Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/45/01. The contractual start date was in December 2012. The draft report began editorial review in May 2013 and was accepted for publication in January 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Fleeman et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
The term chronic kidney disease (CKD) is used to describe abnormal kidney function (or structure) and is defined according to the presence or absence of kidney damage and level of kidney function. There are no obvious symptoms of decreasing kidney function and, hence, diagnosis often occurs when patients present for other conditions related to CKD, such as cardiovascular disease (CVD) and diabetes. As CKD is often asymptomatic in its early stages, patients are often not diagnosed until the disease has reached an advanced stage. 1 People with CKD have an increased prevalence of CVD and are more likely to die from a CVD-related cause than they are to progress to end-stage renal disease (ESRD). 1
Traditionally, serum creatinine measurements were the mainstay for initial identification of CKD. Higher levels of creatinine indicate a lower glomerular filtration rate (GFR) which indicates decreased renal function. However, in the early stages of CKD, creatinine levels may be within the normal range. Partly for this reason, it is commonly recommended that all clinical biochemistry laboratories should provide an estimated GFR (eGFR) calculated using a formula to identify affected people. In the UK, laboratories typically use the Modification of Diet in Renal Disease (MDRD) formula,2 which takes into account a patient’s age, sex and ethnicity alongside serum creatinine levels. It is recognised that eGFR is not a perfect measure. It is most likely to be inaccurate in people at extremes of body type such as those with muscle wasting disorders, amputees and malnourished people. 1 The MDRD formula tends to underestimate normal function3 and eGFR calculations assume that the level of creatinine in the blood is stable. Importantly, eGFR is only valid in adults over 18 years old, is not valid for pregnant women and has not been validated for all ethnic groups. 1 Despite this, eGFR is considered to be the ‘gold standard’ measure and it enables CKD to be classified from stage 1 (minimal or no symptoms) to stage 5 (ESRD) ( Table 1 ). Stage 3 CKD is commonly referred to as moderate CKD.
| Stagea | Description |
|---|---|
| 1 | Normal kidney function as measured by normal or increased GFR ≥ 90 ml/minute/1.73m2 with other evidence of kidney damage (e.g. urine findings, structural abnormalities or genetic trait) |
| 2 | Reduced kidney function as measured by slight decrease in GFR to between 60 and 89 ml/minute/1.73m2 with other evidence of kidney damage (e.g. urine findings, structural abnormalities or genetic trait) |
| 3A | Moderately reduced kidney function as measured by a decrease in GFR to between 45 and 59 ml/minute/1.73m2 with or without other evidence of kidney damage |
| 3B | Moderately reduced kidney function as measured by a decrease in GFR to between 30 and 44 ml/minute/1.73m2 with or without other evidence of kidney damage |
| 4 | Severely reduced kidney function as measured by a decrease in GFR to between 15 and 29 ml/minute/1.73m2 with or without other evidence of kidney damage |
| 5 | Very severe or established renal failure as measured by a GFR < 15 ml/minute/1.73m2 or a patient requiring dialysis |
No specific treatment has been found to unequivocally slow the worsening of CKD, but the control of blood pressure helps5 and there is emerging evidence that treatment with sodium bicarbonate also slows down progression. 6 Thus, where an underlying cause can be identified, treatment tends to focus on this underlying cause in order to attempt to slow progression of renal failure.
Evidence from a large data set of 177,570 patients studied over 25 years (Kaiser Permanente)7 has reported uric acid level to be associated with ESRD. Clinical evidence from randomised controlled trials (RCTs) is now emerging that allopurinol (Zyloric), a drug commonly used to treat abnormally elevated levels of uric acid in patients with gout, may slow the progression of CKD. 8 Abnormally elevated levels of uric acid in the blood are known as hyperuricaemia, which develops when there is an excess production of uric acid, decreased excretion of uric acid or a combination of both. Uric acid is associated with CVD, CKD, metabolic syndrome and hypertension. High concentrations of uric acid in the blood may result in gout. The extent to which uric acid is a cause, effect or indeed a coincidental factor for these diseases remains unknown. 9–12
Evidence is also emerging from RCTs that allopurinol may be effective in reducing cardiovascular risk in people with CKD. 13,14 In the absence of the use of an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) to control hypertension, there is also RCT evidence of a significant worsening of hypertension, a significant decline in kidney function and a significant increase in the urinary excretion of transforming growth factor beta-1 following allopurinol withdrawal. 15
Epidemiology
A systematic review of 26 population studies conducted worldwide (but none from the UK) and published in 2008 found that CKD prevalence varied widely among the study populations investigated, strongly depending on how GFR was measured or calculated. 16 However, in all populations, prevalence rates increased with age with the median prevalence rate being 7.2% in persons aged 30 years and over, while in persons aged 64 years and over it was between 23.4% and 35.8%. 16 The 2009 Health Survey for England has estimated the prevalence of stages 3–5 CKD in persons aged 16 years and over in England to be 6% (male, 5%; female, 7%), ranging from 1% of males and 2% of females aged 16–54 years to 31% of males and 36% of females aged 75 years and over. 17 According to the New Opportunities for Early Renal Intervention by Computerised Assessment project, based on data from three primary care trusts in England, the age-standardised prevalence of stages 3–5 CKD was 8.5% (male, 5.8%; female, 10.6%). 18 It has been recently found in the USA that the prevalence of CKD stages 3–5 is increasing most rapidly in those aged 60 years and over. Between 1988 and 1994 and 2003 and 2006, data from the National Health and Nutrition Examination Survey suggested a rise from 18.8% to 24.5% in this age group, whereas the prevalence of CKD stages 3–5 in people between the ages of 20 years and 39 years remained consistently below 0.5%. 19 To date, incidence estimates have been less commonly reported in the literature.
The incidence and prevalence of hyperuricaemia are not commonly studied. In a relatively recent study of the US population in 2007–8, prevalence rates for hyperuricaemia were reported to be 21.4% (men, 21.2%; women, 21.6%) compared with 3.9% for gout (men, 5.9%, women, 2.0%) in the same study. 20 A recent systematic review conducted in China has estimated the prevalence of hyperuricaemia to be similar to the US rate for men (21.6%) but not women (8.6%). 21
Description of technology under assessment
Allopurinol was developed in 1956 for use as an adjuvant in chemotherapy for leukaemia. 22 It was discovered that allopurinol inhibits conversion of hypoxanthine to xanthine to uric acid, resulting in a decrease in the production of uric acid. Allopurinol was first licensed in 1966. Cumulative sales figures estimate over 22 million patient-years for allopurinol since launch (Mary Cockburn, Medical Information Service, Aspen, 2013, personal communication).
In the UK, allopurinol is indicated for all forms of hyperuricaemia not controllable by diet (adults), secondary hyperuricaemia of differing origin (adults, children and adolescents) and in clinical complications of hyperuricaemic states, particularly manifest gout, urate nephropathy and for the dissolution and prevention of uric acid stones (adults). 23 For adults, it is also approved for the management of recurrent mixed calcium oxalate stones in concurrent hyperuricaemia when fluid, dietary and similar measures have failed. 23 In children and adolescents it is also indicated for uric acid nephropathy during the treatment of leukaemia, hereditary enzyme deficiency disorders, Lesch–Nyhan syndrome and adenine phosphoribosyltransferase deficiency. 24 In the USA, allopurinol is indicated for the management of patients with signs and symptoms of primary or secondary gout (acute attacks, tophi, joint destruction, uric acid lithiasis and/or nephropathy). 24 It is also approved for the management of patients with leukaemia, lymphoma and malignancies who are receiving cancer therapy (which causes elevations of serum and urinary uric acid levels) and the management of patients with recurrent calcium oxalate calculi whose daily uric acid excretion exceeds 800 mg/day in male patients and 750 mg/day in female patients.
The dose required to lower serum uric acid to normal or near-normal levels varies with the type of patient and severity of the disease. In the UK, according to the Medicines and Healthcare products Regulatory Agency (MHRA), a dose of 100–200 mg/day is recommended for mild conditions, 300–600 mg/day for moderately severe conditions and 700–900 mg/day in severe conditions. 23 In the USA, 200–300 mg/day is recommended for people with mild gout, 400–600 mg/day for those with moderately severe tophaceous gout and a maximum dose of 800 mg/day for those with severe conditions. 24 Allopurinol may be taken once daily, preferably after food. 23,24 However, if the daily dosage exceeds 300 mg/day, gastrointestinal intolerance may occur and, so, a divided dosage regimen may be appropriate. 23,24 A maximum dose of 400 mg/day is recommended for children and adolescents for the treatment of malignant conditions. 23
As allopurinol and its metabolites are primarily eliminated by the kidney, accumulation of the drug can occur in renal failure. 23,24 Hence, the US Food and Drug Administration (FDA) recommends that the dose of allopurinol should be reduced in people with CKD. 24 The MHRA, which granted the UK licence, is more explicit and states that in patients with impaired renal function, a starting dose of 100 mg/day should be employed and only be increased if the serum/urinary response is unsatisfactory. 23 Both the MHRA and FDA recommend a dose of 200 mg/day for people with a creatinine clearance of 10–20 ml/minute and when the creatinine clearance is < 10 ml/minute the dose should not exceed 100 mg/day. With extreme renal impairment (creatinine clearance < 3 ml/minute) the interval between doses may also need to be lengthened. 23,24
Usual care and guidelines for the management of chronic kidney disease
The recent National Institute for Health and Care Excellence (NICE) guideline makes recommendations for the treatment of people with CKD. 1 These echo the recommendations of the UK Renal Association,25 which do not differ significantly from guidelines published elsewhere in the world. In general, the aim is to control blood pressure and complications such as diabetes in people with CKD. Hence, it is recommended that treatment should consist of both lifestyle support (e.g. dietary advice, encouragement to exercise and smoking cessation interventions) and drugs, in particular, drugs to control hypertension. In addition, patients are encouraged to avoid nephrotoxic medications such as non-steroidal anti-inflammatory drugs. The precise regimen of treatment will, therefore, depend to some extent on a patient’s albumin creatinine ratio and comorbidities.
In addition to treatment for underlying causes and comorbidities, patients with more advanced stages of CKD may also require treatments for anaemia and bone disease. 1 Severe CKD requires renal replacement therapy, which may involve a form of dialysis or transplantation of a new kidney in suitable patients.
Commonly used to treat hyperuricaemia associated with gout, Lesch–Nyhan syndrome and tumour lysis syndrome (typically seen during the treatment of leukaemia), allopurinol is not commonly used to treat asymptomatic hyperuricaemia as may occur in CKD. Indeed, there are no specific guidelines for using allopurinol to treat people with CKD. According to NICE guidelines issued in 2008, there is insufficient evidence to recommend the routine use of drugs to lower uric acid levels in people with CKD who have asymptomatic hyperuricaemia. 1 Hence, patients are normally only treated if they also have gout and/or hyperuricaemia. As noted above in Chapter 1, Description of technology under assessment, in the UK a starting dose of 100 mg/day is indicated for patients with impaired renal function. 23 Professional guidelines also recommend a medication review for all people with CKD: ‘patients on analgesics, certain β-blockers (including atenolol), digoxin and allopurinol may all need their dose reducing’. 26
According to data presented to NICE in 2008, allopurinol is the most commonly used urate-lowering drug in the UK (89% of gout treatments), with most cases (98%) using doses of ≤ 300 mg/day. 27 Similarly, it was recently reported that in the USA over 90% of urate-lowering prescriptions are for allopurinol but, again, allopurinol is rarely (< 5% of patients) prescribed at doses exceeding 300 mg/day. 28 However, such doses may not be effective for the treatment of gout. 29 While it is generally recommended that doses be increased from a low dose,23,24 it has been reported that, in practice, this rarely happens. 27 According to the British Society for Rheumatology, dosing should be based on achieving a target level of serum uric acid of < 300 µmol/l. 30
Safety profile of allopurinol
Although mostly well tolerated, reports of serious adverse events (SAEs) appeared soon after allopurinol was approved for use and the first death directly related to allopurinol [from toxic epidermal necrosis (TEN)] was reported in 1970. 31 In general, harmful effects from allopurinol have been ascribed to toxicity (bone marrow suppression), hypersensitivity (rash, hepatic injury, renal injury, eosinophilia, leucocytosis), drug interactions (including with ampicillin, warfarin and certain cytotoxic agents such as azathioprine and mercaptopurine), idiopathic reactions and severe reactions resulting from the normal therapeutic effects of allopurinol. 32 Because skin reactions to allopurinol can be severe, and sometimes fatal, treatment with allopurinol should be discontinued immediately if a rash develops.
The term allopurinol hypersensitivity syndrome (AHS) has been coined to encompass the plethora of features caused by hypersensitivity to allopurinol. Singer and Wallace32 have proposed diagnostic criteria for the definition of AHS and following a review of 101 cases of AHS, Arellano and Sacristan33 modified the suggested criteria. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome is another term proposed by Bocquet et al. 34 to describe drug hypersensitivity syndromes. Table 2 summarises the diagnostic criteria proposed by each of these authors.
| Singer and Wallace32 diagnostic criteria for AHS | Arellano and Sacristan33 diagnostic criteria for AHS | Bocquet et al. 34 diagnostic criteria for DRESS |
|---|---|---|
|
|
|
Existing data support the coexistence of three mechanisms for AHS: immunological factors, genetic predisposition and accumulation of the drug. 33 People with the HLA-B*5801 allele who are treated with allopurinol have been identified to be at increased risk of severe cutaneous adverse reactions (SCARs) [AHS/DRESS or Stevens–Johnson syndrome (SJS) or TEN]. 35 It is largely in order to avoid AHS/DRESS that reduced doses of allopurinol, based on creatinine clearance, were first recommended for people with CKD. 36 However, it has been reported that some patients with gout receiving allopurinol at creatinine clearance-adjusted doses do not benefit because the dosage may become too low to be able to effectively reduce serum uric acid levels. 37
According to adverse event (AE) data submitted to the FDA between the first quarter of 2004 and the first quarter of 2012, DRESS, SJS and TEN are some of the most commonly reported AEs, albeit at an incidence of ≤ 4% of all AEs. 38 Other similarly relatively common AEs (at a frequency of 1–2%) include renal failure, pyrexia and rash which, as reported in Table 2, are associated with AHS/DRESS.
Aims and objectives of the current review
The primary aim of this systematic review was to consider the clinical effectiveness of allopurinol for people with CKD. Primarily, the review considers the evidence from RCTs in terms of effects on mortality, progression of CKD, cardiovascular risk and the effects on blood pressure and a number of disease markers. Given the importance of AEs (common and rare), a secondary aim was to consider the evidence from observational studies describing AE and quality-of-life data.
Chapter 2 Methods
Search strategy
The following databases were searched for relevant published literature on 7 January 2013.
-
MEDLINE (1946 to 7 January 2013)
-
EMBASE (1974 to 28 December 2012)
-
The Cochrane Library (Issue 1, 2013)
-
ClinicalTrials.gov (7 January 2013).
The search strategy is presented in Appendix 1 . Search terms included a combination of index terms (for the disease) and free-text words for the technologies involved (generic and trade names of the drugs). No methodological filters were employed to limit results to a specific study design. No date or language limits were applied to the search strategy, although it was recognised that studies in languages other than English would not be translated and, therefore, would be excluded. However, by quantifying the number excluded in this way, a simple if crude assessment of the likelihood of publication bias was made. In addition to the search of electronic databases, bibliographies of retrieved citations were also examined. Two manufacturers [GlaxoSmithKline who are listed as the manufacturer of Zyloric in British National Formulary (BNF) No. 64 (September 2012)39 and Aspen who were identified as the current marketing holder of Zyloric by GlaxoSmithKline] were approached for data. All the identified literature was held in the EndNote X5 software package (Thomson Reuters, NY, New York City).
Study selection
The citations identified by the search strategy were assessed for inclusion through two stages. First, two reviewers (NF and GP) independently screened all relevant titles and abstracts obtained via electronic searching to identify potentially relevant studies for inclusion in the review (screening stage 1). Second, full-text copies of the potentially relevant studies were obtained and assessed independently by the same two reviewers using the inclusion criteria outlined in Table 3 (stage 2). Any disagreements between reviewers were resolved by discussion at each stage. Studies that did not meet the inclusion criteria at stage 2 were excluded.
| Criteria | Included | Excluded |
|---|---|---|
| Population | Studies which include people with CKD | Studies which do not include any people with CKD |
| Intervention | Studies where patients in at least one treatment group are treated with allopurinol of any dose | |
| Setting | Any health-care setting (including the community and the home) | |
| Comparator | Usual care or placebo | Any treatment that cannot be considered to include usual care |
| Outcomes | Primary:
|
No study will be excluded based on its outcomes Included studies may however be excluded from the analysis if they do not report on outcomes specified in the inclusion criteria |
| Study design | RCTs for evidence of efficacy Observational studies for AE and quality-of-life data |
Animal models Preclinical and biological studies Narrative reviews, editorials, opinions For efficacy, reports published as meeting abstracts only, where insufficient methodological details are reported to allow critical appraisal of study quality Letters, commentaries and editorials |
As is evident from Table 3 , systematic reviews were neither explicitly included nor excluded at stage 2. We used identified systematic reviews pragmatically, primarily to identify any relevant studies that we had not identified from our own searches. Data were not extracted from the systematic reviews but from the primary studies included.
Quality assessment and data extraction
Data extraction forms were developed and piloted on a sample of included studies and adapted to reflect the nature of both RCT and observational studies related to AEs and quality of life. Data were extracted on study design, population characteristics and outcomes by one reviewer (NF) and independently checked for accuracy by a second reviewer (GP). Differences were resolved through discussion.
All included RCTs were assessed for risk of bias using criteria based on Centre for Reviews and Dissemination guidance for undertaking reviews in health care. 40 These criteria include key aspects of RCT design and quality. Data relating to quality assessment were extracted by one reviewer (NF) and independently checked for accuracy by a second reviewer (GP). Differences were resolved through discussion.
No universally accepted standardised quality assessment tool exists for use in observational studies. There are a multitude of observational study designs and, so, even where tools exist, applying them is problematic. For example, a review of non-randomised studies conducted by Deeks et al. 41 described seven types of observational study and identified 194 tools that could be or had been used to assess non-randomised studies. Quality assessment of observational studies was planned and piloted using a modified version of the Newcastle-Ottawa Scale;42 however, owing to the heterogeneous study designs of included studies it was impossible to extract or compare information in a meaningful and relevant manner. Therefore, we made the pragmatic decision not to quality assess the observational studies that were used to identify AE data.
Evidence synthesis
The results of the data extraction and quality assessment for each study were presented in structured tables and as a narrative summary. All summary statistics were extracted for each outcome and where possible, data were pooled. 43 Meta-analysis was carried out using a fixed-effects model using Review Manager (RevMan, The Cochrane Collaboration, London, UK) and separating outcomes by the time points at which they were reported (6, 9, 12 and 24 months). Mean differences and confidence intervals (CIs) were presented.
Heterogeneity was explored through consideration of the study populations, methods and interventions and by visualisation of results. Heterogeneity was assessed in statistical terms, by the chi-squared test for homogeneity and the I 2 statistic. 44 Where I 2 was greater than 50%, a random-effects model was employed. Owing to the limited number of studies included, planned subgroup analysis based on stage of CKD, age, sex, ethnicity, concomitant medication and comorbidities were not performed. Limitations in the available data also meant that the planned sensitivity analyses, including the use of meta-regression, were not conducted.
Chapter 3 Quantity and quality of research available
Number of studies identified and included
Electronic searching of databases resulted in 1973 references when duplicates were automatically removed in EndNote. A further 123 duplicates were removed manually; thus, we identified 1850 unique references for screening at stage 1.
Initial screening identified 77 references to obtain as full-text papers, to which inclusion criteria were applied. Twenty studies (reported in 22 citations) were included at stage 2, from which an additional 12 references were identified via hand-searching bibliographies of included studies. Thus, a total of 32 studies (34 references) were included in the review and 55 references excluded (see Appendix 2 ). All of the included references were published in English.
The most common reason for exclusion was that the publication reported only single case reports (< 10 patients). While a number of excluded references were not published in English, only two were primarily excluded for this reason. One of these publications, published in German, appeared to be an overview of the published literature and, so, would have been excluded. 45 The other publication, from Hungary,46 reported on a review of all patients (n = 11) with AHS referred to a dermatology department over a period of 4 years. This paper may have been included had it been possible to translate it. As this small study is the only non-English study that could have been included from our searches, any publication bias resulting from the exclusion of non-English studies is likely to be small.
Included citations consisted of one overview of the literature,8 one systematic review and meta-analysis,35 four RCTs13,14,47,48 and 26 other studies. 32,33,36,49–71 As shown in Figure 1 , data were only synthesised from 21 studies. This is because patients included in the reviews of case reports by Lang,60 as well as the studies of patients by Lupton64 and McInnes et al. ,65 were also included in the reviews of case reports by Hande et al. 36 and Singer and Wallace. 32 The data from these reviews of case reports were also included in Arellano and Sacristan. 33 Hence, only data from Arellano and Sacristan33 were synthesised.
