not their conclusions can be applied to men. There is evidence that since 1999 increasing numbers of both men and women in the UK are failing to recognise that they are overweight or obese. 40 Men may be more likely than women to misperceive their weight, less likely to consider their body weight a risk for their health and less likely to consider managing or be actively trying to manage their weight. 41,42
Men’s attitudes to lifestyle behaviour change
Men may be more reluctant to change their current lifestyle than women43 and may be cynical about government health messages. 44 Media and other sociocultural influences may also encourage men to maintain a larger, more muscular, masculine body size. 45 Men could be less interested in gaining an ideal body weight, according to the medical definition, and more interested in physical activity and regaining fitness and a masculine body shape. 46 There may also be differences in the way that men and women view physical activity as a means of becoming stronger, fitter and healthier. 47
Weight-loss programmes and facilities, including commercial weight-loss programmes, could be seen as feminised spaces,46,47 and there is some evidence to suggest that men may prefer masculine spaces, such as their workplace, for such programmes. 48,49 Fear and embarrassment may particularly deter men from taking part in weight-loss programmes and could mean that talking to an advisor on a one-to-one basis, rather than working in a group, is preferred. 49 Some men have also cited that having a male advisor for lifestyle change is important in the health-care setting. 50
Men could be less interested in undertaking weight-loss diets, which are perceived as tasting poor and failing to satisfy the appetite. 44 Men could distance themselves from the feminised realm of dieting, in which women are viewed as the experts. 51
Previous evidence associated with weight-loss management programmes and men
Given that there are difficulties in encouraging men to undertake weight management, what is the evidence for improving their engagement in services and should weight-loss programmes be designed differently for men and women?
The National Institute for Health and Care Excellence3 and the Scottish Intercollegiate Guidelines Network (SIGN)52 have not provided specific guidance for men, as opposed to women, for the prevention and treatment of obesity. NICE guidance on behaviour change interventions called for research on the cost-effectiveness of behaviour change interventions for men and women separately, but did not provide evidence on the effectiveness of lifestyle interventions separately by sex. 53
The Men’s Health Forum convened a conference in 2005 with 23 health and social policy researchers to discuss men and weight issues; the outcomes of this conference were subsequently published in a book entitled Hazardous Waist: Tackling Male Weight Problems. 54 Evidence of effective interventions was not reviewed, although several examples of innovative approaches in the UK were presented. The conference conclusions included a need to invest in ‘male-sensitive approaches’, that ‘men’s attitudes to weight and weight loss need to be more fully understood’ and that the ‘existing, broadly “unisex”, approach is failing men’ (pp. 218–19). 54
A systematic review was conducted by Robertson and colleagues55 in 2008 to explore the effectiveness of male-specific health-promoting interventions covering a wide range of health behaviours. However, it did not identify any intervention studies (at the time that the review was conducted) that had focused on men and weight management or weight loss.
More recently, Young and colleagues56 systematically reviewed men-only weight-loss or weight-maintenance interventions of any duration, limiting their review to the 18–65 years age group and people without obesity-related morbidity, for example diabetes. Only 12 of the 23 identified studies were RCTs and six included a follow-up of approximately a year or longer. Thirty-one different interventions were identified with a median weight loss of 6.25%. A high frequency of contact (three or more per month), group programmes, a mean age of ≤ 43 years in the sample and prescribing an energy-restricted diet were associated with greater programme effectiveness. Only five of the studies tested interventions that were specifically designed for men.
Aims of this project
The evidence briefly discussed in this chapter suggests that methods to engage men in services, and the services themselves, are currently not optimal. We set out to systematically review evidence-based management strategies for treating obesity in men and how to engage men with obesity in weight management programmes. Where we use the term ‘engagement’, this is to denote obese men deciding to start using services to help them lose weight.
We asked the following questions:
-
What works for obesity management for men?
-
How can men be engaged with services?
-
Should services for men and women be different?
Our overarching objective was to integrate the quantitative and qualitative evidence base by systematically reviewing:
-
the clinical effectiveness and cost-effectiveness of interventions for obesity in men, and in men compared with women
-
the clinical effectiveness and cost-effectiveness of interventions to engage men in their weight reduction
-
qualitative research with men about obesity management and with providers of such services for men.
This report is structured in the following way:
-
Chapter 2 presents the methods for the quantitative and qualitative systematic reviews and the mixed-methods synthesis of these reviews.
-
Chapter 3 presents the systematic review of RCTs of interventions [lifestyle and/or the UK licensed medication orlistat (a pharmaceutical agent to aid weight loss)] in any setting with men only who are obese with a BMI of ≥ 30 kg/m2 (or overweight with a BMI of ≥ 28 kg/m2 and with cardiac risk factors based on orlistat guidance) and with follow-up of at least 1 year. Chapter 3 also presents the systematic review of RCTs of interventions (as above) in any setting including both men and women who are obese with a BMI of ≥ 30 kg/m2 (or overweight with a BMI of ≥ 28 kg/m2 and with cardiac risk factors based on orlistat guidance) in which the results are presented separately for men and women in the same trial. We use trials with both men and women to look for differences in effectiveness.
-
Chapter 4 presents the results of the systematic review of interventions for men with obesity in the UK, of any setting, study design or duration. This includes data from UK studies with men and women in which data are presented separately for men and women. This chapter also contains details of the search carried out for the systematic review of studies to increase the engagement of men with obesity services; however, no studies fitting the inclusion criteria were found. However, information on engaging men with services is available and discussed in the first review in this chapter.
-
Chapter 5 presents the systematic review of economic evaluations of obesity interventions, including studies in which data were presented either for men only or for men compared with women.
-
Chapter 6 presents the systematic review of qualitative studies that have explored men’s engagement and experiences associated with weight management interventions linked to RCTs and other intervention studies. We also included qualitative studies on obesity from the UK that were not linked to interventions. This review focused on questions relating to the context of these interventions and well as their mechanisms and outcomes. The findings from the qualitative studies were combined with the findings from all of the quantitative reviews in a mixed-methods synthesis.
-
Chapter 7 presents our overall discussion of the results from all of the reviews.
-
Chapter 8 draws out the implications for health care and makes recommendations for future research.
Chapter 2 Methods
We undertook six systematic reviews as follows:
-
a systematic review of RCTs of interventions with men only
-
a systematic review of RCTs of interventions in which the results were presented separately for men and women in the same trial
-
a systematic review of interventions for men, or men and women compared, with obesity in the UK in any setting, using any study design and of any duration
-
a systematic review of interventions to increase the engagement of men with services for obesity management, using any study design
-
a systematic review of the cost-effectiveness of alternative strategies for the management of obesity in adult men
-
a systematic review of qualitative research with obese men, or obese men compared with obese women, and with health professionals and commercial organisations managing obesity.
We prepared a priori protocols detailing the objectives; types of study design, participants, interventions and outcomes considered; and the inclusion/exclusion criteria for all reviews. For quantitative reviews we followed methodological guidance recommended by The Cochrane Collaboration57 and Centre for Reviews and Dissemination. 58 Details of the methods used for the cost-effectiveness review are provided in Chapter 5 .
Inclusion and exclusion criteria
Types of study
The systematic reviews of men only and men and women compared included RCTs or quasi-randomised trials (including trials with a cluster design) with a mean or median duration of ≥ 52 weeks for all groups. This duration of follow-up for data was to ensure that long-term weight-loss and weight-maintenance interventions were evaluated for their associated effects on weight- and obesity-related morbidities. 23 This was also the minimum duration of studies adopted by NICE for its review of obesity. 3
For the systematic review of UK interventions, any study design and duration were considered to include and evaluate as much UK-relevant research as possible. We included studies of men only and studies of men and women if data were presented separately for men.
For the systematic review of interventions to increase engagement, we included any study design that examined interventions to increase the engagement of men with services for obesity management.
Types of participants
Men aged ≥ 16 years were included, with no upper age limit. Participants in studies included in the systematic reviews of men only, men and women compared and UK interventions had to have a mean or median BMI of ≥ 30 kg/m2 (or ≥ 28 kg/m2 with cardiac risk factors based on criteria for receiving orlistat). When body weight was reported instead of BMI, we calculated BMI using relevant population data for heights to assess study eligibility. 23 We recognised that the BMI of men targeted in systematic reviews of engagement and cost-effectiveness may not have been clearly stated.
Types of interventions and comparators
Systematic reviews of studies of men only, men and women compared and the UK interventions considered interventions in the form of orlistat (but not sibutramine or rimonabant, which no longer have UK licences), diet, physical activity, behaviour change techniques or combinations of any of these. For the reviews of men-only RCTs and RCTs of men and women compared, we considered any of these interventions along with placebo and ‘no treatment’ as comparators. For the systematic review of engagement we considered any form of intervention to increase the engagement of men with services for obesity management.
Setting
We considered all settings for the lifestyle and drug interventions, including hospitals, primary care, the community (including a community pharmacy), commercial organisations, the voluntary sector, leisure centres, workplaces, the internet and other digital domains, for example mobile phone networks. This is because there is increasing collaboration between the NHS and non-NHS organisations in the delivery of services. It may also be the case that increasing the participation of men in obesity services requires their engagement in settings outside primary and secondary care.
Types of outcome measures
We developed our rationale for outcome measurement from our existing knowledge of the topic area and in consultation with the project advisory group. Studies had to explicitly mention weight loss or weight-loss maintenance as a main outcome to be eligible for inclusion.
We considered the following types of outcome:
-
Primary outcome: weight change
-
Secondary outcomes:
-
waist circumference
-
cardiovascular risk factors [decreases in these are generally beneficial for cardiovascular risk, with the exception of high-density lipoprotein (HDL) cholesterol]: total cholesterol, HDL cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, fasting glucose, glycated haemoglobin (HbA1c), systolic and diastolic blood pressure
-
disease-specific outcomes (e.g. erectile function)
-
adverse events
-
quality of life outcomes
-
process outcomes (e.g. staff involvement, setting, type of intervention, timing, frequency, individual and/or group setting, couple or family setting, proportion recruited and dropping out, participants’ evaluations)
-
economic costs.
-
Exclusion criteria
We did not consider interventions including complementary therapy, for example acupuncture, or non-diet products promoted for weight loss available solely over the counter. Studies evaluating bariatric surgery or examining a combination of interventions, for example smoking cessation and weight loss at the same time, or examining men with obesity receiving psychotropic medication, with learning disabilities or with a diagnosed eating disorder, were also excluded.
Search strategies
For the search strategies there were no language restrictions and studies had to be set in societies relevant to the UK setting. We maintained the comprehensive electronic search conducted in MEDLINE and EMBASE in our previous systematic review23 of RCTs of lifestyle interventions for weight loss in obese adults with 1 year of follow-up. From our existing searches for this review and subsequent updates we identified approximately 800 potentially relevant reports, in any language, for full-text assessment for the review of men-only RCTs and RCTs of men and women compared. In addition to this search we conducted comprehensive electronic searches based on our existing search strategy to identify RCTs of interventions for obesity in men. To avoid unnecessary overlap with the pre-existing results from the review by Avenell and colleagues,23 the search strategy for reviews of men-only RCTs and RCTs of men and women compared excluded studies published before 2001.
Highly sensitive electronic searches were undertaken to inform the reviews of UK interventions and interventions to increase engagement. The searches were designed to identify studies of interventions for obese men in the UK, studies of interventions to increase the engagement of men with obesity management services and qualitative research with obese men or obese men compared with obese women. The searches for all reviews were designed to identify systematic reviews and other background information relevant to the management of obesity in men. Additionally, a separate search was undertaken to identify studies examining the cost-effectiveness of interventions for obese men. The searches for each of the reviews were designed to be mutually exclusive, with the results of each new search being deduplicated against the results of the previous searches. Table 2 details the databases searched for each review.
| Database | Men-only RCTs | RCTs of men and women compared | UK interventions | Engagement | Cost-effectiveness | Qualitative research |
|---|---|---|---|---|---|---|
| MEDLINE | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| EMBASE | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cumulative Index to Nursing and Allied Health Literature (CINAHL) | ✓ | ✓ | – | ✓ | – | ✓ |
| PsycINFO | ✓ | ✓ | ✓ | ✓ | – | ✓ |
| Cochrane Central Register of Controlled Trials (CENTRAL) | ✓ | ✓ | – | – | – | – |
| Cochrane Database of Systematic Reviews (CDSR) | ✓ | ✓ | – | – | – | – |
| Database of Abstracts of Reviews of Effects (DARE) | ✓ | ✓ | – | – | – | – |
| NHS Economic Evaluation Database (NHS EED) | ✓ | ✓ | – | – | ✓ | – |
| Health Technology Assessment (HTA) database | ✓ | ✓ | – | – | – | – |
| Applied Social Sciences Index and Abstracts (ASSIA) | – | – | – | ✓ | – | ✓ |
| Education Resources Information Center (ERIC) | – | – | – | – | – | ✓ |
| Anthropology Plus | – | – | – | – | – | ✓ |
| British Nursing Index | – | – | – | – | – | ✓ |
| Social Sciences Citation Index (SSCI) | – | – | ✓ | – | – | ✓ |
| Health Management Information Consortium (HMIC) | – | – | – | – | ✓ | – |
| Conference Proceedings Citation Index – Social Science & Humanities (CPCI-SSH) | – | – | ✓ | – | – | ✓ |
| Cost-Effectiveness Analysis Registry (CEA Registry) | – | – | – | – | ✓ | – |
| Research Papers in Economics (RePEc) | – | – | – | – | ✓ | – |
| ClinicalTrials.gov | ✓ | ✓ | – | – | – | – |
| CenterWatch | ✓ | ✓ | – | – | – | – |
| Current Controlled Trials | ✓ | ✓ | – | – | – | – |
| International Clinical Trials Registry | ✓ | ✓ | – | – | – | – |
The database searches were conducted over the following time periods:
-
MEDLINE In-Process & Other Non-Indexed Citations: 1948 to 30 July 2012
-
MEDLINE: 1948 to 2012 Week 31
-
EMBASE: 1980 to 2012 Week 31
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL): 1981 to July 2012
-
PsycINFO: 1800s to July 2012
-
Social Sciences Citation Index (SSCI): 1970 to July 2012
-
Conference Proceedings Citation Index – Social Science & Humanities (CPCI-SSH): 1990 to July 2012
-
Cochrane Central Register of Controlled Trials (CENTRAL): The Cochrane Library, Issue 5, 2012
-
Cochrane Database of Systematic Reviews (CDSR): The Cochrane Library, Issue 5, 2012
-
ClinicalTrials.gov: September 2011
-
CenterWatch: September 2011
-
Current Controlled Trials: September 2011
-
World Health Organization International Clinical Trials Registry: September 2011
-
Applied Social Sciences Index and Abstracts (ASSIA): 1987 to July 2012
-
Education Resources Information Center (ERIC): 1966 to July 2012
-
Anthropology Plus: 1957 to July 2012
-
British Nursing Index: 1994 to October 2011
-
NHS Economic Evaluation Database (NHS EED): July 2012
-
Health Technology Assessment (HTA) database: July 2012
-
Database of Abstracts of Reviews of Effects (DARE): July 2012
-
Health Management Information Consortium (HMIC): 1979 to November 2011
-
Cost-Effectiveness Analysis Registry (CEA Registry): January 2012
-
Research Papers in Economics (RePEc): January 2012.
Hand searching
Reference lists of all included studies were scanned to identify any additional potentially relevant reports. We also searched the internet for online weight-loss programmes specifically targeted at men (e.g. www.fatmanslim.com) and the Picker Institute and Joanna Briggs Institute websites for grey literature.
Other methods of ascertaining relevant information sources
When contact details were available, we contacted all authors of men-only RCTs to identify any qualitative or other relevant published or unpublished reports. Our advisory group members provided details of potentially relevant reports and further potentially useful contacts and information sources. Each of the Men’s Health Forum representatives included articles in their newsletters highlighting our project and providing details for readers to contact us with any relevant reports. The English Men’s Health Forum also publicised the project through its Twitter account. Furthermore, we contacted the Association for the Study of Obesity, Dietitians in Obesity Management and other commercial organisations to identify published and unpublished UK studies (see Appendix 1 ).
Quantitative reviews of randomised controlled trials and other intervention studies
Data extraction strategy
One reviewer (CR) independently screened the titles and abstracts of all identified items. Full-text copies of all potentially relevant reports were obtained and independently assessed for eligibility (CR assessed reviews of men-only RCTs, RCTs of men and women compared and interventions to increase engagement; AA assessed the review of UK interventions). One reviewer (CR) extracted details of study design, methods, participants, interventions and outcomes using a data extraction form (see Appendix 2 ). The data extraction was then checked by a second reviewer (AA) and any errors were corrected.
Quality assessment strategy
We assessed the methodological quality of included RCTs using The Cochrane Collaboration’s tool for assessing risk of bias57 (see Appendix 3 ). We assessed the methodological quality of non-randomised comparative studies using a 17-item checklist, with the same checklist minus four questions used to assess the quality of case series (see Appendix 4 ). The checklist was developed for NICE through the Review Body for Interventional Procedures (ReBIP) and was adapted from several sources, including the NHS Centre for Reviews and Dissemination’s guidance for those conducting or commissioning systematic reviews,58 Verhagen and colleagues,59 Downs and Black60 and the Generic Appraisal Tool for Epidemiology (GATE). 61 Individual items within these tools were rated as ‘yes’, ‘no’ or ‘unclear’ so that a rating of ‘yes’ denoted the optimal rating for methodological quality. Two reviewers independently assessed the quality of all included full-text primary studies. In addition, we used an adapted version of the Campbell & Cochrane Equity Methods Group checklist62 for each of the reviews to assess the effect of interventions reported in the included studies on disadvantaged groups and/or their impact on reducing socioeconomic inequalities (see Appendices 8 , 10 and 12 ). Individual items were worded as ‘yes’, ‘no’ or ‘unclear/not reported.’ Conference abstracts and poster presentations were excluded unless sufficient details were reported to carry out quality, equity and sustainability assessments (e.g. protocols, internal reports). Any disagreements or uncertainty were resolved by discussion between the two reviewers. A third reviewer acted as an arbitrator when consensus could not be reached.
Data analysis
We imported data into Review Manager (RevMan) software (version 5.1; The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) for data synthesis. We reported means or changes in means or proportions between groups. For continuous outcomes we reported the mean difference or standardised mean difference (different scales for the same outcome) and for dichotomous data we presented the risk ratio with 95% confidence intervals (CIs). Because of the inherent heterogeneity in studies of obesity interventions, when study results could be quantitatively pooled we used random-effects meta-analysis throughout. For meta-analysis plots of only one study we used fixed effects. We used visual inspection and the I 2 statistic to assess heterogeneity in forest plots57 and planned funnel plot analysis to investigate reporting biases for forest plots with ≥ 10 studies.
We planned to explore the role of sex as a treatment modifier by conducting a meta-analysis of the treatment by sex interaction effect across trials in which outcomes were presented separately by sex,63 but this was not possible because of the heterogeneous nature of the interventions, particularly in terms of dietary calorie prescription. For statistics on the proportion of participants completing the study, only studies that reported the rate of dropout were included. The risk difference and its CI between men and women were calculated with the p-value.
For the analysis of mean weight difference between men and women, the weighted mean difference (WMD) was calculated for both men and women when more than one group was reported. The standard deviation (SD) for the WMD was calculated using the formulae for calculating SD for grouped data. Studies with no baseline weight values were excluded from the analysis of weight difference. In the analysis of percentage weight loss the WMD was divided by the baseline weight. For each study the number of participants, the WMD of weight or percentage weight loss from baseline and its SD were entered into RevMan software. The random-effects model was used.
Subgroup analyses were planned to explore whether the effectiveness of interventions differed according to whether participants were selected on the basis of newly diagnosed or pre-existing obesity-related comorbidities (e.g. diabetes, hypertension). This was not possible because of the limited quantity of data and the heterogeneity of the studies. Sufficient data were not available to explore the effect of deprivation, age and ethnicity on effectiveness nor were there sufficient data to explore the effect of assumed values for weight on meta-analyses.
The methods for incorporating economics evidence into the reviews followed those recommended in The Cochrane Handbook. 57 A narrative synthesis is presented.
Integrated qualitative and quantitative evidence synthesis
We undertook a realist integrated qualitative and quantitative evidence synthesis to investigate what weight management interventions work for men, with which men and under what circumstances. From a realist perspective, it is important to conceptualise any intervention intended to improve health by considering the:
-
context that an intervention/programme will be situated within so that factors that might inhibit or enhance its effectiveness can be identified
-
mechanisms of the intervention/programme and how the intended programme beneficiaries will interact and react to the intervention processes and mechanisms
-
outcomes, both positive and negative, that may arise from an individual’s engagement with the proposed intervention.
A body of literature has emerged over recent years that has stressed the importance of considering public health problems (such as obesity) from a so-called socioecological perspective. 5,64 – 69 Hence, our methodological approach investigated issues relating to the macro-, meso- and micro-level influences that shape and influence men’s perspectives and experiences related to engaging with weight management programmes. By macro-level influences we mean the wider social, cultural, economic and political factors that overarch and influence the meso level of workplace, community, family, friends and peers, whereas micro level refers to the individual psychological and biological determinants of health and well-being.
A priori research questions
The primary aim of the evidence synthesis was to uncover how effective interventions work and to describe key intervention ingredients, processes and environmental and contextual factors that contribute to effectiveness. 70 We also aimed to identify the barriers and facilitators that men experience when engaging with a weight management intervention. Both deductive and inductive analytical approaches were employed throughout the review process and as such the following a priori research questions were developed to guide our initial investigation:
-
What are the best evidence-based management strategies for treating obesity in men?
-
How can men’s engagement in obesity services be improved?
In addition to these a priori research questions we also developed a series of 10 more detailed research questions that emerged inductively from the initial findings of the review of men-only RCTs (see Chapter 3 ) and also the expertise, knowledge and previous research of the chief investigator (AA) and principal investigators (FD, PH, EvT). Generating inductive research questions in this way is an inherent property of qualitative research and particularly of a grounded theory approach in which data collection and analysis proceed iteratively to confirm or refute an emerging theory:
-
How are men initially motivated to lose weight?
-
How are men attracted to taking part in the trial/intervention?
-
Are men consulted in the design of the intervention?
-
If it is found that interventions for men should be different from those for women, how should they be different and why?
-
Are group-based interventions for men found to be more effective for weight loss than those delivered to individual men?
-
Are certain features of diets found to be more attractive for obese men?
-
Are certain features of physical activity stated to be more attractive for obese men? How and why are these features more attractive?
-
What efforts are made to help men continue with the programme?
-
Do men state who they believe to be the best person/persons to deliver the intervention?
-
Are programmes deliberately involving partners/families more effective?
These questions were incorporated into our data extraction form (see Appendix 15 ) to code the data from studies linked to interventions to identify a priori themes. A full description of the data analysis cycle is provided in The analysis cycle and thematic synthesis.
Inclusion and exclusion criteria
We included any study reporting qualitative research with obese men, or obese men in contrast to obese women. In addition we included qualitative data from both health professionals and commercial organisations involved in managing obesity. The search included studies published from 1990 onwards and no language restrictions were placed on any of the searches.
As stated above in the description of methods for quantitative reviews, the studies included men who were 16 years or over, with no upper age limit, who had a mean or median BMI of 30 kg/m2. We included data from qualitative and mixed method studies linked to the identified RCTs and linked to RCTs not included in the quantitative reviews for this report. We also included any qualitative data reported as part of papers reporting quantitative outcomes. Furthermore, we included data from qualitative studies linked to non-randomised intervention studies and qualitative data from studies that were not linked to any specific UK-based, men-only interventions that had reported on men’s experiences of weight-loss attempts.
Studies conducted in developed countries were included if they contributed relevance to the UK context and all settings for lifestyle and drug interventions were considered. These included workplaces, football and rugby clubs, primary care, the internet, and religious and community settings.
We did not consider studies where men were not included and where obesity and weight management were not the prime focus. In addition, studies that did not contain primary qualitative research with obese men were not considered.
Identification of studies
The search methods for the review of qualitative studies have been reported earlier (see Search strategies). Two researchers independently screened abstracts for inclusion and all eligible study reports were entered into NVivo 9 qualitative data management software (QSR International, Southport, UK). Two researchers then grouped the final included studies into three categories:
-
qualitative and mixed-method studies linked to eligible RCTs, including any qualitative data reported as part of papers reporting quantitative outcomes
-
qualitative and mixed-method studies linked to ineligible RCTs and identified non-randomised intervention studies, including any qualitative data reported
-
UK-based qualitative studies not linked to any specific interventions that contained men-only samples.
Although it could be argued that separation of the studies into groups could cause further decontextualisation, as the focus of this project is to assess the evidence for weight management interventions, grouping the studies in this way assisted in the integration of the quantitative and qualitative review processes.
Data extraction strategy
For the studies linked to interventions, one reviewer (DA) used a data extraction form (see Appendix 15 ) to extract details of study design, methods, participants, interventions, findings, data pertaining to area and setting, and quality. Completed data extraction forms were checked by a second reviewer (either FD or EvT). Any disagreements over the interpretation of extracted data were discussed at group meetings. After agreement was reached the extraction forms were imported into NVivo 9 for analysis.
Following the analysis of the intervention study data, a further process of data extraction was applied to the nine theoretical studies not linked to interventions to investigate whether these studies contained data to confirm, refute or add any new thematic insights. Three researchers (DA, FD and EvT) screened three of the non-intervention studies each and extracted and inserted data into a Microsoft Excel spreadsheet (2007; Microsoft Corporation, Redmond, WA, USA) containing our interpretive themes derived from the intervention study data as headings. Data extraction of the non-intervention studies was cross-checked by other researchers in the group.
Quality assessment strategy
There is a great diversity of approaches to data collection and data analysis within qualitative research and also a multiplicity of theoretical perspectives. This has made it difficult to develop consensus over which criteria are the most useful when assessing the quality of qualitative studies. 1,71,72 At present, some qualitative synthesis methods such as framework synthesis and thematic synthesis undertake highly specified forms of quality appraisal that can result in the exclusion of studies that are judged to be of poor methodological quality. However, other methods such as critical interpretive synthesis do not exclude papers as long as they meet basic relevance criteria. 73
With this in mind, there appears to be a growing argument amongst certain researchers72,74 – 77 that qualitative studies should not be excluded from qualitative evidence syntheses based on quality assessment. They argue that excluding studies because of methodological flaws or incomplete reporting may result in the loss of valuable new insights, whereas studies that are methodologically sound may suffer from poor interpretation of data, leading to an insufficient insight into the phenomenon under study. 76 In addition, Carroll and colleagues74 contend that a quality appraisal instrument can assess only what is reported in a publication; thus, aspects such as the style of journal or word limits may have a bearing on whether or not studies have adequate space to describe fully certain elements of a study that a quality assessment tool may be investigating. For example, Garip and Yardley39 note that papers published in medical journals were often rated poorly using the Critical Appraisal Skills Programme (CASP) tool78 because of the lack of space to provide full methodological details. We found these arguments against excluding studies to be convincing and therefore did not exclude any of the 13 qualitative studies linked to interventions on the basis of quality. Instead, we elected to formulate and apply a quality appraisal tool during the process of data extraction.
Our quality assessment tool was formulated following a consultation of the following critical appraisal checklists: CASP,78 the Consolidated Criteria for Reporting Qualitative Research79 and the Joanna Briggs Institute Qualitative Assessment and Review Instrument. 80 We included criteria from these that we considered were key in terms of methodological rigour and also in terms of importance for our a priori research questions, which are specifically concerned with informing policy and practice.
The criteria that we selected were:
-
Aims and methods:
-
Research questions – stated explicitly or implicitly within the general text/topic guide? In what section(s) of the paper are questions mentioned? Are they prospective or retrospective?
-
Theoretical and epistemological perspective underpinning the qualitative research?
-
Theoretical perspective underpinning the intervention?
-
Qualitative methods used?
-
Data analysis technique and procedure?
-
-
Sample:
-
Sample size?
-
Sample characteristics?
-
Sample selection process?
-
Sample inclusion and exclusion criteria?
-
-
Reflexivity:
-
Evidence of researcher reflexivity?
-
-
Ethics:
-
Evidence of attention to ethical issues?
-
-
General criteria:
-
Are the findings adequately supported by the data presented?
-
Is there potential for a ‘charisma effect’ with this study? (this relates to the potential influence of the principal investigator)
-
Any other quality issues not covered by previous items?
-
The quality appraisal tool was integrated into the data extraction form and was applied by one researcher (DA). The quality assessments were subsequently cross-checked by another researcher (either FD or EvT). The findings and conclusions of our quality assessment are discussed in Chapter 6 .
The analysis cycle and thematic synthesis
We developed an analysis cycle that started with coding of the qualitative data from studies linked to interventions followed by the development of initial descriptive themes and finally the development of higher-order analytical and interpretive themes and concepts. This cyclical and iterative process was conducted to identify the promising ‘ingredients’ of interventions that are likely to be effective in male weight reduction, both in terms of essential and necessary contextual/environmental variables and intervention processes.
Development of a thematic index
To develop a thematic index, one researcher (DA) coded the findings reported in the included qualitative studies linked to interventions line by line for content and meaning and categorised these according to whether they corresponded to the a priori themes or whether they appeared to represent emergent themes unconnected to the a priori themes.
After undertaking this process for all studies linked to interventions the coding was cross-checked by another researcher (FD). The data then underwent a second importing process within NVivo 9 into framework matrices for comparison of the a priori and emergent themes for effective and non-effective interventions. Framework matrices were used to facilitate the use of the constant comparative method to search for patterns and relationships and assist with developing theory. All qualitative researchers (DA, FD, PH and EvT) then developed the descriptive thematic index over a series of meetings, with a tree structure of themes and subthemes to enable us to remain close to the reported study findings. The thematic framework was discussed at a meeting with the Men’s Health Forum representatives to ascertain service users’ perspectives. The qualitative researchers decided that one of the 10 a priori themes (‘Are programmes deliberately involving partners/families more effective?’) was not supported by the data and it was rejected. To develop a thematic index, one researcher (DA) coded the findings reported in the qualitative studies line by line.
The development of interpretive themes
We then generated a more refined set of interpretive themes from the a priori and emergent themes for the effective management of obesity in men and the barriers to and facilitators of engaging in weight management programmes. In meta-ethnography these are described as ‘third-order interpretations’. 81 Following the completion of the analytical cycle for studies linked to interventions, the theoretical studies not linked to interventions were then read to ascertain whether they provided disconfirming evidence or added any new perspectives and all relevant data from these studies were extracted. This process was conducted to test the robustness of the synthesis and has been recommended in previous narrative synthesis methods literature. 82
The final stage of the analysis involved integrating the qualitative findings with findings from the quantitative reviews. This was achieved through a process of in-depth reading of each results chapter by all members of the research group to identify where qualitative findings were supported or refuted by quantitative findings. The supporting or disconfirming quantitative data were then integrated into the findings.
Researcher perspectives
It is important to be aware of the researchers’ backgrounds and associated perspectives when interpreting our findings. DA is a health services researcher with a background in sociology and mixed-methods research methodologies. FD is a public health researcher with a background in health promotion and nursing, with an interest in health inequalities and the social determinants of health outcomes and behaviours. EvT is a medical sociologist with a background in qualitative and mixed-methods research and an interest in public health in general and health promotion in particular. PH is an academic general practitioner (GP) with expertise in qualitative and mixed-methods research, particularly when applied to RCTs of complex interventions. None of the qualitative researchers can be considered obese. Throughout the study reflexivity took place through weekly research team discussions until a consensus was reached.
Chapter 3 Systematic reviews of men-only randomised controlled trials and randomised controlled trials with data for men and women compared
In this chapter we present two systematic reviews. The first is a systematic review of RCTs of interventions (lifestyle and/or the UK-licensed medication orlistat) in any setting with men only who are obese with a BMI of ≥ 30 kg/m2 (or overweight with a BMI of ≥ 28 kg/m2 and cardiac risk factors based on orlistat guidance) and for which there are follow-up data for at least 1 year.
The second is a systematic review of RCTs of interventions (as above) in any setting with both men and women who are obese with a BMI of ≥ 30 kg/m2 (or overweight with a BMI of ≥ 28 kg/m2 and cardiac risk factors based on orlistat guidance) in which the results are presented separately for men and women in the same trial. We use trials with both men and women to look for differences in effectiveness.
Quantity of evidence
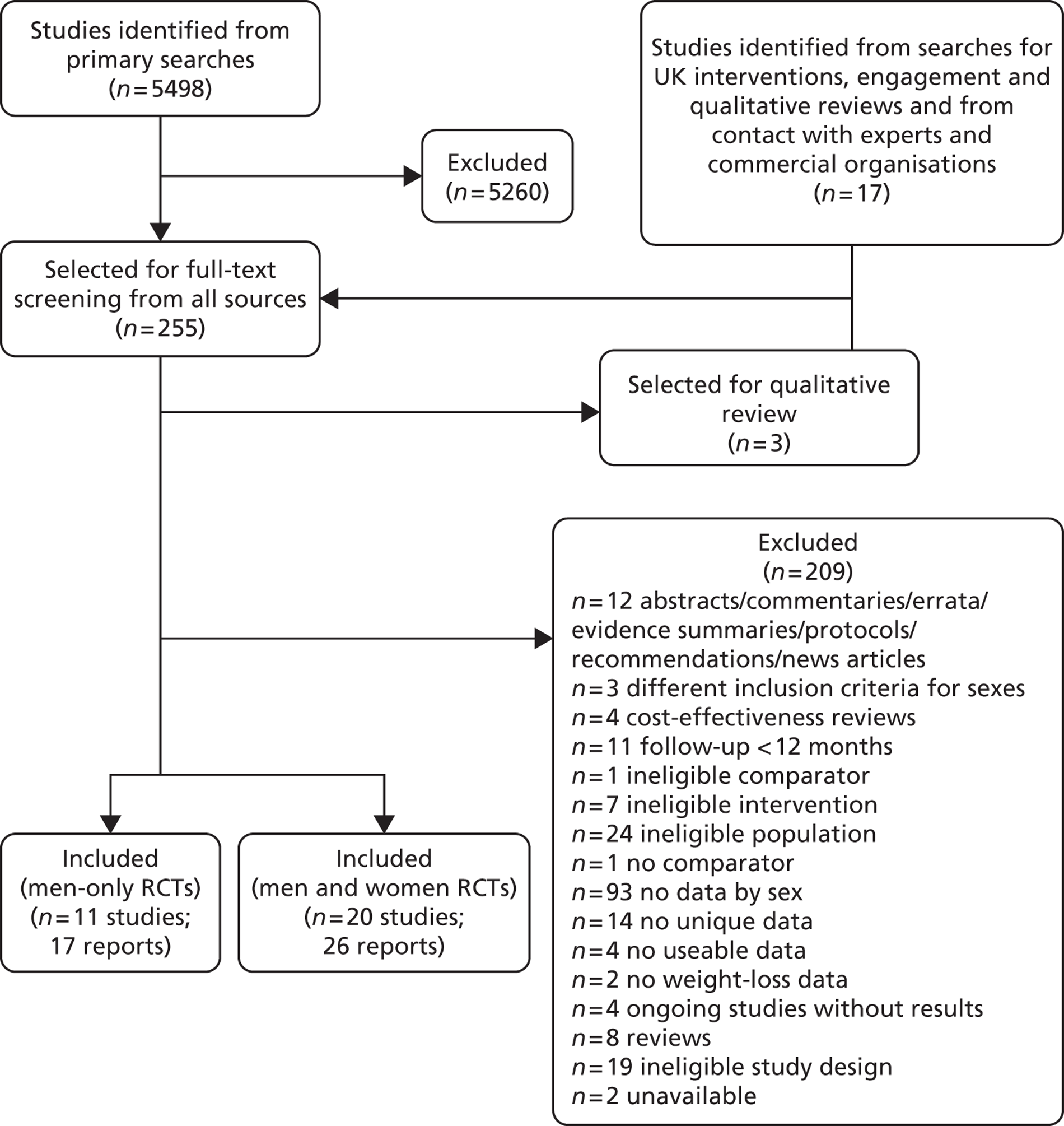
Our primary literature search identified 5498 potentially relevant titles and abstracts ( Figure 1 ). In addition to this, we identified 17 potentially relevant reports from other sources, such as commercial organisations and expert opinion. We selected 255 reports for full-text assessment, of which 11 RCTs83 – 93 were included in the review of men-only RCTs and 20 RCTs94 – 113 were included in the review of RCTs of men and women, along with six reports114 – 119 linked to the review of men-only RCTs and six reports120 – 125 linked to the review of RCTs of men and women.
FIGURE 1.
Flow chart of the number of potentially relevant reports and the numbers subsequently included and excluded from the reviews.
Review of men-only randomised controlled trials
Number and type of studies
Eleven RCTs including men only were identified as eligible for inclusion, nine of which investigated weight-loss interventions. 83,85 – 87,89 – 93 One trial examined a reducing diet for weight loss. 93 Three trials investigated the type of reducing diet to use. 83,87,91 Three trials looked at the use of physical activity in weight reduction. 91 – 93 Three trials examined a diet plus behaviour therapy and exercise advice for weight loss. 85,89,90 Jeffery and colleagues86 investigated the use of various monetary contracts for individual or group weight loss. Two trials investigated exercise or behaviour change training for weight maintenance. 84,88 The weight-maintenance trial conducted by King and colleagues88 randomised men who had received active weight-loss interventions in the trial by Wood and colleagues. 93 This was the only weight-maintenance trial found that was linked to one of the eligible weight-loss intervention trials identified by our screening process. Details of the interventions investigated by the individual trials are presented in Table 3 . None of the trials reported involving male service users in the design of the intervention. The period of follow-up for all of the trials ranged from 12 to 36 months (median 15 months), with five trials83,88,89,92,93 having a follow-up period of 1 year only.
| Study ID | Participants | Interventions | Outcomes | Notes |
|---|---|---|---|---|
| Benassi-Evans 200983 | Location: One nutrition clinic in Adelaide, Australia Period of study: NR Inclusion criteria: Male, age 20–65 years, BMI 27–40 kg/m2, at least one cardiovascular disease risk factor other than obesity Exclusion criteria: History of metabolic or coronary disease, type 1 or 2 diabetes Age (years), mean (SEM): a: 54.94 (1.17); b: 52.94 (1.5) BMI (kg/m2), mean (SEM): a: 32.42 (0.79); b: 31.47 (0.96) Weight (kg), mean (SEM): a: 99.84 (2.45); b: 99.58 (3.61) Baseline comparability: Yes |
Description of interventions: a: High-protein (red meat) diet comprising 35% protein, 40% carbohydrate, 25% fat, 7 MJ with some adjustment in energy to achieve approximate weight loss of 1 kg per week b: High-carbohydrate diet comprising 17% protein, 58% carbohydrate, 25% fat, 7 MJ with some adjustment in energy to achieve approximate weight loss of 1 kg per week Timing of active intervention: a + b: 0–12 weeks intensive weight loss with fortnightly clinic visits followed by monthly weight-maintenance visits up to 1 year No. of times contacted: a + b: 15 No. allocated: a: 16; b: 17 No. completed: a: 16; b: 17 Dropout (%): 0 No. assessed: a: 16; b: 17 |
Length of follow-up: 1 year Outcome: Weight |
|
| Borg 2002,84 Kukkonen-Harjula 2005116 | Location: Single research clinic, Finland Period of study: NR Inclusion criteria: age 35–50 years, BMI 30–40 kg/m2, waist circumference > 100 cm, clinically healthy other than obesity Exclusion criteria: Regular medication, participation in leisure time exercise more than twice weekly, smoker, resting blood pressure > 160/105 mmHg Age (years), mean (SD): a + b + c: 42.6 (4.6) BMI (kg/m2) (after 2 months of very low-calorie diet and before randomisation), mean (SD): a: 33.1 (2.7); b: 33.3 (2.8); c: 32.4 (2.4) Weight (kg), mean (SD): a + b + c: 106.0 (9.9) Baseline comparability: Yes |
Description of interventions: All men participated in a 2-month weight reduction programme consisting of a very low energy diet (Nutrilett, Leiras Oy, Turku, Finland) of 2 MJ per day for 8 weeks followed by a low energy diet of 5 MJ per day. Men attended small group weekly meetings led by a nutritionist. Men were then randomised to groups a, b and c: a: Control: Men advised not to increase their physical activity b: Walking: An exercise instructor supervised one group training session per week – 10-minute warm-up + 45 minutes’ training + 5-minute cool down. Heart rate monitors used to ensure target training intensity of 60–70% maximum oxygen consumption. Energy expenditure per exercise session 1.7 MJ c: Resistance exercise: An exercise instructor supervised one group training session per week – 10-minute warm-up + 45 minutes’ training + 5-minute cool down. Resistance load set at 60–80% of one repetition maximum with eight repetitions and three sets in each exercise. Each session included six exercises aimed at large muscle groups. Energy expenditure per exercise session 1.2 MJ Men continued to meet weekly in small groups in their intervention group throughout the 6-month weight-maintenance phase. Men in all groups were given the same instruction to follow ad libitum a high-carbohydrate, low-fat diet and received written material. No special instructions for diet or physical activity were given during follow-up after the weight-maintenance period Timing of active intervention: 6 months (preceded by 2-month pretreatment phase) No. of times contacted: a: 24 times, weekly for 6 months; b + c: 96 times, with exercise sessions up to three times per week for 6 months No. allocated: a: 30; b: 30; c: 30 No. completed: a: 22; b: 20; c: 26 Dropout (%): a: 27; b: 33; c: 13 No. assessed: a: 22; b: 20; c: 26 |
Length of follow-up: 21 months Outcomes: BMI, WHR, LDL and HDL cholesterol, systolic and diastolic blood pressure, fasting plasma glucose |
|
| Esposito 200485 | Location: One university hospital, Naples, Italy Period of study: 2000–3 Inclusion criteria: Age 35–55 years with erectile dysfunction (IIEF-5 score < 22), no participation in diet reduction programmes within previous 6 months, sedentary (< 1 hour per week physical activity), BMI ≥ 30 kg/m2 Exclusion criteria: Diabetes mellitus/impaired glucose tolerance, impaired renal function/macroalbuminuria, pelvic trauma, prostatic disease, peripheral neuropathy, hypertension, cardiovascular disease, psychiatric problems, drug/alcohol abuse, taking medication for erectile dysfunction Age (years), mean (SD): a: 43 (5.1); b: 43.5 (4.8) BMI (kg/m2), mean (SD): a: 36.4 (2.3); b: 36.9 (2.5) Baseline comparability: Yes |
Description of interventions: a: General oral and written advice regarding healthy food choices and exercise given at baseline b: Group sessions led by a nutritionist and exercise trainer providing individually tailored advice about reducing calorie intake, goal setting and self-monitoring to achieve a 10% reduction in body weight. Dietary advice and guidance for increasing physical activity tailored to each individual man. Behavioural and psychological counselling offered. Diet composition per 1000 kcal comprised carbohydrate 50–60%, protein 15–20%, total fat < 30% and fibre 18 g Timing of active intervention: a + b: 2 years – monthly visits for year 1, bimonthly visits for year 2 No. of times contacted: a + b: 18 No. allocated: a: 55; b: 55 No. completed: a: 52; b: 52 Dropout (%): a: 5.5; b: 5.5 No. assessed: a: 55; b: 55 |
Length of follow-up: 2 years Outcomes: Weight, BMI, total cholesterol, HDL cholesterol, triglycerides, systolic and diastolic blood pressure, erectile function, glucose |
The trial objective was to determine the effect of weight loss and increased physical activity on erectile function in obese men |
| Jeffery 1983,86,114 Jeffery 1984117 | Location: One university research centre, MN, USA Period of study: 1974–5 Inclusion criteria: Age 35–75 years, self-reported body weight > 30 lb (13.6 kg) above ideal weight Exclusion criteria: Uncontrolled diabetes, heart disease, concurrent dietary or psychological treatment, self-report of six or more alcoholic drinks per day Age (years), mean: a: (i) 52.0, (ii) 53.8, (iii) 52.4; b: (i) 54.1, (ii) 50.5, (iii) 53.8 BMI (kg/m2), mean: a: (i) 30.5, (ii) 31.8, (iii) 32.8; b: (i) 31.0, (ii) 32.3, (iii) 32.7 Weight (kg), mean: a: (i) 93.07, (ii) 99.38, (iii) 104.83; b: (i) 96.17, (ii) 102.87, (iii) 107.86 Baseline comparability: Yes |
Description of interventions: All groups participated in a 15-week educational programme emphasising reduced eating and increased exercise equally. Three levels of monetary deposit were made at the first meeting a: Individual monetary contracts: (i) US$30, (ii) US$150, (iii) US$300 b: Group monetary contracts: (i) US$30, (ii) US$150, (iii) US$300 Refunds at a rate of US$1, US$5 or US$10 per pound, up to a maximum cumulative weight loss of 2 lb per week. Individual contracts based on individual weight loss. Group contracts based on average group weight loss Timing of active intervention: 0–15 weeks No. of times contacted: a + b: 16 No. allocated: a: (i) 16, (ii) 15, (iii) 14; b: (i) 17, (ii) 14, (iii) 13 No. completed: a: (i) 16, (ii) 14, (iii) 14; b: (i) 17, (ii) 13, (iii) 12 Dropout (%): a: 2.2; b: 4.5 No. assessed: a: (i) 16, (ii) 15, (iii) 14; b: (i) 17, (ii) 14, (iii) 13 |
Length of follow-up: 2 years Outcome: Weight |
1-year data reported in Jeffery et al.;86 2-year data reported in Jeffery et al. 117 |
| Khoo 201187 | Location: Community, Adelaide, Australia Period of study: June 2007–May 2008 Inclusion criteria: BMI > 30 kg/m2, waist circumference ≥ 102 cm, type 2 diabetes mellitus, HbA1c on diet or oral medication stable for 3 months ≤ 7% Exclusion criteria: Smoker, previous or current treatment for sexual problems or lower urinary tract symptoms, glomerular filtration rate < 60 ml per minute, alcohol > 500 g per week in previous 12 months Age (years), mean (SD): a: 58.1 (11.4); b: 62.3 (5.9) BMI: NR Weight (kg), mean (SD): a: 112.7 (19.2); b: 109.6 (14.9) Baseline comparability: Poorer IIEF-5 score in a |
Description of interventions: a: Low-calorie diet: Total intake of 900 kcal per day from two liquid meal replacements consumed daily (Kicstart, Pharmacy Health Solutions), providing a maximum of 450 kcal, 0.8 g protein per kg of ideal body weight and the recommended daily allowance of vitamins, minerals and omega 3 and 6 fatty acids, plus one other small meal. After 8 weeks men changed to follow b b: High-protein, low-fat diet: Daily energy reduction of approximately 600 kcal per day. Daily consumption of 300 g lean meat, poultry or fish, three servings of cereals/breads and low-fat dairy and two servings of fruit and vegetables All men received a written plan with diet information, a menu plan, recipes and advice for cooking and eating out and all maintained their usual daily activity levels Timing of active intervention: a + b: 1 year No. of times contacted: a + b: 16–29 No. allocated: a: 19; b:12 No. completed: a: 9; b: 7 Dropout (%): a: 52.63; b: 41.67 No. assessed: a: 9; b:7 |
Length of follow-up: 1 year Outcomes: Weight, waist circumference, erectile function, adverse events |
|
| Morgan 201189 | Location: One university centre, University of Newcastle, Australia Period of study: 2007–8 Inclusion criteria: BMI 25–37 kg/m2 Exclusion criteria: History of major medical problems preventing physical activity, recent weight loss of ≥ 4.5 kg, taking medications that might affect body weight Age (years), mean (SD): a: 34 (11.6), b: 37.5 (10.4) BMI (kg/m2), mean (SD): a: 30.5 (3.0), b: 30.6 (2.7) Weight (kg), mean (SD): a: 99.2 (13.7); b: 99.1 (12.2) Baseline comparability: Yes |
Description of interventions: a: Researcher delivered one 60-minute face-to-face weight-loss information session. Participants given a self-help weight-loss programme booklet b: Researcher delivered one 75-minute information session (60 minutes on weight loss + 15 minutes’ internet instruction). Participants given self-help weight-loss programme booklet and 3 months’ online support from the study website, Calorie King. Participants received personalised online feedback and responses to any questions posted on the website noticeboard, including anecdotes and weight-loss strategies for men Weight-loss information sessions in both groups covered instruction relating to the modification of diet and physical activity habits and behaviour change, based on Bandura’s social cognitive theory Timing of active intervention: 3 months No. of times contacted: a: four times for 3-monthly assessments; b: 11 times for 3-monthly assessments and seven feedback sessions No. allocated: a: 31; b: 34 No. completed: a: 20; b: 26 Dropout (%): a: 35.5; b: 23.5 No. assessed: a: 31; b: 34 |
Length of follow-up: 12 months Outcomes: Weight change, waist circumference, BMI, systolic and diastolic blood pressure |
|
| Patrick 201190 | Location: Universities of California, and San Diego, San Diego State, USA Period of study: February 2004–March 2005 Inclusion criteria: BMI ≥ 25 kg/m2, age 25–55 years Exclusion criteria: NR Age (years), mean (SD): a: 42.8 (8.0); b: 44.9 (7.8) BMI (kg/m2), mean (SD): a: 34.3 (4.0); b: 34.2 (4.2) Weight (kg), mean (SD): a: 104.6 (15.3); b: 104.7 (15.3) Baseline comparability: Yes |
Description of interventions: a: Wait list/general internet advice: Participants given access to a website giving general male health advice that was unlikely to produce a change in diet or physical activity (e.g. stress, hair loss, worksite injury prevention). Men were given the option to swap to the weight-loss intervention after 12 months b: Internet-based diet and physical activity advice and behavioural support; based on social cognitive theory and informed by the behavioural determinants model and designed to improve diet and physical activity in five key areas to promote weight loss, rather than directly targeting calorie restriction: increase fruit and vegetable intake to five to nine servings per day, decrease saturated fat intake to ≤ 20 g per day, increase wholegrain intake to three or more servings per day, increase physical activity to > 10,000 steps per day using a pedometer for at least 5 days per week and participate in upper and lower body strength training at least twice per week Men met with a case manager to set goals at baseline and completed weekly web-based activities, including behaviour change skills and reading diet and physical activity information. Men also had the opportunity to e-mail study experts (dietitian, physical activity expert and a clinical psychologist). Both groups paid US$20 for completing 6 months and US$100 for completing 12 months Timing of active intervention: 12 months No. of times contacted: a: 3; b: 55 No. allocated: a: 217; b: 224 No. completed: a + b: 309 Dropout (%): a + b: 29.9 No. assessed: a: 217; b: 224 |
Length of follow-up: 12 months Outcomes: weight, BMI |
Trial conducted focus groups with men and two weight-loss experts to tailor the intervention for men (not published) |
| Pavlou 198991 (main trial) | Location: One university centre, Boston University Medical Centre, MA, USA Period of study: NR Inclusion criteria: Male, age 26–52 years, euthyroid, free from any physical, psychological or metabolic impairment Exclusion criteria: NR Age (years), mean (SD): a: 41.5 (7.59); b: 42.9 (6.63); c: 45.1 (10.0); d: 49.6 (8.4); e: 41.8 (10.44); f: 41.8 (7.57); g: 46.1 (9.33); h: 44.5 (9.6) (completers) BMI (kg/m2), mean: a: 32.54; b: 32.4; c: 32.07; d: 31.5; e: 30.13; f: 34.82; g: 31.89; h: 33.78 (completers) Weight (kg), mean, SEM (SD): a: 103.1, 3.1 (9.80); b: 105.0, 4.4 (14.59); c: 100.8, 2.3 (9.2); d: 98.8, 2.6 (10.4); e: 96.1, 3.3 (10.44); f: 103.0, 3.7 (13.34); g: 100.8, 2.3 (9.76); h: 105.7, 3.4 (13.6) (completers) Baseline comparability: Yes |
Description of interventions: Subjects were randomly assigned to four diets and exercise and non-exercise groups for 8 weeks Exercise consisted of a 90-minute supervised exercise programme three times a week from baseline to week 8, which consisted of 35–60 minutes of aerobic activity, e.g. walk–jog–run (70–85% max. heart rate), calisthenics and relaxation techniques. Non-exercise groups were instructed to continue normal daily activity and not to participate in any form of additional supervised and/or unsupervised physical activity during the initial 8 weeks a: Balanced caloric-deficit, low-calorie diet (BCDD). 1000 kcal per day selected from usual four food groups in quantities thought to meet basic requirements b: BCDD + exercise c: Protein-sparing modified fast, low carbohydrate (PSMF). Ketogenic diet of meat, fish and fowl used as only dietary source to provide equivalent of 1.2 g high biological value protein per kg of ideal body weight or 1000 kcal per day, no carbohydrate and all fat ingested from meat, fish and fowl. Participants prescribed 2.8 g potassium chloride daily d: PSMF + exercise e: DPC-70: A very low-calorie diet of 420 kcal powdered protein carbohydrate mix derived from calcium caseinate, egg albumin and fructose, formulated with vitamins and minerals to meet the US recommended dietary allowances (RDA) dissolved in water or other non-caloric liquid. Fat content zero. Participants instructed to consume five packets a day and to consume no other nutrients. Participants prescribed 2.8 g potassium chloride daily f: DPC-70 + exercise g: DPC 800: A very low-calorie diet of 800 kcal per day provided in powdered form consumed similarly to DPC-70, providing a complete mixture of nutrients and similar nutritionally to BCDD except for fewer calories h: DPC-800 + exercise All participants attended weekly education sessions up to week 8 that included behaviour modification, diet and general nutrition and exercise education. All participants were given multivitamins and daily food and activity records up to week 8. Non-caloric liquids, including coffee, were allowed in unrestricted amounts Timing of active intervention: 8 weeks + 18 months’ follow up No. of times contacted: a–h: 11 times, weekly 0–8 weeks then at 8 and 18 months No. allocated: 160 men (20 in each intervention group) No. completed: a: 10; b: 11; c: 16; d: 16; e: 10; f: 13; g: 18; h: 16 (18 months post treatment) Dropout (%): 31 (18 months) No. assessed: a: 10; b: 11; c: 16; d: 16; e: 10; f: 13; g: 18; h: 16 (18 months; completers) |
Length of follow-up: 18 months Outcomes: Weight, total cholesterol, HDL cholesterol, triglycerides, systolic and diastolic blood pressure |
|
| Pavlou 198991 (pilot) | Location: As above Period of study: As above Inclusion criteria: As above Exclusion criteria: As above Age (years), mean (SD): a: 49.2 (6.48); b: 44.8 (7.84); c: 46.1 (5.14); d: 48.1 (4.65) (data for completers) BMI (kg/m2), mean: a: 31.75; b: 31.92; c: 31.11; d: 30.4 (completers) Weight (kg), mean (SEM): a: 102.3 (2.1); b: 99.2 (4.2); c: 101.7 (3.1); d: 97.3 (4.1) Baseline comparability: Yes |
Description of interventions: As above but a, b, c and d only Timing of active intervention: 12 weeks No. of times contacted: 16 times, weekly 0–12 weeks and then at 6, 18 and 36 months No. allocated: a–d: 24 No. completed: a: 5; b: 6; c: 5; d: 5 (36 months post treatment) Dropout (%): 13 (36 months post treatment) No. assessed: a: 5; b: 6; c: 5; d: 5 (36 months post treatment) |
Length of follow-up: 162 weeks Outcome: Weight |
|
| van Aggel-Leijssen 2001,92 van Aggel-Leijssen 2002,118 Lejeune 2003119 | Location: One university research department, Maastricht, the Netherlands Period of study: Not reported Inclusion criteria: Good health, ≤ 2 hours per week of sports activities, stable body weight over previous 3 months (< 3 kg change) Exclusion criteria: Physically demanding job, taking medication known to influence measured variables Age (years), mean (SD): a: 38.6 (6.5); b: 39.3 (7.7) BMI (kg/m2), mean (SD): a: 32.0 (2.1); b: 32.6 (2.5) Weight (kg), mean (SD): a: 103.6 (11.7); b: 102.6 (9.8) Baseline comparability: Yes |
Description of interventions: a: 12-week energy restriction period followed by 40-week weight-maintenance period: Weeks 1–6: Very low energy diet of 2.1 MJ per day protein-enriched formula diet (Modifast, Novartis) consisting of 50 g carbohydrate, 52 g protein, 7 g fat Weeks 7–8: 1.4 MJ per day formula + 3.5 MJ per day free choice Weeks 9–10: 0.7 MJ per day formula + 4.9 MJ per day free choice Weeks 11–12: Participants received dietary instruction to stabilise their body weight and not to change their habitual activity pattern b: 12-week energy restriction period (as in a) combined with an exercise training programme [40% maximal oxygen consumption (VO2 max.)] for 1 hour, four times a week – cycling, walking or aqua-jogging (three sessions supervised by a personal trainer in the lab and one session at home). Exercise training programme continued during the weight-maintenance period to week 52 Timing of active intervention: a: weeks 0–12; b: weeks 0–12 No. of times contacted: a: 12 times at weekly intervals; b: 168 times up to four times per week for 52 weeks No. allocated: a: 20; b: 20 No. completed: a: 15; b: 14 Dropout (%): a: 25; b: 30 No. assessed: a: 15; b: 14 |
Length of follow-up: 52 weeks Outcomes: Weight, BMI |
|
| Wood 1988,93 Fortmann 1988115 | Location: One research centre, Stanford, CA, USA Period of study: NR Inclusion criteria: 120–160% ideal body weight, non-smoker, plasma total cholesterol < 8.28 mmol/l, triglycerides < 5.65 mmol/l, normal electrocardiogram, alcohol intake less than four drinks per day, sedentary activity, weight stable (±5 lb) over the past year Exclusion criteria: Blood pressure > 160/100 mmHg, taking medications affecting lipids, plasma cholesterol > 300 mg/dl, triglycerides > 500 mg/dl, exercise three or more times per week Age (years), mean (SD): a: 45.2 (7.2); b: 44.2 (8.2); c: 44.1 (7.8) BMI: NR Weight (kg), mean (SD): a: 95.4 (10.6); b: 93.0 (8.8); c: 94.1 (8.6) Baseline comparability: Yes |
Description of interventions: a: Control: Weight stable, no added energy restriction or exercise b: Diet: Energy restriction (reduced food quantity but not proportions of fat, carbohydrate, protein and alcohol). Energy intake reduced by 300–500 kcal per day depending on weight-loss goals, energy needs and baseline food intake to achieve approximately 0.3–0.6 kg fat loss per week. No added exercise weight-loss goals c: Exercise: Increased activity with instruction to maintain exercising heart rate of 65–85% of peak heart rate (approx. caloric output of 8–10 cal per minute). Supervised sessions three times per week starting with fast walking for 25 minutes, gradually adding jogging for 0–3 months with continuous jogging increasing to 40–50 minutes. Two additional days of unsupervised walking or jogging added by month 6. Programme aimed to decrease body fat by 2–3 kg in the first 3 months, 4–5 kg in months 3–6 and the remainder in months 6–9 Timing of active intervention: b + c: 0–9 months No. allocated: a: 52; b 51; c: 52 No. completed: a: 51; b: 49; c: 49 Dropout (%): a 1.9%; b: 5.8%; c: 3.9% No. assessed: a: 44; b: 41; c: 36 |
Length of follow-up: 1 year Outcomes: Mean change in weight, mean change in systolic and diastolic blood pressure |
|
| King 198988 (continuation of Wood 198893) | At the end of the 1-year trial phase93 participants from groups a and b were randomised within intervention groups to weight-maintenance follow-up interventions Age (years), mean (SD): a: (i): 45.5 (9.6), (ii): 44.4 (4.8); b: (i) 46.1 (7.6); (ii) 42.9 (7.3) BMI: NR Weight (kg), mean (SD): a: (i): 85.7 (9.1), (ii): 83.4 (8.8); b: (i): 91.0 (10.6), b (ii): 86.2 (7.6) Baseline comparability: Yes |
Description of interventions: a (i): Diet mail and telephone contact. Participants received monthly mailings consisting of a supportive letter, self-scored assessment of an energy restriction problem area and list of energy restriction coping suggestions (e.g. holiday eating, emotional eating, eating away from home, stress eating). Mailings supplemented by telephone calls monthly for the first 3 months, then at 6, 9 and 12 months a (ii): Diet control. Participants received no mailings or telephone calls b (i): Exercise mail and telephone contact. As in a (i) but self-scored assessment of an exercise problem area and list of exercise coping strategies (e.g. time pressure, boredom, illness or injury) b (ii): Exercise control [as in a (ii)] At the beginning of the weight-maintenance phase, participants in all groups were given written information for the weight control method that they received during the weight-loss trial period and were encouraged to seek support from members of their original intervention group Timing of active intervention: 1-year weight-maintenance phase following end of 1-year weight-loss phase No. of times contacted: a: three times at 6-monthly intervals; b: 21 times at monthly intervals No. allocated: a: (i) 24, (ii) 20; b: (i) 24, (ii) 22 No. completed: a: (i) 20, (ii) 16; b: (i) 21, (ii) 15 Dropout (%): a: (i) 16.7, (ii) 20%; b: (i) 12.5, (ii) 31.8 No. assessed: a: (i) 20, (ii) 16; b: (i) 21, (ii) 15 |
Length of follow-up: 1 year Outcome: Mean change in weight |
The trials conducted by Jeffery and colleagues,86 Patrick and colleagues,90 Pavlou and colleagues91 and Wood and colleagues88,93 were carried out in the USA. Trials conducted by Benassi-Evans and colleagues,83 Khoo and colleagues87 and Morgan and colleagues89 were carried out in Australia; and studies conducted by Borg and colleagues,84 Esposito and colleagues85 and van Aggel-Leijssen and colleagues92 were conducted in Finland, Italy and the Netherlands respectively. All trials were single-centre RCTs. Seven trials reported interventions aimed at men individually. 83,87 – 92 Men received group interventions in three trials. 84,85,93 Only one trial86 directly compared the effectiveness of group and individual interventions. Details of the exact settings for the trials were generally not well described; most appeared to be in a research setting. Only the trial by Esposito and colleagues85 appears to have been conducted in a outpatient health service setting. One trial was conducted amongst university staff89 whereas Pavlou and colleagues91 recruited public sector workers, including those in the police department.
When e-mail addresses were valid,84 – 86,89 we contacted the authors requesting any additional relevant reports and publications as well as any process or qualitative evaluations that they may have published elsewhere. Of these, only the SHED-IT (Self-Help, Exercise and Diet using Information Technology)89 study authors replied. We did not identify any additional eligible publications for inclusion in any of our reviews, other than those we had already identified.
Characteristics of the men
Men were mainly recruited through mass media advertising. 83,84,87,90,92,93 Two studies recruited men through the workplace (university staff in the SHED-IT trial89 and the police department and Metropolitan District Commission in the trial by Pavlou and colleagues91) and one study85 recruited men from a hospital outpatient clinic for weight loss. The studies conducted by Jeffery and colleagues86 and King and colleagues88 recruited men from ongoing RCTs. 93,114 All studies reported mean age, weight and, with the exception of that by King and colleagues,88 BMI at baseline. Excluding data from the trial conducted by King and colleagues,88 because of overlap of the participants with those in the trial by Wood and colleagues,93 the majority of men were middle-aged with trials’ median (range) age of 46 years (36–62 years), weight of 101.5 kg (93.0–112.7 kg) and BMI of 32.4 kg/m2 (30.1–36.9 kg/m2). A total of 1238 men were allocated to an intervention with 1098 included in the trial analyses. Details of the inclusion/exclusion criteria for the individual trials are presented in Table 3 .
Overview of types of outcomes reported
Quantitative outcomes
All studies reported either baseline and end weights or changes in weight. Four studies reported change in BMI. 84,85,89,92 Three studies reported waist circumference87,89,90 and one reported waist-to-hip ratio. 85
For cardiovascular risk factors, only two studies reported total cholesterol,85,91 three studies reported HDL cholesterol,84,85,91 one study reported LDL cholesterol,84 two studies reported triglycerides85,91 and three studies reported systolic and diastolic blood pressure. 84,85,89 Systolic and diastolic blood pressure data for the trial by Wood and colleagues93 were reported in a linked publication. 115 The trials by Borg and colleagues84 and Esposito and colleagues85 reported results for fasting plasma glucose.
Only two trials reported a male-specific disease outcome: erectile function following weight loss. 85,87 One trial92 reported adverse events and one trial84 reported lack of adverse events. The trials did not report quality of life, HbA1c levels or any economic outcomes.
Quality of the evidence
Risk of bias
The risk of bias assessment for the individual trials is shown in Appendix 8 (see Table 56 ). Figure 2 summarises the assessment.
FIGURE 2.
Summary of risk of bias assessment of trials included in the systematic review of men-only RCTs.
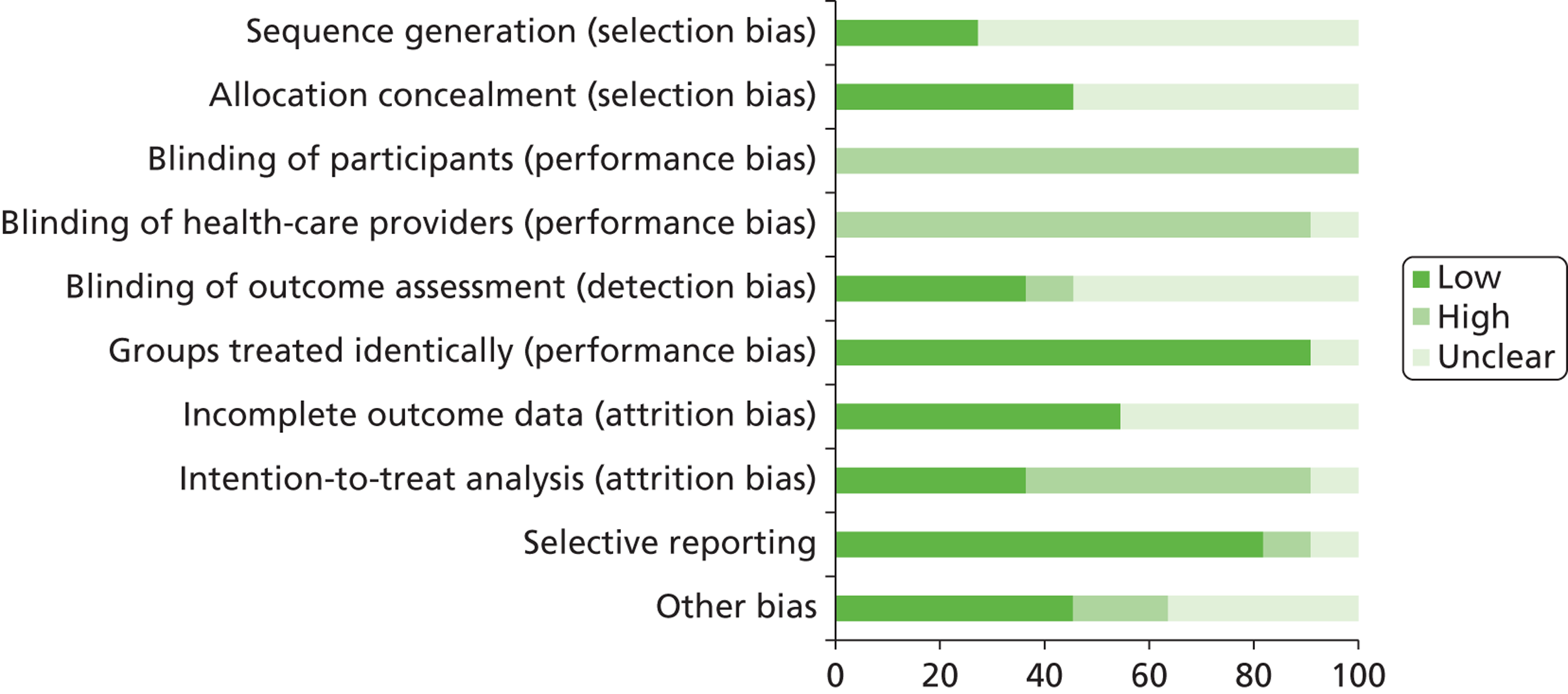
It was unclear whether random sequence generation was adequate in all but three studies,89,90,93 which were judged to have adequately randomised participants. Five studies84,85,90,91,93 were judged to have successfully concealed allocation, whereas this was unclear in the remaining trials. No trial was able to carry out blinding of participants and the majority did not blind health-care providers, although this item was uncertain in the trial by Benassi-Evans and colleagues83. This is because blinding of participants and health-care providers would have largely been impossible because of the nature of the interventions considered by the trials. Four trials did blind outcome assessors,85,88 – 90 whereas the trial conducted by Borg and colleagues84 did not. It was unclear whether outcome assessors were blinded in the remaining trials.
The majority of the trials (10/11, 90.9%) were judged to have treated participants similarly apart from the given intervention in each arm of the trial, with only the trial conducted by Esposito and colleagues85 causing uncertainty for this item. Over half of the trials were judged to be at low risk for incomplete outcome data,84 – 87,89,92 with this item being unclear in the other trials. 83,88,90,91,93 Only four trials85,86,89,90 carried out intention-to-treat analysis with the majority (6/11, 54.5%) of the trials analysing data for trial completers only. It was unclear whether or not an intention-to-treat analysis was carried out in the trial conducted by Benassi-Evans and colleagues. 83
The trial by Khoo and colleagues87 was judged to be at high risk of selective reporting as the authors did not report HbA1c outcomes. This was unclear for the SHED-IT trial89 as outcomes considered in the trial protocol were not reported in the 6- and 12-month reports [e.g. Short Form questionnaire-12 items (SF-12) and sexual function data]. All other trials were judged to be at low risk for this item.
It was unclear for four studies83,84,91,93 whether or not the trials were at risk of any other bias. Five trials85,87,88,90,92 were judged to be at low risk for this item, with the trial by Jeffery and colleagues86 considered to be at high risk as the participants paying larger monetary contracts in both the individual arm and the group arm had a higher mean baseline weight than the participants paying smaller deposits. The authors acknowledged that randomisation may have failed to equally distribute participants with respect to their weight. The authors also experienced difficulty recruiting people to this trial, as only 50% of those eligible signed monetary contracts. The SHED-IT trial89 was also judged to be at high risk of other bias because of the limited generalisability of the participants, who were all staff or students recruited from a single university, and because of the potential for contamination between participants because of the work/student environment. It was unclear if the trial conducted by Pavlou and colleagues91 was at a similar risk of workplace contamination.
Assessment of equity and sustainability
Figure 3 summarises the equity assessment. Results for the individual trials are detailed in Appendix 8 (see Table 57 ).
FIGURE 3.
Summary of the equity and sustainability assessment of trials included in the systematic review of men-only RCTs.
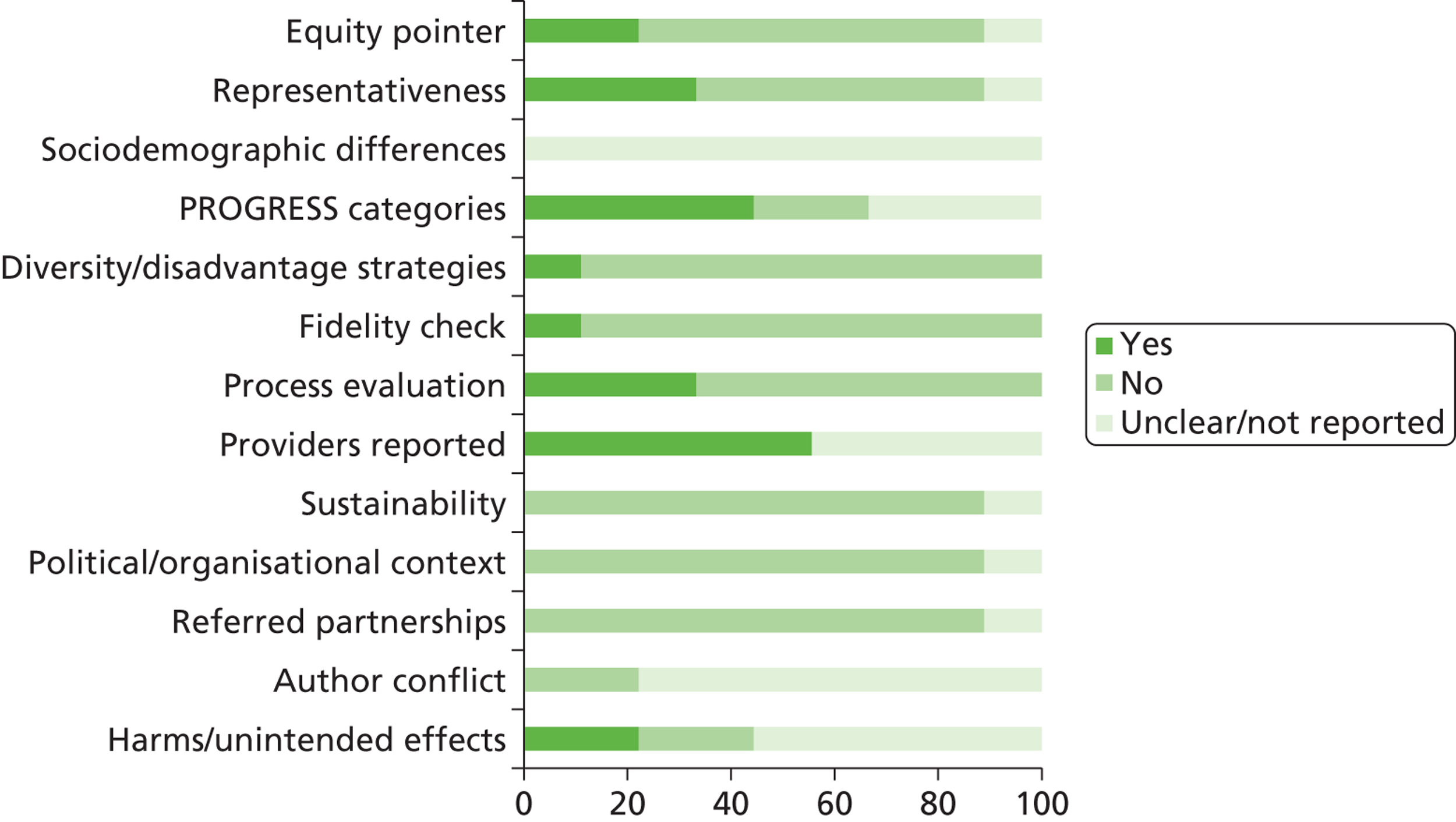
The majority of equity items were not considered, were unclear or were not reported by the trial authors. The trials did not report on diversity, sustainability or political context and did not describe any partnerships.
Only three trials were considered to have been conducted in a way that could have excluded specific groups of men, therefore influencing equity of access. The trial conducted by Jeffery and colleagues86 was considered to have excluded men who could not afford to pay a financial deposit. The SHED-IT trial89 was also judged to have potentially limited the inclusion of non-academic men because of the university setting and the use of internet and mobile phone technologies. Similarly, the trial by Patrick and colleagues90 required men to have internet access and to be computer literate.
Three trials considered unrepresentative samples of obese men: the trial by Esposito and colleagues85 recruited men from a weight-loss outpatient clinic who all had erectile dysfunction; men in the SHED-IT trial89 were all university staff or students; and men in the Pavlou trial91 were all police officers. Similarly, the trials by Wood and colleagues93 and King and colleagues88 had very narrow inclusion criteria and therefore excluded a wide number of potentially relevant men from their samples. It was unclear whether or not the trial by Jeffery and colleagues86 included a representative sample of obese men as there was insufficient detail in the report. Inclusion criteria were not reported by Patrick and colleagues. 90
The SHED-IT trial89 reported differences in compliance by occupation and age, with non-academic staff members and older men showing greater compliance. It is unclear whether or not any socioeconomic differences existed between withdrawals from each of the intervention groups. Patrick and colleagues90 reported that younger, non-white men were more likely to withdraw from their trial. The remaining trials did not report on any socioeconomic differences between withdrawals and exclusions.
Only three trials86,89,90 reported on categories relating to place of residence, race/ethnicity, occupation, gender, religion, education, socioeconomic status or social capital (PROGRESS). It was unclear in the trial by Patrick and colleagues90 whether or not the authors had attempted to address diversity or disadvantage with their trial intervention. All other trials did not address or report these items.
Four studies84,91 – 93 carried out fidelity checks for physical activity by monitoring heart rate or maximal oxygen consumption (VO2 max.) of the men during the exercise sessions, thus ensuring that the men exercised at the required level of training intensity. The remaining studies did not report on fidelity checks, although the SHED-IT trial89 reported qualitative data concerning the enthusiasm of men for the internet and control interventions.
Most trials83,85,89,90,92,93 (6/11, 54.5%) reported details of who provided the interventions. The other trials did not report this item86 – 88,91 or it was unclear. 84 Providers were dietitians, exercise instructors or research staff.
The trial conducted by Borg and colleagues84 was partly funded by the Finnish pharmaceutical company Leiras Oy. As this company manufactures a weight-loss product it is unclear whether or not this created any author conflict. Patrick and colleagues90 also reported that three of the trial authors were co-owners of Santech, Inc. (San Diego, CA, USA), which developed products related to the described research at the time of publication. Both San Diego State University and the University of California approved this arrangement in accordance with their conflict of interest policies. The other trials did not report whether or not there was potential for author conflict.
Assessment of effectiveness
An exercise programme compared with control
Wood and colleagues93 compared men in an exercise programme with a control group of men who made no change to their diet or level of physical activity. Men in the exercise programme participated in supervised exercise three times per week in 1-hour sessions with the aim of reducing their total body fat by one-third over a 9-month period. Exercise activities included calisthenics, muscle stretching, brisk walking and jogging. At 1 year the authors reported a significant difference in weight in favour of a programme of exercise compared with the control group (no diet or exercise) (mean difference –4.60 kg, 95% CI –6.18 kg to –3.02 kg) ( Figure 4 ).
FIGURE 4.
Effect of an exercise programme vs. control on weight (kg).
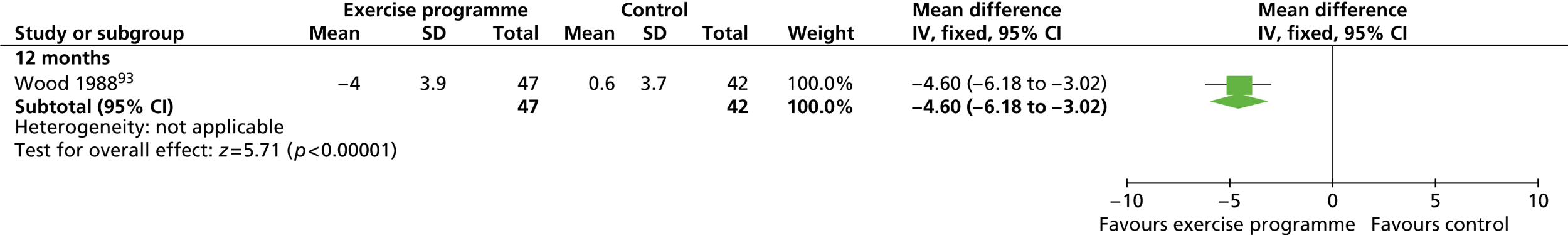
At 1 year, exercisers significantly lowered their triglyceride levels compared with the control group (reported p < 0.05) and significantly improved their HDL cholesterol compared with the control group (reported p < 0.01) ( Table 4 ). No other differences for cholesterol, LDL cholesterol or systolic or diastolic blood pressure (measured in the clinic) were reported as significant, although changes in the exercise group were all more beneficial than those in the control group.
| Outcome | Exercise (n = 47) | Control (n = 42) |
|---|---|---|
| Total cholesterol (mmol/l) | –0.25 (0.64) | –0.23 (0.65) |
| LDL cholesterol (mmol/l) | –0.25 (0.61) | –0.21 (0.67) |
| HDL cholesterol (mmol/l) | 0.11 (0.15) | –0.02 (0.11) (n = 41) |
| Triglycerides (mmol/l) | –0.16 (0.53) | +0.08 (0.6) |
| Systolic blood pressure (mmHg) | –6.6 (8.4) (n = 42) | –4.1 (8.0) (n = 35) |
| Diastolic blood pressure (mmHg) | –4.1 (8.0) (n = 42) | –2.6 (8.1) (n = 35) |
A reducing diet compared with control
Wood and colleagues93 also compared the control group with men who followed a reducing diet (300–500 kcal per day deficit) aimed at reducing total body fat by one-third over 9 months; this group also made no alteration to their level of physical activity. At 1 year the diet group had lost significantly more weight than the control group, with a mean difference of –7.80 kg (95% CI –9.38 kg to –6.22 kg) ( Figure 5 ).
FIGURE 5.
Effect of a reducing diet vs. control on weight (kg).
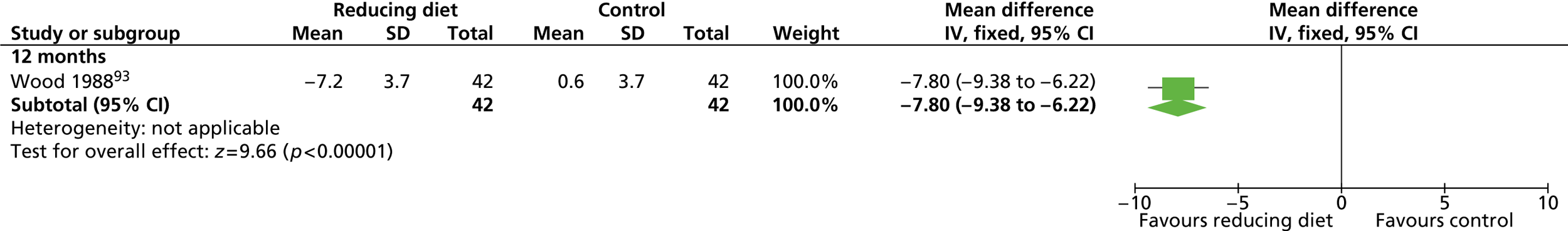
At 1 year, dieters showed significantly lower triglyceride levels (reported p < 0.05) and HDL cholesterol levels (reported p < 0.001) than the control group ( Table 5 ). No other risk factor changes were reported as significantly different, although changes in the diet group were all more beneficial than changes in the control group.
| Outcome | Diet (n = 42) | Control (n = 42) |
|---|---|---|
| Total cholesterol (mmol/l) | –0.36 (0.56) | –0.23 (0.65) |
| LDL cholesterol (mmol/l) | –0.31 (0.64) | –0.21 (0.67) |
| HDL cholesterol (mmol/l) | 0.12 (0.16) (n = 41) | –0.02 (0.11) (n = 41) |
| Triglycerides (mmol/l) | –0.27 (0.72) | +0.08 (0.6) |
| Systolic blood pressure (mmHg) | –5.7 (7.9) (n = 38) | –4.1 (8.0) (n = 35) |
| Diastolic blood pressure (mmHg) | –5.6 (7.3) (n = 38) | –2.6 (8.1) (n = 35) |
An exercise programme compared with a reducing diet
A reducing diet was shown to produce a significant reduction in weight compared with an exercise programme at 1 year, with a mean difference of –3.20 kg (95% CI –4.78 kg to –1.62 kg) ( Figure 6 ). 93
FIGURE 6.
Effect of an exercise programme vs. a reducing diet on weight (kg).
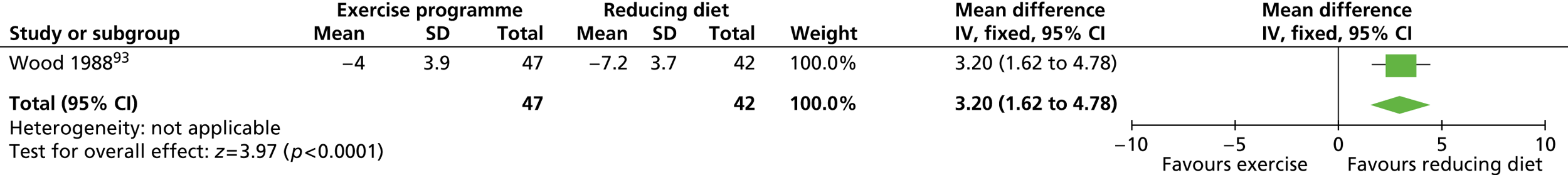
None of the risk factor differences between the reducing diet group and the exercise programme group ( Table 6 ) were reported as statistically significant.
| Outcome | Exercise (n = 47) | Diet (n = 42) |
|---|---|---|
| Total cholesterol (mmol/l) | –0.25 (0.64) | –0.36 (0.56) |
| LDL cholesterol (mmol/l) | –0.25 (0.61) | –0.31 (0.64) |
| HDL cholesterol (mmol/l) | 0.11 (0.15) | 0.12 (0.16) (n = 41) |
| Triglycerides (mmol/l) | –0.16 (0.53) | –0.27 (0.72) |
| Systolic blood pressure (mmHg) | –6.6 (8.4) (n = 42) | –5.7 (7.9) (n = 38) |
| Diastolic blood pressure (mmHg) | –4.1 (8.0) (n = 42) | –5.6 (7.3) (n = 38) |
A low-fat reducing diet with behaviour therapy and exercise advice compared with control
Three studies examined behavioural therapy, exercise advice and low-fat reducing diets compared with a control group. In the SHED-IT trial by Morgan and colleagues,89 participants were given support and advice through a free study website. The control group received an information booklet only. At 12 months the internet group had lost more weight than the control group, with a mean difference of –2.20 kg (95% CI –5.65 to 1.25 kg), but the difference in weight reduction was not significant.
Similarly, Patrick and colleagues90 used the internet to deliver dietary and physical activity advice and behavioural therapy. The trial authors held interviews with two male weight-loss experts and held focus groups with men to tailor the intervention specifically for men described as overweight. The results of this developmental work showed that men wanted an intervention that was individualised, fact based, flexible and simple to understand. Men also indicated that they preferred the use of ‘businesslike’ language. Pedometers were provided to encourage physical activity and were enjoyed by the men for their novelty and assistance with self-monitoring of their behaviour. Men in the control group were given access to a website detailing general male-related health advice that was unlikely to lead to lifestyle changes that would promote weight loss (e.g. dealing with stress, hair loss, worksite injury prevention). Men receiving the weight-loss intervention lost more weight than men in the control group but the difference between the groups at 12 months was also not significant.
The trial by Esposito and colleagues85 examined behavioural therapy, advice on how to increase physical activity and a low-fat reducing diet. The men were recruited because they were obese and had erectile dysfunction [determined by a score of ≤ 21 on the International Index of Erectile Function (IIEF)126]. The men met in groups but received advice tailored to their individual requirements. The control group in this trial received general oral and written advice regarding healthy food choices and exercise at baseline only. At 2 years the intervention group had lost significantly more weight than the control group, with a mean difference of –13.00 kg (95% CI –16.18 kg to –9.82 kg) ( Figure 7 ).
FIGURE 7.
Effect of a low-fat reducing diet with behaviour therapy and exercise advice vs. control on weight (kg).
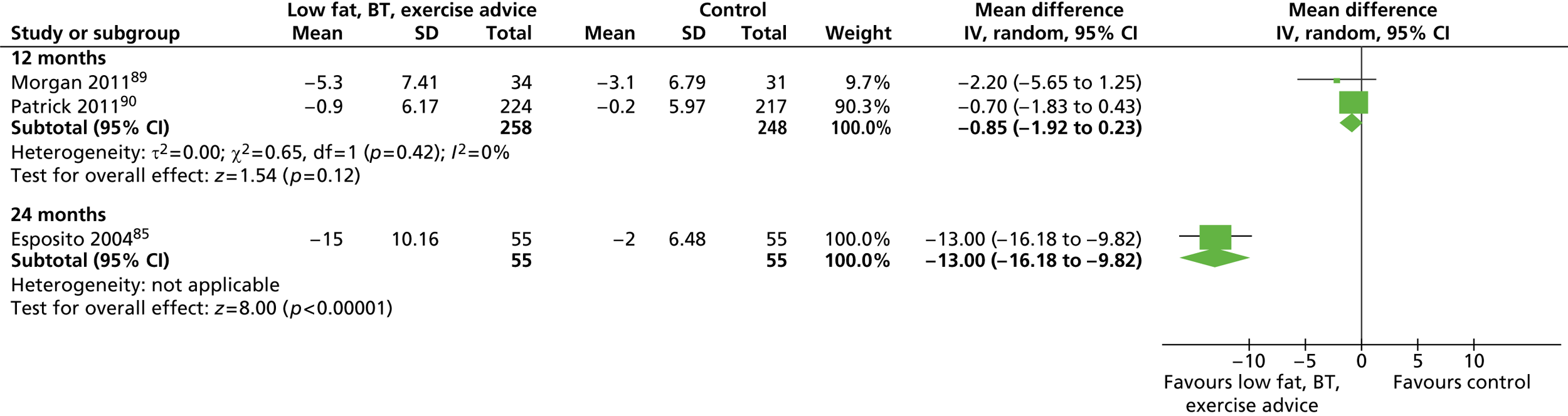
At 12 months the only significant difference between groups with regard to risk factors was in systolic blood pressure in favour of the intervention group (reported p < 0.03)89 ( Table 7 ).
| Outcome | Low-fat reducing diet, behaviour therapy and exercise advice | Total no. of men | Control | Total no. of men |
|---|---|---|---|---|
| BMI (kg/m2) | ||||
| 12 months | –1.7 (–2.4 to –1.0) | 34 | –0.9 (–1.7 to –0.2) | 31 |
| 24 months | –5.7 | 55 | –0.7 | 55 |
| Systolic blood pressure (mmHg) | ||||
| 12 months | –11 (–14 to –7) | 34 | –6 (–10 to –2) | 31 |
| 24 months | –3 | 55 | –1 | 55 |
| Diastolic blood pressure (mmHg) | ||||
| 12 months | –6 (–10 to –2) | 34 | –4 (–9 to –1) | 31 |
| 24 months | –4 | 55 | 0 | 55 |
| Waist circumference (cm) | ||||
| 12 months | –5.8 (–7.9 to –3.6) | 34 | –3.8 (–6.1 to –1.6) | 31 |
| Total cholesterol (mmol/l) | ||||
| 24 months | –0.29 | 55 | 0.05 | 55 |
| HDL cholesterol (mmol/l) | ||||
| 24 months | 0.23 | 55 | 0.03 | 55 |
| Triglycerides (mmol/l) | ||||
| 24 months | –0.22 | 55 | –0.05 | 55 |
| Fasting plasma glucose (mmol/l) | ||||
| 24 months | –0.44 | 55 | –0.06 | 55 |
| Waist-to-hip ratio | ||||
| 24 months | –0.09 | 55 | 0 | 55 |
At 24 months the intervention group in the trial by Esposito and colleagues85 showed significant improvements compared with the control group for total cholesterol, triglycerides and fasting plasma glucose (reported p ≤ 0.05). BMI, HDL cholesterol, systolic and diastolic blood pressure and waist-to-hip ratio were also significantly improved (reported p ≤ 0.01) (see Table 7 ).
Esposito and colleagues85 also reported that 17 out of 55 men in the intervention group compared with three out of 55 men in the control group reported an IIEF score of ≥ 22 (indicating regained sexual function; reported p = 0.001).
A diet and exercise programme compared with a diet only
Two trials reported the effect of adding an exercise programme to a diet compared with diet only. The trial by van Aggel-Leijssen and colleagues92 was a small trial in which men were randomised to a diet only or a diet and exercise programme. All men followed a 10-week diet. For the first 6 weeks men followed a very low energy (500 kcal per day) formula diet (Modifast) of 50 g of carbohydrate, 52 g of protein and 7 g of fat. For weeks 7–8, men consumed 330 kcal per day of the formula diet and 840 kcal per day from foods of their choice. During weeks 9–10, men consumed 170 kcal per day of the formula diet and 1170 kcal per day from their chosen food. The men were then instructed to stabilise their body weight for weeks 11–12. Men in the diet and exercise group also participated in a low-intensity exercise programme (40% VO2 max.) for 12 weeks, which was then continued to week 52. The men trained four times per week in 1-hour sessions. Three of these sessions were supervised by a personal trainer in the research laboratory and the other session was unsupervised at home. The exercise sessions consisted of cycling, walking and aqua-jogging. Attendance for supervised exercise sessions was 57% (SD 20%). Reasons for non-attendance included illness, holidays and work commitments. Two of the men in the exercise group had to withdraw from the study because of knee injuries. There were no other reported adverse events. Weight data for men at 12 months were reported in a linked report by Lejeune and colleagues. 119 Men in the diet and exercise group did not lose as much weight as men in the diet-only group (mean difference 4.20 kg, 95% CI –1.47 to 9.87 kg).
The study by Pavlou and colleagues91 consisted of a pilot trial and the main trial. The pilot trial compared a low-calorie diet of 1000 kcal per day coupled with an exercise programme with a low-calorie diet only. The pilot trial also compared a low-calorie, low-carbohydrate diet of 1000 kcal per day plus an exercise programme with a very low-calorie diet only. The main trial compared the same two diets, with and without an exercise programme, as the pilot trial. The main trial additionally compared the effect of adding exercise to two types of very low-calorie diet (420 kcal per day and 800 kcal per day).
Combining results for all diet groups, the effect of adding an exercise programme to a diet was highly significant at 18 months (mean difference –7.63 kg, 95% CI –10.33 to –4.92 kg) and at 36 months (mean difference –8.22 kg, 95% CI –15.27 to –1.16 kg). There were no significant differences between the 1000 kcal per day low-carbohydrate diet and the 1000 kcal per day low-calorie diet, however, or for the two forms of very low-calorie diet (420 and 800 kcal per day) at 18 or 36 months ( Figure 8 ).
FIGURE 8.
Effect of a diet and exercise programme vs. a diet on weight (kg, SDs assumed).
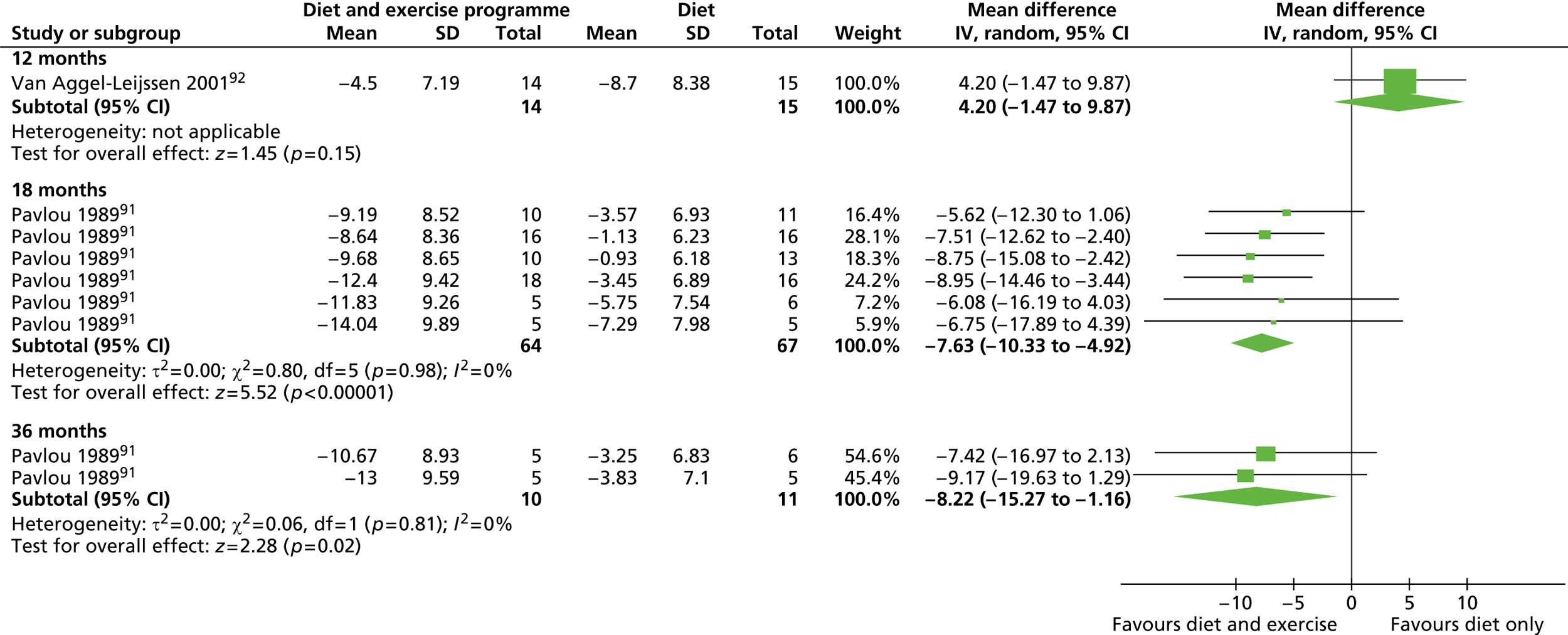
In the main trial,91 systolic (mean difference –8.90 mmHg, 95% CI 13.65 to –4.15 mmHg) and diastolic (mean difference –12.10 mmHg, 95% –15.20 to –9.00 mmHg) blood pressure were significantly lower in the diet and exercise groups compared with the diet only groups at 18 months.
Type of diet
A high-protein reducing diet compared with a high-carbohydrate reducing diet
The trial by Benassi-Evans and colleagues83 compared a high-protein diet with a high-carbohydrate diet. Both diets consisted of 1670 kcal per day with some adjustment in energy to achieve an approximate weight loss of 1 kg per week. At 12 months the high-protein diet group had not lost as much weight as the high-carbohydrate diet group, although the difference between groups was not statistically significant (mean difference 1.55 kg, 95% CI –4.70 to 7.80 kg kg) ( Figure 9 ).
FIGURE 9.
Effect of a high-protein diet vs. a high-carbohydrate diet on weight (kg) at 12 months.
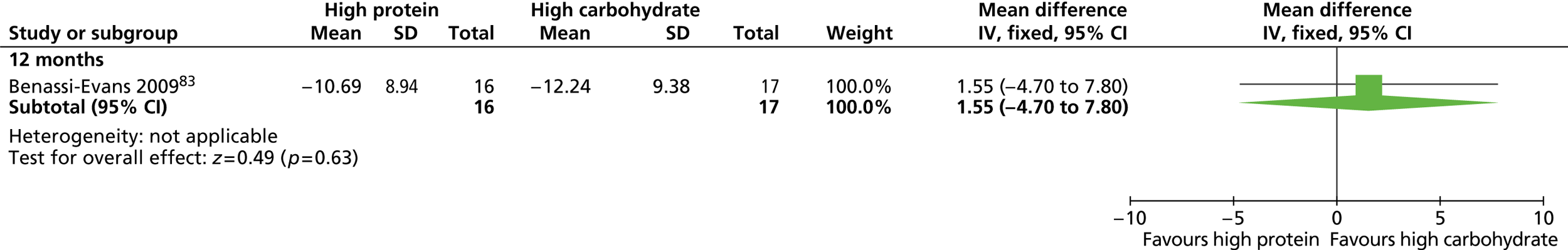
A high-protein, low-fat diet compared with a low-calorie diet
Khoo and colleagues87 randomised men with type 2 diabetes mellitus to a modified low-calorie diet or a high-protein, low-fat diet. By 12 months men in the low-calorie diet group had lost slightly more weight than men in the high-protein diet group (–9.5 kg vs. –9.0 kg). For both groups the men lost a significant amount of weight, reduced their waist circumference measurement and saw improvements in erectile function as measured by the IIEF-5, but the differences between groups were not statistically significant.
Monetary contracts: individual compared with group
The trial conducted by Jeffery and colleagues86 recruited 89 men to receive a 15-week behaviourally oriented weight reduction programme with a goal of achieving a total weight loss of 30 lb (13.6 kg) at a rate of 2 lb (0.2 kg) per week. Using a factorial design, men in the trial were randomised to pay monetary deposits of US$30, US$150 or US$300 and to either a group contract or an individual contract. Refunds were made at a rate of US$1, US$5 or US$10 per lb lost up to a maximum cumulative weight loss of 2 lb per week. Men in the individual contract groups received refunds based on individual weight loss, whereas those with group contracts were refunded based on the mean weight loss of their group. Group contracts produced significantly more weight loss than individual contracts both at 1 year and 2 years (reported p < 0.05). Effects of contract size at 1 year86 and 2 years117 were reported as not significant. Table 8 shows the mean weight loss for group and individual contracts by contract size at 1 and 2 years.
| Contract size | Calculated mean weight loss (kg) | |
|---|---|---|
| Group contract | Individual contract | |
| 1 year | ||
| US$30 | –8.55 (n = 17) | –5.35 (n = 16) |
| US$150 | –10.02 (n = 13) | –7.39 (n = 15) |
| US$300 | –6.60 (n = 13) | –6.24 (n = 14) |
| 2 years | ||
| US$30 | –6.40 (n = 17) | –3.69 (n = 16) |
| US$150 | –7.02 (n = 13) | –3.78 (n = 15) |
| US$300 | –6.31 (n = 13) | –3.24 (n = 14) |
Weight maintenance
Diet and exercise compared with diet for weight maintenance
The trial conducted by Borg and colleagues84 examined whether or not adding walking or resistance training to diet compared with diet alone improved weight maintenance following a weight reduction period. During the weight reduction period, participants followed a very low-calorie diet (Nutrilett) of 500 kcal per day for 2 months. The mean weight loss at the end of the weight reduction period was 14.2 kg. Participants were then randomised to follow a low-fat diet of 1200 kcal per day only or to diet and walking or diet and resistance training exercise groups. Other than reduced fat and calorie intake, the diet was unrestricted in terms of choice or amount of food consumed. Exercise sessions were held three times a week and lasted 45 minutes each. The walking exercise was designed to produce an energy expenditure of 400 kcal per session, whereas the resistance training produced an expenditure of 300 kcal per session. Men in the diet-only group were advised not to increase their physical activity during the 6-month weight-maintenance phase. At 31 months the walking group showed the greatest increase in weight with an average weight gain of 10.1 kg. The resistance training and diet-only groups increased their weight by an average of 9.1 and 8.4 kg respectively. Differences between groups for weight gain were not statistically significant, although the waist-to-hip ratio for the resistance training group increased significantly less than that of the walking group despite the larger weight gain in this group (reported p = 0.04). Outcomes for waist-to-hip ratio, LDL cholesterol, HDL cholesterol, fasting plasma glucose and systolic and diastolic blood pressure were not significantly different between the exercise groups and the diet-only group at 13 months. HDL cholesterol improved significantly more in the resistance training group than in the walking group (reported p = 0.03) at 31 months ( Table 9 ). The energy expenditure of both physical activities in the exercise groups decreased during the weight-maintenance period because of poor long-term adherence to the prescribed regimen.
| Outcome | Diet and resistance training (n = 24) | Diet and walking (n = 18) | Diet only (control) (n = 19) |
|---|---|---|---|
| BMI (kg/m2) | 32.0 (3.3) | 32.0 (4.1) | 31.3 (3.1) |
| Mean difference vs. control (95% CI) | –0.2 (–2.0 to 1.5) | 0.2 (–1.6 to 2.1) | |
| Waist-to-hip ratio | 0.98 (0.07) | 1.01 (0.06) | 1.00 (0.06) |
| Mean difference vs. control (95% CI) | –0.04 (–0.07 to –0.00) | 0.01 (–0.03 to 0.04) | |
| Systolic blood pressure (mmHg) | 136 (15) | 131 (19) | 132 (15) |
| Mean difference vs. control (95% CI) | 1 (–8 to 10) | –2 (–11 to 8) | |
| Diastolic blood pressure (mmHg) | 87 (10) | 84 (10) | 84 (10) |
| Mean difference vs. control (95% CI) | –0 (–6 to 6) | 1 (–6 to 7) | |
| LDL cholesterol (mmol/l) | 3.56 (0.58) | 3.53 (0.93) | 3.47 (0.74) |
| Mean difference vs. control (95% CI) | 0.04 (–0.30 to 0.38) | –0.11 (–0.47 to 0.25) | |
| HDL cholesterol (mmol/l) | 1.24 (0.31) | 1.25 (0.20) | 1.27 (0.27) |
| Mean difference vs. control (95% CI) | 0.01 (–0.09 to 0.11) | –0.01 (–0.11 to 0.10) | |
| Fasting plasma glucose (mmol/l | 5.01 (0.38) | 5.00 (0.52) | 5.12 (0.49) |
| Mean difference vs. control (95% CI) | –0.05 (–0.30 to 0.21) | –0.09 (–0.36 to 0.18) |
A behavioural intervention for weight maintenance compared with control
The trial conducted by King and colleagues88 randomised men from the diet and exercise arms of the trial by Wood and colleagues93 at the end of the 1-year trial period. These men were randomised within their original intervention groups to receive behavioural support based on their original weight-loss method or to two assessment-only control groups. The behavioural support intervention comprised monthly mailed information packs including a supportive letter and a list of coping strategies for problems relevant to their original intervention, for example holiday eating for dieters or finding time to engage in physical activity for exercisers. The men were also telephoned regularly to discuss any concerns or questions related to their problem areas and were weighed at 6-monthly intervals. Men in the assessment-only groups were given written information about their original weight-loss method at the start of the weight-maintenance period. The men received no other contact apart from the 6-monthly weight assessments.
The behavioural therapy intervention produced greater weight-maintenance success for the exercise group compared with the control group than it did for dieters. After 1 year, exercisers who received the behavioural intervention weighed significantly less than control participants (–3.10 kg, 95% CI –5.04 kg to –1.16 kg). Dieters in the behavioural intervention group were not significantly different from control participants after 1 year (0.60 kg, 95% –1.27 kg to 2.47 kg) ( Figure 10 ).
FIGURE 10.
Effect of a behavioural intervention for weight maintenance vs. control on weight (kg) at 12 months.
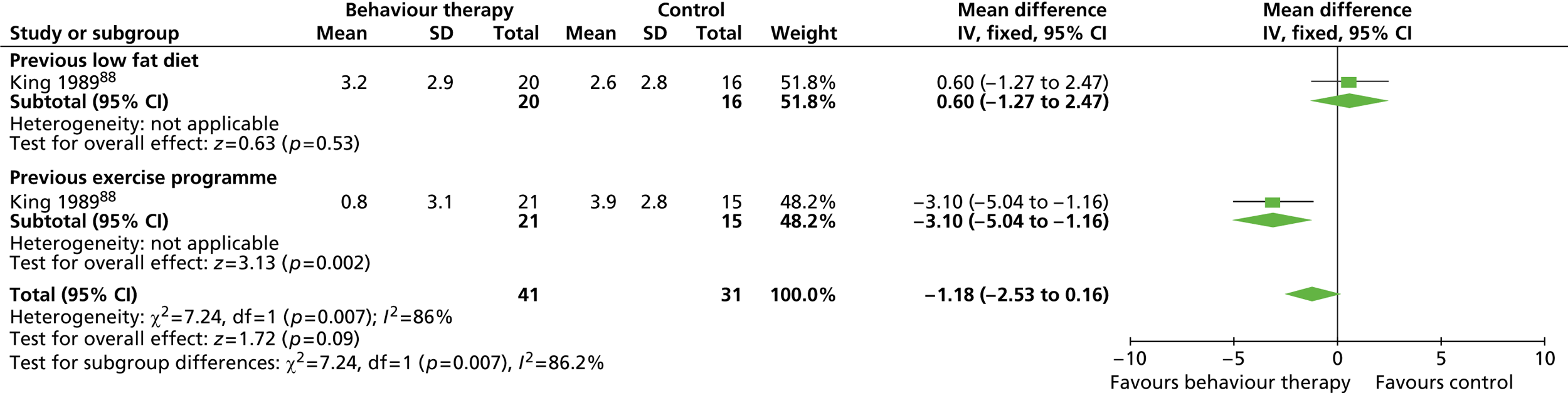
Discussion of results from the review of men-only trials
We identified very few long-term men-only randomised trials investigating interventions for reducing male obesity. Only two of our 11 included trials investigated weight maintenance in men. Results from this systematic review should therefore be treated with caution because of the limited evidence base. Nevertheless, it is possible to conclude that interventions containing a prescribed dietary regimen will produce greater reductions in weight for obese men than interventions that do not, for example interventions including exercise alone. 93 The type of reducing diet prescribed did not affect the amount of weight reduction, and higher protein intakes could not be demonstrated to be more effective for weight loss. 83,87,91 Adding an exercise component to a dietary regimen produced a marked effect in the long term, as reported in the trial conducted by Pavlou and colleagues (follow-up at 18 and 36 months). 91 Shorter-term results reported in the small trial by van Aggel-Leijssen and colleagues92 seem to contradict this, although it should be noted that the exercise protocols for these studies differed greatly.
When frequency of contact varied between interventions, the intervention with the greatest frequency of contact (often supervised exercise classes) usually produced more favourable results. This is contradicted in the trial by van Aggel-Leijssen and colleagues92 in which the diet and exercise group received more contacts but did not lose as much weight as the diet-only group.
Only one trial, that conducted by Jeffery and colleagues,86 directly examined the effect of group compared with individual interventions for weight loss. This trial suggested that men lose more weight if they attend weight-loss sessions in groups, with group monetary contracts compared with individual contracts as incentives. This is in keeping with the findings of a systematic review127 comparing group and individual treatments for obesity in both men and women. This review also found that group-based interventions were more effective, although the reviewed population was predominantly female.
Few interventions included a structured formal behaviour change programme although many included elements of behaviour change, for example self-monitoring and goal setting. Trials conducted by Esposito and colleagues,85 Khoo and colleagues87 and Morgan and colleagues89 included behavioural support as part of a dietary-based intervention. The trials did not compare different types of behaviour change activity and consequently it is impossible to assess whether or not one type of activity is most effective. The trial by King and colleagues88 showed that behavioural support can be beneficial for men during weight maintenance, although this seemed to be more effective at reducing weight regain by encouraging men to continue to engage in physical activity rather than restricting their dietary intake. The trial by Borg and colleagues84 did not clearly demonstrate that the type of physical activity was important for weight maintenance, as resistance training was not associated with significantly less weight gain than walking, and neither type of exercise was significantly better than diet only for preventing weight gain.
Few reports gave details of the method of randomisation. It is therefore not possible to judge the success of randomisation for these trials. Similarly, few authors used intention-to-treat analysis, choosing instead to present data for completers only both at baseline and for final outcome measurements. It is therefore difficult to judge the level of attrition bias in these studies. The methodological quality of future trials could be improved by adopting an intention-to-treat approach, carrying out assiduous follow-up of participants and providing a conservative estimate of results by assuming weight regain to baseline for those who drop out. Reporting could also be improved by following the standards outlined in the Consolidated Standards of Reporting Trials (CONSORT) statement. 128,129 Similarly, few trials reported details concerning the equity or sustainability of the considered interventions. Providers of the interventions were described at an occupational level, for example nutritionist or physical activity trainer, but the sex of providers was not reported. It is subsequently unclear from the included studies whether the sex of the person providing a weight-loss intervention to men, either individually or in groups, is an important factor in the effectiveness of that intervention.
Future trials should also gather information on patient-reported quality of life and clinical and economic outcomes to assess the full value of an intervention other than for amount of weight lost. Future research is also required to develop effective interventions to prevent men regaining weight in the long term following successful weight loss.
Review of randomised controlled trials of men and women compared
This systematic review was of RCTs of interventions in any setting for men and women who are obese with a BMI of ≥ 30 kg/m2 (or overweight with a BMI of ≥ 28 kg/m2 and cardiac risk factors based on orlistat guidance) in which the results are presented separately for men and women in the same trial. We used trials including both men and women to look for differences in effectiveness.
Number and type of studies
Twenty RCTs94 – 113 including both men and women, with six linked reports,120 – 125 were identified as eligible for inclusion. Nine trials94,97 – 99,109 – 113 were conducted in the USA; six95,96,101,102,104,108 were conducted in Finland and one was conducted in each of the following locations: Canada,106 Israel,107 Scandinavia,105 Sweden103 and the UK. 100 The trial by Jolly and colleagues100 in the UK evaluated commercial and NHS weight-loss services in primary care and the community. The study by Ross and colleagues106 was conducted in a primary care setting in Canada. The remaining studies appeared to be conducted in research settings.
