Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/07/01. The contractual start date was in February 2006. The draft report began editorial review in June 2013 and was accepted for publication in October 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Neil Marlow reports personal fees from Novartis, personal fees from Elsevier, outside the submitted work. Tim Coleman reports personal fees from Pierre Fabre Laboratories, France, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Cooper et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
The problem
Maternal smoking during pregnancy is the most important, and preventable, cause of adverse pregnancy outcomes including placental abruption, miscarriage, birth before 37 weeks’ gestation (pre-term birth) and low birthweight (LBW). 1 Pre-term birth is the principal cause of neonatal death and morbidity, with up to 50% of infants’ neurodevelopmental problems being attributable to this. 2 Similarly, LBW births are a marker of ill health and are associated with the future development of coronary heart disease, type 2 diabetes and obesity. 3
Tobacco smoking in pregnancy is a worldwide public health problem. The UK’s estimated rate of pre-natal smoking is 12%4 and rates are similar in most developed countries, including Australia (17%),5 Denmark (16%),6 the USA (11%)7 and Germany (13%). 8 Some other European countries, such as Spain9 and Poland,10 have considerably higher rates of maternal smoking in pregnancy, reaching around 30%. Although the prevalence of smoking in pregnancy appears generally to be reducing in high-income countries, in low- and middle-income countries, rates of maternal smoking in pregnancy are believed to be increasing. 11 It is predicted that in the future, higher rates of maternal smoking in low- and middle-income countries will substantially transfer the health burden from smoking during pregnancy to these nations. 12 Additionally, in high-income countries, rates of smoking in pregnancy remain highest among younger women and those who are more socially disadvantaged. 4 As the children of mothers who smoke are twice as likely to become smokers,13 smoking in pregnancy perpetuates cycles of deprivation and health inequalities across generations that permanent smoking cessation initiated in pregnancy could reduce.
Treatments for smoking cessation in pregnancy
Stopping smoking in pregnancy not only benefits maternal health but has positive impacts on infant outcomes and effective cessation interventions which are used by pregnant women reduce numbers of LBW and pre-term births. 14 Presumably, because the harms of smoking and the benefits of stopping are widely known, many smokers stop when they are planning a pregnancy or soon after conceiving; for example, in England and Wales, around 50% of smokers manage to stop smoking during at least part of their pregnancy. 4 For pregnant women who are unable to stop smoking without assistance, there are only two proven cessation interventions which could help them with this: behavioural14 and self-help15 smoking cessation support. Intensive behavioural support, which is delivered outside women’s routine antenatal care, can reduce smoking in later pregnancy14 [pooled risk ratio (RR) for reduction in smoking prevalence after behavioural support 0.94, 95% confidence interval (CI) 0.93 to 0.96], as can self-help cessation interventions15 [odds ratio (OR) for cessation following self-help intervention 1.83, 95% CI 1.23 to 2.73]. Behavioural support usually involves psychologically orientated counselling that can broadly be described as ‘cognitive–behavioural therapy’. However, for this intervention to have an effect, women must attend appointments with health professionals in addition to their routine antenatal care; however, in countries such as England, where such support has been freely available for some time, relatively few pregnant smokers have made use of this. Using the NHS Stop Smoking Service (SSS)16 and maternity statistics17 combined with national survey data,4 one can estimate that, in 2011, only 14% of English pregnant smokers set quit dates using such support and as few as 6% subsequently managed to stop smoking for at least 4 weeks. Self-help support, including books, manuals, text messaging and DVDs, involves structured interventions that may be introduced briefly to smokers by health professionals, but are primarily designed for motivated quitters to work through on their own. Self-help interventions are not likely to appeal to all smokers and do require a certain level of cognitive ability for successful use.
Outside of pregnancy there are more cessation interventions of proven efficacy available to assist smokers, including nicotine replacement therapy (NRT),18 bupropion (Zyban®, GSK)19 and varenicline (Champix®, Pfizer). 20 NRT works by substituting the nicotine inhaled in tobacco smoke, which is accompanied by many other toxins, for ‘clean’ medicinal nicotine (e.g. transdermal patches or lozenges). Using NRT after becoming abstinent permits the smoker to lessen or avoid withdrawal symptoms and, eventually, as the amount or dose of NRT reduces, these are eliminated. Bupropion is an antidepressant with an uncertain mechanism of action, but is thought to promote smoking cessation by antagonising nicotinic acetylcholine receptors. Varenicline is an alpha-4 beta-2 nicotinic acetylcholine receptor partial agonist; it binds to nicotinic receptors and is thought to act by preventing the pleasurable sensations that smokers experience after smoking, making them less likely to do so and, hence, more likely to achieve cessation. Unfortunately, neither varenicline nor bupropion is approved for use in pregnancy. Also, possibly owing to fears that either drug might cause fetal harm, there are insufficient studies in pregnancy to draw any conclusions about the efficacy of safety of either drug for use by pregnant smokers.
Nicotine is the active ingredient of NRT and its impacts in pregnancy have been much more thoroughly researched. 21 Nicotine is a known neurotoxin and may be expected to affect developing fetal nerve tissues. This may explain observed associations between behavioural problems and attention deficit disorder among smokers’ children. 22,23 However, while tobacco combustion creates and releases many potential fatal toxins in addition to nicotine, NRT delivers nicotine alone. Consequently, there is an international, expert consensus that maternal use of NRT in pregnancy should be safer for the fetus than continued smoking. 24 It should be noted that this consensus, while logical, is theoretically based and is not underpinned by research evidence. Nevertheless, this consensus has had an impact internationally on the use of NRT in pregnancy and has contributed to a relaxation in indications for NRT prescribing during pregnancy in some countries. For example, since 2003 in the UK, the British National Formulary25 (the manual used to guide prescribing in the UK NHS) has listed pregnancy as a ‘caution’ rather than a ‘contraindication’ to using NRT. Additionally, in 2005, the UK Medicines and Healthcare products Regulatory Agency (MHRA) issued guidance that specifically stated that pregnant women who had not managed to stop smoking using other means could be prescribed NRT. Many other countries take similar approaches; the authoritative website, www.treatobacco.net, hosts all nations’ smoking cessation guidelines and the vast majority of those written in English recommend cautious use of NRT for smoking cessation in pregnancy. 26
Current evidence for nicotine-replacement therapy use in pregnancy
Health policy recommendations for NRT use in pregnancy have developed in the absence of scientific evidence. In 2004, when the study described in this report [the Smoking Nicotine And Pregnancy (SNAP) trial] was commissioned, only three trials had investigated NRT for smoking cessation in pregnancy27–29 and the largest of these29 was excluded from a later meta-analysis30 and Cochrane review31 because its design made it impossible to attribute treatment effects to NRT. Pooling of data from these three studies, including the trial which was not included in later reviews, suggested that NRT used in pregnancy had borderline effectiveness for reducing smoking in later pregnancy (pooled RR 0.94, 95% CI 0.89 to 1.00). 32 In addition, one of these studies had found that slightly heavier and, therefore, potentially healthier, infants were born to women who had been randomised to NRT. 27 While the SNAP trial was running, three further trials investigating the efficacy and safety of NRT for smoking cessation in pregnancy were published. 33–35 However, a systematic review and meta-analysis that included these three more recent trials and also the two previous relevant studies27,28 found no evidence that that NRT was effective for smoking cessation in pregnancy (pooled RR for cessation after NRT 1.63, 95% CI 0.85 to 3.14). 30 This lack of evidence for the use of NRT in pregnancy comes from trials conducted in Canada, the USA, Denmark and Australia, which randomised a total of 695 women. 27,28,33–35
Unfortunately, despite the knowledge that nicotine is potentially fetotoxic, there is little evidence available to assess whether or not using NRT in pregnancy is safe. Of the five trials above that reported before the SNAP trial concluded, only three monitored maternal or infant birth outcomes27,34,35 and none collected data on infants’ outcomes after delivery. Given this paucity of empirical data, meta-analyses investigating the impact of NRT on infants’ birth outcomes have been inconclusive30 and more data are required. In addition, as nicotine could be one of the tobacco smoke constituents responsible for the cognitive and behavioural problems seen in infants born to smokers,22 studies that assess the impact of NRT used for cessation in pregnancy on early infant outcomes are also needed. It remains likely that nicotine is not solely responsible for these adverse effects; indeed, it may have no such impacts, and other toxins in tobacco smoke could be partially, or perhaps even completely responsible for them. However, this should not be assumed.
In summary, smoking in pregnancy is an extremely harmful behaviour and an increasing public health problem internationally. There are only two cessation interventions of proven efficacy for use in pregnancy and no licensed drug cessation treatments have been shown to be safe or effective in pregnancy. There is a consensus in favour of using NRT in pregnancy, but NRT remains of unproven efficacy and its impacts on infants born to mothers who use it in pregnancy require determining. Consequently, the SNAP trial, a double-blind, randomised placebo-controlled trial of NRT used for smoking cessation in pregnancy, was planned and is described within this report.
Objectives
The overall aim of the study was to investigate whether or not NRT is more effective than placebo in achieving smoking cessation for women between 12 and 24 weeks pregnant, who currently smoke five or more cigarettes per day and who smoked at least 10 cigarettes per day before pregnancy.
The specific study objectives were to compare:
-
the clinical effectiveness and cost-effectiveness for achieving biochemically validated smoking cessation of 15 mg per 16 hours transdermal nicotine patches with placebo patches in women at delivery
-
the effects of maternal NRT patch use with placebo patch use during pregnancy on (1) disability, behaviour and development and (2) respiratory symptoms in infants at 2 years of age.
Chapter 2 Methods
Trial design
This was a phase IV, multicentre, double-blind, randomised (1 : 1 allocation and stratified by site), placebo-controlled, parallel-group trial of standard-dose (15 mg per 16 hours) NRT patches. Participants were monitored from their recruitment at between 12 and 24 weeks’ gestation until the delivery of their babies and then followed up by questionnaire for a further 2 years.
Participants and recruitment
Eligibility criteria
Eligible women were aged 16–50 years, between 12 and 24 weeks’ pregnant, smoked at least 10 cigarettes per day before pregnancy and continued to smoke at least five cigarettes per day. The eligible women also had an exhaled carbon monoxide (CO) concentration of at least 8 parts per million (p.p.m.). They were excluded if they had contraindications to the use of NRT including severe cardiovascular disease, unstable angina, cardiac arrhythmias, recent cardiovascular accident or transient ischaemic attack, chronic generalised skin disorders or known sensitivity to nicotine patches, chemical or alcohol dependence, known major fetal abnormalities, or were unable to give informed consent. Women could enrol in the trial only once but could participate in other non-conflicting research projects.
Recruiting centres
Participants were recruited from seven hospital antenatal clinics in the Midlands and north-west England. Initially, these were at Nottingham University Hospitals NHS Trust City Hospital campus, Nottingham University Hospitals NHS Trust Queen’s Medical Centre (QMC) campus, Sherwood Forest Hospitals NHS Foundation Trust (King’s Mill Hospital) and University Hospital of North Staffordshire NHS Trust (City General Site). Three further sites were added later to improve recruitment rates: Mid Cheshire Hospitals NHS Foundation Trust (Leighton Hospital), East Cheshire NHS Trust (Macclesfield District General Hospital) and Derby Hospitals NHS Foundation Trust (Derby City General, later to become Royal Derby Hospital).
Research midwives
In each centre, research midwives (RMs) undertook all trial-related procedures. RMs were trained in research procedures by the trial manager with input from the chief investigator and attended monthly staff update meetings. In addition, Clare Mannion, one of the trial co-investigators and a UK expert trainer of smoking cessation professionals, provided the RMs with training, to English national standards, in delivery of behavioural support to pregnant women. 36
Recruitment and consent
We used three methods of identifying and recruiting eligible women who were interested in stopping smoking.
-
Community midwives usually ask women about smoking status at their booking appointment and then refer those who would like help to stop smoking to their local NHS SSS. In some recruiting areas, women referred to NHS SSS were asked if they would be interested in finding out about the trial and the RM contacted any who expressed interest. Those who were not interested or eligible for enrolment, but who wanted to stop smoking, were seen by the NHS SSS as per normal practice.
-
Leaflets containing brief information about the trial were sent to women before their antenatal clinic or routine ultrasonography scan appointments. Women attending these clinics were then asked to complete a screening questionnaire to identify those who were eligible and interested (see Appendix 1).
-
Some women who had seen information leaflets or posters advertising the study in hospitals contacted the RMs directly.
Potentially eligible women who expressed interest in the trial were given a participant information sheet (PIS) and, after having chance to consider this for at least 24 hours and discuss with a RM, gave their written informed consent before trial data were collected. In addition to trial participation, women were asked to give consent for researchers to have access to their and their child’s medical records, for information held by the NHS to be used to keep in touch with them and to follow their health status, and also for storage of blood samples for possible use in future research. For most trial participants, consent and baseline data collection took place in their homes.
Interventions
The only difference in interventions delivered to trial groups was in the type of transdermal patches allocated to women. In the intervention group, these were active nicotine patches (15 mg per 16 hours NRT transdermal patches), whereas women in the control group received visually identical placebo patches.
At enrolment, RMs delivered behavioural support lasting up to 1 hour. During counselling, RMs applied techniques to encourage cognitive and behavioural changes among smokers such that smoking cessation could be successfully achieved. The initial session focused on behavioural advice and tips for smokers, including preparation for quitting and how to avoid smoking lapses once a quit attempt had begun in addition to instruction and advice on how to use patches (which could be either placebo or nicotine). During the session, participants were required to set a quit date within 2 weeks from which follow-up was timed. A manual used by RMs to guide the support sessions (‘The SNAP trial’s guide to stopping smoking during pregnancy’: see Appendix 2) was written by Clare Mannion and included some techniques from the US ‘Smoking Cessation and Reduction in Pregnancy Treatment’ trials37 that were believed to be relevant to UK smokers. As is consistent with good smoking cessation practice, this manual, which contained tips and suggestions for becoming smoke-free, was left with women so that they could refer to it after their support session. Subsequently, participants were randomised to equal-sized groups receiving either a 4-week supply of 15 mg per 16 hours NRT transdermal patches or visually identical placebos (United Pharmaceuticals, Amman, Jordan), which women were instructed to start on their quit dates. One month after quitting, those not smoking, validated by CO measurement of < 8 p.p.m.,38 were issued with another 4-week patch supply if they wanted it. In addition to behavioural support delivered at enrolment, RMs provided three further behavioural support sessions to all participants. Telephone behavioural support was delivered on participants’ quit dates, at 3 days afterwards and at 1 month afterwards; those women who collected a second month’s supply of NRT also received face-to-face support from the RM at the time this was delivered to them. These sessions involved reinforcement of earlier behavioural sessions, with an added focus on ways of avoiding relapse now that quit attempts had begun.
Provision of additional behavioural support and nicotine-replacement therapy to trial participants and availability of nicotine-replacement therapy to non-participants
Prior to starting the trial, we visited local NHS SSSs in the recruiting areas and discussed their service provision, as it was intended that these would provide behavioural support to women enrolled onto the trial. Primary care trusts (PCTs) in all of the trial’s recruiting areas had NHS SSSs for pregnant women, but several PCTs had recently undergone local reorganisations and there was considerable variation in the delivery of cessation services. Some services were already issuing NRT to pregnant women, despite the absence of evidence for its effectiveness. We hoped to get agreement from PCTs that, for the duration of the trial, NRT would be issued only to pregnant women within the trial, thereby ensuring that local availability did not jeopardise recruitment and/or retention of participants or interpretation of trial findings. The outcome of these visits meant that, in most trial areas (all except Derby), women’s contact details were shared with their local NHS SSS, which agreed to contact participants and offer them additional behavioural support. They also agreed that they would not normally offer participants any non-trial NRT products. Women were encouraged to ask for further behavioural support as necessary, and RMs or NHS SSS staff, guided by the manual, delivered any additional support that participants required. In Derby, where participants’ contact details were not shared with the NHS SSS, RMs provided additional support. The provision of additional behavioural support to participants and the availability of NRT to participants and non-participants who contacted the NHS SSS are shown in Table 1, which illustrates the context in which trial recruitment occurred.
| Trial centre | PCTs hosting NHS SSS within the area of each trial centre | Provision of additional behavioural support | NRT availability within PCT | |
|---|---|---|---|---|
| Trial participants | Non-participants | |||
| Nottingham University Hospitals NHS Trust (City Hospital and QMC campuses) | Nottingham City PCT (Nottingham City New Leaf) | Nottingham City New Leaf (Stop Smoking) Service | Not offered or prescribed NRT; if any enquired about NRT, referred back to a trial researcher for further discussion | NRT still offered as judged appropriate to non-trial pregnant women |
| Nottinghamshire County PCT (Nottinghamshire County New Leaf) | Nottinghamshire County New Leaf (Stop Smoking) Service | NRT not prescribed to any pregnant women through the service for the duration of the trial | NRT not prescribed to any pregnant women through the service for the duration of the trial | |
| Derbyshire County PCT (few participants only from the Nottingham University Hospitals NHS Trust centres) | By RMs | No agreements made; NRT available via local NHS SSS | No changes to NRT provision; available via local NHS SSS | |
| Sherwood Forest Hospitals NHS Foundation Trust (King’s Mill Hospital) | Nottinghamshire County PCT (Nottinghamshire County New Leaf) | Nottinghamshire County New Leaf (Stop Smoking) Service | NRT not prescribed to any pregnant women through the service for the duration of the trial | NRT not prescribed to any pregnant women through the service for the duration of the trial |
| University Hospital of North Staffordshire NHS Trust (City General site) | Stoke PCT (North Staffordshire NHS SSS) | RMs until 1 month, then passed to North Staffordshire NHS SSS for further support if required | No NRT prescribed to women enrolled onto the trial | NRT prescribed only to pregnant women not eligible or not interested in participating in the trial |
| Mid Cheshire Hospitals NHS Foundation Trust (Leighton Hospital) | Central and Eastern Cheshire PCT (Central and Eastern Cheshire NHS SSS) | Central and Eastern Cheshire NHS SSS | All eligible women informed and referred to trial. NRT not offered or prescribed to any pregnant women enrolled in the trial | NRT initially not prescribed to any pregnant women; later changed so available to women not eligible or interested in participating in the trial |
| East Cheshire NHS Trust (Macclesfield District General Hospital) | Central and Eastern Cheshire PCT (Central and Eastern Cheshire NHS SSS) | Central and Eastern Cheshire NHS SSS | All eligible women informed and referred to trial. NRT not offered or prescribed to any pregnant women enrolled in the trial | NRT initially not available to any pregnant women; later changed so available to women not eligible or interested in participating in the trial |
| Derby Hospitals NHS Foundation Trust (Derby City General Hospital) | Derby City and Derbyshire County PCTs (Fresh Start) | By RMs | No agreements made; trial participants not referred to NHS SSS | No changes to NRT provision; available via local NHS SSS |
Randomisation and blinding
Eligibility criteria were entered into a secure online database before internet-based randomisation that was stratified by recruiting site and used a computer-generated, pseudorandom code using random permuted blocks of randomly varying size, with a 1 : 1 allocation ratio, which was created by the Nottingham Clinical Trials Unit (NCTU) and held on a secure server in accordance with their standard operating procedures. Following randomisation, the database issued participants with a unique identifier and allocated a trial treatment-pack number to them. Identically packaged treatments, previously prepared by one central pharmacy (QMC pharmacy, Nottingham University Hospitals NHS Trust), were dispensed by local pharmacies. All pharmacists, research staff and trial participants were blinded to treatment allocations.
In addition, during the follow-up period in the 2 years after ascertainment of the primary outcome at delivery, participants and anyone involved in following them up and entering data remained blind to the treatment allocation.
Data collection to delivery
Baseline
At baseline, RMs collected women’s demographic and contact details: ‘Heaviness of Smoking Index’,39 a measure of nicotine addiction recorded on a scale of 0 (lower) to 6 (higher nicotine addiction); number of daily cigarettes smoked before pregnancy; partner’s smoking status; gestation; ethnicity; age completed full-time education; parity; use of NRT in current pregnancy; height and weight. Women were also asked to provide an exhaled CO measurement, blood and saliva samples for cotinine estimation and a blood sample for future deoxyribonucleic acid (DNA) extraction (any studies using the DNA samples would require further funding and relevant ethical approvals).
One month
One month after the quit date, RMs telephoned participants and asked for details about their smoking status. This included whether or not they had smoked since their agreed quit date and, if so, how often this had occured and whether or not they had smoked in the previous 24 hours. RMs also asked about the number of times they had received additional behavioural support and whether this had been face to face, by telephone or by mobile telephone text message; the number of trial patches they had used (i.e. adherence); and whether or not they had obtained and used any additional NRT outside of the trial. Those who reported not smoking were visited for CO validation and a saliva sample (for cotinine measurement) was obtained from any women who were still using trial patches and not smoking. Non-contactable women were sent a postal questionnaire.
Delivery
When participants were admitted to hospital in established labour prior to childbirth, or as soon as possible afterwards, as at the 1-month contact, RMs or delivery suite staff ascertained smoking status, use of trial and ‘non-trial’ NRT, including reported numbers of patches used and additional behavioural support received. Exhaled CO measurements and saliva cotinine samples were obtained from women who reported not smoking for at least 24 hours before delivery. RMs telephoned those missed while in hospital, and any who reported abstinence were visited at home for biochemical validation within a maximum of 4 weeks after delivery. Maternal and infant birth outcomes were obtained from medical records. Maternal outcomes included hypertension (> 140/90 mmHg) measured on two or more occasions during pregnancy, miscarriage or stillbirth, labour onset (spontaneous, induced or no labour), mode of delivery (spontaneous vaginal, assisted vaginal or caesarean section) and antenatal or postnatal hospital admissions. Infant data and outcomes collected at delivery or after discharge included date of birth, name, hospital and NHS numbers, sex, birthweight, gestational age at birth, number of births (with number and birth order if multiple birth), live birth or stillbirth, arterial cord-blood pH (either ≥ 7 or < 7), Apgar score (either ≥ 7 or < 7), intraventricular haemorrhage, neonatal convulsions, admissions to neonatal intensive care units (NICUs) and any congenital abnormalities.
Adverse event monitoring
During each contact with participants, RMs enquired about adverse events (AEs) or symptoms the participant had experienced. RMs also obtained this information from monthly examination of medical records. They then summarised the descriptions in the case report forms and on the study database. Descriptions were used to code the AEs according to standard terms in the Medical Dictionary for Regulatory Activities (MedDRA®) version 13.1 [www.meddra.org/; MedDRA is the international medical terminology developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA trademark is owned by International Federation of Pharmaceutical Manufacturers and Associations on behalf of ICH]. The incidence of events was analysed according to the MedDRA System Organ Class categorisation and preferred terms. Information about deaths after delivery was obtained from the NHS Health and Social Care Information Centre’s Data Linkage Service (previously Office for National Statistics) with whom all trial participants and their infants had been flagged. After starting the trial, it was realised that many relatively common pregnancy-related events were being reported as serious adverse events (SAEs), including pregnancy-related hospital admissions, premature birth and LBW and, on clinical review, none of these had been considered to be related to the study drug. The Trial Steering Committee (TSC) and the sponsor, therefore, advised that it would be appropriate to amend the protocol so that only maternal and fetal/infant deaths and hospital admissions unrelated to the underlying pregnancy would be reported as SAEs, and ethical approval was received for this. We continued to collect and monitor these along with other AEs, and the Data Monitoring Committee (DMC) reviewed data showing the distribution of all AEs and SAEs within trial groups at their meetings.
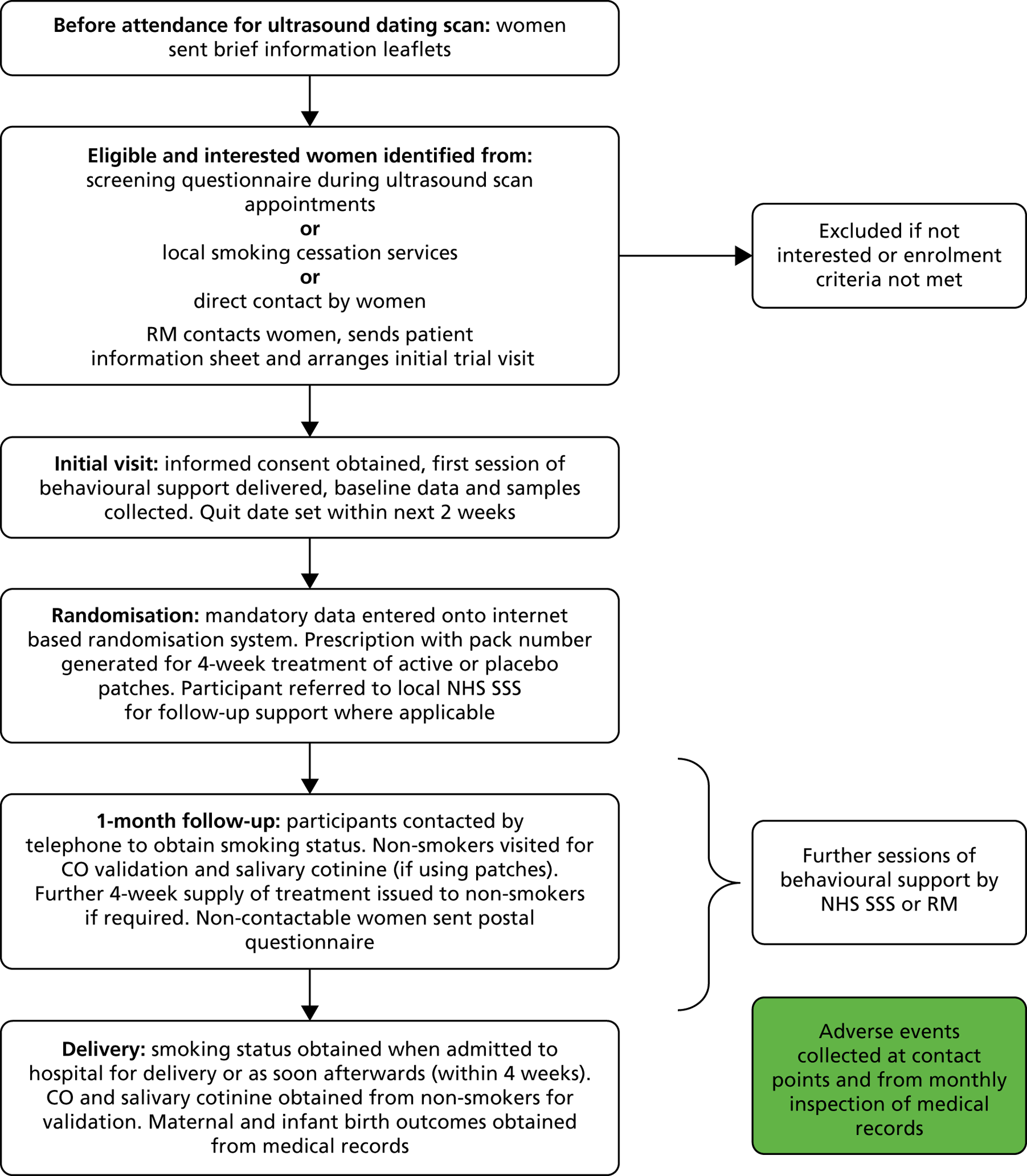
Figure 1 is a flow chart showing the data collection process from recruitment until delivery.
