Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 04/35/08. The contractual start date was in October 2006. The draft report began editorial review in May 2012 and was accepted for publication in August 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Andrew McCaddon is a scientific advisor and shareholder of COBALZ Ltd – a private limited company developing novel B vitamin and antioxidant supplements.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Bedson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Plain English summary
Depression is common and serious. Only half of sufferers respond well to antidepressants. There is reason to hope that folic acid, which helps mothers and babies in pregnancy, will help. We conducted the clinical trial known as FolATED to test whether adding folic acid to antidepressants makes them work better and also gives good value for money. We also studied genetic and other scientific aspects of depression.
We aimed to recruit 450 adults from across Wales with confirmed moderate or severe depression for which they were taking or about to start antidepressants, but without other serious illness. Our target was for 360 (80%) of them to complete carefully designed questionnaires about their mental health on three occasions over 6 months. We actually recruited 475, and analysed 440 (93%) of them. Once a day for 12 weeks these participants added an extra pill to their antidepressants. For half of them, chosen at random, this pill contained 5 mg of folic acid. For the other half this pill looked the same but did not contain any folic acid. Only one person knew who had which pill.
Unfortunately the reported health of those who received active pills did not improve any more than the health of those who took inactive pills. So there is now no reason to believe that folic acid strengthens antidepressants. Fortunately recent research suggests that methylfolate may be better at this. So FolATED has undermined guidelines that advocate folic acid for depression, but suggested another way forward.
Chapter 1 Introduction
Background
Prevalence of depression
Depression is one of the main mental health disorders presenting in primary care. 1 The prevalence of major depression in the general population ranges from 3% to 10% with more than 150 million people at a time suffering from depression across the world. 2 In the UK prevalence of depression in 2009–10 was 11% in England, 11.5% in Northern Ireland, 8.6% in Scotland, and 7.9% in Wales. 3 Unipolar depression leads to 12.15% of years lived with disability, and is ranked as the third leading contributor to the global burden of diseases. 2 Indeed depression is currently the second cause of disability worldwide for males and females between the ages of 15 and 44 years and is predicted to reach second place for all ages by 2020. 4 Depression has become the leading cause of disability in Europe, leading to a loss of one in every 10 healthy years of life, and the leading cause of early retirement. 5 Depression and stress are now the commonest reported causes for sickness absence from work in the UK6 with over 100 million working days lost across Europe at a cost of 1% Gross Domestic Product (GDP). 7 A higher prevalence of depression is observed in women than men across the 18- to 64-year age range with women up to 2.5 times more likely to develop depression. 8
Characteristics of depression
The core symptoms of depression are low mood and loss of interest or enjoyment in usually pleasurable activities. Associated symptoms include disturbances to sleep and appetite, reduced energy and concentration, negative thoughts of guilt or worthlessness and suicidal ideation. The International Statistical Classification of Diseases & Related Health Problems (ICD-10) states that for a diagnosis of depression at least five symptoms need to be present, including at least one of the core symptoms, at an intensity that causes functional impairment and for a minimum duration of 2 weeks. Depression is classified as mild, moderate or severe according to the number of symptoms present and degree of functional impairment, and the grading of severity is of direct relevance to the treatment approaches recommended. 9 Depression is associated with increased mortality linked to suicide, alcohol and drug misuse, and increased rates of cardiovascular disease. 10 Depression thus burdens individuals, families, the NHS, and the national economy. 11 One UK study estimated the total cost of depression to the UK in 2000 at £9 billion; at that time, before the introduction of Improving Access to Psychological Therapies (also known as IAPT) in NHS England, the direct cost of treatment, mainly antidepressant medication (ADM), was £370 million; and indirect costs of 110 million working days lost to depression accounted for the vast majority of the total cost. 12 The sub-optimal treatment of depressive disorders is therefore of great public health concern.
Treatments for depression
In accordance with the joint report of the World Health Organization (WHO) and World Organization of National Colleges & Associations of Family Doctors (WONCA)1 and the National Institute for Health and Care Excellence (NICE) guidance9 the majority of people with depression are identified, treated and managed within primary care. Treatment aims to relieve symptoms, restore functioning and, in the long term, prevent relapse. The goal of treatment is complete remission which is associated with better functioning and reduced risk of relapse. 13 While there is some evidence that people with depressive symptoms improve over time without treatment,14,15 a significant proportion follow a chronic course with significant levels of depressive symptoms and functional continuing for several years. 16,17
Antidepressants are recommended as a treatment option for moderate to severe depression either in combination with psychotherapy9 or as monotherapy. 18 Owing to their greater tolerability selective serotonin reuptake inhibitors (SSRIs) are recommended as first-line treatment in primary care. 9 Patients treated with an SSRI are seven times more likely to complete a therapeutic course than those treated with tricyclic antidepressants (TCAs). 19 In clinical trials of antidepressants typically 50% of patients with depression respond to active treatment, while one-third respond to placebo20 with the placebo response appearing to increase over time in clinical trials. 21 With first-line treatments about one-third of patients achieve remission from depression, increasing to two-thirds with refinement of treatment. 22 A study of mental disorders in 14 centres worldwide found that 50% of patients continued to have a diagnosis of depression after 1 year23 with at least 10% having persistent or chronic depression. 24 Furthermore, at least 50% of people will go on to have at least one further episode of depression following their first episode of major depression. 25 The risk of further recurrences after second and third episodes rises to 70% and 90% respectively. 25 Cumulative rates of recurrence remain linear over long periods of follow-up (30–40 years), indicating a constant risk of recurrence over the lifespan. 26 Therefore recurrence rates increase with length of follow-up, and for the majority of patients depression is a recurrent condition.
Depression is a prevalent global health problem resulting in high levels of disability. While effective treatments are available outcomes remain sub-optimal with a significant proportion of patients failing to achieve remission and experiencing chronic illness, early relapse and multiple recurrences across the lifespan. There remains a pressing need for research to optimise outcomes from antidepressant treatment.
Review of the literature
Depression and folate
Over recent years there has been a growing interest and an increasing body of evidence exploring the relationship between B vitamins, in particular folate, and depression. 27–30 Folate is a naturally occurring B vitamin and can be found in leafy green vegetables, fruits, dried beans and peas. 31 Folic acid is the synthetic form of folate, which is inexpensive and found in supplements and fortified food. 31,32
There is evidence to suggest that low folate intake is associated with symptoms of depression. 33–36 Studies report that up to one-third of patients with depressive illness have decreased serum and red cell folate levels. 37 Many people with depression have lower concentrations of folate than people with other psychiatric disorders or no psychiatric disorder. 34,38,39 Associations between folate deficiency and depressive symptoms, symptom severity and treatment outcomes in adults and the older adult population have been reported. 38,40–44 Low folate intake may also increase the risk of recurrent depression33 and depression in later life. 43 Gilbody and colleagues conducted a systematic review of observational epidemiological studies investigating the relationship between low folate status and depression. 45 They concluded that low folate status was associated with depression but could not conclude that that was a causal relationship. Though low folate may result from poor nutrition or socio-economic disadvantage, confounders are common in chronic mental illness. Other recent evidence suggests that low folate may be a consequence rather than a cause of depressive symptoms. 46 While there is weak evidence that increased folate intake may prevent depressive symptoms,35,47 this is not a consistent finding. 48 In one randomised controlled trial (RCT), for example, giving folate (2 mg) with vitamins B12 and B6 did not reduce severity of depressive symptoms. 49
The role of homocysteine
Folate is absorbed and transported in the blood in the form of 5-methyltetrahydrofolate (5-MTHF)50 and is measured in the blood as either serum folate or red cell folate. 50
Homocysteine is a highly sensitive marker of folate status51 and functional folate deficiency is indicated by elevated homocysteine. Tiemeier et al. found a significant relationship between depression and hyperhomocysteinemia, folate and B12 deficiency. 52 Observational studies indicate that patients with depression have increased plasma total homocysteine concentrations. 53,54 A recent meta-analysis showed that older adults with a high total homocysteine concentration have an increased risk of depression [odds ratio (OR) = 1.70; 95% confidence interval (CI) from 1.38 to 2.08]. 55
There are important relationships between folic acid and vitamin B12 that have implications for how folate can be used therapeutically. For people who are deficient in vitamin B12, exposure to high levels of folate can result in subacute combined degeneration of the spinal cord, which may be linked to impaired methionine biosynthesis. 56 As methylmalonyl CoA mutase, a vitamin B12-dependent enzyme, converts methylmalonyl-CoA to succinyl-CoA, blood methylmalonic acid (MMA) levels increase with suboptimal B12 status. The cross-sectional study of > 10,000 participants in the US National Health and Nutrition Examination Survey (NHANES) observed a novel relationship between MMA and folate:57 in patients with low B12 (< 148 pmol/l ≈ 200 ng/l) MMA increased significantly with increasing serum folate. This finding is due, either to adverse oxidative effects of unmetabolised folic acid on B12 homeostasis, or inability of patients with low B12 to retain intracellular folate. 58
Antidepressant treatment and folate
Folate is an essential cofactor for the biosynthesis of both serotonin (5-hydroxytryptamine or 5-HT) and noradrenaline. Thus folate deficiency leads to impaired serotonin synthesis in the human brain. 59 This may provide a theoretical model for claims that folate can play a role in the treatment and prevention of depression. 60 Virtually all antidepressants are thought to act by prolonging the activity of serotonin or noradrenaline in neurotransmission or by modulating monoamine receptor sensitivity. 61 Lower folate levels have been associated with poorer antidepressant response. 62 Further evidence suggests that baseline levels of folate within the normal range predict antidepressant response. 63
This raises the possibility of folate being used to augment antidepressants. Initial small feasibility studies seemed to confirm the potential of this augmentation strategy. 32 A Cochrane review and meta-analysis50,64 explored the role of folate augmentation in depression. Only two RCTs were identified (combined n = 151), both of which suggest possible beneficial effects of folate augmentation. 65,66 Two further small trials have given contradictory results. One study, 67 with a clinical sample of 42 patients with major depressive disorder, reported that 10 mg of escitalopram (Cipralex®, Lundbeck) alone produced greater improvement than the combination of escitalopram and folic acid (2.5 mg/day). The other study, 68 with a clinical sample of 27, reported a greater reduction in depressive symptoms when 20 mg of fluoxetine (non-proprietary) was augmented with folic acid (10 mg/day) than when augmented with placebo. However, emerging evidence within an older depressed population suggests there may be no benefit from folate augmentation of antidepressants. 69 Despite this negative finding in the elderly there is still interest in a potential augmentation role for the B vitamins in general, with the currently recruiting B-VITAGE trial exploring B vitamin supplementation in later life. 70 These data convey mixed messages about the use of folic acid to improve ADM. To advance this debate about the clinical effectiveness of folic acid, we plan to update the Cochrane systematic review to include this and other recently reported studies of augmentation by folic acid. 50
Folate, depression and genetics
There has recently been much research aimed at identifying genetic aspects of depression and antidepressant therapy. Indeed, a number of large genome-wide association studies, and some subsequent meta-analyses, have identified genetic polymorphisms associated with both risk of depression71–73 and response to antidepressant therapy. 74,75 However, these studies have been unable to demonstrate consistent and reproducible genetic associations.
Only a few studies have described an association between risk of depression and genetic variation of genes of the one-carbon folate and methionine biosynthesis pathways. Most have focused on, and identified, an association with depression and the frequently characterised c.677C > T polymorphism (rs1801133) of the methyltetrahydrofolate reductase (MTR) gene. 76,77 This variant encodes a valine to alanine amino acid substitution at residue 222. The variant protein has reduced catalytic activity and thermolability and is associated with elevated homocysteine levels under conditions of impaired folate status. Others, in addition to MTR c.677C > T, have also described the p.D919G variant (rs1805087) of the MTR gene as a statistically significant risk factor for moderate and severe depression in postmenopausal women. 78 The MTR gene encodes the protein methyltetrahydrofolate reductase, which is a key enzyme in the biosynthesis of homocysteine to methionine.
Furthermore, it has recently been reported that the MTR c.677C > T polymorphism modifies the protective effect of folic acid against depression after pregnancy. 79 Others have demonstrated an association between c.677C > T and folate and homocysteine concentrations. 80 These observations further support the hypothesis that genetic variation in the one-carbon folate pathway may affect folic acid efficacy as an adjuvant to antidepressant therapy by altering folate bioavailability and increasing homocysteine levels.
Summary
Depression is a prevalent and debilitating mental health disorder. It often follows a chronic or recurrent course across the lifespan. Antidepressants are the recommended treatment for moderate to severe depression. Only half of people will respond to first-line treatment, and only one-third will achieve remission. Further research is needed to investigate ways of augmenting antidepressants to boost treatment response and rates of remission.
Evidence suggests that folic acid may be a useful adjunct to antidepressant treatment for four reasons:
-
Patients with depression often have a functional folate deficiency.
-
The severity of deficiency, indicated by elevated homocysteine, correlates with depression severity.
-
Low folate is associated with poor antidepressant response.
-
Folate is required for the synthesis of neurotransmitters in the pathogenesis and treatment of depression.
Objectives
The Cochrane review by Taylor and colleagues concluded that adequately powered randomised trials were needed to investigate the therapeutic potential of folate augmentation of antidepressants. 50 The National Institute of Health Research Health Technology Assessment (NIHR HTA) programme commissioned FolATED to address this gap in the literature.
The main objectives of FolATED were to assess the clinical effectiveness and cost-effectiveness of adding folic acid to the antidepressant treatment of moderate to severe depression. Our secondary objectives were to explore whether baseline folate and homocysteine predict response to treatment, and investigate whether response to treatment depends on genetic polymorphisms related to folate metabolism.
Chapter 2 Methods
Trial design
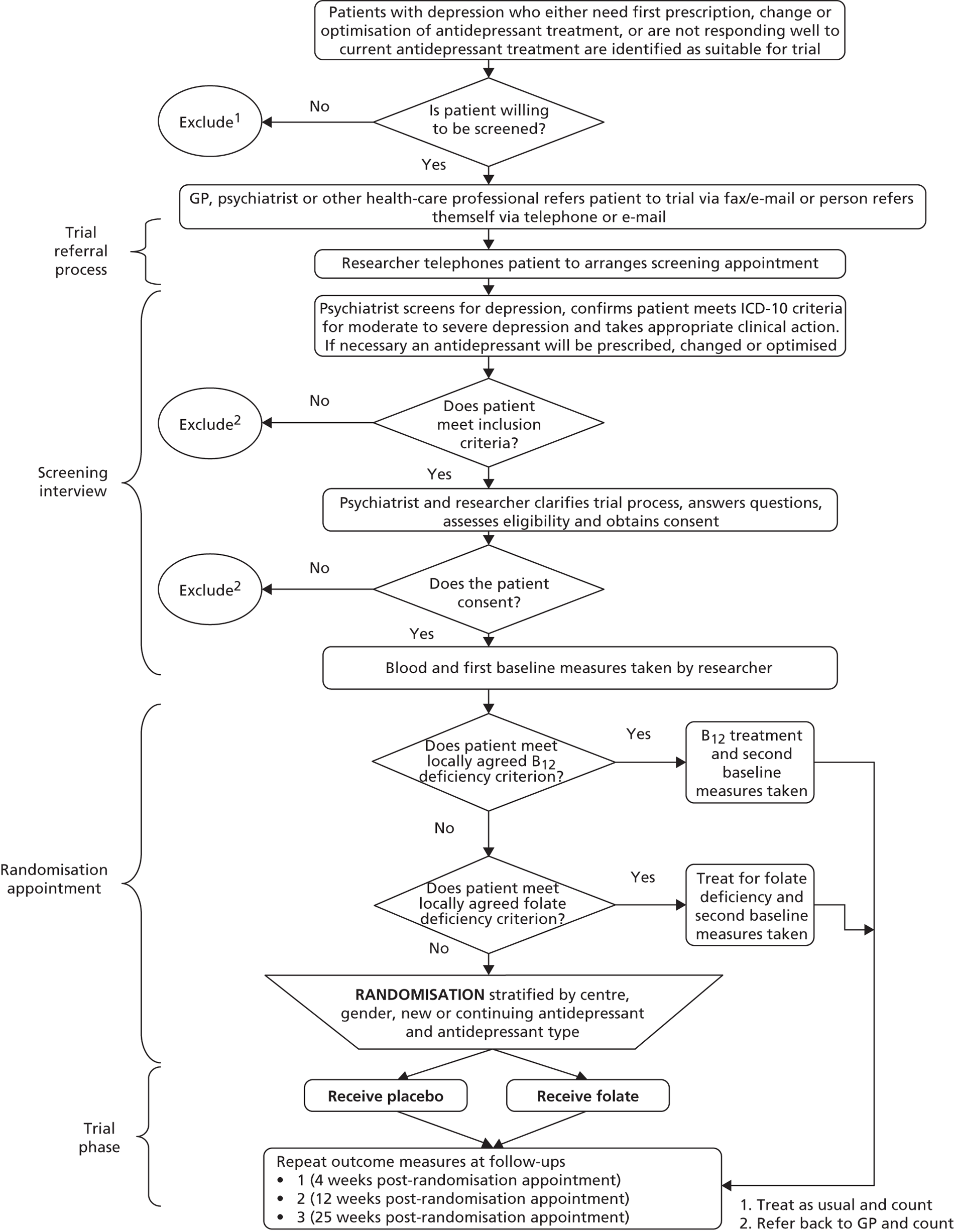
FolATED was a three-centre, double-blind and placebo-controlled, but otherwise pragmatic, randomised trial of folic acid augmentation of antidepressant treatment for people with moderate to severe depression. Participants were allocated to folic acid or matching placebo in equal proportions. Assessment took place at weeks –2 (to screen for eligibility and initiate ADM if needed); –1 (to check by telephone for tolerability of antidepressants); 0 (baseline – to randomise to folate or placebo); and 4, 12 and 25 weeks (to assess outcomes). Figure 1 shows the flow diagram for the trial. 81,82
FIGURE 1.
Flow diagram of the trial.
Participants
Settings
We recruited participants from primary and secondary care at three centres across Wales between July 2007 and November 2010. The sites were North East Wales, North West Wales and Swansea; and covered a population of about 1.35 million people in 2009. 83 We screened potential participants referred by their primary or secondary care clinicians or themselves for eligibility – in a variety of settings including general practice, secondary mental health services, research clinics, and patients’ homes.
Informed consent
All potential participants received a copy of the information sheet and consent form (see Appendix 1) from their referring clinician or the research team at least 24 hours before screening to ensure they had time to consider the study. Trial psychiatrists or screening nurses checked that eligible patients fully understood the study and gave them the opportunity to ask questions. To all potential participants we stressed that taking part in the study was voluntary and that their clinical care would not change if they did not want to take part in the trial.
Inclusion and exclusion criteria
Trial psychiatrists could assess eligibility of any potential participant. For self-referred participants registered mental health nurses liaising with a trial psychiatrist could also assess eligibility.
Potential participants were eligible to take part in the trial if they met all these criteria:
-
presenting with moderate to severe depressive symptoms confirmed by a trial psychiatrist during the screening interview and reporting a score of at least 19 on the Beck Depression Inventory version 2 (BDI-II) at screening, and at least 17 at baseline84
-
being treated with ADM, or about to commence ADM treatment
-
aged at least 18 years
-
able to give informed consent, and
-
able to complete the research assessments.
We excluded potential participants from the trial if they:
-
a. were folate deficient
-
b. were B12 deficient
-
c. had taken supplements containing folic acid within 2 months
-
d. suffered from psychosis
-
e. suffered from bipolar disorder
-
f. were participating in other clinical research
-
g. were pregnant or planning to become pregnant
-
h. were taking anticonvulsants
-
i. had a serious, advanced or terminal illness with a life expectancy of less than 1 year
-
j. had recently started treatment for a medical condition that had not yet been stabilised, or
-
k. had a diagnosis of, or treatment for, any malignant disease or related condition like intestinal polyposis.
Sample size
We originally powered FolATED to detect a difference between the two treatment groups of three points on the BDI-II at 25 weeks, judging that a clinically important difference. As we estimated the standard deviation (SD) of BDI-II scores in the trial population at 10.7, our protocol proposed a completed sample size of 400 at 25 weeks to yield 80% power to detect this difference using a significance level of 5%. As interim analysis of baseline BDI-II scores showed that their SD was about 10, we revised the target completed sample size to 358 at 25 weeks. The original protocol allowed 10% loss at each of the three follow-up assessments, thus requiring to randomise 549 to achieve 400 completers at 25 weeks. As interim analysis also showed that retention at 25 weeks was 79% rather than the 73% expected, the new target of 358 completers needed a randomised sample of 453.
Randomisation
At screening the screener took a blood sample to assess B12 and folate status, and arranged a further appointment within 14 days to confirm the B12 and folate results. We excluded participants who were B12 or folate deficient from the main trial but offered them the opportunity to continue in the ‘comprehensive cohort’ of recruited patients.
Eligible participants completed the baseline assessments and the recruiting centre telephoned the randomisation centre at NWORTH, Bangor University. NWORTH used dynamic allocation to protect against subversion while ensuring that each arm of the trial was balanced for the stratification variables. For each participant the adaptive algorithm recalculated the likelihood of their allocation between treatment groups from the distribution of stratification variables among participants already recruited and allocated. This process keeps the balance between strata within acceptable limits of the target allocation ratio of 1 : 1 while maintaining unpredictability. 85 The selected stratification variables were:
-
centre (North East Wales, North West Wales or Swansea)
-
sex (male or female)
-
timing of ADM (new or continuing)
-
type of ADM (SSRI or other)
-
whether participant had received counselling for depression (ever or never).
Intervention
By the time participants entered the trial, we ensured they were receiving antidepressant treatment optimised to therapeutic dosages – equivalent to SSRI of at least 20 mg per day or TCA of at least 150 mg per day. Most had received an antidepressant prescription from their general practitioner (GP) before referral to the trial. For patients not on ADM, trial psychiatrists initiated ADM to meet clinical need and patient preference in accordance with routine practice. For patients on sub-therapeutic ADM, trial psychiatrists optimised the treatment regime according to the British National Formulary (BNF),86 namely citalopram dose of at least 20 mg per day or equivalent. For participants who had been receiving a therapeutic dose of ADM, we encouraged trial psychiatrists to optimise dose according to the BNF, for example by increasing citalopram dosage to 40 mg per day, or change the antidepressant, again depending on clinical need and patient preference.
Participants received a 12-week supply of either 5 mg folic acid or placebo in addition to their ADM. We selected the 5-mg dose of folic acid because that is effective for other indications,87 carries a low risk of adverse events (AEs), and is routinely used to treat folate deficiency. 31
Bilcare (formerly DHP Ltd) supplied the trial drugs and achieved the blinding needed by the trial by the process of over-encapsulation. They placed each tablet inside a size ‘1’ opaque hard gelatin capsule and added lactose BP, an ingredient of the tablet, to fill the capsule. To produce placebo for the trial they filled the same capsules with lactose. They packed capsules into high-density polyethylene bottles with tamper-evident child-resistant screw caps. They tested to confirm that the over-encapsulated tablet complied with the British Pharmacopoeia88 standard for disintegration in vitro. They also checked each batch for the presence or absence of 5 mg folic acid.
Blinding
The North Wales Organisation for Randomised Trials in Health coded the identically packaged folic acid and placebo randomly for each stratification group. Each patient’s prescription indicated his or her trial number and package serial number generated by NWORTH, thus determining the appropriate trial package. NWORTH and the local pharmacies held the key to the randomisation codes. The telephone numbers of those pharmacies were available to break codes in emergency.
This ensured that throughout recruitment treatment allocations were unknown to participants, healthcare professionals, investigators, and researchers. We broke randomisation codes for two participants. One was diagnosed with lower oesophageal cancer, and the other collapsed with agitation, breathing difficulty, raised pulse, and reduced consciousness. On both occasions the local pharmacist revealed the code from a scratch card. This ensured that we revealed only the individual allocation, only to those who needed to know.
An independent GP monitored blood results, notably to check for B12 and folate deficiency at follow-up. To avoid accidental unblinding researchers engaged in clinical data collection or analysis did not have access to these blood results. Furthermore we separated pharmacogenetic and biochemistry analysis from clinical effectiveness analysis, and combined these results only when analyses were complete. Formal unblinding of the randomisation codes took place at the joint final meeting of the Trial Steering Committee (TSC) and the Data Monitoring and Ethics Committee (DMEC) on 10 October 2011.
Data collection
We collected data at screening, baseline and randomisation, and 4, 12 and 25 weeks after randomisation, using the trial case report forms (CRFs). Though we aimed to collect the data on the day due, this was not always possible. Hence there was a window for each data collection (Table 1).
| Data collection | Due date (days since randomisation) | Window |
|---|---|---|
| Screening for eligibility | –14 days | ± 10 days |
| Randomisation | 0 | Origin |
| 4-week follow-up | 28 days | + 14 days |
| 12-week follow-up | 84 days | ± 14 days |
| 25-week follow-up | 175 days | ± 28 days |
We designed some questionnaires for completion by researchers or clinicians, and others by participants. The preferred mode was face to face. When that was not possible, we permitted completion over the telephone and mailed the questionnaire to the participant in advance.
Primary outcome measure
The main outcome measure was self-rated symptom severity as measured by the Beck Depression Inventory version 2 (BDI-II). 84 The BDI-II consists of 21 items, each rated on a four-point scale ranging from 0 to 3; a total score of 1–13 indicates no depression, 14–18 mild depression, 19–28 moderate depression, and 29–63 severe depression. As BDI-II scores at 25 weeks are useful in assessing participants’ medium-term recovery, that was the basis of our original power calculation (see Sample size, above). When updating that calculation, we also designated the primary outcome as the area under the curve (‘AUC’ for short) of mean BDI-II scores between randomisation and the 25-week follow-up, because this summarises participants’ recovery across the whole of that period. 84 Though there was less prior information on AUCs, notably on clinically important differences, we judged that the combination of well-behaved BDI-II scores and the mathematically robust trapezium method of estimating AUCs89 would make 358 an appropriate target sample size (see Sample size, above). In the event we were able to analyse many more than 358 trial participants. Because the actual follow-up time could vary by up to 4 weeks from the target of 25 weeks (see Table 1), we converted the area under the curve to a more meaningful ‘AUC average’, which represents a participant’s BDI-II (or other outcome) score averaged over that participant’s follow-up period.
Secondary outcome measures
Symptom severity
Clinicians rated symptom severity using the Montgomery–Åsberg Depression Rating Scale (MADRS) and the Clinical Global Impression (CGI) of change at baseline and 4, 12 and 25 weeks. The MADRS90 consists of 10 items each rated on a seven-point scale (0–6), which yield a total score between 0 and 60. The CGI91 comprises three separate clinician-rated items: an ordinal scale of current severity of illness between 1 and 7; an ordinal scale of global improvement since recruitment between 1 and 7; and an efficacy index ranging from 0.25 to 4.0 derived from a 4 × 4 matrix plotting therapeutic effect against side effects.
Researchers at all centres received training in completing the rated scales (MADRS and CGI) from standard training videos. To estimate inter-rater reliability, we collated researchers’ ratings of these videos on several occasions.
Health status
Participants reported their mental and physical health by version 2 of the UK 12-item Short Form Health Survey (SF-12) and their quality of life by the EQ-5D, both at baseline, 4, 12 and 25 weeks. The SF-12 is a functional measure of quality of life comprising 10 five-point items and two three-point items. 92 Using scoring algorithms designed to achieve standardisation to a mean of 50 and a SD of 10 in the 1998 general US population, these items yield separate physical and mental component scores (PCS-12 and MCS-12). Though we used EQ-5D as a secondary measure of clinical effectiveness, its main purpose was to measure health utility for economic analysis. 93
Proportion of participants with moderate depression
Though we adopted the standard definition of moderate depression as a BDI-II score of 19 or more, we estimated the proportion of participants with moderate depression by statistical inference from the observed distribution of BDI-II scores. This technique is more robust to random variation than mere counting.
Adverse events and side effects
Though we asked centres to report all AEs, we focused on serious adverse events (SAEs) including inpatient admissions, attempted or completed suicide, and other mortality. We asked centre principal investigators to assess whether folic acid could possibly have caused each SAE, and whether it was an expected consequence. The chief investigators reviewed these data blind to the random allocations.
We assessed side effects through the UKU side effects scale,94 which sums scores on 48 distinct side effects – 10 ‘psychic’, 8 ‘neurologic’, 11 ‘autonomic’ and 19 other – and adds two global items. It rates each item on a four-point scale ranging from 0 to 3, yielding system-specific scores out of 30, 24, 33 and 57, two global scores out of three, and a total score out of 150.
Adherence to the trial drug
We assessed adherence to the trial medication from dispensing records; returned tablet count at 12 weeks; folate and homocysteine levels at 12 weeks; and the Morisky Questionnaire95 administered only at 12 weeks. This instrument asks four binary questions about adherence, and reports the number of positive responses as a score between 0 and 4.
Folate status and B12 status
We measured red cell folate at baseline; and serum folate, homocysteine and B12 from blood samples collected at baseline, 12 and 25 weeks. We sent all of those samples to local NHS laboratories on the day of collection. For homocysteine analysis we centrifuged venous blood within 30 minutes of collection and stored the plasma at –20 °C until analysis. We assayed all samples from individual participants in the same batch to minimise the effect of inter-batch variation. We measured plasma total homocysteine using a one-step immunoassay following reduction with dithiothreitol, commercially available from the Abbott Diagnostics ARCHITECT system. The average intra-batch coefficient of variation (CV) for homocysteine was less than 3%.
Suicidality
We rated suicidality by Section C of the Mini International Neuropsychiatric Interview (MINI)96 at baseline and 4, 12 and 25 weeks. Summing the scores allocated to ‘yes’ responses yields a total score between 0 and 33, which the MINI criteria categorise into low risk (0–5), moderate risk (6–9) or high risk (≥ 10).
Other data
We collected basic demographic information for each participant including sex, age, ethnicity, employment status, marital status and number of dependent children. We also recorded smoking and alcohol consumption, which are known to affect homocysteine levels.
Follow-up
Thus we thoroughly assessed participants at 4, 12 and 25 weeks after randomisation. Antidepressants show a delayed and variable onset of clinical improvements in depression. 97–99 Previous trials suggest that 50% of those who eventually respond to ADM start to respond within 2 weeks, 75% within 4 weeks, and almost all within 6 weeks. 100 Hence we scheduled the first assessment at week 4, 6 weeks after the start or optimisation of antidepressant treatment. Non-response at 4 weeks may lead to changes in the ADM in accordance with the BNF and NICE guidelines. Hence the second assessment at 12 weeks could measure both continuing and late responses to ADM and folate augmentation. The third assessment at 25 weeks addressed any changes in effectiveness after the end of folic acid therapy, but during ADM, since that is the minimum duration of maintenance antidepressant treatment. 18
Quality assurance
The conduct of this trial followed the principles of good clinical practice (GCP) outlined by the ICH-GCP and complied with EU directive 2001/20/EC. 101 The research also adhered to the Medical Research Council (MRC) guidelines for clinical trials102–104 and the Research Governance Frameworks for England and Wales. 105–107 In particular we anonymised all research data and stored them securely. All research team members received general training in GCP and trial-specific training in the protocol, recruiting participants, taking blood, completing CRFs, conducting assessments, and reporting AEs. We also developed a fieldworker’s manual to maintain consistency between sites.
Independent Trial monitoring
We established a TSC and a DMEC to oversee FolATED through biannual meetings or telephone conferences. The TSC comprised an independent chair, three independent members, and five members of the FolATED trial management team. The DMEC comprised an independent chair and two independent members, with the trial statistician in attendance (see Appendix 2).
Approvals
The Multicentre Research Ethics Committee (MREC) for Wales gave initial ethical approval on 6 November 2006, and the Medicines and Healthcare products Regulatory Agency (MHRA) issued the Clinical Trial Authorisation (CTA) on 21 December 2006. Appendix 3 lists the dates of approvals for individual centres.
Summary of changes to the project protocol
Appendix 4 lists all substantial changes to the protocol approved by TSC, DMEC, MHRA, MREC, and primary and secondary care R&D Departments.
Statistical methods
Statistical analysis plan
Before starting analysis we developed our analysis plan for approval by the DMEC (see Appendix 5).
Trial populations
‘Analysed’ population
Randomisation allocated all participants to one of the two treatments. The CONSORT guidelines require that the main analysis be ‘by treatment allocated’. Ideally, therefore, this population should comprise all randomised participants. In practice only participants who contributed at least one BDI-II measured after baseline can usefully contribute. To get the most from their data, we used established methods to impute their missing data.
Complete case population
This population comprises only those participants whose outcome data are complete. It provides a sensitivity analysis of two issues: whether primary and secondary findings are sensitive to the absence of missing data, and the methods we used to impute those missing data.
‘Randomised’ population
At first sight it is difficult to draw inferences about this population because some contributed no data after baseline, even on the BDI-II. Because we know the baseline characteristics of all these participants, however, it is possible to reweight the ‘analysed population’ so that they match the characteristics of the randomised population, notably allocated treatment, stratifying variables and baseline BDI-II.
Imputation of missing data for ‘analysis by treatment allocated’
We excluded participants without follow-up data from the primary analysis ‘by treatment allocated’. For each variable we summarised missing data by reason (mainly participant withdrew; questionnaire not returned; page missing; item missing). Where < 10% of data were missing, we treated them as if they were missing completely at random (MCAR). 108 If > 10% of data were missing, we explored the missing data and tabulated them by the stratification variables both as reported at randomisation and as validated after quality assurance; by participant demographics; and by other important covariates. Rather than exclude participants missing some data, we chose to impute these data (see Appendix 5).
Missing items within a subscale
For missing items within a subscale we took account of methodological publications about the instrument. To impute missing items we used the principle that, if < 25% of the items within a subscale were missing for a participant at a time point, one should impute them by the weighted mean of the completed items, but if > 50% of the items within a subscale were missing for a participant at a time point, one should treat that subscale as wholly missing and impute it accordingly.
Missing subscales
Where between 25% and 50% of the items within a subscale were missing, we proceeded thus: if < 40% of the subscales for a participant at a time point were missing, we imputed all missing subscales by a single application of the general regression model for missing data imputation used in SPSS (Statistical Product and Service Solutions, SPSS Inc., Chicago, IL, USA),109 taking account of all validated stratification variables. If > 40% of the subscales for a participant at a time point were missing, but < 20% of participants experienced that problem, we imputed all missing subscales by a single multivariate imputation across all time points that also took account of all validated stratification variables. Fortunately these rules covered the whole of FolATED.
Missing time points
If one of the four time points for a participant was missing, we imputed all subscales within that time point by five iterations of the repeated-measures model for missing data imputation used in SPSS using all other subscales at all time points together with age, gender, centre and group. 107
Data description and transformation
Initially we summarised data by allocated treatment and centre. Rather than test for statistical differences between allocated groups at baseline, we adjusted for any imbalance by analysis of covariance. Our analysis plan assumed that residual variation from our statistical models follows Normal distributions. This is a robust assumption in the sense that only a substantial deviation would invalidate each analysis. So we plotted and reviewed residual distributions. As none of these was substantial, we did not need to transform data to improve consistency with the assumption of Normality. Hence we present all data as collected.
Methods for analysing outcomes
All of our statistical tests were two-sided with a significance level of 5%.
Continuous outcomes with baseline and more than one follow-up
We used the AUC average, not only to summarise treatment outcome across the whole of the 25 weeks of data collection, but also to take account of the correlation between successive measurements for the same participant. We calculated the AUC average by using the trapezium rule89,110 to weight the outcome scores at baseline and the three actual follow-up points. From the imputed data set we estimated the average score for each participant over his or her total follow-up period as the area under the EQ-5D utility curve divided by the duration of follow-up, using the trapezoidal rule specified by the formula:
where Uj is the utility attributed to the jth measurement, T is the duration in days of the participant’s study period, tj is the time in days at which the jth measurement takes place for that participant,111 and values j = 0, 1, 2 and 3 correspond to the baseline and three subsequent follow-ups respectively. We used similar formulae to calculate AUC averages for BDI-II, MADRS and SF-12 physical and mental component scores.
As covariates in these analyses we used the validated stratification variables – centre, sex, new or continuing case, type of antidepressant and previous counselling. For the individual time points, which contribute to and illustrate the AUC, we used analysis of covariance to adjust for the corresponding baseline score.
Continuous outcome with no baseline and only one follow-up (Morisky scale)
We used analysis of covariance with baseline depression scores and validated stratification variables as covariates, to test whether medication adherence, measured on the Morisky scale, differs significantly between the two treatment arms. If so, we would have added the Morisky score and ADMs recorded by GPs to the usual covariates.
Dichotomous outcomes (serious adverse events and adverse events)
We used logistic regression of (S)AE compared with no (S)AE over each participant’s time in the trial to test whether the proportion of (S)AEs differs between treatment arms, using baseline scores and validated stratification variables as covariates. We transformed all estimated fixed effects back from their logistic form and summarised them by OR, standard error, 95% CI, and significance level.
Covariates to be adjusted within the statistical model
We kept baseline depression scores and validated stratification variables as covariates throughout. We also explored covariates of potential scientific relevance, including demographic (notably age, ethnicity, marital status, number of dependants and employment status, coded in accordance with usual demographic practice) and clinical (e.g. referral source, smoking, alcohol consumption and medication adherence, measured by both Morisky scale and recorded prescriptions). We fitted and retained these if they showed evidence of an effect at a significance level of 10%.
Interactions to be tested
Within each analysis we tested for interaction between treatment and centre, not least because of substantial differences in psychiatric practice and recruitment policy. On finding no evidence of interaction we estimated the treatment effect for each centre. We also tested for interactions between treatment and significant covariates.
Deviations from protocol
During the trial there were two protocol deviations that resulted in systematic missing data – one within a centre at one time point, the other within a single instrument early in the trial. First, early in the trial 13 participants in one centre did not receive appointments for visits at 4 weeks as the centre was under pressure from a large number of referrals; fortunately preventive action prevented any recurrence. Second, early in the trial 83 participants completed an incorrect version of the MADRS instrument: 40 at screening; 29 at randomisation; eight at 4 weeks; and six at 12 weeks. As both were administrative errors balanced between treatment groups, however, sensitivity analysis suggested that neither resulted in systematic bias. We therefore invoked our standard missing data procedures (see Imputation of missing data for ‘analysis by treatment allocated’, above).
Sensitivity analyses
We applied three main sensitivity analyses – to the BDI-II as primary outcome in the first instance, with the intention of applying them to other outcome measures if the BDI-II proved sensitive to alternative assumptions. First we used ‘complete case’ analysis to test the sensitivity of findings to the absence of missing data; and the methods we used to impute those missing data. Secondly we used multi-level modelling with the same covariates, also known as repeated measures analysis of variance, to test the sensitivity of findings to our choice of AUC as main method of analysis; we estimated parameters for three fixed factors – the three time points (4, 12 and 25 weeks), centre and treatment group. Finally we reweighted the ‘analysed population’ to match the characteristics of the ‘randomised population’, and test the sensitivity of findings to non-response. To do so, we matched the participants completely lost to follow-up to participants from the analysed population. First we linked them by allocated treatment, centre and gender. Then we used a hierarchical cluster analysis to identify the best set of variables to match the non-responders to members of the responding trial population – age, marital status, reported alcohol intake, and BDI-II at screening and at baseline. We conducted this procedure both on raw data and on imputed data.
Biochemical analyses
The first of our secondary objectives was to explore whether baseline folate and homocysteine predict response to treatment – the difference between baseline and follow-up. We followed participants at 12 weeks, as they completed the trial medication, and at 25 weeks, the usual endpoint of antidepressant trials. Though many of our analyses of effectiveness use simple linear regression, this is less well suited to analyse topics where multicollinearity, that is multiple correlation, plays a major role. Instead we use repeated measures analysis of variance, which examines all four time points (i.e. baseline and 4, 12 and 25 weeks) simultaneously by fitting all four measures and adjusting for stratification variables, baseline measurements, biochemistry, demography and other covariates.
Health economics methods
Introduction
There are no economic evaluations of folic acid in managing depression. If shown to be effective, however, folic acid could represent a highly cost-effective treatment option. As it costs only 3 pence a day, the main cost drivers are likely to be hospital admissions, use of health and personal social services, ADM, and other aspects of care which might change following therapeutic benefit.
The aim of the economic analysis was therefore to assess whether the addition of 5 mg folic acid, once daily for 12 weeks, in new or existing users of antidepressants, represents a cost-effective use of healthcare resources. We limited this analysis to trial-generated estimates of costs and benefits, without modelling wider effects.
Perspective
In line with the NICE reference case,112 we adopted the costing perspective of the National Health Service (NHS) and Personal Social Services (PSS). We estimated all costs in 2009–10 prices.
Data sources
Resource use
We derived participants’ use of services from:
-
self-completed questionnaires
-
GPs’ records of prescribed medications, and
-
our register of serious adverse events (SAEs), specifically for hospital admissions.
We based our resource use questionnaire on that used in the Assessing Health Economics of Anti-Depressants (AHEAD) trial of the cost-effectiveness of tricyclic antidepressants, selective serotonin re-uptake inhibitors and lofepramine. 113 It comprises four sections, relating to patients’ use of general practice and generic community nursing services, social services, psychiatric hospital and community services, and other health services, notably hospital admissions and attendances, including at Emergency Departments (Table 2). Research professionals completed the questionnaire by asking participants to recall their use of these services for the 3 months before the baseline visit, and 12 and 25 weeks thereafter.
| Item | Unit | Cost | Comments and assumptions | Reference |
|---|---|---|---|---|
| General practice and community pharmacy and nursing services | ||||
| GP consultation | Visit | £36 | 11.7 minutes/consultation, including direct care staff costs and qualification costs | 119 |
| GP home visit | Visit | £120 | 23.4 minutes/visit, including travel, direct care staff costs and qualification costs | 119 |
| GP telephone contact | Call | £22 | 7.1 minutes/call, including direct care staff costs and qualification costs | 119 |
| Practice nurse at surgery | Visit | £12 | 15.5 minutes/consultation, including qualification costs | 119 |
| District nurse at home | Visit | £27 | 20 minutes/visit, including qualification costs | 119 |
| Counsellor at surgery | Visit | £71 | 96.6 minutes/consultation | 119 |
| Health visitor | Visit | £37 | 20 minutes/visit | 119 |
| Vitamin B12 test | Test | £1.29 | NHS reference cost code DAP841 | 116 |
| Pharmacy dispensing fee | Prescription item | £3.03 | Average NHS cost/item dispensed, assuming all prescribed items dispensed | 115 |
| Social services | ||||
| Social worker (home or office) | Visit | £213 | 1 hour face-to-face contact | 119 |
| Home help | Contact | £75 | 3 hours/week of local authority home care | 119 |
| Care assistant | Contact | £214 | 10 hours/week of local authority community care | 119 |
| Day centre | Day | £36 | Based on community care package | 119 |
| Psychiatric hospital and community services | ||||
| Consultant psychiatrist at hospital | Visit | £205 | NHS reference cost code PS25B | 116 |
| Consultant psychiatrist at home | Visit | £328 | Cost/hour of patient contact, including qualification costs | 119 |
| Clinical psychologist | Visit | £81 | Cost/hour of client contact | 119 |
| Community psychiatric nurse | Visit | £56 | Cost/per hour of client contact | 119 |
| Other services | ||||
| Day hospital | Day | £99 | NHS reference cost code DCF41 | 116 |
| Emergency Department | Visit | £116 | NHS reference cost code 180 | 116 |
| Hospital clinic | Visit | £199 | NHS reference cost code 430 | 116 |
| Mental health inpatient stay | Night | £302 | NHS reference cost code MHIPA2 | 116 |
| Occupational health services | Visit | £46 | Hospital occupational therapist | 119 |
| NHS Direct | Contact | £21.37 | Cost/nurse adviser contact | 120 |
| Ambulance or paramedic | Contact | £246 | NHS reference cost code PS25A | 116 |
We sought details of participants’ prescribed medicines, over the 25 weeks they were in the trial, from their GPs. Two pharmacy technicians compiled a database of prescription data, normally supplied as printouts or screen dumps, and a pharmacist checked it for accuracy. We also checked data on hospital admissions, obtained directly from participants, against our SAE register.
Unit costs
The costs of the intervention were: folic acid 5 mg – 84 tablets costing £2.67;114 dispensing fee equal to NHS average of £3.03;115 and serum vitamin B12 testing from the NHS reference costs for biochemistry. 116
We derived the unit costs of other resources from standard sources (see Table 2). We took drug costs from the Prescription Cost Analysis,117 which derives products’ net ingredient costs (i.e. excluding discounts and dispensing costs) from actual NHS expenditure. 118 We took the cost of pharmacy dispensing from a report commissioned by the Department of Health to estimate the cost of providing community pharmacies. 115 We retrieved the costs of healthcare professionals’ time from the Unit Costs of Health and Social Care 2010. 119 These include salaries and expenses, costs of training and qualifications, and capital and overhead costs. We took hospital costs from the NHS reference costs,117 which underpin the calculation of the tariff for ‘payment by results’ in England.
Health outcomes
The primary measure of health outcome for economic analysis was the quality-adjusted life-year (QALY), estimated from the EQ-5D questionnaire administered at baseline and 4, 12 and 25 weeks. This assesses health-related quality of life on five dimensions – mobility, self-care, usual activities, pain-discomfort and anxiety-depression. The three possible responses on each dimension are ‘no problems’, ‘moderate problems’ and ‘extreme problems’. We converted participants’ responses into a single, preference-based utility using on the UK tariff. 121
Secondary measures of health outcome for economic analysis included the EQ-VAS, the UK Short Form Health Survey – 6 Dimensions (SF-6D) and BDI-II, all completed at the same times as the EQ-5D. The EQ-VAS is a vertical 20-cm visual analogue scale for recording participants’ rating of their current health-related quality of life. The SF-6D derives an alternative preference-based utility from SF-12 responses using weights estimated from a sample of the general population by the standard gamble technique. 122
For the cost-effectiveness analysis, we calculated the number of weeks free from moderate or severe depression (defined as a BDI-II score < 13)123 by statistical inference from the observed distribution of BDI-II scores, assuming linear interpolation between time points (baseline, 4, 12 and 25 weeks).
Data analysis
We combined data on costs and outcomes in the ‘treatment allocated’ population (see Statistical methods, Imputation of missing date for analysis by treatment allocated, Missing items within a subscale, above) over 25 weeks to estimate an incremental cost-effectiveness ratio (ICER) for comparison against accepted thresholds. Primary analysis was on the data set in which we had imputed missing data in the manner described above (see Statistical methods, Trial populations).
Analysis of costs
We estimated costs over 25 weeks for each participant by aggregating across resource categories. To draw inferences from this skewed distribution, we used ‘bootstrapping’, that is resampling with replacement and re-estimating sample means for each replicate. We used 10,000 replicates, corrected for bias and skewness by the technique known as ‘bias correction and acceleration’, and generated 95% CIs. We inferred whether differences in mean costs between treatment and control groups were statistically significant from those bootstrapped CIs. 124 To adjust for differences at baseline and in duration of follow-up, we used the regression model:125
where patient i in treatment group gi has a pre-baseline cost of Ci and a time between randomisation and final follow up of Ti and β1 represents the difference in costs after adjusting for imbalance in mean costs before baseline. We used the logarithmic transformation to address the natural skewness of costs, and transformed the results of the regression back to recover the differential cost. As there were essentially no differences in demographic variables between groups, we did not need to adjust for any other variable.
Analysis of health outcomes
While the AUC average Uav is the measure analysed in effectiveness tables (see Methods for analysing outcomes: Continuous outcomes with baseline and more than one follow-up above), economic analysis uses QALY: the area under the utility curve over the participant’s follow-up period in years. Hence:
where Uav is the participant’s AUC average utility and T is the duration in days of the participant’s study period. However, as this period may vary from 21 to 29 weeks, we adjusted QALYs, like costs, for duration as well as baseline.
To adjust QALYs for differences in baseline utility and duration of follow-up, we used the regression model:126
where patient i in treatment group gi has baseline utility of Bi [equal to U0 in equation (1) above] and time between randomisation and final follow-up of Ti; and β1 represents the difference in QALYs after adjusting for imbalance in mean utility at baseline. As again there were essentially no differences in demographic variables between the two groups, we did not need to adjust for any other variable. We applied the same procedure to other measures of health outcome.
For all economic measures of health outcome we used 10,000 replicates to generate non-parametric bootstrapped 95% CIs, again corrected for bias and skewness, for the differences in means between treatment and control groups.
Cost–utility analysis
Comparing two treatments results in one of four scenarios. The intervention ‘dominates’ if it saves costs and improves health outcomes. The intervention ‘is dominated’ if it increases costs and outcomes deteriorate. More commonly the intervention improves outcomes at greater cost, or saves costs at the expense of outcomes. Then one must estimate an incremental cost-effectiveness ratio (ICER) by dividing the difference in adjusted mean costs (ΔC) by the difference in adjusted mean benefits (ΔB). NICE is more likely to recommend an intervention for use by the NHS if the ICER falls below the threshold for cost-effectiveness, which ranges from £20,000 to £30,000 per QALY. 112
We used non-parametric bootstrapping to map the joint distribution of costs and outcomes on the cost-effectiveness plane and generate cost-effectiveness acceptability curves to show the probability that the intervention was cost-effective across a range of thresholds of cost-effectiveness.
Sensitivity analysis
To examine the extent to which the ICERs are sensitive to basic assumptions, we used two alternative approaches to measuring utility – the EQ-VAS and the SF-6D, and restricted analysis to all participants who gave complete EQ-5D responses. We used R software111 for all analyses.
Genetic methods
Our aim was to test whether genetic polymorphisms affect the efficacy of folic acid in combination with ADM, with a view to using them as predictive markers of adjuvant folic acid efficacy. There is strong evidence to suggests that folic acid can play a role in the treatment and prevention of depression. 60 It is the effect of genetic variability on this role that we aim to investigate. This study focuses on variability in genes encoding proteins and enzymes implicated in the carbon folate and methionine synthesis pathways, rather on genome-wide analysis. 127 We justify this approach by the folic acid intervention in this study and the weight of evidence to suggest decreased folate is associated with depressive illness. 41,77,128
Given the level of complexity of the one-carbon folate pathway,127 the genetic characteristics of FolATED trial participants span a comprehensive set of folate pathway genes beyond the commonly analysed methylenetetrahydrofolate reductase (MTHFR) polymorphisms. Similar pathway-wide candidate gene approaches to genotyping have previously successfully identified genetic risk factors for several clinical phenotypes including colorectal,129,130 breast,131 and bladder cancers,132 and cleft lip or palate. 133
DNA isolation
We extracted genomic DNA from 5 ml of whole blood using the Chemagic Magnetic Module (MSM) 1 system according to the manufacturer’s protocol (Chemagen Biopolymer-Technologie AG, Baesweiler, Germany). We eluted samples in 500 µl of the manufacturer’s elution buffer.
Single nucleotide polymorphism selection
We compiled a list of 25 candidate genes127 associated with either the one-carbon folate or methionine synthesis pathways. We identified 48 non-synonymous single nucleotide polymorphisms (SNPs) within these genes from the Single Nucleotide Polymorpism Database [dbSNP;134 www.ncbi.nlm.nih.gov/SNP (accessed May 2008)] and selected for analysis those with minor allele frequency greater than 5%. We included a further 100 SNPs from the HapMap (http://hapmap.ncbi.nlm.nih.gov) population of Utah residents with ancestry from northern and western Europe which, when analysed by Haploview software version 402 (www.broadinstitute.org/haploview/haploview), tagged at least one other SNP. We added a 19 base pair (bp) deletion polymorphism of intron 1 of the dihydrofolate reductase gene (DHFR) and a 28 base pair double or triple tandem repeat polymorphism of the thymidylate synthase (TYMS) gene because both have been extensively characterised in clinical studies. 127
Genotyping
We designed multiplex assays for the MALDI-TOF-based Sequenom iPLEX system (Sequenom Inc., San Diego, CA, USA) using the software at https://mysequenom.com/default.aspx. We included 140 SNPs in five-assay plexes ranging from 11 to 35 SNPs in size. We excluded five SNPs which we could not incorporate in assays because of proximal nucleotide sequence constraints and another three SNPs which we could not include at a minimum plexing level of > 10 SNPs per assay (see Appendix 6, Table 35).
We genotyped patients for these 140 SNPs according to the manufacturer’s protocol using 40 ng/reaction genomic DNA. We obtained sequence-specific polymerase chain reaction (PCR) and extension reaction oligonucleotides from Metabion GmbH (Martinsried, Germany). Table 36 of Appendix 6 defines the corresponding primer and probe sequences.