Table 10 details the characteristics of the included reports. A very diverse range of interventions was tested in the trials. The majority of trials investigated interventions for weight loss. Three trials investigated different types of reducing diet or when to use such diets. 103,107,113 Eight trials investigated a variety of dietary, physical activity and behaviour change interventions compared with control/usual care interventions. 97,99 – 101,104,108,110,111 The study by Volpe and colleagues109 compared a reducing diet, an exercise programme or both together. One trial examined which type of behaviour change programme to use. 97 One trial examined the effect of modifying the home environment. 94 The study by Jeffery and colleagues evaluated the delivery of a programme by mail or by telephone. 99 One study evaluated the delivery of a programme by doctors or by nurses. 102 Wing and colleagues112 investigated whether or not weight loss was improved if a spouse also attended the programme. Two trials by Hakala and colleagues95,96 examined the benefit of an initial inpatient rehabilitation programme.
| Study ID | Participants | Interventions | Outcomes | Notes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gorin 201394 | Location: Home environment, Providence, RI, USA Period of study: NR Inclusion criteria: Age 21–70 years, BMI 25–50 kg/m2 and have a household member willing to participate in the study as a support partner (support partners had to reside in the same home as the participant and be interested in weight loss). Partner inclusion/exclusion criteria as per participant criteria except for lower age range of 15–70 years Exclusion criteria: Heart condition, chest pain during activity or rest, loss of consciousness, unable to walk two blocks without stopping, current participation in another weight-loss programme, taking weight-loss medication, current pregnancy or planned in the next 18 months, or any condition judged by the research team to impede completion of the study protocol (i.e. plans to relocate, substance abuse). Individuals with joint problems, using prescription medication or other conditions that could limit exercise were required to obtain written physician consent to participate Age (years), mean (SD): Participants: a: 50.4 (9.3), b: 47.5 (11.3); partners: a: 47.9 (13.3), b: 47.8 (13.0) Weight (kg): NR BMI (kg/m2), mean (SD): Participants: a: 36.1 (6.1), b: 36.7 (6.2); partners: a: 33.1 (5.7), b: 32.8 (6.1) Baseline comparability: Yes |
Description of interventions: a: Standard behavioural weight-loss treatment (participants only). Standard calorie- and fat-restricted diet (e.g. 1200–1800 kcal per day and 30% fat, depending on initial weight) to achieve a 10% weight-loss goal. Sample meal plans and a calorie guidebook were provided, and the amount of physical activity was gradually increased until participants achieved ≥ 200 minutes of moderate intensity physical activity per week. Given pedometers and a goal of 10,000 steps per day. Instruction in core behavioural skills was provided through daily diaries for recording all food and beverage intake with corresponding calories, fat grams, minutes of physical activity, daily steps and weight. Interventionists provided weekly written feedback. Stimulus control, problem-solving, goal-setting and cognitive restructuring skills were also taught. The focus of treatment shifted to weight-loss maintenance and relapse prevention in the later months of the programme b: Standard behavioural weight-loss treatment plus modifications to the home environment (participants and partners). Standard treatment as described above but additionally manipulated physical and social aspects within participants’ households. Partners were encouraged to attend all weight-loss groups and make the same diet and exercise changes as participants Modifying type and amount of food consumed: Once monthly participants conducted a ‘cabinet cleanout’ removing any items listed on a high-fat foods checklist. A complementary ‘filling up with fit foods’ exercise using a provided checklist of foods consistent with the dietary prescription was also completed monthly. To increase cues for healthy food choices, participants were provided with a low-calorie cookbook, a subscription to a healthy recipe magazine and motivational posters. Appropriately sized dishware and glasses, a food scale and a set of measuring cups and spoons were provided to limit portions and decrease passive eating. Participants were also encouraged to use a commercially available online grocery order and home delivery service to limit impulse purchases. Participants paid for their own groceries but were reimbursed for the delivery fee Modifying availability of exercise equipment and sedentary activities: A treadmill or stationary bicycle was provided for home use and participants were asked to decrease time spent watching television and restrict viewing to one location. Each television in the home was fitted with a television allowance device that provided feedback about weekly viewing habits. Exercise videotapes, resistance bands, a subscription to an exercise-related magazine and motivational posters were also provided to further increase cues for physical activity Increasing saliency of consequences: Participants were given a digital body weight scale and a full-length mirror with instructions to place these in prominent locations to serve as daily cues to self-weigh and limit overeating/engage in physical activity Both conditions had weekly group meetings for 6 months followed by biweekly meetings for 12 months. Participants and partners in both a and b each received US$25 for completing the 6-month assessment and US$50 for completing the 18-month assessment Timing of active intervention: 18 months No. of times contacted: 51 No. allocated: Participants: a: men: 21, women: 78; b: men: 23, women: 79; partners: a: men: 52, women: 47; b: men: 55, women: 47 No. completed: Participants: a: 86, b: 99; partners: a: 82, b: 99 Dropout (%): Participants: a: 13, b: 3; partners: a: 17, b: 3 No. assessed: Participants: a: men: 21, women: 78; b: men: 23, women: 79; partners: a: men: 52, women: 47; b: men: 55, women: 47 |
Length of follow-up: 18 months Outcomes by sex: Weight |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hakala 199395 | Location: One rehabilitation centre, Finland Period of study: NR Inclusion criteria: At least 50% overweight, age 20–54 years Exclusion criteria: Cardiovascular disease, metabolic disease, psychiatric disease, hypothyroidism Age (years), mean (SD) (range): Men: a: 39 (9) (28–53), b: 40 (10) (27–51); women: a: 41 (8) (25–54), b: 37 (6) (24–52) Weight (kg), mean (SD) (range): Men: a: 121.9 (10.3) (109–141), b: 120.2 (9) (109–131); women: a: 104.0 (12.2) (83–132), b: 104.3 (10.6) (87–126) BMI (kg/m2), mean (SD) (range): Men: a: 42.7 (4.0) (37.4–50.3), b: 41.7 (3.1) (38.3–49.2); women: a: 43.6 (4.8) (36.3–56.7); b: 43.4 (5.4) (33.9–51.9) Baseline comparability: Yes |
Description of interventions: a: 2-week inpatient treatment in a rehabilitation centre. Weight reduction programme consisted of a 1200 kcal per day diet and group counselling sessions led by a nutritionist, physiotherapist and occupational therapist (10 participants per group) including 15 hours of nutrition counselling, 15 hours of physical activity, 12 hours of occupational therapy and 1 hour of individual nutritionist counselling. Physician-led lecture and examination. Followed by 4-monthly individual physician appointments for 2 years b: A 1200 kcal per day diet and individual physician-led counselling in 20-minute sessions, monthly for the first year and 4-monthly over the second year. The physician provided advice and information leaflets concentrating on weight reduction for the first 6 months and changes in body weight and health status after 6 months No anorexigenic drugs were used in either group Timing of active intervention: a: 2-week intensive weight reduction followed by counselling for up to 2 years; b: 2 years of counselling No. of times contacted: a: 40; b:15 No. allocated: Men: a: 10, b: 10; women: a: 20, b: 20 No. completed: Men: a: 9, b: 9; women: a: 19, b: 16 Dropout (%): Men: a: 10, b: 10; women: a: 5, b: 20 No. assessed: Men: a: 9, b: 9; women: a: 19, b: 16 |
Length of follow-up: 5 years Outcomes by sex: Weight |
Weight reduction programme based on that used in Karvetti and Hakala101 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hakala 199496 | Location: One rehabilitation centre and one health centre, Finland Period of study: NR Inclusion criteria: At least 54% overweight, no participation in a weight reduction course in the previous 2 years Exclusion criteria: Epilepsy, cardiac failure Age (years), mean (SD) (range): Men: a: 40 (11) (25–52), b: 44 (6) (38–53); women: a: 40 (7) (26–51), b: 40 (8) (25–52) Weight (kg), mean (SD) (range): Men: a: 143.6 (17.1) (127–174), b: 137.6 (11.0) (120–156); women: a: 120.7 (9.3) (106–146), b: 119.2 (12.6) (101–144) BMI (kg/m2), mean (SD) (range): Men: a: 40.5 (3.9) (36–48), b: 37.7 (2.3) (34–40); women: a: 39.8 (4.3) (35–51), b: 39.2 (3.5) (34–46) Baseline comparability: Yes |
Description of interventions: a: 3-week inpatient treatment in a rehabilitation centre. Programme consisted of a low-fat, high-fibre diet of 1200 kcal per day and group counselling sessions led by a nutritionist, physician and occupational therapist (10 participants per group) including 21 hours of nutrition and behaviour counselling, 16 hours of recreational activity, 15 hours of physical activity, 6 hours of food preparation advice, 6 hours of social counselling, a 1-hour lecture, 1 hour of individual nutritionist-led counselling and one individual physician appointment. Following the intensive weight reduction period, participants had appointments with their GP at 1- to 2-monthly intervals b: A 10-week low-fat, high-fibre diet of 1200 kcal per day and group counselling in a health centre setting led by three specially trained public health nurses. The group leader gave instruction and motivation according to a weight reduction plan based mainly on nutrition education and dietary counselling. Three lectures (one physician led, one psychologist led, one physiotherapist led) provided encouragement and support. Following the intensive weight reduction period, participants had appointments with their GP at 1- to 2-monthly intervals Drug treatment for obesity was not used in any phase of the study Timing of active intervention: a: 3 weeks followed by GP counselling for up to 2 years; b: 10 weeks followed by GP counselling for up to 2 years No. of times contacted: a: 13–21; b: 23–34 No. allocated: Men: a: 9, b: 9; women: a: 21, b: 21 No. completed: Men: a: 7, b: 6; women: a: 16, b: 14 Dropout (%): Men: a: 22, b: 33; women: a: 24, b: 33 No. assessed: Men: a: 7, b: 6; women: a: 16, b: 14 |
Length of follow-up: 5 years Outcomes by sex: Weight |
Weight reduction programme based on that used in Karvetti 1992101 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heitzmann 198797 | Location: California, USA Period of study: Prior to November 1985 Inclusion criteria: Fasting blood glucose > 140 mg/dl or blood glucose > 200 mg/dl 2 hours after administration of 75 g of carbohydrate; medically safe to participate in exercise regimens Exclusion criteria: Significant heart or vascular disease Age (years), mean (SD): Men + women: 52.94 (12.08) Weight (kg), mean: Men: 90.13; women: 72.43 BMI: NR Baseline comparability: Unclear |
Description of interventions: All participants were given dietary advice by a registered nutritionist and a prescribed exercise regimen based on exercise tolerance tests a: Relaxation training (control) – participants were offered muscle relaxation training and factual information about diabetes b: Behaviour modification (self-control) – based on Ferguson and Ferguson’s Habits Not Diets.130 Participants kept daily records of weight, type and amount of food eaten, events surrounding eating, time allocated/time spent exercising and place where exercised c: Cognitive modification (goal setting and role of cognitions) – based on Mahoney and Mahoney.131 Participants were instructed to set reasonable goals and keep a diary of their self-statements during eating and exercise d: Cognitive–behaviour modification (goal setting and behaviour monitoring) – participants instructed to keep daily records, set goals and keep a diary of self-statements as in b and c Timing of active intervention: a–d: 7 weeks No. of times contacted: a–d: 13 No. allocated: Men + women: a: 14; b: 13; c: 13; d: 15 No. completed: Men + women: a: 12; b: 10; c: 10; d: 12 Dropout (%): Men + women: a: 14.3; b: 23.1; c: 10; d: 12 No. assessed: Men + women: a–d: 46 |
Length of follow-up: 18 months Outcomes by sex: Weight, HbA1c |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jeffery 198498 | Location: University of Minnesota, USA Period of study: 1983–4 Inclusion criteria: Men 30 lb (13.6 kg), women 20 lb (9.1 kg) over ideal body weight Exclusion criteria: Medical or behavioural contraindications Age (years), mean: Self-referred group: men: 44.3, women: 44.5; population sample: men: 52.3, women: 50.3 Weight (kg), mean: Self-referred group: men: 127.82, women: 83.96; population sample: men: 106.46, women: 82.46 BMI (kg/m2), mean: Self-referred group: men: 32.61, women: 31.50; population sample: men: 32.97, women: 30.53 Baseline comparability: Men and women in the self-referred group were younger than those in the population sample, a higher number had previously participated in a weight control programme and a higher number had an earlier age at onset of being overweight |
Description of interventions: Weight-loss phase: All groups participated in a 16-week educational programme emphasising reduced eating and increased exercise equally. All paid a US$150 deposit a: Self-referred population – recruited through newspaper advertisement: (i) control – deposit refunded at initial session; (ii) constant contract – deposit refunded in US$30 increments for every 5 lb (2.3 kg) group average weight loss; (iii) increasing contract – deposit refunded for successive 5 lb (2.3 kg) lost in increments of US$5, US$10, US$20, US$40 and US$75 b: Population sample – referred from the population sample in Jeffery et al.:86 (i) control – deposit refunded at initial session; (ii) constant contract – deposit refunded in US$30 increments for every 5 lb (2.3 kg) group average weight loss; (iii) increasing contract – deposit refunded for successive 5 lb (2.3 kg) lost in increments of US$5, US$10, US$20, US$40 and US$75 Weight-maintenance phase: 17 men and 25 women were randomised to either intensive weekly problem-solving sessions or non-specific 3-monthly weight-maintenance sessions. Both groups paid a US$100 deposit, which was returned in US$25 increments for attendance at quarterly sessions. Those remaining participants who were not randomised to the maintenance phase were contacted at the 1-year follow-up assessment only Timing of active intervention: 16 weeks + 8-month weight-maintenance period No. of times contacted: a + b: 20; intensive maintenance 26; non-specific maintenance 22 No. allocated: a: men: (i) 10, (ii) 7, (iii) 11; women: 31 (numbers allocated unclear); b: men: (i) 10, (ii) 9, (iii) 8; women: (i) 11, (ii) 9, (iii) 9 No. completed: a + b: men: 53; women: 57 Dropout (%): a + b: men: 3.6; women: 5.3 No. assessed: NR |
Length of follow-up: 1 year Outcomes by sex: Weight |
1-year data not reported by participants randomised/not randomised to the maintenance phase | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jeffery 200399 | Location: Four managed care organisation clinics, USA Period of study: NR Inclusion criteria: age ≥ 18 years, BMI > 27 kg/m2 Exclusion criteria: NR Age (years), mean (SEM): Men + women: a: 50.8 (0.5); b: 50.7 (0.5); c: 50.6 (0.5) Weight (kg): NR BMI (kg/m2), mean (SEM): Men + women: a: 34.0 (0.2); b: 33.5 (0.2); c: 34.1 (0.2) Baseline comparability: Participants randomised to the telephone group were more likely to report taking medication for depression (p < 0.002) |
Description of interventions: a: Control (usual care): Participants had access to weight management services generally available to members of Health Partners private health insurance b: Telephone group: Participants were given a telephone number to activate the intervention. Materials for 10 lessons were mailed at the beginning of the programme. The telephone counsellor provided guidance for each lesson and gave feedback about progress including discussion of behavioural strategies tried since the last session, advice to improve/maintain lifestyle behaviour and a verbal description of the assignment for the next lesson. Average length of telephone call was 19 minutes c: Mail group: Participants activated the intervention by sending a postcard to the study office. Materials and lessons as for the telephone group but interactions between counselling staff and participants were completed via mailed progress reports detailing behaviour change goals, perceived progress and action steps to achieve goals. The counsellor reviewed the report, made comments and returned them by mail along with the next lesson. The process was repeated until all 10 lessons were completed Lessons were carried out at a rate of one per week or at the participant’s own pace. Follow-up options were available to both groups b and c from a health counsellor. Topics covered included nutrition, physical activity, goal setting, stimulus control, social support and self-motivation. If participants discontinued contact before completing 10 lessons, they were contacted at 30 days, 60 days and then at 6-month intervals for 2 years. Those choosing not to activate the programme were also contacted at 6-month intervals for 2 years. Completers of the 10-week programme were followed up at 6, 12, 18 and 24 months Timing of active intervention: 10 weeks No. of times contacted: a: 5; b: 15; c: 15 No. allocated: Men: a: 163, b: 159, c: 186; women: a: 437, b: 442, c: 414 No. completed: NR Dropout (%): NR No. assessed: Men: a: 163, b:159, c: 186; women: a: 437, b: 442, c: 414 |
Length of follow-up: 24 months Outcomes by sex: Weight loss at 12 months |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jolly 2011100 | Location: 17 general practices, South Birmingham Care Trust, UK Period of study: 2009 Inclusion criteria: Age ≥ 18 years, raised BMI in previous 15 months, BMI ≥ 25 kg/m2 for South Asians and ≥ 30 kg/m2 for participants of all other ethnicities without obesity-related comorbidity, BMI ≥ 23 kg/m2 for South Asians and ≥ 28 kg/m2 for participants of all other ethnicities with obesity-related comorbidity Exclusion criteria: Presence of serious comorbidities, unable to understand English, pregnant, unwilling to be randomised Age (years), mean (SD): Men + women: a: 49.67 (13.83); b: 47.45 (14.35); c: 50.71 (14.56); d: 48.84 (14.91); e: 49.76 (14.51); f: 48.75 (15.63); g: 50.48 (13.79); h: 48.94 (15.82) Weight (kg): NR BMI (kg/m2), men + women, n (%): < 30: a: 14 (14); b: 14 (14); c: 12 (12); d: 11 (11); e: 17 (17); f: 14 (14); g: 11 (16); h: 9 (13) 30–34: a: 48 (48); b: 54 (54); c: 51 (51); d: 51 (51); e: 49 (49); f: 51 (51); g: 39 (56); h: 35 (50) 35–39: a: 25 (25); b: 28 (28); c: 29 (29); d: 32 (32); e: 27 (27); f: 27 (27); g: 18 (26); h: 20 (29) ≥ 40: a: 6 (6); b: 4 (4); c: 8 (8); d: 5 (5); e: 4 (4); f: 5 (5); g: 2 (3); h: 3 (4) Baseline comparability: Yes |
Description of interventions: a: Control – participants were sent vouchers for 12 free sessions at a local authority-run leisure centre. Participants were given no other advice or contact b: Participants were able to choose allocation to one of six interventions (c–h) c: Weight Watchers – group-based programme with one-to-one support. One-hour meetings delivered by a group leader with discussion at community venues. Core programme based on a food points system aiming for a 500-kcal deficit per day, leading to 0.5–1.0 kg weight loss per week. Physical activity was encouraged with the goal of achieving 10,000 steps daily. Rewards were given for every 3.2 kg lost and for 5% and 10% of body weight lost. Behaviour change strategies included stages of change, food and activity diaries, goal setting and evaluation of progress d: Slimming World – group-based programme with one-to-one telephone support available. Meetings lasting for 90 minutes were held in community venues. Members encouraged to eat low energy density foods plus extras rich in calcium and fibre. Weight-loss goals were set by the individual. Physical activity was encouraged with build up to 30 minutes of moderate activity on 5 days a week. Also included access to a website and magazines. Awards given for 3.2 kg lost and loss of 10% of body weight. Behaviour change theory based on transactional analysis, motivational interviewing, weekly weighing, group support, group praise, continued commitment in absence of weight loss, self-monitoring, visualisation techniques and personal eating plans e: Rosemary Conley – group based with one-to-one support. Additional support available by e-mail or telephone. Meetings lasting 90 minutes took place in community venues. Sessions included a 45-minute optional exercise class. Goals were staged: either 1–1.5 kg loss per week with a goal of losing 6.35 kg, or 0.5–1 kg loss per week with a goal of losing 3.2 kg. Behaviour change theory based on role modelling, group support, visualisation and reframing. Rewards given for slimmers who maintained or lost weight, including slimmer of the week and certificates for 3.2 kg and 6.35 kg weight-loss milestones f: NHS Size Down – group-based programme run by support workers trained by the NHS dietetics service. Programme consisted of 2-hour sessions held weekly over 6 weeks, with follow-up sessions at 9 and 12 weeks. Focus on long-term changes in eating behaviour patterns to achieve a balanced diet and increase physical activity g: General practice – one-to-one, client-led sessions in NHS general practice. Initial session lasted 30 minutes with follow-up sessions lasting 15–20 minutes. Included problem-solving approach to explore goals and expectations, weight and dieting history, the ‘eatwell plate’, goal setting, self-monitoring through food diaries and planning strategies to deal with challenging situations and maintaining weight loss. Weight-loss goals were 5–10% of body weight at a rate of 0.5–1 kg per week over 3–6 months followed by maintenance. Physical activity goals were to increase activity levels to 30 minutes of moderate activity on 5 days a week. Homework provided for discussion or personal reflection. Participants were encouraged to reward themselves for success h: Pharmacy – as general practice but delivered from NHS pharmacy setting Timing of active intervention: 12 weeks No. of times contacted: a: 1; b: as chosen provider; c–e, g: 12; f: 8; h: 11 No. allocated: Men: a: 25, b: 30, c: 28, d: 35, e: 31, f: 36, g: 23, h: 19; women: a: 75, b: 70, c: 72, d: 65, e: 69, f: 64, g: 47, h 51 No. completed: Men: a: 22, b: 17, c: 24, d: 24, e: 23, f: 28, g: 14, h: 10; women: a: 24, b: 22, c: 18, d: 28, e: 27, f: 26, g: 15, h: 22 Dropout (%): Men: a: 12, b: 43.3, c: 14.3, d: 31.4, e: 25.8, f: 22.2, g: 39.1, h: 47.4; women: a: 32, b: 31.4, c: 25, d: 43.1, e: 39.1, f: 40.6, g: 31.9, h: 43.1 No. assessed: Men: a: 25, b: 30, c: 28, d: 35, e: 31, f: 36, g: 23, h: 19; women: a: 75, b: 70, c: 72, d: 65, e: 69, f: 64, g: 47, h: 51 |
Length of follow-up: 1 year Outcomes by sex: Weight loss |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Karvetti 1992101 | Location: One research centre, Finland Period of study: NR Inclusion criteria: NR Exclusion criteria: Diabetes, any disease preventing compliance with the weight reduction programme Age (years), mean: Men + women: a: 47.8; b 48.5 Weight (kg), mean: Men: a: 101.83, b: 100.65; women: a: 87.08, b: 90.0 BMI (kg/m2), mean: Men + women: a: 33.5; b: 34.4 Baseline comparability: Yes |
Description of interventions: a: Control group – participants given no instruction but were informed that they had been selected to participate in a weight reduction course to be held after the 1-year follow-up assessment b: Low-fat, low-sugar, high-fibre 1200 kcal per day diet combined with a group-based weight reduction programme (eight subgroups with 12–18 participants in each group) based on nutrition education and counselling to modify counterproductive dietary habits, organised through the 1-year intervention period by seven trained public health nurses. Three lectures (one physician led, one psychologist led, one physiotherapist led) provided encouragement and support. Eventual weight-maintenance diet of 1800 kcal per day Timing of active intervention: 1 year No. of times contacted: a: 2; b: 12 No. allocated: Men + women: a: 117; b: 126 No. completed: Men: a: 20, b: 21; women: a: 76, b: 71 Dropout (% defaulters): Men + women: a: 18; b: 26 No. assessed: Men: a: 20, b: 21; women: a: 76, b: 71 |
Length of follow-up: 1 year Outcomes by sex: Weight, systolic and diastolic blood pressure |
Control group acted as a control for the first year of follow-up only. Paper reports data for 7 years. Weight data derived from graph | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Korhonen 1987102 | Location: One hospital outpatient clinic, Finland Period of study: NR Inclusion criteria: Newly diagnosed non-insulin-dependent diabetes: fasting venous blood glucose ≥ 7.0 mmol/l and/or 2-hour blood glucose ≥ 10.0 mmol/l on oral glucose tolerance test Exclusion criteria: NR Age (years), mean (SEM): Men: a: 54.8 (1.3); b: 53.6 (1.3); women: a: 57.8 (1.0), b: 59.1 (1.2) Weight (kg), mean (SEM): Men: a: 97.5 (3.6), b: 93.4 (3.4); women: a: 81.9 (3.6), b: 78.7 (2.2) BMI (kg/m2), mean (SEM): Men: a: 31.7 (1.0), b 31.3 (0.8); women: a: 32.7 (1.9), 31.8 (0.8) Baseline comparability: Yes |
Description of interventions: Before randomisation, a doctor described the general outline of therapy and stressed the importance of diet and weight reduction in diabetes control to all participants a: Doctor only: Short, written information leaflet giving dietary instruction in weight reduction provided by a doctor; general leaflet used for obese non-diabetic patients. No additional instruction given at follow-up visits b: Specialist nurse: Nurse assessed the diet history of each participant and gave individual instruction for following a hypocaloric diet. Instructions repeated at follow-up visits Timing of active intervention: 12 months No. of times contacted: a: 5; b: 5 No. allocated: Men: a: 20, b: 20; women a: 20, b: 20 No. completed: Men: a: 19, b: 19; women a: 15, b: 18 Dropout (%): Men: a: 5, b: 5; women a: 25, b: 10 No. assessed: Men: a: 19, b: 19; women: a: 15, b: 18 |
Length of follow-up: 1 year Outcomes by sex: Weight change |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lantz 2003103 | Location: One hospital outpatient clinic, Sweden Period of study: January 1996–February 1999 Inclusion criteria: Age 18–60 years, BMI > 30 kg/m2 Exclusion criteria: Participation in other ongoing clinical trial, concomitant serious disease (e.g. type 1 diabetes, renal or hepatic failure, unstable angina, recent myocardial infarction, chronic infections, psychotic disorder and bulimia), previous obesity surgery, drug abuse Age (years), mean (SD): Men + women: a: 41.9 (10.6); b: 41.4 (11.3) Weight (kg), mean: Men: a: 117.65, b: 125; women: a:111.11, b: 110.71 BMI (kg/m2), mean (SD): Men + women: a: 39.9 (5.6); b: 40.1 (5.7) Baseline comparability: Yes |
Description of interventions: Both groups exposed to a 16-week pretreatment phase during which participants consumed a very low-calorie diet (VLCD) of 450 kcal per day supplied by Modifast (Novartis). The treatment phase was followed by a 3-week refeeding phase during which ordinary food was introduced a: Repeated VLCD every 3 months for 2 weeks. Recommended to follow an individualised hypocaloric diet, providing a 500 kcal per day deficit, at other times b: Recommended to follow an individualised hypocaloric diet providing a 500 kcal per day deficit. Advised to use VLCD when body weight passed an individual predetermined cut-off (individual body weight after pretreatment phase plus 3 kg). The cut-off level was reduced during the trial for those who continued to lose weight but remained unchanged for those who regained weight Timing of active intervention: Up to 24 months No. of times contacted: a: 69; b: 69 No. allocated: Men: a: 42, b: 44; women: a: 119, b: 129 No. completed: Men: a: 14, b: 21; women: a: 43, b: 39 Dropout (%): Men: a: 66.7, b: 52.3; women: a: 63.9, b: 69.8 No. assessed: Men: a: 14, b: 21; women: a: 43, b: 39 |
Length of follow-up: 2 years Outcomes by sex: Weight loss |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lindstrom 2008104 | Location: Five diabetes centres, Finland Period of study: 1993–2000 Inclusion criteria: Age 40–65 years, BMI > 25 kg/m2, impaired glucose tolerance [defined as plasma glucose 7.8–11.0 mmol/l 2 hours after administration of 75-g glucose in those with a non-diabetic fasting glucose concentration (plasma glucose < 7.8 mmol/l)], mean value of two glucose tolerance tests Exclusion criteria: Previous diagnosis of diabetes mellitus (other than gestational diabetes mellitus), persons involved regularly in a vigorous exercise programme, subjects receiving treatment to lower blood glucose (other than routine dietary and health advice), chronic disease making 6-year survival improbable, other medical characteristics likely to interfere with study participation, unbalanced clinical conditions such as thyroid and liver disease Age (years), mean (SD): Men + women: a: 55.0 (7.0); b: 55.0 (7.0) Weight (kg): NR BMI (kg/m2), mean (SD): Men: a 29.7 (3.6), b: 30.1 (3.5); women: a: 31.7 (4.7), b: 32.1 (4.9) Baseline comparability: Significant difference in systolic blood pressure (mmHg), mean (SD): a: 136 (17); b: 140 (18) (p = 0.03) |
Description of interventions: a: General advice: At baseline participants were advised to adjust total energy intake to reduce BMI to < 25 kg/m2, consume < 30% of energy intake from fat, reduce alcohol intake and stop smoking. They were also given verbal and written dietary advice, verbal general information regarding health benefits of recreational exercise and additional routine advice at the yearly follow-up when 3-day food record assessed and 2-km walking test performed b: Lifestyle modification: Participants informed at start of risk factors for diabetes. A 3-day food diary at baseline provided the basis for dietary advice in the second session. Participants were advised to reduce weight with a goal of a BMI of < 25 kg/m2 but in practice weight targets were 5–10 kg of weight loss. Advised to consume > 50% carbohydrate, < 10% saturated fat, 20% monosaturated and polyunsaturated fat or up to 25% if surplus is from monounsaturated fat; to consume < 300 mg per day cholesterol and 1 g protein per kg ideal body weight per day; to increase fibre intake to 15 g per 1000 kcal; to use low-fat milk products, low-fat meat products, soft margarine and vegetable oil rich in monounsaturated fatty acids (primarily rapeseed oil). Energy content was re-evaluated if no weight loss at visits; if no weight loss in first 6–12 months and BMI > 30 kg/m2 a very low-calorie diet was considered (6–12 weeks’ duration with group meetings every 1–2 weeks). Dietary advice was individually tailored and the person responsible for preparing meals in the family was invited to attend sessions (if not the participant). Advice was tailored to participants’ educational level. Participants were individually guided to increase endurance exercise (programme differed between study centres); also, when possible, there was a supervised, progressive, individually tailored circuit-type resistance training twice weekly. participants were encouraged to perform 30 minutes of daily moderate exercise. A 3-day food diary and a 24-hour exercise diary were kept every 3 months and a 12-month physical activity history and 2-km walking test were completed at the annual visit Timing of active intervention: 2–6 years No. of times contacted: a: 5; b: 29 No. allocated: Men: a: 81, b: 91; women: a: 176, b: 174 Completed (at 2 years): Men + women: a: 242; b: 240 Dropout (%) (at 2 years): Men + women: a: 6; b: 8 No. assessed: Men: a: 81, b: 91; women: a: 176; b: 174 |
Length of follow-up: median 4 years Outcomes by sex: Diabetes incidence |
Finnish Diabetes Prevention Study (methods from Tuomilehto 2001132) All participants had impaired glucose tolerance |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Richelsen 2007105 | Location: Nine clinical research centres, Scandinavia Period of study: NR Inclusion criteria: Age 18–65 years, abdominal obesity [BMI 30–45 kg/m2 and waist circumference ≥ 102 cm (men) and ≥ 92 cm (women)] and one or more of the following risk factors: impaired fasting glucose (plasma glucose ≥ 6.1 mmol/l), diet-treated type 2 diabetes (plasma glucose ≥ 7.0 mmol/l) or dyslipidaemia [HDL cholesterol ≤ 0.9 mmol/l (men), ≤ 1.1 mmol/l (women) and/or serum triglycerides between ≥ 2.0 mmol/l and ≤ 10.0 mmol/l]; 5% weight loss achieved during the 8-week very low-calorie diet prerandomisation period Exclusion criteria: During the randomised phase, participants with deterioration in glucose control were prescribed metformin. If metformin failed to keep HbA1C level at < 10% the participant was withdrawn Age (years), mean (range): Men + women: a: 46.7 (19–63); b: 47.2 (20–64) Weight (kg), mean: Men + women: a: 97.5; b: 95.7 BMI (kg/m2) (prerandomisation), mean (range): Men + women: a: 37.6 (30.0–45.0); b: 37.4 (30.1–45.2) Baseline comparability: Yes |
Description of interventions: Prerandomisation: All participants prescribed very low-calorie diet [Modifast (Novartis) or Nutrilett] of 600–800 kcal per day for 8 weeks. Those achieving 5% weight loss were randomised as follows: a: Placebo b: Orlistat 120 mg three times daily Both groups were instructed to follow a standard energy-restricted diet consisting of a 600 kcal per day deficit and were advised to reduce fat intake to approx. 30% of total energy, especially saturated fat, and increase fruit and vegetable intake. Participants were also advised to increase daily physical activity Timing of active intervention: a + b: 36 months No. of times contacted: a + b: 24 No. allocated: Men: a: 76, b: 76; women: a: 80, b: 77 No. completed: Men + women: a: 98; b: 102 Dropout (%): Men + women: a: 37.2; b: 33.3 No. assessed: NR |
Length of follow-up: 36 months Outcomes by sex: Weight |
Sponsored by Roche | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ross 2012106 | Location: Three primary health-care clinics, Ontario, Canada Period of study: December 2004–January 2008 Inclusion criteria: Age 25–75 years, sedentary (one or fewer physical activity sessions per week), BMI 27–39.9 kg/m2, abdominally obese (waist circumference ≥ 102 cm for men and ≥ 88 cm for women), weight stable to within 2 kg in last 6 months Exclusion criteria: Serious medical conditions preventing increased daily activity including significant cardiovascular disease, planning for pregnancy in the next 2 years or pregnant Age (years), mean (SD): Men: a: 55.7 (11.5), b: 53.2 (10.7); women: a: 50.9 (11.7), 50.5 (11.1) Weight (kg), mean (SD): Men: a: 98.2 (13.5), b: 101.4 (13.2); women: a: 85.3 (12.5), b: 86.9 (12.1) BMI (kg/m2), mean (SD): Men: a: 32.0 (4.0), b: 32.4 (3.7); women: a: 32.0 (4.3), b: 32.7 (4.3) Baseline comparability: Yes |
Description of interventions: a: Usual care: GP gave advice regarding lifestyle strategies for obesity reduction. GPs followed their usual appointment schedule and counselling approach b: Behavioural intervention: Motivational interviewing and individual stage-based tailored counselling based on transtheoretical model and social cognitive theory. Counselling sessions were provided by health educators who were educated to degree level in kinesiology and had received behavioural counselling training from a clinical psychologist before the start of the trial. During months 0–6, participants were given knowledge and skills to increase physical activity and consume a healthy diet through 15 one-to-one information sessions. During months 7–12, participants attended six sessions in which they were encouraged to maintain healthy eating patterns and 45–60 minutes of physical activity daily. During months 13–24, participants attended 12 sessions but the duration of each was determined by the participant’s waist circumference and physical activity level Timing of active intervention: 2 years No. of times contacted: a: ≥ 5; b: 38 No. allocated: Men: a: 72, b: 74; women: a: 169, b: 175 No. completed: Men: a + b: 121; women: a + b: 275 Dropout (%): Men: a + b: 17.1; women: a + b: 20.0 No. assessed: Men: a: 72, b: 74; women: a: 169, b: 175 |
Length of follow-up: 2 years Outcomes by sex: Weight, BMI, waist circumference, LDL and HDL cholesterol, triglycerides, systolic and diastolic blood pressure, fasting plasma glucose, adverse events |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shai 2008107 | Location: Workplace (one medical research clinic), Israel Period of study: July 2005–June 2007 Inclusion criteria: Age 40–65 years, BMI ≥ 27 kg/m2 or presence of type 2 diabetes or coronary heart disease regardless of age or BMI Exclusion criteria: Pregnant or lactating, serum creatinine ≥ 2 mg/dl, liver dysfunction, gastrointestinal problems preventing patients from following the trial diets, active cancer or participation in another diet trial Age (years), mean (SD): Men + women: a: 51.0 (7.0); b: 53.0 (6.0); c: 52.0 (7.0) Weight (kg), mean (SD): Men: a: 92.9 (11.9), b: 91.5 (13.6), c: 93.2 (14.0); women: a: 81.7 (10.6), b: 89.4 (13.6), c: 77.9 (9.0) BMI (kg/m2), mean (SD): Men + women: a: 30.6 (3.2); b: 31.2 (4.1); c: 30.8 (3.5) Baseline comparability: Yes |
Description of interventions: a: Low-fat, restricted-calorie diet: 1500 kcal per day for women and 1800 kcal per day for men with 30% of calories obtained from fat, 10% from saturated fat and an intake of 300 mg cholesterol per day. Participants were counselled to consume low-fat grains, vegetables, fruit and legumes and to limit additional fats, sweets and high-fat snacks. Based on American Heart Association guidelines133 b: Mediterranean, restricted-calorie diet: 1500 kcal per day for women and 1800 kcal per day for men with a goal of no more than 35% of calories obtained from fat. Main sources of fat were 30–45 g olive oil and < 20 g nuts per day. Participants were counselled to consume a diet rich in vegetables and low in red meat. Based on recommendations of Willett and Skerrett134 c: Low-carbohydrate, non-restricted-calorie diet: Aimed to provide 20 g carbohydrates per day for a 2-month induction phase and immediately after religious holidays with a gradual increase to a maximum of 120 g per day to maintain weight loss. Total calorie, protein and fat intakes were not limited but participants were counselled to choose vegetarian sources of fat and protein to avoid trans fat. Based on the Atkins diet135 Each diet group was assigned a registered dietitian who led all six subgroups of that group. There were 18 group meetings in total lasting 90 minutes each. Another dietitian conducted 10- to 15-minute telephone calls six times over the 2-year trial period with participants experiencing adherence difficulties. A summary of each call was given to the group dietitian A sample of 74 wives of husbands in each group attended support meetings for the first 6 months (assignment not randomised) Timing of active intervention: 2 years No. of times contacted: a–c: 48 No. allocated: Men: a: 89, b: 89, c: 99; women: a: 15, b: 20, c: 10 No. completed: Men + women: a: 94; b: 93; c: 85 Dropout (%): Men + women: a: 9.6; b: 14.7; c: 22 No. assessed: Men: a: 89, b: 89, c: 99; women: a: 15, b: 20, c: 10 |
Length of follow-up: 2 years Outcomes by sex: Weight loss |
Data for wives of trial participants published separately in Golan et al. 120 [Dietary Intervention Randomized Controlled Trial (DIRECT) spousal study] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Golan 2010120 (linked to Shai 2008107) | 74 wives of Dietary Intervention Randomized Controlled Trial (DIRECT) study husbands (who were not part of the trial) were followed up for 2 years Age (years), mean (SD): Wives: a: 50.5 (6.0); b: 52.0 (5.57); c: 49.4 (6.97) Weight (kg), mean (SD): Wives: a: 67.8 (10.51); b: 73.1 (15.92); c: 73.18 (14.07) BMI (kg/m2), mean (SD): Wives: a: 24.9 (3.69); b: 27.81 (4.98); c: 27.79 (5.14) Baseline comparability: Yes |
Interventions as in Shai 2008107. Every 2 months during the first 6 months of the study, participating wives were invited to a 90-minute support group meeting specific to the relevant dietary arm for their husband. The weight of the 74 husbands was compared with the weight of the 248 DIRECT study men whose wives did not take part in the group sessions | Length of follow-up: 2 years Outcomes by sex: Weight loss |
Ancillary spouse study | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vanninen 1992,108 Vanninen 1993125 | Location: One outpatient clinic and five community health centres, Finland Period of study: 1987–9 Inclusion criteria: Age 40–64 years, repeated fasting venous blood glucose > 6.7 mmol/l Exclusion criteria: Chronic disease affecting glucose tolerance, unwilling to participate Age (years), mean (SD): Men: a + b: 53 (7); Women: a + b: 54 (6) Weight (kg), mean (SD): Men: 95 (12); women: 88 (16) BMI (kg/m2), mean (SD): Men: a: 30.1 (3.1), b: 31.1 (3.7); women: a: 34.2 (6.2), b: 33.4 (6.7) Baseline comparability: Women had a higher mean BMI than men. Women in the conventional care group had higher HbA1c and fasting plasma glucose levels than women in the intensified diet and exercise group following the pretreatment phase (baseline) |
Description of interventions: Both groups underwent a 3-month basic education programme pre randomisation giving diet and exercise advice and information a: Conventional care: No further educational materials given. Attended community health centre at intervals of 2–3 months and the outpatient clinic at 6 and 12 months. No access to a dietitian b: Intensified diet and exercise education: Attended diabetes specialist-led outpatient clinics six times every 2 months. A physician provided printed and oral instruction and general motivation and follow-up; a dietitian provided intensified diet education and a nurse was responsible for further patient education and metabolic control follow-up. Goals of dietary education were weight reduction, normoglycaemia, correction of dyslipidaemias, individually planned energy restriction, restricted total fat intake (especially saturated) and dietary cholesterol, a moderate increase in the consumption of unsaturated fats and foods containing complex carbohydrates and to encourage regular eating patterns and moderate consumption. Participants were also encouraged to increase physical activity to 30–60 minutes three to four times per week, with a recommended average heart rate of 110–140 beats per minute. Types of exercise recommended included walking, jogging, cycling, swimming or cross-country skiing. Activity was monitored using daily exercise records. No written exercise instruction or supervision was given Timing of active intervention: 12 months No. of times contacted: a: 7–9; b: 7 No. allocated: Men + women: a + b: 90 No. completed: Men: a: 24, b: 21; women: a: 16, b: 17 Dropout (%): Men + women: a + b: 13.3 No. assessed: Men: a: 24, b: 21; women: a: 16, b: 17 |
Length of follow-up: 12 months Outcomes by sex: BMI, total and HDL cholesterol, triglycerides, HbA1c, fasting plasma glucose |
All participants had non-insulin-dependent type 2 diabetes mellitus at baseline | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Volpe 2008109 | Location: One university research centre, Pennsylvania, USA Period of study: NR Inclusion criteria: Age 24–62 years, sedentary (exercising no more than 1 day per week), non-smoker, BMI 27–35 kg/m2, no acute illness or trauma within previous 6 months, no history of cardiovascular disease, hypertension, hyper/hypothyroidism or any other type of chronic disease Exclusion criteria: Participation in any weight reduction programme within previous 3 months, taking supplements for weight reduction (e.g. physician prescribed or over-the-counter medication) within previous 3 months Age (years): Unclear if data reported for women only Weight (kg): Unclear if data reported for women only BMI: Unclear if data reported for women only Baseline comparability: Unclear |
Description of interventions: All participants underwent a 1- to 2-week prerandomisation phase during which they were habituated to the NordicTrack™ (Chaska, MN, USA) indoor skiing apparatus, fitness levels were measured using the NordicTrack home fitness test and baseline measurements were taken a: Diet: Participants attended intensive nutrition classes advising on adhering to a low-energy, heart-healthy diet with a goal of losing 0.5–1.0 kg body weight per week. After 7 months classes were replaced by monthly telephone/e-mail messages up to month 9 to check dietary adherence b: Exercise: Exercise sessions supervised by trained graduate/undergraduate students 3 days per week for 6 weeks, increasing to five times per week for months 4–6. At 7 months participants were given exercise equipment to continue unsupervised exercise in their own homes. Classes were replaced by monthly telephone/e-mail messages up to month 9 to check exercise adherence c: Diet + Exercise: As a and b Timing of active intervention: 9 months No. of times contacted: a: 19; b: 83; c: 83 No. allocated: Men: a–c: 44; women a: 15, b: 17, c: 14 No. completed: NR Dropout (%): NR No. assessed: Men: a–c: 44; women a: 15, b: 17, c: 14 |
Length of follow-up: 12 months Outcomes by sex: Weight, waist circumference, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, systolic and diastolic blood pressure |
NordicTrack sponsored the study. The authors declare no conflict of interest | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wadden 2011110 | Location: 16 health centres, USA Period of study: NR Inclusion criteria: Age 45–74 years (changed to 55–74 years later and upper range reported as 76 years later), BMI ≥ 25 kg/m2 (≥ 27 kg/m2 if currently taking insulin), type 2 diabetes mellitus (determined by self-report with verification), able to complete 12/14 daily diet and exercise records during 2-week self-monitoring phase Exclusion criteria: Age ≥ 75 years, HbA1c > 11%, blood pressure ≥ 160/100 mmHg, fasting triglycerides ≥ 600 mg/dl, inadequate control of comorbid conditions, factors limiting adherence to/conduct of trial, underlying disease likely to limit lifespan and/or affect safety of interventions, type 1 diabetes Age (years), mean (SD): Men + women: a: 58.9 (6.9); b: 58.6 (6.8) Weight (kg), mean (SD): Men: a: 109.0 (18.0), b: 108.9 (19.0); women: a: 95.4 (17.3), b: 94.8 (17.9) BMI (kg/m2), mean (SD): Men: a: 35.1 (5.2), b: 35.3 (5.7); women: a: 36.6 (6.0), b: 36.3 (6.2) Baseline comparability: Yes |
Description of interventions: All participants required to complete daily diet and exercise records during 2-week self-monitoring phase before randomisation. All subsequent eligible participants received an initial 1-hour diabetes education session including general recommendations for healthy eating, physical activity and diabetes care. Smokers encouraged to quit but did not receive formal smoking cessation counselling a: Diabetes support and education: Participants attended three group education/social support sessions per year. One session covered diet/nutrition, one exercise and one social support. Support sessions allowed participants to discuss issues related to living with diabetes. Attendance at sessions was encouraged but not required b: Intensive lifestyle intervention (ILI): Group lifestyle meetings held for the first 3 weeks of each month with one individual meeting with interventionists (registered dietitians, psychologists and exercise specialists) in the fourth week. Group meetings replaced by individual lifestyle counselling in year 2. Individuals were encouraged to lose ≥ 10% of their initial body weight by 6 months. Participants not meeting this goal or who regained weight were offered orlistat. Each centre had a goal of inducing a minimum mean loss of 7% of initial body weight. Centres achieving < 5% loss were given extra assistance to improve weight-loss outcomes. Participants followed a portion-controlled diet with calorie goals of 1200–1800 kcal per day depending on initial body weight for the first year. Participants consumed meal replacements and structured meal plans for the first 4 months, followed by one meal and one snack replacement in the form of liquid shakes and meal bars for months 5–12. Participants were also given an exercise goal of ≥ 175 minutes per week of unsupervised activity by 6 months. Taught behavioural techniques included problem solving, motivational interviewing, self-regulation theory and relapse prevention Timing of active intervention: 4 years No. of times contacted: a: 16; b: 199 No. allocated: Men: a: 1038, b: 1044; women: a: 1537, b: 1526 No. completed: Ongoing Dropout (%): Ongoing No. assessed: Men: a: 1038, b: 1044; women: a: 1537, b: 1526 |
Length of follow-up: 4 years (year 1 weight reduction, years 2–4 weight maintenance) Outcomes by sex: Weight loss |
Look AHEAD study – outcomes reported by sex for ILI group only | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gorin 2008121 (linked to Wadden 2011110) | Location: 3/16 Look AHEAD centres, USA Period of study: NR Inclusion criteria: Untreated spouse of Look AHEAD participant, willing to participate Exclusion criteria: None Age (years), mean (SD): Men + women: a: 59.8 (9.0); b: 58.6 (7.5) Weight (kg), mean (SD): Men: a: 93.04 (19.03), b: 95.94 (16.76); women: a: 76.97 (14.96), b: 81.52 (18.98) BMI (kg/m2), mean (SD): Men + women: a: 30.1 (6.0); b: 31.0 (6.2) Baseline comparability: Yes |
Description of interventions: As for Wadden 2011110 post randomisation Timing of active intervention: 1 year No. of times contacted: a + b: 2 No. allocated: Men: a: 85, b: 69; women: a: 103, b: 100 No. completed: NR Dropout (%): NR No. assessed: Men: a: 85, b: 69; women: a: 103, b: 100 |
Length of follow-up: 1 year Outcomes by sex: Weight |
Look AHEAD ancillary spouse study | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schwartz 2012124 (linked to Wadden 2011110) | Location: 5/16 Look AHEAD centres, USA Period of study: NR Inclusion criteria: Participating in Look AHEAD study Exclusion criteria: As Wadden 2011110 Age (years), mean (SD): Men: a: 60.0 (6.4), b: 60.4 (6.5); women: a: 57.8 (6.5), b: 57.0 (6.6) Weight (kg), mean (SD): Men: a: 104.9 (14.3), b: 102.9 (15.3); women: a: 93.5 (16.0), b: 92.1 (16.7) BMI (kg/m2), mean (SD): Men: a: 34.0 (4.3), b: 33.9 (4.6); women: a: 36.3 (5.5), b: 35.8 (5.7) Baseline comparability: Women had a higher BMI than men |
Description of interventions: As for Wadden 2011110 post randomisation Timing of active intervention: 1 year No. of times contacted: a + b: 2 No. allocated: Men: a: 246, b: 237; women: a: 386, b: 405 No. completed: NR Dropout (%): NR No. assessed: Men: a: 246, b: 237; women: a: 386, b: 405 |
Length of follow-up: 1 year Outcomes by sex: Weight, bone loss |
Look AHEAD ancillary bone mineral density study | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stewart 2011123 (linked to Wadden 2011110) | Location: 1/16 Look AHEAD centres, USA Period of study: NR Inclusion criteria: Participating in Look AHEAD study Exclusion criteria: As Wadden 2011110 Age (years), mean (SD): Men: a: 61.9 (5.2), b: 61.4 (5.8); women: a: 59.0 (6.1), b: 59.4 (6.9) Weight (kg), mean: Men: a: 105.1, b: 108.0; women: a: 96.5, b: 96.4 BMI (kg/m2), mean (SD): Men: a: 33.1 (4.4), b: 33.9 (5.1); women: a: 36.4 (5.3), b: 36.4 (5.6) Baseline comparability: Women had a higher BMI than men |
Description of interventions: As for Wadden 2011110 post randomisation Timing of active intervention: 1 year No. of times contacted: a + b: 2 No. allocated: Men: a: 33, b: 36; women: a: 43, b: 45 No. completed: Men + women: a: 70; b: 70 Dropout (%): Men + women: a: 7.89; b: 13.58 No. assessed: Men: a: 33, b: 36; women: a: 43, b: 45 |
Length of follow-up: 1 year Outcomes by sex: Weight |
Look AHEAD ancillary body image study | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wing 2010122 (linked to Wadden 2011110) | Location: 5/16 Look AHEAD centres, USA Period of study: NR Inclusion criteria: Male Look AHEAD participants who reported being sexually active in the previous 6 months Exclusion criteria: As Wadden 2011110 Age (years), mean (SD): a: 60.3 (6.6); b: 60.7 (6.5) Weight (kg), mean (SD): a: 109.2 (17.7); b: 110.6 (18.4) BMI (kg/m2), mean (SD): a: 35.1 (5.2); b: 35.6 (5.5) Baseline comparability: Yes |
Description of interventions: As for Wadden 2011110 post randomisation Timing of active intervention: 1 year No. of times contacted: As Wadden 2011110 but with two additional assessments at baseline and 1 year No. allocated: a: 185; b: 187 No. completed: a: 153; b: 153 Dropout (%): a: 17.3; b: 18.2 No. assessed: a: 153; b: 153 |
Length of follow-up: 1 year Outcomes (all male patients): weight loss, total cholesterol, LDL and HDL cholesterol, systolic and diastolic blood pressure, HbA1c change, erectile function |
Subgroup of Look AHEAD study (erectile function) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| West 2008111 | Location: 27 diabetes outpatient clinics, USA Period of study: NR Inclusion criteria: ≥ 25 years, BMI ≥ 24 kg/m2, fasting plasma glucose concentration 5.3–6.9 mmol/l, plasma glucose concentration 12 hours post oral glucose test 7.8–11.0 mmol/l Exclusion criteria: Taking medications known to alter glucose tolerance or had significant illness that could reduce life expectancy or trial participation Age, n (%): Age (years) White Black Hispanic Men < 40 a: 20 (10.9); b: 19 (9.6) a: 6 (10.5); b: 10 (17.2) a: 2 (3.5); b: 6 (12.0) 40–49 a: 43 (23.4); b: 44 (22.1) a: 18 (31.6); b: 20 (34.5) a: 21 (36.8); b: 10 (20.0) 50–59 a: 55 (29.9); b: 54 (27.1) a: 19 (33.3); b: 19 (32.8) a: 23 (40.4); b: 19 (38.0) 60+ a: 66 (35.9); b: 82 (41.2) a: 14 (24.6); b: 9 (15.5) a: 11 (19.3); b: 15 (30.0) Women < 40 a: 51 (12.7); b: 65 (17.1) a: 26 (23.4); b: 27 (22.5) a: 33 (20.3); b: 32 (20.8) 40–49 a: 163 (40.6); b: 138 (36.2) a: 48 (43.2); b: 47 (39.2) a: 61 (37.4); b: 66 (42.9) 50–59 a: 113 (28.1); b: 107 (28.1) a: 29 (26.1); b: 29 (24.2) a: 52 (31.9); b: 30 (19.5) 60+ a: 75 (18.7); b: 71 (18.6) a: 8 (7.2); b: 17 (14.2) a: 17 (10.4); b: 26 (16.9) Weight (kg), mean (SD): Sex White Black Hispanic Men a: 102.8 (18.9); b: 100.5 (20.1) a: 93.0 (18.5); b: 95.7 (17.9) a: 100.5 (17.8); b: 104.4 (22.1) Women a: 93.7 (20.3); b: 95.1 (21.2) a: 85.2 (19.1); b: 82.0 (14.8) a: 98.9 (20.2); b: 97.1 (20.8) BMI (kg/m2), n (%) BMI (kg/m2) White Black Hispanic Men < 30 a: 73 (39.7); b: 92 (46.2) a: 23 (40.4); b: 25 (43.1) a: 20 (35.1); b: 15 (30.0) 30–34.99 a: 60 (32.6); b: 63 (31.7) a: 21 (36.8); b: 17 (29.3) a: 20 (35.1); b: 21 (42.0) 35+ a: 51 (27.7); b: 44 (22.1 a: 13 (22.8); b: 16 (27.6) a: 17 (29.8); b: 14 (28.0) Women < 30 a: 111 (27.6); b: 100 (26.3) a: 32/28.8); b: 32/26.7) a: 32 (19.6); b: 37 (24.0) 30–34.99 a: 104 (25.9); b: 101 (26.5) a: 39/35.1); b: 50/41.7) a: 35 (21.5); b: 46 (29.9) 35+ a: 187 (46.5); b: 180 (47.2) a: 40/36.0); b: 38 (31.7) a: 96 (58.9); b: 71 (46.1) Baseline comparability: Higher proportion of obese participants were female and black. Higher proportion of white participants |
Age (years) | White | Black | Hispanic | Men | < 40 | a: 20 (10.9); b: 19 (9.6) | a: 6 (10.5); b: 10 (17.2) | a: 2 (3.5); b: 6 (12.0) | 40–49 | a: 43 (23.4); b: 44 (22.1) | a: 18 (31.6); b: 20 (34.5) | a: 21 (36.8); b: 10 (20.0) | 50–59 | a: 55 (29.9); b: 54 (27.1) | a: 19 (33.3); b: 19 (32.8) | a: 23 (40.4); b: 19 (38.0) | 60+ | a: 66 (35.9); b: 82 (41.2) | a: 14 (24.6); b: 9 (15.5) | a: 11 (19.3); b: 15 (30.0) | Women | < 40 | a: 51 (12.7); b: 65 (17.1) | a: 26 (23.4); b: 27 (22.5) | a: 33 (20.3); b: 32 (20.8) | 40–49 | a: 163 (40.6); b: 138 (36.2) | a: 48 (43.2); b: 47 (39.2) | a: 61 (37.4); b: 66 (42.9) | 50–59 | a: 113 (28.1); b: 107 (28.1) | a: 29 (26.1); b: 29 (24.2) | a: 52 (31.9); b: 30 (19.5) | 60+ | a: 75 (18.7); b: 71 (18.6) | a: 8 (7.2); b: 17 (14.2) | a: 17 (10.4); b: 26 (16.9) | Sex | White | Black | Hispanic | Men | a: 102.8 (18.9); b: 100.5 (20.1) | a: 93.0 (18.5); b: 95.7 (17.9) | a: 100.5 (17.8); b: 104.4 (22.1) | Women | a: 93.7 (20.3); b: 95.1 (21.2) | a: 85.2 (19.1); b: 82.0 (14.8) | a: 98.9 (20.2); b: 97.1 (20.8) | BMI (kg/m2) | White | Black | Hispanic | Men | < 30 | a: 73 (39.7); b: 92 (46.2) | a: 23 (40.4); b: 25 (43.1) | a: 20 (35.1); b: 15 (30.0) | 30–34.99 | a: 60 (32.6); b: 63 (31.7) | a: 21 (36.8); b: 17 (29.3) | a: 20 (35.1); b: 21 (42.0) | 35+ | a: 51 (27.7); b: 44 (22.1 | a: 13 (22.8); b: 16 (27.6) | a: 17 (29.8); b: 14 (28.0) | Women | < 30 | a: 111 (27.6); b: 100 (26.3) | a: 32/28.8); b: 32/26.7) | a: 32 (19.6); b: 37 (24.0) | 30–34.99 | a: 104 (25.9); b: 101 (26.5) | a: 39/35.1); b: 50/41.7) | a: 35 (21.5); b: 46 (29.9) | 35+ | a: 187 (46.5); b: 180 (47.2) | a: 40/36.0); b: 38 (31.7) | a: 96 (58.9); b: 71 (46.1) | Description of interventions: a: Standard lifestyle + placebo: Placebo tablet given once daily and increased to twice daily after 1 month. Lifestyle recommendations given in written form with annual 20- to 30-minute session with individual participants emphasising a healthy lifestyle, food pyramid, National Cholesterol Education Programme step 1 diet, the need to lose 5–10% of body weight through diet and exercise with an eventual goal of 30 minutes of an activity such as walking 5 days per week and the need to avoid excessive alcohol b: Intensive lifestyle: 16 sessions over 24 weeks with individual participants focusing on dietary changes to promote weight loss of at least 7% of initial body weight including a low-calorie, low-fat diet and increasing physical activity to achieve 150 minutes per week of moderate exercise (e.g. walking and cycling). Sessions included topics and lessons covering lifestyle change, self-monitoring, goal setting, stimulus control, nutrition, environmental change and problem-solving/coping strategies. A toolbox approach was used to add new strategies to help achieve goals. Group sessions were available after the initial 16 sessions were completed. Optional short courses lasting 4–6 weeks offered after 6 months covering nutrition, exercise and behavioural topics. Also, three or four motivational campaigns per year Timing of active intervention: a: one session at baseline, repeated annually; b: at least 16 individual sessions over 24 weeks; two supervised group exercise sessions offered each week; group courses offered quarterly lasting 4–6 weeks to help with weight loss and exercise goals; also seen usually individually once every two months for remainder of the trial and contacted by telephone at least once between visits No. of times contacted: a: 3; b: 30 No. allocated. n (%): Sex White Black Hispanic Men a: 184 (18.9); b: 199 (20.7) a: 57 (5.9); b: 58 (6.0) a: 57 (5.9); b: 50 (5.2) Women a: 402 (41.3); b: 381 (39.6) a: 111 (11.4); b: 120 (12.5) a: 163 (16.7); b: 154 (16.0) Completed (at 30 months), n (%) Sex White Black Hispanic Men a: 124 (13.2); b: 135 (14.4) a: 44 (4.7); b: 41 (4.4) a: 39 (4.2); b: 31 (3.3) Women a: 269 (13.6); b: 261 (13.2) a: 76 (3.8); b: 77 (3.9) a: 104 (5.3); b: 102 (5.2) Dropout (%): Sex White Black Hispanic Men a: 32.6; b: 32.2 a: 22.8; b: 29.3 a: 31.6; b: 38.0 Women a: 33.1; b: 31.5 a: 31.5; b: 35.8 a: 36.2; b: 33.8 No. assessed, (%): Sex White Black Hispanic Men a: 124 (13.2); b: 135 (14.4) a: 44 (4.7); b: 41 (4.4) a: 39 (4.2); b: 31 (3.3) Women a: 269 (13.6); b: 261 (13.2) a: 76 (3.8); b: 77 (3.9) a: 104 (5.3); b: 102 (5.2) |
Sex | White | Black | Hispanic | Men | a: 184 (18.9); b: 199 (20.7) | a: 57 (5.9); b: 58 (6.0) | a: 57 (5.9); b: 50 (5.2) | Women | a: 402 (41.3); b: 381 (39.6) | a: 111 (11.4); b: 120 (12.5) | a: 163 (16.7); b: 154 (16.0) | Sex | White | Black | Hispanic | Men | a: 124 (13.2); b: 135 (14.4) | a: 44 (4.7); b: 41 (4.4) | a: 39 (4.2); b: 31 (3.3) | Women | a: 269 (13.6); b: 261 (13.2) | a: 76 (3.8); b: 77 (3.9) | a: 104 (5.3); b: 102 (5.2) | Sex | White | Black | Hispanic | Men | a: 32.6; b: 32.2 | a: 22.8; b: 29.3 | a: 31.6; b: 38.0 | Women | a: 33.1; b: 31.5 | a: 31.5; b: 35.8 | a: 36.2; b: 33.8 | Sex | White | Black | Hispanic | Men | a: 124 (13.2); b: 135 (14.4) | a: 44 (4.7); b: 41 (4.4) | a: 39 (4.2); b: 31 (3.3) | Women | a: 269 (13.6); b: 261 (13.2) | a: 76 (3.8); b: 77 (3.9) | a: 104 (5.3); b: 102 (5.2) | Length of follow-up: 30 months Outcomes by sex: Weight loss |
Main Diabetes Prevention Program trial included standard lifestyle + metformin 850 mg twice daily treatment arm Methods detailed in other publications136,137 |
||||||||||||
| Age (years) | White | Black | Hispanic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 40 | a: 20 (10.9); b: 19 (9.6) | a: 6 (10.5); b: 10 (17.2) | a: 2 (3.5); b: 6 (12.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 40–49 | a: 43 (23.4); b: 44 (22.1) | a: 18 (31.6); b: 20 (34.5) | a: 21 (36.8); b: 10 (20.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50–59 | a: 55 (29.9); b: 54 (27.1) | a: 19 (33.3); b: 19 (32.8) | a: 23 (40.4); b: 19 (38.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 60+ | a: 66 (35.9); b: 82 (41.2) | a: 14 (24.6); b: 9 (15.5) | a: 11 (19.3); b: 15 (30.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 40 | a: 51 (12.7); b: 65 (17.1) | a: 26 (23.4); b: 27 (22.5) | a: 33 (20.3); b: 32 (20.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 40–49 | a: 163 (40.6); b: 138 (36.2) | a: 48 (43.2); b: 47 (39.2) | a: 61 (37.4); b: 66 (42.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50–59 | a: 113 (28.1); b: 107 (28.1) | a: 29 (26.1); b: 29 (24.2) | a: 52 (31.9); b: 30 (19.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 60+ | a: 75 (18.7); b: 71 (18.6) | a: 8 (7.2); b: 17 (14.2) | a: 17 (10.4); b: 26 (16.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | White | Black | Hispanic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | a: 102.8 (18.9); b: 100.5 (20.1) | a: 93.0 (18.5); b: 95.7 (17.9) | a: 100.5 (17.8); b: 104.4 (22.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | a: 93.7 (20.3); b: 95.1 (21.2) | a: 85.2 (19.1); b: 82.0 (14.8) | a: 98.9 (20.2); b: 97.1 (20.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (kg/m2) | White | Black | Hispanic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 30 | a: 73 (39.7); b: 92 (46.2) | a: 23 (40.4); b: 25 (43.1) | a: 20 (35.1); b: 15 (30.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30–34.99 | a: 60 (32.6); b: 63 (31.7) | a: 21 (36.8); b: 17 (29.3) | a: 20 (35.1); b: 21 (42.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35+ | a: 51 (27.7); b: 44 (22.1 | a: 13 (22.8); b: 16 (27.6) | a: 17 (29.8); b: 14 (28.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| < 30 | a: 111 (27.6); b: 100 (26.3) | a: 32/28.8); b: 32/26.7) | a: 32 (19.6); b: 37 (24.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30–34.99 | a: 104 (25.9); b: 101 (26.5) | a: 39/35.1); b: 50/41.7) | a: 35 (21.5); b: 46 (29.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35+ | a: 187 (46.5); b: 180 (47.2) | a: 40/36.0); b: 38 (31.7) | a: 96 (58.9); b: 71 (46.1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | White | Black | Hispanic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | a: 184 (18.9); b: 199 (20.7) | a: 57 (5.9); b: 58 (6.0) | a: 57 (5.9); b: 50 (5.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | a: 402 (41.3); b: 381 (39.6) | a: 111 (11.4); b: 120 (12.5) | a: 163 (16.7); b: 154 (16.0) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | White | Black | Hispanic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | a: 124 (13.2); b: 135 (14.4) | a: 44 (4.7); b: 41 (4.4) | a: 39 (4.2); b: 31 (3.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | a: 269 (13.6); b: 261 (13.2) | a: 76 (3.8); b: 77 (3.9) | a: 104 (5.3); b: 102 (5.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | White | Black | Hispanic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | a: 32.6; b: 32.2 | a: 22.8; b: 29.3 | a: 31.6; b: 38.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | a: 33.1; b: 31.5 | a: 31.5; b: 35.8 | a: 36.2; b: 33.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex | White | Black | Hispanic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Men | a: 124 (13.2); b: 135 (14.4) | a: 44 (4.7); b: 41 (4.4) | a: 39 (4.2); b: 31 (3.3) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Women | a: 269 (13.6); b: 261 (13.2) | a: 76 (3.8); b: 77 (3.9) | a: 104 (5.3); b: 102 (5.2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wing 1991112 | Location: One university, USA Period of study: NR Inclusion criteria: Age 30–65 years, > 20% over ideal body weight, type 2 diabetes, i.e. fasting glucose ≥ 140 mg/dl or ≥ 200 mg/dl 2 hours after oral glucose load, spouses ≥ 15% above ideal body weight and age 30–70 years, US$150 deposit per couple, which could be earned back in full Exclusion criteria: NR Age (years), mean (SD): Men + women: a: 51.2 (7.3); b: 53.6 (7.7) BMI (kg/m2), mean (SD): Men + women: a: 36.64 (5.77); b: 35.68 (5.76) Baseline comparability: Yes |
Description of interventions: All participants received behavioural weight-loss programme consisting of stimulus control, problem solving, assertion, goal setting and cognitive techniques; participants advised to monitor calorie intake to between 1200 and 1500 kcal per day with a reduction in fat intake and simple carbohydrates and increase in complex carbohydrates and fibre; stepwise goals for walking with final goal to expend 1000 kcal per week; deposit refunded according to weight loss and attendance a: Alone: Participants attended the programme alone. Spouses were not permitted to attend but attended assessment sessions after 20 weeks and at 1-year follow-up. Deposit refund contingent on participant’s weight loss and participant/spouse attendance at assessments b: Together: Spouse participated in all aspects of the programme and no distinction was made in treatment between the participant and the spouse; half of therapy sessions focused on social support and behavioural marital therapy literature, e.g. mutual positive reinforcement. Deposit refunds contingent on participant and spouse weight loss and attendance at assessments Timing of active intervention: 72 weeks No. of times contacted: a + b: 21 No. allocated: Men + women: a: 25; b: 24 No. completed: Men: a: 10, b: 8; women a: 13; b: 12 Dropout (%): Overall patients 12.3; spouses 13.3 No. assessed: Men: a: 10, b: 8; women: a: 13, b: 12 |
Length of follow-up: 72 weeks Outcomes by sex: Weight (participants only) |
All type 2 diabetes, obese spouse | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wing 1994113 | Location: University of Pittsburgh, PA, USA Period of study: Prior to November 1993 Inclusion criteria: Either sex, age 30–70 years, > 30% or > 18 kg over ideal body weight (based on Metropolitan Life Insurance tables), on-insulin-dependent diabetes (criteria according to National Diabetes Data Group) Exclusion criteria: Health problems that would interfere with the use of very low-calorie diets Age (years), mean (SD): Men + women: 51.8 (9.6) BMI (kg/m2), mean (SD): Men + women: 37.9 (6.3) Baseline comparability: Yes |
Description of interventions: a: 1000–1200 kcal per day consisting of < 30% energy intake from fat from baseline to week 50 b: 500 kcal per day either as liquid supplement (Optifast 70; Sandoz Nutrition, Minneapolis, MN, USA) or lean meat, fish or fowl for weeks 0–12 and weeks 24–36; other foods gradually reintroduced over following 4 weeks to consume 1000–1200 kcal per day at weeks 13–23 and weeks 37–50 a + b: All participants kept self-monitoring records, which were reviewed at weekly group meetings. Meetings also included detailed discussion on nutrition, which included focusing on reducing fat content and increasing intake of complex carbohydrate and fibre, and exercise, which stressed walking or behavioural techniques, including stimulus control, goal setting and self-monitoring of intake and exercise, preplanning, relapse prevention and modifying cognitions. Also included role playing and individual discussion and questions. All participants were encouraged to increase walking to 2 miles per day for 5 days per week. All participants kept 3-day food diaries at baseline, 6 months and 12 months. All diabetes medications were discontinued at the start and an algorithm was used to determine if and when to restart medication. All participants were given vitamin/mineral supplements throughout the study. All participants deposited US$150, which was refunded in full for reaching behavioural goals and attending assessments at baseline, 6 months and 50 weeks Timing of active intervention: a + b: 50 weeks plus follow-up 1 year later No. of times contacted: a: 52; b: 78 No. allocated: Men: a: 18, b: 15; women: a: 30, b: 30 Completed at 2 years: Men + women a: 38; b: 36 Dropout (%): Men + women a: 20.8; b: 20 Number assessed at 1 year: Men + women: a: 41; b: 38 |
Length of follow-up: 2 years Outcomes by sex: Weight change |
Baseline weight by sex not reported. Denominator by sex not reported at 1-year follow-up. Weight not reported by sex at 2 years | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
One trial105 solely investigated interventions for weight maintenance, comparing the effectiveness of orlistat with the effectiveness of placebo treatment. The period of follow-up ranged from 1 to 6 years (median 2 years). A trial published in 198498 included a weight-maintenance component in a subset of participants following a weight reduction phase. Both phases of this trial investigated the effect of financial contracts on weight loss and maintenance.
Of the six reports linked to RCTs, five were identified as ancillary studies to main trials. These included two studies examining spousal effects,120,121 one study examining the effects of weight loss on erectile function in a subset of male participants,122 one study investigating differences in body image between men and women123 and one study investigating the effects of weight-loss interventions on bone mineral density. 124 One report provided additional data for risk factors not included in the main trial report. 125
Characteristics of the men and women
Of the included reports we identified three large trials of weight-loss interventions for the prevention or treatment of type 2 diabetes: the Diabetes Prevention Program (DPP);111 the Finnish Diabetes Prevention Study (FDPS)104 and the Look AHEAD (Action for Health in Diabetes) trial. 110 A further five trials targeted participants with type 2 diabetes97,102,108,112,113 and two included some people with diabetes or impaired glucose tolerance. 105,107 In total, 12,934 men and women were enrolled in the trials, with the mean age of participants ranging from 37 to 59 years (median 55 years). The highest reported BMI was 42.7 kg/m2 for men95 and 43.6 kg/m2 for women,95 whereas the lowest was 29.7 kg/m2 104 and 30.53 kg/m2 98 respectively. Only nine trials reported BMI by sex at baseline. In eight studies women had a higher BMI than men,95,96,102,104,106,108,110,111 and in one study BMI was higher in men. 98
Attrition in men compared with women
Eight trials95,96,98,100,102,103,106,111 provided data that could be included in the analysis comparing the numbers of men and women who completed the trials. In total, there were 3813 participants; 1197 were men and 2616 were women ( Tables 11 and 12 ). The results shows that men were 11% (95% CI 8% to 14%, p < 0.001) more likely to complete the trial than women, suggesting men are highly motivated once commencing weight loss.
| Study ID | % men recruited | No. randomised | No. completed | ||
|---|---|---|---|---|---|
| Men | Women | Men | Women | ||
| Hakala 199395 | 33.3 | 20 | 40 | 18 | 35 |
| Hakala 199496 | 30.0 | 18 | 42 | 13 | 30 |
| Jeffery 198498 | 48.7 | 55 | 58 | 53 | 55 |
| Lantz 2003103 | 25.8 | 86 | 248 | 35 | 82 |
| Korhonen 1987102 | 50.0 | 40 | 40 | 38 | 33 |
| Jolly 2011100 | 30.7 | 227 | 513 | 162 | 182 |
| Ross 2012106 | 29.8 | 146 | 344 | 121 | 275 |
| West 2008111 | 31.3 | 605 | 1331 | 416 | 889 |
| Total | 31.4 | 1197 | 2616 | 856 | 1581 |
| Sex | Completed study | Did not complete study | Total | Proportion completing |
|---|---|---|---|---|
| Male | 856 | 341 | 1197 | 0.72 |
| Female | 1581 | 1035 | 2616 | 0.60 |
| Total | 2437 | 1376 | 3813 | 0.64 |
| Difference in proportion between men and women (95% CI) | 0.11 (0.08 to 0.14), p < 0.001 | |||
Overview of types of outcomes reported
Quantitative outcomes
All trials reported either baseline and end weights or changes in weight by sex except for the FDPS,104 which reported incidence of diabetes by sex. Two studies reported waist circumference by sex106,109 and two reported BMI by sex. 106,108 Cardiovascular risk factors were reported by sex in five trials: two reported HbA1c,97,108 three reported systolic and diastolic blood pressure,101,106,109 three reported triglycerides106,108,109 and three reported total and HDL cholesterol. 106,108,109 LDL cholesterol was reported by Volpe and colleagues109 and Ross and colleagues106 and fasting plasma glucose was reported by Vanninen and colleagues108 and Ross and colleagues. 106
Process outcomes
Hakala96 evaluated inpatient rehabilitation and health centre weight-loss programmes. The only reported difference by sex was that men in the health centre group were more likely to find GP appointments useful than women (63% of men vs. 23% of women). Both programmes met the expectations of participants. Participants in the rehabilitation group considered counselling by a nutritionist, physiotherapist and physician to be necessary and counselling from a social worker to be useful, although 62% wanted more individual counselling from the nutritionist. The majority (72%) of the rehabilitation participants felt that the 3-week inpatient period was satisfactory. The remaining 28% felt that it was too short. In comparison, the majority (72%) of the health centre participants felt the longer 10-week course was too short and 77% stated that they wanted more individual counselling with a greater emphasis on practical physical activity and dietary advice. No data were provided by sex for process outcomes.
Similarly, all nurses delivering the 6-week weight reduction programme in the trial by Karvetti and Hakala101 and the majority (58%) of the participants responding to the evaluation assessment (86% response rate) felt that the weight-loss programme in this trial was too short. Most nurses suggested that the programme should be extended to 10 weeks. The programme met the expectations of 66% of the participants. The only reported difference by sex was that most men (80%) and just under half of the women (42%) felt that six weight assessments during the intervention year were adequate. The remainder would have preferred more frequent assessment. Group support was also reported as being important for successful weight reduction.
Of the GPs participating in the trial conducted by Hakala,96 65% responded to the evaluation exercise. Only 41% considered their role as a GP to be suitable for the follow-up of obese participants, with 47% feeling that they were not suitable. Many felt that the task was uninteresting and useless, with only 35% showing that they were motivated to support participants in their weight reduction efforts. Similarly, the authors of the Lighten Up trial100 noted that GPs may have less faith in their ability to produce positive weight change in obese participants. Participants in this trial also noted that difficulty arranging regular appointment times with their general practice contributed to their failure to complete the full programme. Commercial companies, on the other hand, were able to offer weekly meetings at the same time each week. Neither trial reported differences by sex.
Quality of the evidence
Risk of bias
The risk of bias assessment for the individual trials is shown in Appendix 10 (see Table 58 ). Figure 11 summarises the assessment.
FIGURE 11.
Summary of risk of bias assessment of trials included in the systematic review of men and women.
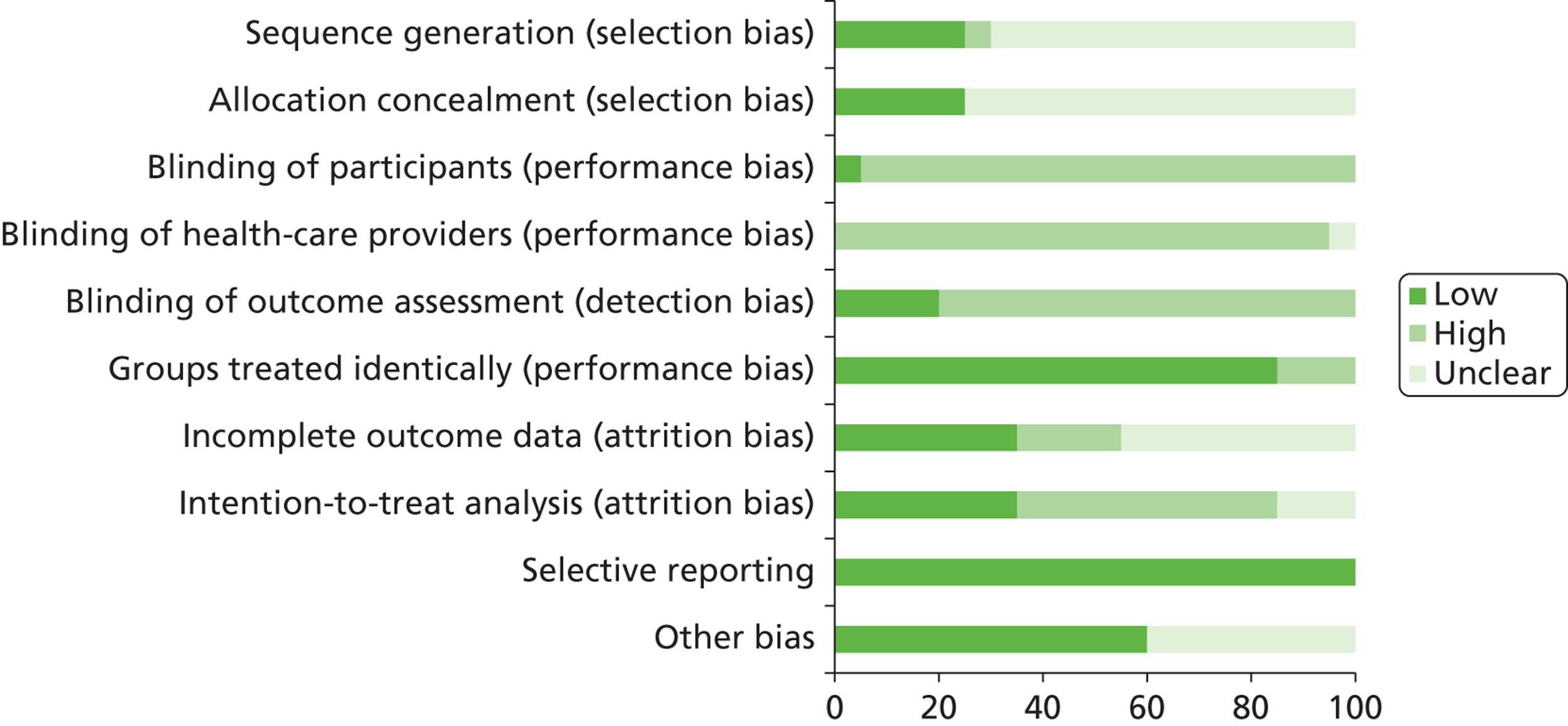
Five trials99,100,104 – 106 were judged to have adequate sequence generation but this was unclear for the majority of trials (14/20, 70%). Only one trial97 was judged as having a high risk for this item. Similarly, only five trials97,99,100,104,106 demonstrated successful allocation concealment and were considered at low risk for selection bias. Richelsen and colleagues105 compared orlistat therapy with placebo for weight maintenance; therefore, blinding was possible in this trial, although it was unclear whether or not those administering treatment were blinded to the nature of the interventions and whether participants were unblinded by gastrointestinal effects or not. Blinding of participants or health-care providers was not possible for any of the remaining trials. Four trials100,104,107,111 carried out blinding of outcome assessors. This was unclear for the remaining trials.
In three trials, groups were treated differently apart from the interventions received. For the trial conducted by Shai and colleagues,107 fewer men in the low-fat diet group participated in the ancillary substudy; in the DPP trial111 the standard lifestyle group received a placebo tablet whereas the intensive lifestyle group received no tablet; and in the trial conducted by Wing and colleagues113 the very low-calorie diet group received physician monitoring, which was not given to the low calorie diet group. Although only four trials95,96,101,111 were judged as being at high risk of attrition bias because of incomplete outcome data, the risk of bias for this item was unclear in just under half of all trial reports (9/20; 45%). 94,97 – 99,103,105,108,109,113 Only seven trials94,99,100,104,106,107,110 carried out a full intention-to-treat analysis and for three trials97,98,109 the method of analysis was unclear. The remaining trials analysed data for trial completers/compliers only. All trials were considered to be at low risk for selective reporting and only two trials were considered to be at unclear risk of other bias. Jeffery and colleagues98 enrolled men from a previous weight-loss trial conducted by the same authors. Gorin and colleagues94 provided free exercise equipment for use in the home and reimbursed participants in the intervention group for home delivery of grocery items; the control group received no similar ‘incentives’ or additional reason to feel obligated to complete the trial.
Assessment of equity and sustainability
Trial-level results are detailed in Appendix 10 (see Table 59 ). Figure 12 summarises the equity assessment.
FIGURE 12.
Summary of equity assessment of trials included in the systematic review of men and women.
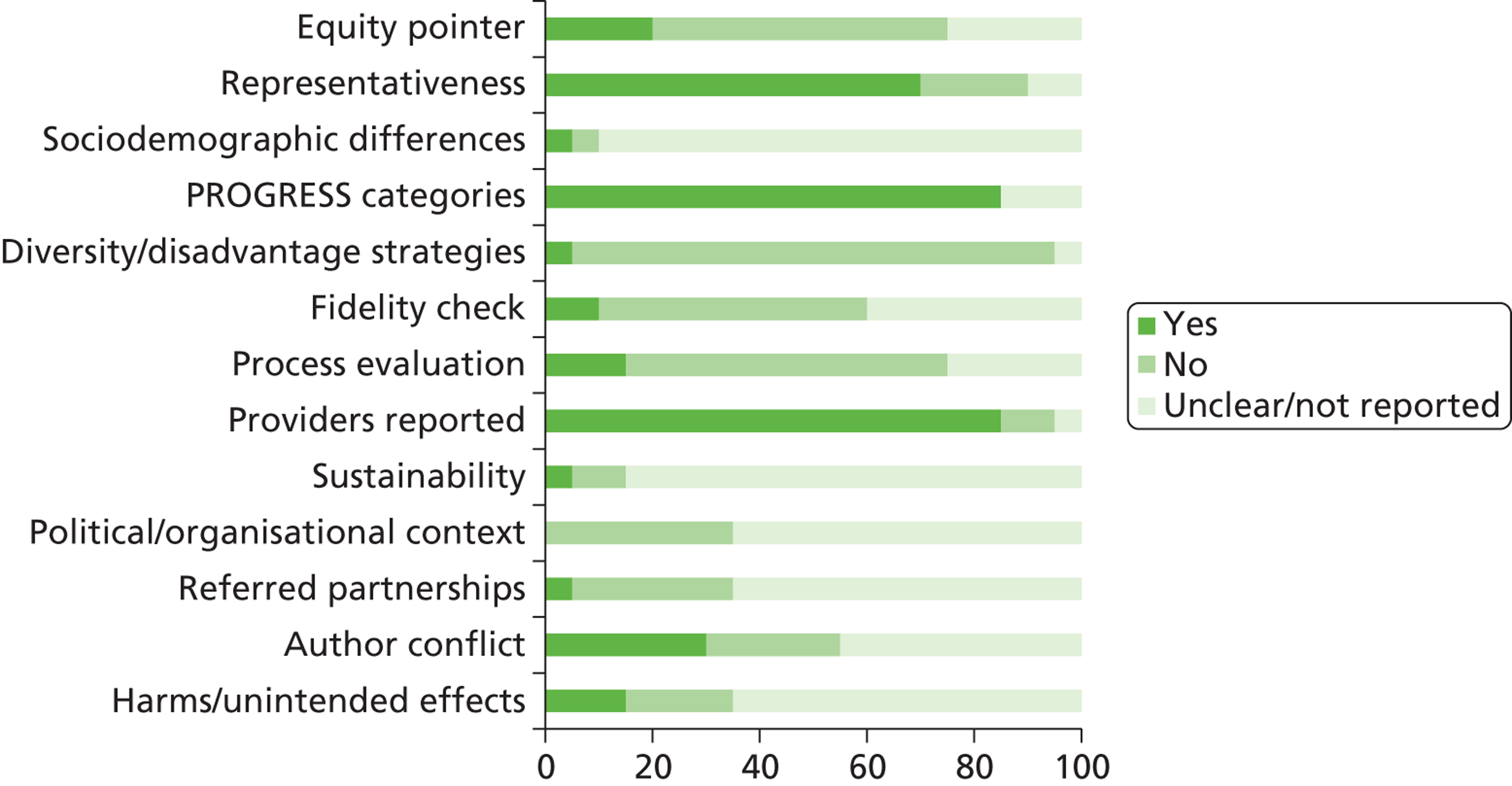
As with our review of men-only RCTs, the majority of equity items were not considered, were unclear or were not reported by the trial authors. The trials mainly did not report on diversity, sustainability or political context and did not describe partnerships. The majority of trials included a representative participant spectrum with the exception of those conducted by Karvetti and Hakala,101 Jeffery and colleagues,98 Volpe and colleagues,109 and Gorin and colleagues,94 but none reported sociodemographic differences between participants withdrawing or excluded and those continuing. Four trials were considered to have occurred in settings that could have excluded specific population groups. The trials conducted by Jeffery and colleagues98 and Wing and colleagues112 both required financial deposits at trial entry, thus potentially excluding less affluent participants. Similarly, Jeffery and colleagues99 recruited participants with private health-care insurance only. Shai and colleagues107 conducted their trial in a work setting with on-site medical clinic facilities. Only two trials100,110 carried out fidelity checks. Three trials reported adverse harms. 100,105,106 As discussed earlier, only three trials evaluated process outcomes. 96,100,101 None of the trial authors were considered to have conflicting interests although this was unclear for five reports. 95,101,103,105,106
Assessment of effectiveness
An exercise programme compared with a low-fat reducing diet
Volpe and colleagues109 compared a supervised exercise programme, a low-fat reducing diet, and a supervised exercise programme plus a low-fat reducing diet. The goal was for participants to lose 0.5–1.0 kg per week, although it is unclear whether this related to the dietary prescription alone or also took account of the exercise programme. By 12 months the exercise group and the diet group had gained weight. Men in the exercise group gained more weight than men in the diet group whereas the reverse was true for women ( Figure 13 ).
FIGURE 13.
Effect of an exercise programme vs. a low-fat reducing diet on weight change in men and women.
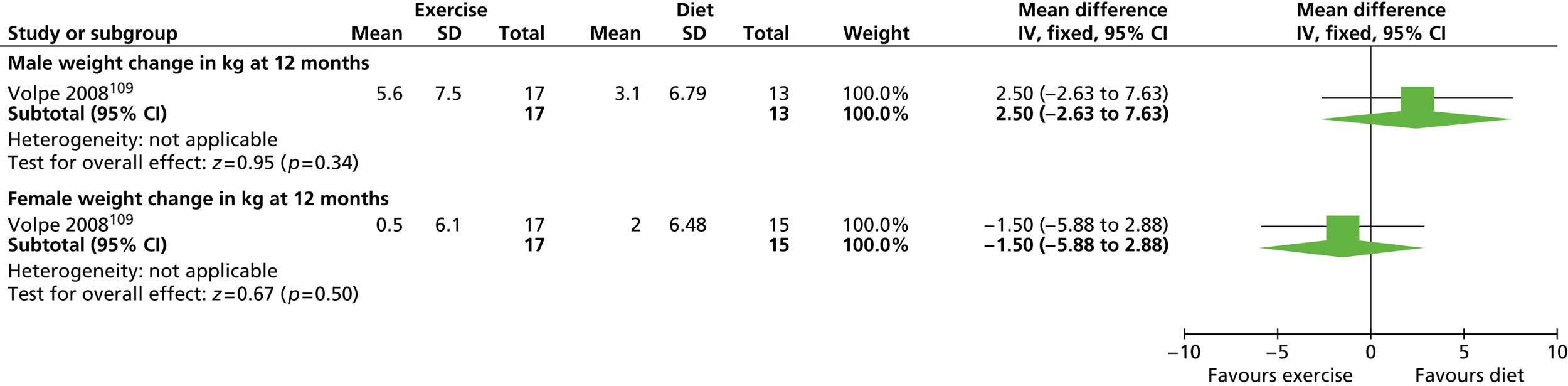
At 1 year, all groups showed little difference in changes to waist circumference. All groups showed a large deterioration in HDL cholesterol and an increase in LDL cholesterol. Similarly, all groups showed increases in triglycerides, with the exception of men in the diet group in which levels were lowered, although changes were more modest. Both men and women in the diet group and men in the exercise group lowered their systolic and diastolic blood pressure, with men showing greater decreases than women. Both types of blood pressure were raised for women in the exercise group, especially diastolic blood pressure. The difference in diastolic pressure between women in the diet group and women in the exercise group was significant after 1 year (p = 0.01) ( Table 13 ).
| Outcome | Exercise programme | Low-fat reducing diet | ||
|---|---|---|---|---|
| Men (n = 17) | Women (n = 17) | Men (n = 13) | Women (n = 15) | |
| Waist circumference (cm) | –1.2 | –3.9 | –0.1 | +0.9 |
| Total cholesterol (mmol/l) | –0.18 | +0.03 | +0.01 | +0.26 |
| LDL cholesterol (mmol/l) | +0.37 | +0.15 | +0.21 | +0.44 |
| HDL cholesterol (mmol/l) | –0.18 | –0.13 | –0.19 | –0.24 |
| Triglycerides (mmol/l) | +0.07 | +0.03 | –0.06 | +0.01 |
| Systolic blood pressure (mmHg) | –14.4 | +3.0 | –13.3 | –1.5 |
| Diastolic blood pressure (mmHg)a | –7.9 | +6.3a | –9.5 | –2.6 |
A low-fat reducing diet plus an exercise programme compared with a low-fat reducing diet
In the same trial109 a combination of a low-fat reducing diet and an exercise programme was compared with the same diet but without the exercise programme. At 12 months, men in the diet and exercise programme group gained less weight than men in the diet group. The protective effect of diet and exercise was repeated for the women ( Figure 14 ).
FIGURE 14.
Effect of a low-fat reducing diet plus an exercise programme vs. a low-fat reducing diet on weight change in men and women.
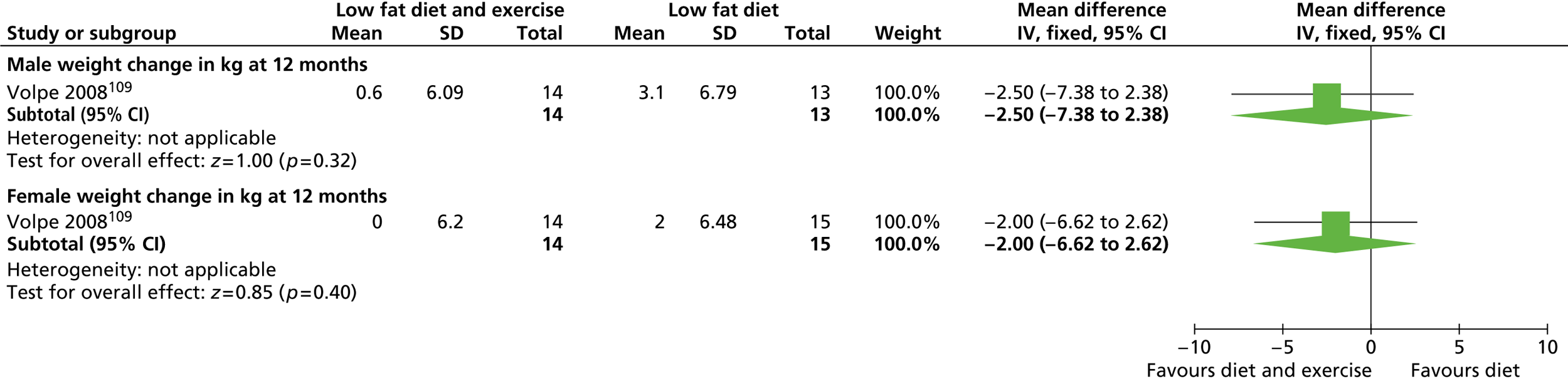
The low-fat reducing diet and exercise programme resulted in modest reductions in waist circumference. Although both groups showed poorer LDL cholesterol levels, the diet and exercise group showed less deterioration than the diet group for both sexes. Women in the diet and exercise group and men in both groups reduced their triglyceride levels, with men in the combined group showing the greatest reduction. Men in both groups reduced their systolic and diastolic blood pressure with the greatest reduction shown in the diet and exercise group. Conversely, both diastolic and systolic blood pressure slightly increased in women in this group, whereas diastolic and systolic blood pressure slightly decreased in women in the diet group ( Table 14 ).
| Outcome | Low-fat reducing diet and exercise programme | Low-fat reducing diet | ||
|---|---|---|---|---|
| Men (n = 14) | Women (n = 14) | Men (n = 13) | Women (n = 15) | |
| Waist circumference (cm) | –1.9 | –2.1 | –0.1 | +0.9 |
| Total cholesterol (mmol/l) | –0.04 | –0.18 | +0.01 | +0.26 |
| LDL cholesterol (mmol/l) | +0.15 | +0.10 | +0.21 | +0.44 |
| HDL cholesterol (mmol/l) | –0.13 | –0.23 | –0.19 | –0.24 |
| Triglycerides (mmol/l) | –0.20 | –0.16 | –0.06 | +0.01 |
| Systolic blood pressure (mmHg) | –15.6 | +2.1 | –13.3 | –1.5 |
| Diastolic blood pressure (mmHg) | –12.3 | +1.2 | –9.5 | –2.6 |
A low-fat reducing diet plus an exercise programme compared with an exercise programme
The trial by Volpe and colleagues109 also compared diet plus exercise with exercise alone. The addition of a diet to exercise again provided greater benefits than exercise alone in terms of less weight gain for both sexes, although the difference was statistically significant for men only (p = 0.04) ( Figure 15 ).
FIGURE 15.
Effect of a low-fat reducing diet plus an exercise programme vs. an exercise programme on weight change in men and women.
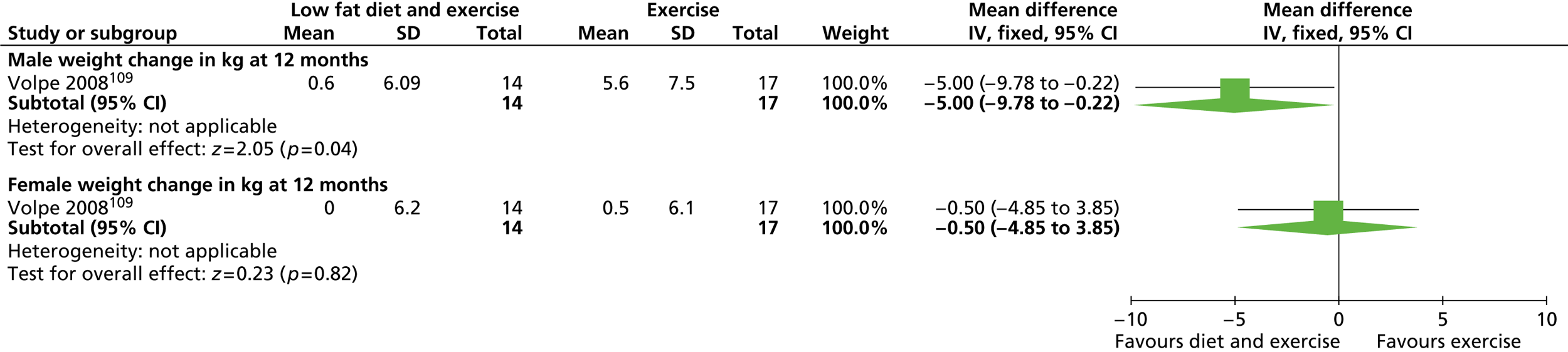
Modest reductions in waist circumference were also found for this comparison. LDL cholesterol levels in all groups worsened. The decrease in triglyceride levels for the diet and exercise group was not repeated in the exercise group, with both sexes showing increased levels. The combination of both diet and exercise produced the greatest reduction for this outcome. Again, only men benefited from a reduction in systolic and diastolic blood pressure, with the greatest reduction occurring in the combined intervention group. Both diastolic and systolic pressure were raised in women in both groups at 1 year ( Table 15 ).
| Outcome | Low-fat reducing diet and exercise programme | Exercise programme | ||
|---|---|---|---|---|
| Men (n = 14) | Women (n = 14) | Men (n = 17) | Women (n = 17) | |
| Waist circumference (cm) | –1.9 | –2.1 | –1.2 | –3.9 |
| Total cholesterol (mmol/l) | –0.04 | –0.18 | –0.18 | +0.03 |
| LDL cholesterol (mmol/l) | +0.15 | +0.10 | +0.37 | +0.15 |
| HDL cholesterol (mmol/l) | –0.13 | –0.23 | –0.18 | –0.13 |
| Triglycerides (mmol/l) | –0.20 | –0.16 | +0.07 | +0.03 |
| Systolic blood pressure (mmHg) | –15.6 | +2.1 | –14.4 | +3.0 |
| Diastolic blood pressure (mmHg) | –12.3 | +1.2 | –7.9 | +6.3 |
A low-fat reducing diet with exercise advice compared with control
Vanninen and colleagues108 investigated a low-fat reducing diet and exercise advice compared with basic conventional educational materials only. Details of the exact dietary prescription were not provided. All participants in this trial were non-insulin-dependent type 2 diabetics. After 1 year men in the intervention group had lost significantly more weight than men in the control group (p = 0.04). Women in the intervention group also lost more weight than women in the control group although the difference was not significant ( Figure 16 ). It should be noted that women had a higher mean BMI than men at baseline (34.2 and 33.4 kg/m2 for women vs. 30.1 and 31.1 kg/m2 for men for the control and intervention groups respectively).
FIGURE 16.
Effect of a low-fat reducing diet with exercise advice vs. control on weight change in men and women.
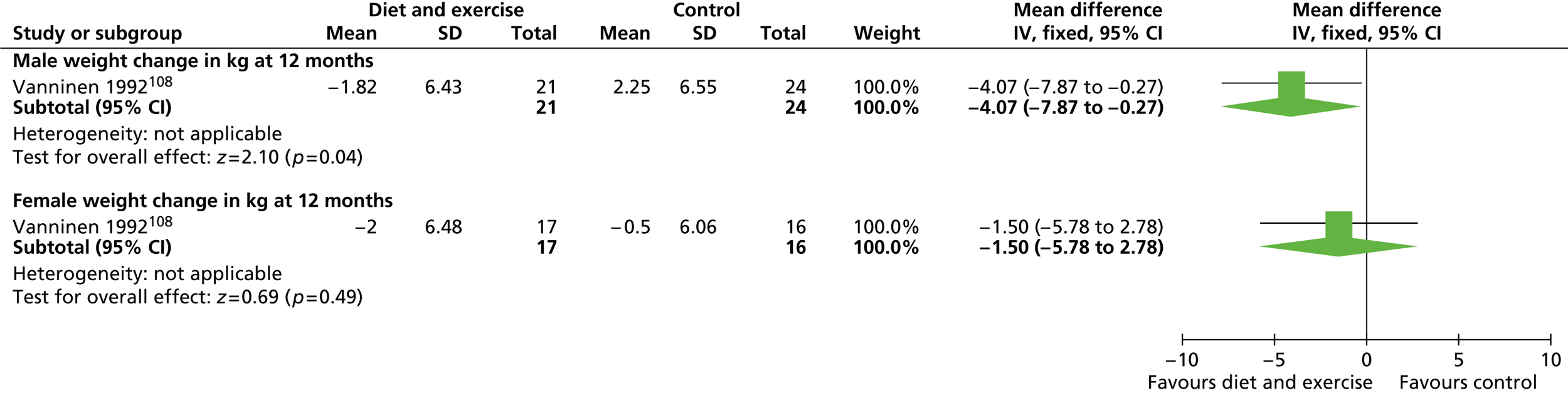
Men in the intervention group showed a greater improvement in total cholesterol, triglyceride and HbA1c levels than men in the control group, although the control group had a greater improvement in HDL cholesterol levels. Fasting plasma glucose levels increased for both groups of men but the control group saw the greatest increase. No significant differences between groups were reported for men ( Table 16 ).
| Outcome | Time (months) | Low-fat reducing diet and exercise advice | Control | ||
|---|---|---|---|---|---|
| Men (n = 21) | Women (n = 17) | Men (n = 24) | Women (n = 16) | ||
| Total cholesterol (mmol/l) | 12 | –0.3 | 0 | +0.1 | +0.2 |
| HDL cholesterol (mmol/l) | 12 | +0.11 | +0.12 | +0.4 | +0.04 |
| Triglycerides (mmol/l) | 12 | –0.9 | –0.1 | 0 | +0.2 |
| HbA1c (%) | 12 | –0.1 | –0.9 | +0.1 | –0.9 |
| Fasting plasma glucose (mmol/l) | 12 | +0.1 | –0.6 | +0.6 | –1.3 |
Women in the intervention group showed greater improvements than women in the control group for total cholesterol and triglycerides and also for HDL cholesterol. The control group showed a greater decrease in fasting plasma glucose levels, but the improvement in HbA1c was the same in both women’s groups. However, women in the control group had higher HbA1c and fasting plasma glucose levels than women in the intensified diet and exercise group at baseline (see Table 16 ). In a later report,125 the authors report a continuing reduction in BMI for the intervention groups at 15 months, with some reduction also seen in the control groups.
Diabetes incidence
The FDPS104 included participants at high risk of developing type 2 diabetes based on a classification of being overweight and having impaired glucose tolerance. Participants were randomised to receive an individually tailored low-fat reducing diet plus an exercise programme to achieve 5% weight loss or to receive general advice. After a median follow-up period of 4 years, the active intervention group showed favourable results in reducing the incidence of diabetes. The incidence rate was 8.6 (95% CI 5.8 to 12.6) per 100 person-years for men in the control group compared with 3.7 (95% CI 2.2 to 6.2) per 100 person-years in the intervention group. For women the incidence rate was 6.9 (95% CI 5.2 to 9.2) in the control group and 4.3 (95% CI 3.0 to 6.2) in the intervention group. The hazard ratio for diabetes incidence was 0.43 (95% CI 0.22 to 0.81) for men and 0.61 (95% CI 0.39 to 0.97) for women, with no statistically significant interaction between sex and intervention. Other risk factors were not reported by sex in this study.
A low-fat reducing diet plus behavioural therapy compared with control
Karvetti and Hakala101 investigated a low-fat reducing diet plus behavioural therapy delivered in the primary health-care setting. The dietary prescription of 1200 kcal per day for weight reduction and 1800 kcal per day for maintenance was not reported to differ by sex. Participants in the intervention group were supported by public health nurses with three lectures from a physician, a psychologist and a physiotherapist. Participants were also given encouragement and support. The control group received no instruction and was contacted for yearly assessment only. After 1 year both men (–11.80 kg, 95% CI –16.86 to –6.74 kg) and women (–5.60 kg, 95% CI –8.74 to –4.57 kg) in the intervention group had lost significantly more weight than men and women in the control group respectively ( Figure 17 ).
FIGURE 17.
Effect of a low-fat reducing diet plus behavioural therapy vs. control on weight change in men and women.
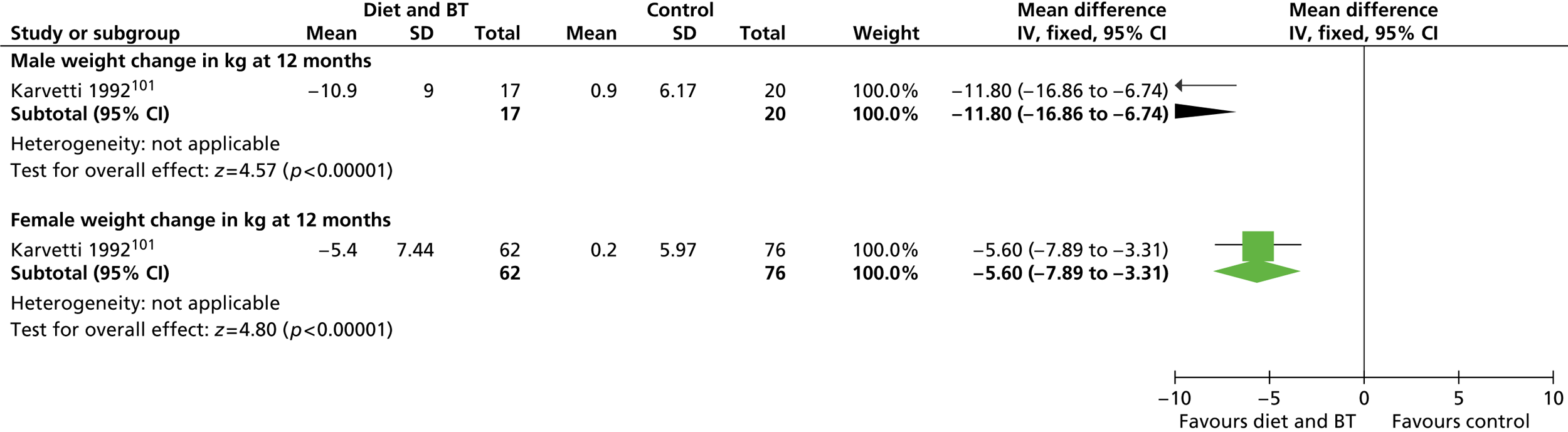
Women in the intervention group showed a significant reduction in systolic blood pressure compared with women in the control group (reported p < 0.05) ( Table 17 ). No other changes in risk factors were reported as being statistically significant between groups according to sex.
| Outcome | Diet and behavioural therapy | Control | ||
|---|---|---|---|---|
| Men (n = 22) | Women (n = 71) | Men (n = 20) | Women (n = 76) | |
| Total cholesterol (mmol/l) | +0.2 | +0.2 | +0.2 | +0.3 |
| HDL cholesterol (mmol/l) | +0.25 | +0.18 | +0.02 | +0.04 |
| Systolic blood pressure (mmHg) | 0 | –6.00a | –1.00 | 0 |
| Diastolic blood pressure (mmHg) | –7.00 | –6.00 | –5.00 | –3.00 |
A low-fat reducing diet plus exercise advice, behavioural therapy and home environment modification compared with a low-fat reducing diet plus exercise advice and behavioural therapy only
The trial by Gorin and colleagues94 randomised overweight and obese participants and an overweight household member, willing to act as a support partner, to a low-fat reducing diet with exercise advice and behavioural therapy or to the same treatment package but with modifications made to the home environment. Only participants received treatment in the standard programme whereas both participants and partners received treatment in the modified programme. Most participant–partner pairs were spouses or significant others (77.2%), with the remainder being participant–adult child (17.4%), participant–other relative (3.0%) and participant–roommate (2.5%) pairings. Modifications targeted physical and social cues in the home. At 18 months, women in the modified programme lost significantly more weight than women in the standard programme [–8.1 kg (SD 1.1 kg) vs. –4.2 kg (SD 1.1 kg), reported p = 0.014]. However, men in the standard programme lost more weight than men in the modified programme [–10.0 kg (SD 2.3 kg) vs. –4.6 kg (SD 2.2 kg)] although differences were not significant (reported p = 0.065). Partners in the modified programme lost more weight than partners in the standard programme at 18 months, regardless of sex. The authors reported that sex did not moderate weight regain for participants or partners, with men and women in both groups regaining weight at equivalent rates.
A low-fat reducing diet plus an exercise programme and behavioural therapy
Wing and colleagues112 randomised obese type 2 diabetic participants to receive a behavioural weight-loss programme either with their obese spouse (together) or without their spouse (alone). All participants received behavioural therapy consisting of stimulus control, problem solving, assertion, goal setting and cognitive techniques. Participants were also advised to monitor calorie intake to between 1200 and 1500 kcal per day and to set stepwise goals for walking. Participants in the together group attended with their spouses and both were targeted for weight loss. Participants in the alone group attended by themselves with spouses attending assessment sessions only. The weight loss of participants treated alone and together was not significantly different after 1 year, although men lost more weight when treated alone whereas women did better when treated together ( Table 18 ). There was no evidence that marital satisfaction affected weight loss. Spouses of both sexes lost more weight in the together group than in the alone group (p < 0.05) but there were no differences between treatment groups for change in calorie intake or exercise.
| Outcome | Together | Alone | ||
|---|---|---|---|---|
| Men (n = 8) | Women (n = 12) | Men (n = 10) | Women (n = 13) | |
| Weight loss at 1 year (kg) | –1.25 | –5.89 | –7.25 | –2.26 |
The Look AHEAD study110 recruited overweight or obese type 2 diabetics to a trial comparing an intensive lifestyle intervention comprising a low-fat reducing diet, some meal replacements and exercise advice and intensive behavioural therapy with diabetes support and education. The intensive lifestyle intervention was designed to produce a minimum weight loss of 7% of initial body weight during the first year, with dietary instructions tailored to initial body weight.
Wadden and colleagues110 reported weight data by sex for the active intervention group. The men in this group consistently lost more weight than the women at each annual assessment up to 4 years’ follow-up ( Table 19 ). The prescribed calorie intake was based on weight but it is not clear whether or not the calorie intake also took account of sex. Attendance and treatment contacts were similar for men and women in the first 4 years.
| Follow-up period (year) | Men (n = 1044) | Women (n = 1526) |
|---|---|---|
| 1 | –9.3 | –8.1 |
| 2 | –7.1 | –5.9 |
| 3 | –5.9 | –4.6 |
| 4 | –5.2 | –4.4 |
Effect on body image of a low-fat reducing diet with exercise advice and behavioural therapy compared with control
Several ancillary studies have reported sex effects in the Look AHEAD study. Stewart and colleagues123 investigated changes in body image in men and women. The authors recruited participants from one centre (Pennington Biomedical Research Centre, Los Angeles, CA, USA) and reported weight data by sex for both groups at 1 year. Women in this study had a higher baseline BMI than men in both treatment groups. Women in the intervention group had a slightly higher discrepancy between their current and their ideal body size (body dissatisfaction) than men (20.9 vs. 19.6), whereas men in the control group were slightly more dissatisfied than women with their body size (20.0 vs. 19.0). Both men and women in the intervention group lost significantly more weight than men and women in the control group respectively (reported p < 0.001) ( Figure 18 ). Both men and women in the intervention group showed a significant reduction in body image dissatisfaction compared with the control group after 1 year (p < 0.05 and p < 0.01 respectively). Men in both the intervention group and the control group showed a greater reduction in dissatisfaction than women [–8.1 (standard error 1.59) vs. –6.3 (standard error 0.94) for the intervention group and –3.3 (standard error 1.66) vs. –2.3 (standard error 0.96) for the control group].
FIGURE 18.
Effect of a low-fat reducing diet with exercise advice and behavioural therapy vs. control on weight change in men and women.
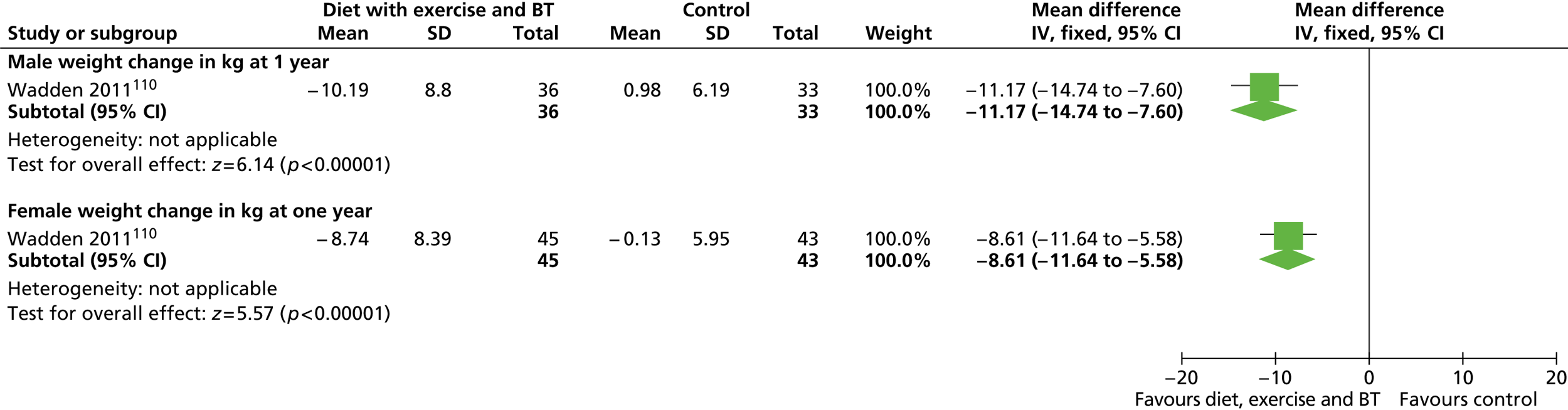
Effect on bone mineral density of a low-fat reducing diet with exercise advice and behavioural therapy compared with control
In a separate substudy Schwartz and colleagues124 investigated the effect of the weight-loss intervention on bone mineral density in participants from five of the Look AHEAD centres. Hip, spine and whole-body dual-energy X-ray absorptiometry scans were obtained for 237 men and 405 women in the intervention group and 246 men and 386 women in the control group. After 1 year, at the total hip the difference in bone loss between the two treatment groups was significantly greater for men (–1.48% intervention vs. 0.02% control) than for women (–1.44% intervention vs. –0.61% control, p = 0.04). The authors reported that there was no evidence of an interaction by sex at the other bone sites.
Effect on erectile dysfunction of a low-fat reducing diet with exercise advice and behavioural therapy compared with control
Wing and colleagues122 investigated the effect of weight loss on erectile function. Men with erectile dysfunction (n = 153 in the intervention group and n = 150 in the control group) were recruited from five of the 16 Look AHEAD centres. Sexual function was evaluated by self-reported completion of the IIEF. 126 The IIEF is a validated scale of erectile function, with five domains, with scores ≤ 10 denoting severe dysfunction, scores of 11–21 denoting moderate dysfunction, scores of 22–25 indicating mild dysfunction and scores ≥ 26 indicating no dysfunction. After 1 year men in the intervention group showed a greater weight change than men in the control group ( Figure 19 ).
FIGURE 19.
Effect of a low-fat reducing diet with exercise advice and behavioural therapy vs. control on weight change (kg) in men with erectile dysfunction.
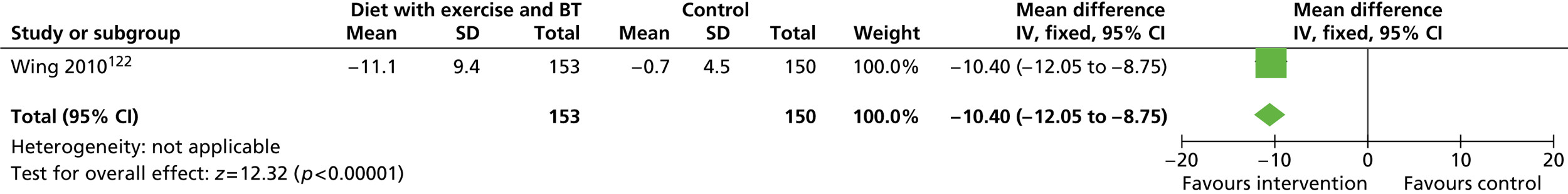
After adjusting for baseline differences in erectile function score, the authors reported an increase (improvement) in erectile function of 1.3 (SD 4.7) with the intensive lifestyle intervention at 1 year and 0.03 (SD 5.7) in the control group at 1 year (reported p = 0.06).
Using the cut-off values of ≤ 21 and ≥ 22 to denote worsening or improvement in erectile function, respectively, the greater weight loss achieved by the intervention group did not produce a significantly greater improvement in erectile function in men with moderate or severe dysfunction compared with the control group. For mild or no dysfunction, significantly fewer men in the intervention group than in the control group reported worsening of function (reported p < 0.001). The intervention group also showed significantly greater changes in HbA1c, systolic and diastolic blood pressure and HDL cholesterol (all reported p ≤ 0.01) ( Table 20 ).
| Outcome | Diet and exercise with behavioural therapy (n = 153) | Control (n = 153) |
|---|---|---|
| Total cholesterol (mmol/l) | –0.26 (n = 148) | –0.22 (n = 144) |
| LDL cholesterol (mmol/l) | –0.18 (n = 148) | –0.14 (n = 144) |
| HDL cholesterol (mmol/l)a | 0.09 (n = 148) | –0.03 (n = 144) |
| Systolic blood pressure (mmHg)a | –7.5 (SD 16.3) | –1.5 (SD 14.9) (n = 150) |
| Diastolic blood pressure (mmHg)a | –4.7 (SD 7.9) | –1.0 (SD 7.6) (n = 150 |
| HbA1c (%)a | –0.7 (SD 1.0) | –0.3 (SD 1.1) (n = 150) |
Effect on spouses of a low-fat reducing diet with exercise advice and behavioural therapy
Gorin and colleagues121 assessed the impact of the intervention and control treatments on the untreated spouses of the Look AHEAD participants from three sites. There were no specific eligibility requirements for spouses other than a willingness to participate in the research. Spouses were not formally involved in either treatment group and were not expected to attend group meetings. Participants in the active intervention group were taught ways to enhance social support to promote their weight-loss efforts (e.g. how to communicate assertively with family members about desired food modifications). Participants in the control group received no such training. As in the full trial, intensive lifestyle participants lost more weight than control participants during the first year [–9.9 kg (SD 7.6 kg) vs. –1.2 kg (SD 4.9 kg), reported p < 0.001].
After 1 year, spouses of the intensive lifestyle participants had a weight change of –2.4 kg (SD 4.5 kg) compared with –0.2 kg (SD 3.3 kg) for spouses of control participants. The authors reported no effect by sex or baseline weight of the spouse.
A low-fat reducing diet with an exercise programme and behavioural therapy compared with placebo
The DPP111 randomised individuals at high risk of diabetes to an intensive low-fat reducing diet with an exercise programme and behavioural therapy, metformin or placebo. For the purposes of this review, we present data for the intensive intervention and placebo groups only. The aim of the intensive lifestyle programme was to lose 7% of initial body weight and maintain this weight loss throughout the trial. The calorie goals were calculated based on initial weight loss and a deficit of 500–1000 kcal per day, together with an increase in physical activity equivalent to 700 kcal per week. By 30 months, both sexes had lost more weight in the intensive group than in the placebo group (reported p < 0.001).
The authors reported that, within the lifestyle treatment group, black women lost significantly less weight than all other race–sex groups (reported p < 0.01) with the exception of black men ( Table 21 ). A higher proportion of women than men were obese at baseline (74% vs. 59%) and a higher proportion of black people than Hispanic and white people were obese at baseline (74% vs. 67% and 68% respectively).
| Ethnic group | Low-fat reducing diet with exercise programme and behavioural therapy | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 year | 18 months | 2 years | 30 months | 1 year | 18 months | 2 years | 30 months | ||
| Men | White | –8.3 (7.9) (n = 192) | –7.4 (7.6) (n = 190) | –7.1 (7.7) (n = 182) | –5.7 (7.6) (n = 135) | –0.8 (4.7) (n = 177) | –0.6 (5.3) (n = 172) | –0.8 (5.8) (n = 169) | –0.7 (5.2) (n = 124) |
| Black | –7.6 (6.9) (n = 43) | –7.5 (8.0) (n = 40) | –6.2 (8.4) (n = 41) | –4.8 (3.5) (n = 31) | –0.1 (5.7) (n = 54) | –0.0 (5.0) (n = 50) | 0.1 (5.2) (n = 50) | 0.5 (5.2) (n = 39) | |
| Hispanic | –7.5 (6.1) (n = 54) | –6.6 (7.0) (n = 53) | –6.8 (6.4) (n = 53) | –6.2 (6.6) (n = 41) | 0.7 (3.5) (n = 55) | 1.0 (3.1) (n = 54) | 1.2 (3.3) (n = 54) | 1.2 (3.3) (n = 44) | |
| Women | White | –7.8 (7.4) (n = 367) | –6.6 (8.2) (n = 352) | –5.7 (8.7) (n = 344) | –4.2 (7.5) (n = 261) | –0.8 (5.2) (n = 385) | –0.5 (5.8) (n = 373) | –0.6 (6.4) (n = 359) | –0.9 (7.0) (n = 269) |
| Black | –4.4 (6.0) (n = 144) | –3.9 (6.1) (n = 136) | –3.2 (5.8) (n = 131) | –2.1 (6.3) (n = 102) | 0.2 (4.3) (n = 151) | 0.5 (5.6) (n = 146) | 0.7 (5.5) (n = 144) | 1.3 (5.3) (n = 104) | |
| Hispanic | –5.8 (6.1) (n = 111) | –6.2 (6.5) (n = 107) | –5.5 (6.9) (n = 106) | –5.1 (8.3) (n = 77) | –1.1 (4.4) (n = 101) | –0.5 (4.7) (n = 100) | 0.2 (4.0) (n = 95) | 0.7 (4.3) (n = 76) | |
Comparisons of different types of diet
Shai and colleagues107 investigated the effectiveness of a low-fat reducing diet (1500 kcal per day for women, 1800 kcal per day for men), a Mediterranean diet with equivalent calories and a low carbohydrate (20 g per day initially increasing to 120 g per day) non-restricted calorie diet in the Dietary Intervention Randomized Controlled Trial (DIRECT). At the end of the 2-year trial, the only significant difference was for women in the Mediterranean reducing diet group, who lost significantly more weight than women in the low-fat reducing diet group (p = 0.01) ( Figures 20–22 ).
FIGURE 20.
Effect of a low-carbohydrate diet vs. a low-fat reducing diet on weight change (kg) in men and women.
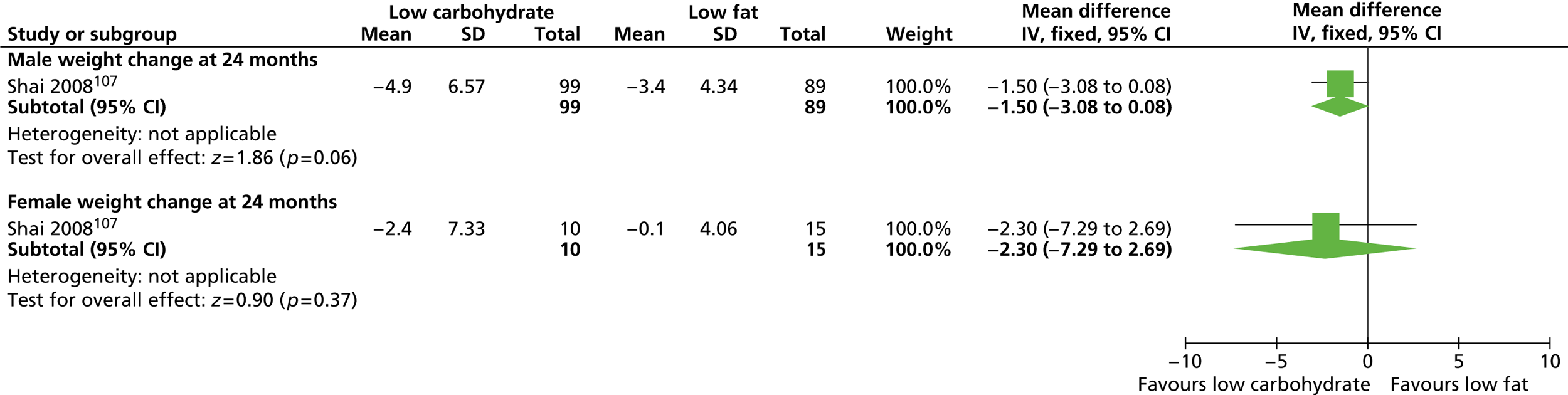
FIGURE 21.
Effect of a low-carbohydrate diet vs. a Mediterranean reducing diet on weight change (kg) in men and women.
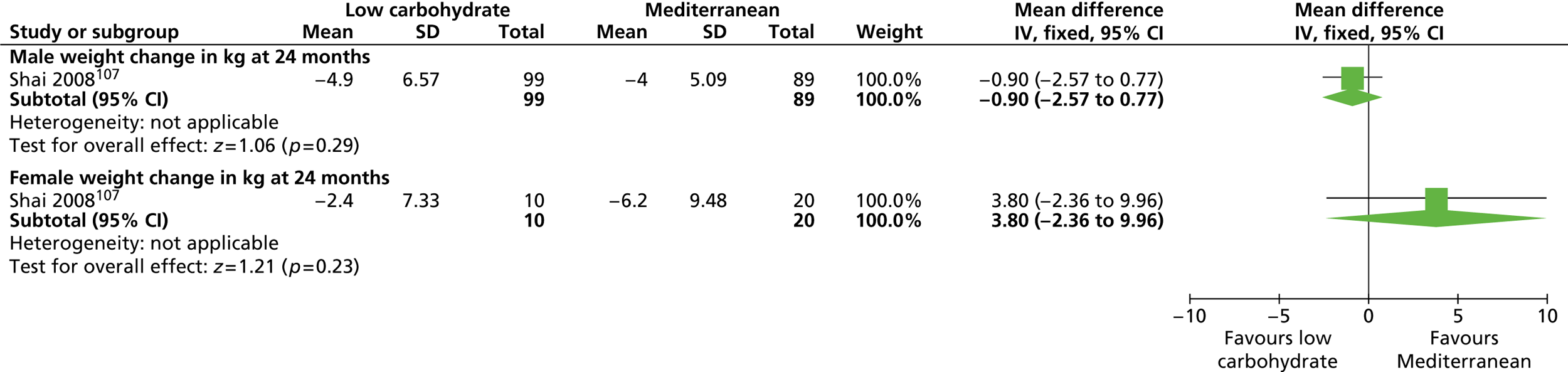
FIGURE 22.
Effect of a Mediterranean reducing diet vs. a low-fat reducing diet on weight change (kg) in men and women.
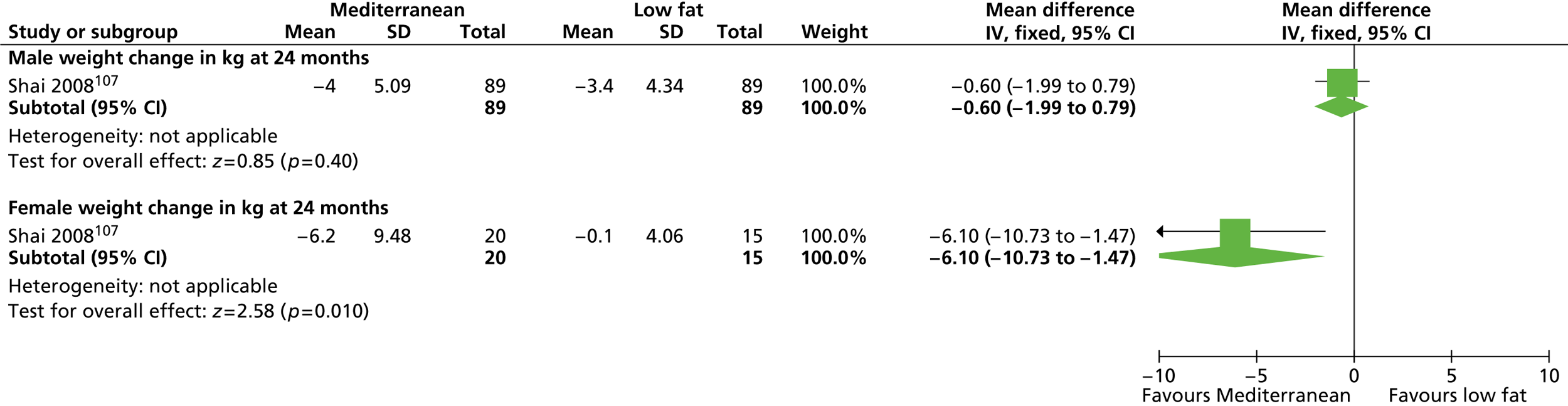
Effect on Dietary Intervention Randomized Controlled Trial wives
Golan and colleagues120 conducted a parallel study describing the effect of the DIRECT dietary interventions on 74 wives of men participating in the trial. The wives were not randomised to any treatment group but were invited to attend the 90-minute support group meetings held every 2 months for the DIRECT participants. The aim of the meetings was to update the wives about the principles of the diet strategy to which their husbands had been randomised rather than treating the wives directly. At the end of the trial, men whose wives had attended support meetings lost more weight than men who did not have spousal support, both as an entire group and within each diet group ( Figure 23 ).