FIGURE 1.
Trial flow from recruitment to delivery.
Data collection and follow-up after delivery
After primary outcome data were collected, participants were followed up by postal questionnaire at 6 months, 1 year and 2 years after delivery of their infants. Questionnaires were not sent after maternal or infant deaths, if no birth details were available, if the participant had withdrawn consent for follow-up, or if no contact details could be obtained for the participant and if they were not registered with a general practitioner (GP). The NHS Health and Social Care Information Centre sent the trial office a report including information on any participant and infant deaths every 3 months. However, because of a delay in receiving the death report, a questionnaire was sent inadvertently to a participant whose infant had died; we subsequently sent the NHS Health and Social Care Information Centre a list of participants and infants who were due to be sent questionnaires for them to check every month. For non-respondents, or if questionnaires had been returned undelivered, the trial office contacted alternative family members using contact details that the participant had provided when they enrolled onto the trial and, if necessary, the participant’s GP or PCT were contacted to obtain their current contact details. During the follow-up period in the 2 years after ascertainment of the primary outcome at delivery, all those involved in following-up participants and in entering data remained blind to the treatment allocation.
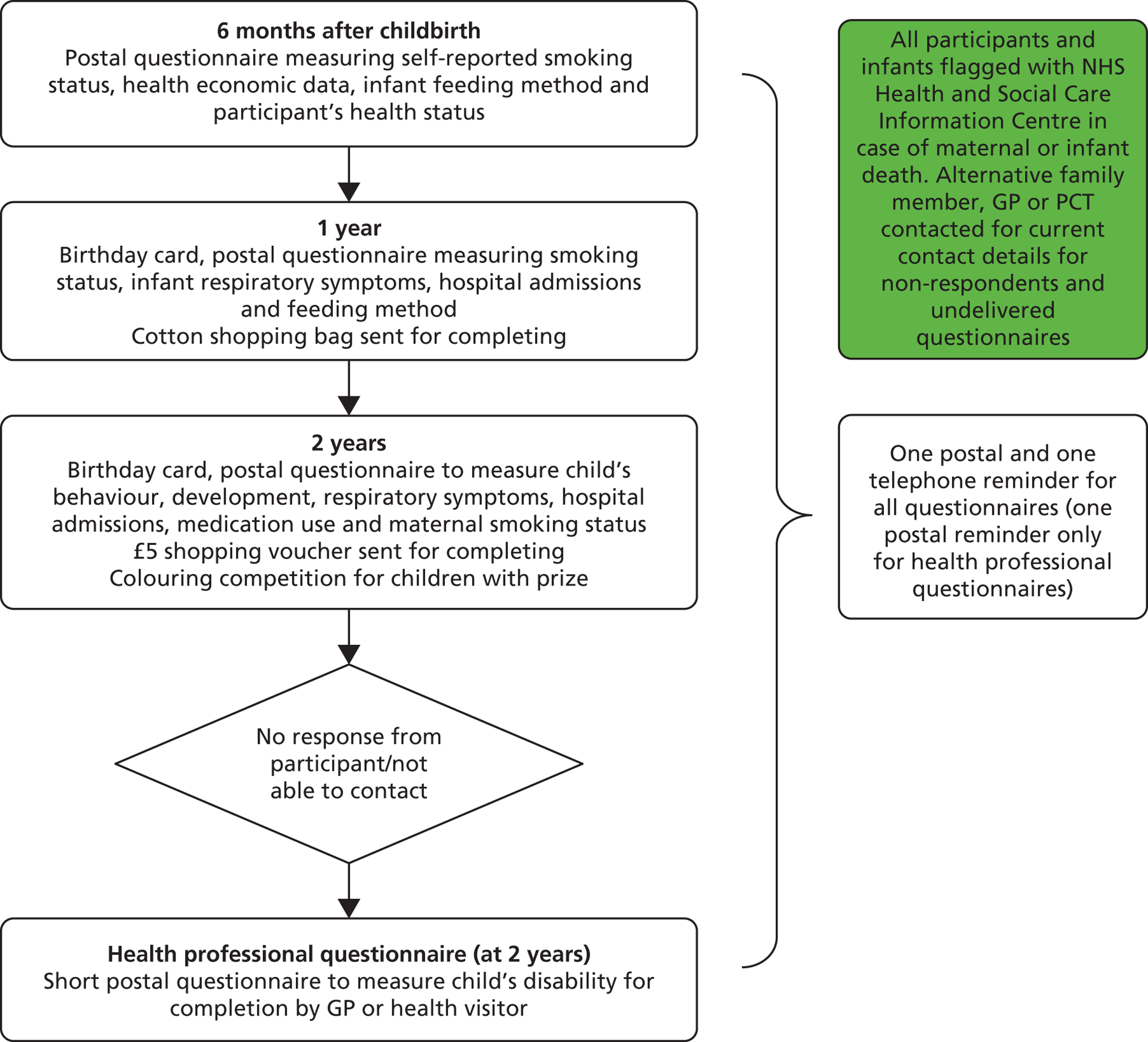
A flow chart outlining the follow-up process from delivery until the infants’ second birthdays is shown in Figure 2.
FIGURE 2.
Trial follow-up from delivery to infants’ second birthdays.
The evidence base for questionnaire design was taken into account when composing instruments40 and the following evidence-based methods to improve postal returns of questionnaires were used to maximise response rates. 41 At each follow-up point, participants were sent a postal questionnaire followed by one postal reminder after 2 weeks. After a further 2 weeks, participants were telephoned and asked to complete the questionnaire over the telephone with an appointment made to call them back when necessary. To maintain contact between researchers and participants, the trial office sent greetings cards following childbirth, at Christmas and on the child’s first and second birthdays and participants were sent cards reminding them to inform the team of any address changes. At 1 year, a cotton shopping bag was sent to participants on completion of the questionnaire; at 2 years, participants were given a £5 shopping voucher for questionnaire completion and there was a colouring competition that children of respondents could enter. The colouring competition had a £50 shopping voucher prize, with a winner chosen three times per year.
Six months after childbirth
The following information was collected from participants 6 months after delivery: current smoking status, smoking status since childbirth, maternal use of NRT and NHS SSSs since childbirth, length of any maternal hospital inpatient stay after delivery lasting > 24 hours, length of any infant inpatient stay in special care after birth, numbers of additional infant hospital admissions for respiratory illness or other causes, infant feeding method and a health status measure – the European Quality of Life-5 Dimensions (EQ-5D). 42
One year after childbirth
A shorter questionnaire was sent at 1 year, primarily to maintain contact with the participant, but also to collect the following information: current smoking status, smoking status since birth of infant, infant’s respiratory symptoms, infant hospital admissions for respiratory illness and other causes, and infant feeding method.
Two years after childbirth
At 2 years, a questionnaire sent to participants, the 2-year ‘participant questionnaire’ (PQ2) asked about maternal smoking behaviour and infant development. The PQ2 used items from the Ages and Stages Questionnaire®, Third Edition (ASQ-3™),43,44 which has been developed for assessing child development at 2 years and is valid for use from 23 months until 25 months and 15 days. It was designed to be completed by parents and to distinguish between children with suspected developmental delay and those for whom development is within the normal range. In a US population, the ASQ-3 has been reported to have a sensitivity of 92.2% and a specificity of 71.9% for detecting developmental delay at 24-months. 45 With permission from the authors and publishers, the wording of some questions was slightly adapted for our UK population. Items 1–30 on the PQ2 consisted of all 30 ASQ-3 items on child development covering five domains: communication, gross motor, fine motor, problem solving and personal–social development. Seven additional PQ2 items (i.e. PQ2 items 31–36 and 44), also taken from the ASQ-3, were mixed ‘yes’ or ‘no’/free text questions investigating both general and specific parental concerns relating to infant health and development. The PQ2 also contained items that were not from the ASQ-3 asking about infant hospital admissions, parental reports of infants’ respiratory symptoms and any medication taken for these.
Health professional questionnaire
If, at 2 years, participants did not respond to the PQ2 questionnaire, the health professional questionnaire (HPQ) was posted to their GPs. This shorter questionnaire was designed to be easily completed using medical or health visitors’ records and health professionals completing HPQs required little knowledge of the infants. If GPs could not complete HPQs, they were asked to forward these to health visitors. The HPQ contained items that corresponded to those on the PQ2 and were also intended to measure children’s disability and general health in a manner consistent with the ASQ-3. 46–48 This included open-response questions that corresponded to ‘non-domain’ ASQ-3 items included on the PQ2.
For any participants for whom we received both the completed PQ2 and HPQ, only responses from PQ2 were used in analyses.
The system we used to map the question responses to outcomes is outlined in the Derivation of composite ‘impairment’ outcome for infants section and in the statistical analysis plan (SAP) (this can be accessed at http://eprints.nottingham.ac.uk/3283/).
Outcome measures and definitions
Primary outcome to delivery
The primary outcome was self-reported, prolonged and total abstinence from smoking between the quit date and delivery, validated by exhaled CO and salivary cotinine (COT) at childbirth. Occasional minor lapses (no more than five cigarettes in total between the quit date and delivery) were not counted as a return to smoking; this is consistent with standard criteria for assessing outcome in cessation studies. 49 CO readings of ≤ 8 p.p.m. and COT of < 10 ng/ml indicated not smoking. 38 The method used for deriving the primary outcome from responses at 1 month and delivery is detailed in Box 1.
For a positive response (i.e. abstinent from smoking), the following were required:
at 1 month: ‘smoked since quit date’ = ‘no’ or ‘missing’ or ‘how often have you smoked’ = ‘five times or less’ or ‘at least weekly but less than daily’ or ‘missing’ (i.e. any response other than ‘on most days or frequently’)
and
at delivery: ‘smoked in last 24 hours’ = ‘no’ and ‘smoked since quit date’ = ‘no’ and CO result is between 0 and 8 and/or COTa,b < 10 ng/ml or ‘how often have you smoked’ = ‘five times or less’ and CO result is between 0 and 8 and/or COT < 10 ng/ml.
a Some participants will only have CO measurements and, for these women, readings in the stated reference range are defined as a positive primary outcome (even without COT). Most trial participants will have both CO and COT measurements and, for these women, BOTH readings must fall within defined ranges to count as having a positive outcome.
b At the outset of the trial, CO only was used to validate abstinence from smoking at delivery, but at DMC/TSC request this was changed and COT was added. Consequently, for most participants, both CO and COT were available at delivery. Therefore, either CO or COT could be used individually for validation, but if both were available then they both needed to indicate abstinence for a positive outcome.
Secondary outcomes
-
Smoking outcomes monitored until delivery
-
self-reported, prolonged abstinence from smoking between the quit date and 1 month
-
self-reported, prolonged abstinence from smoking between the quit date and 1 month with biochemical validation (exhaled CO) (this outcome was not listed in the trial protocol).
-
self-reported, prolonged abstinence from smoking between the quit date and delivery
-
self-reported, prolonged abstinence from smoking between the quit date and delivery, with biochemical validation of this at both 1-month follow-up and delivery
-
self-reported smoking cessation for the previous 24-hour period at delivery validated by exhaled CO and saliva cotinine estimation.
-
-
Smoking outcomes monitored after delivery
-
self-reported, prolonged abstinence from smoking between the quit date and 6 months after delivery
-
self-reported smoking cessation for the previous 7-day period at 6 months after delivery.
-
-
Birth and maternal outcomes
-
miscarriage (non-live birth prior to 24 weeks’ gestation) and stillbirth (non-live birth at 24 weeks’ gestation or later)
-
neonatal death (i.e. from live birth to 28 days)
-
post-neonatal death (29 days to 2 years)
-
individualised birthweight z-score (i.e. birthweight adjust for gestational age, maternal height, maternal weight at booking and ethnic group)
-
unadjusted birthweight and birthweight as z-score
-
Apgar score
-
cord blood pH
-
gestational age at birth
-
intraventricular haemorrhage
-
neonatal enterocolitis
-
neonatal convulsions
-
congenital abnormality
-
NICU admission
-
infant ventilated > 24 hours
-
elective termination
-
elective termination undertaken for fetal morbidity judged incompatible with fetal/infant survival
-
maternal mortality
-
mode of delivery
-
hypertension in pregnancy (> 140/90 mmHg at least twice).
-
Outcomes 2 years after delivery
-
Infant impairment
-
Defined as presence of disability and/or behaviour and development problem(s) and categorised as:
-
survival with no impairment: two of the outcomes in the protocol at 2 years were (1) behaviour and development and (2) disability. These outcomes were combined for analysis and reporting purposes and the mapping of protocol outcomes on to those listed above is fully described in the next section (see Derivation of composite ‘impairment’ outcome for infants) and the SAP (http://eprints.nottingham.ac.uk/3283/)
-
survival with definite impairment (this outcome was not listed in the trial protocol)
-
survival with suspected impairment (this outcome was not listed in the trial protocol).
-
-
Survival with ‘no impairment’ was the primary outcome at 2 years.
-
-
Infant respiratory symptoms
-
Smoking outcomes
-
self-reported, prolonged abstinence from smoking between quit date and 2 years after delivery
-
self-reported smoking cessation for previous 7-day period at 2 years after delivery.
-
Derivation of composite ‘impairment’ outcome for infants
Overview
Full details of how these outcomes were derived from questionnaire responses can be found in the SAP (http://eprints.nottingham.ac.uk/3283/), but a summary follows. This process was discussed with the TSC and the independent TSC statistician approved the SAP before analyses began.
Rationale
At the outset of the trial, we proposed two discrete infant outcomes at 2 years and these were (1) behaviour and development and (2) disability. However, there was no expectation that infants born within the trial would have particularly high rates of disability or developmental problems; therefore, when preparing the SAP for the follow-up analyses, the decision was taken to amalgamate data on both outcomes into one composite outcome. The rationale was that this would permit the trial to demonstrate with greater confidence whether or not NRT is safe for use in pregnancy, as judged in terms of infant development at 2 years.
Impairments were categorised in a mutually exclusive way so that all infants for whom questionnaires had been returned were allocated to just one of the following categories: ‘survival with no impairment’, ‘definite developmental impairment’ or ‘suspected developmental impairment’. This categorisation was based on responses to selected PQ2 and HPQ items, including the ASQ-3 domains. The scoring of the domains is explained in the following section [Scoring of Ages and Stages Questionnaire (2-year participant questionnaire) domain scores], followed by an explanation of how these domain scores and other PQ2/HPQ responses were collated to form outcomes. In some cases, one or more members of the research team inspected hard copies of questionnaires to allocate outcomes after making judgements about free-text responses and, in all such cases, assessors were blind to participants’ treatment allocations.
Scoring of Ages and Stages Questionnaire (2-year participant questionnaire) domain scores
Ages and Stages Questionnaire items from the PQ2 that are components of ASQ-3 domains were scored and these individual item scores were summed to give overall ‘domain’ scores, as described in the ASQ-3 User Guide. 45 Overall ‘domain’ scores were then compared with standard thresholds/cut-off points to determine whether domain scores should be categorised as ‘normal’, ‘abnormal’ or ‘borderline’. In clinical practice, any infants with ‘abnormal’ scores for any ASQ-3 domain would be considered to have ‘failed’ the ASQ-3 and would be recommended to undergo a more detailed assessment for development delay and those with borderline scores would be closely monitored.
Primary outcome: survival with no impairment
We classified infants as having ‘survived with no impairment’ if scores were normal for all ASQ-3 domains included on the PQ2 AND no problems were reported in PQ2 items 31–35 (i.e. free-text response questions also taken from the ASQ-3). If the PQ2 was not completed but a HPQ had been returned, this was used instead and ‘survival with no impairment’ was considered to have occurred when no HPQ responses indicated potential developmental problems.
Definite and suspected impairment
-
‘Definite’ developmental impairment: we classified infants as having developmental impairment if scores were at or below the cut-point in any ASQ-3 domain(s) or, if no participant questionnaire had been completed, if the HPQ indicated severe problems for any of questions 1–4 and/or severe disability was indicated by the response to question 9 and/or severe development delay was indicated in response to question 10.
-
‘Suspected’ developmental impairment: we classified infants as having suspected developmental impairment if all ASQ-3 scores were above the cut-point, but one or more domains scores fell within the borderline range and/or this classification was used if responses to either the PQ2 or HPQ reported concerns about developmental impairment that were judged to represent potential impairment. ‘Yes’ responses to PQ2 items questions 31–37 or HPQ questions 1–6 were examined by the research team; if these were judged to potentially reflect valid developmental impairments, infants were placed in the ‘suspected impairment’ category and/or any response stating that a child had mild or moderate disability on HPQ question 9 and/or mild or moderate development delay on HPQ question 10 was classed as suspected developmental impairment.
It should be noted that HPQ/PQ2 items dealing with feeding and behaviour problems were not used in derivation of these early child outcomes; these kinds of problems are frequently reported and have a variety of causes. A priori, we decided not to consider reports of these problems as indicative of impairment. In addition, as questions 1–4 were yes/no responses with free text, the severity of problems could be difficult to establish. Therefore, we used caution and if there were doubts about the severity of problems we classified them as ‘suspected’ impairment.
Derivation of infant respiratory problems
Infants were judged to have a respiratory problem if, at 2 years, any of questions 38–42 of the PQ2 and/or question 7 of the HPQ indicated this. On the PQ2, these questions included hospital admissions for respiratory problems, problems with chest or breathing (yes/no, free text), wheeze or whistling in chest (yes/no, frequency), doctor diagnosed asthma (yes/no), asthma medications taken (yes/no, inhaler description free text). The HPQ asked whether or not the child has problems with their chest or breathing (yes/no, free text).
Maternal smoking outcomes after delivery
We assessed these in a manner consistent with Russell Criteria49 and with smoking outcomes reported at delivery. The derivation of smoking outcomes is described below:
-
Positive outcome for ‘Self-reported, prolonged abstinence from smoking between quit date and 2 years after delivery’: the participant must have met the criteria for prolonged abstinence at delivery (i.e. positive primary outcome), plus one of the following responses
-
‘smoked since 2-year-old was born’ = ‘No’
-
or
-
‘how often have you smoked’ = ‘five times or less’
-
If any participant questionnaires were completed at 6 and/or 12 months, these should all have had the same responses as above for a positive outcome.
-
-
Smoked in last week (self-reported smoking cessation for previous 7-day period at 2 years after delivery):
-
‘smoked since two year old was born’ = ‘No’
-
or ‘smoked in last week’ = ‘No’
-
-
Smoked in last 2 years (self-reported prolonged abstinence from smoking between delivery and 2 years) (this outcome was not listed in the trial protocol)
-
‘smoked since two year old was born’ = ‘No’
-
or
-
‘how often have you smoked’ = ‘5 times or less’
-
If any participant questionnaires had been completed at 6 and/or 12 months, these should all have had the same responses as above for a positive outcome.
-
Outcomes collected on 6-month and 1-year questionnaires
Smoking status and respiratory outcome items were included on 6-month and 1-year questionnaires. These were not listed in the study protocol and only smoking outcomes are reported later. In addition, at 6 months, the EQ-5D questionnaire items were also used and questionnaire items asked about length of stay in hospital and/or special care unit after delivery, infant hospital admissions and medications taken; these data were intended for the Health economics analysis (see Chapter 4).
The 6-month and 1-year questionnaires included questions about breast and bottle-feeding and, again, these items were not included in the original study protocol and data are not presented in this report.
Sample size
We planned to recruit 525 women into each arm of the study. A trial with 500 women in each arm would detect an absolute difference of 9% in smoking cessation rates between the two groups immediately before childbirth, with a two-sided significance level of 5% and a power of 93%. It was anticipated that up to 5% of women would be lost to follow-up, and the sample size (of 500) was inflated by a factor of 1.05 to allow for this. This size of study allowed smaller treatment effects to be detected with lower power. For example, there would be 80% power to detect an absolute difference in cessation rates of 7%.
A Cochrane review showed that approximately 10% of women who are still smoking at the time of their first antenatal visit stop smoking with usual care, and a further 6–7% will stop as a result of a formal smoking cessation programme using intensive behavioural counselling. 32 Therefore, in our control group (placebo plus intensive behavioural counselling), a smoking cessation rate of around 16% was anticipated. The most recent Cochrane review of the efficacy of NRT outside of pregnancy had reported a treatment effect (OR) for transdermal patches of 1.74 (95% CI 1.57 to 1.93). 50 Consequently, if NRT were similarly effective in pregnancy, one could expect a smoking cessation rate of approximately 25% in the treatment group (NRT plus intensive behavioural counselling).
Tables in the SAP show the consequences to study power if the treatment effects in the trial were smaller or overall quit rate was lower than expected (http://eprints.nottingham.ac.uk/3283/).
Statistical methods
The SAP for the primary analysis was finalised before any analyses started. For analyses to be conducted on follow-up data, the analysis plan was added to and finalised during the follow-up period, before any follow-up analyses commenced. (The SAP containing both primary and follow-up analyses can be found at http://eprints.nottingham.ac.uk/3283/). Data cleaning and preparatory work were performed blind to study arm allocation and all analyses of outcomes recorded at delivery were performed blind to study arm allocation, with treatment codes revealed after these were completed. However, it was not possible to perform all follow-up analyses blind. Statistical analyses were performed using SAS software version 9.1.3 (SAS Institute Inc., Cary, NC, USA) and Stata/SE version 11.2 (StataCorp LP, College Station, TX, USA).
Analysis to primary outcome point (delivery)
Analysis was on an intention-to-treat (ITT) basis and participants who, for any reason, had missing outcome data were assumed to be smoking. The proportion of women who reported prolonged abstinence from smoking immediately before childbirth was compared between treatment groups by logistic regression and adjusted for recruitment centre as a stratification variable. Statistical significance was assessed using the likelihood ratio test. The primary analysis adjusted for no further variables as multivariate analysis results, and therefore overall conclusions, can be sensitive to decisions concerning which variables to adjust for and how these are specified. Nevertheless, we planned a secondary analysis adjusting for baseline COT (continuous variable), maternal education (in years) and partners’ smoking status (binary variable), as adjusting for potentially important prognostic factors can improve the precision of treatment effect estimates. 51 Other smoking cessation outcomes were analysed similarly.
Fetal and maternal birth outcomes were compared on an ITT basis. For binary outcomes, ORs were obtained using logistic regression adjusted for recruitment centre and also using the likelihood ratio test (with Fisher’s exact test used and stratification by centre ignored when numbers of events were small). For continuous outcomes, we compared means between groups with adjustment for recruitment centre using multiple linear regression.
For fetal outcomes, primary analysis was of singleton births only to allow for the fact that observations will be non-independent and that non-singleton births are likely to have different birth outcomes. However, we undertook a sensitivity analysis including multiple births, with clustering of outcomes accounted for using an approach previously published. This adapts the methodology previously created for use with cluster randomised controlled trials (RCTs), assuming that each woman is regarded as the ‘cluster’ and her number of offspring the cluster size. 52
In all analyses, a p-value of < 0.05 was taken to indicate statistical significance and 95% CIs were calculated.
Analysis at the 2-year follow-up point
The ASQ-3 does not require adjustment of an infant’s age to allow for prematurity once he or she reaches 24 months of age; therefore, as the questionnaire was sent shortly before the child’s second birthday, no adjustment to infant ages was made in analyses.
Maternal characteristics at baseline and delivery and infant birth outcomes at delivery were compared between those participants and infants who did and did not have outcomes ascertained at 2 years after delivery. We also compared maternal and infant characteristics according to whether follow-up at 2 years was by PQ2, HPQ or neither.
Analysis of early childhood outcomes was on an ITT basis with participants analysed in the treatment groups to which they were randomised. Participants with no live birth (i.e. miscarriage, stillbirth or elective termination) or those where the pregnancy outcome was unknown were excluded from the ITT analysis, but postnatal infant deaths were included in the denominator for developmental outcomes. The primary analysis was restricted to singleton births to allow for the fact that observations will be non-independent and that multiple births may have very different outcomes. For the primary outcome, survival with no impairment, a complete case analysis was compared with an analysis using multiple imputation to deal with missing values. Multiple imputation was carried out using the ‘mi’ commands in Stata and in our multiple imputation we included all of the complete baseline and the treatment code, and used 20 imputations. Using this approach, multiple imputation was also used for the other developmental outcomes: suspected and definite developmental impairment. The infant impairment and respiratory outcomes at 2 years were analysed as binary indicators of presence or absence of the outcome. The ORs for the effect of treatment group were obtained by logistic regression adjusting for centre as the stratification factor. In a subsidiary analysis, multiple births were included and clustering accounted for by the same method as in analysis at delivery. We also conducted sensitivity analyses comparing the results of analyses based on parental responses only and those based on a combination of parental and health professional responses.
Smoking outcomes were also analysed on an ITT basis, with all women analysed in the treatment groups to which they were randomised and all non-respondents assumed to be smoking. ORs for the effect of treatment group on smoking cessation outcomes were also obtained by logistic regression. As at delivery, the primary analysis adjusted only for centre, but we carried out sensitivity analysis that also adjusted for baseline COT, partner’s smoking status and age at finishing education.
We tested the assumption that those missing at follow-up were smokers by exploring alternative associations for the relationship between smoking status and ‘missingness’. 53 In this analysis, we defined the OR for the association between quitting and being missing as the informatively missing odds ratio (IMOR) and we looked at the effect on the size of the treatment effect on smoking abstinence outcome by varying the size of this OR between 0 and 1. In the main analysis, the assumption that those missing at follow-up are smokers is equivalent to assuming that IMOR equals 0 (i.e. that all those who are missing are smokers). We altered this OR up to IMOR equals 1, which is equivalent to assuming that there is no association between being missing data and smoking status. We carried out this analysis using the mean score method to estimate the treatment effect under the pattern mixture model, logit[E(y|r,my)] = α1 + β1r + myδ, where my is an indicator of whether the outcome is missing or otherwise, r is the treatment effect, and exponential (δ) [the OR between outcome y and my (IMOR)] is varied in the range 0–1. 54 α1 and β1 are estimated using the subgroup with outcome data, missing values of y (the outcome) are replaced by invlogit(α1 + β1r + δ), the mean of this new y variable is calculated for the intervention and control arms (say a1 and a0), and ORs calculated from these mean values accordingly [a1/(1 – a1)]/[a0/1 – a0)].
Secondary analysis
A priori, we planned to investigate whether or not there was any relationship between self-reported nicotine patch use in pregnancy and the presence or absence of developmental impairment in infants at 2 years. For this, we conducted an exploratory regression analysis with absence of impairment at 2 years as the dependent variable and the number of nicotine patches women reported having used when asked at delivery as an explanatory variable. ‘Suspected’ and ‘definite’ impairment categories were combined into one group representing infants who did not have impairment-free survival at 2 years. We investigated the possibility that baseline maternal characteristics may have a confounding effect on any relationship and adjusted for confounders as appropriate. For those in the placebo group, we set adherence with nicotine patches as zero. Additionally, if data on adherence were not reported at delivery, we imputed 0 days use of nicotine patches.
Trial management and conduct
The trial was co-ordinated from a central trial office located within the University of Nottingham, with the day-to-day running supervised and organised by a local trial management group and trial manager. The trial was sponsored by the University of Nottingham and conducted in accordance with good clinical practice (GCP) guidelines. All research staff received GCP and research governance training in addition to the study-specific training. Monthly research staff meetings were held in Nottingham, which all RMs were encouraged to attend; the aim was to keep them motivated, updated and trained, as well as to give them the opportunity to network with each other.
