Notes
Article history
This issue of the Health Technology Assessment journal series contains a project commissioned/managed by the Methodology research programme (MRP). The Medical Research Council (MRC) is working with NIHR to deliver the single joint health strategy and the MRP was launched in 2008 as part of the delivery model. MRC is lead funding partner for MRP and part of this programme is the joint MRC–NIHR funding panel ‘The Methodology Research Programme Panel’.
To strengthen the evidence base for health research, the MRP oversees and implements the evolving strategy for high quality methodological research. In addition to the MRC and NIHR funding partners, the MRP takes into account the needs of other stakeholders including the devolved administrations, industry R&D, and regulatory/advisory agencies and other public bodies. The MRP funds investigator-led and needs-led research proposals from across the UK. In addition to the standard MRC and RCUK terms and conditions, projects commissioned/managed by the MRP are expected to provide a detailed report on the research findings and may publish the findings in the HTA journal, if supported by NIHR funds.
The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by O’Cathain et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
The use of qualitative research with randomised controlled trials (RCTs) in the health field is not new. There are excellent examples and discussions published in the 1990s. 1,2 In the 2000s, the Medical Research Council (MRC) framework for the development and evaluation of complex interventions, although focusing on neither qualitative research nor trials, highlighted the utility of using a variety of methods at different phases of the evaluation process, including qualitative research. 3–5
The contribution of qualitative research to randomised controlled trials
Researchers have internationally described the contribution qualitative research can make to trials in the health field. 2,6–8 These include general statements about ‘harnessing the benefits of trials’ as well as more specific contributions around explaining outcomes or variation in outcomes, verifying outcomes from standardised instruments, explaining how mechanisms work in a theoretical model, developing good recruitment and consent procedures, understanding how an intervention works in practice, drawing attention to outcomes important to patients and practitioners, communicating findings, helping to interpret the trial results, understanding why an intervention was or was not successful, optimising the implementation of an intervention in a pilot trial and challenging the underlying theory of the intervention. Researchers have also described detailed examples of some of these contributions; explicitly showing the value of the qualitative research to the specific trial it was undertaken with. 1,9
Complex interventions
There is an increasing understanding that many of the interventions evaluated in the health field are complex, requiring focus on the fidelity and quality of implementation of the intervention, clarification of the causal mechanisms and exploration of any variation in outcomes. 5 Thus, qualitative research is likely to be essential at some stage of developing or evaluating complex interventions. It may also be useful for simpler interventions such as those in drug trials for which the complexity may not be related to the intervention itself but to the lives of the people who will take the drug or the environment in which the trial of that drug operates.
Context
There is increasing reference in the literature on interventions and trials to the importance of understanding the effect of the context in which an intervention is delivered within a trial10 and the role of qualitative research in facilitating this understanding. 7,11 Those designing interventions need to understand the context in which they will operate and when reporting trials, researchers need to describe the context in which the intervention was evaluated so that users of that research can transfer findings to their own context. 4 Indeed, Hawe et al. 12 describe a ‘process and context evaluation’. Wells et al. 10 explore the role of context in trials rather than the role of qualitative research per se; nonetheless, their study is highly relevant to the role of qualitative research with trials. Through the use of multiple case studies of trials of complex interventions, they identified that context is important to understanding the mechanism and generalisability of complex interventions, for instance, context may shape the intervention and fidelity to the intervention, the scale of the problem the intervention seeks to address may be context specific and practitioners’ enthusiasm for the intervention may affect the quality of the trial as well as the intervention. They also point out that some of the assumptions underpinning trials may be compromised owing to tensions between the ideal of a trial and the reality of implementing it in the real world and propose that the concept of reflexivity would encourage more realistic accounts of trials which may ultimately lead to better understanding of effectiveness studies. This highlights the utility of qualitative research not simply for trials of complex interventions but trials and interventions operating in complex environments.
Process evaluations
Process evaluations have been conducted since the mid-1980s to improve understanding of applied interventions and in particular to consider conditions under which the intervention is effective, for whom it is effective and the key mechanisms by which the intervention works. 13 Linnan and Steckler13 propose that process evaluations can be used for both positive and negative trials to understand which components of the intervention contributed to its effectiveness or to understand why the trial was unsuccessful. They list seven key components of a process evaluation: context, reach, dose delivered, dose received, fidelity, implementation and recruitment. Oakley et al. 14 list a similar range of issues that process evaluations can address alongside trials, including understanding implementation of the intervention, contextual factors that affect an intervention, dose of intervention, variation in outcomes and interpretation of the trial findings. These issues overlap with the contribution qualitative research can make to trials when the qualitative research occurs during rather than before or after the trial. However, it is important to understand that process evaluations can be fully quantitative, fully qualitative or mixed methods, drawing on combinations of surveys, documents, observation and interview and, therefore, they should not be conflated with qualitative research. 14 Because qualitative research can be a key component of process evaluations, and, indeed, Oakley et al. 14 argue that process evaluations should be both qualitative and quantitative, it was important to consider process evaluations when assessing the contribution of qualitative research to trials.
The rationale for the QUAlitative Research in Trials study (maximising the value of QUAlitative Research with Trials)
Researchers have identified the potential value of undertaking qualitative research with trials and there are examples of its application in the literature. 1,9 However, there is a need to take stock of this whole endeavour and consider how qualitative research is being used in practice and whether or not its potential is being maximised. The impetus for the QUAlitative Research in Trials (QUART) study came from a sense that there were problems with gaining value from this work. A member of our team had participated in the commissioning of trials in health care in the UK and raised concerns about how useful the planned qualitative research would be for the trial with which it was being commissioned because researchers sometimes failed to be explicit about the work the qualitative research was doing for the specific trial. For example, qualitative research with the aim of ‘exploring patients’ and health professionals’ experiences of the intervention’ raised the question of how this would enhance, add value or impact on the specific trial it was being commissioned with. This prompted the development of the QUART study, with the aim of assessing the contribution qualitative research undertaken with trials makes to the specific trial. The concern was related to our team’s more general interest in maximising the potential of mixed methods research. 15 However, our perspective in the QUART study was different because, rather than being interested in the whole mixed methods study of a RCT and qualitative research, we were interested in maximising the value of the qualitative research for the specific trial. Our focus was on the utility of the qualitative research,16 viewing qualitative research within an ‘enhancement model’,17 whereby we were interested in how qualitative research enhances the trial, rather than how it makes an independent contribution to knowledge. Flemming et al. 7 describe this as ‘enhancing evidence from trials’.
Existing frameworks for the use of qualitative research with randomised controlled trials
Researchers have described a variety of contributions of qualitative research to trials and intervention studies. Frameworks are useful because they can help novice researchers to learn about these contributions and more experienced researchers to select from the full range of contributions when designing studies. We found two existing frameworks of the use of qualitative research with trials:
-
A temporal framework of the contribution of qualitative research before, during and after a trial. 2,6,8 These authors offer a list of contributions of qualitative research at these three time points of a trial.
-
The MRC framework18 of phases of an evaluation (pre-trial, pilot trial, definitive trial, implementation) has been used by Jansen et al. 11 to document the contribution of qualitative research to the development of community-based interventions in primary care.
Flemming et al. 7 do not put forward a framework but structure their consideration of how mixed methods research can generate evidence for palliative care around study planning, recruitment, conduct and implementation. This draws on a combination of the two frameworks presented above. These frameworks were based on the authors’ research experience, practical examples of the use of qualitative research and trials, and the authors’ perceptions of the potential contribution of qualitative research to trials. We wanted to develop a framework inductively, based on empirical evidence of the contribution made, recognising that the most appropriate framework might be one of the two already in existence.
Focus on specific trials
Qualitative research can be undertaken to explore trials in general. For example, qualitative interviews can be undertaken with key trial stakeholders about strategies to improve recruitment to trials generally. This can be a useful endeavour but was not the focus of the QUART study, for which the interest was the value of qualitative research undertaken with specific trials.
Defining value
In the QUART study, we define the value of qualitative research undertaken with a specific trial as the ‘work’ the qualitative research does for, or the ‘impact’ it has on, that trial. For example, it contributes to optimising the intervention prior to the main trial or it explains the trial findings. However, when we undertook the first component of the QUART study – the systematic mapping review of journal articles reporting the qualitative research – few articles described the impact of the qualitative research on the specific trial. Instead, the authors described the conclusions of the qualitative research, sometimes explicitly articulating the value of the qualitative research for the specific trial or sometimes for future trials or the work that trials are engaged in, namely the provision of evidence of effectiveness of health interventions (we call this ‘the trial endeavour’ within this report). So, although the QUART study focus was on the value of the qualitative research to the specific trial, we had to widen our definition of value when assessing articles reporting the qualitative research. In addition, as the authors of these articles did not necessarily articulate the value for the specific trial or the trial endeavour, or that any value was attained in practice, we sometimes found ourselves having to make a subjective assessment of the ‘potential value’ of that qualitative research.
Maximising value
Jansen et al. 11 identified how the value of qualitative research may not currently be maximised in practice. They studied 33 trials in which qualitative research was used to develop an intervention and identified that it was rarely used to improve or tailor interventions to fit with primary care, so that interventions were delivered as planned rather than adjusted to local circumstances. We took a similar approach to Jansen et al. 11 by considering what researchers were doing and reporting in practice and considering any gaps in this practice that would allow those producing and applying research findings to maximise the value of this qualitative research. We took this approach because there is no agreed consensus on the value that should be attained from undertaking qualitative research with trials.
The boundaries of the QUAlitative Research in Trials study
The QUART study focused on:
-
RCTs in health rather than in other fields such as social or educational research
-
qualitative research undertaken in the context of specific trials rather than the body of research for which qualitative research is used to explore trials as a social process or to explore an aspect of trials more generally
-
the qualitative research component rather than the whole study or the trial
-
all interventions, including complex interventions
-
research published in English.
Aims and objectives
Aim
To identify the range of ways qualitative research is used with specific trials and how to maximise its value to the specific trial.
Objectives
-
To map and quantify the range of ways qualitative research is used with trials.
-
To identify good practice and ways of maximising the value of the qualitative research in this context.
-
To explore researchers’ perspectives on the current practice of combining qualitative research and trials.
Chapter 2 Design and overview of methods
Design
The proposal for the QUART study can be seen in Appendix 1 . We undertook a sequential mixed methods study with four components. First, a systematic mapping review of peer-reviewed journal articles reporting qualitative research undertaken with specific trials; second, a review of the documentation of studies which combined trials and qualitative research; third, a survey of lead investigators of trials that appeared not to have used qualitative research, and these three components were followed by a fourth component of a qualitative interview study of researchers identified from these articles and studies.
Methods
Systematic mapping review of published journal articles
We undertook a database search of peer-reviewed journals between January 2008 and September 2010 for articles reporting qualitative research undertaken with specific trials. The aim was to map the range of contributions of the qualitative research to the specific trial and identify good practice and ways of maximising value.
Review of studies
Not all qualitative research undertaken with trials is published in peer-reviewed journals. 8 We undertook a systematic search of the metaRegister of Controlled Trials (mRCT), a database of registered trials, to identify trials funded in the UK and ongoing between 2001 and 2010 that reported the use of qualitative methods on the trial database. The aim was to explore the potential contribution of the qualitative research to the specific trial and consider good practice in terms of how it was presented in proposals and reports. This also offered insights into recent practice because some studies started in 2010, whereas studies in the mapping review started much earlier than this.
Survey of lead investigators
We anticipated that qualitative research undertaken with trials would not necessarily be described on the mRCT database. Therefore, we undertook a survey of lead investigators of trials with no apparent qualitative research on the mRCT database of registered trials funded in the UK. The aim was to identify ‘invisible’ qualitative research and its contribution to the specific trial.
A qualitative interview study with researchers
We undertook a semistructured telephone interview study with researchers purposively sampled from the first three components of the study. The aim was to explore researchers’ perceptions of how to maximise the value of qualitative research with trials and the facilitators and barriers to this.
Report structure
We report the methods, findings and discussions of each component in separate chapters ( Figure 1 ). Then we integrate these in Chapter 7 and discuss the integrated findings in Chapter 8 .
FIGURE 1.
Mixed methods design as reported in separate chapters.
Public and patient involvement
We held a meeting with two members of the public as part of our public and patient involvement (PPI) for the QUART study. Both were identified because they had been PPI representatives on trials. One person had been an active PPI member of a mixed methods pilot trial and another a PPI member of a drug trial and, it transpired, had also recruited participants into trials as part of their job. We met during the early stages of the mapping review and asked them to read two articles reporting qualitative research undertaken with trials and discuss the content of these articles with the QUART team. They were extremely positive about the need to include qualitative research with trials because, for them, the qualitative research paid attention to the views and needs of people, bringing to the fore the human beings that recruit to, or participate within, trials. This meeting shaped our understanding of the value of the qualitative research and highlighted the need for our team to ensure that we paid full attention to the positive aspects of the research we read.
Chapter 3 A systematic mapping review of published qualitative research undertaken with specific randomised controlled trials
Aim
We undertook a review of peer-reviewed journal articles reporting the qualitative research undertaken with a specific trial in order to map the contribution of aspects of trials addressed by the qualitative research. We then sought to identify good practice and missed opportunities within articles to understand how to maximise the value of qualitative research undertaken with specific trials when reporting the qualitative research in a journal article.
We identified problems with our intended approach early in this process. We had assumed that these articles would explicitly state the contribution the qualitative research had made to the specific trial, for example optimised recruitment processes or explained trial findings. However, we found that this rarely occurred and that the contribution was often directed at future trials and the trial endeavour of generating evidence of effectiveness of health interventions. We also found that authors did not necessarily articulate the contribution and we had to draw this out ourselves. As there was rarely evidence that this value had occurred, we refer to potential value throughout this chapter. In this chapter we address two issues:
-
the aspect of the trial addressed by the qualitative research
-
its potential value.
First, we identified the aspect of the trial addressed by the qualitative research by considering the stated aim of the qualitative research in the article. This was often general rather than specific so we discarded this approach and considered the actual focus of the journal article. For example, a stated aim might be ‘to interview participants who have experienced the intervention in a pilot trial to explore their views of the intervention’ but the focus of the article was ‘identifying problems with the feasibility of the intervention’. Second, we considered the potential value first in relation to the trial and then we attempted to abstract this to consider value to the trial endeavour. For example, the impact on a trial of identifying problems with the feasibility of an intervention might be the intervention was refined for use in the main trial and its abstracted potential value might be ‘optimising the intervention for the main trial’ and ‘saving money by not undertaking a large expensive trial of a suboptimal intervention’.
Methods
Systematic mapping review
We undertook a ‘mapping review’ or ‘systematic map’ of published journal articles reporting qualitative research undertaken with specific RCTs. 19 The aim of this type of review is to map out and categorise existing literature on a particular topic, with further review work expected. Formal quality appraisal is not expected and synthesis is graphical or tabular. We called our review a ‘systematic mapping review’ because labels for different types of reviews are not consistent. 19 This review involved a systematic search for published articles reporting qualitative research undertaken with specific trials and categorisation of the uses made of qualitative research into an inductively developed framework. This was followed by detailed synthesis of articles within each category to identify the potential value and ways in which value had been maximised, followed by synthesis of these issues across all categories. We did not aim to synthesise findings from these studies.
Mapping review search strategy
Our plan was to search for articles published between 2001 and September 2010, which was the last full month prior to our search. We searched the following databases: MEDLINE, PreMEDLINE, EMBASE, The Cochrane Library, Health Technology Assessment (HTA), PsycINFO, CINAHL, British Nursing Index, Social Sciences Citation Index and Applied Social Sciences Index and Abstracts. We used two sets of search terms, one to identify RCTs and one to identify qualitative research, searching for journal articles that included both sets of terms. The search terms for RCTs are well documented. We adapted The Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE. 20 The search terms for qualitative research were more challenging. We started with a qualitative research filter21 but this returned many articles that were irrelevant to our study. We made decisions about the terms to use for the final search in an iterative manner, balancing the need for comprehensiveness and relevance. 20 We tested a range of terms in MEDLINE and held team discussions about the specificity and sensitivity of different terms. The final search strategy is reported in Appendix 2 . We identified 15,208 references, which was reduced to 10,822 after electronic removal of duplicates. We downloaded these references to a data management software program Endnote X5.0.1 for windows (Thomson Reuters, CA, USA).
Mapping review inclusion and exclusion criteria
Our inclusion criteria were English-language articles published between 2001 and September 2010 reporting the findings of empirical qualitative research studies undertaken before, during or after a specific RCT in the field of health. These could include qualitative research reported within a mixed methods article. Our exclusion criteria were:
-
not a journal article (e.g. conference proceedings, book chapter)
-
no abstract available
-
not a specific RCT (e.g. qualitative research about hypothetical RCTs or RCTs in general)
-
not qualitative research
-
not health related
-
not a report of findings of empirical research (e.g. published protocol, methodological paper, editorial)
-
not reported in English
-
not human research.
Screening references and abstracts for the mapping review
We applied the exclusion criteria electronically to the 10,822 references and abstracts. The numbers of references identified increased each year ( Figure 2 ). We do not report 2010 in Figure 2 because we did not search the full year.
FIGURE 2.
Numbers of references identified in an electronic search for qualitative research undertaken with RCTs between 2001 and 2009.
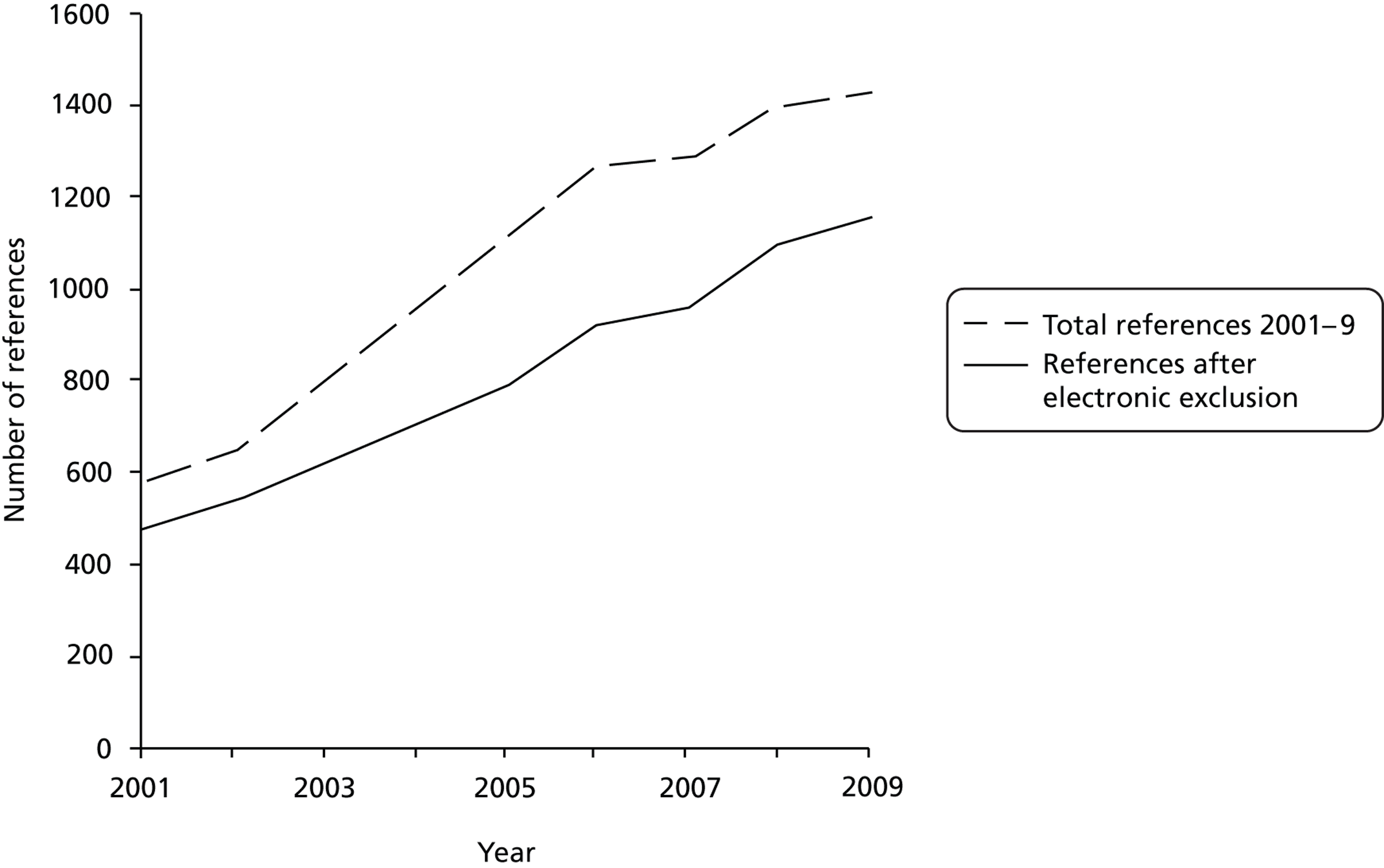
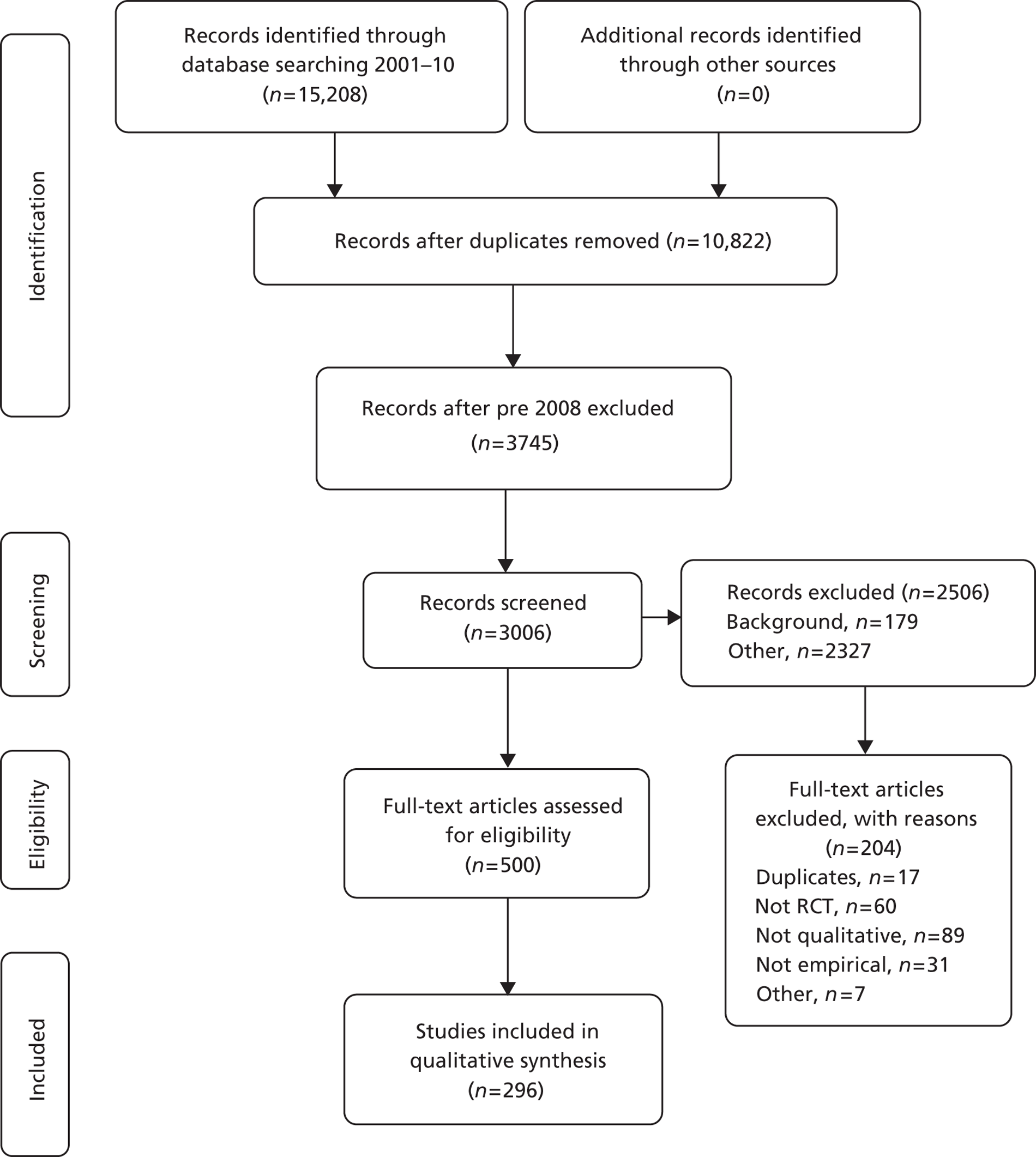
Owing to the large number of references identified, we made the decision to focus on articles published between January 2008 and September 2010. The rationale was that the most recently published articles would offer the most useful insights to future researchers. This resulted in 3745 references and abstracts, with 739 excluded by electronic application of exclusion criteria ( Table 1 ). One of the research team (SJD) read the abstracts of the remaining 3006 references and excluded a further 2506. The most common reasons for exclusion were that the abstract did not refer to a RCT, did not use qualitative research or did not report empirical research ( Figure 3 and see Table 1 ). There were 500 abstracts after this screening process. Two members of the research team (KJT and AO) reviewed a sample of 100 excluded abstracts to check the reliability of the exclusion process. Each read 50 abstracts and placed them into the exclusion categories. Of the 100 abstracts reviewed, all were categorised as exclusions. There was some disagreement about which exclusion category was most appropriate for 16 abstracts. These disagreements were resolved with discussion between team members. Further screening was undertaken on full articles (see Categorisation based on full articles).
| Criterion | Electronic screening of 3745 references | Screening of abstracts of 3006 articles | Screening of 500 full articles |
|---|---|---|---|
| Duplicates | 0 | 8 | 17 |
| Not a journal article | 29 | 2 | 0 |
| No abstract | 65 | 0 | – |
| Not RCT | 2 | 1102 | 60 |
| Not qualitative research | 1 | 609 | 89 |
| Not health related | 2 | 69 | 0 |
| Not empirical | 640 | 708 | 31 |
| Other: not English, not human, full article not found | 0 | 8 | 7 |
| Total exclusions | 739 | 2506 | 204 |
FIGURE 3.
The PRISMA flow diagram for articles 2008–10.
Preliminary categorisation of abstracts using the stated aim of the qualitative research
We did not set out with a plan of how we would develop our map or framework. As we proceeded, we realised that the approach we were taking was similar to the five stages of ‘framework analysis’, an approach to analysing qualitative data. 22 The stages of framework analysis are ‘familiarisation’ with the data, ‘identifying a thematic framework’, ‘indexing’ by applying the framework systematically to the data, ‘charting’ by putting data related to a theme together and ‘mapping and interpretation’ to think about the meaning of the findings in relation to original purpose of the research.
As a starting point for the categorisation of qualitative research we undertook the first three stages of ‘framework analysis’. We familiarised ourselves with the articles by reading a small set of abstracts (n = 122). We identified a thematic framework by using the stated aim of the qualitative research within the abstract to identify categories and subcategories of a ‘preliminary framework’. We also considered the potential of the temporal framework6,8 and the MRC framework for the evaluation of complex interventions3 as our overarching framework. We found that the information given in the abstracts did not include details necessary for mapping onto these frameworks. After team discussions, we finalised our preliminary framework and one team member (SJD) ‘indexed’ by applying it to the stated aim of the qualitative research in our 500 abstracts, open to emergent categories which were then added to the framework ( Table 2 ). The purpose of this initial categorisation was to create meaningful categories to enable a second, more in-depth, process of categorisation at a later stage.
| Category | Subcategory | Numbers of abstracts |
|---|---|---|
| Intervention | Patient views of intervention in RCT | 114 |
| Professional views of intervention in RCT | 35 | |
| Develop the intervention | 94 | |
| Build explanatory model of how intervention works | 12 | |
| Factors influencing intervention delivery | 19 | |
| Evaluation of intervention | 38 | |
| Design and conduct | Recruitment for RCT | 50 |
| Being part of trial | 25 | |
| Would patients participate in trial? | 19 | |
| Trial design (acceptability of some designs) | 11 | |
| Outcomes | Variation in outcomes | 14 |
| Accuracy of outcome measures | 15 | |
| Additional outcomes following trial | 22 | |
| Outcome tool development | 18 | |
| Disease | Patient views of disease | 20 |
| Professional views of disease | 5 | |
| Dissemination | Dissemination of trial results | 4 |
| Unclassifiable | Do not know | 10 |
Categorisation based on full articles
We obtained copies of full articles for the 500 included abstracts. All members of the team read articles from across the categories before selecting a category to lead on and engaging in a process similar to framework analysis:
Step 1: familiarisation – we read the full articles in the category, including those that had been moved from another category by other team members.
Step 2: further exclusion – we excluded some articles on further reading, for example those that did not report qualitative research. Application of exclusion criteria became challenging and these challenges are discussed in Challenges applying the framework. We excluded another 204 articles at this stage (see Table 1 and Figure 3 ).
Step 3: identification of a thematic framework through recategorisation and development of categories and subcategories – we recategorised any articles that, after full reading, did not appear to fit into the allocated category or subcategory. We considered whether subcategories were right and changed their titles, their order in the category and added or collapsed subcategories. We held in-depth discussions about a number of articles to help us define the unique characteristics of a particular subcategory. At this stage we felt that the preliminary categorisation based on the stated aim of the article did not necessarily describe the focus actually taken by the qualitative research presented in the article. For example, articles that were originally categorised as ‘patient view of intervention’ were put into new categories such as identifying the ‘perceived value and benefits of intervention’. This recategorisation stage required a considerable amount of discussion between team members, with all members reading abstracts and articles, and discussing disagreements in terms of allocating articles to categories.
Step 4: ‘indexing’ or allocation of articles – each article was allocated mainly to one subcategory but some had more than one focus and were categorised into two or more subcategories.
Step 5: data extraction or ‘charting’ – we undertook formal data extraction for up to six articles from each subcategory. For subcategories with more than six articles, we randomly selected articles with the exception of the ‘intervention’ category. Because this category had very large subcategories, we undertook purposive sampling to ensure we displayed the range of articles within each subcategory. During data extraction, we described authorship, the qualitative methods, the RCT, the intervention, the stated aim of the qualitative research, the aspect of the trial addressed by the qualitative research and the potential value of the qualitative research for the trial endeavour (see Step 6). Reflections on good practice and ways in which the value of the qualitative research for RCTs could have been maximised were largely based on these data extractions of six articles per subcategory. However, these reflections were also informed by our wider reading of all the articles within that subcategory. This step was similar to the ‘charting’ stage of framework analysis for which data within a theme are studied in depth. Matrices of subthemes and interviewees are created in the standard approach to framework analysis. We created figures based on descriptive data and display them in the findings.
Step 6: identifying potential value for articles in the data extraction – we had intended to extract the value of the qualitative research to the specific trial. During team discussions, we identified that the value of the qualitative research was not necessarily explicitly articulated within the articles. When it was not explicit, we made a subjective assessment of potential value based on reading the article and our own subjective assessment of issues we had identified in background reading in Chapter 1 . In practice, authors of these articles implicitly discussed value through how they framed the article in the introduction and the issues they alluded to in the discussion. We considered these to be potential value rather than actual value because authors rarely evidenced impact that occurred in practice. We developed a tick box set of values for the data extraction form but mainly used a free text box to record potential value.
Step 7: identifying exemplars – for each subcategory, we attempted to identify an article that we judged as undertaking that type of work well. Key considerations were how explicit authors were about the impact of the qualitative research on the specific trial or the message for the wider trial endeavour and the depth of insights described in the article.
Step 8: describing the type of intervention – finally, two members of the team worked together to categorise the intervention for articles in the data extraction (AO and KJT). Each independently categorised the intervention into ‘drug or device’ or ‘complex intervention’. This was a crude categorisation based on a brief description of an intervention in the qualitative article. Our approach was to identify RCTs of drugs and devices (including surgery and acupuncture) and then categorise any others as complex interventions because of the lack of detail required to classify an intervention as complex with any confidence. We then compared our categorisations and discussed disagreements to reach consensus.
Challenges applying the framework
We met four key challenges during this categorisation process and resolved them during team discussions as follows.
Maintaining perspective
Qualitative research generates knowledge and has value outside the context of RCTs. As stated earlier, our goal was to categorise the contribution of qualitative research to the trial endeavour. This felt uncomfortable at times because we were ignoring the strengths and value of qualitative research in its own right. We resolved this difficulty by ensuring that we were transparent about the perspective we were taking.
Defining ‘specific randomised control trial’
We met three challenges here. First, some trials were referred to as a ‘clinical trial’ or ‘trial’ and it was not clear if they were RCTs; therefore, we took a generous approach to including these articles. Second, sometimes the qualitative research was undertaken regarding a set of trials rather than a specific trial, for example a programme of trials on asthma in a single medical centre involved in trial recruitment. We did not want to include the vast literature for which qualitative research is used to explore different aspects of trials in general but decided to include articles based on sets of trials about a specific disease or patient group, for example undertaken in a neonatal trials unit, because of the possibility that the qualitative research could impact on that set of trials. Third, we were clear that hypothetical trials were excluded but made an explicit decision to include any hypothetical trials we identified in our two ‘in principle’ subcategories in which there was evidence of a commitment to undertake a trial if the intervention or trial design were acceptable ( Table 3 shows final categories including ‘acceptability of the intervention in principle’ and ‘acceptability of the trial in principle’). Therefore, there was some blurring of our boundaries and in data extraction we identified these as ‘grey’ articles.
| Category | Subcategory | n (%) |
|---|---|---|
| Intervention content and delivery | 254 (71) | |
| Intervention development | 48 (13) | |
| Intervention components | 10 (3) | |
| Models, mechanisms and underlying theory development | 23 (6) | |
| Perceived value and benefits of intervention | 42 (12) | |
| Acceptability of intervention in principle | 32 (9) | |
| Feasibility and acceptability of intervention in practice | 83 (23) | |
| Fidelity, reach and dose of intervention | 12 (3) | |
| Implementation of the intervention in the real world | 4 (1) | |
| Trial design, conduct and processes | 54 (15) | |
| Recruitment and retention | 11 (3) | |
| Diversity of participants | 7 (2) | |
| Trial participation | 4 (1) | |
| Acceptability of the trial in principle | 5 (1) | |
| Acceptability of the trial in practice | 4 (1) | |
| Ethical conduct | 16 (4) | |
| Adaptation of trial conduct to local context | 2 (1) | |
| Impact of trial on staff, researchers or participants | 5 (1) | |
| Outcomes | 5 (1) | |
| Breadth of outcomes | 1 (< 1) | |
| Variation in outcomes | 4 (1) | |
| Measures | 10 (3) | |
| Accuracy of measures | 7 (2) | |
| Completion of measures | 1 (< 1) | |
| Development of measures | 2 (1) | |
| Condition | Experience of the disease, behaviour or beliefs | 33 (9) |
| Total uses | 356 (100) | |
Defining ‘qualitative research’
We could not rely on the article authors’ use of the term ‘qualitative research’; for example, some authors called surveys a qualitative method. We defined qualitative research as qualitative data collection (interviews, focus groups, observation, documents) and analysis (textual analysis, usually supported by quotes). We found many articles based on open questions in surveys or unstructured interviews reduced to quantitative findings reported as percentages. We generally excluded these but found it difficult to decide on this boundary for some articles. For example, an open question on a survey resulted in a detailed textual analysis so we included it. Again, there was some blurring of our boundaries and in data extraction we identified these as ‘grey’ articles.
Distinguishing the contribution of the qualitative research from other methods and approaches
Some articles reported mixed methods research and we had to work hard to distinguish findings based on the qualitative rather than the quantitative research. We felt we were generally successful but that it was much harder to distinguish the contribution of qualitative research within articles reporting participatory action research.
Quality appraisal
Quality appraisal is not essential for mapping reviews. 19 We stated in our proposal that we would consider quality but our aim was not to judge the quality of the qualitative research. However, we did commit to explicitly reviewing our exemplars using accepted quality criteria. We revisited this during the study and discussed the benefits of either applying a quality assessment checklist to all articles or to only those we selected as exemplars. We identified the Critical Appraisal Skills Programme checklist for the appraisal of qualitative research as a simple and well-regarded checklist. 23 We decided not to apply it to all 296 articles for two reasons. First, the methodological quality of these types of articles has already been assessed for 30 qualitative studies undertaken with RCTs. 8 That study found that there was not sufficient information in one-third of these 30 studies to assess methodological quality and considerable weaknesses were found for the rest, including inadequate description of sampling and analysis. Second, our interest was different – we wanted to assess quality defined as the contribution the qualitative research made to the trial endeavour and identify quality issues specific to this. We decided not to apply quality criteria to our exemplars because we wanted the keep the focus on quality in terms of the impact of the qualitative research on the specific trial or the message for the wider trial endeavour.
Synthesis within and across subcategories
We identified good practice and ways of maximising value within each subcategory by reading the articles and identifying issues that were present in some articles and missing from others. Then we asked questions within the whole subcategory: why did researchers do the qualitative research? Did it seem like a useful thing to do? What did some researchers do that felt helpful? What could have been done to improve the value of the research for the trial endeavour? Finally, we identified commonly occurring themes across the subcategories. This was similar to the final stage of framework analysis, ‘mapping and interpretation’, in which researchers consider relationships between themes in the context of the original research question.
Findings
We present the findings in four sections:
-
framework of the use of qualitative research with trials
-
detailed description of framework subcategories with examples
-
the potential value of qualitative research with trials
-
the rationale for the QUART study (maximising the value of QUAlitative Research with Trials).
Framework of the use of qualitative research with trials
Size and characteristics of the evidence base
We identified 296 articles published between January 2008 and September 2010. The references of these articles are listed in Appendix 3 . There was no evidence of increasing numbers per year in this short time period: 113 articles in 2008, 105 in 2009 and 78 in the first nine months of 2010 (equivalent to 104 in a full year).
Most of the first authors were based in the USA (107 articles) and the UK (97 articles), with others based in Scandinavian countries (21 articles), Australia (20 articles), Canada (15 articles), South Africa (seven articles), New Zealand (five articles), Spain (five articles) and a range of other countries in Africa (six articles), Asia (six articles) and Europe (seven articles). The journals these articles were published in are listed in Appendix 4 .
Framework of the focus of the qualitative research
As we read the full articles and discussed the content within the team, the framework presented in Table 2 developed into five broad categories related to:
-
the intervention being tested
-
the trial design and conduct
-
outcomes
-
measures used within the trial
-
the condition studied in the trial.
Subcategories also developed further; for example, the subcategories of ‘patient views of the intervention’ and ‘professional views of the intervention’ disappeared because we were interested in the insights offered by these views for the trial. For example, we developed a subcategory of ‘acceptability of the intervention in principle’ that was always based on exploring the views of patients and/or professionals. At this stage, some articles were reclassified to a different category when the focus of the full article conflicted with the focus described in the abstract. Some articles were classified into two or more subcategories, usually within the same category. We identified 22 discrete subcategories within the five broad categories and identified 356 incidents of these within the 296 articles ( Table 3 ).
Distribution within subcategories
The majority of the articles focused on the intervention (66%, 196/296), with few articles focusing on measures and outcomes (see Table 3 ). This imbalance between categories may reflect practice or may be due to some activities undertaken in relation to trials not being published or not being identified by our search strategy. The subcategories that each of the 296 articles were allocated to are shown in Appendix 3 at the end of each reference.
Timing of the qualitative research
A total of 28% (82/296) of articles reported qualitative research undertaken at the pre-trial stage, i.e. as part of a pilot, feasibility or early-phase trial or study in preparation for the main trial. Some activities would be expected to occur only prior to the main trial, such as intervention development, and all of these articles were undertaken pre-trial. However, others that may also be expected to occur prior to the trial, such as ‘acceptability of the intervention in principle’, occurred frequently during the main trial. This is discussed in more detail in the next section on the individual subcategories (see Detailed description of framework subcategories with examples).
It was not possible to categorise articles using the temporal framework before, during and after the trial6,8 because it was unclear in many articles when the data collection for the qualitative research was undertaken in terms of during or after the trial. Sometimes data collection was undertaken after the intervention ended, after the last outcome was measured, or after the trial findings were known. Sometimes the data analysis was undertaken at these different stages based on data collected during the trial. Often it was not clear precisely when the data collection or analysis was undertaken.
Detailed description of framework subcategories with examples
In this section we explore each of the 22 subcategories identified in Table 3 in detail. We based this on reading all the articles and the detailed data extraction on up to six articles per subcategory and extracting data on 104 articles in total. These subcategory assessments were subjective, inductive and emergent, shaped by the articles we found and by our values and interests. Within each subcategory we:
-
describe the subcategory
-
give examples of articles within it (see the relevant table within each subcategory)
-
identify which of the examples focused on drugs or devices and which on complex interventions (see the relevant table within each subcategory)
-
indicate an exemplar in the figure of examples
-
explore the potential value of using qualitative research to this purpose
-
offer suggestions for good practice.
Category 1: content and delivery of the trial intervention
The first category focused on the intervention being tested in the trial and was by far the largest category, accounting for 254 of the 356 uses of the qualitative research (71%). Qualitative research was used to explore eight aspects of the content and delivery of trial interventions from development through to implementation in the real world (see Table 3 ):
-
intervention development
-
intervention components
-
models, mechanisms and underlying theory development
-
perceived value and benefits of intervention
-
acceptability of intervention in principle
-
feasibility and acceptability of intervention in practice
-
fidelity, reach and dose of intervention
-
implementation of the intervention in the real world.
Development of the trial intervention
Qualitative research to support intervention development may be undertaken as part of a formal evaluation framework that advocates the use of theoretical and feasibility modelling prior to testing an intervention in a trial,3 or it may be undertaken without a formal framework, with the clear intention to create, or refine, all or part of an intervention for testing within a trial. Our criterion for including studies in this subcategory was that the qualitative findings reported substantive development of the intervention content or delivery. We expected these interventions to be complex interventions involving behavioural aspects of care delivery or receipt. We identified 48 articles, all of which included qualitative research undertaken prior to a planned main trial. Many of these studies used mixed methods research and some described other pre-trial work in conjunction with the intervention development, such as testing possible outcome measures for use in the full trial and trial design issues such as recruitment and retention. We selected six examples purposively to include a range of interventions: clinical, educational and professional. These are described in Table 4 . The focus of the qualitative research was always clearly related to the specific trial and the qualitative methods included interviews, focus groups and non-participant observation. The research subjects were drawn from members of the public, potential trial participants and professionals involved in the delivery of the proposed intervention.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for the specific trial and the trial endeavour |
|---|---|---|---|---|---|
| Davies et al. 200924 | USA | Community-based pragmatic RCT comparing health/social support and parenting interventions for mothers living with HIV (C) | To ensure the intervention met the cultural and situational needs of the target recipients in terms of content and delivery process | Semistructured interviews with seven service providers and two focus groups with clinic patients and clinic advisory group members | Several intervention elements were developed in direct response to the qualitative findings. At time of publishing, the full trial was under way. Strengthens trial relevance by ensuring intervention is culturally appropriate and sensitive to the needs of recipients, carers and those delivering care |
| Gulbrandsen et al. 200825 | Norway | Pragmatic RCT of ‘Four Habits’ a clinical communication tool designed and evaluated in USA for use in Norway (C) | As part of mixed methods research to identify ways to tailor the intervention content to meet the needs of local health-care practice | Three focus groups with local physicians who had been given the intervention training | Confirmed cultural alignment and informed elements of the training programme for use in the planned trial. Strengthens trial relevance by ensuring intervention is sensitive to the needs of those delivering care and locally appropriate. Improves internal validity of trial by reducing the risk of poor implementation affecting the effectiveness of the intervention |
| Jefford et al. 200826 | Australia | RCT of standard correspondence compared with tailored chemotherapy information for GPs (C) | To identify information needs of GPs treating patients receiving chemotherapy | Focus groups (10 GPs as intervention recipients) and semistructured interviews | Directly informed information content and mode of communication in the trial. Strengthens trial relevance by optimising intervention in line with perceived needs of recipients. Engenders stakeholders’ support for the trial |
| Marciel et al. 201027 | USA | Planned multicentre RCT of mobile telephone intervention to improve adherence to treatment by adolescents with cystic fibrosis (C) | As part of mixed methods research to refine the intervention taking account of patient and provider perspectives | Focus group with 17 health-care professionals and semistructured interviews with 18 patients and 12 parents | Changes to the intervention delivery as a result of the focus group concerns. Interviews confirmed acceptability of intervention. Strengthens trial relevance by maximising cultural appropriateness of the intervention. Strengthens trial relevance by optimising intervention in line with perceived needs of recipients, carers and those delivering care. Engenders stakeholders’ support for the trial |
| Nagel et al. 200928 | Australia | RCT of a brief intervention for indigenous people with chronic mental illness and substance dependence (C) | To understand local perspectives of mental illness and mental health to develop a culturally adapted brief intervention | Group and individual interviews, and observation, within a participatory action research model engaging local aboriginal health workers and recovered patients. This continued alongside the trial | Incorporated into the design of the intervention but clear evidence of the process for this not provided in this paper, which reports the trial results. Strengthens trial relevance by maximising cultural appropriateness of the intervention. Strengthens trial relevance by optimising intervention in line with perceived needs of recipients, carers and those delivering care. Engenders stakeholders’ support |
| Redfern et al. 200829 | UK | Planned RCT of prevention advice to improve risk factor management after stroke (C) | Part of mixed methods research exploring patient experiences and understanding of secondary prevention and observational work to understand context and process | 20 semistructured interviews with potential intervention recipients. Non-participant observation at two hospitals to explore delivery, process and context | Development phase of MRC framework. Strengthens trial relevance by optimising intervention in line with perceived needs of recipients. Improves internal validity of trial by reducing the risk of poor implementation affecting the effectiveness of the intervention |
Intervention development undertaken in a pre-trial context can strengthen the relevance of a trial by optimising the intervention and/or its delivery from the perspective of recipients and providers. The rationale for this use of qualitative research fell into three categories:
-
The need to optimise an existing intervention that has some clinical or practice history in order to ensure that the ‘best’ intervention is tested in the trial. Multiple versions of an existing intervention may be in use with no identifiable ‘gold standard’ and the problem may be presented as needing to develop an optimised intervention.
-
The need to adapt an existing intervention for use in a different context:
-
– A new clinical or practice context, for example a similar programme of care for a different patient group or a hospital service delivered in primary care.
-
– A different geographical context for which the goal is to adapt a successful intervention developed in one country for use in another country where health services and health-care delivery differ (e.g. Gulbrandsen’s ‘Four Habits’25).
-
– A different cultural context that may require adapting a mainstream intervention for use with a specific minority group or subgroup of a clinical population (e.g. Nagel’s Torres Strait Islanders28 or Marciel’s adolescents with cystic fibrosis27).
-
-
The need to develop a ‘de novo’ intervention, based on perceived clinical or practice need and, often, a theoretical understanding of the nature of the required intervention. 26,29
The driver for undertaking qualitative research in this context was not clinical appropriateness, but rather the need to explore the intervention as part of a matrix of health-related social behaviour on the part of patients, carers, health professionals or communities. That is to say, articles described participants in terms of their social and behavioural characteristics and explore experiences and beliefs from the perspective of a community of recipients or carers, a community of professionals delivering the intervention, or a particular context of delivery (primary care, community care, web-based care). Key to this was the way in which the research participants were defined and the issues particular to the group that were predicted to be relevant. Examples of this included women living with human immunodeficiency virus (HIV) who were also fulfilling the role of mothers,24 people with mental health problems who were also part of a minority indigenous group with specific cultural characteristics,28 or physicians who delivered care within the context of a particular national culture and its health-care system. 25
In order to achieve the research goal of identifying the best possible (or most appropriate) intervention for testing in a full trial, the need to attend to the health-related behaviours and beliefs of relevant groups (to be accessed via their experiences and perspectives) was the starting point and rationale for most of the qualitative research undertaken in this subcategory, whether the intervention was being designed de novo, being optimised or being adapted for use in a new context. In addition, some studies reported the importance of qualitative research in the development phase of a trial in terms of the opportunity to engender ‘stakeholder support’ for the trial process. 26–28 This was described as operating both at an individual level (patient and professional) and at a community level. 26–28
-
Be clear that changes were made to the intervention
-
In some cases, the qualitative findings resulted in a demonstrated change being made to the intervention content or delivery. In others, confirmation of acceptability was the real objective of the study. Good practice would entail making a clear distinction between these two aims within articles.
-
-
Ensure changes to the intervention are grounded in the data
-
When the aim of the qualitative research was to develop the intervention for a planned trial, it was important for researchers to demonstrate the mechanisms by which the qualitative data influenced the final intervention design. For this to happen it was necessary for the changes to be convincingly grounded in the data. This did not always occur, perhaps owing to limited article word count because in papers reporting the development of the intervention alongside the trial results, very little space was allocated to the qualitative component and its impact was usually reported rather than demonstrated.