FIGURE 23.
Effect of spousal support on weight change (kg) in DIRECT husbands at 2 years.
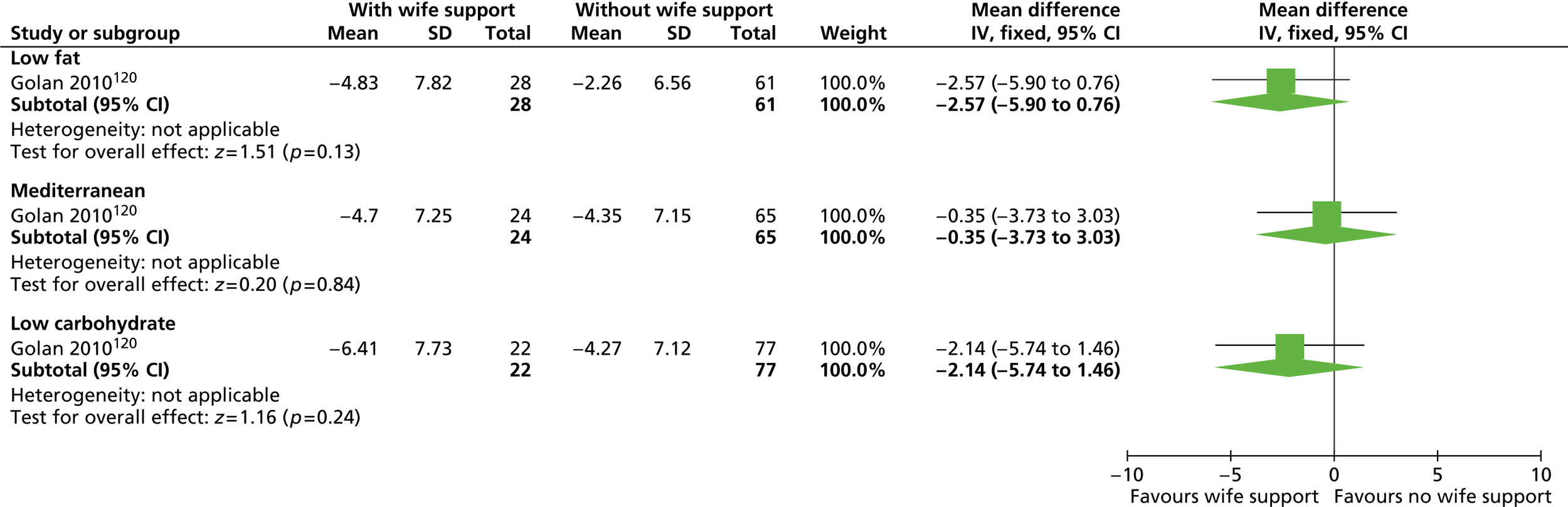
Golan and colleagues120 investigated whether or not the intervention had any indirect influence on the DIRECT wives, termed ‘halo’ effects by the authors ( Figures 24–26 ). Differences in weight loss between groups were statistically significant between the low-carbohydrate diet and low-fat reducing diet groups only (reported p < 0.05).
FIGURE 24.
Effect of a low-carbohydrate diet vs. a low-fat reducing diet on weight change (kg) in DIRECT wives.
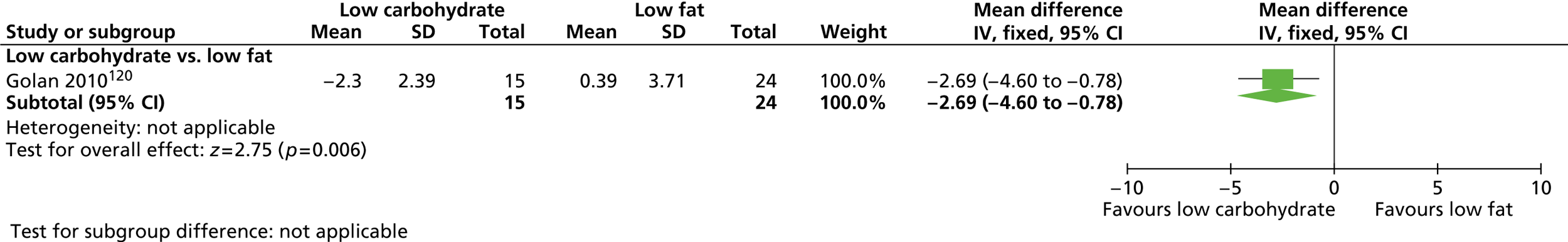
FIGURE 25.
Effect of a Mediterranean reducing diet vs. a low-carbohydrate diet on weight change (kg) in DIRECT wives.
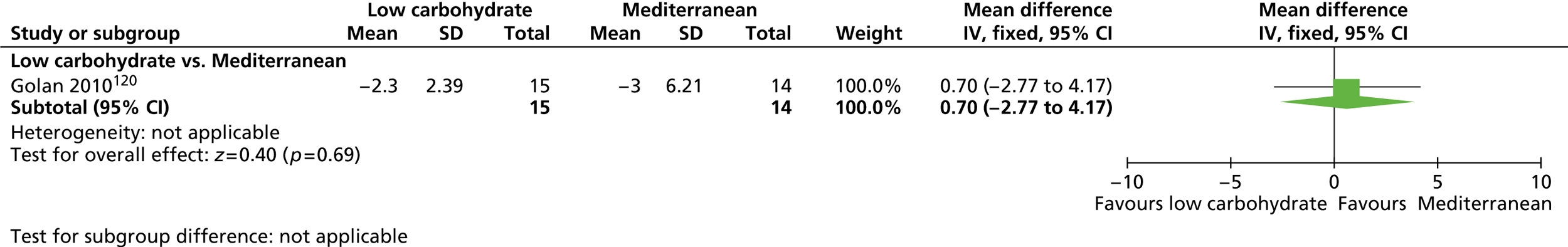
FIGURE 26.
Effect of a Mediterranean reducing diet vs. a low-fat reducing diet on weight change (kg) in DIRECT wives.
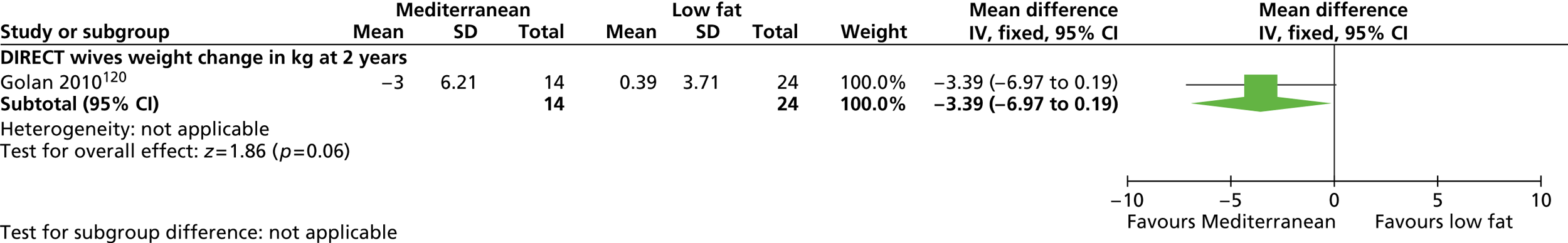
Wing and colleagues113 compared an intermittent very low-calorie diet (400–500 kcal per day) with a low-calorie, low-fat diet (1000–1200 kcal per day) in a 1-year trial including participants with type 2 diabetes. Both groups also received behavioural therapy and exercise advice and deposited US$150, which was refunded depending on compliance. Women in the very low-calorie diet group lost significantly more weight after 1 year than women in the low-calorie, low-fat diet group (14.1 kg vs. 8.6 kg, reported p < 0.023) whereas men showed comparable weight loss in both treatment groups (15.4 kg and 15.5 kg respectively).
An on-demand diet compared with a regularly repeated diet
After 16 weeks of a 450 kcal per day diet, Lantz and colleagues103 randomised participants to receive either an on-demand very low-calorie diet (450 kcal per day) or a regularly repeated diet. After the initial 16 weeks, participants in the intermittent on-demand group followed a 500 kcal per day deficit diet but changed to the 450 kcal per day diet when their individual body weight reached a predetermined cut-off level throughout the trial period. Participants in the regularly repeated group followed the same 500 kcal per day deficit diet but used the 450 kcal per day diet for a fortnight every third month.
At 2 years, men in the on-demand intermittent diet group showed significantly more weight change than men in the regularly repeated diet group (mean difference –10.50 kg, 95% CI –4.84 to –16.16 kg). There was no significant difference in weight loss between diets for women (mean difference 1.80 kg, 95% CI 5.23 to –1.63 kg) ( Figure 27 ).
FIGURE 27.
Effect of intermittent vs. repeated very low-calorie diets on weight change in men and women.
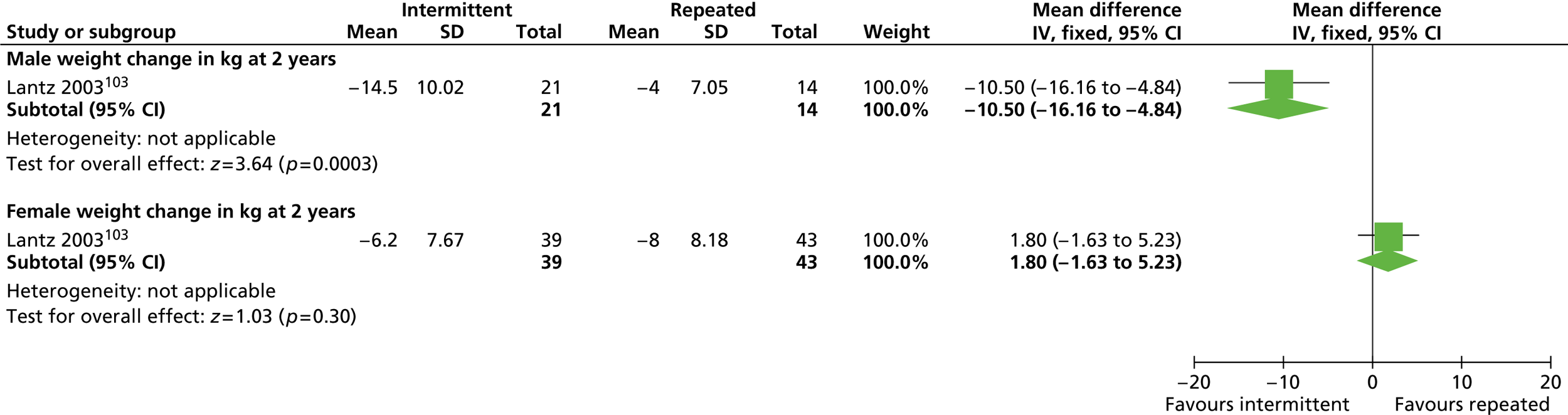
Types of behaviour change for weight loss
Heitzmann and colleagues97 randomised participants with type 2 diabetes to a behavioural, cognitive or cognitive–behavioural therapy or a control group, who received muscle relaxation training and factual diabetes information only. Participants in all groups received dietary advice from a registered nutritionist and were given individual exercise advice. At 18 months across all intervention groups, it was reported that men lost an average of 3.63 kg whereas women gained an average of 0.04 kg ( Table 22 ). That men may benefit more from weight reduction programmes than women was shown by a borderline significant interaction (reported p = 0.057). Men also experienced a significantly greater reduction in HbA1c than women (reported p < 0.05) but this difference was not significant between experimental groups. The effects of the individual programmes by sex were not reported.
| Intervention | Men (n = 22 for all groups) | Women (n = 24 for all groups) |
|---|---|---|
| Control | +0.6 | +1.4 |
| Behavioural | –4.2 | –0.7 |
| Cognitive | –2.5 | –1.5 |
| Cognitive–behavioural | –0.7 | –2.0 |
An intensive inpatient rehabilitation setting compared with a community setting
Hakala and colleagues95,96 investigated the effectiveness of an intervention carried out in an initial inpatient rehabilitation setting compared with an intervention carried out in a community setting for people who were at least 50% overweight. The rehabilitation intervention included intensive behavioural and educational group sessions along with a prescribed physical activity programme and occupational therapy, as well as individual nutritionist (1200 kcal per day) and physician counselling. The community intervention involved the same dietary intervention but included either individual physician counselling95 or group-based counselling delivered in the health centre setting. 96
In the earlier trial,95 men did better in the community setting than in the inpatient setting, possibly because of more individual counselling, although differences were statistically significant only for years 1 and 2 (p < 0.01). There were no significant differences between groups for women ( Figure 28 ).
FIGURE 28.
Effect of an intervention in an intensive rehabilitation setting vs. an intervention in a community setting, including individual counselling, on weight change in men and women.
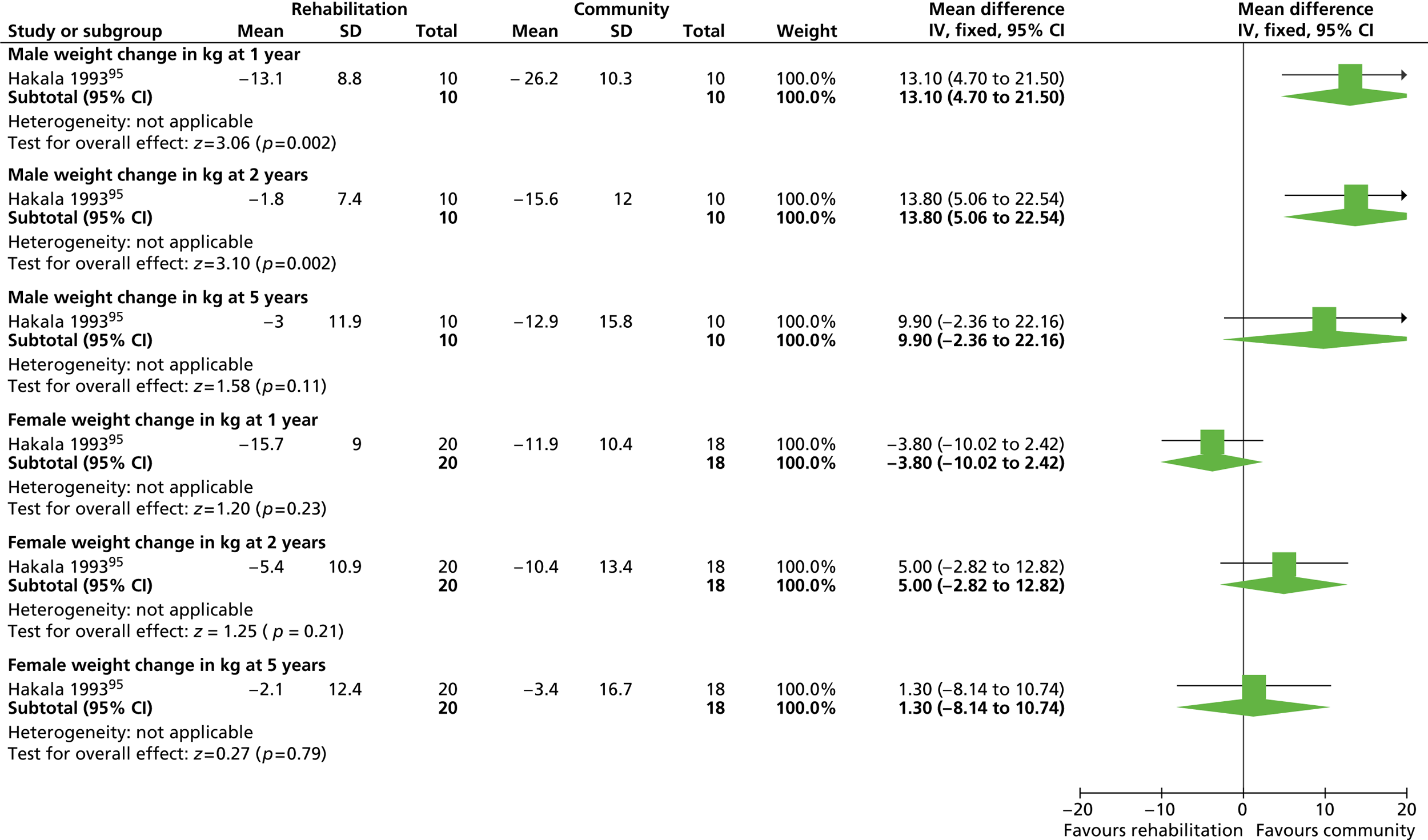
In the later trial by Hakala and colleagues,96 a similar comparison was carried out between an intervention in an initial intensive inpatient rehabilitation setting and an intervention in a community setting, delivered in group format only. When both rehabilitation and community interventions were delivered to men in groups, the rehabilitation setting produced favourable results, although differences were again statistically significant only over the first 2 years (p = 0.02 and p = 0.04 respectively). For women, the rehabilitation setting produced no significant benefit in weight loss over the community intervention for any time point from 1 to 5 years ( Figure 29 ).
FIGURE 29.
Effect of an intervention in an intensive rehabilitation setting vs. an intervention in a community setting on weight change in men and women.
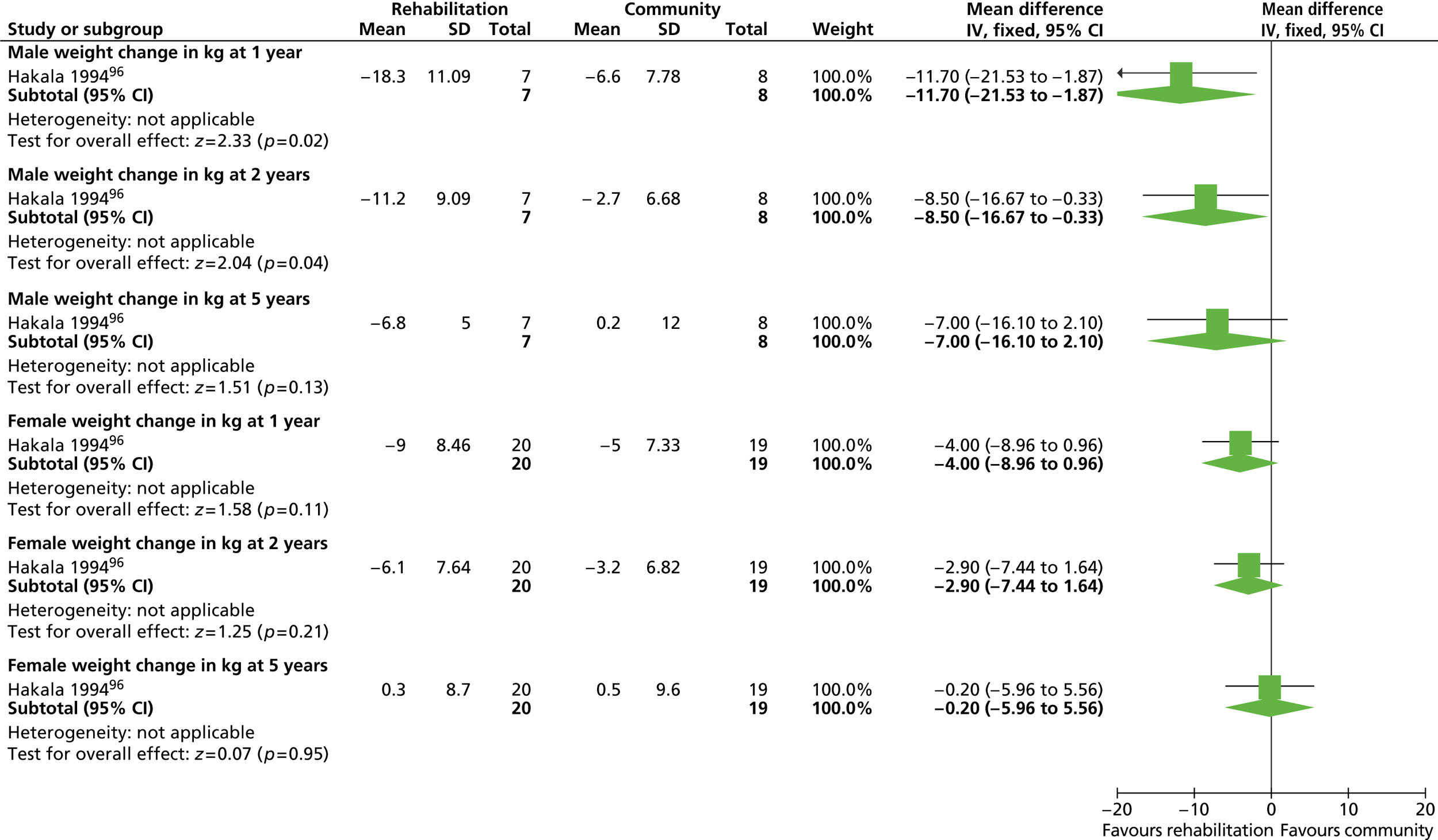
Type of provider and tailoring for dietary intervention
A tailored nurse intervention compared with a doctor-provided leaflet for weight loss
Korhonen and colleagues102 randomised type 2 diabetic patients to receive care from either a doctor or a specialist nurse to investigate the effect of provider and individual tailoring of a diet and weight reduction intervention on achievement of weight loss. Participants randomised to the care of a doctor were given general concise written information on diet and weight reduction with no further instruction thereafter. Participants randomised to the care of the nurse were given individual assessments and tailored dietary instruction. Nurse interventions were repeated at follow-up visits. At 12 months there were no significant differences between groups for either sex for weight change ( Figure 30 ).
FIGURE 30.
A tailored nurse intervention vs. a doctor-provided leaflet for men and women.
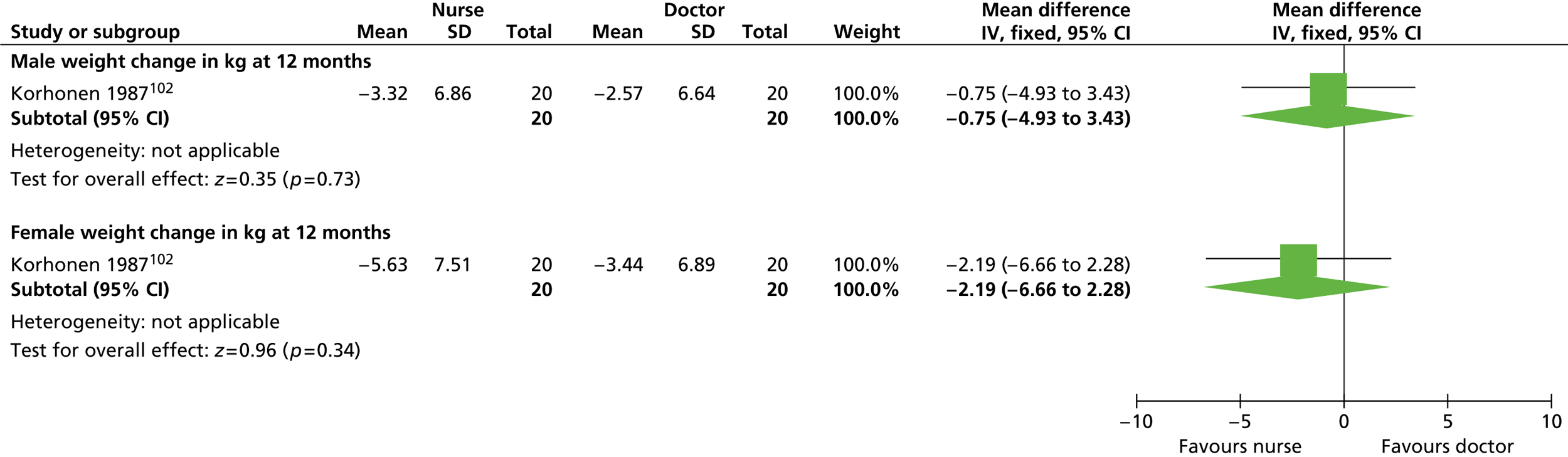
Commercial providers compared with NHS providers
The Lighten Up trial100 randomised participants to one of three weight-loss programmes run by commercial companies (Weight Watchers, Slimming World and Rosemary Conley) or to one of three programmes delivered through the NHS (NHS Size Down, a GP or a pharmacist) or to a choice group in which they were able to choose one of the six programmes depending on their preference. For the control group (minimal interventional) participants received vouchers for 12 free sessions at a council-run leisure centre. All programmes lasted for 12 weeks and provided advice on exercise, but only the Rosemary Conley group had an exercise class provided. The Weight Watchers and Rosemary Conley programmes tailor advice by sex. The investigators did label some of the commercial groups as ‘male friendly’ so that men would know that they would not be the only ones present. For the Rosemary Conley programme, a group walk was available for people who did not want to undertake the group exercise. Women were more likely to choose a commercial provider than men (81% vs. 47%). Men in the choice arm were more likely to choose a NHS programme.
Statistically significant weight loss at 1 year from baseline was found for all groups except for the general practice and pharmacy groups (complete case analysis, baseline observation carried forward and last observation carried forward). Only the Weight Watchers group was significantly different from the control group for men and women combined (adjusted mean difference –2.49 kg, 95% CI –4.15 kg to –0.83 kg). The authors found no statistically significant interaction between sex and weight-loss programme.
Further data supplied by the authors show significant weight loss from baseline for women in the choice, NHS Size Down, Rosemary Conley, Slimming World, Weight Watchers and control groups, for all methods of analysis. For men, the NHS Size Down, Rosemary Conley and Weight Watchers programmes produced significant weight loss from baseline for all analyses. For men, the control and Slimming World programmes also produced significant weight loss from baseline, but only in the last observation carried forward analysis ( Table 23 ).
| Type of analysis | Choice | Minimal intervention | NHS | Commercial | ||||
|---|---|---|---|---|---|---|---|---|
| General practice | Size Down | Pharmacy | Rosemary Conley | Slimming World | Weight Watchers | |||
| Men | ||||||||
| Complete cases | –0.52 (2.49 to –3.52) (n = 17) | –2.23 (–1.22 to 5.68) (n = 22) | –1.27 (1.61 to –4.15) (n = 14) | –4.59 (–2.19 to –6.99) (n = 28)b | –1.32 (2.15 to –4.79) (n = 10) | –2.69 (–0.46 to –4.91) (n = 23)a | –2.91 (0.05 to –5.86) (n = 24) | –6.05 (–2.77 to –9.32) (n = 24)b |
| BOCF | –0.35 (1.62 to –2.32) (n = 25) | –1.64 (0.86 to –4.14) (n = 30) | –0.77 (0.91 to –2.45) (n = 23) | –3.57 (–1.62 to –5.53) (n = 36)b | –0.69 (0.99 to –2.38) (n = 19) | –1.99 (–0.32 to –3.67) (n = 31)a | –1.99 (0.04 to –4.02) (n = 35) | –5.18 (–2.28 to –8.08) (n = 28)a |
| LOCF | –1.09 (1.12 to –3.30) (n = 25) | –2.65 (–0.06 to –5.23) (n = 30)a | –1.25 (0.49 to –3.00) (n = 23) | –4.03 (–2.09 to –5.98) (n = 36)b | –2.53 (0.15 to –5.20) (n = 19) | –2.93 (–0.59 to –5.28) (n = 31)a | –3.52 (–1.29 to –5.76) (n = 35)a | –5.61 (–2.72 to –8.50) (n = 28)b |
| Women | ||||||||
| Complete cases | –2.06 (–0.20 to –3.92) (n = 48)a | –3.25 (–1.22 to –5.28) (n = 51)a | –1.25 (1.16 to –3.68) (n = 32) | –3.06 (–0.55 to –5.56) (n = 38)a | –1.15 (0.09 to –3.49) (n = 29) | –3.58 (–0.90 to –6.27) (n = 42)a | –3.22 (–1.34 to –5.10) (n = 37)a | –3.71 (–1.07 to –5.72) (n = 54)b |
| BOCF | –1.32 (–0.12 to –2.51) (n = 75)a | –2.37 (–0.86 to –3.88) (n = 70)a | –0.86 (0.77 to –2.49) (n = 47) | –1.81 (–0.31 to –3.32) (n = 64)a | –0.65 (0.65 to –1.96) (n = 51) | –2.18 (–0.52 to –3.84) (n = 69)a | –1.84 (–0.71 to –2.96) (n = 65)a | –2.78 (–1.24 to –4.32) (n = 72)b |
| LOCF | –1.41 (–0.09 to –2.72) (n = 75)a | –3.10 (–1.54 to –4.65) (n = 70)b | –1.07 (0.66 to –2.81) (n = 47) | –2.57 (–1.00 to –4.14) (n = 64)a | –1.59 (0.05 to –3.24) (n = 51) | –3.27 (–1.57 to –4.97) (n = 69)b | –3.14 (–1.94 to –4.35) (n = 65)b | –3.86 (–2.31 to –5.42) (n = 72)b |
Telephone compared with mail advice and behaviour change techniques
Jeffery and colleagues99 compared the effectiveness of an intervention including weight reduction advice, physical activity advice and behaviour change techniques delivered by telephone or mail. A control group received usual care. Details of the dietary and exercise advice are unclear. Men in both the telephone group and the mail group had lost significantly more weight at 1 year than men in the control group (p = 0.03). By contrast, there were no significant differences between women in the telephone or mail group and women in the control group. There were no significant differences for either sex for the telephone group or the mail group ( Figures 31–33 ).
FIGURE 31.
Effect of telephone advice and a behaviour change intervention vs. control on weight change in men and women.
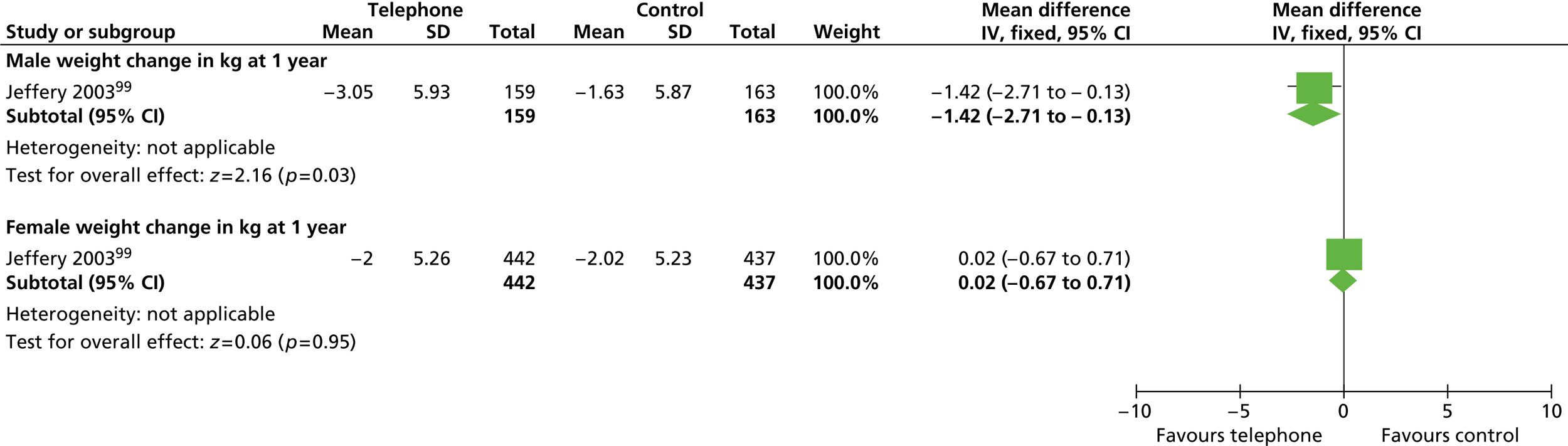
FIGURE 32.
Effect of mail advice and a behaviour change intervention vs. control on weight change in men and women.
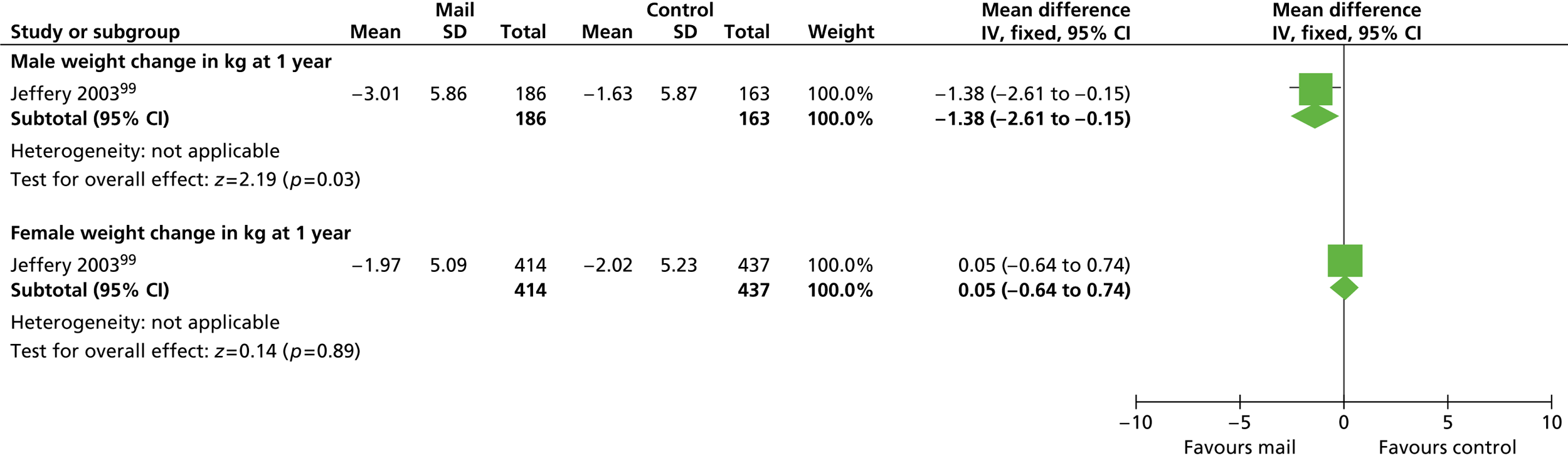
FIGURE 33.
Effect of telephone advice vs. mail advice and a behaviour change intervention on weight change in men and women.
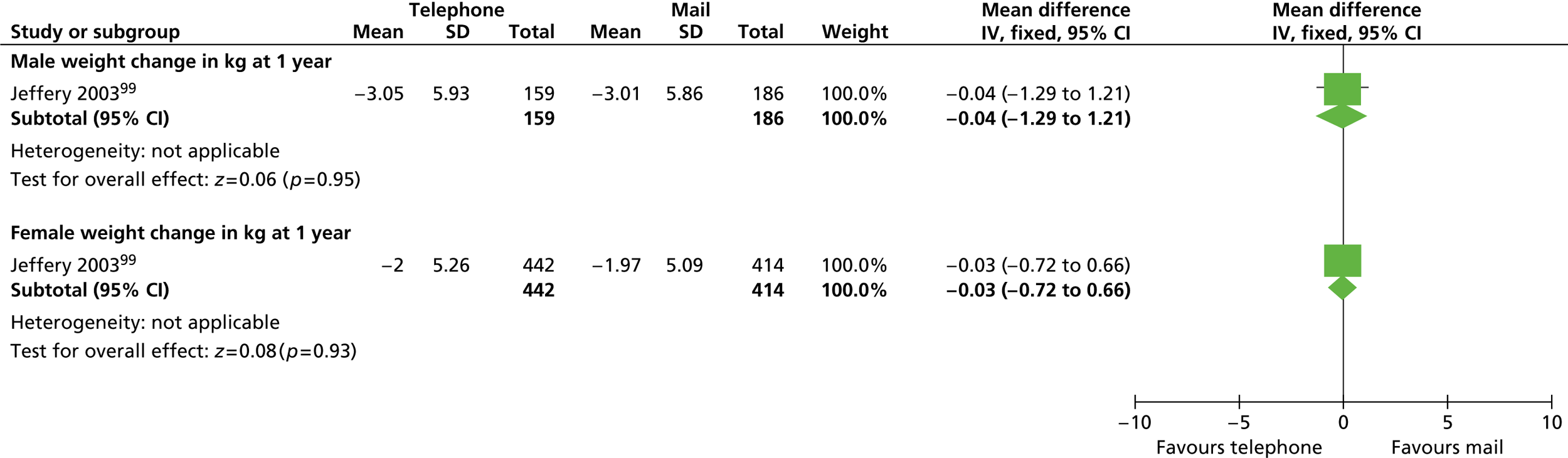
Physical activity advice, a healthy diet and behavioural therapy compared with usual care
The Prevention and Reduction of Obesity Through Active Living (PROACTIVE) trial106 randomised abdominally obese participants to receive an intervention offering physical activity and behavioural therapy or to usual care. Participants in the intervention group received individually tailored counselling based on motivational interviewing to provide the knowledge and skills to increase physical activity and adopt a healthy diet and eating patterns. Calorie reduction was not explicitly mentioned. Participants receiving usual care were given lifestyle advice for reducing obesity from their primary care physician following the usual appointment schedule and counselling approach. After 2 years, men in the intervention group had lost significantly more weight and significantly reduced their BMI and waist circumference compared with men in the usual care group. Women in the intervention group significantly reduced their waist circumference compared with women in the usual care group after 1 year but the effect was lost by 2 years. There were no other significant differences between groups ( Figure 34 and Table 24 ).
FIGURE 34.
Effect of physical activity advice, a healthy diet and behavioural therapy vs. usual care on weight change in men and women.
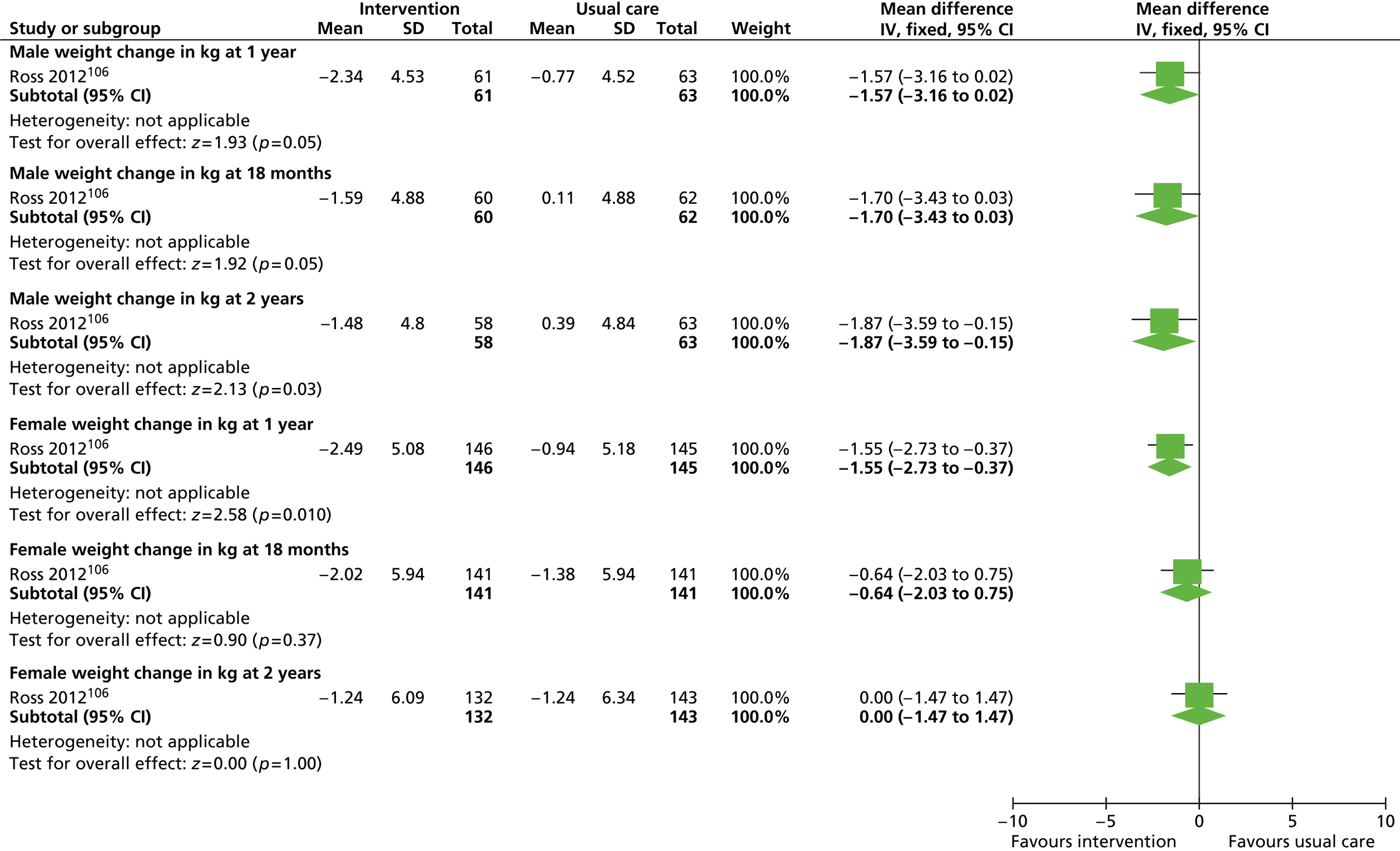
| Outcome | Sex | Follow-up | Healthy diet, physical activity and behavioural therapy | Usual care |
|---|---|---|---|---|
| BMI (kg/m2) | Men | 1 year | –0.72 | –0.23 |
| 18 months | –0.50 | +0.04 | ||
| 2 yearsa | –0.56 | +0.12 | ||
| Women | 1 year | –0.93 | –0.34 | |
| 18 months | –0.73 | –0.49 | ||
| 2 years | –0.50 | –0.45 | ||
| Waist circumference (cm) | Men | 1 year | –2.9 | –1.3 |
| 18 months | –2.2 | –0.5 | ||
| 2 yearsb | –1.6 | +0.1 | ||
| Women | 1 yearc | –2.4 | –0.8 | |
| 18 months | –1.6 | –0.4 | ||
| 2 years | –0.6 | +0.3 | ||
| Total cholesterol (mmol/l) | Men | 1 year | –0.08 | –0.13 |
| 18 months | –0.14 | –0.10 | ||
| 2 years | –0.29 | –0.24 | ||
| Women | 1 year | +0.31 | –0.11 | |
| 18 months | –0.28 | –0.14 | ||
| 2 years | +0.35 | –0.30 | ||
| HDL cholesterol (mmol/l) | Men | 1 year | –0.06 | –0.07 |
| 18 months | –0.09 | –0.13 | ||
| 2 years | –0.20 | –0.19 | ||
| Women | 1 year | –0.14 | –0.13 | |
| 18 months | –0.19 | –0.18 | ||
| 2 years | –0.27 | –0.25 | ||
| LDL cholesterol (mmol/l) | Men | 1 year | +0.15 | –0.004 |
| 18 months | +0.13 | +0.07 | ||
| 2 years | +0.08 | +0.03 | ||
| Women | 1 year | –0.10 | +0.07 | |
| 18 months | –0.01 | +0.12 | ||
| 2 years | +0.02 | +0.04 | ||
| Triglycerides (mmol/l) | Men | 1 year | –0.38 | –0.15 |
| 18 months | –0.38 | –0.13 | ||
| 2 years | –0.35 | –0.20 | ||
| Women | 1 year | –0.17 | –0.08 | |
| 18 months | –0.17 | –0.16 | ||
| 2 years | –0.19 | –0.19 | ||
| Systolic blood pressure (mmHg) | Men | 1 year | –2.92 | –3.46 |
| 18 months | –0.99 | –1.06 | ||
| 2 years | –1.69 | –0.31 | ||
| Women | 1 year | –2.39 | –1.43 | |
| 18 months | –0.89 | –2.67 | ||
| 2 years | –0.01 | –0.70 | ||
| Diastolic blood pressure (mmHg) | Men | 1 year | –2.98 | –2.53 |
| 18 months | –1.81 | –2.27 | ||
| 2 years | –2.26 | –0.88 | ||
| Women | 1 year | –1.63 | –1.02 | |
| 18 months | –1.32 | –1.77 | ||
| 2 years | –0.71 | –0.61 | ||
| Fasting plasma glucose (mmol/l) | Men | 1 year | –0.12 | +0.11 |
| 18 months | +0.27 | +0.05 | ||
| 2 years | +0.07 | +0.23 | ||
| Women | 1 year | –0.06 | +0.09 | |
| 18 months | +0.05 | +0.22 | ||
| 2 years | +0.09 | +0.22 | ||
| No. of musculoskeletal adverse events | Men | Total | 96 | 96 |
| Requiring physician visit | 51 | 50 | ||
| Requiring hospitalisation | 2 | 2 | ||
| Women | Total | 204 | 215 | |
| Requiring physician visit | 124 | 110 | ||
| Requiring hospitalisation | 3 | 10 | ||
| No. of potential cardiovascular adverse events | Men | Total | 46 | 39 |
| Requiring physician visit | 36 | 29 | ||
| Requiring hospitalisation | 7 | 9 | ||
| Women | Total | 69 | 98 | |
| Requiring physician visit | 40 | 54 | ||
| Requiring hospitalisation | 2 | 9 |
Varying monetary contracts for weight loss
Jeffery and colleagues98 investigated the effect of financial contracts on weight loss and weight maintenance in men and women recruited from a previously identified population or people self-referred through newspaper advertisements. All participants paid a US$150 deposit at the start of a 16-week weight-loss phase consisting of nutrition, exercise and behaviour change technique education sessions. Details of the dietary and exercise advice are unclear but the aim was to lose 2 lb (0.9 kg) per week. Participants randomised to the control groups were refunded their entire deposit at the initial session. Participants in the constant contract groups were refunded $30 for each successive group average weight loss of 5 lb (2.27 kg) and participants in the increasing contract groups were refunded US$5, US$10, US$20, US$40 and US$75 for successive 5-lb group weight losses. Following the weight-loss phase, 17 men and 25 women were randomised to receive either intensive or non-specific weight-maintenance sessions.
Those enrolling in the maintenance phase paid a US$100 deposit, which was returned in US$25 increments for attendance at quarterly group sessions. Those not enrolling in the maintenance phase were contacted at the 1-year follow-up assessment only. Table 25 details the weight loss for all participants at 1 year. Eleven participants gave a self-reported weight at this assessment. The trial authors added 5 lb to these weights for their analyses. Two participants who were lost to follow-up were recorded as having lost 0 lb and three participants were excluded from the analyses.
| Sample | Weight change (kg) | n | |
|---|---|---|---|
| Self-referred | |||
| Control | Men | –4.27 | 10 |
| Women | –9.21 | 9 | |
| Constant contract | Men | –4.44 | 7 |
| Women | –8.24 | 10 | |
| Increasing contract | Men | –6.63 | 11 |
| Women | –4.30 | 10 | |
| Population | |||
| Control | Men | –2.82 | 10 |
| Women | –2.71 | 11 | |
| Constant contract | Men | –5.43 | 9 |
| Women | –3.87 | 9 | |
| Increasing contract | Men | –8.93 | 8 |
| Women | –9.54 | 9 | |
The authors reported that weight loss at 1 year was not statistically associated with recruitment source, contract type or sex. Analysis of percentage change in weight showed that women lost significantly more weight than men (reported p < 0.05). During weight maintenance it was reported that the only significant effect was for women in the intensive maintenance condition who outperformed men for this contract type (reported p < 0.006).
Orlistat compared with placebo for weight maintenance
Richelsen and colleagues105 investigated the effect of orlistat in people with type 2 diabetes, impaired fasting glucose or dyslipidaemia. Before randomisation, participants all initially lost at least 5% of their body weight by following a very low-calorie diet of 600–800 kcal per day over an 8-week period. Participants were then randomised to receive lifestyle counselling with either 120 mg of orlistat three times daily or matching placebo capsules. Weight change from the start of the diet to 3 years, analysed using the last observation carried forward for dropouts, was reported as significantly greater for women in the orlistat group than for women in the placebo group [–9.7 kg (–8.4%) vs. –6.3 kg (–5.3%), p < 0.02]. For men the difference between groups was not significant [orlistat vs. placebo: –8.9 kg (–8.3%) vs. –8.1 kg (–7.5%)].
Comparison between weight loss in men and weight loss in women across trials
For the analysis comparing weight loss between men and women a total of 11 studies had data available,95,96,98,101,102,106,108 – 112 including a total of 5519 participants, 3493 women and 2026 men. Two analyses were carried out comparing mean weight change and percentage weight loss between men and women. Both analyses show that there were no significant differences in weight change between men and women recruited to these studies ( Figures 35 and 36 ). However, few studies provided sufficient data to allow us to be sure that men and women were prescribed the same calorie deficit. Whether men or women adhere better to lifestyle prescription is unclear.
FIGURE 35.
Difference in mean weight loss (kg) between men and women.
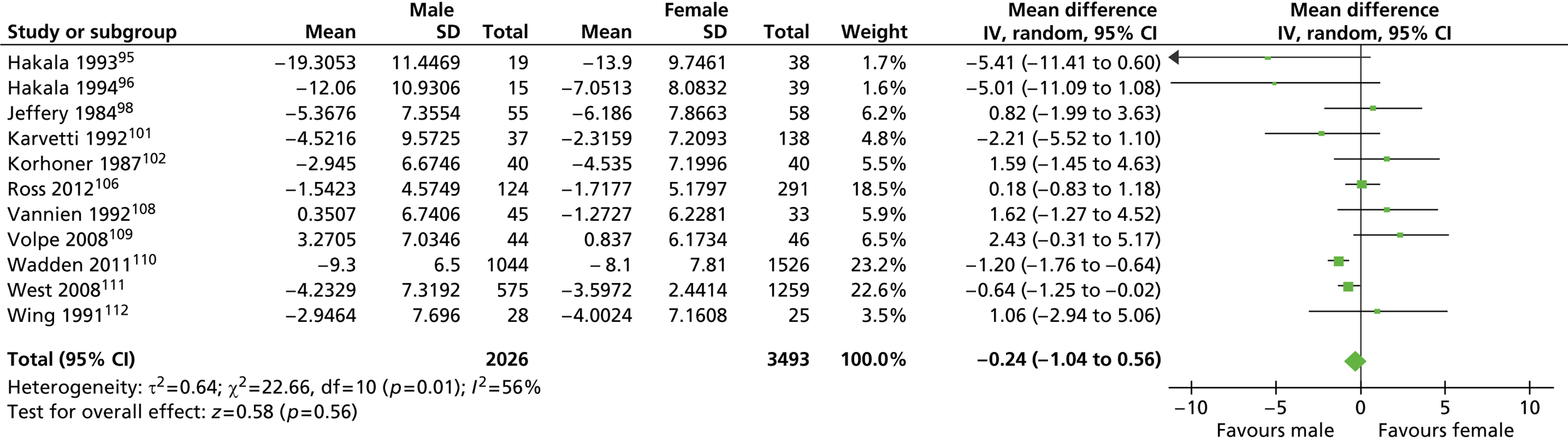
FIGURE 36.
Percentage weight loss from baseline in men and women.
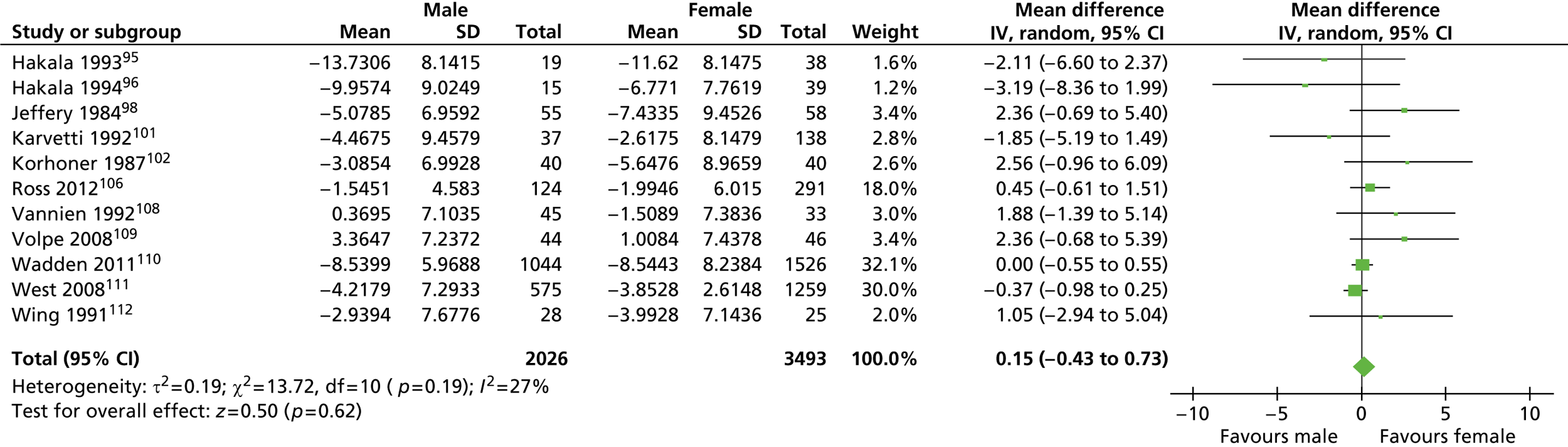
Discussion of the results from the review of trials with data for men and women
We identified a modest number of trials reporting outcomes by sex, the majority of which recruited many more women than men. The variety of different interventions, and the small size of many of the studies, mean that conclusions about the best study design for men, and whether or not services should be different for men and women, can only be tentative. Few of the trials considered truly comparable interventions and, in most cases, data were unsuitable for pooling in a formal meta-analysis.
Our analyses of weight loss showed no significant differences between men and women, although it should be noted that this was based on only a handful of trials. Dietary and physical activity prescriptions were rarely described well, with little evidence of allowances made for the greater body size and muscle mass of obese men. Authors often did not report baseline weight or BMI by sex, reporting only weight change data between the sexes at varying time points. This makes comparisons between men and women problematic as men tend to be heavier with proportionately greater muscle mass than women and will therefore lose more weight than women if prescribed the same calorie intake and if adherence is similar. Only the DIRECT trial107 reported a more generous calorie allowance for men than for women with the Look AHEAD trial110 reporting a 1200–1800 kcal per day diet depending on initial body weight. Similarly, the Weight Watchers and Rosemary Conley programmes also take account of body size and sex for calorie allowances.
From our results it is possible to conclude that interventions encompassing both diet and exercise components are more successful in achieving weight loss and preventing weight gain than interventions including diet or exercise only. 109 Men outperformed women when they had to reduce their calorie intake in response to body weight cues rather than following a very low-calorie diet at regular intervals. 103 Regulating calorie intake by responding to one’s own body may offer a greater sense of personal control over weight loss, which could be more important to men than to women. This could partly be explained by evidence suggesting that physical vulnerability or health issues are often a key motivator for men,44 and so evidence of increased body weight acted as a better motivator for the men in this trial. Alternatively, it could be that this form of weight regulation was seen as less regimented or imposing by the men and was therefore favoured because of the tendency for men to be reluctant to follow formal diet plans. 44
There was no clear evidence that type of diet influenced long-term weight loss in men. 107,113
Men have benefited from training in behaviour change techniques alongside diet and physical activity interventions. 97,99,101,102,110,111 Although men performed well in terms of weight loss in group settings,96,98,100,101,110 more favourable results were produced when individual support or tailored advice was delivered to men as well as the group intervention. This personalising of the intervention could be more important for men than for women. 95,99,106 This may also offer men a greater sense of personal control or men may have greater educational needs in terms of weight-loss reduction techniques than women. Results from the Look AHEAD trial110 suggest that tailoring by ethnicity may be more important for women than men for certain ethnic groups, although whether or not this is true for ethnic groups outside the USA requires further investigation.
When group programmes are offered both sexes lost more weight in an intense rehabilitation setting than in a community setting, with men in particular losing more weight in this setting. 96 Support from a spouse120 and learning how to enhance social support from family members121 may also be favourable for men. Having a partner participate in a weight reduction programme also produces favourable weight-loss results but this effect appears to be greater for women than for men. 94,112 Gorin and colleagues94 suggest that this may be explained in part by households in which women are more likely to be responsible for food shopping and preparation. Having a partner who is following the same diet plan reduces the burden of preparing additional meals and the likelihood of purchasing foods that are inconsistent with weight-loss efforts. Men, on the other hand, may assume more responsibility for their own behaviour change if they attend on their own. The authors admit that their interpretation is speculative and that other factors may act as important moderators. When ‘traditional’ gender roles are less evident, programmes involving participating partners could produce different results.
Altering weight reduction activities to be compatible with perceptions of feminine and masculine behaviours also appears to be important for achieving successful results. In the Lighten up trial100 the authors noted that, although men performed well in the programmes delivered by commercial companies, fewer than half picked these programmes when the choice of provider was freely available. The authors suggest that commercial companies may appear more female orientated. By contrast, NHS-delivered programmes may be perceived by men as purely concerned with improving health rather than physical appearance and may therefore appear more masculine in this regard. Men in the choice group had poorer results than women in this group, which could reflect men’s greater educational needs. Of the NHS programmes, the Size Down programme produced results that were comparable with those of some of the commercial companies. Size Down was the only NHS programme to offer group sessions, again suggesting a benefit from group interactions.
Of the commercial programmes, men appeared to do best with Weight Watchers, but numbers of participants were limited. A specific area of the Weight Watchers website is dedicated to men only. The success of Weight Watchers may be in allowing men to feel that they are following a programme that is targeted to their needs. Having the flexibility to adapt programmes in this way may encourage greater programme engagement among men. Unlike commercial companies, primary care may lack the resources required to provide sufficient support to patients to produce effective weight-loss results. Results from the Hakala96 and Lighten Up100 trials also suggest that general practice staff may perceive themselves as unsuitable for arranging weight-loss care for obese patients. Although the staff in the Lighten Up trial received specific training, they were less experienced in a weight management role than providers of commercial weight-loss programmes. This suggests that health service staff may benefit from greater education and training in weight loss if weight management services are to be delivered by the primary health-care system in this way. Similarly, patients may be too inhibited to discuss problems experienced with weight-loss techniques if they feel that they are constrained to the limited time available during a GP consultation.
Our analyses of trial retention showed that men were significantly more likely to complete a trial than women. We are unable to comment on possible explanations for the differential dropout between men and women from the available data. Nevertheless, this finding suggests that, although fewer men are likely to join weight-loss programmes, once they do join they show the motivation and commitment to ‘stick with’ the programme. This highlights the importance of finding successful strategies to engage men in weight-loss services and will be discussed further in Chapter 4 .
Overall summary from both reviews in this chapter
We summarise in the following sections the main points that have arisen from both reviews in this chapter.
General issues relating to methodology
-
We identified very few randomised trials examining weight loss in men-only groups. Few mixed-sex trials reported weight-loss outcomes by sex, and trials recruited much greater proportions of women. Despite this sex bias, men were rarely consulted beforehand about the design of studies or asked their views on the programmes that they undertook.
-
Male study participants tended to be middle-aged, white and not morbidly obese. Men from minority groups were under-represented. Relatively few interventions involved men who were obese with existing health problems, such as type 2 diabetes, cardiovascular disease or osteoarthritis. Few trials presented data on changes in cardiovascular risk factors, or clinical outcomes.
-
Most of the interventions were not described in sufficient detail such that they could be replicated. Few studies reported conducting fidelity checks for intervention delivery. Interventions tended to be intensive in terms of time required from participants and those delivering the programme.
-
Providers of the interventions were described at an occupational level, for example nutritionist or physical activity trainer, but the sex of providers was not reported. It is unclear from the included studies whether or not the sex of the person providing a weight-loss intervention to men, either individually or in groups, is an important factor in the effectiveness of that intervention.
-
There were particularly few studies that looked at the long-term maintenance of weight loss.
-
There were very few data on quality of life or clinical and economic outcomes to assess the full value of an intervention.
-
Details of trial methodology were often inadequately reported, for example method of randomisation. Reporting could also be improved by following the standards outlined in the CONSORT statement. 128,129 Few authors presented data for the entire cohort (e.g. baseline observation carried forward, last entry carried forward), choosing instead to present data for completers only both at baseline and for final outcome measurement. It is therefore difficult to judge the level of attrition bias in these studies. Similarly, few trials reported details concerning the equity or sustainability of the considered interventions.
Pointers for effective interventions
Although few trials were available, there are some pointers for factors that may contribute to effective programmes for men:
-
The type of reducing diet, for example providing more protein, has not been shown so far to affect long-term weight loss. 83,87,91,107,113 However, intermittent periods of very low-calorie dieting, as required, may be better than regular periods of such dieting. 103
-
Men may do well if physical activity is part of a weight-loss programme and may be more likely to respond to this than women. 93,106,109 Men like using pedometers90 but weight loss is better with a reducing diet than with physical activity alone, and better if both are provided. 91,93,109 However, one small trial did not find that a physical activity programme and a reducing diet were better than the diet alone. 92
-
Behaviour change training improves long-term weight loss, and weight maintenance for men after a physical activity programme. 88,101
-
Health concerns could help motivate men. Intensive programmes with low-fat reducing diets and physical activity with or without behaviour change training can reduce weight and improve erectile dysfunction in men with and without type 2 diabetes,85,110,122 and prevent diabetes,104 although in type 2 diabetes successful weight loss might increase the risk of osteoporosis. 124
-
Once recruited, men appear less likely to drop out from programmes than women. Men may like less monitoring than women. 96 Telephone and mail support could be useful. 99
-
The effect of support from partners to aid weight loss is inconsistent. 94,112,120
-
Men may be particularly less likely to choose a commercial weight-loss programme than women. 100 Health service programmes appear to be favoured by men with obesity. 96,100 The comparative effectiveness of NHS and commercial weight-loss programmes for men in the trial by Jolly and colleagues100 is unclear.
-
Men do well in groups of men, but some individual tailoring of advice or counselling may also aid weight loss. 85,90,95,96 Too many weight-loss sessions may be counterproductive. 101 Group financial contracts were associated with better weight loss than individual contracts, but the size of the contract has not been found to be a significant factor. 86,98
-
Men like individualised, fact-based, flexible and simple to understand information. 90
-
Men are less likely than women to do well using orlistat to help long-term weight-loss maintenance. 105
-
The benefits of internet-based advice for men are presently unclear. 89,90
Chapter 4 Systematic review of UK interventions with data for men or for men and women compared
In this chapter we provide the results of the systematic review of UK interventions for men with obesity, including any setting, study design or duration. This review also includes data from mixed-sex UK studies in which data were provided separately for men and women.
This chapter also contains details of the systematic review of studies that specifically investigated increasing the engagement of men with obesity services (i.e. increasing the take-up of services by men); no studies fitting the inclusion criteria for this review were found. However, information on engaging men with services is available and discussed in the first review in this chapter.
Quantity of evidence
Our primary literature searches identified 2057 potentially relevant titles and abstracts ( Figure 37 ). In addition to this, we identified 20 potentially relevant reports from other sources listed in Appendix 1 , such as commercial organisations, professional organisations, and from grey literature. For our review of UK studies of any design, we selected 140 reports for full-text assessment, of which we identified 15 eligible reports of men-only studies33 – 35,138 – 149 (two of which were RCTs141,146). We also found 11 eligible reports31,36,37,150 – 157 (including one linked report157) of mixed-sex studies in which the results were reported by sex. Of these included reports, one was an abstract141 and four were poster presentations. 148,151 – 153 The remaining reports were full-text publications. Three of the full-text reports were written as evaluation reports of public health initiatives139,147,149 and were not published in an academic journal. One other mixed-sex UK RCT by Jolly and colleagues100 has already been discussed in Chapter 3 in the review of interventions for men and women compared. No eligible reports were identified for inclusion in our review of interventions to promote the engagement of men with weight-loss services.
FIGURE 37.
Flow chart of the number of potentially relevant reports and the numbers of reports subsequently included and excluded from the reviews of UK interventions and interventions to increase engagement (numbers for engagement review are in parentheses).
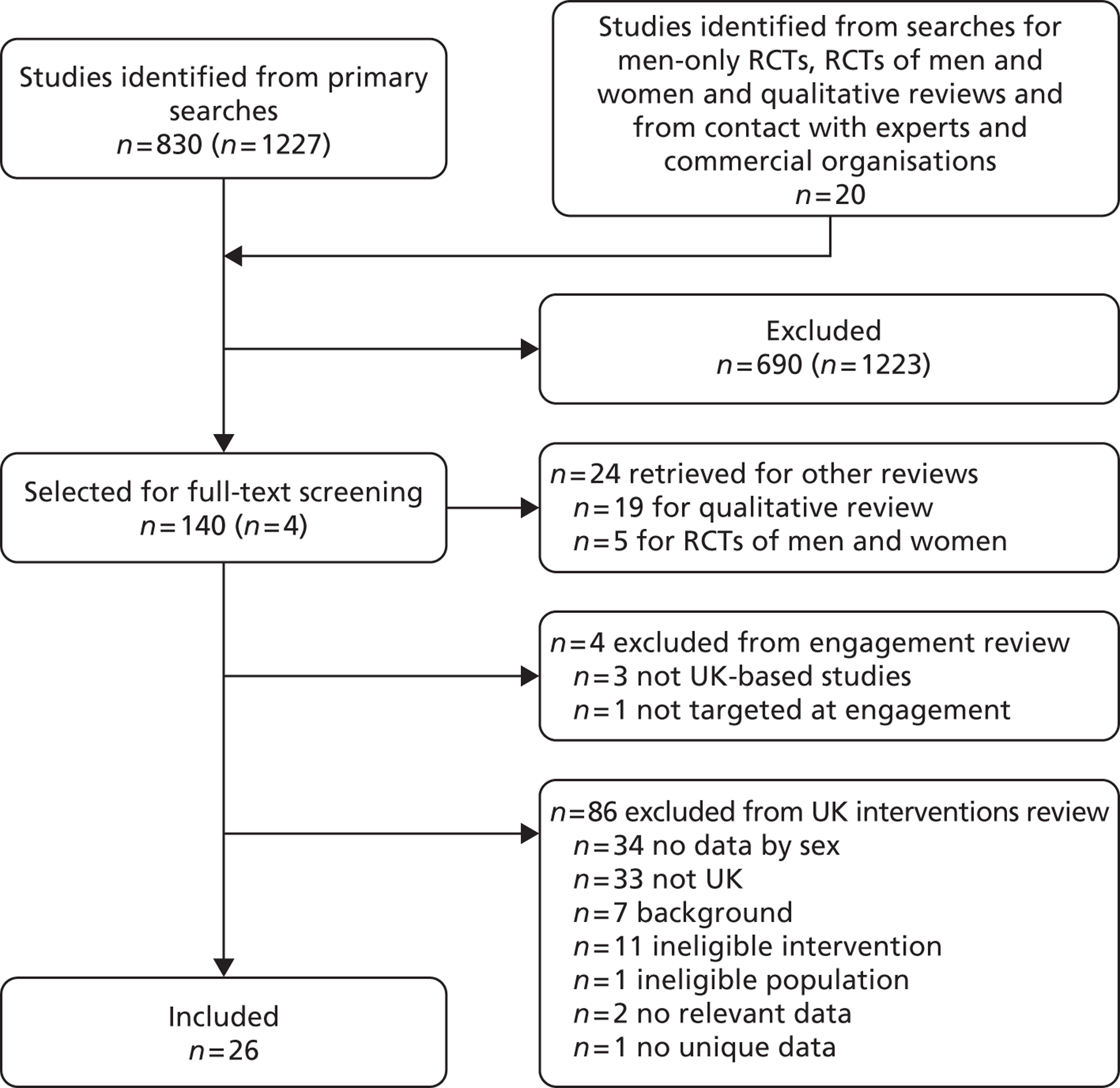
Characteristics of included studies
Tables 26 and 27 detail the characteristics of the included studies. Of the men-only studies, we identified two RCTs with follow-up periods of 12141 and 24 weeks146 and seven prospective cohort studies33,138 – 140,142,147,149 with follow-up ranging from 6 weeks149 to 49 months. 142 There were two retrospective cohort studies that included follow-up periods of 10148 and 2434 weeks. Of the mixed-sex reports, seven were retrospective cohort studies with follow-up periods ranging from 12 weeks150 to 24 months. 31 We included two prospective cohort studies,31,155 which reported data for men at 6 weeks and 12 months respectively.
| Study ID | Participants | Interventions | Outcomes | Notes |
|---|---|---|---|---|
| Brady 2010138 | Study design: Prospective cohort Location: Two SPL football clubs, Glasgow, UK Period of study: Prior to 2010 Inclusion criteria: Age 40–60 years Exclusion criteria: Inability to exercise, overt cardiovascular or any other disease precluding participation Age: NR Weight (kg), mean: 95 BMI: NR Baseline comparability: NA |
Description of intervention: Diet and exercise – dietary adjustment consisted of adopting a Mediterranean-style diet with less red meat and dairy and moderation of alcohol intake. Dietary emphasis was on reducing saturated fat and salt with calorie reduction when required. The exercise programme was designed for each individual using a safe zone of 60–80% of the maximum predicted heart rate. Heart rate monitors were used when exercising to ensure that exercise was carried out in the safe zone. Men were instructed to exercise for 20 minutes at the appropriate heart rate three to four times weekly. Additionally, men attended their respective club stadiums once weekly for a 2-hour session. The first hour consisted of a general health discussion (cardiovascular health, alcohol, obesity, dental health, diabetes, urological and prostatic disease, stress management and diet). For the second hour men rotated between 20 minutes’ pitch-side exercise, a 20-minute cardiovascular workout and 20 minutes of dietary advice and discussion. Exercise classes were run by professional SPL coaching staff. Health lectures were delivered by physicians from Glasgow hospitals. Dietary advice was provided by research dietitians and nurses Duration of active intervention: 10 weeks No. of times contacted: 12 No. allocated: 40 No. completed: 36 Dropout (%): 10 No. assessed: 36 |
Length of follow-up: 15 months Outcomes: Weight, total cholesterol, blood pressure |
Glasgow Rangers and Celtic football clubs |
| Drummond 2004140 | Study design: Prospective cohort Location: Workplace/community, Birmingham, Edinburgh, London, UK Period of study: NR Inclusion criteria: Registered taxi driver, waist circumference ≥ 96 cm, free from illness Exclusion criteria: Special diet for medical/other reason, weight-reducing diet in previous 3 months Age (years), mean (range): 42 (20–64) Weight (kg), mean (SD): 106 (21) BMI (kg/m2), mean (SD): 34.6 (6.2) Baseline comparability: NA |
Description of intervention: Diet – nutritional advice given based on 7-day unweighed diet diaries completed at baseline. Diet consisted of a low-fat, high-carbohydrate (sugar-containing), energy-restricted diet, high in fruit and vegetables. Individualised plans to produce an energy deficit of 600–700 kcal daily, producing an approximate weight loss of 7–8 kg over 3 months (5–10% of body weight). Men were advised to consume small high-carbohydrate, sugar-containing Carb Boosters™ snacks frequently. Each snack contained 20 g of sugar. The aim of the snacks was to increase total carbohydrate intake in a palatable way and prevent a ‘starve and binge’ pattern of dieting Duration of active intervention: 12 weeks No. of times contacted: 5 No. allocated: 107 No. completed: 76 Dropout (%): 29 No. assessed: 76 |
Length of follow-up: 12 weeks Outcomes: Weight |
Study sought to recruit both male and female taxi drivers but only men volunteered to participate In addition to participant informed consent, the partner/wife of each man signed a consent form stating they would support the participant in the study |
| Gray 2009142 | Study design: Prospective cohort Location: Multiple local community men’s health clinics, Grangemouth, UK Period of study: NR Inclusion criteria: BMI ≥ 30 kg/m2 or waist circumference ≥ 102 cm Exclusion criteria: NR Age (years), mean (range): 50.9 (23–74) Weight: NR BMI (kg/m2), n (%): < 30: 7 (7) 30–34.9: 62 (59.3) 35–39.9: 26 (24.4) > 40: 10 (9.3) Baseline comparability: NA |
Description of intervention: Low-fat diet and physical activity advice delivered in men-only groups over 12 weekly sessions. Men were provided with a weight management booklet and informed that they were personally responsible for their own weight management. Men were educated about a healthy balanced diet and healthy portion sizes by comparison between individual diaries and the food plate model. Men were also given examples of daily eating plans and discussed the role of alcohol consumption, physical activity in weight management and potential barriers to exercise. Men could apply for a free pass to a local authority-run sports centre to assist with increasing physical activity. The psychology of behaviour change and value of social support were discussed and men set individual SMART goals for weight loss (aimed to lose 0.5–1 kg per week), exercise and alcohol consumption. Men also met with a previous programme completer midway through the course. Feedback was given in the form of sandbags representing each man’s personal midpoint weight loss and through comparison between week 1 and week 10 food diaries. Advice on dealing with relapses and how to manage weight long term was provided and men were invited to join organised post-programme meetings Duration of active intervention: 12 weeks No. of times contacted: 14 No. allocated: 109 No. completed (at 12 weeks): 80 Dropout (%): Unclear No. assessed: 80 |
Length of follow-up: 49 months Outcomes: Weight, BMI, waist circumference |
BMI entry criteria stated in methods as > 30 kg/m2 but seven men reported to have a BMI < 30 kg/m2 |
| Gray 2011141 | Study design: RCT Location: Two SPL football clubs, UK Period of study: September 2010 Inclusion criteria: Age 35–65 years, BMI ≥ 27 kg/m2, consent to randomisation and weight, height and waist measurements Exclusion criteria: Contraindications for vigorous physical exercise (e.g. systolic blood pressure ≥ 160 mmHg, diastolic blood pressure ≥ 100 mmHg) Age (years), mean (range): a + b: 47.1 (32–68) Weight (kg), mean (SD): a: 107.6 (15.0); b: 107.5 (19.5) BMI: NR Baseline comparability: Yes (personal communication from author) |
Description of interventions: a: ‘Gender-sensitised’ weight management, physical activity and healthy living programme based on control theory158 and components of behavioural change techniques.159,160 Men attended 12 weekly classroom-based group discussions held at their SPL club training ground where they received personalised advice on diet (portion control and healthy eating) to suit individual circumstances and preferences. Following these discussions men engaged in pitch-side/in-stadia structured aerobic, muscle strengthening and flexibility exercise training sessions, which were tailored to individual fitness and ability. Weekly sessions lasted 90 minutes. Outside the weekly sessions men were given an incremental walking programme. Pedometers were issued to the men to monitor individual daily goals and progress was reported at the weekly meetings. Men were encouraged to supplement walking with more strenuous activity if they were able to and to meet up outside the programme to train together. The aim was to achieve 45 minutes of moderate physical activity most days. Avoidance of behaviours that would undermine weight loss was also encouraged b: Waiting list: A comparison group were randomised to receive the FFIT intervention 4 months later Duration of active intervention: 12 weeks No. of times contacted: 13 No. allocated: a: 51; b: 52 No. completed: a: 44; b: 42 Dropout (%): a: 13.7; b: 19.2 No. assessed: a: 44; b: 42 |
Length of follow-up: 12 weeks Outcomes: Weight, waist circumference |
FFIT feasibility pilot RCT. The main trial was ongoing at the time of publication |
| Leslie 2002146 | Study design: RCT Location: One petrochemical worksite, Glasgow, UK Period of study: NR Inclusion criteria: BMI ≥ 25 kg/m2 Exclusion criteria: Under supervision of the worksite medical officer, diabetic requiring insulin, any condition requiring prescribed foods or specialist dietary intervention, intentional weight loss > 3 kg in the previous 3 months Age (years), mean (SD): a: 41.3 (8.1); b: 42.1 (7.8) Weight (kg), mean (SD): a: 98.2 (13.9); b: 94.6 (13.3) BMI (kg/m2), mean (SD): a: 31.5 (3.7); b: 30.4 (3.7) Baseline comparability: Group a heavier with higher triglyceride levels |
Description of interventions: Weight-loss phase: weeks 0–12 a: Energy-deficient diet – individualised energy prescriptions calculated taking age, sex and body weight into account to induce a daily 600-kcal deficit using Schofield equations and 1.3 × basal metabolic rate activity factor b: Generalised low calorie diet – generalised 1500 kcal per day diet Participants within a and b were randomised to meat and no meat groups. Participants in the meat group consumed red meat at least five times per week. Participants in the non-meat group substituted red meat with fish, eggs and cheese Weight-maintenance phase: weeks 12–24: Diet and energy requirements recalculated for weight stability rather than loss Duration of active intervention: 12 weeks No. of times contacted: 12 No. allocated: a: 61; b: 61 No. completed: a: 45; b: 40 Dropout (%): a: 26.2; b: 34.4 No. assessed: a: 61; b: 61 |
Length of follow-up: 24 weeks Outcomes: Weight, BMI, total, LDL and HDL cholesterol, triglycerides |
|
| Witty 2010149 | Study design: Prospective cohort Location: Stadium and training ground for Leeds Rhinos Rugby League Club, Leeds, UK Period of study: 2009 rugby league season Inclusion criteria: NR Exclusion criteria: NR Age: NR Weight (kg), mean (SD): 105.5 (28.5) BMI: NR Baseline comparability: NA |
Description of intervention: The Tackling Men’s Health initiative was developed out of a partnership between the Department of Health, Leeds Rhinos Rugby League Club and Leeds Metropolitan University. The pilot health programme was a multicomponent health promotion intervention designed to be delivered alongside league fixtures and targeted at men attending Leeds Rhinos Headingley Carnegie Stadium with the aim of promoting engagement with local and national health services and promoting health and well-being. The original intervention design consisted of 12 themed match days. Themes included mental health, diet and nutrition, exercise and sexual health. Match-day themes were chosen to link in with a common theme spanning the length of the rugby league season. The theme for the pilot season was obesity and a weight-loss group was run at the Kirkstall Road training ground Duration of active intervention: 6 weeks No. of times contacted: 7 No. allocated: 7 No. completed: 7 Dropout (%): 0 No. assessed: 7 |
Length of follow-up: 6 weeks Outcomes: Weight |
|
| Department of Health and Leeds Metropolitan University 2010139 | Study design: Prospective cohort Location: Stadium and training ground for Leeds Rhinos Rugby League Club, Leeds, UK Period of study: 2010 rugby league season Inclusion criteria: NR Exclusion criteria: NR Age: NR Weight (kg), mean (SD): 108.03 (18.69) BMI (kg/m2), mean (SD): 35.24 (5.75) Baseline comparability: NA |
Description of intervention: In continuation of the Leeds Rhinos programme (described in Witty and White149), NHS Leeds ran an 8-week men-only ‘10% Lifestyle Course’ from 21 April to 9 June 2010. The course consisted of eight weekly sessions each lasting for 90 minutes. The first 45 minutes consisted of a theory session, covering the following issues: session 1 – introduction, physical activity screening, food/activity diaries; session 2 – review of food/activity diaries, goal setting; session 3 – making changes to lifestyle; session 4 – Eatwell plate/portion size; session 5 – physical activity – why do it, how much, how hard?; session 6 – maintaining changes; session 7 – Leeds Rhinos session (nutrition of a rugby player) – had to be postponed; session 8 – way forward/evaluation session. The remaining 45 minutes were for physical activity. Sessions consisted of a mixture of activities such as gym sessions, walking and circuits: session 1 – introduction, physical activity screening; session 2 – circuit-based class, combination of aerobic and resistance training; sessions 3–5 – gym sessions: men spent 5 minutes on four different machines; they were provided with a record sheet so that they could monitor levels worked at and how far they travelled on cardio machines; for resistance the attendees could use either light weights or dynabands; session 6 – following risk assessment a walk from the Kirkstall Road training ground along the canal; a member of Leeds Rhinos staff acted as a back marker and carried a first aid kit; consisted of a 10-minute gradual warm-up, 20 minutes’ walking at moderate intensity, 5–10 minutes’ slowing down (low intensity) and 5 minutes’ stretching on the training ground; session 7 – led by staff from Leeds Rhinos (supported by weight management physical activity staff); took place outside on the training field and included small games and rugby drills; session 8 – poor weather so a final gym session was completed Duration of active intervention: 8 weeks No. of times contacted: 9 No. allocated: 12 No. completed: 10 Dropout (%): 16.7 No. assessed: 10 |
Length of follow-up: 8 weeks Outcomes: Weight, BMI, waist circumference |
Men’s Health Plus provided the names of the six men who attended the weight management group run during the 2009 season who wished to continue attending a group to facilitate further weight loss. Unclear if these men were recruited to the 2010 season programme |
| Holt 2007145 | Study design: Retrospective cohort Location: Multiple community locations, UK Period of study: 1998–2005 Inclusion criteria: BMI > 29 kg/m2, completed brief medical questionnaire during GP consultation Exclusion criterion: Contraindications for a very low-calorie diet (VLCD) Age: NR Weight (kg), mean: 121.5 BMI (kg/m2), mean: 38.5 Baseline comparability: NA |
Description of intervention: VLCD and behavioural therapy delivered to men-only groups by a commercial provider, LighterLife. VLCD formula diet of three food packs per day, 550 kcal per day, 50 g protein, 50 g carbohydrate and 100% recommended daily allowances for vitamin and minerals. Behavioural therapy based on transactional analysis and cognitive–behavioural therapy and addiction/change theory. Men abstained from conventional foods replacing them with fortified ‘Man Plan’ food packs for 8 weeks initially. Men were also advised to avoid alcohol consumption. LighterLife counsellors provided support to help men explore reasons for their overeating, develop practical and psychological strategies for long-term weight management and help them implement and sustain healthy lifestyle changes. Men were required to have GP check-ups every 28 days whilst using a VLCD for total nutrition. Once men had achieved their target weight they were encouraged to attend a weight management group for at least 1 year free of charge (VLCD formula foods were available to purchase if required) Duration of active intervention: 8 weeks No. of times contacted: 11 No. allocated: NR No. completed: 1279 Dropout (%): NR No. assessed: 1279 |
Length of follow-up: 8 weeks Outcomes: Weight, BMI, motivating factors |
|
| Hallam Spencer 2008143 | Study design: Retrospective cohort Location: Multiple community locations, UK Period of study: 2007 Inclusion criteria: BMI > 29 kg/m2, completed brief medical questionnaire during GP consultation Exclusion criterion: Contraindications for a VLCD Age: NR Weight (kg), mean: 121.3 BMI (kg/m2), mean: 38.0 Baseline comparability: NA |
Description of intervention: LighterLife VLCD programme as described in Holt et al. 145 Duration of active intervention: 8 weeks No. of times contacted: 11 No. allocated: NR No. completed: 1000 Dropout (%): NR No. assessed: 1000 |
Length of follow-up: 8 weeks Outcomes: Weight, BMI |
|
| Salsbury 2009148 | Study design: Retrospective cohort Location: Multiple community locations, UK Period of study: 2008 Inclusion criteria: BMI > 29 kg/m2, completed brief medical questionnaire during GP consultation Exclusion criterion: Contraindications for a VLCD Age: NR Weight (kg), mean 119.17 BMI (kg/m2), mean: 37.35 Baseline comparability: NA |
Description of intervention: LighterLife VLCD programme as described in Holt et al. 145 Duration of active intervention: 8 weeks No. of times contacted: 11 No. allocated: NR No. completed: 2200 Dropout (%): NR No. assessed: 2200 |
Length of follow-up: 8 weeks Outcomes: Weight, BMI |
|
| Hallam 2010144 | Study design: Retrospective cohort Location: Multiple community locations, UK Period of study: January–September 2009 Inclusion criteria: BMI > 29 kg/m2, completed brief medical questionnaire during GP consultation Exclusion criterion: Contraindications for a VLCD Age: NR Weight (kg), mean: 123.2 BMI (kg/m2), mean: 38.6 Baseline comparability: NA |
Description of intervention: LighterLife VLCD programme as described in Holt et al. 145 Duration of active intervention: 8 weeks No. of times contacted: 11 No. allocated: NR No. completed: 950 Dropout (%): NR No. assessed: 950 |
Length of follow-up: 8 weeks Outcomes: Weight, BMI |
|
| Hallam 201135 | Study design: Retrospective cohort Location: Multiple community locations, UK Period of study: January–August 2010 Inclusion criteria: BMI > 29 kg/m2, completed brief medical questionnaire during GP consultation Exclusion criterion: Contraindications for a very low-calorie diet (VLCD) Age: NR Weight (kg), mean: 124.0 BMI (kg/m2), mean: 39.0 Baseline comparability: NA |
Description of intervention: LighterLife VLCD programme as described in Holt et al. 145 Duration of active intervention: 8 weeks No. of times contacted: 11 No. allocated: NR No. completed: 1006 Dropout (%): NR No. assessed: 1006 |
Length of follow-up: 8 weeks Outcomes: Weight, BMI |
|
| McFarlane 2006147 | Study design: Prospective cohort Location: One community centre, Arbroath, UK Period of study: March 2006 Inclusion criteria: NR Exclusion criteria: NR Age (years), mean: 59 Weight: NR BMI (kg/m2), mean: 32.4 Baseline comparability: NA |
Description of intervention: Men-only weight management programme (Bloke’s Weigh) jointly developed by the NHS Angus Weight Management Project Facilitator and the MACH4 (Male Checks for Health) Men’s Health Project. Group meetings were held in a church hall where men were given information and advice about weight-loss topics, including healthy eating/basic nutrition, causes of weight gain, benefits of weight loss, fat and sugar content of foods, food labelling advice, the role of alcohol in weight gain, the importance of exercise, exercise referral and the importance of adequate fluid intake. Behaviour change topics such as self-monitoring, hunger scores and decisional balance were also discussed Duration of active intervention: 10 weeks No. of times contacted: 10 No. allocated: 38 No. completed: 18 Dropout (%): 52.6 No. assessed: 23 (regular attenders) |
Length of follow-up: 10 weeks Outcomes: Weight, BMI, waist circumference |
|
| Bye 200534 | Study design: Retrospective cohort Location: Multiple community locations, UK Period of study: NR Inclusion criterion: Membership of a men-only Slimming World group Exclusion criterion: Attendance < 8 weeks Age (years), mean, median (range): 49.4, 47 (29–71) Weight (kg), mean, SD (range) 114.7, 19.9 (79.6–210.7) BMI (kg/m2), mean, SD (range): 35.9, 6.1 (27.9–53.6) Baseline comparability: NA |
Description of intervention: Men-only commercial weight management programme (Slimming World). Standard open-sex programme adapted to offer men-only group meetings. Programme consisted of an individualised diet plan including unlimited ‘free foods’ and advice for satisfying appetite and recipe and menu ideas. Men were also given advice for increasing physical activity and increasing motivation. Support and encouragement obtained through group motivating sessions led by trained Slimming World consultants Duration of active intervention: At least 8 weeks No. of times contacted: ≥ 8 No. allocated: 125 No. completed: Unclear Dropout (%): Unclear No. assessed: 16 in programme at 24 weeks |
Length of follow-up: 24 weeks Outcomes: Weight, BMI |
|
| Poulter 201233 | Study design: Prospective cohort Location: Three NHS primary care trust communities, UK Period of study: NR Inclusion criteria: Overweight/obese, NHS referral to Weight Watchers Exclusion criteria: NR Age (years), mean (SD): 47.6 (12.7) Weight: NR BMI (kg/m2), mean (SD): 36.1 (5.8) Baseline comparability: NA |
Description of intervention: NHS-funded referral to a commercial weight-loss programme (Weight Watchers; as described for Ahern et al. 37) but the standard open-gender model was adapted to provide men-only meetings. No other changes made except in two of three locations a 30-minute ‘add-on’ exercise class led by qualified local authority fitness instructors was delivered after the standard Weight Watchers meeting Duration of active intervention: 12 weeks No. of times contacted: 12 No. allocated: 62 No. completed: 39 Dropout (%): 37.1 No. assessed: 62 |
Length of follow-up: 12 weeks Outcomes: Weight, BMI |
| Study ID | Participants | Interventions | Outcomes | Notes |
|---|---|---|---|---|
| Ross 200831 | Study design: Prospective cohort Location: 56 NHS GP practices, UK Period of study: 1 January 2001–31 December 2004 Inclusion criteria: Age 18–75 years, BMI ≥ 30 kg/m2 (or ≥ 28 kg/m2 with obesity related comorbidities), participant at the contemplative or action stage of the transtheoretical model of behaviour change Exclusion criteria: NR Age (years), mean (SD): Men + women: 49.4 (13.5) Weight: NR BMI (kg/m2), mean (SD): Men + women: 37.1 (6.0) Baseline comparability: NR |
Description of intervention: Primary care weight management programme (Counterweight) based on the transtheoretical model of behaviour change. Programme was delivered by GP practice nurses and health-care assistants usually as part of nurse appointments for managing comorbid conditions or through dedicated weight management clinics. Involved a prescribed eating plan delivered individually or in groups to achieve an energy deficit of ≥ 500 kcal per day and weight loss of 5–10%. Participants were taught behaviour change skills and provided with tools to achieve individual or group goals: daily living diary, healthy eating quizzes, personal weight-loss plans, prescribed number of food group servings, advice for reading food labels, meal planning and eating out. Reasons for emotional eating and social pressure to eat were discussed. Advice for increasing physical activity was also given and participants were offered referral to a GP exercise scheme if this was appropriate/available. Participants were also counselled to reshape negative thoughts and to enable successful weight maintenance and prevent relapse Duration of active intervention: 12 weeks No. of times contacted: 12 No. eligible at 1 year: Men: 438; women: 1468 No. completed at 1 year: Men: 171; women: 471 Dropout (%): Men: 51.0; women: 67.9 No. assessed: Men: 171; women: 471 |
Length of follow-up: 24 months (outcomes by sex reported at 12 months) Outcomes by sex: Weight |
|
| Dixon 2012150 | Study design: Retrospective, non-randomised, comparative cohort (service evaluation) Location: 12 GP surgeries, England, UK Period of study: December 2007–May 2010 Inclusion criteria: Age > 16 years, BMI ≥ 30 kg/m2 with either a raised waist measurement > 94 cm or one or more comorbidity or other reason stated by the clinician, ready to lose weight. Some clinicians referred patients with a BMI < 30 kg/m2 using the ‘other reason’ option. All patients were included in the evaluation Exclusion criterion: Attended a slimming club in the previous 6 months Age (years), mean: Men + women: 47.1 Weight (kg), mean (SD): Men + women: Slimming World: 104.7 (19.8); Weight Watchers: 104.3 (19.8); Rosemary Conley: 100.6 (16.8) BMI (kg/m2), mean (SD): Men + women: Slimming World: 37.7 (5.8); Weight Watchers: 37.9 (6.2); Rosemary Conley: 37.6 (5.7) Baseline comparability: Yes |
Description of intervention: Pilot NHS slimming on referral scheme. Participants were offered 12 free weekly sessions at one of three commercial providers: Weight Watchers, Slimming World or Rosemary Conley Diet and Fitness Clubs. All providers used group sessions lasting 60–90 minutes allowing for brief one-to-one support during weighing. Participants received information plus optional support by telephone or through websites. Weight Watchers and Rosemary Conley aimed for a set amount of weight loss whereas Slimming World set individual weight-loss goals. All providers encouraged a balanced diet and an increase in levels of physical activity. Rosemary Conley also included an optional 45-minute exercise class Duration of active intervention: 12 sessions No. of times contacted: 12 No. allocated: Men: 150; women: 907 Number completed: Men: 63; women: 414 Dropout (%): Men: 58.0; women: 54.4 No. assessed: Men: 150; women: 907 (last observation carried forward) |
Length of follow-up: 12 weeks Outcomes by sex: Likelihood of achieving 5 kg or 5% weight loss |
|
| Johnson 2011154 | Study design: Retrospective cohort/audit Location: UK Period of study: July 2005–November 2008 Inclusion criteria: Paying at least 1 month’s subscription to the Nutracheck internet weight-loss programme (NutraTech Ltd, Nottingham, UK), record of two or more weights in a 28-day period Exclusion criterion: Participation in the Nutracheck programme for too short a time to give reliable weight change data Age (years), mean (SD): Men: 39.2 (10.1); women: 35.5 (10.5) Weight (kg), mean (SD): Men: 99.0 (16.9); women: 78.0 (16.3) BMI (kg/m2), mean (SD): Men: 31.0 (4.7); women: 28.9 (5.8) Baseline comparability: Men were heavier and had a higher BMI than women |
Description of intervention: Internet-based diet and exercise advice – a commercial, online platform for completing food and exercise diaries in return for a monthly subscription charge. Personalised daily calorie targets are set, adjusting for an individual’s activity level and chosen rate of weight loss up to a maximum loss of 0.9 kg per week. A daily online food diary calculates an estimated daily calorie intake by linking to a database of > 40,000 branded and unbranded food items. A daily exercise diary calculates estimated energy expenditure from participant records. A target of 200 kcal per day energy expenditure is encouraged. Behaviour change supported through weight charting, self-monitoring and access to health and nutrition information and an online social community providing support and motivation Duration of active intervention: Mean 186.7 days No. of times contacted: Daily diary completion recommended and participants had voluntary access to the online social forum No. allocated: NR No. completed: Men: 642; women: 2979 Dropout (%): NR No. assessed: Men: 642; women: 2979 |
Length of follow-up: Mean 186.7 days Outcomes by sex: Weight |
|
| Kirk 2000155 | Study design: Prospective cohort Location: One university, Edinburgh, UK Period of study: January–March 1999 Inclusion criteria: Age 20–45 years, in good health, BMI 25–35 kg/m2, not on a special diet for medical reasons, not recently participated in a weight reducing diet, light to moderate habitual physical activity levels Exclusion criteria: Taking prescribed medication that might influence body weight, given up smoking in the previous 3 months Age (years), mean (SD): Men: 35.7 (9.0); women: 41.1 (12.2) Weight (kg), mean (SD): Men: 93.8 (8.0); women: 80.8 (8.9) BMI, mean (SD): Men: 30.6 (2.1); women: 31.2 (3.9) Baseline comparability: Women were older and had a higher BMI than men |
Description of intervention: An initial 2-week period of modest energy restriction followed by a 4-week high-carbohydrate dietary regime. During the initial 2 weeks, participants replaced one main meal, lunch or dinner, with a 45-g serving of a Kellogg’s breakfast cereal of the participant’s choice with 125 ml semi-skimmed milk. Following the energy restriction phase, meal replacement ended but participants were encouraged to eat breakfast cereals as snacks if desired. Participants were also given advice about increasing their carbohydrate intake and how to consume at least five portions of fruit and vegetables per day. Dietary advice was tailored to suit individual eating habits based on data obtained from participant food diaries. Participants were asked to maintain their normal levels of physical activity throughout Duration of active intervention: 6 weeks No. of times contacted: 3 No. allocated: Men + women: 29 No. completed: Men: 6; women: 16 Dropout (%): Men + women: 24 No. assessed: Men: 6; women: 16 |
Length of follow-up: 6 weeks Outcomes by sex: Weight loss |
|
| Rolland 2013156 | Study design: Retrospective cohort Location: Multiple community locations, UK Period of study: 2009–10 Inclusion criteria: Asian men and women recruited to LighterLife with 12-week weight change data available, completed brief medical questionnaire during GP consultation Exclusion criterion: Contraindications for a very low-calorie diet Age (years), mean (SD): Men: Asian: 39.8 (10.9), Caucasian: 39.9 (10.6); women: Asian: 35.9 (11.4), Caucasian: 35.9 (11.3) Weight (kg), mean (SD): Men: Asian: 117.0 (21.5), Caucasian: 126.5 (22.0); women: Asian: 91.7 (12.6), Caucasian: 95.4 (12.0) BMI (kg/m2), mean (SD): Men: Asian: 37.8 (5.8), Caucasian 38.5 (6.1); women: Asian: 35.4 (4.0), Caucasian: 35.4 (4.0) Baseline comparability: Asian men and women matched to a LighterLife Caucasian population for age, BMI and sex |
Description of intervention: As described for Salsbury et al. 148 delivered in same-sex groups Duration of active intervention: 12 weeks No. of times contacted: 15 No. allocated: Men: Asian: 81 (66 Indian, 15 Pakistani); women: 429 (316 Indian, 113 Pakistani). Both sexes matched to a Caucasian population No. completed: Men: 36; women: 166 Dropout (%): Men: 55.6; women: 61.3 No. assessed: Men: 36; women: 166 |
Length of follow-up: 12 weeks Outcomes by sex: Weight, waist circumference |
Substudy analysis |
| Evans 2011a152 | Study design: Retrospective cohort Location: 26 GP practices and four leisure centres within Hertfordshire NHS Primary Care Trust, UK Period of study: January 2009–December 2010 Inclusion criteria: Age ≥ 18 years, BMI ≥ 30 kg/m2 (or ≥ 28 kg/m2 with comorbidities) Exclusion criteria: Pregnant/breastfeeding women, history of eating disorder Age (years), mean (range) (attending five or more appointments): Men: 55 (27–81); women 52 (19–87) Weight (kg), mean (range) (attending five or more appointments): Men: 117.4 (77.0–203.0); women: 97.9 (60.2–164.0) BMI (kg/m2), mean (range) (attending five or more appointments): Men: 37.6 (26.6–71.1); women: 36.9 (25.5–60.3) Baseline comparability: Yes |
Description of intervention: Computer-based programme (ProHealthClinical) involving dietary and physical advice with behavioural therapy available to primary health-care professionals and offered to patients in dedicated sessions or integrated with chronic illness clinics (e.g. diabetes, cardiovascular disease). ProHealthClinical was developed by KasTech Ltd and allows health-care professionals to provide comprehensive, personalised guidance through structured healthy eating plans, physical activity advice, realistic goal setting, self-monitoring, positive reinforcement, personalised feedback and long-term support. The food and activity database can be customised for cultural preferences and to incorporate local community health and well-being initiatives Duration of active intervention: 12 weeks (six appointments) No. of times contacted: 6 No. allocated: NR No. completed (attended five or more appointments within 14 weeks): Men: 246; women: 677 Dropout (%): NR No. assessed: Men: 246; women: 677 |
Length of follow-up: 14 weeks Outcomes by sex: Weight |
Data by gender presented at the National Obesity Forum Eastern Regional Obesity Network meeting, December 2011 |
| Evans 2011b151 | Study design: Retrospective cohort Location: GP practices within NHS Cambridgeshire Primary Care Trust, UK Period of study: 2007–11 Inclusion criteria: NR Exclusion criteria: NR Age (years), mean (range) (attending six or more appointments): Men: 61 (34–80); women: 55 (18–84) Weight (kg), mean (range) (attending six or more appointments): Men: 115.3 (77.5–163.9); women: 95.4 (65.8–169.5) BMI (kg/m2), mean (range) (attending six or more appointments): Men: 37.1 (27.0–53.2); women: 35.6 (24.5–54.1) Baseline comparability: Men slightly older and had a higher BMI than women |
Description of intervention: ProHealthClinical (as described in Evans152) delivered as a group-based community health improvement programme. Lifestyle intervention workshops aimed to help participants achieve permanent changes through gradually changing their eating and activity routines through goal setting, self-monitoring, identifying/discussing barriers to change, positive feedback, problem-solving techniques and 2-week lifestyle challenges (e.g. consuming fruit and vegetable snacks only). Participants met fortnightly in small teams to support individual changes Duration of active intervention: 12 weeks (eight workshops) No. of times contacted: 8 No. allocated: NR No. completed (attended six or more workshops within 12 weeks): Men: 33; women: 123 Dropout (%): NR No. assessed: Men: 33; women: 123 |
Length of follow-up: 12 weeks Outcomes by sex: Weight |
Data not reported by sex in abstract. Received through personal communication from author |
| Evans 2012153 | Study design: Retrospective cohort Location: 23 GP surgeries, Cambridgeshire, UK Period of study: February 2010–October 2011 Inclusion criteria: Age ≥ 18 years, BMI ≥ 30 kg/m2 or ≥ 28 kg/m2 with comorbidities (high blood pressure, impaired glucose tolerance, type 2 diabetes) Exclusion criterion: Participants who did not demonstrate the motivation to lose weight in the first 4 weeks were discharged Age (years), mean (range) (attending five or more appointments): Men: 61 (26–82); women: 56 (20–81) Weight (kg), mean (range) (attending five or more appointments): Men: 117.9 (81.2–213.4); women: 98.1 (70.4–173.7) BMI (kg/m2), mean (range) (attending five or more appointments): Men: 37.5 (27.4–61.4); women: 37.3 (28.4–61.3) Baseline comparability: Yes |
Description of intervention: Peripatetic level 1 weight management services (Weigh2Go). Commissioned by Cambridgeshire Association to Commission Health (CATCH) using ProHealthClinical (as described in Evans152). The Cambridgeshire Community Services NHS Trust Dietetic Business Unit were responsible for training and supporting the primary care staff who delivered the intervention Duration of active intervention: 3 months No. of times contacted: 6 No. allocated (attending four or more appointments): Men: 627; women: 444 No. completed (attending five or more appointments): Men: 118; women: 294 Dropout (%): NR No. assessed (attending five or more appointments): Men: 118; women: 294 |
Length of follow-up: 12 months (outcomes by sex reported at 3-month follow-up) Outcomes by sex: Weight |
|
| Stubbs 201136 | Study design: Retrospective cohort Location: 77 primary care and NHS trust communities, UK Period of study: May 2004–November 2009 Inclusion criteria: Each participating primary care/NHS trust set its own referral criteria, which varied depending on local weight management care pathways; data on weight, height, age and sex available; had sufficient time to complete the 12-week programme Exclusion criteria: Age < 16 years, pregnant Age (years), mean (SD): Men: 51.4 (13.8); women: 46.8 (14.4) Weight (kg), mean (SD): Men: 117.9 (22.4); women 97.2 (18.5) BMI (kg/m2), mean (SD): Men: 37.8 (6.6); women: 36.7 (6.5) Baseline comparability: Men were older and slightly heavier with a higher BMI |
Description of intervention: Primary care and NHS trust referral to commercial weight management provider (Slimming World). Participants issued 12-week voucher packs for Slimming World (funded by primary care/NHS trusts and subsidised by Slimming World). Vouchers could be used to attend local Slimming World group of choice. Participants treated and delivered the usual Slimming World programme in exactly the same way as private members (as described for Bye et al. 34) Duration of active intervention: 12 weeks No. of times contacted: 12 No. allocated: Men: 3651; women: 30,620 No. completed: Men: 2329; women: 17,578 Dropout (%): Men: 36.2; women: 42.6 No. assessed: Men: 3651; women: 30,620 |
Length of follow-up: 12 weeks Outcomes by sex: Weight, BMI |
|
| Stubbs 2012157 | Study design: Retrospective cohort Location: 45 primary care or NHS trusts, UK Period of study: May 2004–November 2009 Inclusion criteria: Participants were offered subsequent referral at the referrer’s discretion. Criteria for this varied between NHS trusts but included the selection of those who were deemed to be motivated by having a higher starting BMI, those making gradual progress towards their treatment objectives, those with comorbidities that would be improved by further weight loss and those unable to self-fund further attendance Exclusion criteria: Age < 16 years, pregnant women Age (years), mean (SD): Men: 53.9 (13.1); women: 49.2 (14.4) Weight kg: Mean (SD) Men: 119.9 (21.8); Women: 100.4 (19.4) BMI (kg/m2), mean (SD): Men: 38.4 (6.3); Women: 37.9 (6.8) Baseline comparability: Men were older and heavier than women |
Description of intervention: As described in Stubbs et al. 36 except that some of the NHS trusts involved in the Stubbs 201136 audit offered a second, consecutive referral of 12 sessions Duration of active intervention: 24 weeks No. of times contacted: Up to 24 No. allocated: Men: 575; women: 4179 No. completed (attended at least 20/24 sessions): Men: 475; women: 3168 Dropout (%): Men: 17.4; women: 24.2 No. assessed: Men: 575; women: 4179 |
Length of follow-up: 24 weeks Outcomes by sex: Weight, BMI |
|
| Ahern 201137 | Study design: Retrospective cohort Location: NHS primary care trust communities, UK Period of study: 2 April–6 October 2009 Inclusion criterion: NHS referral to Weight Watchers Exclusion criteria: NR Age (years), median (IQR): Men + women: 49 (38 to 61) Weight (kg), median (IQR): Men + women: 94.3 (83.7 to 107.7) BMI (kg/m2), median (IQR): Men + women: 35.1 (31.8 to 39.5) Baseline comparability: NR |
Description of intervention: NHS-funded referral to commercial weight-loss programme. Participants received vouchers to attend 12 Weight Watchers group meetings. Participants followed the standard Weight Watchers ProPoints® Plan. The plan allows daily and weekly point allowances for food and drinks. Participants were also provided with information and tips for healthy eating/lifestyle, menus and the ProPoints guide booklet. An online calculator and tools and mobile smart phone apps were also available. A ProPoints calculator and restaurant guide were available to buy at meetings. Social support was provided by attendance at group meetings and through Facebook, Twitter and YouTube Duration of active intervention: 12 sessions No. of times contacted: 12 No. allocated: Men: 3074; women: 26,252 No. completed: Men: 1274; women: 10,577 Dropout (%): Men: 58.6; women: 59.7 No. assessed: Men: 1274; women: 10,577 |
Length of follow-up: > 24 weeks Outcomes by sex: % weight change |
Four reports were of studies using male-orientated sports settings to facilitate recruitment and intervention delivery; two involved four Scottish Premier League (SPL) football clubs138,141 and two involved one Rugby League club. 139,149 Four reports were of interventions provided by commercial organisations34,148,154,156 and five reports were of NHS referrals to commercial organisations. 33,36,37,150,157 A further three reports151 – 153 were of a commercial computer package for use by health-care professionals in the NHS primary and secondary care setting. Two studies were set in men’s health clinics run by NHS primary care142,147 and two were set in the workplace. 140,146 One study was set in the NHS GP setting31 and one examined the promotion of dietary carbohydrate intake in individuals in the community. 155
In terms of the interventions, three studies provided dietary advice only. 140,146,155 It was not clear whether or not any advice on behaviour change was given in these studies. All of the remaining studies discussed in this chapter provided dietary and exercise advice and behaviour change training, and four of these also provided an exercise programme to attend. 138,139,141,149 An exercise programme is also provided in the Rosemary Conley Diet and Fitness Clubs, which was one of the interventions evaluated by Dixon and colleagues. 150
Characteristics of the men
A total of 11,426 men were allocated to an intervention and 8957 were included in the report analyses. Men represented 11.7% of the population of mixed-sex studies ( Table 28 ). The youngest reported mean age for men was 39 years and the oldest was 61 years. The lowest reported mean BMI was 30.6 kg/m2 and the highest was 39.0 kg/m2. The lowest reported mean weight was 93.8 kg and the highest was 126.5 kg.
| Study ID | No. of participants | % men | ||
|---|---|---|---|---|
| Men | Women | All | ||
| Ross 200831 (Counterweight) | 438 | 1468 | 1906 | 23.0 |
| Dixon 2012150 | 150 | 907 | 1057 | 14.2 |
| Kirk 2000155 | 6 | 16 | 22 | 27.3 |
| Rolland 2013156 | 81 | 429 | 510 | 15.9 |
| Johnson 2011154 (Nutracheck) | 642 | 2979 | 3621 | 17.7 |
| Evans 2011152 (ProHealthClinical, Hertfordshire)a | 298 | 846 | 1144 | 26.0 |
| Evans 2011151 (ProHealthClinical, Cambridgeshire)a | 43 | 179 | 222 | 19.4 |
| Evans 2012153 (ProHealthClinical)a | 118 | 294 | 412 | 28.6 |
| Stubbs 201136 (Slimming World) | 3651 | 30,620 | 34,271 | 10.7 |
| Ahern 201137 (Weight Watchers) | 3074 | 26,252 | 29,326 | 10.5 |
| Total | 8501 | 63,990 | 72,491 | 11.7 |
Overview of types of outcomes reported
All studies reported either baseline and end weights or changes in weight. Nine studies provided details of change in BMI33,34,36,139,142,146 – 148,157 and four reported changes in waist circumference. 139,141,147,156 One study reported changes in total cholesterol and blood pressure138 and one reported changes in total, LDL and HDL cholesterol and triglycerides. 146 Twelve studies gave details of percentage change in body weight. 34,36,138,140 – 142,147,151 – 154,156 Nine studies reported achieving 5 kg or 5% or 10% weight loss. 34,36,142,147,150 – 154
Six reports138,139,141,142,147,155 conducted a formal process evaluation.
Quality of the evidence
Risk of bias
The risk of bias assessment for the individual studies is shown in Appendix 12 (see Tables 60 and 61 ). Only one full-text publication of an RCT146 was identified; this study was judged to be at high risk of bias for the sequence generation, allocation concealment and blinding (participant and health-care provider) domains of The Cochrane Collaboration’s risk of bias tool (see Appendix 12 , Table 60 ). 57 It was unclear whether or not outcome assessors were blinded, all groups were treated identically and if the authors carried out intention-to-treat analyses. The trial was, however, judged to be at low risk of bias for incomplete data. The trial by Gray and colleagues141 was excluded from the risk of bias assessment as outcome data were available only in abstract format.
The remaining 14 full-text publications were assessed using the ReBIP quality assessment tool for non-randomised comparative and case series studies (see Appendix 12 , Table 61 ). Figure 38 summarises the risk of bias assessment for these studies. Items in italics are valid for comparative studies only. The majority (57.1%) of studies included a representative participant sample. Rolland and colleagues156 analysed data for participants who had baseline and 12-week weight data only and Kirk and colleagues155 included only healthy overweight participants. Data were largely collected prospectively and interventions were delivered by appropriate people (71.4% and 64.3% of studies respectively). All studies used valid objective outcome measures but few (28.6%) included a sufficient follow-up time (here considered to be at least 1 year) to give meaningful information on the sustainability of weight loss. Just over half of the studies (57.1%) provided information on participant dropouts. Many items were unclear because of insufficient reporting by study authors and it is therefore not possible to summarise the impact of these biases on the overall body of evidence.
FIGURE 38.
Summary of risk of bias assessment of non-RCT studies included in the review of UK interventions.
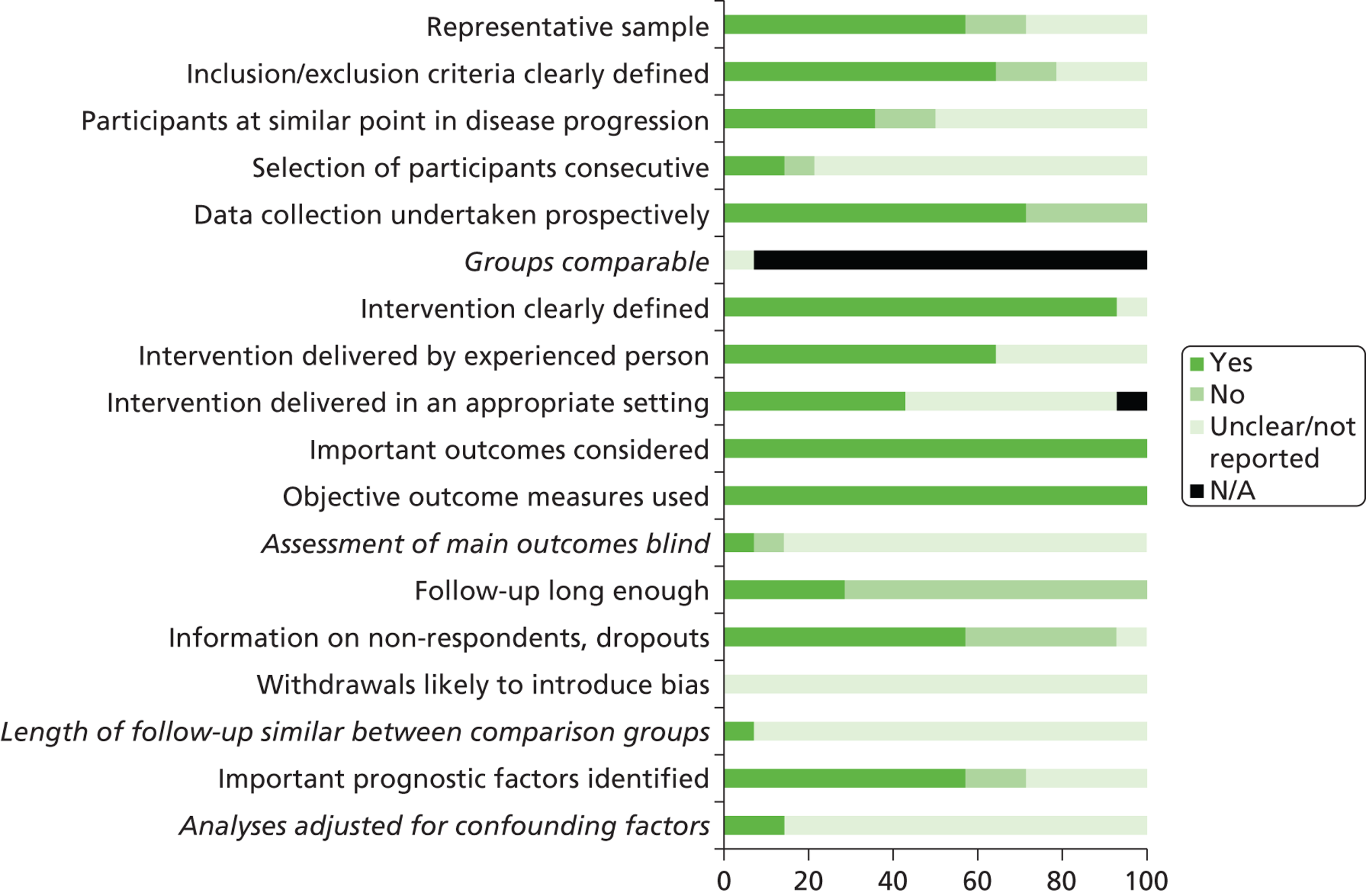
Assessment of equity and sustainability
We assessed 16 studies for equity and sustainability. Results for the individual studies are detailed in Appendix 12 (see Table 62 ). Although the Football Fans in Training (FFIT) pilot RCT141 was excluded from the risk of bias assessment, details of the intervention and intervention delivery were made available to us by the study authors (Kate Hunt, University of Glasgow, February 2012, personal communication). We therefore included this study in our assessment, summarised in Figure 39 . Just over half (56.3%) of the studies were conducted in settings considered to target or exclude specific populations. Three studies targeted a male sports fan base138,139,141,149 and two studies targeted participants in their workplace. 140,146 Three studies of commercial providers34,154,156 were judged to have potentially excluded populations unable to afford their subscription charges. None of the studies reported on sociodemographic differences between participant dropouts and withdrawals, although many (56.3%) reported details for some PROGRESS categories at baseline. Few (25.0%) considered sustainability or discussed their interventions in political or other organisational contexts (31.2%). Four studies described partnerships between academic and sporting institutions and the NHS138,139,141,149 and four described partnerships between commercial organisations and the NHS. 33,36,37,150
FIGURE 39.
Summary of the equity and sustainability assessment of trials included in the review of UK interventions.
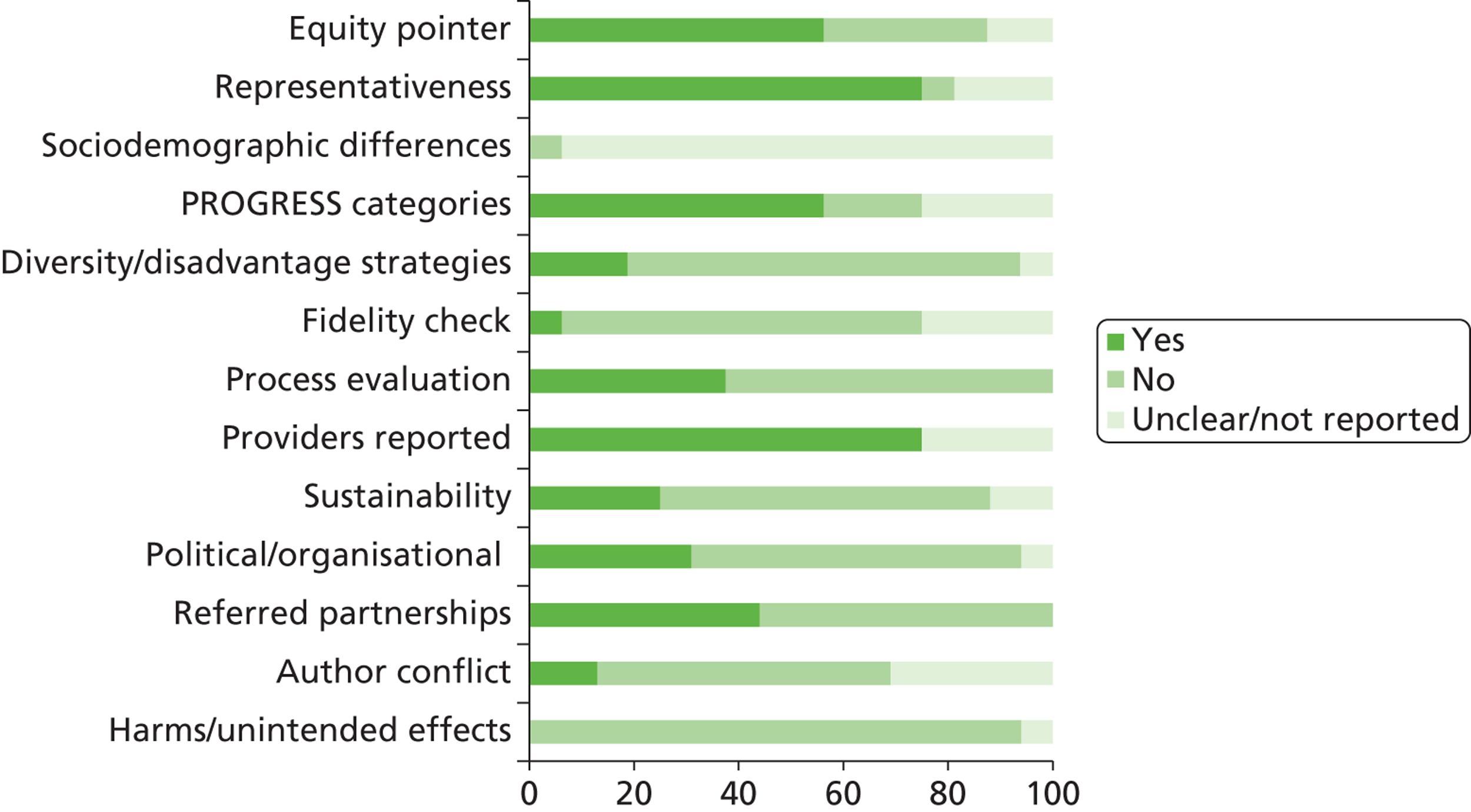
None of the studies indicated whether participants had experienced adverse harms as a result of the interventions. The Slimming World studies34,36 had potential conflicts of interest as the study authors are employed by Slimming World and the research was funded by the organisation. Conflict of interest was less clear for the Rolland and colleagues’ LighterLife study,156 with some of the authors acting as LighterLife consultants and research funding provided by LighterLife. Furthermore, although Johnson and colleagues154 declared that they had no conflict of interest in their analysis of Nutracheck subscribers, the data were supplied by a company representative who also commented on the study analysis. Conflict of interest was also unclear in the study by Drummond and colleagues. 140 Although the authors were independent, the Carb Boosters snacks used in the study are manufactured by the study funder, the Sugar Bureau. Similarly, the study by Leslie and colleagues146 included meat and no meat groups and was funded by the Meat and Livestock Commission, and the study by Kirk and colleagues155 of breakfast cereal was supported by the Kellogg Company of Great Britain. Other items were largely unclear because of inadequate reporting issues.