In addition to GCP and research governance training, all RMs were trained to English national standards in delivering smoking cessation advice, with particular emphasis on the issues faced by pregnant smokers. RMs completed the 2-day training before they started recruiting, with additional refresher training during the trial. The general aim of training was to provide RMs with an understanding of the basic epidemiology of smoking behaviour, the motivations behind this, harm caused by smoking and environmental tobacco smoke exposure, why people smoke and key barriers to quitting experienced by those smokers who attempt cessation. Skills-focused sessions also aimed to equip RMs with the counselling skills required to help smokers to begin thinking differently about their habit (cognitive change) and to apply recognised techniques to overcoming their addiction (behavioural change). RMs were trained to put emphasis on the value of behavioural approaches for cessation in pregnancy, on the uncertainty regarding the efficacy of NRT and the inappropriateness of using either bupropion or varenicline in pregnancy. In addition, they were trained to counsel participants in the appropriate use of patches (i.e. as if all were issued with active patches) and to instruct them not to smoke or use non-trial NRT preparations in addition to trial medications.
The NCTU provided a web-based database and randomisation system, data management reports and MedDRA coding of AEs. The system was held on a secure server in the NCTU, had a full electronic audit trail and full back-ups of the database were made every 24 hours. Baseline and follow-up data were collected on paper case report forms (CRFs) or questionnaires and then inputted onto the database by the RM or trial administrator. The database included validation checks on data fields, whereby responses not meeting expected criteria would be flagged so that data entry errors were minimised. The trial administrator or trial manager checked all database entries contributing to the outcomes at delivery against the CRF and clarified any queries with RMs. In addition, 10% of follow-up questionnaires were checked against database entries.
The independent TSC and the DMC met once or twice per year to monitor and supervise the progress and conduct of the trial and to review any safety or data issues. The DMC received blinded outcome data each time it met. Stopping rules were established such that if quit rates in the whole sample fell below 4%, or if recruitment rates fell below 25% of the target, then they would consider recommending that the trial be stopped.
Oxfordshire Research Committee A granted national research ethical approval, with additional local approvals for each recruitment centre and Clinical Trial Authorisation (CTA) approval from the MHRA (CTA number: 03057/0002/001-0001). The protocol was published,55 with several approved amendments made to the original protocol after the start of recruitment; details of these amendments are given below (see Protocol amendments) and the final protocol can be found in Appendix 3. The trial was registered on the ISRCRN database (ISRCTN07249128) and was assigned a EudraCT number (2004-002621-46). The NHS National Institute for Health Research (NIHR) Primary Care Research Network adopted the study.
Bulk supplies of the NRT and placebo patches were manufactured by United Pharmaceuticals, purchased at market rates and imported into the EU via Almac Ltd, Clinical Trial Services, Craigavon, Northern Ireland. QMC Clinical Trials Pharmacy at Nottingham University Hospitals NHS Trust managed quality control testing, packaging and labelling of participant packs. To ensure stability for the whole study period, the patches needed to be stored refrigerated at 2–8 °C before being dispensed to participants. However, as the drug was stable at temperatures of < 25 °C for 3 months, and only 1 month’s supply of patches was issued at a time, it was not necessary for participants to store them in a refrigerator.
Baseline and 1-month saliva samples were analysed at laboratories within the Centre for Oncology and Molecular Medicine, Division of Medical Sciences at the University of Dundee, UK, under the overall supervision of Professor Michael Coughtrie (a co-investigator). Baseline blood samples were analysed by ABS Laboratories Ltd, Welwyn Garden City, Hertfordshire, UK, and saliva samples taken at delivery were analysed by Salimetrics Europe Ltd, Newmarket, Suffolk, UK.
Protocol amendments
Brief details of protocol amendments made after the start of recruitment, but prior to breaking treatment allocation codes, are listed below.
-
We originally anticipated that women would be sent the PIS before their clinic appointments so that they could then be recruited and consented when they attended for their antenatal scan appointment. However, it was soon realised that, overall, this was not a practical option and that most women were being enrolled on home visits. Therefore, rather than posting the PIS to all women, we added the option of just sending leaflets containing brief information about the trial, with the full PIS later posted to women who had been identified and contacted using the screening questionnaire.
-
Small changes were made to the protocol to clarify trial processes and allow for minor variations in practice in the different centres due to different local arrangements in, for example, prescribing and dispensing practices, local clinic arrangements, follow-up cessation support and time spent in hospital after delivery.
-
Ambiguities in the primary outcome measure were addressed and clarified in the protocol, including the time window in which data collected could be used for analysis, how self-report and biochemical validation data of smoking cessation contributed to a positive primary outcome and what constitutes ‘prolonged abstinence from smoking’. Secondary outcomes were clarified to distinguish and define fetal death at different gestations, i.e. miscarriage and stillbirth.
-
We obtained ethical approval to send a ‘congratulations on the birth of your baby’ card to women after delivery.
-
The content of the questionnaires sent at 6 months, 1 year and 2 years after delivery was finalised and approved, along with a questionnaire to be sent to participants when they could not be contacted 1 month after their quit date. We also decided to include incentives of shopping vouchers and a colouring competition to help improve response rates for the 2-year questionnaires. 41
-
Once the trial started, we realised that as many pregnancy-related conditions required hospital admissions this was resulting in a large number of SAE reports, none of which were felt to be related to the study drug. Therefore, the sponsor and TSC recommended that we should extend the list of conditions in the protocol that did not need to be reported as a SAE (any deaths of the mother or fetus/infant were still reported). All these AEs were still collected and reviewed by the DMC so that unforeseen impacts of NRT could be monitored.
-
Following further stability data from the manufacturer of the nicotine and placebo patches used in the study, the shelf life was extended from 24 to 42 months.
Trial extensions
The HTA granted two time extensions to the application, adding a total of 12 months to the original length of the trial. This was necessary as the start of recruitment was slightly delayed and the overall recruitment period took 10 months longer than the original estimate of 24 months. Careful budgeting of trial resources funded the majority of this extension, but a small addition to the budget was also awarded.
Chapter 3 Results
Recruitment and follow-up of outcomes at delivery
Recruitment and flow of participants through the trial
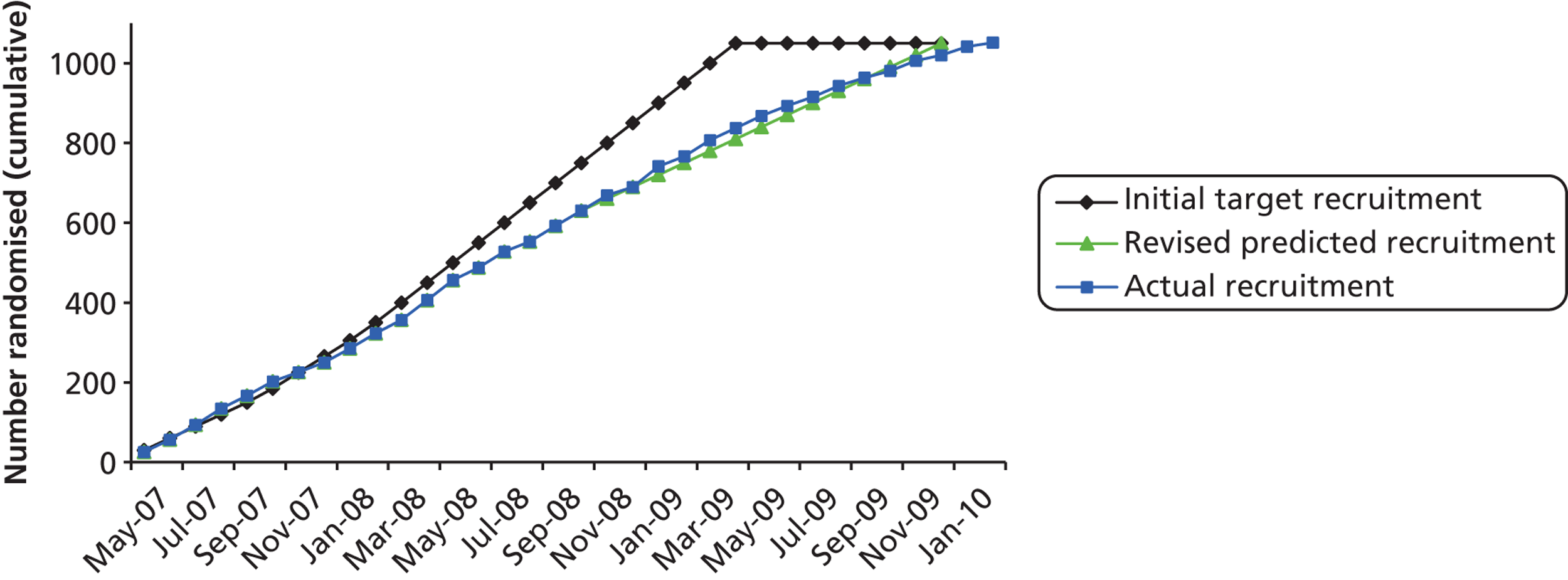
Participants were recruited between May 2007 and February 2010 (Figure 3 and Table 2). We initially estimated that recruitment would take 24 months, but after the first 6 months, target figures were revised in line with actual recruitment figures and the recruitment period extended by 10 months.
FIGURE 3.
Cumulative trial recruitment.
| Centre | NRT (n) | Placebo (n) | Total (N = 1050), n (%) |
|---|---|---|---|
| Nottingham University Hospitals NHS Trust (QMC campus) | 61 | 62 | 123 (11.7) |
| Nottingham University Hospitals NHS Trust (City Hospital campus) | 62 | 66 | 128 (12.2) |
| Sherwood Forest Hospitals NHS Foundation Trust (King’s Mill Hospital) | 108 | 108 | 216 (20.6) |
| University Hospital of North Staffordshire (City General Site) | 127 | 130 | 257 (24.5) |
| Mid Cheshire Hospitals NHS Foundation Trust (Leighton Hospital) | 83 | 84 | 167 (15.9) |
| East Cheshire NHS Trust (Macclesfield District General Hospital) | 40 | 40 | 80 (7.6) |
| Derby Hospitals NHS Foundation Trust (Derby City General – later to become Royal Derby Hospital) | 40 | 39 | 79 (7.5) |
The Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 4) summarises the process of recruitment and flow of participants through the study to the primary follow-up point. Approximately 21,000 women were informed of the trial by questionnaires that were distributed and completed in antenatal clinics. The majority of these (around 18,590) were excluded without further contact either because the screening questionnaire showed that they were not eligible (usually because they were not smokers), or because they had no interest in joining the trial.
FIGURE 4.
The CONSORT diagram showing flow of participants to delivery. From Coleman et al. 56 Copyright © 2012 Massachusetts Medical Society. Reprinted with permission. DNA, did not attend.
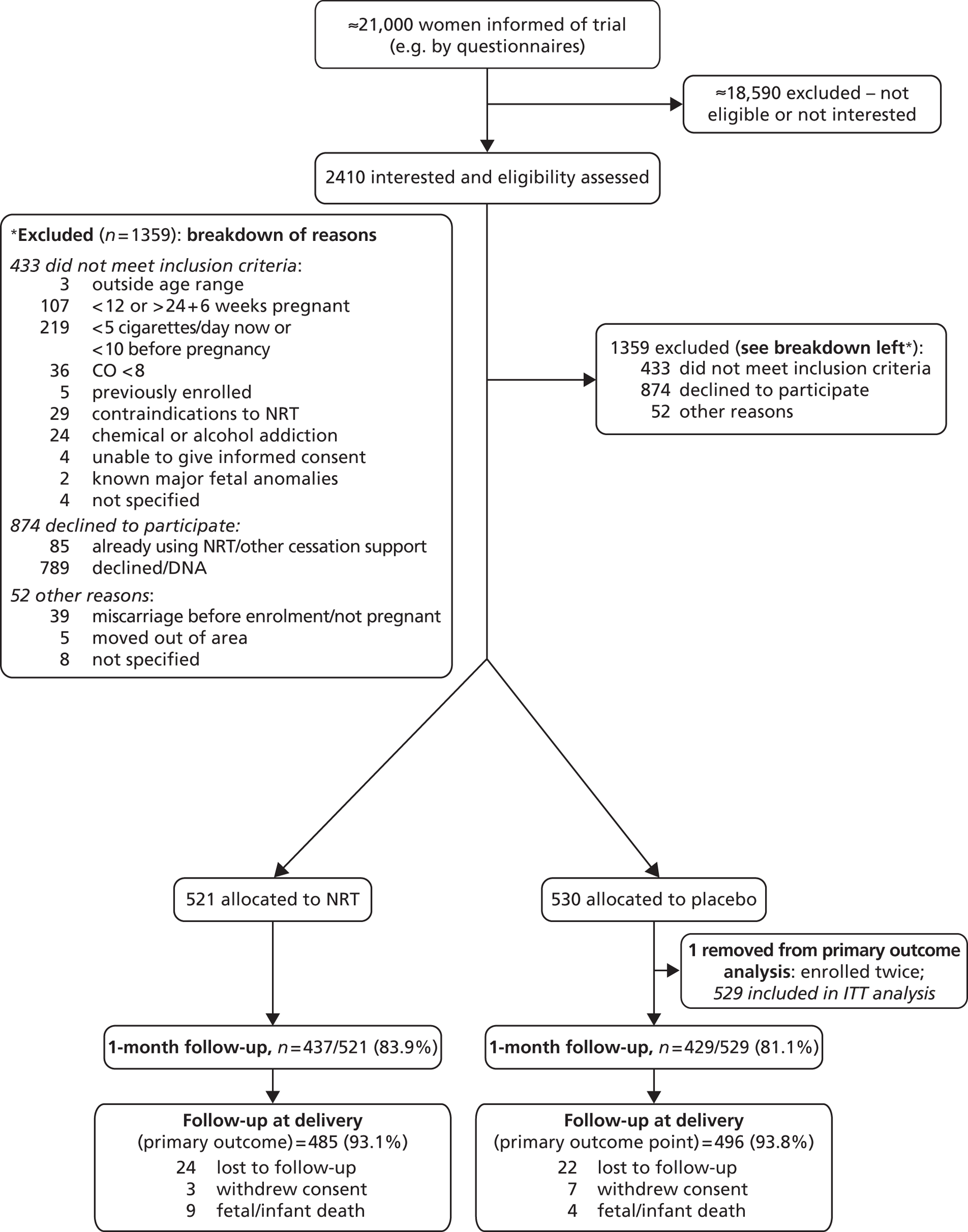
Of the 2410 women who expressed interest in the trial and were assessed for eligibility, 1051 (43.6%) were randomised: 521 were assigned to receive NRT and 530 were assigned to receive placebo patches (see Figure 4). One woman was mistakenly enrolled for a second time in a subsequent pregnancy; her second enrolment in the placebo group was removed from all analyses, giving a final sample size of 1050 (529 in the placebo group).
Protocol breaches were discovered for 13 other participants, but after consideration of violation details it was decided that these were not serious and would have no significant impact on trial participants or the scientific integrity of the trial. These participants, therefore, remained in the trial and their data were used in analyses; details of these protocol breaches are given in Appendix 4.
Two additional problems affecting 27 participants occurred within one site pharmacy, but, again, these were judged to have no significant impact on participants or trial integrity, and details are given in Appendix 4.
Of 1050 pregnancies, 1038 were singleton and 12 were twin.
At 1 month after their quit date, 866 women (82.5%) provided outcome data and, of these, 573 (66.2%) responded by telephone, 19 (2.2%) responded by questionnaire and 274 (31.6%) attended face-to-face consultations with RMs.
At delivery, 981 (93.4%) participants provided smoking outcome data, but 46 (4.4%) who could not be contacted within the necessary time frame, 10 (1.0%) who withdrew consent and 13 (1.2%) who experienced fetal or infant death (including one elective termination) were not asked for their smoking status.
For most participants who reported that they were non-smokers, biochemical validation was obtained. The validation rates at 1 month were 89% (116/131) in the NRT group and 85% (63/74) in the placebo group. At delivery, validation rates were 89% (58/65 women) in the NRT group and 92% (45/49) in the placebo group.
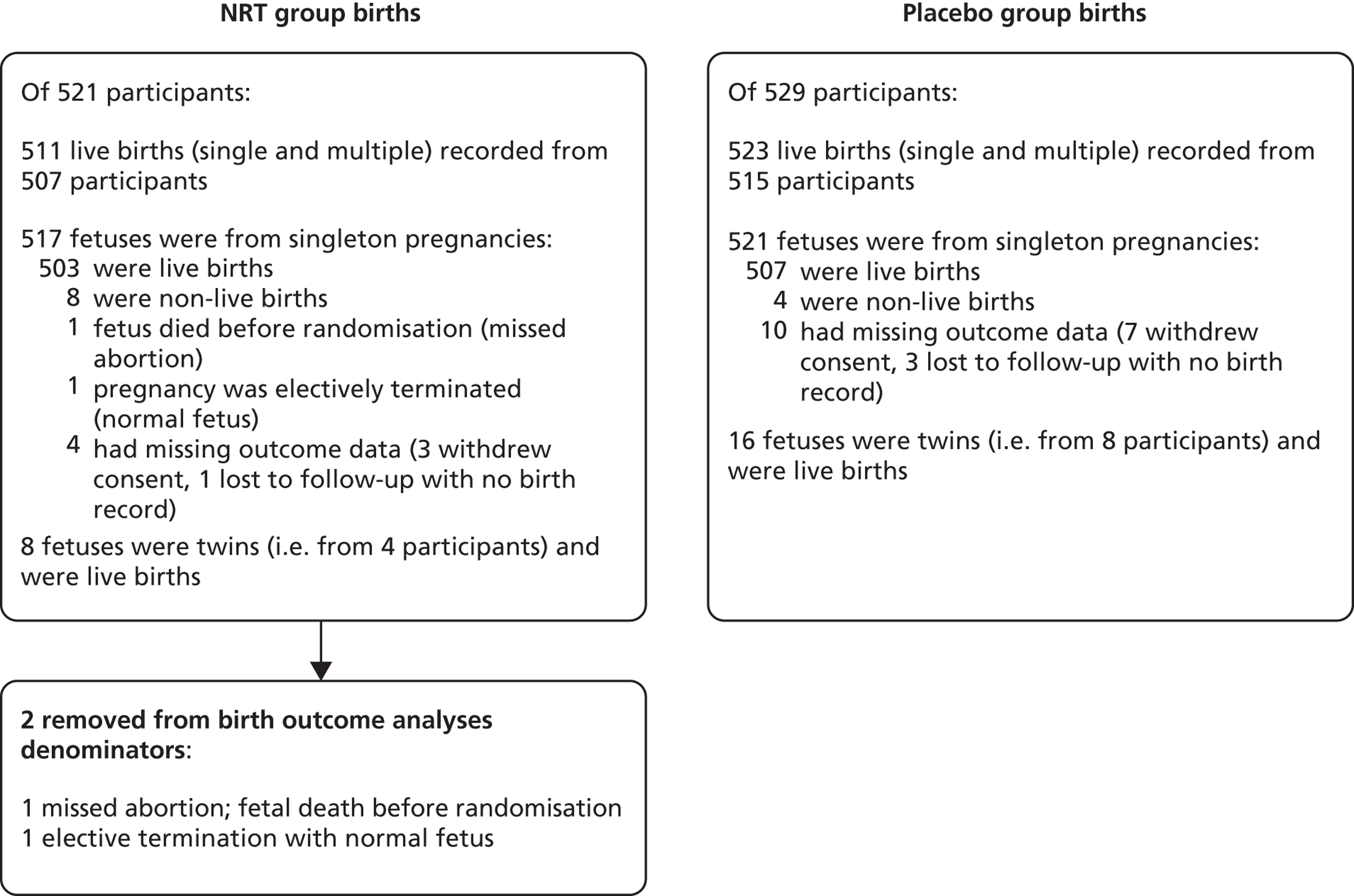
The ascertainment rate for birth outcomes was more complete than smoking outcomes as these were obtained from participants’ hospital notes. Figure 5 summarises numbers of births within participants and completeness of birth outcome ascertainment.
FIGURE 5.
Completeness of birth outcome data. From Coleman et al. 56 Copyright © 2012 Massachusetts Medical Society. Reprinted with permission.
Further details on the numbers of participants who were followed up and for whom outcome data were obtained at 1 month and at delivery are presented by study centre in Table 3.
| Outcome ascertainment | Nottingham – QMC | Nottingham – City | King’s Mill | North Staffs | Leighton | Macclesfield | Derby | Total |
|---|---|---|---|---|---|---|---|---|
| 1-month visit, n (%) | ||||||||
| In person | 36 (29.3) | 33 (25.8) | 41 (19.0) | 52 (20.2) | 75 (44.9) | 20 (25.0) | 17 (21.5) | 274 (26.1) |
| Telephone or returned questionnaire | 71 (57.7) | 80 (62.5) | 123 (56.9) | 156 (60.7) | 68 (40.7) | 41 (51.3) | 53 (67.1) | 592 (56.4) |
| No contact at 1 month | 16 (13.0) | 15 (11.7) | 52 (24.1) | 49 (19.1) | 24 (14.4) | 19 (23.8) | 9 (11.4) | 184 (17.5) |
| Total | 123 | 128 | 216 | 257 | 167 | 80 | 79 | 1050 (100) |
| Final trial status, n (%) | ||||||||
| Outcome data obtained | 115 (93.5) | 118 (92.2) | 210 (97.2) | 232 (90.3) | 158 (94.6) | 72 (90.0) | 76 (96.2) | 981 (93.4) |
| Fetal/infant deatha | 2 (1.6) | 2 (1.6) | 1 (0.5) | 3 (1.2) | 3 (1.8) | 1 (1.3) | 1 (1.3) | 13 (1.2) |
| Lost to follow-up or withdrew consent | 6 (4.9) | 8 (6.3) | 5 (2.3) | 22 (8.6) | 6 (3.6) | 7 (8.8) | 2 (2.5) | 56 (5.3) |
| Total | 123 | 128 | 216 | 257 | 167 | 80 | 79 | 1050 (100) |
Baseline characteristics
Sociodemographic characteristics, smoking behaviour, obstetric history and participants’ prior use of NRT were similar in both trial groups (Table 4). Women had a mean age of 26 years and joined the trial at a mean gestational age of 16 weeks. Participants were heavy smokers and around one-third smoked within 5 minutes of waking and the median number of cigarettes smoked per day at randomisation was 14.
| Characteristic | NRT (N = 521) | Placebo (N = 529) | |
|---|---|---|---|
| Mean age (years), (SD) | 26.4 (6.2) | 26.2 (6.1) | |
| Median number of cigarettes smoked daily before pregnancy (IQR) | 20 (15–20) | 20 (15–20) | |
| Median number of cigarettes smoked daily at randomisation (IQR) | 13 (10–20) | 15 (10–20) | |
| Mean gestational age at baseline (weeks) (SD) | 16.2 (3.6) | 16.3 (3.5) | |
| Ethnic group, n (%) | White British | 503 (96.5) | 515 (97.4) |
| Other | 18 (3.5) | 14 (2.6) | |
| Mean age left full-time educationb (years) (SD) | 16.2 (1.4) | 16.3 (1.7) | |
| Parity,c n (%) | 0–1 | 356 (68.3) | 363 (68.6) |
| 2–3 | 129 (24.8) | 142 (26.9) | |
| ≥ 4 | 36 (6.9) | 24 (4.5) | |
| Median baseline cotinine levels (ng/ml) (IQR) | 123.1 (80.1–179.8) | 121.2 (77.2–175.9) | |
| Time to first cigarette (minutes), n (%) | 0–15 | 281 (54.0) | 285 (53.9) |
| > 15–60 | 199 (38.2) | 198 (37.4) | |
| > 60 | 41 (7.9) | 46 (8.7) | |
| Women with partner who smokes,d n/women with a partner, n (%) | 356/481 (74.0) | 360/482 (74.7) | |
| Mean height (cm)e (SD) | 163.2 (6.8) | 163.0 (6.5) | |
| Mean weight (kg)f (SD) | 71.7 (18.2) | 71.6 (17.2) | |
| Previous preterm birth,g n (%) | 42 (8.1) | 50 (9.5) | |
| Length of first behavioural support session (minutes), n (%) | ≤ 30 | 84 (16.1) | 81 (15.3) |
| 31–60 | 428 (82.1) | 433 (81.9) | |
| > 60 | 9 (1.7) | 15 (2.8) | |
| Use of NRT within pregnancy and prior to enrolment,h n (%) | 23 (4.4) | 24 (4.5) | |
Primary outcome measure at delivery
The rate of prolonged abstinence at delivery with validation was 9.4% in the NRT group and 7.6% in the placebo group (OR for abstinence with NRT 1.26, 95% CI 0.82 to 1.96) (Table 5).
| Outcome | n (%) | OR (95% CI)a | Adjusted OR (95% CI)b | |
|---|---|---|---|---|
| NRT (N = 521) | Placebo (N = 529) | |||
| Primary | ||||
| Prolonged self-reported abstinence from smoking between quit date and delivery with COT and/or CO validationc,d | 49 (9.4) | 40 (7.6) | 1.26 (0.82 to 1.96) | 1.27 (0.82 to 1.98) |
| Secondary | ||||
| Prolonged abstinence from quit date to delivery without validation | 65 (12.5) | 49 (9.3) | 1.40 (0.94 to 2.07) | 1.41 (0.95 to 2.09) |
| Abstinence to 1 month after quit date without validation | 131 (25.1) | 74 (14.0) | 2.07 (1.51 to 2.85) | 2.13 (1.54 to 2.95) |
| Abstinence to 1 month after quit date with CO validatione | 111 (21.3) | 62 (11.7) | 2.05 (1.46 to 2.88) | 2.10 (1.49 to 2.97) |
| Prolonged abstinence to delivery with validation at 1 month after quit date and delivery | 42 (8.1) | 32 (6.0) | 1.36 (0.84 to 2.19) | 1.37 (0.84 to 2.22) |
| Point prevalence cessation (> 24-hour quit) at delivery with CO validation | 63 (12.1) | 53 (10.0) | 1.23 (0.84 to 1.82) | 1.24 (0.84 to 1.85) |
| Point prevalence cessation (> 24-hour quit) at delivery without validation | 104 (20.0) | 89 (16.8) | 1.24 (0.90 to 1.70) | 1.25 (0.90 to 1.72) |
Secondary outcome measures at delivery
Smoking outcomes at delivery
For self-reported (i.e. non-validated) abstinence, there was a slightly larger but still non-significant difference in quit rates: 12.5% with NRT compared with 9.3% with placebo (OR 1.40, 95% CI 0.94 to 2.07) (see Table 5). At 1 month, the validated abstinence rate was significantly higher in the NRT group than in the placebo group (21.3% vs. 11.7%, respectively; OR 2.05, 95% CI 1.46 to 2.88). Similar findings were found for adjusted analyses with all smoking outcomes.