FIGURE 1.
Flow diagram showing number of citations found from search and included in review.
We also contacted GlaxoSmithKline, the manufacturer of Zyloric listed in BNF No. 64 (September 2012),39 to enquire if they had additional data on allopurinol. They informed us they did not and directed us to Aspen, the current manufacturer of Zyloric. Aspen also informed us they did not have any data, but did provide us with a search of the literature (EMBASE and MEDLINE) that they had conducted using the search terms ‘chronic kidney’ and ‘allopurinol’ from 2000 onwards (Mary Cockburn, Medical Information Service, Aspen, 2013, personal communication). Their search identified 24 unique references, all of which had been identified by our searches.
As references reporting on AEs were identified via hand-searching bibliographies of included studies which appeared to be a relatively high number of references, we conducted an additional search of Ovid MEDLINE and Ovid OLDMEDLINE 1946 to 7 January 2013 with Daily Update on 4 March 2013 to ensure that we had not failed to identify any more potentially relevant studies. For this search, we used the following search term: ‘Allopurinol/ae [Adverse Effects]’ with no limits for language, year or type of study. This broad search yielded 651 citations. However, no additional studies were identified for inclusion in the review.
Reviews
Key aspects of both reviews8,35 identified by our electronic searches are presented in Table 4 .
| Study | Aim of review | Sources searched | Eligibility criteria | Included studies |
|---|---|---|---|---|
| Kabul and Shepler 20128 | To provide a brief report on the studies evaluating the use of allopurinol for delay of kidney disease progression and a discussion of the current limitations and future research needed |
|
|
Efficacy studies:
|
| Somkrua et al. 201135 | To systematically accumulate and quantitatively analyse the genetic association between the HLA-B*5801 allele and allopurinol-induced SJS/TEN |
|
Included:
|
AE studies:
|
The overview by Kabul and Shepler,8 in part, had a remit similar to our systematic review. In this review, the methods used to conduct the literature search were described (a search of electronic databases and a search of primary studies in the reference lists of identified reviews), although not in sufficient detail to replicate the searches (e.g. search terms are listed but not how these are combined). No detail is given as to how studies were selected, data extracted or quality assessed (if at all).
The systematic review and meta-analysis conducted by Somkrua et al. 35 aimed to present evidence for the genetic association between the HLA-B*5801 allele and allopurinol-induced SJS/TEN. Bibliographic databases and terms used to search these are described, as are limits (e.g. limited to English-language studies). The authors of this review also examined bibliographies of included citations in order to identify additional studies. The manner in which studies were selected, data extracted and quality assessed was described. Methods for conducting the meta-analysis (DerSimonian and Laird method72 in which statistical heterogeneity was assessed via the Q-statistic and I 2 tests44) and reasons for study exclusion were also described. The authors report that from 94 records, 88 citations were assessed for eligibility (as there were six duplicates). Six studies were included in the analysis.
Randomised controlled trials
Four RCTs13,14,47,48 involving 257 participants are included in the review and summarised in Table 5 . Two of these13,48 were also included in the review by Kabul and Shepler. 8 We also identified two further RCTs by Kao et al. 14 and Shi et al. 47 The former RCT resulted in three publications, including a full article in a peer-reviewed journal published in 201114 and two conference abstracts from 2010. 73,74 One of these abstracts74 included analysis on fewer patients than those reported by the other two citations;14,73 therefore, some findings slightly differ. The data reported in our systematic review are all taken from the most recent publication in 2011. 14
| Study | Aim of study | Size of study | Intervention/comparator | Outcomes | Length of follow-up |
|---|---|---|---|---|---|
| Goicoechea et al. 201013 |
|
113 patients | Allopurinol vs. control (usual care) |
|
Mean (SD) follow-up: 23.4 (7.8) months |
| Kao et al. 201114 |
|
53 patients | Allopurinol vs. control (placebo) |
|
9 months |
| Shi et al. 201247 | To explore the effect of lowering uric acid with allopurinol on the preservation of renal function or use of antihypertensive treatment | 40 patients | Allopurinol vs. control (usual care) |
|
6 months |
| Siu et al. 200648 | To investigate the renal effects of allopurinol treatment in hyperuricaemic people with CKD | 51 patients | Allopurinol vs. control (usual care) |
|
12 months |
As reported in Table 5 , included RCTs were all relatively small in size, including between 40 and 113 randomised patients and relatively short in duration, with trials varying from 6 months47 to a follow-up of 23.4 months (hereafter referred to as 24 months). 13 All the primary and secondary outcomes prespecified for our systematic review were assessed by at least one trial.
Two ongoing studies75,76 were also identified by our review; one of these (NCT01575379)76 was also identified in the review by Kabul and Shepler. 8 Two further studies [CKD-FIX (Controlled trial of slowing of Kidney Disease progression From the Inhibition of Xanthine oxidase)77 and PERL (Preventing Early Renal function Loss )78] not registered at ClinicalTrials.gov were identified via an external peer reviewer. Key features of the four studies are summarised in Table 6 . Two of the identified ongoing trials are also relatively small, intending to recruit between 60 and 80 patients, with an expected follow-up of 12 weeks and 24 months. The final data collection dates for primary outcome measures in these trials are June 2015 (NCT01575379)76 and September 2015 (NCT01228903)75 respectively. It should be noted that NCT0157537976 is a pilot study to inform PERL,78 which, like CKD-FIX,77 is planned to be much larger in size and much longer in duration. In CKD-FIX,77 620 patients with CKD will be randomised 1 : 1 to either allopurinol or control. Treatment will be blinded and patients will be followed up for 2 years. In PERL,78 400 patients with diabetes will be randomised 1 : 1 to either allopurinol or control and followed up for 5 years. The final data collection dates for these two trials are not known.
| Trial identifier | Aim of study | Size of study | Intervention/comparator | Outcomes | Length of follow-up |
|---|---|---|---|---|---|
| NCT0157537976 | To gather preliminary information on whether or not allopurinol can be used to prevent or delay the loss of kidney function that may accompany diabetes | 60 | Allopurinola vs. control (placebo) |
|
24 months |
| NCT0122890375 | To test the hypothesis that uric acid impairs the function of vessels in patients with kidney disease | 80 | Allopurinolb vs. control (placebo) |
|
12 weeks |
| CKD-FIX77 | To test the hypothesis that allopurinol will significantly slow kidney failure progression in patients with moderate CKD (stages 3–4) | 620 | Allopurinolc vs. control (placebo) |
|
24 months |
| PERL78 | To evaluate allopurinol in reducing kidney function loss among people with type 1 diabetes | 400 | Allopurinold vs. control (placebo) |
|
60 months |
The methodological quality of the included trials, as assessed by risk of bias, is summarised in Table 7 .
| Study | Checklist items | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomisation | Potential confounding | Baseline | Blinding | Withdrawals | Analysis | |||||||||||
| Truly random | Allocation concealment | Number stated | Eligibility criteria specified | Co-interventions identified | Comparability presented | Comparability achieved | Assessors | Administration | Participants | Procedure assessed | Imbalances/dropouts | > 80% in final analysis | Reasons stated | Intention to treat | Outcomes | |
| Goicoechea et al. 201013 | ✓ | U | ✓ | ✓ | ✓ | ✓ | pa | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓b | U |
| Kao et al. 201114 | U | U | ✓ | ✓ | ✓ | ✓ | pa | ✓ | Uc | Uc | ✗ | ✓ | ✓ | ✓ | ✓b | U |
| Shi et al. 201247 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | pa | ✓ | ✗ | ✗ | NA | ✓ | ✓ | ✓ | ✓d | U |
| Siu et al. 200648 | ✓ | U | ✓ | ✓ | ✓ | ✓ | pa | U | ✗ | ✗ | U/NA | ✓ | ✓ | ✓ | ✓b | U |
Three trials13,47,48 reported randomisation, but only one trial47 provided any information on treatment allocation. Kao et al. 14 provided no details about either. Kao et al. 14 was the only trial that claimed to be double blind although, aside from the assessors, it is unclear from reading the published paper who was blinded. In Goicoechea et al. 13 and Shi et al. 46 assessors were reported to be blinded.
In terms of dropouts from the study and follow-up, all trials13,14,47,48 accounted for dropouts and followed up > 80% of patients. It was not always clear in Goicoechea et al. 13 how many patients were included in each analysis for all outcomes. However, the principal author did provide this information on request (Dr Marion Goicoechea, Hospital General Universitario Gregori Marañón, 2013, personal communication).
Only Goicoechea et al. 13 reported to have conducted analysis on an intention-to-treat (ITT) basis, although information provided on request revealed the analyses were conducted on patients who completed the study (Dr Marion Goicoechea, Hospital General Universitario Gregori Marañón, 2013, personal communication). According to the Cochrane Collaboration,79 this may still be considered to be an ITT analysis when data can be assumed to be missing at random. There is no evidence to suggest this was not the case. Kao et al. 14 and Siu et al. 48 analysed data only from patients who received and completed their treatment. However, this may also be considered to be an ITT analysis in the same way that the analysis in Goicoechea et al. 12 could be considered to be an ITT. Shi et al. 47 may also be considered to have employed an ITT analysis as there were no reported dropouts.
All trials reported a variety of outcomes, but it was not clear how many of these end points were protocol defined and how many were exploratory. Goicoechea et al. 13 stated that their primary objective was to analyse the effect of allopurinol in patients with moderate CKD in the reduction of inflammatory markers and renal disease progression and, hence, had multiple primary outcomes. In Kao et al. ,14 the primary outcome was left ventricular mass index (LVMI). The primary outcome investigated by Shi et al. 47 was a change in renal function determined by changes in eGFR and by Siu et al,48 renal progression as defined by one of four states: stable, worsening, ESRD or death. Only Kao et al 14 presented details of power calculations required to derive sample size.
Two trials reported the results of subgroup analyses. 47,48 Shi et al. 47 reported repeated values of eGFR during the 6-month therapy for patients with eGFR< 60 ml/minute/1.73 m2. Siu et al. 48 divided the treatment group by uric acid level at the end of the study and correlation analysis was performed among three groups (0.2–0.299 mmol/l, 0.3–0.399 mmol/l and 0.4–0.45 mmol/l) with regard to percentage decrease in systolic blood pressure and serum creatinine levels. It is not clear if any of the subgroup analyses were determined a priori.
Observational studies
Because AE and quality-of-life data may not be adequately captured by RCTs (although some data were available on AEs and hospitalisations in the RCTs), studies reporting non-RCT evidence were also considered to be eligible for inclusion into the systematic review (see Table 3 ). Twenty-six additional citations reporting on AEs were included as summarised in Table 8 . Three of these were not published papers but abstracts only. 57,59,66 No studies which measured the quality of life of patients were identified.
| Study | Aim of study | Study type, size, follow-up/dates |
|---|---|---|
| Arellano and Sacristan 199333 | To review the pathophysiology, pathology and clinical findings of AHS | Retrospective review of 101 cases between 1970 and the end of 1990 |
| Atzori et al. 201249 | As part of the activity of an intensive drug surveillance programme, assessment of allopurinol cutaneous ADR frequency was conducted | Retrospective review of all patients (84 cases) reporting allopurinol cutaneous adverse reactions at a dermatology department from January 2001 until December 2010 |
| Bowie et al. 196750 | To report on renal function in 14 patients receiving allopurinol | Prospective observational study of 14 patients, mean follow-up of 12 months |
| Chiu et al. 201251 | To examine the association between the HLA-B*5801 allele and AHS in Han-Chinese patients in Hong Kong | Retrospective case–control study of 20 patients with AHS and 30 controls, identified from June 2009 to July 2011 |
| Dalbeth and Stamp 200652 | To determine the effect of published allopurinol dosing guidelines on control of hyperuricaemia in patients with gout | Retrospective review of 250 patients with gout attending rheumatology clinics from 2001 to 2004 |
| Hande et al. 198436 | To review the pharmacokinetics of allopurinol in patients with renal insufficiency to determine more appropriate drug dosages in this patient population in hopes of avoiding life-threatening allopurinol toxicity | Retrospective review of 78 cases of severe toxic reactions described in the literature from 1 January 1960 to 1 July 1982 |
| Hung et al. 200553 | To identify genetic markers for allopurinol-induced SCAR | Retrospective case–control association study of 51 patients with allopurinol-induced SJS/TEN and 228 controls (135 allopurinol-tolerant subjects and 93 healthy subjects from the general population) genotyped for HLA-A, HLA-B, HLA-C and DRB1 from 1996 to 2004 |
| Jung et al. 201154 | To determine the incidence of AHS in patients with chronic renal insufficiency according to HLA-B*5801 and clinical implication of HLA-B*5801 as a risk marker for development of allopurinol-induced hypersensitivity | Retrospectively reviewed the medical records of 448 patients with chronic renal insufficiency who took allopurinol and carried out serological HLA typing for kidney transplantation between January 2003 and May 2010 |
| Kang et al. 201155 | To explore genetic markers for allopurinol-induced SCAR | Retrospective case–control study of 25 cases of allopurinol-induced SCAR and 57 allopurinol-tolerant controls genotyped for HLA-A, HLA-B, and HLA-C from the Korean Pharmacogenetic Adverse Drug Reaction Research Network Database from 2002 to 2009 |
| Kaniwa et al. 200856 | To explore genetic biomarkers related to SJS and TEN in Japanese patients living in Japan | Retrospective case–control study of 58 patients with SJS/TEN (10 allopurinol induced) genotyped for HLA-B between July 2006 and April 2008 |
| Khabbal et al. 201257 | To report some cases of AEs attributed to allopurinol notified to a pharmacovigilance unit | Retrospective review of 10 allopurinol-induced AEs (pharmacovigilance study) |
| Khoo 200058 | To document the clinical characteristics and degree of severity of allopurinol adverse reactions in patients admitted to a local tertiary referral dermatological institution and review the indications for allopurinol therapy | Retrospective review of 13 hospital in patients with allopurinol adverse reactions over 3 years (July 1995 and June 1998) |
| Krishnamurthy 201059 | To determine the effect of allopurinol on kidney function in a male veteran population | Retrospective case–control study (50 cases, 50 controls) using pharmacy, medical and laboratory records of veterans enrolled at a health-care centre, from October 2000 to November 2006 |
| Lang 197960 | To determine the frequency and severity of severe reactions to allopurinol | Retrospective study of 20 patients (18 inpatients and 2 outpatients) seen at three teaching hospitals between 1 January 1973 and 1 May 1978 |
| Lee et al. 200861 | To document the clinical presentation of allopurinol hypersensitivity in a local population, examine the indications for urate-lowering therapy and to identify potential associations with such a syndrome | Retrospective review of 28 cases from 3783 inpatient dermatology consultations from September 2002 to September 2006 |
| Levin and Abrahams 196662 | A number of objectives including to determine whether or not the side effects of allopurinol therapy are different in patients with impaired renal function | Prospective single-group, before-and-after-treatment study of 33 patients |
| Lonjou et al. 200863 | To investigate the relationship between SJS/TEN and HLA-B in a large number of patients in a European population | Hybrid prospective and retrospective genotyping study of 150 cases of SJS/TEN (31 taking allopurinol) for HLA-B included in a European study (RegiSCAR) of SJS and TEN |
| Lupton 197964 | To review the reported cases of allopurinol hypersensitivity reactions | Retrospective review of 38 cases of AHS reported in the literature between 1970 and 1979 |
| McInnes et al. 198165 | To describe the adverse effects attributed to allopurinol | Retrospective review of 1835 out of 29,524 (33 cases) hospital inpatients treated with allopurinol from 22 hospitals monitored in a drug surveillance programme from 1966 |
| Paisansinsup and Schousboe 201166 | To consider optimal dosing | Retrospective review of 551 patients who had their serum creatinine measured while on allopurinol from 1 January 2004 to 31 December 2010 |
| Panomvana et al. 200867 | To examine the relationships between plasma oxypurinol concentration and the changes in serum urate level and renal function after taking a standard dose of allopurinol, 300 mg daily, in gout patients with renal insufficiency | Prospective single-group, 6-week follow-up before-and-after-treatment study of 27 patients |
| Singer and Wallace 198632 | To evaluate the indications for allopurinol therapy in patients and to determine whether or not some of the morbidity and mortality resulting from the drug might have been avoided | Retrospective review of 72 patients described in the literature |
| Stamp et al. 201169 | To determine the efficacy and safety of increasing the allopurinol dose above the proposed creatinine clearance-based dose in patients with gout | Prospective observational study of 83 patients recruited between March 2006 and February 2008, follow-up 12 months |
| Stamp et al. 201268 | To determine the relationship between allopurinol dosing and AHS | Retrospective case–control study (54 patients with gout who developed AHS, 157 matched controls) between 1 January 1998 and September 30, 2010 |
| Tassaneeyakul et al. 200970 | To investigate the relationship between SJS/TEN and HLA-B*5801 in a Thai population | Retrospective case–control study of 27 patients with SJS/TEN and 54 allopurinol-tolerant patients genotyped for HLA-B from one of five local hospitals in Thailand from 1995 to 2008 |
| Vazquez-Mellado et al. 200171 | To determine the prevalence of adverse reactions attributable to allopurinol in patients with primary gout according to dose and creatinine clearance rate | Retrospective study comparing (a) 52 patients who received creatinine clearance-adjusted doses of allopurinol to (b) 68 patients who received non-adjusted higher maintenance doses of allopurinol |
For reasons highlighted in Chapter 2, Quality assessment and data extraction, no attempt to assess the quality of observational studies was made.
Chapter 4 Efficacy evidence from randomised controlled trials
Study characteristics
All RCTs were published between 2005 and 2012. Two trials were conducted in Europe (Madrid, Spain,13 and unspecified locations in the UK14) and two trials were conducted in China (Guangdong47 and Hong Kong48). The Kao et al. trial14 was sponsored by the British Heart Foundation and the Shi et al. trial47 by grants from Chinese authorities (the Scientific and Technologic Committee of Guangdong Province, Guangdong Province Health Office and Guangdong Natural Science Foundation); and the other two trials did not report on financial support.
As reported in Table 9 , although each trial had its own eligibility criteria, in general, similar patients were included across the trials with the exception that Shi et al. 47 included people with immunoglobulin A nephropathy (IgAN), serum creatinine < 265 μmol/l and excluded patients receiving ACEIs or ARBs. Patients with more progressive forms of CKD, many of whom were receiving concomitant antihypertensives at baseline, were included in the other three trials. 13,14,48
| Study | Study population/size | Included | Excluded | Allopurinol dose/concomitant medication |
|---|---|---|---|---|
| Goicoechea et al. 201013 | Patients with moderate CKD (eGFR < 60 ml/min). Allopurinol (n = 57); control (n = 56); all (n = 113) | Presence of renal disease, defined as having an eGFR < 60 ml/minute; stable clinical condition in terms of no hospitalisations or cardiovascular events in the 3 months prior to screening; stable renal function (baseline serum creatinine not increased by 50% in the 3 months prior to screening) |
|
Allopurinol: 100 mg/day. The dosage of antihypertensive drugs, lipid-lowering agents, and antiplatelet drugs were continued and adjusted according to the individual patient’s clinical condition |
| Kao et al. 201114 | Patients with stage 3 CKD and LVH. Allopurinol (n = 27); control (n = 26); all (n = 53) | Reports only exclusion criteria |
|
Allopurinol: 100 mg/day for 2 weeks. If tolerated, dose of allopurinol was increased to 300 mg/day. Patients were allowed to continue all of their concomitant treatment. Patients received lifestyle modification and continued their usual care |
| Shi et al. 201247 | Hyperuricaemic IgAN patients. Allopurinol (n = 21); control (n = 19); all (n = 40) |
|
|
Allopurinol: 100 mg/day to 300 mg/day depending on baseline serum creatinine and uric acid.a Patients already taking ACEIs and ARBs (but not other antihypertensives) were excluded. Patients diagnosed with hypertension during the trial follow-up also received antihypertensive drugs with titration of CCB and β-blocker |
| Siu et al. 200648 | Hyperuricaemic patients with chronic kidney disease. Allopurinol (n = 25); control (n = 26); all (n = 51) |
|
|
Allopurinol: 100 mg/day or 200 mg/day depending on baseline serum creatinine.b Dosages of antihypertensive drugs, lipid-lowering agents, and steroid or cytotoxic drugs were continued and adjusted according to the individual patient’s clinical conditions |
In Goicoechea et al. ,13 patients were given a 100 mg/day dose of allopurinol. In Kao et al. ,14 patients also started on 100 mg/day, but this dose was increased to 300 mg/day if tolerated. In Shi et al. ,47 patients started on 200 mg/day or 300 mg/day, and in Siu et al. 48 100 mg/day to 200 mg/day. In the last two trials,47,48 the starting dose depended on their serum creatinine levels and, in both trials, the dose was adjusted according to uric acid levels.