Assessment of effectiveness
Because of the heterogeneity of the included studies, we did not attempt any quantitative synthesis of the results. Instead, summary data from the included studies and a narrative overview of each study are presented.
Men-only programmes
Weight-loss programmes and the workplace
Two studies investigated the delivery of dietary interventions to promote weight loss in a male-orientated workplace setting. The first of these146 was conducted in a single petrochemical worksite in Glasgow over a 24-week period. Men were randomised to receive either an individualised energy-deficient diet or a general low-calorie diet. Men in the energy-deficient diet group received individualised energy prescriptions calculated in accordance with their age, sex and body weight to produce a 600-kcal daily energy deficiency. Men in the general diet group consumed 1500 kcal per day. The weight-loss phase of the trial lasted 12 weeks. During weeks 12–24, diet and energy requirements were recalculated for weight maintenance rather than weight loss. Within each group, men were also randomised to consume meat or no meat. Men in the meat groups consumed red meat at least five times per week. Men in the non-meat groups substituted red meat with fish, eggs and cheese. Intention-to-treat analyses showed no significant difference in weight loss between the energy-deficient diet and the general low-calorie diet during the weight-loss phase [–3.7 kg (SD 3.4 kg) vs. –3.9 kg (SD 3.5 kg) respectively, p = 0.78]. Differences between groups for other risk factors were also non-significant ( Table 29 ). For those who completed the programme, both groups significantly gained weight during the weight-maintenance phase but the difference between groups was not significant [+0.9 kg (SD 2.0 kg) vs. +1.4 kg (SD 1.6 kg), p = 0.27].
| Risk factor | Energy-deficient diet (n = 49) | General low-calorie diet (n = 42) |
|---|---|---|
| Weight loss (kg) | –4.3 (3.4) | –5.0 (3.5) |
| Waist circumference (cm) | –4.7 (3.4) | –5.2 (3.4) |
| Total cholesterol (mmol/l) | –0.20 (0.6) | –0.34 (0.5) |
| LDL cholesterol (mmol/l) | –0.09 (0.5) | –0.20 (0.2) |
| HDL cholesterol (mmol/l) | +0.005 (0.1) | –0.02 (0.2) |
| Triglycerides (mmol/l) | –0.2 (0.8) | –0.2 (0.8) |
Data for meat/non-meat participants were not reported by the original diet group. Meat consumption did not significantly affect weight loss or biochemistry. Average weight loss during the weight-loss phase for those men who completed the meat and non-meat programmes was –4.2 kg (SD 3.7 kg) and –5.0 kg (SD 3.2 kg) respectively. Again, both groups gained weight during the weight-maintenance phase [+0.9 kg (SD 1.6 kg) vs. +1.4 kg (SD 2.0 kg)]. The study authors commented that weight loss was less than expected and the dropout rate was significantly greater in the general low-calorie diet group.
The study conducted by Drummond and colleagues140 aimed to recruit male and female taxi drivers, but only men volunteered. Men followed a low-fat, high-carbohydrate (sugar-containing), energy-restricted diet that was tailored to produce a daily energy deficit of 600–700 kcal for each man over a 12-week period. Men were also advised to consume specialist sugar-containing Carb Boosters snacks to prevent a ‘starve and binge’ dieting pattern. The study was funded by the Sugar Bureau. By 12 weeks both BMI and weight were significantly reduced (p < 0.05). The average weight loss in completers was –5.5 kg, representing a 5.2% reduction in total body weight. The waist-to-hip ratio was reduced from 1.00 to 0.97. The men also perceived that their quality of life had increased over the 12-week period.
Men’s health clinics
Two studies evaluated NHS-delivered, men-only weight management groups. The Bloke’s Weigh programme147 was jointly developed by the NHS Angus Weight Management Project Facilitator and the MACH4 (Male Checks for Health) Men’s Health Project to provide a weight management group to the men of Arbroath, a town in Scotland that is recognised to have high levels of social deprivation. The men met in a local church hall and received advice for following a low-fat diet and increasing levels of physical activity over a 6-week period, extended to 10 weeks because of demand. Causes of weight gain, the role of alcohol, self-monitoring and the benefits of weight loss were also discussed. Four men also approached the Men’s Health Project worker for a health check and advice outside the group environment; areas of concern were weight and diet related but mental health issues were also raised. Twenty-three of the 38 participants attended the sessions regularly. Of these, four men gained weight but the other men showed varying degrees of weight loss (15 men lost 1–6 kg and four men lost 7–11 kg). Reductions in BMI and waist circumference were also achieved ( Table 30 ).
| Outcome | No. of men (n = 23) |
|---|---|
| Weight | |
| Gain | 4 |
| Lost 1–6 kg | 15 |
| Lost 7–11 kg | 4 |
| BMI | |
| Increase | 5 |
| Reduced by 2 kg/m2 | 14 |
| Reduced by up to 4 kg/m2 | 4 |
| % of baseline weight lost | |
| Increase | 4 |
| ≤ 5 | 14 |
| 6–10 | 3 |
| 11–15 | 2 |
| Waist circumference | |
| Increase | 7 |
| Lost ≤ 5 cm | 7 |
| Lost > 5 cm to ≤ 10 cm | 8 |
| Lost > 10 cm to ≤ 16 cm | 1 |
McFarlane and colleagues147 conducted a process evaluation of the Bloke’s Weigh programme. Ten men stated that they would have attended the programme in mixed-sex groups, nine stated that they would not or probably would not have attended and one man stated that he would possibly have attended. The authors reported that the majority of men enjoyed all sessions but that they would have preferred more meetings over a longer time period and the inclusion of an exercise class. Some men stated that a 19:00 start time would be more convenient than 18:00. Blood pressure measurements and relaxation/stress management techniques were also recommended for future programmes.
Gray and colleagues142 evaluated a group-based weight management programme aimed at men in the Camelon and Grangemouth areas of Scotland. Twelve weekly group sessions were held in local community health clinics. The men were also given advice for following a low-fat reducing diet and increasing physical activity with the aim of losing 0.5–1 kg per week. The psychology of behaviour change and value of social support were also discussed. Men were also invited to join organised post-programme meetings to facilitate long-term weight management. Men with a BMI of ≥ 35 kg/m2 were more likely to enrol in the programme than those with a BMI of < 30 kg/m2. The majority of men who enrolled were married and employed (both 73.5%). Almost half (47.9%) of the men came from households classed as deprived according to the Scottish Index of Multiple Deprivation (see www.scotland.gov.uk/Topics/Statistics/SIMD), but none was from an area classified in the most deprived quintile. In total, 80 men completed the 12-week programme. The average weight loss was 5.0 kg (range –17.2 kg to +2.6 kg); the average reduction in BMI was 1.3 kg/m2 (range –5.5 kg/m2 to +2.2 kg/m2); and the average reduction in waist circumference was 7.5 cm (range –27.5 cm to +3.0 cm). At 12 weeks, 35.4% of completers had lost ≥ 5% of their body weight and 8.9% had lost ≥ 10%. Following programme completion, weight-loss records were available for 20 men. These men were between 1 and 49 months post programme and had maintained an average 3.7% weight loss compared with their baseline weight (range –32.6% to +25.6%). In total, 14 men were lighter than their baseline weight, two were stable and four had gained weight compared with their original starting weight. The men’s experiences of being in the programme were evaluated using focus group interviews. The results of this evaluation are discussed in Chapter 6 .
Sports clubs
Four studies138,139,141,149 investigated the use of male-orientated sports clubs to deliver nutritional advice and an exercise programme in men-only groups. The study by Brady and colleagues138 included male season ticket holders at Glasgow Rangers and Celtic football clubs. Both clubs were part of the SPL at the time of the study. Interested men were asked to provide details of their height, weight, general fitness level and approximate level of general health. Men were graded according to their BMI measurement and those with the highest BMI were selected first. A total of 20 men were invited to each club for the first programme cycle. There were two early withdrawals before the initial assessment but these places were readily filled by others. The authors reported that almost all men underestimated their true weight and overestimated their height. The men attended 10 weekly sessions lasting 2 hours at their respective club stadiums. The first hour covered discussion of health issues, with health lectures delivered by a physician. Mediterranean-style low-fat dietary advice was delivered by research dietitians and nurses, with calorie restriction when required. Emphasis was placed on changing lifestyles and adopting healthy behaviours for the men and their families. The second hour consisted of exercise classes run by professional Rangers and Celtic coaching staff. Heart rate monitors were used and the men were instructed to exercise at an appropriate heart rate for 30 minutes three to four times a week. At the start of the programme only six men could jog round the stadium football pitch without stopping (distance of around 350 m). After the 10-week programme all of the men could complete one lap and some were able to complete multiple laps without stopping. The programme attracted 100% attendance. Furthermore, some of the men arranged to exercise in small groups after the programme finished. Others encouraged the setting up of exercise programmes at their workplace. Data for 36 out of 40 men were available 15 months after the programme finished ( Table 31 ).
| Outcome | Baseline | Mean change at 10 weeks (n = 40) | Mean change at 15 months post baseline |
|---|---|---|---|
| Weight (kg) | 95.0 | –2.7 | –3.8 (n = 36) |
| Total cholesterol (mmol/l) | 5.66 | –0.75 | –0.49 (n = NR) |
| Systolic blood pressure (mmHg) | 136.6 | –2.5 | NR |
| Diastolic blood pressure (mmHg) | NR | –1.0 | NR |
The study authors deliberately targeted their intervention at men who had a passion for their football club. Participants ranged from manual workers and office workers to a company director but all shared an enthusiasm for their club. The authors reported that every man considered their participation in the programme to be one of the most rewarding experiences of their lives.
Gray and colleagues141 delivered a similar intervention at two SPL clubs (Hearts and Kilmarnock) as part of a pilot RCT lasting 12 weeks. Men were randomised either to receive the FFIT weight-loss programme or to a waiting list control group. The recruitment target (n = 60) was achieved in the larger city-based club (Hearts) but not in the smaller town-based club. As with the study by Brady and colleagues,138 men in the FFIT programme attended 12 weekly sessions at their club training ground where they received personalised dietary and healthy eating and behaviour change advice followed by structured exercise classes. Men in the waiting list group were told that they would receive the FFIT programme 4 months later. The majority of men were white (99%), married (71.8%) and in full-time employment (76.7%). The authors reported that there were no baseline differences between the FFIT group and the waiting list group (Dr Cindy Gray, University of Glasgow, October 2012, personal communication).
The FFIT men achieved significant weight loss and a significant reduction in waist circumference compared with the waiting list men, who showed increases for both outcomes ( Table 32 ). The FFIT men also reported significant improvements in self-esteem, quality of life (as measured by the SF-12) and physical activity and healthy eating. These changes were also significantly different from those in the waiting list group (reported p = 0.001 to 0.048).
| Outcome | FFIT | Waiting list | ||
|---|---|---|---|---|
| Baseline (n = 51) | 12 weeks (n = 44) | Baseline (n = 52) | 12 weeks (n = 42) | |
| Weight (kg), mean (SD) | 107.6 (15.0) | 101.6 (14.1) | 107.5 (19.5) | 106.2 (18.5) |
| % weight loss from baseline, mean (SD)a | –4.6 (2.8) | +0.6 (0.2) | ||
| Waist circumference (cm), median (IQR)a | 115.5 (111.5 to 124.6) | 112.9 (106.7 to 120.2) | 115.1 (107.4 to 121.7) | 116.6 (108.8 to 121.6) |
The attrition rate was reported as low, with 83.5% of the men completing the FFIT programme. The authors reported that the men were very positive about their participation in the programme. The affiliation with the football clubs was highlighted as being the main incentive as many men indicated that they would not have attended a weight-loss programme in an alternative setting. The professional coaching staff also gave positive feedback about their involvement with the programme.
Similar weight-loss programmes have been aimed at men in the rugby league setting. The Tackling Men’s Health (TMH) initiative was developed out of a partnership between the Department of Health, Leeds Rhinos Rugby League Club and Leeds Metropolitan University. The initiative was promoted in partnership with male health-related projects, the Yorkshire Man Mini Manual (Men’s Health Forum) and Change4Life (Cancer Research UK), and targeted men attending rugby matches at the Leeds Rhinos stadium with the aim of improving various areas of health and well-being (e.g. mental health, diet and nutrition, exercise and sexual health). TMH staff found it difficult to recruit men to the weight-loss group, highlighting difficulties in identifying men in a crowd of supporters as overweight without causing offence. The recruitment strategy was therefore altered for the weight-loss group: men were contacted by the Leeds Rhinos club through existing communication strategies and offered the opportunity to attend the weight-loss programme at the club training ground. Group sessions were split between discussing diet, physical activity and behaviour change and taking part in physical activity involving aerobic and muscle resistance training exercises. The programme was run over a 6-week period during the 2009 rugby league season for a group of seven men149 and was repeated over an 8-week period during the 2010 season for a group of 12 men (10 completed the course). 139 The main facilitator for the theory sessions was male but the intervention was delivered by a mixed-sex team. The average weight loss for the 2009 and 2010 groups was –2.1 kg (SD 3.3 kg) and –2.4 kg (SD 1.4 kg) respectively. Furthermore, data for the 2010 group showed an average 4.3-cm (SD 2.3 cm) reduction in waist circumference and an average BMI reduction of 0.8 kg/m2.
Men in the 2010 group139 gave their evaluation of the course. The key reasons that the 12 men gave for joining the programme were the chance to use the club training facilities (five men); that the sessions were held in the evening (four men); the accessible location (three men); and the men-only group (two men). The men enjoyed the sessions, particularly the inclusion of physical activity exercises and the ability to discuss sensitive issues in a male-only environment. The initiative also provided the opportunity to refer the men to other local health initiatives and two men were referred to a specialist diabetes group.
Commercial weight-loss providers
We identified seven reports of men-only weight-loss groups provided by commercial organisations: Weight Watchers,33 Slimming World34 and LighterLife. 35,143 – 145,148 Both Weight Watchers and Slimming World delivered their standard programme in men-only groups. Men in the Weight Watchers report were referred by their NHS health-care provider. Weight Watchers provide a dietary plan that allocates points to certain foods whereas Slimming World allows unlimited consumption of low-calorie ‘free foods’ and a ‘no hunger’ diet plan. A 30-minute exercise add-on class was provided to two-thirds of the Weight Watchers groups. The Weight Watchers programme is designed to create a caloric deficit for a healthy weight-loss rate of up to 2 lb a week, taking into account an individual’s sex, age, height and current weight. Slimming World does not differentiate by sex in its dietary prescription.
The LighterLife ‘Man Plan’ programme was aimed at men and participants were provided with a booklet using male-friendly language and humour, using a football team analogy to describe the benefits of the programme. The LighterLife men abstained from conventional food and alcohol for 8 weeks and consumed very low-calorie diet formula foods known as ‘Man Plan’ food packs (although the diet is the same for men and women, irrespective of body weight). Abstinence from conventional food is reported by the LighterLife authors as providing clarity around boundaries for food and drink consumption. Group behaviour change work is also used to explore reasons for overeating and develop practical and psychological strategies for future weight maintenance.
Men in the LighterLife, Weight Watchers and Slimming World reports were followed up for a period of 8, 12 and 24 weeks, respectively, although men in the Weight Watchers group were able to complete the 12 sessions over a longer time period if necessary. For the Slimming World programme, data were analysed for men who had attended for at least 8 weeks.
LighterLife data were available at 8 weeks from five reports. 35,143 – 145,148 Details are presented in Table 33 .
| Study ID (number of men) | Mean start weight (kg) | Mean start BMI (kg/m2) | Mean weight change (kg) | Mean BMI change (kg/m2) | % weight loss |
|---|---|---|---|---|---|
| Holt 2007145 (n = 1279) | 121.5 | 38.5 | –17.4 | –5.5 | 14.3 |
| Hallam Spencer 2008143 (n = 1000) | 121.3 | 38.0 | –17.5 | –5.5 | 14.5 |
| Salsbury 2009148 (n = 2200) | 119.2 | 37.4 | –14.8 | –4.7 | 12.5 |
| Hallam 2010144 (n = 950) | 123.2 | 38.6 | –19.5 | –6.1 | 15.8 |
| Hallam 201135 (n = 1006) | 124.0 | 39.0 | –19.4 | –6.1 | 15.6 |
The majority of men completed the Weight Watchers programme (63%, 39/62) and the majority achieved a weight loss of ≥ 5% [77% of programme completers (30/39) and 52% of all participants (32/62)]. 33 Average weight loss for all men was –6.3 kg (SD 4.1 kg) and average BMI reduction was 2.1 kg/m2. The authors reported that the men were positive about the Weight Watchers programme and unanimously preferred the men-only meetings.
Weight-loss data for the Slimming World programme for those men who had been members for at least 24 weeks were available for 16 men only. 34 These men lost an average of 13.2 kg (SD 3.6 kg) representing an 11.4% (SD 4.2%) change in weight (69% achieved 10% weight loss and 31% achieved at least 5% weight loss). At the point of data collection, the average BMI reduction was –3.4 kg/m2. There were no significant differences in weight loss between men employed in shift work and men employed in non-shift work.
Mixed-sex programmes
NHS primary care setting
We identified four reports of weight-loss programmes delivered in the NHS primary care setting. 31,151 – 153 Three of these reports describe an evidence-based computer programme, ProHealthClinical. The programme provides instant access to practical eating plans and strategies for increasing physical activity as well as motivational information, such as behaviour change techniques, and tools for tracking patient progress for health-care practitioners. The Counterweight programme offers patients a prescribed energy-deficient diet, behavioural therapy and advice for increasing physical activity.
ProHealthClinical
ProHealthClinical is a computer toolbox of weight and behaviour modification resources developed by KasTech Ltd. KasTech Ltd is a private, non-NHS company that sells its product and training to health-care professionals. ProHealthClinical provides a range of evidence-based weight and behaviour tools that enhance the skills, knowledge and effectiveness of health-care and non-health-care practitioners providing weight-loss advice to patients (e.g. a lifestyle goal-setting database, meal and snack plans, activity energy expenditure information, progress graphs). Advice takes account of the age and sex of the patient. We identified three studies of GP practices that purchased the programme and training from KasTech Ltd. The first of these evaluated ProHealthClinical in 26 GP practices and four leisure centres in NHS Hertfordshire Primary Care Trust. 152 Centres either offered dedicated weight-loss clinic sessions or integrated patients into existing chronic illness clinics over six appointment sessions. Practitioners were encouraged to discharge patients who were unmotivated to make lifestyle changes within the first 4 weeks. The number of men enrolling in the programme (26.7%) was reported to be higher than is usually seen for commercial weight-loss programmes (10%). Of those attending five or more appointments, 26.7% (n = 246) were male, the majority of whom lost weight (228/246, 92.7%). The average weight loss for men attending five or more appointments was 5.1 kg (average 4.4% change from baseline weight) and 102 (41.5%) men achieved weight loss of ≥ 5% of their baseline weight. For women attending five or more appointments, the average weight loss was 3.9 kg and the average percentage weight loss was 4%, with 245 (36.2%) losing ≥ 5% of their baseline weight.
A similar evaluation of the programme was conducted in 20 GP practices in NHS Cambridgeshire Primary Care Trust. 151 Eight fortnightly workshops were held over 3 months. Dietitians and physical activity instructors delivered four out of eight workshops to patients who worked together in small supportive teams. Participants were also given practical 2-week lifestyle challenges, for example eating only fruit or vegetable snacks. In total, 33 men and 123 women attended six or more workshops within 3 months. The mean reduction in weight was 5.2 kg for men (mean 4.6% of baseline weight) and 3 kg for women (mean 3.1% of baseline weight). The programme produced weight loss of ≥ 5% for 12/33 (36.4%) men and 28/123 (22.8%) women.
In the third study, ProHealthClinical was used within a pilot weight management service, Weigh2Go, commissioned by the Cambridgeshire Association to Commission Health (CATCH). 153 Participants were invited to attend six appointments over a 3-month time frame. Of those attending at least five appointments, 118 (28.6%) were men and 112 (94.9%) lost weight. Mean weight loss was 5.4 kg and mean per cent weight change was 4.7% for men; the equivalent figures for women were 4.1 kg and 4.2%. In total, 52 men (44.1%) and 96 (32.7%) women achieved ≥ 5% weight loss.
Table 34 provides a summary of the data from the various programmes using ProHealthClinical.
| Outcome | Hertfordshire PCT | Cambridgeshire PCT | Weigh2Go |
|---|---|---|---|
| No. of appointments attended | ≥ 5 | ≥ 6 | ≥ 5 |
| Men | |||
| n | 246 | 33 | 118 |
| Weight loss (kg), mean (range) | –5.1 (–17.3 to +4.1) | –5.2 (–17.7 to +3.1) | –5.4 (–18.4 to +2.7) |
| Weight change (%), mean (range) | –4.4 (–12.5 to +2.6) | –4.6 (–15.5 to +2.5) | –4.7 (–14.8 to +2.3) |
| Women | |||
| n | 677 | 123 | 294 |
| Weight loss (kg), mean (range) | –3.9 (–23.2 to +5.5) | –3.0 (–19.7 to +1.6) | –4.1 (–16.5 to +2.1) |
| Weight change (%), mean (range) | –4.0 (–19.9 to +4.4) | –3.1 (–16.6 to +2.0) | –4.2 (–16.8 to +2.2) |
Counterweight
The Counterweight programme31 was delivered by specially trained GP practice nurses and health-care assistants. Their role was to deliver education and advice through discussion and communication of information and through the transfer of behaviour change skills and strategies. Participants were prescribed a > 500 kcal per day energy-deficit low-fat diet plan and aimed to achieve a weight-loss goal of 5–10% through individual or group goal-setting behaviour. The GP exercise referral scheme was also offered to participants when this was available and appropriate for individuals. In total, 22 practices were located in deprived areas of the UK, a further 22 were from intermediately deprived areas and 12 were from affluent areas; these practices contributed 36.4%, 29.5% and 34.2% of the total study population respectively. The programme lasted twelve weeks. At 12 months, data for 171 (49%) men and 471 (32%) women were available. Mean weight loss was –3.4 kg (SD 7.31 kg) for men. There was no significant difference in weight loss between the sexes [mean weight loss for women –2.8 kg (SD 6.38 kg)].
Commercial weight-loss programmes
We identified two eligible studies of commercial weight-loss programmes: LighterLife156 and Nutracheck. 154
In the first of these reports, Rolland and colleagues156 investigated whether or not British Asian men and women differed from a matched Caucasian group in their response to the LighterLife very low-calorie diet. The standard programme was delivered to participants in single-sex groups. Outcomes at 12 weeks are shown in Table 35 . Asian men and Caucasian men lost a similar percentage of weight whereas Asian men showed a greater reduction in mean waist circumference per kg of weight loss.
| Outcome | Asian men (n = 36) | Caucasian men (n = 36) | Asian women (n = 166) | Caucasian women (n = 166) |
|---|---|---|---|---|
| Weight loss (kg) | –21.4 (6.7) | –24.4 (8.2) | –14.5 (4.6) | –19.3 (4.2) |
| Weight loss (%) | 18.1 (4.0) | 18.6 (7.3) | 15.9 (5.0) | 28.1 (7.3) |
| Change in waist circumference (cm) | –20.6 (12.8) | –16.1 (6.7) | –15.9 (6.6) | 18.9 (5.3) |
| Waist circumference change per kg of weight loss (cm) | –1.05 (0.7) | –0.71 (0.4) | –1.19 (0.74) | 1.00 (0.3) |
Nutracheck is a commercial internet-based weight-loss programme offering reducing diet and exercise advice. Nutracheck offers personalised daily calorie and physical activity targets, online food and exercise diaries that calculate calorie intake and energy expenditure, and access to an online social community providing support and motivation. Johnson and Wardle154 carried out a retrospective analysis of self-reported weight loss in men and women joining Nutracheck between July 2005 and November 2008. During the study period the Nutracheck Men service was launched (April 2007). Men registering from this time onwards were free to join either the male or the unisex version of the programme. Data were available for 642 men and 2979 women with a mean follow-up period of 186.7 days (SD 192.6 days). Average (mean) weight loss for the men was 5.6 kg (SD 6.5 kg), equivalent to 5.5% (SD 5.9%) of initial weight. Just under half of the overweight or obese men (47.6%) achieved > 5% weight loss. For women, average weight loss was less than for men [3.7 kg (SD 5.1 kg)], equivalent to 4.5% (SD 5.5%) of initial weight. A smaller percentage of overweight or obese women also achieved 5% weight loss (40.7%). Men remained registered for longer than women (187 vs. 170 days, reported p < 0.05) and made more frequent diary entries (56% vs. 52% of daily entries, reported p < 0.05). Women posted more messages on the online forum (35% vs. 19%, reported p < 0.001) although forum use was not a significant predictor of weight loss. It is unclear whether both the unisex and the Nutracheck Men forums were included in the analysis.
NHS referral to commercial weight-loss programmes
Four reports investigated NHS referral to commercial weight-loss programmes. Ahern and colleagues37 conducted an independent analysis of referral to Weight Watchers and Dixon and colleagues150 reported on referral to Weight Watchers, Slimming World or Rosemary Conley Diet and Fitness Clubs. Stubbs and colleagues36,157 reported on referral to Slimming World, although the research team was not independent of the commercial provider. Participants in all studies were given vouchers for 12 free sessions with the commercial provider. Participants in the study by Dixon and colleagues150 had a free choice of provider. Vouchers for Weight Watchers cost the NHS £45 per participant. 37 Vouchers for Slimming World were funded by the NHS and subsidised by the commercial company. 157
In the study by Ahern and colleagues37 some of the 12-week courses were repeat referrals for the same participant. For those completing a first referral course, median percentage weight change was greater in men than in women (difference between men and women –0.54%, 95% CI –0.81% to –0.27%, reported p < 0.001). For men and women completing their first referral course, median weight change was –5.4 kg [interquartile range (IQR) –7.8 to –3.1 kg)] representing 5.6% (IQR –8.1% to –3.2%) weight loss. Dixon and colleagues150 similarly reported that men were more likely to lose 5 kg than women (OR 0.66, 95% CI 0.46 to 0.96, reported p = 0.03). Fewer men in this report were referred by GPs to the scheme (n = 265, 18.5%). Men were also less likely to return their consent form and receive vouchers than women. There was no difference in attendance and completion rates between sexes.
Stubbs and colleagues157 noted that men represented a greater proportion of their audit than the proportion in the standard commercial Slimming World population (11% (n = 3651) vs. 6%). The mean follow-up time was 9.2 weeks. Using the last observation carried forward, mean weight loss for men was 5.8 kg (SD 4.9 kg) or 4.9% (SD 4.0%) of initial weight, with a reduction in BMI of 1.8 kg/m2 (SD 1.6 kg/m2). Weight loss was less for women: 3.8 kg (SD 3.5 kg) or 3.9% (SD 3.5%) of initial weight, with a reduction in BMI of 1.4 kg/m2 (SD 1.3 kg/m2). Men did not attend a greater number of sessions but significantly more men than women were classed as completers (attended at least 10/12 sessions) (63.8% vs. 57.4%, reported p < 0.001). More men lost 5% (46.3%) and 10% (10.6%) of their initial weight than women (34.6% and 5.2% respectively) by the 12th session.
Some of the NHS trusts involved in the study by Stubbs and colleagues36 offered a second referral, resulting in 4754 participants (575 men and 4179 women) having a 24-session referral period to Slimming World. 157 There was no significant difference between men and women in the percentage classed as completers (attended 20/24 sessions) (82.6% vs. 75.8% respectively). Men continued to lose significantly more weight than women (79.5% vs. 73.8% lost 5% of their weight at baseline and 44.3% vs. 36.4% lost 10% of their weight by the 24th session). In the regression model, male sex was the second greatest predictor of weight loss (reported p = 0.013) after percentage weight loss in the first week (reported p < 0.001). Men in both Slimming World studies were older (reported p < 0.001) and had a higher BMI (p < 0.00136 and p = 0.032157) at baseline than women.
Weight maintenance
One study examined the promotion of dietary carbohydrate for weight maintenance following an initial energy reduction phase. 155 In total, 29 employees of Queen Margaret University College, Edinburgh, enrolled in the Kellogg’s-funded study and, of these, six men and 16 women completed the full 6-week course. All were habitual breakfast eaters and four were regular smokers. For the first 2 weeks participants were asked to replace one main meal, lunch or dinner, with a 45-g serving of a Kellogg’s breakfast cereal of the participant’s choice with 125 ml semi-skimmed milk. Following the energy restriction phase, meal replacement ended but participants were encouraged to eat breakfast cereals as snacks if desired. Participants were also given individually tailored advice on increasing their carbohydrate intake and how to consume at least five portions of fruit and vegetables per day. A participation fee of £75 was paid to everyone completing the study. Of those completing the study, mean weight loss at 2 weeks was 1.6 kg for men and 2.1 kg for women. At 6 weeks, overall mean weight loss was 0.8 kg in men and 2.3 kg in women. It should be noted, however, that women had a slightly higher mean BMI than men at baseline and only six men completed the full regime. In total, 19 of the 22 completers responded to an acceptability feedback questionnaire administered at the end of the study, with 12 (63%) stating that they had found the 2-week meal replacement regime easy to follow and 16 (84%) stating that they would use this method for losing weight again. Only one woman who failed to maintain her initial weight loss gave a negative response.
Comparison between men-only and mixed-sex programmes
Few of the identified reports of men-only and mixed-sex weight management programmes included similar interventions or follow-up periods making comparisons between men-only and mixed-sex groups problematic. Interventions were comparable for two of the commercial providers, Slimming World and Weight Watchers, but differences in numbers of men recruited, follow-up and outcome reporting make comparisons difficult.
Discussion
We identified few studies for inclusion in the systematic review of studies of UK interventions with data for men or for men and women compared and found no eligible studies for inclusion in our review of interventions to promote male engagement with obesity services. The included studies were of moderate quality with limited follow-up and variable reporting of outcomes. Few studies identified were comparable in terms of interventions, timing of outcome assessment and participants recruited. Similarly, we were unable to make comparisons between the effectiveness of men-only and mixed-sex programmes from the available data. The results for this review should therefore be interpreted with caution because of the limited evidence base from which they are drawn.
Only seven reports138,139,141,142,147 – 149 described tailoring intervention delivery to men. Strategies to promote engagement included using male-friendly language, male humour, men-only groups and venues that promoted camaraderie through shared sporting interests. These strategies were reported as being highly successful in attracting and engaging men with programmes, although it should be noted that very few men were recruited to these programmes and therefore robust conclusions cannot be drawn. Nevertheless, the men gave positive evaluations of these interventions, describing them as enjoyable and informative, and welcomed the opportunity to discuss sensitive issues in men-only groups. However, the men-only setting did not appear to be the most important reason for attracting men to join a weight-loss programme. Men particularly enjoyed interventions that were affiliated with their sports club, indicating that this is a potentially useful setting for attracting certain types of men. Indeed, enthusiasm for their chosen football or rugby team appeared to be the biggest driver for motivating men to join the weight-loss programme. These programmes included a structured exercise programme with healthy eating advice, which may suggest that men prefer to lose weight through exercise rather than through a programme requiring adherence to a strict dietary regime. Holding group sessions in the evening was also described as being useful for attendance.
Attrition rates for programmes designed for men tended to be low but programmes were short and numbers of men recruited were low, with the exception of the LighterLife study148 (although the attrition rate for this study is unknown). All other studies were of standard unisex programmes delivered in either male-only groups33,34,140,146 or mixed-sex groups. 31,36,37,150 – 157 As seen in our review of mixed-sex trials, fewer men joined these programmes than women but more men completed them and men tended to show a greater percentage weight loss than women. As some of the programmes required referral from health service staff, we do not know whether or not referral patterns differed for men and women.
It should be noted that studies did not often report adjusting energy allowances for men and women. However, Weight Watchers provide differing calorie allowances by sex37,150 and Nutracheck154 tailor daily calorie targets in accordance with individual participants’ characteristics and chosen rate of weight loss.
Successful programmes generally included some element of individual tailoring, in the form of individualised dietary allowances,33,37,140,154 exercise programmes138,141 and/or personalised feedback on weight loss. 142,154 However, the only study in which an individualised energy-deficient diet and a general low-calorie diet were compared showed no difference in weight loss between the two types of dietary regime. 146 Weight loss for the general diet group was less than expected and the attrition rate was significantly higher in this group than in the individualised diet group, although there were no differences in reasons for withdrawal. This could indicate that men are more likely to adhere to a tailored diet even if it does not produce better weight-loss results. This study also found that diet plans high in red meat were no more beneficial than diets in which meat was excluded.
The LighterLife148,156 very low-calorie formula food diet produced highly favourable results, especially in waist reduction for Asian men, although it should be noted that follow-up details for these participants were very limited. Longer-term results are required to understand whether or not weight loss achieved through replacement food diets is sustainable following reintroduction of normal food during weight maintenance.
Programmes from commercial organisations36,37,154,157 produced results that were as good as those from NHS programmes31,151 – 153 when these were delivered in mixed-sex settings, whether this was for privately subscribing or NHS referral participants. When interventions were delivered in single-sex groups, however, commercial providers33,34,148,150,156 outperformed NHS services, but data were very limited. 142,147 This is in keeping with the results of our reviews of RCTs, which highlighted the resource potential that commercial companies have, enabling them to offer flexible services to their participants compared with the NHS. In a single-sex setting, commercial companies have further opportunities to tailor services for men whilst offering them regular classes at times that can be compatible with work and family commitments. As previously shown, men are, however, less likely to choose a commercial provider than they are to choose a NHS programme. The NHS referral scheme may increase men’s engagement with commercial providers.
Most of the interventions involved group meetings. We lack data within this review to be able to compare these interventions with interventions aimed at individuals but, in keeping with previous findings, it is suggested that men lose more weight in a group setting in which individual advice as well as support and motivation can be provided. As with our previous reviews, authors made little attempt to consult men in the design of the interventions and few were successful in recruiting substantial numbers of men. Men made up a very small proportion of participants recruited to mixed-sex studies, highlighting the problems of engaging men in weight-loss programmes compared with women. As seen in the results of our review of RCTs of men and women ( Chapter 3 ), when men were recruited they were more likely than women to regularly attend and complete programmes. This highlights the importance of engaging men with weight-loss services. Future research should consult individual men or men’s health representatives during intervention development with a view to improving male recruitment. Furthermore, outcome data should be collected at sufficient follow-up intervals to ensure that adequate information is obtained beyond the immediate weight-loss periods and through the difficult weight-maintenance phase. Study authors could also improve outcome reporting by adhering to the CONSORT129 recommendations for scientific reporting and presenting baseline and outcome data consistently by sex and intervention group with clear reporting of numbers of participants enrolled, assigned, withdrawn (with reasons) and analysed. Weight-loss data should be provided for all participants enrolled, preferably using both baseline observation carried forward and last observation carried forward results for handling dropouts. Information concerning energy prescription by sex would also be useful, to allow a direct comparison of responses by sex.
Overall summary
We summarise below the main points that have arisen from the review in this chapter.
General issues relating to methodology
-
We found no studies specifically examining how to improve men’s take-up of obesity services.
-
We identified very few randomised trials examining weight loss in men-only groups or weight loss by sex in the UK. Mixed-sex studies had much lower proportions of men than women, especially in commercial weight-loss programmes. Men were rarely consulted beforehand about the design of studies or asked their views on the programmes that they undertook.
-
Male study participants tended to be middle-aged, white and not morbidly obese. Relatively few interventions involved men who were obese and who were selected as a result of an existing health problem such as type 2 diabetes, cardiovascular disease or osteoarthritis. Very few studies presented data on changes in cardiovascular risk factors, clinical outcomes, quality of life or economic outcomes.
-
Most of the interventions were not described in sufficient detail such that they could be replicated. Few studies reported conducting fidelity checks for intervention delivery.
-
The sex of providers was not reported. It is unclear from the included studies whether or not the sex of the person providing a weight-loss intervention to men is an important factor in the effectiveness of that intervention.
-
Many studies were of short duration. Few authors presented data for the entire cohort (e.g. baseline observation carried forward, last entry carried forward), choosing instead to present data for completers only both at baseline and for final outcome measurement. It is therefore difficult to judge the level of attrition bias in these studies. Similarly, few studies reported details concerning the equity or sustainability of the considered interventions.
Pointers for effective interventions
Although few studies were available, there are some pointers for factors that may contribute to effective programmes for men:
-
Effective interventions in workplaces and with sports fans in sporting venues were able to recruit men, although the scale of these interventions was limited. Although a suitable place and time may aid recruitment, it may also exclude those for whom these are not relevant. Interventions with football fans have had low dropout rates and shown very positive responses from participants, with significant improvements in self-esteem and quality of life. The opportunity to improve physical fitness and discuss issues in a male-only environment was valued.
-
The type of reducing diet followed has not been shown so far to affect weight loss. 140,146
-
Weight-loss programmes specifically for men delivered through the NHS142,147 have so far been small, with limited follow-up. Feedback was generally positive; however, not all participants felt that men-only programmes were needed.
-
Weight Watchers, Slimming World and LighterLife have provided men-only weight-loss groups. LighterLife tailored its programme for men but the extent to which tailoring was carried out by Weight Watchers and Slimming World was unclear. For the Weight Watchers programme, men lost more weight in the men-only groups than in the mixed-sex groups. Short-term data show that all of these programmes were effective in terms of weight loss, and they were probably more effective than the men-only NHS programmes; however, data are very limited.
-
Short-term data also show that men do well in the mixed-sex LighterLife and Nutracheck online commercial programmes.
-
Data show that weight-loss programmes delivered in NHS primary care are also effective (ProHealthClinical and Counterweight) and show that more men join these programmes than commercial mixed-sex weight-loss programmes. The Counterweight study provides much longer follow-up data than the ProHealthClinical studies.
-
NHS referrals have been investigated in the trial by Jolly and colleagues and also for Weight Watchers, Slimming World and Rosemary Conley. The proportions of men attending such programmes are higher than for non-referral schemes.
-
It appears that men may lose more weight than women with the ProHealthClinical, LighterLife, Nutracheck, Weight Watchers and Slimming World programmes. However, not all providers prescribed a calorie deficit that took account of sex.
Chapter 5 Systematic review of economic evaluations
This chapter, evaluating the cost-effectiveness of interventions for obesity in men, consists of three main sections, namely (1) a brief outline and explanation of the principles and methods of the economic evaluation of health-care programmes; (2) the methods of the systematic review process; and (3) the results of the systematic review, including summary results and quality assessment of the included studies. The chapter concludes with a discussion of the results, leading to broad conclusions and recommendations for the conduct of future economic evaluations of obesity interventions in a male subpopulation.
Principles of economic evaluation
The need for economic evaluation of health-care programmes (drugs, interventions, medical devices, diagnostic tools, etc.) is driven by the fact that national health budgets are a scarce resource. Budget limits mean that trade-offs between health-care interventions need to be made. The allocation of resources to one intervention or clinical area means that we are forgoing an opportunity to spend these resources on an alternative health-care programme. This economic concept is commonly referred to as opportunity cost, that is, the highest-valued alternative forgone as a result of a spending allocation decision. Economic evaluation is essentially the comparative analysis of alternative courses of action in terms of both their costs (resource use) and their effectiveness (health effects). It is a method of providing decision-makers with the tools and information necessary to make the allocation decision in a way that maximises benefit and reduces opportunity costs.
There are four main methods of economic evaluation, each of which is summarised briefly in Table 36 . The measurement of costs is similar across all economic evaluation frameworks. Good studies would be expected to consider the direct costs of an intervention together with the costs of downstream complications, such as cardiovascular events, stroke, diabetes and other health conditions related to the clinical area of interest (in the context of this review we are interested in obesity-related complications). Of the four economic evaluation frameworks outlined, the most simplistic is cost-minimisation analysis, which would essentially lead a decision-maker to adopt the least costly intervention. Cost–benefit analysis could be considered the broadest measure of evaluation, accounting for individual preferences and broad outcomes measures that go beyond health outcomes. The wider the measure of benefit used, the more likely the analysis framework is to consider the effects that are of greatest importance to individuals. Cost–utility analysis [cost per quality-adjusted life-year (QALY) gained] and cost-effectiveness analysis (usually cost per life-year gained) are the most commonly used frameworks of economic evaluation. Cost–utility analysis is the approach to decision-making recommended by NICE. 161 QALYs represent a combination of the quality and length of additional life-years attributable to an intervention in one measure. For example, a value of 6 QALYs could mean 6 years in full health or 12 years in half of full health [i.e. 12 life-years gained (LYG) but with a quality of life of 0.5 on a scale from 0 to 1].
| Economic evaluation method | Outcomes measured as: |
|---|---|
| Cost-minimisation analysis | Not applicable; outcomes are assumed equal for all options, hence decisions are made based on the least costly intervention |
| Cost-effectiveness analysis | Outcomes are measured in their natural units (e.g. LYG or kg lost) |
| Cost–utility analysis | Outcomes are measured as QALYs |
| Cost–benefit analysis | Costs and outcomes are measured in monetary terms (benefit often measured as willingness to pay) |
For the purposes of this review, the two frameworks used in the included studies are cost-effectiveness analysis and cost–utility analysis.
The concept of economic evaluation and specifically the comparative analysis of the differences between costs and benefits (incremental costs and incremental benefits) across treatment groups can be summarised on the cost-effectiveness plane ( Figure 40 ).
FIGURE 40.
Cost-effectiveness plane of measures of economic costs and benefits. NE, north-east; NW, north-west; SE, south-east; SW, south-west.
The results of an analysis could be seen to fit onto one of four quadrants in the cost-effectiveness plane, namely:
-
North-west (NW) quadrant – the intervention under consideration is more costly and less effective than the comparator; therefore, the new intervention is dominated and should not be accepted.
-
South-east (SE) quadrant – the intervention under consideration is less costly and more effective than the comparator; therefore, the new intervention is dominant and should be accepted by a decision-maker.
-
South-west (SW) quadrant – the intervention is less costly but also less effective. In this case, a decision-maker would need to weigh up the potential cost savings against the loss in benefit, with a decision being required about the amount of savings that would be needed before a decision-maker could allow a unit loss of benefit to occur.
-
North-east (NE) quadrant – the intervention is more costly and more effective. A decision is required about how much we are willing to pay to achieve an additional single unit of benefit.
The decision rule for the NW and SE quadrants is clear because one treatment dominates (either the comparator or the experimental treatment is less costly and more effective). For the NE and SW quadrants a judgement is required whether the more effective treatment is worth the additional cost (or whether the cost savings are worth the potential loss in QALYs). To inform decision-making in these scenarios, information is provided in terms of the incremental cost-effectiveness ratio (ICER), which is essentially the difference in costs between the two treatments under consideration divided by the difference in benefits. This allows us to calculate the cost per unit change in benefit. In the context of a cost–utility analysis, an intervention might typically be acceptable to a decision-maker if the additional cost of achieving an additional QALY is < £20,000–30,000 per QALY gained. Although NICE does not operate a threshold value of willingness to pay (WTP) for a QALY gain per se, it typically recommends interventions with a cost per QALY gained within this range. However, exceptions to this broad guideline exist. It is not clear what one might consider a typically acceptable value of WTP for a gain in life-years. However, generally speaking, the higher the ICER the greater the health-care expenditure required to achieve a unit gain in benefit and hence the less likely an intervention is to be considered cost-effective. Assuming that additional LYG are in full health, one could reasonably assume a similar decision rule to that mentioned for QALYs.
The results of the studies included in our systematic review will be described and discussed in the context of the cost-effectiveness plane and will include a discussion about which strategies for the management of obesity in men may be acceptable to health-care decision-makers. This process is, however, complicated by the heterogeneity of the included studies, the range of country settings and the uncertainty created through inflation and alternative values of currencies’ purchasing power (purchasing power parity indices). The reader should therefore exercise caution in terms of any comparative conclusions across studies and should instead focus and assess each study on its own individual merits.
Systematic review of cost-effectiveness studies
Aims
To report the costs, outcomes and cost-effectiveness results of alternative strategies for the management of obesity in adult men.
Methods
Search strategy
Studies that reported both costs and outcomes of alternative strategies for weight loss, providing a distinct and interpretable focus on strategies for the management of male obesity, were identified. This included studies alongside RCTs as well as de novo decision-analytical models. An extensive electronic search was carried out to identify reports of relevant published and ongoing studies as well as grey literature. A highly sensitive search strategy using both appropriate subject headings and text word terms to identify reports on costs and weight-loss strategies for the management of male obesity was used (see Appendix 1 ).
The following databases were searched:
-
MEDLINE (1946 to January 2012)
-
MEDLINE-In-Process & Other Non-Indexed Citations (19 January 2012)
-
EMBASE (1974 to January 2012)
-
HMIC (1979 to January 2012)
-
NHS EED
-
CEA Registry
-
RePEc.
No language restrictions were imposed on the search; however, the search was limited to studies published post 1990 in societies relevant to the UK setting.
Eligibility and inclusion criteria for studies
Studies that compared both costs and outcomes for interventions for the management of obesity in adult men were included. Studies were excluded if they did not attempt to relate cost to outcome data. Methodological papers, papers that review economic evaluations (although their reference lists were checked for additional papers to include), discursive analysis of costs/benefits, partial evaluation studies such as cost analysis, efficacy or effectiveness evaluations and cost of treatment/burden of illness papers were all excluded.
Studies included men with a mean or median age of ≥ 16 years, with no upper age limit. Studies particularly examining men with obesity related to psychotropic medication or a diagnosed eating disorder or with learning disabilities were excluded.
A range of interventions were deemed suitable for inclusion in our review, namely orlistat (but not sibutramine or rimonabant, which no longer have UK licences), diet, physical activity, behaviour change techniques or combinations of any of these. Complementary therapy (e.g. acupuncture), non-diet products promoted for weight loss available solely over the counter or bariatric surgery were not included for evaluation. Weight loss or weight gain prevention after weight loss needed to be explicitly stated as the main goal of the intervention undergoing economic evaluation. Studies examining a combination of interventions, for example smoking cessation and weight loss, at the same time were not included in the review.
Data extraction strategy
Data extraction was undertaken by the project health economist. Data extraction forms were checked by a second member of the review team for consistency and accuracy. The data extraction process focused on two key areas: (1) the results of the economic evaluations in terms of estimates of costs and effects and (2) the methods used to derive the results. Detailed data extraction forms for each study are reported in Appendix 13 .
Data synthesis
Because of the heterogeneity of the studies retrieved, we did not attempt any quantitative synthesis of the included studies. Instead, summary data from the included studies and a narrative overview of each study are presented. When incremental costs, incremental effects or ICERs have not been reported, when possible we have undertaken these calculations, based on data included in the studies. The aim of the narrative is to identify common results across broad intervention groups. Common strengths and weaknesses across the studies are identified and used to develop recommendations for future economic evaluation studies of weight-loss interventions for men.
Results
Number of studies retrieved from the searches
Details of study identification are provided in Figure 41 .
FIGURE 41.
Flow chart for identification of studies.
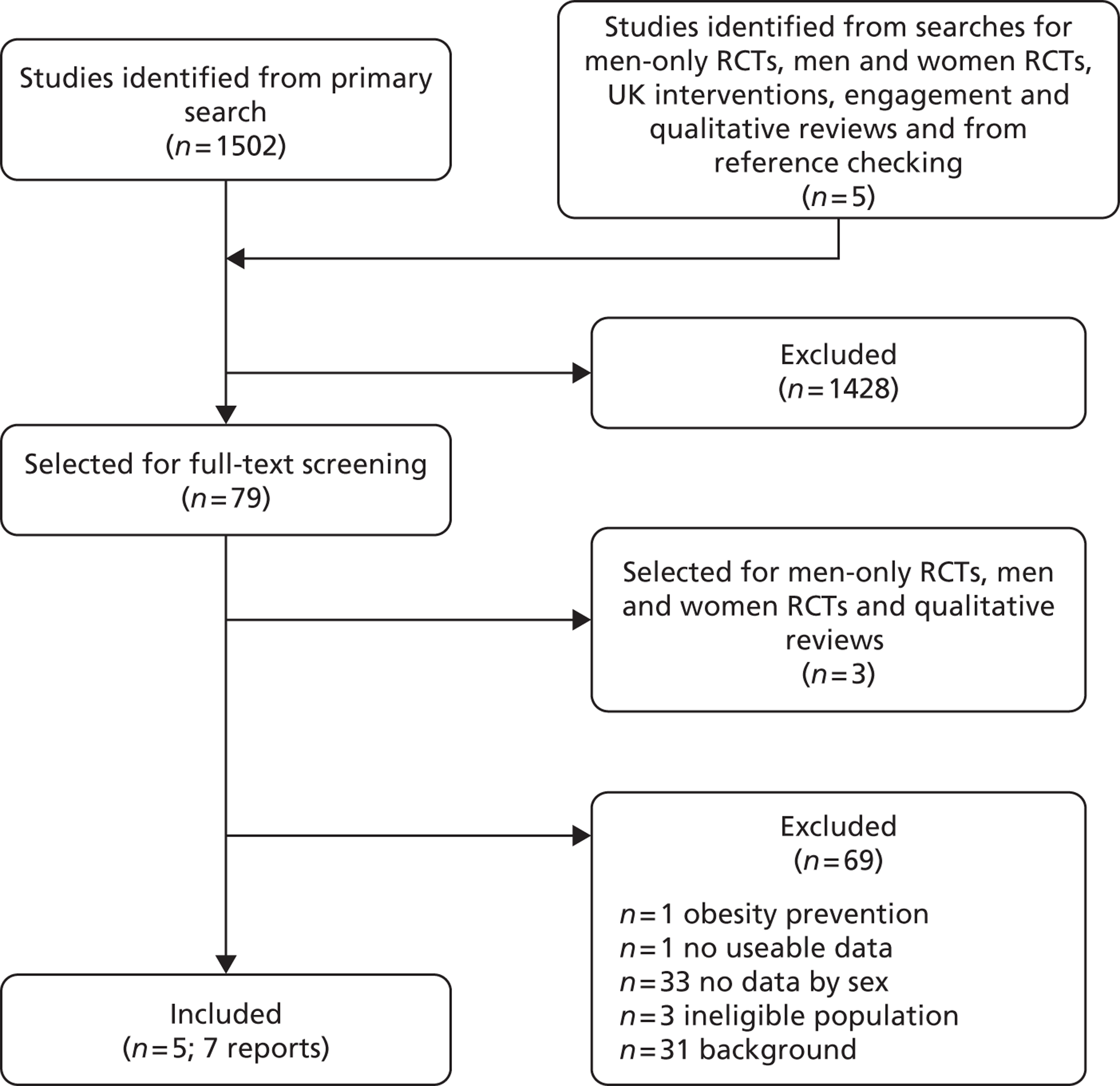
Using the search strategy outlined in Appendix 1 a total of 1502 titles and abstracts were identified as being potentially relevant to our research question. These studies were screened by a project review team member to assess their relevance to the study question, focusing on economic evaluations of interventions for weight management in obese men. Of those initial 1502 screened titles and abstracts, a total of 79 were deemed potentially relevant and/or required further evaluation to assess their eligibility for inclusion and were read in full. On reading all full-text papers, a total of five studies162 – 166 were deemed to fit with our inclusion criteria and were formally included in the review and quality assessment process. In addition, our searches retrieved one further methodological paper167 reporting a value of information analysis alongside one of our included studies. We also retrieved one clinical guideline from NICE53 that assessed the cost-effectiveness of orlistat for use in the UK. The guideline briefly discussed male-specific issues and conducted some brief modelling that showed potentially differential results for male and female subgroups. The additionally retrieved methodological study and clinical guideline have not been data extracted or formally quality assessed; however, they are discussed in the results section of this review as they address important policy and methodological questions, especially in relation to developing a future research agenda for obesity-related weight management.
Full and detailed data extraction forms for each included study as well as completed quality assessment checklists, based on Phillips and colleagues,168 are presented in Appendix 13 .
Description and characteristics of the included studies
Of the five studies that were deemed eligible for inclusion in the review, three162,163,165 assessed lifestyle interventions including components of physical activity, dietary advice, professional counselling and group behavioural modifications. The remaining two studies164,166 evaluated the cost-effectiveness of orlistat (a pharmaceutical agent to aid weight loss). The first evaluated orlistat in addition to standard diabetes management. 166 The second evaluated orlistat in addition to a lifestyle intervention. 164 The comparator group for both studies included a placebo drug, prescribed for the same frequency and duration as orlistat. All studies reported results for male subgroups; however, only one study163 reported results for a wholly male group of participants. Another study166 reported results for adults; however, because of the nature of the baseline data, it could be assumed that the results were most relevant to a very specific subgroup of the population (age 52 years, male).
Studies were conducted in a variety of countries, including Italy,164 Switzerland,162 Denmark,165 Australia163 and the USA. 166 None of the included studies was UK specific.
Description of the interventions included in the review
Lifestyle interventions
Of the five included studies, three162,163,165 evaluated lifestyle interventions/behavioural modification interventions with the aim of reducing participant weight. Because of the heterogeneity of the interventions, it is not possible to make comparisons across the studies.
Segal and colleagues163 evaluated a range of alternative programmes in different population groups in Australia for the prevention of type 2 diabetes. A total of six programmes were evaluated in different settings. For the purposes of this review, however, only one programme reported results for a male population. Programme IV consisted of a group behavioural modification for men based on an empowerment philosophy, involving five to six group sessions, with the aim of reducing waist size through dietary change and increased physical activity, with no further details reported. We were unable to retrieve additional information regarding the detailed methods and results for the male-specific programme IV. Alternative interventions evaluated ranged from diet and counselling to media campaigns to surgery. No results were presented for male-specific subgroups other than for programme IV detailed here.
Olsen and colleagues165 assessed the role of alternative health-care professionals in the delivery of nutritional advice to obese patients, to stimulate weight loss and reduce the risks of ischaemic heart disease and death. The study compared two health professionals providing advice (GP or dietitian) with no active intervention. Although both interventions delivered dietary advice, there were important differences in the content of the interventions. GP counselling delivered over 12 months (one consultation for 30 minutes and a further five consultations of 12 minutes each) included the provision of general lifestyle advice, commercially available written information and leaflets on healthy diet. The dietitian-provided advice focused on the principles of good nutrition, including restricting total dietary energy, reducing fat intake and advice on a cholesterol-lowering diet. Six consultations were delivered over 12 months (one 60-minute consultation and five 30-minute consultations). Results were presented for male specific subgroups of the population.
Galani and colleagues162 estimated the long-term health and economic consequences of preventing and treating obesity with lifestyle interventions in an overweight and obese population subgroup. The lifestyle intervention was the same for both overweight and obese subgroups and was compared against a standard of care deemed to be appropriate and reflective of participants’ BMI. The intervention consisted of regular physical activity advice (to undertake at least 30 minutes of moderate physical activity daily) and detailed dietary recommendations (adapted from the FDPS). 132 Recommendations were to limit intake of fat to < 30% of energy consumed and of saturated fat to < 10% and to increase fibre to at least 15 g per 1000 kcal. Participants attended regular supervised exercise sessions and dietitian consultations over 3 years of follow-up. The intervention was compared with different standards of care for overweight and obese population groups. Overweight participants received no active intervention. Obese patients received basic dietary counselling and physical exercise sessions over the 3-year follow-up period. The study also evaluated cost-effectiveness for a borderline obese group to assess any important transitions to obesity and how these might impact on the cost-effectiveness outcomes.
Orlistat
A further two studies included in our review evaluated the use of orlistat in combination with lifestyle interventions for the prevention of type 2 diabetes.
Maetzel and colleagues166 evaluated the cost-effectiveness of 120 mg of orlistat taken three times daily for 1 year in addition to standard treatment for type 2 diabetes, including pharmacotherapy (e.g. metformin) and weight management in the form of dietary and physical activity advice. This was compared over an 11-year time horizon with adherence to standard treatment guidelines alone. The evaluation thus compared an intervention group who received orlistat and adhered to treatment guidelines in year 1 followed by 10 years of adherence to guidelines with a comparator group who received 11 years of adherence to treatment guidelines alone. The intervention was delivered in a US health-care setting. Detailed information on the components of standard treatment guidelines were not available by treatment group; however, it can be assumed that there are no incremental differences attributable to this.
The second orlistat study164 involved a cost–utility analysis over a 10-year time horizon, which evaluated the longer-term economic impact of the use of orlistat plus a lifestyle intervention compared with a lifestyle intervention alone in Italian obese patients through the long-term projection of XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) study results. 169 The intervention consisted of 120 mg of orlistat three times a day over 4 years in addition to the lifestyle intervention compared with the lifestyle intervention plus a similarly delivered placebo drug. The lifestyle intervention, common to both arms of the study, involved patients being prescribed a low-fat reducing diet and physical activity advice.
Both studies were quite similar in terms of the research question addressed (i.e. both evaluated the cost-effectiveness of orlistat over a similar time period); however, the baseline data sources were different. Maetzel and colleagues166 relied on the UK Prospective Diabetes Study (UKPDS)170 for input data and Iannazzo and colleagues164 relied on the XENDOS RCT. 169 Both studies reported results for a male subgroup of the modelled population. However, in the study by Maetzel and colleagues166 it was indirectly assumed that the results were most relevant to men because cardiovascular risk factors were calculated based on UKPDS data,170 which references a 52-year-old man. The data from Iannazzo and colleagues,164 on the other hand, are directly linked to the XENDOS trial data,169 with extrapolations based on sex-specific data from the trial. There were key differences in the assumptions regarding maintenance of treatment effect and weight loss. Maetzel and colleagues166 focused on HbA1c levels, and tested two scenarios, one in which the treatment effect was maintained for only 1 year and the other in which the treatment effect persisted for 3 years. Iannazzo and colleagues164 assumed, based on data from the XENDOS study,169 that weight returned to baseline levels after 4 years of treatment. NICE has also issued guidance for the use of orlistat in the management of obesity. 53 The guidance issued by NICE is summarised in the following section.
Table 37 presents summary information with regard to the intervention type, reference population modelled and characteristics for all five of the included studies.
| Study ID | Country | Population group evaluated | Male/female breakdown | Study setting | Description of intervention | Description of control/comparison |
|---|---|---|---|---|---|---|
| Galani 2007162 | Switzerland | Overweight or obese adults (by Swiss standards), aged > 25 with a baseline BMI score of ≥ 27 kg/m2 (overweight) or ≥ 33 kg/m2 (obese) | Results reported for men and women together and for male and female subgroups separately | Primary care | Lifestyle intervention consisting of (1) regular physical activity, at least 30 minutes per day, plus detailed dietary advice on a low-fat, high-fibre reducing diet (based on FDPS171); (2) regular dietitian consultations and supervised exercise sessions over 3 years, frequency of classes unclear | Overweight: No active intervention Obese: Basic dietary counselling and physical exercise sessions delivered regularly over 3 years |
| Iannazzo 2008164 | Italy | The population was based on the XENDOS study data169 and the Italian obese population, with a BMI ≥ 30 kg/m2. The model was analysed for the Italian obese population, age 30–60 years, base-case model age 35 years | 47.7% male; 52.3% female Model inputs were sex specific |
Not reported but assumed to be primary care | 4 years of treatment with 120 mg of orlistat three times per day in combination with a low-fat reducing diet and physical activity advice | 4 years of treatment with a placebo drug three times per day in combination with the same low-fat reducing diet and physical activity advice |
| Maetzel 2003166 | USA | Overweight and obese adults with type 2 diabetes | Not reported; however, risk factors used for the model refer to a 52-year-old man, with data sourced from the UKPDS170 | US health-care setting, assumed secondary care | Treatment over an 11-year time horizon: Year 1: orlistat + adherence to guideline therapy;a years 2–11: adherence to guideline therapya only | Treatment over an 11-year time horizon with adherence to guidelinesa only |
| Olsen 2005165 | Denmark | Obese patients with at least one of the following: BMI ≥ 30kg/m2, waist circumference > 102 cm (men) and > 88 cm (women), dyslipidaemia and type 2 diabetes | Not reported but results were presented based on sex-specific cardiovascular risk parameters, generated from weight loss in the study | Primary care (GP/dietitian clinics) | GP nutritional counselling: General lifestyle and healthy diet advice, six counselling sessions over 12 months (one of 30 minutes and five of 12 minutes) Dietitian nutritional counselling: Detailed advice on principles of good nutrition (restricted total dietary energy, reduced-fat and cholesterol-lowering diet). Six consultations over 12 months (one of 60 minutes and five of 30 minutes) |
Standard care – no active intervention |
| Segal 1998163 | Australia | People with impaired glucose tolerance, overweight/obese men, seriously obese people, women with previous gestational diabetes and the general Australian population | Programme IV – men onlyb | Programme IV – primary care | Programme IV: Group behavioural modification for men based on empowerment philosophy (five to six group sessions). The aim was to reduce waist size through diet change and increased activity | Standard care – no active intervention |
National Institute for Health and Care Excellence guidance
The National Institute for Health and Care Excellence in the UK has issued clinical guideline no. 43 (CG43) on obesity. 53 CG43 replaced previous NICE guidelines for individual therapies for the management of obesity, namely orlistat (technology appraisal no. 22, TA22),172 sibutramine (TA31)173 and surgery for morbid obesity (TA46). 174 The last TA is outwith the scope of our review and is not discussed further. In addition, following the suspension of the marketing authorisation for sibutramine (Reductil, Abbott Laboratories) by the Medicines and Healthcare products Regulatory Agency (MHRA) in January 2010,170,175 NICE recommendations were updated to refer health-care professionals to the MHRA advice and its previous recommendation for the use of sibutramine was withdrawn.
The discussion in this section therefore focuses on NICE guidance for orlistat. The original guidance was developed in 2000 as part of TA22,172 informed by an independent review group report. 176 However, this report did not present sex-specific results and so was not included in our review. Guidance was subsequently developed in 2006 (CG43), with the development of a comprehensive guideline document for the management of obesity. 53 The review was subsequently updated in December 2011. 177 In terms of the health economic content and cost-effectiveness case in the review, the guideline development team conducted a large systematic review of options for the treatment of obesity, which included orlistat and lifestyle interventions. However, few studies were found and none was reported to refer to a male subgroup of the population. Therefore, no sex-specific cost-effectiveness analyses were reported for orlistat or for lifestyle interventions for the management of male obesity.
Additional modelling work was therefore undertaken to estimate sex-specific quality-of-life weights to inform QALY calculations and for use in subsequent economic modelling exercises. Data from Macran178 were used to estimate quality-of-life weights for five BMI ranges. Data from Ara and Brennan’s submission to NICE,179 quoted in CG43,53 were used to estimate weight based on BMI for male and female subgroups, given the average height of a typical man and typical woman. Weight-loss figures were ascribed to central values of each range and a linear trend between midpoints was assumed. Taking this midpoint of each BMI range, weight loss and utility gain178 were synthesised into utility gained per kg lost for men and women. Expected quality of life values were estimated for each 0.1 increment of BMI. A diabetes disutility value of 0.8661 was identified from the literature and assumed. 3 Sex-specific QALY and cost per QALY sensitivity analyses were presented based on available effectiveness (weight loss) and cost data for orlistat. Detailed costings were not presented within the analysis. The modelled sensitivity analysis presented indicates little or no sex-specific difference in cost-effectiveness of 12 months’ treatment with orlistat, with ICERs for both male and female subgroups well below a commonly acceptable WTP of £20,000 per QALY gained.
Differences between male and female subgroups appear to be more pronounced when comparing longer-term treatment (48 months) with current practice of 12 months’ treatment. The base-case analysis reports a higher cost per QALY for men (£29,089) than women (£26,917). Within this analysis, in a male subgroup, the data suggest that the greater the initial BMI, the more cost-effective orlistat is, with an ICER of £29,920 when BMI is 38 kg/m2, increasing to £33,134 when initial BMI is 30 kg/m2. The converse appears to be true for women, with an ICER of £30,155 for an initial BMI of 38 kg/m2 and an ICER of £23,982 for an initial BMI of 30 kg/m2. The results show that for the comparison between 48 months of treatment and 12 months of treatment, cost-effectiveness of orlistat is dependent on a number of factors, including sex, baseline BMI, weight trend without orlistat and weight regain after treatment discontinuation. The conclusion of the evaluation was that NICE could not recommend 48 months of treatment with orlistat, given the uncertainty in the ICER presented.
Work by Foxcroft180 and referenced by NICE53 evaluates both the European Medicines Agency (EMA)181,182 and NICE guidelines172 for the use of orlistat. These guidelines recommend different continuation rules for treatment with orlistat. The original NICE guidelines172 (TA22, updated as part of CG4353) recommend continuation only if patients achieve 5% weight loss at 3 months and 10% weight loss at 6 months. The EMA criterion181,182 is slightly more relaxed than that used by NICE, requiring only a minimum of 5% weight loss at 3 months to justify continuation of treatment. Results from the Foxcroft study180 were not reported separately for male and female subgroups. However, for all adults, the results suggest that adoption of the EMA criterion for the continuation of orlistat would result in a lower ICER than that obtained using the NICE guideline criterion. The cost per QALY was £24,400 (range £10,900–77,200) and £19,000 (range £8800–57,800) for the NICE and EMA criteria respectively. The study recommended that future economic evaluations of the cost-effectiveness of orlistat should consider the use of the EMA criterion. The most recent review of the NICE guidelines177 considered the Foxcroft analysis and the less restrictive EMA criterion. The NICE update to the guidance found that, although there was some evidence to support removal of the requirement that at least 10% of body weight is lost by 6 months for continuation of treatment, there are equally reasons for the criterion to remain unchanged. Methodological uncertainty in the estimation approach to QALY gains led NICE to conclude that adoption of wider EMA-based criterion was not recommended at this time.
Quality of the included studies
Model structure
All studies included in the review were based on models with a common goal of extrapolating short-term outcomes, such as weight loss, to longer-term health benefits, either in terms of survival (LYG) or a combination of survival and quality of life (QALYs). All studies reported incremental costs and incremental benefits, for which formal cost-effectiveness analyses were undertaken. For the purposes of assessing the quality of the modelling studies in this review, particular attention was given to model parameter estimation and the methods by which short-term outcomes were extrapolated over a longer-term horizon, accounting for downstream health-care costs and patient health outcomes. Included studies were quality assessed using the criteria of Phillips and colleagues. 168 These criteria provide a platform to quality assess studies based on best practice methods for the conduct of decision modelling studies for use in health-care decision-making. Detailed quality assessment checklists for each of the included studies are presented in Appendix 13 for information.
All included studies used modelling techniques of varying degrees of complexity and sophistication to synthesise cost and effectiveness outcomes. All studies measured cost-effectiveness using some form of extrapolation of lifetime mortality and disease risk. Four out of the five included studies estimated their results using Markov models to extrapolate weight loss and disease risk to longer-term outcomes. 162 – 164,166 Two out of the four Markov models measured outcomes in terms of incremental cost per QALY gained, combining measures of additional life-years with quality-of-life weights applied to disease states in the model. 162,164 The remaining two Markov models163,166 did not report any utility values and hence outcomes were based on survival estimates alone, with the economic evaluation presented as cost per life-year gained163 and cost per event-free/diabetes-free life-year gained. 166 The remaining study165 was based on a Cox regression model using time to event modelling, adjusted for baseline risk factors, to estimate survival associated with treatment. The model predicted life-years and LYG without ischaemic heart disease. Given that the obesity-related health complications are likely to occur into the future, it is important that the dynamic nature of progression between disease states is adequately modelled. For this reason it could be argued that Markov modelling was the most appropriate method to synthesise the dynamic cost and effect relationships for obesity-related disease pathways. Further, Markov models, because of their design, are more appropriate to estimate the impact of more dynamic state transitions associated with alternative courses of disease progression. The models are, however, only as good as the data used to generate the state transitions and there is a trade-off between the level of sophistication of the models and the data available to populate them.
Selection of interventions considered
Only one of the included studies explicitly discussed or justified the intervention strategies compared. 163 However, given the wide variation in possible interventions (both pharmaceutical and lifestyle related), the interventions compared in each study appear to be appropriate, given the decision problem and stated objective of the respective analyses. Segal and colleagues163 conducted a systematic review to determine the most appropriate programmes to compare in a cost-effectiveness framework. Although the review was systematic and six programmes were ultimately evaluated, it was difficult to draw comparisons across programmes given the heterogeneity of the populations and programmes evaluated. For the purposes of this review, our discussion pertaining to this study relates only to the male-specific group behavioural intervention (programme IV). 163
Incorporation of data into economic models
All studies clearly described the methods used to incorporate data into their models and sources were mainly derived from the literature. Although a broad spectrum of literature appears to have been considered for the majority of included studies, literature searches were none the less ad hoc and there was no evidence of systematic literature searching to identify key model parameters. Justification for the use of data is generally poor with little discussion of choices between key data sources. When meta-analyses of treatment effects were carried out there was little information provided on the data synthesis methods used and so quality assessment was not possible. Only one study briefly described the model used to synthesise data as a random-effects meta-analysis. 162 Nevertheless, the data included in the studies were well referenced and data sources were clear for the most part.
Cost data in the models
Four162 – 164,166 of the five studies considered costs over an extended time horizon. The remaining study165 did not extrapolate costs beyond the cost of the intervention and so failed to account for any downstream costs associated with obesity-related complications. This is an important part of the disease process and limits the value of the study results. The costing perspective was described in all studies. Two studies stated that a societal perspective was used;162,164 however, explicit data relating to a societal perspective, beyond health service resource use, were not detailed in the studies. It appears that, given the data included, these studies would more accurately be described as using a health services or health services and personal perspective. One explanation for this could be a lack of consistency in the definition of perspectives across countries. Some countries might consider publicly provided health care to be provided from a societal perspective. It is important that perspective is clearly defined for all studies. Intervention costs were included for all studies; however, detailed calculations were not presented in all studies, rendering it difficult to quality assess the costing methods in general. Costs were incorporated for downstream health events in all but one study;165 however, again this information was reported with varying degrees of completeness and detail. It is imperative that economic evaluation studies include detailed unit costs of interventions, follow-up costs of obesity-related complications and a synthesis of these two cost aspects to generate total cost estimates. It should in theory be possible to reproduce results given the data provided in a study; however, this was not the case in the studies retrieved for this review.
The costing year was explicitly reported in four studies;162,163,165,166 however, it was also possible to source the costing year indirectly for the fifth study through the appropriate references. 164 A range of currency data was reported, with only one study163 converting currency to international dollars. Galani and colleagues162 also converted data to study year euros. Many different currencies and costing years meant that a comparison of the results is subject to considerable uncertainty because of the wide variations in exchange and inflation rates over time. Discount rates applied to costs were clearly detailed for all studies that measured longer-term horizon costs (four162 – 164,166 out of five included studies). The fifth study165 considered costs over a 1-year time horizon only and thus discounting of costs was not necessary. The impact of uncertainty surrounding discount rates was tested in sensitivity analyses, with two162,166 of the five studies reporting cost-effectiveness results associated with varying discount rates. Iannazzo and colleagues164 reported discount rates but did not test assumptions regarding their impact in sensitivity analysis. As Olsen did not consider long-term costs, discounting was considered only for outcome data. 165 The final study did not report any sensitivity analyses for the male-specific programme within the study. 163
Effectiveness, treatment outcomes and linking of evidence
Effectiveness data used for the studies relate to a linked evidence approach. For the majority of studies, the goal was weight loss. For the most part, however, model inputs were predicted based on clinical outcomes such as lipid levels, systolic blood pressure and HbA1c levels and taken from published sources, with the link between weight loss and these outcomes less clear. One study164 used Framingham risk equations183 to determine relative risks of cardiovascular events. These were then linked, using a combination of the literature and modelling exercises, to final health outcomes and complications (e.g. diabetes, stroke, myocardial infarction). This has been completed to varying degrees of complexity and it is not always clear whether male-specific data are used for the model inputs. In terms of the effectiveness of the interventions for weight loss, only one162 of the five studies explicitly reported weight-loss data, despite this being an outcome of importance to the study questions. Instead, studies relied on cardiovascular risk data at a population level, based on weight-loss data from randomised trials, as model inputs.
Galani and colleagues162 estimated weight loss on the basis of a meta-analysis of randomised trials and used Framingham risk equations together with national Swiss data to estimate cardiovascular risk factors and mortality. Methods for the meta-analysis were not clearly reported and it was not possible to quality assess this aspect. Assumptions regarding weight loss were clearly documented and weight loss was maintained over 6 years, with linear regain over 4 years. Therefore, after 10 years it was assumed that weight had returned to baseline levels. This assumption was validated against the FDPS;132 however, alternative assumptions were not explored in sensitivity analysis. Although mortality and cardiovascular risk factors are based on sex-specific data and these are extrapolated to final outcomes, it is not clear whether these were applied to sex-specific weight-loss data or not.
The remaining studies did not report weight loss per se, despite this being an implied goal of the studies in all but one case. 165 Olsen and colleagues165 used a combination of clinical data, including risk factors of age, sex and BMI score, to establish the lifetime mortality risk. It was assumed that the effects of the intervention would be continued over the lifetime of follow-up, an assumption that the authors acknowledge as a key limitation of the study. Despite this, no alternative sensitivity analyses were presented to test the impact of this assumption on cost-effectiveness outcomes.
The model developed by Iannazzo and colleagues164 was based on the extrapolation of results from the XENDOS RCT. 169 Data from the trial were used to predict diabetes incidence and forecast blood pressure and cholesterol variation. The impact on cardiovascular disease was described using Framingham risk equations. Although no other studies were considered, and no data synthesis was undertaken, the randomised and blinded nature of the XENDOS data is likely to ensure that rigorous estimates were used in the model. Methods of data extrapolation were clearly described and were sex specific; however, the use of Framingham equations, which do not explicitly include BMI as a risk parameter, mean that the model was not sufficiently sensitive to changes in weight and BMI. However, data from the original XENDOS trial,169 which did measure weight gain and maintenance, showed that weight returned to almost baseline levels at 4 years’ follow-up.
Maetzel and colleagues166 also did not directly report weight loss in their study. However, weight loss was an important goal of the study. Instead, weight loss impacted on HbA1c scores, which were reported and linked to relative cardiovascular risk reductions. Data from four placebo-controlled trials were used to conduct meta-analyses to synthesise outcomes in terms of HbA1c levels. However, details of the data synthesis methods used were not provided and the quality of the analysis methods was not critiqued within the study. Methods of extrapolation of treatment effects over an extended time horizon were clearly described and it was assumed that patients receiving orlistat would experience weight loss over 1 year of therapy, after which weight regain would be linear over 3 years and weight would then match that of the placebo group. This assumption was tested in sensitivity analysis and was found to have an impact on cost-effectiveness results.
In general, data syntheses for treatment effects were documented but methods were poorly reported. It was not clear whether or not separate weight-loss parameters were included for men and women; however, cardiovascular risk inputs to the model were sex specific. The link between weight loss and cardiovascular outcomes was poorly addressed and this adds uncertainty to the cost-effectiveness results. Although results were often clearly reported separately for male and female subgroups, it would also be useful to see input parameters (including weight-loss data, cardiovascular risks and health state utilities) reported by sex subgroup. This would give a clear picture of how sex-specific inputs were used in the model and would facilitate the hypothetical/theoretical reproduction of the results by sex subgroup. Assumptions regarding maintenance of weight loss were well described in the orlistat studies. 162,166 However, one of the lifestyle intervention studies165 does not deal explicitly with this issue and appears to assume that the treatment effect is continuous based on initial or transient improvement in cardiovascular health parameters. This is a strong assumption and likely inappropriate in the context of the research question addressed. No sensitivity analyses were explored.
Utilities and quality-adjusted life-years
Only two studies reported results in terms of QALYs. 162,164 Although details of mortality risk and hence life-years were clearly described across the studies, methods of utility estimation were not. Utility estimates were taken from the literature, were sourced adequately and were clearly referenced. However, the methods used to derive those utilities were not clearly described (e.g. standard gamble, time trade-off, visual analogue scale or discrete choice experiments or other methods). The estimation method for utilities is an important factor in determining their robustness and theoretical validity, and hence in the quality assessment of this part of the studies. However, neither of the two studies that estimated QALYs provided in-depth descriptions of the utility estimation methods. There was no discussion of different options for utility estimation or of any impact that these would have had on the overall results. This is a key methodological limitation of these otherwise well-conducted and methodologically appropriate studies.
Sensitivity analyses
All studies attempted some form of sensitivity analysis, mainly focusing on issues of parameter uncertainty. However, none addressed all four types of uncertainty (structural uncertainty, methodological uncertainty, heterogeneity and parameter uncertainty).
Two of the included studies addressed structural uncertainty. Segal and colleagues163 calculated both gross costs (intervention programme delivery costs only) and net costs (programme delivery costs less any downstream future costs to health services) in their analysis. Maetzel and colleagues166 explored the impact of alternative assumptions regarding weight regain and duration of treatment effect. This was the only study to consider this important issue and, as expected, the results were found to be sensitive to this assumption.
Methodological uncertainty was generally well addressed. However differences in results, arising from varying discount rates were explicitly presented in only two162,166 of the five studies. Although Segal and colleagues163 reported sensitivity analysis of discount rates for one individual programme, this was not differences in results for the male-specific programme, and so the impact of alternative discount rates on this programme was not clear. Iannazzo and colleagues164 recalculated the cost-estimates assuming that the Italian NHS and not the patient paid for orlistat. Olsen and colleagues 165 addressed methodological uncertainty in terms of alternative costing assumptions and by inclusion of people’s own use of time in sensitivity analysis.
Heterogeneity in the study results was well accounted for across studies, with four out of five studies reporting results for key subgroups (e.g. impaired glucose tolerance, age, sex). All studies apart from that by Maetzel and colleagues166 reported male and female subgroup results separately; in the study by Maetzel and colleagues166 the base-case model results were specific to a male subgroup. Subgroup analyses conducted were appropriate to the study question and were generally clearly reported and interpreted. When multivariable sensitivity analyses were conducted, the results were not always reported separately for male and female subgroups. Sensitivity analyses tended to focus on base-case results for all patients and not individual subgroups. This renders it difficult to interpret any impact that sensitivity analyses may have had on sex-specific cost-effectiveness estimates.
Parameter uncertainty was explored in four out of five studies, the exception being the study by Olsen and colleagues. 165 Most sensitivity analyses were conducted as simplistic one-way analyses (changing one parameter at a time); however, this does not account for the joint dependence of one parameter on another or, indeed, the dependence of one parameter on sex-specific subgroups. However, some two-way deterministic analyses (varying the values of two parameters at the same time) were conducted by Maetzel and colleagues166 and Galani and colleagues. 162 Sensitivity analyses undertaken were clearly described and justified across the studies. Results were presented in a fair and balanced way and the impact of the sensitivity analyses was appropriately discussed. In one study,163 comprehensive sensitivity analyses were presented for some programmes but not for the programme relevant to a male subgroup.
Three studies162,164,166 conducted extensive probabilistic analysis. Results were presented in the form of cost-effectiveness acceptability curves (CEACs) and scatterplots to illustrate uncertainty. However, in only one of the studies were uncertainty illustrations presented separately for male and female subgroups. 162 Maetzel and colleagues166 also conducted probabilistic sensitivity analysis with an extensive range of clinical outcome parameters. The inclusion of cost parameters was less clear. Results were again illustrated in the form of CEACs. Bootstrapping analyses were plotted on a comparative graph of costs and effects from the Cox regression models used in the study by Olsen and colleagues165 and these illustrated the uncertainty and wide variability in the ICERs presented.
Consistency and validation of the results
The mathematical logic behind the models was tested in only one study, that by Iannazzo and colleagues,164 with the convergence and stability of the model being tested. Multiple chains were run and both visual and statistical tests were used to test the model’s reliability. Counterintuitive results were found in one study,162 with cost-effectiveness in a borderline obese group being greater than in an overweight group and poorer than in an obese group. These results were acknowledged and discussed, with justification provided in the article. Four162,164 – 166 out of five studies discussed the results in the context of other literature in the field and comparisons were made with the results of similar studies when appropriate. No in-depth discussion of other studies was presented by Segal and colleagues. 163
Summary
Studies were of variable methodological quality but many were appropriate to and compliant with best practice guidelines at the time of their publication. There are some common themes and issues that could be addressed to improve future economic evaluations of men’s health interventions to induce weight loss:
-
Improving the reporting of sources used to inform utility weights and also the methods used within source studies to derive those weights (e.g. standard gamble, time trade-off, visual analogue scale or discrete choice experiments).
-
Reporting of costs (both intervention and subsequent costs of complications) in detail, with appropriate references, in a manner that would facilitate the theoretical reproduction of the study results.
-
Downstream costs to health services associated with the differential risk of significant health-care events should be incorporated in economic models as standard.
-
Assumptions should be clearly defined and highlighted in the published articles. It should be clear to the reader what assumptions have been used, especially regarding maintenance of weight loss and continuation of treatment effect. These should be comprehensively tested in structural sensitivity analyses.
-
Methods used to model the effect of weight loss on future obesity-related disease should be comprehensively explained. Where a number of potential data sources exists, choices regarding which source to use should be clearly outlined and any potential variation explored in deterministic or probabilistic sensitivity analysis.
-
Specifically for interventions relating to men, data inputs for the model should be clearly detailed on a sex-specific basis. When data for all sexes have been used and applied to male and female subgroups separately, this should be acknowledged and highlighted as a potential limitation.
By addressing these points it would be possible to greatly improve the quality of studies in the area of men’s health in general and in the area of the treatment of male obesity in particular. These points are based on issues arising from the studies included in the review; however, it is acknowledged that the estimation of results separately for male and female subgroups was not the primary goal of the included studies and so sensitivity analyses were not all presented separately for sex-specific subgroups.
A summary of the quality assessment checklists is presented in Table 38 . Detailed comments on individual studies are presented in the detailed quality assessment checklists in Appendix 13 .
| Quality criterion | Dimension of quality | Question | Galani 2007162 | Iannazzo 2008164 | Maetzel 2003166 | Olsen 2005165 | Segal 1998163 |
|---|---|---|---|---|---|---|---|
| Structure | |||||||
| S1 | Statement of decision problem/objective | Is there a clear statement of the decision problem? | Y | Y | Y | Y | Y |
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | Y | Y | Y | Y | Y | ||
| Is the primary decision-maker specified? | N | N | N | N | N | ||
| S2 | Statement of scope/perspective | Is the perspective of the model clearly stated? | Y | Y | Y | ? | Y |
| Are the model inputs consistent with the stated perspective? | N | N | Y | N | Y | ||
| Has the scope of the model been stated and justified? | Y | Y | N | N | Y | ||
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | Y | Y | Y | ? | ? | ||
| S3 | Rationale for structure | Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Y | Y | Y | N | Y |
| Are the sources of data used to develop the structure of the model specified? | Y | Y | N | N | N | ||
| Are the causal relationships described by the model structure justified appropriately? | Y | Y | Y | Y | N | ||
| S4 | Structural assumptions | Are the structural assumptions transparent and justified? | Y | Y | Y | ? | Y |
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | Y | Y | Y | N | Y | ||
| S5 | Strategies/comparators | Is there a clear definition of the options under evaluation? | Y | Y | Y | Y | ? |
| Have all feasible and practical options been evaluated? | Y | Y | Y | Y | Y | ||
| Is there justification for the exclusion of feasible options? | NA | NA | NA | NA | Y | ||
| S6 | Model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | Y | Y | Y | N | Y |
| S7 | Time horizon | Is the time horizon of the model sufficient to reflect all important differences between options? | Y | ? | ? | N | Y |
| Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | Y | Y | Y | Y/N | ? | ||
| S8 | Disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | Y | Y | Y | NA | Y |
| S9 | Cycle length | Is the cycle length defined and justified in terms of the natural history of disease? | Y | Y | Y/N | NA | Y/N |
| Data | |||||||
| D1 | Data identification | Are the data identification methods transparent and appropriate given the objectives of the model? | Y/N | Y/N | Y | Y | ? |
| When choices have been made between data sources, are these justified appropriately? | N | ? | N | ? | ? | ||
| Has particular attention been paid to identifying data for the important parameters in the model? | Y | Y/N | Y | Y | Y | ||
| Has the quality of the data been assessed appropriately? | Y | Y | ? | N | N | ||
| When expert opinion has been used, are the methods described and justified? | NA | NA | NA | NA | N | ||
| D2 | Data modelling | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Y | Y | Y | Y | Y |
| D2a | Baseline data | Is the choice of baseline data described and justified? | Y | Y | Y | Y | Y/N |
| Are transition probabilities calculated appropriately? | ? | Y | ? | NA | ? | ||
| Has a half-cycle correction been applied to both costs and outcomes? | N/Y | N | N | NA | N | ||
| If not, has this omission been justified? | NA | N | N | NA | N | ||
| D2b | Treatment effects | If relative treatment effects have been derived from the trial data, have they been synthesised using appropriate techniques? | Y | Y | ? | ? | ? |
| Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | Y | Y | Y | Y | Y | ||
| Have alternative extrapolation assumptions been explored through sensitivity analysis? | N | N | Y | N | N | ||
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | Y | Y | Y | Y/N | Y | ||
| Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis? | N | N | Y | N | Y | ||
| D2c | Costs | Are the costs incorporated into the model justified? | Y | Y | Y | N | Y |
| Have the sources for all costs been described? | Y | Y | Y | Y | Y | ||
| Have discount rates been described and justified given the target decision-maker? | Y | Y | Y | Y | Y | ||
| D2d | Quality of life weights (utilities) | Are the utilities incorporated into the model appropriate? | ? | Y | NA | NA | NA |
| Is the source for the utility weights referenced? | Y | Y | NA | NA | NA | ||
| Are the methods of derivation for the utility weights justified? | ? | ? | NA | NA | NA | ||
| D3 | Data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | Y | Y | Y | Y | Y/N |
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | NA | NA | NA | NA | NA | ||
| Is the process of data incorporation transparent? | Y | Y | Y | Y | N | ||
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | Y | Y | NA | NA | NA | ||
| If data have been incorporated as distributions, is it clear that second-order uncertainty is reflected? | Y | Y | Y | NA | NA | ||
| D4 | Assessment of uncertainty | Have the four principal types of uncertainty been addressed? | N | N | N | N | N |
| If not, has the omission of particular forms of uncertainty been justified? | N | N | Y | N | N | ||
| D4a | Methodological | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | Y | Y | Y | Y | N |
| D4b | Structural | Is there evidence that structural uncertainties have been addressed through sensitivity analysis? | N | N | Y | N | Y |
| D4c | Heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | Y | Y | N | Y | Y |
| D4d | Parameter | Are the methods of assessment of parameter uncertainty appropriate? | Y | Y | Y | Y | ? |
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | N | Y | Y | NA | Y | ||
| Consistency | |||||||
| C1 | Internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | N | Y | N | N | N |
| C2 | External consistency | Are any counterintuitive results from the model explained and justified? | Y | NA | NA | NA | NA |
| If the model has been calibrated against independent data, have any differences been explained and justified? | Y | Y | N | NA | N | ||
| Have the results of the model been compared with those of previous models and any differences in results explained? | Y | Y | Y | Y | N | ||
Summary of the results of the included studies
As described in Table 37 , three studies focused on lifestyle interventions and two on orlistat. The included studies were, in most cases, broadly similar in terms of patient populations; however, there was wide heterogeneity in terms of the concomitant lifestyle intervention and control group accompanying orlistat treatment. Data were not reported in sufficient detail across studies to assess common outcome measures and the study designs were not sufficiently homogeneous to draw any comparative conclusions across studies. When appropriate, incremental costs, incremental effects and ICERs have been calculated based on data provided within the studies. Because of the wide variation in reported currencies and costing years, we have inflated costs from the study publication dates to 2012 values using appropriate inflation indices for each individual country. Inflation rates were sourced from Eurostat184 (EU and US data) and rate inflation sources185 (Australian historical data). Costs were then converted to common 2012 UK pounds using purchasing power parity indices provided by the Organisation for Economic Co-operation and Development. 186 The purpose of this exercise is not to facilitate a direct comparison between studies but rather to assess them all in a common currency and year of valuation, to aide understanding of the results.
Despite the heterogeneity of the study results we can nonetheless comment on individual study results and interpret these in the context of the decision problem, the objective of the analysis, the structure of the model and the outcome measures reported. Summary cost-effectiveness results are presented in Table 39 , with more detailed information on each study available from the data extraction forms included in Appendix 13 . In this table the key base-case results are presented. In studies in which the base-case analysis was concluded using a cost–utility and cost-effectiveness analysis framework, the former is reported. Results refer to male-only subgroup analysis unless otherwise stated.
| Study ID | Intervention | Comparator | Time horizon of model | Currency (year) | Base-case discount rates | Primary economic outcome measure | Incremental costs, study currency (2012 UK £)a | Incremental outcomesa | ICER, study currency (2012 UK £)a | ICER range from sensitivity analyses, study currency (2012 UK £)a | Results from probabilistic sensitivity analysis (if applicable) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Galani 2007162 | Lifestyle intervention | OW group (standard care); OB group (lifestyle advice) | 60 years or to max. age of 85 years | Swiss francs (2006) | Costs 3%; effects 3% | QALYs | OW: +405 (+196); BL: –6 (–3); OB: +127 (+61) | OW: +0.25; BL: +0.28; OB: +0.29 | NR for all age groups; calculated as: OW: 1620 (784); BL: dominant; OB: 438 (212)b | Dominant to +2014 (974) | BL only presentedc 57% (35-year-old man) to 72% (55-year-old man) probability of cost-effectiveness at a WTP of 0 CHF per QALY gained92% (35-year-old man) to 98% (55-year-old man) probability of cost-effectiveness at a WTP of 1000 CHF per QALY gained |
| Iannazzo 2008164 | Orlistat + lifestyle intervention | Placebo + lifestyle intervention | 10 years | Euros (2007)d | Costs 3.5%; effects 3.5% | QALYs | +2931 (+2739) | +0.046 | 74,290 (69,427) | Men only: NR Men and women: 10,160 (9495)–79,110 (73,931) | Men only: NR Men and women: 15% probability of cost-effectiveness at a WTP of €45,000 per QALY gained (base-case analysis), increasing to 99% probability for a subgroup with IGT |
| Maetzel 2003166 | Orlistat in addition to standard treatment | Standard treatment guidelines alone | 11 years | US dollars (2001) | Costs 3%; effects 3% | Event-free LYG | +1099 (+969) | +0.162 | 8327 (7258) | 8327 (7258)–25,827 (22,511) | 95% probability of cost-effectiveness at a WTP of US$20,000 (i.e. £17,431) per event-free life-year gained, assuming continuation of treatment effect over 3 years95% probability of cost-effectiveness at a WTP of US$68,000 (£59,265) per event-free life-year gained, assuming continuation of treatment effect for 1 year only |
| Olsen 2005165 | GP or dietitian counselling | Standard care | Costs: 1 year; effects: up to age 80 years | Danish kroner (2001) | Costs none; effects 5% | LYG | Diet: +1684 (+182); GP: +774 (+84) | Diet: +0.0002; GP: +0.1210 | Diet: NR, calculated as 8.42 M (912,148); GP: 6399 (615) | Diet: 26,730 (2896)–6,155,000 (660,820); GP: 3240 (351)–24,037 (2604) | NA |
| Segal 1998163,e | Group behavioural modification | Standard care | 25 years post intervention | Australian dollars (1997) | Costs 5%; effects 5% | LYG | Intervention cost 577 (365); total cost NR | Mixed:f +111;g IGT: +138g | (Net costs)Mixed:f dominant; IGT: Dominant | Dominant to 1600 (1013) | NA |
Lifestyle interventions
In relation to the lifestyle intervention studies, Segal and colleagues163 found the group behavioural intervention for men to be cost-effective for both the mixed glucose tolerance subgroup (presumed to be a mix of normal glucose tolerance, impaired glucose tolerance and type 2 diabetes) and the impaired glucose tolerance subgroup. Although the intervention was found to be cost-effective for both groups, the estimates of ICER were slightly lower for the impaired glucose tolerance subgroup, indicating potentially greater value for money associated with intervening with those at greatest risk. The male-specific programme was on average less costly and more effective and was thus dominant over the comparator treatment of no routine intervention. The sensitivity analyses showed that the conclusions were robust to plausible variation in the treatment success rate. Despite a lack of detailed information available for the male-specific programme, the results indicated that a group behavioural modification intervention may be a cost-effective use of health-care resources. Preventative measures in type 2 diabetes thus showed the potential to be either cost saving or highly cost-effective. However, the authors flagged an urgent need for research in the area of diabetes prevention, especially in terms of quality-of-life outcomes. Despite this recommendation being made in 1998, there is still little robust evidence regarding the impact of weight loss on quality of life and cardiovascular risk events within an economic evaluation framework for overweight and obese men.
Olsen and colleagues165 found that GP counselling was more cost-effective than dietitian counselling for encouraging weight loss. The authors concluded that GP counselling is cost-effective, but that the differences are probably the result of GPs offering extra advice beyond the dietary/nutritional counselling prescribed in the protocol. The authors concluded that, despite the lack of data from their study to support dietitian counselling, the role of the dietitian should not be discounted, especially given health-care provider constraints in practice.
Galani and colleagues162 conducted a cost–utility analysis and found that a lifestyle intervention was highly cost-effective, with ICERs well below those typically considered good value for money for health gains. ICERs were dominant for a male subgroup including those who were borderline obese, with the intervention being less costly and more effective than the comparator. Results for other groups (overweight and obese) were also associated with ICERs of < 2000 CHF (Swiss francs) or £1000 (year 2012) per QALY gained. Given usual values of WTP for a QALY gained considered by decision-makers, the intervention evaluated was highly cost-effective. The results remained robust to sensitivity analyses for the male population subgroup, adding strength to the cost-effectiveness conclusions drawn by the authors. The authors recommend that pan-European research in the area should be a priority.
In summary, based on the data presented in this review, there is some evidence to suggest that lifestyle interventions could be a highly cost-effective use of resources in terms of encouraging weight loss and improving health outcomes in overweight and obese men. However, because of the many assumptions made and the heterogeneity of the studies, it is not possible to synthesise a robust comparison of these interventions regarding cost-effectiveness.
Orlistat
Two studies evaluating orlistat for obesity presented data of relevance to a male subgroup of the population. The first164 reported a cost–utility analysis of orlistat in combination with a lifestyle intervention compared with a lifestyle intervention alone. The base-case ICER was €74,290 (£69,427, year 2012), well above a level of WTP for a QALY gain that would typically be accepted as cost-effective. Although sensitivity analyses were not available for a male-specific subgroup, sensitivity analyses were conducted for the wider group of all sexes combined. There was significant uncertainty in the presented ICERs, with the results being particularly sensitive to the level of risk of developing diabetes. Therefore, the authors concluded that, if the drug was targeted at a high-risk group (i.e. impaired glucose tolerance), the treatment had an estimated ICER of €10,160 (year 2007) per QALY gained, equivalent to approximately £9500 (year 2012), indicating that the drug was likely to be cost-effective if targeted at the highest-risk subgroups of the population. Although data were not presented, it is likely that the conclusion on cost-effectiveness in those at greatest risk also holds true for men.
The second study,166 carried out in the USA, reported costs per event-free LYG. In the base-case analysis it appeared that the drug was an appropriate addition to standard guideline treatment for diabetes, resulting in weight loss. The findings suggest that the use of orlistat was cost-effective in the management of overweight and obese patients with diabetes in the USA. The results of the study are not presented separately for male and female subgroups but could be considered representative of a 52-year-old man, based on the relative risk calculations for cardiovascular events used in the model. Although the authors suggested that the intervention may be cost-effective in a US setting, it was not clear whether results would be transferable to a UK setting. There was some uncertainty in the estimates of cost-effectiveness and the results were particularly sensitive to the assumed duration of treatment effect. The greater the duration of benefit, the more likely the drug was to be cost-effective. Observational data to support long-term use of orlistat in this population are needed to validate the results of the study and further studies are required to reproduce the results in male subgroups of different ages in the UK population.
In summary, there is some evidence to suggest that orlistat is a cost-effective add-on to lifestyle interventions in male overweight and obese patients, with a greater likelihood of cost-effectiveness if the drug is targeted towards those at greatest risk of diabetes.
Summary
There is evidence of mixed quality and strength which suggests that lifestyle interventions are cost-effective at reducing weight and improving future health gains. There is also some evidence that orlistat may be cost-effective as an add-on to lifestyle interventions if targeted at the subgroups at greatest risk. Despite these promising results, strong assumptions were made in the studies regarding the continuation of treatment effects and the similarity of weight-loss success across male and female subgroups. There were no clear indications of large differences in cost-effectiveness outputs across studies between male-specific subgroups and their female counterparts.
Discussion
We conducted a systematic search of the literature for studies that assessed the cost-effectiveness of a range of interventions for the treatment of male obesity. To our knowledge, this is the only systematic review of the cost-effectiveness of weight-loss interventions specific to a male subgroup of the population.
However, we were unable to find any studies that specifically set out to model the cost-effectiveness of obesity interventions in men. In total, five studies reporting male-specific subgroup analyses were deemed relevant for inclusion (three evaluating lifestyle interventions and two evaluating orlistat). Of the included studies, one described a male-specific intervention programme, among others;163 however, only limited details of the particular intervention are provided. A second study166 was modelled on risk factors for a 52-year-old man (from the UKPDS170); however, male-specific weight-loss data were not explicitly included in the model. The fact that only subgroup analyses are reported renders it difficult to quality assess the included studies in terms of sex-specific model inputs. The remaining three studies simply presented male subgroup analyses.
There is an indication in the included studies that lifestyle interventions appear to be highly cost-effective and that orlistat has the potential to be cost-effective when prescribed to those at greatest risk of complications of type 2 diabetes. However, it is important to interpret the studies in this review in light of their methodological quality and limitations.
First, as discussed, the studies were not designed to determine cost-effectiveness for a male-only subgroup of the population. The male-specific results presented were instead subgroup analyses of the main studies. It is unclear in a number of studies how sex-specific data were used to populate the economic models. Although results are presented for male subgroups, the use of non-sex-specific baseline data inputs for the models is likely to reduce the ability to show any male/female differences in the cost-effectiveness results.
Second, strong assumptions were made across the studies with regard to the continuation of treatment effects and weight-loss maintenance over time, with no clear consensus about how these assumptions have been incorporated into the economic models. Studies that assumed maintenance of incremental weight loss over time for the experimental group are likely to bias the analysis greatly in favour of the experimental intervention. There is great uncertainty, for example, with regard to the continuation of the treatment effect for orlistat. One study166 investigated this and found substantial variation in the ICER depending on the assumptions used in the model. Although it is accepted that there is a lack of evidence with regard to the continuation of the treatment effect, it is imperative that this aspect is adequately and comprehensively tested in sensitivity analyses to explore the impact of uncertainty in the model parameters on the final results. This will allow decision-makers to decide what level of uncertainty they are willing to accept when making recommendations regarding the provision of various treatments or interventions.
Third, the models have been developed with varying degrees of modelling sophistication and complexity. Although four studies included Markov decision-analytic models, the level of complexity and the number of potentially important health states included varied greatly across the studies, from only three in one study to eight in another. It is important that best practice guidelines and detailed clinical expertise are sought when developing economic models for weight-loss interventions, both in men specifically and in all population groups more generally. The inconsistency across studies in terms of model structure and health states modelled means that results are likely to differ substantially across studies, and common comparisons cannot be made. For example, studies that consider downstream costs of diabetic complications are more likely to accurately reflect the dynamics of the disease pathway than those that do not. This coupled with the fact that outcome measures are not consistent across the studies (some reporting cost per life-year gained, others cost per event-free life-year gained and others QALYs) renders it difficult to disentangle any real trends across the studies.
Fourth, only two of the included models presented detailed cost–utility analyses with dynamic modelling of the treatment pathway and reported results in terms of costs per QALY gained. Cost–utility analyses are recommended by NICE for the evaluation of health-care interventions and have been used previously in numerous cardiovascular and diabetic modelling studies. The use of QALYs as the estimate of choice is important as QALYs measure both the mortality aspect and the quality-of-life aspect of the chronic complications of obesity. However, although methodologically robust, the two studies that reported costs per QALY gained were not designed to answer a male-specific question.
Fifth, broader measures of benefit, which go beyond the QALY measure and value attributes of an intervention beyond health outcomes, are more likely to capture issues of importance to the patients themselves. One suggestion for future health research in this area could be the use of cost–benefit analyses and discrete choice experiments to evaluate the processes and attributes of care that patients, and indeed taxpayers, feel are of greatest value. This would extend the measure of benefit considered beyond quality of life to include a range of attributes of importance.
Finally, the use of older studies with outdated unit costs across different countries and with different control treatments and subtle differences in the interventions delivered renders broad comparisons across studies difficult. The lack of evidence is further complicated by the lack of generalisability of the studies to the UK setting. Although we have endeavoured to generate UK estimates where possible based on inflationary and purchasing power parity assumptions, such an approach generates uncertainty in itself. It is therefore our conclusion that, on the basis of the retrieved literature, there is insufficient evidence available to make clear recommendations regarding the cost-effectiveness of interventions or treatments for male obesity. There is also insufficient evidence to recommend different treatment strategies for male and female subgroups on the grounds of cost-effectiveness.
Overall summary of the cost-effectiveness review
The key conclusions from the review of cost-effectiveness studies are summarised below:
-
There were no studies evaluating the cost-effectiveness of weight reduction interventions exclusively in an overweight and obese male population.
-
Five studies of weight-loss interventions for male and female participants evaluated the cost-effectiveness of the interventions in male subgroups.
-
The evidence suggests that lifestyle interventions combining low-fat, usually calorie-reducing dietary advice and physical activity are likely to be cost-effective; however, the interventions were poorly described in some studies.
-
There is some evidence suggesting that orlistat may be cost-effective in addition to lifestyle interventions, especially when targeted at those with or at greatest risk of developing type 2 diabetes (e.g. those individuals who have impaired glucose tolerance).
-
None of the studies presented clearly differing conclusions for male and female subgroups and there is insufficient evidence to say whether this is because of a real similarity in the groups or the result of methodological complications with regard to sex-specific inputs to the economic models.
-
Studies were of varying methodological quality, especially with regard to modelling methods and assumptions over the continuation of the treatment effect (i.e. the modelling of maintenance of weight loss over a longer time period) and the modelled link between weight loss and final health outcomes.
-
The methodological variability and study heterogeneity, with many key differences across studies, including differences in comparators, interventions, costing methodology, model sophistication, primary economic analysis outcomes and country of study, make it difficult to assess the factors that are of greatest importance in determining cost-effectiveness.
-
Therefore, on the basis of the evidence summarised in the review and the lack of generalisability to a UK setting, it is impossible to draw clear conclusions on the cost-effectiveness of alternative interventions for the treatment of male obesity.
Future research recommendations
There is an urgent need for UK male-specific economic evaluations to assess the value for money of alternative weight reduction strategies in a UK decision-making context. Such studies should systematically consider the available evidence on acceptability, effectiveness, costs and cost-effectiveness of alternative interventions. The following are key requirements for future research:
-
The development of decision-analytical models that model a dynamic disease pathway, using sex-specific model inputs to determine the key drivers of cost-effectiveness in weight-loss interventions for obese men and what, if any, differences exist in terms of cost-effectiveness across sex subgroups.
-
In terms of the parameters of greatest importance, a study conducted by Galani and colleagues167 alongside one of our included studies162 indicated that, based on expected value of information analysis, if further research were to be commissioned it should focus on the effectiveness of lifestyle interventions for cardiovascular risk factors and quality of life of overweight and obese people.
-
An important consideration for future research will be to explore and improve modelling methods to link the effect of weight loss to overall disease risk. Some of our included studies seemed to suggest that small and even transient weight loss may have an impact on future disease risk and therefore could have an important impact on long-term effectiveness and cost-effectiveness outcomes.
-
Future studies should focus on assumptions surrounding the maintenance of weight loss over time, rigorously testing the impact of assumptions on cost-effectiveness outcomes through comprehensive sensitivity analyses.
-
It is important for future research to use a broad measure of benefit and to involve service users in the decision-making process, focusing on the interventional processes and associated outcomes of care that are of greatest importance to individuals to effectively and cost-effectively moderate behaviour.
Chapter 6 Systematic review of qualitative research and mixed-method synthesis of data from men
This chapter is organised into themes based on a logic model ( Figure 42 ) that emerged by applying a combined realist and socioecological approach (for methods see Chapter 2 ). Study characteristics presents a narrative description of the studies that were included in this review. Social, cultural and environmental influences on obesity in men highlights the wider social, cultural, economic and political macro-level themes that have been implicated as predisposing men to gain weight in the first place. Engagement with weight management programmes focuses on men’s engagement with weight management programmes and implementing programme advice within their everyday lives. First, themes that relate to the role and influence of relationships and interactions with family, friends and peers, and various settings like the workplace, home and sports facilities are discussed. We focus on the time period before joining a formal weight management programme to understand factors that motivate men to want to lose weight and decide to engage. In The weight management programme, the themes that relate to men’s perspectives of the relevance and utility of weight-loss programmes and their experiences acquired during their journey through the processes and components delivered as part of weight-loss interventions and how this relates to programme adherence are discussed. The impact and consequences of weight-loss programmes discusses men’s perspectives on the outcomes achieved after they have completed a weight management programme and the consequences for men, their families and their friends. For each theme the qualitative findings are linked to randomised and non-randomised intervention studies aimed at obese men. These findings are then integrated with relevant quantitative systematic review findings (see Chapters 3 and 4 ). Finally, we draw on the wider qualitative literature on aspects of obesity in men. Our analysis is supported by first-order quotations from men with relevant characteristics included in the studies. Author interpretations of primary qualitative data have been described as second-order constructs or second-order themes39 and, finally, third-order interpretations are made as a research team. The chapter finishes with the quality assessment carried out during the data extraction process (see Quality assessment of qualitative studies linked to interventions).
FIGURE 42.
Review Of MEn and Obesity (ROMEO) evidence synthesis logic model.
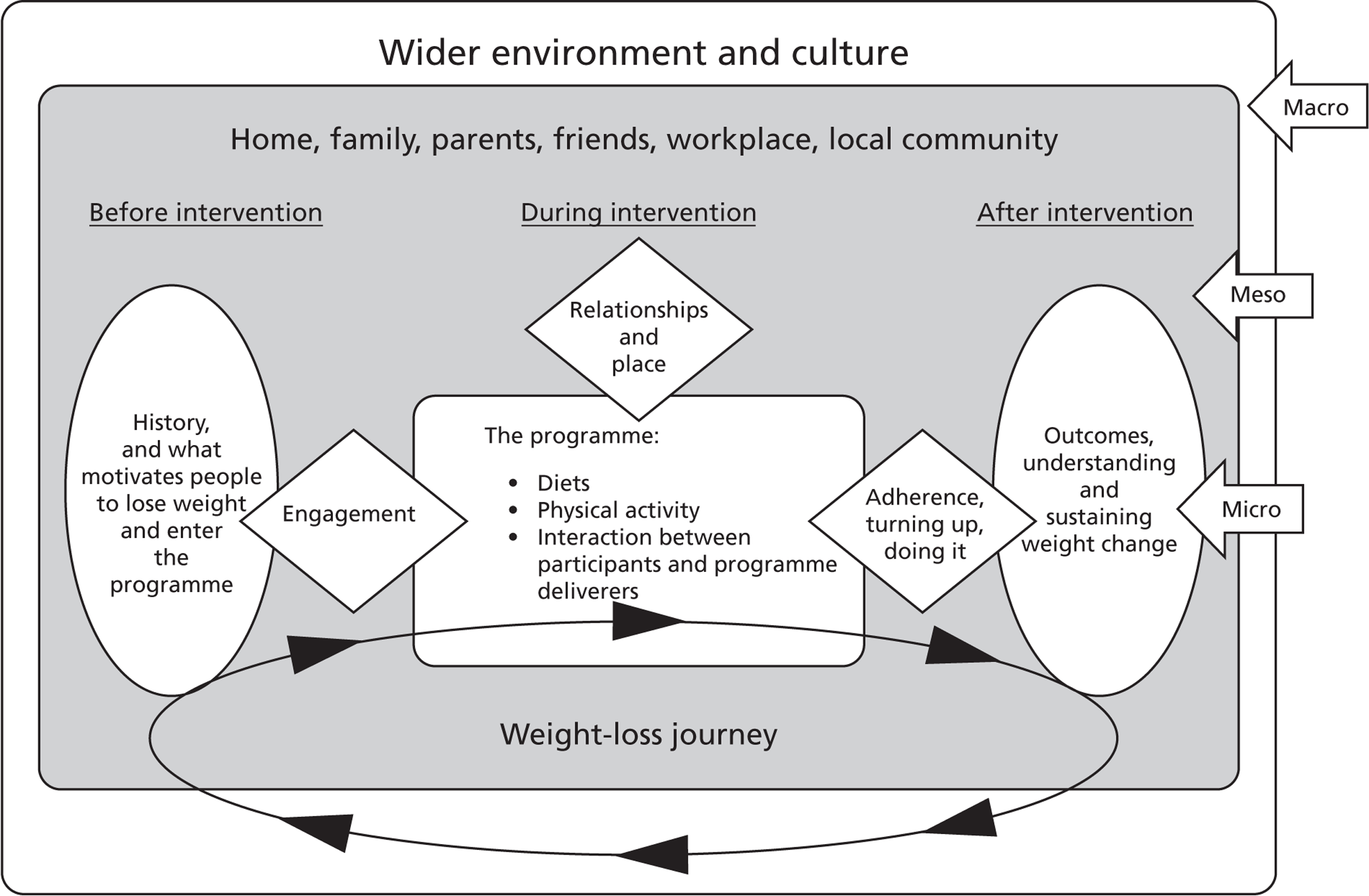
Study characteristics
The primary searches identified 5209 references of which 407 were selected for full-text screening. In addition, 29 studies were identified from other sources, such as commercial organisations and contact with experts. Of these 436 studies, 22 met the inclusion criteria. 44,46,49,89,142,149,187 – 202 Figure 43 provides details of study identification (see Appendix 15 for the data extraction form).
FIGURE 43.
Flow chart for identification of studies.
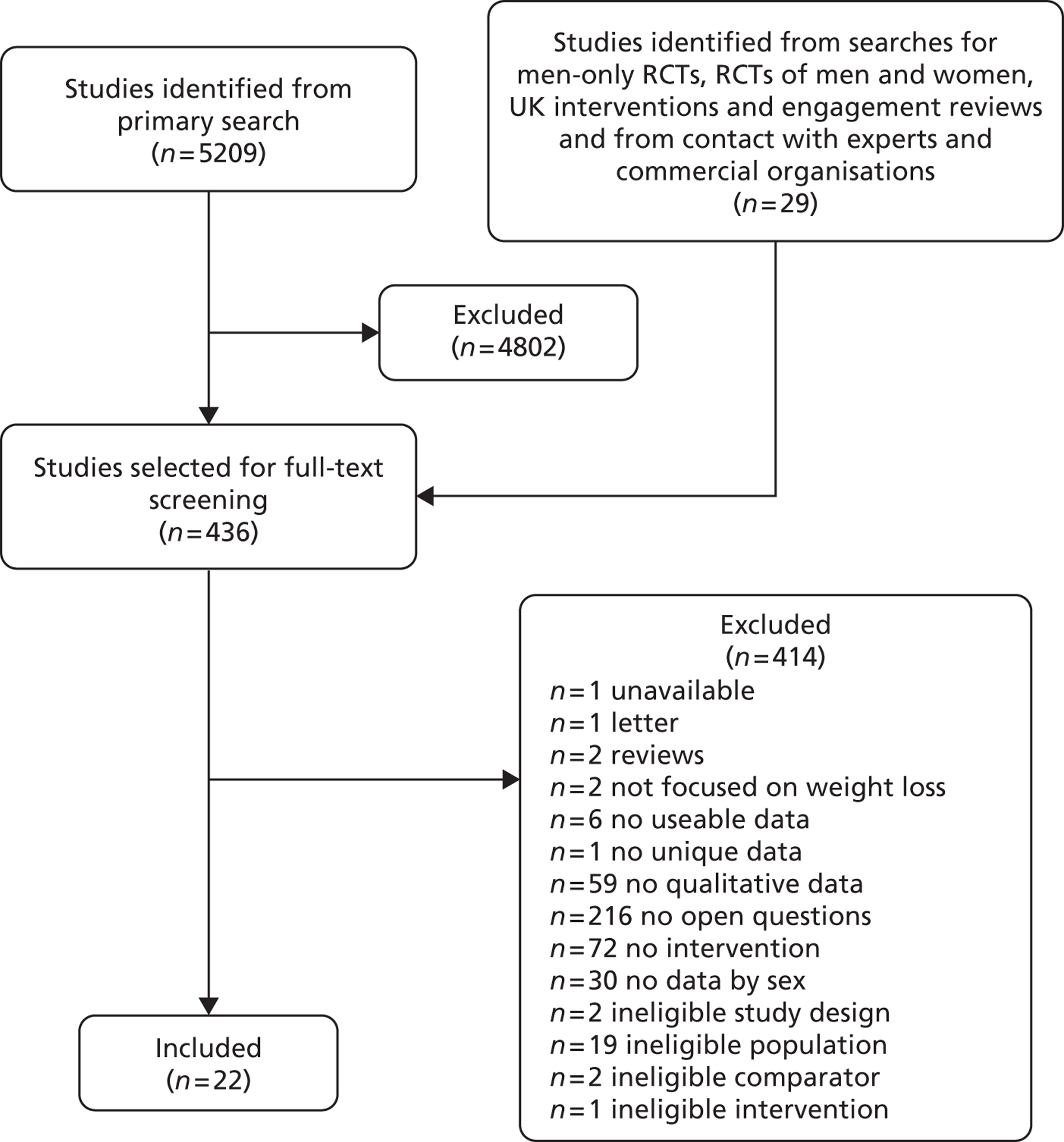
We included 13 studies linked to interventions46,49,89,142,149,187 – 194 and nine studies not linked to interventions. 44,195 – 202 With regard to the studies linked to interventions, five studies46,89,187 – 189 were linked to RCTs and the remaining eight49,142,149,190 – 194 were linked to non-randomised studies.
Four of the included studies linked to interventions46,142,149,189 were linked to studies included in the quantitative reviews in this report and these findings are reported in Chapters 3 and 4 . Eight studies46,49,89,142,149,189,191,193 were linked to interventions that were open to men only. Of these eight, the studies by Gray and colleagues142 and Leishman193 were linked to the same group-based programme (the Camelon model147), which was delivered in men’s health clinics in Scotland, whereas the studies by White and colleagues49 and Harrison191 were linked to the Health of Men workplace-based weight management programme that was delivered in England in a group format. The study by Witty and White149 was linked to a group-based intervention that was delivered at Leeds Rhinos Rugby League Club in England. 139 The studies by Morgan and colleagues89,189 were linked to the SHED-IT trial,189 which was an individual website-based weight management programme delivered in Australia. The study by Hunt and colleagues46 was linked to the group-based FFIT intervention delivered at senior Scottish football club stadiums. 141
The remaining five studies were linked to interventions that were open to both men and women. The study by Gallagher and colleagues187 was linked to a group-based intervention delivered in Australia,203 that by Mallyon and colleagues188 was linked to an individual-based intervention delivered in Australia,204 and the study by Abildso and colleagues190 was linked to a group-based insurance-sponsored weight management scheme delivered in the USA. 205 The study by Kim and colleagues192 reported on a faith-based group weight management programme delivered within a rural African American community, whereas the study by Ogden and Sidhu194 analysed the experiences of individuals taking the medication orlistat for weight loss in England.
Each of the nine studies not linked to interventions was UK based and collected primary data from a male-only sample. These studies drew on the views, attitudes and perceptions of men who taken part in formal weight management programmes and interventions or who had attempted to reduce or manage their weight in other ways. De Souza and Ciclitira195 interviewed men to investigate their views on health and body image. In addition, Gillon196 examined how men talk about body weight. The study by Gough and Conner44 provided an account of meanings that men attach to food and the links between food and health. Gough and Flanders197 conducted research with men who were active members of the gay ‘bear’ community, which is a homosexual subculture in which excess weight is considered sexually attractive. McCullagh198 interviewed long-distance lorry drivers to inform the development of appropriate health education strategies to encourage them to be health aware, access services and attain a healthier lifestyle. One study by Monaghan199 investigated attitudes to obesity and ill health, disease and disease risk and another study by Monaghan200 discussed justifications for levels of body mass that are in the overweight or obese range. A third study by Monaghan201 explored men’s talk about physical activity, weight, health and slimming. Finally, the study by Weaver and colleagues202 investigated how men understand obesity and its relation to the risk of diabetes.
Social, cultural and environmental influences on obesity in men
Most included studies took a predominantly individualistic approach to the factors that may influence weight gain, rather than a more ecological and environmental approach. References to issues in the wider environment that may act to increase the propensity for men to be obese were identified in four45,48,141,148 of the 13 included studies linked to interventions and six43,190,193,197,198,201 of the nine studies that were not linked to interventions.
Two distinct subthemes emerged from the data: Sociostructural determinants of obesity and Obesity and deprivation. No quantitative data from the other systematic reviews were found to support or disconfirm these themes.
Sociostructural determinants of obesity
White and colleagues49 referred to structural changes in society that may predispose individuals to gain weight. They presented this in a second-order construct as a dichotomy between (1) the fecklessness of three-quarters of men in the UK, which the authors thought was unlikely, and (2) vulnerability because of structural changes in the wider society.
No participant quotes were found to indicate that men were thinking about wider society-level determinants of their behaviour when discussing factors that helped or hindered weight-loss attempts. Indeed, it is evident from the data presented in the remainder of this chapter that those issues were almost entirely absent from men’s conceptualisation of the problem, with an emerging prevalent theme of personal responsibility more obviously apparent. White and colleagues49 did not reference specific examples of sociostructural issues that may influence the increasing prevalence of obesity in the UK. However, previous research206 has demonstrated that individuals may interact with environments on many levels in activity-related settings such as the workplace, home, school and the neighbourhood that can be and are influenced by structural factors in the macro environment such as government policy, education and health systems.
Some studies in this review suggested that the role that environment plays in influencing behaviours helped some researchers decide where to locate the delivery of weight management programmes. For example, the Health of Men programme, as described by Harrison191 and White and colleagues,49 was located in the workplace, with the rationale that it was more convenient to access than non-workplace settings. Two other studies were located in sports facilities,46,149 with the rationale that these environments were congruous to male identities and therefore more appealing to men than interventions in more formal clinical settings. Targeting a population through a special interest setting was apparent in the study by Kim and colleagues,192 who located their faith-based weight management intervention in a (predominantly) African American church. The Camelon model142,193 was located in a more formal health-care setting, namely a men’s health clinic. However, in contrast to the other studies mentioned here, the authors emphasised the utility of a group-based setting for men (over the physical location of the intervention) in allowing men to raise sensitive issues that may otherwise be embarrassing to discuss. 142 The remaining studies188,190,194 were not clear about the setting where the intervention took place.
Participants in two studies191,194 made reference to environmental factors relating to work that they believed played a part in the onset of their obesity. A 43-year-old white participant in Ogden and Sidhu’s study194 on the experiences of taking orlistat for weight management stated that his static job played a part in his obesity. This man talked about knowing that he ate far too much of the wrong things, naming bacon, eggs, sausages, chips, meat and bread.
