Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 05/52/03. The contractual start date was in January 2010. The draft report began editorial review in May 2013 and was accepted for publication in October 2013. The project was jointly funded by the HTA programme and the European Commission. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Declared competing interests of authors
Both authors work for the National Institute for Health Research (NIHR) Evaluation, Trials and Studies Co-ordinating Centre. EUnetHTA project is supported by a grant from the European Commission. Both authors were members of the EUnetHTA Executive Committee from April 2008 until December 2012, which includes the period when this evaluation was undertaken. This committee had overall responsibility for the delivery of the EUnetHTA JA, of which the work described in this monograph is a part.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Woodford Guegan et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
General introduction
The National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme [represented by the NIHR Evaluation, Trials and Studies Co-ordinating Centre (NETSCC)] was invited to join the European network for Health Technology Assessment Joint Action (EUnetHTA JA) project 2010–12. Participation in this project was part-funded by the European Union (EU) Commission and the NIHR HTA programme.
The authors from NETSCC took on formal roles in three work packages under two broad activities.
Evaluation of the processes of the European network for Health Technology Assessment Joint Action project
It is important that such a project is appropriately evaluated and lessons learnt for future initiatives. A work package was included in the project to consider this and NETSCC was invited to lead it. This was our primary role within the EUnetHTA JA project.
The Directorate-General of the European Commission for Health and Consumers (DG SANCO) requires a process evaluation (rather than an outcome valuation) for all its funded projects. We undertook this role, but extended it slightly by offering some evaluation work to other work packages.
Informing clinical decision-makers about clinical research studies under development: development of a data set to inform a registry
Broadly speaking there are two methods for performing HTA: prospective clinical studies and retrospective systematic reviews. The majority of EU member organisations of the EUnetHTA JA perform only systematic reviews. In addition to these, the UK NIHR HTA programme has a strong history of conducting prospective clinical studies. The Netherlands also commissions such clinical studies and Norway, France, Italy and Portugal have mechanisms to request them. Having a database registry of planned prospective clinical studies would prevent duplication of effort and enable alignment of trial designs to produce more outcome data. It would also be of benefit to those EUnetHTA JA project participants who only perform systematic reviews – they would know when primary research was due to finish and could align the start of their systematic reviews accordingly. It is important that such a registry contains the appropriate data fields and the UK NIHR HTA programme led an activity to compile such a data set. This workstream was within the work package concerned with the production of additional evidence for new technologies [work package 7 (WP7)].
Both activities were performed by the NETSCC using the principles of project management and are described in detail in the following chapters.
Evaluation of the European network for Health Technology Assessment Joint Action project
Health technology assessment in the United Kingdom
Health technology assessment has been described as ‘the provision for health-care decision-makers of high-quality research information on the cost, clinical effectiveness and broader impact of health technologies. Health technologies are . . . all interventions offered to patients’. 1 The findings of applied research studies are vital in supporting an effective and efficient health system. 2
Early attempts to improve health-care decisions in the UK’s NHS were aided by the publication of Effectiveness and Efficiency: Random Reflections on Health Services by Cochrane in the early 1970s. 3 This influential book identified the lack of good-quality data to guide health decisions and highlighted the randomised controlled trial (RCT) as the most reliable assessment method. The UK’s NIHR Health Technology Assessment programme (formerly the NHS Health Technology Assessment programme) was established in the UK in 1993 with the purpose of producing such information for NHS clinicians. 1 It commissions both evidence syntheses and RCTs.
Previous European Union initiatives in health technology assessment
The EU Commission recognised HTA as a key tool in making health-care decisions. 4 It supported HTA-related studies in the 1980s and this continued throughout the 1990s and 2000s:
-
EUR-ASSESS 1994–7: This represented the first of a number of initiatives which aimed to form a European network for HTA. It investigated harmonisation of HTA methodology, priority-setting processes, strategies for disseminating results and issues on how to link the results of HTA to coverage. 5
-
HTA Europe 1997–8: The main activity of HTA Europe was to describe the HTA processes and health systems of all members of the European Union. These reports were published in the International Journal of Technology Assessment in Health Care. 6
-
ECHTA/ECAHI (the European Collaboration for Assessment of Health Interventions and Technology) 2000–2: This informal network provided the benefits of working together, sharing information, providing education and training and sharing methodologies. 7
European network for Health Technology Assessment 2006–8
In 2005 the EU called for proposals for projects to establish a European network for HTA. A group led by the Danish Board of Health was invited to bid for this work, with the goal of developing a set of tools to facilitate co-operation. Following this the EUnetHTA 2006–8 project was formed based on a contract funded by an EU grant. This project aimed to ‘create an effective and sustainable network for HTA across Europe that could develop and implement practical tools to provide reliable, timely, transparent and transferable information to contribute to HTAs in member states’. 8
Project structure
The project was divided into eight work packages co-ordinated by a secretariat:
-
co-ordination
-
communications
-
evaluation
-
common core HTA
-
adapting existing HTAs from one country into other settings
-
transferability of HTA to health policy
-
monitoring development for emerging new technologies and prioritisation of HTA
-
system to support HTA in member states with limited institutionalisation of HTA.
Thirty-four associated partners (contributing to the budget and receiving a share of the grant) and 30 collaborating partners (participating at their own expense) contributed to the project. A 3-year work plan was devised and followed. 8 Governance was through a steering committee (consisting of all associated partners) and an executive committee (consisting of all work package lead partners).
Project deliverables
The main project deliverables were practical tools for conducting HTA. The two tools of potentially immediate use to most HTA agencies were:
-
The core model: This is intended to serve as a platform to aid co-operation in developing a new HTA report. 9 It describes a number of domains (e.g. clinical, economic, etc.). The underlying idea is that different HTA organisations can prepare each domain then enter their findings into a central library. Each individual national organisation can then prepare its own local report for its own health economy by drawing mainly on material contained within the central library (with minor modifications to fit local idiosyncrasies).
-
The adaptation toolkit: This toolkit helps HTA practitioners convert a report between different health-care settings by working through a number of domains. 10,11
Evaluation of the European network for Health Technology Assessment 2006–8 project
Internal evaluation of the EUnetHTA project was an essential requirement of the EU and was the subject of work package 3. This had three objectives:
-
to provide an audit function during the project with regular feedback to the European Commission and the project organisation
-
to evaluate changes over time during the project period to show development towards the establishment of an effective and sustainable network
-
to summarise lessons learned to support the effectiveness and sustainability of the network in its next phase, from 2009 and onward.
The prospective evaluation consisted of three approaches: annual surveys of project participants, twice-yearly interviews with work package leads and documentary analysis of work packages. 12 It concluded that the project had been successful in developing tools that describe a standard for conducting and reporting HTAs and this should facilitate greater international collaboration. Support was evident for a future network. 12
The evaluation report included nine recommendations for a future sustainable network:
-
Secure funding, and maintain a dedicated co-ordinating secretariat.
-
Improve efficiency through an organisational structure made up of work packages managed by a core of dedicated partners, with less committed partners taking part as a wider review group.
-
Continue developing and evaluating the tools as necessary.
-
Involve people in the work to ensure commitment, a high level of knowledge and a broad basis for decision-making processes.
-
Encourage collaboration and communication among all parties to ensure coherence within groups and EUnetHTA.
-
Continue developing the communication platform and functionality of the clearinghouse to make EUnetHTA a central reference point for HTA in Europe.
-
Arrange face-to-face meetings, particularly at the start of group or committee work to strengthen social coherence and reach a common understanding of the work.
-
Evaluate the tools used in real setting and the technical communication platform.
-
English should continue to be the main language.
Evaluation of the European network for Health Technology Assessment collaboration
After the completion of the project at the end of 2008, a number of the partners decided to maintain the network and relationships which had been established over the previous 3 years. This collaboration was established by 25 founding partner organisations from 13 EU member states, Norway and Switzerland. The main output from this year was an application for funding to DG SANCO’s call for a joint action in the field of public health, which became the first EUnetHTA JA.
European network for Health Technology Assessment Joint Action: description of the project
The EUnetHTA JA was a further project funded by DG SANCO, with an overall aim of producing a self-sustaining ongoing European collaboration in HTA. It aimed to ‘ensure the completion and development of HTA in the EU, including work on relative effectiveness of drugs’. Collaboration between the EU Commission and member state-appointed HTA agencies resulted in the establishment of a 3-year joint action project (2010–12), which EUnetHTA was asked to perform. At its investure it was foreseen that sustainability following the project would be ensured through an EU directive. 8
The EUnetHTA JA project was based on a contract of a funding grant with the EU Commission DG SANCO (2009 23 02 – EUnetHTA Joint Action) which specified what it must achieve. The technical annex of the grant agreement described the action as ‘exchanging knowledge and best practice’ with the subaction, ‘Building on the expertise already developed in the field of health technology assessment, ensure the continuation and development of HTA in the EU, including work on relative effectiveness (RE) of drugs’. It is important to make explicit the context of EUnetHTA within local political procedures. The strategic position of the EUnetHTA JA is that ‘its outputs will be used to inform, but not mandate the content of national/regional/institutional HTA reports’. 13
Aims and objectives of the European network for Health Technology Assessment Joint Action project
The project had three aims:13
-
to facilitate the efficient use of resources available for HTA
-
to create a sustainable system of HTA knowledge sharing
-
to promote good practice in HTA methods and processes.
The overarching objective of the EUnetHTA JA was to ‘establish an effective and sustainable HTA collaboration in Europe that brings added value at the regional, national and European level’.
This was separated into three specific objectives:13
-
Development of a general strategy and a business model for sustainable European collaboration on HTA. Specifically, this involves constructing a business model for collaboration addressing the sustainability of the HTA collaboration within the EU.
-
Development of HTA tools and methods. Specifically, developing principles, methodological guidance and functional online tools and policies.
-
Application and field-testing of developed tools and methods. Specifically, testing and implementation of tools and methods.
Project participant health technology assessment agencies
At its commencement in autumn of 2012 the EUnetHTA JA comprised 38 government-appointed organisations from 26 EU member states, Norway and Croatia. Organisations who were members of the EUnetHTA JA were either associate partners (which received 50% funding from the EU grant and 50% from national resources) or collaborating partners (who participated in the project at their own expense). The main partner was the Danish Health and Medicines Authority, which also led the secretariat.
European network for Health Technology Assessment Joint Action: project structure
The work of the EUnetHTA JA was divided into eight work packages: three cross-cutting and five stand-alone ( Table 1 ).
| Work package | Title | Aim |
|---|---|---|
| 1 | Co-ordination | To facilitate the achievement of the EUnetHTA JA general objective of putting into practice an effective and sustainable HTA collaboration in Europe that brings added value at the European, national and regional level |
| 2 | Communication | To facilitate coherent, effective and sustainable external communication of the EUnetHTA JA, where its aims, objectives, work in progress, results and final products are known to all partners, identified stakeholders and target groups in the EU and at a national regional level |
| 3 | Evaluation | To identify to what extent the individual work packages enable the EUnetHTA JA to meet its objective |
| 4 | Core model | Further development and testing of the core model developed in the project 2006–8 |
| 5 | Relative effectiveness assessment of pharmaceuticals | To apply the concepts developed in the core model to provide methods to test the relative effectiveness of pharmaceuticals |
| 6 | Information platform | Developing the tools and internal communication platform to support the other work packages |
| 7 | New technologies | To support collaboration on new technologies and to contribute to reducing duplication of work by:
|
| 8 | Business plan | Construction of a detailed business model for collaboration addressing the sustainability of the HTA collaboration within the EU |
All work packages had a lead partner agency that was responsible for submitting the 3-year work plan for the work package, delivering its deliverables and reporting via the annual technical reports. WP2, WP4, WP5 and WP7 also had a co-lead partner agency.
The governance structure is outlined in Figure 1 .
FIGURE 1.
The governance structure of the EUnetHTA JA. (Reproduced from www.eunethta.eu with permission from EUnetHTA secretariat).
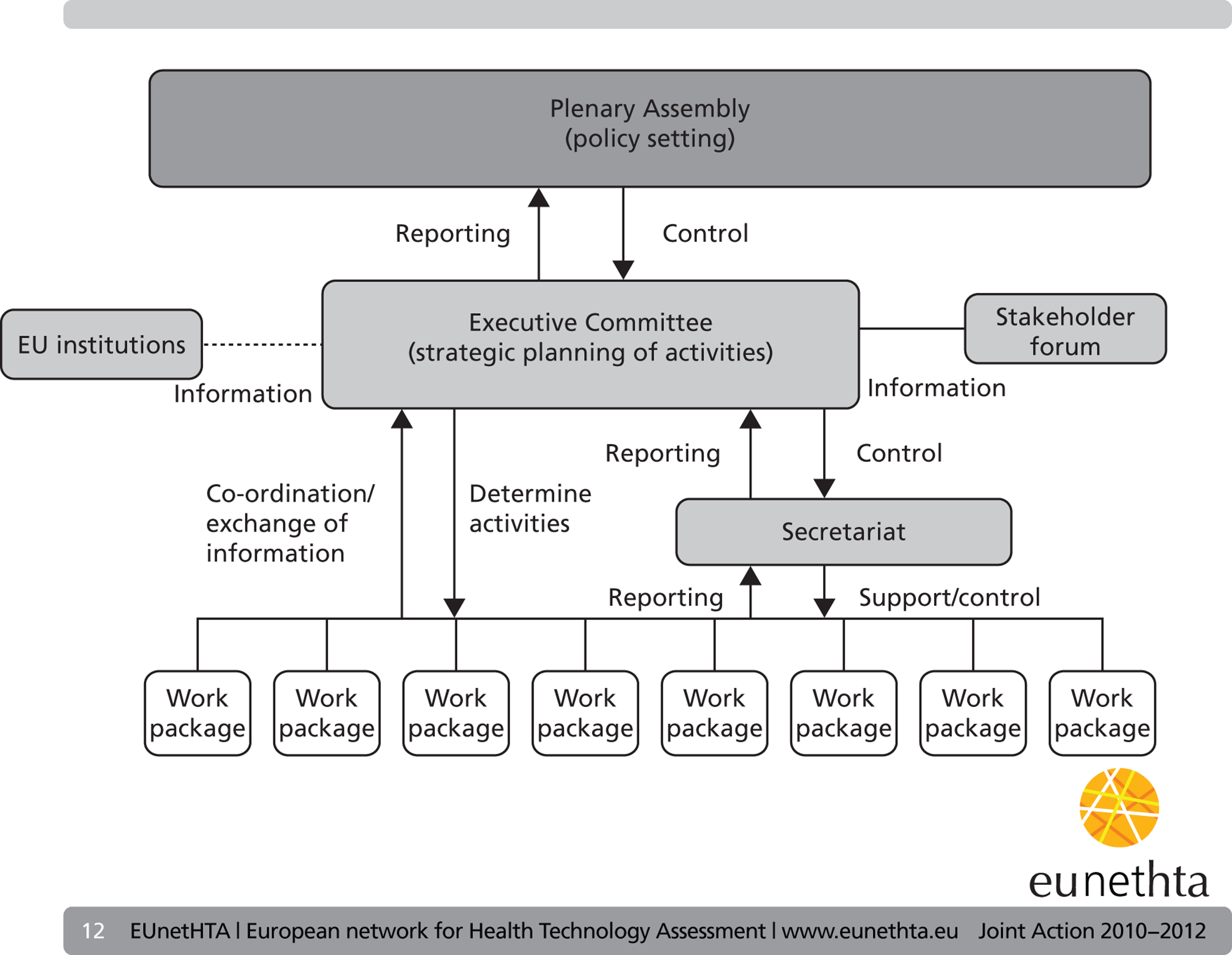
Executive committee
The executive committee was composed of the lead partners of the eight work packages, the chairperson of the plenary assembly (a non-voting member) and three elected members (from the EUnetHTA JA member agencies). This committee was the main executive body involved in strategic leadership of the project. Regular face-to-face meetings were held and e-meetings were held every 2 months.
Plenary assembly
The plenary assembly was the main governance and policy-setting body of the EUnetHTA JA. It was composed of the head of each partner organisation (or their representative). The chairperson was elected by plenary assembly members and ensured liaison between the executive committee and the plenary assembly. This annual meeting was of crucial importance because it represented the only meeting of all project organisations and its function was to agree policy.
The stakeholder forum
In formation of the EUnetHTA JA the EU Commission emphasised the importance of giving a greater focus to stakeholders than had been given during the EUnetHTA 2006–8 project. The stakeholder forum was established in 2010 to facilitate information exchange with the stakeholders and was part of the governance structure for the EUnetHTA JA.
European umbrella organisations, with four types of expertise, were invited to apply to join the stakeholder forum: industry, patients/consumers, providers and payers. Despite several attempts it was not possible to recruit experts to the health media category. Some eligible organisation that were approved for participation in the EUnetHTA JA stakeholder forum were not selected for the final list of the EUnetHTA JA stakeholder forum members because of the limitation of the number of seats per stakeholder group. These organisations received all the information that was circulated to members of the stakeholder forum. They were able to provide written comments on such documents through representative organisations on the forum or via the secretariat. Expertise was represented as follows.
Industry
-
The European Co-ordination Committee of the Radiological Electromedical and Healthcare IT Industry (COCIR).
-
The European Federation of Pharmaceutical Industries and Associations (EFPIA).
-
The European Generic Medicines Association (EGA).
-
Eucomed.
Four organisations applied to be members of the stakeholder forum, but were unsuccessful in gaining a place:
-
Association of the European Self-Medication Industry (AESGP)
-
European Diagnostic Manufacturers Association (EDMA)
-
EuropaBio
-
The European Association of Pharmaceutical Full-line Wholesalers (GIRP).
Patients/consumers
-
The European Consumers’ Organisation (BEUC).
-
The European Cancer Patient Coalition (ECPC).
-
The European Patients’ Forum (EPF).
-
The European Rare Diseases Organisation (EURORDIS).
An additional organisation applied to join the EUnetHTA JA stakeholder forum, but was unsuccessful in gaining a place:
-
The European Federation of Neurological Associations (EFNA).
Providers
-
The Standing Committee of European Doctors (CPME).
-
European Hospital and Healthcare Federation (HOPE).
Payers
-
Association Internationale de la Mutualité (AIM).
-
European Social Insurance Platform (ESIP).
European network for Health Technology Assessment Joint Action: project deliverables
There were eleven project deliverables, which are shown in Table 2 .
| Number | Title | Description | Work package responsible | Month of delivery |
|---|---|---|---|---|
| 1 | An online tool and service for producing, publishing, storing and retrieving HTA information | It facilitates the use of the paper-based HTA Core Model developed previously, allowing to produce, publish, store and retrieve core HTAs and other HTA information not included in core HTAs. It supports production of local reports using core HTAs | WP4 | December 2012 |
| HTA Core Model on screening | A new application of the HTA Core Model | WP4 | March 2011 | |
| 2 | A set of two core HTAs | Two completely new core HTAs on topics that are pertinent to several HTA agencies and that can be utilised when producing local HTA reports on the same topics | WP4 | December 2012 |
| 3 | A methodological guidance that will be appropriate for the assessment of relative effectiveness of pharmaceuticals | A common methodology for REA of pharmaceuticals consisting of a tutorial that describes the fundamental principles of REA and a toolbox that can be used in daily practice for REA in standardised fashion | WP5 | December 2012 |
| 4 | Operational web-based toolkit including database-containing information on evidence generation on new technologies | Database including information on:
|
WP7 | September 2012 |
| 5 | Quarterly communication protocol for information flow on ongoing/planned national assessments of same technologies | Protocols containing information on ongoing/planned national assessments of identical and therefore alerted topics, to facilitate the analysis of hindrances and chances of collaboration on specific topics | WP7 | December 2012 |
| 6 | IMS and the related documentation, processes and policies | The IMS provides a single point of access ensuring compatibility to resources that help to conduct HTA, with emphasis on automation of the content update processes | WP6 | September 2012 |
| 7 | Communication and dissemination plan | Building on the communication strategy developed during EUnetHTA 2006–8 project, an elaborated communication and dissemination plan will be written and implemented as part of the EUnetHTA JA | WP2 | June 2011 |
| 8 | Stakeholder policy | Development of a stakeholder involvement policy | WP1/WP8 | October 2010 |
| 9 | Collaboratively developed business model for sustainability | Development of a collaborative business model for sustainability | WP8 | December 2011 |
| 10 | A relative effectiveness assessment of a (group of) pharmaceutical(s) | As a part of methodological guidance development and in line with the core-HTA development | WP5 | March 2012 |
| 11 | Final report from the EUnetHTA JA | Final report including evaluation results | WP3 | December 2012 |
Tools
Tools were developed during the EUnetHTA JA to help the production of HTA reports and collaboration in HTA:
-
EUnetHTA Planned and Ongoing Projects Database (POP; Ludwig Boltzmann Institute, Vienna, Austria): This database was developed during the new technologies work package (WP7). It aims to reduce duplication in HTA production and to facilitate collaboration between HTA agencies. During 2010 it was available as an Microsoft Excel datasheet (Microsoft Corporation, Redmond, WA, USA) and was converted into a database in 2011. Access to the POP database was restricted to those EUnetHTA Partners who contributed data.
-
HTA Core Model: This online tool aims to help with the production, storage and utilisation of structured HTA information. It was developed during the EUnetHTA 2006–8 project and work continued in EUnetHTA JA in the HTA Core Model work package (WP4).
-
EVIdence DatabasE on New Technologies (EVIDENT): The EVIDENT was developed from the EUnetHTA Interafe to Facilitate Furthering of Evidence Level (EIFFEL) database which was developed during the EUnetHTA 2006–8 project. It allows the sharing of information about promising new health technologies, remaining evidence gaps, recommendations or requests for further information and additional data being collected.
Informing clinical decision-makers about clinical research studies under development: development of a data set to inform a registry
The number of clinical trials is increasing yearly and many are undertaken for regulatory purposes. These are explanatory in nature, thereby identifying whether or not an intervention can produce the desired effect under ideal conditions. 14 Such trials tend to be small in scale or involve investigational interventions which are not in wide clinical use. By their nature they take place within small communities of investigators. They are often funded by manufacturers or research charities – organisations with no wider responsibility beyond their shareholders or donors. 15
However, there is progressive growth and interest in pragmatic trials (and other prospective study designs) which are designed to deliver clinical effectiveness and cost-effectiveness information to directly inform policy, commissioning and clinical decision-makers. Pragmatic studies reflect the actual environment in which clinical practice occurs and create robust evidence to inform these decision-makers,16,17 including organisations such as the National Institute for Health and Care Excellence (NICE) in the UK. These studies are often large and expensive – as they often involve active comparators and ‘soft’ concepts such as ‘usual care’ and ‘best current treatment’. 18 They are often commissioned through publicly funded research management organisations, such as the NIHR in the UK and, hence, these funders must consider taxpayer value for money in their decision-making processes. This is money which if not spent on medical research would, in all likelihood, have been spent on direct clinical care. It behoves such funders to be aware of the activities of their counterparts in other international funding agencies so as not to commission work when the answers would be available elsewhere.
For established, funded clinical trials the scenario is simple. All such studies should be entered into one of a number of international clinical trials registries, such as ClinicalTrials at the National Institute of Health and Current Controlled Trials. Among other benefits, this enables:
-
triallists to see what work is under way in their field
-
clinicians to identify trials in which they might like to take part or enrol their patients
-
reviewers and policy-makers to assess publication bias, by using registered trials as a denominator.
Some funders make this a condition of funding and many journals will only publish studies which have been prospectively registered. 19,20 A funder, therefore, only has to check a limited number of registries for overlapping studies to become aware of what is already under way in a field and decide if it is sufficiently similar to what is planned.
The scenario is much more complex for pragmatic trials when earlier in their gestation. A trial is effectively invisible outside the organisation which may fund it when it is anywhere in its life from a funder having an idea or receiving an application through to a short period of time after a funding decision. Importantly, this runs the risk of multiple funders accidentally spending their limited resource on similar studies – which, perversely, may not be similar enough to allow subsequent meta-analysis of outcomes. Existing registries go part way to addressing this need by allowing funders to identify trials that are under way. However, there remains a gap because there is no widely used registry that tracks trials in development. Therefore, funders run the risk of developing trials in parallel, which may have been avoided or ameliorated if they had been aware of parallel activity.
An example of limited alignment of outcome measures is illustrated by the SOLD (Synergism or Long Duration), PERSEPHONE (Phase III Randomized Study of Neoadjuvant or Adjuvant Trastuzumab (Herceptin®) in Women With HER2-Positive Early Breast Cancer) and PHARE (Protocol of Herceptin Adjuvant With Reduced Exposure, a Randomised Comparison of 6 Months vs 12 Months in All Women Receiving Adjuvant Herceptin) trials, which were all funded around 2006. 21,22 These studies all compared the use of various durations of trastuzumab (Herceptin®, Genentech), using patient relevant outcomes and were, in essence, pragmatic in design. The commissioned trials are comparatively ‘stand alone’. None of their primary outcomes were directly comparable – they used different measures, and those with similar measures assessed them at different end points. It is likely that had the agencies been aware of these trials in parallel development, they could have included appropriate outcome measures to facilitate meta-analysis or other comparison between them.
An example of what should be possible is the interaction between the CATT (Comparison of Age-Related Macular Degeneration Treatments Trials) and IVAN [A randomised controlled trial (RCT) of alternative treatments to Inhibit VEGF in patients with Age-related choroidal Neovascularisation (IVAN)] trials. 23–25 These trials investigated the use of ranibizumab and bevacizumab (Avastin®, Genentech) in the management of wet age-related macular degeneration (AMD). Briefly, both agents are manufactured by the same drug company. Ranibizumab is licensed for the management of wet AMD and bevacizumab is not. Ranibizumab is approximately two orders of magnitude more expensive. There were good biological reasons to believe that both drugs would have similar clinical effectiveness in the management of wet AMD. Discussion between the trial teams during the design and set up of these trials led to an agreed set of primary and secondary outcome measures and a subsequent demonstration that neither drug is superior to the other. Given the hostility of the pharmaceutical industry towards these trials (and the implication of their findings for industry profit margins), the similar results demonstrated against the same outcome measures will give commissioners added confidence should they decide to commission a service based on the cheaper (and, hence, more cost-effective) agent.
There is, therefore, a need for a system to facilitate the identification of pending similar pragmatic studies by international trial funders. This would enable optimisation of scarce public resources – both financial, and in terms of patients and researchers. Desbiens suggested that a ‘Registry of Hypothesis’ should be developed and shared by the medical community. 26 It seems to us that such a registry is too far divorced from the reality of actual funding of a trial – there are many ideas but not all are worth committing resources to. In addition, to a funder, what is important is not who had an idea, but who else might be funding a relevant trial.
It was therefore considered that a registry of ‘trials which funders are considering’ could have potential for filling this gap, and led to several opportunities:
-
Funders could commit to one multinational study, rather than multiple smaller studies.
-
Funders could ensure that at least some outcome data in multiple studies would be directly comparable – thus facilitating meta-analysis.
