Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/127/04. The contractual start date was in January 2012. The draft report began editorial review in September 2013 and was accepted for publication in January 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
All authors received advice, support and in-person tutorials during nurse training programme with AB Mando, Huddinge, Sweden.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Hamilton-Shield et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Childhood obesity is common, affecting approximately one-quarter of all school leavers. 1 Obese children are more likely to remain obese in adult life,2,3 which puts them at high risk of developing a number of chronic health conditions, including diabetes, cardiovascular diseases and cancer. Uncertainty remains about how best to manage childhood obesity in terms of intervention and setting. 4 It has been estimated that the current cost of obesity is approximately £4.2B to the NHS and £16B to the wider economy. 5 The Foresight report6 suggests that these figures will double by 2050. There is a research imperative to identify cost-effective programmes for primary and community care and to identify those that are effective at promoting long-term weight maintenance following initial therapy. 4
Current evidence base for weight loss programmes
Current weight management programmes for children are directed at multicomponent interventions addressing healthy nutrition and improved levels of physical activity. Unfortunately, the evidence for the clinical effectiveness of such programmes remains scarce. The Cochrane meta-analysis of lifestyle interventions for obesity in children under 18 years old, including behavioural interventions, identified a mean difference in body mass index standard deviation score (BMI z-value) above ‘standard or minimal intervention’ of –0.04 [95% confidence interval (CI) –0.12 to 0.04], at termination of the intervention (12 months). 4 Such changes are unlikely to be clinically or metabolically advantageous to improving the health of an obese child. These statistically significant, but clinically unimportant, changes mirror data not included in the Cochrane review for community-based interventions, such as the Watch it and Lifestyle Education for Activity Program (LEAP) studies. 7,8 The LEAP study, the only randomised controlled trial (RCT) published prior to the commencement of the Community Mandolean RCT (ComMando) on primary care management of childhood obesity, found that the intervention did not achieve sustained weight reduction. 8
There is limited evidence that family-based approaches to weight management in childhood are more likely to be effective than those aimed at the child alone. 4 However, there is strong epidemiological evidence that children living in a family with an obese parent are more likely to develop obesity themselves and, thus, an intervention aimed at improving the weight of both obese child and parent seems a logical strategy to more fully engage a family in fundamental changes to lifestyle behaviour. 9,10
Speed of eating and its relation to obesity
There is a growing body of evidence that eating behaviours other than simple nutritional decision processes may be a determinant of obesity. Studies in adults and children suggest that increased speed of eating is associated with an increased risk of obesity. 11,12 Theoretically, eating food swiftly leads to a state of relative ‘satiety unresponsiveness’ by which an individual fails to respond to normal satiety signals such as gastric distension and gut peptide release. 13
Mandometer/Mandolean: a new device for weight management
Mandolean® [previously Mandometer® (Mikrodidakt AB, Lund, Sweden)] is a novel weight management method. It teaches patients how to eat and recognise hunger and satiety with the help of a small computer that receives information from a small scale beneath the patient’s plate of food. The Mandolean system allows the patient to see a rate of eating displayed on the screen that describes the rate at which normal individuals eat that amount of food and feel satiety as they eat. At the same time, the patient’s own eating speed and perception of satiety is shown on the screen. The patient then gradually learns to model his or her pattern of eating to a more normal and slower pattern of eating.
Evidence base for Mandolean
In a pilot study of obese adolescents referred to a hospital, we were able to demonstrate that speed of eating was faster than for normal-weight adult controls and that it was possible to slow down speed of food consumption using a Mandolean. 14 On the basis of these pilot data, we undertook a hospital-based, randomised trial of Mandolean in adolescents (aged 9–17 years), eliciting a mean BMI z-value reduction of –0.4 (95% CI –0.30 to –0.51) and a mean difference of 0.27 (95% CI 0.14 to 0.41) compared with standard care. Average self-determined portion size in the Mandolean group reduced at 12 months post randomisation by 45 g (95% CI 7 to 84 g) with maintenance of satiety compared with baseline. Importantly, the overall BMI z-value reduction was maintained 6 months after termination of therapy. 15 This device proved as effective as an adjunct to standard lifestyle modification in treating obesity in adolescents as pharmacotherapy, with maintenance of benefit post therapy which had not been described for any other weight loss intervention in children.
Aims and rationale
Current multicomponent interventions addressing childhood obesity through behavioural modification of physical activity and nutritional choices have produced very limited benefits in terms of improvement in BMI z-value, the key outcome measure for evaluating obesity interventions in childhood. 16
This aim of the main ComMando trial was to reproduce the greatly improved reduction in BMI z-value seen in the Mandolean hospital trial, but this time in a primary care setting delivered by trained practice nurses to primary school-aged children. If we were able to demonstrate significant changes in adiposity in both primary school-aged children and their obese parents, this was likely to become a central strategy in enabling sustainable weight loss through the learning of healthier eating behaviours.
Objectives
We set out to use both quantitative and qualitative methods to investigate the clinical effectiveness, acceptability and cost-effectiveness of using a computer device, Mandolean, to retrain pro-obesogenic eating behaviours (speed of eating and portion size determination) in obese children and their families as an adjunct to standard lifestyle education in primary care clinics.
The ComMando trial was in two distinct phases. Phase I was a fully randomised pilot to assess the feasibility and acceptability of the trial design. Phase II was the main trial, to be undertaken after further development of the Mandolean intervention, to determine the clinical effectiveness and cost-effectiveness of the Mandolean intervention. The main trial was planned to move seamlessly from the pilot to full trial phase and mirrored that of the pilot trial in terms of the content of the intervention, inclusion criteria, outcome measures and period of follow–up.
Although the main trial was not completed and ran for only 5 months before being terminated, the objectives of both parts of the study are detailed below.
Pilot study objectives
To develop the infrastructure to deliver a full trial including:
-
establishing recruitment methodologies and ensuring strategies were successful in terms of rate of recruitment
-
engaging three hub practices to deliver treatment and a number of spoke general practices to feed into the hubs
-
training nurses at the three hubs to deliver treatment
-
developing a protocol for treatment delivery
-
refining trial paperwork and study design
-
testing the acceptability of the Mandolean intervention for families
-
examining patient and parental views and experience of treatment
-
testing adherence to Mandolean therapy
-
testing adherence to regular consultation attendance.
Success of the pilot study was to be determined by meeting the following criteria:
-
recruitment of at least 36 families who would be eligible for the main study
-
90% of patients randomised to Mandolean would be successfully eating off the device at least five times a week
-
at least 60% of those using Mandolean would demonstrate a decrease in speed of meal consumption (longer meals) since baseline within 3 months of starting therapy
-
at least 80% would attend the 3-month nurse appointment for both study groups.
Main trial objectives
The main trial objectives were as follows:
-
to determine if Mandolean therapy could be delivered in the community by trained nurses in order to obtain a BMI z-score improvement at least 0.25 greater than standard care in obese children
-
to examine the clinical effectiveness of Mandolean therapy in obese adults/parents
-
to explore the effect of parental usage of Mandolean on child and parental weight loss
-
to examine the cost-effectiveness of Mandolean therapy compared with standard care
-
to assess patient and practitioner experience and acceptability of Mandolean and standard care
-
to develop a ‘toolkit’ for future users of this technology in other health-care settings.
Structure of this report
Despite not meeting all of the objectives within the specified time frame, the pilot study was completed and moved into the main trial. As a result of ongoing recruitment issues, and some technical issues relating to the Mandolean, the main trial was closed after 5 months. The main trial objectives as set out above were not completed and are therefore not reported. This report will concentrate on the objectives set out for the pilot study. Data are presented for participants recruited during the pilot phase and also those recruited during the short period of the main trial.
Chapter 2 Methods
It was originally agreed that if the pilot study met its criteria for success the study would move on to Phase II, when a fully powered RCT would be conducted, recruiting an additional 604 families. The data collected from most of the participants in the pilot were to be included in the main analysis, with the exception of participants who had taken part in the nested qualitative study (described below). Primary outcome data from these families would not have been included in the final trial analysis, as the experience of being interviewed and observed may have influenced their views of the trial and the Mandolean.
Trial setting
The study was undertaken in general practices across Bristol, North Somerset and South Gloucestershire (BNSSG) primary care trusts (PCTs) corporately known as BNSSG. The study used a ‘hub and spoke’ model of recruitment whereby certain general practices were identified as ‘hub practices’ and surrounding local practices acted as ‘spoke practices’. Nine strategically placed practices (five in Bristol, one in north Somerset and three in south Gloucestershire) were recruited to provide weight management clinics for the main study. Three of these hubs were recruited during the pilot phase, from April 2012 to December 2012, with a further six during the main trial period from January 2013 to July 2013. These additional six hubs were recruited to enable the study to meet its recruitment targets for the main trial. The roles of the hub practices were to refer to the study, as well as providing clinic space and time to host a study nurse to deliver the intervention. The spoke practices only acted as referral sites and their patients were seen for treatment at their nearest hub practice. We used the Primary Care Research Network (PCRN) (south-west) north hub to identify hub and spoke practices. We targeted hub practices where there was a practice nurse available and willing to undertake Mandolean training to deliver the trial intervention.
Both standard care and Mandolean therapy were delivered by trained practice nurses who attended an adapted Department of Health (DH)-approved ‘weight management training programme for children’ in Bristol. Further training on Mandolean therapy by Swedish collaborators was included. The learning programme utilised a mainly small-group teaching format. 17
Trial design
This was a two-arm, parallel, RCT. Participants were randomised into one of two groups: (1) standard care plus Mandolean therapy or (2) standard care alone. Participants were randomised using the Bristol Randomised Trials Collaboration randomisation service. Allocation of consenting families was stratified by hub and minimised by the age, gender and baseline BMI z-value of the child and by body mass index (BMI) of the study parent (obese/not obese). Embedded within the pilot was a qualitative study to explore the views and experiences of those receiving Mandolean therapy.
Recruitment
A total of five recruitment routes were used, as described below.
General practitioner-initiated recruitment
General practitioners who identified a child as a potential participant were asked to inform the child and their parents of the trial. If the patients were interested in participating, the GP then completed a screening form to determine if the child met the required eligibility criteria. If the child was eligible to take part, the GP asked the parents to sign a ‘permission for researcher contact’ form to confirm that they were happy for their contact details to be released to the research team. The GP also sent the researchers the completed screening information. If the child was not eligible, the GP explained this to the child and his or her parents and an anonymised screening information sheet was sent to the research team. As soon as an eligible patient had been referred, the researcher mailed a copy of the adult information sheet and age-appropriate child information sheet to the potential participant’s home address. Within 1 week of mailing out the information sheet, the researcher contacted the patients to answer any questions they had about the study and to arrange a recruitment appointment, which took place in the patient’s home. If the patient did not attend the appointment or was no longer interested in participating after considering the patient information sheet, a ‘referral outcome’ form indicating this was sent to the patient’s GP. Patients who decided not to take part were asked for their reason for declining, if they were willing to provide this. The researcher made clear that they were under no obligation to provide a reason if they preferred not to. They were also asked if they would be willing to take part in a short telephone interview with a qualitative researcher, to explain their reason for declining in more detail. If they agreed, their contact details were retained. If not, they received no further contact from the research team.
Patients who attended a recruitment appointment had the opportunity to discuss the study further with the local researcher, who went through the patient information sheet with them and obtained consent from the parent for the baseline height and weight measurements of the child to be taken to recheck eligibility. If the child was still eligible, the researcher obtained consent from the parent for them and the child to enter into the trial. Consenting patients were then asked to complete a baseline questionnaire. The local researcher also provided the participants with two ‘blinded’ Mandoleans, which recorded portion size and eating rate, but did not display this information on the screen. The researcher demonstrated how to use the device and asked both parent and child to use them for one meal a day for a week (this was subsequently reduced to 3 days as the families found this task onerous). An appointment to see the practice nurse for treatment to begin was arranged for the end of the baseline data collection week. Participants were asked to return the Mandolean at this appointment. At the initial nurse appointment, the nurse used an online randomisation system to determine treatment allocation while with the participants. The nurse was then able to inform the participants immediately of their allocation and arrange for the appropriate treatment to begin. The outcome of the recruitment and initial nurse appointments were sent to the GP along with details of where the patient was receiving treatment if they had been recruited into the study.
Health-care professional-initiated recruitment
Health-care professionals (HCPs) based at the practice that saw children potentially eligible for the trial could also refer. They were asked to introduce the trial to the child and parent and ask the parent to complete the ‘permission for researcher contact’ form. They also completed the screening and referral form and faxed both the ‘permission to contact’ form and the referral form to the local researcher. The local researcher then let both the GP and the HCP know of the outcome of the referral.
Recruitment through advertisements
Advertisements through various media, including posters in GP practice waiting rooms and a schools magazine (Primary Times), were used to aid recruitment. These advertisements invited patients to approach their GP about the trial or to get in touch directly with the research team. Patients responding to GP waiting room posters who contacted their GP were referred to the study as per routes 1 and 2 (GP- or HCP-initiated recruitment). Potential participants who contacted the research team directly, were briefly screened by telephone to ensure their registered GP practice was within the BNSSG area and that the child was within the correct age range for the study. The parent was asked if they were aware of the child meeting any of the exclusion criteria. The researcher explained that they would still need to be screened for their child’s BMI when they first met with the child, and explained that there was a threshold BMI level which the child must be over before they could enter the trial, as we were unable to offer the intervention to all children.
If, following screening, the patient was likely to be eligible, the researcher arranged a time to meet with the child and their parent to talk further about the study. The potential participants were asked for their contact details and were sent the adult patient information sheet, and an age-appropriate children’s information sheet.
The research meeting would then continue as per recruitment routes 1 and 2. If the patient was eligible and consented to take part, a letter was sent to the participant’s GP informing them that their patient was taking part in the study. The GP letter asked the GP to contact the study team if they knew of any reason the patient should not take part, with specific emphasis on the study’s exclusion criteria.
Record screening
Where there was agreement from the GP, practice staff were asked to review patient records to identify any potential participants from all families known to have children within the age range of the study. All families with children in the appropriate age range were contacted by post. They were sent an invitation letter, brief information sheet, and a ‘permission for researcher contact’ form. On return of a ‘permission for researcher contact’ form, the researcher contacted the potential participants and recruitment continued as per route 3 (recruitment through advertisements).
Recruitment through schools
School nurses in local primary schools were contacted to help identify potential participants. School nurses could send information about the trial to parents of potential participants who were encouraged to contact the study team for enrolment into the trial, as detailed previously in Recruitment through advertisements.
Inclusion/exclusion criteria
Inclusion
The target population was obese children aged 5–11 years with a BMI ≥ 95th percentile [definition of obesity in National Child Measurement Programme (NCMP)].
Exclusion
Parents were required to read and understand information written in English for consent purposes, data collection and for treatment. Therefore, children whose parents were unable to read English were excluded from the study.
General practitioners used an adapted screening tool to identify the need for the child to consult a secondary care paediatrician. This tool was previously developed for the Research for Patient Benefit (RfPB) trial18 (see Appendix 1 ).
Red flag indicators (i.e. need for secondary care evaluation) were:
-
possible genetic cause for obesity – learning difficulties, visual or hearing difficulties, dysmorphic features
-
possible associated endocrine disorder – weight and height disproportionate (height > 50th percentile), features of delayed or precocious puberty, features of Cushing’s syndrome
-
possible comorbidity – recurrent severe headaches (Idiopathic Intracranial hypertension), features of sleep apnoea syndrome, polyuria or polydypsia (type 2 diabetes)
-
features of an overt eating disorder – history of suggestive of bulimia
-
iatrogenic causes of obesity – cranial surgery, anticonvulsant therapy.
Randomisation
Allocation of consenting families was stratified by hub and minimised by the child’s age, gender and baseline BMI z-value, and by BMI of the study parent (obese/not obese). Concealment of allocation was ensured by use of an automated web-based randomisation service hosted by the Bristol Randomised Trials Collaboration, a UKCRC (UK Clinical Research Collaboration)-registered clinical trials unit. Participants were randomised on a 1 : 1 basis, to either the intervention or control group. Baseline data were collected prior to allocation.
Treatment
Standard care
Participants allocated to the control arm received a package of care specifically tailored for the treatment of childhood obesity in a primary care setting. This was referred to as standard care, though this reflected an enhanced level of care to that usually received in primary care settings.
In standard care, emphasis was placed on implementing changes to increase levels of enjoyable physical activity to national recommended levels (60 minutes’ exercise a day for children) alongside a balanced diet, based on the ‘Eatwell Plate’. 19 Families were encouraged to set their own dietary goals and targets, with practical advice and guidance from the practice nurse. In encouraging activity, the approach was one of facilitation rather than prescription. Motivational interviewing techniques were used to engage patients and families in the decision-making process for lifestyle changes which were consistent with self-determination principles and, therefore, more likely to lead to responsibility for long-term change. 20
Standard care appointments were delivered at 3-monthly intervals over a 12-month period, resulting in five appointments in total. In addition, three supportive telephone calls were provided to help patients to engage in behaviours discussed in the face-to-face sessions.
Experimental intervention
Participants allocated to receive the Mandolean intervention received the same standard care package as the control group with the addition of Mandolean therapy. Mandolean therapy required the patient to attend appointments with the nurse specialist fortnightly for the first 2 months of treatment. This meant that there were four additional Mandolean appointments, resulting in a total of nine appointments and three telephone calls for patients allocated to the intervention arm.
What is Mandolean therapy?
Mandolean is a portable weighing scale connected to a small computer which can generate a graphical representation of food removal from the plate with weight of food (grams) on the y-axis and time (minutes) on the x-axis. The patient puts a measured portion of food determined by a therapist on the scale and the computer records and displays, in real-time graphics, the removal of food from the plate as the patient eats; time zero on the graph effectively displaying total portion size. Removing food from the plate generates a gradually developing, red line on screen which is visible to the patient and can be compared and matched to a preset eating line on screen displaying the speed at which the therapist wants the patient to eat. Deviation from the training line by eating too quickly or slowly elicits a spoken request from Mandolean to slow down or eat faster. At regular intervals, a rating scale appears on the monitor of the computer and the patient rates their level of fullness (satiety): from 0 (no satiety) to 100 (maximum satiety). Patient-rated satiety appears as a dot on screen yielding a ‘development of satiety’ curve allowing comparison of the development of fullness to a ‘normal’ fullness curve again preset on screen. During ‘Mandolean training’ the patient gradually adopts a more normal pattern of eating and satiety by following these training lines and curves ( Figure 1 ).
FIGURE 1.
Representation of graphics seen on a Mandolean screen during training. (a) The blue training line is presented to patient to follow while eating. (b) The green line develops as food is consumed. In this case, food is being consumed too quickly, so the computer would encourage the patient to eat a little more slowly to approximate the green line to the training line. (c) At regular intervals during the meal, the patient is asked by the computer screen to rate satiety. (d) Dark green satiety ratings are plotted by the computer on to the screen. The patient learns to associate feeling of ‘fullness’ with the dark green S-shaped satiety training line. When eating quickly, as in this case, satiety is rated low. Note: no numerical values are displayed on the axes during training.
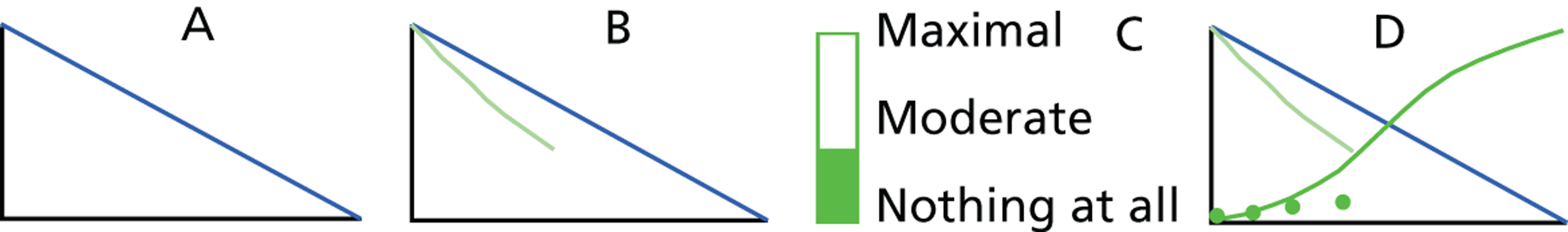
Mandolean training
During week 1, the child and parent were trained in how to use the Mandolean. This was a stepped process and is outlined below.
Step 1: the practice nurse used the ‘blind’ (no visual or oral feedback provided to participant) Mandolean data collected in the previous week to assess baseline food intake, rate of eating and satiety. Participants’ eating behaviours were then compared with previously established normal eating patterns. 14 Individualised training lines for portion size, eating speed (reflecting weight removal from plate on y-axis) and satiety were then programmed on to the participant’s Mandolean.
Step 2: the participants were encouraged to eat at least one cooked meal (usually evening meal) a day from their Mandolean, matching as closely as possible their red eating line to the current preset ‘optimal’ eating line.
Step 3: participants received up to four new training lines over 3 months, effectively reducing total portion size and slowing down food removal from the plate in order to ‘normalise’ portion size, eating rate and satiety response. The treatment goals aimed for the patient to feel ‘full’ after eating 300–350 g of food over 12–15 minutes. Normal-weight healthy volunteers stop eating and rate their level of satiety at about 50–60 on the Mandolean rating scale (random units: 0 having no satiety and 100 being completely satiated) after eating this amount of food in this period of time. 21
Data collection
Qualitative study
A qualitative study was designed alongside the pilot trial. It entailed holding interviews with families randomised to the Mandolean intervention arm, and with individuals who had been approached about the trial but who had declined to take part.