The repeated use of ‘I’ by this participant (in his account of this issue) suggested a strong sense of intrinsic personal responsibility with regard to his engagement in behaviours that he perceived had led to his obesity. Nevertheless, reference to external determinants of behaviour (considered beyond the control of the individual) that had contributed to a person’s weight gain was observed in the study by Harrison,191 linked to the Health of Men workplace-based intervention. Here a participant described how working irregular hours away from home played a role in his gaining weight:
When you work away from home you have no one to prepare nutritious food for you, you live off takeaways, kebabs, pub meals and have a few pints
Mark, age 34 years, p.70191
The reference to ‘you’ as opposed to ‘I’ may thus be viewed as absolving oneself from blame. Finally, a 47-year-old participant quoted by Ogden and Sidhu194 attributed his obesity to his home environment, family behaviour in relation to food availability, finishing his children’s food and his personal coping strategy of turning to food for comfort to improve his general sense of well-being.
These examples illustrate the complexity of the interactions between the individual men’s intrinsic and extrinsic motivation to change and the environmental barriers to and facilitators of instigating and sustaining change. Such interactions, which operate between the macro, meso and micro levels described in ecological models of behaviour, pervade the themes that are presented in the subsequent sections of this chapter.
Referring to the wider qualitative literature on obesity in men, reference is made to the concept of the obesogenic environment. This has been defined as:
The sum of influences that the surroundings, opportunities, or conditions of life have on promoting obesity in individuals or populations
p. 56465
Rayner and Lang,67 Hanlon and colleagues,64 Butland and colleagues,5 Swinburn and colleagues65 and Leeder207 have argued that sociostructural factors have played a very powerful role in creating toxic environmental conditions that make it very difficult for individuals to maintain a healthy weight. They point to relatively recent but radical changes in food supply (in terms of the price and availability of food), transport and urban design (especially walkability) as well as commercial pressures to overconsume food and engage in less than optimal levels of physical activity as key drivers of the obesity problem.
The importance of the sociostructural determinants of obesity as a theme that contrasts with the obesity as individual responsibility standpoint was supported in five of the studies not linked to weight management interventions. 44,197,198,201,202 For example, Weaver and colleagues202 provided direct quotes from men regarding sociostructural determinants of keeping fit and eating healthily. These quotes, related to press and government health promotion messages, such as eating five portions of fruit or vegetables a day, and food labelling, indicated that some men were conscious of the sociocultural and economic aspects of their lives that encourage weight gain. 202
However, government public health messages were described in another study44 as an intrusive health lobby, which can prompt resistance and a will to reclaim eating as personal choice. A quote provided to support this referred to all enjoyable food being bad for you, something which the 47-year-old respondent argued was not the case two decades ago when you could eat what you liked.
Nevertheless, another participant, in the study by Weaver and colleagues,202 appeared to show that government-driven health messages are heeded by some:
There is always fresh fruit in the house . . . I try and keep away from microwave meals . . . we don’t eat a lot of fried food you know, if everything is done, it would be grilled or it would be boiled
Jack, age 34 years, p. 4202
The use of pronouns is interesting, as the impersonal ‘there is always fresh fruit’ represents a detachment from the actual buying or eating of the fruit but also a recognition of the importance of healthy food being always available as a facilitator to healthy behaviour.
The men in McCullagh’s study198 on long-distance lorry drivers also frequently attributed their self-perceived poor diet and low levels of exercise to their obesogenic work environment. They recognised that driving long hours leads to weight gain, with some expressing regrets about gaining a licence. In particular, the drivers lamented the lack of healthy food available at roadside catering establishments in the UK:
The food offered in transport cafes is appalling. The breakfasts have names like ‘Belly Buster’ or ‘Heart Attack’
No individual characteristics provided, p. 5198
The theme pertaining to perceptions of obesogenic environments was also confirmed in Monaghan’s199 field diary, written when observing male members of a slimming club. One excerpt covered the difficulties that men encounter in resisting the temptations of the garage shop when filling up their cars, as well as vending machines at work, with a discussion of strategies of how to avoid these temptations.
Obesity and deprivation
Links between obesity and deprivation were highlighted in the Camelon men’s health clinic group-based intervention of Gray and colleagues,142 who stated in a second-order construct that obesity is linked to socioeconomic status through poorer men eating less well, with the incidence of obesity increasing with deprivation and lower levels of education.
This was supported by author text in five of the non-intervention studies,193,197,198,201,202 in which the relationship between obesity and health inequalities was discussed. For example, Weaver and colleagues202 investigated how men understand obesity and its relation to the risk of diabetes. They concluded that lower social class men were more at risk of obesity and its related diseases, but that they were also a hard-to-reach group for health promotion.
In addition, De Souza and Ciclitira195 referenced findings from the literature which suggested that people with lower levels of education and wealth are at a greater risk of obesity and as a result have a lower life expectancy. 195,208 In addition, De Souza and Ciclitira state that, although people of higher socioeconomic status report higher levels of perceived overweight, they have been found to be more likely to monitor their weight closely, more likely to try to lose weight and more likely to report higher levels of physical activity. 195,209 Furthermore, De Souza and Ciclitira195 cite evidence that men of lower socioeconomic status were less likely than women to have knowledge of healthy eating and that the lower a man’s social class or education level the lower the level of such knowledge.
A debate emerges concerning the relative appropriateness of framing obesity discourses according to gender, health or wider macro sociostructural and socioeconomic perspectives. De Souza and Ciclitira195 argue that an appreciation of socioeconomic factors and education, rather than gender differences, is important when investigating the causes of obesity.
However, Monaghan199 argues that focusing the obesity debate on the perceived unhealthiest individuals occupying lower socioeconomic strata is unhelpful. Monaghan refers to victim blaming, a term generated by the orthodox medicalised view of obesity, whereby obese people from lower social class backgrounds are regarded as being in poor health because of their obesity. Moreover, they are forced into pursuing individualised solutions that, in themselves, may be physiologically or psychologically harmful. It is argued that one of the consequences of the focus on obesity is that it legitimates and perpetuates a society in which many people are dissatisfied with their bodies.
The study by Weaver and colleagues202 also provided direct quotes from men indicating that economic factors constrained their choices for healthy eating and exercise. For example, men described the high cost of membership of clubs and gyms, or the fact that healthy food, especially fruit and vegetables, is more expensive than food high in sugar. Similar economic issues were raised by long-distance lorry drivers in the context of work, with an example given in the study by McCullagh198 of a apple costing an expensive 60p.
Engagement with weight management programmes
Despite the increasing prevalence of male obesity in the UK, and the existence of well-established links between obesity and poor health, men are less likely than women to participate in weight-loss programmes. 142,210 The reasons for this lack of participation are not well understood as little research has been carried out to identify the barriers to and facilitators of the participation and engagement of men in weight-loss programmes. 189 This section discusses themes that have been found to affect men’s engagement with programmes and programme advice, in particular considering the lead-up to participating in a weight-loss programme. These themes relate to the role and influence of programme settings and interactions with family, friends and peers and in particular refer to the initial motivation to lose weight, factors that attract men to participate in interventions, the importance of location and setting in acting as a ‘hook’ to engage men to join weight management programmes and the influence of partners, family and friends on men’s engagement with weight management programmes.
Initial motivation to lose weight
Men may pay less attention to their weight than women and may therefore be less likely to attempt weight loss than women. 41,42 However, little is known about the types of issues that motivate men to either join a formal programme or try to lose weight independently. Leishman193 reported that a diagnosis and label of obesity came as a substantial jolt to men attending the group-based Camelon programme in Scotland, who had not previously thought of themselves as being problematically overweight. This realisation appeared to increase their motivation and commitment to embark on a process of trying to lose weight. For example, a 42-year-old man expected that tests would show that he was a bit overweight but finding out that he was obese hit him very hard. When the facts were staring him in the face he felt motivated to act. 193
Another participant from the study by Leishman193 referred to how men who are big can be normalised within some environmental settings such as the workplace. The authors suggested that presenting information on weight visually can potentially provide the jolt needed to increase motivation. The use of charts to display clinical measurements in relation to norms links to the theme discussed later around men’s preference for more scientific and technological representations of obesity. One 38-year-old man was shocked to be labelled obese because in his workplace most of his colleagues were big, suggesting that obesity and being overweight was a previously unacknowledged social network norm for him:
My size just seemed normal. When the girl (assessing nurse) showed me the chart I was really shocked to see that I was clinically obese. If it had showed me as being fat it wouldn’t have got to me as much and I probably wouldn’t have done anything about it.
p. 79193
The obesity diagnosis bringing about the motivation to act (as opposed to being labelled fat) is also highlighted in the study by Gray and colleagues,142 which was linked to the Camelon programme, delivered to men in groups in a health clinic setting in Scotland. In this study men experienced dissatisfaction with their body image only when they were labelled as obese. In contrast, it was reported that men whose BMI was in the overweight range (< 30 kg/m2) were less likely to enrol in the Camelon model’s weight management programme. Gray and colleagues142 therefore contended that contemporary social norms that place emphasis on the idea that men should be bigger and stronger than women have perhaps influenced men’s perceptions of an ideal weight as being in the overweight range.
Concerns regarding health issues that may be worsened by obesity and the concomitant fear and anxiety related to a medical diagnosis or event, or the realisation of how ageing with obesity affects health, can also motivate obese men to lose weight. For example, a 44-year-old participant in the study by Morgan and colleagues89 reported having had a recent health scare and realising that he was not getting any younger, which motivated him to act.
Motivation seemed to increase particularly if a hospital admission was reported, with accounts of medical procedures interpreted as serious or life threatening involving mechanical deficiencies in the heart or breathing, as detailed by Ogden and Sidhu194 investigating the experiences of individuals taking the medication orlistat for weight loss in England. Typically, men would attend their GP surgery and be referred for tests, which showed that their health was far worse than they had envisaged, and this diagnosis/realisation triggered them to do something. As one 43-year-old (of white ethnic origin) in the study explained:
It got to the stage that I knew I was going to die and that was the turning point. I knew I was going to die unless I did something about it. And then I just got into gear and it turned me right around
p. 548194
A similar picture was seen in the study by Gallagher and colleagues:
Having a heart attack really scared me. I just wanted to feel better, see my kids grow up, and be more in control. I had tried so many things, but being in hospital really brought me to my senses
John, age 38 years187
In addition, the study by White and colleagues49 (which is linked to the Health of Men workplace-based weight management programme delivered in a group format in England) found that some overweight participants who felt unhealthy, as opposed to actually being diagnosed with a health problem, were motivated to lose weight.
A desire to improve personal health was also highlighted in the quantitative evidence base. In our review of men-only trials (see Chapter 3 ), emphasising the link between weight loss and improved erectile dysfunction85,122 produced very favourable reductions in body weight. Similarly, men who participated in the Bloke’s Weigh programme147 expressed concerns over mental health issues in addition to concerns about weight and diet (see Chapter 4 ). The greater take-up of commercial weight-loss programmes by men referred from general practice than from self-referral, as seen in Chapter 4 , also suggests the importance of health issues for motivation.
In the study by Morgan and colleagues189 linked to the web-based SHED-IT trial, a concern over physical appearance was also described as a motivator to take part in a weight-loss programme for younger men in particular. However, the only quote provided to support this is from a middle-aged man who, whilst looking at himself in a mirror, compared his appearance unfavourably to that of his father.
These themes pertaining to the initial motivation to lose weight were largely supported in the studies not linked to weight management interventions. For example, the study by De Souza and Ciclitira195 confirmed the theme that men were likely to be motivated to lose weight for health reasons, which they distinguish from their perceptions about why women attempt to lose weight (for appearances). One 54-year-old man reiterated that women were more likely to want to lose weight to fit into their clothes for reasons of vanity and that men were more likely to want to lose weight for medical reasons. Another 45-year-old man in the same study agreed that women’s weight loss was driven by vanity.
Similarly, the study by Gough and Conner44 reported that men were initially motivated to lose weight for health reasons, for example after being diagnosed with diabetes, or when health professionals advised a diet to lower cholesterol.
The study by De Souza and Ciclitira195 also found that for gay men weight management was thought of as a way to improve appearance. For example, a 33-year-old participant talked about slimness as being something that was socially prized within the gay community and another man of the same age talked about withdrawing from the gay scene as he no longer thought himself attractive enough because of weight gain.
However, evidence from participants self-identifying as gay ‘bears’ in the study by Gough and Flanders197 refuted the notion that being labelled as obese provides motivation to lose weight. In this particular gay subculture, obese bodies are the norm and are viewed as desirable and healthy. A 39-year-old man explains how the bear community encourages him to be ‘meaty’, with a BMI in the overweight to obese range. Moreover, the bear community had an immense appeal to him as he felt that he had gained more friends since increasing his BMI.
Factors that attract men to participate in interventions
It has been argued that the failure of programmes to recognise gender issues in weight management may have a part to play in the apparent lack of enthusiasm that men have for joining weight management programmes. 210 Several studies demonstrated how being able to undertake weight-loss activities in a male-only environment was appealing for some men. In the study from Australia by Morgan and colleagues189 a 21-year-old participant explained that he was attracted to the male-only aspect of the programme.
This was also found in the study linked to the group-based Camelon programme, delivered in a health clinic setting in Scotland, with Gray and colleagues142 quoting a man as saying that he would not attend any intervention with female participants.
However, other research211 has found that male-only features of interventions were less important to most men. Instead, the opportunity to receive a second opinion was more important. In addition, a 19-year-old participant in the study by Morgan and colleagues189 stated that the website-based programme with individual advice sounded achievable and this was more important than the male-only aspect of the intervention.
A non-intervention study195 provided evidence to support the view that not all men prefer male-only weight management environments. One 40-year-old man, who was the only man in a slimming club, appreciated the support that he got from his fellow club members. He was also helped by the fact that he was the manager of 20 women, half of whom attended slimming clubs, leading to a competition at work to lose weight, with him reporting that he had beaten them all.
An example of how the delivery of an intervention can engage men is provided by several studies through the use of humour or banter. 46,49,142,149,189,191,193 For example, a nurse in Witty and White’s study149 noted the sensitive communication issues that arise in identifying obesity and inviting men to join a group. A light-hearted approach with the use of banter was important when attempting to approach and encourage overweight or obese men to take part in a study, to lessen the chance of causing offence.
Morgan and colleagues189 explained how humour was deployed in the SHED-IT website-based weight management programme to aid participation and engagement. Comical language and imagery (such as the picture of a beer glass) were used in the programme’s promotional materials to attract men and get across the message that the internet-based programme allowed treats such as beer. This was said to be particularly successful with regard to recruitment; a 29-year-old man said he saw the glass of beer and signed up immediately. 189
A further reason why men were attracted to take part in the Camelon programme was the knowledge that the programme had been successful for other men. This is illustrated by one participant who had been persuaded to join because he had heard that the groups worked well and were a good laugh. 193
The theme of humour is considered again in the following section (see The weight management programme) when the nature of the interactions within weight-loss programmes are reported.
Engagement is also influenced by perceptions of what the weight management programme will involve. Two of the included studies141,192 indicated that men would be disinclined to undertake any forms of strict dieting. For example, a participant in Leishman’s study193 linked to the Camelon programme delivered in a group format in health clinics in Scotland, explained his feelings of apprehension at joining if strict dieting was involved, as a diet that makes one starve is not appealing. However, the man was pleasantly surprised that Camelon was more of an education programme about healthy eating and exercise not a strict diet. In fact, at first he thought that it would not work as he felt that he was eating the same volume of food, with just more vegetables and fruit.
The absence of an extreme dietary regime appeared to be instrumental in attracting men to participate in several studies. 142,189 Gray and colleagues142 found that men had joined the group-based Camelon programme as it was marketed as not involving dieting. However, no participant quotations are given to support this finding.
The importance of location and setting as a ‘hook’ to engage men
The location and setting of certain programmes acted as an important element to attract men to participate in them. In the study by Hunt and colleagues46 the intervention was located within SPL football club stadiums and targeted obese supporters. The aim of weight loss therefore becomes congruent with a strong personal commitment to being a football supporter. Our interpretation is that associating long-standing loyalty, commitment and pleasure attained from collectively supporting a football team (still predominantly a male activity) with challenging men’s lifestyles to encourage behaviour change, such as losing weight, could hypothetically increase the likelihood of ‘contagious motivation’ whereby motivation for turning up to support the team either consciously or subconsciously transfers to motivation to lose weight with fellow team supporters. To support this interpretation, a participant emphasised having been a lifelong football supporter and the status that being part of an intervention with his club gave him in his circle of friends and how this was an incentive to engage with the intervention and lose weight. 46
Hunt and colleagues46 contended that the setting acted as a ‘hook’ and an additional incentive to attract men to participate in weight-loss activities, which they had felt unable to do in other contexts. The football context confirmed the men’s male identities and made them feel comfortable. Our interpretation is that the football club setting facilitated enrolment into a cohesive group of men with shared characteristics (overweight team supporters) who partook in a task-orientated group to lose weight according to key criteria for delivering health improvement groups. 212 Similarly, Witty and White149 studied a weight-loss programme that was set in a rugby stadium. Certain men seemed more inclined to attend a weight management programme located in such a setting, which might be because of a reduction in men’s anxiety in this setting. One respondent commented that he would recommend this programme to his friends and that he found it more comfortable than traditional health services, because some men still have:
Anxiety or apprehension about consulting a health professional in a building that is very clearly a health oriented building so I think that the opportunity to be able to access some form of health service in an environment that I would imagine feels far less threatening.
Age 35–44 years, p. 25149
The sense of well-being and pleasure associated with attending a football or rugby game contrasts with the anxiety and fear that can be experienced when attending a programme in a health setting. Indeed, a participant in the study by Gallagher and colleagues187 appeared to display a degree of distrust towards health professionals and a lack of confidence that they could offer effective solutions for his problems.
It may be that juxtaposing a challenging task such as weight loss with an activity that increases well-being might help to overcome some of the emotional barriers such as anxiety and fear that could impact on enrolment and engagement with interventions delivered in a health setting.
In Chapter 4 we discussed how Brady and colleagues138 deliberately targeted men who had a shared passion for their football club. The trial attracted limited numbers of men from varying socioeconomic backgrounds but all stated that they found the experience highly rewarding.
White and colleagues49 reported on men’s experiences of a weight-loss intervention based in the workplace, which again appeared to act as an attractor for several of the men who took part. A 54-year-old participant stated that being able to attend an intervention during work hours provided the motivation to attempt weight loss, which is something he would not have attempted in the evening. White and colleagues49 stated that the convenience of having the programme in the workplace played a key part in attracting men; however, they also highlighted that male participation in work-based programmes is to some extent dependent on the creation of a positive environment within the organisation to provide the right climate for the initiative to work. In addition, one aspect that links these three separate contexts (football stadium, rugby stadium, work environment) is that they fit well with masculine identities. Hunt and colleagues46 noted that, when a context is congruent with masculine ideals and not challenging, attempts at engaging in weight loss and health improvement activities are more palatable for men.
Influence of partners, family and friends on men’s participation with weight management programmes
The concept of hegemonic masculinity refers to a culturally normative ideal of male behaviour and is used by Mallyon and colleagues188 to refer to the ways that men think about and do manliness, and specifically as a construct that is the opposite of femininity. In the study by Mallyon and colleagues,188 which is linked to a RCT from Australia, men who engaged in hegemonic masculinity received appropriate dieting support from (female) partners in terms of the preparation of food, which helped them stick to a weight management programme, whereas those less engaged in hegemonic masculinity were more likely to take control of their own dieting practices. Mallyon and colleagues188 explain that men who engage in hegemonic masculinity view dieting as a feminine activity that is about looking slim and pretty, which is linked to vanity. Thus, hegemonically masculine men will look for ways to distance themselves from dieting. For example, a participant who was described by the authors as being more engaged in hegemonic masculinity explained his female partner’s role in his dieting. His wife cooked for him and he acknowledged that his wife did it all for him. 188
The role that female partners play in the preparation of food is also evident in other studies. Leishman193 provided an example in which a man undertaking the Camelon programme set a SMART goal (specific, measurable, achievable, realistic and time limited) for his weight loss in which the importance of his partner’s cooking was illustrated. Instead of having biscuits whilst waiting for his partner to cook for him, he removed himself from that temptation by leaving the house to take the dog for a walk.
Contradictory results were found for the role of female partners in men’s weight loss in our quantitative reviews of men and women94,112,120 (see Chapter 3 ). It is not possible to comment on any causal link between quality of female partner support and weight loss for these studies, but differences could highlight the importance of positive and negative influences of significant others on weight-loss efforts.
The study by Mallyon and colleagues,188 which was linked to a RCT of an intervention delivered individually in Australia, also provided an example of a family member having a negative effect on a man’s engagement with a weight management programme. A quotation described how the man’s mother thought that he was becoming gaunt and too skinny and his perception that her solution to everything was to provide excessive amounts of food, which he blamed for his obesity. Some men undertaking weight management programmes reported having difficulties if they needed to reject their mother’s cooking (which might include large portions or high-calorie food) for dietary reasons, fearing that any rejection would be perceived as a form of insult, which would damage the mother–son relationship. 188
Mallyon and colleagues188 found that being less engaged in hegemonic masculinity could leave a man’s adherence to dieting plans more vulnerable to social sabotage, which in turn could act as a deterrent to adherence. In particular, the encouragement of male peers who were not dieting was found to be an important issue in that, if male peers respond in a way that is perceived by the male dieter to be negative, motivation to stick to a programme may diminish. Choosing against the expected social norm is challenging, for example it was suggested that ordering a healthy meal at a restaurant would often be questioned by male friends. 188 Another participant’s motivation to adhere to a diet was tested and reduced when he ate diet food while his friends ate normal food. 188
Mallyon and colleagues188 suggested that in this context the diet functions as a barrier to normal socialising with friends. Similar issues were presented by Kim and colleagues192 (linked to a faith-based group weight management programme delivered within a rural African American community) with a participant explaining that peer pressure to consume unhealthy food affected his ability to adhere to eating healthily, commenting on how strong you have to be to resist peer pressure:
‘Man, you better get up from here – nobody want that food you’re eating. You know, eat something that’s good for you.’ And it may, if you’re not strong enough, you gonna say, ‘Well, hey, maybe I’ll try a piece’
Age 45 years, p. 641192
Unlike the facilitating influence of cohesive group settings such as sports clubs or the workplace on the engagement of men in weight management interventions, the influence of groups such as family and friends can serve as either a facilitator of or a barrier to engagement with weight management programmes and/or the prescribed advice forthcoming from such programmes. One of the studies not linked to a weight management intervention provided evidence to confirm the crucial role of partners in weight-loss attempts, suggesting that sudden jolts relating to opportune moments or particularly symbolic or important situations, as described earlier for health, can be the trigger for behaviour change for men:
I realized for some time that I was overweight, and I wasn’t as fit as I should be, and the jolt was my wife saying she’s not going on holiday sitting on a coach with a fat man on half a seat you know
Age 52 years, p. 799195
On the other hand, it was found that partners of the homosexual men in the study could be very unsupportive of weight-loss attempts, because weight loss made the man more attractive to others. 195
Gough and Flanders197 provided a new theme on the influence of families, partners and friends, demonstrating that the gay ‘bear’ community actively encourages men to reject established advice on appropriate BMI levels in order to remain obese, which is seen as sexually attractive. The bear community acts as a social milieu in which these men are empowered to reject the advice of health professionals and feel, comfortable and desired in their obese state. This acceptance (of their overweight status) was valued by some participants over and above the value they place on their personal physical health. A 39-year-old man weighing 20 stone who developed type 2 diabetes as a consequence of being obese downplayed obesity as being manageable and claimed to be more active, because of stopping smoking, than when he was lighter. He was more comfortable being obese.
The weight management programme
This section presents and discusses themes that influence and shape men’s perspectives and experiences when participating in weight management programmes. The content, format and delivery processes of weight management programmes are considered, as well as how these relate to the individual, biological and social determinants of health and well-being. The following themes are presented: men and diets, alcohol and obesity, men and physical activity and understanding interactions within a weight management programme. It should be noted that a degree of overlap was observed with the themes relating to engagement described in the previous section. The demarcation of themes is presented here as a dynamic rather than categorical process.
Men and diets
As noted earlier, men may be more inclined to avoid what is perceived as the feminised realm of dieting, in which women are often viewed as the experts. 51 In addition, poor-tasting diets that emphasise smaller portions are also hypothesised as a reason why men may distance themselves from dieting. 44 However, little is known about the subjective experiences of dieting men188 or the meanings that men attach to food,51 or indeed their experiences and understanding of food. 44 The approach in the Camelon weight management programme was to de-emphasise the role of dieting and weight loss and emphasise a personalised approach that accounts for individual needs, to make men feel in control of their weight loss. Gray and colleagues142 reported that this personalised approach gained the approval of some participants who had taken part in the Camelon programme.
Evidence from Gallagher and colleagues187 also appears to support the idea that a personalised approach is more appealing to men. Quotes from this study in which the intervention was delivered in a group format without an individualised component show that men can find a one size fits all approach confusing. Calorie counting was seen as needing time, which one 58-year-old man perceived as a barrier. Another found it hard to put all of the components of losing weight together – what to eat, how to exercise – and found that it could be too much, with a recommendation to break down the information, implying a need to simplify the messages and reduce the burden.
The finding that individualised programmes are preferable to men is substantiated from the quantitative evidence as successful programmes generally included some element of individual tailoring, either in the form of individualised dietary allowances or advice and/or personalised feedback or support (see Chapter 3 ). 88,90,96,99,100,106,110,142,154,213
A similar de-emphasis on the role of extreme dieting was employed in the SHED-IT internet-based study,189 which some men compared positively with imposed crash diets elsewhere.
The SHED-IT programme also encouraged participants to enjoy some treats in moderation, such as beer and junk food, which was welcomed by participants. One man (age 35 years) used a variant of the expression ‘have one’s cake and eat it’ when referring to having your beer and losing weight. A 21-year-old highlighted that:
The most enjoyable aspect [of the SHED-IT programme] was the fact that it allows for those days where you know, if you have a **** day at work you can just go and have a few beers afterwards and not feel ****house for it
p. E245, quote as in the original189
A 50-year-old participant quoted by Morgan and colleagues189 stated that an appealing aspect of the SHED-IT programme was that he did not have to make any significant alterations to his diet, whereas a 19-year-old participant in the same study welcomed the fact that SHED-IT gave him choices and thus facilitated personal control.
Therefore, men from three studies appeared to welcome aspects of programmes that placed less emphasis on strict dieting and emphasised personal control over which foods they consumed. This appears to support the notion that men are reluctant to diet and are attracted to engage in and adhere to programmes that appear realistic and can feasibly be assimilated into their lives.
Alcohol and obesity
According to Gray and colleagues142 alcohol may pose a particular problem for men in relation to weight gain and thus alcohol intake should be managed when attempting weight loss. An alcohol awareness component was built into the Camelon programme, with the role of alcohol in weight gain considered and each participant setting SMART goals to decrease alcohol intake. However, there were no participant quotes in the study by Gray and colleagues142 to shed light on any issues that men may have had with alcohol in relation to their weight. One other study191 provided participant data on alcohol and obesity. Here, a causal link between alcohol consumption and increased appetite is proposed. For example, one 34-year-old man suggested that after a few beers he lowered his defences and ate more. He would have a few cans of beer most nights and put his feet up to watch television and he commented that with the drinking his diet also deteriorated.
Several of the non-intervention studies provided evidence to both confirm and (in one case) refute our other findings on diet and alcohol. For example, the study by De Souza and Ciclitira195 confirmed that men disliked the restrictiveness of strict dieting and having their alcohol intake limited, especially in social situations. This was argued by the authors rather than being obviously evident from the data presented from the men themselves. For example, the authors stated that a feature of maleness was avoiding activities that could be construed as feminine, such as having a soft drink or following a diet. 195
However, De Souza and Ciclitira195 also explained that the men in this study may have been atypical as they admitted after several interviews to multiple weight-loss attempts in the past, including crash diets such as the Cambridge diet or SlimFast, and described the difficulties that they had experienced in maintaining weight loss. The authors argued that this could be the primary reason why they finally decided to use a formal weight-loss programme, with the support of their partners, with it being perceived as including a legitimate and sensible diet.
The study by Monaghan199 confirmed that men undertaking weight management programmes prefer a system in which they can still enjoy treats, which meet the programme goals but can undermine a healthy diet. One of the participants described a point-based system based on energy-dense foods used by a commercial slimming club. The man explained how he can still enjoy some chocolate, crisps and a bottle of vodka at the end of the working week, yet stay within his weekly maximum points.
Men and physical activity
The included qualitative studies contained very little on the views of men about physical activity. Most data were provided by just one of the included studies46 linked to the group-based FFIT trial delivered in Scotland at the stadiums of Scottish football clubs.
It is suggested that men may view physical activity in different ways from women, especially with regard to using physical activity to become stronger, fitter and healthier,47 and also in how they use pedometers for self-monitoring. 46 Men have also been posited to be more likely to use physical exercise than dieting to control their weight. 46,142
The use of pedometers in the men-only FFIT programme appeared to act as a key motivator for many interviewees. 46 In particular, the pedometer appeared to be useful in encouraging men to meet prespecified individualised activity targets. One quote suggested that a man was competing with himself, for example to reach his daily target of recommended steps, and that he changed his perception of walking as a mode of travel:
I’m walking places I’d just never have dreamed of walking
p. 6146
In addition, a 72-year-old participant in the study by Gallagher and colleagues187 (linked to a group-based RCT delivered in Australia) also felt that using a pedometer helped to facilitate weight loss by allowing him to keep track and understand how much physical activity he undertook each day.
However, the perception of walking as enjoyable was not universal amongst participants. For example, one man who wore his pedometer all the time still did not find walking as attractive as attending the gym,46 and another participant in the same study had got bored with the lack of variety in walking routes.
That men like engaging in physical activity is supported by our review of the quantitative evidence (see Chapter 4 ). Men were also more likely to be successful in their weight-loss efforts if they included some element of physical activity in their weight-loss programme, but only if this was combined with some form of dietary regime106,109 (see Chapter 3 ). In our review of men-only RCTs ( Chapter 3 ), the trial by Patrick and colleagues90 reported that pedometers were enjoyed by the men for their novelty and assistance with self-monitoring of their behaviour. The men in this trial were randomised to receive a low-fat diet with behavioural therapy and exercise advice or general health advice only. It should be noted that, although the intervention group initially lost more weight, by 12 months the differences were not statistically significant, indicating that, although men may enjoy using pedometers, their usefulness in losing weight may vary depending on the overall weight-loss programme. Alternatively, it may be the case that the fun aspect of using pedometers declines with repeated use, which may negatively impact on walking behaviour.
With the above in mind, the studies not linked to weight management interventions also displayed limited data on men’s physical activity preferences. The study by Weaver and colleagues202 provided data that confirmed men’s enjoyment of exercise; their participants spoke of experiencing various immediate benefits, such as being more alert or being less stiff.
Nevertheless, pain as a limiting factor for exercise was raised as a new theme by Weaver and colleagues. 202 Many men particularly described having knee pain, which had a debilitating effect on their ability to exercise. Interestingly, different causal relationships were proposed between knee pain, exercise and obesity. Some men thought that their exercise regime had caused the knee pain, which in turn resulted in weight gain. Weaver and colleagues202 weighed up the available evidence and suggested that taking exercise is unlikely to cause osteoarthritis (unless perhaps in top footballers) and that obesity is clearly linked with long-term knee problems. They concluded that men need to be educated about the impact of weight on their knees and about weight loss as a means to prevent knee problems.
Understanding interactions within a weight management programme
The data pertaining to interactions within the weight management programme were diverse. Within this theme we provide five further subheadings: group-based programmes and social support, promoting engagement and the use of humour, scientific appeal, accountability and adherence, and goal setting.
Group-based programmes and social support
Several studies highlighted the importance of group-based weight management programmes. Proponents of this approach argued that it facilitated peer or social support amongst people with similar health problems. This was observed in the study by Leishman,193 which was linked to the group-based Camelon programme, delivered in men’s health clinics in Scotland. Here, men praised the support that they got from each other and valued the ability to talk to other men on a similar programme.
In addition, a 34-year-old participant in the study by Harrison191 was surprised at how supportive his work colleagues were of him taking part in the Health of Men work-based programme, something he had not expected. Nevertheless, the study by Morgan and colleagues89 found that men undertaking an individually based weight management programme that was internet based would have preferred more contact with the instructor as opposed to peer support, but no participant quotes were provided to support this assertion. However, it is difficult to know how generalisable this finding is to interventions not delivered over the internet.
Indeed, the study by Leishman193 emphasised the importance of support offered by health professionals delivering weight management interventions to help men to stay confident about their ability to lose weight and to stay motivated to do so. Leishman193 argued that men should be encouraged to focus less on ideal body weight, which if using the BMI classification would involve losing large (and unrealistic) amounts of weight. Leishman193 argued that this would be unachievable for many men and instead recommended that men should be directed towards smaller, more realistic weight-loss goals of 5–10%, which still have many direct health benefits and seem more achievable.
Other authors suggested that group-based programmes can be logistically difficult with regard to scheduling meetings and are therefore impractical for time-poor men who already consider time a barrier to engagement with physical activity. 190
Although our quantitative data85,86,90,95,96 supported the assertion that group-based programmes produce beneficial results (see Chapter 3 ), men may be less inclined to join these types of programmes depending on their perception of the weight-loss provider, setting or gender mix. 100 Group-based weight management programmes have been stated to produce more weight loss than individual programmes, even for those who expressed a preference for individual treatment. 121,214 Leishman’s study193 linked to the group-based Camelon programme delivered in a Scottish health-care setting147 noted that factors such as group competitiveness and team spirit can motivate men to meet their goals, whereas the potential for group camaraderie to facilitate the sharing of information, tips and humorous banter can help men to meet weight-loss targets and reduce attrition. 215
Promoting engagement and the use of humour
In Engagement with weight management programmes, describing the importance of location and setting as a ‘hook’ to engage men, the role of humour in attracting men to programmes was mentioned. The included studies also found that humour and banter had a valuable function in building positive relationships between group members and promoting adherence to a programme once engaged. For example, a participant quoted in the study by Gray and colleagues142 (linked to the group-based Camelon programme delivered to men in health clinics in Scotland) explained how the camaraderie and enjoyable conversation in the group made men want to come back the following week. Similarly, a participant in the study by Leishman193 enjoyed and was helped by the atmosphere, laughter and support from men who were all there for the same reason.
Of the non-intervention studies, Gillon196 confirmed that humour was used by men when discussing issues related to weight and was often useful. One participant’s quote discussed how being a rugby player in his youth and being very active had helped him maintain a steady body weight, despite the fact that he tended to eat lots.
The experience of a team atmosphere, with men in similar situations attempting to achieve similar goals, was also found to play a part in the perception that group-based programmes were preferred. This connects with the earlier discussion in which we identified the value of employing cohesive, task-oriented groups for men engaging in a process of weight management. For example, a participant in a study that was linked to the Camelon programme delivered to men in groups in a health clinic setting perceived that being with men who were in the same situation as himself made him realise that he was not alone. 142
In addition, the importance of sharing commonalities (i.e. being a rugby fan and being overweight) with fellow participants was also valued by a participant in the group-based programme at a rugby club described in the study by Witty and White. 149
However, in our quantitative review of UK studies, half of the men (10/20, 50%) attending the Bloke’s Weigh programme147 stated that they would have attended a mixed-gender programme and in the Leeds Rhinos study139 the male-only environment was the least important reason for joining the programme (see Chapter 4 ).
Nevertheless, a participant in the study by Morgan and colleagues189 explained that the male-only feature of the SHED-IT programme was important in that it helped to facilitate engagement in male-oriented banter that for him would have been curtailed in a mixed-sex environment.
Several authors offered views on why they believed that interventions for men should be different from those for women; however, these assertions were unsupported by participant quotes. For example, Gray and colleagues142 argue in their study linked to the Camelon programme that the association between femininity and dieting may act as a major barrier for men:
However, the current evaluation suggests that the perceived focus on the ‘feminine domain’ of dieting within the commercial sector may also act as a significant barrier to men
p. 78
Mallyon and colleagues188 also contended in their study linked to an individually delivered intervention in Australia that male weight management programmes should be framed in such a way that they do not threaten masculine identities, but also conceded that more research in this area was required.
Scientific appeal
Mallyon and colleagues188 suggested that it might be possible to enhance the attractiveness of weight management programmes for men by emphasising their scientific appeal. This, they contended, can help to draw attention away from associations of dieting with feminine weakness. Furthermore, Mallyon and colleagues188 stated that emphasis on the scientific nature of a weight management programme can empower dieting men to resist social sabotage by (often well-meaning) friends and family if they try and entice them to eat like a real man.
Accountability and adherence
Adherence is a decisive factor in predicting success for participants undertaking weight management programmes. 216 With this in mind, the included studies detailed various methods to help participants stick to the programmes in terms of being accountable to oneself and having to account for food choices to others within the programmes. One such method involved creating ways to promote self-monitoring and accountability for participants. For example, the study by Abildso and colleagues,190 which was linked to a group-based insurance-sponsored weight management scheme delivered in the USA, encouraged participants to use a daily food log that was checked by programme staff. The accountability that came with staff reviewing the food logs was stated to be central to successful participant weight loss, and statistical analyses revealed that these food logs were more frequently completed by those who lost a large amount of weight than by those who lost a moderate amount of weight. The following participant extract supports this:
What really helped me was having somebody go over the food log every day. That was the big thing; just having staff talk about things I was eating, choices I was making, maybe making a few little suggestions – that was really very helpful.
No individual characteristics provided, p. 286190
Accountability for one’s own actions whilst undertaking a weight-loss programme was also a factor that promoted adherence in the study linked to the SHED-IT website-based programme delivered in Australia. 189 Men felt accountable by keeping track through the weekly weigh-ins.
Morgan and colleagues189 also reported a similar approach to awareness and self-monitoring of food intake to promote adherence. As outlined in Chapter 3 , participants were randomised to either a weight-loss information-only group or to a group who received weight-loss information, use of a weight-loss website and individualised support from programme staff. Both groups received a weight-loss handbook that detailed a simple energy in/energy out equation, allowing participants to keep a record of their energy intake balance. Morgan and colleagues189 stated that this was mentioned as a source of satisfaction and acted as a mechanism that participants used to control their weight. With this in mind, sticking to the mathematical equation was found to aid initial weight loss, which facilitated further adherence. For example, a 43-year-old man was quoted as noticing that his energy count was directly related to weight, with this knowledge acting as a motivating factor. Similarly, two younger participants preferred the regular counting routines in contrast to less concrete aspects of support. The 21-year old had used some support early on to get going and the 19-year-old seemed to prefer the calorie counting and spoke quite derogatively about the ineffective support.
That men welcome simple, fact-based instruction as part of their weight-loss programme is supported by the reported preferences of men who participated in focus groups during the intervention development stage of the trial conducted by Patrick and colleagues90 (see Chapter 3 ).
The internet users in the programme reported by Morgan and colleagues89 were also asked to submit food (and exercise) logs to receive feedback from programme staff, in a similar approach to that successfully employed in the study by Abildso and colleagues. 190 One man was quoted as saying that the website format alone was sufficient to maintain adherence and self-discipline. He described how he did not need or want someone telling him what to do; he had the knowledge and the website provided him with accountability. 89 This acknowledges the intrinsic motivation that is required to engage in such programmes.
In the Nutracheck study154 (see Chapter 4 ) men made more frequent diary entries than women, suggesting that they prefer this type of self-monitoring activity. However, in the study by Morgan and colleagues,89 non-compliers found that keeping a food diary was overly time-consuming and thus operated as a barrier to successful adherence:
It was just constantly having to get to a computer everyday and spend 10/20 min on there trying to plug in everything I ate was a bit tough
No individual characteristics provided89
A further complaint of non-adherers to this internet programme pertained to a lack of face-to-face contact with programme staff, which was stated to act as a barrier to adherence. Certain participants felt that small group meetings or lectures would have given a more human touch to the project, facilitated peer support and helped maintain focus and motivation.
Goal setting
A further way in which participants were encouraged to stick to programmes was by setting weight-loss goals. For example, a participant in the study by White and colleagues49 linked to the Health of Men group-based programme delivered in the workplace described how setting easy weight-loss goals and anticipating the satisfaction of achieving the 1- or 2-stone weight loss 1 year later helped his adherence and motivation. However, this quote also highlights that there may be a mismatch between participants’ expectations about goals and the targets that are found in the weight-loss literature. The published literature suggests goal-setting targets that are much lower than the targets stated by individuals wishing to lose weight. For example, the quantitative systematic reviews in this report have demonstrated that weight loss of 2 stone (13 kg) after 1 year was rarely achieved (see Chapters 3 and 4 ).
Other studies described the use of innovative methods to demonstrate achieved weight loss to men and to help men stick to a weight management programme. The study by Gray and colleagues142 linked to the group-based Camelon programme delivered in Scotland explained how sandbags were used to give men tangible physical evidence of their weight loss at the midway point of the programme. A participant in the study by Leishman193 (which also investigated men’s experiences of the Camelon programme) explained how proud he was when he held the bag of sand and its weight and the motivational effect that this had on him at the half-way stage of the programme.
Mallyon and colleagues188 examined before and after body scans of men who participated in their trial. The ability of men to compare scan images showing differences in body shape/weight was stated to help them stick to the diet and to be a motivational factor for losing weight.
The impact and consequences of weight-loss programmes
Several studies included data from participants reflecting retrospectively on their participation in weight management programmes and the consequences of participating. These reflections centred on the following issues: how programmes impact on partners and family members, the downside for men of losing weight, improvements in health and fears of relapse when programmes end.
How programmes impact on partners and family members
Within the included studies there were many reported reflections on how aspects of programme activities indirectly impacted on the family members of participants. For example, the Camelon group-based programme delivered in men’s health clinics in Scotland142,193 appeared to have a positive impact on the partners/family of participants. To investigate indirect effects of the programme on female partners, Gray and colleagues142 conducted a focus group with female partners of men taking part in the programme. This focus group research indicated that many of the female partners had been influenced by the men’s engagement with the programme. Indeed, some women had followed the programme alongside their male partner, whereas others stated that their family (including children) were snacking less, taking more exercise and eating more fruit and vegetables. Some women observed that their male partner’s involvement in the Camelon programme had made them think more about what they were eating. 142
In addition, a participant in the study by Leishman193 suggested that the Camelon programme had a positive influence on both his own and his female partner’s eating habits, for example they ate smaller portions and fewer unhealthy snacks. Similar indirect effects on close family members of the SHED-IT website programme were found in the study by Morgan and colleagues,189 who described a 36-year-old speaking of his whole family now eating healthier home-cooked food and fewer takeaway meals. A 40-year-old man referred to his wife walking every morning, something she did not do before he got involved in the SHED-IT programme.
A further effect of weight management programmes on partners and family members was observed in the study by Gallagher and colleagues187 that was linked to a group-based intervention in Australia. It was inferred that nutritional knowledge gained by men through undertaking a weight management programme can be passed on to their children. This was supported by a quote from a man who stated that he was actively teaching his 13-year-old son the right things to eat so that he could make informed choices regarding his weight.
The downside for men of losing weight
Gray and colleagues142 found that men attending the Camelon group-based programme felt dissatisfied with their weight only when they were labelled as obese, for example one 15-stone man was quoted as saying that if he lost too much weight he would probably start looking ill. This sentiment regarding weight loss was found to be universal amongst the men interviewed by Gray and colleagues,142 meaning that, for these men, being in the overweight range represented an ideal weight and they did not want to become too thin.
In a second-order construct, Gray and colleagues142 suggested that this phenomenon arises from social norms with regard to the construction of the ideal male. This dictates that men should be bigger and stronger than women, referring to the ideal male bodybuilder’s body. They concluded that such ideal overweight body images may render health promotion interventions to lose weight ineffective.
With regard to the non-intervention studies the study by Gough and Flanders,197 which conducted research with men who were active members of the gay ‘bear’ community, confirmed the view that losing too much weight can result in a perceived unhealthy appearance. A 37-year-old man explained that losing weight as a gay man could be associated with being human immunodeficiency virus positive. When he lost weight he felt uncomfortable, lost confidence and stopped going out to meet people and he recalled how losing weight made him feel unhappy and lonely. Furthermore, Gough and Flanders197 quoted a 44-year-old man who did not want to adhere to a perfect BMI because:
I want to be around 16 stone, my GP wants me to be 12.5 stone, if I was 12.5 stone, I’d look like I’d been incinerated, something out of Jason and the Argonauts, like the skeletons walking around
p. 248197
These statements suggest that men from a range of backgrounds are keen to avoid looking too thin. The study by De Souza and Ciclitira195 also found that gay men may be put off losing too much weight in case it threatens their relationship with their partner. In addition, Monaghan199 noted in his field diary that some men expressed anxieties about the potential adverse health impacts of rapid weight loss and crash diets, with some agreeing that men need to lose weight gradually to give their bodies a chance to adapt and to stabilise the effects on the heart, kidneys and muscles.
Improvements in health
The studies by Abildso and colleagues190 (linked to a group-based insurance-sponsored weight management scheme delivered in the USA) and Hunt and colleagues46 (linked to the group-based FFIT trial delivered at the stadiums of Scottish football clubs) reported that many participants experienced benefits to health beyond weight loss after participating in the programmes. Examples of such health benefits included improved sleep, decreased pain, improved blood pressure, improved cholesterol levels, loss of leg neuralgia and a decrease in headaches associated with coming off some of the medications taken for conditions related to obesity. 46,190
In addition, there were accounts of how physical fitness had improved as a consequence of a weight-loss programme, with positive consequences for health, with some being quoted as having a more positive mindset, feeling younger or being able to walk up stairs again. 46 Feelings of subjective well-being induced by greater fitness levels were also found in the study by Harrison,191 who quoted men who were proud of their physical achievements in the gym.
These accounts of improved perceptions of health and well-being are positively reinforcing for both adhering to the programme and sustaining behaviour change after the programme has ended. They illustrate the sense of achievement and motivation resulting from success.
Fears of relapse when programmes end
The study by White and colleagues,49 which was linked to a workplace-based weight management programme delivered in England in a group format, described how concern was expressed that the group might gradually stop meeting after the programme ended, which would perhaps impact on adherence to the messages of the programme after it ended. In particular, a participant expressed doubts about group members continuing to walk without the group support, in the context of busy working lives. Three- to 6-monthly group reunions were seen as a good idea to review progress. Popping into a group to be weighed or have blood pressure checked was perceived as more likely. 49
This notion that some men would like to continue meeting up with their group after the programme had finished is mentioned further in the study by White and colleagues. 49 A Health of Men support worker expressed surprise and noted that men had initiated a poster on a board to document participants’ weights, with continued regular weighing perceived as important, particularly if the men did not have scales at home. Sustained access to equipment and a dietitian were also considered important; however, concern was expressed about the lack of capacity to sustain ongoing weekly meetings.
The need for more sustained interventions was therefore evident, but this received little attention in the studies that we identified.
Conclusions
The key findings from this chapter are detailed in the following sections.
Social, cultural and environmental influences on obesity in men
-
Men made very little reference to wider social determinants of their behaviour and instead focused on their motivation and own agency to overcome (or attempt to overcome) those secular macro-level changes that have encouraged the overconsumption of energy-dense foods and constrained or reduced opportunities to be physically active.
-
The role that the environment plays in influencing behaviours helped some researchers decide where to locate the delivery of weight management programmes. For example, the programme described by Harrison191 and White and colleagues49 was located in the workplace, with the rationale that it was more convenient to access than a programme in a non-workplace setting.
-
The importance of the theme of sociostructural determinants of obesity in contrast to the obesity as individual responsibility standpoint is supported in four of the studies not linked to weight management interventions.
Engagement with weight management programmes
-
The main reasons that men gave as their primary motivation for losing weight were a diagnosis of obesity, health scares, particularly if hospitalisation was involved, and a desire to improve personal appearance.
-
From the participant data available we did not establish whether or not male-only weight-loss environments were more attractive to men. Data were presented that did indicate that men felt more comfortable in these settings; however, we also observed that a male-only setting was not consistently considered to be an issue that would attract men to participate.
-
The use of humour in promotional materials for weight management programmes (e.g. comical language and imagery, such as a picture of a beer glass) attracted men. In addition, the knowledge that programmes had been successful for other men increased the likelihood that men would participate.
-
Men were reluctant to undertake any forms of strict dieting as part of a weight management programme.
-
Men appeared to prefer community or workplace settings for interventions over hospital or otherhealth-care settings.
-
The pivotal role of female partners emerged as an important aspect of successful weight-loss attempts.
-
Motivation to stick to a programme may diminish if family members and male peers who are not dieting respond in a negative way to men who diet.
-
The included qualitative studies contained very little information on the views of men on physical activity. However, the data indicated that the use of pedometers appeared to act as a key motivator for men. In particular, pedometers appeared to be useful in encouraging men to meet prespecified individualised activity targets.
The weight management programme
-
Group-based weight management programmes were found to facilitate peer or social support amongst people with similar health problems, despite the fact that some men were initially reluctant to take part in a group programme.
-
However, group-based programmes can be logistically difficult with regard to scheduling meetings and therefore impractical for time-poor men who already consider time a barrier to engagement with physical activity. 190
-
The included studies found that humour, banter and camaraderie had a valuable function in building positive relationships between group members and promoting adherence to programmes once engaged.
-
Men found that being accountable to themselves and having to account for food choices to others within the programme facilitated adherence.
-
A further way in which participants were encouraged to stick to programmes was by setting weight-loss goals.
The impact and consequences of weight-loss programmes
-
The weight management activities of men were found to have had an indirectly positive impact on family members in terms of improved nutrition and increased physical activity levels.
-
Men from a range of backgrounds were keen to avoid looking too thin.
-
Men experienced benefits to health beyond weight loss after participating in the programmes.
-
In the case of group-based programmes, concern was expressed that groups may gradually stop meeting after the programmes ended and this might impact negatively on adherence to the messages of the programmes. The need for more sustained interventions was evident.
What is missing from the qualitative data?
-
Experiences and perspectives of men from black or ethnic minority backgrounds, low-income or unemployed men and rural and/or remote-dwelling men.
-
Although we have a little insight into gay men’s perspectives and experiences of weight-loss attempts from the non-intervention studies, these insights are missing from the intervention literature.
-
Qualitative studies that target mixed samples of men and women need to make gender evident in the reporting of data to provide a clearer gender picture in relation to the results.
Quality assessment of qualitative studies linked to interventions
Our quality assessment covered the following five items: aims and methods, sample details, reflexivity, ethics and general criteria.
Aims and methods
Seven studies45,87,141,148,186,188,189 stated an explicit aim of the research with one stating a research objective. 49 Another stated both an explicit aim and an objective,194 whereas four studies188,191 – 193 did not state any explicit aim (or objective). In addition, just two studies188,189 stated explicit research questions in the text and only three188,192,194 were clear about the theoretical perspective underpinning the research. In terms of describing the theoretical perspective underpinning the intervention that the qualitative study was linked to, three studies46,89,192 provided this information. All studies provided an account of the qualitative methods that were used to gather data and all but two studies191,193 provided in-depth accounts of the chosen procedure for data analysis.
Sample details
Only two studies149,193 failed to provide a clear statement pertaining to sample size. In terms of sample characteristics, just three188,189,191 of the male-only studies provided this information. Of the mixed-sample studies, only that by Ogden and Sidhu194 provided sample characteristics clearly by gender, with those by Abildso and colleagues,190 Gallagher and colleagues187 and Kim and colleagues192 providing sample characteristics but not by gender. In addition, nine46,89,142,187 – 190,192,194 of the studies provided a clear description of the sample selection process. Seven46,89,142,187,189,190,192 of the 13 studies provided clear details of sample inclusion and exclusion criteria.
Reflexivity
Reflexivity is a credibility-adding concept that is central to robust qualitative research. It refers to the qualitative researcher’s engagement with self-critique and self-appraisal throughout the research and explains how his or her own experience has or has not influenced the stages of the research process (Koch and Harrington217 cited in Dowling218). We were therefore interested in investigating whether or not any of the studies linked to interventions made reference to this concept. We found that, despite most of the included studies detailing the ways in which the work was limited, only the study by Morgan and colleagues189 considered the role that the researchers may have played in influencing the findings. For example, they drew attention to the fact that, as the researchers and research staff conducted the interviews; this may have led to some socially desirable responses from respondents.
Ethics
We further assessed whether or not the studies linked to interventions provided any details regarding ethical issues such as obtaining approval for the study from an institutional ethics board. Only three191,193,194 of the included studies did not refer to any ethical issues in the text.
General criteria
The final section of the quality assessment investigated more general aspects of quality in the studies linked to interventions. First, we asked whether or not the findings were adequately supported by the data. We found that six studies49,89,188,189,192,194 stated second-order findings that were unsupported by participant data in the study text. However, it should be noted that this issue may occur because of stringent publication word limits. We further investigated whether or not there was the potential for charisma effects in the research regarding the influence of the study principal investigator or a member of the research team. We suggest that there may have been the potential for such a phenomenon to occur in the studies reporting qualitative data from the SHED-IT internet-based trial from Australia. How the intervention is delivered and the qualitative data are collected may influence how feedback on the programme was reported by certain participants, in that favourable responses may have been given. For example, in the study by Morgan and colleagues89 the authors state that the men would have preferred more contact with the instructor rather than peer support (p. 147). However, it is unclear whether or not the principal investigator is the actor referred to by the term ‘instructor’. This would seem to infer that the instructor has a positive motivational influence on the men, which will most probably affect the ability of the programme to be effectively replicated elsewhere if the same charismatic influence is absent.
Finally, we addressed any other issues relating to the quality of the studies that were not covered by the previous items of the quality appraisal tool. In total, five studies89,187,189,190,192 had issues that we considered were important when interpreting the data. For example, the studies by Abildso and colleagues190 and Gallagher and colleagues187 did not address the possibility that men and women may require different things from a weight-loss programme to successfully lose weight. In addition, the faith-based programme detailed by Kim and colleagues192 lasted for just 8 weeks, which may have been too short a time. Furthermore, the studies by Morgan and colleagues89,189 linked to the internet-based SHED-IT trial are limited in that only the most successful participants in terms of losing weight were interviewed. Thus, the data presented suffer from bias as the less successful participants and those who may not have actually liked the programme were not interviewed.
Chapter 7 Discussion
In this chapter we very briefly summarise our findings and current policies and guidelines relating to men’s health and obesity before discussing the findings from all of the reviews in detail, following our evidence synthesis logic model (see Chapter 6 ). Our findings should be interpreted with the knowledge that the evidence base, particularly in the UK setting, is currently limited in the quality and number of studies and mainly reflects white, middle-class, middle-aged men. Few UK studies had long-term data available. Our results may not necessarily be applicable to all men. Some of our findings may also be applicable to women, but our reviews were not designed to answer questions on weight-loss programmes for women. We had difficulties retrieving studies and it is possible that the studies that we found had more promising results than those we were not able to access.
Summary of findings and policy implications
There were some consistent findings across the systematic reviews that may help with the formation of policies to increase men’s uptake of and continuation with weight-loss programmes. Health issues were important intrinsic (because of increased levels of concern) and extrinsic (prompting advice from others) motivators to engage in programmes and change behaviour. Although health service staff can help motivate change, setting programmes in the health service may be far less attractive than using settings, such as a football stadium, that provide long-term social support and an ambience (e.g. humour and banter) that appeals to men.
Physical activity has more appeal for men than women as a means of weight control (although physical activity on its own is unlikely to result in much weight loss). Dieting, particularly strict dieting, is seen as a feminine activity. Thus, although reducing diets are needed for greater weight loss, strict diets seem unpopular and terms such as ‘healthier eating’ (which allows for treats such as alcohol) and ‘portion control’ seem to be more appealing to men.
Men may have particular difficulties perceiving that they or others are overweight or obese, because of the desirability of muscularity and the masculinity of a large body size. Obesity can be conceptualised as a predominantly female issue around image, a viewpoint perhaps reinforced by the greater attendance of women at weight-loss programmes.
A consistent finding was the lack of consultation with men when developing or evaluating interventions, with very little qualitative research, which is surprising as men are under-represented in almost all weight-loss interventions. NICE3 and SIGN52 also have not provided specific guidance for men for the prevention and treatment of obesity. However, our data show that, once committed, men are less likely to withdraw from programmes and might experience relatively more weight loss than women. Therefore, a focus only on lack of engagement with programmes can underestimate the benefits of existing programmes.
The need for men’s health strategies in member states has been highlighted by a European Commission report,219 which called for policy, research and practice to be developed specifically for men, whose health may be even more disadvantaged by deprivation. The Republic of Ireland has a national men’s health policy, which has increased the focus on men’s health and community settings targeting disadvantaged men, but the countries of the UK do not. 220 In the UK the Equality Act 2010221 (applicable to England, Scotland and Wales) has improved men’s health. 222 The introduction of Gender Equality Duty in 2007 placed a legal obligation on the NHS and public bodies to take account of the specific needs of men and women in these countries. 222
The Men’s Health Forum in England and Wales has been given ‘strategic partner’ status by the Department of Health. 222 The Men’s Health Forum in Scotland also works closely with the Scottish government. For example, in 2005 the Scottish government provided funding to support community-based partnerships in developing Well Men’s Services, funding 16 pilots across seven health boards in Scotland, targeting disadvantaged areas. 211 Northern Ireland bases its equality obligations upon Section 75 of the 1998 Northern Ireland Act. 223
The Men’s Health Forum in England and Wales convened a conference about men and weight issues in 2005 with 23 health and social policy researchers. The outcomes of this conference were subsequently published in a book entitled Hazardous Waist: Tackling Male Weight Problems. 54 The need to focus on male-sensitive approaches to weight-loss issues was apparent then and was encompassed by the publication of The HGV Man Manual,224 designed in the format of the Haynes car maintenance manuals. However, in the UK, initiatives specifically to help obese men, particularly from disadvantaged groups, lose weight, both within the NHS and outside the NHS, are still relatively few in number, with few evaluations available. The NHS Health Trainer Service aims to promote behaviour change in socially disadvantaged people in England and Wales. The most recent study225 showed that only 21% of clients were men, although 69% were from the two most deprived quintiles.
The findings from our systematic reviews were limited by the relatively low numbers of included studies. This was especially the case with regard to data from the UK. It is also important to consider that men are not a homogeneous group. We had even fewer data or no data at all on men from deprived, unemployed, ethnic minority, younger age, disabled, gay, bisexual, transgender, rural and other minority groups.
Discussion of the results from the systematic reviews
How are men motivated to lose weight and to participate in weight management programmes?
We found that men were significantly less likely to drop out of weight-loss programmes than women, and there was some evidence to suggest that they might lose relatively more weight than women. Morishko and colleagues30 undertook a systematic review of predictors of dropout in weight-loss interventions and reported narrative findings that sex was not associated with attrition. However, their review did not focus on RCTs only and there was no statistical synthesis of the results. Our results suggest that men may be harder to engage than women, but then show great commitment, emphasising the need to improve engagement without diminishing commitment.
The evidence from the qualitative studies suggests that middle-aged men were attracted to lose weight once they perceived that they had a problem with their health (particularly a health scare or hospitalisation), and were diagnosed as obese by a health professional or labelled with the term ‘obese’, and/or their weight was shown to them on a chart by a health professional. 44,187,189,193 – 195 Feeling unhealthy was also a motivator. 49 These findings could reflect the predominantly white, middle-class and middle-aged men recruited, who were conscious of their mortality, or suffering from some form of chronic condition that they believed, or had been told, might be ameliorated by weight loss. GP referral to a commercial organisation increased the proportion of men taking part compared with self-referral, again suggesting that for some men concerns about their health and health professionals’ advice acted as motivators to engage in a weight-loss programme. However, qualitative research in the UK reports that GPs think that obesity management is not within their professional domain, even if patients would like them to take responsibility for their weight problems. 226 Contacts with primary care can provide ‘teachable moments’,227 which are opportunities to motivate people to change unhealthy behaviours and opportunities for referral or signposting to available services. ‘Teachable moments’ might be of particular benefit to men from disadvantaged backgrounds, providing that they are in contact with health services already. Offers of health screening, such as checking cholesterol or blood pressure, including checks provided outside health service premises, may also be a way of engaging with obese men. We also found that ‘jolts’ from a partner195 and word of mouth recommendations193 could also help with motivation to lose weight and engage with services.
Our systematic reviews of trials showed that weight-loss programmes could help with comorbidities in obese men. Programmes with low-fat reducing diets and/or physical activity, with or without behaviour change training, improved erectile dysfunction in men with and without type 2 diabetes85,110,122 and prevented diabetes,104 although in type 2 diabetes successful weight loss might increase the risk of osteoporosis. 124 These health benefits could also help motivate men, for example the potential benefit on erectile dysfunction is probably not well known to men. Qualitative research also showed benefits for men, including taking fewer medications, decreased morbidity, increased mobility, such as the ability to tie shoelaces,190 increased physical fitness46,191 and improvement in outcomes not traditionally associated with obesity, such as a reduction in headaches.
The desire to improve personal appearance was an important motivator to lose weight in men too, such as the recognition by one man that he was starting to look like his obese father. 189 However, male social norms and expectations about body size mean that men, in contrast to women, may express a desire to gain weight rather than lose it, as a means of living up to bodily ideals emphasising strength and size. 228 The term ‘bigorexia’ has been used to describe large muscular men who do not wish to look small. 195 It was clear from our review that men from a range of backgrounds were keen to avoid looking too thin. 142 The importance of appearance as a motivator was also evidenced from the very small gay sample. We also found evidence that the so-called gay ‘bear’ subculture celebrated and encouraged men to take on a large body size. 197
We found that interventions in community (particularly associated with Premier League football or rugby clubs) or workplace settings were acceptable and attractive to male supporters and preferable to interventions in hospital or health-care settings. The sense of well-being and pleasure associated with attending a football or rugby game contrasts with the anxiety and fear149 that can be experienced when attending a programme in a health-care setting. 187 Interventions situated in sporting contexts46,138,149 may provide men with a strong sense of affiliation and belonging, bolstering male identities and masculine social capital. Our interpretation is that associating long-standing loyalty, commitment and pleasure attained from collectively supporting a football team (still predominantly a male activity) while challenging men’s lifestyles to encourage behaviour change, such as losing weight, could hypothetically increase the likelihood of ‘contagious motivation’ amongst fans. The club setting for the programme, with the kudos of club privileges such as access to team coaches, changing facilities and club tops, seems to reinforce a connection between being a club supporter and losing weight, and appears to fit the theory of associative coherence. 229 The motivation for being a long-standing team supporter could either consciously or subconsciously become associated with the motivation to lose weight with fellow team supporters. The National Institute for Health Research-funded FFIT trial is evaluating the long-term weight outcomes in a weight-loss programme delivered through SPL football clubs. 230
However, the reach of programmes delivered through sporting venues is not clear, both among the club supporters and among obese men generally. Sporting venues may not be the most promising point of contact for the majority of obese men. Workplaces offer another opportunity to engage men49 and have the potential to influence productivity and absenteeism. 231 Clubs in workplaces and for supporters will not reach those who are unemployed or unable to afford the cost of attending sporting events. Venues that are associated with male identities outside the NHS need to be selected to deliver programmes that could reach and engage these disadvantaged groups, such as barbers, pubs and road service stations. 232,233
Careful use of humour in promotional materials in weight management programmes, such as comical language and imagery (e.g. a picture of a beer glass189), attracted men. However, these should be piloted first as humour has the potential to backfire if issues are seen to be trivialised (Paula Carroll, Men’s Health Forum Ireland, 4 December 2012, personal communication).
What makes weight-loss programmes more effective for men?
Reducing diets and alcohol
There was clear evidence from the randomised trials that reducing diets, particularly low-fat reducing diets, were effective for men and were the most important component of any weight-loss intervention. We were unable to establish that the nutrient (such as providing more protein) or calorie content (provided that there is a prescribed calorie deficit) of the reducing diets influenced the amount of weight lost long term by men. 83,87,91,107,113,140,146 Men did not want to undergo strict or extreme diets142,189,193,195,199 and it may be that using ‘healthier eating’ terms can be used to promote reducing diets to men. 193 The ability to have some alcohol and food treats was also valued. 89,199 However, intermittent periods of very low-calorie dieting, as required, may be better than regular periods of such dieting. 103
Men reported valuing the scientific appeal of the energy intake and calorie expenditure equation and liked having the ability to monitor their calorie intake. 188,189
Physical activity interventions
Men do well if physical activity is part of a weight-loss programme and they may be more likely to respond to such a programme than women. 93,106,109 However, weight loss was better with a reducing diet than with physical activity alone, and better still if both were provided,91,93,109 although one small trial did not find that a physical activity programme and reducing diet were better than the diet alone. 92 We found very little qualitative data from men themselves on physical activity interventions.
The success of the interventions in football clubs and rugby clubs, with their focus on physical activity, reinforces the importance of this aspect for men’s weight-loss programmes. However, pain and comorbidities may be limitations. 202 Men reported that they liked using pedometers. 46,90,187 NICE234 presently recommends that pedometers are used only as part of a package that includes monitoring, feedback and support to set realistic goals (whereby the number of steps taken is gradually increased). Walking as a means to exercise was not universally liked and some men preferred the gym. 46 A preference for the more technological aspects of diets and physical activity, both in the content of the interventions and in the monitoring processes, was evident.
Helping to change behaviour
There were few RCTs that specifically examined whether or which behaviour change strategies were effective for men; those that did demonstrated improved long-term weight loss and maintenance. 88,101 Descriptions of the interventions were often limited with regard to the techniques used. NICE235 recommends that interventions at the individual level contain easy steps that can be taken over time such as learning coping strategies, goal setting and sharing these goals, and planning for social situations that might undermine changes. More recently, Greaves and colleagues236 undertook a systematic review of reviews of intervention components that increased effectiveness for changing diet and levels of physical activity. They found that engaging social support, increased contact frequency and using a cluster of self-regulatory behaviour change techniques (e.g. goal setting, prompting self-monitoring, providing feedback, review of goals) increased effectiveness.
With regard to self-monitoring and feedback, the accountability that came with staff reviewing food logs was stated to be central to successful participant weight loss,190 and statistical analyses revealed that these food logs were more frequently completed by those who lost a large amount of weight than by those who lost a moderate amount of weight. Morgan and colleagues189 found that participants liked the accountability of weigh-ins. Computer-linked monitoring was reported to help but also to be time-consuming, with a lack of personal contact. 189 Men may prefer less monitoring than women. 96 Telephone and mail support could be useful. 99
Goal setting and more sustained interventions were also identified as important for men. 49 Interventions delivered in UK health services were often of short, fixed duration, perhaps reflecting the constrained spending on health care.
Social support within the programme
Group and individual support
In a systematic review of male inclusion in RCTs, Pagoto and colleagues29 found a trend towards lower representation of men in group (24%) than in individual (29%) or mail/e-mail/internet programmes (34%). The male/female mix of the groups was not specified. We also found that men were considerably under-represented in commercial weight-loss groups when both men and women were eligible to participate.
We found that men do well in groups of men but that individual tailoring of fact-based, simple-to-understand advice or counselling was wanted too. 49,85,90,95,96,142,187 Jeffery and colleagues86 also found that group monetary contracts were more effective for weight loss than individual monetary contracts for men.
Some men clearly wished to attend a men-only group142,189 whereas for others this was not considered important. 189,195 It could be deduced that being able to identify with the other individuals attending the programme, rather than just men only, is the issue (although these are more likely to be other men). 191,193 Group-based weight management programmes were found to facilitate peer or social support amongst people with similar health problems, despite the fact that some men were initially reluctant to take part in a group programme.
De Souza and Ciclitira195 suggested that men were happy to attend mixed slimming classes as they received support and were ‘cheered on’ by their fellow female slimmers. The authors talked about the ‘competitive’ aspect of this being attractive to men.
Groups provided camaraderie, and the spontaneous humour and banter was important to men. 46,49,142,149,189,191,193,196 These features are used in the FFIT trial, in which the Facebook page is engaging participants in banter and support (Kate Hunt, University of Glasgow, 6 February 2012, personal communication). Men also reported that being accountable to themselves and having to account for food choices to others within a programme facilitated programme adherence. 190
There are many factors to consider when designing group-based programmes. 212 Although groups may require fewer resources, group-based programmes can be logistically difficult with regard to scheduling meetings and may therefore be impractical for time-poor men, who already consider time a barrier to engagement with physical activity. 190
In the case of group-based programmes, concern was expressed that groups may gradually stop meeting after programmes have ended and this may impact negatively on adherence to the messages of the programmes. 49 This anxiety has also been seen with smoking cessation groups (Flora Douglas, University of Aberdeen, 12 March 2013, personal communication). On the other hand, too many weight-loss group sessions may be counterproductive. 101
Commercial organisations
Commercial weight-loss organisations36,37,154,157 were effective at producing weight loss in men, producing results that were as good as those in NHS programmes31,151 – 153 when delivered in mixed-sex settings. Men are much less likely to enrol in mixed-sex programmes run by these organisations than women,100 but some men-only groups and websites are available. When interventions were delivered to single-sex groups,33,34,148,150,156 commercial providers appeared to outperform single-sex NHS services,142,147 but data were very limited. Referral by GPs to commercial providers can improve the low take-up by men, but health service programmes appear to be favoured by men, despite the fact that we also found that health service settings appear to provoke fear and anxiety amongst men. 96,100 The comparative effectiveness of NHS and commercial weight-loss programmes for men in the long-term randomised UK trial by Jolly and colleagues100 was unclear.
Family and friends
In the trials that we reviewed, the effect of support from partners to aid weight loss was inconsistent. 94,112,120 Partners can have a pivotal role in successful weight-loss attempts,188,193 providing support for those choosing against the expected social norms. 188,192 However, the influence of family members and peers who respond in a negative way can be very detrimental to men’s efforts to lose weight. Men reported not wishing to offend mothers and mothers-in-law intent on ‘feeding them up’. 188 Gay partners may not be happy with their partners losing weight, which they can perceive as making them more attractive to others. 195 Conflict over attempts to lose weight arose especially when the giving and receiving of food was regarded as a means of maintaining communication and relationships with family members. 237,238 Comments from family and friends on weight, body size or image or programme participation can either motivate or demotivate behaviour change.
There were no family-based interventions that fit any of the inclusion criteria for our reviews, but there was good evidence that men’s participation in weight-loss programmes had beneficial effects on the weight of other members of the family. 89,112,121,142,187,193 The Healthy Dads, Healthy Kids study from Australia evaluated the effect of primary school children attending some of the weight-loss sessions intended for adult men. 239 After 6 months men had an impressive weight loss of 7.6 kg (baseline observation carried forward), with a significant improvement in the physical activity levels and dietary intake of their children.
Orlistat
We found that men are less likely than women to do well using orlistat to help long-term weight-loss maintenance. 105 Again, this might reflect men’s lesser interest in dieting.
There was some evidence to suggest that orlistat was a cost-effective add-on to lifestyle interventions in male overweight and obese patients, with a greater likelihood of cost-effectiveness if the drug was targeted towards those at greatest risk of diabetes. 164,166 NICE and EMA both recommend the use of orlistat for the treatment of obesity in the UK and Europe respectively. Thresholds of weight loss required to justify continued treatment with the drug differ across the regulatory bodies. The original NICE guidelines172 recommend continuation of treatment only if subjects achieve 5% weight loss at 3 months and 10% at 6 months. The original181 and updated182 EMA criteria are slightly more relaxed than those used by NICE, requiring only a minimum of 5% weight loss at 3 months to justify continuation of treatment.
Innovations
There was some evidence that men found technology and innovations appealing in their weight-loss programmes, for example using sandbags to demonstrate the weight that had been lost193 or using body scans to show changes in body composition. 188 These tangible props with objective measures to support programmes, for example pedometers, could reduce the need for men to discuss their feelings.
Do men state who they believe to be the best person/persons to deliver the intervention?
We found no clear evidence that the sex or profession of the person delivering the intervention affected men’s outcomes. The only evidence came from an economic evaluation of a study from Denmark by Olsen and colleagues,165 which was not eligible for any of our other reviews. This found that men did better with lifestyle counselling from their GP than with the same number of longer sessions with a dietitian who focused on nutritional counselling. The opposite was the case for women. The sex of the GPs and dietitians was not given. These results could reflect men’s lesser enthusiasm for dieting, rather than the influence of the person providing the counselling.
There is evidence that important differences in training, skills and the personal experience of weight-loss advisors may influence engagement with group-based programmes, although whether advisors were men or women was not examined. 240 In the UK, men appear to be more likely to raise weight problems with primary care nurses than with their GPs. 241
Research by Dutton and colleagues242,243 in the USA found that physicians had target BMIs that were significantly lower for obese women than for obese men. Obese women themselves were significantly more likely to endorse unrealistic weight-loss goals than obese men, but female physicians recommended more reasonable goals than male physicians for both male and female patients.
Social, cultural and environmental influences on obesity in men
Normative understandings of masculinity are often associated with risky behaviours (e.g. drinking, smoking, late health care seeking) even after social class is accounted for. 244 Men’s reluctance to seek help is often explained through theories of masculinity. 245 – 247 Theories of masculinity rest on the concept of ‘hegemonic’ or dominant masculinity248 and, in debates on health-related help-seeking, hegemonic masculinity is thought to create patterns of behaviour that are based on resisting contact with formal services to emphasise self-sufficiency and robustness. However, the notion of hegemonic masculinity as the dominant force in health-related decision-making has been criticised. Some have suggested that masculinity has become abstracted and consequently divorced from studying the actual practices of men. 249 Masculinity, it is argued, interconnects with other factors such as social class, age and ethnicity245,246 and, accordingly, from this perspective, it is impossible to pull men out of the social structures in which they are located. Other factors such as social stratification or age are considered more important than gender. 250 One of the theoretical implications of envisaging masculinity as an external pressure is that men are conceptualised as ‘slaves’ to hegemonic discourses. Unfortunately, this perspective has led to ‘men’ being constructed as a universal category. 251
The desire for muscularity could make it harder for men than women to identify that they are overweight. 2 Although BMI tends to be used more than waist circumference in risk prediction and the NHS, waist circumference might be a better measurement for raising awareness of weight issues amongst men, and less susceptible to conflicts over muscularity. There is continuing debate about the health risks of being overweight with a BMI of ≥ 25 kg/m2 and < 30 kg/m2 as opposed to being obese with a BMI of ≥ 30 kg/m2. 15 An evidence synthesis of health risks according to BMI and waist circumference for men and women by age group would be useful to direct policy.
In our qualitative research synthesis men made very little reference to wider social determinants of their behaviour and instead focused on their motivation and own agency to overcome (or attempt to overcome) those secular macro-level changes that had encouraged the overconsumption of energy-dense foods and constrained or reduced opportunities to be physically active. This could reflect the influence of the intrusive health lobby. 44
We would reiterate the importance of environmental influences on men’s weight,191,194,198,199 as exemplified by the Foresight report. 5 Egger and Swinburn252 have long drawn attention to the environmental determinants of obesogenic behaviours. More recently, De Vogli and colleagues253 have found a clear link between economic globalisation, income inequality and BMI in high-income countries.
The role that the environment plays in influencing behaviours helped some researchers to decide where to locate the delivery of weight management programmes. For example, the programme described by Harrison191 and White and colleagues49 was located in the workplace, with the rationale that it was more convenient to access than non-workplace settings, thus removing a barrier to engagement.
Finally, the cost of attending exercise facilities or purchasing healthier food is a deterrent to making lifestyle changes. 198,202
Findings from the systematic review of economic evaluations
The review of cost-effectiveness studies has revealed a real paucity of evidence on the cost-effectiveness of interventions to tackle male obesity. No studies were conducted in a UK setting and there was much heterogeneity across the five studies included in our review, with studies being of variable methodological quality. The evidence suggested that GP counselling may be more cost-effective than dietitian counselling (although more detailed), again suggesting that an emphasis on diet is a turn-off for male participants. 165 It is interesting that this study showed that women tended to do better with the dietitian than their male counterparts. Segal and collegaues163 investigated a group lifestyle intervention for men but reported only limited data and did not fully describe the intervention evaluated. Evidence suggested that this group intervention was cost-effective. Galani and colleagues162 conducted a high-quality economic modelling study, showing that a lifestyle intervention was highly cost-effective, although further work would be required to demonstrate the viability of such an intervention in a UK male population. Orlistat was found to be a cost-effective addition to a lifestyle intervention, but only when targeted at those men with greatest risk of developing type 2 diabetes.
Further information is required to generate more specific evidence on the cost-effectiveness of orlistat in a UK context and also which prescribing guidelines (NICE or EMA) offer the greatest patient benefit and value for money to the NHS.
Although the studies included in our review provide information on the potential cost-effectiveness of different interventions for weight loss, the results should be interpreted in light of their variable methodological quality, their relevance to a UK health-care setting, the assumptions regarding maintenance of weight loss over time and the applicability of model inputs to a male subgroup of the population. We conclude that, on the basis of the retrieved literature, there is insufficient evidence available to make clear recommendations regarding the cost-effectiveness of interventions or treatments for male obesity, nor is there any evidence to recommend for or against different treatment strategies for male and female subgroups on the grounds of cost-effectiveness. It is imperative that future clinical studies should be accompanied by high-quality economic evaluations.
Limitations of our research
We had difficulty identifying the evidence bases for the reviews included here. Current indexing by databases makes searching for male-/female-related issues and qualitative research very challenging. Finding unpublished evaluations of UK studies was even more challenging. Thus, it is likely that we will have missed some of the evidence base. Evaluations with more favourable results are more likely to have been found. Only one of the long-term randomised trials100 came from the UK, so the generalisability of the trial evidence could be limited. Furthermore, no long-term randomised trial provided qualitative and health economic data. In fact, qualitative and quantitative evidence often did not come from the same study. However, the findings were mostly consistent across the randomised trials, non-randomised interventions and qualitative evidence. If resources had allowed we would also have liked to explore the evidence from a systematic review of surveys of men’s views on obesity and weight loss.
The evidence base for black or ethnic minority men, men with low incomes or who are unemployed, men living in rural and/or remote areas or men with a gay, bisexual or transgender background was almost completely absent. It will be important to study how programmes can be readily adapted to encompass these groups.
Our reviews focused on weight-loss interventions for men who were obese. Prevention of obesity in men should also be a focus for research and health care.
Other evidence and resources
One previous systematic review by Young and colleagues56 has examined the effectiveness of male-only weight-loss and maintenance interventions. Included studies were of any duration or study design, and studies with men with comorbidities were excluded. There is little overlap between our reviews and Young’s review. Young and colleagues56 found that younger age, more frequent contact (three or more contacts per month), a prescribed energy restriction and group face-to-face delivery were associated with improved effectiveness. However, older men are more likely to have not been included in their review because of the presence of comorbidities. A systematic review of workplace physical activity interventions for men has also highlighted the need to specifically target men in the development of interventions. 254 Our findings would not disagree with these reviews.
There are resources available to help people to engage men and to design and provide services to help obese men lose weight. 54,224,255 – 260 These resources emphasise the need for commitment, training and adequate long-term resources for services.
There are also resources available to aid with the conduct and reporting of high-quality randomised trials and economic modelling exercises. Glick and colleagues261 have published detailed guidelines that provide a step-by-step guide to carrying out pragmatic economic evaluations alongside randomised trials. Perhaps of more relevance to obesity-related conditions is the requirement for high-quality modelling studies projecting disease progress, costs and benefits over a longer time horizon, including the impact on patient health and risk of future obesity-related complications. A series of seven papers published by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) provides guidance on decision-analytical modelling in drug trials,262 – 268 and Briggs and colleagues269 provide a good grounding in and discussion of the methods of decision modelling and should be used to inform best practice methods for conducting cost-effectiveness analyses of obesity-related interventions.
Chapter 8 Conclusions
Implications for health care
-
Weight reduction for men is best achieved and maintained with the combination of a reducing diet, physical activity advice or a physical activity programme and behaviour change techniques (e.g. self-monitoring, goal setting, prompting self-monitoring, providing feedback, review of goals). These key components differ from those for women in that men prefer more factual information on how to lose weight and more emphasis on providing physical activity programmes. Weight-loss programmes can prevent type 2 diabetes and improve cardiovascular risk factors and erectile dysfunction, self-esteem and quality of life in men.
-
For some men the opportunity to attend men-only groups may enhance intervention effectiveness. Individual tailoring and feedback may also be features of more effective services.
-
Weight-loss programmes for men may be better provided in social settings, such as sports clubs and workplaces, which may be more successful at engaging men than health service settings. Innovative means of delivering services are needed for hard-to-reach groups, such as those men who do not see their weight status as a problem, younger men, unemployed men and those living in remote and rural locations.
Recommendations for research
General recommendations
-
Research is needed to examine the effectiveness and cost-effectiveness of new approaches to engaging men with weight-loss services and the best design for those services.
-
Men (and women) are a heterogeneous group. Rigorous methods are needed to test more complex interventions in recognition of the fact that simple interventions testing a few components are unlikely to motivate all men. Men should be consulted on how to optimise engagement and make interventions more user-friendly, and services need to be formally evaluated. The experiences and perspectives of men (and women) who are black and from ethnic minority backgrounds, who are unemployed and on low incomes, who are gay, bisexual and transgender and who are from rural and/or remote locations need to be addressed. Rigorous feasibility studies and piloting with service user input at all stages is required before undertaking definitive RCTs.
-
Health concerns, which may prompt contact with health service staff, motivate men to address their obesity. Research is required to examine the most effective brief interventions delivered at these pivotal health service encounters when an obesity-related diagnosis is made.
-
Although there are many published evaluations of weight-loss interventions, the lack of data on participation and outcome by sex and gender suggests that sex and gender are not considered important issues. It is essential to report these data in weight-loss programmes, including the proportions of disadvantaged and minority group participants.
-
The influence of the sex of providers and gatekeepers on engagement in, and outcomes from, weight-loss services should be examined. In addition, the importance of personal experience of weight problems in those delivering weight management programmes requires exploration.
-
Clearer reporting of all aspects of interventions, including psychological and ecological theories and behaviour change techniques, is needed to develop the evidence base and allow others to replicate interventions.
-
Systematic reviews should examine the quantitative and qualitative evidence base for the management of obesity in women, whose needs will differ from those of men.
-
Research is needed on programmes that aim to tackle more than one health-related behaviour at once (such as harmful drinking and obesity), the implementation of such services and their reach in underserved groups of men.
Quantitative research
-
We found relatively few long-term RCTs and there were even fewer UK studies that provided outcome data for men of more than a few months’ follow-up. As was clear from our reviews, men would value longer-term support and there is a need to provide longer-term outcome data (at least 1 year of follow-up). There is also a need to look specifically at ways of enhancing the maintenance of long-term weight loss. The majority of the programmes did not make a distinction between support for the initial weight loss and a different or modified programme to help maintain that weight loss.
-
Although weight loss was the primary aim of the programmes that we reviewed, it is also essential to study the impact of weight-loss programmes, with adequate statistical power, on the prevention and treatment of comorbidities and on quality of life. We recommend that data are collected on cardiovascular risk factors to allow the modelling of impacts on cardiovascular disease, or that the direct effects of weight loss are presented for clinical conditions, for example type 2 diabetes and erectile dysfunction. Our data suggest that weight-loss programmes in the right social setting can improve well-being; thus, quality-of-life data should also be collected.
-
An evidence synthesis of health risks according to BMI and waist circumference by sex and age group is needed to direct policy.
-
Although the written reports of RCTs have improved over time, there is still a need for trials to improve the reporting of methods and reporting of the items recommended by the CONSORT statement (e.g. sequence generation, allocation concealment) and provide the items for the Campbell & Cochrane Equity Methods Group checklist, to assess the impact on disadvantaged groups and the sustainability of interventions. Intervention studies that are not RCTs also need to report these items when relevant. Process evaluations and fidelity checks are needed in trials.
-
Identifying reports of sex-specific studies, or reports in which data are reported separately for men and women, is challenging because of suboptimal indexing in databases. Sex-specific index terms are a feature of the controlled vocabularies of several major databases but they are underutilised. Consistent, reliable indexing is needed to facilitate more efficient literature searching.
-
Identifying studies in a UK setting is problematic. Within electronic databases, indexing with country names could be used more frequently to enable efficient identification of studies set in a particular country.
-
There is no consensus yet on reporting weight-loss results in intervention studies, in which dropouts almost inevitably occur. Providing data for completers only, as was the case in many of the studies reviewed here, will inevitably provide optimistic results. Similarly, using the last observation carried forward for dropouts is also likely to provide results indicating better than actually achieved weight loss. Baseline observation carried forward results are likely to provide the worst case scenario, with the last observation carried forward results between these and the complete case results. Researchers and programme providers should attempt to establish body weight at the end of follow-up, even if only self-reported. Complete case, baseline observation carried forward and last observation carried forward results should be provided to allow comparisons between weight-loss programmes.
Health economics
-
UK cost-effectiveness studies in men are needed.
-
Improvements are needed in the reporting of methods for the estimation of utility weights and the methods used to derive them (e.g. standard gamble, time trade-off, visual analogue scale or discrete choice experiments).
-
Costs (both intervention and subsequent costs of complications) should be reported in detail, with appropriate references, in a manner that would facilitate the theoretical reproduction of the study results.
-
Costs and outcomes should be measured over the appropriate time horizon to include the downstream impact on health service utilisation and quality of life. Best practice decision-analytical methods should be used to model the future impact of weight-loss interventions on disease areas such as diabetes and coronary heart disease. The time horizon of the model should be sufficient to capture all differences between study groups and future costs and outcomes should be discounted to present values using appropriate discount rates relative to the study country.
-
Assumptions used for modelling studies should be clearly defined and highlighted. It should be clear to the reader the assumptions that have been used, especially with regard to maintenance of weight loss and continuation of treatment effect. These assumptions should be comprehensively tested in structural sensitivity analyses.
-
Methods used to model the effect of weight loss on future obesity-related disease should be comprehensively explained, with choices for sources of data clearly outlined and any potential variation explored in deterministic or probabilistic sensitivity analysis.
-
Specifically for interventions relating to men, data inputs for models should be clearly detailed for men. When data for men and women have been assumed and applied to male and female subgroups separately, this should be acknowledged and highlighted as a potential limitation.
Qualitative and mixed-methods research
-
Qualitative research with men is needed to inform all aspects of intervention design, including the identification of new intervention settings, optimal recruitment processes, reasons for attrition and how processes might minimise attrition. Process evaluations of intervention studies are needed to seek feedback on the marketing, content and delivery of interventions and how the macro, meso and micro contexts interact with the intervention.
-
We require a better understanding of the barriers to, and facilitators of, the decision to lose weight and subsequent weight maintenance.
-
Integrating qualitative and quantitative research methods both in systematic reviews and in the design and delivery of complex intervention trials is needed to better understand how men can effectively lose weight and sustain weight loss and how the health service can best deliver services that help them to succeed.
-
There were indications in this research that men may be maintaining relationships with family members by accepting food offerings that may be contraindicated when following a weight-loss/weight-maintenance diet. In addition, given the finding about men’s desire for flexibility and treats within weight-loss diets, more research is needed to understand men’s personal food systems and values to understand how different groups of men value and act on the different dimensions that have been found to be instrumental in food choice decision-making. Having a better understanding of these issues may help with the design of future interventions that take account of, or help men find ways of dealing with unintended social sabotage that comes from not wanting to offend mothers, mothers-in-law, girlfriends or boyfriends, etc.
-
The feasibility and acceptability of interventions delivered to families should be explored.
-
There is a need to understand the public perceptions of men who are not yet overweight or obese (to the extent that they have taken action on it) or suffering from a weight-related health condition, to understand how men who have not yet ‘problematised’ obesity and overweight can be engaged in taking action to prevent their weight becoming a problem (i.e. needing medical intervention and incurring costs to the NHS).
-
With regard to qualitative research recommendations associated with qualitative evidence synthesis, in primary research papers there is a need to reduce the instances in papers in which second-order author interpretations are unsupported by first-order evidence. Participant details attached to quotes when reporting qualitative research should provide details of the sex of the respondent.
-
Reports of qualitative research are indexed inadequately in electronic databases. Despite the primary searches for the review of qualitative studies yielding a large number of results, only five studies were found from the databases for this review. Electronic databases need to index qualitative research as a publication type.
Acknowledgements
We would like to thank the study authors and professional organisations who we contacted who provided additional details of their studies, Cynthia Fraser for providing guidance with literature searching and reference management, Lara Kemp for providing secretarial support and Luke Vale for initial advice on the systematic review of economic evaluations.
The Health Services Research Unit and Health Economics Research Unit, Institute of Applied Health Sciences, University of Aberdeen, are both core funded by the Chief Scientist Office of the Scottish Government Health Directorates.
Contributions of authors
Alison Avenell (Chief Investigator and Clinical Chair in Health Services Research) co-ordinated the design of the study, oversaw and co-ordinated all aspects of the study and wrote the first drafts of the background, discussion and conclusion chapters of the report.
Clare Robertson (Research Fellow) reviewed the evidence for clinical effectiveness and wrote the first drafts of the abstract, executive summary and Chapters 3 and 4 of the report and the first draft of the methods chapter in conjunction with Daryll Archibald.
Daryll Archibald (Research Assistant) reviewed the qualitative evidence and wrote the first draft of Chapter 6 of the report and the first draft of the methods chapter in conjunction with Clare Robertson.
Flora Douglas (Lecturer) led the supervision of the qualitative systematic review and the development and integration of the systematic review themes.
Pat Hoddinott (Professor of Primary Care) co-supervised the qualitative systematic review and the development and integration of the systematic review themes and led the development of the logic model.
Edwin van Teijlingen (Professor) co-supervised the qualitative systematic review and the development and integration of the systematic review themes.
Dwayne Boyers (Health Economist) reviewed the economic evidence and wrote the first draft of Chapter 5 .
Fiona Stewart (Information Specialist) developed and ran the search strategies for all systematic reviews and was responsible for obtaining full-text papers and reference management and providing the flow charts from the searches.
Charles Boachie (Statistician) provided statistical advice and wrote the first draft of the statistical results.
Evie Fioratou (Research Fellow) carried out the literature screening for the qualitative systematic review and drafted an initial outline of the qualitative methods section.
David Wilkins, Tim Street, Paula Carroll and Colin Fowler (representatives from the Men’s Health Forums covering England and Wales, Scotland and all of Ireland respectively) provided valuable consumer input and advice throughout the study.
All authors assisted in preparing the manuscript and commenting on drafts.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Mainstreaming Gender Equity in Health: The Need to Move Forward. Madrid Statement. Copenhagen: WHO Regional Office for Europe; 2002.
- Ross R, Shaw KD, Rissanen J, Martel Y, De Guise J, Avruch L. Sex differences in lean and adipose tissue distribution by magnetic resonance imaging: anthropometric relationships. Am J Clin Nutr 1994;59:1277-85.
- Obesity: Guidance on the Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: NICE; 2006.
- Health Survey for England – 2011, Health, Social Care and Lifestyles. Leeds: Health and Social Care Information Centre; 2012.
- Butland B, Jebb S, Kopelman P, McPherson K, Thomas S, Mardell J, et al. Foresight. Tackling Obesities: Future Choices – Project Report. London: Government Office for Science; 2007.
- Welsh Health Survey 2012: Initial Headline Results. Cardiff: Welsh Government; 2013.
- Bradshaw P, Bromley C, Corbett J, Day J, Doig M, Dowling S, et al. The Scottish Health Survey. Volume 1: Adults. Edinburgh: Scottish Government; 2012.
- Health Survey Northern Ireland: First Results from the 2011/12 Survey. Belfast: Department of Health, Social Services and Public Safety; 2012.
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2012;377:557-67.
- Escoto KH, French SA. Unhealthy and healthy weight control behaviours among bus operators. Occup Med 2012;62:138-40. http://dx.doi.org/10.1093/occmed/kqr178.
- Heraclides AM, Chandola T, Witte DR, Brunner EJ. Work stress, obesity and the risk of type 2 diabetes: gender-specific bidirectional effect in the Whitehall II study. Obesity 2012;20:428-33. http://dx.doi.org/10.1038/oby.2011.95.
- Assessing Body Mass Index and Waist Circumference Thresholds for Intervening to Prevent Ill Health and Premature Death among Adults from Black, Asian and Other Minority Ethnic Groups in the UK. London: NICE; 2013.
- Obesity and Ethnicity. Oxford: National Obesity Observatory; 2011.
- Prospective Studies Collaboration . Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083-96.
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71-82. http://dx.doi.org/10.1001/jama.2012.113905.
- Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105-20. http://dx.doi.org/10.1056/NEJMoa0801891.
- Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 2011;377:1085-95.
- Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 2005;81:555-63.
- Cameron AJ, Magliano DJ, Soderberg S. A systematic review of the impact of including both waist and hip circumference in risk models for cardiovascular diseases, diabetes and mortality. Obes Rev 2013;14:86-94. http://dx.doi.org/10.1111/j.1467-789X.2012.01051.x.
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. http://dx.doi.org/10.1016/S0140-6736(08)60269-X.
- Han TS, Tajar S, O’Neill TW, Jiang M, Bartfai G, Boonen S, et al. Impaired quality of life and sexual function in overweight and obese men: the European Male Ageing Study. Eur J Endocrinol 2011;164:1003-11.
- Strandberg TE, Sirola J, Pitkala KH, Tilvis RS, Strandberg AY, Stenholm S. Association of midlife obesity and cardiovascular risk with old age frailty: a 26-year follow-up of initially healthy men. Int J Obes 2012;36:1153-7.
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8.
- Poobalan AS, Aucott LS, Smith WCS, Avenell A, Jung R, Broom J. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev 2007;8:503-13. http://dx.doi.org/10.1111/j.1467-789X.2007.00393.x.
- Ostergaard JN, Gronbaek M, Schnohr P, Sorensen TIA, Heitmann BL. Combined effects of weight loss and physical activity on all-cause mortality of overweight men and women. Int J Obes 2010;34:760-9. http://dx.doi.org/10.1038/ijo.2009.274.
- Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677-86.
- Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334:299-302. http://dx.doi.org/10.1136/bmj.39063.689375.55.
- Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673-9.
- Pagoto SL, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomised controlled trials of lifestyle weight loss interventions. Obesity 2012;20:1234-9.
- Moroshko I, Brennan L, O’Brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obes Rev 2011;12:912-34. http://dx.doi.org/10.1111/j.1467-789X.2011.00915.x.
- Ross HM, Laws R, Reckless J, Lean M, McQuigg M, Noble P, et al. Evaluation of the Counterweight programme for obesity management in primary care: a starting point for continuous improvement. Br J Gen Pract 2008;58:548-54. http://dx.doi.org/10.3399/bjgp08X319710.
- Morrison DS, Boyle S, Morrison C, Allardice G, Greenlaw N, Forde L. Evaluation of the first phase of a specialist weight management programme in the UK National Health Service: prospective cohort study. Public Health Nutr 2012;15:28-3.
- Poulter J, Raine G, Robertson S. Evaluation of a gender-segregated, commercial, community based, weight management pilot. Obes Facts 2012;5.
- Bye C, Avery A, Lavin J. Tackling obesity in men – preliminary evaluation of men-only groups within a commercial slimming organization. J Hum Nutr Diet 2005;18:391-4. http://dx.doi.org/10.1111/j.1365-277X.2005.00642.x.
- Hallam C, Mullins G, Cassidy M, Cox JSA, Hewlett B, Broom J. Mean weight loss achieved in 8 weeks by 1006 obese male patients on the LighterLife total VLCD weight-loss programme in 2010. Obes Rev 2011;12:230-1.
- Stubbs RJ, Pallister C, Whybrow S, Avery A, Lavin J. Weight outcomes audit for 34,271 adults referred to a primary care/commercial weight management partnership scheme. Obes Facts 2011;4:113-20.
- Ahern AL, Olson AD, Aston LM, Jebb SA. Weight Watchers on prescription: an observational study of weight change among adults referred to Weight Watchers by the NHS. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-434.
- Brown I, Gould J. Decisions about weight management: a synthesis of qualitative studies of obesity. Clin Obes 2011;1:99-109.
- Garip G, Yardley L. A synthesis of qualitative research on overweight and obese people’s views and experiences of weight management. Clin Obes 2011;1:110-26.
- Johnson F, Cooke L, Croker H, Wardle J. Changing perceptions of weight in Great Britain: comparison of two population surveys. BMJ 2008;337:270-2.
- Duncan DT, Wolin KY, Scharoun-Lee M, Ding EL, Warner ET, Bennett GG. Does perception equal reality? Weight misperception in relation to weight-related attitudes and behaviors among overweight and obese US adults. Int J Behav Nutr Phys Act 2011;8. http://dx.doi.org/10.1186/1479-5868-8-20.
- Gregory CO, Blanck HM, Gillespie C, Maynard LM, Serdula MK. Perceived health risk of excess body weight among overweight and obese men and women: differences by sex. Prev Med 2008;47:46-52. http://dx.doi.org/10.1016/j.ypmed.2008.01.008.
- Zhang L, Rashad I. Obesity and time preference: the health consequences of discounting the future. J Biosoc Sci 2008;40:97-113.
- Gough B, Conner MT. Barriers to healthy eating amongst men: a qualitative analysis. Soc Sci Med 2006;62:387-95. http://dx.doi.org/10.1016/j.socscimed.2005.05.032.
- McCabe MP, McGreevy SJ. Role of media and peers on body change strategies among adult men: is body size important?. Eur Eat Disord Rev 2011;19:438-46. http://dx.doi.org/10.1002/erv.1063.
- Hunt K, McCann C, Gray CM, Mutrie N, Wyke S. ‘You’ve got to walk before you run’: positive evaluations of a walking program as part of a gender-sensitized, weight-management program delivered to men through professional football clubs. Health Psychol 2013;32:57-65. http://dx.doi.org/10.1037/a0029537.
- Wolfe BL, Smith JE. Different strokes for different folks: why overweight men do not seek weight loss treatment. Eat Disord 2002;10:115-24. http://dx.doi.org/10.1080/10640260290081687.
- Sabinsky MS, Toft U, Raben A, Holm L. Overweight men’s motivations and perceived barriers towards weight loss. Eur J Clin Nutr 2007;61:526-31.
- White A, Conrad D, Branney P. Targeting men’s weight in the workplace. J Mens Health 2008;5:133-40.
- Pickett-Blakely O, Bleich SN, Cooper LA. Patient–physician gender concordance and weight-related counseling of obese patients. Am J Prev Med 2011;40:616-19. http://dx.doi.org/10.1016/j.amepre.2011.02.020.
- Gough B. ‘Real men don’t diet’: an analysis of contemporary newspaper representations of men, food and health. Soc Sci Med 2007;64:326-37. http://dx.doi.org/10.1016/j.socscimed.2006.09.011.
- Management of Obesity. Edinburgh: SIGN; 2010.
- Obesity: Full Guideline, Section 6 – Health Economics: Evidence Statements and Reviews. London: NICE; 2006.
- White A, Pettifer M. Hazardous Waist: Tackling Male Weight Problems. Oxford: Radcliffe; 2007.
- Robertson LM, Douglas F, Ludbrook A, Reid G, Van Teijlingen E. What works with men? A systematic review of health promoting interventions targeting men. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-141.
- Young MD, Morgan PJ, Plotnikoff RC, Callister R, Collins CE. Effectiveness of male-only weight loss and weight loss maintenance interventions: a systematic review with meta-analysis. Obes Rev 2012;13:393-408. http://dx.doi.org/10.1111/j.1467-789X.2011.00967.x.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2011. www.cochrane-handbook.org/ (accessed February 2013).
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York; 2009.
- Verhagen AP, De Vet HCW, De Bie RA, Kessels AGH, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235-41.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84. http://dx.doi.org/10.1136/jech.52.6.377.
- Jackson R, Ameratunga S, Broad J, Connor J, Lethaby A, Robb G, et al. The GATE frame: critical appraisal with pictures. Evid Based Med 2006;11:35-8. http://dx.doi.org/10.1136/ebm.11.2.35.
- Ueffing E, Tugwell P, Welch V, Petticrew M, Kristjansson E. Equity Checklist for Systematic Review Authors. Cochrane and Campbell Equity Methods Group; 2011.
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326. http://dx.doi.org/10.1136/bmj.326.7382.219.
- Hanlon P, Carlisle S, Hannah M, Lyon A, Reilly D. A perspective on the future public health: an integrative and ecological framework. Perspect Public Health 2012;132:313-19. http://dx.doi.org/10.1177/1757913912440781.
- Swinburn B, Egger G, Raza F. Dissecting obesogenic environments: the development and application of a framework for identifying and prioritising environmental interventions for obesity. Prev Med 1999;29:563-70.
- Whitehead M, Dahlgren G. What can be done about inequalities in health?. Lancet 1991;338:1059-63. http://dx.doi.org/10.1016/0140-6736(91)91911-D.
- Rayner G, Lang T. Ecological Public Health: Reshaping the Conditions of Good Health. London: Routledge; 2012.
- McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q 1988;15:351-77. http://dx.doi.org/10.1177/109019818801500401.
- Stokols D. Translating social ecological theory into guidelines for community health promotion. Am J Health Promot 1996;10:282-98. http://dx.doi.org/10.4278/0890-1171-10.4.282.
- Pawson R. Evidence-Based Policy: a Realist Perspective. Sage: London; 2006.
- Attree P, Milton B. Critically appraising qualitative research for systematic reviews: defusing the methodological cluster bombs. Evid Policy 2006;2:109-26. http://dx.doi.org/10.1332/174426406775249688.
- Dixon-Woods M, Sutton A, Shaw R, Miller T, Smith J, Young B, et al. Appraising qualitative research for inclusion in systematic reviews: a quantitative and qualitative comparison of three methods. J Health Serv Res Policy 2007;12:42-7. http://dx.doi.org/10.1258/135581907779497486.
- Barnett-Page E, Thomas J. Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol 2009;9. http://dx.doi.org/10.1186/1471-2288-9-59.
- Carroll C, Booth A, Cooper K. A worked example of ‘best fit’ framework synthesis: a systematic review of views concerning the taking of some potential chemopreventive agents. BMC Med Res Methodol 2011;11. http://dx.doi.org/10.1186/1471-2288-11-29.
- Dixon-Woods M, Bonas S, Booth A, Jones DR, Miller T, Sutton AJ, et al. How can systematic reviews incorporate qualitative research? A critical perspective. Qual Res 2006;6:27-44. http://dx.doi.org/10.1177/1468794106058867.
- Hannes K. Chapter 4. Cochrane Collaboration Qualitative Research Methods Group . Critical Appraisal of Qualitative Research 2011. http://cqrmg.cochrane.org/supplemental-handbook-guidance (accessed March 2013).
- Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008;8. http://dx.doi.org/10.1186/1471-2288-8-45.
- 10 Questions to Help You Make Sense of Qualitative Research. Oxford: Critical Appraisal Skills Programme; 2010.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. http://dx.doi.org/10.1093/intqhc/mzm042.
- Qualitative Assessment and Review Instrument. Adelaide: Joanna Briggs Institute; 2011.
- Noblit GW, Hare RD. Meta-ethnography: Synthesizing Qualitative Studies. London: Sage; 1988.
- Pope K, Mays N, Popay J. Synthesising Qualitative and Quantitative Health Evidence. Maidenhead: Open University Press; 2007.
- Benassi-Evans B, Clifton PM, Noakes M, Keogh JB, Fenech M. High protein–high red meat versus high carbohydrate weight loss diets do not differ in effect on genome stability and cell death in lymphocytes of overweight men. Mutagenesis 2009;24:271-7. http://dx.doi.org/10.1093/mutage/gep006.
- Borg P, Kukkonen-Harjula K, Fogelholm M, Pasanen M. Effects of walking or resistance training on weight loss maintenance in obese, middle-aged men: a randomized trial. Int J Obes 2002;26:676-83. http://dx.doi.org/10.1038/sj.ijo.0801962.
- Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D’Andrea F, et al. Effect of lifestyle changes on erectile dysfunction in obese men. JAMA 2004;291:2978-84. http://dx.doi.org/10.1001/jama.291.24.2978.
- Jeffery RW, Gerber WM, Rosenthal BS, Lindquist RA. Monetary contracts in weight control: effectiveness of group and individual contracts of varying size. J Consult Clin Psychol 1983;51:242-8. http://dx.doi.org/10.1037//0022-006X.51.2.242.
- Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med 2011;8:2868-75. http://dx.doi.org/10.1111/j.1743-6109.2011.02417.x.
- King AC, Frey-Hewitt B, Dreon DM, Wood PD. Diet vs exercise in weight maintenance. The effects of minimal intervention strategies on long-term outcomes in men. Arch Intern Med 1989;149:2741-6. http://dx.doi.org/10.1001/archinte.1989.00390120085017.
- Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. 12-Month outcomes and process evaluation of the SHED-IT RCT: an internet-based weight loss program targeting men. Obesity 2011;19:142-51. http://dx.doi.org/10.1038/oby.2010.119.
- Patrick K, Calfas KJ, Norman GJ, Rosenberg D, Zabinski MF, Sallis JF, et al. Outcomes of a 12-month web-based intervention for overweight and obese men. Ann Behav Med 2011;42:391-40.
- Pavlou KN, Krey S, Steffee WP. Exercise as an adjunct to weight loss and maintenance in moderately obese subjects. Am J Clin Nutr 1989;49:1115-23.
- van Aggel-Leijssen DPC, Saris WHM, Hul GB, van Baak MA. Short-term effects of weight loss with or without low-intensity exercise training on fat metabolism in obese men. Am J Clin Nutr 2001;73:523-31.
- Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med 1988;319:1173-9. http://dx.doi.org/10.1056/NEJM198811033191801.
- Gorin AA, Raynor HA, Fava J, Maguire K, Robichaud E, Trautvetter J, et al. Randomized controlled trial of a comprehensive home environment-focused weight-loss program for adults. Health Psychol 2013;32:128-37. http://dx.doi.org/10.1037/a0026959.
- Hakala P, Karvetti RL, Ronnemaa T. Group vs. individual weight reduction programmes in the treatment of severe obesity – a five year follow-up study. Int J Obes Relat Metab Disord 1993;17:97-102.
- Hakala P. Weight reduction programmes at a rehabilitation centre and a health centre based on group counselling and individual support: short- and long-term follow-up study. Int J Obes Relat Metab Disord 1994;18:483-9.
- Heitzmann CA, Kaplan RM, Wilson DK, Sandler J. Sex differences in weight loss among adults with type II diabetes mellitus. J Behav Med 1987;10:197-211.
- Jeffery RW, Bjornson-Benson WM, Rosenthal BS, Kurth CL, Dunn MM. Effectiveness of monetary contracts with two repayment schedules on weight reduction in men and women from self-referred and population samples. Behav Ther 1984;15:273-9. http://dx.doi.org/10.1016/S0005-7894(84)80029-5.
- Jeffery RW, Sherwood NE, Brelje K, Pronk NP, Boyle R, Boucher JL, et al. Mail and phone interventions for weight loss in a managed-care setting: Weigh-To-Be one-year outcomes. Int J Obes 2003;27:1584-92. http://dx.doi.org/10.1038/sj.ijo.0802473.
- Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ 2011;343.
- Karvetti RL, Hakala P. A seven-year follow-up of a weight reduction programme in Finnish primary health care. Eur J Clin Nutr 1992;46:743-52.
- Korhonen T, Uusitupa M, Aro A, Kumpulainen T, Siitonen O, Voutilainen E, et al. Efficacy of dietary instructions in newly diagnosed non-insulin-dependent diabetic patients. Comparison of two different patient education regimens. Acta Med Scand 1987;222:323-31. http://dx.doi.org/10.1111/j.0954-6820.1987.tb10679.x.
- Lantz H, Peltonen M, Agren L, Torgerson JS. Intermittent versus on-demand use of a very low calorie diet: a randomized 2-year clinical trial. J Intern Med 2003;253:463-71. http://dx.doi.org/10.1046/j.1365-2796.2003.01131.x.
- Lindstrom J, Peltonen M, Eriksson JG, Aunola S, Hamalainen H, Ilanne PP, et al. Determinants for the effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Care 2008;31:857-62. http://dx.doi.org/10.2337/dc07-2162.
- Richelsen B, Tonstad S, Rossner S, Toubro S, Niskanen L, Madsbad S, et al. Effect of orlistat on weight regain and cardiovascular risk factors following a very-low-energy diet in abdominally obese patients: a 3-year randomized, placebo-controlled study. Diabetes Care 2007;30:27-32. http://dx.doi.org/10.2337/dc06-0210.
- Ross R, Lam M, Blair SN, Church TS, Godwin M, Hotz SB, et al. Trial of prevention and reduction of obesity through active living in clinical settings: a randomized controlled trial. Arch Intern Med 2012;172:414-24. http://dx.doi.org/10.1001/archinternmed.2011.1972.
- Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229-41. http://dx.doi.org/10.1056/NEJMoa0708681.
- Vanninen E, Uusitupa M, Siitonen O, Laitinen J, Lansimies E. Habitual physical activity, aerobic capacity and metabolic control in patients with newly-diagnosed type 2 (non-insulin-dependent) diabetes mellitus: effect of 1-year diet and exercise intervention. Diabetologia 1992;35:340-6. http://dx.doi.org/10.1007/BF00401201.
- Volpe SL, Kobusingye H, Bailur S, Stanck E. Effect of diet and exercise on body composition, energy intake and leptin levels in overweight women and men. J Am Coll Nutr 2008;27:195-208. http://dx.doi.org/10.1080/07315724.2008.10719691.
- Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity 2011;19:1987-98. http://dx.doi.org/10.1038/oby.2011.230.
- West DS, Prewitt TE, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity 2008;16:1413-20. http://dx.doi.org/10.1038/oby.2008.224.
- Wing RR, Marcus MD, Epstein LH, Jawad A. A ‘family-based’ approach to the treatment of obese type II diabetic patients. J Consult Clin Psychol 1991;59:156-62. http://dx.doi.org/10.1037//0022-006X.59.1.156.
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome?. Am J Med 1994;97:354-62. http://dx.doi.org/10.1016/0002-9343(94)90302-6.
- Multiple risk factor intervention trial . Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research Group. JAMA 1982;248:1465-77.
- Fortmann SP, Haskell WL, Wood PD. Effects of weight loss on clinic and ambulatory blood pressure in normotensive men. Am J Cardiol 1988;62:89-93. http://dx.doi.org/10.1016/0002-9149(88)91370-7.
- Kukkonen-Harjula KT, Borg PT, Nenonen AM, Fogelholm MG. Effects of a weight maintenance program with or without exercise on the metabolic syndrome: a randomized trial in obese men. Prev Med 2005;41:784-90. http://dx.doi.org/10.1016/j.ypmed.2005.07.008.
- Jeffery RW, Bjornson-Benson WM, Rosenthal BS, Lindquist RA, Johnson SL. Behavioral treatment of obesity with monetary contracting: two-year follow-up. Addict Behav 1984;9:311-13. http://dx.doi.org/10.1016/0306-4603(84)90027-3.
- van Aggel-Leijssen DP, Saris WH, Hul GB, van Baak MA. Long-term effects of low-intensity exercise training on fat metabolism in weight-reduced obese men. Metabolism 2002;51:1003-10. http://dx.doi.org/10.1053/meta.2002.34028.
- Lejeune MPGM, van Aggel-Leijssen DPC, van Baak MA, Westerterp-Plantenga MS. Effects of dietary restraint vs exercise during weight maintenance in obese men. Eur J Clin Nutr 2003;57:1338-44. http://dx.doi.org/10.1038/sj.ejcn.1601697.
- Golan R, Schwarzfuchs D, Stampfer MJ, Shai I. DIRECT Group . Halo effect of a weight-loss trial on spouses: the DIRECT-Spouse study. Public Health Nutr 2010;13:544-9. http://dx.doi.org/10.1017/S1368980009991273.
- Gorin AA, Wing RR, Fava JL, Jakicic JM, Jeffery R, West DS, et al. Weight loss treatment influences untreated spouses and the home environment: evidence of a ripple effect. Int J Obes 2008;32:1678-84. http://dx.doi.org/10.1038/ijo.2008.150.
- Wing RR, Rosen RC, Fava JL, Bahnson J, Brancati F, Gendrano I, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. J Sex Med 2010;7:156-65. http://dx.doi.org/10.1111/j.1743-6109.2009.01458.x.
- Stewart TM, Bachand AR, Han H, Ryan DH, Bray GA, Williamson DA. Body image changes associated with participation in an intensive lifestyle weight loss intervention. Obesity 2011;19:1290-5. http://dx.doi.org/10.1038/oby.2010.276.
- Schwartz AV, Johnson KC, Kahn SE, Shepherd JA, Nevitt MC, Peters AL, et al. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res 2012;27:619-27. http://dx.doi.org/10.1002/jbmr.1483.
- Vanninen E, Uusitupa M, Lansimies E, Siitonen O, Laitinen J. Effect of metabolic control on autonomic function in obese patients with newly diagnosed type 2 diabetes. Diabet Med 1993;10:66-73. http://dx.doi.org/10.1111/j.1464-5491.1993.tb01999.x.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. http://dx.doi.org/10.1016/S0090-4295(97)00238-0.
- Paul-Ebhohimhen V, Avenell A. A systematic review of the effectiveness of group versus individual treatments for adult obesity. Obes Facts 2009;2:17-24. http://dx.doi.org/10.1159/000186144.
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c869.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c332.
- Ferguson JM, Ferguson C. Habits not Diets: the Secret to Lifetime Weight Control. Boulder, CO: Bull Publishing; 2003.
- Mahoney MJ, Mahoney K. Permanent Weight Control. New York, NY: Norton; 1976.
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50. http://dx.doi.org/10.1056/NEJM200105033441801.
- Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, et al. AHA Dietary Guidelines Revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000;102:2284-99. http://dx.doi.org/10.1161/01.CIR.102.18.2284.
- Willett WC, Skerrett PJ. Eat, Drink and Be Healthy: the Harvard Medical School Guide to Healthy Eating. New York, NY: Simon and Schuster; 2001.
- Atkins RC. Dr Atkins’ New Diet Revolution. New York, NY: Avon Books; 2002.
- Diabetes Prevention Program . Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623-34.
- Diabetes Prevention Program . The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25:2165-71.
- Brady AJ, Perry C, Murdoch DL, McKay G. Sustained benefits of a health project for middle-aged football supporters, at Glasgow Celtic and Glasgow Rangers Football Clubs. Eur Heart J 2010;31:2696-8.
- Tackling Men’s Health. Men Only Weight Management Group: NHS Leeds and Leeds Rhinos, 2010 Season. Leeds: Public Health and Social Care Group Yorkshire and the Humber and NHS Leeds; 2010.
- Drummond S, Dixon K, Griffin J, De Looy A. Weight loss on an energy-restricted, low-fat, sugar-containing diet in overweight sedentary men. Int J Food Sci Nutr 2004;55:279-90. http://dx.doi.org/10.1080/09637480412331290495.
- Gray CM, Hunt K, Mutrie N, Anderson AS, Treweek S, Wyke S. Can the draw of professional football clubs help promote weight loss in overweight and obese men? A feasibility study of the Football Fans in Training programme delivered through the Scottish Premier League. J Epidemiol Community Health 2011;65:A37-8.
- Gray CM, Anderson AS, Clarke AM, Dalziel A, Hunt K, Leishman J, et al. Addressing male obesity: an evaluation of a group-based weight management intervention for Scottish men. J Mens Health 2009;6:70-81. http://dx.doi.org/10.1016/j.jomh.2008.11.002.
- Hallam Spencer CL, Holt J, du Plessis J, Cox JSA, Hewlett B. Weight loss results for 5000 women following the LighterLife programme in 2007. Int J Obes 2008;32.
- Hallam CL, Mullins G, Wiggins J, du Plessis J, Cox JSA, Hewlett B. To report on the weight loss achieved in 12 weeks by 432 super-morbidly obese patients on the LighterLife total VLCD weight-loss programme in 2009; a retrospective study. Obes Rev 2010;11.
- Holt J, Horsnell T, Cox J, du Plessis J, Mullins G, Hewlett B. Obese men are at higher risk than obese women. Following a small trial, a long term observational study will report over the next 10 years on weight loss and weight maintenance on a group of self referred obese men. Int J Obes 2007;31.
- Leslie WS, Lean ME, Baillie HM, Hankey CR. Weight management: a comparison of existing dietary approaches in a work-site setting. Int J Obes Relat Metab Disord 2002;26:1469-75. http://dx.doi.org/10.1038/sj.ijo.0802153.
- McFarlane G, Rennie J. Appendix 2: The Bloke’s Weight – Men’s Weight Management Group Arbroath, March–May 2006. Arbroath: Angus Weight Management Project; 2006.
- Salsbury J, Hallam Spencer C, Wiggins J, Dyson L, du Plessis J, Mullins G. Weight-loss results for 2200 male patients with a BMI > 29 kg/m2 following the LighterLife programme. Obes Facts 2009;2.
- Witty K, White A. The Tackling Men’s Health Evaluation Study: Final Report. Leeds: Leeds Metropolitan University, Centre for Men’s Health; 2010.
- Dixon KJ, Shcherba S, Kipping RR. Weight loss from three commercial providers of NHS primary care slimming on referral in north Somerset: service evaluation. J Public Health 2012;34:555-61. http://dx.doi.org/10.1093/pubmed/fds034.
- Evans S. Community Health Improvement Programme n.d.
- Evans S. Primary Care Weight Management in Hertfordshire Using ProHealth n.d.
- Evans S. Weigh2Go: a peripatetic level 1 weight management service in Cambridge City and South 2012.
- Johnson F, Wardle J. The association between weight loss and engagement with a web-based food and exercise diary in a commercial weight loss programme: a retrospective analysis. Int J Behav Nutr Phys Act 2011;8. http://dx.doi.org/10.1186/1479-5868-8-83.
- Kirk T, Cromble N, Cursiter M. Promotion of dietary carbohydrate as an approach to weight maintenance after initial weight loss: a pilot study. J Hum Nutr Diet 2000;13:277-85. http://dx.doi.org/10.1046/j.1365-277x.2000.00237.x.
- Rolland C, Hallam C, Lula S, Wiggins J, Dyson L, Van Gaal LF, et al. Weight loss and ethnicity: a cohort study of the effects induced by a very low calorie diet. Clin Exp Med Sci 2013;1:97-109.
- Stubbs RJ, Brogelli DJ, Pallister CJ, Whybrow S, Avery AJ, Lavin JH. Attendance and weight outcomes in 4754 adults referred over 6 months to a primary care/commercial weight management partnership scheme. Clin Obes 2012;2:6-14. http://dx.doi.org/10.1111/j.1758-8111.2012.00040.x.
- Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality – social, clinical, and health psychology. Psychol Bull 1982;92:111-35. http://dx.doi.org/10.1037//0033-2909.92.1.111.
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690-701. http://dx.doi.org/10.1037/a0016136.
- Michie S, Abraham C, Eccles MP, Francis JJ, Hardeman W, Johnston M. Strengthening evaluation and implementation by specifying components of behaviour change interventions: a study protocol. Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-10.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Galani C, Schneider H, Rutten FF. Modelling the lifetime costs and health effects of lifestyle intervention in the prevention and treatment of obesity in Switzerland. Int J Public Health 2007;52:372-82. http://dx.doi.org/10.1007/s00038-007-7014-9.
- Segal L, Dalton AC, Richardson J. Cost-effectiveness of the primary prevention of non-insulin dependent diabetes mellitus. Health Promot Int 1998;13:197-209. http://dx.doi.org/10.1093/heapro/13.3.197.
- Iannazzo S, Zaniolo O, Pradelli L. Economic evaluation of treatment with orlistat in Italian obese patients. Curr Med Res Opin 2008;24:63-74. http://dx.doi.org/10.1185/030079907X253591.