Birth outcomes at delivery
Table 6 shows outcomes for singleton births including deaths, mean birthweight and rates of preterm birth, LBW and congenital abnormalities, and these were mainly similar in the two study groups. However, there were significantly more deliveries by caesarean section in the NRT group than in the placebo group (20.7% vs. 15.3%). Analyses that included twin births gave very similar findings.
| Fetal outcomes (singleton births only) | NRT (N = 515) n/N (%) |
Placebo (N = 521) n/N (%) |
OR (95% CI)b |
|---|---|---|---|
| Miscarriagec | 3/515 (0.6) | 2/521 (0.4) | 1.52 (0.25 to 9.13) |
| Stillbirthc | 5/512 (1.0) | 2/519 (0.4) | 2.59 (0.50 to 13.4) |
| Neonatal deathc | 0/507 (0) | 2/517 (0.4) | Not calculated |
| Post-neonatal deathc,d | 1/507 (0.2) | 0/517 (0) | Not calculated |
| Mean (SD) | Mean (SD) | Mean difference (95% CI) | |
| Birthweight, unadjusted (kg) | 3.18 (0.61) | 3.20 (0.59) | –0.02 (–0.10 to 0.05) |
| Birthweight (z-score) | –0.36 (0.99) | –0.31 (1.02) | –0.05 (–0.17 to 0.08) |
| Gestational age (weeks) | 39.5 (2.1) | 39.5 (2.1) | 0.0 (–0.2 to 0.3) |
| n/N (%) | n/N (%) | OR (95% CI)b | |
| Preterm birth(< 37 weeks’ gestation) | 40/507 (7.9) | 45/517 (8.7) | 0.90 (0.58 to 1.41) |
| LBW (< 2.5 kg) | 56/507 (11.0) | 43/517 (8.3) | 1.38 (0.90 to 2.09) |
| NICU admission | 33/507 (6.5) | 35/517 (6.8) | 0.96 (0.58 to 1.57) |
| Apgar score at 5 minutes < 7 | 16/507 (3.2) | 18/517 (3.5) | 0.91 (0.45 to 1.80) |
| Cord-blood arterial pH < 7 | 4/507 (0.8) | 7/517 (1.4) | 0.57 (0.17 to 1.97) |
| Intraventricular haemorrhage | 2/507 (0.4) | 3/517 (0.6) | 0.67 (0.11 to 4.05) |
| Neonatal convulsions | 5/507 (1.0) | 5/517 (1.0) | 1.02 (0.29 to 3.54) |
| Congenital abnormalitiese | 9/507 (1.8) | 13/517 (2.5) | 0.70 (0.30 to 1.66) |
| Necrotising enterocolitis | 3/507 (0.6) | 6/517 (1.2) | 0.50 (0.12 to 2.03) |
| Infant ventilated > 24 hours | 10/507 (2.0) | 11/517 (2.1) | 0.93 (0.39 to 2.22) |
| Assisted vaginal delivery | 38/507 (7.5) | 43/517 (8.3) | 0.95 (0.59 to 1.50) |
| Caesarean delivery | 105/507 (20.7) | 79/517 (15.3) | 1.45 (1.05 to 2.01) |
Adverse events
Other AEs are shown in Table 7 and, apart from skin reactions at the patch site (97 participants in NRT group reported skin reactions compared with 28 in placebo group), rates were similar in the two groups. In total, 46 participants in the NRT group and 32 in the placebo group stopped using the patches permanently owing to AEs. Further information on the distribution and nature of AEs is in Appendix 5.
| AE | NRT (N = 521) | Placebo (N = 529) |
|---|---|---|
| SAEs, n (%) | ||
| Maternal mortality | 0 | 0 |
| Other SAEsb | 9 (1.7) | 6 (1.1) |
| Maternal AEs potentially related to treatment, n (%) | ||
| Patch stopped permanently owing to AEc | 46 (8.8) | 32 (6.0) |
| Skin reactions at patch site (but no treatment discontinuation)d | 97 (18.6) | 28 (5.3) |
| Maternal AEs as probable complications of pregnancy, n (%) | ||
| Blood pressure > 140/90 mmHg on at least two occasions | 24 (4.6) | 25 (4.7) |
| Nausea or vomiting | 16 (3.1) | 19 (3.6) |
| Headache | 25 (4.8) | 16 (3.0) |
| Abdominal pain | 54 (10.4) | 50 (9.5) |
| Vaginal bleeding or haemorrhage | 35 (6.7) | 38 (7.2) |
| Premature rupture of membranese | 6 (1.2) | 10 (1.9) |
| Uterine contractions during pregnancye | 24 (4.6) | 30 (5.7) |
| Gestational diabetes | 3 (0.6) | 3 (0.6) |
| Pre-eclampsia or eclampsia | 3 (0.6) | 5 (0.9) |
| Hospital admission for other pregnancy complicationf | 44 (8.4) | 41 (7.8) |
| Other less frequent maternal AEsg | 63 (12.1) | 73 (13.8) |
| Fetal AEs as probable complications of pregnancy, n (%) | ||
| Decreased fetal movements (fetal hypokinesia)e | 58 (11.1) | 46 (8.7) |
| Other AEs affecting fetusg | 5 (1.0) | 5 (0.9) |
| Neonatal AEsg | 32 (6.1) | 29 (5.5) |
| Total AEs, n h | 535 | 450 |
Adherence with patches and use of smoking cessation support
Adherence with trial patches was low in both groups and rates are shown in Table 8. Only 7.2% of women (35/485) assigned to receive NRT and 2.8% (14/496) assigned to placebo reported using trial medications for more than 28 days. Additionally, although 111 NRT group participants were abstinent at 1 month and had this validated by a RM (see Table 5), only 72 of these (65%) accepted a second month’s supply of patches. The corresponding figure for the placebo group was 47% (29/62). Only 2.5% of NRT group participants (12/485) and 2.2% (11/496) of placebo group participants reported using ‘non-study’ NRT for ≥ 20 days.
| Adherence and support | NRT, n (%) | Placebo, n (%) | p-value (by chi-squared test for trend) | |
|---|---|---|---|---|
| Measures reported at 1 month | N = 437a | N = 429a | ||
| Reported days trial patch use in first month | 0–7 | 180 (41.3) | 220 (51.5) | p < 0.001 |
| 8–14 | 82 (18.8) | 108 (25.3) | ||
| 15–21 | 74 (17.0) | 58 (13.6) | ||
| > 21 | 100 (22.9) | 41 (9.6) | ||
| Days of non-trial NRT use | 0 | 425 (97.3) | 406 (94.6) | p < 0.05 |
| 1–4 | 8 (1.8) | 11 (2.6) | ||
| 5–9 | 1 (0.2) | 5 (1.2) | ||
| ≥ 10 | 3 (0.7) | 7 (1.6) | ||
| Additional face-to-face contacts with NHS SSS advisorb (n) | 0 | 409 (93.6) | 407 (94.9) | NS |
| 1 | 22 (5.0) | 17 (4.0) | ||
| ≥ 2 | 6 (1.4) | 5 (1.2) | ||
| Additional telephone contacts with NHS SSS advisorb (n) | 0 | 146 (33.4) | 139 (32.4) | NS |
| 1 | 122 (27.9) | 114 (26.6) | ||
| 2 | 95 (21.7) | 94 (21.9) | ||
| 3 | 41 (9.4) | 43 (10.0) | ||
| ≥ 4 | 33 (7.6) | 39 (9.1) | ||
| Additional SMS ‘text’ contacts with NHS SSS advisorc (n) | 0 | 332 (76.0) | 329 (76.7) | NS |
| 1 | 50 (11.4) | 53 (12.4) | ||
| 2 | 30 (6.9) | 31 (7.2) | ||
| 3 | 10 (2.3) | 8 (1.9) | ||
| ≥ 4 | 15 (3.4) | 8 (1.9) | ||
| Measures reported at delivery | N = 485c | N = 496c | ||
| Days of non-trial NRT use | 0 | 459 (94.6) | 449 (90.5) | p < 0.05 |
| 1–4 | 7 (1.4) | 16 (3.2) | ||
| 5–19 | 7 (1.4) | 20 (4.0) | ||
| ≥ 20 | 12 (2.5) | 11 (2.2) | ||
| Additional face-to-face contacts with NHS SSS advisorb (n) | 0 | 429 (88.5) | 448 (90.3) | NS |
| 1 | 36 (7.4) | 32 (6.5) | ||
| 2 | 15 (3.1) | 11 (2.2) | ||
| ≥ 3 | 5 (1.0) | 5 (1.0) | ||
| Additional telephone contacts with NHS SSS advisorb (n) | 0 | 141 (29.1) | 156 (31.5) | NS |
| 1–2 | 186 (38.4) | 203 (40.9) | ||
| 3–4 | 93 (19.2) | 86 (17.3) | ||
| 5–9 | 57 (11.8) | 49 (9.9) | ||
| ≥ 10 | 8 (1.7) | 2 (0.4) | ||
| Additional SMS ‘text’ contacts with NHS SSS advisorb (n) | 0 | 347 (71.6) | 365 (73.6) | NS |
| 1–2 | 87 (17.9) | 85 (17.1) | ||
| 3–4 | 24 (5.0) | 27 (5.4) | ||
| 5–9 | 24 (5.0) | 17 (3.4) | ||
| ≥ 10 | 3 (0.6) | 2 (0.4) | ||
| Participants issued with a second month’s supply of patchesd (n) | 72 | 29 | ||
| Reported days of trial patch use by participants issued with 2 months’ worth of patchesd | 0–7 | 3 (4.3) | 1 (3.6) | NS |
| 8–14 | 12 (17.1) | 8 (28.6) | ||
| 15–28 | 20 (28.6) | 5 (17.9) | ||
| ≥ 29 | 35 (50.0) | 14 (50.0) | ||
| Behavioural support from RMs | N = 521 | N = 529 | ||
| Successful contacts with RMs (n)e | 0 | 27 (5.2) | 28 (5.3) | |
| 1 | 74 (14.2) | 74 (14.0) | ||
| 2 | 152 (29.2) | 177 (33.5) | ||
| 3 | 268 (51.4) | 250 (47.3) | ||
As per protocol, RMs attempted to contact participants and provide behavioural support on the quit date, 3 days after their quit date and 1 month after their quit date. In the NRT group, 368 (70.6%) were successfully contacted via a telephone call from a RM on their quit date and 386 (74.1%) 3 days after their quit date. A total of 69 (13.2%) were not successfully contacted on either day, while 302 (58.0%) had a call on both days. In the placebo group, 378 (71.5%) were successfully contacted on their quit date and 381 (72.0%) 3 days after their quit date. A total of 67 women (12.7%) were not successfully contacted on either day, while 297 (56.1%) received a call on both days. At 1 month, 428 (82.1%) participants in the NRT group received support from a RM either face to face [164 (31.5%)] or by telephone [264 (50.7%)]. In the placebo group, 419 (79.2%) participants received RM support [110 (20.8%) face to face, 309 (58.4%) telephone only]. The lower rate of face-to-face support delivered to the placebo group at 1 month reflects the fact that this was delivered at consultations arranged to validate abstinence from smoking. Support was received by participants on all three occasions (i.e. quit date, 3 days after their quit date and 1 month after their quit date) for 268 participants (51.4%) in the NRT group and 250 participants (47.3%) in the placebo group (see Table 8). Support was not received on any of these occasions for 27 participants (5.2%) in the NRT group and 28 participants (5.3%) in the placebo group.
Participants also reported little additional face-to-face or text message contact with, or support from, smoking cessation advisors who worked for local NHS SSS (see Table 8). Support by telephone was more common and both groups reported receiving a median of two telephone contacts from advisors.
Follow-up after delivery
Follow-up of participants and infants after delivery
Follow-up of participants and infants began after the first birth in July 2007 and continued until December 2012.
Two CONSORT diagrams are presented for the follow-up period from delivery until infants were 2 years old; participants indicated as withdrawn in either diagram were not sent further questionnaires after their withdrawal.
Participants’ follow-up
The first diagram (Figure 6) is for all participants and summarises questionnaire distribution and response rates at the three follow-up time points, plus reasons for withdrawal. This provides denominators and follow-up data that are relevant to participant outcomes (e.g. smoking behaviour). By the final follow-up point, 150 participants [14.3% of the original study population: 73 out of 521 (14.0%) NRT, 77 out of 529 (14.6%) placebo] had withdrawn from the study, or a completed 2-year questionnaires had not been received from either the participant or their GP. This includes the 122 participants whose reasons for withdrawal are shown in Figure 6, plus 28 participants with fetal deaths or for whom no birth details were obtained. These are described in Infants’ follow-up.
FIGURE 6.
The CONSORT diagram showing flow of all trial participants during the 2-year follow-up period. a, 26 HPQs were returned in participants who had already returned a PQ2 (n = 12 NRT, n = 14 placebo) and these were not included in subsequent analyses. Reproduced with permission. Cooper S, Taggar J, Lewis S, Marlow N, Dickinson A, Whitemore R, et al. Effect of nicotine patches in pregnancy on infant and maternal outcomes at 2 years: follow-up from the randomised, double-blind, placebo-controlled SNAP trial [published online ahead of print 11 August 2014]. Lancet Respir Med 2014. doi:10.1016/S2213-2600(14)70157-2.
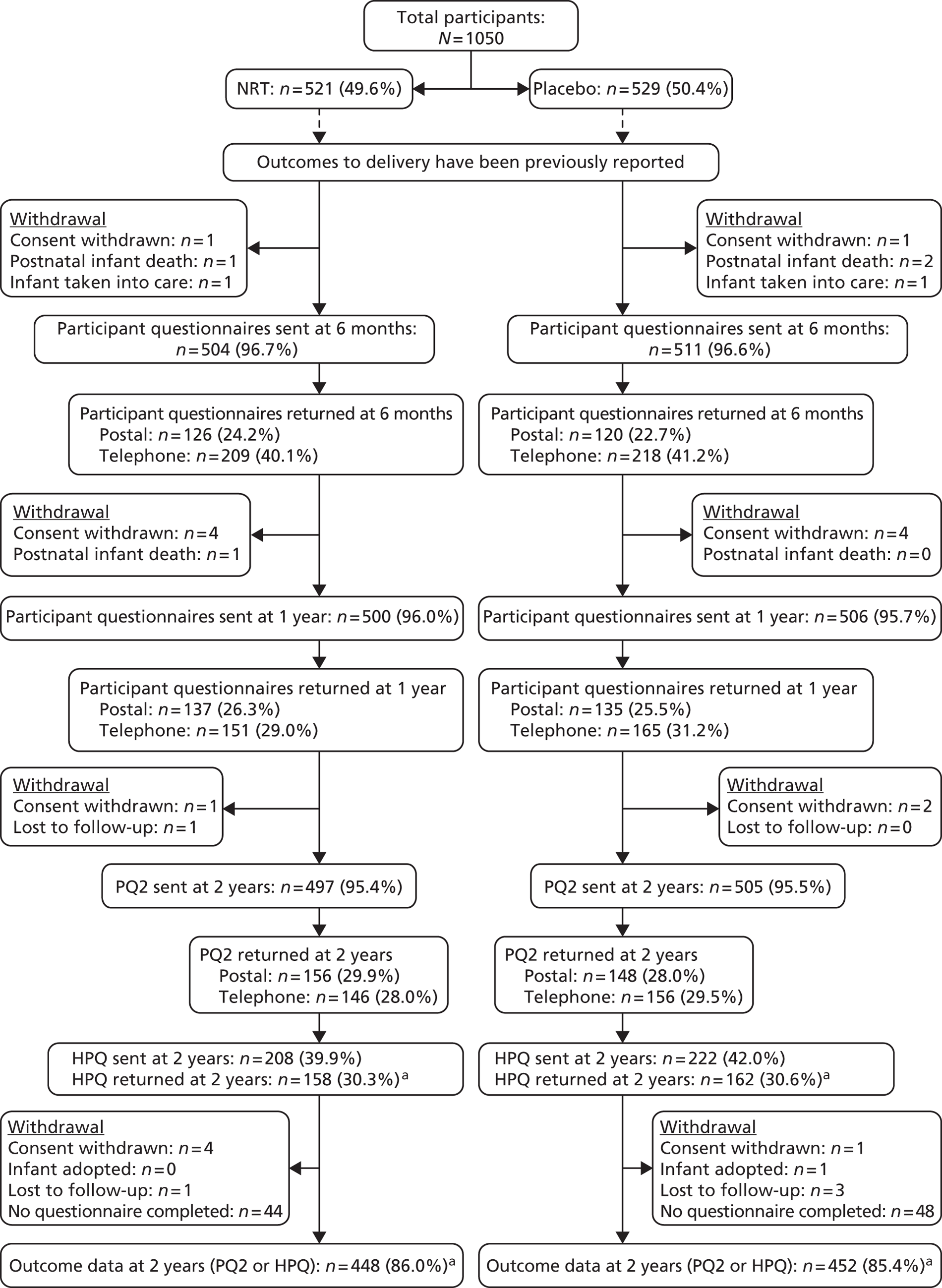
Infants’ follow-up
The second diagram (Figure 7) provides similar details for the 1010 live infants that were known to be born from singleton pregnancies and provides information on follow-up that is relevant to the assessment of infant outcomes at 2 years. This shows the 14 fetal deaths recorded when birth outcome data were analysed (also described in Figure 5), plus 14 participants for whom no infant birth details were obtained. Of those with no birth details, 10 withdrew consent before delivery (three NRT, seven placebo) and four were lost to follow-up (one NRT, three placebo); for ITT analyses of infant outcomes, it has been assumed that 14 live singleton infants were born to these participants. Four singleton infant deaths occurred between birth and the 2-year follow-up (two NRT, two placebo), of which one was known about and recorded when outcomes at delivery were analysed (see Table 6). For simplicity, and as primary analyses for infant outcomes did not include infants carried in multiple birth pregnancies, twin infants are excluded from Figure 5. However, sensitivity analyses included twins and either PQ2 or HPQ responses for 18 twin infants (6 NRT, 12 placebo) from nine of the 12 twin pregnancies were obtained and used in these.
FIGURE 7.
The CONSORT diagram showing flow of participants with live singleton births. These data were used for birth outcome analyses during the 2-year follow-up period. a, 26 HPQs were returned in participants who had already returned a PQ2 (n = 12 NRT, n = 14 placebo) and these were not included in subsequent analyses. Reproduced with permission. Cooper S, Taggar J, Lewis S, Marlow N, Dickinson A, Whitemore R, et al. Effect of nicotine patches in pregnancy on infant and maternal outcomes at 2 years: follow-up from the randomised, double-blind, placebo-controlled SNAP trial [published online ahead of print 11 August 2014]. Lancet Respir Med 2014. doi:10.1016/S2213-2600(14)70157-2.
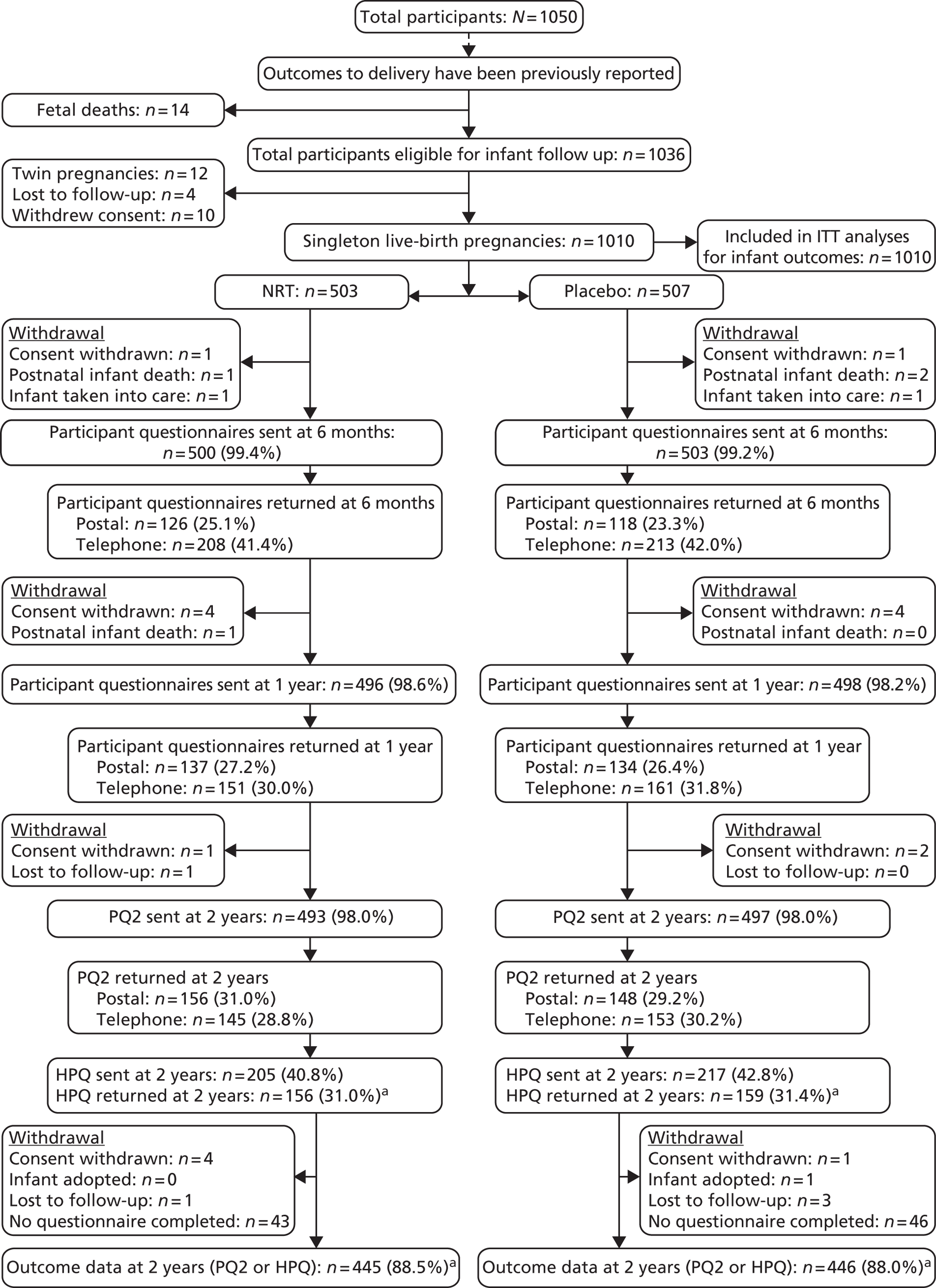
Discrepancies in questionnaire follow-up
One participant in the placebo group was sent neither the 6-month nor the 1-year questionnaire as her birth details were not obtained until 18 months after delivery (i.e. it was not known that a live birth had occurred). Two participants were inadvertently sent questionnaires: one in the placebo group was sent a 6-month questionnaire as the trial team had not been informed that the child had been taken into care at delivery, and one participant in the NRT group whose infant had died was sent a 1-year questionnaire due to a delay in receiving the death report from the NHS Information Centre. There were 26 participants (12 NRT, 14 placebo), all with singleton births, for whom both PQ2 and HPQs were completed and returned, and for these respondents PQ2 responses were used.
Completeness of follow-up for early childhood outcomes
For the 1010 known singleton live births, there were 891 (445 NRT, 446 placebo) (88.2%) responses to either the PQ2 or the HPQ, which could be used in analyses contributing to the primary outcome at the 2-year follow-up (see Figure 7).
Completeness of follow-up for participants’ smoking outcomes
From the full trial cohort of 1050 participants, 606 (302 NRT, 304 placebo) (57.7%) responded to the PQ2 questionnaire providing data that could be used in the analysis of participants’ smoking outcomes at the 2-year follow-up (see Figure 6).
Overall follow-up rates were similar in both groups; the response rates for all follow-up time points can be seen in Figure 6 and Table 9, and in chart form in Figure 8. Most participants required a reminder questionnaire and approximately half of those who provided data did so by completing the questionnaire over the telephone. Around three-quarters of health professionals returned the questionnaire by post, although most needed a postal reminder. The total combined response for participant or HPQs at 2 years was 900 (89.8% of those sent questionnaires at 2 years; 85.7% of all randomised participants). This figure excludes the 26 HPQs that were received for participants who subsequently returned their PQ2.
| Questionnaire | Questionnaire sent (N) | Reminder sent n (% of questionnaires sent) | Returned by post n (% of questionnaires sent) | Completed by telephone n (% of questionnaires sent) | Total response n (% of questionnaire sent) |
|---|---|---|---|---|---|
| Participant 6-month | 1015 | 886 (87.3) | 246 (24.2) | 427 (42.1) | 673 (66.3) |
| Participant 1-year | 1006 | 875 (87.0) | 272 (27.0) | 316 (31.4) | 588 (58.4) |
| PQ2 | 1002 | 864 (86.2) | 304 (30.3) | 302 (30.1) | 606 (60.5) |
| HPQ | 430 | 306 (71.2) | 320 (74.4) | NA | 320a (74.4) |
| Combined 2-year response (i.e. PQ2 or HPQ returned) | 1002 | NA | NA | NA | 900 (89.8) |
FIGURE 8.
Numbers of questionnaires sent and returned during the follow-up period.
