Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/43/01. The contractual start date was in August 2011. The draft report began editorial review in March 2013 and was accepted for publication in October 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
All authors declare that they have no financial or personal relationships with other people or organisations that could inappropriately influence (bias) their work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Livingston et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
The frequency of dementia will rise dramatically over the next 20 years as a result of increased longevity. In the UK, 820,000 people are currently living with dementia (> 1% of the entire UK population) and dementia care is currently estimated to cost £23B per year. 1 The number of people with dementia is projected to reach over 1 million by 2020 and double again in the subsequent 20 years. Costs are expected to treble in the next 30 years as the number of older people increases. 2,3 For comparison, the entire NHS budget was £110B in 2009. 4 Dementia affects not only the person with the illness, but also his or her family and society. The Alzheimer’s Society Dementia UK report found that current levels of services and support for people with dementia and families are inadequate. 2 This impacts on patients and families as well as the UK economy, as it can result in breakdown of care at home and, therefore, in institutionalisation. 5,6 The National Audit Office recently emphasised the need to ‘spend to save’ on dementia care, reducing crises and resultant institutionalisation. The National Dementia Strategy outlines 10-year plans to increase the detection of dementia (currently only 30% of people living with dementia are ever diagnosed) and improve the quality of care for people with dementia and their carers. 3 In the NHS Operating Framework (published 21 June 2010), the Secretary of State for Health named dementia as one of two priority areas for the NHS, with the implementation of the National Dementia Strategy central to these plans. 7 In March 2012, David Cameron launched the Prime Minister’s challenge on dementia. This sets out renewed ambition to build on progress made through the National Dementia Strategy. He cited dementia as his personal priority.
While the core symptom of dementia is cognitive deterioration, agitation is a common, persistent and distressing neuropsychiatric symptom in people with dementia. Agitation may be defined as inappropriate verbal, vocal or motor activity which is not judged by an outside observer to be an outcome of need. 8 The term encompasses physical and verbal aggression. 9 Common symptoms are restlessness, pacing, verbal insults, shouting and physical aggression.
Agitation is one of the most common neuropsychiatric symptoms in dementia, with nearly half of the participants in a representative prevalence study having some symptoms of agitation in the previous month. 9,10 About 80% of those with clinically significant symptoms remained symptomatic 6 months later, and this was more likely where the agitation was initially more severe. 10 In one large study, 41% of people with severe dementia were classified as agitated. 11 A recent review reported that 10–52% of people living in 24-hour care and 19–51% of people with dementia in the community exhibited verbal agitation, one of the most common types of agitation. 12
Three subtypes of agitation have been identified: (1) physically non-aggressive behaviour, such as wandering or trespassing in inappropriate places; (2) physically aggressive behaviour, such as hitting and kicking; and (3) verbally or vocally agitated behaviour, such as repeating words or questions, demanding constant attention, shouting, or verbal aggression. 13 The term ‘agitation’ may also include wandering. 14
The impact of agitation can be devastating for people with dementia, as well as for their family and for paid carers. The socioeconomic impact is also huge. For the person with dementia, it has been associated with poor quality of life. 13,15 This may result directly from the agitated feelings and resultant behaviour, which often occurs several times per hour, occupying a considerable proportion of their day. 16 Agitation also affects relationships within the family and is often associated with feelings of helplessness, anxiety and anger among carers and others. 17 In addition, agitation and associated symptoms predict nursing home admission5 and can also result in greater use of restraint and psychotropic drugs. 18
Reduction in quality of life for the person with dementia may be due not only to the agitation itself, but also to strategies implemented with the intention of managing the agitation. Carers tend to isolate and overmedicate people with agitation in long-term care facilities; the distress caused to the nursing staff can influence the quality of their care to people with agitation and other residents. 16
The currently accepted approach to good management of agitation in dementia begins by considering its underlying cause(s) and treating these (e.g. pain or delirium or constipation) where possible. 19 Psychological and social treatments should be considered before resorting to drug treatments.
Agitation is, however, often difficult to manage and, while the use of psychotropic medication is discouraged, professionals often struggle to implement effective alternative treatment plans. The 2006 National Institute for Health and Care Excellence (NICE) dementia guidelines recommended a range of non-pharmacological interventions, including aromatherapy, music therapy, dance therapy, animal-assisted therapy and multisensory stimulation, but the evidence for many of these is currently unclear. 20 A previous Health Technology Assessment (HTA)-commissioned systematic review found no conclusive evidence to justify recommending any non-pharmacological interventions for reducing wandering behaviour (which, as previously stated, may be regarded as a form of agitation). 14
The potential importance of non-pharmacological approaches has increased because of growing concern regarding the undesirable effects of drug treatments for agitation such as the atypical antipsychotics. In 2004, the Committee on the Safety of Medicines recommended that risperidone (Risperdal®, Janssen-Cilag) and olanzapine (Zyprexa®, Eli Lilly) should not be used for treatment of non-psychotic symptoms in dementia because of increased risk of cerebrovascular adverse events and death. 21 Recent meta-analyses found modest benefits in the treatment of aggression (best evidence for risperidone, followed by aripiprazole) but increased risk of cerebrovascular events and death. 22–24 The 2006 NICE dementia guidelines recommend limiting the use of antipsychotic medication, for treating agitation in people with dementia, to those whose behaviour was causing significant distress. 25 The use of both antipsychotics26 and benzodiazepines27 in dementia has been associated with increased cognitive decline. Both classes of drug are currently commonly prescribed to manage agitation. Cholinesterase inhibitors seem to be ineffective; there was no significant difference between groups when 272 patients with Alzheimer’s disease and agitation unresponsive to psychological treatment were randomised to either donepezil 5–10 mg (Aricept®, Pfizer) or placebo. 28 A 2009 UK government-commissioned review found that only 20% of the 180,000 UK dementia patients prescribed antipsychotics benefited from them, and antipsychotic overprescribing has been linked to 1800 excess deaths per year. 29 The review concluded that it should be an NHS priority to reduce antipsychotic use in people with dementia by two-thirds over the next 3 years.
Our search of core databases of systematic reviews, namely the Database of Abstracts of Reviews of Effects (DARE), HTA and the Cochrane Database of Systematic Reviews (CDSR), identified four reviews focusing on non-pharmacological treatment of agitation in dementia over the past 10 years. These were a recent systematic review of non-pharmacological interventions for agitation in dementia, a review of behavioural interventions and two reviews of music therapy. 30–33 The first of these is a well-conducted review but it included evidence only up to 2004 and limited the review to randomised controlled trials (RCTs) and those written in English or Korean. 31 It therefore did not include recent large RCTs of psychological interventions. It also did not consider cost-effectiveness. It concluded that the trials were small but only sensory interventions showed evidence of benefit. The other three papers did not state predefined inclusion criteria in terms of study design and also did not specify either outcome or validity measures.
Our previous systematic review, considering psychological approaches to all neuropsychiatric symptoms in dementia, included all other such symptoms, as well as agitation. 20 We found that overall psychoeducation for carers and behavioural management techniques for managing neuropsychiatric symptoms were effective treatments whose benefits lasted for months. Music therapy (and possibly other sensory stimulation approaches) were useful during the treatment session but had no longer-term effects; and interventions that changed the visual environment looked promising. A more recent, very broad review of interventions for agitation selected 47 trials of pharmacological and non-pharmacological treatment for consideration and concluded that the best evidence for effective non-drug treatment was for aromatherapy, although all trials were small and of short duration (< 4 weeks). 34
There is an urgent need for an up-to-date systematic synthesis of evidence from studies exploring non-pharmacological management of the broader range of related, and often comorbid, behaviours encompassed by the term ‘agitation’. Consistent evidence-based management of agitation could improve the quality of life of people with dementia and their carers and also be cost-effective. It might relieve the person’s distress, decreasing unnecessary sedation associated with the inappropriate use of medication, and enabling people with dementia to engage in more positive relationships and activities. It could also delay institutionalisation. The National Dementia Strategy anticipated at least a 6% decrease in institutionalisation as a result of early detection and diagnosis of dementia when assessing the cost of implementation. 3 Prompt and effective management of agitation may increase this benefit.
Chapter 2 Review question
Which non-pharmacological interventions are clinically effective for reducing agitation in adults with dementia, considering the following: dementia severity; setting; whether the intervention is with the person with dementia, their carer, or both; and whether any beneficial effects are immediate or longer term?
Chapter 3 Methods
Protocol
We developed a protocol for the review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria. 35 Our protocol is registered with PROSPERO (no. CRD42011001370) and can be accessed at www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42011001370.
Identification of studies
Search dates
Searches were carried out between 9 August 2011 and 12 June 2012.
Search terms
The search terms were agreed in consultation with carer representatives and professionals from a range of older adults disciplines. We searched Web of Knowledge (encompassing MEDLINE); beginning on 9 August 2011; second search: 12 June 2012 using search terms: (agitation OR restless* OR irrita* OR aggression OR “aberrant motor behav*” OR “psychomotor activity” OR “challenging behav*” OR pacing OR sundowning OR wander* OR “walking about” OR “safe walking”) AND (dement* OR alzheimer OR “vascular dement*” OR “pick’s disease” OR huntington OR creutzfeldt OR cjd OR binswanger OR lewy) AND (“randomised control* trial*” OR RCT OR cohort OR observational OR intervention OR “single blind” OR “double blind” OR evaluation OR comparative OR pretest OR “post test”). We then searched Web of Knowledge (incorporating MEDLINE); EMBASE; British Nursing Index; the HTA programme database; PsycINFO; NHS Evidence (16 January 2012); System for Information on Grey Literature (6 December 2011); The Stationery Office Official Documents website; The Stationery National Technical Information Service (27 February 2012); Cumulative Index to Nursing and Allied Health Literature (CINAHL) (17 January 2012); and The Cochrane Library (13 April 2012). Reference lists of included papers and relevant reviews were searched by hand, and all authors of included papers were contacted, where possible, to ask if they knew of other relevant studies.
Study selection
Inclusion and exclusion criteria
We defined agitation as having a behavioural component. Anger, for example, was included only when expressed behaviourally, as opposed to exclusively by report that someone was thought to be feeling angry. We included papers in any language and commissioned translations into English as necessary.
Our inclusion criteria were:
-
studies evaluating a psychological, behavioural, environmental or sensory intervention that aimed to manage agitation
-
studies with a comparator group reported, either a separate group or a before/after comparison (within subjects)
-
studies with agitation results reported as a quantitative outcome, or one could be generated from the data provided
-
studies in which all participants had dementia, or those with dementia were analysed separately
-
studies in which no people with dementia in the sample were aged < 50 years.
We excluded interventions in which every individual was given psychotropic drugs to control agitation or some participants only had medication but not any other type of intervention, unless they were separated out in the analysis.
Data extraction
We assessed reliability of the exclusion procedure by GL and LK independently screening the first 20 papers in our search to decide whether or not they should be included and then comparing their decisions. No paper was excluded incorrectly, thereby confirming satisfactory reliability of our exclusion process. LK and ELH screened all abstracts and extracted the data from the papers. Data extracted included methodological characteristics of the study, descriptors of the intervention, whether the intervention was with the person with dementia, with family carers or with staff, statistical methods used, details of relevant outcome measures, length and time of follow-up, place of intervention, dementia severity and diagnostic details, and summary outcome data (immediate and longer term).
Quality assessment
We assessed validity by operationalising the Centre for Evidence-based Medicine (CEBM) (www.cebm.net/index.aspx?o=1025, accessed on 13 July 2012) RCT evaluation criteria. We have used this method before. 20,36–38 The papers were independently rated for quality by two raters and difficulties in rating were discussed with the senior author or statistician. Each study was given an arithmetic score out of 14 and we have explained how this was done in detail below. Scoring criteria were as follows:
-
Power calculation (yes, 1 point; if yes but not related to agitation, 0.5 points).
-
Were all the full details of the power calculation given (including estimates used, e.g. size of the clinically important effect to be detected, drop-out/non-compliance rates)?; relevant justification (i.e. appropriate references or clinical arguments) – justification should be provided for the effect size considered; chosen levels of significance and power; methods/formula/software used with reference (1 point for all or all excluding last, 0.5 for intermediate information provided, 0 for none).
-
Power of the study to detect a significant effect on agitation (1 point).
-
Blinding of participants (1 point).
-
Blinding of raters (1 point).
-
Intention to treat (1 point) or completer analysis (0 points).
-
Randomised controlled trial (1 point) or non-randomised study (0 points).
-
Description and adequacy of randomisation (if researchers have any control over randomisation) (1 point) OR, if non-randomised, whether the intervention and control group are comparable (possible confounders considered were severity of dementia, whether living at home or in a care home, and severity of agitation) (1 point); follow-up rate at primary outcome time (80% + 1 point; 60 < 80% 0.5 points); whether or not all participants are accounted for (1 point).
-
Validity of outcome measures (0.5 for validated for any population, 1 for validated for this population).
-
Reliability of outcome measures (1 point).
-
Reliability of the dementia diagnosis [Diagnostic and Statistical Manual for Mental Disorders (DSM), International Classification of Diseases, clinical semistructured instrument – do not require scan (1 point)].
-
Were appropriate methods used for statistical analysis? (1 point).
-
Were all participants accounted for? (1 point).
-
Were > 80% of participants retained in the study at 1-month follow-up? (1 point).
Power
We took the conventional measure of 80% power to be adequate. Where papers used a 90% power rating but only achieved between 80% and 90% power, we considered this sufficient power to get a point. If a study achieved sufficient power at baseline and not at the primary follow-up time, but used an intention-to-treat analysis, we awarded the sufficient power point. If participant numbers were within 5% of their power calculation, then we still awarded the sufficient power point.
Reliability and validity of outcome measures
We awarded the reliability point on the basis of inter-rater reliability, and did not mark down if test–retest reliability was not reported. We took an inter-rater reliability of 0.6 to be sufficient, as this is considered substantial by Landis and Koch. 39 We took a liberal view of studies where validated scales had been modified, and reported how they actually used the measure. If, for example, they changed the time period that the scale was used for in order to measure the immediate effect of an intervention, for example changing the Cohen-Mansfield Agitation Inventory (CMAI) to reflect the previous hour rather than the previous 2 weeks in order to assess the effect of a bathing intervention, we regarded this as valid. However, if the instrument was modified so that it could no longer detect change, but only whether or not a symptom was present, for example changing CMAI to yes/no rather than seven-point Likert scale, then we did not consider this as validated. When more than one scale had been used to measure agitation as the primary outcome, and one or more of these were validated and/or reliable, a score of 0.5 was awarded for either or both categories, respectively. Where a study used a validated measure of agitation as their primary outcome, but also reported non-validated measures, then we disregarded the secondary outcome to award the full point for validity.
Randomised controlled trials
We analysed the data in accordance with the design that was actually used rather than the one stated. For example, where a randomised design was used but the intervention was not compared with the control group, we considered this a within-subjects design.
Randomised controlled trials which fulfilled the following criteria were judged to be ‘high-quality RCTs’:
-
randomised
-
at least single blind
-
follow-up rates of 80%
-
intention-to-treat analysis
-
sufficiently powered
-
validity of outcome measures
-
findings reported with relatively narrow confidence intervals (CIs).
Blinding
Where assessor blinding was not possible but the study controlled for systematic bias in other ways (e.g. establishing inter-rater reliability between a subset of blinded and unblinded assessments), then we gave them the point. As blinding is often compromised within psychological studies, we awarded the point as long as blinding had been attempted, and had not completely failed. Where two raters were used and only half were blinded (e.g. a non-blinded rater assessing the immediate effects of an intervention by being present while it was administered, and a blinded rater assessing any follow-up effects), we awarded half a point. We awarded a point for blinding in studies of aromatherapy only where some effort had been made to disguise the smell, for example where raters had to wear nose clips.
Appropriate statistical methods
Where one-tailed statistical tests were used but not justified, and a non-significant result was found, we gave only half a point for appropriate use of statistics. Where results were significant and we had sufficient data, we reanalysed using a two-tailed test, and used the resulting p-value rather than the one in the original paper. As we had reanalysed the data, in this case we gave the full point for appropriate statistics. Where wholly inappropriate statistics were used, for example using nine t-tests without correction with a sample of 12, or an inappropriate statistical test was selected as the primary form of analysis, we did not award a point. If cluster adjustments should have been made but had not been, but otherwise the statistics were appropriate, we awarded half a point for appropriate use of statistics.
Follow-up rates
For the purpose of scoring follow-up rates, we considered patients to be in the study from the time of randomisation for RCTs, or first assessment from non-randomised trials.
The researchers then assigned a level of evidence from the CEBM as follows:
-
Level 1b: high-quality RCTs. These all scored ≥ 10, were single or double blind, with validated outcome measures.
-
Level 2b: lower-quality RCTs and higher-quality non-randomised studies (scoring ≤ 11).
-
Level 2c: moderate-quality non-randomised studies (scoring 6–9).
-
Level 4: these scored < 6. They were not RCTs.
Other levels of evidence were not relevant to our study as they referred to excluded research: 1a, 2a and 3a refer to systematic reviews, 1c and 3b refer to case studies, and level 5 refers to expert opinion.
Risk of bias
Individual study level
The CEBM tool for assessing validity of RCTs, described in detail above in the quality assessment section, allowed us to assess the relative likelihood of a range of biases, including but not limited to selection, attrition and detection bias, in any study; a higher score indicated fewer sources of possible bias than those with lower scores.
Across the studies
We intended to assess publication bias using a funnel plot; however, owing to the heterogeneity of the studies in terms of intervention, outcomes measured and quality, this was not possible. Instead, a comparison between the findings of the higher-quality (levels 2c and above) and the lower-quality (level 4) studies was made.
We attempted to avoid selection bias by:
-
searching both major and minor databases and the grey literature, and doing this twice so as not to miss the most recently published works
-
writing to all authors to enquire about extra papers and unpublished works
-
eliciting expert opinion about extra papers and unpublished works
-
searching references of relevant reviews
-
translating papers into English from other languages.
We attempted to avoid reviewer bias by:
-
working in accordance with our published protocol’s predefined criteria for inclusion of studies
-
assessing inter-rater reliability with regard to inclusion of papers as described above in Data extraction
-
having two independent researchers rate each paper for quality separately.
Categorisation of the intervention
LK, ELH and GL reviewed the details of the interventions, in order to categorise them. If we judged that the interventions were similar but with different labels given, we categorised them together, for example training in person-centred care or communication skills with people with dementia. The categories we used were:
-
activities
-
music therapy (protocol driven and general)
-
sensory interventions (all involved touch, and some included additional sensory stimulation, e.g. light)
-
training paid caregivers in person-centred care or communication skills with supervision (both of which focused on improving communication with the person with dementia and finding out what they wanted)
-
dementia care mapping (DCM): watching an individual with dementia and feeding back what they responded well to and what they did not, and then supervising an implementation of the plan
-
light therapy
-
home-like care
-
aromatherapy
-
training family carers in behavioural management
-
training family carers in cognitive–behavioural therapy (CBT)
-
exercise
-
changing the environment
-
dementia-specific therapies
-
pet therapy.
Table 1 shows how these categories are organised by type of intervention. In many cases, the mode of action of the intervention is not certain, meaning that they could possibly work more than one way and fit into more than one of the ’types’. For example, training family caregivers in CBT encompasses both psychological and behavioural aspects. However, we have organised these into only one type per category for ease of understanding.
| Type of intervention | Categories |
|---|---|
| Psychological: pertaining to mental processes | Training paid caregivers in person-centred care or communication skills with supervision |
| Dementia-specific therapies | |
| Training family caregivers in CBT | |
| Behavioural: pertaining to the person’s actions | Activities |
| DCM | |
| Training family carers in behavioural management | |
| Pet therapy | |
| Exercise | |
| Sensory: pertaining to the person’s senses | Aromatherapy |
| Light therapy | |
| Sensory | |
| Environmental: pertaining to the person’s environment | Home-like care |
| Changing the environment |
Level of agitation
In order to differentiate between interventions treating current agitation and those preventing emergent agitation (new onset, recurrent or increasing agitation), we separated studies according to the level of agitation of recruited participants specified in the inclusion criteria. These levels were as follows.
-
Any level of agitation, including no symptoms.
-
Some symptoms of agitation.
-
A significant level of agitation. Most papers trying to recruit participants with a significant level of agitation used a score of ≥ 39 on the CMAI40 as demonstrating significant agitation, and so we used this as a cut-point. Where another measure of agitation was used, we considered it significant if it would equate to a score of 39 or more on the CMAI, e.g. a score of > 4 on the Neuropsychiatric Inventory (NPI).
-
Those including participants with behavioural disturbance but not specifically agitation were labelled as ‘not specified’.
Data synthesis
The intervention effects, comparing either baseline with post-intervention outcome measurements or the outcome between the control and intervention groups, were estimated for studies with available data. We recalculated some results, for example studies including intervention and control groups but not directly comparing them, or where one-tailed statistical significance tests were used. We were unable to meta-analyse most studies.
Meta-analysis
We decided a priori to meta-analyse where there were at least three studies investigating homogeneous interventions using the same outcome measure which were not of very low quality (score ≥ 6). Light therapy (where three studies met these requirements) was the only intervention which fulfilled these criteria. Because outcome assessment periods differed across these three studies, it was not possible to pool the results using a standard random effects summary statistic method. Thus, we used a Bayesian random effects model with random effects for the intervention and accounting for the time of measurement of the outcome. Independent vague normal prior distributions for the fixed effects and vague uniform prior distributions for the standard deviations (SDs) of the random effects were used; these are relatively standard choices. 41 The sensitivity of the results to the assumptions of the prior distributions was evaluated by using alternative formulations (e.g. gamma and half-Cauchy priors for the SD of the random effects) and the results were not much affected.
Standard effect sizes
As we were unable to meta-analyse studies in the light of our a priori criteria for carrying out such meta-analyses, we estimated the interventions’ standardised effect sizes (SESs) with 95% CI, if data were available,42 to allow comparison across different interventions and outcomes using a common effect measure. In some studies, the outcome was measured at several time points during an intervention. In these papers, the original analyses either used multiple significance tests comparing the outcome at each time point separately, or repeated outcome measurements. As individual patient data were not available to estimate the SES incorporating the repeated measures, we used the outcome data measured at the last time point allowing time for the intervention to work. This also made the SESs estimated from these studies comparable with those where the outcome was only measured at a single time point following intervention. We calculated the SESs and 95% CIs, comparing the outcomes between the control and intervention groups in this paper or baseline with post-intervention outcome measurements in the supplement. We reanalysed some of the studies, for example studies including intervention and control groups but not directly comparing them, or where one-tailed statistical significance tests were used. For these reasons, in some cases we have obtained a result differing from that reported in the original paper.
Chapter 4 Results
Details of included and excluded studies
Figure 1 shows the PRISMA diagram and describes the results of our search.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram.
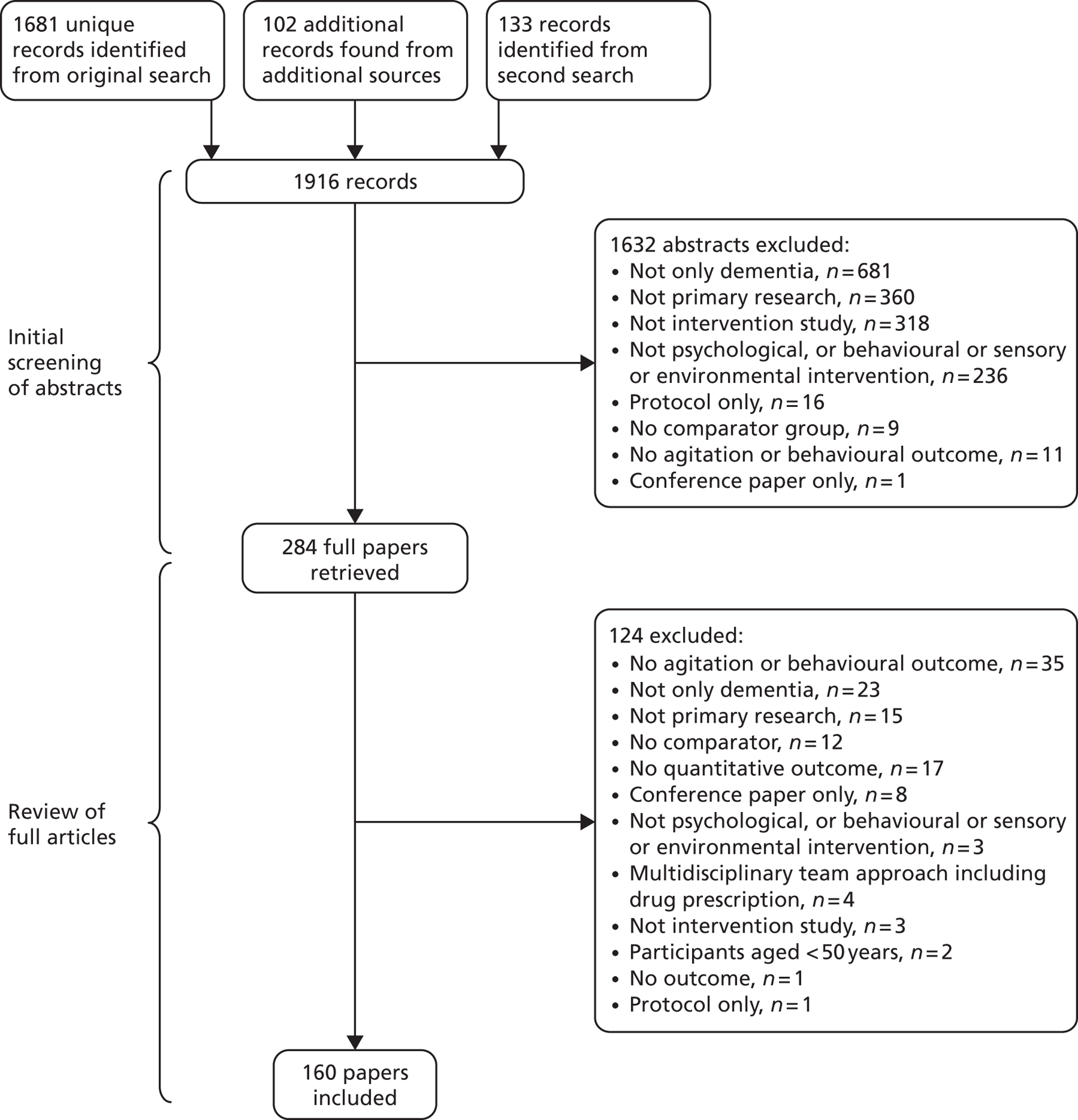
We found 1916 potentially relevant records; we excluded 1632 abstracts, then another 124 full papers and included 160 papers. Of these, eight papers were translated into English: three from Korean, three from German, one from Dutch and one from French. Papers were mostly from English-speaking nations, mainly the USA (n = 77), Australia (n = 13), the UK (n = 13) and Canada (n = 10). Papers were also from the following countries: Italy (n = 7), Taiwan, Province of China (n = 7), the Netherlands (n = 6), Republic of Korea (n = 6), Japan (n = 4), Sweden (n = 4), China/Hong Kong (n = 3), Germany (n = 3), France (n = 2), Islamic Republic of Iran (n = 2), Iceland (n = 1), Israel (n = 1) Norway (n = 1) and Spain (n = 1).
One hundred and four (88 first and 16 second) authors were contacted, who suggested 73 papers, of which 25 were included and 48 were excluded.
The methodological characteristics (whether comparison is between a separate control group or the same people before and after; participants’ agitation level), quality ratings, design, interventions, SESs (95% CIs) for outcomes if calculable or specified as not calculable, and outcomes of the 97 out of 160 (61%) included studies rated as high quality are described in the tables of this chapter. Lower-quality papers are described in Table 13 . Studies demonstrating significantly effective interventions are highlighted in bold and those of interventions that significantly worsened agitation are in green. The results for each category are summarised in the text. Details of each study’s quality score for those rated 2c and above are shown in Appendix 1 . Details of excluded studies and reasons for exclusion are reported in in Appendix 2 .
Seventy-one of the included studies did not record the type of dementia, 54 specified a mix of dementia but did not analyse each type separately, and 35 specified Alzheimer’s disease only. Fifteen studies rated 2c and above had outcome measures that were either invalid (n = 8), unreliable (n = 2) or both (n = 5). The effect of removing these from the analysis is reported in Appendix 3 .
Findings of the review
An overview of the findings of the review is given in Table 2 .
| Findings | SES range |
|---|---|
| Interventions with evidence of efficacy | |
| Working with the person with dementia | |
| Activities | –0.8 to –0.6 |
| Music therapy using a specific protocol | –0.8 to –0.5 |
| Sensory interventions | –1.3 to –0.6 |
| Working through paid caregivers in care homes and assisted living settings, with supervision | |
| Person-centred care and communication skills | –1.8 to –0.3 |
| Dementia care mapping | –1.4 to –0.6 |
| Behavioural management and communication skills | Not calculated |
| Interventions with no evidence of efficacy | |
| Working with the person with dementia | |
| Light therapy | Not applicable |
| Home-like care | |
| Aromatherapy | |
| Interventions with too little evidence to make definitive recommendations | |
| Working with family caregivers in the home of the person with dementia | |
| Training family caregivers in behavioural management | Not applicable |
| Training family caregivers in CBT | |
| Working with the person with dementia in a care home | |
| Music therapy not following a specific protocol | Not applicable |
| Exercise | |
| Dementia-specific therapies | |
| Pet therapy | |
| Working through paid caregivers in care homes, without supervision | Not applicable |
| Changing the environment | Not applicable |
| Mixed interventions | Not applicable |
Interventions with evidence to support their efficacy
Working with the person with dementia
Activities
Details of activity, quality of study and outcome can be seen in Table 3 .
| Author and year | Country of origin | Study design | Degree of participant agitation | Level of evidence | Quality score | Total participants | Therapeutic regime | Separate control group | Immediate outcome | Immediate SES (CI) | Long-term outcome | Long-term SES (CI) | Mean cost per person with agitation (2011 £) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kovach et al., 200343 | USA | RCT | Some | 2b | 7.5 | 78 | Varied activities matched to arousal level (e.g. music, exercise, storytelling) | Usual care | Significant improvement in visual analogue scale of agitation | NC | None | NC | 378 |
| Lin et al., 200944 | Taiwan, Province of China | RCT | Some | 2b | 6 | 133 | 28 sessions of Montessori activities | Presence (having researcher present) | Significant improvement | NC | None | NC | 372 |
| Lee and Kim, 200845 | Republic of Korea | Within subjects | Some | 2c | 7 | 23 | 56 indoor gardening sessions | No | Significantly improvement | –0.8 (–1.4 to –0.2) | None | NC | 274 |
| Buettner and Ferrario, 199746 | USA | RCT | None | 2b | 7 | 66 | 30 weeks of neurodevelopmental sequenced activities (e.g. cooking group), frequency unclear | Usual care including activities | Significantly improved at two time points but not third | NC | None | NC | 696 |
| Fitzsimmons and Buettner, 200247 | USA | RCT | None | 2b | 7 | 29 | Six to 10 sessions of a range of individualised activities (e.g. cooking) | Crossover: usual care | Significant improvement | –0.6 (–1.0 to –0.2) | None | NC | 173 |
| Buettner et al., 199648 | USA | RCT | None | 2b | 7 | 36 | 12 sessions of neurodevelopmental sequenced activities (e.g. cooking group) | Crossover: similar non-sequenced activities | Significantly improved during session, but not on overall weekly measures | NC | NS (4 weeks) | NC | 590 |
| Fitzsimmons and Buettner, 200349 | USA | RCT | None | 2c | 7 | 12 | 10 sessions of a cooking group | Usual care group not analysed | Significant improvement vs. baseline | NC | None | NC | 203 |
| Kolanowski et al., 201150 | USA | RCT | Some | 1b | 13·5 | 128 | 15 sessions activities adjusted or opposite to both skill level (FL) and interest (PSI): FL (+ opposite PSI) PSI (+ opposite FL) FL + PSI |
Opposite FL + PSI | NS | FL: 0.2 (–0.3 to 0.7) to PSI: 1.5 (0.9 to 2) PSI + FL: 1.0 (0.4 to 1.5) |
No differences (1 week) | NC | NC |
| Kolanowski et al., 200551 | USA | Within subjects | None | 2b | 10.5 | 30 | 36 sessions of activities matched to both skill level/interest | Activities matched only to skill level or interest | NS | PSI: –0.2 (–0·7 to 0·4) PSI + FL: –0·1 (–0·6 to 0.4) |
None | NC | NC |
| Cohen-Mansfield et al., 200652 | USA | RCT | None | 2b | 6 | 105 | Five sessions of activity matched to self-identity roles | Standard activities | Significant improvement | NC | None | NC | 80 |
Ten level 1 and 2 studies implemented a group activity, of which three studies tested the additional effect of individualising the activities. All participants were in care homes, except for one study where some participants were recruited from a day centre and others from a care home. 52 No study required participants to have symptoms of agitation to be included.
Some interventions consisted of only one activity, for example cooking groups, while others encompassed a variety of activities. Individualised activities meant that the investigators chose from a list of potential activities and matched them to an individual’s interests and cognitive level, and these could take place either in a group or individually. Two high-quality papers did not find any additional effects of individualising activities,50,51 although one lower-quality paper did. 52 Standard activities reduced emergent (new-onset) symptoms of agitation, and decreased agitation in care homes during the time they were in place. 43–49 The SES was calculable in two studies and was significant in both, with a reduction in symptoms ranging from –0.6 to –0.8. Only two studies measured agitation after the intervention period, at 1-week and 4-week follow-up, and neither showed a difference. 48,50
-
Overall, activities in care homes reduce emergent agitation and decrease symptomatic agitation in care homes during the time they are in place.
-
Individualising activities does not appear to make significant additional reductions in agitation.
-
There is no evidence for those who are severely agitated or who are not in care homes.
Music therapy using a specific protocol
Details of therapy, quality of study and outcome are given in Table 4 .
| Author and year | Country of origin | Study design | Degree of participant agitation | Level of evidence | Quality score | Total participants | Therapeutic regime | Separate control group | Immediate outcome | Immediate SES (CI) | Long-term outcome | Long-term SES (CI) | Mean cost per person with agitation (2011 £) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cooke et al., 201053 | Australia | RCT | Some | 1b | 11.5 | 47 | Music therapy three times per week for 8 weeks | Reading group | NS (total agitation) | –0.9 (–1.2 to –0.6) | NC | NC | NC |
| Sung et al., 201154 | Taiwan, Province of China | RCT | Some | 2b | 10.5 | 55 | Music therapy twice per week for 6 weeks | Usual care | NS | –0.5 (–1.0 to 0.0) | None | NC | NC |
| Sung et al., 200655 | Taiwan, Province of China | RCT | Some | 2b | 7 | 40 | Group music with movement, twice per week for 4 weeks | Usual care | Significant improvement | –0.8 (–1.5 to –0.1) | None | NC | 13 |
| Tuet and Lam, 200656 | Hong Kong | Non-randomised – crossover | Some | 2c | 6 | 16 | Music therapy three times per week for 3 weeks | Usual care (crossover) | Significant worsening | NC | NS (3 weeks) | NC | NC |
| Lin et al., 201157 | Taiwan, Province of China | RCT | None | 2b | 9.5 | 104 | Music therapy twice per week for 6 weeks | Usual care | Significant improvement | –0.6 (–0.9 to –0.4) | Significant improvement (1 month) | –0.6 (–0.9 to –0.3) | 27 |
| Groene, 199358 | USA | RCT | None | 2b | 6a | 30 | Five sessions of 15 minutes of music therapy, two sessions of being read to | Five sessions of 15 minutes of being read to, two sessions of music therapy | NS | NC | None | NC | NC |
| Raglio et al., 200859 | Italy | RCT | Not specific | 2c | 9 | 59 | 30 sessions of music therapy over 16 weeks | No | Significant improvement | NC | NC | NC | NC |
| Svansdottir and Snaedal 200660 | Iceland | Within subjects | None | 2c | 7.5 | 20 | Music therapy three times per week for 6 weeks | No | NS (aggressiveness) | NC | NS (4 weeks) | NC | NC |
| Jennings and Vance, 200261 | USA | Within subjects | None | 2c | 7 | 16 | Music therapy once per week for 4 weeks | No | Significant improvement | NC | None | NC | 24 |
| Suzuki, 200762 | Japan | Non-randomised – case-matched controls | None | 2c | 7 | 16 | Music therapy including short reality orientation, twice per week for 13 weeks | Unclear but presumably usual care | NS | NC | NS | NC | NC |
There were 10 studies of group music therapy following a specific protocol; these were led by a trained therapist and, for example, included a warm-up of a well-known song, and a period of listening to, followed by joining in with, music. 53–62 All took place in care homes, except one which was in a day centre. 61 A reasonable-quality study of music therapy for people with some symptoms of agitation found a significant improvement in the intervention group during the time of the intervention, while two others did not. The largest study included participants irrespective of whether or not they were agitated, and found that music therapy, twice per week for 6 weeks, improved the mean level of agitation symptoms. 44 Three studies considered the longer-term outcome in periods ranging from 3 to 8 weeks, and none found that it continued to be effective. 53,56,60 As the SES was calculated using only the first and last time periods for papers with multiple time points, some of our results differ from the original papers. They ranged from –0.5 to –0.8.
-
In care homes, music therapy by protocol is effective for emergent agitation and decreasing symptomatic agitation, but has no long-term usefulness in agitation.
-
There is no evidence for people with severe agitation. There is minimal evidence outside care homes.
Sensory interventions
Details of sensory interventions are given in Table 5.
| Author and year | Country of origin | Study design | Degree of participant agitation | Level of evidence | Quality score | Total participants | Therapeutic regime | Separate control group | Immediate outcome | Immediate SES (CI) | Long-term outcome | Long-term SES (CI) | Mean cost per person with agitation (2011 £) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moyle et al., 201163 | Australia | Within subjects | Significant | 2c | 6 | 27 | 14 sessions of daily foot massage | No | Significant improvement | –0.6 (–1.2 to 0.0) | Significant reduction (2 weeks) | NC | 128 |
| Yang et al., 200764 | Taiwan, Province of China | Within subjects | Significant | 2c | 6 | 31 | 40 sessions of acupressure over 4 weeks; social contact | No | Significant improvement | –1.2 (–0.7 to –1.7) | None | NC | 527 |
| Woods et al., 200565 | USA | RCT | Some | 2b | 11 | 60 | Therapeutic touch twice per day for 3 days | Placebo therapeutic touch; usual care | NS | NC | None | NC | NC |
| Remington, 200266 | USA | RCT | Some | 2b | 11 | 51 | Hand massage, hand massage and calming music; given once | Usual care | Significant improvement |
Hand massage: –0.6 (–1.1 to –0.1)
plus music: –1.3 (–1.9 to –0.8) |
None | NC | 3, 10 |
| Woods et al., 200967 | USA | RCT | Some | 2b | 10 | 64 | Therapeutic touch twice per day for 3 days | Placebo therapeutic touch; usual care | NS (vs. placebo) | NC | None | NC | NC |
| Hawranik et al., 200868 | USA | RCT | Some | 2b | 7 | 51 | Five sessions of therapeutic touch on consecutive days | Placebo therapeutic touch; usual care | NS (total agitation) | NC | NS (1 week, 2 weeks) | NC | NC |
| Staal et al., 200769 | USA | RCT | Some | 2b | 7 | 24 | Snoezelen, duration unclear | One-to-one structured activity | Significant improvement | –0.3 (–0.9 to –0.3) (Pittsburgh Agitation Scale) | None | NC | 273 |
| Hicks-Moore and Robinson, 200870 | Canada | Within subjects | Some | 2b | 6 | 32 | One session each of hand massage and hand massage plus music, while agitated | No | NS | Music PA: 0.2 (–0.3 to 0.7); PN: –1.8 (–2.4 to –1.2); VA: –1.0 (–1.6 to –0.5) No music PA: –0.4 (–0.9 to 0.1); PN: –1.4 (–1.9 to –0.8); VA: –0.7 (–1.3 to –0.2) |
None | NC | NC |
| Lin et al., 200944 | Taiwan, Province of China | RCT | Some | 2b | 6 | 133 | 28 sessions of acupressure over 4 weeks | Presence (researcher present) | Significant improvement | NC | None | NC | 300 |
| Woods and Dimond, 200271 | USA | Within subjects | Some | 2c | 8 | 10 | Therapeutic touch twice a day for 3 days | No | Significant improvement in subscales | NC | No tests | NC | 32 |
| Gerdner et al., 200872 | USA | Within subjects | Some | 2c | 7 | 9 | 42 sessions of craniosacral massage over 6 weeks | No | Significant improvement | NC | NS total agitation (3 weeks) | NC | |
| Whall et al., 199773 | USA | Quasi-experimental (non-randomised, control groups) | Some | 2c | 7 | 31 | Natural sounds/food while bathing | No | Significant improvement while bathing | NC | None | 30 | |
| van Weert et al., 200574 | Netherlands | RCT | None | 2b | 10 | 125 | Snoezelen over 18 months | No | Significant improvement in aggression. PN and VA NS |
PN –0.1 (–0.3 to –0.1)
PA: –1.4 (–1.7 to –1.0) VA: –3.9 (–4.4 to –3.4) |
None | 109 |
Sensory interventions target perceived understimulation of the person with dementia, and ranged from those focused purely on touch, such as massage, to multisensory interventions involving tactile, light and auditory stimulation, such as ‘snoezelen’. All 13 studies took place in care homes. Some used ‘therapeutic touch,’ which was defined as a healing-based touch intervention designed to focus on the person as a whole. Trials of therapeutic touch found no significant improvements relative to other touch interventions. 65,67,68 There was one large trial that did not specify presence of agitation as an entry criterion, which showed significant improvement. 74 The studies with participants with symptomatic and clinically significant agitation also showed an improvement compared with usual care. 44,63,66,69–73 The SES ranged from –0.6 to –1.3. There were three studies which looked at outcomes between 1 and 3 weeks later: two found no improvement and one found a significant reduction.
Secondary measures
One study assessed the effect of sensory interventions on functioning using the Katz Index of Activities of Daily Living scale, and did not find significant effects. 69
-
Sensory interventions significantly improved emergent agitation, symptomatic agitation, and severe agitation during the time the intervention took place.
-
Therapeutic touch has no added advantages.
-
There is insufficient evidence about long-term effects or in settings outside care homes.
Working through paid caregivers in care homes and assisted living settings
Details of training paid caregivers in person-centred care or communication skills with or without behavioural management training, or DCM with supervision, are given in Table 6 .
| Author and year | Country of origin | Study design | Degree of participant agitation | Level of evidence | Quality score | Total participants | Therapeutic regime | Separate control group | Immediate outcome | Immediate SES (CI) | Long-term outcome | Long-term SES (CI) | Mean cost per person with agitation (2011 £) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chenoweth et al., 200975 | Australia | RCT | Significant | 1b | 11.5 | 180 | Training plus two site visits and telephone-based supervision | Usual care | Significant improvement; restraint use. Prescriptions p.r.n. and CR quality of life NS | –1.4 (–1.5 to –1.3) | Significant improvement (8 weeks) | –1.4 (–1.5 to –1.3) | 99 |
| McCallion et al., 199976 | USA | RCT | Some | 1b | 10 | 66 | Nursing assistants delivered seven communication-focused sessions to family caregiver, with supervision | Usual care | Verbal agitation, physical non-aggression and irritability improved; aggression did not |
PN: –0.6 (–1.0 to 0.3)
VA: –0.7 (–1.0 to –0.3) PA: 0.1 (–0.3 to 0.4) |
Only verbal aggression and irritability remained significant (3 months) | 87 | |
| McCallion et al., 199977 | USA | RCT | Some | 2b | 6 | 105 | Communication skills training with ongoing support | Partial crossover – usual care | Significant improvement in all agitation | –0.4 (–0.7 to –0.2) | Significant improvement; physical restraints improved, PRNs worsened (6 months) | –0.2 (–0.5 to 0.1) | 72 |
| Teri et al., 200578 | USA | RCT | Not specified | 2b | 8 | 31 | Communication and behaviour management skills training, plus training managers to provide supervision | Usual care | Significant improvement in overall agitation 8 weeks | NC | NR | NC | 339 |
| Hoeffer et al., 199779 | USA | Within subjects | Some | 2c | 6 | 11 | Trained in person-focused bathing with support implementing | No | None | Significant improvement in physical and verbal aggression and being upset (combined 1 and 6 months) | 158 | ||
| Sloane et al., 200480 | USA | RCT | None | 2b | 6 | 73 | Trained in person-centred bathing/towel bath with support implementing | Crossover – usual care | Significant improvement for both showering and towel bath conditions | None | 64, 75 | ||
| Deudon et al., 200981 | France | RCT | Not specified | 2b | 7.5 | 306 | Training including issuing ‘staff instruction cards’ on BPSD, ongoing support | Usual care | Significant improvement | –0.32 (–0.48 to –0.16) | Significant improvement (20 weeks) | –0.3 (–0.5 to 1.3) | 31 |
| Chenoweth et al., 200975 | Australia | RCT | Significant | 1b | 11·5 | 191 | 12 hours’ DCM plus support implementing | Usual care | Significant improvement. Prescription of neuroleptic drugs/chemical restraints p.r.n., quality of life and restraint NS | Significant improvement. PRNs and restraint NS (4 months) | 108 | ||
| Chenoweth and Jeon, 200782 | Australia | Within subjects | Significant | 2c | 6 | 35 | 4 days’ DCM plus 2 months of implementing | No | NR | Significant improvement. CG quality of life NS (4 months) | 127 |
As person-centred care, communication skills training and DCM all seek to change the caregiver’s perspective, communication with and thoughts about people with dementia, and to encourage them to see and treat them as individuals rather than being task focused, we grouped them together. They all included supervision during initial training and implementation. Supervision encompasses ongoing practical and theoretical advice in implementation, rather than initial training only.
Person-centred care and communication skills
One large, very high-quality study of training in person-centred care found significant improvements in severe agitation both during the intervention period and 8 weeks later. 75 Three studies of improving communication skills or person-centred care, two for participants with symptomatic agitation and one without this as an entry criterion, found significant improvements in immediate agitation76,77,80 and longer term outcome up to 6 months. 76,77,79 Similarly, in a large study where the inclusion criterion was significant neuropsychiatric problems although not necessarily agitation, there was a significant improvement during the 8 weeks of person-centred care training, and this continued at 20 weeks. 81 The SES ranged from –0.4 to –1.8.
-
There is convincing evidence that training paid caregivers in communication or person-centred care skills is effective for symptomatic and severe agitation, both immediately and up to 6 months, in the care home setting.
-
There is preliminary evidence that it helps to prevent emergent agitation.
-
Evidence for settings other than care homes is limited.
Dementia care mapping
Two studies evaluated DCM in care homes. This is a specific intervention involving individual observation and assessment of each resident’s behaviour, factors improving perceived well-being, and potential environmental triggers. The results are fed back to caregivers, who incorporate them into plan and are supported in implementing any proposed changes. It aims to change the way the unit perceives residents, so that they see them more as individuals. One large, very high-quality study built on a pilot and decreased severe agitation during the intervention period, and the effects continued for 4 months. 75,82 The SES ranged from –0.3 to –1.4.
Secondary measures
Both the pilot82 and the main study75 investigated our secondary measure of quality of life using the QoLAD scale, but found no significant improvement. The pilot study82 assessed our secondary measure of function using the Functional Assessment Staging (FAST) scale but found no significant change.
-
There is some evidence that DCM is effective immediately and over 4 months for severe agitation in care homes.
-
There is little evidence for emergent agitation or symptomatic agitation, or in other settings.
Behavioural management and communication skills
One reasonable-quality RCT addressed behavioural problems rather than specifically agitation as their inclusion criteria, and the intervention was a combined programme of improving communication skills and behavioural management in an assisted living setting. They found significant improvements in agitation immediately after 8 weeks of training. 78
-
There is preliminary evidence that training paid caregivers in behavioural management and communication skills is effective in reducing agitation symptoms in assisted living settings in the short term.
-
There is no evidence in this setting for the longer-term effects.
Interventions with evidence of no efficacy
Working with the person with dementia
Light therapy
Details of light therapy are given in Table 7 .
| Author and year | Country of origin | Study design | Degree of participant agitation | Level of evidence | Quality score | Total participants | Therapeutic regime | Separate control group | Immediate outcome | Immediate SES (CI) | Long-term outcome | Long-term SES (CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burns et al., 200983 | UK | RCT | Some | 1b | 12.5 | 48 | 2 hours’ daily light therapy for 2 weeks | Standard light | NS | –0.2 (–0.6 to 0.2) | NS (4 weeks) | –0.3 (–0.7 to 0.2) |
| Lyketsos et al., 199984 | USA | RCT | Some | 2b | 7.5 | 15 | 1 hour of daily light therapy for 4 weeks | Standard light | NS | –0.3 (–0.8 to 0.2) (BEHAVE-AD) | None | NC |
| Dowling et al., 200785 | USA | RCT | Some | 2b | 6 | 70 | Activities in brightly lit area (outside/lightbox), 1 hour per day/10 weeks | Similar activities in a non-brightly lit area | Significantly worsened | p.m. light: 4.0 (3.1 to 4.9) a.m. light 7.0 (5.8 to 8.3) |
None | NC |
| Thorpe et al., 200086 | Canada | Non-randomised – within subjects ABAa | Some | 2c | 8 | 16 | 5 days of light therapy at 30 minutes per day | No | NS | –0.2 (–0.9 to 0.5) | None | |
| Lovell et al., 199587 | USA | Within subjects | Some | 2c | 7 | 6 | 2 hours’ daily light therapy for 10 days, repeated after 8-day gap | No | Significant improvements vs. non-intervention days | –0.8 (–2.0 to 0.4) (Agitated Behaviour Rating) | None | |
| Satlin et al., 199288 | USA | Within subjects | Some | 2c | 6b | 10 | 2 hours’ daily light therapy for 7 days while restrained | No | NS (agitation, prescription of drugs p.r.n., restraints) | NC | None | |
| Barrick et al., 201089 | USA | Non-randomised weighted control group | None | 2c | 7 | 66 | 3 weeks of a.m. bright light, p.m. bright light, all-day bright light, throughout whole unit | Standard light | Significant worsening in mild/moderate dementia | NC | None | |
| Ancoli-Israel et al., 200390 | USA | RCT | Significant | 2b | 6 | 92 | 2 hours per day of light therapy for 10 days, either a.m. or p.m. | Placebo red light during AM | Verbal agitation worsened | –2.0 (–2.4 to –1.6) | None | –0.3 (–0.6 to 0.1) |
| Skjerve et al., 200491 | Norway | Within subjects | Significant | 2c | 7 | 11 | 4 weeks of daily a.m. light for 45 minutes | No | Unclear: no direct comparisons made | –0.5 (–1.4 to 0.4) | Unclear | –1.0 (–2.0 to –0.1) |
| Haffmans et al., 200192 | Netherlands | Within subjects | Significant | 2c | 6.5 | 10 | 30 minutes’ daily light therapy for 2 weeks | No | Significant improvement (restlessness) | NC | None | NC |
Light therapy’s proposed mechanism for reducing agitation involves manipulating the disrupted circadian rhythms associated with dementia, typically by 30–60 minutes of daily exposure to bright light. All 11 studies took place in care homes. 83–92 None of the high-quality studies (RCTs) found a significant improvement. Among participants with both significant and emergent agitation, the three studies with the largest samples found that agitation was made worse by light therapy. 85,89,90 Most other studies did not report a significant result. A meta-analysis of the three studies using the CMAI found no overall effect of light therapy on the CMAI [0.045 (95% credible interval –1.228 to 1.468)], which is consistent with the findings reported by the individual studies. The between-study variance for the intervention effect was estimated to be 0.83, suggesting a moderate degree of heterogeneity between the studies.
-
Light therapy does not show efficacy for emergent agitation, symptomatic agitation, or severe agitation in care homes.
-
There is preliminary evidence that light therapy worsens agitation.
-
There is no evidence for light therapy in settings other than care homes.
Home-like care
Details of home-like care are given in Table 8 .
| Category of intervention | Author and year | Country of origin | Study design | Degree of participant agitation | Level of evidence | Quality score | Total participants | Therapeutic regime | Separate control group | Immediate outcome | Immediate SES (CI) | Long-term outcome | Long-term SES (CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Home-like care | Verbeek et al., 201093 | USA | Matched controls | Any | 2b | 10.5 | 259 | Small group living in home-like environment | Traditional nursing home | Significant worsening over time (up to 12 months); quality of life NS | –0.1 (–0.2 to 0.1) | None | NC |
| Home-like care | Elmstahl et al., 199794 | Sweden | Within subjects | Any | 2c | 7 | 103 | Corridor-like, ‘L’-shaped or ‘H’-shaped living environment | No | Significantly worsened over 12 months (aggressiveness) | NC | None | NC |
| Home-like care | Reimer et al., 200495 | Canada | Non-randomised-matched groups | Any | 2c | 7 | 185 | Small group living in home-like environment | Traditional nursing home group; plus group recently moved between two traditional nursing homes | NS agitation, quality of life (3, 6, 9 and 12 months) | NC | None | NC |
| Home-like care | Annerstedt et al., 199396 | Sweden | Non-randomised-matched controls | Any | 2c | 6a | 56 | Small group living in home-like environment | Traditional nursing home | Significantly worsened over 12 months (aggressiveness); prescriptions p.r.n. use improved | NC | None | NC |
| Aromatherapy | Burns et al., 201197 | UK | RCT | Significant | 1b | 13.5 | 94 | 168 sessions aromatherapy massage (plus placebo donepezil) | Placebo aromatherapy massage (plus placebo donepezil) | NS | NC | None | NC |
| Aromatherapy | Lin et al., 200798 | Hong Kong | RCT | Significant | 2b | 10 | 35 | 21 sessions lavender aromatherapy | Crossover: odourless sunflower oil | NS | 0.0 (–0.3 to 0.4) | None | NC |
| Aromatherapy | Ballard et al., 200299 | UK | RCT | Significant | 2b | 6 | 72 | 56 sessions of Melissa oil massage | Odourless sunflower oil | Significant improvement | NC | None | NC |
| Aromatherapy | Holmes et al., 2002100 | UK | Within subjects | Some | 2c | 9 | 15 | Aromatherapy oil sprayed on ward for 5 days; also water steam control condition | No | Significant improvement | NC | None | NC |
| Aromatherapy | Akhondzadeh et al., 2003101 | Islamic Republic of Iran | RCT | None | 2b | 7.5 | 42 | Melissa oil aromatherapy, frequency unclear | Placebo aromatherapy oil (details not stated) | ‘Side effect’ of agitation more frequent in placebo group | NC | None | NC |
| Aromatherapy | Cameron, 2012102 | UK | RCT | Not specific | 2b | 7 | 18 | 42 sessions of lemon balm oil massage | Crossover: inert lemon balm oil | NS | NC | None | NC |
Four large studies evaluated small group living for people with dementia, aiming to create a home-like environment. The homes had a maximum of eight residents, and all meals were prepared with staff and residents or family carers. The staff were a small fixed team, and the facilities resembled a domestic environment. None were able to randomise due to ethical and practical considerations, but three used comparisons of traditional nursing homes. None directly recruited participants with agitation. All studies showed increasing agitation in the intervention group over time, with three becoming significantly worse than the comparator over 12 months,93,94,96 and the fourth showing a trend towards more agitated behaviours than control after 6 months (p = 0.087). 95
Secondary measures
Two studies investigated quality of life: one used the QUALIDEM and found no significant change93 and the other measured pleasant activities and found that participation in pleasant activities decreased less in the intervention group than in the control group. 95 This study found a significantly slower decline in functionality as measured by the FAST scale in the intervention group.
-
There is good evidence that moving people with dementia into a home-like care environment does not reduce, and may significantly increase, their risk of developing agitation.
-
There is good evidence that agitation increases in the longer term with home-like care, and this cannot solely be accounted for by the move itself.
Aromatherapy
All six aromatherapy studies took place in care homes. One excellent, large, blinded study found no immediate or long-term improvement for participants with severe agitation. 97 This result is similar to a small, less rigorous blinded study. 102 The results of the non-blinded studies were mixed. 98–101
Secondary measures
Quality of life measured by the Blau Quality of Life (Blau QOL) scale was reported by the large, excellent, blinded study and found no difference between the aromatherapy condition and the control condition. 103 In another study, functionality was measured as the percentage of time spent either constructively, or withdrawn, and the intervention group improved significantly. 99
-
There is good evidence that aromatherapy is not an effective intervention for treating agitation.
-
There is no evidence for settings outside care homes.
Interventions with too little evidence to make definitive recommendations
Working with family caregivers in the home of the person with dementia
Training family caregivers in behavioural management
One very high-quality, large study found no immediate or longer-term effect (3, 6 or 12 months) of 11 sessions of training family caregivers in behavioural management of severe agitation in people with dementia living at home. 104 Of the two smaller studies of symptomatic agitation, the larger found a borderline significant improvement in agitation at 2 weeks, and the other found no effect. 105,106
-
There is preliminary evidence that teaching behavioural management techniques to family caregivers is not an effective intervention for severe agitation, both in the immediate and the longer term.
-
There is not enough evidence to conclude whether it may be helpful in symptomatic or emergent agitation.
Training family caregivers in cognitive–behavioural therapy
Details of training family caregivers in CBT are given in Table 9 .
| Category of intervention | Author and year | Country of origin | Study design | Degree of participant agitation | Level of evidence | Quality score | Total participants | Therapeutic regime | Separate control group | Immediate outcome | Immediate SES (CI) | Long-term outcome | Long-term SES (CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Training family caregivers in behavioural management | Teri et al., 2000104 | USA | RCT | Significant | 1b | 11 | 148 | 11 sessions | Placebo medication (plus two drug arms) | NS (agitation, CG burden) | NC | NS (3, 6 and 12 months) | NC |
| Training family caregivers in behavioural management | Gormley et al., 2001105 | UK | RCT | Some | 1b | 11·5 | 65 | Four sessions | Given non-behavioural advice and signposting | NR | None | NS agitation and CG burden (2 weeks) | –0.6 (–1.0 to –0.2) (RAGE) |
| Training family caregivers in behavioural management | Bourgeois et al., 1997106 | USA | RCT | Some | 2b | 7 | 7 | One workshop and 11 consultations | Home-based support (detail unclear) | NS (vs. control) | NC | Unclear-aggregated with immediate outcome | NC |
| Training family caregivers in CBT | Huang et al., 2003107 | Taiwan, Province of China | RCT | Significant | 2b | 8 | 59 | Two home and 13 telephone consultations | Written educational materials and social telephone calls | Unclear as baseline/change scores not analysed; significantly different at time 2 | –0.3 (–0.6 to –0.0) | Unclear (3 months) | –0.2 (–0.5 to 1.1) |
| Training family caregivers in CBT | Wright et al., 2001108 | USA | RCT | Significant | 2b | 7 | 93 | Three home and two telephone consultations | Usual care | NS | NC | NS agitation, CG well-being (9 months) | NC |
| Training family caregivers in CBT | Haupt et al., 2000109 | Germany | Within subjects | None | 2c | 9 | 14 | 12 groups | No | CG rated agitation NS. Clinician rated agitation improved but not aggression | NC | None | NC |
There were three studies, of reasonable or lower quality, of training family caregivers in CBT in the home: two with people with severe agitation, and one to prevent emergent agitation. None of these found significant improvements. 107–109
-
There is no evidence that teaching family caregivers CBT is effective for treating agitation.
Working with the person with dementia in a care home
Music therapy not following a specific protocol
Details of music therapy not following a specific protocol are given in Table 10 .
| Author | Country of origin | Study design | Degree of participant agitation | Level of evidence | Quality score | Total participants | Therapeutic regime | Separate control group | Immediate outcome | Immediate SES (CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Remington, 200266 | USA | RCT | Some | 2b | 11 | 34 | Calming music, given once | Usual care | Significant improvement in all groups | –0.9 (–1.4 to –0.4) |
| Garland et al., 2007110 | Australia | RCT | Some | 2b | 8 | 30 | Three sessions of preferred music tape | Crossover – usual care | NS | NC |
| Clark et al., 1998111 | USA | RCT | Some | 2b | 6.5 | 18 | Preferred music played while bathing | Usual care | Significant improvement during baths | NC |
| Gerdner, 2000112 | USA | RCT | Some | 2b | 6 | 45 | Individualised music on a cassette, twice per week for 6 weeks | Non-preferred classical music | Significant improvement vs. classical/no music, both during and 15 minutes after treatment | NC |
| Hicks-Moore and Robinson, 200870 | Canada | Within subjects | Some | 2b | 6 | 32 | 10 minutes of preferred music played while agitated | None | Physical non-aggression and verbal agitation improved. Aggression NS | PA: –0.4 (–0.9 to 0) PN: –2.2 (–2.8 to –1.6) VA: –1.0 (–1.5 to –0.4) |
| Park and Specht, 2009113 | USA | Within subjects | Some | 2c | 8.5 | 15 | Family CG played preferred music prior to peak agitation time, twice per week for 2 weeks | No | NS difference between music/no music weeks; however, significant improvements during and immediately after music | NC |
| Gerdner, 2005114 | USA | Within subjects | Some | 2c | 7 | 8 | Music played on CD player every day for 2 months, plus when patient agitated | No | Significant improvement in daytime agitation; mixed result for evening | NC |
| Tabloski, 1995115 | USA | Within subjects | Some | 2c | 7 | 20 | Calming background music for 15 minutes while agitated, repeated once | No | Significant improvement | NC |
| Ragneskog et al., 1996116 | Italy | RCT | None | 2c | 8 | 24 | Soothing music vs. 1920s pop vs. 1980s rock and pop, played at lunchtime | No | NS (restlessness) | NC |
| Chang et al., 2010117 | Taiwan, Province of China | Within subjects | None | 2c | 6 | 47 | Nature sounds played during lunchtime, 7 days per week, repeated four times | No | Significant worsening (total, verbal and physical aggression) | NC |
| Gerdner, 1997118 | USA | Within subjects | None | 2c | 6 | 5 | Individualised music played on a tape, every day for 1 week | No | Significant improvement | NC |
Overall, the 11 studies on music therapy without a specific protocol, which were all in care homes, had small participant numbers, and were typically of a lower quality. Three studies found no improvement in agitation, including the only two blinded studies,66,110,116 and one found an increase in agitation. 117 In general, studies found a significant improvement during the time of the therapy. 67,70,111–115,118 There were no studies of people with severe agitation, and no long-term outcomes were reported.
-
Overall, it is unclear whether or not playing music without a specific protocol is therapeutic for agitation in the short term in care homes.
-
There is no evidence for the long-term usefulness of music therapy without a protocol on agitation, or for treating participants with severe agitation, or for settings outside care homes.
Exercise
Details of exercise, dementia-specific therapies and pet therapy are given in Table 11 .
| Category of intervention | Author and year | Country of origin | Study design | Degree of participant agitation | Level of evidence | Quality score | Total participants | Therapeutic regime | Separate control group | Immediate outcome | Immediate SES (CI) | Long-term outcome | Long-term SES (CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise | Aman and Thomas, 2009119 | USA | Within subjects | Significant | 2c | 8 | 50 | Nine exercise sessions | No | NS (CMAI) | 0.0 (–0.4 to 0.4) | None | NC |
| Exercise | Holmberg, 1997120 | USA | Within subjects | Some | 2c | 6a | 11 | Approximately 156 sessions of a walking group | No | Significantly fewer incidents of aggression on days group met | NC | None | NC |
| Exercise | Eggermont et al., 2010121 | UK | RCT | None | 2b | 6.5a | 112 | 30 sessions of walking | Social visit, outside | NS (restlessness) | NC | NS (7 weeks) | NC |
| Exercise | Buettner and Fitzsimmons, 2004122 | USA | Within subjects | None | 2c | 6.5 | 20 | 28 sessions of either morning or afternoon exercise to music | No | NS | a.m.: –0.1 (–1.0 to 0.8) p.m.: 0.2 (–0.7 to 1) |
None | NC |
| Cognitive stimulation therapy | Robichaud et al., 1994123 | Canada | RCT | Some | 1b | 11.5 | 40 | 20 session group including sensory and social stimulation over 10 weeks | Usual leisure activities | NS | 0.9 (0.3 to 1.5) (Revised Memory and Behaviour Checklist) | None | NC |
| Cognitive stimulation therapy | Hong, 2011124 | Republic of Korea | RCT | None | 2b | 7 | 55 | Culturally familiar environment from youth with sensory activities | Same familiar environment but no activities | NS (total agitation, depression, functional capacity) | –0.3 (–0.7 to 0.1) | None | NC |
| Validation therapy | Toseland125 | USA | RCT | Some | 2b | 6.5 | 33 | Approximately 208 sessions of validation group therapy | Social contact (placebo), usual care (control) | NS (total agitation) | NC | None | NC |
| Pet therapy | Kanamori et al., 2001126 | Japan | Within subjects | None | 2c | 7 | 7 | Six sessions of animal-assisted therapy | No | Mixed: significant improvement in aggressiveness; activity disturbance NS | Aggressiveness: –0.6 (–1.7 to 0.5) Activity: 0.0 (–1.0 to 1.0) (BEHAVE-AD) |
None | NC |
| Pet therapy | Mossello et al., 2011127 | Italy | Within subjects | None | 2c | 7 | 10 | Nine sessions of pet therapy with either dog or plush toy | No | NS | NC | None | NC |
| Pet therapy | Libin and Cohen-Mansfield, 2004128 | USA | Within subjects | None | 2c | 6 | 9 | One session each of robotic cat and plush toy cat | No | Plush cat significantly improved, robotic cat NS | NC | None | NC |
Of the four studies on exercise interventions, all were in care homes. One large, reasonable-quality study of walking (112 participants) and a smaller study of exercise to music found no effect on participants with emergent agitation. 121,122 One study of walking sessions three times per week (11 participants with symptomatic agitation) found that there was less aggression on the days when the group met120 and a study on exercise sessions for people with severe agitation found no effect. 119 Only one of these studies looked at longer-term outcomes, and this was not significant. 121
-
There is no convincing evidence that exercise as an intervention is therapeutic for agitation in care homes.
-
The evidence is of generally low standard, precluding confident conclusions.
-
There is no evidence for other settings, and only minimal evidence (of no effect) in the longer term.
Dementia-specific therapies
Two studies of cognitive stimulation therapy, including one very high-quality study and one study of validation therapy, did not find a significant decrease in agitation. 123–125
Secondary measures
One study investigated functionality using the Activities of Daily Living scale but found no significant change. 124
-
Overall, there is too little evidence to make recommendations on dementia-specific therapies, but they are not designed primarily to improve agitation and do not seem to improve it.
Pet therapy
Three very small, lower-quality studies looked at pet therapy in care homes and a day care centre, including both real and simulated animals (e.g. toys or robots), and found mixed results. 126–128
-
Overall, there is too little evidence, of too low a standard, to make recommendations about the use of pet therapy for agitation.
Training programmes for paid caregivers in care homes
Details of training programmes for paid caregivers in care homes are given in Table 12 .
| Category of intervention | Author and year | Country of origin | Study design | Degree of participant agitation | Level of evidence | Quality score | Total participants | Therapeutic regime | Separate control group | Immediate outcome | Immediate SES (CI) | Long-term outcome | Long-term SES (CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paid unsupervised caregivers | Magai et al., 2002129 | USA | RCT | None | 2b | 6.5 | 91 | Non-verbal communication skills training; no supervision | Educational training (placebo), usual care (control) | NS | NC | NS (9, 12, 15 weeks) | NC |
| Paid unsupervised caregivers | Finnema et al., 2005130 | Netherlands | RCT | None | 2b | 8 | 146 | Whole staff ethos training, selected staff intensive training, groups and supervision on emotion-oriented care | Usual care | NS | NC | None | NC |
| Paid unsupervised caregivers | Galik et al., 2008131 | USA | Within subjects | None | 2c | 6 | 46 | Trained in restoring/promoting functional independence, plus supervision | No | Significant improvement over time (unclear at which time point) | –0.2 (–0.6 to –0.3) | Unclear | NC |
| Paid unsupervised caregivers | Matthews et al., 1996132 | Australia | Within subjects | None | 2c | 6 | 40 | 1-day workshop on person-centred care | No | NS | NC | NS (4, 8 weeks) | NC |
| Changing environment | Detweiler et al., 2008133 | USA | Within subjects | None | 2c | 6 | 34 | Wander-garden available over 1 year | No | Significant improvement. Prescription of neuroleptic drugs/chemical restrains p.r.n. NS | NC | None | NC |
| Changing environment | Perivolaris et al., 2006134 | Canada | Within subjects | None | 2c | 6 | 13 | 12 days of home-like environment for meals with quiet background music | No | NS | NC | None | NC |
| Masking exits | Darby, 1990135 | UK | Within subjects | Some | 2c | 6 | 9 | Full-length and reversed mirror placed over exit door | No | Significantly improved ward exits | NC | None | NC |
| Masking exits | Hussian and Brown, 1987136 | USA | Within subjects | None | 2c | 6 | 8 | Masking tape in front of door | No | Significant improvement; but improvement also found for control condition | NC | None | NC |
| Wayfinding intervention | McGilton et al., 2003137 | Canada | RCT | None | 2b | 7.5 | 32 | 12 sessions of wayfinding intervention | Yes – details not reported | Significant worsening | –0.521 (–1.040 to –0.010) | NS | 0.217 (–0.290 to 0.730) |
| Simulated presence therapy | Camberg et al., 1999138 | USA | RCT | Not specified | 2b | 8 | 54 | Simulated presence tape at least twice per a day while CR agitated | Crossover – neutral tape (placebo), usual care (control) | NS (total agitation) | NC | None | NC |
| Special care unit putting in place a variety of interventions | Bianchetti et al., 1997139 | Italy | Within subjects | Significant | 2c | 6.5 | 16 | 6 months in special care unit with multidisciplinary support | Standard nursing care | Significant improvement in agitation, prescription of neuroleptic drugs/chemical restrains p.r.n. and restraint use |
–0.8 (–1.6 to –0.1)
Restraint –0.4 (–1.1 to 0.3) Psychotropic –0.3 (–1 to 0.4) |
None | NC |
| Mix of activities of daily living, communication skills and psychosocial activities | Beck et al., 2002140 | USA | RCT | Some | 2b | 7 | 96 | 60 sessions promoting either functional independence, psychosocial intervention or both | Social contact (placebo), usual care (control) | NS | NC | NS (1, 2 months) | NC |
The effectiveness of training programmes for paid caregivers depends on what they are trained to do, and the level of supervision provided. Training in both communication skills and person-centred care was not effective when offered without supervision. 129,132 An extensive trial of emotion-oriented care, which aimed to elicit residents’ feelings towards their dementia diagnosis and help them cope with it, alongside promoting staff empathy, failed to find any significant results. 130 However, motivating people with dementia to improve their independence in carrying out activities of daily living (e.g. washing), with supervision, significantly improved agitation in one level 2c study. 131
-
Overall, training staff without supervision seems to be less effective than training with supervision, in both the short and the longer term.
-
The evidence is generally of a low standard, precluding confident conclusions.
Changing the environment
Four small studies tested environmental interventions, with numbers ranging from 8 to 24 participants (see Table 12 ). 133–136 None had a control group. One found significant improvements in agitation by providing a wander garden for care home residents;133 a second found no significant improvements after implementing a more home-like environment at mealtimes. 134 Two small studies tested masking exits to reduce absconding from unlocked doors, and found some improvements. 135,136
-
Overall, the evidence for environmental interventions is limited. Studies lack quality and are too small and disparate to draw conclusions.
Mixed interventions
In one very small study (with 16 participants), special care units compared with standard nursing care improved severe agitation over 6 months (see Table 12 ). 139 In simulated presence therapy, a tape mimicking a telephone conversation with a family member is played at a time when the participant is agitated, and the one study testing this found no significant results. 138 A wayfinding intervention tested whether or not training residents to find their way to their bedroom from the living area of their care home would improve agitation, and found no significant effects initially but, by 3 months, those in the intervention group were significantly more agitated. 139 Finally, one study testing a mix of psychosocial interventions such as massage, and an intervention focusing on improving residents’ independence at activities of daily living, did not find any significant improvements in agitation. 140
-
There is not enough evidence to make recommendations on simulated presence therapy, wayfinding or mixed activities.