In total, 257 patients were analysed in the four RCTs:13,14,47,48 130 patients receiving allopurinol and 127 receiving control (usual treatment/placebo).
Participant characteristics
Key baseline demographic characteristics of participants are presented in Table 10 and key clinical markers at baseline are presented in Table 11 .
| Study | Mean age, (SD) (years) | Male (%) | Indication | Renal pathology (%) | Comorbidities (%) | Concomitant medication (%) |
|---|---|---|---|---|---|---|
| Goicoechea et al. 201013 | Allopurinol, 72.1 (7.9); control, 71.4 (9.5) | Total, 64.6; allopurinol, 64.2; control, 64.9 | Patients with moderate CKD (stage 3+; eGFR < 60 ml/minute) | Allopurinol
|
Allopurinol
|
Allopurinol
|
| Kao et al. 201114 | Allopurinol, 70.6 (6.9) Control, 73.7 (5.3) |
Total, 52.8; allopurinol, 59.2; control, 46.1; p = 0.139 | Patients with stage 3 CKD and left ventricular hypertrophy | Allopurinol
|
Not reported | Allopurinol
|
| Shi et al. 201247 | Allopurinol, 39.7 (10.0); Control, 40.1 (10.8) | Total, 55.0; allopurinol, 61.9; control, 47.3 | Hyperuricaemic patients with IgAN | Not reported | All patients
|
Not reported |
| Siu et al. 200648 | Allopurinol, 42.7 (12.9) Control, 42.8 (16.8) | Total, 41.5; allopurinol, 32.0; control, 53.8; p = 0.09 | Hyperuricaemic people with mild to moderate CKD | Allopurinol
|
Allopurinol
|
Allopurinol
|
| Study | eGFR, mean (SD) (ml/minute) | Serum creatinine, mean (SD) (μmol/l) | Uric acid level, mean (SD) (mmol/l) | Systolic blood pressure, mean (SD) (mmHg) | Diastolic blood pressure, mean (SD) (mmHg) |
|---|---|---|---|---|---|
| Goicoechea et al. 201013 | Allopurinol: 40.6 (11.3) | Allopurinol: 150.28 (35.36)a | Allopurinol: 0.46 (0.12)a | Allopurinol: 147 (20) | Allopurinol: 77 (11) |
| Control: 39.5 (12.4) | Control: 159.12 (53.04)a | Control: 0.43 (0.09)a | Control: 146 (17) | Control: 76 (13) | |
| Kao et al. 201114 | Allopurinol: 44 (11) | Not reported | Allopurinol: 0.44 (0.09) | Allopurinol: 139 (14) | Allopurinol: 70 (8) |
| Control: 46 (9) | Control: 0.42 (0.08) | Control: 145 (18) | Control: 75 (8) | ||
| p = 0.427 | p = 0.575 | p = 0.164 | p = 0.036 | ||
| Shi et al. 201247 | Allopurinol: 69.5 (26.5) | Allopurinol: 114.92 (44.2)a | Allopurinol: 0.47 (0.7)a | Allopurinol: 139.1 (23.8) | Allopurinol: 88.1 (14.31) |
| Control: 63.6 (27.5) | Control: 123.76 (44.2)a | Control: 0.46 (0.07)a | Control: 140.8 (17.1) | Control: 87.36 (11.0) | |
| Siu et al. 200648 | Not reported | Allopurinol: 144.98 (55.69)a | Allopurinol: 0.58 (0.07) | Allopurinol: 138 (20) | Allopurinol: 79 (10) |
| Control: 164.42 (61.00)a | Control: 0.59 (0.10) | Control: 135 (19) | Control: 71 (14) | ||
| p = 0.27 | p = 0.68 | p = 0.25 |
As can be seen from Table 10 , all of the RCTs included patients who were being treated for CKD; none of these studies stated that they included patients with asymptomatic hyperuricaemia. The mean age of patients in all trials was relatively similar between treatment and control groups. However, it was noticeable that in the mean age of patients across trials varied, with the mean age being > 70 years in two trials13,14 and being < 43 years in the other two trials. 47,48 There were proportionately more males in the allopurinol group than in the control group in the Kao et al. 14 and Shi et al. 47 trials, whereas in Siu et al. 48 there were proportionately more females. Conversely, there was a slight minority of males in the control groups in Kao et al. 14 and Shi et al. 47 and a slight majority of males in the control group in Siu et al. 48 Goicoechea et al. 13 did not report the sex of trial participants in the published paper, but provided these data on request (Dr Marion Goicoechea, Hospital General Univesitario Gregori Marañón, 2013, personal communication). The majority of patients were male in this trial, evenly spread between the allopurinol and control groups.
Although no differences between groups were reported to be statistically significant, an examination of renal pathology, comorbidities and concomitant medication suggests some marked differences between groups in each of the trials ( Table 10 ). These included differences in interstitial nephropathy, ischaemic cardiopathy, diabetes mellitus and concomitant medication in Goicoechea et al. ,13 diabetic nephropathy and concomitant medication in Kao et al. 14 and the patients with IgAN, focal segmental glomerulosclerosis, hypertension and concomitant medication in Siu et al. 47
Comparisons across trials are problematic because of the manner in which data were recorded. For example, no patient is reported to have hypertension in Goicoechea et al. 13 (although blood pressure levels were elevated in all trials) whereas it is described as a renal pathology in around half of the patients in Kao et al. 14 and as a comorbidity in 33% of all patients in Shi et al. 47 and 78% of all patients in Siu et al. 48 There also appears to be differences in concomitant medications received across trials. While the majority of patients in all trials received ACEIs or ARBs, the proportions of patients receiving a number of other concomitant medications such as diuretics and statins did appear to be more variable although it is unclear if the same concomitant medications were recorded by all trials. Certainly, based on the reporting of the use of ACEIs or ARBs, differences in the manner of reporting are apparent: Goicoechea et al. 13 appear to include ACEIs and ARBs under the term renin–angiotensin–aldosterone system blockers, Kao et al. 14 combine ACEIs and ARBs together whereas Siu et al. 47 report the use of ACEIs and ARBs separately. Shi et al. 47 do not report on concomitant medication, although it is known that patients receiving ACEIs or ARBs at study enrolment were excluded.
No trial reported the ethnicity of its participants. However, two studies were conducted in Europe13,14 and two were conducted in China. 47,48 It may be assumed, therefore, that the ethnicity of the participants were European and Asian respectively.
Only the trial by Kao et al. 14 presented data on smoking status, an important variable for cardiovascular risk. Smoking status was similar between the two groups (smoker: 15% vs. 12%; ex-smoker: 26% vs. 28%; non-smoker: 59% vs. 60% for allopurinol vs. control respectively; p = 0.118). The only other data relating to a patient’s lifestyle were reported by Goicoechea et al. 13 who noted that all patients were advised about their diet.
Perhaps the most marked differences across trials are in terms of key clinical parameters ( Table 11 ) where differences in baseline eGFR, serum creatinine and diastolic blood pressure are apparent between Shi et al. 47 and the other three trials. 13,14,48 In Shi et al. ,47 the mean eGFR was 69.5 ml/minute/1.73 m2 [standard deviation (SD) 26.5 ml/minute/1.73 m2] in the allopurinol group and 63.6 ml/minute/1.73 m2 (SD 27.5 ml/minute/1.73 m2) in the control group, suggesting many patients with mild CKD were included. These levels were markedly higher than in Goicoechea et al. 13 and Kao et al. 14 Similarly, levels of serum creatinine were markedly lower in Shi et al. 47 than in Goicoechea et al. 13 or Siu et al. 48 In addition, diastolic blood pressure appeared to be higher in both groups in Shi et al. 47 than in patients in the other three trials. 13,14,48 Levels of systolic blood pressure were, on the whole, relatively similar across all trials. 13,14,47,48 Three trials13,14,47 reported uric acid levels, which were similar across all trials and between treatment groups.
The only reported statistical difference between groups in any of the presented clinical markers at baseline was reported in diastolic blood pressure by Kao et al. 14 In this trial, diastolic blood pressure was higher in the control group (p = 0.036). However, in Siu et al. 48 an even larger difference in diastolic blood pressure between treatment groups was reported, but this was not reported to be significant (p = 0.25). Mean diastolic blood pressure was highest in both treatment groups in the trial of IgAN patients. 47 Levels of other clinical parameters were, on the whole, similar between groups.
On balance, we decided that the participant characteristics were sufficiently similar across three trials13,14,48 for these to be considered together for inclusion in meta-analyses. It was considered that Shi et al. ,47 on the other hand, was qualitatively different to these other three trials. 13,14,48 Therefore, we conducted sensitivity analyses by including and excluding this trial from the meta-analysis.
Results of evidence synthesis
All-cause mortality
Two deaths (3.5%) were reported in Goicoechea et al. 13 Both deaths occurred in the control group. No deaths were reported in the other trials. 14,47,48
Progression of chronic kidney disease
Changes in eGFR, were reported by three trials13,14,47 and are presented in Table 12 and Figure 2 . As noted in Table 12 , the method of calculating eGFR was not always the same across studies. No significant differences over time were reported in any study and no significant differences were reported between groups at any point in time except at the end of the study in Goicoechea et al. 13 (p < 0.001) Interestingly, a significant inverse correlation between uric acid levels and eGFR (r = –0375; p = 0001) was also reported at 24 months by Goicoechea et al. 13
| Study | Treatment group | eGFR, mean (SD) (ml/minute/1.73 m2)a | Statistical test for change over time | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 9 months | 12 months | 24 months | |||
| Goicoechea et al. 201013 | Allopurinol (n = 56) | 40.8 (11.2) | 41.1 (12.9) | Not measured | 41.1 (13.2) | 42.2 (13.2) | Not significantb |
| Control (n = 57) | 39.5 (12.4) | 37.2 (14.3) | 35.6 (13.4) | 35.9 (12.3) | Not significantb | ||
| Difference between groups, p < 0.001 | |||||||
| Kao et al. 201114 | Allopurinol (n = 27) | 44 (11) | Not measured | Difference + 0.2 (6.9) | Not measured | Not measured | Not reported |
| Control (n = 26) | 46 (9) | Difference + 0.2 (5.5) | |||||
| Difference between groups, p = 0.427 | Difference between groups, p = 0.997 | ||||||
| Shi et al. 201247 | Allopurinol (n = 21) | 69.5 (26.5) | 73.2 (34.8) | Not measured | Not measured | Not measured | p = 0.2 |
| Control (n = 19) | 63.6 (27.5) | 68.9 (36.6) | p = 0.9 | ||||
| Difference between groups, p = 0.6 | Difference between groups, p = 0.2 | ||||||
Siu et al. 48 reported patients who had stable and worsening of renal function, defined, respectively, as an increase in serum creatinine level at the end of study by < 40% compared with baseline and by > 40% compared with baseline, but not yet requiring dialysis. It was reported that significantly more patients in the control group showed deterioration in kidney function at the end of the study (stable disease, 84% vs. 54%; worsening disease: 12% vs. 42%, for allopurinol and control respectively; p = 0.015).
Regarding progression to ESRD, no trial reported any patient requiring transplantation. Goicoechea et al. 13 reported that one patient in each of the allopurinol and control groups required dialysis, as did Siu et al. 48
Cardiovascular events and cardiovascular risk
We intended to consider cardiovascular events and cardiovascular risk in the following way:
-
mortality directly attributable to cardiovascular events
-
non-fatal cardiovascular events
-
number of patients with risk factors for cardiovascular disease.
No trial reported any cardiovascular mortality. Goicoechea et al. 13 reported cardiovascular events, of which there were twice as many in the control group [15/56 (27%)] as in the allopurinol group [7/57 (12%)] after 24 months. The type of cardiovascular event was not reported by treatment group. According to the authors, Kaplan–Meier survival showed that patients in the allopurinol group had lower cardiovascular risk than patients in the control group (log-rank: 4.25; p = 0.039). Cox regression analysis (adjusted for age, eGFR change and uric acid levels) estimated the decrease in risk attributable to allopurinol to be 71% [hazard ratio (HR) 0.29; 95% CI 0.09 to 0.86; p = 0.026]. The same regression analysis also showed diabetes (HR 4.38; 95% CI 1.59 to 12.09; p = 0.004), previous coronary heart disease (HR 4.49; 95% CI 1.56 to 12.86; p = 0.005) and C-reactive protein (HR 2.83; 95% CI 1.09 to 7.32; p = 0.031) to increase the risk of cardiovascular events.
The other three trials14,47,48 included clinical markers which could be considered to constitute cardiovascular risk ( Table 13 ). In Kao et al. ,14 over 9 months, blood glucose levels were reduced by a slightly greater amount in the allopurinol group than in the control group, but differences were not reported to be significant. Total cholesterol levels fell slightly in both groups in Shi et al. 47 and in the allopurinol group in Siu et al. 48 (where they rose in the control group) while in both groups in both studies, levels of high-density lipoprotein (HDL) remained constant. Levels of low-density lipoprotein (LDL) and triglyceride fell slightly in the allopurinol group in both studies and rose slightly in the control group in both studies. However, no significant differences over time or between groups were reported for any of these markers.
| Study | Levels of total cholesterol, mean (SD) (mmol/l)a | Levels of HDL cholesterol, mean (SD) (mmol/l)a | Levels of LDL cholesterol, mean (SD) (mmol/l)a | Triglyceride levels, mean (SD) (mmol/l)b | Blood glucose levels (diabetes), mean (SD) (mmol/l) |
|---|---|---|---|---|---|
| Goicoechea et al. 201013 | Not reported | Not reported | Not reported | Not reported | Not reported |
| Kao et al. 201114 | Not reported | Not reported | Not reported | Not reported | Allopurinol (n = 27): –0.80 (3.22) |
| Control (n = 26): –0.03 (0.80) | |||||
| p = 0.240 | |||||
| Shi et al. 201247 | Allopurinol (n = 21)
|
Allopurinol (n = 21)
|
Allopurinol (n = 21)
|
Allopurinol (n = 21)
|
Not reported |
Control (n = 19)
|
Control (n = 19)
|
Control (n = 19)
|
Control (n = 19)
|
||
| Difference between groups and over time not reported to be significant | Difference between groups and over time not reported to be significant | Difference between groups and over time not reported to be significant | Difference between groups and over time not reported to be significant | ||
| Siu et al. 200648 | Allopurinol (n = 25)
|
Allopurinol (n = 25)
|
Allopurinol (n = 25)
|
Allopurinol (n = 25)
|
Not reported |
Control (n = 26)
|
Control (n = 26)
|
Control (n = 26)
|
Control (n = 26)
|
||
| Difference over time not reported to be significant No comparison between groups reported | Difference over time not reported to be significant No comparison between groups reported | Difference over time not reported to be significant No comparison between groups reported | Difference over time not reported to be significant No comparison between groups reported |
Change in blood pressure
Change in blood pressure was reported by all four RCTs. 13,14,47,48 The findings from the individual trials in terms of systolic and diastolic blood pressure are presented in Tables 14 and 15 .
| Study | Treatment group | Systolic blood pressure (mmHg), mean (SD) | Statistical test for change over time | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 9 months | 12 months | 24 months | |||
| Goicoechea et al. 201013 | Allopurinol (n = 56) | 147 (20) | 145 (17) | Not measured | 142 (16) | 144 (15) | Not reported |
| Control (n = 57) | 146 (17) | 144 (16) | 141 (15) | 143 (13) | |||
| Kao et al. 201114 | Allopurinol (n = 27) | 139 (14) | –4.9 (17.64) | –6.9 (14.4) | Not measured | Not measured | Not reported |
| Control (n = 26) | 145 (18) | –8.73 (21.36) | –5.1 (15.1) | ||||
| Difference between groups, p = 0.164 | Difference between groups, p = 0.701 | Difference between groups, p = 0.644 | |||||
| Siu et al. 200648 | Allopurinol (n = 25) | 138 (20) | Not measured | Not measured | 127 (21) | Not measured | p = 0.02 |
| Control (n = 26) | 135 (19) | 135 (32) | p = 0.90 | ||||
| Difference between groups, p = 0.68 | Difference between groups, p = 0.31 | ||||||
| Study | Treatment group | Diastolic blood pressure (mmHg), mean (SD) | Statistical test for change over time | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 9 months | 12 months | 24 months | |||
| Goicoechea et al. 201013 | Allopurinol (n = 56) | 77 (11) | 76 (9) | Not measured | 74 (9) | 73 (10) | Not reported |
| Control (n = 57) | 76 (13) | 77 (9) | 75 (8) | 74 (10) | |||
| Kao et al. 201114 | Allopurinol (n = 27) | 70 (8) | –1.85 (11.64) | –3.3 (8.6) | Not measured | Not measured | Not reported |
| Control (n = 26) | 75 (8) | –4.15 (12.95) | –2.5 (9.1) | ||||
| Difference between groups, p = 0.036 | Difference between groups, p = 0.498 | p = 0.741 | |||||
| Siu et al. 200648 | Allopurinol (n = 25) | 79 (10) | Not measured | Not measured | 75 (10) | Not measured | p = 0.12 |
| Control (n = 26) | 71 (14) | 71 (13) | p = 0.89 | ||||
| Difference between groups, p = 0.25 | Difference between groups, p = 0.21 | ||||||
In Shi et al. ,47 diastolic blood pressure levels appeared to be higher in both groups than the other three studies. Changes in blood pressure in this trial were confined to the very small subgroup of patients with normal blood pressure at baseline (n = 17) and were reported as mean arterial pressure. In this subgroup, a significant reduction in mean arterial pressure over time was reported for the allopurinol group [n = 9; baseline: 92.9 mmHg (SD 10.1 mmHg); 6 months: 83.7 mmHg (SD 4.5 mmHg); p < 0.01] but not the control group [n = 8; baseline: 93.7 mmHg (SD 5.4 mmHg); 6 months: 93.8 mmHg (SD 4.1 mmHg); p = 0.9]). A strong correlation was observed between serum uric acid and mean arterial pressure (r = 0.388; p < 0.001).
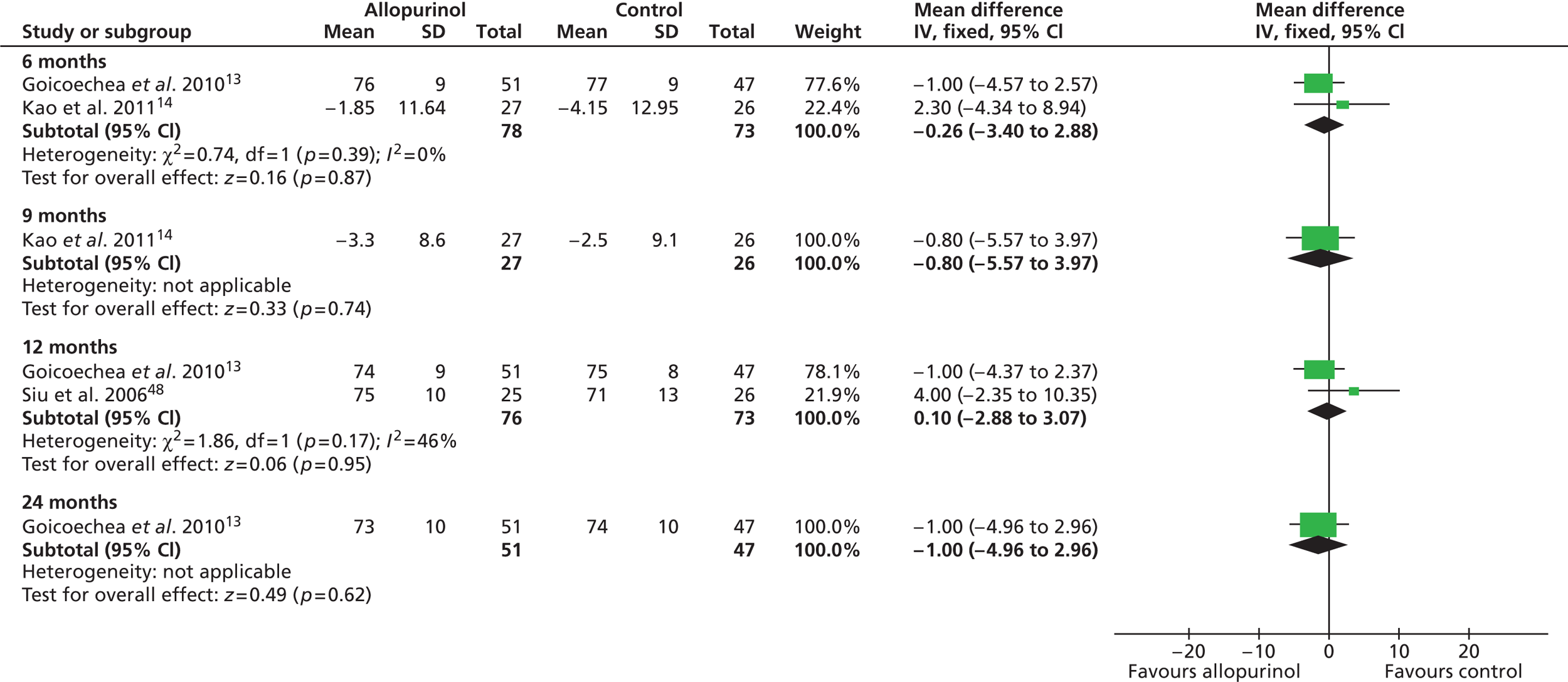
As previously shown in Table 11 and also evident from Table 14 , baseline levels of blood pressure were reasonably similar across three trials13,14,48 which, as highlighted in Chapter 4, Participant characteristics, appeared to include broadly similar types of patients and so these were included in meta-analyses. The findings from these meta-analyses are presented in Figures 3 and 4 respectively. Overall, no significant differences between the treatment groups were found at any time point, both measures of blood pressure remaining largely unaltered over time.