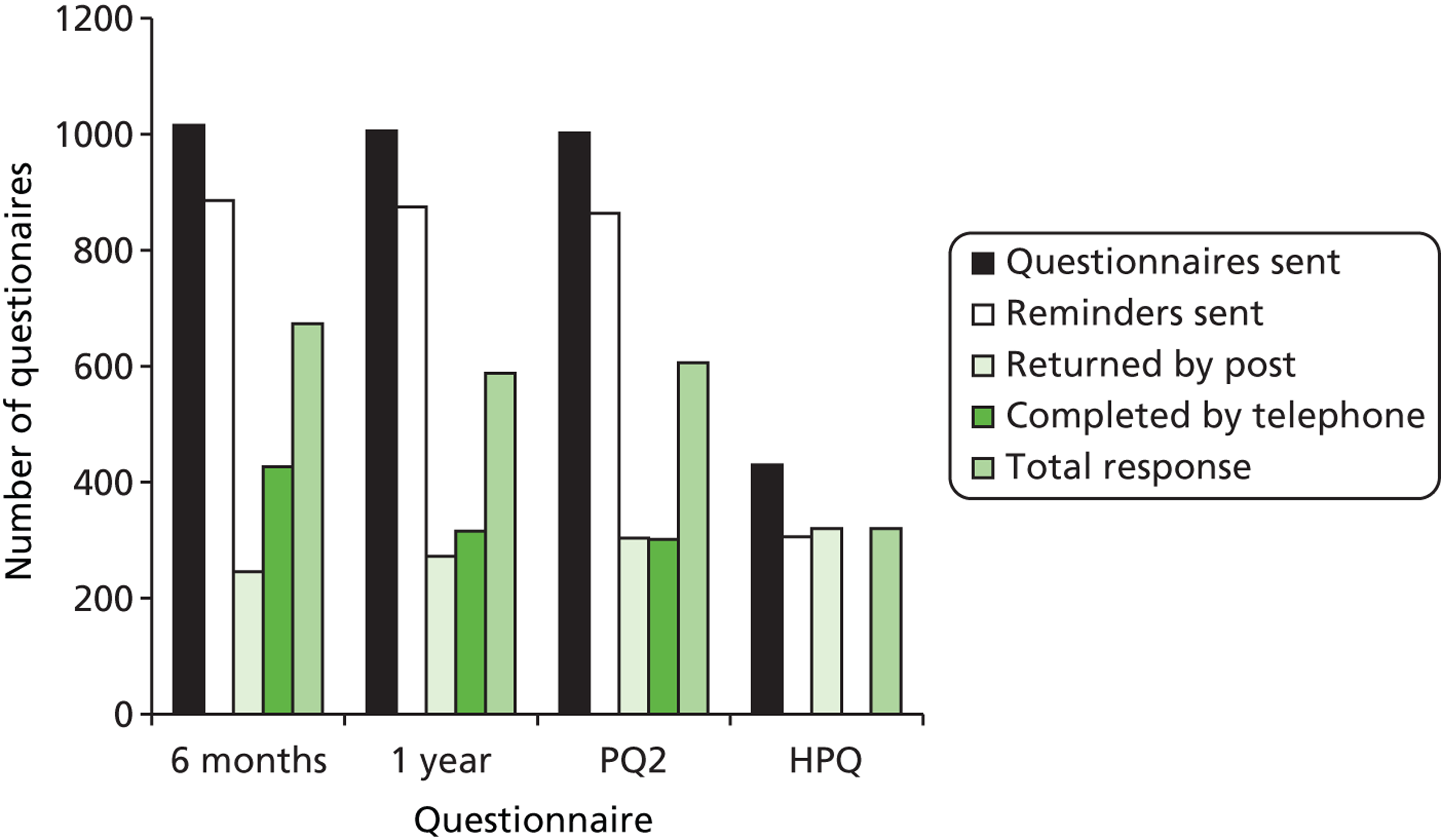
Characteristics of participants and infants who were followed up after delivery
Table 10 shows the maternal baseline characteristics and singleton birth outcomes for the 900 participants who had 2-year outcome data in the NRT and placebo groups. As with the whole trial cohort, these two groups had similar demographic characteristics at enrolment. Singleton infant birth outcomes were also similar in the two groups, apart from delivery by caesarean section, which, as found in the full cohort at primary outcome, was similarly slightly higher in the NRT group than in the placebo group (20.2% compared with 15.5%). The proportion of the participants providing data at 2 years who had prolonged, validated abstinence from smoking at delivery was 10.3% in the NRT group and 8.2% in the placebo group (see Table 10), which compares with 9.4% and 7.6% for NRT and placebo groups, respectively, for the full cohort (see Table 5).
| NRT (N = 448)a | Placebo (N = 452)a | |
|---|---|---|
| Maternal characteristics at study enrolment | ||
| Mean age (years) (SD) | 26.5 (6.2) | 26.3 (6.1) |
| Median number of cigarettes smoked daily before pregnancy (IQR) | 20 (15–20) | 20 (15–20) |
| Median number of cigarettes smoked daily at baseline (IQR) | 13 (10–20) | 15 (10–20) |
| Mean gestational age at baseline (weeks) (SD) | 16.2 (3.5) | 16.3 (3.5) |
| Ethnic group, n (%) | ||
| White British | 434 (96.9) | 442 (97.8) |
| Other | 14 (3.1) | 10 (2.2) |
| Age left full-time education (years) | ||
| Mean (SD) | 16.2 (1.4) | 16.3 (1.7) |
| Missing data (n) | 5 | 8 |
| Index of multiple deprivation | ||
| Mean (SD) | 32.1 (16.7) | 32.4 (16.9) |
| Missing data (n) | 13 | 9 |
| Parity, n (%) | ||
| 0–1 | 306 (68.3) | 311 (68.8) |
| 2–3 | 111 (24.8) | 121 (26.8) |
| ≥ 4 | 31 (6.9) | 20 (4.4) |
| COT at baseline (ng/ml) | ||
| Median (IQR) | 123.7 (80.2–185.4) | 120.9 (75.6–175.9) |
| Missing data (n) | 35 | 33 |
| Time to first cigarette, n (%) | ||
| 0–15 minutes | 245 (54.7) | 243 (53.8) |
| 16–60 minutes | 169 (37.7) | 168 (37.2) |
| > 60 minutes | 34 (7.6) | 41 (9.1) |
| Women with partner who smokes | ||
| n (%) | 306 (68.3) | 306 (67.7) |
| Missing data (n, %) | 34 (7.6) | 38 (8.4) |
| Height (cm) | ||
| Mean (SD) | 162.9 (6.8) | 163.1 (6.4) |
| Missing data (n) | 12 | 13 |
| Weight (kg) | ||
| Mean (SD) | 71.2 (17.8) | 72.3 (17.1) |
| Missing data (n) | 8 | 8 |
| Previous preterm birth, n (%) | 38 (8.5) | 42 (9.3) |
| Length of first behavioural support session, n (%) | ||
| < 30 minutes | 66 (14.7) | 67 (14.8) |
| 31–60 minutes | 376 (83.9) | 371 (82.1) |
| > 60 minutes | 6 (1.3) | 14 (3.1) |
| Use of NRT earlier in pregnancy, n (%) | 19 (4.2) | 23 (5.1) |
| Maternal smoking outcomes at delivery | ||
| Met primary smoking cessation outcome, n (%) | 46 (10.3) | 37 (8.2) |
| Infant birth outcomes at delivery (singleton pregnancies) | NRT (n = 445)b | Placebo (n = 446)b |
| Mean birthweight, unadjusted (kg) (SD) | 3.2 (0.6) | 3.2 (0.6) |
| Mean gestational age (weeks) (SD) | 39.5 (2.1) | 39.5 (2.2) |
| Preterm birth, n (%) | 36 (8.1) | 40 (9.0) |
| LBW (< 2.5 kg), n (%) | 49 (11.0) | 37 (8.3) |
| NICU admission, n (%) | 29 (6.5) | 32 (7.2) |
| Apgar score at 5 minutes < 7, n (%) | 12 (2.7) | 13 (2.9) |
| Congenital abnormalities, n (%) | 7 (1.6) | 12 (2.7) |
| Infant on ventilator > 24 hours, n (%) | 8 (1.8) | 10 (2.2) |
| Assisted vaginal delivery, n (%) | 33 (7.4) | 37 (8.3) |
| Delivery by caesarean section, n (%) | 90 (20.2) | 69 (15.5) |
Table 11 shows the same characteristics within those participants who provided 2-year follow-up data on the PQ2, those for whom a HPQ was completed instead and those for whom no data was obtained. Most of the demographic data are similar in these three groups; however, those with no follow-up data at 2 years had a slightly higher mean index of multiple deprivation (IMD) score (36.8, compared with 32.3 in those with PQ2 data and 32.2 in those with HPQ data). Their first behavioural support session may also have been slightly shorter (21.3% of this group had a behavioural support session of < 30 minutes, compared with 14.4% and 15.7% of those with PQ2 and HPQ data, respectively). Median COT levels at enrolment appear to be slightly higher in those who were followed up by HPQ (131.6 ng/ml, compared with 119.1 ng/ml in the other two groups).
| All participants at randomisation (N = 1050) | Followed up: PQ2 (N = 606) | Followed up: HPQ (N = 294)a | Not followed up (N = 150)b |
|---|---|---|---|
| Maternal characteristics at enrolment/randomisation (all pregnancies) | |||
| Mean age (years) (SD) | 26.9 (6.3) | 25.5 (5.6) | 25.7 (6.3) |
| Median number of cigarettes smoked daily before pregnancy (IQR) | 20 (15–20) | 20 (15–20) | 20 (15–20) |
| Median number of cigarettes smoked daily at baseline (IQR) | 15 (10–20) | 15 (10–18) | 12 (10–15) |
| Mean gestational age at baseline (weeks) (SD) | 16.2 (3.5) | 16.4 (3.5) | 16.2 (3.6) |
| Ethnic group, n (%) | |||
| White British | 588 (97.0) | 288 (98.0) | 142 (94.7) |
| Other | 18 (3.0) | 6 (2.0) | 8 (5.3) |
| Mean age left full-time education (years) (SD) | 16.3 (1.7) | 16.2 (1.3) | 16.3 (1.5) |
| Mean index of multiple deprivation (SD) | 32.3 (17.1) | 32.2 (16.2) | 36.8 (16.0) |
| Parity, n (%) | |||
| 0–1 | 424 (70.0) | 193 (65.7) | 102 (68.0) |
| 2–3 | 144 (23.8) | 88 (29.9) | 39 (26.0) |
| ≥ 4 | 38 (6.3) | 13 (4.4) | 9 (6.0) |
| Median COT level at baseline (ng/ml) (IQR) | 119.1 (72.1–179.5) | 131.6 (88.7–184.7) | 119.1 (80.0–161.3) |
| Time to first cigarette, n (%) | |||
| 0–15 minutes | 329 (54.3) | 159 (54.1) | 78 (52.0) |
| 16–60 minutes | 231 (38.1) | 106 (36.1) | 60 (40.0) |
| > 60 minutes | 46 (7.6) | 29 (9.9) | 12 (8.0) |
| Women with partner who smokes, n (%) | 408 (67.3) | 204 (69.4) | 104 (69.3) |
| Mean height (cm) (SD) | 162.9 (6.8) | 163.2 (6.3) | 163.7 (7.0) |
| Mean weight (kg) (SD) | 72.4 (16.6) | 70.5 (19.1) | 71.0 (18.9) |
| Previous preterm birth, n (%) | 47 (7.8) | 33 (11.2) | 12 (8.0) |
| Length of first behavioural support session, n (%) | |||
| < 30 minutes | 87 (14.4) | 46 (15.7) | 32 (21.3) |
| 31–60 minutes | 505 (83.3) | 242 (82.3) | 114 (76.0) |
| > 60 minutes | 14 (2.3) | 6 (2.0) | 4 (2.7) |
| Use of NRT earlier in pregnancy, n (%) | 33 (5.5) | 9 (3.1) | 5 (3.3) |
| Maternal smoking at delivery (all pregnancies) | |||
| Met primary smoking cessation outcome, n (%) | 66 (10.9) | 17 (5.8) | 6 (4.0) |
| Infant birth outcomes (singleton pregnancies, N = 1010) | (N = 602) | (N = 289)a | (N = 119) |
| Mean birthweight, unadjusted (kg) (SD) | 3.2 (0.58) | 3.1 (0.62) | 3.2 (0.60) |
| Mean gestational age (weeks) (SD) | 39.5 (2.1) | 39.4 (2.3) | 39.6 (2.1) |
| Preterm birth, n (%) | 46 (7.6) | 30 (10.4) | 9 (7.6) |
| LBW (< 2.5 kg), n (%) | 46 (7.6) | 40 (13.8) | 13 (10.9) |
| NICU admission, n (%) | 39 (6.5) | 22 (7.6) | 7 (5.9) |
| Apgar score at 5 minutes < 7, n (%) | 13 (2.2) | 12 (4.2) | 9 (7.6) |
| Congenital abnormalities, n (%) | 8 (1.3) | 11 (3.8) | 3 (2.5) |
| Infant on ventilator > 24 hours, n (%) | 11 (1.8) | 7 (2.4) | 3 (2.5) |
| Assisted vaginal delivery, n (%) | 51 (8.5) | 19 (6.6) | 11 (9.2) |
| Delivery by caesarean section, n (%) | 118 (19.6) | 41 (14.2) | 25 (21.0) |
Participants who completed a PQ2 were more likely to have validated abstinence between quit date and delivery (10.9%, compared with 5.8% of those with HPQ data and 4.0% of those with no 2-year data) (see Table 11).
Outcome measurements after delivery
Infant outcomes at 2 years
Infants’ developmental outcomes and any reports of respiratory problems at 2 years after delivery are shown in Table 12, separated by treatment group for all those with known outcomes, including four postnatal deaths. In Table 13, these results are broken down further to show outcomes by questionnaire and, for comparison purposes, postnatal deaths are not included in the denominators in this table.
| Infant development and respiratory outcomes within treatment groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| NRT, n (%) | Placebo, n (%) | |||||||
| Number of respondentsa | 445 | 446 | ||||||
| Number with development outcomes allocatedb | 443 | 441 | ||||||
| Number of infant deaths (after delivery) | 2 | 2 | ||||||
| Number with known developmental outcomesc | 445 | 443 | ||||||
| Survival with no impairmentd | 323 (72.6) | 290 (65.5) | ||||||
| Definite developmental impairmente | 48 (10.8) | 64 (14.5) | ||||||
| Suspected development impairmentf | 72 (16.2) | 87 (19.6) | ||||||
| Number with respiratory outcomes allocatedb | 444 | 444 | ||||||
| Respiratory problemsg | 132 (29.7) | 111 (25.0) | ||||||
| Findings from analyses investigating effects of treatment allocation on infant development and respiratory outcomes | ||||||||
| Complete case analysis (singleton pregnancies)h | Complete case analyses (adjusted for clustering by twin pregnancies)i,j | Multiple imputation ITT analyses (singleton births) (n = 1010) | ||||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| Survival with no impairmentd | 1.41 (1.05 to 1.87) | 0.020 | 1.43 (1.08 to 1.91) | 0.013 | 1.40 (1.05 to 1.86) | 0.023 | ||
| Definite developmental impairmente | 0.71 (0.48 to 1.06) | 0.093 | 0.73 (0.49 to 1.09) | 0.13 | 0.71 (0.47 to 1.09) | 0.12 | ||
| Respiratory problemsg | 1.28 (0.95 to 1.73) | 0.105 | 1.32 (0.98 to 1.77) | 0.071 | 1.30 (0.97 to 1.74) | 0.083 | ||
| Infant development and respiratory outcomes according to source of data and treatment group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NRT | Placebo | |||||||||
| PQ2, n (%) | HPQ,a n (%) | PQ2 or HPQ,a n (%) | PQ2,a n (%) | HPQ,a n (%) | PQ2 or HPQ,a n (%) | |||||
| Number of respondentsb | 301 | 144 | 445 | 301 | 145 | 446 | ||||
| Number with development outcomes allocateda | 301 | 142 | 443 | 300 | 141 | 441 | ||||
| Survival with no impairmentc | 189 (62.8) | 134 (94.4) | 323 (72.9) | 159 (53.0) | 131 (92.9) | 290 (65.8) | ||||
| Definite developmental impairmentd | 47 (15.6) | 1 (0.7) | 48 (10.8) | 64 (21.3) | 0 (0) | 64 (14.5) | ||||
| Suspected development impairmente | 65 (21.6) | 7 (4.9) | 72 (16.3) | 77 (25.7) | 10 (7.1) | 87 (19.7) | ||||
| Number with respiratory outcomes allocateda | 301 | 143 | 444 | 300 | 144 | 444 | ||||
| Respiratory problemsf | 106 (35.2) | 26 (18.2) | 132 (29.7) | 88 (29.3) | 23 (16.0) | 111 (25.0) | ||||
| Findings from analyses investigating effects of treatment allocation on infant development and respiratory outcomes | ||||||||||
| Complete case analysis (singleton pregnancies)g | Complete case analyses (adjusted for clustering by twin pregnancies)h,i | Multiple imputation ITT analyses (singleton births) (n = 1010) | ||||||||
| PQ2 OR (95% CI)j | PQ2 or HPQ OR (95% CI)j | PQ2 OR (95% CI)k | PQ2 or HPQ OR (95% CI)k | PQ2 or HPQ OR (95% CI)l | ||||||
| Survival with no impairmentc | 1.52 (1.09 to 2.11) | 1.41 (1.06 to 1.88) | 1.58 (1.14 to 2.19) | 1.44 (1.08 to 1.91) | 1.36 (1.02 to 1.82) | |||||
| Definite developmental impairmentd | 0.67 (0.44 to 1.01) | 0.71 (0.47 to 1.06) | 0.66 (0.43 to 1.00) | 0.73 (0.49 to 1.09) | 0.72 (0.48 to 1.07) | |||||
| Respiratory problemsf | 1.38 (0.97 to 1.95) | 1.28 (0.95 to 1.73) | 1.40 (0.99 to 1.98) | 1.32 (0.98 to 1.77) | 1.30 (0.97 to 1.74) | |||||
Of the 1010 singleton infants, 891 (445 NRT, 446 placebo) had information about them returned on either the PQ2 or the HPQ questionnaire; however, owing to missing data, it was not possible to allocate developmental outcomes on one PQ2 (from placebo group) and six returned HPQs (two NRT, four placebo). Within these 891, infants born to women who had been allocated to the NRT group in pregnancy were significantly more likely to have survived with no impairment than those born to women allocated to placebo [323/445 (72.6%) of NRT group infants and 290/443 (65.5%) in the placebo group]. The OR for ‘survival with no impairment’ in the NRT compared with the placebo group obtained in the primary analysis (ITT with multiple imputation analysis) was 1.40 (95% CI 1.05 to 1.86, p = 0.023). Similar statistically significant differences in survival without impairment were also found for other analyses including the clustered analysis with twin births (OR 1.43, 95% CI 1.08 to 1.91, p = 0.013) (see Table 12) and the analysis of singleton infants using just the PQ2 responses (OR 1.52, 95% CI 1.09 to 2.11, p = 0.012) (see Table 13).
There were no significant differences between groups for any of the other infant outcomes (see Tables 12 and 13). Using definitions of developmental impairment described in the methods, ‘definite’ impairment was identified in 48 (10.8%) of the NRT and 64 (14.5%) of the placebo groups, and ‘suspected’ impairment in 72 (16.2%) of NRT and 87 (19.6%) of placebo groups. A greater number of questionnaires that were returned by participants reported potential child development problems than the health professional ones; 253 (42.0%) of the 602 returned PQ2s were categorised with definite or suspected impairment, compared with only 18 (6.2%) of the 289 HPQ responses.
A detailed breakdown of infant developmental outcomes from the PQ2 and HPQs, including ASQ-3 domain scores and problems reported in the supplementary questions, is shown in Table 14. Overall, participants were most likely to report problems with their infant’s talking (23.1%), followed by behaviour (10.3%), but these showed no differences between groups. However, there were significant differences in the number of infants for whom all five ASQ-3 domain scores were normal: 190 (63.1%) in the NRT group compared with 163 (54.2%) in placebo (OR 1.47, 95% CI 1.06 to 2.05, p = 0.02) (see Table 14). For specific items that contributed to ‘survival with no impairment’, the only significant difference between groups was in the ASQ-3 ‘personal-social’ domain; 254 (84.4%) had normal scores in the NRT group compared with 231 (76.7%) in the placebo group. However, although not significantly different, for infants in the NRT group, both the mean scores and the number of infants with normal scores were consistently higher in every ASQ-3 domain. The only other difference was with feeding problems (see Table 14), for which fewer participants allocated to NRT reported problems with their infants’ feeding [18 (6.0%) NRT, 36 (12%) placebo (OR 0.47, 95% CI 0.26 to 0.85, p = 0.011)]. This outcome is not one used by the ASQ-3 questionnaire and did not contribute to ‘survival with no impairment’.
| NRT | Placebo | OR (95% CI)a | p-value | |
|---|---|---|---|---|
| ASQ-3 domain scoresb | ||||
| Number providing data | 301 | 301 | ||
| Number (%) of mothers providing data for at least one domain | 301 (100) | 301 (100) | ||
| Fine motor skills (mean, SD) | 51.3 (8.0) | 50.5 (7.5) | ||
| Fine motor skills normal, n (%) | 264 (87.7) | 255 (84.7) | 1.30 (0.82 to 2.08) | 0.2668 |
| Gross motor skills (mean, SD) | 55.1 (8.9) | 53.4 (11.1) | ||
| Gross motor skills normal, n (%) | 263 (87.4) | 251 (83.4) | 1.40 (0.87 to 2.22) | 0.1469 |
| Communication (mean, SD) | 52.3 (13.2) | 51.0 (14.4) | ||
| Communication normal, n (%) | 265 (88.0) | 259 (86.1) | 1.21 (0.75 to 1.96) | 0.4273 |
| Problem solving (mean, SD)a | 45.6 (10.6) | 44.3 (11.1) | ||
| Problem solving normal, n (%) | 247 (82.1) | 231 (76.7) | 1.34 (0.90 to 2.01) | 0.1479 |
| Personal-social (mean, SD)a | 51.3 (9.7) | 49.1 (10.4) | ||
| Personal-social normal, n (%) | 254 (84.4) | 231 (76.7) | 1.64 (1.08 to 2.48) | 0.0184 |
| All ASQ-3 domains normal | 190 (63.1) | 163 (54.2) | 1.47 (1.06 to 2.05) | 0.0201 |
| Below normal cut-off score in ≥ 1 domains, n (%) | 47 (15.6) | 64 (21.3) | 0.67 (0.44 to 1.02) | 0.0588 |
| Below normal cut-off score in ≥ 2 domains, n (%) | 19 (6.3) | 30 (10.0) | 0.59 (0.32 to 1.08) | 0.0811 |
| ASQ-3 supplementary questions [n (%) reporting problem] | ||||
| Hearing | ||||
| n (%) | 6 (2.0) | 11 (3.7) | 0.54 (0.20 to 1.51) | 0.2326 |
| Missing data | 1 (0.3) | 1 (0.3) | ||
| Speech (talking) | ||||
| n (%) | 72 (23.9) | 67 (22.3) | 1.11 (0.76 to 1.63) | 0.5789 |
| Missing data | 1 (0.3) | 1 (0.3) | ||
| Speech (understanding) | ||||
| n (%) | 21 (7.0) | 22 (7.3) | 0.93 (0.50 to 1.73) | 0.8119 |
| Missing data | 1 (0.3) | 1 (0.3) | ||
| Neuromotor (walking, running, climbing) | ||||
| n (%) | 10 (3.3) | 17 (5.7) | 0.59 (0.27 to 1.33) | 0.1990 |
| Missing data | 1 (0.3) | 1 (0.3) | ||
| Vision | ||||
| n (%) | 11 (3.7) | 18 (6.0) | 0.59 (0.27 to 1.28) | 0.1784 |
| Missing data | 1 (0.3) | 1 (0.3) | ||
| Behaviourc | ||||
| n (%) | 30 (10.0) | 32 (10.6) | 0.91 (0.54 to 1.55) | 0.7362 |
| Missing data | 2 (0.7) | 1 (0.3) | ||
| Feedingc,d | ||||
| n (%) | 18 (6.0) | 36 (12.0) | 0.47 (0.26 to 0.85) | 0.0107 |
| Missing data | 1 (0.3) | 1 (0.3) | ||
| HPQ | ||||
| Number of health professionals providing data | 144 | 145 | ||
| n (%) reporting problems | ||||
| Hearing | 3 (2.1) | 2 (1.4) | 1.52 (0.25 to 9.24) | 0.649e |
| Speech | ||||
| n (%) | 6 (4.2) | 9 (6.2) | 0.57 (0.19 to 1.70) | 0.3110 |
| Missing data | 0 (0) | 5 (3.5) | ||
| Neuromotor | ||||
| n (%) | 3 (2.1) | 5 (3.5) | 0.54 (0.12 to 2.44) | 0.4167 |
| Missing data | 1 (0.7) | 0 (0) | ||
| Vision | 7 (4.9) | 4 (2.8) | 1.93 (0.54 to 6.86) | 0.2988 |
| Behaviourc | ||||
| n (%) | 8 (5.6) | 4 (2.8) | 2.12 (0.62 to 7.21) | 0.229e |
| Missing data | 3 (2.1) | 0 (0) | ||
| Feedingc | ||||
| n (%) | 6 (4.2) | 4 (2.8) | 1.52 (0.42 to 5.51) | 0.523e |
| Missing data | 0 (0) | 1 (0.7) | ||
| Current health status, n (%) | ||||
| No disability | 136 (94.4) | 137 (94.5) | ||
| Mild disability | 3 (2.1) | 5 (3.5) | ||
| Moderate disability | 2 (1.4) | 0 (0) | ||
| Severe disability | 1 (0.7) | 0 (0) | ||
| Missing data | 2 (1.4) | 3 (2.1) | ||
| Concerns about development | ||||
| n (%) | 2 (1.4) | 5 (3.5) | ||
| Missing data | 1 (0.7) | 1 (0.7) | ||
| Formal development assessment carried out (if yes) | ||||
| n (%) | 1 (50.0) | 4 (80.0) | ||
| Missing data | 1 (50.0) | 1 (20.0) | ||
| Overall development delay (if yes), n (%) | ||||
| Mild | 0 (0) | 3 (75.0) | ||
| Moderate | 1 (50.0) | 0 (0) | ||
| Severe | 0 (0) | 0 (0) | ||
| Missing data | 1 (50.0) | 1 (25.0) | ||
Respiratory problems at 2 years were reported in 132 (29.7%) and 111 (25.0%) of infants born in the NRT and placebo groups, respectively (OR 1.30, 95% CI 0.97 to 1.74, p = 0.083) (see Table 12). As with the development questions, more respiratory problems were reported on participant-completed questionnaires [194 (32.2%) of returned PQ2s compared with 49 (17.0%) of HPQs included reports of respiratory symptoms]. A more detailed breakdown of singleton infants’ respiratory problems is given in Table 15, including outcomes that were collected 1 year after delivery. The only significant difference was in the number of participants reporting at 2 years that their child had ever experienced any wheeze or whistling in their chest [74 (24.6%) NRT, 49 (16.3%) placebo (OR 1.72, 95% CI 1.14 to 2.59, p = 0.0099)].