We typed the 19 bp deletion polymorphism from the dihydrofolate reductase gene (DHFR) and the 28 bp tandem repeat polymorphism from the thymidylate synthase (TYMS) gene using previously published protocols and PCR primer sequences21,22 with minor modification. Briefly the 25-µl PCR reaction consisted of 20 ng genomic DNA, 5 pmol each of primer and 18 µl 1.1× ReddyMix™ PCR mastermix (Abgene Ltd, Epsom, UK).
We resolved all PCR products with ethidium bromide staining on a 3% agarose gel. For the DHFR 19 bp deletion, a 92 bp product identified the deletion allele and a 113 bp product identified the insertion allele. For the TYMS tandem repeat a 144 bp product distinguished the triple repeat allele from the double (116 bp).
We undertook all genotyping with 10% of DNA samples duplicated as well as positive and negative controls to confirm genotype calling accuracy and concordance.
Genetics outcomes
For this genetic sub-study, the primary outcome was self-rated symptom severity on the Beck Depression Inventory (BDI-II) at baseline, and 4, 12 and 25 weeks, consistent with the trial’s primary outcome. Secondary outcomes were:
-
symptom severity rated by clinicians on the MADRS and the CGI of change (also at baseline and 4, 12 and 25 weeks)
-
mental and physical aspects of self-reported health status on the SF-12 (ditto)
-
side effects assessed by the UKU side effects scale and reported AEs (ditto), and
-
proportion of patients with self-rated moderate or severe depression (i.e. BDI-II score ≥ 19) at 25 weeks.
Genetics statistical methods
Before the analyses of association, we tested each SNP for Hardy–Weinberg Equilibrium, and excluded those found to deviate at a significance level of 0.1%. We also excluded SNPs which did not meet all our genotype quality criteria:
-
a. minor allele frequency greater than 1%
-
b. genotyping rate greater than 95% per SNP, and
-
c. samples more than 90% of SNPs called.
We fitted three mixed models to all five outcomes for each included SNP. The first (‘baseline model’) included the baseline value of the outcome, covariates representing the three time points (4, 12 and 25 weeks), three validated stratifying variables – centre, type of antidepressant, new or continuing patient – and treatment received, that is whether participants supplemented their medication with folic acid or not. We also tested non-genetic factors known to be generally associated with outcome (age, gender, marital status, employment status, number of dependents, smoking and alcohol consumption, previous counselling and treatment adherence as assessed by the Morisky scale) for univariate association with each outcome and included them in the model if the significance level was less than 10%.
The second (‘SNP’) model was identical to the first with the addition of the SNP as covariate. The third (‘interaction’) model was identical to the second with the addition of interaction between SNP and treatment received. To test for statistical significance of SNP main effects, we used likelihood ratio tests to compare the specific SNP model with the baseline model. To test for statistical significance of the SNP-folated interaction, we again used the likelihood ratio test to compare the specific interaction model with the specific SNP model. Each test tried two models – one making no assumption about the underlying mode of inheritance, the other assuming an additive mode of inheritance – and used the lower significance level for each SNP.
To take account of the multiple comparisons due to four tests on each of more than 100 SNPs, we estimated the false discovery rate (‘FDR’) for each comparison, and treated FDRs less than 5% as statistically significant associations. We used the statistical software packages: R;111 PLINK version 1.07 from http://pngu.mgh.harvard.edu/~purcell/plink/; and PASW version 18135,136 for these analyses.
Systematic review of the effectiveness of folate in augmenting antidepressant medication
Introduction
At the start of the FolATED trial understanding of the benefits of folate augmentation of ADM stemmed from a recent Cochrane systematic review. 50,64 The authors concluded that there was limited evidence that adding folate to ADM was helpful, and recommended larger trials to test this hypothesis thoroughly. That recommendation led directly to the funding of FolATED.
Method
Data sources and study selection
We updated the current Cochrane systematic review50 following analysis of the FolATED trial. The authors of that review searched the Cochrane Central Register of Controlled Trials and MEDLINE from 1966 until May 2005. In August 2012 we reran their search for randomised trials evaluating folate in any form to augment ADM in treating depression. We followed the Cochrane systematic review search strategy in PubMed until December 2011 but without language restrictions. Consistent with the design of FolATED we selected randomised trials evaluating folate to augment antidepressants in treating depressive disorder rather than folate as sole therapy.
Data extraction and synthesis
Two of us (BRC and ITR) independently assessed potential trials for eligibility and quality, and extracted data. The Cochrane systematic review had used the Hamilton Depression Rating Scale (HDRS) as primary outcome. 50 In contrast the FolATED trial used the Beck Depression Index (BDI-II). To compare these instruments we converted both to standard Normal distributions with a SD of 1 and mean equal to the trial effect size, namely the mean difference between trial groups divided by trial SD. We gave each trial a weight inversely proportional to the variance with which it estimated that difference. We used a random-effects model to estimate the standardised mean difference and associated 95% CI. We assessed the heterogeneity of findings by the I2-statistic. 137
Chapter 3 Results
Recruitment: identification and eligibility
We recruited participants in three centres: North East Wales, North West Wales and Swansea. The first randomisations took place in North West Wales in July 2007, in Swansea in August 2007, and in North East Wales in October 2007. We randomised the final participant in November 2010 and completed follow-up in May 2011.
Figure 2 shows that FolATED received 1488 referrals; screened 863, of whom 635 consented to take part; randomised 479, of whom four were in error and removed from analysis; and analysed 440 (92% of the 475 valid randomisations). Though the four randomised in error had BDI-II scores of at least 19 at –2 weeks, these had fallen below 17 at randomisation, so they should have been excluded. The reasons why 625 referred patients did not reach screening were: 44% did not wish to take part; 26% did not respond to the research team; 16.5% were ineligible; and 13.5% did not attend the screening appointment.
FIGURE 2.
‘CONSORT diagram’ of flow of participants through trial. Note: Hence we included 223 + 217 = 440 participants in primary analysis.
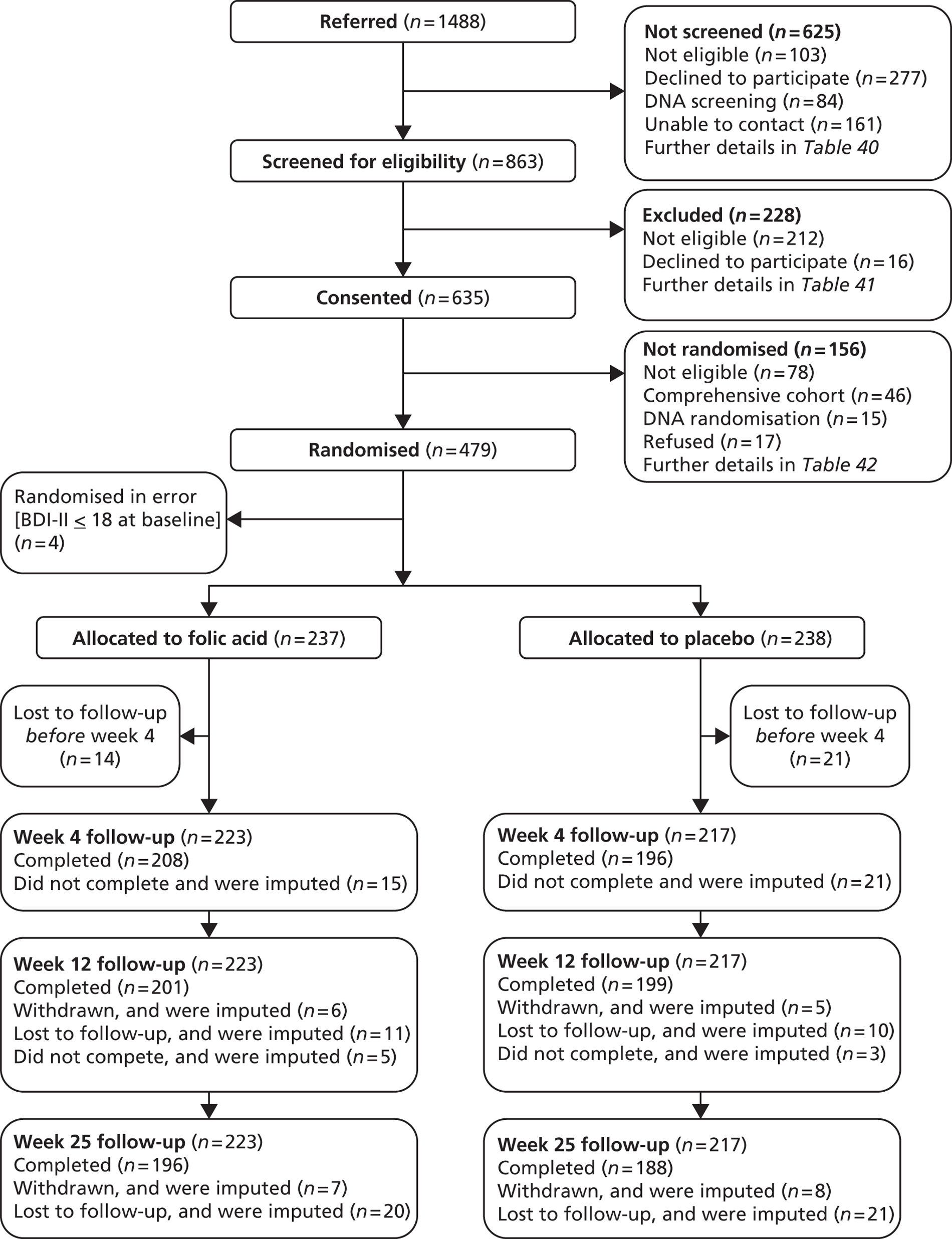
At screening to assess eligibility for the trial, the primary reason for exclusion was that people did not meet the trial specified criteria for moderate to severe depression (54%). The other criteria that excluded more than 5% of those screened were: presence of malignancy or similar disorder (10%); not currently taking antidepressants (9%); and taking anticonvulsants (7%).
Randomisation interviews took place 2 weeks after screening when blood test results were available to verify eligibility to enter the trial. Of the 635 people who had consented to take part, we could not randomise 156: 68 people dropped out between screening and randomisation and a further 42 at the randomisation interview, of whom 36 scored too low on the BDI-II. Forty-six people entered the comprehensive cohort and 20 who were eligible to do so declined. Table 3 cross-tabulates reasons for losses by stage of recruitment and Appendix 7 does so by centre.
| Reason for not randomising | Between referral and screening | Screening | Randomisation | Total |
|---|---|---|---|---|
| Pre-specified exclusion criteria | ||||
| Are under 18 years | 4 | 0 | 0 | 4 |
| Not depressed by ICD – 10 criteria | 0 | 122 | 36 | 158 |
| Folate deficient | 0 | 1 | 15 | 16 |
| B12 deficient | 2 | 0 | 8 | 10 |
| Have taken folate supplementation | 14 | 8 | 2 | 24 |
| Suffered from psychosis | 3 | 2 | 0 | 5 |
| Bipolar disorder | 2 | 4 | 0 | 6 |
| Are already in another research trial | 2 | 0 | 0 | 2 |
| Are pregnant or planning to be so | 9 | 0 | 0 | 9 |
| Taking anticonvulsants | 5 | 16 | 1 | 22 |
| Serious, advanced or terminal illness | 0 | 0 | 0 | 0 |
| Treatment for a medical condition not yet stabilised | 1 | 2 | 0 | 3 |
| Taking lithium | 1 | 0 | 0 | 1 |
| Have had diagnosis of malignant disease | 19 | 22 | 2 | 43 |
| Subtotal | 62 | 177 | 64 | 303 |
| Other exclusions | ||||
| Not on antidepressants | 36 | 21 | 9 | 66 |
| Other | 5 | 14 | 5 | 24 |
| Subtotal | 41 | 35 | 14 | 90 |
| Refusal | 277 | 16 | 17 | 310 |
| Did not attend appointment | 84 | 0 | 15 | 99 |
| Could not contact | 161 | 0 | 0 | 161 |
| Subtotal | 522 | 16 | 32 | 570 |
| Comprehensive cohort | 0 | 0 | 46 | 46 |
| Total | 625 | 228 | 156 | 1009 |
Centre differences in recruitment patterns
North West Wales received 47% of the referrals to the trial and randomised 50% of the final sample. North East Wales and Swansea received and randomised very similar proportions of the total – 27% and 26% respectively of referrals received and 25% of the randomised sample each). Table 4 summarises these flows by centre.
| Reason for not randomising | North East Wales | North West Wales | Swansea | Total |
|---|---|---|---|---|
| Number referred | 400 | 698 | 390 | 1488 |
| Trial exclusion criteria | 15 | 31 | 16 | 62 |
| Other exclusions | 15 | 17 | 9 | 41 |
| Refusal | 91 | 128 | 58 | 227 |
| Did not attend | 21 | 29 | 34 | 84 |
| Could not contact | 26 | 75 | 60 | 161 |
| Number screened | 232 | 418 | 213 | 863 |
| Trial exclusion criteria | 57 | 75 | 45 | 177 |
| Other exclusions | 7 | 25 | 3 | 35 |
| Refusal | 4 | 12 | 0 | 16 |
| Other loss | 0 | 0 | 0 | 0 |
| Number consented | 164 | 306 | 165 | 635 |
| Trial exclusion criteria | 6 | 42 | 16 | 64 |
| Other exclusions | 1 | 9 | 4 | 14 |
| Refusal | 9 | 4 | 4 | 17 |
| Did not attend | 3 | 6 | 6 | 15 |
| To comprehensive cohort | 24 | 7 | 15 | 46 |
| Number randomised | 121 | 238 | 120 | 479 |
Loss to follow-up
We randomised 475 participants (excluding four randomised in error): 237 to receive folic acid and 238 to receive placebo. In the folic acid group 15 people withdrew and 26 were lost to follow-up by 25 weeks. In the placebo group 18 people withdrew and 32 were lost to follow-up by 25 weeks. Table 5 shows the reasons for loss at each stage.
| Type of drop-out | Reason for drop-out | Folate | Placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 weeks | 12 weeks | 25 weeks | Total | 4 weeks | 12 weeks | 25 weeks | Total | ||
| Withdrawals | Too many personal commitments | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 2 |
| Felt better | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | |
| Stopped taking antidepressants | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | |
| Pregnant | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| No reason given or did not want to take part | 3 | 4 | 0 | 7 | 3 | 0 | 0 | 3 | |
| Withdrew owing to adverse side effects | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Had an abnormal electrocardiogram | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Participant elderly and too ill to take part | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Unable to contact because moved out of area | 1 | 0 | 0 | 1 | 0 | 4 | 3 | 7 | |
| Newly diagnosed with bipolar disorder | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Started folic acid supplements – asked to withdraw | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |
| New diagnosis of multiple sclerosis | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Subtotal | 8 | 6 | 1 | 15 | 10 | 5 | 3 | 18 | |
| Lost to follow-up | Did not attend any further follow-up appointments | 6 | 11 | 9 | 26 | 11 | 10 | 11 | 32 |
| DNAd but attended at least one further follow-up | 15 | 5 | 0 | 20 | 21 | 3 | 0 | 24 | |
| Total | 29 | 22 | 10 | 61 | 42 | 18 | 14 | 74 | |
Participant drop-out and missing data
There were no follow-up data for 35 randomised participants; 18 withdrew before the 4-week follow-up (8 folic acid group, 10 placebo group) and 17 did not attend any appointments (6 folic acid group, 11 placebo group). Thus 14 dropped out of the folic acid group and 21 out of the placebo group. We removed these from further analysis. Therefore 440 entered the main analysis, 223 from the folic acid group and 217 from the placebo group. If these evaluable participants missed follow-up appointments, we imputed their data in accordance with Chapter 2, Statistical methods, Trial populations, above (Table 6). However we imputed no baseline measures.
| Follow-up | Folate (n = 223) | Placebo (n = 217) | ||
|---|---|---|---|---|
| Completed | Imputed | Completed | Imputed | |
| 4 weeks | 208 | 15 | 196 | 21 |
| 12 weeks | 201 | 22 | 199 | 18 |
| 25 weeks | 196 | 27 | 188 | 29 |
Of the 440 evaluable participants 36 (8%) missed follow-up at 4 weeks, 40 (9%) at 12 weeks, and 56 (13%) at 25 weeks. Thus 10% of follow-up assessments were missing. Sixty-two participants missed one assessment: 33 at 4 weeks, 6 at 12 weeks and 23 at 25 weeks. Thirty-five participants missed two assessments: 2 at 4 and 12 weeks; 1 at 4 and 25 weeks; and 32 at 12 and 25 weeks. Thus 343 (78%) participants undertook all three assessments.
For the eight main outcome measures (BDI-II, MADRS, CGI, SF-12, EQ-5D, EQ-VAS, MINI and Morisky) a full data set over the four times would have comprised 102,520 data items. Only 2572 (2.5%) were missing, of which 2476 (2.4%) items were in missing subscales or times while 96 (0.1%) items were isolated missing values within otherwise complete subscales. Reassuringly there was no hint of significant differences between trial groups in either respect.
The missing item rate for seven of these measures is fairly consistent: BDI-II, EQ-5D, EQ-VAS, and MINI all 2.3%; MADRS 2.4%; SF-12 3.2% and CGI 3.3%. In contrast the Morisky data had a missing item rate of 10%, not explained by being collected only at 12 weeks. Predictably the pattern of missing data differed very significantly between centres: North West Wales, the best recruiting centre, missed more 4-week data than other centres, but fewer at 25 weeks. This pattern was due to heavy workload early in the trial when several 4-week appointments were missed; fortunately routine monitoring recognised and rectified the problem. Reassuringly there was no significant difference between centres in the proportion followed up.
Validation of stratification variables
The trial used five variables to balance allocation between treatment groups during dynamic randomisation: centre; sex; antidepressant type; whether the participant was new or continuing on treatment; and whether the participant had ever received counselling for depression. Not surprisingly given the speed of the recruitment process, data validation identified a few inconsistencies in these data. However we found no misclassification of centre, sex or counselling, and minimal misclassification of antidepressant type or new patient (Table 7).
| Stratification variable or category | Recorded at randomisation, no. (%) | Amended following validation, no. (%) | ||
|---|---|---|---|---|
| Folate | Placebo | Folate | Placebo | |
| Type of antidepressant | ||||
| SSRI | 157 (70) | 145 (67) | 155 (70) | 143 (66) |
| Other | 66 (30) | 72 (33) | 68 (30) | 74 (34) |
| Previous treatment? | ||||
| No – new ADM | 56 (25) | 52 (24) | 45 (20) | 42 (19) |
| Yes – continuing ADM | 167 (75) | 165 (76) | 178 (80) | 175 (81) |
| Previous counselling? | ||||
| Yes | 101 (45) | 97 (45) | No change | |
| No | 122 (55) | 120 (55) | ||
| Gender | ||||
| Male | 79 (35) | 81 (37) | No change | |
| Female | 144 (65) | 136 (63) | ||
| Centre | ||||
| North East Wales | 57 (26) | 53 (24) | ||
| North West Wales | 110 (49) | 113 (52) | No change | |
| Swansea | 56 (25) | 51 (24) | ||
Baseline characteristics of participants
Table 8 compares the baseline characteristics of those included in the trial analysis with those excluded for lack of follow-up data. Those who dropped out were significantly younger, less likely to have a current partner and more likely to exceed safe limits of alcohol consumption, and had a higher mean BDI-II score. All of these are consistent with these participants having worse depression. However the systematic exclusion of these 35 (7.4%) consented participants from 475 created little risk of bias since they were equally distributed across the arms of the trial. Nevertheless this may have removed those who could have benefited most from the intervention.
| Characteristic | Included (n = 440) | Excluded (n = 35) | Significance test |
|---|---|---|---|
| Age | |||
| Range | 19–81 | 20–66 | |
| Mean (SD) | 45 (13) | 39 (14) | |
| Median (IQR) | 46 (36 to 54) | 40 (26 to 47) | U = 6200, p = 0.005 |
| Gender, no. (%) | |||
| Male | 160 (36) | 11 (31) | χ2 = 0.34, df = 1, p = 0.56 |
| Female | 280 (64) | 24 (69) | |
| Ethnicity, no. (%) | |||
| White | 427 (97) | 34 (97) | Fisher’s: p = 0.46 |
| Other | 5 (1) | 1 (3) | |
| Not stated | 8 (2) | 0 (0) | |
| Marital status, no. (%) | |||
| Single | 109 (25) | 13 (37) | χ2 = 7.0, df = 2, p = 0.031 |
| Had a partner | 91 (21) | 11 (31.5) | |
| Have a partner | 240 (54) | 11 (31.5) | |
| Number of dependent children, no. (%) | |||
| 0 | 269 (61) | 22 (63) | Fisher’s: p = 1 |
| 1 | 70 (16) | 5 (14) | |
| 2 | 61 (14) | 5 (14) | |
| 3 or more | 40 (9) | 3 (9) | |
| Employment, no. (%) | |||
| Full time employed | 121 (28) | 11 (31) | χ2 = 1.1, df = 2, p = 0.58 |
| Part time or in education | 124 (28) | 7 (20) | |
| Smoking status, no. (%) | |||
| Inactive | 195 (44) | 17 (49) | χ2 = 0.093, df = 2, p = 0.96 |
| Smoker | 162 (37) | 14 (40) | |
| Non smoker | 194 (44) | 15 (43) | |
| Ex-smoker | 77 (17) | 6 (17) | |
| Not stated | 7 (2) | ||
| Alcohol consumption per week, no. (%) | |||
| None | 177 (40) | 10 (29) | χ2 = 7.1, df = 2, p = 0.029 |
| Within safe limita | 215 (49) | 16 (45) | |
| Above safe limita | 48 (11) | 9 (26) | |
| Centre, no. (%) | |||
| Bangor | 223 (51) | 15 (43) | χ2 = 1.1, df = 2, p = 0.59 |
| Wrexham | 110 (25) | 9 (26) | |
| Swansea | 107 (24) | 11 (31) | |
| Baseline BDI-II score | |||
| Range | 17–61 | 18–57 | |
| Mean (SD) | 34 (10) | 39 (11) | t = –2.793, df = 461, p = 0.005 |
| Median (IQR) | 33 (26 to 41) | 39 (31 to 48) | |
| Missing | 10 | 2 | |
| Group allocated | |||
| Folate | 223 | 14 | χ2 = 1.5, df = 1, p = 0.22 |
| Placebo | 217 | 21 | |
Differences between centres at baseline
The management of the FolATED trial included a monthly telephone conference between the three clinical centres and NWORTH, the coordinating Clinical Trials Unit. These conferences soon identified substantial differences between centres, notably in psychiatric practice and recruitment policy. Judging that these differences would enhance the generalisability of the trial provided the conduct of the research was consistent across centres, we pursued such consistency, notably by maintaining a rigorous fieldwork handbook and arranging regular inter-centre training.
Table 9 shows that participants differed significantly between centres, notably in:
-
Mean age Those in North West Wales were on average more than 3 years younger than those in the other centres.
-
Numbers of dependent children Half of those in North West Wales had children, but only 30% of those elsewhere.
-
Employment status Swansea had many more students, while North East Wales had more unemployed.
-
Smoking rates Nearly half of those in Swansea smoked, but only 30% of those elsewhere.
| Characteristic | North East Wales (n = 110) | North West Wales (n = 223) | Swansea (n = 107) | Total (n = 440) |
|---|---|---|---|---|
| Age | ||||
| Range | 19–75 | 19–81 | 19–75 | 19–81 |
| Mean (SD) | 46 (11) | 44 (12) | 47 (14) | 45 (13) |
| Gender, no. (%) | ||||
| Male | 30 (27) | 90 (40) | 40 (37) | 160 (36) |
| Female | 80 (73) | 133 (60) | 67 (63) | 280 (64) |
| Ethnicity, no. (%) | ||||
| White | 107 (97) | 220 (99) | 100 (94) | 427 (97) |
| Other | 1 (1) | 2 (1) | 2 (2) | 5 (1) |
| Not stated/missing | 2 (2) | 1 (0) | 5 (4) | 8 (2) |
| Marital status, no. (%) | ||||
| Single | 18 (16) | 62 (28) | 29 (27) | 109 (25) |
| Had a partner | 27 (25) | 40 (18) | 24 (22) | 91 (21) |
| Have a partner | 65 (59) | 121 (54) | 54 (51) | 240 (54) |
| Number of dependent children, no. (%) | ||||
| 0 | 72 (65) | 117 (52) | 80 (75) | 269 (61) |
| 1 | 15 (14) | 40 (18) | 15 (14) | 70 (16) |
| 2 | 18 (16) | 37 (17) | 6 (5.5) | 61 (14) |
| 3 or more | 5 (5) | 29 (13) | 6 (5.5) | 40 (9) |
| Employment status,a no. (%) | ||||
| Full time employed | 38 (35) | 59 (26) | 24 (22) | 121 (28) |
| Part time or in education | 31 (28) | 48 (22) | 45 (42) | 124 (28) |
| Inactive | 41 (37) | 116 (52) | 38 (36) | 195 (44) |
| Smoking status, no. (%) | ||||
| Smoker | 27 (24) | 97 (44) | 38 (35) | 162 (37) |
| Non smoker | 57 (52) | 94 (42) | 50 (47) | 201 (46) |
| Ex-smoker | 26 (24) | 32 (14) | 19 (18) | 77 (17) |
| BDI-II | ||||
| Mean (SD) | 33.7 (9.3) | 34.3 (9.2) | 33.1 (10.8) | 33.7 (9.6) |
| Range | 18–61 | 19–58 | 17–60 | 17–61 |
However there was no significant difference in mean BDI-II scores across centres; or in alcohol consumption.
Another difference between centres identified by our monthly management conferences is that one centre did more to optimise medication than the other two, notably by using reboxetine (Edronax®, Pfizer) to augment basic ADM. Though we plan to analyse the process and outcome of optimisation in detail, we have confirmed that this reboxetine augmentation did not differ between allocated groups; hence there was no danger of bias from differential optimisation.
Baseline demographic profile
Predictably our randomisation algorithm generated similar treatment groups (Tables 10 and 11).
| Characteristic | Folate (n = 223) | Placebo (n = 217) |
|---|---|---|
| Age | ||
| Range | 19–81 | 20–75 |
| Mean (SD) | 45 (14) | 45 (12) |
| Median (IQR) | 47 (35 to 55) | 45 (36 to 53) |
| Gender, no. (%) | ||
| Male | 79 (35) | 81 (37) |
| Female | 144 (65) | 136 (63) |
| Ethnicity, no. (%) | ||
| White | 215 (97) | 212 (98) |
| Other | 5 (2) | 0 (0) |
| Not stated | 3 (1) | 5 (2) |
| Marital status, no. (%) | ||
| Single | 60 (27) | 49 (23) |
| Had a partner | 124 (56) | 116 (53) |
| Have a partner | 39 (17) | 52 (24) |
| Number of dependent children, no. (%) | ||
| 0 | 146 (66) | 123 (56) |
| 1 | 29 (13) | 41 (19) |
| 2 | 27 (12) | 34 (16) |
| 3 or more | 21 (9) | 19 (9) |
| Employment status, no. (%) | ||
| Full time employment | 49 (22) | 72 (33) |
| Part time employment or education | 69 (31) | 55 (25) |
| Inactive | 105 (47) | 90 (42) |
| Smoking status, no. (%) | ||
| Smoker | 82 (37) | 80 (37) |
| Non smoker | 98 (44) | 103 (47) |
| Ex-smoker | 43 (19) | 34 (16) |
| Smoking consumption, no. (%) | ||
| Non-smoker | 141 (63) | 137 (63) |
| Low (≤ 10) | 30 (14) | 34 (16) |
| Medium (between 10 and 20) | 43 (19) | 32 (15) |
| High (≥ 20) | 9 (4) | 14 (6) |
| Alcohol consumption per week, no. (%) | ||
| None | 86 (39) | 82 (38) |
| Below safe limit | 106 (47) | 101 (46) |
| Above safe limit | 31 (14) | 34 (16) |
Baseline clinical profile
| Measure or scale | Folate (n = 223) | Placebo (n = 217) | |
|---|---|---|---|
| Symptom severity instruments | |||
| BDI-II | Range | 17 to 60 | 17 to 61 |
| Mean (SD) | 33 (9) | 34 (10) | |
| MADRS | Range | 1 to 50 | 13 to 53 |
| Mean (SD) | 28 (7) | 29 (7) | |
| CGI: Severity of illness, no. (%) | Normal to mild | 3 (1) | 0 (0) |
| Mild to moderate | 148 (67) | 147 (68) | |
| Moderate to severe | 72 (32) | 70 (32) | |
| Health status and utility | |||
| EQ-5D | Range | –0.2 to 1.0 | –0.3 to 1.0 |
| Mean (SD) | 0.48 (0.30) | 0.51 (0.30) | |
| EQ-VAS | Range | 0 to 100 | 0 to 95 |
| Mean (SD) | 45 (20) | 44 (20) | |
| SF-12: Physical component scale | Range | 17 to 69 | 17 to 71 |
| Mean (SD) | 44 (12) | 44 (13) | |
| SF-12: Mental component scale | Range | 4 to 50 | –1 to 57 |
| Mean (SD) | 26 (9) | 26 (10) | |
| Biochemistry | |||
| Serum folate level | Range | 2 to 20 | 2 to 20 |
| Mean (SD) | 7.1 (4.2) | 7.2 (4.2) | |
| B12 level | Range | 142 to 1019 | 150 to 928 |
| Median (IQR) | 300 (228 to 391) | 306 (248 to 402) | |
Clinical effectiveness results
Is folic acid clinically effective?
Primary clinical effectiveness outcomes
The primary clinical effectiveness outcome measure was self-rated symptom severity as measured by BDI-II scores over 25 weeks, as summarised by the AUC of mean BDI-II scores from randomisation till 25 weeks. This provided no evidence that folic acid was effective (Figure 3). The adjusted difference in AUC between folic acid and placebo was 1.09 (95% CI from –0.48 to 2.66; p = 0.17). The adjusted difference between folic acid and placebo at 25 weeks was 1.27 (95% CI from –0.99 to 3.54; p = 0.27). Similarly there was no significant difference in the proportion of patients who were depressed at 25 weeks (at least moderately, i.e. BDI-II ≥ 19): there were 126/223 (57%) depressed participants in the folate group and 118/217 (54%) in the placebo group. The adjusted OR of being depressed in the folate group compared with placebo group was 1.09 (95% CI from 0.75 to 1.59; p = 0.65).
FIGURE 3.
Estimated mean BDI-II scores over time adjusted for baseline score and stratification variables – by treatment allocated. Baseline score = observed population mean BDI-II score.
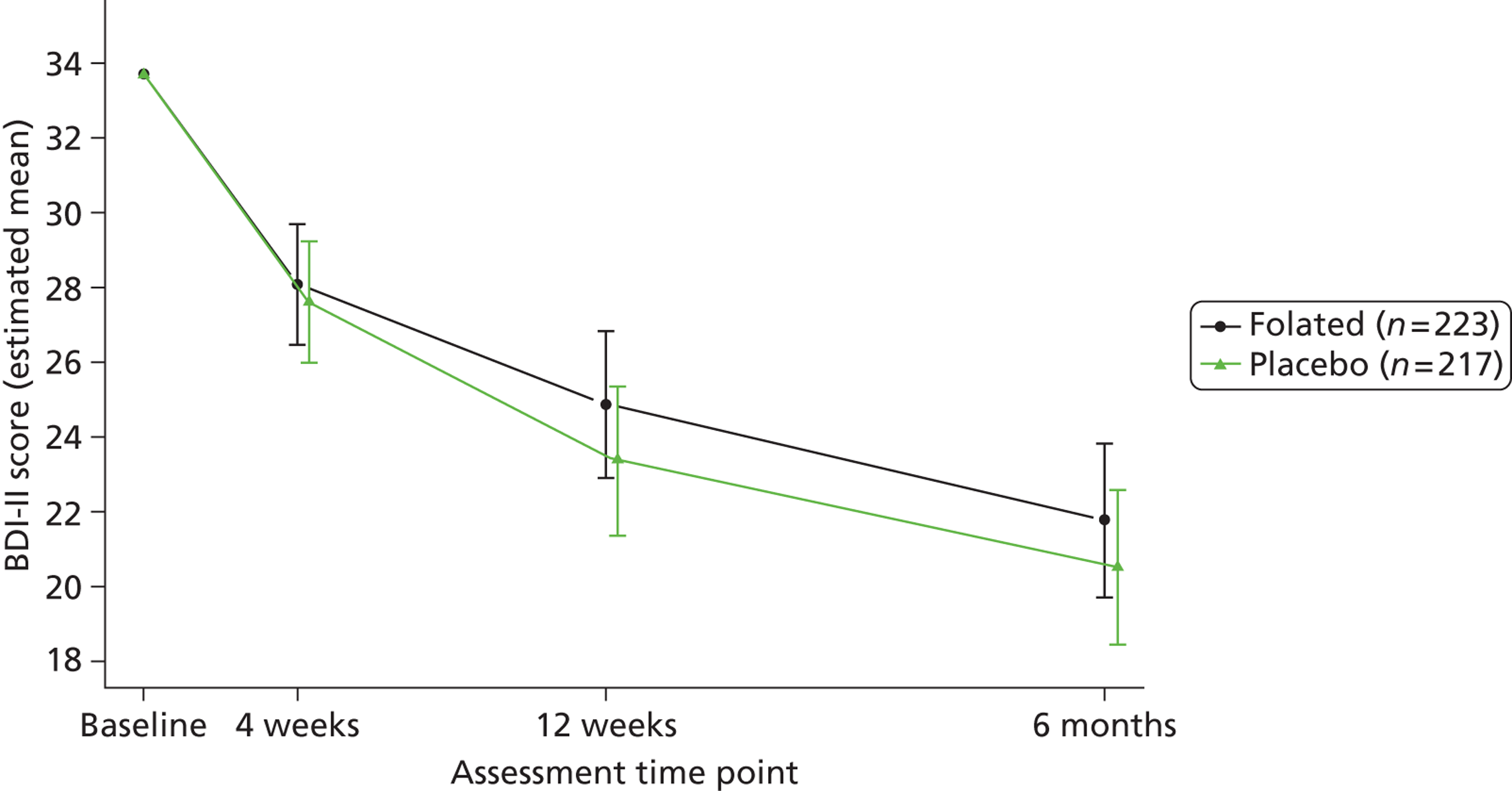
Secondary clinical effectiveness outcomes
Table 12 shows the unadjusted AUC results for all the main outcome measures, together with two-sample t-tests. The only significant result favoured the placebo in the SF-12 mental component. By convention generic outcome measures like EQ-5D and SF-12 show good health by high scores, while condition-specific outcome measures like BDI-II, CGI and MADRS show good health by scores that are low, if not zero. Thus five of the non-significant differences favoured placebo and seven favoured folate. Table 42 of Appendix 10 elaborates on Table 12 by showing the results of unadjusted two-sample t-tests for each variable at each time point separately.
| Outcome variable | Folate | Placebo | Difference (folate minus placebo) | ||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SE) | 95% CI | Significance | |
| BDI-II | 25.70 (10.83) | 25.64 (11.43) | 0.06 (1.06) | −2.02 to 2.15 | 0.953 |
| MADRS | 22.17 (7.59) | 22.39 (8.34) | −0.22 (0.76) | −1.71 to 1.27 | 0.771 |
| Euroqol | |||||
| EQ-5D | 0.573 (0.259) | 0.591 (0.262) | −0.018 (0.025) | −0.067 to 0.031 | 0.476 |
| EQ-VAS | 54.58 (18.38) | 54.34 (17.85) | 0.25 (1.73) | −3.15 to 3.64 | 0.887 |
| SF-12 | |||||
| SF-12 PCS | 44.89 (11.13) | 44.28 (11.48) | 0.61 (1.08) | −1.51 to 2.73 | 0.571 |
| SF-12 MCS * | 31.99 (9.34) | 34.09 (9.01) | −2.09 (0.88) | −3.81 to −0.37 | 0.017 |
| CGI | |||||
| CGI: Severity | 3.60 (0.87) | 3.61 (0.94) | −0.00 (0.09) | −0.17 to 0.17 | 0.980 |
| CGI: Improvement | 3.11 (0.86) | 3.09 (0.95) | 0.02 (0.09) | −0.15 to 0.19 | 0.794 |
| CGI: Efficacya | 0.29 (0.49) | 0.30 (0.50) | −0.01 (0.05) | −0.10 to 0.08 | 0.826 |
| Estimated CGI | 1.34b (–) | 1.35b (–) | 0.99c (–) | 0.90 to 1.08c | |
| UKU | |||||
| UKU: Psychic | 8.21 (3.86) | 8.16 (4.01) | 0.05 (0.38) | −0.68 to 0.79 | 0.894 |
| UKU: Neurologicd | 0.73 (0.67) | 0.79 (0.71) | −0.06 (0.07) | −0.19 to 0.07 | 0.371 |
| Estimated UKU | 0.53e (–) | 0.62e (–) | (–) (–) | (–) | |
| UKU: Autonomic | 2.93 (2.22) | 2.85 (2.51) | 0.08 (0.23) | −0.37 to 0.52 | 0.723 |
| UKU: Other | 3.62 (2.68) | 4.06 (3.11) | −0.44 (0.28) | −0.98 to 0.11 | 0.114 |
Area under the curve analysis adjusted for stratification variables and baseline score of the variable in question gave a very similar picture. Table 13 shows these results and reports all significant stratification and baseline variables. For most variables the baseline score and antidepressant type were significant covariates, but allocated treatment was not. As for the unadjusted AUCs the only outcome on which the allocated treatment had a statistically significant effect was the SF-12 mental component, again favouring the placebo.
| Outcome variable | Difference (folate minus placebo) | Significant covariates | Significance | ||
|---|---|---|---|---|---|
| Mean (SE) | 95% CI | Significance | |||
| BDI-II | 1.09 (0.86) | –0.48 to 2.66 | 0.173 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.028 | ||||
| Centre | 0.025 | ||||
| MADRS | 0.71 (0.62) | –0.51 to 1.93 | 0.252 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.002 | ||||
| Centre | 0.026 | ||||
| EQ-5D | 0.00 (0.017) | –0.034 to 0.033 | 0.982 | Baseline scores | < 0.001 |
| EQ-VAS | –0.48 (1.36) | –3.16 to 2.20 | 0.726 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.001 | ||||
| SF-12 PCS | 0.40 (0.60) | –0.78 to 1.59 | 0.501 | Baseline scores | < 0.001 |
| Previous treatment | 0.002 | ||||
| SF-12 MCS * | –1.97 (0.78) | –3.49 to –0.44 | 0.012 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.001 | ||||
| CGI: Severity | 0.05 (0.08) | –0.11 to 0.2 | 0.553 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.001 | ||||
| Centre | < 0.001 | ||||
| CGI: Improvement | 0.04 (0.08) | –0.13 to 0.21 | 0.649 | Type of antidepressant | 0.002 |
| Centre | 0.009 | ||||
| CGI: Efficacya | 0.98b (0.04) | –0.89 to 1.07 | 0.604 | Type of antidepressant | < 0.001 |
| Centre | < 0.001 | ||||
| UKU: Psychic | 0.31 (0.31) | –0.3 to 0.92 | 0.319 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.001 | ||||
| Centre | 0.029 | ||||
| UKU: Neurologic | –0.01 (0.04) | –0.10 to 0.07 | 0.738 | Baseline scores | < 0.001 |
| Centre | 0.008 | ||||
| UKU: Autonomic | –0.11 (0.15) | –0.42 to 0.19 | 0.458 | Baseline scores | < 0.001 |
| UKU: Other | –0.38 (0.20) | –0.78 to 0.02 | 0.065 | Baseline scores | < 0.001 |
| Centre | 0.002 | ||||
Figures 4–11 display the remaining adjusted analyses, except those relating to the UKU, by individual time points. Though receiving folic acid or placebo does not affect the outcome of treatment in the trial, the pattern of results over time is consistent across measures: ADM achieves major benefit over the first 4 weeks, and continuing though reducing improvement over 25 weeks. In particular Figure 11 shows how the adjusted SF-12 MCSs differ between arms, with the difference favouring placebo: the mean scores diverge by 4 weeks, achieve a substantial gap by 12 weeks, and converge a little by 25 weeks. Table 14 records the statistical analyses underpinning these nine figures, together with the corresponding analyses from the UKU side effects scale.
FIGURE 4.
Estimated mean MADRS scores over time adjusted for baseline score and stratification variables – by treatment allocated. Baseline score = observed population mean MADRS score.
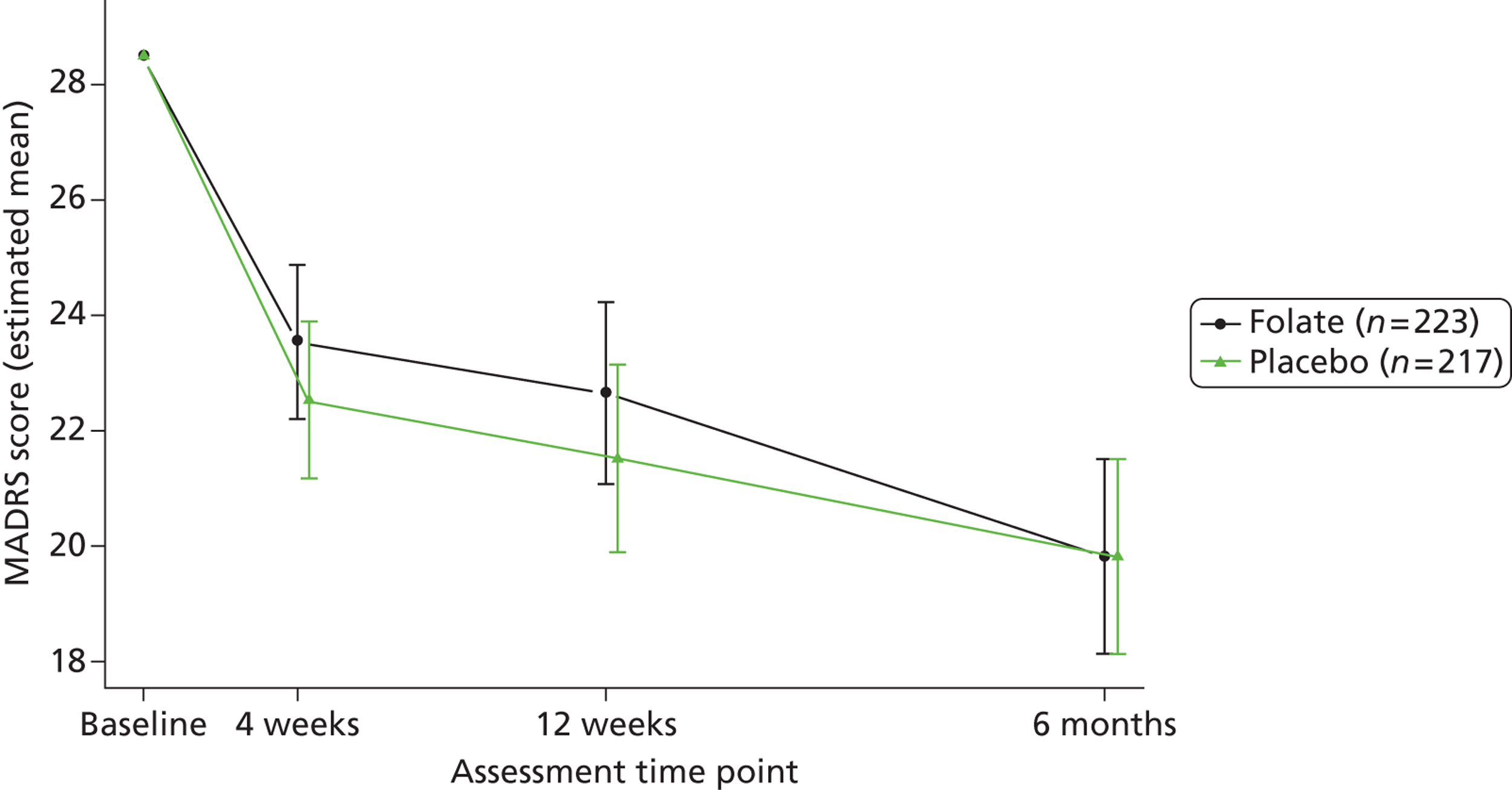
FIGURE 5.
Estimated mean CGI severity scores over time adjusted for baseline score and stratification variables – by treatment allocated. Baseline score = observed population mean CGI severity score.
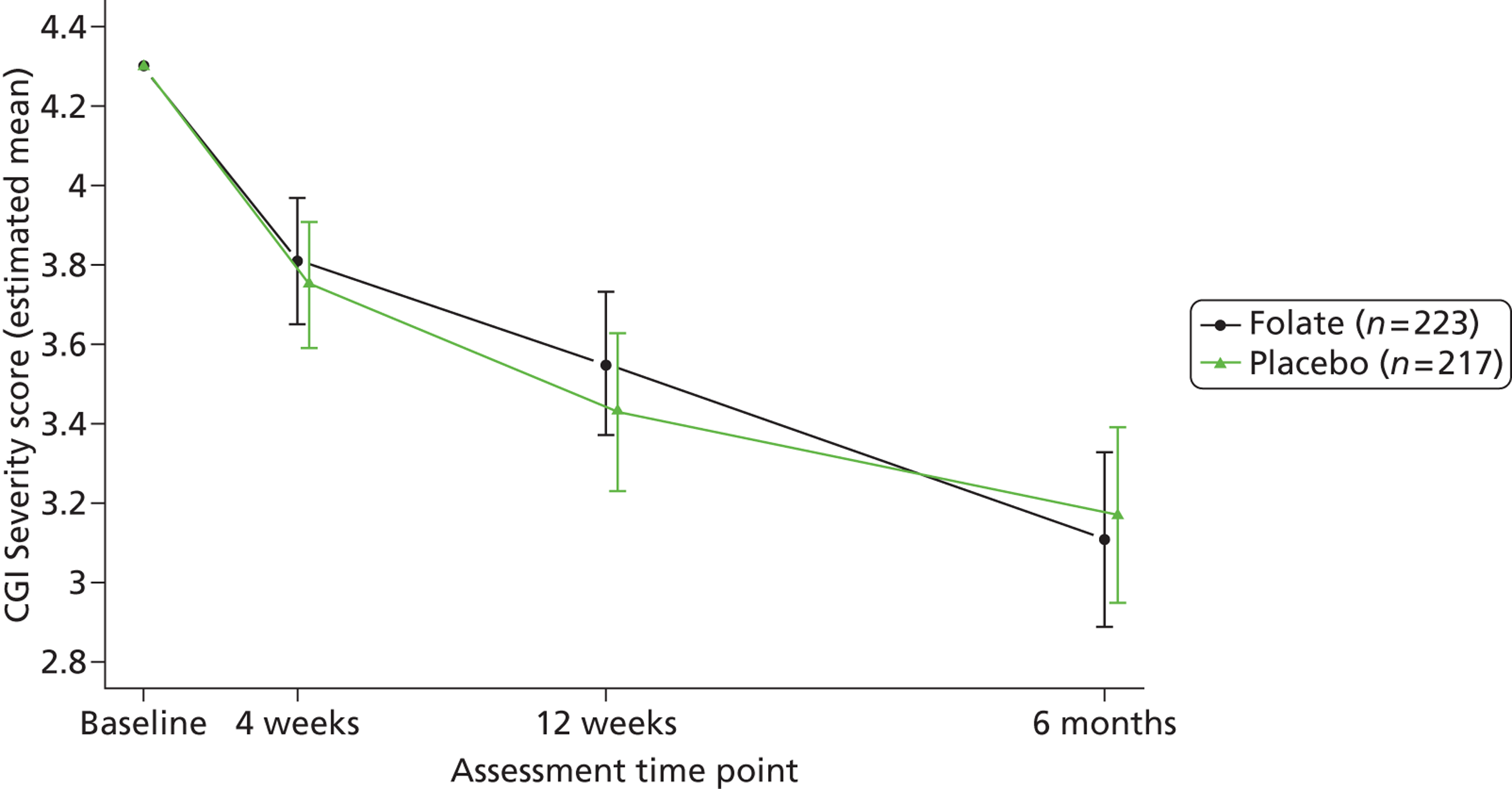
FIGURE 6.
Estimated mean CGI improvement scores at follow up adjusted for stratification variables – by treatment allocated.
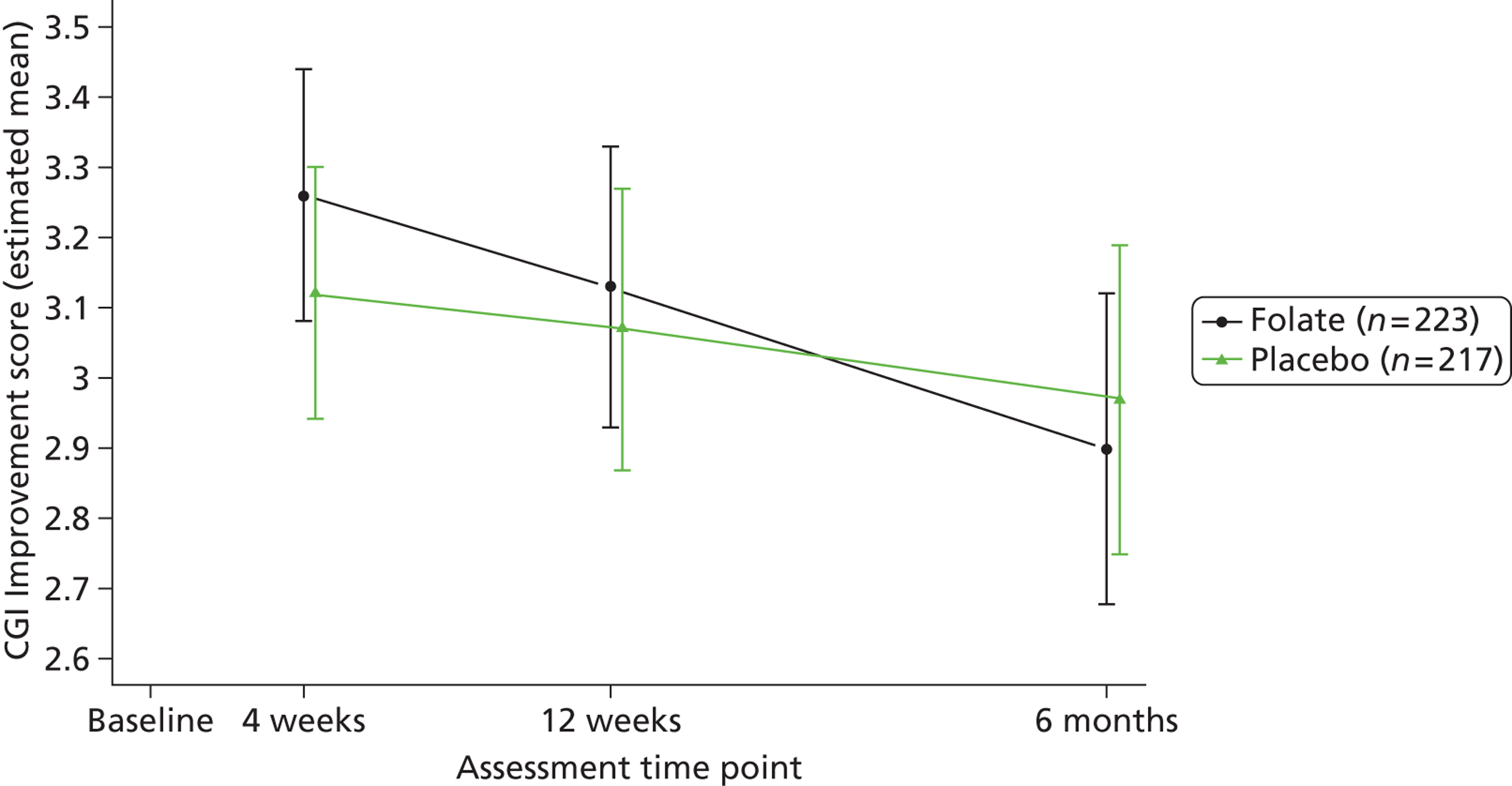
FIGURE 7.
Estimated mean CGI efficacy scores at follow up adjusted for stratification variables – by treatment allocated.