-
On a global basis, this would facilitate an optimum distribution of trial funding, e.g. knowledge of what each funder had planned could facilitate interfunder discussion to obtain maximum value from the resources each had to contribute.
-
With an indicative map of possible trials, systematic reviewers may be able to better plan when to conduct updates of existing reviews.
-
Similarly, policy-makers may be able to plan when to consider updates of their guidance.
Such a registry could (indeed, should) include the hypothesis to be tested, therefore addressing Desbiens’ call for hypotheses to clearly be developed a priori. 26
We considered who such a registry should be aimed at and, specifically, who should register potential studies and who should have read access. The two options are funders and researchers. The incentives and disincentives are different for the two groups.
Funders are motivated to avoid unconscious duplication of funding and maximise the value of the overall research resource they control. They are, therefore, more likely to register studies they are considering with a registry and keep that information up to date.
A researcher might feel this, but is also driven to obtain funding for their own ideas and, hence, avoid the risk of having their research ideas copied by others. We considered that an individual researcher is less likely to submit their ideas to a public registry.
Therefore, we consider that a registry for pragmatic trials in development is more likely to work if the main adopting groups are trial funders.
Should a registry go ahead, consideration should be given to its performance characteristics, but also whether or not it contributes to the originally identified issues of reducing unconscious duplications and promoting discussion and potentially collaboration between funders.
Chapter 2 Methods
Evaluation of the processes of the European network for Health Technology Assessment Joint Action project
Evaluation approach
Internal evaluation process
Evaluation is an important facet of project management and evaluation of the EUnetHTA JA was a requirement of the EU. There are two types of evaluation: internal evaluation (performed by staff directly involved in the project work) and external evaluation (performed by an expert who is not involved in the project). The EU Commission had specified that the former approach should be used, as it had been used in the previous EUnetHTA 2006–8 project. As recommended in conducting evaluations of European projects, the evaluation plan was a key component and integrated within the EUnetHTA JA project from the beginning. 27
These activities were the subject of a specific work package [WP3 (Evaluation)], led by the NETSCC represented by Dr Eleanor Guegan and Dr Andrew Cook.
Strategic evaluation outcome
Rationale
Evaluation has been defined as, the ‘systematic assessment of the operation and/or the outcomes of a program or policy, compared with a set of explicit or implicit standards, as a means of contributing to the improvement of the program or policy’. 28 Such critical reflection can be performed retrospectively (after the programme has ended) or prospectively (designed at the start of the programme). A prospective methodology is the gold standard for evaluation research and was used to evaluate the EUnetHTA JA.
Project evaluation allows monitoring of the processes of the project and achievements against specified criteria for success. This enables assessment of the effectiveness and achievements of the project and the formation of ‘lessons learned’ recommendations to inform future projects. It also ensures accountability against project plans. 29 There are three main types of evaluation for projects:29
-
Formative evaluation: This ongoing evaluation starts early in the project and assesses the nature of the project, the needs that the project addresses and monitors the progress of the project. It identifies gaps in the content and operational aspects.
-
Process evaluation: This monitors the project to ensure it is being completed as designed and to the time schedule.
-
Summative evaluation: This is an overall assessment of the project’s achievements and the effectiveness of its processes. It is completed at the end of the project and provides evidence to support the performance of future projects.
The first stage in the evaluation process was creating a project evaluation plan. The main purpose of the evaluation was to identify to what extent the individual work packages enabled the EUnetHTA JA to meet its objectives. This included project participants’ and external stakeholders’ perception of the project processes and deliverables. Documentary review enabled identification about whether or not the deliverables had been produced by the work streams according to the work plan.
This evaluation was a systematic data collection designed to develop generalisable knowledge and to contribute to quality improvement of the EUnetHTA JA project:
-
The impact of the project was assessed by an outcome evaluation (identifying the success of delivering the stated project deliverables). However, it should be noted that, although it was possible to measure whether or not the outputs had been delivered in accordance with the work plan, assessment of their quality and cost–utility was beyond the scope of the evaluation agreed by the EU Commission.
-
The effectiveness of the project was evaluated by its processes (identifying the effectiveness of the processes employed during the project).
Evaluation questions
There were two primary evaluation questions:
-
Will the EUnetHTA JA achieve its overarching objective, and ultimately did it?
-
The overall objective was defined in the technical annex for the EUnetHTA JA as, ‘The overarching objective of the JA, including work on relative effectiveness of pharmaceuticals, is to put into practice an effective and sustainable HTA collaboration in Europe that brings added value at the European, national and regional level.’
-
This was assessed by whether or not an effective and sustainable HTA collaboration, not reliant on project funding, had been set up at the end of the EUnetHTA JA project.
-
-
Will the EUnetHTA JA achieve its specific objectives, and ultimately did it?
-
Three subobjectives were defined in the technical annex of the grant agreement for the EUnetHTA JA13 as:
-
– Development of a general strategy and a business model for sustainable European collaboration on HTA.
-
– Development of HTA tools and methods.
-
– Application and field-testing of developed tools and methods.
-
Documentary analysis was used to determine whether or not the work packages had produced their deliverables by the end of the EUnetHTA JA project.
-
A prospective evaluation strategy mapped out and evaluated how the activities and deliverables of the individual work packages supported the EUnetHTA JA objectives. The individual work packages within the EUnetHTA JA can themselves be seen as individual projects (with their own objectives, milestones and deliverables) contributing to the overall programme of work of the EUnetHTA JA. This means it was necessary to aggregate evaluation outcomes from the individual projects.
-
-
Questions were included in the questionnaires sent to participants to examine:
-
demographic information about the nature of participants, their organisations and the HTA information they produce
-
the setting-up process for the project
-
progress of the project in the interim year
-
administrative support and communication from the co-ordinating secretariat
-
the role of information technology in supporting the project
-
involvement of external stakeholders
-
the workings of the eight internal work packages
-
the planned follow-up project – European network for Health Technology Assessment Joint action 2 (EUnetHTA JA2).
Questions were included in the questionnaires sent to external stakeholders to examine:
-
the mechanisms of the stakeholder forum
-
the EUnetHTA JA project
-
the workings of the eight internal work packages
-
the planned follow-up project – EUnetHTA JA2.
Various terms were used in the evaluation and these are summarised in Box 1 .
Overarching objective: This was the main objective of the EUnetHTA JA and was assessed by whether or not an effective and sustainable HTA collaboration, not reliant on project funding, had been set up at the end of the EUnetHTA JA project.
Specific objectives: These were the secondary project objectives of the EUnetHTA JA and were assessed by documentary analysis of whether or not the specified deliverables had been produced.
Deliverables: These were the 11 specific deliverables (see Table 2 ) that the project committed to produce. Whether or not these had been delivered according to plan was assessed by documentary analysis of final technical reports submitted by work packages at the project end.
Performance indicators: These are criteria developed for evaluation of the overall success of the project.
Project impact: This was measured by evaluating whether or not the project’s overarching objective and specific objectives had been met and the deliverables produced. These were the specific performance indicators to enable judgement about the project’s impact.
Project effectiveness: This was measured by evaluating how well the project’s processes had worked. The specific performance indicators for this were assessment of communication within the project, administration by the co-ordinating secretariat, involvement of external stakeholders and management of the eight constituent work packages. Whether or not lessons had been learnt and progress made from the preceding EUnetHTA 2006–8 project was also evaluated.
Evaluation methodology
Possible evaluation methods
Several research instruments could have been used to perform the evaluation, such as interviews, focus groups, observations, case studies, questionnaires and document review, which are described below:
-
Interviews: This research methodology enables the probing of a topic with one interviewee to explore meanings and uncover new areas not anticipated at the outset of the research. 30 These can adhere strictly to a formalised interview schedule (structured) or allow divergence from a schedule to pursue an idea in more detail (semistructured). 30 They have the advantage of probing a subject in detail to obtain rich qualitative data, but are expensive and can be difficult to arrange. It would have been useful to have used this methodology in the evaluation to probe the questionnaire data in greater depth, but unfortunately this was beyond the resource agreed by the EU.
-
Focus groups: This is a form of group interview that generates data from the interaction of the group participants. 31 This has particular advantages in exploring the way people think and perceive things, with findings being generated as a result of group discussions. Like individual interviews, they allow the probing of qualitative data. However, they are expensive and it can be difficult to arrange all participants together in one location at a specified time to conduct the focus group. 31 It would have been useful to have employed this methodology in the evaluation, for instance with a group of stakeholders, but unfortunately this was beyond the resource agreed by the EU.
-
Observations: This methodology involves the researcher systematically watching people and events to discover behaviours and interactions in a setting, then describing and analysing what has been observed. 32 This allows identification of any discrepancies between what people say they do and what they actually do. This method was not considered appropriate for the current evaluation.
-
Case studies: This research methodology is used when broad questions need to be addressed in complex circumstances and it typically involves mixed methods of quantitative and qualitative methods. 33 The selection of sites is of key importance to this methodology. This method was not considered appropriate for the current evaluation.
-
Self-completion questionnaires: These confer several advantages for collection of data from project participants and external stakeholders: standardisation of question wording eliminates the possibility of interviewer bias, respondents are allowed to complete the questionnaire at their own convenience and a greater degree of confidentiality is provided than in interviews. 34 However, they also pose several disadvantages; they are difficult to design, are impersonal and inflexible and are dependent on having a valid sampling frame of the correct details of recipients. 27 They are often the only viable survey format when trying to obtain information from a large cohort of respondents that are within a geographically dispersed population. 35
-
Documentary review: This allows review of a project using documents routinely produced without artificially interfering with the project. This enables retrieval of contextual and historical information about a project. However, by definition this means that the data collection is limited and inflexible, and incomplete data might be encountered.
It was necessary to select which evaluation methods would be most appropriate and feasible within the economic, geographic and time restraints of the project. The methods chosen were self-completion questionnaires (owing to the international location and large number of evaluation participants) and documentary review. It would have been ideal to have also used key informant interviews to obtain richer, qualitative data about what was going well in the project, what could be improved and suggestions for improvement, etc. However, unfortunately, this was not possible owing to time and cost restraints (according to the scope of the evaluation agreed by the EU), and this is a major limitation of the methodology. This meant that it was unfortunately not possible to follow up non-respondents to questions with qualitative enquiry to interpret their views. This has meant that the reasons for non-response have been speculated but it was impossible to substantiate this.
Self-completion questionnaires
The evaluation participants were in two survey populations:
-
project participants – members of EUnetHTA JA partner organisations
-
external stakeholders.
This allowed triangulation of information between the project participants and external stakeholders for common topics. Participants at the annual policy-setting plenary assembly meeting were asked to evaluate the meeting.
-
Annual electronic surveys were sent to project participants and external stakeholders. In some cases the same questions were repeated in questionnaires of different years to allow some assessment of longitudinal data. However, evaluation of such longitudinal elements was severely limited because the large turnover of staff meant it was difficult to assess whether an element had changed over time or this change was due to the responses of different respondents.
-
Paper questionnaires were disseminated at each of the annual plenary assembly meetings which asked respondents to evaluate the meeting.
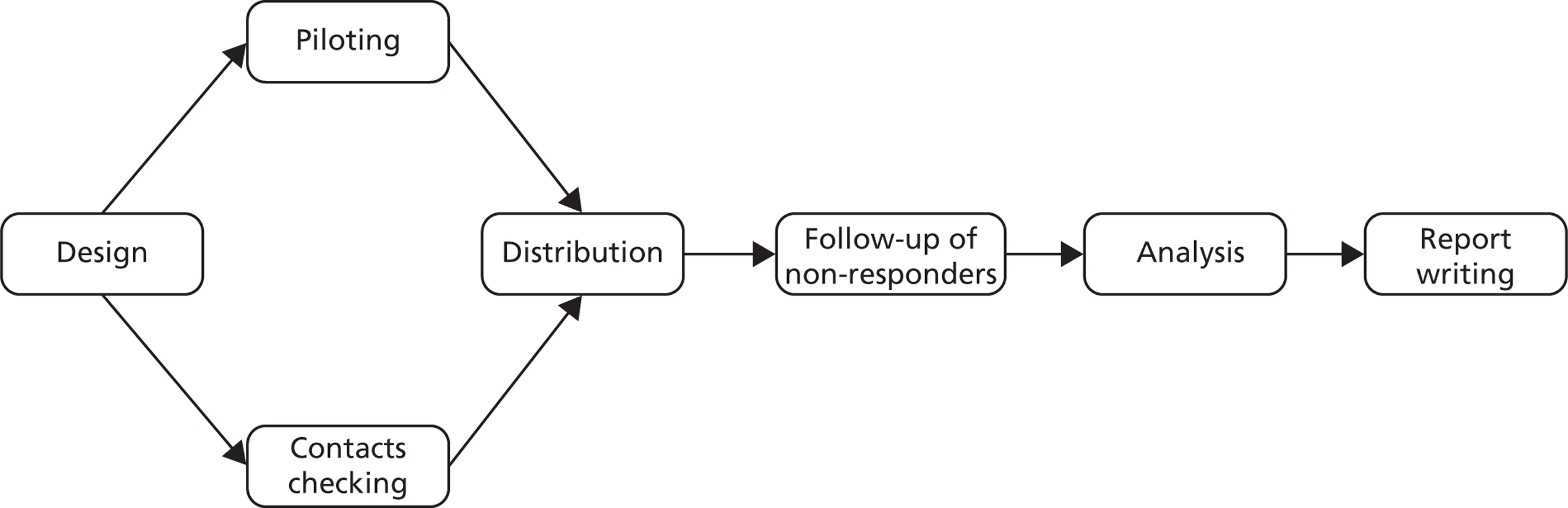
The process for each questionnaire followed the same schedule ( Figure 2 ).
FIGURE 2.
The processes followed for the questionnaires.
Compilation of questionnaire themes
The first stage in the design of a questionnaire is the preparation of a ‘questionnaire specification’ – a comprehensive list of every variable that must be measured. 36 In the current research these were the important aspects, or ‘dimensions of performance’, of the EUnetHTA JA project that needed to be measured. 27 In any evaluation it is impossible to assess all component parts of a project and, therefore, a conscious selection process was performed to consider what needed to be evaluated. Questions were then grouped into a series of ‘question modules’ each concerned with a particular variable, as recommended for survey design. 36 Attention was paid to the order of the individual questions within the question modules to ensure a logical sequence throughout the questionnaire.
Evaluation of the processes of the European network for Health Technology Assessment Joint Action project
A mapping exercise was undertaken to identify which aspects of the project should be assessed at the different time points. One of the objectives of the evaluation was to ‘generate maximum support for the work packages whilst actively seeking evaluation and monitoring solutions that minimise the burden’. This objective was met by decreasing the burden of answering extra questionnaires issued by different work packages. This was achieved by combining questions from other work packages into the questionnaires. The evaluation had been specifically contracted to include questions from the information management work package (WP6). As a courtesy it also included questions from the training strand of the WP8 work package in all three questionnaires and from the dissemination work package (WP2) in 2010. Extensive collaboration was undertaken with the leaders of these other EUnetHTA JA work packages to incorporate their questions. In addition, a questionnaire design workshop was held with participants of the WP6.
Evaluation was performed at three checkpoints within the project, i.e. baseline, interim and towards the end:
-
The formative evaluation started at the baseline of the project (month 6, June 2010). This incorporated the key processes of measuring and monitoring to enable identification of emerging issues, and feedback to the executive committee by evaluation reports. These reports were an appendix to the annual reporting mechanism. The baseline questionnaire captured expectations for the project, experiences during the set-up and identified early concerns about the project.
-
The interim evaluation (mid-term evaluation) of progress against the project plan was performed in month 18 of the project (June 2011). This identified progress against the plan and identified problems requiring corrective action, which were fed back to the executive committee.
-
The final annual questionnaire survey was performed in month 30 (June 2012). This was a towards-the-end evaluation to identify whether or not the objectives of the EUnetHTA JA had been met and flagged-up problems effectively resolved. A limitation of the evaluation was that the EU had requested this be completed before the end of the project.
Evaluation of the plenary assembly meetings
Following the process described above, a standard paper evaluation form was designed to measure participants’ attitudes about attributes of this policy-setting meeting. This was designed to consider:
-
whether or not the meeting met its objectives
-
satisfaction with the conference venue and facilities
-
what the best thing about the meeting was
-
what the worst thing about the meeting was
-
how the next year’s meeting could be improved.
Analysis of the ‘open’ questions from the 2010 survey allowed identification of themes. Therefore, the 2011 and 2012 surveys also included a grid of meeting attributes and Likert answer options to address these themes:
-
receiving meeting documents in advance
-
leadership of the meeting
-
relevance of items discussed
-
meeting and networking with colleagues
-
venue and meeting facilities
-
social event.
At the request of the secretariat, additional questions were included in the 2011 and 2012 surveys. These addressed:
-
when meeting documents were received
-
when meeting documents were read
-
input into developing the meeting agenda.
Question design
Broadly speaking, there are two types of questions that can be used in questionnaires: ‘closed’ questions and ‘open’ questions. Both types of questions were used in the questionnaires.
Closed questions have possible answers predefined by the survey designer and are analysed by frequency measures. They are several different formats: multiple choice, only one choice, Likert scale and matrix. They have the advantage of being easier to analyse than open questions. 37 Multiple-choice questions allowed various choices to be chosen as applicable or may only allow one answer. Likert questions requested respondents to indicate their response according to a predefined scale. However using closed questions means that respondents do not have the ability to provide their own response and, therefore, the richness of potential responses can be limited. 38 There is also the possibility that the answer options are biased and it is important to test this during the piloting phase. A limitation of closed questions is that respondents do not have the ability to explain their answers. 39 This aspect was considered in the design of the questionnaires in the evaluation. To counterbalance this potential problem, most closed questions also included a free-text box to allow respondents to explain their answer, if they wished. This type of question is classified as an ‘expansion’ open question. Such questions act as safety nets, explaining the results of closed questions and identifying new issues not covered by closed questions. 24
Open or free response questions allow respondents to provide their own answers and allow respondents to express their thoughts in their own language. 36 These enable respondents to explain their responses and provide qualitative data. Although these types of questions provide rich data they require more effort to analyse and this was factored into the analysis period. 39 The Cochrane systematic review about methods to increase response to postal and electronic questionnaires identified that the odds of response were reduced by more than half when open questions are used. 40 It was important to be aware of this and to design the questionnaire to contain both open and closed questions. The majority of questions combined a closed question with an open question asking the participant to provide further detail about their response.
The questions included in the participant questionnaires are summarised below in Table 3 .
| Question topic | Question type | Baseline questionnaire (2010) | Interim questionnaire (2011) | Final questionnaire (2012) | Tracker question |
|---|---|---|---|---|---|
| Demographics | |||||
| Professional expertise | Closed | ✓ | ✓ | ✓ | ✓ |
| Gender | Closed | ✓ | ✗ | ✗ | ✗ |
| Age | Closed | ✓ | ✓ | ✓ | ✓ |
| Length in HTA | Open | ✓ | ✗ | ✓ | ✓ |
| Membership of international HTA organisations | Closed | ✓ | ✗ | ✗ | ✗ |
| HTA practitioner | Closed | ✗ | ✓ | ✓ | ✓ |
| Lead person | Closed | ✗ | ✓ | ✓ | ✓ |
| Organisational expertise | Closed | ✗ | ✗ | ✓ | ✗ |
| Organisational and HTA information | |||||
| Difficulties applying | Combined | ✓ | ✗ | ✗ | ✗ |
| Sufficient funding | Combined | ✓ | ✓ | ✓ | ✓ |
| Sufficient staff | Combined | ✓ | ✓ | ✓ | ✓ |
| Succession planning | Combined | ✓ | ✗ | ✗ | ✗ |
| HTA information | Closed | ✓ | ✗ | ✓ | ✓ |
| Setting-up process | |||||
| Achievement of objectives | Combined | ✓ | ✗ | ✗ | ✗ |
| Set-up with EU | Combined | ✓ | ✗ | ✗ | ✗ |
| Organisation into work packages | Combined | ✓ | ✗ | ✗ | ✗ |
| Understand the aims of work packages | Closed | ✓ | ✗ | ✗ | ✗ |
| Foundation as a sustainable collaboration | Combined | ✓ | ✗ | ✗ | ✗ |
| Progress | |||||
| Achievement of objectives | Combined | ✗ | ✓ | ✓ | ✓ |
| Foundation of a sustainable European collaboration | Combined | ✗ | ✓ | ✓ | ✓ |
| Value added | Combined | ✗ | ✓ | ✗ | ✗ |
| Concerns about work packages | Combined | ✗ | ✓ | ✓ | ✓ |
| Evaluation | |||||
| Achievements | Combined | ✗ | ✗ | ✓ | ✗ |
| Personal gain | Combined | ✗ | ✗ | ✓ | ✗ |
| External promotion | Open | ✗ | ✗ | ✓ | ✗ |
| Administration and communication | |||||
| Secretariat leadership | Combined | ✓ | ✓ | ✓ | ✓ |
| Secretariat administration | Combined | ✓ | ✓ | ✓ | ✓ |
| Secretariat other activities | Combined | ✓ | ✗ | ✗ | ✗ |
| Understood information needed | Combined | ✓ | ✗ | ✗ | ✗ |
| Secretariat e-mails | Combined | ✓ | ✗ | ✗ | ✗ |
| Project intranet | Combined | ✓ | ✗ | ✓ | ✓ |
| E-meetings | Combined | ✓ | ✗ | ✗ | ✗ |
| Communication issues | Combined | ✓ | ✓ | ✓ | ✓ |
| Communication methods | Combined | ✗ | ✓ | ✓ | ✓ |
| Project conference | Combined | ✗ | ✗ | ✓ | ✗ |
| Renewal of project identification | Combined | ✗ | ✗ | ✓ | ✗ |
| Improvement of communication | Open | ✗ | ✓ | ✗ | ✗ |
| Project challenges | Open | ✓ | ✓ | ✓ | ✓ |
| Project benefits | Open | ✓ | ✓ | ✓ | ✓ |
| Negative effects of participation | Combined | ✗ | ✓ | ✓ | ✓ |
| Positive effects of participation | Combined | ✗ | ✓ | ✓ | ✓ |
| Information technology | |||||
| Operating system | Closed | ✓ | ✗ | ✗ | ✗ |
| Browser | Closed | ✓ | ✗ | ✗ | ✗ |
| Software packages | Closed | ✓ | ✗ | ✗ | ✗ |
| Communication systems | Closed | ✓ | ✗ | ✓ | ✓ |
| Social media | Closed | ✓ | ✗ | ✓ | ✓ |
| Smart phone/tablet | Closed | ✗ | ✗ | ✓ | ✗ |
| Tools | |||||
| Use/awareness | Closed | ✓ | ✓ | ✓ | ✓ |
| Priority for training | Closed | ✓ | ✓ | ✓ | ✓ |
| Preferred training method | Closed | ✓ | ✗ | ✓ | ✓ |
| Anticipated mobile use | Closed | ✗ | ✗ | ✓ | ✗ |
| Barriers to tools | Closed | ✓ | ✓ | ✓ | ✓ |
| Improvements to tools | Open | ✓ | ✓ | ✓ | ✓ |
| Stopped using tools | Open | ✗ | ✗ | ✓ | ✗ |
| Stakeholders | |||||
| Concerns | Open | ✗ | ✓ | ✓ | ✓ |
| Benefits | Open | ✗ | ✓ | ✗ | ✗ |
| EUnetHTA JA2 | |||||
| Concerns | Combined | ✗ | ✓ | ✓ | ✓ |
| Process | Combined | ✗ | ✓ | ✗ | ✗ |
| Impact of planning | Combined | ✗ | ✓ | ✗ | ✗ |
| Improvement | Open | ✗ | ✓ | ✗ | ✗ |
| Use as a follow-up | Combined | ✗ | ✗ | ✓ | ✗ |
| Main learning point | Open | ✗ | ✗ | ✓ | ✗ |
| Involvement of stakeholders | Open | ✗ | ✗ | ✓ | ✗ |
| Improvement of communication | Open | ✗ | ✗ | ✓ | ✗ |
| Work packages | |||||
| WP1 | Combined | ✓ | ✓ | ✓ | ✓ |
| WP2 | Combined | ✓ | ✓ | ✓ | ✓ |
| WP3 | Combined | ✓ | ✓ | ✓ | ✓ |
| WP4 | Combined | ✓ | ✓ | ✓ | ✓ |
| WP5 | Combined | ✓ | ✓ | ✓ | ✓ |
| WP6 | Combined | ✓ | ✓ | ✓ | ✓ |
| WP7 | Combined | ✓ | ✓ | ✓ | ✓ |
| WP8 | Combined | ✓ | ✓ | ✓ | ✓ |
The questions included in the external stakeholder questionnaires are summarised in Table 4 .
| Question topic | Question type | Baseline questionnaire (2010) | Interim questionnaire (2011) | Final questionnaire (2012) | Tracker question |
|---|---|---|---|---|---|
| Stakeholder forum | |||||
| Purpose of stakeholder forum | Open | ✓ | ✓ | ✗ | ✓ |
| Fulfilment of purpose | Combined | ✗ | ✓ | ✓ | ✓ |
| Awareness of stakeholder forum | Open | ✓ | ✗ | ✗ | ✗ |
| Why applied for membership | Open | ✓ | ✗ | ✗ | ✗ |
| Membership role as expected | Combined | ✗ | ✓ | ✗ | ✗ |
| Role as a member of the forum | Open | ✓ | ✗ | ✗ | ✗ |
| Role represents a good use of organisation’s time | Combined | ✗ | ✓ | ✓ | ✓ |
| Benefits of membership | Open | ✓ | ✗ | ✓ | ✓ |
| Challenges of membership | Open | ✗ | ✗ | ✓ | ✗ |
| Effectiveness of the stakeholder advisory groups | Open | ✗ | ✓ | ✓ | ✓ |
| Contributions to the project | Open | ✗ | ✓ | ✓ | ✓ |
| Offer by membership | Open | ✓ | ✗ | ✗ | ✗ |
| Setting up of the forum | Combined | ✓ | ✗ | ✗ | ✗ |
| Comments about relevant documents | Open | ✓ | ✗ | ✗ | ✗ |
| Comments about the meetings | Open | ✓ | ✓ | ✓ | ✓ |
| Consideration of stakeholders’ views | Combined | ✓ | ✓ | ✓ | ✓ |
| Feedback to stakeholders | Open | ✓ | ✓ | ✓ | ✓ |
| Correct organisations included | Combined | ✗ | ✗ | ✓ | ✗ |
| Concerns about involvement of stakeholders | Combined | ✗ | ✗ | ✓ | ✗ |
| EUnetHTA JA project | |||||
| Achieving objectives | Combined | ✓ | ✓ | ✓ | ✓ |
| Organisational structure | Combined | ✓ | ✗ | ✗ | ✗ |
| A foundation for a European collaboration | Combined | ✓ | ✓ | ✗ | ✓ |
| Functions of a European collaboration | Open | ✓ | ✓ | ✓ | ✓ |
| Added value of a European collaboration | Combined | ✗ | ✗ | ✓ | ✗ |
| Achievement of a European collaboration | Open | ✗ | ✓ | ✗ | ✗ |
| Interactions of a European collaboration with stakeholders | Open | ✗ | ✓ | ✗ | ✗ |
| Attendance at plenary assembly meeting | Combined | ✗ | ✓ | ✗ | ✗ |
| External project promotion | Combined | ✗ | ✗ | ✓ | ✗ |
| Use of tools for HTA producers | Combined | ✗ | ✗ | ✓ | ✗ |
| Would appreciate training in tools | Combined | ✗ | ✗ | ✓ | ✗ |
| Holding a regular conference | Combined | ✗ | ✗ | ✓ | ✗ |
| EUnetHTA JA2 | |||||
| Consultation about planning | Combined | ✗ | ✓ | ✗ | ✗ |
| Concerns about EUnetHTA JA2 | Combined | ✗ | ✓ | ✓ | ✓ |
| Usefulness as a project follow-up | Combined | ✗ | ✗ | ✓ | ✗ |
| Learning from EUnetHTA JA to inform EUnetHTA JA2 | Open | ✗ | ✗ | ✓ | ✗ |
| Improvement of communication | Open | ✗ | ✗ | ✓ | ✗ |
| Work packages | |||||
| WP4 | Combined | ✓ | ✓ | ✓ | ✓ |
| WP5 | Combined | ✓ | ✓ | ✓ | ✗ |
| WP7 | Combined | ✓ | ✓ | ✓ | ✗ |
Attention was paid to the design and wording of the individual questions, according to recognised guidance:36,41
-
avoid long questions
-
avoid double-barrelled questions
-
avoid double negatives
-
ensure inclusion of ‘Don’t Know’ and ‘Not Applicable’ answer options where applicable
-
use simple words, avoid jargon, and avoid abbreviations
-
avoid ambiguous words
-
make questions specific
-
ensure all reasonable response alternatives are included.