- Olsen J, Willaing I, Ladelund S, Jorgensen T, Gundgaard J, Sorensen J. Cost-effectiveness of nutritional counseling for obese patients and patients at risk of ischemic heart disease. Int J Technol Assess Health Care 2005;21:194-202.
- Maetzel A, Ruof J, Covington M, Wolf A. Economic evaluation of orlistat in overweight and obese patients with type 2 diabetes mellitus. Pharmacoeconomics 2003;21:501-12. http://dx.doi.org/10.2165/00019053-200321070-00005.
- Galani C, Al M, Schneider H, Rutten FF. Uncertainty in decision-making: value of additional information in the cost-effectiveness of lifestyle intervention in overweight and obese people. Value Health 2008;11:424-34. http://dx.doi.org/10.1111/j.1524-4733.2007.00284.x.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8.
- Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155-61. http://dx.doi.org/10.2337/diacare.27.1.155.
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-12. http://dx.doi.org/10.1136/bmj.321.7258.405.
- Lindstrom J, Peltonen M, Tuomilehto J. Lifestyle strategies for weight control: experience from the Finnish Diabetes Prevention Study. Proc Nutr Soc 2005;64:81-8. http://dx.doi.org/10.1079/PNS2004412.
- TA22: Orlistat for the Treatment of Obesty in Adults. London: NICE; 2001.
- The Clinical Effectiveness and Cost Effectiveness of Sibutramine for Obesity. London: NICE; 2001.
- The Clinical Effectiveness and Cost Effectiveness of Surgery for People with Morbid Obesity. London: NICE; 2002.
- Sibutramine: Suspension of Marketing Authorisation as Risks Outweigh Benefits. London: Medicine and Healthcare products Regulatory Agency; 2010.
- Foxcroft D, Ludders J. Orlistat for the Treatment of Obesity. Southampton: Wessex Institute for Health Research and Development, Development & Evaluation Committee; 1999.
- Review of Clinical Guideline (CG43) – Obesity: the Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: NICE; 2011.
- Macran S. The Relationship between Body Mass Index and Health-Related Quality of Life. York: Centre for Health Economics, University of York; 2004.
- Ara R, Brennan A. Economic Evaluation of Sibutramine for the Treatment of Obesity in Adults Without Other Co-Morbidities in the UK 2005.
- Foxcroft DR. Orlistat for the treatment of obesity: cost utility model. Obes Rev 2005;6:323-8. http://dx.doi.org/10.1111/j.1467-789X.2005.00211.x.
- London: EMA; 1998.
- Assessment Report for Xenical. London: EMA; 2012.
- Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1991;121:293-8. http://dx.doi.org/10.1016/0002-8703(91)90861-B.
- Annual Average Inflation Rates, 2001–2011. Brussels: European Commission; 2012.
- Rate Inflation . Historical Inflation Rates for Australia (2003 to 2013) 2013. www.rateinflation.com/inflation-rate/australia-historical-inflation-rate (accessed February 2013).
- Prices and Purchasing Power Parities (PPP). Paris: OECD; 2013.
- Gallagher R, Kirkness A, Armari E, Davidson PM. Weight management issues and strategies for people with high cardiovascular risk undertaking an Australian weight loss program: a focus group study. Nurs Health Sci 2012;14:18-24. http://dx.doi.org/10.1111/j.1442-2018.2011.00651.x.
- Mallyon A, Holmes M, Coveney J, Zadoroznyj M. ‘I’m not dieting, I’m doing it for science’: masculinities and the experience of dieting. Health Sociol Rev 2010;19:330-42.
- Morgan PJ, Warren JM, Lubans DR, Collins CE, Callister R. Engaging men in weight loss: experiences of men who participated in the male only SHED-IT pilot study. Obes Res Clin Pract 2011;5. http://dx.doi.org/10.1016/j.orcp.2011.03.002.
- Abildso C, Zizzi S, Gilleland D, Thomas J, Bonner D. A mixed methods evaluation of a 12-week insurance-sponsored weight management program incorporating cognitive-behavioral counseling. J Mix Methods Res 2010;4:278-94. http://dx.doi.org/10.1177/1558689810376949.
- Harrison A, Conrad D, White A. Men’s Health: How to Do It. Oxford: Radcliffe Publishing; 2007.
- Kim KH, Linnan L, Campbell MK, Brooks C, Koenig HG, Wiesen C. The WORD (Wholeness, Oneness, Righteousness, Deliverance): a faith-based weight-loss program utilizing a community-based participatory research approach. Health Educ Behav 2008;35:634-50. http://dx.doi.org/10.1177/1090198106291985.
- Leishman J, White A, Pettifer M. Hazardous Waist: Tackling Male Weight Problems. Oxford: Radcliffe; 2007.
- Ogden J, Sidhu S. Adherence, behavior change, and visualization: a qualitative study of the experiences of taking an obesity medication. J Psychosom Res 2006;61:545-52. http://dx.doi.org/10.1016/j.jpsychores.2006.04.017.
- De Souza P, Ciclitira KE. Men and dieting: a qualitative analysis. J Health Psychol 2005;10:793-804. http://dx.doi.org/10.1177/1359105305057314.
- Gillon E. Can men talk about problems with weight? The therapeutic implications of a discourse analytic study. Counsell Psychother Res 2003;3:25-32. http://dx.doi.org/10.1080/14733140312331384598.
- Gough B, Flanders G. Celebrating ‘obese’ bodies: Gay ‘bears’ talk about weight, body image and health. Int J Mens Health 2009;8:235-53. http://dx.doi.org/10.3149/jmh.0803.235.
- McCullagh J. ‘Tommy the Trucker’: a Consultation and Lifestyle Survey of Lorry Drivers Visiting Sefton. South Sefton: Sefton Health Improvement Support Services; 2005.
- Monaghan LF. McDonaldizing men’s bodies? Slimming, associated (ir)rationalities and resistances. Body Soc 2007;13:67-93. http://dx.doi.org/10.1177/1357034X07077776.
- Monaghan L. Body mass index, masculinities and moral worth: men’s critical understandings of ‘appropriate’ weight-for-height. Sociol Health Illn 2007;29:584-609. http://dx.doi.org/10.1111/j.1467-9566.2007.01007.x.
- Monaghan LF. Men, physical activity, and the obesity discourse: critical understandings from a qualitative study. Sociol Sport J 2008;25:97-129.
- Weaver NF, Hayes L, Unwin NC, Murtagh MJ. ‘Obesity’ and ‘clinical obesity’. Men’s understandings of obesity and its relation to the risk of diabetes: a qualitative study. BMC Public Health 2008;8. http://dx.doi.org/10.1186/1471-2458-8-311.
- Gallagher R, Kirkness A, Zelestis E, Hollams D, Kneale C, Armari E, et al. A randomised trial of a weight loss intervention for overweight and obese people diagnosed with coronary heart disease and/or type 2 diabetes. Ann Behav Med 2012;44:119-28. http://dx.doi.org/10.1007/s12160-012-9369-2.
- Tay J, Brinkworth GD, Noakes M, Keogh J, Clifton PM. Metabolic effects of weight loss on a very-low-carbohydrate diet compared with an isocaloric high-carbohydrate diet in abdominally obese subjects. J Am Coll Cardiol 2008;51:59-67. http://dx.doi.org/10.1016/j.jacc.2007.08.050.
- Abildso CG, Zizzi SJ, Reger-Nash B. Evaluating an insurance-sponsored weight management program with the RE-AIM model, West Virginia, 2004–2008. Prev Chronic Dis 2010;7.
- Lake A, Townshend T. Obesogenic environments: exploring the built and food environments. J R Soc Promot Health 2006;126:262-7. http://dx.doi.org/10.1177/1466424006070487.
- Leeder S. The scope, mission and method of contemporary public health. Aust N Z J Public Health 2007;31:505-8. http://dx.doi.org/10.1111/j.1753-6405.2007.00132.x.
- Wardle J, Steptoe A. Socioeconomic differences in attitudes and beliefs about healthy lifestyles. J Epidemiol Community Health 2003;57:440-3. http://dx.doi.org/10.1136/jech.57.6.440.
- Wardle J, Griffith J. Socioeconomic status and weight control practices in British adults. J Epidemiol Community Health 2001;55:185-90. http://dx.doi.org/10.1136/jech.55.3.185.
- Avery A, White A, Pettifer M. Hazardous Waist: Tackling Male Weight Problems. Oxford: Radcliffe; 2007.
- Douglas F, Amaya M, Greener J, Ludbrook A, Reid G, Robertson L, et al. Research Findings No.62/2008: Well Men Health Service Pilots Evaluation. Edinburgh: Scottish Government; 2008.
- Hoddinott P, Allan K, Avenell A, Britten J. Group interventions to improve health outcomes: a framework for their design and delivery. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-800.
- Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. The SHED-IT randomized controlled trial: evaluation of an internet-based weight-loss program for men. Obesity 2009;17:2025-32. http://dx.doi.org/10.1038/oby.2009.85.
- Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol 2001;69:717-21. http://dx.doi.org/10.1037//0022-006X.69.4.717.
- Minniti A, Bissoli L, Di Francesco V, Fantin F, Mandragona R, Olivieri M, et al. Individual versus group therapy for obesity: comparison of dropout rate and treatment outcome. Eat Weight Disord 2007;12:161-7. http://dx.doi.org/10.1007/BF03327593.
- Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005;293:43-5. http://dx.doi.org/10.1001/jama.293.1.43.
- Koch T, Harrington A. Reconceptualizing rigour: the case for reflexivity. J Adv Nurs 1998;28:882-90. http://dx.doi.org/10.1046/j.1365-2648.1998.00725.x.
- Dowling M. Approaches to reflexivity in qualitative research. Nurse Res 2006;13:7-21. http://dx.doi.org/10.7748/nr2006.04.13.3.7.c5975.
- White A. The State of Men’s Health in Europe: Extended Report. Brussels: European Commission Directorate-General for Health and Consumers; 2011.
- White A, McKee M, Richardson N, De Visser R, Madsen SA, De Sousa BC, et al. Europe’s men need their own health strategy. BMJ 2011;343:1144-7. http://dx.doi.org/10.1136/bmj.d7397.
- Equality Act 2010. London: The Stationary Office; n.d.
- Wilkins D, Savoye E. Men’s Health around the World: a Review of Policy and Progress in 11 Countries. Brussels: European Men’s Health Forum; 2009.
- Section 75 of the Northern Ireland Act 1998: a Guide for Public Authorities. An Outline Guide. Belfast: Equality Commission for Northern Ireland; 2012.
- Banks I. The HGV Man Manual. Yeovil: Haynes; 2005.
- Gardner B, Cane J, Rumsey N, Michie S. Behaviour change among overweight and socially disadvantaged adults: a longitudinal study of the NHS Health Trainer Service. Psychol Health 2012;27:1178-93. http://dx.doi.org/10.1080/08870446.2011.652112.
- Epstein L, Ogden J. A qualitative study of GPs’ views of treating obesity. Br J Gen Pract 2005;55:750-4.
- Cohen DJ, Clark EC, Lawson PJ, Casucci BA, Flocke SA. Identifying teachable moments for health behavior counseling in primary care. Patient Educ Couns 2011;85:e8-15. http://dx.doi.org/10.1016/j.pec.2010.11.009.
- Andersen A, Cohn L, Holbrook T. Making Weight: Men’s Conflicts with Food, Weight, Shape & Appearance. Carlsbad, CA: Gurze Books; 2000.
- Kahneman D. The Associative Machine. Thinking, Fast and Slow. London: Allen Lane; 2011.
- Wyke S, Hunt K. Football Fans in Training (FFIT): a Randomized Controlled Trial of a Gender-Sensitive Weight Loss and Healthy Living Programme Delivered to Men Aged 35–65 by Scottish Premier League (SPL) Football Clubs. Southampton: NIHR Public Health Research Programme; 2011.
- Morgan PJ, Collins CE, Plotnikoff RC, Cook AT, Berthon B, Mitchell S, et al. The impact of a workplace-based weight loss program on work-related outcomes in overweight male shift workers. J Occup Environ Med 2012;54:122-7. http://dx.doi.org/10.1097/JOM.0b013e31824329ab.
- Linnan LA, Reiter PL, Duffy C, Hales D, Ward DS, Viera AJ. Assessing and promoting physical activity in African American barbershops: results of the FITStop pilot study. Am J Mens Health 2011;5:38-46. http://dx.doi.org/10.1177/1557988309360569.
- Driving Men’s Weight Loss Programmes: the ‘How To’ Guide. Little Island, Co. Cork: Get Ireland Active; 2011.
- Walking and Cycling: Local Measures to Promote Walking and Cycling as Forms of Travel or Recreation. London: NICE; 2012.
- Behaviour Change at Population, Community and Individual Levels. London: NICE; 2007.
- Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-119.
- Belasco W. Food: the Key Concepts. Oxford: Berg; 2008.
- Connors M, Bisogni CA, Sobal J, Devine CM. Managing values in personal food systems. Appetite 2001;36:189-200. http://dx.doi.org/10.1006/appe.2001.0400.
- Morgan PJ, Lubans DR, Callister R, Okely AD, Burrows TL, Fletcher R, et al. The ‘Healthy Dads, Healthy Kids’ randomized controlled trial: efficacy of a healthy lifestyle program for overweight fathers and their children. Int J Obes 2011;35:436-47. http://dx.doi.org/10.1038/ijo.2010.151.
- Allan K, Hoddinott P, Avenell A. A qualitative study comparing commercial and health service weight loss groups, classes and clubs. J Hum Nutr Diet 2011;24:23-31. http://dx.doi.org/10.1111/j.1365-277X.2010.01110.x.
- Douglas FCG, Greener J, Van Teijlingen E, Ludbrook A. Services just for men? Insights from a national study of the Well Men Services pilots. BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-425.
- Dutton GR, Perri MG, Dancer-Brown M, Goble M, Van Vessem N. Weight loss goals of patients in a health maintenance organization. Eat Behav 2010;11:74-8. http://dx.doi.org/10.1016/j.eatbeh.2009.09.007.
- Dutton GR, Perri MG, Stine CC, Goble M, Van Vessem N. Comparison of physician weight loss goals for obese male and female patients. Prev Med 2010;50:186-8. http://dx.doi.org/10.1016/j.ypmed.2010.01.014.
- Mahalik JR, Burns SM, Syzdek M. Masculinity and perceived normative health behaviors as predictors of men’s health behaviors. Soc Sci Med 2007;64:2201-9. http://dx.doi.org/10.1016/j.socscimed.2007.02.035.
- Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs 2005;49:616-23. http://dx.doi.org/10.1111/j.1365-2648.2004.03331.x.
- Galdas PM, Broom A, Tovey P. Men’s Health: Body, Identity and Social Context. Chichester: Wiley-Blackwell; 2009.
- O’Brien R, Hunt K, Hart G. ‘It’s caveman stuff, but that is to a certain extent how guys still operate’: men’s accounts of masculinity and help seeking. Soc Sci Med 2005;61:503-16.
- Connell R. Gender and Power: Society, the Person and Sexual Politics. Cambridge: Polity; 1987.
- Scott-Samuel A, Stanistreet D, Crawshaw P. Hegemonic masculinity, structural violence and health inequalities. Crit Public Health 2009;19:287-92. http://dx.doi.org/10.1080/09581590903216420.
- Petersen A. Unmasking the Masculine: ‘Men’ and ‘Identity’ in a Sceptical Age. London: Sage; 1998.
- Crawshaw P. Critical perspectives on the health of men: lessons from medical sociology. Crit Public Health 2009;19:279-85. http://dx.doi.org/10.1080/09581590902941507.
- Egger G, Swinburn B. An ‘ecological’ approach to the obesity pandemic. BMJ 1997;315:477-80. http://dx.doi.org/10.1136/bmj.315.7106.477.
- De Vogli R, Kouvonen A, Elovainio M, Marmot M. Economic globalization, inequality and body mass index: a cross-national analysis of 127 countries. Crit Public Health 2014;24:7-21.
- Wong JY, Gilson ND, van Uffelen JG, Brown WJ. The effects of workplace physical activity interventions in men: a systematic review. Am J Mens Health 2012;6:303-13. http://dx.doi.org/10.1177/1557988312436575.
- Men’s Health Training in Ireland: ‘Engage’. Dublin: Men’s Health Forum in Ireland; 2013.
- Fowler C. Men’s Health: the Engagement Jigsaw – a 12 Point Plan for Effectively Engaging with Men. Dublin: Men’s Health Forum in Ireland; 2012.
- Engaging with Men to Improve their Health: a Toolkit for the Voluntary Sector. London: Men’s Health Forum; 2011.
- McCarthy M, Richardson N. Report on Best Practice Approaches to Tailoring Lifestyle Interventions for Obese Men in the Primary Care Setting. Carlow, Republic of Ireland: Centre for Men’s Health, Institute of Technology Carlow; 2011.
- Robinson M, Robertson S. Health information needs of men. Health Educ J 2014;73:150-8. http://dx.doi.org/10.1177/0017896912471039.
- White A, Cash K, Conrad D, Branney P. The Bradford and Airedale Health of Men Initiative: a Study of its Effectiveness in Engaging with Men. Leeds: Centre for Men’s Health, Leeds Metropolitan University; 2008.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making 2012;32:722-32. http://dx.doi.org/10.1177/0272989X12458348.
- Caro JJ, Briggs AH, Siebert U, Kuntz KM. ISPOR-SMDM Modeling Good Research Practices Task Force . Modeling good research practices – overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med Decis Making 2012;32:667-77. http://dx.doi.org/10.1177/0272989X12454577.
- Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB, et al. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making 2012;32:733-43. http://dx.doi.org/10.1177/0272989X12454579.
- Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Moller J. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-4. Med Decis Making 2012;32:701-11. http://dx.doi.org/10.1177/0272989X12455462.
- Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, et al. Dynamic transmission modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-5. Med Decis Making 2012;32:712-21. http://dx.doi.org/10.1177/0272989X12454578.
- Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M, et al. Conceptualising a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-2. Med Decis Making 2012;32:678-89.
- Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Med Decis Making 2012;32:690-70. http://dx.doi.org/10.1177/0272989X12455463.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
Appendix 1 Search strategies
Clinical effectiveness searches
Review of men-only randomised controlled trials and review of randomised controlled trials of men and women
MEDLINE (1948 to 18 May 2012), MEDLINE In-Process & Other Non-Indexed Citations (18 May 2012) and EMBASE (1980 to 2012 Week 20)
Ovid multifile search: http://shibboleth.ovid.com/
-
obesity/
-
(obesity adj2 (morbid or diabet$)).tw.
-
obesity, morbid/ use prmz
-
morbid obesity/ use emez
-
obes$.tw.
-
weight loss/ use prmz
-
weight reduction/ use emez
-
(weight adj1 (los$ or reduc$ or maint$ or control)).tw.
-
(diet adj5 weight).tw.
-
overweight.tw.
-
or/1-10
-
exp clinical trial/
-
Randomized Controlled Trials as Topic/
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomi?ed.ab.
-
randomization/ use emez
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
or/12-22
-
exp animals/ not humans/
-
23 not 24
-
11 and 25
-
(letter or editorial or comment or note).pt.
-
26 not 27
-
limit 28 to (“all infant (birth to 23 months)” or “all child (0 to 18 years)” or “newborn infant (birth to 1 month)” or “infant (1 to 23 months)” or “preschool child (2 to 5 years)” or “child (6 to 12 years)” or “adolescent (13 to 18 years)”)
-
limit 28 to (embryo or infant or child or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>)
-
28 not 29
-
28 not 30
-
31 or 32
-
limit 33 to yr=”2001 – current”
Cumulative Index to Nursing and Allied Health Literature (1981 to May 2012)
-
(MH “Obesity+”)
-
(MH “Abdominal Fat”)
-
(MH “Body Mass Index”) OR (MH “Body Weight+”)
-
TX Obes* n2 morbid*
-
TX Obes* n2 diabet*
-
TX overweight or obes*
-
TX obes* n3 abdom*
-
TX fat n3 abdom*
-
(S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8)
-
(MH “Weight Loss”)
-
(MH “Diet, Reducing”) OR (MH “Weight Control”) OR (MH “Weight Reduction Programs”) OR (MH “Diet, Fat-Restricted”)
-
TX weight n1 los*
-
TX weight n1 reduc*
-
TX weight n1 maint*
-
TX weight n1 control*
-
TX weight n5 diet
-
TX diet n2 restrict*
-
TX calorie* n3 restrict*
-
TX low n1 calorie
-
(S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19)
-
(MH “Clinical Trials+”)
-
PT randomized controlled trial OR PT clinical trial
-
TX trial* or random* or placebo*
-
S21 or S22 or S23
-
S9 and S20 and S24
-
(MH “Animals+”)
-
S25 not S26
-
PT pamphlet or letter or comment or commentary or editorial
-
S27 not S28
-
S27 not S28 Limiters – Exclude MEDLINE records; Age Groups: Fetus, Conception to Birth, Infant, Newborn: birth-1 month, Infant: 1–23 months, Child, Preschool: 2–5 years, Child: 6–12 years, Adolescent: 13–18 years
-
S29 not S30 Limiters – Published Date from: 20010101–20121231; Exclude MEDLINE records
PsycINFO (1806 to May 2012)
-
DE “Obesity”
-
DE “Body Weight” OR DE “Overweight” OR DE “Obesity”
-
DE “Body Fat”
-
DE “Body Mass Index”
-
TX Obes* n2 morbid*
-
TX Obes* n2 diabet*
-
TX overweight or obes*
-
TX obes* n3 abdom*
-
TX fat n3 abdom*
-
S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9
-
DE “Weight Loss" OR DE “Weight Control”
-
DE “Dietary Restraint”
-
TX weight n1 los*
-
TX weight n1 reduc*
-
TX weight n1 maint*
-
TX weight n1 control*
-
TX weight n5 diet
-
TX diet n2 restrict*
-
TX calorie* n3 restrict*
-
S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19
-
DE “Clinical Trials”
-
TX trial* or random* or placebo*
-
S21 or S22
-
S10 and S20 and S23 Limiters – Age Groups: Childhood (birth–12 yrs), Neonatal (birth–1 mo), Infancy (2–23 mo), Preschool Age (2–5 yrs), School Age (6–12 yrs), Adolescence (13–17 yrs)
-
( S10 and S20 and S23 ) NOT S24
-
PZ pamphlet or letter or comment or commentary or editorial
-
S25 not S26 Limiters – Publication Year from: 2001–2012
Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 5, 2012)
-
MeSH descriptor Obesity explode tree 3
-
MeSH descriptor Weight Loss, this term only
-
(obesity near/2 (morbid or diabet*)):ti or (obesity near/2 (morbid or diabet*)):ab
-
(overweight or obes*):ti or (overweight or obes*):ab
-
(#1 OR #2 OR #3 OR #4)
-
(#5), from 2001 to 2012
Cochrane Database of Systematic Reviews (The Cochrane Library, Issue 5, 2012)
-
MeSH descriptor Obesity explode tree 3
-
MeSH descriptor Weight Loss, this term only
-
(obesity near/2 (morbid or diabet*)):ti or (obesity near/2 (morbid or diabet*)):ab
-
(overweight or obes*):ti or (overweight or obes*):ab
-
(#1 OR #2 OR #3 OR #4)
-
(#5), from 2001 to 2012
Database of Abstracts of Reviews of Effects, NHS Economic Evaluation Database and Health Technology Assessment database (May 2012)
Centre for Reviews and Dissemination: www.crd.york.ac.uk
-
MeSH DESCRIPTOR weight loss
-
MeSH DESCRIPTOR obesity
-
MeSH DESCRIPTOR obesity, morbid
-
(obesity adj2 (morbid or diabet*))
-
(overweight or obes*):TI
-
#1 OR #2 OR #3 OR #4 OR #5
-
FROM 2001 TO 2012
-
#6 AND #7
Trials
ClinicalTrials.gov
CenterWatch
Controlled Trials
International Clinical Trials Registry
Review of UK interventions
MEDLINE (1948 to 17 July 2012), MEDLINE In-Process & Other Non-Indexed Citations (17 July 2012) and EMBASE (1980 to 2012 Week 29)
Ovid multifile search: http://shibboleth.ovid.com/
-
*obesity/
-
obesity hypoventilation syndrome/
-
abdominal obesity/ use emez
-
morbid obesity/ use emez
-
diabetic obesity/ use emez
-
obesity, abdominal/ use prmz
-
obesity, morbid/ use prmz
-
(overweight or obes$).ti.
-
(obes$ adj1 (morbid or diabet$ or abdom$ or central)).tw.
-
or/1–9
-
*weight loss/ use prmz
-
*weight reduction/ use emez
-
(weight adj1 (los$ or reduc$ or maint$ or control or manag*)).tw.
-
(reduc$ adj2 (waist adj3 (ratio or circumference))).tw.
-
(reduc$ adj2 (bmi or body mass index)).tw.
-
(obesity adj1 manag*).tw.
-
(anti obesity or antiobesity).tw.
-
or/11–16
-
comparative study/ use prmz
-
follow-up studies/ use prmz
-
time factors/ use prmz
-
Treatment outcome/ use emez
-
major clinical study/ use emez
-
controlled study/ use emez
-
clinical trial/ use emez
-
(chang$ or evaluat$ or reviewed or baseline).tw.
-
((prospective$ or retrospective$) adj1 (study or studies)).tw.
-
(cohort$ or case series).tw.
-
((compare$ or compara$) adj1 (study or studies)).tw.
-
or/19–29
-
10 and 18 and 30
-
limit 31 to (“all infant (birth to 23 months)” or “all child (0 to 18 years)” or “newborn infant (birth to 1 month)” or “infant (1 to 23 months)” or “preschool child (2 to 5 years)” or “child (6 to 12 years)” or “adolescent (13 to 18 years)”)
-
31 not 32
-
limit 31 to (embryo or infant or child or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>)
-
31 not 34
-
33 or 35
-
exp animals/ not humans/
-
exp nonhuman/ not human/
-
37 or 38
-
36 not 39
-
(letter or editorial or comment or note or review or Video-Audio Media).pt.
-
40 not 41
-
exp great britain/ use prmz
-
united kingdom/ use emez
-
(united kingdom or uk or britain or scotland or england or wales or northern ireland or british or irish or scottish or welsh or english).tw.
-
(United kingdom or uk or Britain or scotland or England or wales or Ireland or London or Birmingham or Leeds or Glasgow or Sheffield or Bradford or Edinburgh or Liverpool or Manchester or Bristol or Wakefield or Cardiff or Coventry or Nottingham or Leicester or Sunderland or Belfast or Newcastle upon Tyne or Brighton or Hull or Plymouth or Stoke-on-Trent or Wolverhampton or Derby or Swansea or Southampton or Salford or Aberdeen or Westminster or Portsmouth or York or Peterborough or Dundee or Lancaster or Oxford or Newport or Preston or Norwich or Chester or Cambridge or Salisbury or Exeter or Gloucester or Lisburn or Chichester or Winchester or Londonderry or Carlisle or Worcester or Bath or Durham or Lincoln or Hereford or Armagh or Inverness or Stirling or Canterbury or Lichfield or Newry or Ripon or Bangor or Truro or Ely or Wells).ad.
-
(United kingdom or uk or Britain or scotland or England or wales or Ireland or London or Birmingham or Leeds or Glasgow or Sheffield or Bradford or Edinburgh or Liverpool or Manchester or Bristol or Wakefield or Cardiff or Coventry or Nottingham or Leicester or Sunderland or Belfast or Newcastle upon Tyne or Brighton or Hull or Plymouth or Stoke-on-Trent or Wolverhampton or Derby or Swansea or Southampton or Salford or Aberdeen or Westminster or Portsmouth or York or Peterborough or Dundee or Lancaster or Oxford or Newport or Preston or Norwich or Chester or Cambridge or Salisbury or Exeter or Gloucester or Lisburn or Chichester or Winchester or Londonderry or Carlisle or Worcester or Bath or Durham or Lincoln or Hereford or Armagh or Inverness or Stirling or Canterbury or Lichfield or Newry or Ripon or Bangor or Truro or Ely or Wells).in.
-
or/43–47
-
42 and 48
-
male/
-
(men or male or males).tw.
-
or/50–51
-
49 and 52
-
(female not male).tw.
-
(women not men).tw.
-
53 not (54 or 55)
PsycINFO (1806 to July 2012)
-
DE “Obesity”
-
TX Obes* n2 morbid*
-
TX Obes* n2 diabet*
-
TX overweight or obes*
-
TX obes* n3 abdom*
-
TX central n3 obes*
-
S1 or S2 or S3 or S4 or S5 or S6
-
DE “Weight Loss”
-
DE “Weight Control”
-
TX weight n1 los*
-
TX weight n1 reduc*
-
TX weight n1 maint*
-
TX weight n1 control*
-
TX reduc* n2 bmi
-
TX reduc* n2 body mass index
-
TX reduc* n2 waist circumference
-
S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16
-
AF United kingdom or uk or Britain or scotland or England or wales or Ireland or London or Birmingham or Leeds or Glasgow or Sheffield or Bradford or Edinburgh or Liverpool or Manchester or Bristol or Wakefield or Cardiff or Coventry or Nottingham or Leicester or Sunderland or Belfast or Newcastle upon Tyne or Brighton or Hull or Plymouth or Stoke-on-Trent or Wolverhampton or Derby or Swansea or Southampton or Salford or Aberdeen or Westminster or Portsmouth or York or Peterborough or Dundee or Lancaster or Oxford or Newport or Preston or Norwich or Chester or Cambridge or Salisbury or Exeter or Gloucester or Lisburn or Chichester or Winchester or Londonderry or Carlisle or Worcester or Bath or Durham or Lincoln or Hereford or Armagh or Inverness or Stirling or Canterbury or Lichfield or Newry or Ripon or Bangor or Truro or Ely or Wells
-
TX women NOT TX men
-
TX female not TX male
-
( TX child* or TX adolescen* ) NOT TX adult*
Social Sciences Citation Index (1970 to July 2012) and Conference Proceedings Citation Index – Social Science & Humanities (1990 to July 2012)
-
TS=(obes* or overweight)
-
TS=(obes* near/2 morbid*)
-
TS=(obes* near/2 diabet*)
-
TS=(obes* near/2 central)
-
TS=(obes* near/2 abdom*)
-
#5 OR #4 OR #3 OR #2 OR #1
-
TS=(weight near/1 los*)
-
TS=(weight near/1 reduc* )
-
TS=(weight near/1 maint*)
-
TS=(weight near/1 control*)
-
TS=(weight near/1 manag*)
-
TS=(reduc* near/2 bmi)
-
TS=(reduc* SAME (body mass index))
-
TS=(reduc* SAME (waist circumference))
-
TS= (obesity near/1 manag*)
-
TS=(antiobesity or anti obesity or anti-obesity)
-
#16 OR #15 OR 14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7
-
AD=(United kingdom or uk or Britain or scotland or England or wales or Ireland or London or Birmingham or Leeds or Glasgow or Sheffield or Bradford or Edinburgh or Liverpool or Manchester or Bristol or Wakefield or Cardiff or Coventry or Nottingham or Leicester or Sunderland or Belfast or Newcastle upon Tyne or Brighton or Hull or Plymouth or Stoke-on-Trent or Wolverhampton or Derby or Swansea or Southampton or Salford or Aberdeen or Westminster or Portsmouth or York or Peterborough or Dundee or Lancaster or Oxford or Newport or Preston or Norwich or Chester or Cambridge or Salisbury or Exeter or Gloucester or Lisburn or Chichester or Winchester or Londonderry or Carlisle or Worcester or Bath or Durham or Lincoln or Hereford or Armagh or Inverness or Stirling or Canterbury or Lichfield or Newry or Ripon or Bangor or Truro or Ely or Wells)
-
#6 and #14 and #15
-
TS=(female not male)
-
TS=(women not men)
-
TS=((child* or adolescen* or teenage* or infant*) not adult*)
-
#16 NOT #17
-
#20 NOT #18
-
#21 NOT #19
Review of engagement
MEDLINE (1948 to 25 July 2012), MEDLINE In-Process & Other Non-Indexed Citations (25 July 2012) and EMBASE (1980 to 2012 Week 30)
Ovid multifile search: http://shibboleth.ovid.com/
-
*obesity/
-
obesity hypoventilation syndrome/
-
abdominal obesity/ use emez
-
morbid obesity/ use emez
-
diabetic obesity/ use emez
-
obesity, abdominal/ use prmz
-
obesity, morbid/ use prmz
-
(overweight or obes$).ti.
-
(obes$ adj1 (morbid or diabet$ or abdom$ or central)).tw.
-
*weight loss/
-
*weight reduction/ use emez
-
(weight adj1 (los$ or reduc$ or maint$ or control or manag$)).tw.
-
(reduc$ adj2 (waist adj3 (ratio or circumference))).tw.
-
(reduc$ adj2 (bmi or body mass index)).tw.
-
(anti obesity or antiobesity).tw.
-
(obesity adj1 manag$).tw.
-
or/1–16
-
*”patient acceptance of health care”/ or *patient compliance/ or *medication adherence/ or *patient participation/ or exp *patient satisfaction/ or treatment refusal/
-
exp health promotion/
-
exp patient attitude/
-
*patient dropouts/ use prmz
-
exp patient compliance/
-
exp Consumer Participation/
-
(uptake or retention or retain or engag$ or particip$ or motivat$ or encourag$ or attrition or dropout or promot$ or recruit$ or involv$).tw.
-
or/18-24
-
((male or men?) adj3 (uptake or retention or retain or engag$ or particip$ or motivat$ or encourag$ or attrition or dropout or promot$ or recruit$ or involv$)).tw.
-
((service$ or program$ or scheme$ or initiative$ or intervention$ or diet$) adj3 ((uptake or retention or retain or engag$ or particip$ or motivat$ or encourag$ or attrition or dropout or promot$ or recruit$ or involv$) adj3 (male or men?))).tw.
-
((uptake or retention or retain or engag$ or particip$ or motivat$ or encourag$ or attrition or dropout or promot$ or recruit$ or involv$) adj3 (men? or male) adj3 ((obesity adj1 manag$) or (weight adj1 (los$ or reduc$ or maint$ or control or manag$)) or (overweight or obes$))).tw.
-
25 and 28
-
17 and 26
-
17 and 27
-
or/29–31
-
limit 32 to (embryo or infant or child or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>)
-
32 not 33
-
limit 32 to (“all infant (birth to 23 months)” or “all child (0 to 18 years)” or “newborn infant (birth to 1 month)” or “infant (1 to 23 months)” or “preschool child (2 to 5 years)” or “child (6 to 12 years)” or “adolescent (13 to 18 years)”)
-
32 not 35
-
34 or 36
-
exp animals/ not humans/
-
37 not 38
-
(female not male).tw.
-
(women not men).tw.
-
39 not (40 or 41)
PsycINFO (1806 to July 2012)
-
DE “Obesity”
-
DE “Overweight”
-
DE “Weight Loss”
-
DE “Weight Control”
-
TX Obes* n2 morbid*
-
TX Obes* n2 diabet*
-
TX overweight or obes*
-
TX obes* n3 abdom*
-
TX central n3 obes*
-
TX weight n1 los*
-
TX weight n1 reduc*
-
TX weight n1 maint*
-
TX weight n1 control*
-
TX reduc* n2 bmi
-
TX reduc* n2 body mass index
-
TX reduc* n2 waist circumference
-
TX obesity n1 manag*
-
TX anti#obesity
-
(S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18)
-
DE “Treatment Compliance” OR DE “Client Participation” OR DE “Treatment Barriers” OR DE “Treatment Dropouts” OR DE “Treatment Refusal”
-
DE ”Client Attitudes” OR DE ”Client Satisfaction”
-
TX (uptake or retention or retain or engag* or particip* or motivat* or encourag* or attrition or dropout* or promot* or recruit* or involv*)
-
S20 or S21 or S22
-
( TX (uptake or retention or retain or engag* or particip* or motivat* or encourag* or attrition or dropout* or promot* or recruit* or involv*) ) N3 ( TX (male or men#) )
-
( TX (uptake or retention or retain or engag* or particip* or motivat* or encourag* or attrition or dropout* or promot* or recruit* or involv*) ) N3 ( TX (male or men#) ) N3 ( TX (service* or program* or scheme* or initiative* or intervention* or diet*) )
-
( TX (uptake or retention or retain or engag* or particip* or motivat* or encourag* or attrition or dropout* or promot* or recruit* or involv*) ) N3 ( TX (male or men#) ) N3 (S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18)
-
S23 and S26
-
S19 and S25
-
S19 and S24
-
S27 or S28 or S29
-
TX women NOT TX men
-
TX female not TX male
-
S30 NOT S31
-
S33 NOT S32
-
( TX child* or TX adolescen* ) NOT TX adult*
-
S34 NOT S35
Cumulative Index to Nursing and Allied Health Literature (1981 to July 2012)
-
(MH “Obesity+”)
-
(MH “Body Mass Index”) OR (MH “Body Weight+”)
-
TX Obes* n2 morbid*
-
TX Obes* n2 diabet*
-
TX overweight or obes*
-
TX obes* n3 abdom*
-
TX fat n3 abdom*
-
(MH “Weight Loss”)
-
TX weight n1 los*
-
TX weight n1 reduc*
-
TX weight n1 maint*
-
TX weight n1 control*
-
TX weight n1 manag*
-
TX reduc* n2 bmi
-
TX reduc* n2 body mass index
-
TX reduc* n2 waist circumference
-
TX obesity n1 manag*
-
TX anti#obesity
-
S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18
-
(MH “Patient Compliance+”) OR (MH “Treatment Refusal”)
-
(MH “Research Dropouts”) OR (MH “Patient Dropouts”)
-
(MH “Consumer Participation”)
-
(MH “Patient Attitudes”)
-
TX (uptake or retention or retain or engag* or particip* or motivat* or encourag* or attrition or dropout* or promot* or recruit* or involv*)
-
S20 or S21 or S22 or S23 or S24
-
( TX (uptake or retention or retain or engag* or particip* or motivat* or encourag* or attrition or dropout* or promot* or recruit* or involv*) ) N3 ( TX (male or men#) )
-
( TX (uptake or retention or retain or engag* or particip* or motivat* or encourag* or attrition or dropout* or promot* or recruit* or involv*) ) N3 ( TX (male or men#) ) N3 ( TX (service* or program* or scheme* or initiative* or intervention* or diet*) )
-
( TX (uptake or retention or retain or engag* or particip* or motivat* or encourag* or attrition or dropout* or promot* or recruit* or involv*) ) N3 ( TX (male or men#) ) N3 S19
-
S25 and S28
-
S19 and S27
-
S19 and S26
-
S29 or S30 or S31
-
TX women NOT TX men
-
TX female not TX male
-
S32 not S33
-
S35 not S34
-
( TX child* or TX adolescen* ) NOT TX adult*
-
S36 NOT S37
Applied Social Sciences Index and Abstracts (1987 to July 2012)
(((uptake or retention or retain or engag* or particip* or motivat* or encourag* or attrition or dropout* or promot* or recruit* or involv*) within 3 (male or males or men or men's)) within 3 ((obes* or overweight or (obes* within 2 control*)) or ((obes* within 2 central) or (obes* within 2 abdom) or (obes* within 2 manag*)) or ((weight within 2 manag*) or (weight within 1 control*) or (weight within 1 los*)) or ((weight within 1 reduc*) or (weight within 1 maint*) or (reduc* within 2 bmi)) or ((reduc* within 2 body mass index) or (reduc* within 5 circumference))))
Or
(((obes* or overweight or (obes* within 2 control*)) or ((obes* within 2 central) or (obes* within 2 abdom) or (obes* within 2 manag*)) or ((weight within 2 manag*) or (weight within 1 control*) or (weight within 1 los*)) or ((weight within 1 reduc*) or (weight within 1 maint*) or (reduc* within 2 bmi)) or ((reduc* within 2 body mass index) or (reduc* within 5 circumference))) And ((uptake or retention or retain or engag* or particip* or motivat* or encourag* or attrition or dropout* or promot* or recruit* or involv*) within 3 (male or males or men or men's)))
Or
(((obes* or overweight or (obes* within 2 control*)) or ((obes* within 2 central) or (obes* within 2 abdom) or (obes* within 2 manag*)) or ((weight within 2 manag*) or (weight within 1 control*) or (weight within 1 los*)) or ((weight within 1 reduc*) or (weight within 1 maint*) or (reduc* within 2 bmi)) or ((reduc* within 2 body mass index) or (reduc* within 5 circumference))) And (((uptake or retention or retain or engag* or particip* or motivat* or encourag* or attrition or dropout* or promot* or recruit* or involv*) within 3 (male or males or men or men's)) within 3 (service* or program* or scheme* or initiative* or intervention* or diet*)))
Review of qualitative evidence
MEDLINE (1948 to 30 July 2012), MEDLINE In-Process & Other Non-Indexed Citations (30 July 2012) and EMBASE (1980 to 2012 Week 31)
Ovid multifile search: http://shibboleth.ovid.com/
-
qualitative research/
-
exp questionnaires/ use prmz
-
exp questionnaire/ use oemezd
-
exp interviews as topic/ use prmz
-
exp interview/ use oemezd
-
(qualitative or interview$ or focus group$ or questionnaire$ or survey$).tw.
-
(ethno$ or grounded or thematic or interpretive or narrative or realist$ or meta stud$ or experience?).tw.
-
or/1-7
-
*obesity/
-
obesity hypoventilation syndrome/
-
abdominal obesity/ use oemezd
-
morbid obesity/ use oemezd
-
diabetic obesity/ use oemezd
-
obesity, abdominal/ use prmz
-
obesity, morbid/ use prmz
-
(overweight or obes$).ti.
-
(obes$ adj1 (morbid or diabet$ or abdom$ or central)).tw.
-
or/9–17
-
*weight loss/
-
*weight reduction/ use oemezd
-
(weight adj1 (los$ or reduc$ or maint$ or control or manag$)).tw.
-
(reduc$ adj2 (waist adj3 (ratio or circumference))).tw.
-
(reduc$ adj2 (bmi or body mass index)).tw.
-
anti obesity.tw.
-
(obesity adj1 manag$).tw.
-
or/19–25
-
8 and 18 and 26
-
limit 27 to (“all infant (birth to 23 months)” or “all child (0 to 18 years)” or “newborn infant (birth to 1 month)” or “infant (1 to 23 months)” or “preschool child (2 to 5 years)” or “child (6 to 12 years)” or “adolescent (13 to 18 years)”)
-
27 not 28
-
limit 27 to (embryo or infant or child or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>)
-
27 not 30
-
29 or 31
-
(female not male).tw.
-
(women not men).tw.
-
33 or 34
-
32 not 35
-
or/19-21,24-25
-
8 and 37
-
(men or male or males).tw.
-
38 and 39
-
36 not 40
-
exp animals/ not humans/
-
remove duplicates from 36
-
limit 43 to yr=”2012 -Current”
-
(20111$ or 2012$).ed. use prmz
-
(20114$ or 20115$ or 2012$).em. use oemezd
-
43 and 45
-
43 and 46
-
44 or 47 or 48
Cumulative Index to Nursing and Allied Health Literature (1981 to July 2012)
Search 1:
-
(MH “Obesity”) OR (MH “Obesity, Morbid”)
-
TX Obes* n2 morbid*
-
TX Obes* n2 diabet*
-
TX overweight or obes*
-
TX obes* n3 abdom*
-
TX central n3 obes*
-
S1 or S2 or S3 or S4 or S5 or S6
-
(MH “Weight Loss”)
-
(MH “Weight Control”)
-
TX weight n1 los*
-
TX weight n1 reduc*
-
TX weight n1 maint*
-
TX weight n1 control*
-
TX reduc* n2 bmi
-
TX reduc* n2 body mass index
-
TX reduc* n2 waist circumference
-
S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16
-
(MH “Qualitative Studies+”)
-
(MH “Interviews+”)
-
(MH “Narratives”)
-
(MH “Projective Techniques+”)
-
(MH “Questionnaires+”)
-
(MH “Focus Groups”)
-
TX qualitative or interview* or focus group* or questionnaire* or survey* or ethno* or grounded or thematic or interpretive or narrative or realist* or meta stud*
-
S18 or S19 or S20 or S21 or S22 or S23 or S24
-
S7 and S17 and S25
-
TX female not male
-
TX women not men
-
S27 or S28
-
S26 not S29
-
( (MH “Child+”) OR (MH “Infant+”) ) NOT (MH “Adult+”)
-
S30 not S31
Search 2:
-
(MH “Qualitative Studies+”)
-
(MH “Interviews+”)
-
(MH “Narratives”)
-
(MH “Projective Techniques+”)
-
(MH “Questionnaires+”)
-
(MH “Focus Groups”)
-
TX qualitative or interview* or focus group* or questionnaire* or survey* or ethno* or grounded or thematic or interpretive or narrative or realist* or meta stud*
-
S1 or S2 or S3 or S4 or S5 or S6 or S7
-
(MH “Weight Loss”)
-
(MH “Weight Control”)
-
TX weight n1 los*
-
TX weight n1 reduc*
-
TX weight n1 maint*
-
TX weight n1 control*
-
TX weight n1 manag*
-
TX obesity n1 manag*
-
TX anti#obesity
-
S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17
-
TX men or male or males
-
S8 and S18 and S19
-
( (MH “Child+”) OR (MH “Infant+”) ) NOT (MH “Adult+”)
-
S20 not S21 Exclude MEDLINE records Published Date from: 19900101-20121231
Social Sciences Citation Index (1970 to July 2012) and Conference Proceedings Citation Index – Social Science & Humanities (1990 to July 2012)
Search 1:
-
TS=(obes* or overweight)
-
TS=(obes* near/2 morbid*)
-
TS=(obes* near/2 diabet*)
-
TS=(obes* near/2 central)
-
TS=(obes* near/2 abdom*)
-
#5 OR #4 OR #3 OR #2 OR #1
-
TS=(weight near/1 los*)
-
TS=(weight near/1 reduc* )
-
TS=(weight near/1 maint*)
-
TS=(weight near/1 control*)
-
TS=(reduc* near/2 bmi)
-
TS=(reduc* SAME (body mass index))
-
TS=(reduc* SAME (waist circumference))
-
#13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7
-
TS=(qualitative or survey* or questionnaire* or focus group* or interview* or ethno* or grounded theory or interpretive or narrative or realist* or meta stud*)
-
#15 AND #14 AND #6
-
TS=(female not male)
-
TS=(women not men)
-
#18 OR #17
-
#16 not #19
-
TS=((child* or adolescen* or teenage* or infant*) not adult*)
-
#20 not #21
Search 2:
-
TS=(qualitative or survey* or questionnaire* or focus group* or interview* or ethno* or grounded theory or interpretive or narrative or realist* or meta stud*)
-
TS=(weight near/1 los*)
-
TS=(weight near/1 reduc* )
-
TS=(weight near/1 maint*)
-
TS=(weight near/1 control*)
-
TS=(weight near/1 manag*)
-
TS=(anti-obesity or anti obesity or antiobesity)
-
TS=(obesity near/1 manag*)
-
#8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2
-
TS=(male or males or men)
-
#10 AND #9 AND #1
PsycINFO (1806 to July 2012)
Search 1:
-
DE “Obesity”
-
TX Obes* n2 morbid*
-
TX Obes* n2 diabet*
-
TX overweight or obes*
-
TX obes* n3 abdom*
-
TX central n3 obes*
-
S6 or S5 or S4 or S3 or S2 or S1
-
DE “Weight Loss”
-
DE “Weight Control”
-
TX weight n1 los*
-
TX weight n1 reduc*
-
TX weight n1 maint*
-
TX weight n1 control*
-
TX reduc* n2 bmi
-
TX reduc* n2 body mass index
-
TX reduc* n2 waist circumference
-
S16 or S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8
-
TX qualitative or interview* or focus group* or questionnaire* or survey* or ethno* or grounded or thematic or interpretive or narrative or realist* or meta stud*
-
DE “Questionnaires” OR DE “Surveys” OR DE “Consumer Surveys” OR DE “Mail Surveys” OR DE “Telephone Surveys” OR DE “Questionnaires” OR DE “General Health Questionnaire” OR DE “Qualitative Research” OR DE "Grounded Theory” OR DE “Ethnography” OR DE “Narratives” OR DE “Projective Techniques” OR DE “Holtzman Inkblot Technique” OR DE “Projective Personality Measures”
-
S19 or S19
-
S7 and S17 and S20
-
TX female NOT TX male
-
TX women NOT TX men
-
S21 not S22
-
S24 not S23
-
( TX child* or TX adolescen* ) NOT TX adult*
-
S25 NOT S26
Search 2:
-
DE “Questionnaires” OR DE “Surveys” OR DE “Consumer Surveys” OR DE “Mail Surveys” OR DE “Telephone Surveys” OR DE “Questionnaires” OR DE “General Health Questionnaire” OR DE “Qualitative Research” OR DE “Grounded Theory” OR DE “Ethnography” OR DE “Narratives” OR DE “Projective Techniques” OR DE “Holtzman Inkblot Technique” OR DE “Projective Personality Measures”
-
TX qualitative or interview* or focus group* or questionnaire* or survey* or ethno* or grounded or thematic or interpretive or narrative or realist* or meta stud*
-
S1 or S2
-
DE “Weight Loss”
-
DE “Weight Control”
-
TX weight n1 los*
-
TX weight n1 reduc*
-
TX weight n1 maint*
-
TX weight n1 control*
-
TX weight n1 manag*
-
TX obesity n1 manag*
-
TX anti#obesity
-
S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12
-
TX men or male or males
-
S3 and S13 and S14 Publication Year from: 1990-2012
Education Resources Information Center (1966 to July 2012)
(((cabs(weight near/1 los*)) OR (cabs(weight near/1 reduc*) or cabs(weight near/1 maint*))) OR (cabs(weight near/1 control*) or cabs(reduc* near/2 bmi))) OR ((cabs(reduc* near/2 body mass index) or cabs(reduc* near/2 waist circumference))) AND (((cabs(obes*)) OR (cabs(overweight) or cabs(obes* near/2 morbid*))) OR (cabs(obes* near/2 diabet*) or cabs(obes* near/2 central))) OR ((cabs(obes* near/2 abdom*) or cabs(fat near/2 abdom)))
Education Resources Information Center (1966 to July 2012)
((DE=((qualitative research) or (qualitative methods) or interviews) or DE=((cognitive interviews) or (exit interviews) or (fitness for interview)) or DE=((focus group interviews) or (semistructured interviews) or (structured interviews)) or DE=((structured behavioural interviews) or (structured clinical interviews) or (videotaped interviews)) or DE=(questionnaires or (mail surveys) or (multiple choice questionnaires)) or DE=(polls or (exit polls) or (opinion polls)) or DE=(semistructured questionnaires)) or((qualitative or interview* or questionnaire*) or (survey* or ethno* or grounded) or (thematic or interpretive or narrative) or (realist* or (meta stud*)))) and (((weight within 1 los*) or (weight within 1 control*) or (weight within 1 reduc*)) or ((weight within 1 maint*) or (weight within 1 manag*) or (obesity within 1 manag*) or (anti*obesity))) and(men or males or male)
1990–2012
Applied Social Sciences Index and Abstracts (1987 to July 2012)
Search 1:
-
obes* or overweight
-
((obes* within 2 morbid*) or (obes* within 2 diabet*) or (obes* within 2 control*)) or ((obes* within 2 central) or (obes* within 2 abdom))
-
#1 or #2
-
((weight within 1 los*) or (weight within 1 control*) or (weight within 1 reduc*)) or ((weight within 1 maint*) or (reduc* within 2 bmi) or (reduc* within 2 body mass index)) or (reduc* within 5 circumference))
-
(DE=((qualitative research) or (qualitative methods) or interviews) or DE=((cognitive interviews) or (exit interviews) or (fitness for interview)) or DE=((focus group interviews) or (semistructured interviews) or (structured interviews)) or DE=((structured behavioural interviews) or (structured clinical interviews) or (videotaped interviews)) or DE=(questionnaires or (mail surveys) or (multiple choice questionnaires)) or DE=(polls or (exit polls) or (opinion polls)) or DE=(semistructured questionnaires)) or((qualitative or interview* or questionnaire*) or (survey* or ethno* or grounded) or (thematic or interpretive or narrative) or (realist* or (meta stud*)))
-
#3 and #4 and #5
Search 2:
((DE=((qualitative research) or (qualitative methods) or interviews) or DE=((cognitive interviews) or (exit interviews) or (fitness for interview)) or DE=((focus group interviews) or (semistructured interviews) or (structured interviews)) or DE=((structured behavioural interviews) or (structured clinical interviews) or (videotaped interviews)) or DE=(questionnaires or (mail surveys) or (multiple choice questionnaires)) or DE=(polls or (exit polls) or (opinion polls)) or DE=(semistructured questionnaires)) or((qualitative or interview* or questionnaire*) or (survey* or ethno* or grounded) or (thematic or interpretive or narrative) or (realist* or (meta stud*)))) and(((weight within 1 los*) or (weight within 1 control*) or (weight within 1 reduc*)) or ((weight within 1 maint*) or (weight within 1 manag*) or (obesity within 1 manag*) or (anti*obesity))) and(men or males or male)
1990–2012
Anthropology Plus (1957 to July 2012)
Search 1:
(kw: overweight or kw: obes*) or (kw: obes* n2 morbid*) or (kw: obes* n2 abdomin*) or (kw: obes* n2 diabet*) or (kw: obes* n2 central) and ((kw: weight n2 los*) or (kw: weight n2 reduc*) or (kw: weight n2 control*))
Search 2:
(kw: weight n2 los*) or (kw: weight n2 reduc*) or (kw: weight n2 control*) or (kw: weight n2 manag*) or (kw: obesity n2 manag*) or (kw: anti and kw: obesity) or (kw: anti-obesity) or (kw: antiobesity)
British Nursing Index (1994 to July 2012)
Ovid multifile search: http://shibboleth.ovid.com/
-
obesity/
-
(overweight or obes$).ti.
-
(obes$ adj1 (morbid or diabet$ or abdom$ or central)).tw.
-
or/1-3
-
research methods/ or “interviews and interviewing”/
-
(qualitative or interview$ or focus group$ or questionnaire$ or survey$).tw.
-
(ethno$ or grounded or thematic or interpretive or narrative or realist$ or meta stud$ or experience$).tw.
-
or/5–7
-
(female not male).tw.
-
(women not men).tw.
-
9 or 10
-
(child$ or adolescent$ or infant$).tw,hw.
-
4 and 8
-
13 not 11
-
14 not 12
Websites consulted
Picker Institute
Joanna Briggs Institute
Men’s Health Forum
Association for the Study of Obesity
fatmanslim.com
Cost-effectiveness searches
MEDLINE (1946 to 19 January 2012), MEDLINE In-Process & Other Non-Indexed Citations (19 January 2012) and EMBASE (1974 to 2012 Week 02)
Ovid multifile search: http://shibboleth.ovid.com/
-
exp “costs and cost analysis”/
-
cost-benefit analysis/
-
quality-adjusted life years/
-
economics,pharmaceutical/
-
exp budgets/
-
exp models, economic/
-
exp decision theory/
-
monte carlo method/
-
markov chains/
-
exp health status indicators/
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimis$)).ab.
-
economic$ model$.tw.
-
(economic$ or pharmacoeconomic$ or pharmaco-economic$).tw.
-
(price$ or pricing).tw.
-
(financial or finance or finances or financed).tw.
-
((value adj2 money) or monetary).tw.
-
markov$.tw.
-
monte carlo.tw.
-
(decision$ adj2 (tree? or analy$ or model$)).tw.
-
(standard adj1 gamble).tw.
-
trade off.tw.
-
or/1–22
-
*obesity/
-
*overweight/
-
obesity, morbid/ use prmz
-
morbid obesity/ use oemez
-
(obes$ or overweight).tw.
-
weight loss/ use prmz
-
weight reduction/ use oemez
-
(weight adj1 (los$ or reduc$ or maint$ or control or manag$)).tw.
-
(obesity adj1 management).tw.
-
(anti obesity or antiobesity).tw.
-
or/24–32
-
(men or male or males).tw.
-
*obesity/ec
-
*overweight/ec
-
or/36-37
-
(women not men).tw.
-
(female not male).tw.
-
38 not (39 or 40)
-
23 and 34 and 35
-
41 or 42
-
exp animals/ not humans/
-
43 not 44
-
(rat or rats).tw.
-
45 not 46
-
limit 47 to (“all infant (birth to 23 months)” or “all child (0 to 18 years)” or “all adult (19 plus years)” or “newborn infant (birth to 1 month)” or “infant (1 to 23 months)” or “preschool child (2 to 5 years)” or “child (6 to 12 years)” or “adolescent (13 to 18 years)”)
-
limit 47 to (embryo or infant or child or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>)
-
47 not 48
-
47 not 49
-
50 or 51
-
limit 52 to yr=”2009 -Current”
-
remove duplicates from 53
-
(letter or editorial or comment or note).pt.
-
54 not 55
NHS Economic Evaluation Database (January 2012)
Centre for Reviews and Dissemination: www.crd.york.ac.uk
-
MeSH DESCRIPTOR Obesity EXPLODE ALL TREES
-
MeSH DESCRIPTOR weight loss
-
MeSH DESCRIPTOR obesity
-
MeSH DESCRIPTOR obesity, morbid
-
(obesity adj2 (morbid or diabet*))
-
(overweight or obes*):TI
-
#2 OR #3 OR #4 OR #5 OR #6
-
IN NHSEED FROM 1990 TO 2012
-
#7 AND #8
Health Management Information Consortium (1979 to November 2011)
-
exp obesity/
-
weight loss/
-
(overweight or obes$).tw.
-
(weight adj1 (los$ or reduc$ or maint$ or control or manag$)).tw.
-
(obesity adj1 management).tw.
-
(anti obesity or antiobesity).tw.
-
or/1-6
-
exp “cost effectiveness”/
-
exp economic evaluation/
-
“cost benefit analysis”/
-
monte carlo methods/
-
cost$.tw.
-
((value adj2 money) or monetary).tw.
-
quality adjusted life years/
-
economic$ model$.tw.
-
(decision$ adj2 (tree? or analy$ or model$)).tw.
-
(standard adj1 gamble).tw.
-
trade off.tw.
-
or/8–18
-
7 and 19
-
limit 20 to yr=”1990 -Current”
-
(female not male).tw
-
(women not men).tw
-
21 not (22 or 23)
Cost-Effectiveness Analysis Registry (January 2012)
https://research.tufts-nemc.org/cear4/SearchingtheCEARegistry/SearchtheCEARegistry.aspx
obesity OR weight loss OR overweight
Research Papers in Economics (January 2012)
obesity or overweight or weight loss
List of professional and commercial organisations contacted
Association for the Study of Obesity
Boots Pharmacy
British Dietetic Association
Cambridge Weight Plan
Counterweight
Diet Chef
Dietitians in Obesity Management
fatmanslim
Go Lower
Jenny Craig
LighterLife
Men’s Health Forums of England and Wales, Scotland, and Ireland
Motivation
Nestlé
Online Slimming Club
ProHealthClinical
Rosemary Conley
Rotherham Institute for Obesity
Scottish Slimmers
Slimming World
Sure Slim
The Lose-It Coach
Trim Up Shape Down
Weight Care
Weight Medics
Weight Watchers
Appendix 2 Data extraction form (reviews of clinical effectiveness)
Version 2 October 2011
| Included in systematic review number | |||
| Trial author and date | |||
| First author institution | |||
| Study ID of any linked reports | |||
| Reference Manager number | |||
| Extracted by | |||
| Checked by | |||
| Location/setting | |||
| Period of study and duration of follow-up | |||
| Method of recruitment and sampling | |||
| Yes | No | Details (include whether single or multicentre, prospective/retrospective, etc.) | |
|---|---|---|---|
| RCT design? | |||
| Yes | No | Details (include whether single or multicentre, prospective/retrospective, etc.) | |
| Quasi-RCT design? | |||
| Yes | No | Details (include whether single or multicentre, prospective/retrospective, etc.) | |
| Other study design? | |||
| Yes | No | Details | |
| Pretreatment phase? | |||
| Yes | No | Details | |
| Subgroup analysis? | |||
| Yes | No | Details | |
| Groups comparable at baseline? | |||
| Participants’ general description (e.g. cardiac risk factors, etc.): Targeted particularly at: men Y/N, diabetics Y/N, impaired glucose tolerance Y/N, impaired fasting glucose Y/N, glycaemia Y/N, hypertensive Y/N, hyperlipidaemia Y/N |
|||
| Inclusion criteria | Exclusion criteria | ||
| Control group | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | |
|---|---|---|---|---|---|
| Type of intervention | |||||
| Individual/group/both | |||||
| Couple/family | |||||
| Lifestyle/drug/both | |||||
| Behaviour change (and underlying theory) | |||||
| Description of intervention (include length of meeting, duration, etc.) | |||||
| Timing of active intervention | |||||
| Duration | |||||
| Number of times contacted | |||||
| Frequency of contact | |||||
| Maximum length of follow-up | |||||
| Health professional involvement (role, timing) | |||||
| Other details of care | |||||
| Baseline characteristics | Control | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | Overall |
|---|---|---|---|---|---|---|
| Number of men enrolled | ||||||
| Number randomised (RCTs only) | ||||||
| Age (mean/median, SD/range) | ||||||
| Social class | ||||||
| Ethnic group | ||||||
| Smoking status | ||||||
| Weight (kg) | ||||||
| Height (m) | ||||||
| Reported BMI (kg/m2) (mean/median, SD/range) | ||||||
| Calculated BMI (kg/m2) | ||||||
| Waist circumference (give units) |
| Baseline characteristics | Control | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | Overall |
|---|---|---|---|---|---|---|
| Total cholesterol (give units) | ||||||
| LDL cholesterol (give units) | ||||||
| HDL cholesterol (give units) | ||||||
| Triglycerides (give units) | ||||||
| Systolic blood pressure (mmHg) | ||||||
| Diastolic blood pressure (mmHg) | ||||||
| HbA1c (%) | ||||||
| Fasting plasma glucose (give units) | ||||||
| Erectile dysfunction (specify measure and whether validated or not) | ||||||
| Quality of life | ||||||
| Generic (specify measure) | ||||||
| Disease specific (specify measure) | ||||||
| Other (specify) | ||||||
| Control | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | Overall | |
|---|---|---|---|---|---|---|
| Number eligible | ||||||
| Number assigned/selected to each group | ||||||
| Number withdrawn/lost to follow-up (with reasons) | ||||||
| Number present for weight at time = | ||||||
| Number withdrawn/lost to follow-up (with reasons) | ||||||
| Number present for weight at time = | ||||||
| Number withdrawn/lost to follow-up (with reasons) | ||||||
| Number present for weight at time = | ||||||
| Number withdrawn/lost to follow-up (with reasons) | ||||||
| Number assessed at end of study, with details | ||||||
| Number completed at end of study | ||||||
| % dropout at end of study | ||||||
| Number dead at end of study | ||||||
| Period of active intervention | ||||||
| Maximum period of follow-up |
| Outcome | Statistics | Timing (e.g. 1 year, 18 months, 2 years) | Control (n/N = ) | Treatment 1 (n/N = ) | Treatment 2 (n/N = ) | Treatment 3 (n/N = ) | Treatment 4 (n/N = ) |
|---|---|---|---|---|---|---|---|
| Weight (kg) | |||||||
| Height (m) | |||||||
| BMI (kg/m2) | |||||||
| % ideal body weight | |||||||
| Waist circumference (give units) | |||||||
| Total cholesterol (give units) | |||||||
| LDL cholesterol (give units) | |||||||
| HDL cholesterol (give units) |
| Outcome | Statistics | Timing (e.g. 1 year, 18 months, 2 years) | Control (n/N = ) | Treatment 1 (n/N = ) | Treatment 2 (n/N = ) | Treatment 3 (n/N = ) | Treatment 4 (n/N = ) |
|---|---|---|---|---|---|---|---|
| Triglycerides (give units) | |||||||
| Systolic blood pressure (mmHg) | |||||||
| Diastolic blood pressure (mmHg) | |||||||
| HbA1c (%) | |||||||
| Fasting plasma glucose (give units) | |||||||
| Erectile dysfunction (specify measure and whether validated or not) |
| Outcome | Statistics | Timing (e.g. 1 year, 18 months, 2 years) | Control (n/N = ) | Treatment 1 (n/N = ) | Treatment 2 (n/N = ) | Treatment 3 (n/N = ) | Treatment 4 (n/N = ) |
|---|---|---|---|---|---|---|---|
| Quality of life | |||||||
| Generic (specify measure) | |||||||
| Disease specific (specify measure) | |||||||
| Other (specify) | |||||||
| Deaths | |||||||
| Morbidity | |||||||
| Adverse events | |||||||
| Compliance |
| Baseline characteristics | Control | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | Overall |
|---|---|---|---|---|---|---|
| Number of women enrolled | ||||||
| Number randomised (RCTs only) | ||||||
| Age (mean/median, SD/range) | ||||||
| Social class | ||||||
| Ethnic group | ||||||
| Smoking status | ||||||
| Weight (kg) | ||||||
| Height (m) | ||||||
| Reported BMI (kg/m2) (mean/median, SD/range) | ||||||
| Calculated BMI (kg/m2) | ||||||
| Waist circumference (give units) |
| Baseline characteristics | Control | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | Overall |
|---|---|---|---|---|---|---|
| Total cholesterol (give units) | ||||||
| LDL cholesterol (give units) | ||||||
| HDL cholesterol (give units) | ||||||
| Triglycerides (give units) | ||||||
| Systolic blood pressure (mmHg) | ||||||
| Diastolic blood pressure (mmHg) | ||||||
| HbA1c (%) | ||||||
| Fasting plasma glucose (give units) | ||||||
| Quality of life | ||||||
| Generic (specify measure) | ||||||
| Disease specific (specify measure) | ||||||
| Other (specify) | ||||||
| Baseline characteristics | Control | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | Overall |
|---|---|---|---|---|---|---|
| Number eligible | ||||||
| Number assigned/selected to each group | ||||||
| Number withdrawn/lost to follow-up (with reasons) | ||||||
| Number present for weight at time = | ||||||
| Number withdrawn/lost to follow-up (with reasons) | ||||||
| Number present for weight at time = | ||||||
| Number withdrawn/lost to follow-up (with reasons) | ||||||
| Number present for weight at time = | ||||||
| Number withdrawn/lost to follow-up (with reasons) | ||||||
| Number assessed at end of study, with details | ||||||
| Number completed at end of study | ||||||
| % dropout at end of study | ||||||
| Number dead at end of study | ||||||
| Period of active intervention | ||||||
| Maximum period of follow-up |
| Outcome | Statistics and who measured | Timing (e.g. 1 year, 18 months, 2 years) | Control (n/N =) | Treatment 1 (n/N =) | Treatment 2 (n/N =) | Treatment 3 (n/N =) | Treatment 4 (n/N =) |
|---|---|---|---|---|---|---|---|
| Weight (kg) | |||||||
| Height (m) | |||||||
| BMI (kg/m2) | |||||||
| % ideal body weight | |||||||
| Waist circumference (give units) | |||||||
| Total cholesterol (give units) | |||||||
| LDL cholesterol (give units) | |||||||
| HDL cholesterol (give units) |
| Outcome | Statistics and who measured | Timing (e.g. 1 year, 18 months, 2 years) | Control (n/N =) | Treatment 1 (n/N =) | Treatment 2 (n/N =) | Treatment 3 (n/N =) | Treatment 4 (n/N =) |
|---|---|---|---|---|---|---|---|
| Triglycerides (give units) | |||||||
| Systolic blood pressure (mmHg) | |||||||
| Diastolic blood pressure (mmHg) | |||||||
| HbA1c (%) | |||||||
| Fasting plasma glucose (give units) |
| Outcome | Statistics and who measured | Timing (e.g. 1 year, 18 months, 2 years) | Control (n/N =) | Treatment 1 (n/N =) | Treatment 2 (n/N =) | Treatment 3 (n/N =) | Treatment 4 (n/N =) |
|---|---|---|---|---|---|---|---|
| Quality of life | |||||||
| Generic (specify measure) | |||||||
| Disease specific (specify measure) | |||||||
| Other (specify) | |||||||
| Deaths | |||||||
| Morbidity | |||||||
| Adverse events | |||||||
| Compliance | |||||||
| Timing (e.g. 1 year, 18 months, 2 years) | Control | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | |
|---|---|---|---|---|---|---|
| Measure of health benefits used in the economic analysis | ||||||
| Direct costs | ||||||
| Indirect costs | ||||||
| Currency | ||||||
| Statistical analysis of quantities/costs | ||||||
| Sensitivity analysis | ||||||
| Estimated benefits used in the economic evaluation | ||||||
| Costs results | ||||||
| Synthesis of costs and benefits | ||||||
| Authors’ conclusions |
| Formally evaluated (Y/N) | Control | Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | |
|---|---|---|---|---|---|---|
| Explicit strategies used to promote recruitment | ||||||
| Explicit strategies used to promote attendance/compliance or weight loss | ||||||
| Explicit strategies used to promote attendance/compliance/weight maintenance | ||||||
| Sex of programme facilitator and health-care professionals reported (give details) | ||||||
| Programme environment | ||||||
| Use of technology |
Appendix 3 Risk of bias form (review of men-only randomised controlled trials and randomised controlled trials of men and women compared)
RCT quality assessment form, version 1, January 2012
Study ID: Reviewer initials: Date:
| Question | Judgement (L = low, U = unclear, H = high) | A description that explains how the judgement was reached | ||
|---|---|---|---|---|
| L | U | H | ||
| Potential for selection bias at trial entry (quality of random allocation concealment) | ||||
1. Was allocation adequately concealed?
|
||||
2. Was random sequence generation adequately generated? (selection bias)
|
||||
| Potential for bias around time of treatment or during outcome assessment (blinding) (performance and detection bias) | ||||
3. Were participants ‘blind’ to treatment status?
|
||||
4. Were health-care providers ‘blind’ to treatment status? (performance bias)
|
||||
5. Were outcome assessors ‘blind’ to treatment status? (detection bias)
|
||||
| 6. Were the groups treated identically other than for the named interventions? (performance bias) | ||||
| Incomplete outcome data: potential for selection bias in analysis (attrition bias) | ||||
7. Was there a description of withdrawals, dropouts and those lost to follow-up?
|
||||
| 8. Was the analysis on an intention-to-treat basis (or is it possible to do so on available data)? i.e. (A) Are results reported for everyone who entered the trial?, (B) are participants analysed in the groups they were originally allocated to? If low for both, intention-to-treat analysis has been performed | ||||
9. Are reports of the study free of the suggestion of selective outcome reporting?
|
||||
10. Was the study apparently free of other bias that could put it at a high risk of bias? (validity, topic/design specific)
|
||||
Appendix 4 Review Body for Interventional Procedures quality assessment form (review of UK interventions and engagement)
Checklist for quality assessment of non-randomised studies (comparative and cohort studies)
Version 1, August 2012
Items specific to comparative studies are in italics
Assessor initial: Date evaluated: Study ID:
| Criteria | Yes | No | Unclear | Comments |
|---|---|---|---|---|
| 1. Were participants a representative sample selected from a relevant patient population, e.g. randomly selected from those seeking treatment despite age, duration of disease, primary or secondary disease and severity of disease? | ||||
| 2. Were the inclusion/exclusion criteria for participants clearly described? | ||||
| 3. Were participants entering the study at a similar point in their disease progression, i.e. severity of disease? | ||||
| 4. Was the selection of patients consecutive? | ||||
| 5. Was data collection undertaken prospectively? | ||||
| 6. Were the groups comparable on demographic characteristics and clinical features? | ||||
| 7. Was the intervention (and comparison) clearly defined? | ||||
| 8. Was the intervention undertaken by someone experienced at performing the procedure?a | ||||
| 9. Were the staff, place and facilities where the patients were treated appropriate for performing the procedure? (e.g. access to back-up facilities in hospital or special clinic) | ||||
| 10. Were any of the important outcomes considered, i.e. weight, clinical effectiveness, cost-effectiveness? | ||||
| 11. Were objective (valid and reliable) outcome measures used, including satisfaction scale? | ||||
| 12. Was the assessment of the main outcomes blind? | ||||
| 13. Was follow-up long enough (≥ 1 year) to detect important effects on outcomes of interest? | ||||
| 14. Was information provided on non-respondents, dropouts?b | ||||
| 15. Did the withdrawals/dropouts have similar characteristics to those of participants who completed the study and were they therefore unlikely to cause bias?c | ||||
| 16. Was the length of follow-up similar between comparison groups? | ||||
| 17. Were the important prognostic factors identified, e.g. age, duration of disease, disease severity?d | ||||
| 18. Were the analyses adjusted for confounding factors? |
Appendix 5 Campbell & Cochrane Equity Methods Group equity checklist (reviews of clinical effectiveness)
| Item | Yes | No | Unclear | Not reported | Notes |
|---|---|---|---|---|---|
| Equity pointer: social context of the study, e.g. was the study conducted in a particular setting that might target/exclude specific populations? | |||||
| Representativeness of sample: are participants in the study likely to be representative of the target population? | |||||
| Sociodemographic differences between withdrawals and exclusions? | |||||
| PROGRESS categories reported at baseline (indicate letters of those reported: place of residence, race, occupation, gender, religion, education and literacy, socioeconomic status, social capital) | |||||
| Did the intervention include strategies to address diversity/disadvantage? | |||||
| Was there a fidelity check? | |||||
| Were process measures taken? | |||||
| Providers (who, number, education/training in intervention delivery, ethnicity, etc., if potentially relevant to acceptance and uptake by participants) | |||||
| Was sustainability discussed by the authors? Was it a consideration in study development? | |||||
| Do the authors describe any political or organisational context? | |||||
| Were any partnerships described? | |||||
| Potential for author conflict, i.e. evidence that author or data collectors would benefit if results favoured the intervention under study or the control? | |||||
| Were outcomes relating to harms/unintended effects of the intervention described? |
Appendix 6 Statistical methods for the reviews of clinical effectiveness
Introduction
The following provides an equation for deriving the SD for the change in weight from baseline given the absolute value of the mean change in weight from baseline, as used in our previous systematic review. 23
Method
Summary statistics were provided from a series of trials representing 62 trial–treatment combinations of which four had no data. A linear regression was made of the SD of the mean change on the absolute mean change for weight.
Results
Of the 58 trial–treatment combinations, 43 reported both the mean change and the standard error of the mean change in body weight from baseline to the end of the first treatment phase whereas eight reported only the mean and seven reported neither. The plot of SD by the absolute value of the mean change ( Figure 44 ) shows two points for which both the absolute mean and the SD of the mean are close to zero; both were excluded from the linear regression, giving n = 41. The linear regression was also repeated with observation 13, which was influential, excluded, to see whether or not the regression coefficients changed ( Table 55 ).
| n | R 2 | Constant | Slope | ||||
|---|---|---|---|---|---|---|---|
| 41 | 53.7% | SD | = | 5.915 | + | 0.283 | × abs(mean) |
| 40 | 63.4% | SD | = | 5.694 | + | 0.328 | × abs(mean) |
FIGURE 44.
Scatterplot of the SD of the mean change in weight by the absolute mean change in weight. Observation 13 is labelled.
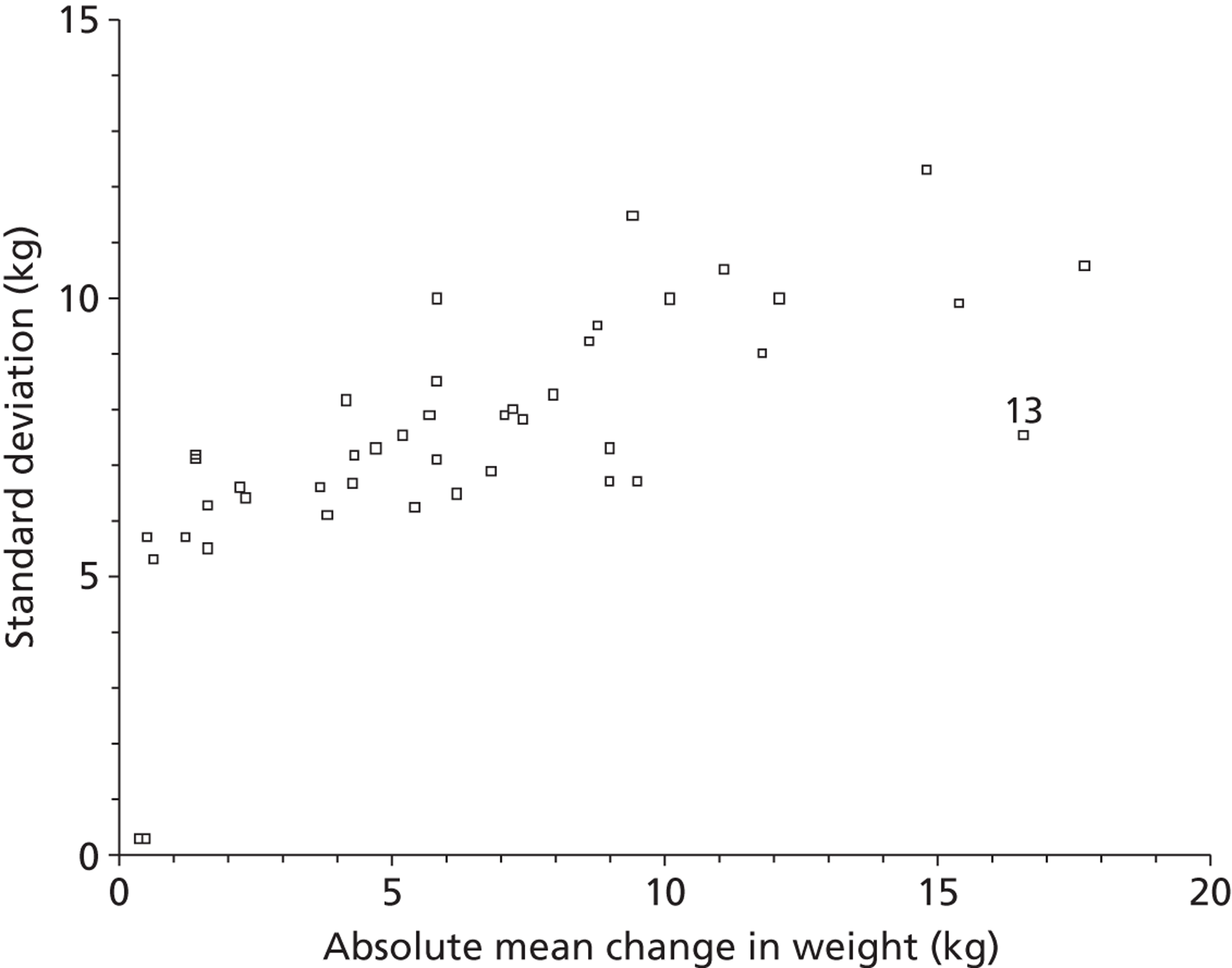
Discussion
The results from the two linear regressions were similar. Diagnostic plots (not shown) suggested that the regression could be improved by allowing for the increase in variation of the SD with increasing mean; however, this is unlikely to change the results.
Conclusion
When the mean change in weight from baseline (mean) is known but its SD is unknown then the SD of the mean change can be derived using the following equation:
Appendix 7 List of included studies: review of men-only randomised controlled trials
Benassi-Evans 2010
Benassi-Evans B, Clifton PM, Noakes M, Keogh JB, Fenech M. High protein–high red meat versus high carbohydrate weight loss diets do not differ in effect on genome stability and cell death in lymphocytes of overweight men. Mutagenesis 2009;24:271–7.
Borg 2002
Borg P, Kukkonen-Harjula K, Fogelholm M, Pasanen M. Effects of walking or resistance training on weight loss maintenance in obese, middle-aged men: a randomized trial. Int J Obes 2002;26:676–83.
Secondary publication
Kukkonen-Harjula KT, Borg PT, Nenonen AM, Fogelholm MG. Effects of a weight maintenance program with or without exercise on the metabolic syndrome: a randomized trial in obese men. Prev Med 2005;41:784–90.
Esposito 2004
Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D’Andrea F, et al. Effect of lifestyle changes on erectile dysfunction in obese men. JAMA 2004;291:2978–84.
Jeffery 1983
Jeffery RW, Gerber WM, Rosenthal BS, Lindquist RA. Monetary contracts in weight control: effectiveness of group and individual contracts of varying size. J Consult Clin Psychol 1983;51:242–8.
Secondary publications
Jeffery RW, Bjornson-Benson WM, Rosenthal BS, Lindquist RA, Johnson SL. Behavioral treatment of obesity with monetary contracting: two-year follow-up. Addict Behav 1984;9:311–13.
Multiple risk factor intervention trial. Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research Group. JAMA 1982;248:1465–77.
Khoo 2011
Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med 2011;8:2868–75.
King 1989
King AC, Frey-Hewitt B, Dreon DM, Wood PD. Diet vs exercise in weight maintenance. The effects of minimal intervention strategies on long-term outcomes in men. Arch Intern Med 1989;149:2741–6.
Morgan 2011
Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. 12-Month outcomes and process evaluation of the SHED-IT RCT: an internet-based weight loss program targeting men. Obesity 2011;19:142–51.
Patrick 2011
Patrick K, Calfas KJ, Norman GJ, Rosenberg D, Zabinski MF, Sallis JF, et al. Outcomes of a 12-month web-based intervention for overweight and obese men. Ann Behav Med 2011;42:391–401.
Pavlou 1989
Pavlou KN, Krey S, Steffee WP. Exercise as an adjunct to weight loss and maintenance in moderately obese subjects. Am J Clin Nutr 1989;49:1115–23.
van Aggel-Leijssen 2001
van Aggel-Leijssen DPC, Saris WHM, Hul GB, van Baak MA. Short-term effects of weight loss with or without low-intensity exercise training on fat metabolism in obese men. Am J Clin Nutr 2001;73:523–31.
Secondary publications
van Aggel-Leijssen DP, Saris WH, Hul GB, van Baak MA. Long-term effects of low-intensity exercise training on fat metabolism in weight-reduced obese men. Metabolism 2002;51:1003–10.
Lejeune MPGM, van Aggel-Leijssen DPC, van Baak MA, Westerterp-Plantenga MS. Effects of dietary restraint vs exercise during weight maintenance in obese men. Eur J Clin Nutr 2003;57:1388–44.
Wood 1988
Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med 1988;319:1173–9.
Secondary publication
Fortmann SP, Haskell WL, Wood PD. Effects of weight loss on clinic and ambulatory blood pressure in normotensive men. Am J Cardiol 1988;62:89–93.
Appendix 8 Detailed quality assessment for individual studies: review of men-only randomised controlled trials
| Item | Benassi-Evans 200983 | Borg 200284 | Esposito 200485 | Jeffrey 198386 | King 198988 a | Khoo 201187 | Morgan 201189 | Patrick 201190 | Pavlou 198991 | van Aggel-Leijssen 200192 | Wood 198893 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence generation (selection bias) | ? | ? | ? | ? | ? | ? | ✓ | ✓ | ? | ? | ✓ |
| Allocation concealment (selection bias) | ? | ✓ | ✓ | ? | ? | ? | ✓ | ✓ | ? | ? | ✓ |
| Blinding of participants (performance bias) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Blinding of health-care providers (performance bias) | ? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Blinding of outcome assessment (detection bias) | ? | ✗ | ✓ | ? | ✓ | ? | ✓ | ✓ | ? | ? | ? |
| Groups treated identically (performance bias) | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Incomplete outcome data (attrition bias) | ? | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ? | ? | ✓ | ? |
| Intention to treat (attrition bias) | ? | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ |
| Selective reporting (reporting bias) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ? | ✓ | ✓ | ✓ | ✓ |
| Other bias | ? | ? | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✗ |
| Item | Benassi-Evans 200983 | Borg 200284 | Esposito 200485 | Jeffrey 198386 | King 198988 a | Khoo 201187 | Morgan 201189 | Patrick 201190 | Pavlou 198991 | van Aggel-Leijssen 200192 | Wood 198893 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Equity pointer: social context of the study, e.g. was the study conducted in a particular setting that might target/exclude specific populations? | ? | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ |
| Representativeness of the sample: are participants in the study likely to be representative of the target population? | ✓ | ✓ | ✗ | ? | ✗ | ✓ | ✗ | ? | ✗ | ✓ | ✗ |
| Sociodemographic differences between withdrawals and exclusions? | ? | ? | ? | ? | ? | ✗ | ? | ✓ | ? | ? | ? |
| PROGRESS categories reported at baseline (indicate letters of those reported: place of residence, race, occupation, gender, religion, education and literacy, socioeconomic status, social capital) | ✗ | ✗ | ? | ✓ | ✗ | ✗ | ✓ | ✓ | ? | ? | ✗ |
| Did the intervention include strategies to address diversity/disadvantage? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ? | ✗ | ✗ | ✗ |
| Was there a fidelity check? | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ |
| Were process measures taken? | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ |
| Providers (who, number, education/training in intervention delivery, ethnicity, etc. if potentially relevant to acceptance and uptake by participants) | ✓ | ? | ✓ | ? | ? | ✗ | ✓ | ✓ | ? | ✓ | ✓ |
| Was sustainability discussed by the authors? Was it a consideration in study development? | ✗ | ✗ | ✗ | ? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Do the authors describe any political or organisational context? | ✗ | ✗ | ✗ | ? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Were any partnerships described? | ✗ | ✗ | ✗ | ? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Potential for author conflict, i.e. evidence that author or data collectors would benefit if results favoured the intervention under study or the control? | ? | ? | ? | ✗ | ? | ✗ | ✗ | ✓ | ? | ? | ? |
| Were outcomes relating to harms/unintended effects of the intervention described? | ? | ✓ | ? | ✗ | ? | ✓ | ✗ | ✗ | ? | ✓ | ? |
Appendix 9 List of included studies: review of randomised controlled trials of men and women compared
Gorin 2013
Gorin AA, Raynor HA, Fava J, Maguire K, Robichaud E, Trautvetter J, et al. Randomized controlled trial of a comprehensive home environment-focused weight-loss program for adults. Health Psychol 2013;32:128–37.
Hakala 1993
Hakala P, Karvetti RL, Ronnemaa T. Group vs. individual weight reduction programmes in the treatment of severe obesity – a five year follow-up study. Int J Obes Relat Metab Disord 1993;17:97–102.
Hakala 1994
Hakala P. Weight reduction programmes at a rehabilitation centre and a health centre based on group counselling and individual support: short- and long-term follow-up study. Int J Obes 1994;18:483–9.
Heitzmann 1987
Heitzmann CA, Kaplan RM, Wilson DK, Sandler J. Sex differences in weight loss among adults with type II diabetes mellitus. J Behav Med 1987;10:197–211.
Jeffery 1984
Jeffery RW, Bjornson-Benson WM, Rosenthal BS, Kurth CL, Dunn MM. Effectiveness of monetary contracts with two repayment schedules of weight reduction in men and women from self-referred and population samples. Behav Ther 1984;15:273–9.
Jeffery 2003
Jeffery RW, Sherwood NE, Brelje K, Pronk NP, Boyle R, Boucher JL, et al. Mail and phone interventions for weight loss in a managed-care setting: Weigh-To-Be one-year outcomes. Int J Obes 2003;27:1584–92.
Jolly 2011
Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ 2011;343:d6500.
Karvetti 1992
Karvetti RL, Hakala P. A seven-year follow-up of a weight reduction programme in Finnish primary health care. Eur J Clin Nutr 1992;46:743–52.
Korhonen 1987
Korhonen T, Uusitupa M, Aro A, Kumpulainen T, Siitonen O, Voutilainen E, et al. Efficacy of dietary instructions in newly diagnosed non-insulin-dependent diabetic patients. Comparison of two different patient education regimens. Acta Med Scand 1987;222:323–31.
Lantz 2003
Lantz H, Peltonen M, Agren L, Torgerson JS. Intermittent versus on-demand use of a very low calorie diet: a randomized 2-year clinical trial. J Intern Med 2003;253:463–71.
Lindstrom 2008
Lindstrom J, Peltonen M, Eriksson JG, Aunola S, Hamalainen H, Ilanne PP, et al. Determinants for the effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Care 2008;31:857–62.
Richelsen 2007
Richelsen B, Tonstad S, Rossner S, Toubro S, Niskanen L, Madsbad S, et al. Effect of orlistat on weight regain and cardiovascular risk factors following a very-low-energy diet in abdominally obese patients: a 3-year randomized, placebo-controlled study. Diabetes Care 2007;30:27–32.
Ross 2012
Ross R, Lam M, Blair SN, Church TS, Godwin M, Hotz SB, et al. Trial of prevention and reduction of obesity through active living in clinical settings: a randomized controlled trial. Arch Intern Med 2012;172:414–24.
Shai 2008
Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229–41.
Secondary publication
Golan R, Schwarzfuchs D, Stampfer MJ, Shai I, DIRECT Group. Halo effect of a weight-loss trial on spouses: the DIRECT-Spouse study. Public Health Nutr 2010;13:544–9.
Vanninen 1992
Vanninen E, Uusitupa M, Siitonen O, Laitinen J, Lansimies E. Habitual physical activity, aerobic capacity and metabolic control in patients with newly-diagnosed type 2 (non-insulin-dependent) diabetes mellitus: effect of 1-year diet and exercise intervention. Diabetologia 1992;35:340–6.
Secondary publication
Vanninen E, Uusitupa M, Lansimies E, Siitonen O, Laitinen J. Effect of metabolic control on autonomic function in obese patients with newly diagnosed Type 2 diabetes. Diabet Med 1993;10:66–73.
Volpe 2008
Volpe SL, Kobusingye H, Bailur S, Stanck E. Effect of diet and exercise on body composition, energy intake and leptin levels in overweight women and men. J Am Coll Nutr 2008;27:195–208.
Wadden 2011
Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity 2011;19:1987–98.
Secondary publications
Gorin AA, Wing RR, Fava JL, Jakicic JM, Jeffery R, West DS, et al. Weight loss treatment influences untreated spouses and the home environment: evidence of a ripple effect. Int J Obes 2008;32:1678–84.
Schwartz AV, Johnson KC, Kahn SE, Shepherd JA, Nevitt MC, Peters AL, et al. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res 2012;27:619–27.
Stewart TM, Bachand AR, Han H, Ryan DH, Bray GA, Williamson DA. Body image changes associated with participation in an intensive lifestyle weight loss intervention. Obesity 2011;19:1290–5.
Wing RR, Rosen RC, Fava JL, Bahnson J, Brancati F, Gendrano I, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. J Sex Med 2010;7:156–65.
West 2008
West DS, Prewitt TE, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity 2008;16:1413–20.
Wing 1991
Wing RR, Marcus MD, Epstein LH, Jawad A. A ‘family-based’ approach to the treatment of obese type II diabetic patients. J Consult Clin Psychol 1991;59:156–62.
Wing 1994
Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 1994;97:354–62.
Appendix 10 Detailed risk of bias assessment for individual studies: review of randomised controlled trials of men and women compared
| Item | Gorin 201394 | Hakala 199395 | Hakala 199496 | Heitzmann 198797 | Jeffery 198498 | Jeffery 200399 | Jolly 2011100 | Karvetti 1992101 | Korhonen 1987102 | Lantz 2003103 | Lindstrom 2008104 | Richelsen 2007105 | Ross 2012106 | Shai 2008107 | Vannine n 1992108 | Volpe 2008109 | Wadden 2011110 | West 2008111 | Wing 1991112 | Wing 1994113 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence generation (selection bias) | ? | ? | ? | ✗ | ? | ✓ | ✓ | ? | ? | ? | ✓ | ✓ | ✓ | ? | ? | ? | ? | ? | ? | ? |
| Allocation concealment (selection bias) | ? | ? | ? | ✓ | ? | ✓ | ✓ | ? | ? | ? | ✓ | ? | ✓ | ? | ? | ? | ? | ? | ? | ? |
| Blinding of participants (performance bias) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Blinding of health-care providers (performance bias) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Blinding of outcome assessment (detection bias) | ? | ? | ? | ? | ? | ? | ✓ | ? | ? | ? | ✓ | ? | ? | ✓ | ? | ? | ? | ✓ | ? | ? |
| Groups treated identically (performance bias) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ |
| Incomplete outcome data (attrition bias) | ? | ✗ | ✗ | ? | ? | ? | ✓ | ✗ | ✓ | ? | ✓ | ? | ✓ | ✓ | ? | ? | ✓ | ✗ | ✓ | ? |
| Intention to treat (attrition bias) | ✓ | ✗ | ✗ | ? | ? | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ? | ✓ | ✗ | ✗ | ✗ |
| Selective reporting (reporting bias) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Other bias | ? | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ? | ? | ? | ✓ | ? | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ? | ✓ |
| Item | Gorin 201394 | Hakala 199395 | Hakala 199496 | Heitzman 198797 | Jeffery 198498 | Jeffery 200399 | Jolly 2011100 | Karvetti 1992101 | Korhonen 1987102 | Lindstrom 2008104 | Lantz 2003103 | Richelsen 2007105 | Ross 2012106 | Shai 2008107 | Vannine n 1992108 | Volpe 2008109 | Wadden 2011110 | West 2008111 | Wing 1991112 | Wing 1994113 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equity pointer: social context of the study, e.g. was the study conducted in a particular setting that might target/exclude specific populations? | ✗ | ? | ? | ✗ | ✓ | ✓ | ✗ | ? | ✗ | ? | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ? |
| Representativeness of the sample: are participants in the study likely to be representative of the target population? | ✗ | ? | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Sociodemographic differences between withdrawals and exclusions? | ✓ | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ✗ | ? | ? | ? |
| PROGRESS categories reported at baseline (indicate letters of those reported: place of residence, race, occupation, gender, religion, education and literacy, socioeconomic status, social capital) | ✓ | ? | ? | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Did the intervention include strategies to address diversity/disadvantage? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ? | ✗ | ✗ |
| Was there a fidelity check? | ✗ | ? | ? | ✗ | ✗ | ✗ | ✓ | ? | ✗ | ✗ | ✗ | ? | ✗ | ✗ | ? | ? | ✓ | ? | ? | ✗ |
| Were process measures taken? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ? | ✗ | ✗ | ? | ? | ✗ | ? | ? | ✗ |
| Details of intervention providers given | ✓ | ✓ | ✓ | ? | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✓ |
| Sustainability of the intervention discussed? | ✗ | ? | ? | ? | ? | ? | ✓ | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ✗ |
| Authors described any political/organisational context? | ✗ | ✗ | ✗ | ? | ? | ? | ✗ | ✗ | ? | ? | ? | ? | ✗ | ? | ? | ? | ? | ? | ? | ✗ |
| Were any partnerships described? | ✗ | ✗ | ✗ | ? | ? | ? | ✓ | ✗ | ? | ? | ? | ? | ✗ | ? | ? | ? | ? | ? | ? | ✗ |
| Was there potential for author conflict | ✗ | ? | ? | ? | ? | ? | ✗ | ? | ? | ? | ? | ? | ? | ✗ | ✗ | ✗ | ✗ | ? | ? | ? |
| Harms/unintended effects of the intervention described? | ? | ✗ | ✗ | ? | ? | ? | ✓ | ✗ | ? | ✗ | ? | ✓ | ✓ | ? | ✗ | ? | ? | ? | ? | ? |
Appendix 11 List of included studies: review of UK interventions
Ahern 2011
Ahern AL, Olson AD, Aston LM, Jebb SA. Weight Watchers on prescription: an observational study of weight change among adults referred to Weight Watchers by the NHS. BMC Public Health 2011;11:434.
Brady 2010
Brady AJ, Perry C, Murdoch DL, McKay G. Sustained benefits of a health project for middle-aged football supporters, at Glasgow Celtic and Glasgow Rangers Football Clubs. Eur Heart J 2010;31:2696–8.
Bye 2005
Bye C, Avery A, Lavin J. Tackling obesity in men – preliminary evaluation of men-only groups within a commercial slimming organization. J Hum Nutr Diet 2005;18:391–4.
Department of Health and Leeds Metropolitan University 2010
Department of Health and Leeds Metropolitan University. Tackling Men’s Health. Men Only Weight Management Group: NHS Leeds and Leeds Rhinos, 2010 Season. Leeds: Public Health and Social Care Group Yorkshire and the Humber and NHS Leeds; 2010.
Dixon 2012
Dixon KJ, Shcherba S, Kipping RR. Weight loss from three commercial providers of NHS primary care slimming on referral in North Somerset: service evaluation. J Public Health 2012;34:555–61.
Drummond 2004
Drummond S, Dixon K, Griffin J, De Looy A. Weight loss on an energy-restricted, low-fat, sugar-containing diet in overweight sedentary men. Int J Food Sci Nutr 2004;55:279–90.
Evans 2011a
Evans S. Primary care weight management in Hertfordshire using ProHealthClinical. National Obesity Forum (NOF) Eastern Regional Obesity Network meeting, December 2011.
Evans 2011b
Evans S. Community health improvement programme. Cambridgeshire Countywide Joint Obesity Strategy meeting, April 2011.
Evans 2012
Evans S. Evans S. Weigh2Go: a peripatetic level 1 weight management service in Cambridge City and South, interim report 2012. Treatment of Obesity Conference, Birmingham, June 2012.
Gray 2009
Gray CM, Anderson AS, Clarke AM, Dalziel A, Hunt K, Leishman J, et al. Addressing male obesity: an evaluation of a group-based weight management intervention for Scottish men. J Mens Health 2009;6:70–81.
Gray 2011
Gray CM, Hunt K, Mutrie N, Anderson AS, Treweek S, Wyke S. Can the draw of professional football clubs help promote weight loss in overweight and obese men? A feasibility study of the Football Fans in Training programme delivered through the Scottish Premier League. J Epidemiol Community Health 2011;65(Suppl. 2):A37–8.
Hallam Spencer 2008
Hallam Spencer CI, Holt J, du Plessis J, Cox JSA, Hewlett B. Weight loss results for 5000 women following the LighterLife Programme in 2007. Int J Obes 2008;32(Suppl. 1):S178.
Hallam 2011
Hallam C, Mullins G, Cassidy M, Cox JSA, Hewlett B, Broom J. Mean weight loss achieved in 8 weeks by 1006 obese male patients on the LighterLife total VLCD weight-loss programme in 2010. Obes Rev 2011;12(Suppl. 1):230–1.
Hallam 2010
Hallam CL, Mullins G, Wiggins J, du Plessis J, Cox JSA, Hewlett B. To report on the weight loss achieved in 12 weeks by 432 super-morbidly obese patients on the LighterLife Total VLCD weight-loss programme in 2009; a retrospective study. Obes Rev 2010;11(Suppl. 1):244.
Holt 2007
Holt J, Horsnell T, Cox J, du Plessis J, Mullins G, Hewlett B. Obese men are at higher risk than obese women. Following a small trial, a long term observational study will report over the next 10 years on weight loss and weight maintenance on a group of self referred obese men. Int J Obes 2007;31(Suppl. 1):S169.
Johnson 2011
Johnson F, Wardle J. The association between weight loss and engagement with a web-based food and exercise diary in a commercial weight loss programme: a retrospective analysis. Int J Behav Nutr Phys Act 2011;8:83.
Kirk 2000
Kirk T, Cromble N, Cursiter M. Promotion of dietary carbohydrate as an approach to weight maintenance after initial weight loss: a pilot study. J Hum Nutr Diet 2000;13:277–85.
Leslie 2002
Leslie WS, Lean ME, Baillie HM, Hankey CR. Weight management: a comparison of existing dietary approaches in a work-site setting. Int J Obes Relat Metab Disord 2002;26:1469–75.
McFarlane 2006
McFarlane G, Rennie J. Appendix 2: The Bloke’s Weight – Men’s Weight Management Group Arbroath, March–May 2006. Arbroath: Angus Weight Management Project; 2006.
Poulter 2012
Poulter J, Raine G, Robertson S. Evaluation of a gender-segregated, commercial, community based, weight management pilot. Obes Facts 2012;5(Suppl. 1):67.
Rolland 2013
Rolland C, Hallam C, Lula S, Wiggins J, Dyson L, Van Gaal LF, et al. Weight loss and ethnicity: a cohort study of the effects induced by a very low calorie diet. Clin Exp Med Sci 2013;1:97–109.
Ross 2008
Ross HM, Laws R, Reckless J, Lean M, McQuigg M, Noble P, et al. Evaluation of the Counterweight programme for obesity management in primary care: a starting point for continuous improvement. Br J Gen Pract 2008;58:548–54.
Salsbury 2009
Salsbury J, Hallam Spencer C, Wiggins J, Dyson L, du Plessis J, Mullins G. Weight-loss results for 2200 male patients with a BMI > 29 kg/m2 following the LighterLife programme. Obes Facts 2009;2(Suppl. 2):246.
Stubbs 2011
Stubbs RJ, Pallister C, Whybrow S, Avery A, Lavin J. Weight outcomes audit for 34,271 adults referred to a primary care/commercial weight management partnership scheme. Obes Facts 2011;4:113–20.
Stubbs 2012
Stubbs RJ, Brogelli DJ, Pallister CJ, Whybrow S, Avery AJ, Lavin JH. Attendance and weight outcomes in 4754 adults referred over 6 months to a primary care/commercial weight management partnership scheme. Clin Obes 2012;2:6–14.
Witty 2010
Witty K, White A. The Tackling Men’s Health Evaluation Study: Final Report. Leeds: Leeds Metropolitan University, Centre for Men’s Health; 2010. URL: www.leedsmet.ac.uk/hss/docs/Tackling_Men_Health_Final_Report.pdf (accessed November 2011).