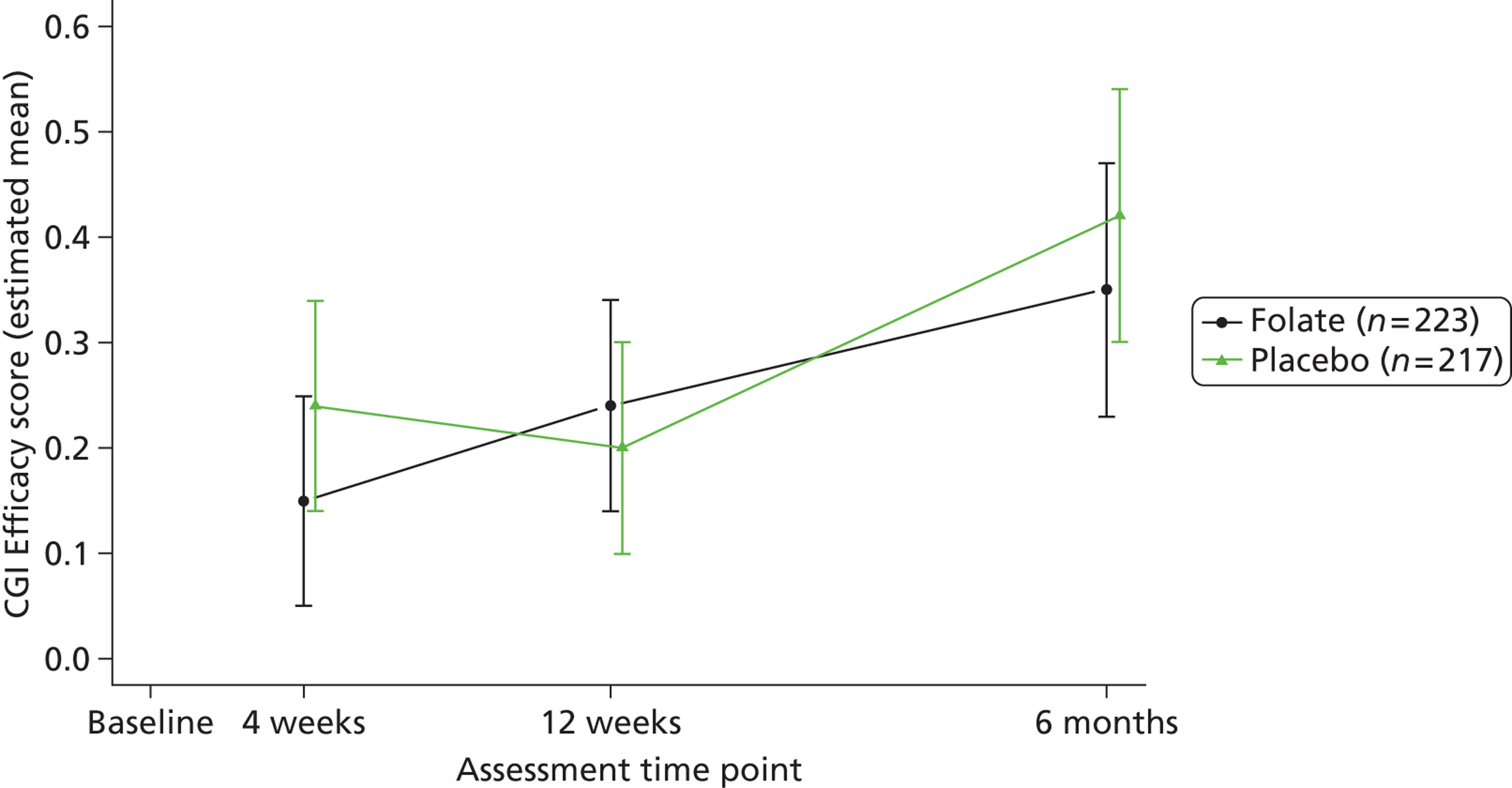
FIGURE 8.
Estimated mean EQ-5D scores over time adjusted for baseline score and stratification variables – by treatment allocated. Baseline score = observed population mean EQ-5D score.
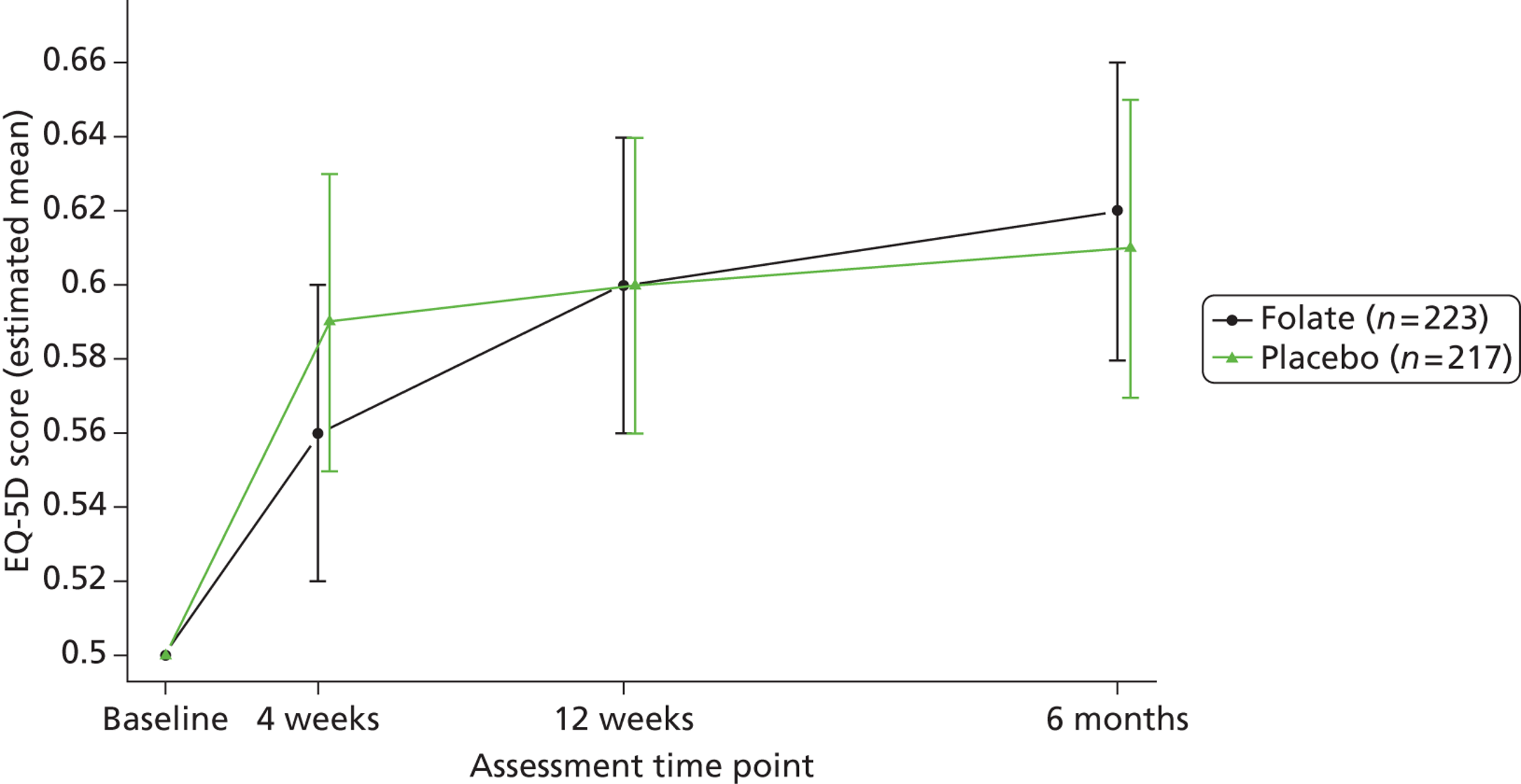
FIGURE 9.
Estimated mean EQ-VAS scores over time adjusted for baseline score and stratification variables – by treatment allocated. Baseline score = observed population mean EQ-VAS score.
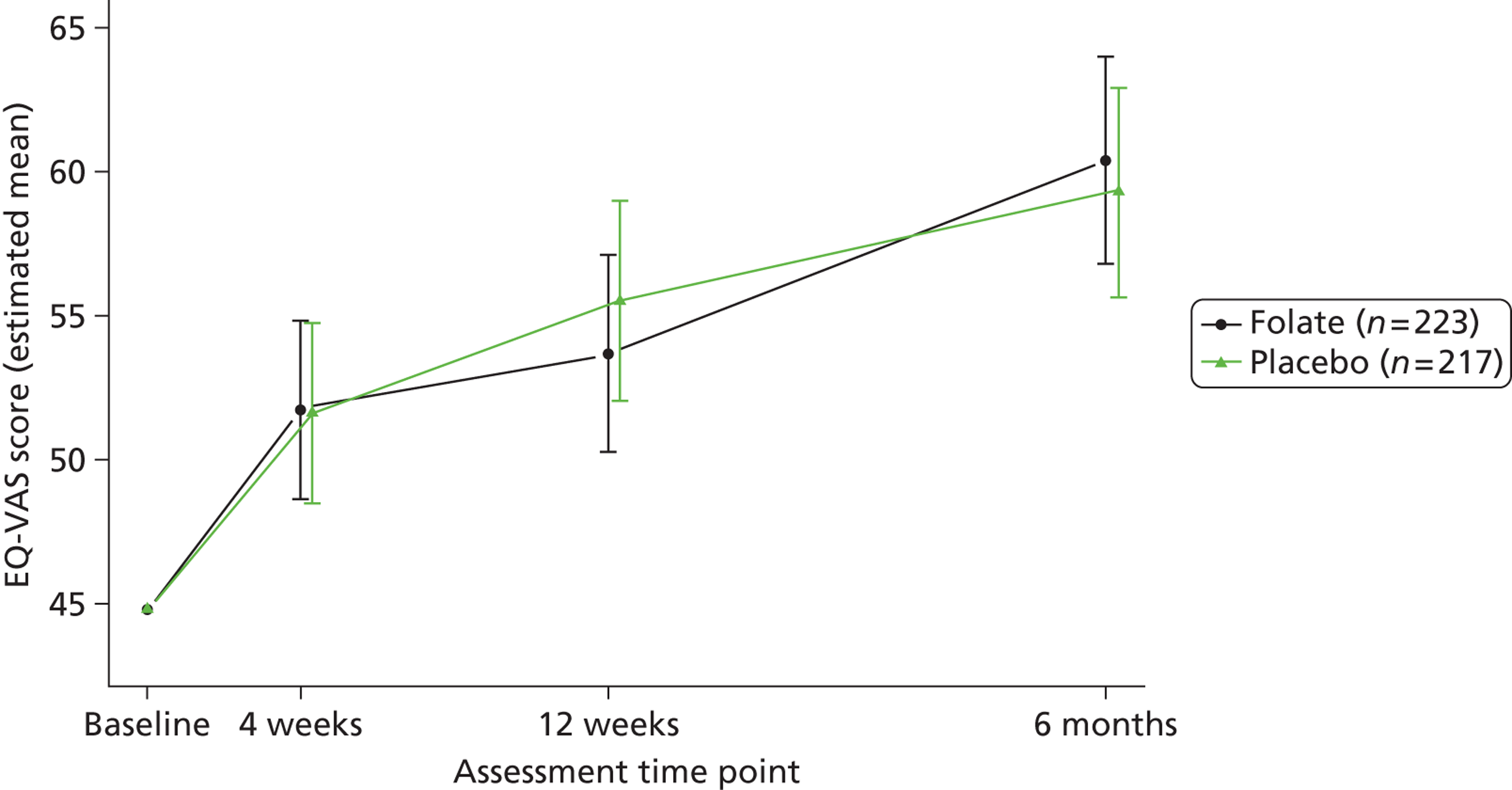
FIGURE 10.
Estimated mean SF-12 Physical Component Scale scores over time adjusted by baseline score and stratification variables – by treatment allocated. Baseline score = observed population mean SF-12 Physical Component Scale score.
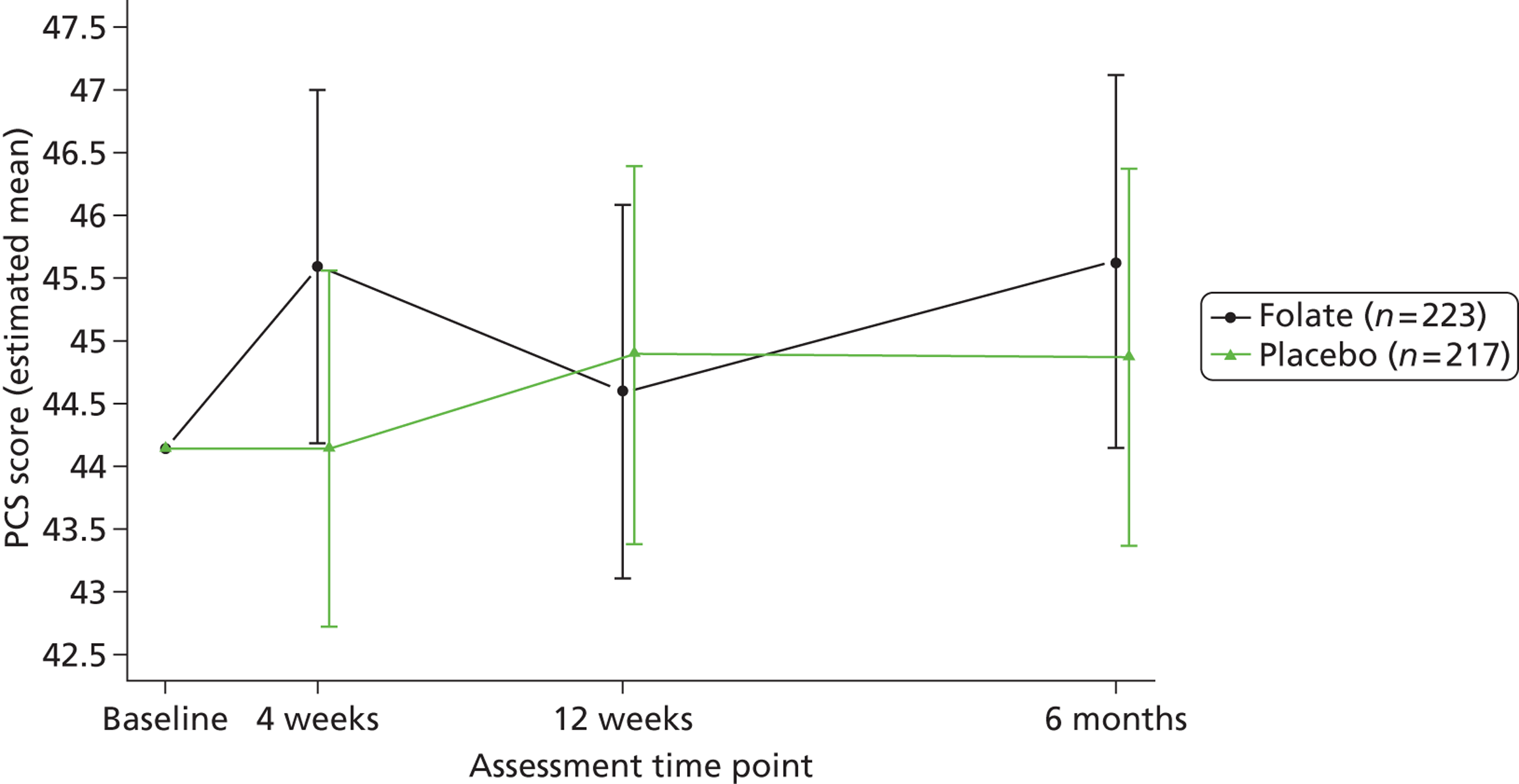
FIGURE 11.
Estimated mean SF-12 Mental Component Scale scores over time adjusted for baseline score and stratification variables – by treatment allocated. Baseline score = observed population mean SF-12 Mental Component Scale score.
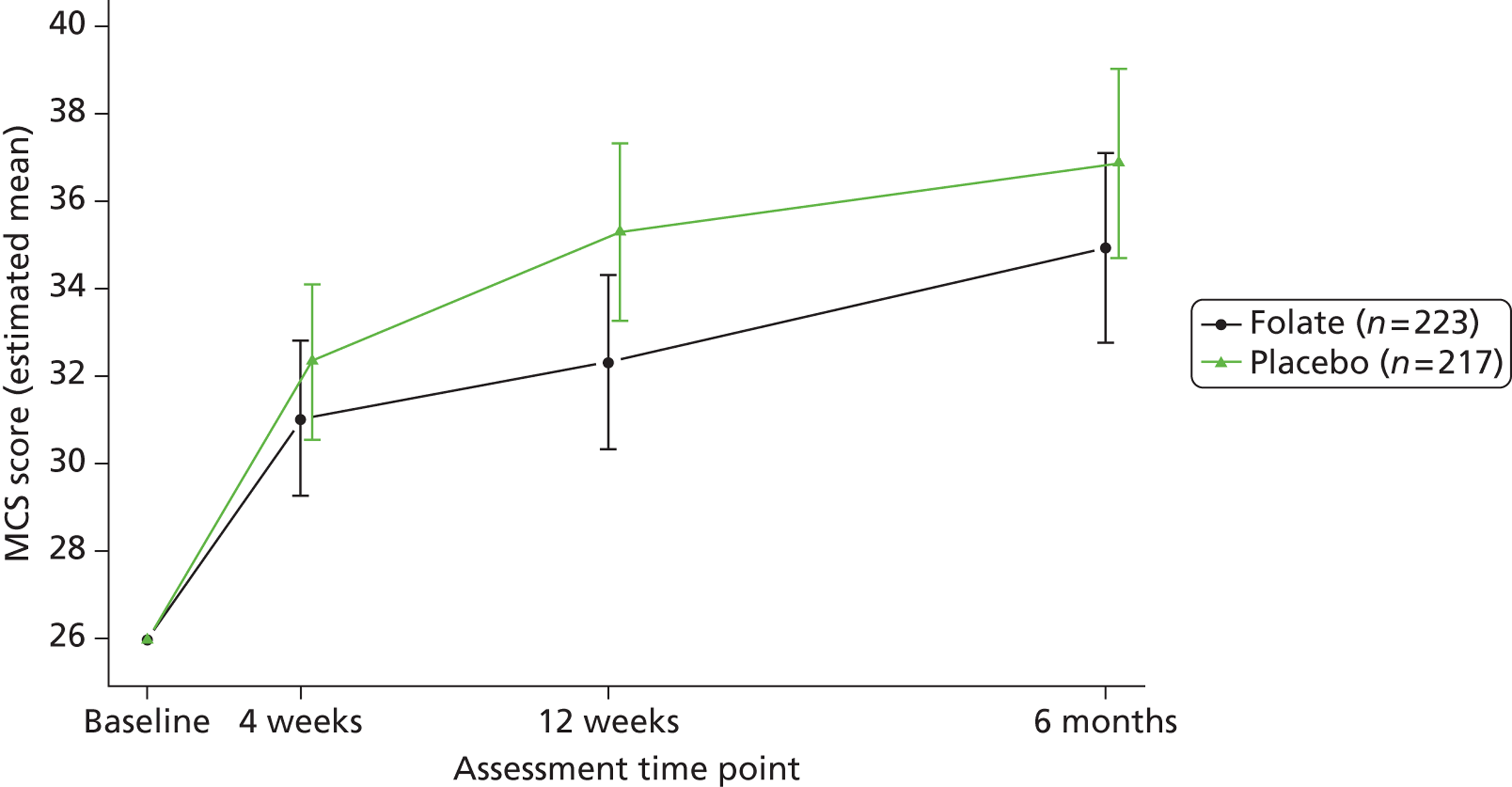
| Outcome variable and time point | Difference (folate minus placebo) | Significant covariates | Significance | ||
|---|---|---|---|---|---|
| Mean (SE) | 95% CI | Significance | |||
| BDI-II | |||||
| BDI-II (4 weeks) | 0.45 (0.91) | –1.34 to 2.23 | 0.623 | Baseline scores | < 0.001 |
| BDI-II (12 weeks) | 1.52 (1.10) | –0.64 to 3.69 | 0.168 | Baseline scores | < 0.001 |
| Centre | 0.013 | ||||
| BDI-II (25 weeks) | 1.27 (1.15) | –0.99 to 3.54 | 0.270 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.004 | ||||
| Centre | 0.011 | ||||
| MADRS | |||||
| MADRS (4 weeks) | 1.02 (0.75) | –0.45 to 2.49 | 0.174 | Baseline scores | < 0.001 |
| MADRS (12 weeks) | 1.14 (0.89) | –0.62 to 2.89 | 0.204 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.012 | ||||
| MADRS (25 weeks) | 0.02 (0.95) | –1.84 to 1.88 | 0.981 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.003 | ||||
| EQ-5D | |||||
| EQ-5D (4 weeks) | –0.028 (0.024) | –0.074 to 0.017 | 0.247 | Baseline scores | < 0.001 |
| Previous treatment | 0.005 | ||||
| EQ-5D (12 weeks) | 0.010 (0.024) | –0.037 to 0.057 | 0.681 | Baseline scores | < 0.001 |
| EQ-5D (25 weeks) | 0.019 (0.024) | –0.027 to 0.065 | 0.450 | Baseline scores | < 0.001 |
| EQ-VAS | |||||
| EQ-VAS (4 weeks) | 0.15 (1.74) | –3.28 to 3.58 | 0.932 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.004 | ||||
| EQ-VAS (12 weeks) | –1.83 (1.90) | –5.57 to 1.91 | 0.337 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.026 | ||||
| EQ-VAS (25 weeks) | 1.11 (2.01) | –2.85 to 5.07 | 0.582 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.002 | ||||
| Previous treatment | 0.018 | ||||
| SF12 PCS | |||||
| SF-12 PCS (4 weeks) | 1.45 (0.78) | –0.08 to 2.98 | 0.064 | Baseline scores | < 0.001 |
| Previous treatment | 0.008 | ||||
| SF-12 PCS (12 weeks) | –0.28 (0.83) | –1.91 to 1.35 | 0.733 | Baseline scores | < 0.001 |
| Previous treatment | 0.019 | ||||
| Centre | 0.063 | ||||
| SF-12 PCS (25 weeks) | 0.76 (0.83) | –0.87 to 2.38 | 0.360 | Baseline scores | < 0.001 |
| Previous counselling | 0.080 | ||||
| Previous treatment | 0.009 | ||||
| SF-12 MCS | |||||
| SF-12 MCS (4 weeks) | –1.28 (0.99) | –3.22 to 0.67 | 0.198 | Baseline scores | < 0.001 |
| SF-12 MCS (12 weeks) * | –2.95 (1.12) | –5.16 to –0.75 | 0.009 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.002 | ||||
| SF-12 MCS (25 weeks) | –1.93 (1.21) | –4.30 to 0.45 | 0.112 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.005 | ||||
| Previous counselling | 0.02 | ||||
| CGI: Severity | |||||
| CGI: Severity (4 weeks) | 0.06 (0.09) | –0.12 to 0.23 | 0.517 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.008 | ||||
| CGI: Severity (12 weeks) | 0.12 (0.11) | –0.09 to 0.32 | 0.276 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.035 | ||||
| Centre | < 0.001 | ||||
| CGI: Severity (25 weeks) | –0.06 (0.12) | –0.30 to 0.18 | 0.616 | Baseline scores | < 0.001 |
| Type of antidepressant | < 0.001 | ||||
| Centre | < 0.001 | ||||
| CGI: Improvement | |||||
| CGI: Improvement (4 weeks) | 0.14 (0.10) | –0.05 to 0.33 | 0.139 | Type of antidepressant | 0.030 |
| CGI: Improvement (12 weeks) | 0.06 (0.11) | –0.16 to 0.28 | 0.594 | Type of antidepressant | 0.052 |
| CGI: Improvement (25 weeks) | –0.07 (0.13) | –0.32 to 0.18 | 0.596 | Type of antidepressant | 0.005 |
| Centre | 0.012 | ||||
| CGI: Efficacy | |||||
| CGI: Efficacy (4 weeks)a | –0.10 (0.05) | –0.20 to 0.01 | 0.079 | Type of antidepressant | 0.003 |
| Centre | < 0.001 | ||||
| CGI: Efficacy (12 weeks)a | 0.04 (0.06) | –0.08 to 0.15 | 0.560 | Type of antidepressant | 0.007 |
| Centre | 0.005 | ||||
| CGI: Efficacy (25 weeks)a | –0.07 (0.06) | –0.19 to 0.05 | 0.272 | Type of antidepressant | 0.004 |
| Centre | < 0.001 | ||||
| UKU: psychic | |||||
| UKU: psychic (4 weeks) | 0.81 (0.47) | –0.11 to 1.73 | 0.084 | Baseline scores | < 0.001 |
| UKU: psychic (12 weeks) | 0.08 (0.47) | –0.84 to 1.00 | 0.869 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.005 | ||||
| Centre | 0.003 | ||||
| UKU: psychic (25 weeks) | 0.37 (0.46) | –0.54 to 1.28 | 0.426 | Baseline scores | < 0.001 |
| Type of antidepressant | 0.002 | ||||
| Centre | 0.013 | ||||
| UKU: neurologic | |||||
| UKU: neurologic (4 weeks)b | –0.00 (0.06) | –0.12 to 0.12 | 0.989 | Baseline scores | < 0.001 |
| Centre | < 0.001 | ||||
| UKU: neurologic (12 weeks)b | –0.03 (0.06) | –0.16 to 0.10 | 0.642 | Baseline scores | < 0.001 |
| Centre | 0.039 | ||||
| UKU: neurologic (25 weeks)b | –0.02 (0.07) | –0.15 to 0.12 | 0.821 | Baseline scores | < 0.001 |
| UKU: autonomic | |||||
| UKU: autonomic (4 weeks) | –0.13 (0.22) | –0.55 to 0.30 | 0.565 | Baseline scores | < 0.001 |
| Centre | 0.004 | ||||
| UKU: autonomic (12 weeks) | –0.17 (0.23) | –0.63 to 0.28 | 0.460 | Baseline scores | < 0.001 |
| UKU: autonomic (25 weeks) | 0.03 (0.24) | –0.43 to 0.49 | 0.907 | Baseline scores | < 0.001 |
| UKU: other | |||||
| UKU: other (4 weeks) | –0.11 (0.28) | –0.65 to 0.44 | 0.694 | Baseline scores | < 0.001 |
| Centre | < 0.001 | ||||
| UKU: other (12 weeks) ** | –0.63 (0.30) | –1.22 to –0.04 | 0.037 | Baseline scores | < 0.001 |
| UKU: other (25 weeks) | –0.39 (0.30) | –0.98 to 0.20 | 0.195 | Baseline scores | < 0.001 |
| Previous treatment | 0.047 | ||||
| Gender | 0.018 | ||||
The absence of the binary variable ‘Previous treatment?’ from the significant covariates in all but the SF-12 Physical Component Score of the 13 rows of Table 13, and from all but four non-psychiatric rows of the 39 rows of Table 14, confirms that this does not seem to predict the clinical outcome of ADM or of adjunctive folate.
Tables 43–46 of Appendix 10 complement Table 14, specifically the BDI-II row, by reporting the analogous logistic regression analyses for two levels of response to treatment – 50% or full improvement – both at 12 and at 25 weeks, thus illustrating the primary analysis in clinical terms.
Side effects, adverse events and suicidality
Side effects
There were 33 reported AEs in the folic acid arm of the trial and 45 in the placebo arm – difference not statistically significant (χ2 = 2.66; df = 1; p = 0.10; OR = 0.66; 95% CI from 0.40 to 1.09). We adjudged six of the AEs in the intervention arm to be serious, compared with 14 in the control arm – difference also not statistically significant (χ2 = 3.59; df = 1; p = 0.058; OR = 0.40; 95% CI from 0.15 to 1.06). We classified seven of the 78 AEs as adverse reactions because folic acid (if prescribed) was a possible cause; unblinding revealed that four had received folic acid and three not – difference again not statistically significant (χ2 = 0.12; df = 1; p = 0.73; OR = 1.30; 95% CI from 0.29 to 5.89). None of these was serious or unexpected.
Suicidality using the MINI and UKU
We measured side effects by the UKU side effects scale. Comparison of the areas under subscale curves over 25 weeks showed no significant differences between treatment groups (Table 15). When we examined differences at each of the three follow-up times for each of the four subscales, we found a marginally significant difference at 12 weeks in the ‘other’ subscale, which includes mostly sexual side effects (p = 0.037). As this is one of many comparisons for the UKU, we treat this finding with caution.
| UKU subscale | Difference (folate minus placebo) | |||
|---|---|---|---|---|
| Mean | (SE) | 95% CI | Significance | |
| Psychic | 0.31 | (0.31) | –0.30 to 0.92 | 0.319 |
| Neurologica | –0.01 | (0.04) | –0.10 to 0.07 | 0.738 |
| Autonomic | –0.11 | (0.15) | –0.42 to 0.19 | 0.458 |
| Other (mostly sexual) | –0.38 | (0.20) | –0.78 to 0.02 | 0.065 |
Table 16 tabulates the numbers and percentages of patients classified in each of three suicidal risk categories from the MINI suicidality scale by follow-up time and treatment arm. All four differences are small and non-significant.
| Week | Folate (n = 223) | Placebo (n = 217) | ||||||
|---|---|---|---|---|---|---|---|---|
| Suicide risk: number (%) | Suicide risk: number (%) | |||||||
| None | Low | Medium | High | None | Low | Medium | High | |
| 0 | 0 (0) | 138 (62) | 36 (16) | 49 (22) | 0 (0) | 143 (66) | 34 (16) | 40 (18) |
| 4 | 0 (0) | 169 (76) | 21 (9) | 33 (15) | 0 (0) | 155 (71) | 32 (15) | 30 (14) |
| 12 | 0 (0) | 152 (68) | 35 (16) | 36 (16) | 0 (0) | 168 (77) | 21 (10) | 28 (13) |
| 25 | 0 (0) | 163 (73) | 35 (16) | 25 (11) | 0 (0) | 156 (72) | 33 (15) | 28 (13) |
Adherence to trial medication
We assessed adherence to the trial medication at 12 weeks in four ways: scores on the Morisky Questionnaire; counting returned trial medication; serum folate levels; and homocysteine levels. We followed the published instructions for calculating respondents’ scores on the Morisky Questionnaire. We defined the biochemical criteria for adherence to folic acid treatment as: serum folate at 12 weeks greater than 15 µg/ml, and reduction of at least 15% in serum homocysteine between baseline and 12 weeks. Table 17 shows no significant difference between randomised groups in Morisky score or tablet count. Of the biochemical criteria, serum folate shows better adherence than homocysteine.
| Adherence at 12 weeks | Folate (n = 223) | Placebo (n = 217) |
|---|---|---|
| Morisky score – no. (%) | ||
| 0 (best) | 4 (2) | 6 (3) |
| 1 | 18 (8) | 17 (8) |
| 2 | 43 (19) | 36 (17) |
| > 2 (worst) | 158 (71) | 158 (73) |
| Missing | Nil | Nil |
| Tablet count – median (IQR) | 14 (7 to 20) | 14 (7 to 20) |
| Missing | 43 | 45 |
| Serum folate > 15 µg/ml – no. (%) | 163 (73) | Not applicable |
| Missing | 29 (13) | |
| Homocysteine reduction of 15% – no. (%) | 103 (46) | Not applicable |
| Missing | 51 (23) | |
Sensitivity analyses of clinical effectiveness outcomes
Complete case analysis
Table 18 comparing the main and complete case analyses of BDI-II found no essential differences between the alternative estimates of the AUC of folic acid minus placebo: in particular the complete case analysis’s estimate of the adjusted AUC was 1.26 (95% CI from –0.56 to 3.08; p = 0.18), very close to that of the main analysis.
| BDI-II scores | Difference (folate minus placebo) | |||||
|---|---|---|---|---|---|---|
| Main analysis (imputing missing) (n = 440) | Complete case analysis (n = 323) | |||||
| Mean | 95% CI | Significance | Mean | 95% CI | Significance | |
| AUC average: unadjusted | –0.06 | –2.02 to 2.15 | 0.953 | 0.11 | –2.30 to 2.52 | 0.930 |
| AUC average: adjusted | 1.09 | –0.48 to 2.66 | 0.173 | 1.26 | –0.56 to 3.08 | 0.175 |
| ANCOVA: adjusted | ||||||
| 4 weeks | 0.45 | –1.34 to 2.23 | 0.623 | 0.91 | –1.14 to 2.96 | 0.384 |
| 12 weeks | 1.52 | –0.64 to 3.69 | 0.168 | 1.75 | –0.68 to 4.19 | 0.157 |
| 25 weeks | 1.27 | –0.99 to 3.54 | 0.270 | 1.24 | –1.44 to 3.91 | 0.364 |
Multi-level repeated-measures analysis
We fitted a mixed-effects multi-level model with the same covariates as the AUC analysis. Table 19 shows that both analyses reported the same general findings; in particular the repeated measures analysis’s estimate of the AUC was 0.67 (95% CI from –1.00 to 2.34; p = 0.429), close to that of the main analysis.
| Outcome variable | Difference (folate minus placebo) | |||||
|---|---|---|---|---|---|---|
| Main analysis (AUC) | Repeated measures analysis | |||||
| Mean | 95% CI | Significance | Mean | 95% CI | Significance | |
| BDI-II | 1.09 | –0.48 to 2.66 | 0.173 | 0.67 | –1.00 to 2.34 | 0.429 |
| MADRS | 0.71 | –0.51 to 1.93 | 0.252 | 0.49 | –0.80 to 1.78 | 0.452 |
| EQ-5D | 0.001 | –0.033 to 0.031 | 0.982 | 0.013 | –0.062 to 0.087 | 0.760 |
| EQ-VAS | –0.48 | –3.16 to 2.20 | 0.726 | 0.03 | –0.02 to 0.08 | 0.230 |
| SF-12 PCS | 0.40 | –0.78 to 1.59 | 0.501 | 0.64 | –0.61 to 1.90 | 0.313 |
| SF-12 MCS * | –1.97 | –3.49 to –0.44 | 0.012 | –1.83 | –3.48 to –0.19 | 0.029 |
| CGI: Severity | 0.05 | –0.11 to 0.20 | 0.553 | 0.00 | –0.16 to 0.16 | 0.995 |
| CGI: Improvement | 0.04 | –0.13 to 0.21 | 0.649 | 0.01 | –0.15 to 0.16 | 0.942 |
| CGI: Efficacya | –0.02 | –0.11 to 0.07 | 0.604 | –0.05 | –0.17 to 0.08 | 0.482 |
| Estimated CGI | 0.98b | 0.90b | 1.07b | 0.95b | 0.84 to 1.08b | |
Analysis reweighted to adjust for participants who did not respond after baseline
Table 20 comparing the main and reweighted analyses of BDI-II found no essential differences between the alternative estimates of the AUC of folic acid minus placebo: in particular the reweighted analysis’s estimate of the adjusted AUC was 1.17 (95% CI from –0.36 to 2.69; p = 0.135), very close to that of the main analysis. Table 47 in Appendix 10 shows that the 35 participants completely lost to follow-up were very similar to their ‘nearest neighbours’ in the analysed population. For 30 participants the matches were unambiguous; we resolved the ambiguity for the remaining five participants by matching them with the candidate with the closest BDI-II at baseline.
| BDI-II | Folate minus placebo | |||||
|---|---|---|---|---|---|---|
| Main analysis (responders) (n = 440) | Reweighted analysis (n = 475) | |||||
| Mean | 95% CI | Significance | Mean | 95% CI | Significance | |
| AUC: unadjusted | –0.06 | –2.02 to 2.15 | 0.953 | 1.02 | –1.83 to 2.18 | 0.864 |
| AUC: adjusted | 1.09 | –0.48 to 2.66 | 0.173 | 1.17 | –0.36 to 2.69 | 0.135 |
| ANCOVA: adjusted 4 weeks |
0.45 | –1.34 to 2.23 | 0.623 | 0.52 | –1.19 to 2.22 | 0.554 |
| 12 weeks | 1.52 | –0.64 to 3.69 | 0.168 | 1.51 | –0.59 to 3.62 | 0.158 |
| 25 weeks | 1.27 | –0.99 to 3.54 | 0.270 | 1.36 | –0.83 to 3.55 | 0.222 |
Biochemistry analysis
Adjusting clinical effectiveness outcomes for biochemistry
Table 21 shows that baseline BDI-II was a powerful predictor of subsequent BDI-II scores, to the exclusion of all biochemical covariates in stepwise linear regression.
| Difference (folate minus placebo) | Significant covariates | Significance | ||
|---|---|---|---|---|
| Mean (SE) | 95% CI | |||
| Unadjusted | ||||
| BDI-II (12 weeks) | 1.21 (1.14) | –1.02 to 3.44 | Baseline BDI-II score | < 0.001 |
| BDI-II (25 weeks) | 0.71 (1.20) | –1.65 to 3.07 | Baseline BDI-II score | < 0.001 |
| Adjusted by stratification variables and allocated treatment | ||||
| BDI-II (12 weeks) | 1.43 (1.12) | –0.79 to 3.65 | Baseline BDI-II score | < 0.001 |
| Centre | 0.019 | |||
| BDI-II (25 weeks) | 0.90 (1.18) | –1.42 to 3.22 | Baseline BDI-II score | < 0.001 |
| Type of ADM | 0.007 | |||
| Centre | 0.012 | |||
The alternative repeated measures analysis adjusted for baseline biochemistry but still found no consistent evidence of differences between folate and placebo groups in any outcome (Table 22). For example after adjustment for serum folate the estimated difference between the folate and placebo groups in the BDI-II after treatment was 0.68 (95% CI from –0.96 to 2.32; p = 0.413). Again only the SF-12 mental health component score (MCS) showed statistical significance with an estimated difference adjusted for baseline serum folate of –1.85 (95% CI from –3.48 to –0.21; p = 0.027).
| Outcome variable | Covariate | Difference (folate minus placebo) | ||
|---|---|---|---|---|
| Mean (SE) | 95% CI | Significance | ||
| BDI-II | Serum folate | 0.682 (0.833) | –0.96 to 2.32 | 0.413 |
| Red cell folate | –0.045 (0.990) | –1.99 to 1.90 | 0.964 | |
| MADRS | Serum folate | 0.530 (0.649) | –0.75 to 1.81 | 0.415 |
| Red cell folate | 0.351 (0.744) | –1.11 to 1.82 | 0.638 | |
| EQ-5Da | Serum folate | 0.012 (0.038) | –0.064 to 0.068 | 0.763 |
| Red cell folate | 0.028 (0.022) | –0.015 to 0.070 | 0.202 | |
| EQ-VASa | Serum folate | 1.019 (0.850) | –0.65 to 2.69 | 0.231 |
| Red cell folate | –0.026 (1.016) | –2.03 to 1.97 | 0.980 | |
| MCS | Serum folate | –1.848 (0.832) | –3.48 to –0.21 | 0.027 |
| Red cell folate | –0.610 (0.991) | –2.56 to 1.34 | 0.538 | |
| PCS | Serum folate | 0.657 (0.635) | –0.59 to 1.91 | 0.302 |
| Red cell folate | 0.996 (0.743) | –0.47 to 2.45 | 0.181 | |
| CGI: Severity | Serum folate | 0.003 (0.082) | –0.16 to 0.16 | 0.971 |
| Red cell folate | –0.085 (0.095) | –0.27 to 0.10 | 0.368 | |
| CGI: Improvement | Serum folate | 0.059 (0.109) | –0.15 to 0.27 | 0.586 |
| Red cell folate | –0.022 (0.124) | –0.27 to 0.22 | 0.859 | |
The estimated change in BDI-II after a unit increase in baseline serum folate was –0.07 (95% CI from –0.24 to 0.10; p = 0.431). Only in the CGI Improvement scale did any baseline biochemical variables predict clinical outcome. There was some evidence that red cell folate predicts outcome and strong evidence for homocysteine: a single unit of baseline homocysteine increased CGI improvement by 0.05 (95% CI from 0.03 to 0.08; p < 0.001).
The repeated measures analysis adjusted for biochemical measures while on treatment in week 12 found no evidence of difference between folate and placebo groups in any instrument (Table 23). However there was clear evidence across most instruments except SF-12 showing that homocysteine measured in week 12 while on treatment predicted clinical outcomes like MADRS, CGI (severity and improvement) and EQ-5D. In particular a unit increase in homocysteine at week 12 increased BDI-II by 0.34 (95% CI from 0.15 to 0.52; p = 0.001). Tables 48 and 49 of Appendix 10 elaborate on Tables 22 and 23 by reporting more extensive models in more detail.
| Outcome variable | Covariate | Difference (folate minus placebo) | ||
|---|---|---|---|---|
| Mean (SE) | 95% CI | Significance | ||
| BDI-II | Serum folate | 0.107 (1.093) | –2.04 to 2.26 | 0.922 |
| Red cell folate | –1.794 (1.490) | –4.73 to 1.14 | 0.230 | |
| MADRS | Serum folate | 0.260 (0.894) | –1.50 to 2.02 | 0.772 |
| Red cell folate | –0.623 (1.188) | –2.97 to 1.72 | 0.601 | |
| EQ-5D | Serum folate | 0.021 (0.022) | –0.011 to 0.074 | 0.363 |
| Red cell folate | 0.059 (0.031) | –0.003 to 0.121 | 0.060 | |
| EQ-VAS | Serum folate | 0.107 (1.093) | –2.04 to 2.26 | 0.922 |
| Red cell folate | –1.794 (1.490) | –4.73 to 1.14 | 0.230 | |
| MCS | Serum folate | –1.172 (1.146) | –3.42 to 1.08 | 0.307 |
| Red cell folate | 0.096 (1.565) | –2.99 to 3.18 | 0.951 | |
| PCS | Serum folate | 1.136 (0.825) | –0.49 to 2.76 | 0.170 |
| Red cell folate | 1.502 (1.114) | –0.69 to 3.70 | 0.179 | |
| CGI: Severity | Serum folate | –0.042 (0.112) | –0.26 to 0.18 | 0.706 |
| Red cell folate | –0.233 (0.144) | –0.52 to 0.05 | 0.107 | |
| CGI: Improvement | Serum folate | –0.063 (0.119) | –0.30 to 0.17 | 0.597 |
| Red cell folate | –0.116 (0.165) | –0.44 to 0.21 | 0.480 | |
Biochemistry outcomes
Despite the lack of clinical response to folic acid, it was effective in increasing participants’ folate. Table 24 shows that the folate group had higher serum folate by 15.1 (95% CI from 12.4 to 17.8) at 12 weeks, and by 15.6 (95% CI from 13.3 to 17.8) at 25 weeks. The difference in red cell folate was 272 (95% CI from 210 to 334) at 12 weeks, but only 82 (95% CI from 26 to 139) at 25 weeks. Baseline scores enhanced the prediction of all four biochemical outcomes. Each unit of baseline serum folate increased serum folate on treatment by 0.49 (95% CI from 0.30 to 0.68); each unit of baseline red cell folate increased red cell folate on treatment by 0.34 (95% CI from 0.22 to 0.45). Age was another good predictor for red cell folate: each year increased mean red cell folate by 2.17 (95% CI from 0.58 to 3.75). The reduction in homocysteine caused by folic acid was –0.93 (95% CI from –1.66 to –0.19). Prediction of homocysteine improved after adjustment for baseline homocysteine and serum folate at 12 weeks. Age and gender were also good predictors for homocysteine: for every year of life homocysteine reduced by 0.048 (95% CI from 0.021 to 0.076); and females had higher homocysteine by 1.64 (95% CI from 0.91 to 2.34).
| Outcome variable | Difference (folate minus placebo) | Statistical significance of other variables | |||
|---|---|---|---|---|---|
| Mean (SE) | 95% CI | Significance | Covariate | Significance | |
| Serum folate | 15.1 (1.4) | 12.4 to 17.8 | < 0.001 | Centre | 0.034 |
| Time | < 0.001 | ||||
| Baseline serum folate | < 0.001 | ||||
| Type of antidepressant | 0.209 | ||||
| Previous treatment | 0.856 | ||||
| Red cell folate at 12 weeks | < 0.001 | ||||
| Allocated treatment by time | < 0.001 | ||||
| Red cell folate | 272.4 (31.4) | 210.5 to 334.3 | < 0.001 | Centre | 0.504 |
| Time | 0.004 | ||||
| Baseline red cell folate | < 0.001 | ||||
| Type of antidepressant | 0.914 | ||||
| Previous treatment | 0.118 | ||||
| Age | 0.008 | ||||
| Serum folate at 12 weeks | < 0.001 | ||||
| Allocated treatment by time | 0.066 | ||||
| Homocysteine | 0.93 (0.37) | 0.19 to 1.66 | 0.014 | Centre | 0.726 |
| Time | 0.703 | ||||
| Baseline homocysteine | < 0.001 | ||||
| Type of antidepressant | 0.285 | ||||
| Previous treatment | 0.773 | ||||
| Age | 0.002 | ||||
| Gender | < 0.001 | ||||
| Serum folate at 12 weeks | < 0.001 | ||||
| Vitamin B12 | 6.20 (8.28) | –10.1 to 22.5 | 0.454 | Centre | 0.005 |
| Time | 0.036 | ||||
| Baseline B12 | < 0.001 | ||||
| Type of antidepressant | 0.017 | ||||
| Previous treatment | 0.998 | ||||
| Homocysteine at 12 weeks | 0.005 | ||||
Many expected that patients deficient in folate and randomised to the folated group would achieve improvements in biochemical and clinical outcomes. So we analysed the subset of patients who at baseline had a serum folate concentration of less than 3 µg/l, a red cell folate less than 200 µg/l, or homocysteine greater than 15.3 µmol/l. Table 25 found no clinical or statistical difference between folate and placebo groups among those with deficiency in serum folate or homocysteine. For example the estimated difference in BDI-II in patients who had less than 3 µg/l serum folate was –1.67 (95% CI from –9.85 to 6.51; p = 0.68). In contrast the few patients who were deficient in baseline red cell folate (RCF) yielded necessarily weak but consistent evidence across multiple instruments that augmenting ADM with folic acid improved clinical outcome. Since high scores in EQ-5D and SF-12, and low scores in depression scales are all good, folic acid achieved (near) significant improvements in six of the eight criteria, specifically in the RCF-deficient group.
| Outcome variable | Deficiency | n | Difference (folate minus placebo) | ||
|---|---|---|---|---|---|
| Mean (SE) | 95% CI | Significance | |||
| BDI-II | Serum folate < 3 µg/l | 27 | –1.67 (3.99) | –9.85 to 6.51 | 0.679 |
| Red cell folate < 200 µg/l | 9 | –18.4 (8.2) | –36.8 to 0.2 | 0.052 | |
| Homocysteine > 15.3 µmol/l | 53 | 3.51 (2.63) | –1.77 to 8.78 | 0.188 | |
| MADRS | Serum folate < 3 µg/l | 27 | 2.51 (2.40) | –2.42 to 7.43 | 0.305 |
| Red cell folate < 200 µg/l | 9 | –7.49 (3.66) | –15.8 to 0.79 | 0.071 | |
| Homocysteine > 15.3 µmol/l | 53 | 1.04 (1.94) | –2.85 to 4.94 | 0.593 | |
| EQ-5D | Serum folate < 3 µg/l | 27 | –0.080 (0.074) | –0.226 to 0.072 | 0.281 |
| Red cell folate < 200 µg/l | 9 | 0.189 (0.131) | –0.109 to 0.488 | 0.179 | |
| Homocysteine > 15.3 µmol/l | 53 | 0.03 (0.07) | –0.11 to 0.17 | 0.661 | |
| EQ-VAS | Serum folate < 3 µg/l | 27 | 0.5 (6.0) | –11.8 to 12.8 | 0.937 |
| Red cell folate < 200 µg/l | 9 | 34.5 (15.5) | –0.6 to 69.6 | 0.054 | |
| Homocysteine > 15.3 µmol/l | 53 | –1.9 (5.9) | –13.6 to 9.9 | 0.753 | |
| MCS | Serum folate < 3 µg/l | 27 | 0.83 (3.69) | –6.75 to 8.40 | 0.824 |
| Red cell folate < 200 µg/l | 9 | 15.9 (6.2) | 2.0 to 29.9 | 0.029 | |
| Homocysteine > 15.3 µmol/l | 53 | –0.38 (2.47) | –5.33 to 4.56 | 0.877 | |
| PCS | Serum folate < 3 µg/l | 27 | 2.26 (2.65) | –3.18 to 7.69 | 0.402 |
| Red cell folate < 200 µg/l | 9 | –1.88 (3.94) | –10.8 to 7.02 | 0.644 | |
| Homocysteine > 15.3 µmol/l | 53 | –3.13 (2.04) | –7.21 to 0.94 | 0.129 | |
| CGI: Severity | Serum folate < 3 µg/l | 27 | –0.19 (0.34) | –0.88 to 0.50 | 0.581 |
| Red cell folate < 200 µg/l | 9 | –1.40 (0.52) | –2.57 to –0.23 | 0.024 | |
| Homocysteine > 15.3 µmol/l | 53 | –0.14 (0.23) | –0.61 to 0.33 | 0.549 | |
| CGI: Improvement | Serum folate < 3 µg/l | 27 | 0.09 (0.32) | –0.56 to 0.73 | 0.787 |
| Red cell folate < 200 µg/l | 9 | –1.35 (0.63) | –2.75 to 0.05 | 0.058 | |
| Homocysteine > 15.3 µmol/l | 53 | –0.06 (0.23) | –0.52 to 0.40 | 0.802 | |
Cost-effectiveness
Resource use
Table 26 presents participants’ use of NHS and PSS resources over their 25 weeks in the trial. The majority of participants, 78% and 81% in folic acid and placebo groups respectively, visited their GP on at least one occasion. The mean number of visits was 3.3 and 3.9. Many participants reported telephone contact with their GPs – 21% of those in the folic acid group and 27% in the placebo group; and visits to practice nurses – 35% and 39% respectively. Despite the NICE recommendation that people with moderate to severe depression should receive psychological therapy, only 7% of intervention participants and 6% of controls did so (0.4 times on average). However 22% of those randomised to folic acid consulted psychiatrists at hospital clinics (0.6 times on average), compared with 29% of control participants (0.8 times on average). Other hospital visits were reported by similar numbers of participants – 22% and 25% respectively; and only 6% of patients in each group were admitted to hospital. All patients received prescribed medication during the trial, with a mean of 6.5 and 7.2 antidepressant items in folic acid and placebo groups respectively. Total prescribing was comparable at 21.0 and 21.9 items respectively. Participants’ use of social services was low. Thus there were no significant differences in resource use between the two groups.
| Type of resource | Folate (n = 223) | Placebo (n = 217) | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Non-zero responses | Mean (SD) | Non-zero responses | |||
| no. (%) | [Median]a | no. (%) | [Median]a | |||
| General practice, community pharmacy, and nursing services | ||||||
| GP surgery visits | 3.3 (3.3) | 173 (78) | [3] | 3.9 (4.1) | 176 (81) | [3] |
| GP home visits | 0.1 (0.6) | 11 (5) | [2] | 0.0 (0.2) | 3 (1) | [1] |
| GP telephone contacts | 0.4 (1.3) | 46 (21) | [1] | 0.6 (1.4) | 59 (27) | [1] |
| Visits to practice nurse | 0.7 (1.2) | 79 (35) | [1] | 1.3 (3.9) | 84 (39) | [2] |
| District nurse home visits | 0.0 (0.1) | 1 (0) | [2] | 0.0 (0.2) | 2 (1) | [2] |
| Counsellor at surgery | 0.6 (1.8) | 31 (14) | [3] | 0.4 (1.5) | 27 (12) | [2] |
| Vitamin B12 testing | 1.0 (0) | 223 (100) | [1] | 0.0 (0) | 0 (0) | [0] |
| Folic acid dispensing fee | 1.0 (0) | 223 (100) | [1] | 0.0 (0) | 0 (0) | [0] |
| Antidepressant items dispensed | 6.5 (3.9) | 223 (100) | [6] | 7.2 (5.7) | 217 (100) | [6] |
| All prescription items dispensed | 21.0 (17.8) | 223 (100) | [17] | 21.9 (27.3) | 217 (100) | [14] |
| Other | 0.2 (0.9) | 19 (9) | [3] | 0.1 (0.6) | 11 (5) | [2] |
| Health visitor | 0.2 (2.6) | 4 (2) | [4] | 0.1 (0.5) | 7 (3) | [1] |
| Social services | ||||||
| Social worker | 0.3 (1.4) | 15 (7) | [2] | 0.3 (1.4) | 14 (6) | [3] |
| Home help | 0.2 (3.2) | 1 (0) | [48] | 1.8 (19.1) | 3 (1) | [162] |
| Care assistant | 0.3 (3.3) | 3 (1) | [8] | 0.9 (10.0) | 5 (2) | [6] |
| Day centre visits | 0.1 (0.8) | 2 (1) | [6.5] | 0.2 (1.8) | 5 (2) | [12] |
| Other (social services) | 0.0 (0.4) | 5 (2) | [2] | 0.1 (1.3) | 6 (3) | [2.5] |
| Psychiatric hospital and community services | ||||||
| Psychiatrist at hospital clinic | 0.6 (1.7) | 50 (22) | [2] | 0.8 (1.9) | 63 (29) | [2] |
| Psychiatrist at home | 0.0 (0.2) | 3 (1) | [2] | 0.0 (0.3) | 2 (1) | [2.5] |
| Psychologist | 0.4 (2.2) | 15 (7) | [4] | 0.4 (2.3) | 14 (6) | [2] |
| Community psychiatric nurse | 0.5 (2.0) | 25 (11) | [3] | 1.4 (4.0) | 35 (16) | [5] |
| Other (psychiatric services) | 0.4 (1.6) | 25 (11) | [2] | 0.3 (2.2) | 16 (7) | [2.5] |
| Other services | ||||||
| Day hospital | 0.2 (0.9) | 18 (8) | [1.5] | 0.3 (1.3) | 17 (8) | [2] |
| Accident and Emergency | 0.2 (0.6) | 31 (14) | [1] | 0.3 (1.1) | 38 (18) | [1] |
| Hospital clinic | 0.5 (1.5) | 48 (22) | [1] | 0.6 (1.5) | 55 (25) | [2] |
| Nights spent on hospital ward | 0.8 (5.2) | 13 (6) | [6] | 0.4 (2.5) | 13 (6) | [3] |
| Occupational or employment health services | 0.1 (0.5) | 12 (5) | [1.5] | 0.3 (1.0) | 21 (10) | [2] |
| Other (hospital) | 0.2 (0.9) | 14 (6) | [1] | 0.1 (0.7) | 9 (4) | [1] |
| NHS Direct | 0.2 (1.7) | 19 (9) | [1] | 0.1 (1.0) | 12 (6) | [1.5] |
| Ambulance or paramedic | 0.1 (0.4) | 14 (6) | [1] | 0.1 (0.5) | 11 (5) | [1] |
The mean percentage of responses missing from the resource use questionnaire, across all questions and time points, was 10.1% in the folic acid group and 11.0% in the control group. The level of missing data was not related to the treatment allocation at any time point (Student’s t-test: p > 0.1 for each point). The lowest rate of missing data was in response to consultations at general practices (8.1% across both treatment groups) while the highest rate of missing data related to contact with health visitors (23.5%). The high response rate is in marked contrast to the AHEAD study, which used the same questionnaire, but required patients to return their completed forms by mail, leaving 73.8% of questionnaires incomplete. 138
Costs
Table 27 presents unadjusted costs incurred during the 25 weeks of follow-up. There were no statistically significant differences in categorised or total cost between treatment groups, although there was a tendency towards lower costs in the control group for all categories. Psychiatric services in hospital or community services took more than half the total cost – £797 in the folic acid group, and £886 in the placebo control group. Participants’ attendances at hospital clinics for consultations with psychiatrists was the main driver of this. The costs of prescribed medicines were the second highest cost category at £240 and £257 in intervention and control groups respectively. Antidepressants accounted for around 30% of total medication costs.
| Type of cost | Folate | Placebo | Difference (folate minus placebo) |
|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| GP costs | 164.20 (144.72 to 185.02) | 186.46 (163.60 to 210.61) | –22.26 (–53.61 to 8.64) |
| Social care costs | 148.46 (84.63 to 233.98) | 324.86 (144.51 to 569.11) | –176.40 (–428.14 to 19.63) |
| Psychiatric hospital and community services costs | 797.37 (562.94 to 1090.52) | 886.40 (712.65 to 1089.37) | –89.03 (–404.23 to 249.42) |
| Antidepressant drug costs | 72.93 (62.39 to 84.47) | 75.30 (63.84 to 88.44) | –2.37 (–19.37 to 13.96) |
| All medication costs | 239.95 (206.94 to 275.14) | 256.79 (201.15 to 324.42) | –16.84 (–91.66 to 49.17) |
| Other costs | 60.72 (44.60 to 78.85) | 66.44 (48.50 to 86.49) | –5.72 (–31.42 to 19.50) |
| Total cost | 1410.21 (1147.28 to 1729.31) | 1719.12 (1398.10 to 2088.25) | –308.94 (–764.14 to 155.18) |
There were differences in baseline costs, evident from the resource use questionnaire relating to the 3 months before baseline visits. Baseline costs in the folic acid group were £514 compared with £746 in the placebo group (a difference of £232, 95% CI from –£9 to £487). Although baseline costs do not contribute to the total costs, imbalances at baseline may reflect an imbalance in patient or disease characteristics. Hence they may bias cost estimates, particularly if previous use of health and social care predicts future use. The primary cost analysis therefore corrected for baseline differences by regression. 125
Adjusting for these differences at baseline reduced the mean difference in total costs from £309 (95% CI from –£155 to £764) to £48 (95% CI from –£292 to £389), still not significant.