Formatting
It is important that questionnaires are designed to be visually attractive as this has been shown to encourage high response rates. 39 In this respect, the layout and flow of the questionnaires was important and the ordering of questions needed to be logical.
Checking of recipient details
It was vital to have an accurate sampling frame for the questionnaires. This ensures that all relevant recipients have been surveyed and ensures integrity of the response rate calculation. It is difficult, but necessary, to differentiate between genuine non-responders and those recipients for whom an incorrect name or e-mail address has been used; therefore, the use of an accurate mailing is essential. 42
Evaluation of the processes of the European network for Health Technology Assessment Joint Action project
It was feasible to survey the entire population and, therefore, no selected sampling was required. An Excel database was obtained from the EUnetHTA JA secretariat which contained details of the EUnetHTA JA project participants in the individual project work packages. This was restructured into a comprehensive database of all the project participants using the following data fields:
-
first name
-
surname
-
e-mail address
-
organisation
-
membership of individual work packages.
The heads of each organisation were asked to confirm the validity of the information of their staff working for EUnetHTA JA. This feedback resulted in a fairly substantial refinement of the contact database.
A pre-notification e-mail was sent to all project participants in advance of sending them a questionnaire. This enabled the correction of any incorrect information and querying of e-mails for which ‘bounce backs’ were obtained. Pre-notification of survey recipients prior to the questionnaire send-out is a strategy that has been shown to increase response rate by 50%. 40 It emphasises the legitimacy of the questionnaire and communicates the value of response. 43 For the current study each recipient of a questionnaire was sent an e-mail 1 week before the send-out notifying them when the questionnaire would be sent to them, the importance of their completing it and the deadline for completion. This also served the purpose of checking e-mail addresses and correcting any errors about work package membership (in the case of project participants) or sending the survey to a nominated colleague instead (in the case of stakeholders).
Details of the representatives of the stakeholder organisations were also obtained from the EUnetHTA JA secretariat.
Evaluation of the plenary assembly meetings
All participants who attended a meeting were surveyed. Therefore, it was unnecessary to perform this stage because the recipients were physically in the room.
Piloting
One disadvantage of the use of self-completion questionnaires is that questions may remain non-completed, without the possibility of explanation. Therefore, time and effort must be spent on designing useful, unambiguous questions. It is important that both the reliability and validity of the questionnaire instrument is assured:
-
Reliability concerns how different people will interpret a question. For a question to be classified as ‘reliable’ respondents must interpret it in the same way and it must therefore be repeatable. 36
-
Validity concerns whether or not the questionnaire actually measures the data it intends to. 27
Before sending out the questionnaire to the recipients it is essential to pilot it on a representative sample first. This is a ‘quality assurance’ method to ensure that the questionnaire contains the correct spelling, is grammatically correct and has a good layout. It also aims to prevent any problems with comprehension and to ensure that the format of the overall survey instrument and individual questions are appropriate. 39 It was important to consider that this was a European project with participants communicating in the common language of English. This was therefore not the native language of the vast majority of participants. Special considerations must be given to the interpretation of question wording and answer options by respondents who are not native English speakers, ensuring the concept is properly understood. This meant that it was necessary to pilot the questionnaires with people who were non-native speakers of English, including those whose primary language was French, German or Spanish.
Evaluation of the processes of the European network for Health Technology Assessment Joint Action project
Questionnaires were piloted with members of the lead partner organisation who were non-native English speakers. Comments were also sought from members of the EUnetHTA JA executive committee and the European Commission. A design workshop was held with members of WP6 in 2010 and the lead partners of WP6 (French) and WP8 (Spanish) also piloted the questionnaires prior to the send-out.
Evaluation of the plenary assembly meetings
The form was piloted by members of the lead partner organisation.
Distribution
When disseminating a questionnaire it is necessary to choose between the possible distribution methods of postal, telephone or internet. Until the 2000s, the primary means of distribution of questionnaires was by face-to-face interviews or postal questionnaires and the advantages and disadvantages of these approaches have been discussed. 36,40,41 However, the 2000s saw the advent of new distribution methods such as e-mail and the internet. The earliest electronic questionnaires involved the distribution of a document [designed in Microsoft Word® (Microsoft Corporation, Redmond, WA, USA) or similar] by e-mail. The EUnetHTA 2006–8 project was also evaluated by questionnaire. However, these were sent by e-mail and the respondent required to record their responses on a Microsoft Word document and e-mail this back to the questionnaire administrator. The effort required in e-mailing the survey back may have contributed to the low response rates received for that questionnaire (23–26% over the years 2006, 2007 and 2008).
Evaluation of the processes of the European network for Health Technology Assessment Joint Action project
The choice of mode of transmission of the questionnaires in the current research was between postal mail, telephone, e-mail and web based. For this study the participants were geographically dispersed over Europe and, therefore, postal distribution was not a practical possibility owing to financial and logistical limitations. Telephone questionnaires were also impractical. A web-based questionnaire that could be completed at the convenience of the respondent was preferable and the recipients were familiar to e-mail communications and using the internet.
Web-based surveys offered several advantages compared with post for the current research: there was a significant cost reduction when considering the international survey population and a quicker turnaround time. 44 In addition to electronic transmission of surveys by e-mail, web-based applications enable automatic data collection. The design of web surveys may be more important than for print surveys. This is primarily because a web-based survey can be displayed differently to a respondent as a result of computer-related glitches and the coding system offers more design capabilities than print. 44 However, the increased ability for design capabilities must be used with some caution because too many design features may lead to overcomplication and a decreased response rate.
Different possible online survey platforms were investigated for the distribution of the questionnaires and SurveyMonkey.com® (Palo Alto, CA, USA) was selected for ease of use and function capability. A paid, professional account was selected from SurveyMonkey.com. 45 This enabled:
-
an unlimited number of questions and responses
-
a custom uniform resource locator (URL)
-
a branded survey with a logo – use of the EUnetHTA logo and colours
-
a survey completion progress bar
-
a custom redirect on survey completion – to the eunethta.net project page
-
a printable PDF version for sharing during design collaboration
-
importing e-mails into the ‘survey manager’ send-out function
-
easy tracking of non-respondents and distribution of follow-up e-mails.
Care was taken that the questionnaire invitations were not sent during the weekends and that the public holidays in the different European countries were avoided. As far as possible these invitations were not sent during the summer holiday season (particularly avoiding the month of August).
Evaluation of the plenary assembly meetings
The analysis of the annual EUnetHTA JA plenary assembly meetings was performed by anonymous self-completion paper questionnaires. These were included as part of the agenda pack given to the meeting participants. They were reminded to complete the questionnaire and hand it in at the end of the meeting.
Follow-up of non-respondents
It is important that as many recipients as possible submit their replies because the non-respondents might differ significantly to the respondents, thereby introducing bias into the results. 36 There seems to be no generalisable recommendation for an acceptable survey response rate. However, this should aim for the highest rate possible and above 50% has been deemed adequate. 34,46
It should be noted that the questionnaire recipients were all associated with the EUnetHTA JA project – either project participants or external stakeholders. Therefore, they had an implicit duty to respond to the questionnaires. However, it was still necessary to employ appropriate strategies to obtain a high response rate to limit bias. A review of methods to increase the response to postal and electronic questionnaires revealed that the odds of response were increased by more than one-quarter when non-respondents were adequately followed up. 40
Evaluation of the processes of the European network for Health Technology Assessment Joint Action project
The recipients had been given a guarantee of confidentiality but not anonymity. This meant that through using the SurveyMonkey.com online platform it was possible to send targeted reminder e-mails to non-respondents. These were personalised e-mails that contained a personal weblink to the questionnaire. E-mails were designed to include two strategies that have been shown to be effective in increasing response rate: each e-mail included a statement that indicated others had responded and provided a deadline for response. 40 Reminders were generally sent 1 week after the date requested for the questionnaire completion and non-respondents were requested to complete the questionnaire within 3 weeks. Two follow-up reminders were sent at 3-weekly intervals.
Evaluation of the plenary assembly meetings
Survey response was anonymous and there was no possibility of tracking non-respondents. Therefore, it was not possible to follow up non-respondents.
Analysis
The overall percentage response rate to questionnaires was calculated as:
It is important that the response rate to individual questions was also defined. The National Center for Education Statistics has stated that key items should achieve a response rate of at least 90%. 47 Accordingly, actual completion rates were shown for each individual question. Data from the two types of question were analysed:
-
The computer software program Statistical Product and Service Solutions (SPSS®, version 19; IBM, New York, NY, USA) was used for the analysis of the quantitative questions. Descriptive statistics were included for categorical data, showing frequency and percentages.
-
Thematic analysis was performed on the qualitative data responses provided to the open questions. Such inductive reasoning involves analysing the data to generate ideas. 48 The grounded theory methodology was used to compare pieces of data to develop conceptualisations to create descriptive knowledge. 48 Topics were identified and clustered into themes. This qualitative analysis was performed by only one researcher owing to the resource limitation of the evaluation agreed with the EU. This has a limitation because it might have biased the results as only one person’s opinion was used. The software program NVivo® (Version 10; QSR International, Victoria, Australia) was used to help analyse the qualitative comments. When analysing open-ended questions it is important to outline the main themes and illustrate them as necessary with quotes36 and this was done in the individual survey reports that were submitted to the secretariat annually.
Report writing
Individual reports were written for each questionnaire. These individual questionnaire reports formed appendices to the technical report submitted to the EUnetHTA JA secretariat each year.
-
2010:
-
plenary assembly evaluation survey
-
EUnetHTA JA participants’ 2010 baseline survey
-
EUnetHTA JA stakeholder forum 2010 baseline survey
-
EUnetHTA JA for those that applied to join the stakeholder forum but were not successful 2010 baseline survey.
-
-
2011:
-
plenary assembly 2011 evaluation survey
-
EUnetHTA JA participants’ 2011 interim survey
-
EUnetHTA JA stakeholder forum 2011 interim survey.
-
-
2012:
-
plenary assembly 2012 evaluation survey
-
EUnetHTA JA participants’ 2012 final survey
-
EUnetHTA JA stakeholders’ 2012 final survey.
-
These reports were uploaded to the EUnetHTA JA members only intranet website. Reports were written as appropriate for work package leaders and tool developers. Articles were submitted to the EUnetHTA JA project e-newsletters to encourage response rates and to report questionnaire results.
Documentary analysis
Each of the eight individual work packages was responsible for writing a final technical report about its performance in the EUnetHTA JA and these were submitted to the secretariat in mid-January 2013. The secretariat was requested to send a copy of each report for analysis in this evaluation. The documentary analysis involved one of the researchers reading the report submitted by each work package and identifying whether or not the work package stated it had produced its deliverable (in accordance with the EUnetHTA JA Grant agreement). 13 This analysis was limited because budgetary restraints of the evaluation meant that it was performed by only one researcher. In addition, it consisted solely of whether or not a work package had self-reported that it had produced the deliverable, rather than an actual observation of the existence of a deliverable.
Informing clinical decision-makers about clinical research studies under development: development of a data set to inform a registry
The project was divided into three phases:
-
development of a data set on which to base a registry
-
assessment of the potential accuracy of that data set
-
building an electronic registry based on the developed data set.
These first two phases are reported below; these are the stages of most relevance to the NIHR Health Technology Assessment programme. The final phase was performed by the French organisation, and fellow member of EUnetHTA, Haute Autorité de Santé (HAS; French National Authority for Health).
Developing the data set
The data set was developed through using the Delphi survey technique. 49–52 This is a method designed to facilitate a decision-making process by groups. It is a technique that aims to reach a consensus of a group by distributing several rounds of anonymous structured questionnaires. 50 The responses from each round are fed back to participants and the responses obtained from a round are used to formulate the next round. 50 Opinions are collected and systematically analysed to obtain consensus. 52
Identifying participants
It was important that the data set should be of a minimum practical size, and limited to contact details and a high-level project description. It appeared evident that for a registry to be both widely accepted and useful the elements in it would need to be widely supported. Therefore, participants to our Delphi process were recruited as widely as possible.
Recruited participants fell into four groups:
-
personal contacts
-
organisations identified through the International Network of Agencies for Health Technology Assessment (INAHTA)53
-
organisations identified through EUnetHTA54
-
organisations identified through contacting groups 1–3.
We built our sampling frame by initially contacting organisations in groups 1–3. We asked each organisation which responded if it was aware of other organisations which may be interested in participating in this project. We then contacted all newly identified organisations (group 4), and repeated this process until we had exhausted all suggestions.
We initially contacted organisations that were known to relevant individuals and organisations that relevant individuals were aware had reputation as a funder or requester of pragmatic studies. A ‘funder of pragmatic studies’ was defined as an organisation which has a remit to allocate funds (either by grant or through a contract) for the research costs associated with pragmatic primary research. The ‘study requester’ is more interesting. Some organisations have a statutory power to request studies to be delivered from certain private sector entities, for example HAS can request drug studies from the pharmaceutical industry.
Organisations within two international membership organisations for HTA [INAHTA (global) and EUnetHTA (European)] were also approached. They were asked for contact details of organisations within members’ countries which may fund clinical studies of this type. Each organisation that responded was also asked about other groups that they were aware of which may have been interested in this project. These groups were then contacted and, where they responded, the process was repeated (i.e. a snowball technique).
At the end of this process 17 organisations from eight countries on three continents had agreed to participate. Some countries handled their responses centrally, pooling responses from several organisations. In other countries organisations participated individually. This meant that 12 separate entities responded to the Delphi rounds (Table 5 ).
| Country | Organisation |
|---|---|
| UK | Programme Grants for Applied Research |
| Research for Patient Benefit | |
| NETSCC | |
| France | Haute Autorite de Santé |
| Institut du Cancer | |
| The Netherlands | College voor Zorgverzekeringen |
| ZonMW | |
| Norway | Combined Norwegian response, co-ordinated by the Norwegian Knowledge Centre for the Health Services |
| Israel | The Israeli Centre for Technology Assessment in Health Care |
| Australia | Australian Safety and Efficacy Register of New Interventional Procedures – Surgical |
| USA | Blue Cross Blue Shield Association Technology Evaluation Centre (also responding on behalf of the Veterans’ Administration, Agency for Healthcare Research and Quality, and the Veterans’ Administration) |
| Canada | Institute of Health Economics |
| Canadian Institutes of Health Research |
Delphi rounds
All participants were asked to take part in up to three Delphi rounds: two were initially planned, with a third held in reserve in case any issues were unresolved at the end of the second.
For each round, a background paper was prepared by the authors at NETSCC, outlining the issues to be addressed in the round and the group’s thoughts so far. For the first round the authors prepared an initial draft of the data set in order to give the group something to start critiquing and changing. A questionnaire was prepared using SurveyMonkey.com for participants to complete. The principles of questionnaire design and the use of SurveyMonkey.com has been described in detail in Chapter 2 (Identifying participants). Copies of the background documents and questionnaires are included in Appendices 12–15 .
Validating the data set
After agreeing the elements of the data set, the next step was to validate the data set. This was done by assessing the data set’s potential accuracy, its ability to match similar studies and identify dissimilar studies as dissimilar. If the data set could not do this, then it would not be fit for purpose. The number of trials we had to consider was small – the aim of this phase was to look for a signal that the data set could be fit for purpose, not to definitively demonstrate so.
As a first part of this phase we identified a set of three potential matching rules, based on matching one or more of the elements of the PICO [population, intervention, comparison and outcome; definition of a research question (e.g. did two elements in the database match on one, two or three elements of population, intervention, and a union of the intervention and comparator conditions]. The intervention and comparator fields were amalgamated for this matching process because a match should not depend on which role a technology is fulfilling, just that it is present in the study.
For this phase Delphi participants were firstly asked to identify clinical and technological topics where they thought that multiple agencies may have undertaken work. The four work areas that were most commonly suggested were selected. For each clinical area we selected an exemplar study from the NIHR HTA programme portfolio. Participants were then asked to submit descriptions of projects that they had undertaken or considered which were similar to the exemplar studies, structured according to the proposed data set. They were also asked to provide studies in similar areas which should not generate a match with the index study when tested. Next, the previously identified matching rules were applied to determine which had the best performance, as defined by Delphi participants.
Chapter 3 Summary results and discussion
This chapter presents a summary of the results and accompanying discussion. For each section the results are presented followed by interpretation and recommendations.
Evaluation of the processes of the European network for Health Technology Assessment Joint Action project
Evaluation of international projects
A project has been defined as ‘directed work that is aimed at achieving specific goals within a defined budget and schedule’. An international project ‘involves multiple locations, entities, organizations and business units’ across different countries. 55 Therefore, the EUnetHTA JA was classified as an international project. Such projects are complicated, owing to the large number of organisations, wide purpose and scope and high cost. 55 At its inception the EUnetHTA JA project had 35 government-appointed organisations. Each of these organisations could have several employees working in a number of work package projects. This meant that communication provided by the co-ordinating secretariat and the specific work packages was important. The EUnetHTA JA had a wide purpose, which necessitated the work to be divided into eight separate work packages. International projects operate in a unique context, where different countries have different economic and political systems,46 all of which have an impact on the management and success of the project.
The evaluation of a large project should include the traditional measures of whether or not the work was completed according to time, cost and quality standards. This should also be expanded to include delivery of new capabilities and business objectives, and assessment of the project’s long-term impact. 56 Project performance can be measured during the lifetime of a project. However, the success or failure of a project can usually only be evaluated months or years after its finish, when the resultant impact can be measured. 57 Consideration of whether or not the deliverables were produced on time was within the scope of the evaluation of the EUnetHTA JA. Unfortunately, the scope of the evaluation was limited by the resources committed by the EU – this meant that evaluation of the project’s delivery according to cost and quality standards and its long-term impact were outside the evaluation’s scope. Owing to limitations mandated by the major funder (the EU), it was only possible to prepare the evaluation 6 months before the end of the project. This is a major limitation of this evaluation process.
Different individuals can have varying measures of project success, depending on their relationship to the project. Project team member’s perspective often includes whether or not they had a satisfactory experience with the project and if it met their needs, while the sponsor considers if the project has provided the desired performance improvement. 56 Therefore, evaluation of participants’ perceptions of the challenges and benefits of the project was included in the evaluation. Project success can also be indicated by high team satisfaction, good morale, increased skill, retention and growth of team members and avoidance of burnout. Therefore, staff members were asked about their perceptions of the project. It was interesting that there was a pattern of approximately one-third of project participants changing between each year of the project. However, consideration about whether or not this might have been related to poor experience on the project or natural movement of the employees was out of the scope of the evaluation. Consideration of the views of external stakeholders were also included in the evaluation.
To achieve project success there must be a clear purpose, specific plans, commitment, open communication, respect and trust, collaboration, political support, clear roles and responsibility and an effective leadership style. 56
Factors leading to the success of a complex project include:58
-
clarity of the goals and commitment to them by the project team
-
establishing smooth communications with supporting infrastructure
-
recruiting project team members with sufficient technical capabilities
-
context of the project considered
-
a supportive project culture.
The EUnetHTA JA is an example of an international project composed of virtual work teams. Such projects are complex and have specific implications. Project performance can be evaluated during a project. However, evaluation can only be performed months or years after its completion. Therefore, the necessity of performing the final evaluation 6 months before the end of the project was a limitation.
Response rate to questionnaires
It is important that high response rates are received to evaluation questionnaires. It is impossible to conjecture about the opinions of non-respondents and how they might differ from those of respondents. Therefore, it is essential to decrease the proportion of non-respondents to ensure the true validity of the results.
The first stage in achieving a high response rate is ensuring that the population survey list is complete and valid. For the stakeholders’ surveys this was relatively straightforward because the secretariat maintains a contact list for these organisations, which are few in number. However, this was more problematic for the project participants’ surveys, perhaps because of the large number of project participants and the complexity of the eight different work packages. This meant that a large degree of ‘data cleaning’ was necessary, for example by contacting the leads of organisations and work packages to receive information about their colleagues.
There is a need for the secretariat to maintain complete and up-to-date details about the staff working on this large international project.
Disappointingly low proportions of questionnaires were completed during the internal evaluation of the previous EUnetHTA 2006–8 project. The response rates for the three annual questionnaires were 23% (2006), 23% (2007) and 26% (2008). 12 Some possible factors that might have contributed towards this low response rate could have included:
-
The questionnaire needed to be completed as a Microsoft Word® document and then e-mailed back. This required some effort from the respondents and there might have been concerns about the confidentiality of results.
-
The questionnaire asked for insertion of the name of the respondent. This again might have caused worries about the confidentiality of data provided.
-
The authors had intended individual participants to complete the questionnaires, but in some instances organisational responses were provided.
It is important that lessons were learnt from the previous evaluation of the EUnetHTA 2006–8 project and resultant mitigating interventions taken for the evaluation of the EUnetHTA JA. Therefore, a structured strategy was implemented to encourage a high response rate, which included targeted follow-up of non-respondents as well as inclusion of both inducing and punitive factors ( Box 2 ).
Certificates were awarded at the annual plenary assembly meeting.
A prize was awarded to the organisation with the highest response rates over the annual surveys.
Punitive factorsNon-respondents were reported to the secretariat and at the plenary assembly.
Non-respondents were reported to organisational leads.
Project participants’ surveys
The following survey codes have been used throughout this report for ease of reporting:
-
project participants’ 2010 survey; p2010
-
project participants’ 2011 survey; p2011
-
project participants’ 2012 survey; p2012.
The response rates to the project participants' questionnaires ranged from 86% to 88% ( Table 6 ). The number of project participants who answered the questionnaires is shown in Figure 3 .
| Questionnaire | Number of questionnaires distributed | First mailing | Second mailing | Third mailing | Total received (n) |
|---|---|---|---|---|---|
| P2010 | 175 | 67% | 83% | 88% | 154 |
| P2011 | 201 | 78% | 84% | 86% | 172 |
| P2012 | 204 | 72% | 79% | 88% | 179 |
FIGURE 3.
Breakdown of responses to the project participants’ questionnaires.
For the project participants’ surveys it was interesting to note that there was a small increase in response rate by using a third send-out in 2010 and 2011 (a 5% and 2% increase respectively). Although the increase was larger in 2012 (9%), a satisfactory response rate of 79% had already been achieved after the second send-out.
If the same strategies are used to maximise response rate, consider in EUnetHTA JA2 that a third send-out may not be required for participants.
Stakeholders’ questionnaires
For ease of reporting reference to the following survey codes have been used throughout this report:
-
stakeholder forum 2010 survey; s2010
-
stakeholder forum 2011 survey; s2011
-
stakeholders’ 2012 survey (for members of the stakeholder forum); s2012a
-
stakeholders’ 2012 survey (for those not successful in gaining a place on the forum); S2012b
-
‘other’ stakeholders’ survey 2010; O2010.
The response rates to the stakeholders’ questionnaire range from 60% to 83% ( Table 7 ). The number of project participants per year of the project is shown in Figure 4 .
| Questionnaire | Number of questionnaires distributed | First mailing | Second mailing | Third mailing | Total received (n) | Non-respondents |
|---|---|---|---|---|---|---|
| S2010 | 12 | 58% | 58% | 83% | 10 | ECPC, EGA |
| O2010 | 5 | 80% | 100% | – | 5 | – |
| S2011 | 12 | 25% | 50% | 67% | 8 | COCIR, EURODIS, EPF, Eucomed |
| S2012a | 12 | 42% | 58% | 75% | 8 | ECPC, EPF, EGA, Eucomed |
| S2012b | 5 | 40% | 40% | 60% | 3 | EFNA, Europabio |
FIGURE 4.
Project participants in the different years of the project.
Project participant turnover
The stakeholder organisations were appointed to the stakeholder forum, or were not appointed but were kept informed by their representative, for the lifetime of the EUnetHTA JA project. This meant that, although the individual contact person sometimes changed, the number and type of organisations did not change.
However, it was interesting to observe that the number of project participants changed during the project. The largest overall increase in members was seen from 2010 to 2011. Approximately one-quarter of participants left the project after the initial year and one-third of the 2011 population was new. A similar pattern was observed for the 2012 questionnaire when one-quarter of recipients were new and one-fifth had left after the 2011 questionnaire.
This has important implications for project management and induction of new members. This suggests that organisational knowledge about the project might be lost and extra time required to initiate new members. It should also be considered that the new members might differ in some facet compared with the ones who have left, for example in outlook and knowledge and this might be reflected in the differences in responses to the longitudinal questions.
It is recommended that concise induction material about the project is provided to facilitate the induction progress of new project members.
Project participant demographics
These results were mostly obtained from closed questions. A range of demographic questions were included in all three annual questionnaires.
As well as evaluating the EUnetHTA JA, the questionnaire also served a dual function of enabling data capture of further information about the nature of member organisations and the project participants. Knowledge of such information about network members could be important in fostering increasing collaboration in the future.