-
-
Place within an evaluative framework
-
The MRC framework for the development and evaluation of complex interventions offers endorsement for the practice of undertaking qualitative research to support intervention development. 3 A conceptual framework for the qualitative research, such as the MRC framework, was not used in the majority of articles in this subcategory. One advantage of using such a framework may be to raise the profile and status of the qualitative research contributing to intervention development and to ensure that its contribution is documented. For example, referencing and using the MRC framework, the authors of one paper were able to claim that their goal was the development of ‘the definitive risk-factor management intervention’. 29
-
Describing the trial intervention and elaborating its components
In this subcategory, the qualitative research supported a description of a trial intervention, or one or more components of a complex intervention. We included articles for which qualitative methods were employed to generate essential data for the description, usually taking into account recipients’ experiences during the trial. These descriptive accounts could be based on mixed methods research, combining qualitative data about patients’ experiences with quantitative data relating to satisfaction measures. We identified 10 articles, all undertaken at the full trial stage. Some of them also reported qualitative research undertaken to meet other objectives such as assessing the acceptability of the intervention. We selected six examples purposively to include a range of interventions: clinical, educational and professional ( Table 5 ). The qualitative methods included interviews and audio recordings of consultations. The research subjects were drawn from members of the public, intervention recipients, and professionals involved in the delivery of the intervention.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for the specific trial and the trial endeavour |
|---|---|---|---|---|---|
| Foster et al. 201030 | USA/UK/Uganda | Cluster RCT of home-based antiretroviral therapy compared with facility-based antiretroviral therapy (DD) | As part of mixed methods research, to describe one component of the intervention in both arms of the trial (the use of medicine companions to encourage adherence) | Longitudinal interviews with 40 participants from both arms | Described the role of medicine companions as a component of the intervention from the perspective of participants. Describes an over-looked or taken-for-granted component of a complex intervention. Informs intervention content for future trials |
| Gambling and Long 201031 | UK | RCT of telephone-based support for behavioural change amongst patients with type 2 diabetes (C) | To explore recipients’ experiences of the ‘packaging’ of advice | Taped samples of telephone counselling sessions and interviews with nine intervention recipients | Explored the way advice was given and received and described the personalised and responsive dynamics as generic components of telephone counselling. Describes generic components within a complex intervention. Informs intervention content for future trials |
| Macpherson and Thomas 200832 | UK | Pragmatic RCT of acupuncture care compared with usual care for persistent lower back pain (DD) | To describe the intervention as delivered from the perspective of the providers | Semistructured interviews with six acupuncturists who delivered care in the trial | Disaggregated components of the intervention and identified self-help advice as an integral process of acupuncture care. Describes a potentially hidden component of a complex intervention. Informs intervention content for future trials |
| McQueen et al. 200933 | USA | RCT of a tailored interactive intervention compared with use of a generic website to increase colorectal cancer screening in primary care (C) | To explore the content and process of physician–patient discussions about screening during a wellness visit | Audio-taped consultations involving 76 patients and eight physicians, all participating in the RCT | Described process of shared decision-making as a component of the intervention delivery. Describes a generic component within a complex intervention. Informs intervention content for future trials |
| Romo et al. 200934 | Spain | Open RCT of hospital-based heroin prescription compared with methadone prescription for long-term socially excluded opiate addicts for whom other treatments have failed (DD) | To explore patients’ and relatives’ experience of the intervention as delivered within the trial | In-depth semistructured interviews with 21 patients receiving intervention and paired family members | Explored the experience of receiving prescription heroin in a hospital setting and described the resulting medicalisation of addiction as a separate component of the intervention. Describes a potentially hidden component of a complex intervention. Informs intervention content for future trials |
| Teti et al. 201035 | USA | RCT of group education + peer-led support groups compared with brief education messages to increase HIV status disclosure and condom use amongst women living with HIV/AIDS (C) | To examine how women experienced the intervention | Semistructured interviews with 18 participants after the intervention was delivered | Described the content of components of the intervention (e.g. group discussions) and explored the relationship between components. Describes components of a novel and complex intervention to allow replicability in the real world |
A lot of space may be needed within a report to describe in detail the components of a complex or multifaceted intervention delivered to recipients. This may not be possible within the structure of a conventional trial report, or valued enough to ensure that a limited word count includes intervention description. The MRC framework for complex interventions3 supports this process at an early stage of evaluation, but all 10 articles undertook this description alongside the main trial (see Table 5 ). Qualitative research undertaken alongside a trial can provide a rich account of elements of the intervention in detail, enhancing understanding of how the intervention plays out in the experimental delivery context of the trial. In the articles reviewed here, there were two main reasons for using qualitative research:
-
to describe and make available an unfamiliar or complex intervention using the experiences and perspectives of trial participants35
-
to increase understanding of individual components of a complex intervention and make them available for further exploration or testing. 30,32–34
Researchers chose to focus on a particular component of a complex intervention for a number of reasons. Macpherson and Thomas32 focused on an underexplored component, looking at acupuncture practitioners’ intention to deliver self-care advice alongside needling. This article raised a question regarding the integral nature of this aspect of the intervention as delivered and the implications of this for future trials of acupuncture care. Romo et al. 34 explored patient and family experiences of receiving prescription heroin in a hospital setting, and identified the perceived medicalisation of addiction (in contrast to criminalisation) as a component of the intervention in its own right. In contrast, Foster et al. 30 focused on an element of the intervention that was common to both arms of the trial (the use of medication companions) and highlighted the fact that this ‘taken-for-granted‘ component was integral to both home- and facility-based delivery of antiretroviral therapy and that its mechanisms of action would benefit from further research. Qualitative research was also used to explore components of complex interventions relating to what might be described as generic strategies, such as ‘shared-decision making’ in the context of advising about different cancer screening options within a consultation33 or the way health behaviour advice is ‘packaged’, rather than its specific content. 31
Accounts of complex interventions used in trials run the risk of reflecting the research protocol, rather than what was delivered in practice. Quantitative research methods may be employed to verify measurable elements of delivery, but qualitative methods, such as non-participant observation or in-depth interviews, can provide rich descriptive accounts of how the intervention was delivered on the ground during the trial, from the perspectives of recipients or providers. This descriptive function may form part of what is described as a ‘process evaluation’, although none of the articles we describe here used that term.
In each of our examples, the qualitative research appeared to provide an opportunity to focus attention on one or more components of a complex intervention, the significance of which might otherwise remain hidden or over-looked in a pragmatic trial. Although some of these articles discussed their findings in terms of possible mechanisms of action, the main purpose was to provide a rich and detailed account of different components of the intervention, based on the participants’ experiences. Using qualitative research data in this way is sometimes seen as ‘poor’ qualitative research because it does not extend itself into interpretive analysis or theory building. However, this tendency to undervalue the potential contribution of rich description has been recognised,36 especially in terms of its ability to ‘challenge taken-for-granted assumptions about the nature of the setting or group under study’. 37
-
Be clear about the origin of the research question
-
In our sample of articles, the genesis of the qualitative research question was sometimes unclear. The stronger articles gave a clear account of the reason for the focus on particular components and indicated whether this was an emergent issue or one that was identified as a research focus early in the trial.
-
-
Place within an evaluative framework
-
Describing, elucidating and highlighting hidden or overlooked components of a complex intervention using qualitative methods is a useful research output that has value for future intervention development, but many of the articles lacked a wider framework for presenting this type of analysis. Use of the MRC framework for the development and evaluation of complex interventions was rare and it may be that this framework could help provide this type of research with a useful rationale and contextual reference point.
-
-
Consider findings in relation to mechanisms of action
-
Descriptive accounts developed through qualitative data may also lend themselves to further development relating to an exploration of mechanisms of action or the explicit development of theoretical concepts underpinning interventions. Some of these articles moved towards this type of analysis, but this was not their aim.
-
-
Make explicit links to the main trial
-
Links to the main trial tended to be limited to the background and methods sections of articles, with little or no attempt to link this research back to the trial in the discussion or conclusion. This raised concerns about whether or not the research had any impact on the trial and also limited its utility for future trials.
-
Models, mechanisms and underlying theory development of the intervention
This subcategory was distinguished from the ‘intervention components’ focus described above by going one step further and attempting to develop underlying concepts and behavioural theories, as well as exploring mechanisms of action. Articles in this subcategory described an explicit aim to develop the conceptual thinking behind the intervention, using the rich data provided by the experience of delivering an intervention within a trial context. In practice, a few articles sat between this subcategory and the previous one, making some reference to theory or mechanisms of action, but falling short of developing these ideas using the qualitative data collected. This subcategory was also distinguished from the next subcategory Exploring perceived value and benefits of the trial intervention, which identifies additional (unmeasured) benefits from the perspective of the trial participants. When articles in this subcategory identify unmeasured benefits, they go on to explore their role as (sometimes hidden) mechanisms for change, and locate these findings in a theoretical framework, thus attempting to integrate any such additional benefits into an explanatory model of the mechanism of action of the intervention.
We identified 23 articles in this subcategory, with only one undertaken at the pre-trial stage. We selected six examples purposively to include examples of the range of topics: treatment, process and context mechanisms. The six articles focused on a range of interventions: clinical, educational and professional ( Table 6 ). The qualitative methods used in these six examples included interviews, focus groups and observation, and the research subjects included trial participants and wider stakeholders.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for the specific trial and the trial endeavour |
|---|---|---|---|---|---|
| Byng et al. 200838 | UK | Cluster RCT of a multifaceted facilitation process (Mental Health Link) to improve care of patients with long-term mental illness (C) | To investigate how a complex health services intervention led to developments in shared care for people with long-term mental illness | Interviews with 46 practitioners and managers from 12 cluster sites to create 12 case studies, analysed using a Realistic Evaluation framework | Identified core functions of shared care and developed a theoretical model linking intervention specific, external and generic mechanisms to improve health care. Unpicks the complexity of an intervention and develops a theoretically informed model of mechanisms of action. Develops an explanatory model of the intervention and/or its delivery to inform the interpretation of the trial results |
| aHoddinott et al. 201039 | UK | Cluster RCT of community breastfeeding support groups to increase breastfeeding rates (C) | To support the development of an explanatory model of the intervention mechanisms and why success was or was not achieved. Part of a mixed methods study to explore anticipated variations in intervention success at cluster level | 64 ethnographic, in-depth interviews, 13 focus groups and 17 observations to produce seven locality case studies, informed by a realist approach | Aided the development of a model of the intervention located in context that helped to explain the observed variation in outcomes at cluster level. Develops a model showing how context and the complex systems within which an intervention occurs can be an important mechanism of change. Develops an explanatory model of the intervention and/or it’s delivery to inform the interpretation of the trial results |
| Liu et al. 200840 | Taiwan | RCT of a body–mind–spirit therapy (including positive psychology and qi-gong exercises) compared with usual care for cancer patients with symptoms of depression and anxiety (C) | To explore treatment mechanisms and provide a more comprehensive understanding of the therapy | Focus group with 12 intervention group participants | Identified eight theoretically informed domains of the treatment effects of group therapy and highlighted the importance of culturally sensitive approaches to therapy. Develops a theoretically informed model of treatment effects from a patient perspective |
| Kaptchuk et al. 200941 | USA | RCT of the placebo effect in patients undergoing acupuncture for symptoms of irritable bowel syndrome (C) | To explore the experiences of patients undergoing placebo-enhanced treatment in the context of a trial | Repeated semistructured interviews with 12 patients in the intervention group | Identified a spectrum of factors associated with the placebo effect and concluded that any single theory or model of placebo is inadequate to explain its reported benefits. Evaluates existing theories of mechanisms of action and develops new insights or models |
| O’Sullivan et al. 201042 | Canada | RCT of theory-based physical activity counsellor to increase activity as a preventative strategy (C) | To identify aspects of the intervention perceived to be instrumental in eliciting desired behaviour changes | Repeated semistructured interviews with 15 patients in the intervention group | Identified aspects of the intervention seen as helpful and linked these to the underlying theory informing the intervention design (Self Determination Theory). Explores perceived mechanisms of action to endorse the theory underlying the intervention |
| Rogers et al. 200943 | UK | RCT of self-management group-based intervention (The Expert Patients Programme) for people with long-term conditions (C) | To explore the concept of social comparison as a mechanism that facilitates or limits the success of self-skills training groups | In-depth interviews with 23 participants before and after the intervention | Described the importance of social comparison as an underlying feature of group-based self-care skills training. Develops a concept or underlying theory that underpins the operation of an intervention |
Qualitative research with this focus appeared to be motivated by a desire to acknowledge the role of complexity in many interventions being trialled, owing either to the multifaceted nature of the intervention or to the complex social structures and contexts in which interventions took place. The qualitative research was intrinsically evaluative and entailed an exploration of the intervention using the perspective of the participants and wider stakeholders. When the goal was an explanatory model of the intervention,38,39 the qualitative research was part of a broader mixed methods approach. Alternatively, the focus was on ‘generic’ mechanisms rather than on those deemed to be specific to the trial intervention, in which case the message was less for the trial itself and more for a ‘class’ of intervention, such as ‘self-management’43 or ‘placebo effects’. 41 Additionally, the research was sometimes designed to address a recognised body of theoretical work, such as theories of behavioural change. Researchers undertaking research in this subcategory reported a number of related reasons for doing so:
-
To unpick the complexity of an intervention and develop a theoretically informed model of mechanisms of action by modelling the relationship between the elements.
-
To refine interventions by identifying the most ‘effective’ components from participants’ accounts, with reference to underlying theories from the social sciences.
-
To create conceptual clarity around an aspect or element of the intervention (e.g. patient-centred care).
-
To develop a theoretically informed model of treatment effects from a patient perspective.
-
To better understand elements of the process of care (e.g. modelling the conditions for ‘well-functioning teams’).
-
To develop empirically based and theoretically grounded explanations of wider treatment effects, especially those related to beliefs and behaviour (e.g. placebo effects).
-
To evaluate existing theories of mechanisms of action and develop new insights or models.
-
To develop existing theories of the mechanisms by which generic outcomes (e.g. ‘self-efficacy’) are achieved through the intervention as a whole or through specific elements of the intervention.
-
To identify mediating factors and ‘hidden mechanisms’ relating to the intervention. The work of Ray Pawson was frequently referenced in relation to this type of work. 44
-
To create explanatory models of the impact of the intervention using a mixed methods approach showing how the context and the complex social systems within which an intervention occurs can be an important mechanism of change.
Research in this subcategory ranged from simple linking of existing theories to the accounts given by participants, to the development of sophisticated and complex models of mechanisms of action that may encompass treatment, process and context. Understanding the impact of ‘context’ on the outcomes of an intervention could have particular relevance to the transferability of the results or the external validity of the trial. The value to the original trial was high, especially where unexpected outcomes were observed, or where measured outcomes were uniformly negative but patient satisfaction with the intervention or the process of care was high. The work by Hoddinott et al. 39 was particularly interesting as the intention to develop an explanatory model of the intervention incorporating contextual effects was designed at the outset of the trial in anticipation of expected context-driven variation in outcomes in the different cluster sites in which the intervention was delivered. This work was therefore able to directly inform the interpretation of the trial results. Alternatively, this research was sometimes reported with little reference in the discussion or conclusions of the trial in which the data were collected, with the key message from the article being directed to clinical practice or to the body of literature that would form the theoretical basis of intervention development in the future.
-
Be explicit about messages for transferability of trial findings
-
Successful research in this subcategory was able to generate new knowledge that had the potential for deepening our understanding of how interventions achieve the results observed in the context of a particular trial and how these lessons may be transferred to other contexts. When appropriate, a commitment to taking a wider view by incorporating aspects of delivery and context would help this type of research to contribute substantially to our understanding of the transferability of the trial findings and identify likely barriers to the successful roll-out of practice based on the trial results.
-
-
Extend current thinking by challenging as well as endorsing theories
-
Research addressing underlying generic concepts and theories underpinning interventions have the potential to inform a wide range of possible interventions in different contexts. Weaker articles missed the opportunity to extend current thinking because external constructs, concepts or theories were employed more for the purposes of referencing or endorsing the findings than for challenging existing ideas or developing new knowledge.
-
Exploring perceived value and benefits of the trial intervention
Articles in this subcategory focused on experiences of giving or receiving the intervention. They were different from articles related to the acceptability or feasibility of an intervention (subcategory Feasibility and acceptability of the trial intervention in practice) in that they were not seeking to assess the viability of the intervention but rather to capture important aspects of the range of outcomes experienced and to understand the ways in which these outcomes were valued by the providers or recipients of an intervention. Judgements about value were part of a process that might include the assessment of alternatives and context. Some of the benefits may be described as indirect benefits, derived from the processes associated with the intervention delivery. In some cases, the value of the wider benefits identified was linked to their role as mechanisms of desired behavioural change within a complex intervention (see earlier subcategory Models, mechanisms and underlying theory development of the intervention). In other cases, perceived benefits were tangential or unrelated to the target outcome (e.g. increased self-esteem or confidence in self-management of long-term conditions), presented as examples of ‘added-value’ for the trial intervention. There was some overlap between this subcategory and a later subcategory on the breadth of outcomes used in a trial (see Breadth of outcomes to be measured in the trial).
We identified 42 articles in this subcategory, only 7% of which reported qualitative research undertaken at the pre-trial stage (3/42). We undertook detailed data extraction on six of them, which were selected purposively to include examples of the different research subject perspectives (intervention recipients and/or intervention providers). They focused on a range of interventions: clinical, educational and professional ( Table 7 ). The qualitative methods used included interviews, focus groups and content analysis of diaries completed as part of the intervention itself. The research subjects included intervention recipients and intervention providers.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| Drahota et al. 200845 | UK | Pragmatic RCT of audio-visual distraction technique for reducing pain and anxiety during minor surgery (C) | To explore attitudes to and experiences of the intervention | Two focus groups with intervention recipients and 10 interviews with clinic staff | Used qualitative findings to support the (negative) findings of the trial. Adds depth to the quantitative findings of the trial in order to make these more accessible to service providers |
| Dowrick et al. 200846 | UK | RCT of reattribution training in general practice for use with patients with medically unexplained symptoms (C) | To explore attitudes to reattribution training amongst practitioners, as part of nested mixed methods study | Semistructured interviews with 12 practitioners participating in the trial | Identified perceived direct and indirect benefits (e.g. increased confidence in working with this group of patients and crossover into chronic disease management). Understanding what GPs valued about the intervention was seen as a potential mechanism for increasing the successful roll out of the intervention. Understands the range of potential benefits and how the intervention is valued in order to encourage take-up or implementation |
| Lindell et al. 201047 | USA | Pilot RCT of a supportive disease management intervention for patients with idiopathic pulmonary fibrosis and their carers (C) | To explore the experiences of patient/carer dyads in the intervention group after receipt of the intervention | Repeated semistructured interviews with 12 patients in the intervention group | Explored reasons for the trial finding that the intervention had a negative impact on the patients, but a positive impact on the care partners. Found hidden (unmeasured benefits amongst patients). Describes unanticipated and/or unmeasured benefits by trial participants in order to better understand the trial findings |
| Lipman et al. 201048 | Canada | RCT of community-based education/support group for lone mothers at risk of social isolation and mental health morbidity (C) | To provide rich descriptive data to explore the impact (benefits and limitations) of a community-based group programme from the participants’ perspective | In-depth, semistructured interviews with eight intervention group mothers | Despite negative RCT findings, found examples of positive value and direct benefits described by participants (e.g. improved communication with their children that were not measured in the RCT outcome measures). The authors argue that these findings have ‘conceptual utility’, allowing service providers to better understand the experiences of service users. Describes unanticipated and/or unmeasured benefits by trial participants in order to improve the intervention or its delivery |
| Malpass et al. 200949 | UK | RCT of usual care compared with single intervention (diet) or multiple intervention (diet plus physical activity) for the management of type 2 diabetes (C) | To explore patients’ experiences of making behavioural changes and assess whether patients found making multiple lifestyle changes counterproductive or beneficial | Telephone interviews at 6 and 9 months post randomisation with 12 participants in each of the intervention arms | Participants found undertaking multiple lifestyle changes provided added benefits, reporting the use of physical activity to support dietary changes and control blood glucose levels. Explores anticipated value and benefit in order to understand the way the intervention works for the recipients |
| Morone et al. 200850 | USA | RCT of mindfulness meditation for older adults with lower back pain (C) | To identify the range of beneficial effects of mindfulness | Content analysis of data from diary entries about experiences of the intervention of 27 participants who completed the programme. The reflective diaries were part of the intervention | Identified several benefits beyond pain relief, including better sleep, increased energy and enhanced well-being. Describes unanticipated and/or unmeasured benefits by trial participants in order to increase understanding of a novel intervention and to generate hypotheses for future research |
Articles were largely focused on complex interventions with behavioural and/or psychological components. Although often not stated, the rationale for conducting the qualitative research appeared to be a desire to understand the experiences of trial participants in terms of the perceived value of the intervention and its benefits, with an underlying assumption that the quantitative measures employed in the trial outcomes were likely to fail to capture some aspect(s) of the intervention valued by patients or participants. This allowed some researchers to identify benefits that were not anticipated at the outset of the trial (and, therefore, not measured) and others to explore benefits that were anticipated but not measured, either because they were not amenable to measurement or because they were considered peripheral to the primary outcome. For example, Morone et al. 50 undertook a qualitative study expecting to find benefits relating to the intervention that were broader than the trial’s primary outcome of pain reduction. For other researchers, a rich account of the meaning of the experience, which allows them to explore why the intervention is valued by participants, may be used to support the case for further research or the adoption of an intervention in practice. In the article by Dowrick et al. ,46 the exploration of the perceived benefits relating to the management of medically unexplained symptoms identified its potential value as a transferable skill in the management of chronic illness. In the article by Lipman et al. ,48 the exploration of benefits was understood in the context of the lives of the trial participants (lone mothers), the qualitative work undertaken during the trial therefore had the potential to increase understanding of the needs of this group. Finally, data collected relating to the ways in which participants experience benefits from a trial intervention may be used to illustrate quantitative findings and give them more immediacy, or they may be used to explore contradictory findings in the trial outcomes, such as high satisfaction scores alongside small changes in other measured outcomes.
-
Consider using qualitative research at the pilot phase
-
This research was most frequently conducted with the main trial rather than as part of any formal pre-trial or piloting work. This restricted the potential impact of the research on the trial itself. If undertaken pre-trial, unexpected benefits could then be considered as candidates for formal outcome measurement (see subcategory on outcomes Breadth of outcomes to be measured in the trial).
-
-
Give more consideration of findings in relation to trial results
-
Because most of this research was conducted during the main trial phase, impact was more likely to be achieved in terms of giving the trial results a ‘human context’, making recommendations for future research or suggestions for clinical practice. Opportunities appeared to be missed within these articles for using the findings of the qualitative research to support the interpretation or presentation of the trial results.
-
-
Give more consideration to messages for implementation in the real world
-
Researchers could have been more explicit about the utility of their findings for encouraging take-up or implementation of an intervention following positive trial results, especially when the intervention was perceived as novel or involved the active collaboration of health staff or patients in its delivery.
-
Gauging the ‘in principle’ acceptability of the trial intervention
We distinguish between acceptability in principle (this subcategory) and acceptability in practice (the next subcategory Feasibility and acceptability of the trial intervention in practice). Acceptability in principle is about beliefs held by people who will either receive or deliver the intervention, which may impact on their willingness to co-operate with the intervention process. This is best understood as principles or beliefs held as standards for guiding or shaping conduct, decisions or choices. We would expect these beliefs to be formed prior to the trial. In contrast, acceptability in practice is experiential and can only be explored following an enactment of the intervention delivery. In order to be classified in this subcategory, articles had to demonstrate that clear ‘in principle’ beliefs about the intervention or aspects of its delivery were being explored, beyond the experience of receiving the intervention.
We identified 32 articles in this subcategory. Only one-quarter reported qualitative research undertaken at the pre-trial stage (8/32). About half of the articles considered patients’ views, slightly fewer considered professionals’ views and some explored both viewpoints in the same study. We purposively selected six examples to include a range of interventions: clinical, educational and professional ( Table 8 ). The qualitative research focus was always clearly related to the trial. The qualitative methods used included interviews and diaries. The research subjects were drawn from members of the public, trial participants, trial decliners and professionals involved in the delivery of the proposed intervention.
| Paper | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| Avery et al. 200851 | UK | ProtecT: a primary care-based pragmatic trial to evaluate the effectiveness and cost-effectiveness of screening and treatment for men at risk of localised prostate cancer (C) | To explore (actual and potential) intervention recipients’ decision-making about screening, including biopsy and how beliefs influence health behaviour | 58 interviews with men accepting screening, not responding to invitation for screening, having received a prostate biopsy or refusing biopsy | Identified good practice in informed decision-making for clinical practice |
| Buzza et al. 201052 | USA | RCT of three patient activation strategies (information, financial incentives and phone contact) to encourage discussion of appropriate prescribing with their provider (C) | To explore provider attitudes to the intervention following a positive trial (includes both acceptability in principle and in practice) | Telephone interviews with 21 providers involved in the trial | May shape similar intervention for different target behaviours in future trials. Findings regarding acceptability may also have value for dissemination/uptake of the positive trial results. Increases likelihood of uptake of intervention in the real world |
| Mihaylov et al. 200853 | UK | STooL: pragmatic factorial multicentred RCT to investigate the clinical and cost-effectiveness of stepped laxative management strategies for chronic constipation in older people (C) | An ‘add-on’ study to explore the meaning of the target condition and beliefs about appropriate treatment amongst intervention recipients and care providers | In-depth interviews with 24 potential intervention recipients and nine GP providers, and group interviews with 17 nurse providers | The RCT closed early owing to low recruitment and the qualitative research demonstrated poor acceptability of the intervention to recipients and low priority amongst providers in the context of current beliefs and practices. Explains trial failure and why similar trials will fail in the future |
| Ockleford et al. 200854 | UK | DESMOND: primary care-based cluster RCT of a structured group education programme for newly diagnosed diabetic patients vs. usual care (C) | To explore experience of being diagnosed with diabetes and its impact on the acceptability of a behavioural intervention | In-depth interviews with 19 intervention recipients and 17 control group participants | Key message related directly to clinical practice rather than to trials |
| Reid et al. 200855 | UK | Cancer pain management RCT comparing two pain relief regimes, one including opioids being offered for the first time (C) | To explore factors influencing intervention recipients’ decisions to accept or reject morphine when first offered as pain relief within the trial | In-depth interviews with 17 trial participants and 12 patients who were invited but declined to enter the trial | Trial was an opportunity to explore the beliefs of cancer patients who were being offered opioids, and assess the in principle acceptability of drugs in this context. The key message was for clinical practice rather than the trial |
| Zhang et al. 201056 | China/USA | Community-based RCT of reduction of risk of diabetes through long-term dietary change from white to brown rice (C) | Pre-trial to explore cultural acceptability and prior beliefs about brown rice consumption amongst potential intervention recipients | Mixed methods study with focus groups of 32 non-trial participants | Identified the beliefs held about brown rice that made it an unacceptable intervention. Results provided valuable insights to guide the design of patient information for the planned trial. Improves viability of trial |
The authors of these articles offered a range of reasons for conducting research into the in principle acceptability of the trial intervention. Some started with this as a purpose but for others it emerged as an issue during the main trial and it then became the focus of the article. In each latter case, the statement supporting the need to undertake the study indicated that there was reason to believe that the idea of the intervention might not be received entirely positively by the recipients or the providers. The given rationale for exploring the in principle acceptability of a trial intervention tended to fall into one of three trial-related categories:
-
The need to explore the impact of individual and wider cultural beliefs on behaviours, such as the likely take-up of a trial intervention by participants.
-
The need to explore the potential impact of individual and wider profession/cultural beliefs on the optimal delivery of the trial intervention.
-
The opportunity to understand changes in existing beliefs about acceptability that emerge during the course of the trial and their implications for the trial results and/or for future clinical practice. In these cases, retrospectively reported acceptability in principle may be contrasted with reported acceptability in practice.
The qualitative research aimed to understand the relationship between beliefs and behaviour or choices made prior to, or during, the trial. A minority of the 32 articles explored this issue in isolation and prior to the trial itself and these articles focused on patients or the general population rather than professionals involved in the delivery of the intervention. They were characterised by a cultural distance between the trial organisers and the recipients of the intervention. An example is Zhang et al. ,56 in which the trial lead was based in the USA and the trial intervention was in China.
The potential impact of this use of qualitative research on the trial itself is likely to depend on its timing. When a concern exists about the acceptability of an intervention to be trialled, doing this work prior to the main trial may impact on trial recruitment, long-term participation or adherence to the intervention, as well as the effective or optimal delivery of the intervention in the context of the trial. An example of this is provided by Zhang et al. 56 who explored the acceptability of dietary changes in advance of a planned trial and found strongly held negative beliefs about the acceptability of brown rice in terms of beliefs about taste and quality. They were able to identify nutritional messages that could increase acceptability and encourage participation in the trial, but these did not change the intervention itself. This highlights why this subcategory differs from what we have called ‘intervention development’ because here it is beliefs about the intervention that are important. In the 32 articles, pre-trial work was relatively rare, with most studies being conducted after commencement of the full trial. This may be due in part to our study inclusion criteria, which required the qualitative research to be linked explicitly to a planned or ongoing trial.
The qualitative research was undertaken alongside the main trial for many of our articles. These explored acceptability in principle alongside acceptability in practice and some also addressed the perceived value and benefits of the intervention as part of a broadly specified rationale for the qualitative research such as ‘to explore the experiences of patients participating in the trial’. This limited the potential impact of the research on the trial because it could not shape the intervention delivered in the trial. However, it did have the potential to relate the qualitative findings to the outcome of the trial, poor implementation of the intervention or low recruitment. 53 Mihaylov et al. 53 were able to explain the failure to recruit in a trial of constipation treatment according to the strongly held beliefs about appropriate or favourite existing treatment and a reluctance to change behaviour as required by entering the trial. Buzza et al. 52 described how negative prior beliefs that existed about some aspects of the intervention (e.g. patient activation seen as a threat to professional autonomy) did not appear to impact on behaviour during the trial. Ockleford et al. 54 were able to demonstrate important relationships between acceptance of a diabetic diagnosis and willingness to engage with self-management groups. They suggested that engagement with the intervention programme might help some trial participants who were struggling with accepting this change to their identity.
Many articles concluded with messages for clinical practice, without reference to the trial findings. The trial appeared to present an opportunity to explore issues relating to beliefs and acceptability relevant to clinical care, some relating to the likely success of the intervention in a real-world context, others making explicit suggestions for improving the intervention or its delivery in practice. For example, Reid et al. 55 concluded with the need to address the ‘in principle’ fears and beliefs about opioid analgesics among cancer patients in order to improve clinical care. In this case, the trial was identified as an opportunity to interview patients who were being offered opioids for the first time and found that patients strongly associated them with end-stage care and death and therefore discounted them as helpful pain relief at earlier stages of treatment. A few articles took their discussion beyond the immediate clinical or public health interventions that were the subject of the trial, raising issues about the transferability of the lessons learnt to other clinical contexts requiring behavioural interventions with similar underpinnings and principles for success, for example patient activation strategies. 52
-
Consider the timing of the qualitative research in relation to the trial
-
When a concern exists about the acceptability of an intervention to be trialled, qualitative research is best used prior to the main trial to allow it to impact on trial recruitment, long-term participation or adherence to the intervention, as well as the effective or optimal delivery of the intervention in the context of the trial. If the qualitative research is undertaken within an ongoing trial, the obvious limitation is that, once in the trial, participants have already made a decision that reflects some degree of acceptance and the sample will reflect this range of beliefs. Some designs overcame this problem by exploring in principle acceptability among those invited but declining to participate in the trial. 51,55
-
-
Be clear about messages for the trial
-
Research in this subcategory had the potential to produce messages of relevance to the trial and beyond in clinical practice or the delivery of population-based public health programmes. Overall, we found a considerable divorce between the trial itself and the qualitative findings. This may have been due to the qualitative articles being published in advance of the trial findings but sometimes the main trial results article did not reference the qualitative research (e.g. Ockleford et al. 54).
-
Feasibility and acceptability of the trial intervention in practice
In this subcategory, the focus of the qualitative research was the feasibility and acceptability in practice of the intervention itself or its mode of delivery within a trial context. Feasibility and acceptability in practice were often considered together, with acceptability treated as a component of feasibility. However, for clarity, it is worth considering them separately at a conceptual level. The feasibility of an intervention depends on a number of factors and includes two distinct perspectives: those involved in delivery of the intervention and those receiving it. From the perspective of those delivering the intervention, feasibility includes the ability to do so in an effective manner that is compatible with their personal skills and work practice, individual and team-based workloads, managerial and contextual constraints and prevailing cultural norms. From the perspective of those in receipt of the intervention, data may be obtained from patients, trial participants, caregivers and other involved parties in order to explore the practical consequences of engaging with the intervention in terms of the demands made on those involved, especially those in addition to the demands imposed on patients and carers by the illness itself. If feasibility asks the question ‘Can it be done within existing contextual constraints?’, acceptability asks the question ‘Are the “costs” of doing it such that people are willing to play their part in the effective delivery or receipt of the intervention?’.
This subcategory contained a large number of articles (83), accounting for over one-fifth of the 356 different uses of qualitative research (23%). Almost one-quarter of the articles (20/83) reporting the use of qualitative research were undertaken at the pre-trial stage. All but one article presented the analysis of data collected from either the providers of the trial intervention or recipients of the trial intervention or control. The exception collected data from managerial staff in the local sites who did not deliver the intervention itself, but who contributed to the context in which the intervention took place. Intervention providers included health professionals, public health practitioners, and teachers. Intervention recipients included patients, carers and members of the public (for public health/prevention trials). Occasionally, the distinction between these two groups was blurred when carers (especially parents) were asked to deliver the trial intervention, or when health-care providers were the recipients of a trial intervention (usually one designed to change their professional behaviour). Complex interventions frequently required intervention recipients to engage in behaviour change, such that they were, in some sense, ‘providers’ of their own intervention.
We present the details of six articles, selected purposively to include examples of the range of research subjects (intervention recipients and providers) and the timing of the data collection (i.e. pre-trial, during the trial). They focused on a range of interventions: clinical, educational and professional ( Table 9 ). The qualitative methods used included interviews, focus groups, diaries and observation, and the research subjects reflected the range of research questions being drawn from members of the public, trial participants, carers and parents and the health professionals involved in the delivery of the proposed intervention.
| Paper | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| Behets et al. 200857 | USA/Madagascar | Pilot RCT of the effectiveness of continuous diaphragm use amongst low-income women highly exposed to STIs in Madagascar (DD) | To explore intervention recipients’ beliefs about effective STI protection and perceived barriers to the continuous use of the diaphragm | Part of mixed methods formative study in preparation for a RCT with focus group discussion with intervention recipients, following use of the intervention | Identified potential behavioural obstacles to the effectiveness of the intervention, stemming from a wider perspective on how recipients lived their day-to-day lives, and exposed a risk of bias in the planned intention-to-treat analysis resulting from differential use of condom protection in the two arms the trial. Exposes a risk of bias in the planned analysis resulting from differential behaviours in the two arms of a trial |
| Ferrer et al. 200958 | USA | RCT of medical assistant driven programme of screening and referral for four health-related behaviours (C) | To explore perceptions of feasibility of delivery, particularly the programme’s effect on the workload, work-flow and job perceptions of the intervention providers | Semistructured interviews with 15 intervention providers (high and low adopters) | Provided insights into contextual factors affecting the programme adoption rate by medical assistants within the trial, which were low overall and variable between practices. Explains trial findings. Explains intervention adoption rates |
| McPherson et al. 200959 | New Zealand | Pilot study for a three-arm RCT of two self-regulation informed goal setting interventions for people with traumatic brain injury (C) | To explore recipient and provider experiences of novel interventions to assess acceptability in practice and identify barriers to the integration of the intervention into current clinical practice | Clinic observation, semistructured interviews with 15 intervention recipients and 11 intervention providers and focus groups with intervention providers | Provided evidence of acceptability, but identified challenges and costs associated with the process of delivering the interventions in practice. Strengthens the case for a definitive trial. Improves viability of main trial |
| Pope et al. 201060 | USA/South Africa | Cluster RCT of provider-initiated (opt-out) HIV counselling and testing of tuberculosis patients in South Africa (C) | To explore the structural and personal factors that may have reduced the acceptability or feasibility of the intervention delivery by the tuberculosis clinic nurses | Focus groups involving 18 trial intervention providers, undertaken after the trial results were known | RCT showed smaller than expected effect and qualitative research provided insights into contextual factors that could have reduced the uptake of HIV testing and counselling, including a lack of space and privacy within the clinic itself. Explains trial findings |
| Sandler et al. 200861 | USA | Open-label trial of stimulant medication reduction for children with ADHD using partial dose with placebo as the experimental intervention (C) | To explore the meaning and acceptability of a placebo intervention from the perspective of recipients and their parents | Interviews with 58 children and 64 parents and formal observation of clinic visits | Demonstrated the acceptability of using full-disclosure placebo prescription in a medication reduction regime in a context of the trial having positive outcomes. Gives patient-centred credence to the findings of a positive trial by demonstrating the acceptability of a controversial intervention. Explains trial findings |
| White and Winstanley 200962 | Australia | RCT of benefits of introducing clinical supervision for mental health nurses (C) | To explore the intervention providers’ beliefs about the acceptability of clinical supervision within the prevailing managerial culture | Diary data from 22 intervention recipients | Offered insights into why the intervention failed in anticipation of a negative trial outcome. Explains trial findings |
The potential value of this use of qualitative research depended on when it was undertaken in relation to the trial. We discussed its potential value at the pre-trial and full trial stages. First, feasibility and acceptability in practice can be explored as part of a pre-trial pilot or feasibility study with the explicit aim of impacting on the way the intervention is delivered in the main trial. For example, the rationale for using qualitative research at this stage was that members of the intervention group were required to make substantial changes to their sexually related behaviour in order to comply with the intervention57 and the use of theoretically informed, but untested, interventions gave rise to practical concerns about the feasibility of delivering them optimally within the context of the rehabilitation clinic. 59 The potential value to the trial endeavour was scope for radical redesign of the intervention, or its mode of delivery, or to raise concerns about the design or value of the main trial being conducted. In this sense, these studies had something in common with those aimed at intervention development; however, intervention development was not an explicit research aim. Second, feasibility and acceptability in practice can be explored during the main trial and indeed the majority of the 83 articles focused on this stage. The impetus for this was not always clear. The initial qualitative research question was ‘to capture the experiences of the participants’ stemming from the recognition that the intervention was either complex or novel or both. In some articles, the focus of the analysis was broad and diffuse whereas in others, the data collected under the banner of ‘participant experiences’ were used selectively in the analysis to address a particular issue arising from the trial conduct or the trial results. The qualitative research was used to describe the intervention itself or introduce it to a clinical community that would not get a full picture of its potential impact on recipients or providers from the main trial results alone. Potential value also lay in providing evidence of acceptability of an effective, but controversial, intervention. 61
A subset of articles presented findings on feasibility and acceptability as part of a broader ‘process evaluation’ that sought to capture and evaluate a range of contextual and behavioural issues relating to the trial conduct and intervention process. Two articles reported qualitative research undertaken after the trial results were known to understand low or variable intervention adoption rates within a trial. The importance of this issue was only identified as the trial progressed. 58,60
-
Identify a specific research question.
-
The research question was very broad in many articles in this subcategory. The apparent lack of focus of some articles may have reflected an underlying methodological tension between a perceived need to identify a priori questions for the qualitative research and a desire to allow emergent issues to be identified during data collection and analysis. However, articles could be strengthened by having a clearer rationale for the analysis, rather than an assumption that patient experiences are important, per se.
-
-
Be explicit about the message for trials.
-
There was a clear sense that it was important to gain an understanding of the way an intervention was received and/or delivered in practice, but not why this deeper understanding was important to the trial itself. Articles with a narrower, trial-linked focus to the analysis carried a stronger message than those trying to present a broad brush analysis of all the factors (positive and negative) that constituted the experience of delivering or receiving an intervention under trial conditions.
-
-
Consider the timing of qualitative research in relation to the trial.
-
Surprisingly few studies looked at delivery issues at the pre-trial stage. There is scope for more work to be carried out at that early stage to shape the practical delivery of the intervention and to ensure that the main trial is not jeopardised by a failure to implement the intervention optimally.
-
Intervention fidelity, reach and dose
In this subcategory of work relating to the trial intervention, the qualitative research supported assessment of the degree to which an intervention was delivered or implemented as planned during a trial, as well as reasons for any variation or deviation. This included issues relating to both provider delivery and recipient adherence or compliance, including intervention quality, dose received, and population reach of the intervention within the trial, as well as local variations in implementation in cluster trials. Because it was often part of a mixed methods process evaluation, we noted how and why the qualitative research was added and what it contributed beyond the output of any quantitative measures employed.
We identified 12 articles in this subcategory, accounting for 3% of the 356 uses of qualitative research, none of which were undertaken pre-trial. We selected six for detailed data extraction ( Table 10 ). The six articles covered a range of interventions: clinical, educational and professional. The qualitative data collection methods used included interviews, focus groups and observation, and the research subjects were drawn from members of the public, trial participants and professionals involved in the delivery of the proposed intervention.
| Paper | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| Ferguson et al. 200963 | Malawi | Open-label community-based RCT of programme to prevent mother-to-child transmission of HIV (C) | To explore protocol implementation (fidelity and reach) relating to breast feeding counselling element of programme | Direct observation of 123 counselling sessions; interviews with 6 nurse counsellors after observations completed. Part of mixed methods process evaluation | Observation used to generate quantitative measure of adherence. In light of high adherence, interview data identified requests for more training and better patient–counsellor ratios. Explores ways of building on successful implementation in future clinical practice |
| Holliday et al. 200964 | UK | Schools-based pragmatic cluster RCT of stop smoking intervention using peer supporters (C) | To identify how the intervention was adapted to meet challenges such as lack of time and the consequences of any variations for integrity (fidelity) of the intervention | 21 direct non-participant observation sessions and postintervention semistructured interviews with 11 trainers responsible for delivering intervention. Part of mixed methods ‘nested process evaluation’ | Identified examples of variation in fidelity of implementation, including student recruitment and reasons for this happening across sites. Explores anticipated variations in intervention implementation in a cluster trial. In the light of positive trial results, identifies ways to safeguard against avoidable and unacceptable variation during implementation |
| McNamara et al. 200965 | New Zealand | RCT with crossover design of two humidification techniques applied by parents in the care of children with tracheostomies (DD) | To explore experiences of parents with strong expressed preferences for one treatment and those who withdrew from the trial owing to concerns with either treatment | Semistructured interviews with nine mothers and four nurses | Found evidence of non-adherence owing to preferences. These were seen as active decisions by caregivers to improve their own coping and the care of the child. Explores ways of building on successful implementation in future clinical practice |
| aMukoma et al. 200966 | South Africa/Norway | Schools-based cluster RCT of HIV education programme to delay onset of sexual intercourse and increase appropriate condom use (C) | To explore whether or not the intervention was implemented as planned, to assess quality and variation of intervention at a local level and to explore the relationship between fidelity of implementation and observed outcomes | Direct classroom observations (26 in 13 intervention schools), 25 semistructured interviews with teachers (intervention deliverers) and 12 focus groups with pupils (recipients). Part of ‘embedded’ mixed methods process evaluation | Showed that intervention was not implemented with high fidelity at many schools and that the quality of delivery, and therefore the extent to which students were exposed to the intervention (dose), varied considerably. Observation and interview data did not always concur with quantitative assessment of fidelity (teachers’ logs). Explores perceptions of fidelity of implementation and reasons for non-compliance. Creates the opportunity for understanding how and why interventions and trial outcomes may be related |
| Palinkas et al. 200867 | USA | RCT of manualised evidence-based treatments vs. usual care for anxiety and depression in children (C) | To explore longer-term clinician intentions to implement treatments | Observation and semistructured interviews with professionals involved in delivery, described as ‘an ethnographic study of implementation’ | Identified three patterns relating to intention to sustain treatments: application with fidelity, abandonment of treatments and partial application of treatments. Explored factors relating to these patterns. Explores ways of building on successful implementation in future clinical practice |
| Plummer et al. 201068 | UK/Tanzania | Placebo-controlled RCT of herpes suppression therapy (DD) | To investigate reasons for poor patient adherence to intervention in a negative trial, recognising that poor adherence may contribute to lack of impact in HIV prevention trials | In-depth interviews with 20 intervention and control (placebo) participants, at final outcome point. Sample selected according to quantitative measures of adherence (‘under users’, ‘good users’ and ‘over users’) | Identified a range of barriers to adherence for under-users including poor understanding of the trial and family crisis. Less successful in explaining ‘over use’ or why some placebo recipients had positive lab results. Explores reasons for non-adherence to treatment. Recognises that biomedical interventions also have strong behavioural components and that adherence patterns must be measured and understood if the trial results are to be reliable |
The most direct impact of this qualitative research on the trial was interpretation of the trial results. If an intervention was not delivered with adequate integrity (i.e. as planned), the results of that trial could reflect the quality of the intervention as delivered and produce false-negative results. Triallists can use quantitative measures of intervention fidelity, dose and reach, but these articles showed the utility of qualitative methods such as observation and interviews in gauging the way an intervention was delivered or implemented within a trial. Observational data were used to create a checklist of quantifiable actions that comprised adequate delivery, showing variation or deviation from protocol. In contrast, qualitative data comprising first-hand accounts and observations of delivery in context explored questions relating to how or why variations and deviations from protocol occurred. Interventions as planned often included a concrete, measurable element defined in a protocol, but the implementation of this was subject to external influences (context) and individual preferences (behaviours and beliefs) on the part of intervention recipients and/or those delivering the intervention. Using qualitative methods to explore these issues was identified as useful in assisting the interpretation of the relationship between implementation and observed outcomes. 66 Cluster trials in particular were seen as vulnerable to a powerful potential context effect, as local structures and practices were likely to impact on delivery. 64,66 Complex interventions frequently required behavioural changes on the part of recipients and/or those delivering the intervention which were sensitive to individual preferences, beliefs and circumstances. 65 Less complex interventions, such as biomedical or drug interventions, were also acknowledged to have strong behavioural components that required attention. The drug trial reported by Plummer et al. 68 attempted to measure adherence to drug therapy among the trial participants, but recognised the limitations of these data for explaining the observed patterns of adherence. Indeed, the qualitative research raised questions regarding the reliability of the quantitative measures of adherence. 66,68
In a pragmatic trial, it could be argued that some variation in the integrity of intervention delivery would be found in practice and, therefore, acceptable in terms of the external validity of the trial results. However, messages for (clinical) practice could emerge as a by-product of assessing fidelity of implementation that had relevance beyond the trial results in terms of understanding potential moderating factors and identifying those that may be amenable to local modification in order to optimise the effectiveness of the intervention in practice. Thus, a study by Holliday et al. 64 was usefully able to identify variations that the authors considered to be both ‘unacceptable’ and ‘avoidable’ in the future implementation of a schools-based smoking cessation intervention, as well as identifying examples of acceptable, and anticipated, variation.
-
Link findings back to the trial.
-
Despite a strong case being made for the potential of qualitative research with this focus being used to aid interpretation of trial results, we found few examples of this in practice in the qualitative articles here. Some articles announced the intention to do this once the trial results were known and these trial articles were not included here. However, separate publication strategies may militate against this happening in practice. When trials assess interventions that are dependent on successful implementation, building this type of work into the trial design at the outset, with clear strategies for incorporating findings into the trial analysis, if appropriate, may increase the utility of undertaking this type of qualitative research with trials as well as enhance the value of the trial itself in terms of the dissemination of successful practice.
-
Implementation of the trial intervention in the real world
In this subcategory, the qualitative research focused on issues relevant to the likely success or failure of future attempts to implement effective interventions, or to embed them in clinical practice. This focused on what was special or unique about the trial conditions or context (i.e. time, space, participants) and how this might impact on any attempt to roll out or implement the intervention as trialled. Exploration of the real-world application of interventions requires that the qualitative data be analysed in conjunction with trial results in an explicit attempt to identify lessons that can be learned about the likely transferability of the trial results to a non-experimental setting. To minimise overlap with other subcategories, we only included articles here for which analysis was undertaken following trial results that supported the roll-out or adoption of the intervention as delivered in the trial. The lessons to be learned from the qualitative research were directed at the possible barriers to successful implementation of the (evidence-based) intervention beyond the trial. This was a small subcategory with only four articles, none of which were undertaken at the pre-trial stage, as was expected. We undertook detailed data extraction on all four articles ( Table 11 ).
| Paper | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| Cals et al. 200969 | Netherlands/UK | Cluster RCT of effectiveness of point-of-care physician testing (and communication training) to reduce antibiotic use in lower respiratory tract infections in primary care (C) | To explore the impact of direct experience of delivering the intervention(s) on subsequent adoption, following positive trial findings | Semistructured interviews with 10 GPs randomised to the intervention(s) and 10 GPs from the control arm. Qualitative data came from the process evaluation which entailed interviewing all participating GPs after the intervention period | Demonstrated how exposure to the intervention positively influenced attitudes and beliefs, which, in turn, impacted on adoption behaviour. This also contributed to the wider debate on how to facilitate the adoption of evidence-based interventions. Supports the interpretation of (positive) trial findings in a way which informs future clinical practice. Contributes to theoretical/explanatory models of health-related behaviour |
| Cals et al. 201070 | Netherlands/UK | Cluster RCT of effectiveness of point-of-care physician testing (and communication training) to reduce antibiotic use in lower respiratory tract infections in primary care (C) | Attitudes and experiences of using the test to support a strategy for encouraging sustained evidence-based use of the intervention following positive trial results | Semistructured interviews with 20 GPs. Qualitative data came from the process evaluation which entailed interviewing all participating GPs after the intervention period | Identified ways in which GPs valued the test and training in practice, for example by enhancing confidence in their prescribing decisions. This also explored lessons learned for near-site testing as a generic technology. Identifies contextually based strategies to support the implementation of the intervention in practice. Contributes to contextual/explanatory models of the implementation of generic health technologies |
| Carnes et al. 200871 | UK | RCT comparing advice to use topical or oral NSAIDs for knee pain in older people (with parallel preference study) (DD) | To explore patient reports of adverse events and expressed preferences for using one mode of analgesia administration over the other | Nested qualitative study of telephone interviews with purposive sample of 30 trial participants | Trial showed equivalence of effect of topical and oral NSAIDs for knee pain. In the light of these findings, the qualitative research provided a model incorporating trial findings and patient preferences into decision-making advice for use in practice. This also contributed to an empirically informed lay model for understanding the use of NSAIDs as pain relief. Supports the interpretation of (positive) trial findings in a way that informs future clinical practice. Contributes to theoretical/explanatory models of health-related behaviour |
| Escoffery et al. 200972 | USA | ‘Diffusion trial’ following RCT of evidence-based skin cancer prevention programme evaluating two strategies for facilitating adoption of the intervention (C) | To explore factors that facilitated and hindered implementation | Mixed methods process evaluation in which qualitative research played a relatively small part. Included open-ended questions to ‘contacts’ at multiple sites | Contributed to a description of implementation processes and identified local barriers to the utilisation and sustainability of the programme. Identifies contextually based strategies to support the roll-out of the intervention in practice |
Articles here addressed the broad question of how confident users of research evidence can be that an intervention delivered successfully in a trial will be transferable outside the experimental trial setting. They were linked to the emerging discipline of ‘implementation science’, which recognises that positive trial results will undergo a process of adoption and incorporation into real-world practice. Murphy et al. 37 describe this evidence gap as the result of an inevitable ‘context stripping’ that occurs in a hypothesis-driven experimental research design. They also suggest that qualitative research is particularly useful for putting context back into the picture in terms of behaviours, beliefs and local circumstances that do not form part of the original quantitative trial evidence.