Level 4 studies
Details of level 4 studies are given in Table 13 .
| Category of Intervention | Author | Degree of agitation for participation in study | Quality score | 24-hour care | Total participants | Therapeutic regime | Separate control group | Immediate outcome | Long-term outcome |
|---|---|---|---|---|---|---|---|---|---|
| Activities | Cohen-Mansfield et al., 201040 | Significant | 5 | Yes | 143 | Short presentation of eight stimuli: live social, stimulated social, task, reading, self-identity, music, work, manipulative | No | Significant improvement in agitation for all stimuli except manipulation | None |
| Activities | Putman and Wang, 2007141 | Significant | 5 | Yes | 16 | 2 years of a psychosocial group, 5 days per week | No | Significant improvement | None |
| Activities | Vespa et al., 2002142 | None | 5 | Yes | 18 | Daily psychosocial activities for at least 1 year | No | Significant improvement | None |
| Activities | Schalek et al., 2004143 | Some | 4.5 | Yes | 20 | 10 sessions of recreational air-mat therapy | Usual care group not analysed | Significant improvement vs. baseline (wandering) | None |
| Activities | Buettner, 1999144 | None | 3 | Yes | Unclear | Recreational items made available over 6 months | Crossover – usual care | Mixed: one group found significant improvement, one did not | None |
| Activities | Volicer et al., 2006145 | None | 0 | Yes | 90 | Club providing continuous meaningful activity; frequency unclear | No | Agitation measurement unclear. Prescription of neuroleptic drugs/chemical restraints p.r.n. less prescribed | None |
| Activities | Luk et al., 2011146 | Some | 5 | Yes | 14 | 12 sessions of outdoor horticultural activities | Sensory and social activities | NS | None |
| Activities – pet therapy | Churchill et al., 1999147 | Some | 4 | Yes | 28 | One session with therapy dog | No | NS | |
| Activities plus validation therapy | Kim et al., 2002148 | None | 5 | No | 22 | Day care with validation therapy principles (50 days) | No | Significantly worsened | None |
| Aromatherapy | Lee, 200548 | None | 5 | Yes | 63 | 12 sessions each of hand massage with lavender oil and jojoba oil | Crossover – usual care | NS | None |
| Aromatherapy | Smallwood et al., 2001150 | None | 5 | Yes | 21 | Aromatherapy massage group; conversation and aromatherapy group (frequency unclear) | Massage only | NS (vs. control) | None |
| DCM plus behavioural intervention | Kuiper et al., 2009151 | None | 5 | Mixed | 67 | Two cycles of 2 weeks’ DCM | No | NS (total agitation, cognition) | NS (6 months) |
| Exercise | Numazu et al., 1995152 | None | 4 | Yes | 11 | 28 exercise sessions to promote sleeping | Control group not analysed | Significant improvement | None |
| Home-like care | Wilkes et al., 2005153 | None | 5.5 | Yes | 23 | Small group living in home-like environment | Traditional nursing home | Significant improvement at 3 but not 6 months | None |
| Individualised activity | Gori et al., 2001154 | None | 4.5 | Yes | 25 | Approximately 49 activities matched to preference | No | NS (total agitation) | None |
| Individualised intervention – aimed at cause of the problem | Cohen-Mansfield et al., 2007155 | Significant | 5.5 | Yes | 167 | 10 sessions aimed at cause of agitation (e.g. thirst) | Education session for staff | Significant improvement | None |
| Individualised intervention – aimed at cause of the problem | Bedard et al., 2011156 | Some | 5 | Yes | 33 | Six sessions targeted at comfort, attention and stimulation whilst agitated | No | NS (total agitation) | Frequency but not duration of agitation improved (2, 4 weeks) |
| Individualised intervention delivered through paid caregiver | Ballard et al., 2009157 | Significant | 5 | No | 387 | Researcher trained paid CG to deliver four sessions of social, music or behavioural intervention | No | Significant improvement | None |
| Light therapy | Calkins et al., 2007158 | Some | 4 | Yes | 18 | Daily outdoor activities in summer for 2 weeks; control: summer no activity, winter indoor activity, winter no activity | No | Individual subscale items showed both significant improvements and significant worsening | None |
| Light therapy | van Hoof et al., 2009159 | None | 3.5 | Yes | 26 | 10 hours’ daily overhead blue or yellow lighting for 3 weeks | Usual care | NS | NS (8 weeks) |
| Masking exits | Dickinson, 1995160 | None | 5 | Yes | 7 | Blind, cloth barrier, and both were used to mask exit door | No | Significant improvement in exiting | None |
| Masking exits | Kincaid and Peacock, 2003161 | None | 5 | Yes | 12 | Wall mural painted over exit door | No | Significant improvement in door testing | None |
| Masking exits | Chafetz, 1990162 | None | 2 | Yes | 30 | Masking tape in front of door | No | NS effect on door opening | None |
| Multidisciplinary care plus activities | Davison et al., 2007163 | Significant | 5.5 | Yes | 34 | Median 90 days of inpatient multidisciplinary care; activities provided | No | Significant improvement | None |
| Multidisciplinary care plus activities | Chapman and Toseland, 2007164 | None | 5 | Yes | 118 | 8 weeks’ inpatient multidisciplinary care, activities provided | Traditional nursing home care | NS (total agitation) | None |
| Music therapy | Ledger and Baker, 2007165 | None | 5.5 | Yes | 60 | Music therapy over 12 months, up to 42 sessions | Usual care | Significant effect on verbal aggression but unclear if improvement/worsening | None |
| Music therapy | Choi et al., 2009166 | None | 5 | No | 10 | Music therapy three times per week for 5 weeks | Usual care, structured therapeutic sessions disallowed, weekly telephone call to maintain engagement | NC | NC |
| Music therapy | Lesta and Petocz, 2006167 | Some | 5 | Yes | 4 | Singing group at peak agitation time, over 4 consecutive days | No | NS (wandering) | None |
| Music therapy | Sherratt et al., 2004168 | Not specific | 5 | Mixed | 29 | One session each of live music, vs. taped music by a musician, vs. taped commercial music | No | NS (challenging behaviour, wandering) | None |
| Music therapy | Ziv et al., 2007169 | None | 5 | Yes | 28 | Taped popular music played to participants, once per week for 3 weeks | No | Significant improvement (negative behaviours) | None |
| Music therapy | Brotons and PickettCooper, 1996170 | Some | 4 | Yes | 27 | Five sessions of music therapy, time period unclear | No | Significant improvement | None |
| Music therapy | Cox et al., 2011171 | Some | 4 | Yes | 7 | Three individual violin recitals over 4 weeks | No | NS | None |
| Music therapy | Brotons and Marti, 2003172 | None | 3.5 | No | 14 | Music therapy plus interdisciplinary care in rural house over 12 days | No | Significant improvement (agitation, CG anxiety); CG burden and depression NS | Improved but unclear if significantly (2 months) |
| Music therapy | Zare, 2010173 | None | 3.5 | Yes | 16 | Individual preferred music, vs. group listening preferred music, vs. group listening of non-preferred music, and group singing of preferred music | Usual care | Significant effect but unclear if improvement/worsening | None |
| Music therapy | Suzuki et al., 2004174 | None | 3 | Yes | 23 | Music therapy twice per week for 8 weeks | Therapeutic physical activities (e.g. games) | NS (irritability vs. control) | None |
| Music therapy | Thomas et al., 1997175 | Some | 2.5 | Yes | 14 | Music played once while bathing | No | NS (total agitation) | None |
| Music therapy | Clair and Bernstein, 1994176 | Some | 2 | Yes | 28 | Stimulative music vs. sedative music in background, every day for 8 weeks | No | NS | NC |
| Music therapy or dementia specific therapy, plus support for the family | Berger et al., 2004177 | None | 4 | No | 36 | 1 year of carers’ support group plus either music therapy or cognitive stimulation therapy | Seen regularly at memory clinic | NS (agitation, CG burden and depression) | None |
| Other – activities, dementia specific therapy, and support for family | Droes et al., 2000178 | None | 3.5 | No | 66 | Approximately 91 day-care centre visits, supporting both CR and CG with dementia diagnosis | Usual care at daycentres | Significant improvement in non-social behaviour but not aggression | None |
| Other – TEACCH | Fischer-Terworth and Probst, 2011179 | None | 3 | Yes | 49 | 6 months of combination music therapy, cognitive stimulation, staff training and CBT principles | Non-specific, occupational interventions | Significant improvement | None |
| Reminiscence | Hall and Hare, 2011180 | None | 5 | Yes | 36 | Interactive reminiscence video | No | NS | None |
| Sensory | Burgio et al., 1996181 | Significant | 5 | Yes | 16 | Eight sessions of white noise over 10 days | No | Significant improvement in verbal agitation for nine selected ‘high responders’ | None |
| Sensory | Suzuki et al., 2010182 | None | 5 | Yes | 40 | 25 sessions of hand massage over 6 weeks | Usual care group not analysed | Significant improvement (aggression vs. baseline); activity disturbance NS | None |
| Sensory | Wang and Hermann, 2006183 | None | 5 | Yes | 6 | 28 sessions of healing touch over 4 weeks | No | Inappropriate analysis | None |
| Sensory | Milev et al., 2008184 | None | 4 | Yes | 18 | Snoezelen: either once or three times per week for 12 weeks | Usual care | Significant improvement in agitation/sleep | Significant improvement (12 weeks) |
| Sensory | Snyder et al., 1995185 | None | 4 | Yes | 18 | 10 daily sessions of hand massage; 10 sessions therapeutic touch | Crossover – usual care | NS | None |
| Sensory | Sutherland et al., 1999186 | Some | 3 | Yes | 10 | 10 daily sessions of foot acupressure and massage | Details unreported | NS (wandering) | NS wandering (3 days) |
| Simulated presence therapy | Woods and Ashley, 1995187 | Some | 4 | Yes | 9 | Simulated presence therapy while CR agitated (frequency NR) | No | Significant improvement | None |
| Simulated presence therapy | Miller et al., 2001188 | Some | 3 | Yes | 13 | One session simulated presence tape | No | Significant improvement | None |
| Special care unit putting in place a variety of interventions | Frisoni et al., 1998189 | Not specific | 4.5 | Yes | 66 | 3 months in special care unit with multidisciplinary support | Standard nursing care | NS (total agitation) | None |
| Special care unit putting in place a variety of interventions | Lawton et al., 1998190 | None | 4.5 | Yes | 182 | Special care unit with focus on appropriate levels of stimulation | Physically identical unit | NS | None |
| Special care unit putting in place a variety of interventions | Bellelli et al., 1998191 | Not specific | 4 | Yes | 55 | 6 months in special care unit with multidisciplinary support | No | Significant improvement in agitation, prescription of neuroleptic drugs/chemical restrains p.r.n. and restraint use; functional capacity NS | None |
| Training paid caregivers in care skills, with support and supervision | Davison et al., 2007192 | None | 5.5 | Yes | 113 | One group trained in care skills only; one group additionally given peer support | Usual care | NS | NS (6 months) |
| Training paid caregivers in challenging behaviour, without supervision | Asthill et al., 2004193 | Some | 2.5 | Yes | Unclear | Four training sessions on challenging behaviour, for two non-random groups | Usual care | Mixed – one group improved, one NS | Mixed – one group improved, one NS (4 weeks) |
| Training paid caregivers in CBT, without supervision | Ousset et al., 2003194 | None | 3 | Yes | 26 | Trained in CBT-based approach to behavioural symptoms | No | NR | Significant improvement (1 month) |
| Training paid caregivers in person-centred care, with supervision | Edberg and Hallberg, 2001195 | None | 4 | Yes | 22 | Trained to use individual care planning, 26 sessions of biweekly supervision | Usual care | Significant improvement; less sad and secluded in rooms than control | None |
| Training paid caregivers in person-centred care, without supervision | Sidani et al., 2012196 | None | 5.5 | Yes | 102 | Staff nurses given 2-hour training on person-centred care, cascaded to nursing assistants | No | NS | None |
| Training paid caregivers in person-centred care, without supervision | Cohen-Mansfield and Jensen, 2006197 | None | 4 | Yes | 20 | Changed care plan to be more similar to premorbid self-care routine | Crossover (usual care) – but only analysed within subjects | NS | None |
| Training paid caregivers to motivate PWD to improve their activities of daily living | Wells et al., 2000198 | None | 4.5 | Yes | 60 | Trained in restoring/promoting functional independence, plus reinforcement sessions | Usual care | Unclear | Mixed: one measure improved, one worsened (6 months) |
| Training paid caregivers to motivate PWD to improve their activities of daily living | Rogers et al., 1999199 | None | 2 | Yes | 84 | Trained in restoring/promoting functional independence, plus habit training follow-up | No | Disruptive behaviour improved | Unclear but disruptive behaviour did increase again (10 days) |
| Training paid givers in behavioural management, with supervision | Visser et al., 2008200 | Some | 4 | Yes | 76 | Trained in behavioural management, one group additionally given peer support | Usual care | NS (agitation, quality of life) | NS (3 and 6 months) |
| Training to allow and make wandering safe, without supervision | Cohen-Mansfield et al., 1997201 | None | 3 | Yes | Unclear | One session on safe management of wandering | No | Agitation NS; physical restraints worsened | Agitation NS; physical restraints worsened (4 weeks) |
Most level 4 studies which fitted our criteria had findings which were inconclusive or in line with the higher-quality studies. They are listed in Table 13 . Many of these tested activities, including pet therapy, in care home settings. 40,141–148 Only four had more than 30 participants and, as with the higher-quality studies, they found for most activities a significant improvement in some aspects of agitation or a reduction in the prescription of psychotropic medication. 40,145,163,164 Low-quality studies of sensory therapies had mixed results. 181–186 As expected, masking exits meant that people tried to exit less often, although putting tape in front of the exit to make a ‘two-dimensional barrier’ did not change the behaviour. 160–162
Two studies of aromatherapy158,159 found no significant effect on agitation. Light therapy remained ineffective in lower-quality studies. 149,150 Unlike the better-quality studies, a lower-quality study of DCM did not find a significant effect. 151 In contrast, one low-quality, low number study of exercise,152 two of simulated presence187,188 and one of home-like care153 were effective, unlike the higher-quality studies. Special care units had mixed results, with the larger studies not showing an effect. 189–191
In high-quality studies, training paid caregivers with supervision was effective, but this was not so in low-quality studies. 192,200 The small, low-quality studies of training paid caregivers with supervision had mixed results but most were negative. 193–199,201 Those of music therapy were small and not blinded to raters, sometimes incorporated other interventions, and had mixed results. 165–177,179
Low-quality studies of individualised interventions were ineffective,155–157 as was individualised activity154 and dementia-specific therapies, which were sometimes used in combination with other therapies. 177,178,180
Chapter 5 Health economic analysis
Introduction
In the study protocol we planned to include the following in the health economic analyses:
-
A systematic review of cost and cost-effectiveness studies of non-pharmacological interventions for reducing agitation in adults with dementia.
-
A detailed costing exercise to evaluate the NHS costs incurred by the provision of each of the interventions considered in the effectiveness review.
-
Construction of a de novo cost-effectiveness model to assess the cost-effectiveness of non-pharmacological interventions to reduce agitation in dementia. To do this we would:
-
– Design an appropriate model to characterise the health states of agitated patients. The proposed design was a Markov model allowing transition between agitation states.
-
– Populate the model using data from published studies and routine sources.
-
– Relate intermediate agitation outcomes to final outcomes, ideally expressed in terms of quality-adjusted life-years (QALYs).
-
– Identify which parameters are uncertain and which are important drivers of cost-effectiveness.
-
As explained below, we identified few economic studies in the systematic review for number 1.
As described in the effectiveness review, we identified a large number of interventions that had been evaluated and many of them were found to have no impact on agitation. In the costing exercise we undertook for number 2, we included only interventions that were shown to have a significant impact on agitation in our effectiveness review. This was because it is unlikely that additional data on the cost of ineffective interventions would affect whether or not these interventions would be implemented.
During the completion of number 3, we encountered a number of problems that limited our ability to meet the original aims described above.
First, as evidenced from our literature review, we found no studies that evaluated the impact of interventions in terms of QALYs. As a consequence we undertook two analyses. The first was a cost-effectiveness analysis measuring cost-effectiveness using intermediate outcome measures, that is to say measures of agitation reported in the effectiveness studies included in the systematic review (e.g. incremental cost per unit improvement in CMAI score). This allows us to examine which interventions for managing agitation represent the best value for money among all such interventions, but it does not allow us to say whether or not any of these interventions are good value for money for the NHS because cost-effectiveness thresholds for these outcomes do not exist. The second analysis we undertook was a cost–utility analysis, seeking to measure the impact of interventions on QALYs. We did this using a two-stage process. At the first stage, we evaluated the impact of the interventions on measures of agitation (e.g. NPI agitation scores), as in our effectiveness review. At the second stage, we used supplementary data to model the impact of changes in agitation on QALYs. We identified a single data source we could use for this analysis – the LASER-AD (London and the South-East Region – Alzheimer’s Disease) study. 10 This was a cohort study of patients with Alzheimer’s disease from London and the south-east region of the UK, who were followed for 54 months. At the last follow-up point in the study, data were collected on NPI agitation scores and a measure of health-related quality of life for people with dementia – the dementia quality of life (DEMQOL) system. We used recently published methods to convert DEMQOL-Proxy values submitted by carers into utilities suitable for estimating QALYs. This approach provided a method for estimating the impact of interventions on QALYs, but it limited the studies we could include in the cost-effectiveness analysis because we could use only studies that evaluated intermediate outcomes using the agitation measure recorded in the LASER-AD study, that is to say NPI agitation scores.
Second, we found no studies that evaluated the impact of non-pharmacological interventions on the full range of health and social care costs associated with agitation that would be relevant in a UK context. As above, we undertook two analyses. In the cost-effectiveness analysis, which we undertook for all effective interventions, we only included intervention costs, assuming that the impact of the interventions on the costs of managing agitation would be zero. For the cost–utility analyses, we adopted the same two-stage process we used to estimate QALYs, first evaluating the impact of interventions on agitation, and then using the LASER-AD study to model the impact of changes in agitation on health and social care costs (which are covered comprehensively in that study). This allowed us to account for the impact of interventions on a comprehensive range of health and social care costs but, as above, it limited the interventions we could evaluate.
Third, with regards the time horizon of our model, as evidenced in the effectiveness review, we found very little evidence supporting the long-term effectiveness of interventions. In the effectiveness review, four types of intervention had a significant effect on agitation. In the case of activities, only two studies measured agitation after the intervention period, at 1 week50 and 4 weeks48 afterwards, and neither continued to be effective at those times. For music therapy, three studies measured longer-term outcomes, at 3 to 8 weeks beyond the end of the intervention,56,60,62 and none continued to be effective. For sensory interventions, three studies evaluated outcomes beyond the end of the intervention period, between 1 and 3 weeks later;63,64,68 there was some evidence that the interventions continued to be effective at that time. In terms of person-centred care and communication skills and DCM, six studies found the improvements in agitation that persisted up to 6 months beyond the end of the intervention. 75–77,79,81,82 In the cost-effectiveness analysis, we limited the time horizon to the time period used to measure outcomes in each study included in the review; where short- and long-term outcomes were reported, we focused on the long-term outcomes. In the cost–utility analysis, given that we found no evidence of effect of interventions beyond 1 year, we limited our time horizon to a 1-year period. In this period we accounted for the time it takes from the start of when the intervention is delivered until the benefits are achieved, and for the time it takes beyond the end of the delivery of the intervention for the effects to diminish.
Fourth, we were unable to find any data on the movement between agitation states over time. This meant that it was not possible to measure value for money in the cost–utility analysis using a Markov model, as originally planned. In the LASER-AD study, NPI agitation scores were measured at repeated points in time, but the time period between measurements was 12–18 months, and what happened between those times was unknown. In our cost–utility analysis, we used a different modelling approach that accounted for the impact of interventions on agitation levels over a 12-month period, and consequent improvements in utility and decreases in health and social care costs achieved before agitation levels were assumed to return to the original levels.
The upshot is that we completed number 3, but we did it using a different approach than originally anticipated in the study protocol. For the interventions identified as being effective in the systematic review, we undertook a cost-effectiveness analysis measuring the incremental cost per unit improvement in agitation. We also undertook a cost–utility analysis measuring the incremental cost per QALY gained, but we were limited in the interventions we could evaluate using this approach.
Aims
Accounting for the above, the aims of the health economic analysis were to:
-
review cost and cost-effectiveness studies of non-pharmacological interventions for reducing agitation in adults with dementia
-
undertake analyses of the cost of non-pharmacological interventions for reducing agitation in adults with dementia
-
undertake a cost-effectiveness analysis of non-pharmacological interventions for reducing agitation in adults with dementia, measuring the incremental cost per unit improvement in agitation
-
construct a cost-effectiveness model to undertake a cost–utility analysis of non-pharmacological intervention for reducing agitation in adults with dementia, measuring the incremental cost per QALY gained.
To achieve the fourth aim we were required to:
-
5. undertake an analysis of health and social care costs associated with agitation
-
6. undertake an analysis of the change in health-related quality of life associated with agitation.
The methods and results for each of these components are described separately below.
Systematic review of cost and cost-effectiveness studies
Methods
We undertook a systematic review of cost and cost-effectiveness studies of non-pharmacological interventions for reducing agitation in adults with dementia. Studies with analyses of costs and cost-effectiveness identified using the strategy described for the effectiveness review were included. In addition, we searched (on 13 March 2012) the NHS Economic Evaluation Database, the HTA programme database and the DARE using the search terms agitat* and dement*. Reference lists of included papers were searched by hand. Our inclusion and exclusion criteria were as described for the effectiveness review above, plus we only included studies that examined the cost and/or cost-effectiveness of interventions. In particular, we rejected studies that were not attempting to reduce agitation, that were not undertaken in people with dementia, that were not non-pharmacological, and that had no health economics component.
NP and SM devised the search strategy. NP conducted the searches and screened the abstract and full text of identified papers. Where inclusion was unclear, this was discussed with SM. NP extracted the data from the included papers.
The quality of the included studies was assessed by applying them to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist. 202
Data were extracted from the included papers on:
-
type of analysis (e.g. cost analysis, cost-effectiveness analysis)
-
descriptors of the intervention and comparator
-
categorisation of the intervention, using the same taxonomy that was used in the effectiveness review
-
country in which the analysis was undertaken, the currency and the study year
-
cost components included in the analysis
-
time horizon
-
summary economic measure
-
results, and
-
how uncertainty was analysed.
To inform our subsequent analysis, we were particularly interested in studies that had measured outcomes in terms of QALYs, that measured health and social care costs comprehensively from a UK perspective, and that measured cost-effectiveness over a long time period.
Results
Cost-effectiveness data from pre-existing studies were limited. We found 688 potentially relevant records, excluded 679 abstracts and seven full papers, and included two papers in the final review ( Figure 2 ). 203,204
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram for review of cost and cost-effectiveness studies.
These studies were, first, a US blended inpatient and outpatient programme to reduce hospitalisation,203 and second, an Australian comparison of DCM and person-centred care programmes ( Table 14 ). 204 The first study is a standalone study. The second study is linked with a clinical trial included in the effectiveness review.
| Authors | Type of analysis | Intervention | Category | Country (currency; year) | Cost components | Time horizon | Summary economic measure | Results | Analysis of uncertainty |
|---|---|---|---|---|---|---|---|---|---|
| Mintzer et al., 1997203 | CEA | Continuum-of-care programme vs. inpatient programme | Changing the environment; DCM | USA (USD; NR) | Inpatient and partial hospitalisation | 21 days | Change in total CMAI for $1000 expenditure | Continuum-of-care programme 0.89; inpatient programme 0.27 | None |
| Norman et al., 2008204 | CEA | Training paid caregivers in person-centred care or dementia-care mapping vs. usual care | Training paid caregivers in person-centred care; DCM | Australia (AUD; NR) | Trainer time to educate and support staff, time staff spent on activities specific to intervention, replacement costs of backfilling positions while staff received training, pharmaceutical use | 8 months | Cost per CMAI score change | The cost per CMAI point averted for person-centred care relative to usual care was A$8.01 immediately after intervention and A$6.43 at follow-up; for DCM these values were A$48.95 and A$46.89 respectively | Univariate sensitivity analysis |
Comparing each study with the CHEERS checklist (see Appendix 4 , Tables 29 and 30 ), the first study was deemed to be low quality due to the extent of missing details about the analysis, and its limited scope. The second study was deemed to be of medium quality; the study was well described but limited in scope.
The range of cost components included in both studies was narrow; the first study included only hospital costs, and found that for $1000 of expenditure, the blended inpatient and outpatient programme produced a change of 0.89 in the CMAI scale, compared with 0.27 for the inpatient programme. The second study included intervention costs and pharmaceutical use and a univariate sensitivity analysis. This measured the cost per CMAI point averted, compared with usual care immediately and at follow-up. Person-centred care costs were A$8.01 immediately, and A$6.43 at follow-up, and DCM costs were A$48.95 and A$46.78, respectively. In both cases, it was difficult to identify if the intervention was cost-effective because the cost-effectiveness threshold for the summary economic measure is unknown. In terms of informing our de novo cost-effectiveness model, neither study measured cost-effectiveness in terms of QALYs, neither considered a comprehensive range of health and social care costs, and neither was UK based. In both studies, the time horizon was < 1 year.
Analysis of the cost of non-pharmacological interventions
Methods
We calculated the NHS and Personal Social Services (PSS) costs in 2011 GBP incurred in the provision of every intervention identified in the effectiveness review that had a significant impact on agitation, where there was evidence that the intervention type had a positive impact on agitation (30 interventions in total). We did not calculate costs for ineffective interventions, as it is unlikely that they would be implemented and so further analysis would be redundant.
Resource use was determined by the intervention descriptors in the included studies. The cost components included in the analysis were limited to the components of the intervention described in each study. The main cost components were staff costs, usually nursing staff. For UK studies, it was usually possible to identify the type of staff involved. For non-UK studies, we made assumptions about the type of staff who would typically be involved if the intervention were provided in the UK context. Durable items were generally inexpensive (e.g. a CD player), and we assumed that these items did not last beyond the end of the intervention described in the study. For group interventions, we calculated the total cost of the intervention and then divided this by the group size as reported in the study. Unit costs were taken from market prices and routine sources. Where cost components were not adequately described, or unit costs were not directly available, we selected the unit cost based on the closest similar service or item from available published unit cost estimates.
Results
We calculated the costs of 30 interventions from 26 studies that had a significant impact on agitation (see Appendix 5 , Tables 31–34 and Box 1 for further details). 43–49,52,54,57,61,63,64,66,69,71–82 Costs ranged from £80 to £696 for activities, from £13 to £27 for music therapy, from £3 to £527 for sensory interventions and from £31 to £339 for training and supervising paid caregivers in person-centred care or communication skills, with or without behavioural management training, and DCM. Costs for individual studies are reported alongside the results of the effectiveness review in Tables 1–5 .
Cost-effectiveness analysis of non-pharmacological interventions for managing agitation
Methods
We calculated the cost-effectiveness of non-pharmacological interventions for managing agitation using the results of the cost analysis described in the previous section. These costs were combined with the outcomes obtained from the effectiveness review to measure the incremental cost per unit improvement in agitation. Effectiveness was measured using difference-in-differences, that is to say the before-and-after change in outcomes in the intervention group minus the before-and-after change in outcomes in the control group. We limited the time horizon to the time period used to measure outcomes in each study included in the review; where short- and long-term outcomes were reported, we focused on the long-term outcomes. As in the effectiveness review, we recalculated some study outcomes, for example for studies including intervention and control groups but not directly comparing them. Cost-effectiveness was computed by dividing the unit cost of each intervention by the change in agitation outcome. While providing a measure of cost-effectiveness, the analysis is limited in its usefulness for the following reasons:
-
Incremental costs are measured in terms of the costs of the intervention only; costs incurred from managing agitation are not included. As discussed above, this is because data were not available on the health and social care costs associated with managing agitation. We provide new estimates for these costs (see Results, below), but their application is limited because they can be applied only to interventions that are evaluated intermediate outcomes using the agitation measure recorded in the LASER-AD study, that is to say NPI agitation scores. If, as is shown below, health and social care costs decline when agitation is reduced, then measuring the incremental costs of an intervention in terms of the direct costs of the intervention only is likely to overestimate the incremental costs of that intervention.
-
Incremental effectiveness is measured in terms of the unit improvement in agitation. As discussed above, this is because data were not available on the QALYs associated with managing agitation. As with the health and social care costs, we provide new estimates of the utility associated with different levels of agitation (see below), but the application of these is also limited because they can be applied only to interventions that are evaluated intermediate outcomes using NPI agitation scores. Focusing on improvements in agitation means that comparisons with other interventions are limited: first, it is not possible to compare non-pharmacological interventions for managing agitation with interventions for other health problems because the outcomes are not commensurate; second, several agitation measures are used in the studies included in the effectiveness review, and it is not possible to make judgements about the relative effectiveness of interventions that are evaluated using different agitation measures because the measures are not commensurate.
-
Third, incremental cost-effectiveness is measured in terms of the incremental cost per unit improvement in agitation. This allows us to make limited judgements about which interventions for managing agitation represent the best value for money among the interventions included in the effectiveness review – the interventions with the lowest incremental cost per unit improvement in agitation, where agitation is measured using the same outcome measure – but it does not allow us to say whether or not any of these interventions are good value for money to the NHS because cost-effectiveness thresholds for these outcomes do not exist.
As explained, in the following sections we present a framework for undertaking cost–utility analyses of interventions for managing agitation that includes the full range of health and social care costs and measures outcomes in terms of QALYs.
Results
The results of the cost-effectiveness analyses are reported in Table 15 . Of the 30 effective interventions for which we calculated costs in the previous section, relevant outcomes were quantitatively reported in the published studies for 23 interventions. 43–49,52,54,57,61,63,64,66,69,71–82 Outcomes were measured in terms of the CMAI (11 interventions), the modified CMAI (five interventions), CMAI subscales (two interventions) and the CMAI-Short form,40 Pittsburgh Agitation Scale,205 Agitated Behaviour Rating Scale,206 Agitated Behaviour in Dementia207 and the Ryden Aggression Scale (all one study each). 208 Table 14 reports the change (difference-in-difference) in these outcome measures, computed for each intervention.
| Author | Unit cost (2011 £) | Outcome measure(s) | Change in outcome | Incremental cost per unit improvement in outcome |
|---|---|---|---|---|
| Activities | ||||
| Lin et al.44 | 372 | CMAI | –2.3 | 162 |
| Lee and Kim45 | 274 | Modified CMAI | –1.9 | 144 |
| Buettner and Ferrario46 | 696 | CMAI | –0.2 | 3480 |
| Fitzsimmons and Buettner47 | 173 | CMAI | –0.3 | 577 |
| Music therapy using a specific protocol | ||||
| Sung et al. 55 | 13 | Modified CMAI | –1.0 | 13 |
| Lin et al.57 | 27 | CMAI | –7.7 | 4 |
| Sensory interventions | ||||
| Moyle et al. 63 | 128 | CMAI-Short Form | –4.6 | 28 |
| Yang et al.64 | 527 | CMAI | –21.6 | 24 |
| Remington66 | ||||
| Hand massage | 3 | Modified CMAI | –6.24 | 1 |
| Hand massage + calming music | 10 | Modified CMAI | –13.5 | 1 |
| Staal et al. 69 | 273 | Pittsburgh Agitation Scale | –0.8 | 341 |
| Lin et al.44 | 300 | CMAI | –2.1 | 143 |
| Woods and Dimond71 | 32 | Agitated Behaviour Rating Scale | –0.03 | 1067 |
| Whall et al. 73 | 30 | Modified CMAI | –6.7 | 4 |
| van Weert et al. 74 | 109 | CMAI subscales (aggressive behaviour; physically non-aggressive behaviour; verbally agitated) | Aggressive behaviour = –3.0; physically non-aggressive behaviour = –0.03; verbally agitated = –0.8 | Aggressive behaviour = 36; physically non-aggressive behaviour = 3633; verbally agitated = 136 |
| Training paid caregivers in person-centred care or communication skills, with supervision, or in DCM with supervision | ||||
| Chenoweth et al.75 | 99 | CMAI | –17.7 | 6 |
| McCallion et al. 76 | 87 | CMAI subscales (physically aggressive behaviour; physically non-aggressive behaviour) | Physically aggressive behaviour = +0.5; physically non-aggressive behaviour = –5.2 | Physically aggressive behaviour = DOM;a physically non-aggressive behaviour = 17 |
| McCallion et al.77 | 72 | CMAI | –1.7 | 42 |
| Teri et al. 78 | 339 | Agitated Behaviour In Dementia | –3.4 | 100 |
| Hoeffer et al. 79 | 158 | Ryden Aggression Scale physical aggression; Ryden Aggression Scale verbal aggression | Ryden Aggression Scale physical aggression = –1.4; Ryden Aggression Scale verbal aggression = –0.3 | Ryden Aggression Scale physical aggression = 113; Ryden Aggression Scale verbal aggression = 527 |
| Deudon et al.81 | 31 | CMAI | –0.5 | 62 |
| Chenoweth et al.75 | 108 | CMAI | –9.8 | 11 |
| Chenoweth and Jeon82 | 127 | CMAI | –11.9 | 11 |
Cost-effectiveness is reported as the incremental cost per unit improvement in outcome. It is not appropriate to compare interventions that are evaluated using different outcome measures because the outcomes are not commensurate. As noted, the most common outcome measure was the CMAI. Among the 11 interventions that were evaluated using this measure, the incremental cost per unit reduction in CMAI score ranged from £162 to £3480 for activities, £4 for music therapy, from £24 to £143 for sensory interventions and from £6 to £62 for training paid caregivers in person-centred care or communication skills with or without behavioural management training, with supervision, and for DCM.
These findings suggest that, among the different types of interventions included in the effectiveness review that measured outcomes in terms of the CMAI, music therapy and training paid caregivers in person-centred care or communication skills with or without behavioural management training, with supervision, and for DCM appear to be the most cost-effective options. However, as we have pointed out above, it is not possible to say whether or not they represent good value for money to the NHS, because we do not know what the NHS is willing to pay is for a one-unit reduction in CMAI score (i.e. we do not know what the cost-effectiveness threshold is). We explore this issue in more detail the following sections.
Analysis of health and social care costs associated with agitation
Methods
We calculated the NHS and PSS costs associated with different levels of agitation measured by the NPI209 agitation scale using data from a cohort study of patients with Alzheimer’s disease. 210 The LASER-AD study recruited 224 people with Alzheimer’s disease between July 2002 and January 2003 from London and the south-east region, UK, and followed them for 54 months. Data on NPI agitation scores and health and social care resource use were collected at baseline (recruitment) and 18, 30, 42 and 54 months. One hundred and eleven participants had died by 54 months; our data set had 695 data points (person follow-ups).
Resource use was measured using the Client Service Receipt Inventory, amended for use with older people,211 for the previous 3 months from participant responses and caregiver reports on where the person was living (at home; in residential respite care; in day respite care; in a residential home; in a nursing home; in sheltered housing; in supported (extra care sheltered) housing; or in hospital awaiting placement), and their contacts with health and social care services [general practitioner (GP), practice nurse, district nurse, dietitian, community psychiatric nurse, home help, meals on wheels, physiotherapist, chiropodist, optician, dentist, audiologist, psychologist, psychiatrist, day centre, hospital outpatient visits and inpatient stays].
We applied unit costs from routine sources212 in 2011 GBP and calculated 3-month costs for each participant at each follow-up point. We then pooled these data and analysed the 3-month costs by agitation level, controlling for a range of covariates including the follow-up point, and adjusting for individual-level clustering.
Agitation was measured according to the NPI agitation score at each follow-up point, which could take one of nine values (0, 1, 2, 3, 4, 6, 8, 9, 12), with higher values indicating more severe levels of agitation.
To account for the skewness of the cost data, we regressed the 3-month total cost variable against the agitation measures using a generalised linear model with gamma family and log link,213 controlling for sex, age at baseline (50–59 years, 60–60 years, 70–79 years, 80–89 years, 90–99 years), cognitive impairment [measured according to Mini-Mental State Examination (MMSE);214 severe ≤ 9 points, moderate 10–20 points, mild 21–30 points], and follow-up point (baseline, 18 months, 30 months, 42 months, 54 months). We adjusted for clustering by participant. We calculated predictive margins for NPI agitation scores; these are the predicted 3-month health and social care costs by NPI agitation score, controlling for the covariates.
Results
The unit costs we applied to the resource use data in the LASER-AD study are in Table 16 . Unadjusted mean SD and median (interquartile range) 3-month costs across all 695 observations were £7749 (£10,273) and £6814 (£1073 to £9757), respectively ( Table 17 ). Unadjusted costs increased with agitation. The 3-month cost data were highly skewed ( Figure 3 ).
| Cost component | Unit cost (£) | Unit | Source |
|---|---|---|---|
| GP | 36.00 | Per surgery consultation | Curtis212 |
| Practice nurse/district nurse | 60.00 | Per hour | Curtis212 |
| Dietitian | 35.00 | Per hour | Curtis212 |
| Community psychiatric nurse | 50.00 | Per hour | Curtis212 |
| Occupational therapist | 82.00 | Per hour | Curtis212 |
| Home help | 27.00 | Per hour | Curtis212 |
| Meals on wheels | 6.14 | Per meal | Curtis212 |
| Physiotherapist | 34.00 | Per hour | Curtis212 |
| Chiropodist | 31.00 | Per hour | Curtis212 |
| Optician | 57.00 | Per contact | NHS Reference Costs215 |
| Dentist | 92.00 | Per contact | NHS Reference Costs215 |
| Audiologist | 67.00 | Per contact | NHS Reference Costs215 |
| Psychiatrist | 418.00 | Per contact | Curtis212 |
| Psychologist | 135.00 | Per hour | Curtis212 |
| Day centre | 34.00 | Per visit | Curtis212 |
| Hospital outpatient visit | 100.00 | Per visit | Curtis212 |
| Hospital inpatient stay | 321.00 | Per day | Curtis212 |
| Residential respite care | 104.71 | Per overnight stay | Curtis212 |
| Day respite care | 95.71 | Per day | Curtis212 |
| Part III residential home | 519.00 | Per week | Curtis212 |
| Nursing home | 741.00 | Per week | Curtis212 |
| Part II sheltered housing | 155.00 | Per week | Curtis212 |
| Supported living part II and a half | 418.00 | Per week | Curtis212 |
| Hospital awaiting placement | 321.00 | Per day | Curtis212 |
| NPI agitation score | Total 3-month cost per person (2011 £) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Median | 25th percentile | 75th percentile | Observations | |
| 0 | 7054 | 10,833 | 3490 | 762 | 9111 | 314 |
| 1 | 5649 | 6066 | 5588 | 667 | 8037 | 68 |
| 2 | 7386 | 8607 | 6832 | 2137 | 9772 | 60 |
| 3 | 7413 | 5770 | 7054 | 2272 | 9769 | 51 |
| 4 | 6977 | 5838 | 6892 | 1699 | 9682 | 60 |
| 6 | 8831 | 10,222 | 6912 | 2430 | 9757 | 57 |
| 8 | 10,572 | 12,174 | 7769 | 5904 | 10,633 | 45 |
| 9 | 11,647 | 10,325 | 9847 | 4504 | 16,182 | 12 |
| 12 | 15,266 | 19,018 | 9198 | 6781 | 11,117 | 28 |
| All | 7749 | 10,273 | 6814 | 1073 | 9757 | 695 |
FIGURE 3.
Distribution of total 3-month costs per person in LASER-AD (695 observations).
Descriptive statistics for the variables used in regression analysis are given in Table 18 . The mode and median NPI agitation score are 0 and 17, respectively. The majority of the sample was female, and aged ≥ 70 years. In 21% of observations, the patient had mild cognitive impairment, compared with 35% for moderate cognitive impairment and 44% for severe cognitive impairment.
| Variables | Frequency | Per cent |
|---|---|---|
| NPI agitation scores | ||
| 0 | 314 | 45 |
| 1 | 68 | 9 |
| 2 | 60 | 8 |
| 3 | 51 | 7 |
| 4 | 60 | 8 |
| 6 | 57 | 8 |
| 8 | 45 | 6 |
| 9 | 12 | 1 |
| 12 | 28 | 4 |
| Sex | ||
| Male | 195 | 28 |
| Female | 500 | 72 |
| Age category (years) | ||
| 50–59 | 10 | 1 |
| 60–69 | 52 | 7 |
| 70–79 | 258 | 37 |
| 80–89 | 307 | 44 |
| 90–99 | 68 | 9 |
| Cognitive impairment | ||
| Mild (MMSE 21–30) | 148 | 21 |
| Moderate (MMSE 10–20) | 243 | 35 |
| Severe (MMSE ≤ 9) | 304 | 44 |
| Follow-up | ||
| Baseline | 203 | 29 |
| 18 months | 165 | 23 |
| 30 months | 132 | 19 |
| 42 months | 110 | 16 |
| 54 months | 85 | 12 |
Based on the regression results, after adjusting for sex, age, cognitive impairment, follow-up and individual clustering, NHS and PSS costs increase with NPI agitation scores, from around £7000 over a 3-month period with clinically non-significant agitation symptoms (NPI agitation score = 0) up to around £15,000 at the most severe levels of agitation (NPI agitation score = 12; Table 19 ). The CIs are wider at higher NPI agitation scores, possibly due to the smaller number of observations with these values. Costs are also higher for females than males, are highest in the youngest age category (50–59 years), increase with cognitive impairment, and do not vary by follow-up point.
| Clinical variables | Mean (95% CI) | Standard error |
|---|---|---|
| NPI agitation scores | ||
| 0 | 7266 (6012 to 8521) | 640 |
| 1 | 7006 (5186 to 8827) | 929 |
| 2 | 8761 (6220 to 11,301) | 1296 |
| 3 | 7298 (5507 to 9089) | 914 |
| 4 | 8557 (6021 to 11,093) | 1294 |
| 6 | 6762 (4871 to 8653) | 965 |
| 8 | 7802 (5956 to 9647) | 942 |
| 9 | 12,806 (5618 to 19,994) | 3667 |
| 12 | 15,148 (4592 to 25,705) | 5386 |
| Sex | ||
| Male | 6215 (4803 to 7627) | 720 |
| Female | 8587 (7250 to 9924) | 682 |
| Age category (years) | ||
| 50–59 | 17,832 (–2947 to 38,611) | 10,602 |
| 60–69 | 5373 (2902 to 7843) | 1261 |
| 70–79 | 6997 (5512 to 8482) | 758 |
| 80–89 | 8617 (6938 to 10,297) | 857 |
| 90–99 | 8958 (5839 to 12,077) | 1592 |
| Cognitive impairment | ||
| Mild (MMSE 21–30) | 2442 (1633 to 3250) | 413 |
| Moderate (MMSE 10–20) | 5720 (4585 to 6854) | 579 |
| Severe (MMSE ≤ 9) | 12,124 (10,152 to 14,096) | 1006 |
| Follow-up | ||
| Baseline | 8558 (6782 to 10,335) | 906 |
| 18 months | 8107 (6493 to 9721) | 823 |
| 30 months | 7926 (6452 to 9399) | 752 |
| 42 months | 7958 (6220 to 9696) | 887 |
| 54 months | 6906 (5683 to 8129) | 624 |
Analysis of health-related quality of life associated with agitation
Methods
We calculated health-related quality of life associated with different levels of agitation measured by the NPI also using the LASER-AD study. We wished to use a preference-based single index measure suitable for calculating QALYs. In the last follow-up in the LASER-AD study (at 54 months), data were collected for the DEMQOL system. This system was developed to generate a measure of health-related quality of life for people with dementia by using patient self-report and carer proxy report. It consists of two interviewer-administered instruments: DEMQOL, which is self-reported by the patient, and DEMQOL-Proxy, which is reported by a carer. 216,217 The system was developed to be used across all types of dementia, care arrangements and levels of severity, and it has been shown to be reliable and valid. DEMQOL data were available for only 28 study participants in the 54-month follow-up in LASER-AD; DEMQOL-Proxy data were available for 84 study participants, and so we focused on the latter. The DEMQOL-Proxy contains 31 items, scored from 1 to 4.
We converted the DEMQOL-Proxy data into a preference-based single index measure suitable for calculating QALYs using a recently published method. This has two stages. At the first stage, the DEMQOL-Proxy was converted into a carer-reported health state classification system using Rasch analysis. A four-dimension classification system was developed using four items of the DEMQOL-Proxy, classifying positive emotion, cognition, appearance and negative emotion. At the second stage, a valuation study of the classification system was undertaken, using 287 members of the general population and the time-trade-off technique. 216 Regression analysis was used to derive preference weights for the carer-reported health state classification system based on the DEMQOL-Proxy, called the DEMQOL-Proxy-U. 216,217
We used the classification system developed by Mulhern et al. ,218 and calculated DEMQOL-Proxy-U values using the preferred regression model (7) reported by Rowen et al. 216
Results
Once we had computed the DEMQOL-Proxy-U scores, we regressed this variable against the agitation measures using a Tobit regression model, controlling for sex, age at baseline and cognitive impairment. We used Tobit regression to account for the censoring of DEMQOL-Proxy-U scores; the feasible range of values using the preferred regression model in Rowen et al. 216 is 0.357 to 0.918; hence, these values were set as the lower and upper limits for censoring in the Tobit model, respectively. We adjusted for clustering by participant. We calculated predictive margins for NPI agitation scores; this is the predicted DEMQOL-Proxy-U score by NPI agitation score, controlling for the covariates.
Unadjusted mean (SD) and median (interquartile range) DEMQOL-Proxy-U scores across the 84 observations were 0.743 (0.118) and 0.730 (0.646 to 0.845), respectively ( Table 20 ). Unadjusted DEMQOL-Proxy-U scores were lower at the highest levels of agitation. The range of values was 0.468 to 0.937 ( Figure 4 ).
| NPI agitation score | DEMQOL-Proxy-U score | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Median | 25th percentile | 75th percentile | Observations | |
| 0 | 0.748 | 0.132 | 0.808 | 0.646 | 0.845 | 26 |
| 1 | 0.720 | 0.111 | 0.679 | 0.655 | 0.845 | 11 |
| 2 | 0.798 | 0.081 | 0.819 | 0.730 | 0.860 | 12 |
| 3 | 0.747 | 0.085 | 0.754 | 0.672 | 0.819 | 10 |
| 4 | 0.844 | 0.079 | 0.844 | 0.788 | 0.900 | 2 |
| 6 | 0.696 | 0.116 | 0.679 | 0.599 | 0.800 | 12 |
| 8 | 0.777 | 0.166 | 0.874 | 0.646 | 0.900 | 5 |
| 9 | 0.696 | 0.107 | 0.646 | 0.624 | 0.819 | 3 |
| 12 | 0.672 | 0.162 | 0.646 | 0.525 | 0.845 | 3 |
| All | 0.743 | 0.118 | 0.730 | 0.646 | 0.845 | 84 |
FIGURE 4.
Distribution of DEMQOL-Proxy-U scores (84 observations). The feasible range of values is 0.357 to 0.918.
Descriptive statistics for the variables used in regression analysis are in Table 21 . In these data, the mode and median NPI agitation scores are 0 and 2, respectively. As before, the majority of the sample were female and aged 70 years or over. In 42% of observations, the patient had mild cognitive impairment, compared with 36% for moderate cognitive impairment and 23% for severe cognitive impairment.
| Clinical variables | Frequency | Per cent |
|---|---|---|
| NPI agitation scores | ||
| 0 | 26 | 31 |
| 1 | 11 | 13 |
| 2 | 12 | 14 |
| 3 | 10 | 12 |
| 4 | 2 | 2 |
| 6 | 12 | 14 |
| 8 | 5 | 6 |
| 9 | 3 | 4 |
| 12 | 3 | 4 |
| Sex | ||
| Male | 24 | 29 |
| Female | 60 | 71 |
| Age category (years) | ||
| 50–59 | 2 | 2 |
| 60–69 | 8 | 10 |
| 70–79 | 32 | 38 |
| 80–89 | 36 | 43 |
| 90–99 | 6 | 7 |
| Cognitive impairment | ||
| Mild (MMSE 21–30) | 35 | 42 |
| Moderate (MMSE 10–20) | 30 | 36 |
| Severe (MMSE ≤ 9) | 19 | 23 |
Based on the regression results, after adjusting for sex, age, cognitive impairment, and individual clustering, there is some evidence that DEMQOL-Proxy-U decline with NPI agitation scores, from a score of around 0.75 with clinically non-significant agitation symptoms (NPI agitation score = 0) to around 0.65 at the most severe levels of agitation (NPI agitation score = 12; Table 22 and Figure 5 ) but the 95% CIs overlap, and there is not a clear trend between the lowest and highest NPI agitation scores, possibly due to the relatively small number of observations.
| Clinical variables | Mean (95% CI) | Standard error |
|---|---|---|
| NPI agitation scores | ||
| 0 | 0.757 (0.711 to 0.803) | 0.023 |
| 1 | 0.729 (0.658 to 0.800) | 0.036 |
| 2 | 0.805 (0.755 to 0.854) | 0.025 |
| 3 | 0.733 (0.682 to 0.784) | 0.026 |
| 4 | 0.869 (0.774 to 0.964) | 0.048 |
| 6 | 0.677 (0.619 to 0.735) | 0.030 |
| 8 | 0.780 (0.674 to 0.886) | 0.054 |
| 9 | 0.679 (0.589 to 0.769) | 0.046 |
| 12 | 0.654 (0.501 to 0.808) | 0.078 |
| Sex | ||
| Male | 0.733 (0.689 to 0.777) | 0.022 |
| Female | 0.748 (0.721 to 0.775) | 0.014 |
| Age category (years) | ||
| 50–59 | 0.581 (0.498 to 0.664) | 0.042 |
| 60–69 | 0.786 (0.732 to 0.840) | 0.028 |
| 70–79 | 0.757 (0.719 to 0.795) | 0.019 |
| 80–89 | 0.724 (0.686 to 0.761) | 0.019 |
| 90–99 | 0.788 (0.732 to 0.844) | 0.028 |
| Cognitive impairment | ||
| Mild (MMSE 21–30) | 0.717 (0.679 to 0.755) | 0.019 |
| Moderate (MMSE 10–20) | 0.774 (0.734 to 0.814) | 0.020 |
| Severe (MMSE ≤ 9) | 0.744 (0.698 to 0.791) | 0.024 |
FIGURE 5.
Adjusted mean DEMQOL-Proxy-U score by NPI agitation score.
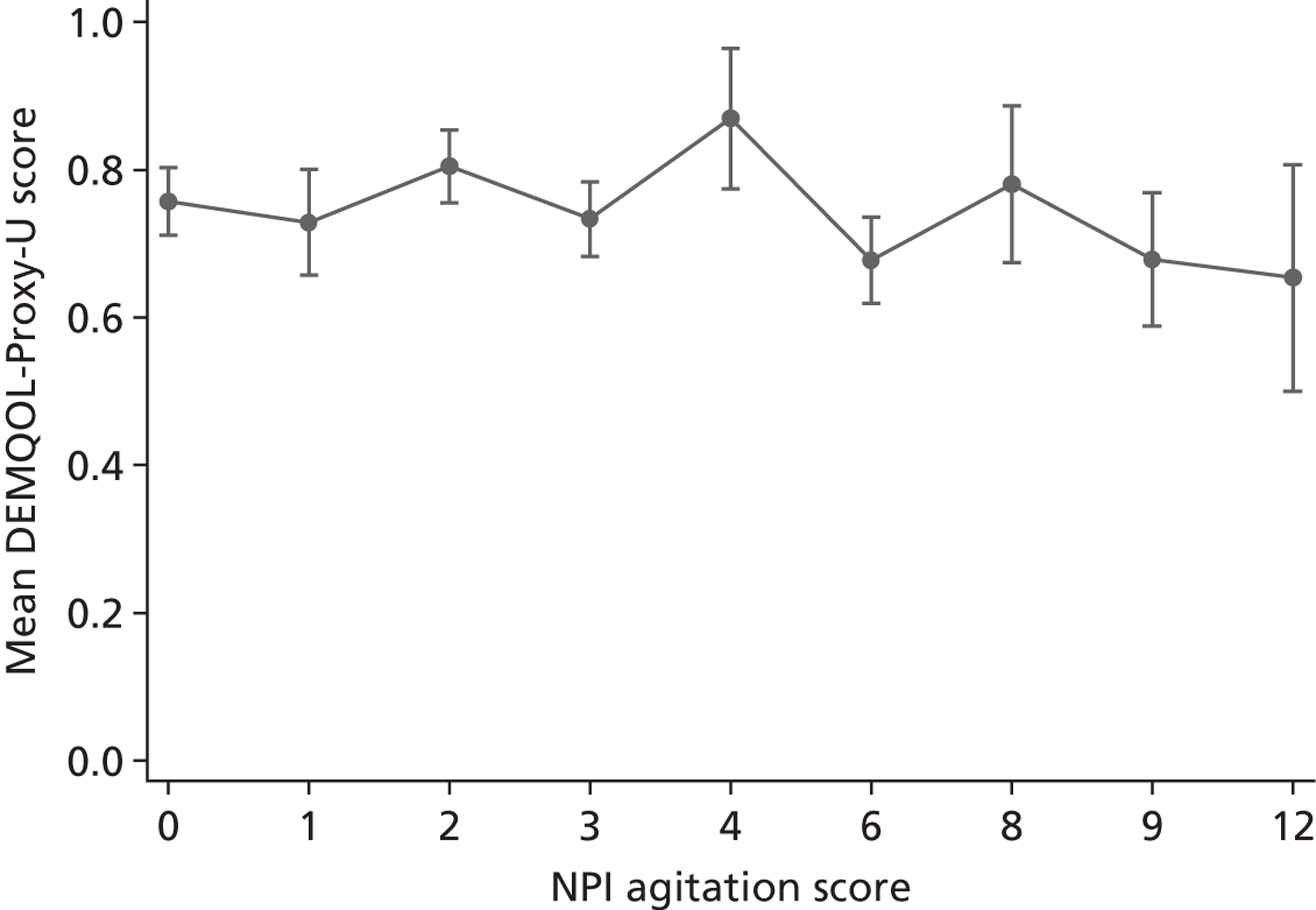
Cost–utility analysis of non-pharmacological interventions for managing agitation
Introduction
In this section, we describe how we constructed an economic model to undertake a cost–utility analysis of a non-pharmacological intervention for reducing agitation in adults with dementia. As discussed, our initial plans as described in the study protocol were not feasible due to lack of data about the impact of non-pharmacological interventions for reducing agitation on QALYs; the impact of non-pharmacological interventions on the full range of health and social care costs associated with agitation that would be relevant in a UK context; time horizon beyond 12 months; and transition probabilities necessary to construct a Markov model. This analysis addresses the limitations of the cost-effectiveness analysis reported above because it includes the full range of costs (intervention costs plus health and social care costs associated with managing agitation), measuring outcomes in terms of QALYs permits comparisons with other interventions, and it is possible to identify whether or not an intervention represents good for money to the NHS, that is to say if the incremental cost per QALY gained is below the NHS’s maximum willingness to pay for a QALY.
We used a different approach to measuring cost-effectiveness, primarily based on data from the LASER-AD study described above, and using only a 12-month time horizon.
Methods
The aim was to use model-based cost–utility analysis to estimate the incremental cost per QALY gained of non-pharmacological interventions for reducing agitation in adults with dementia. The analysis was undertaken from the perspective of the UK NHS and PSS. Costs were calculated in 2011 GBP. As explained, we used a time horizon of 1 year for costs and outcomes. This effectively assumes that after 1 year there were no differences in the costs and QALYs associated between receiving the interventions and not receiving them.
Model structure
The structure of the economic model is in Figure 6 . Using the data and methods described previously, we evaluated the relationship between agitation and health and social care costs associated (see Figure 6a ) and relationship between agitation and utility (see Figure 6b ). Using the data generated by the effectiveness review, we then evaluated the impact of an intervention on agitation over time (see Figure 6c ). The intervention is delivered at the start of the 12-month time horizon and reduces agitation during the period t 1 to t 2. Combining this with data on the relationship between agitation and health and social care costs and utility, we calculated the impact of the intervention on health and social care costs (see Figure 6d ) and utility (see Figure 6e ), assuming that the time period during which the intervention affects agitation is the period during which health and social care costs and utility are also affected. We combined the health and social care costs associated with managing agitation with the costs of the intervention to calculate the incremental costs of the intervention. We then combined the incremental costs with the change in utility to estimate cost-effectiveness.
FIGURE 6.
Model structure. (a) Association between agitation and health and social care costs; (b) association between agitation and utility; (c) impact of intervention on agitation over time; (d) impact of intervention on health and social care costs; (e) impact of intervention on utility.
Intervention
The model requires information on the relationship between the intervention and agitation. It is necessary that agitation is measured in the same units, that is to say using the same instrument, that are used to quantify the relationship between agitation and health and social care costs and agitation and utility. We used the LASER-AD study to quantify these relationships, as this was the only source of data available. In the LASER-AD study, as discussed, agitation was measured in terms of NPI agitation scores. In order to be able to use these data, we required an intervention that was evaluated in terms of its impact on NPI agitation scores. We are not aware of any mapping studies that relate NPI agitation scores to other measures of agitation, such as the CMAI, which was the most commonly used outcome measure in the studies included in our effectiveness review. Further research linking different agitation measures would be beneficial.
Two studies in our effectiveness review had a significant favourable effect on agitation, measured using NPI agitation scores. 179 The study by Ousset et al. 194 provided insufficient information on the details of the intervention, and so we were not able to compute the costs of it. Hence, we evaluated the intervention tested in the study by Fischer-Terworth and Probst. 179
The authors evaluated a multicomponent intervention involving a combination of music therapy, structured teaching, psychoeducational staff training and intensive family member–staff communication in participants with mild to moderate dementia. The intervention was compared with non-specific occupational therapy. Twenty-six participants received the intervention and 22 received the control. The study used a non-randomised design. The intervention was provided for 6 months. NPI agitation scores were measured at baseline and at the 6-month point. The change in NPI agitation scores over time was measured in both groups (on a linear scale), and between-group differences in the change were evaluated. In the effectiveness review, the study was judged to be a level 4 study (i.e. with quality rating < 6) due to the non-randomised design and the poor description of the statistical methods used.
Impact of intervention on agitation
We measured the impact of the intervention in terms of the difference-in-difference in NPI agitation scores using the NPI agitation scores reported at baseline and 6 months in the intervention and control groups. The standard error of the difference-in-difference was computed using the SDs and sample sizes reported at baseline and 6 months in both groups.
Association between agitation and health and social care costs and between agitation and utility
The associations between agitation and health and social care costs and agitation and utility were quantified using the data and methods described above, that is to say using regression analysis of data on health and social care costs, utility, NPI agitation scores, age, sex, cognitive impairment and follow-up from the LASER-AD study. The only difference is that we included NPI agitation scores in the regression models as a linear term rather than categorical indicators for each value to match the effectiveness measure reported by Fischer-Terworth and Probst179 (change in NPI agitation score). We did not evaluate the impact of the change in NPI agitation scores on the change in health and social care costs and the change in utility over time directly in the LASER-AD data due to the relatively small numbers of transitions and the length of time between follow-ups; in addition, the DEMQOL system was measured at only one point in time (the 54-month follow-up). Ideally, we would have had data on the proportion of participants at each level of agitation before and after the intervention, in which case we could have applied the results of the previous analysis of the LASER-AD study directly, but this information was not reported in the study.
Duration of effect of intervention
To measure the incremental costs and QALYs gained due to the intervention, we combined data on the change in health and social care costs and utility associated with the intervention and the time period over which the change occurs. In the Fischer-Terworth and Probst study,179 the intervention was delivered over a 6-month period and the outcomes were assessed only at that point. We assumed the following.
First, the impact of the intervention on agitation was maximised at the 6-month assessment. Second, the transition between agitation levels at baseline and 6 months occurred during the 6-month period, and agitation levels returned to their baseline levels during the period from 6 to 12 months. Third, in the absence of evidence to the contrary, the transitions between agitation levels (baseline values to 6-month values, 6-month values back to baseline values) were instantaneous. Fourth, in the base case, the transitions occur exactly halfway through each 6-month period, that is to say at 3 and 9 months. The sensitivity of the results to the duration of effect of the intervention was assessed in the univariate sensitivity analysis and probabilistic sensitivity analysis (PSA).
Cost of intervention
Based on the information provided in the study, the intervention comprised the following:
-
music-based group therapy once per week for 26 weeks for 45 minutes with a mean group size of seven participants
-
structured teaching with a therapist once per week for 26 weeks for 45 minutes with a mean group size of seven participants
-
psychoeducational staff training by a psychologist through a programme of 12 lessons
-
intensive family member–staff communication comprising provision of basic information about dementia to family members, everyday availability of professional caregivers to answer family members’ questions, and a 1-hour session of psychoeducational counselling by a psychologist to a close family member of each participant.
To compute the unit cost of the intervention per participant, we assumed the following. First, music-based group therapy was provided by a NHS community occupational therapist and, for each 26-week programme, costs were incurred for a CD player (£30) and music (£30). Second, structured teaching was provided by a NHS community occupational therapist. Third, psychoeducational staff training was provided by a psychologist to three members of staff at a time and each of these staff members was responsible for the care of seven participants. Fourth, the provision of basic information about dementia to family members and the everyday availability of professional caregivers to answer family members’ questions incurred zero cost on the basis that these activities provided no increase in workload. Psychoeducation counselling was provided by a clinical psychologist on a one-to-one basis to a close family member of each patient. Fifth, no additional costs were incurred for venue hire. Sixth, given the unspecified but seemingly low-intensity nature of the control intervention (non-specific occupational therapy), we assumed that this was provided at zero additional cost over usual care received by all participants.
Unit costs for staff were taken from published sources. 212 Non-staff unit costs were valued as described.
The sensitivity of the results to the costs of the intervention was assessed in the univariate sensitivity analysis and PSA.
Measuring cost-effectiveness
Cost-effectiveness was measured using monetary net benefits (MNBs). The MNB is calculated as the mean QALYs gained per patient accruing to the intervention multiplied by decision-makers’ maximum willingness to pay for a QALY (also referred to as the cost-effectiveness threshold, which in the UK is approximately £20,000 to £30,000 per QALY gained217,219), minus the mean incremental cost per patient for the intervention. This approach converts the gain in outcomes associated with the intervention into monetary terms and then subtracts the incremental costs of the intervention from the monetised benefits, calculating the net benefit in monetary terms. The incremental costs included the costs of the intervention plus the change on health and social care costs associated with the intervention (positive or negative). MNBs were calculated using the base-case parameter values; these are referred to as the deterministic results as they do not depend on chance. If the MNB for the intervention is > 0, it represents good value for money and is preferred to the comparator on cost-effectiveness grounds.
Sensitivity analysis
One-way sensitivity analysis was undertaken, varying the key parameters one at a time within a prespecified range. The aim was to identify the threshold value for each parameter, where one exists, where the MNB became negative, so the intervention was no longer cost-effective.
We also undertook a PSA as recommended by NICE. Distributions were assigned to parameters to reflect the uncertainty with each parameter value. A random value from the corresponding distribution for each parameter was selected. This generated an estimate of the mean incremental cost and mean QALYs gained and the MNB associated with the intervention. This was repeated 5000 times and the results for each simulation were noted. The mean value for each model parameter and the mean costs and QALYs associated with each treatment option were calculated from the 5000 simulations and these were used to calculate the MNBs for each treatment; these are referred to as the probabilistic results because they depend on chance. The MNB was also calculated for each of the 5000 simulations, and the proportion of times the intervention had a positive MNB was calculated for a range of values for the maximum willingness to pay for a QALY. These were summarised graphically using a cost-effectiveness acceptability curve.
In the PSA, we used gamma distributions to model uncertainty in the cost of the intervention, the impact of the intervention on agitation, the impact of changes in agitation on health and social care costs, and the impact of changes in agitation on utility. 220 To model the duration of effect of the intervention, we used uniform distributions as there are no data to inform the time point at which the transition occurs and so each time was judged to be equally likely. In cases where standard errors were required for the PSA, these were computed from the figures reported in the study (in the case of the impact of the intervention on agitation) or from the statistical analysis of the LASER-AD data (the impact of changes in agitation on health and social care costs, and the impact of changes in agitation on utility). Where standard errors were not obtainable (cost of the intervention), it was assumed that these were equal to the mean. 220
Results
In the base-case analysis, the cost per participant was £406 ( Table 23 ). In the study by Fischer-Terworth and Probst,195 the intervention was calculated to reduce NPI agitation scores by 1.8. Based on the analysis of data from the LASER-AD study, a one-unit increase in NPI agitation scores was associated with a statistically significant increase in health and social care costs over a 3-month period of £306 per patient (95% CI £7 to £606; Table 24 ). Note that we controlled for cognitive impairment in the regression model. If we limit the sample to those with mild or moderate dementia in order to reflect the study population, a one-unit increase in NPI agitation scores is associated with an increase in costs of £394 (95% CI £79 to £708).
| Clinical variables | Base case value (SE) | Distribution | Range | Value at which MNB becomes negative |
|---|---|---|---|---|
| Cost of intervention per participant | 406 (406) | Gamma | 100 to 1000 | – |
| Impact of intervention on reduction on NPI agitation scores | 1.8 (0.813) | Gamma | 0.5 to 4.0 | < 0.6 |
| Impact of a one-unit increase in NPI agitation scores on 3-month health and social care costs | 306 (153) | Gamma | 7 to 606 | < 79 |
| Impact of a one-unit decrease in NPI agitation scores on utility | 0.00661 (0.00413) | Gamma | –0.001485 to 0.0147 | – |
| Proportion of time during 12-month time horizon until intervention takes effect | 0.25 | Uniform | 0.0–0.5 | – |
| Proportion of time during 12-month time horizon after which intervention is no longer effective | 0.75 | Uniform | 0.5–1.0 | – |
| Dependent variable | Regression model | Marginal effect (95% CI) | SE | Observations |
|---|---|---|---|---|
| Three-month health and social care costs per persona | Generalised linear model | 306b (7 to 606) | 153 | 695 |
| DEMQOL-Proxy-U scorec | Tobit | 0.00661d (–0.001485 to 0.0147) | 0.00413 | 84 |
A one-unit decrease in NPI agitation scores was associated with a non-significant increase in DEMQOL-Proxy-U scores of 0.00661 (95% CI –0.001485 to 0.0147). If we limit the sample to those with mild or moderate dementia, the marginal effect is 0.010247 (95% CI 0.00156 to 0.01893).
Using base-case values, the intervention was associated with a cost saving of £711 per patient compared with the comparator (deterministic results, Table 25 ). The savings were due to the reduction in the costs of managing agitation, which more than offset the intervention costs. The QALY gain associated with the intervention was 0.005949. The intervention was less costly and more effective than the comparator, and the MNB is positive at a maximum willingness to pay for a QALY of £20,000 and £30,000, indicating that this option was preferred to the comparator on cost-effectiveness grounds. As expected, the probabilistic results were broadly the same, and the values of the mean costs and mean QALYs per patient were similar.
| Clinical variables | QALYs gained | Incremental cost (£) | MNB | |
|---|---|---|---|---|
| £20,000 | £30,000 | |||
| Deterministic results | 0.005949 | –711 | 830 | 889 |
| Probabilistic results | 0.005829 | –716 | 832 | 891 |
In the one-way sensitivity analysis, the key parameters were varied one at a time within the ranges listed in Table 22 . The results were not sensitive to change, that is to say the MNB did not become negative within the ranges used for the cost of the intervention per participant, the associated between NPI agitation scores and utility, and the duration of effect. If the intervention reduced NPI agitation scores by less than 0.6 units, and if an increase in NPI agitation scores reduced 3-month health and social care costs by less than £79, then the MNB became negative and the intervention no longer represented good value for money.
The cost-effectiveness acceptability curve shows that the intervention had an 82.2% probability of being cost-effective at a maximum willingness to pay for a QALY of £20,000 and an 83.18% probability at a value of £30,000 ( Figure 7 ).
FIGURE 7.
Cost-effectiveness acceptability curves showing the probability that each option is cost-effective at different values of the maximum willingness to pay for a QALY.
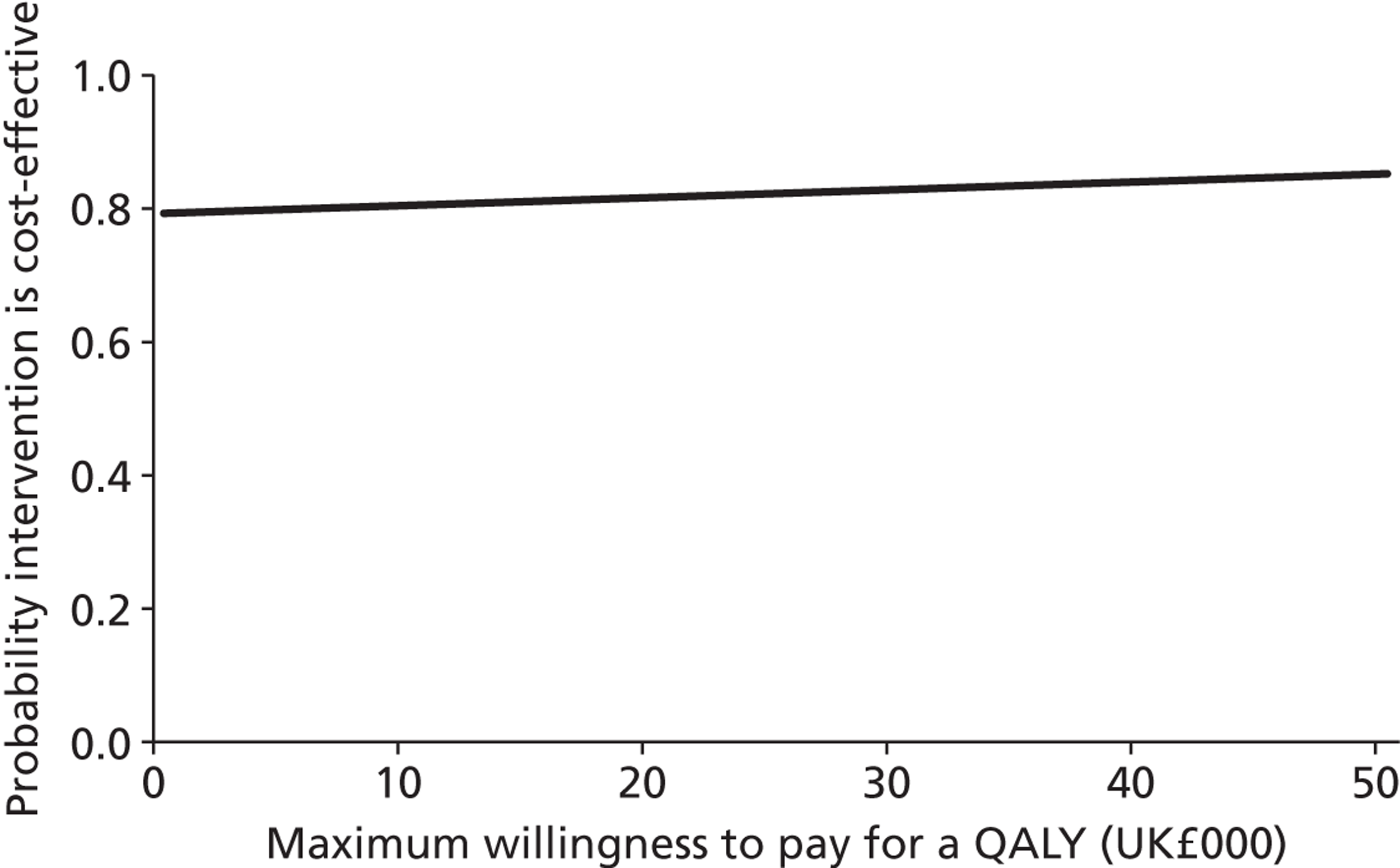
Chapter 6 Discussion
The main findings in this report are clinical, economic and conceptual. We are able to recommend the use of some interventions in specific groups of people with dementia to manage agitation and to recommend that others are not useful. We detail these overall conclusions and their implications below.
Effective interventions
There is convincing evidence that training paid caregivers in communication or person-centred care skills or DCM (all with supervision during implementation) is effective, for symptomatic and severe agitation, during the intervention and for 6 months afterwards, and preliminary evidence that it helps prevent emergent agitation. This form of DCM is somewhat different to that used in everyday practice and so could be regarded as modified. All of these interventions seek to communicate with people with dementia, to understand and fulfil their wishes and needs. The SESs suggest they are similarly efficacious, although DCM is more expensive. A 30% decrease in agitation is clinically significant and these changes are in this range. 99 Another way of considering clinical significance is through effect size; a SES of 0.2 is usually regarded as clinically small, of 0.5 as medium and of 0.8 as large. 221 This suggests that they are clinically significant interventions but there remains substantial variability in the interventions’ statistical significance.
We also found replicated evidence from good-quality studies that sensory interventions, activities and music therapy by protocol reduce emergent agitation and decrease symptomatic agitation in care homes while they are in place. The theory-based activities (neurodevelopmental and Montessori) were more expensive (£590–696) than those promoting pleasant activities (£173–274). There is no evidence for those who are severely agitated.
More evidence is required for the implementation of group activities in care homes over longer time periods to prevent agitation. We were surprised that individualised activities were no more effective than prescribed activities but they may still be important in increasing the number of people accessing the intervention. There is no evidence of the effect of activity provision for people with dementia in their own homes. This is an important area of future research as decreasing emergent agitation could prolong staying at home.
Sensory interventions significantly improved emergent agitation, symptomatic agitation and severe agitation, while they were in place. ‘Therapeutic touch’ had no added advantages. There is no evidence about long-term effects or in settings outside care homes.
Ineffective interventions
Light therapy and aromatherapy do not work for agitation and should not be used. Earlier non-blinded interventions with aromatherapy seemed to be effective, but this was probably the effect of rater bias. Home-like care sounds appealing but was ineffective, possibly because people tend to move to care homes when they have not been able to stay safe in a home-like environment or perhaps the environment was not similar enough to the home they knew.
Interventions with insufficient evidence to draw definitive conclusions
Training family carers in behavioural or CBT interventions for the person with dementia was ineffective in severe agitation and there was a lack of evidence of efficacy at other levels of agitation; perhaps these are difficult to combine with a family relationship. Changing the environment is promising but the evidence is not definitive and we presume that different changes would have different effects. Hiding the exit door could be construed as restraint. Results for music therapy without protocol are equivocal and we would recommend using it with a protocol. Pet therapy results are also varied and it may appeal to some people and not others. Exercise, dementia-specific therapies and wayfinding do not appear to show promise but the studies are unclear because they are of low quality.
Quality of life
Studies in which agitation was an outcome also considered quality of life. Although DCM with supervision improved agitation, it did not improve quality of life in the two studies in which it was measured. Other studies of staff education without supervision, aromatherapy, unstructured music therapy and changing the environment that found no improvement in agitation also found no improvement in quality of life. As there are only two studies which improve agitation and measure quality of life, the evidence is relatively sparse, but we have certainly not demonstrated a clear link between improvement in agitation and quality of life. This may partially be because global measures, by definition, encompass many domains, some of which would not be improved by improving agitation, for example finances, activities of daily living and cognition. Further work and larger studies are needed to consider the link between agitation and quality of life.
Health economic analysis
Our systematic review identified two previous cost and cost-effectiveness studies of non-pharmacological interventions for reducing agitation in adults with dementia. There were judged to be of low to middle quality and provided little information on the cost-effectiveness of interventions in a UK context. The review revealed little pre-existing evidence that could inform our cost-effectiveness model.
We calculated the costs of 30 interventions that had a significant impact on agitation. Costs ranged from £80 to £696 for activities, £13 to £27 for music therapy, £3 to £527 for sensory interventions, £31 to £339 for training paid caregivers in person-centred care or communication skills with or without behavioural management training, with supervision, and for DCM.
Among 11 interventions that were evaluated using the CMAI, the incremental cost per unit reduction in CMAI score ranged from £162 to £3480 for activities, £4 for music therapy, £24 to £143 for sensory interventions, and £6 to £62 for training paid caregivers in person-centred care or communication skills with or without behavioural management training, with supervision, and for DCM.
Based on data from the LASER-AD study, we found that after adjusting for sex, age, cognitive impairment, follow-up and individual clustering, NHS and PSS costs increase with NPI agitation scores from around £7000 over a 3-month period with clinically non-significant agitation symptoms up to around £15,000 at the most severe levels of agitation. The 95% CIs are wider at higher NPI agitation scores, possibly due to the smaller number of observations taking these values.
Again based on data from the LASER-AD study, we found that after adjusting for sex, age, cognitive impairment and individual clustering, there is some evidence that DEMQOL-Proxy-U scores decline with NPI agitation scores, from a score of around 0.75 with clinically non-significant agitation symptoms to around 0.65 at the most severe levels of agitation. However, the 95% CIs overlap, and there is not a clear trend between the lowest and highest NPI agitation scores, possibly due to the relatively small number of observations.
We constructed a new cost-effectiveness model to evaluate the impact of interventions for non-pharmacological interventions for reducing agitation in adults with dementia. The model can evaluate interventions that have been shown to impact on agitation measured using NPI agitation scores. In an illustrative example, we found that a multicomponent intervention in participants with mild to moderate dementia had a positive MNB and had a 82.2% probability of being cost-effective at a maximum willingness to pay for a QALY of £20,000 and a 83.18% probability at a value of £30,000.
Emergent and clinically significant interventions
Some interventions may be helpful in preventing clinically significant agitation or decreasing mean agitation levels but not practical for someone who already has clinically significant agitation and who may be unco-operative and unable to concentrate. Previous reviews have not considered potential differences between preventative interventions and those for clinically significant symptoms. We think this is conceptually important and have specified which interventions are helpful in each category.
Implementation
Implementation of the findings will require considerably more work. Implementing them into everyday practice will involve observing and interviewing a wide range of people with dementia and those who care for them at home, in care homes and in hospitals (including at the end of life), in order to better understand how agitation is currently managed, barriers to good practice and ways in which care could be improved.
In care homes, a manual for training staff in interventions known to be effective would then be developed, tested and implemented, with ‘champions’ within each home charged with ensuring change in care-home culture and continuous implementation. This would require testing in terms of practicality, effectiveness and cost-effectiveness. Our model suggests that the intervention may well be affordable in terms of improvement in agitation, but its impact on QALYs is unclear.
Strengths and limitations
This is an exhaustive systematic review; two independent raters evaluated studies to ensure reliability in study inclusion and quality ratings. We searched all databases in health and social sciences, as well as the grey literature, translated non-English publications and asked authors about any other known studies, and then repeated our searches. We searched not only the Cochrane reviews about agitation and dementia but also the Cochrane reviews about behavioural problems in dementia, to try to ensure that we did not miss papers in which agitation was one of a number of outcome measures. The quality ratings enabled us to systematically consider sources of bias in carrying out and measuring the outcomes of individual studies. We reduced bias by prioritising higher-quality studies. We reduced publication bias by searching the grey literature and asking experts about other studies. Our quality rating tool was derived by assessing validity by operationalising the CEBM RCT evaluation criteria. 222 This meant that cohort studies, for example, would not be able to score as highly as very well-conducted RCTs. While RCTs are conventionally regarded as the highest level of evidence because they reduce bias by minimising systematic differences between groups, this approach is not agreed on by all commentators. In addition, giving one point for every criterion suggests that all are equally important. If the outcome measure is not validated and reliable, the whole study results, however well conducted in other ways, may be questionable. We have reanalysed our results (detailed in Appendix 3 ) and excluding these studies would not change our conclusions, with the possible exception of the evidence for activities which we would have to couch more cautiously. Our team, with clinical, statistical, health economic and carer expertise, have assessed them. As we have separated studies according to the setting, the severity of the participants’ agitation symptoms and the immediate versus long-term results, we are able to consider both the evidence for each of these and the gaps in the evidence.
In terms of the health economic analysis, the main strength is that this is the first study to comprehensively assess the cost-effectiveness of non-pharmacological interventions for reducing agitation in adults with dementia. The main weakness is the paucity of health economic data. Focusing specifically on the model used in the cost–utility analysis, the main strength is that we have developed a model, using new data, that is capable of evaluating the cost-effectiveness of non-pharmacological interventions for reducing agitation in adults with dementia, using a framework that can determine whether or not an intervention is cost-effective using current guidelines. The weakness of the cost–utility analysis is that it is, at present, limited in the interventions that it can evaluate.
‘Active ingredients’
Individual studies had multicomponent interventions and we could not find out which of the components were the ‘active’ ingredients. Nonetheless, we have tried to contrast studies; noting, for example, that differences in results with and without supervision suggest that supervision may be a necessary ‘active ingredient’ in changing care-home culture. Similarly, we have contrasted sensory interventions based on different models and found that there is no significant advantage offered by therapeutic touch over other sensory activities. Activities are an interesting category. It seems likely that, as is the case with people who do not have dementia, some activities are liked by some residents some of the time and, thus, it would be interesting to consider a menu of activities, say for example animal-assisted therapy, dance or cooking, rather than consider any one of them the answer, and understand that this may vary not just with cognition level but with mood, physical illness and other factors. We did not find that individualised activity was better than just offering an activity, but that may be because those who did not like the activity on offer did not enter the study and, thus, activities were effectively individualised.