Health outcomes
Figure 12 shows the distribution of EQ-5D scores by time, treatment group and dimension. At baseline the majority of patients described themselves as having either moderate or severe problems in relation to anxiety or depression (97% in both groups), pain or discomfort (61% and 59% for folic acid and placebo groups respectively), and usual activities (77% and 80% respectively). Improvements in the states of anxiety or depression (to 75% in both groups), and ability to perform usual activities (to 62% and 55% respectively) were evident between baseline and 25 weeks. The corresponding changes in unadjusted mean utilities from baseline to 25 weeks were 0.481 to 0.605 for folic acid and 0.514 to 0.607 for placebo.
FIGURE 12.
Participants responding to each dimension of the EQ-5D – by time and treatment allocated. n shows the number of completed responses within each treatment group. Level 3 represents the most severe problems.
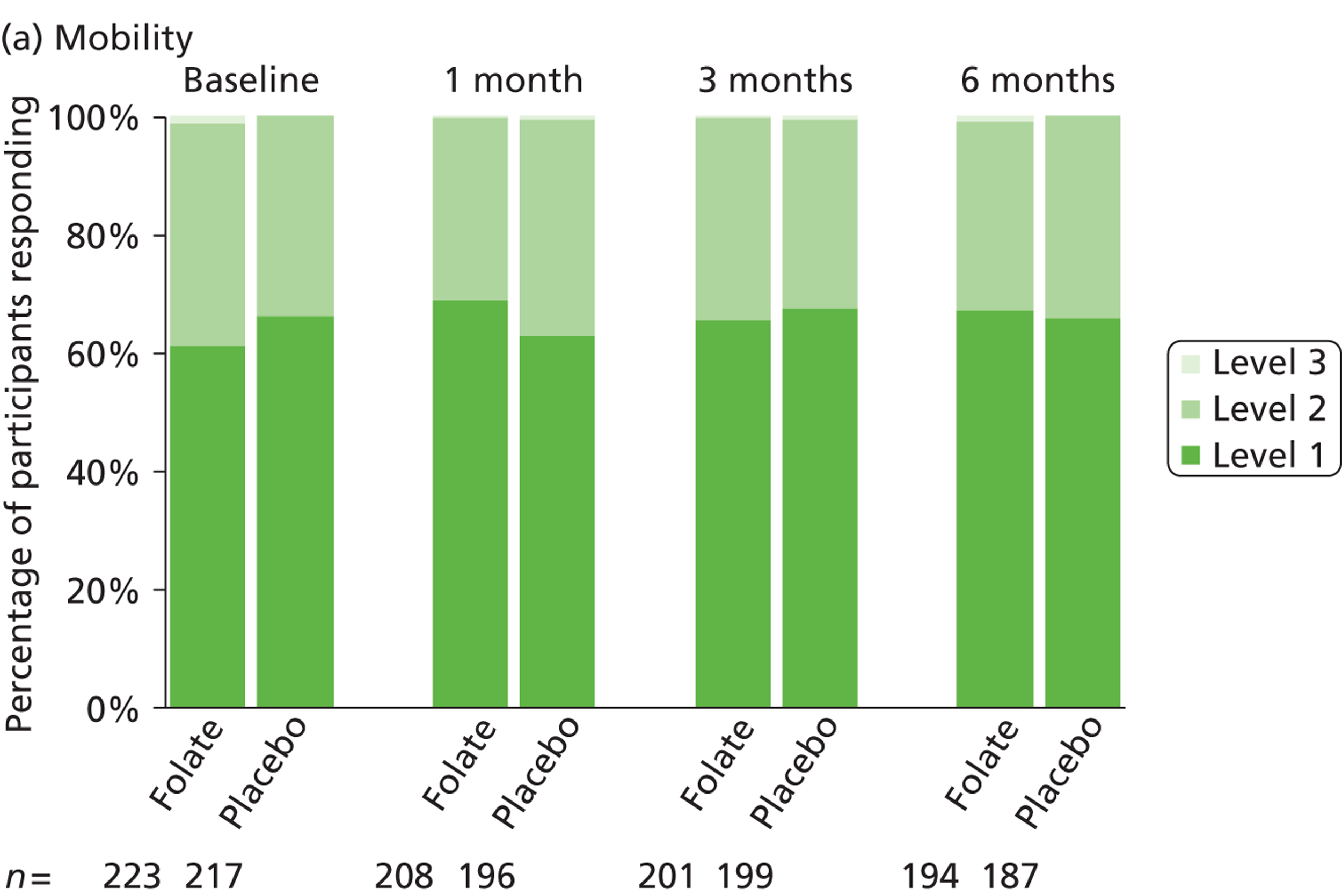
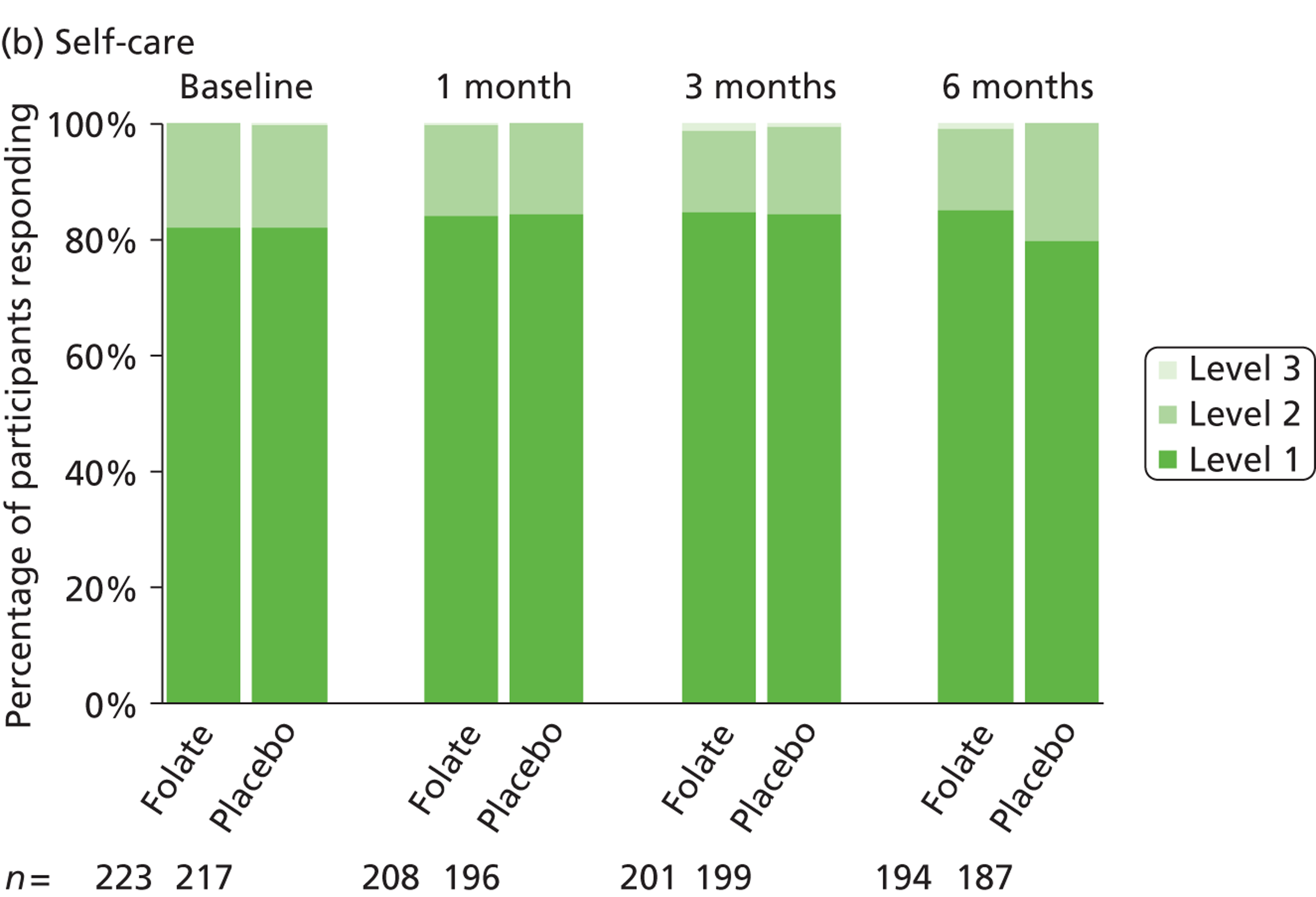
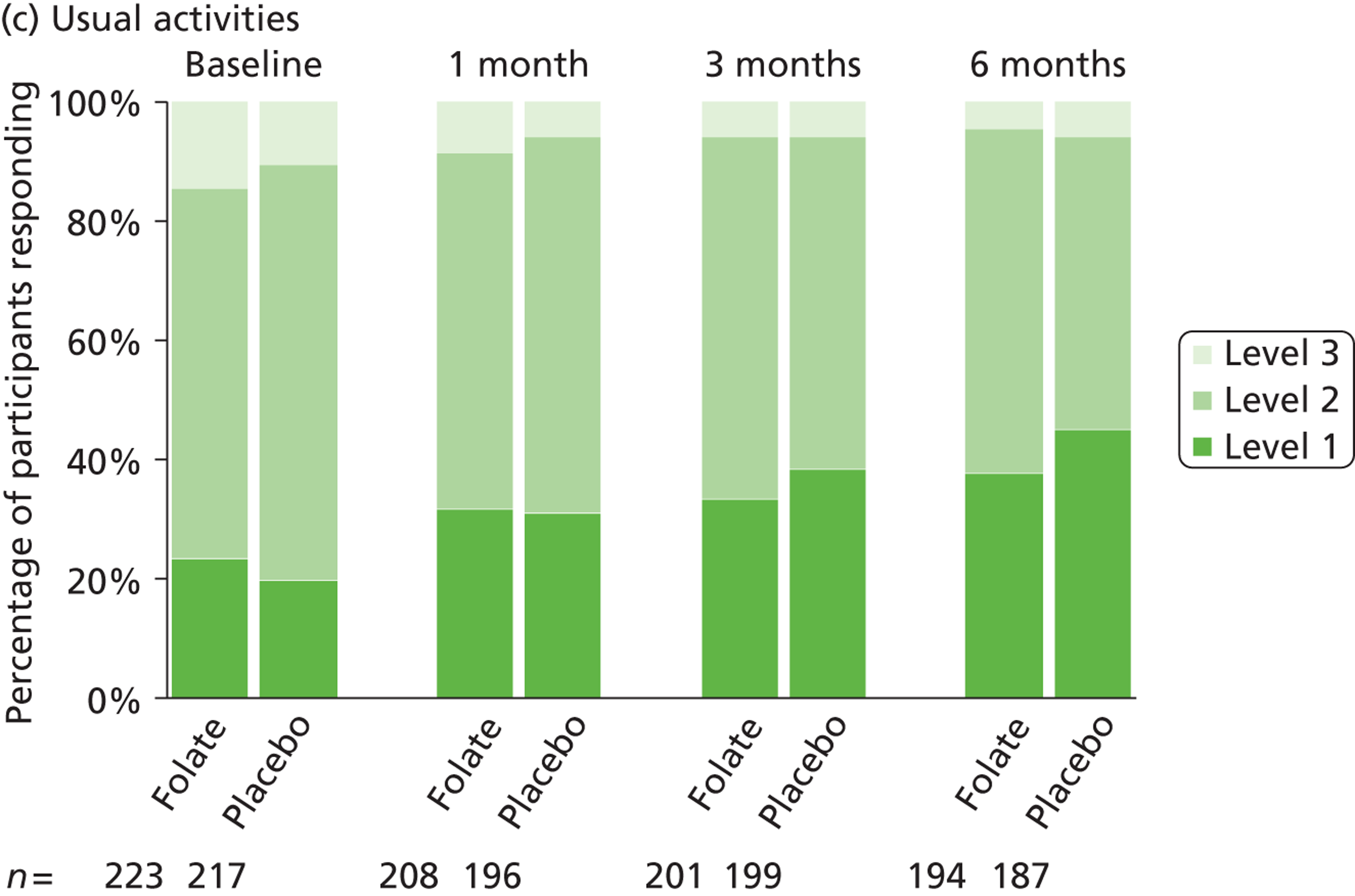
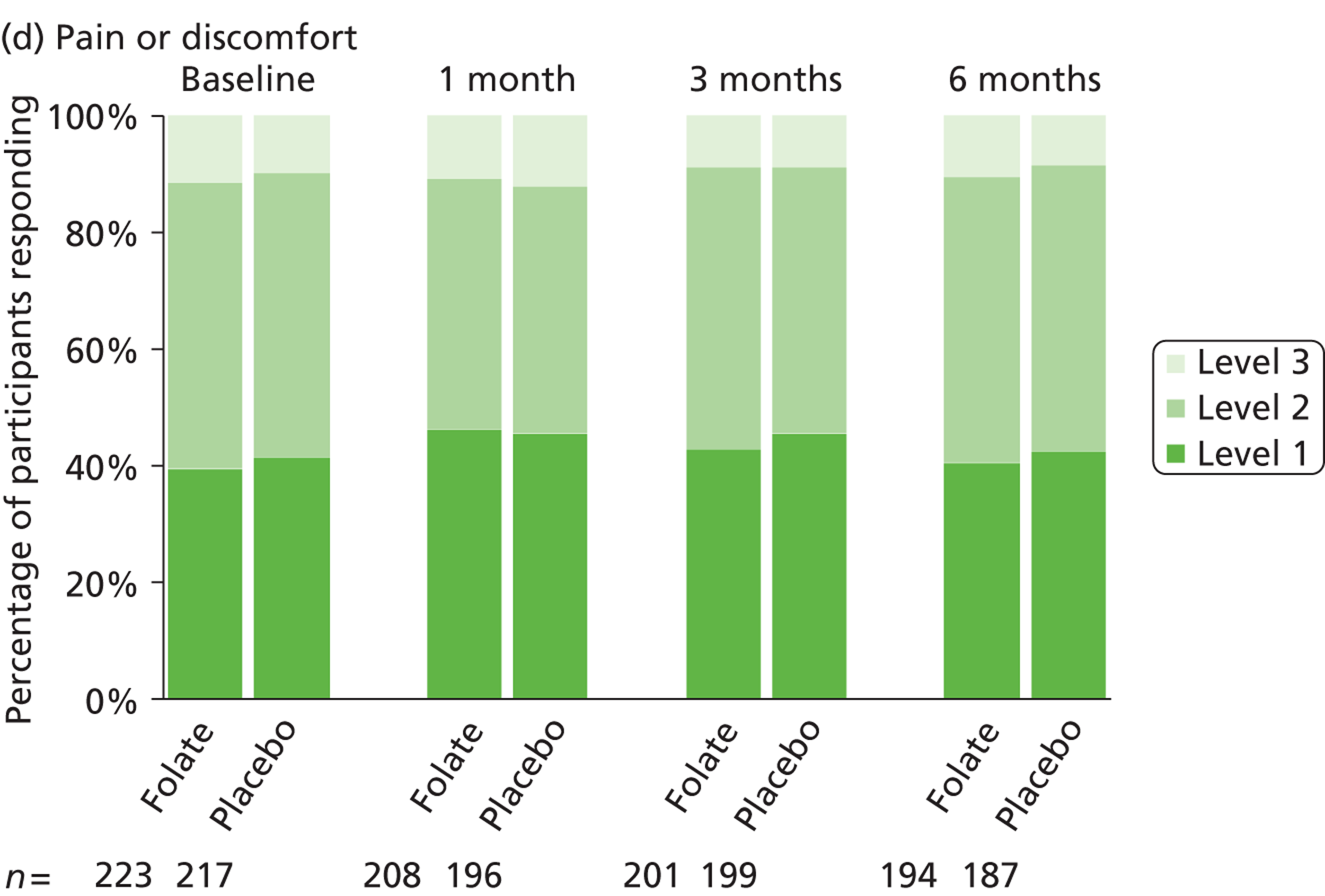
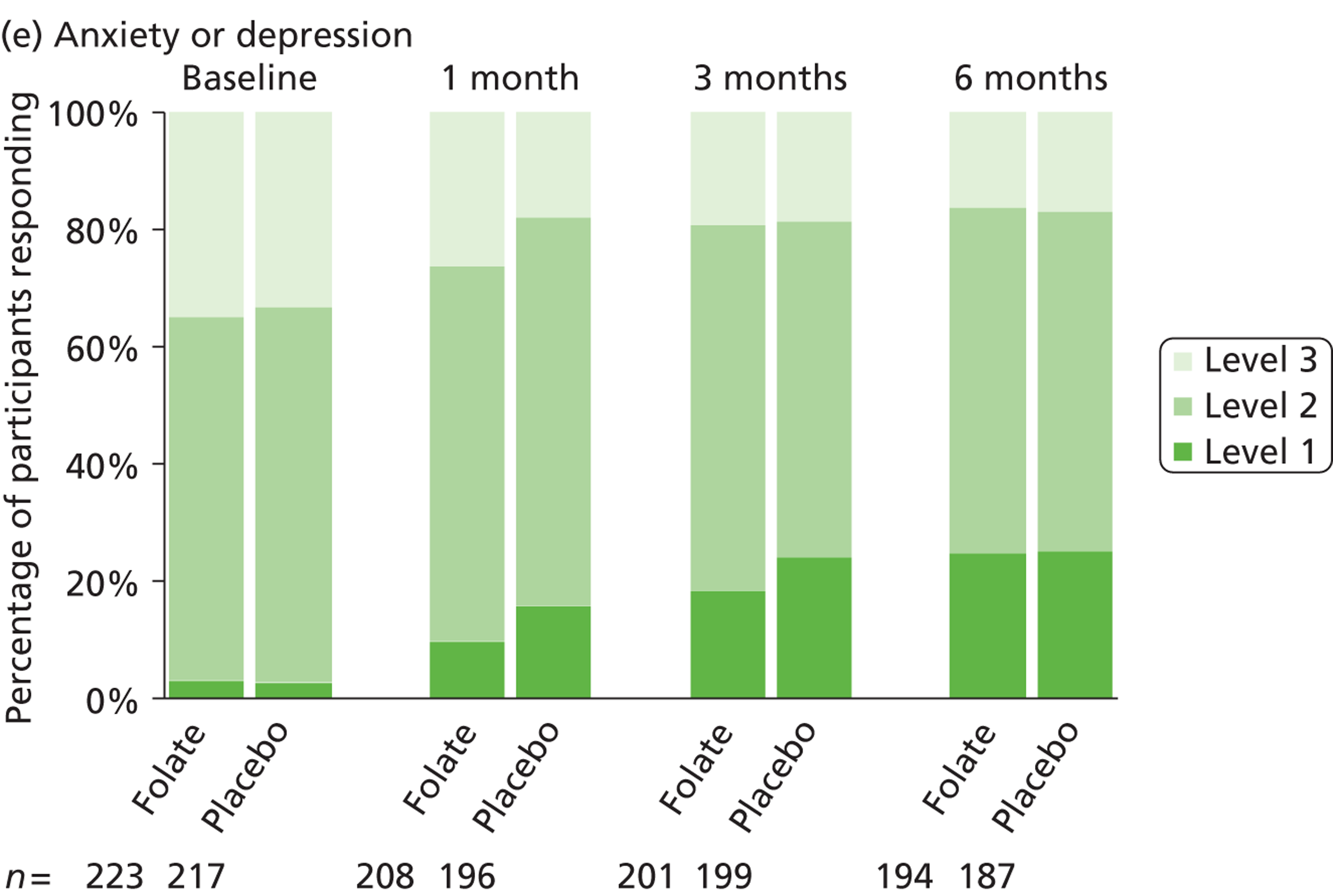
Table 28 presents the numbers of gross and net QALYs gained, as measured by the EQ-5D (primary analysis), EQ-VAS and SF-6D. There were no statistically significant differences between treatment groups. Similarly there were no differences in either outcome measure in the cost-effectiveness analyses – the AUC for BDI-II scores and the number of ‘depression-free weeks’ (when participants’ BDI-II scores were less than 13). The number of participants reporting time free from depression was low – 18 (11%) in the folate group and 23 (15%) in the placebo group at 4 weeks, rising to 56 (33%) and 63 (41%), respectively, at 25 weeks.
| Outcome measure | Full responses no. (%) | Mean (95% CI) including inputed response | |||
|---|---|---|---|---|---|
| Folate | Placebo | Folate (n = 223) | Placebo (n = 217) | Difference | |
| QALYs (EQ-5D) | 172 (77.1) | 165 (76.0) | 0.290 (0.270 to 0.308) | 0.298 (0.279 to 0.316) | –0.0079 (–0.0346 to 0.0191) |
| QALYs (EQ-5D – complete cases) | 0.294a (0.274 to 0.314) | 0.290b (0.269 to 0.309) | 0.0046 (–0.0254 to 0.0328) | ||
| QALYs (EQ-VAS) | 173 (77.6) | 162 (74.7) | 0.276 (0.262 to 0.290) | 0.275 (0.262 to 0.288) | 0.0008 (–0.0187 to 0.0197) |
| QALYs (SF-6D) | 157 (70.4) | 141 (65.0) | 0.292 (0.273 to 0.311) | 0.303 (0.284 to 0.322) | –0.0113 (–0.0378 to 0.0156) |
| AUC (BDI-II)c | 169 (75.8) | 154 (71.0) | 12.91 (12.19 to 13.66) | 12.95 (12.16 to 13.76) | –0.030 (–1.12 to 1.06) |
Cost-effectiveness and uncertainty
In the primary analysis after baseline adjustment following Manca et al. ,126 folic acid is on average £48 less expensive than the placebo group, and more effective by 0.0012 QALYs. As those findings put folic acid in the south-east quadrant of the cost-effectiveness plane (Figure 13) it is therefore the dominant strategy. However there is considerable uncertainty surrounding these estimates, shown by the distribution of costs and QALYs over all four quadrants of the cost-effectiveness plane. The estimated probability of folic acid saving costs is 64%, and that of it gaining QALYs is 55% (Table 29). The resulting cost-effectiveness acceptability curve (Figure 14) shows that the probability of folic acid being cost-effective is 0.62 at the threshold of £20,000 per QALY and 0.61 at the £30,000 threshold (see Table 29).
FIGURE 13.
Cost-effectiveness plane showing joint distribution of costs and QALYs. Note: Confidence ellipses represent the 5%, 50% and 95% levels of confidence.
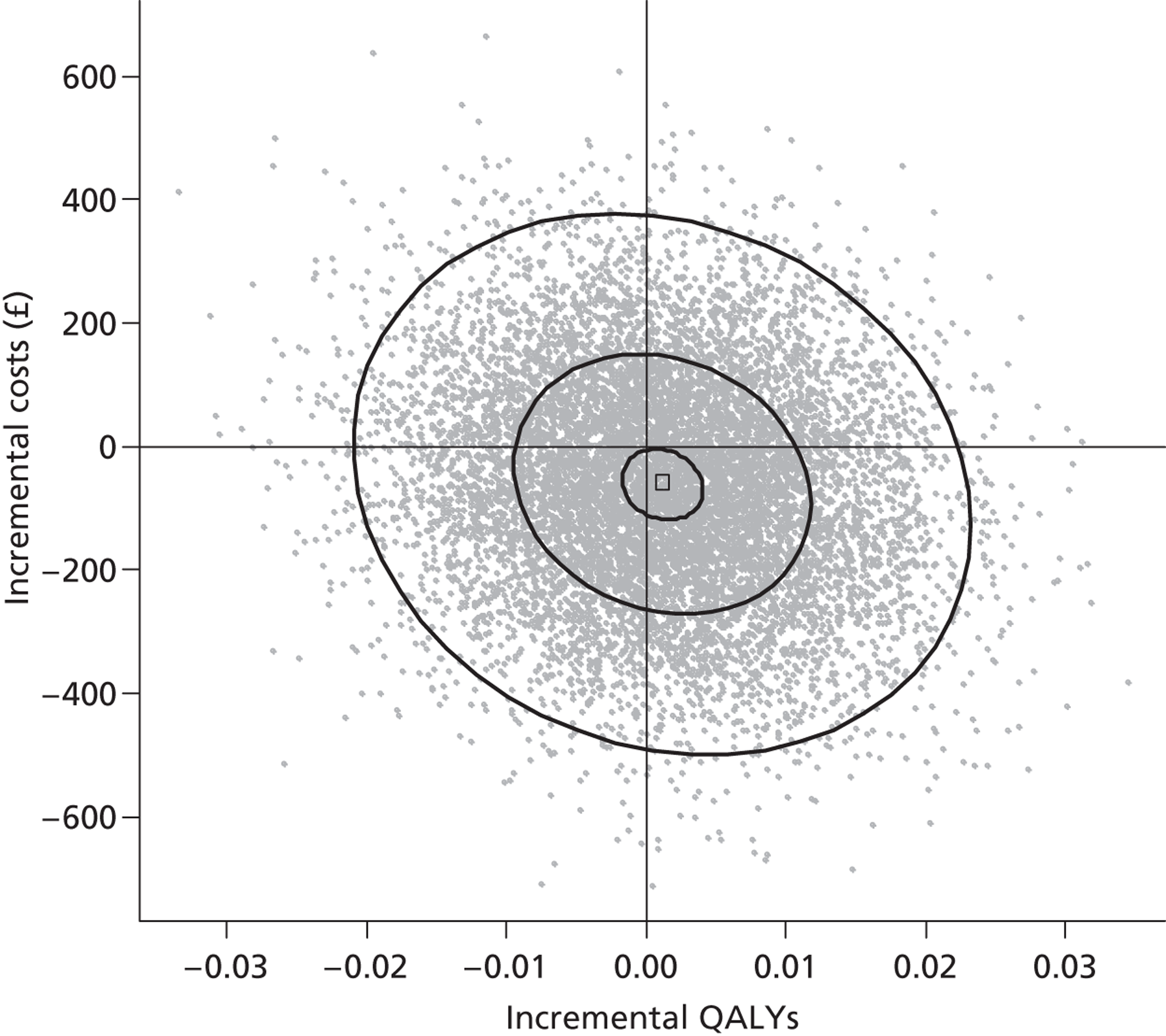
FIGURE 14.
Cost-effectiveness acceptability curve showing probability of folate being cost-effective – by cost-effectiveness threshold (£ per QALY). WTP, willingness to pay.
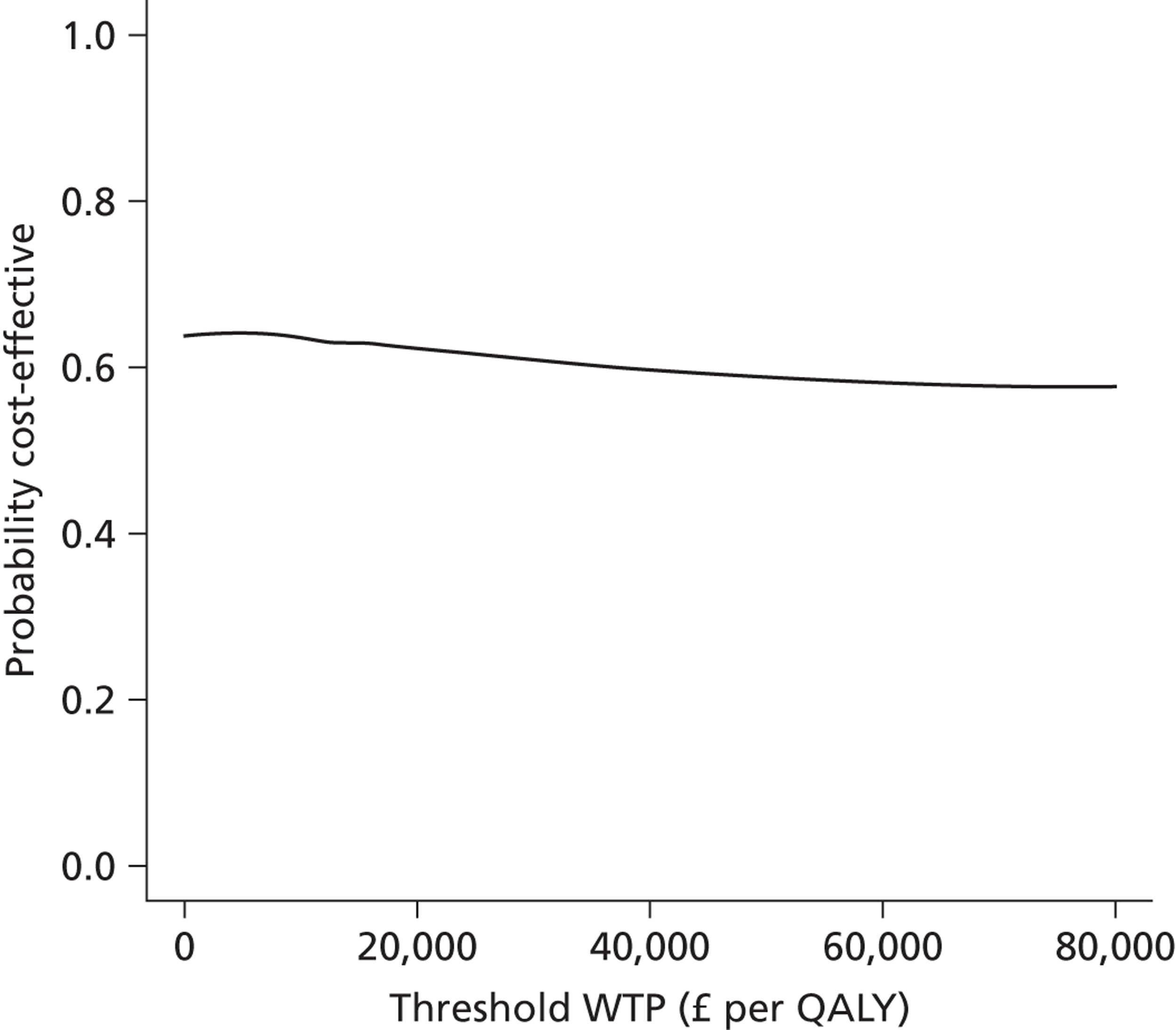
| Outcome | Difference | 95% CI | ICER | Pr (cost saving) | Pr (clinically superior) | Pr (cost-effective at £20,000/QALY) | Pr (cost-effective at £30,000/QALY) |
|---|---|---|---|---|---|---|---|
| Costs | –£48.40 | –£389 to £292 | – | – | – | – | – |
| QALYs (EQ-5D) | 0.0012 | –0.0132 to 0.0186 | Folate is dominant | 0.6373 | 0.5480 | 0.6240 | 0.6093 |
| QALYs (EQ-VAS) | –0.0017 | –0.0155 to 0.0121 | £28,110 per QALYa | 0.6316 | 0.4024 | 0.5420 | 0.5135 |
| QALYs (SF-6D) | 0.0008 | –0.0167 to 0.0183 | Folate is dominant | 0.6251 | 0.5314 | 0.6064 | 0.5926 |
| QALYs (EQ-5D complete cases)b | 0.0129 | –0.0062 to 0.0319 | Folate is dominant | 0.7847 | 0.9011 | 0.9176 | 0.9226 |
| BDI-II (AUC) | 0.4702 | –0.3240 to 1.2643 | Folate is dominant | 0.6276 | 0.8789 | ||
| Depression-free weeks (BDI-II < 13) | –0.8673 | –2.1471 to 0.4124 | £56 per depression-free weeka | 0.6278 | 0.0857 | ||
As this ICER is close to the origin of the cost-effectiveness plane, comparison with other QALY outcomes is labile. For example QALYs derived from both the EQ-VAS and SF-6D located the ICER in the south-west quadrant, where folic acid is both less effective and less expensive than placebo.
The interpretation of cost-effectiveness results suffers from lack of a benchmark for economic efficiency. The unstable direction of differences in mean effect exacerbates this. The primary clinical outcome of area under the BDI-II curve shows folic acid dominating placebo, being more effective and less costly. However the cost per depression-free week averted suggests that folic acid is less effective by an average of 6 depression-free days over 25 weeks, though less expensive. In short the proximity of all possible criteria to the origin of the cost-effectiveness plane, and associated uncertainty suggests that folic acid is no more effective, but no more expensive than placebo.
Genetics
Introduction
Of the 440 patients included in the main analysis by treatment allocated, we excluded from genetic analysis: five with low genotype call rates; 14 non-Caucasians; and 38 who did not consent to the genetic study. Thus we included 383 patients in genetic analysis.
Of the 142 variants genotyped, 38 were omitted as they failed to meet minimum inclusion criteria. These included 19 SNPs owing to quality controls issues with the call rate of the specific SNP assay; 13 SNPs due to a minor allele frequency < 0.01, and six with a Hardy–Weinberg p-value of < 0.0001. In total 104 genetic variants were carried forward for analysis.
Results of the analysis of association between each SNP and each of the seven outcome measures (BDI-II, MADRS, CGI1 severity of illness, EQ-5D, EQ-5D visual analogue score (VAS), SF-12 mental, and SF-12 physical) are given in Table 30. Two associations gave a FDR < 0.05. Results of assessing for the statistical significance of a SNP-treatment group interaction term for each SNP and each outcome are given in Table 31 – for this only one SNP gave a FDR < 0.05.
| SNP | Gene symbol | Associated outcome measure | Significance |
|---|---|---|---|
| rs10380 | MTRR | PCS | 0.0491 |
| rs1051266 | SLC19A1 | EQ-VAS | 0.0103 |
| rs10640 | AMT1 | MCS | 0.0023 |
| rs1127717 | ALDH1L1 | EQ-5D | 0.0064 |
| rs11545078 | GGH | EQ-VAS | 0.0162 |
| rs11627525 | MTHFD1 | MADRS | 0.0004* |
| rs11995525 | GGH | BDI-II | 0.0382 |
| MADRS | 0.0337 | ||
| EQ-VAS | 0.0383 | ||
| rs12347 | MTRR | CGI1 | 0.0329 |
| rs1532268 | MTRR | PCS | 0.0489 |
| rs16837178 | ALDH1L1 | BDI-II | 0.0493 |
| MADRS | 0.0460 | ||
| CGI1 | 0.0356 | ||
| rs1801133 | MTHFR | PCS | 0.0297 |
| MCS | 0.0481 | ||
| rs2236225 | MTHFD1 | MADRS | 0.0354 |
| MCS | 0.0322 | ||
| rs2273026 | SHMT1 | MADRS | 0.0147 |
| rs2273028 | SHMT1 | BDI-II | 0.0431 |
| MADRS | 0.0195 | ||
| rs2330183 | SLC19A1 | EQ-VAS | 0.0060 |
| rs2461838 | SHMT1 | BDI-II | 0.0159 |
| MADRS | 0.0338 | ||
| rs2853532 | TYMS | PCS | 0.0494 |
| rs3772426 | ALDH1L1 | MADRS | 0.0407 |
| MCS | 0.0180 | ||
| rs383028 | FOLH1 | MADRS | 0.0303 |
| EQ-VAS | 0.0143 | ||
| rs4817577 | GART | MCS | 0.0181 |
| rs4920037 | CBS | MADRS | 0.0361 |
| EQ-5D | 0.0195 | ||
| rs4933327 | MAT1A | MADRS | 0.0071 |
| rs588458 | FOLH1 | EQ-5D | 0.0003* |
| EQ-VAS | 0.0414 | ||
| MCS | 0.0140 | ||
| rs6435899 | ATIC | BDI-II | 0.0202 |
| MADRS | 0.0046 | ||
| CGI1 | 0.0275 | ||
| EQ-5D | 0.0212 | ||
| EQ-VAS | 0.0023 | ||
| MCS | 0.0488 | ||
| rs6494509 | MTFMT | PCS | 0.0077 |
| rs6668344 | MTR | BDI-II | 0.0489 |
| rs6800400 | ALDH1L1 | PCS | 0.0035 |
| EQ-5D | 0.0090 | ||
| rs7010484 | GGH | BDI-II | 0.0423 |
| CGI1 | 0.0087 | ||
| MCS | 0.0342 | ||
| rs7553194 | MTHFR | PCS | 0.0138 |
| rs8012229 | MTHFD1 | MADRS | 0.0023 |
| TSER | TYMS | PCS | 0.0088 |
| MCS | 0.0369 |
| SNP | Gene | Interacting outcome measure | Significance |
|---|---|---|---|
| rs1004474 | TYMS | BDI-II | 0.0456 |
| EQ-VAS | 0.0369 | ||
| rs1127717 | ALDH1L1 | EQ-5D | 0.0368 |
| rs13268472 | GGH | PCS | 0.0100 |
| rs16837171 | ALDH1L1 | MADRS | 0.0402 |
| MCS | 0.0038 | ||
| rs17102596 | MAT1A | BDI-II | 0.0135 |
| MADRS | 0.0151 | ||
| CGI1 | 0.0086 | ||
| MCS | 0.0002* | ||
| rs1801394 | MTRR | MCS | 0.0336 |
| rs1950902 | MTHFD1 | CGI1 | 0.0097 |
| rs2236225 | MTHFD1 | PCS | 0.0023 |
| EQ-5D | 0.0164 | ||
| EQ-VAS | 0.0224 | ||
| rs2276726 | ALDH1L1 | EQ-5D | 0.0175 |
| rs2372536 | ATIC | EQ-5D | 0.0069 |
| MCS | 0.0016 | ||
| rs2586154 | MTHFS | BDI-II | 0.0371 |
| rs2586183 | MTHFS | BDI-II | 0.0073 |
| CGI1 | 0.0111 | ||
| EQ-VAS | 0.0179 | ||
| MCS | 0.0026 | ||
| rs2733107 | MTHFS | CGI1 | 0.0347 |
| rs2853532 | TYMS | MCS | 0.0491 |
| rs3862534 | MAT1A | MADRS | 0.0443 |
| MCS | 0.0022 | ||
| rs4779165 | MTHFS | PCS | 0.0057 |
| rs4920037 | CBS | BDI-II | 0.0246 |
| CGI1 | 0.0333 | ||
| MCS | 0.0315 | ||
| rs535112 | CTH | EQ-VAS | 0.0350 |
| rs7010484 | GGH | MADRS | 0.0025 |
| rs8042012 | MTHFS | CGI1 | 0.0262 |
| MCS | 0.0061 | ||
| TSER | TYMS | MCS | 0.0182 |
Statistically significant main single nucleotide polymorphism effects
The rs11627525 SNP in the methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 1 (MTHFD1) gene was associated with MADRS (p = 0.0004, FDR = 0.0467). This association was not replicated with any of the other six outcome measures analysed (p > 0.05; FDR > 0.05).
A plot of mean MADRS at each study time point compared with rs11627525 genotype, stratifying by genotype [CC wild-type group and a combined CT and TT group since the homozygote variant allele frequency was so low (n = 4)], is given in Figure 15. At baseline, there was no difference observed [28.3 ± 0.4 (CC) compared with 29.0 ± 0.7 (CT/TT)]. The data suggest a more dramatic reduction in MADRS between baseline and 12 weeks between CC individuals (mean difference between baseline and 12 weeks: 6.11) and the combined CT/TT group (mean difference between baseline and 12 weeks: 9.82).
FIGURE 15.
Montgomery–Åsberg Depression Rating Scale depression scores by MTHFD1 rs11627525 genotype. Note: Graphs plot mean MADRS scores at each point with vertical bars showing standard errors of means.
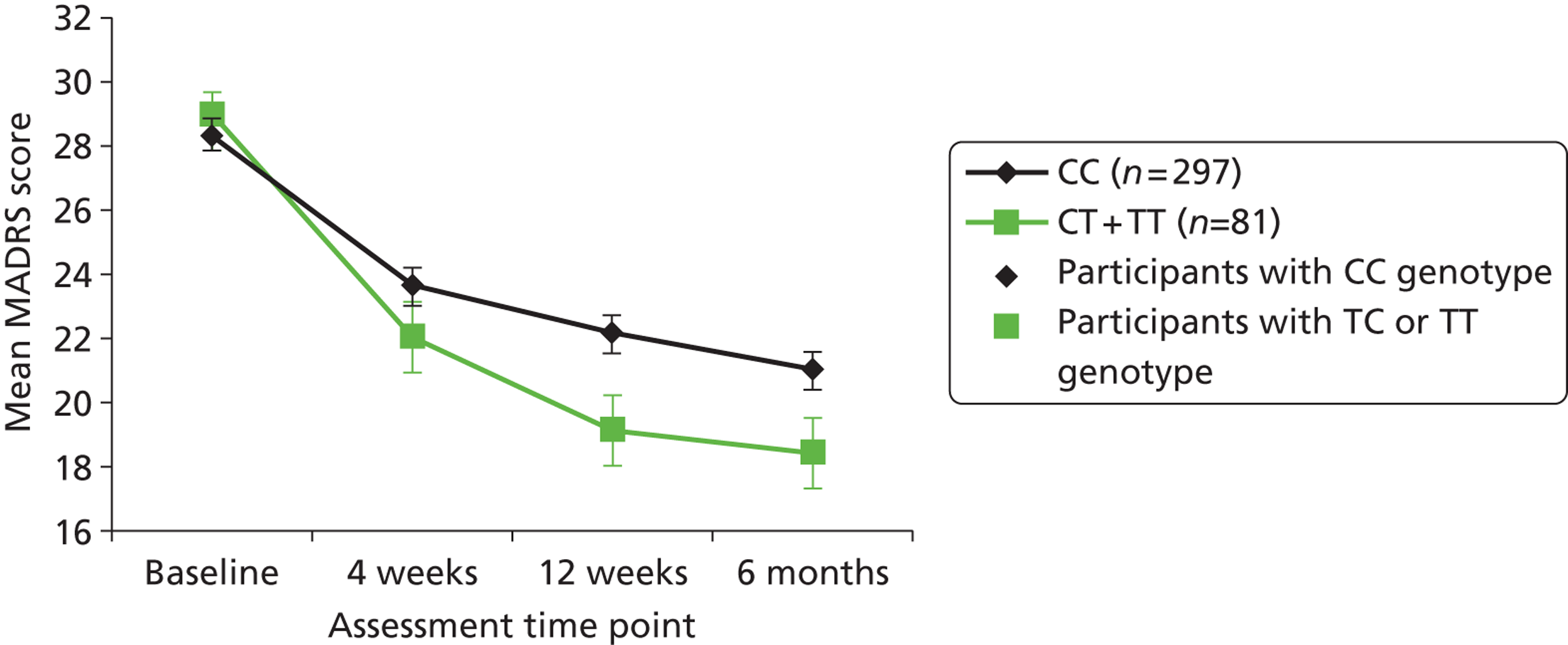
The rs588458 SNP in the folate hydrolase 1 (FOLH1) gene was associated with EQ-5D (p = 0.0003, FDR = 0.0337). This association was not replicated with any of the other six outcomes (FDR > 0.05), although both EQ-VAS (p = 0.0414) and SF-12 mental (p = 0.0140).
A plot of mean EQ-5D scores at baseline, 4 12 and 25 weeks, stratified into the three genotype groups, is given in Figure 16a. At baseline, TT carriers had a mean EQ-5D of 0.534 (± 0.024) – the mean scores for heterozygotes (TC carriers) were 0.049 lower, while they were 0.102 points lower for CC carriers. Similar differences were observed at 4 weeks with TC carriers being 0.078 lower than TT (0.625 ± 0.023) with CC carriers being 0.140 lower than TT. A significant difference in score was apparent at 12 weeks where the mean score (± SE) for TT carriers was 0.645 (± 0.023) compared with 0 for TC genotype (0.058 lower) and (0.140 lower than baseline) for individuals with the CC genotype. Similarly, at 25 weeks, mean EQ-5D for TT individuals (0.673 ± 0.023) was significantly higher (0.09 points) than TC which, in turn, was significantly higher (0.192 points) than for CC genotype). The overall trajectory of improvement (by EQ-5D) appears to stabilise first in the CC group (at around 4 weeks); then the TC group at around 12 weeks; whilst the wild-type TT group appear to continue to improve.
FIGURE 16.
Quality-of-life-scores by FOLH1 rs588458 genotype. Note: Graphs plot mean quality-of-life scores at each point with vertical bars showing standard errors of means.
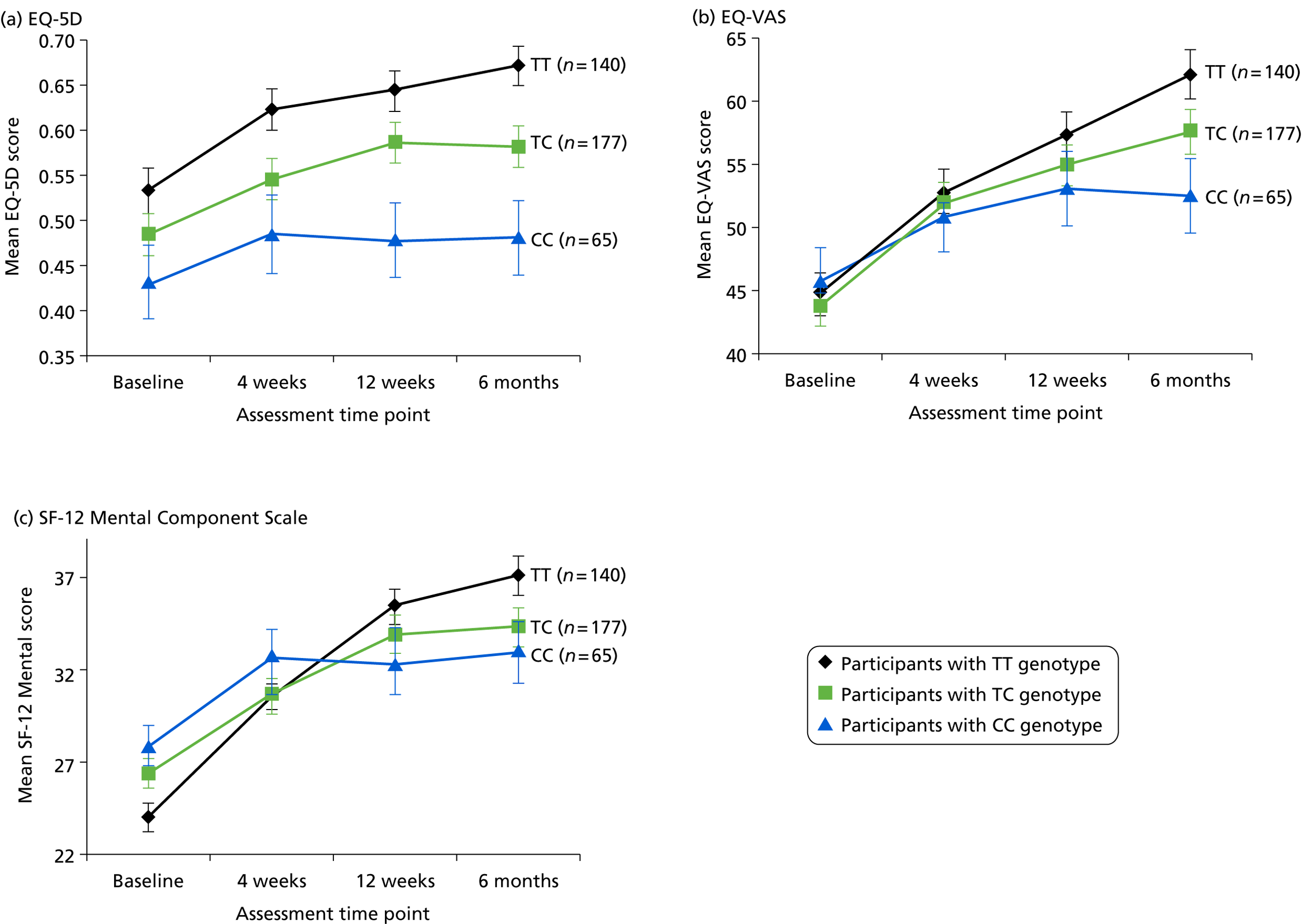
Plots for EQ-VAS and SF-12 Mental Component Scale (MCS) against rs588458 genotype are also provided (see Figure 16b and c, respectively) since the associations with rs588458 in the main SNP effect model gave p < 0.05. For mean EQ-VAS, there was a significant divergence between genotypes at 12 weeks with TT genotype (62.2 ± 1.9) significantly higher (difference of means = 4.5) than CT (57.7 ± 1.8) and CC (52.6 ± 2.8) (difference in means = 9.6). No difference was observed at any earlier time points. Mean SF-12 did not show an obvious divergence between genotypes at any of the time points though a moderate difference was seen at 12 weeks between TT (37.1 ± 1.0) and TC (34.3 ± 1.0).