Thirteen families randomised to the intervention arm were interviewed. These families were to be interviewed at three time points throughout the 12 months they were receiving treatment. The first interview was conducted within the first 2 weeks of the family receiving the Mandolean. The second interview occurred 4 weeks later (when the Mandolean training line should have been established) and the last interview took place at around 10 weeks (when the study parent and child should have been eating to an established training line for a month). The purpose of the interviews was to explore the views and experiences of families who were using the Mandolean and receiving standard care. Five patients who declined to take part in the study were interviewed to identify possible barriers to participation. These patients were provided with a ‘decliner’ form by their GP or this form was mailed to them by a member of the study team. The form asked the participants for details of their reason for declining to take part and also asked if they would be willing to be contacted by a researcher for a brief telephone interview to explore these reasons in more detail. Patients were asked to return the form directly to the research team using a prepaid envelope. Patients did not have to release their contact details and could choose to complete the form anonymously if they did not want to be contacted further.
Sample size
The calculation set out below describes how the sample size required for the main trial was calculated.
In the adolescent trial,15 a difference in mean BMI z-value of –0.27 was obtained between those receiving Mandolean augmented care and those receiving standard care (BMI z-value change –0.4 vs. –0.13). We expected an effect of the same size or larger in the proposed trial, as younger children are more open to change than adolescents. 22 Detection of a minimum difference in reduction of BMI z-value of 0.25 with 80% power and 5% two-sided alpha, required n = 252/arm for analysis. Assuming non-collection of the primary outcome among 20% of participants overall (14% in the Mandometer trial15/23% in RfPB trial23), we planned to recruit a total of 640 children (and families). This included the 10 participants enrolled at the start of the pilot phase who would not be subject to randomisation and would not be included in the final analysis. A minimum reduction of 0.25 in BMI z-value is clinically relevant as we have shown this is the minimum reduction required to improve key metabolic parameters such as body fat, insulin resistance and blood pressure. 24
Outcome measures and their collection
Pilot trial outcomes
The outcomes for the pilot study consisted of a series of process measures: recruitment of practices, families and training nurses; adherence to protocol in terms of attending appointments; and altering Mandolean training lines.
A further objective of the pilot trial was to review the feasibility and acceptability of the secondary outcome measures for use in the main trial (see Main trial outcomes).
Main trial outcomes
The primary outcome measure was the child’s BMI – accurate height and weight – converted to BMI z-values at 12 months. In weight research in children it is important to use BMI z-values. A z-value is defined as the number of standard deviations (SDs) above or below the mean, determined using age- and gender-matched population reference data, and is calculated as (x – mean)/SD, where x is the case value and the mean and SD are derived from control data. 25 The expected mean z-value for a normal population is 0. It is vital to evaluate where an individual lies on the age–gender distribution of BMI curves rather than using absolute values for BMI such as used in adult studies, as children grow both linearly and in terms of weight at differing rates throughout normal childhood.
All height and weight measures for the trial were obtained at the recruitment location and follow-up visits, usually in the child’s home, by a Criminal Records Bureau-checked trained researcher. Weight was measured without shoes in light clothing to the nearest 0.1 kg, using a portable Tanita floor scales (WB 100 S MA, Tanita Europe BV, the Netherlands). The scales were calibrated on a quarterly basis. Height was measured without shoes to the nearest 0.1 cm, using a Seca Leicester stadiometer (Seca, UK).
The secondary outcome measures were:
-
Height and weight of parents of index child converted to BMI for adiposity analysis.
-
Maintained BMI or BMI z-value improvement at 12 months post therapy (24 months after study entry).
-
Quality-of-life measures in child [Pediatric Quality of Life Inventory (PedsQL),26 Child Health Utility 9-Dimensions (CHU9D)27 and European Quality of Life 5-Dimensions – youth (EQ-5D-Y)28] and parents [European Quality of Life 5-Dimensions (EQ-5D)]29 for self-completion at 0, 3, 6, 9, 12 and 24 months.
-
Resource-use questionnaire, including child’s use of primary and secondary care services, to be completed by parent at 3, 6, 9, 12 and 24 months, to be used to inform economic analysis. These were to be given out at scheduled nurse appointments or posted with a prepaid envelope for return to the research team should the participant withdraw from treatment and, therefore, no longer be attending nurse appointments.
-
Change in eating speed and self-determined portion size in ‘blinded’ test meals for both child and parent at 0, 12 months (end of intervention) and 24 months (12 months post intervention). These were to be measured using a ‘blind’ Mandolean, which acted solely as a measuring device and did not provide any feedback on eating rate or portion size choice. Blind Mandoleans were issued at the same time as the anthropometric measurements were taken and collected a week later by the researcher. Parents and children were instructed how to use the blind Mandolean by the researcher. They were asked to eat three meals using the device over the course of 1 week.
-
Precise measures of changes in ‘ideal portion size’ and ‘expected satiety levels’ across a range of commonly consumed foods for both child and parent were to be compared between treatment groups at 0, 12 and 24 months. These were measured using a computer-based program that allows the child or parent to manipulate a photograph of a meal to increase or decrease the volume of food on the plate to represent the portion size they would choose. The measure was presented on a laptop in the patient’s home. The meal photographs were advised by a paediatric dietitian as foods likely to be consumed by children in that age range.
-
Changes in physical activity levels, measured as number of steps per day for 1 week, were collected at baseline using the New Lifestyles NL-800 pedometers (New Lifestyles Inc., MO, USA) which store 7 days of step-count data for a sample of children taking part in the pilot study (this was expected to be measured at 12 and 24 months also in the main trial). Children were instructed to wear the pedometer during the day for 7 consecutive days (except when bathing or swimming) and the pedometers were sealed so that the participant received no feedback on number of steps taken per day. The pedometers were issued at the same time as the anthropometric measurements and it was intended that they would be collected a week later by the researcher.
During the pilot phase it became apparent that it was not possible to collect the pedometer from every child a week later. Because the pedometer was able to store only 7 days of data in a loop-back memory, for every day after the 7 days the child wore the pedometer, one day of data was deleted in order to record a new day.
The study team identified an alternative pedometer, the Nl-2000i (New-Lifestyles Inc., USA), which would allow the research team to lock the memory in order to define the 7-day period. This would enable the research team to collect the pedometer at any time after the 7-week defined period. These new pedometers were not utilised before the main trial terminated.
-
(h) For children aged 8 years and over, dietary restraint measures were to be collected at 0, 12 and 24 months. This is a self-complete measure adapted by Brunstrom et al. 30 from the Dutch Eating Behavior Questionnaire,31 which was presented on the laptop screen following completion of the ideal portion size task.
-
(i) A measure of children’s diets over the last 2–3 months was collected using a paper food frequency questionnaire (FFQ) at baseline (this was also planned for completion at 12 and 24 months). During the pilot phase of the study, two FFQs were trialled: the Scottish Collaborative Group (SCG) FFQ version C2: Diet questionnaire for children32 and Frémeaux et al. ’s33 FFQ for children. As participants entered the study, they were provided with either the SCG FFQ or Frémeaux’s FFQ. The two FFQs were assessed for completeness, and informal assessment of acceptability of the different scales was carried out by the researcher by eliciting feedback from the parents after completion. It was identified that the Frémeaux’s FFQ was more acceptable for parents to complete.
A questionnaire and outcome measure schedule can be found in Appendix 2 detailing time of delivery of each measure for both parents and child.
Process evaluation
A process evaluation was conducted throughout the course of the study to monitor delivery of both the standard care and Mandolean intervention as per protocol. Study nurses kept an appointment log for each participant, recording the date of the appointment, attendance (attended/cancelled/did not attend), type of contact (telephone/face to face/e-mail/letter), duration of contact, topics covered and resources provided (as chosen from a checklist based on the manualised standard care package) and anthropometric measures for the child.
The appointment log enabled the research team to monitor that the content of the sessions were as expected, but also that the spacing and number of appointments were as per protocol for each group. In addition to the appointment log, nurses were also asked to complete a checklist for each appointment to ensure they had completed relevant study procedures. These include checking continued consent to participate, randomisation group, collection of Mandolean data, adjustment of Mandolean training lines, checking for adverse events since the previous appointment, completion of appointment log, record of appointment in patient’s medical notes, measurements of children’s height and weight, usage of other health services and quality-of-life questionnaires at the 3-, 6- and 9-month appointments and completion of a final progress report for the referring GP at the end of treatment. All study nurses were provided with a research manual and training in the study’s standard operating procedures to ensure effective delivery.
Peer meetings were arranged for the study nurses and were facilitated by a senior research nurse from the Western Comprehensive Local Research Network (WCLRN) who had received Mandolean training and was familiar with the study protocol. These meetings provided a further opportunity for nurses to share good practice and to discuss clinical issues arising.
Analysis plan
The quantitative data were to be analysed and the study reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines for RCTs. 34 The summary statistics describe the group of individuals recruited to the trial in relation to those eligible, and investigate comparability of the trial arms at baseline. Descriptive statistics are presented as mean and SD or numbers and percentages.
Chapter 3 Results
Recruitment
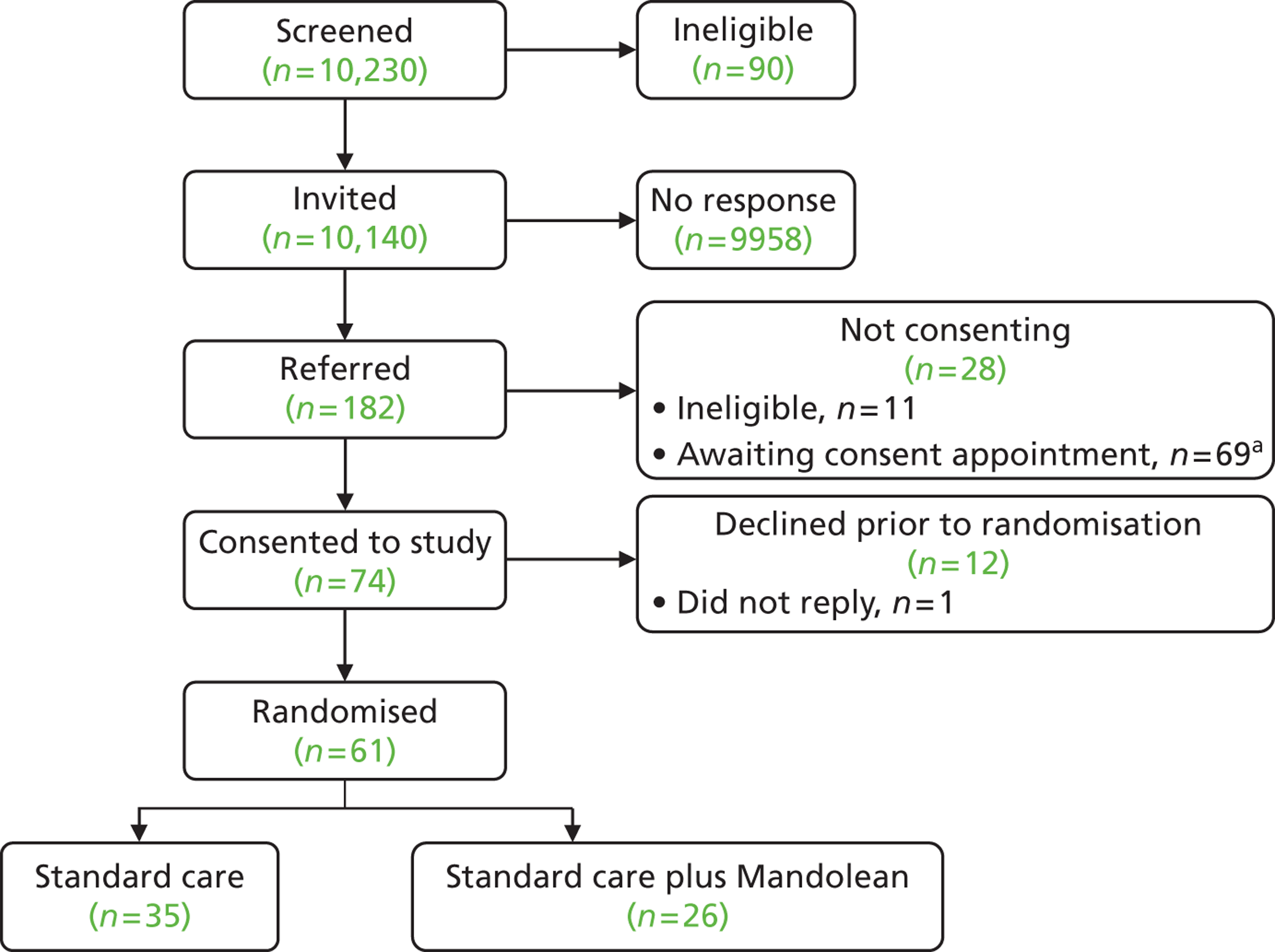
A total of 61 families with an obese child aged between 5 and 11 years took part in the trial; 21 of these families were recruited during the pilot phase (end of December 2012) and 40 were recruited during the main trial phase ( Tables 1 and 2 and Figures 2 and 3 ).
| Referral route | Number of mail-outs | Number of responses | Number consented | Number randomised |
|---|---|---|---|---|
| GP mail-outs | 10,077 | 116 | 42 | 37 |
| GP/HCP referral | n/a | 35 | 18 | 12 |
| Other referral routes | 14 | 12 | ||
| Primary Times advertising | n/a | 17 | – | – |
| Website | n/a | 3 | – | – |
| Word of mouth | n/a | 2 | – | – |
| Children’s hospital poster | n/a | 1 | – | – |
| School mail-outs | n/a | 6 | – | – |
| School nurse | n/a | 2 | – | – |
| GP waiting room advertising | n/a | 0 | – | – |
| Local community locations | n/a | 0 | – | – |
| Total | – | 182 | 74 | 61 |
| Month | Recruitment per month | Cumulative recruitment rate | Cumulative recruitment target | % of target |
|---|---|---|---|---|
| April 2012 | 0 | 0 | 4 | 0 |
| May 2012 | 0 | 0 | 8 | 0 |
| June 2012 | 1 | 1 | 12 | 8 |
| July 2012 | 3 | 4 | 16 | 25 |
| August 2012 | 3 | 7 | 20 | 35 |
| September 2012 | 2 | 9 | 24 | 38 |
| October 2012 | 3 | 12 | 28 | 43 |
| November 2012 | 8 | 20 | 32 | 63 |
| December 2012 | 1 | 21 | 36 | 58 |
| January 2013 | 3 | 24 | 62 | 39 |
| February 2013 | 9 | 33 | 88 | 38 |
| March 2013 | 11 | 44 | 114 | 39 |
| April 2013 | 16 | 60 | 140 | 43 |
| May 2013a | 1 | 61 | 166 | 37 |
| Total | 61 | 61 | 166 | 37 |
FIGURE 2.
Numbers of patients randomised and consented vs. monthly recruitment target.
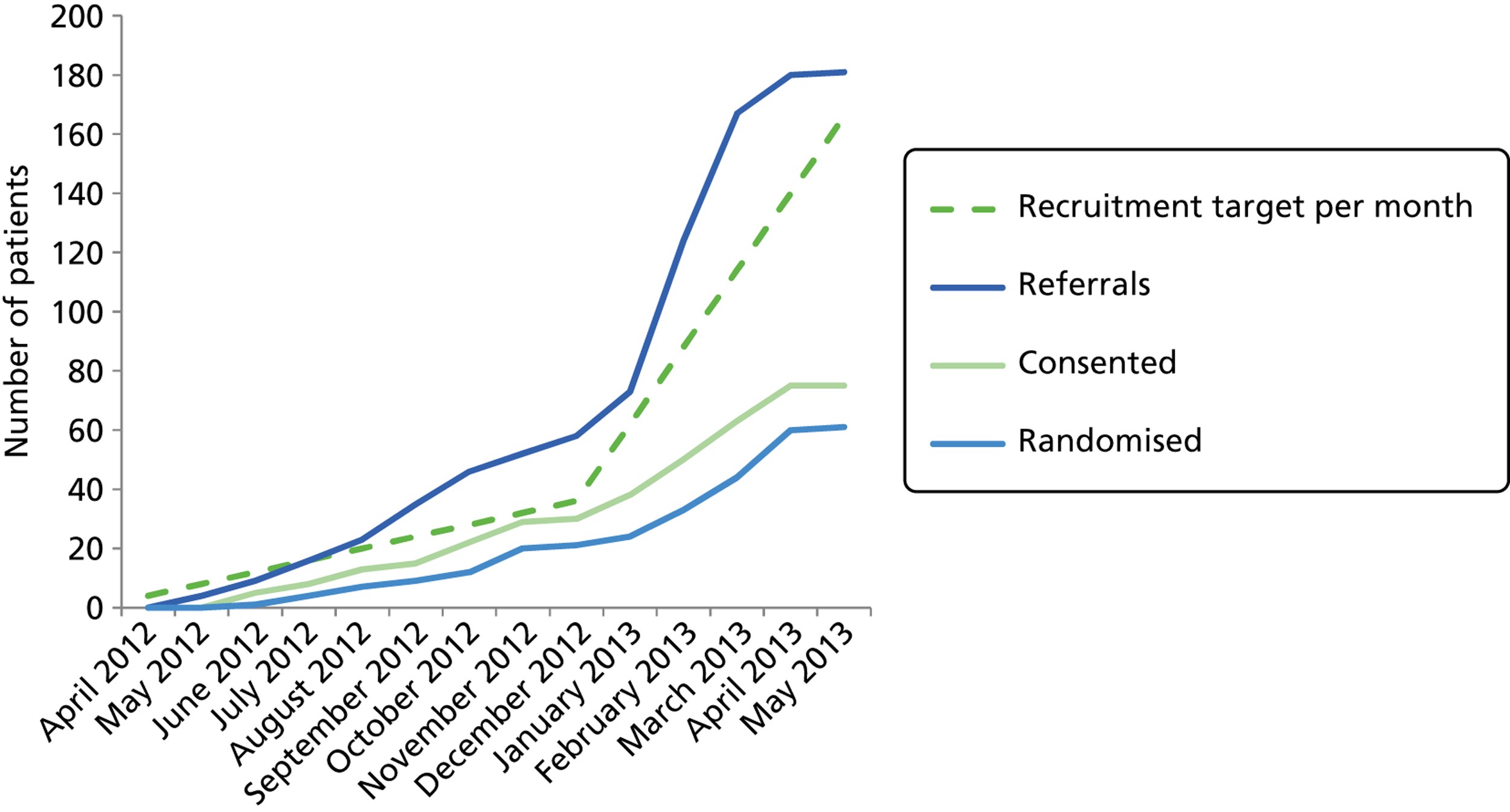
FIGURE 3.
Consolidated Standards of Reporting Trials diagram. a, 69 patients were referred to the study, but were awaiting a consent appointment with the research team at the time of the Health Technology Assessment programme decision to terminate the trial.
Baseline data
In total, 182 children were referred to the study ( Table 3 ), with 40 declining further participation either before or after baseline data collection but prior to randomisation ( Table 4 ). From those families that had consented to participate (n = 74), 61 children were randomised to either the standard or Mandolean arm ( Table 5 ). In those families in which a child was allocated a Mandolean, a parent was also provided with a device to use with their child at mealtimes. Their baseline characteristics are detailed in Table 6 .
| Variable | n = 182 |
|---|---|
| Mean age (years) (SD), range | 9.4 (1.9), 4.8–12.7 |
| Female (%) | 51.7 |
| Missing age data (%) | 3.3 |
| Missing gender data (%) | 6.6 |
| Variable | n = 40a |
|---|---|
| Mean age (years) (SD), range | 9.5 (1.5), 5.8–12.0 |
| Female (%) | 68.4 |
| Missing age data (%) | 7.5 |
| Missing gender data (%) | 5 |
| Variable | Group 1, Mandolean (n = 26) | Group 2, standard care (n = 35) |
|---|---|---|
| Mean age (years) (SD), range | 9.1 (1.6), 6.6–12.0 | 9.6 (1.9), 5.9–12.0 |
| Female (%) | 50 | 60 |
| Non-white ethnicity, n (%) | 0 (0)a | 3 (8.6) |
| Mean BMI (SD), range | 25.4 (3.4), 19.0–31.5 | 25.7 (3.6), 19.9–37.0 |
| z–score (SD), range | 2.7 (0.5), 1.9–3.9 | 2.7 (0.7), 1.8–4.7 |
Retention and adherence
Withdrawals from treatment
There were eight withdrawals from treatment: four from the intervention arm and four from the control arm. All agreed to be recontacted for the 12-month follow-up.
Withdrawals from study
There were seven full withdrawals from the study: six from the intervention arm and one from the control arm.
Lost to follow-up
One additional patient was lost to follow-up, as contact details held by research team and registered practice were no longer current.
Appointment attendance
The standard weight management programme involved four follow-up treatment appointments at 3, 6, 9 and 12 months. These were attended by both control and intervention participants ( Table 7 ).
| Time point | Patients expected to attend, n | Attended, n (%) |
|---|---|---|
| 3 months | 45 | 20 (44) |
| 6 months | 13 | 5 (38) |
| 9 months | 3 | 1 (33) |
| 12 months | 0 | 0 (0)a |
| Total | 61 | 26 (43) |
In addition to the standard weight management programme, intervention participants were required to attend a further four appointments to train them to use the Mandoleans at 2, 4, 6 and 8 weeks after their initial nurse appointment (week 1) ( Table 8 ).
| Time point | Patients expected to attend, n | Attended, n (%) |
|---|---|---|
| 2 weeks | 26a | 20 (77) |
| 4 weeks | 26b | 13 (50) |
| 6 weeks | 26c | 11 (42) |
| 8 weeks | 26d | 9 (35) |
| Total | 104 | 54 (51) |
Non-attendance and cancellation rates
First nurse appointments
A considerable number of patients (38%) failed to attend or cancelled their first nurse appointment after having consented to take part in the trial ( Table 9 ).
| Outcome | Appointments offered | Attended, n (%) | DNA, n (%) | Cancelled, n (%) |
|---|---|---|---|---|
| Consented but declined prior to randomisation | 24 | 0 (0) | 15 (63) | 9 (36) |
| Consented to study | 74 | 61 (82) | 4 (5) | 9 (12) |
| Total | 98 | 61 (62) | 19 (19) | 18 (18) |
Follow-up appointments
Table 10 shows the total number of follow-up treatment appointments offered to participants (both Mandolean and standard weight management appointments) and the number of appointments attended, not attended and cancelled. These numbers include rebookings that were made if a patient did not attend or cancelled an appointment.
| Appointments offered | Attended, n (%) | DNA, n (%) | Cancelled, n (%) |
|---|---|---|---|
| 140 | 80 (57) | 40 (29) | 16 (11)a |
Mandolean adherence
This section will present data to address objectives 1–3 of the pilot phase:
-
recruitment of at least 36 families who would be eligible for the main study
-
at least 90% of patients randomised to Mandolean will successfully be eating off the device at least five times a week
-
at least 60% of those using Mandolean will have demonstrated a decrease in speed of meal consumption (longer meals) from baseline measures within 3 months of starting therapy.