Subtypes of dementia
Most studies recruited people with a variety of types of dementia and none of these analysed the participants according to their type of dementia. The only type of dementia which has any data considering it separately is Alzheimer’s disease. The epidemiology of dementia suggests, as do the papers which describe the numbers in each group, that the vast majority of people in each intervention had Alzheimer’s disease and, thus, we have not analysed those separately, reducing our already limited data, as the positive results are all likely to be applicable to people with Alzheimer’s disease. It is a limitation that we are unable to comment on, as we do not know whether or not individuals with different types of dementias responded differently.
Chapter 7 Intervention setting
Care homes and hospitals
As people with agitation are disproportionately more likely to move to care homes, it is perhaps unsurprising that most interventions have been tried only in care homes, and we do not know their effect or practicality in people’s own homes, or in hospitals. All sensory intervention, aromatherapy, exercise and light-therapy studies were in care homes and, similarly, for both music therapies and activities intervention, all except one study took place in a care home. The exceptions in both cases were studies situated in day centres. All staff interventions (DCM, communication skills and person-centred care) were also in care homes, except one which was in an assisted-living facility. Although there is a lack of good evidence, it is plausible and reasonable at present to build on the evidence available and, in hospital settings, to try interventions that work in care homes, such as staff training with supervision in communication skills as well as music and massage.
Community and domestic interventions
The few interventions in domestic environment comprised teaching family carers behavioural management for severe agitation, or cognitive–behavioural management. The former was unsuccessful in one large RCT of severe agitation and inconclusive in a group with less severe symptoms. The latter was unsuccessful in two large RCT studies of severe agitation and in one small study. The reasons for this are unclear, but it may be the case that family carers do not have the capacity to learn and implement these strategies while looking after someone who is agitated. While we do not know why there is so little evidence in the community, it may be due to difficulties regarding recruitment; people who are agitated and have dementia are often admitted to a care home, and those who do remain at home may be unco-operative and their families may be reluctant to take part in research, particularly as they may not receive the intervention. It is usually easier to recruit in a care home, and researchers may feel that this is a better setting to begin studies.
Country of origin and ethnicity of participants
Nearly all of the research papers come from English-speaking countries or other countries in the developed world; none was sufficiently powered to consider or report the results according ethnicity of the participants. Thus, we are unable to comment about the effect of ethnicity and culture.
Acceptability
Studies often involve only a relatively small number of participants and we speculate that this may be because many of the residents were unwilling or unable to access the interventions.
Lack of evidence
Many of the studies were underpowered and low quality. There were only eight level 1 studies and, while lack of evidence is not evidence of lack of efficacy, there were a number of interventions with insufficient evidence to draw conclusions. There were no studies which targeted agitation at the end of life in dementia, although one-third of people aged > 65 years die with dementia in the UK.
Comparison with other studies
A previous systematic review, focusing on non-pharmacological treatment of agitation in dementia at a time when there was less evidence, concluded that the trials were small but only sensory interventions showed evidence of benefit. 31 This positive review is in line with our findings and contrasts with the earlier Cochrane review, which found only two studies to be of sufficient quality to include and judged that there were not enough data to draw conclusions about massage and touch interventions. 223 These differences may be explained by the several more recent studies. After these, a broader review of interventions for agitation selected 47 trials of pharmacological and non-pharmacological treatment for consideration and concluded that the best evidence for effective non-drug treatment was for aromatherapy, although all trials were small and of short duration (< 4 weeks). Since then, further blinded work has not found aromatherapy to be of benefit. 34 A Cochrane review of light therapy for behavioural symptoms including agitation did not find evidence of benefit. 224 Another Cochrane review focused on special care units for agitation and noted the lack of RCTs and the lack of convincing evidence of benefit for behaviour in general. 225 A recent study, including overall neuropsychiatric symptoms, in contrast to our review about agitation specifically, found that working with family caregivers is effective; it would be useful to examine which symptoms contributed to this effect. 226
Early studies did not have the opportunity to use valid instruments to measure agitation. These now exist but, while several instruments for measuring agitation perform similarly in detecting agitation, they vary in their sensitivity of detecting change. Differences in effect sizes between study results may, therefore, sometimes be due to the difference between the instruments used. Thus, while our study’s strength is the integration of the literature, it underlines how much more work is needed in this field. There are a number of RCTs currently in progress which should add to the evidence base. While agitation in dementia has been regarded as due to organic brain damage, our findings that it is improved by, for example, communication, sensory experiences and activities suggest that the behaviour also arises from unmet needs in someone whose dementia makes them unable to explain or understand this. This is in line with Algase’s Need-Driven Dementia-Compromised Behaviour (NDB)227 theory and Kitwood’s hypothesis that behaviours are in response to need and more likely to occur when care is task driven rather than person centred (although the latter applied to all neuropsychiatric symptoms and we are commenting only on agitation). 228
In the health economic analysis, as identified in the systematic review, there is very little economic evidence.
Chapter 8 Conclusions
Implications for health care
At present, agitation is often managed pharmacologically with antipsychotics, about which there is growing concern, as their use is linked with an increased risk of cerebrovascular adverse events and death while they provide only limited benefit. A range of behavioural techniques are also recommended by NICE but with a limited evidence base. These include aromatherapy, music therapy and dance therapy. Restraint is also used in some countries. People are admitted to care homes (often specialist facilities), as they cannot be looked after at home.
This review has found several interventions which are effective for severe agitation in care homes. Person-centred care, communication, and feedback of DCM with a plan implemented all seem similar and, with supervision, efficacious for more severe agitation, and the effects last over a few months. In addition, activities, sensory intervention and music therapy using a protocol were all effective during the intervention, and activities and music therapy helped to prevent agitation symptoms during the intervention when compared with usual care. Sensory interventions also were helpful for clinically significant agitation. All of these improvements were clinically as well as statistically significant. These are very different from the current model of health care, which, in response to referrals for people with dementia and agitation, assesses individuals and then recommends treatment strategies, often working with the home staff individually. The interventions assessed here work at the level of the whole home instead, and suggest a need for a change of culture rather than individual treatment. There are no studies demonstrating the long-term implementation effect of these interventions, as the longest follow-up periods have been about 6 months. There is currently very little cost-effectiveness evidence. The model that we have generated suggests that there is high chance of benefit at low cost, though data limitations preclude a definitive analysis. If treatments are to be funded then this evidence is necessary. Despite NICE’s recommendation, we found that the better-quality studies did not demonstrate that aromatherapy was effective and that only specific types of music therapy were useful. There is a need for more evidence about dance therapy but it could be subsumed under the rubric of activities. Aromatherapy, light therapy and home-like care do not show evidence of being effective. The results of interventions in people’s own homes working through their family are very disappointing, as there is no high-quality evidence of effectiveness and much more work needs to be done.
Recommendations for research
We recommend a research programme evaluating the implementation of interventions that we have found to be effective. There is most evidence for effective interventions during the time they are being actively implemented. It is logical that effects may not carry over long after implementation has stopped, in an environment where many residents have memory problems and staff turnover is high. Thus, future programmes should focus on changing care-home culture to permanently implement change, and on evaluating the effect on all residents (unless they are unwilling to be evaluated) whether or not they are able or willing to co-operate with the intervention. This real-life implementation will indicate which interventions are likely to be useful in everyday practice for decreasing agitation. We suggest the use of a manual and training of ‘champions’ in each home, as well as the provision of continuing supervision, to evaluate the effect of interventions longer term. This would be manual based so that it can be consistently implemented in wider areas. There are very few studies which demonstrate the effect on quality of life and the work presented here shows that it is unclear whether or not the link exists in intervention studies. The questions to be addressed would regard effectiveness and cost-effectiveness.
In addition, it is clear that much more work is required to consider what interventions are most effective for people with dementia living in their own homes. The lack of evidence and lack of success of interventions which have been tried (despite an enormous need, with 70% or more of people with dementia living at home) suggests that further research in home settings should start with qualitative interviews considering how agitation is experienced by people with moderate and severe dementia living at home, how their families manage, and what interventions would be acceptable and practical. It may be that family carers who are finding it difficult to manage someone with dementia and agitation say that they are not able to learn a new and cognitively complex strategy during this period. This, together with the evidence above from other settings, can help to answer the questions about what are the important components of an intervention to reduce agitation.
As there are no studies at the end of life, we recommend an initial ethnographic approach involving people with dementia, family and paid carers to explore their interactions in end-of-life care and the barriers to and facilitators of compassion and quality of life. This will provide new knowledge about agitation management during the often-distressing terminal phases of dementia. We already know that dying people often do not have their physical and emotional needs attended to as they cannot express them,229 that their families find it difficult to make decisions230 and that the paid carers are often new to the individuals with dementia and do not understand them. This evidence could be used to develop and pilot acceptable and appropriate interventions.
Within individual categories of interventions, it was sometimes difficult to draw conclusions, given the wide variety of interventions and outcome measures. There are several interventions which require more research to indicate if they are effective. There are, for example, no good studies of exercise for agitation in dementia, although a recent study of moderate exercise for depressive symptoms in care-home residents was ineffective and, while not a panacea, it would be good to have an answer. 231
In terms of the health economic analysis, further research is recommended in the following (in decreasing order of priority):
-
Inclusion of health economic analyses in clinical trials of non-pharmacological interventions for reducing agitation in adults with dementia. Such analyses should evaluate the impact of interventions using final outcomes such as QALYs, for example using new approaches based on the DEMQOL system. Such analyses should also include comprehensive cost analyses, including health and social care costs associated with managing agitation.
-
Evaluation of the long-term effects of interventions.
-
Mapping studies to evaluate the relationship between different measures of agitation, for example CMAI scores and NPI agitation scores.
Acknowledgements
This article presents independent research commissioned by the National Institute for Health Research (NIHR) HTA programme: HTA 10/43/01. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. The review was designed, conducted, analysed and interpreted by the authors entirely independently of the funding sources.
Patient/public involvement
We consulted Shirley Nurock, dementia carer, regarding the design, conduct and interpretation of the study.
Contributions of authors
Gill Livingston and Claudia Cooper initiated the review and helped to design the methodology.
Gill Livingston, Lynsey Kelly and Elanor Lewis-Holmes located references, extracted data, assisted with analyses and results interpretation, and drafted the report.
Nishma Patel ran the database searches and quality assessments for the review of economic studies.
Stephen Morris and Nishma Patel undertook the economic analyses.
Gianluca Baio and Rumana Z Omar undertook the statistical analyses. All authors helped to interpret findings and write the final report.
Gill Livingston is guarantor.
Cornelius Katona participated in interpretation of data and revising the paper critically for important intellectual content.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Luengo-Fernandez R, Leal J, Gray A. Dementia 2010. The Economic Burden of Dementia and Associated Research Funding in the United Kingdom. University of Oxford: Alzheimer’s Research Trust 35; 2010.
- Knapp M, Prince M, Albanese E, Banerjee S, Dhanasiri S, Fernández JL, et al. Dementia UK. London: Alzheimer’s Society; 2007.
- Living Well with Dementia: A National Dementia Strategy. London: Department of Health; 2009.
- Chantrill C. Public Expenditure Statistical Analysis 2009. www.ukpublicspending.co.uk/uk_health_care_budget_2009_1.html (accessed 4 April 2014).
- Morris LW, Morris RG, Britton PG. The relationship between marital intimacy, perceived strain and depression in spouse caregivers of dementia sufferers. Br J Med Psychol 1988;61:231-6. http://dx.doi.org/10.1111/j.2044-8341.1988.tb02784.x.
- Cohen-Mansfield J. Agitated behavior in persons with dementia: the relationship between type of behavior, its frequency, and its disruptiveness. J Psychiatr Res 2008;43:64-9. http://dx.doi.org/10.1016/j.jpsychires.2008.02.003.
- ADH/NHS Finance P&O . Revision to the Operating Framework for the NHS in England 2010 11. 2010. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/documents/digitalasset/dh_116860.pdf (accessed 4 April 2014).
- Cohen-Mansfield J, Billig N. Agitated behaviors in the elderly. I. A conceptual review. J Am Geriatr Soc 1986;34:711-21.
- Sommer OH, Kirkevold O, Cvancarova M, Engedal K. Factor analysis of the Brief Agitation Rating Scale in a large sample of Norwegian nursing home patients. Dementia Geriatr Cogn Disord 2010;29:55-60. http://dx.doi.org/10.1159/000265542.
- Ryu SH, Katona C, Rive B, Livingston G. Persistence of and changes in neuropsychiatric symptoms in Alzheimer disease over 6 months – the LASER-AD study. Am J Geriatr Psychiatry 2005;13:976-83. http://dx.doi.org/10.1176/appi.ajgp.13.11.976.
- Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the aging, demographics, and memory study. J Am Geriatr Soc 2010;58:330-7. http://dx.doi.org/10.1111/j.1532-5415.2009.02680.x.
- Lemay M, Landreville P. Verbal agitation in dementia: the role of discomfort. Am J Alzheimers Dis Other Demen 2010;25:193-201. http://dx.doi.org/10.1177/1533317509356687.
- Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol 1989;44:M77-84. http://dx.doi.org/10.1093/geronj/44.3.M77.
- Robinson L, Hutchings D, Corner L, Dickinson HO, Finch T, Hughes J, et al. A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use. Health Technol Assess 2006;10.
- Wetzels RB, Zuidema SU, de Jonghe JFM, Verhey FRJ, Koopmans RTCM. Determinants of quality of life in nursing home residents with dementia. Dementia Geriatr Cogn Disord 2010;29:189-97. http://dx.doi.org/10.1159/000280437.
- Burgio LD, Scilley K, Hardin JM, Janosky J, Bonino P, Slater SC, et al. Studying disruptive vocalization and contextual factors in the nursing home using computer-assisted real-time observation. J Gerontol 1994;49:230-9. http://dx.doi.org/10.1093/geronj/49.5.P230.
- Draper B, Snowdon J, Meares S, Turner J, Gonski P, McMinn B, et al. Case–controlled study of nursing home residents referred for treatment of vocally disruptive behavior. Int Psychogeriatr 2000;12:333-44. http://dx.doi.org/10.1017/S1041610200006438.
- Testad I, Ballard C, Bronnick K, Aarsland D. The effect of staff training on agitation and use of restraint in nursing home residents with dementia: a single-blind, randomized controlled trial. J Clin Psychiatry 2010;71:80-6. http://dx.doi.org/10.4088/JCP.09m05486oli.
- Livingston G, Cooper C, Ames D, Burns A, O’Brien J. Dementia. London: Hodder Arnold; 2010.
- Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021. http://dx.doi.org/10.1176/appi.ajp.162.11.1996.
- Committee for the Safety of Medicines . Atypical Antipsychotics and Stroke 2004. www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON1004298 (accessed 4 April 2014).
- Ballard C, Howard R. Neuroleptic drugs in dementia: benefits and harm. Nat Rev Neurosci 2006;7:492-500. http://dx.doi.org/10.1038/nrn1926.
- Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14:191-210. http://dx.doi.org/10.1097/01.JGP.0000200589.01396.6d.
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934-43. http://dx.doi.org/10.1001/jama.294.15.1934.
- National Institute for Health and Care Excellence . SCIE: Dementia – Supporting People With Dementia and Their Carers in Health and Social Care n.d. www.scie.org.uk/publications/misc/dementia/ (accessed 4 April 2014).
- Ballard C, Margallo-Lana M, Juszczak E, Douglas S, Swann A, Thomas A, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38369.459988.8F.
- Bierman EJ, Comijs HC, Gundy CM, Sonnenberg C, Jonker C, Beekman AT. The effect of chronic benzodiazepine use on cognitive functioning in older persons: good, bad or indifferent?. Int J Geriatr Psychiatry 2007;22:1194-200. http://dx.doi.org/10.1002/gps.1811.
- Howard RJ, Juszczak E, Ballard CG, Bentham P, Brown RG, Bullock R, et al. Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med 2007;357:1382-92. http://dx.doi.org/10.1056/NEJMoa066583.
- Banerjee S. The Use of Antipsychotic Medication for People With Dementia: Time for Action A Report 2010. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_108302.pdf (accessed 4 April 2014).
- Cohen-Mansfield J, Werner P, Marx MS. Screaming in nursing home residents. J Am Geriatr Soc 1990;38:785-92.
- Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health 2009;13:512-20. http://dx.doi.org/10.1080/13607860902774394.
- Spira AP, Edelstein BA. Behavioral interventions for agitation in older adults with dementia: an evaluative review. Int Psychogeriatr 2006;18:195-22. http://dx.doi.org/10.1017/S1041610205002747.
- Sung HC, Chang AM. Use of preferred music to decrease agitated behaviours in older people with dementia: a review of the literature. J Clin Nurs 2005;14:1133-40. http://dx.doi.org/10.1111/j.1365-2702.2005.01218.x.
- Ballard CG, Gauthier S, Cummings JL, Brodaty H, Grossberg GT, Robert P, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol 2009;5:245-55. http://dx.doi.org/10.1038/nrneurol.2009.39.
- David M, Alessandro L, Jennifer T, Douglas GA. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339.
- Paradise M, Cooper C, Livingston G. Systematic review of the effect of education on survival in Alzheimer’s disease. Int Psychogeriatr 2009;21:25-32. http://dx.doi.org/10.1017/S1041610208008053.
- Pitfield C, Shahriyarmolki K, Livingston G. A systematic review of stress in staff caring for people with dementia living in 24-hour care settings. Int Psychogeriatr 2010;23:1-6.
- Cooper C, Balamurali TBS, Selwood A, Livingston G. A systematic review of intervention studies about anxiety in caregivers of people with dementia. Int J Geriatr Psychiatry 2007;22:181-88. http://dx.doi.org/10.1002/gps.1656.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. http://dx.doi.org/10.2307/2529310.
- Cohen-Mansfield J, Marx MS, Dakheel-Ali M, Regier NG, Thein K, Freedman L. Can agitated behavior of nursing home residents with dementia be prevented with the use of standardized stimuli?. J Am Geriatr Soc 2010;58:1459-64. http://dx.doi.org/10.1111/j.1532-5415.2010.02951.x.
- Welton N, Sutton A, Cooper N, Abrams K, Ades A. Evidence Synthesis for Decision Making in Healthcare (Statistics in Practice). Hoboken, NJ: Wiley-Blackwell; 2012.
- Hedges L. Distribution theory for Glass’s Estimator of effect size and related estimators. J Educ Behav Stat 1981;6:107-28. http://dx.doi.org/10.3102/10769986006002107.
- Kovach C, Cashin S, Taneli Y, Dohearty P, Schlidt A, Silva-Smith A. Effects of the BACE intervention on agitation of demented residents in longterm care. Gerontologist 2003;43.
- Lin LC, Yang MH, Kao CC, Wu SC, Tang SH, Lin JG. Using acupressure and Montessori-based activities to decrease agitation for residents with dementia: a cross-over trial. J Am Geriatr Soc 2009;57:1022-9. http://dx.doi.org/10.1111/j.1532-5415.2009.02271.x.
- Lee Y, Kim S. Effects of indoor gardening on sleep, agitation, and cognition in dementia patients–a pilot study. Int J Geriatr Psychiatry 2008;23:485-9. http://dx.doi.org/10.1002/gps.1920.
- Buettner LL, Ferrario J. Therapeutic recreation-nursing team: a therapeutic intervention for nursing home residents with dementia. Annu Ther Recreation 1997;7:21-8.
- Fitzsimmons S, Buettner LL. Therapeutic recreation interventions for need-driven dementia-compromised behaviors in community-dwelling elders. Am J Alzheimers Dis Other Demen 2002;17:367-81. http://dx.doi.org/10.1177/153331750201700603.
- Buettner LL, Lundegren H, Lago D, Farrell P, Smith R. Therapeutic recreation as an intervention for persons with dementia and agitation: an efficacy study. Am J Alzheimers Dis 1996;11:4-12. http://dx.doi.org/10.1177/153331759601100503.
- Fitzsimmons S, Buettner LL. A therapeutic cooking program for older adults with dementia: effects on agitation and apathy. Am J Recreation Ther 2003;2:23-3.
- Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT. A randomized clinical trial of theory-based activities for the behavioral symptoms of dementia in nursing home residents. J Am Geriatr Soc 2011;59:1032-41. http://dx.doi.org/10.1111/j.1532-5415.2011.03449.x.
- Kolanowski AM, Litaker M, Buettner L. Efficacy of theory-based activities for behavioural symptoms of dementia. Nurs Res 2005;54:219-28. http://dx.doi.org/10.1097/00006199-200507000-00003.
- Cohen-Mansfield J, Parpura-Gill A, Golander H. Utilization of self-identity roles for designing interventions for persons with dementia. J Gerontol Series B 2006;61B:202-12. http://dx.doi.org/10.1093/geronb/61.4.P202.
- Cooke ML, Moyle W, Shum DH, Harrison SD, Murfield JE. A randomized controlled trial exploring the effect of music on agitated behaviours and anxiety in older people with dementia. Aging Ment Health 2010;14:905-16. http://dx.doi.org/10.1080/13607861003713190.
- Sung HC, Lee WL, Li TL, Watson R. A group music intervention using percussion instruments with familiar music to reduce anxiety and agitation of institutionalized older adults with dementia. Int J Geriatr Psychiatry 2012;27:621-7. http://dx.doi.org/10.1002/gps.2761.
- Sung HC, Chang SM, Lee WL, Lee MS. The effects of group music with movement intervention on agitated behaviours of institutionalized elders with dementia in Taiwan. Complement Ther Med 2006;14:113-19. http://dx.doi.org/10.1016/j.ctim.2006.03.002.
- Tuet RWK, Lam LCW. A preliminary study of the effects of music therapy on agitation in Chinese patients with dementia. Hong Kong J Psychiatry 2006;16:87-91.
- Lin Y, Chu H, Yang CY, Chen CH, Chen SG, Chang HJ, et al. Effectiveness of group music intervention against agitated behavior in elderly persons with dementia. Int J Geriatr Psychiatry 2011;26:670-8. http://dx.doi.org/10.1002/gps.2580.
- Groene RW. Effectiveness of music-therapy 1/1 intervention with individuals having senile dementia of the Alzheimers type. J Music Ther 1993;30:138-57. http://dx.doi.org/10.1093/jmt/30.3.138.
- Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, Villani D, et al. Efficacy of music therapy in the treatment of behavioral and psychiatric symptoms of dementia. Alzheimer Dis Assoc Disord 2008;22:158-62. http://dx.doi.org/10.1097/WAD.0b013e3181630b6f.
- Svansdottir HB, Snaedal J. Music therapy in moderate and severe dementia of Alzheimer’s type: a case–control study. Int Psychogeriatr 2006;18:613-21. http://dx.doi.org/10.1017/S1041610206003206.
- Jennings B, Vance B. The short-term effects of music therapy on different types of agitation in adults with Alzheimer’s. Activities Adap Aging 2002;6:27-33. http://dx.doi.org/10.1300/J016v26n04_03.
- Suzuki M, Kanamori M, Nagasawa S, Tokiko I, Takayuki S. Music therapy induced changes in behavioral evaluations, and saliva chromogranin A and immunoglobulin A concentrations in elderly patients with senile dementia. Geriatr Gerontol Int 2007;7:61-7. http://dx.doi.org/10.1111/j.1447-0594.2007.00374.x.
- Moyle W, Burne, O’Dwyer S. Exploring the effect of foot massage on agitated behaviours in older people with dementia: a pilot study. Aust J Ageing 2011;30:159-61. http://dx.doi.org/10.1111/j.1741-6612.2010.00504.x.
- Yang MH, Wu SC, Lin JG, Lin LC. The efficacy of acupressure for decreasing agitated behaviour in dementia: a pilot study. J Clin Nurs 2007;16:308-15. http://dx.doi.org/10.1111/j.1365-2702.2006.01428.x.
- Woods DL, Craven RF, Whitney J. The effect of therapeutic touch on behavioral symptoms of persons with dementia. Alt Ther Health Med 2005;11:66-74.
- Remington R. Calming music and hand massage with agitated elderly. Nurs Res 2002;51:317-23. http://dx.doi.org/10.1097/00006199-200209000-00008.
- Woods DL, Beck C, Sinha K. The effect of therapeutic touch on behavioral symptoms and cortisol in persons with dementia. Forschende Komplementarmedizin 2009;16:181-9. http://dx.doi.org/10.1159/000220479.
- Hawranik P, Johnston P, Deatrich J. Therapeutic touch and agitation in individuals with Alzheimer’s disease. West J Nurs Res 2008;30:417-34. http://dx.doi.org/10.1177/0193945907305126.
- Staal JA, Sacks A, Matheis R, Collier L, Calia T, Hanif H, et al. The effects of snoezelen (multi-sensory behavior therapy) and psychiatric care on agitation, apathy, and activities of daily living in dementia patients on a short term geriatric psychiatric inpatient unit. Int J Psychiatry Med 2007;37:357-70. http://dx.doi.org/10.2190/PM.37.4.a.
- Hicks-Moore SL, Robinson BA. Favorite music and hand massage. Dementia 2008;7:95-108. http://dx.doi.org/10.1177/1471301207085369.
- Woods DL, Dimond M. The effect of therapeutic touch on agitated behavior and cortisol in persons with Alzheimer’s disease. Biol Res Nurs 2002;4:104-14. http://dx.doi.org/10.1177/1099800402238331.
- Gerdner LA, Hart LK, Zimmerman M. Craniosacral still point technique – exploring its effects in individuals with dementia. J Gerontol Nurs 2008;34:36-45. http://dx.doi.org/10.3928/00989134-20080301-04.
- Whall AL, Black ME, Groh CJ, Kupferschmid BJ, Foster NL. The effect of natural environments upon agitation and aggression in late stage dementia patients. Am J Alzheimers Dis Other Demen 1997;12:216-20. http://dx.doi.org/10.1177/153331759701200506.
- van Weert JCM, van Dulmen AM, Spreeuwenberg PMM, Ribbe MW, Bensing JM. Behavioral and mood effects of Snoezelen integrated into 24-hour dementia care. J Am Geriatr Soc 2005;53:24-33. http://dx.doi.org/10.1111/j.1532-5415.2005.53006.x.
- Chenoweth L, King MT, Jeon YH, Brodaty H, Stein-Parbury J, Norman R, et al. Caring for Aged Dementia Care Resident Study (CADRES) of person-centred care, dementia-care mapping, and usual care in dementia: a cluster-randomised trial. Lancet Neurol 2009;8:317-25. http://dx.doi.org/10.1016/S1474-4422(09)70045-6.
- McCallion P, Toseland RW, Lacey D, Banks S. Educating nursing assistants to communicate more effectively with nursing home residents with dementia. Gerontologist 1999;39:546-58. http://dx.doi.org/10.1093/geront/39.5.546.
- McCallion P, Toseland RW, Freeman K. An evaluation of a family visit education program. J Am Geriatr Soc 1999;47:203-14.
- Teri L, Huda P, Gibbons L, Young H, van Leynseele J. STAR: a dementia-specific training program for staff in assisted living residences. Gerontologist 2005;45:686-93. http://dx.doi.org/10.1093/geront/45.5.686.
- Hoeffer B, Rader J, McKenzie D, Lavelle M, Stewart B. Reducing aggressive behavior during bathing cognitively impaired nursing home residents. J Gerontol Nurs 1997;23:16-23.
- Sloane PD, Hoeffer B, Mitchell CM, McKenzie DA, Barrick AL, Rader J, et al. Effect of person-centered showering and the towel bath on bathing-associated aggression, agitation, and discomfort in nursing home residents with dementia: a randomized, controlled trial. J Am Geriatr Soc 2004;52:1795-804. http://dx.doi.org/10.1111/j.1532-5415.2004.52501.x.
- Deudon A, Maubourguet N, Gervais X, Leone E, Brocker P, Carcaillon L, et al. Non-pharmacological management of behavioural symptoms in nursing homes. Int J Geriatr Psychiatry 2009;24:1386-95. http://dx.doi.org/10.1002/gps.2275.
- Chenoweth L, Jeon YH. Determining the efficacy of dementia care mapping as an outcome measure and a process for change: a pilot study. Aging Ment Health 2007;11:237-45. http://dx.doi.org/10.1080/13607860600844226.
- Burns A, Allen H, Tomenson B, Duignan D, Byrne J. Bright light therapy for agitation in dementia: a randomized controlled trial. Int Psychogeriatr 2009;21:711-21. http://dx.doi.org/10.1017/S1041610209008886.
- Lyketsos CG, Lindell VL, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry 1999;14:520-5. http://dx.doi.org/10.1002/(SICI)1099-1166(199907)14:7%3C520::AID-GPS983%3E3.3.CO;2-D.
- Dowling GA, Graf CL, Hubbard EM, Luxenberg JS. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. West J Nurs Res 2007;29:961-75. http://dx.doi.org/10.1177/0193945907303083.
- Thorpe L, Middleton J, Russell G, Stewart N. Bright light therapy for demented nursing home patients with behavioral disturbance. Am J Alzheimers Dis Other Demen 2000;15:18-26. http://dx.doi.org/10.1177/153331750001500109.
- Lovell BB, Ancoliisrael S, Gevirtz R. Effect of bright light treatment on agitated behavior in institutionalized elderly subjects. Psychiatry Res 1995;57:7-12. http://dx.doi.org/10.1016/0165-1781(95)02550-G.
- Satlin A, Volicer L, Ross V, Herz L, Campbell S. Bright light treatment of behavioral and sleep disturbances in patients with Alzheimer’s disease. Am J Psychiatry 1992;149:1028-32.
- Barrick AL, Sloane PD, Williams CS, Mitchell C, Connell BR, Wood W, et al. Impact of ambient bright light on agitation in dementia. Int J Geriatr Psychiatry 2010;25:1013-21. http://dx.doi.org/10.1002/gps.2453.
- Ancoli-Israel S, Martin JL, Gehrman P, Shochat T, Corey-Bloom J, Marler M, et al. Effect of light on agitation in institutionalized patients with severe Alzheimer disease. Am J Geriatr Psychiatry 2003;11:194-203. http://dx.doi.org/10.1176/appi.ajgp.11.2.194.
- Skjerve A, Holsten F, Aarsland D, Bjorvatn B, Nygaard HA, Johansen IM. Improvement in behavioral symptoms and advance of activity acrophase after short-term bright light treatment in severe dementia. Psychiatry Clin Neurosci 2004;58:343-7. http://dx.doi.org/10.1111/j.1440-1819.2004.01265.x.
- Haffmans PMJ, Sival RC, Lucius SAP, Cats Q, Van Gelder L. Bright light therapy and melatonin in motor restless behaviour in dementia: a placebo-controlled study. Int J Geriatr Psychiatry 2001;16:106-10. http://dx.doi.org/10.1002/1099-1166(200101)16:1<106::AID-GPS288>3.0.CO;2-9.
- Verbeek H, Zwakhalen SM, van Rossum E, Ambergen T, Kempen GI, Hamers JP. Dementia care redesigned: effects of small-scale living facilities on residents, their family caregivers, and staff. J Am Med Direct Assoc 2010;11:662-70. http://dx.doi.org/10.1016/j.jamda.2010.08.001.
- Elmstahl S, Annerstedt L, Ahlund O. How should a group living unit for demented elderly be designed to decrease psychiatric symptoms?. Alzheimer Dis Assoc Disorders 1997;11:47-52. http://dx.doi.org/10.1097/00002093-199703000-00008.
- Reimer MA, Slaughter S, Donaldson C, Currie G, Eliasziw M. Special care facility compared with traditional environments for dementia care: a longitudinal study of quality of life. J Am Geriatr Soc 2004;52:1085-92. http://dx.doi.org/10.1111/j.1532-5415.2004.52304.x.
- Annerstedt L, Gustafson L, Nilsson K. Medical outcome of psychosocial intervention in demented patients – one-year clinical follow-up after relocation into group living units. Int J Geriatr Psychiatry 1993;8:833-41. http://dx.doi.org/10.1002/gps.930081006.
- Burns A, Perry E, Holmes C, Francis P, Morris J, Howes MJ, et al. A double-blind placebo-controlled randomized trial of Melissa officinalis oil and donepezil for the treatment of agitation in Alzheimer’s disease. Dementia Geriatr Cogn Disorders 2011;31:158-64. http://dx.doi.org/10.1159/000324438.
- Lin PW, Chan WC, Ng BF, Lam LC-W. Efficacy of aromatherapy (Lavandula angustifolia) as an intervention for agitated behaviours in Chinese older persons with dementia: a cross-over randomized trial. Int J Geriatr Psychiatry 2007;22:405-10. http://dx.doi.org/10.1002/gps.1688.
- Ballard CG, O’Brien JT, Reichelt K, Perry EK. Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind, placebo-controlled trial with Melissa. J Clin Psychiatry 2002;63:553-8. http://dx.doi.org/10.4088/JCP.v63n0703.
- Holmes C, Hopkins V, Hensford C, MacLaughlin V, Wilkinson D, Rosenvinge H. Lavender oil as a treatment for agitated behaviour in severe dementia: a placebo controlled study. Int J Geriatr Psychiatry 2002;17:305-8. http://dx.doi.org/10.1002/gps.593.
- Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi AH, Khani M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: a double blind, randomised, placebo controlled trial. J Neurol Neurosurg Psychiatry 2003;74:863-6. http://dx.doi.org/10.1136/jnnp.74.7.863.
- Cameron H. Using lemon balm oil to reduce aggression and agitation in dementia: results of a pilot study. J Dementia Care 2012;19:36-9.
- Blau TH. Quality of life, social indicators, and predictors of change. Prof Psychol 1977;8:464-73.
- Teri L, Logsdon RG, Peskind E, Raskind M, Weiner MF, Tractenberg RE, et al. Treatment of agitation in AD – a randomized, placebo-controlled clinical trial. Neurology 2000;55:1271-8. http://dx.doi.org/10.1212/WNL.55.9.1271.
- Gormley N, Lyons D, Howard R. Behavioural management of aggression in dementia: a randomized controlled trial. Age Ageing 2001;30:141-5. http://dx.doi.org/10.1093/ageing/30.2.141.
- Bourgeois MS, Burgio LD, Schulz R, Beach S, Palmer B. Modifying repetitive verbalizations of community-dwelling patients with AD. Gerontologist 1997;37:30-9. http://dx.doi.org/10.1093/geront/37.1.30.
- Huang HL, Shyu YIL, Chen MC, Chen ST, Lin LC. A pilot study on a home-based caregiver training program for improving caregiver self-efficacy and decreasing the behavioral problems of elders with dementia in Taiwan. Int J Geriatr Psychiatry 2003;18:337-45. http://dx.doi.org/10.1002/gps.835.
- Wright LK, Litaker M, Laraia MT, DeAndrade S. Continuum of care for Alzheimer’s disease: a nurse education and counseling program. Issues Mental Health Nurs 2001;22:231-52. http://dx.doi.org/10.1080/01612840152053084.
- Haupt M, Karger A, Janner M. Improvement of agitation and anxiety in demented patients after psychoeducative group intervention with their caregivers. Int J Geriatr Psychiatry 2000;15:1125-9. http://dx.doi.org/10.1002/1099-1166(200012)15:12<1125::AID-GPS257>3.0.CO;2-F.
- Garland K, Beer E, Eppingstall B, O’Connor DW. A comparison of two treatments of agitated behavior in nursing home residents with dementia: simulated family presence and preferred music. Am J Geriatr Psychiatry 2007;15:514-21. http://dx.doi.org/10.1097/01.JGP.0000249388.37080.b4.
- Clark ME, Lipe AW, Bilbrey M. Use of music to decrease aggressive behaviors in people with dementia. J Gerontol Nurs 1998;24:10-7.
- Gerdner LA. Effects of individualized versus classical “relaxation” music on the frequency of agitation in elderly persons with Alzheimer’s disease and related disorders. Int Psychogeriatr 2000;12:49-65. http://dx.doi.org/10.1017/S1041610200006190.
- Park H, Specht JK. Effect of individualized music on agitation in individuals with dementia who live at home. J Gerontol Nurs 2009;35:47-55. http://dx.doi.org/10.3928/00989134-20090706-01.
- Gerdner LA. Use of individualized music by trained staff and family: translating research into practice. J Gerontol Nurs 2005;31:22-30.
- Tabloski PA. Effects of calming music on the level of agitation in cognitively impaired nursing home residents. Am J Alzheimers Dis Other Demen 1995;10:10-5. http://dx.doi.org/10.1177/153331759501000105.
- Ragneskog H, Brane G, Karlsson I, Kihlgren M. Influence of dinner music on food intake and symptoms common in dementia. Scand J Caring Sci 1996;10:11-7. http://dx.doi.org/10.1111/j.1471-6712.1996.tb00304.x.
- Chang F, Huang H, Lin K, Lin L. The effect of a music programme during lunchtime on the problem behaviour of the older residents with dementia at an institution in Taiwan. J Clin Nurs 2010;19:939-48. http://dx.doi.org/10.1111/j.1365-2702.2009.02801.x.
- Gerdner L. An individualized music intervention for agitation. J Am Psychiatric Nurs Assoc 1997;3:177-84. http://dx.doi.org/10.1016/S1078-3903(97)90043-4.
- Aman E, Thomas DR. Supervised exercise to reduce agitation in severely cognitively impaired persons. J Am Med Direct Assoc 2009;10:271-6. http://dx.doi.org/10.1016/j.jamda.2008.12.053.
- Holmberg SK. Evaluation of a clinical intervention for wanderers on a geriatric nursing unit. Arch Psychiatr Nurs 1997;11:21-8. http://dx.doi.org/10.1016/S0883-9417(97)80046-5.
- Eggermont LH, Blankevoort CG, Scherder EJ. Walking and night-time restlessness in mild-to-moderate dementia: a randomized controlled trial. Age Ageing 2010;39:746-9. http://dx.doi.org/10.1093/ageing/afq115.
- Buettner LL, Fitzsimmons S. Recreational therapy exercise on the special care unit: impact on behaviors. Am J Recreation Ther 2004;3:8-24.
- Robichaud L, Hebert R, Desrosiers J. Efficacy of a sensory integration program on behaviors of inpatients with dementia. Am J Occup Ther 1994;48:355-60. http://dx.doi.org/10.5014/ajot.48.4.355.
- Hong GR. Effects of multisensory stimulation using familiarity: persons with dementia in long-term care facility in Korea. J Korean Acad Nurs 2011;41:528-38. http://dx.doi.org/10.4040/jkan.2011.41.4.528.
- Toseland RW, Diehul M, Freeman K, Manzanares T, Naleppa M, McCallion P. The impact of validation group therapy on nursing home residents with dementia. South Gerontol Soc 1997;16:31-50.
- Kanamori M, Suzuki M, Yamamoto K, Kanda M, Matsui Y, Kojima E, et al. A day care program and evaluation of animal-assisted therapy (AAT) for the elderly with senile dementia. Am J Alzheimers Dis Other Demen 2001;16:234-9. http://dx.doi.org/10.1177/153331750101600409.
- Mossello E, Ridolfi A, Mello AM, Lorenzini G, Mugnai F, Piccini C, et al. Animal-assisted activity and emotional status of patients with Alzheimer’s disease in day care. Int Psychogeriatr 2011;23:899-905. http://dx.doi.org/10.1017/S1041610211000226.
- Libin A, Cohen-Mansfield J. Therapeutic robocat for nursing home residents with dementia: preliminary inquiry. Am J Alzheimers Dis Other Demen 2004;19:111-16. http://dx.doi.org/10.1177/153331750401900209.
- Magai C, Cohen CI, Gomberg D. Impact of training dementia caregivers in sensitivity to nonverbal emotion signals. Int Psychogeriatr 2002;14:25-38. http://dx.doi.org/10.1017/S1041610202008256.
- Finnema E, Dres RM, Ettema T, Ooms M, Adr H, Ribbe M, et al. The effect of integrated emotion-oriented care versus usual care on elderly persons with dementia in the nursing home and on nursing assistants: a randomized clinical trial. Int J Geriatr Psychiatry 2005;20:330-43. http://dx.doi.org/10.1002/gps.1286.
- Galik EM, Resnick B, Gruber-Baldini A, Nahm ES, Pearson K, Pretzer-Aboff I. Pilot testing of the restorative care intervention for the cognitively impaired. J Am Med Direct Assoc 2008;9:516-22. http://dx.doi.org/10.1016/j.jamda.2008.04.013.
- Matthews EA, Farrell GA, Blackmore AM. Effects of an environmental manipulation emphasizing client-centred care on agitation and sleep in dementia sufferers in a nursing home. J Adv Nurs 1996;24:439-47. http://dx.doi.org/10.1046/j.1365-2648.1996.02102.x.
- Detweiler MB, Murphy PF, Myers LC, Kim KY. Does a wander garden influence inappropriate behaviors in dementia residents. Am J Alzheimers Dis Other Demen 2008;23:31-45. http://dx.doi.org/10.1177/1533317507309799.
- Perivolaris A, LeClerc CM, Wilkinson K, Buchanan S. An enhanced dining program for persons with dementia. Alzheimers Care Q 2006;7:258-67.
- Darby SJ. Does a Mirror Deter Wandering in Demented Older People?. Int J Geriatr Psychiatry 1990;6:607-9.
- Hussian RA, Brown DC. Use of two-dimensional grid patterns to limit hazardous ambulation in demented patients. J Gerontol 1987;42:558-60. http://dx.doi.org/10.1093/geronj/42.5.558.
- McGilton KS, Rivera TM, Dawson P. Can we help persons with dementia find their way in a new environment?. Aging Ment Health 2003;7:363-71. http://dx.doi.org/10.1080/1360786031000150676.
- Camberg L, Woods P, Ooi WL, Hurley A, Volicer L, Ashley J, et al. Evaluation of simulated presence: a personalized approach to enhance well-being in persons with Alzheimer’s disease. J Am Geriatr Soc 1999;47:446-52.
- Bianchetti A, Benvenuti P, Ghisla KM, Frisoni GB, Trabucchi M. An Italian model of dementia special care unit: results of a pilot study. Alzheimer Dis Assoc Disord 1997;11:53-6. http://dx.doi.org/10.1097/00002093-199703000-00009.
- Beck CK, Vogelpohl TS, Rasin JH, Uriri JT, O’Sullivan P, Walls R, et al. Effects of behavioral interventions on disruptive behavior and affect in demented nursing home residents. Nurs Res 2002;51:219-28. http://dx.doi.org/10.1097/00006199-200207000-00002.
- Putman L, Wang JT. The Closing Group: Therapeutic recreation for nursing home residents with dementia and accompanying agitation and/or anxiety. Am J Alzheimers Dis Other Demen 2007;22:167-75. http://dx.doi.org/10.1177/1533317507300514.
- Vespa A, Gori G, Spazzafumo L. Evaluation of non-pharmacological intervention on antisocial behavior in patients suffering from Alzheimer’s disease in a day care center. Arch Gerontol Geriatr 2002;34:1-8. http://dx.doi.org/10.1016/S0167-4943(01)00106-6.
- Schalek M, Richeson NE, Buettner LL. Air mat therapy for treatment of agitated wandering: an evidence-based recrational therapy intervention. Am J Recreation Ther 2004;3:18-26.
- Buettner LL. Simple pleasures: a multilevel sensorimotor intervention for nursing home residents with dementia. Am J Alzheimers Dis 1999;14:41-52. http://dx.doi.org/10.1177/153331759901400103.
- Volicer L, Simard J, Pupa JH, Medrek R, Riordan ME. Effects of continuous activity programming on behavioral symptoms of dementia. J Am Med Direct Assoc 2006;7:426-31. http://dx.doi.org/10.1016/j.jamda.2006.02.003.
- Luk KY, Lai KY, Li CC, Cheung WH, Lam SM, Li HY, et al. The effect of horticultural activities on agitation in nursing home residents with dementia. Int J Geriatr Psychiatry 2011;26:435-6. http://dx.doi.org/10.1002/gps.2493.
- Churchill M, Safaoui J, Mccabe BW, Baun MM. Using a therapy dog to alleviate the agitation and desocialization of people with Alzheimer’s disease. J Psychosoc Nurs Mental Health Serv 1999;37:16-22.
- Kim NC, Kim HS, Yoo YS, Hahn SW, Yeom HA. Outcomes of day care: a pilot study on changes in cognitive function and agitated behaviors of demented elderly in Korea. Nurs Health Sci 2002;4:3-7. http://dx.doi.org/10.1046/j.1442-2018.2002.00094.x.
- Calkins M, Szmerekovsky JG, Biddle S. Effect of increased time spent outdoors on individuals with dementia residing in nursing homes. J Housing Elderly 2007;21:211-28. http://dx.doi.org/10.1300/J081v21n03_11.
- van Hoof J, Aarts M, Rense C, Schoutens A. Ambient bright light in dementia: effects on behaviour and circadian rhythmicity. Build Environ 2009;44:146-55. http://dx.doi.org/10.1016/j.buildenv.2008.02.005.
- Kuiper D, Dijkstra GJ, Tuinstra J, Groothoff JW. The influence of Dementia Care Mapping (DCM) on behavioural problems of persons with dementia and the job satisfaction of caregivers: a pilot study. Tijdschrift Gerontol Geriatr 2009;40:102-12. http://dx.doi.org/10.1007/BF03079572.
- Namazi KH, Zadorozny CA, Gwinnup PB. The influences of physical-activity on patterns of sleep behavior of patients with Alzheimers-disease. Int J Ageing Hum Develop 1995;40:145-53. http://dx.doi.org/10.2190/Q1PQ-8MKY-XWHN-JH8M.
- Wilkes L, Fleming A, Wilkes BL, Cioffi JM, Le Miere J. Environmental approach to reducing agitation in older persons with dementia in a nursing home. Aus J Ageing 2005;24:141-5. http://dx.doi.org/10.1111/j.1741-6612.2005.00105.x.
- Gori G, Pientini S, Vespa A. The selection of meaningful activities as a treatment for day-care in dementia. Arch Gerontol Geriatr Suppl 2001;7:207-12. http://dx.doi.org/10.1016/S0167-4943(01)00141-8.
- Cohen-Mansfield J, Libin A, Marx MS. Nonpharmacological treatment of agitation: a controlled trial of systematic individualized intervention. J Gerontol Series A 2007;62:908-16. http://dx.doi.org/10.1093/gerona/62.8.908.
- Bedard A, Landreville P, Voyer P, Verreault R, Vezina J. Reducing verbal agitation in people with dementia: evaluation of an intervention based on the satisfaction of basic needs. Ageing Mental Health 2011;15:855-65. http://dx.doi.org/10.1080/13607863.2011.569480.
- Ballard C, Brown R, Fossey J, Douglas S, Bradley P, Hancock J, et al. Brief psychosocial therapy for the treatment of agitation in Alzheimer disease (the CALM-AD trial). Am J Geriatr Psychiatry 2009;17:726-33. http://dx.doi.org/10.1097/JGP.0b013e3181b0f8c0.
- Lee SY. The effect of lavender aromatherapy on cognitive function, emotion, and aggressive behavior of elderly with dementia. Taehan Kanho Hakhoe Chi 2005;35:303-12.
- Smallwood J, Brown R, Coulter F, Irvine E, Copland C. Aromatherapy and behaviour disturbances in dementia: a randomized controlled trial. Int J Geriatr Psychiatry 2001;16:1010-13. http://dx.doi.org/10.1002/gps.473.
- Dickinson JI, McLain-Kark J, Marshall-Baker A. The effects of visual barriers on exiting behavior in a dementia care unit. Gerontologist 1995;35:127-30. http://dx.doi.org/10.1093/geront/35.1.127.
- Kincaid C, Peacock JR. The effect of a wall mural on decreasing four types of door-testing behaviours. J Appl Gerontol 2003;22:76-88. http://dx.doi.org/10.1177/0733464802250046.
- Chafetz PK. Two-dimensional grid is ineffective against demented patients’ exiting through glass doors. Psychol Aging 1990;5:146-7. http://dx.doi.org/10.1037/0882-7974.5.1.146.
- Davison TE, Hudgson C, McCabe MP, George K, Buchanan G. An individualized psychosocial approach for “treatment resistant” behavioral symptoms of dementia among aged care residents. Int Psychogeriatr 2007;19:859-73. http://dx.doi.org/10.1017/S1041610206004224.
- Chapman DG, Toseland RW. Effectiveness of advanced illness care teams for nursing home residents with dementia. Soc Work 2007;52:321-9. http://dx.doi.org/10.1093/sw/52.4.321.
- Ledger AJ, Baker FA. An investigation of long-term effects of group music therapy on agitation levels of people with Alzheimer’s disease. Aging Ment Health 2007;11:330-8. http://dx.doi.org/10.1080/13607860600963406.
- Choi AN, Lee MS, Cheong KJ, Lee JS. Effects of group music intervention on behavioral and psychological symptoms in patients with dementia: a pilot-controlled trial. Int J Neurosci 2009;119:471-81. http://dx.doi.org/10.1080/00207450802328136.
- Lesta B, Petocz P. Familiar group singing: addressing mood and social behaviour of residents with dementia displaying sundowning. Aus J Music Ther 2006;17:2-17.
- Sherratt K, Thornton A, Hatton C. Emotional and behavioural responses to music in people with dementia: an observational study. Aging Ment Health 2004;8:233-41. http://dx.doi.org/10.1080/13607860410001669769.
- Ziv N, Granot A, Hai S, Dassa A, Haimov I. The effect of background stimulative music on behavior in Alzheimer’s patients. J Music Ther 2007;44:329-43. http://dx.doi.org/10.1093/jmt/44.4.329.
- Brotons M, Pickett-Cooper PK. The effects of music therapy intervention on agitation behaviors of Alzheimer’s disease patients. J Music Ther 1996;33:2-18. http://dx.doi.org/10.1093/jmt/33.1.2.
- Cox E, Nowak M, Buettner P. Managing agitated behaviour in people with Alzheimer’s disease: the role of live music. Br J Occup Ther 2011;74:517-24. http://dx.doi.org/10.4276/030802211X13204135680866.
- Brotons M, Marti P. Music therapy with Alzheimer’s patients and their family caregivers: a pilot project. J Music Ther 2003;40:138-50. http://dx.doi.org/10.1093/jmt/40.2.138.
- Zare M, Ebrahimi AA, Birashk B. The effects of music therapy on reducing agitation in patients with Alzheimer’s disease, a pre–post study. Int J Geriatr Psychiatry 2010;25.
- Suzuki M, Kanamori M, Watanabe M, Nagasawa S, Kojima E, Ooshiro H, et al. Behavioral and endocrinological evaluation of music therapy for elderly patients with dementia. Nurs Health Sci 2004;6:11-8. http://dx.doi.org/10.1111/j.1442-2018.2003.00168.x.
- Thomas DW, Heitman RJ, Alexander T. The effects of music on bathing cooperation for residents with dementia. J Music Ther n.d.;4:246-59.
- Clair AA, Bernstein B. The effect on no music, stimulative background music and sedative background music on agitated behaviours in persons with severe dementia. Activities Adapt Ageing 1994;19:61-70. http://dx.doi.org/10.1300/J016v19n01_05.
- Berger G, Bernhardt T, Schramm U, Muller R, Landsiedel-Anders S, Peters J, et al. No effects of a combination of caregivers support group and memory training/music therapy in dementia patients from a memory clinic population. Int J Geriatr Psychiatry 2004;19:223-31. http://dx.doi.org/10.1002/gps.1055.
- Droes RM, Breebaart E, Ettema TP, van Tilburg W, Mellenbergh GJ. Effect of integrated family support versus day care only on behavior and mood of patients with dementia. Int Psychogeriatr 2000;12:99-115. http://dx.doi.org/10.1017/S1041610200006232.
- Fischer-Terworth C, Probst P. Evaluation of a TEACCH- and music therapy-based psychological intervention in mild to moderate dementia: a controlled trial. J Gerontopsychol Geriatr Psychiatry 2011;24.
- Hall L, Hare J. Video respite for cognitively impaired persons in nursing homes. Am J Alzheimers Dis 2011;12:117-21. http://dx.doi.org/10.1177/153331759701200304.
- Burgio L, Scilley K, Hardin JM, Hsu C, Yancey J. Environmental “white noise”. An intervention for verbally agitated nursing home residents. J Gerontol Series B 1996;51:364-73. http://dx.doi.org/10.1093/geronb/51B.6.P364.
- Suzuki M, Tatsumi A, Otsuka T, Kikuchi K, Mizuta A, Makino K, et al. Physical and psychological effects of 6-week tactile massage on elderly patients with severe dementia. Am J Alzheimers Dis Other Demen 2010;25:680-6. http://dx.doi.org/10.1177/1533317510386215.
- Wang KL, Hermann C. Pilot study to test the effectiveness of healing touch on agitation in people with dementia. Geriatr Nurs 2006;27:34-40. http://dx.doi.org/10.1016/j.gerinurse.2005.09.014.
- Milev RV, Kellar T, McLean M, Mileva V, Luthra V, Thompson S, et al. Multisensory stimulation for elderly with dementia: a 24-week single-blind randomized controlled pilot study. Am J Alzheimers Dis Other Demen 2008;23:372-6. http://dx.doi.org/10.1177/1533317508316681.
- Snyder M, Egan EC, Burns KR. Interventions for decreasing agitation behaviors in persons with dementia. J Gerontol Nurs 1995;21:34-40.
- Sutherland JA, Reakes J, Bridges C. Foot acupressure and massage for patients with Alzheimer’s disease and related dementias. J Nurs Scholar 1999;31:347-8. http://dx.doi.org/10.1111/j.1547-5069.1999.tb00516.x.
- Woods P, Ashley J. Simulated presence therapy: using selected memories to manage problem behaviors in Alzheimer’s disease patients. Geriatr Nurs 1995;16:9-14. http://dx.doi.org/10.1016/S0197-4572(05)80072-2.
- Miller S, Vermeersch PEH, Bohan K, Renbarger K, Kruep A, Sacre S. Audio presence intervention for decreasing agitation in people with dementia. Geriatr Nurs 2001;22:66-70. http://dx.doi.org/10.1067/mgn.2001.115200.
- Frisoni GB, Gozzetti A, Bignamini V, Vellas BJ, Berger A-K, Bianchetti A, et al. Special care units for dementia in nursing homes: a controlled study of effectiveness. Arch Gerontol Geriatr 1998;6:215-24. http://dx.doi.org/10.1016/S0167-4943(98)80031-9.
- Lawton MP, Van Haitsma K, Klapper J, Kleban MH, Katz IR, Maize J. A stimulation-retreat special care unit for elders with dementing illness. Int Psychogeriatr 1998;10:379-95. http://dx.doi.org/10.1017/S104161029800547X.
- Bellelli G, Frisoni GB, Bianchetti A, Boffelli S, Guerrini GB, Scotuzzi A, et al. Special care units for demented patients: a multicenter study. Gerontologist 1998;38:456-62. http://dx.doi.org/10.1093/geront/38.4.456.
- Davison TE, McCabe MP, Visser S, Hudgson C, Buchanan G, George K. Controlled trial of dementia training with a peer support group for aged care staff. Int J Geriatr Psychiatry 2007;22:868-73. http://dx.doi.org/10.1002/gps.1754.
- Asthill LF. Staff training and challenging behaviour in a day hospital. Dementia 2004;3:384-92.
- Ousset PJ, Sorel H, Cazard JC, Vellas B. Behavioral disorders in dementia: intervention study in a long-term care unit. Psychol Neuropsychiatr Vieil 2003;1:207-12.
- Edberg AK, Hallberg IR. Actions seen as demanding in patients with severe dementia during one year of intervention. Comparison with controls. Int J Nursing Studies 2001;38:271-85. http://dx.doi.org/10.1016/S0020-7489(00)00076-6.
- Sidani S, Streiner D, Leclerc C. Evaluating the effectiveness of the abilities-focused approach to morning care of people with dementia. Int J Older People Nurs 2012;7:37-45. http://dx.doi.org/10.1111/j.1748-3743.2011.00273.x.
- Cohen-Mansfield J, Jensen B. Do interventions bringing current self-care practices into greater correspondence with those performed premorbidly benefit the person with dementia? A pilot study. Am J Alzheimers Dis Other Demen 2006;21:312-17. http://dx.doi.org/10.1177/1533317506291135.
- Wells DL, Dawson P, Sidani S, Craig D, Pringle D. Effects of an abilities-focused program of morning care on residents who have dementia and on caregivers. J Am Geriatr Soc 2000;48:442-9.
- Rogers JC, Holm MB, Burgio LD, Granieri E, Hsu C, Hardin JM, et al. Improving morning care routines of nursing home residents with dementia. J Am Geriatr Soc 1999;47:1049-57.
- Visser S, Mccabe M, Hudgson C, Buchanan G, Davison T, George K. Managing behavioural symptoms of dementia: effectiveness of staff education and peer support. Aging Ment Health 2008;12:47-55. http://dx.doi.org/10.1080/13607860701366012.
- Cohen-Mansfield J, Werner P, Culpepper WJ, Barkley D. Evaluation of an inservice training program on dementia and wandering. J Gerontol Nurs 1997;23:40-7.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f1049.
- Mintzer JE, Colenda C, Waid LR, Lewis L, Meeks A, Stuckey M, et al. Effectiveness of a continuum of care using brief and partial hospitalization for agitated dementia patients. Psychiatric Services 1997;48:1435-9.
- Norman R, Haas M, Chenoweth L, Jeon Y-H, King M, Brodaty H, et al. Dementia Care Mapping and Patient-centred Care in Australian Residential Homes: an Economic Evaluation of the Care Study. Sydney, NSW: Centre for Health Economics Research and Evaluation, University of Technology, Sydney; 2008.
- Rosen J, Burgio L, Kollar M, Cain M, Allison M, Fogleman M, et al. A user-friendly instrument for rating agitation in dementia patients. Am J Geriatr Psychiatry 1994;2:52-9. http://dx.doi.org/10.1097/00019442-199400210-00008.
- Bogner J, Corrigan JD, Stange M, Rabold D. Reliability of the Agitated Behavior Scale. J Head Trauma Rehabil 1999;14:91-6. http://dx.doi.org/10.1097/00001199-199902000-00012.
- Logsdon RG, Teri L, Weiner MF, Gibbons LE, Raskind M, Peskind E, et al. Assessment of agitation in Alzheimer’s disease: the agitated behavor in dementia scale. Alzheimer’s Diseases Cooperative Study. J Am Geriatr Soc 1999;47:1354-8.
- Ryden MB, Bossenmaier M, McLachlan C. Aggressive behavior in cognitively impaired nursing home residents. Res Nurs Health 1991;14:87-95. http://dx.doi.org/10.1002/nur.4770140203.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. http://dx.doi.org/10.1212/WNL.44.12.2308.
- Livingston G, Katona C, Roch B, Guilhaume C, Rive B. A dependency model for patients with Alzheimer’s disease: its validation and relationship to the costs of care – the LASER-AD Study. Curr Med Res Opin 2004;20:1007-16. http://dx.doi.org/10.1185/030079904125003980.
- Beecham JKM, Thornicroft G. Measuring Mental Health Needs Costing Psychiatric Interventions. London: Gaskell; 2001.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy 2004;9:197-204. http://dx.doi.org/10.1258/1355819042250249.
- Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- 2010–2011 NHS Reference Costs. London: Department of Health; 2011.
- Rowen D, Mulhern B, Banerjee S, Hout B, Young TA, Knapp M, et al. Estimating preference-based single index measures for dementia using DEMQOL and DEMQOL-Proxy. Value Health 2012;15:346-56. http://dx.doi.org/10.1016/j.jval.2011.10.016.
- Smith SC, Lamping DL, Banerjee S, Harwood RH, Foley B, Smith P, et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol Med 2007;37:737-46. http://dx.doi.org/10.1017/S0033291706009469.
- Mulhern B, Smith SC, Rowen D, Brazier JE, Knapp M, Lamping DL, et al. Improving the measurement of QALYs in dementia: developing patient- and carer-reported health state classification systems using Rasch analysis. Value Health 2012;15:323-33. http://dx.doi.org/10.1016/j.jval.2011.09.006.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Briggs A, Schulpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Long PW. When Is A Difference Between Two Groups Significant? n.d. www.mentalhealth.com/dis-rs/rs-effect_size.html (accessed 16 March 2013).
- Oxford Centre for Evidence-based Medicine . Levels of Evidence 2009. www.cebm.net/index.aspx?o=1025 (accessed 13 July 2012).
- Hansen N, Jorgensen T, Ortenblad L. Massage and touch for dementia. Cochrane Database Syst Rev 2006;4.
- Forbes D, Morgan DG, Bangma J, Peacock S, Pelletier N, Adamson J. Light therapy for managing sleep, behaviour, and mood disturbances in dementia. Cochrane Database Syst Rev 2004;2.
- Lai CK, Yeung JH, Mok V, Chi I. Special care units for dementia individuals with behavioural problems. Cochrane Database Syst Rev 2009;4.
- Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry 2012;169:946-53. http://dx.doi.org/10.1176/appi.ajp.2012.11101529.
- Algase DL, Beck C, Kolanowski A, Whall A, Berent S, Richards K, et al. Need-driven dementia-compromised behavior: an alternative view of disruptive behavior. Am J Alzheimers Disease Other Demen 1996;11:10-9. http://dx.doi.org/10.1177/153331759601100603.
- Kitwood TBK. Towards a theory of dementia care: personhood and well-being. Ageing Soc 1992;12:269-87. http://dx.doi.org/10.1017/S0144686X0000502X.
- Sampson EL, Ritchie CW, Lai R, Raven PW, Blanchard MR. A systematic review of the scientific evidence for the efficacy of a palliative care approach in advanced dementia. Int Psychogeriatr 2005;17:31-40. http://dx.doi.org/10.1017/S1041610205001018.
- Livingston G, Leavey G, Manela M, Livingston D, Rait G, Sampson E, et al. Making decisions for people with dementia who lack capacity: qualitative study of family carers in UK. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4184.
- Underwood M, Lamb SE, Eldridge S, Sheehan B, Slowther AM, Spencer A, et al. Exercise for depression in elderly residents of care homes: a cluster-randomised controlled trial. Lancet 2013;382:41-9. http://dx.doi.org/10.1016/S0140-6736(13)60649-2.
- Weiner MF, Tractenberg RE, Sano M, Logsdon R, Teri L, Galasko D, et al. No long-term effect of behavioral treatment on psychotropic drug use for agitation in Alzheimer’s diease patients. J Geriatr Psychiatry Neurol 2002;15:95-8. http://dx.doi.org/10.1177/089198870201500208.
Appendix 1 Scoring of studies for quality of evidence
Studies were given a score of 0, 0.5 or 1 for each of the following criteria, with a maximum score of 14.
-
whether or not the study was a RCT
-
whether or not it had rater blinding
-
whether or not it had participant blinding
-
whether or not the outcome measure was valid
-
whether or not the outcome measure was reliable
-
whether or not a power analysis had been carried out
-
whether or not enough details had been given of the power analysis
-
whether or not sufficient power had been achieved
-
whether or not all participants were accounted for
-
whether or not > 80% of participants remained in the study at 1 month
-
whether or not intention-to-treat analyses had been used
-
whether or not the randomisation procedure, or in non-randomised studies, the control of the participants, was adequate to prevent bias
-
whether or not the statistical methods used were appropriate
-
whether or not a reliable diagnosis of dementia had been given.
Studies were assessed as being ‘high-quality RCTs’ if they had the following attributes:
-
RCT
-
rater or participant blinding or both
-
valid outcome measure
-
sufficient power
-
intention to treat
-
follow-up rates of > 80%
-
findings with reasonably small CIs.
Studies were then rated according to the CEBM levels of evidence as follows:
-
Level 1b: high-quality RCTs. These all scored ≥ 10, were single or double blind, with validated outcome measures.
-
Level 2b: lower-quality RCTs and higher-quality non-randomised studies (scoring ≤ 11).
-
Level 2c: moderate-quality non-randomised studies (scoring 6–9)
-
Level 4: these scored < 6. They were not RCTs.
Table 26 below shows details of the scoring for each study that was rated as 2c or above.