There were a large number of medical doctors working in the HTA organisations, along with other health-care professionals, economists, researchers and project managers. There was an apparent clustering of participants having worked within HTA for between only 0 and 4 years. Approximately two-thirds of agencies had organisational expertise available in health economics, clinical effectiveness research and clinical expertise. These could be considered key expertise needed for preparation of HTA reports. Less frequent, and possibly more specialist, skills that are not intrinsic to HTA were also available in some agencies. This included skills in information technology (IT), communication, legal services, organisational science and development of surveys. It could be important for members of the network to identify fellow members who have a specialist skill and could help them with a particular task.
A list of ongoing HTA projects, and a final published report, was produced by the majority of organisations: 88% and 81% respectively. An English summary was commonly produced. This would help collaboration to a certain degree. However, a final report completely available in English was always available in only one-fifth of organisations. This could hamper collaboration because the details of the findings and methods sections would be inaccessible to persons not speaking the same native language. A list of planned HTA projects was only publicly available in less than half of cases, but in addition approximately one-third of organisations would share it within the EUnetHTA JA project. This demonstrates the importance of the EUnetHTA JA project and would, hopefully, facilitate collaboration in future projects.
This evaluation has contributed to awareness within the network of types of expertise available and the nature of HTA information produced by individual organisations.
Key performance indicators
Success criteria are measures by which the success or failure of a project can be judged. This extends beyond the traditional measure of whether or not deliverables have been produced according to the project plan. 57
Key performance indicators were developed for the EUnetHTA JA project and these are shown in Box 3 .
-
Production of deliverables according to the 3-year work plan and grant agreement.
-
Objectives (as defined in the Grant agreement) met.
-
Additional added value generated.
-
Effective communication within the project.
-
Effective project administration by the secretariat.
-
Optimal involvement of external stakeholders.
-
Good management of the constituent work packages.
-
Progress from the predecessor EUnetHTA 2006–8 project.
Project impact
The impact of the project was evaluated by assessing the deliverables of the project. These are the results, or products, of the project. Some of these deliverables were tools and methods for conducting HTA. For these cases the potential use in practice of these tools, and training requirements prior to use, were also evaluated. Production of deliverables, according to the 3-year work plan and grant agreement,13 are indicators of project management success. They allow assessment of the performance of the project with respect to time (although considerations of quality and cost were beyond the scope of the present evaluation).
Production of deliverables
These results were obtained documentary analysis of work packages’ final technical reports about whether or not the deliverable actually had been produced.
-
1a. An online tool and service for producing, publishing, storing and retrieving HTA information; December 2012 (WP4 strand A)
-
The HTA Core Model was developed as part of the EUnetHTA 2006–8 project. The development of an online format of the tool aimed to make it easier to use and to enable easier access to information (Ms Julia Chamova, EUnetHTA, Copenhagen, 2010, personal communication).
-
The final technical report submitted by WP4 in January 2013 indicated that this had been delivered.
-
-
-
1b. HTA Core Model on screening; March 2011 (WP4 strand A)
-
Applications of the HTA Core Model tool already existed for medical and surgical interventions and diagnostic technologies, developed during the EUnetHTA 2006–8 project (Ms Julia Chamova, EUnetHTA, Copenhagen, personal communication). An additional application of screening was planned for the EUnetHTA JA project.
-
The final technical report submitted by WP4 in January 2013 indicated that this had been delivered. The report specified that this had been validated during autumn 2012.
-
-
-
2. A set of two core HTAs; December 2012 (WP4 strand B)
-
It was planned that two core HTAs would be produced by HTA agencies using the online tool. This enabled application and field-testing of the core model. The final technical report submitted by WP4 in January 2013 indicated that these had been delivered.
-
-
3. A methodological guidance that will be appropriate for the assessment of relative effectiveness of pharmaceuticals; December 2012 (WP5)
-
This was a new activity of the EUnetHTA JA project, stemming from the wish of DG SANCO and DG Enterprise to assimilate interest in joint assessments of relative effectiveness of pharmaceuticals into existing networks, like EUnetHTA (Ms Julia Chamova, EUnetHTA, Copenhagen, personal communication).
-
The final technical report from WP5 indicated that methodological guidelines would be finalised and published at the end of February 2013 (therefore missing the December 2012 target). There was a plan that this would be published in the planned special joint action edition of the International Journal of Technology Assessment in Health Care in 2013. It was noted that this was a model for rapid assessment. Owing to the high workload in WP5 it had been decided to not pursue the model for full relative effectiveness of pharmaceuticals.
-
-
-
4.Operational web-based toolkit including database-containing information on evidence generation on new technologies; September 2012 (WP7a)
-
This activity continued from the previous project when the EIFFEL platform was developed to share evidence on new technologies. This was refined in the current project to create the EVIDENT database (Ms Julia Chamova, personal communication).
-
The final technical report submitted by WP7 in January 2013 indicated that this had been delivered. It was not possible to identify in what month this had been delivered and if this had met the target of September 2012.
-
-
-
5. Quarterly communication protocol for information flow on ongoing/planned national assessments of same technologies; December 2012 (WP7b)
-
This was a new activity – of developing a database for information on ongoing and planned assessment, with the aim of providing alerts about topics with the potential for collaboration.
-
The final technical report submitted by WP7 in January 2013 indicated that this had been delivered.
-
-
-
6. Information management system (IMS) and the related documentation, processes and policies; September 2012 (WP6)
-
Following on from the project, WP6 aimed to further develop the project intranet site to provide a single point of access to resources that help with conducting HTA, with emphasis on automating content update processes (Ms Julia Chamova, EUnetHTA, Copenhagen, personal communication).
-
The final technical report submitted by WP6 in January 2013 indicated that this had been delivered. However, it was not possible to identify in which month this was produced.
-
-
-
7. Communication and dissemination plan; June 2011 (WP2)
-
Building on the communication strategy developed during the previous project it was planned to write and implement a further elaborated plan.
-
According to the final technical report of WP2 this was delivered according to the work plan in month 18 (June 2011).
-
-
-
8. Stakeholder policy; October 2010 (WP8)
-
Stakeholder involvement was considered during the 2006–8 project and formed part of the internal evaluation during the final year of the project. However, the EU Commission emphasised that there must be greater involvement in the EUnetHTA JA project. This policy was required, for implementation through a formal stakeholder forum (Ms Julia Chamova, EUnetHTA, Copenhagen, personal communication).
-
The final technical report of WP8 indicated that this had been delivered (although it was not possible to identify whether or not this had been in the target month).
-
-
-
9. Collaboratively developed business model for sustainability; December 2011 (WP8)
-
This detailed business model would address sustainable European collaboration on HTA. The final technical report of WP8 indicated that this had been delivered (although it was not possible to identify whether or not this had been in the target month).
-
-
10. A relative effectiveness assessment of a (group of) pharmaceutical(s); March 2012
-
The final technical report from WP5 indicated that the ‘pilot of rapid assessment of a pharmaceutical’ had been published on the EUnetHTA website in January 2013.
-
-
11. Interim and final technical and financial reports from the EUnetHTA JA project; various (WP1)
-
This evaluation report is part of the final technical report of the EUnetHTA JA compiled by the secretariat. Therefore, it was not possible to evaluate whether or not this report would be submitted to the EU Commission by the deadline.
-
Anticipated use of deliverables
These results were obtained from a combination of closed and open questions.
It was important to consider whether or not project participants and stakeholders thought that these tools would actually be helpful in day-to-day HTA work. In the final year of the project, both participants and stakeholders were asked how useful they thought they would find deliverables, and activities, developed as part of the project in their professional life. One of the limitations of performing the evaluation 6 months before the end of the project was that respondents were asked to make predictions – a notoriously difficult concept for questionnaire respondents and one that should be avoided as much as possible.
The facet with the highest proportion rating as ‘very useful’ for both participants and stakeholders was ‘networking with contacts made from participating in the EUnetHTA JA’, with three-fifths of participants rating this very highly and over two-thirds of stakeholders reporting it to be very useful. The vast majority of respondents found the networking they had experienced had been of at least some use.
It is recommended that evaluation of the EUnetHTA JA2 includes consideration about the tangible benefits of networking. This could include a case-study approach to demonstrate the practical benefits of networking.
One-third of respondents did not think they would find the HTA Core Model on screening useful or did not know if they would or not. Over one-quarter of respondents did not think they would find the following tools useful, or did not know if they would or not; ‘A methodological guidance that will be appropriate for the assessment of relative effectiveness of pharmaceuticals’, ‘Operational web based toolkit including database-containing information on evidence generation on new technologies (EVIDENT)’ and ‘Accessing the EUnetHTA tools by a single sign on through the MO (members-only) site’.
Almost half of respondents thought that the HTA Core Model and the POP database would be ‘very useful’. A slightly lower proportion of two-fifths of respondents thought the EVIDENT database would be ‘very useful’.
It should be considered that participant respondents have different types of professional activity, for example in respect to whether or not they prepare HTA reports. Analysis of qualitative comments also identified that the access to tools could vary for different organisations and be dependent on the access policy for specific tools.
It is still too early to assess the benefit of the tools in practice, although prediction of use is encouraging. This suggests that the EUnetHTA JA’s third objective of ‘application and field-testing of tools’ has not been met. However, results at this stage are positive and suggest that they will be of future benefit. The EUnetHTA JA2 project will follow on with further real-life application and testing of the tools.
Tools
The main deliverables of the EUnetHTA JA were a series of structures to help in the production process of HTA reports – the tools. There were also tools to help communication within the project;
-
tools for production of HTA reports: adaptation glossary, adaptation toolkit, core HTA model, EVIDENT (formerly known as EIFFEL), POP workroom/database
-
tools for communication: contact database, e-meetings, EUnetHTA toolbar, mailing list, MO website, MO workrooms, news aggregator, workroom bulletin board.
Use of tools
These results were obtained from a closed question included in all three of the questionnaires.
Unsurprisingly, the most often used tools were those for project communication – the MO intranet website, workrooms and e-meetings. Not all members used the project intranet site (the MO website), although the proportion of respondents using it increased from 2010 to 2012. However, the missing proportion said they were aware of it and are likely to use it in the future.
Workrooms were areas assigned within the project intranet for the individual work packages. Although approximately three-fifths of respondents used them, they rated them poorly. It is, therefore, suggested that their use is helpful, because they were used by a large proportion of participants. However, there is obviously an important need for them to be improved in EUnetHTA JA2. Workroom bulletin boards were less often used, with only approximately 15% of project participants reporting use. It seems that respondents used the website, but not the subsplit workroom provisions. Further evaluation needs to be performed in EUnetHTA JA2 to identify whether these workroom bulletin boards could be useful if they were improved or if participants considered them superfluous and they communicate by other means, such as e-mail.
There was increasing use of the following other communication tools from 2010 to 2012 (although they were still only used by less than half of participants): contact database (a database of details of all the project participants), EUnetHTA toolbar (used on the internet) and mailing list (a list of all project participants).
Unsurprisingly, the results for use of the HTA tools were significantly lower. This can be explained because most of the tools were still in a development stage, even in summer 2012 (which again illustrates the limitations of performing evaluation before the end of the project). The HTA Core Model was the tool that the most participants had used, and the proportion that had used it doubled during the lifetime of the project to three-fifths. In each year, approximately 90% indicated they had used it or might use it in the future. The adaptation glossary and adaptation toolkit were under development, and this is reflected in the results of limited use. Although being developed as part of the project, the EVIDENT database had been used in the previous project (as EIFFEL) and, therefore, a number said they had used it. This database was seen as being relatively less popular because approximately 60% saw a future use for it. The POP tool was converted from a static datasheet to an interactive site during the project and this was reflected in the results; the proportion of participants using it doubled to 50%. Roughly 80% had either used it or saw future use for it.
The tools had not been delivered by the final evaluation and, therefore, it was too early to assess their use in practice. The majority of respondents saw future use for the tools, particularly in the HTA Core Model and the POP database. The actual use of the developed tools is an important topic for evaluation in EUnetHTA JA2.
Priority for training
These results were obtained from a closed question included in all three of the questionnaires.
The HTA tools were consistently seen as being a higher priority for training throughout the lifetime of the project than the project communication tools (which were mostly cited as low priority or of no importance). Therefore, the ‘content’ tools were seen as of greater importance than the ‘process’ tools of the project. Possible reasons for this include that there was greater need within the project for more methodological tools, that such type of tools are more complex to use or that communication tools were easy to navigate. In each year the tool most often cited as ‘top priority’ for training by project participants was the core HTA model. The importance of training both partners and stakeholders in EUnetHTA tools and methods was emphasised in the plans for the EUnetHTA JA2 project. One of the deliverables of the JA2 project is ‘Report on yearly training courses on EUnetHTA tools and methodology’. It is planned that three face-to-face training workshops will be held on EUnetHTA tools and methodology. It is also planned that three additional training courses will be held for the core HTA model. These plans were proposed as a result of evaluation questionnaire findings of EUnetHTA JA.
Training method
These results were obtained from a closed question included in the 2010 and 2012 questionnaires.
In general, the most preferred training method for tools was self-directed with a manual. However, a ‘face-to-face workshop’ was the preferred training method for the HTA Core Model, with over half of respondents preferring this option. As a consequence of the 2010 survey results, a face-to-face workshop about the HTA Core Model was organised as part of the project. It was interesting that the proportion of respondents requesting a face-to-face workshop about the EVIDENT and the POP databases noticeably increased from 2010 to 2012. It is difficult to conjecture the reason for this, but this could have been in part due to satisfaction of respondents with the workshop they had attended about the HTA Core Model. One advantage of using a manual is that learning is self-directed and can be undertaken at a time and place to suit the convenience of the learner. Self-directed manual use is also a cheaper method and negates the need to travel to attend a face-to-face workshop; however, it has the disadvantage that queries and difficulties using a tool cannot be as easily resolved as they could be at a training workshop.
Self-directed manuals are considered a suitable training method for the project-focused tools. Face-to-face workshops are preferred for the HTA methodology tools and this should be considered and developed in the EUnetHTA JA2.
Barriers to using the tools
These results were obtained from a closed question included in all three of the questionnaires.
Respondents were asked whether or not any barriers had prevented them using the tools from ‘organisational’, ‘training’, ‘the tool itself’ and ‘IT’. Encouragingly there was an overall general trend that the frequencies of all barriers decreased during the lifetime of the project. This may have been because problems were resolved during the project – informally or formally. There was a general trend that the effect of training being a barrier to tool use decreased during the project throughout the lifetime of the project from 2010 to 2012. It is possible that as the project progressed, participants picked up knowledge about using the tools either informally or formally (i.e. by the training course for the HTA Core Model). IT was seen as a significant barrier to the performance of e-meetings and training was a noticeable barrier to the HTA Core Model (and is reflected in the decision to hold face-to-face workshops in EUnetHTA JA and EUnetHTA JA2). When asked in 2012 why they had stopped using a tool, few responses were obtained. Reported problems included tools not yet being active, problems with passwords and IT problems with e-meetings.
Perceived ability of the European network for Health Technology Assessment Joint Action project achieving its objectives
These results were obtained from a combined closed and open question included in the 2011 and 2012 questionnaires.
The difficulty of managing the diversity of project members, and formulating a goal that all members remain committed to, over the long duration of an international project has been recognised. 58 One factor that can mitigate this is developing objectives at the start of the project. Achievement of defined objectives can also be used to measure the success of the project. 57 Definition of the project objectives is also important for managing the commitment of external stakeholders.
According to the grant agreement13 the overarching objective of the EUnetHTA JA was to ‘establish an effective and sustainable HTA collaboration in Europe that brings added value at the regional, national and European level’.
Project participants and stakeholders were asked in year 2 of the project what would indicate that this had been achieved. They thought that this would have been achieved when a formal EU HTA agency/network not dependent on project funding had been formed, collaboration was achieved, EUnetHTA tools were adopted at a regional or national level, a library of HTA reports and topics was available, HTA was included in decision-making and impact was evaluated. Stakeholders also cited reduction in duplication of effort, evaluation of measurable objectives, consistent exchange of information and collaboration outputs using national and regional levels. The majority of participants and stakeholders thought that such a sustainable EU collaboration would bring added value to both the national and European level. However, there was less confidence about whether or not this would bring added value to the regional level, with about one-third not knowing whether or not this would be achieved. Comments included that such added value would be seen when there is a reduction in redundant work at the regional and national level and better European leverage.
The fact that such a sustainable European collaboration (as defined by the project participants and stakeholders) had not been established following the EUnetHTA JA (necessitating a second joint action) indicates that the project had not been successful at meeting its overarching objective.
Almost three-quarters of the project participants and external stakeholders thought that the planned EUnetHTA JA2 project was a useful follow-up to the EUnetHTA JA project. This suggests that there was an apparent gap between the JA project and the permanent collaboration on HTA. Comments included that the EUnetHTA JA2 was required to build on the EUnetHTA JA, the tools required further development, there was a need to test the efficacy/effectiveness of join report production, that the EUnetHTA JA2 would act as a bridge to a permanent network for HTA, that it will have a different focus of operational work which will identify feasible co-operation between HTA agencies and allow the testing of models of iterative stakeholder involvement, it is a necessary follow-up to EUnetHTA JA and will provide the occasion to examine in practice the methods developed.
The anticipated timeline of achieving a permanent European HTA network is shown in Figure 5 .
FIGURE 5.
The anticipated timeline of achieving a permanent HTA network in Europe. (Reproduced from www.eunethta.eu with permission from EUnetHTA secretariat.)
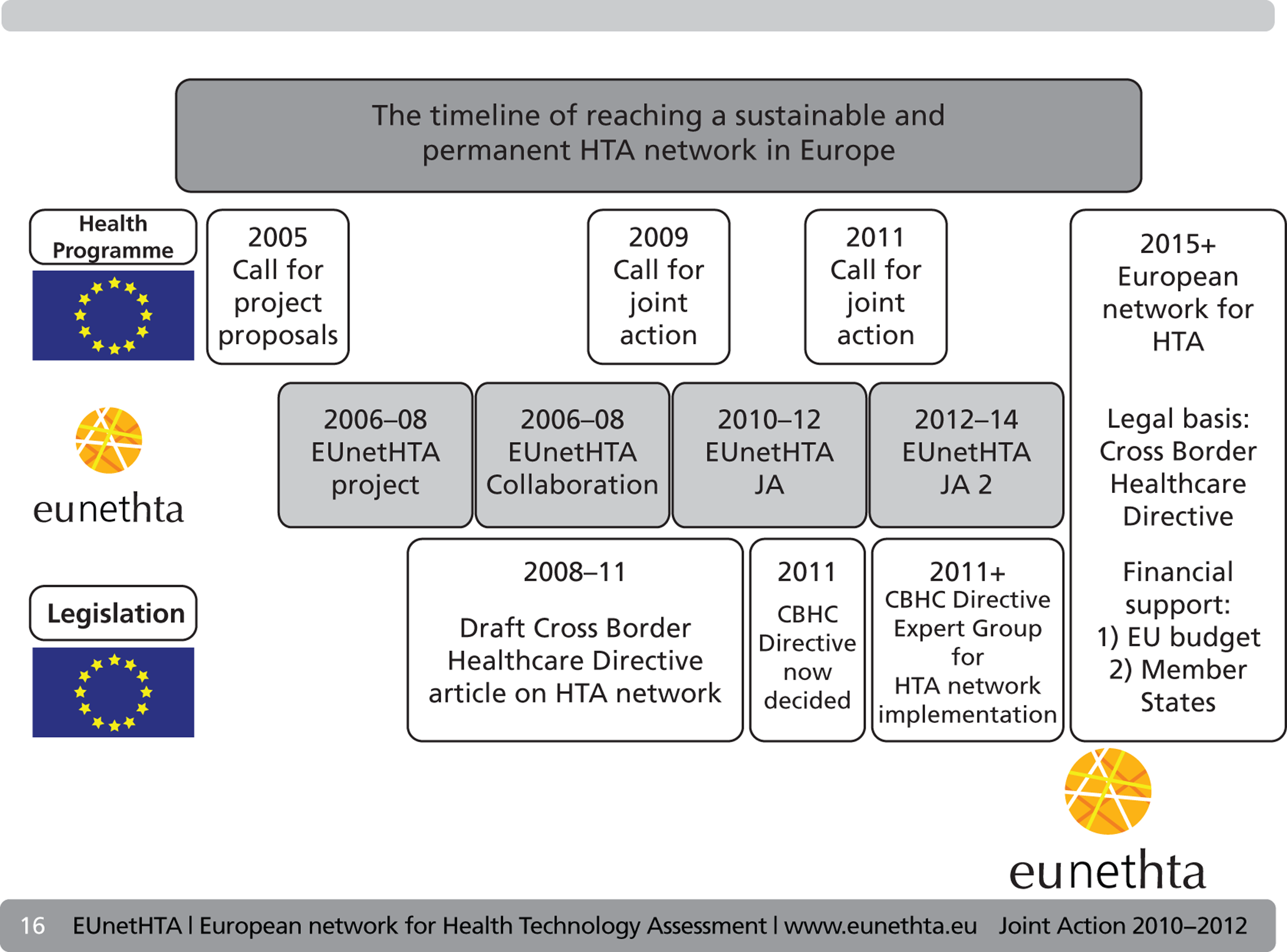
The EUnetHTA JA had three defined objectives, and participants and stakeholders were asked annually whether or not they thought these would be achieved. These objectives were tangible, i.e. production of a strategy business model document, delivery of tools and field-testing of the tools. Documentary analysis was also undertaken to assess whether or not these objectives had indeed been met.
Development of a general strategy and business model for sustainable European collaboration on health technology assessment
The majority of participants and stakeholders had confidence that this objective would be achieved. Indeed, this was the objective that participants and stakeholders had the highest confidence would be met. However, it was interesting that approximately one-tenth of stakeholder forum members in 2011 and 2012 did not think this would be achieved. According to the final technical report, of WP8 this general strategy and business model had been delivered by the end of the project.
This strand of activity is planned to continue in the EUnetHTA JA2 project, ‘Thus, an overarching objective is to develop the background for a general strategy, the principles and the proposal for implementation of a sustainable European Collaboration on HTA in the light of the Directive on CBHC . . .’. 13
The general strategy and business model were developed according to the work plan during the EUnetHTA JA. However, further work is planned during the EUnetHTA JA2 project to further develop this towards a sustainable European HTA Collaboration.
Development of health technology assessment tools and methods
The majority of project participants thought this objective would be met. However, it is interesting to note that participants’ confidence in this being achieved apparently dropped from 2010 to 2012 by approximately 10% and there was a corresponding rise in the number of respondents who indicated they did not know if this would be achieved. There was a similar pattern of apparent doubt by stakeholders because two-fifths were unsure whether or not this would be achieved when asked in 2012.
The proposed HTA tools and methods were all developed according to the grant agreement and the work plan (see Chapter 1, Project deliverables for further details): HTA Core Model, EVIDENT and POP database. The methodological guidelines for relative effectiveness assessment of pharmaceuticals were predicted to be delivered by the end of February 2013. It is notable that further work is planned in the EUnetHTA JA2 project to develop the HTA Core Model. Deliverable 10 will be an ‘Upgraded and updated package of HTA Core Model’. This is to include ‘updated applications on medical/surgical interventions, diagnostic and screening technologies and pharmaceuticals’. 13
All HTA tools and methods were delivered according to the work plan and hence it appears that this objective was achieved. However, further work is planned in EUnetHTA JA2 to further develop the HTA Core Model.
Application and field-testing of developed tools and methods
The majority of participants and stakeholders again showed some apparent confidence that this objective would be achieved. However, a large number of respondents indicated ‘don’t know’. This might have been because they thought that an objective would not be met but were reluctant to choose the negative option choice, but it is difficult to conjecture in the absence of probing respondents to explain their response in greater depth. The apparent confidence of the participants decreased over the years and the proportion who did not know increased. In contrast the number of stakeholders who thought this would be achieved increased over time whilst the number who did not know decreased. However, the number of stakeholders in the forum who did not know whether or not tools would be applied and field-tested was large: three–fifths in 2010 and a half in 2012.
The general objective of the EUnetHTA JA2 is stated in the grant agreement as being ‘to strengthen the practical application of tools and approaches to cross-border HTA collaboration . . .’ (page 35). The first part of this aim indicates that application and field-testing of tools had not been fully completed in the EUnetHTA JA as a follow-up project was necessary. This was specified in the grant agreement as ‘The JA2 will complement the current JA1 by testing the implementation of structures and tools developed previously by way of conducting a set of pilot assessments on technologies to be selected according to common information needs’. 13
It appears that this objective of application and field-testing of tools had not been completely fulfilled by the EUnetHTA JA project and a follow-up EUnetHTA JA2 project was necessary.
Perceived achievements of European network for Health Technology Assessment Joint Action
These results were obtained from a combined closed and open question in the 2012 questionnaire.
Consideration of benefits provided to team members by participation in a project has often been overlooked, or combined into consideration of meeting the project’s objectives. 48 However, it is an important measure of the success of a project. It was a positive finding that the majority of participants thought that the project had achieved what their organisation had hoped for. However, it was concerning that less than half of project participants said that the EUnetHTA JA had achieved what they personally had hoped it would. There was also a large proportion of participants who did not know whether or not it had achieved what they wanted. This, therefore, poses the suggestion that they went into the project not knowing what they wanted it to achieve.
Those who felt achievements had been made cited collaborations and connections developed, successful project progress, network development, the tools and methods developed by the project, information exchange between project members and the effect of greater awareness of policy-makers about HTA in general. In addition to delivering according to the project objectives, this indicates added value of the project. Those who felt that achievements had not been made cited slow progress, lack of change at their local agency, lack of collaborative HTAs produced, difficulties inherent in an international project, lack of cohesion and participation, tools not fit for purpose and that a self-sustaining collaboration had not yet been established. A noticeably larger proportion of participants indicated that they had personally got what they hoped from the project. Reasons included networking, HTA knowledge, information sharing, collaboration, involvement in HTA report production, usefulness of the POP database, ability to act as an ambassador for EUnetHTA and support for establishment of HTA in their own country. One-tenth had not personally got what they hoped from the EUnetHTA JA and reasons included unclear localisation of work, lack of interaction with academia, they had been instructed to take part, tools not being used and difficulties in collaboration.
The main success criteria of a project are meeting its objectives and producing its deliverables according to plan. However, added-value gains are also important. These included networking and information exchange. Such tangible benefits should be measured by the evaluation of the EUnetHTA JA2.
Benefits
These results were obtained from an open question asked in all three of the questionnaires.
Benefits of the EUnetHTA JA from participants’ perspectives included information sharing, collaboration, forming a network, development of methods and tools for doing HTA, networking and greater awareness of recent developments. After analysis of the emergent themes from the P2010 questionnaire results the prevalent project benefits were categorised into a quantitative question using a three-point Likert scale. ‘Networking with colleagues’ and ‘sharing information’ were the two most common project benefits classified as ‘very useful’. When combined into frequencies for all benefits of some use, the vast majority (nine-tenths) of participants found networking, information sharing and awareness of HTA developments of greatest benefit.