In our examples, the qualitative data were analysed after the results of the trial were known, in order to identify specific factors relating to behaviour, beliefs or context that might impact on the future adoption of the intervention. Cals et al. 69,70 demonstrated how qualitative interview data, collected prior to the trial results being known, were analysed retrospectively and in the light of positive trial findings to address explicit issues relating to the likely adoption of the intervention. A particular strength of this work was its ability to describe the perceived value of the intervention (near-site testing) to a sample of clinicians similar to those who would be considering its adoption in the future. For Carnes et al. ,71 the trial message was that neither oral nor topical administration of non-steroidal anti-inflammatory drugs was superior in controlling knee pain. However, the choice between them still has to be made in everyday clinical practice and, using the qualitative research, these researchers were able to describe how prescribing decisions might be enhanced if patient preferences, and the reasons for these preferences, were incorporated into the prescribing process.
All the trials here were positive. However, when trial findings indicated a lack of effectiveness of an intervention used routinely in clinical practice, qualitative enquiry could be used to explore contextual and behavioural barriers to cessation of that intervention. We found no examples of this type of enquiry in the sample of literature reviewed.
-
Undertake analysis with a clear, trial-related, focus.
-
Qualitative research in this subcategory overlapped substantially with that undertaken to explore the feasibility or acceptability of an intervention and to assess the fidelity of its implementation. However, the strength of research in this subcategory lay in its ability to address focused and practical questions relating to the translation of trial findings into practice.
-
-
Be clear about the aim of the analysis in the abstract.
-
Articles by Cals et al. 69,70 were examples of how abstracts sometimes describe the aims and objectives of the type of data collected (views and attitudes based on experiences) rather than the rationale for conducting a particular analysis (to identify factors that may support the process by which an intervention found to be effective is adopted in practice). In these articles, data were collected for a process evaluation and then analysed retrospectively to explore particular issues, given the results of the trial. This fact emerges from reading the full article only. The value of research in this subcategory would be more apparent and accessible if a distinction was made between the original (broad) objectives of data collection and the specific aims of the analysis being presented throughout the article, including the abstract.
-
Category 2: trial design, conduct and process
The second broad category of the framework focused on the design, conduct and process of the specific trial (see Table 3 ). We identified 54 articles with this focus, accounting for 15% of the 356 uses described. One-fifth of articles in this category were based on qualitative research undertaken at the pre-trial stage (22%, 12/54). There were eight aspects of the design and conduct of trials addressed by the qualitative research:
-
recruitment and retention
-
diversity of participants
-
trial participation
-
acceptability of the trial in principle
-
acceptability of the trial in practice
-
ethical conduct
-
adaptation of trial conduct to local context
-
impact of trial on staff, researchers or participants.
Trial recruitment and retention
Randomised controlled trials can fail because of poor recruitment, or be suboptimal owing to lack of statistical power, or cost more money because additional funds are required to address slow recruitment. There is an evidence base on reasons for poor recruitment to trials, based mainly on quantitative research,73 and an evidence base on the effectiveness of interventions for improving recruitment. 74 A survey of trials in primary care in the UK found inefficiency and cost consequences, with trials running past planned recruitment timetables and additional funds needed to address poor recruitment. 75 Within the literature there is an innovative approach to using qualitative research to improve trial recruitment9 in which a trial is embedded within qualitative research to identify trial-specific recruitment issues and take action on them to increase recruitment rates within that trial. Retention can also be a problem once people are recruited. The quality of trials can be damaged if there are large numbers of dropouts, particularly if dropout rates are different for intervention and control arms, leading to concerns about internal validity.
We identified 11 articles focusing on recruitment or retention, 18% (2/11) of which reported qualitative research undertaken at the pre-trial stage. Four articles were from the same team that specialises in the use of qualitative research to increase recruitment for a specific trial. We selected six articles for full data extraction: five unique articles and one from the linked articles. The main method used was interviews, although there was also use of focus groups, observation and documents in some of the studies. 76,77 One article was about recruitment to the intervention rather than the trial itself ( Table 12 ). 77
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| Bill-Axelson et al. 200878 | Sweden/UK | Trial of radical prostatectomy vs. watchful waiting (DD) | To investigate experiences of randomisation with aim of facilitating trial participation in the future | Interviews with 14 participants, non-participants and recruiting clinicians | Identified how to overcome barriers to recruitment. Improves viability of trial |
| De Salis et al. 2008 (grey article: is this an empirical study?)76 | UK | Five different trials, for example social intervention for mentally ill patients (DD and C) | To consider whether or not qualitative research could be used to improve trial recruitment | Interviews with trial leads and recruiters, observation, focus groups, documents | Identified why a successful approach to recruitment was not necessarily transferable to other trials. Improves viability of trial |
| Dormandy et al. 200879 | UK | Cluster trial of screening for haemoglobinopathies (C) | To explore why general practices joined the trial and stayed in it | Interviews with 20 GPs in trial | Identified facilitators and barriers to recruitment and retention. Improves viability of trial |
| Garcia et al. 201077 | USA | Pre-trial feasibility study of stress reduction strategy in Latino school girls (C) | To examine the feasibility and acceptability of the intervention | One focus group of participants after the intervention | Identified the importance of the group leader in encouraging participation and retention. Improves viability of trial |
| Perkins et al. 2008 (grey article: is it qualitative research?)80 | Australia | Cluster trial of chronic disease intervention to improve quality of care in general practice (C) | To examine key issues experienced in recruiting and retaining practices | 155 telephone interviews with general practice staff who participated, did not participate and were not retained in the trial | Identified facilitators and barriers to recruitment. Improves viability of trial |
| Potter et al. 200981 | UK | Trial of telephone support for type 2 diabetes to improve adherence (C) | To explore experiences of recruiting for a trial | Telephone interviews with 10 nurses recruiting for a trial in general practice | Identified facilitators and barriers to recruitment. Improves viability of trial |
A considerable amount of research has been undertaken about barriers to trial recruitment and this has been synthesised. 73 Most of the studies in the review by Ross et al. 73 were quantitative and the articles we identified in this subcategory were building on this evidence base. Rather than attempting to identify barriers as in the review by Ross et al. ,73 they focused on strategies for overcoming the barriers,78 filling gaps in the knowledge base such as barriers to recruiting health professionals rather than patients,79 focusing on retention as well as recruitment,77,79 considering barriers in new research environments80 and considering barriers faced by different types of recruiters. 81 Most of the articles included here undertook the qualitative research to help recruitment in future trials. The main goal of De Salis et al. 76 was the use of qualitative research to increase recruitment rates for the specific trial. These endeavours were extremely useful because researchers were building on the evidence base and addressing an issue for improving future trial viability.
-
Consider the depth of the qualitative research required.
-
Some of the qualitative research reported here was descriptive, listing reasons for poor recruitment or retention as if the data had been obtained from surveys. 77,80,81 The authors were able to identify important issues even when they themselves pointed out the limitation of their content analysis that did not ‘allow a deeper analytic approach’. 78 This raises the issue about whether the strength of qualitative research to move beyond accounts and identify underlying issues, as opposed to creating lists of issues, is needed here or whether descriptive qualitative research is enough. There was also an issue about the labelling of some approaches as qualitative research when in some articles it read as if researchers took advantage of information collected as part of the recruitment process to write up about barriers and solutions. This was a useful endeavour but perhaps wrongly labelled as qualitative research.
-
-
Consider including those who do not participate as well as those who do.
-
Some authors included in their samples people who had not participated or stayed in the trial as well as those who had. This was a useful thing to do but more clarity was needed about the different insights offered by non-participants and participants.
-
-
Look beyond interviews.
-
Most of the studies included here used interviews. De Salis et al. 76 used observation to identify changes to make to a specific trial and were able to draw on more than accounts.
-
-
Impact on the current trial
-
The focus was on learning from current trials to inform future trials. De Salis et al. 76 main goal was the use of qualitative research to increase recruitment rates for the specific trial. This is an approach that should be considered more often.
-
Diversity of trial participants
Concerns have been raised about the external validity of trials if an intervention is tested on a narrow group of individuals who do not reflect the population which would receive the treatment in practice. 82 We identified seven articles focused on broadening diversity of participation in trials, with one undertaken at the pre-trial stage. Two were by the same authors so the six unique ones were selected for data extraction ( Table 13 ). Methods mainly involved focus groups, with one study making use of observation. 85
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| Goode et al. 2008 (grey article: two trials and mainly survey)83 | USA | RCTs of colpopexy and urinary reduction efforts (DD) | To identify themes for a questionnaire about barriers to recruiting older people to surgical trials | Two focus groups with trial coordinators | Identified ways of broadening participation in future surgical trials. Improves external validity of trials |
| Jaspan et al. 2010 (grey article: a group of trials)84 | South Africa | Planned Phase III HIV vaccine trials (DD) | To identify facilitators and barriers to including young people in planned trials | 18 focus groups with 200 young people, parents and community groups | Identified ways of broadening participation in planned trials. Improved communication with community about planned trials. Improves external validity of trials. Improves viability of trials |
| Joseph and Dohan 2009 (grey article: not specific trial)85 | USA | Academic medical centre recruiting to cancer trials (DD) | To explore how barriers impede recruitment of minority ethnic groups | Ethnographic study including observation and interviews with eight staff | Identified selection of ‘good’ patients as barrier to broad recruitment. Improves external validity of trials |
| Kogan et al. 2009 (grey article: not specific trial)86 | USA | Trials embedded in the STEP-BD programme for bipolar disorder (C) | To identify barriers to recruiting a more diverse sample of participants when adding community recruitment centres to academic ones | ‘Derivation of standard qualitative methods’. Meeting and telephone calls with staff from recruitment sites | Identified ways of broadening participation in this set of trials. Improves external validity of trials |
| Russell et al. 200887 | USA | Community-based RCT of behavioural interventions for increasing mammography screening (C) | To examine barriers to recruiting African American women for a trial and generate strategies to address barriers | Three focus groups of people involved in recruitment procedures | Checklist for future trials to facilitate recruitment of this group. Improves external validity of trials |
| Velott et al. 200888 | USA | Trial of community-based behavioural intervention in interconceptional women (C) | To document strategies used and offer perceptions of success of strategies to recruit low-income rural participants | Two focus groups with 4–6 facilitators and 13 interviews with trial recruitment facilitators | Ensured inclusion of hard-to-reach group in the trial. Promoted recruitment for specific trial. Improves external validity of trials. Improves viability of trials |
Authors of these articles were usually building on the evidence base about barriers to broadening trial recruitment by focusing on strategies for overcoming the barriers84,88 and understanding how these barriers impeded diverse recruitment. 85 Some of the studies appeared to be driven by the use of community-based trials,86–88 for which there was a need to include hard-to-reach groups such as those in rural areas, with low income, or from ethnic minority groups. The qualitative research by Jaspan et al. 84 had a high utility for the specific trial because it was identified as a way of interacting with the community and seen as a way of communicating and consulting with the community about the planned trial. There was an aspect of action research in the study by Velott et al. 88 in that they were trying to promote recruitment for their specific trial. This endeavour felt extremely useful because it was addressing the external validity of trials but also the viability of community-based trials. These kinds of articles could also challenge the interpretation of trial findings by highlighting problematic recruitment practices. 85
-
Consider the depth of the qualitative research required.
-
Some of these articles described lists of issues,83 which were useful, but some authors went beyond this to develop understanding. Russell et al. 87 used content analysis but embedded it in known literature to move beyond a simple list. While one of the authors was an anthropologist and observation was used, the question was not ‘what are the barriers’ but ‘how do barriers work’, looking for underlying explanatory rather than descriptive issues. 85 These authors identified the desire of recruiters to recruit good patients and described how the definition of a ‘good patient’ in their research setting excluded minority groups. There was also an example here of an approach which was labelled as qualitative research but was a meeting of recruiters to discuss problems. 86 It produced useful insights but there was also lack of credibility owing to lack of detail about who attended, how many attended and who was able to speak at the meeting.
-
-
Do not be tempted to downplay the strength of qualitative research.
-
There was some evidence of qualitative research being judged from a quantitative perspective in the discussion section of some articles. 87 For example, references were made to small numbers affecting generalisability and how obtaining ‘just perceptions’ would need to be validated. 87 This may have been related to the use of content analysis of the qualitative data in this article, but there is a need to be careful about the inferences drawn from any study and this can be done based on the strengths of the methodology used. In contrast, Joseph and Dohan85 dealt with this extremely well. They recognised that what constitutes a ‘good patient’ is likely to depend on context and that they had studied one context. They attended to the transferability of their findings by requesting that future trial leaders articulate what they think is a good patient to recruit and consider its effect on breadth of recruitment.
-
-
Be explicit about learning for trials.
-
Russell et al. 87 were explicit about the learning for future trials by producing a checklist and being clear about this in the abstract of the article.
-
-
Look beyond interviews and focus groups.
-
Focus groups were commonly used in this subcategory. The one study using observation was extremely insightful. 85 This may have been related to the anthropological background of one of the authors rather than the observation, per se.
-
Trial participation: the experience of being in a trial
The focus of articles in this subcategory was on understanding the challenges people face when in a trial, particularly during recruitment. Articles with a focus on recruitment or strengthening ethical conduct were classified elsewhere. We identified four articles, two of which were undertaken in the context of a group of trials and, thus, could have been excluded. We excluded other articles that were in this territory but appeared to make use of trials as a way of recruiting participants for an interview study rather than focusing on the trials themselves. We extracted data from all four articles ( Table 14 ). Only one study was undertaken at the pre-trial stage. The studies used interviews and two also used observation.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| Jollye 2009 (grey article: three trials)89 | UK | Three trials in a neonatal intensive care unit (DD) | To explore parents’ thoughts on participating in trials | Interviews with seven families of participants and non-participants in trials | Identified how recruiters could be more sensitive to parents. Improves sensitivity to human beings |
| Kohara and Inoue 201090 | Japan | Cancer Phase I clinical trial of an anticancer drug (DD) | To reveal decision-making processes of patients participating or declining a trial | Interviews with 25 people who did and did not participate and observation of six recruitments | Identified how recruiters could be more sensitive. Improves sensitivity to human beings |
| aMcCann et al. 201091 | UK | REFLUX trial of medical and surgical intervention for gastro-oesophageal reflux disease (DD) | To explore perspectives on recruitment and decision making processes | Interviews with 13 people asked to participate and observation of 25 people being recruited for a trial | Identified conditional altruism as a reason for participating in the trial. Improves sensitivity to human beings |
| Ward 2009 (grey article: not specific trial)92 | USA | A neonatal intensive care unit recruiting for trials where there is more than minimal risk to the neonate (DD) | To explore views of experience of being recruited for a trial | Qualitative descriptive study of telephone interviews with 27 parents who participated and did not participate in trials | Identified how recruiters could be more sensitive. Improves sensitivity to human beings |
The articles were about understanding trial participation, focusing on the decision-making processes people went through when asked to join a trial. They focused on sensitive trial recruitment practices, such as when parents were asked to put sick infants in a trial89,92 and cancer trials that recruited patients at the end of their life. 90 Their value was in understanding how trials can be more sensitive to human beings by avoiding misconceptions and improving communication as opposed to improving ethical procedures.
-
Consider the depth of the qualitative research required.
-
This subcategory lent itself to a more in-depth approach to analysis. McCann et al. 91 produced a sophisticated qualitative analysis based on observation and interview to develop the concept of ‘conditional altruism’ rather than simply list themes. Kohara and Inoue90 also provided an in-depth analysis using a grounded theory approach.
-
-
Be explicit about learning for future trials.
-
Good practice was stating clearly how the findings of a study were useful, and to whom, in both the abstract and body of the article. Some of these articles could have been much more explicit about how triallists or recruiters could use their research, for example, those recruiting for trials could understand how to be more sensitive to the journey a parent is on. 89,92 This contrasted with McCann et al. 91 who placed emphasis on the utility of their research by including a section in the discussion on implications for triallists.
-
-
Consider including those who do not participate as well as those who do.
-
Consider looking beyond interviews and focus groups.
The ‘in principle’ acceptability of the trial
This subcategory was about trials that researchers or policy-makers wanted to undertake but they were concerned that the research itself was not acceptable to health professionals or patients, or actual trials where concerns arose about the trial in principle. We excluded hypothetical trials from our review but they were relevant to this subcategory when there was a reason for undertaking a specific trial and researchers were exploring its acceptability in principle. 93 We found five articles, three of which were undertaken at the pre-trial stage, and undertook formal data extraction on all of them ( Table 15 ). Interestingly, studies used content and discourse analysis of newspapers as well as interviews and focus groups with health professionals and patients.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| aCampbell et al. 201094 | UK | Proposed trial of arthroscopic lavage vs. placebo surgical procedure for osteoarthritis of the knee (DD) | To describe attitudes of stakeholders to a trial | Four focus groups and 21 interviews with health professionals and patients | In principle the trial was acceptable but placebo trials were not acceptable to some stakeholders. Ensures viability of trials |
| Cooper et al. 200995 | South Africa/USA | Multicentre trial of microbicides in South Africa (article refers to trials but appears to be a specific trial undertaken in many centres) (DD) | To analyse how trials were portrayed in local newspapers | Content analysis of 13 newspaper articles | That media may shape public perceptions of trials. Ensures viability of trials |
| Lavender and Kingdon 200993 | UK | Hypothetical trial of planned caesarean vs. planned vaginal birth (DD) | To explore women’s views of participating in a planned trial | Interviews with 64 women a year after birth | A trial would not be acceptable. Saves time and money by not setting up a trial that would be unacceptable |
| aMack et al. 201096 | USA | Trial of oral pre-exposure prophylaxis for HIV prevention (DD) | To understand how and why negative media coverage was persuasive in the context to the premature closure of a trial | Critical discourse analysis of media coverage of trial | Identified ethical concerns about the specific trial and concerns about exploitation. Ensures viability of trials by addressing concerns using communication strategies |
| Rosenthal et al. 200997 | Australia/USA | Phase I trial of tolerance and safety of a microbicide gel for men (DD) | To explore experiences of trial participation | 36 longitudinal interviews with men in both arms of the trial | The requirement of abstinence for the trial was acceptable. Ensures viability of trials by showing acceptability |
There were two drivers for using qualitative research to explore the acceptability of a trial in principle. The first was concern about the viability of a proposed trial because key service providers or target participants may be unwilling to participate in a trial which required doing something difficult, for example abstaining from sex,97 the randomisation of options may be unacceptable93 or the control controversial (Campbell et al. ,94 with placebo surgery). Qualitative research for this purpose seemed extremely useful by saving time and money and avoiding the low morale that might be involved in setting up a trial that failed. 93,94 Of course, it could be argued that the qualitative research itself cost time and money and that a cost–benefit comparison may be necessary. However, there was utility of the qualitative findings beyond the proposed trial because it identified why there was a problem so that other researchers could consider the viability of related trials. There was also explicit consideration of the implications for wider types of trials, for example placebo trials and surgical trials in general. 94 The second driver was the closure of a trial and how media portrayal influenced the closure or could influence the viability of future trials by raising the issue of these trials being unacceptable in principle. 95,96
-
Be clear about the research questions.
-
Some studies focused only on the acceptability of a trial in principle and offered very clear findings. 93,94 However, some addressed so many issues concerning both the intervention and the trial itself that it was difficult to understand the purpose of the study and, therefore, engage with the findings. 97
-
-
Focus on in-depth understanding as well as description.
-
The critical discourse analysis by Mack et al. 96 stood out as an exemplar of qualitative research used to understand rather than describe an issue. They offered a sophisticated analysis focusing on how and why the media coverage was persuasive rather than simply describing the media coverage and its effect.
-
-
Make the implications for different audiences clear.
-
Campbell et al. 94 offered an exemplary approach to thinking about the implications of their research for a number of audiences in both the abstract and the conclusion section of their publication, that is, implications for researchers undertaking trials of surgery and placebo surgery trials as well as those interested in the specific trial under consideration. The longer report format of their publication may have helped by offering more space, which might encourage authors to consider the wider implications of their work. However they should be given full credit for doing this in a thorough and clear manner. Others had limited conclusions and reflections for trials, leaving the reader wondering what action triallists might take in response to the findings. 95
-
-
Be explicit about the origin of the research question.
The acceptability of a trial in practice
There were four articles that focused on the acceptability of the trial rather than the intervention in practice. We undertook detailed data extraction on all four articles ( Table 16 ). One study was undertaken at the pre-trial stage. Interviews and focus groups were used.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| Beal et al. 200998 | USA | Wellness intervention for women with fibromyalgia syndrome (C) | To explore how the RCT was experienced by participants, particularly the controls and any placebo effect | Interviews with 16 people who had received intervention or control in a RCT | Showed how the control offered can affect the results of the trial. Challenges what control is offered so that trial results are more informative |
| Jonvallen 2009 (grey article: two trials)99 | Sweden | Multicentre clinical trial of a potential obesity drug (DD) | To explore compliance with protocol when external trial management is used | 19 interviews with staff managing the trial and observation | Staff motivated participants to stay in the trial even when they did not comply with the treatment. Challenges validity of trial findings |
| Thorstensson et al. 2009100 | Sweden/Denmark/UK | Training vs. surgical reconstruction with training for acute injury (C) | To understand participants’ decision to cross over in a trial | Interviews with 34 trial participants who crossed over in the trial | People joined the trial to get the intervention. Offers guidance for future trials where there is strong preference for a treatment to expect crossover and build it into the design to improve the internal validity of future trials |
| Tutton and Grey 2009101 | UK | Feasibility trial of fluid optimisation after hip fracture (DD) | To increase knowledge of implementation of intervention and feasibility of trial | Two focus groups with 17 staff and interview with the research nurse | Identified difficulty recruiting for trial in busy environment and other solution to deal with health problem. Saves money by not doing full trial. Identifies a better intervention |
One of these studies was undertaken in the context of a feasibility trial, for which the researchers wanted to know whether or not it was feasible to run a trial in a busy emergency environment. 101 The main trial was not feasible owing to difficulties recruiting, ability to consent in a busy ward and emergency situation and ethical issues of carer consent when patients were frail. The other three were undertaken in the context of the main trial, for example to consider the acceptability of the trial design to researchers because the control arm offered a service which the researchers believed did not contain the active ingredient but could have offered what they term placebo effects. 98 It was not clear whether or not this was prompted by the trial results, whereas Thorstensson et al. 100 undertook the qualitative research in response to something going wrong with the trial because a large number of participants were moving from one arm to the other and there was a need to understand why Jonvallen99 did not present an explicit motivation for her study. Using qualitative research to consider the acceptability of a trial in practice felt like a useful thing to do. It identified why the trial was not feasible in a busy emergency situation,101 questioned whether or not some controls offer interventions in themselves,98 identified lessons for future trials of interventions that were likely to have strong user preferences100 and questioned whether or not ensuring compliance with trial protocols could cover up the lack of compliance with the intervention. 99
-
Make the implications for different audiences clear.
-
All the articles would have benefited from making the implications for different audiences clear in both the abstract and the conclusions. This was certainly an issue for Thorstensson et al. ,100 for which conclusions could have been directed at triallists to help them deal with strong preferences for treatments by perhaps using different designs in this situation. In other articles, there was opportunity to maximise the value of the qualitative research by going beyond description of the results in the abstract and including implications. For example, Beal et al. 98 could have discussed whether or not triallists should use a control comparison comprising large amounts of education, what other types of controls might be more appropriate and what types of trials and interventions their findings were applicable to. Instead, it was framed as a general inquiry about placebo effects and trial participation.
-
-
Do more of this at the pre-trial stage.
-
Only one of the four studies was undertaken at the pre-trial stage so that lessons were for future trials rather than the specific trial with which the qualitative research was undertaken.
-
Ethical conduct in a trial
Informed consent for participating in trials is a well-researched area. 73,102 Participant concerns about informed consent can be a barrier to recruitment. 73 Interventions to improve participant understanding of trials have had limited success, with the exception of someone offering one-to-one information sessions. 102 Qualitative research has been used to refine trial consent procedures to ensure an ethically acceptable trial design. 103 We found 16 articles that focused on the ethical conduct of the trial, with most of the qualitative studies (n = 14) undertaken during the main trial and very few at the pre-trial stage. We ordered them alphabetically and selected every second one for detailed data extraction ( Table 17 ). Three of these six articles made use of observation as well as interviews and focus groups.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| Gikonyo et al. 2008104 | Africa and UK | Double-blind RCT of safety and efficacy of malaria vaccine (DD) | To explore community understanding of the trial in a rural low-income setting | Interviews and focus groups with three trial recruiters, nine community leaders and two workers | Identified locally appropriate ethical practice, respect for communities who participate in trials and ensured participation in the trial. Challenges established ethical practice. Improves sensitivity to human beings. Ensures viability of future trials |
| Instone et al. 2008 (grey article: because not specific trial)105 | USA | A series of Phase I to Phase III placebo drug trials for people with HCV occurring in a single medical centre (DD) | To examine the processes of informed consent and enrolment into HCV clinical trials | Ethnographical study using interviews with 19 researchers and 25 patients, observation and documents | Identified promotion of therapeutic misconception. Improves ethics for future trials. Improves sensitivity to human beings |
| Mangset et al. 2008106 | Norway | Trial of thrombolytic drug treatment within 6 hours of a stroke (DD) | To explore critically ill patients’ experience of informed consent | Longitudinal interviews with 11 people (26 interviews) who had completed the consent processes for a trial | Identified that disclosure of too much information may be damaging and that there is a need to individualise not standardise informed consent. Challenges ethics paradigms. Improves sensitivity to human beings |
| Penn and Evans 2009107 | South Africa | Community vs. clinic-based antiretroviral medication in multisite trial in South Africa (DD) | To understand the effectiveness of using a modified informed consent process rather than a standard one | Observation and interviews with 13 recruiters and 19 students going through two different informed consent processes | Identified ways of improving ethics and reducing anxiety when enrolling in trials. Improves sensitivity to human beings. Improves accessibility to trials if people understand more about them |
| Timmermans and McKay 2009 (grey article: two trials)108 | USA | Two Phase III randomised clinical trials for methamphetamine dependency (DD) | To examine experiences of participating in trials | Observation and interviews with 10 staff and 60 trial participants | Identified that the trials offered treatment to people without access to treatment. Challenges beliefs about ethics and trials using empirical data |
| Wasan et al. 2009109 | USA/Canada | Double-blind trial of analgesia for back pain (DD) | To explore experiences of trial participation in the context of informed consent and therapeutic misconception | Telephone interviews with 52 trial completers | Identified that the complexities of reasons for participation does not equate to therapeutic misconception. Ensures ethical trials. Challenges beliefs about ethics and trials using empirical data |
Researchers appeared to be driven by concerns that informed consent for participation in trials was problematic in practice. Some were driven by concerns about therapeutic misconception – the conflation of research with clinical care105,108,109 – and whether or not it was actually occurring in a trial. For example, Wasan et al. 109 seemed to be concerned about whether patients joined the trial for clinical benefit when there was no likely benefit. Others were concerned about complicated informed consent processes that appeared to pay no attention to important communication issues relevant to different groups. 107 Gikonyo et al. 104 were part of a Social and Behavioural Research Group independent of the trial, funded to consider a wide range of issues around the trial. They were interested in whether or not everything was working as planned, including informed consent. They produced multiple articles about how to help RCTs work in low-income or diverse communities. Mangset et al. 106 were funded by an ethics group and focused on people who had just had a stroke and for whom informed consent was difficult.
This appeared to be a valuable contribution to the trial endeavour because it challenged ethical assumptions by both ethicists and triallists, showing that informed consent could be more complicated than ethicists present. For example, people do not participate in trials for one simple reason but for a mix of altruism, desire for clinical benefit, access to treatments they do not have access to, or to help health professionals who are there to help patients. 105,108,109 They identified that a benefit of participating in a trial could be better health care when trials are undertaken in contexts in which people pay for health care. 104 This body of research also showed that gold standard informed consent procedures could be problematic and that a simpler informed consent process, which paid attention to language and communication, could improve people’s understanding and, therefore, access to trials. 107 This research also showed that emphasis on formal information giving procedures rather than attention to building relations with a low-income community could be problematic104 and showed the need for individualised rather than standardised informed consent. 106 There was an overlap here with the next subcategory of adaptation of the trial conduct to the local context. As well as considering how to improve informed consent, researchers focused on making trials work for diverse groups, paying attention to local rules and appropriateness104,107 and respect for communities. 104
-
Consider undertaking an in-depth analysis.
-
Be explicit about learning for the trial endeavour.
-
The message for the trial endeavour was very clear in these articles. There was only one example in the six articles where the researchers failed to offer direction and recommendations for that type of trial with that type of patient.
-
-
Be prepared to challenge standard practice and accepted beliefs.
-
One strength of this set of articles was that the authors were prepared to challenge ethicists’ theories using empirical evidence as well as challenge what is considered to be gold standard ethical practice in trials.
-
Adaptation of trial conduct to local context
Interventions may be community based and targeted at ‘hard-to-reach’ communities that are vulnerable or stigmatised. A trial may only be viable within such communities if attention is paid to adapting the trial to the complex context in which it occurs. We found two articles in this subcategory and both used participatory or community action research ( Table 18 ). One of these was undertaken at the pre-trial stage. Both articles reported using a range of methods within this methodological approach: focus groups, interviews, community forums, community councils and participatory mapping. Only some methods were qualitative research and it was difficult to disentangle the contribution of the qualitative research from the community/participatory action research. It is likely that we are discussing the contribution of the latter here.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| Balcazar et al. 2009 (grey article: is it qualitative research?)110 | USA | Pragmatic RCT of health education classes and contact with community health workers for cardiovascular disease prevention vs. educational materials (C) | To present lessons learnt from using community-based participatory research with this trial | Community-based participatory research, including three focus groups with 24–30 community members | Communicates importance of intervention and research to the local community. Community empowerment. Improves quality of intervention and specific trial. Ensures viability or acceptability of future trials |
| Shagi et al. 2008 (grey article: is it qualitative research?)111 | Tanzania/UK | Feasibility study for efficacy and safety Phase III trial of vaginal microbicide (DD) | To explore feasibility of community liaison system | Participatory action research, including interviews and workshops | Improved ethical conduct, recruitment and retention in local context. Ensures viability of the specific trial. Facilitates ethically sound trials |
The core activity in both articles appeared to be the establishment of a liaison network and a council/committee of local people to facilitate two-way communication about the trial. The key driver appeared to be ensuring the trial was feasible in the local context. Other aims were at play, with specific reference to increasing participation within the trial,111 empowerment of a community, dialogue between community and researchers so that research is undertaken with people and not on people,110,111 reduction of the probability of negative reactions to the trial111 and good participatory practice. 111
Taking a participatory approach to making trials appropriate to local contexts seemed like a very important thing to do for community-based trials in ‘hard-to-reach’ communities. Shagi et al. 111 made the point that this was not only essential to the successful conduct of their feasibility study for the trial and would also be used in the main trial. Balcazar et al. 110 reported that the process helped disseminate to the community the need for the trial. The two-way communication seemed key to improving the trial quality and trial sensitivity to human beings.
-
Consider the utility of an action or participatory research paradigm/methodology.
-
Researchers used participatory research rather than qualitative methods to improve local appropriateness. This approach could be used in community-based trials as a matter of course.
-
-
Measure success of participatory approaches.
-
Participatory approaches may be expensive and cost-effectiveness must be considered. Balcazar et al. 110 felt that it was important to take that approach but also say ‘unfortunately, specific data to measure success of this interaction were not collected’. Shagi et al. 111 did collect quantitative data on the goal of the action research, for example, 1600 women were recruited and follow-up rates > 80% were achieved for all routine clinics, but the authors could not attribute this to their participatory approach. If people use a participatory approach they may wish to provide evidence of its success to convince others to use it.
-
Impact of trial on staff, researchers or participants
Trials can affect staff delivering the intervention or collecting data for the trial as well as those participating in the trial. We found five articles in this subcategory and extracted data for them all ( Table 19 ). There was potential overlap between the subcategory Trial participation: the experience of being in a trial and articles in this subcategory, which focused only on trial participants. The articles included in this subcategory went beyond experiences of participation and explored the impact of being in the trial (rather than having the intervention) on participants’ treatment or wellbeing. Only one study was undertaken pre-trial. The qualitative methods were interviews, focus groups and two used a longitudinal design.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trial and the trial endeavour |
|---|---|---|---|---|---|
| aGrbich et al. 2008112 | USA | Factorial cluster trial of different models of palliative care including educational outreach and case conferences (C) | To explore the effect of the trial on staff, for example whether or not staff felt burdened | Longitudinal focus groups (11 in total) with staff delivering the intervention and collecting the data at three time points during the trial | Improved trial procedures. Kept people on board with the trial. Indicated how to improve future trials in research-novice organisations. Ensures sensitivity to human beings. Ensures viability of trials |
| Kyle and Marks-Maran 2008113 | UK | Multicentre pilot RCT of efficacy of aromatherapy in reducing levels of anxiety amongst palliative care patients (C) | To learn how the aromatherapists felt about practice changes as a result of participating in the RCT | Three focus groups with 14–19 therapists who had provided treatment in the trial and reflective diaries | Offered empirical evidence that both supported and refuted concerns held within a practitioner community. Improves acceptability of future trials |
| Paterson et al. 2008114 | Australia/UK | Single blind trial of acupuncture vs. sham acupuncture for migraine (DD) | To explore the impact of the trial context on participants | Longitudinal interviews with 10 participants and a single service provider | Interpreted results of specific trial. Interprets trial results |
| Stadler et al. 2008115 | South Africa | Feasibility study of a Phase III trial of a microbicide (DD) | To explore subjective experience of women in RCT | 13 focus groups with the community and 10 focus groups and 21 interviews with participants | Identified sense of empowerment of trial participants. Ensures viability of future trials by ensuring people participate. Ensures sensitivity to human beings |
| Yawn et al. 2010116 | USA | Pragmatic practice-based trial of Translating Research into Practice for Postpartum Depression (C) | To explore the impact of participating in a trial | Interviews with 48 nurses and doctors in family practice participating in a trial as part of a research network | Showed benefits of running trials for family practices. Ensures viability of future trials |
Researchers were not explicit about why they used qualitative research for this purpose. It seemed to be driven by concerns about the viability of the specific trial and future trials: concern that the practitioner community had little experience with trials and thus might be burdened by the trial,112 concern about the viability of trials in the context of the closure of trials in a particular community,115 or concern that practitioners might not be able to practice normally within a trial. 113 A different concern was that trials might impact on the content of the very treatment they were trying to test. 114 It appeared to be useful work for both the specific trial and future trials. For example, Grbich et al. 112 reported that the qualitative research improved communication so that adversaries of the trial turned into advocates, sorted out problems at an early stage, resulted in improvements in some trial procedures and identified issues about neglecting non-trial patients to offer extra care to trial patients. There was also evidence of challenging assumptions. Kyle and Marks-Maran113 challenged what appeared to be strong concern within a community about RCTs and with empirical evidence showed that although that concern existed, there were other positive aspects of RCTs. Paterson et al. 114 challenged the assumption that treatments offered in RCTs reflect practice.
-
Be clear about the rationale for the research.
-
These articles had very helpful messages for trials, but these were not necessarily clear because the rationale for the research question was not clear.
-
-
Balance the needs of the specific trial and the stakeholders.
-
Grbich et al. 112 had to pay attention to what they could change within the specific trial and what needed to stay the same. They could not change the intervention too much within their trial by dispensing with case conferences because staff did not like them but could change some issues around the delivery of the intervention.
-
-
Be explicit about the implications for trials.
-
Grbich et al. 112 paid attention to the implications of the qualitative research for both the specific and futures trials, detailing these in a large section in the discussion. Other articles in this subcategory could have been clearer about this.
-
Category 3: outcomes
The third broad category of qualitative research focused on the outcomes of the trial. It was a very small category, with only five articles, accounting for 1% of the 356 uses of qualitative research. This was surprising given that this use of qualitative research is included in other frameworks. 6,8 None of the qualitative research was undertaken at the pre-trial stage. There were two subcategories:
-
breadth of outcomes
-
variation of outcomes.
Breadth of outcomes to be measured in the trial
It is usual to have a number of outcomes measured within a trial. These outcomes may be selected by the intervention developers or the triallists, based on a theoretical model of how the intervention is intended to work, with identification of a pathway of expected outcomes. For example, someone receiving a healthy eating intervention may be expected to eat less fat in order to reduce their weight, which would result in measurement of both fat intake and weight within the trial. Alternatively, qualitative research can be used to determine the range of outcomes relevant to the intervention. One might expect this to be used at the first two stages of the MRC framework for developing and evaluating complex interventions. As the intervention is being developed, or a feasibility study or pilot trial is being undertaken, the target group for the intervention can participate in qualitative research to describe the outcomes they perceived they gained after using the intervention and this can be used to consider the outcomes to be measured in the main trial. We identified one article with this focus ( Table 20 ). It differed from the articles focusing on ‘perceived value and benefits of the intervention’ because of the focus on challenging the outcomes which were actually measured in the trial. This article was a candidate for exclusion because the data collection of using an open question on a data collection instrument in the trial was not qualitative. We included it as a ‘grey’ article because the analysis and reporting of the findings was more in-depth than other articles using open questions on surveys.
| Paper | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research to trial and the trial endeavour |
|---|---|---|---|---|---|
| Alraek and Malterud 2009 (grey article: open questions in survey)117 | Norway | Pragmatic RCT of acupuncture to reduce symptoms of the menopause (DD) | To describe changes in health in acupuncture arm of trial | Written answers to open question on questionnaire to 127 patients in intervention arm | Range of outcomes in the trial was not comprehensive. Ensures right outcomes measured in future trials |
The researchers did not appear to set out to address the breadth of outcomes best measured in the main trial. As part of the main trial, they collected data on the patient experience of the intervention and identified the opportunity to consider the breadth of outcomes used in the trial compared with the perceived benefits identified within the open comments. 117 They asked all 127 trial participants receiving acupuncture for menopausal symptoms to complete a written statement about changes they attributed to the intervention. The participants identified a variety of health changes that the authors were concerned may not be revealed by limited outcome measures in acupuncture studies. The utility felt high in terms of questioning whether trials could be missing benefits important to patients or perceived as being achieved by patients,117 although the potential value was for future trials.
-
Consider timing in the context of the MRC framework for Evaluation of Complex Interventions.
-
Alraek and Malterud117 took advantage of a data set to explore this issue. However, the utility of using qualitative research for this purpose lies at the early stages of the MRC framework to ensure that an optimum set of measures is used in the full trial.
-
-
Consider the value of perceived outcomes identified.
-
This approach has the potential to identify many possible outcomes. Alraek and Malterud117 asked respondents to identify ‘even issues that might be considered as less important’. Qualitative research could consider the problem of respondent burden during data collection in trials and give attention to the value of perceived outcomes by participants so that only outcomes valued by participants were measured.
-
-
Be explicit about the message for the current trial or future trials.
-
Articles could do more than problematise the outcome measurement of the trial and identify explicitly what outcomes trials should measure, or what important outcomes they failed to measure and the implication of this for the conclusions of the trial.
-
Variation of outcomes
Qualitative research can be used to explore variation in the health outcomes of individuals receiving an intervention within a trial, or variation in outcomes for different clusters in a trial. We found four articles published between 2008 and 2010 that used qualitative research to explore variation in outcomes within a trial ( Table 21 ). We expected these to be undertaken with the main trial and, indeed, all four were undertaken at that stage. Two of the articles addressed variation explicitly, exploring variation in outcomes between the clusters in cluster trials. 39,118 The article by Hoddinott et al. 39 also described an explanatory model and appears in Table 6 because it is such an excellent example of both subcategories. The other two articles addressed variation between individuals in a trial and also fit elsewhere in the framework. In one article, the trial was an opportunity to explore a research gap and was treated as so peripheral to the qualitative research that this article might belong in Experience of the disease, health behaviour or beliefs. 120 In another article, successful outcomes were the focus, with unsuccessful outcomes included in the sample in a minor way and the article might better fit under Development of the trial intervention. 119 We included them here because they helped to illustrate the potential and practice of using qualitative research to explore variation in outcomes.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research to trial and the trial endeavour |
|---|---|---|---|---|---|
| Aagaard et al. 2010118 | USA | Cluster trial of educational intervention to reduce antibiotic prescribing for acute respiratory infections in emergency departments (C) | To identify organisational factors influencing the effectiveness of the intervention and explain variability in trial outcomes | Mixed methods study including 19 telephone interviews and seven focus groups with staff in seven emergency departments | Leadership explained why the intervention was effective in some clusters and not others. Explains trial results. Facilitates transferability of results by identifying contextual issues important for success |
| aHoddinott et al. 201039 | UK | Cluster RCT of community breastfeeding support groups to increase breastfeeding rates (C) | 64 ethnographic, in-depth interviews, 13 focus groups and 17 observations to produce seven locality case studies, informed by a realist approach | 64 ethnographic, in-depth interviews, 13 focus groups and 17 observations to produce seven locality case studies, informed by a realist approach | Explained variation in seven communities and why rates decreased in some as well increased in others. Explains trial results. Facilitates transferability of results by identifying contextual issues important for success |
| Penn et al. 2008119 | UK | European Diabetes Prevention Study of diet and education for people with impaired glucose tolerance (C) | To understand the experience of participants who maintained behaviour change, in order to inform future intervention design | Interviews with 15 intervention and control patients who were successful at maintaining behaviour, including three who did not achieve success | Context affects success and must be considered for future interventions. Optimises intervention for future trials |
| Peterson et al. 2010120 | USA | Healthy Behaviour Trial of education on modifying risk factors after angioplasty (C) | To document value, attitudes and beliefs that influence behaviour change | Mixed methods including 61 interviews with trial participants who had successfully changed behaviour or not | Identified that interventions need to be culturally relevant and adapted to physical abilities. Optimises intervention for future trial. Helps interpret trial results |
Variation in outcomes measured is likely to occur in any trial. Some variation will be random but some may be explained by differences between individuals receiving the intervention or differences between groups or communities in a cluster trial. Researchers from the two cluster trials were driven to explore variation in outcome because they expected it to occur39 or identified the variation and wanted to understand why it had occurred;118 they used qualitative data collected during the trial. We wondered if this would be driven by null trials but this was not the case because Hoddinott et al. 39 planned to do their analysis prior to the trial and the trail reported by Aagaard et al. 118 trial was positive. The researchers seemed aware of the different contexts in which the intervention operated within the different clusters. Understanding the effect of context on outcome could then lead to understanding the transferability of findings to different contexts, offering textured conclusions from the trial rather than a simple ‘yes/no’ answer. 118 The utility of exploration of variation in outcomes for individual patients was less obvious in the two articles included here and this may have been because they did not absolutely fit this subcategory. 119,120 Both had useful messages for future intervention developers and interestingly, both identified the importance of the individual’s context to making or maintaining successful lifestyle change. The findings could also have been used to interpret the trial results but were not.
-
Pay attention to validity and credibility.
-
Blinding was identified as an important issue in the two cluster trials for which the outcomes of a small number of clusters were compared with qualitative findings from each cluster. Hoddinott et al. 39 undertook seven ‘embedded case studies’ using the clusters as cases and emphasised the importance of building their model of how organisations used the intervention prior to knowing the trial outcomes ‘to minimise bias’. Aagaard et al. 118 identified the importance of blinding the researchers to the seven emergency departments in their study when analysing the qualitative data, but stated it was not possible, and this, therefore, was a limitation of the study. For individual-level studies, it was blinding of individuals during the interviews to their categorisation of ‘benefited from intervention’ that researchers identified as important. 119,120
-
There was a need for transparency when defining an individual as having benefited from the intervention or when producing ratings based on outcomes and processes, stating how this was done and who was involved. Aagaard et al. 118 used their qualitative research to produce a ‘qualitative rating’ on three key issues, including leadership of quality improvement. Then they compared scores on outcomes with scores on processes across the seven clusters. They hypothesised the direction of effect for their three process variables and found it for one, found nothing for another, and found the opposite to the hypothesised direction in the third. The authors drew due attention to the limitations of doing this, rightly reporting that their results were formative, but perhaps still drew too firm a conclusion based on the data they had.
-
-
Clarify implications for practice.
-
Penn et al. ,119 Peterson et al. 120 and Hoddinott et al. 39 were clear about the implications of their findings, although Aagaard et al. 118 could have considered the implications for transferability of findings and mechanisms of action and Peterson et al. 120 could have considered implications for the specific trial.
-
-
Consider using ethnography.
-
Hoddinott et al. 39 produced an in-depth and convincing analysis, highlighting the strength of ethnography for that the outcomes would vary and planned to investigate this. This prospective the work they were doing.
-
-
Plan for variation.
-
Hoddinott et al. 39 expected approach was likely to ensure they had the information and resources they needed to explore any variation.
-
Category 4: measures used in a trial
The fourth broad category focused on the measures used within the trial. Leidy and Vernon121 reflect on the role of qualitative research for ensuring the clarity and content validity of patient reported outcomes in clinical trials. They focus on the instruments used to measure outcomes and identify the role of qualitative research in selecting the right instrument, ensuring the selected instrument matches issues important to patients, constructing a new instrument or adapting an existing one and considering the validity of using validated outcome measures in different groups. We identified 10 articles focusing on the measures used in the trial, accounting for 3% of the 356 uses of qualitative research. One-third of the articles (3/10) reported qualitative research undertaken at the pre-trial stage. There were three subcategories which aligned to some extent with those of Leidy and Vernon121 but which focused on process as well as outcome measures:
-
accuracy of measures
-
completion of measures
-
development of measures.
Accuracy of measures
We identified seven articles related to assessing the accuracy of both process and outcome measures used in the trial and selected six for data extraction ( Table 22 ). Three studies were undertaken at the pre-trial stage and interviews were the main approach used, including cognitive interviews.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research to trial and the trial endeavour |
|---|---|---|---|---|---|
| aFarquhar et al. 2010122 | UK | Phase II pilot RCT of breathlessness intervention for COPD (C) | To explore the feasibility of using an outcome measure for the main trial | Longitudinal interviews with 13 patients in the intervention arm on 51 occasions. Recording of participants completing a questionnaire | Rejected use of outcome measure for the main trial owing to lack of validity in this patient group. Ensures right measures used in specific and in future trials |
| Penman-Aguilar et al. 2009123 | USA/Madagascar | Pilot RCT of diaphragm with microbicide for STIs (DD) | To obtain views of adherence and acceptability of intervention | Interviews with 47 patients in the intervention arm | Identified that a process measure in the pilot RCT was not measured accurately. Ensures right measures used in specific and future trials |
| Pool et al. 2009124 | The Netherlands | Neck pain (no detail given) (C) | To explore patient understanding of a scale used in a RCT | Cognitive ‘think aloud’ interviews with 13 patients in RCT | Identified problems with an outcome measure used in the trial. Ensures right measures used in future trials |
| Pool et al. 2010125 | Various | Double-blind placebo trial of efficacy of gel in preventing HIV infection (DD) | To consider adherence with intervention | 725 interviews, 219 focus groups and observation with participants in the RCT, staff in the RCT and the community | Identified way of collecting more accurate adherence data. Ensures right measures used in future trials. Ensures right interpretation of future trial results |
| Robertson et al. 2009126 | UK | RCT of two treatments for Paget’s disease: intensive therapy vs. symptomatic treatment (DD) | To explore how people evaluate quality of life when completing outcome measures | Cognitive interviews with 21 participants in the RCT | Identified implications for the use of health-related quality-of-life instruments in trials. Ensures right measures used in future trials |
| Sloboda et al. 2008127 | USA | Cluster RCT of a drug prevention education programme (C) | To explore the meaning of drug prevention education | Interviews with 51 students in the RCT | Survey used to identify exposure to the intervention was worded in a misleading way. Ensures right measures used in future trials. Ensures right interpretation of future trial results |
Researchers did not necessarily plan to explore the accuracy of measures as the aim of their qualitative research pre-trial. It appeared to occur in response to problems arising during data collection and analysis of the trial. An exception was an exploration of the performance of a validated instrument for a particular patient group in a pilot trial in preparation for the main trial. 122 In other studies, issues occurred within the trial and qualitative research was established to explore them, for example, during the trial the research assistants were being asked to clarify how a key outcome measure should be completed. 124 The reason for undertaking the qualitative research was not always clear,123 with some suggestion that a team member had a particular interest and explored it using the trial as opportunity. 126
Even though there was only one example of the planned use of qualitative research on the measures used in the trials prior to the main trial in our six extracted articles, and three in total in this subcategory, there appeared to be merit in making this the planned objective of future qualitative research with trials. The utility felt high by providing extra instructions to allow valid completion of validated measures,126 not using an inappropriate instrument in the full trial,122 and facilitating the development of accurate measures using quantitative data in the full trial. 123 The potential value was improving the internal validity of the specific or future trials.
-
Increase depth of qualitative research.
-
Reflect on the relationship between the qualitative and quantitative research.