Objective 1: recruitment of at least 36 families who would be eligible for the main study
A total of 182 children (families) contacted the study team, of who 85 were assessed for eligibility. A total of 74 were eligible and consented to the study and 61 were randomised over a period of 12 months ( Table 11 ).
This report will focus on the 26 children randomised to the standard care plus Mandolean arm.
| Number of families | Number of families | ||
|---|---|---|---|
| Randomised | 61 | Not randomised | 13 |
| Standard care | 35 | Declined | 12 |
| Standard care plus Mandolean | 26 | DNA | 1 |
Objective 2: at least 90% of patients randomised to Mandolean will successfully be eating off the device at least five times a week
This estimate involves two parameters:
-
the number of meals recorded
-
the number of days between randomisation and end of the study or withdrawal from treatment, whichever came first.
Children randomised to Mandolean therapy did not use the device as frequently as instructed ( Table 12 ). Use of the Mandolean device on at least 5 days each week equated to 71% usage. Only 5 of the 26 (19%) participants randomised to the Mandolean arm achieved this target.
| Variable | No of childrenb | Mean | SD | Median | 25th, 75th centiles | Range |
|---|---|---|---|---|---|---|
| Meals | 22 | 24.7 | 27.3 | 16 | 10, 31 | 1–131 |
| Days | 22 | 70.9 | 52.8 | 59 | 28, 84 | 14–216 |
| Per cent usage | 22 | 43.8 | 33.3 | 40 | 11, 63 | 1–100 |
As each participant has a number of observations (meals) in the data set, it is useful to present summary statistics both between and within individuals ( Table 13 ).
| Variable | Summary level | Mean | SD | Minimum | Maximum | Observations, n |
|---|---|---|---|---|---|---|
| Duration prescribed (minutes) | Overall | 11.5 | 2.6 | 4.0 | 18.0 | Meals = 543 |
| Between | – | 3.1 | 5.3 | 18.0 | Participants = 22 | |
| Within | – | 1.6 | 6.6 | 16.6 | Mean meals/participant = 24.7 | |
| Duration actual (minutes) | Overall | 10.0 | 4.4 | 1.3 | 33.1 | Meals = 543 |
| Between | – | 3.0 | 2.5 | 18.5 | Participants = 22 | |
| Within | – | 3.5 | −3.7 | 24.6 | Mean meals/participant = 24.7 | |
| Weight of meal consumed (g) | Overall | 219.9 | 80.6 | 1.0 | 499.0 | Meals = 543 |
| Between | – | 65.1 | 81.5 | 334.7 | Participants = 22 | |
| Within | – | 62.8 | −92.8 | 448.3 | Mean meals/participant = 24.7 | |
| Meal consumption rate (g/minute) | Overall | 25.2 | 12.9 | 0.1 | 107.2 | Meals = 543 |
| Between | – | 8.6 | 10.0 | 41.8 | Participants = 22 | |
| Within | – | 11.0 | −10.8 | 99.2 | Mean meals/participant = 24.7 |
The overall and within individual statistics are calculated over 543 meals. The between individual statistics are calculated over 22 individuals and the average number of meals per person (24.7).