Appendix 12 Detailed quality assessment for individual studies: review of UK interventions
| Item | Leslie 2002146 |
|---|---|
| Sequence generation (selection bias) | ✗ |
| Allocation concealment (selection bias) | ✗ |
| Blinding of participants (performance bias) | ✗ |
| Blinding of health-care providers (performance bias) | ✗ |
| Blinding of outcome assessment (detection bias) | ? |
| Groups treated identically (performance bias) | ? |
| Incomplete outcome data (attrition bias) | ✓ |
| Intention to treat (attrition bias) | ? |
| Selective reporting (reporting bias) | ✓ |
| Other bias | ? |
| Ahern 201137 | Brady 2010138 | Bye 200534 | Department of Health 2010139 | Dixon 2012150 | Drummond 2004140 | Gray 2009142 | Johnson 2011154 | Kirk 2000155 | McFarlane 2006147 | Rolland 2013156 | Ross 200831 | Stubbs 201136 | Witty 2010149 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Representative sample | ✓ | ✓ | ? | ? | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ? | ? |
| Inclusion/exclusion criteria clearly defined | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ? | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ? |
| Participants at similar point in disease progression | ? | ✓ | ? | ? | ✗ | ✓ | ✓ | ? | ✗ | ? | ✓ | ✓ | ? | ? |
| Selection of participants consecutive | ? | ? | ? | ? | ? | ? | ? | ✗ | ? | ✓ | ? | ✓ | ? | ? |
| Data collection undertaken prospectively | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Groups comparable | NA | NA | NA | NA | ? | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Intervention clearly defined | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Intervention delivered by an experienced person | ✓ | ✓ | ✓ | ✓ | ? | ? | ✓ | ✓ | ? | ? | ✓ | ✓ | ✓ | ? |
| Intervention delivered in an appropriate setting | ? | ✓ | ? | ✓ | ? | ? | ✓ | NA | ✓ | ? | ? | ✓ | ? | ✓ |
| Important outcomes considered | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Objective outcome measures used | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Assessment of main outcomes blind | NA | NA | NA | NA | ✗ | NA | NA | ✓ | NA | NA | NA | NA | NA | NA |
| Follow-up long enough | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ |
| Information on non-respondents, dropouts | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ? | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ |
| Withdrawals likely to introduce bias | ? | ? | ? | ? | ? | ? | ? | ? | ✓ | ? | ? | ? | ? | ? |
| Length of follow-up similar between comparison groups | NA | NA | NA | NA | ✓ | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Important prognostic factors identified | ? | ✓ | ? | ? | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ? |
| Analyses adjusted for confounding factors | NA | NA | NA | NA | ✓ | NA | NA | NA | NA | NA | NA | NA | ✓ | NA |
| Ahern 201137 | Brady 2012138 | Bye 200534 | Department of Health 2010139 | Dixon 2012150 | Drummond 2004140 | Gray 2009142 | Gray 2011141 | Johnson 2011154 | Kirk 200087 | Leslie 2002146 | McFarlane 2006147 | Rolland 2013156 | Ross 200831 | Stubbs 201136 | Witty 2010149 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Equity pointer: social context of the study, e.g. was the study conducted in a particular setting that might target/exclude specific populations? | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ? | ✗ | ? | ✓ |
| Representativeness of the sample: are participants in the study likely to be representative of the target population? | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ | ? | ✓ |
| Sociodemographic differences between withdrawals and exclusions? | ? | ? | ? | ? | ? | ? | ? | ? | ✗ | ? | ? | ? | ? | ? | ? | ? |
| PROGRESS categories reported at baseline (indicate letters of those reported: place of residence, race, occupation, gender, religion, education and literacy, socioeconomic status, social capital) | ? | ✗ | ✓ | ✗ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ? | ? | ✓ | ✓ | ✓ | ✗ |
| Did the intervention include strategies to address diversity/disadvantage? | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ? | ✓ | ✗ |
| Was there a fidelity check? | ✗ | ✗ | ? | ? | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ? | ✗ | ✗ | ✗ | ? |
| Were process measures taken? | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✓ |
| Details of intervention providers given | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ? | ? | ✓ | ? | ✓ | ✓ | ✓ | ✓ |
| Sustainability of the intervention discussed? | ? | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ? | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ |
| Do the authors describe any political or organisational context? | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ? | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ |
| Were any partnerships described? | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ |
| Was there potential for author conflict | ✗ | ✗ | ✓ | ✗ | ✗ | ? | ✗ | ✗ | ? | ? | ? | ✗ | ? | ✗ | ✓ | ✗ |
| Harms/unintended effects of the intervention described? | ✗ | ✗ | ✗ | ✗ | ? | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
Appendix 13 Supplementary material for the review of cost-effectiveness
Quality assessment checklists for included economic evaluations
Galani 2007162
| Quality criterion | Dimension of quality | Question | Response | Comments |
|---|---|---|---|---|
| Structure | ||||
| S1 | Statement of decision problem/objective | Is there a clear statement of the decision problem? | Y | The decision problem was to quantify the lifetime health and economic consequences of preventing and treating obesity with lifestyle interventions in Switzerland |
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | Y | The objective of the model was to measure the lifetime effects of a 3-year lifestyle intervention compared with standard care in an overweight and obese Swiss population using a Markov decision-analytic model. This was consistent with the decision problem | ||
| Is the primary decision-maker specified? | N | None stated; however, given that the project was funded by the Swiss Federal Office of Health, it might be assumed that Swiss health-care decision-making bodies would be the primary decision-makers using the results of this study | ||
| S2 | Statement of scope/perspective | Is the perspective of the model clearly stated? | Y | Perspective of the model is stated as societal |
| Are the model inputs consistent with the stated perspective? | N | It is not clear how society’s wider costs have been included. Given the parameters detailed in the analysis, it would appear that costs are measured from a health services provider’s perspective | ||
| Has the scope of the model been stated and justified? | Y | The scope of the model is not explicitly discussed; however, it is clear from the objectives detailed | ||
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | Y | Outcomes are measured as LYG and QALYs gained. The economic evaluation is reported as incremental cost per life-year gained and cost per QALY gained and is appropriate to the decision problem and objectives | ||
| S3 | Rationale for structure | Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Y | A comprehensive model structure has been described detailing the progression through key health states linked to obesity |
| Are the sources of data used to develop the structure of the model specified? | Y | The model structure is based on key health states linked to obesity, which were chosen based on published data. A detailed diagram of the model structure is also provided | ||
| Are the causal relationships described by the model structure justified appropriately? | Y | Causal relationships and methods to derive links between treatment effects, cardiovascular risks, etc., are clearly outlined and discussed | ||
| S4 | Structural assumptions | Are the structural assumptions transparent and justified? | Y | Structural assumptions are acknowledged and discussed. It is assumed that patients enter a state and remain in that state unless they develop cardiovascular disease or die |
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | Y | The structural assumptions of the model are clearly acknowledged and justified in the context of the complexity of the decision problem (e.g. comorbidities have not been modelled because of a lack of Swiss prevalence data to inform the model) | ||
| S5 | Strategies/comparators | Is there a clear definition of the options under evaluation? | Y | Interventions that are considered are clearly detailed |
| Have all feasible and practical options been evaluated? | Y | All practical options are considered given the decision problem and scope of the analysis. The authors clearly discuss their reasons for including standard care as the comparator, most notably because it is current practice in Switzerland | ||
| Is there justification for the exclusion of feasible options? | NA | See above | ||
| S6 | Model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | Y | The Markov decision model with a lifetime horizon is appropriate to link intermediate outcomes to longer-term health outcomes and costs. The choice of model structure and health states is appropriate |
| S7 | Time horizon | Is the time horizon of the model sufficient to reflect all important differences between options? | Y | A lifetime model horizon of 60 years’ duration (up to age 85 years) is appropriate to measure all potential differences between modelled interventions over a patient’s lifetime |
| Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | Y | Time horizon, treatment effect and duration of effect are all clearly described. It is assumed that weight lost as a result of the intervention is maintained up to 6 years, with a linear weight regain each year thereafter for 4 years. The assumption was sustained by data from the FDPS171 | ||
| S8 | Disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | Y | A total of five key health states related to obesity are included in the Markov model. The authors also acknowledge and justify the omission of a number of other health states for which the link between obesity and health state is less clear/less data rich |
| S9 | Cycle length | Is the cycle length defined and justified in terms of the natural history of disease? | Y | A cycle length of 1 year is described and is appropriate to the decision problem |
| Data | ||||
| D1 | Data identification | Are the data identification methods transparent and appropriate given the objectives of the model? | Y/N | All data included in the model are clearly identified and sources are clearly referenced throughout. To estimate the efficacy of the intervention, a random-effects meta-analysis of a range of trials was carried out. However, it is not clear how the trials used for the meta-analysis were identified (e.g. whether or not a systematic literature search was carried out). Sources of utility data are clearly reported; however, identification methods and methods used to measure the utility weights in the source studies are not discussed or justified |
| When choices have been made between data sources, are these justified appropriately? | N | Alternative sources of data are not systematically explored in the study; however, a meta-analysis of treatment effectiveness is carried out | ||
| Has particular attention been paid to identifying data for the important parameters in the model? | Y | Disease-related mortality used in the model is age and sex specific and meta-analyses are conducted for treatment effectiveness | ||
| Has the quality of the data been assessed appropriately? | Y | Limitations of the data are discussed and, when appropriate, they are tested in comprehensive probabilistic sensitivity analysis | ||
| When expert opinion has been used, are the methods described and justified? | NA | No expert opinion has been referred to in the study | ||
| D2 | Data modelling | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Y | Modelling methodology follows well-known best practice Markov modelling methodology |
| D2a | Baseline data | Is the choice of baseline data described and justified? | Y | There is a clear description and justification of all baseline data used for the model. Sources are clearly referenced. Baseline data, cardiovascular risk and transition probabilities between health states are estimated using sex-specific data; however, data for inputs are not presented for male and female subgroups separately |
| Are transition probabilities calculated appropriately? | ? | Not clear – transition probabilities are calculated based on two large American prospective epidemiological studies. Detailed calculation methods are not provided, neither are the transition probabilities themselves | ||
| Has a half-cycle correction been applied to both costs and outcomes? | N/Y | A half-cycle correction has not been applied to costs but has been applied to life expectancy data assuming transition midway through the cycle | ||
| If not, has this omission been justified? | NA | |||
| D2b | Treatment effects | If relative treatment effects have been derived from the trial data, have they been synthesised using appropriate techniques? | Y | A random-effects meta-analysis of a range of studies has been used to estimate treatment effect. A large number of studies have been included in the meta-analysis for the estimation of both BMI and other key clinical outcome data |
| Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | Y | Methods and assumptions for extrapolation are clearly described and justified | ||
| Have alternative extrapolation assumptions been explored through sensitivity analysis? | N | No alternative extrapolation assumptions have been explored | ||
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | Y | Assumptions regarding maintenance of weight loss are clearly described | ||
| Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis? | N | None detailed | ||
| D2c | Costs | Are the costs incorporated into the model justified? | Y | Costs incorporated into the model appear to be appropriate. In general, the reporting of cost data is lacking in detail and it is unclear which, if any, indirect costs are included. The perspective is stated as societal but it appears more like a health services provider perspective, given the data included |
| Have the sources for all costs been described? | Y | Sources are reported in the study although details are limited. Both direct costs and indirect costs of complications were obtained from published literature | ||
| Have discount rates been described and justified given the target decision-maker? | Y | Discount rates for costs and QALYs are 3% and are appropriate. Discount rates are varied in sensitivity analysis | ||
| D2d | Quality of life weights (utilities) | Are the utilities incorporated into the model appropriate? | ? | Unclear as methods of utility estimation are not clearly described in the study, although references for the source studies are provided |
| Is the source for the utility weights referenced? | Y | References for utility weights are provided throughout | ||
| Are the methods of derivation for the utility weights justified? | ? | Not clear as no details of the methods for calculation of utility weights are provided | ||
| D3 | Data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | Y | Data incorporated in the model are described and referenced throughout. Greater detail for unit costs and utility data would have been helpful |
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | NA | No mutually inconsistent data presented | ||
| Is the process of data incorporation transparent? | Y | Data incorporation is clearly explained | ||
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | Y | Distributions are detailed for key model parameters. No justifications are provided; however, the distributions used are standard practice for probabilistic sensitivity analysis | ||
| If data have been incorporated as distributions, is it clear that second-order uncertainty is reflected? | Y | Monte Carlo simulations were used | ||
| D4 | Assessment of uncertainty | Have the four principal types of uncertainty been addressed? | N | Structural uncertainty has not been accounted for. The model has not been rerun to test the impact of structural assumptions |
| If not, has the omission of particular forms of uncertainty been justified? | N | No discussion of structural uncertainty is provided | ||
| D4a | Methodological | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | Y | Discount rate varied between 0% and 3% for the base case and all subgroup analyses |
| D4b | Structural | Is there evidence that structural uncertainties have been addressed through sensitivity analysis? | N | No structural uncertainty addressed in sensitivity analysis |
| D4c | Heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | Y | Model has been rerun for a number of subgroups, including age- and sex-specific results |
| D4d | Parameter | Are the methods of assessment of parameter uncertainty appropriate? | Y | Probabilistic sensitivity analysis has been undertaken |
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | N | Ranges for potential parameter values are not reported. Extended details of inputs for distributions for the probabilistic sensitivity analysis are reported in a subsequent study167 | ||
| Consistency | ||||
| C1 | Internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | N | There are no details of piloting/validation or testing of the model; however, it would appear that the model is logical and generates results which have good face validity. The modelling method used is well established in the economic evaluation field |
| C2 | External consistency | Are any counterintuitive results from the model explained and justified? | Y | Counterintuitive results for the borderline obese group compared with the obese group are discussed and justified with discussion of the possible reasons for the dominant ICERs in the borderline obese group. This is because lifestyle intervention costs are offset by longer-term treatment outcomes and reduced costs associated with treating cardiovascular disease in later life from obesity complications |
| If the model has been calibrated against independent data, have any differences been explained and justified? | Y | Data from the model have been compared against and justified with independent data whenever possible | ||
| Have the results of the model been compared with those of previous models and any differences in results explained? | Y | A comprehensive literature search was conducted for other economic models in this clinical area and the results of these models were compared with those of the present study. Anomalies and inconsistencies are explored and reasons for differences discussed | ||
Iannazzo 2008164
| Quality criterion | Dimension of quality | Question | Response | Comments |
|---|---|---|---|---|
| Structure | ||||
| S1 | Statement of decision problem/objective | Is there a clear statement of the decision problem? | Y | The decision problem was to evaluate the economic impact of the use of orlistat plus a lifestyle intervention compared with the lifestyle intervention alone in an Italian obese population |
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | Y | The model simulated clinical and economic outcomes in a representative Italian obese cohort using a Bayesian probabilistic Markov model to extrapolate the clinical results from the XENDOS double-blind RCT169 in terms of diabetes progression and associated mortality | ||
| Is the primary decision-maker specified? | N | The primary decision-maker is not explicitly specified; however, the study was funded by a grant from Roche. Publication of the study results was not dependant on agreement or input from the funders and the authors’ results were independent of the company funding the study | ||
| S2 | Statement of scope/perspective | Is the perspective of the model clearly stated? | Y | A societal perspective is stated |
| Are the model inputs consistent with the stated perspective? | N | The authors state that a societal perspective was considered. However, costs considered appear only to be applicable to a health services and patient perspective (assuming patients pay for orlistat directly in Italy). Indirect costs (e.g. productivity losses) associated with a typical analysis from a societal perspective to not appear to have been considered | ||
| Has the scope of the model been stated and justified? | Y | The scope of the model is clear from the information included in the objective and methods | ||
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | Y | Outcomes are measured as QALYs and are consistent with the perspective, scope and objectives of the model | ||
| S3 | Rationale for structure | Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Y | The structure is consistent with the development of diabetes from weight gain and associated risk factors. A more dynamic model may have presented a better picture of diabetes disease progression and this is acknowledged by the study authors |
| Are the sources of data used to develop the structure of the model specified? | Y | The model is broadly structured around the extrapolation of the XENDOS study results.169 There is no evidence of any structured literature searching to inform the model structure | ||
| Are the causal relationships described by the model structure justified appropriately? | Y | Data are sourced from the XENDOS study169 and are used to estimate risks of cardiovascular disease/stroke, etc. Risks and data were age and sex specific and the key clinical end point was transition from obese health state to diabetes. Data are clearly linked from weight loss to cardiovascular risk outcomes and hence mortality and QALYs gained. The authors acknowledge the limitations of the modelling processes that were used to model long-term outcomes, and in particular that the model was insensitive to changes in weight-loss data | ||
| S4 | Structural assumptions | Are the structural assumptions transparent and justified? | Y | Structural assumptions are clear; however, a comprehensive list of all model assumptions would have been helpful |
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | Y | The model structure used appears to be appropriate | ||
| S5 | Strategies/comparators | Is there a clear definition of the options under evaluation? | Y | Options evaluated are clearly defined and comprehensive data on the content of the interventions are provided |
| Have all feasible and practical options been evaluated? | Y | Given that the model evaluates the long-term costs and economic consequences of the interventions in the XENDOS trial,169 the use of these options for evaluation is appropriate | ||
| Is there justification for the exclusion of feasible options? | NA | No details of exclusions presented | ||
| S6 | Model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | Y | The analysis was based on best practice modelling techniques and a Bayesian probabilistic Markov decision model was used to answer the decision problem. This modelling approach is appropriate in the context of the decision problem and is a key strength of the analysis |
| S7 | Time horizon | Is the time horizon of the model sufficient to reflect all important differences between options? | ? | The time horizon of the model is 10 years (4 years of treatment plus 6 years of follow-up). Although it is likely that this is sufficient to establish the cost-effectiveness of the intervention, a longer time horizon might have been appropriate to estimate lifetime diabetes-related outcomes. The choice of a 10-year time horizon is not discussed; however, the authors acknowledge that a more dynamic model of diabetes might generate more relevant results. A longer time horizon would rely on stronger assumptions about weight-loss maintenance in the long term, for which data are sparse |
| Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | Y | The time horizon is clearly described, as is treatment duration. The duration of treatment effect is based on the XENDOS follow-up results at 4 years.169 The weight reduction trend was stronger in the orlistat group at 2 years but weight returned almost entirely to baseline levels at 4 years’ follow-up. The lack of sufficient modelling sensitivity to weight changes is discussed as a limitation of the analysis | ||
| S8 | Disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | Y | The disease states broadly fit with the decision problem; however, the authors acknowledge a key limitation, the inadequately modelled relationship between weight loss and cardiovascular risk. Further, a more dynamic modelling of health states would be helpful; however, this is subject to data availability to inform such a process |
| S9 | Cycle length | Is the cycle length defined and justified in terms of the natural history of disease? | Y | The cycle length is 1 year and is appropriate to the decision problem and the history of diabetic disease |
| Data | ||||
| D1 | Data identification | Are the data identification methods transparent and appropriate given the objectives of the model? | Y/N | Data identification methods are clear in terms of cardiovascular risk, cost inputs and mortality calculations. However, there is little information on the methods used to identify appropriate utility weights for the model |
| When choices have been made between data sources, are these justified appropriately? | ? | Unclear – alternative data sources for populating the model are not discussed and there is no evidence of systematic literature searches to populate the model. However, the data that have been included appear appropriate to the decision problem | ||
| Has particular attention been paid to identifying data for the important parameters in the model? | Y/N | Adequate attention is given to the identification of data for the key model parameters (mortality, cardiovascular risks, costs, etc.). However, more information could have been supplied on the identification of utility weights for QALY calculations. When weights are sourced from the literature, a brief critical appraisal of calculation methods would have been beneficial | ||
| Has the quality of the data been assessed appropriately? | Y | Although there is no systematic critique of the data used in the model, the limitations of the data are clearly outlined and discussed in the discussion section of the paper | ||
| When expert opinion has been used, are the methods described and justified? | NA | There is no evidence that expert opinion has been used to inform the model structure and design or parameter inputs | ||
| D2 | Data modelling | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Y | A Bayesian probabilistic Markov model is used and this is a key strength of the analysis. The Markov model included two Bayesian statistical models fitted on the trial data, to predict diabetes incidence and cardiovascular disease risk factors. Parameters were drawn directly from their posterior distributions. Risk and mortality inputs are age and sex specific. The comprehensiveness of the model used is a key strength of the study |
| D2a | Baseline data | Is the choice of baseline data described and justified? | Y | The main data source to populate the model was the XENDOS study.169 This is appropriate and justified given the double-blind randomised nature of the study. Other secondary data sources are used and justified appropriately |
| Are transition probabilities calculated appropriately? | Y | Transition probabilities between the three key health states are clearly described and justified, including detailed information on their calculations | ||
| Has a half-cycle correction been applied to both costs and outcomes? | N | No half-cycle correction has been included | ||
| If not, has this omission been justified? | N | No justification given | ||
| D2b | Treatment effects | If relative treatment effects have been derived from the trial data, have they been synthesised using appropriate techniques? | Y | Treatment effects and clinical input data are derived from a range of sources, most notably the XENDOS trial,169 which ensures the use of rigorous data because of the blinded randomised approach. No detailed data syntheses are reported in the study |
| Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | Y | Methods of extrapolation are clearly described in the study for each relevant parameter (e.g. cardiovascular risk, mortality, QALYs) | ||
| Have alternative extrapolation assumptions been explored through sensitivity analysis? | N | None documented – it would have been helpful to explore a lifetime model horizon | ||
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | Y | It appears that no continuing effect is assumed beyond the pivotal trial follow-up period, with weight seen to return to baseline levels at 4 years | ||
| Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis? | N | Assessments of alternative treatment effects have not been explicitly modelled | ||
| D2c | Costs | Are the costs incorporated into the model justified? | Y | Costs are appropriate to the decision model. Costs of the lifestyle intervention are not included; however, the authors point out that there would be little or no difference in costs between the intervention group and the control group as all study participants received the lifestyle intervention. Diabetes costs are incorporated and referenced but are not broken down into specific components of cost. The authors acknowledge this as a limitation of their analysis |
| Have the sources for all costs been described? | Y | Sources are clearly referenced throughout | ||
| Have discount rates been described and justified given the target decision-maker? | Y | Discount rates for costs and QALYs are 3.5% per annum and are appropriate given best practice guidelines for discounting | ||
| D2d | Quality of life weights (utilities) | Are the utilities incorporated into the model appropriate? | Y | Utility weights used appear to be appropriate and are taken from published literature; however, methods of utility estimation are not clearly described |
| Is the source for the utility weights referenced? | Y | Sources are clearly referenced | ||
| Are the methods of derivation for the utility weights justified? | ? | Not clear – methods of utility estimation from the source studies are not described in detail | ||
| D3 | Data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | Y | All important data sources are sufficiently referenced |
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | NA | There do not appear to be any mutually inconsistent data in the study | ||
| Is the process of data incorporation transparent? | Y | Data incorporation is clearly explained and transparent. It is clear that sex-specific inputs run throughout the model from input to risk calculation to final mortality rate estimation. Sex-specific utilities are also used | ||
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | Y | Choices of distributions are clearly presented and are appropriate | ||
| If data have been incorporated as distributions, is it clear that second-order uncertainty is reflected? | Y | Monte Carlo simulation of the Markov model is clearly undertaken | ||
| D4 | Assessment of uncertainty | Have the four principal types of uncertainty been addressed? | N | Structural uncertainties are not considered in the sensitivity analysis |
| If not, has the omission of particular forms of uncertainty been justified? | N | None reported | ||
| D4a | Methodological | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | Y | The model has been rerun on the basis of alternative payers for orlistat [Italian patients – base case; Italian NHS – sensitivity (scenario) analysis]. No sensitivity analysis was carried out around the discount rate used in the model |
| D4b | Structural | Is there evidence that structural uncertainties have been addressed through sensitivity analysis? | N | No structural sensitivity analyses were undertaken |
| D4c | Heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | Y | The model is rerun for age- and sex-specific subgroups. Data inputs are age and sex specific and risks, etc., flow the whole way from model input to final output analysis. A separate model is run for an impaired glucose tolerance subgroup |
| D4d | Parameter | Are the methods of assessment of parameter uncertainty appropriate? | Y | Comprehensive probabilistic analyses are undertaken to investigate parameter uncertainty |
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | Y | Ranges are sampled from the appropriate distributions for the probabilistic sensitivity analysis | ||
| Consistency | ||||
| C1 | Internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | Y | Convergence and overall stability of the model have been thoroughly checked. Multiple chains were run and both visual and Gelman–Rubin–Brooks diagnostics were performed |
| C2 | External consistency | Are any counterintuitive results from the model explained and justified? | NA | Results do not appear to be counterintuitive and have good face validity |
| If the model has been calibrated against independent data, have any differences been explained and justified? | Y | All data are incorporated into the model and are in broad agreement with similar independent data sources | ||
| Have the results of the model been compared with those of previous models and any differences in results explained? | Y | A comprehensive comparison with other similar models is carried out and hypotheses drawn up as to why differences may exist across the studies considered | ||
Maetzel 2003166
| Quality criterion | Dimension of quality | Question | Response | Comments |
|---|---|---|---|---|
| Structure | ||||
| S1 | Statement of decision problem/objective | Is there a clear statement of the decision problem? | Y | The decision problem was to evaluate the economic value of pharmacological treatment of type 2 diabetes mellitus in overweight and obese patients using orlistat in conjunction with standard diabetes therapies and weight-loss management, compared with standard therapy and weight-loss management alone |
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | Y | The objective of the model was to evaluate the cost-effectiveness of orlistat in addition to standard diabetes treatment (including sulphonylureas, metformin or insulin plus diet and physical activity). The model objective is thus consistent with the decision problem | ||
| Is the primary decision-maker specified? | N | Not explicitly stated; however, the study was funded by Roche pharmaceuticals and two of the authors are employees of the company | ||
| S2 | Statement of scope/perspective | Is the perspective of the model clearly stated? | Y | Perspective was that of a US health-care provider |
| Are the model inputs consistent with the stated perspective? | Y | Costs are included that are relevant to a health-care provider in the US setting (both drug and secondary care costs) and consistent with the perspective stated. Indirect costs for a range of health states are also included | ||
| Has the scope of the model been stated and justified? | N | Not explicitly stated; however, this is clear from the objectives | ||
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | Y | Outcomes are measured as event-free LYG. Outcomes in the form of QALYs may have been preferable; however, the authors address this in the limitations section | ||
| S3 | Rationale for structure | Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Y | A range of health states linked to diabetes are included and are relevant given the decision problem |
| Are the sources of data used to develop the structure of the model specified? | N | The comparators for the model are clearly explained and referenced. There is, however, no evidence of systematic searching to inform the most important health states for the model | ||
| Are the causal relationships described by the model structure justified appropriately? | Y | Causal relationships between weight loss, HbA1c level and risk of developing complications are clearly described, as is the link to health states | ||
| S4 | Structural assumptions | Are the structural assumptions transparent and justified? | Y | Structural assumptions are clearly outlined and justified in a separate section of the article |
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | Y | Given the data availability, the structural assumptions are clear and valid. When assumptions have been made, these are discussed in detail in the discussion with clear acknowledgement of the likely negative implications for the study results of simplifying assumptions | ||
| S5 | Strategies/comparators | Is there a clear definition of the options under evaluation? | Y | Intervention and comparator are broadly described. Specific details of the exercise and diet advice are not provided; however, given that these are the same across groups, there is no impact on cost-effectiveness results |
| Have all feasible and practical options been evaluated? | Y | Given the decision problem to evaluate orlistat, all practical options are considered | ||
| Is there justification for the exclusion of feasible options? | NA | |||
| S6 | Model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | Y | A Markov state transition model is appropriate for the evaluation and modelling of complications and dynamics of a chronic disease such as diabetes |
| S7 | Time horizon | Is the time horizon of the model sufficient to reflect all important differences between options? | ? | Probably, although it is not clear whether the 11-year time horizon for the model is sufficient to capture all of the health-related effects of diabetes linked to improving HbA1c levels and weight loss |
| Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | Y | All are clearly described and justified. Limitations are acknowledged because of uncertainties of weight regain and duration of treatment effect, including transient weight loss and its impact on longer-term health outcomes. The choice of time horizon is justified on the basis of a single study which found that intensive blood glucose control for 10 years substantially reduced diabetes-related complications | ||
| S8 | Disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | Y | The states modelled appear to be appropriate for the condition that the model has been developed for |
| S9 | Cycle length | Is the cycle length defined and justified in terms of the natural history of disease? | Y/N | Cycle length of 1 year is clearly defined; however, no explicit justification is given. A cycle length of 1 year is probably sufficient to capture the dynamics of a disease such as diabetes and the annualised risks of developing complications related to diabetes |
| Data | ||||
| D1 | Data identification | Are the data identification methods transparent and appropriate given the objectives of the model? | Y | All data used in the model are clearly defined. Although there is no evidence of systematic searching of the literature, the effectiveness of the drug was estimated using a meta-analysis of key RCTs and risks of complications adapted from the well-known UKPDS170 |
| When choices have been made between data sources, are these justified appropriately? | N | Choices between alternative data sources have not been discussed in the paper, although it would appear that the data used are appropriate to the objective of the model. It is not clear why the UKPDS data are specific only to a 52-year-old man. There is no discussion presented around this assumption/interpretation of the results from the UKPDS170 | ||
| Has particular attention been paid to identifying data for the important parameters in the model? | Y | A meta-analysis was undertaken of four placebo-controlled RCTs in overweight or obese adults to measure the effectiveness of orlistat in terms of reduced HbA1c levels. Reduced HbA1c levels were used to derive the reduction in diabetes incidence attributable to treatment, using the UKPDS170 | ||
| Has the quality of the data been assessed appropriately? | ? | It is not clear how the quality of the data inputs was assessed | ||
| When expert opinion has been used, are the methods described and justified? | NA | There is no reference to the use of expert opinion in the study, although one would assume that expert opinion might have been sought to inform some of the key model assumptions, including the health states included | ||
| D2 | Data modelling | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Y | Markov modelling is used and is appropriate given the decision problem and objective of the evaluation |
| D2a | Baseline data | Is the choice of baseline data described and justified? | Y | Baseline relative risk data are taken from the UKPDS170 and are appropriate to the study decision problem |
| Are transition probabilities calculated appropriately? | ? | The calculation of transition probabilities is not explicitly addressed. Risk data are taken from the UKPDS170 and are based on a selective demographic of the population. Alternative methods of selecting risks were not addressed | ||
| Has a half-cycle correction been applied to both costs and outcomes? | N | No half-cycle correction is mentioned | ||
| If not, has this omission been justified? | N | No justification given | ||
| D2b | Treatment effects | If relative treatment effects have been derived from the trial data, have they been synthesised using appropriate techniques? | ? | Treatment effects have been synthesised using meta-analysis; however, details of the methods of the data synthesis are not clearly described. Methods are not described in sufficient detail to quality assess the meta-analysis used |
| Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | Y | Methods of extrapolation are detailed in the study and these are justified in the text. Alternative assumptions regarding the maintenance of treatment effect are explored in sensitivity analysis | ||
| Have alternative extrapolation assumptions been explored through sensitivity analysis? | Y | Alternative assumptions around the duration of treatment effect and the length of continued weight loss resulting from orlistat treatment are explored in sensitivity analysis and are found to have an impact on the cost-effectiveness results | ||
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | Y | Uncertainty surrounding the continued effect of orlistat on blood glucose levels and thus risk of complications related to diabetes is discussed | ||
| Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis? | Y | Key assumptions are described and tested in sensitivity analysis when appropriate. The authors explore a 1-year vs. a 3-year continuation of response based on alternative methods of calculating HbA1c data outcomes | ||
| D2c | Costs | Are the costs incorporated into the model justified? | Y | All costs included in the model are justified. It appears that given the perspective of the analysis all relevant costs have been included |
| Have the sources for all costs been described? | Y | All sources for costs are clearly referenced and individual cost elements for each diabetic complication were taken into account | ||
| Have discount rates been described and justified given the target decision-maker? | Y | Discount rates are 3% as appropriate | ||
| D2d | Quality of life weights (utilities) | Are the utilities incorporated into the model appropriate? | NA | Utilities were not considered as part of this model. This is a shortcoming of the analysis, which is acknowledged in the limitations section. No attempt was made to estimate utility weights for the various disease health states, at least some of which could have been retrieved from the literature and supplemented with author assumptions to obtain a broad idea of QALY outcomes |
| Is the source for the utility weights referenced? | NA | |||
| Are the methods of derivation for the utility weights justified? | NA | |||
| D3 | Data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | Y | All data sources are clearly described and referenced throughout |
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | NA | There are no mutually inconsistent data | ||
| Is the process of data incorporation transparent? | Y | Data are all incorporated clearly into the model, with clear links between source studies and final model outcomes. Understanding is aided by the diagram of the decision tree | ||
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | NA | Distributions were not explicitly assigned to individual parameters. Instead, sampling was conducted with 1000 estimates taken from CIs and focused around the mean estimate | ||
| If data have been incorporated as distributions, is it clear that second-order uncertainty is reflected? | Y | Yes, through Monte Carlo simulation with sampling from the reported CIs, with more sampling from around the mean of the distributions | ||
| D4 | Assessment of uncertainty | Have the four principal types of uncertainty been addressed? | N | Heterogeneity has not been accounted for, given that the results are applicable only to 52-year-old men, reflective of the results of the UKPDS.170 These limitations are acknowledged by the authors |
| If not, has the omission of particular forms of uncertainty been justified? | Y | Justification is provided based on the data availability from the source study; however, the results could have been rerun for other hypothetical inputs into the model and based on other published literature discussed by the authors | ||
| D4a | Methodological | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | Y | A range of alternative discounting rates is explored. Other methodoligical uncertainties are not considered in the sensitivity analyses |
| D4b | Structural | Is there evidence that structural uncertainties have been addressed through sensitivity analysis? | Y | Alternative assumptions surrounding the continued effect of treatment are explored in sensitivity analysis |
| D4c | Heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | N | Heterogeneity has not been addressed (see above) |
| D4d | Parameter | Are the methods of assessment of parameter uncertainty appropriate? | Y | Probabilistic sensitivity analysis is conducted. The inclusion of parameters is extensive in terms of effectiveness data. Parameter uncertainty is reflected through sampling from CIs of the key clinical outcome parameters. It is stated that cost data are also included in the probabilistic sensitivity analysis, although ranges/CIs are not included. It would appear that sampling may have been conducted around total cost estimates although this is not explicitly clear from the article |
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | Y | CIs for distributions are provided. No other analyses focused on ranges apart from those considered in the probabilistic sensitivity analysis | ||
| Consistency | ||||
| C1 | Internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | N | No evidence of validation of the mathematical logic of the model was provided; however, the modelling process used is well known and widely validated in the health economics field |
| C2 | External consistency | Are any counterintuitive results from the model explained and justified? | NA | Counterintuitive results did not emerge from the model. A detailed discussion of the model results is given in the discussion section |
| If the model has been calibrated against independent data, have any differences been explained and justified? | N | No evidence of model calibration | ||
| Have the results of the model been compared with those of previous models and any differences in results explained? | Y | The authors engage in a detailed comparison of their results with the results of similar studies. Differences between the results of this study and those of a previously published model were discussed. Problems associated with generalisability (especially to a female group of the population) were addressed and limitations appropriately acknowledged | ||
Olsen 2005165
| Quality criterion | Dimension of quality | Question | Response | Comments |
|---|---|---|---|---|
| Structure | ||||
| S1 | Statement of decision problem/objective | Is there a clear statement of the decision problem? | Y | The decision problem is to determine the costs and effects in terms of LYG of providing nutritional counselling by a GP or dietitian |
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | Y | The objective of the valuation is to compare nutritional counselling by a GP with that provided by a dietitian to patients with obesity and a high risk of ischaemic heart disease. The objective of the model was to measure LYG and LYG without IHD, accounting for a range of risk factors | ||
| Is the primary decision-maker specified? | N | It is not clear who funded the study; however, it is stated that the study was supposed to form the basis for future decisions on the organisation of nutritional counselling in primary care | ||
| S2 | Statement of scope/perspective | Is the perspective of the model clearly stated? | ? | The perspective of the model is not explicitly stated, although a patient, health services and even a societal perspective appear to be considered. The final results appear to be from a health services perspective |
| Are the model inputs consistent with the stated perspective? | N | Although the perspective is not explicitly stated, it appears that the costing data used refer to a health services perspective for the base-case analysis. Patients’ use of time was identified in the methods and reported in the sensitivity analysis results. It was also stated that production losses were considered, although the model inputs and results were not reported for this perspective | ||
| Has the scope of the model been stated and justified? | N | Not explicitly stated but appears to be clear from the objective of the study and model | ||
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | ? | Given the lack of clarity regarding the perspective, outlined above, it is not clear whether or not costs are consistent with the analysis perspective. For example, productivity costs and the methods to derive their estimates are not clearly stated | ||
| S3 | Rationale for structure | Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | N | The modelling of life-years is appropriate; however, costs are considered only over a short time horizon, which is insufficient to measure the impact of future obesity-related health events. The use of QALYs would provide a more robust analysis for decision-makers |
| Are the sources of data used to develop the structure of the model specified? | N | The model was developed by the authors and no data have been identified to inform the model structure specifically. No explicit justification was given for the chosen model structure over any plausible alternatives. There is no evidence of any systematic approach to deciding on the most appropriate model structure | ||
| Are the causal relationships described by the model structure justified appropriately? | Y | Causal relationships within the model appear justified and appropriate, given the model design and decision problem. Mathematical models are clearly defined and explained. There is no clear link specified between weight loss and final outcomes. Weight-loss data are not reported | ||
| S4 | Structural assumptions | Are the structural assumptions transparent and justified? | ? | There is no clear list of assumptions. It is assumed that the effect of the lifestyle intervention is maintained throughout the study period |
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | N | Structural assumptions are not clear. The assumption of maintenance of effect is unreasonable and likely introduces bias to the study results in favour of the intervention being evaluated | ||
| S5 | Strategies/comparators | Is there a clear definition of the options under evaluation? | Y | GP or dietitian advice compared with no routine advice |
| Have all feasible and practical options been evaluated? | Y | Other options may be of relevance but, given the scope and objective of the model, the interventions included could be deemed appropriate. No other plausible interventions have been explored for inclusion | ||
| Is there justification for the exclusion of feasible options? | NA | None reported | ||
| S6 | Model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | N | The decision problem is to estimate the costs and effects; however, lifelong costs are omitted. The model is inadequate to fully capture the long-term dynamics of a lifestyle intervention |
| S7 | Time horizon | Is the time horizon of the model sufficient to reflect all important differences between options? | N | The time horizon is appropriate for estimation of differences in life-years; however, it is inappropriate for the long-term cost implications in terms of reduced cardiovascular events |
| Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | Y/N | The time horizon of the model is clearly described, as is the duration of treatment. The assumption that treatment effect is sustained for the duration of the study is not appropriate. This is acknowledged as a limitation; however, no attempt was made to remedy this or investigate its impact through sensitivity analysis | ||
| S8 | Disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | NA | Dynamic state transition models were not considered |
| S9 | Cycle length | Is the cycle length defined and justified in terms of the natural history of disease? | NA | The model did not contain cycles |
| Data | ||||
| D1 | Data identification | Are the data identification methods transparent and appropriate given the objectives of the model? | Y | Baseline risk scores are taken from two Danish population studies. Effects were estimated from a meta-analysis of nine RCTs. References are clear throughout. Costing of the intervention is clearly described |
| When choices have been made between data sources, are these justified appropriately? | ? | It is not clear what other choices for data sources were available. There is no clear systematic discussion of the available evidence so it is not possible to assess the appropriateness of any choices that were made | ||
| Has particular attention been paid to identifying data for the important parameters in the model? | Y | Key baseline data and data used in the model are clearly described and referenced | ||
| Has the quality of the data been assessed appropriately? | N | The authors do not present a comprehensive discussion of all of the shortcomings of their data, despite acknowledging some of the limitations | ||
| When expert opinion has been used, are the methods described and justified? | NA | No expert opinion is referred to in the study | ||
| D2 | Data modelling | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Y | The methods used to develop the model appear to be appropriate given the model structure chosen; however, this form of model is not ideal to measure the lifetime cost and effect dynamics of a lifestyle intervention to encourage weight loss |
| D2a | Baseline data | Is the choice of baseline data described and justified? | Y | Baseline data are clearly identified and are appropriate inputs for the model used. Further details on the methods used for the meta-analysis of treatment effect would have improved the study |
| Are transition probabilities calculated appropriately? | NA | |||
| Has a half-cycle correction been applied to both costs and outcomes? | NA | |||
| If not, has this omission been justified? | NA | |||
| D2b | Treatment effects | If relative treatment effects have been derived from the trial data, have they been synthesised using appropriate techniques? | ? | It is not clear whether or not the synthesis of treatment effects from the nine RCTs meta-analysed was appropriate as details of the methods used in the meta-analysis are not reported |
| Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | Y | Extrapolation of risk factors to LYG is appropriate given the model used to answer this decision problem. However, alternative models may have been more appropriate for the disease pathway | ||
| Have alternative extrapolation assumptions been explored through sensitivity analysis? | N | The model for extrapolation to LYG was not explored in sensitivity analysis | ||
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | Y/N | It is assumed that the effects of the lifestyle intervention will be maintained over a lifetime. This is acknowledged by the authors as a key limitation of the study. Although the assumption is documented, it is not necessarily justified and is inappropriate given the disease pathway and known poor maintenance of treatment effect once support is removed | ||
| Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis? | N | No alternative sensitivity analyses have been presented for assumptions relating to the continuation of treatment effects | ||
| D2c | Costs | Are the costs incorporated into the model justified? | N | Costs were described and included for the intervention only. Longer-term cost implications have not been explored. It is not clear how productivity costs and/or patient-related costs are incorporated |
| Have the sources for all costs been described? | Y | The sources for all costs that were included in the model are clearly referenced and described; however, detailed unit costs are not presented, rendering a theoretical reproduction of the study results difficult | ||
| Have discount rates been described and justified given the target decision-maker? | Y | Discount rates of 5% were applied to life-years only. This may be appropriate but was not tested in sensitivity analysis. Costs were not discounted as all costs considered occurred within a 1-year time frame | ||
| D2d | Quality of life weights (utilities) | Are the utilities incorporated into the model appropriate? | NA | No utilities were incorporated into the model |
| Is the source for the utility weights referenced? | NA | |||
| Are the methods of derivation for the utility weights justified? | NA | |||
| D3 | Data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | Y | All data are referenced and described appropriately |
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | NA | No mutually inconsistent data | ||
| Is the process of data incorporation transparent? | Y | Data are clearly incorporated | ||
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | NA | Data are not incorporated as distributions | ||
| If data have been incorporated as distributions, is it clear that second-order uncertainty is reflected? | NA | Data are not incorporated as distributions | ||
| D4 | Assessment of uncertainty | Have the four principal types of uncertainty been addressed? | N | No structural uncertainty was addressed |
| If not, has the omission of particular forms of uncertainty been justified? | N | No justification given | ||
| D4a | Methodological | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | Y | A range of methodological uncertainties was explored in the analysis. Sensitivity analysis, including a value for patients’ own use of time, was considered. Outcomes were presented in terms of LYG without ischaemic heart disease as well as the base case of LYG. Calculations were rerun based on interchanging GP and dietitian costs of delivering the respective interventions |
| D4b | Structural | Is there evidence that structural uncertainties have been addressed through sensitivity analysis? | N | The model has not been revised to use any alternatively plausible structures, such as a Markov model structure |
| D4c | Heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | Y | Heterogeneity is addressed and results are reported for male/female subgroups |
| D4d | Parameter | Are the methods of assessment of parameter uncertainty appropriate? | Y | Uncertainty in costs and effects is addressed using bootstrapping of cost and effect differences to determine the precision of the ICERs and to calculate CIs. No alternative values of input parameters were explored in the model |
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | NA | Point estimates are presented alongside CIs of cost and effect outcomes. The impact of uncertainty was not included in a sensitivity analysis | ||
| Consistency | ||||
| C1 | Internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | N | The model structure and mathematical equations were not tested for consistency within the study; however, the model used is a common approach adopted for survival analysis |
| C2 | External consistency | Are any counterintuitive results from the model explained and justified? | NA | No counterintuitive results have been reported |
| If the model has been calibrated against independent data, have any differences been explained and justified? | NA | No calibration undertaken | ||
| Have the results of the model been compared with those of previous models and any differences in results explained? | Y | The model results are compared with those of a range of other similar studies. However, no comparison is made to sex-specific subgroups from other studies in the literature. The study results appear to be broadly aligned with the literature for both sexes together, considering the differing assumptions in some of the models | ||
Segal 1998163
| Quality criterion | Dimension of quality | Question | Response | Comments |
|---|---|---|---|---|
| Structure | ||||
| S1 | Statement of decision problem/objective | Is there a clear statement of the decision problem? | Y | The decision problem is to determine the cost-effectiveness of methods for the prevention of type 2 diabetes and to determine if some approaches are more cost-effective than other uses of health-care resources |
| Is the objective of the evaluation and model specified and consistent with the stated decision problem? | Y | The key objective of the evaluation is to use cost-effectiveness analysis to determine the role of type 2 diabetes prevention and to ascertain whether or not programmes for primary prevention are cost-effective and whether or not certain programmes are more cost-effective than others. The model objective was to track states of impaired glucose tolerance/normal glucose tolerance/type 2 diabetes for intervention and control cohorts | ||
| Is the primary decision-maker specified? | N | None stated; however, the project was funded by the Department of Human Services, Victoria, Australia. It could be assumed that the target audience for the article is an Australian decision-making body | ||
| S2 | Statement of scope/perspective | Is the perspective of the model clearly stated? | Y | Although not explicitly stated, the perspective is clearly that of the health services provider |
| Are the model inputs consistent with the stated perspective? | Y | Costs relate to intervention cost and downstream cost savings associated with each intervention group and are thus consistent with the assumed perspective | ||
| Has the scope of the model been stated and justified? | Y | The scope of the model is to evaluate life-years, diabetic state and short- and long-term costs of a range of interventions | ||
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | ? | Yes; however, not all results and outcomes were comprehensively reported for each individual programme considered. Outcomes would be better reported as QALYs although this was not possible because of a lack of information on utility weights for the health states | ||
| S3 | Rationale for structure | Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Y | Given the objective to look at only diabetes as a health condition, the structure of the model is appropriate. However, a more dynamic model of diabetes may be required to fully address progression between diabetes health states |
| Are the sources of data used to develop the structure of the model specified? | N | It is not clear what, if any, data sources were used to develop the structure of the model | ||
| Are the causal relationships described by the model structure justified appropriately? | N | Causal relationships are not clearly presented for each individual programme. There are inadequate data specific to each programme to make a judgement on how causal relationships are generated. Further data have been requested from the study authors but were not available at the time of publication | ||
| S4 | Structural assumptions | Are the structural assumptions transparent and justified? | Y | Key assumptions are noted in the methods section, especially with regard to the definition of success or not in terms of continuation of weight loss within the model. The authors acknowledge that a more dynamic structure would have been better; however, because of a lack of data this was not possible |
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | Y | The assumptions stated are reasonable; however, it was not possible to assess the appropriateness of all assumptions and their application to each individual programme | ||
| S5 | Strategies/comparators | Is there a clear definition of the options under evaluation? | ? | There is mixed information available for each of the six programmes and further systematic information is required for all six interventions in terms of who delivered the intervention, how long it was delivered for, in what setting, the frequency of classes, etc. Information summarising key trials for each programme are available from the authors on request; details were requested but were not available at the time of publication |
| Have all feasible and practical options been evaluated? | Y | Interventions were included based on a systematic review of the literature and a detailed process of selection of the most appropriate interventions | ||
| Is there justification for the exclusion of feasible options? | Y | Diabetes drug therapies were excluded as they refer to management of disease rather than prevention | ||
| S6 | Model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | Y | The broad model type is appropriate for measuring longer-term costs and outcomes of the disease pathway |
| S7 | Time horizon | Is the time horizon of the model sufficient to reflect all important differences between options? | Y | A time horizon of 25 years post intervention seems appropriate to capture relevant cost and mortality differences |
| Are the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | ? | The time horizon is described clearly but not justified. The duration of treatment is clear for programme IV but is not justified explicitly. Assumptions regarding the duration of treatment effect are clear and tested in sensitivity analysis through the use of alternative success rates | ||
| S8 | Disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in question and the impact of interventions? | Y | States of impaired glucose tolerance and normal glucose tolerance are appropriate given the decision problem and objective of the model; however, the inclusion of other states could have improved the relevance of the model, were the data available |
| S9 | Cycle length | Is the cycle length defined and justified in terms of the natural history of disease? | Y/N | Cycle length is defined as 5-yearly but this appears to be an arbitrary choice with no clear justification given |
| Data | ||||
| D1 | Data identification | Are the data identification methods transparent and appropriate given the objectives of the model? | ? | Transparent for some but not all programmes. Data were well defined for the men-only programme |
| When choices have been made between data sources, are these justified appropriately? | ? | No clear evidence of systematic searches for data and evidence and no documentation of key choices that were made nor any justification for such choices. However, the choice of some data inputs was validated against peer-reviewed studies (e.g. the impact of weight loss on type 2 diabetes incidence) | ||
| Has particular attention been paid to identifying data for the important parameters in the model? | Y | A range of sources is referenced for key data inputs, especially in relation to mortality estimates. Details of downstream costs are clearly referenced. Although systematic searches are not described, it appears as if the most relevant data are included | ||
| Has the quality of the data been assessed appropriately? | N | There is no critical assessment/critique/quality assessment of the data used to inform the model beyond implied quality as data are from RCTs | ||
| When expert opinion has been used, are the methods described and justified? | N | It is stated that expert clinical advice is used in combination with studies from the literature to inform the process of identifying transition matrices; however, no further details are provided. It is not clear where model inputs (e.g. transition probabilities) are sourced from (e.g. published literature or clinical expert opinion, nor is it clear if model inputs are programme specific or if similar input data were used for all programmes | ||
| D2 | Data modelling | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Y | Extrapolation methodology is broadly appropriate and appears justifiable and to reflect best practice at the time that the study was conducted |
| D2a | Baseline data | Is the choice of baseline data described and justified? | Y/N | Baseline data are described but not clearly justified |
| Are transition probabilities calculated appropriately? | ? | Unclear. Expert opinion was used to inform transition matrices; however, it is unclear how this was used | ||
| Has a half-cycle correction been applied to both costs and outcomes? | N | No half-cycle correction reported | ||
| If not, has this omission been justified? | N | No justification or mention of half-cycle correction being omitted | ||
| D2b | Treatment effects | If relative treatment effects have been derived from the trial data, have they been synthesised using appropriate techniques? | ? | Not clear – insufficient information provided for each individual programme with regard to baseline trial data. No syntheses of outcome data were reported and it is assumed that no such meta-analyses were conducted |
| Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | Y | The method of extrapolation uses 5-yearly transitions between impaired glucose tolerance and normal glucose tolerance and appears appropriate given the decision problem. A comprehensive list of references for mortality estimation, including national sources, is provided | ||
| Have alternative extrapolation assumptions been explored through sensitivity analysis? | N | No alternative extrapolation approaches are considered | ||
| Have assumptions regarding the continuing effect of treatment once treatment is complete been documented and justified? | Y | Model assumptions have been clearly documented. There were no data on continued treatment effect over time, which limits the usefulness of the cost-effectiveness conclusions | ||
| Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis? | Y | Sensitivity analyses are conducted for programme IV based on altering success rates and hence mortality vectors within the model. A range of other sensitivity analyses was undertaken and reported only for another programme, for which data were not male specific | ||
| D2c | Costs | Are the costs incorporated into the model justified? | Y | Cost data are included. There is no detail of how the costs of each individual intervention are calculated and unit costs are not presented. Total net costs over follow-up are not presented for each intervention |
| Have the sources for all costs been described? | Y | Sources are described although details are not thoroughly reported | ||
| Have discount rates been described and justified given the target decision-maker? | Y | 5% discount rate used for costs and life-years | ||
| D2d | Quality of life weights (utilities) | Are the utilities incorporated into the model appropriate? | NA | Utilities were not included in the model. The authors acknowledge the value of QALYs; however, information to inform such a calculation was not available at the time of the study |
| Is the source for the utility weights referenced? | NA | |||
| Are the methods of derivation for the utility weights justified? | NA | |||
| D3 | Data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | Y/N | Data sources are referenced although it is not clear how each source is applied to each programme. Further details are required to adequately quality assess this point |
| Has the use of mutually inconsistent data been justified (i.e. are assumptions and choices appropriate)? | NA | No mutually inconsistent data | ||
| Is the process of data incorporation transparent? | N | Because of the large number of programmes considered, it is not clear how data are incorporated on a programme-specific basis. Details are, however, provided for programme IV (men-only group) | ||
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | NA | Data were not incorporated as distributions | ||
| If data have been incorporated as distributions, is it clear that second-order uncertainty is reflected? | NA | |||
| D4 | Assessment of uncertainty | Have the four principal types of uncertainty been addressed? | N | Uncertainty was addressed through extensive sensitivity analysis only for one programme; this was not related to the men-only intervention (programme IV) |
| If not, has the omission of particular forms of uncertainty been justified? | N | Only one of the programmes evaluated report sensitivity analyses in detail. No sensitivity analyses are reported for the male-specific programme. There is no justification given as to why this programme was selected as the only one on which to report sensitivity analyses. A sensitivity analysis of treatment success rate was carried out for all programmes | ||
| D4a | Methodological | Have methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | N | Not for all programmes – methodological uncertainty was investigated for the behavioural programme for the seriously obese only, in terms of adjusting the discount rate. Details and results were not presented for the male-specific programme IV |
| D4b | Structural | Is there evidence that structural uncertainties have been addressed through sensitivity analysis? | Y | Gross costs (intervention costs only) and net costs (intervention delivery and downstream costs) were provided |
| D4c | Heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | Y | Analyses were rerun for impaired glucose tolerance only and mixed glucose tolerance subgroups for all programmes |
| D4d | Parameter | Are the methods of assessment of parameter uncertainty appropriate? | ? | Assumed patient success is varied within a sensible range of values; however, no other results were reported for other parameters specific to programme IV |
| If data are incorporated as point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | Y | Ranges are based on arbitrary numbers but are clear in their objective of characterising the uncertainty in the results | ||
| Consistency | ||||
| C1 | Internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | N | There are no reports of mathematical consistency checks applied to the model |
| C2 | External consistency | Are any counterintuitive results from the model explained and justified? | NA | Results do not appear counterintuitive and no such results were identified in the study |
| If the model has been calibrated against independent data, have any differences been explained and justified? | N | No discussion of any attempt to calibrate the results against independent data | ||
| Have the results of the model been compared with those of previous models and any differences in results explained? | N | Results of the overall study of six interventions were compared against other those of studies in the field and cost-effectiveness estimates were found to compare favourably with those in other studies. A detailed comparative discussion of the methods and associated results is not undertaken for programme IV | ||
Individual study data extraction forms
Galani 2007162
| Title: Modelling the lifetime costs and health effects of lifestyle intervention in the prevention and treatment of obesity in Switzerland | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study charateristics | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country of study | Switzerland | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Setting | Primary care | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Research question/objective | To quantify the lifetime health and economic consequences of preventing and treating obesity with a lifestyle intervention in Switzerland | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study design (model/RCT) | Markov model | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inclusion criteria | For the economic model inclusion criteria were overweight and obese by Swiss standards; minimum age 25 years; ages modelled 25, 35, 45 and 55 years. In the economic model, subjects have a BMI of 27 kg/m2 (overweight group) or 33 kg/m2 (obese group) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exclusion criteria | None specified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intervention | Overweight and obese subjects received the same lifestyle intervention of regular physical activity and low-fat reducing diet, including diets rich in fruit and vegetables (adapted from the FDPS171). Detailed dietary recommendations, including limiting intake of fat to < 30% of energy consumed and saturated fat to < 10% and increasing fibre to at least 15 g per 1000 kcal as well as advice about specific food types. Also asked to undertake moderate exercise for at least 30 minutes per day. Participants attended dietitian and supervised exercise sessions over the 3 years: seven dietitian visits in the first year with four visits per year thereafter over the remaining 2 years; four exercise sessions per month in the first year and two sessions per month thereafter in each subsequent year for a total of 3 years | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Control/comparison | Overweight subjects: no active intervention. Obese subjects: basic dietary counselling (three visits to the dietitian in year 1 and one visit per year thereafter) and physical exercise sessions (two sessions per month for the first year and one session per month thereafter) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Methods: costing | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Perspective | Societal perspective stated | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Currency | Swiss francs, converted to euros in the analysis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Currency year of reporting | All costs were updated to year 2006 using the consumer price index | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discount rate for costs | 3% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other costing information | The inclusion of indirect costs was not entirely clear, except that they were derived from the literature | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Methods: outcome measures | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Primary economic evaluation outcome | LYG and QALYs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discount rate for outcomes | 3% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Time horizon over which outcomes were assessed | 60 years, to a maximum age of 85 years | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Methods: modelling | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type of model used | Markov decision model | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Size of model cohort | 10,000 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Model time horizon | 60 years (for a 25-year-old) or to a maximum age of 85 years | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health states modelled | A total of seven health states were modelled: (1) overweight/obese (overweight or obese and free of complications); (2) hypertension (overweight or obese and BP > 140/90 mmHg); (3) hypercholesterolaemia (overweight or obese and total serum cholesterol ≥ 6.2 mmol/l); (4) diabetes (overweight or obese and type 2 diabetes: fasting glucose at least 7.8 mmol/l or 2-hour glucose at least 11.1 mmol/l); (5) stroke (overweight or obese and developed stroke); (6) coronary heart disease (overweight or obese and developed coronary heart disease); (7) death | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Methods: economic evaluations alongside RCTs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age group | NA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sex breakdown | NA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period of intervention | NA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period of follow-up | NA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n randomised to intervention | NA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n randomised to control | NA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Data completeness intervention | NA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Data completeness control | NA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Results, base-case costs: deterministic model analysis results | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intervention costs (male only) | Total cost data from the base-case model were not reported in detail. Unit costs (CHF) included were: Lifestyle intervention 1268 Standard care obese 573 Hypertension 1653 Type 2 diabetes 2890 Hypercholesterolaemia 1245 Coronary heart disease 6242 Stroke 11,495 |
Lifestyle intervention | 1268 | Standard care obese | 573 | Hypertension | 1653 | Type 2 diabetes | 2890 | Hypercholesterolaemia | 1245 | Coronary heart disease | 6242 | Stroke | 11,495 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lifestyle intervention | 1268 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard care obese | 573 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hypertension | 1653 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type 2 diabetes | 2890 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hypercholesterolaemia | 1245 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Coronary heart disease | 6242 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stroke | 11,495 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Comparator group costs (male only) | Cost of delivering the control arm of the study estimated as 573 CHF. No further details regarding total comparative costs reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Incremental costs (male only) | Total incremental costs (CHF) were reported as follows (Incremental data refer to long-term costs and effects per person per year.): Weight category 0% discounting 3% discounting Overweight +156 +405 Borderline obese –260 –6 Obese –70 +127 |
Weight category | 0% discounting | 3% discounting | Overweight | +156 | +405 | Borderline obese | –260 | –6 | Obese | –70 | +127 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight category | 0% discounting | 3% discounting | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overweight | +156 | +405 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Borderline obese | –260 | –6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Obese | –70 | +127 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Results, base-case primary economic outcome: deterministic model analysis results | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intervention group outcomes (male only) | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Comparator group outcomes (male only) | Individual results by intervention/control group not explicitly reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Incremental outcomes (male only) | Weight category 0% discounting 3% discounting Life-years (intervention – control) Overweight +0.03 +0.01 Borderline obese +0.03 +0.01 Obese +0.02 +0.01 QALYs (intervention – control) Overweight +0.29 +0.25 Borderline obese +0.32 +0.28 Obese +0.33 +0.29 | Weight category | 0% discounting | 3% discounting | Life-years (intervention – control) | Overweight | +0.03 | +0.01 | Borderline obese | +0.03 | +0.01 | Obese | +0.02 | +0.01 | QALYs (intervention – control) | Overweight | +0.29 | +0.25 | Borderline obese | +0.32 | +0.28 | Obese | +0.33 | +0.29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight category | 0% discounting | 3% discounting | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Life-years (intervention – control) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overweight | +0.03 | +0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Borderline obese | +0.03 | +0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Obese | +0.02 | +0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| QALYs (intervention – control) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overweight | +0.29 | +0.25 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Borderline obese | +0.32 | +0.28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Obese | +0.33 | +0.29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Results, primary economic analysis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Incremental results are as follows (all of these results are based on probabilistic analysis) | Weight category Age (years) 0% discounting 3% discounting ICERs (cost per life-year gained) (costs reported in CHF) Overweight 25 –2840 (D) 34,291 35 3006 30,934 45 3054 24,473 55 2787 17,149 Borderline obese 25 –19,496 (D) –14,886 (D) 35 –14,196 (D) –52,927 (D) 45 –14,158 (D) –9595 (D) 55 –13,282 (D) –10,417 (D) Obese 25 –12,657 (D) 58 35 –7373 (D) 5580 45 –8912 (D) 185 55 –8048 (D) –1745 (D) ICERs (cost per QALY gained) (costs reported in CHF) Overweight 25 –374 (D) 1854 35 395 2014 45 324 1457 55 237 914 Borderline obese 25 –2560 (D) –781 (D) 35 –283 (D) –331 (D) 45 –1373 (D) –523 (D) 55 –1027 (D) –508 (D) Obese 25 –1453 (D) 3 35 395 276 45 –741 (D) 9 55 –502 (D) –69 (D) | Weight category | Age (years) | 0% discounting | 3% discounting | ICERs (cost per life-year gained) (costs reported in CHF) | Overweight | 25 | –2840 (D) | 34,291 | 35 | 3006 | 30,934 | 45 | 3054 | 24,473 | 55 | 2787 | 17,149 | Borderline obese | 25 | –19,496 (D) | –14,886 (D) | 35 | –14,196 (D) | –52,927 (D) | 45 | –14,158 (D) | –9595 (D) | 55 | –13,282 (D) | –10,417 (D) | Obese | 25 | –12,657 (D) | 58 | 35 | –7373 (D) | 5580 | 45 | –8912 (D) | 185 | 55 | –8048 (D) | –1745 (D) | ICERs (cost per QALY gained) (costs reported in CHF) | Overweight | 25 | –374 (D) | 1854 | 35 | 395 | 2014 | 45 | 324 | 1457 | 55 | 237 | 914 | Borderline obese | 25 | –2560 (D) | –781 (D) | 35 | –283 (D) | –331 (D) | 45 | –1373 (D) | –523 (D) | 55 | –1027 (D) | –508 (D) | Obese | 25 | –1453 (D) | 3 | 35 | 395 | 276 | 45 | –741 (D) | 9 | 55 | –502 (D) | –69 (D) | ||||||||||
| Weight category | Age (years) | 0% discounting | 3% discounting | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ICERs (cost per life-year gained) (costs reported in CHF) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overweight | 25 | –2840 (D) | 34,291 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35 | 3006 | 30,934 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45 | 3054 | 24,473 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 55 | 2787 | 17,149 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Borderline obese | 25 | –19,496 (D) | –14,886 (D) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35 | –14,196 (D) | –52,927 (D) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45 | –14,158 (D) | –9595 (D) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 55 | –13,282 (D) | –10,417 (D) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Obese | 25 | –12,657 (D) | 58 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35 | –7373 (D) | 5580 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45 | –8912 (D) | 185 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 55 | –8048 (D) | –1745 (D) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ICERs (cost per QALY gained) (costs reported in CHF) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Overweight | 25 | –374 (D) | 1854 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35 | 395 | 2014 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45 | 324 | 1457 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 55 | 237 | 914 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Borderline obese | 25 | –2560 (D) | –781 (D) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35 | –283 (D) | –331 (D) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45 | –1373 (D) | –523 (D) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 55 | –1027 (D) | –508 (D) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Obese | 25 | –1453 (D) | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35 | 395 | 276 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45 | –741 (D) | 9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 55 | –502 (D) | –69 (D) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Results, secondary analysis (1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No other secondary analyses reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Results, sensitivity analysis (1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No other deterministic sensitivity analyses reported | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Results, subgroup analyses (1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No other subgroup analyses reported beyond those reported in the tables above | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uncertainty | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Probabilistic analysis undertaken? | Yes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Results reported for male subgroup? | Yes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Distribution for costs | Normal (intervention costs); gamma (costs of obesity complications) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parameter a | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parameter b | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Distribution for utilities | Beta | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parameter a | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parameter b | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Distribution for other | Normal (cardiovascular risk scores) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parameter a | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parameter b | NR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Results illustrated using (e.g. CEAC, scatter plot) | CEACs and scatter plots of the cost-effectiveness plane; scatter plot of cost-effectiveness results was not presented for a male-only subgroup | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key results of PSA (mean estimates of ICER) | See above | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Probability of cost-effectiveness | Men and women together: Overweight 5%, borderline obese 78%, obese 47% at WTP = 0 CHF; overweight 35%, borderline obese 99%, obese 92% at WTP = 1000 CHF Men only, borderline obese patients reported only (estimated from the graphs presented): Male age 25 years 70%, male age 35 years 57%, male age 45 years 68%, male age 55 years 72% at WTP = 0 CHF; male age 25 years 93%, male age 35 years 92%, male age 45 years 96%, male age 55 years 98% at WTP = 1000 CHF Results are on average (according to the graph) better for men than for women in terms of cost-effectiveness, especially for young men compared with young women. Results for borderline obese male and female subgroups, 3% discount rate |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other methodological issues | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Expected value of perfect information (EVPI) analysis undertaken? | No – a related study provides value of information analysis167 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Type | NA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Results | NA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Conclusions of EVPI analysis/recommendations for future research | NA – no EVPI analysis conducted as part of this study | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study strengths | The separate analysis of the borderline obese group allows the researchers to study the transition from overweight to obesity | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Study limitations (as identified by authors) | (1) The availability of additional Swiss-specific data would have improved the model (e.g. epidemiological data such as the correlation between BMI and risk of complications, obesity-related mortality and changes in utility for weight loss have not been recorded for Swiss populations); (2) future work should account for other complications of obesity such as metabolic syndrome, colorectal cancer, gall bladder disease, sleep apnoea and depression; (3) this study does not account for the impact of smoking as a risk factor for disease, although it has been documented that obese smokers have an increased risk of mortality; (4) the costs of obesity are estimated using secondary sources (the cost of stroke in this study is very high, although this was confirmed through a number of cost-of-illness studies) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Conclusions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key issues noted with regard to sex: (1) the average LYG using the lifestyle intervention compared with standard care in overweight, borderline obese and moderately obese patients were between 0.02 and 0.05 for men and women; (2) the average QALYs gained using the lifestyle intervention compared with usual care are greater for men than for women: 0.29 vs. 0.27 (overweight); 0.32 vs. 0.29 (borderline obese); 0.33 vs. 0.29 (morbidly obese) respectively; (3) incremental costs tended to be slightly lower for men than for women; (4) for overweight subjects, the ICER tended to favour younger (age 25 years) and older (age 85 years) men, with ICERs consistently but only marginally favourable in comparison to the female group (this was consistent across weight groups); (5) the intervention is highly cost-effective for all subgroups of age and sex with ICERs often dominant and always falling below 2014 CHF per QALY (men) and 6286 CHF per QALY (women) The key study conclusion was that lifestyle interventions are cost-effective for the long-term prevention and treatment of obesity |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Recommendations for future research | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| There is a need for pan-European research into the cost-effectiveness of interventions to tackle obesity | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Iannazzo 2008164
| Title: Economic evaluation of treatment with orlistat in Italian obese patients | |||||||||||||||||||||||
| Study characteristics | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country of study | Italy | ||||||||||||||||||||||
| Study setting | Not reported, presumably primary care | ||||||||||||||||||||||
| Research question/objective | To evaluate the economic impact of the use of orlistat plus a lifestyle intervention compared with the lifestyle intervention alone in Italian obese patients through the long-term projection of XENDOS study results169 | ||||||||||||||||||||||
| Study design (model/RCT) | Bayesian probabilistic Markov model extrapolating the findings of the XENDOS study169 | ||||||||||||||||||||||
| Inclusion criteria | XENDOS study: BMI ≥ 30 kg/m2; Italian obese patients, age 30–60 years (modelled cohort age ≥ 35 years) Age (SD): 57.5 (13.3) years (men only); 47.7% male, 52.3% female (Institute for Statistics data) |
||||||||||||||||||||||
| Exclusion criteria | None explicitly reported in this study, although the main study report will include exclusion criteria | ||||||||||||||||||||||
| Intervention | Orlistat (120 mg three times a day over 4 years) + lifestyle intervention. The lifestyle intervention consisted of a prescribed calorie-lowering diet with 30% of calories from fat and no more than 300 mg of cholesterol per day. Also encouraged to walk at least an extra 1 km per day | ||||||||||||||||||||||
| Control/comparison | Lifestyle intervention as described above in combination with a placebo drug given three times per day over 4 years | ||||||||||||||||||||||
| Methods: costing | |||||||||||||||||||||||
| Perspective | Societal perspective considered as orlistat is not available on the Italian NHS | ||||||||||||||||||||||
| Currency | Euros | ||||||||||||||||||||||
| Currency year of reporting | Not reported although assumed to be 2007 from reference list | ||||||||||||||||||||||
| Discount rate for costs | 3.5% | ||||||||||||||||||||||
| Methods: outcome measures | |||||||||||||||||||||||
| Primary economic evaluation outcome | QALYs | ||||||||||||||||||||||
| Discount rate for outcomes | 3.5% | ||||||||||||||||||||||
| Time horizon over which outcomes were assessed | 10 years (4 years of treatment and 6 years of follow-up); cycle length was 1 year | ||||||||||||||||||||||
| Methods: modelling | |||||||||||||||||||||||
| Type of model used | Bayesian probabilistic Markov model derived using random distributions from a Monte Carlo simulation | ||||||||||||||||||||||
| Size of model cohort | 30,000 iterations and excluding the first 15,000 to ensure the convergence of the two Bayesian fitting models. | ||||||||||||||||||||||
| Model time horizon | 10 years | ||||||||||||||||||||||
| Health states modelled/model description | Health states modelled: (1) obese; (2) diabetes; (3) death. The Markov model included two Bayesian statistical models fitted to the outcomes from the XENDOS data:169 (1) to predict diabetes incidence; (2) to forecast blood pressure and cholesterol changes from baseline (cardiovascular risk parameters). Mortality – transition to the death state in the model estimated using cardiovascular disease mortality Framingham risk equations and non-cardiovascular disease mortality. Utility for obese state extrapolated from the literature. Disutilities were associated with the risk of major cardiovascular events | ||||||||||||||||||||||
| Methods, economic evaluations alongside RCTs: XENDOS RCT data169 (baseline data for model) | |||||||||||||||||||||||
| Age group | 30–60 years, mean 43 years | ||||||||||||||||||||||
| Sex breakdown | XENDOS: NR; Italian population: 47.7% (men), 52.3% (women) | ||||||||||||||||||||||
| Period of intervention | 4 years | ||||||||||||||||||||||
| Period of follow-up | 4 years only (no additional follow-up in the RCT, hence the need for a model) | ||||||||||||||||||||||
| n randomised to intervention | NR in the modelling study | ||||||||||||||||||||||
| n randomised to control | NR in the modelling study | ||||||||||||||||||||||
| n randomised total | NR in the modelling study | ||||||||||||||||||||||
| Data completeness intervention | NR in the modelling study | ||||||||||||||||||||||
| Data completeness control | NR in the modelling study | ||||||||||||||||||||||
| Results, base-case costs | |||||||||||||||||||||||
| Intervention group costs (95% CI) | Men only: NR; men and women: €15,530 (€3342 to €117,000) | ||||||||||||||||||||||
| Comparator group costs (95% CI) | Men only: NR; men and women: €12,580 (€51 to €116,600) | ||||||||||||||||||||||
| Incremental costs (95% CI) | Men only: +€2931 (€383 to €3351); men and women: +€2948 (€369 to €3353) | ||||||||||||||||||||||
| Results, base-case primary economic outcome (QALYs) | |||||||||||||||||||||||
| Intervention group outcomes (95% CI) | Men only: NR; men and women: 6.13 (2.44 to 8.718) | ||||||||||||||||||||||
| Comparator group outcomes (95% CI) | Men only: NR; men and women: 6.084 (2.417 to 8.666) | ||||||||||||||||||||||
| Incremental outcomes (95% CI) | Men only: +0.046 (0.018 to 0.074); men and women: +0.046 (0.017 to 0.076) | ||||||||||||||||||||||
| Results, base-case results | |||||||||||||||||||||||
| Incremental costs (95% CI) | Men only: +€2931 (€383 to €3351); men and women: +€2948 (€369 to €3353) | ||||||||||||||||||||||
| Incremental outcomes (95% CI) | Men only: +0.046 (0.018 to 0.074); men and women: +0.046 (0.017 to 0.076) | ||||||||||||||||||||||
| ICER (95% CI) | Men only: €74,290 (€8408 to €179,600); men and women: €75,310 (€7613 to €180,600) | ||||||||||||||||||||||
| Results, subgroup analysis (1): impaired glucose tolerance | |||||||||||||||||||||||
| Incremental costs (95% CI) | Men only: NR; men and women: +€2237 (–€5601 to €3239) | ||||||||||||||||||||||
| Incremental outcomes (95% CI) | Men only: NR; men and women: +0.123 (0.048 to 0.199) | ||||||||||||||||||||||
| ICER (95% CI) | Men only: NR; men and women: €21,230 (dominant to €62,050) | ||||||||||||||||||||||
| Results, subgroup analysis (2): age < 60 years | |||||||||||||||||||||||
| Incremental costs (95% CI) | Men only: NR; men and women: +€2933 (–€312 to €3355) | ||||||||||||||||||||||
| Incremental outcomes (95% CI) | Men only: NR; men and women: +0.043 (0.017 to 0.072) | ||||||||||||||||||||||
| ICER (95% CI) | Men only: NR; men and women: €79,110 (dominant to €194,500) | ||||||||||||||||||||||
| Results, subgroup analysis (3): age ≥ 60 years | |||||||||||||||||||||||
| Incremental costs (95% CI) | Men only: NR; men and women: +€2918 (€895 to €3327) | ||||||||||||||||||||||
| Incremental outcomes (95% CI) | Men only: NR; men and women: +0.048 (0.019 to 0.077) | ||||||||||||||||||||||
| ICER (95% CI) | Men only: NR; men and women: €70,720 (€18,090 to €162,900) | ||||||||||||||||||||||
| Results, sensitivity analysis (1): orlistat is given to every obese patient and the NHS pays an estimated ex-factory price for it | |||||||||||||||||||||||
| Incremental costs (95% CI) | Men only: NR; men and women: NR | ||||||||||||||||||||||
| Incremental outcomes (95% CI) | Men only: NR; men and women: NR | ||||||||||||||||||||||
| ICER (95% CI) | Men only: NR; men and women: €42,300 (dominant to €108,700) | ||||||||||||||||||||||
| Results, sensitivity analysis (2): orlistat is given only to obese patients with impaired glucose tolerance | |||||||||||||||||||||||
| Incremental costs (95% CI) | Men only: NR; men and women: NR | ||||||||||||||||||||||
| Incremental outcomes (95% CI) | Men only: NR; men and women: NR | ||||||||||||||||||||||
| ICER (95% CI) | Men only: NR; men and women: €10,160 (dominant to €38,760) | ||||||||||||||||||||||
| Uncertainty | |||||||||||||||||||||||
| Probabilistic analysis undertaken? | Yes | ||||||||||||||||||||||
| Results reported for male subgroup? | No – results reported only for men and women together | ||||||||||||||||||||||
| Probabilistic distributions used in the model: | Distribution for Distribution Costs per patient per year (DIA) Gamma Cost per case (diabetes) Normal Cost per case (control) Normal Utilities, obese state (male) Beta Disutilities (DIA) Beta Disutilities (myocardial infarction) Beta Disutilities (stroke) Beta Non-cardiovascular disease mortality OB (male) Gamma Non-cardiovascular disease mortality DIA (male) Gamma DIA, obese patients with diabetes; OB, obese patients without diabetes. | Distribution for | Distribution | Costs per patient per year (DIA) | Gamma | Cost per case (diabetes) | Normal | Cost per case (control) | Normal | Utilities, obese state (male) | Beta | Disutilities (DIA) | Beta | Disutilities (myocardial infarction) | Beta | Disutilities (stroke) | Beta | Non-cardiovascular disease mortality OB (male) | Gamma | Non-cardiovascular disease mortality DIA (male) | Gamma | DIA, obese patients with diabetes; OB, obese patients without diabetes. | |
| Distribution for | Distribution | ||||||||||||||||||||||
| Costs per patient per year (DIA) | Gamma | ||||||||||||||||||||||
| Cost per case (diabetes) | Normal | ||||||||||||||||||||||
| Cost per case (control) | Normal | ||||||||||||||||||||||
| Utilities, obese state (male) | Beta | ||||||||||||||||||||||
| Disutilities (DIA) | Beta | ||||||||||||||||||||||
| Disutilities (myocardial infarction) | Beta | ||||||||||||||||||||||
| Disutilities (stroke) | Beta | ||||||||||||||||||||||
| Non-cardiovascular disease mortality OB (male) | Gamma | ||||||||||||||||||||||
| Non-cardiovascular disease mortality DIA (male) | Gamma | ||||||||||||||||||||||
| DIA, obese patients with diabetes; OB, obese patients without diabetes. | |||||||||||||||||||||||
| Results illustrated using (e.g. CEAC, scatter plot) | CEACs and scatter plots used to illustrate the data; however, none of the illustrations are presented separately for a male subgroup | ||||||||||||||||||||||
| Key results of PSA (mean estimates of ICER) | NR | ||||||||||||||||||||||
| Probability of cost-effectiveness | Base case: ∼15% at €45,000 per QALY gained; scenario 2 (impaired glucose tolerance only subgroup): 99.2% at €45,000 per QALY gained | ||||||||||||||||||||||
| Other methodological issues | |||||||||||||||||||||||
| Expected value of perfect information (EVPI) analysis undertaken? | No | ||||||||||||||||||||||
| Type | NA | ||||||||||||||||||||||
| Results: | NA | ||||||||||||||||||||||
| Conclusions of EVPI analysis/recommendations for future research | NA | ||||||||||||||||||||||
| Study strengths | (1) The authors use a novel modelling approach, based on detailed clinical data from the XENDOS trial;169 (2) the modelling includes Monte Carlo simulation within a probabilistic sensitivity analysis to address uncertainty; (3) integrated WinBUGS modelling – linking posterior distributions of each modelled parameter within a probabilistic framework; (4) the modelling process follows best practice ISPOR guidelines | ||||||||||||||||||||||
| Study limitations | (1) The cost of diabetes – to make a more accurate estimate a submodel to represent the dynamics of diabetes progression, complications and related costs would probably have been necessary; (2) the costs of the lifestyle intervention have not been included; this would impact on the budget but not on the incremental results for the economic evaluation given that the resource use was assumed to be equal across the randomised groups; (3) the model has a lack of predictive power for estimating cardiovascular risk impacts based on weight loss – the effects of orlistat were therefore lower than initially expected; (4) the model simulates cardiovascular disease risk parameters independently (e.g. blood pressure and cholesterol risk); however, these risk parameters could be cross-linked, resulting in a higher overall risk than that modelled; (5) Framingham equations are intrinsically conservative for the estimation of cardiovascular risks and the relationships between risk and weight reduction | ||||||||||||||||||||||
| Conclusions | |||||||||||||||||||||||
| If orlistat treatment is targeted at patients at high risk of developing diabetes, this treatment has an estimated cost–utility ratio of about €10,160 per QALY and is therefore highly cost-effective at usual threshold values of the maximum WTP for a QALY gain. There appears to be little or no difference between a male-only subgroup and both sexes (men and women considered together). However, this could be because the model is insensitive to links between weight loss and cardiovascular disease outcomes and therefore any potential differences by sex at the weight-loss stage might be missed using the model | |||||||||||||||||||||||
| Recommendations for future research | |||||||||||||||||||||||
| None explicitly made | |||||||||||||||||||||||
Maetzel 2003166
| Title: Economic evaluation of orlistat in overweight and obese patients with type 2 diabetes mellitus | |||||||||||||||||||||||||||||||||||||
| Study characteristics | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country of study | USA | ||||||||||||||||||||||||||||||||||||
| Research question/objective | To evaluate the cost-effectiveness of orlistat in addition to standard type 2 diabetes treatment in the treatment of overweight and obese people with diabetes (type 2) in a US-based health-care setting | ||||||||||||||||||||||||||||||||||||
| Study design (model/RCT) | Markov state transition model | ||||||||||||||||||||||||||||||||||||
| Inclusion criteria | Not specifically stated but the model refers to overweight and obese patients with type 2 diabetes mellitus | ||||||||||||||||||||||||||||||||||||
| Exclusion criteria | None stated | ||||||||||||||||||||||||||||||||||||
| Study setting | US health-care setting. Not explicitly stated whether secondary or primary care delivery | ||||||||||||||||||||||||||||||||||||
| Intervention | 11 years of standard diabetes therapy (ATG) + treatment with orlistat (120 mg three times per day for the first year), i.e. year 1 (orlistat + ATG), years 2–11 (ATG only). ATG = standard pharmacotherapy for type 2 diabetes (e.g. metformin) and weight management (diet and physical activity) | ||||||||||||||||||||||||||||||||||||
| Control/comparison | 11 years of therapy with ATG | ||||||||||||||||||||||||||||||||||||
| Methods: costing | |||||||||||||||||||||||||||||||||||||
| Perspective | US health-care provider | ||||||||||||||||||||||||||||||||||||
| Currency | US dollars | ||||||||||||||||||||||||||||||||||||
| Currency year of reporting | All costs inflated to 2001 US dollars using appropriate component of the consumer price index | ||||||||||||||||||||||||||||||||||||
| Discount rate for costs | 3% | ||||||||||||||||||||||||||||||||||||
| Other costing information | Costs included in the analysis were for (1) study drugs (orlistat, metformin, sulphonylurea and insulin) and (2) management of complications (included a variety of cost categories depending on the complication). Drug costs came from the National Prescription Audit. Costs were reported as costs for one-time treatment and follow-up for the specific complication considered in the model. Assumptions are described and justified | ||||||||||||||||||||||||||||||||||||
| Methods: outcome measures | |||||||||||||||||||||||||||||||||||||
| Primary economic evaluation outcome | Incremental cost per event-free life-year gained | ||||||||||||||||||||||||||||||||||||
| Discount rate for outcomes | 3% | ||||||||||||||||||||||||||||||||||||
| Time horizon over which outcomes were assessed | 11 years | ||||||||||||||||||||||||||||||||||||
| ATG, adherence to guidelines. | |||||||||||||||||||||||||||||||||||||
| Methods: modelling | |||||||||||||||||||||||||||||||||||||
| Type of model used | Markov state transition model | ||||||||||||||||||||||||||||||||||||
| Size of model cohort | 100,000 were simulated and run through the model | ||||||||||||||||||||||||||||||||||||
| Model time horizon | 11 years | ||||||||||||||||||||||||||||||||||||
| Health states modelled | (1) fatal or non-fatal myocardial infarction; (2) fatal or non-fatal stroke; (3) fatal or non-fatal microvascular disease; (4) amputation or death from peripheral vascular disease; (5) heart failure; (6) cataract extraction; (7) no complication | ||||||||||||||||||||||||||||||||||||
| Methods: economic evaluations alongside RCTs | |||||||||||||||||||||||||||||||||||||
| Age group: | NA | ||||||||||||||||||||||||||||||||||||
| Sex breakdown | NA | ||||||||||||||||||||||||||||||||||||
| Period of intervention | NA | ||||||||||||||||||||||||||||||||||||
| Period of follow-up | NA | ||||||||||||||||||||||||||||||||||||
| n randomised to intervention | NA | ||||||||||||||||||||||||||||||||||||
| n randomised to control | NA | ||||||||||||||||||||||||||||||||||||
| Data completeness intervention | NA | ||||||||||||||||||||||||||||||||||||
| Data completeness control | NA | ||||||||||||||||||||||||||||||||||||
| Results, primary economic analysis and sensitivity analysis | |||||||||||||||||||||||||||||||||||||
| Discount rate (%) Incremental costs [(ATG + orlistat) vs. ATG] (US$)a Incremental outcome [(ATG + orlistat) vs. ATG]a ICER (US$)a Mean updated HbA1c (3-year persistence of effect)b 3 +1122 +0.135 8327 0 +1099 +0.162 6791 5 +1136 +0.12 9462 Raw annual HbA1c (1-year persistence of effect)c 3 +1352 +0.057 23,574 0 +1365 +0.065 20,899 5 +1348 +0.052 25,827 | Discount rate (%) | Incremental costs [(ATG + orlistat) vs. ATG] (US$)a | Incremental outcome [(ATG + orlistat) vs. ATG]a | ICER (US$)a | Mean updated HbA1c (3-year persistence of effect)b | 3 | +1122 | +0.135 | 8327 | 0 | +1099 | +0.162 | 6791 | 5 | +1136 | +0.12 | 9462 | Raw annual HbA1c (1-year persistence of effect)c | 3 | +1352 | +0.057 | 23,574 | 0 | +1365 | +0.065 | 20,899 | 5 | +1348 | +0.052 | 25,827 | |||||||
| Discount rate (%) | Incremental costs [(ATG + orlistat) vs. ATG] (US$)a | Incremental outcome [(ATG + orlistat) vs. ATG]a | ICER (US$)a | ||||||||||||||||||||||||||||||||||
| Mean updated HbA1c (3-year persistence of effect)b | 3 | +1122 | +0.135 | 8327 | |||||||||||||||||||||||||||||||||
| 0 | +1099 | +0.162 | 6791 | ||||||||||||||||||||||||||||||||||
| 5 | +1136 | +0.12 | 9462 | ||||||||||||||||||||||||||||||||||
| Raw annual HbA1c (1-year persistence of effect)c | 3 | +1352 | +0.057 | 23,574 | |||||||||||||||||||||||||||||||||
| 0 | +1365 | +0.065 | 20,899 | ||||||||||||||||||||||||||||||||||
| 5 | +1348 | +0.052 | 25,827 | ||||||||||||||||||||||||||||||||||
| Uncertainty | |||||||||||||||||||||||||||||||||||||
| Probabilistic analysis undertaken? | Yes. PSA was applied by sampling from the CIs for clinical and cost outcomes (n = 1000 samples). Distributions were not specified for the parameters but sampling was more frequent around mean estimates | ||||||||||||||||||||||||||||||||||||
| Results reported for male subgroup? | Results are based on the UKPDS170 and are therefore based on specific risk factors for a 52-year-old male referent group | ||||||||||||||||||||||||||||||||||||
| Distribution for costs | Not specified | ||||||||||||||||||||||||||||||||||||
| Parameter a | Not specified | ||||||||||||||||||||||||||||||||||||
| Parameter b | Not specified | ||||||||||||||||||||||||||||||||||||
| Distribution for utilities | Not specified | ||||||||||||||||||||||||||||||||||||
| Parameter a | Not specified | ||||||||||||||||||||||||||||||||||||
| Parameter b | Not specified | ||||||||||||||||||||||||||||||||||||
| Distribution for other | Not specified | ||||||||||||||||||||||||||||||||||||
| Parameter a | Not specified | ||||||||||||||||||||||||||||||||||||
| Parameter b | Not specified | ||||||||||||||||||||||||||||||||||||
| Results illustrated using (e.g. CEAC, scatterplot) | CEACs | ||||||||||||||||||||||||||||||||||||
| Key results of PSA (including probability of cost-effectiveness) | Sensitivity analysis for the base case (i.e. mean imputed HbA1c – 3-year persistence of effect) showed that 95% of cost-effectiveness ratios fell under a threshold of slightly less than US$20 000 per event-free life-year gained. For the raw annual HbA1c (1-year persistence scenario), 95% of the cost-effectiveness ratios fell below a threshold of US$68,000 per event-free life-year gained. By studying graphically presented data it appears that the probability of cost-effectiveness is ∼30% for a 1-year persistence of effect and > 95% for a 3-year persistence of effect at a WTP of US$20,000 per event-free life-year gained; and ∼80–90% for a 1-year persistence of effect and > 95% for a 3-year persistence of effect at a WTP of US$50,000 per event-free life-year gained. Overall, it is clear that less benefit is achieved with a shorter persistence of orlistat effect (maintenance of weight-loss effect) | ||||||||||||||||||||||||||||||||||||
| Conclusions of sensitivity analysis | Use of raw annual HbA1c but 3-year persistence of effect led to an ICER of US$21,962 per event-free life-year gained, which is higher than the ICER observed using mean updated HbA1c. The assumption of 0% and 5% discounting led to ICERs of US$6791 and US$9462 per event-free life-year gained respectively | ||||||||||||||||||||||||||||||||||||
| Conclusions of subgroup analysis | Main subgroup analysis undertaken was based on raw/imputed (1–3-year persistence) HbA1c levels, showing as expected that a longer persistence of effect yields greater benefits and is more cost-effective | ||||||||||||||||||||||||||||||||||||
| Conclusions drawn from PSA | Longer persistence of effect (mean imputed HbA1c) is more likely to be cost-effective than 1-year persistence of effect (raw data) | ||||||||||||||||||||||||||||||||||||
| Other methodological issues | |||||||||||||||||||||||||||||||||||||
| Expected value of perfect information (EVPI) analysis undertaken? | No | ||||||||||||||||||||||||||||||||||||
| Type | NA | ||||||||||||||||||||||||||||||||||||
| Results | NA | ||||||||||||||||||||||||||||||||||||
| Conclusions of EVPI analysis/recommendations for future research | NA | ||||||||||||||||||||||||||||||||||||
| Study strengths (as identified by authors) | Results were based on the combined experience of orlistat from four RCTs of 1 years’ duration in obese adult patients with type 2 diabetes | ||||||||||||||||||||||||||||||||||||
| Study limitations (as identified by authors) | (1) The results are likely to be conservative as the authors did not account for a reduction in lipid parameters or blood pressure and their effect on other known conditions that were not modelled; (2) the costs included for treating congestive heart failure were likely too low, representing only one episode of inpatient care; (3) the results are limited by the short-term nature of the supporting clinical trials; (4) there is limited evidence supporting the efficacy of orlistat beyond treatment duration; (5) it is not known how long it takes for body weight to return to baseline levels; (6) the long-term clinical impact of even a transient reduction in HbA1c or body weight is unknown; (7) relative risk reductions used in the model are sourced from the UKPDS170 and as such are applicable to a cohort of 52-year-old men only; (8) quality of life measures were not reported in the supporting clinical trials, preventing a full-scale cost–utility (cost per QALY) analysis | ||||||||||||||||||||||||||||||||||||
| Key author conclusions | |||||||||||||||||||||||||||||||||||||
| Orlistat complements traditional type 2 diabetes medication by causing weight loss. The findings suggest that orlistat is a cost-effective therapy in the management of overweight and obese patients with type 2 diabetes in the USA | |||||||||||||||||||||||||||||||||||||
| Recommendations for future research | |||||||||||||||||||||||||||||||||||||
| Observational data to support long-term use of orlistat in this population are needed to validate the results of the study | |||||||||||||||||||||||||||||||||||||
Olsen 2005165
| Title: Cost-effectiveness of nutritional counseling for obese patients and patients at risk of ischemic heart disease | |
| Study characteristics | |
|---|---|
| Country of study | Denmark (not directly stated but assumed) |
| Study setting | Primary care (GP/dietitian clinics) |
| Research question/objective | To compare the costs and effects (in terms of LYG) of nutritional counselling provided by a GP or a dietitian |
| Study design (model/RCT) | Randomised study used to inform costs and a Cox regression model used to estimate LYG |
| Inclusion criteria | RCT inclusion criteria included BMI ≥ 30 kg/m2, waist circumference > 102 cm (male) or > 88 cm (female), dyslipidaemia, type 2 diabetes |
| Exclusion criteria | None reported |
| Intervention | GPs were randomised to deliver nutritional counselling or to refer to a dietitian GP counselling: Initial counselling session of 30 minutes plus five follow-on sessions of 12 minutes each over a 12-month period. Counselling consisted of general lifestyle advice and the delivery of commercially available information on a healthy diet Dietitian referrals: Dietitian provided individual counselling based on indication from referral, dietary history and routine. Focus was on principles of good nutrition, food shopping, cooking methods, meal planning and exercise. Recommendations included reduction of fat component of diet and/or a cholesterol-lowering diet. Initial counseling session of 1 hour plus five follow-up sessions of 30 minutes each |
| Control/comparison | Both interventions (GP and dietitian) were compared against ‘do nothing’ (no active intervention). This was based on assumption, rather than a randomised ‘do nothing’ arm |
| Methods: costing | |
| Perspective | Health services perspective. Patient perspective considered as a sensitivity analysis. Article methods section indicates a societal perspective may also have been considered but no results are reported |
| Currency | Danish kroner |
| Currency year of reporting | 2001 |
| Discount rate for costs | No discounting as time horizon was 1 year |
| Other costing information | Direct intervention costs (time spent with dietitian and GP), patients’ use of time and potential changed consumption of medicine because of intervention were all considered. Long-term costs were not considered |
| Methods: outcome measures | |
| Primary economic outcome measure | LYG and LYG without ischaemic heart disease |
| Discount rate for outcomes | 5% |
| Time horizon for outcomes | Time to event of death, model run up to 80 years of age. Time horizon was therefore 80 years minus current age |
| Methods: modelling | |
| Type of model used | Cox regression model for LYG/survival; non-parametric bootstrapping used to estimate CIs |
| Size of model cohort | NA |
| Model time horizon | 80 years of age – current age of the modelled participant |
| Health states modelled/model description | Primary modelled outcomes were LYG and LYG without ischaemic heart disease, so the main health states modelled were survival and death. The risk factors included in the model were sex, cholesterol (including HDL), systolic blood pressure, smoking, BMI, diabetes, familial predisposition and previous heart disease. Two Danish population studies (n = 11,765) were used to estimate the risk scores in the model and nine RCTs were used to estimate the effect of the intervention. The Cox regressions described time to event (i.e. death) based on these risk factors and an underlying survival function, adjusting for current age. For each included patient, the prognostic index, absolute risk of dying by the 80th year and absolute risk of ischaemic heart disease and survival were estimated before and after the intervention to estimate LYG. Therefore, the comparator group was essentially no intervention as it was assumed that the prognostic index remained unchanged without intervention |
| Methods: economic evaluations alongside RCTs | |
| Age group | NR |
| Sex breakdown | Results available for men = 121/401 (70/243 dietitian, 51/158 GP) |
| Period of intervention | 1 year |
| Period of follow-up | Costs over 1 year, life-years long term using Cox regression model |
| n randomised to intervention 1 (dietitian) | Total randomised: n = 503; n = 312 randomised to dietitian, full data available for n = 243 (n = 70 male) |
| n randomised to intervention 2 (GP) | Total randomised: n = 503; n = 191 randomised to GP, full data available for n = 158 (n = 51 male) |
| Data completeness intervention 1 (dietitian) | Life-years estimated for men: 243/312 |
| Data completeness intervention 2 (GP) | Life-years estimated for men: 158/191 |
| Results, base-case costs (results based on LYG) | |
| Intervention group costs (men only) (range) | Dietitian: 1684 DKK (720–2971 DKK); GP: 774 DKK (416–818 DKK) |
| Comparator group costs (men only) | Essentially do nothing, cost 0 DKK |
| Incremental costs (men only) (range) | Dietitian: 1684 DKK (720–2971 DKK); GP: 774 DKK (416–818 DKK) |
| Results, base-case primary economic outcome | |
| Outcomes measure used | LYG |
| Discount rate used | 5% |
| Intervention group outcomes (men only) (95% CI) | Dietitian: ΔLYG: 0.0002 (–0.053 to 0.0531); GP: ΔLYG: 0.1210 (0.0424 to 0.1997) |
| Comparator group outcomes (men only) | Essentially do nothing (ΔLYG = 0) |
| Incremental outcomes (men only) (95% CI) | Dietitian: ΔLYG: 0.0002 (–0.053 to 0.0531); GP: ΔLYG: 0.1210 (0.0424 to 0.1997) |
| Results, base-case analysis (LYG) | |
| Incremental costs (men only) (range) | Dietitian: 1684 DKK (720–2971 DKK); GP: 774 DKK (416–818 DKK) |
| Incremental outcomes (men only) (95% CI) | Diet: ΔLYG: 0.0002 (–0.053 to 0.0531); GP: ΔLYG: 0.1210 (0.0424 to 0.1997) |
| ICER (men only) (95% CI) | Dietitian: NR, calculated as 8,420,000 DKK (NR); GP: 6399 DKK (bias-corrected 95% CI 3911 DKK to 16,787 DKK) |
| Results, secondary analysis (1): outcomes measured as LYG without ischaemic heart disease | |
| Incremental costs (men only) (range) | Dietitian: 1684 DKK (720–2971 DKK); GP: 770 DKK (416–818 DKK) |
| Incremental outcomes (men only) (95% CI) | Dietitian: 0.0630 (–0.0140 to 0.1400); GP: 0.2376 (0.1015 to 0.3737) |
| ICER (men only) | Dietitian: NR, calculated as 26,730 DKK (bias-corrected 95% CI NR); GP: 3240 DKK (bias-corrected 95% CI 2069 DKK to 6841 DKK) |
| Results, sensitivity analysis (1): costs and ICERs calculated based on the estimated use of GP time (identical time estimates for dietitians and GPs) | |
| Incremental costs (men only) (95% CI) | Dietitian: 541 DKK (NR); GP: 774 DKK (NR) |
| Incremental outcomes (men only) (95% CI) | Dietitian: 0.0002 (–0.0530 to 0.0531); GP: 0.1210 (0.0424 to 0.1997) |
| ICER (men only) (95% CI) | Dietitian: 2,705,000 DKK (NR); GP: 6399 DKK (NR) |
| Results, sensitivity analysis (2): costs and ICERs calculated based on registered use of dietitian time (identical time estimates for dietitians and GPs) | |
| Incremental costs (men only) (95% CI) | Dietitian: 1231 DKK (NR); GP: 2278 DKK (NR) |
| Incremental outcomes (men only) (95% CI) | Dietitian: 0.0002 (–0.0530 to 0.0531); GP: 0.1210 (0.0424 to 0.1997) |
| ICER (men only) (95% CI) | Dietitian: NR, calculated as 6,155,000 DKK (NR); GP: 18,821 DKK (NR) |
| Results, sensitivity analysis (3): base-case analysis – outcomes measured as LYG and reported for men and women together | |
| Incremental costs (men and women) (range) | Dietitian: 1642 DKK (720–3204 DKK); GP: 755 DKK (416–818 DKK) |
| Incremental outcomes (men and women) (95% CI) | Dietitian: 0.0274 (0.0013 to 0.0534); GP: 0.0919 (0.0569 to 0.1269) |
| ICER (men and women) | Dietitian: 59,987 DKK (bias-corrected 95% CI 30,545 DKK to 996,368 DKK); GP: 8213 DKK (bias-corrected 95% CI 5910 DKK to 12,850 DKK) |
| Results, sensitivity analysis (4): outcomes measured as LYG without ischaemic heart disease and reported for men and women together | |
| Incremental costs (men and women) (range) | Dietitian: 1642 DKK (720–3204 DKK); GP: 751 DKK (416–818 DKK) |
| Incremental outcomes (men and women) (95% CI) | Dietitian: 0.0700 (0.0388 to 0.1011); GP: 0.1608 (0.1054 to 0.2162) |
| ICER (men and women) | Dietitian: 23,469 DKK (bias-corrected 95% CI 16,223 DKK to 41,912 DKK); GP: 4670 DKK (bias-corrected 95% CI 3480 DKK to 6905 DKK) |
| Results, sensitivity analysis (5): costs and ICERs calculated based on the estimated use of GP time (identical time estimates for dietitians and GPs); outcomes measured as LYG and reported for men and women together | |
| Incremental costs (men and women) (range) | Dietitian: 533 DKK (NR); GP: 755 DKK (NR) |
| Incremental outcomes (men and women) (95% CI) | Dietitian: 0.0274 (0.0013 to 0.0534); GP: 0.0919 (0.0569 to 0.1269) |
| ICER (men and women) (95% CI) | Dietitian: 19,472 DKK (NR); GP: 8213 DKK (NR) |
| Results, sensitivity analysis (6): costs and ICERs calculated based on the estimated use of dietitian time (identical time estimates for dietitians and GPs); outcomes measured as LYG and reported for men and women together | |
| Incremental costs (men and women) (range) | Dietitian: 1204 DKK (NR); GP: 2209 DKK (NR) |
| Incremental outcomes (men and women) (95% CI) | Dietitian: 0.0274 (0.0013 to 0.0534); GP: 0.0919 (0.0569 to 0.1269) |
| ICER (men and women) | Dietitian: 43,987 DKK (NR); GP: 24,037 DKK (NR) |
| Uncertainty | |
| Probabilistic analysis undertaken? | NA – bootstrapped estimates used to describe uncertainty in the cost–effect pairs |
| Results reported for male subgroup? | No |
| Distribution for costs | NA |
| Parameter a | NA |
| Parameter b | NA |
| Distribution for utilities | NA |
| Parameter a | NA |
| Parameter b | NA |
| Distribution for other | NA |
| Parameter a | NA |
| Parameter b | NA |
| Results illustrated using (e.g. CEAC, scatterplot) | CEACs and scatterplots of 10,000 bootstrapped iterations |
| Key results of PSA | Probability of cost-effectiveness: Dietitian ∼0% and GP ∼100% at a WTP of 25,000 DKK per life-year gained; dietitian ∼80% and GP ∼100% at a WTP of 100,000 DKK per life-year gained |
| Conclusions of sensitivity analysis | GP counselling tends to be more cost-effective. Using identical time estimates for dietitian and GP counselling services resulted in lower intervention costs for the dietitian group; however, GP counselling was still more cost-effective. Male participants tended to have a lower ICER for GP counselling and female participants tended to have a lower ICER for dietitian counselling |
| Conclusions of subgroup analysis | Information based on subgroups showed that, in general, ICERs were lower when the outcome measured was LYG without ischaemic heart disease, that is, the cost of gaining 1 extra life-year without ischaemic heart disease was lower than the cost of gaining 1 extra life-year. The GP group tended to be more cost-effective here also, especially for the male subgroup |
| Conclusions drawn from PSA | The probability of acceptance of GP counselling would be much greater than the probability of acceptance of dietitian counselling. Further, there was much greater statistical uncertainty for the dietitian group than the GP group |
| Other methodological issues | |
| Expected value of perfect information (EVPI) analysis undertaken? | No |
| Type | NA |
| Results | NA |
| Conclusions of EVPI analysis/recommendations for future research | NA |
| Study strengths (as identified by authors) | None explicitly referenced in the text |
| Study limitations (as identified by authors) | (1) Simplifying model assumptions are in their own right a potential limitation (e.g. it was assumed that the improvement in lifestyle was maintained beyond the intervention period); (2) it should be noted that the greater effect in the GP group could be caused by other factors, for example GPs may give wider lifestyle advice than dietitians (e.g. on smoking cessation); (3) the applied costing method can give rise to methodological concerns because time estimates are compared with standard agreed salaries; transferability of results may thus be limited; (4) the GPs that partook in the study may not be representative as they may have an unrepresentative interest in preventative care |
| Key author conclusions | |
| GP counselling appears more cost-effective than dietitian counselling. Both dietitian- and GP-provided interventions were considered potentially cost-effective. The results were not so clear for the male-only group with some ICERs excessively high because of small outcome differences. For all age groups the base-case analysis showed both counselling strategies to be cost-effective. Although GP counselling was more cost-effective and had a lower ICER than the dietitian intervention, the dietitian intervention could also be considered potentially cost-effective, costing an additional 59,987 DKK per life-year gained. Basing conclusions on the assumption that the WTP for a life-year and a QALY gained is similar, then both GP and dietician advice could offer good value for money, given that society typically places the WTP for a QALY gain at 88,000 DKK | |
| Recommendations for future research | |
| The results indicate that nutritional counselling should be combined with advice regarding other lifestyle changes. No sex-specific recommendations were made | |
Segal 1998163
| Title: Cost-effectiveness of the primary prevention of non-insulin dependent diabetes mellitus | |||||||||||||||||||||||||||||||
| Study characteristics | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country of study | Australia | ||||||||||||||||||||||||||||||
| Study setting | Hospital, community and primary care, depending on programme delivered; programme IV appears to be delivered in a primary care/community setting | ||||||||||||||||||||||||||||||
| Research question/objective | To investigate whether the prevention of NIDDM is cost-effective compared with other possible uses of health-care resources and whether some approaches to NIDDM prevention are more cost-effective than others | ||||||||||||||||||||||||||||||
| Study design (model/RCT) | Markov model (specific Markov submodel for each of the six programmes considered) | ||||||||||||||||||||||||||||||
| Inclusion criteria | Those with IGT, overweight/obese men, seriously obese people, women with previous gestational diabetes and the general Australian population. Detailed inclusion criteria for each programme are not reported. For programme IV the target was obese and overweight men, a mixed group (10% impaired glucose tolerance, 90% normal glucose tolerance) and those with impaired glucose tolerance only | ||||||||||||||||||||||||||||||
| Exclusion criteria | Not reported. The use of antidiabetic drugs was not included as a programme for evaluation as this was deemed management rather than prevention of diabetes and was outwith the scope of the study | ||||||||||||||||||||||||||||||
| Intervention | A total of six programmes were considered of which only one was male specific. Programme IV was a group behavioural modification for men (five to six group sessions; aim to reduce waist size through diet change and increased activity; empowerment philosophy). Information regarding potentially relevant male-specific data from other programmes was not available | ||||||||||||||||||||||||||||||
| Control/comparison | Standard care – no active intervention in the control cohort of the models/submodels | ||||||||||||||||||||||||||||||
| Methods: costing | |||||||||||||||||||||||||||||||
| Perspective | Health-care providers | ||||||||||||||||||||||||||||||
| Currency | Australian dollar. Conversion to US dollars was carried out using a median exchange rate for August/September/October 1997 of AUS$1 = US$0.72 | ||||||||||||||||||||||||||||||
| Currency year of reporting | 1997 | ||||||||||||||||||||||||||||||
| Discount rate for costs | 5%, applied to downstream costs | ||||||||||||||||||||||||||||||
| Other information on costing | Average programme costs were reported; however, individual cost components or detailed resource use was not. Costs were reported in the study results as gross costs (i.e. programme costs) and net costs (i.e. programme/intervention delivery costs less any downstream cost savings from the treatment of diabetes). The cost of managing NIDDM was AUS$1800 per diabetic per year in the model | ||||||||||||||||||||||||||||||
| Methods: outcome measures | |||||||||||||||||||||||||||||||
| Primary economic outcome measures | LYG, reduction in diabetes years | ||||||||||||||||||||||||||||||
| Discount rate for outcomes | 5% | ||||||||||||||||||||||||||||||
| Time horizon for outcomes | Mortality vectors applied to intervention and control cohorts for 25 years post intervention. Remaining life expectancy calculated by adjusting the population life expectancy for the diabetic state and whether or not weight loss was achieved | ||||||||||||||||||||||||||||||
| Methods: modelling | |||||||||||||||||||||||||||||||
| Type of model used | Markov models (a set of sub Markov models for each individual programme) | ||||||||||||||||||||||||||||||
| Size of model cohort | NR | ||||||||||||||||||||||||||||||
| Model time horizon | 25 years post intervention | ||||||||||||||||||||||||||||||
| Health states modelled/model description | NIDDM, normal glucose tolerance, impaired glucose tolerance | ||||||||||||||||||||||||||||||
| Methods: economic evaluations alongside RCTs | |||||||||||||||||||||||||||||||
| Age group | NA | ||||||||||||||||||||||||||||||
| Sex breakdown | NA | ||||||||||||||||||||||||||||||
| Period of intervention | NA | ||||||||||||||||||||||||||||||
| Period of follow-up | NA | ||||||||||||||||||||||||||||||
| n randomised to intervention | NA | ||||||||||||||||||||||||||||||
| n randomised to control | NA | ||||||||||||||||||||||||||||||
| Data completeness intervention | NA | ||||||||||||||||||||||||||||||
| Data completeness control | NA | ||||||||||||||||||||||||||||||
| Results, base-case costs | |||||||||||||||||||||||||||||||
| Intervention group programme IV (men only) | Gross cost: AUS$577 (AUS$195 excluding screening cost per case detected); net cost: NR | ||||||||||||||||||||||||||||||
| Comparator group programme IV (men only) | Assumed AUS$0 | ||||||||||||||||||||||||||||||
| Incremental costs | Final discounted incremental costs were not directly reported; therefore, incremental gross cost for programme IV can only be extracted as AUS$577 (AUS$195 excluding screening cost per case detected) | ||||||||||||||||||||||||||||||
| Results, base-case primary economic outcome | |||||||||||||||||||||||||||||||
| Programme IV (per 100) Group Diabetes years prevented LYG Intervention group Mixed 135 3016 IGT 484 2750 Comparator group Mixed 172 2905 IGT 639 2888 Incremental outcomes Mixed 37 111 IGT 155 138 |
Group | Diabetes years prevented | LYG | Intervention group | Mixed | 135 | 3016 | IGT | 484 | 2750 | Comparator group | Mixed | 172 | 2905 | IGT | 639 | 2888 | Incremental outcomes | Mixed | 37 | 111 | IGT | 155 | 138 | |||||||
| Group | Diabetes years prevented | LYG | |||||||||||||||||||||||||||||
| Intervention group | |||||||||||||||||||||||||||||||
| Mixed | 135 | 3016 | |||||||||||||||||||||||||||||
| IGT | 484 | 2750 | |||||||||||||||||||||||||||||
| Comparator group | |||||||||||||||||||||||||||||||
| Mixed | 172 | 2905 | |||||||||||||||||||||||||||||
| IGT | 639 | 2888 | |||||||||||||||||||||||||||||
| Incremental outcomes | |||||||||||||||||||||||||||||||
| Mixed | 37 | 111 | |||||||||||||||||||||||||||||
| IGT | 155 | 138 | |||||||||||||||||||||||||||||
| Results, primary economic analysis (base-case results) | |||||||||||||||||||||||||||||||
| Incremental costs (programme IV) | Gross cost: AUS$577 (AUS$195 excluding screening cost per case detected); net cost: NR | ||||||||||||||||||||||||||||||
| Incremental outcomes (programme IV, per 100) | Group Diabetes years prevented LYG Mixed 37 111 IGT 155 138 | Group | Diabetes years prevented | LYG | Mixed | 37 | 111 | IGT | 155 | 138 | |||||||||||||||||||||
| Group | Diabetes years prevented | LYG | |||||||||||||||||||||||||||||
| Mixed | 37 | 111 | |||||||||||||||||||||||||||||
| IGT | 155 | 138 | |||||||||||||||||||||||||||||
| ICER, reported only as incremental cost per life-year gained | Group Gross cost per life-year gained Net cost per life-year gained IGT 500 NS IGTa 1600 NS Mixed 700 NS | Group | Gross cost per life-year gained | Net cost per life-year gained | IGT | 500 | NS | IGTa | 1600 | NS | Mixed | 700 | NS | ||||||||||||||||||
| Group | Gross cost per life-year gained | Net cost per life-year gained | |||||||||||||||||||||||||||||
| IGT | 500 | NS | |||||||||||||||||||||||||||||
| IGTa | 1600 | NS | |||||||||||||||||||||||||||||
| Mixed | 700 | NS | |||||||||||||||||||||||||||||
| Results, secondary analysis | |||||||||||||||||||||||||||||||
| None applicable to programme IV | |||||||||||||||||||||||||||||||
| Results, sensitivity analysis (1): the impact of a change in the programme success rate on cost-effectiveness (programme IV, men only) | |||||||||||||||||||||||||||||||
| Incremental net cost per life-year saved: Programme type Analysis Assumed success rate (%) Net cost per life-year saved IV (men only) Low 20 100 Base case b 33 NS High 45 NS |
Programme type | Analysis | Assumed success rate (%) | Net cost per life-year saved | IV (men only) | Low | 20 | 100 | Base case b | 33 | NS | High | 45 | NS | |||||||||||||||||
| Programme type | Analysis | Assumed success rate (%) | Net cost per life-year saved | ||||||||||||||||||||||||||||
| IV (men only) | Low | 20 | 100 | ||||||||||||||||||||||||||||
| Base case b | 33 | NS | |||||||||||||||||||||||||||||
| High | 45 | NS | |||||||||||||||||||||||||||||
| Results, sensitivity analysis (2) | |||||||||||||||||||||||||||||||
| A range of other sensitivity analyses was carried out on various programmes but none of those reported was for the male-specific programme IV. Additional male-specific data from other programmes were requested from the study authors but were not available at the time of publication | |||||||||||||||||||||||||||||||
| Uncertainty | |||||||||||||||||||||||||||||||
| Probabilistic analysis undertaken? | No | ||||||||||||||||||||||||||||||
| Results reported for male subgroup? | NA | ||||||||||||||||||||||||||||||
| Distribution for costs | NA | ||||||||||||||||||||||||||||||
| Parameter a | NA | ||||||||||||||||||||||||||||||
| Parameter b | NA | ||||||||||||||||||||||||||||||
| Distribution for utilities | NA | ||||||||||||||||||||||||||||||
| Parameter a | NA | ||||||||||||||||||||||||||||||
| Parameter b | NA | ||||||||||||||||||||||||||||||
| Distribution for other | NA | ||||||||||||||||||||||||||||||
| Parameter a | NA | ||||||||||||||||||||||||||||||
| Parameter b | NA | ||||||||||||||||||||||||||||||
| Results illustrated using (e.g. CEAC, scatterplot) | NA | ||||||||||||||||||||||||||||||
| Key results of PSA | NA | ||||||||||||||||||||||||||||||
| Conclusion of sensitivity analysis | Net cost per life-year gained is sensitive to the rate of programme success. However, despite varying the success rate of the male-only programme IV, the intervention remained highly cost-effective and there was a net saving for all but the lowest success rate considered | ||||||||||||||||||||||||||||||
| Conclusions of subgroup analysis | The group behavioural programme was cost-effective for both the impaired glucose tolerance subgroup and the mixed levels of glucose tolerance subgroup of men. Cost-effectiveness appeared even more definitive in those with impaired glucose tolerance | ||||||||||||||||||||||||||||||
| Conclusions drawn from PSA | NA | ||||||||||||||||||||||||||||||
| Other methodological issues | |||||||||||||||||||||||||||||||
| Expected value of perfect information (EVPI) analysis undertaken? | None | ||||||||||||||||||||||||||||||
| Type | NA | ||||||||||||||||||||||||||||||
| Results | NA | ||||||||||||||||||||||||||||||
| Conclusions of EVPI analysis/recommendations for future research | NA | ||||||||||||||||||||||||||||||
| Study strengths (as identified by authors) | NR | ||||||||||||||||||||||||||||||
| Study limitations (as identified by authors) | (1) A single transition matrix for most programmes has been used to progress each cohort between diabetic states; a more dynamic model of progression between diabetic states might have been preferred; (2) a further simplification is the selection of a single target age group; (3) costs have been limited to direct costs; no account is taken of other programme costs (e.g. time commitment of participants); (4) the results do not account for quality of life; the authors state that this was not possible given the poor evidence base at the time of the study | ||||||||||||||||||||||||||||||
| Key author conclusions | |||||||||||||||||||||||||||||||
| For the prevention of NIDDM, the interventions considered may be cost saving or highly cost-effective relative to other possible uses of health-care resources. The behavioural diet programmes for high-risk groups were found to be highly cost-effective relative to other health-care programmes. The male-specific group behavioural programme was particularly cost-effective or cost saving when accounting for downstream cost savings associated with the prevention of and lesser need for costly management of NIDDM | |||||||||||||||||||||||||||||||
| Recommendations for future research | |||||||||||||||||||||||||||||||
| (1) There is a need to focus on NIDDM prevention; if the incidence of NIDDM is not contained, the cost to the community in terms of illness, loss of quality of life, premature death and allocation of scarce health-care resources will be an ever-increasing burden; (2) broader population-based programmes targeting high-risk groups; (3) the application of studies to a more international context; (4) the pilot introduction of programmes is recommended | |||||||||||||||||||||||||||||||
Appendix 14 List of included studies: qualitative review
Abildso 2010
Abildso C, Zizzi S, Gilleland D, Thomas J, Bonner D. A mixed methods evaluation of a 12-week insurance-sponsored weight management program incorporating cognitive-behavioral counseling. J Mix Methods Res 2010;4:278–94.
De Souza 2005
De Souza P, Ciclitira KE. Men and dieting: a qualitative analysis. J Health Psychol 2005;10:793–804.
Gallagher 2012
Gallagher R, Kirkness A, Armari E, Davidson PM. Weight management issues and strategies for people with high cardiovascular risk undertaking an Australian weight loss program: a focus group study. Nurs Health Sci 2012;14:18–24.
Gillon 2003
Gillon E. Can men talk about problems with weight? The therapeutic implications of a discourse analytic study. Counsell Psychother Res 2003;3:25–32.
Gough 2006
Gough B, Conner MT. Barriers to healthy eating amongst men: a qualitative analysis. Soc Sci Med 2006;62:387–95.
Gough 2009
Gough B, Flanders G. Celebrating ‘obese’ bodies: Gay ‘bears’ talk about weight, body image and health. Int J Mens Health 2009;8:235–53.
Gray 2009
Gray CM, Anderson AS, Clarke AM, Dalziel A, Hunt K, Leishman J, et al. Addressing male obesity: an evaluation of a group-based weight management intervention for Scottish men. J Mens Health 2009;6:70–81.
Harrison 2007
Harrison A. Weight management in the workplace. In Conrad D, White A, editors. Men’s Health: How To Do It. Oxford: Radcliffe Publishing; 2007. pp. 59–72.
Hunt 2013
Hunt K, McCann C, Gray CM, Mutrie N, Wyke S. ‘You’ve got to walk before you run’: positive evaluations of a walking program as part of a gender-sensitized, weight-management program delivered to men through professional football clubs. Health Psychol 2013;32:57–65.
Kim 2008
Kim KH, Linnan L, Campbell MK, Brooks C, Koenig HG, Wiesen C. The WORD (Wholeness, Oneness, Righteousness, Deliverance): a faith-based weight-loss program utilizing a community-based participatory research approach. Health Educ Behav 2008;35:634–50.
Leishman 2007
Leishman J. Working with men in groups – experience from a weight management programme in Scotland. In White A, Pettifer M, editors. Hazardous Waist: Tackling Male Weight Problems. Oxford: Radcliffe; 2007. pp. 75–86.
Mallyon 2010
Mallyon A, Holmes M, Coveney J, Zadoroznyj M. ‘I’m not dieting, I’m doing it for science’: masculinities and the experience of dieting. Health Sociol Rev 2010;19:330–42.
McCullagh 2005
McCullagh J. ‘Tommy the Trucker’: a Consultation and Lifestyle Survey of Lorry Drivers Visiting Sefton. South Sefton: Sefton Health Improvement Support Services; 2005.
Monaghan 2007a
Monaghan LF. McDonaldizing men’s bodies? Slimming, associated (ir)rationalities and resistances. Body Soc 2007;13:67–93.
Monaghan 2007b
Monaghan L. Body mass index, masculinities and moral worth: men’s critical understandings of ‘appropriate’ weight-for-height. Sociol Health Illn 2007;29:584–609.
Monaghan 2008
Monaghan LF. Men, physical activity, and the obesity discourse: critical understandings from a qualitative study. Sociol Sport J 2008;25:97–129.
Morgan 2011a
Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. 12-Month outcomes and process evaluation of the SHED-IT RCT: an internet-based weight loss program targeting men. Obesity 2011;19:142–51.
Morgan 2011b
Morgan PJ, Warren JM, Lubans DR, Collins CE, Callister R. Engaging men in weight loss: experiences of men who participated in the male only SHED-IT pilot study. Obes Res Clinical Pract 2011;5:e169–266.
Ogden 2006
Ogden J, Sidhu S. Adherence, behavior change, and visualization: a qualitative study of the experiences of taking an obesity medication. J Psychosom Res 2006;61:545–52.
Weaver 2008
Weaver NF, Hayes L, Unwin NC, Murtagh MJ. ‘Obesity’ and ‘clinical obesity’. Men’s understandings of obesity and its relation to the risk of diabetes: a qualitative study. BMC Public Health 2008;8:311.
White 2008
White A, Conrad D, Branney P. Targeting men’s weight in the workplace. J Mens Health 2008;5:133–40.
Witty 2010
Witty K, White A. The Tackling Men’s Health Evaluation Study: Final Report. Leeds: Leeds Metropolitan University, Centre for Men’s Health; 2010. URL: www.leedsmet.ac.uk/hss/docs/Tackling_Men_Health_Final_Report.pdf (accessed November 2011).
Appendix 15 Data extraction form: qualitative review
| 1. Bibliographic information | |
|---|---|
| Article title: | |
| Extracted by: | |
| Checked by: | |
| Type of publication, date and page numbers (if journal article also give title of the journal): | |
| Linked to RCT or non-randomised intervention: | |
| Country of setting: | |
| 2. Researcher details | |
| Authors and affiliations (list as presented on paper): | |
| Gender of researchers who collect the qualitative data: | |
| Academic discipline of authors: | |
| 3. Aims and methods | |
| Study aims: | |
| Research questions – Stated explicitly or implicit within the general text/topic guide? In what section(s) of the paper are questions mentioned? Are they prospective or retrospective? | |
| Theoretical and epistemological perspective underpinning the qualitative research (if not explicitly stated the extractor should offer an interpretation): | |
| Theoretical perspective underpinning the intervention: | |
| Qualitative methods used: | |
| Data analysis technique and procedure: | |
| 4. Findings | |
| (a) Themes | |
| Which key themes are stated to have emerged from the qualitative research? | |
| (b) Engagement | |
| How are men attracted to taking part in the trial/intervention? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| How are men motivated to lose weight? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| (c) Intervention | |
| Characteristics of the intervention: | |
| Timing of the intervention (when, how often, for how long): | |
| Who delivers the intervention? (discipline, gender) | |
| Focus of the programme (i.e. diet, exercise, behavioural counselling): | |
| Results: | |
| Demographic information: | |
| Dropout rate: | |
| Sex breakdown (if programme not for men only): | |
| (d) Intervention (communication) process | |
| Are communication processes referred to in the protocol? | Notes: |
| Any specific training provided as part of the intervention? (e.g. psychological behaviour change techniques). If so, are certain features of behaviour change found to be more attractive for obese men? How and why are these features more attractive? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| Is fidelity to protocol mentioned? | |
| What are men’s perceptions of the communication process? | Notes: |
| Participant quotes (first order): | |
| Author statements (second order): | |
| Summary (third order): | |
| (e) Central research questions derived from quantitative review to guide data extraction | |
| How are men consulted in the design of the intervention (if they are)? Should also include which literature is consulted to aid conception of the design. Was the literature gender specific? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| If it is found that interventions for men should be different from those for women, how should they be different and why? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| Are group-based interventions for men found to be more effective for weight loss than interventions delivered to individual men? How and why are they more effective? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| Are certain features of diets found to be more attractive for obese men? How and why are these features more attractive? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| Are certain features of physical activity stated to be more attractive for obese men? How and why are these features more attractive? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| What is stated with regard to participant attrition and what efforts are made to help men continue with the programme? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| Do men state who they believe to be the best person/persons to deliver the intervention? If so, why are they preferred? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| Are programmes deliberately involving partners/families more effective? How, why and at what stage? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| Were there any other emergent themes or gaps/omissions not covered by the above? | Notes: |
| Summary (third order): | |
| Participant quotes (first order): | |
| Author statements (second order): | |
| 5. Area and context (micro, meso and macro levels) | |
| Rationale for setting choice (for intervention and where qualitative research is conducted): | |
| Meaning attributed to where the intervention is delivered: | |
| Perceptions about the venue (micro) and area of setting (meso): | |
| What else is going on at the time of the intervention (e.g. clinic appointment) (meso): | |
| Does the setting potentially exclude/target populations? (meso) | |
| Are there any wider media, cultural, political or contextual factors that might be influencing the intervention? (macro) | |
| 6. Quality | |
| (a) Sample | |
| Sample size: | |
| Sample characteristics: | |
| Sample selection process: | |
| Sample inclusion and exclusion criteria: | |
| (b) Reflexivity | |
| Evidence of researcher reflexivity: | |
| (c) Ethics | |
| Evidence of attention to ethical issues: | |
| (d) General | |
| Are the findings adequately supported by the data presented? | |
| Is there potential for a ‘charisma effect’ in this study? (this relates to the potential influence of the principal investigator) | |
| Any other quality issues not covered above? | |
Appendix 16 List of excluded studies
Reviews of men-only randomised controlled trials and randomised controlled trials of men and women compared
Abstracts, commentaries, errata, evidence summaries, protocols, recommendations and news articles
First year of Look AHEAD trial yields encouraging results. Diabetes Dateline spring/summer 2008, pp. 7–8.
Collins CE, Morgan PJ, Jones P, Fletcher K, Martin J, Aguiar EJ, et al. Evaluation of a commercial web-based weight loss and weight loss maintenance program in overweight and obese adults: a randomized controlled trial. BMC Public Health 2010;10:669.
Cudjoe S, Moss S, Nguyen L. How do exercise and diet compare for weight loss? J Fam Pract 2007;56:841–4.
Donaldson E, Fallows S. A text message-based weight management intervention for overweight adults. J Hum Nutr Diet 2011;24:385–6.
Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain in adults. Med Sci Sports Exerc 2009;41:459–71.
Gordon J, Dibble T, Adams C, McGee E. An evaluation of the long-term effectiveness of two adult community-based group weight management interventions. J Hum Nutr Diet 2011;24:388.
Kumanyika SK, Shults J, Fassbender J, Whitt MC, Brake V, Kallan MJ, et al. Corrigendum to ‘Outpatient weight management in African-Americans: the Healthy Eating and Lifestyle Program (HELP) study’. Prev Med 2006;42:1
Miyachi M, Ohmori Y, Morita A, Aiba N. Effects of pedometer-based physical activity intervention on abdominal fat and blood pressure: Saku community-based randomized crossover intervention study. J Clin Hypertens 2010;12:A14.
Morgan PJ, Collins CE, Plotnikoff RC, McElduff P, Burrows T, Warren JM, et al. The SHED-IT community trial study protocol: a randomised controlled trial of weight loss programs for overweight and obese men. BMC Public Health 2010;10:701.
Resch KL. Dietary Intervention Randomized Controlled Trial (DIRECT) group: weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. Forsch Komplementmed 2008;15:351–2.
Rossner S. Atkins – the superior slimming diet? Scand J Food Nutr 2007;51:4.
Wycherley TP, Brinkworth GD, Clifton PM, Noakes M. A one year high protein, low fat weight loss diet improves body composition and cardiometabolic risk factors in overweight males with features of the metabolic syndrome. Australas Med J 2011;4:731.
Different inclusion criteria for men and women
Kiernan M, King AC, Stefanick ML, Killen JD. Men gain additional psychological benefits by adding exercise to a weight-loss program. Obes Res 2001;9:770–7.
Torgerson JS, Lissner L, Lindroos AK, Kruijer H, Sjostrom L. VLCD plus dietary and behavioural support versus support alone in the treatment of severe obesity. A randomised two-year clinical trial. Int J Obes 1997;21:987–94.
Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N Engl J Med 1991;325:461–6.
Cost-effectiveness reviews
Bogers RP, Barte JC, Schipper CM, Vijgen SM, de Hollander EL, Tariq L, et al. Relationship between costs of lifestyle interventions and weight loss in overweight adults. Obes Rev 2010;11:51–61.
Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo-Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet 2010;376:1775–84.
Cobiac L, Vos T, Veerman L. Cost-effectiveness of Weight Watchers and the Lighten Up to a Healthy Lifestyle program. Aust N Z J Public Health 2010;34:240–7.
Joo NS, Park YW, Park KH, Kim CW, Kim BT. Cost-effectiveness of a community-based obesity control programme. J Telemed Telecare 2010;16:63–7.
Follow-up < 12 months
Apekey T, Morris A, Fagbemi S, Griffiths G. Benefits of moderate-intensity exercise during a calorie-restricted low-fat diet. Health Educ J 2012;71:154–64.
Azadbakht L, Parvin M, Ahmad E, Tohid A, Fereidoun A. Beneficial effects of a Dietary Approaches to Stop Hypertension eating plan on features of the metabolic syndrome. Diabetes Care 2005;28:2823–31.
Brownell KD, Cohen RY, Stunkard AJ, Felix MR, Cooley NB. Weight loss competitions at the work site: impact on weight, morale and cost-effectiveness. Am J Public Health 1984;74:1283–5.
Janiszewski PM, Ross R. Effects of weight loss among metabolically healthy obese men and women. Diabetes Care 2010;33:1957–9.
LeCheminant JD, Jacobsen DJ, Hall MA, Donnelly JE. A comparison of meal replacements and medication in weight maintenance after weight loss. J Am Coll Nutr 2005;24:347–53.
Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. The SHED-IT randomized controlled trial: evaluation of an internet-based weight-loss program for men. Obesity 2009;17:2025–32.
Ross LJ, Tapsell LC, Probst Y. Optimizing dietary fat in a weight-loss trial requires advice based on a structured ‘whole-of-diet’ model. Nutr Res 2011;31:683–90.
Schroder KEE. Computer-assisted dieting: effects of a randomized nutrition intervention. Am J Health Behav 2011;35:175–88.
Sofer S, Eliraz A, Kaplar S, Voet H, Fink G, Kima T, et al. Greater weight loss and hormonal changes after 6 months diet with carbohydrates eaten mostly at dinner. Obesity 2011;19:2006–14.
Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, Ward DS. The Weigh Study: a randomized trial focusing on daily self-weighing for weight loss among overweight adults. Ann Behav Med 2012;43:S272.
Takada A, Nakamura R, Furukawa M, Takahashi Y, Nishimura S, Kosugi S. The relationship between weight loss and time and risk preference parameters: a randomized controlled trial. J Biosoc Sci 2011;43:481–503.
Ineligible comparator
Hofmann M, Rodriguez JE, Shearer B. Are group visits effective for the treatment of obesity? Evid Based Pract 2010;13:6–7.
Ineligible intervention
Buse JB, Drucker DJ, Taylor KL, Kim T, Walsh B, Hu H, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 2010;33:1255–61.
Caterson I, Coutinho W, Finer N, Van Gaal L, Maggioni A, Torp PC, et al. Early response to sibutramine in patients not meeting current label criteria: preliminary analysis of SCOUT lead-in period. Obesity 2010;18:987–94.
Derosa G, Maffioli P, Ferrari I, D’Angelo A, Fogari E, Palumbo I, et al. Orlistat and L-carnitine compared to orlistat alone on insulin resistance in obese diabetic patients. Endocr J 2010;57:777–86.
Groeneveld IF, Proper KI, Absalah S, van der Beek AJ, van Mechelen W. An individually based lifestyle intervention for workers at risk for cardiovascular disease: a process evaluation. Am J Health Promot 2011;25:396–401.
Groeneveld IF, van Wier MF, Proper KI, Bosmans JE, van Mechelan W, van der Beek AJ. Cost-effectiveness and cost–benefit of a lifestyle intervention for workers in the construction industry at risk for cardiovascular disease. J Occup Environ Med 2011;53:610–7.
Jeffery RW, Gerber WM. Group and correspondence treatment for weight reduction used in the Multiple Risk Factor Intervention Trial. Behav Ther 1982;13:24–30.
Jeffery RW, French SA. Preventing weight gain in adults: design, methods and one year results from the Pound of Prevention study. Int J Obes Relat Metab Disord 1997;21:457–64.
Libardi C, Souza GV, Gáspari A, Dos Santos CF, Leite S, Dias R, et al. Effects of concurrent training on interleukin-6, tumour necrosis factor-alpha and C-reactive protein in middle-aged men. J Sports Sci 2011;29:1573–81.
Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O’Neil PM, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity 2011;19:110–20.
Ineligible population
Dekkers JC, van Wier MF, Ariëns GA, Hendriksen IJ, Pronk NP, Smid T, et al. Comparative effectiveness of lifestyle interventions on cardiovascular risk factors among a Dutch overweight working population: a randomized controlled trial. BMC Public Health 2011;11:49.
Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med 2003;163:1343–50.
Imayama I, Alfano CM, Bertram LAC, Wang C, Xiao L, Duggan C, et al. Effects of 12-month exercise on health-related quality of life: a randomized controlled trial. Prev Med 2011;52:344–51.
Kamioka H, Nakamura Y, Okada S, Kitayuguchi J, Kamada M, Honda T, et al. Effectiveness of comprehensive health education combining lifestyle education and hot spa bathing for male white-collar employees: a randomized controlled trial with 1-year follow-up. J Epidemiol 2009;19:219–30.
King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation 1995;91:2596–604.
Kirk EP, Jacobsen DJ, Gibson C, Hill JO, Donnelly JE. Time course for changes in aerobic capacity and body composition in overweight men and women in response to long-term exercise: the Midwest Exercise Trial (MET). Int J Obes Relat Metab Disord 2003;27:912–19.
Leichtle AB, Helmschrodt C, Ceglarek U, Shai I, Henkin Y, Schwarzfuchs D, et al. Effects of a 2-y dietary weight-loss intervention on cholesterol metabolism in moderately obese men. Am J Clin Nutr 2011;94:1189–95.
McAuley KA, Taylor RW, Farmer VL, Hansen P, Williams SM, Booker CS, et al. Economic evaluation of a community-based obesity prevention program in children: the APPLE project. Obesity 2010;18:131–6.
McTiernan A, Sorensen B, Irwin ML, Morgan A, Yasui Y, Rudolph RE, et al. Exercise effect on weight and body fat in men and women. Obesity 2007;15:1496–512.
Nakajima Y, Sato K, Sudo M, Nagao M, Kano T, Harada T, et al. Practical dietary calorie management, body weight control and energy expenditure of diabetic patients in short-term hospitalization. J Atheroscler Thromb 2010;17:558–67.
Nakata Y, Okada M. [Effects of weight-loss tools and a group-based weight-loss support program: rationale and study design of a randomized controlled trial]. Jpn J Public Health 2010;57:835–42.
Novotny R, Chen C, Williams A, Albright C, Nigg C, Oshiro C, et al. US acculturation is associated with health behaviors and obesity, but not their change, with a hotel-based intervention among Asian-Pacific Islanders. J Acad Nutr Diet 2012;112:649–56.
Potteiger JA, Jacobsen DJ, Donnelly JE, Hill JO, Midwest ET. Glucose and insulin responses following 16 months of exercise training in overweight adults: the Midwest Exercise Trial. Metabolism 2003;52:1175–81.
Pritchard JE, Nowson CA, Wark JD. Bone loss accompanying diet-induced or exercise-induced weight loss: a randomised controlled study. Int J Obes 1996;20:513–20.
Pritchard JE, Nowson CA, Wark JD. A worksite program for overweight middle-aged men achieves lesser weight loss with exercise than with dietary change. J Am Diet Assoc 1997;97:37–42.
Saito H, Kimura Y, Tashima S, Takao N, Nakagawa A, Baba T, et al. Psychological factors that promote behavior modification by obese patients. Biopsychosoc Med 2009;3:9
Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E, Cook NR, Hebert P, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. Arch Intern Med 1993;153:849–58.
Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith WD, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001;134:1–11.
ter Bogt NC, Bemelmans WJ, Beltman FW, Broer J, Smit AJ, van der Meer K. Preventing weight gain. One-year results of a randomized lifestyle intervention. Am J Prev Med 2009;37:270–7.
ter Bogt NC, Bemelmans WJ, Beltman FW, Broer J, Smit AJ, van der Meer K. Preventing weight gain by lifestyle intervention in a general practice setting: three-year results of a randomized controlled trial. Arch Intern Med 2011;171:306–13.
ter Bogt NC, Milder IE, Bemelmans WJ, Beltman FW, Broer J, Smit AJ, et al. Changes in lifestyle habits after counselling by nurse practitioners: 1-year results of the Groningen Overweight and Lifestyle study. Public Health Nutr 2011;14:995–1000.
van Wier MF, Dekkers JC, Hendriksen IJ, Heymans MW, Ariëns GA, Pronk NP, et al. Effectiveness of phone and e-mail lifestyle counseling for long term weight control among overweight employees. J Occup Environ Med 2011;53:680–6.
Ineligible study design
Atlantis E, Barnes EH, Ball K. Weight status and perception barriers to healthy physical activity and diet behavior. Int J Obes 2008;32:343–52.
Clifford PA, Tan SY, Gorsuch RL. Efficacy of a self-directed behavioral health change program: weight, body composition, cardiovascular fitness, blood pressure, health risk, and psychosocial mediating variables. J Behav Med 1991;14:303–23.
Forster JL, Jeffery RW, Snell MK. One-year follow-up study to a worksite weight control program. Prev Med 1988;17:129–33.
Han TS, Tajar S, O’Neill TW, Jiang M, Bartfai G, Boonen S, et al. Impaired quality of life and sexual funciton in overweight and obese men: the European Male Ageing Study. Eur J Endocrinol 2011;164:1003–11.
Hankonen N, Vollmann M, Renner B, Absetz P. What is setting the stage for abdominal obesity reduction? A comparison between personality and health-related social cognitions. J Behav Med 2010;33:415–22.
Hughes MC, Girolami TM, Cheadle AD, Harris JR, Patrick DL. A lifestyle-based weight management program delivered to employees: examination of health and economic outcomes. J Occup Environ Med 2007;49:1212–17.
Johansson K, Hemmingsson E, Harlid R, Trolle LY, Granath F, Rossner S, et al. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. BMJ 2011;342:d3017.
Jonasson J, Linné Y, Neovius M, Rössner S. An Internet-based weight loss programme: a feasibility study with preliminary results from 4209 completers. Scand J Public Health 2009;37:75–82.