| Study | Overall category | 1. RCT | 2. Participant blinding | 3. Rater blinding | 4. 80% follow-up rate at 1 month | 5. Intention-to-treat analysis | 6. Sufficiently powered to measure agitation | 7. Valid outcome measure | 8. Power calculation carried out | 9. Power calculation – full details given | 10. Randomisation/control procedures adequate | 11. Reliable outcome measure | 12. All participants accounted for | 13. Statistical methods appropriate | 14 Reliable diagnosis of dementia | QS (range 1–14, sum of 14 individual quality scores) | Good-quality RCTa | Total participants | Level of evidence allocated | Reason for allocation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akhondzadeh et al., 2003101 | Aromatherapy | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0.5 | 1 | 0 | 0 | 1 | 1 | 7.5 | 0 | 42 | 2b | RCT with QS ≥ 6 |
| Aman and Thomas, 2009119 | Exercise | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 | 0 | 50 | 2c | Not a RCT, QS = 6–9 inclusive |
| Ancoli-Israel et al., 200390 | Light therapy | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 | 0 | 92 | 2b | RCT with QS ≥ 6 |
| Annerstedt et al., 199396 | Changing environment | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 6 | 0 | 56 | 2c | Not a RCT, QS = 6–9 inclusive |
| Ballard et al., 200299 | Aromatherapy | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 6 | 0 | 72 | 2b | RCT with QS ≥ 6 |
| Barrick et al., 201089 | Light therapy | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 66 | 2c | Not a RCT, QS = 6–9 inclusive |
| Beck et al., 2002140 | Other | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 7 | 0 | 96 | 2b | RCT with QS ≥ 6 |
| Bianchetti et al., 1997139 | Changing environment | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0.5 | 0 | 6.5 | 0 | 16 | 2c | Not a RCT, QS = 6–9 inclusive |
| Bourgeois et al., 1997106 | Behaviour therapy | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | 0 | 7 | 2b | RCT with QS ≥ 6 |
| Buettner et al., 199648 | Activities | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 36 | 2b | RCT with QS ≥ 6 |
| Buettner and Ferrario, 199746 | Activities | 1 | 0 | 1 | 0.5 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0.5 | 0 | 7 | 0 | 66 | 2b | RCT with QS ≥ 6 |
| Buettner and Fitzsimmons, 2004122 | Activities | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0.5 | 0 | 6.5 | 0 | 20 | 2c | Not a RCT, QS = 6–9 inclusive |
| Burns et al., 201197 | Aromatherapy | 1 | 1 | 1 | 0.5 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 12.5 | 1 | 94 | 1b | ‘Good-quality RCT’* with QS ≥ 10 |
| Burns et al., 200983 | Light therapy | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 | 12.5 | 1 | 48 | 1b | ‘Good-quality RCT’* with QS ≥ 10 |
| Camberg et al., 1999138 | Simulated Presence therapy | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 8 | 0 | 54 | 2b | RCT with QS ≥ 6 |
| Cameron 2012102 | Aromatherapy | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 7 | 0 | 18 | 2b | RCT with QS ≥ 6 |
| Chang et al., 2010117 | Music therapy | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | 0 | 47 | 2c | Not a RCT, QS = 6–9 inclusive |
| Chenoweth and Jeon, 200782 | DCM plus behavioural intervention | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | 0 | 35 | 2c | Not a RCT, QS = 6–9 inclusive |
| Chenoweth et al., 200975 | Education | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 0 | 11.5 | 1 | 180 | 1b | ‘Good-quality RCT’* with QS ≥ 10 |
| Clark et al., 1998111 | Music therapy | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0.5 | 0 | 6.5 | 0 | 18 | 2b | RCT with QS ≥ 6 |
| Cohen-Mansfield et al., 2006197 | Individualised intervention | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 6 | 0 | 105 | 2b | RCT with QS ≥ 6 |
| Cooke et al., 201053 | Music therapy | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 0 | 11.5 | 1 | 47 | 1b | ‘Good-quality RCT’* with QS ≥ 10 |
| Darby 1990135 | Changing environment | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | 0 | 9 | 2c | Not a RCT, QS = 6–9 inclusive |
| Detweiler et al., 2008133 | Changing environment | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | 0 | 34 | 2c | Not a RCT, QS = 6–9 inclusive |
| Deudon et al., 200981 | Education | 1 | 0 | 0.5 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7.5 | 306 | 2b | RCT with QS ≥ 6 | |
| Dowling et al., 200784 | Light therapy | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 | 0 | 70 | 2b | RCT with QS ≥ 6 |
| Eggermont et al., 2010121 | Exercise | 1 | 0 | 0 | 0.5 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 6.5 | 0 | 112 | 2b | RCT with QS ≥ 6 |
| Elmstahl et al., 199795 | Changing environment | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 | 0 | 103 | 2c | Not a RCT, QS = 6–9 inclusive |
| Finnema et al., 2005130 | Education | 1 | 0 | 0 | 0.5 | 0 | 0 | 1 | 1 | 0.5 | 0 | 1 | 1 | 1 | 1 | 8 | 0 | 146 | 2b | RCT with QS ≥ 6 |
| Fitzsimmons and Buettner, 200247 | Individualised intervention | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 29 | 2b | RCT with QS ≥ 6 |
| Fitzsimmons and Buettner, 200349 | Activities | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 12 | 2c | Not a RCT, QS = 6–9 inclusive |
| Galik et al., 2008131 | Education | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | 0 | 46 | 2c | Not a RCT, QS = 6–9 inclusive |
| Garland et al., 2007110 | Music therapy | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 8 | 0 | 30 | 2b | RCT with QS ≥ 6 |
| Gerdner 2000112 | Music therapy | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 6 | 0 | 45 | 2b | RCT with QS ≥ 6 |
| Gerdner et al., 200872 | Sensory | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 9 | 2c | Not a RCT, QS = 6–9 inclusive |
| Gerdner 2005114 | Music therapy | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 8 | 2c | Not a RCT, QS = 6–9 inclusive |
| Gerdner 1997118 | Music therapy | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 6 | 0 | 5 | 2c | Not a RCT, QS = 6–9 inclusive |
| Gormley et al., 2001105 | Behaviour therapy | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 0 | 1 | 1 | 1 | 1 | 11.5 | 1 | 65 | 1b | ‘Good-quality RCT’* with QS ≥ 10 |
| Groene 199358 | Music therapy | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 | 0 | 30 | 2b | RCT with QS ≥ 6 |
| Haffmans et al., 200192 | Light therapy | 0 | 0 | 0 | 0.5 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 6.5 | 0 | 10 | 2c | Not an RCT, QS = 6–9 inclusive |
| Haupt et al., 2000109 | Education | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 9 | 0 | 14 | 2c | Not a RCT, QS = 6–9 inclusive |
| Hawranik et al., 200868 | Sensory | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 7 | 0 | 51 | 2b | RCT with QS ≥ 6 |
| Hicks-Moore and Robinson, 200870 | Music therapy | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 6 | 0 | 32 | 2b | RCT with QS ≥ 6 |
| Hoeffer et al., 199779 | Education | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | 0 | 11 | 2c | Not a RCT, QS = 6–9 inclusive |
| Holmberg 1997120 | Exercise | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 6 | 0 | 11 | 2c | Not a RCT, QS = 6–9 inclusive |
| Holmes et al., 2002100 | Aromatherapy | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 9 | 0 | 15 | 2c | Not a RCT, QS = 6–9 inclusive |
| Hong 2011124 | Sensory | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 55 | 2b | RCT with QS ≥ 6 |
| Huang et al., 2003107 | Education | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 8 | 0 | 59 | 2b | RCT with QS ≥ 6 |
| Hussian and Brown, 1987136 | Changing environment | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | 0 | 8 | 2c | Not a RCT, QS = 6–9 inclusive |
| Jennings and Vance, 200261 | Music therapy | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 16 | 2c | Not a RCT, QS = 6–9 inclusive |
| Kanamori et al., 2001126 | Pet therapy | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 7 | 0 | 7 | 2c | Not a RCT, QS = 6–9 inclusive |
| Kolanowski et al., 200551 | Individualised intervention | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0.5 | 0 | 1 | 1 | 1 | 1 | 11.5 | 1 | 30 | 1b | ‘Good-quality RCT’* with QS ≥ 10 |
| Kolanowski et al., 201150 | Individualised intervention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 | 13.5 | 1 | 128 | 1b | ‘Good-quality RCT’* with QS ≥ 10 |
| Kovach et al., 200343 | Individualised intervention | 1 | 0 | 1 | 0.5 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7.5 | 0 | 78 | 2b | RCT with QS ≥ 6 |
| Lee and Kim, 200845 | Activities | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 7 | 0 | 23 | 2c | Not a RCT, QS = 6–9 inclusive |
| Libin and Cohen-Mansfield, 2004128 | Pet therapy | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 6 | 0 | 9 | 2c | Not a RCT, QS = 6–9 inclusive |
| Lin et al., 200944 | Activities | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 6 | 0 | 133 | 2b | RCT with QS ≥ 6 |
| Lin et al., 200798 | Aromatherapy | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 10 | 0 | 35 | 2b | RCT with QS ≥ 6 |
| Lin et al., 201157 | Music therapy | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 | 9.5 | 0 | 104 | 2b | RCT with QS ≥ 6 |
| Lovell et al., 199587 | Light therapy | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 6 | 2c | Not a RCT, QS = 6–9 inclusive |
| Lyketsos et al., 199984 | Light therapy | 1 | 0 | 1 | 0.5 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 7.5 | 0 | 15 | 2b | RCT with QS ≥ 6 |
| Magai et al., 2002129 | Education | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0.5 | 0 | 6.5 | 0 | 91 | 2b | RCT with QS ≥ 6 |
| Matthews et al., 1996132 | Education | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | 0 | 40 | 2c | Not a RCT, QS = 6–9 inclusive |
| McCallion et al., 199976 | Education | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 10 | 1 | 66 | 1b | ‘Good-quality RCT’* with QS ≥ 10 |
| McCallion et al., 199977 | Education | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 6 | 0 | 105 | 2b | RCT with QS ≥ 6 |
| McGilton et al., 2003137 | Other – wayfinding intervention | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0.5 | 1 | 0 | 0 | 1 | 0 | 7.5 | 0 | 32 | 2b | RCT with QS ≥ 6 |
| Mossello et al., 2011127 | Pet therapy | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 10 | 2c | Not a RCT, QS = 6–9 inclusive |
| Moyle et al., 201163 | Sensory | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | 0 | 27 | 2c | Not a RCT, QS = 6–9 inclusive |
| Park and Specht, 2009113 | Music therapy | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 0 | 8.5 | 0 | 15 | 2c | Not a RCT, QS = 6–9 inclusive |
| Perivolaris et al., 2006134 | Changing environment | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | 0 | 13 | 2c | Not a RCT, QS = 6–9 inclusive |
| Raglio et al., 200859 | Music therapy | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 9 | 0 | 59 | 2c | Not a RCT, QS = 6–9 inclusive |
| Ragneskog et al., 1996116 | Music therapy | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 | 0 | 24 | c2 | Not an RCT, QS = 6–9 inclusive |
| Reimer et al., 200495 | Changing environment | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 185 | 2c | Not a RCT, QS = 6–9 inclusive |
| Remington, 200266 | Music therapy | 1 | 0 | 0.5 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 0 | 11 | 0 | 68 | 2b | RCT with QS ≥ 6 |
| Robichaud et al., 1994123 | Sensory | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 0 | 1 | 1 | 1 | 1 | 11.5 | 1 | 40 | 1b | ‘Good-quality RCT’* with QS ≥ 10 |
| Satlin et al., 199288 | Light therapy | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 6 | 0 | 10 | 2c | Not a RCT, QS = 6–9 inclusive |
| Skjerve et al., 200491 | Light therapy | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 | 0 | 11 | 2c | Not a RCT, QS = 6–9 inclusive |
| Sloane et al., 200480 | Education | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 6 | 0 | 73 | 2b | RCT with QS ≥ 6 |
| Staal et al., 200769 | Sensory | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 7 | 24 | 2b | RCT with QS ≥ 6 | |
| Sung et al., 200655 | Music therapy | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 40 | 2b | RCT with QS ≥ 6 |
| Sung et al., 201254 | Music therapy | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 0 | 10.5 | 0 | 55 | 2b | No RCT, QS > 9 ≤ 11 |
| Suzuki, 200762 | Music therapy | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 | 0 | 16 | 2c | Not a RCT, QS = 6–9 inclusive |
| Svansdottir and Snaedal, 200660 | Music therapy | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0.5 | 1 | 7.5 | 0 | 20 | 2c | Not a RCT, QS = 6–9 inclusive |
| Tabloski 1995115 | Music therapy | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 7 | 0 | 20 | 2c | Not a RCT, QS = 6–9 inclusive |
| Teri et al., 200578 | Education | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 8 | 0 | 31 | 2b | RCT with QS ≥ 6 |
| Thorpe et al., 200086 | Light therapy | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 | 0 | 16 | 2c | Not a RCT, QS = 6–9 inclusive |
| Toseland 1997125 | Dementia specific therapy | 1 | 0 | 1 | 0.5 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 6.5 | 0 | 33 | 2b | RCT with QS ≥ 6 |
| Tuet and Lam, 200656 | Music therapy | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 6 | 0 | 16 | 2c | Not a RCT, QS = 6–9 inclusive |
| van Weert et al., 200547 | Sensory | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 10 | 0 | 125 | 2b | RCT with QS ≥ 6 |
| Verbeek et al., 201093 | Changing environment | 0 | 0 | 0 | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10.5 | 0 | 259 | 2b | Not a RCT, QS> 9 ≤ 11 |
| Weiner et al., 2002232 | Behaviour therapy | 1 | 0 | 1 | 0.5 | 1 | 1 | 1 | 1 | 0.5 | 0 | 1 | 1 | 1 | 1 | 11 | 1 | 77 | 1b | ‘Good-quality RCT’* with QS ≥ 10 |
| Whall et al., 199773 | Sensory | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | 0 | 31 | 2c | Not a RCT, QS = 6–9 inclusive |
| Woods et al., 200565 | Sensory | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 11 | 0 | 60 | 2b | RCT with QS ≥ 6 |
| Woods and Dimond, 200271 | Sensory | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 | 0 | 10 | 2c | Not a RCT, QS = 6–9 inclusive |
| Woods et al., 200967 | Sensory | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 11 | 0 | 64 | 2b | RCT with QS ≥ 6 |
| Wright et al., 2001108 | Education | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 7 | 0 | 93 | 2b | RCT with QS ≥ 6 |
| Yang et al., 200764 | Sensory | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 6 | 0 | 31 | 2c | Not a RCT, QS = 6–9 inclusive |
Appendix 2 List of excluded studies and reasons for exclusion
| Reference | Reason for exclusion |
|---|---|
| 100th Anniversary of H Kh Buniatian/International Symposium on Actual Problems in Neurochemistry and Neuroimmunology, Yerevan, Armenia, September 24–28, 2007. Neurochem Res 2008;33:1150–67 | No participants with dementia/not separately analysed |
| 82nd Congress of the Deutschen-Gesellschaft-fur-Neurologie, Nurnberg, Germany, September 23–26, 2009. Akt Neurol 2009;36(Suppl. 2):S47–215 | No participants with dementia/not separately analysed |
| Abernethy AP, Farrell TW. Pain and palliative care pharmacotherapy literature summaries and analyses. J Pain Palliative Care Pharmacother 2009;23:62–8 | No participants with dementia/not separately analysed |
| Abuhamdah S, Huang L, Elliott MS, Howes MJ, Ballard C, Holmes C, et al. Pharmacological profile of an essential oil derived from Melissa officinalis with anti-agitation properties: focus on ligand-gated channels. J Pharmacy Pharmacol 2008;60:377–84 | No participants with dementia/not separately analysed |
| Adib-Samii P, Brice G, Martin RJ, Markus HS. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype study in 200 consecutively recruited individuals. Stroke 2010;41:630–4 | No participants with dementia/not separately analysed |
| Adriani W, Ognibene E, Heuland E, Ghirardi O, Caprioli A, Laviola G. Motor impulsivity in APP-SWE mice: a model of Alzheimer’s disease. Behav Pharmacol 2006;17:525–33 | No participants with dementia/not separately analysed |
| Agarwal V, O’Neill PJ, Cotton BA, Pun BT, Haney S, Thompson J, et al. Prevalence and risk factors for development of delirium in burn intensive care unit patients. J Burn Care Res 2010;31:706–15 | No participants with dementia/not separately analysed |
| Agueera-Ortiz LF. Memantine in the pharmacologic treatment of moderately severe to severe Alzheimer’s disease in Spain (MEMORY study). Rev Neurol 2010;51:525–34 | Not a psychological, behavioural, sensory or environmental intervention |
| Ahmed MB. Alzheimer’s disease: recent advances in aetiology, diagnosis, and management. Texas Med 2001;97:50–8 | Not primary research |
| Akashi T, Arima K, Maruyama N, Ando S, Inose T. Severe cerebral atrophy in progressive supranuclear palsy – a case-report. Clin Neuropathol 1989;8:195–9 | No participants with dementia/not separately analysed |
| Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi AH, Khani M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: a double blind, randomised and placebo-controlled trial. J Clin Pharmacy Therap 2003;28:53–9 | No outcome measuring agitation |
| Akil M, Schwartz JA, Dutchak D, Yuzbasiyangurkan V, Brewer GJ. The psychiatric presentations of Wilsons-Disease. J Neuropsychiatry Clin Neurosci 1991;3:377–82 | No participants with dementia/not separately analysed |
| Aleman A, Kahn RS. Effects of the atypical antipsychotic risperidone on hostility and aggression in schizophrenia: a meta-analysis of controlled trials. Eur Neuropsychopharmacol 2001;11:289–93 | No participants with dementia/not separately analysed |
| Alessi CA, Schnelle JF. Approach to sleep disorders in the nursing home setting. Sleep Med Rev 2000;4:45–56 | No participants with dementia/not separately analysed |
| Alessi CA, Yoon EJ, Schnelle JF, Al Samarrai NR, Cruise PA. A randomised trial of a combined physical activity and environmental intervention in nursing home residents: Do sleep and agitation improve? J Am Geriatr Soc 1999;47:784–91 | No participants with dementia/not separately analysed |
| Alexander G, Hanna A, Serna V, Younkin L, Younkin S, Janus C. Increased aggression in males in transgenic Tg2576 mouse model of Alzheimer’s disease. Behav Brain Res 2011;216:77–83 | No participants with dementia/not separately analysed |
| Alexopoulos GS, Jeste DV, Chung H, Carpenter D, Ross R, Docherty JP. The expert consensus guideline series. Treatment of dementia and its behavioural disturbances. Introduction: methods, commentary, and summary. Postgrad Med 2005;Spec No.:6–22 | No participants with dementia/not separately analysed |
| Alexopoulos GS, Streim J, Carpenter D, Docherty JP. Introduction: methods, commentary, and summary. J Clin Psychiatry 2004;65:5–99 | Not primary research |
| Algase D, Son GR, Beel-Bates C, Song J, Yao L, Beattie E, et al. Initial psychometric evaluation of the wayfinding effectiveness scale. West J Nurs Res 2007;29:1015–32 | No participants with dementia/not separately analysed |
| Algase DL, Antonakos C, Yao L, Beattie ER, Hong GRS, Beel-Bates CA. Are wandering and physically nonaggressive agitation equivalent? Am J Geriatr Psychiatry 2008;16:293–9 | No participants with dementia/not separately analysed |
| Algase DL, Antonakos CL, Beattie E, Beel-Bates CA, Yao L. New parameters for daytime wandering. Res Gerontol Nurs 2009;2:58–68 | Not intervention study |
| Algase DL, Beattie ER, Antonakos C, Beel-Bates CA, Yao L. Wandering and the physical environment. Am J Alzheimers Dis Other Demen 2010;25:340–6 | Not intervention study |
| Algase DL, Beattie ER, Bogue EL, Yao L. The Algase Wandering Scale: initial psychometrics of a new caregiver reporting tool. Am J Alzheimers Dis Other Demen 2001;16:141–52 | No participants with dementia/not separately analysed |
| Algase DL, Beattie ERA. Training allies to reduce variance in a nursing home study: a communication intervention. Int J Geriatr Psychiatry 1996;11:889–93 | No participants with dementia/not separately analysed |
| Algase DL. Wandering in dementia. Annu Rev Nurs Res 1999;17:185–217 | Not primary research |
| Algase DL. What’s new about wandering behaviour? An assessment of recent studies. Int J Older People Nurs 2006;1:226–34 | Not primary research |
| Algozzine TW, Pitner JK, Mintzer JE, Jackson CW. Evaluation of the effectiveness of lorazepam for the treatment of agitation in the demented elderly. Pharmacotherapy 1996;16:499 | Not a psychological, behavioural, sensory or environmental intervention |
| Allain H, Schuck S, Mauduit N, Djemai M. Comparative effects of pharmacotherapy on the maintenance of cognitive function. Eur Psychiatry 2001;16:35S–41S | Not primary research |
| Allain H, Tessier C, Bentue-Ferrer D, Tarral A, Le Breton S, Gandon JM, et al. Effects of risperidone on psychometric and cognitive functions in healthy elderly volunteers. Psychopharmacology 2003;165:419–29 | No participants with dementia/not separately analysed |
| Allegri RF, Sarasola D, Serrano CM, Taragano FE, Arizaga RL, Butman J, et al. Neuropsychiatric symptoms as a predictor of caregiver burden in Alzheimer’s disease. Neuropsychiatr Dis Treat 2006;2:105–10 | No participants with dementia/not separately analysed |
| Allen RS, Burgio LD, Fisher SE, Hardin JM, Shuster JL. Behavioural characteristics of agitated nursing home residents with dementia at the end of life. Gerontologist 2005;45:661–6 | Not intervention study |
| Allen-Burge R, Stevens AB, Burgio LD. Effective behavioural interventions for decreasing dementia-related challenging behaviour in nursing homes. Int J Geriatr Psychiatry 1999;14:213–28 | Not primary research |
| Altes MK, Kurz A. Antidepressants for demented patients. Z Gerontol Geriatr 2000;33:396–400 | Not primary research |
| Alves G, Forsaa EB, Pedersen KF, Gjerstad MD, Larsen JP. Epidemiology of Parkinson’s disease. J Neurol 2008;255:18–32 | No participants with dementia/not separately analysed |
| Amano N, Matsuishi T, Akagi M, Yokoi S. The outpatient clinic for the aged in the department of psychiatry of Yokohama City University Hospital Japan. Yokohama Med J 1986;37:527–34 | No participants with dementia/not separately analysed |
| Ambree O, Touma C, Goertz N, Keyvani K, Paulus W, Palme R, et al. Activity changes and marked stereotypic behaviour precede A beta pathology in TgCRND8 Alzheimer mice. Neurobiol Ageing 2006;27:955–64 | No participants with dementia/not separately analysed |
| Amer-Ferrer G, de la Pena A, Soriano MTG, Martin AG. Main components of neuropsychiatric inventory in Alzheimer’s disease. Definition of behavioural syndromes. Neurologia 2005;20:9–16 | Not intervention study |
| Aminoff BZ, Adunsky A. Dying dementia patients: too much suffering, too little palliation. Am J Alzheimers Dis Other Demen 2004;19:243–7 | Not intervention study |
| Ancill RJ, Carlyle WW, Liang RA, Holliday SG. Agitation in the demented elderly – a role for benzodiazepines. Int Clin Psychopharmacol 1991;6:141–6 | Not a psychological, behavioural, sensory or environmental intervention |
| Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003;26:342–92 | No participants with dementia/not separately analysed |
| Ancoliisrael S, Klauber MR, Gillin JC, Campbell SS, Hofstetter CR. Sleep in noninstitutionalized Alzheimers-disease patients. Ageing 1994;6:451–8 | Not intervention study |
| Anderson KE, Marshall FJ. Behavioural symptoms associated with Huntington’s disease. Adv Neurol 2005;96:197–208 | No participants with dementia/not separately analysed |
| Anderson MA, Wendler MC, Congdon JC. Entering the world of dementia: CNA interventions for nursing home residents. J Gerontol Nurs 1998;24:31–7 | No quantitative outcome |
| Andre VM, Cepeda C, Levine MS. Dopamine and glutamate in Huntington’s disease: a balancing act. CNS Neurosci Ther 2010;16:163–78 | No participants with dementia/not separately analysed |
| Andrianov AM, Akhrem AA. Spatial structure of peptide rp142 containing the immunodominant epitope of the HIV-1 protein gp120. Theoretical study. Biofizika 1999;44:10–17 | No participants with dementia/not separately analysed |
| Angelini A, Bendini C, Neviani F, Neri M. Behavioural and psychological symptoms of dementia (BPSD) in elderly demented subjects: is the long lasting use of atypical antipsychotic drugs useful and safe? Arch Gerontol Geriatr 2007;44:35–43 | Not a psychological, behavioural, sensory or environmental intervention |
| Ansell BJ. Visual tracking behaviour in low functioning head-injured adults. Arch Phys Med Rehabil 1995;76:726–31 | No participants with dementia/not separately analysed |
| Antai-Otong D. Managing geriatric psychiatric emergencies: delirium and dementia. Nurs Clin North Am 2003;38:123 | Not primary research |
| Antai-Otong D. Pharmacological management of psychosis in Alzheimer’s disease: clinical challenges associated with second-generation antipsychotic medications. Persp Psychiatr Care 2008;44:120–3 | Not primary research |
| Antonini A. The role of I-ioflupane SPECT dopamine transporter imaging in the diagnosis and treatment of patients with dementia with Lewy bodies. Neuropsychiatr Dis Treat 2007;3:287–92 | Not primary research |
| Antonsson H, Graneheim U, Lundstrom M, Astrom S. Caregivers’ reflections on their interactions with adult people with learning disabilities. J Psychiatr Mental Health Nurs 2008;15:484–91 | No participants with dementia/not separately analysed |
| Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dementia Geriatr Cogn Disord 2008;25:115–26 | No participants with dementia/not separately analysed |
| Arbus C, Gardette V, Bui E, Cantet C, Andrieu S, Nourhashemi F, et al. Antidepressant use in Alzheimer’s disease patients: results of the REAL.FR cohort. Int Psychogeriatr 2010;22:120–8 | Not intervention study |
| Areosa SA, Sheriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005;2:CD003154 | Not primary research |
| Arlien-Soborg P, Bruhn P, Christensen EL, Gyldensted C, Damgaard M. Chronic painter’s disease: a follow-up investigation of 26 former house painters with occupational toxic encephalopathy. Ugeskr Laeger 1981;143:3069–74 | No participants with dementia/not separately analysed |
| Arney D. Sheep behaviour, needs, housing and care. Scand J Lab Animal Sci 2009;36:69–73 | No participants with dementia/not separately analysed |
| Aronson MK, Post DC, Guastadisegni P. Dementia, agitation, and care in the nursing-home. J Am Geriatr Soc 1993;41:507–12 | Not intervention study |
| Aronstein Z, Olsen R, Schulman E. The nursing assistants use of recreational interventions for behavioural management of residents with Alzheimer’s disease. Am J Alzheimers Dis Other Demen 1996;11:26–31 | No outcome measuring agitation |
| Arrieta-Cruz I, Pfaff DW. Definition of arousal and mechanistic studies in intact and brain-damaged mice. Disord Consciousness 2009;1157:24–31 | No participants with dementia/not separately analysed |
| Arrigo AP. sHsp as novel regulators of programmed cell death and tumorigenicity. Pathologie Biologie 2000;48:280–8 | No participants with dementia/not separately analysed |
| Arriola E, Ignacio Diago J, Antonio Buron J, Gallego R. Open-label, observational study of the effects of risperidone on the behavioural and psychological symptoms of dementia and caregiver stress in the community setting. Am J Geriatr Pharmacother 2005;3:8–16 | Not a psychological, behavioural, sensory or environmental intervention |
| Arsland D. Drug treatment of emotional and cognitive dysfunctions in Alzheimer’s disease. Tidssk Nor Laegeforen 1998;118:560–5 | Not primary research |
| Askenasy JJM. Sleep disturbances in Parkinsonism. J Neural Transmission 2003;110:125–50 | No participants with dementia/not separately analysed |
| Astell AJ. The Impact of Relaxation on Stress and Agitation in Dementia. National Research Register; 2006 | Conference presentation only |
| Aszalos Z. Some neurological and psychiatric complications in the disorders of the hypothalamus and the pituitary gland. Orvosi Hetilap 2007;148:723–30 | No participants with dementia/not separately analysed |
| Ata T, Terada S, Yokota O, Ishihara T, Fujisawa Y, Sasaki K, et al. Wandering and faecal smearing in people with dementia. Int Psychogeriatr 2010;22:493–500 | Not intervention study |
| Auchus AP, Bissey-Black C. Pilot study of haloperidol, fluoxetine, and placebo for agitation in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 1997;9:591–3 | Not a psychological, behavioural, sensory or environmental intervention |
| Aud MA, Parker-Oliver D, Bostick J, Schwarz B, Tofle RB. Social model care units for persons with dementia: the Missouri Demonstration Project. Alzheimers Care Q 2005;6:306–15 | No quantitative outcome |
| Ayalon L, Arean P, Bornfeld H, Beard R. Long term care staff beliefs about evidence based practices for the management of dementia and agitation. Int J Geriatr Psychiatry 2009;24:118–24 | No participants with dementia/not separately analysed |
| Ayalon L, Bornfeld H, Gum AM, Arean PA. The use of problem-solving therapy and restraint-free environment for the management of depression and agitation in long-term care. Clin Gerontologist 2009;32:77–90 | No comparator |
| Ayalon L, Gum AM, Feliciano L, Arean PA. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia – a systematic review. Arch Intern Med 2006;166:2182–8 | Not primary research |
| Bacalman S, Farzin F, Bourgeois JA, Cogswell J, Goodlin-Jones BL, Gane LW, et al. Psychiatric phenotype of the fragile X-associated tremor/ataxia syndrome (FXTAS) in males: newly described fronto-subcortical dementia. J Clin Psychiatry 2006;67:87–94 | No participants with dementia/not separately analysed |
| Bachinskaya N, Hoerr R, Ihl R. Alleviating neuropsychiatric symptoms in dementia: the effects of Ginkgo biloba extract EGb 761. Findings from a randomised controlled trial. Neuropsychiatr Dis Treat 2011;7:209–15 | Not a psychological, behavioural, sensory or environmental intervention |
| Bachman D, Rabins P. “Sundowning” and other temporally associated agitation states in dementia patients. Annu Rev Med 2006;57:499–511 | Not primary research |
| Bachman DL. Sleep disorders with ageing – evaluation and treatment. Geriatrics 1992;47:53 | No participants with dementia/not separately analysed |
| Bachurin S, Bukatina E, Lermontova N, Tkachenko S, Afanasiev A, Grigoriev V, et al. Antihistamine agent dimebon as a novel neuroprotector and a cognition enhancer. Neuroprotective Agents 2001;939:425–35 | Not primary research |
| Baillon S, Van Diepen E, Prettyman R, Redman J, Rooke N, Campbell R. A comparison of the effects of Snoezelen and reminiscence therapy on the agitated behaviour of patients with dementia. Int J Geriatr Psychiatry 2004;19:1047–52 | No quantitative outcome |
| Baiyewu O, Smith-Gamble V, Akinbiyi A, Lane KA, Hall KS, Ogunniyi A, et al. Behavioural and caregiver reaction of dementia as measured by the neuropsychiatric inventory in Nigerian community residents. Int Psychogeriatr 2003;15:399–409 | No participants with dementia/not separately analysed |
| Baker F. The effects of live, taped, and no music on people experiencing posttraumatic amnesia. J Music Therapy 2001;38:170–92 | No participants with dementia/not separately analysed |
| Baker J, Keady J, Hardman P, Kay J, Jones L, Jolley D. Psychotropic PRN use among older people’s inpatient mental health services. J Psychiatr Mental Health Nurs 2010;17:463–8 | No participants with dementia/not separately analysed |
| Bakey AA, Kunik ME, Orengo CA, Molinari VA, Workman RH, Hamilton JD. Outcome of psychiatric hospitalisation for very low-functioning demented patients. J Geriatr Psychiatry Neurol 1997;10:55–7 | Not intervention study |
| Bakke BL, Kvale S, Burns T, McCarten JR. Multicomponent intervention for agitated behaviour in a person with Alzheimers-disease. J Appl Behav Anal 1994;27:175–6 | No comparator |
| Bakker TJEM, Duivenvoorden HJ, van der Lee J, Trijsburg RW. Prevalence of psychiatric function disorders in psychogeriatric patients at referral to nursing home care – the relation to cognition, activities of daily living and general details. Dementia Geriatr Cogn Disord 2005;20:215–24 | Not intervention study |
| Balas MC, Deutschman CS, Sullivan-Marx EM, Strumpf NE, Alston RP, Richmond TS. Katz index of independence in activities of daily living. J Nurs Scholar 2007;39:147–54 | Not intervention study |
| Ball V, Snow A, Steele AB, Morgan RO, Davila JA, Wilson N, et al. Quality of relationships as a predictor of psychosocial functioning in patients with dementia. J Geriatr Psychiatry Neurol 2010;23:109–14 | Not intervention study |
| Ball VL, Hudson S, Davila J, Morgan R, Walder A, Graham DP, et al. Post-traumatic stress disorder and prediction of aggression in persons with dementia. Int J Geriatr Psychiatry 2009;24:1285–90 | Not intervention study |
| Ballard C, Corbett A, Chitramohan R, Aarsland D. Management of agitation and aggression associated with Alzheimer’s disease: controversies and possible solutions. Curr Opin Psychiatry 2009;22:532–40 | Not primary research |
| Ballard C, Corbett A. Management of neuropsychiatric symptoms in people with dementia. CNS Drugs 2010;24:729–39 | Not primary research |
| Ballard C, Creese B, Corbett A, Aarsland D. Atypical antipsychotics for the treatment of behavioural and psychological symptoms in dementia, with a particular focus on longer term outcomes and mortality. Ex Opin Drug Saf 2011;10:35–43 | Not primary research |
| Ballard C, Day S, Sharp S, Wing G, Sorensen S. Neuropsychiatric symptoms in dementia: Importance and treatment considerations. Int Rev Psychiatry 2008;20:396–404 | Not primary research |
| Ballard C, Grey A, Ayre G. Psychotic symptoms, aggression and restlessness in dementia. Revue Neurologique 1999;155(Suppl. 4):44–52 | Not primary research |
| Ballard C, Margallo-Lana M, Juszczak E, Douglas S, Swann A, Thomas A, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ 2005;330:874–7 | Not a psychological, behavioural, sensory or environmental intervention |
| Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev 2006;1 | Not primary research |
| Ballard C. Agitation and psychosis in dementia. Am J Geriatr Psychiatry 2007;15:913–17 | Not primary research |
| Ballard CG, Gauthier S, Cummings JL, Brodaty H, Grossberg GT, Robert P, et al. Management of agitation and aggression associated with Alzheimer disease. Nature Rev Neurol 2009;5:245–55 | Not primary research |
| Ballard CG, Margallo-Lana M, Fossey J, Reichelt K, Myint P, Potkins D, et al. A 1-year follow-up study of behavioral and psychological symptoms in dementia among people in care environments. J Clin Psychiatry 2001;62:631–6 | Not intervention study |
| Ballard CG, Thomas A, Fossey J, Lee L, Jacoby R, Lana MM, et al. A 3-month, randomized, placebo-controlled, neuroleptic discontinuation study in 100 people with dementia: the neuropsychiatric inventory median cutoff is a predictor of clinical outcome. J Clin Psychiatry 2004;65:114–19 | Not a psychological, behavioural, sensory or environmental intervention |
| Ban TA, Morey LC, Fjetland OK, Rengo F, Ferrara N, Agnetti V, et al. Early manifestations of dementing illness – treatment with glycosaminoglycan polysulfate. Progr Neuro-Psychopharmacol Biol Psychiatry 1992;16:661–76 | Not a psychological, behavioural, sensory or environmental intervention |
| Banerjee S, Samsi K, Petrie CD, Alvir J, Treglia M, Schwam EM, et al. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. Int J Geriatr Psychiatry 2009;24:15–24 | Not primary research |
| Baquero M, Blasco R, Campos-Garcia A, Garces M, Fages EM, Andreu-Catala M. Descriptive study of behavioural disorders in mild cognitive impairment. Rev Neurol 2004;38:323–6 | No participants with dementia/not separately analysed |
| Barba R, Garay J, Martin-Alvarez H, Herrainz C, Castellanos V, Gonzalez-Anglada I, et al. Use of neuroleptics in a general hospital. BMC Geriatr 2002;2:2 | Not intervention study |
| Barone P, Amboni M, Vitale C, Bonavita V. Treatment of nocturnal disturbances and excessive daytime sleepiness in Parkinson’s disease. Neurology 2004;63:S35–8 | No participants with dementia/not separately analysed |
| Bartels SJ, Horn SD, Smout RJ, Dums AR, Flaherty E, Jones JK, et al. Agitation and depression in frail nursing home elderly patients with dementia – treatment characteristics and service use. Am J Geriatr Psychiatry 2003;11:231–8 | Not intervention study |
| Bartko G. [New formulations of olanzapine in the treatment of acute agitation.] Neuropsychopharmacol Hung 2006;8:171–8 | No participants with dementia/not separately analysed |
| Barton S, Findlay D, Blake RA. The management of inappropriate vocalisation in dementia: a hierarchical approach. Int J Geriatr Psychiatry 2005;20:1180–6 | Not primary research |
| Baskys A, Fang L. Antipsychotic quetiapine at low concentrations reduces N–methyl–D–aspartate–induced cell death in organotypic hippocampal cultures without altering N–methyl–D–aspartate–mediated responses: implications for treatment of Lewy body dementia. Soc Neurosci Abstract View Itinerary Plan 2003 | No participants with dementia/not separately analysed |
| Baskys A, Segal J, Fang LW. Neuroprotective properties of topiramate in organotypic hippocampal cultures: implications for treatment of vascular and other dementias. Drug Devel Res 2002;56:393–400 | No participants with dementia/not separately analysed |
| Bassetti CL. Nonmotor disturbances in Parkinson’s disease. Neurodegen Dis 2011;8:95–108 | No participants with dementia/not separately analysed |
| Battaglia J, Lindborg SR, Alaka K, Meehan K, Wright P. Calming versus sedative effects of intramuscular olanzapine in agitated patients. AmJ Emerg Med 2003;21:192–8 | No participants with dementia/not separately analysed |
| Battaglia J. Pharmacological management of acute agitation. Drugs 2005;65:1207–22 | No participants with dementia/not separately analysed |
| Bavazzano A, Magnolfi SU, Calvani D, Valente C, Boni F, Baldini A, et al. Functional evaluation of Alzheimer patients during clinical trials: a review. Arch Gerontol Geriatr 1998;27–32 | Not primary research |
| Bayer E, Goettsch S, Mueller JW, Griewel B, Guiberman E, Mayr LM, et al. Structural analysis of the mitotic regulator hPin1 in solution – insights into domain architecture and substrate binding. J Biol Chem 2003;278:26183–93 | No participants with dementia/not separately analysed |
| Beattie ERA, Algase DL, Song J. Keeping wandering nursing home residents at the table: improving food intake using a behavioural communication intervention. Ageing Mental Health 2004;8:109–16 | No comparator |
| Beattie ERA, Algase DL. Improving table-sitting behaviour of wanderers via theoretic substruction. Designing an Intervention. J Gerontol Nurs 2002;28:6–11 | Not primary research |
| Beck C, Richards K, Lambert C, Doan R, Landes RD, Whall A, et al. Factors associated with problematic vocalizations in nursing home residents with dementia. Gerontologist 2011;51:389–405 | Not intervention study |
| Beck CK, Shue VM. Interventions for treating disruptive behaviour in demented elderly people. Nurs Clin North Am 1994;29:143–55 | Not primary research |
| Beck S, Paton C, Euba R, Goddard C. Atypical antipsychotics in the elderly. Int J Psychiatry Clin Prac 2001;5:257–61 | No participants with dementia/not separately analysed |
| Becker PM, Feussner JR, Mulrow CD, Williams BC, Vokaty KA. The role of lumbar puncture in the evaluation of dementia – the Durham-Veterans-Administration Duke-University study. J Am Geriatr Soc 1985;33:392–6 | Not a psychological, behavioural, sensory or environmental intervention |
| Bedard A, Landreville P. Preliminary studies of non-pharmacological intervention to reduce verbal agitation in people affected by dementia. Can J Aging 2005;24:319–28 | No comparator |
| Bedrosian TA, Herring KL, Weil ZM, Nelson RJ. Altered temporal patterns of anxiety in aged and amyloid precursor protein (APP) transgenic mice. Proc Natl Acad Sci U.S.A. 2011;108:11686–91 | No participants with dementia/not separately analysed |
| Beeri MS, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry 2002;17:403–8 | Not intervention study |
| Behavior Genetics Association. 30th Annual Meeting of the Behavior Genetics Association, Burlington, VT, USA, 30 June 2000. Behav Genet 2000;30:397–423 | Not primary research |
| Beier MT. Pharmacotherapy for behavioral and psychological symptoms of dementia in the elderly. Am J Health-Syst Pharm 2007;64:S9–17 | Not primary research |
| Bekelman DB, Black BS, Shore AD, Kasper JD, Rabins PV. Hospice care in a cohort of elders with dementia and mild cognitive impairment. J Pain Symptom Manag 2005;30:208–14 | Not intervention study |
| Belanger HG, King-Kallimanis B, Nelson AL, Schonfeld L, Scott SG, Vanderploeg RD. Characterizing wandering behaviors in persons with traumatic brain injury residing in Veterans Health Administration nursing homes. Arch Phys Med Rehabil 2008;89:244–50 | No participants with dementia/not separately analysed |
| Bellnier TJ. Continuum of care: stabilizing the acutely agitated patient. Am J Health-Syst Pharm 2002;59:S12–18 | Not primary research |
| Belzie LR. Risperidone for AIDS-associated dementia: a case series. AIDS Patient Care STDs 1996;10:246–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Benoit M, Arbus C, Blanchard F, Camus V, Cerase V, Clement JP, et al. Professional consensus on the treatment of agitation, aggressive behaviour, oppositional behaviour and psychotic disturbances in dementia. J Nutr Health Aging 2006;10:410–5 | Not primary research |
| Benoit M, Robert PH, Staccini P, Brocker P, Guerini O, Lechowshi L, et al. One-year longitudinal evaluation of neuropsychiatric symptoms in Alzheimer’s disease. The REAL.FR study. J Nutr Health Aging 2005;9:95–9 | Not intervention study |
| Berg D. Movement disorders associated with disturbances of brain iron metabolism. Eur J Neurol 2006;13:304 | No participants with dementia/not separately analysed |
| Berger K. Non-opioid analgesics and the risk of restless leg syndrome – a spurious association? Sleep Med 2003;4:351–2 | No participants with dementia/not separately analysed |
| Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intens Care Med 2001;27:859–64 | No participants with dementia/not separately analysed |
| Bergman J, Lerner V. Successful use of donepezil for the treatment of psychotic symptoms in patients with Parkinson’s disease. Clin Neuropharmacol 2002;25:107–10 | No participants with dementia/not separately analysed |
| Berlow YA, Wells WM, Ellison JM, Sung YH, Renshaw PF, Harper DG. Neuropsychiatric correlates of white matter hyperintensities in Alzheimer’s disease. Int J Geriatr Psychiatry 2010;25:780–8 | Not intervention study |
| Bernardo CG, Singh V, Thompson PM. Safety and efficacy of psychopharmacological agents used to treat the psychiatric sequelae of common neurological disorders. Exp Opin Drug Saf 2008;7:435–45 | Not primary research |
| Berrios GE, Wagle AC, Markova IS, Wagle SA, Rosser A, Hodges JR. Psychiatric symptoms in neurologically asymptomatic Huntington’s disease gene carriers: a comparison with gene negative at risk subjects. Acta Psychiatr Scand 2002;105:224–30 | No participants with dementia/not separately analysed |
| Bezzant K. Practice development: providing benefits for both managers and older patients with delerium and dementia. J Nurs Manag 2008;16:141–6 | Not primary research |
| Bhana N, Spencer CM. Risperidone – a review of its use in the management of the behavioural and psychological symptoms of dementia. Drugs Aging 2000;16:451–71 | Not primary research |
| Bharani N, Snowden M. Evidence-based interventions for nursing home residents with dementia-related behavioral symptoms. Psychiatric Clin North Am 2005;28:985 | Not primary research |
| Bharucha AJ, Vasilescu M, Dew MA, Begley A, Stevens S, Degenholtz H, et al. Prevalence of behavioral symptoms: comparison of the minimum data set assessments with research instruments. J Am Med Direct Assoc 2008;9:244–50 | Not intervention study |
| Bianchetti A, Trabucchi M. Behavioural and psychological symptoms of dementia: clinical aspects. Neurosci Res Comm 2004;35:173–83 | Not primary research |
| Bidzan L, Bidzan M. [Assessment of behavioural and psychotic symptoms and functional status of nursing home residents with a diagnosis of dementia.] Psychiatria Polska 2006;40:833–43 | Not intervention study |
| Bidzan L, Bidzan M. Reliability of The Cohen-Mansfield Agitation Inventory Polish version among demented and non-demented nursing home residents. Psychiatria Polska 2007;41:789–97 | No participants with dementia/not separately analysed |
| Billig N. Management of agitation in nursing home patients – treatment options. Drugs Aging 1996;9:93–100 | Not primary research |
| Bird M, Jones RH, Kortent A, SmIthers H. A controlled trial of a predominantly psychosocial approach to BPSD: treating causality. Int Psychogeriatr 2007;19:874–91 | Multidisciplinary team input including pharmacological intervention |
| Bird M, Llewellyn-Jones R, Smithers H, Andrews C, Cameron I, Cottee A, et al. Challenging behaviours in dementia: a project at Hornsby Ku-Ring-Gai Hospital. Aus J Ageing 1998;17:10–15 | Protocol only |
| Bird M, Llewellyn-Jones R. Psychosocial approaches to challenging behaviour in dementia: results of an intervention study. Austr J Ageing 2000;19:64–5 | Conference Presentation Only |
| Bird M, Llewellyn-Jones RH, Korten A. An evaluation of the effectiveness of a case-specific approach to challenging behaviour associated with dementia. Aging Mental Health 2009;13:73–83 | No outcome measuring agitation |
| Bird M. Challenging behaviour in dementia: a critical role for psychology. Aus Psychol 1999;34:144–8 | Not primary research |
| Birks J, Flicker L. Selegiline for Alzheimer’s disease. Cochrane Database Syst Rev 2003;1:CD000442 | Not primary research |
| Bishara D, Taylor D, Howard RJ, Abdel-Tawab R. Expert opinion on the management of behavioural and psychological symptoms of dementia (BPSD) and investigation into prescribing practices in the UK. Int J Geriatr Psychiatry 2009;24:944–54 | No participants with dementia/not separately analysed |
| Biswas J, Jayachandran M, Thang PV, Fook VFS, Choo TS, Qiang Q, et al. Agitation monitoring of persons with dementia based on acoustic sensors, pressure sensors and ultrasound sensors: a feasibility study. Able Data 2006;8:3–14 | Not intervention study |
| Bittner DM, Gron G. Alzheimer’s disease – diagnostic and therapeutic approaches. Nervenheilkunde 2005;24:591 | Not primary research |
| Blair DT. Assaultive behavior. Does provocation begin in the front office? J Psychosoc Nurs Mental Health Serv 1991;29:21–6 | No participants with dementia/not separately analysed |
| Blanco-Munez O, Suarez-Gauthier A, Martin-Garcia H, Diaz-Konrad V, Antonio-Roman V, Cabello A. Unusual cortical compromise in a case of Wernicke’s encephalopathy. Rev Neurol 2006;42:596–9 | No participants with dementia/not separately analysed |
| Bliwise DL, Greenaway MC. Will APPLES hit a ceiling? Sleep 2011;34:249–50 | No participants with dementia/not separately analysed |
| Bliwise DL, Yesavage JA, Tinklenberg JR. Sundowning and rate of decline in mental function in Alzheimers-disease. Dementia 1992;3:335–41 | Not intervention study |
| Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias. Clin Cornerstone 2004;6(Suppl. 1A):S16–28 | Not primary research |
| Boada M, Cejudo JC, Tarraga L, Lopez OL, Kaufer D. Neuropsychiatric Inventory Questionnaire (NPI-Q): Spanish validation of a brief clinical form of the Neuropsychiatric inventory (NPI). Neurologia 2002;17:317–23 | No participants with dementia/not separately analysed |
| Boada M, Tarraga L, Modinos G, Diego S, Reisberg B. Behavioural pathology in Alzheimer’s disease rating scale (BEHAVE-AD): Spanish validation. Neurologia 2006;21:19–25 | No participants with dementia/not separately analysed |
| Bobin SA, Currie JR, Merz PA, Miller DL, Styles J, Walker WA, et al. The comparative immunoreactivities of brain amyloids in Alzheimers disease and Scrapie. Acta Neuropathologica 1987;74:313–23 | No participants with dementia/not separately analysed |
| Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioural symptoms in Alzheimer disease. Arch Neurol 1997;54:465–73 | Not a psychological, behavioural, sensory or environmental intervention |
| Bodick NC, Offen WW, Shannon HE, Satterwhite J, Lucas R, van Lier R, et al. The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioural symptoms of Alzheimer disease. Alzheimer Dis Assoc Disord 1997;11:S16–22 | Not a psychological, behavioural, sensory or environmental intervention |
| Boillet D, Szoke A. Psychiatric symptoms as single manifestation of hypothyroidism. Encephale Rev Psychiatr Clin Biol Therap 1998;24:65–8 | No participants with dementia/not separately analysed |
| Bolea-Alamanac BM, Davies SJ, Christmas DM, Baxter H, Cullum S, Nutt DJ. Cyproterone to treat aggressivity in dementia: a clinical case and systematic review. J Psychopharmacol 2011;25:141–5 | Not primary research |
| Bolivar VJ, Ganus JS, Messer A. The development of behavioural abnormalities in the motor neuron degeneration (MND) mouse. Brain Res 2002;937:74–82 | No participants with dementia/not separately analysed |
| Bonelli RM, Hofmann P. A review of the treatment options for Huntington’s disease. Exp Opin Pharmacother 2004;5:767–76 | No participants with dementia/not separately analysed |
| Boockvar KS, Lachs MS. Predictive value of nonspecific symptoms for acute illness in nursing home residents. J Am Geriatr Soc 2003;51:1111–15 | No participants with dementia/not separately analysed |
| Borbasi S, Emmanuel E, Farrelly B, Ashcroft J. A nurse practitioner initiated model of service delivery in caring for people with dementia. Contemp Nurse 2010;36:49–60 | No participants with dementia/not separately analysed |
| Borbasi S, Emmanuel E, Farrelly B, Ashcroft J. Report of an evaluation of a Nurse-led Dementia Outreach Service for people with the behavioural and psychological symptoms of dementia living in residential aged care facilities. Perspect Public Health 2011;131:124–30 | No outcome measuring agitation |
| Borgenicht K, Carty E, Feigenbaum LZ. Community resources for frail older patients. West J Med 1997;167:291–4 | No participants with dementia/not separately analysed |
| Borroni B, Agosti C, Padovani A. Behavioral and psychological symptoms in dementia with Lewy-bodies (DLB): frequency and relationship with disease severity and motor impairment. Arch Gerontol Geriatr 2008;46:101–6 | Not intervention study |
| Bouman A, I, Ettema T, Wetzels R, van Beek A, de Lange J, Droes R. Evaluation of Qualidem: a dementia-specific quality of life instrument for persons with dementia in residential settings; scalability and reliability of subscales in four Dutch field surveys. Int J Geriatr Psychiatry 2011;26:711–22 | No participants with dementia/not separately analysed |
| Bourbonnais A, Ducharme F. The meanings of screams in older people living with dementia in a nursing home. Int Psychogeriatr 2010;22:1172–84 | Not intervention study |
| Bowen JD, Malter AD, Sheppard L, Kukull WA, McCormick WC, Teri L, et al. Predictors of mortality in patients diagnosed with probable Alzheimer’s disease. Neurology 1996;47:433–9 | Not intervention study |
| Bower FL, McCullough CS, Pille BL. Synthesis of research findings regarding the care of people with Alzheimer’s disease – part II. Online J Knowledge Synth Nurs 2002;9(4) | Not primary research |
| Boyer P, Ondo W, Allen R, Earley C, Menzies S, Chen XL, et al. Neuropathologic evaluation of the central nervous system in restless legs syndrome: case report and review of literature. Soc Neurosci Abstracts 2000;26 | No participants with dementia/not separately analysed |
| Bozeat S, Gregory CA, Ralph MAL, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry 2000;69:178–86 | Not intervention study |
| Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica 1991;82:239–59 | No participants with dementia/not separately analysed |
| Branconnier RJ, Cole JO. Memory assessment technique for use in geriatric psychopharmacology – drug efficacy trial with naftidrofuryl. J Am Geriatr Soc 1977;25:186–8 | No participants with dementia/not separately analysed |
| Brenner HD, Alberti L, Keller F, Schaffner L. Pharmacotherapy of agitational states in psychiatric gerontology – double-blind-study – Febarbamat-Pipamperon. Neuropsychobiology 1984;11:187–90 | Not a psychological, behavioural, sensory or environmental intervention |
| Bridges-Parlet S, Knopman D, Steffes S. Withdrawal of neuroleptic medications from institutionalized dementia patients: results of a double-blind, baseline-treatment-controlled pilot study. J Geriatr Psychiatry Neurol 1997;10:119–26 | Not a psychological, behavioural, sensory or environmental intervention |
| Bridges-Parlet S, Knopman D, Thompson T. A descriptive study of physically aggressive behavior in dementia by direct observation. J Am Geriatr Soc 1994;42:192–7 | Not intervention study |
| Broadway J, Mintzer J. The many faces of psychosis in the elderly. Curr Opin Psychiatry 2007;20:551–8 | Not primary research |
| Brodaty H, Ames D, Snowdon J, Woodward M, Kirwan J, Clarnette R, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry 2003;64:134–43 | Not a psychological, behavioural, sensory or environmental intervention |
| Brodaty H, Ames D, Snowdon J, Woodward M, Kirwan J, Clarnette R, et al. Risperidone for psychosis of Alzheimer’s disease and mixed dementia: results of a double-blind, placebo-controlled trial. Int J Geriatr Psychiatry 2005;20:1153–7 | Not a psychological, behavioural, sensory or environmental intervention |
| Brodaty H, Draper B, Saab D, Low LF, Richards V, Paton H, et al. Psychosis, depression and behavioural disturbances in Sydney nursing home residents: prevalence and predictors. Int J Geriatr Psychiatry 2001;16:504–12 | Not intervention study |
| Brodaty H, Draper BM, Low LF. Behavioural and psychological symptoms of dementia: a seven-tiered model of service delivery. Med J Aus 2003;178:231–4 | No participants with dementia/not separately analysed |
| Brodaty H, Low LF. Aggression in the elderly. J Clin Psychiatry 2003;64:36–43 | Not primary research |
| Brodaty H, Woodward M, Boundy K, Barnes N, Allen G. A naturalistic study of galantamine for Alzheimer’s disease. CNS Drugs 2006;20:935–43 | Not a psychological, behavioural, sensory or environmental intervention |
| Broniatowski M, Grundfest-Broniatowski S, Tyler DJ, Scolieri P, Abbass F, Tucker HM, et al. Dynamic laryngotracheal closure for aspiration: a preliminary report. Laryngoscope 2001;111:2032–40 | No participants with dementia/not separately analysed |
| Brooker DJ, Woolley RJ, Lee D. Enriching opportunities for people living with dementia in nursing homes: an evaluation of a multi-level activity-based model of care. Aging Mental Health 2007;11:361–70 | No outcome measuring agitation |
| Brooker DJ, Woolley RJ. Enriching opportunities for people living with dementia: the development of a blueprint for a sustainable activity-based model. Aging Mental Health 2007;11:371–83 | No participants with dementia/not separately analysed |
| Brooker DJR, Snape M, Johnson E, Ward D, Payne M. Single case evaluation of the effects of aromatherapy and massage on disturbed behaviour in severe dementia. Br J Clin Psychol 1997;36:287–96 | No comparator |
| Brotons M, Koger SM, Pickett-Cooper P. Music and dementias: a review of literature. J Music Ther 1997;34:204–45 | Not primary research |
| Brown P, Gajdusek DC. Survival of Scrapie virus after 3 years internment. Lancet 1991;337:269–70 | No participants with dementia/not separately analysed |
| Brummel-Smith K, London MR, Drew N, Krulewitch H, Singer C, Hanson L. Outcomes of pain in frail older adults with dementia. J Am Geriatr Soc 2002;50:1847–51 | Not intervention study |
| Brusco LI, Fainstein I, Marquez M, Cardinali DP. Effect of melatonin in selected populations of sleep-disturbed patients. Biol Signals Receptors 1999;8:126–31 | Not a psychological, behavioural, sensory or environmental intervention |
| Brusco LI, Marquez M, Cardinali DP. Melatonin treatment stabilizes chronobiologic and cognitive symptoms in Alzheimer’s disease. Neuroendocrinol Lett 2000;21:39–42 | Not a psychological, behavioural, sensory or environmental intervention |
| Brusco LI, Marquez M, Cardinali DP. Monozygotic twins with Alzheimer’s disease treated with melatonin: case report. J Pineal Res 1998;25:260–3 | Not a psychological, behavioural, sensory or environmental intervention |
| Buck D, Gregson BA, Bamford CH, McNamee P, Farrow GN, Bond J, et al. Psychological distress among informal supporters of frail older people at home and in institutions – the resource implications study group of the MRC cognitive function and ageing study. Int J Geriatr Psychiatry 1997;12:737–44. | No participants with dementia/not separately analysed |
| Buckwalter KC, Stolley JM, Farran CJ. Managing cognitive impairment in the elderly: conceptual, intervention and methodological issues. Online J Knowledge Synth Nurs 1999;6:10 | Not primary research |
| Buerger K, Mergner R, Arbusow V, Padberg F, Hampel H. Late onset Huntington’s disease: a differential diagnosis of Alzheimer’s disease. Nervenarzt 2002;73:870–3 | No participants with dementia/not separately analysed |
| Buettner L, Fitzsimmons S. Recreational therapy interventions: a fresh approach to treating apathy and mixed behaviors in dementia. Non-Pharmacol Ther Dementia 2011;1:27–42 | No participants with dementia/not separately analysed |
| Buettner L, Fitzsimmons S. Mixed behaviors in dementia – the need for a paradigm shift. J Gerontol Nurs 2006;32:15–22 | Not intervention study |
| Buettner L, Kolanowski A. Practice guidelines for recreation therapy in the care of people with dementia (CE). Geriatr Nurs 2003;24:18–25 | Not primary research |
| Buettner LL, Fitzsimmons S, Atav AS. Predicting outcomes of therapeutic recreation interventions for older adults with dementia and behavioral symptoms. Ther Recreation J 2006;40:33–47 | No quantitative outcome |
| Buettner LL, Fitzsimmons S, Dudley WN. Impact of underlying depression on treatment of neuropsychiatric symptoms in older adults with dementia. Res Gerontol Nurs 2010;3:221–32 | Not primary research |
| Buffington J, Chapman LE, Stobierski MG, Hierholzer JC, Gary HE, Guskey LE, et al. Epidemic keratoconjunctivitis in a chronic care facility – risk-factors and measures for control. J Am Geriatr Soc 1993;41:1177–81 | No participants with dementia/not separately analysed |
| Buldyrev SV, Cruz L, Gomez-Isla T, Gomez-Tortosa E, Havlin S, Le R, et al. Description of microcolumnar ensembles in association cortex and their disruption in Alzheimer and Lewy body dementias. Proc Natl Acad Sci USA 2000;97:5039–43 | No participants with dementia/not separately analysed |
| Bungener C, Jouvent R, Derouesne C. Affective disturbances in Alzheimer’s disease. J Am Geriatr Soc 1996;44:1066–71 | Not intervention study |
| Burgio LD, Butler FR, Roth DL, Hardin JM, Hsu CC, Ung K. Agitation in nursing home residents: the role of gender and social context. Int Psychogeriatr 2000;12:495–511 | Not intervention study |
| Burgio LD, Scilley K, Hardin JM, Hsu C. Temporal patterns of disruptive vocalization in elderly nursing home residents. Int J Geriatr Psychiatry 2001;16:378–86 | Not intervention study |
| Burgio LD, Sinnott J. Behavioral treatments and pharmacotherapy – acceptability ratings by elderly individuals in residential settings. Gerontologist 1990;30:811–16 | No participants with dementia/not separately analysed |
| Burgio LD, Stevens A, Burgio KL, Roth DL, Paul P, Gerstle J. Teaching and maintaining behavior management skills in the nursing home. Gerontologist 2002;42:487–96 | No participants with dementia/not separately analysed |
| Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Ex Opin Pharmacother 2009;10:2407–14 | Not primary research |
| Burney-Puckett M. Sundown syndrome: etiology and management. J Psychosoc Nurs Mental Health Serv 1996;34:40–3 | Not primary research |
| Burns A, De Deyn PP. Risperidone for the treatment of neuropsychiatric features in dementia. Drugs Aging 2006;23:887–96 | Not primary research |
| Burns A, Mittelman M, Cole C, Morris J, Winter J, Page S, et al. Transcultural influences in dementia care: observations from a psychosocial intervention study. Dementia Geriatr Cogn Disord 2010;30:417–23 | No participants with dementia/not separately analysed |
| Buron B. Levels of personhood: a model for dementia care. Geriatr Nurs 2008;29:324–32 | No participants with dementia/not separately analysed |
| Byrne J. A Randomised Controlled Trial of Bright Light Therapy for Agitation and Sleep Disturbance in Dymptoms of Dementia. National Research Register; 2000 | No outcome reported |
| Caballero J, Hitchcock M, Scharre D, Beversdorf D, Nahata MC. Cognitive effects of atypical antipsychotics in patients with Alzheimer’s disease and comorbid psychiatric or behavioral problems: a retrospective study. Clin Ther 2006;28:1695–700 | Not a psychological, behavioural, sensory or environmental intervention |
| Cacabelos R, Franco-Maside A, Alvarez XA. Influence of the somatotropinergic system on mental function and psychomotor activity: Environmental factors, development, cognition, and neuropsychiatric disorders. Int Congress Series 1992;973:161–72 | No participants with dementia/not separately analysed |
| Cacabelos R, Rodriguez B, Carrera C, Caamano J, Beyer K, Lao JI, et al. APOE-related frequency of cognitive and noncognitive symptoms in dementia. Methods Findings Exp Clin Pharmacol 1996;18:693–706 | Not intervention study |
| Cahn LA, Diesfeld HF. Use of neuroleptics in treatment of dementia in old-age – critical analysis with reference to an experiment with a long-acting oral neuroleptic (Penfluridol Janssen). Psychiatria Neurologia Neurochirurgia 1973;76:411–20 | Not a psychological, behavioural, sensory or environmental intervention |
| Caine ED. Clinical perspectives on atypical antipsychotics for treatment of agitation. J Clin Psychiatry 2006;67:22–31 | Not primary research |
| Camberg L, Woods P, Ooi WL, Hurley A, Volicer L, Ashley J, et al. Evaluation of simulated presence: a personalized approach to enhance well-being in persons with Alzheimer’s disease. J Am Geriatr Soc 1999;47:446–52 | Protocol only |
| Campbell NL, Khan BA, Farber M, Campbell T, Perkins AJ, Hui SL, et al. Improving delirium care in the intensive care unit: the design of a pragmatic study. Trials 2011;12 | No participants with dementia/not separately analysed |
| Cantillon M, Brunswick R, Molina D, Bahro M. Buspirone vs haloperidol – a double-blind trial for agitation in a nursing home population with Alzheimer’s disease. Am J Geriatr Psychiatry 1996;4:263–7 | Not a psychological, behavioural, sensory or environmental intervention |
| Cantillon M, Molina D, Brunswick R. Anxiety as a factor in agitation in the demented institutionalized elderly – randomized single-blind treatment with azapirones versus neuroleptics. Biol Psychiatry 1994;35:629 | Not a psychological, behavioural, sensory or environmental intervention |
| Caparros-Lefebvre D, Dewailly D. Preliminary PILOTE study of cyproterone acetate for the treatment of aggressive behavior associated with severe dementia. Revue Neurologique 2005;161:1071–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Capote B, Parikh N. Cyclandelate in treatment of senility – controlled-study. J Am Geriatr Soc 1978;26:360–2 | Not a psychological, behavioural, sensory or environmental intervention |
| Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Curr Neuropharmacol 2010;8:218–27 | Not primary research |
| Carlyle W, Ancill RJ, Sheldon L. Aggression in the demented patient – a double-blind study of loxapine versus haloperidol. Int Clin Psychopharmacol 1993;8:103–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Cassidy EL, Sheikh JI. Pre-intervention assessment for disruptive behaviour problems: a focus on staff needs. Aging Mental Health 2002;6:166–71 | No participants with dementia/not separately analysed |
| Cassimjee N, Stuart AD, Marchetti-Mercer M. Non-cognitive disturbances and patient characteristics: prevalence and relationship in Alzheimer’s disease. South Afr J Psychol 2005;35:225–43 | Not intervention study |
| Ceraso D, Duenas-Castel C, Raimondi N, Celis E, Carrillo R, Ugarte Ubiergo S, et al. Latin American survey on delirium in critical patients. Medicina Intensiva 2010;34:495–505 | No participants with dementia/not separately analysed |
| Chafetz PK. Behavioral and cognitive outcomes of SCU care. Clin Gerontol 1991;11:19–38 | No outcome measuring agitation |
| Chan A, Shea TB. Apolipoprotein E3 as a risk factor for Alzheimer’s disease under conditions of nutritional imbalance. J Alzheimers Dis 2010;21:49–55 | No participants with dementia/not separately analysed |
| Chan DC, Kasper JD, Black BS, Rabins PV. Prevalence and correlates of behavioral and psychiatric symptoms in community-dwelling elders with dementia or mild cognitive impairment: the Memory and Medical Care Study. Int J Geriatr Psychiatry 2003;18:174–82 | Not intervention study |
| Chan S, Fung MY, Tong CW, Thompson D. The clinical effectiveness of a multisensory therapy on clients with developmental disability. Res Develop Disabil 2005;26:131–42 | No participants with dementia/not separately analysed |
| Chan WC, Lam LC, Tam CW, Lui VW, Leung GT, Lee AT, et al. Neuropsychiatric symptoms are associated with increased risks of progression to dementia: a 2-year prospective study of 321 Chinese older persons with mild cognitive impairment. Age Ageing 2011;40:30–5 | No participants with dementia/not separately analysed |
| Chan WC, Lam LCW, Choy CNP, Leung VPY, Li SW, Chiu HFK. A double-blind randomised comparison of risperidone and haloperidol in the treatment of behavioural and psychological symptoms in Chinese dementia patients. Int J Geriatr Psychiatry 2001;16:1156–62 | Not a psychological, behavioural, sensory or environmental intervention |
| Chan WC, Lam LC-W, Tam CW-C, Lui VW-C, Chan SS-M, Chan WM, et al. Prevalence of neuropsychiatric symptoms in Chinese older persons with mild cognitive impairment–a population-based study. Am J Geriatr Psychiatry 2010;18:948–54 | No participants with dementia/not separately analysed |
| Chandler JD. Geriatric psychiatry. Primary Care 1987;14:761–72 | Not primary research |
| Chapman SB, Weiner ME, Rackley A, Hynan LS, Zientz J. Effects of cognitive-communication-stimulation for Alzheimer’s disease patients treated with donepezil. J Speech Language Hearing Res 2004;47:1149–63 | Not a psychological, behavioural, sensory or environmental intervention |
| Chappell NL, Reid RC. Dimensions of care for dementia sufferers in long-term care institutions: are they related to outcomes? J Gerontol Series B 2000;55:S234–44 | Not intervention study |
| Charles E, Bouby-Serieys V, Thomas P, Clement J. Links between life events, traumatism and dementia; an open study including 565 patients with dementia. Encephale Rev Psychiatr Clin Biol Therap 2006;32:746–52 | Not intervention study |
| Chatterton W, Baker F, Morgan K. The singer or the singing: who sings individually to persons with dementia and what are the effects? Am J Alzheimers Dis Other Demen 2010;25:641–9 | Not primary research |
| Chazot PL. Drug evaluation: safinamide for the treatment of Parkinson’s disease, epilepsy and restless legs syndrome. Curr Opin Invest Drugs 2007;8:570–9 | No participants with dementia/not separately analysed |
| Chela CM, Campbell ID, Siankanga Z. Clinical care as part of integrated Aids management in a Zambian rural community. AIDS Care 1989;1:319–26 | No participants with dementia/not separately analysed |
| Chemerinski E, Petracca G, Manes F, Leiguarda R, Starkstein SE. Prevalence and correlates of anxiety in Alzheimer’s disease. Depression Anxiety 1998;7:166–70 | Not intervention study |
| Chemerinski E, Petracca G, Teson A, Sabe L, Leiguarda R, Starkstein SE. Prevalence and correlates of aggressive behavior in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 1998;10:421–5 | Not intervention study |
| Chemerinski E, Sabe L, Petracca G, Teson A, Starkstein S. Prevalence and clinical correlates of aggressive behavior in Alzheimer’s disease (AD). J Neurol Sci 1997;150:S19 | Not intervention study |
| Chen ST, Sultzer DL, Hinkin CH, Mahler ME, Cummings JL. Executive dysfunction in Alzheimer’s disease: association with neuropsychiatric symptoms and functional impairment. J Neuropsychiatry Clin Neuroscie 1998;10:426–32 | Not intervention study |
| Chen YH, Lin LC, Watson R. Evaluation of the psychometric properties and the clinical feasibility of a Chinese version of the Doloplus-2 scale among cognitively impaired older people with communication difficulty. Int J Nurs Studies 2010;47:78–88 | No participants with dementia/not separately analysed |
| Chengappa KNR, Levine J, Ulrich R, Parepally H, Brar JS, Atzert R, et al. Impact of risperidone on seclusion and restraint at a state psychiatric hospital. Can J Psychiatry 2000;45:827–32 | No participants with dementia/not separately analysed |
| Cherry DL, Vickrey BG, Schwankovsky L, Heck E, Plauche M, Yep R. Interventions to improve quality of care: the Kaiser Permanente – Alzheimer’s Association Dementia Care Project. Am J Managed Care 2004;10:553–60 | No participants with dementia/not separately analysed |
| Cheung DS, Chien WT, Lai CK. Conceptual framework for cognitive function enhancement in people with dementia. J Clin Nurs 2011;20:1533–41 | No participants with dementia/not separately analysed |
| Chiabrando G, Bianchi S, Poluzzi E, Montanaro N, Scanavacca P. Profile of atypical-antipsychotics use in patients affected by dementia in the University Hospital of Ferrara. Eur J Clin Pharmacol 2010;66:661–9 | Not intervention study |
| Chibnall JT, Tait RC, Harman B, Luebbert RA. Effect of acetaminophen on behavior, well-being, and psychotropic medication use in nursing home residents with moderate-to-severe dementia. J Am Geriatr Soc 2005;53:1921–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Chilukoti N, Early K, Sandhu S, Riley-Doucet C, Debnath D. Assistive technology for promoting physical and mental exercise to delay progression of cognitive degeneration in patients with dementia. 2007 IEEE Biomedical Circuits and Systems Conference 2007;235–8 | No participants with dementia/not separately analysed |
| Chiu YC, Algase D, Whall A, Liang L, Liu HC, Lin KN, et al. Getting lost: directed attention and executive functions in early Alzheimer’s disease patients. Dementia Geriatr Cogn Disord 2004;17:174–80 | Not intervention study |
| Choi SH, Park KW, Na DL, Han HJ, Kim EJ, Shim YS, et al. Tolerability and efficacy of memantine add-on therapy to rivastigmine transdermal patches in mild to moderate Alzheimer’s disease: a multicenter, randomized, open-label, parallel-group study. Curr Med Res Opin 2011;27:1375–83 | Not a psychological, behavioural, sensory or environmental intervention |
| Chokroverty S. Sleep and neurodegenerative diseases. Semin Neurol 2009;29:446–67 | No participants with dementia/not separately analysed |
| Chouza C, Romero S, Lorenzo J, Camano JL, Fontana AP, Alterwain P, et al. Clinical-trial of tiapride in patients with dyskinesia. Semaine des Hopitaux 1982;58:725–33 | No participants with dementia/not separately analysed |
| Chrispal A, Mathews KP, Surekha V. The clinical profile and association of delirium in geriatric patients with hip fractures in a tertiary care hospital in India. J Assoc Phys India 2010;58:15–19 | No participants with dementia/not separately analysed |
| Christensen DB, Benfield WR. Alprazolam as an alternative to low-dose haloperidol in older, cognitively impaired nursing facility patients. J Am Geriatr Soc 1998;46:620–5 | Not a psychological, behavioural, sensory or environmental intervention |
| Chui HC, Lyness SA, Sobel E, Schneider LS. Extrapyramidal signs and psychiatric-symptoms predict faster cognitive decline in Alzheimers-disease. Arch Neurol 1994;51:676–81 | Not intervention study |
| Chung JA, Cummings JL. Neurobehavioral and neuropsychiatric symptoms in Alzheimer’s disease – characteristics and treatment. Neurol Clin 2000;18:829 | Not primary research |
| Chung JC, Lai CK, Chung PM, French HP. Snoezelen for dementia. Cochrane Database Syst Rev 2002;4:CD003152 | Not primary research |
| Cifu DX, Anderson JC, Lopez E. Agitation in the older adult with traumatic brain injury. Neurorehabilitation 1995;5:245–54 | No participants with dementia/not separately analysed |
| Citrome L. Schizophrenia and valproate. Psychopharmacol Bull 2003;37(Suppl. 2):74–88 | No participants with dementia/not separately analysed |
| Ciurli P, Formisano R, Bivona U, Cantagallo A, Angelelli P. Neuropsychiatric disorders in persons with severe traumatic brain injury: prevalence, phenomenology, and relationship with demographic, clinical, and functional features. J Head Trauma Rehabil 2011;26:116–26 | No participants with dementia/not separately analysed |
| Clark WS, Street JS, Feldman PD, Breier A. The effects of olanzapine in reducing the emergence of psychosis among nursing home patients with Alzheimer’s disease. J Clin Psychiatry 2001;62:34–40 | Not a psychological, behavioural, sensory or environmental intervention |
| Clarke DE, Ko JY, Kuhl EA, van Reekum R, Salvador R, Marin RS. Are the available apathy measures reliable and valid? A review of the psychometric evidence. J Psychosom Res 2011;70:73–97 | No participants with dementia/not separately analysed |
| Clarke DE, van Reekum R, Simard M, Streiner DL, Conn D, Cohen T, et al. Apathy in dementia: clinical and sociodemographic correlates. J Neuropsychiatry Clin Neurosci 2008;20:337–47 | Not intervention study |
| Close Kirkwood S, Siemers E, Viken RJ, Hodes ME, Conneally PM, Christian JC, et al. Evaluation of psychological symptoms among presymptomatic HD gene carriers as measured by selected MMPI scales. J Psychiatric Res 2002;36:377–82 | No participants with dementia/not separately analysed |
| Coccaro EF, Kramer E, Zemishlany Z, Thorne A, Rice CM, Giordani B, et al. Pharmacological treatment of noncognitive behavioral disturbances in elderly demented patients. Am J Psychiatry 1990;147:1640–5 | Not a psychological, behavioural, sensory or environmental intervention |
| Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell 2009;139:1157–69 | No participants with dementia/not separately analysed |
| Cohen-Mansfield J, Dakheel-Ali M, Marx MS. Engagement in persons with dementia: the concept and its measurement. Am J Geriatr Psychiatry 2009;17:299–307 | No participants with dementia/not separately analysed |
| Cohen-Mansfield J, Garfinkel D, Lipson S. Melatonin for treatment of sundowning in elderly persons with dementia – a preliminary study. Arch Gerontol Geriatr 2000;31:65–76 | Not a psychological, behavioural, sensory or environmental intervention |
| Cohen-Mansfield J, Jensen B. Assessment and treatment approaches for behavioral disturbances associated with dementia in the nursing home: self-reports of physicians’ practices. J Am Med Direct Assoc 2008;9:406–13 | No participants with dementia/not separately analysed |
| Cohen-Mansfield J, Jensen B. Nursing home physicians knowledge of and attitudes toward nonpharmacological interventions for treatment of behavioral disturbances associated with dementia. J Am Med Direct Assoc 2008;9:491–8 | No participants with dementia/not separately analysed |
| Cohen-Mansfield J, Libin A. Verbal and physical non-aggressive agitated behaviors in elderly persons with dementia: robustness of syndromes. J Psychiatr Res 2005;39:325–32 | Not intervention study |
| Cohen-Mansfield J, Lipson S, Werner P, Billig N, Taylor L, Woosley R. Withdrawal of haloperidol, thioridazine, and lorazepam in the nursing home – A controlled, double-blind study. Arch Intern Med 1999;159:1733–40 | No participants with dementia/not separately analysed |
| Cohen-Mansfield J, Marx MS, Dakheel-Ali M, Regier NG, Thein K. Can persons with dementia be engaged with stimuli? Am J Geriatr Psychiatry 2010;18:351–62 | No outcome measuring agitation |
| Cohen-Mansfield J, Marx MS, Lipson S, Werner P. Predictors of mortality in nursing home residents. J Clin Epidemiol 1999;52:273–80 | Not intervention study |
| Cohen-Mansfield J, Marx MS, Regier NG, Dakheel-Ali M. The impact of personal characteristics on engagement in nursing home residents with dementia. Int J Geriatr Psychiatry 2009;24:755–63 | Not intervention study |
| Cohen-Mansfield J, Marx MS, Rosenthal AS. Dementia and agitation in nursing-home residents – how are they related. Psychol Aging 1990;5:3–8 | Not intervention study |
| Cohen-Mansfield J, Marx MS, Thein K, Dakheel-Ali M. The impact of past and present preferences on stimulus engagement in nursing home residents with dementia. Aging Mental Health 2010;14:67–73 | No outcome measuring agitation |
| Cohen-Mansfield J, Marx MS, Thein K, Dakheel-Ali M. The impact of stimuli on affect in persons with dementia. J Clin Psychiatry 2011;72:480–6 | No outcome measuring agitation |
| Cohen-Mansfield J, Mintzer JE. Time for change: the role of non-pharmacological interventions in treating behavior problems in nursing home residents with dementia. Alzheimer Dis Assoc Disord 2005;19:37–40 | Not primary research |
| Cohen-Mansfield J, Parpura-Gill A. Bathing: a framework for intervention focusing on psychosocial, architectural and human factors considerations. Arch Gerontol Geriatr 2007;45:121–35 | No comparator |