The ultimate aim of the European HTA projects is to lead to the formation of a sustainable network. It is encouraging that participants found benefit from networking with others. This might mean that they have built connections with counterparts in different organisations and are able to learn from them and share their own experiences, and it would be useful to investigate this further in EUnetHTA JA2. As well as sharing local information, participants were also able to increase their knowledge about specific HTA-related developments. Other benefits were cited as capacity building, training and face-to-face meetings.
Networking was an added benefit of the project process of the EUnetHTA JA project. It is important that this is further evaluated in the EUnetHTA JA2 project to identify what tangible benefits this has led to, e.g. in terms of topic identification, collaboration on HTA reports, etc.
Project effectiveness
The effectiveness was evaluated by the processes of the project. These are the inputs into the project that may lead directly or indirectly to the success of the project. 57 These project processes can be used to subjectively evaluate the performance of a project.
Set-up of the project
Project start-up
These results were obtained from a combined closed and open question included in the 2010 questionnaire.
Approximately three-fifths of participants were satisfied with the set-up of the project, but one-fifth thought it could have been better. Suggestions included better transparency, decreased bureaucracy, better communication from the JA secretariat and the EU Commission, greater preparation time and maintenance of constant personnel dealing with the project.
The initiation stage of a project is crucial. The most important factor is gaining consensus from project participants about what is to be achieved, and how this should be done, at the outset. 55 A well-planned project will avoid scope creep further down the line by defining the purpose, scope, roles, costs and schedule.
More time should be factored in for this crucial design stage for complex projects than was allowed for the EUnetHTA JA. It is important that individual participants feel included in this formative stage and communication is clear at the outset. This stage is important in ensuring all participants are clear at the outset and it should not be rushed.
Organisation into work packages
These results were obtained from a combined closed and open question included in the 2010 questionnaire.
In common with other such international projects, the EUnetHTA JA project was structured by division into eight distinct work packages. The majority of participants and stakeholders who expressed an opinion thought this structuring was positive, but approximately one-quarter of participants thought there would also be negative aspects associated with this division of work. Concerns centred around possible overlap between the aims and work of individual work packages and the importance of effective communication between them. Most projects funded by the EU are formed in this structure of individual work packages that can be viewed as discrete projects. As one participant responded, ‘it is difficult to see how else the project could have been arranged’. However, this structure means that communication was required between the individual work packages to avoid duplication of work and, wherever possible, enable collaboration. This was done by bimonthly meetings of the executive committee, which included the leaders of all work packages along with the secretariat and the representative of the European Commission. Such a steering committee is recommended for complex, international projects. These committees prevent tension between the individual projects and allow for greater flexibility at the operational level. 58 In their consideration of international project management Lientz and Rea considered having two committees: an overseeing committee and one composed of more senior work package leaders. 55
Consideration could be given to establish a second committee in the EUnetHTA JA2. This could be made up of an individual worker of each work package and could meet virtually by e-meetings to strengthen links between the subproject teams.
Sufficient resources
These results were obtained from a combined closed and open question included in all of the three questionnaires.
Participants provided broadly similar results between 2010 and 2012, with only about a half indicating that their organisation had sufficient funding and three-fifths indicating that there was sufficient staff. The proportion that thought they did not have enough resources was fairly constant at approximately one-fifth over the 3 years. A large number of participants indicated that they did not know whether or not their organisation had sufficient funding or staff to fulfil their obligations to the EUnetHTA JA project. This may be because not all respondents were senior enough to know details about financing, but a lack of staff might be more apparent to them. In hindsight, it might have been better to have asked this question to the organisational lead to obtain views from only senior staff (although this might be biased).
It is obviously important that organisations had sufficient staff and resources to be able to fulfil their commitment to the EUnetHTA JA. From the data collected it is unclear whether it was actually the case that organisations did not have sufficient resources or this was the perception of their staff. Implications of insufficient funding cited by project participants were that the work required extra time for completion than had been estimated or organisations were subsidising the work themselves by working at weekends, devoting more staff to EUnetHTA than were paid by project funding. Lack of staff meant increased workload within the team, and this could have a negative effect on staff morale. For an international project it is important to realise the importance of the self-interest and resources of each of the individual organisations at the start. 55 It is important that the individual participant organisations feel that they will benefit from the project. It cannot be assumed that organisations will contribute their best resources or effort to the project, to make up any shortfall. This can mean that the work was conducted to a basic standard, with the project work being viewed as a non-priority task. Conflict with normal work of a project participant is also an important factor. Most international team members are not solely dedicated to the project, but must fulfil requirements of their regular job in addition. 55 This could also lead to decreased enthusiasm within an organisation for participation in future international projects. This seemed to be particularly important in this project because the funding was obtained in a 50 : 50 ratio from the EU and the member state.
Cultural factors and different styles of work in an international project should also not be overlooked. Business needs are diverse in each country involved in the project. The project should address such local issues otherwise it will be seen as negative – requiring resources but not delivering benefits. 55 Also, in the EUnetHTA JA the individual work packages can be seen as inter-related individual projects. It is important to realise that these projects can share the same resources and together can exact too high a stress level from workers. 55
There should be greater use of project management and budgeting techniques in the EUnetHTA JA2 to ensure sufficient resources are allocated to organisations and specific tasks.
Difficulty joining the project
These results were obtained from a combined closed and open question included in the 2010 questionnaire.
Hearteningly, less than one-tenth of respondents reported a problem with their organisation joining the project. Problems reported included lack of a plan, lack of transparency and lack of distinction in work between performed in the preceding collaboration year and the start of the EUnetHTA JA project. It is important that the process for organisations joining a project is straightforward and easily manageable. Almost one-quarter of project participants in 2010 had a suboptimal or mixed understanding about the information that was required from the secretariat before the project started. The proportion who understood requirements increased from before the project began (two-fifths) to after the project started (three-fifths). Concerns included that the information was not always clear and recognition about the complexity of the project. The relative scarcity of problems can be seen as a good indicator of success.
It should also be borne in mind that the number and types of organisations within the EUnetHTA was a fluid situation, with organisations able to leave and join during the project. It would be useful to have a type of induction process for organisations that joined part-way through the project. It could also be useful to produce a map of the characteristics and expertise of the different organisations joining the project, to enhance collaboration opportunities within the network.
Organisations had mostly had a good experience of joining the project, although communication from the secretariat during this period could have been improved somewhat. However, other organisations subsequently joined the EUnetHTA JA project when it was already established. It would be useful to produce an induction pack for such organisations for the EUnetHTA JA2.
Succession planning
These results were obtained from a combined closed and open question included in the 2010 questionnaire.
Almost two-fifths of organisations had no succession plan in place in case a member of staff became ill or left the organisation, with a further one-fifth not knowing whether or not they had one. It is vital that there is a plan in place in case members of staff leave, to ensure that this does not affect the work of the project. Loss of team members can mean that experience and knowledge is lost and activity in an organisation can grind to a halt. 55 In combination with the fluid nature of high turnover of project staff, there was an apparent lack of succession planning. This again reinforces the need for communication and induction of new staff. Examples of this included the secretariat administrator going on maternity leave and the individual leading the contribution of the lead partner of WP2 leaving mid-way through the project. For an international project, there needs to be a local commitment that the project team is stable and committed, and members are not pulled off the project by local management to perform other work. 55 However, in practice little can be done to mitigate the effect of individuals leaving to take up other jobs, going on maternity or sick leave or dying.
In future international HTA projects all participant organisations should be responsible for outlining who will be locally responsible for an organisation’s commitment to the project if key staff become unavailable.
Challenges
These results were obtained from an open question included in all of the three questionnaires.
Qualitative comments from participants about the challenges inherent in the project included the large scope of the project, its international nature, high workload, limited time and resources, the work itself, communication, administration, national/organisational conflict, requirements of individual work packages, imbalance between contributions of participants and problems with collaborative working. A specific problem in 2010 and 2011 was orientation within the project.
After analysis of the emergent themes from the P2010 results, the prevalent project challenges were categorised into a quantitative question using a three-point Likert scale. A large effect was assigned to conflict with other work activities and insufficient organisational funding and staff. Combining the frequencies for the effects showed that approximately three-quarters were affected to some degree by conflict with other work and insufficient staff and funding. The effects of insufficient organisational funding and staff, and conflict with other work have been discussed in earlier sections.
The effects of the large project scale and demands of the individual work packages were small. Interestingly, the demands of individual work packages seemed to increase between 2011 and 2012. The large project scale is one of the inherent problems of international projects and this leads to a complex nature of subprojects. A large proportion of project participants were members of more than one work package, which meant they had to juggle various work demands.
Hearteningly, more than half of respondents experienced no effect from difficulty in communicating in English and difficulties in communicating generally. Communication is affected by various types of culture and it can be difficult to manage different styles in an international project. 58 In addition, project participants had various natural and functional languages. In the EUnetHTA JA project it was necessary to use a common language, which was English. However, it must be considered that the use of a language by non-native speakers can result in insufficient project feasibility, loss of speed, loss of creativity, poor decisions, underuse of resources and unexpected misunderstandings. It is possible that such problems were experienced by participants, but were not deemed sufficiently important to affect the project work significantly.
Broadly speaking, communication within the EUnetHTA JA project was successful. However, coping with the large project scale and conflicts from work of the subprojects was more of a challenge. The greatest challenge was from conflict from other work and insufficient staff and funding. This could be helped by greater use of project planning techniques.
Support from the secretariat
Secretariat leadership
These results were obtained from a combined closed and open question included in all of the three questionnaires.
Approximately three-fifths of respondents thought the secretariat had offered effective leadership. The number of respondents who thought the leadership was OK (but could be better) increased from less than one-tenth in 2010 to one-fifth in 2012 suggesting an apparent slight decrease in confidence as the project progressed. The qualitative data revealed that concerns included negative aspects relating to communication from the secretariat, implications of changes in the secretariat staff, recognition of the difficulty of the task, a distant style used, and a lack of direction provided by the secretariat and that this needs to evolve in the future.
Important attributes for leaders of international projects have been defined. 55 These include problem-solving ability, ability to cope in multiple cultures with diverse political problems, tenacity and the capability of pursuing issues, ability to communicate, a sense of humour, familiarity and knowledge of the business and prior experience in projects. It is also important that the project leader is aware of the intra- and inter-organisational links in the project and is aware of the hidden agenda of individual organisations.
Owing to the specific nature of the EUnetHTA JA project, it was also important that the leader had a strong steer for HTA in Europe. It has been recommended that there are two leaders for international projects. 58 In the EUnetHTA JA project, as in the EUnetHTA 2006–8 project, the leadership was provided by Finn Borlum Kristensen. The secretariat manager was Julia Chamova. From the comments above, it would seem that the leadership was sometimes perceived as somewhat distant and this could be helped by more regular communication about ‘leadership issues’ with participants throughout the project. There was some concern expressed about the over-reliance on Finn Borlum Kristensen and what would happen if he became ill.
Consideration could be given in future projects to have a deputy leader, possibly based in another country.
Secretariat administration
These results were obtained from a combined closed and open question included in all of the three questionnaires.
Effective project administration is essential for any international project. Such a co-ordination role can organise the project files and project history, oversee the lessons learned and issues log, and support and mentor the project leaders. As such, the secretariat functioned as the project management office and it had an important role in both the internal project processes (e.g. monitoring the performance of individual work package projects) and connection to the external world (e.g. by interacting with external stakeholders). For the EUnetHTA JA project, as for the EUnetHTA 2006–8 project, this function was performed by the secretariat, based at the National Board of Health, Denmark.
Approximately three-fifths of respondents thought the administration had been effective. The proportion who thought secretariat leadership was OK (but could be better) rose from one-tenth in 2010 to one-fifth in 2012. This indicates that there was a slight increase in dissatisfaction with the administration as the project progressed. The qualitative data showed that concerns included, problems with e-mail, general communication problems, too short timelines, quicker feedback required, problems with work packages and the belief that more staff were needed in the secretariat.
The main method by which the project secretariat communicated with work package leaders and individual project participants was by e-mail. Therefore, participants were asked about their opinions about the nature of this communication. Approximately three-quarters of respondents thought the e-mails from the secretariat were acceptable, in terms of frequency and content. Comments related to clarity, frequency and length, that e-mails had improved from the previous EUnetHTA project, the importance of ensuring relevant addressee and suggestions for improvement (e.g. to use a standard template and include the work package number and deadline in the subject line). Although e-mail is a practical mode of communication, it is low down the hierarchy of communication methods because of its inherent problems. Suggestions have been made to improve e-mail communications in international projects. These include using specific titles, making the important point(s) in the first six to eight lines and avoiding overloading participants with e-mails. 55
Additional support from the secretariat
These results were obtained from a combined closed and open question included in the 2010 and 2011 questionnaires.
Approximately one-fifth of respondents in 2011 thought there were other activities that the secretariat could do to support the project, and this proportion had risen from 2010. Suggestions included facilitating relationships, providing greater feedback, providing advice about monthly budgeting, being more customer orientated, facilitating development of effective tools, facilitating information exchange, providing project management and enabling effective communication.
Overall, participants were positive about the assistance offered by the secretariat. Various additional activities were suggested, and it is hoped that in EUnetHTA JA2 with greater funding the activities can be expanded, for example to include support with project budgeting and project management.
Communication
Communicating in English
These results were obtained from a combined closed and open question included in all of the three questionnaires.
In any international project, communication is likely to be difficult because all members do not share a common language. It is important that there is a common language for communication. 58 Encouraging, approximately three-quarters of respondents had not experienced any significant problems when communicating in English during the project. The qualitative data showed that concerns included recognition of the inherent nature of communication problems, difficulty with English text, difficulty with audio, difficulty with speech, the need for greater time when communicating in English, recognition that language barriers exist, differences between nationalities, causes problems during meetings, difficulty in finding staff with good English-language skills and the suggestion of checking of documents by native English speakers. It should also be considered that there can be cultural aspects of communication. For example, Scandinavians and north Europeans tend to use a low-context style, where communication is explicit and unambiguous. In comparison in high-context styles (typical of southern Europeans), meanings of words can be hidden. 58
Attention must be given to the difficulty of communicating in a common language. This should include consideration of strategies to overcome this, such as factoring in more time for dialogue and considering the possibility of getting documents checked by native English speakers.
Communication methods
These results were obtained from a combined closed and open question included in the 2011 and 2012 questionnaires.
Owing to the large and complex structure of the project, it was essential that communication was optimal. Participants working on tasks in the individual work packages were examples of virtual teams, ‘. . . teams of workers who are dispersed across geographical, temporal, and organizational boundaries, yet collaborate using information and telecommunications technology’. 55 To help facilitate this, a large number of different types of communication methods were used. Of these, the apparent most useful mechanism was face-to-face meetings. This method was most frequently described as very useful in all years (although this decreased from three-quarters in 2011 to two-thirds in 2012). In the hierarchy of communication methods, in person communication has been ranked as the gold standard method because it is possible to see a person’s body language and catch the tone of voice and any specific nuances. 55 This method is also the best mode for establishing trust59 and discussing any controversial issues. 58 The observed inclination for face-to-face meetings might indicate that the EUnetHTA JA is a ‘relationship-orientated culture’ as such cultures prefer face-to-face meetings because of the benefits of physical, social and situation context. 58 A preference for face-to-face meetings goes hand-in-hand with the importance of networking and reinforces the importance of participants meeting in person as opposed to working solely in virtual teams. However, the benefits of this communication method need to be balanced with the inherent implications in terms of financial and logistical costs. Therefore, it is important that face-to-face meetings are conducted in an optimal manner and this demonstrates the importance of evaluating the policy-setting annual plenary assembly meetings.
Other popular methods for communication were by using the project intranet (the MO website) and by secretariat e-mails. These e-mails were a synchronous mode of communication, in that all (relevant) participants received the information at the same time. This is a communication better suited to task-orientated communications. 58 The MO website acted as the project intranet and was a central repository of project information. A large proportion of members did not know about the effectiveness of the plenary assembly. This was to be expected, because only one representative per organisation was permitted to attend this meeting.
Participants considered that communication could be improved in the EUnetHTA JA2 by having a formal communications plan, devoting more resources to it, improving the current systems (e-meetings, secretariat, website), using social networks, online tools, expanding networking opportunities, using project management techniques and improving communication with stakeholders. Stakeholders mostly considered this from the perspective of their receiving more information themselves rather than communication between workers on the project. Social interactions and networking is especially important for geographically dispersed teams. 59 In this respect, use of social networks such as Facebook (San Francisco, USA), Twitter (San Francisco, USA), LinkedIn (San Francisco, USA). may be useful. This point will be further investigated in the EUnetHTA JA2.
The most popular form of interaction was from face-to-face meetings and these should be used wherever appropriate in the EUnetHTA JA2. It is important that lessons are learned about how such meetings should be conducted and evaluation of the plenary assembly meetings is important in this respect. The project intranet site was very important and should be improved in the EUnetHTA JA2, particularly with respect to the workroom areas.
External promotion
These results came from an open question included in the 2012 questionnaire.
For projects generally little attention has been given to project marketing, which is concerned with communicating the long-term consequences of a project. The relationships between internal and external stakeholders are important. Suggestions for improvement included the need for a greater presence at conferences, need for more published information, improvement of the public website, national relevance should be communicated to countries, a need to target relevant stakeholders, social networks should be employed, missing an individual approach, more training needed and that promotion will be demonstrated best by collaborative HTA reports. There should be a distinction between whether the project has been insufficiently promoted externally or if that is a perception. This workstrand was the subject of the dissemination work package, WP2. Detailed comments about the functioning of this work package are described below.
External promotion of the project should be improved for the EUnetHTA JA2 project. Strategies that have been suggested include better public website, advertisement of timelines and achievements, use of social media, etc.
Operation of work packages
Participants were asked questions in each questionnaire about the work packages they were involved with.
All work packages
All project participants, both members and non-members, were asked general questions about the individual work packages. External stakeholders were also asked for their opinions about the work packages.
Notably concern was expressed from the participants about the tool-generating work packages: WP4 (by almost one-quarter), WP5 (by almost one-fifth) and WP7 (by almost one-fifth). One-fifth of respondents had concerns about WP8. Almost half of respondents did not know if they had concerns about WP2 or WP3, and it is speculated that the fact these respondents did not know if they had concerns or not indicated a general lack of knowledge about these work packages. In contrast, external stakeholders had a higher rate of concerns about work packages which were not associated with developing HTA tools, i.e. WP1, WP2, WP3, WP6 and WP8.
This apparent concern about HTA tool-generating work packages by participants was apparently not reflected in whether or not participants thought their objectives would be met. However, for most work packages over half of the respondents did not know whether or not the objectives would be met. It is possible that respondents who were not part of a specific work package did not know whether or not the objectives of other work packages would be met or it could be that they thought the objectives would not be met but were unwilling to provide a negative response. The proportion of respondents who did not know was also very high for the stakeholders, again over 50%. This could suggest a lack of confidence in work packages being able to meet their objectives. Stakeholders showed lack of knowledge about objective meeting by work packages not associated with developing HTA-tools.
Approximately one-fifth of participants thought they had not received appropriate communications from the majority of work packages. However, a higher proportion thought they had not been adequately informed about WP2 and WP8. Two-fifths of stakeholders thought they had received insufficient communication from WP1. One-quarter of stakeholders thought they had received insufficient communication from WP2, WP6 and WP8.
There were minimal proportion of participants who expressed an opinion that work packages were not worth having. However, approximately half of respondents did not know if WP2 and WP8 were worth having in the project.
There was an indication from stakeholders that they had received insufficient communications from work packages in which they were not members of. They requested greater involvement of stakeholders in other work packages (e.g. by stakeholder advisory groups being set up) and involvement of professionals with specialist expertise in dissemination and business development.
Specific work packages
For the following section, project participants who were members of the individual work packages were asked detailed questions about them.
The individual work packages of the EUnetHTA JA can be viewed as individual projects in a portfolio. A virtual organisation is formed of virtual groups of workers. 59 A virtual work place forms a barrier to the richness of face-to-face interactions and for this reason the usefulness of face-to-face meetings have been used to jump-start an effective team. 59 Three factors are important for the performance of successful virtual teams: shared understanding, integration (working together in a way that creates value) and mutual trust. 59 A list of attributes of team members of international projects has been developed, which includes experience in similar projects, previous experience in international projects, ability to work with other people on tasks, ability to solve problems and work within the organisation, sensitivity to issues and potential problems, availability from their other work to perform tasks on the project, communication skills, ambition and energy, ability to cope with different cultures, ability and willingness to travel, and the ability to work hard at understanding and getting ideas across. 55 All project members have a responsibility for building trust, communicating, using and adhering to common ground rules and managing conflicts. 58
Duties of a project manager of an international project include defining the project by dealing with ambiguity and politics, organising the project, dealing with the routine work, addressing issues and crises, leading by example, co-ordinating administration (e.g. logistical arrangements of face-to-face meetings. 55 Trust between project members is of vital importance in international projects, alongside the belief that fellow team members are putting their best efforts into the work and everyone is working towards the project goal. 58 Such trust is one of the prerequisites of managing global teams. This is difficult to establish in an international project because of knowledge of collaborating partners being low and reliance on virtual working practices. 58 It is also helpful to have informal interaction opportunities for each team.
Some of the main sources of conflict in international projects include ambiguity of project objective, insufficient authority of the project manager, manpower resources, costs, equipment and facilities, priorities and responsibilities. 58 One factor that can be important in the on-time delivery of a project is ‘adequacy of documentation of organisational responsibilities on the project’. 57
Work package 1: co-ordination
-
There appeared to be a slight depreciation over time as the proportion who agreed increased and proportion who strongly disagreed decreased for leadership and communication being effective within the work package and deliverables being clear.
-
The proportion who disagreed increased over time about the objectives of the work package being clear and the amount of work being manageable, which was one-tenth in 2012.
-
Approximately one-fifth disagreed that the work package had benefited from the inclusion of stakeholders and one-quarter that all partners had contributed to the work adequately (2012 questionnaire).
-
Almost one-third did not know how this work package had progressed in 2011, but this decreased in 2012 where approximately half thought it had been OK and half that it had progressed well.
-
The proportion of respondents having concerns about the work package increased over time to one-fifth in 2011. The qualitative data showed that concerns included continuity, high dependency on the lead, too many face-to-face meetings, increased workload, overlap with EUnetHTA JA2 and about the business model.
Overall, this work package appeared to perform well over time with respect to effective leadership, clear objectives and manageable workload. However, concerns included the benefit of stakeholder inclusion and equal contribution of partners. There appeared to be a strong dependency on the lead partner. Concerns about the delivery of the business model and overlap with the EUnetHTA JA2 project have been addressed previously.
Work package 2: dissemination
-
The proportion of respondents who disagreed about leadership and communication being effective within the work package increased over time to one-quarter and two-fifths respectively.
-
The proportion of respondents who did not know was two-fifths or more for the planning timeline being clear, objectives being clear (P2011), progression of the work package over the past year (P2011), frequency of e-meetings, frequency of face-to-face meetings and whether or not the work package had communicated adequately with other work packages.
-
Four-fifths did not know about the benefit of involving stakeholders in the work package.
-
One-fifth of respondents thought the progression of the work package was poor. The qualitative data showed that concerns included the need for more communication, internally and externally, and more support from the co-lead partner.
Overall, this work package appeared to have performed somewhat poorly, with respect to effective leadership and communication. Particular concern was about the leadership, communication and benefit from the involvement of stakeholders. It was relevant that the representative from the colead organisation had died and the representative from the lead partner organisation had left part way through the project. This emphasises the importance of continuity of a project and the need for induction processes so that the project can continue seamlessly.
Work package 3: evaluation
This work package only contained one organisation (NETSCC) and two members of staff and, therefore, the evaluation questions were not asked for this work package.
Work package 4: core health technology assessment model
This was one of the core work packages of the EUnetHTA JA, with the aim of producing a tool for performing HTA. Therefore, stakeholders were also asked about how they thought it had performed.
-
The proportion of respondents who agreed increased, but the proportion who strongly agreed decreased for leadership being effective, objectives being clear, and deliverables being clear.
-
The proportion of respondents who disagreed about communication between members being effective increased over time to one-fifth in 2012.
-
Approximately one-fifth of respondents disagreed about the amount of work being manageable, collaboration between members to produce core HTAs and benefit from the involvement of stakeholders. About two-fifths disagreed about whether or not all members had contributed to the work adequately.
-
The proportion with general concerns about this work package decreased over time from one-quarter to one-fifth. The qualitative data showed that concerns included the applicability of the model, co-ordination issues, communication issues, workload and that stakeholders had been of little use
-
By the final year approximately half of respondents thought it had progressed OK and half that it had progressed well. The qualitative data showed that concerns included slow progress, lack of participation in pilots and would form the basis for EUnetHTA JA2.
-
Stakeholder concerns included limited applicability of the model in practice.
Overall, this work package appeared to perform well, with respect to effective leadership, and clear objectives and deliverables. However, some concerns were expressed about the amount of work, collaboration on the work, contribution of members and involvement of stakeholders. This was one of the work package that contained a larger amount of participants, which could have magnified intrinsic difficulties in communicating, dividing the work evenly and working together.
Work package 5: relative effectiveness assessment of pharmaceuticals
This was one of the core work packages of the EUnetHTA JA, with the aim of producing a tool for performing HTA. Therefore, stakeholders were also asked about how they thought it had performed.
-
There appeared to be some degree of improvement in the leadership being effective and communication between members being effective because one-tenth disagreed in 2010, but this decreased to zero in 2011 (when one-tenth did not know).
-
The proportion of respondents who disagreed about the amount of work being manageable increased to approximately one-third and a similar proportion disagreed about the contribution of staff.
-
Almost one-fifth disagreed about the division of work and one-fifth disagreed about the number of face-to-face meetings being appropriate.
-
Approximately half did not know about the benefits of stakeholder involvement, but half agreed involvement of stakeholders had benefited the work package.
-
Concerns about the work package’s progress decreased from two-fifths in 2010 to one-fifth in 2011. The qualitative data showed that concerns included that there had been a change in focus from developing a full and rapid model for assessment to only a rapid model, high workload beyond the schedule, applicability concerns, insufficient number of face-to-face meetings, and difficulty in managing stakeholder and political interest.
-
The qualitative data showed that stakeholder concerns included lack of involvement, lack of information exchange and inappropriate influence of industry.
Overall, this work package appeared to perform well, with respect to effective leadership and communication. However, there were concerns about the amount of work, division of work, contributions of members and the number of face-to-face meetings (in 2010). This was again one of the work packages with a large number of members, which could cause intrinsic difficulties and the need for face-to-face meetings is highlighted. There was an apparent perception of high workload. It is difficult to conjecture whether there was too much work that had grown from the work plan or if this was members’ perceptions. It is important that workers contribute as equally as possible so that the burden can be shared.
Work package 6: information management system
-
There appeared to be improvement over the lifetime of the project, with the proportion of respondents who agreed decreasing and the proportion of those who strongly agreed increasing for communication between members being effective, objectives being clear, the planning timeline is clear and number of meetings was appropriate.