FIGURE 3.
Systolic blood pressure at 6, 12 and 24 months and difference in systolic blood pressure at 9 months.
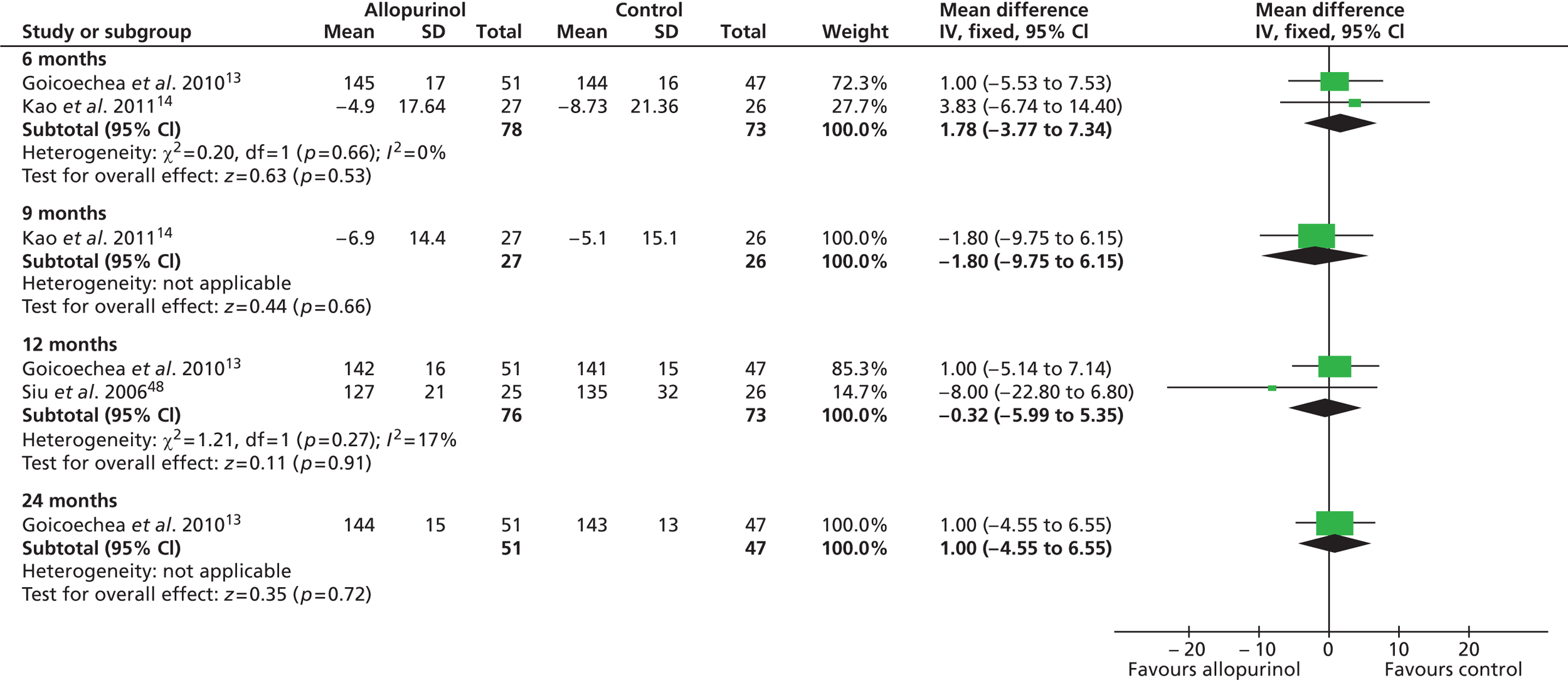
FIGURE 4.
Change in diastolic blood pressure at 6, 12 and 24 months and difference in diastolic blood pressure at 9 months.
Secondary outcomes
The following secondary outcomes were specified in the systematic review protocol:
-
change in uric acid levels
-
change in serum creatinine levels
-
change in albuminuria levels
-
number of patients with endothelial dysfunction
-
number of patients with left ventricular hypertrophy
-
change in number of blood pressure medications
-
AEs
-
quality of life.
Change in uric acid levels
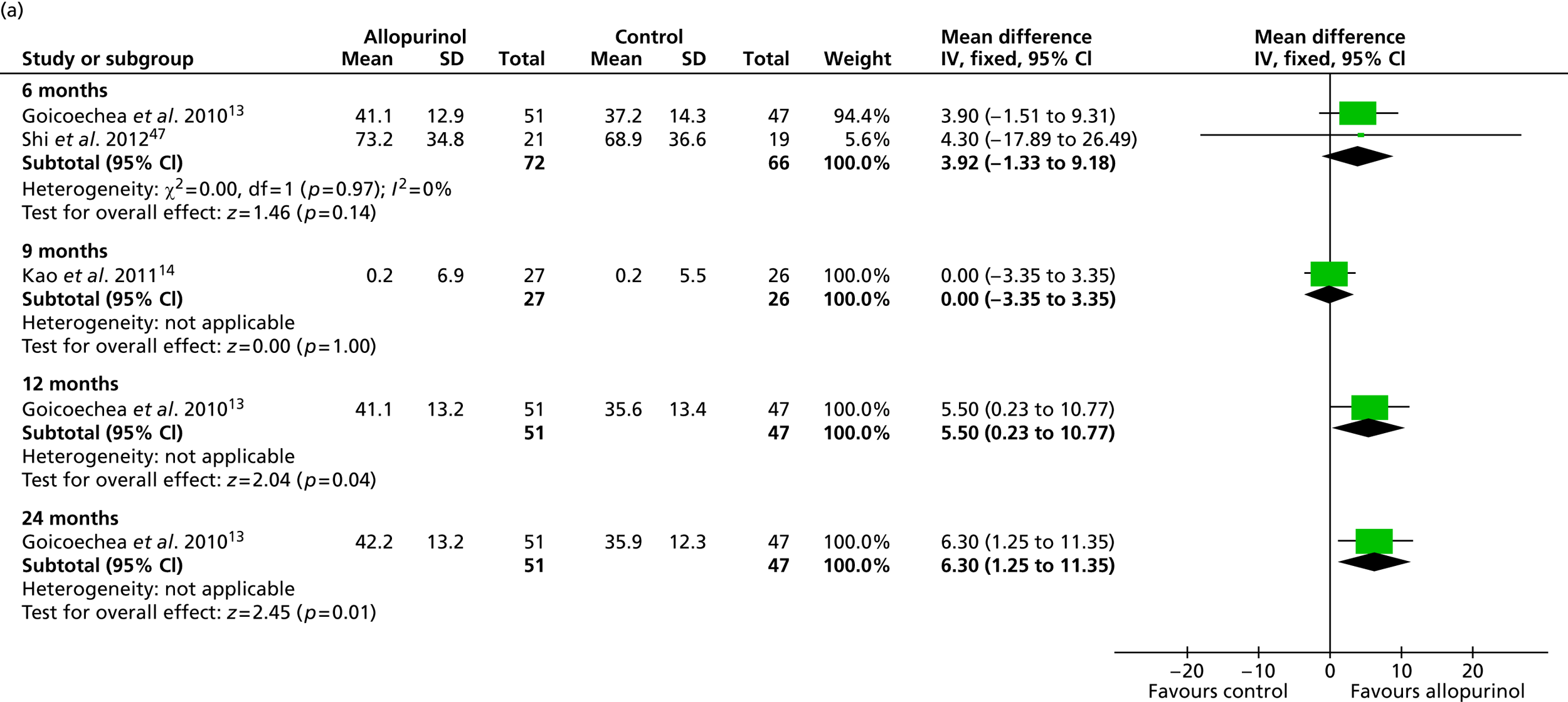
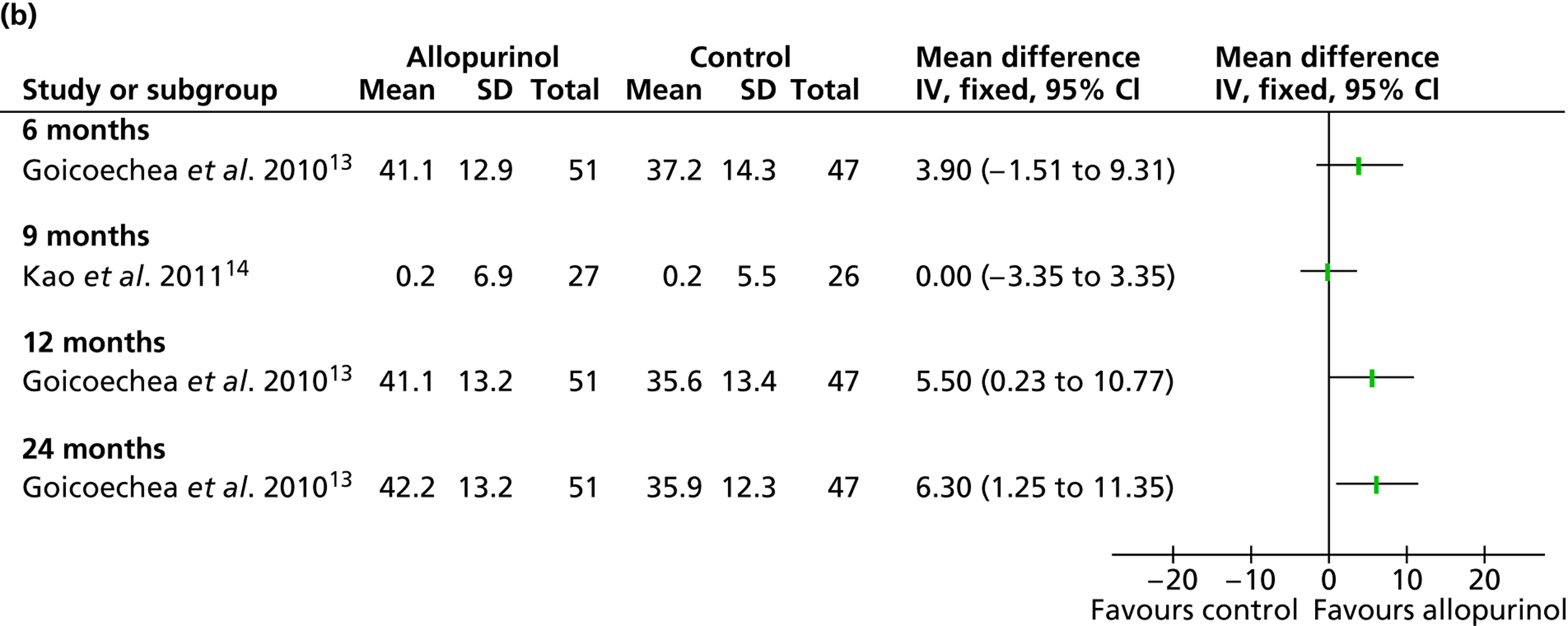
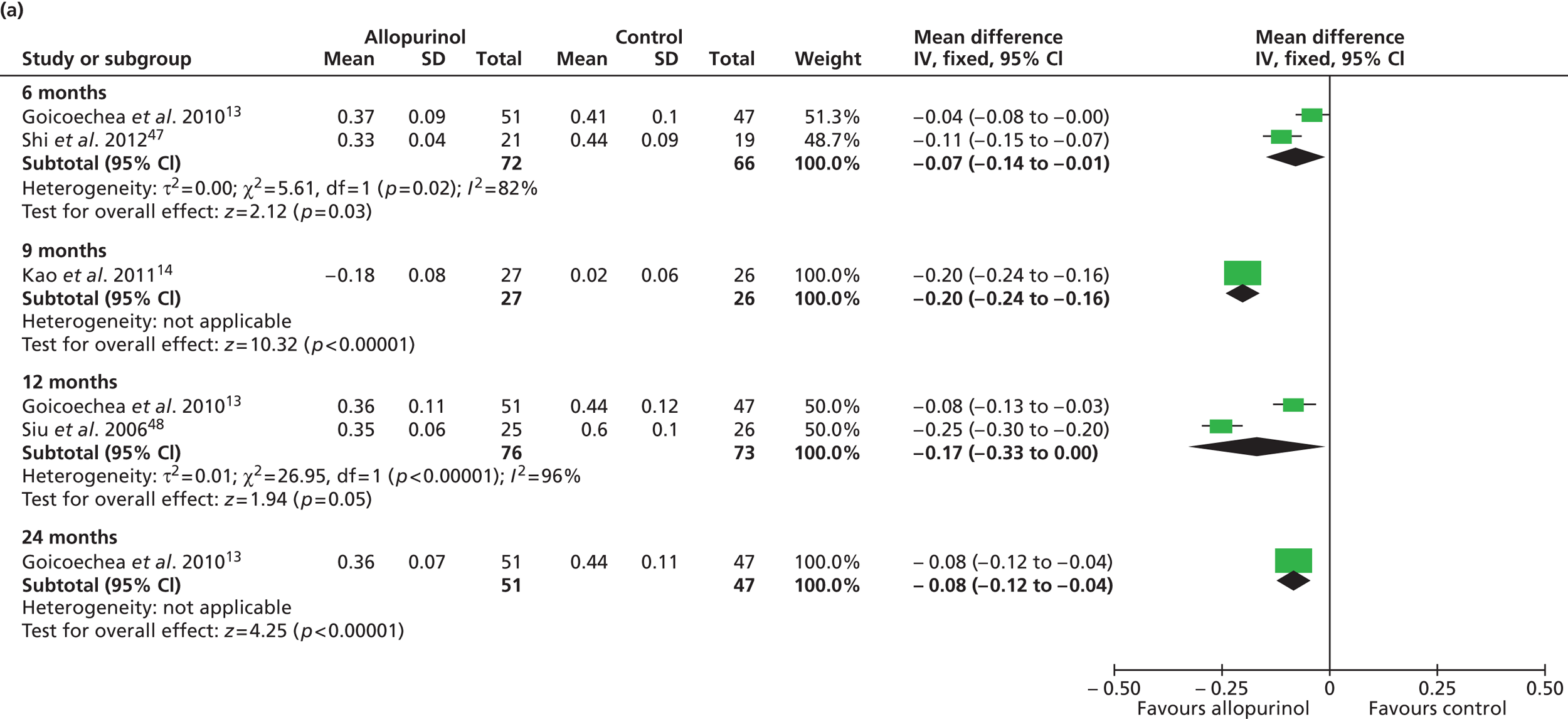
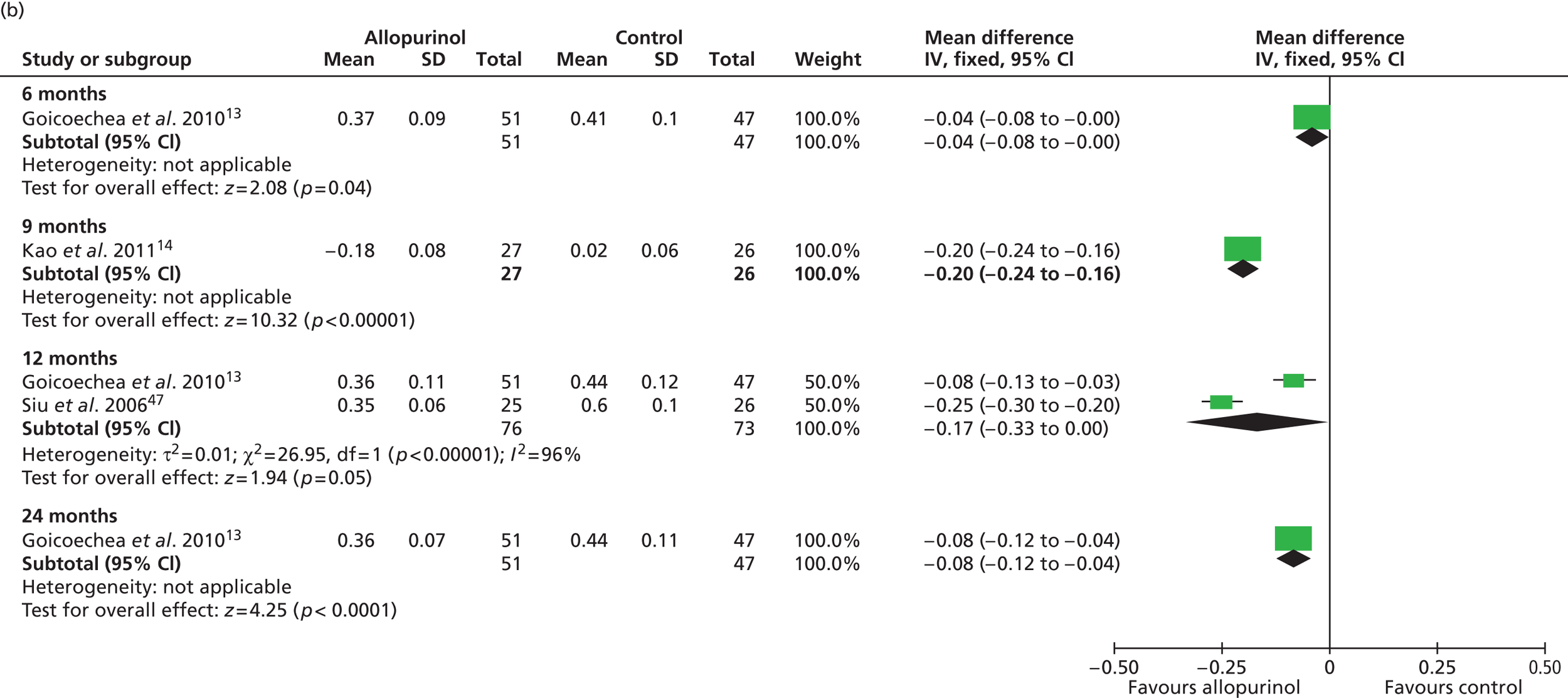
Uric acid level was the only secondary outcome measured in all of the included RCTs. As summarised in Table 16 , changes over time were reported to be significantly improved in the allopurinol group by all four trials that reported this measure. 13,14,47,48 Pooled data from Goicoechea et al. 13 and Shi et al. 47 resulted in a significant difference in uric acid levels favouring allopurinol at 6 months [mean difference –0.07 mmol/l (95% CI –0.14 to –0.01 mmol/l)]. Pooled data from Goicoechea et al. 13 and Siu et al. 48 show a borderline significant improvement at 12 months [mean difference –0.17 mmol/l (95% CI –0.33 to 0.00 mmol/l)] ( Figure 5 ). However, a large amount of statistical heterogeneity was observed.
| Study | Treatment group | Uric acid levels (mmol/l), mean (SD) | Statistical test for change over time | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 9 months | 12 months | 24 months | |||
| Goicoechea et al. 201013 | Allopurinol (n = 56) | 0.47 (0.12)a | 0.37 (0.09)a | Not measured | 0.36 (0.11)a | 0.36 (0.07)a | p < 0.001 |
| Control (n = 57) | 0.43 (0.10)a | 0.41 (0.10)a | 0.44 (0.12)a | 0.44 (0.1)a | Not significant | ||
| Differences between groups, p < 0.016 | |||||||
| Kao et al. 201114 | Allopurinol (n = 27) | 0.47 (0.7)a | Not measured | –0.18 (0.08)a | Not measured | Not measured | Not reported |
| Control (n = 26) | 0.46 (0.07)a | +0.02 (0.06)a | |||||
| Difference between groups in change over time, p < 0.001 | |||||||
| Shi et al. 201247 | Allopurinol (n = 21) | 0.47 (0.07)a | 0.33 (0.04)a | Not measured | Not measured | Not measured | p < 0.001 |
| Control (n = 19) | 0.46 (0.07)a | 0.44 (0.09)a | p = 0.03 | ||||
| Difference between groups at start of study, p = 0.7 | Difference between groups at end of study, p < 0.001 | ||||||
| Siu et al. 200648 | Allopurinol (n = 25) | 0.58 (0.07) | Not measured | Not measured | 0.35 (0.06) | Not measured | p < 0.001 |
| Control (n = 26) | 0.59 (0.10) | 0.60 (0.10) | Not significant | ||||
Significant differences in uric acid levels were also reported in Kao et al. 14 at 9 months and by Goicoechea et al. 13 at 24 months. Interestingly, Shi et al. 47 reported significant differences over time (6 months) in the control group as well as in the allopurinol group. However, differences between groups at the end of study were also reported to be significantly in favour of allopurinol.