| Cohen-Mansfield J, Thein K, Dakheel-Ali M, Marx MS. Engaging nursing home residents with dementia in activities: the effects of modeling, presentation order, time of day, and setting characteristics. Aging Mental Health 2010;14:471–80 | No outcome measuring agitation |
| Cohen-Mansfield J, Waldhorn R, Werner P, Billig N. Validation of sleep observations in a nursing-home. Sleep 1990;13:512–25 | No participants with dementia/not separately analysed |
| Cohen-Mansfield J, Werner P. Predictors of aggressive behaviors: a longitudinal study in senior day care centers. J Gerontol Series B 1998;53:300–10 | Not intervention study |
| Cohen-Mansfield J, Werner P. Typology of disruptive vocalizations in older persons suffering from dementia. Int J Geriatr Psychiatry 1997;12:1079–91 | No participants with dementia/not separately analysed |
| Cohen-Mansfield J, Werner P, Freedman L. Sleep and agitation in agitated nursing-home residents – an observational study. Sleep 1995;18:674–80 | Not intervention study |
| Cohen-Mansfield J, Werner P. Management of verbally disruptive behaviors in nursing home residents. J Gerontol Series A 1997;52:M369–77 | Not a psychological, behavioural, sensory or environmental intervention |
| Cohen-Mansfield J, Werner P. Outdoor wandering parks for persons with dementia: a survey of characteristics and use. Alzheimer Dis Assoc Disord 1999;13:109–17 | No participants with dementia/not separately analysed |
| Cohen-Mansfield J. Assessment of agitation. Int Psychogeriatr 1996;8:233–45 | No participants with dementia/not separately analysed |
| Cohen-Mansfield J. Heterogeneity in dementia: challenges and opportunities. Alzheimer Dis Assoc Disord 2000;14:60–3 | Not primary research |
| Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia – a review, summary, and critique. Am J Geriatr Psychiatry 2001;9:361–81 | Not primary research |
| Cohen-Mansfield J. Use of patient characteristics to determine nonpharmacologic interventions for behavioral and psychological symptoms of dementia. Int Psychogeriatr 2000;12:373–80 | Not primary research |
| Colantonio A, Cohen C, Corlett S. Support needs of elderly caregivers of persons with dementia. Can J Ageing 1998;17:330–45 | No participants with dementia/not separately analysed |
| Colenda CC, Hamer RM. Antecedents and interventions for aggressive-behavior of patients at a geropsychiatric state-hospital. Hosp Community Psychiatry 1991;42:287–92 | No quantitative outcome |
| Colenda CC, Rapp SR, Leist JC, Poses RM. Clinical variables influencing treatment decisions for agitated dementia patients: survey of physician judgments. J Am Geriatr Soc 1996;44:1375–9 | No participants with dementia/not separately analysed |
| Colombo M, Vitali S, Cairati M, Vaccaro R, Andreoni G, Guaita A. Behavioral and psychotic symptoms of dementia (BPSD) improvements in a special care unit: a factor analysis. Arch Gerontol Geriatr 2007;44:113–20 | No participants with dementia/not separately analysed |
| Comella CL. Sleep disorders in Parkinson’s disease: an overview. Movement Disord 2007;22:S367–73 | No participants with dementia/not separately analysed |
| Como PG, Rubin AJ, Obrien CF, Lawler K, Hickey C, Rubin AE, et al. A controlled trial of fluoxetine in nondepressed patients with Huntington’s disease. Movement Disord 1997;12:397–401 | No participants with dementia/not separately analysed |
| Conn D, Thorpe L. Assessment of behavioural and psychological symptoms associated with dementia. Can J Neurol Sci 2007;34:S67–71 | No participants with dementia/not separately analysed |
| Conn DK, Lee V, Steingart A, Silberfeld M. Psychiatric-services – a survey of nursing-homes and homes for the aged in Ontario. Can J Psychiatry 1992;37:525–30 | No participants with dementia/not separately analysed |
| Conn DK, Seitz DP. Advances in the treatment of psychiatric disorders in long-term care homes. Curr Opin Psychiatry 2010;23:516–21 | Not primary research |
| Conn LM, Lion JR. Pharmacologic approaches to violence. Psychiatr Clin North Am 1984;7:879–86 | No participants with dementia/not separately analysed |
| Conney J, Kaston B. Pharmacoeconomic and health outcome comparison of lithium and divalproex in a VA geriatric nursing home population: influence of drug-related morbidity on total cost of treatment. Am J Manag Care 1999;5:197–204 | No participants with dementia/not separately analysed |
| Coogle CL, Parham IA, Cotter JJ, Welleford EA, Netting FE. A professional development program in geriatric interdisciplinary teamwork: implications for managed care and quality of care. J Appl Gerontol 2005;24:142–59 | No participants with dementia/not separately analysed |
| Cooney C, Howard R, Lawlor B. Abuse of vulnerable people with dementia by their carers: can we identify those most at risk? Int J Geriatr Psychiatry 2006;21:564–71 | Not intervention study |
| Cooper C, Selwood A, Blanchard M, Livingston G. Abusive behaviour experienced by family carers from people with dementia: the CARD (caring for relatives with dementia) study. J Neurol Neurosurg Psychiatry 2010;81:592–6 | Not intervention study |
| Cooper C, Selwood A, Blanchard M, Walker Z, Blizard R, Livingston G. The determinants of family carers’ abusive behaviour to people with dementia: results of the CARD study. J Affect Disord 2010;121:136–42 | No participants with dementia/not separately analysed |
| Cooper JK. Drug-treatment of Alzheimers-disease. Arch Intern Med 1991;151:245–9 | Not primary research |
| Cooper JW. Nonpharmacologic and pharmacologic treatment of dementia-associated agitation, aggression and disruptive behavior. J Geriatr Drug Ther 1999;12:5–28 | Not primary research |
| Cooper T. Who manages the managers. J Neurosurg 1989;71:311–15 | No participants with dementia/not separately analysed |
| Coppin AK, Shumway-Cook A, Saczynski JS, Patel KV, Ble A, Ferrucci L, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing 2006;35:619–24 | No participants with dementia/not separately analysed |
| Coreybloom J, Galasko D. Adjunctive therapy in patients with Alzheimers disease – a practical approach. Drugs Aging 1995;7:79–8 | Not primary research |
| Corrigan PW, Yudofsky SC, Silver JM. Pharmacological and behavioral treatments for aggressive psychiatric inpatients. Hosp Community Psychiatry 1993;44:125–33 | Not primary research |
| Cott CA, Dawson P, Sidani T, Wells T. The effects of a walking/talking program on communication, ambulation, and functional status in residents with Alzheimer disease. Alzheimer Dis Assoc Disord 2002;16:81–7 | No outcome measuring agitation |
| Cotter VT. Restraint free care in older adults with dementia. Keio J Med 2005;54:80–4 | Not primary research |
| Cotter VT. The burden of dementia. Am J Manag Care 2007;13:S193–7 | Not primary research |
| Coulson BS, Fenner SG, Almeida OP. Successful treatment of behavioural problems in dementia using a cholinesterase inhibitor: the ethical questions. Aus N Z J Psychiatry 2002;36:259–62 | Not a psychological, behavioural, sensory or environmental intervention |
| Covington JS. Alleviating agitation, apprehension, and related symptoms in geriatric patients – double-blind comparison of a phenothiazine and a benzodiazepine. South Med J 1975;68:719–24 | Not a psychological, behavioural, sensory or environmental intervention |
| Covinsky KE, Eng C, Lui LY, Sands LP, Yaffe K. The last 2 years of life: functional trajectories of frail older people. J Am Geriatr Soc 2003;51:492–8 | Not intervention study |
| Craig D, Hart DJ, Carson R, McIlroy SP, Passmore AP. Allelic variation at the A218C tryptophan hydroxylase polymorphism influences agitation and aggression in Alzheimer’s disease. Neurosci Lett 2004;363:199–202 | Not intervention study |
| Craig D, Hart DJ, Carson R, McIlroy SP, Passmore AP. Psychotic symptoms in Alzheimer’s disease are not influenced by polymorphic variation at the dopamine receptor DRD3 gene. Neurosci Lett 2004;368:33–6 | Not intervention study |
| Craufurd D, Thompson JC, Snowden JS. Behavioral changes in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol 2001;14:219–26 | No participants with dementia/not separately analysed |
| Cronin-Stubbs D. Interventions for cognitive impairment and neurobehavioral disturbances of older adults. Annu Rev Nurs Res 1997;15:35–56 | Not primary research |
| Crotty M, Halbert J, Rowett D, Giles L, Birks R, Williams H, et al. An outreach geriatric medication advisory service in residential aged care: a randomised controlled trial of case conferencing. Age Ageing 2004;33:612–17 | No participants with dementia/not separately analysed |
| Crystal HA, Dickson DW, Lizardi JE, Davies P, Wolfson LI. Antemortem diagnosis of Diffuse Lewy Body disease. Neurology 1990;40:1523–8 | Not intervention study |
| Cubit K. Informed consent for research involving people with dementia: a grey area. Contemp Nurse 2010;34:230–6 | Not intervention study |
| Cubo E, Leurgans S, Goetz CG. Short-term and practice effects of metronome pacing in Parkinson’s disease patients with gait freezing while in the ‘on’ state: randomized single blind evaluation. Parkinsonism Related Disord 2004;10:507–10 | No participants with dementia/not separately analysed |
| Cuellar NG, Strumpf NE, Ratcliffe SJ. Symptoms of restless legs syndrome in older adults: outcomes on sleep quality, sleepiness, fatigue, depression, and quality of life. J Am Geriatr Soc 2007;55:1387–92 | No participants with dementia/not separately analysed |
| Culebras A. Update on disorders of sleep and the sleep-wake cycle. Psychiatr Clin North Am 1992;15:467–89 | No participants with dementia/not separately analysed |
| Cummings JL, Back C. The cholinergic hypothesis of neuropsychiatric symptoms in Alzheimer’s disease. Am J Geriatr Psychiatry 1998;6:S64–78 | Not primary research |
| Cummings JL, Farlow MR, Meng X, Tekin S, Olin JT. Rivastigmine transdermal patch skin tolerability results of a 1-year clinical trial in patients with mild-to-moderate Alzheimer’s disease. Clin Drug Invest 2010;30:41–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Cummings JL, Kaufer D. Neuropsychiatric aspects of Alzheimer’s disease: the cholinergic hypothesis revisited. Neurology 1996;47:876–83 | Not primary research |
| Cummings JL, Mackell J, Kaufer D. Behavioral effects of current Alzheimer’s disease treatments: a descriptive review. Alzheimers Demen 2008;4:49–60 | Not primary research |
| Cummings JL, McPherson S. Neuropsychiatric assessment of Alzheimer’s disease and related dementias. Aging Clin Exp Res 2001;13:240–6 | Not primary research |
| Cummings JL, McRae T, Zhang R. Effects of donepezil on neuropsychiatric symptoms in patients with dementia and severe behavioral disorders. Am J Geriatr Psychiatry 2006;14:605–12 | Not a psychological, behavioural, sensory or environmental intervention |
| Cummings JL, Nadel A, Masterman D, Cyrus PA. Efficacy of metrifonate in improving the psychiatric and behavioral disturbances of patients with Alzheimer’s disease. J Geriatr Psychiatry Neurol 2001;14:101–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Cummings JL, Schneider E, Tariot PN, Graham SM. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology 2006;67:57–63 | Not a psychological, behavioural, sensory or environmental intervention |
| Cummings JL, Street J, Masterman D, Clark WS. Efficacy of olanzapine in the treatment of psychosis in dementia with Lewy bodies. Demen Geriatr Cogn Disord 2002;13:67–73 | Not a psychological, behavioural, sensory or environmental intervention |
| Cummings JL, Tractenberg RE, Gamst A, Teri L, Masterman D, Thal LJ. Regression to the mean: Implications for clinical trials of psychotropic agents in dementia. Curr Alzheimer Res 2004;1:323–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Cummings JL. Behavioral manifestations of Alzheimer’s disease. 2001. pp. 84–8 | Not primary research |
| Cummings JL. Cognitive and behavioral heterogeneity in Alzheimer’s disease: seeking the neurobiological basis. Neurobiol Aging 2000;21:845–61 | Not primary research |
| Cummings JL. Neuropsychiatric and behavioral alterations and their management in moderate to severe Alzheimer disease. Neurology 2005;65:S18–24 | Not primary research |
| Cummings JL. Use of cholinesterase inhibitors in clinical practice – evidence-based recommendations. Am J Geriatr Psychiatry 2003;11:131–45 | Not primary research |
| Cunningham J, Williams KN. A case study of resistiveness to care and elderspeak. Res Theory Nurs Prac 2007;21:45–56 | Not intervention study |
| Currier GW, Chou JCY, Feifel D, Bossie CA, Turkoz I, Mahmoud RA, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry 2004;65:386–94 | No participants with dementia/not separately analysed |
| Currier GW, Simpson GM. Risperidone liquid concentrate and oral lorazepam versus intramuscular haloperidol and intramuscular lorazepam for treatment of psychotic agitation. J Clin Psychiatry 2001;62:153–7 | No participants with dementia/not separately analysed |
| Daiello LA, Ott BR, Lapane KL, Reinert SE, Machan JT, Dore DD. Effect of discontinuing cholinesterase inhibitor therapy on behavioral and mood symptoms in nursing home patients with dementia. Am J Geriatr Pharmacother 2009;7:74–83 | Not intervention study |
| Daiello LA. Atypical antipsychotics for the treatment of dementia-related behaviors: an update. Medicine Health 2007;90:191–4 | Not primary research |
| Daniel DG. Antipsychotic treatment of psychosis and agitation in the elderly. J Clin Psychiatry 2000;61:49–52 | Not primary research |
| Darreh-Shori T, Jelic V. Safety and tolerability of transdermal and oral rivastigmine in Alzheimer’s disease and Parkinson’s disease dementia. Ex Opin Drug Saf 2010;9:167–76 | Not primary research |
| Daselaar SM, Veltman DJ, Rombouts SARB, Raaijmakers JGW, Lazeron RHC, Jonker C. Medial temporal lobe activity during semantic classification using a flexible fMRI design. Behav Brain Res 2002;136:399–404 | No participants with dementia/not separately analysed |
| Dauvilliers Y. Insomnia in patients with neurodegenerative conditions. Sleep Med 2007;8:S27–34 | Not primary research |
| Davidson M. Other options in pharmacological management of behavioural disorders. Int J Neuropsychopharmacol 2004;7:S110 | Not primary research |
| Davidson PW, Cain NN, Sloanereeves JE, Vanspeybroech A, Segel J, Gutkin J, et al. Characteristics of community-based individuals with mental-retardation and aggressive behavioral-disorders. Am J Mental Retard 1994;98:704–16 | No participants with dementia/not separately analysed |
| Davis LL, Buckwalter K, Burgio LD. Measuring problem behaviors in dementia: developing a methodological agenda. Adv Nurs Sci 1997;20:40–55 | No participants with dementia/not separately analysed |
| De Deyn PP, Buitelaar J. Risperidone in the management of agitation and aggression associated with psychiatric disorders. Eur Psychiatry 2006;21:21–8 | Not primary research |
| De Deyn PP, Katz IR, Brodaty H, Lyons B, Greenspan A, Burns A. Management of agitation, aggression, and psychosis associated with dementia: a pooled analysis including three randomized, placebo-controlled double-blind trials in nursing home residents treated with risperidone. Clin Neurol Neurosurg 2005;107:497–508 | Not a psychological, behavioural, sensory or environmental intervention |
| De Deyn PP, Katz IR. Control of aggression and agitation in patients with dementia: efficacy and safety of risperidone. Int J Geriatr Psychiatry 2000;15:S14–29 | Not primary research |
| De Deyn PP, Rabheru K, Rasmussen A, Bocksberger JP, Dautzenberg PLJ, Eriksson S, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology 1999;53:946–55 | Not a psychological, behavioural, sensory or environmental intervention |
| de Jonghe A, Korevaar J, van Munster B, de Rooij S. Effectiveness of melatonin treatment on circadian rhythm disturbances in dementia. Are there implications for delirium? A systematic review. Int J Geriatr Psychiatry 2010;25:1201–8 | Not primary research |
| de Medeiros K, Robert P, Gauthier S, Stella F, Politis A, Leoutsakos J, et al. The Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr 2010;22:984–94 | No participants with dementia/not separately analysed |
| de Rooij SE, van Munster BC, Korevaar JC, Casteelen G, Schuurmans MJ, van der Mast RC, et al. Delirium subtype identification and the validation of the Delirium Rating Scale – Revised-98 (Dutch version) in hospitalized elderly patients. Int J Geriatr Psychiatry 2006;21:876–82 | No participants with dementia/not separately analysed |
| de Tommaso M, Difruscolo O, Sciruicchio V, Specchio N, Livrea P. Abnormalities of the contingent negative variation in Huntington’s disease: correlations with clinical features. J Neurol Sci 2007;254:84–9 | No participants with dementia/not separately analysed |
| de Vasconcelos Cunha UG, Lopes Rocha F, Avila de Melo R, Alves Valle E, de Souza Neto JJ, Mendes Brega R, et al. A placebo-controlled double-blind randomized study of venlafaxine in the treatment of depression in dementia. Dementia Geriatr Cogn Disord 2007;24:36–41 | Not a psychological, behavioural, sensory or environmental intervention |
| de Vugt ME, Stevens F, Aalten P, Lousberg R, Jaspers N, Winkens I, et al. Do caregiver management strategies influence patient behaviour in dementia? Int J Geriatr Psychiatry 2004;19:85–92 | Not intervention study |
| de Winter C, Jansen A, Evenhuis H. Physical conditions and challenging behaviour in people with intellectual disability: a systematic review. J Intellectual Disabil Res 2011;55:675–98 | No participants with dementia/not separately analysed |
| Dealberto MJ, Pajot N, Courbon D, Alperovitch A. Breathing disorders during sleep and cognitive performance in an older community sample: the EVA study. J Am Geriatr Soc 1996;44:1287–94 | No participants with dementia/not separately analysed |
| Deberdt WG, Dysken MW, Rappaport SA, Feldman PD, Young CA, Hay DP, et al. Comparison of olanzapine and risperidone in the treatment of psychosis and associated behavioral disturbances in patients with dementia. Am J Geriatr Psychiatry 2005;13:722–30 | Not a psychological, behavioural, sensory or environmental intervention |
| Dechamps A, Jutand MA, Onifade C, Richard-Harston S, Bourdel-Marchasson I. Co-occurrence of neuropsychiatric syndromes in demented and psychotic institutionalized elderly. Int J Geriatr Psychiatry 2008;23:1182–90 | Not intervention study |
| Degner D, Bleich S, Kropp S, Landen H, Ruther E. Tolerability and efficacy of zuclopenthixol in the treatment of gerontopsychiatric patients – a prospective study. Psychopharmakotherapie 2001;8:152–7 | No participants with dementia/not separately analysed |
| Deguchi A, Suzumura E, Nakamura S, Kawamura N, Kawamura K, Hamaguchi H, et al. Night Spa bathing for patients with senile dementia. J Japan Assoc Phys Med Balneol Climatol 2001;64:71–5 | No outcome measuring agitation |
| Delong GR, Rosenberger PB, Hildreth S, Silver I. The 14-Associated and 6-Associated Clinical Complex – a rejected hypothesis revisited. J Child Neurol 1987;2:117–27 | No participants with dementia/not separately analysed |
| Dening TR, Berrios GE. Wilsons-Disease – a longitudinal-study of psychiatric-symptoms. Biol Psychiatry 1990;28:255–65 | No participants with dementia/not separately analysed |
| Denney A. Quiet music. An intervention for mealtime agitation? J Gerontol Nurs 1997;23:16–23 | No quantitative outcome |
| Derouesne C, Piquard A, Thibault S, Baudouin-Madec V, Lacomblez L. Noncognitive symptoms in Alzheimer’s disease. A study of 150 community-dwelling patients using a questionnaire completed by the caregiver. Revue Neurologique 2001;157:162–77 | Not intervention study |
| Deslauriers S, Landreville P, Dicaire L, Verreault R. Validity and reliability of the French version of the Cohen-Mansfield agitation. Can J Aging 2001;20:373–84 | No participants with dementia/not separately analysed |
| Dettmore D, Kolanowski A, Boustani M. Aggression in persons with dementia: use of nursing theory to guide clinical practice. Geriatr Nurs 2009;30:8–17 | Not primary research |
| Deutsch LH, Bylsma FW, Rovner BW, Steele C, Folstein MF. Psychosis and physical aggression in probable Alzheimers disease. Am J Psychiatry 1991;148:1159–63 | Not intervention study |
| Deutsch LH, Rovner BW. Agitation and other noncognitive abnormalities in Alzheimers disease. Psychiatr Clin North Am 1991;14:341–51 | Not primary research |
| Devanand DP, Levy SR. Neuroleptic treatment of agitation and psychosis in dementia. J Geriatr Psychiatry Neurol 1995;8:S18–27 | Not primary research |
| Devanand DP, Marder K, Michaels KS, Sackeim HA, Bell K, Sullivan MA, et al. A randomized, placebo-controlled dose-comparison trial of haloperidol for psychosis and disruptive behaviors in Alzheimer’s disease. Am J Psychiatry 1998;155:1512–20 | Not a psychological, behavioural, sensory or environmental intervention |
| Devanand DP, Pelton GH. A 6-month, Randomized, double-blind, placebo-controlled pilot discontinuation trial following response to haloperidol treatment of psychosis and agitation in Alzheimer’s disease. Biol Psychiatry 2011;69:35S-6S | Not a psychological, behavioural, sensory or environmental intervention |
| Devanand DP, Sackeim HA, Mayeux R. Psychosis, behavioral disturbance, and the use of neuroleptics in dementia. Comprehens Psychiatry 1988;29:387–401 | Not primary research |
| Devanand DP. Use of the Columbia University Scale to assess psychopathology in Alzheimer’s disease. Int Psychogeriatr 1997;9(Suppl. 1):137 | No participants with dementia/not separately analysed |
| Dewing J. Care for older people with a dementia in acute hospital settings. Nurs Older People 2001;13:18–20 | Not primary research |
| Dewing J. Responding agitation in people with dementia. Nurs Older People 2010;22:18–25 | Not primary research |
| Dewing J. Screening for wandering among older persons with dementia. Nurs Older People 2005;17:20–4 | Not primary research |
| Dewing J. Sundowning in older people with dementia: evidence base, nursing assessment and interventions. Nurs Older People 2003;15:24–32 | Not primary research |
| Dewing J. Wandering into the future: reconceptualizing wandering ‘a natural and good thing’. Int J Older People Nurs 2006;1:239–49 | Not primary research |
| DeYoung S, Just G, Harrison R. Decreasing aggressive, agitated, or disruptive behavior: perticipation in a behavior management unit. J Gerontol Nurs 2002;28:22–31 | Some participants < 50 years old |
| Di Carlo A, Baldereschi M, Amaducci L, Maggi S, Grigoletto F, Scarlato G, et al. Cognitive impairment without dementia in older people: Prevalence, vascular risk factors, impact on disability. The Italian longitudinal study on aging. J Am Geriatr Soc 2000;48:775–82 | No participants with dementia/not separately analysed |
| Di Iulio F, Palmer K, Blundo C, Casini AR, Gianni W, Caltagirone C, et al. Occurrence of neuropsychiatric symptoms and psychiatric disorders in mild Alzheimer’s disease and mild cognitive impairment subtypes. Int Psychogeriatr 2010;22:629–40 | Not intervention study |
| Dickson TC, Chuckowree JA, Chuah MI, West AK, Vickers JC. alpha-internexin immunoreactivity reflects variable neuronal vulnerability in Alzheimer’s disease and supports the role of the beta-amyloid plaques in inducing neuronal injury. Neurobiol Dis 2005;18:286–95 | No participants with dementia/not separately analysed |
| Diehl J, Mayer T, Kurz A, Forstl H. Features of frontotemporal dementia from the perspective of a special support group. Nervenarzt 2003;74:445 | No participants with dementia/not separately analysed |
| Diesfeldt HFA. [Behavior that is difficult to manage and psychological interventions.] Tijdschr Gerontol Geriatr 2005;36:98–9 | Not primary research |
| Ditzler K. Efficacy and tolerability of memantine in patients with dementia syndrome – a double-blind, placebo controlled trial. Arzneimittel-Forschung 1991;41–2:773–80 | Not a psychological, behavioural, sensory or environmental intervention |
| Diwan S, Phillips VL. Agitation and dementia-related problem behaviors and case management in long-term care. Int Psychogeriatr 2001;13:5–21 | Not intervention study |
| do Prado RCP, Barbosa ER. Depression in Parkinson’s disease – study of 60 cases. Arquivos de Neuro-Psiquiatria 2005;63:766–71 | No participants with dementia/not separately analysed |
| Dobrohotoff JT, Llewellyn-Jones RH. Psychogeriatric inpatient unit design: a literature review. Int Psychogeriatr 2011;23:174–89 | No participants with dementia/not separately analysed |
| Dobson CM. Experimental investigation of protein folding and misfolding. Methods 2004;34:4–14 | No participants with dementia/not separately analysed |
| Dolder CR, Davis LN, McKinsey J. Use of psychostimulants in patients with dementia. Ann Pharmacother 2010;44:1624–32 | Not primary research |
| Dombrovski AY, Mulsant BH, Ferrell RE, Lotrich FE, Rosen JI, Wallace M, et al. Serotonin transporter triallelic genotype and response to citalopram and risperidone in dementia with behavioral symptoms. Int Clin Psychopharmacol 2010;25:37–45 | Not a psychological, behavioural, sensory or environmental intervention |
| Doody RS, Stevens JC, Beck C, Dubinsky RM, Kaye JA, Gwyther L, et al. Practice parameter: management of dementia (an evidence-based review) – report of the quality standards subcommittee of the American Academy of Neurology. Neurology 2001;56:1154–66 | Not primary research |
| dos Santos-Neto LL, de Vilhena Toledo MA, Medeiros-Souza P, de Souza GA. The use of herbal medicine in Alzheimer’s disease – a systematic review. Evidence-Based Complement Alt Med 2006;3:441–5 | Not primary research |
| Draper B, Meares S, McIntosh H. A psychogeriatric outreach service to nursing homes in Sydney. Aus J Ageing 1998;17:184–6 | Not intervention study |
| Druckenbrod RW, Rosen J, Cluxton RJ. As-needed dosing of antipsychotic-drugs – limitations and guidelines for use in the elderly agitated patient. Ann Pharmacother 1993;27:645–8 | Not primary research |
| Dubois B, McKeith I, Orgogozo JM, Collins O, Meulien D. A multicentre, randomized, double-blind, placebo-controlled study to evaluate the efficacy, tolerability and safety of two doses of metrifonate in patients with mild-to-moderate Alzheimer’s disease: the malt study. Int J Geriatr Psychiatry 1999;14:973–82 | Not a psychological, behavioural, sensory or environmental intervention |
| Duckett S. Managing the sundowning patient. J Rehabil 1993;59:24–9 | Not primary research |
| Duff K, Beglinger LJ, O’Rourke ME, Nopoulos P, Paulson HL, Paulsen JS. Risperidone and the treatment of psychiatric, motor, and cognitive symptoms in Huntington’s disease. Ann Clin Psychiatry 2008;20:1–3 | No participants with dementia/not separately analysed |
| Duignan D, Hedley L, Milverton R. Exploring dance as a therapy for symptoms and social interaction in a dementia care unit. Nurs Times 2009;105:19–22 | No quantitative outcome |
| Dunkin JJ, Anderson-Hanley C. Dementia caregiver burden – a review of the literature and guidelines for assessment and intervention. Neurology 1998;51:S53–60 | Not primary research |
| Dunn JC, Thiru-Chelvam B, Beck CHM. Interdisciplinary care. Bathing: pleasure or pain? J Gerontol Nurs 2002;28:6–13 | No outcome measuring agitation |
| Dunn NR, Pearce GL, Shakir SAW. Adverse effects associated with the use of donepezil in general practice in England. J Psychopharmacol 2000;14:406–8 | Not intervention study |
| Dvir H, Silman I, Harel M, Rosenberry TL, Sussman JL. Acetylcholinesterase: from 3D structure to function. Chemico-Biol Interact 2010;187:10–22 | No participants with dementia/not separately analysed |
| Eagles JM, Gilleard CJ. The demented elderly admitted to a psychogeriatric assessment unit – changes in disability and outcome from 1977–1982. Br J Psychiatry 1984;144:314–16 | Not intervention study |
| Earley CJ. Disorders associated with difficulty in initiating or maintaining sleep. Neurologist 1997;3:77–94 | No participants with dementia/not separately analysed |
| Edell WS, Tunis SL. Antipsychotic treatment of behavioral and psychological symptoms of dementia in geropsychiatric inpatients. Am J Geriatr Psychiatry 2001;9:289–97 | Not a psychological, behavioural, sensory or environmental intervention |
| Edvardsson D, Sandman P, Noy R, Karlsson S. Associations between the working characteristics of nursing staff and the prevalence of behavioral symptoms in people with dementia in residential care. Int Psychogeriatr 2008;20:764–76 | Not intervention study |
| Edwards AM, Phillips RA, Watkins NW, Freeman MP, Murphy EJ, Afanasyev V, et al. Revisiting Levy flight search patterns of wandering albatrosses, bumblebees and deer. Nature 2007;449:1044-U5 | No participants with dementia/not separately analysed |
| Eeles EM, Stephens M, Benedict C, Beaverstock J, Gupta M, Page M. Sleep in dementia assessment may require a multidisciplinary approach. Am J Geriatr Psychiatry 2006;14:986–7 | Not a psychological, behavioural, sensory or environmental intervention |
| Egan MY, Munroe S, Hubert C, Rossiter T, Gauthier A, Eisner M, et al. Caring for residents with dementia and aggressive behavior – impact of life history knowledge. J Gerontol Nurs 2007;33:24–30 | No comparator |
| Eichelman B, Hartwig A. The Carolina Nosology of Destructive Behavior (CNDB). J Neuropsychiatry Clin Neurosci 1990;2:288–96 | No participants with dementia/not separately analysed |
| Elie D, Gagnon P, Gagnon B, Giguere A. Using psychostimulants in end-of-life patients with hypoactive delirium and cognitive disorders: a literature review. Can J Psychiatry 2010;55:386–93 | No participants with dementia/not separately analysed |
| Elie M, Boss K, Cole M, McCusker J, Belzile E, Ciampi A. A retrospective, exploratory, secondary analysis of the association between antipsychotic use and mortality in elderly patients with delirium. Int Psychogeriatr 2009;21:588–92 | No participants with dementia/not separately analysed |
| Ellison JM, Harper DG, Berlow Y, Zeranski L. Beyond the “C” on MCI: noncognitive symptoms in amnestic and non-amnestic mild cognitive impairment. CNS Spectrums 2008;13:66–72 | No participants with dementia/not separately analysed |
| Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291:1753–62 | No participants with dementia/not separately analysed |
| Engelborghs S, Maertens K, Nagels G, Vloeberghs E, Marien P, Symons A, et al. Neuropsychiatric symptoms of dementia: cross-sectional analysis from a prospective, longitudinal Belgian study. Int J Geriatr Psychiatry 2005;20:1028–37 | Not intervention study |
| Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Holman A, Witteman JCM, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002;287:3223–9 | No participants with dementia/not separately analysed |
| Ernst E. Is reflexology an effective intervention? A systematic review of randomised controlled trials. Med J Aus 2009;191:263–6 | Not primary research |
| Ersek M, Herr K, Neradilek MB, Buck HG, Black B. Comparing the psychometric properties of the Checklist of Nonverbal Pain Behaviors (CNPI) and the Pain Assessment in Advanced Dementia (PAIN-AD) instruments. Pain Med 2010;11:395–404 | No participants with dementia/not separately analysed |
| Estes ML, Chimowitz MI, Awad IA, Mcmahon JT, Furlan AJ, Ratliff NB. Sclerosing vasculopathy of the central-nervous-system in nonelderly demented patients. Arch Neurol 1991;48:631–6 | Not intervention study |
| Ettema TP, Droes RM, de Lange J, Mellenbergh GJ, Ribbe MW. QUALIDEM: Development and evaluation of a dementia specific quality of life instrument. Scalability, reliability and internal structure. Int J Geriatr Psychiatry 2007;22:549–56 | No participants with dementia/not separately analysed |
| Eustace A, Coen R, Walsh C, Cunningham CJ, Walsh JB, Coakley D, et al. A longitudinal evaluation of behavioural and psychological symptoms of probable Alzheimer’s disease. Int J Geriatr Psychiatry 2002;17:968–73 | Not intervention study |
| Evers MM, Marin DB. Mood disorders – effective management of major depressive disorder in the geriatric patient. Geriatrics 2002;57:36–40 | No participants with dementia/not separately analysed |
| Fabrega H, Mezzich J, Ulrich RF. Black-white differences in psychopathology in an urban psychiatric population. Comprehens Psychiatry 1988;29:285–97 | No participants with dementia/not separately analysed |
| Factor SA, Molho ES, Brown DL. Acute delirium after withdrawal of amantadine in Parkinson’s disease. Neurology 1998;50:1456–8 | No participants with dementia/not separately analysed |
| Factor SA, Molho ES. Emergency department presentations of patients with Parkinson’s disease. Am J Emerg Med 2000;18:209–15 | No participants with dementia/not separately analysed |
| Falsetti AE. Risperidone for control of agitation in dementia patients. Am J Health-Syst Pharm 2000;57:862–74 | Not primary research |
| Farlow MR, Graham SM, Alva G. Memantine for the treatment of Alzheimer’s disease. Drug Saf 2008;31:577–85 | Not a psychological, behavioural, sensory or environmental intervention |
| Farran CJ, Gilley DW, McCann JJ, Bienias JL, Lindeman DA, Evans DA. Efficacy of behavioral interventions for dementia caregivers. West J Nurs Res 2007;29:944–60 | No participants with dementia/not separately analysed |
| Farrell Miller M. Physical aggressive resident behavior during hygienic care. J Gerontol Nurs 1997;23:24–39 | No participants with dementia/not separately analysed |
| Fauth E, Zarit S, Femia E, Hofer S, Stephens M. Behavioral and psychological symptoms of dementia and caregivers’ stress appraisals: Intra-individual stability and change over short-term observations. Aging Mental Health 2006;10:563–73 | Not intervention study |
| Feinberg MV, Michocki RJ. Clinical and regulatory concerns in Alzheimer’s disease management: role of the pharmacist. Am J Health-Syst Pharm 1998;55:S26–31 | No participants with dementia/not separately analysed |
| Feldt KS, Ryden MB. Aggressive behavior. Educating nursing assistants. J Gerontol Nurs 1992;18:3–12 | No participants with dementia/not separately analysed |
| Feliciano L, Steers ME, Elite-Marcandonatou A, Mclane M, Arean PA. Applications of preference assessment procedures in depression and agitation management in elders with dementia. Clin Gerontol 2009;32:239–59 | No comparator |
| Ferini-Strambi L, Fantini ML, Zucconi M, Castronovo V, Marelli S, Oldani A, et al. REM sleep behaviour disorder. Neurol Sci 2005;26:S186–92 | No participants with dementia/not separately analysed |
| Fernandez HH, Lapane KL, Ott BR, Friedman JH. Gender differences in the frequency and treatment of behavior problems in Parkinson’s disease. Movement Disord 2000;15:490–6 | No participants with dementia/not separately analysed |
| Fernandez-Martinez M, Castro J, Molano A, Zarranz JJ, Rodrigo RM, Ortega R. Prevalence of neuropsychiatric symptoms in Alzheimer’s disease and vascular dementia. Curr Alzheimer Res 2008;5:61–9 | Not intervention study |
| Ferran J, Wilson K, Doran M, Ghadiali E, Johnson F, Cooper P, et al. The early onset dementias: a study of clinical characteristics and service use. Int J Geriatr Psychiatry 1996;11:863–9 | Not intervention study |
| Ferre Jodra A, Capdevila Ordonez M, Garcia Lidon E, Almenar Monforte C. Evaluation of the activity in a mean stay unit of psychogeriatrics. Revista Espanola de Geriatria y Gerontologia 2002;37:190–7 | No participants with dementia/not separately analysed |
| Ferris SH, Mackell JA, Mohs R, Schneider LS, Galasko D, Whitehouse PJ, et al. A multicenter evaluation of new treatment efficacy instruments for Alzheimer’s disease clinical trials: overview and general results. Alzheimer Dis Assoc Disord 1997;11(Suppl. 2):S1–12 | No participants with dementia/not separately analysed |
| Ferris SH, Sathananthan G, Gershon S, Clark C. Senile dementia – treatment with deanol. J Am Geriatr Soc 1977;25:241–4 | Not a psychological, behavioural, sensory or environmental intervention |
| Ferris SH, Steinberg G, Shulman E, Kahn R, Reisberg B. Institutionalisation of Alzheimer’s disease patients: reducing precipitating factors through family counseling. Home Health Care Serv Q 1987;8:23–51 | No outcome measuring agitation |
| Filali M, Lalonde R, Rivest S. Cognitive and non-cognitive behaviors in an APPswe/PS1 bigenic model of Alzheimer’s disease. Genes Brain Behav 2009;8:143–8 | No participants with dementia/not separately analysed |
| Finfgeld-Connett D. Management of aggression among demented or brain-injured patients. Clin Nurs Res 2009;18:272–87 | Not primary research |
| Finkel SI, Lyons J. Nursing home research from investigators’ perspective. Int Psychogeriatr 1996;8(Suppl. 3):371 | Not primary research |
| Finkel SI, Lyons JS, Anderson RL, Sherrell K, Davis J, Cohen-Mansfield J, et al. A randomized, placebo-controlled trial of thiothixene in agitated, demented nursing-home patients. Int J Geriatr Psychiatry 1995;10:129–36 | Not a psychological, behavioural, sensory or environmental intervention |
| Finkel SI, Mintzer JE, Dysken M, Krishnan KRR, Burt T, McRae T. A randomized, placebo-controlled study of the efficacy and safety of sertraline in the treatment of the behavioral manifestations of Alzheimer’s disease in outpatients treated with donepezil. Int J Geriatr Psychiatry 2004;19:9–18 | Not a psychological, behavioural, sensory or environmental intervention |
| Finkel SI. Effects of rivastigmine on behavioral and psychological symptoms of dementia in Alzheimer’s disease. Clin Ther 2004;26:980–90 | Not primary research |
| Finkel SI. Managing the behavioral and psychological signs and symptoms of dementia. Int Clin Psychopharmacol 1997;12:S25–8 | Not primary research |
| Fischer T, Spahn C, Kovach C. [Targeted management of challenging behavior in persons with dementia: the “Serial Trial Intervention” (STI).] Pflege Zeitschrift 2007;60:370–3 | Not primary research |
| Fischer-Terworth C, Probst P, Glanzmann PG, Knorr CC. Psychological interventions in dementia: an evaluative review. Zeitschrift fur Psychiatrie Psychologie und Psychotherapie 2009;57:195–206 | Not primary research |
| Fisher JE, Swingen DN. Contextual factors in the assessment and management of aggression in dementia patients. Cogn Behav Prac 1997;4:171–90 | Not primary research |
| Fisher R, Blair M, Shedletsky R, Lundell A, Napoliello M, Steinberg S. An open dose finding study of melperone in treatment of agitation and irritability associated with dementia. Can J Psychiatry 1983;28:193–6 | Not a psychological, behavioural, sensory or environmental intervention |
| Fitten LJ, Ortiz F, Siembieda DW, O’Neill J, Halgren E, Fisher A. Reduction of motoric agitation and restlessness by AF102B and tacrine in the macaque. J Neuropsychiatry Clin Neurosci 1999;11:79–85 | No participants with dementia/not separately analysed |
| Fitzpatrick AL, Buchanan CK, Nahin RL, DeKosky ST, Atkinson HH, Carlson MC, et al. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol Series A 2007;62:1244–51 | No participants with dementia/not separately analysed |
| Flannery RB, Peterson B, Walker AP. Precipitants of elderly psychiatric patient assaults on staff: preliminary empirical inquiry. Psychiatr Q 2005;76:167–75 | No participants with dementia/not separately analysed |
| Flannery RBJ. Characteristics of assaultive psychiatric inpatients: updated review of findings, 1995–2000. Am J Alzheimers Dis Other Demen 2001;16:153–6 | No participants with dementia/not separately analysed |
| Flannery RBJ. Domestic violence and elderly dementia sufferers. Am J Alzheimers Dis Other Demen 2003;18:21–3 | Not primary research |
| Fleming KC, Evans JM. Pharmacological therapies in dementia. Mayo Clin Proc 1995;70:1116–23 | Not primary research |
| Fleminger S. Long-term psychiatric disorders after traumatic brain injury. Eur J Anaesthesiol 2008;25:123–30 | No participants with dementia/not separately analysed |
| Flint AJ, van Reekum R. The pharmacologic treatment of Alzheimer’s disease: a guide for the general psychiatrist. Can J Psychiatry 1998;43:689–97 | Not primary research |
| Flynn BL, Ranno AE. Pharmacologic management of Alzheimer disease part II: antioxidants, antihypertensives, and ergoloid derivatives. Ann Pharmacother 1999;33:188–97 | Not primary research |
| Fook VFS, Tay SC, Jayachandran M, Biswas J, Zhang DQ. An ontology-based context model in monitoring and handling agitation behaviour for persons with dementia. Fourth Annual IEEE International Conference on Pervasive Computing and Communications Workshops, Proceedings 2006;560–4 | No participants with dementia/not separately analysed |
| Forbes DA. Review: sparse evidence supports non-pharmacological interventions for preventing wandering in people with dementia. Evidence-Based Nurs 2007;10:15 | Not primary research |
| Ford GR, Goode KT, Barrett JJ, Harrell LE, Haley WE. Gender roles and caregiving stress: an examination of subjective appraisals of specific primary stressors in Alzheimer’s caregivers. Aging Mental Health 1997;1:158–65 | No participants with dementia/not separately analysed |
| Forlenza OV, Cretaz E, de Oliveira Diniz BS. The use of antipsychotics in patients with dementia. Revista Brasileira de Psiquiatria 2008;30:265–70 | Not primary research |
| Fossey J, Ballard C, Juszczak E, James I, Aldler N, Jacoby R, et al. Effect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia: cluster randomised trial. BMJ 2006;332:756–8A | No participants with dementia/not separately analysed |
| Foster HG, Hillbrand M, Chi CC. Efficacy of carbamazepine in assaultive patients with frontal-lobe dysfunction. Prog Neuro-Psychopharmacol Biol Psychiatry 1989;13:865–74 | Not a psychological, behavioural, sensory or environmental intervention |
| Fountoulakis KN, Tsolaki M, Kazis A. Target symptoms for fluvoxamine in old age depression. Int J Psychiatry Clin Prac 2000;4:127–34 | No participants with dementia/not separately analysed |
| Franco KN, Messinger-Rapport B. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. J Am Med Direct Assoc 2006;7:201–2 | Not primary research |
| Frank L, Kleinman L, Ciesla G, Rupnow MFT, Brodaty H. The effect of risperidone on nursing burden associated with caring for patients with dementia. J Am Geriatr Soc 2004;52:1449–55 | No participants with dementia/not separately analysed |
| Frenchman IB. Risperidone, haloperidol, and olanzapine for the treatment of behavioral disturbances in nursing home patients: a retrospective analysis. Curr Ther Res 2000;61:742–50 | Not a psychological, behavioural, sensory or environmental intervention |
| Freudenreich O. Drug-induced sialorrhea. Drugs Today 2005;41:411–8 | No participants with dementia/not separately analysed |
| Freund LY, Basun H, Cederholm T, Faxén IG, Garlind A, Grut M, et al. Omega-3 supplementation in mild to moderate Alzheimer’s disease: effects on neuropsychiatric symptoms. Int J Geriatr Psychiatry 2008;23:161–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Freund-Levi Y, Basun H, Cederholm T, Faxén-Irving G, Garlind A, Grut M, et al. Omega-3 supplementation in mild to moderate Alzheimer’s disease: effects on neuropsychiatric symptoms. Int J Geriatr Psychiatry 2008;23:161–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Freyne A, Wrigley M. Aggressive incidents towards staff by elderly patients with dementia in a long-stay ward. Int J Geriatr Psychiatry 1996;11:57–63 | No participants with dementia/not separately analysed |
| Frost RE, Messiha FS. Clinical uses of lithium-salts. Brain Res Bull 1983;11:219–31 | Not a psychological, behavioural, sensory or environmental intervention |
| Frumin M, Chisholm T, Dickey CC, Daffner KR. Psychiatric and behavioral problems. Neurol Clin 1998;16:521 | No participants with dementia/not separately analysed |
| Fuchs-Lacelle S, Hadjistavropoulos T. Development and preliminary validation of the pain assessment checklist for seniors with limited ability to communicate (PACSLAC). Pain Manag Nurs 2004;5:37–49 | Not primary research |
| Fuentes P, Slachevsky A, Reyes P, Cartier L. Frontotemporal dementia non familial and generalized epilepsy. Arq Neuropsiquiatr 2005;63:1016–20 | Not intervention study |
| Fuglum E, Schillinger A, Andersen JB, Belstad BE, Jensen D, Muller F, et al. Zuclopenthixol and haloperidol levomepromazine in the treatment of elderly patients with symptoms of aggressiveness and agitation – a double-blind, multi-centre study. Pharmatherapeutica 1989;5:285–91 | Not a psychological, behavioural, sensory or environmental intervention |
| Fuh JL, Lam L, Hirono N, Senanarong V, Cummings JL. Neuropsychiatric Inventory workshop: behavioral and psychologic symptoms of dementia in Asia. Alzheimer Dis Assoc Disord 2006;20:314–7 | No participants with dementia/not separately analysed |
| Fuh JL. Study of behavioral and psychological symptoms of dementia in Taiwan. Acta Neurol Taiwan 2006;15:154–60 | Not primary research |
| Fujikawa T, Takahashi T, Kinoshita A, Kajiyama H, Kurata A, Yamashita H, et al. Quetiapine treatment for behavioral and psychological symptoms in patients with senile dementia of Alzheimer type. Neuropsychobiology 2004;49:201–4 | Not a psychological, behavioural, sensory or environmental intervention |
| Fujimoto C, Ito K, Iwasaki S, Nakao K, Sugasawa M. Reversible impairment of auditory callosal pathway in 5-fluorouracil-induced leukoencephalopathy: parallel changes in function and imaging. Otol Neurotol 2006;27:716–19 | No participants with dementia/not separately analysed |
| Fujita T, Kurihara M, Hasegawa K. Short-term therapeutic prognosis of cognitive impairment with cerebrovascular diseases in chronic stages. Japan J Geriatr 1989;26:499–506 | No participants with dementia/not separately analysed |
| Futrell M, Melillo KD, Remington R, Schoenfelder DP. Evidence-based guideline. Wandering. J Gerontol Nurs 2010;36:6–16 | Not primary research |
| Gaber S, Ronzoni S, Bruno A, Biagi A. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr 2001;159–62 | Not a psychological, behavioural, sensory or environmental intervention |
| Gagnon J, Petit D, Latreille V, Montplaisir J. Neurobiology of sleep disturbances in neurodegenerative disorders. Curr Pharmaceut Design 2008;14:3430–45 | No participants with dementia/not separately analysed |
| Galasko D. An integrated approach to the management of Alzheimer’s disease: assessing cognition, function and behaviour. Eur J Neurol 1998;5:S9–17 | Not primary research |
| Galinsky T, Feng HA, Streit J, Brightwell W, Pierson K, Parsons K, et al. Risk factors associated with patient assaults of home healthcare workers. Rehabil Nurs 2010;35:206–15 | No participants with dementia/not separately analysed |
| Gallagher D, Coen R, Kilroy D, Belinski K, Bruce I, Coakley D, et al. Anxiety and behavioural disturbance as markers of prodromal Alzheimer’s disease in patients with mild cognitive impairment. Int J Geriatr Psychiatry 2011;26:166–72 | No participants with dementia/not separately analysed |
| Gallagher M. Evaluating a protocol to train hospice staff in administering individualized music. Int J Palliative Nurs 2011;17:195–201 | Protocol only |
| Gallagher-Thompson D, Brooks JO, Bliwise D, Leader J, Yesavage JA. The relations among caregiver stress, sundowning symptoms, and cognitive decline in Alzheimers disease. J Am Geriatr Soc 1992;40:807–10 | Not intervention study |
| Gallarda T, Olie JP. An update on biological treatment of behavioral and psychological signs and symptoms of dementia. Encephale Rev Psychiatr Clin Biol Therap 2000;26:72–80 | Not primary research |
| Garcia-Alberca J, Pablo Lara J, Gonzalez-Baron S, Barbancho M, Porta D, Berthier M. Prevalence and comorbidity of neuropsychiatric symptoms in Alzheimer’s disease. Actas Esp Psiquiatr 2008;36:265–70 | Not intervention study |
| Garcia-Alloza M, Hirst WD, Chen CPLH, Lasheras B, Francis PT, Ramirez MJ. Differential involvement of 5-HT1B/1D and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer’s disease. Neuropsychopharmacology 2004;29:410–16 | No participants with dementia/not separately analysed |
| Gareri P, Cotroneo A, Lacava R, Seminara G, Marigliano N, Loiacono A, et al. Comparison of the efficacy of new and conventional antipsychotic drugs in the treatment of behavioral and psychological symptoms of dementia (BPSD). Arch Gerontol Geriatr 2004;207–15 | Not a psychological, behavioural, sensory or environmental intervention |
| Gareri P, Cotroneo A, Marchisio U, Curcio M, De Sarro G. Risperidone in the treatment of behavioral disorders in elderly patients with dementia. Arch Gerontol Geriatr 2001;173–82 | Not a psychological, behavioural, sensory or environmental intervention |
| Gareri P, De Fazio P, Cotroneo A, Lacava R, Gallelli L, De Fazio S, et al. Anticholinergic drug-induced delirium in an elderly Alzheimer’s dementia patient. Arch Gerontol Geriatr 2007;44:199–206 | No participants with dementia/not separately analysed |
| Gareri P, Lacava R, Cotroneo A, Bambara V, Marigliano N, Castagna A, et al. Valproate-induced delirium in a demented patient. Arch Gerontol Geriatr 2009;49:113–18 | Not intervention study |
| Garre-Olmo J, Lopez-Pousa S, Vilalta-Franch J, Gracia Blanco M, Bulbena Vilarrasa A. Grouping and trajectories of neuropsychiatric symptoms in patients with Alzheimer’s disease, part II: two-year patient trajectories. J Alzheimers Dis 2010;22:1169–80 | Not intervention study |
| Garre-Olmo J, Lopez-Pousa S, Vilalta-Franch J, Gracia Blanco M, Bulbena Vilarrasa A. Grouping and trajectories of the neuropsychiatric symptoms in patients with Alzheimer’s disease, part I: symptom clusters. J Alzheimers Dis 2010;22:1157–67 | No participants with dementia/not separately analysed |
| Garvey DS, Saenz de Tejada I, Earl RA, Khanapure SP, inventors; NitroMed I, assignee. Nitrosated and nitrosylated phosphodiesterase inhibitors, compositions and methods of use. US 6462044. 2002 Oct. 8 | No participants with dementia/not separately analysed |
| Gates DM, Fitzwater E, Meyer U. Violence against caregivers in nursing homes. Expected, tolerated, and accepted. J Gerontol Nurs 1999;25:12–22 | No participants with dementia/not separately analysed |
| Gatto E, Parisi V, Persi G, Luis Etcheverry J, Martin C. Restless legs: evaluation of sensitivity, specificity of a self-administered survey. Neurology 2011;76:A585 | No participants with dementia/not separately analysed |
| Gauthier S, Feldman H, Hecker J, Vellas B, Ames D, Subbiah P, et al. Efficacy of donepezil on behavioral symptoms in patients with moderate to severe Alzheimer’s disease. Int Psychogeriatr 2002;14:389–404 | Not a psychological, behavioural, sensory or environmental intervention |
| Gauthier S, Juby A, Dalziel W, Rehel B, Schecter R. Effects of rivastigmine on common symptomatology of Alzheimer’s disease (EXPLORE). Curr Med Res Opin 2010;26:1149–60 | Not a psychological, behavioural, sensory or environmental intervention |
| Gauthier S, Juby A, Rehel B, Schecter R. EXACT: rivastigmine improves the high prevalence of attention deficits and mood and behaviour symptoms in Alzheimer’s disease. Int J Clin Prac 2007;61:886–95 | Not a psychological, behavioural, sensory or environmental intervention |
| Gauthier S, Loft H, Cummings J. Improvement in behavioural symptoms in patients with moderate to severe Alzheimer’s disease by memantine: a pooled data analysis. Int J Geriatr Psychiatry 2008;23:537–45 | Not a psychological, behavioural, sensory or environmental intervention |
| Gauthier S, Wirth Y, Mobius HJ. Effects of memantine on behavioural symptoms in Alzheimer’s disease patients: an analysis of the Neuropsychiatric Inventory (NPI) data of two randomised, controlled studies. Int J Geriatr Psychiatry 2005;20:459–64 | Not primary research |
| Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging – population-based study. Arch Gen Psychiatry 2008;65:1193–8 | No participants with dementia/not separately analysed |
| Gedye A. Serotonergic treatment for aggression in a Downs syndrome adult showing signs of Alzheimers disease. J Mental Defic Res 1991;35:247–58 | No participants with dementia/not separately analysed |
| Gehrman PR, Connor DJ, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. Am J Geriatr Psychiatry 2009;17:166–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Genazzani AR, Spinetti A, Gallo R, Bernardi F. Menopause and the central nervous system: intervention options. Maturitas 1999;31:103–10 | No participants with dementia/not separately analysed |
| Georgotas A. Affective disorders in the elderly – diagnostic and research considerations. Age Ageing 1983;12:1–10 | No participants with dementia/not separately analysed |
| Gerdner LA. Individualized music intervention protocol. J Gerontol Nurs 1999;25:10–16 | Protocol only |
| Gerritsen D, Achterberg W, Steverink N, Pot A, Frijters D, Ribbe M. The MDS challenging behavior profile for long-term care. Aging Mental Health 2008;12:116–23 | No participants with dementia/not separately analysed |
| Gianni S, Ivarsson Y, De Simone A, Travaglini-Allocatelli C, Brunori M, Vendruscolo M. Structural characterization of a misfolded intermediate populated during the folding process of a PDZ domain. Nature Struct Mol Biol 2010;17:1431–U57 | No participants with dementia/not separately analysed |
| Giasson BI, Van Deerlin VM. Mutations in LRRK2 as a cause of Parkinson’s disease. Neurosignals 2008;16:99–105 | No participants with dementia/not separately analysed |
| Giehm L, Otzen DE. Strategies to increase the reproducibility of protein fibrillization in plate reader assays. Anal Biochem 2010;400:270–81 | No participants with dementia/not separately analysed |
| Gilbert AM, Stack GP. 8-aza-bicyclo[3.2.1]octan-3-ol derivatives of 2,3-dihydro-1,4-benzodioxan as 5-HT1A antagonists. Official Gazette of the United States Patent and Trademark Office Patents 2003;1277(1) | No participants with dementia/not separately analysed |
| Gilley DW, Bienias JL, Wilson RS, Bennett DA, Beck TL, Evans DA. Influence of behavioral symptoms on rates of institutionalization for persons with Alzheimer’s disease. Psychol Med 2004;34:1129–35 | Not intervention study |
| Gilley DW, Wilson RS, Beckett LA, Evans DA. Psychotic symptoms and physically aggressive behavior in Alzheimer’s disease. J Am Geriatr Soc 1997;45:1074–9 | Not intervention study |
| Gillioz AS, Villars H, Voisin T, Cortes F, Gillette-Guyonnet S, Andrieu S, et al. Spared and impaired abilities in community-dwelling patients entering the severe stage of Alzheimer’s disease. Dementia Geriatr Cogn Disord 2009;28:427–32 | Not intervention study |
| Gitlin LN, Corcoran MA. Managing dementia at home: the role of home environmental modifications. Topics Geriatr Rehabil 1996;12:28–39 | No participants with dementia/not separately analysed |
| Gitlin LN, Hodgson N, Jutkowitz E, Pizzi L. The cost-effectiveness of a nonpharmacologic Intervention for individuals with dementia and family caregivers: the Tailored Activity Program. Am J Geriatr Psychiatry 2010;18:510–19 | No participants with dementia/not separately analysed |
| Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. Am J Geriatr Psychiatry 2008;16:229–39 | No outcome measuring agitation |
| Gleason RP, Schneider LS. Carbamazepine treatment of agitation in Alzheimers outpatients refractory to neuroleptics. J Clin Psychiatry 1990;51:115–18 | Not a psychological, behavioural, sensory or environmental intervention |
| Glenn MB. Sudden cardiac death and stroke with the use of antipsychotic medications: implications for clinicians treating individuals with traumatic brain injury. J Head Trauma Rehabil 2010;25:68–70 | No participants with dementia/not separately analysed |
| Glick ID, Murray SR, Vasudevan P, Marder SR, Hu RJ. Treatment with atypical antipsychotics: new indications and new populations. J Psychiatr Res 2001;35:187–91 | Not primary research |
| Glueckauf RL, Ketterson TU, Loomis JS, Dages P. Online support and education for dementia caregivers: overview, utilization, and initial program evaluation. Telemed J E-Health 2004;10:223–32 | No participants with dementia/not separately analysed |
| Glueckauf RL, Loomis JS. Alzheimer’s Caregiver Support Online: lessons learned, initial findings and future directions. Neurorehabilitation 2003;18:135–46 | No participants with dementia/not separately analysed |
| Gobbi G, Gaudreau PO, Leblanc N. Efficacy of topiramate, valproate, and their combination on aggression/agitation behavior in patients with psychosis. J Clin Psychopharmacol 2006;26:467–73 | No participants with dementia/not separately analysed |
| Goddaer J, Abraham IL. Effects of relaxing music on agitation during meals among nursing home residents with severe cognitive impairment. Arch Psychiatr Nurs 1994;8:150–8 | No participants with dementia/not separately analysed |
| Goff DC, Cather C, Freudenreich O, Henderson DC, Evins A, Culhane MA, et al. A placebo-controlled study of sildenafil effects on cognition in schizophrenia. Psychopharmacology 2009;202:411–17 | No participants with dementia/not separately analysed |
| Gohier B, Verny C, Ricalens J, Allain P, Bouneau D, Garre J. Evaluation of depression in Huntington disease: a crossing semiology. Int J Neuropsychopharmacol 2004;7:S205 | No participants with dementia/not separately analysed |
| Gokalsing E, Robert PH, Lafont V, Medecin I, Baudu C, Boyer P, et al. Evaluation of the supervisory system in elderly subjects with and without disinhibition. Eur Psychiatry 2000;15:407–15 | No participants with dementia/not separately analysed |
| Golini L, Colangeli R, Tranquillo V, Mariscoli M. Association between neurologic and cognitive dysfunction signs in a sample of aging dogs. J Vet Behav-Clin Applic Res 2009;4:25–30 | No participants with dementia/not separately analysed |
| Goodnick PJ, Barrios CA. Use of olanzapine in non-psychotic psychiatric disorders. Ex Opin Pharmacother 2001;2:667–80 | Not primary research |
| Gotell E, Brown S, Ekman SL. The influence of caregiver singing and background music on vocally expressed emotions and moods in dementia care: a qualitative analysis. Int J Nurs Stud 2009;46:422–30 | No outcome measuring agitation |
| Grau-Veciana JM. Treatment of non cognitive symptoms of Alzheimer’s disease. Rev Neurol 2006;42:482–8 | Not primary research |
| Grey KF. Managing agitation and difficult behavior in dementia. Clin Geriatr Med 2004;20:69 | Not primary research |
| Green BH, Dewey ME, Copeland JRM, Saunders PA, Sharma V, Larkin B, et al. Prospective data on the prevalence of abnormal involuntary movements among elderly people living in the community. Acta Psychiatr Scand 1993;87:418–21 | No participants with dementia/not separately analysed |
| Greenberg SM, Tennis MK, Brown LB, Gomez-Isla T, Hayden DL, Schoenfeld DA, et al. Donepezil therapy in clinical practice – a randomized crossover study. Arch Neurol 2000;57:94–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Greendyke RM, Kanter DR, Schuster DB, Verstreate S, Wootton J. Propranolol treatment of assaultive patients with organic brain disease – a double-blind crossover, placebo-controlled study. J Nervous Mental Dis 1986;174:290–4 | Not a psychological, behavioural, sensory or environmental intervention |
| Greendyke RM, Kanter DR. Therapeutic effects of pindolol on behavioral disturbances associated with organic brain disease – a double-blind study. J Clin Psychiatry 1986;47:423–6 | Not a psychological, behavioural, sensory or environmental intervention |
| Gregg TR. Cortical and limbic neural circuits mediating aggressive behavior. Neurobiol Aggression 2003;1–20 | No participants with dementia/not separately analysed |
| Gregory CA, Serra-Mestres J, Hodges JR. Early diagnosis of the frontal variant of frontotemporal dementia: how sensitive are standard neuroimaging and neuropsychologic tests? Neuropsychiatry Neuropsychol Behav Neurol 1999;12:128–35 | No participants with dementia/not separately analysed |
| Greve M, O’Connor D. A survey of Australian and New Zealand old age psychiatrists’ preferred medications to treat behavioral and psychological symptoms of dementia (BPSD). Int Psychogeriatr 2005;17:195–205 | Not intervention study |
| Grossberg G, Manes F, Allegri R, Gutierrez Robledo LM, Gloger S, Xie L, et al. Benefits of extended-release memantine (28 mg, once daily) on caregiver distress: results of a multinational, double-blind, placebo-controlled trial in moderate to severe Alzheimer’s disease. Neurology 2010;74:A271 | Not a psychological, behavioural, sensory or environmental intervention |
| Grossberg GT, Grossberg AL. Treating psychiatric disorders in the nursing home: a focus on Alzheimer’s disease. Bull Menninger Clin 1999;63:A22–30 | Not primary research |
| Grossberg GT, Lake JT. The role of the psychiatrist in Alzheimer’s disease. J Clin Psychiatry 1998;59:3–6 | No participants with dementia/not separately analysed |
| Grossberg GT, Pejovic V, Miller ML, Graham SM. Memantine therapy of behavioral symptoms in community-dwelling patients with moderate to severe Alzheimer’s disease. Dementia Geriatr Cogn Disord 2009;27:164–72 | Not primary research |
| Grossman F. A review of anticonvulsants in treating agitated demented elderly patients. Pharmacotherapy 1998;18:600–6 | Not primary research |
| Grossman M, Armstrong C, Onishi K, Thompson H, Schaefer B, Robinson K, et al. Patterns of cognitive impairment in relapsing-remitting and chronic progressive multiple-sclerosis. Neuropsychiatry Neuropsychol Behav Neurol 1994;7:194–210 | No participants with dementia/not separately analysed |
| Gruber-Baldini AL, Boustani M, Sloane PD, Zimmerman S. Behavioral symptoms in residential care/assisted living facilities: prevalence, risk factors, and medication management. J Am Geriatr Soc 2004;52:1610–17 | No participants with dementia/not separately analysed |
| Guay DR. Inappropriate sexual behaviors in cognitively impaired older individuals. Am J Geriatr Pharmacother 2008;6:269–88 | Not primary research |
| Guay DRP. Drug forecast: memantine, prototype of a new approach to treatment of dementia. Consult Pharm 2003;18:625–34 | Not primary research |
| Guerin O, Andrieu S, Schneider SM, Cortes F, Cantet C, Gillette-Guyonnet S, et al. Characteristics of Alzheimer’s disease patients with a rapid weight loss during a six-year follow-up. Clin Nutr 2009;28:141–6 | Not intervention study |
| Gurevitz SL, Costakis T, Leiter J. Do atypical antipsychotics cause weight gain in nursing home dementia residents? Consult Pharm 2004;19:809–12 | Not a psychological, behavioural, sensory or environmental intervention |
| Gustafson L. Clinical picture of frontal-lobe degeneration of non-Alzheimer type. Dementia 1993;4:143–8 | Not primary research |
| Gustafson Y, Lundstrom M, Bucht G, Edlund A. [Delirium in old age can be prevented and treated.] Tidsskr Nor Lageforen 2002;122:810–14 | No participants with dementia/not separately analysed |
| Guttman R, Altman RD, Nielsen NH. Alzheimer disease – report of the council on scientific affairs. Arch Fam Med 1999;8:347–53 | No participants with dementia/not separately analysed |
| Gutzmann H, Kuhl KP, Kanowski S, Khan-Boluki J. Measuring the efficacy of psychopharmacological treatment of psychomotoric restlessness in dementia: clinical evaluation of tiapride. Pharmacopsychiatry 1997;30:6–11 | Not a psychological, behavioural, sensory or environmental intervention |
| Ha TM, Cho DM, Park SW, Joo MJ, Lee BJ, Kong BG, et al. Evaluating associations between 5-HTTLPR polymorphism and Alzheimer’s disease for Korean patients. Dementia Geriatr Cogn Disord 2005;20:31–4 | Not intervention study |
| Haber S. Anatomical relationship between the basal ganglia and the basal nucleus of meynert in human and monkey forebrain. Proc Natl Acad Sci USA 1987;84:1408–12 | No participants with dementia/not separately analysed |
| Hachinski V. Stroke and vascular cognitive impairment – a transdisciplinary, translational and transactional approach. Stroke 2007;38:1396–403 | No participants with dementia/not separately analysed |
| Haddad PM, Dursun SM. Neurological complications of psychiatric drugs: clinical features and management. Hum Psychopharmacol 2008;23:15–26 | Not primary research |
| Hagen BF, Sayers D. When caring leaves bruises: the effects of staff education on resident aggression. J Gerontol Nurs 1995;21:7–16 | No participants with dementia/not separately analysed |
| Hall GR, Buckwalter KC, Stolley JM, Gerdner LA, Garand L, Ridgeway S, et al. Standardized care plan. Managing Alzheimer’s patients at home. J Gerontol Nurs 1995;21:37–47 | Not primary research |
| Hall KA, Keks NA, O’Connor DW. Transdermal estrogen patches for aggressive behavior in male patients with dementia: a randomized, controlled trial. Int Psychogeriatr 2005;17:165–78 | Not a psychological, behavioural, sensory or environmental intervention |
| Hallberg IR, Norberg A. Strain among nurses and their emotional-reactions during 1 year of systematic clinical supervision combined with the implementation of individualized care in dementia nursing. J Adv Nurs 1993;18:1860–75 | No participants with dementia/not separately analysed |
| Hamazaki T, Sawazaki S, Itomura M, Asaoka E, Nagao Y, Nishimura N, et al. The effect of docosahexaenoic acid on aggression in young adults – a placebo-controlled double-blind study. J Clin Invest 1996;97:1129–33 | No participants with dementia/not separately analysed |
| Handratta V, Hsu E, Vento J, Yang C, Tanev K. Neuroimaging findings and brain-behavioral correlates in a former boxer with chronic traumatic brain injury. Neurocase 2010;16:125–34 | No participants with dementia/not separately analysed |
| Happe S. Excessive daytime sleepiness and sleep disturbances in patients with neurological diseases – epidemiology and management. Drugs 2003;63:2725–37 | No participants with dementia/not separately analysed |
| Harinath M, Rosen J, Marin R. Neuropsychiatric sequelae of cerebrovascular disease. Neurologist 1995;1:219–31 | No participants with dementia/not separately analysed |
| Harris MK, Shneyder N, Borazanci A, Korniychuk E, Kelley RE, Minagar A. Movement disorders. Med Clin North Am 2009;93:371 | No participants with dementia/not separately analysed |
| Hart BD, Wells DL. The effects of language used by caregivers on agitation in residents with dementia. CNS 1997;11:20–3 | Not a psychological, behavioural, sensory or environmental intervention |
| Hart DJ, Craig D, Compton SA, Critchlow S, Kerrigan BM, McIlroy SP, et al. A retrospective study of the behavioural and psychological symptoms of mid and late phase Alzheimer’s disease. Int J Geriatr Psychiatry 2003;18:1037–42 | Not intervention study |
| Harwood DG, Kalechstein A, Barker WW, Strauman S, George-Hyslop PS, Iglesias C, et al. The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer’s disease. Int J Geriatr Psychiatry 2010;25:511–18 | Not intervention study |
| Harwood DG, Ownby RL, Barker WW, Duara R. The factor structure of the Cornell Scale for depression in dementia among probable Alzheimer’s disease patients. Am J Geriatr Psychiatry 1998;6:212–20 | Not intervention study |
| Harwood DG, Sultzer DL, Wheatley MV. Impaired insight in Alzheimer disease: association with cognitive deficits, psychiatric symptoms, and behavioral disturbances. Neuropsychiatry Neuropsychol Behav Neurol 2000;13:83–8 | Not intervention study |
| Harwood DG, Sultzer DL. “Life is not worth living”: hopelessness in Alzheimer’s disease. J Geriatr Psychiatry Neurol 2002;15:38–43 | Not intervention study |
| Haspel T. Beta-blockers and the treatment of aggression. Harvard Rev Psychiatry 1995;2:274–81 | Not primary research |
| Hastings SN, Thompson-Heisterman A, Farrell SP. Identifying and treating agitated behaviors in the long-term care setting. Lippincott’s Prim Care Prac 1999;3:204–15 | Not primary research |
| Hattori H. [Depression in the elderly.] Nihon Ronen Igakkai Zasshi 2008;45:451–61 | No participants with dementia/not separately analysed |
| Haupt M, Cruz-Jentoft A, Jeste D. Mortality in elderly dementia patients treated with risperidone. J Clin Psychopharmacol 2006;26:566–70 | Not a psychological, behavioural, sensory or environmental intervention |
| Haupt M, Kurz A, Greifenhagen A. Depression in Alzheimer’s disease – phenomenological features and association with severity and progression of cognitive and functional impairment. Int J Geriatr Psychiatry 1995;10:469–76 | Not intervention study |
| Haupt M, Kurz A, Janner M. A 2-year follow-up of behavioural and psychological symptoms in Alzheimer’s disease. Dementia Geriatr Cogn Disord 2000;11:147–52 | Not intervention study |
| Haupt M. The course of behavioural symptoms and their psychosocial treatment in dementia sufferers. Z Gerontol Geriatr 1999;32:159–66 | Not primary research |
| Hausdorff JM, Lertratanakul A, Cudkowicz ME, Peterson AL, Kaliton D, Goldberger AL. Dynamic markers of altered gait rhythm in amyotrophic lateral sclerosis. J Appl Physiol 2000;88:2045–53 | No participants with dementia/not separately analysed |
| Haveman MJ, Maaskant MA, Lantman HMV, Urlings HFJ, Kessels AGH. Mental-health problems in elderly people with and without Down’s syndrome. J Intellect Disabil Res 1994;38:341–55 | No participants with dementia/not separately analysed |
| Haviv H, Wong DM, Greenblatt HM, Carlier PR, Pang YP, Silman I, et al. Crystal packing mediates enantioselective ligand recognition at the peripheral site of acetylcholinesterase. J Am Chem Soc 2005;127:11029–36 | No participants with dementia/not separately analysed |
| Hayashi Y, Ishida Y, Inoue T, Udagawa M, Takeuchi K, Yoshimuta H, et al. Treatment of behavioral and psychological symptoms of Alzheimer-type dementia with Yokukansan in clinical practice. Prog Neuro-Psychopharmacol Biol Psychiatry 2010;34:541–5 | Not a psychological, behavioural, sensory or environmental intervention |
| Hebert LE, Scherr PA, Beckett LA, Funkenstein HH, Albert MS, Chown MJ, et al. Relation of smoking and alcohol consumption to incident Alzheimers-disease. Am J Epidemiol 1992;135:347–55 | No participants with dementia/not separately analysed |
| Held C. [Management of behavioral disorders in patients with dementia. Significance, diagnosis, non-medicamentous and medicamentous therapy.] Praxis 2000;89:1376–85 | Not primary research |
| Helmes E, Csapo KG, Short JA. Standardization and validation of the multidimensional observation scale for elderly subjects (Moses). J Gerontol 1987;42:395–405 | No participants with dementia/not separately analysed |
| Herman RE, Williams KN. Elderspeak’s influence on resistiveness to care: focus on behavioral events. Am J Alzheimers Dis Other Demen 2009;24:417–23 | Not intervention study |
| Hermans D, Hla HU, McShane R. Non-pharmacological interventions for wandering of people with dementia in the domestic setting. Cochrane Database Syst Rev 2007;1 | Not primary research |
| Hernandez-Vara J. Brain parenchyma sonography in the study of movement disorders. Rev Neurol 2010;50:486–94 | No participants with dementia/not separately analysed |
| Herrmann F, Grandjean R, Izard I, Giannakopoulos P, Vaucher M. Incidence of behavioral disturbances in geriatric psychiatry. Med Hyg 2004;62:1433 | Not primary research |
| Herrmann N, Cappell J, Eryavec GM, Lanctot KL. Changes in nursing burden following memantine for agitation and aggression in long-term care residents with moderate to severe Alzheimer’s disease: an open-label pilot study. CNS Drugs 2011;25:425–33 | Not a psychological, behavioural, sensory or environmental intervention |
| Herrmann N, Gauthier S, Lysy PG. Clinical practice guidelines for severe Alzheimer’s disease. Alzheimers Demen 2007;3:385–97 | Not primary research |
| Herrmann N, Gauthier S. Diagnosis and treatment of dementia: 6. Management of severe Alzheimer disease. Can Med Assoc J 2008;179:1279–87 | No participants with dementia/not separately analysed |
| Herrmann N, Lanctot KL, Rothenburg LS, Eryavec G. A placebo-controlled trial of valproate for agitation and aggression in Alzheimer’s disease. Dementia Geriatr Cogn Disord 2007;23:116–19 | Not a psychological, behavioural, sensory or environmental intervention |
| Herrmann N, Lanctot KL. From transmitters to treatment: the pharmacotherapy of behavioural disturbances in dementia. Can J Psychiatry 1997;42:S51–64 | Not primary research |
| Herrmann N, Lanctot KL. Pharmacologic management of neuropsychiatric symptoms of Alzheimer disease. Can J Psychiatry 2007;52:630–46 | No participants with dementia/not separately analysed |
| Herrmann N, Li A, Lanctot K. Memantine in dementia: a review of the current evidence. Ex Opin Pharmacother 2011;12:787–800 | Not primary research |
| Herrmann N, Rabheru K, Wang J, Binder C. Galantamine treatment of problematic behavior in Alzheimer disease – post-hoc analysis of pooled data from three large trials. Am J Geriatr Psychiatry 2005;13:527–34 | Not a psychological, behavioural, sensory or environmental intervention |
| Herrmann N, Rothenburg LS, Black SE, Ryan M, Liu BA, Busto UE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol 2008;28:296–301 | Not a psychological, behavioural, sensory or environmental intervention |
| Herrmann N. Recommendations for the management of behavioral and psychological symptoms of dementia. Can J NeurolSci 2001;28:S96–107 | Not primary research |
| Herrmann N. Valproic acid treatment of agitation in dementia. Can J Psychiatry 1998;43:69–72 | Not a psychological, behavioural, sensory or environmental intervention |
| Herz LR, Volicer L, Ross V, Rheaume Y. Pharmacotherapy of agitation in dementia. Am J Psychiatry 1992;149:1757–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Hewawasam L. Floor patterns limit wandering of people with Alzheimer’s. Nursing Times 1996;92:41–4 | No comparator |
| Hicks S. Relaxing music at mealtime in nursing homes: effects on agitated patients with dementia. J Gerontol Nurs 2005;31:26–32 | No participants with dementia/not separately analysed |
| Hilbe J, Schulc E, Linder B, Them C. Development and alarm threshold evaluation of a side rail integrated sensor technology for the prevention of falls. Int J Med Inform 2010;79:173–80 | No participants with dementia/not separately analysed |
| Hock C, Wettstein A, Giannakopoulos P, Schupbach B, Muller-Spahn F. [Diagnosis and therapy of behavior disorders in dementia.] Praxis 2000;89:1907–13 | Not primary research |
| Hodgkinson B, Koch S, Nay R, Lewis M. Managing the wandering behaviour of people living in a residential aged care facility. Int J Evid Based Healthc 2007;5:406–36 | Not primary research |
| Hodgson NA, Andersen S. The clinical efficacy of reflexology in nursing home residents with dementia. J Alt Complement Med 2008;14:269–75 | No outcome measuring agitation |
| Hoe J, Katona C, Orrell M, Livingston G. Quality of life in dementia: care recipient and caregiver perceptions of quality of life in dementia: the LASER-AD study. Int J Geriatr Psychiatry 2007;22:1031–6 | Not intervention study |
| Hoeffer B, Talerico KA, Rasin J, Mitchell C, Stewart BJ, McKenzie D, et al. Assisting cognitively impaired nursing home residents with bathing: effects of two bathing interventions on caregiving. Gerontologist 2006;46:524–32 | No participants with dementia/not separately analysed |
| Hoffman D, Ryan JP. An animal model of Alzheimer’s disease implications for spatial disorientation memory loss and wandering behavior. Soc Neurosci Abstracts 1991;17:1065 | No participants with dementia/not separately analysed |
| Hofmann N. Understanding the neuropsychiatric symptoms of Huntington’s disease. J Neurosci Nurs 1999;31:309–13 | No participants with dementia/not separately analysed |
| Hokkanen L, Rantala L, Remes AM, Harkonen B, Viramo P, Winblad I. Dance/movement therapeutic methods in management of dementia. J Am Geriatr Soc 2003;51:576–7 | No outcome measuring agitation |
| Holliday-Welsh DM, Gessert CE, Renier CM. Massage in the management of agitation in nursing home residents with cognitive impairment. Geriatr Nurs 2009;30:108–17 | No participants with dementia/not separately analysed |
| Holm A, Michel M, Stern GA, Hung TM, Klein T, Flaherty L, et al. The outcomes of an inpatient treatment program for geriatric patients with dementia and dysfunctional behaviors. Gerontologist 1999;39:668–76 | Not a psychological, behavioural, sensory or environmental intervention |
| Holmberg SK. A walking program for wanderers: volunteer training and development of an evening Walker’s group. Geriatr Nurs 1997;18:160–5 | No outcome measuring agitation |
| Holmes C, Smith H, Ganderton R, Arranz M, Collier D, Powell J, et al. Psychosis and aggression in Alzheimer’s disease: the effect of dopamine receptor gene variation. J Neurol Neurosurg Psychiatry 2001;71:777–9 | Not intervention study |
| Holmes C, Wilkinson D, Dean C, Clare C, El Okl M, Hensford C, et al. Risperidone and rivastigmine and agitated behaviour in severe Alzheimer’s disease: a randomised double blind placebo controlled study. Int J Geriatr Psychiatry 2007;22:380–1 | Not a psychological, behavioural, sensory or environmental intervention |
| Holroyd S, Currie LJ, Abraham IL. Aggression in a rural psychogeriatric outreach program. Int J Geriatr Psychiatry 1996;11:529–33 | Not intervention study |
| Holtzer R, Tang MX, Devanand DP, Albert SM, Wegesin DJ, Marder K, et al. Psychopathological features in Alzheimer’s disease: course and relationship with cognitive status. J Am Geriatr Soc 2003;51:953–60 | Not intervention study |
| Homma A, Niina R, Ishii T, Hasegawa K. Behavioral-evaluation of Alzheimer-disease in clinical-trials – development of the Japanese version of the Gbs scale. Alzheimer Dis Assoc Disord 1991;5:S40–8 | No participants with dementia/not separately analysed |
| Hong GRS, Song JA. Relationship between familiar environment and wandering behaviour among Korean elders with dementia. J Clin Nurs 2009;18:1365–73 | Not intervention study |