-
There was disagreement about two approaches to ensuring accurate measures were taken in RCTs. The first was that having explored accuracy using the qualitative research, then quantitative measures could be used. 123 The second was that qualitative research should be used to collect difficult to measure processes and outcomes within the trial, even if numbers were large and it was time consuming and expensive. 124,125 The best option might be dependent on the situation, with attention needing to be paid to the trade-off between efficiency and accuracy.
-
-
Link with the current trial or future trials.
-
Messages for future studies and trials were sometimes explicit in the abstract. 123,124,126,127 For example, Penman-Aguilar et al. 123 was clear that they had learnt a lot for the main trial using the qualitative research in their mixed methods study in their pilot trial and developed a measure for the main trial. Farquhar et al. 122 concluded that they would not use the instrument in the main trial, although the abstract focused more on the learning for people using the instrument in general.
-
-
Timing of research: more pre-trial work needed.
-
In an ideal world, all of this type of work would occur at the pre-trial stage and there were examples of this. 122,123 However, some of the articles used qualitative research during or after the main trial,124–127 so that lessons learnt were relevant only to future trials rather than the specific trial.
-
Completion of process and outcome measures
We identified only one article exploring completion of outcome measures ( Table 23 ). This was related to retention of participants within a trial (see earlier subcategory Trial recruitment and retention) but here retention was affected by how the outcome measures were administered. This study was not undertaken at the pre-trial stage and used interviews with trial participants.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research to trial and the trial endeavour |
|---|---|---|---|---|---|
| Nakash et al. 2008128 | UK | RCT of mechanical supports for severe ankle sprains (DD) | To examine factors affecting response and non-response | Interviews with 22 participants, eight of whom did not respond | Identified reasons for non-response such as not understanding trial and fully recovered. Improves internal and external validity of future trials. Helps interpretation of specific and future trials |
Non-completion of outcome measures affects the internal and external validity of a RCT. Nakash et al. 128 undertook a useful study exploring why some participants did not return their postal survey within the RCT. They argued that the usual methodological issues about survey response might not apply within a RCT. Indeed, they found that a lack of understanding of the trial resulted in non-return of questionnaires, identifying ways of improving completion of measures in future trials.
-
Timing of research: more pre-trial work needed.
-
Nakash et al. 128 undertook this useful work during the main trial with lessons for future trials, when it would have been more beneficial at the pre-trial stage in order to impact on the specific trial.
-
Development and validation of measures
When reading abstracts and full articles, we identified a subcategory of the use of qualitative research for developing and validating instruments for use in trials. They were identified by our search strategy because they explicitly reported that the instrument was being developed for use in trials and they used secondary analysis of data from a trial to test the quantitative psychometric properties of a newly developed instrument.
We identified two articles that described the development of an instrument for measuring a secondary outcome in a trial ( Table 24 ). These studies were not completed prior to the start of trial to allow the use of the instruments to measure processes or outcomes in the trial. Both articles used mixed methods research, with the qualitative research undertaken prior to the trial to develop the instrument and the quantitative research undertaken during the trial. Focus groups or interviews were used to identify items for a questionnaire and cognitive interviews to explore content and face validity of that questionnaire. We found other articles doing this work but excluded them because the qualitative research was not described in enough detail. There are likely to be more articles describing the development of an instrument for use within a future trial, but these two were included because some of the quantitative psychometric testing took place using trial participants.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research to trial and the trial endeavour |
|---|---|---|---|---|---|
| Abetz et al. 2009129 | UK/USA/France | Double-blind placebo RCT of patch treatment in Alzheimer’s disease (DD) | To identify items for an instrument for use in the specific RCT and check acceptability of developed questionnaire on carer satisfaction | Three focus groups with 24 carers prior to trial to identify items and 10 cognitive interviews during trial | Ensured use of validated instrument within trial. Improves credibility of the findings |
| Leidy et al. 2008 (grey article: is it qualitative research?)130 | USA | Phase III trial of two products for mild to moderate asthma (DD) | To develop instrument to measure patient perceptions of immediate effect of medication | 53 interviews to develop instrument before the trial and 50 cognitive interviews | Ensured use of validated instrument within trial. Improves credibility of the findings |
Trials need validated measures and these are not necessarily available for secondary outcomes in a trial. Resources may not be available to develop validated secondary measures prior to the main trial. The two articles included here faced this situation and used a mix of qualitative research before the main trial and quantitative psychometric testing during the main trial to develop validated outcome measures. The potential value was to ensure validated instruments were available for future trials, or to improve the credibility of the trial findings by being able to report that these were based on validated instruments.
-
Describe the qualitative research in more detail.
-
These articles reported mixed methods studies. Articles reporting mixed methods research have space limitations that affect the reporting of details of methods and findings. The authors here chose to limit the space given to reporting the qualitative research, but this can be problematic when the basis of the instrument is the qualitative research.
-
-
Explicitly consider the utility of instruments beyond the specific trial.
-
Some instruments may be useful for a range of interventions or may be very specific to the intervention for which it was developed. Researchers should be more explicit about the utility of their instrument beyond their specific trial and the extent to which an instrument has been shaped by the needs of a trial, which might make it difficult to use in other trials. 129
-
Category 5: target condition
The fifth broad category focused on the condition that the intervention was aimed at within the trial, for example depression, with 33 articles accounting for 9% of the 356 uses. Only 6% (2/33) articles reported qualitative research undertaken at the pre-trial stage. We identified only one subcategory within this category:
-
experience of the disease, behaviour or beliefs.
Experience of the disease, health behaviour or beliefs
For detailed data extraction, we put the 33 articles in alphabetical order and selected every sixth article. These focused on a range of health issues: the meaning of a service to users, help-seeking behaviour, health service utilisation, the nature of a symptom and self-management ( Table 25 ). The topics were always related to the trial intervention in some way, either exploring the issue that the intervention was attempting to affect or something related to that issue. All the studies used interviews or focus groups with patients participating in the trial, carers of trial participants, or health professionals who were trial participants or service providers within the trial.
| Study | Country | Trial | Aim of qualitative research | Qualitative methods and sample | Value of qualitative research for trials and the trial endeavour |
|---|---|---|---|---|---|
| Bair et al. 2009131 | USA | Pragmatic RCT of antidepressives and pain management vs. usual care (C) | To identify the facilitators and barriers to self-management of chronic pain for patients with pain and depression | Four focus groups with 18 patients in the intervention group | Implications for the design of the intervention. Optimises the intervention for future trials |
| Chew-Graham et al. 2009132 | UK | Pragmatic RCT of antidepressants vs. counselling for postnatal depression (C) | To explore patient and health professional views about disclosure of symptoms of post natal depression | Interviews with 61 staff and patients from both arms of the trial | The implications were for clinical practice. Optimises the intervention for future trials |
| Cook et al. 2009133 | Canada | Parallel placebo RCT of drug for Alzheimer’s (DD) | To characterise the nature of verbal repetition, a reduction in which carers were keen to obtain from any treatment | Analysis of 130 video interviews collected to measure trial outcomes of all participants | Identified important outcomes clinical trials may not measure. Improves ability to identify benefit by ensuring right outcomes measured in future trials |
| Holtrop et al. 2008134 | USA | RCT of effectiveness of feedback on clinicians’ referrals to a smoking cessation quit line (C) | To understand the facilitators and barriers to referral to smoking cessation telephone help lines | Interviews with 31 clinicians referring patients in the trial | Showed that the focus on the intervention in the trial was only one factor influencing behaviour. Develops other interventions which may be better than the one in the trial |
| Mavhu et al. 2010135 | Zimbabwe/UK/South Africa/Malawi | Cluster RCT of two methods of community-level case finding for tuberculosis (C) | To explore why people who report a cough do not access services | Interviews and two focus groups with 32 trial participants who reported a cough but had not accessed services | None: implications were for clinical practice for controlling tuberculosis. Optimises the intervention for future trials |
| Sekelja et al. 2010136 | Australia | RCT of early or late referral to palliative care (C) | To explore the meaning of palliative care to carers | Telephone interviews with 30 carers of participants in a trial | None: implications were for service users. Develops or improves an intervention for future trials |
The authors did not offer an explicit rationale for using trial participants to explore wider health issues. This appeared to be an opportunistic endeavour, with researchers taking advantage of an identified group of people with a disease, or experiencing a health service, to address a research gap. In some studies, the intention was to collect data on the intervention and other interesting issues arose during the interviews, which then became the focus of an article. 132 In other studies, we could see that the trial offered access to a group of people that might otherwise be hard to identify for qualitative research,135 that the data collected in the trial were rich enough to allow secondary analysis to explore issues of concern to carers133 and that researchers had an unanswered question when the trial ended which was addressed by returning to trial participants. 134
There was no doubt that researchers were attempting to address research gaps and made convincing arguments for the need for their research. These articles contributed to the wider knowledge base in terms of understanding more about users’ experiences of a service136 and offering implications for clinical practice. 132,135 There was even an indication in one article that drawing on the trial as a sampling frame for the qualitative research strengthened the research because of the ability to use the large geographical spread of trial participants which would not be feasible in a separately funded qualitative study. 132 Some articles also offered contributions to the trial endeavour by identifying other potential interventions for testing134 and understanding more about a health problem that was important but was not being measured in clinical trials. 133
The authors themselves often drew attention to concerns, within the discussion section of their articles, related to the generalisability or transferability of their findings beyond the trial. First, health professionals or patients in a trial might not be representative of those in the real world; for example, clinicians in a trial may have had more of an interest in smoking cessation than the general population of clinicians134 or areas participating in the trial may have done so because they had poorer services. 132 Second, being in a trial may have affected the views of participants. 132 Third, the need to fit in with a trial may have affected data collection; for example, the need to collect qualitative data after the last trial outcome measurement may have introduced recall issues for the qualitative research. 132 It was extremely helpful to read researchers’ reflections on the strengths and limitations of this approach to identify suggestions for good practice.
-
State clearly whether or not the intervention and control group are included in the qualitative study and consider the implication of this sampling strategy.
-
In some of the articles, the qualitative research had been undertaken with participants from both arms of the trial whereas, in others, only those offered or completing the intervention had been included. Some authors stated their sampling frame clearly in their methods sections131–133 whereas in other articles we had to work this out ourselves because it was mentioned only in passing in later parts of the article. This information was important for interpretation of findings because experiences in different arms of a trial may not be the same. It may also be important to reflect on sampling from different arms of the RCT both when planning the best sampling strategy for the study and when analysing the qualitative data because one group of authors who used both intervention and control participants expressed concerns about having two groups within their sample with very different experiences. 136
-
-
Consider the influence of the trial on the findings.
-
In some articles, the researchers described the trial and its participants in some detail. 132–134,136 This offered an in-depth description of the context of the research participants, which is important for the credibility of qualitative research. The detail of the inclusion and exclusion criteria for the trial, by Bair et al., 131 was particularly helpful because of the degree to which this shaped the qualitative sample. Description of the content of the intervention and the control was also important because this affected interviewees’ recent experiences of health care and treatment. The discussion section of these types of articles would benefit from an explicit subsection considering the influence of the trial on findings and interpretation.
-
-
Consider implications of the findings for the trial endeavour.
-
We acknowledge that the articles considered here may be one of a number written from the qualitative research undertaken with the RCT and that relating findings to the trial endeavour may be better undertaken within other articles. Even so, we think it is important to consider inferences for the trial endeavour because the qualitative study participants were sampled from trial participants. Holtrop et al. 134 did this by showing that the intervention in the trial was one of many influences on the behaviour they wished to change and they were able to identify other future interventions that might be more useful. Some researchers discussed this issue as a brief point in the discussion section of their article rather than in the abstract or conclusion. For example, Cook et al. 133 point out in their discussion that clinical trials are not measuring an important outcome for carers because they do not understand how to measure it. This was an important message for triallists that could have been made more explicitly within the article.
-
-
Pay attention to the clarity of the key message for different audiences.
-
More attention could have been paid to implications of the findings for different audiences. This seemed to be more important for articles in this category because of the opportunity for confusion. Authors had to describe the RCT and the intervention within these articles and then go on and describe qualitative findings that related to neither of these issues. This could lead to problems understanding the message of the article because it might be unclear if the implications were for the trial intervention or the health condition.
-
The potential value of qualitative research with trials
We considered the lists of potential value of the qualitative research for the trial endeavour documented in the final column of the tables describing examples from each subcategory in Detailed description of framework subcategories with examples. We listed and grouped them into categories of potential value ( Table 26 ). The range of potential values was impressive, from improving the science and ethics of trials to reducing the costs of trials and making trials more sensitive to the people who participate in them.
| Category | Potential value | Examples |
|---|---|---|
| Bias | Avoids measurement bias | Helps test face and content validity of instruments in the relevant patient group |
| Efficiency | Ensures faster recruitment | Uses observation and interviews to identify problems with recruitment in a specific trial |
| Saves money | Stops attempts at full trials of poor or unacceptable interventions, or unacceptable trials designs | |
| Ethics | Ensures sensitivity of trials to human beings in trials | Ensures that recruitment and communication strategies can pay attention to health professionals and patients so that the experience is positive for them |
| Improves informed consent | Challenges current assumptions about gold standard informed consent, or value of information vs. value of communication | |
| Implementation | Facilitates replicability of intervention in the real world | Describes components of the intervention so that others can make use of the full intervention in the real world |
| Facilitates transferability of findings in the real world | Identifies contextual issues important for success | |
| Interpretation | Explains trial findings | Explains why trials were null; this may prevent another trial of a similar intervention. Contextualises results of successful trial to support dissemination and transferability in real world |
| Relevance | Ensures interventions meet the needs of health professionals and patients | Identifies value of intervention to important stakeholders. Ensures intervention is culturally appropriate |
| Success | Makes a trial successful, feasible, viable | Engenders stakeholder support for the trial. Makes a trial locally appropriate to cultural needs |
| Validity | Improves internal validity | Ensures the correct measures are used to measure the correct outcomes |
| Improves external validity | Helps to understand how to broaden recruitment to include hard-to-reach groups |
General suggestions for good practice and maximising value
The trial article
By studying the qualitative article only, we realised that we were missing a crucial part of the picture – the article reporting the trial. This trial article might include the actual impact of the qualitative research on the specific trial, for example optimising the intervention, explaining the trial findings, addressing implementation in the real world; therefore, we took a 1 in 10 sample of our qualitative articles by randomly selecting 30 of our 296 articles and searched for the related trial article in order to summarise the actual value of the qualitative research for a specific trial as documented in the trial article. We found the trial article for only 20 out of 30 studies. Two qualitative articles were in our ‘grey’ area and used more than one trial, for two the trial was still in progress, for two the study was a pilot/feasibility and we could not locate the full trial, for one the trial had been abandoned and the qualitative research undertaken to understand why and, finally, we had three for which we could not locate the article or we were unsure if the trial article we found was the same trial as described in the qualitative article. For a further article we could not access the trial article, leaving 19 trial articles for analysis. Only 4 out of 19 (21%) of the trial articles identified the impact the qualitative research had on the trial ( Table 27 ).
| Impact of the research described in the qualitative article | Number of trial articles |
|---|---|
| Trial article DID NOT reference qualitative research | 9 |
| Trial article published BEFORE qualitative article | 4 |
| Trial article published same year as qualitative article | 4 |
| Other | 1 |
| The trial article DID reference the qualitative article | 8 |
| Mentions it but no impact | 6 |
| (Mentions qualitative research to be published) | 3 |
| Explicit impact | 2 |
| (Explained findings) | 1 |
| (Developed intervention) | 1 |
| A mixed methods article of both the trial and qualitative research | 2 |
| Explicit impact | 2 |
| Explained/modified findings | 1 |
| Lack of feasibility of main trial | 1 |
Good practice
We identified some issues repeatedly and bring these together to consider good practice and ways of maximising value for this whole body of research.
-
Be clear about the impact on the current trial or about the implications for future trials.
-
Given that qualitative research was undertaken in the context of a specific trial, we expected it to impact on that trial in some way by optimising the intervention, improving recruitment within the trial, explaining findings, identifying the most appropriate set of outcomes, or facilitating transferability of trial findings by considering contextual issues important to effectiveness. We found some evidence of this reported in the qualitative research articles but generally our expectation was not met. This was related to two issues, first, some of the contribution that qualitative research can make requires that it is undertaken at the pre-trial stage in order to have an impact on the main trial and much of it was undertaken with the main trial in practice. Second, qualitative research undertaken with the main trial, which had the potential to impact on the trial, did not appear to do this in the qualitative article. A key example of this was the lack of evidence of qualitative research explaining trial findings. We acknowledge that this may have been reported elsewhere, such as the trial article but could not see much evidence of this in our small sample when we explored this.
-
The learning from this body of work was largely for future trials but this learning was rarely explicit within articles. Researchers need to be explicit about the implications of their qualitative research for the trial endeavour. The abstract of an article is the window to the article and is read by more people than the full article so we recommend that researchers report this in the abstract as well as the conclusion of the article. Implications may exist for different stakeholders and it is worth being explicit about whether the implications are for the triallists, intervention developers, policy-makers, patients or service providers who may be designing new interventions or trials, or attempting to implement trial findings in the real world.
-
-
Undertake qualitative research at the pre-trial stage if possible.
-
Almost three-quarters of the qualitative research in our sample was undertaken with the full trial rather than pre-trial, even in situations in which we would expect it to be undertaken only at a pre-trial stage, such as the acceptability of the intervention in principle, recruitment, diversity of participation, acceptability of the trial in principle, breadth of outcomes, accuracy and development of measures. We understand that some of the issues that emerged from qualitative research were unexpected and that researchers were taking the opportunity to identify these for future trials. However, qualitative research undertaken at the feasibility/pilot stage of a trial can impact on the full trial as well as on future trials so we recommend that researchers consider how to optimise interventions and recruitment prior to undertaking expensive full trials. We recognise that obtaining funding for pre-trial research might be challenging and that changes may be necessary to how research is funded.
-
-
Consider depth of analysis.
-
As a team, we value both descriptive and explanatory qualitative research. Describing interventions as practised, for example, may be very important for replicability of interventions in the real world. However, some of the articles we read were like reports of mini surveys and, although useful for identifying lists of issues, did not generate understanding. Some researchers went beyond studying what happened and considered why it happened. This appeared to be facilitated by considering a single focus within an article rather than attempting to describe a whole dataset and the use of observation and ethnography (see next point, Make use of the range of qualitative methods available). Qualitative research can also challenge as well as describe and explain and some of the in-depth analysis we read here challenged beliefs, for example about good ethical practice.
-
-
Make use of the range of qualitative methods available.
-
The most usual approaches taken within these articles were interviews with a sample of trial participants and focus groups with intervention deliverers. The use of diaries and recorded consultations, which were part of the intervention, proved useful to researchers. Observation and ethnography was rarely used and seemed a powerful approach when used. This may be related to the fact that anthropologists are more likely to use observation and they might be interested in ‘what is really going on here’ types of questions that lead to explanatory analyses. We are not recommending that researchers without experience undertake more observation when doing this research, but that they consider the benefits of observation or a disciplinary perspective on the quality of research produced.
-
-
Consider utility of participatory or ‘dynamic’ approaches.
-
There were a few examples of researchers working at the pre-trial stage who focused on the viability of a trial, either focusing on recruitment for a challenging trial or making trials in complex and challenging contexts locally appropriate. Public health trials are likely to fall into this ‘challenging’ category because they focus on difficult behaviour changes or are based in vulnerable communities. These participatory or dynamic approaches, which are open to adapting the trial conduct and intervention at the feasibility phase so that full trials are successfully completed, may facilitate viability of these complex trials.
-
-
Improve reporting of articles.
-
We read some excellent articles and highlighted the best of these when describing examples of subcategories in Detailed description of framework subcategories with examples. We felt that some articles could have reported the research more clearly and we identify ways in which this could be done ( Table 28 ).
-
| Aspect of the research | Guidance |
|---|---|
| Research question | Explain the origin of the analysis focus/research question. Why was it important, how did it emerge and when did it emerge in relation to the trial? Articulating the origin of the research question contextualises the findings |
| Do not be afraid to select a focus rather than try to report all the findings. A single message powerfully told can have more meaning that 20 issues described in broad brush | |
| Be clear about the aim of the analysis in the abstract and main paper. Sometimes research starts with a broad aim of ‘exploring the views of patients’ but the focused analysis for the paper is the feasibility of the intervention. Making this clear helps the reader to understand the findings | |
| Framing | Place the research within an evaluative framework such as the MRC framework for the development and evaluation of complex interventions |
| Describe the trial, considering the information that is relevant to the qualitative research | |
| Data collection and analysis | Be clear about when the qualitative data collection AND analysis was undertaken – after trial data collection was complete? When the trial results were known? |
| Be clear about whether the research was undertaken with participants experiencing the intervention or the control or both of these groups | |
| Balance | Give the qualitative research space in mixed methods articles |
| Conclusions and implications | Report implications for the specific trial and future trials in both the abstract and the discussion |
| Make the implications for different stakeholders clear: triallists, intervention developers, service providers, patients |
Drug trials versus trials of complex interventions
We classified interventions in the data extraction articles as drug or device, or complex. We found that researchers used qualitative research with RCTs for drugs or devices as well as complex interventions within each of the five categories of the framework ( Table 29 ). Bearing in mind that articles for data extraction were selected purposively for the ‘intervention’ category of our framework and by systematic random sample for other categories, the use of qualitative research with a focus on the intervention tended to be undertaken on complex interventions (only 15% of data extracted articles were for drugs or devices). When the focus was the category ‘design and conduct of trial’ and ‘measures’, the interventions were much more likely to be drugs or devices (65% and 67%, respectively). A total of 38% (40/104) of data extracted articles focused on drugs or devices, but this percentage cannot be extrapolated to the full 296 articles. A crude estimate for the 296 articles, based on applying percentages of drug or device data extracted articles within each category and then summing totals across categories, is that 27% of interventions were drugs or devices.
| Category | Subcategory | n focused on DD/n data extracted (%) |
|---|---|---|
| Intervention | Intervention development, N = 48 | 0/6 (0) |
| Intervention components, N = 10 | 3/6 (50) | |
| Models, mechanisms and underlying theory development, N = 23 | 0/6 (0) | |
| Perceived value and benefits of intervention, N = 42 | 0/6 (0) | |
| Acceptability of intervention in principle, N = 32 | 0/6 (0) | |
| Feasibility and acceptability of intervention in practice, N = 83 | 1/6 (17) | |
| Fidelity, reach and dose of intervention, N = 12 | 2/6 (33) | |
| Implementation of the intervention in the real world, N = 4 | 1/4 (25) | |
| Trial design, conduct and processes | Recruitment and retention, N = 11 | 2/6 (33) |
| Diversity of participants, N = 7 | 3/6 (50) | |
| Trial participation, N = 4 | 4/4 (100) | |
| Acceptability of the trial in principle, N = 5 | 5/5 (100) | |
| Acceptability of the trial in practice, N = 4 | 2/4 (50) | |
| Ethical conduct, N = 16 | 6/6 (100) | |
| Adaptation of trial conduct to local context, N = 2 | 1/2 (50) | |
| Impact of trial on staff, researchers or participants, N = 5 | 2/5 (40) | |
| Outcomes | Breadth of outcomes, N = 1 | 1/1 (100) |
| Variation in outcomes, N = 4 | 0/4 (0) | |
| Measures | Accuracy of measures, N = 7 | 3/6 (50) |
| Completion of measures, N = 1 | 1/1 (100) | |
| Development of measures, N = 2 | 2/2 (100) | |
| Condition | Experience of the disease, behaviour or beliefs, N = 33 | 1/6 (17) |
| Total | N = 296 | 40/104 (38) |
It is interesting to consider why researchers used qualitative research to explore the intervention in a drugs or devices trial given that this was a minority event. These studies were about adherence to drugs or devices, different settings for administering drugs or devices, or there was acknowledgement that the use of the drug or device required behaviour change. Across all categories, qualitative research was used in drugs or devices trials undertaken in complex environments (e.g. the community rather than hospital) or complex patient groups (e.g. substance abuse).
Discussion and conclusions
Summary of findings from the systematic mapping review
We found a large number of articles based on qualitative research undertaken with a specific trial. Numbers appeared to be increasing between 2001 and 2010, although, when exclusion criteria were applied, there was no evidence of an increase over the shorter time period of 2008–10. The range of contributions was extensive, with more activity in some areas than others, for example exploring the feasibility and acceptability of an intervention in practice. This body of work had the potential to offer a wide range of value to trials but this appeared to be potential value rather than actual value because of the lack of explicit attention within these articles to how the research impacted on the specific trial or the implications for future trials. Value could be maximised by being clear about the impact of the qualitative research on the specific trial or its implications for the trial endeavour, including future trials, doing more of this work at the pre-trial stage, undertaking in-depth analysis with an explanatory or challenging focus that attends to ‘what is really going on here’, making more use of non-participant observation, considering the use of participatory or dynamic approaches in pre-trial research for challenging trials and better reporting of the qualitative research. Qualitative research was undertaken with trials of drugs or devices as well as trials of complex interventions.
The systematic mapping review in the context of other research
Lewin et al. 8 detail how qualitative research can contribute to the development and evaluation of complex interventions and then go on to identify how it actually contributes in practice. The list of ways in which it can be used overlapped with ours to a large extent to develop and refine the intervention, to develop or select appropriate outcome measures, to examine whether or not the intervention was delivered as intended (including describing the intervention as delivered), to unpack processes of implementation, to explore delivers’ and recipients’ responses to the intervention, to explore reasons for findings of the trial, and to explain variations in effectiveness within the sample. Our research looked beyond complex interventions and by doing so added a new category of the design and conduct of the trial. The framework Lewin et al. 8 used, of qualitative research undertaken before, during and after the trial, did not fit well with our findings because we found that researchers used qualitative research with the same focus at different times of the trial process. For example, qualitative research undertaken at the same time as the main trial was used to explain trial findings, whereas Lewin et al. 8 report that this occurred after a trial. Lewin et al. 8 also identified issues we did not, for example the use of qualitative research to generate hypotheses. Their objectives of the qualitative research in practice aligned much more with our findings: developing the intervention, experiences of the intervention, reasons for refusing participation, developing outcome measures and illness experience. Creswell et al. 6 list 20 reasons for including qualitative research with a trial using the before, during and after the trial framework. Again, the items overlap with our subcategories: understand impact of intervention, understand mechanisms, fidelity of implementation, develop recruiting and consent practice, validate outcome measures, develop an instrument and explain variation in outcomes. As with Lewin et al. ,8 the framework did not reflect what happens in practice in terms of when the qualitative research is undertaken. Creswell et al. 6 also identified issues we did not, for example, to document the need for an intervention, identify mediating and moderating factors and understand how participants view the trial results. This means that our framework is unlikely to be comprehensive.
Lewin et al. 8 found reports and publications of 30 trials of complex interventions that used qualitative research. They found that in the trial and qualitative publications, most had no evidence of integration at the level of interpretation and few qualitative studies were used to explain the trial findings. Our findings concurred with this; however, they also found something that was contradictory to our findings: there was more use of qualitative research before than during the trial. We found the opposite but this may have been because Lewin et al. 8 included unpublished qualitative research whereas our review used only published qualitative research. Finally, Lewin et al. 8 also identified problems with reporting the qualitative research, as authors could have been more explicit about how qualitative research explained findings or developed the intervention. In the wider literature, there are ‘trial articles’ reporting how the qualitative research explained the trial findings. 137 The message that emerges from both our own research and that of Lewin et al. 8 is that this may be something researchers expect to happen more than it actually happens in practice.
Noyes et al. 138 discuss the relevance of qualitative research for systematic reviews in The Cochrane Handbook for Systematic Reviews of Interventions. They highlight the importance of qualitative studies undertaken with trials to add value to systematic reviews rather than simply the specific trial, including research about the intervention implementation and the illness experience. They identify the value of this research in enhancing the relevance and utility of the systematic review of trials to potential research users and in explaining heterogeneity of findings in a review; however they also highlight the problem of retrieving these articles. Our research shows that they will be able to locate some of these articles but systematic reviewers themselves will have to do the work in terms of thinking about the relevance of these articles to the trial-based evidence because the authors themselves may not have been explicit about this.
Strengths and limitations of the systematic mapping review
The strengths of this part of the study were twofold. First, it was grounded in published research that is available for other researchers and research users to utilise. Second, there was a considerable amount of team discussion to ensure clarity and consistency of decision-making. However, there were eight limitations. First, this component includes only part of the picture and some qualitative research undertaken with trials is not published. In addition, we did not include some terms in our search strategy that may have identified qualitative research, for example documentary analysis and participatory research, although some articles based on these approaches were identified using other terms. Second, the impact of the qualitative research on the trial may be published elsewhere, particularly the final report of the study or the trial article. We did a small substudy of trial articles and were not convinced that the impact of the qualitative research was published there either, a finding supported by other researchers. 8 Third, our review was based on publications between 2008 and 2010 and is unlikely to be comprehensive. We had originally planned to identify articles over the much longer time period of 2001–9 but changed our strategy when our more comprehensive search strategy identified many more articles than indicated by our pilot search in our research proposal. The short time frame used may have limited the range of aspects of a trial we identified in our framework. We acknowledge that the framework is not comprehensive and present it as likely to evolve in the future. Fourth, our search identified journal articles explicitly connected to a trial. Some qualitative research undertaken with a specific trial in mind may not mention the trial and this may occur more with some contributions than others, for example developing interventions, developing outcome measures and implementation in the real world. Therefore, we may have underestimated the use of qualitative research for some purposes. Fifth, although we tried to be consistent in our decision-making about inclusion of articles, we struggled to draw a clean line around some decisions such as ‘Is it qualitative research according to our definition that both qualitative data collection and analysis were used’. Sixth, we did not apply a quality assessment checklist to articles to consider the relationship between quality and maximising the value of qualitative research for RCTs. Seventh, we made judgements here from a narrow perspective and did not necessarily give suitable recognition that when researchers write a stand-alone article based on qualitative research, they also have to make a stand-alone coherent story. Also, researchers do not necessarily start out with the intention of exploring some of the issues that they focused on here. Rather they may take advantage of the trial as an opportunity to do this research or the issue emerges unexpectedly during the trial. Eighth, this review was based on published research and this may not reflect the breadth of qualitative research that is undertaken in practice.
Conclusions and implications of the mapping review
A considerable amount of qualitative research undertaken with trials has been published in the past few years, carried out in relation to trials of both drugs or devices and complex interventions. Qualitative research may be undertaken with trials but not published, so we have addressed here only part of the work undertaken by qualitative research with trials. Based on what is published in peer reviewed journal articles, qualitative research is undertaken for a wide range of purposes with implications for the specific trial, future trials and the endeavour of generating evidence of effectiveness of health interventions. There are excellent examples of this qualitative research and a wide range of benefits it can offer. However, our review suggests that this value is more about potential rather than actual impact and there is a need to improve the reporting of this research so that stakeholders with an interest in trials and the evidence produced from trials can learn from this extensive body of work. We published the framework in a journal article. 139
Chapter 4 Review of proposals and reports of studies combining qualitative research and trials
Aim
Not all qualitative research undertaken with trials is published;8 therefore, simply focusing on journal articles offers a limited view of how qualitative research is used and the impact it has on the specific trial. The aim of this component was to identify studies that combined qualitative research and RCTs and explore how qualitative research was used and ways of maximising its value. We aimed to consider this using two key study documents: proposals to funders and final reports to funders. We limited this part of the study to the UK in order to identify lessons for UK-based funding bodies.
Methods
Identifying trials with qualitative research
The mRCT located at www.controlled-trials.com/ was set up in 1998. It is an international searchable database containing information about the study hypothesis, design, funders, sponsors and contact details of predominantly ongoing trials. UK funding bodies have their own subregisters within the mRCT. In 2011, we searched for UK-based RCTs that used qualitative research and were ongoing between 2001 and 2010. We searched four active registers: UK Trials, MRC, National Institute for Health Research (NIHR) HTA and the Wellcome Trust. The search terms used to identify qualitative research are listed in Appendix 5 . Owing to the limited search facility in this database, we entered each term individually and then removed any duplicate studies identified.
A total of 122 studies (3%) from the 3812 trials listed in the four UK subregisters appeared to use qualitative research. From these 122, we excluded seven trials that were too old (end date before 2001) and 26 for which the search terms we had used, for example ‘qualitative’ did not relate to qualitative research, leaving 89 trials with qualitative research. Of these 89 trials, most (n = 69) were identified from the subregister UK Trials with the rest from HTA (n = 10), MRC (n = 7) and the Wellcome Trust (n = 3). The lead researcher of each study was contacted via email and a request made for the full proposal, the final report and a list of any relevant publications in peer-reviewed journals. One reminder was sent.
Data extraction and analysis
SJD read the proposals and formally extracted data using a data extraction form that consisted of general information about the proposal, such as the funder and the year it was written; the type of RCT; the type of intervention; the stated aim and the rationale given for undertaking the qualitative research; the qualitative design, methods, and participants; and information about how the qualitative research was described within the structure of the proposal (e.g. which section of the proposal the qualitative research appeared in, how much space was given to the description, details of costings and resources, and the type of language used to describe the relationship between the qualitative research and the trial). Space for open comments was available when the assessor (SJD) recorded any aspects of the proposal that seemed interesting or unusual. The structured aspects of the data were entered into IBM Statistical Product and Service Solutions (SPSS) Statistics v20 (IBM Corporation, Armonk, NY, USA). The main analysis was descriptive, displaying the proportion of proposals within each category of each item. The chi-squared test or Fisher’s exact test was used to compare different types of proposals. Some sections, such as the aim and rationale of the qualitative research, required verbatim extraction and SJD grouped these extracts into categories using a process similar to thematic analysis.
AR read the reports to identify whether the authors reported how the qualitative research had an impact on the specific trial, making written notes on the type of impact described. We had planned to undertake a more thorough analysis of these reports but had limited time owing to the unexpectedly large size of the systematic mapping review in Chapter 3 .
Findings
Description of studies
We obtained proposals for 41 out of 89 studies (46%). The proposals had start dates between 1999 and 2010, with three studies commencing prior to 2001, 17 studies between 2001 and 2005 and 21 studies between 2006 and 2010. The proposals addressed a wide variety of topics including cancer, mental health, complementary medicine, public health issues such as obesity and diabetes, and the delivery of health care. We had requested ‘proposals’ from lead researchers rather than specifying that we wanted the original proposals sent to funders, and we received 53 different documents: 36 proposals to funders and 17 published protocols.
We obtained reports for 22 out of 76 studies (29%) that had been completed at the time we requested study documentation.
Proposals
Further exclusions
We excluded 17 published protocols because they were written after researchers obtained the funding. On further reading of the remaining proposals, we excluded 4 out of 36 because they did not describe qualitative research. Of these, three were qualitative follow-up studies added into the mRCT database after the registration of the trial and one study used the term qualitative in the database, but it used neither qualitative methods nor qualitative analysis and was thus excluded, leaving a sample of 32 proposals. The studies were funded by HTA (n = 19), the MRC (n = 5), the Department of Health (n = 3) and others including the Scottish Executive Health Department and the Wellcome Trust (n = 5).
Stated aim of the qualitative research
Statements relating to the aim of the qualitative research were extracted from the proposals and subjected to a thematic analysis shaped by the framework of the focus of qualitative research (see Table 4 ) from the systematic mapping review in order to identify the intended focus of the qualitative research for the trial (stated aim). All proposals stated at least one aim of the qualitative research and some proposals stated more than one aim, for example, different aims for the start-up and main phase of the trial. We identified 68 aims in total from 32 proposals ( Table 30 ). These aims fitted our framework in Chapter 3 with the exception of a large subcategory (n = 11) related to obtaining the experiences and views of the intervention in which it was difficult to identify anything more specific than general experiences of different groups about the intervention. Similar to our framework in Chapter 3 , the majority of stated aims focused on the intervention, with 39 of the 68 relating to the intervention either solely (24 aims) or in combination with other categories (15 aims). Most intervention-related aims were about acceptability and implementation of the intervention either specifically (10 aims) or more generically (six aims) including feasibility and usefulness of the intervention. Two of the aims were unclear about which aspect of the intervention they would focus on. Other stated aims addressed trial design, conduct and processes (15 aims); the outcomes being measured (five aims); measures used in the trial (two aims); and the disease or target condition (two aims).
| Framework categorya | Framework subcategory | Examples of stated aims of the qualitative work from proposals |
|---|---|---|
| Intervention (39) | Intervention development (3) | Develop [intervention] |
| Focus group to identify range of possible interventions | ||
| Intervention components (2) | Subsidiary aim is to find out what ‘support as usual’ means | |
| Describe ‘usual care’ for this patient group | ||
| Models, mechanisms and underlying theory development (2) | To better understand women’s decisions about [intervention] and build a conceptual model of preferences | |
| Develop theoretical framework regarding subjective experiences of service users | ||
| Feasibility and acceptability of intervention in practice (8) | Examine acceptability of [intervention] for those > 75 years and to ascertain the views of various stakeholders | |
| Treatment acceptability and usefulness of intervention | ||
| Assess the acceptability of the intervention to patients and healthcare providers | ||
| Explore factors associated with success or failure of the intervention: feasibility, acceptability of different models of [intervention] | ||
| Intervention fidelity, reach and dose (3) | Identify patients’ reasons for completing or not completing [intervention] | |
| Establish patient costs and how they affect adherence, gain insights into reasons for poor uptake and lack of adherence | ||
| Compliance with intervention | ||
| Intervention implementation (2) | [Healthcare professionals]: experiences of applying new intervention, impressions of ‘climate’ in group and impact on wider service. Managers: understanding of service policies and practices for [treatment group] – perceived influence of [this type of trial] on clinical practice | |
| Assess the impact of the new [intervention] on the workforce, other key stakeholders and national leads | ||
| Generic acceptability/implementation (6) | Identify factors (organisational, professional and patient related) that influence successful implementation | |
| Views of [healthcare professionals] concerning implementation of the service. Understand how the intervention is delivered in practice | ||
| Experiences and views about the intervention (11) | Main trial: assess experience of receiving [intervention] | |
| To qualitatively explore participants’ experiences of the two [interventions] | ||
| Explore patients’ views and experiences of [intervention] | ||
| Patient experiences about the process and effects of [intervention] | ||
| Unclear (2) | Identify additional factors influencing the uptake of [intervention] and the way it is used | |
| Explore how [intervention] influences beliefs and behaviours | ||
| Trial design, conduct and processes (15) | Recruitment and retention (4) | Improve trial recruitment ‘to recommend recruitment strategies most likely to promote recruitment into the main trial’ |
| Process evaluation will see barriers to recruitment | ||
| Start up: assess parent and clinician attitudes to recruitment methods | ||
| Reasons for rates of recruitment on trial | ||
| Trial participation (5) | What it is like to take part in a trial | |
| Start up: assess parent and clinician attitudes to participation | ||
| Understand why some men consent to randomisation | ||
| Acceptability of trial in principle (1) | Explore attitudes towards possible trial; focus groups with patients to explore attitudes towards proposed trial, initial suggestions around participation | |
| Acceptability of trial in practice (1) | Understand the reasons for acceptance and refusal of randomisation’ | |
| Ethical conduct (2) | Ethical and practical issues of consent and assent, for example feasibility and acceptability of different consent models, views on participation, merits of conducting a trial | |
| Consent and assent | ||
| PPI (1) | Phase 1: develop qualitative methods for main study to engage service users and carers in driving the research process and to elicit views of NHS services | |
| Unclear (1) | The empirical investigation of the social organisation, production and effects of the RCT in practice | |
| Outcomes (5) | Breadth of outcomes (2) | Ensure that the most relevant [outcome] factors are assessed by the questionnaires |
| To access important aspects of care not available from standardised clinical outcome measures | ||
| Variation of outcomes (2) | To examine the implementation process in order to understand and explain any differences in outcome between intervention sites | |
| Phase 2: determine users’ and carers’ views on the process and effects of [intervention] compared with the views of those who received the control | ||
| Unclear (1) | Start-up: assess parent and clinician attitudes to outcomes | |
| Measures (2) | Completion of measures (1) | Ensure the feasibility of daily assessment |
| Development of measures (1) | Look at ways of asking about [outcome measures] | |
| Target condition (2) | Experience of the disease, health behaviour and beliefs (2) | Main trial: explore parent and clinician attitudes and knowledge to [health behaviour] |
| Explore issues related to [disease] | ||
| Unclear (5) | Non-attendance (1) | Provide insights about non-attendance |
| Unclear (4) | Understanding of processes underlying changes in patients’ beliefs and attitudes | |
| To provide a richer understanding of patient and carer perceptions to complement quantitative data | ||
| Differences in experience between two treatment groups | ||
| Process evaluation (monitor effect on outcomes) |
Stated rationale for the qualitative research
Statements relating to the rationale for undertaking the qualitative research were extracted and subjected to an inductive thematic analysis to identify why researchers felt it was important to undertake the qualitative research (we call this the stated rationale). Only 19 out of 32 proposals (59%) included some statement about the value for the trial of doing the qualitative research. Of those 19 proposals, 14 (74%) gave one rationale and five gave more than one rationale. Rationales included interpreting the trial findings (n = 7), optimising implementation into clinical practice (n = 5), optimising the trial process (n = 3), improving recruitment and consent procedures for the main trial (n = 3), and generating theories and models to guide future interventions (n = 3) ( Table 31 ). These mapped onto the potential value we identified in the journal articles in Table 26. Other rationales for doing the qualitative research included engaging users in driving the research process, giving a voice to trial participants, understanding the process and outcomes of care, exploring the range of resources for economic analysis and generating theory to guide the trial and health community.
| Rationalea | Statements from proposals |
|---|---|
| Patient voice or engagement (2) | Engage service users in driving research process |
| Bring together the views of different research participants | |
| Optimise the trial process/develop the best processes to maximise the success of the trial (3) | Optimise overall trial process |
| Develop qualitative model to understand perceptions and inform strategies for full trial | |
| Modify procedures and documentation in feasibility phase | |
| Improve recruitment and consent procedures for main trial (3) | Develop training programme to improve recruitment strategies for main trial |
| Develop consent procedures for main trial | |
| Generate theory of attendance at [intervention site] | |
| Generate theories and models to guide intervention development (3) | Build conceptual model of preferences for intervention |
| Provide new insights into patients’ views and experiences of [intervention] and usual care | |
| Develop theoretical framework regarding subjective experience of service users | |
| Generate theories to guide the trial and health community (2) | Provide generalisable information for wider health community about acceptability |
| Develop theoretical model of HTA practice/develop critical understanding of social processes and practices implicated in development, implementation and dissemination of RCT in field of HTA | |
| Optimise implementation into clinical practice (5) | Inform future development of services of this intervention |
| Inform roll out of intervention to wider community | |
| Inform commissioners and service providers to contribute to maximisation of quality and uptake | |
| Assess feasibility of/learn lessons for delivering [intervention] in NHS | |
| Interpret trial findings (especially unexpected findings) (8) | Understand and explain differences in outcomes between intervention sites |
| Provide a richer understanding of patient and carer perceptions to complement quantitative data | |
| Help interpret post-trial findings | |
| Insight into/possible explanations for differential success of intervention | |
| Interpret trial results to understand why intervention did work/work to further interpret and illuminate the findings from the trial itself | |
| Influence interpretation of trial findings (especially unexpected findings)/identify unexpected outcomes | |
| Other (2) | Understand, as well as quantify, the process and outcome of care |
| Explore range of resource use for economic analysis |
Space allocated to the qualitative research
Proposals ranged from 4 to 73 pages long, with a mean of 23 pages. From reading each of the proposals, the amount of space dedicated to the qualitative research in the proposal was categorised as one sentence, more than one sentence but less than a paragraph, more than one paragraph but not in its own section, or having its own section with its own heading. In 8 out of 32 proposals (25%) in which qualitative research occupied less than a paragraph, the same information or sentence was often repeated in several sections of the proposal. In four proposals (13%) there was more than one paragraph about the qualitative research, while in 20 of the proposals (63%) there was a section dedicated to the qualitative research, although the quality of what was written varied across proposals. This was not simply that short proposals had short descriptions – the shortest proposal had a section on the qualitative research.
Type and size of qualitative study
Methods were described in 30 out of 32 proposals (94%). Interviews were the most common method, used in all but one study, followed by focus groups, sometimes in combination with interviews. Two studies also used observations and one study also used diaries. Only 21 studies (66%) included any information about how many interviews or focus groups were planned. Of these, 10 proposals stated up to 50 interviews, nine studies proposed between 50 and 100, and two studies intended to undertake between 150 and 200 interviews. For those combining interviews and focus groups, two studies proposed up to six focus groups and 15–30 interviews, while one study proposed 24 focus groups and 60 interviews.
Analysis was described in 23 out of 32 (72%), simply stating the type of analysis they would use. These included constant comparative analysis (19%), thematic analysis (19%), content analysis (13%), framework analysis (9%), with interpretative phenomenological analysis mentioned in one proposal. The eight proposals that gave less space to the qualitative research (less than one paragraph) were less likely to include an analysis section (Fisher’s exact test, p = 0.002).
Expertise and resources
A total of 75% (24/32) of proposals included some information about who would do the research. However, only nine proposals (28%) stated that they would use qualitative researchers, with another 11 (34%) stating that they would use researchers but not specifying whether or not they were qualitative researchers, or stating that the trial or project managers or the lead researcher would do the qualitative research. Four proposals (13%) did not provide any information. One proposal stated that the researchers would train community advisers to do the qualitative research. In terms of resources, 19 out of 32 proposals (59%) included information about resources to undertake the qualitative work. These included transcription (53%), travel (47%), equipment (26%) and training (21%).
Language used to identify the relationship of the qualitative research to the trial
A total of 20 proposals (63%) described the relationship between the qualitative research and the trial: 10 described a relationship in which the qualitative study was subordinate to the trial, represented by language such as embedded, incorporating, nested, sample from, and substudy. Six proposals positioned the qualitative study on a par with the trial, using language such as alongside, concurrent, in combination with, linked, and parallel. Four did not use specific terms, simply saying that they would also carry out semi-structured interviews or a RCT with a qualitative evaluation. Studies with less than one paragraph for the qualitative research were less likely to describe this relationship (Fisher’s exact test, p = 0.01).
Reports
The 22 final reports varied in length, ranging from 2 to over 220 pages. Some reports required by funders only address a list of activities and outputs rather than detailed information about the research project. Ten of the reports were HTA reports, some of which we included as peer-reviewed articles in the systematic mapping review (see Chapter 3 ). Three reports did not mention qualitative research. We had the proposal for one of these and it described qualitative research. The lead researcher informed us that the study design had changed after initial funding had been agreed. Fourteen of the reports (64%) explicitly identified that the qualitative research had impacted on the specific trial ( Table 32 ). The type of impact mapped onto the uses of potential value of qualitative research to trials identified in Chapter 3 .
| Impact | Number |
|---|---|
| Yes, impact on the trial | 14 |
| Facilitated recruitment or explained why poor | 3 |
| Identified lack of acceptability or acceptability of intervention | 2 |
| Identified lack of acceptability of trial design | 2 |
| Qualified or explained trial findings | 6 |
| Modified way intervention was implemented | 1 |
| Impact unclear | 1 |
| Report too short | 4 |
| No mention of qualitative research | 3 |
| Total | 22 |
Discussion
Summary of findings from review of studies
Around 3% of UK trials on the mRCT database reported using qualitative research. In proposals to funders, we recognise that researchers are often constrained by the necessity of providing detailed information about how the trial will be conducted and the lack of space available on application forms. That being said, there were still some concerns about how the qualitative research was described. The stated aim and rationale for using qualitative research in proposals broadly aligned with our findings in Chapter 3 . The framework of uses of qualitative research was sometimes difficult to apply because of the vagueness of statements in some proposals. In terms of stated aims, we identified an additional category of patient and public involvement that was not found in the systematic mapping review and more generic statements about the acceptability and implementation of the intervention, and the experiences and views of participants that we were unable to categorise more specifically. We did not find examples of the following subcategories found in the systematic mapping review: perceived value and benefits of the intervention, acceptability of the intervention in principle, diversity of participants, adaptation of the trial to local context, impact of the trial on staff, researchers and participants, and the accuracy of measures.
Suggested improvements to proposals include giving an explicit rationale for using the qualitative research, giving space to describe the sample size and analysis and resources, and specifying the use of experienced qualitative researchers. The number of interviews or focus groups planned in some of the proposals seemed extremely high, highlighting a potential feasibility issue for some studies in completing the planned data collection or analysing a large volume of data successfully. It may be important for funders to question whether or not the qualitative data is being subject to quantitative criteria when large samples are proposed. If the trial or project manager and general researchers on the trial are to undertake the qualitative work, this suggests that there may be a lack of training and expertise for the qualitative work, indeed, particularly in the case of the trial manager the qualitative research risks being subsumed by the trial. Even though all the proposals we included were funded, we suggest that the lack of information about costs and resources means that it may be difficult for funders to see that the qualitative research is adequately resourced, whether or not it is feasible to complete, or if it provides value for money.
The impact of the qualitative research on the trial was much more obvious in final reports than in qualitative articles reported in Chapter 3 . This component of the study also highlighted the complex context of doing qualitative research with trials, showing how some qualitative research is added after the trial is funded, some is not conducted as planned and some qualitative research is not published in the final report.
Review of studies in context of other research
Lewin et al. 8 concluded from a review of reports and publications of 100 trials of complex interventions that few used qualitative research. Their study found qualitative research for 30% of trials, which was much higher than our study. This may be explained by their focus on complex interventions because qualitative research may be more commonly undertaken with these types of trials than clinical trials, or because they undertook more strategies to identify the qualitative research compared with our electronic search of a database. Our low percentage of 3% was much more similar to Flemming et al. ,7 who found that less than 1% of 146 trials in palliative care in a systematic review used qualitative research.