Siu et al. 48 report results of a subgroup analysis in which the treatment group was divided into three categories according to uric acid level at the end of the study (0.2–0.299 mmol/l; 0.3–0.399 mmol/l; 0.4–0.45 mmol/l). In these very small subgroups, no clinical correlation could be shown for the three categories of target uric acid levels in relation to change in systolic blood pressure (p = 0.24). Similarly, no clinical correlation could be shown in relation to change in serum creatinine level (p = 0.32).
Change in serum creatinine levels
Siu et al. 48 was the only trial to report on changes in serum creatinine. In the allopurinol group, these increased over 12 months from 146 µmol/l (SD 56 µmol/l) to 176 µmol/l (SD 81 µmol/l). This increase was not statistically significant (p = 0.15). In the control group, serum creatinine levels increased from 164 µmol/l (SD 61 µmol/l) to 255 µmol/l (85 µmol/l), a change over time that was statistically significant (p = 0.003). However, the difference between groups at 12 months was not significant (p = 0.08).
Change in albuminuria levels
Change in albuminuria levels were reported only by Goicoechea et al. 13 In the allopurinol group the median decreased from 36 mg/day at baseline to 16 mg/day at 12 months. In the control group the median (interquartile range) value increased from 32 mg/day to 51 mg/day. Despite these apparent large differences over time and between groups, no significant differences were reported either in the change over time or between groups (p-values not presented).
Number of patients with endothelial dysfunction and number of patients with left ventricular hypertrophy
End points relating to endothelial dysfunction and left ventricular hypertrophy were addressed only by Kao et al. 14 In this trial, the primary outcome was change in LVMI at 9 months which in the allopurinol group was –1.42 g/m2 (SD 4.67 g/m2) and in the control +1.28 g/m2 (4.45 g/m2), a significant difference (p = 0.036). Endothelial dysfunction was measured by a change in flow-mediated dilatation (FMD) of the brachial artery. In the allopurinol group, the percentage change in FMD response to hyperaemia was 1.72% (SD 2.95%) at 6 months and 1.26% (SD 3.06%) at 9 months, compared with 0.03% (SD 2.84%) and 1.05% (SD 2.84%) respectively in the control group. At both points in time, differences between groups were statistically significant (p = 0.053 and p = 0.009 respectively). The authors state that no correlations were found between urate levels (either its baseline or its change) and the changes seen in LVMI or FMD.
Change in number of blood pressure medications
Three of the trials14,47,48 reported change in blood pressure medications ( Table 17 ). In Kao et al. ,14 a greater proportion of control group patients commenced medication and a greater proportion of allopurinol group patients stopped medication. However, no significant differences between groups were reported. In Siu et al. ,48 the number of patients who were receiving antihypertensives at the end of the study remained the same as that at the beginning of the study in both groups (although proportionately, there was an increase in the allopurinol group and a decrease in the control group); no significant differences between groups were reported at the beginning or end of study. In Shi et al. ,47 however, significant differences between treatment groups were reported at the end of the study (p = 0.003). In this trial, no patients commenced antihypertensive medication in the allopurinol group but 78% reduced treatment (including one patient who stopped) whereas in the control group, no patient reduced their medication but 33% increased it. However, the numbers of patients receiving medication in this trial are very small.
| Study | Measure of blood pressure medication |
|---|---|
| Goicoechea et al. 201013 | Not reported |
| Kao et al. 201114 | Allopurinol
|
Control
|
|
| Difference between groups in commencing medication, p = 0.150 | |
| Difference between groups in stopping medication, p = 0.258 | |
| Shi et al. 201247 | Allopurinol
|
Control
|
|
| Differences between groups, p = 0.003 | |
| Siu et al. 200648 | Allopurinol
|
Control
|
|
| Difference between groups at start of study: ACEI, p = 0.90; ARB, p = 0.38 | |
| Difference between groups at end of study: ACEI, p = 0.77; ARB, p = 0.11 |
Chapter 5 Adverse event evidence
Study characteristics
Adverse event data were available from 25 studies of patients being treated for various reasons, not just CKD: four RCTs13,14,47,48 and 21 observational studies (four prospective observational cohort studies,50,62,67,69 a hybrid prospective/retrospective study,63 eight retrospective case–control studies,51,53–56,59,68,70 six retrospective cohort studies,49,52,58,61,66,71 one review of case reports33 and a pharmacovigilance study57). In total, the 25 studies reported 2629 patients treated with allopurinol (including 433 patients chosen as allopurinol-tolerant controls) and 200 patients not treated with allopurinol. Study characteristics are summarised in Appendix 3, Table 23 .
Participant characteristics
In terms of the indications for allopurinol, these varied across and, in some cases, within studies. In seven studies,13,14,47,48,50,62,67 all patients had CKD and, in three of these,50,62,67 all patients also had gout. Gout was also an indication in nine other studies. 52,55,58,61,66,68–71 Hyperuricaemia was stated to be an indication in six studies,53–55,59,63,70 while patients with asymptomatic hyperuricaemia were known to be included in four others. 33,58,61 Two studies56,57 provided no information on the indication for allopurinol.
The mean age of patients varied considerably across the trials from 39 years in the allopurinol group of Shi et al. 47 to 74 years in Atzori et al. 49 The range of ages in studies also varied considerably across studies; the largest range was in Stamp et al. ,68 in which the age ranged between 23 and 92 years. Overall, the youngest patient was 8 years62 and the oldest patient was 96 years. 49
The proportion of males included in studies also varied by study. Most studies reported that the majority of patients were male, with males being a minority in only three studies 48,49,61 For studies that included a control group,13,14,47,48,51,53–55,59,68,70 the proportion of males was well balanced in both arms in only two. 13,68 The majority of studies had a greater proportion of males in the control group48,51,53–55,59,70 than the allopurinol group. 14,47
Thirteen studies50–56,58,61,63,68–70 explicitly reported the ethnicity of their subjects, although the ethnicity is implied (to be European) in another. 49 Four of these studies50,52,68,69 were conducted in New Zealand, where the ethnicity of patients varied across studies. In Stamp et al. ,69 the majority (82%) of patients were of European ancestry and this ethnic group also made up a large proportion of cases (48%) in Stamp et al. 68 In this latter study, there were significant differences between cases and controls, largely as a result of differences in the proportion of patients of Chinese origin (19% cases vs. < 1% controls) and Maori and Pacific Islanders (30% cases vs. 48% controls). While only 14% of patients were Maori and Pacific Islanders in Stamp et al. ,69 these accounted for 72% of patients in Dalbeth and Stamp (Maori: 26%; Pacific: 46%). 52 Reporting of ethnic origin was incomplete in Bowie et al. 50 Eight studies were conducted in Asian countries and consisted of patient populations primarily (> 90%) of Singaporean-Chinese origin in two studies,58,61 Han-Chinese in two studies, 51,53 Korean in two studies,54,55 and, in the remaining two, Thai70 and Japanese. 56 The remaining study which reported on ethnicity was conducted in Europe63 and the majority (> 85%) were described as European.
Baseline demographic characteristics are summarised in Appendix 3, Table 24 .
Adverse event data
A broad range of AE data were collected, as summarised in Table 18 .
| AE of any type or severity | SCARs only | Allopurinol dose and AEs | Genetics and SCARs | Other AE data |
|---|---|---|---|---|
Total (n = 11)
|
Total (n = 13)
|
Total (n = 4) | Total (n = 8) | Total (n = 4) |
Adverse events of any type or severity
Eleven studies, comprising four RCTs,13,14,47,48 four prospective studies,50,62,67,69 two retrospective cohort studies66,71 and one case–control study,59 reported a broad range of AEs not limited to SCARs. The findings from the 11 studies are summarised in Table 19 .
| Study type | Number of studies/subjects | Any type of AE (%) | Any type of SAE (%) | Withdrawal of allopurinol due to AE (%) | Deaths due to AEs (%) |
|---|---|---|---|---|---|
| RCT | 4/257 | ||||
| Allopurinol: 130 | Allopurinol: 0.8 | Allopurinol: 0.8a | 4.6 | Allopurinol: 0 | |
| Control: 127 | Control: 0 | Control: 0a | Control: 0 | ||
| Prospective observational | 4/157 | ||||
| Allopurinol: 157 | Allopurinol: 12.1 | Allopurinol: 0 | 0.8 | 0 | |
| Retrospective cohort and case–control | 3/771 | ||||
| Allopurinol: 721 | Allopurinol: 10.1 | Allopurinol: 0.1 | 0 | Allopurinol: 0 | |
| Control: 50 | Control: 0 | Control: 0 | |||
| All studies | 11/1185 | ||||
| Allopurinol: 1108 | Allopurinol: 9.2 | Allopurinol: 0.2 | 0.8 | Allopurinol: 0 | |
| Control: 177 | Control: 0 | Control: 0 | Control: 0 |
Across all 11 studies, the proportion of AEs reported by patients receiving allopurinol was 9.2%, but the proportion varied across studies and by study type. In RCTs, only one patient (0.8%) treated with allopurinol reported an AE;14 however, the type of AE was not reported. The proportion of AEs reported by patients receiving allopurinol in observational studies was higher than in RCTs (prospective: 12.1%; retrospective: 10.1%). Where specified, the most common AEs reported were rash,50,62,69,71 and gastrointestinal problems. 50,59,62,67,69 No AEs were reported by patients who did not receive allopurinol. Only two (< 1%) SAEs were reported by patients taking allopurinol. In Siu et al. ,48 the type of SAE was not specified, whereas in Vasquez-Mellado et al. 71 the SAE was identified as AHS. In RCTs, three patients in Kao et al. ,14 two patients in Goicoechea et al. 13 and one patient in Siu et al. 48 stopped taking allopurinol because of AEs. Two patients in the prospective observational study by Stamp et al. 69 also had to withdraw because of an AE. With the exception of Goicoechea et al. ,13 in which both patients stopped allopurinol because of gastrointestinal problems, the AEs resulting in study withdrawal were rash in five instances12,48,69 and arthralgia in another. 14 No deaths from AEs were reported in the 11 studies.
Severe cutaneous adverse reactions
Thirteen studies33,49,51–56,58,61,63,68,70 reported SCARs experienced by patients. As shown in Table 20 , types of SCARs reported on varied from study to study. With the exception of Lonjou et al. ,63 which utilised both prospective and retrospective study designs, all studies reporting on AEs were retrospective. It was possible to estimate the incidence of SCARs from only two studies. 52,54 In Dalbeth and Stamp,52 the incidence of SCARs was 2%. Focusing only on AHS, Jung et al. 54 reported the incidence to be 2% in patients with chronic renal insufficiency who were taking allopurinol and who carried out serological human leucocyte antigen (HLA) typing for future kidney transplantation.
| Study | Type of SCAR reported | Time to SCAR | SJS (%) | SJS/TEN (%) | TEN (%) | AHS (%) | Death from SCAR (%) |
|---|---|---|---|---|---|---|---|
| Arrellano and Sacristan 199333 | AHS | Mean 47 days (SD 109 days) (range 1–728 days) | 9a | Not reported | 26 | 100 | 27 |
| Atzori et al. 201249 | Any SCAR | Lesion onset generally occurred 2–4 weeks after drug intake | 21 | 5 | 11 | 4 | 12 |
| Chiu et al. 201251 | AHS | Median | 30b | Not reported | 35 | 30 | 10 |
| Case: 24.5 days (range 10–56 days) | |||||||
| Control: 4.5 years (range 1–15 years) | |||||||
| p < 0.001 | |||||||
| Dalbeth and Stamp 200752 | AHS | Not reported | Not reported | Not reported | Not reported | 100 | 0 |
| Hung et al. 200553 | Any SCAR | Median | 25 | 10 | 6 | 59 | Not reported |
| Cases: 26 days (range 1–56 days) | |||||||
| Control: 22 months (range 6–107 months) | |||||||
| p < 0.0001 | |||||||
| Jung et al. 201154 | Any SCAR | Mean | Not reported | 13 | 0 | 44 | Not reported |
| Case: 59.1 days (SD 45.5 days) | |||||||
| Control: 887.1 days (SD 821.3 days) | |||||||
| p < 0.001 | |||||||
| Kang et al. 201155 | Any SCAR | Median | 0 | 20 | 0 | 80 | Not reported |
| All SCAR cases: 1.0 month (range 0.2–5.3) months | |||||||
| SJS/TEN cases: 0.7 months (range 0.2–1.1 months) | |||||||
| AHS cases: 1.0 month (range 0.7–5.3 months) | |||||||
| Control: 29.1 months (range 6–72 months) | |||||||
| Kaniwa et al. 200856 | SJS or TEN | Not reported | 70 | 0 | 30 | Not reported | Not reported |
| Khoo and Leow 200058 | Any SCAR | Mean: 21 days (range 4 to 54 days) | 31a | Not reported | Not reported | Not reported | 0 |
| Lee et al. 200861 | Any SCAR | Mean: 30 days | 7 | 11 | Not reported | Not reported | 18 |
| Median: 30 days (range 13–42 days) | |||||||
| Lonjou et al. 200863 | SJS or TEN | Not reported | 52 | 35 | 13 | Not reported | Not reported |
| Stamp et al. 201268 | AHS | Median: 30 days (range 1–1080 days) | 7a | Not reported | 19 | Not reported | 6 |
| Tassaneeyakul et al. 200970 | SJS or TEN | Median | 81 | 4 | 15 | Not reported | Not reported |
| Case: 14 days (range 3–50 days) | |||||||
| Control: 26 days (range 3–600 days) | |||||||
| p < 0.05 |
The most common types of SCARs were SJS, SJS/TEN, TEN and AHS. Other symptoms reported across studies included fever,33,51,58,61,68 leucocytosis, eosinophilia,33,51,58,61,68 transaminitis33,58,61 and renal impairment61,68 which are all symptoms associated with AHS (see Table 2 ). Severe cutaneous adverse reactions typically occurred within the first 2 months of commencing allopurinol, although this did vary from study to study. Mortality rates from SCARs varied across studies from 0%58 to 27%. 33 Interestingly, in this last study, mortality was reported to be slightly higher in patients with TEN than among those who exhibited other types of SCARs, although no figure was provided. However, the mortality rate was not increased in patients with history of renal failure, or in those who developed renal failure or had an exacerbation of their renal failure.
Five13,49,51,58,61,68 of the included studies reported on hospitalisation from AEs: two case–control studies51,68 and three retrospective cohorts. 48,58,61 In Chiu et al. ,51 all patients with SCARs required hospitalisation, whereas in Stamp et al. 68 80% of patients with AHS required hospitalisation [of which 14% were admitted to the intensive care unit (ICU)]. Controls in both these studies were patients receiving allopurinol who did not develop SCARs. Atzori et al. 49 reported that, in a dermatology unit over 10 years,49 hospitalisation was required for 96% of all patients with an allopurinol-induced SCAR. In these patients, the length of stay ranged from 7 days to 43 days. Two further studies58,61 were studies of inpatients and, hence, all patients were hospitalised. In Khoo and Leow58 the length of stay in the hospital ranged from 3 days to 14 days, with a mean of 7 days. In Lee et al. 61 the average length of stay was 16 days (range 5–48 days). In this last study, it was reported that 14% required ICU care and emergent haemodialysis for multiorgan failure.
Allopurinol dose and adverse events
Two studies59,66 reported on the relationship between the allopurinol dose and relatively minor AEs. The mean dose for all patients was reported to be between 221 mg/day59 and 227 mg/day (with a median of 300 mg/day). 66 In these studies the proportion of patients reporting an AE was 6% and 12% respectively.
Three studies66,69,71 further considered the relationship between AEs and recommended allopurinol doses based on creatinine clearance according to guidelines developed by Hande et al. 36 In an analysis adjusted for age, sex, diabetes mellitus, ischaemic heart disease, hypertension, use of diuretics and use of aspirin, Paisansinsup and Schousboe66 reported no significant difference in AEs between those treated with higher than recommended doses of allopurinol and those treated with doses within or below the recommended dose [odds ratio (OR) 0.84, 95% CI 0.49 to 1.46]. Stamp et al. 69 reported that all [3/34 (9%)] patients with an AE (all rashes) occurred when allopurinol was increased above the recommended level. In two (8%) of these patients, allopurinol needed to be stopped; for the other patient, lowering the dose to 300mg/day was sufficient. Vasquez-Mellado et al. 71 reported the prevalence of any type of AE in a cohort of patients who received creatinine clearance-adjusted maintenance doses of allopurinol (group A) or non-adjusted higher maintenance doses of allopurinol (group B). Only patients in group B were reported to have renal failure [30/68 (44%)]. The prevalence of AEs was similar between groups (4% in group A, 3% in group B). The only SAE (AHS) was reported in group A. Despite adjustments for dose based on creatinine clearance, the majority of patients in both groups received a dose of 300 mg/day (88% and 91% respectively).
The mean or median dose in relation to patients with and without SCAR was reported by seven studies. 33,51–55,68 Mean or median doses in groups of patients experiencing a SCAR varied across the studies from 100 mg/day to 300 mg/day. In five studies51,53–55,68 where patients with SCARs were compared with controls, in four instances51,54,55,68 the mean or median dose was higher in the group of cases.
In relation to recommended doses of allopurinol based on creatinine clearance according to the Hande et al. 36 guidelines, Dalbeth and Stamp,52 reported that no patient with AHS was taking a higher than recommended dose. Stamp et al. 68 provided comprehensive data on both starting dose and allopurinol dose at the time of occurrence of AHS. In this study, cases were receiving a significantly higher allopurinol dose than controls both at the beginning of the study and at the time AHS occurred and the authors also report that there was a significant increase in the proportion of patients developing AHS as the allopurinol dose corrected for the estimated GFR increased. However, 10 out of 53 cases (19%) had their allopurinol dose increased compared with 50 out of 150 controls (33%). In cases, the mean increase was 197.5 mg/day, and in controls the mean increase in allopurinol was 110.5 mg/day. Allowing for matching between cases and controls, the mean increase in dosage was significantly greater in the cases (p = 0.002). At the time of the drug reaction, 12% of cases and 26% of controls were receiving the creatinine clearance-based allopurinol dose. Multivariate analysis, allowing for the effects of ethnicity and tophi with matching between cases and controls, revealed a strong dose–response relationship between the starting dose of allopurinol adjusted for eGFR and the risk of AHS (overall dose effect p < 0.001).
Genetics and adverse events
The findings from eight studies49,51,53–56,63,70 that examined the association between the HLA-B*5801 allele and SCAR are summarised in Table 21 . In populations of all ethnicities, the HLA-B*5801 allele was found to be strongly associated with SCAR, particularly in Chinese and Korean populations where >90% to 100% of patients with SCAR possessed this allele. Odds ratios varied from 80 (95% CI 34 to 187; p < 0.0001) in a European study63 to 580.3 (95% CI 34.4 to 9780.9; p < 0.0001) in Han–Chinese patients residing in Taiwan. In addition to the primary studies, the meta-analysis by Somkrua et al. 35 which included four studies53–55,70 of Asian populations reported 54 out of 55 SJS/TEN cases with the HLA-B*5801 allele compared with 74 out of 678 matched controls (OR 96.60, 95% CI 24.49 to 381.00, p < 0.001).
| Study | Frequency of HLA-B*5801 allele in patients reporting AEs | Association of HLA-B*5801 allele and AEs |
|---|---|---|
| Atzori et al. 201249 | SJS/TEN: 4/4 (100%) | Not estimated |
| Chiu et al. 201251 | SJS: 6/6 (100%) | Association of HLA-B*5801 allele and allopurinol-induced SCAR: OR 123.5 (95% CI 12.8 to 1195.1; p < 0.0001) |
| TEN: 7/7 (100%) | ||
| DRESS: 6/6 (100%) | Association of HLA-B*5801 allele and allopurinol SCAR excluding patient with erythema multiforme major: OR 229.7 (95% CI 11.7 to 4520.4; p < 0.0001) | |
| Erythema multiforme major: 0/1 | ||
| Hung et al. 200553 | Case: 51/51 (100%) | Association of HLA-B*5801 and SJS/TEN: OR 580.3 (95% CI 34.4 to 9780.9; p < 0.0001)a |
| Control: 20/135 (15%) | ||
| Jung et al. 201154 | SCAR: 9/9 (100%) | Association of HLA-B*5801 and SCAR: OR 179.3 (95% CI 10.2 to 3151.7; p < 0.0001) |
| No SCAR: 41/432 (9%) | ||
| Kang et al. 201155 | SCAR: 23/25 (92%) | Association of HLA-B*5801 and SCAR: OR 97.8 (95% CI 18.3 to 521.5; p < 0.0001) |
| AHS: 19/20 (95%) | Association of HLA-B*5801 and AHS: OR 161.5 (95% CI 18.2 to 1430.9; p < 0.0001) | |
| SJS/TEN: 4/5 (80%) | Association of HLA-B*5801 and SJS/TEN: OR 34.0 (95% CI 3.2 to 356.1; p < 0.0001) | |
| Kaniwa et al. 200856 | SJS/TEN: 4/20 (20%) | Association of HLA-B*5801 and SJS/TEN: OR 40.83 (95% CI 10.5 to 158.9; p < 0.0001) |
| Japanese population: 6/986 (< 1%) | ||
| Lonjou et al. 200863 | SJS/TEN: 15/27 (55%) | Association of HLA-B*5801 and SJS/TEN: OR 80 (95% CI 34 to 187; p < 0.0001) |
| European population: 28/1822 (2%) | ||
| Tassaneeyakul et al. 200970 | Case: 27/27 (100%) | Association of HLA-B*5801 and SJS/TEN: OR 348.3 (95% CI 19.2 to 6336.9; p < 0.0001) |
| Control: 7/54 (13%) |
Other adverse event data
Risk factors for adverse events
In Chiu et al. ,51 60% of SCAR cases had chronic renal insufficiency compared with 23% in the control group (p < 0.01). Similarly, Hung et al. 53 reported that patients with chronic renal insufficiency had an increased risk for SCARs (55% vs. 21%, OR 4.7, 95% CI 2.3 to 9.3; p < 0.001). In Stamp et al. ,68 multivariate analysis allowing for matching between cases and controls showed that the presence of tophi was associated with a reduced risk of AHS (OR 0.29, 95% CI 0.01 to 0.83; p = 0.021). This study also reported ethnicity to be associated with risk of AHS. Compared with New Zealanders of European descent, there was a decreased risk of AHS in patients of Maori or Pacific Island descent (OR 0.24; p = 0.02) and an increased risk of AHS in those of Chinese descent (OR 70.8; p = 0.005).