| Hongratanaworakit T, Buchbauer G. Relaxing effect of ylang ylang oil on humans after transdermal absorption. Phytother Res 2006;20:758–63 | No participants with dementia/not separately analysed |
| Hoogendijk WJ, Meynen G, Feenstra MG, Eikelenboom P, Kamphorst W, Swaab DF. [Increased activity of stress-regulating systems in Alzheimer disease.] Tijdschr Gerontol Geriatr 2001;32:17–23 | No participants with dementia/not separately analysed |
| Hopman-Rock M, Staats PGM, Tak ECPM, Droes RM. The effects of a Psychomotor Activation Programme for use in groups of cognitively impaired people in homes for the elderly. Int J Geriatr Psychiatry 1999;14:633–42 | No participants with dementia/not separately analysed |
| Hou JGG, Lai EC. Non-motor symptoms of Parkinson’s disease. Int J Gerontol 2007;1:53–64 | No participants with dementia/not separately analysed |
| Howard R, Ballard C, O’Brien J, Burns A. Guidelines for the management of agitation in dementia. Int J Geriatr Psychiatry 2001;16:714–17 | Not primary research |
| Howard RJ, Juszczak E, Ballard CG, Bentham P, Brown RG, Bullock R, et al. Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med 2007;357:1382–92 | Not a psychological, behavioural, sensory or environmental intervention |
| Howes K, Schmidt B, Church-Kopish J, Pulukuri S, Frederick J, Sitaramayya A, et al. AMD-Like Retinal Degeneration in S100B Transgenic Mice. ARVO Annual Meeting Abstract Search and Program Planner 2002;2812 | No participants with dementia/not separately analysed |
| Hozumi S, Hori H, Okawa M, Hishikawa Y, Sato K. Favorable effect of transcranial electrostimulation on behavior disorders in elderly patients with dementia: a double-blind study. Int J Neurosci 1996;88:1–10 | Not a psychological, behavioural, sensory or environmental intervention |
| Hsieh CJ, Chang CC, Lin CC. Neuropsychiatric profiles of patients with Alzheimer’s disease and vascular dementia in Taiwan. Int J Geriatr Psychiatry 2009;24:570–7 | Not intervention study |
| Huang HL, Shyu YIL, Chen ST, Hsu WC. Caregiver self-efficacy for managing behavioural problems of older people with dementia in Taiwan correlates with care receivers’ behavioural problems. J Clin Nurs 2009;18:2588–95 | Not intervention study |
| Huang L, Abuhamdah S, Howes MJ, Elliot MS, Ballard C, Holmes C, et al. Pharmacological profile of essential oils derived from Lavandula angustifolia and Melissa officinalis with anti-agitation properties: focus on ligand-gated channels. J Pharm Pharmacol 2008;60:1515–22 | No participants with dementia/not separately analysed |
| Huang ZW, Gabriel JM, Baldwin MA, Fletterick RJ, Prusiner SB, Cohen FE. Proposed 3-dimensional structure for the cellular prion protein. Proc Natl Acad Sci USA 1994;91:7139–43 | No participants with dementia/not separately analysed |
| Huber CG, Lambert M, Naber D, Schacht A, Hundemer HP, Wagner TT, et al. Validation of a Clinical Global Impression Scale for Aggression (CGI-A) in a sample of 558 psychiatric patients. Schizophrenia Res 2008;100:342–8 | No participants with dementia/not separately analysed |
| Huell M, Voigt-Radloff S. Nonmedical treatment of dementia. Nervenarzt 2008;79:159–64 | Not primary research |
| Huertas D, Alino JJ, Molina JD, Chamorro L, Balanza J, Jimenez MP, et al. Antiaggressive effect of cyproterone versus haloperidol in Alzheimer’s disease: a randomized double-blind pilot study. J Clin Psychiatry 2007;68:439–44 | Not a psychological, behavioural, sensory or environmental intervention |
| Hughes TL, Medina-Walpole AM. Implementation of an interdisciplinary behavior management program. J Am Geriatr Soc 2000;48:581–7 | Multidisciplinary team input including pharmacological intervention |
| Hurt C, Bhattacharyya S, Burns A, Camus V, Liperoti R, Marriott A, et al. Patient and caregiver perspectives of quality of life in dementia – an investigation of the relationship to behavioural and psychological symptoms in dementia. Dementia Geriatr Cogn Disord 2008;26:138–46 | Not intervention study |
| Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ 2011;343 | Not a psychological, behavioural, sensory or environmental intervention |
| Hwang JP, Tsai SJ, Yang CH, Liu KM, Lirng JF. Persecutory delusions in dementia. J Clin Psychiatry 1999;60:550–3 | Not intervention study |
| Iijima S. Prevention and treatment of dementia what should we do today? Japan J Geriatr 1991;28:465–9 | Not intervention study |
| Ikeda K, Akiyama H, Arai T, Matsushita M, Tsuchiya K, Miyazaki H. Clinical aspects of argyrophilic grain disease. Clin Neuropathol 2000;19:278–84 | Not intervention study |
| Nelson JC, Tune LE, Daiello LA, Porsteinsson A. Interactive grand rounds: mood stabilizers in dementia. Geriatrics 2002;57:A2–5 | Not a psychological, behavioural, sensory or environmental intervention |
| Iqbal MM, Rahman A, Husain Z, Mahmud SZ, Ryan WG, Feldman JM. Clozapine: a clinical review of adverse effects and management. Ann Clin Psychiatry 2003;15:33–48 | No participants with dementia/not separately analysed |
| Irvine A, Bourgeois M, Billow M, Seeley JR. Internet training for nurse aides to prevent resident aggression. J Am Med Direct Assoc 2007;8:519–26 | No participants with dementia/not separately analysed |
| Ishii S, Streim JE, Saliba D. Potentially reversible resident factors associated with rejection of care behaviors. J Am Geriatr Soc 2010;58:1693–700 | No participants with dementia/not separately analysed |
| Ismail M, Dagerman K, Tariot PN, Abbott S, Kavanagh S, Schneider LS. National Institute of Mental Health clinical antipsychotic trials of intervention effectiveness- Alzheimer’s disease (CATIE-AD): baseline characteristics. Curr Alzheimer Res 2007;4:325–35 | Not intervention study |
| Ismail MS, Schneider LS, Tariot P, Dagerman KS, Davis S. NIMH comparative effectiveness of antipsychotic medications in patients with Alzheimer’s disease (CATIE-AD): clinical course. Neurobiol Aging 2004;25:S188 | Not a psychological, behavioural, sensory or environmental intervention |
| Issa AM, Keyserlingk EW. Current and future clinical trials for Alzheimer’s disease: evolving ethical concerns. Prog Neuro-Psychopharmacol Biol Psychiatry 2000;24:1229–49 | Not primary research |
| Jackson CW, Pitner JK, Mintzer JE. Zolpidem for the treatment of agitation in elderly demented patients. J Clin Psychiatry 1996;57:372–3 | Not a psychological, behavioural, sensory or environmental intervention |
| Jacobs HE, Lynch M, Cornick J, Slifer K. Behavior management of aggressive sequela after Reyes syndrome. Arch Phys Med Rehabil 1986;67:558–63 | No participants with dementia/not separately analysed |
| Jacquy J. [Treatment of Alzheimer’s disease: the current situation?] Revue Med Brux 2010;31:357–62 | Not primary research |
| Jagmin MG. Postoperative mental status in elderly hip surgery patients. Orthopaedic Nurs 1998;17:32–42 | No participants with dementia/not separately analysed |
| Jahangir A, Shen WK, Neubauer SA, Ballard DJ, Hammill SC, Hodge DO, et al. Relation between mode of pacing and long-term survival in the very elderly. J Am Coll Cardiol 1999;33:1208–16 | No participants with dementia/not separately analysed |
| Jahnel M. [The case of a 86-years old woman first diagnosed with Huntington’s disease.] Psychiatr Prax 2004;31:S134–6 | No participants with dementia/not separately analysed |
| James IA. Stuff and nonsense in the treatment of older people: essential reading for the over-45s. Behav Cogn Psychother 2008;36:735–47 | No participants with dementia/not separately analysed |
| Jankovic J. Treatment of hyperkinetic movement-disorders with tetrabenazine – a double-blind crossover study. Ann Neurol 1982;11:41–7 | No participants with dementia/not separately analysed |
| Jarrott SE, Kwack HR, Relf D. An observational assessment of a dementia-specific horticultural therapy program. Horttechnology 2002;12:403–10 | No outcome measuring agitation |
| Jarzebska E. [Stroke patients’ apathy.] Pol Merkur Lekarski 2007;22:280–2 | No participants with dementia/not separately analysed |
| Javadpour A, Ahmadzadeh L, Bahredar MJ. An educative support group for female family caregivers: impact on caregivers’ psychological distress and patient’s neuropsychiatry symptoms. Int J Geriatr Psychiatry 2009;24:469–71 | No outcome measuring agitation |
| Jehel L, Paterniti S, Brunet A, Louville P, Guelfi J. Peritraumatic distress prospectively predicts PTDS symptoms in assault victims. Encephale Rev Psychiatr Clin Biol Therap 2006;32:953–6 | No participants with dementia/not separately analysed |
| Jentoft AJC. Older patients agitation treatment. Rev Clin Esp 2003;203:346–8 | Not primary research |
| Jessen F, Kaduszkiewicz H, Daerr M, Bickel H, Pentzek M, Riedel-Heller S, et al. Anticholinergic drug use and risk for dementia: target for dementia prevention. Eur Arch Psychiatry Clin Neuroscie 2010;260:S111–15 | Not intervention study |
| Jeste DV, Blazer D, Casey D, Meeks T, Salzman C, Schneider L, et al. ACNP white paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008;33:957–70 | Not primary research |
| Johnson JC. Delirium in the elderly. Emerg Med Clin North Am 1990;8:255–66 | No participants with dementia/not separately analysed |
| Johnston JE. Sleep problems in the elderly. J Am Acad Nurse Pract 1994;6:161–6 | No participants with dementia/not separately analysed |
| Jones R. A review comparing the safety and tolerability of memantine with the acetylcholinesterase inhibitors. Int J Geriatr Psychiatry 2010;25:547–53 | Not primary research |
| Josephs KA, Whitwell JL, Weigand SD, Senjem ML, Boeve BF, Knopman DS, et al. Predicting functional decline in behavioural variant frontotemporal dementia. Brain 2011;134:432–48 | Not intervention study |
| Jost BC, Grossberg GT. The evolution of psychiatric symptoms in Alzheimer’s disease: a natural history study. J Am Geriatr Soc 1996;44:1078–81 | Not intervention study |
| Juszczak LJ. Comparative vibrational spectroscopy of intracellular tau and extracellular collagen I reveals parallels of gelation and fibrillar structure. J Biol Chem 2004;279:7395–404 | No participants with dementia/not separately analysed |
| Kalapatapu RK, Schimming C. Update on neuropsychiatric symptoms of dementia: antipsychotic use. Geriatrics 2009;64:10–18 | Not primary research |
| Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry 1999;56:713–18 | No participants with dementia/not separately analysed |
| Kalman J, Kalman S, Pakaski M. [Recognition and treatment of behavioral and psychological symptoms of dementias: lessons from the CATIE-AD study.] Neuropsychopharmacol Hung 2008;10:233–49 | Not primary research |
| Kamboj SK, Curran HV. Scopolamine induces impairments in the recognition of human facial expressions of anger and disgust. Psychopharmacology 2006;185:529–35 | No participants with dementia/not separately analysed |
| Kamei J, Miyata S, Ohsawa M. Involvement of the benzodiazepine system in the anxiolytic-like effect of Yokukansan (Yi-gan San). Prog Neuro-Psychopharmacol Biol Psychiatry 2009;33:1431–7 | No participants with dementia/not separately analysed |
| Kamei S, Oishi M, Takasu T. Evaluation of fasudil hydrochloride treatment for wandering symptoms in cerebrovascular dementia with P-31-magnetic resonance spectroscopy and Xe-computed tomography. Clin Neuropharmacol 1996;19:428–38 | Not a psychological, behavioural, sensory or environmental intervention |
| Kanno H, Sekiguchi K, Yamaguchi T, Terawaki K, Yuzurihara M, Kase Y, et al. Effect of yokukansan, a traditional Japanese medicine, on social and aggressive behaviour of para-chloroamphetamine-injected rats. J Pharm Pharmacol 2009;61:1249–56 | No participants with dementia/not separately analysed |
| Kant R, Bogyi AM, Carosella NW, Fishman E, Kane V, Coffey CE. ECT as a therapeutic option in severe brain injury. Convuls Ther 1995;11:45–50 | No participants with dementia/not separately analysed |
| Karki SD. Evaluation of the cost impact of different atypical antipsychotics in management of agitation in residents with dementia in New York State nursing homes. J Am Geriatr Soc 2003;51:S103 | No participants with dementia/not separately analysed |
| Karlsson I. Pharmacologic treatment of noncognitive symptoms of dementia. Acta Neurologica Scand 1996;93:101–4 | Not primary research |
| Karp BPI, Juliano DM, Berman KF, Weinberger DR. Neurological outcome of patients with dorsolateral prefrontal leukotomy. J Neuropsychiatry Clin Neurosci 1992;4:415–21 | No participants with dementia/not separately analysed |
| Kasckow JW, McElroy SL, Cameron RL, Mahler LL, Fudala SJ. A pilot study on the use of divalproex sodium in the treatment of behavioral agitation in elderly patients with dementia: assessment with the BEHAVE-AD and CGI rating scales. Curr Ther Res 1997;58:981–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Kasckow JW, Mulchahey JJ, Mohamed S. The use of novel antipsychotics in the older patient with neurodegenerative disorders in the long-term care setting. J Am Med Direct Assoc 2004;5:242–8 | Not primary research |
| Katona C, Livingston G, Manela M, Leek C, Mullan E, Orrell M, et al. The symptomatology of depression in the elderly. Int Clin Psychopharmacol 1997;12:S19–23 | No participants with dementia/not separately analysed |
| Katz I, De Deyn PP, Mintzer J, Greenspan A, Zhu Y, Brodaty H. The efficacy and safety of risperidone in the treatment of psychosis of Alzheimer’s disease and mixed dementia: a meta-analysis of 4 placebo-controlled clinical trials. Int J Geriatr Psychiatry 2007;22:475–84 | Not primary research |
| Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psychiatry 1999;60:107 | Not a psychological, behavioural, sensory or environmental intervention |
| Katz IR, Rupnow M, Kozma C, Schneider L. Risperidone and falls in ambulatory nursing home residents with dementia and psychosis or agitation – secondary analysis of a double-blind, placebo-controlled mal. Am J Geriatr Psychiatry 2004;12:499–508 | Not primary research |
| Kaufer A. Treatment of neuropsychiatric symptoms in Alzheimer’s disease. Rev Neurol 2002;35:846 | Not primary research |
| Kaufer D. Beyond the cholinergic hypothesis: The effect of metrifonate and other cholinesterase inhibitors on neuropsychiatric symptoms in Alzheimer’s disease. Dementia Geriatr Cogn Disord 1998;9:8–14 | Not a psychological, behavioural, sensory or environmental intervention |
| Kaufer DI, Cummings JL, Christine D. Effect of tacrine on behavioral symptoms in Alzheimer’s disease: an open-label study. J Geriatr Psychiatry Neurol 1996;9:1–6 | Not a psychological, behavioural, sensory or environmental intervention |
| Keady J, Jones L. Investigating the causes of behaviours that challenge in people with dementia. Nurs Older People 2010;22:25–9 | Not primary research |
| Keatinge D, Scarfe C, Bellchambers H, McGee J, Oakham R, Probert C, et al. The manifestation and nursing management of agitation in institutionalised residents with dementia. Int J Nurs Prac 2000;6:16–25 | Not intervention study |
| Keller HH, Gibbs AJ, Boudreau LD, Goy RE, Pattillo MS, Brown HM. Prevention of weight loss in dementia with comprehensive nutritional treatment. J Am Geriatr Soc 2003;51:945–52 | No outcome measuring agitation |
| Keller HH, Gibbs-Ward A, Randall-Simpson A, Bocock MA, Dimou E. Meal rounds: an essential aspect of quality nutrition services in long-term care. J Am Med Direct Assoc 2006;7:40–5 | Not primary research |
| Kellison IL, Kirsch-Darrow L, Fernandez H, Okun MS, Bowers D. Apathy in non-demented Parkinson disease: relationship to executive dysfunction? Neurology 2007;68:A20 | No participants with dementia/not separately analysed |
| Kennedy DO, Scholey AB. The psychopharmacology of European herbs with cognition-enhancing properties. Curr Pharm Design 2006;12:4613–23 | Not primary research |
| Kennedy J, Deberdt W, Siegal A, Micca J, Degenhardt E, Ahl J, et al. Olanzapine does not enhance cognition in non-agitated and non-psychotic patients with mild to moderate Alzheimer’s dementia. Int J Geriatr Psychiatry 2005;20:1020–7 | Not a psychological, behavioural, sensory or environmental intervention |
| Kennedy JS, Bymaster FP, Schuh L, Calligaro DO, Nomikos G, Felder CC, et al. A current review of olanzapine’s safety in the geriatric patient: from pre-clinical pharmacology to clinical data. Int J Geriatr Psychiatry 2001;16:S33–61 | No participants with dementia/not separately analysed |
| Kennedy JS, Zagar A, Bymaster F, Nomikos G, Trzepacz PT, Gilmore JA, et al. The central cholinergic system profile of olanzapine compared with placebo in Alzheimer’s disease. Int J Geriatr Psychiatry 2001;16:S24–32 | Not a psychological, behavioural, sensory or environmental intervention |
| Khachaturian ZS. A chapter in the development of Alzheimer’s disease research: a case study of public policies on the development & funding of research program. Alzheimers Demen 2007;3:243–58 | No participants with dementia/not separately analysed |
| Kheterpal I, Williams A, Murphy C, Bledsoe B, Wetzel R. Structural features of the A beta amyloid fibril elucidated by limited proteolysis. Biochemistry 2001;40:11757–67 | No participants with dementia/not separately analysed |
| Kidd PM. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Alt Med Rev 2007;12:207–27 | No participants with dementia/not separately analysed |
| Kiely DK, Morris JN, Algase DL. Resident characteristics associated with wandering in nursing homes. Int J Geriatr Psychiatry 2000;15:1013–20 | Not intervention study |
| Kim H, Whall AL. Factors associated with psychotropic drug usage among nursing home residents with dementia. Nurs Res 2006;55:252–8 | Not intervention study |
| Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia – A review of the evidence. Drugs Aging 2008;25:187–96 | Not primary research |
| Kindermann SS, Dolder CR, Bailey A, Katz IR, Jeste DV. Pharmacological treatment of psychosis and agitation in elderly patients with dementia – four decades of experience. Drugs Aging 2002;19:257–76 | Not primary research |
| King T, Mallet L. Brachial-plexus palsy with the use of haloperidol and a geriatric chair. DICP Ann Pharmacother 1991;25:1072–4 | Not intervention study |
| King-Kallimanis B, Schonfeld L, Molinari VA, Algase D, Brown LM, Kearns WD, et al. Longitudinal investigation of wandering behavior in department of veterans affairs nursing home care units. Int J Geriatr Psychiatry 2010;25:166–74 | Not intervention study |
| Kinon BJ, Stauffer VL, McGuire HC, Kaiser CJ, Dickson RA, Kennedy JS. The effects of antipsychotic drug treatment on prolactin concentrations in elderly patients. J Am Med Direct Assoc 2003;4:189–94 | Not a psychological, behavioural, sensory or environmental intervention |
| Kinoshita T, Hanabusa H. Issues facing home-based medical support services. Psychogeriatrics 2010;10:90–4 | Not a psychological, behavioural, sensory or environmental intervention |
| Kirbach S, Simpson K, Nietert PJ, Mintzer J. A Markov model of the cost effectiveness of olanzapine treatment for agitation and psychosis in Alzheimer’s disease. Clin Drug Invest 2008;28:291–303 | Not primary research |
| Kirkwood SC, Siemers E, Viken R, Hodes ME, Conneally PM, Christian JC, et al. Longitudinal personality changes as measured by the MMPI in presymptomatic HD gene carriers. Am J Hum Genet 2000;67:138 | No participants with dementia/not separately analysed |
| Kirkwood SC, Siemers E, Viken R, Hodes ME, Conneally PM, Christian JC, et al. Longitudinal personality changes among presymptomatic Huntington disease gene carriers. Neuropsychiatry Neuropsychol Behav Neurol 2002;15:192–7 | No participants with dementia/not separately analysed |
| Kittur SD, Ruskin P. Environmental modification for treatment of agitation in Alzheimer’s patients. Neurorehabilitation 1999;12:211–14 | No comparator |
| Kleijer B, van Marum R, Egberts A, Jansen P, Frijters D, Heerdink E, et al. The course of behavioral problems in elderly nursing home patients with dementia when treated with antipsychotics. Int Psychogeriatr 2009;21:931–40 | Not intervention study |
| Klein DA, Steinberg M, Galik E, Steele C, Sheppard JM, Warren A, et al. Wandering behaviour in community-residing persons with dementia. Int J Geriatr Psychiatry 1999;14:272–9 | Not intervention study |
| Kleinman L, Frank L, Ciesla G, Rupnow M, Brodaty H. Psychometric performance of an assessment scale for strain in nursing care: the M-NCAS. Health Qual Life Outcomes 2004;2:62 | No participants with dementia/not separately analysed |
| Klosterkötter J, Ebel H, Schultzeö-Lutter F, Steinmeyer EM. Diagnostic validity of basic symptoms. Eur Arch Psychiatry Clin Neurosci 1996;246:147–54 | No participants with dementia/not separately analysed |
| Kluger A, Ferris SH. Scales for the assessment of Alzheimer’s disease. Psychiatric Clin North Am 1991;14:309–26 | No participants with dementia/not separately analysed |
| Knecht S, Wersching H, Lohmann H, Berger K, Ringelstein EB. How much does hypertension affect cognition? Explained variance in cross-sectional analysis of non-demented community-dwelling individuals in the SEARCH study. J Neurol Sci 2009;283:149–52 | No participants with dementia/not separately analysed |
| Knopman DS, Morris JC. An update on primary drug therapies for Alzheimer disease. Arch Neurol 1997;54:1406–9 | Not primary research |
| Knorr U, Vinberg M, Klose M, Feldt-Rasmussen U, Hilsted L, Gade A, et al. Rationale and design of the participant, investigator, observer, and data-analyst-blinded randomized AGENDA trial on associations between gene-polymorphisms, endophenotypes for depression and antidepressive intervention: the effect of escitalopram versus placebo on the combined dexamethasone-corticotrophine releasing hormone test and other potential endophenotypes in healthy first-degree relatives of persons with depression. Trials 2009;10:66 | No participants with dementia/not separately analysed |
| Kobayashi DT, Chen KS. Behavioral phenotypes of amyloid-based genetically modified mouse models of Alzheimer’s disease. Genes Brain Behav 2005;4:173–96 | No participants with dementia/not separately analysed |
| Koga H, Yuzuriha T, Yao H, Endo K, Hiejima S, Takashima Y, et al. Quantitative MRI findings and cognitive impairment among community dwelling elderly subjects. J Neurol Neurosurg Psychiatry 2002;72:737–41 | No participants with dementia/not separately analysed |
| Kogoj A, Darovec J, Velikonja I, Kocmur M, Denislic M, Mursec M, et al. Regulatory issues concerning behavioural and psychological symptoms of dementia in Slovenia: guidelines. Eur J Psychiatry 2004;18:171–80 | Not primary research |
| Kohler CG, Pickholtz J, Ballas C. Neurosyphilis presenting as schizophrenialike psychosis. Neuropsychiatry Neuropsychol Behav Neurol 2000;13:297–302 | No participants with dementia/not separately analysed |
| Kolanowski A, Litaker M. Social interaction, premorbid personality, and agitation in nursing home residents with dementia. Arch Psychiatr Nurs 2006;20:12–20 | Not intervention study |
| Kolcaba K, Miller CA. Geropharmacology treatment: behavioral problems extend nursing responsibility. J Gerontol Nurs 1989;15:29–35 | Not primary research |
| Koller D, Eisele M, Kaduszkiewicz H, Schoen G, Steinmann S, Wiese B, et al. Ambulatory health services utilization in patients with dementia – is there an urban–rural difference? Int J Health Geo 2010;9:1–8 | Not intervention study |
| Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Mental Health 2009;13:512–20 | Not primary research |
| Kontsevoy VA, Andrusenko MP, Medvedev AV, Safarova TP. Application of clopixole in therapy of aged mental patients. Zhurnal Nevropatologii I Psikhiatrii Imeni S S Korsakova 2000;100:29–34 | Not intervention study |
| Koopmans RT, van der Molen M, Raats M, Ettema TP. Neuropsychiatric symptoms and quality of life in patients in the final phase of dementia. Int J Geriatr Psychiatry 2009;24:25–32 | Not intervention study |
| Kopecky HJ, Yudofsky SC. Agitation: Conceptualization, measurement, and treatment. Bull Menninger Clin 1999;63:A31–5 | No participants with dementia/not separately analysed |
| Kopetz S, Steele CD, Brandt J, Baker A, Kronberg M, Galik E, et al. Characteristics and outcomes of dementia residents in an assisted living facility. Int J Geriatr Psychiatry 2000;15:586–93 | Not intervention study |
| Kotlercope S, Camp CJ. Anosognosia in Alzheimer-disease. Alzheimer Dis Assoc Disord 1995;9:52–6 | Not intervention study |
| Kovach CR, Logan BR, Simpson MR, Reynolds S. Factors associated with time to identify physical problems of nursing home residents with dementia. Am J Alzheimers Dis Other Demen 2010;25:317–23 | Not intervention study |
| Kovach CR, Schlidt AM. The agitation-activity interface of people with dementia in long-term care. Am J Alzheimers Dis Other Demen 2001;16:240–6 | Not intervention study |
| Kovach CR. Sensoristasis and imbalance in persons with dementia. J Nurs Scholarship 2000;32:379–84 | No participants with dementia/not separately analysed |
| Krasucki C, Howard R, Mann A. The relationship between anxiety disorders and age. Int J Geriatr Psychiatry 1998;13:79–99 | No participants with dementia/not separately analysed |
| Kreutzer JS, Marwitz JH, Witol AD. Interrelationships between crime, substance-abuse, and aggressive behaviors among persons with traumatic brain injury. Brain Inj 1995;9:757–68 | No participants with dementia/not separately analysed |
| Krishnan S, Cairns R, Howard R. Cannabinoids for the treatment of dementia. Cochrane Database Syst Rev 2009;2:CD007204 | Not primary research |
| Kruger G, Haubitz I. Classification of organic brain syndromes by cluster-analysis. Archiv Psychiatr Nervenkrankheiten 1980;228:299–315 | No participants with dementia/not separately analysed |
| Kukoc M. Confessions and the post-communist conflict of civilizations. Drustvena Istrazivanja 1995;4:937–49 | No participants with dementia/not separately analysed |
| Kulisevsky J, Litvan I, Berthier ML, Pascual-Sedano B, Paulsen JS, Cummings JL. Neuropsychiatric assessment of Gilles de la Tourette patients: comparative study with other hyperkinetic and hypokinetic movement disorders. Move Disord 2001;16:1098–104 | No participants with dementia/not separately analysed |
| Kulkarni J, de Castella A, Headey B, Marston N, Sinclair K, Lee S, et al. Estrogens and men with schizophrenia: is there a case for adjunctive therapy? Schizophrenia Res 2011;125:278–83 | No participants with dementia/not separately analysed |
| Kumar V, Durai NB, Jobe T. Pharmacologic management of Alzheimer’s disease. Clin Geriatr Med 1998;14:129 | Not primary research |
| Kunik M. Preventing development of aggression in dementia: epidemiological intervention development. Gerontologist 2010;50:199–200 | Not intervention study |
| Kunik ME, Cully JA, Snow AL, Souchek J, Sullivan G, Ashton CM. Treatable comorbid conditions and use of VA health care services among patients with dementia. Psychiatr Serv 2005;56:70–5 | Not intervention study |
| Kunik ME, Ponce H, Molinari V, Orengo C, Emenaha I, Workman R. The benefits of psychiatric hospitalization for older nursing home residents. J Am Geriatr Soc 1996;44:1062–5 | No participants with dementia/not separately analysed |
| Kunik ME, Snow A, Davila JA, Steele AB, Balasubramanyam V, Doody RS, et al. Causes of aggressive behavior in patients with dementia. J Clin Psychiatry 2010;71:1145–52 | Not intervention study |
| Kunik ME, Walgama JP, Snow A, Davila JA, Schulz PE, Steele AB, et al. Documentation, assessment, and treatment of aggression in patients with newly diagnosed dementia. Alzheimer Dis Assoc Disord 2007;21:115–21 | Not intervention study |
| Kurita A, Katayama K, Morita M, Kurita M, Inoue K. [Relationship between cognitive deficits, behavioral disturbances and falls in patients with dementia.] Nihon Ronen Igakkai zasshi 1997;34:662–7 | Not intervention study |
| Kurlan R, Cummings J, Raman R, Thal L. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology 2007;68:1356–63 | Not a psychological, behavioural, sensory or environmental intervention |
| Kurz A, Schwalen S, Schmitt A. Effects of risperidone on behavioral and psychological symptoms associated with dementia in clinical practice. Int Psychogeriatr 2005;17:605–16 | Not a psychological, behavioural, sensory or environmental intervention |
| Kurz AF, Erkinjuntti T, Small GW, Lilienfeld S, Damaraju CRV. Long-term safety and cognitive effects of galantamine in the treatment of probable vascular dementia or Alzheimer’s disease with cerebrovascular disease. Eur J Neurol 2003;10:633–40 | Not a psychological, behavioural, sensory or environmental intervention |
| Kushnir SL. Pimozide in the management of psychotically agitated demented patients. J Am Geriatr Soc 1987;35:457–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Kutner NG, Brown PJ, Stavisky RC, Clark WS, Green RC. “Friendship” interactions and expression of agitation among residents of a dementia care unit – six-month observational data. Res Aging 2000;22:188–205 | Not intervention study |
| Kverno KS, Black BS, Blass DM, Geiger-Brown J, Rabins PV. Neuropsychiatric symptom patterns in hospice-eligible nursing home residents with advanced dementia. J Am Med Direct Assoc 2008;9:509–15 | Not intervention study |
| Kwok H, Cheung PW. Co-morbidity of psychiatric disorder and medical illness in people with intellectual disabilities. Curr Opin Psychiatry 2007;20:443–9 | No participants with dementia/not separately analysed |
| Kyomen HH, Hennen J, Gottlieb GL, Wei JY. Estrogen therapy and noncognitive psychiatric signs and symptoms in elderly patients with dementia. Am J Psychiatry 2002;159:1225–7 | Not a psychological, behavioural, sensory or environmental intervention |
| Kyomen HH, Satlin A, Hennen J, Wei JY. Estrogen therapy and aggressive behavior in elderly patients with moderate-to-severe dementia – results from a short-term, randomized, double-blind trial. Am J Geriatr Psychiatry 1999;7:339–48 | Not a psychological, behavioural, sensory or environmental intervention |
| Lacasse H, Perreault MM, Williamson DR. Systematic review of antipsychotics for the treatment of hospital-associated delirium in medically or surgically ill patients. Ann Pharmacother 2006;40:1966–73 | No participants with dementia/not separately analysed |
| Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc 2008;56:862–70 | Not a psychological, behavioural, sensory or environmental intervention |
| Lagerstrom M, Magnusson D. Behavior at 10-years and 13-years of age for children with low-birth-weight. Perceptual Motor Skills 1990;71:579–94 | No participants with dementia/not separately analysed |
| Lagomasino I, Daly R, Stoudemire A. Medical assessment of patients presenting with psychiatric symptoms in the emergency setting. Psychiatr Clin North Am 1999;22:819 | No participants with dementia/not separately analysed |
| Lai CK, Yeung JH, Mok V, Chi I. Special care units for dementia individuals with behavioural problems. Cochrane Database Syst Rev 2009;4:CD006470 | Not primary research |
| Lai CKY, Arthur DG. Wandering behaviour in people with dementia. J Adv Nurs 2003;44:173–82 | Not primary research |
| Lai MK, Tsang SW, Esiri MM, Francis PT, Wong PT, Chen CP. Differential involvement of hippocampal serotonin(1A) receptors and re-uptake sites in non-cognitive behaviors of Alzheimer’s disease. Psychopharmacology 2011;213:431–9 | No participants with dementia/not separately analysed |
| Lai MK, Tsang SW, Esiri MM, Keene J, Hope T, Francis PT, et al. Serotonin 5-HT2A receptor alterations in the postmortem neocortex of behaviorally assessed Alzheimer patients. J Neurochem 2003;87:106 | No participants with dementia/not separately analysed |
| Laks J, Miotto R, Morinho V, Engelhardt E. Use of aripiprazole for psychosis and agitation in dementia. Int Psychogeriatr 2006;18:335–40 | Not a psychological, behavioural, sensory or environmental intervention |
| Lancioni GE, Perilli V, Singh NN, O’Reilly MF, Cassano G. A man with severe Alzheimer’s disease stops wandering during a picture colouring activity. Develop Neurorehabil 2011;14:242–6 | No comparator |
| Lanctot KL, Bowles SK, Herrmann N, Best TS, Naranjo CA. Drugs mimicking dementia – dementia symptoms associated with psychotropic drugs in institutionalised cognitively impaired patients. CNS Drugs 2000;14:381–90 | Not intervention study |
| Lanctot KL, Herrmann N, Eryavec G, van Reekum R, Reed K, Naranjo CA. Central serotonergic activity is related to the aggressive behaviors of Alzheimer’s disease. Neuropsychopharmacology 2002;27:646–54 | No participants with dementia/not separately analysed |
| Lanctot KL, Herrmann N, van Reekum R, Eryavec G, Naranjo CA. Gender, aggression and serotonergic function are associated with response to sertraline for behavioral disturbances in Alzheimer’s disease. Int J Geriatr Psychiatry 2002;17:531–41 | Not a psychological, behavioural, sensory or environmental intervention |
| Landreville P, Bedard A, Verreault R, Desrosiers J, Champoux N, Monette J, et al. Non-pharmacological interventions for aggressive behavior in older adults living in long-term care facilities. Int Psychogeriatr 2006;18:47–73 | Not primary research |
| Landreville P, Dicaire L, Verreault R, Levesque L. A training program for managing agitation of residents in long-term care facilities: description and preliminary findings. J Gerontol Nurs 2005;31:34–42 | No participants with dementia/not separately analysed |
| Landreville P, LeBlanc V. Older adults’ acceptability ratings of treatments for verbal agitation in persons with dementia. Am J Alzheimers Dis Other Demen 2010;25:134–41 | No participants with dementia/not separately analysed |
| Landsberg G, Denenberg S, Araujo J. Cognitive dysfunction in cats: a syndrome we used to dismiss as ‘old age’. J Feline Med Surg 2010;12:837–48 | No participants with dementia/not separately analysed |
| Lange RT, Hopp GA, Kang N. Psychometric properties and factor structure of the Neuropsychiatric Inventory Nursing Home version in an elderly neuropsychiatric population. Int J Geriatr Psychiatry 2004;19:440–8 | No participants with dementia/not separately analysed |
| Lann-Wolcott H, Medvene LJ, Williams K. Measuring the person-centeredness of caregivers working with nursing home residents with dementia. Behav Ther 2011;42:89–99 | No participants with dementia/not separately analysed |
| Lantz MS, Marin D. Pharmacologic treatment of agitation in dementia: a comprehensive review. J Geriatr Psychiatry Neurol 1996;9:107–19 | Not primary research |
| Lara DR, Cruz MRS, Xavier F, Souza DO, Moriguchi EH. Allopurinol for the treatment of aggressive behaviour in patients with dementia. Int Clin Psychopharmacol 2003;18:53–5 | Not a psychological, behavioural, sensory or environmental intervention |
| Larue A, Watson J, Plotkin DA. First symptoms of dementia – a study of relatives’ reports. Int J Geriatr Psychiatry 1993;8:239–45 | Not intervention study |
| Lawlor B, Ni Bhriain S. Psychosis and behavioural symptoms of dementia: defining the role of neuroleptic interventions. Int J Geriatr Psychiatry 2001;16:S2–6 | Not primary research |
| Lawlor B. Effective behavioral interventions for decreasing dementia-related challenging behavior in nursing homes – commentary. Int J Geriatr Psychiatry 1999;14:230–2 | Not primary research |
| Lawlor BA, Ryan TM, Schmeidler J, Mohs RC, Davis KL. Clinical symptoms associated with age at onset in Alzheimers disease. Am J Psychiatry 1994;151:1646–9 | Not intervention study |
| Lawlor BA. Behavioral and psychological symptoms in dementia: the role of atypical antipsychotics. J Clin Psychiatry 2004;65:5–10 | Not primary research |
| Lawrence JM, Davidoff DA, Katt-Lloyd D, Connell A, Berlow YA, Savoie JA. Is large-scale community memory screening feasible? Experience from a regional memory-screening day. J Am Geriatr Soc 2003;51:1072–8 | No participants with dementia/not separately analysed |
| Lebert F, Pasquier F, Petit H. Behavioral-effects of trazodone in Alzheimers disease. J Clin Psychiatry 1994;55:536–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Lebert F, Pasquier F. Treatment of psychiatric and behavioural symptoms in Alzheimer’s disease. Revue Neurologique 2003;159:825–30 | Not primary research |
| Lebert F, Stekke W, Hasenbroekx C, Pasquier F. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dementia Geriatr Cogn Disord 2004;17:355–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Lebert F. Selective serotonine reuptake inhibitors for depression in Alzheimer-type and other dementias. Presse Med 2003;32:1181–6 | Not primary research |
| Leblhuber F. Treatment of behavioral abnormalities in demented patients with citalopram. Acta Med Austriaca 1994;21:104–6 | Not a psychological, behavioural, sensory or environmental intervention |
| Lee KS, Cho HS, Hong CH, Kim DG, Oh BH. Differences in neuropsychiatric symptoms according to mild cognitive impairment subtypes in the community. Dementia Geriatr Cogn Disord 2008;26:212–17 | No participants with dementia/not separately analysed |
| Lee SB, Kim KW. Nonpharmacological interventions for Alzheimer’s disease. J Korean Med Assoc 2009;52:1069–76 | Not primary research |
| Leehey MA. Fragile X-Associated Tremor/Ataxia Syndrome: clinical phenotype, diagnosis, and treatment. J Invest Med 2009;57:830–6 | No participants with dementia/not separately analysed |
| Lefroy RB, McHale P, Hyndman J, Hobbs MST. Understanding the behaviour of people in special dementia units: the contribution of rating scales. Aus J Ageing 1996;15:105–10 | No participants with dementia/not separately analysed |
| Leger JM, Moulias R, Robert P, Vellas B, Chapuy PH, Monfort JC, et al. Agitation and aggressiveness among the elderly population living in nursing or retirement homes in France. Int Psychogeriatr 2002;14:405–16 | No participants with dementia/not separately analysed |
| Lehninger FW, Ravindran VL, Stewart JT. Management strategies for problem behaviors in the patient with dementia. Geriatrics 1998;53:55 | Not primary research |
| Lemay M, Landreville P. Verbal agitation in dementia: the role of discomfort. Am J Alzheimers Dis Other Demen 2010;25:193–201 | Not primary research |
| Lemke MR, Stuhlmann W. Carbamazepine treatment of agitated behavior in gerontopsychiatric inpatients. Psychiatr Prax 1994;21:147–50 | Not a psychological, behavioural, sensory or environmental intervention |
| Leocani L, Martinelli V, Santuccio G, Possa F, Magnani G, Comi G. Neurophysiological evaluation of executive functions in multiple sclerosis. Recent Adv Hum Neurophysiol 1998;1162:1081–8 | No participants with dementia/not separately analysed |
| Leonard DP, Kidson MA, Brown JGE, Shannon PJ, Taryan S. Double-blind trial of lithium-carbonate and haloperidol in Huntingtons-Chorea. Aus N Z J Psychiatry 1975;9:115–18 | No participants with dementia/not separately analysed |
| Leonard R, Tinetti ME, Allore HG, Drickamer MA. Potentially modifiable resident characteristics that are associated with physical or verbal aggression among nursing home residents with dementia. Arch Int Med 2006;166:1295–300 | Not intervention study |
| Leone E, Deudon A, Bauchet M, Laye M, Bordone N, Lee JH, et al. Management of apathy in nursing homes using a teaching program for care staff: the STIM-EHPAD study. Int J Geriatr Psychiatry 2013;28:383–92 | No outcome measuring agitation |
| Leroi I, Michalon M. Treatment of the psychiatric manifestations of Huntington’s disease: a review of the literature. Can J Psychiatry 1998;43:933–40 | No participants with dementia/not separately analysed |
| Lesser JM, Hughes SV. Psychosis-related disturbances – psychosis, agitation and disinhibition in Alzheimer’s disease: definitions and treatment options. Geriatrics 2006;61:14 | Not primary research |
| Leverenz JB, Miller MA, Dobie DJ, Peskind ER, Raskind MA. Increased alpha 2-adrenergic receptor binding in locus coeruleus projection areas in dementia with Lewy bodies. Neurobiol Aging 2001;22:555–61 | No participants with dementia/not separately analysed |
| Levitsky AM, Owens NJ. Pharmacologic treatment of hypersexuality and paraphilias in nursing home residents. J Am Geriatr Soc 1999;47:231–4 | Not primary research |
| Levy MA, Burgio LD, Sweet R, Bonino P, Janosky J, Perel J. A trial of buspirone for the control of disruptive behaviours in community-dwelling patients with dementia. Int J Geriatr Psychiatry 1994;9:841–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Levy ML, Cummings JL, Fairbanks LA, Bravi D, Calvani M, Carta A. Longitudinal assessment of symptoms of depression, agitation, and psychosis in 181 patients with Alzheimer’s disease. Am J Psychiatry 1996;153:1438–43 | Not intervention study |
| Levy ML, Cummings JL, Kahn-Rose R. Neuropsychiatric symptoms and cholinergic therapy for Alzheimer’s disease. Gerontology 1999;45:15–22 | Not primary research |
| Levy ML, Miller BL, Cummings JL, Fairbanks LA, Craig A. Alzheimer disease and frontotemporal dementias – behavioural distinctions. Arch Neurol 1996;53:687–90 | Not intervention study |
| Lewis CF. Successfully treating aggression in mentally ill prison inmates. Psychiatr Q 2000;71:331–43 | No participants with dementia/not separately analysed |
| Lewis DO, Shanok SS, Pincus JH, Giammarino M. The medical assessment of seriously delinquent boys – a comparison of pediatric, psychiatric, neurological, and hospital record data. J Adolesc Health 1982;3:160–4 | No participants with dementia/not separately analysed |
| Lewis JA. The effect of the N.E.S.T. approach on a dementia-specific unit. Am J Recreation Ther 200;6:32–8 | No participants with dementia/not separately analysed |
| Li DW, Mohanty S, Irback A, Huo S. Formation and growth of oligomers: a Monte Carlo study of an amyloid tau fragment. PLoS Compu Biol 2008;4:e1000238 | No participants with dementia/not separately analysed |
| Li L, von Bergen M, Mandelkow EM, Mandelkow E. Structure, stability, and aggregation of paired helical filaments from tau protein and FTDP-17 mutants probed by tryptophan scanning mutagenesis. J Biol Chem 2002;277:41390–400 | No participants with dementia/not separately analysed |
| Lian W, Gu YR, Pedersen B, Kukar T, Govindasamy L, Agbandje-McKenna M, et al. Crystallization and preliminary X-ray crystallograph is studies on recombinant rat choline acetyltransferase. Acta Crystallographica Section D 2004;60:374–5 | No participants with dementia/not separately analysed |
| Liberski PP, Yanagihara R, Wells GAH, Gibbs CJ, Gajdusek DC. Comparative ultrastructural neuropathology of naturally-occurring bovine spongiform encephalopathy and experimentally induced Scrapie and Creutzfeldt-Jakob Disease. J Comp Pathol 1992;106:361–81 | No participants with dementia/not separately analysed |
| Liem-Moolenaar M, Rad M, Zamuner S, Cohen AF, Lemme F, Franson KL, et al. Central nervous system effects of the interaction between risperidone (single dose) and the 5-HT(6) antagonist SB742457 (repeated doses) in healthy men. Br J Clin Pharmacol 2011;71:907–16 | No participants with dementia/not separately analysed |
| Lillquist PP. Challenges in surveillance of dementias in New York State. Prevent Chronic Dis 2004;1:A08 | No participants with dementia/not separately analysed |
| Lim PP, Sahadevan S, Choo GK, Anthony P. Burden of caregiving in mild to moderate dementia: an Asian experience. Int Psychogeriatr 1999;11:411–20 | No participants with dementia/not separately analysed |
| Lin ST, Yang P, Lai CY, Su YY, Yeh YC, Huang MF, et al. Mental health implications of music: insight from neuroscientific and clinical studies. Harvard Rev Psychiatry 2011;19:34–46 | Not primary research |
| Lindborg SR, Beasley CM, Alaka K, Taylor CC. Effects of intramuscular olanzapine vs. haloperidol and placebo on QTc intervals in acutely agitated patients. Psychiatry Res 2003;119:113–23 | Not a psychological, behavioural, sensory or environmental intervention |
| Linde K, ter Riet G, Hondras M, Vickers A, Saller R, Melchart D. Systematic reviews of herbal medicines – an annotated bibliography. Forsch Komplement Med 2003;10:17–27 | No participants with dementia/not separately analysed |
| Lindenmayer JP, Kotsaftis A. Use of sodium valproate in violent and aggressive behaviours: a critical review. J Clin Psychiatry 2000;61:123–8 | No participants with dementia/not separately analysed |
| Lindenmayer JP. The pathophysiology of agitation. J Clin Psychiatry 2000;61:5–10 | No participants with dementia/not separately analysed |
| Lindgren C, Hallberg IR, Norberg A. Diagnostic reasoning in the care of a vocally disruptive severely demented patient. A case report. Scand J Caring Sci 1992;6:97–103 | No comparator |
| Liperoti R, Pedone C, Corsonello A. Antipsychotics for the treatment of behavioral and psychological symptoms of dementia (BPSD). Curr Neuropharmacol 2008;6:117–24 | Not primary research |
| Little JT, Satlin A, Sunderland T, Volicer L. Sundown syndrome in severely demented patients with probable Alzheimers disease. J Geriatr Psychiatry Neurol 1995;8:103–6 | Not intervention study |
| Littner M, Kushida CA, Anderson M, Bailey D, Berry RB, Davila DG, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002 – an American academy of sleep medicine report. Sleep 2003;26:337–41 | No participants with dementia/not separately analysed |
| Litvinenko IV, Odinak MM, Mogil’naya VI, Perstnev SV. Use of memantine (akatinol) for the correction of cognitive impairments in Parkinson’s disease complicated by dementia. Neurosci Behav Physiol 2010;40:149–55 | No participants with dementia/not separately analysed |
| Liukkonen A. [Disruptive behavior; what is it, how prevalent is it and how much nursing care does it require in geriatric departments?] Hoitotiede 1993;5:64–71 | No participants with dementia/not separately analysed |
| Livingston G, Katona C. The place of memantine in the treatment of Alzheimer’s disease: a number needed to treat analysis. Int J Geriatr Psychiatry 2004;19:919–25 | Not primary research |
| Livingston G, Walker A, Katona C, Cooper C. Antipsychotics and cognitive decline in Alzheimer’s disease: the LASER-Alzheimer’s disease longitudinal study. J Neurol Neurosurg Psychiatry 2007;78:25–9 | Not intervention study |
| Lobaugh NJ, Karaskov V, Rombough V, Rovet J, Bryson S, Greenbaum R, et al. Piracetam therapy does not enhance cognitive functioning in children with Down syndrome. Arch Pediatr Adolesc Med 2001;155:442–8 | No participants with dementia/not separately analysed |
| Loehle M, Storch A, Reichmann H. Beyond tremor and rigidity: non-motor features of Parkinson’s disease. J Neural Transmission 2009;116:1483–92 | No participants with dementia/not separately analysed |
| Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging 2007;22:28–36 | Not primary research |
| Logsdon RG, Teri L, McCurry SM, Gibbons LE, Kukull WA, Larson EB. Wandering: a significant problem among community-residing individuals with Alzheimer’s disease. J Gerontol Series B 1998;53:294–9 | Not intervention study |
| Lonergan E, Luxenberg J, Colford J. Haloperidol for agitation in dementia. Cochrane Database Syst Rev 2002;2:CD002852 | Not primary research |
| Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, et al. Patterns of change in the treatment of psychiatric symptoms in patients with probable Alzheimer’s disease from 1983 to 2000. J Neuropsychiatry Clin Neurosci 2003;15:67–73 | Not intervention study |
| Lopez OL, Becker JT. Factors that modify the natural course of Alzheimer’s disease. Rev Neurol 2003;37:149–55 | Not primary research |
| Lopez OL, Wisniewski SR, Becker JT, Boller F, DeKosky ST. Psychiatric medication and abnormal behavior as predictors of progression in probable Alzheimer disease. Arch Neurol 1999;56:1266–72 | Not intervention study |
| Lopez-Pousa S, Garre-Olmo J, Vilalta-Franch J, Turon-Estrada A, Pericot-Nierga I. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr 2008;47:207–15 | Not intervention study |
| Lopez-Pousa S, Garre-Olmo J, Vilalta-Franch J. Galanthamine versus donepezil in the treatment of Alzheimer’s disease. Revista de Neurologia 2007;44:677–84 | Not primary research |
| Lou MF. The use of music to decrease agitated behaviour of the demented elderly: the state of the science. Scand J Caring Sci 2001;15:165–73 | Not primary research |
| Louis ED, Benito-Leon J, Bermejo-Pareja F. Population-based prospective study of cigarette smoking and risk of incident essential tremor. Neurology 2008;70:1682–7 | No participants with dementia/not separately analysed |
| Lovera J, Frohman E, Brown T, Bandari D, Nguyen L, Yadav V, et al. Memantine for cognitive impairment in multiple sclerosis: a randomized placebo-controlled trial. Multiple Sclerosis 2010;16:715–23 | Not a psychological, behavioural, sensory or environmental intervention |
| Lu CJ, Tune LE. Chronic exposure to anticholinergic medications adversely affects the course of Alzheimer disease. Am J Geriatr Psychiatry 2003;11:458–61 | Not a psychological, behavioural, sensory or environmental intervention |
| Lucero M, Hutchinson S, Leger-Krall S, Wilson HS. Wandering in Alzheimer’s dementia patients. Clin Nurs Res 1993;2:160–75 | Not intervention study |
| Lucero M, Pearson R, Hutchinson S, Leger-Krall S, Rinalducci E. Products for Alzheimer’s self-stimulatory wanderers. Am J Alzheimer’s Dis Other Demen 2001;16:43–50 | Not intervention study |
| Lucero M. Intervention strategies for exit-seeking wandering behaviour in dementia residents. Am J Alzheimer’s Dis Other Demen 2002;17:277–80 | Not primary research |
| Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment – results from the Cardiovascular Health Study. JAMA 2002;288:1475–83 | Not intervention study |
| Lyketsos CG, Sheppard JME, Steinberg M, Tschanz JAT, Norton MC, Steffens DC, et al. Neuropsychiatric disturbance in Alzheimer’s disease clusters into three groups: the Cache County study. Int J Geriatr Psychiatry 2001;16:1043–53 | Not intervention study |
| Lyketsos CG, Steele C, Galik E, Rosenblatt A, Steinberg M, Warren A, et al. Physical aggression in dementia patients and its relationship to depression. Am J Psychiatry 1999;156:66–71 | Not intervention study |
| Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JCS. Mental and behavioral disturbances in dementia: findings from the Cache County Study on memory in aging. Am J Psychiatry 2000;157:708–14 | Not intervention study |
| Lyons MK. Deep brain stimulation: current and future clinical applications. Mayo Clin Proc 2011;86:662–72 | No participants with dementia/not separately analysed |
| Mack JL, Patterson MB. The evaluation of behavioral disturbances in Alzheimers disease – the utility of 3 rating scales. J Geriatr Psychiatry Neurol 1994;7:99–115 | No participants with dementia/not separately analysed |
| Madhusoodanan S, Brenner R, Cohen CI. Role of atypical antipsychotics in the treatment of psychosis and agitation associated with dementia. CNS Drugs 1999;12:135–50 | Not primary research |
| Madhusoodanan S, Shah P, Brenner R, Gupta S. Pharmacological treatment of the psychosis of Alzheimer’s disease – what is the best approach? CNS Drugs 2007;21:101–15 | Not primary research |
| Mador JE, Giles L, Whitehead C, Crotty M. A randomized controlled trial of a behavior advisory service for hospitalized older patients with confusion. Int J Geriatr Psychiatry 2004;19:858–63 | No participants with dementia/not separately analysed |
| Maeda J, Higuchi M, Suhara T. [Evaluation of imaging biomarker by transgenic mouse models.] Nihon shinkei seishin yakurigaku zasshi 2009;29:73–8 | No participants with dementia/not separately analysed |
| Maeda J, Higuchi M, Suhara T. Evaluation of imaging biomarker by transgenic mouse models. Japan J Neuropsychopharmacol 2009;29:73–8 | No participants with dementia/not separately analysed |
| Mahlberg R, Walther S, Eichmann U, Tracik F, Kunz D. Effects of rivastigmine on actigraphically monitored motor activity in severe agitation related to Alzheimer’s disease: a placebo-controlled pilot study. Arch Gerontol Geriatr 2007;45:19–26 | Not a psychological, behavioural, sensory or environmental intervention |
| Mancini M, Agozzino B, Bompani E. Clinical and therapeutic effects of Ginkgo biloba extract (Egb) compared to placebo in the treatment of patients affected by senile psychoorganic dementia on an arteriosclerotic basis. Gazz Med Ital Arch Sci Med 1993;152:69–80 | Not a psychological, behavioural, sensory or environmental intervention |
| Mann WC, Hurren MD, Charvat BA, Tomita MR. Changes over one year in assistive device use and home modifications by home-based older persons with Alzheimer’s disease. Topics Geriatr Rehabil 1996;12:9–16 | Not intervention study |
| Marcantonio ER, Simon SE, Bergmann MA, Jones RN, Murphy KM, Morris JN. Delirium symptoms in post-acute care: prevalent, persistent, and associated with poor functional recovery. J Am Geriatr Soc 2003;51:4–9 | No participants with dementia/not separately analysed |
| Marder SR, Sorsaburu S, Dunayevich E, Karagianis JL, Dawe IC, Falk DM, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatry 2010;71:433–41 | No participants with dementia/not separately analysed |
| Margallo-Lana M, Swann A, O’Brien J, Fairbairn A, Reichelt K, Potkins D, et al. Prevalence and pharmacological management of behavioural and psychological symptoms amongst dementia sufferers living in care environments. Int J Geriatr Psychiatry 2001;16:39–44 | Not intervention study |
| Marin DB, Green CR, Schmeidler J, Harvey PD, Lawlor BA, Ryan TM, et al. Noncognitive disturbances in Alzheimer’s disease: frequency, longitudinal course, and relationship to cognitive symptoms. J Am Geriatr Soc 1997;45:1331– | Not intervention study |
| Marksteiner J, Schmidt R. Treatment strategies in Alzheimer’s disease with a focus on early pharmacological interventions. Drugs Aging 2004;21:415–26 | Not primary research |
| Marquardt G. Wayfinding for people with dementia: a review of the role of architectural design. HERD 2011;4:75–90 | Not primary research |
| Marshall FJ, Walker F, Frank S, Oakes D, Plumb S, Factor SA, et al. Tetrabenazine as antichorea therapy in Huntington disease – a randomized controlled trial. Neurology 2006;66:366–72 | No participants with dementia/not separately analysed |
| Marshall MC, Soucy MD. Delirium in the intensive care unit. Crit Care Nurs Q 2003;26:172–8 | No participants with dementia/not separately analysed |
| Martin H, Slyk MP, Deymann S, Cornacchione MJ. Safety profile assessment of risperidone and olanzapine in long-term care patients with dementia. J Am Med Direct Assoc 2003;4:183–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Martin J, Marler M, Shochat T, Ancoli-Israel S. Circadian rhythms of agitation in institutionalized patients with Alzheimer’s disease. Chronobiol Int 2000;17:405–18 | Not intervention study |
| Martinon-Torres G, Fioravanti M, Grimley EJ. Trazodone for agitation in dementia. Cochrane Database Syst Rev 2004;4:CD004990 | Not primary research |
| Martinosaltzman D, Blasch BB, Morris RD, McNeal LW. Travel behavior of nursing-home residents perceived as wanderers and nonwanderers. Gerontologist 1991;31:666–72 | Not intervention study |
| Mast BT. Impact of cognitive impairment on the phenomenology of geriatric depression. Am J Geriatr Psychiatry 2005;13:694–700 | Not intervention study |
| Mather JA, Nemecek D, Oliver K. The effect of a walled garden on behavior of individuals with Alzheimer’s. Am J Alzheimers Dis Other Demen 1997;12:252–7 | No outcome measuring agitation |
| Matsui T, Nakaaki S, Murata Y, Sato J, Shinagawa Y, Tatsumi H, et al. Determinants of the quality of life in Alzheimer’s disease patients as assessed by the Japanese version of the quality of life – Alzheimer’s disease scale. Dementia Geriatr Cogn Disord 2006;21:182–91 | No participants with dementia/not separately analysed |
| Matteson MA, Linton A. Wandering behaviors in institutionalized persons with dementia. J Gerontol Nurs 1996;22:39–46 | Not intervention study |
| Mausbach BT, Aschbacher K, Patterson TL, Ancoli-Israel S, von Kanel R, Mills PJ, et al. Avoidant coping partially mediates the relationship between patient problem behaviors and depressive symptoms in spousal Alzheimer caregivers. Am J Geriatr Psychiatry 2006;14:299–306 | No participants with dementia/not separately analysed |
| Mayers K, Griffin M. The play project--use of stimulus objects with demented patients. J Gerontol Nurs 1990;16:32–7 | No outcome measuring agitation |
| Mazeh D, Zemishlani H, Barak Y, Mirecki I, Paleacu D. Donepezil for negative signs in elderly patients with schizophrenia: an add-on, double-blind, crossover, placebo-controlled study. Int Psychogeriatr 2006;18:429–36 | No participants with dementia/not separately analysed |
| McAiney CA, Stolee P, Hillier LM, Harris D, Hamilton P, Kessler L, et al. Evaluation of the sustained implementation of a mental health learning initiative in long-term care. Int Psychogeriatr 2007;19:842–58 | No participants with dementia/not separately analysed |
| McCurry SM, Reynolds CF, Ancoli-Israel S, Teri L, Vitiello MV. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med Rev 2000;4:603–28 | Not primary research |
| Mcdonald WM, Krishnan KRR. Pharmacological management of the symptoms of dementia. Am Fam Phys 1990;42:123–32 | Not a psychological, behavioural, sensory or environmental intervention |
| McGaffigan S, Bliwise DL. The treatment of sundowning – a selective review of pharmacological and nonpharmacological studies. Drugs Aging 1997;10:10–17 | Not primary research |
| McGauran N, Wieseler B, Kreis J, Schueler YB, Koelsch H, Kaiser T. Reporting bias in medical research – a narrative review. Trials 2010;11 | No participants with dementia/not separately analysed |
| McGee SB, Orengo CA, Kunik ME, Molinari VA, Workman RH. Delirium in geropsychiatric patients: patient characteristics and treatment outcomes. J Geriatr Psychiatry Neurol 1997;10:7–10 | Not intervention study |
| McGeer EG, McGeer PL. Mini-review – The importance of inflammatory mechanisms in Alzheimer disease. Exp Gerontol 1998;33:371–8 | Not primary research |
| McGeer P, McGeer E. Mechanisms of cell death in Alzheimer disease: immunopathology. J Neural Trans Suppl 1998;54:159–66 | No participants with dementia/not separately analysed |
| McGeer PL, McGeer EG. Glial cell reactions in neurodegenerative diseases: pathophysiology and therapeutic interventions. Alzheimer Dis Assoc Disord 1998;12:S1–6 | No participants with dementia/not separately analysed |
| McGilton K, Wells J, Teare G, Davis A, Rochon E, Calabrese S, et al. Rehabilitating patients with dementia who have had a hip frature – part I: behavioral symptoms that influence care. Topics Geriatr Rehabil 2007;23:161–73 | Not intervention study |
| McGilton KS, Boscart V, Fox M, Sidani S, Rochon E, Sorin-Peters R. A systematic review of the effectiveness of communication interventions for health care providers caring for patients in residential care settings. Worldviews Evidence-Based Nurs 2009;6:149–59 | No participants with dementia/not separately analysed |
| McGough EL, Kelly VE, Logsdon RG, McCurry SM, Cochrane BB, Engel JM, et al. Associations between physical performance and executive function in older adults with mild cognitive impairment: gait speed and the timed “up & go” test. Phys Ther 2011;91:1198–207 | No participants with dementia/not separately analysed |
| McHenry M, Wilson R. The challenge of unintelligible speech following traumatic brain injury. Brain Injury 1994;8:363–75 | No participants with dementia/not separately analysed |
| McKeage K. Memantine a review of its use in moderate to severe Alzheimer’s disease. CNS Drugs 2009;23:881–97 | Not primary research |
| McKee SA, Harris GT, Rice ME, Silk L. Effects of a Snoezelen room on the behavior of three autistic clients. Res Develop Disabil 2007;28:304–1 | No participants with dementia/not separately analysed |
| McKeith IG. Dementia with Lewy bodies. Clin Manag 2001:175–9 | Not primary research |
| McMinn B, Draper B. Vocally disruptive behaviour in dementia: development of an evidence based practice guideline. Aging Ment Health 2005;9:16–24 | Not primary research |
| McNeal KM, Meyer RP, Lukacs K, Senseney A, Mintzer J. Using risperidone for Alzheimer’s dementia-associated psychosis. Ex Opin Pharmacother 2008;9:2537–43 | Not primary research |
| McShane R, Keene J, Gedling K, Fairburn C, Jacoby R, Hope T. Do neuroleptic drugs hasten cognitive decline in dementia? Prospective study with necropsy follow up. BMJ 1997;314:266–70 | Not intervention study |
| McShane R, Sastre AA, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006;2:CD003154 | Not primary research |
| McWalter G, Toner H, McWalter A, Eastwood J, Marshall M, Turvey T. A community needs assessment: the care needs assessment pack for dementia (CarenapD) – its development, reliability and validity. Int J Geriatr Psychiatry 1998;13:16–22 | No participants with dementia/not separately analysed |
| Meares S, Draper B. Treatment of vocally disruptive behaviour of multifactorial aetiology. Int J Geriatr Psychiatry 1999;14:285–90 | No participants with dementia/not separately analysed |
| Meehan KM, Wang HE, David SR, Nisivoccia JR, Jones B, Beasley CM, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology 2002;26:494–504 | Not a psychological, behavioural, sensory or environmental intervention |
| Mega MS, Dinov ID, Lee L, O’Connor SM, Masterman DM, Wilen B, et al. Orbital and dorsolateral frontal perfusion defect associated with behavioral response to cholinesterase inhibitor therapy in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 2000;12:209–18 | Not intervention study |
| Mega MS, Lee L, Dinov ID, Mishkin F, Toga AW, Cummings JL. Cerebral correlates of psychotic symptoms in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2000;69:167–71 | No participants with dementia/not separately analysed |
| Mega MS, Masterman DM, O’Connor SM, Barclay TR, Cummings JL. The spectrum of behavioral responses to cholinesterase inhibitor therapy in Alzheimer disease. Arch Neurol 1999;56:1388–93 | Not a psychological, behavioural, sensory or environmental intervention |
| Meguro K, Yamaguchi S, Shimada M, Itoh M, Yamadori A. Striatal dopaminergic transmission and neocortical glucose utilization in Alzheimer’s disease: a triple-tracer positron emission tomography study. Arch Gerontol Geriatr 2000;31:147–58 | No participants with dementia/not separately analysed |
| Meiland FJM, Kat MG, van Tilburg W, Jonker C, Droes RM. The emotional impact of psychiatric symptoms in dementia on partner caregivers – do caregiver, patient, and situation characteristics make a difference? Alzheimer Dis Assoc Disord 2005;19:195–201 | No participants with dementia/not separately analysed |
| Meinhold JM, Blake LM, Mini LJ, Welge JA, Schwiers M, Hughes A. Effect of divalproex sodium on behavioural and cognitive problems in elderly dementia. Drugs Aging 2005;22:615–26 | Not intervention study |
| Mendelowitz AJ. The utility of intramuscular ziprasidone in the management of acute psychotic agitation. Ann Clin Psychiatry 2004;16:145–54 | Not primary research |
| Menon AS, Gruber-Baldini AL, Hebel JR, Kaup B, Loreck D, Zimmerman SI, et al. Relationship between aggressive behaviors and depression among nursing home residents with dementia. Int J Geriatr Psychiatry 2001;16:139–46 | Not intervention study |
| Merrick GS, Butler WM, Wallner KE, Galbreath RW, Lief JH. Long-term urinary quality of life after permanent prostate brachytherapy. Int J Rad Oncol Biol Phys 2003;56:454–61 | No participants with dementia/not separately analysed |
| Mervis JR, Ganzell S, Fitten LJ, Daum G, Tripodis K, Takayesu S. Comparison of carbamazepine and trazodone in the control of aggression agitation in demented, institutionalized patients – a randomized double-blind parallel study. J Am Geriatr Soc 1991;39:A75 | Not a psychological, behavioural, sensory or environmental intervention |
| Mestre T, Ferreira J, Coelho MM, Rosa M, Sampaio C. Therapeutic interventions for symptomatic treatment in Huntington’s disease. Cochrane Database Syst Rev (Online) 2009;3:CD006456 | No participants with dementia/not separately analysed |
| Meyer J, Schalock R, Genaidy H. Aggression in psychiatric hospitalized geriatric-patients. Int J Geriatr Psychiatry 1991;6:589–92 | Not intervention study |
| Meyer JS, Welch KMA, Deshmukh VD, Perez F, I, Jacob RH, Haufrect DB, et al. Neuro transmitter precursor amino-acids in the treatment of multi infarct dementia and Alzheimers disease. J Am Geriatr Soc 1977;25:289–98 | Not a psychological, behavioural, sensory or environmental intervention |
| Michaelis ML. Drugs targeting Alzheimer’s disease: some things old and some things new. J Pharmacol Exp Ther 2003;304:897–904 | Not primary research |
| Michel B, Luciani V, Geda Y, Sambuchi N, Paban V, Azorin J. In Alzheimer’s disease, the clinical expression of behavioral and psychological signs and symptoms is early and specific of neuropathological stages. Encephale Rev Psychiatr Clin Biol Therap 2010;36:314–25. | Not intervention study |
| Mihaescu R, Detmar SB, Cornel MC, van der Flier WM, Heutink P, Hol EM, et al. Translational research in genomics of Alzheimer’s disease: a review of current practice and future perspectives. J Alzheimers Dis 2010;20:967–80 | Not primary research |
| Millán-Calenti JC, Gandoy-Crego M, Antelo-Martelo M, López-Martinez M, Riveiro-López MP, Mayán-Santos JM. Helping the family carers of Alzheimer’s patients: from theory... to practice. A preliminary study. Arch Gerontol Geriatr 2000;30:131–8 | No participants with dementia/not separately analysed |
| Miller CA. How to try this: Communication difficulties in hospitalized older adults with dementia. Am J Nurs 2008;108:58–67 | Not primary research |
| Miller EA, Schneider LS, Rosenheck RA. Predictors of nursing home admission among Alzheimer’s disease patients with psychosis and/or agitation. Int Psychogeriatr 2011;23:44–53 | Not intervention study |
| Miller LJ. The use of cognitive enhancers in behavioral disturbances of Alzheimer’s disease. Consult Pharm 2007;22:754–62 | Not primary research |
| Millichap D, Oliver C, McQuillan S, Kalsy S, Lloyd V, Hall S. Descriptive functional analysis of behavioral excesses shown by adults with Down syndrome and dementia. Int J Geriatr Psychiatry 2003;18:844–54 | Not intervention study |
| Mimica N, Glamuzina K, Vucic K, Gatin M, Dajcic M, Dajcic T, et al. Art therapy for people with dementia – case report. 25th International Conference of Alzheimers Disease International 2010;95–9 | No comparator |
| Mimica N, Kalinic D. Art therapy may be benefitial for reducing stress – related behaviours in people with dementia – case report. Psychiatria Danubina 2011;23:125–8 | No comparator |
| Mintzer J, Brawman-Mintzer O, Mirski DF, Unger R, Nietert P, Meeks A, et al. Fenfluramine challenge test as a marker of serotonin activity in patients with Alzheimer’s dementia and agitation. Biol Psychiatry 1998;44:918–21 | Not a psychological, behavioural, sensory or environmental intervention |
| Mintzer J, Faison W, Street JS, Sutton VK, Breier A. Olanzapine in the treatment of anxiety symptoms due to Alzheimer’s disease: a post hoc analysis. Int J Geriatr Psychiatry 2001;16:S71–7 | Not primary research |
| Mintzer JE, Colenda C, Waid LR, Lewis L, Meeks A, Stuckey M, et al. Effectiveness of a continuum of care using brief and partial hospitalization for agitated dementia patients. Psychiatr Serv 1997;48:1435–9 | Multidisciplinary team input including pharmacological intervention |
| Mintzer JE, Hoernig KS, Mirski DF. Treatment of agitation in patients with dementia. Clin Geriatr Med 1998;14:147 | Not primary research |
| Mintzer JE, Tune LE, Breder CD, Swanink R, Marcus RN, McQuade RD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry 2007;15:918–31 | Not a psychological, behavioural, sensory or environmental intervention |
| Mintzer JE. Underlying mechanisms of psychosis and aggression in patients with Alzheimer’s disease. J Clin Psychiatry 2001;62:23–5 | Not primary research |
| Mirza NR, Peters D, Sparks RG. Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists. CNS Drug Rev 2003;9:159–86 | No participants with dementia/not separately analysed |
| Mittelman MS, Roth DL, Haley WE, Zarit SH. Effects of a caregiver intervention on negative caregiver appraisals of behavior problems in patients with Alzheimer’s disease: results of a randomized trial. J Gerontol Series B 2004;59:27–34 | No outcome measuring agitation |
| Miura T, Yoda M, Tsutsumi C, Murayama K, Takeuchi H. Conformational regulation of amyloid beta-peptide by lipid membranes and metal ions. Yakugaku Zasshi 2010;130:495–501 | No participants with dementia/not separately analysed |
| Miyamoto Y, Ito H, Otsuka T, Kurita H. Caregiver burden in mobile and non-mobile demented patients: a comparative study. Int J Geriatr Psychiatry 2002;17:765–73 | Not intervention study |
| Miyaoka T, Furuya M, Yasuda H, Hayashia M, Inagaki T, Horiguchi J. Yi-gan san for the treatment of borderline personality disorder: an open-label study. Prog Neuro-Psychopharmacol Biol Psychiatry 2008;32:150–4 | No participants with dementia/not separately analysed |
| Mizrahi R, Starkstein SE, Jorge R, Robinson RG. Phenomenology and clinical correlates of delusions in Alzheimer disease. Am J Geriatr Psychiatry 2006;14:573–81 | Not intervention study |
| Mizukami K, Asada T, Kinoshita T, Tanaka K, Sonohara K, Nakai R, et al. A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int J Neuropsychopharmacol 2009;12:191–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Mizukami K, Hatanaka K, Ishii T, Iwakiri M, Sodeyama N, Tanaka Y, et al. Effects of sodium valproate on behavioral disturbances in elderly outpatients with dementia. Geriatr Gerontol Int 2010;10:324–6 | Not a psychological, behavioural, sensory or environmental intervention |
| Mizukami K. Kampo therapy as an alternative to pharmacotherapy using antipsychotic medicines for behavioral and psychological symptoms of dementia (BPSD). Psychogeriatrics 2008;8:137–41 | Not primary research |
| Mizuno E, Hosak T, Ogihara R, Higano H, Mano Y. Effectiveness of a stress management program for family caregivers of the elderly at home. J Med Dent Sci 1999;46:145–53 | No participants with dementia/not separately analysed |
| Mogoanta L, Marinescu D, Udristoiu T, Udristoiu I, Pirici D. Neuroprotective effect of cerebrolysin and erythropoietin versus haloperidol in an Alzheimer’s disease – animal model. Eur Neuropsychopharmacol 2010;20:S565–6 | No participants with dementia/not separately analysed |
| Mohamed S, Rosenheck R, Lyketsos CG, Schneider LS. Caregiver burden in Alzheimer disease: cross-sectional and longitudinal patient correlates. Am J Geriatr Psychiatry 2010;18:917–27 | Not intervention study |
| Mohr P, Pecenak J, Svestka J, Swingler D, Treuer T. Treatment of acute agitation in psychotic disorders. Neuroendocrinol Lett 2005;26:327–35 | No participants with dementia/not separately analysed |
| Mohundro BL, Pope K, Shaw V, Hitchcock K. Which drugs are best when aggressive Alzheimer’s patients need medication? J Fam Prac 2010;59:595–604 | Not a psychological, behavioural, sensory or environmental intervention |
| Mok V, Wong A, Ho S, Leung T, Lam WWM, Wong KS. Rivastigmine in Chinese patients with subcortical vascular dementia. Neuropsychiatr Dis Treat 2007;3:943–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Mokhber N, Azarpazhooh MR, Khajehdaluee M, Velayati A, Hopwood M. Randomized, single-blind, trial of sertraline and buspirone for treatment of elderly patients with generalized anxiety disorder. Psychiatry Clin Neurosci 2010;64:128–33 | No participants with dementia/not separately analysed |
| Mollenhauer B, Foerstl H, Deuschl G, Storch A, Oertel W, Trenkwalder C. Lewy Body and Parkinsonian dementia: common, but often misdiagnosed conditions. Deutsches Arzteblatt Int 2010;107:684–U31 | Not primary research |
| Monastero R, Mangialasche F, Camarda C, Ercolani S, Camarda R. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J Alzheimers Dis 2009;18:11–30 | No participants with dementia/not separately analysed |
| Moniz-Cook E, Stokes G, Agar S. Difficult behaviour and dementia in nursing homes: five cases of psychosocial intervention. Clin Psychol Psychother 2003;10:197–208 | No comparator |
| Moniz-Cook E, Woods R, Gardiner E, Silver M, Agar S. The Challenging Behaviour Scale (CBS): development of a scale for staff caring for older people in residential and nursing homes. Br J Clin Psychol 2001;40:309–22 | No participants with dementia/not separately analysed |
| Moniz-Cook E, Woods R, Gardiner E. Staff factors associated with perception of behaviour as ‘challenging’ in residential and nursing homes. Aging Mental Health 2000;4:48–55 | No participants with dementia/not separately analysed |
| Moniz-Cook E, Woods RT, Richards K. Functional analysis of challenging behaviour in dementia: the role of superstition. Int J Geriatr Psychiatry 2001;16:45–56 | No comparator |
| Monsch AU, Giannakopoulos P. Effects of galantamine on behavioural and psychological disturbances and caregiver burden in patients with Alzheimer’s disease. Curr Med Res Opin 2004;20:931–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Montejo A, Majadas S, Mayoral F, Sanjuan J, Ros S, Olivares J, et al. Analysis of prescription patterns of antipsychotic agents in psychiatry. Actas Esp Psiquiatr 2006;34:323–9 | No participants with dementia/not separately analysed |
| Moore D, Algase DL, Powell-Cope G, Applegarth S, Beattie ER. A framework for managing wandering and preventing elopement. AmJ Alzheimers Dis Other Dementias 2009;24:208–19 | Not primary research |
| Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, Bava A. Olanzapine as a possible treatment for anxiety due to vascular dementia: an open study. Am J Alzheimers Dis Other Demen 2004;19:81–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Gabapentin for the treatment of behavioural alterations in dementia – preliminary 15-month investigation. Drugs Aging 2003;20:1035–40 | Not a psychological, behavioural, sensory or environmental intervention |
| Moretti R, Torre P, Antonello RM, Cazzato G, Griggio S, Bava A. An open-label pilot study comparing rivastigmine and low-dose aspirin for the treatment of symptoms specific to patients with subcortical vascular dementia. Curr Ther Res 2002;63:443–58 | Not a psychological, behavioural, sensory or environmental intervention |
| Morinaga A, Hasegawa K, Nomura R, Ookoshi T, Ozawa D, Goto Y, et al. Critical role of interfaces and agitation on the nucleation of A beta amyloid fibrils at low concentrations of A beta monomers. Biochim Biophys Acta 2010;1804:986–95 | No participants with dementia/not separately analysed |
| Moss R, Damico S, Maletta G. Mental dysfunction as a sign of organic illness in the elderly. Geriatrics 1987;42:35 | No participants with dementia/not separately analysed |
| Mowla A, Pani A. Comparison of topiramate and risperidone for the treatment of behavioural disturbances of patients with Alzheimer disease: a double-blind, randomised clinical trial. J Clin Psychopharmacol 2010;30:40–3 | Not a psychological, behavioural, sensory or environmental intervention |
| Mulchahey JJ, Malik MS, Sabai M, Kasckow JW. Serotonin-selective reuptake inhibitors in the treatment of geriatric depression and related disorders. Int J Neuropsychopharmacol 1999;2:121–7 | Not primary research |
| Mullan E, Katona C, Bellew M. Patterns of sleep disorders and sedative hypnotic use in seniors. Drugs Aging 1994;5:49–58 | No participants with dementia/not separately analysed |
| Mulsant BH, Gharabawi GM, Bossie CA, Mao L, Martinez RA, Tune LE, et al. Correlates of anticholinergic activity in patients with dementia and psychosis treated with risperidone or olanzapine. J Clin Psychiatry 2004;65:1708–14 | Not a psychological, behavioural, sensory or environmental intervention |
| Mulsant BH, Mazumdar S, Pollock BG, Sweet RA, Rosen J, Lo K. Methodological issues in characterizing treatment response in demented patients with behavioral disturbances. Int J Geriatr Psychiatry 1997;12:537–47 | Not intervention study |
| Murakami Y, Zhao Q, Harada K, Tohda M, Watanabe H, Matsumoto K. Choto-san, a Kampo formula, improves chronic cerebral hypoperfusion-induced spatial learning deficit via stimulation of muscarinic M-1 receptor. Pharmacol Biochem Behav 2005;81:616–25 | No participants with dementia/not separately analysed |
| Murgod UA, Saleem Q, Anand A, Brahmachari SK, Jain S, Muthane UB. A clinical study of patients with genetically confirmed Huntington’s disease from India. J Neurol Sci 2001;190:73–8 | No participants with dementia/not separately analysed |
| Murphy S, Churchill S, Bry L, Chueh H, Weiss S, Lazarus R, et al. Instrumenting the health care enterprise for discovery research in the genomic era. Genome Res 2009;19:1675–81 | No participants with dementia/not separately analysed |
| Nagaratnam N, Lewis-Jones M, Scott D, Palazzi L. Behavioral and psychiatric manifestations in dementia patients in a community: caregiver burden and outcome. Alzheimer Dis Assoc Disord 1998;12:330–4 | Not intervention study |
| Nagaratnam N, Patel I, Whelan C. Screaming, shrieking and muttering: the noise-makers amongst dementia patients. Arch Gerontol Geriatr 2003;36:247–58 | Not intervention study |
| Nagaratnam N, Phan TA, Barnett C, Ibrahim N. Angular gyrus syndrome mimicking depressive pseudodementia. J Psychiatry Neurosci 2002;27:364–8 | No participants with dementia/not separately analysed |
| Nagaratnam N, Wong M, Gunja N. Dementia-related behavioral changes – a physician’s office-based study. Arch Gerontol Geriatr 2001;32:67–76 | Not intervention study |