-
Approximately one-tenth of respondents thought the technical issues were unclear in 2011.
-
Three-fifths of respondents did not know about the benefits of involving stakeholders in the work package.
-
Approximately one-fifth of members had concerns about the work package, which included that the work was dependent on the other work packages, the inherited IT system from the previous project and they would appreciate e-meetings to discuss the work between the planned face-to-face meetings.
-
The proportion of respondents who thought the project had progressed well increased from approximately one-half to two-thirds in 2012.
Overall, this work package appeared to perform very well, with respect to effective communication between partners, clear objectives, clear planning timeline and an appropriate number of e-meetings. Some concern was shown about the equal involvement of members and of stakeholders. The information technology infrastructure is important for a geographically dispersed project and the other work packages were dependent on it.
Work package 7: new technologies
This was one of the core work packages of the EUnetHTA JA, with the aim of producing a tool for performing HTA. Therefore, stakeholders were also asked how they thought it had been performed.
-
There was a noticeable level of disagreement in 2010, which improved slightly over the project; communication between members being effective (one-quarter in 2010), objectives being clear (one-fifth in 2010), leadership being effective, deliverables being clear and planning timeline being clear.
-
The proportion of respondents who strongly agreed about the leadership being effective increased, while the proportion who disagreed also increased between 2010 and 2012.
-
Three-fifths of participants were unsure about the benefit of involving stakeholders.
-
Only about one-third of respondents agreed or strongly agreed that all members had contributed to the work adequately.
-
The proportion who thought progress had been good increased in 2011 to three-fifths. The qualitative data showed that concerns included that the two strands had developed quite differently: lack of partners populating the POP database and obscure development of EVIDENT.
-
The qualitative data showed that stakeholder concerns included delay in deliverables and lack of involvement of stakeholder advisory groups (SAGs) in the process.
One of the objectives of the process evaluation was to ‘prospectively be vigilant of emerging issues and support the achievement of objectives’. It was identified in the first survey in 2010 that there were concerns about the leadership of WP7. This matter was raised with the lead organisation of WP7 and the executive committee. The WP7 lead partner explained that they had experienced lack of staff to fulfil some of their leadership activities as a result of a staff member going on maternity leave and they had plans to resolve this matter. This was not an apparent problem in the next annual survey of 2011 and so it seemed that the matter had been effectively resolved.
Overall, this work package seemed to progress quite well. After a somewhat disappointing start, it seemed to improve over the life of the project. However, there were some apparent concerns over unequal contribution of partners to the work.
Work package 8: strategy and business model development
-
There was still significant disagreement in 2012 of at least one-fifth for communication being effective and the deliverables and objectives being clear.
-
Two-fifths did not know if all members had contributed to the work adequately.
-
Almost half did not know about communication with other work packages and the benefit of involvement of stakeholders in the work package work.
-
This work package was split into three subsections and by the end of the project about one-tenth of respondents for each section thought it had progressed poorly.
-
Approximately one-third of respondents had concerns in 2010 about the work package, but this decreased to one-fifth in 2011. The qualitative data showed that concerns included confusion about constitution and structure of the work package into three different work streams.
This was a complex work package and was subdivided into three separate work streams. This was reflected in the concerns expressed about the work package’s structure and may have impacted on concerns about the communication, deliverables and objectives.
Plenary assembly meetings
Paper questionnaires were distributed to participants of the annual plenary assembly meetings and the response rates are shown in Table 8 .
| Questionnaire | Number of questionnaires distributed | Response rate | Total n received |
|---|---|---|---|
| Plenary assembly evaluation 2010 | 39 | 74% | 29 |
| Plenary assembly evaluation 2011 | 47 | 77% | 36 |
| Plenary assembly evaluation 2012 | 50 | 78% | 39 |
The plenary assembly was an annual meeting to which a representative of all project participant organisations was invited, alongside members of the stakeholder forum. It had an elected chairperson and was a policy-setting forum. These meetings were costly, in respect of financial cost of members travelling to a foreign city and person-hours involved. Therefore, it was essential that these meetings were as productive as possible. The academic literature shows that it is important that meetings are reviewed at their outset and learning used to improve future events. 60 Therefore, the meetings were evaluated and the results fed back to the secretariat in a detailed report to lead to quality improvement initiatives. The findings will be of use in planning the plenary assembly meetings of the EUnetHTA JA2.
A meeting can be considered a project tool. 61 Meetings can fulfil various functions; information sharing, brainstorming, problem solving, and decision-making and socialising. 61 Characteristics of meetings have been studied and classified as temporal (relating to the use of time in a meeting, e.g. use of a break), physical (relating to the meeting environment, e.g. meeting space), procedural (relating to how the meeting is conducted, e.g. following a formal agenda) and attendee (relating to members, e.g. the role of the meeting facilitator). 61
Objectives
Participants were asked a closed question in each questionnaire about whether or not they thought the meetings’ objectives had been achieved.
Overall, approximately one-tenth of meeting participants did not know whether or not the meetings’ objectives had been met in 2010 and 2012. About one-fifth were not sure for the 2011 meeting. It is recommended that the meeting objectives are made explicit from the outset. It was concerning that one-tenth of participants thought the meeting’s objectives had not been met in 2011. Causes for concern included questions not being answered, lack of useful information and lack of time for debate. When indicating that the meeting objectives had been met, it appeared that participants cited factors that had been important for them personally, such as information gain and endorsement of decisions.
The objectives of the meeting should be made explicit on the agenda before the meeting.
Most preferred aspect
Participants were asked an open question in each questionnaire about what the best aspect of the meeting had been.
Overall, meeting participants seemed to appreciate meeting people face-to-face, discussions, information sharing, social activity and good meeting logistics.
The academic literature has shown that the agenda is crucial in meetings and it has been recommended that meetings begin with a few easy items to settle participants in. 60 Difficult items should be placed around breaks. Frequent breaks should be factored into meetings because concentration typically wanes after about 45 minutes. This also enables participants more opportunities for informal networking. Larger meetings have been correlated with a lower quality and demonstrated the need for a facilitator. Other attributes that have a positive effect include having a break, sufficient lighting and temperature of the room, room space and refreshments. 61
Least preferred aspect
Participants were asked an open question in each questionnaire about what the worst aspect of the meeting had been.
In 2010 problems seemed to centre around technical problems with microphones during the meeting and difficulties getting to the venue. Good meeting logistics are important for a successful interaction and functioning microphones are an important facet of this. In 2011 there were apparent difficulties posed because of the meeting venue (such as seeing the slides) and chairing of the meeting (such as lack of summing up and apparently treating comments at different levels). It is crucial that tables and chairs are positioned so that the chairperson and all the members may participate appropriately. The academic literature has shown that seating the chairperson separately might help them exert their authority over proceedings60 and it is important that participants are able to express their opinions and everyone is heard, and that discussions are respectful. 61 How the participants viewed the meeting can have important attitude and behavioural implications and can impact negatively on the ability of a meeting to reach its goal and support of future meetings. Perception of meetings can also have an effect on employee morale. In 2012 there was an apparent lack of people participating in discussions in the plenary assembly meetings. A common theme throughout the three meetings was lack of time for discussions and participants wishing the opportunity to prepare themselves in greater detail before the meeting. Statistically significant benefits have been found in the academic literature with prior access to an agenda and it is believed that this helps in setting expectations in advance of the meeting and allows for pre-meeting preparation. 61 The working environment of meetings is very important and emphasises the finding that this affects mood and influences behaviour. 61 To make meetings more interesting the format of presentations in the plenary assembly meetings can be changed, for example using group breakout sessions and different visual presentations (e.g. flip charts, video clips, etc.). Other important facets of a meeting include a productive, morale-boosting nature and presentations of adequate content and length. 61
Meeting attributes
In 2011 and 2012 participants were asked to rate attributes of the meeting in a combined closed and open question.
The factor that was rated very good most often was the social event. The second best was meeting or networking with colleagues, with 97% of participants in 2011 and 99% in 2012 rating this as good or very good. An important facet of building trust within an international team is providing informal interaction opportunities. 58 Establishing trust is integral for a successful international project, but is difficult to establish. It cannot be achieved by a one-off meeting but takes time and is a reiterative process in order to cement relationships. 58 The importance of combining a team meeting with a team-building activity, such as having a meal in a restaurant, has been emphasised. 58 Reviews of research have highlighted the importance of initial face-to-face meetings in ‘jump starting’ effective virtual teams. 59
It was noticeable that the meeting venue in 2011 was poor – only 8% rated this as very good compared with 64% in 2012. Leadership of the meeting was also better in 2012 than 2011 with 95% rating this as good or very good in 2012. In decision-making meetings the chairperson should use a participatory style rather than an autocratic one. At the end of a meeting the discussions should be summarised and actions denoted. 62
Involvement of stakeholders
The concept of a project’s value can also be reflected in its worth to different stakeholders. 63 Stakeholders can be defined as ‘actors who have an interest in the issue under consideration, who are affect by the issue, or who – because of their position – have or could have an active or passive influence on the decision-making and implementation process’. 64 Alongside the individual project participants this also included external stakeholders. Such stakeholders for a project can be from heterogeneous organisations, from different cultures and hold different values. 63 Knowledge about relevant external stakeholders is vital for international projects. It is important that their influence level is assessed and their objectives in relation to project strategies and deliverables identified. Stakeholder position in a project can be defined as ally (agree with the need for the project, support the strategies and can influence other stakeholders), oppose (disagree with the strategies and dissent from the project) and neutral (do not have a special interest, but will not suffer from the project’s completion). 65 It is important that an analysis of stakeholders is performed at the start of a project. This allows mapping of an individual stakeholder’s relation to the project, as well as the relationship between stakeholders. 64
Views of project participants about the involvement of stakeholders
Participants were asked open questions in 2011 and 2012 about concerns associated with involvement of stakeholders in the project. They were asked what the benefits of stakeholder involvement had been by an open question included in the 2011 questionnaire.
When participants were asked in 2012, only two-fifths had no concerns about the actual involvement of stakeholders in the EUnetHTA JA. A further two-fifths did not know if they had concerns and one-fifth had concerns. Only one-third of participants had no concerns about the level of commitment of stakeholders, one-fifth had concerns and half did not know. The qualitative data showed that concerns centred around lack of participation and commitment of stakeholders and the heterogeneous nature of stakeholders. Therefore, there was a large proportion of concern or apparent lack of knowledge, which might have indicated a lack of added value.
Response rates to stakeholder questionnaires
For the S2011 survey, only half of the four industry representatives and half of the four patients/consumers responded. The situation was repeated in S2012 (although the individual non-responding organisations were different). It may be that lack of response is itself of value (e.g. leading to the speculated conclusion that the stakeholder organisation does not consider EUnetHTA of sufficient importance to engage with), but it would be preferred to have this opinion explicitly stated. Unfortunately, it was beyond the scope of the evaluation (agreed with the EU Commission) to include follow-up of non-respondents.
Stakeholders must commit to answer evaluation questionnaires in the EUnetHTA JA2, so that their opinions can be considered.
Formation of the stakeholder forum
A combination of closed and open questions were included in the questionnaires about the formation of the forum, its purpose, why stakeholders had applied to join it, what they could contribute and whether or not appropriate organisations had been included.
A prerequisite of the EUnetHTA JA project was an acknowledgement of the importance of communicating with stakeholders. 13 With this aim a formal stakeholder forum was established in 2010. This contained four seats for stakeholder categories of industry, patients/consumers, providers and payers. Stakeholders became aware of this forum from various avenues, including from the EU Commission, the EUnetHTA secretariat and key project personnel and other stakeholders. Stakeholders had applied to be an external stakeholder for various reasons, including contributing to the JA project, to enable reciprocal working patterns and ensuring a balance of stakeholder expertise. Stakeholders believed they could contribute to the project by providing specialist HTA knowledge, providing access to experts, providing a governance role and disseminating the project results. They saw the purpose of the forum as facilitating the stakeholder involvement process by providing an intermediary link between the stakeholders and the JA project, overseeing work package and SAG activities, developing consensus for decision-making and being ‘transparent, responsive, accountable and participative’.
In practice, only two organisations applied for the four seats in both the provider and payer categories. This only represents half-capacity take-up and it is suggested that possibly these were inappropriate types of stakeholder expertise to include in the project or that the call for stakeholders was too limited. There were more participants than spaces for the industry and patients/consumer groups, and this meant that some applicants were excluded. The perceived risk from non-inclusion of these organisations were suggested as missing expertise, decreased scope of JA activities, decreased confidence in the project and inclusion of less experienced representatives. It was notable that only one-fifth thought that the way of setting up the forum was straightforward. The qualitative data showed that concerns included the process itself, that it was organised to fulfil a certain type of stakeholder involvement, restricting stakeholder members prevented equal contributions, lack of clarity about why applicants were rejected and complexity in assigning a representative. However, about three-quarters of stakeholders questioned near the end of the project thought the formation of the forum had been an effective mechanism for involving stakeholders. Over two-thirds of respondents surveyed in 2012 thought the appropriate organisations had been included. Those members who had applied to join the forum but had been excluded from the forum were represented by a representative for their specific group. At the start of the project the majority of these organisations were concerned that they would not be represented adequately. However, these concerns appeared unfounded because by the end of the project all agreed that they had been kept adequately updated by either their forum representative or by the secretariat.
The qualitative data showed that there were apparent initial concerns about the formation of the forum because it was seen as a complicated business and there were concerns about restriction of expertise. However, by the end of the project this was seen as having been an effective mechanism and mostly included the correct stakeholders. Those who had not been successful in gaining a place had been kept informed by their representative.
Overall, the stakeholder forum seemed to be an effective means of involving external stakeholders in the EUnetHTA JA. Non-members appeared to have been adequately informed by their designated representative.
Operation of the stakeholder forum
A combination of closed and open questions were included in the questionnaires to identify stakeholders’ views about the operation of the forum, whether or not it was fulfilling its purpose and comments on the meetings held.
In 2011, all respondents agreed that the forum was fulfilling its purpose, recognising an initial start-up period. It was seen as being inclusive, evolving and that there was increasing trust. Its purpose was cited as being a special advisory group to the executive committee, commentating on work package tasks, overseeing SAGs, gaining consensus for decisions. The majority thought membership was involving what they had envisaged and it seemed to be improving over time. However, in 2012 about one-third thought it was not fulfilling its purpose and about one-third did not know if it was or not. Of the third who thought it was not fulfilling its purpose, half were patient organisations and half were industry. It could be worth probing stakeholders in greater depth to analyse why the functioning of the forum appeared to depreciate between 2011 and 2012 (but unfortunately this was beyond the scope of the evaluation agreed with the EU Commission).
Regular stakeholder meetings were held (both electronically and face-to-face) and almost three-quarters thought these had been useful. In general, these seemed to be appreciated, were well organised and of a suitable frequency. Suggestions for improvement included having pre-preparation materials sent in advance, a stakeholder chairperson, facilitating more contribution from stakeholders and more suitable agenda items following greater dialogue about setting the agenda.
Stakeholder forum meetings were appreciated and should continue in the EUnetHTA JA2. Improvements could be made, for example by having a more participatory nature, greater dialogue about setting the agenda and considering having a stakeholder chairperson.
Half of respondents had concerns about the principles of stakeholder involvement in the EUnetHTA JA (stakeholder policy and standard operating procedures). Of these five organisations all types of stakeholder were included.
There is a need to review the documents and processes for stakeholder involvement in EUnetHTA JA2.
Nature of being a stakeholder of the European Network for Health Technology Assessment Joint Action
A combination of closed and open questions were included in the questionnaires about what it meant to be a stakeholder in the project: how they had been involved, how their opinions and expertise had been used, if adequate feedback had been received and how involvement of stakeholders could be improved in EUnetHTA JA2.
At the start of the project stakeholders thought their involvement and contributions would include general input and specific HTA knowledge/expertise, attending meetings, being involved in work packages, facilitating experts, sharing information, providing a governance role and disseminating project results. In the interim year, almost three-quarters of stakeholders indicated that involvement in the stakeholder forum had involved what they thought it would, although some concerns centred around balance of the forum’s representativeness. During the project and at the end, they commented how they had been involved. This broadly included the activities they had anticipated, along with being a member of SAGs, participation in the plenary assembly, organising a joint industry response, regular contact with work package leaders, participating in the EUnetHTA JA conference and in discussions about the future of EUnetHTA JA2. However, it was of concern that almost three-quarters had worries about the actual involvement of stakeholders in the EUnetHTA JA1. Half did not think that their organisation’s expertise had been appropriately used. Of these five organisations, two were from industry, two were patient organisations and one was a payer organisation.
Half had concerns about the ‘principles of stakeholder involvement in the EUnetHTA JA’. The majority of stakeholders either had concerns about the commitment of stakeholders in the JA or did not know if they had concerns or not. Of the four who had concerns, two were from patient organisations, one was from industry and one was from providers.
About one-third of stakeholders thought that stakeholders’ views were not adequately considered and the proportion of those who thought that were considered increased from one-third to one-half by the final year. The two organisations which disagreed were industry and patient organisations. Suggestions for improvement included involving stakeholders at an earlier stage in iterative planning, producing summaries of contributions and making the forum more participatory in nature.
There was a sense that stakeholders thought they had much to offer the work of the EUnetHTA JA project but that they could have been involved to a greater degree.
In the interim year, the majority thought that being a member of the stakeholder forum had been a good use of their organisation’s time, but this decreased to two-thirds in 2012. The organisations which thought it had not been a good use of time were both patient organisations. In contrast all organisations who were not participants of the forum had ‘got what it hoped by being a Stakeholder of the JA’ and that it had been a useful use of their time. Participation was seen to confer benefits to stakeholders; increasing general HTA knowledge, recognition for the umbrella stakeholder organisation and ability to comment on documents before publication.
Stakeholder advisory groups were formed for WP4, WP5 and WP7 with the purpose of organising stakeholder input into the work of the EUnetHTA JA. This way of working was apparently well received, with about two-thirds of respondents appreciating this style of involvement. However, the qualitative data showed that concerns included short timelines for responding to consultations and the diverse nature of the stakeholder forum made it difficult to balance points of view.
Developed during the project, stakeholder advisory groups appeared to function well. Concerns, such as short timelines for responding to consultation and the difficulties of obtaining a balance view should be addressed in EUnetHTA JA2.
The stakeholders did not know at baseline whether or not they would be provided with adequate feedback about the project and they suggested that a stakeholder chairperson be appointed to better link them to the project. However, these fears seemed to be unfounded because the majority thought they had received adequate feedback in the interim and final years. There were still some suggestions for improvement, such as that having earlier information about work packages would enable better participation. The one organisation that disagreed was a patient organisation.
The provision of information to stakeholders had generally been adequate. Improvements could be made, for example by providing information earlier, etc.
There was some apparent concern by some members that the forum was a homogeneous group of experts who could be biased (particularly towards the pharmaceutical industry). In the future, efforts must be made to ensure greater participation and representation of all groups’ views.
The different natures, interests and level of influence must be recognised in EUnetHTA JA2. Processes and documents must ensure a participatory nature and inclusion of the views of all relative stakeholders.
Improvement in European network for Health Technology Assessment Joint Action 2
Stakeholders considered that positive involvement had been seen through SAGs, that the diversity of different stakeholder groups on the forum needs to be managed and that earlier involvement of stakeholders, such as in formulating the project objectives and evaluation criteria, would be appreciated. Participants also thought that stakeholders should be involved earlier in the process, that the involvement of stakeholders should prevent bias towards industry, that the forum had been a good mechanism and that communication between project participants and stakeholders should be improved.
There was a sense that stakeholders appreciated involvement in the EUnetHTA JA. However, they felt they had more expertise to offer and this could be facilitated by earlier and more inclusive involvement in work package tasks with longer deadlines for review. Participation could also be helped by an inclusive stakeholder forum that appreciated the heterogeneous nature of stakeholders and valued contributions from all groups equally.
European network for Health Technology Assessment Joint Action 2
The EUnetHTA JA2 project has been formed by a grant agreement with the European Commission. Its general objective is to ‘strengthen the practical application of tools and approaches to cross-border HTA collaboration’. 66 It is notable that the main outcome of the EUnetHTA JA2 will be similar to the overarching objective of the EUnetHTA JA, ‘the implementation of the permanent network for HTA in Europe’. 66 This project will operate from 2012 to 2015 and it will be composed of the following work packages:
-
WP1 – co-ordination
-
WP2 – dissemination
-
WP3 – evaluation
-
WP4 – testing collaborative production of HTA information for national adaptation and reporting
-
WP5 – applying the HTA Core Model for rapid assessment for national adaptation and reporting
-
WP6 – information management infrastructure and services
-
WP7 – methodology development and evidence generation: guidelines and pilots production
-
WP8 – maintenance of HTA Core Model infrastructure to support shared production and sharing of HTA information.
Purpose
The majority of stakeholders, and almost three-quarters of participants, thought the EUnetHTA JA2 would serve as a useful follow-up to the EUnetHTA JA project, with the rest not knowing whether or not it would be. Comments revolved around the fact that EUnetHTA JA2 would benefit from the experiences of the EUnetHTA JA, that it is a necessary follow-up project, that it has a different focus, that further development of tools for HTA is required, there is a need to test the effectiveness of joint report production, this would identify co-operation between agencies and enable examination of the methods developed in actual practice. It was seen as a bridge between the EUnetHTA JA and the permanent network. This seemed to be an indication that the EUnetHTA JA had not completely fulfilled its objectives and that it needed a further joint action project to be able to actually apply the tools in practice.
Concerns
The proportion of project participants who had concerns about the EUnetHTA JA2 project decreased from 2011, when over half had concerns, to 2012, when approximately one-fifth had concerns. Different concerns were noted in the 2 years. The qualitative data showed that concerns in 2010 included worries about an overlap with the current JA, HTA reports, poor definition of the project and political aspects. The qualitative data showed that concerns in 2011 included integration of deliverables, scope of the project and involvement of the correct expertise. Lack of resources was noted in both years. Almost one-third of stakeholders had concerns, which included stakeholders not being sufficiently involved or representation of different stakeholders. These also included commitment of governments to implement outputs and the need to have clear obligations and a timetable. About one-third of project participants had concerns about the actual planning process, which included lack of time in submitting the plans, and problems with the overlap between the two projects. About three-fifths thought it was having an impact on the time available for work on the EUnetHTA JA project. Suggestions for how it could have been better included having more time for preparatory work, a bigger focus on capacity building, greater stakeholder input and incorporating recognised project management techniques.
Lessons learned from the European network for Health Technology Assessment Joint Action
Project participants and stakeholders had different perspectives on what could be learnt from the EUnetHTA JA to inform the next project. Participants recognised the importance of collaboration and communication and contribution of participants, better project planning and budgeting, more accurate scoping of work at the start, information sharing, development of tools and appropriate involvement of stakeholders and experts. Stakeholders felt less complexity and greater transparency was needed, a formalised lessons learned document was required from the EUnetHTA JA to build on and stakeholder involvement should be strengthened.
Involvement of stakeholders
Suggestions for improving the involvement of stakeholders included earlier involvement, need for more balanced representation to avoid industry bias, recognition that they have an important role in validating the tools, sharing deliverables with them, the positive effect of SAGs and to share timelines earlier. This indicates the impression that stakeholder involvement was a developing process; it was recognised as needed from the EUnetHTA 2006–8 project, developed through the EUnetHTA JA project with the establishment of the stakeholder forum and SAGs and needs further evolvement in the EUnetHTA JA2 project. In this respect, it is hoped that the lessons learned from the evaluation of the JA project will be helpful.
It was not possible to progress from the EUnetHTA JA to the permanent network, but a bridging project seemed necessary: the EUnetHTA JA2 project. It is important that lessons are learned from the EUnetHTA JA. These included utilising better project planning and budgeting and stakeholder involvement. Stakeholder involvement is seen as an evolving process; recognised as needed by the EUnetHTA 2006–8 project, developed during the EUnetHTA JA project and requiring further development in the EUnetHTA JA2 project.
Lessons learned from the European network for Health Technology Assessment 2006–8 project
The objective of the EUnetHTA 2006–8 project was:
. . . to establish an effective and sustainable European network of Health Technology Assessment to inform policy decisions. The overall strategic objective is to connect public national health technology assessment (HTA) agencies, research institutions and health ministries, enabling an effective exchange of information and support to policy decisions by member states.
A key part of effective project management is learning from previous experiences, in a quality improvement process. This should ensure that learning is embedded into continuous improvement of project processes. 57 Therefore, it was important to identify the recommendations made in the evaluation report of the EUnetHTA 2006–8 project and to assess whether or not these had been acted on for the EUnetHTA JA. Similarly to the EUnetHTA JA project, one work package was responsible for the internal evaluation of the EUnetHTA 2006–8 projects and delivering a final report. This included the following recommendations ‘for a future sustainable network’67 shown in Box 4 below.
-
Secure funding and maintain a dedicated co-ordinating secretariat.
-
Ensure efficiency through an organisational structure made up of work packages managed by a core of dedicated partners, with less committed partners taking part as a wider review group.;
-
Continue developing and evaluating the tools as necessary and in real settings.
-
Involve people in the work to ensure commitment, a high level of knowledge, and a broad basis for decision-making processes.
-
Encourage collaboration and communication among all parties to ensure coherence within groups and within the EUnetHTA collaboration.
-
Continue developing the communication platform and clearinghouse functionality to make the EUnetHTA collaboration the central reference point for HTA in Europe.
-
Arrange face-to-face meetings at the outset of group or committee work to strengthen social coherence and reach a common understanding of the work.
-
Evaluate the technical communication platform.
-
English has been the main language and should continue to be so.
It is important to note that the recommendations that were made from the evaluation of the EUnetHTA 2006–8 project were for ‘the future sustainable network’ which, at the time of writing the report, was thought to be established in 2009. However, this was not achieved as anticipated directly following the 2006–8 project. Instead, it was necessary to have the EUnetHTA JA project and the EUnetHTA JA2 project to bridge the gap between the initial EUnetHTA 2006–8 project and the sustainable network which is currently expected to be established by 2015.
Secure funding and maintain a dedicated co-ordinating secretariat
The EUnetHTA 2006–8 project was cofunded by an EU grant and the individual participating organisations in a ratio of 1 : 1. For the EUnetHTA JA this was also part funded, in a 1 : 1 ratio from the EU and from Associate partner organisations. 13 As for the EUnetHTA 2006–8 project, the main partner was the Danish Health and Medicines Authority (formerly the National Board of Health). It acted as a dedicated co-ordinating secretariat and led the co-ordination work package (WP1). One aim of this work package was to ensure the submission of technical reports to the EU Commission on time and meeting deadlines of deliverables.
In the EUnetHTA JA project, the co-ordinating secretariat was maintained. Evaluation results showed that approximately three-fifths of project participants thought they had provided effective administration and three-quarters thought their communication by e-mails was adequate. Other activities that could be performed by the secretariat included a greater role in facilitating relationships, providing project management guidance and providing greater feedback about the project. It is suggested that this be considered for EUnetHTA JA2.