Pharmacovigilance
Presented only as an abstract,57 a pharmacovigilance study from Morocco in which all 10 patients were included because they experienced a SAE was identified. Eight patients were reported to have chronic renal failure, six with a creatinine clearance < 30 ml/minute/1.73 m2. This study reports four instances of DRESS and three instances each of acute intestinal nephritis and acute renal failure. Two patients were reported to have died, but it is not reported which SAEs resulted in death.
Chapter 6 Discussion
Principal findings
We have conducted a systematic review of the literature to summarise the clinical effectiveness of allopurinol for people with CKD. Evidence for efficacy was determined solely from RCTs. The majority of patients in all trials started at a dose of 100mg/day as recommended by the MHRA. 23 This ensures that the trials are relevant to current clinical practice. All trials included patients with stage 3 CKD, although Shi et al. 47 appeared to include a majority of patients with milder CKD. Therefore, we conducted sensitivity analyses by including and excluding this trial from the meta-analysis. This did not result in differences in the overall findings.
None of the trials reported a worsening of CKD progression over time in the allopurinol group. Only Goicoechea et al. 13 reported a significant improvement in mean eGFR compared with the control group. It is therefore not possible to conclude that allopurinol is effective in decreasing the progression of the disease.
Only Goicoechea et al. 13 explicitly considered cardiovascular events as an end point. The findings from this study suggest that allopurinol may result in fewer cardiovascular events. However, such findings clearly need replicating in other studies.
No changes over time or between groups were reported in systolic or diastolic blood pressure, a risk factor for both CKD and CVD, from our meta-analysis of three trials. However, examination of the results also needs to consider that patients in the trials were receiving other treatments for their hypertension. It is therefore worth exploring changes in antihypertensive use further in future studies.
No significant differences were reported for the majority of other risk factors for CVD measured by the trials. However, significant (but small) improvements in left ventricular hypertrophy and endothelial dysfunction were reported in the allopurinol group in Kao et al. 14 Such improvements would unlikely have resulted in immediate differences in cardiovascular events in the short to medium term and their effects on CKD progression are unclear.
Given allopurinol is a drug which is designed to lower urate levels, the improvements in the allopurinol but not control groups in reducing uric acid levels in three trials13,14,48 is reassuring, suggesting that the drug was being taken at adequate doses and adequately adhered to. The improvement in uric acid levels reported in the control group in Shi et al. ,47 on the other hand, is surprising.
It is not clear how changes in uric acid levels are related to improvements in the other end points, if at all. Interestingly, in Siu et al. ,48 in a subgroup analyses, no clinical correlation could be shown between uric acid levels and change in serum creatinine or blood pressure while linear regression analysis conducted by Kao et al. 14 reported endothelial function to be independently related to uric acid levels. However, in both trials, subgroups were extremely small. Therefore, the mechanism of action through which allopurinol could result in the observed improvements in efficacy discussed above remains unexplained, mirroring the conundrum of whether uric acid is a cause, effect or indeed a coincidental factor for CKD and CVD. 9–12
Given the importance of AEs (both common and rare) and quality of life, in addition to the RCT evidence,13,14,47,48 we also considered evidence from 21 observational studies describing AE data;33,49–59,61–63,66–71 no studies were identified reporting on quality of life. Patients included in these studies had a variety of diseases (e.g. gout) and it is unclear whether or not the incidence and type of AEs and SAEs may therefore differ in people with CKD. Nevertheless, findings suggest that AEs may occur in 9.2% of patients and that no more than 2% of all patients who take allopurinol will experience a SCAR52 or, more specifically, AHS. 54 The evidence appears to be conflicting whether or not SCARs are dose related. A particularly interesting finding from the studies of SCARs was the association of the HLA-B*5801 allele with SCARs. However, as many patients with the HLA-B*5801 allele do not develop SCARs and, since it is not clear how to prevent SCARs, the utility of HLA-B*5801 testing is likely to be limited. This is particularly true in predominantly non-Asian populations, in which this allele is particularly rare.
Strengths and limitations
The main strength of our review is that we have systematically identified and presented evidence from RCTs assessing the efficacy of allopurinol in people with CKD. In addition, we have also examined evidence for the incidence of allopurinol-related AEs reported in the literature.
There are a number of limitations to our review. First, while the methodological quality of the included trials is acceptable, there are substantive limitations. None of the included studies included reported concealment of allocation, one of the greatest risks to study validity. There are relatively few (< 115) patients enrolled in any given trial, which limits the applicability of the results to the larger population in clinical practice. Even if a comprehensive meta-analysis could have been carried out, the total population of patients is only 257. In addition, the populations are clinically heterogeneous in terms of stage of disease, age, ethnicity and concomitant therapies. Trials other than Goicoechea et al. 13 are also limited by their relatively short follow-up period.
Arguably a greater limitation is that important confounding variables were not adequately described or controlled for. For example, information on diet and alcohol intake, which could influence all end points, is lacking. Although data are provided on concomitant medication, more insight may have been gained if more information had been provided on drugs which have been reported to lower levels of uric acid, such as losartan potassium,80 which may or may not have been taken by patients in studies (ARBs were permitted in three trials13,14,48). No trial attempted to adjust for these in factors their analysis.
In terms of end points, it would have been preferable if all trials had included eGFR to measure CKD progression as this is considered to be the best currently available measure. This would have enabled comparison of data from all included trials. With regard to cardiovascular risk, the end points measured by three trials,13,46,47 are surrogate measures and, in this respect, it is a limitation that only Goicoechea et al. 12 reported on cardiovascular events.
Finally, there is a dearth of studies examining information on quality of life using validated measures. In the absence of studies directly measuring quality of life in patients with CKD commencing allopurinol, we can only assume that the quality of life is not impaired unless they experience a SAE and/or are hospitalised. Studies utilising validated quality-of-life measures in people with CKD being treated with allopurinol would therefore be illuminating.
Given the above, the results from the ongoing CKD-FIX77 trial are eagerly awaited. Additional RCT evidence addressing many of the limitations of the studies identified by this systematic review and designed along the lines of CKD-FIX77 could also be warranted. The challenges of conducting such a trial have recently been explored by Badve et al. ,81 who argue that a pilot study should initially be conducted at a few sites in a single country. Based on power calculations, they estimate that this would need to include 620 patients (which is the same number of patients being enrolled into the CKD-FIX77 trial). Badve et al. 81 also argue that the pilot study should then be followed by an international multicentre trial of 7470 patients.
Alongside RCT evidence, additional supporting data are required from observational studies of patients with CKD and using allopurinol. If derived from, or linked to, a database or register such as the Kaiser Permanente data set used to assess risk factors for ESRD,7 such studies could collect data on the relationship between allopurinol and a number of risk factors and outcomes (efficacy and AEs).
Chapter 7 Conclusions
Based on results from four RCTs, there is limited evidence that allopurinol reduces CKD progression, or reduces the incidence of cardiovascular events or the prevalence of cardiovascular risk factors. However, the evidence is derived from a relatively small number of trials with limited numbers of patients, relatively short follow-up and inconsistencies in outcome measures. Furthermore, it is not clear whether the findings are attributable to allopurinol or other potentially confounding factors that were not controlled for in the trials. No evidence for a significant change in blood pressure, a risk factor for both CKD and CVD, was reported from any of the trials or from our meta-analysis. However, this finding may be confounded by other changes in treatment protocols and this requires further investigation.
Based on evidence from RCTs and 21 observational studies, it appears that AEs and, in particular, SAEs attributable to allopurinol are rare. However, the exact incidence is unknown. Based on data extracted from observational studies, it is speculated that the incidence of SCARs may be no more than 2% of patients treated. However, this estimate is derived from evidence of patients treated with allopurinol for any indication and not for CKD. Direct evidence for the impact of allopurinol on quality of life is lacking.
Suggested research priorities
Given the uncertainties in the evidence base highlighted above, there is a need for a further RCT to be conducted, comparing allopurinol with usual care. Ideally, a double-blind trial design should be employed and, hence, usual care will also include placebo. The dose of allopurinol should be in accordance with guidelines for current practice. Ideally, such a trial would also be adequately powered to assess for CKD progression and also to consider stratification of key factors such as age, ethnicity, stage of CKD, comorbidities and concomitant medication (particularly other urate-lowering medications). However, the feasibility of enrolling enough patients to be included in a suitably large trial may be questioned given it has been estimated the sample size would need to be 620 patients for a pilot trial and 7470 patients for a gold standard trial. Nevertheless, a RCT larger in size than the trials conducted to date and included in this systematic review is required. This should include many of the same outcomes as previous RCTs and given the chronic nature of the disease, with a minimum overall follow-up of 24 months. As a minimum, end points should include measures of eGFR (primary outcome), cardiovascular events, cardiovascular risk factors and AEs (including SAEs, particularly SCARs). It could also include a composite end point (e.g. halving eGFR, ESRD requiring renal replacement therapy or renal death and a composite cardiovascular outcome). End points could also include changes in concomitant medication (e.g. antihypertensives) and, ideally, disease-specific quality-of-life measures. In order to inform analysis, it is important to collect information on the following baseline characteristics: age, sex, ethnicity, comorbidities and concomitant medication. The feasibility of collecting data on other lifestyle factors such as smoking, diet and alcohol intake (which are all cardiovascular risk factors and/or impact on levels of uric acid) should also be considered. Many of these requirements may be met by the ongoing CKD-FIX trial.
Additional supporting data are required from observational studies of patients with CKD and using allopurinol. Ideally observational data will include many of the data reported in RCTs, for example patient characteristics, allopurinol dose (and changes in dose), concomitant medication, change in eGFR, cardiovascular events, cardiovascular risk factors and AEs (including SAEs, particularly SCARs).
Acknowledgements
We would like to thank to Janet Atkinson for administrative support. In addition, we also thank GlaxoSmithKline, Aspen Pharma, Dr Marian Goicoechea and Dr Michelle Kao who all responded to our requests for additional information. Finally, we thank the anonymous peer reviewers of this report who drew our attention to two ongoing trials we had not identified from our searches.
Contributions of authors
Nigel Fleeman Project management, data management, systematic review of clinical effectiveness data and preparation of report.
Gerlinde Pilkington Second reviewer contributing to processes of study selection, data extraction and quality assessment and provided comments on all drafts of the report.
Yenal Dundar Development of search strategies.
Kerry Dwan Statistical expertise.
Angela Boland Provided comments on the draft report.
Rumona Dickson Contributed to the development of the review protocol and provided comments on the draft report.
Hameed Anijeet Contributed to the development of the review protocol and clinical advice.
Tom Kennedy Contributed to the development of the review protocol and clinical advice.
Jason Pyatt Contributed to the development of the review protocol and clinical advice.
All contributors took part in the editing and production of this report.
Rider on responsibility for report
The views in the report are those of the authors and not necessarily those of the NIHR HTA programme. Any errors are the responsibility of the authors.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- National Institute for Health and Clinical Excellence . Chronic Kidney Disease: Early Identification and Management of Chronic Kidney Disease in Adults in Primary and Secondary Care 2008. http://www.nice.org.uk/CG073 (accessed September 2012).
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-70. http://dx.doi.org/10.7326/0003-4819-130-6-199903160-00002.
- Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 2007;18:2749-57. http://dx.doi.org/10.1681/ASN.2007020199.
- The Renal Association . CKD Stages 2013. www.renal.org/information-resources/the-uk-eckd-guide/ckd-stages#sthash.6RIgjA2q.dpbs (accessed 16 April 2014).
- Ripley E. Complementary effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in slowing the progression of chronic kidney disease. Am Heart J 2009;157:S7-16. http://dx.doi.org/10.1016/j.ahj.2009.04.008.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009;20:2075-84. http://dx.doi.org/10.1681/ASN.2008111205.
- Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med 2009;169:342-50. http://dx.doi.org/10.1001/archinternmed.2008.605.
- Kabul S, Shepler B. A review investigating the effect of allopurinol on the progression of kidney disease in hyperuricemic patients with chronic kidney disease. Clin Ther 2012;34:2293-6. http://dx.doi.org/10.1016/j.clinthera.2012.10.008.
- Puddu P, Puddu GM, Cravero E, Vizioli L, Muscari A. The relationships among hyperuricemia, endothelial dysfunction, and cardiovascular diseases: molecular mechanisms and clinical implications. J Cardiol 2012;59:235-42. http://dx.doi.org/10.1016/j.jjcc.2012.01.013.
- Nakagawa T, Kang DH, Feig D, Sanchez-Lozada LG, Srinivas TR, Sautin Y, et al. Unearthing uric acid: an ancient factor with recently found significance in renal and cardiovascular disease. Kidney Int 2006;69:1722-5. http://dx.doi.org/10.1038/sj.ki.5000391.
- Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med 2008;75:S13-16. http://dx.doi.org/10.3949/ccjm.75.Suppl_5.S13.
- Edwards NL. The role of hyperuricemia in vascular disorders. Curr Opin Rheumatol 2009;21:132-7. http://dx.doi.org/10.1097/BOR.0b013e3283257b96.
- Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 2010;5:1388-93. http://dx.doi.org/10.2215/CJN.01580210.
- Kao M, Ang D, Gandy S, Nadir M, Houston J, Lang C, et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol 2011;22:1382-9. http://dx.doi.org/10.1681/ASN.2010111185.
- Talaat KM, el-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol 2007;27:435-40. http://dx.doi.org/10.1159/000105142.
- Zhang Q-L, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 2008;8. http://dx.doi.org/10.1186/1471-2458-8-117.
- Roderick P, Roth M, Mindell J. Prevalence of chronic kidney disease in England: Findings from the 2009 Health Survey for England. J Epidemiol Community Health 2011;65.
- Stevens PE, O’Donoghue DJ, de Lusignan S, Van Vlymen J, Klebe B, Middleton R, et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int 2007;72:92-9. http://dx.doi.org/10.1038/sj.ki.5002273.
- National Kidney and Urologic Diseases Information Clearinghouse (National Institute of Diabetes and Digestive and Kidney Diseases and National Institutes of Health) . Kidney Disease Statistics for the United States 2012. http://kidney.niddk.nih.gov/kudiseases/pubs/kustats/#4 (accessed September 2012).
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011;63:3136-41. http://dx.doi.org/10.1002/art.30520.
- Liu B, Wang T, Zhao H, Yue W, Yu H, Liu C, et al. The prevalence of hyperuricemia in China: a meta-analysis. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-832.
- Robins RK. Potential Purine Antagonists. I. Synthesis of some 4,6-substituted pyrazolo [3,4-d] pyrimidines1. J Am Chem Soc 1956;78:784-90. http://dx.doi.org/10.1021/ja01585a023.
- Medicines and Healthcare products Regulatory Agency . Public Assessment Report. Allopurinol 100mg Tablets. Allopurinol 200mg Tablets. Allopurinol 300mg Tablets 2010. www.mhra.gov.uk/home/groups/par/documents/websiteresources/con093782.pdf (accessed September 2012).
- DailyMed . Allopurinol Tablet n.d. http://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=allopurinol&x=0&y=0 (accessed September 2012).
- Taal M, Tomson C. Clinical Practice Guidelines for the Care of Patients with Chronic Kidney Disease. UK Renal Association Clinical Practice Guidelines 4th Edition 2007 2007. www.renal.org/pages/media/download_gallery/CKDfinalMar07.pdf (accessed October 2012).
- British Medical Assocation & NHS Employers . Chronic Kidney Disease Frequently Asked Questions 2011. www.nhsemployers.org/SiteCollectionDocuments/Chronic_kidney_disease_FAQs%20-%20ja040711.pdf (accessed September 2012).
- Stevenson M, Pandor A. Febuxostat for the treatment of hyperuricaemia in people with gout: a single technology appraisal. Health Technol Assess 2008;12.
- Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010;12. http://dx.doi.org/10.1186/ar2978.
- Chao J, Terkeltaub R. A critical reappraisal of allopurinol dosing, safety, and efficacy for hyperuricemia in gout. Curr Rheumatol Rep 2009;11:135-40. http://dx.doi.org/10.1007/s11926-009-0019-z.
- Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J, et al. British Society for Rheumatology and British Health Professionals in Rheumatology Guideline for the Management of Gout. Rheumatology 2007;46:1372-4. http://dx.doi.org/10.1093/rheumatology/kem056a.
- Kantor GL. Toxic epidermal necrolysis, azotemia, and death after allopurinol therapy. JAMA 1970;212:478-9. http://dx.doi.org/10.1001/jama.1970.03170160066019.
- Singer JZ, Wallace SL. The allopurinol hypersensitivity syndrome. Unnecessary morbidity and mortality. Arthritis Rheum 1986;29:82-7. http://dx.doi.org/10.1002/art.1780290111.
- Arellano F, Sacristan JA. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother 1993;27:337-43.
- Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg 1996;15:250-7. http://dx.doi.org/10.1016/S1085-5629(96)80038-1.
- Somkrua R, Eickman EE, Saokaew S, Lohitnavy M, Chaiyakunapruk N. Association of HLA-B*5801 allele and allopurinol-induced Stevens–Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. BMC Med Genet 2011;12. http://dx.doi.org/10.1186/1471-2350-12-118.
- Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med 1984;76:47-56. http://dx.doi.org/10.1016/0002-9343(84)90743-5.
- Perez-Ruiz F, Hernando I, Villar I, Nolla JM. Correction of allopurinol dosing should be based on clearance of creatinine, but not plasma creatinine levels: another insight to allopurinol-related toxicity. J Clin Rheum 2005;11:129-33. http://dx.doi.org/10.1097/01.rhu.0000164822.98163.22.
- DrugCite . Allopurinol Adverse Events and Side Effects Reported to the FDA 2012. www.drugcite.com/?q=ALLOPURINOL (accessed October 2012).
- British National Formulary . BNF 63 2012. www.bnf.org/bnf/ (accessed 16 January 2013).
- CRD’s Guidance for Undertaking Reviews in Healthcare: Systematic Reviews (3rd Edition). York: CRD, University of York; 2008.
- Deeks J, Dinnes J, D’Amico R, Sowden A, Sakarovitch C, Song F. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7.
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses 2006. www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 31 October 2011).
- Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care – Meta-analysis in Context. London: BMJ books; 2001.
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. http://dx.doi.org/10.1136/bmj.327.7414.557.
- Russmann S, Lauterburg B. Life-threatening adverse effects of pharmacologic antihyperuricemic therapy. Ther Umsch 2004;61:575-7. http://dx.doi.org/10.1024/0040-5930.61.9.575.
- Kinyo A, Lakatos A, Varga A, Gyulai R, Varga E, Bata-Csorgo Z, et al. Allopurinol-induced hypersensitivity syndrome. Orv Hetil 2012;153:586-91.
- Shi Y, Chen W, Jalal D, Li Z, Chen W, Mao H, et al. Clinical outcome of hyperuricemia in IgA nephropathy: a retrospective cohort study and randomized controlled trial. Kidn Blood Press Res 2012;35:153-60. http://dx.doi.org/10.1159/000331453.
- Siu Y-P, Leung K-T, Tong MK-H, Kwan T-H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 2006;47:51-9. http://dx.doi.org/10.1053/j.ajkd.2005.10.006.