| Nakaoka A, Suto S, Makimoto K, Yamakawa M, Shigenobu K, Tabushi K. Pacing and lapping movements among institutionalized patients with dementia. Am J Alzheimers Dis Other Demen 2010;25:167–72 | Not intervention study |
| Nakimuli-Mpungu E, Musisi S, Mpungu SK, Katabira E. Primary mania versus HIV-related secondary mania in Uganda. Am J Psychiatry 2006;163:1349–54 | No participants with dementia/not separately analysed |
| Namazi KH, Rosner TT, Calkins MP. Visual barriers to prevent ambulatory Alzheimers patients from exiting through an emergency door. Gerontologist 1989;29:699–702 | No quantitative outcome |
| Narevic E, Giles GM, Rajadhyax R, Managuelod E, Monis F, Diamond F. The effects of enhanced program review and staff training on the management of aggression among clients in a long-term neurobehavioral rehabilitation program. Aging Mental Health 2011;15:103–12 | No participants with dementia/not separately analysed |
| Narumoto J, Miya H, Shibata K, Nakamae T, Okamura A, Matsuoka T, et al. Challenging behavior of patients with frontal dysfunction managed successfully with behavioral intervention. Psychogeriatrics 2009;9:147–50 | No comparator |
| Nassisi D, Korc B, Hahn S, Bruns J Jr, Jagoda A. The evaluation and management of the acutely agitated elderly patient. Mount Sinai J Med 2006;73:976–84 | No participants with dementia/not separately analysed |
| Navarrete F, Perez-Ortiz J, Femenia T, Garcia-Gutierrez M, Garcia-Paya M, Leiva-Santana C, et al. Methods to evaluate cognitive disorders in animal models. Rev Neurol 2008;47:137–45 | No participants with dementia/not separately analysed |
| Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nature Rev Neurosci 2007;8:536–46 | No participants with dementia/not separately analysed |
| Neugroschl J. Agitation – how to manage behavior disturbances in the older patient with dementia. Geriatrics 2002;57:33–7 | No participants with dementia/not separately analysed |
| Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thompson K, Mellow AM, et al. The effects of acute scopolamine in geriatric depression. Arch Gen Psychiatry 1988;45:906–12 | No participants with dementia/not separately analysed |
| Ng B, Camacho A, Lara DR, Brunstein MG, Pinto OC, Akiskal HS. A case series on the hypothesized connection between dementia and bipolar spectrum disorders: bipolar type VI? J Affect Disord 2008;107:307–15 | Not intervention study |
| Nguyen Qa, Paton C. The use of aromatherapy to treat behavioural problems in dementia. Int J Geriatr Psychiatry 2008;23:337–46 | Not primary research |
| Nguyen VT, Love AR, Kunik ME. Preventing aggression in persons with dementia. Geriatrics 2008;63:21–6 | Not primary research |
| Nickel C, Labmann C, Tritt K, Muehlbacher M, Kaplan P, Kettler C, et al. Topiramate in treatment of depressive and anger symptoms in female depressive patients: a randomized, double-blind, placebo-controlled study. J Affect Disord 2005;87:243–52 | No participants with dementia/not separately analysed |
| Niederhofer H. Acetylcholinesterase inhibitors may improve efficacy and reduce adverse effects of tricyclic antidepressants for depression. Drugs Aging 2008;25:715 | No participants with dementia/not separately analysed |
| Niemeijer AR, Frederiks BJ, Riphagen II, Legemaate J, Eefsting JA, Hertogh CM. Ethical and practical concerns of surveillance technologies in residential care for people with dementia or intellectual disabilities: an overview of the literature. Int Psychogeriatr 2010;22:1129–42 | Not primary research |
| Nishtala PS, McLachlan AJ, Bell J, Chen TF. Determinants of antipsychotic medication use among older people living in aged care homes in Australia. Int J Geriatr Psychiatry 2010;25:449–57 | Not intervention study |
| Nobili A, Riva E, Tettamanti M, Lucca U, Liscio M, Petrucci B, et al. The effect of a structured intervention on caregivers of patients with dementia and problem behaviours – a randomised controlled pilot study. Alzheimer Dis Assoc Disord 2004;18:75–82 | No outcome measuring agitation |
| Nolan BAD, Mathews RM. Facilitating resident information seeking regarding meals in a special care unit: an environmental design intervention. J Gerontol Nurs 2004;30:12 | No participants with dementia/not separately analysed |
| Nooyens AC, van Gelder BM, Verschuren W. Smoking and cognitive decline among middle-aged men and women: the Doetinchem Cohort Study. Am J Public Health 2008;98:2244–50 | No participants with dementia/not separately analysed |
| Nordheim J, Liebich M. Dementia and challenging behavior: results of a study on the structured care concept “Serial Trial Intervention”. Z Gerontol Geriatr 2010;43:70–1 | Conference Presentation Only |
| Nordheim J. Reduction of challenging behavioural pattern in people with dementia: serial trial intervention as strategy for nurses. Development and examination of a German frame with the STI-D-study. Z Gerontol Geriatr 2008;41:41–2 | Conference Presentation Only |
| Norton MJ, Allen RS, Snow A, Hardin J, Burgio LD. Predictors of need-driven behaviors in nursing home residents with dementia and associated certified nursing assistant burden. Aging Mental Health 2010;14:303–9 | Not intervention study |
| Norwitz ER, Repke JT. Obstetric issues in women with neurologic diseases. Curr Problems Obstet Gynecol Fertil 1997;20:191–230 | No participants with dementia/not separately analysed |
| Nygaard HA, Bakke K, Brudvik E, Elgen K, Lien GK. Dosing of neuroleptics in elderly demented patients with aggressive and agitated behavior – a double-blind study with zuclopenthixol. Curr Med Res Opin 1994;13:222–32 | Not a psychological, behavioural, sensory or environmental intervention |
| Nygaard HA, Fuglum E, Elgen K. Zuclopenthixol, Melperone and haloperidol levomepromazine in the elderly – metaanalysis of 2 double-blind trials at 15 nursing-homes in Norway. Curr Med Res Opin 1992;12:615–22 | No participants with dementia/not separately analysed |
| Nyman S, Almqvist EW. Aggression in Huntington’s disease: measure instrument for assessment of interventions and guidelines for strategies in aggressive behaviour. J Neurol Neurosurg Psychiatry 2005;76:A27–A54 | No participants with dementia/not separately analysed |
| Nyth AL, Gottfries CG. The clinical efficacy of citalopram in treatment of emotional disturbances in dementia disorders – a Nordic multicenter study. Br J Psychiatry 1990;157:894–901 | Not a psychological, behavioural, sensory or environmental intervention |
| O’Connor DW, Ames D, Gardner B, King M. Psychosocial treatments of psychological symptoms in dementia: a systematic review of reports meeting quality standards. Int Psychogeriatr 2009;21:241–51 | Not primary research |
| Ohadinia S, Noroozian M, Shahsavand S, Saghafi S. Evaluation of insomnia and daytime napping in Iranian Alzheimer disease patients – relationship with severity of dementia and comparison with normal adults. Am J Geriatr Psychiatry 2004;12:517–22 | Not intervention study |
| Okura T, Plassman BL, Steffens DC, Llewellyn D, Potter GG, Langa KM. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the Aging, Demographics, and Memory study. J Am Geriatr Soc 2010;58:330–7 | Not intervention study |
| Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Neuropsychiatric symptoms and the risk of institutionalization and death: the Aging, Demographics, and Memory study. J Am Geriatr Soc 2011;59:473–81 | Not intervention study |
| Olafsson K, Jorgensen S, Jensen HV, Bille A, Arup P, Andersen J. Fluvoxamine in the treatment of demented elderly patients – a double-blind, placebo-controlled study. Acta Psychiatrica Scandinavica 1992;85:453–6 | Not a psychological, behavioural, sensory or environmental intervention |
| Olin JT, Fox LS, Pawluczyk S, Taggart NA, Schneider LS. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry 2001;9:400–5 | Not a psychological, behavioural, sensory or environmental intervention |
| Onega LL, Abraham IL. Factor structure of the Dementia Mood Assessment Scale in a cohort of community-dwelling elderly. Int Psychogeriatr 1997;9:449–57 | No participants with dementia/not separately analysed |
| Onor ML, Saina M, Trevisiol M, Cristante T, Aguglia E. Clinical experience with risperidone in the treatment of behavioural and psychological symptoms of dementia. Prog Neuro-Psychopharmacol Biol Psychiatry 2007;31:205–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Opie J, Doyle C, O’Connor DW. Challenging behaviours in nursing home residents with dementia: a randomized controlled trial of multidisciplinary interventions. Int J Geriatr Psychiatry 2002;17:6–13 | Multidisciplinary team input including pharmacological intervention |
| Orengo CA, Kunik ME, Molinari VA, Teasdale TA, Workman RH, Yudofsky SC. Association of serum cholesterol and triglyceride levels with agitation and cognitive function in a geropsychiatry unit. J Geriatr Psychiatry Neurol 1996;9:53–6 | No participants with dementia/not separately analysed |
| Orsulic-Jeras S, Schneider NM, Camp CJ. Special Feature: Montessori-based activities for long-term care residents with dementia. Topics Geriatr Rehabil 2000;16:78–91 | No quantitative outcome |
| Orth M, Handley OJ, Schwenke C, Dunnett SB, Craufurd D, Ho AK, et al. Observing Huntington’s Disease: the European Huntington’s Disease Network’s REGISTRY. PLOS Curr 2010;2 | No participants with dementia/not separately analysed |
| Osborn GG, Saunders AV. Current treatments for patients with Alzheimer disease. J Am Osteopath Assoc 2010;110(Suppl. 8):S16–26 | Not primary research |
| O’Shea E, Devane D, Murphy K, Cooney A, Casey D, Jordan F, et al. Effectiveness of a structured education reminiscence-based programme for staff on the quality of life of residents with dementia in long-stay units: a study protocol for a cluster randomised trial. Trials 2011;12:41 | Protocol only |
| Osterkamp L, Mathews RM, Burgio LD, Hardin JM. Social workers’ acceptability ratings of behavioral treatments and pharmacotherapy for the management of geriatric behavior problems. Educ Gerontol 1997;23:425–35 | No participants with dementia/not separately analysed |
| Ott A, Andersen K, Dewey ME, Letenneur L, Brayne C, Copeland JRM, et al. Effect of smoking on global cognitive function in nondemented elderly. Neurology 2004;62:920–4 | No participants with dementia/not separately analysed |
| Ott BR, Lapane KL, Gambassi G. Gender differences in the treatment of behavior problems in Alzheimer’s disease. Neurology 2000;54:427–32 | Not intervention study |
| Ottaviani M, Mazzeo R, Cangiotti M, Fiorani L, Majoral JP, Caminade AM, et al. Time evolution of the aggregation process of peptides involved in neurodegenerative diseases and preventing aggregation effect of phosphorus dendrimers studied by EPR. Biomacromolecules 2010;11:3014–21 | No participants with dementia/not separately analysed |
| Ouldred E, Bryant C. Dementia care. Part 2: understanding and managing behavioural challenges. Br J Nurs 2008;17:242–7 | Not primary research |
| Overall KL. Natural animal models of human psychiatric conditions: assessment of mechanism and validity. Prog Neuro-Psychopharmacol Biol Psychiatry 2000;24:727–76 | No participants with dementia/not separately analysed |
| Overshott R, Byrne J, Burns A. Nonpharmacological and pharmacological interventions for symptoms in Alzheimer’s disease. Ex Rev Neurother 2004;4:809–21 | Not primary research |
| Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev 2008;1:CD005317 | No participants with dementia/not separately analysed |
| Ownby RL. Quetiapine and rivastigmine for agitation in Alzheimer’s disease. Curr Psychiatry Rep 2006;8:10 | Not a psychological, behavioural, sensory or environmental intervention |
| Ozakbas S, Ormeci B, Akdede BBK, Alptekin K, Idiman E. Utilization of the auditory consonant trigram test to screen for cognitive impairment in patients with multiple sclerosis: comparison with the paced auditory serial addition test. Multiple Sclerosis 2004;10:686–9 | No participants with dementia/not separately analysed |
| Ozdemir L, Karabulut E. Nurse education regarding agitated patients and its effects on clinical practice. Contemp Nurse 2009;34:119–28 | No participants with dementia/not separately analysed |
| Padberg F, Stubner S, Buch K, Boetsch T, Ehrhardt T, Moller HJ, et al. Current therapeutic strategies in Alzheimer’s dementia. Medizinische Welt 1999;50:105–13 | Not primary research |
| Padovani A, Agosti C, Premi E, Bellelli G, Borroni B. Extrapyramidal symptoms in frontotemporal dementia: prevalence and clinical correlations. Neurosci Lett 2007;422:39–42 | Not intervention study |
| Paleacu D, Mazeh D, Mirecki I, Even M, Barak Y. Donepezil for the treatment of behavioral symptoms in patients with Alzheimer’s disease. Clin Neuropharmacol 2002;25:313–17 | Not a psychological, behavioural, sensory or environmental intervention |
| Palijan TZ, Radeljak S, Kovac M, Kovacevic D. Relationship between comorbidity and violence risk assessment in forensic psychiatry – the implicaton of neuroimaging studies. Psychiatr Danub 2010;22:253–6 | No participants with dementia/not separately analysed |
| Palmstierna T, Wistedt B. Staff Observation Aggression Scale, SOAS – presentation and evaluation. Acta Psychiatrica Scand 1987;76:657–63 | No participants with dementia/not separately analysed |
| Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol 2009;66:435–40 | Not primary research |
| Pancrazi MP, Metais P. Clinical characteristics. Presse Med 2003;32:742–9 | No participants with dementia/not separately analysed |
| Pancrazi MP, Metais P. Treatment of the psychological and behavioural disorders of Alzheimer’s disease. Presse Med 2005;34:667–72 | Not primary research |
| Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma-Injury Infect Crit Care 2008;65:34–41 | No participants with dementia/not separately analysed |
| Paniagua MA, Paniagua EW. The demented elder with insomnia. Clin Geriatr Med 2008;24:69 | Not primary research |
| Panov A, Kubalik N, Brooks BR, Shaw CA. In vitro effects of cholesterol beta-d-glucoside, cholesterol and cycad phytosterol glucosides on respiration and reactive oxygen species generation in brain mitochondria. J Membr Biol 2010;237:71–7 | No participants with dementia/not separately analysed |
| Papageorgiou C, Grapsa E, Christodoulou NG, Zerefos N, Stamatelopoulos S, Christodoulou GN. Association of serum nitric oxide levels with depressive symptoms: a study with end-stage renal failure patients. Psychother Psychosomat 2001;70:216–20 | No participants with dementia/not separately analysed |
| Pariel-Madjlessi S, Madjlessi A, Fremont P, Belmin J. Typology of the old patients hospitalised in psychiatry: importance of the psychiatric antecedents before the age of 60 years. Encephale Rev Psychiatr Clin Biol Therap 2001;27:423–8 | Not intervention study |
| Park H. Effect of music on pain for home-dwelling persons with dementia. Pain Manag Nurs 2010;11:141–7 | No outcome measuring agitation |
| Parke B, Beaith A, Slater L, Clarke AM. Contextual factors influencing success or failure of emergency department interventions for cognitively impaired older people: a scoping and integrative review. J Adv Nurs 2011;67:1426–48 | No participants with dementia/not separately analysed |
| Parnetti L, Brooks JO, Pippi M, Caputo N, Chionne F, Senin U, et al. Diagnosing Alzheimer’s disease in very elderly patients – Relevance of some functional and psychobehavioral aspects assessed by the Gottfries-Brane-Steen Rating Scale for dementia. Gerontology 1997;43:335–42 | No participants with dementia/not separately analysed |
| Pasman HRW, Onwuteaka-Philipsen BD, Kriegsman DMW, Onms ME, Ribbe MW, Van der Wal G. Discomfort in nursing home patients with severe dementia in whom artificial nutrition and hydration is forgone. Arch Intern Med 2005;165:1729–35 | Not intervention study |
| Pasman HRW, Onwuteaka-Philipsen BD, Kriegsman DMW, Ooms ME, Ribbe MW, Van der Wal G. [Degree of discomfort following the decision to discontinue artificial nutrition and hydration in institutionalised psychogeriatric patients with severe dementia who no longer or scarcely eat or drink.] Ned Tijdschr Geneeskd 2006;150:243–8 | Not intervention study |
| Pasman HRW, Onwuteaka-Philipsen BD, Kriegsman DMW, Ooms ME, van der Wall G, Ribbe MW. Predictors of survival in nursing home patients with severe dementia in whom artificial nutrition and hydration forgone. Int Psychogeriatr 2006;18:227–40 | Not intervention study |
| Pasquier F, Lebert F, Lavenu I, Guillaume B. The clinical picture of frontotemporal dementia: diagnosis and follow-up. Dementia Geriatr Cogn Disord 1999;10:10–14 | Not intervention study |
| Passmore MJ, Gardner DM, Polak Y, Rabheru K. Alternatives to atypical antipsychotics for the management of dementia-related agitation. Drugs Aging 2008;25:381–98 | Not primary research |
| Patat A, Alberini H, Bonhomme D, Soubrane C, Allain H, Gandon JM. Effects of tiapride on electroencephalograms and cognitive functions in the elderly. Int Clin Psychopharmacol 1999;14:199–208 | No participants with dementia/not separately analysed |
| Peak JS, Cheston RIL. Using simulated presence therapy with people with dementia. Aging Mental Health 2002;6:77–81 | No comparator |
| Pelton GH, Devanand DP, Bell K, Marder K, Marston K, Liu XH, et al. Usefulness of plasma haloperidol levels for monitoring clinical efficacy and side effects in Alzheimer patients with psychosis and behavioral dyscontrol. Am J Geriatr Psychiatry 2003;11:186–93 | Not a psychological, behavioural, sensory or environmental intervention |
| Pepersack T. [End of life of demented patients: ethical aspects.] Revue Med Brux 2010;31:333–41 | Not primary research |
| Perls TT, Herget M. Higher Respiratory-infection rates on an Alzheimers special care unit and successful intervention. J Am Geriatr Soc 1995;43:1341–4 | Not intervention study |
| Perneczky R. The therapy of frontotemporal dementia. Zeitschr Psychiatr Psychol Psychotherap 2008;56:47–9 | Not primary research |
| Perri R, Koch G, Carlesimo GA, Serra L, Fadda L, Pasqualetti P, et al. Alzheimer’s disease and frontal variant of frontotemporal dementia – a very brief battery for cognitive and behavioural distinction. J Neurol 2005;252:1238–44 | No participants with dementia/not separately analysed |
| Peskind ER, Tsuang DW, Bonner LT, Pascualy M, Riekse RG, Snowden MB, et al. Propranolol for disruptive behaviors in nursing home residents with probable or possible Alzheimer disease – a placebo-controlled study. Alzheimer Dis Assoc Disord 2005;19:23–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Peters KR, Rockwood K, Black SE, Bouchard R, Gauthier S, Hogan D, et al. Characterizing neuropsychiatric symptoms in subjects referred to dementia clinics. Neurology 2006;66:523–8 | Not intervention study |
| Peters KR, Rockwood K, Black SE, Hogan DB, Gauthier SG, Loy-English I, et al. Neuropsychiatric symptom clusters and functional disability in cognitively-impaired-not-demented individuals. Am J Geriatr Psychiatry 2008;16:136–44 | No participants with dementia/not separately analysed |
| Petersen RC. Aging, mild cognitive impairment, and Alzheimer’s disease. Neurol Clin 2000;18:789 | Not primary research |
| Peters-Libeu CA, Newhouse Y, Hall SC, Witkowska H, Weisgraber KH. Apolipoprotein E center dot dipalmitoylphosphatidylcholine particles are ellipsoidal in solution. J Lipid Res 2007;48:1035–44 | No participants with dementia/not separately analysed |
| Petkova AT, Yau WM, Tycko R. Experimental constraints on quaternary structure in Alzheimer’s beta-amyloid fibrils. Biochemistry 2006;45:498–512 | No participants with dementia/not separately analysed |
| Petrie WM, Ban TA. Psycho-pharmacology for the elderly. Prog Neuro-Psychopharmacol 1981;5:335–42 | Not a psychological, behavioural, sensory or environmental intervention |
| Petrovic M, Hurt C, Collins D, Burns A, Camus V, Liperoti R, et al. Clustering of behavioural and psychological symptoms in dementia (BPSD): a European Alzheimer’s disease consortium (EADC) study. Acta Clinica Belgica 2007;62:426–32 | No participants with dementia/not separately analysed |
| Philipsen M, Rosenbeck-Hansen JV, Waldemar G. [Behavioral disorders in nursing home residents. 147 consecutive referrals to an interdisciplinary team of consulting specialists.] Ugeskr Laeger 1999;161:5915–19 | Not intervention study |
| Phillips C, Polakoff D, Maue SK, Mauch R. Assessment of constipation management in long-term care patients. J Am Med Direct Assoc 2001;2:149–54 | No participants with dementia/not separately analysed |
| Phillips LR. Abuse of aging caregivers – test of a nursing intervention. Adv Nurs Sci 2008;31:164–81 | No participants with dementia/not separately analysed |
| Phillips VL, Diwan S, Egner A. Development of a tool for assessment and care planning for dementia-related problem behaviors in home and community-based services programs: the Problem Behavior Inventory. Home Health Care Serv Q 2002;21:29–45 | No participants with dementia/not separately analysed |
| Piani A, Brotini S, Dolso P, Budai R, Gigli GL. Sleep disturbances in elderly: a subjective evaluation over 65. Arch Gerontol Geriatr 2004;325–31 | Not intervention study |
| Picardi A, Pasquini P, Abeni D, Fassone G, Mazzotti E, Fava GA. Psychosomatic assessment of skin diseases in clinical practice. Psychother Psychosomat 2005;74:315–22 | No participants with dementia/not separately analysed |
| Pieper MJC, Achterberg WP, Francke AL, van der Steen JT, Scherder EJA, Kovach CR. The implementation of the serial trial intervention for pain and challenging behaviour in advanced dementia patients (STA OP!): a clustered randomized controlled trial. BMC Geriatr 2011;11:12 | Protocol only |
| Pinheiro D. Anticonvulsant mood stabilizers in the treatment of behavioral and psychological symptoms of dementia (BPSD). Encephale Rev Psychiatr Clin Biol Therap 2008;34:409–15 | Not primary research |
| Pirttila T, Wilcock G, Truyen L, Damaraju CV. Long-term efficacy and safety of galantamine in patients with mild-to-moderate Alzheimer’s disease: multicenter trial. Eur J Neurol 2004;11:734–41 | Not a psychological, behavioural, sensory or environmental intervention |
| Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Behavioral symptoms and the administration of psychotropic drugs to aged patients with dementia in nursing homes and in acute geriatric wards. Int Psychogeriatr 2004;16:61–74 | Not intervention study |
| Plosker GL, Gauthier S. Cerebrolysin: a review of its use in dementia. Drugs Aging 2009;26:893–915 | Not primary research |
| Politis AM, Vozzella S, Mayer LS, Onyike CU, Baker AS, Lyketsos CG. A randomized, controlled, clinical trial of activity therapy for apathy in patients with dementia residing in long-term care. Int J Geriatr Psychiatry 2004;19:1087–94 | No outcome measuring agitation |
| Pollak P. Psychic disorders. Revue Neurologique 2002;158:S125–31 | No participants with dementia/not separately analysed |
| Pollero A, Gimenez M, Allegri RF, Taragano FE. [Neuropsychiatric symptoms in patients with Alzheimer disease.] Vertex (Buenos Aires, Argentina) 2004;15:5–9 | Not intervention study |
| Pollock BG, Mulsant BH, Rosen J, Mazumdar S, Blakesley RE, Houck PR, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry 2007;15:942–52 | Not a psychological, behavioural, sensory or environmental intervention |
| Pollock BG, Mulsant BH, Rosen J, Sweet RA, Mazumdar S, Bharucha A, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry 2002;159:460–5 | Not a psychological, behavioural, sensory or environmental intervention |
| Pollock BG, Mulsant BH. Behavioral disturbances of dementia. J Geriatr Psychiatry Neurol 1998;11:206–12 | Not primary research |
| Pontius AA. Fastest fight/flight reaction via amygdalar visual pathway implicates simple face drawing as its marker: neuroscientific data consistent with neuropsychological findings. Aggression Violent Behav 2005;10:363–73 | No participants with dementia/not separately analysed |
| Porsteinsson AP, Tariot PN, Erb R, Cox C, Smith E, Jakimovich L, et al. Placebo-controlled study of divalproex sodium for agitation in dementia. Am J Geriatr Psychiatry 2001;9:58–66 | Not a psychological, behavioural, sensory or environmental intervention |
| Porsteinsson AP, Tariot PN, Jakimovich LJ, Kowalski N, Holt C, Erb R, et al. Valproate therapy for agitation in dementia – open-label extension of a double-blind trial. Am J Geriatr Psychiatry 2003;11:434–40 | Not a psychological, behavioural, sensory or environmental intervention |
| Porsteinsson AP. Divalproex sodium for the treatment of behavioural problems associated with dementia in the elderly. Drugs Aging 2006;23:877–86 | Not primary research |
| Pot AM, van Dyck R, Jonker C, Deeg DJH. Verbal and physical aggression against demented elderly by informal caregivers in the Netherlands. Soc Psychiatry Psychiatr Epidemiol 1996;31:156–62 | Not intervention study |
| Priano L, Gasco MR, Mauro A. Transdermal treatment options for neurological disorders – impact on the elderly. Drugs Aging 2006;23:357–75 | No participants with dementia/not separately analysed |
| Price JD, Hermans DG, Grimley EJ. Subjective barriers to prevent wandering of cognitively impaired people. Cochrane Database Syst Rev 2000;4:CD001932 | Not primary research |
| Pritchard A, Harris J, Pritchard C, Coates J, Haque S, Holder R, et al. The effect of the apolipoprotein E gene polymorphisms and haplotypes on behavioural and psychological symptoms in probable Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2007;78:123–6 | No participants with dementia/not separately analysed |
| Pritchard AL, Harris J, Pritchard CW, Coates J, Haque S, Holder R, et al. Role of 5HT(2A) and 5HT(2C) polymorphisms in behavioural and psychological symptoms of Alzheimer’s disease. Neurobiol Aging 2008;29:341–7 | Not primary research |
| Pritchard AL, Pritchard CW, Bentham P, Lendon CL. Investigation of the role of the dopamine transporter in susceptibility to behavioural and psychological symptoms of patients with probable Alzheimer’s disease. Dementia Geriatr Cogn Disord 2008;26:257–60 | Not intervention study |
| Pritchard AL, Pritchard CW, Bentham P, Lendon CL. Role of serotonin transporter Polymorphisms in the behavioural and psychological symptoms in probable Alzheimer disease patients. Dementia Geriatr Cogn Disord 2007;24:201–6 | Not intervention study |
| Pritchard AL, Ratcliffe L, Sorour E, Haque S, Holder R, Bentham P, et al. Investigation of dopamine receptors in susceptibility to behavioural and psychological symptoms in Alzheimer’s disease. Int J Geriatr Psychiatry 2009;24:1020–5 | Not intervention study |
| Profenno L, Loy R, Ryan M, Tariot P, Federoff H, Coleman P. Preliminary expression analysis of valproate-regulated serum proteins in Alzheimer’s disease using SELDI protein chips. Soc Neurosci Abstracts 2001;27:2567 | No participants with dementia/not separately analysed |
| Profenno LA, Jakimovich L, Holt CJ, Porsteinsson A, Tariot PN. A randomized, double-blind, placebo-controlled pilot trial of safety and tolerability of two doses of divalproex sodium in outpatients with probable Alzheimer’s disease. Curr Alzheimer Res 2005;2:553–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Profenno LA, Tariot PN. Pharmacologic management of agitation in Alzheimer’s disease. Dementia Geriatr Cogn Disord 2004;17:65–77 | Not primary research |
| Proitsi P, Hamilton G, Tsolaki M, Lupton M, Daniilidou M, Hollingworth P, et al. A Multiple Indicators Multiple Causes (MIMIC) model of Behavioural and Psychological Symptoms in Dementia (BPSD). Neurobiol Aging 2011;32:434–42 | No participants with dementia/not separately analysed |
| Pugh PL, Ahmed SF, Smith MI, Upton N, Hunter AJ. A behavioural characterisation of the FVB/N mouse strain. Behav Brain Res 2004;155:283–9 | No participants with dementia/not separately analysed |
| Pugh PL, Richardson JC, Bate ST, Upton N, Sunter D. Non-cognitive behaviours in an APP/PS1 transgenic model of Alzheimer’s disease. Behav Brain Res 2007;178:18–28 | No participants with dementia/not separately analysed |
| Pulsford D, Duxbury J. Aggressive behaviour by people with dementia in residential care settings: a review. J Psychiatr Mental Health Nurs 2006;13:611–18 | Not primary research |
| Rabinowicz AL, Starkstein SE, Leiguarda RC, Coleman AE. Transient epileptic amnesia in dementia: a treatable unrecognized cause of episodic amnestic wandering. Alzheimer Dis Assoc Disord 2000;14:231–3 | Not intervention study |
| Rabinowitz J, Katz I, De Deyn PP, Greenspan A, Brodaty H. Treating behavioral and psychological symptoms in patients with psychosis of Alzheimer’s disease using risperidone. Int Psychogeriatr 2007;19:227–40 | Not primary research |
| Rabins PV. Developing treatment guidelines for Alzheimer’s disease and other dementias. J Clin Psychiatry 1996;57:37–8 | Not primary research |
| Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio M, Gentile S, et al. Efficacy of music therapy treatment based on cycles of sessions: a randomised controlled trial. Aging Mental Health 2010;14:900–4 | No outcome measuring agitation |
| Ragneskog H, Asplund K, Kihlgren M, Norberg A. Individualized music played for agitated patients with dementia: analysis of video-recorded sessions. Int J Nurs Prac 2001;7:146–55 | No comparator |
| Ragneskog H, Kihlgren M, Karlsson I, Norberg A. Dinner music for demented patients: analysis of video-recorded observations. Clin Nurs Res 1996;5:262 | No outcome measuring agitation |
| Ragneskog H, Kihlgren M. Music and other strategies to improve the care of agitated patients with dementia – interviews with experienced staff. Scand J Caring Sci 1997;11:176–82 | Not intervention study |
| Rainer M, Anderle E, Masching A, Sepandj A, Haushofer M. Prevalence and management of non-cognitive symptoms of Alzheimer’s disease. Neuropsychiatrie 1996;10:161–3 | Not intervention study |
| Rainer M, Haushofer M, Pfolz H, Struhal C, Wick W. Quetiapine versus risperidone in elderly patients with behavioural and psychological symptoms of dementia: efficacy, safety and cognitive function. Eur Psychiatry 2007;22:395–403 | Not a psychological, behavioural, sensory or environmental intervention |
| Rainer MK, Mucke HAM, Kruger-Rainer C, Haushofer M, Kasper S. Zotepine for behavioural and psychological symptoms in dementia – an open-label study. CNS Drugs 2004;18:49–55 | Not a psychological, behavioural, sensory or environmental intervention |
| Ramadan FH, Naughton BJ, Bassanelli AG. Treatment of verbal agitation with a selective serotonin reuptake inhibitor. J Geriatr Psychiatry Neurol 2000;13:56–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Ranen NG, Peyser CE, Folstein SE. ECT as a treatment for depression in Huntingtons-Disease. J Neuropsychiatry Clin Neurosci 1994;6:154–9 | No participants with dementia/not separately analysed |
| Rao V, Rosenberg P, Bertrand M, Salehinia S, Spiro J, Vaishnavi S, et al. Aggression after traumatic brain injury: prevalence and correlates. J Neuropsychiatry Clin Neurosci 2009;21:420–9 | No participants with dementia/not separately analysed |
| Rapp M, Treusch Y, Heinz A, Gutzmann H. “Challenging behavior in dementia” in nursing homes: evaluation of a project tandem nursing and physician guides prepared VIDEANT. Z Gerontol Geriatr 2010;43:71 | Conference Presentation Only |
| Rappaport SA, Marcus RN, Manos G, McQuade RD, Oren DA. A randomized, double-blind, placebo-controlled tolerability study of intramuscular aripiprazole in acutely agitated patients with Alzheimer’s, vascular, or mixed dementia. J Am Med Direct Assoc 2009;10:21–7 | Not a psychological, behavioural, sensory or environmental intervention |
| Raskind MA, Peskind ER. Alzheimer’s disease and related disorders. Med Clin North Am 2001;85:803 | Not primary research |
| Raskind MA, Peskind ER. Neurobiological bases of noncognitive behavioral problems in Alzheimer disease. Alzheimer Dis Assoc Disord 1994;8:54–60 | No participants with dementia/not separately analysed |
| Raskind MA, Sadowsky CH, Sigmund WR, Beitler PJ, Auster SB. Effect of tacrine on language, praxis, and noncognitive behavioral problems in Alzheimer disease. Arch Neurol 1997;54:836–40 | Not primary research |
| Raskind MA. Evaluation and management of aggressive behavior in the elderly demented patient. J Clin Psychiatry 1999;60:45–9 | Not primary research |
| Raskind MA. Psychopharmacology of noncognitive abnormal behaviors in Alzheimer’s disease. J Clin Psychiatry 1998;59:28–32 | Not primary research |
| Ratey JJ, Gutheil CM. The measurement of aggressive behavior: reflections on the use of the Overt Aggression Scale and the Modified Overt Aggression Scale. J Neuropsychiatry Clin Neurosci 1991;3:S57–60 | No participants with dementia/not separately analysed |
| Ravona-Springer R, Beeri MS, Goldbourt U. Repetitive thinking as a psychological cognitive style in midlife is associated with lower risk for dementia three decades later. Dementia Geriatr Cogn Disord 2009;28:513–20 | No participants with dementia/not separately analysed |
| Rayner AV, O’Brien JG, Shoenbachler B. Behavior disorders of dementia: recognition and treatment. Am Fam Phys 2006;73:647–52 | Not primary research |
| Reichman WE. Alzheimer’s disease: Clinical treatment options. Am J Manag Care 2000;6:S1125–38 | Not primary research |
| Reilly DF, McNeely MJ, Doerner D, Greenberg DL, Staiger TO, Geist MJ, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med 1999;159:2185–92 | No participants with dementia/not separately analysed |
| Reimer MA, Slaughter S, Donaldson C, Currie G, Eliasziw M. Special care facility compared with traditional environments for dementia care: a longitudinal study of quality of life. J Am Geriatr Soc 2004;52:1085–92 | Not intervention study |
| Reitz C, den Heijer T, van Duijn C, Hofman A, Breteler M. Relation between smoking and risk of dementia and Alzheimer disease – the Rotterdam Study. Neurology 2007;69:998–1005 | No participants with dementia/not separately analysed |
| Renwick S, Fox C, Edwards C. Operationalising anger and aggression in mental health settings. The application of validated theories of anger to interventions for people with dementia. Neurobiol Aging 2002;23:S545 | Not intervention study |
| Rhodes-Kropf J, Cheng H, Castillo EH, Fulton AT. Managing the patient with dementia in long-term care. Clin Geriatr Med 2011;27:135 | Not primary research |
| Rhodes-Kropf J, Lantz MS. Alternative medicine – achieving balance between herbal remedies and medical therapy. Geriatrics 2001;56:44 | Not a psychological, behavioural, sensory or environmental intervention |
| Rhymes JA, McCullough LB, Luchi RJ, Teasdale TA, Wilson N. Withdrawing very low-burden interventions in chronically ill patients. JAMA 2000;283:1061–3 | Not primary research |
| Rialle V, Ollivet C, Guigui C, Herve C. What do family caregivers of Alzheimer’s disease patients desire in smart home technologies? Methods Inform Med 2008;47:63–9 | No participants with dementia/not separately analysed |
| Richeson NE, Neill DJ. Therapeutic recreation music intervention to decrease mealtime agitation and increase. Am J Recreation Ther 2004;3:37–41 | No quantitative outcome |
| Richeson NE. Effects of animal-assisted therapy on agitated behaviors and social interactions of older adults with dementia. Am J Alzheimers Dis Other Demen 2003;18:353–8 | No outcome measuring agitation |
| Richter JM, Roberto KA, Bottenberg DJ. Communicating with persons with Alzheimers disease – experiences of family and formal caregivers. Arch Psychiatr Nurs 1995;9:279–85 | Not intervention study |
| Richy F, Makaroff L, Pietri G, Moorcroft E, Winkelman J. Restless Legs Syndrome (RLS) and Subsequent Cardiovascular (CV) Risk in a US observational setting. Neurology 2011;76:A549 | No participants with dementia/not separately analysed |
| Riemersma-van der Lek R, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities – a randomized controlled trial. JAMA 2008;299:2642–55 | No participants with dementia/not separately analysed |
| Rigaud AS, Pino M, Wu YH, De Rotrou J, Boulay M, Seux ML, et al. Support for patients with Alzheimer’s disease and their caregivers by gerontechnology. Geriatr Psychol Neuropsychiatr Vieil 2011;9:91–100 | Not primary research |
| Rikkert MGMO, Rigaud ASP. Melatonin in elderly patients with insomnia – a systematic review. Z Gerontol Geriatr 2001;34:491–7 | No participants with dementia/not separately analysed |
| Risse SC, Lampe TH, Cubberley L. Very low-dose neuroleptic treatment in 2 patients with agitation associated with Alzheimers disease. J Clin Psychiatry 1987;48:207–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Rivail L, Chipot C, Maigret B, Bestel I, Sicsic S, Tarek M. Large-scale molecular dynamics of a G protein-coupled receptor, the human 5-HT4 serotonin receptor, in a lipid bilayer. J Mol Structure-Theochem 2007;817:19–26 | No participants with dementia/not separately analysed |
| Rivas-Vazquez RA, Carrazana EJ, Rey GJ, Blais MA, Racher DA. Alzheimer’s disease: pharmacological treatment and management. Clin Neuropsychol 2000;14:93–109 | Not primary research |
| Riveravazquez AB, Noriegasanchez A, Ramirezgonzalez R, Martinezmaldonado M. Acute hypercalcemia in hemodialysis patients – distinction from dialysis dementia. Nephron 1980;25:243–6 | No participants with dementia/not separately analysed |
| Roberge RF. Agitation in elderly people – diagnostic and therapeutic approach. Can Fam Phys 1996;42:2392–8 | No participants with dementia/not separately analysed |
| Robert PH, Allain H. Clinical management of agitation in the elderly with tiapride. Eur Psychiatry 2001;16:42S-7S | Not primary research |
| Roberts C. The management of wandering in older people with dementia. J Clin Nurs 1999;8:322–3 | No quantitative outcome |
| Robinson L, Bamford C, Briel R, Spencer J, Whitty P. Improving patient-centered care for people with dementia in medical encounters: an educational intervention for old age psychiatrists. Int Psychogeriatr 2010;22:129–38 | No participants with dementia/not separately analysed |
| Robinson L, Brittain K, Lindsay S, Jackson D, Olivier P. Keeping In Touch Everyday (KITE) project: developing assistive technologies with people with dementia and their carers to promote independence. Int Psychogeriatr 2009;21:494–502 | Not intervention study |
| Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al. A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use. Health Technol Assess 2006;10(26) | Not primary research |
| Robinson L, Hutchings D, Corner L, Finch T, Hughes J, Brittain K, et al. Balancing rights and risks: conflicting perspectives in the management of wandering in dementia. Health Risk Soci 2007;9:389–406 | Not intervention study |
| Robinson L, Hutchings D, Dickinson H, Corner L, Beyer F, Finch T, et al. Effectiveness and acceptability of non-pharmacological interventions to reduce wandering in dementia: a systematic review. Int J Geriatr Psychiatry 2007;22:9–22 | Not primary research |
| Editorial Staff. Robotherapy in dementia care: a pilot project using artificial reality in dementia care. Can Nurs Home 2005;16:19–22 | No quantitative outcome |
| Rockwell E, Jackson E, Vilke G, Jeste DV. A study of delusions in a large cohort of Alzheimers disease patients. Am J Geriatr Psychiatry 1994;2:157–64 | Not intervention study |
| Rockwood K, Black SE, Robillard A, Lussier I. Potential treatment effects of donepezil not detected in Alzheimer’s disease clinical trials: a physician survey. Int J Geriatr Psychiatry 2004;19:954–60 | Not intervention study |
| Rockwood K, Dobbs AR, Rule BG, Howlett SE, Black WR. The impact of pacemaker implantation on cognitive-functioning in elderly patients. J Am Geriatr Soc 1992;40:142–6 | No participants with dementia/not separately analysed |
| Rockwood K, Moorhouse PK, Song X, MacKnight C, Gauthier S, Kertesz A, et al. Disease progression in vascular cognitive impairment: cognitive, functional and behavioural outcomes in the Consortium to Investigate Vascular Impairment of Cognition (CIVIC) cohort study. J Neurol Sci 2007;252:106–12 | Not intervention study |
| Rodda J, Morgan S, Walker Z. Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer’s disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine. Int Psychogeriatr 2009;21:813–24 | Not primary research |
| Roger M, Gerard D, Leger JM. Agitation in elderly: interest of tiapride. A review. Encephale Rev Psychiatr Clin Biol Therap 1998;24:462–8 | No participants with dementia/not separately analysed |
| Rohde A, Marneros A. Psychoses during puerperium – symptoms, course and long-term prognosis. Geburtshilfe Frauenheilkunde 1993;53:800–10 | No participants with dementia/not separately analysed |
| Rojas-Fernandez CH, Lanctot KL, Allen DD, MacKnight C. Pharmacotherapy of behavioral and psychological symptoms of dementia: time for a different paradigm? Pharmacotherapy 2001;21:74–102 | Not primary research |
| Rolland Y, Andrieu S, Cantet C, Morley JE, Thomas D, Nourhashemi F, et al. Wandering behavior and Alzheimer disease. The REAL.FR prospective study. Alzheimer Dis Assoc Disord 2007;21:31–8 | Not intervention study |
| Rolland Y, Payoux P, Lauwers-Cances V, Voisin T, Esquerre JP, Vellas B. A SPECT study of wandering behavior in Alzheimer’s disease. Int J Geriatr Psychiatry 2005;20:816–20 | Not intervention study |
| Rolland Y, Van Kan G, Hermabessiere S, Gerard S, Guyonnet-Gillette S, Vellas B. Descriptive study of nursing home residents from the REHPA network. J Nutr Health Aging 2009;13:679–83 | No participants with dementia/not separately analysed |
| Romero B, Wenz M. Results of a multimodal treatment programme for persons with dementia and family caregivers in the Alzheimer Therapy Centre Bad Aibling. Z Gerontol Geriatr 2002;35:118–28 | No outcome measuring agitation |
| Romero B, Wenz M. Self-maintenance therapy in Alzheimer’s disease. Neuropsychol Rehabil 2001;11:333–55 | Not a psychological, behavioural, sensory or environmental intervention |
| Rongve A, Boeve BF, Aarsland D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J Am Geriatr Soc 2010;58:480–6 | Not intervention study |
| Rosato-Siri M, Cattaneo A, Cherubini E. Nicotine-induced enhancement of synaptic plasticity at CA3-CA1 synapses requires GABAergic interneurons in adult anti-NGF mice. J Physiol 2006;576:361–77 | No participants with dementia/not separately analysed |
| Rose KM, Beck C, Tsai PF, Liem PH, Davila DG, Kleban M, et al. Sleep disturbances and nocturnal agitation behaviors in older adults with dementia. Sleep 2011;34:779–86 | Not intervention study |
| Rosen H, Swigar ME. Depression and normal pressure hydrocephalus – dilemma in neuropsychiatric differential-diagnosis. J Nervous Mental Dis 1976;163:35–40 | No participants with dementia/not separately analysed |
| Rosen I. Electroencephalography as a diagnostic tool in dementia. Dementia Geriatr Cogn Disord 1997;8:110–16 | No participants with dementia/not separately analysed |
| Rosen J, Bohon S, Gershon S. Antipsychotics in the elderly. Acta Psychiatrica Scand Suppl 1990;358:170–5 | No participants with dementia/not separately analysed |
| Rosen J, Mittal V, Mulsant BH, Degenholtz H, Castle N, Fox D. Educating the families of nursing home residents: a pilot study using a computer-based system. J Am Med Direct Assoc 2003;4:128–34 | No participants with dementia/not separately analysed |
| Rosen J, Mulsant BH, Kollar M, Kastango KB, Mazumdar S, Fox D. Mental health training for nursing home staff using computer-based interactive video: a 6-month randomized trial. J Am Med Direct Assoc 2002;3:291–6 | No participants with dementia/not separately analysed |
| Rosen T, Lachs MS, Bharucha AJ, Stevens SM, Teresi JA, Nebres F, et al. Resident-to-resident aggression in long-term care facilities: Insights from focus groups of nursing home residents and staff. J Am Geriatr Soc 2008;56:1398–408 | No participants with dementia/not separately analysed |
| Rosen T, Lachs MS, Pillemer K. Sexual aggression between residents in nursing homes: literature synthesis of an underrecognized problem. J Am Geriatr Soc 2010;58:1970–9 | Not primary research |
| Rosen T, Pillemer K, Lachs M. Resident-to-resident aggression in long-term care facilities: an understudied problem. Aggression Violent Behav 2008;13:77–87 | No participants with dementia/not separately analysed |
| Rosenheck RA, Leslie DL, Sindelar JL, Miller EA, Tariot PN, Dagerman KS, et al. Cost–benefit analysis of second-generation antipsychotics and placebo in a randomized trial of the treatment of psychosis and aggression in Alzheimer disease. Arch Gen Psychiatry 2007;64:1259–68 | Not a psychological, behavioural, sensory or environmental intervention |
| Rosenthal GE, Fortinsky RH. Differences in the treatment of patients with acute myocardial-infarction according to patient age. J Am Geriatr Soc 1994;42:826–32 | No participants with dementia/not separately analysed |
| Rosler M, Frey U. Influence of treatment with acetyl-cholinesterase inhibitors on psychopathological symptoms in Alzheimer’s disease. Fortschr Neurol Psychiatr 2002;70:78–83 | Not primary research |
| Roth DL, Stevens AB, Burgio LD, Burgio KL. Timed-event sequential analysis of agitation in nursing home residents during personal care interactions with nursing assistants. J Gerontol Series B 2002;57:461–8 | No participants with dementia/not separately analysed |
| Roth J. Huntington’s Disease. Ceska Slov Neurologie Neurochirurgie 2010;73:107–23 | No participants with dementia/not separately analysed |
| Rothman M, Dubin WR. Patients released after psychiatric commitment evaluation – comparison with the committed. J Clin Psychiatry 1982;43:90–3 | No participants with dementia/not separately analysed |
| Rott HD. Chorea huntington – demonstration of extrapyramidal muscular activity by M-mode ultrasound. Ultraschall in der Medizin 1986;7:193–4 | No participants with dementia/not separately analysed |
| Rouquet JP, Bezaury JP. Treatment of agitation in dementia – a comparative open trial of tiapride versus lorazepam. Semaine des Hopitaux 1984;60:3086–8 | No participants with dementia/not separately analysed |
| Rovner BW, Steele CD, Shmuely Y, Folstein MF. A randomized trial of dementia care in nursing homes. J Am Geriatr Soc 1996;44:7–13 | No outcome measuring agitation |
| Rowe MA, Vandeveer SS, Greenblum CA, List CN, Fernandez RM, Mixson NE, et al. Persons with dementia missing in the community: is it wandering or something unique? BMC Geriatr 2011;11:28 | Not intervention study |
| Rowland T, Depalma L. Current neuropharmacologic interventions for the management of brain injury agitation. Neurorehabilitation 1995;5:219–32 | No participants with dementia/not separately analysed |
| Rubin EH, Morris JC, Berg L. The progression of personality changes in senile dementia of the Alzheimer’s type. J Am Geriatr Soc 1987;35:723–5 | Not intervention study |
| Rummans TA, Lauterbach EC, Coffey CE, Royall DR, Cummings JL, Salloway S, et al. Pharmacologic efficacy in neuropsychiatry: a review of placebo-controlled treatment trials – a report of the ANPA committee on research. J Neuropsychiatry Clin Neurosci 1999;11:176–89 | No participants with dementia/not separately analysed |
| Rusanen M, Kivipelto M, Quesenberry CP, Zhou J, Whitmer RA. Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Intern Med 2010;170 | No participants with dementia/not separately analysed |
| Ruths S, Straand J, Nygaard HA, Bjorvatn B, Pallesen S. Effect of antipsychotic withdrawal on behavior and sleep/wake activity in nursing home residents with dementia: a randomized, placebo-controlled, double-blinded study the Bergen District Nursing Home study. J Am Geriatr Soc 2004;52:1737–43 | Not a psychological, behavioural, sensory or environmental intervention |
| Ryan JM. Pharmacologic approach to aggression in neuropsychiatric disorders. Semin Clin Neuropsychiatry 2000;5:238–49 | No participants with dementia/not separately analysed |
| Sachdev P, Kruk J. Restlessness: the anatomy of a neuropsychiatric symptom. Aus N Z J Psychiatry 1996;30:38–53 | No participants with dementia/not separately analysed |
| Sachs GS. A review of agitation in mental illness: Burden of illness and underlying pathology. J Clin Psychiatry 2006;67:5–12 | Not primary research |
| Sajatovic M, Ramsay E, Nanry K, Thompson T. Lamotrigine therapy in elderly patients with epilepsy, bipolar disorder or dementia. Int J Geriatr Psychiatry 2007;22:945–50 | Not primary research |
| Saletu B, Grunberger J, Anderer R. On brain protection of co-dergocrine mesylate (hydergine) against hypoxic hypoxidosis of different severity – double-blind placebo-controlled quantitative EEG and psychometric studies. Int J Clin Pharmacol Ther 1990;28:510–24 | No participants with dementia/not separately analysed |
| Saletu M, Grunberger J, Saletu B, Mader R. Accelerated remission of alcoholic organic brain-syndrome with Emd-21657 – double-blind clinical and psychometric trials. Arzneimittel-Forschung 1978;28–2:1525–7 | No participants with dementia/not separately analysed |
| Salvador MTG, Lopez CA, Lyketsos CG. Treatment of agitation in dementia patients. Med Clin 1999;113:592–7 | Not primary research |
| Salzman C, Jeste DV, Meyer RE, Cohen-Mansfield J, Cummings J, Grossberg GT, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry 2008;69:889–98 | Not intervention study |
| Salzman C. Psychiatric Medications for Older Adults: The Concise Guide. New York, NY: The Guildford Press: 2001 | No participants with dementia/not separately analysed |
| Sanchez-Barcelo E, Mediavilla M, Tan D, X, Reiter R. Clinical uses of melatonin: evaluation of human trials. Curr Med Chem 2010;17:2070–95 | Not primary research |
| Sander K, Bickel H, Horn C, Huntgeburth U, Poppert H, Sander D. Peripheral arterial disease: predictors and treatment, based on the two-year data of the INVADE study. Deutsche Medizinische Wochenschrift 2008;133:455–9 | No participants with dementia/not separately analysed |
| Sands LP, Xu H, Craig BA, Eng C, Covinsky KE. Predicting change in functional status over quarterly intervals for older adults enrolled in the PACE community-based long-term care program. Aging Clin Exp Res 2008;20:419–27 | No participants with dementia/not separately analysed |
| Sano M, Mackell JA, Ponton M, Ferreira P, Wilson J, Pawluczyk S, et al. The Spanish instrument protocol: design and implementation of a study to evaluate treatment efficacy instruments for Spanish-speaking patients with Alzheimer’s disease. Alzheimer Dis Assoc Disord 1997;11:S57–64 | Protocol only |
| Sasaki M, Dawson VL, Dawson TM. The NO signaling pathway in the brain: neural injury, neurological disorders, and aggression. Contemporary Neuroscience Cerebral signal transduction: from first to fourth messengers 2000;151–74 | No participants with dementia/not separately analysed |
| Areosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005;3:CD003154 | Not primary research |
| Sato S, Mizukami K, Moro K, Tanaka Y, Asada T. Efficacy of perospirone in the management of aggressive behavior associated with dementia. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:679–83 | Not a psychological, behavioural, sensory or environmental intervention |
| Sattar SP, Padala PR, McArthur-Miller D, Roccaforte WH, Wengel SP, Burke WJ. Impact of problem alcohol use on patient behavior and caregiver burden in a geriatric assessment clinic. J Geriatr Psychiatry Neurol 2007;20:120–7 | Not intervention study |
| Saunders K, Brain S, Ebmeier KP. Diagnosing and managing psychosis in primary care. Practitioner 2011;255:17 | No participants with dementia/not separately analysed |
| Savage T, Crawford I, Nashed Y. Decreasing assault occurrence on a psychogeriatric ward: an agitation management model. J Gerontol Nurs 2004;30:30–7 | No participants with dementia/not separately analysed |
| Savaskan E, Schnitzler C, Schroeder C, Cajochen C, Mueller-Spahn F, Wirz-Justice A. Treatment of behavioural, cognitive and circadian rest-activity cycle disturbances in Alzheimer’s disease: haloperidol vs. quetiapine. Int J Neuropsychopharmacol 2006;9:507–16 | Not a psychological, behavioural, sensory or environmental intervention |
| Scarmeas N, Brandt J, Blacker D, Albert M, Hadjigeorgiou G, Dubois B, et al. Disruptive behavior as a predictor in Alzheimer disease. Arch Neurol 2007;64:1755–61 | Not intervention study |
| Schaefer PW. Diffusion-weighted imaging as a problem-solving tool in the evaluation of patients with acute strokelike syndromes. TMRI 2000;11:300–9 | No participants with dementia/not separately analysed |
| Scheifes A, Stolker J, Egberts A, Nijman H, Heerdink E. Representation of people with intellectual disabilities in randomised controlled trials on antipsychotic treatment for behavioural problems. J Intellect Disabil Res 2011;55:650–64 | No participants with dementia/not separately analysed |
| Schmidt R, Assem-Hilger E, Benke T, Dal Bianco P, Delazer M, Ladurner G, et al. Sex differences in Alzheimer disease. Neuropsychiatrie 2008;22:1–15 | Not primary research |
| Schmitt JAJ, Wingen M, Ramaekers JG, Evers EAT, Riedel WJ. Serotonin and human cognitive performance. Curr Pharm Design 2006;12:2473–86 | No participants with dementia/not separately analysed |
| Schmitz WD, Brenner AB, Bronson JJ, Ditta JL, Griffin CR, Li YW, et al. 5-Arylamino-1,2,4-triazin-6(1H)-one CRF(1) receptor antagonists. Bioorgan Med Chem Lett 2010;20:3579–83 | No participants with dementia/not separately analysed |
| Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14:191–210 | Not primary research |
| Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia – meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934–43 | Not primary research |
| Schneider LS, Dagerman KS. Psychosis of Alzheimer’s disease: clinical characteristics and history. J Psychiatr Res 2004;38:105–11 | Not primary research |
| Schneider LS, Ismail MS, Dagerman K, Davis S, Olin J, McManus D, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE): Alzheimer’s disease trial. Schizophrenia Bull 2003;29:57–72 | Protocol only |
| Schneider LS, Katz IR, Park S, Napolitano J, Martinez RA, Azen SP. Psychosis of Alzheimer disease – validity of the construct and response to risperidone. Am J Geriatr Psychiatry 2003;11:414–25 | Not primary research |
| Schneider LS, Pollock VE, Lyness SA. A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc 1990;38:553–63 | Not primary research |
| Schneider LS, Pollock VE, Zemansky MF, Gleason RP, Palmer R, Sloane RB. A pilot study of low-dose L-deprenyl in Alzheimer’s disease. J Geriatr Psychiatry Neurol 1991;4:143–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail M, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525–38 | Not a psychological, behavioural, sensory or environmental intervention |
| Schneider LS, Tariot PN, Lyketsos CG, Dagerman KS, Davis KL, Davis S, et al. National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) – Alzheimer disease trial methodology. Am J Geriatr Psychiatry 2001;9:346–60 | Protocol only |
| Schneider LS. Efficacy of treatment for geropsychiatric patients with severe mental illness. Psychopharmacol Bull 1993;29:501–24 | Not primary research |
| Schoenmakers B, Buntinx F, De Lepeleire J. Can pharmacological treatment of behavioural disturbances in elderly patients with dementia lower the burden of their family caregiver? Fam Prac 2009;26:279–86 | No participants with dementia/not separately analysed |
| Schonfeld L, King-Kallimanis B, Brown LM, Davis DM, Kearns WD, Molinari VA, et al. Wanderers with cognitive impairment in department of veterans affairs nursing home care units. J Am Geriatr Soc 2007;55:692–9 | Not intervention study |
| Schreinzer D, Ballaban T, Brannath W, Lang T, Hilger E, Fasching P, et al. Components of behavioral pathology in dementia. Int J Geriatr Psychiatry 2005;20:137–45 | No participants with dementia/not separately analysed |
| Schrijnemaekers VJ, van Rossum E, Candel M, Frederiks CM, Derix MM, Sielhorst H, et al. [Effects of emotion-oriented care on elderly people with cognitive impairment and behavioral problems in residential homes.] Tijdschr Gerontol Geriatr 2003;34:151–61 | No participants with dementia/not separately analysed |
| Schuck S, Allain H, Bentue-Ferrer D, Gerard D. Double blind study of tiapride versus haloperidol and placebo in agitation and aggressiveness in elderly patients with cognitive impairment. Fund Clin Pharmacol 2000;14:286 | Not a psychological, behavioural, sensory or environmental intervention |
| Schulte-Herbrueggen O, Heuser I. Affective and behavioral disorders in dementia: diagnosis and treatment. Nervenheilkunde 2007;26:663 | Not primary research |
| Scripnikov A, Khomenko A, Napryeyenko O. Effects of Ginkgo biloba extract EGb 761 on neuropsychiatric symptoms of dementia: findings from a randomised controlled trial. Wiener medizinische Wochenschrift (1946) 2007;157:295–300 | Not a psychological, behavioural, sensory or environmental intervention |
| Segatore M, Adams D. Managing delirium and agitation in elderly hospitalized orthopaedic patients: part 2--interventions. Orthopaedic Nurs 2001;20:61 | No participants with dementia/not separately analysed |
| Seidl U, Lueken U, Voelker L, Re S, Becker S, Kruse A, et al. Non-cognitive symptoms and psychopharmacological treatment in demented nursing home residents. Fortschr Neurol Psychiatr 2007;75:720–7 | Not intervention study |
| Seidl UW, Re S, Voelker L, Marin R, Kruse A, Schrober J. Apathy and non-cognitive symptoms in a population of demented nursing home residents in Germany. Neurobiol Aging 2004;25:S338 | Not intervention study |
| Seitz D, Adunuri N, Gill S, Gruneir A, Herrmann N, Rochon P. Antidepressants for agitation and psychosis in dementia. Cochrane Database Syst Rev 2011;2 | Not primary research |
| Seki Y, Takahashi H, Aizawa Y. [Hemostatic evaluation of a patient with haloperidol-induced neuroleptic malignant syndrome associated with disseminated intravascular coagulation.] Rinsho ketsueki 1998;39:374–8. | Not intervention study |
| Selbaek G, Kirkevold O, Engedal K. The course of psychiatric and behavioral symptoms and the use of psychotropic medication in patients with dementia in Norwegian nursing homes – a 12-month follow-up study. Am J Geriatr Psychiatry 2008;16:528–36 | Not intervention study |
| Seow D, Gauthier S. Pharmacotherapy of Alzheimer disease. Can J Psychiatry 2007;52:620–9 | Not primary research |
| Serrano C, Martelli M, Harris P, Tufro G, Ranalli C, Taragano F, et al. Primary progressive aphasia: its clinical variability. An analysis of 15 cases. Rev Neurol 2005;41:527–33 | No participants with dementia/not separately analysed |
| Sevier S, Gorek B. Cognitive evaluation in care planning for people with Alzheimer disease and related dementias. Geriatr Nurs 2000;21(2):92–7 | Not primary research |
| Shadlen MF, Larson EB, Gibbons L, McCormick WC, Teri L. Alzheimer’s disease symptom severity in blacks and whites. J Am Geriatr Soc 1999;47:482–6 | Not intervention study |
| Shaffer DR, Dooley W, Williamson GM. Endorsement of proactively aggressive caregiving strategies moderates the relation between caregiver mental health and potentially harmful caregiving behavior. Psychol Aging 2007;22:494–504 | No participants with dementia/not separately analysed |
| Shah A, Evans H, Parkash N. Evaluation of three aggression/agitation behaviour rating scales for use on an acute admission and assessment psychogeriatric ward. Int J Geriatr Psychiatry 1998;13:415–20 | No participants with dementia/not separately analysed |
| Shankle WR, Nielson KA, Cotman CW. Low-dose propranolol reduces aggression and agitation resembling that associated with orbitofrontal dysfunction in elderly demented patients. Alzheimer Dis Assoc Disord 1995;9:233–7 | Not a psychological, behavioural, sensory or environmental intervention |
| Shannon KM, Moore CG. Sleep disruption in Huntington’s Disease: relationship to clinical disease features. Neurology 2001;56(Suppl. 3):A214 | No participants with dementia/not separately analysed |
| Shea TB. Effects of dietary supplementation with N-acetyl cysteine, acetyl-L-carnitine and S-adenosyl methionine on cognitive performance and aggression in normal mice and mice expressing human ApoE4. Neuromol Med 2007;9:264–9 | No participants with dementia/not separately analysed |
| Shega JW, Ellner L, Lau DT, Maxwell TL. Cholinesterase inhibitor and N-methyl-D-aspartic acid receptor antagonist use in older adults with end-stage dementia: a survey of hospice medical directors. J Palliative Med 2009;12:779–83 | No participants with dementia/not separately analysed |
| Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs GA. Management of noncancer pain in community-dwelling persons with dementia. J Am Geriatr Soc 2006;54:1892–7 | Not intervention study |
| Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs GA. Pain in community-dwelling persons with dementia: frequency, intensity, and congruence between patient and caregiver report. J Pain Symp Manag 2004;28:585–92 | Not intervention study |
| Shelton PS, Brooks VG. Estrogen for dementia-related aggression in elderly men. Ann Pharmacother 1999;33:808–12 | Not primary research |
| Shen WK, Hayes DL, Hammill SC, Bailey KR, Ballard DJ, Gersh BJ. Survival and functional independence after implantation of a permanent pacemaker in octogenarians and nonagenarians – a population-based study. Ann Intern Med 1996;125:476–80 | No participants with dementia/not separately analysed |
| Sherratt K, Thornton A, Hatton C. Music interventions for people with dementia: a review of the literature. Aging Mental Health 2004;8:3–12 | Not primary research |
| Shevitz S. Agitated patient. J Family Prac 1979;9:305–11 | Not primary research |
| Shiga Y, Saito H, Mochizuki H, Chida K, Tsuburaya K. [A case of adrenoleukodystrophy having progressed from the frontal lobes.] Rinsho shinkeigaku 1992;32:600–5 | No participants with dementia/not separately analysed |
| Shinno H, Inami Y, Inagaki T, Nakamura Y, Horiguchi J. Effect of Yi-Gan San on psychiatric symptoms and sleep structure at patients with behavioral and psychological symptoms of dementia. Prog Neuro-Psychopharmacol Biol Psychiatry 2008;32:881–5 | Not a psychological, behavioural, sensory or environmental intervention |
| Shinoda-Tagawa T, Leonard R, Pontikas J, McDonough JE, Allen D, Dreyer PI. Resident-to-resident violent incidents in nursing homes. JAMA 2004;291:591–8 | Not intervention study |
| Shua-Haim JR, Shua-Haim V, Ross JS. Combination therapy with donepezil and sertraline in the treatment of Alzheimer’s disease. Alzheimers Rep 1998;1:303–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Shub D, Ball V, Abbas AAA, Gottumukkala A, Kunik ME. The link between psychosis and aggression in persons with dementia: a systematic review. Psychiatr Q 2010;81:97–110 | Not primary research |
| Shuster JL. Palliative care for advanced dementia. Clin Geriatr Med 2000;16:373 | Not primary research |
| Siddique H, Hynan LS, Weiner MF. Effect of a serotonin reuptake inhibitor on irritability, apathy, and psychotic symptoms in patients with Alzheimer’s disease. J Clin Psychiatry 2009;70:915–18 | Not a psychological, behavioural, sensory or environmental intervention |
| Siders C, Nelson A, Brown LM, Joseph I, Algase D, Beattie E, et al. Evidence for implementing nonpharmacological interventions for wandering. Rehabil Nurs 2004;29:195–206 | Not primary research |
| Silver BV, Collins L, Zidek KA. Risperidone treatment of motor restlessness following anoxic brain injury. Brain Inj 2003;17:237–44 | No participants with dementia/not separately analysed |
| Simic G, Stanic G, Mladinov M, Jovanov-Milosevic N, Kostovic I, Hof P. Does Alzheimer’s disease begin in the brainstem? Neuropathol Appl Neurobiol 2009;35:532–54 | No participants with dementia/not separately analysed |
| Simuni T, Sethi K. Nonmotor manifestations of Parkinson’s disease. Ann Neurol 2008;64:S65–80 | No participants with dementia/not separately analysed |
| Sink KM, Covinsky KE, Newcomer R, Yaffe K. Ethnic differences in the prevalence and pattern of dementia-related behaviors. J Am Geriatr Soc 2004;52:1277–83 | Not intervention study |
| Sival RC, Albronda T, Haffmans PMJ, Saltet ML, Schellekens CMAM. Is aggressive behaviour influenced by the use of a behaviour rating scale in patients in a psychogeriatric nursing home? Int J Geriatr Psychiatry 2000;15:108–11 | No participants with dementia/not separately analysed |
| Sival RC, Duivenvoorden HJ, Jansen PAF, Haffmans PMJ, Duursma SA, Eikelenboom P. Sodium valproate in aggressive behaviour in dementia: a twelve-week open label follow-up study. Int J Geriatr Psychiatry 2004;19:305–12 | Not a psychological, behavioural, sensory or environmental intervention |
| Sival RC, Haffmans PMJ, Jansen PAF, Duursma SA, Eikelenboom P. Sodium valproate in the treatment of aggressive behavior in patients with dementia – a randomized placebo controlled clinical trial. Int J Geriatr Psychiatry 2002;17:579–85 | Not a psychological, behavioural, sensory or environmental intervention |
| Skjerve A, Bjorvatn B, Holsten F. Light therapy for behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry 2004;19:516–22 | Not primary research |
| Skocic M, Dujmovic J, Jevtovic S, Jakovljevic M. Premorbid combat related PTSD in Huntington’s disease case report. Psychiatria Danubina 2010;22:286–8 | No participants with dementia/not separately analysed |
| Skovdahl K, Sorlie V, Kihlgren M. Tactile stimulation associated with nursing care to individuals with dementia showing aggressive or restless tendencies: an intervention study in dementia care. Int J Older People Nurs 2007;2:162–70 | No comparator |
| Skrobik Y, Ahern S, Leblanc M, Marquis F, Awissi DK, Kavanagh BP. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anaesthes Analges 2010;111:451–63 | No participants with dementia/not separately analysed |
| Slachevsky A, Fuentes P. An update on the treatment of psychological and behavioral symptoms associated to dementia. Revista Medica de Chile 2005;133:1242–51 | Not primary research |
| Slimak K. Use of Tropical Root Crops in Dietary Intervention Strategies. Official Gazette of the United States Patent and Trademark Office Patents 2010 | No participants with dementia/not separately analysed |
| Slimak KM. Use of Tropical Root Crops in Effective Intervention Strategies for Treating Difficult and Complex Cases and Chronic Diseases. Official Gazette of the United States Patent and Trademark Office Patents 2003;1275(2) | No participants with dementia/not separately analysed |
| Sloane PD, Davidson S, Knight N, Tangen C, Mitchell CM. Severe disruptive vocalizers. J Am Geriatr Soc 1999;47:439–45 | Not intervention study |
| Sloane PD, Mitchell CM, Preisser JS, Phillips C, Commander C, Burker E. Environmental correlates of resident agitation in Alzheimer’s disease special care units. J Am Geriatr Soc 1998;46:862–9 | Not intervention study |
| Small DH, Maksel D, Kerr ML, Ng J, Hou X, Chu C, et al. The beta-amyloid protein of Alzheimer’s disease binds to membrane lipids but does not bind to the alpha 7 nicotinic acetylcholine receptor. J Neurochem 2007;101:1527–38 | No participants with dementia/not separately analysed |
| Small GW. Treatment of Alzheimer’s disease: Current approaches and promising developments. Am J Med 1998;104:32S–8S | Not primary research |
| Smallwood J, Irvine E, Coulter F, Connery H. Psychometric evaluation of a short observational tool for small-scale research projects in dementia. Int J Geriatr Psychiatry 2001;16:288–92 | No participants with dementia/not separately analysed |
| Smith DA, Perry PJ. Nonneuroleptic treatment of disruptive behavior in organic mental syndromes. Ann Pharmacother 1992;26:1400–8 | Not primary research |
| Smith G, Vigen V, Evans J, Fleming K, Bohac D. Patterns and associates of hyperphagia in patients with dementia. Neuropsychiatry Neuropsychol Behav Neurol 1998;11:97–102 | Not intervention study |
| Smith GC, Strain JJ, Hammer JS, Wallack JJ, Bialer PA, Schleifer SS, et al. Organic mental disorders in the consultation-liaison psychiatry setting – a multi-site study. Psychosomatics 1997;38:363–73 | No participants with dementia/not separately analysed |
| Smith H, Bruckenthal P. Implications of opioid analgesia for medically complicated patients. Drugs Aging 2010;27:417–33 | No participants with dementia/not separately analysed |
| Smith M, Buckwalter K. Behaviors associated with dementia. Am J Nurs 2005;105:40–52 | Not primary research |
| Smith-Jones SM, Francis GM. Disruptive, institutionalized elderly: a cost-effective intervention. J Psychosoc Nurs Mental Health Serv 1992;30:17–20 | No participants with dementia/not separately analysed |
| Snow AL, Dani R, Souchek J, Sullivan G, Ashton CM, Kunik ME. Comorbid psychosocial symptoms and quality of life in patients with dementia. Am J Geriatr Psychiatry 2005;13:393–401 | Not intervention study |
| Snow AL, Hovanec L, Brandt J. A controlled trial of aromatherapy for agitation in nursing home patients with dementia. J Alt Complement Med 2004;10:431–7 | No comparator |
| Snowdon J. Reporting changes in scores on the Cohen-Mansfield Agitation Inventory subscales. J Clin Psychiatry 2003;64:732 | No participants with dementia/not separately analysed |
| Snowdon J. The meaning of words. Int Psychogeriatr 2003;15:219–22 | No participants with dementia/not separately analysed |
| Snyder M, Tseng Y, Brandt C, Croghan C, Hanson S, Constantine R, et al. A glider swing intervention for people with dementia. Geriatr Nurs 2001;22:86–90 | Some participants < 50 years old |
| Snyder S, Strain JJ, Fulop G. Evaluation and treatment of mental disorders in patients with Aids. Comprehens Ther 1990;16:34–41 | No participants with dementia/not separately analysed |
| Sokol DK, Chen D, Farlow MR, Dunn DW, Maloney B, Zimmer JA, et al. High levels of Alzheimer beta-amyloid precursor protein (APP) in children with severely autistic behavior and aggression. J Child Neurol 2006;21(6):444–9 | No participants with dementia/not separately analysed |
| Sokol DK, Long J, Maloney B, Chen D, Lahiri DK. Potential Alzheimer’s disease markers for autism? Beta-amyloid precursor protein and acetylcholinesterase correlate with aggression in autism. Neurology 2009;72:A53 | No participants with dementia/not separately analysed |
| Sommer BR, Fenn HH, Ketter TA. Safety and efficacy of anticonvulsants in elderly patients with psychiatric disorders: oxcarbazepine, topiramate and gabapentin. Ex Opin Drug Saf 2007;6:133–45 | Not primary research |
| Sommer OH, Aga O, Cvancarova M, Olsen IC, Selbaek G, Engedal K. Effect of oxcarbazepine in the treatment of agitation and aggression in severe dementia. Dementia Geriatr Cogn Disord 2009;27:155–63 | Not a psychological, behavioural, sensory or environmental intervention |
| Song JA, Algase D. Premorbid characteristics and wandering behavior in persons with dementia. Arch Psychiatr Nurs 2008;22:318–27 | Not intervention study |
| Song JA, Algase DL, Beattie ERA, Milke DL, Duffield C, Cowan B. Comparison of U.S., Canadian, and Australian participants’ performance on the Algase Wandering Scale-Version 2 (AWS-V2). Res Theory Nurs Prac 2003;17:241–56 | Not intervention study |
| Song JH. Effects of a robot pet-assisted program for elderly people with dementia. J Korean Acad Nurs 2009;39:562–73 | No outcome measuring agitation |
| Souder E, Heithoff K, O’Sullivan PS, Lancaster AE, Beck C. Identifying patterns of disruptive behavior in long-term care residents. J Am Geriatr Soc 1999;47:830–6 | Not intervention study |
| Sourial R, McCusker J, Cole M, Abrahamowicz M. Agitation in demented patients in an acute care hospital: Prevalence, disruptiveness, and staff burden. Int Psychogeriatr 2001;13:183–97 | Not intervention study |
| Spagnolo C, Dallasta D, Iannuccelli M, Cucinotta D, Passeri M. A controlled double-blind trial comparing etoperidone with thioridazine in the management of severe senile dementia. Drugs Exp Clin Res 1983;9:873–80 | Not a psychological, behavioural, sensory or environmental intervention |
| Spalletta G, Musicco M, Padovani A, Rozzini L, Perri R, Fadda L, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry 2010;18:1026–35 | Not intervention study |
| Sparks MB. Inpatient care for persons with Alzheimer’s disease. Crit Care Nurs Q 2008;31:65–72 | Not primary research |
| Spear J, Chawla S, O’Reilly M, Rock D. Does the HoNOS 65+ meet the criteria for a clinical outcome indicator for mental health services for older people? Int J Geriatr Psychiatry 2002;17:226–30 | No participants with dementia/not separately analysed |
| Speziale J, Black E, Coatsworth-Puspoky R, Ross T, O’Regan T, Ed C. Moving forward: evaluating a curriculum for managing responsive behaviours in a geriatric psychiatry inpatient population. Gerontologist 2009;49:570–6 | No participants with dementia/not separately analysed |
| Spira AP, Edelstein BA. Behavioral interventions for agitation in older adults with dementia: an evaluative review. Int Psychogeriatr 2006;18:195–225 | Not primary research |
| Spira AP, Edelstein BA. Operant conditioning in older adults with Alzheimer’s disease. Psychol Record 2007;57:409–27 | No outcome measuring agitation |
| Spiridigliozzi GA, Heller JH, Crissman BG, Sullivan-Saarela JA, Eells R, Dawson D, et al. Preliminary study of the safety and efficacy of donepezil hydrochloride in children with Down syndrome: a clinical report series. Am J Med Genet Part A 2007;143A:1408–13 | No participants with dementia/not separately analysed |
| Sposaro F, Danielson J, Tyson G. iWander: An Android application for dementia patients. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference 2010;2010:3875–8 | Not primary research |
| Squitieri F, Cannella M, Porcellini A, Brusa L, Simonelli M, Ruggieri S. Short-term effects of olanzapine in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol 2001;14:69–72 | No participants with dementia/not separately analysed |
| Srikanth S, Nagaraja A, Ratnavalli E. Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer’s disease, vascular dementia and frontotemporal dementia. J Neurol Sci 2005;236:43–8 | Not intervention study |
| Staekenborg SS, Su T, van Straaten EC, Lane R, Scheltens P, Barkhof F, et al. Behavioural and psychological symptoms in vascular dementia; differences between small- and large-vessel disease. J Neurol Neurosurg Psychiatry 2010;81:547–51 | Not intervention study |
| Starkstein SE, Jorge R, Mizrahi R, Robinson RG. The construct of minor and major depression in Alzheimer’s disease. Am J Psychiatry 2005;162:2086–93 | Not intervention study |
| Starkstein SE, Jorge R, Petracca G, Robinson RG. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry 2007;15:42–9 | Not intervention study |
| Starkstein SE, Mizrahi R, Garau L. Specificity of symptoms of depression in Alzheimer disease – a longitudinal analysis. Am J Geriatr Psychiatry 2005;13:802–7 | Not intervention study |
| Steele C, Lucas MJ, Tune L. Haloperidol versus thioridazine in the treatment of behavioral symptoms in senile dementia of the Alzheimers type – preliminary findings. J Clin Psychiatry 1986;47:310–12 | Not a psychological, behavioural, sensory or environmental intervention |
| Steers M, Feliciano L. A Behavioral intervention to reduce agitation in individuals with Down’s syndrome and dementia. Gerontologist 2009;49:395 | Conference Presentation Only |
| Steffens DC, Maytan M, Helms MJ, Plassman BL. Prevalence and clinical correlates of neuropsychiatric symptoms in dementia. Am J Alzheimers Dis Other Demen 2005;20:367–73 | Not intervention study |
| Steinberg M, Munro CA, Samus Q, Rabins PV, Brandt J, Lyketsos CG. Patient predictors of response to treatment of depression in Alzheimer’s disease: the DIADS study. Int J Geriatr Psychiatry 2004;19:144–50 | Not a psychological, behavioural, sensory or environmental intervention |
| Steinberg M, Sheppard JM, Tschanz JT, Norton MC, Steffens DC, Breitner JCS, et al. The incidence of mental and behavioral disturbances in dementia: the cache county study. J Neuropsychiatry Clin Neurosci 2003;15:340–5 | Not intervention study |
| Steinert T, Bergk J. Aggressive and violent behaviour. Diagnosis, prevention, and treatment. Nervenarzt 2008;79:359–68 | No participants with dementia/not separately analysed |
| Stelzner G, Riedel-Heller SG, Sonntag A, Matschinger H, Jakob A, Angermeyer MC. Determinants of psychotropic drug use in residential and nursing homes. Z Gerontol Geriatr 2001;34:306–12 | No participants with dementia/not separately analysed |
| Sterniczuk R, Antle MC, LaFerla FM, Dyck RH. Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: part 2. Behavioral and cognitive changes. Brain Res 2010;1348:149–55 | No participants with dementia/not separately analysed |
| Sterniczuk R, Dyck RH, LaFerla FM, Antle MC. Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: part 1. Circadian changes. Brain Res 2010;1348:139–48 | No participants with dementia/not separately analysed |
| Stevenson GS, Khan MA, Perumal N. A psychiatric intensive care unit for older adults: an interval comparison of admissions. Int Psychogeriatr 2009;21:278–85 | No participants with dementia/not separately analysed |
| Stewart R. Neuroimaging in dementia and depression. Curr Opin Psychiatry 2001;14:371–5 | Not primary research |
| Stoessl A. Potential therapeutic targets for Parkinson’s disease. Ex Opin Ther Targets 2008;12:425–36 | No participants with dementia/not separately analysed |
| Stoppe G, Brandt CA, Staedt JH. Behavioural problems associated with dementia – the role of newer antipsychotics. Drugs Aging 1999;14:41–54. | Not primary research |