Research commissioners make decisions about funding studies based on application forms with detailed proposals. A search of the literature revealed few articles related to writing proposals for qualitative research in general or specifically related to qualitative research combined with trials. Sandelowski and Barroso140 refer to the process of writing qualitative research proposals as ‘artful design’ that requires ‘reflexivity, elegant expression, imaginative rehearsal, and strategic disarmament’ in order to neutralise any anticipated concerns reviewers may have. They argue that the proposal must demonstrate the researchers’ knowledge of the methods and field while demonstrating awareness and respect for their audience. They point out a tension between the emergent nature of the qualitative research and the planning of the research, with the need to show the significance of the research to be undertaken. That is, they put store by the rationale for doing the work. Connelly and Yoder141 identify a number of common failings in qualitative proposals which we also found in the proposals in our study such as inadequate explanation of methodological techniques and lack of rationale for the use of qualitative methodology. The lack of description of sample size and analysis has been found for mixed methods research more generally,142 highlighting the difficulties research commissioners may face when deciding on the quality of qualitative research.
Strengths and limitations of review of studies
It is unusual to assess the research proposals submitted to funders. Yet the proposal is the starting point of any study and shapes the utility of the research. We assessed the proposals of funded studies and cannot say how they compare with those of unfunded studies but would suggest they are likely to be superior. Our study had three limitations. First, qualitative research undertaken with trials may not be identified on the database we used, underestimating the amount of this type of research that occurs. Second, our response rate was poor, particularly for the final reports, leaving us with small numbers and the likelihood of non-response bias. Third, requesting full application forms with proposals would have given us a more comprehensive and consistent set of documents for use in our study.
Conclusions and implications of review of studies
A proposal without a solid rationale for undertaking the qualitative research with the trial suggests that the qualitative research and its relationship to the trial have not been properly thought through. All the proposals included in our study were successful in that they were funded. However, we would still suggest that it is insufficient to state the aims and rationale of the qualitative research without reference to the details of who will carry out the qualitative research or how it will be conducted and resourced, all of which are important to enable reviewers and research commissioners to make a confident decision about whether or not to fund the trial with qualitative research. We summarise our findings here to offer guidance for researchers and research commissioners for the content of proposals relating to the qualitative research undertaken with trials ( Table 33 ).
| Aspect of design | Recommended details |
|---|---|
| Aim and rationale | Describe the aim of the qualitative research and its rationale. Include a statement addressing the ways in which it ‘adds value’ to the trial |
| As far as possible, make aims specific to the trial, for example ‘to explore patient views on adherence’ rather than general, for example ‘to explore patient experiences’ | |
| Methods | Provide a clear account of the proposed methods of data collection including the location and timing of data collection, the skills and seniority of the person who will undertake data collection and the process of obtaining consent from participants |
| Describe the status and scale of the proposed qualitative research including a clear rationale for the sampling method, sample size and how participants will be selected | |
| Describe and reference the proposed analysis methods and give a clear rationale for the approach to be taken | |
| Integration with trial | Identify the skills and seniority of the person who will undertake the analysis and write-up |
| Outline suggestions for integrating and synthesising qualitative data/findings with the trial results | |
| Costs | Describe the broad marginal costs of the qualitative research and highlight any dedicated equipment, software, staff and transcription costs |
| Leadership | Identify which of the co-applicants will take overall responsibility for the qualitative research and describe their role in the design, data collection, analysis and write-up of the study |
Chapter 5 A survey to identify ‘invisible’ qualitative research undertaken with randomised controlled trials
Aim
In Chapter 3 , we reported on qualitative research undertaken with trials and subsequently published in peer-reviewed journals. In Chapter 4 we reported on studies that used both qualitative research and trials to understand how these studies are proposed and reported to funders. We identified these studies using abstracts on a trials database. These abstracts may not have reported the use of some qualitative research so we undertook a survey of lead investigators of studies registered in the mRCT database, which we had not identified as using qualitative research in our review in Chapter 4 , in order to measure the amount of qualitative research which is ‘invisible’ when searching trials databases and thereby gauge how much of the picture we missed in Chapter 4 .
Methods
We undertook two email surveys of lead investigators of studies registered on the mRCT database www.controlled-trials.com/. The first was a survey of a random sample of lead investigators and the second was a survey of lead investigators of trials identified as likely to be of complex interventions because we hypothesised that qualitative research was more likely to be used with trials of complex interventions. Both samples were identified by applying a random number generator formula to record numbers in the mRCT database.
Random sample
To obtain a random sample of 100 studies, we identified 120 records, from which we excluded 18 studies that we had identified as using qualitative research (see Chapter 4 ) or with an end date prior to 2001. We excluded a further 13 studies without a valid email address for the lead investigator, which left 89 studies. We did not apply further exclusion criteria.
Complex interventions sample
It proved impossible to apply a formal definition of a complex intervention5 to the brief abstracts on the mRCT database; therefore, we had to find a way of only using the limited information in an abstract to screen for trials which were of complex interventions. We identified trials with patient-reported primary outcome measures as a proxy for trials of complex interventions. This removed clinical trials, to create a sample likely to be ‘rich in complex intervention trials’. To identify a sample of 100 studies, it was necessary to generate 400 random records. SJD excluded studies that had used qualitative research and applied our definition of complex intervention to abstracts, discussing any ambiguous entries with the wider team. We identified 104 studies and excluded a further five studies without a valid email address for the lead investigator, which left 99 studies.
Data collection
We developed a short questionnaire to find out whether or not any qualitative research had been undertaken, or was being undertaken, with the trial (see Appendix 6 ). We piloted the survey with six colleagues who had led trials and made some minor modifications to the survey process and questionnaire based on their feedback. The questionnaire asked if any qualitative research was undertaken with the trial and, if so, to describe the objectives and methods of the research. Two further questions were included for completed trials only about details of reports and publications from the qualitative research. Finally, an open question asked for ‘any other comments’.
We sent an email and attached electronic questionnaire to the lead investigator. If we had no response after 2 weeks, we sent a reminder email. We entered the answers to structured questions into IBM SPSS v20 to produce descriptive statistics and undertook a descriptive thematic analysis of comments to the open question.
Results
Response rates
We received 38 completed questionnaires from the random sample, giving a response rate of 43% (38/89). We excluded a further five questionnaires in which respondents reported that the trial had not been undertaken, leaving 33 responses. From the complex intervention sample, we received 34 completed questionnaires, giving a response rate of 34% (34/99).
Prevalence of qualitative research
For the random sample, 8 out of 33 respondents [24%, 95% confidence interval (CI) 10% to 39%] reported that qualitative research was conducted with the trial and described methods that we classed as qualitative research. Three respondents reported using qualitative research but described interview-administered surveys or meetings and e-mails with researchers and we classified these as trials not using qualitative research. For the complex intervention sample, 15 out of 34 respondents (44%, 95% CI 27% to 61%) reported that qualitative research was used and described methods that we classed as qualitative research.
Methods used
Interviews were the most common method used. Of the eight random sample studies, three used interviews only, two used interviews and focus groups, one used interviews and non-participant observation, one used only focus groups and one used only non-participant observation. Of the 15 complex intervention studies, seven used interviews only, three used interviews and non-participant observation, four used interviews and focus groups, and one used only non-participant observation.
Focus of the qualitative research
The qualitative research focused on a range of issues that we were able to classify using the framework described in Chapter 3 , with the intervention as the most common focus. Of the eight random sample studies, six focused on the intervention in terms of developing the intervention, acceptability of the intervention, how the intervention was implemented and treatment fidelity. One focused on participation in the trial and one did not give enough information to classify the focus of the research. Of the 15 complex interventions sample studies, nine focused on the intervention in terms of developing the intervention, acceptability of the intervention and treatment fidelity. Other uses included trial design (n = 1), recruitment (n = 1), ethical issues (n = 1) and the target condition (n = 2).
Reporting the qualitative research
Most of the studies had submitted a final report by the time of completing the questionnaire: 6 out of 8 of the random sample and 10 out of 15 of the complex intervention sample. Of the eight random sample studies, five reported that the qualitative research had been, or would be, reported in a PhD thesis (n = 3) or a peer-reviewed journal (n = 2). Ten of the 15 complex intervention sample responded that the qualitative research had been or would be reported in a PhD thesis (n = 1) or peer-reviewed journal (n = 8) or the final report (n = 1). One participant stated that the qualitative work would not appear in the final report.
Comments made by respondents
We identified three themes from the comments of all respondents (numbers of respondents in brackets):
Funding
Different points were made by different respondents relating to funding: there was a bias against funding qualitative research with trials (n = 1), the qualitative research was unfunded (n = 1) and qualitative research had been undertaken prior to the trial and funded separately (n = 1).
Publishing
With regards to publishing the qualitative research, it was stated that the research assistant/PhD student would publish it (n = 1), they would not publish because they ran out of time and money and the research assistant had moved on to another study (n = 1), they did not publish it but the authors report that the interviews were exceptionally valuable in understanding the results of the trial (n = 1), they do not bother to publish applied qualitative research because it is not valued academically (n = 1), it was not part of the core message and would distract from it (n = 1) and the qualitative research was still ongoing when the trial finished (n = 1).
Perceived value of the qualitative research
Four respondents did not include qualitative research because they felt it was unscientific (n = 1), unnecessary (n = 1), the study was designed a long time ago (n = 1) or they had learnt about qualitative research since getting the study funded (n = 1). Two respondents who had undertaken qualitative research had recommended in the final report that more qualitative research was needed or had undertaken more of it in relation to the original trial. Three respondents described the utility of the qualitative research for the trial in explaining the trial findings (n = 2) or making a limited contribution (n = 1).
Discussion
The surveys were small with low response rates but nonetheless offered useful data for estimating the prevalence of the use of qualitative research with trials in conjunction with findings reported in Chapter 4 and identifying perceptions of researchers that can be viewed in conjunction with findings from our interview study reported in Chapter 6 . The surveys showed that our review of studies in Chapter 4 considerably underestimated the percentage of trials with qualitative research, particularly for trials of complex interventions. Of 3812 UK trials, we identified 3% (122 trials) that appeared to include qualitative research. Our random sample survey found 8 out of 38 respondents had used qualitative research with their trials. If we assume that non-respondents did not use qualitative research then 8 out of 89 trials (9%) of trials used qualitative research without recording this fact in the trials database. If this is combined with recorded qualitative research rates, we estimate that at least 12% of UK based trials in the mRCT database ongoing in the time period 2001 to 2010 used qualitative research. As we anticipated, the same calculation applied to complex intervention trials suggests a higher rate of qualitative research use (18%). However, this is still lower than the 30% reported by Lewin et al. 8 Our estimates are likely to be conservative because we assumed that all non-responses were negative and the wording on the questionnaire about the use of qualitative research – ‘when conducting your trial’ – may have encouraged responses about concurrent rather than sequential use of qualitative research.
The surveys offered some insights into why the qualitative research was not included in the trials database: because it was unfunded, was funded separately or was perceived as peripheral to the trial because it was an opportunity for researchers to obtain their PhDs. Although the qualitative research was invisible in the trials database, some of it was published in peer-reviewed journals so could have been included in Chapter 3 if it was explicitly linked with the trial and published between 2008 and 2010. The objectives of the qualitative research described in the surveys aligned with those described in our framework based on our systematic mapping review in Chapter 3 and did not identify new categories or subcategories. The surveys did however identify another value of the qualitative research – helping researchers obtain further degrees – albeit not a value to the trial endeavour. Researchers mainly used interview studies and this concurs with findings in Chapter 3 . Some of the issues arising in the open comments are similar to findings from the interviews with researchers reported next in Chapter 6 .
Chapter 6 Researchers’ perceptions of combining qualitative research and trials: a qualitative telephone interview study
Aim
To explore researchers’ perceptions of how to maximise the value of qualitative research undertaken with specific trials.
Methods
Design
We undertook a qualitative telephone semistructured interview study of researchers with experience of working on studies combining qualitative research and trials. We used telephone rather than face-to-face interviews for practical reasons. The strengths of this was that they are less time-consuming to undertake across a wide geographical area, and their main weakness – difficulty in generating a rapport with an interviewee – was minimised because researchers are likely to be comfortable with this medium as they use telephones in their daily work.
Sample
We wanted to explore the views of researchers with a wide range of experience. We sampled from the sources described earlier in the report: authors of articles reporting qualitative research undertaken with trials, researchers of studies from the mRCT database and researchers from our survey of ‘invisible’ qualitative research. We limited our sample to UK-based researchers because facilitators and barriers may differ by country and we wanted to gain a more in-depth picture that was relevant to the UK. We undertook purposive sampling of chief/principal investigators and qualitative researchers because we felt they would offer different perspectives. We attended to maximum variation by including researchers who had worked on studies covering the range of uses of the qualitative research in relation to the trial (see Table 3 ). We used an iterative approach for selecting potential interviewees: we selected 10 interviewees, invited them for interview and interviewed the six that agreed; then we selected another set of potential interviewees to complement the first six interviewees. We read transcripts after each interview and met as a team to discuss sampling. After the first 15 interviews, we paid attention to data saturation for the aim of our study. At this stage we felt that we were hearing similar issues and decided to interview another three to six researchers in order to fill the gaps within our sample. We were particularly keen to ensure we addressed all the framework categories (see Table 3 ) and, wherever possible, authors of the most recently published articles in our framework to ensure we included researchers’ more recent experiences.
Interviews
The interview guide was semistructured, focusing both on the specific study we had used to select interviewees and on more general views based on wider experiences of doing this type of research (see Appendix 7 for final interview guide). The interview guide addressed the objectives of the qualitative component within their study and how these arose, how the qualitative research was undertaken in conjunction with the trial, what made it successful (asking interviewees for their understanding of ‘success’) or how it could have been more successful, the visibility and status of the qualitative component, decisions about reporting findings, and perceptions of whether or not it was worth doing the qualitative research. We viewed our first interview as a pilot and refined the interview guide, continuing to do so as we read transcripts of early interviews. For instance, for later interviews we added a more direct question about what constituted ‘success’ for the qualitative research. The interviews were conducted during 2011 by AR and lasted between 39 and 81 minutes. They were digitally recorded with permission from interviewees.
Analysis
Interviews were transcribed verbatim and checked for accuracy by AR. Transcripts were returned to interviewees to allow them to identify extracts they preferred were not used in verbatim quotes. For the analysis, we followed stages of the ‘framework’ approach22 because we had a priori issues to explore as well as wanting to allow for emergent issues. Our team read transcripts for familiarisation, developed an initial thematic framework based on the interview guide and early transcripts and AR coded all transcripts to this thematic framework using NVivo 9 (QSR International, Warrington, UK). Our team discussed the content of codes and connections between codes, returning to read some transcripts to ensure that we kept a case focus while thinking about themes. The analysis was continued by JG who wrote up the themes for discussion within the team. The final analysis and write up was undertaken by AO in discussion with KJT and SJD. We selected quotes to evidence and illustrate our findings and we identify interviewees by the roles they had within the research team of the study they were selected from.
Our focus within these interviews was on how the value of qualitative research is maximised for the specific trial with which it has been commissioned. As we reported earlier, this was the focus of our whole study but we widened it for the systematic mapping review (see Chapter 3 ) because so many articles had implications for future trials.
Findings
Description of interviewees
We approached 26 researchers and interviewed the 18 who agreed. The interviewees were researchers who may be known by readers of this report and we have taken care throughout the presentation of findings to protect anonymity. For this reason, we do not present a table of characteristics with a row for each interviewee, but simply summarise characteristics. We sampled interviewees from the mapping review (n = 9), review of studies (n = 6) and surveys (n = 3), and selected them as the lead researcher (n = 11) or the qualitative researcher (n = 7) on the selected study, although two lead researchers identified themselves as both. They were male (n = 5), female (n = 13) and had worked on trial designs such as cluster (n = 6), early phase (n = 2) and pragmatic trials (n = 7), and had undertaken qualitative research to address the intervention (n = 11), the trial conduct (n = 6), outcomes (n = 1), measures (n = 1) and the target condition of the trial (n = 2). Interviewees described themselves as a quantitative researcher (n = 4), qualitative researcher (n = 5), mixed methods researcher (n = 1), principal/chief investigator (n = 6) and both lead investigator and qualitative researcher (n = 2).
Overview of themes
Our focus was on how to maximise the value of the qualitative research for the specific trial in terms of the impact it had on the intervention, trial conduct, outcomes and measures used, interpretation of trial findings or implementation of the trial findings in the real world. The focus was different from the focus in Chapter 3 , which considered the value to the more general trial endeavour of producing evidence of effectiveness of health interventions.
Interviewees were clear that qualitative research could, and did, have value to the specific trial. Maximising that value required two things: that high-quality qualitative research was undertaken and that integration of the quantitative and qualitative components of these studies occurred. As we analysed the data, we developed and tested the ‘theory’ that the value of the qualitative research to the specific trial was dependent on how it was valued by the lead investigator, the team and prevailing research structures. Consequently, the presentation of findings shows how aspects of the social organisation and implementation of these studies contributed to, or inhibited, the quality of the qualitative research and its integration with the trial.
Theme 1: the value of qualitative research to the specific trial
Interviewees were clear that qualitative research did have, or could have, value to the specific trial. We describe here the range of ways in which the interviewees articulated this value and the extent to which our definition of success for the qualitative research had an impact on the specific trial aligned with interviewees’ views. First of all, we describe how interviewees defined qualitative research because this shaped their beliefs about its value in this context.
Different ways of defining qualitative research
Implicit within the interviews was that qualitative research does not comprise a single approach but a number of different approaches employed by a number of different disciplines. Interviewees referred to a range of methods used within their studies. The variety of methods was matched by the variety of ways in which they described how they analysed their qualitative data: thematic analysis, inductive thematic analysis, interpretive phenomenological analysis, realist evaluation, using an iterative process, using NVivo, interrogating the data using key words, or using a combination of techniques that made definition difficult. Some attempts at definition involved reference to a set of qualitative skills, including conceptual and interpretive skills. Others approached definition in terms of highlighting the purposes or functions served by qualitative research which are not fit for quantitative approaches. Qualitative research addressed ‘how’ and ‘why’ questions, with some qualitative interviewees suggesting that these were more complex than questions addressed by RCTs and that, consequently, qualitative research was much more difficult to undertake.
We expect RCTs to come up with an answer, don’t we? We always expect that, you know, this trial will be able to solve the answer to this question, and I don’t think that’s what qualitative research is about. I think it’s more about trying to gain a broader understanding . . .
T16, qualitative researcher
Quantitative research is . . . much easier because it is much more logical, structured, rule-bound, I suppose, and not as conceptual, and I think qualitative research is much more difficult, because it’s more conceptual, because the theoretical frameworks are less well developed and there are conflicting . . . theories . . .
T6, lead investigator
A belief in the complexity and depth of qualitative research, illustrated by the quote above, was contrasted by some interviewees who described their research colleagues’ belief of qualitative research as simply the use of closed questionnaires, or in fact anything that was not a trial. Finally, interviewees defined qualitative research by its quality of flexibility to emergent situations – that it could be undertaken with one specific purpose in mind but end up producing unanticipated findings, or that it may reveal useful things in relation to the particular intervention under study but also illuminate phenomena in a way that could shape future interventions or services. They also described the need for qualitative research to evolve over time within a study rather than be constrained by the original research design, for example, in order to incorporate additional interests or to address difficulties that arise within the wider study. This openness, flexibility and responsiveness to needs was discussed as the reality, and as a strength, that was different from qualitative research that was unplanned and not thought through.
The value of qualitative research to the specific trial
Examples were cited in which qualitative research was not seen to have any potential to add value to a trial, for example, drug trials or trials with clinical rather than behavioural outcomes. For trials of complex interventions, interviewees described a variety of ways in which qualitative research did add value to the trial and in some cases described it as best practice, citing the MRC guidance for developing and evaluating complex interventions. The ways in which interviewees described qualitative research adding value to the specific trial aligned with our framework based on our systematic mapping review (see Chapter 3 ). Interviewees described using qualitative research to identify problems and solutions prior to the main trial by exploring trial feasibility, informing the intervention or developing data collection instruments. This was seen as much more routine practice than in previous years, addressing problems that had occurred previously with trials. It was described as saving the effort of undertaking trials which proved unfeasible, making viable a trial by ensuring its ability to recruit or retain participants, or optimising interventions, instruments and procedures to ensure the best RCT of the best intervention. Therefore, the language was both overcoming barriers faced by RCTs and improving RCTs.
It’s a given really that we ought to consider using qualitative research methods to uncover the barriers for trials etc. in advance, before trying to do them . . . that’s quite routine now that people are really into . . . looking at the feasibility of whether interventions are acceptable to the users before you actually try and implement them in an experimental setting. So I think the value of qualitative research in trials is, there are many, but certainly (1) looking at the barriers of actually undertaking a trial, (2) looking at the acceptability of interventions and procedures, are, through qualitative methods, enormously useful.
T2, lead investigator
The qualitative research was also used with the main trial to understand the intervention implementation and context in order to facilitate use of the evidence in the real world. This was described sometimes, but not frequently, as process evaluation. The use of the qualitative research to understand context was valuable because it increased the utility of the evidence generated by the trial for changing practice in the real world. It was seen to be invaluable not only in relation to the specific trial, but for future interventions, trials and clinical practice.
. . . the qualitative side will always be successful in helping you to contextualise the findings. Without the qualitative side, the context is always going to be much more poorly described. In some types of trial that may not matter hugely, in other types of trial it may be absolutely essential to understand more about the context of those delivering the intervention, those receiving the intervention, how they have experienced it, in order to understand the feasibility of wider use of the intervention . . . let’s say we had demonstrated that the [X] intervention was effective, there would still be lots of questions – ‘well how would you successfully roll it out, how would you apply it widely across the NHS?’ . . . so in terms of success – how can you tell whether it’s been successful? I suppose that that for me is one of the key things, is it helping an audience, whoever’s trying to understand what the trial has shown . . . is it helpful in terms of explaining the context within which the implications of a trial can be considered.
T12, lead investigator
Qualitative research was also valued for explaining trial results that were null, disappointing, surprising or confusing. In one study, the researchers were puzzled as to why an intervention that had been highly valued by many members of the group receiving it was not effective. The qualitative data revealed variability in the quality of the intervention provided, which may have contributed to the unexpected trial result. Explanations could be pragmatic and specific to the trial or be more generalisable through use of theory. It was not simply about explaining trial results, but also offering complementary findings which supplemented or modified the conclusion of the trial, for example by identifying benefits of the intervention that had not been measured quantitatively in the trial, or identifying that although the intervention proved effective within the trial, it would meet implementation difficulties that would limit its adoption in routine practice.
. . . some of the findings were coming out that were perhaps difficult to understand, and it allowed us to work out why those findings, what was behind those [. . .] there might be more similarity between the control site and intervention site than you actually anticipated, because people change the practice and they take on different methods over time. So the qualitative methods could tease out that kind of information and allow us to look more deeply into why the findings might not be so different.
T1, qualitative researcher
It was pretty much informed by a grounded theory approach all the way through, in that what we wanted to do was to develop theory about why the intervention outcomes were as they were.
T9, lead investigator and qualitative researcher
In addition to the qualitative research being a problem solver, explainer and translator of evidence into the real world, there were also some unexpected benefits of using qualitative research. Some interviewees highlighted the importance of the qualitative research in securing stakeholder engagement to ensure trial viability. In relation to the issue of ‘buy in’, interviewees described how those responsible for delivering the intervention felt that being interviewed or taking part in workshops to discuss the eligibility of potential trial participants had ‘given them a voice’ and created a positive relationship with the trial researchers which helped them to remain enthusiastic about the trial. This had not been an explicit aim of the qualitative research but was what may be termed a ‘secondary gain’.
Impact on the specific trial is not what it is all about
A key belief held within our research team was that qualitative research should have an impact on the specific trial and we explored this explicitly within the interviews. We asked interviewees to reflect on how successful the qualitative research had been and define success from their perspective. Some interviewees equated success with impact on the specific trial (see The value of qualitative research to the specific trial) but felt that there were other benefits to using the qualitative research. They discussed the value to future trials by informing the researcher’s future practice in developing and evaluating complex interventions. The qualitative research could also offer benefits to the research team rather than the specific trial by offering an opportunity for a junior researcher to undertake his or her PhD. They described success in terms of it having ‘answered the question that it’s addressing’, having valued the participants by incorporating and representing their experiences faithfully and having achieved publications. Success for them included offering insights into ‘patient experience’ that had the potential to improve service delivery generally.
It depends what you’ve set out to do, if you've set out to enrich your data from your main trial, and you’ve been able to achieve that then that would be success. If you get publications out of it, obviously that is, we’ve all got to think about that, that's success. If you’ve managed to enrich the experience of people taking part – I mean certainly my experience is that people who were taking part in the main trial really valued having the qualitative research because they liked talking about their story, and it was more personal for them and enriched their experience of taking part in the main trial – so I think that’s success as well. And I think probably if their experiences then being adequately written up and adequately recognised and not, you know, it is then getting put on a back burner, which is unfair to the participants . . . being seen as, like, secondary or less important to the main findings, that probably is not successful . . . I think qualitative research as part of RCTs is a very valuable thing to do, and that I think it’s happening more and more, which is a good thing, and I think a mixed methods approach is happening more, which is again a good thing, and I think everybody benefits from it, particularly the participants. And at the end of the day that’s why we are doing research, isn’t it? It’s to help benefit people.
T16, qualitative researcher
There were also negative views about the qualitative research having an impact on the specific trial. Qualitative research directed at assessing the feasibility of a trial, which resulted in the trial not proceeding, may be viewed by some as a success but viewed by others as a failure because the triallist could not proceed along their planned route of undertaking the main trial. Our early interviews, when we asked about impact on the trial the question, were sometimes met with denial that impact had occurred because this was considered to be problematic. One interviewee was insistent that allowing the qualitative research to have any impact at all on the trial would constitute ‘contamination’. This was brought about partly through how we expressed the question but also highlighted the fear that qualitative researchers should be careful not to damage the trial as an experiment. As well as this reaction of fear, there was genuine concern about the potential for qualitative research to offer a therapeutic effect and thereby damage the RCT experiment, particularly if the interviews and observation were more intensive than the intervention under study: ‘. . . particularly where the intervention you’re evaluating has got a psychosocial component, you do worry a little bit about interviewer effect . . . a therapeutic effect which can water down the impact of the actual intervention within the trial.’ (T17, qualitative researcher.)
Theme 2: there is value but it needs to be maximised
Even though the position of our interviewees was that qualitative research was valuable and many of them perceived it to have been successful within their studies, they identified problems with attaining full value from it and identified solutions required to do so. They expressed concerns about the quality of the qualitative research undertaken and the integration between the qualitative research and the trial. Attention to both of these issues was required to maximise the value of the qualitative research to the specific trial.
Do ‘proper’ qualitative research
Interviewees rarely offered specific quality criteria but were, instead, critical of what they perceived to be poor-quality qualitative research undertaken in this context, identifying a lack of conceptual thinking, analytical thinking or what the interviewee below describes as ‘intellectual intensity’. These criticisms were made by both quantitative study leads and qualitative researchers.
I think one of the problems that we face with qualitative research is that it is talked up a lot, but quite often the product that is delivered isn’t nearly as good as the aspirations for the discipline [. . .] I would say that most of it falls very far short of this sort of conceptual framework, real contribution to understanding that we think it will be, so that a very common process is for people to use expressions like, ‘we did a phenomenological analysis,’ ‘we extracted the themes and I’m going to present them to you’ and then they present to you four themes, each of which is named, and then they give some quotes, in a box or in the text, and you come out the other end and you think, ‘I don’t know if I’ve learnt anything from this’. And when you meet really good qualitative research, you know what [. . .] we can achieve with it, but so often, I think, the intellectual intensity that’s required to do it well isn’t applied.
T8, lead investigator
Interestingly, this interviewee, who identified himself or herself as ‘not a specialist qualitative researcher’, described a process of undertaking qualitative research that contributed to poor quality, i.e. large numbers of interviews, a lack of an iterative approach and overly optimistic expectations of what it could deliver. It felt like a model that this interviewee worked hard to resist, although they did not explicitly indicate exactly who or what was imposing this model:
I’m becoming much more directive about not saying ‘well, let’s interview 40 people’, but say, ‘let’s just start with one and . . . then do another and do another, that that would be a better way to do it’, so what we tend to do now is much less quantity of qualitative interviews . . . what is also better practice, which is really look at them as we go along . . .
T8, lead investigator
Although some interviewees discussed the need for ‘proper’ qualitative research, they also identified the problem that qualitative researchers from different disciplines have different understandings of the meaning of quality. One of the consequences of a lack of consensus of the meaning of quality was that qualitative research might be criticised and labelled as poor, damaging its ability to obtain funding, or its credibility and, by means of this, its utility. This was in contrast with the general acceptance of the meaning of a quality RCT. Of course, there are debates about quality criteria for RCTs, but researchers were working in the context of combining two contrasting methodologies of the ‘varied and contested’ qualitative research and the ‘singular and accepted’ RCT.
Integrate the qualitative research and trial: essential to the study or an ‘add-on’?
When interviewees perceived a lack of impact of the qualitative research on the specific trial, they explained it in terms of a failure of the two methods to be integrated.
. . . it can be used in much more creative ways than it is being I think, or it has been doing, because I think there’s still this idea that it’s just something on its own whereas it can be integrated.
T1, lead investigator
There were various descriptions of the integration or separation of the qualitative research and the trial. A few interviewees described the qualitative research as essential to the study and as essential as the trial:
I think the key to it is using qualitative methods because you really need to. [. . .] Sometimes qualitative methods get put in because they feel qualitative methods ought to be in there these days . . . so I think it very important that the qualitative methods are actually needed, so there is a research question there that qualitative methods would answer . . . So, having them there for the right reasons
T17, qualitative researcher
Three of our interviewees who described integrated studies were driven by the need to undertake research that was applicable to the complex world in which health care operated and could not conceive of undertaking a trial of the types of issues they were interested in without using qualitative research. A sign of how integral the qualitative research was to the study was never more telling than when the trial publication waited for the delayed qualitative research.
. . . for example, we’ve had the quantitative data pretty much ready [. . .] for about 6 months, but because of various delays the qualitative has lagged behind a bit, so we’re only just catching up with that now. So you could argue that we could have published 4 or 5 months ago, but we’re making it wait for the qualitative, which has not been a problem in the team, but I could imagine in another team where they weren’t so keen or so understanding of qualitative methods and what it does bring to the final paper, that could be a challenge.
T17, qualitative researcher
The clearest expression of the essential status of the qualitative research to the study came from a lead investigator who viewed the trial as embedded within the qualitative research rather than the more usual description of the qualitative research embedded within the trial:
It’s a qualitatively motivated trial really . . . I don’t know whether ‘motivated’ is the right word there, but, as I said, I very much see the qualitative as coming – the trial as being embedded within qualitative research, so it’s very, very visible.
T9, lead investigator and qualitative researcher
Much more common was a description of the qualitative research as important to the study rather than essential, with the qualitative research ‘embedded’, ‘nested’, ‘integral/integrated/integration’ and ‘built into’ the study. This contrasted with other interviewees who described ‘the trial’ and the qualitative research as an ‘added extra’ using terms such as ‘separate’, ‘bought in’, a qualitative ‘element’, ‘detached from’, an ‘adjunct’ to, ‘secondary/second tier’, not ‘joined up’, an ‘added extra rather than core’. More ambiguous descriptions sought to describe a degree of separateness but with an attempt to keep the two in juxtaposition: ‘in parallel/on a parallel track’, ‘side by side’, and ‘alongside’. More clearly pejorative descriptions referred to qualitative research being ‘bolted on’, ‘squeezed in’ or as constituting ‘tokenism’.
Theme 3: the significance of research teams for maximising the value of the qualitative research
The quality and integration of the qualitative research and trial were dependent to a large extent on the structural composition and social organisation of research teams for these studies. Important aspects of the team included who was part of the team prior to obtaining funding and during the study, the stability of team members, the status of different team members, the distribution of resources within the team, communication within the team and how differences within the team were handled.
Who is in the team?
Both the quality of the qualitative research and the integration with the trial were affected by who was in the team from planning through to completion. The integration of the qualitative research and the trial was dependent on how essential the qualitative research was perceived as being to the study. If qualitative research was perceived as essential or important then it was designed in the original research proposal, with senior qualitative expertise on the team from the outset. One interviewee suggested that locating someone committed to qualitative research permanently within a clinical trials unit could facilitate this early understanding of the need for qualitative research and expertise in designing it. In practice, the qualitative researcher mirrored the status of the qualitative research by being a key member of the team, such as the joint principal investigator with a quantitative colleague, or by being an ‘add-on’. An interviewee had been the ‘added on’ junior team member in the past:
I was brought in to help finish off those interviews and then I led the focus groups [. . .] Because I was brought in at a late stage, and I basically came in, finished off a bit of a job and then wrote the sections for the report, but didn’t really bring it together, I’m not sure how the links were made between the two.
T13, qualitative researcher
On another study, they felt themselves to be a ‘full’ team member, which they felt enhanced the quality of research produced by contributing to better research proposals. The risk of failure to integrate the qualitative research and the trial could also be exacerbated by what social scientists refer to as ‘Taylorisation’ in which externally appointed qualitative researchers were only around for discrete ‘bits’ of the work rather than being in a position to have an overview of the relation of the parts to the whole. They arrived on the study when it was designed and left the study prior to publications being written.
. . . there’s no mention of the qualitative component in the trial results paper . . .
That’s right, linking the trial results with the qualitative, that just hasn’t really happened at all, and I think that’s probably because the discussions about having some sort of qualitative component to the trial [. . . ] wasn’t the PI’s [principle investigator’s] main interest, because he’s a clinician . . . when I became attached to a different project, given the kind of, not so much lack of communication, that’s probably the wrong way to describe it, but if you’re still on the same site as the people running the trial, you can keep in touch better and remind each other that we should be meeting up and discussing how everything fits together . . . it was harder to keep that going. And so it got written up as my qualitative sub-study (laughs).
Status of team members
The trial was often described as the primary aspect of a study, which also included qualitative research. This status of qualitative research as an ‘add-on’ inevitably set up an organisationally hierarchical structure in which, characteristically, the lead researcher was a ‘quantitatively oriented’ professor who ran the trial and qualitative researchers were co-applicants or on fixed term contracts. When this was not the case, it was seen as being atypical: ‘I was the PI [principle investigator] and I’m a qualitative researcher, so it’s quite unusual in that most complex intervention trials are designed by the quantitative person and the qualitative researcher is sort of bought in.’ (T9, lead investigator and qualitative researcher.) Another interviewee who described a very integrated approach to their study was a quantitative lead investigator who shared the study leadership with a qualitative lead investigator.
In other studies, the presence of senior qualitative researchers could ensure good-quality qualitative research was undertaken by facilitating in-depth analysis and write-up. Problems could occur when there were only junior researchers on the team, without the support of experienced qualitative researchers. The qualitative research could have a very quantitative approach imposed on it to make it acceptable to the team, for example, the topic guide being highly structured and resembling an interviewer-administered questionnaire. Even when senior researchers were on the team, supervision may not be available to junior researchers owing to time constraints.
. . . there certainly are an awful lot of grant applications I see where it is quite clear that the little bit on process evaluation is just there as a bit of a token, maybe got a junior qualitative Research Assistant from a sociology department down the road to write a paragraph to go in the bid and then they cost it in for a few hours a week for the duration of the trial and clearly they are not an integral and powerful part of the study team
T18, lead investigator
Resources available indicates value of qualitative research and affects quality
There was certainly an issue about resources within the team, with the value of a study component evident through the resources it attracted. Some studies had senior qualitative researchers as applicants with enough of their time funded to ensure good-quality research was undertaken and qualitative researchers to undertake the research rather than trial managers doing it alongside running the trial: ‘That’s the ideal situation – where the work is properly funded’ (T3, qualitative researcher). Time as well as staff was an important resource, with enough time allocated to allow for an in-depth analysis.
. . . allowing adequate time for analysis of qualitative data is important because it does take much longer than analysing quantitative so therefore there is a cost implication, so that’s the only thing that could be challenging when working with people who are from a more quantitative background is helping them understand the process of qualitative analysis and the time that it takes.
T17, qualitative researcher
Teamworking: the importance of communication in integration and feeling valued
In addition to the way teams were structured, the way they operated on a day-to-day basis and how members communicated with each other was seen to contribute to the way qualitative research could add value to the trial. Engaging the whole team and working in a collaborative way was described as facilitative to integrating different parts of the study. Leadership was identified as important for bringing people together: ‘it did take a strong leader to bring everybody together [. . .] he did bring people together and had a big discussion’ (T15, lead investigator). The meetings encouraged communication and often involved everyone attending all the team meetings, especially at the beginning of the project, so that good relationships could be forged from the start. There was also a cost to communication, therefore, researchers described getting the balance right by having task-oriented groups of staff in between ‘full’ meetings and bringing people together at certain stages in the project. Key stages were ‘the beginning, the results, what do they mean especially, and dissemination.’ (T6, lead investigator.) What seemed to be important was that some thought was given to how meetings and other forms of communication could be designed to make inclusiveness and team integration more effective. Importantly, this contributed to qualitative researchers feeling that their work was valued, particularly if they were part of the highest meeting in the hierarchy of meetings: ‘I think we felt valued because the trial steering group valued that work stream’ (T3, qualitative researcher).
. . . the main thing was openness of communication, fostering an environment that everybody's views counts and everybody’s methodology is on the same level, and about each member of the team facilitating qualitative or quantitative research to be done, at the time that it was required, so our team meetings were very open, we all knew we didn’t know everything, so we were very open about asking questions from each other, because none of us could cover the full spectrum that everybody else had, and, keeping those channels of communication open was seen to be very good.
T4, lead investigator
So I think that’s why I would say it did work . . . because maybe people felt part of that whole process of bringing the qualitative together.
T13, qualitative researcher
Teams could be close or distant in their working practices. Close teams did not necessarily occur by accident – some qualitative researchers could, and did, choose to work with respectful colleagues who would be collaborative team members. The team could also be bound by shared values around the intervention and context of the research. Closeness could develop over time, for example, as a colleague who was described as not ‘overly impressed’ with the qualitative research became more interested once the qualitative findings came in (T10, qualitative researcher). However, communication and team interest did not automatically result in integration:
More quantitatively oriented triallists [. . .] might be interested in the qualitative results in their own right, but I don’t know how interested they’re going to be in thinking about what it tells us about the trial. [. . .] They provide a path of least resistance, if you want
T11, qualitative researcher
Other researchers described an experience of distance being created by colleagues perceiving their research as unimportant in comparison with the trial.
. . . there wasn’t a high degree of interest in the qualitative study in the wider research group, but it had to happen so it did, and I would even say that some of the key researchers involved in the wider study just wanted to distance themselves from it [. . .] 'well that’s over there, that’s something soft that you’re doing, but we're important and we’re over here and we’re doing all this important stuff
T10, qualitative researcher
Disciplinary backgrounds and differences
The disciplinary background of team members and the associated research paradigms were seen to be significant for the value placed on the qualitative research. Some clinical specialties were seen to be more sympathetic to qualitative research than others – palliative care, public health and primary care – for which one might argue the complexity of interventions is more obvious than in other specialties. A lack of expertise in qualitative research by the quantitative researchers could not only affect the quality of the qualitative research by imposing quantitative ideals on it, for example, wanting large numbers, but could also affect the confidence clinicians had in the quality of the qualitative research and therefore their willingness to consider interaction with the trial. For an interviewee who felt that both the trial manager and trial co-ordinator were receptive to emerging qualitative findings and willing to take them on board, the fact that these colleagues had some qualitative experience of their own was seen to be a significant contributory factor (T11, qualitative researcher).
It was clear to me that [name of professor] didn’t know a great deal [about how the qualitative investigation would be undertaken] and he was looking to me to say, ‘is this methodologically sound, am I going to get panned when I go to X conference or Y conference, because you haven’t done something?’ [. . .] They want the qualitative research to be of a standard that they feel able to defend, but they’re not familiar enough with qualitative approaches to know that you can actually defend something completely different if you know how to make the argument
T10, qualitative researcher
These differences were due to a lack of understanding of qualitative research by quantitative researchers, or a lack of understanding that there was anything to understand. A qualitative interviewee pointed out that in contrast with discussions about sample size, for which people will defer to the statisticians or triallists who are seen as the experts in this respect, ‘when it comes to the qualitative stuff, everyone’s got an opinion, even if they’re informed or not’ (T11, qualitative researcher). The effect of not having expertise, or constraining that expertise, on the quality of the qualitative research was not necessarily evident until too late, as in a case for which, despite a ‘few workshops to do with training’ with interviewers beforehand, transcripts of in-depth interviews ‘read like clinic interviews’ in which the participant ‘only got the choice of saying “yes” or “no”.’ (T15, lead investigator.)
Theme 4: how structural issues shape practice: what is valued in academia does not necessarily facilitate the value of the qualitative research for the trial
Our interviewees presented a number of structural issues as barriers to producing good-quality applied qualitative research, which, in turn, reduced the value of the qualitative research for the trial. What was valued within academia and traditional disciplines, what they perceived funding agencies were willing to fund, research governance structures and the current culture of learning could limit the quality of the qualitative research. Structural issues were also barriers to integrated research and publishing qualitative research was presented as a particular problem.
The drive to pursue academic careers and, therefore, the value placed on some journals and articles
The qualitative research was described as integral to some studies, driven by the need to find effective treatments and increase patient benefit. However, a qualitative researcher reflected on how trials could also be vehicles for the career progression of some researchers rather than ways of identifying effective treatments for patients: ‘They are not driven to find a treatment that is acceptable to patients, are they? They are essentially driven by the task of undertaking a highbrow RCT, getting publications on it and moving on to the next big thing’ (T10, qualitative researcher). However, interviewees who spoke with passion about the essential contribution of qualitative research to producing evidence of use in the real world were also driven by the need to be seen within their own institutions as someone doing ‘valuable research’. Valuable research produced high-impact papers, termed ‘three-star’ papers by the current national exercise within the UK, which allocates resources based on research quality. If the qualitative research could not produce these valued papers then it was viewed as less valuable, or even worthless, within academia.
. . . if it’s not a three-star paper, which they define as something like obviously high impact and is top 10% of Web of Science categories, then it doesn’t count for anything at all, and you’re literally in their eyes wasting your time writing it. So, it’s difficult for me to justify the time writing up qualitative papers for minor journals when I could be writing high impact papers or getting more grant money in.
T5, lead investigator
Pursuit of an academic career within current structures also included the need to bring in more funding and move on to the next project, resulting in teams breaking up before ‘non-priority’ papers were written, which, in this case, were the qualitative papers unlikely to be published in three-star journals. Given that we found 97 examples of UK publications in less than a 3-year period (see Chapter 3 ), we could see that our interviewees were making these points about maximising value rather than attaining any value. Nonetheless, there was evidence that more of this research was happening and perceived to be of value than was being published:
. . . our priority now, at the moment, is just to get the trial paper published. [. . .] I’m being honest here, there is an issue of will here. Because we have learnt a lot by doing this, including by doing the qualitative research and we’ve moved on to new projects, and we’ve just started another massive project, and so the question is where is the appetite when we’ve moved on. Who in our team is going to develop the qualitative publications from the work that we did before?
T8, lead investigator, underlines indicate interviewee emphasis
The value of applied qualitative research within academic disciplines
The academic career is shaped by academic discipline. Combining qualitative research and trials is a multidisciplinary endeavour and the qualitative research can be undertaken by researchers from disciplines such as psychology, sociology, anthropology and subdisciplines such as public health or health services research. Qualitative and mixed methods researchers described the tensions between applied research and their academic discipline. Qualitative research, applied research or applied qualitative research may not be valued within a discipline, making it difficult to publish in a high-impact journal within their discipline or a ‘decent qualitative journal’ (T5, lead investigator). The option available for publication here was a lower-status applied journal because high-status applied journals valued the trial publication rather than the qualitative research publication.
The challenges are more to do with [. . .] convincing traditional triallists and traditional medics who think in terms of experimental designs only, and really don’t think that qualitative research is any more than something that is a bit woolly. So that the latest challenge is presenting and selling the importance of doing qualitative research and what it actually achieves
T2, lead investigator
Funding agencies: do triallists value it enough to ask them to pay for it?
Interviewees noted that qualitative and mixed methods researchers were now on research commissioning panels, which they perceived as creating a favourable condition for obtaining funding. The MRC framework for the evaluation of complex interventions was identified as shaping the environment within which researchers worked, promoting the value of qualitative research to funding agencies to the point that they would sometimes request that qualitative research was included within a bid.
I think the commissioning process has really changed quite significantly since this was set up [. . .] now the MRC has its famous complex trials framework. And that’s just been part of the general change across the whole community with the interdisciplinary understanding of applied health researchers coming to bear on the broader community to show that qualitative research is important, has important roles to play in trials . . . And so the commissions have improved dramatically.
T2, lead investigator
However, the availability of funds clearly exercised interviewees. One interviewee described the funder’s commitment to the qualitative research as integral to the trial and their willingness to fund it fully as a key contributing factor to maximising its value. They then went on to say that ‘to get a donor who’s willing to put the extra money in is exceptional’ (T15, lead investigator) and indeed another regarded having dedicated staff to undertake the qualitative research as ‘quite a luxury’ (T3, qualitative researcher). It was not clear to what extent the funding agencies were contributing actively to this perceived lack of funding for fully resourced qualitative research and to what extent lead applicants were contributing to it by trying to keep the costs of a bid down, either by ‘second-guessing’ their chances of securing a large enough grant to fund the qualitative research properly or by trading on the goodwill of qualitative researchers to ‘squeeze it in’ without proper funding. This ‘minimal cost’ approach was felt to compromise the depth and quality and, therefore, the value of the qualitative research:
Because it wasn’t that the funder said you cannot have this money, but we underestimated in order to keep the costs of the study down, and hence didn’t have the time or the resource in terms of the researcher to gather the data and to analyse the data as fully as you would have liked. So I suppose that’s the take-home message really [laugh], [. . .] we shouldn’t have been quite so ambitious in the original proposal and should have tried to seek more funding.
T12, lead investigator
This contrasted with a lead investigator who felt that qualitative research was central to the study and believed so much in the research that they made sure it was properly funded.
Related to this was a sense that applicants were not necessarily communicating the worth of the qualitative research by not being sufficiently explicit about the aims and objectives that the qualitative research would fulfil for the trial. This was perceived as problematic if a large amount of money was requested from funders because it raised the question of value for money:
. . . it’s a difficult one for funders because you know, I sit on funding panels, and I sometimes think you can look at proposals and see that they just squeeze something in and it’s not really clear what the linkages are and the value, so I think researchers have a real job to sell the value of these different methods in their proposals … and then funders make a decision of whether to fund it or not, don’t they?
T13, qualitative researcher
Overall, there was a sense that a range of parties needed to change their approach to funding qualitative research and trials to maximise the value of this endeavour. If the funder and the applicants viewed the qualitative research as an ‘add-on’ then this determined its value in terms of impact on the trial. This meant that the space in the funding application form was used to give detail about the central endeavour – the trial – with the recognition that if this was not detailed fully then funding would not occur. Interviewees suggested that funders could change application forms to offer explicit space for the qualitative research:
The quantitative side tends to take predominance very often in terms of the application [. . .] and the qualitative side is much often less fully described, because there’s less space left on the application form.
T12, lead investigator
As a grant reviewer, you tend to get very tired of people saying ‘Oh yes, we’ll do a mixed methods evaluation, including a process evaluation, because it’s very important, blah blah blah’ and then they say absolutely nothing about what exactly they’re going to do [. . .] there are very few applications you get where they can say a lot because of the fact that the forms aren’t helpful to support that.
T18, lead investigator
This contrasted with an example in which the qualitative research was far from ‘add-on’. Funding for the full trial was dependent on the qualitative research at the development stage of the trial, which focused the applicant’s mind on ensuring that this phase of the project followed the best possible practice (including, in their view, publishing a mixed methods paper).
Research governance: not conducive to emergent qualitative research
Research in the NHS takes place within a governance structure. The demands of research governance, in relation to ethics committees and research and development permissions, were cited by some interviewees as representing an indirect barrier to maximising the value of the qualitative research by reducing the strengths of the qualitative research. This was due to such demands not being compatible with the requirements of some approaches to qualitative research. Topic guides needed to change iteratively during a study but some ethics committees required notification of change to any documentation:
Quite a few of the ethics committees asked to see the in-depth interview guides and the focus group discussion guides and it meant if we wanted to change anything, we’d have to go back and resubmit all of these again.
T15, lead investigator
The time taken to secure approval meant that insufficient time was left towards the end of the project to complete the planned number of qualitative interviews and to do justice to the qualitative data that had been collected. The time taken to complete what were described as bureaucratic procedures was felt particularly acutely in studies for which the qualitative research had not been incorporated in the original proposal.
Gaining expertise and developing a culture of learning
A number of interviewees drew attention to the importance of the quality of the qualitative research and the extent to which this could depend on the calibre of the researcher. As well as expertise in qualitative research, expertise was also required in relevant evaluative frameworks and in combining RCTs and qualitative research. Reach, Efficacy, Adoption, Implementation, and Maintenance (RE-AIM), MRC and realist evaluation frameworks were mentioned as useful by some interviewees. There was a view that the literature on qualitative methods in health services research is very broad in its focus and that it could be adapted to be applicable to RCTs. There were also suggestions that concrete examples of good practice in the form of case studies would be helpful (showing not only how qualitative methods were used but also the insights that would have been unavailable from the trial alone). Helpful literature, was cited, for example from the Cochrane group and Jenny Donovan and colleagues, University of Bristol. However, this was not just about availability of relevant literature but about a culture of learning from this literature and the value given to such learning: ‘I used to say, “when you started this project, how many other people’s things like that did you read?” And the answer is none.’ (T8, lead investigator.) This contrasted with the learning that occurred within some experienced teams, members of which felt that they undertook more sophisticated studies as they learned from previous experience. There was a sense that this whole endeavour needed more guidance, networking and profile in order for learning to occur within the research community.