Statistically significant single nucleotide polymorphism–treatment interaction
For the model incorporating the treatment interaction term, the association of the rs17102596 SNP in the methionine adenosyltransferase I alpha (MAT1A) gene and SF-12 mental status was statistically significant (p = 0.0002, FDR = 0.0255).
A plot of mean SF-12 Mental Component Score at each study time point stratified by genotype and treatment group (folic acid or placebo) is given in Figure 17. At baseline, no difference in SF-12 is observed between TT (25.0 ± 0.8) and TC + CC (25.2 ± 1.2) groups in the folate arm of the trial. In the placebo arm, there was a modest difference at baseline between TT (25.8 ± 0.9) and TC + CC (28.2 ± 1.3). In both arms of the trial, wild-type (TT) individuals showed a similar trajectory for increase in SF-12 with similar mean SF-12 at 25 weeks (folic acid = 25.0 ± 0.8; placebo = 25.8 ± 0.9). In individuals with TC or CC genotypes in the placebo group, there was a marginally steeper trajectory of the ΔSF-12 resulting in a significantly higher mean value at 12 weeks (39.6 ± 1.7) compared with TT individuals though much of this difference could be explained by the variability in baseline measure. This is considerably higher than those individuals with TC or CC genotype receiving folic acid (31.2 ± 1.7) (difference in mean = 8.4) whose Δ mean SF-12 trajectory stabilises after 4 weeks. The SNP effect model was run on the two arms of the trial separately (data not shown) with resulting statistically significant difference in both the folate group (p = 0.02) and the placebo group (p = 0.00006).
FIGURE 17.
SF-12 Mental Component Scores by MAT1A rs17102596 genotype – by treatment allocated. Note: Graphs plot mean SF-12 MCS scores at each point with vertical bars denoting standard error of the mean.
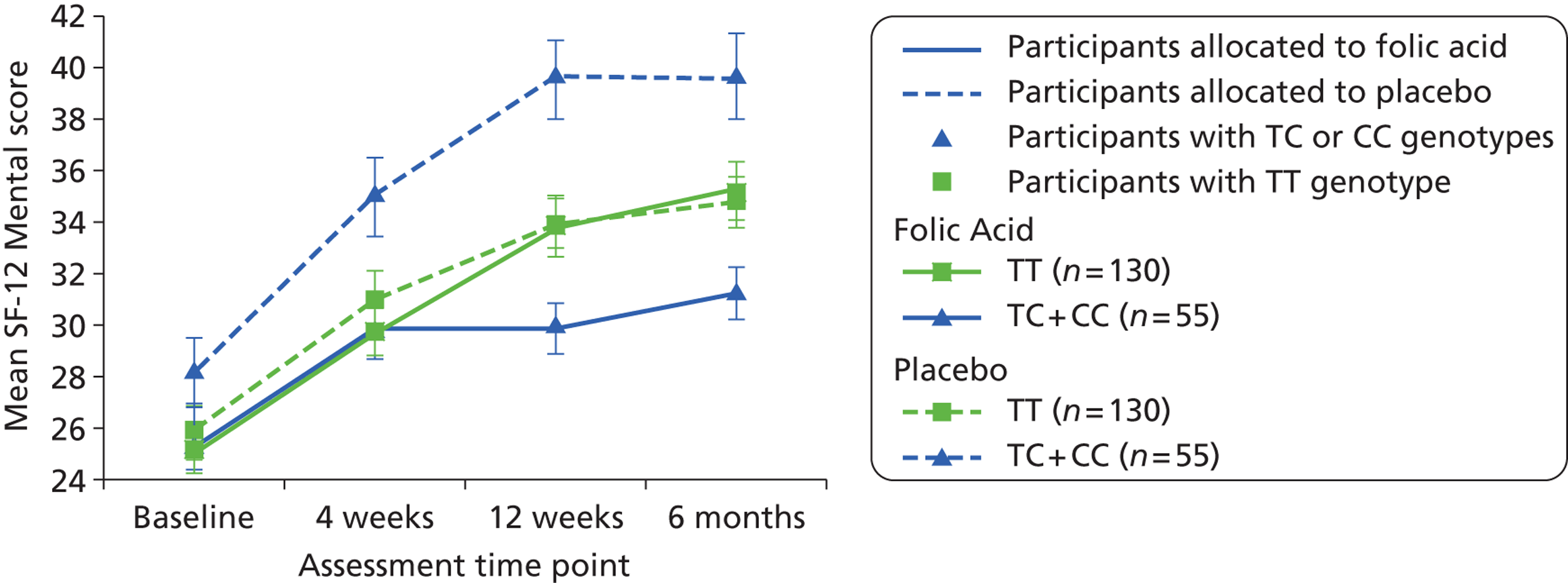
As three other outcomes measure (BDI-II, MADRS, and CGI severity of illness) gave p < 0.05 for tests of association with rs17102596-treatment interaction (see Table 31), these too were plotted (not shown). In all three instances the pattern demonstrated with SF-12 was not replicated and no clear distinction in the trajectory of the improvement determined by each outcome was seen.
Comprehensive cohort
We recruited participants to this cohort as part of the basic trial process until screening results were known. During the screening appointment the screener took a blood sample to assess B12 and serum folate status, and arranged a further research appointment within 14 days to confirm these results. As we then needed to treat patients who were B12 or folate deficient, we had to exclude them from the main trial. Nevertheless we invited them to continue in the ‘comprehensive cohort’ of recruited patients. Though they could not contribute to our evaluation of folic acid as adjunct to ADM, they help us to describe the experiences of the entire cohort of patients invited to join FolATED.
Recruitment and baseline characteristics of the comprehensive cohort
Of the 635 people who originally consented to take part, we could not randomise 156 (see Figure 2). Of these 68 dropped out between screening and randomisation, and another 42 at the randomisation interview, 36 because their BDI-II scores were too low for the trial. The remaining 46 people continued as ‘residual’ members of the comprehensive cohort alongside the 475 properly randomised into the trial. Table 3 tabulates the reasons for losses by stage of recruitment; and Appendix 7 does so by centre. Table 32 compares the baseline characteristics of the ‘residual’ cohort with those of trial completers: though residual members included more economically inactive females, the most notable difference is that, while Bangor recruited most to the trial, Wrexham recruited most to the residual cohort.
| Participant characteristic | Residual cohort (n = 46) | Trial completers (n = 440) |
|---|---|---|
| Age: mean (SD) in years | 47 (12) | 45 (13) |
| Gender: female | 35 (76%) | 280 (64%) |
| Race: white | 44 (96%) | 427 (97%) |
| Marital status | ||
| Single | 9 (20%) | 109 (25%) |
| Had a partner | 18 (39%) | 91 (21%) |
| Have a partner | 19 (41%) | 240 (54%) |
| No dependent children | 30 (65%) | 269 (61%) |
| Employment | ||
| Full time employed | 7 (15%) | 121 (28%) |
| Part time or in education | 10 (22%) | 124 (28%) |
| Inactive | 29 (63%) | 195 (44%) |
| Smoker? Never | 17 (37%) | 194 (44%) |
| Alcohol? None | 14 (30%) | 177 (40%) |
| Centre | ||
| Bangor | 7 (15%) | 223 (51%) |
| Wrexham | 24 (52%) | 110 (25%) |
| Swansea | 15 (33%) | 107 (24%) |
| Baseline BDI-II score | ||
| Mean (SD) | 34 (13) | 34 (10) |
Methods of analysis and results from the residual cohort
We followed the residual cohort like trial participants, but limited data collection to BDI-II at baseline, 4, 12 and 25 weeks. We also used similar methods of analysis. As the findings of fully imputed and complete case analyses were very similar, Table 33 displays the former. Though both residual and control groups show steady improvement in BDI-II scores from baseline over 25 weeks, the residual members start slower but finish with greater net improvement. We attribute this to the delayed effects of therapy to correct known deficiencies in B12 or folate.
| Patient sample | Time of analysis | n | Change from baseline | |||
|---|---|---|---|---|---|---|
| Mean | (SD) | 95% CI | Significance | |||
| Residual cohort (imputed) | 4 weeks | 45 | 4.33 | (10.82) | 1.00 to 7.65 | 0.012 |
| 12 weeks | 45 | 7.22 | (9.41) | 4.08 to 10.40 | < 0.001 | |
| 25 weeks | 45 | 14.53 | (13.50) | 9.67 to 19.40 | < 0.001 | |
| Control participants (imputed) | 4 weeks | 217 | 6.06 | (9.96) | 4.72 to 7.39 | < 0.001 |
| 12 weeks | 217 | 9.54 | (11.81) | 7.96 to 11.12 | < 0.001 | |
| 25 weeks | 217 | 11.33 | (12.56) | 9.65 to 13.01 | < 0.001 | |
| Patient sample | Time of analysis | Change in residual cohort minus change in control | ||||
| Mean | (SE) | 95% CI | Significance | |||
| Residual cohort (imputed) minus control participants (imputed) | 4 weeks | −1.73 | (1.75) | −5.18 to 1.72 | 0.323 | |
| 12 weeks | −2.32 | (1.62) | −5.50 to 0.86 | 0.151 | ||
| 25 weeks | 3.20 | (2.19) | −1.11 to 7.51 | 0.143 | ||
Systematic review of the effectiveness of folate in augmenting antidepressant medication
Introduction
There is general consensus in current clinical guidelines for depression that the augmentation of ADM with folate improves patient symptoms. However the supporting evidence is little more than biochemical theory and two small trials. 65,66 Indeed the authors of the current Cochrane systematic review concluded that there was limited evidence that adding folate to ADM was helpful, and recommended a trial like FolATED to test this hypothesis thoroughly. 50
Effects of interventions
Our updated search found no additional trials that met the criteria of the Cochrane review. 50 Hence our updated review analysed three trials – FolATED with 440 analysable participants, Coppen and Bailey with 100,65 and Godfrey et al. with 24. 66 As the primary analysis of the much larger FolATED trial favours the placebo (Figure 18), our updated review reverses the findings of the Cochrane review. As higher scores on both alternative outcomes – HDRS and BDI-II – show more depression, the resulting standardised mean difference of 0.05 (95% CI from –0.11 to 0.22; p = 0.52) also favours placebo. Furthermore there was no heterogeneity between studies. Hence there is no evidence to support the use of folic acid as an adjunct to ADM.
FIGURE 18.
Updated systematic review of the effectiveness of folic acid augmentation of antidepressants.
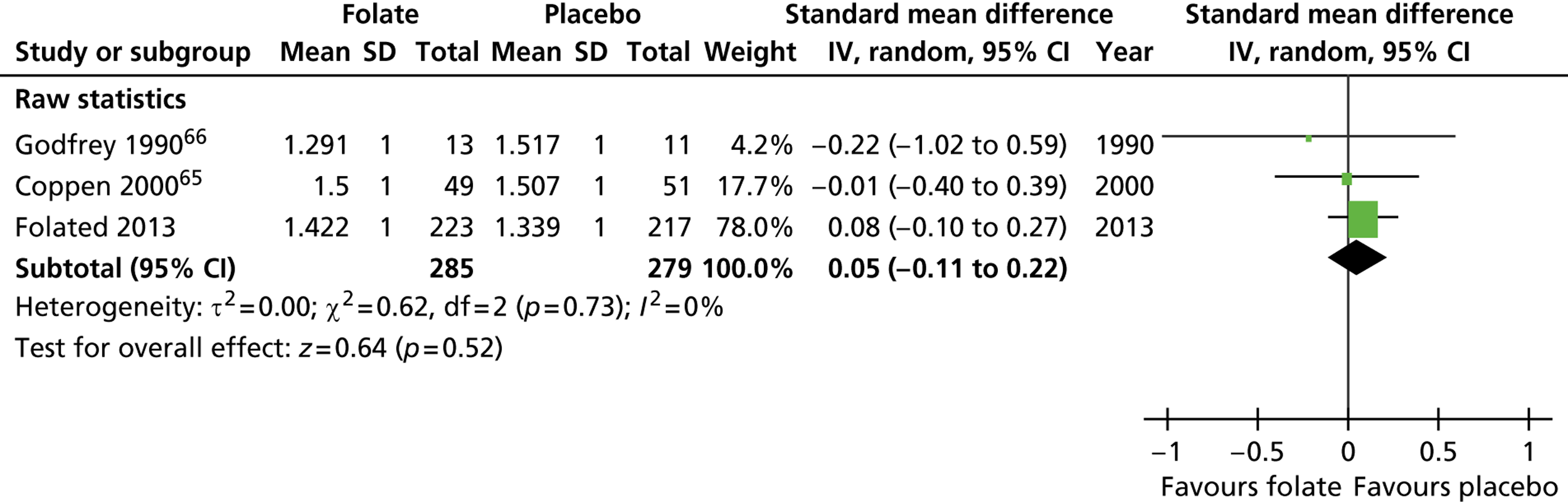
Chapter 4 Discussion
Summary of findings
Clinical effectiveness
FolATED shows that on average routinely adding 5 mg of folic acid to antidepressants prescribed at therapeutic dosages has no clinical benefits. The lack of any mean treatment effect is highly consistent across all outcome measures and time points. The trajectory of response across time is also consistent between measures. The one exception to this is the MCS of the SF-12, which shows a significant difference favouring placebo, especially at 12 weeks. It is not immediately clear whether this single statistically significant adverse result in a secondary outcome measure has any clinical significance. As we used many secondary measures in the trial, this may be a statistical artefact of multiple testing. Given high levels of correlation between the MCS and measures of depression;139,140 however, it seems unlikely that the MCS detected a real treatment effect not detected by the other outcome measures.
To put this disappointing finding into context, we record that the biochemical outcomes confirmed that folic acid was delivered successfully, as in other studies. 141 Fortunately the few patients who were deficient in baseline red cell folate yielded consistent evidence that augmenting ADM with folic acid improved their clinical outcomes. Furthermore folic acid appeared well tolerated, resulting in no more reports of side effects, AEs or SAEs than placebo.
Cost-effectiveness
The FolATED trial showed no significant clinical effect of 5 mg folic acid once daily for 12 weeks in new or existing users of antidepressants. The economic analysis suggested that folic acid might save costs of about £48 per patient (equivalent to about two-thirds of the cost of antidepressants). According to economic theory, one might therefore conclude that folic acid has positive net benefits, and should be recommended for use. However it seems unlikely that the prescribing of an additional, ineffective medicine would result in reduced costs, since the probability of folic acid being cost saving was not high. A more appropriate conclusion, therefore, is that the economic evaluation was unable to demonstrate cost-effectiveness. We interpret results suggestive of dominance in the primary analysis with caution, as the mean difference in effect was less than half a quality-adjusted day (or six depression-free days) over the 25 weeks of the trial. Furthermore complete case analysis and alternative methods of calculating QALYs did not contradict the principal findings given the small differences in costs and effects, and their associated uncertainty.
Although it is not generally possible for an intervention to be cost-effective when clinical effectiveness has not been established, the application of a cost minimisation analysis would be inappropriate for several reasons. 142,143 First, lack of significant effects does not confirm equivalence. 144 Second, equivalence in one clinical end point does not necessarily mean equivalence in others. Third, preference-based measures of outcome may reveal differences unrelated to the effect of the intervention on depression. Finally it is arguable that hypothesis testing is arbitrary and irrelevant to decision making because the intervention with the highest net benefit should be adopted whether or not difference in benefit reach conventional levels of statistical significance. 145
We considered it inappropriate to extend the economic analysis to evaluate the cost-effectiveness of pharmacogenetic testing, as the evidence had not supported the clinical utility of testing in relation to the predicting patients’ responsiveness to folic acid.
Genetics
Our study has identified two polymorphisms within genes of the one-carbon folate and methionine metabolism pathways associated with outcome regardless of treatment arm (a main SNP effect), and one polymorphism–treatment interaction which is associated with outcome. However the well characterised c.677C > T (rs1801133) polymorphism of the MTHFR associated in previous studies with depression risk or outcome76,77,79,128 did not modify the effect of either the antidepressant therapy or the folic acid supplementation. This seems to correlate with the study finding that folic acid and modification of homocysteine levels does not influence antidepressant outcome in treatment of moderate to severe depression.
MTHFD1 genetic variation association with outcome measures
Our data suggest an association between the rs11627525 SNP of the MTHFD1 gene locus and outcome. Though statistically significant only for the MADRS outcome, the trend was also present in the BDI-II and CGI measures of severity of illness. The main difference in MADRS between genotypes appears to occur between 4 and 12 weeks with the trajectory of improvement similar after the end of folic acid supplementation at 12 weeks. Given that the effect is seen in the study cohort as a whole regardless of arm, however, any relationship to folic acid supplementation is questionable. Further support comes from the lack of an association of the SNP with any outcome when analysed using the model incorporating the treatment interaction term.
The MTHFD1 gene encodes an enzyme which catalyses three sequential reactions in the inter-conversion of derivatives of one-carbon tetrahydrofolate, key substrates for methionine, thymidylate, and purine synthesis de novo. Given its function within the one-carbon folate metabolism, MTHFD1 is a very good candidate. As only one outcome measure reached statistical significance, however, this finding does not provide compelling evidence that the SNP in question might be a reliable predictive marker of antidepressant therapy outcome. Furthermore a difference of three points on MADRS scale after 12 weeks between the two genetics groups, as well as a decrease of 10.5 (variant carriers) and 7.3 (wild-type) is not clinically significant; so prognostic utility is debatable. However previous studies have estimated the minimal clinically relevant change in the MADRS as 1.6 to 1.9. 146
FOLH1 genetic variation association with outcome measures
In addition to the MTHFD1 SNP, our data suggest that the rs588458 SNP in the FOLH1 gene locus is significantly associated with outcome, particularly the SF-12 mental component score (MCS) well-being tool. This is an intronic variant with no obvious consequence for the functionality of the FOLH1 protein. Within the HapMap (http://hapmap.ncbi.nlm.nih.gov) CEPH population of Utah residents with ancestry from northern and western Europe, however, rs588458 is in high linkage disequilibrium (D″ = 1) with rs202676, a c.484T>C polymorphism, encoding a tyrosine to histidine substitution at amino acid residue 60 (p.Y60H). This variant has recently been identified as a potential risk factor for anencephaly, a neural tube defect, in a Chinese population. 147
FOLH1 acts as a glutamate carboxypeptidase which performs the initial hydrolysis of glutamate residues of the main dietary form of folates, folylpoly-γ-glutamates. Thus FOLH1 is a key regulator of intestinal absorption of dietary folate. Studies have demonstrated that the presence of a p.H475Y amino acid substitution polymorphism in FOLH1 is associated with impaired absorption of dietary folate, with associated low blood levels and hyperhomocystinuria. 148 Indeed studies have identified an association between the c.1561C>T (rs61886492) polymorphism and depressive symptoms on the Center for Epidemiologic Studies Depression scale (CES-D),149 and cognitive function. 150
The association with rs588458 is seen in our study group as a whole regardless of treatment arm. Folic acid is likely to neutralise any effect of FOLH1 genetic variation on folate levels, and subsequent improvement in outcome measures. As folic acid supplementation will swamp dietary folate levels, any effect of FOLH1 variation is likely to occur before or after administration of folic acid or placebo. From our data, baseline EQ-5D, as well as the trajectory of increase after baseline is greatest in wild-type (TT) and is still increasing between 12 and 25 weeks when both heterozygote (TC) and homozygote variant (CC) appear to plateau. It is plausible that the difference between genotypes is due to variability in the availability of dietary folates. Observations for two other outcome measures (EQ-VAS and SF-12 mental) appear to support the differences in outcome trajectory, particularly between 12 and 25 weeks.
However these observations need caution. Analysis of correlation between serum and red cell folate levels and genotype suggested that there was no effect on folate levels driven by FOLH1 genetic variation. It is possible that the association with outcome is independent of folate status. FOLH1, or GCPII as it is also known, is expressed in brain tissue and hydrolyses extracellular, N-acetylaspartylglutamate, N-acetylaspartate and glutamate thus affecting glutamatergic transmission. Protein expression and functionality of FOLH1 may therefore play an important role in the pathophysiology of psychiatric disorders. 151 It is possible that worse outcomes in patients with variant FOLH1 may be due to a GCPII functionality in the brain and its effect on the depressive symptoms rather than the FOLH1 effect on folate levels. So this is an encouraging observation that requires considerable further investigation.
MAT1A genetic variation and treatment group interaction with outcome measures
A single interaction between SNP rs17102596 and treatment group was associated with the SF-12 Mental Scale. This SNP lies within an intronic region of the MAT1A gene. MAT1A encodes the enzyme, methionine adenosyltransferase I alpha, which catalyses the transfer of adenosyl moiety of ATP to methionine, forming S-adenosylmethionine, the source of methyl groups for most biological methylations. Mutations of the MAT1A have been identified in patients with methionine adenosyltransferase deficiency, also known as hypermethionaemia. 152,153 So it is unlikely that variability in this gene would have any direct effect on either folate or homocysteine levels. This requires further investigation.
Curiously this association suggests that participants with the variant for the MAT1A polymorphism have better outcomes on the SF-12 Mental Component Scale when receiving placebo, and worse outcomes when receiving folate. However no other outcome analysed was able to demonstrate a similar effect. Thus we suspect this is a spurious observation. As such it should be viewed with caution until such time as it is independently validated.
Strengths and limitations of FolATED
FolATED is by far the largest trial to evaluate the clinical effectiveness and cost-effectiveness of folic acid to augment ADM for depression. We powered it to detect a clinically small difference between treatment groups, and followed rigorous procedures for randomisation and blinding. We recruited a wide range of patients being treated for moderate or severe depression in primary or secondary care. There were few exclusion criteria, and our sample included common comorbidities like substance misuse, often excluded from less pragmatic trials.
The reported response to ADM was within the range expected from a mixed study of new and continuing treatment episodes. After 25 weeks 36% of our sample had achieved ‘response to treatment’ defined as a 50% reduction in BDI-II score from baseline; this is consistent with clinical response rates of about 50% reported in the literature for new patients. 18 Also after 25 weeks 27% had achieved ‘remission’, defined as a BDI-II score less than or equal to 12;123 this is consistent with remission rates between 35% and 50% reported in the literature for new patients. 18 Again after 25 weeks 46% had improved to the point where they reported BDI-II scores less than 19 (i.e. depression so mild that they would not have been eligible for FolATED), also consistent with reported recovery rates.
This suggests that the lack of treatment effect for folic acid is not attributable to unusual treatment resistance to antidepressants in our sample. Indeed the trajectory of response to ADM in our sample, with a clear reduction in depressive symptoms over the first few weeks followed by slower improvement up to 25 weeks, is entirely consistent with the model reported in the large GENDEP sample of 807 patients with major depressive disorder treated with escitalopram or nortriptyline. 154
Also in line with the published literature, baseline severity of depressive symptoms predicted greater response to ADM. 155 Treatment with SSRIs also led to better outcomes over 25 weeks than treatment with other antidepressants. This supports previous reviews suggesting better practical outcomes for SSRIs are due to much better completion of therapeutic courses for SSRIs compared with other antidepressants like TCAs. 156 Other findings also accord with observations from antidepressant trials, with both continuing, rather than new, antidepressant treatment, and economic inactivity like unemployment or long-term sickness, leading to poorer response. In short FolATED was both robust and representative.
Interpretation
Clinical effectiveness
Our negative outcomes contrast with positive findings in two small, selective trials. Coppen and Bailey reported a significantly greater reduction in HDRS scores in females treated with fluoxetine and 400 µg of folic acid, compared with fluoxetine and placebo. 65 They suggested that males required higher doses of folic acid. In response we selected 5 mg of folic acid for FolATED to cover the entire dose–response curve They achieved very high clinical response rates – 82% in the folic acid arm and 62% in the placebo arm – surpassing those in the general literature, because their sample was much less representative than that recruited by FolATED. For example they excluded patients with continuing episodes of depression, previous poor response to fluoxetine, or comorbid substance use. One must also question the robustness of their blinding, especially in handling blood results, whereas FolATED took great care at all stages to avoid unblinding clinicians, patients and researchers. While females treated with folic acid by Coppen showed a 21% reduction in homocysteine levels, similar to the FolATED sample, the males did not. In FolATED, however, there was no gender difference in homocysteine levels following treatment with folic acid, suggesting that we covered the dose–response curve, yet without treatment effect.
In contrast Godfrey and colleagues recruited only from secondary care, specifically patients with folate deficiency (viz. red cell folate less than 200 µg) but suffering from a wide range of psychiatric disorders including depression and schizophrenia; hence they had to adapt clinical outcomes to diagnosis. 66 This makes comparison difficult as FolATED examined the routine use of folic acid augmentation in depressed patients recruited predominantly from primary care. Though Godfrey used 15 mg of the biologically active MTHF, the gross differences between studies generate no evidence for the superiority of MTHF over folic acid.
Hence, after updating the systematic review50 by including FOLATED, we found no evidence to support the previous suggestion that folic acid could improve the clinical benefits of ADM.
Cost-effectiveness
Our economic evaluation showed that folic acid, clinically ineffective, could not be a cost-effective use of resources. With no other economic evaluations of folic acid in managing depression, we assessed external validity in comparison with other studies of depression. For example the AHEAD trial pragmatically compared TCAs, SSRIs and lofepramine in UK primary care, and provided the prototype for our resource use questionnaire. Though AHEAD followed patients for 12 months, their rates of contact with healthcare professionals was comparable pro rata with FolATED findings. AHEAD trial participants visited GPs on 9.1 occasions over 12 months, compared with 3.6 over 25 weeks in FolATED; the mean length of inpatient stay was 1.05 days in AHEAD, compared with 0.58 in FolATED. With one exception we consider our participants’ use of NHS and PSS resources to be generally representative of UK practice, and typical of locations other than the recruiting sites. The exception lies in divergence from the NICE recommendation that people with moderate to severe depression receive psychological therapy, as only 6.5% of participants did so. Fortunately there is no evidence that greater use of psychotherapy, engendered for example by the English ‘Improving Access to Psychological Therapy’ programme would have changed any of our insignificant findings. As our genetic analysis found no support for pharmacogenetic testing in this field, that too could not be a cost-effective use of resources.
Genetic implications
Our study has identified three polymorphisms associated with modification of depression outcomes. For MTHFD1 rs11627525 and FOLH1 rs588458 this association was independent of treatment allocated; in particular the new link between EQ-5D and the rs588458 genotype needs further study. The MAT1A rs17102596 SNP modified the SF-12 Mental Component Scale through interaction between SNP and treatment.
In general, however, there is little evidence that any of the variants analysed can predict patients’ response to ADM or the efficacy of folic acid. Even the three SNPs showing association could not replicate this with any other outcome measure. Above all none of the polymorphisms analysed could achieve statistically significant modification of BDI-II, the primary outcome measure.
However we limited the variants analysed to genes within the one-carbon folate or methionine synthesis pathways. It is possible that other factors, genetic or otherwise, may influence patient response to ADM or folic acid. Thus it is conceivable that in future a whole-genome approach to the FolATED data set could identify markers of efficacy beyond those already analysed.
Biochemical interpretation
Folate is a naturally occurring B vitamin, needed in the brain to synthesise serotonin, noradrenaline and dopamine. In humans the biologically active form is 5-methyltetrahydrofolate (5-MTHF), which is derived from ingested folates and is the form taken up by cells and transported to the cerebrospinal fluid (CSF) via folate receptors. Folic acid is an inactive, stable, synthesised, oxidised form of folate, not naturally found in the human body; it has to undergo transformation to 5-MTHF – an inefficient process as unmetabolised folic acid remains in the blood even after an intake of only 400 µg, the recommended daily intake of folate. Folinic acid (calcium folinate) is a stable reduced form of 5-MTHF that needs no transformation before entering the CSF. 157 There is evidence that commercial preparations of folic acid can compete with 5-MTHF for the folate receptor and thus exacerbate a folate deficiency in the central nervous system (CNS). 158 Hence we wonder whether our finding that folic acid had a statistically significant negative effect on the widely used SF-12 MCS was a manifestation of such a folate deficiency rather than the type 1 error that we first supposed.
Thus better understanding of the one-carbon folate and methionine biosynthesis pathways have raised questions about the most appropriate formulation of folate to use clinically for functional folate deficiency. Stahl argues that 5-MTHF is therapeutically better than folic acid as it bypasses conversion of folic acid to the biologically active 5-MTHF, which may be difficult for some patients. 159 Furthermore high doses of inactive folic acid may compete with 5-MTHF for transport across the blood–brain barrier, potentially reducing active 5-MTHF in the brain. Other reasons why 5-MTHF synthesis may be difficult include inhibition of the enzyme by anticonvulsants. Fortunately we avoided this potential confounding factor by excluding patients on anticonvulsant medication.
Against this biomedical background our rigorous and powerful trial has established that folic acid has no general role as adjunct in antidepressant therapy. However studies in patients with cardiovascular disease have shown that higher doses of folic acid produce greater concentrations of 5-MTHF in plasma. 160 Moreover the reductions in homocysteine in FolATED participants on folic acid relative to those on placebo suggests that they were successfully metabolising folic acid to 5-MTHF. Nevertheless we suspect that the beneficial increase in 5-MTHF was masked by the excess folic acid that competed with 5-MTHF for the folate receptors and led to the negative results.
Recommendations for research
Folate and depression
Our inclusive multi-disciplinary trial has shown beyond doubt that folic acid is an ineffective adjunct in antidepressant therapy. Furthermore better understanding of the one-carbon pathway offers a plausible explanation why. 158 Before we can write off folic acid entirely, however, we recommend updating the Cochrane systematic review and meta-analysis,64 and summarising the systematic review data more thoroughly than was possible in Chapter 3 (see Systematic review of the effectiveness of folate in augmenting antidepressant medication).
At the time of our initial grant proposal little information was available on the use of 5-MTHF or folinic acid in patients with depression. Now there is evidence that 5-MTHF given as adjunct or monotherapy reduces depressive symptoms in patients with low folate levels or alcoholism, and improves cognitive function and reduces depressive symptoms in elderly patients with dementia and folate deficiency. 161 Furthermore there are long-standing concerns that folate may increase cancer risk, mask B12 deficiency and exacerbate depressive symptoms. As 5-MTHF may reduce some of these risks, we judge that that is now a candidate for a large multi-centre trial. There is also evidence that adjunctive 5-MTHF reduces depressive symptoms in patients who were partially responsive or non-responsive to a selective SSRI. 161
In that context we offer the design of FolATED as a proven model. With the benefit of hindsight, however, we judge that a trial recruiting for 1 year in 10 centres would yield better value for money than one recruiting for 3 years in three centres.
Recruitment
Like many trials FolATED recruited more slowly than expected. We based the original target of randomising 550 participants in three centres over 2 years – slightly less than 24 per month across all three centres – on experience with a previously successful trial in one of the three centres. Initially, however, the complex design of FolATED, designed to exclude patients suffering from folate or B12 deficiency while allowing psychiatric teams to optimise antidepressant treatment in normal clinical practice (Figure 1), restricted randomisation across all three centres to eight a month. We attribute our success in eventually increasing these initial rates by 50% to 12 a month across all three centres to a lot of hard work, a combination of creative protocol changes summarised in Appendix 4, and the mutual support engendered by joint training and joint monthly telephone conferences about management and recruitment.
Late in the conduct of FolATED we used a qualitative reflective data collection tool to undertake a retrospective study of recruitment and retention issues (see Appendix 11). We sought, not to evaluate which methods were the most effective in increasing recruitment and retention, but rather to gain insights into problems we faced and methods we used to overcome recruitment and retention problems within FolATED. We found little research into participants’ preferences between different approaches. This leads us to recommend that future large trials include smaller trials or qualitative studies or both to assess the effectiveness of materials used in recruitment such as posters, leaflets, patient information sheets and newsletters to participating practitioners.
Chapter 5 Conclusions
This rigorous and adequately powered trial has established that folic acid is not an effective adjunct to antidepressant therapy. The NIHR Health Technology Assessment programme commissioned FolATED at a time when there was considerable scientific interest in the role of folate in the aetiology of depression and in treating depression. During the lifetime of FolATED this interest has grown, with increasing international pressure to use folate as an adjunct to antidepressants and in algorithms for treating depression.
The unequivocally negative findings of FolATED demand reappraisal of this consensus and associated treatment guidelines. Thus there is a strong case for research to investigate whether future trials of 5-MTHF would yield value for money.
Acknowledgements
Contributions of authors
All 19 authors contributed to design and data collection or analysis and interpretation, commented on successive drafts, and approved the version to be published. More specifically:
Emma Bedson (Research Officer, Clinical Trials) was co-applicant and trial manager; she contributed to developing and implementing the design, management and quality assurance of the trial; and coordinated this report.
Diana Bell (Research Nurse, Clinical Trials) was local trial coordinator for North West Wales; she coordinated recruitment, data collection, local reporting and administration.
Daniel Carr (Tenure Track Fellow Pharmacogenetics) contributed to developing and implementing the genetic component of the study and interpreting data; he led the writing of the pharmacogenetics sections of this report.
Ben Carter (Lecturer, Statistics) was senior trial analyst; he undertook validatory statistical analysis and contributed to interpreting data and drafting this report.
Dyfrig Hughes (Professor of Pharmacoeconomics) was applicant and principal investigator for health economics.
Andrea Jorgensen (Lecturer, Medical Statistics and Pharmacogenetics) undertook the pharmacogenetic analysis, and contributed to interpreting data and drafting this report.
Helen Lewis (Research Officer, Clinical Trials) was local trial coordinator for North East Wales; she contributed to developing and coordinating recruitment, data collection, local reporting and administration, and led the writing of Appendix 11 describing recruitment into FolATED.
Keith Lloyd (Professor of Psychological Medicine) was co-applicant, clinical chief investigator and principal investigator for Swansea.
Andrew McCaddon (Principal in General Practice) was principal investigator for the sub-study of MMA.
Stuart Moat (Director, Medical Biochemistry) was co-applicant and principal investigator for medical biochemistry.
Joshua Pink (Doctoral Student, Health Economics) contributed to analysing and interpreting the economic data, and drafting this report.
Munir Pirmohamed (NHS Professor of Pharmacogenetics) was co-applicant and principal investigator for pharmacogenetics.
Seren Roberts (Research Fellow, Psychology) was co-applicant and principal investigator for North East Wales; she coordinated the development and implementation of the study design, led the writing of the published protocol, and contributed to interpreting data and drafting this report.
Ian Russell (Professor of Clinical Trials) was co-applicant, methodological chief investigator and chair of the trial management group; he edited this report.
Yvonne Sylvestre (Research Officer, Statistics) was assistant trial statistician; she undertook primary statistical analysis and contributed to interpreting data and drafting this report.
Richard Tranter (Senior Lecturer, Psychological Medicine and Clinical Trials) was co-applicant and principal investigator for North West Wales; he contributed to the development and implementation of the study design, and led contributed to interpreting data and drafting this report.
Rhiannon Whitaker (Associate Director, Clinical Trials) was senior trial statistician; she led the development and implementation of data management, analysis and reporting.
Clare Wilkinson (Professor of General Practice) was co-applicant and principal investigator for general practice.
Nefyn Williams (Senior Lecturer in General Practice) contributed to developing and implementing recruitment from primary care and prescribing data for economic evaluation; assessing laboratory test results and communicating abnormal results to clinicians; and interpreting data.
Profound thanks to:
The National Institute for Health Research Health Technology Assessment Programme for funding FolATED; and their staff for generous and patient support.
The trial participants, especially for completing a high proportion of responses.
General practitioners, psychiatrists and other healthcare practitioners for generously referring participants to the study.
Psychiatrists who generously screened participants and consented them into the study – Yinka Abiodun, Ahmed Alastal, Angela Ambrose, Aneeba Anwar, Mohammed Butt, Padmaja Chalsani, Jude Chukwuma, Alexis Economou, Fiona Farquahar, Qamar Jabeen, Archana Jauhari, Kristy Khonji, Ravi Krishnaiah, Stefanie Linden, Danielle Loveday, Richard Mellor, Jorge Diaz-Monoz, Emma Morgan, Jisha Mukundan, Askey Nair, Subhash Pinnaka, Mohtasim Qamruddan, Yusuf Ragoonwala, Javier Rodriguez-Mendieta, Divya Sakuja, Saurabh Saxena, Adeel Siddiqui, Nick Smith, Mujahid Ali Syed, Faye Tarrant, Ranjit Tatineni, Johannes Thome and Joe John Vattakatuchery.
Researchers who assiduously recruited participants and completed trial documentation – Kim Davies (North West Wales), Azzam Farroha and Faye Griffith-Noble (Swansea), and Diane Pasterfield (North East Wales).
Research professionals from the National Institute for Social Care and Health Research – Clinical Research Centre (NISCHR CRC) who energetically supporting the trial in many ways – Nathan Bray, Theresa Gill, Michelle Grey, Jenny Griffiths, Martin Jones, Adwoa Hughes-Morley, Vaughn Price, Leanne Quinn, Julia Roberts, Farah Shiraz, Sue Thomson, Melissa Van Der Bijl and Sally Williams.
Staff from the biochemistry, haematology and pharmacy departments of Singleton Hospital in Swansea, Wrexham Maelor Hospital and Ysbyty Gwynedd in Bangor– Keith Dadds, Gareth Davies, Robert Jones, Christine Lloyd, Sue Lord, Dewi Morris, Adele Sparks, Rachel Still, Lisa Wakeman, Robert Walters, David Watson and Chris Wight.
Angela Hollett and Stephanie Rodger (pharmacy technicians) who helped to analyse prescription data.
Vittorio Simeon of the Wolfson Centre for Personalised Medicine at the University of Liverpool, who helped to analyse genetic data.
Daphne Russell of Swansea University College of Medicine for scientific proofreading.
And not least the staff of the North Wales Organisation for Randomised Trials in Health (NWORTH) – Darren Baker, Zoe Hoare and Rashesh Mehta for data management, Debbie Skelhorn for quality assurance, and Victoria Buckley for administering FolATED.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Roberts SH, Bedson E, Hughs D, Lloyd K, Moat S, Pirmohamed M, et al. Folate Augmentation of Treatment – Evaluation for Depression (FolATED: protocol of a randomised controlled trial). BMC Psychiatry 2007;15:65.
Carr DF, Whiteley G, Alfirevic A, Pirmohamed M. Investigation of inter-individual variability of the one-carbon folate pathway: a bioinformatic and genetic review. Pharmacogenomics J 2009;9:291–305.
References
- Integrating Mental Health into Primary Care: A Global Perspective. Geneva, Switzerland: WHO; 2008.
- Investing in Mental Health. Geneva, Switzerland: WHO; 2003.
- Sweet D, Beaumont J. Health Clinical Research Centre. London: Office for National Statistics; 2011.
- Depression. Geneva, Switzerland: WHO; 2011.
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011;21:655-79. http://dx.doi.org/10.1016/j.euroneuro.2011.07.018.
- Kemp PA, Davidson J. Routes onto Incapacity Benefit: Findings from a Survey of Recent Claimants. Leeds: Department for Work and Pensions; 2007.
- Henderson J, Henderson G, Lavikainen J, McDaid D. Actions Against Depression: Improving Mental Health and Well Being by Combating the Adverse Health, Social and Economic Consequences of Depression. Luxembourg: European Commission; 2004.
- Waraich P, Goldner EM, Somers JM, Hsu L. Prevalence and incidence studies of mood disorders: a systematic review of the literature. Can J Psychiatry 2004;49:124-38.
- Depression: The NICE Guidance on the Treatment and Management of Depression in Adults. London: British Psychological Society and Royal College of Psychiatrists; 2010.
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 1998;55:580-92. http://dx.doi.org/10.1001/archpsyc.55.7.580.
- The Psychological Care of Medical Patients: Practical Guide. London: Royal College of Psychiatrists; 2003.
- Thomas CM, Morris S. Cost of depression among adults in England in 2000. Br J Psychiatry 2003;183:514-19. http://dx.doi.org/10.1192/bjp.183.6.514.
- Kennedy N, Foy K. The impact of residual symptoms on outcome of major depression. Curr Psychiatry Rep 2005;7:441-6. http://dx.doi.org/10.1007/s11920-005-0065-9.
- Coryell W, Akiskal HS, Leon AC, Winokur G, Maser JD, Mueller TI, et al. The time course of nonchronic major depressive disorder: uniformity across episodes and samples. Arch Gen Psychiatry 1994;51:405-10. http://dx.doi.org/10.1001/archpsyc.1994.03950050065007.
- Posternak MA, Miller I. Untreated short-term course of major depression: meta-analysis of outcomes from studies using wait-list control groups. J Affect Disord 2001;66:139-46. http://dx.doi.org/10.1016/S0165-0327(00)00304-9.
- Keller MB, Klerman GL, Lavori PW, Coryell W, Endicott J, Taylor J. Long-term outcome of episodes of major depression: clinical and public health significance. JAMA 1984;252:788-92. http://dx.doi.org/10.1001/jama.1984.03350060032024.
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry 1998;55:694-700. http://dx.doi.org/10.1001/archpsyc.55.8.694.
- Anderson IM, Ferrier IN, Baldwin RC, Cowen PJ, Howard L, Lewis G, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol 2008;22:343-96. http://dx.doi.org/10.1177/0269881107088441.
- Donoghue J. Antidepressant use patterns in clinical practices: comparisons among tricyclic antidepressants and selective serotonin reuptake inhibitors. Acta Psychiatr Scand 2000;101:57-61. http://dx.doi.org/10.1111/j.1600-0447.2000.tb10949.x.
- Mulrow CD, Williams JWJ, Trivedi M, Chiquette E, Aguilar C, Cornell JE, et al. Treatment of Depression-newer Pharmacotherapies: Summary. Rockville, MD: US Agency for Healthcare Research and Quality; 1999.
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA 2002;287:1840-7. http://dx.doi.org/10.1001/jama.287.14.1840.
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: STAR*D report. Am J Psychiatry 2006;163:1905-17. http://dx.doi.org/10.1176/appi.ajp.163.11.1905.
- Simon GE, Goldberg DP, von Korff M, Ustun TB. Understanding crossnational differences in depression prevalence. Psycholog Med 2002;32:585-94.
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095-105. http://dx.doi.org/10.1001/jama.289.23.3095.
- Kupfer DJ. Long-term treatment of depression. J Clin Psychiatry 1991;52:28-34.
- Angst J, Gamma A, Sellaro R, Lavori PW, Zhang H. Recurrence of bipolar disorders and major depression: life-long perspective. Eur Arch Psychiatry Clin Neurosci 2003;253:236-40.
- Roberts SH, Bedson E, Tranter R. Half-baked? B vitamins and depression (editorial). Am J Clin Nutr 2010;92:269-70.
- Pauwels EKJ. The folate puzzle: IV – folate and depression. Drugs Future 2008;33:591-5. http://dx.doi.org/10.1358/dof.2008.033.07.1236397.
- Sarris J, Schoendorfer N, Kavanagh DJ. Major depressive disorder and nutritional medicine: review of monotherapies and adjuvant treatments. Nutr Rev 2009;67:125-31. http://dx.doi.org/10.1111/j.1753-4887.2009.00180.x.
- Robinson DS. Vitamins, monoamines, and depression. Prim Psychiatry 2009;16:19-21.
- Folate: Dietary Supplement Fact Sheet. Bethesda, MD: National Institutes of Health; 2009.
- Morris DW, Trivedi M, Rush AJ. Folate and unipolar depression. J Altern Complement Med 2008;14:277-85. http://dx.doi.org/10.1089/acm.2007.0663.
- Astorg P, Couthouis A, De Courcy GP, Bertrais S, Arnault N, Meneton P, et al. Association of folate intake with the occurrence of depressive episodes in middle-aged French men and women. Br J Nutr 2008;100:183-7. http://dx.doi.org/10.1017/S0007114507873612.
- Sanchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B6 and vitamin B12 intake and depression in the SUN cohort study. J Hum Nutr Diet 2009;22:122-33.
- Wantanabe H, Konno Y, Matsumato M, Nomachi S, Masaki K, Okayama H. Relationship of dietary folate intake, serum folate, and homocysteine with depression in young women. J Obstet Gynecol Neonatal Nurs 2011;40:S92-3.
- Tolmunen T, Hintikka J, Ruusunen A, Voutilainen S, Tanskanen A, Valkonen VP, et al. Dietary folate and the risk of depression in Finnish middle-aged men: prospective follow-up study. Psychother Psychosom 2004;74:334-9.
- Carney MWP. Serum folate values in 423 psychiatric patients. BMJ 1967;4:512-16. http://dx.doi.org/10.1136/bmj.4.5578.512.
- Ng T, Feng L, Niti M, Kua E, Yap K. Folate, vitamin B12, homocysteine, and depressive symptoms in a population sample of older Chinese adults. J Am Geriatr Soc 2009;57:871-6. http://dx.doi.org/10.1111/j.1532-5415.2009.02229.x.
- Abou-Saleh MT, Coppen A. Serum and red blood cell folate in depression. Acta Psychiatr Scand 1989;80:78-82. http://dx.doi.org/10.1111/j.1600-0447.1989.tb01303.x.
- Alpert JE, Mischoulon D, Nierenberg AA, Fava M. Nutrition and depression: focus on folate. Nutrition 2000;16:544-6. http://dx.doi.org/10.1016/S0899-9007(00)00327-0.
- Morris MS, Fava M, Jacques PF, Selhub J, Rosenberg IH. Depression and folate status in the US population. Psychother Psychosom 2003;72:80-7. http://dx.doi.org/10.1159/000068692.
- Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol 2005;19:59-65. http://dx.doi.org/10.1177/0269881105048899.
- Kim J-M, Stewart R, Kim S-W, Yang S-J, Shin I-S, Yoon J-S. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br J Psychiatry 2008;192:268-74. http://dx.doi.org/10.1192/bjp.bp.107.039511.
- Skarupski KA, Tangney C, Li H, Ouyang B, Evans DA, Morris MC. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr 2010;92:330-5. http://dx.doi.org/10.3945/ajcn.2010.29413.
- Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health 2007;61:631-7. http://dx.doi.org/10.1136/jech.2006.050385.
- Kendrick T, Dunn N, Robinson S, Oestmann A, Godfrey K, Cooper C, et al. A longitudinal study of blood folate levels and depressive symptoms among young women in the Southampton Women’s Survey. J Epidemiol Community Health 2008;62:966-72. http://dx.doi.org/10.1136/jech.2007.069765.
- Payne M, Jamerson BD, Potocky CF, . Natural food folate and late-life depression. J Nutr Elder 2009;28:348-58. http://dx.doi.org/10.1080/01639360903417181.
- Walker JG, Mackinnon AJ, Batterham P, Jorm AF, Hickie I, McCarthy A, et al. Mental health literacy, folic acid and vitamin B12, and physical activity for the prevention of depression in older adults: randomised controlled trial. Br J Psychiatry 2010;197:45-54. http://dx.doi.org/10.1192/bjp.bp.109.075291.
- Ford AH, Flicker L, Thomas J, Norman P, Jamrozik K, Almeida OP. Vitamins B12, B6, and folic acid for onset of depressive symptoms in older men: results from a 2-year placebo-controlled randomized trial. J Clin Psychiatry 2008;69:1203-9. http://dx.doi.org/10.4088/JCP.v69n0801.
- Taylor MJ, Carney S, Geddes J, Goodwin G. Folate for depressive disorders. Cochrane Database Syst Rev 2003;2. http://dx.doi.org/10.1002/14651858.CD003390.
- Bottiglieri T. Homocysteine and folate metabolism in depression. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:1103-12. http://dx.doi.org/10.1016/j.pnpbp.2005.06.021.
- Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan AJ, Breteler MM. Vitamin B12, folate, and homocysteine in depression: the Rotterdam Study. Am J Psychiatry 2002;159:2099-101. http://dx.doi.org/10.1176/appi.ajp.159.12.2099.
- Sachdev PS, Parslow RA, Lux O, Salonikas C, Wen W, Naidoo D, et al. Relationship of homocysteine, folic acid and vitamin B12 with depression in a middle-aged community sample. Psycholog Med 2005;35:529-38. http://dx.doi.org/10.1017/S0033291704003721.
- Tolmunen T, Hintikka J, Voutilainen S, Ruusunen A, Alfthan G, Nyyssonen K, et al. Association between depressive symptoms and serum concentrations of homocysteine in men: population study. Am J Clin Nutr 2004;80:1574-8.
- Almeida OP, McCaul K, Hankey GJ, Norman P, Jamrozik K, Flicker L. Homocysteine and depression in later life. Arch Gen Psychiatry 2008;65:1286-94. http://dx.doi.org/10.1001/archpsyc.65.11.1286.
- Scott J, Dinn J, Wilson P, Weir D. Pathogenesis of subacute combined degeneration: result of methyl group deficiency. Lancet 1981;2:334-7. http://dx.doi.org/10.1016/S0140-6736(81)90649-8.
- Selhub J, Morris MS, Jacques PF. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci U S A 2007;104:1995-2000. http://dx.doi.org/10.1073/pnas.0709487104.
- Quinlivan EP. In vitamin B12 deficiency, higher serum folate is associated with increased homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci U S A 2008;105:1387-8. http://dx.doi.org/10.1073/pnas.0711541105.
- Botez MI, Young SN, Bachevalier J, Gauthier S. Folate deficiency and decreased brain 5-hydroxytryptamine synthesis in man and rat. Nature 1979;278:182-3. http://dx.doi.org/10.1038/278182a0.
- Lazarou C, Kapsou M. The role of folic acid in prevention and treatment of depression: an overview of existing evidence and implications for practice. Complement Ther Clin Pract 2010;16:161-6. http://dx.doi.org/10.1016/j.ctcp.2010.01.003.
- Parker G, Mitchell P, Wilhelm K, Menkes DB, Snowdon J, Schweitzer I, et al. Are the newer antidepressant drugs as effective as established physical treatments? Results from an Australasian clinical panel review. Aus N Z J Psychiatry 1999;33:874-81. http://dx.doi.org/10.1046/j.1440-1614.1999.00648.x.
- Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry 1997;154:426-8.
- Alpert M, Silva RR, Pouget ER. Prediction of treatment response in geriatric depression from baseline folate level: interaction with SSRI or tricyclic antidepressant. J Clin Psychopharmacol 2003;23:309-13. http://dx.doi.org/10.1097/01.jcp.0000084024.22282.cd.
- Taylor MJ, Carney SM, Goodwin GM, Geddes JR. Folate for depressive disorders: systematic review and meta-analysis of randomised controlled trials. J Psychopharmacol 2004;18:251-6.
- Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: randomised, placebo controlled trial. J Affect Disord 2000;60:121-30. http://dx.doi.org/10.1016/S0165-0327(00)00153-1.
- Godfrey PSA, Toone BK, Carney MWP, Flynn TG, Bottiglieri T, Laundy M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet 1990;336:392-5. http://dx.doi.org/10.1016/0140-6736(90)91942-4.
- Basoglu C, Ates M, Algul A, Ipcioglu OM, Gecici O, Yilmaz O, et al. Adjuvant folate with escitalopram treatment and homocysteine, folate, vitamin B-12 levels in patients with major depressive disorder. Bull Clin Psychopharmacol 2009;19:135-42.
- Resler G, Lavie R, Campos J, Mata S, Urbina M, Garcia A, et al. Effect of folic acid combined with fluoxetine in patients with major depression on plasma homocysteine and vitamin B12, and serotonin levels in lymphocytes. Neuroimmunomodulation 2008;15:145-52.
- Christensen H, Aiken A, Batterham PJ, Walker J, Mackinnon AJ, Fenech M, et al. No clear potentiation of antidepressant medication effects by folic acid and vitamin B12 in a large community sample. J Affect Disord 2011;130:37-45. http://dx.doi.org/10.1016/j.jad.2010.07.029.
- Ford AH, Flicker L, McCaul K, van Bockxmeer F, Hegarty S, Hirani V, et al. The B-VITAGE trial: a randomized trial of homocysteine lowering treatment of depression in later life. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-8.
- Shi J, Potash JB, Knowles JA, Weissman MM, Coryell W, Scheftner WA, et al. Genome-wide association study of recurrent early-onset major depressive disorder. Mol Psychiatry 2011;16:193-201. http://dx.doi.org/10.1038/mp.2009.124.