- Atzori L, Pinna AL, Mantovani L, Ferreli C, Pau M, Mulargia M, et al. Cutaneous adverse drug reactions to allopurinol: 10 year observational survey of the dermatology department--Cagliari University (Italy). J Eur Acad Dermatol Venereol 2012;26:1424-30. http://dx.doi.org/10.1111/j.1468-3083.2011.04313.x.
- Bowie EA, Simmonds HA, North JD. Allopurinol in treatment of patients with gout and chronic renal failure. N Z Med J 1967;66:606-11.
- Chiu MLS, Hu M, Ng MHL, Yeung CK, Chan JCY, Chang MM, et al. Association between HLA-B*58:01 allele and severe cutaneous adverse reactions with allopurinol in Han Chinese in Hong Kong. Br J Dermatol 2012;167:44-9. http://dx.doi.org/10.1111/j.1365-2133.2012.10894.x.
- Dalbeth N, Stamp L. Allopurinol dosing in renal impairment: walking the tightrope between adequate urate lowering and adverse events. Semin Dial 2007;20:391-5. http://dx.doi.org/10.1111/j.1525-139X.2007.00270.x.
- Hung S, Chung W, Liou L. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA 2005;102:4134-9. http://dx.doi.org/10.1073/pnas.0409500102.
- Jung J-W, Song W-J, Kim Y-S, Joo KW, Lee KW, Kim S-H, et al. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant 2011;26:3567-72. http://dx.doi.org/10.1093/ndt/gfr060.
- Kang HR, Jee YK, Kim YS, Lee CH, Jung JW, Kim SH, et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genom 2011;21:303-7. http://dx.doi.org/10.1097/FPC.0b013e32834282b8.
- Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens–Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics 2008;9:1617-22. http://dx.doi.org/10.2217/14622416.9.11.1617.
- Khabbal Y, Mernissi F, Arrayhani M, Houssaini TS. Acute renal failure induced by allopurinol. Fundam Clin Pharmacol 2012;26.
- Khoo BP, Leow YH. A review of inpatients with adverse drug reactions to allopurinol. Singapore Med J 2000;41:156-60.
- Krishnamurthy A, Lazaro DM, Blumenthal DR, Gerber DA, Flom PL. The effect of allopurinol on renal function in patients with hyperuricemia: a case–control study. Arthritis Rheum 2010;62.
- Lang PG. Severe hypersensitivity reactions to allopurinol. South Med J 1979;72:1361-8. http://dx.doi.org/10.1097/00007611-197911000-00004.
- Lee HY, Ariyasinghe JT, Thirumoorthy T. Allopurinol hypersensitivity syndrome: a preventable severe cutaneous adverse reaction?. Singapore Med J 2008;49:384-7.
- Levin NW, Abrahams OL. Allopurinol in patients with impaired renal function. Ann Rheum Dis 1966;25:681-7.
- Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. A European study of HLA-B in Stevens–Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genom 2008;18:99-107. http://dx.doi.org/10.1097/FPC.0b013e3282f3ef9c.
- Lupton GP, Odom RB. The allopurinol hypersensitivity syndrome. J Am Acad Dermatol 1979;1:365-74. http://dx.doi.org/10.1016/S0190-9622(79)70031-4.
- McInnes GT, Lawson DH, Jick H. Acute adverse reactions attributed to allopurinol in hospitalised patients. Ann Rheum Dis 1981;40:245-9. http://dx.doi.org/10.1136/ard.40.3.245.
- Paisansinsup T, Schousboe JT. Adverse reactions to allopurinol are not increased in patients exposed to doses higher than recommended for creatinine clearance: a retrospective study of a large urban multispecialty group. Arthritis Rheum 2011;63.
- Panomvana D, Sripradit S, Angthararak S. Higher therapeutic plasma oxypurinol concentrations might be required for gouty patients with chronic kidney disease. J Clin Rheum 2008;14:6-11. http://dx.doi.org/10.1097/RHU.0b013e318164dceb.
- Stamp L, Taylor W, Jones P. Starting dose is a risk factor for allopurinol hypersensitivity syndrome. Arthritis Rheum 2012;64:2529-36. http://dx.doi.org/10.1002/art.34488.
- Stamp LK, O’Donnell JL, Zhang M, James J, Frampton C, Barclay ML, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum 2011;63:412-21. http://dx.doi.org/10.1002/art.30119.
- Tassaneeyakul W, Jantararoungtong T, Chen P, Lin PY, Tiamkao S, Khunarkornsiri U, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens–Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genom 2009;19:704-9. http://dx.doi.org/10.1097/FPC.0b013e328330a3b8.
- Vazquez-Mellado J, Meono-Morales E, Pacheco-Tena C. Relationship between adverse events associated with allopurinol and renal function in patients with gout. Ann Rheum Dis 2001;60:981-3. http://dx.doi.org/10.1136/ard.60.10.981.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. http://dx.doi.org/10.1016/0197-2456(86)90046-2.
- Kao MP, Ang DS, Gandy SJ, Lang CC, Struthers AD. Allopurinol reduces both left ventricular hypertrophy and endothelial dysfunction in patients with chronic kidney disease. NDT Plus 2010;3.
- Kao MP, Ang DS, Struthers AD. Allopurinol improves vascular function in patients with chronic kidney disease. NDT Plus 2010;3.
- ClinicalTrials.gov . Uric Acid and the Endothelium Is CKD n.d. http://clinicaltrials.gov/ct2/show/NCT01228903 (accessed 19 April 2013).
- ClinicalTrials.gov . A Pilot Study of Allopurinol to Prevent Kidney Function Loss in Type 1 Diabetes (PERL) 2012. http://clinicaltrials.gov/ct2/show/NCT01575379 (accessed 19 April 2013).
- University Australasian Kidney Trials Network (University of Queensland) . The CKD-FIX Trial: Controlled Trial of Slowing of Kidney Disease Progression from the Inhibition of Xanthine Oxidase n.d. http://apps.who.int/trialsearch/trial.aspx?trialid=ACTRN12611000791932 (accessed 29 November 2013).
- Maahs D, Caramori L, Cherney DI, Galecki A, Gao C, Jalal D, et al. Uric Acid Lowering to Prevent Kidney Function Loss in Diabetes: The Preventing Early Renal Function Loss (PERL) Allopurinol Study. Curr Diab Rep 2013;13:550-9. http://dx.doi.org/10.1007/s11892-013-0381-0.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2011. www.cochrane-handbook.org (accessed 1 November 2012).
- Miao Y, Ottenbros SA, Laverman GD, Brenner BM, Cooper ME, Parving HH, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension 2011;58:2-7. http://dx.doi.org/10.1161/HYPERTENSIONAHA.111.171488.
- Badve SV, Brown F, Hawley CM, Johnson DW, Kanellis J, Rangan GK, et al. Challenges of conducting a trial of uric-acid-lowering therapy in CKD. Nat Rev Nephrol 2011;7:295-300. http://dx.doi.org/10.1038/nrneph.2010.186.
- Andrade RJ, de la Mata M, Lucena MI, Lopez-Rubio F, Corrales MA. Severe acute hepatitis due to allopurinol in a patient with asymptomatic hyperuricemia and kidney failure. A review of the literature and an analysis of the risk factors. Gastroenterol Hepatol 1997;20:353-6.
- Arbeteta J, Ledo L, Teruel JL, Ortuno J. Severe adverse reaction to allopurinol. Med Clin 1987;88:125-6.
- Badkoobeh RS, Nozari Y, Larti F, Safari S, Ahmadi F, Emami M. Allopurinol effects on diastolic dysfunction in ESRD patients with hyperuricemia. Tehran Univ Med J 2011;68:618-23.
- Biagioni EB, Rinaldi S, Marietta L, Girardis MM. Acute renal failure and liver necrosis associated to allopurinol therapy. Anaesth Intens Care 2012;40:190-1.
- Bruguera M, Libre J, Aubia J, Serrano S. Allopurinol-induced cholestasis. Gastroenterol Hepatol 1983;6:253-5.
- Buna D. Allopurinol hypersensitivity syndrome: a case report and review. Can J Hosp Pharm 2000;53:36-9.
- Carella G, Mancini S, Marra L, Bevilacqua E. Allopurinol-induced hepatitis. Progr Med 1989;45:9-13.
- Casas E, Michan A, Gonzalez Sanz A, Garcia Puig J. Prevention of the adverse effects of allopurinol. Ann Med Intern 1989;6.
- Chen YC, Chiu HC, Chun CY. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol 2010;146:1373-9. http://dx.doi.org/10.1001/archdermatol.2010.198.
- Chan JCY, Yeung CK. Drug reaction with eosinophilia and systemic symptoms (DRESS): a retrospective analysis of 21 cases in a tertiary teaching hospital in Hong Kong. J Am Acad Dermatol 2012;66.
- Craveiro N, Alves C, Ribeiro CF, Marques FB. Hypersensitivity syndrome due to drug exposure – ten years case review. Pharmacoepidemiol Drug Saf 2011;20:S332-3.
- Dashti-Khavidaki S, Shiemorteza M, Ahmadi F. Allopurinol induced hypersensitivity syndrome: case report and a literature review. Int J Clin Pharm 2011;33:293-4.
- Elasy T, Kaminsky D, Tracy M, Mehler PS. Allopurinol hypersensitivity syndrome revisited. West J Med 1995;162:360-1.
- Fernandez G, Garcia JE, Ahijado F, Diaz-Tejeiro R, Gonzalez E, Gomez E, et al. Allopurinol and bone marrow aplasia. Nephron 1993;64. http://dx.doi.org/10.1159/000187341.
- Handa SP. Drug-induced acute interstitial nephritis: report of 10 cases. Can Med Assoc J 1986;135:1278-81.
- Hande KR. Evaluation of a thiazide-allopurinol drug interaction. Am J Med Sci 1986;292:213-16. http://dx.doi.org/10.1097/00000441-198610000-00006.
- Hanger HC, Pillans PI. Death following allopurinol hypersensitivity syndrome. N Z Med J 1994;107.
- Hu H, Kao K, Huang C, Yang C, Hung C. Risk factors of mortality in severe cutaneous adverse reactions patients with pulmonary involvement. Crit Care 2012;16.
- Hung C-C, Liu W-C, Kuo M-C, Lee C-H, Hwang S-J, Chen H-C. Acute renal failure and its risk factors in Stevens–Johnson syndrome and toxic epidermal necrolysis. Am J Nephrol 2009;29:633-8. http://dx.doi.org/10.1159/000195632.
- Iakunina IA, Barskova VG, Nasonova VA. Allopurinol efficacy in patients with tophus gout and chronic renal failure. Ter Arkh 2006;78:88-91.
- Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol 2007;39:1227-33. http://dx.doi.org/10.1007/s11255-007-9253-3.
- Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I, et al. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol 2011;6:1887-94. http://dx.doi.org/10.2215/CJN.11451210.
- Kumar A, Edward N, White MI, Johnston PW, Catto GR. Allopurinol, erythema multiforme, and renal insufficiency. BMJ 1996;312:173-4. http://dx.doi.org/10.1136/bmj.312.7024.173.
- Kwon KS, Kim BS, Jang BS, Kim MB, Oh CK, Kwon YW, et al. Clinical study of drug rash with eosinophilia and systemic symptoms (DRESS) on drug eruption patients over the last 10 years (1995–2004). Korean J Dermatol 2005;43:1164-9.
- Lee HY, Pang SM, Thamotharampillai T. Allopurinol-induced Stevens–Johnson syndrome and toxic epidermal necrolysis. J Am Acad Dermatol 2008;59:352-3. http://dx.doi.org/10.1016/j.jaad.2008.02.021.
- Loffler W, Seibke W, Seibke E, Reiter S, Jahn M, Hehlmann R, et al. Non-responsiveness to allopurinol in renal hypouricaemia. Klin Wochenschr 1989;67. http://dx.doi.org/10.1007/BF01736535.
- Markel A. Allopurinol-induced DRESS syndrome. Isr Med Assoc J 2005;7:656-60.
- Morel D, Guez S, Merville P, Deminiere C, Tamisier JM, Potaux L. Recurrent renal failure associated with hypersensitivity to allopurinol. Nephrol Dial Transplant 1999;14:780-1. http://dx.doi.org/10.1093/ndt/14.3.780.
- Ng KP, Stringer S, Jesky M, Dutton M, Ferro C, Cockwell P. Use of allopurinol is associated with reduced arterial stiffness in progressive chronic kidney disease (CKD). Nephrol Dial Transplant 2012;27.
- Park KS, Chang JW, Yang WS, Kim SB, Park SK, Lee SK, et al. Natural correction of hyperuricemia, not by allopurinol, could slow down the progression of renal disease in patients with chronic kidney disease stage 3. Clin Nutr Suppl 2011;6:67-8. http://dx.doi.org/10.1016/S1744-1161(11)70169-7.
- Park KS, Chang JW. Natural correction of hyperuricemia, not by allopurinol, could slow down the progression of renal disease in the patient with chronic kidney disease stage 3. Kidney Res Clin Pract 2012;31. http://dx.doi.org/10.1016/j.krcp.2012.04.516.
- Querings K, Balda BR, Bachter D. Treatment of acquired reactive perforating collagenosis with allopurinol. Br J Dermatol 2001;145:174-6. http://dx.doi.org/10.1046/j.1365-2133.2001.04310.x.
- Rapado A. Allopurinol in thiazide-induced hyperuricaemia. Ann Rheum Dis 1966;25:660-6.
- Renwick IG. Asymptomatic hyperuricemia and allopurinol induced toxic epidermal necrolysis. Br Med J 1985;291. http://dx.doi.org/10.1136/bmj.291.6493.485.
- Rivas Gonzalez P, Calvo Hernandez R, Molinelli Barranco M, Diaz Curiel M. Allopurinol hypersensitivity syndrome. Rev Clin Esp 2001;201.
- San Andres Rebollo F, Gonzalez Rubio M, Postigo C, Garfia C, Cabeza Alvarez C, Viana Alonso A. Hypersensitivity syndrome caused by allopurinol: report of 2 cases and review of the literature. Rev Clin Esp 1992;191:426-9.
- Sauve C, Pinquier JL, Boissonnad A, Sicard D, Christoforov B. Systemic allopurinol toxicity. Eur J Internal Med 1992;3:78-81.
- Shalom R, Rimbroth S, Rozenman D, Markel A. Allopurinol-induced recurrent DRESS syndrome: pathophysiology and treatment. Ren Fail 2008;30:327-9. http://dx.doi.org/10.1080/08860220701861045.
- Shelmadine B, Bowden RG, Wilson RL, Beavers D, Hartman J. The effects of lowering uric acid levels using allopurinol on markers of metabolic syndrome in end-stage renal disease patients: a pilot study. Anadolu Kardiyol Derg 2009;9:385-9.
- Simmons FF, Gerety D. Granulomatous hepatitis in a patient receiving allopurinol. Gastroenterology 1972;62:101-4.
- Sjoberg KH. Allopurinol therapy of gout with renal complications. Ann Rheum Dis 1966;25:688-90.
- Stevens SL, Goldman MH. Cyclosporine toxicity associated with allopurinol. South Med J 1992;85:1265-6. http://dx.doi.org/10.1097/00007611-199212000-00032.
- Tausche AK, Aringer M, Schroeder HE, Bornstein SR, Wunderlich C, Wozel G. The Janus faces of allopurinol-allopurinol hypersensitivity syndrome. Am J Med 2008;121:e3-4. http://dx.doi.org/10.1016/j.amjmed.2007.10.028.
- Vinciullo C. Allopurinol hypersensitivity: a potentially life threatening reaction. Australas J Dermatol 1984;25:59-67. http://dx.doi.org/10.1111/j.1440-0960.1984.tb00628.x.
- Vinciullo C. Allopurinol hypersensitivity. Med J Aus 1984;141:449-50.
- Wilkinson DG. Allopurinol and agranulocytosis. Lancet 1977;2:1282-3. http://dx.doi.org/10.1016/S0140-6736(77)92683-6.
- Woss EN. Hypersensitivity angiitis following allopurinol therapy. Fortschr Med 1988;106:730-1.
- Yelken B, Gorgulu N, Altun I, Yilmaz A, Caliskan YK, Yazici H, et al. Reduction of uric acid levels with allopurinol treatment improves endothelial function in the patients with chronic renal failure. NDT Plus 2010;3:177-8.
- Yelken BC, Gorgulu N, Altun I, Yilmaz A, Yazici H, Oflaz H, et al. Reduction of uric acid levels with allopurinol treatment improves endothelial function in patients with chronic kidney disease. Clin Nephrol 2012;77:275-82. http://dx.doi.org/10.5414/CN107352.
- Yiğiner O, Ozcelik F, Aparci M, Isilak Z, Uz O. The beneficial effects of allopurinol in cardiology practice: decrease in uric acid and vascular oxidative stress/the effects of lowering uric acid levels using allopurinol on markers of metabolic syndrome in end-stage renal disease patients: a pilot study. Anadolu Kardiyol Derg 2010;10:294-5. http://dx.doi.org/10.5152/akd.2010.079.
- Zagaria MAE. Cutaneous adverse reactions: Stevens–Johnson syndrome. US Pharm 2008;33:20-6.
Appendix 1 Literature search strategies
All databases were searched on 7 January 2013.
Ovid MEDLINE(R) In-Process and Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to 7 January 2013
| Search terms | Results | |
|---|---|---|
| 1 | (allopurinol or allohexal or allosig or milurit or alloril or progout or zyloprim or zyloric or zyrik or aluron).af. | 8218 |
| 2 | exp Allopurinol/ | 6398 |
| 3 | exp Kidney Failure, Chronic/ | 74,699 |
| 4 | exp Renal Insufficiency, Chronic/ | 77,022 |
| 5 | (chronic$ adj3 (nephrop$ or kidney or renal)).tw. | 49,251 |
| 6 | ((endstage or end-stage) adj2 (renal or kidney$)).tw. | 24,949 |
| 7 | (proteinuri$ or albuminuri$ or uremia$ or uraemia$).tw. | 41,516 |
| 8 | ((kidney or renal) adj2 (disease$ or failure$ or sufficien$ or insufficien$)).ti,ab. | 150,279 |
| 9 | (esrd or eskd or esrf or ckf or ckd or crf or crd).ti,ab. | 30,318 |
| 10 | exp Renal Insufficiency/ or *Kidney Diseases/ | 161,247 |
| 11 | or/1-2 | 8218 |
| 12 | or/3-10 | 265,647 |
| 13 | 11 and 12 | 676 |
| 14 | Animals/ not Humans/ | 3,735,310 |
| 15 | 13 not 14 | 603 |
EMBASE 1974 to 28 December 2012
| Search terms | Results | |
|---|---|---|
| 1 | (allopurinol or allohexal or allosig or milurit or alloril or progout or zyloprim or zyloric or zyrik or aluron).af. | 17,739 |
| 2 | (chronic$ adj3 (nephrop$ or kidney or renal)).tw. | 64,274 |
| 3 | ((endstage or end-stage) adj2 (renal or kidney$)).tw. | 31,278 |
| 4 | (proteinuri$ or albuminuri$ or uremia$ or uraemia$).tw. | 52,296 |
| 5 | (esrd or eskd or esrf or ckf or ckd or crf or crd).ti,ab. | 39,763 |
| 6 | ((kidney or renal) adj2 (failure$ or suffcien$ or insufficien$)).ti,ab. | 118,561 |
| 7 | exp kidney failure/ | 211,324 |
| 8 | or/2-7 | 310,195 |
| 9 | 1 and 8 | 2079 |
| 10 | limit 9 to human | 1747 |
The Cochrane Library Issue 1, 2013
| Search terms | Results | |
|---|---|---|
| 1 | allopurinol or allohexal or allosig or milurit or alloril or progout or zyloprim or zyloric or zyrik or aluron:ti,ab,kw (Word variations have been searched) | 489 |
| 2 | MeSH descriptor: [Renal Insufficiency] explode all trees | 3988 |
| 3 | #1 and #2 | 13 |
ClinicalTrials.gov was searched using the term ‘allopurinol’.
As a relatively large number of studies of AEs were identified through the reference lists of studies already identified and included, an additional search of Ovid MEDLINE was conducted to identify AEs in order to ensure no studies had in fact been missed. The reason why so many AE studies had not been identified from the electronic searches already conducted was because the majority of AE studies were not specific to only patients with CKD, whereas the electronic searches conducted to date had the specific aim of only identifying patients with CKD. Therefore, the following broad search was conducted.