Let us examine the second block of results, actual meal duration, as an example. The average meal duration across all meals for all individuals was 10 minutes, with a range of 1.3–33.1 minutes. The lowest mean meal duration for each individual was 2.5 minutes and the highest 18.5 minutes. ‘Meal duration within’ varied between –3.7 minutes and 24.6 minutes. This tells us about deviation from each individual’s average, and we would expect some of these deviations to be negative. We have to take account of the global mean of 10 minutes and, when we do this, we find that one individual deviated from their average by 13.7 minutes (range –3.7 to 10 minutes), and one individual deviated from their average by 14.6 minutes (range 24.6–10 minutes). Finally, the reported SDs tell us that the variation in mean meal duration between individuals (3.0 minutes) is slightly less than the variation in meal duration within individuals over time (3.5 minutes). The intraclass correlation coefficient (between-participant variance divided by between- plus within-participant variance) indicates that around 40% of the variation in meal duration is because of differences between individuals.
It should also be noted that data for a number of meals suggested problems with use of the Mandolean device. For example, a meal starting weight that is less than meal end weight gives rise to a negative weight consumed and negative consumption rate. Similarly, extreme values of either weight consumed or meal duration (such as when the device was left on after the user had finished eating) or both gives rise to implausible meal consumption rates. Therefore, after examining distributions of these variables, it was decided to include only meals lasting between 1 and 40 minutes and with weight of meal consumed between 1 g and 700 g. This resulted in data from 17 out of 560 meals being discarded.
Objective 3: at least 60% of those using Mandolean will have demonstrated a decrease in speed of meal consumption (longer meals) from baseline measures within 3 months of starting therapy
The following graphs present participant-level data for each of the 24 participants randomised to Mandolean for weight consumed and consumption rate for every meal ( Figure 4 ). The treatment goals were to help the participant feel ‘full’ after eating 300–350 g of food over 12–15 minutes.
FIGURE 4.
Graphs displaying participant-level data for each of the 24 patients randomised to Mandolean for weight consumed and consumption rate for every meal. (a)–(x) Individual patients. Patient identification codes (IDs) are included in each figure part.
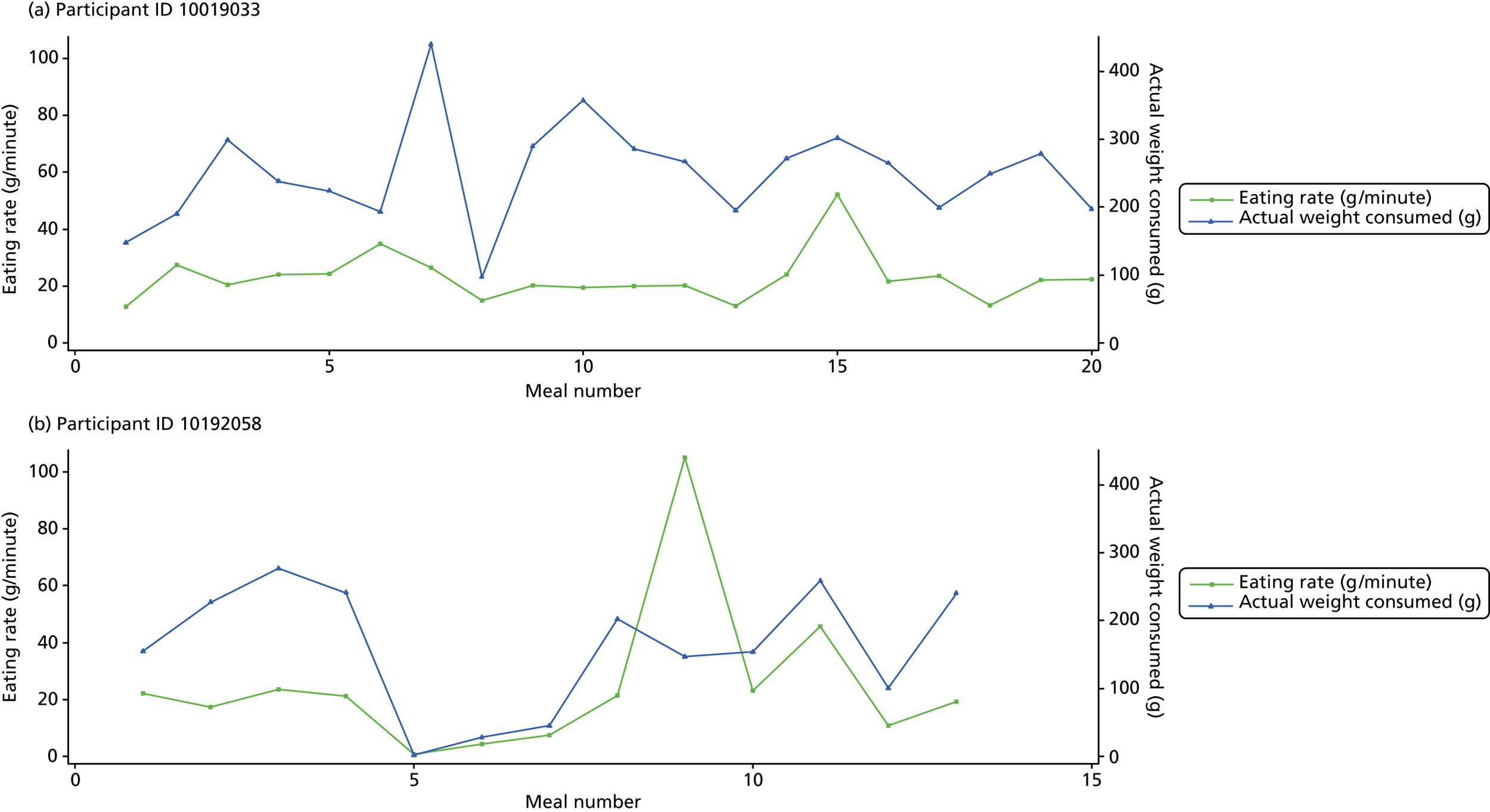
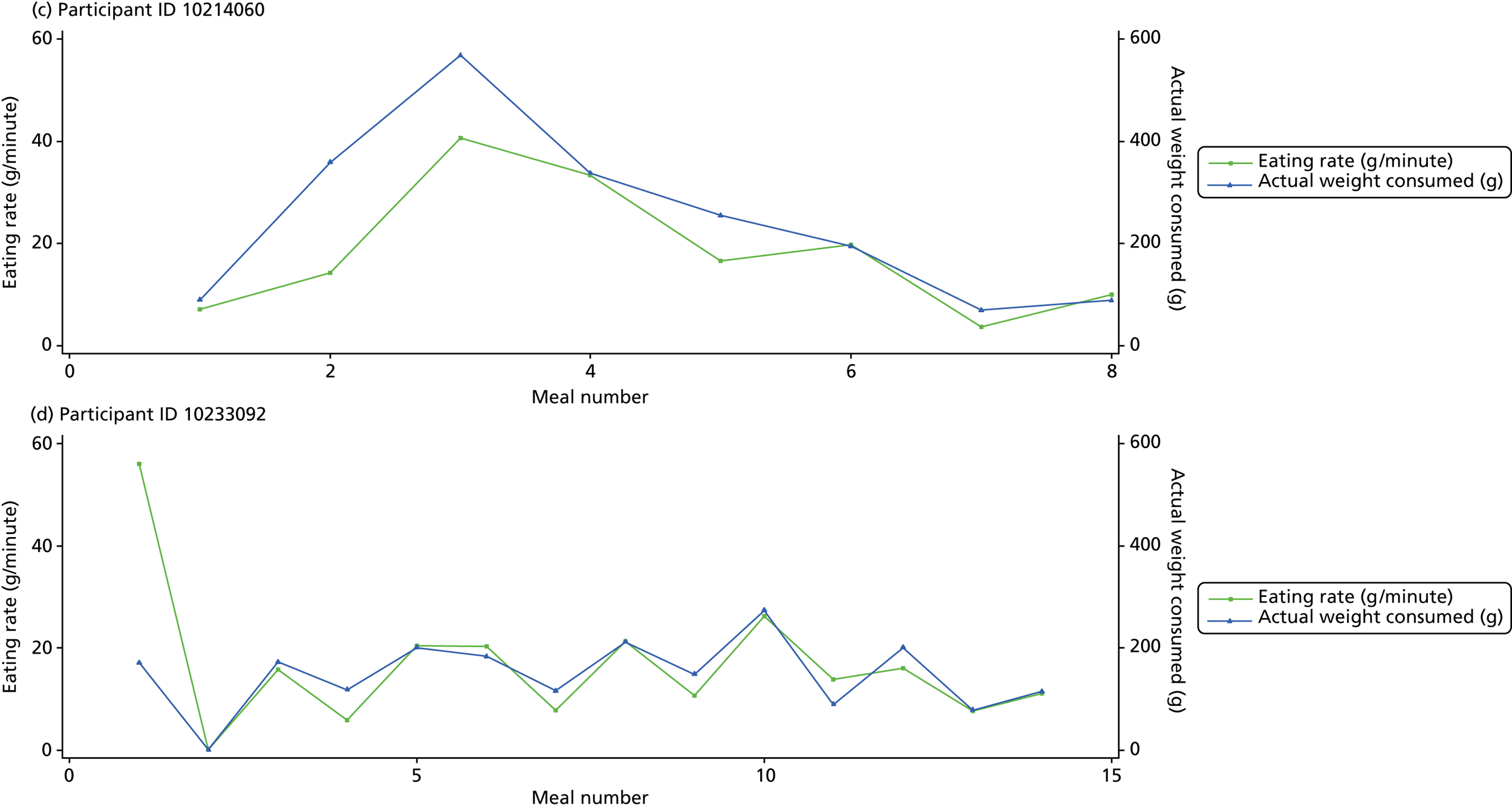
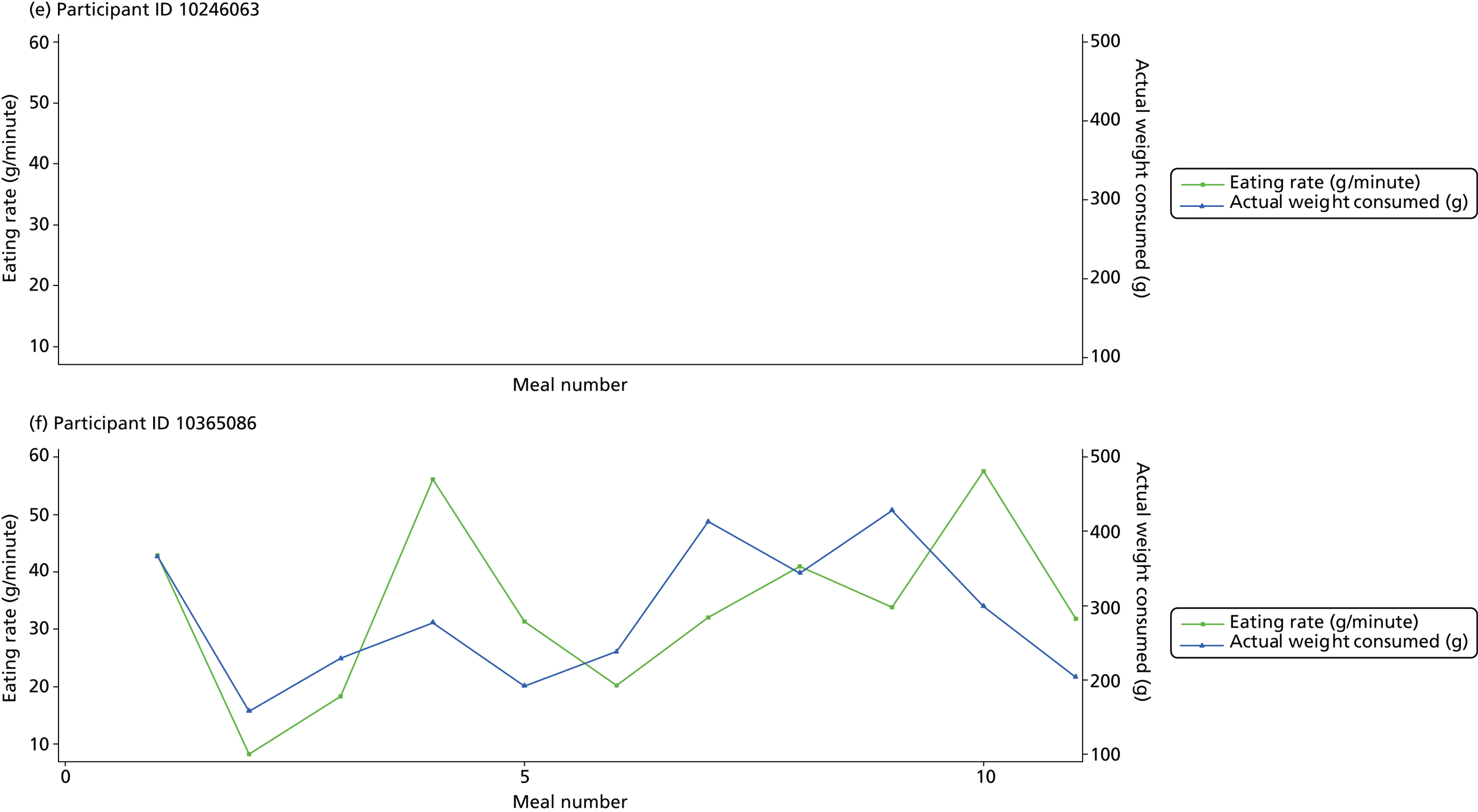
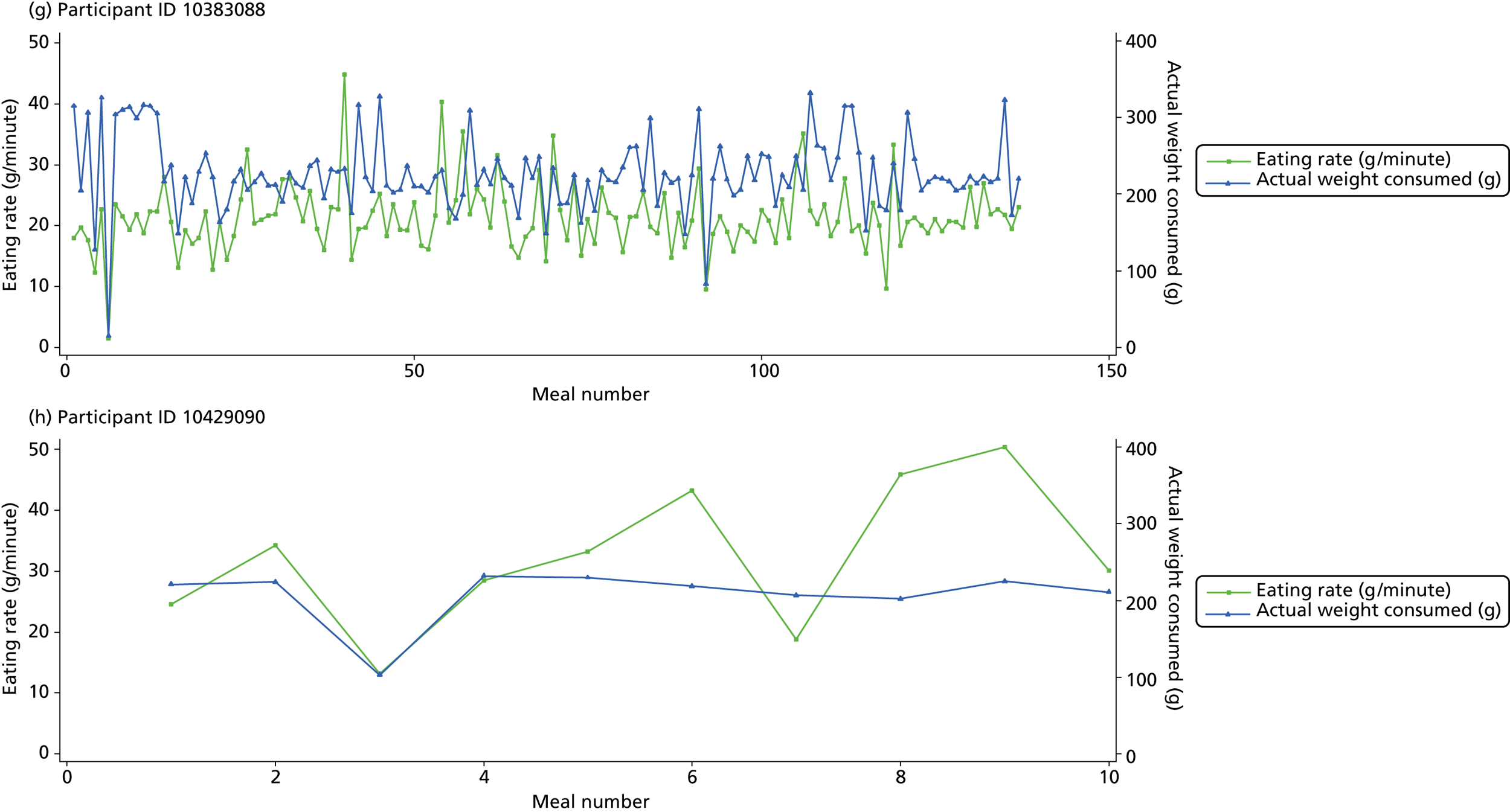
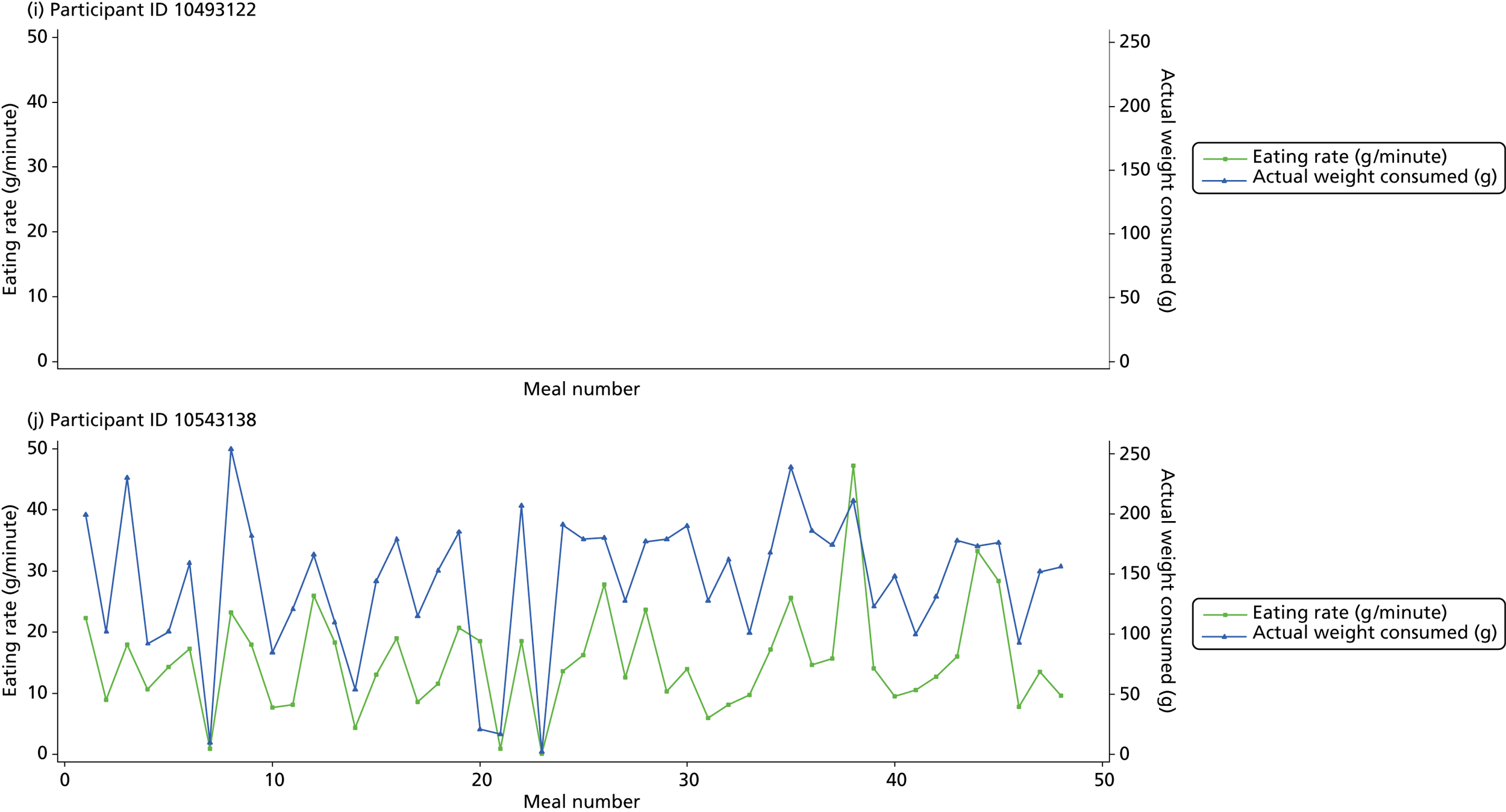
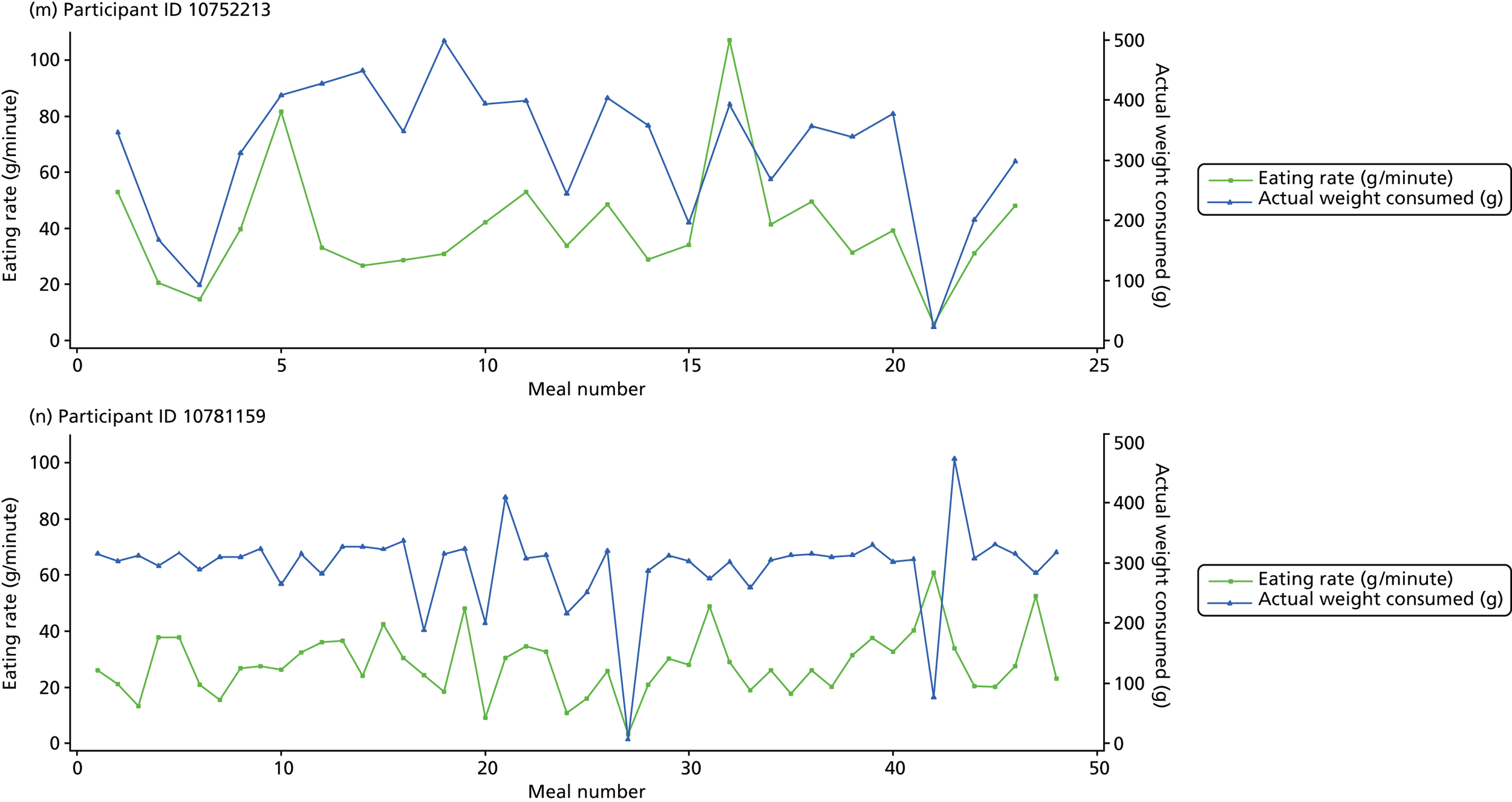
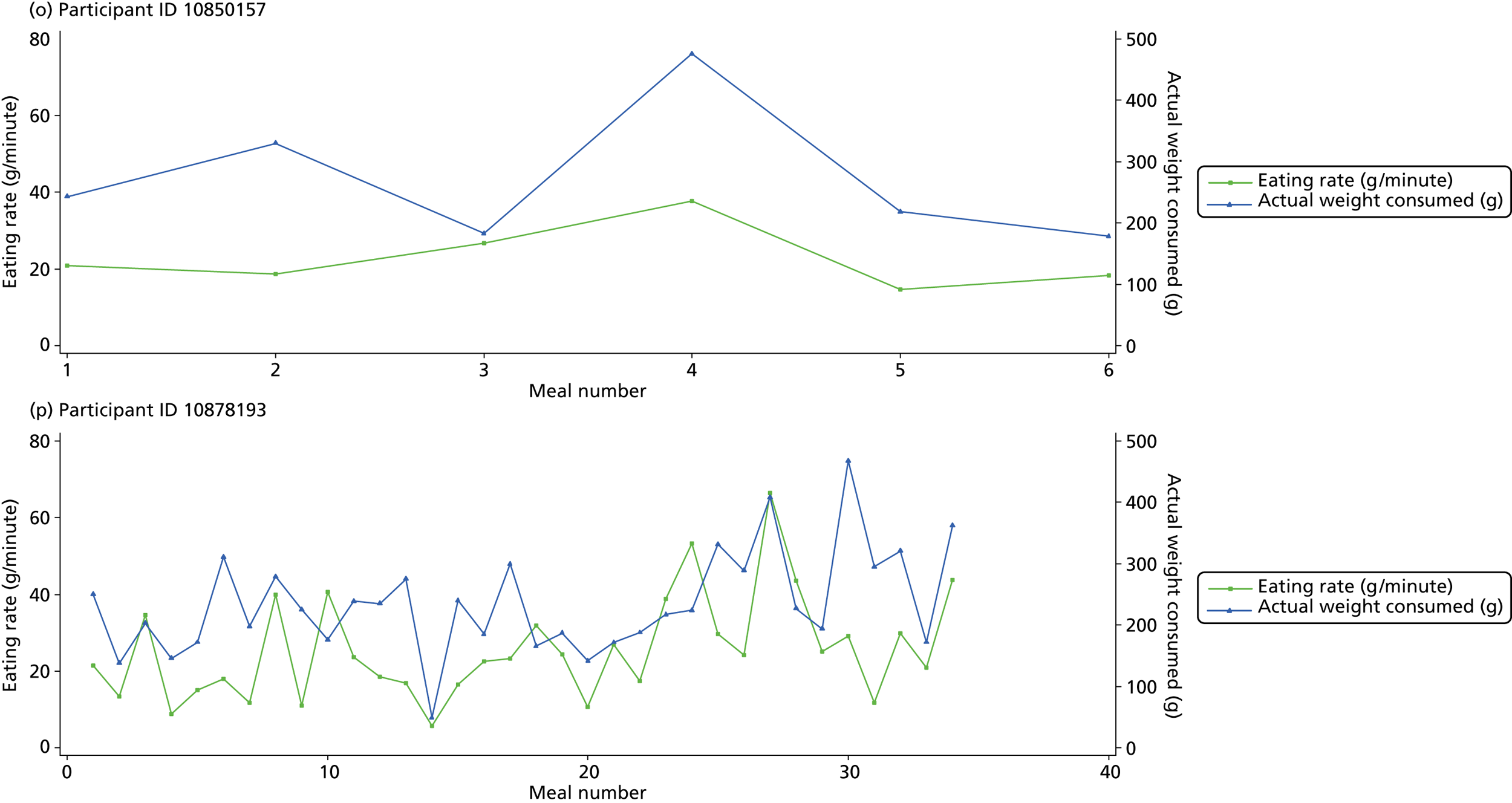
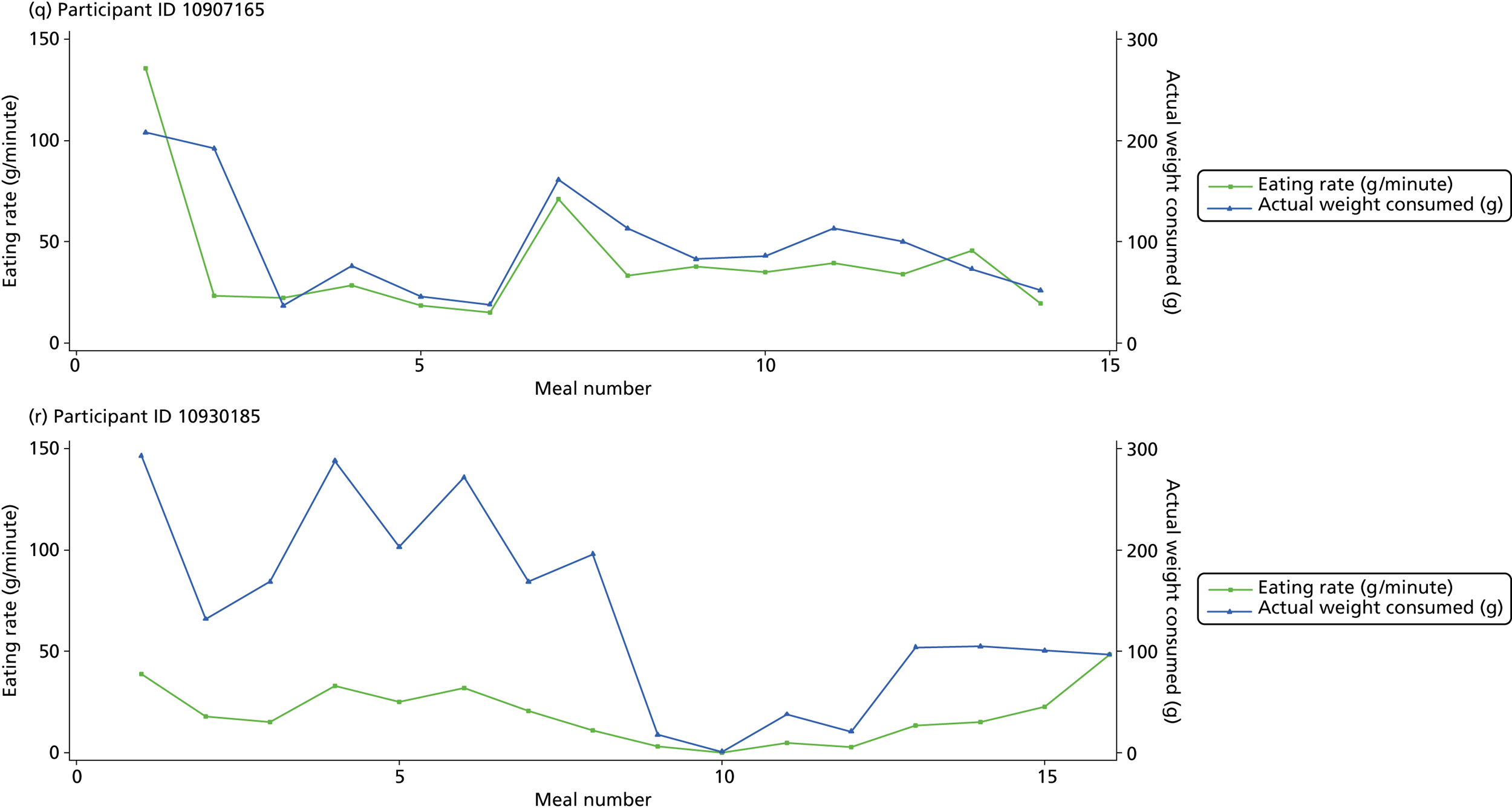
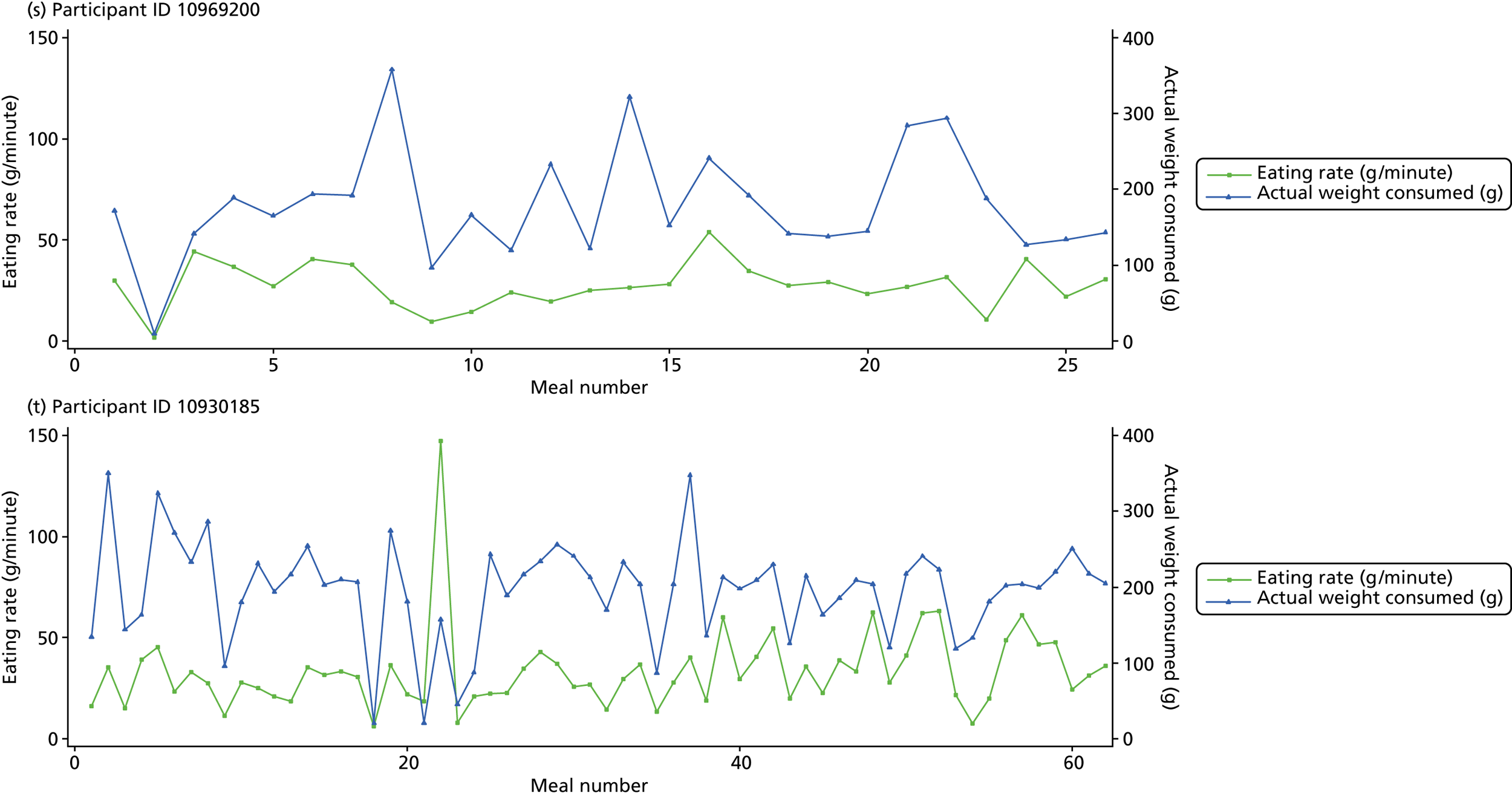
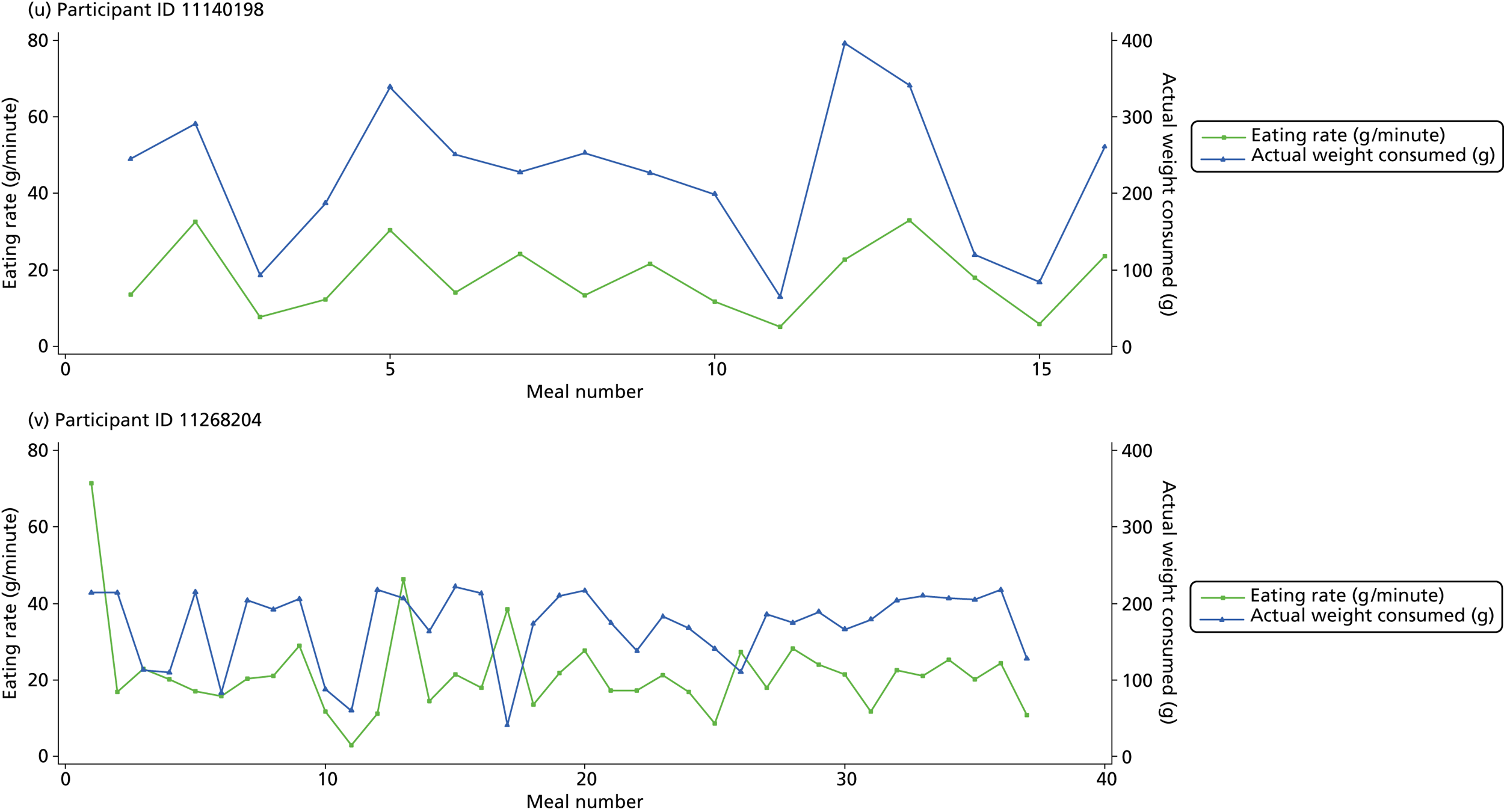
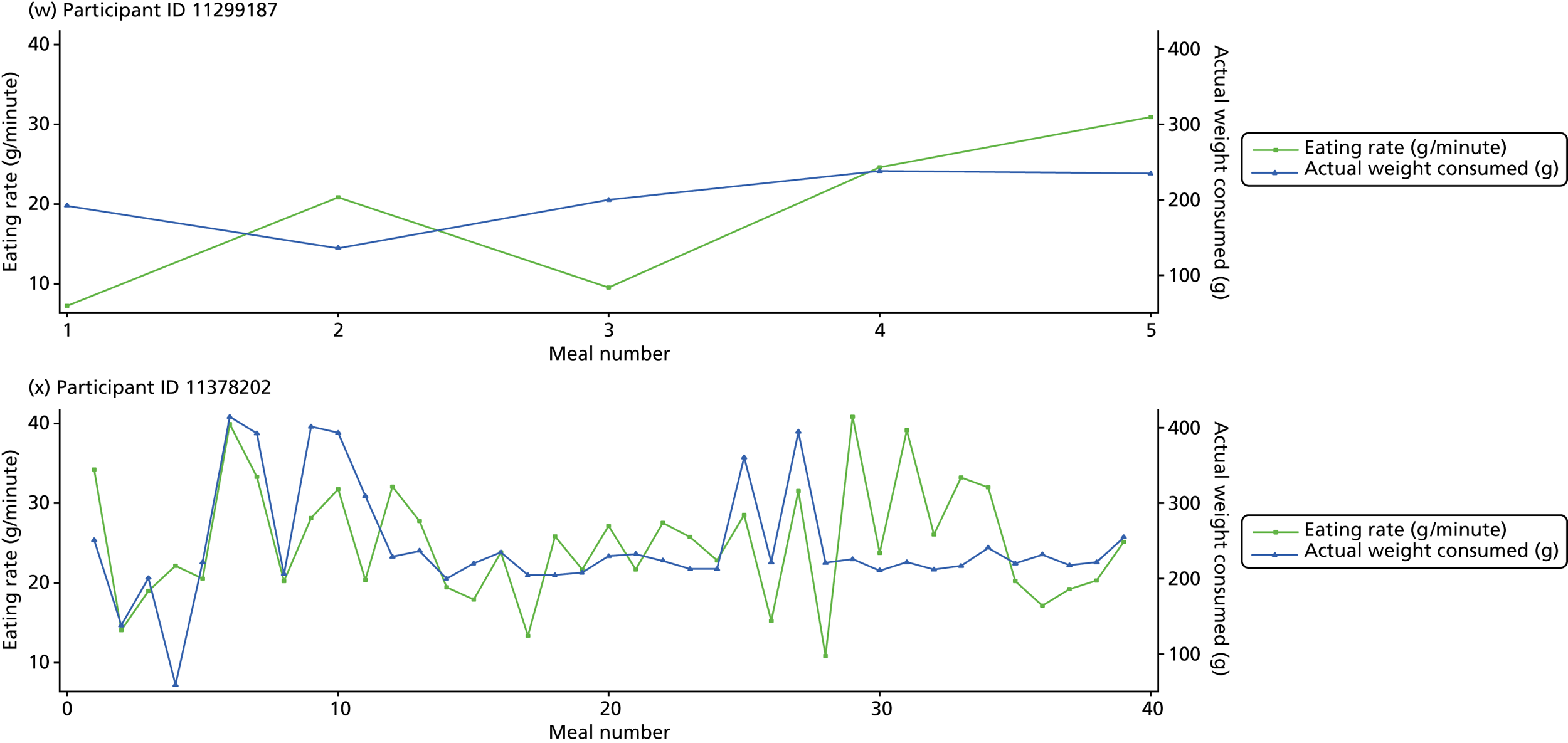
Participants 10246063, 10493122 and 10554133 did not complete any training or treatment meals (see Figure 4e , i and k ). Participant 10850157 completed six training meals, but no treatment meals. These four participants are, therefore, excluded from Tables 12 and 13 .