Publication structures do not facilitate integration
As described earlier, interviewees felt that more integration of the quantitative and qualitative components of studies was necessary to maximise the value of the qualitative research for the specific trial. However, regardless of the degree of integration of the two during the trial, capitalising on the value of the qualitative research to trials at the publication stage seemed to present a final challenge that proved insurmountable for many. Typically, two separate sets of journal articles were published. Publishing the quantitative and qualitative findings separately mirrored the social organisation of the teams more generally. However, it was also the case when qualitative and quantitative researchers worked closely together because of publication ‘norms’:
So that’s going to be just purely qualitative spin-off papers if you like. I would be very, very surprised if we willfind ourselves in a situation where the trial results didn’t go out in a paper which was just the trial results, in the normal way that you would expect, in a main journal . . .
T11, qualitative researcher
Note the use of the terms ‘spin-off’ and ‘main’ in this quote, continuing to emphasise the trial as the main endeavour and the qualitative research as ‘add-on’. A barrier to publishing integrated results was word length for journal articles. Alternatives were found such as publishing quantitative and qualitative findings alongside each other in the same issue of a single journal or writing an integrated article after publication of the qualitative and trial results separately in order to ‘bring it all together’ (T7, lead investigator and qualitative researcher). Some researchers were ‘very keen to have the qualitative integrated with the quantitative, rather than just publish them separately’ (T17, qualitative researcher) and wanted the ‘norm’ to be integrated papers.
. . . ideally we would have put more stuff from the process evaluation in the main trial paper but we didn’t . . . because of the word length, you know, word count restriction meant that just by reporting all the usual stuff for the primary and secondary outcome analyses, there wasn’t really room for much else.
T18, lead investigator
However, this was not about simply changing publishing practices. There were several factors militating against ‘integration’ within publications: the fragmentation of teams which leaves the lower-priority paper unpublished, the demands of qualitative data analysis sometimes meant that the production of the findings of the qualitative and the quantitative research got ‘out of synch’, and other priorities and pressures on academics, for example, to publish in high-impact journals unsympathetic to qualitative research or to generate further income, both of these resulting in the ‘down-grading’ of the qualitative findings to the status of ‘minor’ articles which are then never written.
. . . the thing that’s difficult is the integrating the two at the end and trying not to just publish them separately, and I don’t think that's necessarily been done that successfully so far. There are some good examples of where it's been done very well, but not all trials, it doesn’t always happen, I mean, there can be a lot of qualitative data that doesn’t make it into publication, as we found with our own work, and partly that’s to do with time.
T17, qualitative researcher
Theme 5: an improving climate but action is required for further improvement
Interviewees who had been engaged in combining RCTs and qualitative research for many years felt that the climate for the endeavour had become more facilitative: ‘Every statistician who I work with now has a mutual respect for qualitative research . . . thirty years ago they would have said “Well, that’s not science, so what are you doing that for? It’s just story-telling” ’ (T2, lead investigator). This progress was in part attributed to changing priorities in health-care research, in which RCTs were being used for more complex or behavioural interventions. A major funding body, the MRC, had also contributed by showing the value of qualitative research within an evaluative framework for these complex interventions. However, interviewees who were less experienced researchers, who found themselves in lower-status positions within teams, had experiences that indicated that progress may be patchy and that there is still room for improvement. This patchiness was evident in more experienced voices too. One interviewee expressed the view that it is less frequent these days to come up against overt antagonism towards qualitative research because there is more of a climate of acceptability, but that, nevertheless, some funding agencies and researchers are not convinced:
[Two named boards] have got quite a lot of mixed methods people in, so . . . kind of quite well attuned to it really . . . but my perception is that across the whole (wider funding landscape) it’s probably not quite very well appreciated.
T18, lead investigator
Some structural issues are also changing in ways that may facilitate maximising the value of qualitative research undertaken with trials. For example, the progression of electronic journals with no word limitations may encourage more integrated reporting of studies, as might the increasing use by the NIHR of publishing final reports within peer-reviewed journals. However, some interviewees wanted to see more agency from individual researchers, for example, they wanted the research community to actively learn from practice rather than continuing to do more of the same.
Discussion
Summary of findings from interview study
The value of undertaking qualitative research with trials was considered to be high by researchers engaged in this endeavour but this did not necessarily equate to the QUART team definition of value to the specific trial. Impact on the specific trial was not viewed as essential by some researchers. It was described as central and essential for some studies but as an ‘add-on’ to the trial for other studies. Lead researchers who described their focus as undertaking research to change practice in the real world, who were highly aware of the complexity of their interventions, the environment the intervention would be tested in and the environment the intervention would need to operate in outside the context of a trial, viewed qualitative research as essential and its impact on the trial as necessary. For other studies, the qualitative research was an ‘add-on’ to the trial, sometimes viewed as an interesting but ultimately separate endeavour, or sometimes as a distant ‘something funders like to see’, which could result in under-resourced qualitative research that would not impact on the trial. Structural issues supported the trial as the most valued component of these studies.
Interview study in the context of other research
The findings of this interview study were similar to findings from interviews with researchers from mixed methods studies. Researchers valued mixed methods research and saw integration of the qualitative and quantitative findings as a mark of quality but tended not to engage in it because of dysfunctional or multidisciplinary teamworking142 and structural issues such as publication norms. 143 Our findings were also similar to issues raised in a discussion of a methodological paper about the use of mixed methods in palliative care in which the authors identified a lack of skill mix and experience in mixed methods research, problems obtaining funding and under-resourced studies resulting in poor quality and difficulties in publishing integrated findings. 7 An editorial about health services research also highlighted the need to develop skills and train people in qualitative research and mixed methods and the need for structural changes in journal publications, such as longer word lengths and skilled reviewers, to facilitate qualitative research. 144 Simons145 reported her personal experience of being a trial co-ordinator and carrying out a qualitative case study within the trial for a PhD, offering a different, but complementary, perspective. Simons145 highlighted the difficulties of both being engaged with a trial and undertaking qualitative research that challenged the trial, taking care not to damage the experiment while doing the qualitative research, being very rigid in her interviewing technique compared with a sociologist she worked with. The author reported how publishing the trial took precedence and the inability to show the impact of the qualitative research on the trial because the qualitative research was not complete at the time of publishing the trial. Her work identified a view that was expressed by one of our interviewees and perhaps did not receive much attention in our write-up. This view challenged the basic assumptions of trials and identified the naivety of some trials of socially complex interventions that work under the assumption that context can be controlled. 11
Strengths and limitations and reflexivity of interview study
This interview study drew on the range and depth of experience of researchers. The sample was taken from three sources to ensure we had the views of researchers currently undertaking this endeavour, who had seen projects through to completion and who had worked on projects for which the qualitative research was not necessarily visible. The sample was drawn from the UK, where the use of qualitative research with trials is acceptable to significant funding bodies such as the NIHR and MRC but may be less acceptable to other funders of more clinical research. Researchers from other countries will have to consider the transferability of these findings to their specific contexts. The interview topic guide and the analysis and interpretation were shaped by the QUART team’s belief that qualitative research undertaken with a specific trial should have an impact on that trial. We were open to the possibility that researchers would disagree with this position, and indeed researchers offered other reflections on value, but our presentation of the findings has been shaped by our research question and stance. The QUART study grant holders have undertaken trials themselves and are supportive of trials for generating evidence of effectiveness. We are critical of how some trials have been undertaken, including our own research, but do not challenge the need for trials. Again, this stance shaped our research question, sampling, analysis and interpretation.
Conclusions and implications of the interview study
One conclusion that could be drawn from this research is that all trials should be undertaken with qualitative research that has equal status with the trial and is viewed as essential to the generation of evidence of effectiveness. The conclusion we draw, as the QUART study team, is different, however. We do not necessarily want to see an increase in the use of qualitative research but rather an increase in thinking about whether and how it can be of use within a particular study and how it can be resourced to maximise its value. We would like to see trial teams thinking about the problems their interventions and trials are likely to face and the value of qualitative research in addressing problems such as poor recruitment, a lack of understanding of why interventions work or not, a lack of use of evidence generated by trials in practice, or the failure of interventions and trials in complex environments. Where these problems are likely to be significant, well-resourced and expertly executed qualitative research may enhance the production of evidence of effectiveness as well as enhance its relevance and usability in the real world. The issue that appeared to be at play within our interviews was the sense that some qualitative research is being undertaken in the UK research community because it’s the ‘done thing’, without valuing its potential to impact on the specific trial. If that impact is important to the research lead, this shapes the team structure, team communication, resources and dissemination strategy. There was also an indication that impact of the qualitative research occurs within some studies but is invisible to others owing to structural constraints, such as publishing norms, which limits its value to other researchers working on similar interventions or in similar environments.
Chapter 7 Integration of findings from different components
Aim
The aim was to bring together the findings from the different study components to help to draw meta-inferences from the study.
Methods
We identified findings from each method: systemic mapping review of articles, review of studies, survey and interviews. We identified themes within these findings and displayed them together on a grid ( Table 34 ). We undertook a partial ‘triangulation protocol’ approach. 146 Within each theme we considered where findings from different components of the study converged, offered complementary information, were contradictory or did not exist. Then we drew conclusions or meta-inferences within each theme. 147 During this process, we paid attention to overlap in the samples of different components, for example we had interviewed people who had published articles or undertaken studies so some convergence between components was expected.
| Themes | Review (international) | Studies (UK) | Surveys (UK) | Interviews (UK) | Conclusions |
|---|---|---|---|---|---|
| Prevalence of the use of qualitative research with trials | We found 296 articles reporting qualitative research in fewer than 3 years, but we did not count the number of trials published in this time period | Around 3% of UK funded trials ongoing between 2001 and 2010 used qualitative research | 3% is an underestimate and it is more likely to be of the order of 12% for trials and at least 18% for trials of complex interventions | – | Qualitative research is used with a minority of trials |
| Our electronic search identified an increase in references over the years 2001–9 but this may have been related to an increased use of terms we searched for. There was no increase in articles published between 2008 and 2010 | – | – | The perception that using qualitative research with trials is more common now was expressed by researchers with many years of experience in research | There is a perception that the use of qualitative research with trials is on the increase but this could be using baselines of many years ago. There is no evidence that it has increased over the last few years | |
| Range of applications of qualitative research with trials | We identified 22 subcategories of use, with some uses appearing to be much more common than others. This lack of balance may be due to not being able to link some types of uses back to the trial, our searching or our categorising processes. Based on published research only | We identified a range of uses that reflected our framework and findings in Chapter 3 . When planning to use qualitative research with trials, the stated aim was sometimes vague, rather than reflecting the more specific uses we identified in publications | Numbers were small but we identified a range of uses that reflected our framework and findings in Chapter 3 . Respondents identified another use: that trial staff got their PhDs by doing qualitative research with the trial | Interviewees described a range of uses that reflected our framework in Chapter 3 . This was expected because they had been sampled from the review as well as other components. They highlighted further uses, for example, for staff on the trial to get PhD, stakeholder engagement to help the trial viability. They highlighted the value of the flexibility of qualitative research and its ability to respond to emergent issues | Qualitative research is used to address a wide range of aspects of trials and may have secondary gains, for example educational qualifications of research staff, stakeholder engagement. The possible imbalance between different uses may not be a problem but the research community could reflect on this |
| Value of qualitative research to the trial | The QUART team could see the utility of the qualitative research for the specific trial and for the wider trial endeavour as we read these articles. Authors were not always explicit about actual or potential value to the trial | Some researchers stated a rationale in their proposals that aligned with values identified in Chapter 3 . Rationales for using qualitative research with a trial were missing for a large minority of proposals for studies | Some researchers stated they did not use qualitative research because they saw no value in using it | Interviewees with experience of using qualitative research with trials perceived that it was of value not just to the specific trial but to future trials (which we addressed in Chapter 3 ) and service delivery more generally. Interviewees also perceived value around qualitative findings supporting the use of evidence in the real world. This came across more strongly in the interviews than other study components | Qualitative research is being used to improve the value of specific trials and is considered to be valuable for future trials and the generation of evidence of effectiveness in the real world |
| 38% of our data extracted articles were based on DDs. The value of qualitative research with trials was apparent for drug trials as well as complex interventions for which the participants or the intervention delivery environment were complex | – | – | Many of our interviewees described complex interventions and complex trial environments | Qualitative research is of value not simply for complex interventions. Some drug trials take place in complex environments with participants living complex lives | |
| With the exception of the literature focusing on intervention development, we rarely saw evidence within the articles of actual impact on the specific trial | Researchers often did not describe the rationale for using qualitative research in proposals. However, researchers did describe the impact on the specific trial in many final reports | – | Interviewees identified a problem around integrating the qualitative research and trial and the barrier to this at the publication stage of a study. Some published their qualitative research and some did not because it was not a priority | The value of qualitative research to trials is often potential value because the value is being lost at the publication stage. Researchers do not publish all of this work and are not always explicit about the impact on the trial or the trial endeavour in journal articles | |
| How to maximise value | Many articles failed to include explicit statements about the implications of the qualitative research for the trial and for evidence of effectiveness | Many proposals did not state the expected value of the qualitative research. This was not an issue for final reports in which the impact of the qualitative research on the trial was described | Sometimes the triallist or the qualitative researcher is not interested in the qualitative research impacting on the trial. Sometimes it is essential to both the triallist and the qualitative researcher | At all stages of the research process, researchers need be explicit about the value of the qualitative research to the trial, so others can see that value and make use of it | |
| Only 25% of articles based on qualitative research were undertaken at the pre-trial stage. This was mostly intervention development work | – | – | Researchers described the value of qualitative research at the pre-trial stage but did not say they wanted more of this to be done. The role of qualitative research at this stage was ‘problem solver’ | Do more qualitative research at the pre-trial stage rather than the main trial stage in order to increase the potential for impact on the trial itself | |
| The QUART team could see the potential value of explaining trial findings but did not see much evidence of this in the qualitative articles or the small number of trial articles looked at. The largest subcategory of use explored intervention feasibility and acceptability alongside the main trial. This had the most value for explaining trial findings yet these articles were particularly vague about implications for the trial | There was evidence of the qualitative research explaining trial findings in the final reports and this was a stated rationale in some proposals | – | Researchers valued qualitative research as explainer of trial findings and described examples of it explaining null or unusual findings. They also described modifying and supplementing trial findings. However, they felt that some of their colleagues did not understand qualitative research and lack of communication within the team could act as a barrier to this occurring | If a rationale for the qualitative research is explaining trial findings, then ensure that this is described at the publication stage. This is a weak finding because we did not identify a large number of trial articles for which we expected to see this occurring in order to assess whether or not it had occurred | |
| Some articles asked ‘what is really going on here’ whereas others read like mini surveys. Articles taking an explanatory or challenging stance were powerful | Some qualitative research was not described in the proposal in any detail to show it had been thought through. Proposals did not specify the use of experienced qualitative researchers | – | Improving the quality of the qualitative research was identified as one of two ways to maximise value. Barriers to this were imposing of quantitative ideals of large numbers, lack of resources, bringing in the qualitative researcher late, low status of the qualitative researcher on team and lack of skilled qualitative researchers. Analytical and interpretative skills were part of interviewees’ understanding of the strength of qualitative research | Attention to depth of analysis can maximise value, but this does not just miraculously appear. It can be produced by junior researchers learning on the job, but planning the research, resourcing it and using skilled qualitative researchers can help to facilitate depth. Funding bodies can facilitate it by changing application forms and researchers can ask for appropriate resources | |
| Interviews and focus groups were commonly used. Many of the exemplars we identified had used observation or taken an ethnographical approach. There were some examples of dynamic and participatory approaches that seemed powerful at the feasibility stage for making the main trials viable | Interviews and focus groups were planned in the proposals. There was only one example of observation in the proposals | Interviews and focus groups were commonly used | – | Expanding a narrow focus on interviews and focus groups to include observation or dynamic or participatory methods, when appropriate | |
| We found many more publications than expected and had to reduce the time frame of the review study to make workload manageable | – | There were examples of the qualitative research not necessarily getting published | Even though we found many more articles than we expected in Chapter 3 , interviewees expressed concerns that publishing was difficult owing to the status of qualitative research, disciplinary disrespect for applied research and publication in low-impact journals | There is a need to address structural issues affecting publication of this endeavour | |
| There were some examples of very large qualitative studies but this was not common in our data extracted articles | Some proposals had very large amounts of data collection proposed. Two out of 32 had > 100 interviews proposed | Some interviewees described a model of large numbers of interviews reducing the quality of qualitative research | There may be a tendency to impose a quantitative framework, resulting in studies with very large numbers, which works against the strength of qualitative research offering depth | ||
| Some articles lacked explicit implications for the trial endeavour | – | – | Researchers do not necessarily read about how others have undertaken qualitative research with trials prior to doing this themselves. There is ‘team learning’ that is taken on to future projects by some teams | The use of qualitative research with trials is an emerging methodology and training is needed for individuals engaging in it | |
| Climate | – | – | – | Researchers who have been in the field a long time say the climate for undertaking qualitative research with trials is improving | The climate for undertaking qualitative research with trials may be getting better |
Chapter 8 Discussion
Summary of findings
Qualitative research is used with trials. At least one in eight trials funded in the UK and ongoing in the 2000s used qualitative research, increasing to at least one in five for trials of complex interventions. A large number of articles reporting qualitative research undertaken with trials, almost 300, were published between 2008 and 2010. We used these articles to develop an empirically based framework of the aspects of trials addressed by qualitative research. This framework consisted on five broad categories (intervention, trial design and conduct, outcomes, measures, and target health condition), with 22 subcategories. Qualitative research was used with both trials of drugs or devices and trials of complex interventions. When the focus of the qualitative research was the intervention or outcomes, the trial intervention was likely to be a more complex intervention than a drug or device. This qualitative research had implications for the specific trial it was undertaken with, future trials and the endeavour of generating evidence of effectiveness of health interventions. There were excellent examples of this qualitative research and a wide range of ways in which it could bring value to the trial and trial endeavour; however, this value was likely to be potential rather than actual value. This was partly to do with how researchers do this work and partly to do with how they publish it. Researchers work within structures that encourage obtaining less value from this approach than is likely to be possible. Researchers driven by the need to provide evidence of effectiveness for real-world application have overcome some of these structural barriers.
Comparison with other research
Each of the study components has been considered in the context of relevant research at the end of each chapter. Here, we summarise the unique contribution of our study and how it supports or contradicts previous research. Both Lewin et al. 8 and Creswell et al. 6 identified lists of uses of qualitative research with trials within a temporal framework. Our research complements these studies by presenting an empirically developed framework based on different aspects of a trial. By studying what researchers have actually published, we show that some of the contributions that qualitative research can make are not occurring in practice and that much of the endeavour occurs during the trial, even when ideally it should occur before the trial. Lewin et al. 8 took a complementary approach of studying trial and qualitative research articles as well as unpublished research for 30 trials of complex interventions. Their conclusions of a lack of integration between the trial and the qualitative research, and methodological shortcomings of the qualitative research, were largely supported by our findings, with details of differences discussed in Chapter 3 . The findings from our qualitative research largely concurred with a similar study of mixed methods researchers in health services research,142,143 although this is not too surprising given that some of those interviewees in the earlier study had worked on studies of trials and qualitative research and the research was undertaken by the same lead researcher. Its added value to the evidence base was identifying how the ‘add-on’ status of qualitative research to trials limits its ability to impact on trials and how the way in which research is published contributes to this.
Some literature reflecting on this methodological development takes a more challenging stance than our study. It addresses the philosophical differences between RCTs and qualitative research,148 the tensions between undertaking qualitative research and delivering a RCT,145 and the practical challenges of undertaking qualitative research in the context of a RCT. 149 Munro and Bloor149 express concern that the burden of expectation on process evaluations may be too great. They cite the range of questions that process evaluations could address to show that much can be expected and that this may lead to too much data being collected within them. Indeed, our framework might add to this problem by identifying the wide range of ways in which qualitative research can be used with trials. We think that Munro and Bloor149 make an important point and that researchers will need to consider the most important aspects of an intervention or trial to be addressed by any qualitative research. Munro and Bloor’s149 experience of a process evaluation alongside a feasibility trial highlighted two problems: the timing of the process evaluation compared with the trial and the challenges of generalisation of findings. First, the process evaluation data collection may be complete when unexpected findings emerge from the outcome evaluation, which the process evaluation is then asked to explain without the option of further data collection. Interviewees in our study did not identify this specific issue but did raise the wider challenge of the trial and qualitative research findings being available at different times. Second, they highlight the tension between the inductive nature of the qualitative research and the expectation that the process evaluation will pronounce on generalisability. They recognise the value of process evaluation but highlight the need to attend to some problems emerging from practice. The reflections of Munro and Bloor149 bring to mind the debates about the challenges of identifying causal relationships with qualitative research. 150 Qualitative researchers will need to balance attending to the uncertainties in their research and the claims being made, while also attending to the strengths of qualitative research to facilitate understanding of complex issues.
Strengths, limitations and reflexivity
Our study has three key strengths. First, it is timely in that a considerable amount of this research has been undertaken without much methodological reflection of this rapidly developing area. Second, the study focuses on what researchers are actually doing rather than debates about what can be done in theory. Third, it combines evidence that is based on the lasting end products of research – publications – and researchers’ views of this field. Our study has four main limitations. First, the review focused on publications from 2008 to 2010, which arose from studies that may have begun as early as 2000. Thus, they reflect recent history rather than current practice. However, the interviews were undertaken in 2011 with researchers who discussed their current work as well as their past work, so recent practice is included. Second, although the review was international, the rest of the study focused on the UK. Third, each method had its own limitations, which we discussed at the end of each chapter. It is worth bearing in mind that the mapping review was based on published research and that this might not reflect all the ways that qualitative research is used with trials. Fourth, our study focused on the value to the trial with which the qualitative research was undertaken. The assessment of potential value in our mapping review was subjective but did concur with views expressed by our interviewees. The researchers we interviewed and our PPI participants identified a further value of qualitative research for bringing patients and health professionals into the picture, a value that we identified in some parts of our values framework (that is, a potential value of ensuring the trial was sensitive to human beings) but perhaps on reflection did not fully appreciate.
The study focus, methods and inferences are shaped by our research team. Three of the authors (AO, KJT and JH) are the grant holders on the original bid for this study, they are (or have been) members of research commissioning boards in the UK, have undertaken studies combining trials and qualitative research and would call themselves health services researchers. Three of the authors are qualitative researchers who have not worked on trials (SJD, JG and AR). Disciplinary backgrounds of the team include psychology (JH and SJD), sociology (KJT, AR and JG) and health services research (AO). A driver for undertaking this study was a belief that qualitative research can be an important addition to trials and the evidence produced from trials, and concerns that the value was not being maximised. A different set of researchers might have been less accepting of the utility of trials or of qualitative research and may have developed a different framework from the one we present in Chapter 3 .
Generalisability and transferability
The review was based on international publications written in English between 2008 and 2010 and its geographical transferability will be to countries that publish academic research in English. Publications in later years may be different, but, given that researchers in the UK identified publishing as a major structural barrier, one might surmise that the same picture would emerge from a review of 2012–14 unless researchers make a conscious effort to change practice. The only issue that may affect temporal transferability is the increasing use of electronic publication and its accompanying tolerance of longer articles. The interview study was UK based and undertaken in 2011 and, therefore, has geographical and temporal specificity. The context was one of combining qualitative research and trials being a generally accepted endeavour. Researchers from outside the UK may wish to consider the findings from the UK-based components of our study in the context of how health research is undertaken in their own countries. As our study focused on health research, its findings may not be transferable to trials undertaken in social, educational and other fields of research. However, it is interesting to note that the 2010 paper by Spillane et al. 151 from education describes using qualitative research with a trial in ways that are very similar to those found in our study: to monitor the fidelity of treatment implementation in the trial, identify variables that mediate relations between treatment and outcomes, and validate measures and instruments.
A rapidly developing field
Researchers are developing strategies for undertaking qualitative research with trials. This involves qualitative research being included in procedures and practices associated with trials, for example having standard operating procedures within clinical trials units152 and publishing protocols for the process evaluation as well as the trial. 153 These developments highlight the growing status of combining qualitative research with trials and its establishment as a normal and acceptable practice. These developments are welcome if they attend to maintaining the emergent and flexible aspects of qualitative research in contrast to the highly protocolised world of RCTs. Established trial methodology may also be affected by accumulated understanding from qualitative research about how interventions and trials operate in the real world. A very helpful contribution to the literature is researcher reflections on undertaking this type of research, based on personal experience145,149 or methodological perspective. 154 Finally, systematic reviewers interested in explaining the heterogeneity of trial results want to study the qualitative research undertaken with included trials. 155
Conclusions and recommendations for different stakeholders
If qualitative research is undertaken with specific trials, should we not expect that research to impact on the trial in some way by optimising the intervention or trial conduct, explaining trial findings or facilitating transferability of trial findings in the real world? In Chapter 3 , we identified detailed suggestions for good practice and how to maximise value for each of the 22 ways in which qualitative research was used with trials. In Chapters 4–6 , we identified further ways of maximising value when writing proposals, reporting and conducting this type of research. In Chapter 6 , we explored how wider structural issues affect the value gained from qualitative research undertaken with trials by supporting and valuing the trial as the key endeavour. Below we offer summary recommendations to maximise the value of undertaking qualitative research with trials:
Researchers
Plan the qualitative research
It is not possible or necessary to use qualitative research to address all the aspects identified in our framework in the context of a single trial. Researchers will need to think about the problems and challenges their planned trial is likely to face and design the qualitative research to address these, while allowing for a degree of openness and flexibility to address possible emerging issues as the trial progresses.
Design and implement studies not trials
Some lead researchers we interviewed identified the qualitative research as essential to their desire to produce evidence relevant to the real world, whereas others described it as an addition to the trial and even as peripheral to the trial. When it was perceived as essential to the trial, impact on the trial was central to the team’s thinking. Powerful examples in the literature have embedded the trial in the qualitative research and ‘contravened conventional approaches by being driven not by the RCT design but by the qualitative research’9 or viewed their research as an integrated methods approach. 1
Use qualitative research at the feasibility and pilot stage of randomised controlled trials
Qualitative research undertaken at the feasibility or pilot phases of a RCT can impact on the main trial by optimising the intervention, improving the conduct of the trial, or identifying the appropriate outcomes and measures. This can result in successful and efficient trials that, we would argue, would be more likely to have positive results if shown to be feasible. At the pre-trial stage the research can be iterative, feeding into the feasibility trial and seeing improvements in recruitment or the intervention during this time. Bradley et al. 1 and Donovan et al. 9 took this approach and the latter describes it as a ‘sociological iterative approach’.
Think about the impact on the trial and implications for the trial endeavour
One of the most problematic things we came across here was the lack of explicit articulation of the effect of the qualitative research on the trial or the lessons for that trial or future trials. We do not think that the qualitative research and the trial have to be published in the same article for this to happen. Some authors show that these lessons can be articulated in the qualitative article. There is an issue about the timing of the two components working against this.
A related finding was researcher concern about the contamination of the experiment by the qualitative research, with concern that some intensive techniques, such as diary keeping and interviews, can offer a therapeutic effect. 156 This requires thought at the planning stage so that intensive qualitative data collection on a large proportion of the trial participants can be undertaken after the collection of important outcome data. There is also the potential for bias for some endeavours, for example using qualitative research to study variation of outcomes when the outcomes are already known. Again, the potential for this bias can be considered at the planning stage. There is some evidence in the wider literature that the qualitative research can take on procedures associated with trials, such as having a published protocol for the process evaluation or qualitative research,153 which would ensure planning and thinking about contamination and bias and how to minimise these in the context of a specific study.
Undertake depth qualitative research
Sandelowski and Barroso,140 in the context of the synthesis of qualitative research, describe articles that present summaries of data organised as themes rather than presenting findings in the form of a model or integrating concept. They did not want to discard this research because of its utility for health-care practice. We felt the same about some of the research we included here, which was descriptive and sometimes read as a list of issues. We felt that some of these studies were useful, for example list of solutions to the problem of trial recruitment in a deprived community. Yet other research required a more explanatory approach with in-depth analysis (e.g. asking why the trial finding was null or positive) and this could be lacking.
Some researchers propose the need to attend more to the epistemological roots of qualitative research to maximise its potential. 157 There are concerns in educational evaluation that qualitative research has been assigned to second-class status when used with RCTs, with undermining of its epistemological roots. We found some evidence of this in our interviews in which some researchers described implicit pressure to undertake large samples and structured interviews. We recommend that researchers engage expertise in qualitative research and resource the qualitative research in terms of the time, money and staffing required. There is a lot of activity in this field yet researchers present their findings without necessarily building on learning in the field. May158 presents the ‘normalisation process model‘ to facilitate service providers and researchers in understanding the factors that help or hinder the embedding of complex interventions in routine practice. He constructs this model using qualitative research, some of which was undertaken with RCTs, and then suggests that it can be used as a framework for prospective qualitative research. This approach to building a conceptual framework from existing qualitative research can help learning from disparate qualitative research undertaken with trials. There may be other opportunities to develop such models or make use of existing ones.
Allow qualitative research to take a challenging role
We identified qualitative research as a describer, explainer and translator of evidence, with some evidence for qualitative research as a challenger of current practices. There are examples in the wider literature of qualitative research challenging the underlying theory of the intervention,1 challenging whether or not the trial should continue159 and being used to provide evidence for discontinuing a trial. 160
Develop a learning environment about this issue
Some researchers have developed considerable expertise in undertaking qualitative research with trials and could share this expertise by running training courses and writing about how to undertake this work well. Researchers new to the field of combining qualitative research and trials – both qualitative researchers and triallists – would benefit from attending these courses and reading some of the exemplars we identify in this report.
Funders
Some of our interviewees identified funding application forms as a structural barrier to giving a detailed description of the qualitative research to be undertaken with a trial. These application forms could include requirements for the detail of the qualitative research, for example specifying the rationale for its use and detail of methods, sample size, analysis and resources required.
Ethics committees
Some of our interviewees identified the practice of ethics committees as a barrier to the iterative approach often taken with qualitative research. Ethics committees could recognise the need for qualitative research to evolve throughout studies without the need for submission of substantial amendments.
Journal editors
Publication norms were identified by some of our interviewees as a barrier to continuing to publish applied qualitative research and to addressing the impact of the qualitative research on the trial within journal articles, for example explaining trial results. Journal editors could consider the importance of applied qualitative research to their readership and, where it is undertaken with a trial, request that the lessons for trials are made explicit. Electronic publication, with its associated longer article length, may facilitate this.
Acknowledgements
We would like to thank:
All the participants of the study for sending documentation, returning questionnaires and participating in interviews. We understand how busy our participants were and thank them for their help, enthusiasm and insights.
Two members of the public, Leonie Martin and Christine McLoughlin, who met with us to offer their views of the value of qualitative research to trials.
Richard Campbell for administrative support throughout the whole study including locating copies of articles, administering the survey and transcribing the interviews.
Contributions of authors
Professor Alicia O’Cathain (Chair in Health Services Research, Applied Health Research) designed the study, obtained the funding, guided data collection, analysed all components, and wrote the first draft of the report.
Professor Kate J Thomas (Honorary Chair in Health Services Research, Applied Health Research) designed the study, obtained the funding, guided data collection, analysed all components, led the survey and the ‘intervention’ component of the mapping review, and edited the report.
Dr Sarah J Drabble (Research Associate, Psychology) undertook data collection on the mapping review and survey, analysed the qualitative study and study documents, and edited the report.
Dr Anne Rudolph (Research Associate, Sociology) undertook data collection for all components, analysed the study documents and edited the report.
Dr Jackie Goode (Senior Research Fellow, Sociology) analysed the qualitative study and wrote the first draft of that chapter.
Professor Jenny Hewison (Chair in the Psychology of Healthcare, Applied Health Research) obtained the funding, commented on data collection and analysis, and edited the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the MRC, NETSCC, the HTA programme or the Department of Health.
Publications
O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3:e002889.
Drabble SJ, O’Cathain A, Thomas KJ, Rudolph A, Hewison J. Describing qualitative research undertaken with randomised controlled trials in grant proposals: a documentary analysis. BMC Med Res Methodol 2014;14:24.
O’Cathain A, Goode J, Drabble SJ, Thomas KJ, Rudolph A, Hewison J. Getting added value from using qualitative research with randomised controlled trials: a qualitative interview study. Trials 2014; in press.
References
- Bradley F, Wiles R, Kinmonth AL, Mant D, Gantley M. SHIP Collaborative Group . Development and evaluation of complex interventions in health services research: case study of the Southampton heart integrated care project (SHIP). BMJ 1999;318:711-15. http://dx.doi.org/10.1136/bmj.318.7185.711.
- Sandelowski M. Using qualitative methods in intervention studies. Res Nurs Health 1996;19:359-64. http://dx.doi.org/10.1002/(SICI)1098-240X(199608)19:4%3C359::AID-NUR9%3E3.3.CO;2-8.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. http://dx.doi.org/10.1136/bmj.321.7262.694.
- Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455-9. http://dx.doi.org/10.1136/bmj.39108.379965.BE.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1655.
- Creswell JW, Fetters MD, Plano Clark VL, Morales A, Andrew S, Halcomb EJ. Mixed Methods Research for Nursing and the Health Sciences. Oxford: Wiley-Blackwell; 2009.
- Flemming K, Adamson J, Atkin K. Improving the effectiveness of interventions in palliative care: the potential role of qualitative research in enhancing evidence from randomized controlled trials. Palliat Med 2008;22:123-31. http://dx.doi.org/10.1177/0269216307087319.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3496.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report – Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. BMJ 2002;325:766-9. http://dx.doi.org/10.1136/bmj.325.7367.766.
- Wells M, Williams B, Treweek S, Coyle J, Taylor J. Intervention description is not enough: evidence from an in-depth multiple case study on the untold role and impact of context in randomised controlled trials of seven complex interventions. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-95.
- Jansen YFM, Foets MME, de Bont AA. The contribution of qualitative research to the development of tailor-made community-based interventions in primary care: a review. Eur J Public Health 2010;20:220-6. http://dx.doi.org/10.1093/eurpub/ckp085.
- Hawe P, Shiell A, Riley T. Complex interventions: how ‘out of control’ can a randomised controlled trial be?. BMJ 2004;328:1561-3. http://dx.doi.org/10.1136/bmj.328.7455.1561.
- Linnan L, Steckler A, Steckler A, Linnan L. Process Evaluations for Public Health Interventions and Research. San Francisco, CA: Jossey-Bass; 2002.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. RIPPLE Study team . Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. http://dx.doi.org/10.1136/bmj.332.7538.413.
- O’Cathain A, Murphy E, Nicholl J. Integration and publications as indicators of ‘yield’ from mixed methods studies. J Mixed Methods Res 2007;1:147-63.
- Sandelowski M. Using qualitative research. Qual Health Res 2004;14:1366-86. http://dx.doi.org/10.1177/1049732304269672.
- Popay J, Williams G. Qualitative research and evidence-based healthcare. J R Soc Med 1998;91:32-7.
- A Framework for the Development and Evaluation of RCTs for Complex Interventions to Improve Health. London: MRC; 2000.
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inform Libraries J 2009;26:91-108. http://dx.doi.org/10.1111/j.1471-1842.2009.00848.x.
- Lefebvre C, Manheimer E, Glanville J, Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, Ltd; 2008.
- Grant MJ. Searching for Qualitative Research Studies on the MEDLINE Database n.d.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. London: Routledge; 1994.
- CASP . Making Sense of Evidence About Clinical Effectiveness 2010. www.casp-uk.net/wp-content/uploads/2011/11/CASP_Qualitative_Appraisal_Checklist_14oct10.pdf (accessed September 2012).
- Davies SL, Horton TV, Williams AG, Martin MY, Stewart KE. MOMS: formative evaluation and subsequent intervention for mothers living with HIV. AIDS Care 2009;21:552-60. http://dx.doi.org/10.1080/09540120802301832.
- Gulbrandsen P, Krupat E, Benth JS, Garratt A, Safran DG, Finset A, et al. ‘Four Habits’ goes abroad: report from a pilot study in Norway. Patient Educ Counsel 2008;72:388-93. http://dx.doi.org/10.1016/j.pec.2008.05.012.
- Jefford M, Baravelli C, Dudgeon P, Dabscheck A, Evans M, Moloney M, et al. Tailored chemotherapy information faxed to general practitioners improves confidence in managing adverse effects and satisfaction with shared care: results from a randomized controlled trial. J Clin Oncol 2008;26:2272-7. http://dx.doi.org/10.1200/JCO.2007.14.7710.
- Marciel KK, Saiman L, Quittell LM, Dawkins K, Quittner AL. Cell phone intervention to improve adherence: cystic fibrosis care team, patient, and parent perspectives. Pediatr Pulmonol 2010;45:157-64. http://dx.doi.org/10.1002/ppul.21164.
- Nagel T, Robinson G, Condon J, Trauer T. Approach to treatment of mental illness and substance dependence in remote Indigenous communities: results of a mixed methods study. Aus J Rural Health 2009;17:174-82. http://dx.doi.org/10.1111/j.1440-1584.2009.01060.x.
- Redfern J, Rudd AD, Wolfe CDA, Mckevitt C. Stop Stroke: development of an innovative intervention to improve risk factor management after stroke. Patient Educ Counsel 2008;72:201-9. http://dx.doi.org/10.1016/j.pec.2008.03.006.
- Foster SDN, Nakamanya S, Kyomuhangi R, Amurwon J, Namara G, Amuron B, et al. The experience of ‘medicine companions’ to support adherence to antiretroviral therapy: Quantitative and qualitative data from a trial population in Uganda. AIDS Care 2010;22:35-43.
- Gambling T, Long A. Tailoring advice and optimizing response: a case study of a telephone-based support for patients with type 2 diabetes. Fam Prac 2010;27:179-85. http://dx.doi.org/10.1093/fampra/cmp097.
- Macpherson H, Thomas K. Self-help advice as a process integral to traditional acupuncture care: implications for trial design. Complement Ther Med 2008;16:101-6.
- Mcqueen A, Bartholomew LK, Greisinger AJ, Medina GG, Hawley ST, Haidet P, et al. Behind closed doors: physician-patient discussions about colorectal cancer screening. J Gen Intern Med 2009;24:1228-35. http://dx.doi.org/10.1007/s11606-009-1108-4.
- Romo N, Poo M, Ballesta R. From illegal poison to legal medicine: a qualitative research in a heroin-prescription trial in Spain. Drug Alcohol Rev 2009;28:186-95. http://dx.doi.org/10.1111/j.1465-3362.2008.00015.x.
- Teti M, Bowleg L, Cole R, Lloyd L, Rubinstein S, Spencer S, et al. A mixed methods evaluation of the effect of the protect and respect intervention on the condom use and disclosure practices of women living with HIV/AIDS. AIDS Behav 2010;14:567-79. http://dx.doi.org/10.1007/s10461-009-9562-x.
- Sandelowski M. Whatever happened to qualitative description?. Res Nurs Health 2000;23:334-40. http://dx.doi.org/10.1002/1098-240X(200008)23:4%3C334::AID-NUR9%3E3.0.CO;2-G.
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess 1998;2.
- Byng R, Norman I, Redfern S, Jones R. Exposing the key functions of a complex intervention for shared care in mental health: case study of a process evaluation. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-274.
- Hoddinott P, Britten J, Pill R. Why do interventions work in some places and not others: a breastfeeding support group trial. Soc Sci Med 2010;70:769-78. http://dx.doi.org/10.1016/j.socscimed.2009.10.067.
- Liu CJ, Hsiung PC, Chang KJ, Liu YF, Wang KC, Hsiao FH, et al. A study on the efficacy of body-mind-spirit group therapy for patients with breast cancer. J Clin Nurs 2008;17:2539-49. http://dx.doi.org/10.1111/j.1365-2702.2008.02296.x.
- Kaptchuk TJ, Shaw J, Kerr CE, Conboy LA, Kelley JM, Csordas TJ, et al. ‘Maybe I made up the whole thing’: placebos and patients’ experiences in a randomized controlled trial. Cult Med Psychiatry 2009;33:382-411.
- O’sullivan TL, Fortier MS, Faubert C, Culver D, Blanchard C, Reid R, et al. Interdisciplinary physical activity counseling in primary care: a qualitative inquiry of the patient experience. J Health Psychol 2010;15:362-72. http://dx.doi.org/10.1177/1359105309350516.
- Rogers A, Gately C, Kennedy A, Sanders C. Are some more equal than others? Social comparison in self-management skills training for long-term conditions. Chronic Illn 2009;5:305-17. http://dx.doi.org/10.1177/1742395309350384.
- Pawson R, Tilley N. Realistic Evaluation. London: Sage; 1997.
- Drahota A, Galloway E, Stores R, Ward D, Severs M, Dean T. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot 2008;18:211-19. http://dx.doi.org/10.1016/j.foot.2008.06.001.
- Dowrick C, Gask L, Hughes JG, Charles-Jones H, Hogg JA, Peters S, et al. General practitioners’ views on reattribution for patients with medically unexplained symptoms: a questionnaire and qualitative study. BMC Fam Prac 2008;9.
- Lindell KO, Olshansky E, Song MK, Zullo TG, Gibson KF, Kaminski N, et al. Impact of a disease-management program on symptom burden and health-related quality of life in patients with idiopathic pulmonary fibrosis and their care partners. Heart Lung 2010;39:304-13. http://dx.doi.org/10.1016/j.hrtlng.2009.08.005.
- Lipman EL, Kenny M, Jack S, Cameron R, Secord M, Byrne C. Understanding how education/support groups help lone mothers. BMC Publ Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-4.
- Malpass A, Andrews R, Turner KM. Patient perception, preference and participation: patients with type 2 diabetes experiences of making multiple lifestyle changes: a qualitative study. Patient Educ Counsel 2009;74:258-63.
- Morone NE, Lynch CS, Greco CM, Tindle HA, Weiner DK. ‘I felt like a new person.’ the effects of mindfulness meditation on older adults with chronic pain: qualitative narrative analysis of diary entries. J Pain 2008;9:841-8. http://dx.doi.org/10.1016/j.jpain.2008.04.003.
- Avery KNL, Blazeby JM, Lane JA, Neal DE, Hamdy FC, Donovan JL. Decision-making about PSA testing and prostate biopsies: a qualitative study embedded in a primary care randomised trial. Eur Urol 2008;53:1186-93. http://dx.doi.org/10.1016/j.eururo.2007.07.040.
- Buzza CD, Williams MB, Weg MWV, Christensen AJ, Kaboli PJ, Reisinger HS. Part II, Provider perspectives: should patients be activated to request evidence-based medicine? A qualitative study of the VA project to implement diuretics (VAPID). Implement Sci 2010;5. http://dx.doi.org/10.1186/1748-5908-5-24.
- Mihaylov S, Stark C, Mccoll E. Steeped treatment of older adults on laxatives: the STOOL trial. Health Technol Assess 2008;12.
- Ockleford E, Shaw RL, Willars J, Dixon-Woods M. Education and self-management for people newly diagnosed with type 2 diabetes: a qualitative study of patients’ views. Chron Illn 2008;4:28-37. http://dx.doi.org/10.1177/1742395307086673.
- Reid CM, Gooberman-Hill R, Hanks GW. Opioid analgesics for cancer pain: symptom control for the living or comfort for the dying? A qualitative study to investigate the factors influencing the decision to accept morphine for pain caused by cancer. Ann Oncol 2008;19:44-8. http://dx.doi.org/10.1093/annonc/mdm462.
- Zhang G, Malik VS, Pan A, Kumar S, Holmes MD, Spiegelman D, et al. Substituting brown rice for white rice to lower diabetes risk: a focus-group study in Chinese adults. J Am Diet Assoc 2010;110:1216-21. http://dx.doi.org/10.1016/j.jada.2010.05.004.
- Behets FMTF, Van Damme K, Turner AN, Rabenja NL, Ravelomanana NLR, Raharinivo MSM, et al. Evidence-based planning of a randomized controlled trial on diaphragm use for prevention of sexually transmitted infections. Sex Transmit Dis 2008;35:238-42. http://dx.doi.org/10.1097/OLQ.0b013e31815abaa2.
- Ferrer RL, Mody-Bailey P, Jaen CR, Gott S, Araujo S. A medical assistant-based program to promote healthy behaviors in primary care. Ann Fam Med 2009;7:504-12. http://dx.doi.org/10.1370/afm.1059.
- Mcpherson KM, Kayes N, Weatherall M. Members of the Goals SRRG. A pilot study of self-regulation informed goal setting in people with traumatic brain injury. Clin Rehabil 2009;23:296-309. http://dx.doi.org/10.1177/0269215509102980.
- Pope DS, Atkins S, Deluca AN, Hausler H, Hoosain E, Celentano DD, et al. South African TB nurses’ experiences of provider-initiated HIV counseling and testing in the Eastern Cape Province: a qualitative study. AIDS Care 2010;22:238-45. http://dx.doi.org/10.1080/09540120903040594.
- Sandler A, Glesne C, Geller G. Children’s and parents’ perspectives on open-label use of placebos in the treatment of ADHD. Child Care Health Develop 2008;34:111-20. http://dx.doi.org/10.1111/j.1365-2214.2007.00743.x.
- White E, Winstanley J. Implementation of clinical supervision: educational preparation and subsequent diary accounts of the practicalities involved, from an Australian mental heath nursing innovation. J Psychiatr Mental Health Nurs 2009;16:895-903. http://dx.doi.org/10.1111/j.1365-2850.2009.01466.x.
- Ferguson YO, Eng E, Bentley M, Sandelowski M, Steckler A, Randall-David E, et al. Evaluating nurses’ implementation of an infant-feeding counseling protocol for HIV-infected mothers: the BAN Study in Lilongwe, Malawi. AIDS Educ Prevent 2009;21:141-55. http://dx.doi.org/10.1521/aeap.2009.21.2.141.
- Holliday J, Audrey S, Moore L, Parry-Langdon N, Campbell R. High fidelity? How should we consider variations in the delivery of school-based health promotion interventions?. Health Educ J 2009:44-62. http://dx.doi.org/10.1177/0017896908100448.
- McNamara DG, Dickinson AR, Byrnes CA. The perceptions and preferences of parents of children with tracheostomies in a study of humidification therapy. J Child Health Care 2009;13:179-97. http://dx.doi.org/10.1177/1367493509336686.
- Mukoma W, Flisher AJ, Ahmed N, Jansen S, Mathews C, Klepp KI, et al. Process evaluation of a school-based HIV/AIDS intervention in South Africa. Scand J Public Health 2009;37:37-4.
- Palinkas LA, Schoenwald SK, Hoagwood K, Landsverk J, Chorpita BF, Weisz JR, et al. An ethnographic study of implementation of evidence-based treatments in child mental health: first steps. Psychiatr Serv 2008;59:738-46.
- Plummer ML, Watson-Jones D, Lees S, Baisley K, Matari S, Changalucha J, et al. A qualitative study of participant adherence in a randomized controlled trial of herpes suppressive therapy for HIV prevention in Tanzania. AIDS Care 2010;22:499-508.
- Cals JWL, Butler CC, Dinant GJ. ‘Experience talks’: physician prioritisation of contrasting interventions to optimise management of acute cough in general practice. Implement Sci 2009;4.
- Cals JWL, Chappin FHF, Hopstaken RM, van Leeuwen ME, Hood K, Butler CC, et al. C-reactive protein point-of-care testing for lower respiratory tract infections: a qualitative evaluation of experiences by GPs. Fam Prac 2010;27:212-18. http://dx.doi.org/10.1093/fampra/cmp088.
- Carnes D, Anwer Y, Underwood M, Harding G, Parsons S, Team TS. Influences on older people’s decision making regarding choice of topical or oral NSAIDs for knee pain: qualitative study. BMJ 2008;336:142-5. http://dx.doi.org/10.1136/bmj.39401.699063.BE.
- Escoffery CG, Glanz K, Hall D, Elliott T. A multi-method process evaluation for a skin cancer prevention diffusion trial. Eval Health Prof 2009;32:184-203. http://dx.doi.org/10.1177/0163278709333154.
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999;52:1143-56.
- Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002360.
- Bower P, Wilson S, Mathers N. Short report: how often do UK primary care trials face recruitment delays?. Fam Prac 2007;24:601-3. http://dx.doi.org/10.1093/fampra/cmm051.
- De Salis I, Tomlin Z, Toerien M, Donovan J. Using qualitative research methods to improve recruitment to randomized controlled trials: the Quartet study. J Health Serv Res Policy 2008;13:92-6. http://dx.doi.org/10.1258/jhsrp.2008.008028.
- Garcia C, Pintor JK, Lindgren S. Feasibility and acceptability of a school-based coping intervention for Latina adolescents. J School Nurs 2010;26:42-5. http://dx.doi.org/10.1177/1059840509351021.
- Bill-Axelson A, Christensson A, Carlsson M, Norlen BJ, Holmberg L. Experiences of randomization: interviews with patients and clinicians in the SPCG-IV trial. Scand J Urol Nephrol 2008;42:358-63. http://dx.doi.org/10.1080/00365590801950253.
- Dormandy E, Kavalier F, Logan J, Harris H, Ishmael N, Marteau TM, et al. Maximising recruitment and retention of general practices in clinical trials: a case study. Br J Gen Prac 2008;58:759-66. http://dx.doi.org/10.3399/bjgp08X319666.
- Perkins D, Harris MF, Tan J, Christl B, Taggart J, Fanaian M. Engaging participants in a complex intervention trial in Australian General Practice. BMC Med Res Method 2008;8. http://dx.doi.org/10.1186/1471-2288-8-55.
- Potter R, Dale J, Caramlau I. A qualitative study exploring practice nurses’ experience of participating in a primary care-based randomised controlled trial. J Res Nurs 2009;14:439-47. http://dx.doi.org/10.1177/1744987108098228.
- Rothwell PM. External validity of randomised controlled trials: ‘to whom do the results of this trial apply?’. Lancet 2005;365:82-93. http://dx.doi.org/10.1016/S0140-6736(04)17670-8.