- Shyn SI, Shi J, Kraft JB, Potash JB, Knowles JA, Weissman MM, et al. Novel loci for major depression identified by genome-wide association study of Sequenced Treatment Alternatives to Relieve Depression and meta-analysis of three studies. Mol Psychiatry 2011;16:202-15. http://dx.doi.org/10.1038/mp.2009.125.
- Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry 2009;14:359-75. http://dx.doi.org/10.1038/mp.2008.125.
- Ising M, Lucae S, Binder EB, Bettecken T, Uhr M, Ripke S, et al. A genome-wide association study points to multiple loci that predict antidepressant drug treatment outcome in depression. Arch Gen Psychiatry 2009;66:966-75.
- Uher R, Perroud N, Ng MY, Hauser J, Henigsberg N, Maier W, et al. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry 2010;167:555-64. http://dx.doi.org/10.1176/appi.ajp.2009.09070932.
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol 2007;165:1-13. http://dx.doi.org/10.1093/aje/kwj347.
- Lewis SJ, Lawlor DA, Davey Smith G, Araya R, Timpson N, Day IN, et al. The thermolabile variant of MTHFR is associated with depression in the British Women’s Heart and Health Study and a meta-analysis. Mol Psychiatry 2006;11:352-60. http://dx.doi.org/10.1038/sj.mp.4001790.
- Slopien R, Jasniewicz K, Meczekalski B, Warenik-Szymankiewicz A, Lianeri M, Jagodzinski PP. Polymorphic variants of genes encoding MTHFR, MTR, and MTHFD1 and the risk of depression in postmenopausal women in Poland. Maturitas 2008;61:252-5.
- Lewis SJ, Araya R, Leary S, Smith GD, Ness A. Folic acid supplementation during pregnancy may protect against depression 21 months after pregnancy, an effect modified by MTHFR C677T genotype. Eur J Clin Nutr 2012;66:97-103. http://dx.doi.org/10.1038/ejcn.2011.136.
- Crider KS, Zhu JH, Hao L, Yang QH, Yang TP, Gindler J, et al. MTHFR 677C→T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. Am J Clin Nutr 2011;93:1365-72. http://dx.doi.org/10.3945/ajcn.110.004671.
- Roberts SH, Bedson E, Hughes DA, Lloyd KR, Moat S, Pirmohamed M, et al. Folate Augmentation of Treatment – Evaluation for Depression (FolATED): protocol of a randomised controlled trial. BMC Psychiatry 2007;7. http://dx.doi.org/10.1186/1471-244X-7-65.
- Roberts SH, Bedson E, Hughes DA, Lloyd KR, Menkes DB, Moat SJ, et al. Erratum: Folate Augumentation of Treatment – Evaluation for Depression (FolATED): protocol of a randomised controlled trial. BMC Psychiatry 2009;9. http://dx.doi.org/10.1186/1471-244X-9-14.
- Data for the areas identified in the Wales spatial plan, 2010 update. Statistical Bulletin. 2010.
- Beck AT, Brown GK, Steer RA. BDI-II Manual. San Antonio, TX: Psychological Corporation; 1996.
- Russell D, Hoare ZSJ, Whitaker R, Whitaker CJ, Russell IT. Generalised method for adaptive randomisation in clinical trials. Stat Med 2011;30:922-34.
- British National Formulary. British Medical Association and Royal Pharmaceutical Society: biannual; n.d.
- Moat SJ, Doshi SN, Lang D, McDowell IFW, Lewis MJ, Goodfellow J. Treatment of coronary heart disease with folic acid: is there a future?. Am J Physiol 2004;287:H1-H7.
- Bailer J, Peigorsch W. Estimating integrals using quadrature methods with an application in pharmacokinetics. Biometrics 1990;46:1201-11. http://dx.doi.org/10.2307/2532462.
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382-9. http://dx.doi.org/10.1192/bjp.134.4.382.
- Guy W. Clinical Global Impression (CGI). Taken from ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: Department of Health, Education, and Welfare; 1976.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand 1987;334:1-100. http://dx.doi.org/10.1111/j.1600-0447.1987.tb10566.x.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67-74. http://dx.doi.org/10.1097/00005650-198601000-00007.
- Sheehan D, Janavs J, Baker R, Harnett-Sheehan K, Knapp E, Sheehan M, et al. Mini International Neuropsychiatric Interview (MINI). Tampa, FL: University of South Florida; 2006.
- Parker G, Roy K, Menkes DB, Snowdon J, Boyce P, Grounds D, et al. How long does it take antidepressant therapies to work?. Aus N Z J Psychiatry 2000;34:65-70.
- Nierenberg AA, Farabaugh AH, Alpert JE, Gordon J, Worthington JJ, Rosenbaum JF, et al. Timing of onset of antidepressant response with fluoxetine treatment. Am J Psychiatry 2000;157:1423-8. http://dx.doi.org/10.1176/appi.ajp.157.9.1423.
- Gershon S. Antidepressants: can we determine how quickly they work?. Psychopharmacol Bull 1995;31:21-2.
- Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry 2006;63:1217-23. http://dx.doi.org/10.1001/archpsyc.63.11.1217.
- Directive 2001/20/EC of European Parliament and the Council of 4 April 2001. n.d.
- MRC Guidelines for Good Clinical Practice in Clinical Trials. London: Medical Research Council; 1998.
- A framework for development and evaluation of RCTs for complex interventions to improve health. London: MRC; 2000.
- Good Research Practice. London: MRC; 2000.
- Research Governance Framework for Health and Social Care. London: Department of Health; 2005.
- Research Governance Framework for Health and Social Care in Wales. Cardiff: Welsh Assembly Government; 2001.
- Research Governance Framework for Health and Social Care in Wales. Cardiff: WORD; 2009.
- Fayers PM, Curran D, Machin D. Incomplete quality of life data in randomized trials: missing items. Stat Med 1998;17:679-96. http://dx.doi.org/10.1002/(SICI)1097-0258(19980315/15)17:5/7%3C679::AID-SIM814%3E3.3.CO;2-O.
- Little R, Rubin D. Statistical Analysis with Missing Data. Chichester: Wiley; 2002.
- Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230-5. http://dx.doi.org/10.1136/bmj.300.6719.230.
- R Project for Statistical Computing n.d. www.r-project.org/ (accessed March 2013).
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al. A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine. Health Technol Assess 2005;9.
- British National Formulary. London: RPSGB; 2012.
- Cost of Service Inquiry for Community Pharmacy: Report. London: Pricewaterhouse Coopers LLP; 2011.
- NHS Reference Costs 2009–10. London: DH; 2010.
- Prescription Cost Analysis, England 2010. London: DH; 2011.
- Hughes DA, Tilson L, Drummond M. Estimating drug costs in economic evaluations in Ireland and the UK: an analysis of practice and research recommendations. Pharmacoeconomics 2009;27:635-43. http://dx.doi.org/10.2165/10899570-000000000-00000.
- Curtis L. Unit Costs of Health and Social Care 2010. University of Kent at Canterbury: Personal Social Services Research Unit; 2010.
- Board Scorecard 2010/11. London: NHSD; 2011.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: University of York; 1999.
- Brazier JE, Roberts JR. Estimation of a preference-based index from the SF-12. Med Care 2004;42:851-9.
- Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychol Assess 1998;10:83-9. http://dx.doi.org/10.1037//1040-3590.10.2.83.
- Mihaylova B, Briggs A, O’Hagan A, Thompson S. Review of statistical methods for analysing healthcare resources and costs. Health Econ 2011;20:897-916. http://dx.doi.org/10.1002/hec.1653.
- van Asselt AD, van Mastrigt GA, Dirksen CD, Arntz A, Severens JL, Kessels AG. How to deal with cost differences at baseline. Pharmacoeconomics 2009;27:519-28. http://dx.doi.org/10.2165/00019053-200927060-00007.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Carr DF, Whiteley G, Alfirevic A, Pirmohamed M. Investigation of inter-individual variability of the one-carbon folate pathway: a bioinformatic and genetic review. Pharmacogenom J 2009;9:291-305. http://dx.doi.org/10.1038/tpj.2009.29.
- Bjelland I, Tell GS, Vollset SE, Refsum H, Ueland PM. Folate, vitamin B12, homocysteine, and the MTHFR 677C→T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry 2003;60:618-26. http://dx.doi.org/10.1001/archpsyc.60.6.618.
- Koushik A, Kraft P, Fuchs CS, Hankinson SE, Willett WC, Giovannucci EL, et al. Nonsynonymous polymorphisms in genes in the one-carbon metabolism pathway and associations with colorectal cancer. Cancer Epidemiol Biomarkers Prev 2006;15:2408-17. http://dx.doi.org/10.1158/1055-9965.EPI-06-0624.
- Levine AJ, Figueiredo JC, Lee W, Conti DV, Kennedy K, Duggan DJ, et al. A candidate gene study of folate-associated one carbon metabolism genes and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 2010;19:1812-21. http://dx.doi.org/10.1158/1055-9965.EPI-10-0151.
- Lissowska J, Gaudet MM, Brinton LA, Chanock SJ, Peplonska B, Welch R, et al. Genetic polymorphisms in the one-carbon metabolism pathway and breast cancer risk: population-based case-control study and meta-analyses. Int J Cancer 2007;120:2696-703. http://dx.doi.org/10.1002/ijc.22604.
- Moore LE, Malats N, Rothman N, Real FX, Kogevinas M, Karami S, et al. Polymorphisms in one-carbon metabolism and trans-sulfuration pathway genes and susceptibility to bladder cancer. Int J Cancer 2007;120:2452-8. http://dx.doi.org/10.1002/ijc.22565.
- Blanton SH, Henry RR, Yuan Q, Mulliken JB, Stal S, Finnell RH, et al. Folate pathway and nonsyndromic cleft lip and palate. Birth Defects Res A Clin Mol Teratol 2011;91:50-6. http://dx.doi.org/10.1002/bdra.20740.
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005;21:263-5.
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559-75. http://dx.doi.org/10.1086/519795.
- Predictive Analytic SoftWare (first known as Statistical Package for Social Sciences, then Statistical Product and Service Solutions) Version 18. Chicago IL: SPSS Inc; 2009.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collaboration; 2011.
- Mistry H, Buxton M, Longworth L, Chatwin J, Peveler R. Assessing Health Economics of Anti-Depressants team. Comparison of general practitioner records and patient self-report questionnaires for estimation of costs. Eur J Health Econ 2005;6:261-6.
- Hurst NP, Ruta DA, Kind P. Comparison of the MOS short form-12 (SF-12) health status questionnaire with the SF36 in patients with rhematoid arthritis. Br J Rheumatol 1998;37:862-9. http://dx.doi.org/10.1093/rheumatology/37.8.862.
- Mystakidou K, Tsilika E, Parpa E, Pathiaki M, Galanos A, Vlahos L. The relationship between quality of life and levels of hopelessness and depression in palliative care. Depress Anxiety 2008;25:730-6. http://dx.doi.org/10.1002/da.20319.
- Moat SJ, Madhavan A, Taylor SY, Payne N, Allen RH, Stabler SP, et al. High – but not low-dose folic acid improves endothelial function in coronary artery disease. Eur J Clin Invest 2006;36:850-9. http://dx.doi.org/10.1111/j.1365-2362.2006.01739.x.
- Briggs AH, O’Brien BJ. The death of cost-minimization analysis?. Health Econ 2001;10:179-84. http://dx.doi.org/10.1002/hec.584.
- Dakin H, Wordsworth S. Cost-minimisation analysis versus cost-effectiveness analysis – revisited. Health Econ 2013;22:22-34. http://dx.doi.org/10.1002/hec.1812.
- Altman DG, Bland JM. Statistics notes: absence of evidence is not evidence of absence. BMJ 1995;311. http://dx.doi.org/10.1136/bmj.311.7003.485.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64. http://dx.doi.org/10.1016/S0167-6296(98)00039-3.
- Duru G, Fantino B. The clinical relevance of changes in the Montgomery–Åsberg Depression Rating Scale using the minimum clinically important difference approach. Curr Med Res Opin 2008;24:1329-35. http://dx.doi.org/10.1185/030079908X291958.
- Xie H, Guo J, Wang J, Wang F, Zhao H, Liu C, et al. Glutamate carboxypeptidase II gene polymorphisms and neural tube defects in a high-risk Chinese population. Metab Brain Dis 2012;27:59-65. http://dx.doi.org/10.1007/s11011-011-9272-8.
- Devlin AM, Ling EH, Peerson JM, Fernando S, Clarke R, Smith AD, et al. Glutamate carboxypeptidase II: a polymorphism associated with lower levels of serum folate and hyperhomocysteinemia. Hum Mol Genet 2000;9:2837-44. http://dx.doi.org/10.1093/hmg/9.19.2837.
- Ye X, Lai CQ, Crott JW, Troen AM, Ordovas JM, Tucker KL. The folate hydrolase 1561C>T polymorphism is associated with depressive symptoms in Puerto Rican adults. Psychosom Med 2011;73:385-92. http://dx.doi.org/10.1097/PSY.0b013e31821a1ab4.
- Halsted CH, Wong DH, Peerson JM, Warden CH, Refsum H, Smith AD, et al. Relations of glutamate carboxypeptidase II (GCPII) polymorphisms to folate and homocysteine concentrations and to scores of cognition, anxiety, and depression in a homogeneous Norwegian population: the Hordaland Homocysteine Study. Am J Clin Nutr 2007;86:514-21.
- Guilarte TR, Hammoud DA, McGlothan JL, Caffo BS, Foss CA, Kozikowski AP, et al. Dysregulation of glutamate carboxypeptidase II in psychiatric disease. Schizophr Res 2008;99:324-32. http://dx.doi.org/10.1016/j.schres.2007.11.013.
- Chamberlin ME, Ubagai T, Mudd SH, Levy HL, Chou JY. Dominant inheritance of isolated hypermethioninemia is associated with a mutation in the human methionine adenosyltransferase 1A gene. Am J Hum Genet 1997;60:540-6.
- Hazelwood S, Bernardini I, Shotelersuk V, Tangerman A, Guo J, Mudd H, et al. Normal brain myelination in a patient homozygous for a mutation that encodes a severely truncated methionine adenosyltransferase I/III. Am J Med Genet 1998;75:395-400. http://dx.doi.org/10.1002/(SICI)1096-8628(19980203)75:4%3C395::AID-AJMG9%3E3.0.CO;2-P.
- Uher R, Muthen B, Souery D, Mors O, Jaracz J, Aitchison KJ, et al. Trajectories of change in depression severity during treatment with antidepressants. Psychol Med 2010;40:1367-77. http://dx.doi.org/10.1017/S0033291709991528.
- Khan A, Leventhal RM, Khan SR, Brown WA. Severity of depression and response to antidepressants and placebo: An analysis of the Food and Drug Administration database. J Clin Psychopharmacol 2002;22:40-5. http://dx.doi.org/10.1097/00004714-200202000-00007.
- Donoghue J, Hylan TR. Antidepressant use in clinical practice: efficacy v. effectiveness. Br J Psychiatry 2001;179:s9-17. http://dx.doi.org/10.1192/bjp.179.42.s9.
- Hyland K, Shoffner J, Heales SJ. Cerebral folate deficiency. Inherit Metab Dis 2010;33:563-70. http://dx.doi.org/10.1007/s10545-010-9159-6.
- Wu D, Partridge WM. Blood–brain barrier transport of reduced folic acid. Pharm Res 2009;16:415-19.
- Stahl SM. Novel therapeutics for depression: L-methylfolate as a trimonamine modulator and antidepressant-augmenting agent. CNS Spectr 2007;12:739-44.
- Doshi SN, McDowell IFW, Moat SJ, Payne N, Durant HJ, Lewis MJ, et al. Folic acid improves endothelial function in coronary artery disease via mechanism(s) largely independent of homocysteine lowering. Circulation 2002;105:22-6.
- Fava M, Mischoulon D. Folate in depression: efficacy, safety, differences in formulations and clinical issues. J Clin Psychiatr 2009;70:12-7. http://dx.doi.org/10.4088/JCP.8157su1c.03.
- Campbell NR. How safe are folic acid supplements?. Arch Intern Med 1996;156:1638-44. http://dx.doi.org/10.1001/archinte.156.15.1638.
- Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr 2007;85:193-200.
- Standard Operating Procedure for Data Management (NWORTH 6.01). Bangor: NWORTH; 2010.
- Standard Operating Procedure for Statistics (NWORTH 5.02). Bangor: NWORTH; 2010.
- Edwards RT, Hounsome B, Linck P, Russell IT. Economic evaluation alongside pragmatic randomised trials: developing a standard operating procedure for clinical trials units. Trials 2008;9. http://dx.doi.org/10.1186/1745-6215-9-64.
- Hood K, Robling M, Russell IT, . Mode of data elicitation, acquisition and response to surveys: a systematic review. Health Technol Assess 2012;16.
- The Measurement and Valuation of Health. University of York: Centre for Health Economics; 1995.
- Gnanadesikan R, Wilk MB. Probability plotting methods for the analysis of data. Biometrika 1965;55:1-17.
- Shapiro SS, Wilk MB. An analysis of variance test for normality. Biometrika 1965;52:591-61. http://dx.doi.org/10.2307/2333709.
- Shapiro SS, Francia RS. Approximate analysis of variance test for normality. J Am Stat Assoc 1972;67:215-25. http://dx.doi.org/10.2307/2284728.
- Sculpher M. Subgroups and heterogeneity in cost-effectiveness analysis. Pharmacoeconomics 2008;26:799-806. http://dx.doi.org/10.2165/00019053-200826090-00009.
- Bowling A. Research Methods in Health: Investigating Health and Health Services. Buckinghamshire, Philadelphia, PA: Open University Press; 2002.
- St Pierre RG, Wholey JS, Hatry HP, Newcomer KE. Handbook of Practical Program Evaluation. San Francisco, CA: Jossey-Bass; 2004.
- Bower P, Wilson S, Mathers N. How often do UK primary care trials face recruitment delays?. Fam Pract 2007;24:601-3. http://dx.doi.org/10.1093/fampra/cmm051.
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7.
- Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess 1999;3.
- Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: A review of randomised controlled trials. BMC Med Res Method 2006;6.
- Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess 2007;11.
- McDonald AM, Treweek S, Shakur H, Free C, Knight R, Speed C, et al. Using a business model approach and marketing techniques for recruitment to clinical trials. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-74.
- Barnard KD, Dent L, Cook A. A systematic review of models to predict recruitment to multicentre clinical trials. BMC Med Res Method 2010;10. http://dx.doi.org/10.1186/1471-2288-10-63.
- Bower P, Wallace P, Ward E, Graffy J, Miller J, Delaney B, et al. Improving recruitment to health research in primary care. Fam Pract 2009;26:391-7. http://dx.doi.org/10.1093/fampra/cmp037.
- Goodyear-Smith F, York D, Petousis-Harris H, Turner N, Copp J, Kerse N, et al. Recruitment of practices in primary care research: the long and the short of it. Fam Pract 2009;26:128-36. http://dx.doi.org/10.1093/fampra/cmp015.
- Thomas KS, Koller K, Foster K, Perdue J, Charlesworth L, Chalmers JR. The UK clinical research network: has it been a success for dermatology clinical trials?. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-153.
- National Institute for Social Care and Health Research . NISCHR Clinical Research Centre (CRC) 2011. www.wales.nhs.uk/sites3/page.cfm?orgid=952&pid=52012 (accessed March 2013).
- Cox K AM, Wilson E, Elkan R. An evaluation of the introduction of Clinical Trial Officer roles into the cancer clinical trial setting in the UK. Eur J Cancer Care 2005;14:448-56. http://dx.doi.org/10.1111/j.1365-2354.2005.00611.x.
- Stead M, Cameron D, Lester N, Parmar M, Haward R, Kaplan R, et al. Strengthening clinical cancer research in the United Kingdom. Br J Cancer 2011;104:1529-34. http://dx.doi.org/10.1038/bjc.2011.69.
Appendix 1 Participant Information Leaflet and Informed Consent Form
Appendix 2 Independent members of Trial Steering Committee and Data Monitoring and Ethics Committee
Trial Steering Committee independent members
Allan House (Chair) – Professor of Liaison Psychiatry, University of Leeds
Ian Anderson – Professor of Psychiatry, University of Manchester
Glyn Lewis – Professor of Psychiatric Epidemiology, University of Bristol
Lynne Pike – patient representative
Data Monitoring and Ethics Committee independent members
Francis Creed (Chair 2006–8) – Professor of Psychological Medicine, Manchester University
David Baldwin (Chair 2008–11) – Professor of Psychiatry, University of Southampton
Chris Dowrick – Professor of Primary Medical Care, University of Liverpool
Gareth Griffiths – Scientific Director of Wales Cancer Trials Unit
Appendix 3 Dates of initial approvals
| Regulatory body | Date of approval | |
|---|---|---|
| Multi-centre Research Ethics Committee for Wales | 6 November 2006 | |
| Medicines & Healthcare products Regulatory Authority | 21 December 2006 | |
| Secondary Care | Swansea NHS Trust | 21 September 2006 |
| North East Wales NHS Trust | 16 October 2006 | |
| North West Wales NHS Trust | 17 November 2006 | |
| Primary Care | Swansea Local Health Board | 13 November 2006 |
| North Wales Local Health Boards | 12 June 2007 | |
Appendix 4 Summary of protocol changes approved by DMEC, TSC, MHRA, MREC and NCCHTA
Referrals
Initially we accepted referrals from GPs and psychiatrists only. To be more inclusive, and to improve recruitment into the trial, on 15 May 2009 we started to accept referrals from other healthcare professionals including primary care liaison workers, social workers and mental health nurses.
To improve recruitment in the Swansea centre, we amended our protocol to accept self referrals from people already taking antidepressants. From 26 June 2010 we recruited participants through advertisements in the community, including posters, local newspapers, pharmacies, clinics and general practices. To help with the expected increase in referrals we gained permission for registered mental health nurses to screen people who refer themselves to the study.
Our original protocol stated that we would reimburse general practices £50 to cover administration costs for every patient recruited. In November 2010 we gained permission to extend reimbursement to secondary care teams.
Recruitment
We initially planned to complete all randomisations by August 2008. However unforeseen delays in research governance delayed the start of recruitment by 6 months for reasons including: change of sponsor; delays in appointing researchers; delays in obtaining regulatory approval; and delays in obtaining honorary contracts. We also encountered obstacles to recruitment including: shortage of psychiatrists to screen patients; fewer referrals from secondary care than expected; fewer referrals of newly diagnosed patients than expected; limited space in general practices for screening; and difficulty in contacting referred patients. The NIHR HTA programme therefore granted us a 24-month costed extension to extend recruitment by 18 months.
On 25 June 2009 we gained permission to extend the Wrexham centre to include Conwy and Denbighshire.
Exclusion criteria
In July 2007 we removed substance misuse from the list of exclusion criteria for three reasons:
-
to make the trial more pragmatic and inclusive
-
to increase recruitment, and
-
because we could detect folate deficiency from alcohol use through blood tests at screening.
We added malignancy and related conditions like intestinal polyposis as exclusion criteria, following advice from the TSC in January 2007 based on evidence from rat studies that high folate intake may increase growth of existing tumours.
In response to our original application for a CTA the MHRA asked us to add taking lithium as an exclusion criterion, which we did. However we found no evidence of adverse interactions between lithium and folate. Furthermore we identified that this exclusion criterion was impeding recruitment into the trial, particularly from secondary care. In April 2008, we therefore amended the exclusion criterion for taking lithium to exclude patients with bipolar disorder whether taking lithium or not. This ensured that only patients with unipolar depression entered the trial.
Data collection
It had been our intention for the FolATED trial to be paperless. However IT constraints led us had to substitute a rigorous paper-based system to collect data and we amended the protocol accordingly.
We identified a need for researchers to make home visits to conduct the research assessments. Depression can often make it difficult for patients to attend hospital and many potential participants had requested that researchers go to their homes for appointments. In May 2009 we amended the protocol to allow researchers to visit homes when needed.
Assessment and follow-up
Following the initial application for approval in October 2006, we received further requests from the MREC, local R&D Departments and Trial Management Group to amend the protocol, including:
-
taking extra samples of blood for homocysteine analysis, to guard against losing samples in the post
-
collecting red cell folate at screening to ensure that we did not randomise patients with folate deficiency
-
collecting serum folate at weeks 12 and 25 to check for compliance
-
measuring plasma vitamin B12 concentrations at minus 2, 12 and 25 weeks, to guard against participants becoming B12 deficient during treatment with folic acid and resulting in a neuropathy, and
-
asking patients at each follow-up whether they are taking additional supplements.
We found that the initial screening appointment with patients took longer than expected owing to the number of assessments. In April 2008 we gained permission to remove the majority of the assessments including the MADRS, UKU, SF12, EQ5D and CGI from this appointment. The full battery of assessments at randomisation, which provided the true baseline, continued as planned. This increased the number of patients be screened by psychiatrists and reduced the burden on participants.
After the MHRA recommended following up any pregnancy that occurs during a trial, we gained permission in April 2008 to alter the information sheet to allow us to track any such pregnancy.
On 17 September 2008, the TSC identified the need to ask participants explicitly about attempts at self-harm. Though we define self-harm as a SAE, this relies on participants reporting it without prompts. We therefore added the question: ‘Since we last saw you, have you tried to harm yourself? For example, have you taken any extra tablets or cut yourself or done anything else to injure yourself?’
Additional analyses
In December 2009 we gained permission to investigate the interaction between folic acid and MMA, a metabolic marker of the enzymatic function of vitamin B12 and a more sensitive marker of B12 status. High-dose folate is generally considered safe, provided that B12 deficiency is excluded. 162 However, there is evidence that high folate levels combined with low B12 levels are associated with significant cognitive impairment in the elderly. 163 Furthermore the US National Health and Nutrition Examination Survey observed a relationship between folic acid and MMA (57). The FolATED trial offers a rare opportunity to observe the effects of folic acid supplementation on MMA concentration (not reported in this monograph).
To ensure the safety of participants in the trial fieldworkers informed their Principal Investigator (PI) of any participant who attempted suicide or was at high risk. For this purpose they completed the MINI suicidality scale at the end of the MADRS. If there was a marked increase in suicidal risk, they reported this as a SAE. Though we had approval to monitor suicidality through our safety reporting system, we did not name the MINI suicidality explicitly in our protocol. The MREC therefore asked us not to analyse these data. However our TSC were concerned that not to report fully on the safety of our intervention would be unscientific. So they recommended that we seek permission to publish this information in the public domain. We therefore gained permission in May 2011 to publish analyses comparing differences between the two treatment groups in MINI suicidality scores at follow-up.
Clarification of protocol
Following meetings of TSC and Data Monitoring Committee early in 2007 we replaced the lay summary with a technical abstract at the beginning of the protocol. 81 We elaborated on screening, informed consent, randomisation, withdrawal and safety reporting. We improved clarity, not least by integrating the flow diagram and outcome measures table into the main text of the protocol. 81,82
Review of power calculation (approved by DMEC, TSC and NCCHTA)
We originally powered FolATED to detect a difference between the two treatment groups of three points on the Beck Depression Inventory (BDI-II) at 25 weeks, judging that a clinically important difference. As we estimated the SD of BDI-II scores in the trial population at 10.7, our protocol proposed a completed sample size of 400 at 25 weeks to yield 80% power to detect this difference using a significance level of 5%. As interim analysis of baseline BDI-II scores showed that their SD was about 10, we revised the target completed sample size to 358 at 25 weeks. The original protocol allowed 10% loss at each of the three follow-up assessments, thus requiring a randomised sample of 549 to achieve 400 completers at 25 weeks. As interim analysis also showed that retention at 25 weeks was 79% rather than the 73% expected, the new target of 358 completers needed a randomised sample of 453 participants.
Appendix 5 Analysis plan
Introduction
Trial design
FolATED is a three-centred, double-blind, placebo-controlled, pragmatic randomised trial of folic acid augmentation of moderate-to-severe depression. It investigates the effect of augmentation on new and continuing ADM. Assessments take place 2 weeks before randomisation (‘week –2’) to screen for eligibility and initiate antidepressant if required; 1 week before randomisation (‘week –1’) by telephone to check for tolerability of antidepressant; baseline (‘week 0’) to randomise to folate or placebo; and at weeks 4, 12 and 25 to assess outcomes. To estimate the effectiveness of folic acid in augmenting ADM, the trial uses standardised instruments to measure changes in depressive symptoms from two perspectives – clinical and participants’.
Primary outcome measures
The primary clinical effectiveness outcome measure is self-rated symptom severity as measured by the Beck Depression Inventory (BDI-II). Though BDI-II scores at 25 weeks are useful in assessing participants’ medium-term recovery, the primary outcome is the ‘AUC’ of mean BDI-II scores between randomisation and the 25-week follow-up, to aggregate participants’ paths to recovery over that timeframe. The primary economic measure is ICER, namely cost per QALY gained.
Secondary outcome measures
-
Symptom severity as measured by clinicians using the MADRS and the CGI of change.
-
Health status (mental and physical components) as measured by SF-12.
-
Health utility as measured by the EuroQoL (EQ-5D).
-
Proportion of patients with moderate depression (defined as BDI-II score ≥ 19) at week 25 – estimated by statistical inference from observed distribution of BDI-II scores rather than statistically weaker technique of counting cases.
-
Side effects as measured by the UKU side effects scale and reported AEs – serious examples include psychiatric inpatient admission, attempted or completed suicide, and other mortality.
-
Compliance and adherence of patients to take the medication as prescribed.
-
Suicidality as measured by the MINI suicidality scale.
-
Folate status as measured by homocysteine derived from blood samples taken at baseline, 12, and 25 weeks; and B12 status as measured by MMA at 12 weeks only.
-
Resource use as measured by the self-completed health and social care resource use questionnaire, and
-
Genetic analysis of SNPs between the two arms.
Scope of statistical analysis plan
The statistical analysis plan focuses on clinical effectiveness as measured by its primary outcome and secondary outcomes a to g. Annexes 2 and 3 summarise the assessment timetable and outcome measures. Annex 4 outlines the methods for economic analyses; Annex 5 those for genetic analysis; and Annex 6 those for biochemical analysis.
Management of analysis of trial
The trial data manager will coordinate the preparation and provision of suitable data sets for the various analysts, and the transfer of data between them. He will manage all data in accordance with the NWORTH data management standard operating procedure (SOP) – 6.01. 164 Statistical and health economic analysis will follow the principles set out in the corresponding NWORTH SOPs – 5.02165 and 7.01. 166 The genetic analyses will follow the relevant SOPs in use in Liverpool University.
Data collection
Visit windows
Though we aim to complete questionnaires 2 weeks before randomisation and 12 and 25 weeks thereafter, we have defined an advisory window for each. We monitor reasons for collecting the data outside these windows.
| Data collection | Day due (days since randomisation) | Window |
|---|---|---|
| Screening for eligibility (week –2) | –14 days | ± 10 days |
| Telephone monitoring | –7 days | ± 3 days |
| Randomisation (baseline) | 0 | Origin |
| 4-week follow-up | 28 days | + 14 days |
| 12-week follow-up | 84 days | ± 14 days |
| 25-week follow-up | 175 days | ± 28 days |
Mode of data collection
Some of the trial instruments are administered by researcher or clinician, and others by participant. There is evidence from the MODE-ARTS systematic review that pragmatic changes of administrator ‘at random’ are unlikely to bias outcomes within placebo-controlled trials. 167
Allocation concealment and unblinding the data
In principle throughout recruitment to the trial we conceal treatment allocation from participants, healthcare professionals, investigators, and study team. We shall continue this during data analysis. The trial report will specify occasions when blinding was broken for specific participants.
We shall unblind the data at the joint final meeting of the TSC and the DMEC meeting on 10 October 2011. The chair of this meeting will have a sealed envelope to unblind the data when the TSC and DMEC members are content. After correcting errors detected up to, and as a consequence of, the TSC-DMEC meeting, the trial statistician (RhW) will freeze the database. No longer than a week before that meeting an independent senior statistician (CJW) will check the allocations and thus become unblinded to the treatment allocation. However the trial statisticians (RhW, YS as the assistant trial statistician, and BRC as senior trial analyst responsible for the second, validating analysis) will continue to analyse clinical effectiveness blind to allocated treatment.
Delivery of data sets to biochemistry, genetics and health economics teams
These teams will receive cleaned data from NWORTH. Annexes 4–6 describe the analysis of these data.
Statistical methodology
Populations
‘Analysed’ population
This population – the subject of the principal analyses – comprises all randomised participants with at least one post-baseline BDI-II outcome. If they fail to return any questionnaire or to complete all items for each instrument, we shall impute these data using the principles and methods described under Missing Data above.
‘Complete case’ population
This population comprises only those participants whose outcome data are complete. It will provide a useful sensitivity analysis of primary and secondary findings, especially whether they are sensitive to the presence of missing data and the methods of imputation we use to augment them.
‘Randomised’ population
At first sight it is difficult to draw inferences about this population because some contributed no data after baseline, even on the BDI-II. Because we know the baseline characteristics of all these participants, however, it is possible to reweight the analysed population so they match the characteristics of the randomised population, notably allocated treatment, stratifying variables and baseline BDI-II.
Instruments
BDI-II
We shall compare the effects of adding folic acid or placebo on self-rated symptom severity by comparing the total scores of the BDI-II. 84
Scoring: This instrument collected data at: screening; baseline; 4; 12; and 25 weeks. We shall score it according to the validated manual, namely by summing the ratings of the 21 items. Each item is rated on a four-point scale ranging from 0 to 3, yielding a total possible score of 63. If a participant ticks more than one category, we shall score the highest.
MADRS
We shall compare the effects of adding folic acid or placebo on researcher-rated depression severity by comparing total MADRS scores. 90
Scoring: This instrument collected data at: baseline; 4; 12; and 25 weeks. We shall score it according to the validated manual by adding the ratings of the 10 items. Each item is rated on a seven-point scale ranging from 0 to 6, yielding a possible score of 60. If a rater selects more than one category, we shall score the highest.
CGI
We shall compare the effects of adding folic acid or placebo on the researcher-rated CGI. 91
Scoring: This instrument collected data at: baseline; 4; 12; and 25 weeks. It comprises three separate clinician-rated items: an ordinal scale of current severity of illness using a range of responses from 1 to 7; an ordinal scale of global improvement since recruitment ranging from 1 to 7; and an efficacy index ranging from 0.25 to 4.0 derived from a 4 × 4 matrix plotting therapeutic effect against side effects.
SF-12 version 2
We shall compare the effects of adding folic acid or placebo on self-rated quality of life in the form of SF-12 scores for mental and physical components. 91
Scoring: We collected SF-12 data at baseline 4, 12, and 25 weeks. The SF-12 comprises ten × 5-point items and three 3-point items. We shall score it according to the validated method, namely by applying Norm-Based Scoring (NBS) algorithms using the recommended standardisation (mean = 50, SD = 10 in the 1998 general US population) to calculate the physical and mental component scores PCS-12 and MCS-12.
Missing items: We shall use the SF-12 missing data software which uses Missing Data Estimation.
EQ-5D
We shall compare the effects of adding folic acid or placebo on self-rated health utility in the form of the EQ-5D (also known as EuroQol). This consists of a self-reported matrix and a self-rated visual analogue scale (EQ-VAS). 93 The self-reported matrix comprises five dimensions: mobility, self-care, usual activities, pain-discomfort and anxiety-depression.
Scoring: This instrument collected data at: 4, 12, and 25 weeks. The five dimensions use three-point scales ranging from 0 to 2. If a participant ticks more than one category, we shall score the highest. In the clinical effectiveness section we shall analyse this as an outcome in its own right, rather than convert it to QALYs. In contrast the health economics section will convert these data to QALYs.
UKU side-effects scale
We shall compare the effects of adding folic acid or placebo on four UKU researcher-rated system-specific side-effect scores and their total. 94
Scoring: Participants completed the instrument at telephone monitoring 1 week before baseline; baseline; 4; 12; and weeks. The instrument sums scores on 48 distinct side effects – 10 ‘psychic’, 8 ‘neurologic’, 11 ‘autonomic’ and 19 other, of which 11 are gender-free, four are male-specific and four are female-specific. It finishes with three generic items, of which we used two – global assessments by ‘patient’ and researcher – for imputation. It rates each item on a four-point scale ranging from 0 to 3, yielding possible system-specific scores of 30, 24, 33 and 45 (different questions for each sex, but same total).
Missing items: If respondents fail to answer system-specific items in the instrument, we shall consider them informative missing items and impute by assuming they did not experience those items If raters score above the range of the instrument, we shall censor at the upper limit.
Analysis: As the authors developed the UKU scale specifically for psychotropic drugs, they planned mainly to analyse specific side effects, but acknowledged the case for planned analyses of system-specific scores. 93 Believing that folate does not have independent psychotropic properties, however, we shall test whether it is safe by analysing the four system-specific scores.
Morisky compliance scale
We shall use this four-item instrument to compare the effects of adding folic acid or placebo on self-rated compliance with medication. 95
Scoring: Participants completed the instrument at 12 weeks. We shall score it as the number of ‘yes’ responses, yielding a score between 0 and 4.
MINI suicidality scale
We shall use this six-item instrument to compare the effects of folic acid and placebo on self-reported suicidality. 96
Scoring: Participants completed the instrument at all six data collection points: screening; monitoring; baseline; 4; 12 and 25 weeks. Though we selected it primarily to fulfil our duty of care to participants, the TSC and DMEC asked us to analyse it as an outcome in its own right, and the Wales MREC agreed. We shall score it by summing the scores allocated to ‘yes’ responses, which range between 0 and 10, yielding a total score between 0 and 33.
Missing data
We shall adopt a consistent approach to missing data relating to both effectiveness and cost-effectiveness except where individual outcome measures require some variation in that approach; for example the SF12 has its own missing data software. In particular we shall exclude participants without follow-up data. Then for each variable we shall summarise the frequency of missing data by type (e.g. participant withdrew; questionnaire not returned; page missing; item missing). Where < 10% of data are missing, we shall assume they are missing completely at random in the sense that there is no systematic reason for absences (MCAR). 108 Where > 10% of data are missing, we shall explore the missing data and tabulate them by stratification variables [namely centre (Swansea, North East Wales or North West Wales); sex (male or female); new or continuing prescription (where participants in the second category have taken the same daily antidepressant for at least 2 months with a stable dose in the therapeutic range reported in the BNF for at least 1 month); type of antidepressant prescribed (SSRI or other) and whether or not they have ever received counselling for depression], both as reported at randomisation and as validated after quality assurance; patient demographics; and other important scientific covariates. If there is no reason to suspect that the data are not MCAR, we shall impute values to be used in the main analyses by the following methods. If there is reason to suspect that the missing data are not MCAR, the trial statistician and CI will discuss the findings.
Missing items within a subscale
If a subscale comprises three or fewer items, we shall treat each as a separate subscale. In addressing missing items within a subscale thus defined, we shall take account of methodological publications about the validated instrument. In principle we seek to impute missing items to complete instruments thus:108
-
a. If < 25% of the items within a subscale are missing for a participant at a time point, we shall impute them by the weighted mean of the completed items.
-
b. If > 50% of the items within a subscale are missing for a participant at a time point, we shall treat that subscale as missing and impute it.
Missing subscales
Where between 25% and 50% of the items within a subscale are missing, we shall proceed thus:
-
If < 40% of the subscales for a participant at a time point are missing, we shall impute all missing subscales by a single application at that point of the SPSS multivariate imputation algorithm that also takes account of all validated stratification variables.
-
If > 40% of the subscales for a participant at a time point are missing, but < 20% of participants experience that problem, we shall impute all missing subscales by a single multivariate imputation that also takes account of all validated stratification variables across all time points.
-
If > 40% of the subscales for a participant at a time point had been missing, and > 20% of participants had experienced that problem, we would have used multiple multivariate imputations; in the event, however, this never happened.
Missing time points
-
If < 15% of all time points are missing, we shall impute all subscales within each time point by one iteration of the SPSS multiple imputation algorithm using all other subscales at all time points, together with age, gender, centre and group.
-
If > 15% but < 30% of all time points are missing, we shall impute all subscales within each time point by five iterations of the SPSS multiple imputation algorithm using all other subscales at all time points, together with age, gender, centre and group.
Preliminary data description
We shall summarise baseline and demographic data by both treatment allocation and centre. Conscious that our three centres differed in many respects notably psychiatric practice and recruitment policy, we shall present outcomes believed to follow a Normal distribution in the form: number of responses; mean and SD. If Normal plots show evidence of non-normality however, we shall them present them in the form: number of responses; median and first and third quartiles.
Data transformations
Our analysis plan assumes that residual variation from our statistical models will follow approximately Normal distributions. This is a robust assumption in the sense that only a substantial deviation would invalidate each analysis. Hence the trial statistician will plot all residual distributions and discuss any evidence against normality, as shown for example by Normal plots, with the CI. If necessary we shall seek an optimal transformation to improve approximation to Normality.
Analytical methods
All tests will be two-sided with a significance level of 5% but no correction for multiple testing.
Continuous outcomes with baseline and more than one follow-up (for example BDI-II)
We shall use ‘AUC’ to combine outcomes over all time points to create the primary outcome. As covariates in the AUC analysis we shall use validated stratification variables – centre, gender, new or old patient, type of antidepressant and previous counselling. For individual time points we shall use analysis of covariance to adjust for the corresponding baseline score.
As a sensitivity analysis we shall use multi-level modelling with the same covariates, also known as repeated measures analysis of variance. We shall estimate parameters for three fixed factors – the three time points (4, 12 and 25 weeks), centre and treatment group. We shall also include interactions between treatment and both time point and centre. We shall summarise all effects by parameter estimate, standard error, significance level, and 95% confidence level.
Continuous outcome with no baseline and only one follow-up (Morisky scale)
We shall use analysis of covariance, with baseline depression scores and validated stratification variables as covariates, to test whether medication adherence, measured on the Morisky scale, is significantly different between the treatment groups. If so, or if there is other evidence that the Morisky score influences the main psychological outcomes, we shall test in secondary analysis whether adding it to the usual covariates improves the fit of each model and refine those models accordingly. In these circumstances we shall test whether also to add prescribed medications recorded by GPs to the usual covariates.
Dichotomous outcomes (serious adverse effects and adverse events)
We shall use logistic regression of the binary response of (S)AE or no (S)AE over each participant’s time in the trial to test whether the proportion of participants who experienced (S)AEs differs between treatment arms. Covariates will include baseline scores and validated stratification variables. We shall transform all estimated fixed effects back from their logistic form and summarise them by OR; standard error; significance level and 95% CI.
Covariates for adjustment within statistical model
We shall keep baseline depression scores and validated stratification variables as covariates throughout. We shall explore covariates of potential scientific relevance, including: demographic (e.g. age, ethnicity, marital status, number of dependants and employment status, coded in accordance with usual demographic practice); and clinical (e.g. referral source, smoking, alcohol consumption and medication adherence, measured by both Morisky scale and recorded prescriptions). We shall retain these if they achieve significance levels of 10%.
Interactions to be tested
Within each analysis we shall test for interaction between treatment and centre, not least because our three centres differed in many respects, notably psychiatric practice and recruitment policy. Any evidence of interaction between treatment and centres will lead to exploratory analysis to explain the effects, initially by covariates within the ‘treatment allocated’ population. Failing that, we shall estimate the treatment effect for each centre separately. We shall also test interactions with significant covariates and include these interactions within the model if significant at the 10% level.
Sensitivity analyses
In addition to the planned sensitivity analyses described in Chapter 2 (see Statistical methods, Methods for analysing outcomes, Sensitivity analyses), we shall use sensitivity analysis ad hoc to test whether the validity of the trial is at risk, for example to protocol deviations that result in systematic missing data or potentially differential reasons for withdrawal from the trial and loss to follow-up.
Annex 2: Assessment timetable
| Assessment type | Outcome measure | Respondent | Screening | Baseline | Follow-ups | |||
|---|---|---|---|---|---|---|---|---|
| Week 2 (eligibility) | Week 1 (telephone contact) | Week 0 | Week 4 | Week 12 | Week 25 | |||
| Screening tool for ICD-10 | Symptoms: BDI-II | Patient | ✓ | |||||
| Screening tool | MINI depression | Clinician | ✓ | |||||
| Blood testing | FBC | Researcher | ✓ | |||||
| Serum folate | Researcher | ✓ | ✓ | ✓ | ||||
| Red cell folate | Researcher | ✓ | ||||||
| B12 | Researcher | ✓ | ✓ | ✓ | ||||
| Homocysteine | Researcher | ✓ | ✓ | ✓ | ||||
| MMA | Researcher | ✓ | ✓ | |||||
| Genetics | Researcher | ✓ (needs extra consent) | ||||||
| Depression status | BDI-II | Patient | ✓ | ✓ | ✓ | ✓ | ✓ | |
| MADRS | Researcher | ✓ | ✓ | ✓ | ✓ | |||
| Health status and quality of life | CGI | Researcher | ✓ | ✓ | ✓ | ✓ | ||
| SF-12 | Patient | ✓ | ✓ | ✓ | ✓ | |||
| EQ-5D | Patient | ✓ | ✓ | ✓ | ✓ | |||
| Health economics | Resource usage | Patient | ✓ | ✓ | ✓ | |||
| Suicidality | MINI suicidality | Researcher | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Compliance and side effects | Morisky Questionnaire | Patient | ✓ | |||||
| UKU side effects scale | Researcher | ✓ | ✓ | ✓ | ✓ | ✓ | ||
Annex 3: Outcome measures
Depression outcomes
| Tool | Purpose | Time points (weeks) |
|---|---|---|
| BDI-II | Primary outcome for clinical effectiveness: Self-rated measure of symptom severity | –2 |
| 0 | ||
| 4 | ||
| 12 | ||
| 25 | ||
| 0 | ||
| MADRS | Secondary outcome: Researcher-rated measure of symptom severity | 0 |
| 4 | ||
| 12 | ||
| 25 | ||
| CGI | Secondary outcome: Researcher-rated measure of three dimensions – illness severity, global improvement and response to treatment | 0 |
| 4 | ||
| 12 | ||
| 25 | ||
General quality-of-life outcomes
| Tool | Purpose | Time points (weeks) |
|---|---|---|
| SF-12 | Secondary outcome: Self-rated measure of health status in two dimensions – mental and physical | 0 |
| 4 | ||
| 12 | ||
| 25 | ||
| EQ-5D | Primary outcome for cost-effectiveness: Self-rated measure of health-related quality of life | 0 |
| 4 | ||
| 12 | ||
| 25 | ||
Biochemistry outcomes
| Tool | Purpose | Time points (weeks) |
|---|---|---|
| Serum folate | Clinical outcome: Blood concentration level | –2 |
| 12 | ||
| 25 | ||
| Vitamin B12 | Clinical outcome: Blood concentration level | –2 |
| 12 | ||
| 25 | ||
| Homocysteine | Clinical outcome: Blood concentration level | –2 |
| 12 | ||
| 25 | ||
| MMA | Clinical outcome: Blood concentration level (not reported in this monograph) | –2 |
| 12 | ||
Side effects and compliance
| Tool | Purpose | Time points (weeks) |
|---|---|---|
| UKU side effects scale | Secondary outcome: Self-rated measure of side effects on four main dimensions – psychic, neurologic, autonomic and other | –1 |
| 0 | ||
| 4 | ||
| 12 | ||
| 25 | ||
| Morisky Questionnaire | Self-rated measure of drug compliance | 12 |
Annex 4: Economic analysis plan
Aim
To assess whether the use of folic acid supplementation is cost-effective by estimating the incremental cost–utility and cost-effectiveness ratios of ADM plus folic acid relative to ADM alone.
Data
Healthcare resources: We measure participants’ use of health and social care services by:
-
a. self-completed questionnaires (collected by research professionals at baseline, and weeks 12 and 25), which ask patients to recall their use of general practice, community services and social services over previous 3 months
-
b. general practice records of prescribed medications over 25 weeks of follow-up, and
-
c. data on hospitalisations, triggered by notified SAEs.
Unit costs: We shall derive the cost of most resources used from national sources. 114,116,119 We shall estimate specialised costs like pharmacogenetic testing from appropriate local sources. The cost year will be 2010 and the perspective will be that of the NHS and PSS.
Health outcomes: Participants completed the EQ5D instrument at: baseline, 4, 12, and 25 weeks. We shall convert their responses into a single, preference-based utility value based on the UK tariff. 168 They completed the SF12 at: baseline, 4, 12, and 25 weeks. To generate the SF6D utility score from their responses we shall apply a valuation algorithm using preference weights obtained from a sample of the general population using the standard gamble technique. 122 For the cost-effectiveness analysis, we shall estimate the number of weeks free from moderate or severe depression from participants’ responses to the BDI-II.
Analysis
Cost analysis: In principle we shall impute missing data on resource use according to the section of the statistical analysis plan on ‘Missing items within an instrument’. We shall estimate the mean cost per patient over 25 weeks across both arms of the trial together with their respective bootstrapped 95% CIs using 10,000 replicates. We shall analyse cost data by assuming that the large samples generate nearly Normal distributions of sample means, thus justifying Student’s t-test and ordinary least squares (OLS) linear regression. If quantile–quantile plots,169 and Shapiro-Wilk170 and Shapiro-Francia171 tests for normality show problems like skewness and excess zeros (at least 20% of samples),124 we shall develop an appropriate generalised linear model. To gain precision in estimating mean costs, we shall include covariates in the cost regression models. Selection of covariates will accord with the section of the statistical analysis plan on ‘Covariates for adjustment within statistical model’.
Analysis of health outcomes: We shall impute data missing from EQ5D responses according to the section of the statistical analysis plan on ‘Missing items within an instrument’. We shall present descriptive statistics of fully imputed responses to individual items within the EQ5D (mobility, self-care, usual activity, pain-discomfort and anxiety-depression) and the derived utility scores and Visual Analogue Scale scores for each time point across treatment groups. We shall estimate the number of QALYs experienced by each patient over 25 weeks as the area under the EQ5D utility curve, using the trapezoidal rule and adjusting for baseline utility score. 170 We shall derive non-parametric 95% CIs from 10,000 bootstrapped replicates. We shall analyse these QALY data by Student’s t-test and OLS linear regression, after testing by quantile-quantile plots169 and the Shapiro-Wilk170 and Shapiro-Francia171 tests that the large samples generate Normal distributions. To gain precision in estimating mean QALYs, we shall include covariates in the QALY regression models. Selection of covariates will include baseline utility score126 and accord with the section of the statistical analysis plan on ‘Covariates for adjustment within statistical model’).
Cost-effectiveness analysis: We shall estimate the number of weeks free from moderate or severe depression (when BDI-II scores are less than 13) by statistical inference from the observed distribution of BDI-II scores and assuming linear interpolation between time points – baseline and at 4, 12 and 25 weeks.
Incremental and uncertainty analysis: We shall derive ICERs by dividing differences in adjusted mean costs by differences in adjusted mean effects. We shall explore uncertainty around ICERs using non-parametric bootstrapping. We shall display results on cost-effectiveness planes and as cost-effectiveness acceptability curves showing when the results fall below given cost-effectiveness thresholds. We shall conduct sensitivity analyses to test the robustness of our findings and examine the extent to which ICERs are sensitive to key assumptions in the analysis, notably on unit costs and SF6D utilities and through complete case analysis.
Secondary analysis: Depending on the analysis of clinical data, secondary analyses will assess relationships between cost-effectiveness and potential predictors of response, notably adverse reactions and high-cost episodes by including them in generalised linear regression models. 172 These are likely to include baseline disease severity and class of prescribed ADM, along with gender, age and genetic factors. If the results of the genetic analysis suggest clinical value, we shall undertake exploratory analysis of the cost-effectiveness of pharmacogenetic testing.
Annex 5: Genetic analysis plan
We shall analyse baseline samples for 140 single nucleotide polymorphisms and two tandem repeat polymorphisms within the genomic loci of 25 genes related to either one-carbon folate metabolism or methionine biosynthesis pathways. Initially we shall examine each single nucleotide polymorphism (‘SNP’) in turn for association with participants’ response to antidepressant medication (ADM).
We shall fit two mixed models. The first will include baseline values, the three time points (4, 12 and 25 weeks), centre, genetic factors potentially associated with outcome a priori [age, gender, body mass index (BMI), co-medications, type of antidepressant and new or continuing patient] as well as an indicator variable to indicate whether a patient supplemented their treatment with folic acid or not. This indicator variable will specify treatment received rather than treatment allocated.
The second model will be the same as the first except for a covariate representing the SNP. We shall compare both models by the likelihood ratio test, and record the significance level. Initially we shall assume an additive mode of inheritance for each SNP, with patients having the wild-type genotype coded ‘0’, those having the heterozygous genotype coded ‘1’, and those having the homozygous variant genotype coded ‘2’. Later sensitivity analysis will test whether a dominant allele model provides a better fit to the data.