| Infant respiratory outcomes (singleton pregnancies)a | NRT (n = 503) | Placebo (n = 507) | OR (95% CI)b | p-value | Adjusted OR (95% CI)c | p-value |
|---|---|---|---|---|---|---|
| 1 year after deliveryd | ||||||
| Number (%) of respondents | 288 (57.3) | 295 (58.2) | ||||
| Wheeze or whistling | ||||||
| n (%) | 62 (21.5) | 57 (19.3) | 1.18 (0.78 to 1.77) | 0.4356 | 1.00 (0.65 to 1.55) | 0.9820 |
| Missing data | 0 (0) | 1 (0.3) | ||||
| If yes, how many attacks in last year? n (%) | ||||||
| 0 | 6 (9.7) | 8 (14.0) | ||||
| 1–3 | 41 (66.1) | 33 (57.9) | ||||
| 4–12 | 12 (19.4) | 10 (17.5) | ||||
| > 12 | 1 (1.6) | 4 (7.0) | ||||
| Missing data | 2 (3.2) | 2 (3.5) | ||||
| How often has sleep been disturbed due to wheezing? n (%) | ||||||
| Never | 38 (61.3) | 38 (66.7) | ||||
| < 1 night/week | 14 (22.6) | 12 (21.1) | ||||
| ≥ 1 night/week | 7 (11.3) | 5 (8.8) | ||||
| Missing data | 3 (4.8) | 2 (3.5) | ||||
| Doctor diagnosed asthma, n (%)e | 13 (4.5) | 15 (5.1) | 0.95 (0.44 to 2.06) | 0.9005 | 0.97 (0.42 to 2.24) | 0.9421 |
| Dry cough at night | ||||||
| n (%) | 27 (9.4) | 20 (6.8) | 1.46 (0.79 to 2.69) | 0.2219 | 1.26 (0.65 to 2.42) | 0.4906 |
| Missing data | 1 (0.4) | 1 (0.3) | ||||
| Seen by paediatrician or chest specialist about chest or breathing problems | ||||||
| n (%) | 28 (9.7) | 28 (9.5) | 1.06 (0.61 to 1.86) | 0.8263 | 0.99 (0.55 to 1.78) | 0.9680 |
| Missing data | 1 (0.4) | 1 (0.3) | ||||
| 2 years after delivery (maternal questionnaire: PQ2) | ||||||
| Number (%) of respondents | 301 (59.8) | 301 (59.4) | ||||
| Respiratory related hospital admissions | ||||||
| n (%) | 39 (13.0) | 34 (11.3) | 1.22 (0.74 to 2.01) | 0.4340 | 1.47 (0.85 to 2.53) | 0.1615 |
| Missing data | 0 (0) | 1 (0.3) | ||||
| Problems with chest or breathing | ||||||
| n (%) | 53 (17.6) | 48 (16.0) | 1.21 (0.78 to 1.88) | 0.3881 | 1.22 (0.76 to 1.95) | 0.4079 |
| Missing data | 1 (0.3) | 1 (0.3) | ||||
| Wheeze or whistling | ||||||
| n (%) | 74 (24.6) | 49 (16.3) | 1.72 (1.14 to 2.59) | 0.0087 | 1.79 (1.14 to 2.79) | 0.0099 |
| Missing data | 0 (0) | 2 (0.7) | ||||
| If yes, how frequently? n (%) | ||||||
| Every day | 5 (6.8) | 3 (6.1) | ||||
| Every week | 10 (13.5) | 3 (6.1) | ||||
| ≤ once/month | 58 (78.4) | 42 (85.7) | ||||
| Missing data | 1 (1.4) | 1 (2.0) | ||||
| Doctor diagnosed asthmae | ||||||
| n (%) | 31 (10.3) | 19 (6.3) | 1.79 (0.98 to 3.28) | 0.0547 | 1.74 (0.91 to 3.32) | 0.0889 |
| Missing data | 0 (0) | 1 (0.3) | ||||
| Medicines taken for cough/wheeze/chest problems | ||||||
| n (%) | 51 (16.9) | 39 (13.0) | 1.43 (0.90 to 2.27) | 0.1231 | 1.42 (0.86 to 2.32) | 0.1653 |
| Missing data | 0 (0) | 2 (0.7) | ||||
| 2 years after delivery (HPQ)f | ||||||
| Number (%) of respondents | 144 (28.6) | 145 (28.6) | ||||
| Problems with chest or breathing | ||||||
| n (%) | 26 (18.1) | 23 (15.9) | 1.04 (0.57 to 1.89) | 0.8953 | 1.05 (0.56 to 1.98) | 0.8730 |
| Missing data | 1 (0.7) | 1 (0.7) | ||||
Maternal smoking outcomes after delivery
After delivery, both point prevalence and prolonged abstinence from smoking was low and relapse to smoking gradually increased (Table 16). During the 2-year follow-up, the numbers of participants in the NRT and placebo groups reporting abstinence from smoking since their quit dates were 28 (5.4%) and 17 (3.2%), respectively, at 6 months, and 19 (3.7%) and 11 (2.1%), respectively, at 1 year. By 2 years after delivery, 15 (2.9%) allocated to NRT and nine (1.7%) allocated to placebo remained abstinent (OR 1.71, 95% CI 0.74 to 3.94, p = 0.20). Table 17 shows that participants who did not provide any smoking data at the three postnatal follow-up time points were more likely to have been smokers at delivery, which is consistent with the assumption used in analyses of smoking behaviour, i.e. that participants who were lost to follow-up should be counted as smokers.
| Maternal smoking outcomesa | NRT (n = 521)a | Placebo (n = 529)a | OR (95% CI)b | p-value | Adjusted OR (95% CI)c | p-value |
|---|---|---|---|---|---|---|
| 6 months after delivery | ||||||
| Number (%) of respondents | 335 (64.3) | 338 (63.9) | ||||
| Self-reported prolonged abstinence since delivery, n (%)d | 57 (10.9) | 50 (9.5) | 1.18 (0.79 to 1.76) | 0.4254 | 1.23 (0.81 to 1.87) | 0.3251 |
| Self-reported 7-day cessation, n (%) | 56 (10.8) | 52 (9.8) | 1.11 (0.74 to 1.65) | 0.6228 | 1.15 (0.76 to 1.74) | 0.5032 |
| Prolonged abstinence from smoking between quit date and 6 months after delivery, n (%)e | 28 (5.4) | 17 (3.2) | 1.71 (0.92 to 3.17) | 0.0836 | 1.84 (0.98 to 3.46) | 0.0547 |
| 1 year after delivery | ||||||
| Number (%) of respondents | 288 (55.3) | 300 (56.7) | ||||
| Self-reported prolonged abstinence since delivery, n (%)d,f | 33 (6.3) | 29 (5.5) | 1.16 (0.70 to 1.95) | 0.5640 | 1.18 (0.69 to 2.04) | 0.5437 |
| Self-reported 7-day cessation, n (%)f | 55 (10.6) | 37 (7.0) | 1.57 (1.01 to 2.43) | 0.0413 | 1.55 (0.98 to 2.46) | 0.0574 |
| Prolonged abstinence from smoking between quit date and 1 year after delivery, n (%)e,f | 19 (3.7) | 11 (2.1) | 1.78 (0.84 to 3.78) | 0.1273 | 2.20 (0.98 to 4.92) | 0.0475 |
| 2 years after delivery | ||||||
| Number (%) of respondents (PQ2 only)g | 302 (58.0) | 304 (57.5) | ||||
| Self-reported prolonged abstinence since delivery, n (%)d | 23 (4.4) | 21 (4.0) | 1.11 (0.61 to 2.04) | 0.7274 | 1.03 (0.53 to 1.98) | 0.9409 |
| Self-reported 7-day cessation, n (%) | 45 (8.6) | 43 (8.1) | 1.06 (0.69 to 1.65) | 0.7789 | 0.98 (0.62 to 1.56) | 0.9483 |
| Prolonged abstinence from smoking between quit date and 2 years after delivery, n (%)e | 15 (2.9) | 9 (1.7) | 1.71 (0.74 to 3.94) | 0.2036 | 1.96 (0.82 to 4.70) | 0.1204 |
| Smoking status at delivery | Smoking data reporteda | No smoking data reportedb | p-valuec |
|---|---|---|---|
| 6-month follow-up | N = 656 | N = 325 | |
| Smoking at delivery, n (%) | 579 (88.3) | 313 (96.3) | < 0.001 |
| Abstinent at delivery, n (%) | 77 (11.7) | 12 (3.7) | |
| 1-year follow-up | N = 571 | N = 410 | |
| Smoking at delivery, n (%) | 511 (89.5) | 381 (92.9) | 0.065 |
| Abstinent at delivery, n (%) | 60 (10.5) | 29 (7.1) | |
| 2-year follow-up | N = 589 | N = 392 | |
| Smoking at delivery, n (%) | 523 (88.8) | 369 (94.1) | 0.004 |
| Abstinent at delivery, n (%) | 66 (11.2) | 23 (5.9) |
Additionally, the sensitivity analysis that further investigated this assumption found that varying the relationship between missingness and smoking status had almost no impact on ORs that compared smoking cessation rates between trial groups at 2 years. Varying the IMOR between 0 and 1 (in an unadjusted analysis) altered the OR for the effect of treatment group on self-reported prolonged abstinence since delivery from 1.117 (IMOR = 0) to 1.107 (IMOR = 1), the OR for self-reported 7-day cessation from 1.068 to 1.059, and the OR for prolonged abstinence from smoking between quit date and 2 years after delivery from 1.713 to 1.707. This provides added reassurance that treating those with missing outcome data as smokers had no substantial impact on the study findings for smoking outcomes.
Secondary analysis: adherence with nicotine patches and impairment
Data on the presence or absence of impairment at 2 years of age were available for 884 singleton infants and this was the sample size for analysis. Reported adherence with nicotine patches was heavily skewed and skewness was increased by coding placebo group participants’ adherence with nicotine patches as zero. We analysed adherence with nicotine-containing patches in three categories: 0 days’ use and, for participants reporting at least use of one patch (1 day’s use), we used a median split within these participants (median = 10 days’ use) to create two categories of ‘up to median use’ (1–10 days) and ‘above median use’ (11–56 days). Table 18 shows the numbers in each adherence category, the distribution of survival with no impairment within these categories and crude and adjusted OR for the association between adherence categories and survival with no impairment, using 0 days’ adherence as a reference category. Adjustment was made for partner’s smoking status, which was found to be associated with outcome in the multivariable model. Results in Table 18 suggest that participants in the highest category of adherence with nicotine-containing patches were more likely to have infants who had survived without impairments (adjusted OR 1.72, 95% CI 1.22 to 2.57).
| Reported number of days on which nicotine patches used (adherence category) | Number (%) participants in adherence category (N = 884a) | Number (%) infants from each adherence category that survived with no impairment (N = 613) | Crude OR (95% CI) | Adjusted OR (95% CI)b |
|---|---|---|---|---|
| 0 | 474 (53.6) | 313 (66.0) | 1.00 (not estimable) | 1.00 (not estimable) |
| 1–10 (≤ median) | 197 (22.3) | 136 (69.0) | 1.15 (0.80 to 1.64) | 1.12 (0.78 to 1.60) |
| 11–56 (> median) | 213 (24.1) | 164 (77.0) | 1.72 (1.19 to 2.50) | 1.77 (1.22 to 2.57) |
Chapter 4 Health economics analysis report
Introduction
The cost-effectiveness of NRT use by the general population has been established57,58 and a number of studies have investigated the potential cost saving of smoking cessation interventions in pregnancy,59 but few have used empirical data on costs to calculate the incremental cost-effectiveness of smoking interventions. 60
This chapter reports on an economic evaluation conducted alongside the SNAP trial, addressing the value for money and cost-effectiveness of NRT patches and behavioural support compared with behavioural support alone.
The objectives were:
-
to compare the costs associated with the control and intervention strategies
-
to estimate the consequences of these alternatives
-
to assess cost-effectiveness of NRT patches used in addition to behavioural support on smoking cessation at delivery
-
to use EQ-5D data collected at 6 months after delivery to model longer-term cost–utility of NRT used for smoking cessation in pregnancy
-
to explore the potential for providing monetary estimates of the long-term impacts on the child of their differential birth outcomes.
Methods
Overview
A cost-effectiveness analysis was undertaken to compare NRT patches and behavioural support to behavioural support only, for women who were smoking during pregnancy. The main outcome for the economic evaluation was biochemically validated abstinence from smoking between a quit date and delivery. As recommended by the National Institute for Health and Care Excellence (NICE),61 analyses were conducted from a NHS and personal-social services viewpoint, including direct health effects (maternal smoking cessation) and costs (or cost savings) to the NHS. Mothers were eligible for inclusion in the SNAP trial if they were between 12 and 24 weeks’ gestation and outcomes were collected at delivery (up to 42 weeks’ gestation); therefore, the time horizon of the trial was up to 7 months.
Cost estimation
There were two main components to the costing of the control and intervention strategies: first, the costs of the inputs required for the interventions and second, the resources used to care for each woman and her infant during the period between randomisation and delivery.
Intervention costs
The cost inputs for the interventions included training and staff time to deliver the behavioural support (band 7 midwives), CO monitors (breath testing equipment), consumables (NRT patches and consumables associated with CO breath testing) and overheads for premises.
Training costs were calculated for 2 days of training for 10 midwives (salary costs at a mid-point of band 7), provided by an NHS SSS advisor (salary costs an average of band 5 and 6) at an NHS SSS, including overheads62 and the cost of 1060 15-page manuals that were used both to guide intervention delivery and as information for participants.
At baseline, participants were given a 4-week supply of NRT patches at a dose of 15 mg per 16 hours. Participants in the placebo arm were given identical patches, without the active ingredients; however, these placebo patches represent a research cost and were therefore excluded from the costing. The placebo group will therefore be referred to as the control group throughout Chapter 4. At the baseline hospital antenatal visit, midwives also provided up to 1 hour of behavioural support. The time midwives spent providing face-to-face behavioural support was recorded and multiplied by salary and overhead costs to calculate a cost per session. All women provided a CO reading at baseline.
Three sessions of behavioural support were provided over the telephone on the quit date and at 3 days and 1 month after this. For each woman, successful calls were logged and these varied in length and although call times were not recorded, call lengths were estimated by a trial midwife to be the following: 2 minutes for the quit date call, 3.5 minutes for the call 3 days after the quit date, 4 minutes for the call 1 month after the quit date to self-reported smokers and 2 minutes for 1 month calls to self-reported non-smokers. The call for self-reported non-smokers was shorter as, during this, midwives would arrange a home visit to validate smoking cessation and provide face-to-face behavioural support. Midwives would try to contact participants several times if they did not get through; therefore, the cost of failed call attempts was also estimated. Failed calls were assumed to be 30 seconds per call attempt, with three call attempts per woman who did not speak to the midwife on the appropriate day, and for each successful call we also assumed a 30 second failed call attempt.
Women who self-reported not smoking at 1 month after the quit date were visited at home to have a session of behavioural support and for CO validation. Women biochemically verified as abstinent at 1 month were offered a second 4-week supply of patches. At delivery, midwives used CO monitors to verify smoking status, which took approximately 10 minutes of midwife time.
To calculate the costs of CO monitoring per use at baseline, 1 month and delivery, the costs of the equipment and associated consumables were totalled and divided by the total number of uses. The cost of four CO monitors was not depreciated as the life expectancy was estimated to be around 5 years (the length of the trial). One calibration kit was required for every two monitors. Based on usage evidence from the trial we assumed semidisposable mouthpiece adaptors were changed after 60 uses, batteries were changed every 240 uses and one disposable mouth piece and alcohol-free wipe was required for each use.
Resource-use costs
Resource-use data were collected from trial participants and from medical records. We collected data from participants on their use of NHS SSS (either face to face or by telephone). Information about antenatal hospital admissions and mode of delivery was collected from maternal medical records and data on admissions to neonatal special care came from infant medical records.
Valuation of costs
All data were valued in monetary terms and unit costs were reported in pounds sterling for the financial year 2009–10 (representing the mid-point of the trial). Any costs occurring in prior or later price years were inflated or deflated using the Hospital and Community Health Services pay and prices index. 63 As for the economic evaluation, we considered trial follow-up until to 7 months post randomisation only and no discounting was required. For standard NHS health care, UK unit costs were applied from national sources, increasing the generalisability of the results. Table 19 presents a summary of resource use and unit costs, with the calculation of the costs detailed in Calculating costs.
| Unit cost (£) | Unit | Source of unit cost | |
|---|---|---|---|
| Interventions | |||
| 15 mg per 16 hours NRT patches | 1.28 | Patch | The Health and Social Care Information Centre64 |
| Dispensing cost | 2.14 | Prescription | www.psnc.org.uk/pages/archive.html 65 |
| Band 7 midwife time (including overheads) | 35.28 | Per hour | The NHS Staff Council,66 Curtis63 |
| Antenatal midwife home visit | 45.00 | Visit | Department of Health67 |
| CO monitors and consumables | 0.47 | Per use | Estimated from the SNAP trial |
| Printing | 0.96 | 15-page manual | Estimated from the SNAP trial |
| Resource use | |||
| NHS SSS | |||
| Face to face (individual or group session) | 11.69 | Session | NICE62 |
| Telephone call (4-minute call) | 1.27 | Call | NICE62 |
| Text message | 0.16 | Message | NHS Connecting for Health68 |
| Maternal antenatal admission | 1180.48 | Admission | Department of Health67 |
| Mode of delivery | |||
| Unassisted vaginal delivery | 1454.28 | Obstetric delivery | Department of Health67 |
| Assisted vaginal delivery | 2095.06 | ||
| Caesarean section | 3028.66 | ||
| Baby admission to neonatal unit | 7532.31 | Admission | Department of Health,67 The Health and Social Care Information Centre69 |
Calculating costs
In order to calculate the costs of face-to-face NHS SSS sessions, a weighted average cost of individual and group sessions was calculated based on information from a NICE costing report. 62 Salary and overheads information from the same report were used to calculate the cost per minute of a phone call (assumed to be the same length as the calls reported for providing behavioural support).
The cost of mode of delivery was established by calculating a weighted average of unit costs of the different modes of delivery activities recorded in NHS reference costs. A similar method was used to calculate an average cost of a maternal antenatal admission, based on antenatal observations and investigations.
To calculate the cost of the admission of a baby to neonatal care, a weighted average of bed-day costs for neonatal critical care from NHS reference costs (£618) was multiplied by a weighted average length of stay for neonates with major diagnoses (12.2 days) according to Hospital Episode Statistics for 2009–10. 69
Quantities of services used were multiplied by the relevant unit costs to estimate overall cost profiles for women in the trial.
Outcome measures
The measure of health benefit for the economic evaluation was the same as the primary measure of clinical effectiveness in the SNAP trial: self-reported and biochemically validated maternal smoking cessation from quit date to immediately before delivery. Temporary smoking lapses of up to a total of five cigarettes (on up to five occasions) were permitted.
The National Institute of Health and Care Excellence (2008)61 recommend that health outcomes should be measured in quality-adjusted life-years (QALYs) to facilitate comparisons between different health-care programmes. However, QALYs, commonly calculated from the generic health-related quality-of-life tool the EQ-5D,70 may be inappropriate in a study including pregnant women. Generic quality of life studies have shown poorer quality-of-life in early pregnancy compared with population norms for women of child-bearing age71 and substantial changes in quality of life, particularly declining physical functioning and vitality, occur over the course of pregnancy. 72 These dramatic changes in quality of life between pregnancy and the post-partum period would likely mask any potential short-term quality-of-life gains from smoking cessation. Nevertheless, EQ-5D data were collected at 6 months after delivery, within the postal questionnaire sent to participants, which is described in Chapter 2, Methods.
Analysis
An incremental cost-effectiveness analysis was undertaken, following the NICE guidance for health-care evaluations,61 comparing the additional costs of NRT patches with behavioural support alone, as well as the additional benefits, to give a cost per additional quitter.
The incremental cost-effectiveness ratio (ICER) calculates the mean cost of the intervention group over and above the control and divides by the mean difference in health benefits. The following formula is for ICER, for which Δ represents change, C represents the costs, E represents the effects and subscript I and C refer to the intervention and control, respectively.
All analyses were conducted on an ITT basis in which all randomised participants were included and analysed in the groups to which they were randomised. Analyses were conducted in Microsoft Excel, 2010, version 14.0.7113.5005 (32-bit) (Microsoft Corporation, Redmond, WA, USA).
There were no missing data on the effectiveness outcome (validated smoking cessation from quit date to delivery), as any women without validated cessation were assumed to be smokers. Missing data for cost items were imputed using average costs for the appropriate arm of the trial, allowing the base-case analysis to be completed for all women in the trial and, therefore, with the same quit rate as the main effectiveness analyses in Chapter 3.
Cost data were bootstrapped to account for skewness, sampling with replacement observations 1000 times to generate a new population of sample means with an approximate normal distribution. Bootstrap results were presented graphically using a cost-effectiveness plane73 to show the uncertainty around the mean estimates of incremental costs and effects.
The EQ-5D data collected at 6 months after delivery were converted into a single index summary score after applying UK population values. 74 Descriptive statistics for EQ-5D data are presented by trial group. We planned to use 6-month EQ-5D data in conjunction with smoking status utility values based on quit rates at 2 years after delivery to calculate the longer-term costs and benefits of differential quit rates between the intervention and the control arms.
Results
A total of 1050 women were recruited to the SNAP trial: 521 and 529 in the NRT and placebo arms, respectively.
Costs
The breakdown of intervention costs for the trial arms are presented in Table 20. Costs for providing behavioural support and CO monitoring were relatively equal in both arms, although costs of the 4-week home visits were slightly higher in the NRT group than the control group (£14.31 compared with £9.46, respectively) because rates of self-reported smoking cessation were higher in the NRT group at 4 weeks (25.1% compared with 14.0%). The mean total intervention cost in the control group was £47.75. The comparative cost of providing behavioural support and CO monitoring was £52.24 in the NRT group, and the total mean cost including NRT patches was £98.31.
| Intervention | NRT group, n = 521, mean (SD) |
Control, n = 529, mean (SD) |
|---|---|---|
| Training cost (per face-to-face session) | ||
| Training costs for midwives (per hospital behavioural support session) | 4.18 | 4.18 |
| Treatment cost (per participant) | ||
| NRT patches | 46.07 (15.57) | 0.00 |
| Behavioural support session at hospital (including CO monitoring) | 21.72 (4.18) | 22.00 (4.62) |
| Telephone callsa | ||
| Calls on quit date | 1.30 (0.27) | 1.30 (0.27) |
| Calls on quit date + 3 days | 1.97 (0.64) | 1.94 (0.66) |
| Calls on quit date + 1 month (self-reported smokers) | 1.78 (0.88) | 1.91 (0.87) |
| Calls on quit date + 1 month (self-reported non-smokers) | 1.07 (0.27) | 1.00 (0.24) |
| Home visit at 4 weeks for behavioural support for self-reported non-smokers (including CO monitoring) | 14.31 (21.14) | 9.46 (18.47) |
| 10 minutes to monitor CO levels at delivery | 5.92 (1.61) | 5.96 (1.54) |
| Average intervention costs (per participant) | 98.31 (35.21) | 47.75 (19.03) |
Table 21 reports the resources utilised in the two arms of the trial. Use of NHS SSS, maternal antenatal hospital admissions and admissions to neonatal care were similar in the two groups, although more women had a caesarean section in the NRT group than in the control group: 20.9% and 16.1%, respectively.
| NRT, N = 521 | Placebo, N = 529 | |
|---|---|---|
| NHS SSS | ||
| Missing data, mean n (%) | 36 (6.9) | 33 (6.2) |
| Face-to-face session, mean n (SD) | 0.18 (0.6) | 0.16 (0.6) |
| Telephone call, mean n (SD) | 2.06 (2.3) | 1.84 (2.0) |
| Text message, mean n (SD) | 0.80 (1.8) | 0.71 (1.7) |
| Maternal antenatal hospital admission | ||
| Missing data, mean n (%) | 10 (1.9) | 12 (2.3) |
| Admissions, mean n (%) | 79 (15.2) | 82 (15.5) |
| Mode of birth | ||
| Missing data, mean n (%) | 10 (1.9) | 14 (2.7) |
| Unassisted vaginal birth, mean n (%) | 362 (69.5) | 386 (73.0) |
| Assisted vaginal birth, mean n (%) | 40 (7.7) | 44 (8.3) |
| Caesarean section, mean n (%) | 109 (20.9) | 85 (16.1) |
| Baby admitted to neonatal unit | ||
| Missing data, mean n (%) | 10 (1.9) | 14 (2.7) |
| Admissions,a mean n (%) | 37 (7.1) | 39 (7.3) |
Quantities of services used were multiplied by the relevant unit costs in Table 19 to calculate the cost of resources used for each woman and total costs were calculated by adding resource-use costs to intervention costs. Table 22 summarises total mean costs for the trial groups. Mean intervention costs were significantly higher in the NRT group, at a mean difference of £50.56. Total mean resource-use costs were £40.26 higher in the NRT group and overall costs were, therefore, £90.81 higher for this group; however, these differences were not statistically significant.
| Type of cost | NRT group, n = 521, mean (SD) | Control group, n = 529, mean (SD) | Differencea (95% CI) |
|---|---|---|---|
| Intervention costs | £98.31 (35.21) | £47.75 (19.03) | £50.56 (47.13 to 53.99) |
| Resource-use costs | £2571.56 (2393.63) | £2531.31 (2384.34) | £40.26 (–248.76 to 329.27) |
| Total costs | £2669.87 (2394.09) | £2579.06 (2385.68) | £90.81 (–198.31 to 379.94) |
Outcomes
Any women without biochemical validation of quitting were assumed to be smokers. In the NRT group, 49 out of 521 women (9.4%) were abstinent with biochemical validation from quit date to delivery and in the control group this was true for 40 out of the 529 women (7.6%), a non-significant difference of 1.8% (see Chapter 3).
Cost-effectiveness analysis and uncertainty
Table 23 presents the ICER, combining the differential costs of the treatment groups, with the differential quit rates. NRT plus behavioural support was found to be somewhat more costly than behavioural support alone, but with a slightly higher quit rate. This generates an ICER of £4926 per quitter. If decision-makers are willing to pay > £4926 for an additional quitter, then NRT would be the preferred option; otherwise, behavioural support alone should be adopted.
| NRT group, n = 521 | Control group, n = 529 | |
|---|---|---|
| Cost (SD) | £2669.87 (£2394.09) | £2579.06 (£2385.68) |
| Quit rate | 9.4% | 7.6% |
| ICER | £4926 per quitter | |
| Bootstrapped 95% CI of ICER | –£114,128 to £126,747 | |
A cost-effectiveness scatter plot was produced based on the bootstrapping, including 1000 resamples of the costs and effects data. The bootstrapped results were plotted on a cost-effectiveness plane, visually displaying the uncertainty around the mean differences in costs and benefits between the trial arms (Figure 9). The majority of the plots in the scatter plot fall in the north-east quadrant, indicating that NRT is likely to be more effective but more costly than behavioural support alone. However, the scatter plot also shows the uncertainty around the cost estimates.
FIGURE 9.
Cost-effectiveness plane.