Note that the blue line (square symbols if printed in black and white) on the graphs represents eating rate and should be read against the left y-axis, and the red line (triangle symbols) represents weight of food consumed and should be read against the right y-axis. Up to seven meals (the first meals on each graph) were considered as training meals where duration and food weight was not prescribed, the purpose was to assess baseline food intake, rate of eating and satiety. This information alongside previously established normal eating patterns provided the patient with an individualised training line (see Chapter 2, Mandolean training). It also helped to familiarise the participant with using the device.
The data were not formally analysed to assess objective 3. However, it is apparent from the graphs that there was no obvious systematic reduction in eating speed during the study for these participants.
Health Technology Assessment programme progress evaluation of pilot study
With insufficient recruitment of families to trial, at a meeting with a Health Technology Assessment programme monitoring board in London on 27 March 2013, a recommendation was made to terminate the trial early and cease progression to the main study. All study activities ceased on 31 July 2013.
Adverse events
There were no adverse events regarded as serious, unexpected or suspected to be related to the study treatment.
Chapter 4 Nested qualitative study
Qualitative study aims
The aims of the qualitative study conducted during the ComMando feasibility trial were to (1) assess individuals’ reasons for not taking part in the trial and (2) explore the views and experiences of families who had been randomised to the intervention arm (i.e. Mandolean plus standard care). In order to address these aims, in-depth interviews were held with individuals who had declined the invitation to take part in the pilot trial and with families who had been recruited to the trial and allocated to the intervention arm. The data gathered from ‘decliners’ provided insight into how the recruitment process, materials and presentation of the trial to potential participants could be improved to ensure adequate recruitment to the main trial. The accounts of those receiving treatment highlighted the extent to which Mandolean and standard care were acceptable to families and indicate any difficulties families were experiencing with the treatments or trial. This insight provides the researchers with an opportunity to consider whether or not treatment delivery or content needed to be changed prior to the main trial.
This section has been structured so that the methods of recruitment, data collection and analysis used with decliners, and families participating in the trial are detailed first. Findings from analysis of the material gathered are then presented, and followed by a discussion on the implications in terms of future trials and use of the Mandolean.
Qualitative study methods
Recruitment and sampling of participants in qualitative study
Recruitment of decliners
There were three groups of decliners: one group who declined when being approached by their GP, one group who declined between seeing the GP and having the baseline appointment, and one group who declined between baseline and randomisation (the first nurse appointment) and had used the Mandolean for 1 week. All families that declined were asked to complete a decliner form and to indicate if they would be willing to take part in an interview to discuss their reasons for declining. Details of parents who indicated that they were willing to take part in an interview were given to the qualitative researcher, who then contacted the parents by telephone to organise a suitable day and time to be interviewed. Willingness to take part in the interview was taken as consent. Decliner data were collected throughout the duration of the qualitative study.
Recruitment of families in the intervention arm
Details of the qualitative study were given at the baseline appointment (week 0) to all families recruited to the pilot study. At the start of the first nurse appointment (week 1), families were randomised to either standard care or the intervention arm. Details of families randomised to the intervention, and who had agreed to be approached about the qualitative study, were sent to the qualitative team. The intention was then to purposefully sample families who varied in relation to age and gender of the study child, and whether or not the study parent was obese. Within this sampling approach, we aimed for maximum variation in relation to social class and ethnicity.
Qualitative study data collection
Interviews with decliners
The aim of the decliner interviews was to gain understanding of these parents’ views of the trial information, their reasons for not wanting to take part in the trial, and suggestions for improvement of the materials and enhancing the recruitment of parents and children. All decliner interviews were conducted by telephone. A topic guide was used to ensure consistency across the interviews. All interviews were audio recorded and fully transcribed.
Interviews with families
The aim was to interview each family recruited to the qualitative study on three occasions: within 2 weeks of being allocated to the intervention (i.e. shortly after the first nurse appointment), then again at 4 weeks (when the Mandolean training line should have been established) and, finally, at 10 weeks (when the study parent and child should have been eating to an established training line for a month). This longitudinal design was adopted because we wanted to assess how families used and incorporated the Mandolean into their daily lives over this time period, and because there would be issues specific to certain weeks which we wanted to assess (e.g. families’ experiences of adapting to changes in the training line).
Topic guides were used to ensure consistency across the interviews. There was a topic guide for each time point, as the issues to explore during each interview varied ( Table 14 ). The guides were developed by the two qualitative researchers on the research team and were based on relevant literature and the aims of the main trial and the qualitative study.
| Time point 1 (2–3 weeks) | Time point 2 (4–6 weeks) | Time point 3 (weeks 7–10) |
|---|---|---|
| Reasons for taking part (including information received about the study) | Nurse support | Nurse support |
| Previous and other current weight management treatments | Mandolean experiences | Mandolean experiences |
| Dietary habits, food shopping and choices, mealtimes, school lunches | Changes to dietary and eating habits, mealtimes, school lunches, food choices, speed of eating, portion sizes | Changes to dietary and eating habits, mealtimes, school lunches, food choices, speed of eating, portion sizes |
| Mandolean: initial experiences and views | Barriers to taking part and improving the experience | |
| Initial changes to dietary and eating habits, food choices |
At time point 1, families were asked to describe previous and current eating habits, portion sizes and the structure of family mealtimes, and to detail their views and experiences of being in the trial and using the Mandolean. They were also asked to discuss the information and treatment received to date for them and their child’s weight management. At time points 2 and 3, families were asked to describe their experiences and views of using the Mandolean, eating to different training lines and nurse appointments, and to detail any changes made to food shopping, food choices, dietary habits, speed of eating and portion sizes. At time point 3, families were also asked about any barriers parents and children may have to taking part in the trial and using the Mandolean, and suggestions to how their experiences of taking part in the trial may be improved.
Parents, and children aged 8 years and over, were asked if they would like to be interviewed together or separately. All children aged 6–7 years were interviewed with their parents. To ensure that children interviewed with their parents felt able to contribute, each guide included questions directly targeted at the study children, and appropriate for their age group, using prompts when necessary, for example, ‘Tell me what happens when you visit the nurse’, ‘Does the nurse talk to you about healthy foods?’ (prompt) and ‘Tell me what it’s like to use the scales at mealtimes’ (scales referred to the Mandolean).
Prior to commencement of the interview, the qualitative researcher again explained the purpose of the interview and asked families (parent and study child) to sign a consent form. All parents and children were reassured that they could withdraw at any time during the interview, without giving a reason, and that their withdrawal would not impact on their participation in the trial. All the interviews were audio recorded and fully transcribed.
Analysis of the interview data
All interview data (decliner and participating families) were transcribed and checked for accuracy. When checking for accuracy, all names and locations were deleted and replaced with appropriate markers (e.g. nurse, child, area) to ensure anonymity. The qualitative researcher then read each transcript to gain familiarity with the data. All data were then coded and organised using computer qualitative software, NVivo 8 (QSR International Pty Ltd, Victoria, Australia). Data were initially coded to reflect sections of the data relating to key topics for exploration, such as views of the Mandolean and eating to a training line. Once all the data were coded, summaries were produced and the data further analysed using thematic content analysis. This method of data analysis allowed patterns and themes within the data to be identified across the three time points. Analysing data longitudinally (i.e. across the three time points) allowed for a rich account of families’ experiences of a weight management intervention (i.e. using a Mandolean and its integration into family mealtimes) and specific issues relating to certain weeks (e.g. adapting to changes to training lines).
To ensure confidentiality and anonymity, all decliner and participating families were given identification codes (IDs) on entry into the trial, and these codes were used on all transcriptions to ensure no one family, nurse, GP practice, and location, could be identified by name. In this chapter, quotes are used to illustrate key findings and tagged according to participants’ ID code and age of the study child.
Qualitative study results
Interviews with decliners
Eight parents who declined to take part in the pilot or main trial agreed to take part in a telephone interview to discuss their views further. However, the researcher was unable to contact three of these parents and, thus, in the end, only five parents were interviewed. All the parents interviewed were mothers of children, four girls and one boy, aged 9–10 years, who fulfilled the entry criteria (BMI ≥ 95th percentile). Interviews lasted approximately 25 minutes and took place during the months of August and September 2012. All parents who took part in the interviews had declined between baseline and randomisation (the first nurse appointment) and had used the Mandolean for 1 week.
None of the parents interviewed had tried any other form of weight management treatment for their child. During the interviews, they talked about their views of the trial information and materials, their reasons for initially wanting to take part in the trial and then their reasons for declining.
Trial information and presentation of materials
All parents interviewed were happy with the information provided about the trial and understood what would be involved for them and their children if they took part. On the whole, parents felt that the information was clear and easy to understand, and they had the opportunity to ask questions if necessary.
Reasons for initially wanting to take part in the main trial
-
Parents were encouraged by their children’s initial enthusiasm to use a ‘plate’ (referring to the Mandolean) that talked to them.
-
Parents were concerned about their children’s weight, dietary habits and food choices, particularly snack foods (e.g. crisps and fizzy drinks).
-
Parents said their children were teased about their weight by older siblings and peers at school.
-
Parents said their children liked the idea of doing something together with their parent to help with weight management and were happy to take part.
-
There was a general view that few programmes target weight management in younger children and this was an opportunity to take part in such a programme.
Reasons why families then declined to take part
-
Timing of the trial had coincided with busy life schedules, making it difficult for parents and children to give their full commitment.
-
The constant technical problems with the Mandoleans deterred parents and children from wanting to continue taking part. Typically, the Mandoleans repeatedly turned on and off during their use. This became frustrating for parents and their children.
-
After using the Mandolean for a week, the practicalities of using the Mandolean at home for the duration of the trial became daunting for parents. For example, incorporating the Mandolean into family mealtimes required setting up of the device, carefully serving food onto plate positioned on scales and ensuring availability of plug sockets within reach of the lead (even though the Mandoleans had batteries, the batteries did not always work, and the leads were sometimes too short to reach the wall sockets). In addition, parents sometimes forgot to use the Mandolean, and having to replate food in this case often meant that cooked food became cold.
-
One parent had misunderstood the purpose of the main trial. She had thought that taking part meant receiving a ‘tailor-made diet’ for her child, and was surprised to receive a ‘plate thing’. On this basis, this parent felt taking part was not going to be of benefit to her child in terms of making healthy food choices.
Despite the initial enthusiasm of children to take part and use the Mandoleans, parents talked about the reasons their children did not want to take part. These were:
-
Children were teased by siblings when using the Mandolean.
-
One child preferred to diet with her parent instead of using the Mandolean.
-
One child would take the plate off the Mandolean and said they did not want to use it, they felt embarrassed.
-
One child thought there was some group work involved and this worried them, and did not want to take part.
Improving the experience
Parents suggested that involving a sibling may help to reduce sibling teasing, and that more nutritional and dietary advice specific to their child should be given.
The intervention families interviewed
Although the intention was to purposefully sample families, as recruitment to the trial was very slow, in the end, details of the first 13 families eligible to take part in the qualitative study were sent to the qualitative researcher. The qualitative researcher then contacted these 13 families by telephone to provide further details about the qualitative study and to ask if they were still willing to be interviewed. All were still happy to be interviewed.
All 13 families were interviewed at time point 1 ( Tables 15 and 16). At time point 2, two parents withdrew from the main trial and the qualitative study, one parent withdrew from the qualitative study only, and one parent was unable to be contacted. Thus, nine parents were interviewed at time point 2. At time point 3, three families withdrew from the main trial and the qualitative study. Thus, six families were interviewed at time point 3.
| Time point 1 (week 2–3) (n = 13) | Time point 2 (weeks 4–6) (n = 9) | Time point 3 (weeks 7–10) (n = 6) | |
|---|---|---|---|
| Completed | 6 girls, 7 boys | 4 girls, 5 boys | 4 girls, 2 boys |
| Withdrew | – | 3 | 3 |
| Unable to contact | – | 1 | – |
| ID | Parent | Age and sex of child | Time point 1 (weeks 2–3) (n = 13) |
Time point 2 (weeks 4–6) (n = 9) |
Time point 3 (weeks 7–10) (n = 6) |
|---|---|---|---|---|---|
| 10192 | Mother | Girl aged 9 years | ✓ | ✓ | ✓ |
| 10246 | Mother | Boy aged 11 years | ✓ | ✓ | W MT QS |
| 10365 | Mother | Boy aged 12 years | ✓ | W MT QS | – |
| 10493 | Mother | Girl aged 7 years | ✓ | W MT QS | – |
| 10558 | Mother | Girl aged 7 years | – | W QS | – |
| 11036 | Mother | Girl aged 10 years | ✓ | ✓ | ✓ |
| 10781 | Mother | Girl aged 8 years | ✓ | ✓ | ✓ |
| 10930 | Mother | Boy aged 6 years | ✓ | Unable to contact parent | – |
| 11268 | Mother | Boy aged 6 years | ✓ | ✓ | ✓ |
| 11378 | Mother | Girl aged 9 years | ✓ | ✓ | ✓ |
| 10878 | Mother | Boy aged 7 years | ✓ | ✓ | ✓ |
| 10752 | Father | Boy aged 10 years | ✓ | ✓ | W MT QS |
| 10747 | Mother | Boy aged 9 years | ✓ | ✓ | W MT QS |
All 13 parents interviewed at time point 1 (12 mothers and one father) were white European/British. Age at randomisation ranged from 26.7 to 60.0 years (mean 41.4 years, SD 8.24 years). Four parents were divorced or separated, eight parents were married or living with partner and one was a single parent. Twelve parents had previously tried other treatments and diet plans in order to try and lose weight. The remaining parent had previously received treatment at a weight management clinic, as she had been very underweight prior to, during and after the birth of her child. One parent reported experiencing depressive symptoms.
Of the 13 parents interviewed, 12 parents had experienced, or were currently experiencing, problems with weight management. Parents had tried various slimming diets available; two parents had had gastric bands fitted and one parent was currently taking a prescribed diet pill and following a strict diet.
All 13 children (six girls and seven boys) were white European/British, and at randomisation were aged 6.2–11.6 years (mean 8.8 years, SD 1.71 years). Twelve children attended primary school and one child was in first year of senior school.
Eleven parents and their children were interviewed together and one parent and child (aged 11 years) were interviewed separately, with the parent available in the next room. While various attempts were made to direct questions to children, parents often interrupted and on occasion answered on their child’s behalf.
At each time point, interviews were conducted within the planned period, i.e. weeks 2–3, 4–6 and 7–10. All interviews at time points 1 and 3 were conducted face to face in parents’ own homes, with the exception of one parent at time point 3, who was interviewed by telephone. At time point 2, five interviews were conducted by telephone. All of these interviews had been arranged and then cancelled for various reasons. Thus, in order to keep to the timetable, interviews were rescheduled to be conducted by telephone with the parent’s agreement. The other time point 2 interviews were held on a face-to-face basis. When conducting the interview by telephone, in cases where both the parent and child were being interviewed, the participant’s telephone was placed on speaker mode so that both individuals could participant in the interview.
All interviews lasted between 35 and 55 minutes and took place between August 2012 and July 2013.
Reasons for withdrawal from the main trial and the qualitative study included personal reasons undisclosed, too busy and problems with the Mandolean; one family felt uncomfortable with the interview because of questions regarding their child’s weight management, and two children (ages 9 and 10 years) were getting ‘fed up’ of using the Mandolean and did not want to continue. The parents of these two children (who withdrew at time point 3) were very positive about the trial and had made some changes to food shopping, portion sizes, speed of eating (i.e. they said their children were feeling full and eating less at mealtimes), and had lost a little bit of weight. They also said that using the Mandolean had helped to make these changes and hoped they would maintain them.
Results of qualitative interviews with families randomised to intervention arm
This section starts with families’ reasons for taking part in the trial. It then details key findings across the three time points (time points 1, 2 and 3), relating to:
-
views of the Mandolean
-
portion sizes and weighing food
-
eating to the training line
-
voice commands
-
measuring fullness
-
nurse appointments
-
changes to dietary and eating habits
-
barriers to taking part, and improving the experience of taking part in the trial.
Reasons for taking part in the trial
All parents and children interviewed were happy with the information received regarding the trial and felt they had received enough information to make an informed decision about whether or not to take part in the trial. The reasons for parents taking part in the trial related to three main areas: weight management, views of the Mandolean and education on eating habits. Parents hoped that taking part would have long-term health and dietary benefits for their children. Parents also said that taking part would contribute to helping other parents and children in a similar situation.
However, one parent said she had initially been a little upset at receiving the invite letter about the trial and so her reason for taking part was very different: to prove her child was not fat:
So I think a little bit of it was to prove them [researchers] wrong as well. So really just curiosity and I wanted to prove my point that he’s not the word I’m not allowed to say. (Child does not like the word ‘fat’.)
Parent 10930, child aged 6 years
Weight management
The reasons parents gave for wanting to take part in the trial included being concerned about their children’s weight, longer-term health and self-esteem. They viewed the trial as a chance to help their child’s weight management, having tried other treatments in the past which had been unsuccessful:
It’s like when I rang up and I spoke to the first person I said look, I’ve seen the advert I’m really interested in doing this, my daughter is overweight and we’ve tried everything, I said I’ve tried everything, this is my last type of hope. I don’t know what else to do.
Parent 10781, child aged 8 years
Children talked about being teased by siblings and peers about their weight, and wanting to take part to help ‘lose a few pounds’ and change their eating habits and become healthier. Children were also concerned about their future health:
Do you worry about your weight at all?
A bit.
What do you worry about?
That I’m gonna die like, so I want to live like as long as someone else.
Views of the Mandolean
Children appeared interested in the Mandolean, and initially were keen to use it. They were interested in the technology, and having a ‘machine’ (Mandolean) that talked to you seemed like ‘fun’. For some children, the colour of the Mandolean was quite important and they seemed pleased when given a colour they liked. However, one child (ID 11036, age 10 years) felt ‘singled out’ as the only ‘child’ in the family having to use the Mandolean, and described it as being ‘unfair’ when siblings were eating ‘normally’.