Before the analyses of association, we shall test each SNP for Hardy–Weinberg Equilibrium. We shall flag those found to deviate at the 1% significance level but include them in analysis. We shall include in the association analyses only SNPs passing the following genotype quality criteria:
-
minor allele frequency > 0.01
-
genotypes call > 95% of SNPs
-
samples call > 90% of SNPs.
In addition we shall exclude any patient samples with > 5% missing genotypes from analysis. Also before the analyses of association, we shall reduce the number of SNPs investigated by assessing the extent of linkage disequilibrium (‘LD’) between SNPs. Where the LD is significant (r2 > 0.81) for a group of two or more SNPs, we shall include only the SNP with the least missing data in the analyses.
If the study includes only a small proportion of self-reported non-Caucasian patients (5%), we shall exclude them from the genetic association analyses. If the proportion exceeds 5%, we shall adjust for ethnic origin in analysis by including additional covariates in the regression models.
Once all genetic analyses of association with participants’ response to ADMs are complete, we shall estimate the FDR for each association. We shall treat FDRs less than 0.05 as statistically significant associations. For marginally significant SNPs (i.e. FDR < 0.10), we shall fit a further regression model including a folic acid × SNP interaction term to identify genetic predictors of the efficacy of folic acid adjuvant to ADM. We shall compare this model with the model without interaction, and re-estimate the FDR with 5% again the criterion of significance.
We shall also undertake exploratory analyses for association between each SNP and changes from baseline in the biochemical markers specified in Annex 6 below, following the same format as for the primary analyses. We shall use the statistical packages R, SPSS and PLINK. 111,135,136
Hence the genetic component of the study will need the clinical and biochemical data specified in Annex 2 including the following stratifying variables: centre, age, gender, BMI (i.e. weight/height2), co-medications, type of antidepressant, and whether new or continuing patient.
Annex 6: Biochemistry analysis plan
Homocysteine
Folate and vitamin B12 concentrations are major determinants of one-carbon metabolism, in which the essential methyl donor S-adenosylmethionine (SAM) is formed. SAM is essential for neurologic function. Low plasma folate concentrations are associated with poor response to ADM, and treatment with folic acid can improve that response. Plasma total homocysteine (tHcy) is a sensitive marker of folate (and B12) status. We measure this at baseline and following treatment with folic acid or placebo primarily to assess the response to folate therapy. It will be informative to characterise how response to folate augmentation depends upon baseline homocysteine levels. We will assay homocysteine by an automated analyser (Abbott Architect) using one-step immunoassay with chemiluminescence detection (Axis-Shield). This assay is standardised using the National Institute of Standards and Technology (NIST) Standard Reference Material. It is used to assess patient samples routinely, and is subject to rigorous internal and external quality assurance assessments.
Biochemistry analysis
As patients with depression have increased homocysteine concentrations, the primary analysis of homocysteine will follow essentially the same analysis plan as for genetics in Annex 5. We shall compare the effects of folic acid and placebo on tHcy using the mixed model approach to repeated-measures analysis of variance. As potential covariates we shall use baseline Hcy, age, gender, BMI (weight/height2), co-medications, type of ADM, and new or continuing patient. We shall estimate parameters for three fixed factors – the three time points (4, 12 and 25 weeks), centre (especially important as tHcy sample handling differs between centres) and treatment group. We shall also include the interaction between treatment group and time point to test whether differences between treatments vary over time.
We shall add reported compliance with folate therapy or placebo to this model and test whether this leads to a fall in tHcy. We shall investigate how type of ADM, lifestyle factors (e.g. smoking, BMI and alcohol) and genetic polymorphisms in the folate pathway affect patients’ baseline homocysteine concentrations; and how folic acid affects BDI-II scores, the primary outcome measure. We shall also test the interaction between biochemical variables and the final set of genetic polymorphisms analysed by the genetics analysis plan (see Annex 5). We shall explore the extent to which tHcy and MMA, both baseline and subsequent, predict BDI-II, MADRS and CGI scores.
Methylmalonic acid
Methylmalonic acid is a sensitive marker of B12 status. Hence the trial affords a unique opportunity to estimate the effects of folic acid supplementation on MMA concentration specifically in individuals with low-to-normal B12 levels, and thus assist in elucidating the nature of the relationship, if any, between folic acid and MMA. If folic acid is clearly shown to influence MMA concentrations, this will be of considerable importance for public health decisions relating to the issue of food folate fortification in the UK and elsewhere. There is evidence from cross-sectional studies that the combination of high plasma folate and low B12 status is associated with cognitive decline. Hence we shall later explore the extent to which MMA acts as a prognostic variable for other outcomes.
Thus the non-genetic data required for the biochemical component of the study are the same as those listed in Annex 2 for the genetic component of the study.
Imputing serum folate measurements censored at 20
We shall impute separately for groups A and B without using demographic variables like age or sex.
-
Step 1:
-
a. Regress log (serum folate) on log (red cell folate – RCF) using raw data where both values are present; and derive slope and intercept.
-
b. Replace serum folates recorded as > 20 by 20.
-
c. Regress log (serum) on log(RCF) after including the extra data points added in step 1b, expecting the intercept to increase and the slope to decrease.
-
-
Step 2:
-
a. Use slope and intercept from step 1c to impute serum folates recorded as > 20.
-
b. Retain all imputations that yield serum folates > 20; and replace by 20 all imputations that yield serum folates < 20 in 2.1.
-
c. Regress log(serum) on log(RCF) after including the extra data points imputed in step 2b, expecting the intercept to increase and the slope to decrease.
-
-
Final steps:
-
a. Repeat steps 2a to 2c till slope changes by < 0.1 × SD of slope or no imputed folates < 20.
-
b. Impute all serum folates recorded as > 20 using slope and intercept from final iteration of step 2c; add normally distributed residual with mean zero and SD = residual SD of final model.
-
c. Repeat entire process after replacing RCF by B12 to impute missing values when RCF is missing but B12 is present; however imputation is not possible when folate, RCF and B12 are all missing.
-
d. Assess imputation process by scatter plots of serum folate against RCF and B12.
-
Appendix 6 Supplementary tables for single nucleotide polymorphisms
| Gene (HUGO) | Accession number | Chromosome position |
|---|---|---|
| AHCY | rs866027 | chr20:32874310 |
| rs13043752 | chr20:32883308 | |
| ALDH1L1 | rs4646760 | chr3:125822871 |
| rs4646756 | chr3:125824256 | |
| rs1127717 | chr3:125826059 | |
| rs2276726 | chr3:125826287 | |
| rs3772426 | chr3:125829830 | |
| rs1868130 | chr3:125837819 | |
| rs12106789 | chr3:125838351 | |
| rs4646739 | chr3:125844079 | |
| rs6763254 | chr3:125848067 | |
| rs6799991 | chr3:125856916 | |
| rs6800400 | chr3:125857342 | |
| rs11923466 | chr3:125859367 | |
| rs10934751 | chr3:125868513 | |
| rs16837171 | chr3:125871773 | |
| rs16837178 | chr3:125872293 | |
| rs3796191 | chr3:125872384 | |
| rs6774807 | chr3:125883082 | |
| rs4679102 | chr3:125885196 | |
| rs9842910 | chr3:125896572 | |
| AMD1 | rs7768897 | chr6:111212283 |
| AMT1 | rs10640 | chr3:49454277 |
| rs1464568 | chr3:49458266 | |
| ATIC | rs7585489 | chr2:216181868 |
| rs2372536 | chr2:216190020 | |
| rs10932606 | chr2:216198374 | |
| rs6737407 | chr2:216207121 | |
| rs6435899 | chr2:216207271 | |
| rs4672768 | chr2:216214124 | |
| BHMT | rs6875201 | chr5:78410564 |
| rs506500 | chr5:78414337 | |
| rs3733890 | chr5:78421959 | |
| CBS CTH | rs12613 | chr21:44473691 |
| rs4920037 | chr21:44481891 | |
| rs234706 | chr21:44485350 | |
| rs535112 | chr1:70889476 | |
| rs525276 | chr1:70893391 | |
| rs17131305 | chr1:70896165 | |
| rs473334 | chr1:70896967 | |
| rs1021737 | chr1:70904800 | |
| DHFR | rs1677692 | chr5:79937014 |
| rs10072026 | chr5:79945140 | |
| DNMT1 | rs10407514 | chr19:10255112 |
| rs8111085 (merged with rs2228612) | chr19:10273372 | |
| rs6511677 | chr19:10277799 | |
| FOLH1 | rs16906158 | chr11:49170774 |
| rs383028 | chr11:49174141 | |
| rs202687 | chr11:49180269 | |
| rs7126892 | chr11:49198799 | |
| rs202712 | chr11:49198924 | |
| rs588458 | chr11:49214048 | |
| FPGS | rs2230270 | chr9:130570894 |
| rs34354111 | chr9:130575702 | |
| FTCD | rs17004505 | chr21:47571209 |
| rs28941768 | chr21:47571859 | |
| GART | rs8971 | chr21:34883618 |
| rs6517178 | chr21:34888621 | |
| rs2834234 | chr21:34894623 | |
| rs4817577 | chr21:34894797 | |
| GGH | rs11995525 | chr8:63934988 |
| rs7010484 | chr8:63937675 | |
| rs11545078 | chr8:63938764 | |
| rs3780130 | chr8:63940776 | |
| rs13268472 | chr8:63942016 | |
| rs10957267 | chr8:63944251 | |
| rs17194931 | chr8:63944344 | |
| rs13270305 (merged with rs11545077) | chr8:63951237 | |
| rs1800909 | chr8:63951312 | |
| MAT1A | rs4933327 | chr10:82033683 |
| rs17102596 | chr10:82035173 | |
| rs2236568 | chr10:82035923 | |
| rs873395 | chr10:82037215 | |
| rs1143693 | chr10:82040484 | |
| rs3862534 | chr10:82048978 | |
| MTFMT | rs34507711 | chr15:65295441 |
| rs6494509 | chr15:65307463 | |
| rs35302908 | chr15:65308791 | |
| rs11638255 | chr15:65321037 | |
| MTHFD1 | rs8006686 | chr14:64868671 |
| rs1950902 | chr14:64882380 | |
| rs10133855 | chr14:64890227 | |
| rs34181110 | chr14:64892470 | |
| rs11627525 | chr14:64894362 | |
| rs10498514 | chr14:64899055 | |
| rs2236225 | chr14:64908845 | |
| rs8012229 | chr14:64911562 | |
| rs2281603 | chr14:64926097 | |
| MTHFR | rs2184226 | chr1:11847436 |
| rs35737219 | chr1:11850750 | |
| rs2274974 | chr1:11851319 | |
| rs13306556 | chr1:11852110 | |
| rs1801131 | chr1:11854476 | |
| rs1801133 | chr1:11856378 | |
| rs4846052 | chr1:11857951 | |
| rs17367504 | chr1:11862778 | |
| rs2066472 | chr1:11862971 | |
| rs7553194 | chr1:11864149 | |
| rs2244976 | chr8:19122545 | |
| MTHFS | rs8923 | chr15:80137560 |
| rs4779165 | chr15:80151013 | |
| rs2586154 | chr15:80165368 | |
| rs12899781 | chr15:80168282 | |
| rs7166189 | chr15:80172385 | |
| rs2733107 | chr15:80178612 | |
| rs8042012 | chr15:80179847 | |
| rs2586183 | chr15:80180106 | |
| rs12898642 | chr15:80182050 | |
| rs8040104 | chr15:80183342 | |
| MTR | rs12749581 | chr1:236966848 |
| rs6668344 | chr1:237001326 | |
| rs4659727 | chr1:237006914 | |
| rs1805087 | chr1:237048500 | |
| rs10158222 | chr1:237050682 | |
| rs1252252 | chr1:237056002 | |
| rs11799647 | chr1:237060921 | |
| MTRR | rs1801394 | chr5:7870973 |
| rs326124 | chr5:7877178 | |
| rs161869 | chr5:7877831 | |
| rs1532268 | chr5:7878179 | |
| rs10380 | chr5:7897191 | |
| rs12347 | chr5:7897283 | |
| rs716537 | chr5:7899419 | |
| rs8659 | chr5:7900833 | |
| SHMT1 | rs1979277 | chr17:18232096 |
| rs2273028 | chr17:18239012 | |
| rs9910090 | chr17:18250399 | |
| rs2273026 | chr17:18256979 | |
| rs2461838 | chr17:18265264 | |
| SLC19A1 | rs1051296 | chr21:46934861 |
| rs12659 | chr21:46951556 | |
| rs2330183 | chr21:46953292 | |
| rs1051266 | chr21:46957794 | |
| rs3177999 (merged with rs1131596) | chr21:46957916 | |
| rs35789560 | chr9:130575515 | |
| TYMS | rs1004474 | chr18:660383 |
| rs11540152 | chr18:662215 | |
| rs596909 | chr18:669087 | |
| rs11540153 | chr18:669117 | |
| rs2853532 | chr18:670414 | |
| Unable to plex owing to sequence constraints | ||
| Gene (HUGO) | rs number | Chromosome position |
| ALDH1L1 | rs4646750 | chr3:125826003 |
| CBS | rs1801181 | chr21:44480616 |
| DNMT1 | rs8112801 | chr19:10253099 |
| MTHFR | rs3927589 | chr1:11854493 |
| TYMS | rs2853542 | chr18:657685 |
| SNPs excluded as incompatible with assay containing ≤ 10 SNP plex | ||
| MTHFS | rs16971449 | chr15:80152997 |
| SLC19A1 | rs35786590 | chr21:46935675 |
| SLC19A1 | rs7278825 | chr21:46935942 |
| rs number | Gene (HUGO) | Forward PCR primer (5′ to 3′) | Reverse PCR primer (5′ to 3′) | Extension probe sequence (5′ to 3′) |
|---|---|---|---|---|
| Assay 1 (35-plex) | ||||
| rs10072026 | DHFR | ACGTTGGATGGCAGCTTCATCAATAGCTCC | ACGTTGGATGTGTCTCATAGTGGAGATCAG | gAGTGGAGATCAGTATATGATAA |
| rs1127717 | ALDH1L1 | ACGTTGGATGCGGGCTTTTATCTCTCTTGC | ACGTTGGATGCCTTGGCTATGAACATGTGG | ACATGTGGTCTTCCACG |
| rs1143693 | MAT1A | ACGTTGGATGCATCCTCCTCATTTCTGTCC | ACGTTGGATGTCAAGACTTGCAACGTGCTG | acTCCCCAGATATTGCCCA |
| rs11540152 | TYMS | ACGTTGGATGAGTCCCCTTCTTCTCTGGTG | ACGTTGGATGGGGAGTGAAAATCTGGGATG | ggtgaCAATGGATCCCGAGAC |
| rs11540153 | TYMS | ACGTTGGATGCCAAGCGCACATGATGATTC | ACGTTGGATGAGTTGACCAACTGCAAAGAG | gccaAACTGCAAAGAGTGATTGACA |
| rs11638255 | MTFMT | ACGTTGGATGAAAAGTGGAAGGAGGACTGC | ACGTTGGATGCGTATATACTTCTCTGCCTC | CTGCCTCTTTTTCCCAATT |
| rs11799647 | MTR | ACGTTGGATGTGGCTGAGGTTGAGAAATGG | ACGTTGGATGCCTTGAGGATCATCAAGAAT | TTAGTCTGTATCATATCCCAAAA |
| rs11923466 | ALDH1L1 | ACGTTGGATGTTATGCACTTGCCTATTGTC | ACGTTGGATGAAGGCCACTACATAGGTAAG | gATGAAAGTCCATATTATTGTATTTTA |
| rs12106789 | ALDH1L1 | ACGTTGGATGTGACACCTCCCTTTTCCATC | ACGTTGGATGGAGTGGTGGAAGATGGAGG | cGATGGAGGGACTGGA |
| rs12347 | MTRR | ACGTTGGATGTGGCCATGAAGCTGGATGTT | ACGTTGGATGTCCTTCTCAAGAGATGCTCC | tggtgGCCCCAGCAAAGTATGT |
| rs1252252 | MTR | ACGTTGGATGGGGCTGGAACTTAACATTAG | ACGTTGGATGAAATCCTCACTGTATGCGCC | atGCTCGGACGCCACAGAAT |
| rs12659 | SLC19A1 | ACGTTGGATGAGCACTGAGTCCCCACAGG | ACGTTGGATGTGCGAAACCTCGGCTTCGGA | gtCTGGAGCGCATGAATCC |
| rs12749581 | MTR | ACGTTGGATGGCGGGAGAAGCTAAACGAAG | ACGTTGGATGGTCATTGTTGCCTTTCAGCG | ccttGCATGATCTTTAAATTCCTGACCT |
| rs16906158 | FOLH1 | ACGTTGGATGATCTGGATCATTATACCGAG | ACGTTGGATGAAATGACGAGTCCTCTGTGG | tcACAAACTACCAAACAAATTAAA |
| rs17102596 | MAT1A | ACGTTGGATGACAAAGGCATTAGGGAGTGG | ACGTTGGATGTTGAAAACAGGTAACCTGCC | CCTGCCAAAGCTTCA |
| rs1801131 | MTHFR | ACGTTGGATGGAGCTGCTGAAGATGTGGG | ACGTTGGATGACCATTCCGGTTTGGTTCTC | ttggCAAAGACTTCAAAGACACTT |
| rs1801394 | MTRR | ACGTTGGATGGTGAAGATCTGCAGAAAATCC | ACGTTGGATGATATGCTACACAGCAGGGAC | agtCAAAGGCCATCGCAGAAGAAAT |
| rs202687 | FOLH1 | ACGTTGGATGATAGGAATAGCACTGAATC | ACGTTGGATGCCATGCTCATGGATAGGAAG | cccccAACGGCCATATTGCCC |
| rs2066472 | MTHFR | ACGTTGGATGTGGAATCTGGTGACAAGTGG | ACGTTGGATGGAGATGAGATTGACAGCTCC | ttgcCAGCTCCCTCAGCAGTT |
| rs2236225 | MTHFD1 | ACGTTGGATGCACTAACCTACAAACCCTTC | ACGTTGGATGATCGCACATGGCAATTCCTC | gttaCTCCATCATTGCAGACC |
| rs2236568 | MAT1A | ACGTTGGATGAGTGGAGGAATCCTAGGGAC | ACGTTGGATGCATTTAGTGCACTCTCTGGG | aggaGTCTGACAGTGTGCAAGAAAT |
| rs2330183 | SLC19A1 | ACGTTGGATGGCCTCTTCCTCTGGACTGT | ACGTTGGATGACTACAGGCAGGCACCAAAC | GCACCAAACGTCCTCAG |
| rs234706 | CBS | ACGTTGGATGTCACACTGCTGCAGGATCTC | ACGTTGGATGTTCTATGCAGTACCGCAACG | ctcccCGCCAGCAACCCCCTGGCTCACTA |
| rs2834234 | GART | ACGTTGGATGTCACTTGGGATATCTTCTGC | ACGTTGGATGTTAAAAACAAGTCATCACC | cttTTAAAAACAAGTCATCACCTAAACG |
| rs2853532 | TYMS | ACGTTGGATGTCAGAGCTGAAGGGATCTGG | ACGTTGGATGTACCAGAAGCACCAGTTTCC | cccTTATTCCTGCTGTATTTGTAAT |
| rs34354111 | FPGS | ACGTTGGATGGACTAGGTGGCTGGAAGATG | ACGTTGGATGCTCGTCTTCAGCTGCATTTC | ggggaATGCCTTGCAATGGATCA |
| rs35737219 | MTHFR | ACGTTGGATGTCAGGACGCAGGGTCATGGA | ACGTTGGATGTGGTGGAAGACACATTGGAG | CCCAGAATGCGAGAGAAA |
| rs3733890 | BHMT | ACGTTGGATGTTGTTGCAGTCAGGAGTGTG | ACGTTGGATGAGTGAAGCTCATGAAGGAGG | GCTTGGAGGCTGCCC |
| rs3862534 | MAT1A | ACGTTGGATGCCAAGCCTATACTGAGGGA | ACGTTGGATGCTCATGGATCAAAACAACAC | tcataGATCAAAACAACACACGGTAC |
| rs4646739 | ALDH1L1 | ACGTTGGATGCTGCTTCTCTCCAGGACTTC | ACGTTGGATGACAGCCTGAGTAGTCTATCC | tttttATGTCCCTTATGTGGCTTCC |
| rs4817577 | GART | ACGTTGGATGAATAGCGGTTCTCAGGGATG | ACGTTGGATGTTTAACTGGTCTAGGGCAGG | ggcgGGGAATTGAAATGAGTTTGAAC |
| rs506500 | BHMT | ACGTTGGATGTGACAGAATGAGACTCCATC | ACGTTGGATGAGATTTCCAAGCACAGTTCC | aaaaCAAGCACAGTTCCATGGATGAAT |
| rs6494509 | MTFMT | ACGTTGGATGGTTCCTTGGAATATCAGGGC | ACGTTGGATGGAGTACCTATTCTGTGCCAG | ACTGTACAAGATGCTGTAAATA |
| rs6763254 | ALDH1L1 | ACGTTGGATGTCTCTAGGGCTCTAAGGGTC | ACGTTGGATGGCAGATGAACAACCAACTCC | CCAACTCCTCAAGCA |
| rs8111085 | DNMT1 | ACGTTGGATGCCTTTACCTTTTCATCCTCG | ACGTTGGATGGAGGCCCGAAGAAAAAGAAC | ggagGAACCTGAAAAAGTAAATCCACAG |
| Assay 2 (35-plex) | ||||
| rs1004474 | TYMS | ACGTTGGATGTAAAACTGTGACTCTCCCCC | ACGTTGGATGGGGAAAGGCTGACATACATC | GATGGTGATGTTCGTCTA |
| rs1021737 | CTH | ACGTTGGATGGCAGCTTCTAATAGCAGCTC | ACGTTGGATGGCACTGTTATTATAGCACCC | ACCCTCCAAGTGGAA |
| rs1051266 | SLC19A1 | ACGTTGGATGCGTAGAAGCAAAGGTAGCAC | ACGTTGGATGAGAAGCAGGTGCCCGTGGAA | cccaaCCCGAGCTCCGGTCCTGGCGGC |
| rs13268472 | GGH | ACGTTGGATGTTCCCACCTTGCATGAAGAC | ACGTTGGATGTCTCACCTGATGGATTACGG | ctccGGATTACGGGGTGACT |
| rs13306556 | MTHFR | ACGTTGGATGTCAAGTTCAGGAGAGGATGC | ACGTTGGATGACAGATGACCCTCTGAGAAG | GGTGAGATATCCCTCCT |
| rs1464568 | AMT1 | ACGTTGGATGTCCCCACTGGATGGACAAAA | ACGTTGGATGAGACCTGCTGAATCAGAACC | aAGAACCTTCATTTTAACAAGATC |
| rs161869 | MTRR | ACGTTGGATGACCGTAGCTGAAAACATGAG | ACGTTGGATGGGAGGAATTCTATTCCTGTC | tgCCTATCCCTCTTCATTTCTT |
| rs16837171 | ALDH1L1 | ACGTTGGATGAACTCACGGTACCAGCAAAC | ACGTTGGATGCTCATAGTGTTGTTAGGCAG | ATTGTTCTGGTATAAAAGGTG |
| rs1805087 | MTR | ACGTTGGATGCTTTGAGGAAATCATGGAAG | ACGTTGGATGTCTACCACTTACCTTGAGAG | CCTTGAGAGACTCATAATGG |
| rs2184226 | MTHFR | ACGTTGGATGACCAGCTCTGTGGCCTGTGT | ACGTTGGATGCCAGGAAGTCCAAGCCCAT | cGCTCCTCTCTGGCTA |
| rs2281603 | MTHFD1 | ACGTTGGATGGACCCTTTGATATCCTTGTC | ACGTTGGATGATCCTGTGTGGAACAGGTTG | ggggCTGAGAAAAACTAGAAAAAGC |
| rs2372536 | ATIC | ACGTTGGATGGTTGCCTGCAATCTCTATCC | ACGTTGGATGTCAATTTGCTCCACAGCCTC | ACAGCCTCCTCAACA |
| rs2461838 | SHMT1 | ACGTTGGATGAGGTGACTTCACTTACTAGG | ACGTTGGATGTTTCTGTAGAGGCACTGTTG | GTGGCATGATCCTCAAC |
| rs2586154 | MTHFS | ACGTTGGATGCTAGCTGACCATTAGGGAGA | ACGTTGGATGGCTGTTCTCACTCTGTTTCC | ttatGTTTTACTGAGTGCCCTG |
| rs2586183 | MTHFS | ACGTTGGATGTCTACCTCCTTCATGTCCTG | ACGTTGGATGCTTGCAATCTTTCCCCTTGA | ATCTTTCCCCTTGAAAATATG |
| rs2733107 | MTHFS | ACGTTGGATGCATCACACTCTGTACTGTGG | ACGTTGGATGTGTCCCAAACACTGTAAAGC | gacaAAACACTGTAAAGCTAGATG |
| rs3177999 | SLC19A1 | ACGTTGGATGATCTTCCAAGGTGCCCTGAC | ACGTTGGATGACCATCCTGCTCAGGCCAC | ctgcGGGGACGAAGGTGAC |
| rs34181110 | MTHFD1 | ACGTTGGATGGAATCATCCACTTTCCTGGC | ACGTTGGATGGCTTAGAGCACAGTAGAGAG | ctccCAGTAGAGAGTGCCAAGC |
| rs3772426 | ALDH1L1 | ACGTTGGATGTGCACACAAGGTCCTGTCTG | ACGTTGGATGGAATTATCCCTGGGACTCTG | cccgCTGTGGAAGTCTCATGACTCC |
| rs4646756 | ALDH1L1 | ACGTTGGATGTGCTACCCAGACCTGCATAG | ACGTTGGATGCTGAACCCTCAGCCAGAAAC | ccttGCCCCTGGGTCTTCA |
| rs525276 | CTH | ACGTTGGATGACAGAGCAAGACTCCATTTC | ACGTTGGATGTGTAAAGGGAAAGATGTTG | AGGGAAAGATGTTGATAATGTAT |
| rs6435899 | ATIC | ACGTTGGATGGGTGGAAGCCATATCAAGTG | ACGTTGGATGTGAAGACACAGGGCATTTCC | tccaaTGCAGTGATCCCTCATGTTCTT |
| rs6511677 | DNMT1 | ACGTTGGATGAGTCTTCACCTCCCACTCTG | ACGTTGGATGTTGAGACTGAGCCTGAATCC | AGGTGGGCAGAATACC |
| rs6517178 | GART | ACGTTGGATGGAACAGTCCAAAGTAGTGGG | ACGTTGGATGAATCCCATGTTGGTTTGATG | tTTGGTTTGATGATAATACTTTTACA |
| rs6668344 | MTR | ACGTTGGATGAGACATCCCTGATCTGACTC | ACGTTGGATGCTAAAGGGAAGGTTGAATTTG | TGGCTAGAGGGCTGT |
| rs7010484 | GGH | ACGTTGGATGTCGGAGATCAAGTAACCCAC | ACGTTGGATGCCAGGACAAAGACCAGATAG | gaAGGACAAAGACCAGATAGATTCTTA |
| rs7126892 | FOLH1 | ACGTTGGATGTTCCCTTTTTCACTAAGGAG | ACGTTGGATGGCTTTATACGTGGCATTTT | agTACATTACTTGAAATTCTGTTTAAT |
| rs716537 | MTRR | ACGTTGGATGTCCGACGTTAGAAACGTCTG | ACGTTGGATGGTGAGTTACACTTCCTACCC | gCCTACCCTCAAACAACTTA |
| rs7166189 | MTHFS | ACGTTGGATGCGAGACTCCGTCTTAAAAAG | ACGTTGGATGCCATCTCATGATTTGCTGCC | TGCTGCCTACTTCATCC |
| rs7585489 | ATIC | ACGTTGGATGAAGGGTCAAGTGAAGGACAG | ACGTTGGATGAAAGTCTGCCAGAGTGTTCG | gaggCTGCCAGAGTGTTCGTGGTTA |
| rs8012229 | MTHFD1 | ACGTTGGATGGGCCCATGTGGAAATGAATG | ACGTTGGATGAGCACACTGCAGGCCTTTTG | ctCCTTTTGGCACCCTGC |
| rs8040104 | MTHFS | ACGTTGGATGATGGGATTTGAGGAAGGAAG | ACGTTGGATGGATCGAGAGTTGAATAAGCAG | gggaAGTTAACACTCAATGTGAGACTGT |
| rs8042012 | MTHFS | ACGTTGGATGCATCTCTATAATGTGCCTGC | ACGTTGGATGGCTGCCTCTAGAGGAATTAG | ACTGGAGGAAACCAAGTAAAATT |
| rs8923 | MTHFS | ACGTTGGATGGGTCCCAGTGAATGAAAACG | ACGTTGGATGCACTGATTATTTGGCTGTAG | gggaCTGTAGTAATCCAGATTTAAGCTG |
| rs9842910 | ALDH1L1 | ACGTTGGATGAAGGTACAACACCAAGAGGG | ACGTTGGATGGCCTGGCCAATTTTGTTATAC | aACTTGGTGAGCCAATAT |
| Assay 3 (34-plex) | ||||
| rs10133855 | MTHFD1 | ACGTTGGATGAAATCAGCTGGGCTTGGTAG | ACGTTGGATGCCTCCCGGGTTCAAGTGAGTC | cgtgCAAGTGAGTCTCCTGC |
| rs1051296 | SLC19A1 | ACGTTGGATGATACCAAGGCCAGCACGTC | ACGTTGGATGAGTGTGTCCATCCTGACCTG | ggggCTCAGCTGCTCCCACACT |
| rs10957267 | GGH | ACGTTGGATGCCTTCCCCTGTGACAATTAG | ACGTTGGATGTAAATGGCTCCCACACTGTC | ccgcCTATAGTTTTGGTCCCCATTTATC |
| rs11545078 | GGH | ACGTTGGATGAGTGAAGTTCAGCGGCATTG | ACGTTGGATGGAGCTTTCACTGCTGATTAG | gaATTAGTGGAGAGTGCTTATTAA |
| rs11995525 | GGH | ACGTTGGATGGGAAACATTCAGAATCCAAC | ACGTTGGATGCCTCCAGATTCCAACCTTTC | gaccCATTTGGCATTTACATTTACAT |
| rs12613 | CBS | ACGTTGGATGTTGAGAGAGAAGTCGGCCAG | ACGTTGGATGGGACTCTTCTCTCTTTTGCC | TGCCTTTAATCCACTCTG |
| rs12899781 | MTHFS | ACGTTGGATGGAAACAATCATTGGCTCACC | ACGTTGGATGTATTTTTTGGCCTGGCTTCC | ctttCTGCTCCTATGTCCATAT |
| rs13043752 | AHCY | ACGTTGGATGTGGACATTGCTGAGAACGAG | ACGTTGGATGGCCCTTCAGTGGCTTGGAG | cctaTTGGAGGCCGAGTACC |
| rs13270305 | GGH | ACGTTGGATGAGCCTCGAGCTGTCTAGACC | ACGTTGGATGCAGCCTCCTTACCGATGATG | TGATGGGCTTCTTGG |
| rs1532268 | MTRR | ACGTTGGATGAGCAGCTCTGACTTCACAAG | ACGTTGGATGGGACAAGAGGAGATAAGTGG | ttgtCATCACCTGCATCCT |
| rs17004505 | FTCD | ACGTTGGATGGGGATTTTCCAGGGAACGAG | ACGTTGGATGAAGTCGCTGTTTCCCAGCAG | GGAAATGCTGCCACA |
| rs17367504 | MTHFR | ACGTTGGATGAGGAAGGAGAAGGCAGAGTG | ACGTTGGATGCACTGTGGAGGGACTTTTAC | tttttGAGGGACTTTTACAGGCAAC |
| rs1801133 | MTHFR | ACGTTGGATGCTTGAAGGAGAAGGTGTCTG | ACGTTGGATGCTTCACAAAGCGGAAGAATG | cccaTGCGTGATGATGAAATCG |
| rs2244976 | MTHFR | ACGTTGGATGGTGGACCACTCCCCTTAAAA | ACGTTGGATGTGAGAAATCTCTGATTTCC | TCCAAATCAATTCTGAAACCTTC |
| rs2273026 | SHMT1 | ACGTTGGATGGCCCTAGGCTCTTGCTTAAA | ACGTTGGATGCCTATGACTGTGTCAGAACC | gggCAGAACCAGGCCTTTGAAGATATT |
| rs2273028 | SHMT1 | ACGTTGGATGCTTTTCACTCCTGGAGGAAG | ACGTTGGATGCTGGGTTTGAGCCTAAAAAG | GCCCCTATGATCCCA |
| rs2274974 | MTHFR | ACGTTGGATGAGCCGAATGCTGTCACTTGG | ACGTTGGATGTCCAGAACATGAAGCTGACG | ggggtCTGGATGATCTCTCGC |
| rs35789560 | SLC19A1 | ACGTTGGATGTTCCAGTGCTGCTGGTGTTC | ACGTTGGATGCCACAGACCAACAGAACTTC | ctGACCAGGTCCTGCTC |
| rs3780130 | GGH | ACGTTGGATGTCTGCTTGGATTTGTGGTAG | ACGTTGGATGGGAAGATCACAGTCTGGTAG | GGTAGAAAACATATAAGCATCTG |
| rs3796191 | ALDH1L1 | ACGTTGGATGTCCCCTTGACAGAAACTGAC | ACGTTGGATGATGGGCAAAGCGTCTCCCTC | ccTCTCCCTCGGGCACC |
| rs383028 | FOLH1 | ACGTTGGATGGCCATGTTTCTTTGCAGGTG | ACGTTGGATGGAACCAGCTGAAAATATCGG | GGTAAGAAACAAGACAAATAATTTTA |
| rs4659727 | MTR | ACGTTGGATGACCTCTCTGGTTCTTTGTGG | ACGTTGGATGCCTAACCATCACCATATCTC | CATCACCATATCTCATCTGAA |
| rs473334 | CTH | ACGTTGGATGGAAACTAAAAATTCACTAGG | ACGTTGGATGGGCTTTATGATATGATATAAG | GTCCATCTTAATTATAATCATAATTTG |
| rs4933327 | MAT1A | ACGTTGGATGTGTTACAGTTCGTTGCTCCC | ACGTTGGATGAGGAAGGGCATTGGAAGATG | tGCATTGGAAGATGGAACAG |
| rs535112 | CTH | ACGTTGGATGCGTTTTCTGTTGTGGGTGTC | ACGTTGGATGATGCCAGAAACTCTAGAAAC | TCTAGAAACTATAGAATAAGTATCTAAG |
| rs588458 | FOLH1 | ACGTTGGATGCTCTGGGGTATGTAGAGTTG | ACGTTGGATGTGGGTGATCCAAATCCCATC | tcccCCCATCAATGTACAGCATAT |
| rs6774807 | ALDH1L1 | ACGTTGGATGAGGGTGAGGCAGAAAAGAAG | ACGTTGGATGAGAGAAAGCTACACCAAAAC | acatAGAAAGCTACACCAAAACCTGAAT |
| rs6799991 | ALDH1L1 | ACGTTGGATGATCTGAGTCCACAGCCTGAG | ACGTTGGATGAAACACCCCAGAGAGGCTCC | ccccGAGAGGCTCCATCCCAGCACCG |
| rs6875201 | BHMT | ACGTTGGATGAAGAGTCAGGAAGCCCTATG | ACGTTGGATGGAAATTAAGACCCAGAGTCC | ggatAGTCCCTGAGTAATTTATACA |
| rs7553194 | MTHFR | ACGTTGGATGGGCTGCTCTTCTTACACATC | ACGTTGGATGTCAGGCAATCCTTCTGCCTC | CTTCTGCCTCAGCCTT |
| rs7768897 | AMD1 | ACGTTGGATGCTTCATCTGGCCATTCACTC | ACGTTGGATGCCTGGGAGACAAAGTGAAAC | GAACCACTGAGTGAGACA |
| rs8006686 | MTHFD1 | ACGTTGGATGCTACACATCTGTATGAAGCC | ACGTTGGATGATCTGCCTCTGCATTCAGTC | GCTGTGTGCTGGTTTGA |
| rs8659 | MTRR | ACGTTGGATGCACTCTGGCATATGATTTATC | ACGTTGGATGGTACGTACTGGTACCTGTAA | CCCAAAATTCTGAAATTGTGACTT |
| rs8971 | GART | ACGTTGGATGCCTTGTGGTATCAAAGGAGC | ACGTTGGATGCCAATCACCCAGGCTTCTTC | tgCCTTGTGCTGCTGGATA |
| Assay 4 (25-plex) | ||||
| rs10158222 | MTR | ACGTTGGATGGTAACTCCTTTTTTTACGTGG | ACGTTGGATGACTCTGACTCACGGAGTGAC | TTGAGCAGCTTGCTTCCA |
| rs10380 | MTRR | ACGTTGGATGTGACAACCTTTTAGTGATCC | ACGTTGGATGGATGAGTTAAGATCCCATGC | gggtATCCCATGCTTAAGGAAAT |
| rs10407514 | DNMT1 | ACGTTGGATGTGACAGAGCAAGACTCCATC | ACGTTGGATGTGCGTGCTTCAAACTGTGAG | CTTCAAACTGTGAGACTAAATCT |
| rs10498514 | MTHFD1 | ACGTTGGATGTCAGGTGGTATGGTGTATGC | ACGTTGGATGGGGTCTGTGTTAAACAGTTA | gTTTATTTGAATTTCTGTAACAAATAC |
| rs10640 | AMT1 | ACGTTGGATGTCCTCCCAGACTTGCCTTAC | ACGTTGGATGTTTCAGGATTCAGTGGAGTC | ttgcGTCCATTAATGCATACCAGG |
| rs10934751 | ALDH1L1 | ACGTTGGATGATGCCTCATGTCCATCCTTG | ACGTTGGATGAGAGTCAGGTGGGCACAGC | GGGGACCTTGGGTGT |
| rs12898642 | MTHFS | ACGTTGGATGGTAGTCTTCGGTCATTTCAC | ACGTTGGATGAAGGAGTGGAGAAGCTTGTG | AGGTAAAATAAAGCATACTCTAGG |
| rs17131305 | CTH | ACGTTGGATGCCTCTATTTTTCATTTCTTC | ACGTTGGATGCCTATGACACTCTCAGTGAC | gcgCTAAGTGATGTGAAGTGATG |
| rs17194931 | GGH | ACGTTGGATGCTCATACCTTGTTGAAAGGG | ACGTTGGATGCCCTGGCAGAGAATACAGC | gtgtCAGAGAATACAGCTGTTAGTT |
| rs1800909 | GGH | ACGTTGGATGGCAGAGCTTTTGAAAGGCGG | ACGTTGGATGCAGAGTAGCAGGCCCAGCA | tccccGCACGCACAGCAGGC |
| rs1868130 | ALDH1L1 | ACGTTGGATGTCAACGAGACTTCAGAGTGG | ACGTTGGATGCAAGATAATGGCTTAAGTGGG | aAGTCTGGGAGGAGGCAG |
| rs1950902 | MTHFD1 | ACGTTGGATGAGATTGACTAGCATCAATG | ACGTTGGATGCCTTAGGCGTACAAGGAATG | GGTCACCTCTAGCAAGT |
| rs202712 | FOLH1 | ACGTTGGATGGCCTTTTGCAAATTTTCTG | ACGTTGGATGATCAAGCAACATGAAGAGGC | TGGTAGAAAGCACAATACATAG |
| rs2230270 | FPGS | ACGTTGGATGTATACCTGTGTCCGATGCTG | ACGTTGGATGAAGGCCAAGGCGGCGTTGGA | ctccaCCTCCAGGCCCAGGG |
| rs326124 | MTRR | ACGTTGGATGTAGTGTACCACATGAGCACC | ACGTTGGATGAAGAGGAGGCAGATTTCAGG | TGTGTCATTATGACTCAGG |
| rs35302908 | MTFMT | ACGTTGGATGAGAACTGCAACCTGACTTCC | ACGTTGGATGGGAGGAACAAACTTCAGAAC | CTTCAGAACAAATATTCAGACTTTAC |
| rs4646760 | ALDH1L1 | ACGTTGGATGAAATGGCTGCCTCAGATTGG | ACGTTGGATGCGAGGTCATGAATCTTACCC | CCTGGCAGTGGATAG |
| rs4672768 | ATIC | ACGTTGGATGGAGAACCATTTGACTTCTCC | ACGTTGGATGGATACACTCAGTCAAAAAGGC | TAACAACAGGATTTGGGTT |
| rs4679102 | ALDH1L1 | ACGTTGGATGCCCTCTCATTATCACAGGGA | ACGTTGGATGAAGGTAAGGGTGGATTTTGG | CAAGTTAAATGAAAGGATTGAGTTAT |
| rs4779165 | MTHFS | ACGTTGGATGATCTGTCTCTGTGGACTAAC | ACGTTGGATGGGCTATTTATGAGATGGATTG | gggagTTCCAGCAGATGTCAG |
| rs4846052 | MTHFR | ACGTTGGATGCCAGCACTCCATGTAGTTTC | ACGTTGGATGGACTAAACTTACTAGCCGCC | gggctGTCAGGCAAGCAGGA |
| rs4920037 | CBS | ACGTTGGATGATGCCACTGACTAGCCACAC | ACGTTGGATGTTAGAAGCTGGTGTGTGCTC | ccccCCCCAGAGGTCTAGATCAACT |
| rs6737407 | ATIC | ACGTTGGATGGAGATAAAAGTTGTAGATTC | ACGTTGGATGTAGGAGAAATAGGGTAACGG | GTTACATGGGAACTCTTACAA |
| rs6800400 | ALDH1L1 | ACGTTGGATGAGATTGAACAGATGCCTCTC | ACGTTGGATGCCCAGTGCCTAATGATGTTG | cTATTCTTATTTGCCGTCCATA |
| rs9910090 | SHMT1 | ACGTTGGATGTGGTTTTGGTGCTGGAGCTG | ACGTTGGATGTGGTGACATCTCTGCTCGG | aAGCAGCAGCACAAGGA |
| Assay 5 (11-plex) | ||||
| rs10932606 | ATIC | ACGTTGGATGAAGGACTTCTATCTCTATG | ACGTTGGATGAAATAGGAAATCCTATAATG | AGGAAATCCTATAATGAAACAAA |
| rs11627525 | MTHFD1 | ACGTTGGATGATCACCCATCCCAGAGAATC | ACGTTGGATGCCTCTGTAGTCATGCTTTTG | ATGAAGGCTGTCATTGGAT |
| rs1677692 | DHFR | ACGTTGGATGAAGGCTCAGTATGAAGGGTC | ACGTTGGATGCCCTGAAATGGAATAGTGTC | GGAATAGTGTCTTAACCAGAA |
| rs16837178 | ALDH1L1 | ACGTTGGATGTCACCAAAGCAGGACTCATC | ACGTTGGATGTTTGAGCAGGCAAGTGACCC | CGTGCCCTTACCATTTT |
| rs1979277 | SHMT1 | ACGTTGGATGGGAGGAGGTTGAGAGCTTC | ACGTTGGATGCTCCTTTAGAAGTCAGGCAG | aaCAGGCAGAGGGAAGA |
| rs2276726 | ALDH1L1 | ACGTTGGATGCATTTCCCTCAACAGCACTC | ACGTTGGATGCCCACCTGGCTTGTTCTTTG | CTTTGATGTGGGTCTCA |
| rs28941768 | FTCD | ACGTTGGATGTTCTTAGGGAGGGCCTCGTA | ACGTTGGATGGTGCCAGTTTACCTGTACGG | GCCAGGATGGACAGT |
| rs34507711 | MTFMT | ACGTTGGATGAAGCTCAACCAAGCCAATGC | ACGTTGGATGGCATTGTTGCATAGCAACAG | GCAACAGTTTTTTTCTGCT |
| rs596909 | TYMS | ACGTTGGATGGGGTTGGTTTTGATGGTGTC | ACGTTGGATGGTACCTGTCCTCTCTTTTTG | gggACAGATTATTCAGGACAGG |
| rs866027 | AHCY | ACGTTGGATGAGCTTCTGAGGTGATTCCAA | ACGTTGGATGAGGACAGATCCCATGTTCTC | ttAGCTGAATGCCGTGGC |
Appendix 7 Loss between referral and randomisation
| Exclusion criteria | North East Wales | North West Wales | Swansea | Total |
|---|---|---|---|---|
| Aged under 18 years | 0 | 4 | 0 | 4 |
| B12 deficient | 0 | 0 | 2 | 2 |
| Have taken folate supplementation | 4 | 6 | 4 | 14 |
| Suffered from psychosis | 1 | 1 | 1 | 3 |
| Bipolar disorder | 1 | 0 | 1 | 2 |
| Already in another research trial | 1 | 1 | 0 | 2 |
| Pregnant or planning to be | 3 | 4 | 2 | 9 |
| Taking anticonvulsants | 1 | 2 | 2 | 5 |
| Treatment for medical condition not yet stabilised | 0 | 1 | 0 | 1 |
| Taking lithium | 0 | 0 | 1 | 1 |
| Diagnosed with malignant disease | 4 | 12 | 3 | 19 |
| Subtotal | 15 | 31 | 16 | 62 |
| Not on antidepressants | 13 | 16 | 7 | 36 |
| Other | 2 | 2 | 1 | 5 |
| Subtotal | 15 | 17 | 9 | 41 |
| Self exclusion: refused | 91 | 128 | 58 | 277 |
| Did not attend screening | 21 | 29 | 34 | 84 |
| Could not be contacted | 26 | 75 | 60 | 161 |
| Subtotal | 138 | 235 | 152 | 522 |
| Total | 168 | 280 | 177 | 625 |
| Exclusion criteria | North East Wales | North West Wales | Swansea | Total |
|---|---|---|---|---|
| Aged under 18 years | 0 | 0 | 0 | 0 |
| Not depressed by ICD-10 criteria or low BDI-II | 42 | 45 | 35 | 122 |
| Folate deficient | 0 | 1 | 0 | 1 |
| Have taken folate supplementation | 4 | 3 | 1 | 8 |
| Suffered from psychosis | 1 | 0 | 1 | 2 |
| Bipolar disorder | 1 | 3 | 0 | 4 |
| Taking anticonvulsants | 6 | 6 | 4 | 16 |
| Treatment for medical condition not yet stabilised | 1 | 0 | 1 | 2 |
| Diagnosis with malignant disease | 2 | 17 | 3 | 22 |
| Subtotal | 57 | 75 | 45 | 177 |
| Not on antidepressants | 1 | 20 | 0 | 21 |
| Other | 6 | 5 | 3 | 14 |
| Subtotal | 7 | 25 | 3 | 35 |
| Self exclusion: refused | 4 | 12 | 0 | 16 |
| Did not attend or could not be contacted | 0 | 0 | 0 | 0 |
| Subtotal | 4 | 12 | 0 | 16 |
| Total | 68 | 112 | 48 | 228 |
| Exclusion criteria | North East Wales | North West Wales | Swansea | Total |
|---|---|---|---|---|
| Not depressed by ICD-10 criteria or low BDI-II | 6 | 23 | 7 | 36 |
| Folate deficient | 0 | 8 | 7 | 15 |
| B12 deficient | 0 | 7 | 1 | 8 |
| Have taken folate supplementation | 0 | 1 | 1 | 2 |
| Taking anti convulsants | 0 | 1 | 0 | 1 |
| Diagnosed with malignant disease | 0 | 2 | 0 | 2 |
| Subtotal | 6 | 42 | 16 | 64 |
| Not on antidepressants | 0 | 7 | 2 | 9 |
| Other | 1 | 2 | 2 | 5 |
| Subtotal | 1 | 9 | 4 | 14 |
| Self exclusion: refused | 9 | 4 | 4 | 17 |
| Did not attend or could not be contacted | 3 | 6 | 6 | 15 |
| Subtotal | 12 | 10 | 10 | 32 |
| Randomised in error | 2 | 0 | 2 | 4 |
| Recruited to comprehensive cohort | 24 | 7 | 15 | 46 |
| Subtotal | 26 | 7 | 17 | 50 |
| Total | 45 | 68 | 47 | 160 |
Appendix 8 Follow-ups completed and imputed
| North East Wales | North West Wales | Swansea | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | no. (%)a | no. (%)a | no. (%)a | no. (%)a | |||||
| Total randomised | 119 | 238 | 118 | 475 | |||||
| No follow-up (n = 35) | Folate | 3 | 2.5 | 8 | 3.4 | 3 | 2.5 | 14 | 2.5 |
| Placebo | 6 | 6.8 | 7 | 2.9 | 8 | 6.8 | 21 | 5.0 | |
| Analysed ( n = 440) | Folate | 57 | 47.5 | 110 | 46.2 | 56 | 47.5 | 223 | 47.9 |
| Placebo | 53 | 43.2 | 113 | 47.5 | 51 | 43.2 | 217 | 44.5 | |
| 4 weeks imputed | Folate | 2 | 3.5 | 10 | 9.1 | 3 | 5.4 | 15 | 6.7 |
| Placebo | 1 | 1.9 | 16 | 14.2 | 4 | 7.8 | 21 | 9.7 | |
| 12 weeks imputed | Folate | 4 | 7.0 | 5 | 4.5 | 13 | 23.2 | 22 | 9.9 |
| Placebo | 7 | 13.2 | 6 | 5.3 | 5 | 9.8 | 18 | 8.3 | |
| 25 weeks imputed | Folate | 8 | 14.0 | 6 | 5.5 | 13 | 23.2 | 27 | 12.1 |
| Placebo | 12 | 22.6 | 12 | 10.6 | 5 | 9.8 | 29 | 13.4 | |
| Two time points imputed | Folate | 4 | 8.5 | 4 | 1.7 | 10 | 8.5 | 18 | 3.4 |
| Placebo | 8 | 2.5 | 6 | 2.5 | 3 | 2.5 | 17 | 6.7 | |
Appendix 9 Elaborated demographic data
| Participant characteristic | North East Wales (n = 110) | North West Wales (n = 223) | Swansea (n = 107) | |
|---|---|---|---|---|
| number (%) | number (%) | number (%) | ||
| Ethnicitya | White | 8 (7) | 88 (40) | 14 (14) |
| White British | 89 (81) | 123 (55) | 79 (74) | |
| White Irish | 1 (1) | 5 (2) | ||
| Other white background | 9 (8) | 4 (2) | 7 (6) | |
| White and black Caribbean | 1 (0) | |||
| Black (British) | 1 (1) | 1 (1) | ||
| Caribbean | 1 (1) | |||
| Other Asian background | 1 (0.5) | |||
| Not stated | 2 (2) | 1 (0.5) | 5 (5) | |
| Marital statusb | Single (never married) | 17 (16) | 60 (27) | 24 (24) |
| Married (first marriage) | 43 (39) | 73 (33) | 42 (41) | |
| Divorced | 19 (17) | 27 (12) | 16 (16) | |
| Separated | 4 (4) | 6 (3) | 4 (4) | |
| Widowed | 4 (4) | 7 (3) | 4 (4) | |
| Cohabiting | 12 (11) | 37 (17) | 5 (5) | |
| Remarried | 10 (9) | 11 (5) | 7 (7) | |
| Number of dependent childrenc | 0 | 51 (57) | 87 (45) | 31 (53) |
| 1 | 15 (17) | 40 (21) | 15 (26) | |
| 2 | 18 (20) | 37 (19) | 6 (10) | |
| 3 | 3 (3) | 13 (7) | 4 (7) | |
| 4 | 1 (1) | 7 (4) | 2 (3) | |
| 5 | 1 (1) | 2 (1) | ||
| 6 | 3 (2) | |||
| 7 | 3 (2) | |||
| 8 | 1 (1) | |||
| Employment statusd | Full time employed | 35 (32) | 52 (23) | 22 (22) |
| Part time employed | 14 (13) | 22 (10) | 7 (7) | |
| Self-employed full time | 3 (3) | 7 (3) | 2 (2) | |
| Self-employed part time | 4 (4) | 4 (2) | 1 (1) | |
| Unemployed | 8 (7) | 47 (21) | 14 (14) | |
| Retired | 10 (9) | 16 (7) | 22 (22) | |
| Student | 2 (2) | 5 (2) | 10 (10) | |
| Looking after family/home | 4 (4) | 10 (5) | 4 (4) | |
| Permanently sick/disabled | 18 (17) | 32 (14) | 11 (11) | |
| Temporarily sick/disabled | 11 (10) | 27 (12) | 9 (9) | |
| Smoking statuse | Smoker | 27 (25) | 97 (44) | 38 (37) |
| Non-smoker | 56 (51) | 93 (42) | 45 (44) | |
| Ex-smoker | 26 (24) | 32 (14) | 19 (19) | |
| Drinking (units/week)f | None | 52 (48) | 78 (35) | 38 (38) |
| 1–7 | 30 (28) | 79 (36) | 27 (27) | |
| 8–14 | 16 (15) | 20 (9) | 15 (15) | |
| 15–21 | 6 (6) | 12 (5) | 10 (10) | |
| 22–35 | 3 (3) | 12 (5) | 7 (7) | |
| 36–50 | 2 (2) | 6 (3) | 1 (1) | |
| 51 or more | 14 (6) | 3 (3) | ||
Appendix 10 Elaborated clinical effectiveness results
| Outcome variable | Folate | Placebo | Difference (folate minus placebo) | Favours | Significance | |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | 95% CI | |||
| BDI-II (4 week) | 27.30 (11.56) | 27.84 (12.83) | –0.54 (12.20) | –2.83 to 1.75 | FOLATE | 0.643 |
| BDI-II (12 week) | 25.13 (13.56) | 24.66 (13.6) | 0.47 (13.58) | –2.07 to 3.02 | PLACEBO | 0.714 |
| BDI-II (25 week) | 22.61 (13.53) | 22.33 (14.09) | 0.28 (13.81) | –2.31 to 2.87 | PLACEBO | 0.832 |
| MADRS (4 week) | 23.29 (8.92) | 23.24 (9.49) | 0.05 (9.21) | –1.67 to 1.78 | PLACEBO | 0.953 |
| MADRS (12 week) | 21.83 (10.06) | 21.62 (10.73) | 0.21 (10.39) | –1.74 to 2.16 | PLACEBO | 0.832 |
| MADRS (25 week) | 19.77 (9.99) | 20.54 (11.24) | –0.77 (10.62) | –2.76 to 1.22 | FOLATE | 0.449 |
| EQ-5D (4 week) | 0.54 (0.31) | 0.59 (0.31) | –0.04 (0.31) | –0.10 to 0.01 | PLACEBO | 0.133 |
| EQ-5D (12 week) | 0.58 (0.3) | 0.59 (0.3) | –0.01 (0.30) | –0.06 to 0.05 | PLACEBO | 0.807 |
| EQ-5D (25 week) | 0.60 (0.3) | 0.6 (0.31) | 0.00 (0.30) | –0.05 to 0.06 | EQUAL | 0.949 |
| EQ-VAS (4 week) | 52.50 (21.69) | 51.66 (20.41) | 0.84 (21.07) | –3.11 to 4.79 | FOLATE | 0.677 |
| EQ-VAS (12 week) | 54.61 (23) | 55.76 (22.2) | –1.15 (22.61) | –5.39 to 3.09 | PLACEBO | 0.594 |
| EQ-VAS (25 week) | 59.98 (22.71) | 58.13 (23.57) | 1.85 (23.14) | –2.48 to 6.19 | FOLATE | 0.401 |
| SF-12 PCS (4 week) | 45.39 (12.56) | 43.71 (12.49) | 1.68 (12.52) | –0.66 to 4.03 | FOLATE | 0.159 |
| SF-12 PCS (12 week) | 44.67 (12.03) | 44.78 (13.05) | –0.11 (12.54) | –2.46 to 2.24 | PLACEBO | 0.925 |
| SF-12 PCS (25 week) | 45.20 (12.05) | 44.21 (12.34) | 0.98 (12.20) | –1.30 to 3.27 | FOLATE | 0.398 |
| SF-12 MCS (4 week) | 30.61 (11.35) | 32.13 (11.61) | –1.52 (11.48) | –3.67 to 0.63 | PLACEBO | 0.165 |
| SF-12 MCS (12 week) * | 32.66 (12.34) | 35.64 (12.19) | –2.97 (12.27) | –5.27 to –0.68 | PLACEBO | 0.011 |
| SF-12 MCS (25 week) | 34.56 (13.18) | 36.47 (12.87) | –1.90 (13.03) | –4.34 to 0.54 | PLACEBO | 0.126 |
| CGI: Severity (4 week) | 3.79 (0.98) | 3.78 (1.01) | 0.01 (0.99) | –0.17 to 0.20 | PLACEBO | 0.875 |
| CGI: Severity (12 week) | 3.59 (1.13) | 3.52 (1.24) | 0.07 (1.18) | –0.16 to 0.29 | PLACEBO | 0.555 |
| CGI: Severity (25 week) | 3.21 (1.34) | 3.33 (1.35) | –0.12 (1.34) | –0.37 to 0.14 | FOLATE | 0.363 |
| CGI: Improvement (4 week) | 3.30 (1.02) | 3.17 (1.01) | 0.13 (1.01) | –0.06 to 0.32 | PLACEBO | 0.179 |
| CGI: Improvement (12 week) | 3.14 (1.16) | 3.09 (1.21) | 0.05 (1.18) | –0.17 to 0.27 | PLACEBO | 0.678 |
| CGI: Improvement (25 week) | 2.95 (1.31) | 3.04 (1.4) | –0.09 (1.35) | –0.34 to 0.16 | FOLATE | 0.482 |
| CGI: Efficacy (4 week)a | 0.09 (0.59) | 0.17 (0.59) | –0.08 (0.59) | –0.19 to 0.03 | FOLATE | 0.159 |
| CGI: Efficacy (12 week)a | 0.22 (0.64) | 0.17 (0.64) | 0.05 (0.64) | –0.07 to 0.17 | PLACEBO | 0.447 |
| CGI: Efficacy (25 week)a | 0.27 (0.66) | 0.32 (0.7) | –0.05 (0.68) | –0.18 to 0.08 | FOLATE | 0.425 |
| UKU: PSYCHIC (4 WEEK) | 8.98 (5.43) | 8.38 (5.28) | 0.60 (5.36) | –0.40 to 1.61 | PLACEBO | 0.238 |
| UKU: PSYCHIC (6 WEEK) | 7.91 (5.2) | 8.09 (5.5) | –0.18 (5.35) | –1.18 to 0.82 | FOLATE | 0.728 |
| UKU: PSYCHIC (12 week) | 6.83 (5.11) | 6.72 (5.38) | 0.11 (5.24) | –0.87 to 1.09 | PLACEBO | 0.825 |
| UKU: Neurologic (4 week)b | 0.63 (0.85) | 0.68 (0.83) | –0.04 (0.84) | –0.20 to 0.12 | FOLATE | 0.605 |
| UKU: Neurologic (12 week)b | 0.55 (0.78) | 0.62 (0.83) | –0.07 (0.80) | –0.22 to 0.08 | FOLATE | 0.381 |