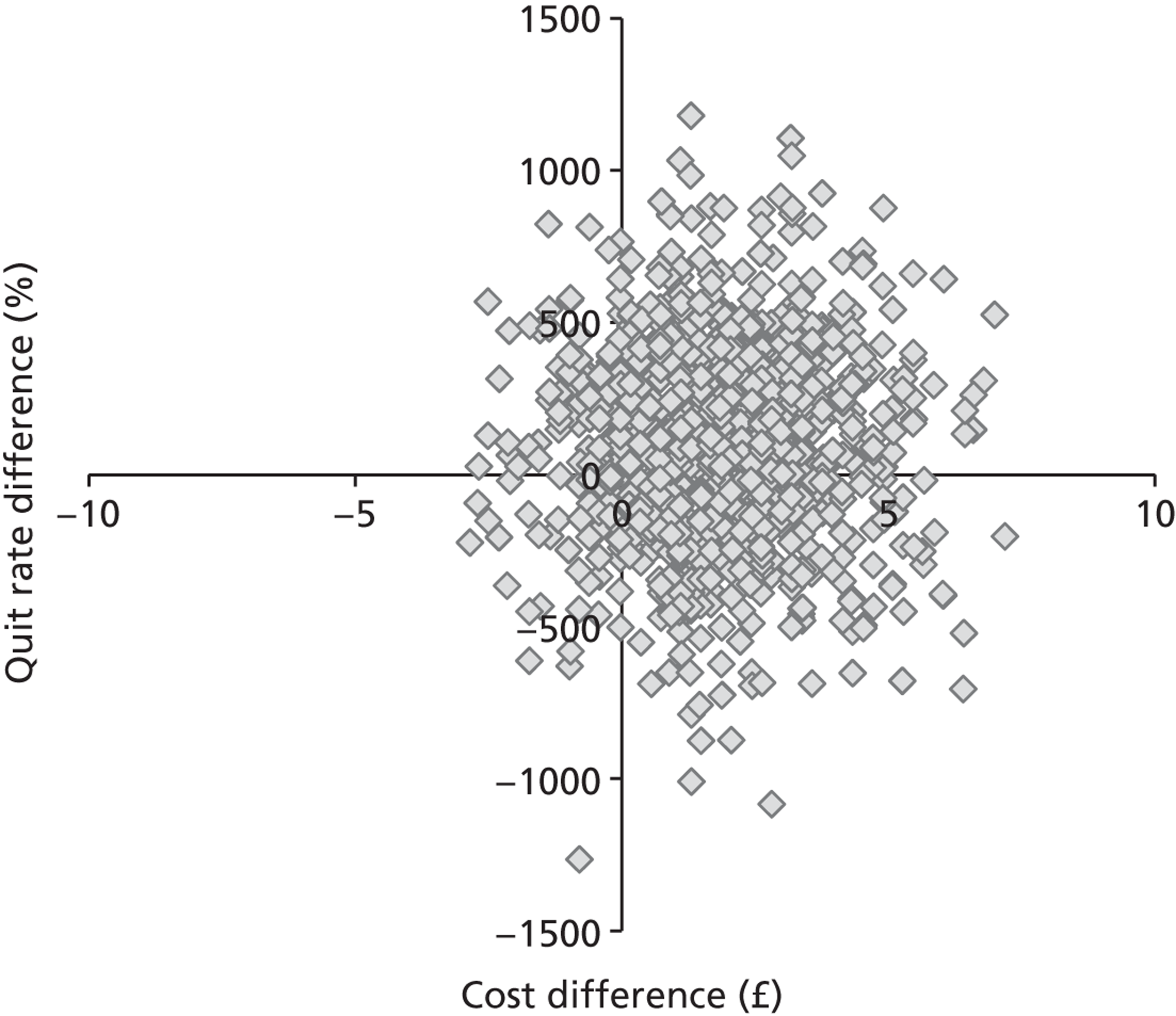
Sensitivity analyses
To allow for uncertainty in our estimates of costs and consequences, we conducted a sensitivity analysis excluding 12 women who had multiple births (four twin births in the NRT group and eight in the control group) who are more likely to have complicated pregnancies and deliveries. Table 24 shows the total costs and quit rates in the singleton-only analysis. Quit rates were very similar (+ 0.08% in the NRT group and –0.46% in the control group); total costs were lower in the singleton-only analysis, but by a similar amount in both groups (–£64.37 in the NRT group and –£72.32 in the control group). The corresponding ICER generated for singleton births was £4156 per quitter.
| NRT group, n = 517 | Control group, n = 521 | |
|---|---|---|
| Cost (SD) | £2604.73 (£2198.98) | £2505.97 (£2184.43) |
| Quit rate | 9.48% | 7.10% |
| ICER | £4156 | |
| Bootstrapped 95% CI of ICER | (–£65,994 to £82,059) | |
Projecting longer-term costs and benefits (objectives 4 and 5)
We had planned to use EQ-5D data collected 6 months after delivery in conjunction with smoking status utility values based on quit rates at 2 years to calculate the longer-term costs and benefits of differential quit rates between the intervention and control arms. These longer-term analyses were impeded for a number of reasons. First, there was only a small, non-significant difference in quit rates between groups. Second, there was no difference in EQ-5D scores between the groups at 6 months (Table 25). Third, there was great uncertainty in cost estimates in the alongside trial economic analysis, which would be amplified in any longer-term projections, generating non-robust estimates. Fourth, there were no significant differences in birth outcomes, precluding monetary estimates of the long-term impacts on the child.
| NRT group, n = 335 | Control group, n = 338 | |
|---|---|---|
| Missing data | 3 | 11 |
| Mean (95% CI) | 0.896 (0.875 to 0.917) | 0.894 (0.873 to 0.916) |
Summary
-
Total mean costs were £90.81 higher in the NRT group, representing around a 3% difference in costs between trial groups. The higher costs in the NRT group were mainly attributable to the cost of the NRT patches (mean = £46.07).
-
The ICER associated with NRT patch use was estimated at £4926 per quitter. Sensitivity analyses including only singleton births resulted in an ICER of £4156 per quitter; however, there were very wide CIs around these estimates, indicating a high level of uncertainty. This uncertainty occurred because there were only small differences in total costs between the groups, but the total cost for each group was affected by high within-group variability, which was particularly influenced by costs attributable to antenatal or neonatal admissions. For example, in both groups, approximately 7% of babies were admitted to neonatal care and each had an admission cost of £7532, compared with an average between-group difference in costs of only £91. Therefore, as between-group cost differences are small, if women with these high resource-use costs happen to fall in one arm by chance, this could change the result of the ICER.
-
If decision-makers are willing to pay > £4156 for an additional quitter, then NRT should be adopted; otherwise, behavioural support alone should be adopted.
Chapter 5 Discussion
Principal findings
Smoking outcomes
This trial demonstrates that, at 12–24 weeks’ gestation, supplementing behavioural support with a 15 mg per 16 hours nicotine patch was no more effective than a placebo in promoting sustained smoking cessation throughout pregnancy. Clinically and statistically significant higher biochemically validated cessation rates were obtained with NRT at 1 month, but this effect did not persist into later pregnancy. After childbirth, self-reported, prolonged cessation since quit dates agreed in pregnancy were between 1% and 2% higher in women who had been randomised to NRT, but these small differences were not statistically significant.
Maternal and fetal birth outcomes
Maternal and fetal birth outcomes were generally very similar with almost no statistically significant differences between groups. Caesarean section births were more frequent in the NRT group and this difference was statistically significant (OR 1.45, 95% CI 1.05 to 2.01).
Infant outcomes at 2 years
At 2 years of age, singleton infants born to trial participants who had been allocated NRT were significantly more likely to have no developmental impairment than those born to participants in the placebo group. Very similar findings occurred whether using data obtained from only participant-completed questionnaires (PQ2) or data from both PQ2 and HPQ questionnaires combined. Additionally, very similar findings were noted in pre-specified analyses, which (1) included twin births and adjusted for clustering of outcomes and (2) applied multiple imputation methods to investigate the assumption that data missing were missing at random (i.e. that missingness was associated with baseline characteristics but not with the outcome itself). No significant difference in reported rates of infants’ respiratory problems was noted.
Economic outcomes
Total mean costs were £90.81 higher in the NRT group, with the excess largely attributable to the cost of NRT patches (mean cost of patches = £46.07). For singleton births only, an ICER of £4156 per quitter was derived; therefore, if decision-makers are willing to pay this amount for each additional quitter, then NRT, used in addition to behavioural support, would be the preferred option. The results showed no differences between groups in birth outcomes or health status as measured by EQ-5D at 6 months postnatal. Therefore, it was not possible to estimate a cost per QALY or model long-term QALYs.
There has been little research investigating the cost-effectiveness of smoking cessation interventions in pregnancy. Although a number of studies have investigated the potential cost saving of smoking interventions for pregnant women,59 these studies have mainly been conducted in the USA with restricted perspectives, particularly omitting data relating to infant outcomes. Only two studies have thus far reported QALYs: one being a simple model based on an American trial60 and another a hypothetical model constructed for NICE guidance. 75 Although these studies suggest that smoking cessation in pregnancy may be cost-effective, they are mainly poor quality and the settings and methods used preclude comparison with our results. Furthermore, with the exception of the NICE model,75 none of the studies has explored the cost-effectiveness of NRT.
Adherence
Adherence to both types of patch was low. Although adherence was not a trial outcome, the pattern of adherence may be related to, and at least partially explain, smoking cessation and birth outcomes and, consequently, requires discussion.
Limitations and strengths
Overall comments
This study is by far the largest of its type. Prior to the SNAP trial being completed, in the five previous RCTs of NRT for smoking cessation in pregnancy, only 695 women had been randomised. 30 We believe that this is the first trial to test whether or not a smoking cessation intervention delivered in pregnancy can affect infant outcomes. The study is also original in that in previous trials of NRT in pregnancy, maternal smoking behaviour has been investigated for only up to 3 months after childbirth,34,35 whereas we followed up participants and infants until 2 years after birth. We can find only one trial testing any smoking cessation intervention (i.e. a non-NRT intervention) for pregnant women that attempted to monitor smoking behaviour up to and beyond 2 years after childbirth. 76 However, in this study, smoking data were not sought from all trial participants at predetermined time points, but was obtained opportunistically at multiple, different times between 8 and 54 months after childbirth, rendering smoking behaviour data difficult to interpret.
Outcomes recorded at and before delivery
By carefully implementing a double-blind, placebo-RCT design, most of the biases that could have influenced outcomes at these time points have been minimised. However, we did not ask women which treatment they perceived they had been allocated to and hence have no data to confirm whether participants remained blinded to their treatment allocations or, indeed, guessed their allocation. Consequently, it is possible that some participants may have correctly determined their treatment allocation and, if this had occurred, we would be unable to quantify its extent. However, unblinded trials of NRT in pregnancy tend to overestimate the treatment effect from NRT30 and we found no effect at delivery. Additionally, loss of blinding among trial participants would not explain the very different pattern of findings with respect to efficacy at 1 month and delivery.
The strengths of the study appear to outweigh its weaknesses and overall findings reported are likely to be valid. Target sample size was achieved; therefore, the study was adequately powered to detect the anticipated 9% difference in cessation rates between trial arms. However, it remains possible that NRT could work for smoking cessation in pregnancy, but with a lesser impact because smaller treatment effects (i.e. < 9% absolute difference) would not necessarily have been detected by a trial of this size. At baseline, groups were well balanced for all variables recorded, including those with potential to influence findings; therefore, chance differences between groups are unlikely to explain these. Additionally, the high outcome ascertainment rates, which were very similar in both groups, reduce the likelihood that ascertainment bias influenced study outcomes. Birth outcome ascertainment rates were particularly high and, as RMs who extracted data on birth outcomes and AEs from medical records were blinded to patch allocation, these data are particularly likely to be free from bias. Similarly, when participants reported abstinence from smoking, equally high biochemical verification rates were obtained from participants in both trial groups, minimising any bias in the reporting of outcomes that may have occurred.
The validity of trial findings could have been affected by unintentional variation in treatments provided for participants, or in any support or treatment sought by them that was additional to trial interventions. For example, differences between NRT and placebo group outcomes would have been minimised if trial participants had also been prescribed NRT from their GPs, issued with it by local NHS SSSs or had bought it from pharmacies. However, only 2.2% in the placebo group (and 2.5% allocated NRT) reported using NRT obtained outside of the trial for more than 20 days and it seems unlikely that this level of usage, which was very similar in both trial arms, would have unduly affected findings. Finally, if participants were inadequately prepared for, or inadequately instructed about using, NRT, then this could have affected their ability to use NRT sufficiently well for any impacts of this treatment to become apparent. However, the trial RMs, trained to English national cessation standards, provided accompanying behavioural support and, in both trial groups, participants’ rates of accessing subsequent additional support were similarly low. This low level of additional support may have affected overall quit rates. However, the level of behavioural support was comparable with that used in ‘low intensity support’ nicotine patch trials conducted among non-pregnant subjects in which NRT has been found effective (RR 1.78, 95% CI 1.49 to 2.12),18 for which NRT caused a near doubling of cessation at the 1-month follow-up point. Taken together, these points suggest that the behavioural support delivered in the trial probably gave women appropriate instruction in using NRT and, as no differences were seen between groups in additional support received by trial participants, this is unlikely to explain trial findings.
Infant outcomes at 2 years
Key strengths are that no previous trials have tested the impact of a smoking cessation intervention in pregnancy on infant outcomes and we achieved high (around 88%) outcome ascertainment rates combined with low withdrawal and missing data rates at 2 years. Follow-up rates were very similar in both trial groups, reflecting the fact that staff conducting follow-up were blind to participants’ treatment allocations. Additionally, we used caution when classifying questionnaires reporting infants’ development and respiratory symptoms and manually checked individual questionnaire responses, when necessary. We were cautious when categorising open-response questionnaire items, generally allocating infants as experiencing ‘suspected’, rather than ‘definite’ impairment, based on responses to these. A similar approach was also taken with open response items on respiratory problems. Consequently, it is unlikely that infants classed as having ‘no impairment’ were actually impaired or that infants categorised as experiencing ‘respiratory problems’ had not had these. Finally, with respect to the primary outcome monitored at 2 years, ‘survival with no impairment’, we obtained the same pattern of findings in all pre-planned analyses, irrespective of whether or not parental or health professional reports of infant health were used, or whether or not twins were included. This consistency of findings suggests that the overall finding, that NRT used in pregnancy has a beneficial effect on child development as measured by ASQ-3, is likely to be valid and unlikely to have occurred by chance.
A limitation is that the ASQ-3 is generally used as a screening tool to identify potential impairments that are subsequently confirmed or refuted by detailed face-to-face assessment. 43 We cannot, therefore, be certain that the parent-reported ‘snapshot’ of child development obtained via completed ASQ-3 questionnaires is perfectly valid for allocating infants as experiencing ‘definite’ or ‘no impairment’. However, a previous UK obstetric trial used 30-item ASQ questionnaires to detect neurosensory disability and, in a subgroup of infants, compared a range of failed and non-failed ASQ scores with outcomes from ‘gold standard’ face-to-face developmental assessments. 77 This found that, when used for detecting neurodevelopmental problems in a cohort of participants and infants that was similar to those in this study, the 30-item ASQ compared well with standard face-to-face assessments and had a negative predictive value of 99.5% (95% CI 98.3% to 99.9%). 77 If, in our study, the ASQ-3 performed similarly and if we had employed gold standard face-to-face assessments, one would expect very few infants categorised by ASQ-3 as having no impairment to then be diagnosed with developmental problems in face-to-face assessments. Our use of a self-report questionnaire completed by health professionals to help decide whether or not infants had developmental impairments could also be criticised. Using this method of data collection, we do not know whether or not those completing questionnaires did so with reference to medical records or other knowledge of infants. However, very similar questionnaires have been used in previous authoritative cohort studies78 and incorporation of such health professional data into analyses in this trial did not affect outcomes.
Smoking outcomes at 2 years
This was the first trial of a smoking cessation intervention to monitor longitudinal smoking rates within pregnant trial participants for as long as 2 years after delivery; therefore, the data are novel. Although data were self-reported and we obtained no information on smoking behaviour for around 40% of participants, sensitivity analysis suggested that our assumption that non-respondents at 2 years were smokers was appropriate.
Economic analyses
We present a within trial incremental cost-effectiveness analysis, calculating the cost per additional quitter associated with NRT patch use. Owing to the issues around measuring the EQ-5D in a population of pregnant women, we did not propose to calculate QALYs, which reduces the comparability of our cost-effectiveness outcome with different health-care programmes. The trial did not find a difference in outcomes that would have enabled the projection of longer-term costs and benefits of NRT use, thus precluding conclusions about the long-term cost-effectiveness of NRT patch use in pregnancy.
Interpretation and generalisability
Smoking outcomes
In contrast to the negative primary outcome recorded at delivery, the increased cessation until 1 month after randomisation was of a similar magnitude to that seen following NRT use by non-pregnant smokers. 18 The lack of a statistically significant longer-term effect from NRT may be explained by the low adherence rates in the trial. One reason for the apparently low adherence is that many participants were specifically instructed to not use NRT when smoking and many failed to quit and restarted smoking. However, this does not explain why only 58% (101/173) of participants who were abstinent at 1 month accepted a second month’s supply of NRT. Other NRT trials in pregnancy have reported similarly low rates of adherence: two studies of NRT patches found median durations of NRT use of 2 weeks34 and 3 weeks,27 and, in a trial that tested 2-mg nicotine gum, this was used for just over 5 weeks. 35 Outside pregnancy, most smokers who attempt to quit with NRT discontinue this within 1 month because they either start smoking again or they believe it is not working. 79 Nevertheless, despite such reports of adherence with NRT being lower than recommended,79 trials18 and cohort data from routine clinical care80 both demonstrate that NRT used by non-pregnant smokers is effective for smoking cessation. Adherence with NRT is potentially an important influence on the efficacy of this treatment in pregnancy; NRT cannot have an effect if it is not used and reduced adherence with NRT in later pregnancy could explain the lack of effect shown at delivery. Therefore, potential influences on adherence in the trial require further exploration.
Trial participants often discontinue treatments after experiencing AEs or side effects; however, only 8.8% of women in the NRT group reported stopping patches after AEs. In previous similar trials, the discontinuation rate was 12% for nicotine gum35 and 4.4% for nicotine patches or placebos. 27 These AE rates are much lower than participants’ rates of early treatment discontinuation and hence can only partially explain this. Treatment discontinuation could be explained by increases in maternal nicotine and cotinine clearance in pregnancy; increases of 60% and 140%, respectively, have been reported to occur by 25 weeks’ gestation. 81 Such increases would be expected to reduce NRT-generated nicotine levels and increase users’ withdrawal symptoms. It is possible that, for NRT to consistently ameliorate women’s nicotine withdrawal symptoms and be effective throughout pregnancy, a higher dose is required. 82 However, this trial did not include assessment of nicotine metabolism and did not assess withdrawal symptoms. In addition, factors other than increases in metabolism may explain low NRT adherence rates in this and previous trials.
In summary, this trial provides no evidence that NRT, as the 15 mg per 16 hours transdermal patch, is effective for smoking cessation in pregnancy. Women used the patches for shorter periods than recommended and this could explain negative trial findings. It is possible that, even with the low adherence displayed by trial participants, NRT actually does have a positive effect and, with a larger sample size, the 1.8% absolute difference in favour of treatment with NRT would have become statistically significant. However, a recent meta-analysis of this trial’s findings with all previous similar studies (n = 1745) does not suggest this. In this analysis, the RR for cessation in later pregnancy after using NRT compared with control was 1.33 (95% CI 0.93 to 1.91). 31 Even if the small difference between groups in this trial does represent a real effect of NRT that has not been statistically proven, it seems unlikely that, based solely on this small effect on maternal smoking behaviour, NRT used in pregnancy would be considered clinically useful. The number needed to treat (calculated using trial data) is 54, which means that if the magnitude of effect observed in this trial were to be found statistically significant (e.g. in a future meta-analysis), 54 women would require treatment with NRT to produce one successful quitter. This success rate would be unlikely to impress clinicians who would probably be inclined to use other methods of cessation support for pregnant smokers.
Other outcomes measured at delivery
Rates of adverse outcomes were similar between groups with the exception of caesarean deliveries, which were unexpectedly more frequent in the NRT group. This is difficult to explain and is likely to be a chance occurrence. However, caution is warranted when interpreting the overall similarity in birth outcomes between trial groups. Because many adverse birth outcomes are quite rare, some comparisons may have limited power and the low rate of treatment adherence makes it difficult to attribute the presence or absence of differences in birth outcomes to NRT.
Outcomes measured after delivery
This study has provided the first evidence that a smoking cessation intervention delivered to pregnant women can influence the development of their offspring. We found that NRT, used in pregnancy for smoking cessation, without having any statistically significant, long-term effect on maternal smoking in pregnancy, had a positive impact on subsequent child development. The most likely explanation for this impact lies in the altered smoking behaviour of women who were randomised to NRT. For example, the transient doubling of quit rates in the first month after trial enrolment and until around 20 weeks’ gestation could have occurred at a crucial time for infants’ brain maturation, resulting in the greater survival to 2 years of age with no developmental impairment. The slightly higher, but non-significant, quit rates observed from delivery until 2 years and the reduced accompanying exposure of infants to domestic environmental tobacco smoke may also have had additional positive effects. If the impact of NRT on infants is mediated through increased maternal smoking cessation, any effective smoking cessation intervention used by pregnant women would be expected to have similar effects. However, it remains possible that any protective impact of NRT on infants’ development arises directly from the impact of nicotine itself and is not mediated by reduced fetal or infant exposure to tobacco smoke toxins. Irrespective of whether the impact of NRT on infants is direct or mediated (indirect), this trial is reassuring about NRT use in pregnancy. Although in controlled laboratory studies, nicotine has been shown to cause fetal tachycardia, albeit to a lesser extent than smoking,21,83,84 this trial provides no evidence that NRT is harmful. Similarly, as nicotine is a neurotoxin and can cause behavioural problems in young rodents,21,83 there has previously been concern that NRT might harm infants’ developing nervous systems and that it could cause behavioural problems and poorer academic achievement in smokers’ children. 22 However, study findings clearly suggest that nicotine is unlikely to be responsible for these problems.
As mentioned above, findings from this trial require confirmation; future studies of NRT and other smoking cessation interventions in pregnancy should follow-up infants and trial participants for at least 2 years after delivery to facilitate this. Additionally, the cohort of trial infants from this trial should be followed after 2 years, to determine whether or not impacts on child development attributable to NRT persist into childhood. If further studies can replicate findings or confirm that these benefits persist later into childhood, then this would provide reassurance that these findings did not occur by chance, which is important both scientifically and economically. Intervention costs were relatively low (< £100 per participant) and, should the impacts on infants identified in this study be confirmed, it is likely that NRT used in pregnancy for smoking cessation would be viewed as cost-effective, potentially generating cost savings for the NHS.
The trial had a relatively pragmatic design, with reasonably broad inclusion and few exclusion criteria, therefore, results are likely to be generalisable to most pregnant smokers. However, women recruited to the trial were between 12 and 24 weeks’ gestation and, therefore, our results may be less applicable to those trying to quit using NRT earlier or later in their pregnancy. For example, at earlier gestations, nicotine metabolism may not yet have increased and so the dose of NRT used in this trial may be sufficient to help such women to quit successfully. Our 1-month findings suggest that this could be likely and as we found no safety issues, future studies could consider including women earlier in pregnancy. Similarly, as we only included women who smoked at least 5 cigarettes a day, the results cannot necessarily be extrapolated to those who are very light smokers and less addicted to nicotine and they may also be more likely to be able to successfully quit with the dose of NRT used in this trial.
Conclusions
The NRT transdermal nicotine patches (15 mg per 16 hours) used in pregnancy for smoking cessation caused a transient, doubling of cessation that disappeared by delivery. Infants born to women in the NRT group were more likely to survive without impairment to 2 years of age, but there were no differences in the experience of respiratory symptoms between groups.
Recommendations for research (in priority order)
-
Randomised controlled trials investigating the efficacy and safety of NRT when used for smoking cessation in pregnancy should test higher than standard dose NRT such as:
-
patches delivering more than 15 mg nicotine in 16 hours (e.g. 21 mg in 24 hours)
-
4-mg gum used as required or
-
a NRT patch combined with any ‘on demand’ short-acting NRT (e.g. gum or nasal spray).
-
-
To investigate whether or not apparent differences in infants’ outcomes persist into childhood. RCTs investigating NRT for smoking cessation in pregnancy should assess infants’ clinical and economic outcomes after 2 years of age.
-
RCTs investigating the efficacy of NRT or other interventions for smoking cessation used in pregnancy should include an assessment of impacts on infants using outcomes similar to those employed in SNAP.
-
Reasons for pregnant women’s low levels of adherence with NRT should be investigated; findings could be used in future trials to enhance participants’ adherence with NRT.
-
Increases in nicotine metabolism, occurring as pregnancy progresses, could explain the reduced efficacy that NRT has in later pregnancy. Further research should investigate this hypothesis.
Implications for health care
In the UK and some other health-care systems, NRT has become an established component of cessation support for pregnant women. Although the SNAP trial found no evidence that standard dose NRT is effective for smoking cessation, there was also no evidence that this is less safe than smoking; indeed, the study suggests that NRT use in pregnancy is safe in terms of infant outcomes assessed at 2 years and may have a protective effect on infant development. Although this is the first time that a smoking cessation intervention has been observed to have a beneficial effect on pregnant smokers’ offspring, this finding provides support for interventions involving NRT in pregnancy. Overall, our findings provide no evidence that NRT should not be used in pregnancy and instead suggest that NRT might be beneficial in this setting.
Effects of NRT on infant development are likely to be mediated through the small, observed changes in maternal smoking. There are good reasons to believe that NRT used at higher doses might affect both maternal smoking and infant development more substantially and trials of higher-dose NRT are indicated. Other cessation interventions delivered in pregnancy may have similar impacts on infants, but this requires confirmation. Choosing between interventions for use with pregnant smokers is, therefore, difficult; both ‘self-help’ and behavioural smoking cessation support promote maternal smoking cessation and improve birth outcomes. However, while there is no evidence that NRT has these effects, NRT does appear to have a potentially important protective effect on infant development. Therefore, this study supports offering NRT to pregnant women who smoke; however, any such offer should take account of the somewhat stronger research evidence from other studies indicating that behavioural or ‘self-help’ support both have beneficial effects on smoking behaviour in pregnancy.
Acknowledgements
We wish to thank the many people listed below who contributed to the success of this study including all the participants and their families for their time and involvement.
Contributions of authors
All authors made substantial contributions to conception and design and/or to acquisition of data and/or to analysis and interpretation of data as listed below.
All authors were involved in drafting the manuscript or revising it critically for important intellectual content.
All authors approved the final version.
Sue Cooper (SNAP Trial Manager, Senior Research Fellow) was involved in design, conduct, acquisition of data, analysis and report writing phases.
Sarah Lewis (Professor of Medical Statistics) was involved in design, conduct, analysis and report writing phases.
James G Thornton (Professor of Obstetrics and Gynaecology, Clinical Trials) was involved in design, conduct, analysis and report writing phases.
Neil Marlow (Professor of Neonatology) was involved in design, conduct, analysis and report writing phases, in particular the follow-up of infants after delivery.
Kim Watts (Midwife Lecturer) was involved in design, conduct, analysis and report writing phases.
John Britton (Professor of Epidemiology) was involved in design, conduct, analysis and report writing phases.
Matthew J Grainge (Lecturer, statistics) was involved in the analysis and interpretation of data to delivery and in the report writing phase.
Jaspal Taggar (Clinical Lecturer, statistics) was involved in the analysis and interpretation of follow-up data and in the report writing phase.
Holly Essex (Research Fellow, health economics) was involved in the conduct, analysis, interpretation and report writing for the health economics chapter.
Steve Parrott (Senior Research Fellow, health economics) was involved in the conduct, analysis, interpretation and report writing for the health economics chapter.
Anne Dickinson (Clinical Trial Research Assistant) was involved in the design, conduct and acquisition of data and in the report writing phase.
Rachel Whitemore (Administrative Assistant) was involved in the design, conduct and acquisition of data during the follow-up phase and in the report writing phase.
Tim Coleman (SNAP Trial Chief Investigator and Professor of Primary Care) was involved in design, conduct, analysis and report writing phases.
SC, SL, JB, MJG, HE, SP, AD, RW and TC are members of the UK Centre for Tobacco and Alcohol Studies (UKCTAS). Funding from the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Medical Research Council and the National Institute of Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged.
SC, JT, AD, RW and TC are members of the NIHR School for Primary Care Research.
TC acknowledges the support of the East Midlands Collaboration for Leadership in Applied Health Research and Care (CLAHRC).
NM receives part funding from the Department of Health’s NIHR Biomedical Research Centre’s funding scheme at UCLH/UCL.
Trial team
Trial applicants
Chief investigator: Tim Coleman.
Co-applicants: James G Thornton, John Britton, Kim Watts, Michael Coughtrie, Clare Mannion, Neil Marlow, Christine Godfrey.
Trial manager
Sue Cooper.
Trial management group
Tim Coleman, Sue Cooper, James Thornton, John Britton, Sarah Lewis, Kim Watts, Neil Marlow.
Trial administrators
Anne Dickinson, Rachel Whitemore.
Trial Steering Committee
Peter Brocklehurst (chair), Carol Coupland (independent statistician), Peter Hajek, Sue Maguire (PPI/lay member), Michael Murphy.
Data Monitoring and Ethics Committee
Janet Peacock (chair), Christopher Butler (from January 2009), David Field, Khalid Khan (until October 2008).
Statisticians
Sarah Lewis, Matthew J Grainge, Jaspal Taggar.
Health economists
Christine Godfrey, Holly Essex, Steve Parrott.