| Stotsky B. Multicenter Study Comparing thioridazine with diazepam and placebo in elderly, nonpsychotic patients with emotional and behavioural disorders. Clin Ther 1984;6:546–59 | Not a psychological, behavioural, sensory or environmental intervention |
| Street JS, Clark WS, Gannon KS, Cummings JL, Bymaster FP, Tamura RN, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities – a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2000;57:968–76 | Not a psychological, behavioural, sensory or environmental intervention |
| Street JS, Clark WS, Kadam DL, Mitan SJ, Juliar BE, Feldman PD, et al. Long-term efficacy of olanzapine in the control of psychotic and behavioral symptoms in nursing home patients with Alzheimer’s dementia. Int J Geriatr Psychiatry 2001;16:S62–70 | Not a psychological, behavioural, sensory or environmental intervention |
| Streim JE, Porsteinsson AP, Breder CD, Swanink R, Marcus R, McQuade R, et al. A randomized, double-blind, placebo-controlled study of aripiprazole for the treatment of psychosis in nursing home patients with Alzheimer disease. Am J Geriatr Psychiatry 2008;16:537–50 | No outcome measuring agitation |
| Strydom A, Romeo R, Perez-Achiaga N, Livingston G, King M, Knapp M, et al. Service use and cost of mental disorder in older adults with intellectual disability. Br J Psychiatry 2010;196:133–8 | No participants with dementia/not separately analysed |
| Stubbs B, Yorston G, Knight C. Physical intervention to manage aggression in older adults: how often is it employed? Int Psychogeriatr 2008;20:855–7 | No participants with dementia/not separately analysed |
| Stubbs B. Physical intervention in older adult psychiatry: an audit of physical ailments identified by physiotherapists and the implications for managing aggressive behavior. Int Psychogeriatr 2009;21:1196–7 | No participants with dementia/not separately analysed |
| Suedfeld P, Borrie RA. Health and therapeutic applications of chamber and flotation restricted environmental stimulation therapy (REST). Psychol Health 1999;14:545–66 | Not primary research |
| Suh GH, Greenspan AJ, Choi SK. Comparative efficacy of risperidone versus haloperidol on behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry 2006;21:654–60 | Not a psychological, behavioural, sensory or environmental intervention |
| Suh GH, Kim JK, Cho MJ. Community study of dementia in the older Korean rural population. Aus N Z J Psychiatry 2003;37:606–12 | No participants with dementia/not separately analysed |
| Suh GH, Son HG, Ju YS, Jcho KH, Yeon BK, Shin YM, et al. A randomized, double-blind, crossover comparison of risperidone and haloperidol in Korean dementia patients with behavioral disturbances. Am J Geriatr Psychiatry 2004;12:509–16 | Not a psychological, behavioural, sensory or environmental intervention |
| Suh GH, Yeon BK, Shah A, Lee JY. Mortality in Alzheimer’s disease: a comparative prospective Korean study in the community and nursing homes. Int J Geriatr Psychiatry 2005;20:26–34 | Not intervention study |
| Suh GH. Agitated behaviours among the institutionalized elderly with dementia: validation of the Korean version of the Cohen-Mansfield Agitation Inventory. Int J Geriatr Psychiatry 2004;19:378–85 | No participants with dementia/not separately analysed |
| Suhr J, Anderson S, Tranel D. Progressive muscle relaxation in the management of behavioural disturbance in Alzheimer’s disease. Neuropsychol Rehabil 1999;9:31–44 | No outcome measuring agitation |
| Sultzer DL, Davis SM, Tariot PN, Dagerman KS, Lebowitz BD, Lyketsos CG, et al. Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: Phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry 2008;165:844–54 | Not a psychological, behavioural, sensory or environmental intervention |
| Sultzer DL, Gray KF, Gunay I, Berisford MA, Mahler ME. A double-blind comparison of trazodone and haloperidol for treatment of agitation in patients with dementia. Am J Geriatr Psychiatry 1997;5:60–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Sultzer DL, Gray KF, Gunay I, Wheatley MV, Mahler ME. Does behavioral improvement with haloperidol or trazodone treatment depend on psychosis or mood symptoms in patients with dementia? J Am Geriatr Soc 2001;49:1294–300 | Not a psychological, behavioural, sensory or environmental intervention |
| Sultzer DL, Levin HS, Mahler ME, High WM, Cummings JL. Assessment of cognitive, psychiatric, and behavioral disturbances in patients with dementia – the Neurobehavioral Rating-Scale. J Am Geriatr Soc 1992;40:549–55 | No participants with dementia/not separately analysed |
| Sultzer DL. Psychosis and antipsychotic medications in Alzheimer’s disease: clinical management and research perspectives. Dementia Geriatr Cogn Disord 2004;17:78–90 | Not primary research |
| Sultzer DL. Selective serotonin reuptake inhibitors and trazodone for treatment of depression, psychosis, and behavioral symptoms in patients with dementia. Int Psychogeriatr 2000;12:245–51 | Not a psychological, behavioural, sensory or environmental intervention |
| Sunderland T, Silver MA. Neuroleptics in the Treatment of Dementia. Int J Geriatr Psychiatry 1988;3:79–88 | Not a psychological, behavioural, sensory or environmental intervention |
| Sunderland T. Treatment of the elderly suffering from psychosis and dementia. J Clin Psychiatry 1996;57:53–6 | Not primary research |
| Sung HC, Chang AM. Use of preferred music to decrease agitated behaviours in older people with dementia: a review of the literature. J Clin Nurs 2005;14:1133–40 | Not primary research |
| Sutor B, Rummans TA, Smith GE. Assessment and management of behavioral disturbances in nursing home patients with dementia. Mayo Clin Proc 2001;76:540–50 | Not primary research |
| Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry 1997;58:212–16 | Not a psychological, behavioural, sensory or environmental intervention |
| Swearer JM, Hoople NE, Kane KJ, Drachman DA. Predicting aberrant behavior in Alzheimer’s disease. Neuropsychiatry Neuropsychol Behav Neurol 1996;9:162–70 | Not intervention study |
| Sweet RA, Nimgaonkar VL, Kamboh MI, Lopez OL, Zhang F, DeKosky ST. Dopamine receptor genetic variation, psychosis, and aggression in Alzheimer disease. Arch Neurol 1998;55:1335–40 | Not intervention study |
| Swerdlow RH, Khan SM. A “mitochondrial cascade hypthesis” for sporadic Alzheimer’s disease. Med Hypoth 2004;63:8–20 | No participants with dementia/not separately analysed |
| Swett C. Inpatient seclusion: description and causes. Bull Am Acad Psychiatry Law 1994;22:421–30 | No participants with dementia/not separately analysed |
| Swift RH, Harrigan EP, Cappelleri JC, Kramer D, Chandler LP. Validation of the behavioural activity rating scale (BARS)(TM): a novel measure of activity in agitated patients. J Psychiatr Res 2002;36:87–95 | No participants with dementia/not separately analysed |
| Szulik J. [Antipsychotics in geriatric institutions.] Vertex (Buenos Aires, Argentina) 2007;18:454–60 | Not primary research |
| Szymczynska P, Innes A. Evaluation of a dementia training workshop for health and social care staff in rural Scotland. Rural Remote Health 2011;11:1611 | No participants with dementia/not separately analysed |
| Tadaka E, Kanagawa K. Effects of reminiscence group in elderly people with Alzheimer disease and vascular dementia in a community setting. Geriatr Gerontol Int 2007;7:167–73 | No outcome measuring agitation |
| Taft LB, Barkin RL. Drug abuse? Use and misuse of psychotropic drugs in Alzheimer’s care. J Gerontol Nurs 1990;16:4–10 | Not primary research |
| Taft LB, Delaney K, Seman D, Stansell J. Dementia care creating a therapeutic milieu. J Gerontol Nurs 1993;19:30–9 | Not primary research |
| Takahashi H, Yoshida K, Sugita T, Higuchi H, Shimizu T. Quetiapine treatment of psychotic symptoms and aggressive behavior in patients with dementia with Lewy bodies: a case series. Prog Neuro-Psychopharmacol Biol Psychiatry 2003;27:549–53 | Not a psychological, behavioural, sensory or environmental intervention |
| Talerico KA, Evans LK, Strumpf NE. Mental health correlates of aggression in nursing home residents with dementia. Gerontologist 2002;42:169–77 | Not intervention study |
| Talerico KA, Evans LK. Responding to safety issues in frontotemporal dementias. Neurology 2001;56:S52–5 | Not primary research |
| Talwalker S. The cardinal features of cognitive and noncognitive dysfunction and the differential efficacy of tacrine in Alzheimer’s disease patients. J Biopharm Stat 1996;6:443–56 | Not intervention study |
| Tardiff K. Unusual diagnoses among violent patients. Psychiatr Clin North Am 1998;21:567 | No participants with dementia/not separately analysed |
| Targum SD, Abbott JL. Psychoses in the elderly: a spectrum of disorders. J Clin Psychiatry 1999;60:4–10 | No participants with dementia/not separately analysed |
| Tariot P, Gaile SE, Castelli NA, Porsteinsson AP. Treatment of agitation in dementia. N Direct Mental Health Serv 1997;76:109–23 | Not primary research |
| Tariot PN, Erb R, Leibovici A, Podgorski CA, Cox C, Asnis J, et al. Carbamazepine treatment of agitation in nursing-home patients with dementia – a preliminary study. J Am Geriatr Soc 1994;42:1160–6 | Not a psychological, behavioural, sensory or environmental intervention |
| Tariot PN, Jakimovich LJ, Erb R, Cox C, Lanning B, Irvine C, et al. Withdrawal from controlled carbamazepine therapy followed by further carbamazepine treatment in patients with dementia. J Clin Psychiatry 1999;60:684–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Tariot PN, Loy R, Ryan JM, Porsteinsson A, Ismail S. Mood stabilizers in Alzheimer’s disease: symptomatic and neuroprotective rationales. Adv Drug Deliv Rev 2002;54:1567–77 | Not primary research |
| Tariot PN, Mack JL, Patterson MB, Edland SD, Weiner MF, Fillenbaum G, et al. The Behavior Rating-Scale for Dementia of the consortium to establish a registry for Alzheimers disease. Am J Psychiatry 1995;152:1349–57 | No participants with dementia/not separately analysed |
| Tariot PN, Profenno LA, Ismail MS. Efficacy of atypical antipsychotics in elderly patients with dementia. J Clin Psychiatry 2004;65:11–15 | Not primary research |
| Tariot PN, Raman R, Jakimovich L, Schneider L, Porsteinsson A, Thomas R, et al. Divalproex sodium in nursing home residents with possible or probable Alzheimer disease complicated by agitation – a randomized, controlled trial. Am J Geriatr Psychiatry 2005;13:942–9 | Not a psychological, behavioural, sensory or environmental intervention |
| Tariot PN, Ryan JM, Porsteinsson AP, Loy R, Schneider LS. Pharmacologic therapy for behavioral symptoms of Alzheimer’s disease. Clin Geriatr Med 2001;17:359 | Not primary research |
| Tariot PN, Schneider L, Katz IR, Mintzer JE, Street J, Copenhaver M, et al. Quetiapine treatment of psychosis associated with dementia: a double-blind, randomized, placebo-controlled clinical trial. Am J Geriatr Psychiatry 2006;14:767–76 | Not a psychological, behavioural, sensory or environmental intervention |
| Tariot PN, Schneider LS, Katz IR. Anticonvulsant and other non-neuroleptic treatment of agitation in dementia. J Geriatr Psychiatry Neurol 1995;8:S28–39 | Not primary research |
| Tariot PN, Schneider LS, Mintzer JE, Cutler AJ, Cunningham MR, Thomas JW, et al. Safety and tolerability of divalproex sodium in the treatment of signs and symptoms of mania in elderly patients with dementia: results of a double-blind, placebo-controlled trial. Curr Ther Res 2001;62:51–67 | Not a psychological, behavioural, sensory or environmental intervention |
| Tariot PN, Sunderland T, Weingartner H, Murphy DL, Cohen MR, Cohen RM. Naloxone and Alzheimers disease. Arch Gen Psychiatry 1986;43:727–32 | Not a psychological, behavioural, sensory or environmental intervention |
| Tariot PN, Thal L, Jakimovich L, Thomas R, Raman R. A multicenter, randomized, double-blind, placebo-controlled trial of valproate for agitation associated with dementia. Neurobiol Aging 2004;25:S189 | Not a psychological, behavioural, sensory or environmental intervention |
| Tariot PN. Medical management of advanced dementia. J Am Geriatr Soc 2003;51:S305–13 | Not primary research |
| Tariot PN. Treatment strategies for agitation and psychosis in dementia. J Clin Psychiatry 1996;57:21–9 | Not primary research |
| Taylor JL, DuQueno L, Novaco RW. Piloting a ward anger rating scale for older adults with mental health problems. Behav Cogn Psychother 2004;32:467–79 | No participants with dementia/not separately analysed |
| Terawaki K, Ikarashi Y, Sekiguchi K, Nakai Y, Kase Y. Partial agonistic effect of yokukansan on human recombinant serotonin 1A receptors expressed in the membranes of Chinese hamster ovary cells. J Ethnopharmacol 2010;127:306–12 | No participants with dementia/not separately analysed |
| Teri L, Hughes JP, Larson EB. Cognitive deterioration in Alzheimers disease – behavioural and health factors. J Gerontol 1990;45:58–63 | Not intervention study |
| Teri L, Larson EB, Reifler BV. Behavioral disturbance in dementia of the Alzheimers type. J Am Geriatr Soc 1988;36:1–6 | Not intervention study |
| Teri L, Logsdon R. Assessment and management of behavioral disturbances in Alzheimer’s disease. Comprehens Ther 1990;16:36–42 | Not primary research |
| Teri L, Logsdon RG, McCurry SM. Nonpharmacologic treatment of behavioral disturbance in dementia. Med Clin North Am 2002;86:641 | Not primary research |
| Teri L, Logsdon RG. Methodologic issues regarding outcome measures for clinical drug trials of psychiatric complications in dementia. J Geriatr Psychiatry Neurol 1995;8:S8–17 | No participants with dementia/not separately analysed |
| Testad I, Aasland AM, Aarsland D. The effect of staff training on the use of restraint in dementia: a single-blind randomised controlled trial. Int J Geriatr Psychiatry 2005;20:587–90 | Conference Presentation Only |
| Testad I, Auer S, Mittelman M, Ballard C, Fossey J, Donabauer Y, et al. Nursing home structure and association with agitation and use of psychotropic drugs in nursing home residents in three countries: Norway, Austria and England. Int J Geriatr Psychiatry 2010;25:725–31 | Not intervention study |
| Testad I, Ballard C, Bronnick K, Aarsland D. The effect of staff training on agitation and use of restraint in nursing home residents with dementia: a single-blind, randomized controlled trial. J Clin Psychiatry 2010;71:80–6 | No participants with dementia/not separately analysed |
| Testad I, Mikkelsen A, Ballard C, Aarsland D. Health and well-being in care staff and their relations to organizational and psychosocial factors, care staff and resident factors in nursing homes. Int J Geriatr Psychiatry 2010;25:789–97 | No participants with dementia/not separately analysed |
| Thal LJ, Ferguson JM, Mintzer J, Raskin A, Targum SD. A 24-week randomized trial of controlled-release physostigmine in patients with Alzheimer’s disease. Neurology 1999;52:1146–52 | Not a psychological, behavioural, sensory or environmental intervention |
| Thaut MH, Miltner R, Lange HW, Hurt CP, Hoemberg V. Velocity modulation and rhythmic synchronization of gait in Huntington’s disease. Movement Disord 1999;14:808–19 | No participants with dementia/not separately analysed |
| Theison AK, Geisthoff UW, Foerstl H, Schroeder SG. Agitation in the morning: symptom of depression in dementia? Int J Geriatr Psychiatry 2009;24:335–40 | Not intervention study |
| Theuring F, Thunecke M, Kosciessa U, Turner JD. Transgenic animals as models of neurodegenerative diseases in humans. Trends Biotechnol 1997;15:320–5 | No participants with dementia/not separately analysed |
| Thomas P, Thomas C, Billon R, Peix R, Faugeron P, Clement J. Depression and frontal dysfunction: risks for the elderly? Encephale Rev Psychiatr Clin Biol Therap 2009;35:361–9 | Not intervention study |
| Thorgrimsen L, Spector A, Wiles A, Orrell M. Aroma therapy for dementia. Cochrane Database Syst Rev 2003;3:CD003150 | Not primary research |
| Thorpy MJ. Sleep disorders in Parkinson’s disease. Clin Cornerstone 2004;6(Suppl. 1A):S7–15 | No participants with dementia/not separately analysed |
| Thyer J, Unal A, Thomas P, Eaton B, Bhashyam R, Ortenburg J, et al. Prion-removal capacity of chromatographic and ethanol precipitation steps used in the production of albumin and immunoglobulins. Vox Sanguinis 2006;91:292–300 | No participants with dementia/not separately analysed |
| Tiberti C, Sabe L, Kuzis G, Cuerva AG, Leiguarda R, Starkstein SE. Prevalence and correlates of the catastrophic reaction in Alzheimer’s disease. Neurology 1998;50:546–8 | Not intervention study |
| Tobias CR, Turns DM, Lippmann S, Pary R, Embry CK. Psychiatric-disorders in the elderly – psychopharmacologic management. Postgrad Med 1988;83:313–19 | Not a psychological, behavioural, sensory or environmental intervention |
| Togo T, Isojima D, Akatsu H, Suzuki K, Uchikado H, Katsuse O, et al. Clinical features of argyrophilic grain disease – a retrospective survey of cases with neuropsychiatric symptoms. Am J Geriatr Psychiatry 2005;13:1083–91 | No participants with dementia/not separately analysed |
| Tollefson GD, Taylor CC. Olanzapine: Preclinical and clinical profiles of a novel antipsychotic agent. CNS Drug Rev 2000;6:303–63 | No participants with dementia/not separately analysed |
| Toro P, Schoenknecht P, Schroeder J. Type II diabetes in mild cognitive impairment and Alzheimer’s disease: results from a prospective population-based study in Germany. J Alzheimers Dis 2009;16:687–91 | Not intervention study |
| Torta R, Badino E, Scalabrino A. Therapeutic strategies for behavioral and psychological symptoms (BPSD) in demented patients. Arch Gerontol Geriatr 2004;443–54 | Not primary research |
| Tractenberg RE, Gamst A, Weiner MF, Koss E, Thomas RG, Teri L, et al. Frequency of behavioral symptoms characterizes agitation in Alzheimer’s disease. Int J Geriatr Psychiatry 2001;16:886–91 | Not intervention study |
| Tractenberg RE, Jin S, Patterson M, Schneider LS, Gamst A, Thomas RG, et al. Qualifying change: a method for defining clinically meaningful outcomes of change score computation. J Am Geriatr Soc 2000;48:1478–82 | No participants with dementia/not separately analysed |
| Tractenberg RE, Weiner MF, Patterson MB, Teri L, Thal LJ. Comorbidity of psychopathological domains in community-dwelling persons with Alzheimer’s disease. J Geriatr Psychiatry Neurol 2003;16:94–9 | Not intervention study |
| Traissard N, Herbeaux K, Cosquer B, Jeltsch H, Ferry B, Galani R, et al. Combined damage to entorhinal cortex and cholinergic basal forebrain neurons, two early neurodegenerative features accompanying Alzheimer’s disease: effects on locomotor activity and memory functions in rats. Neuropsychopharmacology 2007;32:851–71 | No participants with dementia/not separately analysed |
| Treatment of special populations with the atypical antipsychotics. Collaborative working group on clinical trial evaluations. J Clin Psychiatry 1998;59(Suppl. 12):46–52 | No participants with dementia/not separately analysed |
| Treloar AJ, MacDonald AJD. Outcome of delirium.1. Outcome of delirium diagnosed by DSM-III-R, ICD-10 and CAMDEX and derivation of the reversible cognitive dysfunction scale among acute geriatric inpatients. Int J Geriatr Psychiatry 1997;12:609–13 | No participants with dementia/not separately analysed |
| Treloar AJ, MacDonald AJD. Outcome of delirium.2. Clinical features of reversible cognitive dysfunction – are they the same as accepted definitions of delirium? Int J Geriatr Psychiatry 1997;12:614–18 | No participants with dementia/not separately analysed |
| Treusch Y, Jerosch D, Majic T, Heinz A, Gutzmann H, Rapp MA. How can we provide better services for demented nursing home residents suffering from apathy? Psychiatr Prax 2010;37:84–8 | Not primary research |
| Tsai SJ, Hwang JP, Yang CH, Liu KM. [Characteristics of dementia patients with aggressive behaviors.] Changgeng yi xue za zhi/Changgeng ji nian yi yuan 1995;18:361–4 | Not intervention study |
| Tsoi T, Baillon S, Lindesay J. Early frontal executive impairment as a predictor of subsequent behavior disturbance in dementia. Am J Geriatr Psychiatry 2008;16:102–8 | Not intervention study |
| Tsolaki M. Evaluation of tiapride in agitated elderly outpatients: an open study. Hum Psychopharmacol 2001;16:417–22 | No participants with dementia/not separately analysed |
| Tsuang D, Larson EB, Li G, Shofer JB, Montine KS, Thompson ML, et al. Association between lifetime cigarette smoking and Lewy Body accumulation. Brain Pathol 2010;20:412–18 | No participants with dementia/not separately analysed |
| Tueth MJ. Dementia: Diagnosis and emergency behavioral complications. J Emerg Med 1995;13:519–25 | Not primary research |
| Tulloch KJ, Zed PJ. Intramuscular olanzapine in the management of acute agitation. Ann Pharmacother 2004;38:2128–35 | Not primary research |
| Turner J, Snowdon J. An innovative approach to behavioral assessment and intervention in residential care: a service evaluation. Clin Gerontol 2009;32:260–75 | No participants with dementia/not separately analysed |
| Turner S. Behavioural symptoms of dementia in residential settings: a selective review of non-pharmacological interventions. Aging Mental Health 2005;9:93–104 | Not primary research |
| Tyas SL, White LR, Petrovitch H, Ross GW, Foley DJ, Heimovitz HK, et al. Mid-life smoking and late-life dementia: the Honolulu-Asia Aging Study. Neurobiol Aging 2003;24:589–96 | No participants with dementia/not separately analysed |
| Uchida N, Egashira N, Iwasaki K, Ishibashi A, Tashiro R, Nogami A, et al. Yokukansan inhibits social isolation-induced aggression and methamphetamine-induced hyperlocomotion in rodents. Biol Pharm Bull 2009;32:372–5 | No participants with dementia/not separately analysed |
| Ueki A, Morita Y, Miyoshi K. Changes in symptoms after the great Hanshin earthquake in patients with dementia. Japan J Geriatr 1996;33:573–9 | Not intervention study |
| Uttl B, Santacruz P, Litvan I, Grafman J. Caregiving in progressive supranuclear palsy. Neurology 1998;51:1303–9 | No participants with dementia/not separately analysed |
| Van de Ven L, Hectors R. [The support of relatives of the demented elderly.] Tijdschr Gerontol Geriatr 1983;14:149–56 | No participants with dementia/not separately analysed |
| Van de WA, Feys H, De Weerdt W, Dom R. Cognitive and behavioural effects of music-based exercises in patients with dementia. Clin Rehabil 2004;18:253–60 | No quantitative outcome |
| van der Geer E, Vink A, Schols J, Slaets J. Music in the nursing home: hitting the right note! The provision of music to dementia patients with verbal and vocal agitation in Dutch nursing homes. Int Psychogeriatr 2009;21:86–93 | Not intervention study |
| van der Linde R, Stephan BCM, Matthews FE, Brayne C, Savva GM. Behavioural and psychological symptoms in the older population without dementia – relationship with socio-demographics, health and cognition. BMC Geriatr 2010;10:87 | No participants with dementia/not separately analysed |
| van der Ploeg ES, Eppingstall B, O’Connor DW. The study protocol of a blinded randomised-controlled cross-over trial of lavender oil as a treatment of behavioural symptoms in dementia. BMC Geriatr 2010;10:49 | Protocol only |
| van der Ploeg ES, O’Connor DW. Evaluation of personalised, one-to-one interaction using Montessori-type activities as a treatment of challenging behaviours in people with dementia: the study protocol of a crossover trial. BMC Geriatr 2010;10:3 | Protocol only |
| van Diepen E, Baillon SF, Redman J, Rooke N, Spencer DA, Prettyman R. A pilot study of the physiological and behavioural effects of Snoezelen in dementia. Br J Occup Ther 2002;65 | No quantitative outcome |
| Van Dijck A, Van Dam D, De Deyn PP. Species, strain, and gender issues in the development and validation of animal models of dementia. Animal Models Dementia 2011;48:53–75 | No participants with dementia/not separately analysed |
| van Duijn E, Kingma E, van der Mast R. Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci 2007;19:441–8 | No participants with dementia/not separately analysed |
| van Duijn E. Treatment of irritability in Huntington’s disease. Curr Treat Options Neurol 2010;12:424–33 | No participants with dementia/not separately analysed |
| van Hoof J, Kort H, Rutten P, Duijnstee M. Ageing-in-place with the use of ambient intelligence technology: perspectives of older users. Int J Med Inform 2011;80:310–31 | No participants with dementia/not separately analysed |
| van Hout HP, Vernooij-Dassen MJ, Stalman WA. Diagnosing dementia with confidence by GPs. Fam Pract 2007;24:616–21 | No participants with dementia/not separately analysed |
| Van Leuven F, Dewachter I, Terwel D, Lasrado R, Muyllaert D, Van der Auwera I, et al. Multiple transgenic mice, better models for AD? Neurobiol Ageing 2004;25:S10 | No participants with dementia/not separately analysed |
| van Marum RJ. Update on the use of memantine in Alzheimer’s disease. Neuropsychiatr Dis Treat 2009;5:237–47 | Not primary research |
| van Reekum R, Clarke D, Conn D, Herrmann N, Eryavec G, Cohen T, et al. A randomized, placebo-controlled trial of the discontinuation of long-term antipsychotics in dementia. Int Psychogeriatr 2002;14:197–210 | Not a psychological, behavioural, sensory or environmental intervention |
| Vance DE, Burgio LD, Roth DL, Stevens AB, Fairchild JK, Yurick A. Predictors of agitation in nursing home residents. J Gerontol Series B 2003;58:129–37 | Not intervention study |
| Vanderzeypen F, Bier JC, Genevrois C, Mendlewicz J, Lotstra F. Frontal dementia or dementia praecox? The case report of a psychotic disorder with a severe decline. Encephale Rev Psychiatr Clin Biol Therap 2003;29:172–80 | Not intervention study |
| Vandijk PTM, Dippel DWJ, Habbema JDF. A behavioral rating scale as a predictor for survival of demented nursing-home patients. Arch Gerontol Geriatr 1994;18:101–13 | Not intervention study |
| Vargas A, Rojas-Ruiz MT, Roman SS, Salin-Pascual RJ. Development of bulimia nervosa after bariatric surgery in morbid obesity patients. Salud Mental 2003;26:28–32 | No participants with dementia/not separately analysed |
| Vasile D, Vasiliu O, Patriche D, Stanescu B, Bancescu R. Oral risperidone efficacy in Alzheimer dementia with psychotic features. Int J Neuropsychopharmacol 2008;11:176 | Not a psychological, behavioural, sensory or environmental intervention |
| Vecchierini MF. Sleep disturbances in Alzheimer’s disease and other dementias. Psychol Neuropsychiatr Vieil 2010;8:15–23 | Not primary research |
| Vega UM, Marinho V, Engelhardt E, Laks J. Neuropsychiatric symptoms in dementias: preliminary report of a prospective outpatient evaluation in Brazil. Arq Neuropsiquiatr 2007;65:498–502 | Not intervention study |
| Veinbergs I, Friberg M, Rodriguez M, Hubbard D, Trotter C, LaPaglia A, et al. The effects of AC-90222, a m1 selective agonist with weak D2 antagonist properties, in cognitive and antipsychotic behavioral assays. Soc Neurosci Abstracts 2001;27:1457 | No participants with dementia/not separately analysed |
| Verghese J, Mahoney J, Ambrose AF, Wang C, Holtzer R. Effect of cognitive remediation on gait in sedentary seniors. J Gerontol Series A 2010;65:1338–43 | No participants with dementia/not separately analysed |
| Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry 2007;78:929–35 | Not intervention study |
| Verhey FRJ, Verkaaik M, Lousbert R. Olanzapine versus haloperidol in the treatment of agitation in elderly patients with dementia: results of a randomized controlled double-blind trial. Dementia Geriatr Cogn Disord 2006;21:1–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Verhoeff NPLG. Radiotracer imaging of dopaminergic transmission in neuropsychiatric disorders. Psychopharmacology 1999;147:217–49 | No participants with dementia/not separately analysed |
| Verkaik R, Francke AL, van Meijel B, Ribbe MW, Bensing JM. Comorbid depression in dementia on psychogeriatric nursing home wards: which symptoms are prominent? Am J Geriatr Psychiatry 2009;17:565–73 | Not intervention study |
| Verkaik R, Francke AL, van Meijel B, Spreeuwenberg PM, Ribbe MW, Bensing JM. The introduction of a nursing guideline on depression at psychogeriatric nursing home wards: effects on certified nurse assistants. Int J Nurs Stud 2011;48:710–19 | Not primary research |
| Verma SD, Davidoff DA, Kambhampati KK. Management of the agitated elderly patient in the nursing home: the role of the atypical antipsychotics. J Clin Psychiatry 1998;59:50–5 | No participants with dementia/not separately analysed |
| Vernooij-Dassen M, Vasse E, Zuidema S, Cohen-Mansfield J, Moyle W. Psychosocial interventions for dementia patients in long-term care. Int Psychogeriatr 2010;22:1121–8 | Not primary research |
| Vida S, Desrosiers P, Carrier L, Gauthier S. Prevalence of depression in Alzheimers disease and validity of research diagnostic-criteria. J Geriatr Psychiatry Neurol 1994;7:238–44 | Not intervention study |
| Vidovich MR, Lautenschlager NT, Flicker L, Clare L, Almeida OP. The PACE Study: a randomised clinical trial of cognitive activity (CA) for older adults with mild cognitive impairment (MCI). Trials 2009;10 | No participants with dementia/not separately analysed |
| Vidovich MR, Shaw J, Flicker L, Almeida OP. Cognitive activity for the treatment of older adults with mild Alzheimer’s Disease (AD) – PACE AD: study protocol for a randomised controlled trial. Trials 2011;12 | Protocol only |
| Viggo Hansen N, Jorgensen T, Ortenblad L. Massage and touch for dementia. Cochrane Database Syst Rev 2006;4:CD004989 | Not primary research |
| Vilalta Franch J, Lopez Pousa S, Llinas Regla J. [Non cognitive symptoms in dementias.] Actas Esp Psiquiatr 1999;27:334–40 | Not primary research |
| Vilalta-Franch J, Garre-Olmo J, Lopez-Pousa S, Turon-Estrada A, Lozano-Gallego M, Hernandez-Ferrandiz M, et al. Comparison of different clinical diagnostic criteria for depression in Alzheimer disease. Am J Geriatr Psychiatry 2006;14:589–97 | Not intervention study |
| Vilalta-Franch J, Lopez-Pousa S, Turon-Estrada A, Lozano-Gallego M, Hernandez-Ferrandiz M, Pericot-Nierga I, et al. Syndromic association of behavioral and psychological symptoms of dementia in Alzheimer disease and patient classification. Am J Geriatr Psychiatry 2010;18:421–32 | Not intervention study |
| Vilalta-Franch J, Lozano-Gallego M, Hernandez-Ferrandiz M, Llinas-Regla J, Lopez-Pousa S, Lopez OL. Neuropsychiatric inventory. The psychometric properties of its adaptation to Spanish. Rev Neurol 1999;29:15–19 | No participants with dementia/not separately analysed |
| Vitiello MV, Borson S. Sleep disturbances in patients with Alzheimer’s disease – epidemiology, pathophysiology and treatment. CNS Drugs 2001;15:777–96 | Not primary research |
| Vloeberghs E, Coen K, Van Dam D, De Deyn PP. Validation of the APP23 transgenic mouse model of Alzheimer’s disease through evaluation of risperidone treatment on aggressive behaviour. Arzneimittel-Forschung-Drug Res 2008;58:265–8 | No participants with dementia/not separately analysed |
| Vloeberghs E, Van Dam D, Coen K, Staufenbiel M, De Deyn PP. Aggressive male APP23 mice modeling behavioral alterations in dementia. Behav Neurosci 2006;120:1380–3 | No participants with dementia/not separately analysed |
| Vloeberghs E, Van Dam D, Franck F, Staufenbiel M, De Deyn PP. Mood and male sexual behaviour in the APP23 model of Alzheimer’s disease. Behav Brain Res 2007;180:146–51 | No participants with dementia/not separately analysed |
| Volavka J. Can aggressive behavior in humans be modified by beta blockers? Postgrad Med 1988;Spec No:163–8 | No participants with dementia/not separately analysed |
| Volicer L, Camberg L, Hurley AC, Ashley J, Woods P, Ooi WL, et al. Dimensions of decreased psychological well-being in advanced dementia. Alzheimer Dis Assoc Disord 1999;13:192–201 | No participants with dementia/not separately analysed |
| Volicer L, Hurley AC. Management of behavioral symptoms in progressive degenerative dementias. J Gerontol Series A 2003;58:837–45 | No participants with dementia/not separately analysed |
| Volicer L, van der Steen JT, Frijters DH. Modifiable factors related to abusive behaviors in nursing home residents with dementia. J Am Med Direct Assoc 2009;10:617–22 | Not intervention study |
| Volicer L. Can dietary intervention help in management of problem behaviors in dementia? J Nutr Health Aging 2009;13:499–501 | Not a psychological, behavioural, sensory or environmental intervention |
| von Bergen M, Li L, Mandelkow E. Conformation and stability of tau and Alzheimer paired helical filaments probed by tryptophan scanning mutagenesis. Soc Neurosci Abstract Viewer Itinerary Planner 2003 | No participants with dementia/not separately analysed |
| von Gunten A, Alnawaqil AM, Abderhalden C, Needham I, Schupbach B. Vocally disruptive behavior in the elderly: a systematic review. Int Psychogeriatr 2008;20:653–72 | No participants with dementia/not separately analysed |
| von Gunten A, Favre M, Gurtner C, Abderhalden C. Vocally disruptive behavior (VDB) in the institutionalized elderly: a naturalistic multiple case report. Arch Gerontol Geriatr 2011;52:E110–16 | Not intervention study |
| Wagenaar DB, Mickus M, Luz C, Kreft M, Sawade J. An administrator’s perspective on mental health in assisted living. Psychiatr Serv 2003;54:1644–6 | Not intervention study |
| Waldemar G, Gauthier S, Jones R, Wilkinson D, Cummings J, Lopez O, et al. Effect of donepezil on emergence of apathy in mild to moderate Alzheimer’s disease. Int J Geriatr Psychiatry 2011;26:150–7 | Not a psychological, behavioural, sensory or environmental intervention |
| Walker J, Fowler S, Miller D, Sun A, Weisman G, Wood W, et al. Spatial learning and memory impairment and increased locomotion in a transgenic amyloid precursor protein mouse model of Alzheimer’s disease. Behav Brain Res 2011;222:169–75 | No participants with dementia/not separately analysed |
| Walsh JS, Welch HG, Larson EB. Survival of outpatients with Alzheimer-type dementia. Ann Intern Med 1990;113:429–34 | Not intervention study |
| Walsh PN. Ageing and health issues in intellectual disabilities. Curr Opin Psychiatry 2005;18:502–6 | No participants with dementia/not separately analysed |
| Walter LC, Lui LY, Eng C, Covinsky KE. Risk of hip fracture in disabled community-living older adults. J Am Geriatr Soc 2003;51:50–5 | No participants with dementia/not separately analysed |
| Walzer T, Chemerinski E, Sabe L, Herrmann M, Starkstein S. Anosognosia for behavioral problems in Alzheimer’s disease (AD). J Neurol Sci 1997;150:S22–3 | Not intervention study |
| Wang CC, Lu TH, Liao WC, Yuan SC, Kuo PC, Chuang HL, et al. Cigarette smoking and cognitive impairment: a 10-year cohort study in Taiwan. Arch Gerontol Geriatr 2010;51:143–8 | No participants with dementia/not separately analysed |
| Wang LY, Shofer JB, Rohde K, Hart KL, Hoff DJ, McFall YH, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry 2009;17:744–51 | Not a psychological, behavioural, sensory or environmental intervention |
| Ward T, Murphy E, Procter A, Weinman J. An observational study of 2 long-stay psychogeriatric wards. Int J Geriatr Psychiatry 1992;7:211–17 | No participants with dementia/not separately analysed |
| Ward-Smith P, Llanque SM, Curran D. The effect of multisensory stimulation on persons residing in an extended care facility. Am J Alzheimers Dis Other Demen 2009;24:450–5 | No quantitative outcome |
| Watson CG, Klett WG. Prediction of Wais scores from group ability tests. J Clin Psychol 1973;29:46–9 | No participants with dementia/not separately analysed |
| Watson PL, Shintani AK, Tyson R, Pandharipande PP, Pun BT, Ely EW. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med 2008;36:3171–7 | No participants with dementia/not separately analysed |
| Wei Z, Qi J, Dai Y, Bowen WD, Mousseau DD. Haloperidol disrupts Akt signalling to reveal a phosphorylation-dependent regulation of pro-apoptotic Bcl-XS function. Cell Signal 2009;21:161–8 | No participants with dementia/not separately analysed |
| Weiner MF, Hynan LS, Bret ME, White C. Early behavioral symptoms and course of Alzheimer’s disease. Acta Psychiatr Scand 2005;111:367–71 | Not intervention study |
| Weiner MF, Tractenberg R, Teri L, Logsdon R, Thomas RG, Gamst A, et al. Quantifying behavioral disturbance in Alzheimer’s disease patients. J Psychiatr Res 2000;34:163–7 | No participants with dementia/not separately analysed |
| Weiner MF, Williams B, Risser RC. Assessment of behavioral symptoms in community-dwelling dementia patients. Am J Geriatr Psychiatry 1997;5:26–30 | No participants with dementia/not separately analysed |
| Weinrich S, Egbert C, Eleazer GP, Haddock KS. Agitation – measurement, management, and intervention research. Arch Psychiatr Nurs 1995;9:251–60 | No participants with dementia/not separately analysed |
| Weintraub D, Katz IR. Pharmacologic interventions for psychosis and agitation in neurodegenerative diseases: evidence about efficacy and safety. Psychiatr Clin North Am 2005;28:941 | Not primary research |
| Weisskopf MG, Grodstein F, Ascherio A. Smoking and cognitive function in Parkinson’s disease. Move Disord 2007;22:660–5 | No participants with dementia/not separately analysed |
| Weitzel T, Robinson S, Barnes MR, Berry TA, Holmes JM, Mercer S, et al. The special needs of the hospitalized patient with dementia. Medsurg Nurs 2011;20:13–19 | Not primary research |
| Wells Y, Jorm AF. Evaluation of a special nursing home unit for dementia sufferers: a randomised controlled comparison with community care. Aus N Z J Psychiatry 1987;21:524–31 | No outcome measuring agitation |
| Werner P, Tabak N, Alpert R, Bergman R. Interventions used by nursing staff members with psychogeriatric patients resisting care. Int J Nurs Stud 2002;39:461–7 | No participants with dementia/not separately analysed |
| Werner S. [Physical activity for patients with dementia. Respecting autonomy.] Pflege Zeitschrift 2011;64:205 | Not primary research |
| Wersinger S, Ginns EI, O’Carroll A, Lolait S, Young W III. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry 2002;7:975–84 | No participants with dementia/not separately analysed |
| Wessels AM, Pollock BG, Anyama NG, Schneider LS, Lieberman JA, Marder SR, et al. Association of 9-hydroxy risperidone concentrations with risk of switching or discontinuation in the clinical antipsychotic trial of intervention effectiveness – Alzheimer’s disease trial. J Clin Psychopharmacol 2010;30:683–7 | Not a psychological, behavioural, sensory or environmental intervention |
| Westbury J, Jackson S, Peterson G. Psycholeptic use in aged care homes in Tasmania, Australia. J Clin Pharm Ther 2010;35:189–93 | Not intervention study |
| Westrich L, Sprouse J. Circadian rhythm dysregulation in bipolar disorder. Curr Opin Invest Drugs 2010;11:779–87 | No participants with dementia/not separately analysed |
| Wetzels R, Zuidema S, de Jonghe J, Verhey F, Koopmans R. Determinants of quality of life in nursing home residents with dementia. Dementia Geriatr Cogn Disord 2010;29:189–97 | Not intervention study |
| Wetzels R, Zuidema S, Jansen I, Verhey F, Koopmans R. Course of neuropsychiatric symptoms in residents with dementia in long-term care institutions: a systematic review. Int Psychogeriatr 2010;22:1040–53 | Not primary research |
| Wetzels RB, Zuidema SU, de Jonghe JF, Verhey FR, Koopmans RT. Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2-year period. Am J Geriatr Psychiatry 2010;18:1054–65 | Not intervention study |
| Weyerer S, Schaeufele M, Hendlmeier I. Evaluation of special and traditional dementia care in nursing homes: results from a cross-sectional study in Germany. Int J Geriatr Psychiatry 2010;25:1159–67 | Not intervention study |
| Whall AL, Colling KB, Kolanowski A, Kim HJ, Hong GRS, DeCicco B, et al. Factors associated with aggressive behavior among nursing home residents with dementia. Gerontologist 2008;48:721–31 | Not intervention study |
| Wheeler NL, Oyebode JR. Dementia care. 2: exploring how nursing staff manage challenging behaviour. Nurs Times 2010;106:20–2 | No participants with dementia/not separately analysed |
| White HK, McConnell ES, Bales CW, Kuchibhatla M. A 6-month observational study of the relationship between weight loss and behavioral symptoms in institutionalized Alzheimer’s disease subjects. J Am Med Direct Assoc 2004;5:89–97 | Not intervention study |
| Whyte S. Life-enhancing dance for elders with dementia. J Dementia Care 2010;18:37–9 | No outcome measuring agitation |
| Wiegand MH. Antidepressants for the treatment of insomnia: a suitable approach? Drugs 2008;68:2411–17 | No participants with dementia/not separately analysed |
| Wiener PK, Kiosses DN, Klimstra S, Murphy C, Alexopoulos GS. A short-term inpatient program for agitated demented nursing home residents. Int J Geriatr Psychiatry 2001;16:866–72 | No participants with dementia/not separately analysed |
| Wiener PK, Kiosses DN, Klimstra S, Murphy C, Alexopoulos GS. A short-term inpatient program for agitated demented nursing home residents. Int J Geriatr Psychiatry 2001;16:866–72 | Not intervention study |
| Wierman HR, Wadland W, Walters M, Kuhn C, Farrington S. Nonpharmacological management of agitation in hospitalized patients with late-stage dementia: a pilot study. J Gerontol Nurs 2011;37:44–8 | No quantitative outcome |
| Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008;69:341–8 | Not a psychological, behavioural, sensory or environmental intervention |
| Wilden BM, Wright NE. Concept of pre-death. Restlessness in dementia. J Gerontol Nurs 2002;28:24 | Not intervention study |
| Wilkinson D, Gauthier S, Rive B. Memantine decreases, and prevents the emergence of, key behavioural symptoms of Alzheimer’s disease. Eur Neuropsychopharmacol 2008;18:S507 | Not a psychological, behavioural, sensory or environmental intervention |
| Williams A. What bothers caregivers of stroke victims? J Neurosci Nurs 1994;26:155–61 | No participants with dementia/not separately analysed |
| Williams BR, Nazarians A, Gill MA. A review of rivastigmine: a reversible cholinesterase inhibitor. Clin Ther 2003;25:1634–53 | Not primary research |
| Williams DP. An in-service workshop for nursing personnel on the management of catastrophic reactions in dementia victims. Clin Gerontol 1994;14:47 | No participants with dementia/not separately analysed |
| Williams GO, Gjerde CL, Haugland S, Darnold D, Simonton LJ, Woodward PJ. Patients with dementia and their caregivers 3 years after diagnosis. A longitudinal study. Arch Fam Med 1995;4:512–17 | Not intervention study |
| Williams GO. Management of depression in the elderly. Primary Care 1989;16:451–74 | No participants with dementia/not separately analysed |
| Williams KN, Herman RE. Linking resident behavior to dementia care communication: effects of emotional tone. Behav Ther 2011;42:42–6 | Not intervention study |
| Williams TI. Evaluating effects of aromatherapy massage on sleep in children with autism: a pilot study. Evidence-Based Complement Alt Med 2006;3:373–7 | No participants with dementia/not separately analysed |
| Williams-Burgess C. Agitation in older persons with dementia: a research synthesis. Online J Knowledge Synth Nurs 1996;3:U4–22 | Not primary research |
| Williams-Grey CH, Foltynie T, Lewis SJ, Barker RA. Cognitive deficits and psychosis in Parkinson’s disease – a review of pathophysiology and therapeutic options. CNS Drugs 2006;20:477–505 | No participants with dementia/not separately analysed |
| Willson RA. The benefit of long-term interferon alfa therapy for symptomatic mixed cryoglobulinemia (cutaneous vasculitis/membranoproliferative glomerulonephritis) associated with chronic hepatitis C infection. J Clin Gastroenterol 2001;33:137–40 | No participants with dementia/not separately analysed |
| Winblad B, Cummings J, Andreasen N, Grossberg G, Onofrj M, Sadowsky C, et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease – rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007;22:456–67 | Not a psychological, behavioural, sensory or environmental intervention |
| Wingenfeld K, Seidl N, Ammann A. Preventing disruptive behavior of nursing home residents. Z Gerontol Geriatr 2011;44:27–32 | No participants with dementia/not separately analysed |
| Wisniewski HM, Silverman W. Diagnostic criteria for the neuropathological assessment of Alzheimer’s disease: current status and major issues. Neurobiol Aging 1997;18:S43–50 | Not primary research |
| Witzke J, Rhone RA, Backhaus D, Shaver NA. How sweet the sound – research evidence for the use of music in Alzheimer’s dementia. J Gerontol Nurs 2008;34:45–52 | Not primary research |
| Woelk H, Arnoldt K, Kieser M, Hoerr R. Ginkgo biloba special extract EGb 761 (R) in generalized anxiety disorder and adjustment disorder with anxious mood: a randomized, double-blind, placebo-controlled trial. J Psychiatr Res 2007;41:472–80 | No participants with dementia/not separately analysed |
| Woerner MG, Correll CU, Alvir JM, Greenwald B, Delman H, Kane JM. Incidence of tardive dyskinesia with risperidone or olanzapine in the elderly: results from a 2-year, prospective study in antipsychotic-naive patients. Neuropsychopharmacology 2011;36:1738–46 | No participants with dementia/not separately analysed |
| Wolfklein GP. New Alzheimers drug expands your options in symptom management. Geriatrics 1993;48:26 | Not primary research |
| Wood LD, Neumiller JJ, Setter SM, Dobbins EK. Clinical review of treatment options for select nonmotor symptoms of Parkinson’s disease. Am J Geriatr Pharmacother 2010;8:294–315 | Not primary research |
| Woodcock JH. A neuropsychiatric approach to impulse disorders. Psychiatr Clin North Am 1986;9:341–52 | No participants with dementia/not separately analysed |
| Woodruff-Pak DS. Animal models of Alzheimer’s disease: therapeutic implications. J Alzheimers Dis 2008;15:507–21 | No participants with dementia/not separately analysed |
| Woods DL, Rapp CG, Beck C. Escalation/de-escalation patterns of behavioral symptoms of persons with dementia. Aging Mental Health 2004;8:126–32 | Not primary research |
| Woods RT. Discovering the person with Alzheimer’s disease: cognitive, emotional and behavioural aspects. Aging Mental Health 2001;5:S7–16. | Not primary research |
| Wooten V. Evaluation and management of sleep disorders in the elderly. Psychiatr Ann 1990;20:466–73 | No participants with dementia/not separately analysed |
| Wooten V. Sleep disorders in geriatric patients. Clin Geriatr Med 1992;8:427–39 | No participants with dementia/not separately analysed |
| Workman RH, Molinari V, Rezabek P, McCullough LB, Khalsa DK, Trivedi S, et al. An ethical framework for understanding patients with antisocial personality disorder who develop dementia: diagnosing and managing disorders of autonomy. J Ethics Law Aging 1997;3:79–90 | No participants with dementia/not separately analysed |
| Wright MT, Cummings JL. Neuropsychiatric disturbances Alzheimer’s disease and other dementias: recognition and management. Neurologist 1996;2:207–18 | Not primary research |
| Wroblewski BA, Joseph AB, Kupfer J, Kalliel K. Effectiveness of valproic acid on destructive and aggressive behaviours in patients with acquired brain injury. Brain Inj 1997;11:37–47 | No participants with dementia/not separately analysed |
| Wultsch T, Chourbaji S, Fritzen S, Kittel S, Grimblatt E, Gerlach M, et al. Behavioural and expressional phenotyping of nitric oxide synthase-I knockdown animals. J Neural Transmis 2007;72:69–85 | No participants with dementia/not separately analysed |
| Wynn ZJ, Cummings JL. Cholinesterase inhibitor therapies and neuropsychiatric manifestations of Alzheimer’s disease. Dementia Geriatr Cogn Disord 2004;17:100–8 | Not primary research |
| Yamamoto Ki, Shinba T. [Central noradrenergic system in psychiatry.] Seishin shinkeigaku zasshi 2009;111:741–61 | Not primary research |
| Yamashita M, Amagai M. Family caregiving in dementia in Japan. Appl Nurs Res 2008;21:227–31 | No participants with dementia/not separately analysed |
| Yamazaki T, Blinov N, Wishart D, Kovalenko A. Hydration effects on the HET-s prion and amyloid-beta fibrillous aggregates, studied with three-dimensional molecular theory of solvation. Biophys J 2008;95:4540–8 | No participants with dementia/not separately analysed |
| Yao L, Algase D. Emotional intervention strategies for dementia-related behavior: a theory synthesis. J Neurosci Nurs 2008;40:106–15 | No participants with dementia/not separately analysed |
| Yao L, Algase D. Environmental ambiance as a new window on wandering. West J Nurs Res 2006;28:89–104 | Not intervention study |
| Young KWH, Greenwood CE, van Reekum R, Binns MA. A randomized, crossover trial of high-carbohydrate foods in nursing home residents with Alzheimer’s disease: associations among intervention response, body mass index, and behavioral and cognitive function. J Gerontol Series A 2005;60:1039–45 | Not intervention study |
| Young KWH, Greenwood CE, van Reekum R, Binns MA. Providing nutrition supplements to institutionalized seniors with probable Alzheimer’s disease is least beneficial to those with low body weight status. J Am Geriatr Soc 2004;52:1305–12 | Not a psychological, behavioural, sensory or environmental intervention |
| Young R, Batkai S, Dukat M, Glennon RA. TDIQ (5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinoline) exhibits anxiolytic-like activity in a marble-burying assay in mice. Pharmacol Biochem Behav 2006;84:62–73 | No participants with dementia/not separately analysed |
| Yousef MI. Aluminium-induced changes in hemato-biochemical parameters, lipid peroxidation and enzyme activities of male rabbits: protective role of ascorbic acid. Toxicology 2004;199:47–57 | No participants with dementia/not separately analysed |
| Yu F, Kolanowski AM, Litaker M. The association physical function with agitation and passivity in nursing home residents with dementia. J Gerontol Nurs 2006;32:30–6 | Not intervention study |
| Yu X, Zheng J. Polymorphic structures of Alzheimer’s beta-amyloid globulomers. PLOS ONE 2011;6 | No participants with dementia/not separately analysed |
| Yuen HK, Benzing P. Guiding of behaviour through redirection in brain injury rehabilitation. Brain Inj 1996;10:229–38 | No participants with dementia/not separately analysed |
| Zanetti O, Frisoni GB, Deleo D, Dellobuono M, Bianchetti A, Trabucchi M. Reality orientation therapy in Alzheimer-disease – useful or not – a controlled study. Alzheimer Dis Assoc Disord 1995;9:132–8 | No outcome measuring agitation |
| Zannino G, Gargiulo A, Lamenza F, Marotta MG, Barzotti T, Silvestri A, et al. The management of psychogeriatric patient. Arch Gerontol Geriatr 2004;465–70 | No participants with dementia/not separately analysed |
| Zaraa AS. Pharmacologic management of agitation and psychosis in older demented patients. Geriatrics 2003;58:48 | Not a psychological, behavioural, sensory or environmental intervention |
| Zaremba PD, Bialek M, Blaszczyk B, Cioczek P, Czuczwar SJ. Non-epilepsy uses of antiepileptic drugs. Pharmacol Rep 2006;58:1–12 | No participants with dementia/not separately analysed |
| Zarros AC, Kalopita KS, Tsakiris ST. Serotoninergic impairment and aggressive behavior in Alzheimer’s disease. Acta Neurobiologiae Experimentalis 2005;65:277–86 | Not primary research |
| Zaudig M. A risk-benefit assessment of risperidone for the treatment of behavioural and psychological symptoms in dementia. Drug Safety 2000;23:183–95 | Not primary research |
| Zayas EM, Grossberg GT. Treating the agitated Alzheimer patient. J Clin Psychiatry 1996;57:46–54 | Not primary research |
| Zebenholzer K, Oder W. Neurological and psychosocial sequelae 4 and 8 years after severe head injury: a catamnestic study. Wiener Klinische Wochenschrift 1998;110:253–61 | No participants with dementia/not separately analysed |
| Zec RF, Burkett NR. Non-pharmacological and pharmacological treatment of the cognitive and behavioral symptoms of Alzheimer disease. Neurorehabilitation 2008;23:425–38 | Not primary research |
| Zetteler J. Effectiveness of simulated presence therapy for individuals with dementia: a systematic review and meta-analysis. Aging Mental Health 2008;12:779–85 | Not primary research |
| Zhao JH, Liu HL, Liu YF, Lin HY, Fang HW, Ho Y, et al. Molecular dynamics simulations to investigate the aggregation behaviors of the A beta(17–42) Oligomers. J Biomol Struct Dynamics 2009;26:481–90 | No participants with dementia/not separately analysed |
| Zhong K, Tariot P, Minkwitz MC, Devine NA, Mintzer JE. Quetiapine for the treatment of agitation in elderly institutionalized patients with dementia: a randomized, double-blind trial. Neuropsychopharmacology 2004;29:S130 | Not a psychological, behavioural, sensory or environmental intervention |
| Zhong KX, Tariot P, Mintzer J, Minkwitz M, Devine N. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr Alzheimer Res 2007;4:81–93 | Not a psychological, behavioural, sensory or environmental intervention |
| Zhou FC, Buchwald N. Connectivities of the striatal grafts in adult-rat brain – a rich afference and scand striatonigral efference. Brain Res 1989;504:15–30 | No participants with dementia/not separately analysed |
| Ziemba C, Foster G, Neufeld R, Breuer B. Haloperidol holiday: Is it a beneficial vacation for some nursing home residents? Clin Gerontol 1997;17:15–24 | No participants with dementia/not separately analysed |
| Zieschang T, Dutzi I, Mueller E, Hestermann U, Gruenendahl K, Braun AK, et al. Improving care for patients with dementia hospitalized for acute somatic illness in a specialized care unit: a feasibility study. Int Psychogeriatr 2010;22:139–46 | No participants with dementia/not separately analysed |
| Zinetti J, Daraux J, Ploskas F. [Psychiatric emergencies in the elderly.] Rev Prat 2003;53:1197–200 | No participants with dementia/not separately analysed |
| Zisselman MH, Rovner BW, Shmuely Y, Ferrie P. A pet therapy intervention with geriatric psychiatry inpatients. Am J Occup Ther 1996;50:47–51 | No participants with dementia/not separately analysed |
| Zucconi M, Lanuzza B, Cosentino F, I, Iero I, Tripodi M, Marelli S, et al. A single question for the rapid screening of restless legs syndrome in the neurological clinical practice. J Sleep Res 2006;15:91 | No participants with dementia/not separately analysed |
| Zuidema SU, de Jonghe JF, Verhey FR, Koopmans RT. Agitation in Dutch institutionalized patients with dementia: factor analysis of the Dutch version of the Cohen-Mansfield Agitation Inventory. Dementia Geriatr Cogn Disord 2007;23:35–41 | No participants with dementia/not separately analysed |
| Zuidema SU, de Jonghe JF, Verhey FR, Koopmans RT. Neuropsychiatric symptoms in nursing home patients: factor structure invariance of the dutch nursing home version of the neuropsychiatric inventory in different stages of dementia. Dementia Geriatr Cogn Disord 2007;24:169–76 | No participants with dementia/not separately analysed |
| Zuidema SU, de Jonghe JF, Verhey FR, Koopmans RT. Predictors of neuropsychiatric symptoms in nursing home patients: influence of gender and dementia severity. Int J Geriatric Psychiatry 2009;24:1079–86 | Not intervention study |
| Zuidema SU, Derksen E, Verhey FR, Koopmans RT. Prevalence of neuropsychiatric symptoms in a large sample of Dutch nursing home patients with dementia. Int J Geriatr Psychiatry 2007;22:632–8 | Not intervention study |
| Zuidema SU, van Iersel MB, Koopmans RTCM, Verhey FRJ, Olde Rikkert MGM. [Efficacy and adverse reactions of antipsychotics for neuropsychiatric symptoms in dementia: a systematic review.] Ned Tijdschr Geneeskd 2006;150:1565–73 | Not primary research |
| Zwijsen SA, Smalbrugge M, Zuidema SU, Koopmans RT, Bosmans JE, van Tulder MW, et al. Grip on challenging behaviour: a multidisciplinary care programme for managing behavioural problems in nursing home residents with dementia. Study protocol. BMC Health Serv Res 2011;11 | Protocol only |
Appendix 3 Effect of removing studies where the outcome measures are invalid or unreliable or both
| Category of intervention | Effect of removing invalid and/or unreliable outcome measures studies |
|---|---|
| Activities | Decrease by two in number of studies showing significant improvement at short term, no change at long term (Kovach 2003,43 Lee and Kim 200845). This slightly weakens the case for activities as an intervention with evidence of efficacy |
| Music therapy with a specific protocol | Decrease by one in number of studies showing no significant effects at short term, no change at long term (Groene 199358). This does not affect the conclusions about this intervention |
| Light therapy | Decrease by one in number of studies showing significant worsening of agitation at short term, no findings at long term (Barrick 201089), decrease by one in number of studies showing no significant effects at short term, no findings at long term (Satlin 199288). This does not affect the conclusions about this intervention |
| Aromatherapy | Decrease by one in number of studies showing significant worsening of agitation at short term, no findings at long term (Akondzadeh 2003101). This does not affect the conclusions about this intervention |
| Education in behavioural management for family caregivers | Decrease by one in the number of studies showing no significant effects in short term and no findings at long term (Bourgeois 1997106). This does not affect the conclusions about this intervention, except to underline the lack of evidence in this area |
| Exercise | Decrease by two (50%) in the number of studies, one of which showed the only significant improvement at short term (Holmberg 1997120) and no findings in long term, the other having no significant findings at short term and no findings at long term (Eggermont 2010121). This does not affect the conclusions about this intervention, except to underline the lack of evidence in this area |
| Music therapy without a specific protocol | Decrease by one in number of studies showing significant improvement in agitation at short term and no findings at long term (Clark 1998111), and decrease by one in number of studies showing no significant findings at short term and no findings at long term (Garland 2007110). This does not affect the conclusions about this intervention, except to underline the lack of evidence in this area |
| Changing environment (masking exits) | Decrease by two (100%) of studies exploring changing the environment by masking exits, one of which found a short-term improvement (Darby 1990135) and one was not significant (Hussian and Brown 1987136). This does not affect the conclusions about this intervention, except to underline the lack of evidence in this area |
| Wayfinding | Decrease by one (100%) of studies, showing non-significant results at short term and worsening of agitation at long term (McGilton 2003137). This does not affect the conclusions about this intervention, except to underline the lack of evidence in this area |
| Simulated presence therapy | Decrease by one of studies showing no significant results at short term and no long-term findings (Camberg 1999138). This does not affect the conclusions about this intervention, except to underline the lack of evidence in this area |
Effect of removing studies with outcome measures that are neither reliable nor valid
Two studies with quality scores of exercise, and one each of aromatherapy, light therapy and music therapy with a specific protocol, with quality ratings > 4, used outcome measures that were neither shown to be valid nor shown to be reliable.
Removing these from the overall results has the following result:
-
Music therapy with a specific protocol has one fewer insignificant study.
-
Light therapy has one fewer insignificant study.
-
Aromatherapy has one fewer study where agitation worsened.
-
Exercise loses 50% of its evidence (two out of four studies), one of which had shown improvement in the short term but no long-term findings, leaving two studies with no significant findings.
Effect of removing studies which are not valid but are reliable
Two studies of music therapy, two studies of masking exits and one each of education for family caregivers, activities, simulated presence therapy, and light therapy, with quality ratings > 4, had non-validated but reliable outcome measures.
The effect upon the category of changing the environment is that it removed studies relating to masking exits, which increases the homogeneity of the category slightly.
Music therapy without a specific protocol loses one study showing significant improvement in the short term, and one study with no significant results in the short term, and so makes little impact.
Education for family caregivers loses one study showing no significant results at short and long term, which makes little difference to the conclusions already made.
Activities loses one reasonable-quality (2b) study with good participant numbers (78), showing significant improvement in the short term but not in the long term, which slightly decreases the effectiveness of the intervention overall at short term.
Removing one simulated presence study with no significant results at short term and no long term results makes little difference to the conclusions already made.
Light therapy loses one moderate-quality (2c) study with reasonable participant numbers (n = 66).
Effect of removing studies which are valid but are not reliable
One study looking at wayfaring and one looking at activities, with quality ratings > 4, did not have reliable outcomes.
The effect upon activities would be that one 2c study with 23 participants, which showed improvement in the short term but did not examine long-term results, would be excluded, slightly weakening the conclusions about activities in the short term.
The effect upon wayfaring would be that there would be no studies investigating this intervention in the study.
Appendix 4 Health economics: CHEERS statements
| Section/item | Recommendation | Reported on page(s) |
|---|---|---|
| Title and abstract | ||
| Title | This study was not identified as an economic evaluation based on the title | 1435 |
| Abstract | A structured abstract was provided, but contained few details of the economic evaluation | 1435 |
| Introduction | ||
| Background and objectives | The broader context for the study was discussed. The study question was presented, and the relevance for practice decisions was discussed | 1435–6 |
| Methods | ||
| Target population and subgroups | The characteristics of the study population were discussed and justified | 1437 |
| Setting and location | The setting and location were described | 1436 |
| Study perspective | The study perspective was not stated | |
| Comparators | The comparators were clearly described | 1436 |
| Time horizon | The time horizon was not specifically described, and it is unclear if the time horizon was the same for the two comparators | 1437–8 |
| Discount rate | Discounting was unnecessary since costs and outcomes were measured over a period of less than 1 year | |
| Choice of health outcomes | The outcome measure was described and justified | 1437 |
| Measurement of effectiveness | The study design used to measure effectiveness was clearly described | 1437–8 |
| Measurement and valuation of preference based outcomes | Not applicable | |
| Estimating resources and costs | The approach used to measure resource use was described. Sources of unit cost data were not provided | 1438 |
| Currency, price date, and conversion | The currency was stated. The cost base year was not provided | 1438 |
| Choice of model | The choice of model structure was not justified | |
| Assumptions | The assumptions used to measure costs were stated | 1438 |
| Analytical methods | The analytical methods were simply described | 1438 |
| Results | ||
| Study parameters | Study parameters were described. Ranges and references were not reported. Probability distributions were not used | |
| Incremental costs and outcomes | Costs and outcomes were reported separately, and incremental cost-effectiveness ratios were reported | 1438–9 |
| Characterising uncertainty | There was no analysis of uncertainty | |
| Characterising heterogeneity | Cost analyses were reported for subgroups | 1438 |
| Discussion | ||
| Study findings, limitations, generalisability, and current knowledge | There was little discussion of the economic evaluation | 1439 |
| Other | ||
| Source of funding | Sources of funding were not stated | |
| Conflicts of interest | Conflicts of interest were not stated | |
| Section/item | Recommendation | Reported on page(s) |
|---|---|---|
| Title and abstract | ||
| Title | This study was identified as an economic evaluation based on the title | Page numbers were not reported |
| Abstract | A structured abstract was provided | |
| Introduction | ||
| Background and objectives | The broader context for the study was discussed. The study question was presented, and the relevance for practice decisions was discussed. The economic evaluation was linked to a clinical trial (Chenoweth et al.) | |
| Methods | ||
| Target population and subgroups | The characteristics of the study population were discussed and justified | |
| Setting and location | The setting and location were described | |
| Study perspective | The study perspective was not stated | |
| Comparators | The comparators were clearly described | |
| Time horizon | The time horizon was stated | |
| Discount rate | Discounting was unnecessary since costs and outcomes were measured over a period of less than one year | |
| Choice of health outcomes | The outcome measure was described and justified | |
| Measurement of effectiveness | The study design used to measure effectiveness was clearly described | |
| Measurement and valuation of preference based outcomes | Not applicable | |
| Estimating resources and costs | The approach used to measure resource use was described. Sources of unit cost data were provided | |
| Currency, price date, and conversion | The currency was stated. The cost base year was not provided | |
| Choice of model | The choice of model structure was justified | |
| Assumptions | The assumptions used to measure costs were stated | |
| Analytical methods | The analytical methods were described | |
| Results | ||
| Study parameters | Study parameters were described. Ranges and references were not always reported. Probability distributions were not used | |
| Incremental costs and outcomes | Costs and outcomes were reported separately, and incremental cost-effectiveness ratios were reported | |
| Characterising uncertainty | A univariate sensitivity analysis was reported | |
| Characterising heterogeneity | Results were not reported for subgroups | |
| Discussion | ||
| Study findings, limitations, generalisability, and current knowledge | The study findings, limitations, generalisability and addition to knowledge were discussed | |
| Other | ||
| Source of funding | Sources of funding were reported in the clinical trial paper | |
| Conflicts of interest | Conflicts of interest were reported in the clinical trial paper | |
Appendix 5 Health economics: intervention costs
| Author | Resource item | Unit cost (£) | Source | Assumptions |
|---|---|---|---|---|
| Kovach et al. 43 | Geriatric nurse practitioner (GNP) to configure activities daily | 189.00 | Curtis212 | Takes 30 minutes’ planning per patient |
| Staff nurse to configure activities daily | 189.00 | Curtis212 | Takes 30 minutes’ planning per patient | |
| Total | 378.00 | |||
| Lin et al. 44 | Montessori method (nursing staff time) | 22.09 | Curtis212 | |
| Rhythmic music | 0.34 | a | ||
| Clay | 5.99 | b | ||
| Paints | 15.99 | c | ||
| Nursing staff | 324.00 | Curtis212 | ||
| Pedometer | 3.99 | d | ||
| Total | 372 | |||
| Lee et al. 45 | Edible dropwort | 3.09 | e | |
| Bean sprout | 1.48 | f | ||
| Nursing assistant | 262.96 | Curtis212 | Two nursing staff a.m./p.m. | |
| Watering cans | 1.78 | f | ||
| Dust cloths | 0.36 | g | ||
| Sleep diary | 3.99 | h | ||
| Total | 274 | |||
| Buettner et al. 46 | Specialist designed programmes | 102.52 | i, j | 187.5 hours over 10 weeks designing programmes |
| Specialist training activities department (nurse staff) | 102.52 | i, j | 187.5 hours over 10 weeks working with departments | |
| Nurse staff took over from specialist for 10 weeks | 153.41 | i, j | Takes 30 minutes to plan and implement each plan, per patient, 187.5 hours spent approximately over 10 weeks | |
| Air mat | 43.64 | k | ||
| Stim box | 4.99 | l | ||
| Relaxation therapy | 288.00 | m | £18 for 30 minutes per relaxation therapy. Relaxation therapy given 4 days per week for 4 weeks | |
| Herb garden | 1.18 | n | ||
| Total | 696 | |||
| Fitzsimmons et al. 49 | Nursing assistant supervising | 135.00 | Curtis212 | 10 sessions |
| Index cards | 0.24 | o | ||
| Cookbooks | 8.98 | p | ||
| Magazines | 3.70 | q | ||
| Apples | 1.50 | r | Items bought for 5/10 sessions | |
| Cream | 5.00 | s | Items bought for 5/10 sessions | |
| Potatoes | 1.35 | t | ||
| Bananas | 1.30 | u | Items bought for 5/10 sessions | |
| Eggs | 0.40 | v | Items bought for 5/10 sessions | |
| Quiche | 2.20 | w | ||
| Apple sauce | 3.45 | x | Items bought for 5/10 sessions | |
| Butter | 8.00 | y | Items bought for 5/10 sessions | |
| Custard | 2.35 | z | Items bought for 5/10 sessions | |
| Total | 173 | |||
| Buettner et al. 48 | Air mat | 43.64 | k | |
| Stim box | 4.99 | l | ||
| Relaxation therapy | 288.00 | m | £18 for 30 minutes per relaxation therapy. Relaxation therapy given 4 days per week for 4 weeks | |
| Herb garden | 1.18 | n | ||
| Nursing staff implementing programme | 252.00 | Curtis212 | 3 hours of activity supervised 7 days per week for 4 weeks | |
| Total | 590 | |||
| Fitzsimmons et al. 49 | Nursing staff | 202.50 | Curtis212 | Received 5 days per week |
| Total | 203 | |||
| Cohen-Mansfield et al. 52 | Nursing staff | 33.75 | Curtis212 | |
| Cookie ingredients | 2.00 | aa | ||
| Scrap book | 5.99 | ab | ||
| Portable CD player | 6.25 | ac | ||
| Camera | 5.62 | ad | ||
| Bible audio tapes | 0.38 | ae | ||
| Golf equipment | 57.49 | af | ||
| Travel magazines | 1.88 | ag | ||
| Total | 80 |
| Author | Resource item | Unit cost (£) | Source | Assumption(s) |
|---|---|---|---|---|
| Sung et al. 54 | Nursing practitioner | 12.00 | Curtis212 | |
| Music CD | 0.83 | a | ||
| Total | 13 | |||
| Lin et al. 57 | Music therapists time | 2.64 | b, c | Band 7 mid-point £35,184 |
| University music therapy course | 24.39 | £1000 per music therapy course, group sessions | ||
| Total | 27 | |||
| Jennings et al. 61 | Nursing assistant | 13.50 | Curtis212 | Observed for 1 hour across all 16 participants |
| Therapist | 10.50 | Curtis212 | ||
| Total | 24 |
| Author | Resource item | Unit cost (£) | Source | Assumption(s) |
|---|---|---|---|---|
| Moyle et al. 63 | Foot massage | 128.33 | a | |
| Total | 128 | |||
| Yang et al. 64 | Acupressure | 500.00 | b | £35–60, mid-point £50 |
| Nursing staff offering companionship and conversation | 27.00 | Curtis212 | Nurse offered 30 minutes of conversation per patient | |
| Total | 527 | |||
| Remington66 | CD | 0.88 | ||
| Portable CD player | 1.47 | |||
| Nursing staff present | 0.53 | Curtis212 | Nursing staff present to play calm music | |
| Total | 3 | |||
| Hand massage | 7.00 | a | ||
| Hand massage plus calm music | 9.35 | a | ||
| Nursing staff present | 0.53 | Curtis212 | ||
| Total | 10 | Nursing staff present to play calm music | ||
| Staal et al. 69 | Bead mazes | 12.95 | c | |
| Puzzles | 6.60 | d | ||
| Occupational community therapist | 84.00 | Curtis212 | 1 hour with occupational therapist | |
| Nursing staff | 162.00 | Curtis212 | Six sessions of 30–180 minutes | |
| Snoezelen room per day | 7.22 | Curtis212 | ||
| Total | 273 | |||
| Lin et al. 44 | Acupressure | 300.00 | b | £35–60, mid-point £50 |
| Total | 300 | |||
| Woods et al. 71 | Nursing staff | 32.40 | Curtis212 | 12 minutes, over 2 days for 1 week |
| Total | 32 | |||
| Gerdner et al. 72 | Craniosacral therapy | 298.10 | e | |
| Nursing assistant | 189.38 | Curtis212 | ||
| Total | 487 | |||
| Whall et al. 73 | Animal CD | 0.49 | f | |
| Picture | 4.00 | g | ||
| Pudding | 1.86 | h, i | ||
| Nursing staff giving shower | 9.00 | Curtis212 | 10 minutes in the bath | |
| Dietitian devising food plan | 5.83 | Curtis212 | Devising food plan takes 10 minutes per patient | |
| Nursing devising food plan | 9.00 | Devising food plan takes 10 minutes per patient | ||
| Total | 30 | |||
| van Weert et al. 74 | Certified nursing assistant | 54.00 | Curtis212 | 1 hour |
| Certified nursing assistant | 14.40 | Curtis212 | 16 hours × 4 weeks = 960 minutes | |
| Stimulus screening offered to patients | 8.67 | |||
| Certified nursing assistant wrote plan | 27.00 | Curtis212 | Takes 30 minutes to plan each plan, per patient | |
| Snoezelen room | 5.28 | Curtis212 | Revenue costs, land costs and capital charges related to land | |
| Total | 109 |
| Author | Resource item | Unit cost (£) | Source | Assumption(s) |
|---|---|---|---|---|
| Chenoweth et al. 75 | Researcher’s time | 11.05 | a, b | 12 hours of training in total |
| (2 days’) training by mapper to nurse/care staff | 4.42 | a, b | Band 7, mid-point £35,184, 2 days’ training course consists of 12 hours of training in total | |
| Nurse staff develop and implement care practices | 27.00 | Curtis212 | Takes 30 minutes to plan each plan, per patient | |
| Review of patients’ histories | 9.02 | Takes 30 minutes to plan each plan, per patient | ||
| Researcher visited each site (five sites) twice to help change practices | 2.76 | 3 hours per site. Total 5 × 15 | ||
| Support over 4 months | 44.78 | 2 hours per day over 4 months = 121.6 (days) × 2 = 243.33/98 patients | ||
| Total | 99 | |||
| McCallion et al. 76 | Nursing assistant time | 75.68 | a, b | 35 minutes’ observation per patient × 7 |
| Trainer observation | 10.53 | a, b | Band 7, mid-point £35,184 | |
| Videotapes | 1.20 | c | £5.99 for pack of five | |
| Total | 87 | |||
| McCallion et al. 77 | Two nurses’ time | 15.92 | Offered to two nurses | |
| Social worker delivered programme | 31.55 | Curtis212 | ||
| Social worker participated in training nurses 4.5 days | 24.69 | |||
| Total | 72 | |||
| Hoeffer et al. 79 | Nurses | 104.26 | a, b | Band 7, mid-point £35,184 30 minutes for four baths over 3 weeks |
| Specialist nurse observation | 26.33 | Curtis212 | £316 per patient-related hour; four baths average 5 minutes per observation, four baths over three weeks |
|
| Written plan by specialist, nurse for each resident | 26.33 | Curtis212 | £316 per patient-related hour; four baths average Time spent per plan: 10 minutes |
|
| Videotape to train nursing assistant | 1.20 | c | £5.99 for pack of five | |
| Total | 158 | |||
| Sloane et al. 80 | Three certified nursing assistants | 6.75 | Curtis212 | 1 hour per bath |
| Two bath towels | 11.99 | d | ||
| No rinse soap | 0.08 | e | ||
| No rinse soap | 0.08 | e | ||
| Clinical psychologist | 45.00 | Curtis212 | ||
| Total | 64 | |||
| Three certified nursing assistants | 6.48 | Curtis212 | 1 hour per bath | |
| Bath blankets | 11.99 | d | ||
| Two bath towels | 11.99 | d | ||
| No rinse soap | 0.08 | e | ||
| Book | 1.65 | f | ||
| Clinical psychologist | 43.20 | Curtis212 | ||
| Total | 75 | |||
| Deudon et al. 81 | Nursing staff | 0.47 | Curtis212 | |
| Teaching session conducted by another nurse | 27.06 | a, b | ||
| Trainer’s time | 2.49 | a, b | ||
| Refreshing wipes | 0.07 | g | 10 packs needed | |
| Moisturising hand cream | 0.66 | h | 50 tubes needed | |
| Total | 31 | |||
| Chenoweth et al. 82 | Two researchers (2 days’) training, five sites | 9.93 | a, b | Band 7, mid-point £35,184 |
| Two nursing assistant (2 days’), five sites | 29.72 | Curtis212 | ||
| Reported back to nurses | 1.66 | Researchers reported back to staff nurse at each site (five sites) too 2 hours at each site | ||
| Nurse developing care plans | 27.00 | Curtis212 | Takes 30 minutes to plan each plan, per patient | |
| Support over 4 months | 40.26 | 2 hours per day over 4 months = 121.6 (days) × 2 = 243.33/98 patients | ||
| Total | 108 | |||
| Chenoweth et al. | Training per staff (two nurses, 3 days) | 55.54 | Curtis212 | Band 7, mid-point £35,184, assumption 6 hours of training over 3 days |
| DCM trainers (two trainers, 3 days) | 9.28 | a, b | Band 7, mid-point £35,184 | |
| 8 hours of continuous 5-minute DCM observations of each participant | 4.12 | |||
| Observations completed in 3 hours by researchers at each site, total 6 hours | 3.27 | |||
| Nurse developing care plans + researchers helping | 36.02 | Curtis212 | Takes 30 minutes to plan each plan, per patient | |
| 2 hours of researcher’s time to give DCM trained/managers support | 4.12 | 2 hours per week for 1 month. Total 8 hours | ||
| 20 minutes per chart reviewed by researcher | 6.01 | Per chart 20 minutes | ||
| Researchers feeding back | 9.02 | Feedback 30 minutes | ||
| Total | 127 |
List of abbreviations
- BPSD
- behavioural and psychological symptoms of dementia
- CBT
- cognitive–behavioural therapy
- CEBM
- Centre for Evidence-based Medicine
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CMAI
- Cohen-Mansfield Agitation Inventory
- DARE
- Database of Abstracts of Reviews of Effects
- DCM
- dementia care mapping
- DEM
- dementia quality of life
- FAST
- Functional Assessment Staging
- GP
- general practitioner
- HTA
- Health Technology Assessment
- LASER-AD
- London and the South-East Region – Alzheimer‘s Disease
- MMSE
- Mini-Mental State Examination
- MNB
- monetary net benefit
- NICE
- National Institute for Health and Care Excellence
- NPI
- Neuropsychiatric Inventory
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SES
- standardised effect size