Educating children on healthy eating and dietary habits
Having external help was perceived by parents to be beneficial; they felt that children were more likely to accept advice from someone in ‘authority’ (trial nurses), and that trial nurses would support parents in any dietary changes they were going to implement. Importantly, parents were happy to take part in a trial that focused on the re-education of children, as opposed to over emphasis on ‘losing weight’; in particular, they didn’t want their children to become ‘food obsessed’. Parents hoped that by taking part their children would learn about making healthy food choices and would develop healthy dietary habits for the longer term.
We wanted to take part, well it was really because we’ve always had a problem with [child]. He was a very fast eater and he would pack food away pretty quick, and he would put it away. [Child: In less than 5 minutes.]. Yeah he could put it away pretty quick but he’s slowed down now. Yeah so the signals hadn’t gone right, so he was sort of thinking he was still hungry and then started to overeat, and if we told him no, that’s enough, sometimes he would throw a strop about it.
Parent 10752, child aged 10 years
Some parents talked about mealtimes being a family activity and quality time together, and some felt ‘guilty’ in that they may unnecessarily provide too much food at mealtimes. Thus, parents too were hoping to learn about good dietary habits that were age appropriate to their children, and to receive advice on alternative and healthier snacks and breakfasts, and ideas for school lunch boxes:
I’m really conscious of like in-between meals, but I think the plate [referring to the Mandolean] is going to show me where I go wrong, because I do tend to treat them all the same. [Child] is eight, her brother’s 10, and her sister’s nearly 12, so I just pile it on, pack it in yeah, feed them up, so I think that’s where I’m going to, over time that’s going to be a learning curve for me definitely.
Parent 10558, child aged 8 years
For some parents, who had struggled with their own weight, it was hard to educate and guide their children in making healthy food choices. They hoped that receiving external support and guidance would prevent their children from going through a similar experience:
. . . and I can see and I know what it was like for me as a child and I can see myself in him and thinking oh how I struggled, I don’t want him to struggle the same. So any help that I can get.
Parent 10365, child aged 11 years
Children also said that taking part meant having support from a nurse regarding healthy eating, and said it was good that their parents were taking part and supporting them too. In addition to the challenge of healthy eating, losing weight was mentioned by a few children.
Well I hope I lose quite a few – weight by using it . . ., and not put that much on, and the only reason I wanted to do this was because it’s kind of challenging like to be healthy, and stuff like that.
Child 10752, aged 10 years
Views of the Mandolean
Across the three time points, families talked about their views and experiences of using the Mandolean at home. These centred on the themes of incorporating the Mandolean into family mealtimes and frequency of use, challenges of using the Mandolean, and children telling friends. In addition, parents said that the Mandolean was incompatible with other diets and weight loss treatments they were using, for example ‘diet pills’, a gastric band, and following a Slimming World diet. Unfortunately, there were a number of technical problems with the Mandolean throughout the trial, and families had up to three replacements. This disrupted the frequency and consistency of their use. Typical problems encountered included Mandoleans turning on and off during their use at home, voice commands often stuttering and seeming to malfunction, nurses being unable to download data that initially delayed the setting of training lines and, on occasion, the Mandoleans failing to recognise plates used when weighing food.
Incorporating the Mandolean into family mealtimes and frequency of use
The families interviewed reported that they tried to use the Mandoleans every day for one meal, usually the evening meal, with some families opting for the breakfast meal, and a few families using it for both breakfast and evening meals. However, frequency of use varied, as in reality using the Mandoleans every day was not practical because of family, school and social activities, eating out, holidays, and type of meal, (e.g. light sandwich or finger-type food). On these occasions the Mandolean was not used:
I think it’s hard, certain meals you have are harder to try and use it, or if we go out.
And what sort of meals do you think it’s harder?
Obviously sandwiches and things. I try and use it every day, like I said to [researcher] and [nurse], there are occasions that we will not be able to use them, like when she goes to stay away at someone else’s house on a sleep over, I can’t ask that person to do it.
Parent 10781, child aged 8 years, time point 1
For one parent, using the Mandolean was infrequent and was dependent on how much time she had, and how she and her child were feeling in the morning, although she did appreciate that it did help to encourage her child to eat breakfast at the table:
Well I suppose it depends on what mood he’s in and what mood I’m in and how much time we’ve got in the morning, it does make me stop him and sit him down and make him eat the breakfast, rather than in the car with something as we’re driving off, so yeah it does make you think.
Parent 10930, child aged 6, time point 1
The majority of parents were surprised at how well their children had adapted to using the Mandolean and, for most families, as the trial progressed, using the Mandolean became a routine at the chosen mealtimes and became less hard work than at the start of the trial:
I think it’s really good. I think it’s a positive thing. It’s not a huge disruption or anything. It took a bit of getting used to, certainly just remembering to do it and then remembering to serve the food onto the plate. You get the prompts – how full are you, that kind of thing – so it’s just remembering to do it. But the first, I’d say, couple of weeks I thought, ‘Oh God, we’ve got to do that again,’ but now it’s absolutely fine. It’s just second nature, really.
Parent 11268, child aged 6 years, time point 3
As this parent’s child says:
Well, just doing it. I like just doing it, I like eating from the plate.
Child 11268, aged 6 years, time point 3
Initially children interviewed enjoyed using the Mandoleans; they liked using the technology, pressing buttons and following the training line. One child said:
I like eating off it because I like the electronic stuff.
Child 10192, aged 9 years
Some children said that using the Mandolean made them sit upright, as opposed to slouching, because the Mandolean made the plate higher than normal. This might have helped with digestion.
A few of the older children (i.e. ages 9–11 years) interviewed were able to use the Mandoleans themselves for breakfast meals, for example setting up the Mandoleans and weighing food to the required proportions. Some children would set up the Mandoleans ready for the evening meal, although parents prepared and plated food to the required weight and proportions.
Challenges of using the Mandolean
Despite some of the positive views about using the Mandolean, families also experienced a number of teething problems and challenges incorporating the Mandolean into family mealtimes. For example, parents described the Mandolean as annoying and time-consuming in terms of setting up, preparation, and plating food; it was something else to think about and they often had to replate food because they had forgotten to use it. Two parents said that the Mandoleans were cumbersome and took up what little space they had in the kitchen and the dining table. Reliance on an electric power source could also be problematic if the power sockets were not in a nearby location or the cable was not long enough to reach them. In addition, even though the Mandolean is fitted with a rechargeable battery, the battery sometimes did not work. For some parents the challenges of using the Mandolean spanned the three time points, while, for others, using the Mandolean became less of a challenge and they had accepted it as part of the family mealtime routine.
Typically parents said the buttons on the screen were too small, that it was difficult to see the instructions on the screen, and that they often had to rely on their children for help. In addition, some parents commented that the Mandolean dominated family mealtimes, which was frustrating, as this was the family’s social time together.
By time points 2 and 3, some children were fed up of using the Mandolean, and this made it ‘harder work’ for parents to implement using the Mandolean. A few parents also tired of using the Mandolean; setting up, dishing food onto a plate, and weighing food. Some children were also less likely to help set up the Mandoleans as they had been doing at the start of the trial:
Well just like before they would set it up and they would set it up and make sure it’s all done when we’re serving up dinner and now we’re kind of having to remind her or we’re having to do it ourselves . . . I just think that to start with it was really exciting and now it’s not really so exciting. Just more novelty, it’s not that she doesn’t want to do it or anything.
Parent 11378, child aged 9 years, time point 3
I think enthusiasm’s gone off. In the beginning it was a lot easier. Even though it’s a quick thing to do, I am finding it difficult to fit it in. It’s only just putting it on the scales but it’s so . . . well the enthusiasm, it’s hard to keep going.
Parent 11036, child aged 10 years, time point 3
One child described how he thought using the Mandolean for 1 year might get ‘boring’ and interfere with his social life, as he would have to come home to use it. This child suggested a shorter period might be better:
It’s going to be really annoying, coz I have to get up, set it up in my home for a year, it’d be really limiting.
Child 10365, aged 12 years
Telling friends
One child (aged 6 years) was excited about using the Mandolean and told his friends at school and three children said they had only told their ‘best friend’. However, the majority of children preferred to keep the Mandolean a secret and not tell their friends; they felt a little embarrassed and were worried the whole school would find out. Some just forgot about it when they left the house in the morning to go to school.
Portion sizes and weighing food
There was general agreement among all the parents interviewed that using the Mandoleans had been helpful in terms of determining portion sizes and what percentage of each food type should be included in a meal, appropriate to their child’s age, and children were adapting to smaller portions:
I like the scales when I’m dishing out her food because actually you’ve got a percentage so when you’re putting the vegetables on you know what percentage of vegetables has gone on to the plate. It obviously goes up to a hundred and you can . . . like so I put . . . if you think, I don’t know, like a third, a third should be vegetables, you know, so thirty-three per cent you should be able to see at least as vegetables. Now when I’m dishing up and I can see the per cent, I quite like that now that I, I almost know that I’m not overloading her plate with, and you know, it, it’s sort of controlling me really what I put down so.
Parent 11036, child aged 10 years, time point 3
I don’t really care what the portion size is any more, I’ve got used to it.
Child 11036, aged 10 years, time point 3
Interestingly, one parent talked about the different ages of her children and portion sizes being more of a problem than the actual appropriate portion size. For example, giving younger siblings more food than the study child, that may appear a little unfair and difficult for children to understand. In this instance, the study child felt that it was because she was ‘fat’:
Yeah, and [sibling] needs it and that’s quite tricky because it kind of seems unfair that I’m almost like not letting her eat and letting him eat. I think it’s hard when it’s so close to home it’s almost like me saying to him ‘yes you can have seconds and to her, like no you can’t’, although obviously yeah long term it is in her best interests, I think it’s quite hard for a child to see and understand that and rationalise it really . . . Even if it’s something that she doesn’t necessarily want, I think then it becomes a kind of a, you’re letting him and you’re not letting me, so why is that? And then its like, ‘well do you think that I’m fat’, sometimes she’ll say that.
Parent 11378, child aged 9 years, time point 3
Weighing food and what is eaten
Across the three time points, most parents reported that the weighing of food and achieving the 100% target was a continual challenge. Parents described getting the required 100% target weight as tricky, and there were times when they had to put extra food on the plate, or remove food from the plate, and this became annoying and time-consuming:
I would only say that at the moment that is the frustrating thing, is getting the weight thing right because it does, you know it’s not a case of getting the scales [Mandolean] out and putting the plate on and doing that, you’ve then got to faff around with, okay, well I’m 20% short so what can I put on it.
Parent 10878, child aged 7 years, time point 3
Depending on the type of food being served, some parents found it difficult to gauge whether to set the Mandoleans to weigh a light or a heavier meal, (e.g. breakfast/lunch or evening meal respectively). Parents said the Mandolean did not account for the seasons and variety of foods eaten, for example salads in the summer can be very light:
We had like a few things where we found it, we had the weights redone last time we went to see the nurse because we were finding that we were both using it for the lunch rather than the dinner because the dinner when we put the food on the plate it was like enormous it was like three times what we would normally have eaten. [Nurse] changed it to make it less . . . it’s quite tricky to gauge whether the food we’re eating is going to be light or heavy, which sounds a bit silly but there are some things that I think oh it’s not going to be that heavy and then it is, and if you plate it all up with the lunch and then thing that’s like a tiny amount, you know you’re still going to be hungry after that, you then have to take it all off and then reset it for like a dinner, and then put like the plates back on and then add a bit more food to it, because then . . . that can become a bit of a faff.
Parent 11378, child aged 9 years, time point 2
I don’t think the weight thing works. I understand the portion control how, you know, we’re known as a nation for having too bigger portions and so I understand the theory of having smaller portions and stuff but I do think it needs to take into account what food you’re eating.
Parent 10878, child aged 7 years, time point 2
One child used the Mandolean for breakfast and detailed how he found it confusing and annoying in terms of getting foods to the 100% weight limit:
Not really I think it’s actually quite adding to it [adds more food than would normally have] Cos now it says on the portions I have to have one, two Weetabix and that was like my limit cos they were really big, they were like that big and then it said like fifty per cent so I’d have to have four and I obviously can’t do that, so I had to press down on it [uses hands to reach the 100% target weight] and then click on it, but it gets really annoying because it says you’re eating too fast slow down, cos you take off your hand and it thinks that you’ve eaten half of it.
Child 10365, aged 11 years, time point 1
There was also some doubt in parents’ minds about how the Mandolean was going to help children change eating habits and food choices. Although they receive a ‘healthy eat plate’ (The Eatwell Plate19), any type of food can be served as long as it reaches the required weight:
I don’t really understand how it works, but as to the effect of how it . . . well I could eat anything basically couldn’t I? I could have a plate of chocolate every day. But all they’re looking at is how long you’ve taken to eat it and how much it weighs. So for me that is a bit of a sort of like it’s quite hard to get my head round that one. I mean the other thing is they don’t . . . it doesn’t take into consideration if you have a pudding . . . that again is another thing, what do you eat around that meal is not even considered.
Parent 10878, child aged 7 years, time point 2
Eating to a training line
Between time points 1 and 3, parents and children seemed happier and more confident about following the training line and parents noticed significant changes to their own and their children’s pace of eating and quantity of food consumed:
The majority of the time he’s basically following the line that it wants him to without trying too hard. He seems to be eating at the right speed and eating the right amount.
Parent 11268, child aged 6 years, time point 3
Well because if you’re like, sometimes it tells you to eat slower, you kind of eat slower, but if you’re eating normally off the scales nobody like tells you to go faster or eat slower, so then you just like do it and you feel not full up and then keep eating more, but with the ComMando thing [Mandolean], it’s eating the amount in a reasonable time.
Child 10752, aged 10 years, time point 1
The majority of children were pleased that their parent was taking part and using the Mandolean with them. This helped them to grasp how to use the Mandolean and made the experience something to share, helping their parents and ensuring they too were following the training line:
. . . with having someone else doing it and watching [them] doing it as well, I get the hang of it a lot more.
Child 10752, aged 10 years, time point 1
Importantly, sharing the experience with someone else felt less isolating for children, as one child’s parent explains:
Like she’ll tell me if I’ve got my lines right on the machine, it’s like mummy you’re supposed to be doing it to that one, and things like that. I think it’s nice having someone else doing it with you from the point of view of other children, like if I’m doing it kind of gives it a bit more credibility, if it was just her doing it I think she would feel a bit more self-conscious about it.
Parent 11378, child aged 9 years, time point 2
A key problem reported by parents was the constant need to adjust the training line as a result of the weight setting being inappropriate for their children, that is, they had to add more food items to the plate, which made portion sizes larger than would normally serve, or remove food items from the plate in order to get the 100% target, and having to wait for the next nurse appointment for this to be done.
Voice commands
There were contradictory views regarding the voice commands among parents and children. Some parents reported them as helpful in terms of raising awareness about speed of eating:
It’s always been an issue I think since he started school with how quick he can eat. It frightens me sometimes that he’s cleared his plate in 5 minutes and we’re all not even halfway through and he’s finished his tea. And I’m thinking, ‘Where’s that food gone?’ And he’s finished and he wants to go and play – that type of thing. But now the voice is saying, ‘Can you eat a little bit slower?’ ‘Can you eat a little bit slower?’ And by about the third or fourth time, he is eating slower. It’s become recognisable in all his meals, not just the one where we’re using the scales [Mandolean]. He’s been eating breakfast, lunch and things, he will sit and eat it properly without thinking, ‘Ooh, I’ve got 5 minutes to eat this then I can go and do something else.
Parent 11268, child aged 6 years, time point 3
Other parents, however, described the voice commands as ‘irritating and annoying’, and often parents and children would turn them off, turn the volume down, or just ignore them and follow just the training line. Getting the balance between eating too quickly and eating too slowly was a continual frustration for all families:
Yeah it’s . . . but sort of it always tell us that we’re eating, you know, you’re eating too slow and it’s like well actually if I eat any slower I wouldn’t be eating and then at other times it tells us we’re eating too fast and it’s like well we don’t ever seem to get it right and there’s not one meal we’ve had where it doesn’t tell us we’re eating too fast or too slow and it’s quite funny really. (Laughter) And you don’t want to encourage indigestion . . . So I tend not to listen to it. You’ve got to eat at your own pace.
Parent 10878, child aged 7 years, time point 2
Children interviewed also said that the voice on the Mandolean was very annoying, describing it as too ‘robotic’, an ‘American voice’ and a male adult, and that it became a bit ‘boring’. Children would prefer a cartoon voice, a voice they could choose and download, use their own voice, or even have a child speaking. Whatever the voice, they would prefer it was more fun and child friendly. Parents and children tended to turn the sound off, turn the volume down, and one child said:
I put my fingers in my ears.
Child 10781, aged 8 years
For younger children the concept of eating too quickly or too slowly was confusing and hard to follow:
What’s difficult about using the Mandolean?
Quite a lot of the time it’s just . . . you slow down eating and . . . because I’m eating at my perfect rate, although I . . . sometimes I’m putting red sauce on and it says ‘Can you hurry up a bit’, and it’s like annoying, I’m putting red sauce on. It’s really confusing really.
Mum gives another example of times when the Mandolean is confusing for child:
For example, one evening we cut a pizza in big triangles, and he lifted the whole thing up and then it got a bit confused, and it was saying ‘You’re eating too fast’ and he didn’t think he was eating fast because he still had the bit of pizza in his hand. But maybe we need to cut it into smaller pieces.
Parent 11268, child aged 6 years, time point 3
Parents also said that, because the Mandolean did not account for the type of food being consumed, it did not take into account that foods varied in how much time they took to eat, for example soup and soft-textured meals require less chewing than other foods, such as meat. Further, when children were chewing foods, such as meat, the voice would say they were eating too slowly. Parents reported this as confusing for their child.
Measuring fullness
Parents said that, although the training line was easy to follow, the concept of how hungry you are and ‘feeling full’ was hard to gauge, and thought the wording for younger children might be difficult. They suggested that it may be better to ask how hungry children feel rather than how full they feel:
It says please rate your fullness and it’s nothing at all at the bottom, extremely weak, very weak, weak, moderate, strong, very strong, extremely strong or maximal, ‘please rate your fullness’. That’s quite a hard concept but you know, especially for the children and with that vocabulary. I mean I don’t know how much [child] actually understands I think she just gauged that that’s what you have to do, because we said to her you have to see how full you are, but I don’t know that she’s actually read all of it and understood it in great detail and I think well she’s nearly 10, I don’t know if you were doing it with like younger children you know. I think that would be really hard for a 6 year old to comprehend unless their parents are saying like you have to, the fuller you get the more you have to put the arrow up, unless parents are simplifying for the children. I just kind of do it quite randomly I have to be honest because I don’t know if I’m moderately full, I really don’t know, and what my version of moderately full would be, could be completely different to someone else.
Parent 11378, child aged 9 years time point 3
However, as the trial progressed, some of the older children said they were more aware of how full they were because they were now eating a little slower:
It’s just when I’m eating too fast, I’m getting full up, like before I was like, I was eating, I was full up and then I was eating more because I just wanted to finish it because it’s so nice . . . I used to have more and now I’m having less. I’m having the right amount for my age. I’m eating more at a maximum speed, and thinking . . . do I want to eat more?
Child 11036, aged 10 years, time point 3
Standard care: the nurse appointments
Key issues that parents raised when talking about their experiences of standard care related to themes of frequency of appointments, education on healthy dietary and eating habits, and receiving feedback on their children’s progress. As mentioned earlier, the technical problems with the Mandoleans caused some disruption to their use at home. Similarly, nurses were often unable to download data from the Mandoleans and this often took up a large proportion of the nurses time scheduled to give to families.
Appointments
Despite any problems with the Mandoleans, all parents and children were happy with the nurses and attending appointments, and the majority of children enjoyed going to see the nurse. They looked forward to telling the nurse about any changes they had made to food choices, and liked receiving stickers for doing well. However, some parents were not happy that the nurse appointments were going to be less often as the study progressed. There was also an issue with how the training line would be altered at the appropriate time, as the gap widened between appointments:
At the moment I think the next one is in 2 weeks and then it’s a month after that. So if they’re don’t get it [training line] right in the next 2 weeks, it’s a month before we get seen again so if the measurements and the times aren’t right, it’s a long time to have to go.
Parent 10878, child aged 7 years, time point 2
Education on dietary and eating habits
Parents found the nurse support invaluable and necessary to provide reassurance they were doing the right things at home, to give advice on alternative healthy food choices, snacking options and dietary habits and to answer any questions that arose during the trial. Importantly, parents felt that children listened to the nurse’s advice regarding diet and food choices, rather than their parents’ advice:
Well like I think overall the whole things been good, and being kind of positive for everyone, because some of the stuff it has made us, you know it’s made us think oh good we are doing the right thing or heading in the right direction and there are other things that we could improve on and having like the third party of the nurse saying yes, ‘[child], you’re doing really well, that’s really good, but how about you look at doing this’ and in terms of like breakfast, we used to kind of have probably about seven or eight cereals, depending on which ones the kids were going through their phase of all liking, and she’s sort of said porridge is really great and they’re all eating a lot more porridge now, and I said ‘right once the other cereals have gone, you know I’m only going to keep porridge in’ so that’s kind of the choice.
Parent 11378, child aged 9 years, time point 3
Some children said that the information packs provided by the nurse were helpful and were keen to show the qualitative researcher the healthy eat plate, booklets on healthy eating and their sticker chart. One parent felt that getting children involved in this way was essential and, again, made it easier for them, as parents, to implement the changes:
So she gives us a lot of support and encouragement, like that’s it [child], come on [child] can you do this, can you write down what you’re snacking on, and keep a list of what, so we can see what you’re doing, just to get her, to get [child] involved a bit more not just listening to us all the time.
Parent 11378, child aged 9 years, time point 2
Some parents would have liked more advice regarding diet and healthy options, other than the healthy eating plate, with some suggestions for meal plans, and some would have liked to receive more direction regarding exercise:
I think there could be for both because part of the healthy diet and losing weight and stuff is exercise as well. So obviously this, the styles and the advice are given and the portion size and everything is all to do with food. But I do think there needs to be a balance of exercise as well. And although they’re trying to make [us do] more exercise they don’t actually point you in the right direction to get that because some people don’t know where to start.