Research team
In addition to those listed above, the complete trial team includes:
Research staff: Janet Brown, Yvette Davis, Anne Dickinson, Caroline Dixon, Fiona Holloway, Joanne Lakin, Jayne Platts, Farzana Rashid, Amanda Redford, Cara Taylor, Rachel Whitemore.
Principal investigators (in recruiting centres): Jonathan Allsop (Derby Hospitals NHS Foundation Trust), Simon Cunningham (Mid Cheshire Hospitals NHS Foundation Trust), Karen Glass (Sherwood Forest Hospitals NHS Foundation Trust), Vince Hall (East Cheshire NHS Trust), Khaled Ismail (University Hospital of North Staffordshire NHS Trust), Margaret Ramsay (Nottingham University Hospitals NHS Trust – QMC campus), James Thornton (Nottingham University Hospitals NHS Trust – City Campus).
Midwife leads (in recruiting centres): Sheena Appleby, Denise Bailey, Linda Gustard, Emma Haworth, Grace Hopps, Amanda Lindley, Chris Kettle, Colleen Pearce, Dymphna Sexton-Bradshaw, Julia Savage, Sandra Smith, Sheila Taylor, Alison Whitham.
Primary care trust and NHS SSS staff: Barbara Brady, Michelle Battlemuch, Wendy Dudley, Rochelle Edwards, Lorraine Frith, Indu Hari, Catriona Holden, Linda Hoskyns, Paul Jackson, Giri Rajaratnam, Deborah Richardson, Lucy Wade, Maureen Whittaker.
QMC pharmacy: Bernie Cook, Sheila Hodgson (lead pharmacist), Lisa Humphries, Bernie Sanders (quality controller).
University of Nottingham: Dan Simpkins (Clinical Trials Unit – database), Luis Vaz (assisting with secondary adherence analyses), Yvanna Kurlak, Clare Randall and John Taylor (assisted with trial administration).
University of Dundee: Sheila Sharp (sample analysis – baseline and 1-month COT).
We would also like to thank the following for their contributions to the study:
Dr Mira Doig and ABS Laboratories Ltd (Welwyn Garden City, UK), for analysis of cotinine in baseline blood samples.
Salimetrics Europe Ltd (Newmarket, Suffolk, UK) for analysis of cotinine in delivery saliva samples.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Bowker KA, Lewis S, Coleman T, Vaz LR, Cooper S. Comparison of cotinine levels in pregnant women whilst smoking and when using nicotine replacement therapy. Nicotine Tob Res 2014;16:895–8.
Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, Lewis S. A randomized trial of nicotine-replacement therapy patches in pregnancy. N Engl J Med 2012;366:808–18.
Coleman T, Thornton J, Britton J, Lewis S, Watts K, Coughtrie MWH, et al. Protocol for the smoking, nicotine and pregnancy (SNAP) trial: double-blind, placebo-randomised, controlled trial of nicotine replacement therapy in pregnancy. BMC Health Serv Res 2007;7:2.
Cooper S, Taggar J, Lewis S, Marlow N, Dickinson A, Whitemore R, et al. Effect of nicotine patches in pregnancy on infant and maternal outcomes at 2 years: follow-up from the randomised, double-blind, placebo-controlled SNAP trial [published online ahead of print 11 August 2014]. Lancet Respir Med 2014. doi:10.1016/S2213-2600(14)70157-2
Kwok TC, Taggar J, Cooper S, Lewis S, Coleman T. Nicotine dependence and biochemical exposure measures in the second trimester of pregnancy. Nicotine Tob Res 2014;16:145–54.
Vaz LR, Leonardi-Bee J, Aveyard P, Cooper S, Grainge M, Coleman T, on behalf of the SNAP trial team. Factors associated with smoking cessation in early and late pregnancy in the Smoking, Nicotine, and Pregnancy Trial: a trial of nicotine replacement therapy. Nicotine Tob Res 2014;16:381–9.
References
- Smoking and the Young. London: Royal College of Physicians; 1992.
- Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, et al. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol 2005;193:626-35. http://dx.doi.org/10.1016/j.ajog.2005.02.106.
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New Engl J Med 2008;359:61-73. http://dx.doi.org/10.1056/NEJMra0708473.
- The NHS Information Centre . Infant Feeding Survey 2010. Early Results 2011. www.hscic.gov.uk/catalogue/PUB00648 (accessed 9 April 2014).
- Mohsin M, Bauman AE. Socio-demographic factors associated with smoking and smoking cessation among 426,344 pregnant women in New South Wales, Australia. BMC Public Health 2005;5. http://dx.doi.org/10.1186/1471-2458-5-138.
- Jensen KE, Jensen A, Nohr B, Kjaer SK. Do pregnant women still smoke? A study of smoking patterns among 261,029 primiparous women in Denmark 1997–2005. Acta Obstet Gyn Scand 2008;87:760-7. http://dx.doi.org/10.1080/00016340802179814.
- Centers for Disease Control and Prevention . Smoking during pregnancy – United States, 1990–2002. MMWR Morb Mortal Wkly Rep 2004;53:911-15.
- Schneider S, Maul H, Freerksen N, Potschke-Langer M. Who smokes during pregnancy? An analysis of the German Perinatal Quality Survey 2005. Public Health 2008;122:1210-16. http://dx.doi.org/10.1016/j.puhe.2008.02.011.
- Martinez-Frias ML, Rodriguez-Pinilla E, Bermejo E. Tobacco smoking during pregnancy in Spain: an analysis according to years, autonomous communities and maternal characteristics. Med Clin (Barc) 2005;124:86-92.
- Polanska K, Hanke W, Sobala W, Broszkiewicz M. Smoking cessation intervention during pregnancy in a Polish urban community – what is the target population?. Tob Induced Dis 2002;1:121-8. http://dx.doi.org/10.1186/1617-9625-1-2-121.
- Oncken CA, Dietz PM, Tong VT, Belizan JM, Tolosa JE, Berghella V, et al. Prenatal tobacco prevention and cessation interventions for women in low- and middle-income countries. Acta Obstet Gynecol Scand 2010;89:442-53. http://dx.doi.org/10.3109/00016341003678450.
- WHO Report on the Global Tobacco Epidemic, 2008 – the MPOWER Package. Geneva: World Health Organization; 2008.
- Leonardi-Bee J, Jere ML, Britton J. Exposure to parental and sibling smoking and the risk of smoking uptake in childhood and adolescence: a systematic review and meta-analysis. Thorax 2011;66:847-55. http://dx.doi.org/10.1136/thx.2010.153379.
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.CD001055.pub3.
- Naughton F, Prevost AT, Sutton S, Naughton F, Prevost AT, Sutton S. Self-help smoking cessation interventions in pregnancy: a systematic review and meta-analysis. Addiction 2008;103:566-79. http://dx.doi.org/10.1111/j.1360-0443.2008.02140.x.
- Statistics on NHS Stop Smoking Services – England, April 2011 to March 2012. Health and Social Care Information Centre: Department of Health; 2013.
- NHS Maternity Statistics – England, 2010–2011. Health and Social Care Information Centre: Department of Health; 2013.
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.CD000146.pub3.
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007;1.
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2012;4.
- Dempsey DA, Benowitz NL. Risks and benefits of nicotine to aid smoking cessation in pregnancy. Drug Saf 2001;24:277-322. http://dx.doi.org/10.2165/00002018-200124040-00005.
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early Hum Develop 2003;75:21-33. http://dx.doi.org/10.1016/j.earlhumdev.2003.09.001.
- Thapar A, Fowler T, Rice F, Scourfield J, van den BM, Thomas H, et al. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am J Psychiatry 2003;160:1985-9. http://dx.doi.org/10.1176/appi.ajp.160.11.1985.
- Benowitz NL, Dempsey DA, Goldenberg RL, Hughes JR, Dolan-Mullen P, Ogburn PL, et al. The use of pharmacotherapies for smoking cessation during pregnancy. Tob Control 2000;9:iii91-4. http://dx.doi.org/10.1136/tc.9.suppl_3.iii91.
- British National Formulary. London: BMA and RPS; 2003.
- Anonymous . National Treatment Guidelines 2013. www.treatobacco.net/en/page_224.php (accessed 15 August 2013).
- Wisborg K, Henriksen TB, Jespersen LB, Secher NJ. Nicotine patches for pregnant smokers: a randomized controlled study. Obstet Gynecol 2000;96:967-71. http://dx.doi.org/10.1016/S0029-7844(00)01071-1.
- Kapur B, Hackman R, Selby P, Klein J, Koren G. Randomized, double-blind, placebo-controlled trial of nicotine replacement therapy in pregnancy. Cur Ther Res 2001;62:274-8. http://dx.doi.org/10.1016/S0011-393X(01)80011-4.
- Hegaard HK, Kjaergaard H, Moller LF, Wachmann H, Ottesen B. Multimodal intervention raises smoking cessation rate during pregnancy. Acta Obstet Gyn Scand 2003;82:813-19. http://dx.doi.org/10.1034/j.1600-0412.2003.00221.x.
- Coleman T, Chamberlain C, Cooper S, Leonardi-Bee J. Efficacy and safety of nicotine replacement therapy for smoking cessation in pregnancy: systematic review and meta-analysis. Addiction 2011;106:52-61. http://dx.doi.org/10.1111/j.1360-0443.2010.03179.x.
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2012;9. http://dx.doi.org/10.1002/14651858.CD010078.
- Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2004;4.
- Hotham ED, Gilbert AL, Atkinson ER. A randomised-controlled pilot study using nicotine patches with pregnant women. Addict Behav 2006;31:641-8. http://dx.doi.org/10.1016/j.addbeh.2005.05.042.
- Pollak KI, Oncken CA, Lipkus IM, Lyna P, Swamy GK, Pletsch PK, et al. Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. Am J Prevent Med 2007;33:297-305. http://dx.doi.org/10.1016/j.amepre.2007.05.006.
- Oncken C, Dornelas E, Greene J, Sankey H, Glasmann A, Feinn R, et al. Nicotine gum for pregnant smokers: a randomized controlled trial. Obstet Gynecol 2008;112:859-67. http://dx.doi.org/10.1097/AOG.0b013e318187e1ec.
- Standard for Training in Smoking Cessation Treatments. London: Health Development Agency; 2003.
- Windsor RA, Woodby LL, Miller TM, Hardin JM, Crawford MA, DiClemente CC. Effectiveness of Agency for Health Care Policy and Research clinical practice guideline and patient education methods for pregnant smokers in medicaid maternity care. Am J Obstet Gynecol 2000;182:68-75. http://dx.doi.org/10.1016/S0002-9378(00)70492-3.
- SRNT Subcommittee on Biochemical Verification . Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4:149-59. http://dx.doi.org/10.1080/14622200210123581.
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict 1989;84:791-9. http://dx.doi.org/10.1111/j.1360-0443.1989.tb03059.x.
- McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al. Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients. Health Technol Assess 2001;5.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Increasing response rates to postal questionnaires: systematic review. BMJ 2002;324. http://dx.doi.org/10.1136/bmj.324.7347.1183.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. http://dx.doi.org/10.1136/bmj.316.7133.736.
- Squires J, Bricker D, Potter L. Revision of a parent-completed development screening tool: Ages and Stages Questionnaires. J Pediatr Psychol 1997;22:313-28. http://dx.doi.org/10.1093/jpepsy/22.3.313.
- Squires J, Bricker D. Ages and Stages Questionnaire: A Parent-Completed Child-Monitoring System. Baltimore, MD: Paul H Brookes Publishing Co.; 2009.
- Squires J, Twombly E, Bricker D, Potter L. ASQ-3 User’s Guide for the Ages and Stages Questionnaires. Baltimore, MD: Paul H Brookes Publishing Co.; 2009.
- Disability and Perinatal Care: Measurement of Health Status at Two Years. Oxford, England: National Perinatal Epidemiology Unit and Oxford Health Authority; 1995.
- Jones HP, Guildea ZE, Stewart JH, Cartlidge PH. The Health Status Questionnaire: achieving concordance with published disability criteria. Arch Dis Child 2002;86:15-20. http://dx.doi.org/10.1136/adc.86.1.15.
- Marlow N. Pulmonary outcomes for the extremely preterm infant. Biol Neonate 2000;78:239-40.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. http://dx.doi.org/10.1111/j.1360-0443.2004.00995.x.
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2004;3.
- Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med 2002;21:2917-30. http://dx.doi.org/10.1002/sim.1296.
- Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed?. BJOG 2004;111:213-19. http://dx.doi.org/10.1111/j.1471-0528.2004.00059.x.
- Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction 2007;102:1564-73. http://dx.doi.org/10.1111/j.1360-0443.2007.01946.x.
- Handling missing data in randomised trials. Cambridge: MRC Biostatistics Unit; 2012.
- Coleman T, Thornton J, Britton J, Lewis S, Watts K, Coughtrie MW, et al. Protocol for the Smoking, Nicotine and Pregnancy (SNAP) trial: double-blind, placebo-randomised, controlled trial of nicotine replacement therapy in pregnancy. BMC Health Serv Res 2007;7. http://dx.doi.org/10.1186/1472-6963-7-2.
- Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, et al. A randomized trial of nicotine-replacement therapy patches in pregnancy. New Engl J Med 2012;366:808-18. http://dx.doi.org/10.1056/NEJMoa1109582.
- Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al. The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation. Health Technol Assess 2002;6.
- Ruger JP, Lazar CM. Economic evaluation of pharmaco- and behavioral therapies for smoking cessation: a critical and systematic review of empirical research. Annu Rev Public Health 2012;33:279-305. http://dx.doi.org/10.1146/annurev-publhealth-031811-124553.
- Ruger JP, Emmons KM. Economic evaluations of smoking cessation and relapse prevention programs for pregnant women: a systematic review. Value Health 2008;11:180-90. http://dx.doi.org/10.1111/j.1524-4733.2007.00239.x.
- Ruger JP, Weinstein MC, Hammond SK, Kearney MH, Emmons KM. Cost-effectiveness of motivational interviewing for smoking cessation and relapse prevention among low-income pregnant women: a randomized controlled trial. Value Health 2008;11:191-8. http://dx.doi.org/10.1111/j.1524-4733.2007.00240.x.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed 8 August 2013).
- National Institute for Health and Clinical Excellence . Smoking Cessation Services: Costing Report Implementing NICE Guidance 2008. www.nice.org.uk/nicemedia/live/11925/39719/39719.pdf (accessed 8 August 2013).
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit; 2012.
- Prescription Cost Analysis England 2009. Leeds: The Health and Social Care Information Centre, Prescribing Support Unit; 2010.
- NHS Monthly Remuneration Statistics. London: Pharmaceutical Services Negotiating Committee; 2013.
- NHS Terms and Conditions of Service Handbook: Pay Circulars (AforC) 1 and 2/2013, Amendment Number 28. London: The NHS Staff Council; 2013.
- NHS Reference Costs 2009–2010: Appendix NSRC01: NHS Trust Reference Cost Schedules. London: Department of Health; 2011.
- Savings Realised by Different Types of Organisations Across the NHS Through the Use of the NHSmail SMS feature: GP Practice, Ayrshire. London: NHS Connecting for Health 2013; n.d.
- Hospital Episode Statistics, Admitted Patient Care – England, 2009–10. Leeds: The Health and Social Care Information Centre; 2010.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Chan OK, Sahota DS, Leung TY, Chan LW, Fung TY, Lau TK. Nausea and vomiting in health-related quality of life among Chinese pregnant women. Aust N Z J Obstet Gynaecol 2010;50:512-18. http://dx.doi.org/10.1111/j.1479-828X.2010.01216.x.
- Haas JS, Jackson RA, Fuentes-Afflick E, Stewart AL, Dean ML, Brawarsky P, et al. Changes in the health status of women during and after pregnancy. J Gen Intern Med 2005;20:45-51. http://dx.doi.org/10.1111/j.1525-1497.2004.40097.x.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-14. http://dx.doi.org/10.1177/0272989X9001000308.
- Dolan P, Gudex C, Kind P, Williams A. The time trade-off method: results from a general population study. Health Econ 1996;5:141-54. http://dx.doi.org/10.1002/(SICI)1099-1050(199603)5:2<141::AID-HEC189>3.0.CO;2-N.
- Taylor M. Economic Analysis of Interventions for Smoking Cessation Aimed at Pregnant Women 2009. http://guidance.nice.org.uk/PH26 (accessed 31 March 2014).
- Seckerwalker R, Solomon L, Flynn B, Skelly J, Lepage S, Goodwin G, et al. Individualized smoking cessation counseling during prenatal and early postnatal care. Am J Obstet Gynecol 1994;171:1347-55. http://dx.doi.org/10.1016/0002-9378(94)90159-7.
- Yu L, Hey E, Doyle L, Farrell B, Spark P, Altman D, et al. Evaluation of the Ages and Stages Questionnaires in identifying children with neurosensory disability in the Magpie Trial follow-up study. Acta Paediatrica 2007;96:1803-8. http://dx.doi.org/10.1111/j.1651-2227.2007.00517.x.
- Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. EPICure Study Group . Neurologic and developmental disability after extremely preterm birth. N Engl J Med 2000;343:378-84. http://dx.doi.org/10.1056/NEJM200008103430601.
- Balmford J, Borland R, Hammond D, Cummings KM. Adherence to and reasons for premature discontinuation from stop-smoking medications: data from the ITC Four-Country Survey. Nicotine Tob Res 2011;13:94-102. http://dx.doi.org/10.1093/ntr/ntq215.
- West R, Zhou X. Is nicotine replacement therapy for smoking cessation effective in the ‘real world’? Findings from a prospective multinational cohort study. Thorax 2007;62:998-1002. http://dx.doi.org/10.1136/thx.2007.078758.
- Dempsey D, Jacob P, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Therapeut 2002;301:594-8. http://dx.doi.org/10.1124/jpet.301.2.594.
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res 2003;5:13-25. http://dx.doi.org/10.1093/ntr/5.1.13.
- Benowitz NL, Dempsey DA. Pharmacotherapy for smoking cessation during pregnancy. Nicotine Tob Res 2004;6:S189-202. http://dx.doi.org/10.1080/14622200410001669169.
- Ogburn PL, Hurt RD, Croghan IT, Schroeder DR, Ramin KD, Offord KP, et al. Nicotine patch use in pregnant smokers: nicotine and cotinine levels and fetal effects. Am J Obstet Gynecol 1999;181:736-43. http://dx.doi.org/10.1016/S0002-9378(99)70521-1.
Appendix 1 Antenatal screening questionnaire
Appendix 2 Smoking cessation manual
Appendix 3 Smoking, Nicotine and Pregnancy Trial protocol
Appendix 4 Protocol breaches
Of 2410 women who expressed an interest in the trial and were assessed for eligibility, 1051 (43.6%) were randomised: 521 were assigned to receive NRT and 530 to receive placebo patches (see Figure 2). One woman was mistakenly enrolled for a second time in a subsequent pregnancy; thus, her second enrolment in the placebo group was removed from all analyses, giving a final sample size of 1050 (529 in the placebo group).
Protocol breaches were discovered for 13 other participants, but after consideration of violation details it was decided that these were not serious and would have no significant impact on trial participants or the scientific integrity of the trial. Therefore, these participants remained in the trial and their data were used in analyses. Two participants had minor chemical dependence problems and three participants were enrolled 4–5 days before they reached 12 weeks’ gestation; however, they were not randomised until 12 weeks. Seven participants did not receive their investigational medicinal product by their quit date owing to pharmacy problems, meaning that they needed to set a new quit date that was then > 2 weeks after their baseline visit. One participant received and used a second supply of patches after the first set appeared to have been lost in the post, however, as she was still smoking at 1 month she would not have been eligible to receive a second set.
Two additional problems affecting 27 participants occurred within one site pharmacy, but again these were judged to have no significant impact on participants or trial integrity. The temperature recorded in the pharmacy fridge at one site was in excess of 8 °C (this was the maximum temperature specified for patch storage before dispensing) for 12 days in a 1-month period and, during this time, 25 subjects had been assigned patches from the pharmacy. On discovery of the problem, unallocated packs were withdrawn, but as patches could be stored for 3 months at ambient temperature without the potency being reduced, and there were no safety issues, it was felt that other than informing participants that patches should not be used for longer than 1 month after issue, no further action needed to be taken with those packs that had been issued. In another incident, due to a mix-up by the site pharmacy, two participants were posted each others treatment pack for the first treatment period and had started to use the patches before the mistake was discovered. The treatment code was not broken, but the trial manager was informed that the participants had received the same treatment allocation and no further action was taken.
Appendix 5 Supplementary data on adverse events
See Table 7 for information on AEs by treatment group.
Adverse events resulting in women permanently discontinuing treatment
Nicotine replacement therapy: 50 adverse events in 46 women
Application site reactions (n = 33), nausea (n = 7), headache (n = 3), dizziness (n = 2), palpitations (n = 2), dyspnoea, oropharyngeal pain, sensory disturbance (all n = 1)
Placebo: 38 adverse events in 32 women
Application site reactions (n = 15), nausea (n = 7), dizziness (n = 5), headache (n = 3), irritability (n = 3), dyspepsia (n = 2), fetal hypokinesia, influenza like illness, palpitations (all n = 1)
Overnight hospital admissions for other pregnancy complications
In total there were 143 events with hospital admissions in 95 women (NRT group) and 146 events with hospital admissions in 94 women (placebo group) (admission for any reason, not only pregnancy related).
Hospital admission for less frequent pregnancy-related events
Nicotine replacement therapy: 44 events
Miscellaneous hospitalisation for pregnancy-related events for which the outcome was no abnormality detected (n = 7), urinary tract infection (n = 7), maternal condition affecting fetus (n = 3), postpartum haemorrhage (n = 3), proteinuria (n = 3), antepartum haemorrhage (n = 2), dizziness (n = 2), polyhydramnios (n = 2), premature separation of placenta (n = 2), chest pain, constipation, disseminated intravascular coagulation, hepatic failure, pruritus generalised, pyelonephritis, renal failure, syncope, tachycardia, ultrasound Doppler abnormal, vision blurred, visual impairment, vulvovaginal candidiasis (all n = 1).
Placebo: 41 events
Miscellaneous hospitalisation for pregnancy-related events for which the outcome was no abnormality detected (n = 5), urinary tract infection (n = 4), maternal condition affecting fetus (n = 4), premature labour (n = 2), premature separation of placenta (n = 2), anaemia, anaphylactic reaction after iron infusion, antepartum haemorrhage, back pain, body temperature increased, cervix cerclage procedure, chest pain, cholestasis of pregnancy, deep-vein thrombosis, diarrhoea, dizziness, dyspnoea, haemoglobin decreased, migraine, musculoskeletal pain, oedema, placenta praevia, proteinuria, pyelonephritis, scar pain, threatened labour, abnormal ultrasound Doppler, urinary retention, visual impairment (all n = 1).
Other, less frequent, adverse events that occurred in < 3% of women or infants and are not logically grouped together
Nicotine replacement therapy
Maternal adverse events (63 events)
Dizziness (n = 6), fall (n = 4), abnormal dreams (n = 3), back pain (n = 3), dyspnoea (n = 3), urinary tract infection (n = 3), diarrhoea (n = 2), hypoaesthesia (n = 2), oedema (n = 2), oropharyngeal pain (n = 1), pruritus (n = 2), chest pain, antepartum haemorrhage, blindness transient, blood pressure decreased, cholelithiasis, hypothyroidism, influenza like illness, intervertebral disc protrusion, kidney infection, malaise, migraine, oligohydramnios, overdose, pain in extremity, palpitations, parvovirus infection, photopsia, pleural effusion, pollakiuria, polyhydramnios, premature separation of placenta, proteinuria, road traffic accident, sensory disturbance, skin disorder, symphysiolysis, type 1 diabetes mellitus, vaginal discharge, vaginal infection, vasodilatation, vision blurred, visual impairment (all n = 1).
Fetal adverse events (total 5 events)
Growth restriction (n = 2), heart rate deceleration, large for dates baby, hospital admission for fetal growth restriction (all n = 1).
Neonatal adverse events (total 32 events)
Jaundice (n = 4), feeding disorder (n = 3), hypoglycaemia (n = 3), hypothermia (n = 2), maternal condition affecting fetus (n = 2), tachypnoea (n = 2), sepsis (n = 2), shoulder dystocia (n = 2), arrhythmia, C-reactive protein increased, clavicle fracture, dehydration, Erb's palsy, infantile apnoeic attack, lower respiratory tract infection, aspiration, respiratory distress syndrome, pyelocaliectasis, temperature regulation disorder, unresponsive to stimuli (all n = 1).
Placebo
Maternal adverse events (total 73 events)
Fall (n = 8), dizziness (n = 7), abnormal dreams (n = 4), lower respiratory tract infection (n = 3), palpitations (n = 3), proteinuria (n = 3), visual impairment (n = 3), back pain (n = 2), malaise (n = 2), musculoskeletal pain (n = 2), oedema (n = 2), physical assault (n = 2), pruritus (n = 2), syncope (n = 2), vaginal discharge (n = 2), cholestasis of pregnancy, diarrhoea, influenza like illness, maternal condition affecting fetus, Bartholin’s cyst, chlamydia test positive, cholestasis, dysgeusia, dysuria, emotional disorder, feeling abnormal, generalised oedema, ocular hyperaemia, pain in extremity, restless legs syndrome, road traffic accident, hyperhidrosis, injury, intentional self-injury, lethargy, meningitis, thrombophlebitis, toothache, umbilical hernia, unstable fetal lie, vulvovaginal discomfort (all n = 1).
Fetal adverse events (total 5 events)
Tachycardia (n = 2), heart rate decreased, small for dates baby, admission for fetal growth restriction (all n = 1).
Neonatal adverse events (total 29 events)
Jaundice (n = 5), hypoglycaemia (n = 5), feeding disorder (n = 3), grunting (n = 3), anaemia (n = 1), convulsion neonatal (n = 2), infantile apnoeic attack (n = 2), respiratory disorder (n = 2), Erb’s palsy, fever, hypoxia, aspiration, respiratory distress syndrome, shoulder dystocia (all n = 1).
List of abbreviations
- AE
- adverse event
- ASQ-3™
- Ages and Stages Questionnaire®, third edition
- CI
- confidence interval
- CO
- carbon monoxide
- CONSORT
- Consolidated Standards of Reporting Trials
- COT
- salivary cotinine
- CRF
- case report form
- CTA
- Clinical Trial Authorisation
- DMC
- Data Monitoring Committee
- DNA
- deoxyribonucleic acid
- EQ-5D
- European Quality of Life-5 Dimensions
- GCP
- good clinical practice
- GP
- general practitioner
- HPQ
- health professional questionnaire
- ICER
- incremental cost-effectiveness ratio
- ICH
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- IMOR
- informatively missing odds ratio
- ITT
- intention to treat
- LBW
- low birthweight
- MedDRA®
- Medical Dictionary for Regulatory Activities
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NCTU
- Nottingham Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NICU
- neonatal intensive care unit
- NIHR
- National Institute for Health Research
- NRT
- nicotine replacement therapy
- OR
- odds ratio
- p.p.m.
- parts per million
- PCT
- primary care trust
- PIS
- participant information sheet
- PQ2
- 2-year participant questionnaire
- QALY
- quality-adjusted life-year
- QMC
- Queen’s Medical Centre
- RCT
- randomised controlled trial
- RM
- research midwife
- RR
- risk ratio
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SNAP
- Smoking, Nicotine And Pregnancy trial
- SSS
- Stop Smoking Service
- TSC
- Trial Steering Committee