- Goode PS, Fitzgerald MP, Richter HE, Whitehead WE, Nygaard I, Wren PA, et al. Enhancing participation of older women in surgical trials. J Am Coll Surg 2008;207:303-11. http://dx.doi.org/10.1016/j.jamcollsurg.2008.03.012.
- Jaspan HBS, Soka NF, Mathews C, Flisher AJ, Mark D, Middelkoop K, et al. A qualitative assessment of perspectives on the inclusion of adolescents in HIV vaccine trials in South Africa. Int J STD AIDS 2010;21:172-6. http://dx.doi.org/10.1258/ijsa.2009.008484.
- Joseph G, Dohan D. Diversity of participants in clinical trials in an academic medical center: the role of the ‘good study patient?’. Cancer 2009;115:608-15. http://dx.doi.org/10.1002/cncr.24028.
- Kogan JNB. Increasing minority research participation through collaboration with community outpatient clinics: the STEP-BD Community Partners Experience. Clin Trials 2009;6:344-54. http://dx.doi.org/10.1177/1740774509338427.
- Russell KM, Maraj MS, Wilson LR, Shedd-Steele R, Champion VL. Barriers to recruiting urban African American women into research studies in community settings. Appl Nurs Res 2008;21:90-7. http://dx.doi.org/10.1016/j.apnr.2006.05.001.
- Velott DL, Baker SA, Hillemeier MM, Weisman CS. Participant recruitment to a randomized trial of a community-based behavioral intervention for pre- and interconceptional women findings from the Central Pennsylvania Women’s Health Study. Womens Health Issues 2008;18:217-24.
- Jollye S. An exploratory study to determine how parents decide whether to enrol their infants into neonatal clinical trials. J Neonatal Nurs 2009;15:18-24. http://dx.doi.org/10.1016/j.jnn.2008.07.012.
- Kohara I, Inoue T. Searching for a way to live to the end: decision-making process in patients considering participation in cancer phase I clinical trials. Oncol Nurs Forum 2010;37:E124-32. http://dx.doi.org/10.1188/10.ONF.E124-E132.
- McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-31.
- Ward FR. Chaos, vulnerability and control: parental beliefs about neonatal clinical trials. J Perinatol 2009;29:156-62. http://dx.doi.org/10.1038/jp.2008.139.
- Lavender T, Kingdon C. Primigravid women’s views of being approached to participate in a hypothetical term cephalic trial of planned vaginal birth versus planned cesarean birth. Birth 2009;36:213-19. http://dx.doi.org/10.1111/j.1523-536X.2009.00325.x.
- Campbell MK, Skea ZC, Sutherland AG, Cuthbertson BH, Entwistle VA, McDonald AM, et al. Effectiveness and cost-effectiveness of arthroscopic lavage in the treatment of osteoarthritis of the knee: a mixed methods study of the feasibility of conducting a surgical placebo-controlled trial (the KORAL study). Health Technol Assess 2010;14.
- Cooper SA, Anafi P, Sun C, Naidoo N, Reddy P, Buchanan D. Content analysis of local newspaper coverage of the microbicide trials in South Africa. Int Q Commun Health Educ 2009;29:105-21. http://dx.doi.org/10.2190/IQ.29.2.b.
- Mack N, Robinson ET, Macqueen KM, Moffett J, Johnson LM. The exploitation of ‘Exploitation’ in the tenofovir prep trial in Cameroon: lessons learned from media coverage of an HIV prevention trial. J Empir Res Hum Res Ethics 2010;5:3-19.
- Rosenthal SL, Holmes W, Maher L. Australian men’s experiences during a microbicide male tolerance study. AIDS Care 2009;21:125-30. http://dx.doi.org/10.1080/09540120802084958.
- Beal CC, Stuifbergen A, Volker D, Becker H. Women’s experiences as members of attention control and experimental intervention groups in a randomized controlled trial. Can J Nurs Res 2009;41:16-31.
- Jonvallen P. Compliance revisited: pharmaceutical drug trials in the era of the contract research organization. Nurs Inq 2009;16:347-54. http://dx.doi.org/10.1111/j.1440-1800.2009.00455.x.
- Thorstensson CA, Lohmander LS, Frobell RB, Roos EM, Gooberman-Hill R. Choosing surgery: patients’ preferences within a trial of treatments for anterior cruciate ligament injury. A qualitative study. BMC Musculoskel Disord 2009;10. http://dx.doi.org/10.1186/1471-2474-10-100.
- Tutton E, Gray B. Fluid optimisation using a peripherally inserted central catheter (PICC) following proximal femoral fracture: lessons learnt from a feasibility study. J Orthopaed Nurs 2009;13:11-8. http://dx.doi.org/10.1016/j.joon.2008.10.007.
- Flory J, Emanuel E. Interventions to improve research participants' understanding in informed consent for research: a systematic review. JAMA 2004;292:1593-601. http://dx.doi.org/10.1001/jama.292.13.1593.
- Koops L, Lindley R. Thrombolysis for acute ischaemic stroke: consumer involvement in design of new randomised controlled trial. BMJ 2002;325. http://dx.doi.org/10.1136/bmj.325.7361.415.
- Gikonyo C, Bejon P, Marsh V, Molyneux S. Taking social relationships seriously: lessons learned from the informed consent practices of a vaccine trial on the Kenyan Coast. Soc Sci Med 2008;67:708-20. http://dx.doi.org/10.1016/j.socscimed.2008.02.003.
- Instone SLM, Mueller MR, Gilbert TL. Therapeutic discourse among nurses and physicians in controlled clinical trials. Nurs Ethics 2008;15:803-12. http://dx.doi.org/10.1177/0969733008095388.
- Mangset M, Forde R, Nessa J, Berge E, Wyller TB, Wyller TB. I don’t like that, it’s tricking people too much. . .: acute informed consent to participation in a trial of thrombolysis for stroke. J Med Ethics 2008;34:751-6.
- Penn C, Evans M. Recommendations for communication to enhance informed consent and enrolment at multilingual research sites. African J AIDS Res 2009;8:285-94. http://dx.doi.org/10.2989/AJAR.2009.8.3.5.926.
- Timmermans S, Mckay T. Clinical trials as treatment option: bioethics and health care disparities in substance dependency. Soc Sci Med 2009;69:1784-90. http://dx.doi.org/10.1016/j.socscimed.2009.09.019.
- Wasan AD, Taubenberger SP, Robinson WM. Reasons for participation in pain research: can they indicate a lack of informed consent?. Pain Med 2009;10:111-19. http://dx.doi.org/10.1111/j.1526-4637.2008.00481.x.
- Balcazar H, Rosenthal L, De Heer H, Aguirre M, Flores L, Vasquez E, et al. Use of community-based participatory research to disseminate baseline results from a cardiovascular disease randomized community trial for Mexican Americans living in a U.S.-Mexico border community. Educ Health 2009;22.
- Shagi C, Vallely A, Kasindi S, Chiduo B, Desmond N, Soteli S, et al. A model for community representation and participation in HIV prevention trials among women who engage in transactional sex in Africa. AIDS Care 2008;20:1039-49. http://dx.doi.org/10.1080/09540120701842803.
- Grbich C, Abernethy AP, Shelby-James T, Fazekas B, Currow DC. Creating a research culture in a palliative care service environment: a qualitative study of the evolution of staff attitudes to research during a large longitudinal controlled trial (ISRCTN81117481). J Palliat Care 2008;24:100-9.
- Kyle G, Marks-Maran D. Focus group interviews: how aromatherapists feel about changing their practice through undertaking a randomised controlled trial?. Complement Ther Clin Prac 2008;14:204-11. http://dx.doi.org/10.1016/j.ctcp.2008.01.003.
- Paterson C, Zheng Z, Xue C, Wang Y. ‘Playing their parts’: the experiences of participants in a randomized sham-controlled acupuncture trial. J Alt Complement Med 2008;14:199-208.
- Stadler JJ, Delany S, Mntambo M. Women’s perceptions and experiences of HIV prevention trials in Soweto, South Africa. Soc Sci Med 2008;66:189-200.
- Yawn BPP, Pace W, Dietrich A, Bertram S, Kurland M, Graham D, et al. Practice benefit from participating in a practice-based research network study of postpartum depression: a National Research Network (NRN) report. J Am Board Fam Med 2010;23:455-64. http://dx.doi.org/10.3122/jabfm.2010.04.090246.
- Alraek TM, Malterud K. Acupuncture for menopausal hot flashes: a qualitative study about patient experiences. J Altern Complement Med 2009;15:153-8. http://dx.doi.org/10.1089/acm.2008.0310.
- Aagaard EM, Gonzales R, Camargo CAJ, Auten R, Levin SK, Maselli J, et al. Physician champions are key to improving antibiotic prescribing quality. Joint Commission J Qual Patient Saf 2010;36:109-16.
- Penn L, Moffatt SM, White M. Participants’ perspective on maintaining behaviour change: a qualitative study within the European Diabetes Prevention Study. BMC Public Health 2008;8. http://dx.doi.org/10.1186/1471-2458-8-235.
- Peterson JC, Allegrante JP, Pirraglia PA, Robbins L, Lane KP, Boschert KA, et al. Living with heart disease after angioplasty: A qualitative study of patients who have been successful or unsuccessful in multiple behavior change. Heart Lung 2010;39:105-15. http://dx.doi.org/10.1016/j.hrtlng.2009.06.017.
- Leidy NK, Vernon M. Perspectives on patient-reported outcomes: content validity and qualitative research in a changing clinical trial environment. Pharmacoeconomics 2008;26:363-70. http://dx.doi.org/10.2165/00019053-200826050-00002.
- Farquhar M, Ewing G, Higginson IJ, Booth S. The experience of using the SEIQoL-DW with patients with advanced chronic obstructive pulmonary disease (COPD): issues of process and outcome. Qual Life Res 2010;19:619-29.
- Penman-Aguilar A, Swezey T, Turner AN, Bell AJ, Ramiandrisoa FN, Legardy-Williams J, et al. Promoting continuous use as a strategy for achieving adherence in a trial of the diaphragm with candidate microbicide. AIDS Educ Prevent 2009;21:512-25. http://dx.doi.org/10.1521/aeap.2009.21.6.512.
- Pool JJM, Hiralal S, Ostelo RWJG, Van Der Veer K, Vlaeyen JWS, Bouter LM, et al. The applicability of the Tampa Scale of Kinesiophobia for patients with sub-acute neck pain: a qualitative study. Qual Quant 2009;43:773-80. http://dx.doi.org/10.1007/s11135-008-9203-x.
- Pool RM. Assessing the accuracy of adherence and sexual behaviour data in the MDP301 Vaginal microbicides trial using a mixed methods and triangulation model. PLOS ONE 2010;5:1-9.
- Robertson C, Langston AL, Stapley S, Mccoll E, Campbell MK, Fraser WD, et al. Meaning behind measurement: self-comparisons affect responses to health-related quality of life questionnaires. Qual Life Res 2009;18:221-30. http://dx.doi.org/10.1007/s11136-008-9435-1.
- Sloboda Z, Pyakuryal A, Stephens PC, Teasdale B, Forrest D, Stephens RC, et al. Reports of substance abuse prevention programming available in schools. Prevent Sci 2008;9:276-87. http://dx.doi.org/10.1007/s11121-008-0102-0.
- Nakash R, Hutton J, Lamb S. Response and non-response to postal questionnaire follow-up in a clinical trial: a qualitative study of the patient’s perspective. J Eval Clin Prac 2008;14:226-35. http://dx.doi.org/10.1111/j.1365-2753.2007.00838.x.
- Abetz L, Rofail D, Mertzanis P, Heelis R, Rosa K, Tellefsen C, et al. Alzheimer’s disease treatment: assessing caregiver preferences for mode of treatment delivery. Adv Ther 2009;26:627-44. http://dx.doi.org/10.1007/s12325-009-0034-5.
- Leidy NK, Mathias SD, Parasuraman BM, Patrick DL, Pathak D. Development and validation of an onset of effect questionnaire for patients with asthma. Allergy Asthma Proc 2008;29:590-9. http://dx.doi.org/10.2500/aap.2008.29.3164.
- Bair MJ, Matthias MS, Nyland KA, Huffman MA, Stubbs DL, Kroenke K, et al. Barriers and facilitators to chronic pain self-management: a qualitative study of primary care patients with comorbid musculoskeletal pain and depression. Pain Med n.d.;10:1280-90. http://dx.doi.org/10.1111/j.1526-4637.2009.00707.x.
- Chew-Graham CA, Sharp D, Chamberlain E, Folkes L, Turner KM. Disclosure of symptoms of postnatal depression, the perspectives of health professionals and women: a qualitative study. BMC Fam Prac 2009;10. http://dx.doi.org/10.1186/1471-2296-10-7.
- Cook C, Fay S, Rockwood K. Verbal repetition in people with mild-to-moderate Alzheimer Disease: a descriptive analysis from the VISTA clinical trial. Alzheimer Dis Assoc Disord 2009;23:146-51. http://dx.doi.org/10.1097/WAD.0b013e318193cbef.
- Holtrop JS, Malouin R, Weismantel D, Wadland WC. Clinician perceptions of factors influencing referrals to a smoking cessation program. BMC Fam Prac 2008;9. http://dx.doi.org/10.1186/1471-2296-9-18.
- Mavhu W, Dauya E, Bandason T, Munyati S, Cowan FM, Hart G, et al. Chronic cough and its association with TB-HIV co-infection: factors affecting help-seeking behaviour in Harare, Zimbabwe. Trop Med Intl Health 2010;15:574-9. http://dx.doi.org/10.1111/j.1365-3156.2010.02493.x.
- Sekelja N, Butow PN, Tattersall MHN. Bereaved cancer carers’ experience of and preference for palliative care. Support Care Cancer 2010;18:1219-28.
- Hoddinott P, Britten J, Prescott GJ, Tappin D, Ludbrook A, Godden DJ. Effectiveness of policy to provide breastfeeding groups (BIG) for pregnant and breastfeeding mothers in primary care: cluster randomised controlled trial. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.a3026.
- Noyes J, Popay J, Pearson A, Hannes K, Booth A, Higgins JPT, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, Ltd; 2011.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3.
- Sandelowski M, Barroso J. Creating metasummaries of qualitative findings. Nurs Res 2003;52:226-33. http://dx.doi.org/10.1097/00006199-200307000-00004.
- Connelly LM, Yoder LH. Improving qualitative proposals: common problem areas. Clin Nurse Spec 2000;14:69-74. http://dx.doi.org/10.1097/00002800-200003000-00009.
- O’Cathain A, Murphy E, Nicholl J. The quality of mixed methods studies in health services research. J Health Serv Res Policy 2008;13:92-8. http://dx.doi.org/10.1258/jhsrp.2007.007074.
- O’Cathain A, Nicholl J, Murphy E. Structural issues affecting mixed methods studies in health research: a qualitative study. BMC Med Res Method 2009;9. http://dx.doi.org/10.1186/1471-2288-9-82.
- Devers KJ. Qualitative methods in health services and management research: pockets of excellence and progress, but still a long way to go. Med Care Res Rev 2011;68:41-8. http://dx.doi.org/10.1177/1077558710384269.
- Simons L. Moving from collision to integration: reflecting on the experience of mixed methods. J Res Nurs 2007;12:73-8. http://dx.doi.org/10.1177/1744987106069514.
- Farmer T, Robinson K, Elliott SJ, Eyles J. Developing and implementing a triangulation protocol for qualitative health research. Qual Health Res 2006;16:377-94. http://dx.doi.org/10.1177/1049732305285708.
- Tashakkori A, Teddlie C. Handbook of mixed methods in social & behavioral research. CA: Sage; 2003.
- Blackwood B, O’Halloran P, Porter S. On the problems of mixing RCTs with qualitative research: the case of the MRC framework for the evaluation of complex healthcare interventions. J Res Nurs 2010;15:511-21. http://dx.doi.org/10.1177/1744987110373860.
- Munro A, Bloor M. Process evaluation: the new miracle ingredient in public health research?. Qual Res 2010;10:699-713. http://dx.doi.org/10.1177/1468794110380522.
- Maxwell JA. Using qualitative methods for causal explanation. Field Methods 2004;16:243-64. http://dx.doi.org/10.1177/1525822X04266831.
- Spillane JP, Pareja AS, Dorner L, Barnes C, May H, Huff J, et al. Mixing methods in randomized controlled trials (RCTs): validation, contextualization, triangulation, and control. Educ Asse Eval Acc 2010;22:5-28. http://dx.doi.org/10.1007/s11092-009-9089-8.
- Rapport F, Storey M, Porter A, Snooks H, Jones K, Peconi J, et al. Qualitative research within trials: developing a standard operating procedure for a clinical trials unit. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-54.
- Grant A, Dreischulte T, Treweek S, Guthrie B. Study protocol of a mixed methods evaluation of a cluster randomized trial to improve the safety of NSAID and antiplatelet prescribing: data-driven quality improvement in primary care. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-154.
- Hesse-Biber S. Weaving a multimethodology and mixed methods praxis into randomized control trials to enhance credibility. Qual Inq 2012;18:876-89. http://dx.doi.org/10.1177/1077800412456964.
- Glenton C, Lewin S, Scheel IB. Still too little qualitative research to shed light on results from reviews of effectiveness trials: a case study of a Cochrane review on the use of lay health workers. Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-53.
- Hawe P, Shiell A, Riley T, Gold L. Methods for exploring implementation variation and local context within a cluster randomised community intervention trial. J Epidemiol Community Health 2004;58:788-93. http://dx.doi.org/10.1136/jech.2003.014415.
- Howe KR. A critique of experimentalism. Qual Inq 2004;10:42-61. http://dx.doi.org/10.1177/1077800403259491.
- May C. A rational model for assessing and evaluating complex interventions in health care. BMC Health Serv Res 2006;6.
- Riley T, Hawe P, Shiell A. Contested ground: how should qualitative evidence inform the conduct of a community intervention trial?. J Health Serv Res Policy 2005;10:103-10. http://dx.doi.org/10.1258/1355819053559029.
- Murtagh MJ, Thomson RG, May CR, Rapley T, Heaven BR, Graham RH, et al. Qualitative methods in a randomised controlled trial: the role of an integrated qualitative process evaluation in providing evidence to discontinue the intervention in one arm of a trial of a decision support tool. Qual Saf Health Care 2007;16:224-9. http://dx.doi.org/10.1136/qshc.2006.018499.
Appendix 1 Proposal
Appendix 2 Search terms used in systematic mapping review
| Original terms identified in study proposal | Additional search terms added to the search | |
|---|---|---|
| Terms to identify RCT | Terms to identify qualitative research | |
| randomised control$ trial$.mp | qualitative research.mp. OR qualitative research/ | |
| clinical trial.mp OR clinical trial/ | (qualitative ADJ3 method$).mp | |
| pragmatic trial.mp | ((qualitative ADJ3 study) OR (qualitative ADJ3 studies)).mp | |
| complex intervention.mp | (focus group$ OR focus-group$).mp | |
| (controlled trial$ OR controlled-trial$).mp | narrative analysis.mp | |
| grounded theory.mp | ||
| process evaluation.mp | ||
| (mixed method$ OR mixed-method$).mp | ||
| observation$.mp (EXCLUDED) | ||
| interview$ (EXCLUDED) | (in-depth ADJ4 interview$).mp | |
| (((((semi structured ADJ5 interview$) OR semistructured) ADJ5 interview$) OR semi-structured) ADJ5 interview$).mp | ||
| qualitative interview$.mp | ||
| (interview$ AND theme$).mp | ||
| (interview$ AND (audio recorded OR audio-recorded)).mp | ||
| case studies (EXCLUDED) | (qualitative case study OR qualitative case studies OR qualitative case-study OR qualitative case-studies).mp | |
| (descriptive case study OR descriptive case studies OR descriptive case-study OR descriptive case-studies).mp | ||
| qualitative (EXCLUDED) | qualitative exploration.mp | |
| (qualitative analysis OR qualitative analyses OR qualitatively analy?ed).mp | ||
| (qualitative ADJ3 data).mp | ||
| qualitative evaluation.mp | ||
| qualitative intervention.mp | ||
| qualitative approach.mp | ||
| qualitative inquiry.mp | ||
| discourse analysis.mp | ||
| discursive.mp | ||
| phenomenological.mp | ||
| thematic analysis.mp | ||
| ethnograph$.mp | ||
| action research.mp | ||
| (ethno methodology OR ethnomethodology).mp | ||
| social construction$.mp | ||
| NOT phenomenological characteristics.mp | ||
| NOT phenomenological model.mp | ||
| NOT action research arm test.mp | ||
| NOT protocol.ti | ||
Appendix 3 References of 296 articles in Chapter 3
Aagaard EM, Gonzales R, Camargo CAJ, Auten R, Levin SK, Maselli J, et al. Physician champions are key to improving antibiotic prescribing quality. Joint Commission J Qual Patient Saf 2010;36:109–16. (See Chapter 3 , Variation of outcomes.)118
Abetz L, Rofail D, Mertzanis P, Heelis R, Rosa K, Tellefsen C, et al. Alzheimer’s disease treatment: assessing caregiver preferences for mode of treatment delivery. Adv Ther 2009;26:627–44. (See Chapter 3 , Development and validation of measures.)129
Ahmad F, Skinner HA, Stewart DE, Levinson W. Perspectives of family physicians on computer-assisted health-risk assessments. J Med Internet Res 2010;12:e12. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Aiarzaguena JM, Gaminde I, Grandes G, Salazar A, Alonso I, Sanchez A. Somatisation in primary care: experiences of primary care physicians involved in a training program and in a randomised controlled trial. BMC Fam Prac 2009;10:73. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Alraek TM, Malterud K. Acupuncture for menopausal hot flashes: a qualitative study about patient experiences. J Altern Complement Med 2009;15:153–8. (See Chapter 3 , Breadth of outcomes to be measured in the trial.)117
Angus JE, Rukholm E, Michel I, Larocque S, Seto L, Lapum J, et al. Context and cardiovascular risk modification in two regions of Ontario, Canada: a photo elicitation study. Int J Environ Res Public Health 2009;6:2481–99. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Audrey SH. Commitment and compatibility: teachers’ perspectives on the implementation of an effective school-based, peer-led smoking intervention. Health Educ J 2008;67:74–90. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Avery KNL, Blazeby JM, Lane JA, Neal DE, Hamdy FC, Donovan JL. Decision-making about PSA testing and prostate biopsies: a qualitative study embedded in a primary care randomised trial. Eur Urol 2008;53:1186–93. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )51
Bair MJ, Matthias MS, Nyland KA, Huffman MA, Stubbs DL, Kroenke K, et al. Barriers and facilitators to chronic pain self-management: a qualitative study of primary care patients with comorbid musculoskeletal pain and depression. Pain Med 2009:10:1280–90. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)131
Bakas T, Farran CJ, Austin JK, Given BA, Johnson EA, Williams LS. Content validity and satisfaction with a stroke caregiver intervention program. J Nurs Scholar 2009;41:368–75. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice.)
Balcazar H, Rosenthal L, De Heer H, Aguirre M, Flores L, Vasquez E, et al. Use of community-based participatory research to disseminate baseline results from a cardiovascular disease randomized community trial for Mexican Americans living in a U.S.-Mexico border community. Educ Health 2009;22:279. (See Chapter 3 , Adaptation of trial conduct to local context. )110
Banek K, Kilian A, Allan R. Evaluation of Interceptor long-lasting insecticidal nets in eight communities in Liberia. Malaria J 2010;9:84. (See Chapter 3 , Development of the trial intervention)
Barlow JH, Turner AP, Gilchrist M. A randomised controlled trial of lay-led self-management for myocardial infarction patients who have completed cardiac rehabilitation. Eur J Cardiovasc Nurs 2009;8:293–301. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Bazzano AN, Kirkwood BR, Tawiah-Agyemang C, Owusu-Agyei S, Adongo PB. Beyond symptom recognition: care-seeking for ill newborns in rural Ghana. Trop Med Int Health 2008;13:123–8. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Beal CC, Stuifbergen A, Volker D, Becker H. Women’s experiences as members of attention control and experimental intervention groups in a randomized controlled trial. Can J Nurs Res 2009;41:16–31. (See Chapter 3 , The acceptability of a trial in practice. )98
Beattie A, Shaw A, Kaur S, Kessler D. Primary-care patients’ expectations and experiences of online cognitive behavioural therapy for depression: a qualitative study. Health Expect 2009;12:45–59. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Behets FMTF, Van Damme K, Turner AN, Rabenja NL, Ravelomanana NLR, Raharinivo MSM, et al. Evidence-based planning of a randomized controlled trial on diaphragm use for prevention of sexually transmitted infections. Sex Transmit Dis 2008;35:238–42. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )57
Bekkers MJ, Simpson SA, Dunstan F, Hood K, Hare M, Evans J, et al. Enhancing the quality of antibiotic prescribing in primary care: qualitative evaluation of a blended learning intervention. BMC Fam Prac 2010;11:34. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Bhandari S, Levitch AH, Ellis KK, Ball K, Everett K, Geden E, et al. Comparative analyses of stressors experienced by rural low-income pregnant women experiencing intimate partner violence and those who are not. J Obstetr Gynecol Neonatal Nurs 2008;37:492–501. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Bill-Axelson A, Christensson A, Carlsson M, Norlen BJ, Holmberg L. Experiences of randomization: interviews with patients and clinicians in the SPCG-IV trial. Scand J Urol Nephrol 2008;42:358–63. (See Chapter 3 , Trial recruitment and retention. )78
Bleijlevens MHC, Hendriks MRC, Van Haastregt JCM, Van Rossum E, Kempen GIJM, Diederiks JPM, et al. Process factors explaining the ineffectiveness of a multidisciplinary fall prevention programme: a process evaluation. BMC Public Health 2008;8:332. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice, and Chapter 3 , Intervention fidelity, reach and dose. )
Bradley-Ewing A, Thomson D, Pinkston M, Goggin KJ. A qualitative examination of the indirect effects of modified directly observed therapy on health behaviors other than adherence. AIDS Patient Care STDs 2008;22:663–8. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Brooks DM, Bradt J, Eyre L, Hunt A, Dileo C. Creative approaches for reducing burnout in medical personnel. Arts Psychother 2010;37:255–63. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Brown AH, Cohen AN, Chinman MJ, Kessler C, Young AS. EQUIP: implementing chronic care principles and applying formative evaluation methods to improve care for schizophrenia: QUERI Series. Implement Sci 2008;3:9. (See Chapter 3 , Development of the trial intervention. )
Bunik M, Shobe P, O’Connor ME, Beaty B, Langendoerfer S, Crane L, et al. Are 2 weeks of daily breastfeeding support insufficient to overcome the influences of formula? Acad Pediatr 2010;10:21–8. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Buzza CD, Williams MB, Weg MWV, Christensen AJ, Kaboli PJ, Reisinger HS. Part II, Provider perspectives: should patients be activated to request evidence-based medicine? A qualitative study of the VA project to implement diuretics (VAPID). Implement Sci 2010;5:24. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention; Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )52
Byng R, Norman I, Redfern S, Jones R. Exposing the key functions of a complex intervention for shared care in mental health: case study of a process evaluation. BMC Health Serv Res 2008;8:274. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )38
Cabassa LJ, Hansen MC, Palinkas LA, Ell K. Azucar y nervios: explanatory models and treatment experiences of Hispanics with diabetes and depression. Soc Sci Med 2008;66:2413–24. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Cals JWL, Chappin FMF, Hopstaken RM, van Leeuwen ME, Hood K, Butler CC, et al. C-reactive protein point-of-care testing for lower respiratory tract infections: a qualitative evaluation of experiences by GPs. Fam Prac 2010;27:212–18. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention, and Chapter 3 , Implementation of the trial intervention in the real world. )70
Cals JWL, Butler CC, Dinant GJ. ‘Experience talks’: physician prioritisation of contrasting interventions to optimise management of acute cough in general practice. Implement Sci 2009;4:57. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Implementation of the trial intervention in the real world. )69
Campbell MK, Skea ZC, Sutherland AG, Cuthbertson BH, Entwistle VA, McDonald AM, et al. Effectiveness and cost-effectiveness of arthroscopic lavage in the treatment of osteoarthritis of the knee: a mixed methods study of the feasibility of conducting a surgical placebo-controlled trial (the KORAL study). Health Technol Assess 2010;14(5). (See Chapter 3 , The ‘in principle’ acceptability of the trial.)94
Carin-Levy G, Kendall M, Young A, Mead G. The psychosocial effects of exercise and relaxation classes for persons surviving a stroke. Can J Occup Ther 2009;76:73–80. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Carnes D, Anwer Y, Underwood M, Harding G, Parsons S, Team TS. Influences on older people’s decision making regarding choice of topical or oral NSAIDs for knee pain: qualitative study. BMJ 2008;336:142–5. (See Chapter 3 , Implementation of the trial intervention in the real world, and Chapter 3 , Experience of the disease, health behaviour or beliefs.)71
Chatterjee SC. Integrating evidence-based treatments for common mental disorders in routine primary care: Feasibility and acceptability of the MANAS intervention in Goa, India. World Psychiatry 2008;7:39–46. (See Chapter 3 , Development of the trial intervention. )
Chew-Graham C, Chamberlain E, Turner K, Folkes L, Caulfield L, Sharp D. GPs’ and health visitors’ views on the diagnosis and management of postnatal depression: a qualitative study. Br J Gen Prac 2008;58:169–76. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Chew-Graham C, Dowrick C, Wearden A, Richardson V, Peters S. Making the diagnosis of chronic fatigue syndrome/myalgic encephalitis in primary care: a qualitative study. BMC Fam Prac 2010;11:16. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Chew-Graham C, Slade M, Montana C, Stewart M, Gask L. Loss of doctor-to-doctor communication: lessons from the reconfiguration of mental health services in England. J Health Serv Res Policy 2008;13:6–12. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Chew-Graham CA, Cahill G, Dowrick C, Wearden A, Peters S. Using multiple sources of knowledge to reach clinical understanding of chronic fatigue syndrome. Ann Fam Med 2008;6:340–8. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Chew-Graham CA, Sharp D, Chamberlain E, Folkes L, Turner KM. Disclosure of symptoms of postnatal depression, the perspectives of health professionals and women: a qualitative study. BMC Fam Prac 2009;10:7. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)132
Coghill N, Cooper AR. Motivators and de-motivators for adherence to a program of sustained walking. Prevent Med 2009;49:24–7. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice, and Chapter 3 , Intervention fidelity, reach and dose. )
Coleman CL, Jemmott L, Jemmott JB, Strumpf N, Ratcliffe S. Development of an HIV Risk Reduction Intervention for Older Seropositive African American Men. AIDS Patient Care STDs 2009;23:647–55. (See Chapter 3 , Development of the trial intervention. )
Conboy L, Quilty MT, Kerr C, Shaw J, Wayne P. A qualitative analysis of adolescents’ experiences of active and sham Japanese-style acupuncture protocols administered in a clinical trial. J Alt Complement Med 2008;14:699–705. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention; Chapter 3 , Feasibility and acceptability of the trial intervention in practice, and Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Connor K, Mcneese-Smith D, Van Servellen G, Chang B, Lee M, Cheng E, et al. Insight into dementia care management using social-behavioral theory and mixed methods. Nurs Res 2009;58:348–58. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )
Cook C, Fay S, Rockwood K. Decreased initiation of usual activities in people with mild-to-moderate Alzheimer’s disease: a descriptive analysis from the VISTA clinical trial. Int Psychogeriatr 2008;20:952–63. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Cook C, Fay S, Rockwood K. Verbal repetition in people with mild-to-moderate Alzheimer Disease: a descriptive analysis from the VISTA clinical trial. Alzheimer Dis Assoc Disord 2009;23:146–51. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)133
Cooper SA, Anafi P, Sun C, Naidoo N, Reddy P, Buchanan D. Content analysis of local newspaper coverage of the microbicide trials in South Africa. Int Q Commun Health Educ 2009;29:105–21. (See Chapter 3 , The ‘in principle’ acceptability of the trial. )95
Cummings E, Turner P. Patient self-management and chronic illness: evaluating outcomes and impacts of information technology. Stud Health Technol Informat 2009;143:229–34. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Daley AJ, Copeland RJ, Wright NP, Wales JKH. ‘I can actually exercise if I want to; it isn’t as hard as I thought’: a qualitative study of the experiences and views of obese adolescents participating in an exercise therapy intervention. J Health Psychol 2008;13:810–19. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Davidson P, Digiacomo M, Zecchin R, Clarke M, Paul G, Lamb K, et al. A cardiac rehabilitation program to improve psychosocial outcomes of women with heart disease. J Womens Health 2008;17:123–34. (See Chapter 3 , Development of the trial intervention. )
Davies SL, Horton TV, Williams AG, Martin MY, Stewart KE. MOMS: formative evaluation and subsequent intervention for mothers living with HIV. AIDS Care 2009;21:552–60. (See Chapter 3 , Development of the trial intervention. )24
De Salis I, Tomlin Z, Toerien M, Donovan J. Qualitative research to improve RCT recruitment: issues arising in establishing research collaborations. Contemp Clin Trials 2008;29:663–70. (See Chapter 3 , Trial recruitment and retention. )
De Salis I, Tomlin Z, Toerien M, Donovan J. Using qualitative research methods to improve recruitment to randomized controlled trials: the Quartet study. J Health Serv Res Policy 2008;13(Suppl. 3):92–6. (See Chapter 3 , Trial recruitment and retention. )76
Decker C, Arnold SV, Olabiyi O, Ahmad H, Gialde E, Luark J, et al. Implementing an innovative consent form: the PREDICT experience. Implement Sci 2008;3:58. (See Chapter 3 , Development of the trial intervention. )
Deegan PE, Rapp C, Holter M, Riefer M. Best practices: a program to support shared decision making in an outpatient psychiatric medication clinic. Psychiatr Serv 2008;59:603–5. (See Chapter 3 , Development of the trial intervention. )
Dennison L, Stanbrook R, Moss-Morris R, Yardley L, Chalder T. Cognitive behavioural therapy and psycho-education for chronic fatigue syndrome in young people: Reflections from the families’ perspective. Br J Health Psychol 2010;15:167–83. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Donovan JL, Lane JA, Peters TJ, Brindle L, Salter E, Gillatt D, et al. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. J Clin Epidemiol 2009;62:29–36. (See Chapter 3 , Trial recruitment and retention. )
Dormandy E, Kavalier F, Logan J, Harris H, Ishmael N, Marteau TM, et al. Maximising recruitment and retention of general practices in clinical trials: a case study. Br J Gen Prac 2008;58:759–66. (See Chapter 3 , Trial recruitment and retention. )79
Dow B, Moore K, Scott P, Ratnayeke A, Wise K, Sims J, et al. Rural carers online: a feasibility study. Aus J Rural Health 2008;16:221–5. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Dowrick C, Gask L, Hughes JG, Charles-Jones H, Hogg JA, Peters S, et al. General practitioners’ views on reattribution for patients with medically unexplained symptoms: a questionnaire and qualitative study. BMC Fam Prac 2008;9:46. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )46
Drahota A, Galloway E, Stores R, Ward D, Severs M, Dean T. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot 2008;18:211–19. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )45
Duncan LG, Bardacke N. Mindfulness-based childbirth and parenting education: promoting family mindfulness during the perinatal period. J Child Fam Stud 2010;19:190–202. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Eley DS, Del Mar CB, Patterson E, Synnott RL, Baker PG, Hegney D. A nurse led model of chronic disease care – an interim report. Aus Fam Phys 2008;37:1030–2. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Escoffery CG, Glanz K, Hall D, Elliott T. A multi-method process evaluation for a skin cancer prevention diffusion trial. Eval Health Prof 2009;32:184–203. (See Chapter 3 , Implementation of the trial intervention in the real world. )72
Farquhar M, Ewing G, Higginson IJ, Booth S. The experience of using the SEIQoL-DW with patients with advanced chronic obstructive pulmonary disease (COPD): issues of process and outcome. Qual Life Res 2010;19:619–29. (See Chapter 3 , Accuracy of measures.)122
Farzanfar R, Stevens A, Pham Q, Friedman R. A formative qualitative evaluation of usability and acceptability of a workplace mental health assessment and intervention system. Int J Mental Health Prom 2008;10:17–25. (See Chapter 3 , Development of the trial intervention. )
Ferguson YO, Eng E, Bentley M, Sandelowski M, Steckler A, Randall-David E, et al. Evaluating nurses’ implementation of an infant-feeding counseling protocol for HIV-infected mothers: the BAN Study in Lilongwe, Malawi. AIDS Educ Prevent 2009;21:141–55. (See Chapter 3 , Intervention fidelity, reach and dose. )63
Ferrer RL, Mody-Bailey P, Jaen CR, Gott S, Araujo S. A medical assistant-based program to promote healthy behaviors in primary care. Ann Fam Med 2009;7:504–12. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )58
Forrester D, Mccambridge J, Waissbein C, Emlyn-Jones R, Rollnick S. Child risk and parental resistance: can motivational interviewing improve the practice of child and family social workers in working with parental alcohol misuse? Br J Soc Work 2008;38:1302–19. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Forster DA, Mclachlan HL. Women’s views and experiences of breast feeding: positive, negative or just good for the baby? Midwifery 2010;26:116–25. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Foster SDN, Nakamanya S, Kyomuhangi R, Amurwon J, Namara G, Amuron B, et al. The experience of ‘medicine companions’ to support adherence to antiretroviral therapy: Quantitative and qualitative data from a trial population in Uganda. AIDS Care 2010;22(Suppl. 1):35–43. (See Chapter 3 , Describing the trial intervention and elaborating its components, and Chapter 3 , Intervention fidelity, reach and dose. )30
Frost J, Shaw A, Montgomery A, Murphy DJ. Women’s views on the use of decision aids for decision making about the method of delivery following a previous caesarean section: qualitative interview study. BJOG 2009;116:896–905. (See Chapter 3 , Describing the trial intervention and elaborating its components. )
Frye V, Fortin P, Mackenzie S, Purcell D, Edwards LV, Mitchell SG, et al. Managing identity impacts associated with disclosure of HIV status: a qualitative investigation. AIDS Care 2009;21:1071–8. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Gambling T, Long A. The realisation of patient-centred care during a 3-year proactive telephone counselling self-care intervention for diabetes. Patient Educ Counsel 2010;80:219–26. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )
Gambling T, Long A. Tailoring advice and optimizing response: a case study of a telephone-based support for patients with type 2 diabetes. Fam Prac 2010;27:179–85. (See Chapter 3 , Describing the trial intervention and elaborating its components, and Chapter 3, Exploring perceived value and benefits of the trial intervention. )31
Garcia C, Pintor JK, Lindgren S. Feasibility and acceptability of a school-based coping intervention for Latina adolescents. J School Nurs 2010;26:42–52. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice, and Chapter 3 , Trial recruitment and retention. )77
Garcia E, Timmermans DR, Van LE. Reconsidering prenatal screening: an empirical-ethical approach to understand moral dilemmas as a question of personal preferences. J Med Ethics 2009;35:410–14. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention. )
Garvie PAL. Development of a directly observed therapy adherence intervention for adolescents with human immunodeficiency virus-1: application of focus group methodology to inform design, feasibility, and acceptability. J Adolesc Health 2009;44:124–32. (See Chapter 3 , Development of the trial intervention. )
Gask L, Bower P, Lovell K, Escott D, Archer J, Gilbody S, et al. What work has to be done to implement collaborative care for depression? Process evaluation of a trial utilizing the normalization process model. Implement Sci 2010;5:15. (See Chapter 3 , Development of the trial intervention. )
Gensichen J, Jaeger C, Peitz M, Torge M, Guthlin C, Mergenthal K, et al. Health care assistants in primary care depression management: role perception, burdening factors, and disease conception. Ann Fam Med 2009;7:513–19. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Gibson CAS. Physical activity across the curriculum: year one process evaluation results. Int J Behav Nutr Phys Activ 2008;36:36. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Gibson F, Aldiss S, Taylor RM, Maguire R, Kearney N. Involving health professionals in the development of an advanced symptom management system for young people: the ASyMS-YG study. Eur J Oncol Nurs 2009;13:187–92. (See Chapter 3 , Development of the trial intervention. )
Gikonyo C, Bejon P, Marsh V, Molyneux S. Taking social relationships seriously: lessons learned from the informed consent practices of a vaccine trial on the Kenyan Coast. Soc Sci Med 2008;67:708–20. (See Chapter 3 , Ethical conduct in a trial. )104
Gilson N, Mckenna J, Cooke C. Experiences of route and task-based walking in a university community: qualitative perspectives in a randomized control trial. J Phys Activ Health 2008;5(Suppl. 1):S176–82. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Goode PS, Fitzgerald MP, Richter HE, Whitehead WE, Nygaard I, Wren PA, et al. Enhancing participation of older women in surgical trials. J Am Coll Surg 2008;207:303–11. (See Chapter 3 , Diversity of trial participants. )83
Goodman H, Davison J, Preedy M, Peters E, Waters P, Persaud-Rai B, et al. Patient and staff perspective of a nurse-led support programme for patients waiting for cardiac surgery: participant perspective of a cardiac support programme. Eur J Cardiovasc Nurs 2009;8:67–73. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Gorman JR, Usita PM, Madlensky L, Pierce JP. A qualitative investigation of breast cancer survivors’ experiences with breastfeeding. J Cancer Survivor 2009;3:181–91. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Gowers SG, Clark AF, Roberts C, Byford S, Barrett B, Griffiths A, et al. A randomised controlled multicentre trial of treatments for adolescent anorexia nervosa including assessment of cost-effectiveness and patient acceptability – the TOuCAN trial. Health Technol Assess 2010;14(15). (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Grbich C, Abernethy AP, Shelby-James T, Fazekas B, Currow DC. Creating a research culture in a palliative care service environment: a qualitative study of the evolution of staff attitudes to research during a large longitudinal controlled trial (ISRCTN81117481). J Palliat Care 2008;24:100–9. (See Chapter 3 , Impact of trial on staff, researchers or participants.)112
Greene E, Batona G, Hallad J, Johnson S, Neema S, Tolley EE. Acceptability and adherence of a candidate microbicide gel among high-risk women in Africa and India. Cult Health Sex 2010;12:739–54. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Griffiths FP. Group versus individual sessions delivered by a physiotherapist for female urinary incontinence: an interview study with women attending group sessions nested within a randomised controlled trial. BMC Womens Health 2009;9:25. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Guest GS. Acceptability of PrEP for HIV prevention among women at high risk for HIV. J Womens Health 2010;19:791–8. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Gulbrandsen P, Krupat E, Benth JS, Garratt A, Safran DG, Finset A, et al. ‘Four Habits’ goes abroad: report from a pilot study in Norway. Patient Educ Counsel 2008;72:388–93. (See Chapter 3 , Development of the trial intervention. )25
Guldberg TL, Vedsted P, Lauritzen T, Zoffmann V. Suboptimal quality of type 2 diabetes care discovered through electronic feedback led to increased nurse-GP cooperation. A qualitative study. Prim Care Diabet 2010;4:33–9. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Gysels MP. Community response to intermittent preventive treatment of malaria in infants (IPTi) delivered through the expanded programme of immunization in five African settings. Malaria J 2009;8:191. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Hallett J, Maycock B, Kypri K, Howat P, Mcmanus A. Development of a web-based alcohol intervention for university students: processes and challenges. Drug Alcohol Rev 2009;28:31–9. (See Chapter 3 , Development of the trial intervention. )
Hamilton AB, Cohen AN, Young AS. Organizational readiness in specialty mental health care. J Gen Intern Med 2010;25(Suppl. 1):27–31. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention. )
Harley AE, Devine CM, Beard B, Stoddard AM, Hunt MK, Sorensen G. Multiple health behavior changes in a cancer prevention intervention for construction workers, 2001–2003. Prevent Chron Dis 2010;7:A55. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )
Hartley S, Murira G, Mwangoma M, Carter J, Newton CRJC. Using community/researcher partnerships to develop a culturally relevant intervention for children with communication disabilities in Kenya. Disabil Rehabil 2009;31:490–9. (See Chapter 3 , Development of the trial intervention, and Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )
Hauser M, Lautenschlager M, Gudlowski Y, Ozgurdal S, Witthaus H, Bechdolf A, et al. Short Communication: Psychoeducation with patients at-risk for schizophrenia-An exploratory pilot study. Patient Educ Counsel 2009;138–42. (See Chapter 3 , Development of the trial intervention. )
Herriot AM, Thomas DE, Hart KH, Warren J, Truby H. A qualitative investigation of individuals’ experiences and expectations before and after completing a trial of commercial weight loss programmes. J Hum Nutr Diet 2008;21:72–80. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Hill Z, Tawiah-Agyemang C, Odei-Danso S, Kirkwood B. Informed consent in Ghana: what do participants really understand? J Med Ethics 2008;34:48–53. (See Chapter 3 , Ethical conduct in a trial. )
Hoddinott P, Britten J, Pill R. Why do interventions work in some places and not others: a breastfeeding support group trial. Soc Sci Med 2010;70:769–78. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention, and Chapter 3 , Variation of outcomes. )39
Holliday J, Audrey S, Moore L, Parry-Langdon N, Campbell R. High fidelity? How should we consider variations in the delivery of school-based health promotion interventions? Health Educ J 2009;44–62. (See Chapter 3 , Intervention fidelity, reach and dose. )64
Holmes WRM. Attitudes of men in an Australian male tolerance study towards microbicide use. Sex Health 2008;5:273–8. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention. )
Holtrop JS, Malouin R, Weismantel D, Wadland WC. Clinician perceptions of factors influencing referrals to a smoking cessation program. BMC Fam Prac 2008;9:18. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)134
Howard L, De Salis I, Tomlin Z, Thornicroft G, Donovan J. Why is recruitment to trials difficult? An investigation into recruitment difficulties in an RCT of supported employment in patients with severe mental illness. Contemp Clin Trials 2009;30:40–6. (See Chapter 3 , Trial recruitment and retention. )
Hurley MV, Walsh N, Bhavnani V, Britten N, Stevenson F. Health beliefs before and after participation on an exercised-based rehabilitation programme for chronic knee pain: doing is believing. BMC Musculoskel Disord 2010;11:31. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Innes NPT, Marshman Z, Vendan RE. A group of general dental practitioners’ views of preformed metal crowns after participation in the Hall technique clinical trial: a mixed-method evaluation. Prim Dent Care 2010;17:33–7. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Instone SLM, Mueller MR, Gilbert TL. Therapeutic discourse among nurses and physicians in controlled clinical trials. Nurs Ethics 2008;15:803–12. (See Chapter 3 , Ethical conduct in a trial. )105
Jack SM, Jamieson E, Wathen CN, Macmillan HL. The feasibility of screening for intimate partner violence during postpartum home visits. Can J Nurs Res 2008;40:150–70. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Jackson CC. Evaluating a web-based MMR decision aid to support informed decision-making by UK parents: a before-and-after feasibility study. Health Educ J 2010;69:74–83. (See Chapter 3 , Development of the trial intervention. )
Jaspan HBS, Soka NF, Mathews C, Flisher AJ, Mark D, Middelkoop K, et al. A qualitative assessment of perspectives on the inclusion of adolescents in HIV vaccine trials in South Africa. Int J STD AIDS 2010;21:172–6. (See Chapter 3 , Diversity of trial participants. )84
Jefford M, Baravelli C, Dudgeon P, Dabscheck A, Evans M, Moloney M, et al. Tailored chemotherapy information faxed to general practitioners improves confidence in managing adverse effects and satisfaction with shared care: results from a randomized controlled trial. J Clin Oncol 2008;26:2272–7. (See Chapter 3 , Development of the trial intervention. )26
Johnson MG. Patients’ opinions of acute chest pain care: a qualitative evaluation of chest pain units. J Adv Nurs 2009;65:120–9. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Jollye S. An exploratory study to determine how parents decide whether to enrol their infants into neonatal clinical trials. J Neonatal Nurs 2009;15:18–24. (See Chapter 3 , Trial participation: the experience of being in a trial.)89
Jonvallen P. Compliance revisited: pharmaceutical drug trials in the era of the contract research organization. Nurs Inq 2009;16:347–54. (See Chapter 3 , The acceptability of a trial in practice. )99
Joseph G, Dohan D. Diversity of participants in clinical trials in an academic medical center: the role of the ‘good study patient?’. Cancer 2009;115:608–15. (See Chapter 3 , Diversity of trial participants. )85
Joseph G, Dohan D. Recruiting minorities where they receive care: institutional barriers to cancer clinical trials recruitment in a safety-net hospital. Contemp Clin Trials 2009;30:552–9. (See Chapter 3 , Diversity of trial participants. )
Juraskova I, Butow P, Lopez A, Seccombe M, Coates A, Boyle F, et al. Improving informed consent: pilot of a decision aid for women invited to participate in a breast cancer prevention trial (IBIS-II DCIS). Health Expectations 2008;11:252–62. (See Chapter 3 , Ethical conduct in a trial. )
Kahan NR, Kahan E, Waitman DA, Kitai E, Chintz DP. The tools of an evidence-based culture: implementing clinical-practice guidelines in an Israeli HMO. Acad Med 2009;84:1217–25. (See Chapter 3 , Development of the trial intervention. )
Kanter JWR. The use and nature of present-focused interventions in cognitive and behavioral therapies for depression. Psychotherapy 2009;46:220–32. (See Chapter 3 , Intervention fidelity, reach and dose. )
Kaptchuk TJ, Shaw J, Kerr CE, Conboy LA, Kelley JM, Csordas TJ, et al. ‘Maybe I made up the whole thing’: placebos and patients’ experiences in a randomized controlled trial. Cult Med Psychiatry 2009;33:382–411. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )41
Kebaabetswe P, Ndase P, Mujugira A, Sekoto T, Ntshimane M, Owor A, et al. Educational/counseling model health care: perceptions of couple HIV counseling and testing in Botswana: a stakeholder analysis. Patient Educ Counsel 2010;120–3. (See Chapter 3 , Development of the trial intervention. )
Kennedy HP, Farrell T, Paden R, Hill S, Jolivet R, Willetts J, et al. ‘I wasn’t alone’ – a study of group prenatal care in the military. J Midwif Womens Health 2009;54:176–83. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Killaspy H, Johnson S, Pierce B, Bebbington P, Pilling S, Nolan F, et al. Successful engagement: a mixed methods study of the approaches of assertive community treatment and community mental health teams in the REACT trial. Soc Psychiatry Psychiatr Epidemiol 2009;44:532–40. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )
King KM, Mcfetridge-Durdle J, Leblanc P, Anzarut A, Tsuyuki RT. A descriptive examination of the impact of sternal scar formation in women. Eur J Cardiovasc Nurs 2009;8:112–18. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Knifed E, Lipsman N, Mason W, Bernstein M. Patients’ perception of the informed consent process for neurooncology clinical trials. Neuro-Oncology 2008;10:348–54. (See Chapter 3 , Ethical conduct in a trial. )
Kogan JNB. Increasing minority research participation through collaboration with community outpatient clinics: the STEP-BD Community Partners Experience. Clin Trials 2009;6:344–54. (See Chapter 3 , Diversity of trial participants. )86
Kohara I, Inoue T. Searching for a way to live to the end: decision-making process in patients considering participation in cancer phase I clinical trials. Oncol Nurs Forum 2010;37:E124–32. (See Chapter 3 , Trial participation: the experience of being in a trial.)90
Kotz D, Vos R, Huibers MJ, Huibers MJH. Ethical analysis of the justifiability of labelling with COPD for smoking cessation. J Med Ethics 2009;35:534–40. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Kyle G, Marks-Maran D. Focus group interviews: how aromatherapists feel about changing their practice through undertaking a randomised controlled trial? Complement Ther Clin Prac 2008;14:204–11. (See Chapter 3 , Impact of trial on staff, researchers or participants.)113
Lavender T, Bedwell C, Tsekiri-O’brien E, Hart A, Turner M, Cork M. A qualitative study exploring women’s and health professionals’ view of newborn bathing practices. Evidence Based Midwif 2009;7:112–21. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention. )
Lavender T, Kingdon C. Primigravid women’s views of being approached to participate in a hypothetical term cephalic trial of planned vaginal birth versus planned cesarean birth. Birth 2009;36:213–19. (See Chapter 3 , The ‘in principle’ acceptability of the trial. )93
Lees S, Desmond N, Allen C, Bugeke G, Vallely A, Ross D. Sexual risk behaviour for women working in recreational venues in Mwanza, Tanzania: considerations for the acceptability and use of vaginal microbicide gels. Cult Health Sex 2009;11:581–95. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention. )
Lees SC. Comparison of sexual behavior data collected using a coital diary and a clinic-based interview during a microbicide pilot study in Mwanza, Tanzania. Sex Transmit Dis 2010;37:497–501. (See Chapter 3 , Accuracy of measures.)