Kerr J, Norman GJ, Adams MA, Ryan S, Frank L, Sallis JF, et al. Do neighborhood environments moderate the effect of physical activity lifestyle interventions in adults? Health Place 2010;16:903–8.
Mann RA. The behavior-therapeutic use of contingency contracting to control an adult behavior problem: weight control. J Appl Behav Anal 1972;5:99–109.
Mann RA. The use of contingency contracting to facilitate durability of behavior change: weight loss maintenance. Addict Behav 1976;1:245–9.
Michie S. Talking to primary care patients about weight: a study of GPs and practice nurses in the UK. Psychol Health Med 2008;12:521–5.
O’Neil PM, Currey HS, Hirsch AA, Riddle FE, Taylor CI, Malcolm RJ, et al. Effects of sex of subject and spouse involvement on weight loss in a behavioral treatment program: a retrospective investigation. Addict Behav 1979;4:167–77.
Rozensky RH, Bellack AS. Individual differences in self-reinforcement style and performance in self- and therapist-controlled weight reduction programs. Behav Res Ther 1976;14:357–64.
Stanforth D, Mackert M. Social undermining of healthy eating and exercise behaviors. ACSMS Health Fitness J 2009;13:14–19.
Timko CA, Perone J, Crossfield A. Are you currently on a diet? What respondents mean when they say ‘yes’. Eat Disord 2006;14:157–66.
Trent LK, Stevens LT. Evaluation of the Navy’s obesity treatment program. Mil Med 1995;160:326–30.
Van Wormer JJ, Martinez AM, Martinson BC, Crain AL, Benson GA, Cosentino DL, et al. Self-weighing promotes weight loss for obese adults. Am J Prev Med 2009;36:70–3.
Villanova N, Pasqui F, Burzacchini S, Forlani G, Manini R, Suppini A, et al. A physical activity program to reinforce weight maintenance following a behavior program in overweight/obese subjects. Int J Obes 2006;30:697–703.
No comparator
Wassertheil-Smoller S, Langford HG, Blaufox MD. Effective dietary intervention in hypertensives: sodium restriction and weight reduction. J Am Diet Assoc 1985;85:423–30.
No data by sex
Ahmed GM, Mostafa M, Farouk M, El-Gohary A. Efficacy of weight reduction program on obese patients with diabetic peripheral neuropathy. Egypt J Neurol Psychiatry Neurosurg 2010;47:673–9.
Amati F, Barthassat V, Miganne G, Hausman I, Monnin D, Constanza MC, et al. Enhancing regular physical activity and relapse prevention through a 1-day therapeutic patient education workshop: a pilot study. Patient Educ Couns 2007;68:70–8.
Andrews RC, Cooper AR, Montgomery AA, Norcross AJ, Peters TJ, Sharp DJ, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011;378:129–39.
Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med 2011;365:1959–68.
Assuncao MC, Gigante DP, Cardoso MA, Sartorelli DS, Santos IS. Randomized, controlled trial promotes physical activity and reduces consumption of sweets and sodium among overweight and obese adults. Nutr Res 2010;30:541–9.
Avila JJ, Gutierres JA, Sheehy ME, Lofgren IE, Delmonico MJ. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur J Appl Physiol 2010;109:517–25.
Barham K, West S, Trief P, Morrow C, Wade M, Weinstock RS. Diabetes prevention and control in the workplace: a pilot project for county employees. J Public Health Manag Pract 2011;17:233–41.
Bartfield JK, Stevens VJ, Jerome GJ, Batch BC, Kennedy BM, Vollmer WM, et al. Behavioral transitions and weight change patterns within the PREMIER trial. Obesity 2011;19:1609–15.
Belalcazar LM, Reboussin DM, Haffner SM, Hoogeveen RC, Kriska AM, Schwenke DC, et al. A 1-year lifestyle intervention for weight loss in individuals with type 2 diabetes reduces high C-reactive protein levels and identifies metabolic predictors of change: from the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care 2010;33:2297–303.
Belalcazar LM, Reboussin DM, Haffner SM, Reeves RS, Schwenke DC, Hoogeveen RC, et al. Marine omega-3 fatty acid intake: associations with cardiometabolic risk and response to weight loss intervention in the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care 2010;33:197–9.
Ben-Avraham S, Harman-Boehm I, Shwarzfuchs D, Shai I. Dietary strategies for patients with type 2 diabetes in the era of multi-approaches; review and results from the Dietary Intervention Randomized Controlled Trial (DIRECT). Diabetes Res Clin Pract 2009;86S:S41–8.
Berry D, Savoye M, Melkus G, Grey M. An intervention for multiethnic obese parents and overweight children. Appl Nurs Res 2007;20:63–71.
Bladbjerg EM, Larsen TM, Due A, Jespersen J, Stender S, Astrup A. Long-term effects on haemostatic variables of three ad libitum diets differing in type and amount of fat and carbohydrate: a 6-month randomised study in obese individuals. Br J Nutr 2010;104:1824–30.
Bliddal H, Leeds AR, Stigsgaard L, Astrup A, Christensen R. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomised controlled trial. Ann Rheum Dis 2011;70:1798–803.
Burke LE, Conroy MB, Sereika SM, Elci OU, Styn MA, Acharya SD, et al. The effect of electronic self-monitoring on weight loss and dietary intake: a randomized behavioral weight loss trial. Obesity 2011;19:338–44.
Castelnuovo G, Manzoni GM, Cuzziol P, Cesa GL, Corti S, Tuzzi C, et al. TECNOB study: ad interim results of a randomized controlled trial of a multidisciplinary telecare intervention for obese patients with type-2 diabetes. Clin Pract Epidemiol Ment Health 2011;7:44–50.
Castro L, Rachlin H. Self-reward, self-monitoring, and self-punishment as feedback in weight control. Behav Ther 1980;11:38–48.
Castro L, de Pérez GC, de Albanchez DB, de León EP. Feedback properties of self-reinforcement: further evidence. Behav Ther 1983;14:672–81.
Champagne CM, Broyles ST, Moran LD, Cash KC, Levy EJ, Lin PH, et al. Dietary intakes associated with successful weight loss and maintenance during the Weight Loss Maintenance trial. J Am Diet Assoc 2011;111:1826–35.
Chapman SL, Jeffrey DB. Situational management, standard setting, and self-reward in a behavior modification weight loss program. J Consult Clin Psychol 1978;46:1588–9.
Chen L, Caballero B, Mitchell DC, Loria C, Lin PH, Champagne CM, et al. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: a prospective study among United States adults. Circulation 2010;121:2398–406.
Choo J, Elci OU, Yang K, Turk MW, Styn MA, Sereika SM, et al. Longitudinal relationship between physical activity and cardiometabolic factors in overweight and obese adults. Eur J Appl Physiol 2010;108:329–36.
Cocco G, Pandolfi S, Rousson V. Sufficient weight reduction decreases cardiovascular complications in diabetic patients with the metabolic syndrome: a randomized study of orlistat as an adjunct to lifestyle changes (diet and exercise). Heart Drug 2005;5:68–74.
Colvin RH, Zopf KJ, Myers JH. Weight control among coworkers. Effects of monetary contingencies and social milieu. Behav Modif 1983;7:64–75.
Cooper KL, Meng Y, Harnan S, Ward SE, Fitzgerald P, Papaioannou D, et al. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health Technol Assess 2011;15(4).
Davis NJ, Tomuta N, Isasi CR, Leung V, Wylie-Rosett J. Diabetes-specific quality of life after a low-carbohydrate and low-fat dietary intervention. Diabetes Educ 2012;38:250–5.
DiMarco ID, Klein DA, Clark VL, Wilson GT. The use of motivational interviewing techniques to enhance the efficacy of guided self-help behavioral weight loss treatment. Eat Behav 2009;10:134–6.
Earles JE, Kerr B, James LC, Folen RA. Clinical effectiveness of the LE3AN Program: a military healthy lifestyle program. J Clin Psychol Med Settings 2007;14:51–7.
Forslund HB, Klingström S, Hagberg H, Löndahl M, Torgerson JS, Lindroos AK. Should snacks be recommended in obesity treatment? A 1-year randomized clinical trial. Eur J Clin Nutr 2008;62:1308–17.
Gagnon C, Brown C, Couture C, Kamga-Ngande CN, Hivert MF, Baillargeon JP, et al. A cost-effective moderate-intensity interdisciplinary weight-management programme for individuals with prediabetes. Diabetes Metab 2011;37:410–18.
Galani C, Schneider H, Rutten FF. Modelling the lifetime costs and health effects of lifestyle intervention in the prevention and treatment of obesity in Switzerland. Int J Public Health 2007;52:372–82.
Goetzel RZ, Roemer EC, Pei X, Short ME, Tabrizi MJ, Wilson MG, et al. Second-year results of an obesity prevention program at the Dow Chemical Company. J Occup Environ Med 2010;52:291–302.
Gohner W, Schlatterer M, Seelig H, Frey I, Berg A, Fuchs R. Two-year follow-up of an interdisciplinary cognitive-behavioral intervention program for obese adults. J Psychol 2012;146:371–91.
Gorin AA, Pinto AM, Tate DF, Raynor HA, Fava JL, Wing RR. Failure to meet weight loss expectations does not impact maintenance in successful weight losers. Obesity 2007;15:3086–90.
Groeneveld IF, Proper KI, van der Beek AJ, van Mechelen W. Sustained body weight reduction by an individual-based lifestyle intervention for workers in the construction industry at risk for cardiovascular disease: results of a randomized controlled trial. Prev Med 2010;51:240–6.
Haapala I, Barengo NC, Biggs S, Surakka L, Manninen P. Weight loss by mobile phone: a 1-year effectiveness study. Public Health Nutr 2009;12:2382–91.
Harris J, Felix L, Miners A, Murray E, Michie S, Ferguson E, et al. Adaptive e-learning to improve dietary behaviour: a systematic review and cost-effectiveness analysis. Health Technol Assess 2011;15(37).
Haynes SM, Lyons GF, McCombie EL, McQuigg MS, Mongia S, Noble PA, et al. Long-term cost-effectiveness of weight management in primary care. Int J Clin Pract Suppl 2010;64:775–83.
Holme I, Høstmark AT, Anderssen SA. ApoB but not LDL-cholesterol is reduced by exercise training in overweight healthy men. Results from the 1-year randomized Oslo Diet and Exercise Study. J Intern Med 2007;262:235–43.
Iqbal N, Vetter ML, Moore RH, Chittams JL, Dalton-Bakes CV, Dowd M, et al. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity 2010;18:1733–8.
Jacobs DR Jr, Sluik D, Rokling-Andersen MH, Anderssen SA, Drevon CA. Association of 1-y changes in diet pattern with cardiovascular disease risk factors and adipokines: results from the 1-y randomized Oslo Diet and Exercise Study. Am J Clin Nutr 2009;89:509–17.
Jakicic JM, Otto AD, Lang W, Semler L, Winters C, Polzien K, et al. The effect of physical activity on 18-month weight change in overweight adults. Obesity 2011;19:100–9.
Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stoll J, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011;378:1485–92.
Jeffery RW, Thompson PD, Wing RR. Effects on weight reduction of strong monetary contracts for calorie restriction or weight loss. Behav Res Ther 1978;16:363–9.
Jeffrey DB. A comparison of the effects of external control and self-control on the modification and maintenance of weight. J Abnorm Psychol 1974;83:404–10.
John LK, Loewenstein G, Troxel AB, Norton L, Fassbender JE, Volpp KG. Financial incentives for extended weight loss: a randomized, controlled trial. J Gen Intern Med 2011;26:621–6.
Katula JA, Vitolins MZ, Rosenberger EL, Blackwell CS, Morgan TM, Lawlor MS, et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care 2011;34:1451–7.
Keränen AM, Strengell K, Savolainen MJ, Laitinen JH. Effect of weight loss intervention on the association between eating behaviour measured by TFEQ-18 and dietary intake in adults. Appetite 2011;56:156–62.
Keyhan G, Rahme E, Grover S, Dasgupta K. Weight change during diet and exercise interventions among overweight adults with type 2 diabetes: results of a chart review. Can J Diabetes 2008;32:255–9.
Kim SI, Kim HS. Effectiveness of mobile and internet intervention in patients with obese type 2 diabetes. Int J Med Inform 2008;77:399–404.
Kumanyika SK, Shults J, Fassbender J, Whitt MC, Brake V, Kallan MJ, et al. Outpatient weight management in African-Americans: the Healthy Eating and Lifestyle Program (HELP) study. Prev Med 2005;41:488–502.
Levy RL, Jeffery RW, Langer SL, Graham DJ, Welsh EM, Flood AP, et al. Maintenance-tailored therapy vs. standard behavior therapy for 30-month maintenance of weight loss. Prev Med 2010;51:457–9.
Linde JA, Rothman AJ, Baldwin AS, Jeffery RW. The impact of self-efficacy on behavior change and weight change among overweight participants in a weight loss trial. Health Psychol 2006;25:282–91.
Look AHEAD Research Group, Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007;30:1374–83.
Madsen EL, Rissanen A, Bruun JM, Skogstrand K, Tonstad S, Hougaard DM, et al. Weight loss larger than 10% is needed for general improvement of levels of circulating adiponectin and markers of inflammation in obese subjects: a 3-year weight loss study. Eur J Endocrinol 2008;158:179–87.
Mahoney MJ, Moura NG, Wade TC. Relative efficacy of self-reward, self-punishment, and self-monitoring techniques for weight loss. J Consult Clin Psychol 1973;40:404–7.
Manning RM, Jung RT, Leese GP, Newton RW. The comparison of four weight reduction strategies aimed at overweight diabetic patients. Diabet Med 1995;12:409–15.
Manning RM, Jung RT, Leese GP, Newton RW. The comparison of four weight reduction strategies aimed at overweight patients with diabetes mellitus: four-year follow-up. Diabet Med 1998;15:497–502.
Marinilli PA, Gorin AA, Raynor HA, Tate DF, Fava JL, Wing RR. Successful weight-loss maintenance in relation to method of weight loss. Obesity 2008;16:2456–61.
Martin CK, Talamini L, Johnson A, Hymel AM, Khavjou O. Weight loss and retention in a commercial weight-loss program and the effect of corporate partnership. Int J Obes 2010;34:742–50.
McConnon A, Kirk SF, Cockroft JE, Harvey EL, Greenwood DC, Thomas JD, et al. The Internet for weight control in an obese sample: results of a randomised controlled trial. BMC Health Serv Res 2007;7:206.
Melin I, Reynisdottir S, Berglund L, Zamfir M, Karlstrom B. Conservative treatment of obesity in an academic obesity unit. Long-term outcome and drop-out. Eat Weight Disord 2006;11:22–30.
Moore SD, King AC, Kiernan M, Gardner CD. Outcome expectations and realizations as predictors of weight regain among dieters. Eat Behav 2011;12:60–3.
Murphy JK, Williamson DA, Buxton AE, Moody SC, Absher N, Warner M. The long-term effects of spouse involvement upon weight loss and maintenance. Behav Ther 1982;13:681–93.
Murphy JK, Bruce BK, Williamson DA. A comparison of measured and self-reported weights in a 4-year follow-up of spouse involvement in obesity treatment. Behav Ther 1985;16:524–30.
Nakata Y, Okada M, Hashimoto K, Harada Y, Sone H, Tanaka K. Comparison of education-only versus group-based intervention in promoting weight loss: a randomised controlled trial. Obes Facts 2011;4:222–8.
Oenema A, Brug J, Lechner L. Web-based tailored nutrition education: results of a randomized controlled trial. Health Educ Res 2001;16:647–60.
Prochaska JO, Norcross JC, Fowler JL, Follick MJ, Abrams DB. Attendance and outcome in a work site weight control program: processes and stages of change as process and predictor variables. Addict Behav 1992;17:35–45.
Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2011;124:563–71.
Racette SB, Deusinger SS, Inman CL, Burlis TL, Highstein GR, Buskirk TD, et al. Worksite Opportunities for Wellness (WOW): effects on cardiovascular disease risk factors after 1 year. Prev Med 2009;49:108–14.
Rejeski WJ, Brubaker PH, Goff DC, Bearon LB, McClelland JW, Perri MG, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med 2011;171:880–6.
Rejeski WJ, Mihalko SL, Ambrosius WT, Bearon LB, McClelland JW. Weight loss and self-regulatory eating efficacy in older adults: the cooperative lifestyle intervention program. J Gerontol B Psychol Sci Soc Sci 2011;66:279–86.
Rejeski WJ, Ip EH, Bertoni AG, Bray GA, Evans G, Gregg EW, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med 2012;366:1209–17.
Reseland JE, Anderssen SA, Solvoll K, Hjermann I, Urdal P, Holme I, et al. Effect of long-term changes in diet and exercise on plasma leptin concentrations. Am J Clin Nutr 2001;73:240–5.
Soureti A, Murray P, Cobain M, Chinapaw M, van Mechelen W, Hurling R. Exploratory study of web-based planning and mobile text reminders in an overweight population. J Med Internet Res 2011;13:e118.
Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008;299:1139–48.
Tanumihardjo SA, Valentine AR, Zhang Z, Whigham LD, Lai HJ, Atkinson RL. Strategies to increase vegetable or reduce energy and fat intake induce weight loss in adults. Exp Biol Med 2009;234:542–52.
Thorpe MP, Jacobson EH, Layman DK, He X, Kris-Etherton PM, Evans EM. A diet high in protein, dairy, and calcium attenuates bone loss over twelve months of weight loss and maintenance relative to a conventional high-carbohydrate diet in adults. J Nutr 2008;138:1096–100.
Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50.
van Weir MF, Dekkers JC, Hendriksen IJ, Heymans MW, Ariens GA, Pronk NP, et al. Effectiveness of phone and e-mail lifestyle counseling for long term weight control among overweight employees. J Occup Environ Med 2011;53:680–6.
Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–29.
Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011;365:1969–79.
Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL, Machan J. STOP regain: are there negative effects of daily weighing? J Consult Clin Psychol 2007;75:652–6.
Woo J, Sea MMM, Tong P, Ko GTC, Lee Z, Chan J, et al. Effectiveness of a lifestyle modification programme in weight maintenance in obese subjects after cessation of treatment with orlistat. J Eval Clin Pract 2007;13:853–9.
Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults – a randomized clinical trial. J Gerontol A Biol Sci Med Sci 2009;64:90–5.
Yates T, Davies MJ, Sehmi S, Gorely T, Khunti K. The Pre-diabetes Risk Education and Physical Activity Recommendation and Encouragement (PREPARE) programme study: are improvements in glucose regulation sustained at 2 years? Diabet Med 2011;28:1268–71.
No unique data
Albu JB, Heilbronn LK, Kelley DE, Smith SR, Azuma K, Berk ES, et al. Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes 2010;59:627–33.
Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403.
Diabetes Prevention Program Research Group. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res 2004;12:1426–34.
Frey-Hewitt B, Vranizan KM, Dreon DM, Wood PD. The effect of weight loss by dieting or exercise on resting metabolic rate in overweight men. Int J Obes 1990;14:327–34.
Linde JA, Jeffery RW, Levy RL, Pronk NP, Boyle RG. Weight loss goals and treatment outcomes among overweight men and women enrolled in a weight loss trial. Int J Obes 2005;29:1002–5.
Lindstrom J, Tuomilehto J. The diabetes risk score. A practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725–31.
Linna MS, Borg P, Kukkonen-Harjula K, Fogelholm M, Nenonen A, Ahotupa M, et al. Successful weight maintenance preserves lower levels of oxidized LDL achieved by weight reduction in obese men. Int J Obes 2007;31:245–53.
Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 2005;142:611–19.
Perreault L, Ma Y, Dagogo-Jack S, Horton E, Marrero D, Crandall J, et al. Sex differences in diabetes risk and the effect of intensive lifestyle modification in the Diabetes Prevention Program. Diabetes Care 2008;31:1416–21.
Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF, Diabetes Prevention Program Research Group. Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care 2009;32:1583–8.
Perri MG, McAllister DA, Gange JJ, Jordan RC, McAdoo G, Nezu AM. Effects of four maintenance programs on the long-term management of obesity. J Consult Clin Psychol 1988;56:529–34.
Petrofsky J, Batt J, Berk L, Bains G, Wong J, Radabaugh S, et al. A combination of exercise with a mini medicine ball and diet with a meal replacement shake as a synergistic program to increases fitness and produces weight loss. J Appl Res 2011;11:20–8.
Petrofsky J, Batt J, Berk L, Bains G, Wong J, Radabaugh S, et al. A video exercise and diet program using a nutritional meal replacement shake for weight loss. J Appl Res 2011;11:29–36.
Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J, for the RIO-North America Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight and obese patients. RIO-North America: a randomized controlled trial. JAMA 2007;295:761–75.
Sherwood NE, Jeffery RW, Pronk NP, Boucher JL, Hanson A, Boyle R, et al. Mail and phone interventions for weight loss in a managed-care setting: weigh-to-be 2-year outcomes. Int J Obes 2006;30:1565–73.
Williams PT, Krauss RM, Vranizan KM, Wood PD. Changes in lipoprotein subfractions during diet-induced and exercise-induced weight loss in moderately overweight men. Circulation 1990;81:1293–304.
Williams PT, Krauss RM, Vranizan KM, Albers JJ, Wood PD. Effects of weight-loss by exercise and by diet on apolipoproteins A-I and A-II and the particle-size distribution of high-density lipoproteins in men. Metabolism 1992;41:441–9.
Williams PT, Krauss RM, Stefanick ML, Vranizan KM, Wood PD. Effects of low-fat diet, calorie restriction, and running on lipoprotein subfraction concentrations in moderately overweight men. Metabolism 1994;43:655–63.
No useable data
Ash S, Reeves MM, Yeo S, Morrison G, Carey D, Capra S. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with type II diabetes: a randomised trial. Int J Obes Relat Metab Disord 2003;27:797–802.
Dunn CL, Hannan PJ, Jeffery RW, Sherwood NE, Pronk NP, Boyle R. The comparative and cumulative effects of a dietary restriction and exercise on weight loss. Int J Obes 2006;30:112–21.
Lappalainen TJ, Tolppanen AM, Kolehmainen M, Schwab U, Lindström J, Tuomilehto J, et al. The common variant in the FTO gene did not modify the effect of lifestyle changes on body weight: the Finnish Diabetes Prevention Study. Obesity 2009;17:832–6.
Shea MK, Nicklas BJ, Houston DK, Miller ME, Davis CC, Kitzman DW, et al. The effect of intentional weight loss on all-cause mortality in older adults: results of a randomized controlled weight-loss trial. Am J Clin Nutr 2011;94:839–46.
No weight loss data
Bleich SN, Huizinga MM, Beach MC, Cooper LA. Patient use of weight-management activities: a comparison of patient and physician assessments. Patient Educ Couns 2010;79:344–50.
Cizza G, Marincola P, Mattingly M, Williams L, Mitler M, Skarulis M, et al. Treatment of obesity with extension of sleep duration: a randomized, prospective, controlled trial. Clin Trials 2010;7:274–85.
Ongoing trials, no results
Eakin EG, Reeves MM, Marshall AL, Dunstan DW, Graves N, Healy GN, et al. Living Well with Diabetes: a randomized controlled trial of a telephone-delivered intervention for maintenance of weight loss, physical activity and glycaemic control in adults with type 2 diabetes. BMC Public Health 2010;10:452.
Elder C, Gallison C, Lindberg NM, DeBar L, Funk K, Ritenbaugh C, et al. Randomized trial of Tapas Acupressure Technique for weight loss maintenance: rationale and study design. J Altern Complement Med 2010;16:683–90.
Ross R, Blair SN, Godwin M, Hotz S, Katzmarzyk PT, Lam M, et al. Prevention and Reduction of Obesity through Active Living (PROACTIVE): rationale, design and methods. Br J Sports Med 2009;43:57–63.
Wadden TA. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity 2006;14:737–52.
Reviews
Anderson LM, Quinn TA, Glanz K, Ramirez G, Kahwati LC, Johnson DB, et al. The effectiveness of worksite nutrition and physical activity interventions for controlling employee overweight and obesity. Am J Prev Med 2009;37:340–57.
Baulch J, Chester A, Brennan L. Treatment alternatives for overweight and obesity: the role of online interventions. Behav Change 2008;1:1–14.
Catenacci VA, Wyatt HR. The role of physical activity in producing and maintaining weight loss. Nat Clin Pract Endocrinol Metab 2007;518–29.
Christian JG, Tsai AG, Bessesen DH. Interpreting weight losses from lifestyle modification trials: using categorical data. Int J Obes 2010;34:207–9.
Esposito K, Kastorini CM, Panagiotakos DB, Giugliano D. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab Syndr Relat Disord 2011;9:1–12.
Foreyt J, Scott L, Gotto A. Weight control and nutrition education programs in occupational settings. Public Health Rep 1980;95:127–36.
Kremers S, Reubsaet A, Martens M, Gerards S, Jonkers R, Candel M, et al. Systematic prevention of overweight and obesity in adults: a qualitative and quantitative literature analysis. Obes Rev 2010;11:371–9.
Sanderson PW, Clemes SA, Biddle SJ. The correlates and treatment of obesity in military populations: a systematic review. Obes Facts 2011;4:229–37.
Unavailable
Archer WR, Batan MC, Ramsey Buchanan L, Soler RE, Ramsay DC, Kirchhofer A, et al. Promoting practices for the prevention and control of obesity in the workforce. Am J Health Promot 2011;25:e12–26.
Makoundou V, Bobbioni HE, Gachoud JP, Habicht F, Pataky Z, Golay A. A 2-year multifactor approach of weight loss maintenance. Eat Weight Disord 2010;15:e9–14.
Reviews of UK interventions and engagement
Background
DeVille-Almond J, Tahrani AA, Grant J, Gray M, Thomas GN, Taheri S. Awareness of obesity and diabetes: a survey of a subset of British male drivers. Am J Mens Health 2011;5:30–7.
Lee CG, Boyko EJ, Nielson CM, Stefanick ML, Bauer DC, Hoffman A, et al. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc 2011;59:233–40.
Lorimer K, Gray CM, Hunt K, Wyke S, Anderson A, Benzeval M. Response to written feedback of clinical data within a longitudinal study: a qualitative study exploring the ethical implications. BMC Med Res Methodol 2011;11:10.
McCabe MP, McGreevy SJ. Role of media and peers on body change strategies among adult men: is body size important? Eur Eat Disord Rev 2011;19:438–46.
Morrison DS, Boyle S, Morrison C, Allardice G, Greenlaw N, Forde L. Evaluation of the first phase of a specialist weight management programme in the UK National Health Service: prospective cohort study. Public Health Nutr 2012;15:28–38.
Pagoto SL, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity 2012;20:1234–9.
Zajacova A, Dowd JB, Burgard SA. Overweight adults may have the lowest mortality – do they have the best health? Am J Epidemiol 2011;173:430–7.
Ineligible intervention
Bush A, Wood DF, Parrott D, Johnston DG. Management of severe obesity by a short period of intensive in-patient dietary restriction and counselling: an evaluation of effectiveness. J Hum Nutr Diet 1993;6:125–30.
Drummond S, Kirk T. The effect of different types of dietary advice on body composition in a group of Scottish men. J Hum Nutr Diet 1998;11:473–85.
Erfurt JC, Foote A, Heirich MA, Gregg W. Improving participation in worksite wellness programs: comparing health education classes, a menu approach, and follow-up counseling. Am J Health Promot 1990;4:270–8.
Gibbs H, Lee L, McFarlane L, Hughes N, Aubry C, Huntly M, et al. Weigh2go – a peripatetic level 1 weight management service in Cambridge City and South Cambridgeshire. Treatment of Obesity Conference, Birmingham, June 2012.
Hallam CL, Mullins G, du Plessis J, Cox JSA, Hewlett B. To report on the weight loss achieved in 8 weeks by 950 obese male patients on the LighterLife Total for Men VLCD weight-loss programme in 2009; a retrospective study. Obes Rev 2010;11:243.
Kamel EG, McNeill G, Van Wijk MC. Change in intra-abdominal adipose tissue volume during weight loss in obese men and women: correlation between magnetic resonance imaging and anthropometric measurements. Int J Obes 2000;24:607–13.
Leslie WS, Hankey CR, McCombie L, Lean ME. Weight management: a survey of current practice in secondary care NHS settings in 2004. J Eval Clin Pract 2005;11:462–7.
McCrea C, Summerfield, AB. A pilot study of the therapeutic usefulness of videofeedback for weight loss and improvement of body image in the treatment of obesity. Behav Psychother 1988;16:269–84.
McQuigg M, Brown JE, Broom JI, Laws RA, Reckless JPD, Noble PA, et al. Engaging patients, clinicians and health funders in weight management: the Counterweight programme. Fam Pract 2008;25:i79–86.
O’Kane M, Wales JK. The effect of the composition of very low- and low-calorie diets on 1 week’s weight loss in obese patients. J Hum Nutr Diet 1994;7:3–10.
Strain GW, Zumoff B, Miller LK, Rosner W, Levit C, Kalin M, et al. Effect of massive weight loss on hypothalamic-pituitary-gonadal function in obese men. J Clin Endocrinol Metab 1988;66:1019–23.
Webber J, Donaldson M, Allison SP, Fukagawa NK, Macdonald IA. The effects of weight loss in obese subjects on the thermogenic, metabolic and haemodynamic responses to the glucose clamp. Int J Obes 1994;18:725–30.
Ineligible population
Holt J, Horsnell T, Mullins G, du Plessis J, Cox J, Hewlett B. What motivates men to lose weight? Int J Obes 2007;31:S169.
No data by sex
The implementation of the Counterweight programme in Scotland, UK. Fam Pract 2012;29:I139–44.
Bryant EJ, Caudwell P, Hopkins ME, King NA, Blundell JE. Psycho-markers of weight loss. The roles of TFEQ disinhibition and restraint in exercise-induced weight management. Appetite 2012;58:234–41.
Caudwell P, Hopkins M, King NA, Stubbs RJ, Blundell JE. Exercise alone is not enough: weight loss also needs a healthy (Mediterranean) diet? Public Health Nutr 2009;12:1663–6.
Collinson A, Lindley R, Campbell A, Waters I, Lindley T, Wallace A. An evaluation of an internet-based approach to weight loss with low glycaemic load principles. J Hum Nutr Diet 2011;24:192–5.
Connolly S, Holden A, Turner E, Fiumicelli G, Stevenson J, Hunjan M, et al. MyAction: an innovative approach to the prevention of cardiovascular disease in the community. Br J Cardiol 2011;18:171–6.
Crisp AH, Stonehill E. Treatment of obesity with special reference to seven severely obese patients. J Psychosom Res 1970;14:327–45.
DasGupta P, Ramhanmdany E, Brigden G, Lahiri A, Baird IM, Raftery EB. Improvement in left ventricular function after rapid weight loss in obesity. Eur Heart J 1992;13:1060–6.
Dhindsa P, Scott AR, Donnelly R. Metabolic and cardiovascular effects of very-low-calorie diet therapy in obese patients with type 2 diabetes in secondary failure: outcomes after 1 year. Diabet Med 2003;20:319–24.
Finlayson G, Caudwell P, Gibbons C, Hopkins M, King N, Blundell J. Low fat loss response after medium-term supervised exercise in obese is associated with exercise-induced increase in food reward. J Obes 2011:615624. doi: 10.1155/2011/615624.
Fletcher B, Hanson J, Page N, Pine K. FIT – do something different: a new behavioral program for sustained weight loss. Swiss J Psychol 2011;70:25–34.
Frost G. Comparison of two methods of energy prescription for obese non-insulin-dependent diabetics. Pract Diabetes Int 1989;6:273–5.
Frost G, Masters K, King C, Kelly M, Hasan U, Heavens P, et al. A new method of energy prescription to improve weight loss. J Hum Nutr Diet 2007;20:152–6.
Grace C, Summerbell C, Kopelman P. An audit of dietary treatment modalities and weight loss outcomes in a specialist obesity clinic. J Hum Nutr Diet 2007;11:197–202.
Hajek P, Humphrey K, McRobbie H. Using group support to complement a task-based weight management programme in multi-ethnic localities of high deprivation. Patient Educ Couns 2010;80:135–7.
Hallam CL, Broom J, Mullins G, Cox JSA, Hewlett B. Weight loss commensurate with reversal of type 2 diabetes using a VLCD approach. Appetite 2011;58:1176.
Hankey CR, Lean MEJ, Lowe GDO, Rumley A, Woodward M. Effects of moderate weight loss on anginal symptoms and indices of coagulation and fibrinolysis in overweight patients with angina pectoris. Eur J Clin Nutr 2002;56:1039–45.
Hickson M, Macqueen C, Frost G. Evaluation of attendance and weight loss in an intensive weight management clinic compared to standard dietetic care. J Hum Nutr Diet 2009;22:72–6.
Hosker JP, Kumar S, Gordon C, Bhatnagar D, France M, Boulton AJM. Diet treatment of newly presenting type-2 diabetes improves insulin secretory capacity, but has no effect on insulin sensitivity. Diabet Med 1993;10:509–13.
Jones K, Bone A, Gill G, Martin S, Padmore E, Dolan M, et al. Factors affecting weight loss in obese type 2 diabetes – the vital role of the dietitian. Pract Diabetes Int 1989;6:18–19.
Lloyd A, Khan R. Evaluation of Healthy Choices: a commercial weight loss programme commissioned by the NHS. Perspect Public Health 2011;131:177–83.
McQuigg M, Brown J, Broom J, Laws RA, Reckless JPD, Noble PA, et al. Empowering primary care to tackle the obesity epidemic: the Counterweight programme. Eur J Clin Nutr 2005;59(Suppl. 1):S93–100.
Mengham LH, Morris BF, Palmer CR, White AJS. Is intensive dietetic intervention effective for overweight patients with diabetes mellitus? A randomised controlled study in a general practice. Pract Diabetes Int 1999;16:5–8.
Molokhia M. Obesity wars: a pilot study of very low calorie diets in obese patients in general practice. Br J Gen Pract 1998;48:1251–2.
Paisey RB, Harvey P, Rice S, Belka I, Bower L, Dunn M, et al. An intensive weight loss programme in established type 2 diabetes and controls: effects on weight and atherosclerosis risk factors at 1 year. Diabet Med 1998;15:73–9.
Paisey RB, Frost J, Harvey P, Paisey A, Bower L, Paisey RM, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 2002;15:121–7.
Paxman JR, Hall AC, Harden CJ, O’Keeffe J, Simper TN. Weight loss is coupled with improvements to affective state in obese participants engaged in behavior change therapy based on incremental, self-selected ‘small changes’. Nutr Res 2011;31:327–37.
Relton C, Strong M, Li J. The ‘Pounds for Pounds’ weight loss financial incentive scheme: an evaluation of a pilot in NHS Eastern and Coastal Kent. J Public Health 2011;33:536–42.
Silverstone JT, Cooper RM. Short-term weight loss in refractory obesity. J Psychosom Res 1972;16:123–8.
Stubbs J, Brogelli D, Whybrow S, Avery A, Pallister C, Lavin J. 24-week referral to Slimming World from primary care: weight outcomes for 4,754 adults. Obesity Facts 2012;5(Suppl. 1):215–16.
Stubbs J, Brogelli D, Avery A, Pallister C, Lavin J. Weight outcomes of 497,777 participants in Slimming World’s programme during their first 12 weeks of membership. Obesity Facts 2012;5(Suppl. 1):232–3.
Swift JA, Glazebrook C, Anness A, Goddard R. Obesity-related knowledge and beliefs in obese adults attending a specialist weight-management service: implications for weight loss over 1 year. Patient Educ Couns 2009;74:70–6.
Trueman P, Haynes SM, Felicity L, Louise M, McQuigg MSA, Mongia S, et al. Long-term cost-effectiveness of weight management in primary care. Int J Clin Pract 2010;64:775–83.
Ware LJ, Hurling R, Bataveljic O, Fairley BW, Hurst TL, Murray P, et al. Rates and determinants of uptake and use of an internet physical activity and weight management program in office and manufacturing work sites in England: cohort study. J Med Internet Res 2008;10:e56.
No relevant data
Harvey EL, Glenny AM, Kirk SFL, Summerbell CD. A systematic review of interventions to improve health professionals’ management of obesity. Int J Obes 1999;23:1213–22.
Harwell TS, Vanderwood KK, Hall TO, Butcher MK, Helgerson SD. Factors associated with achieving a weight loss goal among participants in an adapted diabetes prevention program. Prim Care Diabetes 2011;5:125–9.
McLean N, Griffin S, Toney K, Hardeman W. Family involvement in weight control, weight maintenance and weight-loss interventions: a systematic review of randomised trials. Int J Obes 2003;27:987–1005.
No unique data
Pallister C, Avery A, Stubbs J, Lavin J. Slimming World on referral: evaluation of weight management outcomes when working in partnership with a commercial organisation. Obes Rev 2010;11:237.
Not targeted at engagement
Lavin JH, Avery A, Whitehead SM, Rees E, Parsons J, Bagnall T, et al. Feasibility and benefits of implementing a slimming on referral service in primary care using a commercial weight management partner. Public Health 2006;120:872–81.
Not a UK study
Anandacoomarasamy A, Leibman S, Smith G, Caterson I, Giuffre B, Fransen M, et al. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Ann Rheum Dis 2012;71:26–32.
Baba NH, Sawaya S, Torbay N, Habbal Z, Azar S, Hashim SA. High protein vs high carbohydrate hypoenergetic diet for the treatment of obese hyperinsulinemic subjects. Int J Obes 1999;23:1202–6.
Bauman WA, Schwartz E, Rose HG, Eisenstein HN, Johnson DW. Early and long-term effects of acute caloric deprivation in obese diabetic patients. Am J Med 1988;85:38–46.
Bess BE, Marlin RL. A pilot study of medication and group therapy for obesity in a group of physicians. Hillside J Clin Psychiatry 1984;6:171–87.
Blacket RB, Leelarthaepin B, McGilchrist CA. The synergistic effect of weight loss and changes in dietary lipids on the serum cholesterol of obese men with hypercholesterolaemia: implications for prevention of coronary heart disease. Aust N Z J Med 1979;9:521–9.
Borel AL, Nazare JA, Smith J, Almeras N, Tremblay A, Bergeron J, et al. Sustained improvement in insulin sensitivity despite moderate body weight regain: results of a three year lifestyle intervention program. Obesity Facts 2012;12(Suppl. 1):21.
Burnette JL, Finkel EJ. Buffering against weight gain following dieting setbacks: an implicit theory intervention. J Exp Soc Psychol 2012;48:721–5.
Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab 2001;86:355–62.
Ellsworth GA, Strain GW, Strain JJ, Vaillant GE. Defensive maturity ratings and sustained weight loss in obesity. Psychosomatics 1986;27:772–81.
Haapala I, Barengo NC, Biggs S, Surakka L, Manninen P. Weight loss by mobile phone: a 1-year effectiveness study. Public Health Nutr 2009;12:2382–91.
Handjieva-Darlenska T, Handjiev S, Larsen TM, van Baak MA, Jebb S, Papadaki A, et al. Initial weight loss on an 800-kcal diet as a predictor of weight loss success after 8 weeks: the Diogenes study. Eur J Clin Nutr 2010;64:994–9.
Harrison RL, Mattson LK, Durbin DM, Fish AF, Bachman JA. Wellness in community living adults: the Weigh to Life Program. Patient Educ Couns 2012;86:270–6.
Hoy MK, Heshka S, Allison DB, Grasset E, Blank R, Abiri M, et al. Reduced risk of liver-function-test abnormalities and new gallstone formation with weight loss on 3350-kJ (800-kcal) formula diets. Am J Clin Nutr 1994;60:249–54.
Kraemer WJ, Volek JS, Clark KL, Gordon SE, Puhl SM, Koziris LP, et al. Influence of exercise training on physiological and performance changes with weight loss in men. Med Sci Sports Exerc 1999;31:1320–9.
LeCheminant JD, Gibson CA, Sullivan DK, Hall S, Washburn R, Vernon MC, et al. Comparison of a low carbohydrate and low fat diet for weight maintenance in overweight or obese adults enrolled in a clinical weight management program. Nutr J 2007;6:36.
Libardi CA, Souza GV, Gáspari AF, Dos Santos CF, Leite S, Dias R, et al. Effects of concurrent training on interleukin-6, tumour necrosis factor-alpha and C-reactive protein in middle-aged men. J Sports Sci 2011;29:1573–81.
Luszczynska A, Haynes C. Changing nutrition, physical activity and body weight among student nurses and midwives: effects of a planning intervention and self-efficacy beliefs. J Health Psychol 2009;14:1075–84.
Maraki MI, Aggelopoulou N, Christodoulou N, Anastasiou CA, Toutouza M, Panagiotakos DB, et al. Lifestyle intervention leading to moderate weight loss normalizes postprandial triacylglycerolemia despite persisting obesity. Obesity 2011;19:968–76.
Neve M, Morgan PJ, Collins CE. Weight change in a commercial web-based weight loss program and its association with website use: cohort study. J Med Internet Res 2011;13:110–19.
Niemeier HM, Leahey T, Reed KP, Brown RA, Wing RR. An acceptance-based behavioral intervention for weight loss: a pilot study. Behav Ther 2012;43:427–35.
Pollak KI, Alexander SC, Coffman CJ, Tulsky JA, Lyna P, Dolor RJ, et al. Physician communication techniques and weight loss in adults: Project CHAT. Am J Prev Med 2010;39:321–8.
Schroder KEE. Computer-assisted dieting: effects of a randomized nutrition intervention. Am J Health Behav 2011;35:175–88.
Schuster I, Vinet A, Karpoff L, Startun A, Jourdan N, Dauzat M, et al. Diastolic dysfunction and intraventricular dyssynchrony are restored by low intensity exercise training in obese men. Obesity 2012;20:134–40.
Shapiro JR, Stout AL, Musante GJ. ‘Structure-size me:’ weight and health changes in a four week residential program. Eat Behav 2006;7:229–34.
Sofer S, Eliraz A, Kaplar S, Voet H, Fink G, Kima T, et al. Greater weight loss and hormonal changes after 6 months diet with carbohydrates eaten mostly at dinner. Obesity 2011;19:2006–14.
Sorensen G, Stoddard A, Quintiliani L, Ebbeling C, Nagler E, Yang M, et al. Tobacco use cessation and weight management among motor freight workers: results of the Gear Up for Health Study. Cancer Causes Control 2010;21:2113–22.
Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, Ward DS. The Weigh Study: a randomized trial focusing on daily self-weighing for weight loss among overweight adults. Ann Behav Med 2012;43(Suppl.):S272.
Vezina WC, Grace DM, Hutton LC, Alfieri MH, Colby PR, Downey DB, et al. Similarity in gallstone formation from 900 kcal/day diets containing 16 g vs 30 g of daily fat: evidence that fat restriction is not the main culprit of cholelithiasis during rapid weight reduction. Dig Dis Sci 1998;43:554–61.
Wachholtz A, Binks M, Eisenson H, Kolotkin R, Suzuki A. Does pain predict interference with daily functioning and weight loss in an obese residential treatment-seeking population? Int J Behav Med 2010;17:118–24.
Watson PM, Dugdill L, Pickering K, Bostock S, Hargreaves J, Staniford L, et al. A whole family approach to childhood obesity management (GOALS): relationship between adult and child BMI change. Ann Hum Biol 2011;38:445–52.
Winick C, Rothacker DQ, Norman RL. Four worksite weight loss programs with high-stress occupations using a meal replacement product. Occup Med (Lond) 2002;52:25–30.
Wood ER. Weight loss maintenance 1 year after individual counseling. J Am Diet Assoc 1990;90:1256–60.
Qualitative review
No qualitative data
Alahuhta MA, Korkiakangas EE, Keranen AM, Kyngas HA, Laitinen JH. Using pictures as vignettes to assess stages of change in weight management. Scand J Public Health 2011;39:403–9.
Barbarin OA, Tirado M. Enmeshment, family processes, and successful treatment of obesity. Fam Relat 1985;34:115–21.
Beresford SAA, Locke E, Bishop S, West B, McGregor BA, Bruemmer B, et al. Worksite study promoting activity and changes in eating (PACE): design and baseline results. Obesity 2007;15:4S–15S.
Bjork CC, Rossner S. ‘Extravagances’ during weight reduction, an analysis of food preference and selection. Appetite 1998;31:93–9.
Bleich SN, Pickett-Blakely O, Cooper LA. Physician practice patterns of obesity diagnosis and weight-related counseling. Patient Educ Couns 2011;82:123–9.
Bocchieri LE, Meana M, Fisher BL. Perceived psychosocial outcomes of gastric bypass surgery: a qualitative study. Obes Surg 2002;12:781–8.
Brown J, Wimpenny P. Determining factors required for a holistic approach to weight management of those with obesity. Adv Nurs Sci 2011;34:136–50.
Brumley DW. FitBlue: a multidimensional weight management initiative. Dis Manag 2007;10(Suppl. 1):S18–22.
Carels RA, Cacciapaglia HM, Rydin S, Douglass OM, Harper J. Can social desirability interfere with success in a behavioral weight loss program? Psychol Health 2006;21:65–78.
Carels RA, Harper J, Konrad K. Qualitative perceptions and caloric estimations of healthy and unhealthy foods by behavioral weight loss participants. Appetite 2006;46:199–206.
Carels RA, Young KM, Wott CB, Harper J, Gumble A, Hobbs MW, et al. Internalized weight stigma and its ideological correlates among weight loss treatment seeking adults. Eat Weight Disord 2009;14:e92–7.
Chatterjee N, Blakely DE, Barton C. Perspectives on obesity and barriers to control from workers at a community centre serving low-income Hispanic children and families. J Community Health Nurs 2005;22:23–36.
Cioffi J. Clients’ experiences of a weight-management programme: a qualitative study. Health Educ 2002;102:16–22.
Colvin RH, Olson SB. A descriptive analysis of men and women who have lost significant weight and are highly successful at maintaining the loss. Addict Behav 1983;8:287–95.
Curran AE, Caplan DJ, Lee JY, Paynter L, Gizlice Z, Champagne C, et al. Dentists’ attitudes about their role in addressing obesity in patients: a national survey. J Am Dent Assoc 2010;141:1307–16.
Davis NJ, Shishodia H, Taqui B, Dumfeh C, Wylie-Rosett J. Resident physician attitudes and competence about obesity treatment: need for improved education. Med Educ Online 2007;13:5.
Economos CD, Hildebrandt L, Hyatt RR. College freshman stress and weight change: differences by gender. Am J Health Behav 2008;32:16–25.
Egger G, Bolton A, O’Neill M, Freeman D. Effectiveness of an abdominal obesity reduction programme in men: the GutBuster ‘waist loss’ programme. Int J Obes 1996;20:227–31.
Eisenstein AR, Prohaska TR, Kruger J, Satariano WA, Hooker S, Buchner D, et al. Environmental correlates of overweight and obesity in community residing older adults. J Aging Health 2011;23:994–1009.
Garaulet M, Perez-Llamas F, Zamora S, Tebar FJ. Weight loss and possible reasons for dropping out of a dietary behavioural programme in the treatment of overweight patients. J Hum Nutr Diet 1999;12:219–27.
Glucksman ML, Hirsch J. The response of obese patients to weight reduction: a clinical evaluation of behavior. Psychosom Med 1968;30:1–11.
Glucksman ML. The response of obese patients to weight reduction. II: a quantitative evaluation of behavior. Psychosom Med 1968;30:359–73.
Greaney ML, Less FD, White AA, Dayton SF, Riebe D, Blissmer B, et al. College students’ barriers and enablers for healthful weight management: a qualitative study. J Nutr Educ Behav 2009;41:281–6.
Green KL, Cameron R, Polivy J, Cooper K, Liu LY, Leiter L, et al. Weight dissatisfaction and weight loss attempts among Canadian adults. Can Med Assoc J 1997;157:S17–25.
Grilo CM, Shiffman S, Wing RR. Relapse crises and coping among dieters. J Consult Clin Psychol 1989;57:488–95.
Hankey CR, Leslie WS, Lean MEJ. Why lose weight? Reasons for seeking weight loss by overweight but otherwise healthy men. Int J Obes 2002;26:880–2.
Harvey EL, Summerbell CD, Kirk SFL, Hill AJ. Dietitians’ views of overweight and obese people and reported management practices. J Hum Nutr Diet 2002;15:331–47.
Hoefkens C, Lachat C, Kolsteren P, Van Camp J, Verbeke W. Posting point-of-purchase nutrition information in university canteens does not influence meal choice and nutrient intake. Am J Clin Nutr 2011;94:562–70.
Huang J, Yu H, Marin E, Brock S, Carden D, Davis T. Physicians’ weight loss counseling in two public hospital primary care clinics. Acad Med 2004;79:156–61.
Imayama I, Alfano CM, Bertram LAC, Wang C, Xiao L, Duggan C, et al. Effects of 12-month exercise on health-related quality of life: a randomized controlled trial. Prev Med 2011;52:344–51.
Jackson C, Coe A, Cheater FM, Wroe S. Specialist health visitor-led weight management intervention in primary care: exploratory evaluation. J Adv Nurs 2007;58:23–34.
Janda LH, Rimm DC. Covert sensitization in the treatment of obesity. J Abnorm Psychol 1972;80:37–42.
Jeffery RW, Bjornson-Benson WM, Rosenthal BS, Lindquist RA, Kurth CL, Johnson SL. Correlates of weight loss and its maintenance over two years of follow-up among middle-aged men. Prev Med 1984;13:155–68.
Jeffery RW, French SA, Schmid TL. Attributions for dietary failures: problems reported by participants in the Hypertension Prevention Trial. Health Psychol 1990;9:315–29.
Knauss MR, Jeffrey DB, Knauss CS, Harowski K. Therapeutic contact and individual differences in a comprehensive weight loss program. Behav Ther 1983;6:124–8.
Kolotkin RL, Binks M, Crosby RD, Østbye T, Gress RE, Adams TD. Obesity and sexual quality of life. Obesity 2006;14:472–9.
Lemon SC, Rosal MC, Zapka J, Borg A, Andersen V. Contributions of weight perceptions to weight loss attempts: differences by body mass index and gender. Body Image 2009;6:90–6.
Leon GR, Chamberlain K. Emotional arousal, eating patterns, and body image as differential factors associated with varying success in maintaining a weight loss. J Consult Clin Psychol 1973;40:474–80.
Lynch E, Liu K, Wei GS, Spring B, Kiefe C, Greenland P. The relation between body size perception and change in body mass index over 13 years. Am J Epidemiol 2009;169:857–66.
McCabe MP, McGreevy SJ. Role of media and peers on body change strategies among adult men: is body size important? Eur Eat Disord Rev 2011;19:438–46.
Neve MJ, Morgan PJ, Collins CE. Participant characteristics and reach of a commercial web-based weight loss program. Nutr Diet 2010;67:267–74.
Ogden J, Clementi C, Aylwin S. The impact of obesity surgery and the paradox of control: a qualitative study. Psychol Health 2006;21:273–93.
O’Sullivan HL, Alexander E, Ferriday D, Brunstrom JM. Effects of repeated exposure on liking for a reduced-energy-dense food. Am J Clin Nutr 2010;91:1584–9.
Pleas J. Long-term effects of a lifestyle-change obesity treatment program with minorities. J Natl Med Assoc 1988;80:747–52.
Pollak KI, Alexander SC, Coffman CJ, Tulsky JA, Dolor RJ, Lyna P, et al. Physician communication techniques and weight loss in adults: Project CHAT. Am J Prev Med 2010;39:321–8.
Popkess-Vawter S, Yoder E, Gajewski B. The role of spirituality in holistic weight management. Clin Nurs Res 2005;14:158–74.
Presnell K, Pells J, Stout A, Musante G. Sex differences in the relation of weight loss self-efficacy, binge eating, and depressive symptoms to weight loss success in a residential obesity treatment program. Eat Behav 2008;9:170–80.
Rejeski WJ, Mihalko SL, Ambrosius WT, Bearon LB, McClelland JW. Weight loss and self-regulatory eating efficacy in older adults: the Cooperative Lifestyle Intervention Program. J Gerontol B Psychol Sci Soc Sci 2011;66:279–86.
Rohrer JE, Cassidy HD, Dressel D, Cramer B. Effectiveness of a structured intensive weight loss program using health educators. Dis Manag Health Outcomes 2008;16:449–54.
Sarlio-Lahteenkorva S. Weight loss and quality of life among obese people. Soc Indic Res 2001;54:329–54.
Schwartz MB, Brownell KD. Matching individuals to weight-loss treatments – a survey of obesity experts. J Consult Clin Psychol 1995;63:149–53.
Stalonas PM, Perri MG, Kerzner AB. Do behavioral treatments of obesity last? A five-year follow-up investigation. Addict Behav 1984;9:175–83.
Tamers SL, Beresford SAA, Cheadle AD, Zheng Y, Bishop SK, Thompson B. The association between worksite social support, diet, physical activity and body mass index. Prev Med 2011;53:53–6.
Thompson RL, Thomas DE. A cross-sectional survey of the opinions on weight loss treatments of adult obese patients attending a dietetic clinic. Int J Obes 2000;24:164–70.
Thuan J-F, Avignon A. Obesity management: attitudes and practices of French general practitioners in a region of France. Int J Obes 2005;29:1100–6.
Tinker JE, Tucker JA. Environmental events surrounding natural recovery from obesity. Addict Behav 1997;22:571–5.
Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–29.
Wolfe BL, Smith JE. Different strokes for different folks: why overweight men do not seek weight loss treatment. Eat Disord 2002;10:115–24.
Wysoker A. The lived experience of choosing bariatric surgery to lose weight. J Am Psychiatr Nurses Assoc 2005;11:26–34.
No open questions
Amati F, Barthassat V, Miganne G, Hausman I, Monnin D, Constanza MC, et al. Enhancing regular physical activity and relapse prevention through a 1-day therapeutic patient education workshop: a pilot study. Patient Educ Couns 2007;68:70–8.
Andreyeva T, Michaud P, van Soest A. Obesity and health in Europeans aged 50 years and older. Public Health 2007;121:497–509.
Arrebola E, Gomez-Candela C, Fernandez-Fernandez C, Loria V, Munoz-Perez E, Bermejo LM. Evaluation of a lifestyle modification program for treatment of overweight and nonmorbid obesity in primary healthcare and its influence on health-related quality of life. Nutr Clin Pract 2011;26:316–21.
Bachofner M, Stransky M. Experience in a group of overweight persons. Soz Praventivmed 1977;22:139–41.
Bakhshi E, Mohammad K, Eshraghian MR, Seifi B. Factors related to obesity among Iranian men: results from the National Health Survey. Public Health Nutr 2010;13:1389–94.
Baradel LA, Gillespie C, Kicklighter JR, Doucette MM, Penumetcha M, Blanck HM. Temporal changes in trying to lose weight and recommended weight-loss strategies among overweight and obese Americans, 1996–2003. Prev Med 2009;49:158–64.
Barichella M, Malavazos AE, Fatati G, Cereda E. Awareness and knowledge about weight status and management: results from the 1 d sensitization campaign ‘Obesity Day’ in northern Italy. Public Health Nutr 2011;14:1813–22.
Bas M, Donmez S. Self-efficacy and restrained eating in relation to weight loss among overweight men and women in Turkey. Appetite 2009;52:209–16.
Berg C, Lappas G, Wolk A, Strandhagen E, Toren K, Rosengren A, et al. Eating patterns and portion size associated with obesity in a Swedish population. Appetite 2009;52:21–6.
Bocquier A, Verger P, Basdevant A, Andreotti G, Baretge J, Villani P, et al. Overweight and obesity: knowledge, attitudes, and practices of general practitioners in France. Obes Res 2005;13:787–95.
Bodor JN, Rice JC, Farley TA, Swalm CM, Rose D. The association between obesity and urban food environments. J Urban Health 2010;87:771–81.
Borg P, Fogelholm M, Kukkonen-Harjula K. Food selection and eating behaviour during weight maintenance intervention and 2-y follow-up in obese men. Int J Obes 2004;28:1548–54.
Boutelle KN, Kirschenbaum DS, Baker RC, Mitchell ME. How can obese weight controllers minimize weight gain during the high risk holiday season? By self-monitoring very consistently. Health Psychol 1999;18:364–8.
Brown A, Siahpush M. Risk factors for overweight and obesity: results from the 2001 National Health Survey. Public Health 2007;121:603–13.
Brown I, Stride C, Psarou A. Management of obesity in primary care: nurses’ practices, beliefs and attitudes. J Adv Nurs 2007;59:329–41.
Brug J, Wammes B, Kremers S, Giskes K, Oenema A. Underestimation and overestimation of personal weight status: associations with socio-demographic characteristics and weight maintenance intentions. J Hum Nutr Diet 2006;19:253–62.
Buclin-Thiebaud S, Pataky Z, Bruchez V, Golay A. New psycho-pedagogic approach to obesity treatment: a 5-year follow-up. Patient Educ Couns 2010;79:333–7.
Burke MA, Heiland FW, Nadler CM. From ‘overweight’ to ‘about right’: evidence of a generational shift in body weight norms. Obesity 2010;18:1226–34.
Burroughs VJ, Nonas C, Sweeney CT, Rohay JM, Harkins AM, Kyle TK, et al. Self-reported weight loss practices among African American and Hispanic adults in the United States. J Natl Med Assoc 2010;102:469–75.
Butryn ML, Thomas JG, Lowe MR. Reductions in internal disinhibition during weight loss predict better weight loss maintenance. Obesity 2009;17:1101–3.
Chapman K, Ogden J. The prevalence of mechanisms of dietary change in a community sample. Appetite 2010;55:447–53.
Chourdakis M, Tzellos T, Papazisis G, Toulis K, Kouvelas D. Eating habits, health attitudes and obesity indices among medical students in northern Greece. Appetite 2010;55:722–5.
Chung M, Melnyk P, Blue D, Renaud D, Breton M. Worksite health promotion: the value of the Tune Up Your Heart program. Popul Health Manag 2009;12:297–304.
Clemens H, Thombs D, Olds S, Gordon KL. Normative beliefs as risk factors for involvement in unhealthy weight control behavior. J Am Coll Health 2008;56:635–41.
Clune A, Fischer JG, Lee JS, Reddy S, Johnson MA, Hausman DB. Prevalence and predictors of recommendations to lose weight in overweight and obese older adults in Georgia senior centers. Prev Med 2010;51:27–30.
Collins CE, Morgan PJ, Warren JM, Lubans DR, Callister R. Men participating in a weight-loss intervention are able to implement key dietary messages, but not those relating to vegetables or alcohol: the Self-help, Exercise and Diet using Internet Technology (SHED-IT) study. Public Health Nutr 2011;14:168–75.
Conlon KE, Ehrlinger J, Eibach RP, Crescioni AW, Alquist JL, Gerend MA, et al. Eyes on the prize: the longitudinal benefits of goal focus on progress toward a weight loss goal. J Exp Soc Psychol 2011;47:853–5.
Corbalan T, Morales F, Baraza L, Canteras J, Garaulet A. Major barriers to weight loss in patients attending a Mediterranean diet-based behavioural therapy: the Garaulet method. Rev Esp Obes 2009;7:144–54.
Craigie AM, Barton KL, Macleod M, Williams B, van Teijlingen E, Belch JJF, et al. A feasibility study of a personalised lifestyle programme (HealthForce) for individuals who have participated in cardiovascular risk screening. Prev Med 2011;52:387–9.
Crawford D, Owen N, Broom D, Worcester M, Oliver G. Weight-control practices of adults in a rural community. Aust N Z J Public Health 1998;22:73–9.
Davison KK, Birch LL. Lean and weight stable: behavioral predictors and psychological correlates. Obes Res 2004;12:1085–93.
DeBate R, Lewis M, Zhang Y, Blunt H, Thompson SH. Similar but different: sociocultural attitudes towards appearance, body shape dissatisfaction, and weight control behaviors among male and female college students. J Health Educ 2008;39:296–302.
DeVille-Almond J, Tahrani AA, Grant J, Gray M, Thomas GN, Taheri S. Awareness of obesity and diabetes: a survey of a subset of British male drivers. Am J Mens Health 2011;5:30–7.
DiBonaventura MD, Chapman GB. The effect of barrier underestimation on weight management and exercise change. Psychol Health Med 2008;13:111–22.
DiMarco ID, Klein DA, Clark VL, Wilson GT. The use of motivational interviewing techniques to enhance the efficacy of guided self-help behavioral weight loss treatment. Eat Behav 2009;10:134–6.
Dohm FA, Beattie JA, Aibel C, Striegel-Moore RH. Factors differentiating women and men who successfully maintain weight loss from women and men who do not. J Clin Psychol 2001;57:105–17.
Donath SM. Who’s overweight? Comparison of the medical definition and community views. Med J Aust 2000;172:375–7.
Drapeau V, Provencher V, Lemieux S, Despres JP, Bouchard C, Tremblay A. Do 6-y changes in eating behaviors predict changes in body weight? Results from the Quebec Family Study. Int J Obes 2003;27:808–14.
Drapkin RG, Wing RR, Shiffman S. Responses to hypothetical high-risk situations – do they predict weight-loss in a behavioural treatment program or the context of dietary lapses. Health Psychol 1995;14:427–34.
Drummond S, Kirk T. The effect of different types of dietary advice on body composition in a group of Scottish men. J Hum Nutr Diet 1998;11:473–85.
Duncan DT, Wolin KY, Scharoun-Lee M, Ding EL, Warner ET, Bennett GG. Does perception equal reality? Weight misperception in relation to weight-related attitudes and behaviors among overweight and obese US adults. Int J Behav Nutr Phys Act 2011;8:20.
Dutton GR, Perri MG, Dancer-Brown M, Goble M, Van Vessem N. Weight loss goals of patients in a health maintenance organization. Eat Behav 2010;11:74–8.
Dutton GR, Perri MG, Stine CC, Goble M, Van Vessem N. Comparison of physician weight loss goals for obese male and female patients. Prev Med 2010;50:186–8.
Dwyer T, Hosmer D, Hosmer T, Venn AJ, Blizzard CL, Granger RH, et al. The inverse relationship between number of steps per day and obesity in a population-based sample – the AusDiab study. Int J Obes 2007;31:797–804.
Ely AC, Greiner KA, Born W, Hall S, Rhode PC, James AS, et al. Concordance of patient–physician obesity diagnosis and treatment beliefs in rural practice settings. J Rural Health 2006;22:364–7.
Fabricatore AN, Wadden TA, Womble LG, Sarwer DB, Berkowitz RI, Foster GD, et al. The role of patients’ expectations and goals in the behavioral and pharmacological treatment of obesity. Int J Obes 2007;31:1739–45.
Felix H, West DS, Bursac Z. Impact of USPSTF practice guidelines on clinician weight loss counseling as reported by obese patients. Prev Med 2008;47:394–7.
Ferrante JM, Piasecki AK, Ohman-Strickland PA, Crabtree BF. Family physicians’ practices and attitudes regarding care of extremely obese patients. Obesity 2009;17:1710–16.
Finkelstein EA, Brown DS, Evans WD. Do obese persons comprehend their personal health risks? Am J Health Behav 2008;32:508–16.
Fogelholm M, Kujala U, Kaprio J, Sarna S. Predictors of weight change in middle-aged and old men. Obes Res 2000;8:367–73.
Foster GD, Wadden TA, Phelan S, Sarwer DB, Sanderson RS. Obese patients’ perceptions of treatment outcomes and the factors that influence them. Arch Intern Med 2001;161:2133–9.
Foster GD, Wadden TA, Makris AP, Davidson D, Sanderson RS, Allison DB, et al. Primary care physicians’ attitudes about obesity and its treatment. Obes Res 2003;11:1168–77.
Friedman KE, Reichmann SK, Costanzo PR, Zelli A, Ashmore JA, Musante GJ. Weight stigmatization and ideological beliefs: relation to psychological functioning in obese adults. Obes Res 2005;13:907–16.
Fuhrman J, Sarter B, Glaser D, Acocella S. Changing perceptions of hunger on a high nutrient density diet. Nutr Perspect 2011;34:5–17.
Georgiadis MM, Biddle SJH, Stavrou NA. Motivation for weight-loss diets: a clustering, longitudinal field study using self-esteem and self-determination theory perspectives. Health Educ J 2006;65:53–72.
Goulis DG, Giaglis GD, Boren SA, Lekka I, Bontis E, Balas EA, et al. Effectiveness of home-centered care through telemedicine applications for overweight and obese patients: a randomized controlled trial. Int J Obes 2004;28:1391–8.
Granlund B, Sojka P, Johansson H. Towards individual profile analysis for obesity treatment: motivational factors. Scand J Behav Ther 1988;17:3–23.
Grave RD, Melchionda N, Calugi S, Centis E, Tufano A, Fatati G, et al. Continuous care in the treatment of obesity: an observational multicentre study. J Intern Med 2005;258:265–73.
Grave RD, Calugi S, Corica F, Di Domizio S, Marchesini G, Quovadis SG. Psychological variables associated with weight loss in obese patients seeking treatment at medical centers. J Am Diet Assoc 2009;109:2010–16.
Green SM, McCoubrie M, Cullingham C. Practice nurses’ and health visitors’ knowledge of obesity assessment and management. J Hum Nutr Diet 2000;13:413–23.
Greene GW, Schembre SM, White AA, Hoerr SL, Lohse B, Shoff S, et al. Identifying clusters of college students at elevated health risk based on eating and exercise behaviors and psychosocial determinants of body weight. J Am Diet Assoc 2011;111:394–400.
Gregory CO, Blanck HM, Gillespie C, Maynard LM, Serdula MK. Perceived health risk of excess body weight among overweight and obese men and women: differences by sex. Prev Med 2008;47:46–52.
Gripeteg L, Karlsson J, Torgerson J, Lindroos AK. Predictors of very-low-energy diet outcome in obese women and men. Obes Facts 2010;3:159–65.
Grover VP, Keel PK, Mitchell JP. Gender differences in implicit weight identity. Int J Eat Disord 2003;34:125–35.
Gucciardic E, Wang SC, Badianic T, Stewart DE. Beyond adolescence: exploring Canadian women and men’s perception of overweight. Womens Health Issues 2007;17:374–82.
Hare SW, Price JH, Flynn MG, King KA. Attitudes and perceptions of fitness professionals regarding obesity. J Community Health 2000;25:5–21.
Harring HA, Montgomery K, Hardin J. Perceptions of body weight, weight management strategies, and depressive symptoms among US college students. J Am Coll Health 2010;59:43–50.
Harris CL, George VA. Dietary restraint influences accuracies in estimating energy expenditure and energy intake among physically inactive males. Am J Mens Health 2010;4:33–40.
Harvey-Berino J, Pintauro S, Buzzell P, DiGuilio M, Gold BC, Moldovan C, et al. Does using the internet facilitate the maintenance of weight loss? Int J Obes 2002;26:1254–60.
Hawks SR, Madanat HN, Merrill RM, Goudy MB, Miyagawa T. A cross-cultural analysis of ‘motivation for eating’ as a potential factor in the emergence of global obesity: Japan and the United States. Health Promot Int 2003;18:153–62.
Hawley L, Harker D, Harker M. A social cognitive approach to tackle inactivity and obesity in young Australians. J Bus Res 2010;63:116–20.
Hilbert A, Rief W, Braehler E. Stigmatizing attitudes toward obesity in a representative population-based sample. Obesity 2008;16:1529–34.
Holm JE, Vogeltanz-Holm N, Poltavski D, McDonald L. Assessing health status, behavioral risks, and health disparities in American Indians living on the northern plains of the US. Public Health Rep 2010;125:68–78.
Holm L, Hoff A, Erichsen L, Mohl M, Toubro S, Astrup A. Social and cultural acceptability of fat reduced diets among Danish overweight subjects: high-protein versus high-carbohydrate diets. Food Qual Prefer 2008;19:43–50.
Hope AA, Kumanyika SK, Shults J, Holmes WC. Changes in health-related quality of life among African-Americans in a lifestyle weight loss program. Qual Life Res 2010;19:1025–33.
Hoppe R, Ogden J. Practice nurses’ beliefs about obesity and weight related interventions in primary care. Int J Obes 1997;21:141–6.
James LC, Folen RA, Noce MA. A healthy lifestyle program for the treatment of obesity in minority men. J Clin Psychol Med Settings 1998;5:259–73.
Jay M, Gillespie C, Ark T, Richter R, McMacken M, Zabar S, et al. Do internists, pediatricians, and psychiatrists feel competent in obesity care? J Gen Intern Med 2008;23:1066–70.
Jay M, Gillespie C, Schlair S, Sherman S, Kalet A. Physician’s use of the 5As in counseling obese patients: is the quality of counseling associated with patients’ motivation and intention to lose weight? BMC Health Serv Res 2010;10:159.
Jay M, Schlair S, Caldwell R, Kalet A, Sherman S, Gillespie C. From the patient’s perspective: the impact of training on resident physician’s obesity counseling. J Gen Intern Med 2010;25:415–22.
Jeffery RW. Behavioral treatment of obesity with monetary contracting: two-year follow-up. Addict Behav 1984;9:311–13.
Jeffery RW, Folsom AR, Luepker RV, Jacobs DRJ, Gillum RF, Taylor HL, et al. Prevalence of overweight and weight loss behavior in a metropolitan adult population: the Minnesota Heart Survey experience. Am J Public Health 1984;74:349–52.
Jeffery RW, Wing RR. Long-term effects of interventions for weight-loss using food provision and monetary incentives. J Consult Clin Psychol 1995;63:793–6.
Jeffery RW, Wing RR, Thorson C, Burton LR. Use of personal trainers and financial incentives to increase exercise in a behavioral weight-loss program. J Consult Clin Psychol 1998;66:777–83.
Jeffery RW, French SA. Preventing weight gain in adults: the Pound of Prevention study. Am J Public Health 1999;89:747–51.
Johnson F, Cooke L, Croker H, Wardle J. Changing perceptions of weight in Great Britain: comparison of two population surveys. BMJ 2008;337:270–2.
Jordan HA, Canavan AJ, Steer RA. Long-term follow-up of patients who lost weight in a cognitive-behavioral treatment program. Psychol Addict Behav 1987;1:14–21.
Kading CL, Wilder RS, Vann WFJ, Curran AE. Factors affecting North Carolina dental hygienists’ confidence in providing obesity education and counseling. J Dent Hyg 2010;84:94–102.
Karlsson J, Taft C, Sjostrom L, Torgerson JS, Sullivan M. Psychosocial functioning in the obese before and after weight reduction: construct validity and responsiveness of the Obesity-related Problems scale. Int J Obes 2003;27:617–30.
Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Health-related quality of life in WHO Class II-III obese men losing weight with very-low-energy diet and behaviour modification: a randomised clinical trial. Int J Obes Relat Metab Disord 2002;26:487–95.
Kaul L, Nidiry JJ. Management of obesity in low-income African Americans. J Natl Med Assoc 1999;91:139–43.
Keranen AM, Savolainen MJ, Reponen AH, Kujari ML, Lindeman SM, Bloigu RS, et al. The effect of eating behavior on weight loss and maintenance during a lifestyle intervention. Prev Med 2009;49:32–8.
Keranen AM, Strengell K, Savolainen MJ, Laitinen JH. Effect of weight loss intervention on the association between eating behaviour measured by TFEQ-18 and dietary intake in adults. Appetite 2011;56:156–62.
Kiernan M, King AC, Stefanick ML, Killen JD. Men gain additional psychological benefits by adding exercise to a weight-loss program. Obes Res 2001;9:770–7.
Kim Y, Pike J, Adams H, Cross D, Doyle C, Foreyt J. Telephone intervention promoting weight-related health behaviors. Prev Med 2010;50:112–17.
Klemenc-Ketis Z, Bulc M, Kersnik J. Attitudes of Slovenian family practice patients toward changing unhealthy lifestyle and the role of family physicians: cross-sectional study. Croat Med J 2011;52:205–11.
Ko JY, Brown DR, Galuska DA, Zhang J, Blanck HM, Ainsworth BE. Weight loss advice US obese adults receive from health care professionals. Prev Med 2008;47:587–92.
Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-Lite) in a community sample. Qual Life Res 2002;11:157–71.
Konttinen H, Haukkala A, Sarlio-Lahteenkorva S, Silventoinen K, Jousilahti P. Eating styles, self-control and obesity indicators. The moderating role of obesity status and dieting history on restrained eating. Appetite 2009;53:131–4.
Kotecki JE, Clayton BD. Educating pharmacy students about nutrition and physical activity counseling. J Health Educ 2003;34:34–40.
Kruger J, Lee CD, Ainsworth BE, Macera CA. Body size satisfaction and physical activity levels among men and women. Obesity 2008;16:1976–9.
Krukowski RA, Harvey-Berino J, Ashikaga T, Thomas CS, Micco N. Internet-based weight control: the relationship between web features and weight loss. Telemed J E Health 2008;14:775–82.
Kushner RF, Choi SW. Prevalence of unhealthy lifestyle patterns among overweight and obese adults. Obesity 2010;18:1160–7.
Langer SL, Flood AP, Welsh EM, Levy RL, Jaeb MA, Laqua PS, et al. Mood, weight, and physical activity among obese individuals enrolled in a long-term weight-loss program: trajectories and associations with gender. Internet J Ment Health 2009;6:1–11.
Laska MN, Pasch KE, Lust K, Story M, Ehlinger E. Latent class analysis of lifestyle characteristics and health risk behaviors among college youth. Prev Sci 2009;10:376–86.
Latner JD, Wilson GT, Jackson ML, Stunkard AJ. Greater history of weight-related stigmatizing experience is associated with greater weight loss in obesity treatment. J Health Psychol 2009;14:190–9.
Leidy HJ, Tang M, Armstrong CLH, Martin CB, Campbell WW. The effects of consuming frequent, higher protein meals on appetite and satiety during weight loss in overweight/obese men. Obesity 2011;19:818–24.
Levy AS, Heaton AW. Weight control practices of US adults trying to lose weight. Ann Intern Med 1993;119:661–6.
Lichwala-Zyla C, Price JH, Dake JA, Jordan T, Price JA. Psychiatrists’ perceptions and practices in treating patients’ obesity. Acad Psychiatry 2009;33:370–6.
Lillis J, Hayes SC. Measuring avoidance and inflexibility in weight related problems. Int J Behav Consult Ther 2008;4:348–54.
Lillis J, Luoma JB, Levin ME, Hayes SC. Measuring weight self-stigma: the Weight Self-stigma Questionnaire. Obesity 2010;18:971–6.
Lillis J, Levin ME, Hayes SC. Exploring the relationship between body mass index and health-related quality of life: a pilot study of the impact of weight self-stigma and experiential avoidance. J Health Psychol 2011;16:722–7.
Linde JA, Jeffery RW, Levy RL, Pronk NP, Boyle RG. Weight loss goals and treatment outcomes among overweight men and women enrolled in a weight loss trial. Int J Obes 2005;29:1002–5.
Linné Y, Hemmingsson E, Adolfsson B, Ramsten J, Rössner S. Patient expectations of obesity treatment – the experience from a day-care unit. Int J Obes 2002;26:739–41.
Loeppke R, Nicholson S, Taitel M, Sweeney M, Haufle V, Kessler RC. The impact of an integrated population health enhancement and disease management program on employee health risk, health conditions, and productivity. Popul Health Manag 2008;11:287–96.
Lutfiyya MN, Nika B, Ng L, Tragos C, Won R, Lipsky MS. Primary prevention of overweight and obesity: an analysis of national survey data. J Gen Intern Med 2008;23:821–3.
Lyons MA, Crow C, Dunn L, Edwards B, Graves A, Shelton M, et al. Partnership for healthier rural communities. Online J Rural Nurs Health Care 2001;2:9.
Mannucci E, Petroni ML, Villanova N, Rotella CM, Apolone G, Marchesini G, et al. Clinical and psychological correlates of health-related quality of life in obese patients. Health Qual Life Outcomes 2010;8:90.
Marcellini F, Giuli C, Papa R, Tirabassi G, Faloia E, Boscaro M, et al. Obesity and body mass index (BMI) in relation to life-style and psycho-social aspects. Arch Gerontol Geriatr 2009;49:195–206.
Marquis M. Exploring convenience orientation as a food motivation for college students living in residence halls. Int J Consum Stud 2005;29:55–63.
Marzocchi R, Moscatiello S, Villanova N, Suppini A, Marchesini G. Psychological profile and quality of life of morbid obese patients attending a cognitive behavioural program. Psihologijske Teme 2008;17:349–60.
Matheson FI, Moineddin R, Glazier RH. The weight of place: a multilevel analysis of gender, neighborhood material deprivation, and body mass index among Canadian adults. Soc Sci Med 2008;66:675–90.
Mattfeldt-Beman MK, Corrigan SA, Stevens VJ, Sugars CP, Dalcin AT, Givi MJ, et al. Participants’ evaluation of a weight-loss program. J Am Diet Assoc 1999;99:66–71.
McCabe MP, Ricciardelli LA, James T. A longitudinal study of body change strategies of fitness center attendees. Eat Behav 2007;8:492–6.
McConnon A, Kirk SFL, Ransley JK. Process evaluation of an internet-based resource for weight control: use and views of an obese sample. J Nutr Educ Behav 2009;41:261–7.
McLaren L, Godley J. Social class and BMI among Canadian adults: a focus on occupational prestige. Obesity 2009;17:290–9.
McPherson KE, Turnbull JD. Body image satisfaction in Scottish men and its implications for promoting healthy behaviors. Int J Mens Health 2005;4:3–12.
Melin I, Karlstrom B, Lappalainen R, Berglund L, Mohsen R, Vessby B. A programme of behaviour modification and nutrition counselling in the treatment of obesity: a randomised 2-y clinical trial. Int J Obes 2003;27:1127–35.
Metz U, Welke J, Esch T, Renneberg B, Braun V, Heintze C. Perception of stress and quality of life in overweight and obese people – implications for preventive consultancies in primary care. Med Sci Monit 2009;15:H1–6.
Meyer AH, Weissen-Schelling S, Munsch S, Margraf J. Initial development and reliability of a motivation for weight loss scale. Obes Facts 2010;3:205–11.
Miles A, Rapoport L, Wardle J, Afuape T, Duman M. Using the mass-media to target obesity: an analysis of the characteristics and reported behaviour change of participants in the BBC’s ‘Fighting Fat, Fighting Fit’ campaign. Health Educ Res 2001;16:357–72.
Miller SK, Alpert PT, Cross CL. Overweight and obesity in nurses, advanced practice nurses, and nurse educators. J Am Acad Nurse Pract 2008;20:259–65.
Millstein RA, Carlson SA, Fulton JE, Galuska DA, Zhang J, Blanck HM, et al. Relationships between body size satisfaction and weight control practices among US adults. Medscape J Med 2008;10:119.
Moore TJ, Alsabeeh N, Apovian CM, Murphy MC, Coffman GA, Cullum-Dugan D, et al. Weight, blood pressure, and dietary benefits after 12 months of a web-based nutrition education program (DASH for Health): longitudinal observational study. J Med Internet Res 2008;10:e52.
Mullaney MI, Corish CA, Loxley A. Exploring the nutrition and lifestyle knowledge, attitudes and behaviour of student home economics teachers: baseline findings from a 4-year longitudinal study. Int J Consum Stud 2008;32:314–22.
Muller SM, Gorrow TR, Schneider SR. Enhancing appearance and sports performance: are female collegiate athletes behaving more like males? J Am Coll Health 2009;57:513–20.
Murphy JK, Bruce BK, Williamson DA. A comparison of measured and self-reported weights in a 4-year follow-up of spouse involvement in obesity treatment. Behav Ther 1985;16:524–30.
Mussap AJ. Reinforcement sensitivity theory (RST) and body change behaviour in males. Pers Individ Dif 2006;40:841–52.
Nawaz H, Adams ML, Katz DL. Weight loss counseling by health care providers. Am J Public Health 1999;89:764–7.
Nebel IT, Bluher M, Starcke U, Muller UA, Haak T, Paschke R. Evaluation of a computer based interactive diabetes education program designed to train the estimation of the energy or carbohydrate contents of foods. Patient Educ Couns 2002;46:55–9.
Neighbors LA, Sobal J. Weight and weddings: expectations about wedding-specific body weight and shape ideals and dieting and exercise behavior among university students. Eat Behav 2008;9:430–7.
Neighbors L, Sobal J, Liff C, Amiraian D. Weighing weight: trends in body weight evaluation among young adults, 1990 and 2005. Sex Roles 2008;59:68–80.
Neumark-Sztainer D, Jeffery RW, French SA. Self-reported dieting: how should we ask? What does it mean? Associations between dieting and reported energy intake. Int J Eat Disord 1997;22:437–49.
Nothwehr F, Snetselaar L, Wu H. Age group differences in diet and physical activity-related behaviors among rural men and women. J Nutr Health Aging 2008;12:169–74.
O’Dea JA, Abraham S. Knowledge, beliefs, attitudes, and behaviors related to weight control, eating disorders, and body image in Australian trainee home economics and physical education teachers. J Nutr Educ 2001;33:332–40.
Ogden J, Hoppe R. Changing practice nurses’ management of obesity. Proceedings from the ASO and BDA symposium held on 25 November 1997 at St. Bartholomew’s Hospital, London. J Hum Nutr Diet 1998;11:249–56.
Okonkwo O, While A. University students’ views of obesity and weight management strategies. Health Educ J 2010;69:192–9.
Ousley L, Cordero ED, White S. Fat talk among college students: how undergraduates communicate regarding food and body weight, shape and appearance. Eat Disord 2008;16:73–84.
Paap CE, Gardner RM. Body image disturbance and relationship satisfaction among college students. Pers Individ Dif 2011;51:715–19.
Paxman JR, Hall AC, Harden CJ, O’Keeffe J, Simper TN. Weight loss is coupled with improvements to affective state in obese participants engaged in behavior change therapy based on incremental, self-selected ‘small changes’. Nutr Res 2011;31:327–37.
Petersen R, Sill S, Lu C, Young J, Edington DW. Effectiveness of employee internet-based weight management program. J Occup Environ Med 2008;50:163–71.
Phelan S, Wing RR, Raynor HA, Dibello J, Nedeau K, Peng W. Holiday weight management by successful weight losers and normal weight individuals. J Consult Clin Psychol 2008;76:442–8.
Phelan S, Liu T, Gorin A, Lowe M, Hogan J, Fava J, et al. What distinguishes weight-loss maintainers from the treatment-seeking obese? Analysis of environmental, behavioral, and psychosocial variables in diverse populations. Ann Behav Med 2009;38:94–104.
Phelan S, Nallari M, Darroch FE, Wing RR. What do physicians recommend to their overweight and obese patients? J Am Board Fam Med 2009;22:115–22.
Pickett-Blakely O, Bleich SN, Cooper LA. Patient–physician gender concordance and weight-related counseling of obese patients. Am J Prev Med 2011;40:616–19.
Pillitteri JL, Shiffman S, Rohay JM, Harkins AM, Burton SL, Wadden TA. Use of dietary supplements for weight loss in the United States: results of a national survey. Obesity 2008;16:790–6.
Pinto AM, Gorin AA, Raynor HA, Tate DF, Fava JL, Wing RR. Successful weight-loss maintenance in relation to method of weight loss. Obesity 2008;16:2456–61.
Plourde H, Nolin B, Receveur O, Ledoux M. Psychosocial correlates of body mass index in four groups of Quebec adults. J Biosoc Sci 2010;42:601–18.
Potter MB, Vu JD, Croughan-Minihane M. Weight management: what patients want from their primary care physicians. J Fam Pract 2001;50:513–18.
Puhl RM, Brownell KD. Confronting and coping with weight stigma: an investigation of overweight and obese adults. Obesity 2006;14:1802–15.
Puhl R, Wharton C, Heuer C. Weight bias among dietetics students: implications for treatment practices. J Am Diet Assoc 2009;109:438–44.
Ramirez EM, Rosen JC. A comparison of weight control and weight control plus body image therapy for obese men and women. J Consult Clin Psychol 2001;69:440–6.
Rejeski W, Mihalko S, Ambrosius W, Bearon L, McClelland J. Weight loss and self-regulatory eating efficacy in older adults: the Cooperative Lifestyle Intervention Program. J Gerontol B Psychol Sci Soc Sci 2011;66B:279–86.
Robinson RG, Folstein MF, Simonson M, McHugh PR. Differential antianxiety response to caloric intake between normal and obese subjects. Psychosom Med 1980;42:415–27.
Rodriguez JE, Burg JR, Brown LS. Mobile health unit for minority obesity education: local residents’ attitudes and perceptions. J Natl Med Assoc 2006;98:1792–6.
Rothert K, Strecher VJ, Doyle LA, Caplan WM, Joyce JS, Jimison HB, et al. Web-based weight management programs in an integrated health care setting: a randomized, controlled trial. Obesity 2006;14:266–72.
Ruelaz AR, Diefenbach P, Simon B, Lanto A, Arterburn D, Shekelle PG. Perceived barriers to weight management in primary care – perspectives of patients and providers. J Gen Intern Med 2007;22:518–22.
Ryden A, Sullivan M, Torgerson JS, Karlsson J, Lindroos AK, Taft C. Severe obesity and personality: a comparative controlled study of personality traits. Int J Obes 2003;27:1534–40.
Salmons PH. Weight control in university students. J R Soc Med 1987;80:6–8.
Saloumi C, Plourde H. Differences in psychological correlates of excess weight between adolescents and young adults in Canada. Psychol Health Med 2010;15:314–25.
Salvador M, Garcia-Galvez C, de la Fuente M. Creencias y estrategias para el control del peso, satisfaccion con la imagen corporal y autoestima. Eur J Educ Psychol 2010;3:257–73.
Sarkin JA, Johnson SS, Prochaska JO, Prochaska JM. Applying the transtheoretical model to regular moderate exercise in an overweight population: validation of a stages of change measure. Prev Med 2001;33:462–9.
Sarlio-Lahteenkorva S, Rissanen A, Kaprio J. A descriptive study of weight loss maintenance: 6 and 15 year follow-up of initially overweight adults. Int J Obes Relat Metab Disord 2000;24:116–25.
Schmalz DL. ‘I feel fat’: weight-related stigma, body esteem, and BMI as predictors of perceived competence in physical activity. Obes Facts 2010;3:15–21.
Schwartz MB, Chambliss HO, Brownell KD, Blair SN, Billington C. Weight bias among health professionals specializing in obesity. Obes Res 2003;11:1033–9.
Sciamanna CN, Kiernan M, Rolls BJ, Boan J, Stuckey H, Kephart D, et al. Practices associated with weight loss versus weight-loss maintenance results of a national survey. Am J Prev Med 2011;41:159–66.
Seppanen-Nuijten E, Lahti-Koski M, Mannisto S, Knekt P, Rissanen H, Aromaa A, et al. Fat free mass and obesity in relation to educational level. BMC Public Health 2009;9:448.
Serdula MK, Collins ME, Williamson DF, Anda RF, Pamuk E, Byers TE. Weight control practices of US adolescents and adults. Ann Intern Med 1993;119:667–71.
Sheets V, Ajmere K. Are romantic partners a source of college students’ weight concern? Eat Behav 2005;6:1–9.
Shiffman S, Sweeney CT, Pillitteri JL, Sembower MA, Harkins AM, Wadden TA. Weight management advice: what do doctors recommend to their patients? Prev Med 2009;49:482–6.
Smith AW, Borowski LA, Liu B, Galuska DA, Signore C, Klabunde C, et al. US primary care physicians’ diet-, physical activity-, and weight-related care of adult patients. Am J Prev Med 2011;41:33–42.
Smith CF, Williamson DA, Bray GA, Ryan DH. Flexible vs. rigid dieting strategies: relationship with adverse behavioral outcomes. Appetite 1999;32:295–305.
Smith T, Hawks SR. Intuitive eating, diet composition, and the meaning of food in healthy weight promotion. J Health Educ 2006;37:130–6.
Sobal J, Rauschenbach BS, Frongillo EA. Obesity and marital quality – analysis of weight, marital unhappiness, and marital problems in a US national sample. J Fam Issues 1995;16:746–64.
Steenhuis IHM, Bos AER, Mayer B. (Mis)interpretation of body weight in adult women and men. J Hum Nutr Diet 2006;19:219–28.
Stotland SC, Larocque M. Convergent validity of the Larocque Obesity Questionnaire and self-reported behavior during obesity treatment. Psychol Rep 2004;95:1031–42.
Surtees PG, Wainwright NWJ, Khaw KT. Obesity, confidant support and functional health: cross-sectional evidence from the EPIC-Norfolk cohort. Int J Obes 2004;28:748–58.
Taggart M. The attitudes and activities of registered nurses towards health promotion and patient education in the emergency department. NENA Outlook 2009;32:15–19.
Takada A, Nakamura R, Furukawa M, Takahashi Y, Nishimura S, Kosugi S. The relationship between weight loss and time and risk preference parameters: a randomized controlled trial. J Biosoc Sci 2011;43:481–503.
Tan D, Zwar NA, Dennis SM, Vagholkar S. Weight management in general practice: what do patients want? Med J Aust 2006;185:73–5.
Tantleff-Dunn S, Barnes R, Larose J. It’s not just a ‘woman thing:’ the current state of normative discontent. Eat Disord 2011;19:392–402.
Thompson SC, Schwankovsky L, Pitts J. Counseling patients to make life-style changes – the role of physician self-efficacy, training and beliefs about causes. Fam Pract 1993;10:70–5.
Tiggemann M, Williamson S. The effect of exercise on body satisfaction and self-esteem as a function of gender and age. Sex Roles 2000;43:119–27.
Timperio A, Cameron-Smith D, Burns C, Crawford D. The public’s response to the obesity epidemic in Australia: weight concerns and weight control practices of men and women. Public Health Nutr 2000;3:417–24.
Torgerson JS, Lissner L, Lindroos AK, Kruijer H, Sjostrom L. VLCD plus dietary and behavioural support versus support alone in the treatment of severe obesity. A randomised two-year clinical trial. Int J Obes 1997;21:987–94.
Tur JA, Serra-Majem LS, Romaguera D, Pons A. Profile of overweight and obese people in a Mediterranean region. Obes Res 2005;13:527–36.
Turner-McGrievy GM, Campbell MR, Tate DF, Truesdale KP, Bowling JM, Crosby L. Pounds Off Digitally study: a randomized podcasting weight-loss intervention. Am J Prev Med 2009;37:263–9.
Van Dyck D, Cardon G, Deforche B, Owen N, De Cocker K, Wijndaele K, et al. Socio-demographic, psychosocial and home-environmental attributes associated with adults’ domestic screen time. BMC Public Health 2011;11:668.
van Wier MF, Ariens GAM, Dekkers JC, Hendriksen IJM, Pronk NP, Smid T, et al. ALIFE@Work: a randomised controlled trial of a distance counselling lifestyle programme for weight control among an overweight working population [ISRCTN04265725]. BMC Public Health 2006;6:140.
Vandelanotte C, Sugiyama T, Gardiner P, Owen N. Associations of leisure-time internet and computer use with overweight and obesity, physical activity and sedentary behaviors: cross-sectional study. J Med Internet Res 2009;11:1–8.
Vernay M, Malon A, Oleko A, Salanave B, Roudier C, Szego E, et al. Association of socioeconomic status with overall overweight and central obesity in men and women: the French Nutrition and Health Survey 2006. BMC Public Health 2009;9:215.
Walfish S, Brown TA. Self-assessed emotional factors contributing to increased weight in presurgical male bariatric patients. Bariatr Nurs Surg Patient Care 2009;4:49–52.
Wammes B, French S, Brug J. What young Dutch adults say they do to keep from gaining weight: self-reported prevalence of overeating, compensatory behaviours and specific weight control behaviours. Public Health Nutr 2007;10:790–8.
Wammes B, Oenema A, Brug J. The evaluation of a mass media campaign aimed at weight gain prevention among young Dutch adults. Obesity 2007;15:2780–9.
Wamsteker EW, Geenen R, Zelissen PMJ, van Furth EF, Iestra J. Unrealistic weight-loss goals among obese patients are associated with age and causal attributions. J Am Diet Assoc 2009;109:1903–8.
Wang WC, Worsley A, Cunningham EG, Hunter W. Investigation of population heterogeneity of diet use among middle-aged Australians. Br J Nutr 2011;105:1091–9.
Wannamethee SG, Shaper AG. Alcohol, body weight, and weight gain in middle-aged men. Am J Clin Nutr 2003;77:1312–17.
Wardle J, Griffith J, Johnson F, Rapoport L. Intentional weight control and food choice habits in a national representative sample of adults in the UK. Int J Obes 2000;24:534–40.
Wardle J, Haase AM, Steptoe A. Body image and weight control in young adults: international comparisons in university students from 22 countries. Int J Obes 2006;30:644–51.
Wee CC, Davis RB, Phillips RS. Stage of readiness to control weight and adopt weight control behaviors in primary care. J Gen Intern Med 2005;20:410–5.
Weiss EC, Galuska DA, Khan LK, Serdula MK. Weight-control practices among US adults, 2001–2002. Am J Prev Med 2006;31:18–24.
Wen LM, Rissel C. Inverse associations between cycling to work, public transport, and overweight and obesity: findings from a population based study in Australia. Prev Med 2008;46:29–32.
Westenhoefer J, Stunkard AJ, Pudel V. Validation of the flexible and rigid control dimensions of dietary restraint. Int J Eat Disord 1999;26:53–64.
Wiczinski E, Doering A, John J, von Lengerke T, Kora SG. Obesity and health-related quality of life: does social support moderate existing associations? Br J Health Psychol 2009;14:717–34.
Wilson DB, Johnson RE, Jones RM, Krist AH, Woolf SH, Flores SK. Patient weight counseling choices and outcomes following a primary care and community collaborative intervention. Patient Educ Couns 2010;79:338–43.
Yaemsiri S, Slining MM, Agarwal SK. Perceived weight status, overweight diagnosis, and weight control among US adults: the NHANES 2003–2008 study. Int J Obes 2011;35:1063–70.
No intervention
Alexander SC, Ostbye T, Pollak KI, Gradison M, Bastian LA, Brouwer RJN. Physicians’ beliefs about discussing obesity: results from focus groups. Am J Health Promot 2007;21:498–500.
Barker R, Cooke B. Diet, obesity and being overweight: a qualitative research study. Health Educ J 1992;51:117–21.
Bidgood J, Buckroyd J. An exploration of obese adults’ experience of attempting to lose weight and to maintain a reduced weight. Counsel Psychother Res 2005;5:221–9.
Bottamini G, Ste-Marie DM. Male voices on body image. Int J Mens Health 2006;5:109–32.
Bove CF, Sobal J. Body weight relationships in early marriage. Weight relevance, weight comparisons, and weight talk. Appetite 2011;57:729–42.
Brink PJ, Ferguson K. The decision to lose weight. West J Nurs Res 1998;20:84–102.
Callen BL, Pemberton G. Weight gain in overweight and obese community dwelling old-old. J Nutr Health Aging 2008;12:233–7.
Chambers JA, Swanson V. Stories of weight management: factors associated with successful and unsuccessful weight maintenance. Br J Health Psychol 2012;17:223–43.
Chan RSM, Lok KYW, Sea MMM, Woo J. Clients’ experiences of a community based lifestyle modification program: a qualitative study. Int J Environ Res Public Health 2009;6:2608–22.
Chang CT, Chang KH, Cheah WL. Adults’ perceptions of being overweight or obese: a focus group study. Asia Pac J Clin Nutr 2009;18:257–64.
Chavez-Martinez A, Cason KL, Mayo R, Nieto-Montenegro S, Williams JE, Haley-Zitin V. Assessment of predisposing, enabling, and reinforcing factors toward food choices and healthy eating among Hispanics in South Carolina. Top Clin Nutr 2010;25:47–59.
Ciblis A, Dooley B, Eldin N. One size fits all? Gender as an issue in obesity. Clin Obes 2011;1:168–74.
Cluskey M, Grobe D. College weight gain and behavior transitions: male and female differences. J Am Diet Assoc 2009;109:325–9.
Derksen RE, Brink-Melis WJ, Westerman MJ, ten Dam JJM, Seidell JC, Visscher TLS. A local consensus process making use of focus groups to enhance the implementation of a national integrated health care standard on obesity care. Fam Pract 2012;29:i177–84.
Diaz VA, Mainous AG III, Pope C. Cultural conflicts in the weight loss experience of overweight Latinos. Int J Obes 2007;31:328–33.
Escoffery C, Kegler MC, Alcantara I, Wilson M, Glanz K. A qualitative examination of the role of small, rural worksites in obesity prevention. Prev Chronic Dis 2011;8:A75.
Forsyth G, Handcock P, Rose E, Jenkins C. Fitness instructors: how does their knowledge on weight loss measure up? Health Educ J 2005;64:154–67.
Fukuoka Y, Kamitani E, Bonnet K, Lindgren T. Real-time social support through a mobile virtual community to improve healthy behavior in overweight and sedentary adults: a focus group analysis. J Med Internet Res 2011;13:e49.
Galli N, Reel JJ. Adonis or Hephaestus? Exploring body image in male athletes. Psychol Men Masc 2009;10:95–108.
Gorton D, Dixon R, Maddison R, Mhurchu CN, Jull A. Consumer views on the potential use of mobile phones for the delivery of weight-loss interventions. J Hum Nutr Diet 2011;24:616–19.
Grant PG, Boersma H. Making sense of being fat: a hermeneutic analysis of adults’ explanations for obesity. Counsel Psychother Res 2005;5:212–20.
Gray CM, Hunt K, Lorimer K, Anderson AS, Benzeval M, Wyke S. Words matter: a qualitative investigation of which weight status terms are acceptable and motivate weight loss when used by health professionals. BMC Public Health 2011;11:513.
Green AR, Larkin M, Sullivan V. Oh stuff it! The experience and explanation of diet failure: an exploration using interpretative phenomenological analysis. J Health Psychol 2009;14:997–1008.
Greener J, Douglas F, van Teijlingen E. More of the same? Conflicting perspectives of obesity causation and intervention amongst overweight people, health professionals and policy makers. Soc Sci Med 2010;70:1042–9.
Gunther S, Guo F, Sinfield P, Rogers S, Baker R. Barriers and enablers to managing obesity in general practice: a practical approach for use in implementation activities. Qual Prim Care 2012;20:93–103.
Hansson LM, Rasmussen F, Ahlstrom GI. General practitioners’ and district nurses’ conceptions of the encounter with obese patients in primary health care. BMC Fam Pract 2011;12:7.
Hardcastle S, Hagger MS. ‘You can’t do it on your own’: experiences of a motivational interviewing intervention on physical activity and dietary behaviour. Psychol Sport Exerc 2011;12:314–23.
Haslam C, Sherratt E, Holdsworth M, Beardsworth A, Keil T, Goode J. Social factors associated with self-reported dietary change. J Nutr Educ 2000;32:296–303.
Heading G. Rural obesity, healthy weight and perceptions of risk: struggles, strategies and motivation for change. Aust J Rural Health 2008;16:86–91.
Heintze C, Metz U, Hahn D, Niewoehner J, Schwantes U, Wiesner J, et al. Counseling overweight in primary care: an analysis of patient–physician encounters. Patient Educ Couns 2010;80:71–5.
Heintze C, Sonntag U, Brinck A, Huppertz M, Niewöhner J, Wiesner J, et al. A qualitative study on patients’ and physicians’ visions for the future management of overweight or obesity. Fam Pract 2012;29:103–9.
James DCS. Factors influencing food choices, dietary intake, and nutrition-related attitudes among African Americans: application of a culturally sensitive model. Ethn Health 2004;9:349–67.
Kegler MC, Escoffery C, Alcantara I, Ballard D, Glanz K. A qualitative examination of home and neighborhood environments for obesity prevention in rural adults. Int J Behav Nutr Phys Act 2008;5:65.
Kwan S. Competing motivational discourses for weight loss: means to ends and the nexus of beauty and health. Qual Health Res 2009;19:1223–33.
Leppanen V, Linne S. Meanings of overweight. Sociologisk Forskning 2007;44:6–28.
Leverence RR, Williams RL, Sussman A, Crabtree BF. Obesity counseling and guidelines in primary care: a qualitative study. Am J Prev Med 2007;32:334–9.
Lewis S, Thomas SL, Blood RW, Hyde J, Castle DJ, Komesaroff PA. Do health beliefs and behaviors differ according to severity of obesity? A qualitative study of Australian adults. Int J Environ Res Public Health 2010;7:443–59.
Lewis S, Thomas SL, Blood RW, Castle D, Hyde J, Komesaroff PA. ‘I’m searching for solutions’: why are obese individuals turning to the Internet for help and support with ‘being fat’? Health Expect 2011;14:339–50.
Lewis S, Thomas SL, Blood RW, Castle DJ, Hyde J, Komesaroff PA. How do obese individuals perceive and respond to the different types of obesity stigma that they encounter in their daily lives? A qualitative study. Soc Sci Med 2011;73:1349–56.
Lewis S, Thomas SL, Hyde J, Castle DJ, Komesaroff PA. A qualitative investigation of obese men’s experiences with their weight. Am J Health Behav 2011;35:458–69.
Lindelof A. Men stuff themselves – women psychologise. Sygeplejersken 2005;105:40–3.
Luevorasirikul K, Boardman HF, Anderson CW. A study of university students and pharmacists’ perspectives on weight management. Int J Health Promot Educ 2010;48:42–5.
Malterud K, Ulriksen K. Obesity in general practice: a focus group study on patient experiences. Scand J Prim Health Care 2010;28:205–10.
Mercer SW, Tessier S. A qualitative study of general practitioners’ and practice nurses’ attitudes to obesity management in primary care. Health Bull (Edinb) 2001;59:248–53.
Michie S. Talking to primary care patients about weight: a study of GPs and practice nurses in the UK. Psychol Health Med 2007;12:521–5.
Monaghan LF. Weighty words: expanding and embodying the accounts framework. Soc Theory Health 2006;4:128–67.
Naslindh-Ylispangar A, Sihvonen M, Kekki P. Health, utilisation of health services, ‘core’ information, and reasons for non-participation: a triangulation study amongst non-respondents. J Clin Nurs 2008;17:2972–8.
Ogden J, Hills L. Understanding sustained behavior change: the role of life crises and the process of reinvention. Health 2008;12:419–37.
Ogden J, Clementi C. The experience of being obese and the many consequences of stigma. J Obes 2010:429098. doi:10.1155/2010/429098.
Olson EA, Visek AJ, McDonnell KA, DiPietro L. Thinness expectations and weight cycling in a sample of middle-aged adults. Eat Behav 2012;13:142–5.
Overgaard D. Being obese is paradoxical living – an exploratory study of five persons lived experiences of being overweight. Theoria J Nurs Theory 2002;11:3–12.
Owen TA. Weight in Wales. Nutr Bull 2004;29:85–91.
Payne N, Jones F, Harris PR. The role of perceived need within the theory of planned behaviour: a comparison of exercise and healthy eating. Br J Health Psychol 2004;9:489–504.
Puhl RM, Moss-Racusin CA, Schwartz MB, Brownell KD. Weight stigmatization and bias reduction: perspectives of overweight and obese adults. Health Educ Res 2008;23:347–58.
Ricciardelli R, Clow K. Men, appearance, and cosmetic surgery: the role of self-esteem and comfort with the body. Can Rev Sociol 2009;34:105–34.
Ristovski-Slijepcevic S, Bell K, Chapman GE, Beagan BL. Being ‘thick’ indicates you are eating, you are healthy and you have an attractive body shape: perspectives on fatness and food choice amongst black and white men and women in Canada. Health Sociol Rev 2010;19:317–29.
Rogge M, Greenwald M, Golden A. Obesity, stigma, and civilized oppression. Adv Nurs Sci 2004;27:301–15.
Rowland AL. The health challenges of urban Latino college students as revealed through student journaling. J Hispanic High Educ 2008;7:131–43.
Sabinsky MS, Toft U, Raben A, Holm L. Overweight men’s motivations and perceived barriers towards weight loss. Eur J Clin Nutr 2007;61:526–31.
Sant’Anna SC, Ferriani M. Group work: reflections on the daily work, report on an experience. Rev Lat Am Enfermagem 2000;8:97–101.
Sarlio-Lahteenkorva S. Relapse stories in obesity. Eur J Public Health 1998;8:203–9.
Smith LH, Holm L. Obesity in a life-course perspective: an exploration of lay explanations of weight gain. Scand J Public Health 2011;39:396–402.
Sonntag U, Brink A, Renneberg B, Braun V, Heintze C. GPs’ attitudes, objectives and barriers in counselling for obesity – a qualitative study. Eur J Gen Pract 2012;18:9–14.
Stuckey HL, Boan J, Kraschnewski JL, Miller-Day M, Lehman EB, Sciamanna CN. Using positive deviance for determining successful weight-control practices. Qual Health Res 2011;21:563–79.
Swift JA, Glazebrook C, Novak N, Anness A. Beliefs regarding the consequences of obesity and ideal weight: an instrument development study. Patient Educ Couns 2007;68:200–7.
Tang JW, Mason M, Kushner RF, Tirodkar MA, Khurana N, Kandula NR. South Asian American perspectives on overweight, obesity, and the relationship between weight and health. Prev Chronic Dis 2012;9:E107.
Thomas SL, Hyde J, Karunaratne A, Herbert D, Komesaroff PA. Being ‘fat’ in today’s world: a qualitative study of the lived experiences of people with obesity in Australia. Health Expect 2008;11:321–30.
Thomas SL, Lewis S, Hyde J, Castle D, Komesaroff P. ‘The solution needs to be complex.’ Obese adults’ attitudes about the effectiveness of individual and population based interventions for obesity. BMC Public Health 2010;10:420.
Thompson JL, Jago R, Brockman R, Cartwright K, Page AS, Fox KR. Physically active families – de-bunking the myth? A qualitative study of family participation in physical activity. Child Care Health Dev 2010;36:265–74.
Throsby K. ‘How could you let yourself get like that?’: stories of the origins of obesity in accounts of weight loss surgery. Soc Sci Med 2007;65:1561–71.
Walsh JR, White AA, Greaney ML. Using focus groups to identify factors affecting healthy weight maintenance in college men. Nutr Res 2009;29:371–8.
Ziebland S, Robertson J, Jay J, Neil A. Body image and weight change in middle age: a qualitative study. Int J Obes 2002;26:1083–91.
No data by sex
Befort CA, Stewart EE, Smith BK, Gibson CA, Sullivan DK, Donnelly JE. Weight maintenance, behaviors and barriers among previous participants of a university-based weight control program. Int J Obes 2008;32:519–26.
Beneke WM. Long-term results of two behavior modification weight loss programs using nutritionists as therapists. Behav Ther 1978;9:501–7.
Brown I. Nurses’ attitudes towards adult patients who are obese: literature review. J Adv Nurs 2006;53:221–32.
Burke LE, Swigart V, Turk MW, Derro N, Ewing LJ. Experiences of self-monitoring: successes and struggles during treatment for weight loss. Qual Health Res 2009;19:815–28.
Cotugna N, Mallick A. Following a calorie-restricted diet may help in reducing healthcare students’ fat-phobia. J Community Health 2010;35:321–4.
Eley DS, Eley RM. How do rural GPs manage their inactive and overweight patients? A pilot study of rural GPs in Queensland. Aust Fam Physician 2009;38:747–8.
Gallagher R, Kirkness A, Armari E, Davidson PM. Participants’ perspectives of a multi-component, group-based weight loss programme supplement for cardiac rehabilitation: a qualitative study. Int J Nurs Pract 2012;18:28–35.
Garip G, Yardley L. A synthesis of qualitative research on overweight and obese people’s views and experiences of weight management. Clin Obes 2011;1:110–26.
Gerber L. My body is a testimony: appearance, health, and sin in an evangelical weight-loss program. Social Compass 2009;56:405–18.
Herriot AM, Thomas DE, Hart KH, Warren J, Truby H. A qualitative investigation of individuals’ experiences and expectations before and after completing a trial of commercial weight loss programmes. J Hum Nutr Diet 2008;21:72–80.
Johannessen KB, Berntsen D. Motivation for weight loss affects recall from autobiographical memory in dieters. Memory 2009;17:69–83.
Jones N, Furlanetto DL, Jackson JA, Kinn S. An investigation of obese adults’ views of the outcomes of dietary treatment. J Hum Nutr Diet 2007;2007:486–94.
Kennedy BM, Ard JD, Harrison L Jr, Conish BK, Kennedy E, Levy EJ, et al. Cultural characteristics of African Americans: implications for the design of trials that target behavior and health promotion programs. Ethn Dis 2007;17:548–54.
Kiesewetter S, Kopsel A, Kopp W, Kallenbach-Dermutz B, Pfeiffer AF, Spranger J, et al. Psychodynamic mechanism and weight reduction in obesity group therapy – first observations with different attachment styles. Psychosoc Med 2010;7:Doc04. doi: 10.3205/psm000066.
Maldonato A, Piana N, Bloise D, Baldelli A. Optimizing patient education for people with obesity: possible use of the autobiographical approach. Patient Educ Couns 2010;79:287–90.
Marchessault G, Thiele K, Armit E, Chapman GE, Levy-Milne R, Barr SI. Canadian dietitians’ understanding of non-dieting approaches in weight management. Can J Diet Pract Res 2007;68:67–72.
McQuigg M, Brown JE, Broom JI, Laws RA, Reckless JPD, Noble PA, et al. Engaging patients, clinicians and health funders in weight management: the Counterweight programme. Fam Pract 2008;25(Suppl. 1):i79–86.
McTigue KM, Bhargava T, Bryce CL, Conroy M, Fischer GS, Hess R, et al. Patient perspectives on the integration of an intensive online behavioral weight loss intervention into primary care. Patient Educ Couns 2011;83:261–4.
Murphree D. Patient attitudes toward physician treatment of obesity. J Fam Pract 1994;38:45–8.
Ostberg AL, Wikstrand I, Bostrom KB. Group treatment of obesity in primary care practice: a qualitative study of patients’ perspectives. Scand J Public Health 2011;39:98–105.
Patrick K, Raab F, Adams MA, Dillon L, Zabinski M, Rock CL, et al. A text message-based intervention for weight loss: randomized controlled trial. J Med Internet Res 2009;11:12–19.
Perry K, Hickson M, Thomas J. Factors enabling success in weight management programmes: systematic review and phenomenological approach. J Hum Nutr Diet 2011;24:301–2.
Psarou A, Brown I. Patients’ experiences of prescribed anti-obesity drugs and perceptions of support from primary care: a qualitative study. Prim Health Care Res Dev 2010;11:250–9.
Roberts A, Ashley G. What are the characteristics of overweight and obese patients who achieve weight loss and what factors are most helpful? A quantitative and qualitative study of patients and interventions in a rural general practice. J Hum Nutr Diet 1999;12:20–7.
Romney M, Thomson E, Kash K. Population-based worksite obesity management interventions: a qualitative case study. Popul Health Manag 2011;14:127–32.
Tod AM, Lacey A. Overweight and obesity: helping clients to take action. Br J Community Nurs 2004;9:59–66.
Visram S, Crosland A, Cording H. Triggers for weight gain and loss among participants in a primary care-based intervention. Br J Community Nurs 2009;14:495–501.
Ward SH, Gray AM, Paranjape A. African Americans’ perceptions of physician attempts to address obesity in the primary care setting. J Gen Intern Med 2009;24:579–84.
White SL, Maloney SK. Promoting healthy diets and active lives to hard-to-reach groups: market research study. Public Health Rep 1990;105:224–31.
Wiggins S. Managing blame in NHS weight management treatment: psychologizing weight and ‘obesity’. J Community Health Nurs 2009;19:374–87.
Ineligible comparator
Andronicou A, Hackett A, Richards J, Krska J. Views and use of over-the-counter weight loss products among the general public. Int J Health Promot Educ 2009;47:63–8.
Lorimer K, Gray CM, Hunt K, Wyke S, Anderson A, Benzeval M. Response to written feedback of clinical data within a longitudinal study: a qualitative study exploring the ethical implications. BMC Med Res Methodol 2011;11:10.
Ineligible population
Allan K, Hoddinott P, Avenell A. A qualitative study comparing commercial and health service weight loss groups, classes and clubs. J Hum Nutr Diet 2011;24:23–31.
Brown I, Thompson J. Primary care nurses’ attitudes, beliefs and own body size in relation to obesity management. J Adv Nurs 2007;60:535–43.
Burke LE, Choo J, Warziski M, Music E, Novak J, Sereika S. Self-monitoring among subjects in a weight loss study. Eur J Cardiovasc Nurs 2005;4:1444.
Chang P. Effects of interviewer questions and response type on compliance – an analog study. J Couns Psychol 1994;41:74–82.
Coupar AM, Kennedy T. Running a weight control group: experiences of a psychologist and a general practitioner. J R Coll Gen Pract 1980;30:41–8.
Cox JS, Kreitzman SN, Coxon AY, Walls J. Long-term outcome of a self-help very-low-calorie-diet weight-loss program. Am J Clin Nutr 1992;56:279–80S.
Fox NJ, Ward KJ, O’Rourke AJ. The ‘expert patient’: empowerment or medical dominance? The case of weight loss, pharmaceutical drugs and the internet. Soc Sci Med 2005;60:1299–309.
Gormally J, Rardin D, Black S. Correlates of successful response to a behavioral weight control clinic. J Couns Psychol 1980;27:179–91.
Haines J, Neumark-Sztainer D, Thiel L. Addressing weight-related issues in an elementary school: what do students, parents, and school staff recommend? Eat Disord 2007;15:5–21.
Harvey-Berino J, Gold EC, West DS, Shuldiner AR, Walston J, Starling RD, et al. Does genetic testing for obesity influence confidence in the ability to lose weight? A pilot investigation. J Am Diet Assoc 2001;101:1351–3.
Hester JR, McKenna J, Gately PJ. Obese young people’s accounts of intervention impact. Patient Educ Couns 2010;79:306–14.
Kennedy F. Teaching dietitians to use psychological techniques with obese clients. Behav Psychother 1987;15:88–99.
Kincey J. Target setting, self-reinforcement pattern and locus of control orientation as predictors of outcome in a behavioural weight-loss programme. Behav Res Ther 1980;18:139–45.
Loro AD, Fisher EB, Levenkron JC. Comparison of established and innovative weight-reduction treatment procedures. J Appl Behav Anal 1979;12:141–55.
Mitchell C, Stuart RB. Effect of self-efficacy on dropout from obesity treatment. J Consult Clin Psychol 1984;52:1100–1.
Mobbs O, Crepin C, Thiery C, Golay A, Van der Linden M. Obesity and the four facets of impulsivity. Patient Educ Couns 2010;79:372–7.
Murphy K, Brennan L, Walkley J, Reece J, Little E. Primary goals for weight loss questionnaire (PGWLQ): development and psychometric evaluation in overweight and obese adults. Behav Change 2011;28:29–44.
Neumark-Sztainer D, Story M. Recommendations from overweight youth regarding school-based weight control programs. J Sch Health 1997;67:428–33.
Webber LS, Johnson CC, Rose D, Rice JC. Development of ACTION! Wellness program for elementary school personnel. Obesity 2007;15:48–56S.
No useable data
Adolfsson B, Carlson A, Unden A, Rossner S. Treating obesity: a qualitative evaluation of a lifestyle intervention for weight reduction. Health Educ J 2002;61:244–58.
Gough B. ‘Real men don’t diet’: an analysis of contemporary newspaper representations of men, food and health. Soc Sci Med 2007;64:326–37.
Harvey EL, Hill AJ. Health professionals’ views of overweight people and smokers. Int J Obes 2001;25:1253–61.
Ogden J, Flanagan Z. Beliefs about the causes and solutions to obesity: a comparison of GPs and lay people. Patient Educ Couns 2008;71:72–8.
Reyes N, Oliver T, Klotz A, LaGrotte CS, Virus A, et al. Similarities and differences between weight loss maintainers and regainers: a qualitative analysis. J Acad Nutr Diet 2012;112:499–505.
Witty K, White AK. ‘Tackling Men’s Health’ – establishing a men’s health intervention within a large sports stadium. J Mens Health 2010;7:305.
Not focused on weight loss
Boucher JL, Benson GA, Kovarik S, Solem B, Van Wormer JJ. Current trends in weight management: what advice do we give to patients? Diabetes Spectr 2007;20:153–8.
Kelders SM, van Gemert-Pijnen JEWC, Werkman A, Seydel ER. Evaluation of a web-based lifestyle coach designed to maintain a healthy bodyweight. J Telemed Telecare 2010;16:3–7.
No unique data
Harrison A. Health of Men – weight management partnership. Community Pract 2007;80:31–4.
Ineligible study design
Knutsen I, Foss C. Caught between conduct and free choice: a field study of an empowering programme in lifestyle change for obese patients. Scand J Caring Sci 2011;25:126–33.
Wysoker A. A conceptual model of weight loss and weight regain: an intervention for change. J Am Psychiatr Nurses Assoc 2002;8:168–73.
Reviews
Sarwer DB, Thompson JK, Cash TF. Body image and obesity in adulthood. Psychiatr Clin North Am 2005;28:69–87.
Wadden TA, Phelan S. Assessment of quality of life in obese individuals. Obes Res 2002;10:50–7S.
Letter
Shah K, Wingkun NJG, Lambert CP, Villareal DT. Weight-loss therapy improves endurance capacity in obese older adults. J Am Geriatr Soc 2008;56:1157–9.
Unavailable
Bozkurt S, Zayim N, Gulkesen KH, Samur MK, Karaagaoglu N, Saka O. Usability of a web-based personal nutrition management tool. Inform Health Soc Care 2011;36:190–2.
Glossary
- Glycated haemoglobin
- Glucose sticks to the haemoglobin in red blood cells to make a ‘glycated haemoglobin’ molecule called haemoglobin A1c (HbA1c). The higher the level of glucose in the blood long term, the higher the level of HbA1c.
List of abbreviations
- BMI
- body mass index
- CASP
- Critical Appraisal Skills Programme
- CEA Registry
- Cost-Effectiveness Analysis Registry
- CEAC
- cost-effectiveness acceptability curve
- CG
- clinical guideline
- CHF
- Swiss francs
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DIRECT
- Dietary Intervention Randomized Controlled Trial
- DPP
- Diabetes Prevention Program
- EMA
- European Medicines Agency
- FDPS
- Finnish Diabetes Prevention Study
- FFIT
- Football Fans in Training
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HDL
- high-density lipoprotein
- HMIC
- Health Management Information Consortium
- ICER
- incremental cost-effectiveness ratio
- IIEF-5
- International Index of Erectile Function – 5
- IQR
- interquartile range
- ISPOR
- International Society for Pharmacoeconomics and Outcomes Research
- LDL
- low-density lipoprotein
- Look AHEAD
- Action for Health in Diabetes
- LYG
- life-year gained
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NE
- north-east (quadrant of the cost-effectiveness plane)
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NW
- north-west (quadrant of the cost-effectiveness plane)
- PROGRESS
- place of residence, race/ethnicity, occupation, gender, religion, education, socioeconomic status or social capital
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- ReBIP
- Review Body for Interventional Procedures
- RePEc
- Research Papers in Economics
- RevMan
- Review Manager
- SD
- standard deviation
- SE
- south-east (quadrant of the cost-effectiveness plane)
- SF-12
- Short Form questionnaire-12 items
- SHED-IT
- Self-Help, Exercise and Diet using Information Technology
- SIGN
- Scottish Intercollegiate Guidelines Network
- SPL
- Scottish Premier League
- SW
- south-west (quadrant of the cost-effectiveness plane)
- TA
- technology appraisal
- TMH
- Tackling Men’s Health
- UKPDS
- UK Prospective Diabetes Study
- VO2 max.
- maximal oxygen consumption
- WMD
- weighted mean difference
- WTP
- willingness to pay
- XENDOS
- XENical in the prevention of Diabetes in Obese Subjects