Conclusion: Funding was secured for the JA project. The secretariat was maintained as a dedicated co-ordinating support.
Assure efficiency through an organisational structure made up of work packages managed by a core of dedicated partners, with less committed partners taking part as a wider review group
The evaluation of the 2006–8 project concluded that ‘work packages have proven to be a good working model’, while recognising that clarification was needed about how each partner could contribute. The structure of work packages had been shown to be effective by the previous project and is a common method of organising the structure of international projects. 58 Therefore, this structure was chosen again for organisation of the EUnetHTA JA project.
The majority of participants and stakeholders who expressed an opinion in the evaluation of the EUnetHTA JA thought this method of organising the work was positive. However, approximately one-quarter of participants thought there would also be negative aspects associated with this division. Concerns centred around possible overlap between the aims and work of individual work packages and the importance of effective communication between them.
Each work package was led by a designated lead partner organisation and the executive committee brought these organisations together, along with the plenary assembly chairperson and EU representative, every 2 months.
In the EUnetHTA JA project there were some apparent suggested cases of unequal contributions from partners. Going forward to the EUnetHTA JA2 project there are plans to manage contributions by classifying organisations as either ‘active’ or ‘less active’ and managing financial reimbursement accordingly.
Conclusion: The same work package format (common to DG SANCO-funded projects) was used for the EUnetHTA JA. Some concerns about apparent unequal contributions means that organisations will be graded for the level of their activity in the EUnetHTA JA2.
Continue developing and evaluating the tools as necessary
Evaluation of the previous project identified that tools had been produced within the project lifecycle according to the scheduled timeframe. However, it identified that adjustments and further development of ideas was needed and, therefore, tools had not been piloted in a real-life setting (although two pilots had been performed for the HTA Core Model; ‘core HTA on drug-eluting stents’ and ‘core HTA on multislice computerised tomography angiography’). One of the three specific objectives of the EUnetHTA JA project was ‘application and field-testing of developed tools and methods’.
The documentary analysis revealed that most of the 11 project deliverables had been produced by the end of the EUnetHTA JA project. These included the following tools; HTA Core Model on screening, an ‘operational web-based toolkit including database-containing information on evidence generation on new technologies’ and a ‘quarterly communication protocol for information flow on ongoing/planned national assessments of same technologies’. It was predicted that the ‘methodological guidance that will be appropriate for the assessment of relative effectiveness of pharmaceuticals’ would be delivered after the project end, in February 2013.
Although the HTA tools had been further tested in the EUnetHTA JA project there seemed little opportunity to evaluate their use in practice. Therefore, one of the main tasks of EUnetHTA JA2 must be to pilot the tools in actual practice of preparing HTAs.
Conclusion: The HTA tools and methods had been further developed in the EUnetHTA JA project. However, there had been little opportunity to evaluate their use in practice and this appears to be the task of the EUnetHTA JA2 project.
Involve people in the work to ensure commitment, a high level of knowledge and a broad basis for decision-making processes
One of the apparent added values of the EUnetHTA JA project was the opportunity for project participants to network and share information with each other. This contributed to achieving a high level of knowledge within the project.
Some considerable turnover in project participants was seen during the lifecycle of the project and it is important to develop a suite of induction materials to involve new staff in the work quickly and efficiently. In view of this turnover it was of concern that only two-fifths of respondents were aware that their organisation had a sufficient plan to mitigate this in succession planning. Commitment to the work might be affected by lack of staff or resources and careful budgeting and project planning would help this in the EUnetHTA JA2.
Conclusion: One of the added values of participation in the project included information sharing. Commitment to the work was demonstrated by the fact that all deliverables were produced according to the work plan. Potential turnover of staff must be managed and processes implemented to manage resources.
Encourage collaboration and communication among all parties to ensure coherence within groups and within European network for Health Technology Assessment
It is slightly ambiguous what the evaluation considered as ‘all parties’ and ‘groups’. Members seemed to collaborate on the work effectively within the separate constituent work packages. It was notable that one of the main benefits of the EUnetHTA JA project was seen by participants as networking. There seemed to be little evidence of tangible collaboration on HTA during the EUnetHTA JA, although some was performed using the HTA Core Model. More collaboration is planned during the EUnetHTA JA2. Communication within the EUnetHTA JA appeared to be effective and the mechanism most preferred by project participants was face-to-face meetings.
Conclusion: Members appeared to collaborate on the tasks within work packages. There was less evidence on collaboration on HTA reports, but this will be progressed in the EUnetHTA JA2 project. Communication seemed to be effective and face-to-face meetings were the preferred mechanism.
Continue developing the communication platform and clearinghouse functionality to make European Network for Health Technology Assessment the central reference point for HTA in Europe
This was not continued during the JA project.
Conclusion This was not continued in the JA project.
Arrange face-to-face meetings at the start of group or committee work to strengthen social coherence and reach a common understanding of the work
Networking was highly valued by project participants and face-to-face meetings were the preferred means of communication. It has been suggested that such meetings are important in fostering trust between virtual teams of an international project.
Conclusion: Face-to-face meetings seemed highly valued by project participants and should be arranged for the start of group work in the EUnetHTA JA2.
Evaluate the tools in real work settings and the technical communication platform
As discussed previously, the focus of the EUnetHTA JA seemed to be on the development of the tools. The focus of the EUnetHTA JA2 will be on evaluating the tools in real work settings. The technical communication platform of the information management system performed well. The intranet site was used by most members. However, some improvements in the workrooms and for the e-meeting system were required.
Conclusion: There appeared little chance to evaluate the tools in real-life work settings, and this will be the focus of EUnetHTA JA2. The information management system supported the communications of the project well.
English has been the main language and should continue to be so
In an international project it is practical if a common language is used. It was encouraging that the majority of participants had not experienced a serious difficulty with either written or spoken English.
Conclusion: Communicating within the project in English has worked well and this should continue for the EUnetHTA JA2 project.
Conclusions
Nature of the project and the evaluation
-
The EUnetHTA JA was a complex international project with a 3-year lifecycle.
-
This evaluation has contributed to the awareness within the network about types of expertise available in, and HTA information produced by, member organisations.
-
There was a high degree of project participant turnover within the lifecycle of the project. This necessitates the provision of high-quality induction materials.
-
The evaluation methodology of repeat cross-sectional population surveying by online questionnaires worked well. The strategy for optimising response rates was effective.
-
One of the requirements of the EUnetHTA JA project was to have this internal evaluation. However the final evaluation results had to be collated 6 months before the end of the project, which limits the evaluation. To be most effective, final evaluation of a project should be done following its end, to enable assessment of impact.
Project impact
-
The impact of the project was measured by assessing whether or not the deliverables and objectives of the project had been met.
-
The majority of deliverables were produced by the end of the project, according to the final technical reports submitted. The methodological guidance for the assessment of relative effectiveness of pharmaceuticals was predicted to be delivered in the month following the project close (by the end of February 2013).
-
The project’s overarching objective was to ‘establish an effective and sustainable HTA collaboration in Europe that brings added value at the regional, national and European level’. Using the definitions provided by project participants and stakeholders it appeared the JA had not been successful in meeting this objective. The foundations laid by the EUnetHTA 2006–8 and the EUnetHTA JA project will be followed by the EUnetHTA JA2 project before evolving into the permanent network in 2015.
-
The business model and strategy was delivered as planned. This strand of activity is being continued in the EUnetHTA JA2.
-
The HTA tools and methods were produced as planned. It is notable that further development of the HTA Core Model is planned during EUnetHTA JA2.
-
The objective of application and field-testing of HTA tools and methods was apparently not met and this will be further explored in EUnetHTA JA2.
-
Only half of respondents thought the EUnetHTA JA had achieved what they wanted, but a larger proportion had personally benefited from the project. Such added value included networking, information sharing and improved awareness of HTA developments.
-
The tools had not been delivered by the time that the final evaluation was conducted and, therefore, it was too early to assess their quality and use in practice. Project participants had used the tools for communication, including the project intranet. Future benefit was anticipated from use in practice of the HTA Core Model, the POP database and, to a slightly lesser extent, the EVIDENT database. The highest priority for training was the HTA Core Model. Face-to-face workshops seemed to be the training method of choice for these ‘content’ tools. Self-directed training with a manual was preferred for the ‘process’ tools – the project communication tools. There is a need to evaluate the effectiveness of the HTA methodology tools in EUnetHTA JA2.
Project effectiveness
-
Effectiveness was measured by evaluating the processes of the project.
-
The set-up of the project, including structure into eight work packages, seemed to be effective. The need for communication between the work packages was emphasised to prevent duplication of effort and harmonisation of processes.
-
Only about half of respondents thought funding was sufficient and budgeting and project planning processes should be used in EUnetHTA JA2 to help with this. Succession planning within organisations is also essential.
-
The main challenges of being involved in the project were insufficient funding or staffing and conflicts with other work. Other problems included the large project scale and demands of individual work packages.
-
Overall the leadership and administration from the secretariat had been effective. There was some apparent concern about the over-reliance on the project’s leader.
-
The majority of participants had not experienced a significant problem in communicating in English, the official language of the project. The most effective method of communicating was by meeting face to face.
-
There seemed to be a lack of knowledge from participants about the work packages that they were not involved in and this could be improved for the EUnetHTA JA2.
-
The plenary assembly meetings had generally worked well. There seemed to be some problems due to the room lay out in 2011 and lack of involvement of participants in 2012.
-
Involvement of stakeholders seemed to be an evolving process that began in the EUnetHTA 2006–8 project, progressed during the EUnetHTA JA project and requires improvement in the EUnetHTA JA2 project. Analysis of stakeholders’ views was hampered by the fact that not all organisations had completed their questionnaire. All organisations must commit to answer evaluation questionnaires in the EUnetHTA JA2, so that their opinions can be considered.
-
Overall, the stakeholder forum seemed to be an effective means of involving external stakeholders in the EUnetHTA JA. Non-members appeared to have been adequately informed by their designated representative. Stakeholder forum meetings were appreciated and should continue in the EUnetHTA JA2. Improvements could be made, such as by having a more participatory nature, greater dialogue about setting the agenda and considering having a stakeholder chairperson. Participation could also be helped by an inclusive stakeholder forum that appreciated the heterogeneous nature of stakeholders and valued contributions from all types of members equally. There is a need to review the documents and processes for stakeholder involvement in EUnetHTA JA2.
-
Developed during the project, SAGs appeared to function well. Concerns, such as short timelines for responding to consultation and the difficulties of obtaining a balanced view should be addressed in EUnetHTA JA2. The different natures, interests and level of influence of different stakeholder categories must be recognised in EUnetHTA JA2. Processes and documents must ensure a participatory nature of inclusion of the views of all relative stakeholders. There was a sense that stakeholders appreciated involvement in the EUnetHTA JA. However, they felt they had more expertise to offer and this could be facilitated by earlier and more inclusive involvement in work package work with longer deadlines for work review.
-
EUnetHTA JA2 is the follow-up project to the EUnetHTA JA and there was an overlap between the two. It was not possible to progress from the EUnetHTA JA to the permanent network, but a ‘bridging’ project seemed necessary – the EUnetHTA JA2 project. It is important that lessons are learned from the EUnetHTA JA. These included utilising better project planning and budgeting and stakeholder involvement.
Lessons learned from European network for Health Technology Assessment 2006–8 project
Overall the EUnetHTA JA project seems to have used the recommendations made by the internal evaluation of the EUnetHTA 2006–8 project with the following being maintained: a co-ordinating secretariat, structure made up of work packages, development of tools, involvement of participants, development of the communication platform, holding face-to-face meetings and using English as a common language. In addition, there seemed to have been some improvement in involving stakeholders in the EUnetHTA JA compared with the EUnetHTA 2006–8 project, aided by the formation of a stakeholder forum. However, there seemed to be limited progress on collaboration between partners and evaluating the tools in real-work settings. The clearinghouse functionality developed during the EUnetHTA 2006–8 project had been apparently abandoned.
Key project success criteria
Success criteria were developed for the project and have been previously defined. These were individually assessed and comments have been provided in Table 9 .
| Project success criteria | Comments |
|---|---|
| Production of deliverables according to the 3-year work plan and grant agreement | Most deliverables were produced by the end of the project. One was anticipated to be delivered in the month following the project close (February 2013) and was delivered March 2013 |
| Objectives (as defined in the grant agreement) met | More work in EUnetHTA JA2 will enable meeting the overarching agreement of establishing a permanent network. The three subobjectives were met |
| Additional added value generated | Additional value included networking, information sharing and increased knowledge of HTA developments |
| Effective communication within the project | No serious communication problems were reported. Face–to-face meetings were the preferred communication method |
| Effective project administration by the secretariat | Leadership and administration by the secretariat was effective. Possible additional functions were cited by respondents and these could be considered for EUnetHTA JA2 |
| Optimal involvement of external stakeholders | Stakeholder involvement had evolved from the EUnetHTA 2006–8 project with respect to the formation of the stakeholder forum. This can be continued in the EUnetHTA JA2 project, particularly in managing the interests of the heterogeneous stakeholders |
| Good management of the constituent work packages | Overall, work packages seemed to perform well. There were some concerns about WP2 and WP8. There were some intrinsic difficulties in managing of the large number of participants in WP4 and WP5 |
| Progress from the predecessor EUnetHTA 2006–8 project | Recommendations from the EUnetHTA 2006–8 project seemed to have been acted on |
Recommendations
-
The final part of the internal evaluation had to be performed 6 months before the end of the EUnetHTA JA project. For future projects the final evaluation should be performed after the end of the project, thereby allowing assessment of the project’s impact.
-
Owing to the large turnover of participants in the project, it is recommended that an induction pack be produced to orientate new staff.
-
It is important that a valid database of all project participants is maintained by the secretariat and kept up to date. It is also the responsibility of leads or organisations, and work package leads, to provide details of any changes of staff in a timely manner.
-
The evaluation strategy was effective for project participants. If the same inductive and punitive factors are used, it might not be necessary to deliver a third send-out to participants.
-
Half of patient groups and half of industry did not complete their evaluation questionnaires. All stakeholders must commit to answering their evaluation questionnaires in the EUnetHTA JA2 so that their views may be considered.
-
During the evaluation it was possible to identify whether or not deliverables had been produced according to the 3-year work plan. However, it was beyond scope to assess the quality of these deliverables.
-
There is a need to investigate the quality and usability of the HTA methodology tools in real-world HTA practice. There should be an assessment of the quality, usability and cost-effectiveness of the HTA Core Model compared with other methods of HTA report production within the EU. Similarly, the effectiveness and cost-effectiveness of the POP database should be assessed. In regard to the POP database, it is recommended that it be assessed how many collaborations have been undertaken as a result of its use.
-
In future HTA collaborations, the involvement of stakeholders needs to evolve. This process had begun in the EUnetHTA 2006–8 project and developed during the EUnetHTA JA with the establishment of the stakeholder forum. Involvement of stakeholders in the EUnetHTA JA2 needs to expand, particularly in regard to managing heterogeneous stakeholder groups.
-
Some progress seems to have been made since the EUnetHTA 2006–8 project in areas such as refining the HTA tools. However, there has been some suggestion that other aspects (including the communication plan and the business model and application of the tools) need further development. This is planned during the EUnetHTA JA2.
-
The HTA tools had not been delivered by the final evaluation and, therefore, it was too early to assess their use in practice. The majority of respondents saw future use in the HTA Core Model and the POP database. These tools should be evaluated in real-life practice in the EUnetHTA JA2.
-
It is recommended that evaluation of the EUnetHTA JA2 includes consideration about the tangible benefits of networking and information exchange. This could include a case-study approach to demonstrate the practical benefits of networking, such as collaborations initiated and topics identified. Any benefits should be compared with the costs that face-to-face meetings entail.
-
Efforts should be made during the EUnetHTA JA2 to improve the project intranet (the MO website), especially with regard to the workrooms and bulletin boards.
-
Face-to-face training workshops about the HTA methodological tools (HTA Core Model), POP and EVIDENT should be performed in the EUnetHTA JA2. Self-directed training with a manual is more appropriate for the project-focused tools.
-
More time should be factored in for the crucial design stage of large, complicated HTA projects. This enables learning from previous similar projects. It is important that individual participants feel included in this formative stage and communication is clear at the outset. This stage is important in ensuring all participants are clear at the outset and should not be rushed.
-
Consideration could be given to establish a second ‘operational’ committee in the EUnetHTA JA2. This could be made up of an individual worker of each work package and could meet virtually to strengthen links between the subprojects.
-
The greater use of project management and budgeting techniques could be used in the EUnetHTA JA2 to ensure sufficient resources are allocated to organisations and specific tasks.
-
In future international HTA projects all participant organisations should be responsible for outlining who will be locally responsible for an organisation’s commitment to the project if key staff become unavailable.
-
Consideration should be given in future projects to have a deputy leader, possibly based in another country.
-
Various additional activities that could be performed, and it is hoped that in EUnetHTA JA2 with greater funding the activities can be expanded, such as to include support with project budgeting and project management.
-
The most popular form of interaction was face-to-face meetings, and this should be considered in the EUnetHTA JA2. It is important that lessons are learned about how such meetings should be conducted and evaluation of the plenary assembly meetings is important in this respect.
-
External promotion of the project should be improved for the EUnetHTA JA2 project. Strategies that have been suggested include a better public website, advertisement of timelines and achievements and greater use of social media.
-
Overall, the stakeholder forum seemed to be an effective means of involving external stakeholders in the EUnetHTA JA.
-
The objectives of plenary assembly meetings should be made explicit on the agenda before the meeting.
Informing clinical decision-makers about clinical research studies under development: development of a data set to inform a registry
Delphi round 1
Response rate
Thirteen organisations had agreed to participate in the Delphi round. The first-round questionnaire was distributed in October 2010. Following a targeted follow-up strategy, 12 of the organisations responded: a 92% response rate.
Language
All organisations from countries where English is not the common language of everyday use supported English being the main language of the data set.
One-quarter of respondents suggested some kind of native (i.e. non-English) language involvement would be useful. Therefore, this issue was carried forward to the second Delphi Round.
Coding systems
Half of respondents recommended using a coding system/controlled vocabulary in addition to English.
All those who recommended using a coding system supported medical subject headings (MeSH). Overwhelmingly the use of terms was supported over codes. The use of ATC (Anatomical Therapeutic Chemical Classification System) was suggested by one respondent and this issue was carried forward to the second Delphi Round.
Population Intervention Comparison and Outcome
The majority of respondents recommended keeping the intervention and control as separate headings, rather than combining the two. This was seen to confer the advantages of:
-
clarity in the designs of trials, and the use of technologies in different countries
-
searching within the database for technologies which are being compared
-
the absence of comparator indicating that a study may not be comparative.
Contact
All respondents indicated that e-mail is an appropriate default contact method.
Study title and research question
There was a mixed result about whether or not a study’s title should be recorded in a country’s native language as well as English. This issue was carried forward to the second Delphi round.
Unit of registration
Having a unique identifier for each record was essential. This would be further explored in the second Delphi round.
Source of research idea
Giving a fully referenced source for a research idea was recommended by the majority of respondents.
Outcomes
It was recommended that a menu of outcomes was developed (including a subsequent trial or registry number).
Types of other information
Types of other information were recommended and this will be explored in the second Delphi.
Delphi Round 2
Response Rate
The second Delphi Round was sent to the 12 organisations that had responded to the first Delphi round. The questionnaire was sent in November 2010 and a targeted follow-up strategy resulted in 10 organisations responding, giving an 83% response rate.
Language
There had been indication from the first Delphi round that consideration should be given to including native language.
The majority agreed that adding optional non-English-language fields for the study title and research question was appropriate.
Coding system
A respondent had suggested including ATC codes.
Only a minority of respondents were able to provide ATC codes for their projects. Therefore, it was decided not to add a specific field for this. However, it may be appropriate for the database programmer to consider encouraging inclusion of these codes in a descriptive free-text field.
Unique identifier
It had been identified from the first Delphi Round that not all organisations maintain unique identifiers for their projects.
Respondents indicated that it would be suitable for either an organisation to allocate a unique identifier for a project, or the registry would generate one.
Outcomes
A list of outcome possibilities was derived from the responses to the second Delphi round questions.
Study went ahead:
-
type of study (required)
-
funded as a trial (include trial reference number from registry, or similar information)
-
funded as another type of study (include reference number and registry, if any)
-
results likely to be available on <date>
-
study going ahead (if no other outcome appropriate)
-
-
funding arrangements (optional)
-
cofunded with specified public sector funder(s)
-
cofunded with specified private sector funder(s)
-
not cofunded
-
cofunding arrangements not available.
-
Study did not go ahead:
-
lack of clinical need for knowledge
-
lack of researcher capacity
-
competing studies already under way leading to a lack of clinical capacity to deliver the research
-
lack of research funding.
Unit of registration
We suggested the unit of registration should be the project, which may contain one or more substudies in parallel or series. The majority of respondents agreed with this suggestion. Therefore, the unit of registration will be the overarching project, with details of substudies recorded in the summary field.
Other information
-
Based on the results received from the respondents it was decided that an optional summary field would be included in the data set. A country field would also be included, with the possibility of recording multiple countries against this field.
-
All respondents supported the inclusion of the planned country, where a planned study would be undertaken. A country field will be included, with the ability of recording multiple countries against the field.
The process of validating the data set
Respondents were asked what clinical areas should be used to test the data set. Suggestions received from more than one organisation were:
-
trastuzumab for breast cancer
-
transcatheter aortic valve implantation (TAVI) compared with other surgery for aortic stenosis
-
vertebroplasty and kyphoplasty compared with conservative therapy (e.g. physiotherapy, occupational therapy) for compression fractures in osteoporosis
-
bevacizumab for macular degeneration compared with bevacizumab for other indications.
Therefore, these indication and intervention combinations were used for efficacy testing of the data set.
Potential accuracy testing
The Delphi participant organisations were asked to complete the data set for studies they were aware of from their own organisations which may be similar to the exemplar studies listed above.
Both authors reviewed the submitted data and made decisions on whether or not the elements for any pair of studies were sufficiently similar that a funder would consider them a close match. Disagreements were resolved by discussion. Had discussion not resolved disagreements we would have referred the disagreement to an identified third person within our organisation, but this did not arise.
The results of the comparisons are shown in Tables 10–13 below.
| Study | Intended match | Population | Intervention/control | Outcomes |
|---|---|---|---|---|
| Reference Study: UK (PERSEPHONE)68 |
Women with HER-2+ breast cancer | Trastuzumab (different durations) | Quality of life Disease-free survival | |
| Netherlands (real-world efficiency of trastuzumab in early breast cancer) (Benien Vingerhoed Van Aken, ZonMW, 2011, personal communication) | Y | ✓ | ✓ | ✗ |
| France (protocol of herceptin adjuvant with reduced exposure)69 | Y | ✓ | ✓ | ? |
| UK and others (Synergistic Or Long Duration; SOLD)70 | Y | ✓ | ✓ | ? |
| UK [an international RCT to compare TARGeted Intra-operative radioTherapy (TARGIT) with conventional post-operative radiotherapy for women with early breast cancer]71 | N | ✗ | ✗ | ✓ |
| Study | Intended match | Population | Intervention/control | Outcomes |
|---|---|---|---|---|
| Reference Study: UK (IVAN)72 | Patients with wet AMD | Bevacizumab Ranibizumab |
Visual acuity Quality of life |
|
| Netherlands [comparing the effectiveness and costs of bevacizumab to ranibizumab in patients with exudative age-related macular degeneration (COSD)] (Benien Vingerhoed Van Aken, ZonMW, 2011, personal communication) | Y | ✓ | ✓ | ? |
| Netherlands (cost-effectiveness and outcome of current treatment strategies in exudative age-related macular degeneration)73 | Y | ✓ | ✓ | ✓ |
| UK (a multi-centre RCT comparing the efficacy, safety and cost-effectiveness of intraocular telescopic devices compared with standard intraocular lens implants in visual impairment in age-related macular degeneration) (Internal NETSCC data) | N | ✓ | ✗ | ✓ |
| UK (assessment of the effectiveness of the Acrysof natural intraocular lens in retarding the progression of age-related macular degeneration) (Internal NETSCC data) | N | ? | ✗ | ? |
| UK (verteprofin photodynamic therapy for neovascular age-related macular degeneration: cohort study for the UK)74 | N | ✓ | ✗ | ✓ |
| UK [Macular EpiRetinal brachytherapy versus Lucentis Only Treatment (MERLOT). A randomised controlled trial of epiretinal brachytherapy for previously treated neovascular age-related macular degeneration]75 | Y | ✓ | ✓ | ✓ |
| UK (regenerative laser therapy in the treatment of early age-related macular degeneration) (Internal NETSCC data) | N | ? | ✗ | ✗ |
| Study | Intended match | Population | Intervention/control | Outcomes |
|---|---|---|---|---|
| Reference Study: UK (UK-TAVI)76 | Patients with moderate and severe aortic stenosis | Transcatheter aortic valve replacement Open aortic valve replacement |
Quality of life Survival |
|
| Norway [a randomised clinical trials of transcatheter aortic valve implementation compared with surgery in patients with high risk aortic valve stenosis (tentative title)] (Inger Natvig Noderhaug, Kunnskapssenteret, 2011, personal communication) | Y | ✓ | ✓ | ✓ |
| France (medical and economic assessment of aortic valves)77 | Y | ✓ | ✓ | ✓ |
| USA (the PARTNER trial: Placement of AoRTic TraNscatheter Valve Trial)78 | Y | ✓ | ✓ | ✓ |
| Study | Intended match | Population | Intervention/control | Outcomes |
|---|---|---|---|---|
| Reference study: UK (vertebroplasty versus physiotherapy for osteoporotic vertebral collapse) (Internal NETSCC data) | Patients with symptomatic osteoporotic vertebral collapse | Vertebroplasty Kyphoplasty Physiotherapy Usual care |
Quality of life Pain |
|
| Netherlands (percutaneous vertebroplasty in the treatment of osteoporotic vertebral fractures)79 | Y | ✓ | ✓ | ✓ |
| UK [SPinal Osteoporotic Fracture Exercise Trial (SPOFET)] (Internal NETSCC data) | N | ✓ | ✓ | ✓ |
| UK [Local Anaesthetic with BupivacaineE and Lidocaine for vertebral fracture trial (LABEL)]80 | Y | ✓ | ✓ | ✗ |
| UK [a pragmatic randomised controlled trial of screening for osteoporosis in older women (SCOOP)]81 | N | ✗ | ✗ | ✗ |
We then derived the sensitivity, specificity, positive and negative predictive values for each matching rule by constructing a 2 × 2 table of whether or not a project was intended to match the exemplar study on one axis, and whether or not the reviewers considered that it matched the other and then applying a standard technique using the statistical computing package R® (version 2.15.3). 82
Sensitivity and specificity calculations
All figures followed by 95% confidence interval, calculated using the statistical computer package R. 82
Sensitivity and specificity calculations were computed and these are shown in Table 14 . The data set is provided in Table 15 .
| Rule | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) |
|---|---|---|---|---|
| Match at least one element in P(IC)O | 1.0 (0.62 to 1.0) | 0.43 (0.10 to 0.82) | 0.73 (0.54 to 0.92) | 1.0 (0.19 to 1.0) |
| Match at least two elements in P(IC)O | 1.0 (0.62 to 1.0) | 0.57 (0.18 to 0.9) | 0.79 (0.49 to 0.95) | 0.79 (0.49 to 0.95) |
| Match at least three elements in P(IC)O | 0.5 (0.19 to 0.81) | 0.86 (0.42 to 1.0) | 0.83 (0.36 to 1.0) | 0.55 (0.23 to 0.83) |
| Number | Item | Definition | Mandatory | Example |
|---|---|---|---|---|
| 1 | Funder | The name of the funder considering the study | Y | NIHR Health Technology Assessment programme |
| 2 | Contact e-mail of funder | A contact e-mail for the funder | Y | hta@hta.ac.uk |
| 3 | Title | The English title for the study | Y | Multi-centre randomised controlled trial of the cost-effectiveness of intra-inguinal percutaneous transluminal angioplasty (PTA) vs. reconstructive surgery for severe limb ischaemia (BASIL) |
| 4 | Native language title | The native language title for the study, expressed in the preferred language of the funder | Y | Multi-centre randomised controlled trial of the cost-effectiveness of intra-inguinal percutaneous transluminal angioplasty (PTA) compared with reconstructive surgery for severe limb ischaemia (BASIL) |
| 5 | Unique ID | A unique ID for the study. This will be a unique code for the study either provided by the funder or generated by the database. In combination with item 1 it will uniquely identify a study within the database | Y | 96/05/01 |
| 6 | Country | One or more countries where the study is planned to take place | Y | UK |
| 7 | Source of question | Where the question came from, e.g. was it specified by a national policy-making or research co-ordinating body | N | URL of NICE guidance specifying the question |
| 8 | Research question (English language) | The primary research question of the project, expressed in English language | Y | What is the clinical effectiveness and cost-effectiveness of angioplasty compared with surgery in the management of severe limb ischaemia? |
| 9 | Native language research question | The primary research question of the project, expressed in the preferred language of the funder | N | What is the clinical effectiveness and cost-effectiveness of angioplasty compared with surgery in the management of severe limb ischaemia? |
| 10 | P | English-language description of the population eligible for study inclusion | N | Patients with limb ischaemia |
| 11 | P-MESH | MeSH codes or terms for the populations eligible for study inclusion (may need to refer to anatomy and disease process) | Y | Femoral artery (A07.231.114.351) |
| 12 | I | English-language description of the new intervention(s) considered in the study | N | Angioplasty |
| 13 | I-MeSH | MeSH codes or terms for the new intervention(s) considered in the study | Y | Angioplasty (E02.148.050) |
| 14 | C | English-language description of the control intervention(s) considered in the study | N | Surgery |
| 15 | C-MeSH | MeSH codes or terms for the control intervention(s) considered in the study | Y | Blood vessel prosthesis implantation (E04.100.814.868.500) |
| 16 | O | English-language description of the key outcome of interest | N | Mortality, amputation, quality of life, cost-effectiveness |
| 17 | O-MeSH | MeSH codes or terms for the key outcomes of interest | Y | Fatal outcome (E05.318.308.985.550.325) Amputation (E04.555.080) Quality-adjusted life-years (E05.318.740.100.500.700) Cost–benefit analysis (N03.219.151.125) |
It was demonstrated that a registry for matching pragmatic clinical studies under consideration by funding agencies could be built on a very small data set. This would include 10 unique items, of which only five are required to describe a study and the rest are metadata.