Parent 10878, child aged 7 years, time point 3
Feedback on children’s progress
Parents said that having nurse appointments was important in providing immediate feedback to children on how well they were doing. They felt that positive feedback could greatly enhance their children’s self-esteem and motivation to continue using the Mandolean, and to follow the dietary advice suggested by the nurse:
And also [child] was kind of worried about her weight and like after the first 2 weeks she’d lost weight which again the nurse handled really well, she said ‘it’s not just about losing weight you know it’s obviously good if you have lost some’. But you see [child] saw that as a really positive thing for her because she was concerned that other children were thinking that she was fat. And that was quite nice for her self-esteem and things.
Parent 11378, child aged 9 years, time point 2
However, parents commented that feedback on their child’s progress was limited and, based on the accounts given by different parents, it seemed to vary between families. Often this was a result of the technical difficulties of downloading data, which often took up a large proportion of the scheduled time nurses gave to families, the widening gap between nurse appointments and cancelled appointments (by both families and nurses).
Changes to dietary and eating habits
Between time point 1 and time point 3, parents and children talked about the changes to school lunch boxes, food choices and food shopping, snacking behaviour, and how they were feeling about these changes. However, parents acknowledged that such changes were not going to happen overnight, and that any long-lasting change may take some time.
Some parents had found it difficult to introduce new foods to their child because their child was a ‘fussy eater’, and others specifically talked about the importance of involving their children. They talked about encouraging their children to take ownership of the changes they were trying to implement, for example choosing fruit for school lunch boxes, and using examples of good food choices from the information booklets provided by the trial nurse:
She’s read a lot of the information that she’s been given by the nurse and she’s like you’re better to eat this rather than that, and she’s taking ownership for lots of it, which is good.
Parent 11378, child aged 9 years, time point 2
School lunch boxes
During the school term, the majority of children took a lunch box to school prepared by their parent or had school lunches; none of the children came home for lunch. Parents said they struggled with choosing healthy foods, as well as maintaining variety suitable for lunch boxes (bearing in mind that food needed to stay ‘fresh’ for 4–5 hours, and often in warm conditions). However, major changes to the contents of children’s school lunch boxes were made, with sugary items, crisps, chocolate bars and fizzy drinks being replaced with water, fruit juices, and more fresh fruit. Parents encouraged their children to take ownership of choosing healthier foods, for example choosing the fruits they would like for their lunch box when they went shopping. This seemed to work, and most of the children were trying fruits previously disliked. One parent had stopped her child having school dinners and now gave her a lunch box so that she could monitor what was being eaten during the day. The child, unfortunately, was not happy with this change:
Well a few times she gave me a packed lunch full of fruit, which is like really boring because then all my friends had all these chocolates and everything.
Child 10246, aged 11 years, time point 1
Food choices
Some parents had made changes to evening meal options, for example they had banned takeaway meals during the week, though for the majority there were no significant changes to evening meals. Some families reduced the quantities of food bought, and one parent had changed shops, converting to a food retailer with less choice and temptation. Some families went food shopping together, and since taking part in the trial were buying more fruit and vegetables, and were more conscious of what was in foods, thus reading food labelling more closely, particularly the fat content and number of calories, and just generally trying to choose the ‘healthier options’.
We go as a family, sometimes we choose what we want to eat and we normally help get stuff, so if dad says like go and get something, say like cereals, we go and find like the lowest cereals, so we find the cheapest one and the lowest in calories.
Child 10752, aged 10 years, time point 1
Snacking behaviour
Children’s snacking behaviour was a major concern for most parents interviewed. In order to help change this behaviour, parents were buying fewer crisps and other snack-type foods and replaced them with fruits and raw vegetables, and healthier cereal-type snack bars. Some children said they had reduced snacking in between meals since taking part in the trial.
I used to come back and I used to, like, just go in the fridge and, like, eat some stuff, but now I don’t, I just come back and I go up in my room, but sometimes if I’m hungry I have, like, an apple . . . It’s like now I don’t, like, have a snack after dinner either.
Child 10192, aged 9 years, time point 3
However, one parent continued to have difficulties with curbing her child’s snacking behaviour. This was becoming increasingly concerning, even though this parent had for some time kept only fruit in the house to snack on. In these instances nurse support was viewed as essential.
She [child] says she’s snacking a bit more lately which we’ve noticed and we’ve tried to curb it now, because [nurse] talked to her and hopefully that will make her realise she can’t, because [nurse] asked her to write things down that she eats now. It’s when she comes in from school is the worst bit; she’s absolutely starving when she comes in from school. She only ever snacks on fruit that is it; I don’t have anything else in the house for her to snack on. But I mean we have to watch the amount of fruit she eats, because she’ll eat an apple and then 5 minutes later oh mummy I’m hungry again, no, no, I’m hungry.
Parent 10781, child aged 8 years, time point 2
Barriers to taking part, and improving the experience of taking part in the trial
Some parents mentioned that the trial’s time line (i.e. 2 years, including 1 year of using the Mandolean) may ‘scare’ some parents and children, ‘putting them off’ to making such a commitment. It was also mentioned that the target group, i.e. children who are ‘clinically obese’, was too narrow, as there may be lots of children who are borderline obese, and who would benefit from such a programme. However, targeting children was viewed as a positive thing. Parents commented that they felt making any change was easier to do as a child than as an adult.
Yeah and I think it’s much easier to do as a child when you’ve got the support, but as an adult, once you’re an adult it’s much harder because it’s your choice but then you’ve got to have a lot of will power.
Parent 11378, child aged 9 years
Parents also offered a number of suggestions regarding how to improve the trial’s presentation and the treatment given. These were:
-
Clarify who to ring if and when any problems arise with either implementing changes or with the Mandolean.
-
Change the voice on the Mandolean to one that is more child friendly.
-
Change the verbal instructions for measuring fullness to be more age appropriate.
-
Form a social network with other local parents and children taking part in the trial to act as an information-sharing, tips and ideas support network. This was viewed as something which could be particularly beneficial for single parents taking part.
-
Include a food diary for parents and children to record what they are eating during the whole day as this might help people get into the habit of eating healthily.
-
Include suggested meal plans.
-
Improve the technology of Mandoleans, i.e. make them more child friendly and appealing, as younger children are used to mobile telephones, iPods, computers, etc.
-
Provide more contact time with trial nurses, either through appointments, or telephone calls in between appointments.
Strengths and weaknesses of the qualitative study
Interviewing on three different occasions enabled exploration of changes over time and detailed assessment of issues that were relevant at specific time points during a family’s experiences of incorporating and using a Mandolean during their daily lives. It also helped the qualitative researcher build rapport with families, which may have led to them giving a more open and honest interview. Another strength of the study was that both parents and children were interviewed. This enabled triangulation of their accounts and a clear understanding of how childhood obesity was being managed within the family context. Furthermore, there has been very little research undertaken with children who are obese, particularly young children, to assess their views of weight interventions, so the study gave new and important insights into the perspectives of these individuals.
Interviews were conducted both by telephone and on a face-to-face basis. As all time point 1 interviews were held on a face-to-face basis and, thus, the participant ‘knew’ the researcher when taking part in later interviews, and well-planned telephone interviews can gather the same material as those conducted on a face-to-face basis,35 this should not have greatly affected what material was gathered.
The fact that all the interviewees were white British may limit the generalisability of some of the findings. However, despite not purposefully sampling participants because of limited trial recruitment, interviews were held with parents of children who varied in terms of both gender and age, and the fact that most of the parents interviewed struggled themselves with their own weight reflects the fact that children who are obese usually have obese parents. 36 With hindsight, it might have been preferable to have interviewed all the children taking part in the study on their own, and not with a parent. Although the qualitative researcher directed questions to children during the interviews, parents often dominated the interview. Thus, interviewing children on their own may have led to a greater understanding of their views and experiences.
Discussion of qualitative findings
It was evident that children often experience teasing from siblings and peers about their weight and that this may affect their self-esteem. Taking part in a trial in which there is less emphasis on weight loss and more on the education of healthy eating and dietary habits may, therefore, be more appealing to families. Trials which involve both parents and children might also be more appealing, as it was apparent that parents and children ‘sharing’ the experience could provide each other with moral support and information, and that children felt less singled out when others in the family were using the same intervention.
In terms of the clinical effectiveness of the intervention, the Mandolean appeared to inform appropriate portion size and the percentage of food type served, change speed of eating (even when not being used) and encourage parents and children to be more conscious about what they were eating, how much they were eating and, for some children, how full they were. It also changed eating habits such as sitting down at the table to eat, taking time specifically to have a meal, and sitting up straighter whilst eating.
In was clear that families in the standard-care arm of the trial greatly valued their progress being monitored and receiving feedback, as they wanted to check that what they were doing was correct and they enjoyed reporting ’successes’ such as weight loss. Parents also valued the advice and information provided by nurses, and support in terms of the nurses being a ‘voice of authority’, and perhaps being someone whose advice and praise of children carried more weight than that given themselves. Child-friendly booklets, sticker charts (receiving stickers as a reward for good progress) and easy-to-read materials for use at home appeared important to children, as they were personal to them, made them feel important and charted their progress and successes.
Chapter 5 Economic evaluation
Aim and design
The aim of the economic evaluation was to investigate the cost-effectiveness of Mandolean therapy compared with standard care in treating obese children aged between 5 and 11 years. The evaluation was designed as a cost–consequence analysis, comparing cost from the perspectives of (i) the health-care provider (the NHS) and (ii) the families of the children participating with BMI z-value; the CHUI9D;37 the EQ-5D-Y;38 and quality-adjusted life-years (for parents) using the European Quality of life 5-Dimensions 5-Levels (EQ-5D-5L). 39
In this report we describe progress up to the point that the trial was stopped. We report on the methods of data collection, the completeness of data gathered and lessons learned.
Data collection
The intervention
The number of clinic appointments associated with using the Mandolean, including details of who was seen and for how long, were recorded as part of the trial.
Outcomes
The CHU9D and the EQ-5D-Y were completed by the children at each of their 3-monthly clinic visits. At the same time, the parents were asked to complete the EQ-5D-5L.
Resource use
Data on NHS and personal resource use associated with the child’s weight were collected at the 3-monthly appointments face to face using a questionnaire designed specifically for this trial, based on one previously used in a similar patient group. 22 The questionnaire addressed the use of primary care, secondary care and personal expenditure as follows:
-
Primary care resources included all face-to-face, telephone and home visit consultations with a GP or a practice nurse. We also collected data on any use of NHS Direct, walk-in centres, out-of-hours services and prescribed medication.
-
Secondary care resources included accident and emergency (A&E) attendances, outpatient appointments and inpatient stays.
-
Cost to the families included travel, expenditure on other weight-related services (e.g. counselling, complementary and alternative therapies), private health care, expenditure on exercise/physical activity and associated travel, extra expenditure on food as advised in the clinics, over-the-counter remedies, loss of earnings, carer support.
Data completeness
Sixty-one children were randomised into the study: 25 to the intervention arm and 36 in the control group. The numbers followed up at each stage (3, 6, 9 and 12 months) are a combination of those who had reached that stage by the time the trial closed and those still engaged with the trial.
At 3 months, 41 families were still involved, though only 20 (49%) attended their follow-up appointment and provided any data. At 6 months there were 11 families involved, of which five (45%) attended their appointment, and at 9 months only one of the three remaining families (33%) attended. No child reached the 12-month follow-up.
The intervention
Information on the number of appointments attended by the children in the intervention group to train them in the use of the Mandolean and to monitor their progress was documented by the nurses involved; these data were complete.
Outcomes
We report here the response rates for the CHU9D, the ED-5D-Y and the parent-completed ED-5D-5L. Table 17 gives the number of families responding to each item of each instrument at each follow-up.
| Baseline | 3 months | 6 months | 9 months | |
|---|---|---|---|---|
| Number in the trial | 61 | 41 | 11 | 3 |
| Number attended appointment | 61 | 20 | 5 | 1 |
| CHU9 | 61 | 18 | 5 | 1 |
| EQ5D-Y | ||||
| 5 items | 59 | 18 | 4 | 1 |
| VAS | 57 | 18 | 4 | 1 |
| EQ-5D–5L | ||||
| 5 items | 56 | 13 | 3 | 1 |
| VAS | 53 | 15 | 4 | 1 |
At baseline all 61 children completed the CHU9D and provided data for all nine items. At the 3-month follow-up, 19 of the 20 children who attended their appointment provided some data, although data were complete in only 18 cases. This represents 44% of those still involved in the trial. Data were complete for all children who attended at both 6 and 9 months.
Fifty-nine (97%) children who were randomised completed all five items of the EQ-5D-Y at baseline, although two did not respond to the visual analogue scale (VAS). At 3, 6 and 9 months the response rates for those attending were 18 out of 20 (90%), four out of five (80%), and one out of one (100%) respectively. Fewer parents completed the EQ-5D-5L. At baseline, 56 (92%) answered the five items and 53 (88%) gave a VAS score; 13 out of 20 (65%) responded at 3 months; three out of five (60%) at 6 months; and one out of one (100%) at 9 months.
Resource use
At 3 months, 19 out of the remaining 41 participants involved in the trial completed resource-use questionnaires. At 6 and 9 months, data were provided by all families still involved, i.e. five and one respectively. The following analysis of missing data is based on the 19 questionnaires returned at the 3-month follow-up.
Full details of the completeness of each requested resource-use item are given in Table 18 . From an NHS perspective, details for a total of nine key questions covering hospital-based services, primary care services and medication use were requested. Sixteen respondents returned complete data sets that could be analysed. Of the 171 (19 × 9) possible answers, four (2%) were missing. Although the medication section was poorly completed, sufficient detail was supplied in all cases to enable a cost to be applied with some basic assumptions.
| Number of complete responses | |
|---|---|
| Number in trial | 41 |
| Number of questionnaires completed | 19 |
| NHS perspective | |
| GP contact | 19 |
| Practice nurse contact | 18 |
| NHS Direct telephone call | 19 |
| NHS walk-in centre visit | 19 |
| Out-of-hours service contact | 19 |
| A&E or minor injuries unit visit | 18 |
| Outpatient appointments | 17 |
| Inpatient stays | 19 |
| Prescribed medication | 19 |
| Patient and carer perspective | |
| Hospital travel | 16 |
| Over-the-counter medication | 19 |
| Private doctor | 19 |
| Counsellor or complementary therapist | 18 |
| Exercise or physical activity | 18 |
| Equipment | 18 |
| Food/diet | 18 |
| Time off work | 18 |
| Childcare | 18 |
| Treats | 18 |
| Other costs | 19 |
| GP travel | 19 |
| ComMando nurse travel | 14 |
From the perspective of the child and parent, 13 questions covering travel and other financial costs to parents and carers were asked. Only 10 participants provided complete data sets for analysis; data in one or more areas were missing in nine. Overall, 15 of the possible 247 (19 × 13) responses (6%) were missing. In an additional five participants, GP travel data that might have been required at later follow-ups were missing.
Observations and lessons learned
In general, resource-use and outcome data were fairly well completed by those families who attended their clinic appointments. However, given that research nurses were in attendance, supervising the completion of questionnaires, any missing data are disappointing.
The CHU9D and the two EQ-5D instruments are not onerous and there was very little item missingness.
Although the resource use reported by the patients and carers has not been validated by comparison with an alternative data source, this study suggests that NHS resource use collected in this way was reported reasonably comprehensively by patients or carers, while personal costs were reported less thoroughly. It seemed that questions about out-of-hours services were prone to misinterpretation by participants and future studies might need to consider how better to request this information. Medication questions were particularly poorly completed and, although costing could be carried out on the basis of information supplied, this would be a labour-intensive process if scaled up to a larger number of patients and consulting medical records would be preferable.
Chapter 6 Discussion
The ComMando main trial terminated early after an in-depth analysis of the findings from the pilot study. Disappointingly, none of the pilot trial objectives were met. Recruitment to this weight management intervention was suboptimal, as was device usage among those randomised to the intervention arm. Neither families randomised to the intervention arm nor those randomised to the standard-care arm attended the minimum clinic appointments deemed necessary to adequately evaluate the trial’s primary or secondary objectives. The remaining discussion will elaborate on aspects of the trial that proved particularly difficult.
Funding
Issues with establishing funding streams for excess treatment costs delayed the start of the pilot study significantly. The PCT seemed unwilling to acknowledge that it had insufficient funds to pay for the initial purchase of Mandoleans and the DH was unable to help until this lack of funds was formally acknowledged. Consequently, funds from another study were used, in the short term, to purchase the first Mandoleans to get the study started.
Recruitment
In retrospect, we should probably have opened more than three sites for the pilot phase to recruit families at a faster rate. We chose three sites closely affiliated to the university, but these practices were in areas of significant deprivation with a tendency to have practice lists with a higher number of hard-to-reach families. Once new practice sites were opened within more varied areas of affluence, recruitment picked up.
We felt that the decision of the Bristol PCT not to report data from the NCMP to families on their children’s weight status denied the study a major impetus for families to seek help with weight issues that may have boosted recruitment at a crucial time. It is our opinion that such surveillance is a wasted opportunity to engage families who may not be fully aware of the advantages of seeking help with childhood obesity.
Very few children on GP lists had a BMI recorded in last 2 years. GPs see very few children aged 5–11 years in regular clinics, so opportunities to engage families are limited. A practical way to improve GP data would be to feed back NCMP data to the child’s GP as well as to their parents.
There appears to be a genuine ambivalence within the population towards engaging with interventions designed to address childhood obesity. Of the estimated 1512 (15% of the total mail shots) potentially eligible patients in an unselected, direct mailing system using practice-based lists asking families if they would consider taking part in an obesity trial, only 98 (6.5%) replied. Targeted mailing has met with some success in the USA,40 but UK GP databases have too little information on children’s BMI to be able to target families with children known to be obese. Before embarking on further primary care-based interventions, it seems necessary to try and establish exactly how the population perceives childhood obesity and whether or not it is considered a real risk to health. Ambivalence towards the recognition,41–43 diagnosis and treatment of childhood obesity44–47 seems to be a real barrier to engagement. Some data suggest that referral from a doctor, such as a GP, may increase the perception that ‘something should be done’:48 this was not really borne out by our recruitment from GPs. Only 38% of those referred directly in to ComMando by their GP were randomised.
Staffing
Identifying staff with the right skills and attitudes to deliver an intervention is important. We initially mainly targeted practice-based, health-care assistants and/or nurses, identifying a single practitioner in most hubs. Although we supplied an excellent course for training, this decision to have a single member of staff from each practice was probably a mistake, but financial constraints dictated this approach. Having only one staff member in each practice able to deliver a complex intervention proved problematic, especially as some staff were off sick for significant periods of time during the pilot. We were able to ‘plug the gaps’, but only by using much higher-qualified staff from the Non-Medicines for Children Research Network based in Bristol Children’s Hospital. Nothing similar was available from the PCRN.
Our Swedish colleagues have pointed out that, in the successful, hospital-based intervention, the nurse had spent a considerable amount of time in Sweden learning Mandometer methods and technology before starting therapy. Some technical issues may have been ameliorated by a more prolonged training period but, pragmatically, this intensive training would not be financially viable across the NHS and we considered the training period provided in ComMando reasonable for a primary care intervention.
Technical issues
There were undoubted issues with Mandolean and its interface with general practice database systems. Although technical support was forthcoming from Stockholm, the technicians took a long time to identify a simple cable issue that was preventing the nurses downloading previous meal data that they needed in order to change training lines. Our colleagues in Stockholm perhaps had not entirely grasped that this was a major barrier to study progress despite frequent requests for further ‘hands-on’ input. They had not encountered similar problems in their clinics in the USA and Australia. We should have prioritised getting this system working earlier, but funding issues and staff recruitment and development were felt to be more important in the initial phases of the study.
Adherence
Data from the 22 patients randomised to Mandolean who used the device regularly indicate that only one-fifth of patients used the device for at least five main meals per week, which was a major pilot study objective. This disappointing percentage may in part reflect problems with the Mandolean device itself, but also probably reflects the fact that the device is not as user friendly for the younger age group examined in this study as for adolescents and adults studied in previous trials. Our qualitative work demonstrates that, although the basics of eating more slowly were easy enough for children to understand, the concept of fullness was difficult to envisage for these younger patients. Furthermore, using the device daily did not engage them as perhaps it might have done if the mechanics of the retraining had used a more child-friendly voice, had cartoon characters involved and perhaps had more to engage younger children such as visual feedback in a fun and playful way, such as stars when a meal had been eaten as directed. As interactive games are so familiar to this younger age group, the rather rudimentary feedback offered by the current Mandolean was probably too mundane to maintain interest over time.
Chapter 7 Conclusion
The fundamental issue that prevented this pilot study from progressing to a completed full trial was poor recruitment. Despite a nearly 20% prevalence of obesity in Bristol at year 6 of primary school49 we were unable to convince enough families to engage in a general practice-based trial which aimed to change children’s eating behaviour despite our previous work demonstrating that families found primary care-based obesity interventions less of a commitment and easier to access compared with hospital-based clinics. 22 There are many reasons why primary care interventions are unlikely to present a convincing solution to the continuing pandemic of childhood obesity. It is undoubtedly the case that parents42,50 and HCPs51 struggle to successfully identify obesity in these children. In addition, HCPs are unwilling to address the issue for fear of being seen as judgemental and derogatory. 52,53 Furthermore, a significant proportion of the population simply does not believe that childhood obesity is an important health issue. 41,50,54,55
Engagement is not the only hurdle to effective intervention. Weight management is a complex issue with a need to evolve healthier diets and activity patterns while addressing economic food cost constraints and the belief systems of not only the children but also their extended families. 56–58 Multicomponent, primary care, weight loss interventions have been tried in the UK,45 Australia44,46 and the USA,58 with a similar remarkable lack of efficacy. The largest, school-based, community programme in the USA also had resoundingly indifferent results. 59 Addressing an additional component of excessive weight gain such as the structure of eating behaviour may thus be a useful adjunct to interventions that have proved unsuccessful before. This certainly was the case in the more structured intervention we tested using Mandolean in the hospital setting. 15 However, we were unable to convince the population in general that such an intervention might prove to be the answer and, furthermore, many of the recruits randomised to Mandolean found the intervention too difficult to adhere to in terms of both commitment to clinic visits and having a more structured eating regimen.