Ovid MEDLINE(R) In-Process and Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to 4 March 2013
| Search terms | Results | |
|---|---|---|
| 1 | Allopurinol/ae [Adverse Effects] | 651 |
Appendix 2 Table of excluded studies with rationale
The list of citations excluded at stage 2 with reasons is presented in Table 22 . It is evident from this table that the most common reason for exclusion was that citations only reported on single case reports (n = 23). These were in addition to 50 case reports already identified and excluded at stage 1 (based on the title/abstract only).
| Study | Reason for exclusion |
|---|---|
| Andrade 199782 | Case report (not in English) |
| Arbeteta 198783 | Case report (not in English) |
| Badkoobeh 201184 | Not a RCT and no reporting of AEs (subjects had ESRD; not in English) |
| Biagioni 201285 | Case report |
| Bruguera 198386 | Could not be obtained within the review time frame (not in English) |
| Buna 200087 | Case report |
| Carella 198988 | Case reports (not in English) |
| Casas 198989 | Could not be obtained within the review time frame (not in English) |
| Chao 200929 | Overview of allopurinol dosing |
| Chen 201090 | Not allopurinol specific (study of DRESS from any drug) |
| Chun-Yin Chan 201291 | Not allopurinol specific (study of DRESS from any drug; abstract only) |
| Craveiro 201192 | Not allopurinol specific (study of hypersensitivity syndrome from any drug; abstract only) |
| Dashti-Khavidaki 201193 | Case report |
| Elasy 199594 | Case report |
| Fernandez 199395 | Case report |
| Handa 198696 | Not allopurinol specific (study of allergic interstitial nephritis from any drug) |
| Hande 198697 | Not relevant population (subjects were healthy volunteers) |
| Hanger 199498 | Case report |
| Hu 201299 | Not allopurinol specific (study of DRESS from any drug; abstract only) |
| Hung 2009100 | Not allopurinol specific (study of Stevens–Johnson syndrome and toxic epidermal necrolysis from any drug) |
| Iakunina 2006101 | Case reports (not in English) |
| Kanbay 2007102 | Not RCT and no reporting of AEs |
| Kanbay 2011103 | Not relevant population (subjects were patients with normal renal function) |
| Kinyo 201246 | Not in English (retrospective review of patients with AHS) |
| Kumar 1996104 | Case report |
| Kwon 2005105 | Not allopurinol specific (study of DRESS from any drug; not in English) |
| Lee et al 2008106 | Letter |
| Loffler 1989107 | Case report |
| Markel 2005108 | Overview of DRESS |
| Morel 1999109 | Case report |
| Ng 2012110 | Not a RCT and no reporting of AEs (abstract only) |
| Park 2011111 | Not a RCT and no reporting of AEs (abstract only) |
| Park 2012112 | Not a RCT and no reporting of AEs (abstract only) |
| Querings 2001113 | Case report |
| Rapado 1966114 | Not relevant population (subjects were patients with thiazide-induced hyperuricaemia) |
| Renwick 1985115 | Case report |
| Rivas Gonzalez 2001116 | Letter (not in English) |
| Russmann 200445 | Not in English (overview of AHS) |
| San andres Rebollo 1992117 | Case reports (not in English) |
| Sauve 1992118 | Case report |
| Shalom 2008119 | Case report |
| Shelmadine 2009120 | Not a RCT and no reporting of AEs (subjects had ESRD) |
| Simmons 1972121 | Case report |
| Sjoberg 1966122 | Case report |
| Stevens 1992123 | Case report |
| Talaat 200715 | Examines effects from withdrawal of allopurinol |
| Tausche 2008124 | Case report |
| Vinciullo 1984125 | Case report |
| Vinciullo 1984126 | Letter |
| Wilkinson 1977127 | Case report |
| Woss 1988128 | Case report (not in English) |
| Yelken 2010129 | Not a RCT and no reporting of AEs (abstract only) |
| Yelken 2012130 | Not a RCT and no reporting of AEs |
| Yiğiner 2010131 | Letter (not in English) |
| Zagaria 2008132 | Not allopurinol specific (overview of SJS from any drug) |
Appendix 3 Characteristics of studies that reported on adverse event data
Study characteristics and baseline demographic characteristics of studies reporting on AEs are summarised in Tables 23 and 24 .
| Studya | Type of study | Allopurinol (n) | Controls | AE data collected | |
|---|---|---|---|---|---|
| Allopurinol tolerant (n) | Other (n) | ||||
| Arellano and Sacristan 199333 | Retrospective review of case reports | 101 | 0 | 0 | AHS |
| In relation to allopurinol dose | |||||
| Atzori et al. 201249 | Retrospective cohort study | 84 | 0 | 0 | SCAR |
| Patients requiring hospitalisation | |||||
| Bowie et al. 196750 | Prospective observational study | 14 | 0 | 0 | Any AE |
| Chiu et al. 201251 | Retrospective case–control study (not matched) | 20 | 30 | 0 | SCAR |
| In relation to HLA-B allele | |||||
| In relation to allopurinol dose | |||||
| Patients requiring hospitalisation | |||||
| Dalbeth and Stamp 200652 | Retrospective cohort study | 227b | 23b | 0 | AHS |
| Goicoechea et al. 201013 | RCT | 57 | 0 | 56 | Any AE |
| Patients requiring hospitalisation | |||||
| Hung et al. 200553 | Retrospective case–control study | 51 | 135c | 0 | SCAR |
| In relation to HLA-B allele | |||||
| In relation to allopurinol dose | |||||
| Jung et al. 201154 | Retrospective case–control study | 432 | 16 | 0 | SCAR |
| In relation to HLA-B allele | |||||
| In relation to allopurinol dose | |||||
| Kang et al. 201155 | Retrospective case–control study | 25 | 57 | 0 | SCAR |
| In relation to HLA-B allele | |||||
| In relation to allopurinol dose | |||||
| Kaniwa et al. 200856 | Retrospective case–control study | 10 | 0 | 0d | SJS/TEN |
| In relation to HLA-B allele | |||||
| Kao et al. 201114 | RCT | 27 | 0 | 26 | Any AE |
| Khabbal et al. 201257 | Pharmacovigilance study | 10 | 0 | 0 | Any AE |
| Khoo 200058 | Retrospective cohort study | 13 | 0 | 0 | AHS |
| In relation to allopurinol dose | |||||
| Average length of stay in hospital | |||||
| Krishnamurthy 201059 | Retrospective case–control study (matched) | 50 | 0 | 50 | Any AE |
| In relation to allopurinol dose | |||||
| Lee et al. 200861 | Retrospective cohort study | 28 | 0 | 0 | AHS |
| Patients requiring ICU care and emergent haemodialysis and average length of stay | |||||
| Levin and Abrahams 196662 | Prospective observational study | 33 | 0 | 0 | Any AE |
| Lonjou et al. 200863 | Hybrid prospective and retrospective genotyping study | 120 | 0 | 0e | SJS/TEN |
| In relation to HLA-B allele | |||||
| Paisansinsup and Schousboe 201164 | Retrospective cohort study | 551 | 0 | 0 | Any AE |
| Only in relation to allopurinol dose | |||||
| Panomvana et al. 200867 | Prospective observational study | 27 | 0 | 0 | Any AE |
| Shi et al. 201247 | RCT | 21 | 0 | 19 | Any AE |
| Siu et al. 200648 | RCT | 25 | 0 | 26 | Any AE |
| Stamp et al. 201169 | Prospective observational study | 83 | 0 | 0 | Any AE |
| In relation to allopurinol dose | |||||
| Stamp et al. 201268 | Retrospective case–control study (matched) | 54 | 157 | 0 | AHS |
| In relation to allopurinol dose | |||||
| Patients requiring hospitalisation | |||||
| Tassaneeyakul et al. 200970 | Retrospective case–control study | 27 | 54 | 0 | SJS/TEN |
| In relation to HLA-B allele | |||||
| Vazquez-Mellado et al. 200171 | Retrospective cohort study | 120 | 0 | 0 | AHS |
| In relation to allopurinol dose | |||||
| Studya | Mean age (range – unless stated otherwise) (years) | Male (%) | Ethnicity (%) | Indications |
|---|---|---|---|---|
| Arellano and Sacristan 199333 | Median: 57.5 (25–89) | 67.3 | Not reported | Patients were included for various indications, mostly asymptomatic hyperuricaemia (75%) |
| Atzori et al. 201249 | 74 (62–96) | 40.5 | European (implied from text of study) | Patients were mostly asymptomatic hyperuricaemia (95%) |
| Bowie et al. 196750 | 47 (33–74) | 78.6 | Maori: 14 | Patients with gout and chronic renal failure |
| Polynesian: 7 | ||||
| Other (not reported): 69 | ||||
| Chiu et al. 201251 | Case: 68.5 (33–96) | 74.0 | Han Chinese: 100 | Not reported |
| Control: 71.5 (41–97) | Case: 55.0 | |||
| p = 0.383 | Control: 86.7 | |||
| p = 0.012 | ||||
| Dalbeth and Stamp 200652 | 56 (26–86) | 82.0 | Pacific: 46 | Patients with gout |
| Maori: 26 | ||||
| European: 21 | ||||
| Other/not stated: 7 | ||||
| Goicoechea et al. 201013 | Allopurinol: 72.1 (SD 7.9) | 64.6 | Not reported | Patients with moderate CKD |
| Allopurinol: 64.2 | ||||
| Control: 71.4 (SD 9.5) | Control: 64.9 | |||
| Hung et al. 200553 | Case: median 66 (18–91) Control: median 56 (21–84) |
Case: 47.9 Control: 92.6 |
Han Chinese: 100 | Hyperuricaemia: 100% |
| Jung et al. 201154 | Case: 41.4 (SD 14.4) | Case: 43.8 | Korean: 100 | Patients with severe CKD being considered for transplantation |
| Control: 35.9 (SD 18.1) | Control: 73.6 | |||
| Kang et al. 201155 | Case: median 58 (35–80) | Case: 56.0 | Korean: 100 | Case
|
| Control: median 51 (20–76) | Control: 64.9 | Control
|
||
| Kaniwa et al. 200856 | 70.9 (SD 9.7) (53–83) | 80.0 | Japanese: 100 | Not reported |
| Kao et al. 201114 | Allopurinol: 70.6 (SD 6.9) | 52.8 | Not reported | Patients with stage 3 CKD and LVH |
| Control: 73.7 (SD 5.3) | Allopurinol: 59.2 | |||
| p = 0.070 | Control: 46.1 | |||
| p = 0.139 | ||||
| Khabbal et al. 201257 | 51 (SD 7) (30–73) | 60.0 | Not reported | Not reported |
| Khoo and Leow 200058 | 52 (29–86) | 69.2 | Chinese: 92 | Patients were included for various indications, mostly gout (46%) and including asymptomatic hyperuricaemia (23%) |
| Malay: 8 | ||||
| Krishnamurthy 201059 | Case: 65.3 Control: 64.8 |
100 | Not reported | Patients with hyperuricaemia who were newly started on allopurinol for any reason and who had evidence of treatment compliance |
| Lee et al. 200861 | 69 (36–91) | 32.1 | Chinese: 96 | Patients were included for various indications, mostly gouty attacks (50%) and including asymptomatic hyperuricaemia (25%) |
| Malay: 4 | ||||
| Levin and Abrahams 196662 | (8–68) | 87.8 | Not reported | Patients with renal disease |
| Lonjou et al. 200863 | 55.4 (SD 18.3) (21–83) | 58.1 | European: 87 | Prospective patients (n = 26); hyperuricaemia 88% |
| African: 3 | ||||
| Asian: 7 | ||||
| South American: 3 | ||||
| Paisansinsup and Schousboe 201166 | Not reported | Not reported | Not reported | Patients with gout |
| Panomvana et al. 200867 | 60.37 (SD 10.76) (42–79) | 88.9 | Not reported | Gout patients with renal insufficiency |
| Shi et al. 201247 | Allopurinol: 39.7 (SD10.0) | 55.0 | Not reported | Hyperuricaemic IgAN patients |
| Control: 40.1 (SD 10.8) | Allopurinol: 61.9 | |||
| Control: 47.3 | ||||
| Siu et al. 200647 | Allopurinol: 42.7 (SD 12.9) | 41.5 | Not reported | Hyperuricaemic patients with mild to moderate CKD |
| Control: 42.8 (SD 16.8) | Allopurinol: 32.0 | |||
| p = 0.78 | Control: 53.8 | |||
| p = 0.09 | ||||
| Stamp et al. 201169 | 58.7 (27–83) | 87.9 | European: 82 | Patients with uncontrolled gout |
| Maori or Pacific Islander: 14 | ||||
| Other: 4 | ||||
| Stamp et al. 201268 | Case: 64.8 (24–87) | 55.5 | Case
|
Patients with gout |
| Control: 64.1 (23–92) | Case: 55.6 | Control
|
||
| p = 0.79 | Control: 55.4 | Difference between groups, p < 0.001 | ||
| p = 1.0 | ||||
| Tassaneeyakul et al. 200970 | Case: median 65 (38–81) | Case: 55.6 | Case
|
Case
|
| Control: median 63.5 (46–90) | Control: 79.6 | Control
|
Control
|
|
| Vazquez-Mellado et al. 200171 | 52.7 (SD 2.4) Median: 55 | 98.3 | Not reported | Patients with gout |
Glossary
- Acute generalised exanthematous pustulosis
- A primarily drug-induced acute eruption, with some similarities to pustular psoriasis. It is characterised by numerous, small, non-follicular pustules with widespread oedema and erythema. Unlike Stevens–Johnson syndrome, the mucus membranes are not affected.
- Albuminuria
- The most common kind of proteinuria, albuminuria describes the presence of albumin in the urine, sometimes indicating kidney disease.
- Allopurinol hypersensitivity syndrome
- An adverse reaction specific to allopurinol. Hypersensitivity syndrome may also occur from taking other drugs (e.g. abacavir, azathioprine or carbamazepine). It is a potentially life-threatening reaction characterised by fever, rash and internal organ involvement.
- Cardiovascular disease
- A group of disorders of the heart and blood vessels. These include, but are not limited to, coronary heart disease, cerebrovascular disease and peripheral arterial disease.
- Case–control study
- An observational study, usually conducted retrospectively, in which cases (e.g. subjects with a particular drug reaction) are selected and compared with controls (e.g. subjects which did not experience the drug reaction). Controls may be matched (e.g. for age, sex, ethnicity, location) or unmatched.
- Cohort study
- An observational study conducted either prospectively or retrospectively in which subjects are studied over a period of time in relation to a particular outcome (e.g. a group of patients may be followed up prospectively to see if they develop a drug reaction, patients who experienced a particular drug reaction may be analysed for retrospective risk factors, e.g. age, sex, drug dose, duration of exposure to a drug).
- Chronic kidney disease
- Chronic kidney disease is defined according to the presence or absence of kidney damage and level of kidney function. Its severity can be classified as ranging from stage 1 to stage 5, with stage 5 being the most severe condition (end-stage renal failure). Chronic kidney disease is also referred to by interchangeable terms such as renal disease or renal insufficiency, chronic kidney disease is the preferred term as it can be defined by its severity as measured by glomerular filtration rate.
- Drug rash with eosinophilia and systemic symptoms
- Another term for drug hypersensitivity syndrome (see Allopurinol hypersensitivity syndrome).
- Eosinophilia
- Eosinophils are a type of white blood cell and eosinophilia is a type of leucocytosis.
- Erythema multiforme
- Sometimes considered to be a milder form of Stevens–Johnson syndrome without mucosal involvement.
- Glomerular filtration rate
- The best overall measure of kidney function. Serum creatinine is a proxy for glomerular filtration rate, but the best measure of glomerular filtration rate is estimated glomerular filtration rate, which uses a formula to take into account factors such as a patient’s age, sex and ethnicity alongside serum creatinine levels.
- Gout
- A disease in which defective metabolism of uric acid results in painful inflammation of the joints, deposits of urates in and around the joints, and usually an excessive amount of uric acid in the blood (serum uric acid).
- Gouty arthritis
- Painful joint inflammation (or attack) that is a manifestation of gout.
- Heterogeneity
- Variability or differences between studies in the estimates of effects.
- HLA-B
- A gene with a critical role in the human immune system. HLA-B belongs to the human leucocyte antigen family of genes. Other genes belonging to this family include HLA-A and HLA-C.
- HLA-B*5801
- An allele (variant) of the human leucocyte antigen gene (major histocompatibility complex, class I, B). Other alleles beginning with HLA-B belong to this gene, those beginning HLA-A to the human leucocyte antigen (major histocompatibility complex, class I, A) and HLA-C to the human leucocyte antigen (major histocompatibility complex, class I, C) genes.
- Hypertension
- Abnormally high blood pressure. Hypertension is a risk factor for both cardiovascular disease and chronic kidney disease.
- Hyperuricaemia
- A condition in which a person has high levels of uric acid in the blood. Hyperuricaemia is associated with both chronic kidney disease and cardiovascular disease. However, it is not known if hyperuricaemia is a cause, consequence or coincidental to either disease.
- Immunoglobulin A nephropathy
- A kidney disorder that occurs when IgA – a protein that helps the body fight infections – settles in the kidneys, causing scarring and inflammation within the kidney.
- Intention to treat
- A method of data analysis in which all patients are analysed in the group they were assigned to at randomisation regardless of treatment adherence.
- Leucocytosis
- A condition characterised by an elevated number of leucocytes (white cells) in the blood. Leucocytes are a vital part of the immune system, enabling the individuals to fight infections. Types of leucocytosis include neutrophilia, monocytosis, basophilia, eosinophilia and lymphocytosis.
- Major histocompatibility complex
- Molecules which mediate interactions of leucocytes with other leucocytes or body cells.
- Meta-analysis
- A quantitative method for combining the results of many trials into one set of conclusions.
- Proteinuria
- The presence of excess of serum proteins in the urine, which may indicate damage to the kidneys.
- Randomised controlled trial
- Considered to be the gold standard for a clinical trial in which prior to receiving an intervention (e.g. a drug) patients are randomly allocated to an intervention group (e.g. to receive the drug) or control group (e.g. to receive usual care or a placebo).
- Serum creatinine
- A chemical waste molecule that is generated from muscle metabolism and transported through the bloodstream to the kidneys. The kidneys filter out most of the creatinine and dispose of it in the urine. Thus, abnormally high levels of creatinine warn of possible damage to the kidneys. Creatinine levels may be used to calculate the creatinine clearance, which reflects the glomerular filtration rate.
- Severe cutaneous adverse reaction
- Adverse reactions to drugs which include hypersensitivity syndrome; Stevens–Johnson syndrome; Stevens–Johnson syndrome/toxic epidermal necrosis overlap and toxic epidermal necrosis: a spectrum of disorders which can be differentiated by the degree of skin and mucous membrane involvement. Classification is dependent on the percentage of skin involvement: Stevens–Johnson syndrome has < 10% total body surface area involvement; Stevens–Johnson syndrome/toxic epidermal necrosis overlap overlap has 10–30% total body surface area involvement; and toxic epidermal necrosis has > 30% total body surface involvement. Sometimes severe cutaneous adverse reactions may also be considered to include the following reactions: erythema multiforme, allopurinol hypersensitivity syndrome (or drug reaction with eosinophilia and systemic symptoms) and acute generalised exanthematous pustulosis.
- Stevens–Johnson syndrome
- A hypersensitivity skin disorder which ranges from mild skin and mucous membrane lesions to a severe, sometimes fatal systemic illness.
- Tophi
- An abnormal mineral deposit at the surface of joints or in skin or cartilage caused by high levels of uric acid in the blood occurring in gout.
- Toxic epidermal necrosis
- Also known as ‘Lyell’s syndrome’, toxic epidermal necrosis is a more severe form of Stevens–Johnson syndrome where internal organs (respiratory and urinary tracts) are often affected and which carries a high mortality.
- Uric acid
- Uric acid is created when the body breaks down a substance called purines. It develops when there is an excess production of uric acid (e.g. a diet rich in purines, metabolic complications that can occur after cancer), decreased excretion of uric acid (e.g. drugs) or a combination of both (e.g. beer).
List of abbreviations
- ACEI
- angiotensin-converting enzyme inhibitor
- AE
- adverse event
- AHS
- allopurinol hypersensitivity syndrome
- ARB
- angiotensin II receptor blocker
- BNF
- British National Formulary
- CI
- confidence interval
- CKD
- chronic kidney disease
- CKD-FIX
- Controlled trial of slowing of Kidney Disease progression From the Inhibition of Xanthine oxidase trial
- CVD
- cardiovascular disease
- DRESS
- drug rash with eosinophilia and systemic symptoms
- eGFR
- estimated glomerular filtration rate
- ESRD
- end-stage renal disease
- FDA
- US Food and Drug Administration
- FMD
- flow-mediated dilatation
- GFR
- glomerular filtration rate
- HDL
- high-density lipoprotein
- HLA
- human leucocyte antigen
- HR
- hazard ratio
- ICU
- intensive care unit
- ITT
- intention to treat
- IgAN
- immunoglobulin A nephropathy
- LDL
- low-density lipoprotein
- LVMI
- left ventricular mass index
- MDRD
- Modification of Diet in Renal Disease formula
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PERL
- Preventing Early Renal function Loss trial
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SCAR
- severe cutaneous adverse reaction
- SD
- standard deviation
- SJS
- Stevens–Johnson syndrome
- SJS/TEN
- Stevens–Johnson syndrome/toxic epidermal necrosis overlap
- TEN
- toxic epidermal necrosis