By the end of the first Delphi round the participants had agreed on the overall shape of the data set, honing down to the most important information to be contained.
-
demographics/epidemiology – funder, contact details
-
trial specific – title, PICO, outcome.
Discussions had been initiated about the most appropriate use of language; whether entering data in English alone was acceptable or if users who usually worked in other languages should have the option to include data in that language too.
The trade-offs between human-readable language and coding systems for the elements of the data set that would describe the key elements of a study were also discussed. A small selection of coding systems were considered.
In round 2, several smaller issues which had arisen in round one were addressed. This included the way organisations group and refer to their studies internally. By the end of the round the coding system, the role of non-English entries and a menu of possible outcomes to record had been agreed. Another important element of round 2 was establishing clinical and technology areas within which to validate the data set. Responders were asked to suggest areas where they might have appropriate studies to carry out some efficacy testing.
By the end of the second round it was clear that participants agreed about the elements of the data set, so the efficacy-testing phase was initiated. Participants were asked what the characteristics of a good matching algorithm would be. There was general agreement that the best algorithm would minimise false negatives, and that false-positive matches were more acceptable than false-negative ones.
The characteristics of the matching rules assessed vary as might have been expected. The more elements a test tries to match before declaring two studies are similar, the higher the specificity and positive predictive value, and the lower the sensitivity and negative predictive value.
It is likely that the most important characteristic of a future database to a funder is the negative predictive value. The funder wants to ensure that they know about all potentially similar studies, and a high negative predictive value (ideally 100%) would provide this reassurance. They also do not want be flooded with false matches – although we know from the Delphi survey that the groups who responded would prefer false-positive matches to missing potential similar studies – although their attitude may change if any data set implemented is unable to minimise false positives. Unfortunately, as the predictive values of a diagnostic test are dependent on population-level frequencies, it is not possible to calculate these without implementing an actual registry. Useful estimates of positive and negative predictive values can only be obtained from a live database containing real-life examples of studies, so this should be done for any subsequent database which may be developed from this data set.
It was envisaged that such a registry could be built progressively as subscribers query the database. Studies entered to search for possible matches would be entered into the database as well as used for searching. Should a match be identified, then the registry would alert both the organisations who entered the matched studies. Those organisations would then discuss between themselves whether the match was true or false, and what (if anything) to do about it. Using this approach would result in minimal extra work for funders searching for similar studies. It would be useful if they could return when a final decision is made on their proposed study. This would enable entry of information on when the study is no longer under consideration to record what its fate was, and more importantly, so the study is no longer flagged up as a possible match for other organisations. However, there could be a default time to retire a study from matches after a particular time should a data owner not return to update their records. There were insufficient data to decide on efficacy regarding diagnostic studies. Depending on the future use of this data set, it would probably be worth investigating in future in a live database registry.
Overall, it seems that a database based on this data set is plausible. Success will depend on the willingness of the research community to engage with it. It will be necessary to develop matching rules within a live database, but we have suggested some starting points.
Chapter 4 Summary
We describe in detail the activities that the authors led on in the EUnetHTA JA, and that were part-funded by both the NIHR Health Technology Assessment programme and the European Commission.
The impact and effectiveness of the project was evaluated by self-completion questionnaires of project participants and external stakeholders, and documentary analysis. One of the best things was the opportunity for networking with HTA experts from other countries. Many learning points were identified which will be helpful to the follow-up EUnetHTA JA2 project.
A data set was developed to inform the creation of a registry for prospective clinical studies. It is hoped that it will help identification of similar studies that are being planned and enable alignment of outcome measures.
Acknowledgements
The preparation of this report was made possible by a grant from the NIHR Health Technology Assessment programme, whose support is gratefully acknowledged.
The authors would like to thank NETSCC colleagues including Professor Ruairidh Milne, Dr Matt Westmore and Elaine Williams and other members of NETSCC’s International Strategy Group (for advice), Dr Sheetal Bhurke (for some analysis of the section about IT from project participants), Hazel Church and Carole Baker (for booking our travel to the EUnetHTA meetings), Dr Sofia Araujo-Betanco and Rafael Pinedo (for piloting of the language of questionnaires), Stephanie Baker, Jo Fraser and Sarah Clements (for help with preparing the certificates for project participants who completed questionnaires).
Specifically thanks are due to members of the EUnetHTA JA executive committee and representatives from the EU for piloting the questionnaires.
Grateful thanks is also extended to all the project participants and external stakeholders who kindly completed their evaluation questionnaires. Similarly, it is extended to those who contributed to the Delphi process and to respondents who provided details about their planned clinical studies.
Contributions of authors
Eleanor Woodford Guegan (Senior Research Fellow) had primary responsibility for performing the evaluation survey; design of the survey, communication with partners, sending the survey, analysis of results and drafting of the chapters. Andrew Cook (Consultant Advisor) contributed to this process.
Andrew Cook had primary responsibility for performing the data set Delphi exercise; design of the survey, analysis of results and drafting of the chapters. Eleanor Woodford Guegan contributed to this process.
Both authors have reviewed the entire contents of the manuscript.
The sole responsibility for the content of this report lies with the authors and the European Commission is not responsible for any use that may be made of the information contained therein www.eunethta.net
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Stevens A, Milne R, Burls A. Health technology assessment: history and demand. J Public Health Med 2003;25:98-101. http://dx.doi.org/10.1093/pubmed/fdg022.
- Drummond M, Banta D. Health technology assessment in the United Kingdom. Int J Technol Assess Health Care 2009;25:178-81. http://dx.doi.org/10.1017/S0266462309090618.
- Cochrane A. Effectiveness and Efficiency: Random Reflections on Health Services. Leeds: Nuffield Provincial Hospitals Trust; 1972.
- Banta D, Borlum Kristensen F. A history of health technology assessment at the European level. Int J Technol Assess Health Care 2009;25:68-73. http://dx.doi.org/10.1017/S0266462309090448.
- Banta D. Introduction to the EUR-ASSESS Report. Int J Technol Assess Health Care 1997;13:133-43.
- Banta D, Oortwijn W. Health technology assessment and health care in the European Union. Int J Technol Assess Health Care 2000;16:626-35.
- Jonsson E, Banta D. Executive Summary of the ECHTA/ECAHI project. Int J Technol Assess Health Care 2002;18:213-17. http://dx.doi.org/10.1017/S0266462302000235.
- Borlum Kristensen F, Makela M, Allgurin Neikter S, Rehnqvist N, Lund Haheim L, Morland B, et al. Planning, development and implementation of a sustainable European network for Health Technology Assessment. Int J Technol Assess Health Care 2009;25:107-16.
- Lampe K, Makela M, Velasco Garrido M, Antila H, Autti-Ramo I. The HTA Core Model: A novel method for producing and reporting health technology assessments. Int J Technol Assess Health Care 2009;25:9-20. http://dx.doi.org/10.1017/S0266462309990638.
- Turner S, Chase DL, Milne R, Cook A, Hicks NJ, Rosten C, et al. The adaptation of health technology assessment reports: identification of the need for, and development of, a toolkit to aid the process. Int J Technol Assess Health Care 2009;25:28-36. http://dx.doi.org/10.1017/S0266462309990651.
- Turner S, Chase DL, Milne R, Cook A, Hicks NJ, Rosten C, et al. The health technology assessment adaptation toolkit: Description and use. Int J Technol Assess Health Care 2009;25:37-41. http://dx.doi.org/10.1017/S0266462309990663.
- Haheim LL, Imaz I, Loud ML, Gasparetto T, Gonzalez-Enriquez J, Dahlgren H, et al. Internal evaluation of the European network for Health Technology Assessment. Int J Technol Assess Health Care 2009;25:99-106. http://dx.doi.org/10.1017/S0266462309990742.
- European Commission . EUnetHTA Joint Action. 2009 23 02 Description of the Action n.d. www.eunethta.eu/sites/5026.fedimbo.belgium.be/files/EUnetHTA%20JA1%20Technical%20Annex.pdf (accessed 20 October 2013).
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009;59:1125-6.
- Zwarenstein M, Oxman AD. Pragmatic Trials in Health Care Systems (PRACTIHC). Why are so few randomized trials useful, and what can we do about it?. J Clin Epidemiol 2006;87:351-5.
- Brass EP. The gap between clinical trials and clinical practice: the use of pragmatic clinical trials to inform regulatory decision making. Clin Pharmacol Ther 2010;87:351-5. http://dx.doi.org/10.1038/clpt.2009.218.
- Maclure M. Explaining pragmatic trials to pragmatic policymakers. J Clin Epidemiol 2009;59:1125-6.
- McMahon AD. Study control, violators, inclusion criteria and defining explanatory and pragmatic trials. Stat Med 2002;21:1365-76. http://dx.doi.org/10.1002/sim.1120.
- De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Kotzin S, et al. Clinical Trial Registration: A Statement from the International Committee of Medical Journal Editors. N Engl J Med 2004;351:1250-1. http://dx.doi.org/10.1056/NEJMe048225.
- De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Is this clinical trial fully registered? A statement from the International Committee of Medical Journal Editors. Lancet 2005;365:1827-9. http://dx.doi.org/10.1016/S0140-6736(05)66588-9.
- Trastuzumab for 6 Months or 1 Year in Treating Women With Nonmetastatic Breast Cancer That Can Be Removed By Surgery n.d. http://clinicaltrialsgov/show/NCT00381901 (accessed 20 October 2013).
- NIHR HTA programme . Details of HTA Project in Progress: PERSEPHONE – Duration of Trastuzumab Study With Chemotherapy in Early Breast Cancer: Six Versus Twelve Months n.d. www.hta.ac.uk/1607 (accessed 20 October 2013).
- Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, . CATT Research Group . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897-908.
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, . CATT Research Group . Ranibizumab and Bevacizumab for Treatment of Neovascular Age-related Macular Degeneration: Two-Year Results. Ophthalmology 2012;119:1388-98.
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, . The IVAN study investigators . Ranibizumab versus Bevacizumab to Treat Neovascular Age-related Macular Degeneration: One-Year Findings from the IVAN Randomized Trial. Ophthalmology 2012;119:1399-411.
- Desbiens NA. Let medicine lead the way beyond the registration of clinical trials: Registering hypotheses. Am J Med Sci 2008;335:137-40. http://dx.doi.org/10.1097/MAJ.0b013e3181572096.
- Hughes J, Nieuwenhuis L. A Project Manager’s Guide to Evaluation. Evaluate Europe Handbook Series. Bremen: ITB Institute Technology and Education; 2005.
- Weiss CH. Evaluation: Methods for Studying Programs and Policies. Upper Saddle River, NJ: Prentice Hall; 1998.
- Zarinpoush F. Project Evaluation Guide for Nonprofit Organizations: Fundamental Methods and Steps for Conducting Project Evaluation. Toronto, ON: Imagine Canada; 2006.
- Britten N. Qualitative Research: Qualitative interviews in medical research. BMJ 1995;311. http://dx.doi.org/10.1136/bmj.311.6999.251.
- Kitzinger J. Qualitative Research: Introducing focus groups. BMJ 1995;311. http://dx.doi.org/10.1136/bmj.311.7000.299.
- Maysa N, C. P. Qualitative Research: Observational methods in health care settings. BMJ 1995;311. http://dx.doi.org/10.1136/bmj.311.6998.182.
- Keen J, Packwooda T. Qualitative Research: Case study evaluation. BMJ 1995;311. http://dx.doi.org/10.1136/bmj.311.7002.444.
- Gillham B. Developing a Questionnaire. London: Continuum International Publishing Group; 2000.
- Edwards P. Increasing response rates to postal questionnaires: systematic review. BMJ 2002;324. http://dx.doi.org/10.1136/bmj.324.7347.1183.
- Oppenheim AN. Questionnaire Design, Interviewing and Attitude Measurement. London: Continuum; 2000.
- O’Cathain A, Thomas KJ. ‘Any other comments?’ Open questions on questionnaires – a bane or a bonus to research?. BMC Med Res Methodol 2004;4.
- Boynton PM, Greenhalgh T. Selecting, designing and developing your questionnaire. BMJ 2004;328:1312-15. http://dx.doi.org/10.1136/bmj.328.7451.1312.
- Boynton PM. Administering, analysing, and reporting your questionnaire. BMJ 2004;328:1372-5. http://dx.doi.org/10.1136/bmj.328.7452.1372.
- Edwards PJ, Roberts I, Clarke MJ, DiGuiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009. http://dx.doi.org/10.1002/14651858.MR000008.pub4.
- McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Stten N, et al. Design and use of questionnaires: a review of best practice applicable to surveys of health survey staff and patients. Health Technol Assess 2001;5.
- Fowler FJ. Survey Research Methods. Sage Publications; 2002.
- de Leeuw E, Callegaro M, Hox J, Korendijk E, Lensvelt-Mulders G. The influence of advance letters on response in telephone surveys: a meta-analysis. Public Opin Q 2007;71:413-43.
- Parsons C. Web-Based Surveys: Best Practices Based on the Research Literature. Visitor Stud 2007;10:13-3. http://dx.doi.org/10.1080/10645570701263404.
- SurveyMonkey.com n.d. www.surveymonkey.com (accessed 20 October 2013).
- Coons SJ. Responses to Survey Research: Transparency and Representativeness Are Key. Clin Ther 2007;29:466-8. http://dx.doi.org/10.1016/S0149-2918(07)80084-1.
- National Center for Education Statistics . Statistical Standards n.d. http://nces.ed.gov/statprog/standards.asp (accessed 25 August 2013).
- Thorne S. Data analysis in qualitative research. Evidence Based Nurs 2000;3:68-70. http://dx.doi.org/10.1136/ebn.3.3.68.
- Chase D, Rosten C, Turner S, Hicks N, Milne RHt. Development of a toolkit and glossary to aid in the adaptation of health technology assessment (HTA) reports for use in different contexts. Health Technol Assess 2009;13.
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000;32:1008-15. http://dx.doi.org/10.1046/j.1365-2648.2000.01567.x.
- Linstone HA, Turoff M. The Delphi Method: Techniques and Applications 2002. http://is.nijt.edu/pubs/delphibook (accessed 20 October 2013).
- Steurer J. The Delphi method: an efficient procedure to generate knowledge. Skeletal Radiol 2011;40:959-61. http://dx.doi.org/10.1007/s00256-011-1145-z.
- INAHTA . INAHTA. Global Networking For Effective Healthcare n.d. www.inahta.net (accessed 20 October 2013).
- EUnetHTA . EUnetHTA n.d. www.eunethta.eu/ (accessed 25 August 2013).
- Lientz BP, Rea KP. International Project Management. San Diego, CA: Elsevier Science; 2003.
- Turner R, Zolin R. Forecasting Success on Large Projects: Developing Reliable Scales to Predict Multiple Perspectives by Multiple Stakeholders Over Multiple Time Frames. Project Manag J 2012;43:87-99. http://dx.doi.org/10.1002/pmj.21289.
- Cooke-Davies T. The ‘real’ success factors on projects. Int J Project Manag 2002;20:185-90. http://dx.doi.org/10.1016/S0263-7863(01)00067-9.
- Koster K. International Project Management. London: Sage Publications Ltd.; 2010.
- Baskerville R, Nanhakumar J. Activating and Perpetuating Virtual Teams: Now That We’re Mobile, Where Do We Go?. IEE Transact Prof Commun 2007;50:17-34.
- O’Dea NA, de Chazal P, Saltman DC, Kidd MR. Running effective meetings: a primer for doctors. Postgrad Med J 2006;82:454-61. http://dx.doi.org/10.1136/pgmj.2005.042424.
- Cohen MA, Allen JA, Rogelberg SG, Luong A. Meeting Design Characteristics and Attendee Perceptions of Staff/Team Meeting Quality. Group Dynamics 2011;15:90-104. http://dx.doi.org/10.1037/a0021549.
- Harolds JA. Planning and conducting meetings effectively, part II: Some component aspects of a meeting. Clin Nucl Med 2012;37:71-3. http://dx.doi.org/10.1097/RLU.0b013e318238c24b.
- Aubry M, Hobbs B. A fresh look at the contribution of project management to organizational performance. Project Manag J 2011;42:3-16. http://dx.doi.org/10.1002/pmj.20213.
- Varvasovszky Z, Brugha R. How to do (or not to do) . . . a stakeholder analysis. Health Policy Plan 2000;15:338-45.
- Binder J. Global Project Management: Communication, Collaboration and Management Across Borders. Aldershot: Gower Publishing Ltd; 2007.
- EUnetHTA JA2 . Description of the Action 2011. www.eunethta.eu/sites/5026.fedimbo.belgium.be/files/Technical%20Annex1b%20of%20the%20EUnetHTA%20JA%202%20Grant%20Agreement.pdf (accessed 20 October 2013).
- Haheim LL, Iglesia II, Laubli M, Gasparetto T, Gonzalez-Enriquez J, Trofimovs I, et al. EUnetHTA Internal Evaluation Report (2006-2008) 2008. www.eunethta.eu/outputs/eunethta-internal-evaluation-report-2006-2008 (accessed March 2014).
- Trastuzumab in Treating Women With HER2-Positive Early Breast Cancer n.d. www.clinicaltrials.gov/ct2/show/NCT00712140?term=Persephone&rank=1 (accessed 20 October 2013).
- Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 2013;14. http://dx.doi.org/10.1016/S1470-2045(13)70225-0.
- The Synergism Or Long Duration (SOLD) Study n.d. www.clinicaltrials.gov/ct2/show/NCT00593697?term=SOLD&rank=1 (accessed 20 October 2013).
- Comparison of Intra-Operative Radiotherapy With Post-Operative Radiotherapy for Women With Early Breast Cancer (TARGIT) n.d. www.clinicaltrials.gov/ct2/show/NCT00983684?term=TARGIT&rank=1 (accessed 20 October 2013).
- The Effectiveness of Community Vs. Hospital Eye Service Follow-up for Patients With Neovascular Age-Related Macular Degeneration With Controlled Disease n.d. www.controlled-trials.com/ISRCTN07479761 (accessed 20 October 2013).
- van der Reis MI, La Heij EC, De Jong-Hesse Y, Ringens PJ, Hendrikse F, Schouten JS. A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina 2011;38:1449-69. http://dx.doi.org/10.1097/IAE.0b013e3182278ab4.
- Reeves BC, Harding SP, Langham J, Grieve R, Tomlin K, Walker J, et al. Verteporfin photodynamic therapy for neovascular age-related macular degeneration: cohort study for the UK. Health Technol Assess 2012;16.
- Macular EpiRetinal Brachytherapy Versus Lucentis® Only Treatment (MERLOT) n.d. http://clinicaltrials.gov/ct2/show/NCT01006538 (accessed 20 October 2013).
- Abrams K, Altman D, Conroy S, Daniel T, Fancourt G, Gray A, et al. The United Kingdom Transcatheter Aortic Valve Implantation (UK TAVI) Trial n.d. www.nets.nihr.ac.uk/projects/hta/095563.
- Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med 2012;366:1705-15. http://dx.doi.org/10.1056/NEJMoa1114705.
- THE PARTNER TRIAL: Placement of AoRTic TraNscathetER Valve Trial n.d. http://clinicaltrials.gov/ct2/show/NCT00530894?term=The+PARTNER+trial&rank=1 (accessed 20 October 2013).
- Klazen CAH, Verhaar HJJ, Lampmann LEH, Juttmann JR, Blonk MC, Jansen FH. VERTOS II: Percutaneous vertebroplasty versus conservative therapy in patients with painful osteoporotic vertebral compression fractures; rationale, objectives and design of a multicenter randomized controlled trial. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-33.
- Brinjikji W, Cornstock BA, Gray L, Kallmes DF. Local Anesthesia with Bupivacaine and Lidocaine for Vertebral Fracture Trial (LABEL): A Report of Outcomes and Comparison with the Investigational Vertebroplasty Efficacy and Safety Trial (INVEST). Am J Neuroradiol 2010;31:1631-4. http://dx.doi.org/10.3174/ajnr.A2145.
- Shepstone L, Fordham R, Lenaghan E, Harvey I, Cooper C, Gittoes N, et al. A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of screening older women for the prevention of fractures: rationale, design and methods for the SCOOP study. Osteoporos Int 2012;23:2507-15. http://dx.doi.org/10.1007/s00198-011-1876-7.
- R Core Team . R: A Language and Environment for Statistical Computing 2013. www.R-project.org (accessed 25 August 2013).
Appendix 1 Participant questionnaire 2010
Appendix 2 Participant questionnaire 2011
Appendix 3 Participant questionnaire 2012
Appendix 4 Stakeholder questionnaire 2010
Appendix 5 Stakeholder questionnaire 2011
Appendix 6 Stakeholder questionnaire 2012
Appendix 7 Questionnaire for stakeholders not admitted to the stakeholder forum 2010
Appendix 8 Questionnaire for stakeholders not admitted to the stakeholder forum 2012
Appendix 9 Plenary assembly questionnaire
Appendix 10 Plenary assembly questionnaire 2011
Appendix 11 Plenary assembly questionnaire 2012
Appendix 12 Introductory note sent to data set Delphi participants
Appendix 13 First data set Delphi questionnaire October 2010
Appendix 14 Report following first Delphi questionnaire
Appendix 15 Second data set Delphi questionnaire December 2010
Glossary
- HTA Core Model ®
- A methodological framework for shared production and sharing of HTA information. It consists of three components: (1) an ontology containing a set of generic questions that define the contents of a HTA; (2) a methodological guidance that assists in answering questions; and (3) a common reporting structure that enables standardised reporting of HTAs. Information is created and presented as assessment elements. Some elements are prioritised over others to support European collaboration through defining them as ‘core elements’.
- Stakeholders
- Those who have an interest in the project or its deliverables. For the EUnetHTA JA project the external stakeholders were in four categories: industry, patients/consumers, providers and payers.
List of abbreviations
- AMD
- age-related macular degeneration
- ATC
- Anatomical and Therapeutic Chemical Classification System
- COCIR
- European Co-ordination Committee of the Radiological Electromedical and Healthcare IT Industry
- DG SANCO
- Directorate-General of the European Commission for Health and Consumers
- ECPC
- European Cancer Patient Coalition
- EFNA
- European Federation of Neurological Associations
- EGA
- European Generic Medicines Association
- EIFFEL
- EUnetHTA Interface to Facilitate Furthering of Evidence Level
- EPF
- European Patients’ Forum
- EU
- European Union
- EUnetHTA JA
- European network for Health Technology Assessment Joint Action
- EUnetHTA JA2
- European network for Health Technology Assessment Joint Action 2
- EURODIS
- The European Rare Diseases Organisation
- EVIDENT
- the EVIdence DatabasE on New Technologies
- HAS
- Haute Autorité de Santé (French National Authority for Health)
- HTA
- Health Technology Assessment
- INAHTA
- International Network of Agencies for Health Technology Assessment
- IT
- information technology
- MeSH
- medical subject heading
- NETSCC
- NIHR Evaluation, Trials and Studies Co-ordinating Centre
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PICO
- population, intervention, comparison and outcome
- POP
- planned and ongoing projects
- RCT
- randomised control trial
- SAG
- stakeholder advisory group
- TAVI
- transcatheter aortic valve implantation
- URL
- uniform resource locator
- WP
- work package