Leidy NK, Mathias SD, Parasuraman BM, Patrick DL, Pathak D. Development and validation of an onset of effect questionnaire for patients with asthma. Allergy Asthma Proc 2008;29:590–9. (See Chapter 3 , Development and validation of measures.)130
Leydon GM, Turner S, Smith H, Little P, Team U. Women’s views about management and cause of urinary tract infection: qualitative interview study. BMJ 2010;340:c279. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Leydon GMT. The journey from self-care to GP care: a qualitative interview study of women presenting with symptoms of urinary tract infection. Br J Gen Pracs 2009;59:e219–25. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Liddy CD. Telehomecare for patients with multiple chronic illnesses: pilot study. Can Fam Phys 2008;54:58–65. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Lin CA, Neafsey PJ, Anderson E. Advanced practice registered nurse usability testing of a tailored computer-mediated health communication program. Comput Informat Nurs 2010;28:32–41. (See Chapter 3 , Development of the trial intervention. )
Lindell KO, Olshansky E, Song MK, Zullo TG, Gibson KF, Kaminski N, et al. Impact of a disease-management program on symptom burden and health-related quality of life in patients with idiopathic pulmonary fibrosis and their care partners. Heart Lung 2010;39:304–13. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )47
Lipman EL, Kenny M, Jack S, Cameron R, Secord M, Byrne C. Understanding how education/support groups help lone mothers. BMC Publ Health 2010;10:4. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )48
Liu CJ, Hsiung PC, Chang KJ, Liu YF, Wang KC, Hsiao FH, et al. A study on the efficacy of body-mind-spirit group therapy for patients with breast cancer. J Clin Nurs 2008;17:2539–49. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )40
Liu ETH, Chen WL, Li YH, Wang CH, Mok TJ, Huang HS. Exploring the efficacy of cognitive bibliotherapy and a potential mechanism of change in the treatment of depressive symptoms among the Chinese: a randomized controlled trial. Cogn Ther Res 2009;33:449–61. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Logsdon C, Foltz MP. Adapting and testing telephone-based depression care management intervention for adolescent mothers. Arch Womens Mental Health 2010;13:307–17. (See Chapter 3 , Development of the trial intervention. )
Lord VMC. Singing teaching as a therapy for chronic respiratory disease – a randomised controlled trial and qualitative evaluation. BMC Pulmon Med 2010;41:41. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Lovell K, Bower P, Richards D, Barkham M, Sibbald B, Roberts C, et al. Developing guided self-help for depression using the Medical Research Council complex interventions framework: a description of the modelling phase and results of an exploratory randomised controlled trial. BMC Psychiatry 2008;8:91. (See Chapter 3 , Development of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Mack N, Robinson ET, Macqueen KM, Moffett J, Johnson LM. The exploitation of ‘Exploitation’ in the tenofovir prep trial in Cameroon: lessons learned from media coverage of an HIV prevention trial. J Empir Res Hum Res Ethics 2010;5:3–19. (See Chapter 3 , The ‘in principle’ acceptability of the trial. )96
Macpherson H, Thomas K. Self-help advice as a process integral to traditional acupuncture care: implications for trial design. Complement Ther Med 2008;16:101–6. (See Chapter 3 , Describing the trial intervention and elaborating its components. )32
Maguire R, Mccann L, Miller M, Kearney N. Nurse’s perceptions and experiences of using of a mobile-phone-based Advanced Symptom Management System (ASyMS) to monitor and manage chemotherapy-related toxicity. Eur J Oncol Nurs 2008;12:380–6. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Mair F, Hiscock J, Beaton S. Understanding factors that inhibit or promote the utilization of telecare in chronic lung disease. Chron Illn 2008;4:110–17. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Makowsky MJ, Schindel TJ, Rosenthal M, Campbell K, Tsuyuki RT, Madill HM. Collaboration between pharmacists, physicians and nurse practitioners: a qualitative investigation of working relationships in the inpatient medical setting. J Interprof Care 2009;23:169–84. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )
Malpass A, Andrews R, Turner KM. Patient perception, preference and participation: patients with type 2 diabetes experiences of making multiple lifestyle changes: a qualitative study. Patient Educ Counsel 2009;74:258–63. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention, and Chapter 3 , Exploring perceived value and benefits of the trial intervention. )49
Mangset M, Forde R, Nessa J, Berge E, Wyller TB, Wyller TB. I don’t like that, it’s tricking people too much. . .: acute informed consent to participation in a trial of thrombolysis for stroke. J Med Ethics 2008;34:751–6. (See Chapter 3 , Ethical conduct in a trial.)106
Mangset MB. ‘Two per cent isn’t a lot, but when it comes to death it seems quite a lot anyway’: patients’ perception of risk and willingness to accept risks associated with thrombolytic drug treatment for acute stroke. J Med Ethics 2009;35:42–6. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Marciel KK, Saiman L, Quittell LM, Dawkins K, Quittner AL. Cell phone intervention to improve adherence: cystic fibrosis care team, patient, and parent perspectives. Pediatr Pulmonol 2010;45:157–64. (See Chapter 3 , Development of the trial intervention. )27
Marcy TW, Kaplan B, Connolly SW, Michel G, Shiffman RN, Flynn BS. Developing a decision support system for tobacco use counselling using primary care physicians. Inform Prim Care 2008;16:101–9. (See Chapter 3 , Development of the trial intervention. )
Martin SB. Carraguard acceptability among men and women in a couples study in Thailand. J Womens Health 2010;19:1561–7. (See Chapter 3 , Development of the trial intervention. )
Mavhu W, Dauya E, Bandason T, Munyati S, Cowan FM, Hart G, et al. Chronic cough and its association with TB-HIV co-infection: factors affecting help-seeking behaviour in Harare, Zimbabwe. Trop Med Intl Health 2010;15:574–9. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)135
Mayer DK, Ratichek S, Berhe H, Stewart S, Mctavish F, Gustafson D, et al. Development of a health-related website for parents of children receiving hematopoietic stem cell transplant: HSCT-CHESS. J Cancer Survivor 2010;4:67–73. (See Chapter 3 , Development of the trial intervention. )
McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11:31. (See Chapter 3 , Trial participation: the experience of being in a trial.)91
Mckay JW. Walking on prescription: the utility of a pedometer pack for increasing physical activity in primary care. Patient Educ Counsel 2009;76:71–6. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
McNamara DG, Dickinson AR, Byrnes CA. The perceptions and preferences of parents of children with tracheostomies in a study of humidification therapy. J Child Health Care 2009;13:179–97. (See Chapter 3 , Intervention fidelity, reach and dose. )65
McPherson KM, Kayes N, Weatherall M, Members of the Goals SRRG. A pilot study of self-regulation informed goal setting in people with traumatic brain injury. Clin Rehabil 2009;23:296–309. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )59
McQueen A, Bartholomew LK, Greisinger AJ, Medina GG, Hawley ST, Haidet P, et al. Behind closed doors: physician-patient discussions about colorectal cancer screening. J Gen Intern Med 2009;24:1228–35. (See Chapter 3 , Describing the trial intervention and elaborating its components, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )33
Menon U, Szalacha LA, Belue R, Rugen K, Martin KR, Kinney AY. Interactive, culturally sensitive education on colorectal cancer screening. Med Care 2008;46(Suppl. 1):S44–50. (See Chapter 3 , Development of the trial intervention. )
Mihaylov S, Stark C, Mccoll E. Steeped treatment of older adults on laxatives: the STOOL trial. Health Technol Assess 2008;12(13). (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention; Chapter 3 , Trial recruitment and retention; and Chapter 3 , Experience of the disease, health behaviour or beliefs.)53
Mikhael JB. Using a pocket card to improve end-of-life care on internal medicine clinical teaching units: a cluster-randomized controlled trial. J Gen Intern Med 2008;23:1222–7. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Milne JL, Spiers JA, Moore KN. Men’s experiences following laparoscopic radical prostatectomy: a qualitative descriptive study. Int J Nurs Stud 2008;45:765–74. (See Chapter 3 , Accuracy of measures.)
Mittal DO. Antipsychotic adherence intervention for veterans over forty with schizophrenia: results of a pilot study. Clin Schizophr Relat Psychoses 2009;2:317–25. (See Chapter 3 , Development of the trial intervention. )
Montgomery CM, Gafos M, Lees S, Morar NS, Mweemba O, Ssali A, et al. Re-framing microbicide acceptability: findings from the MDP301 trial. Cult Health Sex 2010;12:649–62. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Montgomery CM, Lees S, Stadler J, Morar NS, Ssali A, Mwanza B, et al. The role of partnership dynamics in determining the acceptability of condoms and microbicides. AIDS Care 2008;20:733–40. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Morone NE, Lynch CS, Greco CM, Tindle HA, Weiner DK. ‘I felt like a new person.’ the effects of mindfulness meditation on older adults with chronic pain: qualitative narrative analysis of diary entries. J Pain 2008;9:841–8. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention, and Chapter 3 , Exploring perceived value and benefits of the trial intervention. )50
Mueller MRI and Instone SL. Beyond the informed consent procedure: Continuing consent in human research. Cien Saude Colet 2008;13:381–9. (See Chapter 3 , Ethical conduct in a trial. )
Mukoma W, Flisher AJ, Ahmed N, Jansen S, Mathews C, Klepp KI, et al. Process evaluation of a school-based HIV/AIDS intervention in South Africa. Scand J Publ Health 2009;37(Suppl. 2):37–47. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice, and Chapter 3 , Intervention fidelity, reach and dose. )66
Mullins RM. Can older women be motivated to attend for their final Papanicolaou tests? The use of targeted and general personalised reminder letters. Cancer Epidemiol 2009;33:306–8. (See Chapter 3 , Development of the trial intervention. )
Murphy CA, Cupples ME, Percy A, Halliday HL, Stewart MC. Peer-mentoring for first-time mothers from areas of socio-economic disadvantage: a qualitative study within a randomised controlled trial. BMC Health Serv Res 2008;8:46. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Nagel T, Robinson G, Condon J, Trauer T. Approach to treatment of mental illness and substance dependence in remote Indigenous communities: results of a mixed methods study. Aus J Rural Health 2009;17:174–82. (See Chapter 3 , Development of the trial intervention. )28
Nahm ES, Resnick B, Degrezia M, Brotemarkle R. Use of discussion boards in a theory-based health web site for older adults. Nurs Res 2009;58:419–26. (See Chapter 3 , Describing the trial intervention and elaborating its components. )
Nakash R, Hutton J, Lamb S. Response and non-response to postal questionnaire follow-up in a clinical trial: a qualitative study of the patient’s perspective. J Eval Clin Prac 2008;14:226–35. (See Chapter 3 , Completion of process and outcome measures.)128
Nemeth LS, Feifer C, Stuart GW, Ornstein SM. Implementing change in primary care practices using electronic medical records: a conceptual framework. Implement Sci 2008;3:3. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )
Newcomb PA, Mcgrath KW, Covington JK, Lazarus SC, Janson SL. Barriers to patient-clinician collaboration in asthma management: the patient experience. J Asthma 2010;47:192–7. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Newman PA, Daley A, Halpenny R, Loutfy M. Community heroes or ‘high-risk’ pariahs? Reasons for declining to enroll in an HIV vaccine trial. Vaccine 2008;26:1091–7. (See Chapter 3 , Trial recruitment and retention. )
Nguyen TN, Nilsson S, Hellstrom AL, Bengtson A. Music therapy to reduce pain and anxiety in children with cancer undergoing lumbar puncture: a randomized clinical trial. J Pediatr Oncol Nurs 2010;27:146–55. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention, and Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Ni Mhurchu C, Roberts V, Maddison R, Dorey E, Jiang Y, Jull A, et al. Effect of electronic time monitors on children’s television watching: pilot trial of a home-based intervention. Prevent Med 2009;49:413–17. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Nikolajsen L, Lyndgaard K, Schriver NB, Moller JF. Does audiovisual stimulation with music and nature sights (MuViCure) reduce pain and discomfort during placement of a femoral nerve block? J PeriAnesthes Nurs 2009;24:14–18. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Nilsson S, Kokinsky E, Nilsson U, Sidenvall B, Enskar K. School-aged children’s experiences of postoperative music medicine on pain, distress, and anxiety. Pediatr Anesthes 2009;19:1184–90. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Nutting PA, Gallagber K, Riley K, White S, Dickinson WP, Korsen N, et al. Care management for depression in primary care practice: Findings from the RESPECT-depression trial. Ann Fam Med 2008;6:30–7. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Ockleford E, Shaw RL, Willars J, Dixon-Woods M. Education and self-management for people newly diagnosed with type 2 diabetes: a qualitative study of patients’ views. Chron Illn 2008;4:28–37. (See Chapter 3 , Experience of the disease, health behaviour or beliefs, and Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention.)54
O’Dougherty M, Dallman A, Turcotte L, Patterson J, Napolitano MA, Schmitz KH. Barriers and motivators for strength training among women of color and Caucasian women. Women Health 2008;47:41–62. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Olbort R, Mahler C, Campbell S, Reuschenbach B, Muller-Tasch T, Szecsenyi J, et al. Doctors’ assistants’ views of case management to improve chronic heart failure care in general practice: a qualitative study. J Adv Nurs 2009;65:799–808. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Orford JH. To what factors do clients attribute change? Content analysis of follow-up interviews with clients of the UK Alcohol Treatment Trial. J Subst Abuse Treat 2009;36:49–58. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )
O’Sullivan TL, Fortier MS, Faubert C, Culver D, Blanchard C, Reid R, et al. Interdisciplinary physical activity counseling in primary care: a qualitative inquiry of the patient experience. J Health Psychol 2010;15:362–72. (See Chapter 3 , Describing the trial intervention and elaborating its components, and Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )42
Ovbiagele B. ‘Al pie de la letra’: crafting a report card for elderly Spanish-only-speaking patients with stroke. Stroke 2010;41:771–7. (See Chapter 3 , Development of the trial intervention. )
Palinkas LA, Schoenwald SK, Hoagwood K, Landsverk J, Chorpita BF, Weisz JR, et al. An ethnographic study of implementation of evidence-based treatments in child mental health: first steps. Psychiatr Serv 2008;59:738–46. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention, and Chapter 3 , Intervention fidelity, reach and dose. )67
Paterson C, Zheng Z, Xue C, Wang Y. ‘Playing their parts’: the experiences of participants in a randomized sham-controlled acupuncture trial. J Alt Complement Med 2008;14:199–208. (See Chapter 3 , Impact of trial on staff, researchers or participants.)114
Penman-Aguilar A, Swezey T, Turner AN, Bell AJ, Ramiandrisoa FN, Legardy-Williams J, et al. Promoting continuous use as a strategy for achieving adherence in a trial of the diaphragm with candidate microbicide. AIDS Educ Prevent 2009;21:512–25. (See Chapter 3 , Accuracy of measures.)123
Penn C, Evans M. Recommendations for communication to enhance informed consent and enrolment at multilingual research sites. African J AIDS Res 2009;8:285–94. (See Chapter 3 , Ethical conduct in a trial. )107
Penn C, Frankel T, Watermeyer J, Muller M. Informed consent and aphasia: evidence of pitfalls in the process. Aphasiology 2009;23:3–32. (See Chapter 3 , Ethical conduct in a trial. )
Penn L, Moffatt SM, White M. Participants’ perspective on maintaining behaviour change: a qualitative study within the European Diabetes Prevention Study. BMC Public Health 2008;8:235. (See Chapter 3 , Variation of outcomes. )119
Perkins D, Harris MF, Tan J, Christl B, Taggart J, Fanaian M. Engaging participants in a complex intervention trial in Australian General Practice. BMC Med Res Method 2008;8:55. (See Chapter 3 , Trial recruitment and retention. )80
Peterson JC, Allegrante JP, Pirraglia PA, Robbins L, Lane KP, Boschert KA, et al. Living with heart disease after angioplasty: A qualitative study of patients who have been successful or unsuccessful in multiple behavior change. Heart Lung 2010;39:105–15. (See Chapter 3 , Variation of outcomes. )120
Peterson U, Bergstrom G, Samuelsson M, Asberg M, Nygren A. Reflecting peer-support groups in the prevention of stress and burnout: randomized controlled trial. J Adv Nurs 2008;63:506–16. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Phongsavan P, Merom D, Wagner R, Chey T, Von Hofe B, Silove D, et al. Process evaluation in an intervention designed to promote physical activity among adults with anxiety disorders: evidence of acceptability and adherence. Health Prom J Aus 2008;19:137–43. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Intervention fidelity, reach and dose. )
Pilling SA, Williams MB, Brackett RH, Gourley R, Weg MWV, Christensen AJ, et al. Part I. Patient perspective: activating patients to engage their providers in the use of evidence-based medicine: a qualitative evaluation of the VA Project to Implement Diuretics (VAPID). Implement Sci 2010;5:23. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Plow MA, Resnik L, Allen SM. Exploring physical activity behaviour of persons with multiple sclerosis: a qualitative pilot study. Disabil Rehabil 2009;31:1652–65. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )
Plummer ML, Watson-Jones D, Lees S, Baisley K, Matari S, Changalucha J, et al. A qualitative study of participant adherence in a randomized controlled trial of herpes suppressive therapy for HIV prevention in Tanzania. AIDS Care 2010;22:499–508. (See Chapter 3 , Intervention fidelity, reach and dose. )68
Pontin E, Peters S, Lobban F, Rogers A, Morriss RK. Enhanced relapse prevention for bipolar disorder: a qualitative investigation of value perceived for service users and care coordinators. Implement Sci 2009;4:4. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Pool JJM, Hiralal S, Ostelo RWJG, Van Der Veer K, Vlaeyen JWS, Bouter LM, et al. The applicability of the Tampa Scale of Kinesiophobia for patients with sub-acute neck pain: a qualitative study. Qual Quant 2009;43:773–80. (See Chapter 3 , Accuracy of measures.)124
Pool RM. Assessing the accuracy of adherence and sexual behaviour data in the MDP301 Vaginal microbicides trial using a mixed methods and triangulation model. PLOS ONE 2010;5:1–9. (See Chapter 3 , Accuracy of measures.)125
Pope DS, Atkins S, Deluca AN, Hausler H, Hoosain E, Celentano DD, et al. South African TB nurses’ experiences of provider-initiated HIV counseling and testing in the Eastern Cape Province: a qualitative study. AIDS Care 2010;22:238–45. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )60
Potter R, Dale J, Caramlau I. A qualitative study exploring practice nurses’ experience of participating in a primary care-based randomised controlled trial. J Res Nurs 2009;14:439–47. (See Chapter 3 , Trial recruitment and retention. )81
Power LM. A pilot study evaluating a support programme for parents of young people with suicidal behaviour. Child Adolesc Psychiatry Ment Health 2009;3. (See Chapter 3 , Development of the trial intervention. )
Pronyk PM, Kim JC, Abramsky T, Phetla G, Hargreaves JR, Morison LA, et al. A combined microfinance and training intervention can reduce HIV risk behaviour in young female participants. AIDS 2008;22:1659–65. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )
Redfern J, Rudd AD, Wolfe CDA, Mckevitt C. Stop Stroke: development of an innovative intervention to improve risk factor management after stroke. Patient Educ Counsel 2008;72:201–9. (See Chapter 3 , Development of the trial intervention. )29
Reid CM, Gooberman-Hill R, Hanks GW. Opioid analgesics for cancer pain: symptom control for the living or comfort for the dying? A qualitative study to investigate the factors influencing the decision to accept morphine for pain caused by cancer. Ann Oncol 2008;19:44–8. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )55
Reuter K, I S. Implementation and benefits of psychooncological group interventions in German breast centers: A pilot study on supportive-expressive group therapy for women with primary breast cancer. Breast Care 2010;5:91–6. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Rhodes P, Brown J, Madden S. The Maudsley model of family-based treatment for anorexia nervosa: a qualitative evaluation of parent-to-parent consultation. J Marital Fam Ther 2009;35:181–92. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Richardson LM. Collaborative care for adolescent depression: a pilot study. Gen Hosp Psychiatry 2009;31:36–45. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Roberts CMS. A randomised trial of peer review: The UK National Chronic Obstructive Pulmonary Disease Resources and Outcomes Project. Clin Med 2010;10:223–7. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Robertson C, Langston AL, Stapley S, Mccoll E, Campbell MK, Fraser WD, et al. Meaning behind measurement: self-comparisons affect responses to health-related quality of life questionnaires. Qual Life Res 2009;18:221–30. (See Chapter 3 , Accuracy of measures.)126
Rogers A, Gately C, Kennedy A, Sanders C. Are some more equal than others? Social comparison in self-management skills training for long-term conditions. Chronic Illn 2009;5:305–17. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )43
Romo N, Poo M, Ballesta R. From illegal poison to legal medicine: a qualitative research in a heroin-prescription trial in Spain. Drug Alcohol Rev 2009;28:186–95. (See Chapter 3 , Describing the trial intervention and elaborating its components, and Chapter 3 , Exploring perceived value and benefits of the trial intervention. )34
Roots P, Rowlands L, Gowers SG. User satisfaction with services in a randomised controlled trial of adolescent anorexia nervosa. Eur Eating Disord Rev 2009;17:331–7. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Rose L, Alhusen J, Bhandari S, Soeken K, Marcantonio K, Bullock L, et al. Impact of intimate partner violence on pregnant women’s mental health: mental distress and mental strength. Issues Mental Health Nurs 2010;31:103–11. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Rose SB, Smith MC, Lawton BA. ‘If everyone does it, it’s not a big deal.’ Young people talk about chlamydia testing. N Z Med J 2008;121:33–42. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Rosen RK, Morrow KM, Carballo-Dieguez A, Mantell JE, Hoffman S, Gai F, et al. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: a mixed-methods study. J Womens Health 2008;17:383–92. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Rosenthal SL, Holmes W, Maher L. Australian men’s experiences during a microbicide male tolerance study. AIDS Care 2009;21:125–30. (See Chapter 3 , The ‘in principle’ acceptability of the trial. )97
Russell G, Thille P, Hogg W, Lemelin J. Beyond fighting fires and chasing tails? Chronic illness care plans in Ontario, Canada. Ann Fam Med 2008;6:146–53. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Russell KM, Maraj MS, Wilson LR, Shedd-Steele R, Champion VL. Barriers to recruiting urban African American women into research studies in community settings. Appl Nurs Res 2008;21:90–7. (See Chapter 3 , Diversity of trial participants. )87
Saethre EJ, Stadler J. Gelling medical knowledge: innovative pharmaceuticals, experience, and perceptions of efficacy. Anthropol Med 2010;17:99–111. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Sahin-Hodoglugil NN, Van Der Straten A, Cheng H, Montgomery ET, Kacanek D, Mtetwa S, et al. Degrees of disclosure: a study of women’s covert use of the diaphragm in an HIV prevention trial in sub-Saharan Africa. Soc Sci Med 2009;69:1547–55. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Salter ML, Go VF, Celentano DD, Diener-West M, Nkhoma CM, Kumwenda N, et al. The role of men in women’s acceptance of an intravaginal gel in a randomized clinical trial in Blantyre, Malawi: a qualitative and quantitative analysis. AIDS Care 2008;20:853–62. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Samoutis GA, Soteriades ES, Stoffers HE, Zachariadou T, Philalithis A, Lionis C. Designing a multifaceted quality improvement intervention in primary care in a country where general practice is seeking recognition: the case of Cyprus. BMC Health Serv Res 2008;8:181. (See Chapter 3 , Development of the trial intervention. )
Sampson ELT. Palliative care in advanced dementia; a mixed methods approach for the development of a complex intervention. BMC Palliat Care 2008;7:8. (See Chapter 3 , Development of the trial intervention. )
Sand KL. Lung cancer patients’ perceptions of informed consent documents. Patient Educ Counsel 2008;73:313–17. (See Chapter 3 , Ethical conduct in a trial. )
Sandison R, Macdonald H. Experiences of older women increasing fruit and vegetable intake. Br J Commun Nurs 2008;13:418–22. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Sandler A, Glesne C, Geller G. Children’s and parents’ perspectives on open-label use of placebos in the treatment of ADHD. Child Care Health Develop 2008;34:111–20. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )61
Sarris J, Adams J, Kavanagh DJ. An explorative qualitative analysis of participants’ experience of using kava versus placebo in an RCT. Aus J Med Herbalism 2010;22:12–16. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Schnyer RN, Iuliano D, Kay J, Shields M, Wayne P. Development of protocols for randomized sham-controlled trials of complex treatment interventions: Japanese acupuncture for endometriosis-related pelvic pain. J Alt Complement Med 2008;14:515–22. (See Chapter 3 , Development of the trial intervention. )
Schofield P. Snoezelen within a palliative care day setting: a randomized controlled trial investigating the potential. Int J Disabil Hum Develop 2009;8:59–65. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Schroer S, Macpherson H, Adamson J. Designing an RCT of acupuncture for depression – identifying appropriate patient groups: a qualitative study. Fam Pract 2009;26:188–95. (See Chapter 3 , Development of the trial intervention. )
Sekelja N, Butow PN, Tattersall MHN. Bereaved cancer carers’ experience of and preference for palliative care. Support Care Cancer 2010;18:1219–28. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)136
Shagi C, Vallely A, Kasindi S, Chiduo B, Desmond N, Soteli S, et al. A model for community representation and participation in HIV prevention trials among women who engage in transactional sex in Africa. AIDS Care 2008;20:1039–49. (See Chapter 3 , Adaptation of trial conduct to local context. )111
Sheppard VB, Williams KP, Harrison TM, Jennings Y, Lucas W, Stephen J, et al. Development of decision-support intervention for Black women with breast cancer. Psycho-Oncology 2010;19:62–70. (See Chapter 3 , Development of the trial intervention. )
Simpson AR. Patients’ experiences of receiving collaborative care for the treatment of depression in the UK: a qualitative investigation. Mental Health Fam Med 2008;5:95–104. (See Chapter 3 , Development of the trial intervention. )
Skoro-Kondza L, Tai SS, Gadelrab R, Drincevic D, Greenhalgh T. Community based yoga classes for type 2 diabetes: an exploratory randomised controlled trial. BMC Health Serv Res 2009;9:33. (See Chapter 3 , Development of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Slade M, Gask L, Leese M, Mccrone P, Montana C, Powell R, et al. Failure to improve appropriateness of referrals to adult community mental health services – lessons from a multi–site cluster randomized controlled trial. Fam Pract 2008;25:181–90. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Sloboda Z, Pyakuryal A, Stephens PC, Teasdale B, Forrest D, Stephens RC, et al. Reports of substance abuse prevention programming available in schools. Prevent Sci 2008;9:276–87. (See Chapter 3 , Accuracy of measures.)127
Smith LA, Jones C, Adjei RO, Antwi GD, Afrah NA, Greenwood B, et al. Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: user acceptability. Malaria J 2010;9:18. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Sokunbi O, Cross V, Watt P, Moore A. Experiences of individuals with chronic low back pain during and after their participation in a spinal stabilisation exercise programme – a pilot qualitative study. Manual Ther 2010;15:179–84. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Song MK, Ward SE, Happ MB, Piraino B, Donovan HS, Shields AM, et al. Randomized controlled trial of SPIRIT: an effective approach to preparing African-American dialysis patients and families for end of life. Res Nurs Health 2009;32:260–73. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Sorensen JA, May J, Ostby-Malling R, Lehmen T, Strand J, Stenlund H, et al. Encouraging the installation of rollover protective structures in New York State: the design of a social marketing intervention. Scand J Public Health 2008;36:859–69. [Erratum published in Scand J Public Health 2009;37:109]. (See Chapter 3 , Development of the trial intervention. )
Spilsbury K, Cullum N, Dumville J, O’meara S, Petherick E, Thompson C. Exploring patient perceptions of larval therapy as a potential treatment for venous leg ulceration. Health Expect 2008;11:148–59. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention. )
Stadler JJ, Delany S, Mntambo M. Women’s perceptions and experiences of HIV prevention trials in Soweto, South Africa. Soc Sci Med 2008;66:189–200. (See Chapter 3 , Impact of trial on staff, researchers or participants, and Chapter 3 , Experience of the disease, behaviour or beliefs.)115
Stein J, Lewin S, Fairall L, Mayers P, English R, Bheekie A, et al. Building capacity for antiretroviral delivery in South Africa: a qualitative evaluation of the PALSA PLUS nurse training programme. BMC Health Serv Res 2008;8:240. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Stille CJ, Rifas-Shiman SL, Kleinman K, Kotch JB, Finkelstein JA. Physician responses to a community-level trial promoting judicious antibiotic use. Ann Fam Med 2008;6:206–12. (See Chapter 3 , Describing the trial intervention and elaborating its components. )
Stokes H. Reasons for non-adherence to cardiac rehabilitation programmes included lack of motivation, domestic duties, and other health problems. Evidence-Based Nurs 2008;11:63. (See Chapter 3 , Intervention fidelity, reach and dose. )
Strayer SM, Pelletier SL, Martindale JR, Rais S, Powell J, Schorling JB. A PDA-based counseling tool for improving medical student smoking cessation counseling. Fam Med 2010;42:350–7. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Sturt J. One-to-one structured education using the Diabetes Manual: evidence of effectiveness. J Diabet Nurs 2008;12:368. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Sulmasy DP, Astrow AB, He MK, Seils DM, Meropol NJ, Micco E, et al. The culture of faith and hope patients’ justifications for their high estimations of expected therapeutic benefit when enrolling in early phase oncology trials. Cancer 2010;116:3702–11. (See Chapter 3 , Ethical conduct in a trial. )
Svensson HB. Psychological reactions to progression of metastatic breast cancer – an interview study. Cancer Nurs 2009;32:55–63. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Sy A, Glanz K. Factors influencing teachers’ implementation of an innovative tobacco prevention curriculum for multiethnic youth: Project SPLASH. J School Health 2008;78:264–73. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Tamaki A. Effectiveness of home visits by mental health nurses for Japanese women with post-partum depression. Int J Mental Health Nurs 2008;17:419–27. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Tentler A, Silberman J, Paterniti DA, Kravitz RL, Epstein RM. Factors affecting physicians’ responses to patients’ requests for antidepressants: focus group study. J Gen Intern Med 2008;23:51–7. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Teti M, Bowleg L, Cole R, Lloyd L, Rubinstein S, Spencer S, et al. A mixed methods evaluation of the effect of the protect and respect intervention on the condom use and disclosure practices of women living with HIV/AIDS. AIDS Behav 2010;14:567–79. (See Chapter 3 , Describing the trial intervention and elaborating its components, and Chapter 3 , Exploring perceived value and benefits of the trial intervention. )35
Thomas S, Thomas PW, Nock A, Slingsby V, Galvin K, Baker R, et al. Development and preliminary evaluation of a cognitive behavioural approach to fatigue management in people with multiple sclerosis. Patient Educ Counsel 2010;78:240–9. (See Chapter 3 , Development of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Thorstensson CA, Lohmander LS, Frobell RB, Roos EM, Gooberman-Hill R. Choosing surgery: patients’ preferences within a trial of treatments for anterior cruciate ligament injury. A qualitative study. BMC Musculoskel Disord 2009;10:100. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , The acceptability of a trial in practice. )100
Timmermans S, Mckay T. Clinical trials as treatment option: bioethics and health care disparities in substance dependency. Soc Sci Med 2009;69:1784–90. (See Chapter 3 , Ethical conduct in a trial. )108
Tripp KM. Acceptability and use of iron and iron-alloy cooking pots: implications for anaemia control programmes. Public Health Nutr 2010;13:123–30. (See Chapter 3 , Development of the trial intervention, Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Turner KM, Sharp D, Folkes L, Chew-Graham C. Women’s views and experiences of antidepressants as a treatment for postnatal depression: a qualitative study. Fam Prac 2008;25:450–5. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Tutton E, Gray B. Fluid optimisation using a peripherally inserted central catheter (PICC) following proximal femoral fracture: lessons learnt from a feasibility study. J Orthopaed Nurs 2009;13:11–18. (See Chapter 3 , The acceptability of a trial in practice. )101
Tyrer PC. The assessment of dangerous and severe personality disorder: lessons from a randomised controlled trial linked to qualitative analysis. J Forensic Psychiatry Psychol 2009;20:132–46. (See Chapter 3 , Models, mechanisms and underlying theory development of the intervention. )
Uebelacker LA, Tremont G, Epstein-Lubow G, Gaudiano BA, Gillette T, Kalibatseva Z, et al. Open trial of vinyasa yoga for persistently depressed individuals: evidence of feasibility and acceptability. Behav Modific 2010;34:247–64. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Vallely A, Lees S, Shagi C, Kasindi S, Soteli S, Kavit N, et al. How informed is consent in vulnerable populations? Experience using a continuous consent process during the MDP301 vaginal microbicide trial in Mwanza, Tanzania. BMC Med Ethics 2010;11:10. (See Chapter 3 , Ethical conduct in a trial. )
Van Bokhoven MA, Koch H, Dinant GJ, Bindels PJ, Grol RP, Van Der Weijden T. Exploring the black box of change in improving test-ordering routines. Fam Prac 2008;25:139–45. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Velott DL, Baker SA, Hillemeier MM, Weisman CS. Participant recruitment to a randomized trial of a community-based behavioral intervention for pre- and interconceptional women findings from the Central Pennsylvania Women’s Health Study. Womens Health Issues 2008;18:217–24. (See Chapter 3 , Diversity of trial participants. )88
Ventuneac A, Carballo-Dieguez A, Mcgowan I, Dennis R, Adler A, Khanukhova E, et al. Acceptability of UC781 gel as a rectal microbicide among HIV-uninfected women and men. AIDS Behav 2010;14:618–28. (See Chapter 3 , Development of the trial intervention. )
Vermeulen SJ, Anema JR, Schellart AJ, Van Mechelen W, Van Der Beek AJ. Intervention mapping for development of a participatory return-to-work intervention for temporary agency workers and unemployed workers sick-listed due to musculoskeletal disorders. BMC Public Health 2009;9:216. (See Chapter 3 , Development of the trial intervention. )
Wade J, Donovan JL, Lane JA, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018–28. (See Chapter 3 , Ethical conduct in a trial. )
Waller H, Eiser C, Knowles J, Rogers N, Wharmby S, Heller S, et al. Pilot study of a novel educational programme for 11–16 year olds with type 1 diabetes mellitus: the KICk-OFF course. Arch Dis Child 2008;93:927–31. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Ward FR. Chaos, vulnerability and control: parental beliefs about neonatal clinical trials. J Perinatol 2009;29:156–62. (See Chapter 3 , Trial participation: the experience of being in a trial.)92
Wasan AD, Taubenberger SP, Robinson WM. Reasons for participation in pain research: can they indicate a lack of informed consent? Pain Med 2009;10:111–19. (See Chapter 3 , Ethical conduct in a trial. )109
Weinfurt KP, Seils DM, Tzeng JP, Compton KL, Sulmasy DP, Astrow AB, et al. Expectations of benefit in early-phase clinical trials: implications for assessing the adequacy of informed consent. Med Decis Making 2008;28:575–81. (See Chapter 3 , Ethical conduct in a trial. )
West B, Abatemarco D, Ohman-Strickland PA, Zec V, Russo A, Milic R. Project Northland in Croatia: results and lessons learned. J Drug Educ 2008;38:55–70. (See Chapter 3 , Development of the trial intervention. )
White E, Winstanley J. Implementation of clinical supervision: educational preparation and subsequent diary accounts of the practicalities involved, from an Australian mental heath nursing innovation. J Psychiatr Mental Health Nurs 2009;16:895–903. [Erratum published in J Psychiatr Ment Health Nurs 2010;17:96] (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )62
White E, Winstanley J. A randomised controlled trial of clinical supervision: selected findings from a novel Australian attempt to establish the evidence base for causal relationships with quality of care and patient outcomes, as an informed contribution to mental health nursing practice development. J Res Nurs 2010;15:151–67. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention, and Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Whittemore R, Grey M, Lindemann E, Ambrosino J, Jaser S. Development of an Internet coping skills training program for teenagers with type 1 diabetes. Comput Informat Nurs 2010;28:103–11. (See Chapter 3 , Development of the trial intervention. )
Whittingham KS. Behavioural Family Intervention with parents of children with ASD: what do they find useful in the parenting program Stepping Stones Triple P? Res Autism Spect Disord 2009;3:702–13. (See Chapter 3 , Exploring perceived value and benefits of the trial intervention. )
Wiggins SA. Family exemplars during implementation of a home pain management intervention. Issues Comprehens Pediatr Nurs 2009;32:160–79. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Williams PD, Ridder EL, Setter RK, Liebergen A, Curry H, Piamjariyakul U, et al. Pediatric chronic illness (cancer, cystic fibrosis) effects on well siblings: parents’ voices. Issues Comprehens Pediatr Nurs 2009;32:94–113. (See Chapter 3 , Experience of the disease, health behaviour or beliefs.)
Woodsong C, Alleman P. Sexual pleasure, gender power and microbicide acceptability in Zimbabwe and Malawi. AIDS Educ Prevent 2008;20:171–87. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention. )
Yardley L, Dennison L, Coker R, Webley F, Middleton K, Barnett J, et al. Patients’ views of receiving lessons in the Alexander technique and an exercise prescription for managing back pain in the ATEAM trial. Fam Prac 2010;27:198–204. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Yawn BPP, Pace W, Dietrich A, Bertram S, Kurland M, Graham D, et al. Practice benefit from participating in a practice-based research network study of postpartum depression: a National Research Network (NRN) report. J Am Board Fam Med 2010;23:455–64. (See Chapter 3 , Impact of trial on staff, researchers or participants.)116
Young SL, Blanco I, Hernandez-Cordero S, Pelto GH, Neufeld LM. Organoleptic properties, ease of use, and perceived health effects are determinants of acceptability of micronutrient supplements among poor Mexican women. J Nutr 2010;140:605–11. (See Chapter 3 , Feasibility and acceptability of the trial intervention in practice. )
Zhang G, Malik VS, Pan A, Kumar S, Holmes MD, Spiegelman D, et al. Substituting brown rice for white rice to lower diabetes risk: a focus-group study in Chinese adults. J Am Diet Assoc 2010;110:1216–21. (See Chapter 3 , Gauging the ‘in principle’ acceptability of the trial intervention. )56
Appendix 4 List of journals for 296 articles in Chapter 3
Academic Medicine
Academic Pediatrics
Advances in Therapy
AIDS
AIDS & Behavior
AIDS Care
AIDS Care – Psychological and Socio-Medical Aspects of AIDS/HIV
AIDS Education and Prevention
AIDS Patient Care and STDs
African Journal of Aids Research
Allergy and Asthma Proceedings
Alzheimer Disease & Associated Disorders
Annals of Family Medicine
Annals of Oncology
Anthropology & Medicine
Aphasiology
Applied Nursing Research
Archives of Disease in Childhood
Archives of Women’s Mental Health
Arts in Psychotherapy
Australian Family Physician
Australian Journal of Medical Herbalism
Australian Journal of Rural Health
Behavior Modification
Birth
BJOG: An International Journal of Obstetrics & Gynaecology
BMC Family Practice
BMC Health Services Research
BMC Medical Ethics
BMC Medical Research Methodology
BMC Musculoskeletal Disorders
BMC Palliative Care
BMC Psychiatry
BMC Public Health
BMC Pulmonary Medicine
BMC Women’s Health
BMJ
Breast Care
British Journal of Community Nursing
British Journal of General Practice
British Journal of Health Psychology
British Journal of Social Work
Canadian Family Physician
Canadian Journal of Nursing Research
Canadian Journal of Occupational Therapy – Revue Canadienne d’ergotherapie
Cancer
Cancer Epidemiology
Cancer Nursing
Child and Adolescent Psychiatry and Mental Health
Child: Care, Health and Development
Chronic Illness
Ciência & Saúde Coletiva
Computers Informatics Nursing
Clinical Medicine
Clinical Rehabilitation
Clinical Schizophrenia & Related Psychoses
Clinical Trials
Cognitive Therapy and Research
Complementary Therapies in Clinical Practice
Complementary Therapies in Medicine
Contemporary Clinical Trials
Culture Health & Sexuality
Culture, Medicine and Psychiatry
Disability and Rehabilitation
Drug and Alcohol Review
Education for Health
European Eating Disorders Review
European Journal of Cardiovascular Nursing
European Journal of Oncology Nursing
European Urology
Evaluation & the Health Professions
Evidence Based Midwifery
Evidence-Based Nursing
Family Medicine
Family Practice
General Hospital Psychiatry
Health Education Journal
Health Expectations
Health Promotion Journal of Australia
Health Technology Assessment
Heart & Lung
Implementation Science
Informatics in Primary Care
International Journal of Behavioral Nutrition and Physical Activity
International Journal of Environmental Research and Public Health
International Journal of Mental Health Nursing
International Journal of Nursing Studies
International Journal of STD & AIDS
International Journal on Disability and Human Development
International Psychogeriatrics
International Quarterly of Community Health Education
Issues in Comprehensive Pediatric Nursing
Issues in Mental Health Nursing
Journal of Evaluation in Clinical Practice
Journal of Orthopaedic Nursing
Journal of Research in Nursing
Journal of Obstetric, Gynecologic and Neonatal Nursing
Joint Commission Journal on Quality and Patient Safety
Journal of Adolescent Health
Journal of Advanced Nursing
Journal of Alternative and Complementary Medicine
Journal of Asthma
Journal of Cancer Survivorship
Journal of Child and Family Studies
Journal of Child Health Care
Journal of Clinical Epidemiology
Journal of Clinical Nursing
Journal of Clinical Oncology
Journal of Diabetes Nursing
Journal of Drug Education
Journal of Empirical Research on Human Research Ethics
Journal of Forensic Psychiatry & Psychology
Journal of General Internal Medicine
Journal of Health Psychology
Journal of Health Services & Research Policy
Journal of Human Nutrition and Dietetics
Journal of Interprofessional Care
Journal of Marital and Family Therapy
Journal of Medical Ethics
Journal of Medical Internet Research
Journal of Midwifery & Women’s Health
Journal of Neonatal Nursing
Journal of Nursing Scholarship
Journal of Nutrition
Journal of Palliative Care
Journal of Pediatric Oncology Nursing
Journal of PeriAnesthesia Nursing
Journal of Perinatology
Journal of Physical Activity & Health
Journal of Psychiatric and Mental Health Nursing
Journal of School Health
Journal of Substance Abuse Treatment
Journal of the American Board of Family Medicine
Journal of the American College of Surgeons
Journal of the American Dietetic Association
Journal of Women’s Health
Malaria Journal
Manual Therapy
Medical Care
Medical Decision Making
Mental Health in Family Medicine
Midwifery
Neuro-Oncology
Nursing Ethics
Nursing Inquiry
Nursing Research
Oncology Nursing Forum
Pain Medicine
Patient Education and Counseling
Pediatric Anesthesia
Pediatric Pulmonology
PLOS ONE
Preventing Chronic Disease
Prevention Science
Preventive Medicine
Primary Care Diabetes
Primary Dental Care
Psychiatric Services
Psycho-Oncology
Psychotherapy
Public Health Nutrition
Quality & Quantity
Quality of Life Research
Research in Autism Spectrum Disorders
Research in Nursing & Health
Scandinavian Journal of Public Health
Scandinavian Journal of Urology and Nephrology
Sexual Health
Sexually Transmitted Diseases
Social Psychiatry and Psychiatric Epidemiology
Social Science & Medicine
Stroke
Studies in Health Technology and Informatics
Supportive Care in Cancer
The Foot
The International Journal of Mental Health Promotion
The Journal of Pain
The Journal of School Nursing
The New Zealand Medical Journal
Trials
Tropical Medicine & International Health
Vaccine
Women & Health
Women’s Health Issues
World Psychiatry
Appendix 5 Search terms used to identify trials using qualitative research in the metaRegister of Controlled Trials database
| qualitative study |
| qualitative studies |
| qualitative interview(s) |
| qualitative research |
| qualitative method(s) |
| qualitative analysis |
| qualitative analyses |
| qualitatively analysed |
| qualitatively analyzed |
| qualitative data |
| qualitative approach |
| qualitative evaluation |
| qualitative case study |
| qualitative case studies |
| qualitative case-study |
| qualitative case-studies |
| qualitative inquiry |
| qualitative exploration |
| qualitative intervention |
| qualitative |
| semi structured interview(s) |
| semistructured interview(s) |
| semi-structured interview(s) |
| in-depth interview(s) |
| interview(s) AND theme(s) |
| interview(s) AND audio recorded |
| interview(s) AND audio-recorded |
| descriptive case study |
| descriptive case studies |
| focus group(s) |
| focus-group(s) |
| mixed method(s) |
| mixed-method(s) |
| process evaluation |
| ethnography |
| ethnographic |
| ethno methodology |
| ethnomethodology |
| phenomenological |
| action research |
| content analysis |
| narrative analysis |
| grounded theory |
| thematic analysis |
| conversation analysis |
| discursive |
| discourse analysis |
| social constructionist |
| social construction |
| social constructionism |
Appendix 6 Questionnaire
Appendix 7 Topic guide
Introduction: Thank the interviewee for agreeing to the interview and to the recording of the interview. Check consent and if it is OK to record the interviews. Ask them if they have any questions before the interview begins.
Say that you are here to talk to them because of their involvement in a trial with qualitative research: [STUDY A].
Interview topics to be covered during the interview
The interviewee’s role in the trial (PI or qualitative researcher)
The qualitative components of their trial and the interviewee’s perceptions of the objectives for undertaking each component if more than one (Prompt: Pick up on any elements of the trials that we have not yet identified and explore in more depth)
The reasons why qualitative research was undertaken with this trial
The rationale for qualitative research addressing the objectives above
The success of the qualitative research with this trial from the interviewee’s point of view (explore their meaning of ‘successful’)
The interviewee’s perceptions of the impact of qualitative research on the trial itself (Prompts: Did it do the right ‘work’, status of qualitative components, visibility of qualitative components, reporting of qualitative components, worth having a qualitative component)
Challenges and opportunities for the successful use of qualitative research in the interviewee’s study (Prompt for unintended consequences, both positive and negative)
Challenges and opportunities for the successful use of qualitative research in trials more generally
Issues related to the commissioning of the qualitative research for the trial and more generally
The interviewee’s perceptions of qualitative research in general
Ending the interview:
Is there anything else you’d like to say?
What is the message you’d like me to really take away today?
What would you like to see coming out of our study?
Close the interview and thank the interviewee for their participation.
List of abbreviations
- CI
- confidence interval
- HIV
- human immunodeficiency virus
- HTA
- Health Technology Assessment
- MRC
- Medical Research Council
- mRCT
- metaRegister of Controlled Trials
- NIHR
- National Institute for Health Research
- PPI
- public and patient involvement
- QUART
- QUAlitative Research in Trials
- QUARTER
- QUAlitative Research with Trials: Excellent Reporting
- RCT
- randomised controlled trial
- RE-AIM
- Reach, Efficacy, Adoption, Implementation, and Maintenance
- ScHARR
- School of Health and Related Research
- SPSS
- Statistical Product and Service Solutions