| UKU: Neurologic (25 week)b | 0.53 (0.77) | 0.57 (0.83) | –0.04 (0.80) | –0.19 to 0.11 | FOLATE | 0.567 |
| UKU: Autonomic (4 week) | 3.04 (2.91) | 2.94 (3.15) | 0.10 (3.03) | –0.47 to 0.66 | PLACEBO | 0.74 |
| UKU: Autonomic (12 week) | 2.89 (2.85) | 2.88 (2.92) | 0.00 (2.89) | –0.54 to 0.54 | PLACEBO | 0.991 |
| UKU: Autonomic (25 week) | 2.61 (2.58) | 2.44 (2.92) | 0.17 (2.75) | –0.35 to 0.68 | PLACEBO | 0.524 |
| UKU: Other (4 week) | 3.74 (3.54) | 3.89 (3.58) | –0.15 (3.56) | –0.82 to 0.52 | FOLATE | 0.66 |
| UKU: Other (12 week) ** | 3.42 (3.21) | 4.12 (4.04) | –0.70 (3.64) | –1.38 to –0.02 | FOLATE | 0.045 |
| UKU: Other (25 week) | 3.36 (3.07) | 3.77 (3.81) | –0.41 (3.45) | –1.06 to 0.24 | FOLATE | 0.213 |
| Predictors | β | SE (β) | Wald’s χ2 | Significance | Odds ratio [Exp(β)] | 95% CI for Exp(β) |
|---|---|---|---|---|---|---|
| Unadjusted | ||||||
| Serum folate (baseline) | 0.026 | 0.026 | 0.946 | 0.331 | 1.026 | 0.974 to 1.080 |
| Homocysteine (baseline) | 0.007 | 0.019 | 0.157 | 0.692 | 1.007 | 0.971 to 1.045 |
| Constant | 0.332 | 0.349 | 0.908 | 0.341 | 1.394 | |
| Adjusted by stratification variables | ||||||
| Serum folate (baseline) | 0.027 | 0.027 | 0.946 | 0.331 | 1.027 | 0.973 to 1.084 |
| Homocysteine (baseline) | 0.008 | 0.020 | 0.172 | 0.679 | 1.008 | 0.969 to 1.049 |
| Type of ADM | 0.535 | 0.239 | 5.029 | 0.025 | 1.707 | 1.070 to 2.725 |
| Previous counselling | 0.068 | 0.216 | 0.099 | 0.753 | 1.071 | 0.701 to 1.635 |
| Previous treatment | 0.760 | 0.268 | 8.020 | 0.005 | 2.138 | 1.264 to 3.618 |
| Centre | 4.134 | 0.127 | ||||
| Centre 1 | 0.544 | 0.268 | 4.126 | 0.042 | 1.722 | 1.019 to 2.911 |
| Centre 2 | 0.299 | 0.304 | 0.964 | 0.326 | 1.348 | 0.743 to 2.448 |
| Gender | 0.242 | 0.231 | 1.099 | 0.295 | 1.273 | 0.810 to 2.001 |
| Constant | –0.985 | 0.537 | 3.361 | 0.067 | 0.373 | |
| Adjusted by stratification variables and treatment group | ||||||
| Serum folate (baseline) | 0.027 | 0.027 | 0.950 | 0.330 | 1.027 | 0.973 to 1.084 |
| Homocysteine (baseline) | 0.008 | 0.020 | 0.161 | 0.688 | 1.008 | 0.969 to 1.049 |
| Type of ADM (1) | 0.537 | 0.239 | 5.066 | 0.024 | 1.712 | 1.072 to 2.733 |
| Previous counselling | 0.066 | 0.216 | 0.094 | 0.759 | 1.069 | 0.699 to 1.633 |
| Previous treatment | 0.762 | 0.269 | 8.052 | 0.005 | 2.142 | 1.266 to 3.626 |
| Centre | 4.174 | 0.124 | ||||
| Centre 1 | 0.547 | 0.268 | 4.165 | 0.041 | 1.728 | 1.022 to 2.922 |
| Centre 2 | 0.300 | 0.304 | 0.973 | 0.324 | 1.35 | 0.744 to 2.451 |
| Gender | 0.240 | 0.231 | 1.081 | 0.299 | 1.271 | 0.809 to 1.998 |
| Treatment group | 0.056 | 0.211 | 0.070 | 0.791 | 1.057 | 0.700 to 1.598 |
| Constant | –1.012 | 0.548 | 3.418 | 0.064 | 0.363 | |
| Predictors | β | SE (β) | Wald’s χ2 | Significance | Odds ratio [Exp(β)] | 95% CI for Exp(β) |
|---|---|---|---|---|---|---|
| Unadjusted | ||||||
| Serum folate (baseline) | –0.028 | 0.027 | 1.10 | 0.294 | 0.972 | 0.923 to 1.025 |
| Homocysteine (baseline) | –0.011 | 0.018 | 0.417 | 0.518 | 0.989 | 0.955 to 1.024 |
| Constant | 1.295 | 0.349 | 13.7 | 0 | 3.649 | |
| Adjusted by stratification variables | ||||||
| Serum folate (baseline) | –0.023 | 0.028 | 0.696 | 0.404 | 0.977 | 0.925 to 1.032 |
| Homocysteine (baseline) | –0.014 | 0.019 | 0.547 | 0.459 | 0.986 | 0.951 to 1.023 |
| Type of ADM | 0.379 | 0.254 | 2.228 | 0.136 | 1.461 | 0.888 to 2.403 |
| Previous counselling | 0.245 | 0.228 | 1.155 | 0.283 | 1.277 | 0.817 to 1.996 |
| Previous treatment | 0.252 | 0.29 | 0.75 | 0.386 | 1.286 | 0.728 to 2.272 |
| Centre | 4.09 | 0.129 | ||||
| Centre 1 | 0.535 | 0.279 | 3.664 | 0.056 | 1.707 | 0.987 to 2.952 |
| Centre 2 | 0.132 | 0.311 | 0.181 | 0.671 | 1.141 | 0.621 to 2.098 |
| Gender 1 | –0.158 | 0.246 | 0.410 | 0.522 | 0.854 | 0.527 to 1.384 |
| Constant | 0.644 | 0.545 | 1.397 | 0.237 | 1.904 | |
| Adjusted by stratification variables and treatment group | ||||||
| Serum folate (baseline) | –0.023 | 0.028 | 0.685 | 0.408 | 0.977 | 0.926 to 1.032 |
| Homocysteine (baseline) | –0.015 | 0.019 | 0.603 | 0.437 | 0.986 | 0.950 to 1.022 |
| Type of ADM | 0.386 | 0.254 | 2.303 | 0.129 | 1.471 | 0.894 to 2.422 |
| Previous counselling | 0.240 | 0.228 | 1.105 | 0.293 | 1.271 | 0.813 to 1.987 |
| Previous treatment | 0.258 | 0.291 | 0.788 | 0.375 | 1.295 | 0.732 to 2.29 |
| Centre | 4.187 | 0.123 | ||||
| Centre 1 | 0.543 | 0.280 | 3.762 | 0.052 | 1.722 | 0.994 to 2.981 |
| Centre 2 | 0.136 | 0.311 | 0.191 | 0.662 | 1.146 | 0.623 to 2.107 |
| Gender | –0.162 | 0.246 | 0.432 | 0.511 | 0.850 | 0.525 to 1.379 |
| Treatment group | –0.138 | 0.223 | 0.384 | 0.536 | 0.871 | 0.563 to 1.348 |
| Constant | 0.715 | 0.558 | 1.645 | 0.200 | 2.045 | |
| Predictors | β | SE (β) | Wald’s χ | Significance | Odds Ratio [Exp(β)] | 95% CI |
|---|---|---|---|---|---|---|
| Unadjusted | ||||||
| Serum folate (baseline) | 0.073 | 0.039 | 3.526 | 0.06 | 1.076 | 0.997 to 1.161 |
| Homocysteine (baseline) | 0.011 | 0.026 | 0.191 | 0.662 | 1.011 | 0.961 to 1.064 |
| Constant | 1.036 | 0.479 | 4.668 | 0.031 | 2.817 | |
| Adjusted by stratification variables | ||||||
| Serum folate (baseline) | 0.080 | 0.041 | 3.748 | 0.053 | 1.083 | 0.999 to 1.174 |
| Homocysteine (baseline) | 0.021 | 0.031 | 0.473 | 0.492 | 1.022 | 0.961 to 1.086 |
| Type of ADM (1) | 0.540 | 0.327 | 2.731 | 0.098 | 1.716 | 0.904 to 3.257 |
| Previous counselling | 0.299 | 0.279 | 1.144 | 0.285 | 1.348 | 0.780 to 2.331 |
| Previous treatment | 0.444 | 0.339 | 1.710 | 0.191 | 1.559 | 0.801 to 3.031 |
| Centre | 2.407 | 0.300 | ||||
| Centre 1 | 0.477 | 0.358 | 1.777 | 0.182 | 1.611 | 0.799 to 3.247 |
| Centre 2 | 0.017 | 0.39 | 0.002 | 0.964 | 1.018 | 0.474 to 2.184 |
| Gender | 0.462 | 0.296 | 2.431 | 0.119 | 1.587 | 0.888 to 2.834 |
| Constant | –0.313 | 0.729 | 0.184 | 0.668 | 0.732 | |
| Adjusted by stratification variables and treatment group | ||||||
| Serum folate (baseline) | 0.080 | 0.041 | 3.770 | 0.052 | 1.084 | 0.999 to 1.175 |
| Homocysteine (baseline) | 0.021 | 0.032 | 0.428 | 0.513 | 1.021 | 0.960 to 1.086 |
| Type of ADM (1) | 0.548 | 0.328 | 2.802 | 0.094 | 1.730 | 0.911 to 3.288 |
| Previous counselling | 0.292 | 0.280 | 1.093 | 0.296 | 1.340 | 0.774 to 2.318 |
| Previous treatment | 0.450 | 0.341 | 1.737 | 0.187 | 1.568 | 0.803 to 3.062 |
| Centre | 2.558 | 0.278 | ||||
| Centre 1 | 0.496 | 0.360 | 1.901 | 0.168 | 1.642 | 0.811 to 3.322 |
| Centre 2 | 0.020 | 0.390 | 0.003 | 0.960 | 1.020 | 0.475 to 2.189 |
| Gender | 0.457 | 0.297 | 2.370 | 0.124 | 1.580 | 0.883 to 2.827 |
| Treatment group | –0.245 | 0.275 | 0.789 | 0.374 | 0.783 | 0.456 to 1.343 |
| Constant | –0.191 | 0.747 | 0.065 | 0.798 | 0.826 | |
| Predictors | β | SE (β) | Wald’s χ2 | Significance | Odds Ratio [Exp(β)] | 95% CI for Exp(β) |
|---|---|---|---|---|---|---|
| Homocysteine (baseline) | –0.008 | 0.023 | 0.121 | 0.728 | 0.992 | 0.949 to 1.038 |
| Constant | 2.051 | 0.478 | 18.389 | 0 | 7.774 | |
| Adjusted by stratification variables | ||||||
| Serum folate (baseline) | 0.018 | 0.042 | 0.193 | 0.661 | 1.019 | 0.938 to 1.106 |
| Homocysteine (baseline) | –0.008 | 0.025 | 0.096 | 0.757 | 0.992 | 0.946 to 1.042 |
| Type of ADM | 0.440 | 0.375 | 1.376 | 0.241 | 1.553 | 0.744 to 3.242 |
| Previous counselling | 0.298 | 0.322 | 0.856 | 0.355 | 1.347 | 0.716 to 2.534 |
| Previous treatment | 0.200 | 0.399 | 0.251 | 0.616 | 1.221 | 0.559 to 2.667 |
| Centre | 0.352 | 0.838 | ||||
| Centre 1 | 0.119 | 0.413 | 0.083 | 0.773 | 1.127 | 0.501 to 2.533 |
| Centre 2 | –0.121 | 0.453 | 0.072 | 0.789 | 0.886 | 0.365 to 2.151 |
| Gender | 0.111 | 0.340 | 0.107 | 0.744 | 1.117 | 0.574 to 2.177 |
| Constant | 1.512 | 0.758 | 3.983 | 0.046 | 4.538 | |
| Adjusted by stratification variables and treatment group | ||||||
| Serum folate (baseline) | 0.019 | 0.042 | 0.199 | 0.656 | 1.019 | 0.938 to 1.107 |
| Homocysteine (baseline) | –0.009 | 0.025 | 0.144 | 0.704 | 0.991 | 0.943 to 1.040 |
| Type of ADM (1) | 0.453 | 0.377 | 1.448 | 0.229 | 1.574 | 0.752 to 3.293 |
| Previous counselling | 0.288 | 0.323 | 0.796 | 0.372 | 1.334 | 0.709 to 2.510 |
| Previous treatment | 0.208 | 0.401 | 0.27 | 0.604 | 1.232 | 0.561 to 2.705 |
| Centre | 0.394 | 0.821 | ||||
| Centre 1 | 0.139 | 0.416 | 0.111 | 0.739 | 1.149 | 0.509 to 2.594 |
| Centre 2 | –0.115 | 0.453 | 0.065 | 0.799 | 0.891 | 0.367 to 2.165 |
| Gender | 0.102 | 0.341 | 0.089 | 0.765 | 1.107 | 0.567 to 2.162 |
| Treatment group | –0.319 | 0.318 | 1.004 | 0.316 | 0.727 | 0.389 to 1.357 |
| Constant | 1.691 | 0.784 | 4.651 | 0.031 | 5.424 | |
| Participant characteristic | Nearest neighbours (n = 35) | No follow-up (n = 35) | Significance test |
|---|---|---|---|
| Age in years | |||
| Range | 20–68 | 20–66 | |
| Mean (SD) | 42 (13) | 39 (14) | F(1,68) = 0.98, p = 0.33 |
| Marital status, no. (%) | |||
| Single | 12 (48) | 13 (52) | |
| Had a partner | 13 (54) | 11 (46) | |
| Have a partner | 10 (48) | 11 (52) | χ2 with 2 df = 0.25, p = 0.88 |
| Number of dependent children, no. (%) | |||
| 0 | 21 (49) | 22 (51) | |
| 1 | 6 (55) | 5 (45) | |
| 2 | 1 (17) | 5 (83) | |
| 3 or more | 7 (70) | 3 (30) | Fisher’s p = 0.24 |
| Employment, no. (%) | |||
| Full time employed | 10 (48) | 11 (52) | |
| Part time or in education | 12 (63) | 7 (37) | |
| Inactive | 13 (43) | 17 (57) | χ2 with 2 df = 1.90, p = 0.39 |
| Alcohol units per week, no. (%) | |||
| None | 13 (57) | 10 (43) | |
| Within safe limit* | 17 (52) | 16 (48) | |
| Above safe limit* | 5 (36) | 9 (64) | χ2 with 2 df = 1.56, p = 0.46 |
| BDI-II screening | |||
| Range | 24–56 | 24–57 | |
| Mean (SD) | 39 (09) | 42 (09) | F(1,68) = 1.68, p = 0.20 |
| BDI-II baseline | |||
| Range | 20–61 | 18–57 | |
| Mean (SD) | 37 (11) | 40 (11) | F(1,68) = 1.00, p = 0.321 |
| Outcome | Covariate | Difference (folate minus placebo) | Baseline prediction of outcome per biochemical unit | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (SE) | 95% CI | Significance | Mean | (SE) | 95% CI | Significance | ||
| BDI-II | Serum folate | 0.682 | (0.833) | –0.955 to 2.320 | 0.413 | –0.070 | (0.088) | –0.243 to 0.104 | 0.431 |
| Red cell folate | –0.045 | (0.990) | –1.993 to 1.904 | 0.964 | 0.003 | (0.003) | –0.003 to 0.009 | 0.344 | |
| B12 | 0.437 | (1.012) | –1.554 to 2.427 | 0.666 | –0.004 | (0.004) | –0.012 to 0.004 | 0.279 | |
| Homocysteine | 0.703 | (0.850) | –0.968 to 2.373 | 0.409 | 0.000 | (0.071) | –0.139 to 0.139 | 0.999 | |
| MADRS | Serum folate | 0.530 | (0.649) | –0.746 to 1.806 | 0.415 | 0.057 | (0.069) | –0.077 to 0.192 | 0.404 |
| Red cell folate | 0.351 | (0.744) | –1.113 to 1.815 | 0.638 | 0.003 | (0.002) | –0.001 to 0.008 | 0.140 | |
| B12 | 0.528 | (0.814) | –1.073 to 2.128 | 0.517 | –0.003 | (0.003) | –0.010 to 0.003 | 0.323 | |
| Homocysteine | 0.353 | (0.662) | –0.948 to 1.654 | 0.594 | 0.035 | (0.055) | –0.073 to 0.143 | 0.520 | |
| EQ-5D | Serum folate | 0.012 | (0.038) | –0.063 to 0.086 | 0.763 | 0.002 | (0.002) | –0.002 to 0.006 | 0.315 |
| Red cell folate | 0.351 | (0.744) | –1.113 to 1.815 | 0.638 | 0.001 | (0.002) | –0.003 to 0.005 | 0.683 | |
| B12 | 0.066 | (0.044) | –0.021 to 0.153 | 0.137 | 0.000 | (0.000) | 0.000 to 0.000 | 0.107 | |
| Homocysteine | 0.013 | (0.039) | –0.063 to 0.089 | 0.739 | –0.002 | (0.001) | –0.005 to 0.001 | 0.192 | |
| EQ-VAS | Serum folate | 1.019 | (0.850) | –0.651 to 2.689 | 0.231 | 0.134 | (0.091) | –0.045 to 0.312 | 0.142 |
| Red cell folate | –0.026 | (1.016) | –2.026 to 1.973 | 0.979 | 0.001 | (0.003) | –0.005 to 0.007 | 0.713 | |
| B12 | 0.739 | (1.044) | –1.314 to 2.791 | 0.480 | –0.006 | (0.004) | –0.015 to 0.002 | 0.130 | |
| Homocysteine | 0.997 | (0.865) | –0.704 to 2.697 | 0.250 | 0.015 | (0.072) | –0.127 to 0.158 | 0.833 | |
| MCS | Serum folate | –1.848 | (0.832) | –3.483 to –0.213 | 0.027 | –0.046 | (0.088) | –0.219 to 0.127 | 0.602 |
| Red cell folate | –0.610 | (0.991) | –2.560 to 1.339 | 0.538 | –0.007 | (0.003) | –0.013 to –0.001 | 0.019 | |
| B12 | –1.820 | (1.055) | –3.895 to 0.254 | 0.085 | 0.001 | (0.004) | –0.008 to 0.009 | 0.871 | |
| Homocysteine | –1.627 | (0.855) | –3.308 to 0.053 | 0.058 | 0.125 | (0.071) | –0.015 to 0.264 | 0.080 | |
| PCS | Serum folate | 0.657 | (0.635) | –0.591 to 1.905 | 0.302 | 0.029 | (0.068) | –0.104 to 0.163 | 0.666 |
| Red cell folate | 0.996 | (0.743) | –0.466 to 2.457 | 0.181 | 0.001 | (0.002) | –0.003 to 0.005 | 0.683 | |
| B12 | 0.718 | (0.765) | –0.786 to 2.221 | 0.349 | 0.005 | (0.003) | –0.001 to 0.011 | 0.090 | |
| Homocysteine | 0.583 | (0.656) | –0.706 to 1.872 | 0.375 | 0.052 | (0.055) | –0.057 to 0.160 | 0.348 | |
| CGI: Severity | Serum folate | 0.003 | (0.082) | –0.157 to 0.163 | 0.971 | 0.002 | (0.009) | –0.015 to 0.019 | 0.804 |
| Red cell folate | –0.085 | (0.095) | –0.271 to 0.101 | 0.368 | 0.000 | (0.000) | 0.000 to 0.001 | 0.160 | |
| B12 | –0.009 | (0.103) | –0.210 to 0.193 | 0.933 | 0.000 | (0.000) | –0.001 to 0.001 | 0.473 | |
| Homocysteine | –0.014 | (0.083) | –0.176 to 0.149 | 0.870 | –0.004 | (0.007) | –0.018 to 0.009 | 0.523 | |
| CGI: Improvement | Serum folate | 0.059 | (0.109) | –0.154 to 0.273 | 0.586 | 0.007 | (0.012) | –0.016 to 0.030 | 0.533 |
| Red cell folate | –0.022 | (0.124) | –0.265 to 0.221 | 0.859 | 0.001 | (0.000) | 0.000 to 0.002 | 0.026 | |
| B12 | 0.038 | (0.110) | –0.177 to 0.253 | 0.728 | 0.000 | (0.000) | –0.001 to 0.001 | 0.891 | |
| Homocysteine | 0.075 | (0.110) | –0.142 to 0.292 | 0.498 | 0.052 | (0.012) | 0.029 to 0.075 | <0.0001 | |
| Outcome | Covariate | Folate – placebo | On treatment prediction of outcome per biochemical unit | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (SE) | 95% CI | Significance | Mean | (SE) | 95% CI | Significance | ||
| BDI-II | Serum folate | 0.107 | (1.093) | –2.041 to 2.255 | 0.922 | 0.032 | (0.034) | –0.034 to 0.099 | 0.337 |
| Red cell folate | –1.794 | (1.490) | –4.728 to 1.141 | 0.230 | 0.003 | (0.002) | –0.002 to 0.007 | 0.247 | |
| B12 | 0.503 | (1.012) | –1.488 to 2.493 | 0.620 | –0.001 | (0.003) | –0.008 to 0.006 | 0.823 | |
| Homocysteine | 1.154 | (1.044) | –0.899 to 3.206 | 0.270 | 0.334 | (0.095) | 0.148 to 0.520 | 0.0005 | |
| MADRS | Serum folate | 0.260 | (0.894) | –1.498 to 2.017 | 0.772 | 0.026 | (0.031) | –0.034 to 0.086 | 0.398 |
| Red cell folate | –0.623 | (1.188) | –2.963 to 1.717 | 0.601 | 0.002 | (0.002) | –0.002 to 0.006 | 0.299 | |
| B12 | 0.578 | (0.813) | –1.021 to 2.177 | 0.477 | 0.001 | (0.003) | –0.005 to 0.007 | 0.701 | |
| Homocysteine | 1.025 | (0.824) | –0.595 to 2.645 | 0.214 | 0.265 | (0.081) | 0.106 to 0.423 | 0.001 | |
| EQ-5D | Serum folate | 0.021 | (0.023) | –0.025 to 0.067 | 0.363 | 0.000 | (0.001) | –0.002 to 0.001 | 0.738 |
| Red cell folate | 0.059 | (0.031) | –0.002 to 0.121 | 0.060 | 0.000 | (0.000) | 0.000 to 0.000 | 0.150 | |
| B12 | 0.018 | (0.021) | –0.024 to 0.060 | 0.397 | 0.000 | (0.000) | 0.000 to 0.000 | 0.966 | |
| Homocysteine | 0.003 | (0.022) | –0.041 to 0.046 | 0.904 | –0.006 | (0.002) | –0.010 to –0.002 | 0.007 | |
| EQ-VAS | Serum folate | 0.107 | (1.093) | –2.041 to 2.255 | 0.922 | 0.032 | (0.034) | –0.034 to 0.099 | 0.337 |
| Red cell folate | –1.794 | (1.490) | –4.728 to 1.141 | 0.230 | 0.003 | (0.002) | –0.002 to 0.007 | 0.247 | |
| B12 | 0.503 | (1.012) | –1.488 to 2.493 | 0.620 | –0.001 | (0.003) | –0.008 to 0.006 | 0.823 | |
| Homocysteine | 1.154 | (1.044) | –0.899 to 3.206 | 0.270 | 0.334 | (0.095) | 0.148 to 0.520 | 0.0005 | |
| MCS | Serum folate | –1.172 | (1.146) | –3.424 to 1.081 | 0.307 | –0.053 | (0.038) | –0.127 to 0.020 | 0.155 |
| Red cell folate | 0.096 | (1.565) | –2.986 to 3.178 | 0.951 | –0.002 | (0.002) | –0.006 to 0.003 | 0.522 | |
| B12 | –1.834 | (1.052) | –3.903 to 0.235 | 0.082 | –0.001 | (0.004) | –0.008 to 0.007 | 0.812 | |
| Homocysteine | –2.148 | (1.098) | –4.307 to 0.011 | 0.051 | –0.140 | (0.104) | –0.344 to 0.064 | 0.178 | |
| PCS | Serum folate | 1.136 | (0.825) | –0.485 to 2.757 | 0.169 | –0.042 | (0.025) | –0.091 to 0.007 | 0.090 |
| Red cell folate | 1.502 | (1.114) | –0.693 to 3.696 | 0.179 | –0.003 | (0.002) | –0.006 to 0.000 | 0.081 | |
| B12 | 0.634 | (0.761) | –0.863 to 2.131 | 0.405 | 0.003 | (0.003) | –0.002 to 0.008 | 0.255 | |
| Homocysteine | 0.357 | (0.784) | –1.184 to 1.899 | 0.649 | 0.012 | (0.071) | –0.126 to 0.151 | 0.862 | |
| CGI: Severity | Serum folate | –0.042 | (0.112) | –0.261 to 0.177 | 0.706 | 0.003 | (0.004) | –0.004 to 0.010 | 0.391 |
| Red cell folate | –0.233 | (0.144) | –0.517 to 0.051 | 0.107 | 0.000 | (0.000) | 0.000 to 0.001 | 0.339 | |
| B12 | –0.004 | (0.103) | –0.205 to 0.198 | 0.972 | 0.000 | (0.000) | –0.001 to 0.001 | 0.686 | |
| Homocysteine | 0.040 | (0.105) | –0.166 to 0.247 | 0.702 | 0.029 | (0.010) | 0.010 to 0.049 | 0.003 | |
| CGI: Improvement | Serum folate | –0.063 | (0.119) | –0.298 to 0.172 | 0.596 | 0.009 | (0.004) | 0.000 to 0.017 | 0.038 |
| Red cell folate | –0.116 | (0.165) | –0.440 to 0.207 | 0.480 | 0.000 | (0.000) | 0.000 to 0.001 | 0.481 | |
| B12 | 0.041 | (0.109) | –0.173 to 0.256 | 0.704 | 0.000 | (0.000) | 0.000 to 0.001 | 0.308 | |
| Homocysteine | 0.055 | (0.108) | –0.158 to 0.268 | 0.610 | 0.042 | (0.011) | 0.020 to 0.063 | 0.0001 | |
Appendix 11 Recruitment into clinical trials
Introduction
Randomised controlled trials have been seen in recent decades as the ‘gold standard’ for clinical research. 173 They are accepted as generally the best way to estimate the effectiveness and cost-effectiveness of interventions, as they make strong causal connections between interventions and their effects. 174 However more than two thirds of published trials do not achieve their recruitment targets. 175 McDonald et al. looked at 114 trials funded by the MRC or the NIHR HTA programme and found that 54% required an extension, while 41% experienced delays in starting recruitment. 176 This has large cost implications for funders, resulting in trial extensions, and even complete failure. Why some trials recruit well and other suffer problems remains unclear. 177
Several systematic reviews have sought to improve recruitment in trials. Prescott et al. 177 attempted to identify factors that affected the effective running of trials, suggesting that recruitment problems may be reduced by piloting, using multiple recruitment strategies, making contingency plans for slow recruitment and using recruitment coordinators. However none of these approaches had been rigorously evaluated. Watson and Torgerson concluded that recruitment interventions were both sparse and often of poor methodological quality. 178
Campbell et al. set out to identify factors associated with good and poor recruitment in multi-centred trials from a cohort of studies, a selection of case studies and a single in-depth case study. 179 The main themes associated with success were flexibility, adaptability to unexpected issues and better training. The study suggested that the complex nature of multi-centred trials generated unexpected difficulties.
Recent methods to improve recruitment have achieved some success. One such method is the business model using marketing strategies; however these require further research to establish effectiveness and develop useable tools for medical research where these approaches, concepts and terminology are unfamiliar. 180
Barnard et al. looked at the recruitment of participants into trials from a different perspective and aimed to identify different models that may be useful to RCTs when estimating the recruitment of participants. 181 They noted that most trials use an unconditional model of recruitment and suggested that a new model was needed to predict recruitment to clinical trials which takes account of both centre and patient recruitment, recognising that one drives the other.
Health services research often recruits from primary care. However Bower et al.,175 exploring recruitment difficulties, responses to recruitment problems and the relationship between trial characteristics and recruitment, found that recruitment methods requiring GPs to consent patients into trials were particularly problematic. In a review of current literature, Bower also concludes that recruitment of patients into health research from primary care continues to be a major hurdle. 182
Goodyear-Smith et al. sought to identify barriers to recruitment in primary care,183 including lack of time (exacerbated by the annual influenza vaccinations campaign in general practice), the need to identify staff responsible for decision making, the need to clarify the nature of the study, and the need to be flexible in accommodating practices. Strategies to improve recruitment included providing incentives to practices (both material and educational), using a personal approach, ensuring practices feel engaged, minimising disruption, streamlining processes, and using doctors to recruit doctors. They also suggested that smaller practices were easier to recruit than larger practices.
In the UK in recent years, national research networks have tried to improve some of the difficulties in recruiting to clinical trials and running them. These include specialised networks in key areas of NHS research such as cancer, mental health and stroke. Overarching networks also support research in areas like primary care and children’s research. The aim of these research networks is to improve the process of regulatory approvals, provide infrastructure and research support to trials and facilitate the recruitment of patients into trials. 184 In Wales the NISCHR Clinical Research Centre (NISCHR CRC) was established in 2010 to provide research workforce to support and develop research activity within health and social care. 185 However few reported trials have assessed the effectiveness of research networks. Those that have report that they have been successful in improving recruitment rates into trials. 185–187
So we aimed to identify barriers to recruitment and factors that facilitated recruitment in the FolATED trial.
Recruitment methods
We recruited participants over three years in three centres across Wales. Recruitment methods included direct referrals from GPs, psychiatrists and other healthcare professionals, clinical database searches and self-referral. We reported the number of participants recruited by each method in each centre to identify differences.
Recruitment into the trial from primary care was facilitated by research staff employed directly by the trial or by NISCHR CRC. Their Clinical Studies Officers played a pivotal role in recruitment, including practice recruitment through visits and presentations, development and editing of regular newsletters to recruiting practices, and contributing to local and national meetings and conferences. NISCHR CRC staff also helped with computer searches in practices and invitation mailings from practices.
We collected data from each centre on recruitment, including whether from secondary care, general practices or community mental health services, and method of recruitment, for example whether participants were directly referred by healthcare professionals or as a result of invitations from computer searches in practices.
We sought qualitative feedback from centres through a reflective recruitment tool providing written accounts of recruitment strategies, barriers and facilitators in each site (Table 50). We analysed these data using a thematic approach. We also held monthly recruitment meetings and annual training events to identify recruitment difficulties and develop new strategies.
| Recruitment and retention issues and supporting data |
|---|
| 1. General recruitment Issues. Describe any recruitment issues in your area. Below are some suggested categories but you can alter to your own experiences. What difficulties arose? What worked well? |
| 1.1 Geographical |
| 1.2 Population demographics |
| 1.3 Participant attributes |
| 1.4 The GP–patient relationship |
| 1.5 Appointment booking strategies |
| 1.6 Other issues – e.g. staffing issues, recruiting surgeries and psychiatrists, maintaining psychiatric cover, centre approaches and structure, physical environment and travel. |
| 2. Patient recruitment strategies. What strategies were adopted in your centre? How well did they work? Below are suggested categories; please add or omit as appropriate to your centre. |
| 2.1 Posters, leaflets, newsletters |
| 2.2 Traditional GP referral |
| 2.3 Psychiatrist referral |
| 2.4 Computer searches |
| 2.5 Other strategies |
| 3. Increasing recruitment and retention rates. Other methods used to improve patient recruitment and retention rates. How did these work in practice? |
Recruitment performance
We used a wide variety of strategies to help with the recruitment and retention of both recruiters to the trial such as general practices and secondary care services, and trial participants (Table 51). We adopted a flexible approach to recruitment allowing the three centres to identify and deploy the strategies found to be successful in their area. However some strategies were universal such as the use of trial posters, payment of general practices, feedback to GPs of BDI-II scores, regular training events and research team meetings. Local variations included use of research networks such as NISCHR CRC, access to direct referrals to secondary care through flagging in referral notes, different methods of referral and participant reminders for appointments and venues.
| Recruiting practices |
|---|
| Raise awareness of practices |
| Advertising in local health board bulletin |
| Presentations at local GP meetings |
| Stands at mental health practitioner network meetings |
| Posters at NISCHR CRC primary care research events |
| Letters of invitation to general practices |
| Direct phone calls to general practices |
| Raise awareness of patients |
| Posters in general practices |
| Participant information leaflets in waiting rooms |
| Incentives for primary care |
| Pay £50 per patient consented to cover administration costs |
| Provide GP with BDI-II scores and blood results for Quality & Outcomes Framework (QOF) assessments |
| Provide ‘fast track’ access to psychiatric assessment |
| Use NISCHR CRC |
| Provide primary care recruitment assistance and research support in North East Wales |
| Provide research staff for recruitment in Swansea |
| Flag triage to secondary care in North West Wales |
| Training event e.g. recruitment ‘brainstorming’ |
| Monthly research team meetings to discuss recruitment issues across centres |
| Maintaining recruitment |
| Monthly newsletter to recruiting practices and mental health professionals |
| GP feedback events |
| Regular personal contact with practices and feedback from researchers |
| Maintenance of participation in trial |
| Appointment reminder letters and telephone calls |
| Continuity of researcher for participant |
| Reduction in assessment at screening visit |
| Introduction of home visits when beneficial to participants |
Methods used in recruitment
We recruited participants in three centres – North East Wales, North West Wales and Swansea (Table 52). Although several methods of recruitment were adopted by all centres, different methods were used in different centres. North West Wales, the most successful recruiting centre, acquired their referrals mainly from direct referrals to the psychiatric services, supplemented by computer searches at general practices and other referrals through secondary care. North East Wales acquired the majority of their referrals through computer searches at general practices and direct GP referrals supplemented by other mental health referrals. Swansea acquired the majority of their referrals through direct GP referrals, supplementing this with computer searches and other referrals from secondary care (Figure 19).
| Referral method | North East Wales | North West Wales | Swansea |
|---|---|---|---|
| Direct GP referrals | 97 (24.3%) | 429 (61.5%) | 314 (80.5%) |
| Computer search in general practice | 261 (65.4%) | 129 (18.5%) | 32 (8.2%) |
| Direct psychiatrist referral | 21 (5.3%) | 106 (15.1%) | 12 (3%) |
| Other secondary care professional (e.g. CMHT) | 16 (4%) | 32 (4.6%) | 1 (0.3%) |
| Other primary care professional (e.g. Mental Health Practitioner) | 4 (1%) | 2 (0.3%) | 28 (7.2%) |
| Unknown | 0 (0%) | 0 (0%) | 3 (0.8%) |
| Total referrals | 399 (100%) | 698 (100%) | 390 (100%) |
FIGURE 19.
Number and type of referrals.
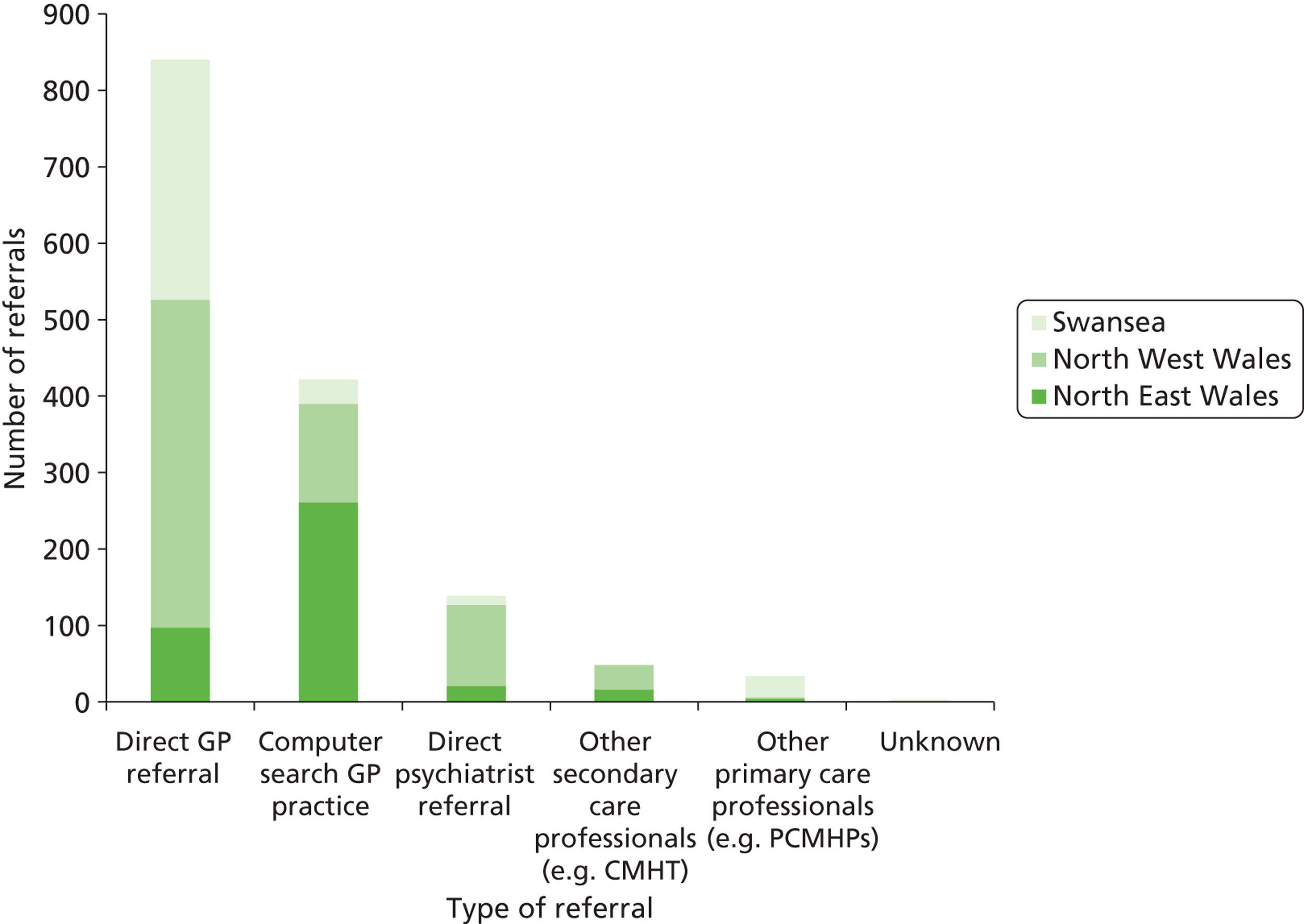
Facilitators to recruitment
Although we adopted different methods of recruitment across sites, we identified several factors as aiding recruitment. A major facilitator was a good relationship with recruiting practices and services. This was seen as a key factor in North West Wales: they had a history of working with general practices and had established good working relationships with them. Elements important in building and maintaining a good relationship included regular contact with practices, fast responses to their queries, regular updates by newsletters and personal contact, and small tokens of appreciation like cards at Christmas.
A perception that participation conferred benefits on patients was also seen as a facilitator. Many GPs were pleased to offer something extra to long-term patients and saw quick access to a psychiatrist as beneficial, and time with the researcher as an alternative or supplement to counselling, which was often difficult to access. Some GPs felt this would be an alternative to patients visiting the surgery.
Relationships were a recurrent theme. A good patient–doctor relationship was viewed as a facilitator to direct referrals, as small surgeries had higher referral rates. Good relationships between participants and researchers were also seen as important in retaining participants. Follow-ups were face to face, helping relationships to develop. Continuity also contributed to the success of relationships with researchers encouraged to follow participants from beginning to end. Also important was personal contact through reminder letters and phone calls.
Awareness raising and networking with gatekeepers like practice managers were important in gaining access to recruiters. Initial contact with Local Health Boards was also seen as a facilitator by providing advice about key contacts, monthly meetings and identifying research-active practices. Furthermore aides-memoire facilitated recruitment by providing reminders to health professionals; these included pens, note paper and trolley tokens with the FolATED logo.
Barriers to recruitment
One common theme was the competing demands on general practice time and resources. Examples of commitments that preoccupied practices included preparing for QOF and the contemporaneous vaccination campaign. Also changes in staff and surgery relocation affected recruitment in some centres.
Barriers included perceptions that research might compromise practice, including concerns about increased visits to GPs, access to the practice database, confidentiality, disruption, expense, inappropriate referrals, consequences of participation for patients, in particular those found not suitable at screening, increased workload, availability of space in surgeries and even the validity of the study.
North East Wales reported that some practices were already taking part in other studies and did not feel they could recruit to another study. Also previous demands by researchers for information had made several practices reluctant to participate, fearing our study was connected in some way.
Staffing problems also reduced recruitment. The Swansea centre lost several research staff within a short time, thus halting participant recruitment during staff recruitment and training. North East Wales had only one researcher for several months before NISCHR CRC could help. North West Wales also had to restrict computer searches and mailings to focus on direct referrals.
Inclement weather also affected recruitment and retention during the winter of 2010. Participants and researchers were often unable to attend appointments, particularly in rural areas.
Discussion
This study has identified facilitators of, and barriers to, recruitment and retention in the FolATED trial. Facilitators included the importance of building good relationships with psychiatric services, practices and participants through the interpersonal skills of researchers and continuing feedback to recruiters. Also important was the potential benefit of the intervention, and participation in the trial, to patients, as perceived both by those recruiting and the participants themselves. Thus raising awareness and networking with gatekeepers like practice managers reportedly improved recruitment.
Barriers included the high demand on practitioners’ time, disruption to the surgery, consequences for ineligible patients, and worries about confidentiality. It is therefore vital that researchers design trials that minimise impact on surgeries and provide reassurance about effects on patients and their confidentiality. It is important that, when dealing with recruiters, researchers tread carefully. If future research is to be successful they need to nurture relationships and not make undue demands. As staffing levels often posed a threat to recruitment, researchers and funders need to be realistic when designing trials. Finally the FolATED trial experienced unforeseen disruption, notably from the volcano eruption in Iceland and the inclement weather of the winter of 2010.
This study also examined the strategies and methods used in the FolATED trial for the recruitment and retention of both those recruiting into the trial and participants in the trial. Recommendations include the need for a flexible approach to such recruitment and retention. An explicit recruitment strategy is essential in any trial; however multi-centred complex interventions present extra challenges which require a flexible and often creative approach. However we suffered from the slow regulatory systems and long waits to implement much needed changes. For example we waited several months to get approval for an improved poster and information leaflet for GP surgeries.
Monthly research team meetings provided the platform for sharing problems and exploring ideas with colleagues. This also encouraged the trial centres to work as a team. Annual training sessions also assisted in exploring and sharing ideas and experiences across centres.
The resources required to recruit and run a complex intervention should not be underestimated. There is a need to acknowledge that recruiting into trials and the day-to-day running of trials is laborous, and that the recruitment phase of a trial is particularly so. Alongside this, trials that employ labour-intensive methods like interviews must be costed accordingly. The competitive nature of bidding for trial funding often results in underfunded studies risking failure of trials owing to a lack of researchers on the ground to coordinate and perform the research. In the FolATED trial assistance from NHS staff and the emerging NISCHR CRC was crucial in centres with limited resources, where even the most successful recruiting centre could have handled more participants but for the limitation of research staff to provide follow-up interviews.
List of abbreviations
- 5-HT
- 5-hydroxytryptamine
- 5-MTHF
- 5-methyltetrahydrofolate
- ADM
- antidepressant medication
- AE
- adverse event
- AHEAD
- Assessing Health Economics of Anti-Depressants trial
- AR
- adverse reaction
- AUC
- area under the curve
- BDI-II
- Beck Depression Inventory (version 2)
- BMI
- body mass index
- BNF
- British National Formulary
- bp
- base pair
- CGI
- Clinical Global Impression
- CI
- confidence interval
- CMHT
- Community Mental Health Team
- CNS
- central nervous system
- CRF
- Case Report Form
- CSF
- cerebrospinal fluid
- CTA
- Clinical Trial Authorisation
- CV
- coefficient of variation
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- European Quality of life scale – 5 Dimensions
- EQ-VAS
- EQ-5D Visual Analogue Scale
- FDR
- false discovery rate
- FolATED
- Folate Augmentation of Treatment – Evaluation for Depression
- GCP
- Good Clinical Practice
- GP
- general medical practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HDRS
- Hamilton Depression Rating Scale
- HTA
- Health Technology Assessment
- ICD-10
- International Statistical Classification of Diseases & Related Health Problems (10th Revision)
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- MADRS
- Montgomery–Åsberg Depression Rating Scale
- MCAR
- missing completely at random
- MCS
- Mental Component Score
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MHRN-Cymru
- Mental Health Research Network for Wales
- MINI
- Mini International Neuropsychiatric Interview
- MMA
- methylmalonic acid
- MRC
- Medical Research Council
- MREC
- Multicentre Research Ethics Committee
- MTHFR
- methylenetetrahydrofolate reductase
- MTR
- methyltetrahydrofolate reductase
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute of Health Research
- NISCHR
- National Institute for Social Care and Health Research (Wales)
- NISCHR CRC
- NISCHR Clinical Research Centre
- NWORTH
- North Wales Organisation for Randomised Trials in Health
- OLS
- ordinary least squares
- OR
- odds ratio
- PCS
- physical component score
- PHQ9
- Patient Health Questionnaire – 9 items
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QOF
- Quality & Outcomes Framework
- RCF
- red cell folate
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SAR
- serious adverse reaction
- SD
- standard deviation
- SF-6D
- UK Short Form Health Survey – 6 Dimensions
- SF-12
- UK 12-item Short Form Health Survey
- SNP
- single nucleotide polymorphism
- SNRI
- selective noradrenaline reuptake inhibitor
- SOP
- standard operating procedure
- SSRI
- selective serotonin reuptake inhibitor
- SUSAR
- suspected unexpected serious adverse reaction
- TCA
- tricyclic antidepressant
- TSC
- Trial Steering Committee
- WHO
- World Health Organization
- WONCA
- World Organization of National Colleges & Associations of Family Doctors