If childhood obesity is characterised by a general public ambivalence to its health consequences and a reticence to radically change behaviours for the better, what is the strategy for progression? In certain circumstances, such as the identification of significant obesity-related comorbidities that can occur in adolescence,60,61 there would seem a cogent argument for continuing to develop effective, personalised interventions that can elicit significant weight loss. However, in these cases, families are clearly aware of the current impact of obesity and, thus, likely to engage. 62 For the general population of families with obesity issues who have not actively sought help, there seems little evidence that they are currently ready to engage in large enough numbers to make a significant public health impact. It appears that future public health strategies and research themes would be better placed in identifying how to radically alter the population’s perceptions of the consequences of childhood obesity. Furthermore, there is possibly a cogent argument for governmental interventions to alter eating behaviours. These might include:
-
taxation on energy-dense drinks and snacks,63 while possibly subsidising healthier options, such as vegetables and fruit, which can prove economically prohibitive to those at the bottom of income scales64
-
banning advertising that aims to increase children’s desire for low-nutrient, energy-dense food products65–67
-
discouraging sedentary behaviours such an excess of ‘screen time’68–70 and encouraging active participation in sport.
There is certainly an argument for looking at France as an exemplar of better practice as rates of childhood obesity have probably decreased or at least stagnated. The French National Nutrition and Health Programme was launched in 2001 and will continue to operate until at least 2015. Among its successes have been the banning of vending machines in schools since 2007 and the introduction of healthy, seated, school meals, now eaten by 60% of school pupils. Furthermore, improving the quality of available processed food has been possible through a voluntary scheme with major food manufacturers backed up by rigorous assessment by the Food Quality Observatory. Some recommendations have not been implemented, such as restricting snacks in schools, but the general wisdom is that this concerted effort has produced significant improvements in the general population’s eating habits. 71
Chapter 8 Implications for future research
Implications for future research include:
-
Consider how to maintain enthusiasm for using the Mandolean, for both parents and children.
-
The Mandolean (technology) needs to be updated to be more child friendly, e.g. by using language (i.e. voice commands and rating scales) appropriate to the children’s age, which may also help to maintain children’s enthusiasm.
-
Perhaps include a short training video, or training session with the trial nurse/researcher, on how to use the Mandolean, which may help overcome some of the challenges initially faced by parents.
-
Providing nutritional advice at the start of the programme and prior to randomisation was viewed as an essential part of the programme (standard-care arm) and may be something to consider in any future trial.
-
Increase contact time with trial nurses, perhaps by means of appointments interspersed with telephone contact, and reduce this gradually over the duration of the trial. This may also help to retain families and maintain their enthusiasm.
-
Widen the target group to include those ‘borderline’ clinically obese.
-
Studies should be conducted on how best to integrate general practice into NCMP data collection and feedback in order that issues around obesity/being overweight can be addressed with less risk of unnecessary stigmatisation. This is probably best done by HCPs already known to families, but is currently impossible in general practice as no useful information by individual family is provided to primary care.
Future outputs
The following topics have been identified for future publications:
-
barriers to treating childhood obesity in primary care and future solutions
-
qualitative findings: patients’ experience and perception of the Mandolean intervention
-
children’s beliefs about food and disparities between the parent and child.
Acknowledgements
We would like to offer huge thanks to the patients and families for taking part in this trial as well as the practice nurses and research nurses for delivering the intervention: Ali Aitchison, Jennie Bradbeer, Rosemary Carpenter, Susan Chesworth, Susan George, Chris Lee, Tracy Mehew, Bronwyn O’Haire, Heather Skudder, Judith Smith, Elizabeth Thomas, Lorraine Thompson and Claire Wilson. The authors would like to acknowledge the help and support of the general practice managers and practice administration staff for hosting the study, recruiting participants and for carrying out practice mail-outs. We would also like to thank the PCRN and the WCLRN for nurse support from paediatric research nurses and their help with recruiting GP practices.
We are thankful for the support and guidance from the Trial Steering Committee and Data Monitoring and Ethics Committee members: Professor Timothy Barrett, Ms Katy Eddolls, Professor Denise Kendrick, Dr Rafael Perera, Dr Sharon Simpson, Dr Shahrad Taheri, Mr Hugh Tregoning and Dr Obioha Ukoumunne.
Contributions of authors
Julian Hamilton-Shield, Sandra Hollinghurst, Alan Montgomery, Katrina Turner and Debbie Sharp designed the study with additional advice from Jon Banks.
Julian Hamilton-Shield, Joanna Goodred, Lesley Powell, Joanna Thorn, Sandra Hollinghurst and Katrina Turner wrote the report.
Joanna Goodred collected the quantitative data.
Lesley Powell undertook the qualitative interviews.
Alan Montgomery undertook the statistical analysis.
All authors edited the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Reilly JJ, Wilson D. Childhood obesity. BMJ 2006;333:1207-10. http://dx.doi.org/10.1136/bmj.39048.503750.BE.
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Racial differences in the tracking of childhood BMI to adulthood. Obes Res 2005;13:928-35. http://dx.doi.org/10.1038/oby.2005.107.
- Guo SS, Chumlea WC. Tracking of body mass index in children in relation to overweight in adulthood. Am J Clin Nutr 1999;70:145S-8S.
- Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O’Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev 2009;1. http://dx.doi.org/10.1002/14651858.CD001872.pub2.
- Obesity General Information. London: DH; 2009.
- Foresight. Tackling Obesity: Future Choices. London: Government Office for Science; 2007.
- Rudolf MC, Christie D, McElhone S, Sahota P, Dixey R, Walker J, et al. Watch it: a community based programme for obese children and adolescents. Arch Dis Child 2006;9:736-9. http://dx.doi.org/10.1136/adc.2005.089896.
- McCallum Z, Wake M, Gerner B, Baur LA, Gibbons K, Gold L, et al. Outcome data from the leap (live, eat and play) trial: a randomized controlled trial of a primary care intervention for childhood overweight/mild obesity. Int J Obes 2007;31:630-6.
- Nowicka P, Flodmark CE. Family in pediatric obesity management: a literature review. Int J Pediatr Obes 2008;3:44-50. http://dx.doi.org/10.1080/17477160801896994.
- Golan M. Parents as agents of change in childhood obesity – from research to practice. Int J Pediatr Obes 2006;1:66-7. http://dx.doi.org/10.1080/17477160600644272.
- Barkeling B, Ekman S, Rossner S. Eating behaviour in obese and normal weight 11-year-old children. Int J Obes Relat Metab Disord 1992;16:355-60.
- Llewellyn CH, Van Jaarsveld CH, Boniface D, Carnell S, Wardle J. Eating rate is a heritable phenotype related to weight in children. Am J Clin Nutr 2008;88:1560-6. http://dx.doi.org/10.3945/ajcn.2008.26175.
- Carnell S, Wardle J. Appetitive traits in children. New evidence for associations with weight and a common, obesity-associated genetic variant. Appetite 2009;53:260-3. http://dx.doi.org/10.1016/j.appet.2009.07.014.
- Bergh CSM, Shield J, Hellers G, Zandian M, Palmberg K, Olofsson B, et al. Obesity: Causes, Mechanisms, and Prevention. Sunderland, MA: Sinauer Associates Inc.; 2008.
- Ford Al, Bergh C, Sodersten P, Sabin Ma, Hollinghurst S, Hunt Lp, et al. Treatment of childhood obesity by retraining eating behaviour: randomised controlled trial. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.b5388.
- Roberts K, Cavill N, Rutter H. Standard Evaluation Framework for Weight Management Interventions. Oxford: National Obesity Observatory; 2009.
- Jaques D. Teaching small groups. BMJ 2003;326:492-4. http://dx.doi.org/10.1136/bmj.326.7387.492.
- Owen SE, Sharp DJ, Shield JP. The bristol online obesity screening tool: experience of using a screening tool for assessing obese children in primary care. Prim Health Care Res Dev 2011;12:293-300. http://dx.doi.org/10.1017/S1463423611000132.
- Food Standards Agency . Eatwell Plate n.d. www.food.gov.uk (accessed 9 September 2013).
- Deci EL, Ryan RM. A motivational approach to self: integration in personality. Nebr Symp Motiv 1990;38:237-88.
- Bergh C, Brodin U, Lindberg G, Sodersten P. Randomized controlled trial of a treatment for anorexia and bulimia nervosa. Proc Natl Acad Sci USA 2002;99:9486-91. http://dx.doi.org/10.1073/pnas.142284799.
- Sabin MA, Ford A, Hunt L, Jamal R, Crowne EC, Shield JP. Which factors are associated with a successful outcome in a weight management programme for obese children?. J Eval Clin Pract 2007;13:364-8. http://dx.doi.org/10.1111/j.1365-2753.2006.00706.x.
- Banks J, Sharp DJ, Hunt LP, Shield JP. Evaluating the transferability of a hospital-based childhood obesity clinic to primary care: a randomised controlled trial. Br J Gen Pract 2012;62:E6-12. http://dx.doi.org/10.3399/bjgp12X616319.
- Ford AL, Hunt LP, Cooper A, Shield JP. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health?. Arch Dis Child 1995;95:256-61. http://dx.doi.org/10.1136/adc.2009.165340.
- Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998;17:407-29. http://dx.doi.org/10.1002/(SICI)1097-0258(19980228)17:4<407::AID-SIM742>3.0.CO;2-L.
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800-12. http://dx.doi.org/10.1097/00005650-200108000-00006.
- Stevens KJ. The Child Health Utility 9D (CHU9D) – a new paediatric preference based measure of health related quality of life. Pro Newsletter 2010;43:11-2.
- Wille N, Badia X, Bonsel G, Burstrom K, Cavrini G, Devlin N, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res 2010;19:875-86. http://dx.doi.org/10.1007/s11136-010-9648-y.
- EuroQol Group . EuroQol – A new facility for the measurement of health related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Brunstrom JM, Davison CJ, Mitchell Gl. Dietary restraint and cognitive performance in children. Appetite 2005;45:235-41. http://dx.doi.org/10.1016/j.appet.2005.07.008.
- Van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord 1986;5:295-31. http://dx.doi.org/10.1002/1098-108X(198602)5:2<295::AID-EAT2260050209>3.0.CO;2-T.
- Kyle J, Masson L, Duthie GG, McNeill G. Estimating dietary flavonoid intake: comparison of a semi-quantitative food frequency questionnaire with 4-day weighed diet records in a Scottish population. Proc Nutr Soc 2002;61.
- Frémeaux AE, Hosking J, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ. Consistency of children’s dietary choices: annual repeat measures from 5 to 13 years (EarlyBird 49). Br J Nutr 2011;106:725-31. http://dx.doi.org/10.1017/S0007114511000705.
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c869.
- Sturges JE, Hanrahan KJ. Comparing telephone and face-to-face qualitative interviewing: a research note. Qual Res 2004;4:107-18. http://dx.doi.org/10.1177/1468794104041110.
- Sabin MA, Ford AL, Hunt LP, Jamal R, Crowne EC, Shield JPH. Which factors are associated with a successful outcome in a weight management programme for obese children?. J Eval Clin Pract n.d.;13:364-8. http://dx.doi.org/10.1111/j.1365-2753.2006.00706.x.
- Stevens K. The Development of a Paediatric Health Related Quality of Life Measure for Use in Economic Evaluation: The Child Health Utility 9D (CHU 9D) n.d. www.shef.ac.uk/scharr/sections/heds/mvh/paediatric (accessed 12 May 2014).
- The EuroQol Group . The EQ-5D-Y n.d. www.euroqol.org/eq-5d-products/eq-5d-y.html (accessed 12 May 2014).
- The EuroQol Group . The EQ-5D-5L n.d. www.euroqol.org/eq-5d-products/eq-5d-5l.html (accessed 12 May 2014).
- Raynor HA, Osterholt KM, Hart CN, Jelalian E, Vivier P, Wing RR. Evaluation of active and passive recruitment methods used in randomized controlled trials targeting pediatric obesity. Int J Pediatr Obes 2009;4:224-32. http://dx.doi.org/10.3109/17477160802596189.
- Park MH, Falconer CL, Saxena S, Kessel AS, Croker H, Skow A, et al. Perceptions of health risk among parents of overweight children: a cross-sectional study within a cohort. Prev Med 2013;57:55-9. http://dx.doi.org/10.1016/j.ypmed.2013.04.002.
- Jones AR, Parkinson KN, Drewett RF, Hyland RM, Pearce MS, Adamson AJ, et al. Parental perceptions of weight status in children: the Gateshead Millennium Study. Int J Obes 2011;35:953-62. http://dx.doi.org/10.1038/ijo.2011.106.
- Newson L, Povey R, Casson A, Grogan S. The experiences and understandings of obesity: families’ decisions to attend a childhood obesity intervention. Psychol Health 2013;28:1287-305. http://dx.doi.org/10.1080/08870446.2013.803106.
- Wake M, Baur La, Gerner B, Gibbons K, Gold L, Gunn J, et al. Outcomes and costs of primary care surveillance and intervention for overweight or obese children: the LEAP 2 randomised controlled trial. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3308.
- Hughes AR, Stewart L, Chapple J, Mccoll JH, Donaldson MD, Kelnar CJ, et al. Randomized, controlled trial of a best-practice individualized behavioral program for treatment of childhood overweight: Scottish Childhood Overweight Treatment Trial (SCOTT). Pediatrics 2008;121:E539-46. http://dx.doi.org/10.1542/peds.2007-1786.
- Wake M, Lycett K, Clifford SA, Sabin MA, Gunn J, Gibbons K, et al. Shared care obesity management in 3–10 year old children: 12 month outcomes of hopscotch randomised trial. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f3092.
- Haisman N, Matyka KA, Stanton A. Lessons from an unsuccessful local attempt to tackle childhood overweight and obesity. Arch Dis Child 2005;90:984-5. http://dx.doi.org/10.1136/adc.2005.073338.
- Stewart L, Chapple J, Hughes AR, Poustie V, Reilly JJ. Parents’ journey through treatment for their child’s obesity: a qualitative study. Arch Dis Child 2008;93:35-9. http://dx.doi.org/10.1136/adc.2007.125146.
- The National Child Measurement Programme. England 2011/12. Leeds: Health and Social Information Centre; 2012.
- Jeffery AN, Voss LD, Metcalf BS, Alba S, Wilkin TJ. Parents’ awareness of overweight in themselves and their children: cross sectional study within a cohort (EarlyBird 21). BMJ 2005;330:23-4. http://dx.doi.org/10.1136/bmj.38315.451539.F7.
- Smith SM, Gately P, Rudolf M. Can we recognise obesity clinically?. Arch Dis Child 2008;93:1065-6. http://dx.doi.org/10.1136/adc.2007.134486.
- Silberberg M, Carter-Edwards L, Murphy G, Mayhew M, Kolasa K, Perrin EM, et al. Treating pediatric obesity in the primary care setting to prevent chronic disease: perceptions and knowledge of providers and staff. N C Med J 2012;73:9-14.
- Turner Km, Shield JP, Salisbury C. Practitioners’ views on managing childhood obesity in primary care: a qualitative study. Br J Gen Pract 2009;59:856-62. http://dx.doi.org/10.3399/bjgp09X472269.
- Etelson D, Brand DA, Patrick PA, Shirali A. Childhood obesity: do parents recognize this health risk?. Obes Res 2003;11:1362-8. http://dx.doi.org/10.1038/oby.2003.184.
- Visram S, Hall TD, Geddes L. Getting the balance right: qualitative evaluation of a holistic weight management intervention to address childhood obesity. J Public Health 2013;35:246-54. http://dx.doi.org/10.1093/pubmed/fds075.
- Barlow SE, Ohlemeyer CL. Parent reasons for nonreturn to a pediatric weight management program. Clin Pediatr 2006;45:355-60. http://dx.doi.org/10.1177/000992280604500408.
- Twiddy M, Wilson I, Bryant M, Rudolf M. Lessons learned from a family-focused weight management intervention for obese and overweight children. Public Health Nutr 2012;15:1310-17. http://dx.doi.org/10.1017/S1368980011003211.
- Raynor HA, Osterholt KM, Hart CN, Jelalian E, Vivier P, Wing RR. Efficacy of U.S. paediatric obesity primary care guidelines: two randomized trials. Pediatr Obes 2012;7:28-3. http://dx.doi.org/10.1111/j.2047-6310.2011.00005.x.
- Group HS, Foster GD, Linder B, Baranowski T, Cooper DM, Goldberg L, et al. A school-based intervention for diabetes risk reduction. N Engl J Med 2010;363:443-53. http://dx.doi.org/10.1056/NEJMoa1001933.
- Nathan BM, Moran A. Metabolic complications of obesity in childhood and adolescence: more than just diabetes. Curr Opin Endocrinol Diabetes Obes 2008;15:21-9. http://dx.doi.org/10.1097/MED.0b013e3282f43d19.
- Sabin MA, Ford AL, Holly JM, Hunt LP, Crowne EC, Shield JP. Characterisation of morbidity in a UK, hospital based, obesity clinic. Arch Dis Child 2006;91:126-30. http://dx.doi.org/10.1136/adc.2005.083485.
- Owen SM, Sharp DJ, Shield JP, Turner KM. Childrens’ and parents’ views and experiences of attendinmg a childhood obesity clinic: a qualitative study. Prim Health Care Res Dev 2009;10:236-44. http://dx.doi.org/10.1017/S1463423609990065.
- Mytton OT, Clarke D, Rayner M. Taxing unhealthy food and drinks to improve health. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2931.
- Banks J, Williams J, Cumberlidge T, Cimonetti T, Sharp DJ, Shield JP. Is healthy eating for obese children necessarily more costly for families?. Br J Gen Pract 2012;62:E1-5. http://dx.doi.org/10.3399/bjgp12X616300.
- Ludwig DS, Nestle M. Can the food industry play a constructive role in the obesity epidemic?. JAMA 2008;300:1808-11. http://dx.doi.org/10.1001/jama.300.15.1808.
- Morley B, Martin J, Niven P, Wakefield M. Public opinion on food-related obesity prevention policy initiatives. Health Promotion J Aus 2012;23:86-91.
- Nestle M. Food marketing and childhood obesity – a matter of policy. N Engl J Med 2006;354:2527-9. http://dx.doi.org/10.1056/NEJMp068014.
- Morley BC, Scully ML, Niven PH, Okely AD, Baur LA, Pratt IS, et al. What factors are associated with excess body weight in australian secondary school students?. Med J Aust 2012;196:189-92. http://dx.doi.org/10.5694/mja11.11184.
- Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38470.670903.E0.
- Reilly JJ. Physical activity, sedentary behaviour and energy balance in the preschool child: opportunities for early obesity prevention. Proc Nutr Soc 2008;67:317-25. http://dx.doi.org/10.1017/S0029665108008604.
- Chauliac M, Hercberg S. Changing the food environment: the French experience. Adv Nutr 2012;3:605S-10S. http://dx.doi.org/10.3945/an.112.001941.
Appendix 1 ComMando screening and referral form
Appendix 2 Questionnaire and outcome measures schedule
| Questionnaire/measure | To be completed by | Baseline (time point 1) | 3 months (time point 2) | 6 months (time point 3) | 9 months (time point 1V) | 12 months (time point V) | 24 months (time point VI) |
|---|---|---|---|---|---|---|---|
| Demographics | Parent and child | ✓ | – | – | – | – | – |
| Child height/weight | Child | ✓ | – | – | – | ✓ | ✓ |
| Parent height/weight | Parent | ✓ | – | – | – | ✓ | ✓ |
| PedsQL | Parent and child | ✓ | – | – | – | ✓ | ✓ |
| EQ-5D-Y | Child | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| CHU9D (7–11 years) | Child | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| EQ-5D | Parent | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Economics questionnaire | Parent | – | ✓ | ✓ | ✓ | ✓ | – |
| Wardle’s Child Eating Behaviours Questionnaire | Parent | ✓ | – | – | – | ✓ | ✓ |
| Dietary restraint questionnaire (8+ years) | Child | ✓ | – | – | – | ✓ | ✓ |
| Blinded eating rate | Parent and child | ✓ | – | – | – | ✓ | ✓ |
| Blinded portion size | Parent and child | ✓ | – | – | – | ✓ | ✓ |
| Ideal portion size | Parent and child | ✓ | – | – | – | ✓ | ✓ |
| FFQ | Parent | ✓ | – | – | – | ✓ | ✓ |
List of abbreviations
- A&E
- accident and emergency
- BMI
- body mass index
- BMI z-value
- body mass index standard deviation score
- BNSSG
- Bristol, North Somerset and South Gloucestershire
- CHU9D
- Child Health Utility 9 Dimensions
- CI
- confidence interval
- ComMando
- Community Mandolean randomised controlled trial
- DH
- Department of Health
- EQ-5D
- European Quality of life 5-Dimensions
- EQ-5D-5L
- European Quality of life 5-Dimensions 5-levels
- EQ-5D-Y
- European Quality of life 5-Dimensions – youth
- FFQ
- food frequency questionnaire
- GP
- general practitioner
- HCP
- health-care professional
- ID
- identification code
- LEAP
- Lifestyle Education for Activity Program
- NCMP
- National Child Measurement Programme
- PCRN
- Primary Care Research Network
- PCT
- primary care trust
- PedsQL
- Pediatric Quality of Life Inventory
- RCT
- randomised controlled trial
- RfPB
- Research for Patient Benefit trial
- SCG
- Scottish Collaborative Group
- SD
- standard deviation
- VAS
- visual analogue scale
- WCLRN
- Western Comprehensive Local Research Network